User login
6 skin disorders of pregnancy: A management guide
The dermatoses of pregnancy are a poorly understood group of conditions. Their only common feature is a tendency to appear during pregnancy.
Only three of these conditions are considered unique to pregnancy, however; the others are probably exacerbations of preexisting conditions triggered by pregnancy. There isn’t even complete agreement on what to call them. To make management even more complex, two patients—mother and fetus—need to be considered in decisions about care.
Who manages these patients is another matter. These conditions fall into overlapping areas of health care, where family physicians, obstetricians, and dermatologists all might have some share in responsibility for diagnosis and treatment. You need to be sufficiently familiar with these conditions so that you can differentiate those that can be treated symptomatically and those that require referral to a specialist. This review and the handy TABLE, will help you toward that end.
TABLE
Skin disorders of pregnancy: What you’ll see, how to treat
| Disorder | Lesions | Diagnosis and sequelae | Treatment | Recurrence |
|---|---|---|---|---|
| Pemphigoid gestationis3,5 | Erythematous papules that progress to vesicles and bullae, in a periumbilical distribution that spares the face, palms, and soles |
|
| Frequent; skips a pregnancy 8% of the time |
| Pruritic urticarial papules and plaques of pregnancy8-10 | Urticarial papules and plaques on the abdomen, legs, arms, buttocks, chest, and back |
| Topical corticosteroids and antihistamines | Uncommon |
| Intrahepatic cholestasis of pregnancy14,17,19-22 | No primary lesions; secondary excoriations in any area that the patient can reach |
| Ursodeoxycholic acid, 450-1,200 mg/d | Frequent |
| Eczema of pregnancy/pruritus of pregnancy4,10,24 | Grouped, crusted erythematous papules, patches, and plaques, most often on extensor surfaces of the arms and legs or on the abdomen |
| Symptomatic treatment with topical corticosteroids or antihistamines | Frequent |
| Acute pustular psoriasis of pregnancy26-28 | Erythematous plaques and pustules that start on the inner thighs and groin and spread to the trunk and extremities |
|
| Unknown |
| Pruritic folliculitis of pregnancy24,28 | Papules and pustules concentrated around hair follicles, often beginning on the abdomen and spreading to the extremities |
| Topical corticosteroids | Unknown |
DERMATOSES UNIQUE TO PREGNANCY
1. Pemphigoid gestationis
Years ago, this disorder was referred to as herpes gestationis, because the lesions are herpetiform. Pemphigoid gestationis (PG) has an incidence of approximately 1 in 10,000 pregnancies.1,2 Time of onset is usually about the 21st week of gestation, although, in about 20% of cases, the eruption appears immediately postpartum.3
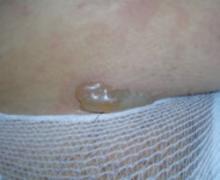
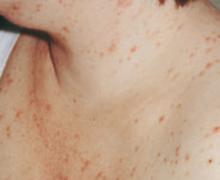
Presentation. The disease usually begins with urticarial papules and plaques around the umbilicus and extremities. Bullous lesions tend to develop as the disease progresses, and are often not present on first presentation (FIGURE 1). Lesions of PG tend to spare the face, palms, and soles. Mucosal surfaces are involved in fewer than 20% of cases. In about 75% of cases, PG flares around the time of delivery, regressing spontaneously after the baby is born.4
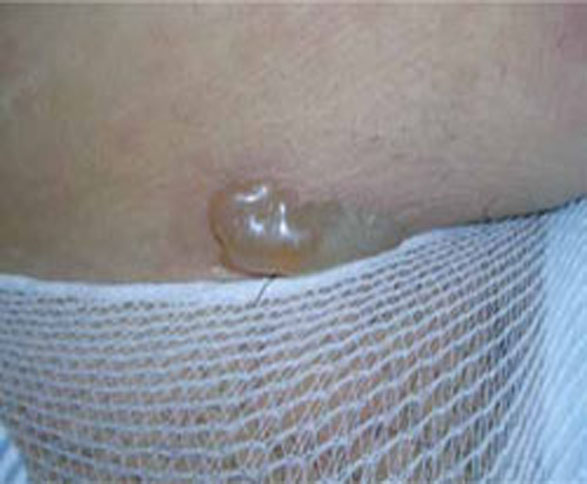
FIGURE 1 Pemphigoid gestationis
As the disease progresses, bullous lesions tend to develop.
Pathophysiology. The pathophysiology of PG is nearly identical to that of bullous pemphigoid, a blistering skin disorder seen more often in elderly patients.5 Pemphigoid disorders are immune processes, involving an immunoglobulin G (IgG) immune response directed at a 180-kDa hemidesmosome transmembrane glycoprotein. This protein is the common target in several subepidermal blistering diseases.
Differential diagnosis. Disorders that may have some of the same features as PG include pruritic urticarial papules and plaques of pregnancy (PUPPP), erythema multiforme, intrahepatic cholestasis of pregnancy (ICP), contact dermatitis, and drug reactions.
Diagnosis. A biopsy is necessary for definitive diagnosis. Direct immunofluorescence (DIF) microscopy of a sample of perilesional skin can show tissue-bound immunoreactants. Linear deposition of the complement component protein C3 along the basement membrane zone is diagnostic for PG. IgG is also deposited about 40% of the time.3
Serum enzyme-linked immunosorbent assay (ELISA) studies are also helpful in diagnosis. They have excellent sensitivity and specificity, as well as the capacity to monitor levels of antibody, which correlate with the severity of disease.1
Treatment. Oral corticosteroids are the first-line treatment for PG, typically 20 to 60 mg/d of prednisone. Oral corticosteroids are generally most effective at ameliorating symptoms. Prednisone at a dosage of 40 to 80 mg/d for a short time has not been associated with congenital abnormalities.6 PG patients can also be treated successfully with intravenous immunoglobulin (IVIG) and cyclosporine in refractory cases.7
Pruritus associated with this condition can interfere with day-to-day activities and with the patient’s ability to sleep. Patients may also complain that the rash is painful, particularly if bullae rupture, leading to superficial ulcerations. Fortunately, the patient’s quality of life can be dramatically improved with systemic corticosteroids—with no significant risk to the fetus.
Sequelae. PG uniformly resolves within a few weeks, but the mother’s autoantibodies can be passively transferred to the fetus, causing vesicles and bullae in the newborn.8 An increased incidence of small-for-gestational age (SGA) infants has also been noted in PG, although no lasting morbidity or mortality in the offspring has been noted.5 The disease tends to recur in future pregnancies.
2. Pruritic urticarial papules and plaques of pregnancy
This condition is known by many names besides its acronym PUPPP: polymorphic eruption of pregnancy, toxemic erythema of pregnancy, and late prurigo of pregnancy.1 It is a pruritic, inflammatory skin disorder that has been variously estimated to occur in anywhere from 1 in 120 to 1 in 240 pregnancies.8 PUPPP is second only to eczema as the most common dermatosis of pregnancy.
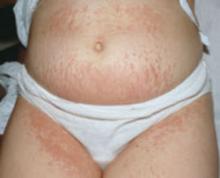
Presentation. As the name suggests, the lesions of PUPPP are itchy, red papules that often coalesce into plaques (FIGURE 2). Lesions usually occur in primigravidas after the 34th week of gestation, although they may be seen at any time from the first trimester through the postpartum period.9
Lesions are classically found on the abdomen, sparing the umbilical area, and are found primarily in the striae. This distribution helps you to differentiate PUPPP from PG, in which lesions typically cluster around the umbilicus. Most PUPPP lesions (80% in one study) are dispersed on the abdomen, legs, arms, buttocks, chest, and back. Another 17% appear only on the abdomen and proximal thighs, and the remaining 3% on the limbs.10 Nearly 50% of the time, lesions also include discrete vesicles.11 There are no reported cases of mucosal involvement.
Patients with this condition are often very uncomfortable. The associated pruritus is severe enough to interfere with sleep. Despite the itching, however, lesions are seldom excoriated.

FIGURE 2 Pruritic urticarial papules and plaques of pregnancy
The itchy, red papules of PUPP often coalesce into plaques.
Pathophysiology. The disorder has been strongly associated with maternal weight gain and multiple gestations. One working hypothesis is that rapid abdominal distention observed in the third trimester leads to damage of the connective tissue, which then releases antigenic molecules, causing an inflammatory reaction.12 Another hypothesis is that increased levels of fetal DNA that have been detected in the skin of PUPPP patients may contribute to the pathology. One study detected male DNA in six of 10 PUPPP sufferers, but found none in any of 26 controls—pregnant women without PUPPP pathology.5 There is some evidence that patients with atopy may be predisposed to PUPPP, as well as patients who are hypertensive or obese.10,13
Differential diagnosis. Initially, PUPPP lesions can be difficult to differentiate from urticarial PG lesions. The distribution of the lesions is the best clue: PG lesions cluster around the umbilicus, whereas PUPPP lesions uniformly spare the umbilical area. Additional disorders in the PUPPP differential are atopic dermatitis, superficial urticarial allergic eruption, viral exanthema, and contact or irritant dermatitis.
Diagnosis. PUPPP can be diagnosed only by clinical observation. None of the available laboratory tests—immunofluorescence, histology, serology—yield findings specific for PUPPP, although histology and immunofluorescence can readily differentiate between this condition and PG.
Treatment. Because the disease holds no real danger for mother or fetus, treatment can be aimed solely at symptomatic relief. Mild-to-potent topical corticosteroids (consider triamcinolone or fluocinonide) should relieve pruritus within 48 to 72 hours.8 Antihistamines and, occasionally, low-dose systemic corticosteroids may also be used. Consider hydroxyzine, although diphenhydramine has the more proven safety profile in pregnancy.
Nonpharmaceutical treatments such as oil baths and emollients should also be considered. If the condition appears classic for PUPPP, it can be managed symptomatically. If there is any question about the diagnosis, however, referral to a dermatologist is prudent.
Sequelae. No increase in maternal or fetal morbidity or mortality is associated with PUPPP. Recurrence is fairly uncommon, as the disease primarily affects women during their first pregnancy.
3. Intrahepatic cholestasis of pregnancy
This condition is also called recurrent or idiopathic jaundice of pregnancy, obstetric cholestasis, and pruritus gravidarum. Intrahepatic cholestasis of pregnancy (ICP) is caused by disruption of hepatic bile flow during pregnancy. It has been recorded at a rate of approximately 10 to 150 of every 10,000 pregnancies in Europe and 70 of every 10,000 in the United States.12 In 80% of patients, time of onset is after the 30th week.14
Although this disorder is not primarily a dermatosis of pregnancy, it is a pruritic condition that often presents with excoriations in pregnant women and is associated with fetal morbidity and mortality. It’s important to be able to identify this disease early to minimize sequelae.
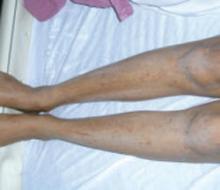
Presentation. There are no primary lesions with ICP. The primary presenting symptom is a generalized pruritus affecting the palms and soles, and sometimes extending to the legs and abdomen (FIGURE 3). This itching is often so severe that it leads to chronic insomnia. You may see secondary skin lesions, such as erythema and excoriations. Observable jaundice occurs in 10% to 20% of patients.3 These patients do not develop the encephalopathy that is associated with cholestasis in the nonpregnant state, however.14
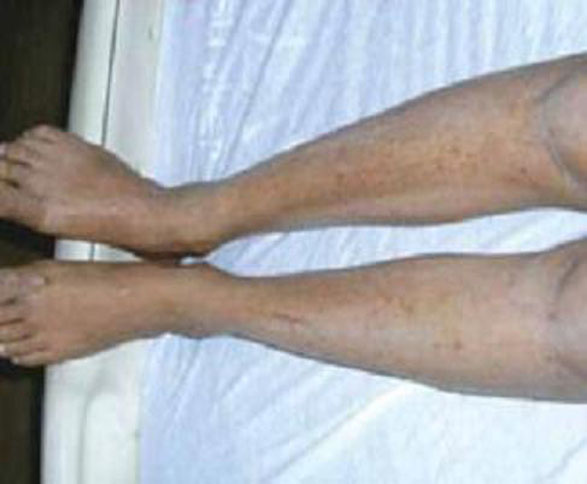
FIGURE 3 Intrahepatic cholestasis of pregnancy
ICP lacks primary lesions. Shown here are the secondary erythema and excoriations that results from scratching the intense pruritis.
Pathophysiology. The genesis of this condition is thought to be a combination of genetic and environmental factors. A family history of the disorder is present in one half of cases; cases with a familial component tend to be more severe.15 ICP may be an exaggerated response to increased estrogen levels in pregnancy, but the mechanism of this response is unknown.16
Differential diagnosis. Other conditions that must be considered in making the diagnosis are viral hepatitis, gallbladder disease, PG, PUPPP, drug hepatotoxicity, primary biliary cirrhosis, and uremia.
Diagnosis. Laboratory values are the definitive diagnostic tool in this condition. Increased levels of serum bile acids are the single most sensitive test. Average levels of serum bile acids in pregnancy are 6.6 µmol/L, with an upper limit of 11 µmol/L. The average value in women who have ICP is 47 µmol/L.17
Although serum bile acids remain the gold standard, a recent study showed that elevated urine bile acids have 100% sensitivity and 83% specificity for ICP.18 In 55% to 60% of cases, the liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are mildly increased. Steatorrhea is often noted by the patient, and is followed by vitamin K deficiency.17
Treatment. The current standard of care for ICP is treatment with ursodeoxycholic acid (UDCA). In four controlled trials, UDCA caused a sustained decrease in serum bile acids.19-22 The dosage used in these trials ranged from 450 to 1,200 mg/d.
Before UDCA treatment was available, ICP was treated with cholestyramine, which could bring about a 70% rate of response. The drawback to cholestyramine treatment is that it precipitates vitamin K, which is already compromised by the disease process. Further, the onset of action of cholestyramine is slow.3
Elective delivery is indicated for ICP, particularly in patients who have a significant clinical presentation.12 Delivery for ICP should be performed around weeks 37 to 38, as stillbirths tend to cluster around weeks 37 to 39.14 Given the significant fetal mortality associated with ICP (see the next paragraph), the condition should be managed by a clinician experienced with the disease—likely, a gastroenterologist.
Sequelae. The impact of this maternal disorder on the fetus can be disastrous: a 10% to 15% rate of perinatal death, and a 30% to 40% rate of premature labor.14 Fortunately, the rate of preterm labor correlates strongly with the level of bile acids, so that as bile acid levels are reduced with UDCA treatment, the rate of preterm labor also falls. Management of ICP has reduced the rate of perinatal death to 3.5%. No evidence of fetal growth retardation has been noted.14
DERMATOSES TRIGGERED BY PREGNANCY
4. Eczema of pregnancy/prurigo of pregnancy
Eczema of pregnancy/prurigo of pregnancy (EP/PP) may not actually be correlated with the pregnant state. Both conditions manifest as eczematous lesions in an atopic distribution. Although they have been described in the literature as separate entities, the lack of clinical distinction between them led Ambros-Rudolph and colleagues to combine them under the umbrella term, atopic eruption of pregnancy.23
In some patients, at least, EP/PP may be preexisting conditions that are exacerbated by pregnancy. One study of 255 patients with the condition found that 20% had had the lesions before they became pregnant.23 The tendency of the condition to be made markedly worse by pregnancy, however, leads us to include it here.
PP has an estimated incidence of 1 in 450 pregnancies.11 Although many authorities consider EP to be the most common dermatosis of pregnancy, no clear estimation of its prevalence has been established.23,24 Taken together, the two conditions have the highest prevalence of all pregnancy-induced dermatoses.
PP is also known as popular dermatitis of Spangler, Nurse’s early prurigo of pregnancy, and linear IgM disease of pregnancy.3,4,23
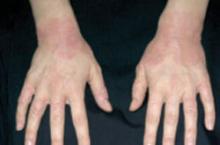
Presentation. The typical presentation is grouped, crusted, erythematous papules, patches, and plaques—frequently with excoriations. Lesions typically present on the extensor surfaces of the arms and legs or on the abdomen (FIGURE 4).4 Recurrence in later pregnancies is common.
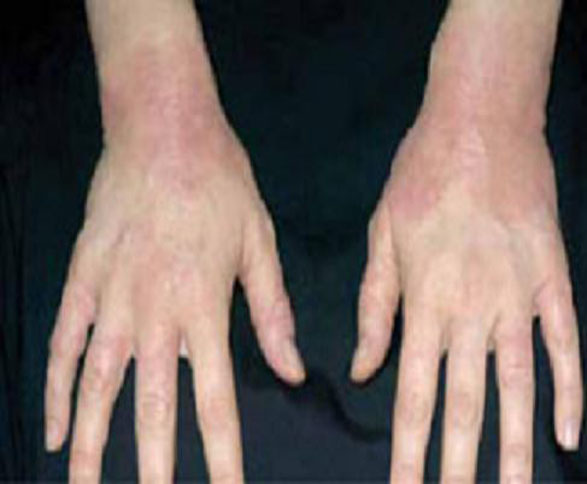
FIGURE 4 Eczema of pregnancy
EP/PP typically manifests as grouped, crusted, erythematous papules, patches, and plaques, often with excoriations.
Pathophysiology. The pathophysiology of EP/PP is not understood. Many patients who develop EP/PP have a history of atopy.10
Differential diagnosis. Conditions that need to be considered in making the diagnosis include Tinea infection, scabies, contact dermatitis, ICP, pruritic folliculitis of pregnancy (PFP), and PG.
Diagnosis. The history and the physical examination determine the diagnosis. Serology, histopathology, and immunofluorescence are nonspecific. Correlation of EP/PP with increased IgE is marginal, at best.24,25
Treatment. These conditions are treated symptomatically with topical corticosteroids or systemic antihistamines.
Sequelae. No increase in maternal or fetal morbidity or mortality is associated with EP/PP.
5. Acute pustular psoriasis of pregnancy
Whether or not acute pustular psoriasis of pregnancy (APPP) is actually a pregnancy-induced dermatosis is subject to debate.
There is evidence that APPP is not unique to pregnancy but simply a manifestation of ordinary psoriasis. Clinically and histologically, APPP is indistinguishable from pustular psoriasis. Unlike most cases of acute psoriasis, however, APPP often appears in pregnancy without any personal or family history of psoriasis and usually ceases when the pregnancy ends. This fact, combined with reports of increased fetal and maternal morbidity and mortality associated with APPP, leads us to include it here.26
Presentation. APPP is a rare condition that may have an onset at any point in pregnancy. Characteristic lesions begin as erythematous plaques with pustules on the inner thighs, flexural areas, and groin and spread to the trunk and extremities. As plaques enlarge, their center becomes eroded and crusted.
The nails may become onycholytic. Hands, feet, and face are usually spared. Oral and esophageal erosions can occur. Pruritus is typically mild, although the lesions are often painful. Flu-like symptoms are often present.27
Pathophysiology. The pathophysiology of APPP is unknown.
