User login
In sight but out of mind
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 44‐year‐old woman was admitted to an Italian hospital with fever and chills that had started approximately 1 week earlier. A few days after onset of fever, she had noticed a red, nonpruritic, confluent, maculopapular rash which began on her face and descended to her body. She also complained of red eyes, photophobia, dyspnea, and watery diarrhea. She denied nausea, vomiting, headache, or neck stiffness. She had seen her primary care physician who had concomitantly prescribed amoxicillin, levofloxacin, and betamethasone. She took the medications for several days without symptomatic improvement.
The salient features of this acute illness include the maculopapular rash, fever, and red eyes with photophobia. The differential diagnosis includes infections, rheumatologic disorders, toxin exposure, and, less likely, hematologic malignancies. In the initial assessment it is crucial to rule out any life‐threatening etiologies of fever and rash such as septicemia from Neisseria meningitidis, bacterial endocarditis, toxic shock syndrome, typhoid fever, and rickettsial diseases. A number of critical components of the history would help narrow the diagnostic considerations, including any history of recent travel, animal or occupational exposure, sexual or medication history, and risk factors for immunosuppression.
The empiric use of antibiotics is indicated when a patient presents with symptoms that suggest life‐threatening illness. For nonemergent conditions, empiric antibiotics may be appropriate when a classic pattern for a given diagnosis is present. In this patient, however, the initial presentation does not appear to be life‐threatening, nor is it easily recognizable as a specific or classic diagnosis. Thus, I would not start antibiotics, because doing so may further disguise the diagnosis by interfering with culture results, or complicate the case by causing an adverse effect such as fever or rash.
One week before the onset of fever she went to the emergency department because of pain in both lower quadrants of her abdomen. The physician removed her intrauterine device (IUD), which appeared to be partially expelled. The patient returned the next day to the emergency department because of severe metrorrhagia.
Complications of IUDs include pelvic inflammatory disease, perforated uterus, myometrial abscess, partial or complete spontaneous abortion, and ectopic pregnancy. Toxic shock syndrome, pelvic inflammatory disease, and retained products from a partial spontaneous abortion can all lead to significant systemic disease and vaginal bleeding.
Her past medical history was unremarkable except for an episode of bacterial meningitis 20 years before. She lived in Florence, Italy, where she worked as a school teacher, and had not traveled outside of Italy in the last year. She was married with 2 children, and denied high‐risk sexual behavior. She did not own any animals.
The patient's lack of travel, high‐risk sexual behavior or animal exposure does not help to alter the differential diagnosis. The prior history of bacterial meningitis raises the question of an immunodeficiency syndrome. At this point, I remain concerned about toxic shock syndrome.
The patient's temperature was 38.2C, her blood pressure was 110/60 mm Hg, respiratory rate was 28 breaths per minute and her heart rate was 108 beats per minute. She was alert and oriented but appeared moderately ill. Her conjunctivae were hyperemic without any drainage, and her oropharynx was erythematous. Lung examination revealed diminished breath sounds in the lower right lung field and crackles bilaterally. Abdominal exam demonstrated mild hepatomegaly, but not splenomegaly. Skin exam showed an erythematous, confluent, maculopapular rash involving her face, torso, back, and extremities; no cutaneous abscesses were noted. Neurological and gynecological exams were both normal, as was the rectal examination.
Her vital signs suggest a progressive illness and possible sepsis. The conjunctival hyperemia could represent several pathologic findings including uveitis with ciliary flush, conjunctival hemorrhage, or hyperemia due to systemic illness. The pulmonary findings could be attributed to pulmonary edema, pneumonia, alveolar hemorrhage, or acute respiratory distress syndrome (ARDS) as a complication of sepsis and systemic inflammation. The hepatomegaly, while non‐specific, may be due to an inflammatory reaction to a systemic illness. If so, I would expect liver tests to be elevated as this can occur in a number of parasitic (eg, toxoplasmosis) and viral (eg, chickenpox, infectious mononucleosis, cytomegalovirus) infections. The lack of concurrent splenomegaly makes lymphoma or other hematologic malignancies less likely. Given the patient's constellation of symptoms, the progressive nature of her illness and the multiple organs involved, I continue to be most concerned about immediately life‐threatening diseases. Toxic shock syndrome secondary to staphylococcal infection can present with many of these signs and symptoms including conjunctival hyperemia, diffuse maculopapular erythema, pharyngitis and sepsis leading to pulmonary edema, pleural effusions and ARDS. Another possibility is leptospirosis, which can be associated with pharyngitis, hepatomegaly, diffuse rash, low‐grade fever, and frequently has conjunctival hyperemia. Moreover, leptospirosis has a markedly variable course and pulmonary hemorrhage and ARDS can occur in severe cases. However, the lack of clear exposure to an environmental source such as contaminated water or soil or animal tissue reduces my enthusiasm for it.
Routine laboratory studies demonstrated: white‐cell count 5210/mm3 (82% neutrophils, 10% lymphocytes, 7% monocytes, and 1% eosinophils); hematocrit 36.3%; platelet count 135,000/mm3; erythrocyte sedimentation rate 49 mm/hour; fibrinogen 591 mg/dL (normal range, 200 ‐ 450 mg/dL); C‐reactive protein 53 mg/L (normal range, <9 mg/L). Serum electrolyte levels were normal. Liver tests demonstrated: aspartate aminotransferase 75 U/L; alanine aminotransferase 135 U/L; total bilirubin within normal limits; gamma glutamyltransferase 86 U/L (normal range, 10‐40 U/L). The urea nitrogen and the creatinine were both normal. The creatine phosphokinase was 381 U/L. Urinalysis was normal. An arterial‐blood gas, obtained while the patient was breathing room air, revealed an oxygen saturation of 87%; pH of 7.45; pCO2 of 38 mm Hg; pO2 of 54 mm Hg; bicarbonate concentration of 27 mmol/L.
