User login
A guide to managing disorders of the ear pinna and canal
Which antibiotics are most useful for infection following ear piercing? When is it safe to attempt removal of a foreign body from the ear canal, and which cerumenolytic agent may be best for ear wax? This review covers common ailments of the outer ear, which are often readily diagnosed given a patient’s history and thorough physical examination. We also address more complicated matters such as deciding when to refer for treatment of suspected malignant otitis externa, and which lab markers to follow when managing it yourself.
A (very) brief review of ear anatomy
Understanding the unique embryology and intricate anatomy of the external ear informs our understanding of predictable infections, growths, and malformations.
The external ear is composed of the external auditory canal and auricle. The external auditory canal has a lateral (external) cartilaginous portion and a medial (internal) bony portion. The auricular structure is complex and formed by the helix, antihelix (crura; scaphoid fossa), tragus, antitragus, conchae, and lobule. The auricle is composed of elastic cartilage covered by skin. The lobule is composed of skin, adipose tissue, and connective tissue.
Embryologically, the auricle, auditory canal, and middle ear form from ectoderm of the first 2 branchial arches during early gestation. The auricle forms from the fusion of soft-tissue swellings (hillocks). Three hillocks arise from the first branchial arch and 3 from the second branchial arch during the fifth and sixth weeks of gestation. Tissues from the second branchial arch comprise the lobule, antihelix, and caudal helix. The cartilage of the tragus forms from the first branchial arch. The ear canal forms from an epithelial invagination of the first branchial arch that also occurs during the fifth week of gestation.1
Infections
Perichondritis
Inflammation or infection of the connective tissue layer surrounding the auricular cartilage (perichondrium) results in perichondritis. Further extension of infection can lead to an auricular abscess. Both of these conditions can have serious consequences.
What you’ll see. The most common risk factor for perichondritis is the popular practice of cosmetic transcartilaginous piercing.2 Piercing of the helix, scapha, or anti-helix (often referred to as “high” ear piercing) causes localized trauma that can strip the adjacent perichondrium, decrease blood supply, create cartilaginous microfractures, and lead to devascularization. Rates of infection as high as 35% have been reported with high-ear piercing.3
The most common microbes associated with perichondritis and pinna abscess formation are Pseudomonas and Staphylococcus species.2 P
Continue to: How to treat
How to treat. The cornerstone of treatment is early detection and antimicrobial coverage with antipseudomonal antibiotics. Ciprofloxacin is the oral antibiotic of choice because of its ability to penetrate the tissue.4 Other options include clindamycin and third- or fourth-generation cephalosporins. If the wound becomes abscessed, perform (or refer for) early surgical incision and drainage.5 A failure to promptly recognize perichondritis or to mistakenly prescribe non-antipseudomonal antibiotics contributes to increased rates of hospitalization.2 Cosmetic deformity is the most common complication of perichondritis. This may require reconstructive surgery.
Otitis externa
Acute otitis externa (AOE; “swimmer’s ear”) is cellulitis of the skin and subdermis of the external ear canal. It is most prevalent in warm, moist climates and almost always associated with acute bacterial infection, most commonly P aeruginosa or S aureus.6 There is also an increased association with poor water quality (containing higher bacterial loads). Anything breaching the integrity of the ear canal can potentially predispose to the development of AOE. This includes trauma from cleaning, cerumen removal, scratching due to allergic conditions, and placement of hearing-aid devices.6
What you’ll see. Suspect AOE when signs or symptoms of ear canal inflammation have appeared rapidly (generally within 2 days) over the past 3 weeks.7 Findings include otalgia, itching, fullness, tragal tenderness, ear canal edema, erythema with or without otorrhea, lymphadenitis, or cellulitis of the pinna or adjacent skin.7 AOE must be distinguished from other causes of otalgia and otorrhea, including dermatitis and viral infection.
How to treat. Topical therapy is recommended for the initial treatment of uncomplicated AOE, usually given over 7 days. Multiple topical preparations are available, such as ciprofloxacin 0.2%/hydrocortisone 1.0%; neomycin/polymyxin B/hydrocortisone; ofloxacin 0.3%; or acetic acid 2.0%.7 Avoid these agents, though, if you suspect tympanic membrane rupture. Quinolone drops are the only topical antimicrobials approved for middle ear use.7
Systemic antibiotics are not recommended for the initial treatment of AOE. Topical agents deliver a much higher concentration of medication than can be achieved systemically. Consider systemic antibiotics if there is extension outside the ear canal, a concern for necrotizing otitis externa (more on this in a bit), or the patient is immunodeficient.8
Continue to: Patient (or parent) education...
Patient (or parent) education is important to ensure proper medication administration. The patient should lie down with the affected ear facing up. After the canal is filled with drops, the patient should remain in this position for 3 to 5 minutes. Gently massaging the tragus can augment delivery. Patients should keep the ear canal as dry as possible and avoid inserting objects (eg, hearing aids, ear buds, cotton-tipped applicators) into the canal for the duration of treatment. The delivery of topical antibiotics can be enhanced by wick placement. Prescribe analgesics (typically nonsteroidal anti-inflammatory agents) based on severity of pain.7
Have patients abstain from water sports for 7 to 10 days. Showering is acceptable with minimal ear exposure to water; bathing is preferred when possible. If there is no clinical improvement in 48 to 72 hours, ask patients to return for re-evaluation.8 Prevention is essential for patients with a history of recurrent otitis externa. Acetic acid solutions create an acidic environment within the canal to help prevent recurrent AOE. Ear plugs and petroleum jelly–soaked cotton plugs prior to water exposure may also help prevent recurrent AOE.
Malignant otitis externa
Malignant, or necrotizing, otitis externa is an aggressive disease form of otitis externa that is most common in individuals with diabetes or other immunodeficiency disorders.9 Most cases are due to infection with P aeruginosa.10 Prior to the availability of effective antibiotics, mortality rates in patients with necrotizing otitis externa were as high as 50%.11
What you’ll see. Patients typically present with severe ear pain, otorrhea, conductive hearing loss, and a feeling of fullness in the external ear canal. Physical examination reveals purulent otorrhea and a swollen, tender ear canal. Exposed bone may be visible, most often on the floor of the canal. The tympanic membrane and middle ear are seldom involved on initial presentation.
The infection often originates at the junction of the bony and cartilaginous portion of the external canal, spreading through the fissures of Santorini to the skull base. If not aggressively treated, the infection spreads medially to the tympanomastoid suture causing intracranial complications—usually a facial nerve neuropathy.
Continue to: Given these clinical findings...
Given these clinical findings, promptly order laboratory studies and imaging to confirm the diagnosis. The erythrocyte sedimentation rate and C-reactive protein level are typically elevated, and either can be used as a marker to follow treatment. Computed tomography (CT) helps to determine the location and extent of disease and is recommended as the initial diagnostic imaging modality for patients with suspected malignant otitis externa.12
Magnetic resonance imaging helps define soft-tissue changes, dural enhancement, and involvement of medullary bone, making this the preferred modality to monitor therapeutic response.12 Technetium bone scanning can also be used for the initial diagnosis (particularly if CT findings are normal and clinical suspicion is high) and for follow-up with treatment.
How to treat. Management involves a team approach with otolaryngology, radiology, neurology, endocrinology, and infectious disease specialists. Long term (6-8 weeks) antipseudomonal antibiotic treatment is typical.
Let culture results guide the choice of antibiotic. Fluoroquinolone therapy, usually ciprofloxacin, is used most often.12 Surgical intervention may be required for local debridement and drainage of abscesses. Close follow-up is necessary due to reports of recurrence up to 1 year after treatment. If left untreated, necrotizing otitis externa can lead to osteomyelitis, meningitis, septic thrombosis, cerebral abscess, and death.11
Cerumen impaction
The relatively small diameter of the external auditory canal increases the risk for impaction of cerumen and foreign bodies. Cerumen impaction, in particular, is a common primary care complaint. Cerumen forms when glandular secretions from the outer two-thirds of the ear canal mix with exfoliated skin. It functions as a lubricant for the ear canal and as a barrier against infection, water accumulation, and foreign bodies.13
Continue to: What you'll see
What you’ll see. You may encounter cerumen impaction in an asymptomatic patient when it prevents visualization of the external auditory canal or tympanic membrane, or when a patient complains of conductive hearing loss, tinnitus, dizziness, ear pain, itching, and cough.13 It is found in 1 in 10 children and 1 in 20 adults.13 There is a higher incidence in patients who are elderly, are cognitively impaired, or wear hearing devices or ear plugs.13,14 Asymptomatic cerumen impaction should not be treated. A recent clinical guideline provides a useful “do and don’t” list for patient education (TABLE).13
How to treat. In asymptomatic patients, the presence of cerumen on examination is not an indication for removal. Based on current guidelines,13 impacted cerumen can safely be removed from the ear canal of symptomatic patients in several ways:
- Manual removal with cerumen loop/spoon or alligator forceps. This method decreases the risk for infection because it limits moisture exposure. However, it should be performed by a health care provider trained in its use because of the risk for trauma to the ear canal and tympanic membrane.
- Irrigation of the ear using tap water or a 50-50 solution of hydrogen peroxide and water. Irrigation can be achieved with a syringe or jet irrigator using a modified tip. This method also has a risk for trauma to the ear canal and tympanic membrane and should only be performed by appropriately trained health care professionals.
- Use of cerumenolytic agents to soften and thin earwax and promote natural extrusion. Several types of cerumenolytic drops (water-based and oil-based) are available and appear to be equally effective. Water-based solutions contain hydrogen peroxide, docusate sodium, acetic acid, and sodium bicarbonate. Oil-based drops may contain peanut, almond, or olive oils. A thorough allergic history should be performed to avoid using products in patients with nut allergies. In head-to-head laboratory comparisons, distilled water appears to be the best cerumenolytic.15
Foreign bodies
Foreign bodies in the external auditory canal (typically beads, cotton tips, and insects) are more common in children than adults.16
What you’ll see. Most foreign bodies are lodged in the bony part of the external auditory canal, and many patients try to remove the object before seeking medical care. Removal requires adequate visualization and skill.17 Although patients may be asymptomatic, most complain of pain, fullness, decreased hearing, or otorrhea.
How to treat. Directly visible objects can often be removed without referral. Suction, irrigation, forceps, probes, and fine hooks have been used. Insect removal can be facilitated by first flooding the canal with xylocaine, alcohol, or mineral oil. Acetone may be used to dissolve foreign bodies containing Styrofoam or to loosen glues. If the object is a button battery, avoid irrigation to prevent liquefaction tissue necrosis.
Continue to: Complications of foreign body removal...
Complications of foreign body removal include pain, otitis externa, otitis media, and trauma to the ear or tympanic membrane. The likelihood of successful removal of the object decreases and the risk for complications increases with each subsequent attempt.17 Consult an otolaryngologist if sedation or anesthesia is required, the foreign body is tightly wedged, there is trauma to the ear canal or tympanic membrane, the foreign body has a sharp edge (eg, glass or wire), or removal attempts have been unsuccessful.
Trauma
Sports injuries, motor vehicle accidents, bites, falls, and burns are the primary causes of trauma to the external ear.18
What you’ll see. Blunt auricular trauma predisposes to infection, necrosis, and scar contracture. One of the most common sequelae is cauliflower ear. Trauma is particularly common with contact sports such as boxing, wrestling, or mixed martial arts. The skin of the auricle attaches directly to the perichondrium. Following blunt or shearing trauma to the auricle, hematomas form within the space between the perichondrium and cartilage of the anterior ear.19
How to treat. Small hematomas can be managed by aspiration, while larger ones generally require open drainage.20 Newer treatments involving pressure dressings and the use of fibrin glue have been proposed.20 Recommend that athletes participating in contact sports wear appropriate protective headgear to prevent auricular hematoma and cauliflower ear.