Differential diagnosis. Conditions with similar presentations include an adverse drug reaction, pityriasis rosea, lichen simplex chronicus, eczema, lupus, and pityriasis rubra pilaris.
Diagnosis. The clinical history and an association with systemic illness are the basis for a diagnosis of APPP. Cultures of pustules are negative for any infective pathology, although, as the disease progresses, pustules may become superinfected. Laboratory testing may show an increased erythrocyte sedimentation rate, hypocalcemia, and a low level of vitamin D.
Treatment. Prednisone, 15 to 60 mg/d, is often sufficient to control the disease.27 Cyclosporine, 100 mg twice daily, has also been shown to be useful.28 Cyclosporine in pregnancy is a Category C drug. Data on fetal malformation associated with cyclosporine therapy are limited, but risk appears minimal.6
Maternal hypocalcemia should be monitored and treated appropriately. If disease progression is judged serious enough, early induction of labor is indicated, because delivery will almost always lead to swift resolution.
Sequelae. A number of case reports link APPP to serious sequelae, including fetal growth retardation, hypocalcemia, and stillbirth.27,29,30 The condition is too rare, however, for good data on specific sequelae. Although APPP does give significant cause for concern, it appears that some of the traditional apprehension comes from older publications reporting a rate of maternal mortality of 70% to 90%.31 This statistic has not been borne out in practice. It does appear that the mother will frequently suffer systemic symptoms, including fever and malaise.
6. Pruritic folliculitis of pregnancy
Accounts of the prevalence of pruritic folliculitis of pregnancy (PFP) vary widely. Some sources report fewer than 30 cases in all of the literature; others indicate that the prevalence is equivalent to that of PG—one in every 10,000 pregnancies.3,11 PFP most often presents in the third trimester. It often resolves before delivery, but uniformly clears within 2 weeks of delivery.
Presentation. PFP presents as papules and pustules concentrated around hair follicles (FIGURE 5). Often, lesions begin on the abdomen and spread to the extremities.24,28 The condition is often, but not always, pruritic. Patients are more likely to be concerned about what the condition means for their health than distressed by the symptoms.
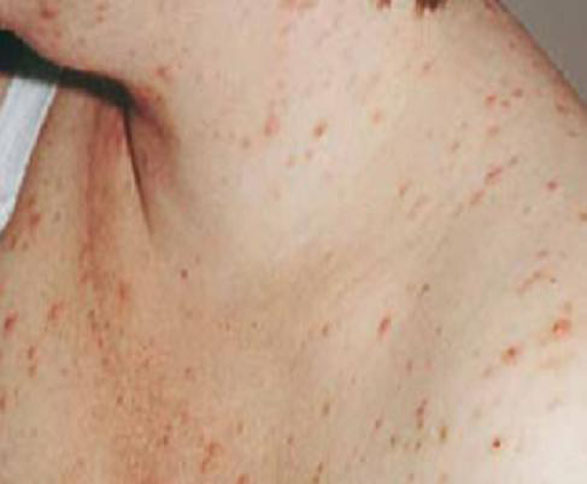
FIGURE 5 Pruritic folliculitis of pregnancy
The papules and pustules of PFP are concentrated around hair follicles.
Pathophysiology. Like many other dermatoses of pregnancy, the pathophysiology of PFP is unknown. There is little evidence that the condition is immunologically or hormonally mediated, and there is no evidence of an infectious component.24,28
Differential diagnosis. PFP must be distinguished from infectious folliculitis, acneiform disorders, HIV-associated eosinophilic folliculitis, and a drug reaction.
Diagnosis. The clinical diagnosis is based on presenting symptoms and third-trimester onset. No specific laboratory or histologic analysis can be used to make a definitive diagnosis.
Treatment. As the condition is, by definition, a nonmicrobial folliculitis, the most effective therapy tends to be with a low- or midpotency topical corticosteroid, such as triamcinolone or desonide. A benzoyl peroxide wash can also be effective.
Sequelae. One study reports an increased incidence of low birth weight, but no associated morbidity or mortality has been reported in recent studies.24
- Pemphigoid gestationis is best managed with oral prednisone at doses from 20 to 60 mg per day to control symptoms
- The pruritus associated with pruritic urticarial papules and plaques of pregnancy can be safely and effectively managed with topical corticosteroids and oral antihistamines
- Treat intrahepatic cholestasis of pregnancy with ursodeoxycholic acid, which likely reduces serum bile acids as well as associated fetal morbidity and mortality
1. Sitaru C, Powell J, Messer G, Bröcker EB, Wojnarowska F, Zillikens D. Immunoblotting and enzyme linked immunosorbent assay for the diagnosis of pemphigoid gestationis. Obstet Gynecol. 2004;103(4):757-763.
2. Engineer L, Bohl K, Ahmed AR. Pemphigoid gestationis: a review. Am J Obstet Gynecol. 2000;183(2):483-491.
3. Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence-based systematic review. Am J Obstet Gynecol. 2003;188(4):1083-1092.
4. Shornick JK. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17(3):172-181.
5. Shornick JK, Bangert JL, Freeman RG, Gilliam JN. Herpes gestationis: clinical and histological features in 28 cases. J Am Acad Dermatol. 1983;8(2):214-224.
6. Leachman SA, Reed BR. The use of dermatologic drugs in pregnancy and lactation. Dermatol Clin. 2006;24(2):167-197, vi.
7. Hern S, Harman K, Bhogal BS, Black MM. A severe persistent case of pemphigoid gestationis treated with intravenous immunoglobulins and cyclosporine. Clin Exp Dermatol. 1998;23(4):185-188.
8. Fitzpatrick TP. Diseases in pregnancy. Color Atlas and Synopsis of Clinical Dermatology. New York, NY: McGraw Hill; 1997:414–419.
9. Aractingi D, Berkane N, Bertheau P, et al. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352(9144):1898-1901.
10. Rudolph CM, Al-Fares S, Vaughan-Jones SA, et al. Polymorphic eruption of pregnancy: clinicopathology and potential trigger factors in 181 patients. Br J Dermatol. 2006;154(1):54-60.
11. Roger D, Vaillant L, Fignon A, et al. Specific pruritic diseases of pregnancy. A prospective study of 3192 pregnant women. Arch Dermatol. 1994;130(6):734-739.
12. McDonald JA. Cholestasis of pregnancy. J Gastroenterol Hepatol. 1999;14(6):515-518.
13. Ohel I, Levy A, Silberstein T, Holcberg G, Sheiner E. Pregnancy outcome of patients with pruritic urticarial papules and plaques of pregnancy. J Maternal Fetal Neonat Med. 2006;19(5):305-308.
14. Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15(17):2049-2066.
15. Shaw D, Frohlich J, Wittmann BA, Willms M. A prospective study of 18 patients with cholestasis of pregnancy. Am J Obstet Gynecol. 1982;142(6 Pt 1):621-625.
16. Reyes H. The spectrum of liver and gastrointestinal disease seen in cholestasis of pregnancy. Gastroenterol Clin North Am. 1992;21(4):905-921.
17. Lammert F, Marschall H, Glantz A, Matern S. Intrahepatic cholestasis of pregnancy; molecular pathogenesis, diagnosis and management. J Hepatol. 2000;33(6):1012-1021.
18. Huang WM, Seubert DE, Donnelly JG, Liu M, Javitt NB. Intrahepatic cholestasis of pregnancy: detection with urinary bile acid assays. J Perinat Med. 2007;35(6):486-491.
19. Palma J, Reyes H, Ribalta J, et al. Ursodeoxycholic acid in the treatment of intrahepatic cholestasis of pregnancy: a randomized, double-blind study controlled with placebo. J Hepatol. 1997;27(6):1022-1028.
20. Diaferia A, Nicastri PL, Tartagni M, et al. Ursodeoxycholic acid therapy in pregnant women with cholestasis. Int J Gynaecol Obstet. 1996;52:133-140.
21. Nicastri PL, Diaferia A, Tartagni M, Loizzi P, Fanelli M. A randomised placebo-controlled trial of ursodeoxycholic acid and S-adenosylmethionine in the treatment of intrahepatic cholestasis of pregnancy. Br J Obstet Gynaecol. 1998;105(11):1205-1207.
22. Glantz A, Marschall HU, Lammert F, Mattsson LA. Intrahepatic cholestasis in pregnancy: a randomized controlled trial comparing dexamethasone and ursodeoxycholic acid. Hepatology. 2005;42(6):1399-1405.
23. Ambros-Rudolph C, Müllegger M, Vaughan-Jones S, et al. The specific dermatoses of pregnancy revisited and reclassified: results of a retrospective two-center study on 505 pregnant patients. J Am Acad Dermatol. 2006;54(3):395-404.
24. Vaughan Jones SA, Hern S, Nelson-Piercy C, Seed PT, Black MM. A prospective study of 200 women with dermatoses of pregnancy correlating clinical findings with hormonal and immunopathological profiles. Br J Dermatol. 1999;141(1):71-81.
25. Holmes RC, Black MM. The specific dermatoses of pregnancy. J Am Acad Dermatol. 1983;8(3):405-412.
26. Bukhari IA. Impetigo herpetiformis in a primagravida: successful treatment with etretinate. J Drugs Dermatol. 2004;3(4):449-451.
27. Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24(2):101-104.
28. Kroumpouzos G, Cohen LM. Pruritic folliculitis of pregnancy. J Am Acad Dermatol. 2000;43(1 Pt 1):132-134.
29. Sahin HG, Sahin HA, Metin A, Zeteroglu S, Ugras S. Recurrent impetigo herpetiformis in a pregnant adolescent: case report. Eur J Obstet Gynecol Reprod Biol. 2002;101:201-203.
30. Aka N, Kuscu NK, Yazicioglu E. Impetigo herpetiformis at the 36th week of gestation. Int J Gynecol Obstet. 2000;69(2):153-154.
31. Wade T, Wade S, Jones H. Skin changes and diseases associated with pregnancy. Obstet Gynecol. 1978;52(2):233-242.
The dermatoses of pregnancy are a poorly understood group of conditions. Their only common feature is a tendency to appear during pregnancy.
Only three of these conditions are considered unique to pregnancy, however; the others are probably exacerbations of preexisting conditions triggered by pregnancy. There isn’t even complete agreement on what to call them. To make management even more complex, two patients—mother and fetus—need to be considered in decisions about care.
Who manages these patients is another matter. These conditions fall into overlapping areas of health care, where family physicians, obstetricians, and dermatologists all might have some share in responsibility for diagnosis and treatment. You need to be sufficiently familiar with these conditions so that you can differentiate those that can be treated symptomatically and those that require referral to a specialist. This review and the handy TABLE, will help you toward that end.
TABLE
Skin disorders of pregnancy: What you’ll see, how to treat
| Disorder | Lesions | Diagnosis and sequelae | Treatment | Recurrence |
|---|---|---|---|---|
| Pemphigoid gestationis3,5 | Erythematous papules that progress to vesicles and bullae, in a periumbilical distribution that spares the face, palms, and soles |
|
| Frequent; skips a pregnancy 8% of the time |
| Pruritic urticarial papules and plaques of pregnancy8-10 | Urticarial papules and plaques on the abdomen, legs, arms, buttocks, chest, and back |
| Topical corticosteroids and antihistamines | Uncommon |
| Intrahepatic cholestasis of pregnancy14,17,19-22 | No primary lesions; secondary excoriations in any area that the patient can reach |
| Ursodeoxycholic acid, 450-1,200 mg/d | Frequent |
| Eczema of pregnancy/pruritus of pregnancy4,10,24 | Grouped, crusted erythematous papules, patches, and plaques, most often on extensor surfaces of the arms and legs or on the abdomen |
| Symptomatic treatment with topical corticosteroids or antihistamines | Frequent |
| Acute pustular psoriasis of pregnancy26-28 | Erythematous plaques and pustules that start on the inner thighs and groin and spread to the trunk and extremities |
|
| Unknown |
| Pruritic folliculitis of pregnancy24,28 | Papules and pustules concentrated around hair follicles, often beginning on the abdomen and spreading to the extremities |
| Topical corticosteroids | Unknown |
DERMATOSES UNIQUE TO PREGNANCY
1. Pemphigoid gestationis
Years ago, this disorder was referred to as herpes gestationis, because the lesions are herpetiform. Pemphigoid gestationis (PG) has an incidence of approximately 1 in 10,000 pregnancies.1,2 Time of onset is usually about the 21st week of gestation, although, in about 20% of cases, the eruption appears immediately postpartum.3
Presentation. The disease usually begins with urticarial papules and plaques around the umbilicus and extremities. Bullous lesions tend to develop as the disease progresses, and are often not present on first presentation (FIGURE 1). Lesions of PG tend to spare the face, palms, and soles. Mucosal surfaces are involved in fewer than 20% of cases. In about 75% of cases, PG flares around the time of delivery, regressing spontaneously after the baby is born.4

FIGURE 1 Pemphigoid gestationis
As the disease progresses, bullous lesions tend to develop.
Pathophysiology. The pathophysiology of PG is nearly identical to that of bullous pemphigoid, a blistering skin disorder seen more often in elderly patients.5 Pemphigoid disorders are immune processes, involving an immunoglobulin G (IgG) immune response directed at a 180-kDa hemidesmosome transmembrane glycoprotein. This protein is the common target in several subepidermal blistering diseases.
Differential diagnosis. Disorders that may have some of the same features as PG include pruritic urticarial papules and plaques of pregnancy (PUPPP), erythema multiforme, intrahepatic cholestasis of pregnancy (ICP), contact dermatitis, and drug reactions.
Diagnosis. A biopsy is necessary for definitive diagnosis. Direct immunofluorescence (DIF) microscopy of a sample of perilesional skin can show tissue-bound immunoreactants. Linear deposition of the complement component protein C3 along the basement membrane zone is diagnostic for PG. IgG is also deposited about 40% of the time.3
Serum enzyme-linked immunosorbent assay (ELISA) studies are also helpful in diagnosis. They have excellent sensitivity and specificity, as well as the capacity to monitor levels of antibody, which correlate with the severity of disease.1
Treatment. Oral corticosteroids are the first-line treatment for PG, typically 20 to 60 mg/d of prednisone. Oral corticosteroids are generally most effective at ameliorating symptoms. Prednisone at a dosage of 40 to 80 mg/d for a short time has not been associated with congenital abnormalities.6 PG patients can also be treated successfully with intravenous immunoglobulin (IVIG) and cyclosporine in refractory cases.7
Pruritus associated with this condition can interfere with day-to-day activities and with the patient’s ability to sleep. Patients may also complain that the rash is painful, particularly if bullae rupture, leading to superficial ulcerations. Fortunately, the patient’s quality of life can be dramatically improved with systemic corticosteroids—with no significant risk to the fetus.
Sequelae. PG uniformly resolves within a few weeks, but the mother’s autoantibodies can be passively transferred to the fetus, causing vesicles and bullae in the newborn.8 An increased incidence of small-for-gestational age (SGA) infants has also been noted in PG, although no lasting morbidity or mortality in the offspring has been noted.5 The disease tends to recur in future pregnancies.
2. Pruritic urticarial papules and plaques of pregnancy
This condition is known by many names besides its acronym PUPPP: polymorphic eruption of pregnancy, toxemic erythema of pregnancy, and late prurigo of pregnancy.1 It is a pruritic, inflammatory skin disorder that has been variously estimated to occur in anywhere from 1 in 120 to 1 in 240 pregnancies.8 PUPPP is second only to eczema as the most common dermatosis of pregnancy.
Presentation. As the name suggests, the lesions of PUPPP are itchy, red papules that often coalesce into plaques (FIGURE 2). Lesions usually occur in primigravidas after the 34th week of gestation, although they may be seen at any time from the first trimester through the postpartum period.9
Lesions are classically found on the abdomen, sparing the umbilical area, and are found primarily in the striae. This distribution helps you to differentiate PUPPP from PG, in which lesions typically cluster around the umbilicus. Most PUPPP lesions (80% in one study) are dispersed on the abdomen, legs, arms, buttocks, chest, and back. Another 17% appear only on the abdomen and proximal thighs, and the remaining 3% on the limbs.10 Nearly 50% of the time, lesions also include discrete vesicles.11 There are no reported cases of mucosal involvement.
Patients with this condition are often very uncomfortable. The associated pruritus is severe enough to interfere with sleep. Despite the itching, however, lesions are seldom excoriated.

FIGURE 2 Pruritic urticarial papules and plaques of pregnancy
The itchy, red papules of PUPP often coalesce into plaques.
Pathophysiology. The disorder has been strongly associated with maternal weight gain and multiple gestations. One working hypothesis is that rapid abdominal distention observed in the third trimester leads to damage of the connective tissue, which then releases antigenic molecules, causing an inflammatory reaction.12 Another hypothesis is that increased levels of fetal DNA that have been detected in the skin of PUPPP patients may contribute to the pathology. One study detected male DNA in six of 10 PUPPP sufferers, but found none in any of 26 controls—pregnant women without PUPPP pathology.5 There is some evidence that patients with atopy may be predisposed to PUPPP, as well as patients who are hypertensive or obese.10,13
Differential diagnosis. Initially, PUPPP lesions can be difficult to differentiate from urticarial PG lesions. The distribution of the lesions is the best clue: PG lesions cluster around the umbilicus, whereas PUPPP lesions uniformly spare the umbilical area. Additional disorders in the PUPPP differential are atopic dermatitis, superficial urticarial allergic eruption, viral exanthema, and contact or irritant dermatitis.
Diagnosis. PUPPP can be diagnosed only by clinical observation. None of the available laboratory tests—immunofluorescence, histology, serology—yield findings specific for PUPPP, although histology and immunofluorescence can readily differentiate between this condition and PG.
Treatment. Because the disease holds no real danger for mother or fetus, treatment can be aimed solely at symptomatic relief. Mild-to-potent topical corticosteroids (consider triamcinolone or fluocinonide) should relieve pruritus within 48 to 72 hours.8 Antihistamines and, occasionally, low-dose systemic corticosteroids may also be used. Consider hydroxyzine, although diphenhydramine has the more proven safety profile in pregnancy.
Nonpharmaceutical treatments such as oil baths and emollients should also be considered. If the condition appears classic for PUPPP, it can be managed symptomatically. If there is any question about the diagnosis, however, referral to a dermatologist is prudent.
Sequelae. No increase in maternal or fetal morbidity or mortality is associated with PUPPP. Recurrence is fairly uncommon, as the disease primarily affects women during their first pregnancy.
3. Intrahepatic cholestasis of pregnancy
This condition is also called recurrent or idiopathic jaundice of pregnancy, obstetric cholestasis, and pruritus gravidarum. Intrahepatic cholestasis of pregnancy (ICP) is caused by disruption of hepatic bile flow during pregnancy. It has been recorded at a rate of approximately 10 to 150 of every 10,000 pregnancies in Europe and 70 of every 10,000 in the United States.12 In 80% of patients, time of onset is after the 30th week.14
Although this disorder is not primarily a dermatosis of pregnancy, it is a pruritic condition that often presents with excoriations in pregnant women and is associated with fetal morbidity and mortality. It’s important to be able to identify this disease early to minimize sequelae.