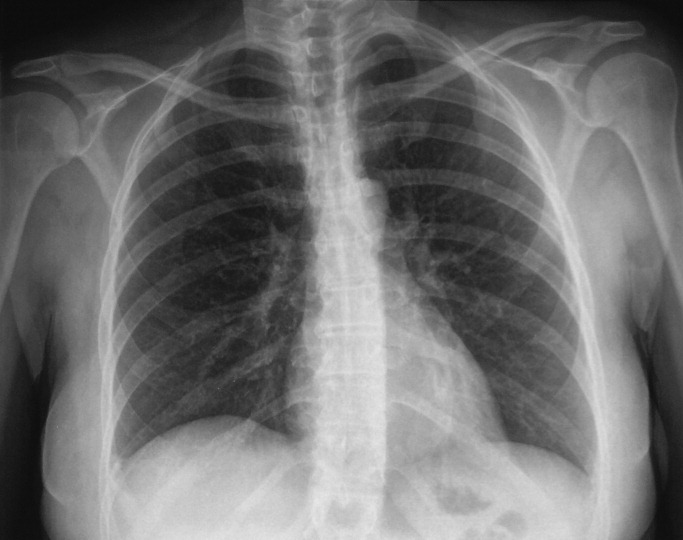
Her electrocardiogram was normal except for sinus tachycardia. Chest film revealed a right‐sided pleural effusion without evidence of parenchymal abnormalities (Figure 1).
Despite the systemic illness, fever, and markedly abnormal inflammatory markers, the white blood cell count remains normal with a slight leftward shift. The most alarming finding is hypoxemia seen on the arterial blood gas. My leading diagnoses for this multisystemic febrile illness with a rash and hypoxia continue to be primarily infectious etiologies, including toxic shock syndrome with Staphylococcus species, leptospirosis, acute cytomegalovirus, and mycobacterial infections. Further diagnostic tests need to be performed but I would begin empiric antibiotics after appropriate cultures have been obtained. Rheumatologic etiologies such as systemic lupus erythematosus (SLE) and sarcoidosis seem less likely. SLE can present with a systemic illness, fever and rash, but the hepatitis, hepatomegaly and hyperemic conjunctivae are less common.
At the time of hospital admission, blood cultures were obtained before azithromycin, meropenem, and vancomycin were initiated for presumed toxic shock syndrome. Transvaginal and abdominal ultrasound studies revealed no abnormalities. She remained febrile but blood cultures returned negative. The results of the following investigations were also negative: immunoglobulin M (IgM) antibodies against Chlamydophila pneumoniae, cytomegalovirus, Epstein‐Barr virus, Legionella pneumophila, parvovirus B19, rubella virus, Coxiella burnetii, Mycoplasma pneumoniae, Chlamydophila psittaci, adenovirus, and coxsackieviruses. Antibodies against human immunodeficiency virus (HIV) 1 and 2 were negative. Tests for hepatitis B (HB surface antigen [HbsAg], HB core antibody [HbcAb] IgM) and C (HCV‐Ab) viruses were negative.
The lack of IgM antibodies for the infections listed markedly reduces their likelihood but does not exclude them. For example, given that the duration of symptoms is nearly 2 weeks at this point, it is possible that IgM has already decreased and IgG titers are now present. The lack of positive cultures does not exclude toxic shock, since in many severe cases the cultures remain negative. Thus, I remain concerned about toxic shock syndrome and would continue broad‐spectrum antibiotics.
After further investigating possible ill contacts to which the patient could have been exposed, it emerged that in the previous weeks there had been a case of measles in the kindergarten where she was working. The patient did not recall her vaccination history.
The recent exposure raises the risk of measles significantly, especially if she was not immunized as a child. Measles typically has an incubation period of 10 to 14 days, thus the prior exposure would fit the time course for the onset of this patient's symptoms. In retrospect, many of this patient's symptoms are classic for measles, including the maculopapular rash that begins on the face and extends downward, the conjunctival hyperemia, the persistent low‐grade fever, and the lack of clinical response to antibiotics.
In adults, measles can be complicated by inflammation in multiple organs resulting in myocarditis, pericarditis, hepatitis, encephalitis, and pneumonia. Thus, elevated transaminases would be consistent with the diagnosis as would a normal abdominal ultrasound. The pneumonia may be due to the measles infection itself or to coexisting viral or bacterial infections. The findings of a mild thrombocytopenia and a low normal leukocyte count can also be seen in measles infections. The diagnosis of measles is based on clinical presentation and by serologic confirmation: IgM antibodies are detectable within 1 or 2 days after the appearance of the rash, whereas the IgG titer rises significantly after 10 days.
I would continue the broad spectrum antibiotics until measles serologies could be confirmed. If the measles serologies are negative, I would continue the evaluation. If the serologies are positive, however, I would continue supportive care and review her pulmonary status to make sure she does not have a secondary bacterial infection. I strongly suspect that she has measles that is complicated by pneumonia and hepatitis.