Neoplasm
Roughly 5% of all skin cancers involve the ear, most frequently the pinna due to chronic sun exposure.21 The most frequently occurring malignancy of the external ear is basal cell carcinoma (BCC), which is responsible for 80% of all nonmelanoma skin cancers.22
Continue to: What you'll see
What you’ll see. BCC of the ear usually involves the preauricular area and the helix. The risk for BCC is related to exposure to ultraviolet radiation. BCC of the ear is more common in men and can be particularly aggressive, highlighting the importance of prevention and prompt recognition. BCC typically presents as a fleshy papule that is often translucent or “pearly’” and has overlying telangiectasia and a “rolled” border. Central ulceration can occur as well.
How to treat. Usual treatment of BCC is surgical excision. Prevention is critical and centers on sun avoidance or the use of appropriate sunscreens.
In addition to BCC, exposure of the external ear to sunlight and ultraviolet radiation predisposes patients to the development of squamous cell carcinoma (SCC) and melanoma. SCC has a variety of presentations including papules, plaques, and nodules. SCC has a higher metastatic potential than does BCC.
Keloid
Keloids are an abnormal healing response to soft-tissue injury: benign fibrocartilaginous growths that extend beyond the original wound.
What you’ll see. Keloids are more common in dark-skinned individuals and tend to result from burns, surgical incisions, infection, trauma, tattooing, injections, piercings, and arthropod bites. In some cases, they arise spontaneously. Keloids are more common in areas of increased skin tension (chest, shoulders, back), but may occur on the ears—most commonly after piercing or trauma. Keloids present clinically as slow-growing rubbery or firm nodules. The diagnosis is typically based on clinical appearance but can be confirmed by histopathology.
Continue to: How to treat
How to treat. Treatments vary and include observation, excision, intralesional injections, cryotherapy, enzyme therapy, silicone gel application, and irradiation.23 Recurrence is common; no therapy has been proven to be universally superior or preferred.
Congenital malformations
Atresia
Disruption of embryologic development (failed invagination of the external auditory canal) can lead to a stenotic or absent ear canal (aural atresia). Aural atresia is also often associated with fusion of the incus and malleus. This condition occurs predominantly in males. Unilateral atresia is more common than bilateral atresia, and the right ear is more often involved than the left.24
Microtia
Microtia is the incomplete development of the pinna leading to a small or deformed pinna. Microtia can be unilateral or bilateral. As with atresia, microtia more commonly affects males and, if unilateral, the right side is more often affected than the left. Microtia can occur in isolation but is often associated with genetic syndromes such as Treacher Collins syndrome and craniofacial microsomia (Goldenhar syndrome). When microtia is identified (typically at birth or early infancy), audiologic testing and a thorough physical examination for evidence of associated defects should be performed. Consult with an audiologist, clinical geneticist, or pediatric otolaryngologist.
Pre-auricular pits
Pre-auricular pits (sinuses) are tiny indentations anterior to the helix and superior to the tragus. While pre-auricular pits are more common on the right side, they are bilateral in 25% to 50% of cases.25 Pre-auricular pits occur in up to 1% of white children, 5% of black children, and 10% of Asian children.25 Children with this condition should undergo formal audiologic testing as their risk for hearing loss is higher compared with the general population.26
The branchio-oto-renal syndrome (associated with pre-auricular pits and hearing loss) also features structural defects of the ear, renal anomalies and/or nasolacrimal duct stenosis or fistulas. If this syndrome is suspected, renal ultrasound imaging is warranted. Other indications for renal ultrasound in patients with a pre-auricular pit are any dysmorphic feature, a family history of deafness, an auricular malformation, or a maternal history of gestational diabetes.27 Pre-auricular pits do not require surgery unless they drain chronically or become recurrently infected. Complete surgical excision is the treatment of choice in these cases.
CORRESPONDENCE
Mark Stephens, MD, 1850 Park Avenue, State College, PA 16801; mstephens3@pennstatehealth.psu.edu
1. Cox TC, Camci ED, Vora S, et al. The genetics of auricular development and malformation: new findings in model systems driving future directions for microtia research. Eur J Med Genet. 2014;57:394-401.
2. Sosin M, Weissler JM, Pulcrano M, et al. Transcartilaginous ear piercing and infectious complications: a systematic review and critical analysis of outcomes. Laryngoscope. 2015;125:1827-1834.
3. Stirn A. Body piercing: medical consequences and psychological motivations. Lancet. 2003;361:1205-1215.
4. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Larygol Oncol. 2013;127:505-508.
5. Mitchell S, Ditta K, Minhas S, et al. Pinna abscesses: can we manage them better? A case series and review of the literature. Eur Arch Otorhinolaryngol. 2015;272:3163-3167.
6. Stone KE. Otitis externa. Pediatr Rev. 2007;28:77-78.
7. Rosenfeld RM, Schwartz SR, Cannon CR, et al. Clinical practice guideline: acute otitis externa. Otolaryngol Head Neck Surg. 2014;150(1 suppl):S1-S24.
8. Prentice P. American Academy of Otolaryngology: Head and Neck Surgery Foundation clinical practice guideline on acute otitis externa. Arch Dis Child Educ Pract Ed. 2015;100:197.
9. Unadkat S, Kanzara T, Watters G. Necrotising otitis externa in the immunocompetent patient. J Laryngol Otol. 2018;132:71-74.
10. Carfrae MJ, Kesser BW. Malignant otitis externa. Otolarngol Clin N Am. 2008;41:537-549.
11. Chandler JR, Malignant otitis externa. Laryngoscope. 1968;78:1257-1294.
12. Hollis S, Evans K. Management of malignant (necrotising) otitis externa. J Laryngol Otol. 2011;125:1212-1217.
13. Schwartz SR, Magit AE, Rosenfeld RM, et al. Clinical practice guideline (update): earwax (cerumen impaction). Otolaryngol Head Neck Surg. 2017;156:S1-S29.
14. Guest JF, Greener MJ, Robinson AC, et al. Impacted cerumen: composition, production, epidemiology and management. QJM. 2004;97:477-488.
15. Saxby C, Williams R, Hickey S. Finding the most effective cerumenolytic. J Laryngol Otol. 2013;127:1067-1070.
16. Awad AH, ElTaher M. ENT foreign bodies: an experience. Int Arch Otorhinolaryngol. 2018;22:146-151.
17. Heim SW, Maughan KL. Foreign bodies in the ear, nose, and throat. Am Fam Physician. 2007;76:1185-1189.
18. Sharma K, Goswami SC, Baruah DK. Auricular trauma and its management. Indian J Otolaryngol Head Neck Surg. 2006;58:232-234.
19. Haik J, Givol O, Kornhaber R, et al. Cauliflower ear–a minimally invasive treatment in a wrestling athlete: a case report. Int Med Case Rep J. 2018;11:5-7.
20. Ebrahimi A, Kazemi A, Rasouli HR, et al. Reconstructive surgery of auricular defects: an overview. Trauma Mon. 2015;20:e28202.
21. Warner E, Weston C, Barclay-Klingle N, et al. The swollen pinna. BMJ. 2017; 359; j5073.
22. Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
23. Ranjan SK, Ahmed A, Harsh V, et al. Giant bilateral keloids of the ear lobule: case report and brief review of the literature. J Family Med Prim Care. 2017;6:677-679.
24. Roland PS, Marple BF. Disorders of the external auditory canal. J Am Acad Audiol. 1997;8:367-378.
25. Scheinfeld NS, Silverberg NB, Weinberg JM, et al. The preauricular sinus: a review of its clinical presentation, treatment, and associations. Pediatr Dermatol. 2004;21:191-196.
26. Roth DA, Hildesheimer M, Bardestein S, et al. Preauricular skin tags and ear pits are associated with permanent hearing impairment in newborns. Pediatrics. 2008;122:e884-890.
27. Tan T, Constantinides H, Mitchell TE. The preauricular sinus: a review of its aetiology, clinical presentation and management. Int J Ped Otorhinolaryngol. 2005;69:1469-1474.
Which antibiotics are most useful for infection following ear piercing? When is it safe to attempt removal of a foreign body from the ear canal, and which cerumenolytic agent may be best for ear wax? This review covers common ailments of the outer ear, which are often readily diagnosed given a patient’s history and thorough physical examination. We also address more complicated matters such as deciding when to refer for treatment of suspected malignant otitis externa, and which lab markers to follow when managing it yourself.
A (very) brief review of ear anatomy
Understanding the unique embryology and intricate anatomy of the external ear informs our understanding of predictable infections, growths, and malformations.
The external ear is composed of the external auditory canal and auricle. The external auditory canal has a lateral (external) cartilaginous portion and a medial (internal) bony portion. The auricular structure is complex and formed by the helix, antihelix (crura; scaphoid fossa), tragus, antitragus, conchae, and lobule. The auricle is composed of elastic cartilage covered by skin. The lobule is composed of skin, adipose tissue, and connective tissue.
Embryologically, the auricle, auditory canal, and middle ear form from ectoderm of the first 2 branchial arches during early gestation. The auricle forms from the fusion of soft-tissue swellings (hillocks). Three hillocks arise from the first branchial arch and 3 from the second branchial arch during the fifth and sixth weeks of gestation. Tissues from the second branchial arch comprise the lobule, antihelix, and caudal helix. The cartilage of the tragus forms from the first branchial arch. The ear canal forms from an epithelial invagination of the first branchial arch that also occurs during the fifth week of gestation.1
Infections
Perichondritis
Inflammation or infection of the connective tissue layer surrounding the auricular cartilage (perichondrium) results in perichondritis. Further extension of infection can lead to an auricular abscess. Both of these conditions can have serious consequences.
What you’ll see. The most common risk factor for perichondritis is the popular practice of cosmetic transcartilaginous piercing.2 Piercing of the helix, scapha, or anti-helix (often referred to as “high” ear piercing) causes localized trauma that can strip the adjacent perichondrium, decrease blood supply, create cartilaginous microfractures, and lead to devascularization. Rates of infection as high as 35% have been reported with high-ear piercing.3
The most common microbes associated with perichondritis and pinna abscess formation are Pseudomonas and Staphylococcus species.2 P
Continue to: How to treat
How to treat. The cornerstone of treatment is early detection and antimicrobial coverage with antipseudomonal antibiotics. Ciprofloxacin is the oral antibiotic of choice because of its ability to penetrate the tissue.4 Other options include clindamycin and third- or fourth-generation cephalosporins. If the wound becomes abscessed, perform (or refer for) early surgical incision and drainage.5 A failure to promptly recognize perichondritis or to mistakenly prescribe non-antipseudomonal antibiotics contributes to increased rates of hospitalization.2 Cosmetic deformity is the most common complication of perichondritis. This may require reconstructive surgery.
Otitis externa
Acute otitis externa (AOE; “swimmer’s ear”) is cellulitis of the skin and subdermis of the external ear canal. It is most prevalent in warm, moist climates and almost always associated with acute bacterial infection, most commonly P aeruginosa or S aureus.6 There is also an increased association with poor water quality (containing higher bacterial loads). Anything breaching the integrity of the ear canal can potentially predispose to the development of AOE. This includes trauma from cleaning, cerumen removal, scratching due to allergic conditions, and placement of hearing-aid devices.6
What you’ll see. Suspect AOE when signs or symptoms of ear canal inflammation have appeared rapidly (generally within 2 days) over the past 3 weeks.7 Findings include otalgia, itching, fullness, tragal tenderness, ear canal edema, erythema with or without otorrhea, lymphadenitis, or cellulitis of the pinna or adjacent skin.7 AOE must be distinguished from other causes of otalgia and otorrhea, including dermatitis and viral infection.
