User login
Impact of Physician Facecards
The patient‐physician relationship is fundamental to safe and effective care. Hospital settings present unique challenges to this partnership, including the lack of a prior relationship for hospital‐based physicians, rapid pace of clinical care, and dynamic nature of inpatient medical teams. Prior studies document that a majority of hospitalized patients are unable to correctly identify their physicians or nurses, and patients in teaching hospitals have difficulty understanding their physicians' level of training.[1, 2, 3, 4] Acknowledging these deficits, professional societies and the Accreditation Council for Graduate Medical Education (ACMGE) have issued policies stating that patients and caregivers need to know who is responsible at every point during patient care.[5, 6] These policies do not, however, make recommendations on methods to achieve better understanding.
Simple interventions improve patients' ability to correctly identify the names and roles of their hospital physicians. Maniaci and colleagues found that patients were better able to identify attending physicians when their names were written on the dry‐erase board in the room.[7] Arora and colleagues asked hospital physicians to give facecards, which included their picture and a description of their role, to patients.[8] Patients were more likely to correctly identify 1 physicians, but, surprisingly, less likely to understand physicians' roles. In a similar study, Francis and colleagues placed photographs with names of the attending and resident physicians on the wall in patient rooms.[9] Patients who had photographs of their physicians on the wall were more likely to correctly identify physicians on their team compared with patients who had no photographs. Additionally, patients who were able to identify more physicians rated satisfaction with physicians higher in 2 of 6 survey questions used. However, the study was limited by the use of a nonvalidated instrument to assess patient satisfaction and the use of an intermediate outcome (ie, ability to identify physicians) as the independent variable rather than the intervention itself (ie, physician photographs).
Beyond satisfaction, lack of familiarity may negatively impact patients' trust and agreement with hospital physicians. Trust and agreement are important predictors of adherence to recommended treatment in outpatient settings[10, 11, 12, 13, 14, 15, 16, 17, 18] but have not been adequately evaluated in hospital settings. Therefore, we sought to pilot the use of physician facecards and assess their potential impact on patients' knowledge of physicians' names and roles as well as patient satisfaction, trust, and agreement with physicians.
METHODS
Setting and Study Design
We performed a cluster randomized controlled trial at Northwestern Memorial Hospital (NMH), an 897‐bed tertiary‐care teaching hospital in Chicago, Illinois. One of 2 similar hospitalist service units and 1 of 2 similar teaching‐service units were randomly selected to implement the use of physician facecards. General medical patients were admitted to the study units by NMH bed‐assignment personnel subject to unit bed availability. No other criteria (eg, diagnosis, severity of illness, or source of patient admission) were used in patient assignment. Each unit consisted of 30 beds, with the exception of 1 hospitalist unit, which had 23. As a result of a prior intervention, physicians were localized to care for patients on specific units.[19] Hospitalist units were each staffed by hospitalists who worked in 7‐day rotations without the assistance of residents or midlevel providers. Teaching units were staffed by physician teams consisting of 1 attending, 1 senior resident, 1 intern, and 1 or 2 third‐year medical students. No fourth‐year students (ie, acting interns) rotated on these services during the study period. Housestaff worked in 4‐week rotations, and attending physicians on the teaching service worked in 2‐week rotations.
Patient rooms included a whiteboard facing the patient with a template prompting insertion of physician name(s). Nurses had the primary responsibility for completing information on the whiteboards.
Physician Facecard
We created draft physician facecards featuring pictures of physicians and descriptions of their roles. We used Lexile analysis, a widely used measure of reading difficulty, to improve readability in an iterative fashion.[20, 21] We then sought feedback at hospitalist and resident meetings. Specifically, we asked for suggested revisions to content and recommendations on reliable methods to deliver facecards to patients. Teaching physicians felt strongly that each team member should be listed and shown on 1 card, which would fit easily into a lab‐coat pocket. We similarly engaged the NMH Patient and Family Advisory Council to seek recommended revisions to content and delivery of the facecards. The Council consists of 18 patient and caregiver members who meet regularly to provide input on hospital programs and proposals. Council members felt strongly that physicians should deliver the cards themselves during their initial introduction, rather than having patients receive cards by other means (eg, as part of unit orientation materials delivered by nonphysician staff members). We incorporated feedback from these stakeholder groups into a final version of the physician facecard and method for delivery (Figure 1).

We implemented the use of facecards from May to June 2012. Physicians on intervention units were informed of the study via email, and one of the co‐investigators (T.C.) distributed a supply of facecards to these physicians at the start of each rotation. This distribution was performed in person, and physicians were instructed to provide a facecard to each new patient during their first encounter. We also placed facecards in easily visible cardholders at the nurses' station on intervention units. Reminder emails were sent once each week to reinforce physician delivery of facecards.
Data Collection and Measures
Each weekday during the study period, we randomly selected patients for structured interviews in the afternoon of their second or third hospital day. We did not conduct interviews on the first day of physicians' rotations and excluded patients whose preferred language was not English and those disoreinted to person, place, or time.
Patients were asked to name the physician(s) primarily responsible for their hospital care and to state the role of each physician they identified. We documented receipt of facecards if one was viewed during the interview and by asking patients if they had received one. We also documented whether 1 correct physician names were written on the whiteboard in the patients' rooms. We used questions from the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey to assess satisfaction with physician communication and overall hospital care. HCAHPS is a validated patient‐satisfaction survey developed by the Agency for Healthcare Research and Quality (AHRQ) to assess hospitalized patients' experiences with care. Physician‐communication questions used ordinal response options of never, sometimes, usually, and always. Overall hospital rating was assessed using a 010 scale with 0=worst hospital possible and 10=best hospital possible. Trust with physicians was assessed using the Wake Forest University Trust Scale.[22] Prior research using this instrument has shown an association between trust and self‐management behaviors.[23] This 10‐item scale uses a 5‐point Likert scale and generates scores ranging from 10 to 50. Agreement with physicians was assessed using 3 questions used in a prior study by Staiger and colleagues showing an association between levels of agreement and health outcomes among outpatients treated for back pain.[17] Specifically, we asked patients to rate their agreement with hospital physicians' (1) explanation for the cause of primary symptoms, (2) plan for diagnostic tests, and (3) suggested plan for treatment using a 5‐point Likert scale. The agreement scale generated scores ranging from 3 to 15.
Approval for the study was obtained from the institutional review board of Northwestern University.
Statistical Analysis
Patient demographic data were obtained from the electronic health record and complemented data from interviews. We used [2] and t tests to compare patient characteristics. We used [2] tests to compare the percentage of patients able to correctly identify 1 of their physicians and 1 of their physicians' roles. We used [2] tests to compare the percentage of patients giving top‐box ratings to all 3 physician‐communicationsatisfaction questions (ie, always) and giving an overall hospital rating of 9 or 10. We used top‐box comparisons, rather than comparison of mean or median scores, because patient‐satisfaction data are typically highly skewed toward favorable responses. This approach is consistent with prior HCAHPS research.[24, 25] We used Mann‐Whitney U tests to compare ratings of trust and agreement. Because delivery of facecards was imperfect, we performed analyses both by intention to treat (ie, intervention vs control units) and based on treatment received (ie, received a facecard vs did not receive a facecard). All analyses were conducted using Stata version 11.2 (StataCorp, College Station, TX).
RESULTS
Study Subjects and Facecard Receipt
Overall, 217 patients were approached for interview. Thirty‐six were excluded because of disorientation, 12 were excluded because their preferred language was not English, and 31 declined to participate in the study. Patient characteristics for the 138 study patients are shown in Table 1. There were no significant differences in patient age, sex, or race. There was no significant difference in the percentage of patients with 1 correct physicians listed on the whiteboard in the room. Delivery of facecards was incomplete, with only 68% of intervention‐unit patients confirmed as having received them. A higher percentage of patients on the hospitalist intervention unit received facecards (23 of 30; 76.7%) than on the teaching intervention unit (22 of 36; 61.1%), but the difference was not statistically significant (P=0.18). There were no significant differences in age, sex, or race between patients who received a facecard compared with those who did not.
| Characteristic | Control Group, N=72 | Intervention Group, N=66 | P Value |
|---|---|---|---|
| |||
| Mean age, years (SD) | 56.8 (18.0) | 55.2 (18.2) | 0.62 |
| Women, n (%) | 35 (48.6) | 28 (42.4) | 0.47 |
| Nonwhite race, n (%) | 35 (50.7) | 36 (57.1) | 0.46 |
| Teaching unit, n (%) | 34 (47.2) | 36 (54.6) | 0.39 |
| Correct physician name on whiteboard, n (%)a | 46 (76.7) | 37 (72.6) | 0.62 |
| Received a facecard, n (%) | 1 (1) | 45 (68.2) | <0.01 |
Patients' Knowledge of Physicians
As shown in Table 2, more patients in the intervention group were able to correctly identify 1 of their treating physicians compared with the control group, but the result was not statistically significant (69.7% vs 58.3%; P=0.17). A significantly larger percentage of patients in the intervention group were able to identify the role of their hospital physicians (51.5% vs 16.7%; P<0.01). When comparing those that received a facecard and those that did not, patients who were given a facecard were more likely to correctly identify their hospital physician (89.1% vs 51.1%; P<0.01). Similarly, patients who had received a facecard were more likely to correctly identify the role of their hospital physician than patients who had not received a facecard (67.4% vs 16.3%; P<0.01).
| Impact | Control Group, N=72, n (%) | Intervention Group, N=66, n (%) | P Value |
|---|---|---|---|
| Patient correctly named 1 hospital physician | 42 (58.3) | 46 (69.7) | 0.17 |
| Patient correctly named role of hospital physician | 12 (16.7) | 34 (51.5) | <0.01 |
| Did Not Receive Facecard, N=92 | Received Facecard, N=46 | P Value | |
| Patient correctly named 1 hospital physician | 47 (51.1) | 41 (89.1) | <0.01 |
| Patient correctly named role of hospital physician | 15 (16.3) | 31 (67.4) | <0.01 |
Levels of Satisfaction, Trust, and Agreement
Overall, patients had high levels of satisfaction, trust, and agreement with hospital physicians. The overall satisfaction with physician communication was 75.6% (mean of top‐box scores across all 3 items), and 81 of 138 (58.7%) patients gave top‐box ratings to all 3 physician‐communicationsatisfaction items. Ninety‐seven of 137 (70.8%) patients rated overall hospital care as 9 or 10. The mean trust score for all patients was 40.77.8 and the median was 41.5 (interquartile range, 3747). The mean agreement score for all patients was 12.42.4 and the median was 12 (interquartile range, 1115). As shown in Table 3, satisfaction, trust, and agreement were similar for patients in the intervention group compared with the control group. Patients who received a facecard rated satisfaction, trust, and agreement slightly higher compared with those who had not received a facecard, but the results were not statistically significant.
| Ratings | Control Group, N=72 | Intervention Group, N=66 | P Value |
|---|---|---|---|
| |||
| Satisfaction with physicians, n (%)a | 39 (54.2) | 42 (63.6) | 0.26 |
| Overall hospital satisfaction, n (%)b | 51 (70.8) | 46 (70.8) | 0.99 |
| Median trust (IQR)c | 42 (3747) | 41 (3746) | 0.81 |
| Median agreement (IQR)c | 12 (1115) | 12 (1215) | 0.72 |
| Did Not Receive Facecard, N=92 | Received Facecard, N=46 | P Value | |
| Satisfaction with physicians, n (%)a | 51 (55.4) | 30 (65.2) | 0.27 |
| Overall hospital satisfaction, n (%)b | 64 (69.6) | 33 (73.3) | 0.65 |
| Median trust (IQR)c | 41 (3547) | 42 (3847) | 0.32 |
| Median agreement (IQR)c | 12 (1114.5) | 12.5 (1215) | 0.37 |
DISCUSSION
We found that receipt of physician facecards significantly improved patients' knowledge of the names and roles of hospital physicians but had little to no impact on satisfaction, trust, or agreement with physicians. Our finding of improved knowledge of the names and roles of physician providers is consistent with prior studies using similar interventions.[7, 8, 9] Facecards may have prompted more effective introductions on the part of physicians and may have served as memory aids for patients to better retain information about their newly introduced hospital physicians.
Patient receipt of the facecard on intervention units was incomplete in our study. Despite engagement of physicians in designing cards that could easily fit into lab coats and a robust strategy to inform and motivate physician delivery of facecards, only 68% of intended patients received them. Although not explicitly reported, prior studies appear to have similarly struggled to deliver interventions consistently. Arora and colleagues reported that facecards were visible in only 59% of patients' rooms among those able to correctly identify 1 of their physicians.[8] A post hoc survey of physicians involved in our study revealed the biggest impediment to delivering facecards was simply forgetting to do so (data not shown). Technologic innovations may help by automating the identification of providers. For example, the University of Pittsburgh Medical Center has piloted smart rooms that use sensor technology to announce the name and role of providers as they enter patients' rooms.[26]
We hypothesized that facecards might improve other important aspects of the patient‐physicians relationship. Although levels of patient satisfaction were slightly higher in patients who had received facecards, the results were not statistically significant. Levels of trust and agreement were minimally higher in patients who received facecards, and the results were not statistically significant. Notably, baseline levels of trust and agreement were higher than we had expected. In fact, levels of trust were nearly identical to those seen in a prior study of outpatients who had been with the same physician for a median of 4 years.[22] Patients in our study may have had high levels of trust in the hospital and transferred this trust to their assigned physicians as representatives of the organization. The high level of agreement may relate to patients' tendency to prefer a more passive role as they encounter serious illness.[27, 28] Paradoxically, these findings may impede optimal patient care. The high levels of trust and agreement in the current study suggest that patients may not question their physicians to clarify plans and the rationale behind them. Prior research has shown that deficits in patients' comprehension of the care plan are often not apparent to patients or their physicians.[4, 29, 30]
Our study has several limitations. First, we assessed an intervention involving 4 units in a single hospital. Generalizability may be limited, as physician‐staffing models, hospitals, and the patients they serve vary. Second, as previously mentioned, patients in the intervention group did not receive physician facecards as consistently as intended. We conducted analyses based on treatment received in an effort to evaluate the impact of facecards if optimally delivered. Third, questions assessing satisfaction, trust, and agreement did not specifically ask patients to reflect on care provided by the primary physician team. It is possible that interactions with other physicians (ie, consultants) may have influenced these results. Fourth, we were underpowered to detect statistically significant improvements in satisfaction, trust, or agreement resulting from our intervention. Assuming the intervention might truly improve satisfaction with physicians from 54.2% to 63.6%, we would have needed 900 patients (ie, 450 each for the intervention and control groups) to have 80% power to detect a statistically significant difference. However, our results show that patients have high levels of trust and agreement with hospital physicians despite the relative lack of familiarity. Therefore, any existing deficits in hospitalized patients' comprehension of the care plan do not appear to be exacerbated by a lack of trust and/or agreement with treating physicians.
CONCLUSION
In summary, we found that physician facecards significantly improved patients' knowledge of the names and roles of hospital physicians but had little to no impact on satisfaction, trust, or agreement with physicians. Baseline levels of satisfaction, trust, and agreement were high, suggesting lack of familiarity with hospital physicians does not impede these important aspects of the patient‐physician relationship. Larger studies are needed to definitively assess the impact of facecards on satisfaction, trust, and agreement with physicians.
Acknowledgments
The authors express their gratitude to members of the NMH Patient and Family Advisory Council for providing input on the design of the physician facecard.
Disclosures: This study was supported by a grant from the Globe Foundation. The authors report no conflicts of interest.
- , , , , , . Ability of hospitalized patients to identify their in‐hospital physicians. Arch Intern Med. 2009;169(2):199–201.
- , . Does your patient know your name? An approach to enhancing patients' awareness of their caretaker's name. J Healthc Qual. 2005;27(4):53–56.
- , , Hospitalized patients' understanding of their plan of care. Mayo Clin Proc. 2010;85(1):47–52.
- , . Communication discrepancies between physicians and hospitalized patients. Arch Intern Med. 2010;170(15):1302–1307.
- Accreditation Council for Graduate Medical Education. Common program requirements. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/CPRs2013.pdf. Revised July 1, 2013.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364–370.
- , , . Increasing a patient's ability to identify his or her attending physician using a patient room display. Arch Intern Med. 2010;170(12):1084–1085.
- , , , et al. Improving inpatients' identification of their doctors: use of FACE cards. Jt Comm J Qual Patient Saf. 2009;35(12):613–619.