The IgM antibody against measles virus returned positive and the patient was diagnosed with measles. By hospital day 5, her fever disappeared, her dyspnea resolved, and her rash had receded. Her oxygen saturation was 97% at the time of discharge.
Commentary
Measles is a highly contagious, acute‐onset, exanthematous disease that affects the respiratory tract and mucous membranes. Measles is clinically characterized by a prodromal stage of cough, conjunctivitis, coryza and high fever, typically lasting between 2 and 4 days.1, 2 The pathognomonic finding on the oral mucosa (Koplik spots) is usually followed by a generalized rash. The characteristic rash of measles is erythematous, nonpruritic, and maculopapular beginning at the hairline and behind the ears, and then spreads down the trunk and limbs and may include the palms and soles.1, 2 Often the patient has diarrhea, vomiting, lymphadenopathy, and splenomegaly; however, the clinical presentation can vary.1, 2 In partially immunized patients, symptoms are often atypical, whereas severe cases are characteristically seen in adults with the most frequent complication being pneumonia. About 3% of young adults with measles have a viral pneumonia that requires hospitalization.24 Adults are much more likely than children to develop hepatitis, bronchospasm and bacterial superinfection.2, 3, 5
The introduction of the measles vaccine initially led to a dramatic decrease in the incidence of measles. However, lack of adherence to vaccination campaigns among some families has been followed by small epidemics. Childhood vaccination rates against measles have recently been reported as 88% in Italy, and even higherover 90%in Tuscany. However, Italy has faced an upsurge of measles since September 2007, with almost 60% of cases occurring in the 15‐ to 44‐year‐old age group.6
Classic presentations of common diseases are easily recognized, but in those cases in which the clinical presentation of uncommon illnesseslike measles in adultsis atypical, the epidemiological data and the clinical history play key roles. In this patient, both the discussant and clinical team focused on the most alarming potential diagnosis: toxic shock syndrome related to the use of the IUD. While appropriate, there were historical clues that this patient had measles that were not specifically soughtthe immunization status and the workplace (school) exposure.
This case highlights 2 important aspects of making a difficult clinical diagnosis. First, the patient did not recall her immunization history, and the clinical team did not clarify it, and thus potential childhood illnesses such as measles and rubella did not remain on the differential diagnosis. Assuming that a patient has had the appropriate vaccinations is done at the clinician'sand the patient'speril. Second, many diseases that commonly afflict children can also occur in adult patients, albeit less frequently. Had this patient been a 5‐year‐old child with the same symptoms, the diagnosis would likely have been made with alacrity. However, maculopapular rashes that begin on the face and spread to the body are quite uncommon in adult medicine. For both discussant and the clinical team, the rash was clearly in sight but the correct diagnosis was out of mind given the rarity of this infection in adults. Fortunately, however, once it became clear that the patient was unlikely to have toxic shock syndrome, the epidemiological detail initially left behind became the sentinel clue necessary to solve the case.
Teaching Points
-
After nearly vanishing in the developed world, measles has shown sporadic signs of resurgence in recent years. The disease needs to be considered in patients presenting with a febrile illness accompanied by an exanthem that begins on the head and spreads inferiorly, especially when accompanied by cough, rhinorrhea, and conjunctival changes.
-
Measles tends to cause relatively severe illness and frequent complications in adults, the most common of which is pneumonia.
- .Measles Virus (Rubeola). In: Mandell GL, Bennett JE, Dolin R, eds.Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases.6th ed.Philadelphia, PA:Elsevier Churchill Livingstone;2005:2031–2038.
- ,.The clinical significance of measles: a review.J Infect Dis.2004;189(Suppl 1):S4–S16.
- ,.Measles in the United Kingdom: can we eradicate it by 2010?Br Med J.2006;333:890–895.
- ,,,,,.Familial cases of severe measles pneumonia.Intern Med.2000;39:670–674.
- ,,, et al.Measles encephalitis and acute pancreatitis in a young adult.Am J Med Sci.2004;327:299–303.
- ,,,, et al.Measles resurges in Italy: preliminary data from September 2007 to May 2008.Euro Surveill.2008;13(29):pii=18928.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 44‐year‐old woman was admitted to an Italian hospital with fever and chills that had started approximately 1 week earlier. A few days after onset of fever, she had noticed a red, nonpruritic, confluent, maculopapular rash which began on her face and descended to her body. She also complained of red eyes, photophobia, dyspnea, and watery diarrhea. She denied nausea, vomiting, headache, or neck stiffness. She had seen her primary care physician who had concomitantly prescribed amoxicillin, levofloxacin, and betamethasone. She took the medications for several days without symptomatic improvement.
The salient features of this acute illness include the maculopapular rash, fever, and red eyes with photophobia. The differential diagnosis includes infections, rheumatologic disorders, toxin exposure, and, less likely, hematologic malignancies. In the initial assessment it is crucial to rule out any life‐threatening etiologies of fever and rash such as septicemia from Neisseria meningitidis, bacterial endocarditis, toxic shock syndrome, typhoid fever, and rickettsial diseases. A number of critical components of the history would help narrow the diagnostic considerations, including any history of recent travel, animal or occupational exposure, sexual or medication history, and risk factors for immunosuppression.
The empiric use of antibiotics is indicated when a patient presents with symptoms that suggest life‐threatening illness. For nonemergent conditions, empiric antibiotics may be appropriate when a classic pattern for a given diagnosis is present. In this patient, however, the initial presentation does not appear to be life‐threatening, nor is it easily recognizable as a specific or classic diagnosis. Thus, I would not start antibiotics, because doing so may further disguise the diagnosis by interfering with culture results, or complicate the case by causing an adverse effect such as fever or rash.