How to treat. Topical therapy is recommended for the initial treatment of uncomplicated AOE, usually given over 7 days. Multiple topical preparations are available, such as ciprofloxacin 0.2%/hydrocortisone 1.0%; neomycin/polymyxin B/hydrocortisone; ofloxacin 0.3%; or acetic acid 2.0%.7 Avoid these agents, though, if you suspect tympanic membrane rupture. Quinolone drops are the only topical antimicrobials approved for middle ear use.7
Systemic antibiotics are not recommended for the initial treatment of AOE. Topical agents deliver a much higher concentration of medication than can be achieved systemically. Consider systemic antibiotics if there is extension outside the ear canal, a concern for necrotizing otitis externa (more on this in a bit), or the patient is immunodeficient.8
Continue to: Patient (or parent) education...
Patient (or parent) education is important to ensure proper medication administration. The patient should lie down with the affected ear facing up. After the canal is filled with drops, the patient should remain in this position for 3 to 5 minutes. Gently massaging the tragus can augment delivery. Patients should keep the ear canal as dry as possible and avoid inserting objects (eg, hearing aids, ear buds, cotton-tipped applicators) into the canal for the duration of treatment. The delivery of topical antibiotics can be enhanced by wick placement. Prescribe analgesics (typically nonsteroidal anti-inflammatory agents) based on severity of pain.7
Have patients abstain from water sports for 7 to 10 days. Showering is acceptable with minimal ear exposure to water; bathing is preferred when possible. If there is no clinical improvement in 48 to 72 hours, ask patients to return for re-evaluation.8 Prevention is essential for patients with a history of recurrent otitis externa. Acetic acid solutions create an acidic environment within the canal to help prevent recurrent AOE. Ear plugs and petroleum jelly–soaked cotton plugs prior to water exposure may also help prevent recurrent AOE.
Malignant otitis externa
Malignant, or necrotizing, otitis externa is an aggressive disease form of otitis externa that is most common in individuals with diabetes or other immunodeficiency disorders.9 Most cases are due to infection with P aeruginosa.10 Prior to the availability of effective antibiotics, mortality rates in patients with necrotizing otitis externa were as high as 50%.11
What you’ll see. Patients typically present with severe ear pain, otorrhea, conductive hearing loss, and a feeling of fullness in the external ear canal. Physical examination reveals purulent otorrhea and a swollen, tender ear canal. Exposed bone may be visible, most often on the floor of the canal. The tympanic membrane and middle ear are seldom involved on initial presentation.
The infection often originates at the junction of the bony and cartilaginous portion of the external canal, spreading through the fissures of Santorini to the skull base. If not aggressively treated, the infection spreads medially to the tympanomastoid suture causing intracranial complications—usually a facial nerve neuropathy.
Continue to: Given these clinical findings...
Given these clinical findings, promptly order laboratory studies and imaging to confirm the diagnosis. The erythrocyte sedimentation rate and C-reactive protein level are typically elevated, and either can be used as a marker to follow treatment. Computed tomography (CT) helps to determine the location and extent of disease and is recommended as the initial diagnostic imaging modality for patients with suspected malignant otitis externa.12
Magnetic resonance imaging helps define soft-tissue changes, dural enhancement, and involvement of medullary bone, making this the preferred modality to monitor therapeutic response.12 Technetium bone scanning can also be used for the initial diagnosis (particularly if CT findings are normal and clinical suspicion is high) and for follow-up with treatment.
How to treat. Management involves a team approach with otolaryngology, radiology, neurology, endocrinology, and infectious disease specialists. Long term (6-8 weeks) antipseudomonal antibiotic treatment is typical.
Let culture results guide the choice of antibiotic. Fluoroquinolone therapy, usually ciprofloxacin, is used most often.12 Surgical intervention may be required for local debridement and drainage of abscesses. Close follow-up is necessary due to reports of recurrence up to 1 year after treatment. If left untreated, necrotizing otitis externa can lead to osteomyelitis, meningitis, septic thrombosis, cerebral abscess, and death.11
Cerumen impaction
The relatively small diameter of the external auditory canal increases the risk for impaction of cerumen and foreign bodies. Cerumen impaction, in particular, is a common primary care complaint. Cerumen forms when glandular secretions from the outer two-thirds of the ear canal mix with exfoliated skin. It functions as a lubricant for the ear canal and as a barrier against infection, water accumulation, and foreign bodies.13
Continue to: What you'll see
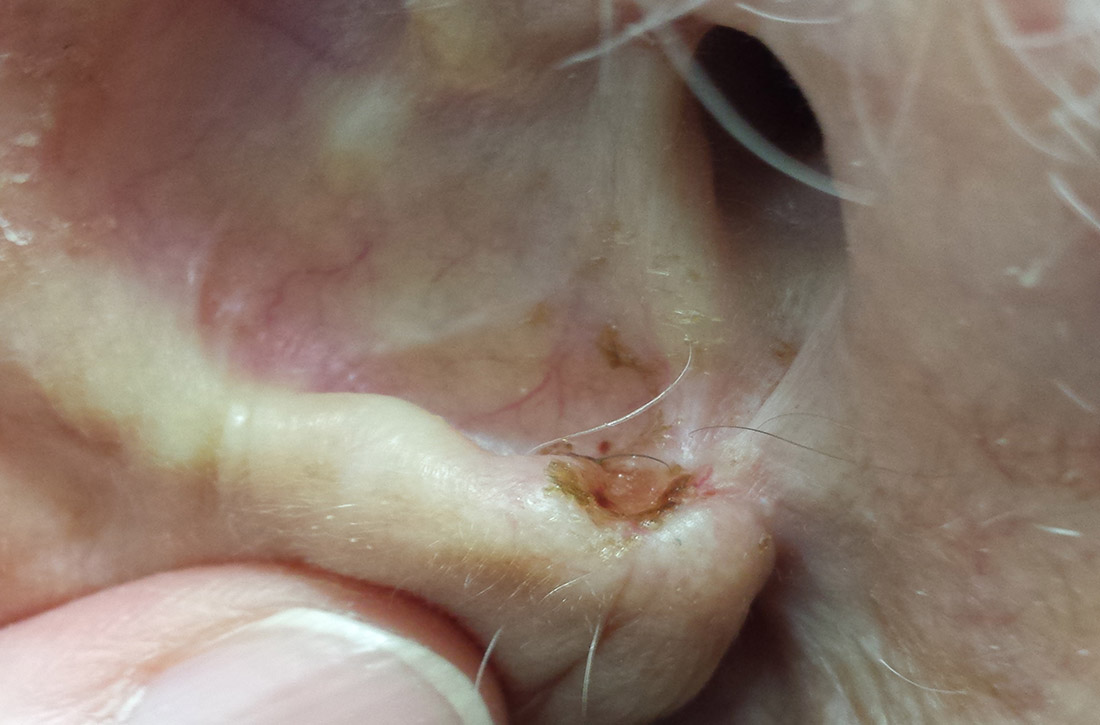
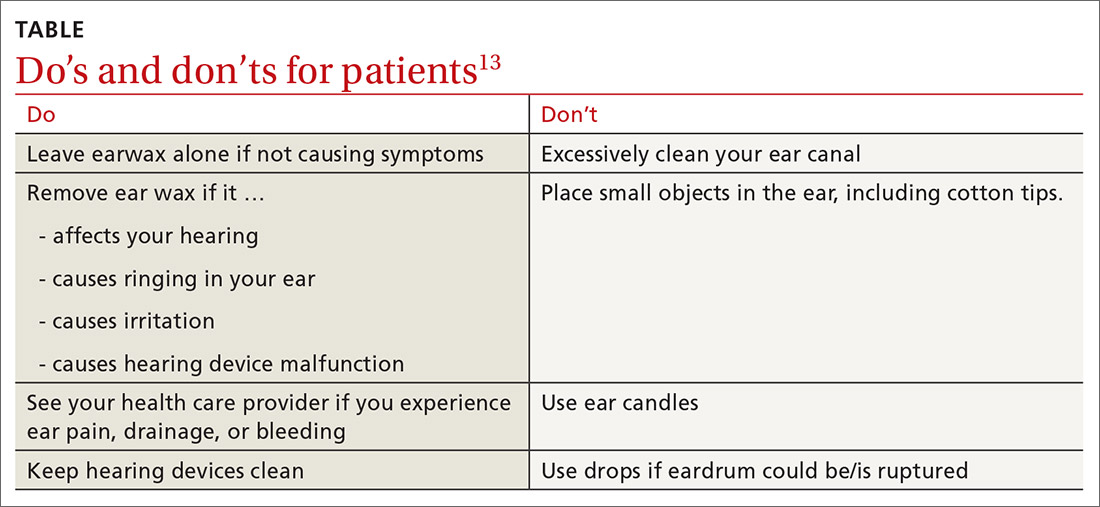
What you’ll see. You may encounter cerumen impaction in an asymptomatic patient when it prevents visualization of the external auditory canal or tympanic membrane, or when a patient complains of conductive hearing loss, tinnitus, dizziness, ear pain, itching, and cough.13 It is found in 1 in 10 children and 1 in 20 adults.13 There is a higher incidence in patients who are elderly, are cognitively impaired, or wear hearing devices or ear plugs.13,14 Asymptomatic cerumen impaction should not be treated. A recent clinical guideline provides a useful “do and don’t” list for patient education (TABLE).13

How to treat. In asymptomatic patients, the presence of cerumen on examination is not an indication for removal. Based on current guidelines,13 impacted cerumen can safely be removed from the ear canal of symptomatic patients in several ways:
- Manual removal with cerumen loop/spoon or alligator forceps. This method decreases the risk for infection because it limits moisture exposure. However, it should be performed by a health care provider trained in its use because of the risk for trauma to the ear canal and tympanic membrane.
- Irrigation of the ear using tap water or a 50-50 solution of hydrogen peroxide and water. Irrigation can be achieved with a syringe or jet irrigator using a modified tip. This method also has a risk for trauma to the ear canal and tympanic membrane and should only be performed by appropriately trained health care professionals.
- Use of cerumenolytic agents to soften and thin earwax and promote natural extrusion. Several types of cerumenolytic drops (water-based and oil-based) are available and appear to be equally effective. Water-based solutions contain hydrogen peroxide, docusate sodium, acetic acid, and sodium bicarbonate. Oil-based drops may contain peanut, almond, or olive oils. A thorough allergic history should be performed to avoid using products in patients with nut allergies. In head-to-head laboratory comparisons, distilled water appears to be the best cerumenolytic.15
Foreign bodies
Foreign bodies in the external auditory canal (typically beads, cotton tips, and insects) are more common in children than adults.16
What you’ll see. Most foreign bodies are lodged in the bony part of the external auditory canal, and many patients try to remove the object before seeking medical care. Removal requires adequate visualization and skill.17 Although patients may be asymptomatic, most complain of pain, fullness, decreased hearing, or otorrhea.
How to treat. Directly visible objects can often be removed without referral. Suction, irrigation, forceps, probes, and fine hooks have been used. Insect removal can be facilitated by first flooding the canal with xylocaine, alcohol, or mineral oil. Acetone may be used to dissolve foreign bodies containing Styrofoam or to loosen glues. If the object is a button battery, avoid irrigation to prevent liquefaction tissue necrosis.
Continue to: Complications of foreign body removal...
Complications of foreign body removal include pain, otitis externa, otitis media, and trauma to the ear or tympanic membrane. The likelihood of successful removal of the object decreases and the risk for complications increases with each subsequent attempt.17 Consult an otolaryngologist if sedation or anesthesia is required, the foreign body is tightly wedged, there is trauma to the ear canal or tympanic membrane, the foreign body has a sharp edge (eg, glass or wire), or removal attempts have been unsuccessful.