Presentation. There are no primary lesions with ICP. The primary presenting symptom is a generalized pruritus affecting the palms and soles, and sometimes extending to the legs and abdomen (FIGURE 3). This itching is often so severe that it leads to chronic insomnia. You may see secondary skin lesions, such as erythema and excoriations. Observable jaundice occurs in 10% to 20% of patients.3 These patients do not develop the encephalopathy that is associated with cholestasis in the nonpregnant state, however.14

FIGURE 3 Intrahepatic cholestasis of pregnancy
ICP lacks primary lesions. Shown here are the secondary erythema and excoriations that results from scratching the intense pruritis.
Pathophysiology. The genesis of this condition is thought to be a combination of genetic and environmental factors. A family history of the disorder is present in one half of cases; cases with a familial component tend to be more severe.15 ICP may be an exaggerated response to increased estrogen levels in pregnancy, but the mechanism of this response is unknown.16
Differential diagnosis. Other conditions that must be considered in making the diagnosis are viral hepatitis, gallbladder disease, PG, PUPPP, drug hepatotoxicity, primary biliary cirrhosis, and uremia.
Diagnosis. Laboratory values are the definitive diagnostic tool in this condition. Increased levels of serum bile acids are the single most sensitive test. Average levels of serum bile acids in pregnancy are 6.6 µmol/L, with an upper limit of 11 µmol/L. The average value in women who have ICP is 47 µmol/L.17
Although serum bile acids remain the gold standard, a recent study showed that elevated urine bile acids have 100% sensitivity and 83% specificity for ICP.18 In 55% to 60% of cases, the liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are mildly increased. Steatorrhea is often noted by the patient, and is followed by vitamin K deficiency.17
Treatment. The current standard of care for ICP is treatment with ursodeoxycholic acid (UDCA). In four controlled trials, UDCA caused a sustained decrease in serum bile acids.19-22 The dosage used in these trials ranged from 450 to 1,200 mg/d.
Before UDCA treatment was available, ICP was treated with cholestyramine, which could bring about a 70% rate of response. The drawback to cholestyramine treatment is that it precipitates vitamin K, which is already compromised by the disease process. Further, the onset of action of cholestyramine is slow.3
Elective delivery is indicated for ICP, particularly in patients who have a significant clinical presentation.12 Delivery for ICP should be performed around weeks 37 to 38, as stillbirths tend to cluster around weeks 37 to 39.14 Given the significant fetal mortality associated with ICP (see the next paragraph), the condition should be managed by a clinician experienced with the disease—likely, a gastroenterologist.
Sequelae. The impact of this maternal disorder on the fetus can be disastrous: a 10% to 15% rate of perinatal death, and a 30% to 40% rate of premature labor.14 Fortunately, the rate of preterm labor correlates strongly with the level of bile acids, so that as bile acid levels are reduced with UDCA treatment, the rate of preterm labor also falls. Management of ICP has reduced the rate of perinatal death to 3.5%. No evidence of fetal growth retardation has been noted.14
DERMATOSES TRIGGERED BY PREGNANCY
4. Eczema of pregnancy/prurigo of pregnancy
Eczema of pregnancy/prurigo of pregnancy (EP/PP) may not actually be correlated with the pregnant state. Both conditions manifest as eczematous lesions in an atopic distribution. Although they have been described in the literature as separate entities, the lack of clinical distinction between them led Ambros-Rudolph and colleagues to combine them under the umbrella term, atopic eruption of pregnancy.23
In some patients, at least, EP/PP may be preexisting conditions that are exacerbated by pregnancy. One study of 255 patients with the condition found that 20% had had the lesions before they became pregnant.23 The tendency of the condition to be made markedly worse by pregnancy, however, leads us to include it here.
PP has an estimated incidence of 1 in 450 pregnancies.11 Although many authorities consider EP to be the most common dermatosis of pregnancy, no clear estimation of its prevalence has been established.23,24 Taken together, the two conditions have the highest prevalence of all pregnancy-induced dermatoses.
PP is also known as popular dermatitis of Spangler, Nurse’s early prurigo of pregnancy, and linear IgM disease of pregnancy.3,4,23
Presentation. The typical presentation is grouped, crusted, erythematous papules, patches, and plaques—frequently with excoriations. Lesions typically present on the extensor surfaces of the arms and legs or on the abdomen (FIGURE 4).4 Recurrence in later pregnancies is common.

FIGURE 4 Eczema of pregnancy
EP/PP typically manifests as grouped, crusted, erythematous papules, patches, and plaques, often with excoriations.
Pathophysiology. The pathophysiology of EP/PP is not understood. Many patients who develop EP/PP have a history of atopy.10
Differential diagnosis. Conditions that need to be considered in making the diagnosis include Tinea infection, scabies, contact dermatitis, ICP, pruritic folliculitis of pregnancy (PFP), and PG.
Diagnosis. The history and the physical examination determine the diagnosis. Serology, histopathology, and immunofluorescence are nonspecific. Correlation of EP/PP with increased IgE is marginal, at best.24,25
Treatment. These conditions are treated symptomatically with topical corticosteroids or systemic antihistamines.
Sequelae. No increase in maternal or fetal morbidity or mortality is associated with EP/PP.
5. Acute pustular psoriasis of pregnancy
Whether or not acute pustular psoriasis of pregnancy (APPP) is actually a pregnancy-induced dermatosis is subject to debate.
There is evidence that APPP is not unique to pregnancy but simply a manifestation of ordinary psoriasis. Clinically and histologically, APPP is indistinguishable from pustular psoriasis. Unlike most cases of acute psoriasis, however, APPP often appears in pregnancy without any personal or family history of psoriasis and usually ceases when the pregnancy ends. This fact, combined with reports of increased fetal and maternal morbidity and mortality associated with APPP, leads us to include it here.26
Presentation. APPP is a rare condition that may have an onset at any point in pregnancy. Characteristic lesions begin as erythematous plaques with pustules on the inner thighs, flexural areas, and groin and spread to the trunk and extremities. As plaques enlarge, their center becomes eroded and crusted.
The nails may become onycholytic. Hands, feet, and face are usually spared. Oral and esophageal erosions can occur. Pruritus is typically mild, although the lesions are often painful. Flu-like symptoms are often present.27
Pathophysiology. The pathophysiology of APPP is unknown.
Differential diagnosis. Conditions with similar presentations include an adverse drug reaction, pityriasis rosea, lichen simplex chronicus, eczema, lupus, and pityriasis rubra pilaris.
Diagnosis. The clinical history and an association with systemic illness are the basis for a diagnosis of APPP. Cultures of pustules are negative for any infective pathology, although, as the disease progresses, pustules may become superinfected. Laboratory testing may show an increased erythrocyte sedimentation rate, hypocalcemia, and a low level of vitamin D.
Treatment. Prednisone, 15 to 60 mg/d, is often sufficient to control the disease.27 Cyclosporine, 100 mg twice daily, has also been shown to be useful.28 Cyclosporine in pregnancy is a Category C drug. Data on fetal malformation associated with cyclosporine therapy are limited, but risk appears minimal.6
Maternal hypocalcemia should be monitored and treated appropriately. If disease progression is judged serious enough, early induction of labor is indicated, because delivery will almost always lead to swift resolution.
Sequelae. A number of case reports link APPP to serious sequelae, including fetal growth retardation, hypocalcemia, and stillbirth.27,29,30 The condition is too rare, however, for good data on specific sequelae. Although APPP does give significant cause for concern, it appears that some of the traditional apprehension comes from older publications reporting a rate of maternal mortality of 70% to 90%.31 This statistic has not been borne out in practice. It does appear that the mother will frequently suffer systemic symptoms, including fever and malaise.
6. Pruritic folliculitis of pregnancy
Accounts of the prevalence of pruritic folliculitis of pregnancy (PFP) vary widely. Some sources report fewer than 30 cases in all of the literature; others indicate that the prevalence is equivalent to that of PG—one in every 10,000 pregnancies.3,11 PFP most often presents in the third trimester. It often resolves before delivery, but uniformly clears within 2 weeks of delivery.
Presentation. PFP presents as papules and pustules concentrated around hair follicles (FIGURE 5). Often, lesions begin on the abdomen and spread to the extremities.24,28 The condition is often, but not always, pruritic. Patients are more likely to be concerned about what the condition means for their health than distressed by the symptoms.

FIGURE 5 Pruritic folliculitis of pregnancy
The papules and pustules of PFP are concentrated around hair follicles.
Pathophysiology. Like many other dermatoses of pregnancy, the pathophysiology of PFP is unknown. There is little evidence that the condition is immunologically or hormonally mediated, and there is no evidence of an infectious component.24,28
Differential diagnosis. PFP must be distinguished from infectious folliculitis, acneiform disorders, HIV-associated eosinophilic folliculitis, and a drug reaction.
Diagnosis. The clinical diagnosis is based on presenting symptoms and third-trimester onset. No specific laboratory or histologic analysis can be used to make a definitive diagnosis.
Treatment. As the condition is, by definition, a nonmicrobial folliculitis, the most effective therapy tends to be with a low- or midpotency topical corticosteroid, such as triamcinolone or desonide. A benzoyl peroxide wash can also be effective.
Sequelae. One study reports an increased incidence of low birth weight, but no associated morbidity or mortality has been reported in recent studies.24
- Pemphigoid gestationis is best managed with oral prednisone at doses from 20 to 60 mg per day to control symptoms
- The pruritus associated with pruritic urticarial papules and plaques of pregnancy can be safely and effectively managed with topical corticosteroids and oral antihistamines
- Treat intrahepatic cholestasis of pregnancy with ursodeoxycholic acid, which likely reduces serum bile acids as well as associated fetal morbidity and mortality
The dermatoses of pregnancy are a poorly understood group of conditions. Their only common feature is a tendency to appear during pregnancy.
Only three of these conditions are considered unique to pregnancy, however; the others are probably exacerbations of preexisting conditions triggered by pregnancy. There isn’t even complete agreement on what to call them. To make management even more complex, two patients—mother and fetus—need to be considered in decisions about care.
Who manages these patients is another matter. These conditions fall into overlapping areas of health care, where family physicians, obstetricians, and dermatologists all might have some share in responsibility for diagnosis and treatment. You need to be sufficiently familiar with these conditions so that you can differentiate those that can be treated symptomatically and those that require referral to a specialist. This review and the handy TABLE, will help you toward that end.
TABLE
Skin disorders of pregnancy: What you’ll see, how to treat
| Disorder | Lesions | Diagnosis and sequelae | Treatment | Recurrence |
|---|---|---|---|---|
| Pemphigoid gestationis3,5 | Erythematous papules that progress to vesicles and bullae, in a periumbilical distribution that spares the face, palms, and soles |
|
| Frequent; skips a pregnancy 8% of the time |
| Pruritic urticarial papules and plaques of pregnancy8-10 | Urticarial papules and plaques on the abdomen, legs, arms, buttocks, chest, and back |
| Topical corticosteroids and antihistamines | Uncommon |
| Intrahepatic cholestasis of pregnancy14,17,19-22 | No primary lesions; secondary excoriations in any area that the patient can reach |
| Ursodeoxycholic acid, 450-1,200 mg/d | Frequent |
| Eczema of pregnancy/pruritus of pregnancy4,10,24 | Grouped, crusted erythematous papules, patches, and plaques, most often on extensor surfaces of the arms and legs or on the abdomen |
| Symptomatic treatment with topical corticosteroids or antihistamines | Frequent |
| Acute pustular psoriasis of pregnancy26-28 | Erythematous plaques and pustules that start on the inner thighs and groin and spread to the trunk and extremities |
|
| Unknown |
| Pruritic folliculitis of pregnancy24,28 | Papules and pustules concentrated around hair follicles, often beginning on the abdomen and spreading to the extremities |
| Topical corticosteroids | Unknown |
DERMATOSES UNIQUE TO PREGNANCY
1. Pemphigoid gestationis
Years ago, this disorder was referred to as herpes gestationis, because the lesions are herpetiform. Pemphigoid gestationis (PG) has an incidence of approximately 1 in 10,000 pregnancies.1,2 Time of onset is usually about the 21st week of gestation, although, in about 20% of cases, the eruption appears immediately postpartum.3
Presentation. The disease usually begins with urticarial papules and plaques around the umbilicus and extremities. Bullous lesions tend to develop as the disease progresses, and are often not present on first presentation (FIGURE 1). Lesions of PG tend to spare the face, palms, and soles. Mucosal surfaces are involved in fewer than 20% of cases. In about 75% of cases, PG flares around the time of delivery, regressing spontaneously after the baby is born.4

FIGURE 1 Pemphigoid gestationis
As the disease progresses, bullous lesions tend to develop.
Pathophysiology. The pathophysiology of PG is nearly identical to that of bullous pemphigoid, a blistering skin disorder seen more often in elderly patients.5 Pemphigoid disorders are immune processes, involving an immunoglobulin G (IgG) immune response directed at a 180-kDa hemidesmosome transmembrane glycoprotein. This protein is the common target in several subepidermal blistering diseases.
Differential diagnosis. Disorders that may have some of the same features as PG include pruritic urticarial papules and plaques of pregnancy (PUPPP), erythema multiforme, intrahepatic cholestasis of pregnancy (ICP), contact dermatitis, and drug reactions.
Diagnosis. A biopsy is necessary for definitive diagnosis. Direct immunofluorescence (DIF) microscopy of a sample of perilesional skin can show tissue-bound immunoreactants. Linear deposition of the complement component protein C3 along the basement membrane zone is diagnostic for PG. IgG is also deposited about 40% of the time.3
Serum enzyme-linked immunosorbent assay (ELISA) studies are also helpful in diagnosis. They have excellent sensitivity and specificity, as well as the capacity to monitor levels of antibody, which correlate with the severity of disease.1
Treatment. Oral corticosteroids are the first-line treatment for PG, typically 20 to 60 mg/d of prednisone. Oral corticosteroids are generally most effective at ameliorating symptoms. Prednisone at a dosage of 40 to 80 mg/d for a short time has not been associated with congenital abnormalities.6 PG patients can also be treated successfully with intravenous immunoglobulin (IVIG) and cyclosporine in refractory cases.7
Pruritus associated with this condition can interfere with day-to-day activities and with the patient’s ability to sleep. Patients may also complain that the rash is painful, particularly if bullae rupture, leading to superficial ulcerations. Fortunately, the patient’s quality of life can be dramatically improved with systemic corticosteroids—with no significant risk to the fetus.
Sequelae. PG uniformly resolves within a few weeks, but the mother’s autoantibodies can be passively transferred to the fetus, causing vesicles and bullae in the newborn.8 An increased incidence of small-for-gestational age (SGA) infants has also been noted in PG, although no lasting morbidity or mortality in the offspring has been noted.5 The disease tends to recur in future pregnancies.
2. Pruritic urticarial papules and plaques of pregnancy
This condition is known by many names besides its acronym PUPPP: polymorphic eruption of pregnancy, toxemic erythema of pregnancy, and late prurigo of pregnancy.1 It is a pruritic, inflammatory skin disorder that has been variously estimated to occur in anywhere from 1 in 120 to 1 in 240 pregnancies.8 PUPPP is second only to eczema as the most common dermatosis of pregnancy.
Presentation. As the name suggests, the lesions of PUPPP are itchy, red papules that often coalesce into plaques (FIGURE 2). Lesions usually occur in primigravidas after the 34th week of gestation, although they may be seen at any time from the first trimester through the postpartum period.9
Lesions are classically found on the abdomen, sparing the umbilical area, and are found primarily in the striae. This distribution helps you to differentiate PUPPP from PG, in which lesions typically cluster around the umbilicus. Most PUPPP lesions (80% in one study) are dispersed on the abdomen, legs, arms, buttocks, chest, and back. Another 17% appear only on the abdomen and proximal thighs, and the remaining 3% on the limbs.10 Nearly 50% of the time, lesions also include discrete vesicles.11 There are no reported cases of mucosal involvement.
Patients with this condition are often very uncomfortable. The associated pruritus is severe enough to interfere with sleep. Despite the itching, however, lesions are seldom excoriated.

FIGURE 2 Pruritic urticarial papules and plaques of pregnancy
The itchy, red papules of PUPP often coalesce into plaques.
Pathophysiology. The disorder has been strongly associated with maternal weight gain and multiple gestations. One working hypothesis is that rapid abdominal distention observed in the third trimester leads to damage of the connective tissue, which then releases antigenic molecules, causing an inflammatory reaction.12 Another hypothesis is that increased levels of fetal DNA that have been detected in the skin of PUPPP patients may contribute to the pathology. One study detected male DNA in six of 10 PUPPP sufferers, but found none in any of 26 controls—pregnant women without PUPPP pathology.5 There is some evidence that patients with atopy may be predisposed to PUPPP, as well as patients who are hypertensive or obese.10,13
Differential diagnosis. Initially, PUPPP lesions can be difficult to differentiate from urticarial PG lesions. The distribution of the lesions is the best clue: PG lesions cluster around the umbilicus, whereas PUPPP lesions uniformly spare the umbilical area. Additional disorders in the PUPPP differential are atopic dermatitis, superficial urticarial allergic eruption, viral exanthema, and contact or irritant dermatitis.
Diagnosis. PUPPP can be diagnosed only by clinical observation. None of the available laboratory tests—immunofluorescence, histology, serology—yield findings specific for PUPPP, although histology and immunofluorescence can readily differentiate between this condition and PG.
Treatment. Because the disease holds no real danger for mother or fetus, treatment can be aimed solely at symptomatic relief. Mild-to-potent topical corticosteroids (consider triamcinolone or fluocinonide) should relieve pruritus within 48 to 72 hours.8 Antihistamines and, occasionally, low-dose systemic corticosteroids may also be used. Consider hydroxyzine, although diphenhydramine has the more proven safety profile in pregnancy.
Nonpharmaceutical treatments such as oil baths and emollients should also be considered. If the condition appears classic for PUPPP, it can be managed symptomatically. If there is any question about the diagnosis, however, referral to a dermatologist is prudent.
Sequelae. No increase in maternal or fetal morbidity or mortality is associated with PUPPP. Recurrence is fairly uncommon, as the disease primarily affects women during their first pregnancy.
3. Intrahepatic cholestasis of pregnancy
This condition is also called recurrent or idiopathic jaundice of pregnancy, obstetric cholestasis, and pruritus gravidarum. Intrahepatic cholestasis of pregnancy (ICP) is caused by disruption of hepatic bile flow during pregnancy. It has been recorded at a rate of approximately 10 to 150 of every 10,000 pregnancies in Europe and 70 of every 10,000 in the United States.12 In 80% of patients, time of onset is after the 30th week.14
Although this disorder is not primarily a dermatosis of pregnancy, it is a pruritic condition that often presents with excoriations in pregnant women and is associated with fetal morbidity and mortality. It’s important to be able to identify this disease early to minimize sequelae.