One week before the onset of fever she went to the emergency department because of pain in both lower quadrants of her abdomen. The physician removed her intrauterine device (IUD), which appeared to be partially expelled. The patient returned the next day to the emergency department because of severe metrorrhagia.
Complications of IUDs include pelvic inflammatory disease, perforated uterus, myometrial abscess, partial or complete spontaneous abortion, and ectopic pregnancy. Toxic shock syndrome, pelvic inflammatory disease, and retained products from a partial spontaneous abortion can all lead to significant systemic disease and vaginal bleeding.
Her past medical history was unremarkable except for an episode of bacterial meningitis 20 years before. She lived in Florence, Italy, where she worked as a school teacher, and had not traveled outside of Italy in the last year. She was married with 2 children, and denied high‐risk sexual behavior. She did not own any animals.
The patient's lack of travel, high‐risk sexual behavior or animal exposure does not help to alter the differential diagnosis. The prior history of bacterial meningitis raises the question of an immunodeficiency syndrome. At this point, I remain concerned about toxic shock syndrome.
The patient's temperature was 38.2C, her blood pressure was 110/60 mm Hg, respiratory rate was 28 breaths per minute and her heart rate was 108 beats per minute. She was alert and oriented but appeared moderately ill. Her conjunctivae were hyperemic without any drainage, and her oropharynx was erythematous. Lung examination revealed diminished breath sounds in the lower right lung field and crackles bilaterally. Abdominal exam demonstrated mild hepatomegaly, but not splenomegaly. Skin exam showed an erythematous, confluent, maculopapular rash involving her face, torso, back, and extremities; no cutaneous abscesses were noted. Neurological and gynecological exams were both normal, as was the rectal examination.
Her vital signs suggest a progressive illness and possible sepsis. The conjunctival hyperemia could represent several pathologic findings including uveitis with ciliary flush, conjunctival hemorrhage, or hyperemia due to systemic illness. The pulmonary findings could be attributed to pulmonary edema, pneumonia, alveolar hemorrhage, or acute respiratory distress syndrome (ARDS) as a complication of sepsis and systemic inflammation. The hepatomegaly, while non‐specific, may be due to an inflammatory reaction to a systemic illness. If so, I would expect liver tests to be elevated as this can occur in a number of parasitic (eg, toxoplasmosis) and viral (eg, chickenpox, infectious mononucleosis, cytomegalovirus) infections. The lack of concurrent splenomegaly makes lymphoma or other hematologic malignancies less likely. Given the patient's constellation of symptoms, the progressive nature of her illness and the multiple organs involved, I continue to be most concerned about immediately life‐threatening diseases. Toxic shock syndrome secondary to staphylococcal infection can present with many of these signs and symptoms including conjunctival hyperemia, diffuse maculopapular erythema, pharyngitis and sepsis leading to pulmonary edema, pleural effusions and ARDS. Another possibility is leptospirosis, which can be associated with pharyngitis, hepatomegaly, diffuse rash, low‐grade fever, and frequently has conjunctival hyperemia. Moreover, leptospirosis has a markedly variable course and pulmonary hemorrhage and ARDS can occur in severe cases. However, the lack of clear exposure to an environmental source such as contaminated water or soil or animal tissue reduces my enthusiasm for it.
Routine laboratory studies demonstrated: white‐cell count 5210/mm3 (82% neutrophils, 10% lymphocytes, 7% monocytes, and 1% eosinophils); hematocrit 36.3%; platelet count 135,000/mm3; erythrocyte sedimentation rate 49 mm/hour; fibrinogen 591 mg/dL (normal range, 200 ‐ 450 mg/dL); C‐reactive protein 53 mg/L (normal range, <9 mg/L). Serum electrolyte levels were normal. Liver tests demonstrated: aspartate aminotransferase 75 U/L; alanine aminotransferase 135 U/L; total bilirubin within normal limits; gamma glutamyltransferase 86 U/L (normal range, 10‐40 U/L). The urea nitrogen and the creatinine were both normal. The creatine phosphokinase was 381 U/L. Urinalysis was normal. An arterial‐blood gas, obtained while the patient was breathing room air, revealed an oxygen saturation of 87%; pH of 7.45; pCO2 of 38 mm Hg; pO2 of 54 mm Hg; bicarbonate concentration of 27 mmol/L.
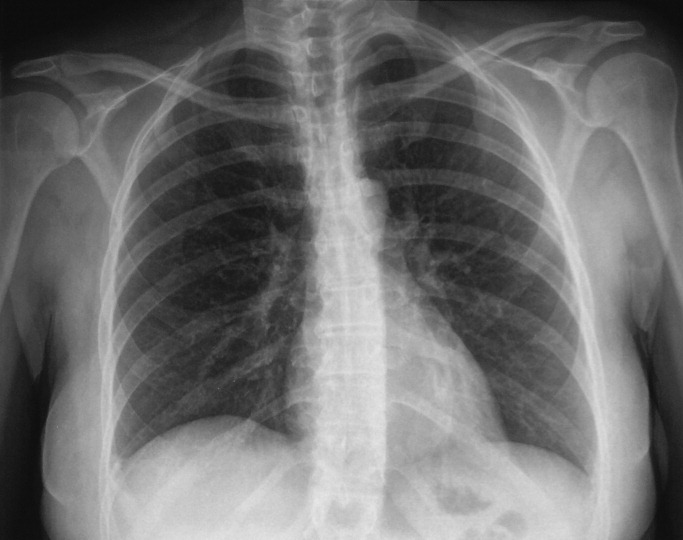
Her electrocardiogram was normal except for sinus tachycardia. Chest film revealed a right‐sided pleural effusion without evidence of parenchymal abnormalities (Figure 1).