Trauma
Sports injuries, motor vehicle accidents, bites, falls, and burns are the primary causes of trauma to the external ear.18
What you’ll see. Blunt auricular trauma predisposes to infection, necrosis, and scar contracture. One of the most common sequelae is cauliflower ear. Trauma is particularly common with contact sports such as boxing, wrestling, or mixed martial arts. The skin of the auricle attaches directly to the perichondrium. Following blunt or shearing trauma to the auricle, hematomas form within the space between the perichondrium and cartilage of the anterior ear.19
How to treat. Small hematomas can be managed by aspiration, while larger ones generally require open drainage.20 Newer treatments involving pressure dressings and the use of fibrin glue have been proposed.20 Recommend that athletes participating in contact sports wear appropriate protective headgear to prevent auricular hematoma and cauliflower ear.
Neoplasm
Roughly 5% of all skin cancers involve the ear, most frequently the pinna due to chronic sun exposure.21 The most frequently occurring malignancy of the external ear is basal cell carcinoma (BCC), which is responsible for 80% of all nonmelanoma skin cancers.22
Continue to: What you'll see
What you’ll see. BCC of the ear usually involves the preauricular area and the helix. The risk for BCC is related to exposure to ultraviolet radiation. BCC of the ear is more common in men and can be particularly aggressive, highlighting the importance of prevention and prompt recognition. BCC typically presents as a fleshy papule that is often translucent or “pearly’” and has overlying telangiectasia and a “rolled” border. Central ulceration can occur as well.
How to treat. Usual treatment of BCC is surgical excision. Prevention is critical and centers on sun avoidance or the use of appropriate sunscreens.
In addition to BCC, exposure of the external ear to sunlight and ultraviolet radiation predisposes patients to the development of squamous cell carcinoma (SCC) and melanoma. SCC has a variety of presentations including papules, plaques, and nodules. SCC has a higher metastatic potential than does BCC.
Keloid
Keloids are an abnormal healing response to soft-tissue injury: benign fibrocartilaginous growths that extend beyond the original wound.
What you’ll see. Keloids are more common in dark-skinned individuals and tend to result from burns, surgical incisions, infection, trauma, tattooing, injections, piercings, and arthropod bites. In some cases, they arise spontaneously. Keloids are more common in areas of increased skin tension (chest, shoulders, back), but may occur on the ears—most commonly after piercing or trauma. Keloids present clinically as slow-growing rubbery or firm nodules. The diagnosis is typically based on clinical appearance but can be confirmed by histopathology.
Continue to: How to treat
How to treat. Treatments vary and include observation, excision, intralesional injections, cryotherapy, enzyme therapy, silicone gel application, and irradiation.23 Recurrence is common; no therapy has been proven to be universally superior or preferred.
Congenital malformations
Atresia
Disruption of embryologic development (failed invagination of the external auditory canal) can lead to a stenotic or absent ear canal (aural atresia). Aural atresia is also often associated with fusion of the incus and malleus. This condition occurs predominantly in males. Unilateral atresia is more common than bilateral atresia, and the right ear is more often involved than the left.24
Microtia
Microtia is the incomplete development of the pinna leading to a small or deformed pinna. Microtia can be unilateral or bilateral. As with atresia, microtia more commonly affects males and, if unilateral, the right side is more often affected than the left. Microtia can occur in isolation but is often associated with genetic syndromes such as Treacher Collins syndrome and craniofacial microsomia (Goldenhar syndrome). When microtia is identified (typically at birth or early infancy), audiologic testing and a thorough physical examination for evidence of associated defects should be performed. Consult with an audiologist, clinical geneticist, or pediatric otolaryngologist.
Pre-auricular pits
Pre-auricular pits (sinuses) are tiny indentations anterior to the helix and superior to the tragus. While pre-auricular pits are more common on the right side, they are bilateral in 25% to 50% of cases.25 Pre-auricular pits occur in up to 1% of white children, 5% of black children, and 10% of Asian children.25 Children with this condition should undergo formal audiologic testing as their risk for hearing loss is higher compared with the general population.26
The branchio-oto-renal syndrome (associated with pre-auricular pits and hearing loss) also features structural defects of the ear, renal anomalies and/or nasolacrimal duct stenosis or fistulas. If this syndrome is suspected, renal ultrasound imaging is warranted. Other indications for renal ultrasound in patients with a pre-auricular pit are any dysmorphic feature, a family history of deafness, an auricular malformation, or a maternal history of gestational diabetes.27 Pre-auricular pits do not require surgery unless they drain chronically or become recurrently infected. Complete surgical excision is the treatment of choice in these cases.
CORRESPONDENCE
Mark Stephens, MD, 1850 Park Avenue, State College, PA 16801; mstephens3@pennstatehealth.psu.edu
Which antibiotics are most useful for infection following ear piercing? When is it safe to attempt removal of a foreign body from the ear canal, and which cerumenolytic agent may be best for ear wax? This review covers common ailments of the outer ear, which are often readily diagnosed given a patient’s history and thorough physical examination. We also address more complicated matters such as deciding when to refer for treatment of suspected malignant otitis externa, and which lab markers to follow when managing it yourself.
A (very) brief review of ear anatomy
Understanding the unique embryology and intricate anatomy of the external ear informs our understanding of predictable infections, growths, and malformations.
The external ear is composed of the external auditory canal and auricle. The external auditory canal has a lateral (external) cartilaginous portion and a medial (internal) bony portion. The auricular structure is complex and formed by the helix, antihelix (crura; scaphoid fossa), tragus, antitragus, conchae, and lobule. The auricle is composed of elastic cartilage covered by skin. The lobule is composed of skin, adipose tissue, and connective tissue.
Embryologically, the auricle, auditory canal, and middle ear form from ectoderm of the first 2 branchial arches during early gestation. The auricle forms from the fusion of soft-tissue swellings (hillocks). Three hillocks arise from the first branchial arch and 3 from the second branchial arch during the fifth and sixth weeks of gestation. Tissues from the second branchial arch comprise the lobule, antihelix, and caudal helix. The cartilage of the tragus forms from the first branchial arch. The ear canal forms from an epithelial invagination of the first branchial arch that also occurs during the fifth week of gestation.1
Infections
Perichondritis
Inflammation or infection of the connective tissue layer surrounding the auricular cartilage (perichondrium) results in perichondritis. Further extension of infection can lead to an auricular abscess. Both of these conditions can have serious consequences.
What you’ll see. The most common risk factor for perichondritis is the popular practice of cosmetic transcartilaginous piercing.2 Piercing of the helix, scapha, or anti-helix (often referred to as “high” ear piercing) causes localized trauma that can strip the adjacent perichondrium, decrease blood supply, create cartilaginous microfractures, and lead to devascularization. Rates of infection as high as 35% have been reported with high-ear piercing.3
The most common microbes associated with perichondritis and pinna abscess formation are Pseudomonas and Staphylococcus species.2 P
Continue to: How to treat
How to treat. The cornerstone of treatment is early detection and antimicrobial coverage with antipseudomonal antibiotics. Ciprofloxacin is the oral antibiotic of choice because of its ability to penetrate the tissue.4 Other options include clindamycin and third- or fourth-generation cephalosporins. If the wound becomes abscessed, perform (or refer for) early surgical incision and drainage.5 A failure to promptly recognize perichondritis or to mistakenly prescribe non-antipseudomonal antibiotics contributes to increased rates of hospitalization.2 Cosmetic deformity is the most common complication of perichondritis. This may require reconstructive surgery.
Otitis externa
Acute otitis externa (AOE; “swimmer’s ear”) is cellulitis of the skin and subdermis of the external ear canal. It is most prevalent in warm, moist climates and almost always associated with acute bacterial infection, most commonly P aeruginosa or S aureus.6 There is also an increased association with poor water quality (containing higher bacterial loads). Anything breaching the integrity of the ear canal can potentially predispose to the development of AOE. This includes trauma from cleaning, cerumen removal, scratching due to allergic conditions, and placement of hearing-aid devices.6
What you’ll see. Suspect AOE when signs or symptoms of ear canal inflammation have appeared rapidly (generally within 2 days) over the past 3 weeks.7 Findings include otalgia, itching, fullness, tragal tenderness, ear canal edema, erythema with or without otorrhea, lymphadenitis, or cellulitis of the pinna or adjacent skin.7 AOE must be distinguished from other causes of otalgia and otorrhea, including dermatitis and viral infection.
How to treat. Topical therapy is recommended for the initial treatment of uncomplicated AOE, usually given over 7 days. Multiple topical preparations are available, such as ciprofloxacin 0.2%/hydrocortisone 1.0%; neomycin/polymyxin B/hydrocortisone; ofloxacin 0.3%; or acetic acid 2.0%.7 Avoid these agents, though, if you suspect tympanic membrane rupture. Quinolone drops are the only topical antimicrobials approved for middle ear use.7
Systemic antibiotics are not recommended for the initial treatment of AOE. Topical agents deliver a much higher concentration of medication than can be achieved systemically. Consider systemic antibiotics if there is extension outside the ear canal, a concern for necrotizing otitis externa (more on this in a bit), or the patient is immunodeficient.8
Continue to: Patient (or parent) education...
Patient (or parent) education is important to ensure proper medication administration. The patient should lie down with the affected ear facing up. After the canal is filled with drops, the patient should remain in this position for 3 to 5 minutes. Gently massaging the tragus can augment delivery. Patients should keep the ear canal as dry as possible and avoid inserting objects (eg, hearing aids, ear buds, cotton-tipped applicators) into the canal for the duration of treatment. The delivery of topical antibiotics can be enhanced by wick placement. Prescribe analgesics (typically nonsteroidal anti-inflammatory agents) based on severity of pain.7
Have patients abstain from water sports for 7 to 10 days. Showering is acceptable with minimal ear exposure to water; bathing is preferred when possible. If there is no clinical improvement in 48 to 72 hours, ask patients to return for re-evaluation.8 Prevention is essential for patients with a history of recurrent otitis externa. Acetic acid solutions create an acidic environment within the canal to help prevent recurrent AOE. Ear plugs and petroleum jelly–soaked cotton plugs prior to water exposure may also help prevent recurrent AOE.
Malignant otitis externa
Malignant, or necrotizing, otitis externa is an aggressive disease form of otitis externa that is most common in individuals with diabetes or other immunodeficiency disorders.9 Most cases are due to infection with P aeruginosa.10 Prior to the availability of effective antibiotics, mortality rates in patients with necrotizing otitis externa were as high as 50%.11
What you’ll see. Patients typically present with severe ear pain, otorrhea, conductive hearing loss, and a feeling of fullness in the external ear canal. Physical examination reveals purulent otorrhea and a swollen, tender ear canal. Exposed bone may be visible, most often on the floor of the canal. The tympanic membrane and middle ear are seldom involved on initial presentation.
The infection often originates at the junction of the bony and cartilaginous portion of the external canal, spreading through the fissures of Santorini to the skull base. If not aggressively treated, the infection spreads medially to the tympanomastoid suture causing intracranial complications—usually a facial nerve neuropathy.
Continue to: Given these clinical findings...
Given these clinical findings, promptly order laboratory studies and imaging to confirm the diagnosis. The erythrocyte sedimentation rate and C-reactive protein level are typically elevated, and either can be used as a marker to follow treatment. Computed tomography (CT) helps to determine the location and extent of disease and is recommended as the initial diagnostic imaging modality for patients with suspected malignant otitis externa.12
Magnetic resonance imaging helps define soft-tissue changes, dural enhancement, and involvement of medullary bone, making this the preferred modality to monitor therapeutic response.12 Technetium bone scanning can also be used for the initial diagnosis (particularly if CT findings are normal and clinical suspicion is high) and for follow-up with treatment.
How to treat. Management involves a team approach with otolaryngology, radiology, neurology, endocrinology, and infectious disease specialists. Long term (6-8 weeks) antipseudomonal antibiotic treatment is typical.