Presentation. There are no primary lesions with ICP. The primary presenting symptom is a generalized pruritus affecting the palms and soles, and sometimes extending to the legs and abdomen (FIGURE 3). This itching is often so severe that it leads to chronic insomnia. You may see secondary skin lesions, such as erythema and excoriations. Observable jaundice occurs in 10% to 20% of patients.3 These patients do not develop the encephalopathy that is associated with cholestasis in the nonpregnant state, however.14

FIGURE 3 Intrahepatic cholestasis of pregnancy
ICP lacks primary lesions. Shown here are the secondary erythema and excoriations that results from scratching the intense pruritis.
Pathophysiology. The genesis of this condition is thought to be a combination of genetic and environmental factors. A family history of the disorder is present in one half of cases; cases with a familial component tend to be more severe.15 ICP may be an exaggerated response to increased estrogen levels in pregnancy, but the mechanism of this response is unknown.16
Differential diagnosis. Other conditions that must be considered in making the diagnosis are viral hepatitis, gallbladder disease, PG, PUPPP, drug hepatotoxicity, primary biliary cirrhosis, and uremia.
Diagnosis. Laboratory values are the definitive diagnostic tool in this condition. Increased levels of serum bile acids are the single most sensitive test. Average levels of serum bile acids in pregnancy are 6.6 µmol/L, with an upper limit of 11 µmol/L. The average value in women who have ICP is 47 µmol/L.17
Although serum bile acids remain the gold standard, a recent study showed that elevated urine bile acids have 100% sensitivity and 83% specificity for ICP.18 In 55% to 60% of cases, the liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are mildly increased. Steatorrhea is often noted by the patient, and is followed by vitamin K deficiency.17
Treatment. The current standard of care for ICP is treatment with ursodeoxycholic acid (UDCA). In four controlled trials, UDCA caused a sustained decrease in serum bile acids.19-22 The dosage used in these trials ranged from 450 to 1,200 mg/d.
Before UDCA treatment was available, ICP was treated with cholestyramine, which could bring about a 70% rate of response. The drawback to cholestyramine treatment is that it precipitates vitamin K, which is already compromised by the disease process. Further, the onset of action of cholestyramine is slow.3
Elective delivery is indicated for ICP, particularly in patients who have a significant clinical presentation.12 Delivery for ICP should be performed around weeks 37 to 38, as stillbirths tend to cluster around weeks 37 to 39.14 Given the significant fetal mortality associated with ICP (see the next paragraph), the condition should be managed by a clinician experienced with the disease—likely, a gastroenterologist.
Sequelae. The impact of this maternal disorder on the fetus can be disastrous: a 10% to 15% rate of perinatal death, and a 30% to 40% rate of premature labor.14 Fortunately, the rate of preterm labor correlates strongly with the level of bile acids, so that as bile acid levels are reduced with UDCA treatment, the rate of preterm labor also falls. Management of ICP has reduced the rate of perinatal death to 3.5%. No evidence of fetal growth retardation has been noted.14
DERMATOSES TRIGGERED BY PREGNANCY
4. Eczema of pregnancy/prurigo of pregnancy
Eczema of pregnancy/prurigo of pregnancy (EP/PP) may not actually be correlated with the pregnant state. Both conditions manifest as eczematous lesions in an atopic distribution. Although they have been described in the literature as separate entities, the lack of clinical distinction between them led Ambros-Rudolph and colleagues to combine them under the umbrella term, atopic eruption of pregnancy.23
In some patients, at least, EP/PP may be preexisting conditions that are exacerbated by pregnancy. One study of 255 patients with the condition found that 20% had had the lesions before they became pregnant.23 The tendency of the condition to be made markedly worse by pregnancy, however, leads us to include it here.
PP has an estimated incidence of 1 in 450 pregnancies.11 Although many authorities consider EP to be the most common dermatosis of pregnancy, no clear estimation of its prevalence has been established.23,24 Taken together, the two conditions have the highest prevalence of all pregnancy-induced dermatoses.
PP is also known as popular dermatitis of Spangler, Nurse’s early prurigo of pregnancy, and linear IgM disease of pregnancy.3,4,23
Presentation. The typical presentation is grouped, crusted, erythematous papules, patches, and plaques—frequently with excoriations. Lesions typically present on the extensor surfaces of the arms and legs or on the abdomen (FIGURE 4).4 Recurrence in later pregnancies is common.

FIGURE 4 Eczema of pregnancy
EP/PP typically manifests as grouped, crusted, erythematous papules, patches, and plaques, often with excoriations.
Pathophysiology. The pathophysiology of EP/PP is not understood. Many patients who develop EP/PP have a history of atopy.10
Differential diagnosis. Conditions that need to be considered in making the diagnosis include Tinea infection, scabies, contact dermatitis, ICP, pruritic folliculitis of pregnancy (PFP), and PG.
Diagnosis. The history and the physical examination determine the diagnosis. Serology, histopathology, and immunofluorescence are nonspecific. Correlation of EP/PP with increased IgE is marginal, at best.24,25
Treatment. These conditions are treated symptomatically with topical corticosteroids or systemic antihistamines.
Sequelae. No increase in maternal or fetal morbidity or mortality is associated with EP/PP.
5. Acute pustular psoriasis of pregnancy
Whether or not acute pustular psoriasis of pregnancy (APPP) is actually a pregnancy-induced dermatosis is subject to debate.
There is evidence that APPP is not unique to pregnancy but simply a manifestation of ordinary psoriasis. Clinically and histologically, APPP is indistinguishable from pustular psoriasis. Unlike most cases of acute psoriasis, however, APPP often appears in pregnancy without any personal or family history of psoriasis and usually ceases when the pregnancy ends. This fact, combined with reports of increased fetal and maternal morbidity and mortality associated with APPP, leads us to include it here.26
Presentation. APPP is a rare condition that may have an onset at any point in pregnancy. Characteristic lesions begin as erythematous plaques with pustules on the inner thighs, flexural areas, and groin and spread to the trunk and extremities. As plaques enlarge, their center becomes eroded and crusted.
The nails may become onycholytic. Hands, feet, and face are usually spared. Oral and esophageal erosions can occur. Pruritus is typically mild, although the lesions are often painful. Flu-like symptoms are often present.27
Pathophysiology. The pathophysiology of APPP is unknown.
Differential diagnosis. Conditions with similar presentations include an adverse drug reaction, pityriasis rosea, lichen simplex chronicus, eczema, lupus, and pityriasis rubra pilaris.
Diagnosis. The clinical history and an association with systemic illness are the basis for a diagnosis of APPP. Cultures of pustules are negative for any infective pathology, although, as the disease progresses, pustules may become superinfected. Laboratory testing may show an increased erythrocyte sedimentation rate, hypocalcemia, and a low level of vitamin D.
Treatment. Prednisone, 15 to 60 mg/d, is often sufficient to control the disease.27 Cyclosporine, 100 mg twice daily, has also been shown to be useful.28 Cyclosporine in pregnancy is a Category C drug. Data on fetal malformation associated with cyclosporine therapy are limited, but risk appears minimal.6
Maternal hypocalcemia should be monitored and treated appropriately. If disease progression is judged serious enough, early induction of labor is indicated, because delivery will almost always lead to swift resolution.
Sequelae. A number of case reports link APPP to serious sequelae, including fetal growth retardation, hypocalcemia, and stillbirth.27,29,30 The condition is too rare, however, for good data on specific sequelae. Although APPP does give significant cause for concern, it appears that some of the traditional apprehension comes from older publications reporting a rate of maternal mortality of 70% to 90%.31 This statistic has not been borne out in practice. It does appear that the mother will frequently suffer systemic symptoms, including fever and malaise.
6. Pruritic folliculitis of pregnancy
Accounts of the prevalence of pruritic folliculitis of pregnancy (PFP) vary widely. Some sources report fewer than 30 cases in all of the literature; others indicate that the prevalence is equivalent to that of PG—one in every 10,000 pregnancies.3,11 PFP most often presents in the third trimester. It often resolves before delivery, but uniformly clears within 2 weeks of delivery.
Presentation. PFP presents as papules and pustules concentrated around hair follicles (FIGURE 5). Often, lesions begin on the abdomen and spread to the extremities.24,28 The condition is often, but not always, pruritic. Patients are more likely to be concerned about what the condition means for their health than distressed by the symptoms.

FIGURE 5 Pruritic folliculitis of pregnancy
The papules and pustules of PFP are concentrated around hair follicles.
Pathophysiology. Like many other dermatoses of pregnancy, the pathophysiology of PFP is unknown. There is little evidence that the condition is immunologically or hormonally mediated, and there is no evidence of an infectious component.24,28
Differential diagnosis. PFP must be distinguished from infectious folliculitis, acneiform disorders, HIV-associated eosinophilic folliculitis, and a drug reaction.
Diagnosis. The clinical diagnosis is based on presenting symptoms and third-trimester onset. No specific laboratory or histologic analysis can be used to make a definitive diagnosis.
Treatment. As the condition is, by definition, a nonmicrobial folliculitis, the most effective therapy tends to be with a low- or midpotency topical corticosteroid, such as triamcinolone or desonide. A benzoyl peroxide wash can also be effective.
Sequelae. One study reports an increased incidence of low birth weight, but no associated morbidity or mortality has been reported in recent studies.24
- Pemphigoid gestationis is best managed with oral prednisone at doses from 20 to 60 mg per day to control symptoms
- The pruritus associated with pruritic urticarial papules and plaques of pregnancy can be safely and effectively managed with topical corticosteroids and oral antihistamines
- Treat intrahepatic cholestasis of pregnancy with ursodeoxycholic acid, which likely reduces serum bile acids as well as associated fetal morbidity and mortality
1. Sitaru C, Powell J, Messer G, Bröcker EB, Wojnarowska F, Zillikens D. Immunoblotting and enzyme linked immunosorbent assay for the diagnosis of pemphigoid gestationis. Obstet Gynecol. 2004;103(4):757-763.
2. Engineer L, Bohl K, Ahmed AR. Pemphigoid gestationis: a review. Am J Obstet Gynecol. 2000;183(2):483-491.
3. Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence-based systematic review. Am J Obstet Gynecol. 2003;188(4):1083-1092.
4. Shornick JK. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17(3):172-181.
5. Shornick JK, Bangert JL, Freeman RG, Gilliam JN. Herpes gestationis: clinical and histological features in 28 cases. J Am Acad Dermatol. 1983;8(2):214-224.
6. Leachman SA, Reed BR. The use of dermatologic drugs in pregnancy and lactation. Dermatol Clin. 2006;24(2):167-197, vi.
7. Hern S, Harman K, Bhogal BS, Black MM. A severe persistent case of pemphigoid gestationis treated with intravenous immunoglobulins and cyclosporine. Clin Exp Dermatol. 1998;23(4):185-188.
8. Fitzpatrick TP. Diseases in pregnancy. Color Atlas and Synopsis of Clinical Dermatology. New York, NY: McGraw Hill; 1997:414–419.
9. Aractingi D, Berkane N, Bertheau P, et al. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352(9144):1898-1901.
10. Rudolph CM, Al-Fares S, Vaughan-Jones SA, et al. Polymorphic eruption of pregnancy: clinicopathology and potential trigger factors in 181 patients. Br J Dermatol. 2006;154(1):54-60.
11. Roger D, Vaillant L, Fignon A, et al. Specific pruritic diseases of pregnancy. A prospective study of 3192 pregnant women. Arch Dermatol. 1994;130(6):734-739.
12. McDonald JA. Cholestasis of pregnancy. J Gastroenterol Hepatol. 1999;14(6):515-518.
13. Ohel I, Levy A, Silberstein T, Holcberg G, Sheiner E. Pregnancy outcome of patients with pruritic urticarial papules and plaques of pregnancy. J Maternal Fetal Neonat Med. 2006;19(5):305-308.
14. Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15(17):2049-2066.
15. Shaw D, Frohlich J, Wittmann BA, Willms M. A prospective study of 18 patients with cholestasis of pregnancy. Am J Obstet Gynecol. 1982;142(6 Pt 1):621-625.
16. Reyes H. The spectrum of liver and gastrointestinal disease seen in cholestasis of pregnancy. Gastroenterol Clin North Am. 1992;21(4):905-921.
17. Lammert F, Marschall H, Glantz A, Matern S. Intrahepatic cholestasis of pregnancy; molecular pathogenesis, diagnosis and management. J Hepatol. 2000;33(6):1012-1021.
18. Huang WM, Seubert DE, Donnelly JG, Liu M, Javitt NB. Intrahepatic cholestasis of pregnancy: detection with urinary bile acid assays. J Perinat Med. 2007;35(6):486-491.
19. Palma J, Reyes H, Ribalta J, et al. Ursodeoxycholic acid in the treatment of intrahepatic cholestasis of pregnancy: a randomized, double-blind study controlled with placebo. J Hepatol. 1997;27(6):1022-1028.
20. Diaferia A, Nicastri PL, Tartagni M, et al. Ursodeoxycholic acid therapy in pregnant women with cholestasis. Int J Gynaecol Obstet. 1996;52:133-140.
21. Nicastri PL, Diaferia A, Tartagni M, Loizzi P, Fanelli M. A randomised placebo-controlled trial of ursodeoxycholic acid and S-adenosylmethionine in the treatment of intrahepatic cholestasis of pregnancy. Br J Obstet Gynaecol. 1998;105(11):1205-1207.
22. Glantz A, Marschall HU, Lammert F, Mattsson LA. Intrahepatic cholestasis in pregnancy: a randomized controlled trial comparing dexamethasone and ursodeoxycholic acid. Hepatology. 2005;42(6):1399-1405.
23. Ambros-Rudolph C, Müllegger M, Vaughan-Jones S, et al. The specific dermatoses of pregnancy revisited and reclassified: results of a retrospective two-center study on 505 pregnant patients. J Am Acad Dermatol. 2006;54(3):395-404.
24. Vaughan Jones SA, Hern S, Nelson-Piercy C, Seed PT, Black MM. A prospective study of 200 women with dermatoses of pregnancy correlating clinical findings with hormonal and immunopathological profiles. Br J Dermatol. 1999;141(1):71-81.
25. Holmes RC, Black MM. The specific dermatoses of pregnancy. J Am Acad Dermatol. 1983;8(3):405-412.
26. Bukhari IA. Impetigo herpetiformis in a primagravida: successful treatment with etretinate. J Drugs Dermatol. 2004;3(4):449-451.
27. Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24(2):101-104.
28. Kroumpouzos G, Cohen LM. Pruritic folliculitis of pregnancy. J Am Acad Dermatol. 2000;43(1 Pt 1):132-134.
29. Sahin HG, Sahin HA, Metin A, Zeteroglu S, Ugras S. Recurrent impetigo herpetiformis in a pregnant adolescent: case report. Eur J Obstet Gynecol Reprod Biol. 2002;101:201-203.
30. Aka N, Kuscu NK, Yazicioglu E. Impetigo herpetiformis at the 36th week of gestation. Int J Gynecol Obstet. 2000;69(2):153-154.
31. Wade T, Wade S, Jones H. Skin changes and diseases associated with pregnancy. Obstet Gynecol. 1978;52(2):233-242.
1. Sitaru C, Powell J, Messer G, Bröcker EB, Wojnarowska F, Zillikens D. Immunoblotting and enzyme linked immunosorbent assay for the diagnosis of pemphigoid gestationis. Obstet Gynecol. 2004;103(4):757-763.
2. Engineer L, Bohl K, Ahmed AR. Pemphigoid gestationis: a review. Am J Obstet Gynecol. 2000;183(2):483-491.
3. Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence-based systematic review. Am J Obstet Gynecol. 2003;188(4):1083-1092.
4. Shornick JK. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17(3):172-181.
5. Shornick JK, Bangert JL, Freeman RG, Gilliam JN. Herpes gestationis: clinical and histological features in 28 cases. J Am Acad Dermatol. 1983;8(2):214-224.
6. Leachman SA, Reed BR. The use of dermatologic drugs in pregnancy and lactation. Dermatol Clin. 2006;24(2):167-197, vi.
7. Hern S, Harman K, Bhogal BS, Black MM. A severe persistent case of pemphigoid gestationis treated with intravenous immunoglobulins and cyclosporine. Clin Exp Dermatol. 1998;23(4):185-188.
8. Fitzpatrick TP. Diseases in pregnancy. Color Atlas and Synopsis of Clinical Dermatology. New York, NY: McGraw Hill; 1997:414–419.
9. Aractingi D, Berkane N, Bertheau P, et al. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352(9144):1898-1901.
10. Rudolph CM, Al-Fares S, Vaughan-Jones SA, et al. Polymorphic eruption of pregnancy: clinicopathology and potential trigger factors in 181 patients. Br J Dermatol. 2006;154(1):54-60.
11. Roger D, Vaillant L, Fignon A, et al. Specific pruritic diseases of pregnancy. A prospective study of 3192 pregnant women. Arch Dermatol. 1994;130(6):734-739.
12. McDonald JA. Cholestasis of pregnancy. J Gastroenterol Hepatol. 1999;14(6):515-518.
13. Ohel I, Levy A, Silberstein T, Holcberg G, Sheiner E. Pregnancy outcome of patients with pruritic urticarial papules and plaques of pregnancy. J Maternal Fetal Neonat Med. 2006;19(5):305-308.
14. Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15(17):2049-2066.
15. Shaw D, Frohlich J, Wittmann BA, Willms M. A prospective study of 18 patients with cholestasis of pregnancy. Am J Obstet Gynecol. 1982;142(6 Pt 1):621-625.
16. Reyes H. The spectrum of liver and gastrointestinal disease seen in cholestasis of pregnancy. Gastroenterol Clin North Am. 1992;21(4):905-921.
17. Lammert F, Marschall H, Glantz A, Matern S. Intrahepatic cholestasis of pregnancy; molecular pathogenesis, diagnosis and management. J Hepatol. 2000;33(6):1012-1021.
18. Huang WM, Seubert DE, Donnelly JG, Liu M, Javitt NB. Intrahepatic cholestasis of pregnancy: detection with urinary bile acid assays. J Perinat Med. 2007;35(6):486-491.
19. Palma J, Reyes H, Ribalta J, et al. Ursodeoxycholic acid in the treatment of intrahepatic cholestasis of pregnancy: a randomized, double-blind study controlled with placebo. J Hepatol. 1997;27(6):1022-1028.
20. Diaferia A, Nicastri PL, Tartagni M, et al. Ursodeoxycholic acid therapy in pregnant women with cholestasis. Int J Gynaecol Obstet. 1996;52:133-140.
21. Nicastri PL, Diaferia A, Tartagni M, Loizzi P, Fanelli M. A randomised placebo-controlled trial of ursodeoxycholic acid and S-adenosylmethionine in the treatment of intrahepatic cholestasis of pregnancy. Br J Obstet Gynaecol. 1998;105(11):1205-1207.