- , , . Patient satisfaction associated with correct identification of physician's photographs. Mayo Clin Proc. 2001;76(6):604–608.
- , , , , , . Physician‐patient relationship and medication compliance: a primary care investigation. Ann Fam Med. 2004;2(5):455–461.
- , , , , . Trust in the health care system and the use of preventive health services by older black and white adults. Am J Public Health. 2009;99(7):1293–1299.
- , , , . The role of patient‐physician trust in moderating medication nonadherence due to cost pressures. Arch Intern Med. 2005;165(15):1749–1755.
- , , . Trust and the acceptance of and adherence to antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;28(1):47–58.
- , , , , , . Linking primary care performance to outcomes of care. J Fam Pract. 1998;47(3):213–220.
- , , , ; The Stanford Trust Study Physicians. Further validation and reliability testing of the Trust in Physician Scale. Med Care. 1999;37(5):510–517.
- , , , , , . The physician's actions and the outcome of illness in family practice. J Fam Pract. 1986;23(1):43–47.
- , , , , . Brief Report: Patient‐physician agreement as a predictor of outcomes in patients with back pain. J Gen Intern Med. 2005;20(10):935–937.
- , , , , , . The influence of patient‐practitioner agreement on outcome of care. Am J Public Health. 1981;71(2):127–131.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , . The Lexile Framework. Durham, NC: Metametrics, Inc.; 1998.
- National Center for Education Statistics; , . Assessing the Lexile Framework: results of a panel meeting. NCES Working Paper Series, No. 2001‐08. Washington, DC: US Department of Education, Office of Educational Research and Improvement; 2001.
- , , , et al. Measuring patients' trust in their primary care providers. Med Care Res Rev. 2002;59(3):293–318.
- , , , , , . The association of patient trust and self‐care among patients with diabetes mellitus. BMC Fam Pract. 2004;5:26.
- , , , , . Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27–37.
- , , , , . Measuring hospital care from the patients' perspective: an overview of the CAHPS Hospital Survey development process. Health Serv Res. 2005;40(6 pt 2):1977–1995.
- . Smart rooms, smart care delivery: UPMC clinician leaders leverage technology for greater effectiveness in patient care. Healthc Inform. 2011;28(9):36, 38–39, 42.
- , . Decision making during serious illness: what role do patients really want to play? J Clin Epidemiol. 1992;45(9):941–950.
- , , , , . The dynamics of change: cancer patients' preferences for information, involvement and support. Ann Oncol. 1997;8(9):857–863.
- , , , et al. Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan. Arch Intern Med. 1997;157(9):1026–1030.
- , , , , , . Patient comprehension of emergency department care and instructions: are patients aware of when they do not understand? Ann Emerg Med. 2009;53(4):454.e15–461.e15.
The patient‐physician relationship is fundamental to safe and effective care. Hospital settings present unique challenges to this partnership, including the lack of a prior relationship for hospital‐based physicians, rapid pace of clinical care, and dynamic nature of inpatient medical teams. Prior studies document that a majority of hospitalized patients are unable to correctly identify their physicians or nurses, and patients in teaching hospitals have difficulty understanding their physicians' level of training.[1, 2, 3, 4] Acknowledging these deficits, professional societies and the Accreditation Council for Graduate Medical Education (ACMGE) have issued policies stating that patients and caregivers need to know who is responsible at every point during patient care.[5, 6] These policies do not, however, make recommendations on methods to achieve better understanding.
Simple interventions improve patients' ability to correctly identify the names and roles of their hospital physicians. Maniaci and colleagues found that patients were better able to identify attending physicians when their names were written on the dry‐erase board in the room.[7] Arora and colleagues asked hospital physicians to give facecards, which included their picture and a description of their role, to patients.[8] Patients were more likely to correctly identify 1 physicians, but, surprisingly, less likely to understand physicians' roles. In a similar study, Francis and colleagues placed photographs with names of the attending and resident physicians on the wall in patient rooms.[9] Patients who had photographs of their physicians on the wall were more likely to correctly identify physicians on their team compared with patients who had no photographs. Additionally, patients who were able to identify more physicians rated satisfaction with physicians higher in 2 of 6 survey questions used. However, the study was limited by the use of a nonvalidated instrument to assess patient satisfaction and the use of an intermediate outcome (ie, ability to identify physicians) as the independent variable rather than the intervention itself (ie, physician photographs).
Beyond satisfaction, lack of familiarity may negatively impact patients' trust and agreement with hospital physicians. Trust and agreement are important predictors of adherence to recommended treatment in outpatient settings[10, 11, 12, 13, 14, 15, 16, 17, 18] but have not been adequately evaluated in hospital settings. Therefore, we sought to pilot the use of physician facecards and assess their potential impact on patients' knowledge of physicians' names and roles as well as patient satisfaction, trust, and agreement with physicians.
METHODS
Setting and Study Design
We performed a cluster randomized controlled trial at Northwestern Memorial Hospital (NMH), an 897‐bed tertiary‐care teaching hospital in Chicago, Illinois. One of 2 similar hospitalist service units and 1 of 2 similar teaching‐service units were randomly selected to implement the use of physician facecards. General medical patients were admitted to the study units by NMH bed‐assignment personnel subject to unit bed availability. No other criteria (eg, diagnosis, severity of illness, or source of patient admission) were used in patient assignment. Each unit consisted of 30 beds, with the exception of 1 hospitalist unit, which had 23. As a result of a prior intervention, physicians were localized to care for patients on specific units.[19] Hospitalist units were each staffed by hospitalists who worked in 7‐day rotations without the assistance of residents or midlevel providers. Teaching units were staffed by physician teams consisting of 1 attending, 1 senior resident, 1 intern, and 1 or 2 third‐year medical students. No fourth‐year students (ie, acting interns) rotated on these services during the study period. Housestaff worked in 4‐week rotations, and attending physicians on the teaching service worked in 2‐week rotations.
Patient rooms included a whiteboard facing the patient with a template prompting insertion of physician name(s). Nurses had the primary responsibility for completing information on the whiteboards.
Physician Facecard
We created draft physician facecards featuring pictures of physicians and descriptions of their roles. We used Lexile analysis, a widely used measure of reading difficulty, to improve readability in an iterative fashion.[20, 21] We then sought feedback at hospitalist and resident meetings. Specifically, we asked for suggested revisions to content and recommendations on reliable methods to deliver facecards to patients. Teaching physicians felt strongly that each team member should be listed and shown on 1 card, which would fit easily into a lab‐coat pocket. We similarly engaged the NMH Patient and Family Advisory Council to seek recommended revisions to content and delivery of the facecards. The Council consists of 18 patient and caregiver members who meet regularly to provide input on hospital programs and proposals. Council members felt strongly that physicians should deliver the cards themselves during their initial introduction, rather than having patients receive cards by other means (eg, as part of unit orientation materials delivered by nonphysician staff members). We incorporated feedback from these stakeholder groups into a final version of the physician facecard and method for delivery (Figure 1).

We implemented the use of facecards from May to June 2012. Physicians on intervention units were informed of the study via email, and one of the co‐investigators (T.C.) distributed a supply of facecards to these physicians at the start of each rotation. This distribution was performed in person, and physicians were instructed to provide a facecard to each new patient during their first encounter. We also placed facecards in easily visible cardholders at the nurses' station on intervention units. Reminder emails were sent once each week to reinforce physician delivery of facecards.
Data Collection and Measures
Each weekday during the study period, we randomly selected patients for structured interviews in the afternoon of their second or third hospital day. We did not conduct interviews on the first day of physicians' rotations and excluded patients whose preferred language was not English and those disoreinted to person, place, or time.
Patients were asked to name the physician(s) primarily responsible for their hospital care and to state the role of each physician they identified. We documented receipt of facecards if one was viewed during the interview and by asking patients if they had received one. We also documented whether 1 correct physician names were written on the whiteboard in the patients' rooms. We used questions from the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey to assess satisfaction with physician communication and overall hospital care. HCAHPS is a validated patient‐satisfaction survey developed by the Agency for Healthcare Research and Quality (AHRQ) to assess hospitalized patients' experiences with care. Physician‐communication questions used ordinal response options of never, sometimes, usually, and always. Overall hospital rating was assessed using a 010 scale with 0=worst hospital possible and 10=best hospital possible. Trust with physicians was assessed using the Wake Forest University Trust Scale.[22] Prior research using this instrument has shown an association between trust and self‐management behaviors.[23] This 10‐item scale uses a 5‐point Likert scale and generates scores ranging from 10 to 50. Agreement with physicians was assessed using 3 questions used in a prior study by Staiger and colleagues showing an association between levels of agreement and health outcomes among outpatients treated for back pain.[17] Specifically, we asked patients to rate their agreement with hospital physicians' (1) explanation for the cause of primary symptoms, (2) plan for diagnostic tests, and (3) suggested plan for treatment using a 5‐point Likert scale. The agreement scale generated scores ranging from 3 to 15.
Approval for the study was obtained from the institutional review board of Northwestern University.
Statistical Analysis
Patient demographic data were obtained from the electronic health record and complemented data from interviews. We used [2] and t tests to compare patient characteristics. We used [2] tests to compare the percentage of patients able to correctly identify 1 of their physicians and 1 of their physicians' roles. We used [2] tests to compare the percentage of patients giving top‐box ratings to all 3 physician‐communicationsatisfaction questions (ie, always) and giving an overall hospital rating of 9 or 10. We used top‐box comparisons, rather than comparison of mean or median scores, because patient‐satisfaction data are typically highly skewed toward favorable responses. This approach is consistent with prior HCAHPS research.[24, 25] We used Mann‐Whitney U tests to compare ratings of trust and agreement. Because delivery of facecards was imperfect, we performed analyses both by intention to treat (ie, intervention vs control units) and based on treatment received (ie, received a facecard vs did not receive a facecard). All analyses were conducted using Stata version 11.2 (StataCorp, College Station, TX).
RESULTS
Study Subjects and Facecard Receipt
Overall, 217 patients were approached for interview. Thirty‐six were excluded because of disorientation, 12 were excluded because their preferred language was not English, and 31 declined to participate in the study. Patient characteristics for the 138 study patients are shown in Table 1. There were no significant differences in patient age, sex, or race. There was no significant difference in the percentage of patients with 1 correct physicians listed on the whiteboard in the room. Delivery of facecards was incomplete, with only 68% of intervention‐unit patients confirmed as having received them. A higher percentage of patients on the hospitalist intervention unit received facecards (23 of 30; 76.7%) than on the teaching intervention unit (22 of 36; 61.1%), but the difference was not statistically significant (P=0.18). There were no significant differences in age, sex, or race between patients who received a facecard compared with those who did not.
| Characteristic | Control Group, N=72 | Intervention Group, N=66 | P Value |
|---|---|---|---|
| |||
| Mean age, years (SD) | 56.8 (18.0) | 55.2 (18.2) | 0.62 |
| Women, n (%) | 35 (48.6) | 28 (42.4) | 0.47 |
| Nonwhite race, n (%) | 35 (50.7) | 36 (57.1) | 0.46 |
| Teaching unit, n (%) | 34 (47.2) | 36 (54.6) | 0.39 |
| Correct physician name on whiteboard, n (%)a | 46 (76.7) | 37 (72.6) | 0.62 |
| Received a facecard, n (%) | 1 (1) | 45 (68.2) | <0.01 |
Patients' Knowledge of Physicians
As shown in Table 2, more patients in the intervention group were able to correctly identify 1 of their treating physicians compared with the control group, but the result was not statistically significant (69.7% vs 58.3%; P=0.17). A significantly larger percentage of patients in the intervention group were able to identify the role of their hospital physicians (51.5% vs 16.7%; P<0.01). When comparing those that received a facecard and those that did not, patients who were given a facecard were more likely to correctly identify their hospital physician (89.1% vs 51.1%; P<0.01). Similarly, patients who had received a facecard were more likely to correctly identify the role of their hospital physician than patients who had not received a facecard (67.4% vs 16.3%; P<0.01).
| Impact | Control Group, N=72, n (%) | Intervention Group, N=66, n (%) | P Value |
|---|---|---|---|
| Patient correctly named 1 hospital physician | 42 (58.3) | 46 (69.7) | 0.17 |
| Patient correctly named role of hospital physician | 12 (16.7) | 34 (51.5) | <0.01 |
| Did Not Receive Facecard, N=92 | Received Facecard, N=46 | P Value | |
| Patient correctly named 1 hospital physician | 47 (51.1) | 41 (89.1) | <0.01 |
| Patient correctly named role of hospital physician | 15 (16.3) | 31 (67.4) | <0.01 |
Levels of Satisfaction, Trust, and Agreement
Overall, patients had high levels of satisfaction, trust, and agreement with hospital physicians. The overall satisfaction with physician communication was 75.6% (mean of top‐box scores across all 3 items), and 81 of 138 (58.7%) patients gave top‐box ratings to all 3 physician‐communicationsatisfaction items. Ninety‐seven of 137 (70.8%) patients rated overall hospital care as 9 or 10. The mean trust score for all patients was 40.77.8 and the median was 41.5 (interquartile range, 3747). The mean agreement score for all patients was 12.42.4 and the median was 12 (interquartile range, 1115). As shown in Table 3, satisfaction, trust, and agreement were similar for patients in the intervention group compared with the control group. Patients who received a facecard rated satisfaction, trust, and agreement slightly higher compared with those who had not received a facecard, but the results were not statistically significant.
| Ratings | Control Group, N=72 | Intervention Group, N=66 | P Value |
|---|---|---|---|
| |||
| Satisfaction with physicians, n (%)a | 39 (54.2) | 42 (63.6) | 0.26 |
| Overall hospital satisfaction, n (%)b | 51 (70.8) | 46 (70.8) | 0.99 |
| Median trust (IQR)c | 42 (3747) | 41 (3746) | 0.81 |
| Median agreement (IQR)c | 12 (1115) | 12 (1215) | 0.72 |
| Did Not Receive Facecard, N=92 | Received Facecard, N=46 | P Value | |
| Satisfaction with physicians, n (%)a | 51 (55.4) | 30 (65.2) | 0.27 |
| Overall hospital satisfaction, n (%)b | 64 (69.6) | 33 (73.3) | 0.65 |
| Median trust (IQR)c | 41 (3547) | 42 (3847) | 0.32 |
| Median agreement (IQR)c | 12 (1114.5) | 12.5 (1215) | 0.37 |
DISCUSSION
We found that receipt of physician facecards significantly improved patients' knowledge of the names and roles of hospital physicians but had little to no impact on satisfaction, trust, or agreement with physicians. Our finding of improved knowledge of the names and roles of physician providers is consistent with prior studies using similar interventions.[7, 8, 9] Facecards may have prompted more effective introductions on the part of physicians and may have served as memory aids for patients to better retain information about their newly introduced hospital physicians.
Patient receipt of the facecard on intervention units was incomplete in our study. Despite engagement of physicians in designing cards that could easily fit into lab coats and a robust strategy to inform and motivate physician delivery of facecards, only 68% of intended patients received them. Although not explicitly reported, prior studies appear to have similarly struggled to deliver interventions consistently. Arora and colleagues reported that facecards were visible in only 59% of patients' rooms among those able to correctly identify 1 of their physicians.[8] A post hoc survey of physicians involved in our study revealed the biggest impediment to delivering facecards was simply forgetting to do so (data not shown). Technologic innovations may help by automating the identification of providers. For example, the University of Pittsburgh Medical Center has piloted smart rooms that use sensor technology to announce the name and role of providers as they enter patients' rooms.[26]
We hypothesized that facecards might improve other important aspects of the patient‐physicians relationship. Although levels of patient satisfaction were slightly higher in patients who had received facecards, the results were not statistically significant. Levels of trust and agreement were minimally higher in patients who received facecards, and the results were not statistically significant. Notably, baseline levels of trust and agreement were higher than we had expected. In fact, levels of trust were nearly identical to those seen in a prior study of outpatients who had been with the same physician for a median of 4 years.[22] Patients in our study may have had high levels of trust in the hospital and transferred this trust to their assigned physicians as representatives of the organization. The high level of agreement may relate to patients' tendency to prefer a more passive role as they encounter serious illness.[27, 28] Paradoxically, these findings may impede optimal patient care. The high levels of trust and agreement in the current study suggest that patients may not question their physicians to clarify plans and the rationale behind them. Prior research has shown that deficits in patients' comprehension of the care plan are often not apparent to patients or their physicians.[4, 29, 30]
Our study has several limitations. First, we assessed an intervention involving 4 units in a single hospital. Generalizability may be limited, as physician‐staffing models, hospitals, and the patients they serve vary. Second, as previously mentioned, patients in the intervention group did not receive physician facecards as consistently as intended. We conducted analyses based on treatment received in an effort to evaluate the impact of facecards if optimally delivered. Third, questions assessing satisfaction, trust, and agreement did not specifically ask patients to reflect on care provided by the primary physician team. It is possible that interactions with other physicians (ie, consultants) may have influenced these results. Fourth, we were underpowered to detect statistically significant improvements in satisfaction, trust, or agreement resulting from our intervention. Assuming the intervention might truly improve satisfaction with physicians from 54.2% to 63.6%, we would have needed 900 patients (ie, 450 each for the intervention and control groups) to have 80% power to detect a statistically significant difference. However, our results show that patients have high levels of trust and agreement with hospital physicians despite the relative lack of familiarity. Therefore, any existing deficits in hospitalized patients' comprehension of the care plan do not appear to be exacerbated by a lack of trust and/or agreement with treating physicians.