Let culture results guide the choice of antibiotic. Fluoroquinolone therapy, usually ciprofloxacin, is used most often.12 Surgical intervention may be required for local debridement and drainage of abscesses. Close follow-up is necessary due to reports of recurrence up to 1 year after treatment. If left untreated, necrotizing otitis externa can lead to osteomyelitis, meningitis, septic thrombosis, cerebral abscess, and death.11
Cerumen impaction
The relatively small diameter of the external auditory canal increases the risk for impaction of cerumen and foreign bodies. Cerumen impaction, in particular, is a common primary care complaint. Cerumen forms when glandular secretions from the outer two-thirds of the ear canal mix with exfoliated skin. It functions as a lubricant for the ear canal and as a barrier against infection, water accumulation, and foreign bodies.13
Continue to: What you'll see
What you’ll see. You may encounter cerumen impaction in an asymptomatic patient when it prevents visualization of the external auditory canal or tympanic membrane, or when a patient complains of conductive hearing loss, tinnitus, dizziness, ear pain, itching, and cough.13 It is found in 1 in 10 children and 1 in 20 adults.13 There is a higher incidence in patients who are elderly, are cognitively impaired, or wear hearing devices or ear plugs.13,14 Asymptomatic cerumen impaction should not be treated. A recent clinical guideline provides a useful “do and don’t” list for patient education (TABLE).13

How to treat. In asymptomatic patients, the presence of cerumen on examination is not an indication for removal. Based on current guidelines,13 impacted cerumen can safely be removed from the ear canal of symptomatic patients in several ways:
- Manual removal with cerumen loop/spoon or alligator forceps. This method decreases the risk for infection because it limits moisture exposure. However, it should be performed by a health care provider trained in its use because of the risk for trauma to the ear canal and tympanic membrane.
- Irrigation of the ear using tap water or a 50-50 solution of hydrogen peroxide and water. Irrigation can be achieved with a syringe or jet irrigator using a modified tip. This method also has a risk for trauma to the ear canal and tympanic membrane and should only be performed by appropriately trained health care professionals.
- Use of cerumenolytic agents to soften and thin earwax and promote natural extrusion. Several types of cerumenolytic drops (water-based and oil-based) are available and appear to be equally effective. Water-based solutions contain hydrogen peroxide, docusate sodium, acetic acid, and sodium bicarbonate. Oil-based drops may contain peanut, almond, or olive oils. A thorough allergic history should be performed to avoid using products in patients with nut allergies. In head-to-head laboratory comparisons, distilled water appears to be the best cerumenolytic.15
Foreign bodies
Foreign bodies in the external auditory canal (typically beads, cotton tips, and insects) are more common in children than adults.16
What you’ll see. Most foreign bodies are lodged in the bony part of the external auditory canal, and many patients try to remove the object before seeking medical care. Removal requires adequate visualization and skill.17 Although patients may be asymptomatic, most complain of pain, fullness, decreased hearing, or otorrhea.
How to treat. Directly visible objects can often be removed without referral. Suction, irrigation, forceps, probes, and fine hooks have been used. Insect removal can be facilitated by first flooding the canal with xylocaine, alcohol, or mineral oil. Acetone may be used to dissolve foreign bodies containing Styrofoam or to loosen glues. If the object is a button battery, avoid irrigation to prevent liquefaction tissue necrosis.
Continue to: Complications of foreign body removal...
Complications of foreign body removal include pain, otitis externa, otitis media, and trauma to the ear or tympanic membrane. The likelihood of successful removal of the object decreases and the risk for complications increases with each subsequent attempt.17 Consult an otolaryngologist if sedation or anesthesia is required, the foreign body is tightly wedged, there is trauma to the ear canal or tympanic membrane, the foreign body has a sharp edge (eg, glass or wire), or removal attempts have been unsuccessful.
Trauma
Sports injuries, motor vehicle accidents, bites, falls, and burns are the primary causes of trauma to the external ear.18
What you’ll see. Blunt auricular trauma predisposes to infection, necrosis, and scar contracture. One of the most common sequelae is cauliflower ear. Trauma is particularly common with contact sports such as boxing, wrestling, or mixed martial arts. The skin of the auricle attaches directly to the perichondrium. Following blunt or shearing trauma to the auricle, hematomas form within the space between the perichondrium and cartilage of the anterior ear.19
How to treat. Small hematomas can be managed by aspiration, while larger ones generally require open drainage.20 Newer treatments involving pressure dressings and the use of fibrin glue have been proposed.20 Recommend that athletes participating in contact sports wear appropriate protective headgear to prevent auricular hematoma and cauliflower ear.
Neoplasm
Roughly 5% of all skin cancers involve the ear, most frequently the pinna due to chronic sun exposure.21 The most frequently occurring malignancy of the external ear is basal cell carcinoma (BCC), which is responsible for 80% of all nonmelanoma skin cancers.22
Continue to: What you'll see
What you’ll see. BCC of the ear usually involves the preauricular area and the helix. The risk for BCC is related to exposure to ultraviolet radiation. BCC of the ear is more common in men and can be particularly aggressive, highlighting the importance of prevention and prompt recognition. BCC typically presents as a fleshy papule that is often translucent or “pearly’” and has overlying telangiectasia and a “rolled” border. Central ulceration can occur as well.
How to treat. Usual treatment of BCC is surgical excision. Prevention is critical and centers on sun avoidance or the use of appropriate sunscreens.
In addition to BCC, exposure of the external ear to sunlight and ultraviolet radiation predisposes patients to the development of squamous cell carcinoma (SCC) and melanoma. SCC has a variety of presentations including papules, plaques, and nodules. SCC has a higher metastatic potential than does BCC.
Keloid
Keloids are an abnormal healing response to soft-tissue injury: benign fibrocartilaginous growths that extend beyond the original wound.
What you’ll see. Keloids are more common in dark-skinned individuals and tend to result from burns, surgical incisions, infection, trauma, tattooing, injections, piercings, and arthropod bites. In some cases, they arise spontaneously. Keloids are more common in areas of increased skin tension (chest, shoulders, back), but may occur on the ears—most commonly after piercing or trauma. Keloids present clinically as slow-growing rubbery or firm nodules. The diagnosis is typically based on clinical appearance but can be confirmed by histopathology.
Continue to: How to treat
How to treat. Treatments vary and include observation, excision, intralesional injections, cryotherapy, enzyme therapy, silicone gel application, and irradiation.23 Recurrence is common; no therapy has been proven to be universally superior or preferred.
Congenital malformations
Atresia
Disruption of embryologic development (failed invagination of the external auditory canal) can lead to a stenotic or absent ear canal (aural atresia). Aural atresia is also often associated with fusion of the incus and malleus. This condition occurs predominantly in males. Unilateral atresia is more common than bilateral atresia, and the right ear is more often involved than the left.24
Microtia
Microtia is the incomplete development of the pinna leading to a small or deformed pinna. Microtia can be unilateral or bilateral. As with atresia, microtia more commonly affects males and, if unilateral, the right side is more often affected than the left. Microtia can occur in isolation but is often associated with genetic syndromes such as Treacher Collins syndrome and craniofacial microsomia (Goldenhar syndrome). When microtia is identified (typically at birth or early infancy), audiologic testing and a thorough physical examination for evidence of associated defects should be performed. Consult with an audiologist, clinical geneticist, or pediatric otolaryngologist.
Pre-auricular pits
Pre-auricular pits (sinuses) are tiny indentations anterior to the helix and superior to the tragus. While pre-auricular pits are more common on the right side, they are bilateral in 25% to 50% of cases.25 Pre-auricular pits occur in up to 1% of white children, 5% of black children, and 10% of Asian children.25 Children with this condition should undergo formal audiologic testing as their risk for hearing loss is higher compared with the general population.26
The branchio-oto-renal syndrome (associated with pre-auricular pits and hearing loss) also features structural defects of the ear, renal anomalies and/or nasolacrimal duct stenosis or fistulas. If this syndrome is suspected, renal ultrasound imaging is warranted. Other indications for renal ultrasound in patients with a pre-auricular pit are any dysmorphic feature, a family history of deafness, an auricular malformation, or a maternal history of gestational diabetes.27 Pre-auricular pits do not require surgery unless they drain chronically or become recurrently infected. Complete surgical excision is the treatment of choice in these cases.
CORRESPONDENCE
Mark Stephens, MD, 1850 Park Avenue, State College, PA 16801; mstephens3@pennstatehealth.psu.edu
1. Cox TC, Camci ED, Vora S, et al. The genetics of auricular development and malformation: new findings in model systems driving future directions for microtia research. Eur J Med Genet. 2014;57:394-401.
2. Sosin M, Weissler JM, Pulcrano M, et al. Transcartilaginous ear piercing and infectious complications: a systematic review and critical analysis of outcomes. Laryngoscope. 2015;125:1827-1834.
3. Stirn A. Body piercing: medical consequences and psychological motivations. Lancet. 2003;361:1205-1215.
4. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Larygol Oncol. 2013;127:505-508.
5. Mitchell S, Ditta K, Minhas S, et al. Pinna abscesses: can we manage them better? A case series and review of the literature. Eur Arch Otorhinolaryngol. 2015;272:3163-3167.
6. Stone KE. Otitis externa. Pediatr Rev. 2007;28:77-78.
7. Rosenfeld RM, Schwartz SR, Cannon CR, et al. Clinical practice guideline: acute otitis externa. Otolaryngol Head Neck Surg. 2014;150(1 suppl):S1-S24.
8. Prentice P. American Academy of Otolaryngology: Head and Neck Surgery Foundation clinical practice guideline on acute otitis externa. Arch Dis Child Educ Pract Ed. 2015;100:197.
9. Unadkat S, Kanzara T, Watters G. Necrotising otitis externa in the immunocompetent patient. J Laryngol Otol. 2018;132:71-74.
10. Carfrae MJ, Kesser BW. Malignant otitis externa. Otolarngol Clin N Am. 2008;41:537-549.
11. Chandler JR, Malignant otitis externa. Laryngoscope. 1968;78:1257-1294.
12. Hollis S, Evans K. Management of malignant (necrotising) otitis externa. J Laryngol Otol. 2011;125:1212-1217.
13. Schwartz SR, Magit AE, Rosenfeld RM, et al. Clinical practice guideline (update): earwax (cerumen impaction). Otolaryngol Head Neck Surg. 2017;156:S1-S29.
14. Guest JF, Greener MJ, Robinson AC, et al. Impacted cerumen: composition, production, epidemiology and management. QJM. 2004;97:477-488.
15. Saxby C, Williams R, Hickey S. Finding the most effective cerumenolytic. J Laryngol Otol. 2013;127:1067-1070.
16. Awad AH, ElTaher M. ENT foreign bodies: an experience. Int Arch Otorhinolaryngol. 2018;22:146-151.
17. Heim SW, Maughan KL. Foreign bodies in the ear, nose, and throat. Am Fam Physician. 2007;76:1185-1189.
18. Sharma K, Goswami SC, Baruah DK. Auricular trauma and its management. Indian J Otolaryngol Head Neck Surg. 2006;58:232-234.
19. Haik J, Givol O, Kornhaber R, et al. Cauliflower ear–a minimally invasive treatment in a wrestling athlete: a case report. Int Med Case Rep J. 2018;11:5-7.
20. Ebrahimi A, Kazemi A, Rasouli HR, et al. Reconstructive surgery of auricular defects: an overview. Trauma Mon. 2015;20:e28202.
21. Warner E, Weston C, Barclay-Klingle N, et al. The swollen pinna. BMJ. 2017; 359; j5073.
22. Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
23. Ranjan SK, Ahmed A, Harsh V, et al. Giant bilateral keloids of the ear lobule: case report and brief review of the literature. J Family Med Prim Care. 2017;6:677-679.