Despite the systemic illness, fever, and markedly abnormal inflammatory markers, the white blood cell count remains normal with a slight leftward shift. The most alarming finding is hypoxemia seen on the arterial blood gas. My leading diagnoses for this multisystemic febrile illness with a rash and hypoxia continue to be primarily infectious etiologies, including toxic shock syndrome with Staphylococcus species, leptospirosis, acute cytomegalovirus, and mycobacterial infections. Further diagnostic tests need to be performed but I would begin empiric antibiotics after appropriate cultures have been obtained. Rheumatologic etiologies such as systemic lupus erythematosus (SLE) and sarcoidosis seem less likely. SLE can present with a systemic illness, fever and rash, but the hepatitis, hepatomegaly and hyperemic conjunctivae are less common.
At the time of hospital admission, blood cultures were obtained before azithromycin, meropenem, and vancomycin were initiated for presumed toxic shock syndrome. Transvaginal and abdominal ultrasound studies revealed no abnormalities. She remained febrile but blood cultures returned negative. The results of the following investigations were also negative: immunoglobulin M (IgM) antibodies against Chlamydophila pneumoniae, cytomegalovirus, Epstein‐Barr virus, Legionella pneumophila, parvovirus B19, rubella virus, Coxiella burnetii, Mycoplasma pneumoniae, Chlamydophila psittaci, adenovirus, and coxsackieviruses. Antibodies against human immunodeficiency virus (HIV) 1 and 2 were negative. Tests for hepatitis B (HB surface antigen [HbsAg], HB core antibody [HbcAb] IgM) and C (HCV‐Ab) viruses were negative.
The lack of IgM antibodies for the infections listed markedly reduces their likelihood but does not exclude them. For example, given that the duration of symptoms is nearly 2 weeks at this point, it is possible that IgM has already decreased and IgG titers are now present. The lack of positive cultures does not exclude toxic shock, since in many severe cases the cultures remain negative. Thus, I remain concerned about toxic shock syndrome and would continue broad‐spectrum antibiotics.
After further investigating possible ill contacts to which the patient could have been exposed, it emerged that in the previous weeks there had been a case of measles in the kindergarten where she was working. The patient did not recall her vaccination history.
The recent exposure raises the risk of measles significantly, especially if she was not immunized as a child. Measles typically has an incubation period of 10 to 14 days, thus the prior exposure would fit the time course for the onset of this patient's symptoms. In retrospect, many of this patient's symptoms are classic for measles, including the maculopapular rash that begins on the face and extends downward, the conjunctival hyperemia, the persistent low‐grade fever, and the lack of clinical response to antibiotics.
In adults, measles can be complicated by inflammation in multiple organs resulting in myocarditis, pericarditis, hepatitis, encephalitis, and pneumonia. Thus, elevated transaminases would be consistent with the diagnosis as would a normal abdominal ultrasound. The pneumonia may be due to the measles infection itself or to coexisting viral or bacterial infections. The findings of a mild thrombocytopenia and a low normal leukocyte count can also be seen in measles infections. The diagnosis of measles is based on clinical presentation and by serologic confirmation: IgM antibodies are detectable within 1 or 2 days after the appearance of the rash, whereas the IgG titer rises significantly after 10 days.
I would continue the broad spectrum antibiotics until measles serologies could be confirmed. If the measles serologies are negative, I would continue the evaluation. If the serologies are positive, however, I would continue supportive care and review her pulmonary status to make sure she does not have a secondary bacterial infection. I strongly suspect that she has measles that is complicated by pneumonia and hepatitis.
The IgM antibody against measles virus returned positive and the patient was diagnosed with measles. By hospital day 5, her fever disappeared, her dyspnea resolved, and her rash had receded. Her oxygen saturation was 97% at the time of discharge.
Commentary
Measles is a highly contagious, acute‐onset, exanthematous disease that affects the respiratory tract and mucous membranes. Measles is clinically characterized by a prodromal stage of cough, conjunctivitis, coryza and high fever, typically lasting between 2 and 4 days.1, 2 The pathognomonic finding on the oral mucosa (Koplik spots) is usually followed by a generalized rash. The characteristic rash of measles is erythematous, nonpruritic, and maculopapular beginning at the hairline and behind the ears, and then spreads down the trunk and limbs and may include the palms and soles.1, 2 Often the patient has diarrhea, vomiting, lymphadenopathy, and splenomegaly; however, the clinical presentation can vary.1, 2 In partially immunized patients, symptoms are often atypical, whereas severe cases are characteristically seen in adults with the most frequent complication being pneumonia. About 3% of young adults with measles have a viral pneumonia that requires hospitalization.24 Adults are much more likely than children to develop hepatitis, bronchospasm and bacterial superinfection.2, 3, 5
The introduction of the measles vaccine initially led to a dramatic decrease in the incidence of measles. However, lack of adherence to vaccination campaigns among some families has been followed by small epidemics. Childhood vaccination rates against measles have recently been reported as 88% in Italy, and even higherover 90%in Tuscany. However, Italy has faced an upsurge of measles since September 2007, with almost 60% of cases occurring in the 15‐ to 44‐year‐old age group.6
Classic presentations of common diseases are easily recognized, but in those cases in which the clinical presentation of uncommon illnesseslike measles in adultsis atypical, the epidemiological data and the clinical history play key roles. In this patient, both the discussant and clinical team focused on the most alarming potential diagnosis: toxic shock syndrome related to the use of the IUD. While appropriate, there were historical clues that this patient had measles that were not specifically soughtthe immunization status and the workplace (school) exposure.