CONCLUSION
In summary, we found that physician facecards significantly improved patients' knowledge of the names and roles of hospital physicians but had little to no impact on satisfaction, trust, or agreement with physicians. Baseline levels of satisfaction, trust, and agreement were high, suggesting lack of familiarity with hospital physicians does not impede these important aspects of the patient‐physician relationship. Larger studies are needed to definitively assess the impact of facecards on satisfaction, trust, and agreement with physicians.
Acknowledgments
The authors express their gratitude to members of the NMH Patient and Family Advisory Council for providing input on the design of the physician facecard.
Disclosures: This study was supported by a grant from the Globe Foundation. The authors report no conflicts of interest.
The patient‐physician relationship is fundamental to safe and effective care. Hospital settings present unique challenges to this partnership, including the lack of a prior relationship for hospital‐based physicians, rapid pace of clinical care, and dynamic nature of inpatient medical teams. Prior studies document that a majority of hospitalized patients are unable to correctly identify their physicians or nurses, and patients in teaching hospitals have difficulty understanding their physicians' level of training.[1, 2, 3, 4] Acknowledging these deficits, professional societies and the Accreditation Council for Graduate Medical Education (ACMGE) have issued policies stating that patients and caregivers need to know who is responsible at every point during patient care.[5, 6] These policies do not, however, make recommendations on methods to achieve better understanding.
Simple interventions improve patients' ability to correctly identify the names and roles of their hospital physicians. Maniaci and colleagues found that patients were better able to identify attending physicians when their names were written on the dry‐erase board in the room.[7] Arora and colleagues asked hospital physicians to give facecards, which included their picture and a description of their role, to patients.[8] Patients were more likely to correctly identify 1 physicians, but, surprisingly, less likely to understand physicians' roles. In a similar study, Francis and colleagues placed photographs with names of the attending and resident physicians on the wall in patient rooms.[9] Patients who had photographs of their physicians on the wall were more likely to correctly identify physicians on their team compared with patients who had no photographs. Additionally, patients who were able to identify more physicians rated satisfaction with physicians higher in 2 of 6 survey questions used. However, the study was limited by the use of a nonvalidated instrument to assess patient satisfaction and the use of an intermediate outcome (ie, ability to identify physicians) as the independent variable rather than the intervention itself (ie, physician photographs).
Beyond satisfaction, lack of familiarity may negatively impact patients' trust and agreement with hospital physicians. Trust and agreement are important predictors of adherence to recommended treatment in outpatient settings[10, 11, 12, 13, 14, 15, 16, 17, 18] but have not been adequately evaluated in hospital settings. Therefore, we sought to pilot the use of physician facecards and assess their potential impact on patients' knowledge of physicians' names and roles as well as patient satisfaction, trust, and agreement with physicians.
METHODS
Setting and Study Design
We performed a cluster randomized controlled trial at Northwestern Memorial Hospital (NMH), an 897‐bed tertiary‐care teaching hospital in Chicago, Illinois. One of 2 similar hospitalist service units and 1 of 2 similar teaching‐service units were randomly selected to implement the use of physician facecards. General medical patients were admitted to the study units by NMH bed‐assignment personnel subject to unit bed availability. No other criteria (eg, diagnosis, severity of illness, or source of patient admission) were used in patient assignment. Each unit consisted of 30 beds, with the exception of 1 hospitalist unit, which had 23. As a result of a prior intervention, physicians were localized to care for patients on specific units.[19] Hospitalist units were each staffed by hospitalists who worked in 7‐day rotations without the assistance of residents or midlevel providers. Teaching units were staffed by physician teams consisting of 1 attending, 1 senior resident, 1 intern, and 1 or 2 third‐year medical students. No fourth‐year students (ie, acting interns) rotated on these services during the study period. Housestaff worked in 4‐week rotations, and attending physicians on the teaching service worked in 2‐week rotations.
Patient rooms included a whiteboard facing the patient with a template prompting insertion of physician name(s). Nurses had the primary responsibility for completing information on the whiteboards.
Physician Facecard
We created draft physician facecards featuring pictures of physicians and descriptions of their roles. We used Lexile analysis, a widely used measure of reading difficulty, to improve readability in an iterative fashion.[20, 21] We then sought feedback at hospitalist and resident meetings. Specifically, we asked for suggested revisions to content and recommendations on reliable methods to deliver facecards to patients. Teaching physicians felt strongly that each team member should be listed and shown on 1 card, which would fit easily into a lab‐coat pocket. We similarly engaged the NMH Patient and Family Advisory Council to seek recommended revisions to content and delivery of the facecards. The Council consists of 18 patient and caregiver members who meet regularly to provide input on hospital programs and proposals. Council members felt strongly that physicians should deliver the cards themselves during their initial introduction, rather than having patients receive cards by other means (eg, as part of unit orientation materials delivered by nonphysician staff members). We incorporated feedback from these stakeholder groups into a final version of the physician facecard and method for delivery (Figure 1).

We implemented the use of facecards from May to June 2012. Physicians on intervention units were informed of the study via email, and one of the co‐investigators (T.C.) distributed a supply of facecards to these physicians at the start of each rotation. This distribution was performed in person, and physicians were instructed to provide a facecard to each new patient during their first encounter. We also placed facecards in easily visible cardholders at the nurses' station on intervention units. Reminder emails were sent once each week to reinforce physician delivery of facecards.
Data Collection and Measures
Each weekday during the study period, we randomly selected patients for structured interviews in the afternoon of their second or third hospital day. We did not conduct interviews on the first day of physicians' rotations and excluded patients whose preferred language was not English and those disoreinted to person, place, or time.
Patients were asked to name the physician(s) primarily responsible for their hospital care and to state the role of each physician they identified. We documented receipt of facecards if one was viewed during the interview and by asking patients if they had received one. We also documented whether 1 correct physician names were written on the whiteboard in the patients' rooms. We used questions from the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey to assess satisfaction with physician communication and overall hospital care. HCAHPS is a validated patient‐satisfaction survey developed by the Agency for Healthcare Research and Quality (AHRQ) to assess hospitalized patients' experiences with care. Physician‐communication questions used ordinal response options of never, sometimes, usually, and always. Overall hospital rating was assessed using a 010 scale with 0=worst hospital possible and 10=best hospital possible. Trust with physicians was assessed using the Wake Forest University Trust Scale.[22] Prior research using this instrument has shown an association between trust and self‐management behaviors.[23] This 10‐item scale uses a 5‐point Likert scale and generates scores ranging from 10 to 50. Agreement with physicians was assessed using 3 questions used in a prior study by Staiger and colleagues showing an association between levels of agreement and health outcomes among outpatients treated for back pain.[17] Specifically, we asked patients to rate their agreement with hospital physicians' (1) explanation for the cause of primary symptoms, (2) plan for diagnostic tests, and (3) suggested plan for treatment using a 5‐point Likert scale. The agreement scale generated scores ranging from 3 to 15.
Approval for the study was obtained from the institutional review board of Northwestern University.
Statistical Analysis
Patient demographic data were obtained from the electronic health record and complemented data from interviews. We used [2] and t tests to compare patient characteristics. We used [2] tests to compare the percentage of patients able to correctly identify 1 of their physicians and 1 of their physicians' roles. We used [2] tests to compare the percentage of patients giving top‐box ratings to all 3 physician‐communicationsatisfaction questions (ie, always) and giving an overall hospital rating of 9 or 10. We used top‐box comparisons, rather than comparison of mean or median scores, because patient‐satisfaction data are typically highly skewed toward favorable responses. This approach is consistent with prior HCAHPS research.[24, 25] We used Mann‐Whitney U tests to compare ratings of trust and agreement. Because delivery of facecards was imperfect, we performed analyses both by intention to treat (ie, intervention vs control units) and based on treatment received (ie, received a facecard vs did not receive a facecard). All analyses were conducted using Stata version 11.2 (StataCorp, College Station, TX).
RESULTS
Study Subjects and Facecard Receipt
Overall, 217 patients were approached for interview. Thirty‐six were excluded because of disorientation, 12 were excluded because their preferred language was not English, and 31 declined to participate in the study. Patient characteristics for the 138 study patients are shown in Table 1. There were no significant differences in patient age, sex, or race. There was no significant difference in the percentage of patients with 1 correct physicians listed on the whiteboard in the room. Delivery of facecards was incomplete, with only 68% of intervention‐unit patients confirmed as having received them. A higher percentage of patients on the hospitalist intervention unit received facecards (23 of 30; 76.7%) than on the teaching intervention unit (22 of 36; 61.1%), but the difference was not statistically significant (P=0.18). There were no significant differences in age, sex, or race between patients who received a facecard compared with those who did not.
| Characteristic | Control Group, N=72 | Intervention Group, N=66 | P Value |
|---|---|---|---|
| |||
| Mean age, years (SD) | 56.8 (18.0) | 55.2 (18.2) | 0.62 |
| Women, n (%) | 35 (48.6) | 28 (42.4) | 0.47 |
| Nonwhite race, n (%) | 35 (50.7) | 36 (57.1) | 0.46 |
| Teaching unit, n (%) | 34 (47.2) | 36 (54.6) | 0.39 |
| Correct physician name on whiteboard, n (%)a | 46 (76.7) | 37 (72.6) | 0.62 |
| Received a facecard, n (%) | 1 (1) | 45 (68.2) | <0.01 |
Patients' Knowledge of Physicians
As shown in Table 2, more patients in the intervention group were able to correctly identify 1 of their treating physicians compared with the control group, but the result was not statistically significant (69.7% vs 58.3%; P=0.17). A significantly larger percentage of patients in the intervention group were able to identify the role of their hospital physicians (51.5% vs 16.7%; P<0.01). When comparing those that received a facecard and those that did not, patients who were given a facecard were more likely to correctly identify their hospital physician (89.1% vs 51.1%; P<0.01). Similarly, patients who had received a facecard were more likely to correctly identify the role of their hospital physician than patients who had not received a facecard (67.4% vs 16.3%; P<0.01).
| Impact | Control Group, N=72, n (%) | Intervention Group, N=66, n (%) | P Value |
|---|---|---|---|
| Patient correctly named 1 hospital physician | 42 (58.3) | 46 (69.7) | 0.17 |
| Patient correctly named role of hospital physician | 12 (16.7) | 34 (51.5) | <0.01 |
| Did Not Receive Facecard, N=92 | Received Facecard, N=46 | P Value | |
| Patient correctly named 1 hospital physician | 47 (51.1) | 41 (89.1) | <0.01 |
| Patient correctly named role of hospital physician | 15 (16.3) | 31 (67.4) | <0.01 |
Levels of Satisfaction, Trust, and Agreement
Overall, patients had high levels of satisfaction, trust, and agreement with hospital physicians. The overall satisfaction with physician communication was 75.6% (mean of top‐box scores across all 3 items), and 81 of 138 (58.7%) patients gave top‐box ratings to all 3 physician‐communicationsatisfaction items. Ninety‐seven of 137 (70.8%) patients rated overall hospital care as 9 or 10. The mean trust score for all patients was 40.77.8 and the median was 41.5 (interquartile range, 3747). The mean agreement score for all patients was 12.42.4 and the median was 12 (interquartile range, 1115). As shown in Table 3, satisfaction, trust, and agreement were similar for patients in the intervention group compared with the control group. Patients who received a facecard rated satisfaction, trust, and agreement slightly higher compared with those who had not received a facecard, but the results were not statistically significant.
| Ratings | Control Group, N=72 | Intervention Group, N=66 | P Value |
|---|---|---|---|
| |||
| Satisfaction with physicians, n (%)a | 39 (54.2) | 42 (63.6) | 0.26 |
| Overall hospital satisfaction, n (%)b | 51 (70.8) | 46 (70.8) | 0.99 |
| Median trust (IQR)c | 42 (3747) | 41 (3746) | 0.81 |
| Median agreement (IQR)c | 12 (1115) | 12 (1215) | 0.72 |
| Did Not Receive Facecard, N=92 | Received Facecard, N=46 | P Value | |
| Satisfaction with physicians, n (%)a | 51 (55.4) | 30 (65.2) | 0.27 |
| Overall hospital satisfaction, n (%)b | 64 (69.6) | 33 (73.3) | 0.65 |
| Median trust (IQR)c | 41 (3547) | 42 (3847) | 0.32 |
| Median agreement (IQR)c | 12 (1114.5) | 12.5 (1215) | 0.37 |
DISCUSSION
We found that receipt of physician facecards significantly improved patients' knowledge of the names and roles of hospital physicians but had little to no impact on satisfaction, trust, or agreement with physicians. Our finding of improved knowledge of the names and roles of physician providers is consistent with prior studies using similar interventions.[7, 8, 9] Facecards may have prompted more effective introductions on the part of physicians and may have served as memory aids for patients to better retain information about their newly introduced hospital physicians.
Patient receipt of the facecard on intervention units was incomplete in our study. Despite engagement of physicians in designing cards that could easily fit into lab coats and a robust strategy to inform and motivate physician delivery of facecards, only 68% of intended patients received them. Although not explicitly reported, prior studies appear to have similarly struggled to deliver interventions consistently. Arora and colleagues reported that facecards were visible in only 59% of patients' rooms among those able to correctly identify 1 of their physicians.[8] A post hoc survey of physicians involved in our study revealed the biggest impediment to delivering facecards was simply forgetting to do so (data not shown). Technologic innovations may help by automating the identification of providers. For example, the University of Pittsburgh Medical Center has piloted smart rooms that use sensor technology to announce the name and role of providers as they enter patients' rooms.[26]
We hypothesized that facecards might improve other important aspects of the patient‐physicians relationship. Although levels of patient satisfaction were slightly higher in patients who had received facecards, the results were not statistically significant. Levels of trust and agreement were minimally higher in patients who received facecards, and the results were not statistically significant. Notably, baseline levels of trust and agreement were higher than we had expected. In fact, levels of trust were nearly identical to those seen in a prior study of outpatients who had been with the same physician for a median of 4 years.[22] Patients in our study may have had high levels of trust in the hospital and transferred this trust to their assigned physicians as representatives of the organization. The high level of agreement may relate to patients' tendency to prefer a more passive role as they encounter serious illness.[27, 28] Paradoxically, these findings may impede optimal patient care. The high levels of trust and agreement in the current study suggest that patients may not question their physicians to clarify plans and the rationale behind them. Prior research has shown that deficits in patients' comprehension of the care plan are often not apparent to patients or their physicians.[4, 29, 30]
Our study has several limitations. First, we assessed an intervention involving 4 units in a single hospital. Generalizability may be limited, as physician‐staffing models, hospitals, and the patients they serve vary. Second, as previously mentioned, patients in the intervention group did not receive physician facecards as consistently as intended. We conducted analyses based on treatment received in an effort to evaluate the impact of facecards if optimally delivered. Third, questions assessing satisfaction, trust, and agreement did not specifically ask patients to reflect on care provided by the primary physician team. It is possible that interactions with other physicians (ie, consultants) may have influenced these results. Fourth, we were underpowered to detect statistically significant improvements in satisfaction, trust, or agreement resulting from our intervention. Assuming the intervention might truly improve satisfaction with physicians from 54.2% to 63.6%, we would have needed 900 patients (ie, 450 each for the intervention and control groups) to have 80% power to detect a statistically significant difference. However, our results show that patients have high levels of trust and agreement with hospital physicians despite the relative lack of familiarity. Therefore, any existing deficits in hospitalized patients' comprehension of the care plan do not appear to be exacerbated by a lack of trust and/or agreement with treating physicians.