22. Glantz A, Marschall HU, Lammert F, Mattsson LA. Intrahepatic cholestasis in pregnancy: a randomized controlled trial comparing dexamethasone and ursodeoxycholic acid. Hepatology. 2005;42(6):1399-1405.
23. Ambros-Rudolph C, Müllegger M, Vaughan-Jones S, et al. The specific dermatoses of pregnancy revisited and reclassified: results of a retrospective two-center study on 505 pregnant patients. J Am Acad Dermatol. 2006;54(3):395-404.
24. Vaughan Jones SA, Hern S, Nelson-Piercy C, Seed PT, Black MM. A prospective study of 200 women with dermatoses of pregnancy correlating clinical findings with hormonal and immunopathological profiles. Br J Dermatol. 1999;141(1):71-81.
25. Holmes RC, Black MM. The specific dermatoses of pregnancy. J Am Acad Dermatol. 1983;8(3):405-412.
26. Bukhari IA. Impetigo herpetiformis in a primagravida: successful treatment with etretinate. J Drugs Dermatol. 2004;3(4):449-451.
27. Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24(2):101-104.
28. Kroumpouzos G, Cohen LM. Pruritic folliculitis of pregnancy. J Am Acad Dermatol. 2000;43(1 Pt 1):132-134.
29. Sahin HG, Sahin HA, Metin A, Zeteroglu S, Ugras S. Recurrent impetigo herpetiformis in a pregnant adolescent: case report. Eur J Obstet Gynecol Reprod Biol. 2002;101:201-203.
30. Aka N, Kuscu NK, Yazicioglu E. Impetigo herpetiformis at the 36th week of gestation. Int J Gynecol Obstet. 2000;69(2):153-154.
31. Wade T, Wade S, Jones H. Skin changes and diseases associated with pregnancy. Obstet Gynecol. 1978;52(2):233-242.
The skin disorders of pregnancy: A family physician’s guide
• Pemphigoid gestationis is best managed with oral prednisone at doses from 20 to 60 mg per day to control symptoms. B
• The pruritus associated with pruritic urticarial papules and plaques of pregnancy can be safely and effectively managed with topical corticosteroids and oral antihistamines. A
• Treat intrahepatic cholestasis of pregnancy with ursodeoxycholic acid, which likely reduces serum bile acids as well as associated fetal morbidity and mortality. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The dermatoses of pregnancy are a poorly understood group of dermatologic conditions. The only thing they have in common is a tendency to appear during pregnancy.
Only 3 are considered unique to pregnancy, however; the others are probably exacerbations of preexisting conditions triggered by pregnancy. There isn’t even complete agreement on what to call them. And to make management even more complex, 2 patients—the mother and the fetus—need to be considered.
Who will manage these patients is another matter. These conditions fall into an overlapping area of health care, where family physicians, obstetricians, and dermatologists all have some share in the responsibility for diagnosis and treatment. As a family physician who probably cares for any number of pregnant patients on a weekly basis, you need to be sufficiently familiar with these conditions so that you can differentiate those that can be treated symptomatically and those that require a referral to a specialist. This review and handy TABLE will help you toward that end.
TABLE
Skin disorders of pregnancy: What you’ll see, how to treat
| Disorder | Lesions | Diagnosis and sequelae | Treatment | Recurrence |
|---|---|---|---|---|
| PG3,5 | Erythematous papules, progressing to vesicles, bullae; periumbilical distribution, sparing face, palms, and soles | Mean onset at 21 weeks; postpartum in 20% of cases. Direct immunofluorescence microscopy shows linear C3 deposition. Newborn may be small for gestational age, but no associated morbidity or mortality | Oral corticosteroids 20-60 mg/d, IVIG, or cyclosporine in refractory cases | Frequent. Skips a pregnancy 8% of the time |
| PUPPP8-10 | Urticarial papules and plaques on abdomen, legs, arms, buttocks, chest, and back | Usually present after 34th week, but can present at any stage. Diagnosis is clinical. No increase in fetal morbidity or mortality | Topical steroids and antihistamines | Uncommon |
| ICP14,17,19-22 | No primary lesions; secondary excoriations in any area patient can reach | Onset after 30th week in 80% of patients. Strongly indicated by serum bile acid >11 mcmol/L. Increased fetal mortality | Ursodeoxycholic acid 450-1200 mg/d | Frequent |
| EP/PP4,10,24 | Grouped, crusted erythematous papules, patches, and plaques, most commonly on extensor surfaces of arms and legs or on abdomen | Onset at any point in pregnancy. Clinical diagnosis. No increase in fetal morbidity or mortality | Symptomatic treatment with topical steroids or antihistamines | Frequent |
| APPP27-29 | Erythematous plaques and pustules starting on inner thighs and groin and spreading to trunk and extremities | Onset at any point in pregnancy. Clinical diagnosis by appearance of lesions and association with systemic illness. Increased incidence of miscarriage and stillbirth, and maternal mortality | Prednisone 15-60 mg/d, cyclosporine 100 mg twice daily in refractory cases, management of associated hypocalcemia | Unknown |
| PFP24,29 | Papules and pustules concentrated around hair follicles, often beginning on abdomen and spreading to extremities | Onset most often in third trimester. Clinical diagnosis. No associated fetal morbidity or mortality | Topical steroids | Unknown |
| APPP, acute pustular psoriasis of pregnancy; EP/PP, eczema of pregnancy/pruritus of pregnancy; ICP, intrahepatic cholestasis of pregnancy; IVIG, intravenous immunoglobulin; PFP, pruritic folliculitis of pregnancy; PG, pemphigoid gestationis; PUPPP, pruritic urticarial papules and plaques of pregnancy. | ||||
Dermatoses unique to pregnancy
Pemphigoid gestationis
Years ago, this disorder was referred to as herpes gestationis, because the lesions are herpetiform. Pemphigoid gestationis (PG) has an incidence of approximately 1 in 10,000 pregnancies.1,2 The time of onset is usually about the 21st week of gestation, although in about 20% of cases, the eruption appears immediately postpartum.3
Presentation. The disease usually begins with urticarial papules and plaques around the umbilicus and extremities. Bullous lesions tend to develop as the disease progresses, and are often not present on first presentation (FIGURE 1). PG lesions tend to spare the face, palms, and soles. Mucosal surfaces are involved in less than 20% of cases. In about 75% of cases, PG flares around the time of delivery, regressing spontaneously after the baby is born.4
Pathophysiology. The pathophysiology is nearly identical to that of bullous pemphigoid, a blistering skin disorder more often seen in elderly patients.5 Pemphigoid disorders are immune processes, involving an immunoglobulin G (IgG) immune response directed at a 180-kDa hemidesmosome transmembrane glycoprotein. This protein is the common target of several subepidermal blistering diseases.
Differential. Disorders that may have some of the same features as PG include pruritic urticarial papules and plaques of pregnancy (PUPPP), erythema multiforme, intrahepatic cholestasis of pregnancy (ICP), contact dermatitis, and drug reactions.
Diagnosis. A biopsy is necessary for the definitive diagnosis. Direct immunofluorescence (DIF) microscopy of a sample of perilesional skin can show tissue-bound immunoreactants. Linear deposition of the complement component protein C3 along the basement membrane zone is diagnostic for PG. IgG is also deposited about 40% of the time.3 Serum enzyme-linked immunosorbent assay (ELISA) studies are also helpful in diagnosis. They have excellent sensitivity and specificity, as well as the capacity to monitor levels of antibody, which correlate with the severity of disease.1
Treatment. Oral corticosteroids are the first-line treatment for PG, typically 20 to 60 mg per day of prednisone. Oral corticosteroids are typically most effective in ameliorating the patient’s symptoms. Prednisone at doses of 40 to 80 mg per day for a short time has not been associated with congenital abnormalities.6 PG patients can also be treated successfully with intravenous immunoglobulin (IVIG) and cyclosporine in refractory cases.7
Pruritus associated with this condition can interfere with day-to-day activity and with the patient’s ability to sleep. Patients may also complain that the rash is painful, particularly if bullae rupture, leading to superficial ulcerations. Fortunately, the patient’s quality of life can be dramatically improved with systemic corticosteroids—with no significant risk to the fetus.
Sequelae. PG uniformly resolves within a few weeks, but the mother’s autoantibodies can passively be transferred to the fetus, causing vesicles and bullae in the newborn.8 An increased incidence of small-for-gestational age (SGA) infants has also been noted in PG, although no lasting morbidity or mortality in the offspring has been noted.5 This disease tends to recur with future pregnancies, but will skip a pregnancy 8% of the time.5
FIGURE 1
Pemphigoid gestationis
Pruritic urticarial papules and plaques of pregnancy
This condition is known by many names besides PUPPP: polymorphic eruption of pregnancy, toxemic erythema of pregnancy, and late prurigo of pregnancy.1 It is a pruritic, inflammatory skin disorder that has been variously estimated to occur in anywhere from 1 in 120 to 1 in 240 pregnancies.8 PUPPP is second only to eczema as the most common dermatosis of pregnancy.
Presentation. As the name implies, the lesions of PUPPP are itchy, red papules that often coalesce into plaques (FIGURE 2). The lesions usually occur in primigravidas after the 34th week of gestation, although they may be seen at any time from the first trimester through the postpartum period.9
Lesions are classically found on the abdomen, sparing the umbilical area, and are found primarily in the striae. This distribution helps to differentiate PUPPP from PG, where the lesions typically cluster around the umbilicus. Most PUPPP lesions (80% in 1 study) are dispersed on the abdomen, legs, arms, buttocks, chest, and back. Another 17% appear only on the abdomen and proximal thighs, and the remaining 3% on the limbs.10 Nearly 50% of the time, lesions also include discrete vesicles.11 There are no reported cases of mucosal involvement.
Patients with this condition are often very uncomfortable. The associated pruritus is severe enough to interfere with sleep. Despite the itching, however, lesions are seldom excoriated.
Pathophysiology. The disorder has been strongly associated with maternal weight gain and multiple gestations. One working hypothesis is that rapid abdominal distention observed in the third trimester leads to damage of the connective tissue, which then releases antigenic molecules, causing an inflammatory reaction.12 Another hypothesis is that increased levels of fetal DNA that have been detected in the skin of PUPPP patients may contribute to the pathology. One study detected male DNA in 6 of 10 PUPPP sufferers, but found none in any of 26 controls— pregnant women without PUPPP pathology.5 There is some evidence that patients with atopy may be predisposed to PUPPP, as well as patients who are hypertensive or obese.10,13
Differential. Initially, PUPPP lesions can be difficult to differentiate from urticarial PG lesions. The distribution of the lesions is the best clue: PG lesions cluster around the umbilicus, whereas PUPPP lesions uniformly spare the umbilical area. Additional disorders in the PUPPP differential are atopic dermatitis, superficial urticarial allergic eruption, viral exanthema, and contact or irritant dermatitis.
Diagnosis. PUPPP can only be diagnosed through clinical observation. None of the available laboratory tests—immunofluorescence, histology, serology—yield findings specific for PUPPP, although histology and immunofluorescence can readily differentiate between this condition and PG.
Treatment. Because the disease holds no real danger for mother or fetus, treatment can be aimed solely at symptomatic relief. Mild to potent topical steroids (consider triamcinolone or fluocinonide) should relieve pruritus within 48 to 72 hours.8 Antihistamines and occasionally low-dose systemic steroids may also be used. Consider hydroxyzine, although diphenhydramine has the more proven safety profile in pregnancy.
Nonpharmacologic treatments such as oil baths and emollients should also be considered. If the condition appears classic for PUPPP, it can be managed symptomatically. However, if there is any question about the diagnosis, referral to a dermatologist is prudent.
Sequelae. No increase in maternal or fetal morbidity or mortality is associated with PUPPP. Recurrence is fairly uncommon, as the disease primarily affects women during their first pregnancy.
FIGURE 2
Pruritic urticarial papules and plaques of pregnancy
Intrahepatic cholestasis of pregnancy
This condition is also called recurrent or idiopathic jaundice of pregnancy, obstetric cholestasis, and pruritus gravidarum. ICP is caused by disruption of hepatic bile flow during pregnancy. It has been recorded at a rate of approximately 10 to 150 per 10,000 pregnancies in Europe and 70 per 10,000 in the United States.12 In 80% of patients, the time of onset is after the 30th week.14 Although this disorder is not primarily a dermatosis of pregnancy, it is a pruritic condition that often presents with excoriations in pregnant women and is associated with fetal morbidity and mortality. It’s important to be able to identify this disease early to minimize sequelae.
Presentation. There are no primary lesions with ICP. The primary presenting symptom is a generalized pruritus affecting the palms and soles, and sometimes extending to the legs and abdomen (FIGURE 3). This itching is often so severe that it leads to chronic insomnia. You may see secondary skin lesions, such as erythema and excoriations. Observable jaundice occurs in 10% to 20% of patients.3 These patients do not develop the encephalopathy that is associated with cholestasis in the nonpregnant state, however.14
Pathophysiology. The genesis of this condition is thought to be a combination of genetic and environmental factors. A family history of the disorder is present in half the cases, and cases with a familial component tend to be more severe.15 ICP may be an exaggerated response to increased estrogen levels in pregnancy, but the mechanism of this response is unknown.16
Differential. Other conditions that must be considered in making the diagnosis are viral hepatitis, gallbladder disease, PG, PUPPP, drug hepatotoxicity, primary biliary cirrhosis, and uremia.
Diagnosis. Laboratory values are the definitive diagnostic tool in this condition. Increased serum bile acids are the single most sensitive test. Average levels of serum bile acids in pregnancy are 6.6 mcmol/L, with an upper limit of 11. The average value in women with ICP is 47 mcmol/L.17
While serum acids remain the gold standard, a recent study showed elevated urine bile acids to have 100% sensitivity and 83% specificity for ICP.18 In 55% to 60% of cases, the liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are mildly increased. Steatorrhea is often noted by the patient, and is followed by vitamin K deficiency.17
Treatment. The current standard of care for ICP is treatment with ursodeoxycholic acid (UDCA). In 4 controlled trials, UDCA showed a sustained decrease in serum bile acids.19-22 Doses used in these trials have varied between 450 and 1200 mg per day.
Before UDCA treatment was available, the disorder was treated with cholestyramine, which could bring about a 70% rate of response. The drawback to cholestyramine treatment is that it precipitates vitamin K, which is already compromised by the disease process. Further, its onset of action is slow.3
Elective delivery is indicated for ICP, particularly in patients with significant clinical presentations.12 Delivery for ICP should be performed around week 37 to 38, as stillbirths tend to cluster around weeks 37 to 39.14 Given the significant fetal mortality associated with this condition (see below), ICP should be managed by a clinician experienced with the disease, likely a gastroenterologist.
Sequelae. The impact of this maternal disorder on the fetus can be disastrous: a 10% to 15% rate of perinatal death, and a 30% to 40% rate of premature labor have been seen with ICP.14 Fortunately, rates of preterm labor are strongly correlated with levels of bile acids, so that as bile acid levels are reduced with UDCA treatment, rates of preterm labor also go down. Currently, management of the condition has reduced rates of perinatal death to 3.5%. There is no evidence of fetal growth retardation.14
FIGURE 3
Intrahepatic cholestasis of pregnancy
Dermatoses triggered by pregnancy
Eczema of pregnancy/prurigo of pregnancy
Eczema of pregnancy/prurigo of pregnancy (EP/PP) may not actually be correlated with the pregnant state. Both conditions manifest as eczematous lesions in an atopic distribution. Although they have been described in the literature as separate entities, the lack of clinical distinction between them led Ambros-Rudolph and colleagues to combine them under the umbrella term, atopic eruption of pregnancy.23 In some patients, at least, they may be preexisting conditions that pregnancy exacerbates. One study of 255 patients with the condition found that 20% had had the lesions before they became pregnant.23 However, the tendency of the condition to be markedly worsened by pregnancy leads us to include it here.
PP has an estimated incidence of 1 in 450 pregnancies.11 But while many authorities consider EP to be the most common dermatosis of pregnancy, no clear estimation of its prevalence has been established.23,24 Taken together, these 2 conditions have the highest prevalence of all pregnancy-induced dermatoses. PP is also known as popular dermatitis of Spangler, Nurse’s early prurigo of pregnancy, and linear IgM disease of pregnancy.3,23,25
Presentation. The typical presentation consists of grouped, crusted, erythematous papules, patches, and plaques—frequently with excoriations. The lesions typically present on the extensor surfaces of the arms and legs or on the abdomen (FIGURE 4).4 Recurrence in later pregnancies is common.
Pathophysiology. The pathophysiology of EP/PP is not understood. Many patients who present with EP/PP have a history of atopy.10
Differential. Conditions that need to be considered in making the diagnosis include tinea infection, scabies, contact dermatitis, ICP, pruritic folliculitis of pregnancy (PFP), and PG.
Diagnosis. History and physical examination determine the diagnosis. Serology, histopathology, and immunofluorescence are not specific, and correlation with increased IgE is marginal, at best.24,26
Treatment. These conditions are treated symptomatically with topical steroids or systemic antihistamines.
Sequelae. No maternal or fetal increase in morbidity or mortality is associated with these conditions.
FIGURE 4
Eczema of pregnancy
Acute pustular psoriasis of pregnancy
Whether or not APPP is actually a pregnancyinduced dermatosis is subject to debate.
There is evidence that APPP is not unique to pregnancy, but is simply a manifestation of ordinary psoriasis. Clinically and histologically, APPP is indistinguishable from pustular psoriasis. Unlike most cases of acute psoriasis, however, APPP often appears in pregnancy without any personal or family history of psoriasis, and usually ceases when the pregnancy is concluded. This fact, combined with reports of increased fetal and maternal morbidity and mortality associated with APPP, lead us to include it here.27
Presentation. APPP is a rare condition that may have an onset at any point in pregnancy. The characteristic lesions begin as erythematous plaques with pustules on the inner thighs, flexural areas, and groin and spread to the trunk and extremities. As the plaques enlarge, the center becomes eroded and crusted. Nails may become onycholytic. The hands, feet, and face are usually spared. Oral and esophageal erosions can occur. Pruritus is typically mild, although the lesions are often painful and flu-like symptoms are often present.28
Pathophysiology. The pathophysiology of this disease is unknown.
Differential. Conditions with similar presentations include an adverse drug reaction, pityriasis rosea, lichen simplex chronicus, eczema, lupus, and pityriasis rubra pilaris.
Diagnosis. Clinical history and association with systemic illness are the basis for a diagnosis of APPP. Cultures of the pustules are negative for any infective pathology, though as the disease progresses, pustules may become superinfected. Lab tests may show an increased erythrocyte sedimentation rate (ESR), hypocalcemia, and low levels of vitamin D.
Treatment. Prednisone 15 to 60 mg per day is often sufficient to control the disease.28 Cyclosporine 100 mg twice daily has also been shown to be useful.29 Cyclosporine in pregnancy is a category C drug. Data on fetal malformation associated with cyclosporine therapy are limited, but the risk appears to be minimal.6 Maternal hypocalcemia should be monitored and treated appropriately. If disease progression is judged serious enough, early induction of labor is indicated, since delivery will almost always lead to swift resolution.