This case highlights 2 important aspects of making a difficult clinical diagnosis. First, the patient did not recall her immunization history, and the clinical team did not clarify it, and thus potential childhood illnesses such as measles and rubella did not remain on the differential diagnosis. Assuming that a patient has had the appropriate vaccinations is done at the clinician'sand the patient'speril. Second, many diseases that commonly afflict children can also occur in adult patients, albeit less frequently. Had this patient been a 5‐year‐old child with the same symptoms, the diagnosis would likely have been made with alacrity. However, maculopapular rashes that begin on the face and spread to the body are quite uncommon in adult medicine. For both discussant and the clinical team, the rash was clearly in sight but the correct diagnosis was out of mind given the rarity of this infection in adults. Fortunately, however, once it became clear that the patient was unlikely to have toxic shock syndrome, the epidemiological detail initially left behind became the sentinel clue necessary to solve the case.
Teaching Points
-
After nearly vanishing in the developed world, measles has shown sporadic signs of resurgence in recent years. The disease needs to be considered in patients presenting with a febrile illness accompanied by an exanthem that begins on the head and spreads inferiorly, especially when accompanied by cough, rhinorrhea, and conjunctival changes.
-
Measles tends to cause relatively severe illness and frequent complications in adults, the most common of which is pneumonia.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 44‐year‐old woman was admitted to an Italian hospital with fever and chills that had started approximately 1 week earlier. A few days after onset of fever, she had noticed a red, nonpruritic, confluent, maculopapular rash which began on her face and descended to her body. She also complained of red eyes, photophobia, dyspnea, and watery diarrhea. She denied nausea, vomiting, headache, or neck stiffness. She had seen her primary care physician who had concomitantly prescribed amoxicillin, levofloxacin, and betamethasone. She took the medications for several days without symptomatic improvement.
The salient features of this acute illness include the maculopapular rash, fever, and red eyes with photophobia. The differential diagnosis includes infections, rheumatologic disorders, toxin exposure, and, less likely, hematologic malignancies. In the initial assessment it is crucial to rule out any life‐threatening etiologies of fever and rash such as septicemia from Neisseria meningitidis, bacterial endocarditis, toxic shock syndrome, typhoid fever, and rickettsial diseases. A number of critical components of the history would help narrow the diagnostic considerations, including any history of recent travel, animal or occupational exposure, sexual or medication history, and risk factors for immunosuppression.
The empiric use of antibiotics is indicated when a patient presents with symptoms that suggest life‐threatening illness. For nonemergent conditions, empiric antibiotics may be appropriate when a classic pattern for a given diagnosis is present. In this patient, however, the initial presentation does not appear to be life‐threatening, nor is it easily recognizable as a specific or classic diagnosis. Thus, I would not start antibiotics, because doing so may further disguise the diagnosis by interfering with culture results, or complicate the case by causing an adverse effect such as fever or rash.
One week before the onset of fever she went to the emergency department because of pain in both lower quadrants of her abdomen. The physician removed her intrauterine device (IUD), which appeared to be partially expelled. The patient returned the next day to the emergency department because of severe metrorrhagia.
Complications of IUDs include pelvic inflammatory disease, perforated uterus, myometrial abscess, partial or complete spontaneous abortion, and ectopic pregnancy. Toxic shock syndrome, pelvic inflammatory disease, and retained products from a partial spontaneous abortion can all lead to significant systemic disease and vaginal bleeding.
Her past medical history was unremarkable except for an episode of bacterial meningitis 20 years before. She lived in Florence, Italy, where she worked as a school teacher, and had not traveled outside of Italy in the last year. She was married with 2 children, and denied high‐risk sexual behavior. She did not own any animals.
The patient's lack of travel, high‐risk sexual behavior or animal exposure does not help to alter the differential diagnosis. The prior history of bacterial meningitis raises the question of an immunodeficiency syndrome. At this point, I remain concerned about toxic shock syndrome.
The patient's temperature was 38.2C, her blood pressure was 110/60 mm Hg, respiratory rate was 28 breaths per minute and her heart rate was 108 beats per minute. She was alert and oriented but appeared moderately ill. Her conjunctivae were hyperemic without any drainage, and her oropharynx was erythematous. Lung examination revealed diminished breath sounds in the lower right lung field and crackles bilaterally. Abdominal exam demonstrated mild hepatomegaly, but not splenomegaly. Skin exam showed an erythematous, confluent, maculopapular rash involving her face, torso, back, and extremities; no cutaneous abscesses were noted. Neurological and gynecological exams were both normal, as was the rectal examination.