CONCLUSION
In summary, we found that physician facecards significantly improved patients' knowledge of the names and roles of hospital physicians but had little to no impact on satisfaction, trust, or agreement with physicians. Baseline levels of satisfaction, trust, and agreement were high, suggesting lack of familiarity with hospital physicians does not impede these important aspects of the patient‐physician relationship. Larger studies are needed to definitively assess the impact of facecards on satisfaction, trust, and agreement with physicians.
Acknowledgments
The authors express their gratitude to members of the NMH Patient and Family Advisory Council for providing input on the design of the physician facecard.
Disclosures: This study was supported by a grant from the Globe Foundation. The authors report no conflicts of interest.
- , , , , , . Ability of hospitalized patients to identify their in‐hospital physicians. Arch Intern Med. 2009;169(2):199–201.
- , . Does your patient know your name? An approach to enhancing patients' awareness of their caretaker's name. J Healthc Qual. 2005;27(4):53–56.
- , , Hospitalized patients' understanding of their plan of care. Mayo Clin Proc. 2010;85(1):47–52.
- , . Communication discrepancies between physicians and hospitalized patients. Arch Intern Med. 2010;170(15):1302–1307.
- Accreditation Council for Graduate Medical Education. Common program requirements. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/CPRs2013.pdf. Revised July 1, 2013.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364–370.
- , , . Increasing a patient's ability to identify his or her attending physician using a patient room display. Arch Intern Med. 2010;170(12):1084–1085.
- , , , et al. Improving inpatients' identification of their doctors: use of FACE cards. Jt Comm J Qual Patient Saf. 2009;35(12):613–619.
- , , . Patient satisfaction associated with correct identification of physician's photographs. Mayo Clin Proc. 2001;76(6):604–608.
- , , , , , . Physician‐patient relationship and medication compliance: a primary care investigation. Ann Fam Med. 2004;2(5):455–461.
- , , , , . Trust in the health care system and the use of preventive health services by older black and white adults. Am J Public Health. 2009;99(7):1293–1299.
- , , , . The role of patient‐physician trust in moderating medication nonadherence due to cost pressures. Arch Intern Med. 2005;165(15):1749–1755.
- , , . Trust and the acceptance of and adherence to antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;28(1):47–58.
- , , , , , . Linking primary care performance to outcomes of care. J Fam Pract. 1998;47(3):213–220.
- , , , ; The Stanford Trust Study Physicians. Further validation and reliability testing of the Trust in Physician Scale. Med Care. 1999;37(5):510–517.
- , , , , , . The physician's actions and the outcome of illness in family practice. J Fam Pract. 1986;23(1):43–47.
- , , , , . Brief Report: Patient‐physician agreement as a predictor of outcomes in patients with back pain. J Gen Intern Med. 2005;20(10):935–937.
- , , , , , . The influence of patient‐practitioner agreement on outcome of care. Am J Public Health. 1981;71(2):127–131.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , . The Lexile Framework. Durham, NC: Metametrics, Inc.; 1998.
- National Center for Education Statistics; , . Assessing the Lexile Framework: results of a panel meeting. NCES Working Paper Series, No. 2001‐08. Washington, DC: US Department of Education, Office of Educational Research and Improvement; 2001.
- , , , et al. Measuring patients' trust in their primary care providers. Med Care Res Rev. 2002;59(3):293–318.
- , , , , , . The association of patient trust and self‐care among patients with diabetes mellitus. BMC Fam Pract. 2004;5:26.
- , , , , . Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27–37.
- , , , , . Measuring hospital care from the patients' perspective: an overview of the CAHPS Hospital Survey development process. Health Serv Res. 2005;40(6 pt 2):1977–1995.
- . Smart rooms, smart care delivery: UPMC clinician leaders leverage technology for greater effectiveness in patient care. Healthc Inform. 2011;28(9):36, 38–39, 42.
- , . Decision making during serious illness: what role do patients really want to play? J Clin Epidemiol. 1992;45(9):941–950.
- , , , , . The dynamics of change: cancer patients' preferences for information, involvement and support. Ann Oncol. 1997;8(9):857–863.
- , , , et al. Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan. Arch Intern Med. 1997;157(9):1026–1030.
- , , , , , . Patient comprehension of emergency department care and instructions: are patients aware of when they do not understand? Ann Emerg Med. 2009;53(4):454.e15–461.e15.
- , , , , , . Ability of hospitalized patients to identify their in‐hospital physicians. Arch Intern Med. 2009;169(2):199–201.
- , . Does your patient know your name? An approach to enhancing patients' awareness of their caretaker's name. J Healthc Qual. 2005;27(4):53–56.
- , , Hospitalized patients' understanding of their plan of care. Mayo Clin Proc. 2010;85(1):47–52.
- , . Communication discrepancies between physicians and hospitalized patients. Arch Intern Med. 2010;170(15):1302–1307.
- Accreditation Council for Graduate Medical Education. Common program requirements. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/CPRs2013.pdf. Revised July 1, 2013.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364–370.
- , , . Increasing a patient's ability to identify his or her attending physician using a patient room display. Arch Intern Med. 2010;170(12):1084–1085.
- , , , et al. Improving inpatients' identification of their doctors: use of FACE cards. Jt Comm J Qual Patient Saf. 2009;35(12):613–619.
- , , . Patient satisfaction associated with correct identification of physician's photographs. Mayo Clin Proc. 2001;76(6):604–608.
- , , , , , . Physician‐patient relationship and medication compliance: a primary care investigation. Ann Fam Med. 2004;2(5):455–461.
- , , , , . Trust in the health care system and the use of preventive health services by older black and white adults. Am J Public Health. 2009;99(7):1293–1299.
- , , , . The role of patient‐physician trust in moderating medication nonadherence due to cost pressures. Arch Intern Med. 2005;165(15):1749–1755.
- , , . Trust and the acceptance of and adherence to antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;28(1):47–58.
- , , , , , . Linking primary care performance to outcomes of care. J Fam Pract. 1998;47(3):213–220.
- , , , ; The Stanford Trust Study Physicians. Further validation and reliability testing of the Trust in Physician Scale. Med Care. 1999;37(5):510–517.
- , , , , , . The physician's actions and the outcome of illness in family practice. J Fam Pract. 1986;23(1):43–47.
- , , , , . Brief Report: Patient‐physician agreement as a predictor of outcomes in patients with back pain. J Gen Intern Med. 2005;20(10):935–937.
- , , , , , . The influence of patient‐practitioner agreement on outcome of care. Am J Public Health. 1981;71(2):127–131.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , . The Lexile Framework. Durham, NC: Metametrics, Inc.; 1998.
- National Center for Education Statistics; , . Assessing the Lexile Framework: results of a panel meeting. NCES Working Paper Series, No. 2001‐08. Washington, DC: US Department of Education, Office of Educational Research and Improvement; 2001.
- , , , et al. Measuring patients' trust in their primary care providers. Med Care Res Rev. 2002;59(3):293–318.
- , , , , , . The association of patient trust and self‐care among patients with diabetes mellitus. BMC Fam Pract. 2004;5:26.
- , , , , . Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27–37.
- , , , , . Measuring hospital care from the patients' perspective: an overview of the CAHPS Hospital Survey development process. Health Serv Res. 2005;40(6 pt 2):1977–1995.
- . Smart rooms, smart care delivery: UPMC clinician leaders leverage technology for greater effectiveness in patient care. Healthc Inform. 2011;28(9):36, 38–39, 42.
- , . Decision making during serious illness: what role do patients really want to play? J Clin Epidemiol. 1992;45(9):941–950.
- , , , , . The dynamics of change: cancer patients' preferences for information, involvement and support. Ann Oncol. 1997;8(9):857–863.
- , , , et al. Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan. Arch Intern Med. 1997;157(9):1026–1030.
- , , , , , . Patient comprehension of emergency department care and instructions: are patients aware of when they do not understand? Ann Emerg Med. 2009;53(4):454.e15–461.e15.
© 2013 Society of Hospital Medicine
Project BOOST
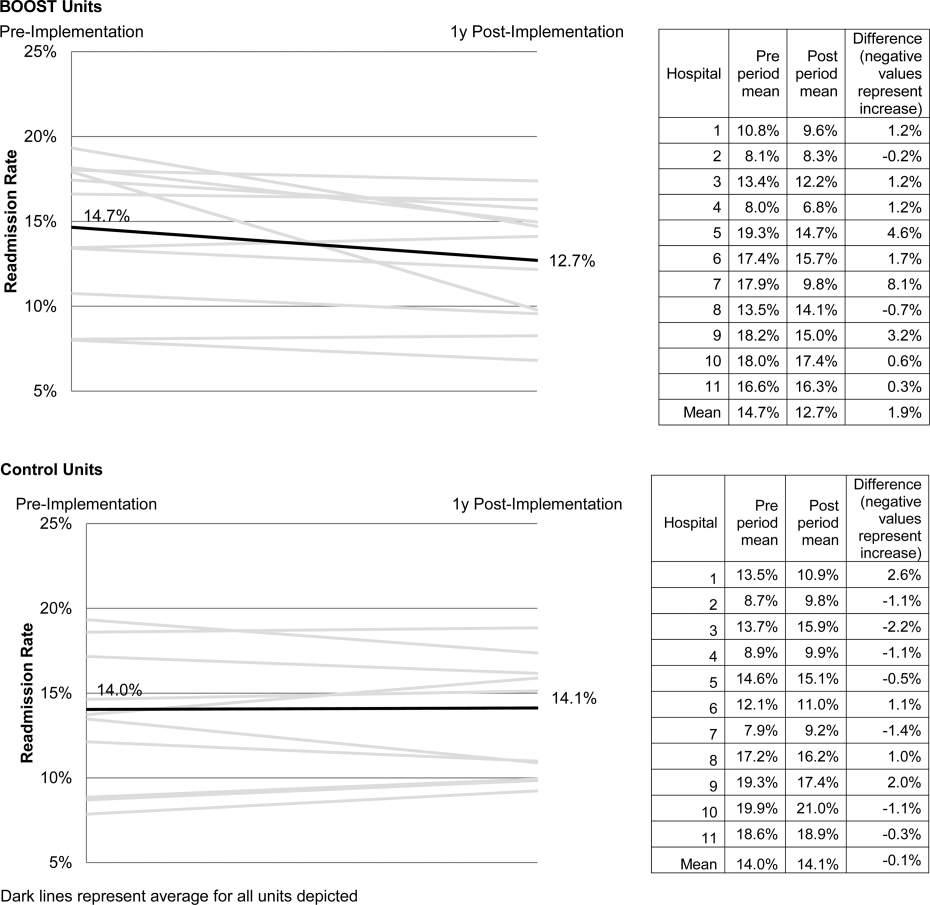
Enactment of federal legislation imposing hospital reimbursement penalties for excess rates of rehospitalizations among Medicare fee for service beneficiaries markedly increased interest in hospital quality improvement (QI) efforts to reduce the observed 30‐day rehospitalization of 19.6% in this elderly population.[1, 2] The Congressional Budget Office estimated that reimbursement penalties to hospitals for high readmission rates are expected to save the Medicare program approximately $7 billion between 2010 and 2019.[3] These penalties are complemented by resources from the Center for Medicare and Medicaid Innovation aiming to reduce hospital readmissions by 20% by the end of 2013 through the Partnership for Patients campaign.[4] Although potential financial penalties and provision of resources for QI intensified efforts to enhance the quality of the hospital discharge transition, patient safety risks associated with hospital discharge are well documented.[5, 6] Approximately 20% of patients discharged from the hospital may suffer adverse events,[7, 8] of which up to three‐quarters (72%) are medication related,[9] and over one‐third of required follow‐up testing after discharge is not completed.[10] Such findings indicate opportunities for improvement in the discharge process.[11]
Numerous publications describe studies aiming to improve the hospital discharge process and mitigate these hazards, though a systematic review of interventions to reduce 30‐day rehospitalization indicated that the existing evidence base for the effectiveness of transition interventions demonstrates irregular effectiveness and limitations to generalizability.[12] Most studies showing effectiveness are confined to single academic medical centers. Existing evidence supports multifaceted interventions implemented in both the pre‐ and postdischarge periods and focused on risk assessment and tailored, patient‐centered application of interventions to mitigate risk. For example Project RED (Re‐Engineered Discharge) applied a bundled intervention consisting of intensified patient education and discharge planning, improved medication reconciliation and discharge instructions, and longitudinal patient contact with follow‐up phone calls and a dedicated discharge advocate.[13] However, the mean age of patients participating in the study was 50 years, and it excluded patients admitted from or discharged to skilled nursing facilities, making generalizability to the geriatric population uncertain.
An integral aspect of QI projects is the contribution of local context to translation of best practices to disparate settings.[14, 15, 16] Most available reports of successful interventions to reduce rehospitalization have not fully described the specifics of either the intervention context or design. Moreover, the available evidence base for common interventions to reduce rehospitalization was developed in the academic setting. Validation of single academic center studies in a broader healthcare context is necessary.
Project BOOST (Better Outcomes for Older adults through Safe Transitions) recruited a diverse national cohort of both academic and nonacademic hospitals to participate in a QI effort to implement best practices for hospital discharge care transitions using a national collaborative approach facilitated by external expert mentorship. This study aimed to determine the effectiveness of BOOST in lowering hospital readmission rates and impact on length of stay.
METHODS
The study of Project BOOST was undertaken in accordance with the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines.[17]
Participants
The unit of observation for the prospective cohort study was the clinical acute‐care unit within hospitals. Sites were instructed to designate a pilot unit for the intervention that cared for medical or mixed medicalsurgical patient populations. Sites were also asked to provide outcome data for a clinically and organizationally similar non‐BOOST unit to provide a site‐matched control. Control units were matched by local site leadership based on comparable patient demographics, clinical mix, and extent of housestaff presence. An initial cohort of 6 hospitals in 2008 was followed by a second cohort of 24 hospitals initiated in 2009. All hospitals were invited to participate in the national effectiveness analysis, which required submission of readmission and length of stay data for both a BOOST intervention unit and a clinically matched control unit.
Description of the Intervention
The BOOST intervention consisted of 2 major sequential processes, planning and implementation, both facilitated by external site mentorsphysicians expert in QI and care transitionsfor a period of 12 months. Extensive background on the planning and implementation components is available at
| Enrollment Sites, n=30 | Sites Reporting Outcome Data, n=11 | Sites Not Reporting Outcome Data, n=19 | P Value for Comparison of Outcome Data Sites Compared to Othersa | |
|---|---|---|---|---|
| ||||
| Region, n (%) | 0.194 | |||
| Northeast | 8 (26.7) | 2 (18.2) | 6 (31.6) | |
| West | 7 (23.4) | 2 (18.2) | 5 (26.3) | |
| South | 7 (23.4) | 3 (27.3) | 4 (21.1) | |
| Midwest | 8 (26.7) | 4 (36.4) | 4 (21.1) | |
| Urban location, n (%) | 25 (83.3) | 11 (100) | 15 (78.9) | 0.035 |
| Teaching status, n (%) | 0.036 | |||
| Academic medical center | 10 (33.4) | 5 (45.5) | 5 (26.3) | |
| Community teaching | 8 (26.7) | 3 (27.3) | 5 (26.3) | |
| Community nonteaching | 12 (40) | 3 (27.3) | 9 (47.4) | |
| Beds number, mean (SD) | 426.6 (220.6) | 559.2 (187.8) | 349.79 (204.48) | 0.003 |
| Number of tools implemented, n (%) | 0.194 | |||
| 0 | 2 (6.7) | 0 | 2 (10.5) | |
| 1 | 2 (6.7) | 0 | 2 (10.5) | |
| 2 | 4 (13.3) | 2 (18.2) | 2 (10.5) | |
| 3 | 12 (40.0) | 3 (27.3) | 8 (42.1) | |
| 4 | 9 (30.0) | 5 (45.5) | 4 (21.1) | |
| 5 | 1 (3.3) | 1 (9.1) | 1 (5.3) | |
Mentor engagement with sites consisted of a 2‐day kickoff training on the BOOST tools, where site teams met their mentor and initiated development of structured action plans, followed by 5 to 6 scheduled phone calls in the subsequent 12 months. During these conference calls, mentors gauged progress and sought to help troubleshoot barriers to implementation. Some mentors also conducted a site visit with participant sites. Project BOOST provided sites with several collaborative activities including online webinars and an online listserv. Sites also received a quarterly newsletter.