Sequelae. There have been a number of case reports that link APPP to serious sequelae, including fetal growth retardation, hypocalcemia, and stillbirth.28,30,31 The condition is too rare, however, for good data on specific sequelae. While the disease does give significant cause for concern, it would appear that some of the traditional apprehension comes from older publications reporting a rate of maternal mortality of 70% to 90%.32 This statistic has not been borne out in clinical practice. It does appear that the mother will frequently suffer from systemic symptoms, including fever and malaise.
Pruritic folliculitis of pregnancy
Accounts of PFP’s prevalence vary widely: Some sources report fewer than 30 cases in all of the literature, while others indicate that the prevalence is equivalent to that of PG, 1 in 10,000.3,11 PFP most commonly presents in the third trimester. It often resolves before delivery, but uniformly clears within 2 weeks of delivery.
Presentation. PFP presents as papules and pustules concentrated around hair follicles (FIGURE 5). Often lesions begin on the abdomen and spread to the extremities.24,29 The condition is often, but not always, pruritic. Patients are more likely to be concerned about what the condition means for their health, rather than being distressed by the symptoms.
Pathophysiology. Like so many of these conditions, the pathophysiology of PFP is unknown. There is little evidence that the condition is immunologically or hormonally mediated, and there is no evidence of an infectious component.24,29
Differential. PFP must be distinguished from infectious folliculitis, acneiform disorders, HIV-associated eosinophilic folliculitis, and a drug reaction.
Diagnosis. The clinical diagnosis is based on presenting symptoms and third trimester onset. No specific lab or histological analysis can be used to make a definitive diagnosis.
Treatment. As the condition is, by definition, a nonmicrobial folliculitis, the most effective therapy tends to be with mid- to lowpotency topical steroids such as triamcinolone or desonide. Additionally, benzoyl peroxide wash can be effective.
Sequelae. One study reports an increased incidence of low birth weight, but currently no associated morbidity or mortality has been reported.24
FIGURE 5
Pruritic folliculitis of pregnancy
CORRESPONDENCE
Matthew Bremmer, MD, 419 W Redwood Street, Department of Dermatology, Baltimore, MD 21230; Mbrem001@umaryland.edu
1. Cassian S, Powell J, Messer G, et al. Immunoblotting and enzymelinked immunosorbent assay for the diagnosis of pemphigoid gestationis. Obstet Gynecol. 2004;103:757-763.
2. Engineer L, Bohl K, Ahmed AR. Pemphigoid gestationis: a review. Am J Obstet Gynecol. 2000;183:483-491.
3. Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence based systematic review. Am J Obstet Gynecol, 2003;188:1083-1092.
4. Shornick JD. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17:172-181.
5. Shornick JK, Bangert JL, Freeman RG, et al. Herpes gestationis: clinical and histological features in 28 cases. J Am Acad Dermatol. 1983;8:214-224.
6. Leachman S, Reed B. The use of dermatologic drugs in pregnancy and lactation. Dermatol Clin. 2004;24:167-197.
7. Hern S, Harman K, Bhogal BS, et al. A severe persistent case of pemphigoid gestationis treated with intravenous immunoglobulins and cyclosporine. Clin Exp Dermatol. 1998;23:185-188.
8. Fitzpatrick TP. Diseases in pregnancy. Color Atlas and Synopsis of Clinical Dermatology. New York, NY: McGraw Hill; 1997:414–419.
9. Aractingi D, Berkane N, Bertheau P, et al. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352:1898-1901.
10. Rudolph CM, Al-Fares S, Vaughan-Jones SA, et al. Polymorphic eruption of pregnancy: clinicopathology and potential trigger factors in 181 patients. Clin Lab Invest. 2006;154:54-60.
11. Roger D, Vaillant L, Fignon A, et al. Specific pruritic diseases of pregnancy. A prospective study of 3192 pregnant women. Arch Dermatol. 1994;130:734-739.
12. McDonald J. Cholestasis of pregnancy. J Gastroenterol Hepatol. 1999;14:515-518.
13. Ohel I, Levy A, Silberstein T, et al. Pregnancy outcome of patients with pruritic urticarial papules and plaques of pregnancy. J Maternal-Fetal Neonat Med. 2006;19:305-308.
14. Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15:2049-2066.
15. Shaw D, Frohlich J, Wittmann BA, et al. A prospective study of 18 patients with cholestasis of pregnancy. Am J Obstet Gynecol. 1982;142:621-625.
16. Reyes H. The spectrum of liver and gastrointestinal disease seen in cholestasis of pregnancy. Gastroenterol Clin North Am. 1992;21:905-921.
17. Lammert F, Marschall H, Glantz A, et al. Intrahepatic cholestasis of pregnancy; molecular pathogenesis, diagnosis and management. J Hepatol. 2000;33:1012-1021.
18. Huang WM, Seubert DE, Donnelly JG, et al. Intrahepatic cholestasis of pregnancy: detection with urinary bile acid assays. J Perinat Med. 2007;35:486-491.
19. Palma J, Reyes H, Ribalta J, et al. Ursodeoxycholic acid in the treatment of intrahepatic cholestasis of pregnancy: a randomized, double blind study controlled with placebo. J Hepatol. 1997;27:1022-1028.
20. Diaferia A, Nicastri PL, Tartagni M, et al. Ursodeoxycholic acid therapy in pregnant women with cholestasis. Int J Gynaecol Obstet. 1996;52:133-140.
21. Nicastri PL, Diaferia A, Tartagni M, et al. A randomised placebocontrolled trial of ursodeoxycholic acid and S-adenosylmethionine in the treatment of intrahepatic cholestasis of pregnancy. Br J Obstet Gynaecol. 1998;105:1205-1207.
22. Glantz A, Marschall HU, Lammert F, et al. Intrahepatic cholestasis in pregnancy: a randomized controlled trial comparing dexamethasone and ursodeoxycholic acid. Hepatology. 2005;42:1399-1405.
23. Ambros-Rudolph CM, Mullegger MM, Vaughan-Jones SA, et al. The specific dermatoses of pregnancy revisited and reclassified: results of a retrospective two-center study on 505 pregnant patients. J Am Acad Dermatol. 2006;54:395-404.
24. Vaughan-Jones SA, Hern S, Nelson-Piercy C, et al. A prospective study of 200 women with dermatoses of pregnancy correlating clinical findings with hormonal and immunopathological profiles. Br J Dermatol. 1999;141:71-81.
25. Shornick JK. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17:172-181.
26. Holmes RC, Black MM. The specific dermatoses of pregnancy. J Am Acad Dermatol. 1983;8:405-412.
27. Bukhari IA. Impetigo herpetiformis in a primagravida: successful treatment with etretinate. J Drugs Dermatol. 2004;3:449-451.
28. Oumeish OY, Parish JL. Impetigo herpetiformis. Clin in Dermatol. 2006;24:101-104.
29. Kroumpouzos G, Cohen LM. Pruritic folliculitis of pregnancy. J Am Acad Dermatol. 2000;43:132-134.
30. Sahin HG, Hasin HA, Metin A, et al. Recurrent impetigo herpetiformis in a pregnant adolescent: a case report. Eur J Obstet Gynecol Reprod Endocrinol. 2002;101:201-203.
31. Aka N, Kuscu NK, Yazicioglu E. Impetigo herpetiformis at the 36th week of gestation. Int J Gynecol Obstet. 2000;69:153-154.
32. Wade TR, Wade SL, Jones HE. Skin changes and diseases associated with pregnancy. Obstet Gynecol. 1978;52:233-242.
• Pemphigoid gestationis is best managed with oral prednisone at doses from 20 to 60 mg per day to control symptoms. B
• The pruritus associated with pruritic urticarial papules and plaques of pregnancy can be safely and effectively managed with topical corticosteroids and oral antihistamines. A
• Treat intrahepatic cholestasis of pregnancy with ursodeoxycholic acid, which likely reduces serum bile acids as well as associated fetal morbidity and mortality. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The dermatoses of pregnancy are a poorly understood group of dermatologic conditions. The only thing they have in common is a tendency to appear during pregnancy.
Only 3 are considered unique to pregnancy, however; the others are probably exacerbations of preexisting conditions triggered by pregnancy. There isn’t even complete agreement on what to call them. And to make management even more complex, 2 patients—the mother and the fetus—need to be considered.
Who will manage these patients is another matter. These conditions fall into an overlapping area of health care, where family physicians, obstetricians, and dermatologists all have some share in the responsibility for diagnosis and treatment. As a family physician who probably cares for any number of pregnant patients on a weekly basis, you need to be sufficiently familiar with these conditions so that you can differentiate those that can be treated symptomatically and those that require a referral to a specialist. This review and handy TABLE will help you toward that end.
TABLE
Skin disorders of pregnancy: What you’ll see, how to treat
| Disorder | Lesions | Diagnosis and sequelae | Treatment | Recurrence |
|---|---|---|---|---|
| PG3,5 | Erythematous papules, progressing to vesicles, bullae; periumbilical distribution, sparing face, palms, and soles | Mean onset at 21 weeks; postpartum in 20% of cases. Direct immunofluorescence microscopy shows linear C3 deposition. Newborn may be small for gestational age, but no associated morbidity or mortality | Oral corticosteroids 20-60 mg/d, IVIG, or cyclosporine in refractory cases | Frequent. Skips a pregnancy 8% of the time |
| PUPPP8-10 | Urticarial papules and plaques on abdomen, legs, arms, buttocks, chest, and back | Usually present after 34th week, but can present at any stage. Diagnosis is clinical. No increase in fetal morbidity or mortality | Topical steroids and antihistamines | Uncommon |
| ICP14,17,19-22 | No primary lesions; secondary excoriations in any area patient can reach | Onset after 30th week in 80% of patients. Strongly indicated by serum bile acid >11 mcmol/L. Increased fetal mortality | Ursodeoxycholic acid 450-1200 mg/d | Frequent |
| EP/PP4,10,24 | Grouped, crusted erythematous papules, patches, and plaques, most commonly on extensor surfaces of arms and legs or on abdomen | Onset at any point in pregnancy. Clinical diagnosis. No increase in fetal morbidity or mortality | Symptomatic treatment with topical steroids or antihistamines | Frequent |
| APPP27-29 | Erythematous plaques and pustules starting on inner thighs and groin and spreading to trunk and extremities | Onset at any point in pregnancy. Clinical diagnosis by appearance of lesions and association with systemic illness. Increased incidence of miscarriage and stillbirth, and maternal mortality | Prednisone 15-60 mg/d, cyclosporine 100 mg twice daily in refractory cases, management of associated hypocalcemia | Unknown |
| PFP24,29 | Papules and pustules concentrated around hair follicles, often beginning on abdomen and spreading to extremities | Onset most often in third trimester. Clinical diagnosis. No associated fetal morbidity or mortality | Topical steroids | Unknown |
| APPP, acute pustular psoriasis of pregnancy; EP/PP, eczema of pregnancy/pruritus of pregnancy; ICP, intrahepatic cholestasis of pregnancy; IVIG, intravenous immunoglobulin; PFP, pruritic folliculitis of pregnancy; PG, pemphigoid gestationis; PUPPP, pruritic urticarial papules and plaques of pregnancy. | ||||
Dermatoses unique to pregnancy
Pemphigoid gestationis
Years ago, this disorder was referred to as herpes gestationis, because the lesions are herpetiform. Pemphigoid gestationis (PG) has an incidence of approximately 1 in 10,000 pregnancies.1,2 The time of onset is usually about the 21st week of gestation, although in about 20% of cases, the eruption appears immediately postpartum.3
Presentation. The disease usually begins with urticarial papules and plaques around the umbilicus and extremities. Bullous lesions tend to develop as the disease progresses, and are often not present on first presentation (FIGURE 1). PG lesions tend to spare the face, palms, and soles. Mucosal surfaces are involved in less than 20% of cases. In about 75% of cases, PG flares around the time of delivery, regressing spontaneously after the baby is born.4
Pathophysiology. The pathophysiology is nearly identical to that of bullous pemphigoid, a blistering skin disorder more often seen in elderly patients.5 Pemphigoid disorders are immune processes, involving an immunoglobulin G (IgG) immune response directed at a 180-kDa hemidesmosome transmembrane glycoprotein. This protein is the common target of several subepidermal blistering diseases.
Differential. Disorders that may have some of the same features as PG include pruritic urticarial papules and plaques of pregnancy (PUPPP), erythema multiforme, intrahepatic cholestasis of pregnancy (ICP), contact dermatitis, and drug reactions.
Diagnosis. A biopsy is necessary for the definitive diagnosis. Direct immunofluorescence (DIF) microscopy of a sample of perilesional skin can show tissue-bound immunoreactants. Linear deposition of the complement component protein C3 along the basement membrane zone is diagnostic for PG. IgG is also deposited about 40% of the time.3 Serum enzyme-linked immunosorbent assay (ELISA) studies are also helpful in diagnosis. They have excellent sensitivity and specificity, as well as the capacity to monitor levels of antibody, which correlate with the severity of disease.1
Treatment. Oral corticosteroids are the first-line treatment for PG, typically 20 to 60 mg per day of prednisone. Oral corticosteroids are typically most effective in ameliorating the patient’s symptoms. Prednisone at doses of 40 to 80 mg per day for a short time has not been associated with congenital abnormalities.6 PG patients can also be treated successfully with intravenous immunoglobulin (IVIG) and cyclosporine in refractory cases.7
Pruritus associated with this condition can interfere with day-to-day activity and with the patient’s ability to sleep. Patients may also complain that the rash is painful, particularly if bullae rupture, leading to superficial ulcerations. Fortunately, the patient’s quality of life can be dramatically improved with systemic corticosteroids—with no significant risk to the fetus.
Sequelae. PG uniformly resolves within a few weeks, but the mother’s autoantibodies can passively be transferred to the fetus, causing vesicles and bullae in the newborn.8 An increased incidence of small-for-gestational age (SGA) infants has also been noted in PG, although no lasting morbidity or mortality in the offspring has been noted.5 This disease tends to recur with future pregnancies, but will skip a pregnancy 8% of the time.5
FIGURE 1
Pemphigoid gestationis
Pruritic urticarial papules and plaques of pregnancy
This condition is known by many names besides PUPPP: polymorphic eruption of pregnancy, toxemic erythema of pregnancy, and late prurigo of pregnancy.1 It is a pruritic, inflammatory skin disorder that has been variously estimated to occur in anywhere from 1 in 120 to 1 in 240 pregnancies.8 PUPPP is second only to eczema as the most common dermatosis of pregnancy.
Presentation. As the name implies, the lesions of PUPPP are itchy, red papules that often coalesce into plaques (FIGURE 2). The lesions usually occur in primigravidas after the 34th week of gestation, although they may be seen at any time from the first trimester through the postpartum period.9
Lesions are classically found on the abdomen, sparing the umbilical area, and are found primarily in the striae. This distribution helps to differentiate PUPPP from PG, where the lesions typically cluster around the umbilicus. Most PUPPP lesions (80% in 1 study) are dispersed on the abdomen, legs, arms, buttocks, chest, and back. Another 17% appear only on the abdomen and proximal thighs, and the remaining 3% on the limbs.10 Nearly 50% of the time, lesions also include discrete vesicles.11 There are no reported cases of mucosal involvement.
Patients with this condition are often very uncomfortable. The associated pruritus is severe enough to interfere with sleep. Despite the itching, however, lesions are seldom excoriated.
Pathophysiology. The disorder has been strongly associated with maternal weight gain and multiple gestations. One working hypothesis is that rapid abdominal distention observed in the third trimester leads to damage of the connective tissue, which then releases antigenic molecules, causing an inflammatory reaction.12 Another hypothesis is that increased levels of fetal DNA that have been detected in the skin of PUPPP patients may contribute to the pathology. One study detected male DNA in 6 of 10 PUPPP sufferers, but found none in any of 26 controls— pregnant women without PUPPP pathology.5 There is some evidence that patients with atopy may be predisposed to PUPPP, as well as patients who are hypertensive or obese.10,13
Differential. Initially, PUPPP lesions can be difficult to differentiate from urticarial PG lesions. The distribution of the lesions is the best clue: PG lesions cluster around the umbilicus, whereas PUPPP lesions uniformly spare the umbilical area. Additional disorders in the PUPPP differential are atopic dermatitis, superficial urticarial allergic eruption, viral exanthema, and contact or irritant dermatitis.
Diagnosis. PUPPP can only be diagnosed through clinical observation. None of the available laboratory tests—immunofluorescence, histology, serology—yield findings specific for PUPPP, although histology and immunofluorescence can readily differentiate between this condition and PG.
Treatment. Because the disease holds no real danger for mother or fetus, treatment can be aimed solely at symptomatic relief. Mild to potent topical steroids (consider triamcinolone or fluocinonide) should relieve pruritus within 48 to 72 hours.8 Antihistamines and occasionally low-dose systemic steroids may also be used. Consider hydroxyzine, although diphenhydramine has the more proven safety profile in pregnancy.
Nonpharmacologic treatments such as oil baths and emollients should also be considered. If the condition appears classic for PUPPP, it can be managed symptomatically. However, if there is any question about the diagnosis, referral to a dermatologist is prudent.
Sequelae. No increase in maternal or fetal morbidity or mortality is associated with PUPPP. Recurrence is fairly uncommon, as the disease primarily affects women during their first pregnancy.
FIGURE 2
Pruritic urticarial papules and plaques of pregnancy
Intrahepatic cholestasis of pregnancy
This condition is also called recurrent or idiopathic jaundice of pregnancy, obstetric cholestasis, and pruritus gravidarum. ICP is caused by disruption of hepatic bile flow during pregnancy. It has been recorded at a rate of approximately 10 to 150 per 10,000 pregnancies in Europe and 70 per 10,000 in the United States.12 In 80% of patients, the time of onset is after the 30th week.14 Although this disorder is not primarily a dermatosis of pregnancy, it is a pruritic condition that often presents with excoriations in pregnant women and is associated with fetal morbidity and mortality. It’s important to be able to identify this disease early to minimize sequelae.
Presentation. There are no primary lesions with ICP. The primary presenting symptom is a generalized pruritus affecting the palms and soles, and sometimes extending to the legs and abdomen (FIGURE 3). This itching is often so severe that it leads to chronic insomnia. You may see secondary skin lesions, such as erythema and excoriations. Observable jaundice occurs in 10% to 20% of patients.3 These patients do not develop the encephalopathy that is associated with cholestasis in the nonpregnant state, however.14
Pathophysiology. The genesis of this condition is thought to be a combination of genetic and environmental factors. A family history of the disorder is present in half the cases, and cases with a familial component tend to be more severe.15 ICP may be an exaggerated response to increased estrogen levels in pregnancy, but the mechanism of this response is unknown.16
Differential. Other conditions that must be considered in making the diagnosis are viral hepatitis, gallbladder disease, PG, PUPPP, drug hepatotoxicity, primary biliary cirrhosis, and uremia.