Her vital signs suggest a progressive illness and possible sepsis. The conjunctival hyperemia could represent several pathologic findings including uveitis with ciliary flush, conjunctival hemorrhage, or hyperemia due to systemic illness. The pulmonary findings could be attributed to pulmonary edema, pneumonia, alveolar hemorrhage, or acute respiratory distress syndrome (ARDS) as a complication of sepsis and systemic inflammation. The hepatomegaly, while non‐specific, may be due to an inflammatory reaction to a systemic illness. If so, I would expect liver tests to be elevated as this can occur in a number of parasitic (eg, toxoplasmosis) and viral (eg, chickenpox, infectious mononucleosis, cytomegalovirus) infections. The lack of concurrent splenomegaly makes lymphoma or other hematologic malignancies less likely. Given the patient's constellation of symptoms, the progressive nature of her illness and the multiple organs involved, I continue to be most concerned about immediately life‐threatening diseases. Toxic shock syndrome secondary to staphylococcal infection can present with many of these signs and symptoms including conjunctival hyperemia, diffuse maculopapular erythema, pharyngitis and sepsis leading to pulmonary edema, pleural effusions and ARDS. Another possibility is leptospirosis, which can be associated with pharyngitis, hepatomegaly, diffuse rash, low‐grade fever, and frequently has conjunctival hyperemia. Moreover, leptospirosis has a markedly variable course and pulmonary hemorrhage and ARDS can occur in severe cases. However, the lack of clear exposure to an environmental source such as contaminated water or soil or animal tissue reduces my enthusiasm for it.
Routine laboratory studies demonstrated: white‐cell count 5210/mm3 (82% neutrophils, 10% lymphocytes, 7% monocytes, and 1% eosinophils); hematocrit 36.3%; platelet count 135,000/mm3; erythrocyte sedimentation rate 49 mm/hour; fibrinogen 591 mg/dL (normal range, 200 ‐ 450 mg/dL); C‐reactive protein 53 mg/L (normal range, <9 mg/L). Serum electrolyte levels were normal. Liver tests demonstrated: aspartate aminotransferase 75 U/L; alanine aminotransferase 135 U/L; total bilirubin within normal limits; gamma glutamyltransferase 86 U/L (normal range, 10‐40 U/L). The urea nitrogen and the creatinine were both normal. The creatine phosphokinase was 381 U/L. Urinalysis was normal. An arterial‐blood gas, obtained while the patient was breathing room air, revealed an oxygen saturation of 87%; pH of 7.45; pCO2 of 38 mm Hg; pO2 of 54 mm Hg; bicarbonate concentration of 27 mmol/L.
Her electrocardiogram was normal except for sinus tachycardia. Chest film revealed a right‐sided pleural effusion without evidence of parenchymal abnormalities (Figure 1).

Despite the systemic illness, fever, and markedly abnormal inflammatory markers, the white blood cell count remains normal with a slight leftward shift. The most alarming finding is hypoxemia seen on the arterial blood gas. My leading diagnoses for this multisystemic febrile illness with a rash and hypoxia continue to be primarily infectious etiologies, including toxic shock syndrome with Staphylococcus species, leptospirosis, acute cytomegalovirus, and mycobacterial infections. Further diagnostic tests need to be performed but I would begin empiric antibiotics after appropriate cultures have been obtained. Rheumatologic etiologies such as systemic lupus erythematosus (SLE) and sarcoidosis seem less likely. SLE can present with a systemic illness, fever and rash, but the hepatitis, hepatomegaly and hyperemic conjunctivae are less common.
At the time of hospital admission, blood cultures were obtained before azithromycin, meropenem, and vancomycin were initiated for presumed toxic shock syndrome. Transvaginal and abdominal ultrasound studies revealed no abnormalities. She remained febrile but blood cultures returned negative. The results of the following investigations were also negative: immunoglobulin M (IgM) antibodies against Chlamydophila pneumoniae, cytomegalovirus, Epstein‐Barr virus, Legionella pneumophila, parvovirus B19, rubella virus, Coxiella burnetii, Mycoplasma pneumoniae, Chlamydophila psittaci, adenovirus, and coxsackieviruses. Antibodies against human immunodeficiency virus (HIV) 1 and 2 were negative. Tests for hepatitis B (HB surface antigen [HbsAg], HB core antibody [HbcAb] IgM) and C (HCV‐Ab) viruses were negative.
The lack of IgM antibodies for the infections listed markedly reduces their likelihood but does not exclude them. For example, given that the duration of symptoms is nearly 2 weeks at this point, it is possible that IgM has already decreased and IgG titers are now present. The lack of positive cultures does not exclude toxic shock, since in many severe cases the cultures remain negative. Thus, I remain concerned about toxic shock syndrome and would continue broad‐spectrum antibiotics.
After further investigating possible ill contacts to which the patient could have been exposed, it emerged that in the previous weeks there had been a case of measles in the kindergarten where she was working. The patient did not recall her vaccination history.
The recent exposure raises the risk of measles significantly, especially if she was not immunized as a child. Measles typically has an incubation period of 10 to 14 days, thus the prior exposure would fit the time course for the onset of this patient's symptoms. In retrospect, many of this patient's symptoms are classic for measles, including the maculopapular rash that begins on the face and extends downward, the conjunctival hyperemia, the persistent low‐grade fever, and the lack of clinical response to antibiotics.
In adults, measles can be complicated by inflammation in multiple organs resulting in myocarditis, pericarditis, hepatitis, encephalitis, and pneumonia. Thus, elevated transaminases would be consistent with the diagnosis as would a normal abdominal ultrasound. The pneumonia may be due to the measles infection itself or to coexisting viral or bacterial infections. The findings of a mild thrombocytopenia and a low normal leukocyte count can also be seen in measles infections. The diagnosis of measles is based on clinical presentation and by serologic confirmation: IgM antibodies are detectable within 1 or 2 days after the appearance of the rash, whereas the IgG titer rises significantly after 10 days.