Outcome Measures
The primary outcome was 30‐day rehospitalization defined as same hospital, all‐cause rehospitalization. Home discharges as well as discharges or transfers to other healthcare facilities were included in the discharge calculation. Elective or scheduled rehospitalizations as well as multiple rehospitalizations in the same 30‐day window were considered individual rehospitalization events. Rehospitalization was reported as a ratio of 30‐day rehospitalizations divided by live discharges in a calendar month. Length of stay was reported as the mean length of stay among live discharges in a calendar month. Outcomes were calculated at the participant site and then uploaded as overall monthly unit outcomes to a Web‐based research database.
To account for seasonal trends as well as marked variation in month‐to‐month rehospitalization rates identified in longitudinal data, we elected to compare 3‐month year‐over‐year averages to determine relative changes in readmission rates from the period prior to BOOST implementation to the period after BOOST implementation. We calculated averages for rehospitalization and length of stay in the 3‐month period preceding the sites' first reported month of front‐line implementation and in the corresponding 3‐month period in the subsequent calendar year. For example, if a site reported implementing its first tool in April 2010, the average readmission rate in the unit for January 2011 through March 2011 was subtracted from the average readmission rate for January 2010 through March 2010.
Sites were surveyed regarding tool implementation rates 6 months and 24 months after the 2009 kickoff training session. Surveys were electronically completed by site leaders in consultation with site team members. The survey identified new tool implementation as well as modification of existing care processes using the BOOST tools (admission risk assessment, discharge readiness checklist, teach back use, mandate regarding discharge summary completion, follow‐up phone calls to >80% of discharges). Use of a sixth tool, creation of individualized written discharge instructions, was not measured. We credited sites with tool implementation if they reported either de novo tool use or alteration of previous care processes influenced by BOOST tools.
Clinical outcome reporting was voluntary, and sites did not receive compensation and were not subject to penalty for the degree of implementation or outcome reporting. No patient‐level information was collected for the analysis, which was approved by the Northwestern University institutional review board.
Data Sources and Methods
Readmission and length of stay data, including the unit level readmission rate, as collected from administrative sources at each hospital, were collected using templated spreadsheet software between December 2008 and June 2010, after which data were loaded directly to a Web‐based data‐tracking platform. Sites were asked to load data as they became available. Sites were asked to report the number of study unit discharges as well as the number of those discharges readmitted within 30 days; however, reporting of the number of patient discharges was inconsistent across sites. Serial outreach consisting of monthly phone calls or email messaging to site leaders was conducted throughout 2011 to increase site participation in the project analysis.
Implementation date information was collected from 2 sources. The first was through online surveys distributed in November 2009 and April 2011. The second was through fields in the Web‐based data tracking platform to which sites uploaded data. In cases where disagreement was found between these 2 sources, the site leader was contacted for clarification.
Practice setting (community teaching, community nonteaching, academic medical center) was determined by site‐leader report within the Web‐based data tracking platform. Data for hospital characteristics (number of licensed beds and geographic region) were obtained from the American Hospital Association's Annual Survey of Hospitals.[18] Hospital region was characterized as West, South, Midwest, or Northeast.
Analysis
The null hypothesis was that no prepost difference existed in readmission rates within BOOST units, and no difference existed in the prepost change in readmission rates in BOOST units when compared to site‐matched control units. The Wilcoxon rank sum test was used to test whether observed changes described above were significantly different from 0, supporting rejection of the null hypotheses. We performed similar tests to determine the significance of observed changes in length of stay. We performed our analysis using SAS 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Eleven hospitals provided rehospitalization and length‐of‐stay outcome data for both a BOOST and control unit for the pre‐ and postimplementation periods. Compared to the 19 sites that did not participate in the analysis, these 11 sites were significantly larger (559188 beds vs 350205 beds, P=0.003), more likely to be located in an urban area (100.0% [n=11] vs 78.9% [n=15], P=0.035), and more likely to be academic medical centers (45.5% [n=5] vs 26.3% [n=5], P=0.036) (Table 1).
The mean number of tools implemented by sites participating in the analysis was 3.50.9. All sites implemented at least 2 tools. The duration between attendance at the BOOST kickoff event and first tool implementation ranged from 3 months (first tool implemented prior to attending the kickoff) and 9 months (mean duration, 3.34.3 months) (Table 2).
| Hospital | Region | Hospital Type | No. Licensed Beds | Kickoff Implementationa | Risk Assessment | Discharge Checklist | Teach Back | Discharge Summary Completion | Follow‐up Phone Call | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
| 1 | Midwest | Community teaching | <300 | 8 | 3 | |||||
| 2 | West | Community teaching | >600 | 0 | 4 | |||||
| 3 | Northeast | Academic medical center | >600 | 2 | 4 | |||||
| 4 | Northeast | Community nonteaching | <300 | 9 | 2 | |||||
| 5 | South | Community nonteaching | >600 | 6 | 3 | |||||
| 6 | South | Community nonteaching | >600 | 3 | 4 | |||||
| 7 | Midwest | Community teaching | 300600 | 1 | 5 | |||||
| 8 | West | Academic medical center | 300600 | 1 | 4 | |||||
| 9 | South | Academic medical center | >600 | 4 | 4 | |||||
| 10 | Midwest | Academic medical center | 300600 | 3 | 3 | |||||
| 11 | Midwest | Academic medical center | >600 | 9 | 2 | |||||
The average rate of 30‐day rehospitalization among BOOST units was 14.7% in the preimplementation period and 12.7% during the postimplementation period (P=0.010) (Figure 1). Rehospitalization rates for matched control units were 14.0% in the preintervention period and 14.1% in the postintervention period (P=0.831). The mean absolute reduction in readmission rates over the 1‐year study period in BOOST units compared to control units was 2.0%, or a relative reduction of 13.6% (P=0.054 for signed rank test comparing differences in readmission rate reduction in BOOST units compared to site‐matched control units). Length of stay in BOOST and control units decreased an average of 0.5 days and 0.3 days, respectively. There was no difference in length of stay change between BOOST units and control units (P=0.966).
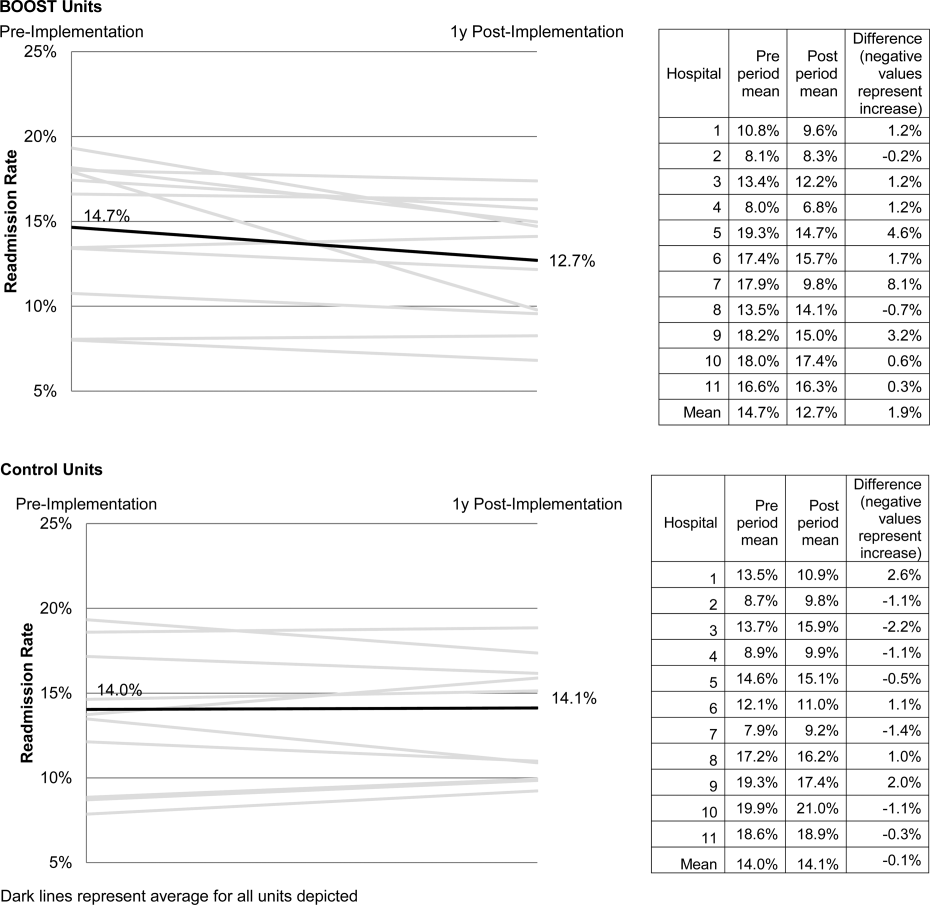
DISCUSSION
As hospitals strive to reduce their readmission rates to avoid Centers for Medicare and Medicaid Services penalties, Project BOOST may be a viable QI approach to achieve their goals. This initial evaluation of participation in Project BOOST by 11 hospitals of varying sizes across the United States showed an associated reduction in rehospitalization rates (absolute=2.0% and relative=13.6%, P=0.054). We did not find any significant change in length of stay among these hospitals implementing BOOST tools.
The tools provided to participating hospitals were developed from evidence found in peer‐reviewed literature established through experimental methods in well‐controlled academic settings. Further tool development was informed by recommendations of an advisory board consisting of expert representatives and advocates involved in the hospital discharge process: patients, caregivers, physicians, nurses, case managers, social workers, insurers, and regulatory and research agencies.[19] The toolkit components address multiple aspects of hospital discharge and follow‐up with the goal of improving health by optimizing the safety of care transitions. Our observation that readmission rates appeared to improve in a diverse hospital sample including nonacademic and community hospitals engaged in Project BOOST is reassuring that the benefits seen in existing research literature, developed in distinctly academic settings, can be replicated in diverse acute‐care settings.
The effect size observed in our study was modest but consistent with several studies identified in a recent review of trials measuring interventions to reduce rehospitalization, where 7 of 16 studies showing a significant improvement registered change in the 0% to 5% absolute range.[12] Impact of this project may have been tempered by the need to translate external QI content to the local setting. Additionally, in contrast to experimental studies that are limited in scope and timing and often scaled to a research budget, BOOST sites were encouraged to implement Project BOOST in the clinical setting even if no new funds were available to support the effort.[12]
The recruitment of a national sample of both academic and nonacademic hospital participants imposed several limitations on our study and analysis. We recognize that intervention units selected by hospitals may have had unmeasured unit and patient characteristics that facilitated successful change and contributed to the observed improvements. However, because external pressure to reduce readmission is present across all hospitals independent of the BOOST intervention, we felt site‐matched controls were essential to understanding effects attributable to the BOOST tools. Differences between units would be expected to be stable over the course of the study period, and comparison of outcome differences between 2 different time periods would be reasonable. Additionally, we could not collect data on readmissions to other hospitals. Theoretically, patients discharged from BOOST units might be more likely to have been rehospitalized elsewhere, but the fraction of rehospitalizations occurring at alternate facilities would also be expected to be similar on the matched control unit.
We report findings from a voluntary cohort willing and capable of designating a comparison clinical unit and contributing the requested data outcomes. Pilot sites that did not report outcomes were not analyzed, but comparison of hospital characteristics shows that participating hospitals were more likely to be large, urban, academic medical centers. Although barriers to data submission were not formally analyzed, reports from nonparticipating sites describe data submission limited by local implementation design (no geographic rollout or simultaneous rollout on all appropriate clinical units), site specific inability to generate unit level outcome statistics, and competing organizational priorities for data analyst time (electronic medical record deployment, alternative QI initiatives). The external validity of our results may be limited to organizations capable of analytics at the level of the individual clinical unit as well as those with sufficient QI resources to support reporting to a national database in the absence of a payer mandate. It is possible that additional financial support for on‐site data collection would have bolstered participation, making the example of participation rates we present potentially informative to organizations hoping to widely disseminate a QI agenda.
Nonetheless, the effectiveness demonstrated in the 11 sites that did participate is encouraging, and ongoing collaboration with subsequent BOOST cohorts has been designed to further facilitate data collection. Among the insights gained from this pilot experience, and incorporated into ongoing BOOST cohorts, is the importance of intensive mentor engagement to foster accountability among participant sites, assist with implementation troubleshooting, and offer expertise that is often particularly effective in gaining local support. We now encourage sites to have 2 mentor site visits to further these roles and more frequent conference calls. Further research to understand the marginal benefit of the mentored implementation approach is ongoing.
The limitations in data submission we experienced with the pilot cohort likely reflect resource constraints not uncommon at many hospitals. Increasing pressure placed on hospitals as a result of the Readmission Reduction Program within the Affordable Care Act as well as increasing interest from private and Medicaid payors to incorporate similar readmission‐based penalties provide encouragement for hospitals to enhance their data and analytic skills. National incentives for implementation of electronic health records (EHR) should also foster such capabilities, though we often saw EHRs as a barrier to QI, especially rapid cycle trials. Fortunately, hospitals are increasingly being afforded access to comprehensive claims databases to assist in tracking readmission rates to other facilities, and these data are becoming available in a more timely fashion. This more robust data collection, facilitated by private payors, state QI organizations, and state hospital associations, will support additional analytic methods such as multivariate regression models and interrupted time series designs to appreciate the experience of current BOOST participants.
Additional research is needed to understand the role of organizational context in the effectiveness of Project BOOST. Differences in rates of tool implementation and changes in clinical outcomes are likely dependent on local implementation context at the level of the healthcare organization and individual clinical unit.[20] Progress reports from site mentors and previously described experiences of QI implementation indicate that successful implementation of a multidimensional bundle of interventions may have reflected a higher level of institutional support, more robust team engagement in the work of reducing readmissions, increased clinical staff support for change, the presence of an effective project champion, or a key facilitating role of external mentorship.[21, 22] Ongoing data collection will continue to measure the sustainability of tool use and observed outcome changes to inform strategies to maintain gains associated with implementation. The role of mentored implementation in facilitating gains also requires further study.
Increasing attention to the problem of avoidable rehospitalization is driving hospitals, insurers, and policy makers to pursue QI efforts that favorably impact readmission rates. Our analysis of the BOOST intervention suggests that modest gains can be achieved following evidence‐based hospital process change facilitated by a mentored implementation model. However, realization of the goal of a 20% reduction in rehospitalization proposed by the Center for Medicare and Medicaid Services' Partnership for Patients initiative may be difficult to achieve on a national scale,[23] especially if efforts focus on just the hospital.
Acknowledgments
The authors acknowledge the contributions of Amanda Creden, BA (data collection), Julia Lee (biostatistical support), and the support of Amy Berman, BS, RN, Senior Program Officer at The John A. Hartford Foundation.
Disclosures
Project BOOST was funded by a grant from The John A. Hartford Foundation. Project BOOST is administered by the Society of Hospital Medicine (SHM). The development of the Project BOOST toolkit, recruitment of sites for this study, mentorship of the pilot cohort, project evaluation planning, and collection of pilot data were funded by a grant from The John A. Harford Foundation. Additional funding for continued data collection and analysis was funded by the SHM through funds from hospitals to participate in Project BOOST, specifically with funding support for Dr. Hansen. Dr. Williams has received funding to serve as Principal Investigator for Project BOOST. Since the time of initial cohort participation, approximately 125 additional hospitals have participated in the mentored implementation of Project BOOST. This participation was funded through a combination of site‐based tuition, third‐party payor support from private insurers, foundations, and federal funding through the Center for Medicare and Medicaid Innovation Partnership for Patients program. Drs. Greenwald, Hansen, and Williams are Project BOOST mentors for current Project BOOST sites and receive financial support through the SHM for this work. Dr. Howell has previously received funding as a Project BOOST mentor. Ms. Budnitz is the BOOST Project Director and is Chief Strategy and Development Officer for the HM. Dr. Maynard is the Senior Vice President of the SHM's Center for Hospital Innovation and Improvement.
References
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- United States Congress. House Committee on Education and Labor. Coe on Ways and Means, Committee on Energy and Commerce, Compilation of Patient Protection and Affordable Care Act: as amended through November 1, 2010 including Patient Protection and Affordable Care Act health‐related portions of the Health Care and Education Reconciliation Act of 2010. Washington, DC: US Government Printing Office; 2010.
- Cost estimate for the amendment in the nature of a substitute to H.R. 3590, as proposed in the Senate on November 18, 2009. Washington, DC: Congressional Budget Office; 2009.