Diagnosis. Laboratory values are the definitive diagnostic tool in this condition. Increased serum bile acids are the single most sensitive test. Average levels of serum bile acids in pregnancy are 6.6 mcmol/L, with an upper limit of 11. The average value in women with ICP is 47 mcmol/L.17
While serum acids remain the gold standard, a recent study showed elevated urine bile acids to have 100% sensitivity and 83% specificity for ICP.18 In 55% to 60% of cases, the liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are mildly increased. Steatorrhea is often noted by the patient, and is followed by vitamin K deficiency.17
Treatment. The current standard of care for ICP is treatment with ursodeoxycholic acid (UDCA). In 4 controlled trials, UDCA showed a sustained decrease in serum bile acids.19-22 Doses used in these trials have varied between 450 and 1200 mg per day.
Before UDCA treatment was available, the disorder was treated with cholestyramine, which could bring about a 70% rate of response. The drawback to cholestyramine treatment is that it precipitates vitamin K, which is already compromised by the disease process. Further, its onset of action is slow.3
Elective delivery is indicated for ICP, particularly in patients with significant clinical presentations.12 Delivery for ICP should be performed around week 37 to 38, as stillbirths tend to cluster around weeks 37 to 39.14 Given the significant fetal mortality associated with this condition (see below), ICP should be managed by a clinician experienced with the disease, likely a gastroenterologist.
Sequelae. The impact of this maternal disorder on the fetus can be disastrous: a 10% to 15% rate of perinatal death, and a 30% to 40% rate of premature labor have been seen with ICP.14 Fortunately, rates of preterm labor are strongly correlated with levels of bile acids, so that as bile acid levels are reduced with UDCA treatment, rates of preterm labor also go down. Currently, management of the condition has reduced rates of perinatal death to 3.5%. There is no evidence of fetal growth retardation.14
FIGURE 3
Intrahepatic cholestasis of pregnancy
Dermatoses triggered by pregnancy
Eczema of pregnancy/prurigo of pregnancy
Eczema of pregnancy/prurigo of pregnancy (EP/PP) may not actually be correlated with the pregnant state. Both conditions manifest as eczematous lesions in an atopic distribution. Although they have been described in the literature as separate entities, the lack of clinical distinction between them led Ambros-Rudolph and colleagues to combine them under the umbrella term, atopic eruption of pregnancy.23 In some patients, at least, they may be preexisting conditions that pregnancy exacerbates. One study of 255 patients with the condition found that 20% had had the lesions before they became pregnant.23 However, the tendency of the condition to be markedly worsened by pregnancy leads us to include it here.
PP has an estimated incidence of 1 in 450 pregnancies.11 But while many authorities consider EP to be the most common dermatosis of pregnancy, no clear estimation of its prevalence has been established.23,24 Taken together, these 2 conditions have the highest prevalence of all pregnancy-induced dermatoses. PP is also known as popular dermatitis of Spangler, Nurse’s early prurigo of pregnancy, and linear IgM disease of pregnancy.3,23,25
Presentation. The typical presentation consists of grouped, crusted, erythematous papules, patches, and plaques—frequently with excoriations. The lesions typically present on the extensor surfaces of the arms and legs or on the abdomen (FIGURE 4).4 Recurrence in later pregnancies is common.
Pathophysiology. The pathophysiology of EP/PP is not understood. Many patients who present with EP/PP have a history of atopy.10
Differential. Conditions that need to be considered in making the diagnosis include tinea infection, scabies, contact dermatitis, ICP, pruritic folliculitis of pregnancy (PFP), and PG.
Diagnosis. History and physical examination determine the diagnosis. Serology, histopathology, and immunofluorescence are not specific, and correlation with increased IgE is marginal, at best.24,26
Treatment. These conditions are treated symptomatically with topical steroids or systemic antihistamines.
Sequelae. No maternal or fetal increase in morbidity or mortality is associated with these conditions.
FIGURE 4
Eczema of pregnancy
Acute pustular psoriasis of pregnancy
Whether or not APPP is actually a pregnancyinduced dermatosis is subject to debate.
There is evidence that APPP is not unique to pregnancy, but is simply a manifestation of ordinary psoriasis. Clinically and histologically, APPP is indistinguishable from pustular psoriasis. Unlike most cases of acute psoriasis, however, APPP often appears in pregnancy without any personal or family history of psoriasis, and usually ceases when the pregnancy is concluded. This fact, combined with reports of increased fetal and maternal morbidity and mortality associated with APPP, lead us to include it here.27
Presentation. APPP is a rare condition that may have an onset at any point in pregnancy. The characteristic lesions begin as erythematous plaques with pustules on the inner thighs, flexural areas, and groin and spread to the trunk and extremities. As the plaques enlarge, the center becomes eroded and crusted. Nails may become onycholytic. The hands, feet, and face are usually spared. Oral and esophageal erosions can occur. Pruritus is typically mild, although the lesions are often painful and flu-like symptoms are often present.28
Pathophysiology. The pathophysiology of this disease is unknown.
Differential. Conditions with similar presentations include an adverse drug reaction, pityriasis rosea, lichen simplex chronicus, eczema, lupus, and pityriasis rubra pilaris.
Diagnosis. Clinical history and association with systemic illness are the basis for a diagnosis of APPP. Cultures of the pustules are negative for any infective pathology, though as the disease progresses, pustules may become superinfected. Lab tests may show an increased erythrocyte sedimentation rate (ESR), hypocalcemia, and low levels of vitamin D.
Treatment. Prednisone 15 to 60 mg per day is often sufficient to control the disease.28 Cyclosporine 100 mg twice daily has also been shown to be useful.29 Cyclosporine in pregnancy is a category C drug. Data on fetal malformation associated with cyclosporine therapy are limited, but the risk appears to be minimal.6 Maternal hypocalcemia should be monitored and treated appropriately. If disease progression is judged serious enough, early induction of labor is indicated, since delivery will almost always lead to swift resolution.
Sequelae. There have been a number of case reports that link APPP to serious sequelae, including fetal growth retardation, hypocalcemia, and stillbirth.28,30,31 The condition is too rare, however, for good data on specific sequelae. While the disease does give significant cause for concern, it would appear that some of the traditional apprehension comes from older publications reporting a rate of maternal mortality of 70% to 90%.32 This statistic has not been borne out in clinical practice. It does appear that the mother will frequently suffer from systemic symptoms, including fever and malaise.
Pruritic folliculitis of pregnancy
Accounts of PFP’s prevalence vary widely: Some sources report fewer than 30 cases in all of the literature, while others indicate that the prevalence is equivalent to that of PG, 1 in 10,000.3,11 PFP most commonly presents in the third trimester. It often resolves before delivery, but uniformly clears within 2 weeks of delivery.
Presentation. PFP presents as papules and pustules concentrated around hair follicles (FIGURE 5). Often lesions begin on the abdomen and spread to the extremities.24,29 The condition is often, but not always, pruritic. Patients are more likely to be concerned about what the condition means for their health, rather than being distressed by the symptoms.
Pathophysiology. Like so many of these conditions, the pathophysiology of PFP is unknown. There is little evidence that the condition is immunologically or hormonally mediated, and there is no evidence of an infectious component.24,29
Differential. PFP must be distinguished from infectious folliculitis, acneiform disorders, HIV-associated eosinophilic folliculitis, and a drug reaction.
Diagnosis. The clinical diagnosis is based on presenting symptoms and third trimester onset. No specific lab or histological analysis can be used to make a definitive diagnosis.
Treatment. As the condition is, by definition, a nonmicrobial folliculitis, the most effective therapy tends to be with mid- to lowpotency topical steroids such as triamcinolone or desonide. Additionally, benzoyl peroxide wash can be effective.
Sequelae. One study reports an increased incidence of low birth weight, but currently no associated morbidity or mortality has been reported.24
FIGURE 5
Pruritic folliculitis of pregnancy
CORRESPONDENCE
Matthew Bremmer, MD, 419 W Redwood Street, Department of Dermatology, Baltimore, MD 21230; Mbrem001@umaryland.edu
• Pemphigoid gestationis is best managed with oral prednisone at doses from 20 to 60 mg per day to control symptoms. B
• The pruritus associated with pruritic urticarial papules and plaques of pregnancy can be safely and effectively managed with topical corticosteroids and oral antihistamines. A
• Treat intrahepatic cholestasis of pregnancy with ursodeoxycholic acid, which likely reduces serum bile acids as well as associated fetal morbidity and mortality. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The dermatoses of pregnancy are a poorly understood group of dermatologic conditions. The only thing they have in common is a tendency to appear during pregnancy.
Only 3 are considered unique to pregnancy, however; the others are probably exacerbations of preexisting conditions triggered by pregnancy. There isn’t even complete agreement on what to call them. And to make management even more complex, 2 patients—the mother and the fetus—need to be considered.
Who will manage these patients is another matter. These conditions fall into an overlapping area of health care, where family physicians, obstetricians, and dermatologists all have some share in the responsibility for diagnosis and treatment. As a family physician who probably cares for any number of pregnant patients on a weekly basis, you need to be sufficiently familiar with these conditions so that you can differentiate those that can be treated symptomatically and those that require a referral to a specialist. This review and handy TABLE will help you toward that end.
TABLE
Skin disorders of pregnancy: What you’ll see, how to treat
| Disorder | Lesions | Diagnosis and sequelae | Treatment | Recurrence |
|---|---|---|---|---|
| PG3,5 | Erythematous papules, progressing to vesicles, bullae; periumbilical distribution, sparing face, palms, and soles | Mean onset at 21 weeks; postpartum in 20% of cases. Direct immunofluorescence microscopy shows linear C3 deposition. Newborn may be small for gestational age, but no associated morbidity or mortality | Oral corticosteroids 20-60 mg/d, IVIG, or cyclosporine in refractory cases | Frequent. Skips a pregnancy 8% of the time |
| PUPPP8-10 | Urticarial papules and plaques on abdomen, legs, arms, buttocks, chest, and back | Usually present after 34th week, but can present at any stage. Diagnosis is clinical. No increase in fetal morbidity or mortality | Topical steroids and antihistamines | Uncommon |
| ICP14,17,19-22 | No primary lesions; secondary excoriations in any area patient can reach | Onset after 30th week in 80% of patients. Strongly indicated by serum bile acid >11 mcmol/L. Increased fetal mortality | Ursodeoxycholic acid 450-1200 mg/d | Frequent |
| EP/PP4,10,24 | Grouped, crusted erythematous papules, patches, and plaques, most commonly on extensor surfaces of arms and legs or on abdomen | Onset at any point in pregnancy. Clinical diagnosis. No increase in fetal morbidity or mortality | Symptomatic treatment with topical steroids or antihistamines | Frequent |
| APPP27-29 | Erythematous plaques and pustules starting on inner thighs and groin and spreading to trunk and extremities | Onset at any point in pregnancy. Clinical diagnosis by appearance of lesions and association with systemic illness. Increased incidence of miscarriage and stillbirth, and maternal mortality | Prednisone 15-60 mg/d, cyclosporine 100 mg twice daily in refractory cases, management of associated hypocalcemia | Unknown |
| PFP24,29 | Papules and pustules concentrated around hair follicles, often beginning on abdomen and spreading to extremities | Onset most often in third trimester. Clinical diagnosis. No associated fetal morbidity or mortality | Topical steroids | Unknown |
| APPP, acute pustular psoriasis of pregnancy; EP/PP, eczema of pregnancy/pruritus of pregnancy; ICP, intrahepatic cholestasis of pregnancy; IVIG, intravenous immunoglobulin; PFP, pruritic folliculitis of pregnancy; PG, pemphigoid gestationis; PUPPP, pruritic urticarial papules and plaques of pregnancy. | ||||
Dermatoses unique to pregnancy
Pemphigoid gestationis
Years ago, this disorder was referred to as herpes gestationis, because the lesions are herpetiform. Pemphigoid gestationis (PG) has an incidence of approximately 1 in 10,000 pregnancies.1,2 The time of onset is usually about the 21st week of gestation, although in about 20% of cases, the eruption appears immediately postpartum.3
Presentation. The disease usually begins with urticarial papules and plaques around the umbilicus and extremities. Bullous lesions tend to develop as the disease progresses, and are often not present on first presentation (FIGURE 1). PG lesions tend to spare the face, palms, and soles. Mucosal surfaces are involved in less than 20% of cases. In about 75% of cases, PG flares around the time of delivery, regressing spontaneously after the baby is born.4
Pathophysiology. The pathophysiology is nearly identical to that of bullous pemphigoid, a blistering skin disorder more often seen in elderly patients.5 Pemphigoid disorders are immune processes, involving an immunoglobulin G (IgG) immune response directed at a 180-kDa hemidesmosome transmembrane glycoprotein. This protein is the common target of several subepidermal blistering diseases.
Differential. Disorders that may have some of the same features as PG include pruritic urticarial papules and plaques of pregnancy (PUPPP), erythema multiforme, intrahepatic cholestasis of pregnancy (ICP), contact dermatitis, and drug reactions.
Diagnosis. A biopsy is necessary for the definitive diagnosis. Direct immunofluorescence (DIF) microscopy of a sample of perilesional skin can show tissue-bound immunoreactants. Linear deposition of the complement component protein C3 along the basement membrane zone is diagnostic for PG. IgG is also deposited about 40% of the time.3 Serum enzyme-linked immunosorbent assay (ELISA) studies are also helpful in diagnosis. They have excellent sensitivity and specificity, as well as the capacity to monitor levels of antibody, which correlate with the severity of disease.1
Treatment. Oral corticosteroids are the first-line treatment for PG, typically 20 to 60 mg per day of prednisone. Oral corticosteroids are typically most effective in ameliorating the patient’s symptoms. Prednisone at doses of 40 to 80 mg per day for a short time has not been associated with congenital abnormalities.6 PG patients can also be treated successfully with intravenous immunoglobulin (IVIG) and cyclosporine in refractory cases.7
Pruritus associated with this condition can interfere with day-to-day activity and with the patient’s ability to sleep. Patients may also complain that the rash is painful, particularly if bullae rupture, leading to superficial ulcerations. Fortunately, the patient’s quality of life can be dramatically improved with systemic corticosteroids—with no significant risk to the fetus.
Sequelae. PG uniformly resolves within a few weeks, but the mother’s autoantibodies can passively be transferred to the fetus, causing vesicles and bullae in the newborn.8 An increased incidence of small-for-gestational age (SGA) infants has also been noted in PG, although no lasting morbidity or mortality in the offspring has been noted.5 This disease tends to recur with future pregnancies, but will skip a pregnancy 8% of the time.5
FIGURE 1
Pemphigoid gestationis
Pruritic urticarial papules and plaques of pregnancy
This condition is known by many names besides PUPPP: polymorphic eruption of pregnancy, toxemic erythema of pregnancy, and late prurigo of pregnancy.1 It is a pruritic, inflammatory skin disorder that has been variously estimated to occur in anywhere from 1 in 120 to 1 in 240 pregnancies.8 PUPPP is second only to eczema as the most common dermatosis of pregnancy.
Presentation. As the name implies, the lesions of PUPPP are itchy, red papules that often coalesce into plaques (FIGURE 2). The lesions usually occur in primigravidas after the 34th week of gestation, although they may be seen at any time from the first trimester through the postpartum period.9
Lesions are classically found on the abdomen, sparing the umbilical area, and are found primarily in the striae. This distribution helps to differentiate PUPPP from PG, where the lesions typically cluster around the umbilicus. Most PUPPP lesions (80% in 1 study) are dispersed on the abdomen, legs, arms, buttocks, chest, and back. Another 17% appear only on the abdomen and proximal thighs, and the remaining 3% on the limbs.10 Nearly 50% of the time, lesions also include discrete vesicles.11 There are no reported cases of mucosal involvement.
Patients with this condition are often very uncomfortable. The associated pruritus is severe enough to interfere with sleep. Despite the itching, however, lesions are seldom excoriated.
Pathophysiology. The disorder has been strongly associated with maternal weight gain and multiple gestations. One working hypothesis is that rapid abdominal distention observed in the third trimester leads to damage of the connective tissue, which then releases antigenic molecules, causing an inflammatory reaction.12 Another hypothesis is that increased levels of fetal DNA that have been detected in the skin of PUPPP patients may contribute to the pathology. One study detected male DNA in 6 of 10 PUPPP sufferers, but found none in any of 26 controls— pregnant women without PUPPP pathology.5 There is some evidence that patients with atopy may be predisposed to PUPPP, as well as patients who are hypertensive or obese.10,13
Differential. Initially, PUPPP lesions can be difficult to differentiate from urticarial PG lesions. The distribution of the lesions is the best clue: PG lesions cluster around the umbilicus, whereas PUPPP lesions uniformly spare the umbilical area. Additional disorders in the PUPPP differential are atopic dermatitis, superficial urticarial allergic eruption, viral exanthema, and contact or irritant dermatitis.
Diagnosis. PUPPP can only be diagnosed through clinical observation. None of the available laboratory tests—immunofluorescence, histology, serology—yield findings specific for PUPPP, although histology and immunofluorescence can readily differentiate between this condition and PG.
Treatment. Because the disease holds no real danger for mother or fetus, treatment can be aimed solely at symptomatic relief. Mild to potent topical steroids (consider triamcinolone or fluocinonide) should relieve pruritus within 48 to 72 hours.8 Antihistamines and occasionally low-dose systemic steroids may also be used. Consider hydroxyzine, although diphenhydramine has the more proven safety profile in pregnancy.
Nonpharmacologic treatments such as oil baths and emollients should also be considered. If the condition appears classic for PUPPP, it can be managed symptomatically. However, if there is any question about the diagnosis, referral to a dermatologist is prudent.
Sequelae. No increase in maternal or fetal morbidity or mortality is associated with PUPPP. Recurrence is fairly uncommon, as the disease primarily affects women during their first pregnancy.
FIGURE 2
Pruritic urticarial papules and plaques of pregnancy
Intrahepatic cholestasis of pregnancy
This condition is also called recurrent or idiopathic jaundice of pregnancy, obstetric cholestasis, and pruritus gravidarum. ICP is caused by disruption of hepatic bile flow during pregnancy. It has been recorded at a rate of approximately 10 to 150 per 10,000 pregnancies in Europe and 70 per 10,000 in the United States.12 In 80% of patients, the time of onset is after the 30th week.14 Although this disorder is not primarily a dermatosis of pregnancy, it is a pruritic condition that often presents with excoriations in pregnant women and is associated with fetal morbidity and mortality. It’s important to be able to identify this disease early to minimize sequelae.