24. Roland PS, Marple BF. Disorders of the external auditory canal. J Am Acad Audiol. 1997;8:367-378.
25. Scheinfeld NS, Silverberg NB, Weinberg JM, et al. The preauricular sinus: a review of its clinical presentation, treatment, and associations. Pediatr Dermatol. 2004;21:191-196.
26. Roth DA, Hildesheimer M, Bardestein S, et al. Preauricular skin tags and ear pits are associated with permanent hearing impairment in newborns. Pediatrics. 2008;122:e884-890.
27. Tan T, Constantinides H, Mitchell TE. The preauricular sinus: a review of its aetiology, clinical presentation and management. Int J Ped Otorhinolaryngol. 2005;69:1469-1474.
1. Cox TC, Camci ED, Vora S, et al. The genetics of auricular development and malformation: new findings in model systems driving future directions for microtia research. Eur J Med Genet. 2014;57:394-401.
2. Sosin M, Weissler JM, Pulcrano M, et al. Transcartilaginous ear piercing and infectious complications: a systematic review and critical analysis of outcomes. Laryngoscope. 2015;125:1827-1834.
3. Stirn A. Body piercing: medical consequences and psychological motivations. Lancet. 2003;361:1205-1215.
4. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Larygol Oncol. 2013;127:505-508.
5. Mitchell S, Ditta K, Minhas S, et al. Pinna abscesses: can we manage them better? A case series and review of the literature. Eur Arch Otorhinolaryngol. 2015;272:3163-3167.
6. Stone KE. Otitis externa. Pediatr Rev. 2007;28:77-78.
7. Rosenfeld RM, Schwartz SR, Cannon CR, et al. Clinical practice guideline: acute otitis externa. Otolaryngol Head Neck Surg. 2014;150(1 suppl):S1-S24.
8. Prentice P. American Academy of Otolaryngology: Head and Neck Surgery Foundation clinical practice guideline on acute otitis externa. Arch Dis Child Educ Pract Ed. 2015;100:197.
9. Unadkat S, Kanzara T, Watters G. Necrotising otitis externa in the immunocompetent patient. J Laryngol Otol. 2018;132:71-74.
10. Carfrae MJ, Kesser BW. Malignant otitis externa. Otolarngol Clin N Am. 2008;41:537-549.
11. Chandler JR, Malignant otitis externa. Laryngoscope. 1968;78:1257-1294.
12. Hollis S, Evans K. Management of malignant (necrotising) otitis externa. J Laryngol Otol. 2011;125:1212-1217.
13. Schwartz SR, Magit AE, Rosenfeld RM, et al. Clinical practice guideline (update): earwax (cerumen impaction). Otolaryngol Head Neck Surg. 2017;156:S1-S29.
14. Guest JF, Greener MJ, Robinson AC, et al. Impacted cerumen: composition, production, epidemiology and management. QJM. 2004;97:477-488.
15. Saxby C, Williams R, Hickey S. Finding the most effective cerumenolytic. J Laryngol Otol. 2013;127:1067-1070.
16. Awad AH, ElTaher M. ENT foreign bodies: an experience. Int Arch Otorhinolaryngol. 2018;22:146-151.
17. Heim SW, Maughan KL. Foreign bodies in the ear, nose, and throat. Am Fam Physician. 2007;76:1185-1189.
18. Sharma K, Goswami SC, Baruah DK. Auricular trauma and its management. Indian J Otolaryngol Head Neck Surg. 2006;58:232-234.
19. Haik J, Givol O, Kornhaber R, et al. Cauliflower ear–a minimally invasive treatment in a wrestling athlete: a case report. Int Med Case Rep J. 2018;11:5-7.
20. Ebrahimi A, Kazemi A, Rasouli HR, et al. Reconstructive surgery of auricular defects: an overview. Trauma Mon. 2015;20:e28202.
21. Warner E, Weston C, Barclay-Klingle N, et al. The swollen pinna. BMJ. 2017; 359; j5073.
22. Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
23. Ranjan SK, Ahmed A, Harsh V, et al. Giant bilateral keloids of the ear lobule: case report and brief review of the literature. J Family Med Prim Care. 2017;6:677-679.
24. Roland PS, Marple BF. Disorders of the external auditory canal. J Am Acad Audiol. 1997;8:367-378.
25. Scheinfeld NS, Silverberg NB, Weinberg JM, et al. The preauricular sinus: a review of its clinical presentation, treatment, and associations. Pediatr Dermatol. 2004;21:191-196.
26. Roth DA, Hildesheimer M, Bardestein S, et al. Preauricular skin tags and ear pits are associated with permanent hearing impairment in newborns. Pediatrics. 2008;122:e884-890.
27. Tan T, Constantinides H, Mitchell TE. The preauricular sinus: a review of its aetiology, clinical presentation and management. Int J Ped Otorhinolaryngol. 2005;69:1469-1474.
PRACTICE RECOMMENDATIONS
› Prescribe topical antibiotics for uncomplicated otitis externa, reserving systemic agents for infection extending outside the ear canal, necrotizing otitis externa, or patients who are immunodeficient. C
› Avoid clearing cerumen if a patient is asymptomatic and advise patients/parents on Do’s and Don’ts for ear wax accumulation. C
› Consider flooding the ear canal with xylocaine, alcohol, or mineral oil before attempting insect removal. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Can you differentiate bacterial from viral pediatric infections based on the CBC?
No—the complete blood count (CBC) alone does not have adequate sensitivity or specificity to tell bacterial from viral infections (strength of recommendation [SOR]: B, cohort studies). When used in conjunction with other clinical parameters in validated decision-making algorithms, the CBC can help detect serious bacterial infections in pediatric patients with fever (SOR: B, cohort studies).
There’s no substitute for history, physical exam, and good judgment
John D. Hallgren, MD
Uniformed Services University of the Health Sciences, RAF Menwith Hill, United Kingdom
Viral vs bacterial—often these are surrogate terms for minor vs serious illness. This review is a great lesson in likelihood ratios. Based on the low likelihood ratio, a CBC alone does not shift our suspicion greatly for serious bacterial infections in intermediate-risk patients; however, if you combine it with a clinical decision rule, it can greatly help decision-making, as evidenced by negative predictive values of 99% and above.
In contrast, we don’t need the CBC to tell us that an adult with the sniffles has a rhino/corona/whatevervirus, nor do we need it to tell us that a febrile, lethargic child with a petechial rash has a life-threatening bacteremia. If you enjoy the muck and the mess of primary care as much as I do, this inquiry should provide you with the validation that there’s no substitute for the history, physical exam, and judgment of a good clinician.
Evidence summary
For acutely febrile patients, the presence of an elevated white blood cell (WBC) count with elevated band forms has dogmatically been thought of as a marker for bacterial infection.1 Current literature, however, does not support this.2
Neisseria meningitides
A retrospective study of 5353 infants ages 3 to 89 days presenting to the emergency department for evaluation of fever showed that 3 of 4 infants ultimately diagnosed with bacterial meningitis would have been missed if the WBC count alone were used to predict which infants need a lumbar puncture.3 A prospective study of 2492 children ages 3 to 24 months presenting to the emergency department with acute fever and an absolute WBC count >15,000/mm3 revealed that neither a polymorphonuclear count of >10,000/mm3 (>66% segmented forms) nor a band count of >500/mm3 was associated with an increased likelihood of occult bacterial infection.4 Other studies show that the WBC alone is poorly discriminatory for identifying either bacteremia or meningitis.5,6
To improve the diagnostic utility of the CBC, other studies have examined individual components of the white blood cell differential count (TABLE 1). In particular, the use of the absolute neutrophil count (ANC) has been proposed as a superior marker of serious bacterial infection.7 A review of 6579 outpatients aged 3 to 36 months presenting to the emergency department with temperatures of 39°C or higher showed an ANC of >10,000/mm3 as more predictive of occult pneumococcal bacteremia than an elevated WBC count (>15,000/mm3) alone.8 Another retrospective review of more than 10,000 patients aged 3 to 36 months presenting to the emergency department used logistic regression to identify predictors of bacteremia. In this study, ANC (>9500/mm3) and WBC (>14,300/mm3) were of equal sensitivity (75%) and specificity (75%) in identifying serious bacterial infection.9 Finally, the band count alone does not accurately predict serious bacterial infection.10
In summary, the CBC cannot be used in isolation to differentiate bacterial from viral illness. The CBC can, however, augment clinical data from the history and physical examination to predict the likelihood of serious bacterial illness. As a result, numerous diagnostic criteria, each incorporating elements of the CBC, have been developed in an attempt to accurately differentiate bacterial from viral illness in acutely febrile patients, most typically children (TABLE 2). These criteria differ by age of the patient, clinical testing recommendations, indications for antibiotic therapy, as well as WBC cutoffs.
TABLE 1
WBC markers: How good are they at predicting serious bacterial infection?9,18,19
| VARIABLE | CUTOFF | SENSITIVITY | SPECIFICITY | LR (95% CI) |
|---|---|---|---|---|
| White blood cell count | 15,000/mm3 | 64%–82% | 67%–75% | 1.9–2.7 (1.1–3.8) |
| Absolute neutrophil count | 10,000/mm3 | 64%–76% | 76%–81% | 3.0–3.3 (1.6–6.2) |
| LR, likelihood ratio; CI, confidence interval. | ||||
TABLE 2
Clinical criteria for predicting serious bacterial infection in febrile children
| CRITERION | ROCHESTER CRITERIA11 | BOSTON CRITERIA12 | PHILADELPHIA CRITERIA13 |
|---|---|---|---|
| Predictive value | 98.9% PV–in ruling out serious bacterial infection | 95% PV+ to identify serious bacterial infection | 100% PV–in ruling out serious bacterial infection |
| Age | <60 days | 1–3 mos Present to emergency dept. with fever ≥38.0°C | 29–56 days Present with fever ≥38.2°C |
| Appearance | Well-appearing Previously healthy No evidence of infection (skin, bone, joint, soft tissue or ear) | Healthy appearing No ear, soft tissue, joint or bone infection on exam | Well-appearing |
| White blood cell count | WBC 5–15,000/mm3 Bands ≤1,500/mm3 | Peripheral WBC ≤20,000/mm3 | WBC ≤15,000/mm3 Band-to-neutrophil ratio of ≤0.2 |
| Urinalysis | ≤10 WBC/hpf of centrifuged urine | Urinalysis ≤10 WBC/hpf | Urinalysis ≤10 WBC/hpf |
| Other tests | If diarrhea, ≤5 WBC/hpf of stool smear | CSF WBC ≤10/hpf | CSF WBC ≤8/hpf with negative gram stain If watery diarrhea, few or no WBC/hpf on stool smear |
| WBC, white blood cell count; hpf, high-powered field; CSF, cerebrospinal fluid; PV, predictive value | |||
Recommendations from others
The American College of Emergency Physicians recommends considering antibiotic therapy for previously healthy, well-appearing children ages 3 to 36 months who present with a fever without a clinical source and a WBC count >15,000/mm3.3,14
The University of Cincinnati Evidence-Based Clinical Practice Guidelines for fever of uncertain source in children ages 2 to 36 months recommends obtaining a CBC for any child who is ill-appearing or at high risk for bacteremia (determined by the clinicians’ judgment). A WBC of ≥15,000/mm3 or ANC >10,000/mm3 provide support for antibiotic therapy.15 The 1993 American Academy of Pediatrics guidelines for fever ≥39°C without a source in children ages 3 months to 3 years recommends a CBC; if the WBC count ≥15,000/mm3, they recommend a blood culture and treatment with antibiotics pending culture results.3,16
It is important to note that in the age of Haemophilus influenza and Streptococcus pneumonia vaccination, the rate of occult bacteremia in febrile children presenting without a source has fallen from 3% to 10% to 1% or less.17 A lower prevalence reduces the utility of routine CBC or blood culture in the evaluation of immunized, febrile children. Parameters such as procalcitonin, interleukin-6, interleukin-8, interleukin-1 receptor antagonist and C-reactive protein show future promise as biochemical markers for identifying serious bacterial infections.18
1. Wile MJ, Homer LD, Gaehler S, Phillips S, Millan J. Manual differential cell counts help predict bacterial infection. A multivariate analysis. Am J Clin Pathol 2001;115:644-649.