- Partnership for Patients, Center for Medicare and Medicaid Innovation. Available at: http://www.innovations.cms.gov/initiatives/Partnership‐for‐Patients/index.html. Accessed December 12, 2012.
- , . Providers have failed to work for continuity. Hospitals. 1979;53(10):79.
- , . Executing high‐quality care transitions: a call to do it right. J Hosp Med. 2007;2(5):287–290.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- , , . Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians. JAMA. 2007;297(8):831–841.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178.
- , , , et al. Advancing the science of patient safety. Ann Intern Med. 2011;154(10):693–696.
- , . From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230.
- , , , et al. Quality improvement projects targeting health care‐associated infections: comparing virtual collaborative and toolkit approaches. J Hosp Med. 2011;6(5):271–278.
- , , , , . Publication guidelines for improvement studies in health care: evolution of the SQUIRE project. Ann Intern Med. 2008;149(9):670–676.
- , , , . Risk stratification and therapeutic decision making in acute coronary syndromes. JAMA. 2000;284(7):876–878.
- , , . Are diagnosis specific outcome indicators based on administrative data useful in assessing quality of hospital care? BMJ. 2004;13(1):32.
- , , , et al. What distinguishes top‐performing hospitals in acute myocardial infarction mortality rates? Ann Intern Med. 2011;154(6):384–390.
- , , , . The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13–20.
- , . Evidence‐based quality improvement: the state of the science. Health Aff (Millwood). 2005;24(1):138–150.
- Center for Medicare and Medicaid Innovation. Partnership for patients. Available at: http://www.innovations.cms.gov/initiatives/Partnership‐for‐Patients/index.html. Accessed April 2, 2012.
Enactment of federal legislation imposing hospital reimbursement penalties for excess rates of rehospitalizations among Medicare fee for service beneficiaries markedly increased interest in hospital quality improvement (QI) efforts to reduce the observed 30‐day rehospitalization of 19.6% in this elderly population.[1, 2] The Congressional Budget Office estimated that reimbursement penalties to hospitals for high readmission rates are expected to save the Medicare program approximately $7 billion between 2010 and 2019.[3] These penalties are complemented by resources from the Center for Medicare and Medicaid Innovation aiming to reduce hospital readmissions by 20% by the end of 2013 through the Partnership for Patients campaign.[4] Although potential financial penalties and provision of resources for QI intensified efforts to enhance the quality of the hospital discharge transition, patient safety risks associated with hospital discharge are well documented.[5, 6] Approximately 20% of patients discharged from the hospital may suffer adverse events,[7, 8] of which up to three‐quarters (72%) are medication related,[9] and over one‐third of required follow‐up testing after discharge is not completed.[10] Such findings indicate opportunities for improvement in the discharge process.[11]
Numerous publications describe studies aiming to improve the hospital discharge process and mitigate these hazards, though a systematic review of interventions to reduce 30‐day rehospitalization indicated that the existing evidence base for the effectiveness of transition interventions demonstrates irregular effectiveness and limitations to generalizability.[12] Most studies showing effectiveness are confined to single academic medical centers. Existing evidence supports multifaceted interventions implemented in both the pre‐ and postdischarge periods and focused on risk assessment and tailored, patient‐centered application of interventions to mitigate risk. For example Project RED (Re‐Engineered Discharge) applied a bundled intervention consisting of intensified patient education and discharge planning, improved medication reconciliation and discharge instructions, and longitudinal patient contact with follow‐up phone calls and a dedicated discharge advocate.[13] However, the mean age of patients participating in the study was 50 years, and it excluded patients admitted from or discharged to skilled nursing facilities, making generalizability to the geriatric population uncertain.
An integral aspect of QI projects is the contribution of local context to translation of best practices to disparate settings.[14, 15, 16] Most available reports of successful interventions to reduce rehospitalization have not fully described the specifics of either the intervention context or design. Moreover, the available evidence base for common interventions to reduce rehospitalization was developed in the academic setting. Validation of single academic center studies in a broader healthcare context is necessary.
Project BOOST (Better Outcomes for Older adults through Safe Transitions) recruited a diverse national cohort of both academic and nonacademic hospitals to participate in a QI effort to implement best practices for hospital discharge care transitions using a national collaborative approach facilitated by external expert mentorship. This study aimed to determine the effectiveness of BOOST in lowering hospital readmission rates and impact on length of stay.
METHODS
The study of Project BOOST was undertaken in accordance with the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines.[17]
Participants
The unit of observation for the prospective cohort study was the clinical acute‐care unit within hospitals. Sites were instructed to designate a pilot unit for the intervention that cared for medical or mixed medicalsurgical patient populations. Sites were also asked to provide outcome data for a clinically and organizationally similar non‐BOOST unit to provide a site‐matched control. Control units were matched by local site leadership based on comparable patient demographics, clinical mix, and extent of housestaff presence. An initial cohort of 6 hospitals in 2008 was followed by a second cohort of 24 hospitals initiated in 2009. All hospitals were invited to participate in the national effectiveness analysis, which required submission of readmission and length of stay data for both a BOOST intervention unit and a clinically matched control unit.
Description of the Intervention
The BOOST intervention consisted of 2 major sequential processes, planning and implementation, both facilitated by external site mentorsphysicians expert in QI and care transitionsfor a period of 12 months. Extensive background on the planning and implementation components is available at
| Enrollment Sites, n=30 | Sites Reporting Outcome Data, n=11 | Sites Not Reporting Outcome Data, n=19 | P Value for Comparison of Outcome Data Sites Compared to Othersa | |
|---|---|---|---|---|
| ||||
| Region, n (%) | 0.194 | |||
| Northeast | 8 (26.7) | 2 (18.2) | 6 (31.6) | |
| West | 7 (23.4) | 2 (18.2) | 5 (26.3) | |
| South | 7 (23.4) | 3 (27.3) | 4 (21.1) | |
| Midwest | 8 (26.7) | 4 (36.4) | 4 (21.1) | |
| Urban location, n (%) | 25 (83.3) | 11 (100) | 15 (78.9) | 0.035 |
| Teaching status, n (%) | 0.036 | |||
| Academic medical center | 10 (33.4) | 5 (45.5) | 5 (26.3) | |
| Community teaching | 8 (26.7) | 3 (27.3) | 5 (26.3) | |
| Community nonteaching | 12 (40) | 3 (27.3) | 9 (47.4) | |
| Beds number, mean (SD) | 426.6 (220.6) | 559.2 (187.8) | 349.79 (204.48) | 0.003 |
| Number of tools implemented, n (%) | 0.194 | |||
| 0 | 2 (6.7) | 0 | 2 (10.5) | |
| 1 | 2 (6.7) | 0 | 2 (10.5) | |
| 2 | 4 (13.3) | 2 (18.2) | 2 (10.5) | |
| 3 | 12 (40.0) | 3 (27.3) | 8 (42.1) | |
| 4 | 9 (30.0) | 5 (45.5) | 4 (21.1) | |
| 5 | 1 (3.3) | 1 (9.1) | 1 (5.3) | |
Mentor engagement with sites consisted of a 2‐day kickoff training on the BOOST tools, where site teams met their mentor and initiated development of structured action plans, followed by 5 to 6 scheduled phone calls in the subsequent 12 months. During these conference calls, mentors gauged progress and sought to help troubleshoot barriers to implementation. Some mentors also conducted a site visit with participant sites. Project BOOST provided sites with several collaborative activities including online webinars and an online listserv. Sites also received a quarterly newsletter.
Outcome Measures
The primary outcome was 30‐day rehospitalization defined as same hospital, all‐cause rehospitalization. Home discharges as well as discharges or transfers to other healthcare facilities were included in the discharge calculation. Elective or scheduled rehospitalizations as well as multiple rehospitalizations in the same 30‐day window were considered individual rehospitalization events. Rehospitalization was reported as a ratio of 30‐day rehospitalizations divided by live discharges in a calendar month. Length of stay was reported as the mean length of stay among live discharges in a calendar month. Outcomes were calculated at the participant site and then uploaded as overall monthly unit outcomes to a Web‐based research database.
To account for seasonal trends as well as marked variation in month‐to‐month rehospitalization rates identified in longitudinal data, we elected to compare 3‐month year‐over‐year averages to determine relative changes in readmission rates from the period prior to BOOST implementation to the period after BOOST implementation. We calculated averages for rehospitalization and length of stay in the 3‐month period preceding the sites' first reported month of front‐line implementation and in the corresponding 3‐month period in the subsequent calendar year. For example, if a site reported implementing its first tool in April 2010, the average readmission rate in the unit for January 2011 through March 2011 was subtracted from the average readmission rate for January 2010 through March 2010.
Sites were surveyed regarding tool implementation rates 6 months and 24 months after the 2009 kickoff training session. Surveys were electronically completed by site leaders in consultation with site team members. The survey identified new tool implementation as well as modification of existing care processes using the BOOST tools (admission risk assessment, discharge readiness checklist, teach back use, mandate regarding discharge summary completion, follow‐up phone calls to >80% of discharges). Use of a sixth tool, creation of individualized written discharge instructions, was not measured. We credited sites with tool implementation if they reported either de novo tool use or alteration of previous care processes influenced by BOOST tools.
Clinical outcome reporting was voluntary, and sites did not receive compensation and were not subject to penalty for the degree of implementation or outcome reporting. No patient‐level information was collected for the analysis, which was approved by the Northwestern University institutional review board.
Data Sources and Methods
Readmission and length of stay data, including the unit level readmission rate, as collected from administrative sources at each hospital, were collected using templated spreadsheet software between December 2008 and June 2010, after which data were loaded directly to a Web‐based data‐tracking platform. Sites were asked to load data as they became available. Sites were asked to report the number of study unit discharges as well as the number of those discharges readmitted within 30 days; however, reporting of the number of patient discharges was inconsistent across sites. Serial outreach consisting of monthly phone calls or email messaging to site leaders was conducted throughout 2011 to increase site participation in the project analysis.
Implementation date information was collected from 2 sources. The first was through online surveys distributed in November 2009 and April 2011. The second was through fields in the Web‐based data tracking platform to which sites uploaded data. In cases where disagreement was found between these 2 sources, the site leader was contacted for clarification.
Practice setting (community teaching, community nonteaching, academic medical center) was determined by site‐leader report within the Web‐based data tracking platform. Data for hospital characteristics (number of licensed beds and geographic region) were obtained from the American Hospital Association's Annual Survey of Hospitals.[18] Hospital region was characterized as West, South, Midwest, or Northeast.
Analysis
The null hypothesis was that no prepost difference existed in readmission rates within BOOST units, and no difference existed in the prepost change in readmission rates in BOOST units when compared to site‐matched control units. The Wilcoxon rank sum test was used to test whether observed changes described above were significantly different from 0, supporting rejection of the null hypotheses. We performed similar tests to determine the significance of observed changes in length of stay. We performed our analysis using SAS 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Eleven hospitals provided rehospitalization and length‐of‐stay outcome data for both a BOOST and control unit for the pre‐ and postimplementation periods. Compared to the 19 sites that did not participate in the analysis, these 11 sites were significantly larger (559188 beds vs 350205 beds, P=0.003), more likely to be located in an urban area (100.0% [n=11] vs 78.9% [n=15], P=0.035), and more likely to be academic medical centers (45.5% [n=5] vs 26.3% [n=5], P=0.036) (Table 1).
The mean number of tools implemented by sites participating in the analysis was 3.50.9. All sites implemented at least 2 tools. The duration between attendance at the BOOST kickoff event and first tool implementation ranged from 3 months (first tool implemented prior to attending the kickoff) and 9 months (mean duration, 3.34.3 months) (Table 2).
| Hospital | Region | Hospital Type | No. Licensed Beds | Kickoff Implementationa | Risk Assessment | Discharge Checklist | Teach Back | Discharge Summary Completion | Follow‐up Phone Call | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
| 1 | Midwest | Community teaching | <300 | 8 | 3 | |||||
| 2 | West | Community teaching | >600 | 0 | 4 | |||||
| 3 | Northeast | Academic medical center | >600 | 2 | 4 | |||||
| 4 | Northeast | Community nonteaching | <300 | 9 | 2 | |||||
| 5 | South | Community nonteaching | >600 | 6 | 3 | |||||
| 6 | South | Community nonteaching | >600 | 3 | 4 | |||||
| 7 | Midwest | Community teaching | 300600 | 1 | 5 | |||||
| 8 | West | Academic medical center | 300600 | 1 | 4 | |||||
| 9 | South | Academic medical center | >600 | 4 | 4 | |||||
| 10 | Midwest | Academic medical center | 300600 | 3 | 3 | |||||
| 11 | Midwest | Academic medical center | >600 | 9 | 2 | |||||
The average rate of 30‐day rehospitalization among BOOST units was 14.7% in the preimplementation period and 12.7% during the postimplementation period (P=0.010) (Figure 1). Rehospitalization rates for matched control units were 14.0% in the preintervention period and 14.1% in the postintervention period (P=0.831). The mean absolute reduction in readmission rates over the 1‐year study period in BOOST units compared to control units was 2.0%, or a relative reduction of 13.6% (P=0.054 for signed rank test comparing differences in readmission rate reduction in BOOST units compared to site‐matched control units). Length of stay in BOOST and control units decreased an average of 0.5 days and 0.3 days, respectively. There was no difference in length of stay change between BOOST units and control units (P=0.966).

DISCUSSION
As hospitals strive to reduce their readmission rates to avoid Centers for Medicare and Medicaid Services penalties, Project BOOST may be a viable QI approach to achieve their goals. This initial evaluation of participation in Project BOOST by 11 hospitals of varying sizes across the United States showed an associated reduction in rehospitalization rates (absolute=2.0% and relative=13.6%, P=0.054). We did not find any significant change in length of stay among these hospitals implementing BOOST tools.
The tools provided to participating hospitals were developed from evidence found in peer‐reviewed literature established through experimental methods in well‐controlled academic settings. Further tool development was informed by recommendations of an advisory board consisting of expert representatives and advocates involved in the hospital discharge process: patients, caregivers, physicians, nurses, case managers, social workers, insurers, and regulatory and research agencies.[19] The toolkit components address multiple aspects of hospital discharge and follow‐up with the goal of improving health by optimizing the safety of care transitions. Our observation that readmission rates appeared to improve in a diverse hospital sample including nonacademic and community hospitals engaged in Project BOOST is reassuring that the benefits seen in existing research literature, developed in distinctly academic settings, can be replicated in diverse acute‐care settings.
The effect size observed in our study was modest but consistent with several studies identified in a recent review of trials measuring interventions to reduce rehospitalization, where 7 of 16 studies showing a significant improvement registered change in the 0% to 5% absolute range.[12] Impact of this project may have been tempered by the need to translate external QI content to the local setting. Additionally, in contrast to experimental studies that are limited in scope and timing and often scaled to a research budget, BOOST sites were encouraged to implement Project BOOST in the clinical setting even if no new funds were available to support the effort.[12]
The recruitment of a national sample of both academic and nonacademic hospital participants imposed several limitations on our study and analysis. We recognize that intervention units selected by hospitals may have had unmeasured unit and patient characteristics that facilitated successful change and contributed to the observed improvements. However, because external pressure to reduce readmission is present across all hospitals independent of the BOOST intervention, we felt site‐matched controls were essential to understanding effects attributable to the BOOST tools. Differences between units would be expected to be stable over the course of the study period, and comparison of outcome differences between 2 different time periods would be reasonable. Additionally, we could not collect data on readmissions to other hospitals. Theoretically, patients discharged from BOOST units might be more likely to have been rehospitalized elsewhere, but the fraction of rehospitalizations occurring at alternate facilities would also be expected to be similar on the matched control unit.
We report findings from a voluntary cohort willing and capable of designating a comparison clinical unit and contributing the requested data outcomes. Pilot sites that did not report outcomes were not analyzed, but comparison of hospital characteristics shows that participating hospitals were more likely to be large, urban, academic medical centers. Although barriers to data submission were not formally analyzed, reports from nonparticipating sites describe data submission limited by local implementation design (no geographic rollout or simultaneous rollout on all appropriate clinical units), site specific inability to generate unit level outcome statistics, and competing organizational priorities for data analyst time (electronic medical record deployment, alternative QI initiatives). The external validity of our results may be limited to organizations capable of analytics at the level of the individual clinical unit as well as those with sufficient QI resources to support reporting to a national database in the absence of a payer mandate. It is possible that additional financial support for on‐site data collection would have bolstered participation, making the example of participation rates we present potentially informative to organizations hoping to widely disseminate a QI agenda.