I would continue the broad spectrum antibiotics until measles serologies could be confirmed. If the measles serologies are negative, I would continue the evaluation. If the serologies are positive, however, I would continue supportive care and review her pulmonary status to make sure she does not have a secondary bacterial infection. I strongly suspect that she has measles that is complicated by pneumonia and hepatitis.
The IgM antibody against measles virus returned positive and the patient was diagnosed with measles. By hospital day 5, her fever disappeared, her dyspnea resolved, and her rash had receded. Her oxygen saturation was 97% at the time of discharge.
Commentary
Measles is a highly contagious, acute‐onset, exanthematous disease that affects the respiratory tract and mucous membranes. Measles is clinically characterized by a prodromal stage of cough, conjunctivitis, coryza and high fever, typically lasting between 2 and 4 days.1, 2 The pathognomonic finding on the oral mucosa (Koplik spots) is usually followed by a generalized rash. The characteristic rash of measles is erythematous, nonpruritic, and maculopapular beginning at the hairline and behind the ears, and then spreads down the trunk and limbs and may include the palms and soles.1, 2 Often the patient has diarrhea, vomiting, lymphadenopathy, and splenomegaly; however, the clinical presentation can vary.1, 2 In partially immunized patients, symptoms are often atypical, whereas severe cases are characteristically seen in adults with the most frequent complication being pneumonia. About 3% of young adults with measles have a viral pneumonia that requires hospitalization.24 Adults are much more likely than children to develop hepatitis, bronchospasm and bacterial superinfection.2, 3, 5
The introduction of the measles vaccine initially led to a dramatic decrease in the incidence of measles. However, lack of adherence to vaccination campaigns among some families has been followed by small epidemics. Childhood vaccination rates against measles have recently been reported as 88% in Italy, and even higherover 90%in Tuscany. However, Italy has faced an upsurge of measles since September 2007, with almost 60% of cases occurring in the 15‐ to 44‐year‐old age group.6
Classic presentations of common diseases are easily recognized, but in those cases in which the clinical presentation of uncommon illnesseslike measles in adultsis atypical, the epidemiological data and the clinical history play key roles. In this patient, both the discussant and clinical team focused on the most alarming potential diagnosis: toxic shock syndrome related to the use of the IUD. While appropriate, there were historical clues that this patient had measles that were not specifically soughtthe immunization status and the workplace (school) exposure.
This case highlights 2 important aspects of making a difficult clinical diagnosis. First, the patient did not recall her immunization history, and the clinical team did not clarify it, and thus potential childhood illnesses such as measles and rubella did not remain on the differential diagnosis. Assuming that a patient has had the appropriate vaccinations is done at the clinician'sand the patient'speril. Second, many diseases that commonly afflict children can also occur in adult patients, albeit less frequently. Had this patient been a 5‐year‐old child with the same symptoms, the diagnosis would likely have been made with alacrity. However, maculopapular rashes that begin on the face and spread to the body are quite uncommon in adult medicine. For both discussant and the clinical team, the rash was clearly in sight but the correct diagnosis was out of mind given the rarity of this infection in adults. Fortunately, however, once it became clear that the patient was unlikely to have toxic shock syndrome, the epidemiological detail initially left behind became the sentinel clue necessary to solve the case.
Teaching Points
-
After nearly vanishing in the developed world, measles has shown sporadic signs of resurgence in recent years. The disease needs to be considered in patients presenting with a febrile illness accompanied by an exanthem that begins on the head and spreads inferiorly, especially when accompanied by cough, rhinorrhea, and conjunctival changes.
-
Measles tends to cause relatively severe illness and frequent complications in adults, the most common of which is pneumonia.
- .Measles Virus (Rubeola). In: Mandell GL, Bennett JE, Dolin R, eds.Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases.6th ed.Philadelphia, PA:Elsevier Churchill Livingstone;2005:2031–2038.
- ,.The clinical significance of measles: a review.J Infect Dis.2004;189(Suppl 1):S4–S16.
- ,.Measles in the United Kingdom: can we eradicate it by 2010?Br Med J.2006;333:890–895.
- ,,,,,.Familial cases of severe measles pneumonia.Intern Med.2000;39:670–674.
- ,,, et al.Measles encephalitis and acute pancreatitis in a young adult.Am J Med Sci.2004;327:299–303.
- ,,,, et al.Measles resurges in Italy: preliminary data from September 2007 to May 2008.Euro Surveill.2008;13(29):pii=18928.
- .Measles Virus (Rubeola). In: Mandell GL, Bennett JE, Dolin R, eds.Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases.6th ed.Philadelphia, PA:Elsevier Churchill Livingstone;2005:2031–2038.
- ,.The clinical significance of measles: a review.J Infect Dis.2004;189(Suppl 1):S4–S16.
- ,.Measles in the United Kingdom: can we eradicate it by 2010?Br Med J.2006;333:890–895.
- ,,,,,.Familial cases of severe measles pneumonia.Intern Med.2000;39:670–674.
- ,,, et al.Measles encephalitis and acute pancreatitis in a young adult.Am J Med Sci.2004;327:299–303.
- ,,,, et al.Measles resurges in Italy: preliminary data from September 2007 to May 2008.Euro Surveill.2008;13(29):pii=18928.