2. Seebach JD, Morant R, Ruegg R, Seifert B, Fehr J. The diagnostic value of the neutrophil left shift in predicting inflammatory and infectious disease. Am J Clin Pathol 1997;107:582-591.
3. Bonsu BK, Harper MB. Utility of the peripheral blood white blood cell count for identifying sick young infants who need lumbar puncture. Ann Emerg Med 2003;41:206-214.
4. Kramer MS, Tange SM, Mills EL, Ciampi A, Bernstein ML, Drummond KN. Role of the complete blood count in detecting occult focal bacterial infection in the young febrile child. J Clin Epidemiol 1993;46:349-357.
5. Brown L, Shaw T, Wittlake WA. Does leucocytosis identify bacterial infections in febrile neonates presenting to the emergency department? Emerg Med J 2005;22:256-259.
6. Garges HP, Moody MA, Cotten CM, et al. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics 2006;117:1094-1100.
7. Gombos MM, Bienkowski RS, Gochman RF, Billett HH. The absolute neutrophil count: is it the best indicator for occult bacteremia in infants? Am J Clin Pathol 1998;109:221-225.
8. Kuppermann N, Fleisher GR, Jaffe DM. Predictors of occult pneumococcal bacteremia in young febrile children. Ann Emerg Med 1998;31:679-687.
9. Isaacman DJ, Shults J, Gross TK, Davis PH, Harper M. Predictors of bacteremia in febrile children 3 to 36 months of age. Pediatrics 2000;106:977-982.
10. Cornbleet PJ. Clinical utility of the band count. Clin Lab Med 2002;22:101-136.
11. Dagan R, Powell KR, Hall CB, Menegus MA. Identification of infants unlikely to have serious bacterial infection although hospitalized for suspected sepsis. J Pediatr 1985;107:855-860.
12. Baskin MN, O’Rourke EJ, Fleisher GR. Outpatient treatment of febrile infants 28 to 89 days of age with intramuscular administration of ceftriaxone. J Pediatr 1992;120:22-27.
13. Baker MD, Bell LM, Avner JR. The efficacy of routine outpatient management without antibiotics of fever in selected infants. Pediatrics 1999;103:627-631.
14. American College of emergency Physicians. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med 2003;42:530-545.
15. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical practice guideline for fever of uncertain source in children in 2 to 36 months of age. Cincinnati, Ohio: Cincinnati Children’s Hospital Medical Center; 2003.
16. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Ann Emerg Med 1993;22:1198-1210.
17. Stoll ML, Rubin LG. Incidence of occult bacteremia among highly febrile young children in the era of the pneumococcal conjugate vaccine. Arch Pediatr Adolesc Med 2004;158:671-675.
18. Pulliam PN, Attia MW, Cronan KM. C-reactive protein in febrile children 1 to 36 months of age with clinically undetectable serious bacterial infection. Pediatrics 2001;108:1275-1279.
19. Pratt A, Attia MW. Duration of fever and markers of serious bacterial infection in young febrile children. Pediatr Int 2007;49:31-35.
No—the complete blood count (CBC) alone does not have adequate sensitivity or specificity to tell bacterial from viral infections (strength of recommendation [SOR]: B, cohort studies). When used in conjunction with other clinical parameters in validated decision-making algorithms, the CBC can help detect serious bacterial infections in pediatric patients with fever (SOR: B, cohort studies).
There’s no substitute for history, physical exam, and good judgment
John D. Hallgren, MD
Uniformed Services University of the Health Sciences, RAF Menwith Hill, United Kingdom
Viral vs bacterial—often these are surrogate terms for minor vs serious illness. This review is a great lesson in likelihood ratios. Based on the low likelihood ratio, a CBC alone does not shift our suspicion greatly for serious bacterial infections in intermediate-risk patients; however, if you combine it with a clinical decision rule, it can greatly help decision-making, as evidenced by negative predictive values of 99% and above.
In contrast, we don’t need the CBC to tell us that an adult with the sniffles has a rhino/corona/whatevervirus, nor do we need it to tell us that a febrile, lethargic child with a petechial rash has a life-threatening bacteremia. If you enjoy the muck and the mess of primary care as much as I do, this inquiry should provide you with the validation that there’s no substitute for the history, physical exam, and judgment of a good clinician.
Evidence summary
For acutely febrile patients, the presence of an elevated white blood cell (WBC) count with elevated band forms has dogmatically been thought of as a marker for bacterial infection.1 Current literature, however, does not support this.2
Neisseria meningitides
A retrospective study of 5353 infants ages 3 to 89 days presenting to the emergency department for evaluation of fever showed that 3 of 4 infants ultimately diagnosed with bacterial meningitis would have been missed if the WBC count alone were used to predict which infants need a lumbar puncture.3 A prospective study of 2492 children ages 3 to 24 months presenting to the emergency department with acute fever and an absolute WBC count >15,000/mm3 revealed that neither a polymorphonuclear count of >10,000/mm3 (>66% segmented forms) nor a band count of >500/mm3 was associated with an increased likelihood of occult bacterial infection.4 Other studies show that the WBC alone is poorly discriminatory for identifying either bacteremia or meningitis.5,6
To improve the diagnostic utility of the CBC, other studies have examined individual components of the white blood cell differential count (TABLE 1). In particular, the use of the absolute neutrophil count (ANC) has been proposed as a superior marker of serious bacterial infection.7 A review of 6579 outpatients aged 3 to 36 months presenting to the emergency department with temperatures of 39°C or higher showed an ANC of >10,000/mm3 as more predictive of occult pneumococcal bacteremia than an elevated WBC count (>15,000/mm3) alone.8 Another retrospective review of more than 10,000 patients aged 3 to 36 months presenting to the emergency department used logistic regression to identify predictors of bacteremia. In this study, ANC (>9500/mm3) and WBC (>14,300/mm3) were of equal sensitivity (75%) and specificity (75%) in identifying serious bacterial infection.9 Finally, the band count alone does not accurately predict serious bacterial infection.10
In summary, the CBC cannot be used in isolation to differentiate bacterial from viral illness. The CBC can, however, augment clinical data from the history and physical examination to predict the likelihood of serious bacterial illness. As a result, numerous diagnostic criteria, each incorporating elements of the CBC, have been developed in an attempt to accurately differentiate bacterial from viral illness in acutely febrile patients, most typically children (TABLE 2). These criteria differ by age of the patient, clinical testing recommendations, indications for antibiotic therapy, as well as WBC cutoffs.
TABLE 1
WBC markers: How good are they at predicting serious bacterial infection?9,18,19
| VARIABLE | CUTOFF | SENSITIVITY | SPECIFICITY | LR (95% CI) |
|---|---|---|---|---|
| White blood cell count | 15,000/mm3 | 64%–82% | 67%–75% | 1.9–2.7 (1.1–3.8) |
| Absolute neutrophil count | 10,000/mm3 | 64%–76% | 76%–81% | 3.0–3.3 (1.6–6.2) |
| LR, likelihood ratio; CI, confidence interval. | ||||
TABLE 2
Clinical criteria for predicting serious bacterial infection in febrile children
| CRITERION | ROCHESTER CRITERIA11 | BOSTON CRITERIA12 | PHILADELPHIA CRITERIA13 |
|---|---|---|---|
| Predictive value | 98.9% PV–in ruling out serious bacterial infection | 95% PV+ to identify serious bacterial infection | 100% PV–in ruling out serious bacterial infection |
| Age | <60 days | 1–3 mos Present to emergency dept. with fever ≥38.0°C | 29–56 days Present with fever ≥38.2°C |
| Appearance | Well-appearing Previously healthy No evidence of infection (skin, bone, joint, soft tissue or ear) | Healthy appearing No ear, soft tissue, joint or bone infection on exam | Well-appearing |
| White blood cell count | WBC 5–15,000/mm3 Bands ≤1,500/mm3 | Peripheral WBC ≤20,000/mm3 | WBC ≤15,000/mm3 Band-to-neutrophil ratio of ≤0.2 |
| Urinalysis | ≤10 WBC/hpf of centrifuged urine | Urinalysis ≤10 WBC/hpf | Urinalysis ≤10 WBC/hpf |
| Other tests | If diarrhea, ≤5 WBC/hpf of stool smear | CSF WBC ≤10/hpf | CSF WBC ≤8/hpf with negative gram stain If watery diarrhea, few or no WBC/hpf on stool smear |
| WBC, white blood cell count; hpf, high-powered field; CSF, cerebrospinal fluid; PV, predictive value | |||
Recommendations from others
The American College of Emergency Physicians recommends considering antibiotic therapy for previously healthy, well-appearing children ages 3 to 36 months who present with a fever without a clinical source and a WBC count >15,000/mm3.3,14
The University of Cincinnati Evidence-Based Clinical Practice Guidelines for fever of uncertain source in children ages 2 to 36 months recommends obtaining a CBC for any child who is ill-appearing or at high risk for bacteremia (determined by the clinicians’ judgment). A WBC of ≥15,000/mm3 or ANC >10,000/mm3 provide support for antibiotic therapy.15 The 1993 American Academy of Pediatrics guidelines for fever ≥39°C without a source in children ages 3 months to 3 years recommends a CBC; if the WBC count ≥15,000/mm3, they recommend a blood culture and treatment with antibiotics pending culture results.3,16
It is important to note that in the age of Haemophilus influenza and Streptococcus pneumonia vaccination, the rate of occult bacteremia in febrile children presenting without a source has fallen from 3% to 10% to 1% or less.17 A lower prevalence reduces the utility of routine CBC or blood culture in the evaluation of immunized, febrile children. Parameters such as procalcitonin, interleukin-6, interleukin-8, interleukin-1 receptor antagonist and C-reactive protein show future promise as biochemical markers for identifying serious bacterial infections.18
No—the complete blood count (CBC) alone does not have adequate sensitivity or specificity to tell bacterial from viral infections (strength of recommendation [SOR]: B, cohort studies). When used in conjunction with other clinical parameters in validated decision-making algorithms, the CBC can help detect serious bacterial infections in pediatric patients with fever (SOR: B, cohort studies).
There’s no substitute for history, physical exam, and good judgment
John D. Hallgren, MD
Uniformed Services University of the Health Sciences, RAF Menwith Hill, United Kingdom
Viral vs bacterial—often these are surrogate terms for minor vs serious illness. This review is a great lesson in likelihood ratios. Based on the low likelihood ratio, a CBC alone does not shift our suspicion greatly for serious bacterial infections in intermediate-risk patients; however, if you combine it with a clinical decision rule, it can greatly help decision-making, as evidenced by negative predictive values of 99% and above.
In contrast, we don’t need the CBC to tell us that an adult with the sniffles has a rhino/corona/whatevervirus, nor do we need it to tell us that a febrile, lethargic child with a petechial rash has a life-threatening bacteremia. If you enjoy the muck and the mess of primary care as much as I do, this inquiry should provide you with the validation that there’s no substitute for the history, physical exam, and judgment of a good clinician.