Nonetheless, the effectiveness demonstrated in the 11 sites that did participate is encouraging, and ongoing collaboration with subsequent BOOST cohorts has been designed to further facilitate data collection. Among the insights gained from this pilot experience, and incorporated into ongoing BOOST cohorts, is the importance of intensive mentor engagement to foster accountability among participant sites, assist with implementation troubleshooting, and offer expertise that is often particularly effective in gaining local support. We now encourage sites to have 2 mentor site visits to further these roles and more frequent conference calls. Further research to understand the marginal benefit of the mentored implementation approach is ongoing.
The limitations in data submission we experienced with the pilot cohort likely reflect resource constraints not uncommon at many hospitals. Increasing pressure placed on hospitals as a result of the Readmission Reduction Program within the Affordable Care Act as well as increasing interest from private and Medicaid payors to incorporate similar readmission‐based penalties provide encouragement for hospitals to enhance their data and analytic skills. National incentives for implementation of electronic health records (EHR) should also foster such capabilities, though we often saw EHRs as a barrier to QI, especially rapid cycle trials. Fortunately, hospitals are increasingly being afforded access to comprehensive claims databases to assist in tracking readmission rates to other facilities, and these data are becoming available in a more timely fashion. This more robust data collection, facilitated by private payors, state QI organizations, and state hospital associations, will support additional analytic methods such as multivariate regression models and interrupted time series designs to appreciate the experience of current BOOST participants.
Additional research is needed to understand the role of organizational context in the effectiveness of Project BOOST. Differences in rates of tool implementation and changes in clinical outcomes are likely dependent on local implementation context at the level of the healthcare organization and individual clinical unit.[20] Progress reports from site mentors and previously described experiences of QI implementation indicate that successful implementation of a multidimensional bundle of interventions may have reflected a higher level of institutional support, more robust team engagement in the work of reducing readmissions, increased clinical staff support for change, the presence of an effective project champion, or a key facilitating role of external mentorship.[21, 22] Ongoing data collection will continue to measure the sustainability of tool use and observed outcome changes to inform strategies to maintain gains associated with implementation. The role of mentored implementation in facilitating gains also requires further study.
Increasing attention to the problem of avoidable rehospitalization is driving hospitals, insurers, and policy makers to pursue QI efforts that favorably impact readmission rates. Our analysis of the BOOST intervention suggests that modest gains can be achieved following evidence‐based hospital process change facilitated by a mentored implementation model. However, realization of the goal of a 20% reduction in rehospitalization proposed by the Center for Medicare and Medicaid Services' Partnership for Patients initiative may be difficult to achieve on a national scale,[23] especially if efforts focus on just the hospital.
Acknowledgments
The authors acknowledge the contributions of Amanda Creden, BA (data collection), Julia Lee (biostatistical support), and the support of Amy Berman, BS, RN, Senior Program Officer at The John A. Hartford Foundation.
Disclosures
Project BOOST was funded by a grant from The John A. Hartford Foundation. Project BOOST is administered by the Society of Hospital Medicine (SHM). The development of the Project BOOST toolkit, recruitment of sites for this study, mentorship of the pilot cohort, project evaluation planning, and collection of pilot data were funded by a grant from The John A. Harford Foundation. Additional funding for continued data collection and analysis was funded by the SHM through funds from hospitals to participate in Project BOOST, specifically with funding support for Dr. Hansen. Dr. Williams has received funding to serve as Principal Investigator for Project BOOST. Since the time of initial cohort participation, approximately 125 additional hospitals have participated in the mentored implementation of Project BOOST. This participation was funded through a combination of site‐based tuition, third‐party payor support from private insurers, foundations, and federal funding through the Center for Medicare and Medicaid Innovation Partnership for Patients program. Drs. Greenwald, Hansen, and Williams are Project BOOST mentors for current Project BOOST sites and receive financial support through the SHM for this work. Dr. Howell has previously received funding as a Project BOOST mentor. Ms. Budnitz is the BOOST Project Director and is Chief Strategy and Development Officer for the HM. Dr. Maynard is the Senior Vice President of the SHM's Center for Hospital Innovation and Improvement.
References
Enactment of federal legislation imposing hospital reimbursement penalties for excess rates of rehospitalizations among Medicare fee for service beneficiaries markedly increased interest in hospital quality improvement (QI) efforts to reduce the observed 30‐day rehospitalization of 19.6% in this elderly population.[1, 2] The Congressional Budget Office estimated that reimbursement penalties to hospitals for high readmission rates are expected to save the Medicare program approximately $7 billion between 2010 and 2019.[3] These penalties are complemented by resources from the Center for Medicare and Medicaid Innovation aiming to reduce hospital readmissions by 20% by the end of 2013 through the Partnership for Patients campaign.[4] Although potential financial penalties and provision of resources for QI intensified efforts to enhance the quality of the hospital discharge transition, patient safety risks associated with hospital discharge are well documented.[5, 6] Approximately 20% of patients discharged from the hospital may suffer adverse events,[7, 8] of which up to three‐quarters (72%) are medication related,[9] and over one‐third of required follow‐up testing after discharge is not completed.[10] Such findings indicate opportunities for improvement in the discharge process.[11]
Numerous publications describe studies aiming to improve the hospital discharge process and mitigate these hazards, though a systematic review of interventions to reduce 30‐day rehospitalization indicated that the existing evidence base for the effectiveness of transition interventions demonstrates irregular effectiveness and limitations to generalizability.[12] Most studies showing effectiveness are confined to single academic medical centers. Existing evidence supports multifaceted interventions implemented in both the pre‐ and postdischarge periods and focused on risk assessment and tailored, patient‐centered application of interventions to mitigate risk. For example Project RED (Re‐Engineered Discharge) applied a bundled intervention consisting of intensified patient education and discharge planning, improved medication reconciliation and discharge instructions, and longitudinal patient contact with follow‐up phone calls and a dedicated discharge advocate.[13] However, the mean age of patients participating in the study was 50 years, and it excluded patients admitted from or discharged to skilled nursing facilities, making generalizability to the geriatric population uncertain.
An integral aspect of QI projects is the contribution of local context to translation of best practices to disparate settings.[14, 15, 16] Most available reports of successful interventions to reduce rehospitalization have not fully described the specifics of either the intervention context or design. Moreover, the available evidence base for common interventions to reduce rehospitalization was developed in the academic setting. Validation of single academic center studies in a broader healthcare context is necessary.
Project BOOST (Better Outcomes for Older adults through Safe Transitions) recruited a diverse national cohort of both academic and nonacademic hospitals to participate in a QI effort to implement best practices for hospital discharge care transitions using a national collaborative approach facilitated by external expert mentorship. This study aimed to determine the effectiveness of BOOST in lowering hospital readmission rates and impact on length of stay.
METHODS
The study of Project BOOST was undertaken in accordance with the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines.[17]
Participants
The unit of observation for the prospective cohort study was the clinical acute‐care unit within hospitals. Sites were instructed to designate a pilot unit for the intervention that cared for medical or mixed medicalsurgical patient populations. Sites were also asked to provide outcome data for a clinically and organizationally similar non‐BOOST unit to provide a site‐matched control. Control units were matched by local site leadership based on comparable patient demographics, clinical mix, and extent of housestaff presence. An initial cohort of 6 hospitals in 2008 was followed by a second cohort of 24 hospitals initiated in 2009. All hospitals were invited to participate in the national effectiveness analysis, which required submission of readmission and length of stay data for both a BOOST intervention unit and a clinically matched control unit.
Description of the Intervention
The BOOST intervention consisted of 2 major sequential processes, planning and implementation, both facilitated by external site mentorsphysicians expert in QI and care transitionsfor a period of 12 months. Extensive background on the planning and implementation components is available at
| Enrollment Sites, n=30 | Sites Reporting Outcome Data, n=11 | Sites Not Reporting Outcome Data, n=19 | P Value for Comparison of Outcome Data Sites Compared to Othersa | |
|---|---|---|---|---|
| ||||
| Region, n (%) | 0.194 | |||
| Northeast | 8 (26.7) | 2 (18.2) | 6 (31.6) | |
| West | 7 (23.4) | 2 (18.2) | 5 (26.3) | |
| South | 7 (23.4) | 3 (27.3) | 4 (21.1) | |
| Midwest | 8 (26.7) | 4 (36.4) | 4 (21.1) | |
| Urban location, n (%) | 25 (83.3) | 11 (100) | 15 (78.9) | 0.035 |
| Teaching status, n (%) | 0.036 | |||
| Academic medical center | 10 (33.4) | 5 (45.5) | 5 (26.3) | |
| Community teaching | 8 (26.7) | 3 (27.3) | 5 (26.3) | |
| Community nonteaching | 12 (40) | 3 (27.3) | 9 (47.4) | |
| Beds number, mean (SD) | 426.6 (220.6) | 559.2 (187.8) | 349.79 (204.48) | 0.003 |
| Number of tools implemented, n (%) | 0.194 | |||
| 0 | 2 (6.7) | 0 | 2 (10.5) | |
| 1 | 2 (6.7) | 0 | 2 (10.5) | |
| 2 | 4 (13.3) | 2 (18.2) | 2 (10.5) | |
| 3 | 12 (40.0) | 3 (27.3) | 8 (42.1) | |
| 4 | 9 (30.0) | 5 (45.5) | 4 (21.1) | |
| 5 | 1 (3.3) | 1 (9.1) | 1 (5.3) | |
Mentor engagement with sites consisted of a 2‐day kickoff training on the BOOST tools, where site teams met their mentor and initiated development of structured action plans, followed by 5 to 6 scheduled phone calls in the subsequent 12 months. During these conference calls, mentors gauged progress and sought to help troubleshoot barriers to implementation. Some mentors also conducted a site visit with participant sites. Project BOOST provided sites with several collaborative activities including online webinars and an online listserv. Sites also received a quarterly newsletter.
Outcome Measures
The primary outcome was 30‐day rehospitalization defined as same hospital, all‐cause rehospitalization. Home discharges as well as discharges or transfers to other healthcare facilities were included in the discharge calculation. Elective or scheduled rehospitalizations as well as multiple rehospitalizations in the same 30‐day window were considered individual rehospitalization events. Rehospitalization was reported as a ratio of 30‐day rehospitalizations divided by live discharges in a calendar month. Length of stay was reported as the mean length of stay among live discharges in a calendar month. Outcomes were calculated at the participant site and then uploaded as overall monthly unit outcomes to a Web‐based research database.
To account for seasonal trends as well as marked variation in month‐to‐month rehospitalization rates identified in longitudinal data, we elected to compare 3‐month year‐over‐year averages to determine relative changes in readmission rates from the period prior to BOOST implementation to the period after BOOST implementation. We calculated averages for rehospitalization and length of stay in the 3‐month period preceding the sites' first reported month of front‐line implementation and in the corresponding 3‐month period in the subsequent calendar year. For example, if a site reported implementing its first tool in April 2010, the average readmission rate in the unit for January 2011 through March 2011 was subtracted from the average readmission rate for January 2010 through March 2010.
Sites were surveyed regarding tool implementation rates 6 months and 24 months after the 2009 kickoff training session. Surveys were electronically completed by site leaders in consultation with site team members. The survey identified new tool implementation as well as modification of existing care processes using the BOOST tools (admission risk assessment, discharge readiness checklist, teach back use, mandate regarding discharge summary completion, follow‐up phone calls to >80% of discharges). Use of a sixth tool, creation of individualized written discharge instructions, was not measured. We credited sites with tool implementation if they reported either de novo tool use or alteration of previous care processes influenced by BOOST tools.
Clinical outcome reporting was voluntary, and sites did not receive compensation and were not subject to penalty for the degree of implementation or outcome reporting. No patient‐level information was collected for the analysis, which was approved by the Northwestern University institutional review board.
Data Sources and Methods
Readmission and length of stay data, including the unit level readmission rate, as collected from administrative sources at each hospital, were collected using templated spreadsheet software between December 2008 and June 2010, after which data were loaded directly to a Web‐based data‐tracking platform. Sites were asked to load data as they became available. Sites were asked to report the number of study unit discharges as well as the number of those discharges readmitted within 30 days; however, reporting of the number of patient discharges was inconsistent across sites. Serial outreach consisting of monthly phone calls or email messaging to site leaders was conducted throughout 2011 to increase site participation in the project analysis.
Implementation date information was collected from 2 sources. The first was through online surveys distributed in November 2009 and April 2011. The second was through fields in the Web‐based data tracking platform to which sites uploaded data. In cases where disagreement was found between these 2 sources, the site leader was contacted for clarification.
Practice setting (community teaching, community nonteaching, academic medical center) was determined by site‐leader report within the Web‐based data tracking platform. Data for hospital characteristics (number of licensed beds and geographic region) were obtained from the American Hospital Association's Annual Survey of Hospitals.[18] Hospital region was characterized as West, South, Midwest, or Northeast.
Analysis
The null hypothesis was that no prepost difference existed in readmission rates within BOOST units, and no difference existed in the prepost change in readmission rates in BOOST units when compared to site‐matched control units. The Wilcoxon rank sum test was used to test whether observed changes described above were significantly different from 0, supporting rejection of the null hypotheses. We performed similar tests to determine the significance of observed changes in length of stay. We performed our analysis using SAS 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Eleven hospitals provided rehospitalization and length‐of‐stay outcome data for both a BOOST and control unit for the pre‐ and postimplementation periods. Compared to the 19 sites that did not participate in the analysis, these 11 sites were significantly larger (559188 beds vs 350205 beds, P=0.003), more likely to be located in an urban area (100.0% [n=11] vs 78.9% [n=15], P=0.035), and more likely to be academic medical centers (45.5% [n=5] vs 26.3% [n=5], P=0.036) (Table 1).
The mean number of tools implemented by sites participating in the analysis was 3.50.9. All sites implemented at least 2 tools. The duration between attendance at the BOOST kickoff event and first tool implementation ranged from 3 months (first tool implemented prior to attending the kickoff) and 9 months (mean duration, 3.34.3 months) (Table 2).
| Hospital | Region | Hospital Type | No. Licensed Beds | Kickoff Implementationa | Risk Assessment | Discharge Checklist | Teach Back | Discharge Summary Completion | Follow‐up Phone Call | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
| 1 | Midwest | Community teaching | <300 | 8 | 3 | |||||
| 2 | West | Community teaching | >600 | 0 | 4 | |||||
| 3 | Northeast | Academic medical center | >600 | 2 | 4 | |||||
| 4 | Northeast | Community nonteaching | <300 | 9 | 2 | |||||
| 5 | South | Community nonteaching | >600 | 6 | 3 | |||||
| 6 | South | Community nonteaching | >600 | 3 | 4 | |||||
| 7 | Midwest | Community teaching | 300600 | 1 | 5 | |||||
| 8 | West | Academic medical center | 300600 | 1 | 4 | |||||
| 9 | South | Academic medical center | >600 | 4 | 4 | |||||
| 10 | Midwest | Academic medical center | 300600 | 3 | 3 | |||||
| 11 | Midwest | Academic medical center | >600 | 9 | 2 | |||||
The average rate of 30‐day rehospitalization among BOOST units was 14.7% in the preimplementation period and 12.7% during the postimplementation period (P=0.010) (Figure 1). Rehospitalization rates for matched control units were 14.0% in the preintervention period and 14.1% in the postintervention period (P=0.831). The mean absolute reduction in readmission rates over the 1‐year study period in BOOST units compared to control units was 2.0%, or a relative reduction of 13.6% (P=0.054 for signed rank test comparing differences in readmission rate reduction in BOOST units compared to site‐matched control units). Length of stay in BOOST and control units decreased an average of 0.5 days and 0.3 days, respectively. There was no difference in length of stay change between BOOST units and control units (P=0.966).

DISCUSSION
As hospitals strive to reduce their readmission rates to avoid Centers for Medicare and Medicaid Services penalties, Project BOOST may be a viable QI approach to achieve their goals. This initial evaluation of participation in Project BOOST by 11 hospitals of varying sizes across the United States showed an associated reduction in rehospitalization rates (absolute=2.0% and relative=13.6%, P=0.054). We did not find any significant change in length of stay among these hospitals implementing BOOST tools.