Presentation. There are no primary lesions with ICP. The primary presenting symptom is a generalized pruritus affecting the palms and soles, and sometimes extending to the legs and abdomen (FIGURE 3). This itching is often so severe that it leads to chronic insomnia. You may see secondary skin lesions, such as erythema and excoriations. Observable jaundice occurs in 10% to 20% of patients.3 These patients do not develop the encephalopathy that is associated with cholestasis in the nonpregnant state, however.14
Pathophysiology. The genesis of this condition is thought to be a combination of genetic and environmental factors. A family history of the disorder is present in half the cases, and cases with a familial component tend to be more severe.15 ICP may be an exaggerated response to increased estrogen levels in pregnancy, but the mechanism of this response is unknown.16
Differential. Other conditions that must be considered in making the diagnosis are viral hepatitis, gallbladder disease, PG, PUPPP, drug hepatotoxicity, primary biliary cirrhosis, and uremia.
Diagnosis. Laboratory values are the definitive diagnostic tool in this condition. Increased serum bile acids are the single most sensitive test. Average levels of serum bile acids in pregnancy are 6.6 mcmol/L, with an upper limit of 11. The average value in women with ICP is 47 mcmol/L.17
While serum acids remain the gold standard, a recent study showed elevated urine bile acids to have 100% sensitivity and 83% specificity for ICP.18 In 55% to 60% of cases, the liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are mildly increased. Steatorrhea is often noted by the patient, and is followed by vitamin K deficiency.17
Treatment. The current standard of care for ICP is treatment with ursodeoxycholic acid (UDCA). In 4 controlled trials, UDCA showed a sustained decrease in serum bile acids.19-22 Doses used in these trials have varied between 450 and 1200 mg per day.
Before UDCA treatment was available, the disorder was treated with cholestyramine, which could bring about a 70% rate of response. The drawback to cholestyramine treatment is that it precipitates vitamin K, which is already compromised by the disease process. Further, its onset of action is slow.3
Elective delivery is indicated for ICP, particularly in patients with significant clinical presentations.12 Delivery for ICP should be performed around week 37 to 38, as stillbirths tend to cluster around weeks 37 to 39.14 Given the significant fetal mortality associated with this condition (see below), ICP should be managed by a clinician experienced with the disease, likely a gastroenterologist.
Sequelae. The impact of this maternal disorder on the fetus can be disastrous: a 10% to 15% rate of perinatal death, and a 30% to 40% rate of premature labor have been seen with ICP.14 Fortunately, rates of preterm labor are strongly correlated with levels of bile acids, so that as bile acid levels are reduced with UDCA treatment, rates of preterm labor also go down. Currently, management of the condition has reduced rates of perinatal death to 3.5%. There is no evidence of fetal growth retardation.14
FIGURE 3
Intrahepatic cholestasis of pregnancy
Dermatoses triggered by pregnancy
Eczema of pregnancy/prurigo of pregnancy
Eczema of pregnancy/prurigo of pregnancy (EP/PP) may not actually be correlated with the pregnant state. Both conditions manifest as eczematous lesions in an atopic distribution. Although they have been described in the literature as separate entities, the lack of clinical distinction between them led Ambros-Rudolph and colleagues to combine them under the umbrella term, atopic eruption of pregnancy.23 In some patients, at least, they may be preexisting conditions that pregnancy exacerbates. One study of 255 patients with the condition found that 20% had had the lesions before they became pregnant.23 However, the tendency of the condition to be markedly worsened by pregnancy leads us to include it here.
PP has an estimated incidence of 1 in 450 pregnancies.11 But while many authorities consider EP to be the most common dermatosis of pregnancy, no clear estimation of its prevalence has been established.23,24 Taken together, these 2 conditions have the highest prevalence of all pregnancy-induced dermatoses. PP is also known as popular dermatitis of Spangler, Nurse’s early prurigo of pregnancy, and linear IgM disease of pregnancy.3,23,25
Presentation. The typical presentation consists of grouped, crusted, erythematous papules, patches, and plaques—frequently with excoriations. The lesions typically present on the extensor surfaces of the arms and legs or on the abdomen (FIGURE 4).4 Recurrence in later pregnancies is common.
Pathophysiology. The pathophysiology of EP/PP is not understood. Many patients who present with EP/PP have a history of atopy.10
Differential. Conditions that need to be considered in making the diagnosis include tinea infection, scabies, contact dermatitis, ICP, pruritic folliculitis of pregnancy (PFP), and PG.
Diagnosis. History and physical examination determine the diagnosis. Serology, histopathology, and immunofluorescence are not specific, and correlation with increased IgE is marginal, at best.24,26
Treatment. These conditions are treated symptomatically with topical steroids or systemic antihistamines.
Sequelae. No maternal or fetal increase in morbidity or mortality is associated with these conditions.
FIGURE 4
Eczema of pregnancy
Acute pustular psoriasis of pregnancy
Whether or not APPP is actually a pregnancyinduced dermatosis is subject to debate.
There is evidence that APPP is not unique to pregnancy, but is simply a manifestation of ordinary psoriasis. Clinically and histologically, APPP is indistinguishable from pustular psoriasis. Unlike most cases of acute psoriasis, however, APPP often appears in pregnancy without any personal or family history of psoriasis, and usually ceases when the pregnancy is concluded. This fact, combined with reports of increased fetal and maternal morbidity and mortality associated with APPP, lead us to include it here.27
Presentation. APPP is a rare condition that may have an onset at any point in pregnancy. The characteristic lesions begin as erythematous plaques with pustules on the inner thighs, flexural areas, and groin and spread to the trunk and extremities. As the plaques enlarge, the center becomes eroded and crusted. Nails may become onycholytic. The hands, feet, and face are usually spared. Oral and esophageal erosions can occur. Pruritus is typically mild, although the lesions are often painful and flu-like symptoms are often present.28
Pathophysiology. The pathophysiology of this disease is unknown.
Differential. Conditions with similar presentations include an adverse drug reaction, pityriasis rosea, lichen simplex chronicus, eczema, lupus, and pityriasis rubra pilaris.
Diagnosis. Clinical history and association with systemic illness are the basis for a diagnosis of APPP. Cultures of the pustules are negative for any infective pathology, though as the disease progresses, pustules may become superinfected. Lab tests may show an increased erythrocyte sedimentation rate (ESR), hypocalcemia, and low levels of vitamin D.
Treatment. Prednisone 15 to 60 mg per day is often sufficient to control the disease.28 Cyclosporine 100 mg twice daily has also been shown to be useful.29 Cyclosporine in pregnancy is a category C drug. Data on fetal malformation associated with cyclosporine therapy are limited, but the risk appears to be minimal.6 Maternal hypocalcemia should be monitored and treated appropriately. If disease progression is judged serious enough, early induction of labor is indicated, since delivery will almost always lead to swift resolution.
Sequelae. There have been a number of case reports that link APPP to serious sequelae, including fetal growth retardation, hypocalcemia, and stillbirth.28,30,31 The condition is too rare, however, for good data on specific sequelae. While the disease does give significant cause for concern, it would appear that some of the traditional apprehension comes from older publications reporting a rate of maternal mortality of 70% to 90%.32 This statistic has not been borne out in clinical practice. It does appear that the mother will frequently suffer from systemic symptoms, including fever and malaise.
Pruritic folliculitis of pregnancy
Accounts of PFP’s prevalence vary widely: Some sources report fewer than 30 cases in all of the literature, while others indicate that the prevalence is equivalent to that of PG, 1 in 10,000.3,11 PFP most commonly presents in the third trimester. It often resolves before delivery, but uniformly clears within 2 weeks of delivery.
Presentation. PFP presents as papules and pustules concentrated around hair follicles (FIGURE 5). Often lesions begin on the abdomen and spread to the extremities.24,29 The condition is often, but not always, pruritic. Patients are more likely to be concerned about what the condition means for their health, rather than being distressed by the symptoms.
Pathophysiology. Like so many of these conditions, the pathophysiology of PFP is unknown. There is little evidence that the condition is immunologically or hormonally mediated, and there is no evidence of an infectious component.24,29
Differential. PFP must be distinguished from infectious folliculitis, acneiform disorders, HIV-associated eosinophilic folliculitis, and a drug reaction.
Diagnosis. The clinical diagnosis is based on presenting symptoms and third trimester onset. No specific lab or histological analysis can be used to make a definitive diagnosis.
Treatment. As the condition is, by definition, a nonmicrobial folliculitis, the most effective therapy tends to be with mid- to lowpotency topical steroids such as triamcinolone or desonide. Additionally, benzoyl peroxide wash can be effective.
Sequelae. One study reports an increased incidence of low birth weight, but currently no associated morbidity or mortality has been reported.24
FIGURE 5
Pruritic folliculitis of pregnancy
CORRESPONDENCE
Matthew Bremmer, MD, 419 W Redwood Street, Department of Dermatology, Baltimore, MD 21230; Mbrem001@umaryland.edu
1. Cassian S, Powell J, Messer G, et al. Immunoblotting and enzymelinked immunosorbent assay for the diagnosis of pemphigoid gestationis. Obstet Gynecol. 2004;103:757-763.
2. Engineer L, Bohl K, Ahmed AR. Pemphigoid gestationis: a review. Am J Obstet Gynecol. 2000;183:483-491.
3. Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence based systematic review. Am J Obstet Gynecol, 2003;188:1083-1092.
4. Shornick JD. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17:172-181.
5. Shornick JK, Bangert JL, Freeman RG, et al. Herpes gestationis: clinical and histological features in 28 cases. J Am Acad Dermatol. 1983;8:214-224.
6. Leachman S, Reed B. The use of dermatologic drugs in pregnancy and lactation. Dermatol Clin. 2004;24:167-197.
7. Hern S, Harman K, Bhogal BS, et al. A severe persistent case of pemphigoid gestationis treated with intravenous immunoglobulins and cyclosporine. Clin Exp Dermatol. 1998;23:185-188.
8. Fitzpatrick TP. Diseases in pregnancy. Color Atlas and Synopsis of Clinical Dermatology. New York, NY: McGraw Hill; 1997:414–419.
9. Aractingi D, Berkane N, Bertheau P, et al. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352:1898-1901.
10. Rudolph CM, Al-Fares S, Vaughan-Jones SA, et al. Polymorphic eruption of pregnancy: clinicopathology and potential trigger factors in 181 patients. Clin Lab Invest. 2006;154:54-60.
11. Roger D, Vaillant L, Fignon A, et al. Specific pruritic diseases of pregnancy. A prospective study of 3192 pregnant women. Arch Dermatol. 1994;130:734-739.
12. McDonald J. Cholestasis of pregnancy. J Gastroenterol Hepatol. 1999;14:515-518.
13. Ohel I, Levy A, Silberstein T, et al. Pregnancy outcome of patients with pruritic urticarial papules and plaques of pregnancy. J Maternal-Fetal Neonat Med. 2006;19:305-308.
14. Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15:2049-2066.
15. Shaw D, Frohlich J, Wittmann BA, et al. A prospective study of 18 patients with cholestasis of pregnancy. Am J Obstet Gynecol. 1982;142:621-625.
16. Reyes H. The spectrum of liver and gastrointestinal disease seen in cholestasis of pregnancy. Gastroenterol Clin North Am. 1992;21:905-921.
17. Lammert F, Marschall H, Glantz A, et al. Intrahepatic cholestasis of pregnancy; molecular pathogenesis, diagnosis and management. J Hepatol. 2000;33:1012-1021.
18. Huang WM, Seubert DE, Donnelly JG, et al. Intrahepatic cholestasis of pregnancy: detection with urinary bile acid assays. J Perinat Med. 2007;35:486-491.
19. Palma J, Reyes H, Ribalta J, et al. Ursodeoxycholic acid in the treatment of intrahepatic cholestasis of pregnancy: a randomized, double blind study controlled with placebo. J Hepatol. 1997;27:1022-1028.
20. Diaferia A, Nicastri PL, Tartagni M, et al. Ursodeoxycholic acid therapy in pregnant women with cholestasis. Int J Gynaecol Obstet. 1996;52:133-140.
21. Nicastri PL, Diaferia A, Tartagni M, et al. A randomised placebocontrolled trial of ursodeoxycholic acid and S-adenosylmethionine in the treatment of intrahepatic cholestasis of pregnancy. Br J Obstet Gynaecol. 1998;105:1205-1207.
22. Glantz A, Marschall HU, Lammert F, et al. Intrahepatic cholestasis in pregnancy: a randomized controlled trial comparing dexamethasone and ursodeoxycholic acid. Hepatology. 2005;42:1399-1405.
23. Ambros-Rudolph CM, Mullegger MM, Vaughan-Jones SA, et al. The specific dermatoses of pregnancy revisited and reclassified: results of a retrospective two-center study on 505 pregnant patients. J Am Acad Dermatol. 2006;54:395-404.
24. Vaughan-Jones SA, Hern S, Nelson-Piercy C, et al. A prospective study of 200 women with dermatoses of pregnancy correlating clinical findings with hormonal and immunopathological profiles. Br J Dermatol. 1999;141:71-81.
25. Shornick JK. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17:172-181.
26. Holmes RC, Black MM. The specific dermatoses of pregnancy. J Am Acad Dermatol. 1983;8:405-412.
27. Bukhari IA. Impetigo herpetiformis in a primagravida: successful treatment with etretinate. J Drugs Dermatol. 2004;3:449-451.
28. Oumeish OY, Parish JL. Impetigo herpetiformis. Clin in Dermatol. 2006;24:101-104.
29. Kroumpouzos G, Cohen LM. Pruritic folliculitis of pregnancy. J Am Acad Dermatol. 2000;43:132-134.
30. Sahin HG, Hasin HA, Metin A, et al. Recurrent impetigo herpetiformis in a pregnant adolescent: a case report. Eur J Obstet Gynecol Reprod Endocrinol. 2002;101:201-203.
31. Aka N, Kuscu NK, Yazicioglu E. Impetigo herpetiformis at the 36th week of gestation. Int J Gynecol Obstet. 2000;69:153-154.
32. Wade TR, Wade SL, Jones HE. Skin changes and diseases associated with pregnancy. Obstet Gynecol. 1978;52:233-242.
1. Cassian S, Powell J, Messer G, et al. Immunoblotting and enzymelinked immunosorbent assay for the diagnosis of pemphigoid gestationis. Obstet Gynecol. 2004;103:757-763.
2. Engineer L, Bohl K, Ahmed AR. Pemphigoid gestationis: a review. Am J Obstet Gynecol. 2000;183:483-491.
3. Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence based systematic review. Am J Obstet Gynecol, 2003;188:1083-1092.
4. Shornick JD. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17:172-181.
5. Shornick JK, Bangert JL, Freeman RG, et al. Herpes gestationis: clinical and histological features in 28 cases. J Am Acad Dermatol. 1983;8:214-224.
6. Leachman S, Reed B. The use of dermatologic drugs in pregnancy and lactation. Dermatol Clin. 2004;24:167-197.
7. Hern S, Harman K, Bhogal BS, et al. A severe persistent case of pemphigoid gestationis treated with intravenous immunoglobulins and cyclosporine. Clin Exp Dermatol. 1998;23:185-188.
8. Fitzpatrick TP. Diseases in pregnancy. Color Atlas and Synopsis of Clinical Dermatology. New York, NY: McGraw Hill; 1997:414–419.
9. Aractingi D, Berkane N, Bertheau P, et al. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352:1898-1901.
10. Rudolph CM, Al-Fares S, Vaughan-Jones SA, et al. Polymorphic eruption of pregnancy: clinicopathology and potential trigger factors in 181 patients. Clin Lab Invest. 2006;154:54-60.
11. Roger D, Vaillant L, Fignon A, et al. Specific pruritic diseases of pregnancy. A prospective study of 3192 pregnant women. Arch Dermatol. 1994;130:734-739.
12. McDonald J. Cholestasis of pregnancy. J Gastroenterol Hepatol. 1999;14:515-518.
13. Ohel I, Levy A, Silberstein T, et al. Pregnancy outcome of patients with pruritic urticarial papules and plaques of pregnancy. J Maternal-Fetal Neonat Med. 2006;19:305-308.
14. Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15:2049-2066.
15. Shaw D, Frohlich J, Wittmann BA, et al. A prospective study of 18 patients with cholestasis of pregnancy. Am J Obstet Gynecol. 1982;142:621-625.
16. Reyes H. The spectrum of liver and gastrointestinal disease seen in cholestasis of pregnancy. Gastroenterol Clin North Am. 1992;21:905-921.
17. Lammert F, Marschall H, Glantz A, et al. Intrahepatic cholestasis of pregnancy; molecular pathogenesis, diagnosis and management. J Hepatol. 2000;33:1012-1021.
18. Huang WM, Seubert DE, Donnelly JG, et al. Intrahepatic cholestasis of pregnancy: detection with urinary bile acid assays. J Perinat Med. 2007;35:486-491.
19. Palma J, Reyes H, Ribalta J, et al. Ursodeoxycholic acid in the treatment of intrahepatic cholestasis of pregnancy: a randomized, double blind study controlled with placebo. J Hepatol. 1997;27:1022-1028.
20. Diaferia A, Nicastri PL, Tartagni M, et al. Ursodeoxycholic acid therapy in pregnant women with cholestasis. Int J Gynaecol Obstet. 1996;52:133-140.
21. Nicastri PL, Diaferia A, Tartagni M, et al. A randomised placebocontrolled trial of ursodeoxycholic acid and S-adenosylmethionine in the treatment of intrahepatic cholestasis of pregnancy. Br J Obstet Gynaecol. 1998;105:1205-1207.
22. Glantz A, Marschall HU, Lammert F, et al. Intrahepatic cholestasis in pregnancy: a randomized controlled trial comparing dexamethasone and ursodeoxycholic acid. Hepatology. 2005;42:1399-1405.
23. Ambros-Rudolph CM, Mullegger MM, Vaughan-Jones SA, et al. The specific dermatoses of pregnancy revisited and reclassified: results of a retrospective two-center study on 505 pregnant patients. J Am Acad Dermatol. 2006;54:395-404.
24. Vaughan-Jones SA, Hern S, Nelson-Piercy C, et al. A prospective study of 200 women with dermatoses of pregnancy correlating clinical findings with hormonal and immunopathological profiles. Br J Dermatol. 1999;141:71-81.
25. Shornick JK. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17:172-181.
26. Holmes RC, Black MM. The specific dermatoses of pregnancy. J Am Acad Dermatol. 1983;8:405-412.
27. Bukhari IA. Impetigo herpetiformis in a primagravida: successful treatment with etretinate. J Drugs Dermatol. 2004;3:449-451.
28. Oumeish OY, Parish JL. Impetigo herpetiformis. Clin in Dermatol. 2006;24:101-104.
29. Kroumpouzos G, Cohen LM. Pruritic folliculitis of pregnancy. J Am Acad Dermatol. 2000;43:132-134.
30. Sahin HG, Hasin HA, Metin A, et al. Recurrent impetigo herpetiformis in a pregnant adolescent: a case report. Eur J Obstet Gynecol Reprod Endocrinol. 2002;101:201-203.
31. Aka N, Kuscu NK, Yazicioglu E. Impetigo herpetiformis at the 36th week of gestation. Int J Gynecol Obstet. 2000;69:153-154.
32. Wade TR, Wade SL, Jones HE. Skin changes and diseases associated with pregnancy. Obstet Gynecol. 1978;52:233-242.