Evidence summary
For acutely febrile patients, the presence of an elevated white blood cell (WBC) count with elevated band forms has dogmatically been thought of as a marker for bacterial infection.1 Current literature, however, does not support this.2
Neisseria meningitides
A retrospective study of 5353 infants ages 3 to 89 days presenting to the emergency department for evaluation of fever showed that 3 of 4 infants ultimately diagnosed with bacterial meningitis would have been missed if the WBC count alone were used to predict which infants need a lumbar puncture.3 A prospective study of 2492 children ages 3 to 24 months presenting to the emergency department with acute fever and an absolute WBC count >15,000/mm3 revealed that neither a polymorphonuclear count of >10,000/mm3 (>66% segmented forms) nor a band count of >500/mm3 was associated with an increased likelihood of occult bacterial infection.4 Other studies show that the WBC alone is poorly discriminatory for identifying either bacteremia or meningitis.5,6
To improve the diagnostic utility of the CBC, other studies have examined individual components of the white blood cell differential count (TABLE 1). In particular, the use of the absolute neutrophil count (ANC) has been proposed as a superior marker of serious bacterial infection.7 A review of 6579 outpatients aged 3 to 36 months presenting to the emergency department with temperatures of 39°C or higher showed an ANC of >10,000/mm3 as more predictive of occult pneumococcal bacteremia than an elevated WBC count (>15,000/mm3) alone.8 Another retrospective review of more than 10,000 patients aged 3 to 36 months presenting to the emergency department used logistic regression to identify predictors of bacteremia. In this study, ANC (>9500/mm3) and WBC (>14,300/mm3) were of equal sensitivity (75%) and specificity (75%) in identifying serious bacterial infection.9 Finally, the band count alone does not accurately predict serious bacterial infection.10
In summary, the CBC cannot be used in isolation to differentiate bacterial from viral illness. The CBC can, however, augment clinical data from the history and physical examination to predict the likelihood of serious bacterial illness. As a result, numerous diagnostic criteria, each incorporating elements of the CBC, have been developed in an attempt to accurately differentiate bacterial from viral illness in acutely febrile patients, most typically children (TABLE 2). These criteria differ by age of the patient, clinical testing recommendations, indications for antibiotic therapy, as well as WBC cutoffs.
TABLE 1
WBC markers: How good are they at predicting serious bacterial infection?9,18,19
| VARIABLE | CUTOFF | SENSITIVITY | SPECIFICITY | LR (95% CI) |
|---|---|---|---|---|
| White blood cell count | 15,000/mm3 | 64%–82% | 67%–75% | 1.9–2.7 (1.1–3.8) |
| Absolute neutrophil count | 10,000/mm3 | 64%–76% | 76%–81% | 3.0–3.3 (1.6–6.2) |
| LR, likelihood ratio; CI, confidence interval. | ||||
TABLE 2
Clinical criteria for predicting serious bacterial infection in febrile children
| CRITERION | ROCHESTER CRITERIA11 | BOSTON CRITERIA12 | PHILADELPHIA CRITERIA13 |
|---|---|---|---|
| Predictive value | 98.9% PV–in ruling out serious bacterial infection | 95% PV+ to identify serious bacterial infection | 100% PV–in ruling out serious bacterial infection |
| Age | <60 days | 1–3 mos Present to emergency dept. with fever ≥38.0°C | 29–56 days Present with fever ≥38.2°C |
| Appearance | Well-appearing Previously healthy No evidence of infection (skin, bone, joint, soft tissue or ear) | Healthy appearing No ear, soft tissue, joint or bone infection on exam | Well-appearing |
| White blood cell count | WBC 5–15,000/mm3 Bands ≤1,500/mm3 | Peripheral WBC ≤20,000/mm3 | WBC ≤15,000/mm3 Band-to-neutrophil ratio of ≤0.2 |
| Urinalysis | ≤10 WBC/hpf of centrifuged urine | Urinalysis ≤10 WBC/hpf | Urinalysis ≤10 WBC/hpf |
| Other tests | If diarrhea, ≤5 WBC/hpf of stool smear | CSF WBC ≤10/hpf | CSF WBC ≤8/hpf with negative gram stain If watery diarrhea, few or no WBC/hpf on stool smear |
| WBC, white blood cell count; hpf, high-powered field; CSF, cerebrospinal fluid; PV, predictive value | |||
Recommendations from others
The American College of Emergency Physicians recommends considering antibiotic therapy for previously healthy, well-appearing children ages 3 to 36 months who present with a fever without a clinical source and a WBC count >15,000/mm3.3,14
The University of Cincinnati Evidence-Based Clinical Practice Guidelines for fever of uncertain source in children ages 2 to 36 months recommends obtaining a CBC for any child who is ill-appearing or at high risk for bacteremia (determined by the clinicians’ judgment). A WBC of ≥15,000/mm3 or ANC >10,000/mm3 provide support for antibiotic therapy.15 The 1993 American Academy of Pediatrics guidelines for fever ≥39°C without a source in children ages 3 months to 3 years recommends a CBC; if the WBC count ≥15,000/mm3, they recommend a blood culture and treatment with antibiotics pending culture results.3,16
It is important to note that in the age of Haemophilus influenza and Streptococcus pneumonia vaccination, the rate of occult bacteremia in febrile children presenting without a source has fallen from 3% to 10% to 1% or less.17 A lower prevalence reduces the utility of routine CBC or blood culture in the evaluation of immunized, febrile children. Parameters such as procalcitonin, interleukin-6, interleukin-8, interleukin-1 receptor antagonist and C-reactive protein show future promise as biochemical markers for identifying serious bacterial infections.18
1. Wile MJ, Homer LD, Gaehler S, Phillips S, Millan J. Manual differential cell counts help predict bacterial infection. A multivariate analysis. Am J Clin Pathol 2001;115:644-649.
2. Seebach JD, Morant R, Ruegg R, Seifert B, Fehr J. The diagnostic value of the neutrophil left shift in predicting inflammatory and infectious disease. Am J Clin Pathol 1997;107:582-591.
3. Bonsu BK, Harper MB. Utility of the peripheral blood white blood cell count for identifying sick young infants who need lumbar puncture. Ann Emerg Med 2003;41:206-214.
4. Kramer MS, Tange SM, Mills EL, Ciampi A, Bernstein ML, Drummond KN. Role of the complete blood count in detecting occult focal bacterial infection in the young febrile child. J Clin Epidemiol 1993;46:349-357.
5. Brown L, Shaw T, Wittlake WA. Does leucocytosis identify bacterial infections in febrile neonates presenting to the emergency department? Emerg Med J 2005;22:256-259.
6. Garges HP, Moody MA, Cotten CM, et al. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics 2006;117:1094-1100.
7. Gombos MM, Bienkowski RS, Gochman RF, Billett HH. The absolute neutrophil count: is it the best indicator for occult bacteremia in infants? Am J Clin Pathol 1998;109:221-225.
8. Kuppermann N, Fleisher GR, Jaffe DM. Predictors of occult pneumococcal bacteremia in young febrile children. Ann Emerg Med 1998;31:679-687.
9. Isaacman DJ, Shults J, Gross TK, Davis PH, Harper M. Predictors of bacteremia in febrile children 3 to 36 months of age. Pediatrics 2000;106:977-982.
10. Cornbleet PJ. Clinical utility of the band count. Clin Lab Med 2002;22:101-136.
11. Dagan R, Powell KR, Hall CB, Menegus MA. Identification of infants unlikely to have serious bacterial infection although hospitalized for suspected sepsis. J Pediatr 1985;107:855-860.
12. Baskin MN, O’Rourke EJ, Fleisher GR. Outpatient treatment of febrile infants 28 to 89 days of age with intramuscular administration of ceftriaxone. J Pediatr 1992;120:22-27.
13. Baker MD, Bell LM, Avner JR. The efficacy of routine outpatient management without antibiotics of fever in selected infants. Pediatrics 1999;103:627-631.
14. American College of emergency Physicians. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med 2003;42:530-545.
15. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical practice guideline for fever of uncertain source in children in 2 to 36 months of age. Cincinnati, Ohio: Cincinnati Children’s Hospital Medical Center; 2003.
16. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Ann Emerg Med 1993;22:1198-1210.
17. Stoll ML, Rubin LG. Incidence of occult bacteremia among highly febrile young children in the era of the pneumococcal conjugate vaccine. Arch Pediatr Adolesc Med 2004;158:671-675.
18. Pulliam PN, Attia MW, Cronan KM. C-reactive protein in febrile children 1 to 36 months of age with clinically undetectable serious bacterial infection. Pediatrics 2001;108:1275-1279.
19. Pratt A, Attia MW. Duration of fever and markers of serious bacterial infection in young febrile children. Pediatr Int 2007;49:31-35.
1. Wile MJ, Homer LD, Gaehler S, Phillips S, Millan J. Manual differential cell counts help predict bacterial infection. A multivariate analysis. Am J Clin Pathol 2001;115:644-649.
2. Seebach JD, Morant R, Ruegg R, Seifert B, Fehr J. The diagnostic value of the neutrophil left shift in predicting inflammatory and infectious disease. Am J Clin Pathol 1997;107:582-591.
3. Bonsu BK, Harper MB. Utility of the peripheral blood white blood cell count for identifying sick young infants who need lumbar puncture. Ann Emerg Med 2003;41:206-214.
4. Kramer MS, Tange SM, Mills EL, Ciampi A, Bernstein ML, Drummond KN. Role of the complete blood count in detecting occult focal bacterial infection in the young febrile child. J Clin Epidemiol 1993;46:349-357.
5. Brown L, Shaw T, Wittlake WA. Does leucocytosis identify bacterial infections in febrile neonates presenting to the emergency department? Emerg Med J 2005;22:256-259.
6. Garges HP, Moody MA, Cotten CM, et al. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics 2006;117:1094-1100.
7. Gombos MM, Bienkowski RS, Gochman RF, Billett HH. The absolute neutrophil count: is it the best indicator for occult bacteremia in infants? Am J Clin Pathol 1998;109:221-225.
8. Kuppermann N, Fleisher GR, Jaffe DM. Predictors of occult pneumococcal bacteremia in young febrile children. Ann Emerg Med 1998;31:679-687.
9. Isaacman DJ, Shults J, Gross TK, Davis PH, Harper M. Predictors of bacteremia in febrile children 3 to 36 months of age. Pediatrics 2000;106:977-982.
10. Cornbleet PJ. Clinical utility of the band count. Clin Lab Med 2002;22:101-136.
11. Dagan R, Powell KR, Hall CB, Menegus MA. Identification of infants unlikely to have serious bacterial infection although hospitalized for suspected sepsis. J Pediatr 1985;107:855-860.
12. Baskin MN, O’Rourke EJ, Fleisher GR. Outpatient treatment of febrile infants 28 to 89 days of age with intramuscular administration of ceftriaxone. J Pediatr 1992;120:22-27.
13. Baker MD, Bell LM, Avner JR. The efficacy of routine outpatient management without antibiotics of fever in selected infants. Pediatrics 1999;103:627-631.
14. American College of emergency Physicians. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med 2003;42:530-545.
15. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical practice guideline for fever of uncertain source in children in 2 to 36 months of age. Cincinnati, Ohio: Cincinnati Children’s Hospital Medical Center; 2003.
16. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Ann Emerg Med 1993;22:1198-1210.
17. Stoll ML, Rubin LG. Incidence of occult bacteremia among highly febrile young children in the era of the pneumococcal conjugate vaccine. Arch Pediatr Adolesc Med 2004;158:671-675.
18. Pulliam PN, Attia MW, Cronan KM. C-reactive protein in febrile children 1 to 36 months of age with clinically undetectable serious bacterial infection. Pediatrics 2001;108:1275-1279.
19. Pratt A, Attia MW. Duration of fever and markers of serious bacterial infection in young febrile children. Pediatr Int 2007;49:31-35.
Evidence-based answers from the Family Physicians Inquiries Network