The tools provided to participating hospitals were developed from evidence found in peer‐reviewed literature established through experimental methods in well‐controlled academic settings. Further tool development was informed by recommendations of an advisory board consisting of expert representatives and advocates involved in the hospital discharge process: patients, caregivers, physicians, nurses, case managers, social workers, insurers, and regulatory and research agencies.[19] The toolkit components address multiple aspects of hospital discharge and follow‐up with the goal of improving health by optimizing the safety of care transitions. Our observation that readmission rates appeared to improve in a diverse hospital sample including nonacademic and community hospitals engaged in Project BOOST is reassuring that the benefits seen in existing research literature, developed in distinctly academic settings, can be replicated in diverse acute‐care settings.
The effect size observed in our study was modest but consistent with several studies identified in a recent review of trials measuring interventions to reduce rehospitalization, where 7 of 16 studies showing a significant improvement registered change in the 0% to 5% absolute range.[12] Impact of this project may have been tempered by the need to translate external QI content to the local setting. Additionally, in contrast to experimental studies that are limited in scope and timing and often scaled to a research budget, BOOST sites were encouraged to implement Project BOOST in the clinical setting even if no new funds were available to support the effort.[12]
The recruitment of a national sample of both academic and nonacademic hospital participants imposed several limitations on our study and analysis. We recognize that intervention units selected by hospitals may have had unmeasured unit and patient characteristics that facilitated successful change and contributed to the observed improvements. However, because external pressure to reduce readmission is present across all hospitals independent of the BOOST intervention, we felt site‐matched controls were essential to understanding effects attributable to the BOOST tools. Differences between units would be expected to be stable over the course of the study period, and comparison of outcome differences between 2 different time periods would be reasonable. Additionally, we could not collect data on readmissions to other hospitals. Theoretically, patients discharged from BOOST units might be more likely to have been rehospitalized elsewhere, but the fraction of rehospitalizations occurring at alternate facilities would also be expected to be similar on the matched control unit.
We report findings from a voluntary cohort willing and capable of designating a comparison clinical unit and contributing the requested data outcomes. Pilot sites that did not report outcomes were not analyzed, but comparison of hospital characteristics shows that participating hospitals were more likely to be large, urban, academic medical centers. Although barriers to data submission were not formally analyzed, reports from nonparticipating sites describe data submission limited by local implementation design (no geographic rollout or simultaneous rollout on all appropriate clinical units), site specific inability to generate unit level outcome statistics, and competing organizational priorities for data analyst time (electronic medical record deployment, alternative QI initiatives). The external validity of our results may be limited to organizations capable of analytics at the level of the individual clinical unit as well as those with sufficient QI resources to support reporting to a national database in the absence of a payer mandate. It is possible that additional financial support for on‐site data collection would have bolstered participation, making the example of participation rates we present potentially informative to organizations hoping to widely disseminate a QI agenda.
Nonetheless, the effectiveness demonstrated in the 11 sites that did participate is encouraging, and ongoing collaboration with subsequent BOOST cohorts has been designed to further facilitate data collection. Among the insights gained from this pilot experience, and incorporated into ongoing BOOST cohorts, is the importance of intensive mentor engagement to foster accountability among participant sites, assist with implementation troubleshooting, and offer expertise that is often particularly effective in gaining local support. We now encourage sites to have 2 mentor site visits to further these roles and more frequent conference calls. Further research to understand the marginal benefit of the mentored implementation approach is ongoing.
The limitations in data submission we experienced with the pilot cohort likely reflect resource constraints not uncommon at many hospitals. Increasing pressure placed on hospitals as a result of the Readmission Reduction Program within the Affordable Care Act as well as increasing interest from private and Medicaid payors to incorporate similar readmission‐based penalties provide encouragement for hospitals to enhance their data and analytic skills. National incentives for implementation of electronic health records (EHR) should also foster such capabilities, though we often saw EHRs as a barrier to QI, especially rapid cycle trials. Fortunately, hospitals are increasingly being afforded access to comprehensive claims databases to assist in tracking readmission rates to other facilities, and these data are becoming available in a more timely fashion. This more robust data collection, facilitated by private payors, state QI organizations, and state hospital associations, will support additional analytic methods such as multivariate regression models and interrupted time series designs to appreciate the experience of current BOOST participants.
Additional research is needed to understand the role of organizational context in the effectiveness of Project BOOST. Differences in rates of tool implementation and changes in clinical outcomes are likely dependent on local implementation context at the level of the healthcare organization and individual clinical unit.[20] Progress reports from site mentors and previously described experiences of QI implementation indicate that successful implementation of a multidimensional bundle of interventions may have reflected a higher level of institutional support, more robust team engagement in the work of reducing readmissions, increased clinical staff support for change, the presence of an effective project champion, or a key facilitating role of external mentorship.[21, 22] Ongoing data collection will continue to measure the sustainability of tool use and observed outcome changes to inform strategies to maintain gains associated with implementation. The role of mentored implementation in facilitating gains also requires further study.
Increasing attention to the problem of avoidable rehospitalization is driving hospitals, insurers, and policy makers to pursue QI efforts that favorably impact readmission rates. Our analysis of the BOOST intervention suggests that modest gains can be achieved following evidence‐based hospital process change facilitated by a mentored implementation model. However, realization of the goal of a 20% reduction in rehospitalization proposed by the Center for Medicare and Medicaid Services' Partnership for Patients initiative may be difficult to achieve on a national scale,[23] especially if efforts focus on just the hospital.
Acknowledgments
The authors acknowledge the contributions of Amanda Creden, BA (data collection), Julia Lee (biostatistical support), and the support of Amy Berman, BS, RN, Senior Program Officer at The John A. Hartford Foundation.
Disclosures
Project BOOST was funded by a grant from The John A. Hartford Foundation. Project BOOST is administered by the Society of Hospital Medicine (SHM). The development of the Project BOOST toolkit, recruitment of sites for this study, mentorship of the pilot cohort, project evaluation planning, and collection of pilot data were funded by a grant from The John A. Harford Foundation. Additional funding for continued data collection and analysis was funded by the SHM through funds from hospitals to participate in Project BOOST, specifically with funding support for Dr. Hansen. Dr. Williams has received funding to serve as Principal Investigator for Project BOOST. Since the time of initial cohort participation, approximately 125 additional hospitals have participated in the mentored implementation of Project BOOST. This participation was funded through a combination of site‐based tuition, third‐party payor support from private insurers, foundations, and federal funding through the Center for Medicare and Medicaid Innovation Partnership for Patients program. Drs. Greenwald, Hansen, and Williams are Project BOOST mentors for current Project BOOST sites and receive financial support through the SHM for this work. Dr. Howell has previously received funding as a Project BOOST mentor. Ms. Budnitz is the BOOST Project Director and is Chief Strategy and Development Officer for the HM. Dr. Maynard is the Senior Vice President of the SHM's Center for Hospital Innovation and Improvement.
References
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- United States Congress. House Committee on Education and Labor. Coe on Ways and Means, Committee on Energy and Commerce, Compilation of Patient Protection and Affordable Care Act: as amended through November 1, 2010 including Patient Protection and Affordable Care Act health‐related portions of the Health Care and Education Reconciliation Act of 2010. Washington, DC: US Government Printing Office; 2010.
- Cost estimate for the amendment in the nature of a substitute to H.R. 3590, as proposed in the Senate on November 18, 2009. Washington, DC: Congressional Budget Office; 2009.
- Partnership for Patients, Center for Medicare and Medicaid Innovation. Available at: http://www.innovations.cms.gov/initiatives/Partnership‐for‐Patients/index.html. Accessed December 12, 2012.
- , . Providers have failed to work for continuity. Hospitals. 1979;53(10):79.
- , . Executing high‐quality care transitions: a call to do it right. J Hosp Med. 2007;2(5):287–290.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- , , . Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians. JAMA. 2007;297(8):831–841.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178.
- , , , et al. Advancing the science of patient safety. Ann Intern Med. 2011;154(10):693–696.
- , . From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230.
- , , , et al. Quality improvement projects targeting health care‐associated infections: comparing virtual collaborative and toolkit approaches. J Hosp Med. 2011;6(5):271–278.
- , , , , . Publication guidelines for improvement studies in health care: evolution of the SQUIRE project. Ann Intern Med. 2008;149(9):670–676.
- , , , . Risk stratification and therapeutic decision making in acute coronary syndromes. JAMA. 2000;284(7):876–878.
- , , . Are diagnosis specific outcome indicators based on administrative data useful in assessing quality of hospital care? BMJ. 2004;13(1):32.
- , , , et al. What distinguishes top‐performing hospitals in acute myocardial infarction mortality rates? Ann Intern Med. 2011;154(6):384–390.
- , , , . The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13–20.
- , . Evidence‐based quality improvement: the state of the science. Health Aff (Millwood). 2005;24(1):138–150.
- Center for Medicare and Medicaid Innovation. Partnership for patients. Available at: http://www.innovations.cms.gov/initiatives/Partnership‐for‐Patients/index.html. Accessed April 2, 2012.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- United States Congress. House Committee on Education and Labor. Coe on Ways and Means, Committee on Energy and Commerce, Compilation of Patient Protection and Affordable Care Act: as amended through November 1, 2010 including Patient Protection and Affordable Care Act health‐related portions of the Health Care and Education Reconciliation Act of 2010. Washington, DC: US Government Printing Office; 2010.
- Cost estimate for the amendment in the nature of a substitute to H.R. 3590, as proposed in the Senate on November 18, 2009. Washington, DC: Congressional Budget Office; 2009.
- Partnership for Patients, Center for Medicare and Medicaid Innovation. Available at: http://www.innovations.cms.gov/initiatives/Partnership‐for‐Patients/index.html. Accessed December 12, 2012.
- , . Providers have failed to work for continuity. Hospitals. 1979;53(10):79.
- , . Executing high‐quality care transitions: a call to do it right. J Hosp Med. 2007;2(5):287–290.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- , , . Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians. JAMA. 2007;297(8):831–841.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178.
- , , , et al. Advancing the science of patient safety. Ann Intern Med. 2011;154(10):693–696.
- , . From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230.
- , , , et al. Quality improvement projects targeting health care‐associated infections: comparing virtual collaborative and toolkit approaches. J Hosp Med. 2011;6(5):271–278.
- , , , , . Publication guidelines for improvement studies in health care: evolution of the SQUIRE project. Ann Intern Med. 2008;149(9):670–676.
- , , , . Risk stratification and therapeutic decision making in acute coronary syndromes. JAMA. 2000;284(7):876–878.
- , , . Are diagnosis specific outcome indicators based on administrative data useful in assessing quality of hospital care? BMJ. 2004;13(1):32.
- , , , et al. What distinguishes top‐performing hospitals in acute myocardial infarction mortality rates? Ann Intern Med. 2011;154(6):384–390.
- , , , . The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13–20.
- , . Evidence‐based quality improvement: the state of the science. Health Aff (Millwood). 2005;24(1):138–150.
- Center for Medicare and Medicaid Innovation. Partnership for patients. Available at: http://www.innovations.cms.gov/initiatives/Partnership‐for‐Patients/index.html. Accessed April 2, 2012.
Copyright © 2013 Society of Hospital Medicine
Hospitalist Communication Training
Hospital settings present unique challenges to patient‐clinician communication and collaboration. Patients frequently have multiple, active conditions. Interprofessional teams are large and care for multiple patients at the same time, and team membership is dynamic and dispersed. Moreover, physicians spend relatively little time with patients[1, 2] and seldom receive training in communication skills after medical school.
The Agency for Healthcare Research and Quality (AHRQ) has developed the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey to assess hospitalized patients' experiences with care.[3, 4, 5] Results are publicly reported on the US Department of Health and Human Services Hospital Compare Web site[6] and now affect hospital payment through the Center for Medicare and Medicaid Services Hospital Value‐Based Purchasing Program.[7]
Despite this increased transparency and accountability for performance related to the patient experience, little research has been conducted on how hospitals or clinicians might improve performance. Although interventions to enhance physician communication skills have shown improvements in observed behaviors, few studies have assessed benefit from the patient's perspective and few interventions have been integrated into practice.[8] We sought to assess the impact of a communication‐skills training program, based on a common framework used by hospitals, on patient satisfaction with doctor communication and overall hospital care.
METHODS
Setting and Study Design
The study was conducted at Northwestern Memorial Hospital (NMH), an 897‐bed tertiary‐care teaching hospital in Chicago, IL, and was approved by the institutional review board of Northwestern University. This study was a preintervention vs postintervention comparison of patient‐satisfaction scores. The intervention was a communication‐skills training program for all NMH hospitalists. We compared patient‐satisfaction survey data for patients admitted to the nonteaching hospitalist service during the 26 weeks prior to the intervention with data for patients admitted to the same service during the 22 weeks afterward. Hospitalists on this service worked 7 consecutive days, usually followed by 7 days free from clinical duty. Hospitalists cared for approximately 1014 patients per day without the assistance of resident physicians or midlevel providers (ie, physician assistants or nurse practitioners). Nighttime patient care was provided by in‐house hospitalists (ie, nocturnists). A majority of nighttime shifts were staffed by physicians who worked for the group for a single year. As a result of a prior intervention, hospitalists' patients were localized to specific units, each overseen by a hospitalist‐unit medical director.[9] We excluded all patients initially admitted to other services (eg, intensive care unit, surgical services) and patients discharged from other services.
Hospitalist Communication Skills Training Program
Northwestern Memorial Hospital implemented a communication‐skills training program in 2009 intended to enhance patient experience and improve patient‐satisfaction scores. All nonphysician staff were required to attend a 4‐hour training session based on the AIDET (Acknowledge, Introduce, Duration, Explanation, and Thank You) principles developed by the Studer Group.[10] The Studer Group is a well‐known healthcare consulting firm that aims to assist healthcare organizations to improve clinical, operational, and financial outcomes. The acronym AIDET provides a framework for communication‐skills behaviors (Table 1).
| AIDET Element | Explanation | Examples |
|---|---|---|
| ||
| Acknowledge | Use appropriate greeting, smile, and make eye contact. | Knock on patient's door. Hello, may I come in now? |
| Respect privacy: Knock and ask for permission before entering. Use curtains/doors appropriately. | Good morning. Is it a good time to talk? | |
| Position yourself on the same level as the patient. | Who do you have here with you today? | |
| Do not ignore others in the room (visitors or colleagues). | ||
| Introduce | Introduce yourself by name and role. | My name is Dr. Smith and I am your hospitalist physician. I'll be taking care of you while you are in the hospital. |
| Introduce any accompanying members of your team. | When on teaching service: I'm the supervising physician or I'm the physician in charge of your care. | |
| Address patients by title and last name (eg, Mrs. Smith) unless given permission to use first name. | ||
| Explain why you are there. | ||
| Do not assume patients remember your name or role. | ||
| Duration | Provide specific information on when you will be available, or when you will be back. | I'll be back between 2 and 3 pm, so if you think of any additional questions I can answer them then. |
| For tests/procedures: Explain how long it will take. Provide a time range for when it will happen. | In my experience, the test I am ordering for you will be done within the next 12 to 24 hours. | |
| Provide updates to the patient if the expected wait time has changed. | I should have the results for this test when I see you tomorrow morning. | |
| Do not blame another department or staff for delays. | ||
| Explanation | Explain your rationale for decisions. | I have ordered this test because |
| Use terms the patient can understand. | The possible side effects of this medication include | |
| Explain next steps/summarize plan for the day. | What questions do you have? | |
| Confirm understanding using teach back. | What are you most concerned about? | |
| Assume patients have questions and/or concerns. | I want to make sure you understood everything. Can you tell me in your own words what you will need to do once you are at home? | |
| Do not use acronyms that patients may not understand (eg, PRN, IR, ICU). | ||
| Thank you | Thank the patient and/or family. | I really appreciate you telling me about your symptoms. I know you told several people before. |
| Ask if there is anything else you can do for the patient. | Thank you for giving me the opportunity to care for you. What else can I do for you today? | |
| Explain when you will be back and how the patient can reach you if needed. | I'll see you again tomorrow morning. If you need me before then, just ask the nurse to page me. | |
| Do not appear rushed or distracted when ending your interaction. | ||
We adapted the AIDET framework and designed a communication‐skills training program, specifically for physicians, to emphasize reflection on current communication behaviors, deliberate practice of enhanced communication skills, and feedback based on performance during simulated and real clinical encounters. These educational methods are consistent with recommended strategies to improve behavioral performance.[11] During the first session, we discussed measurement of patient satisfaction, introduced AIDET principles, gave examples of specific behaviors for each principle, and had participants view 2 short videos displaying a range of communication skills followed by facilitated debriefing.[12] The second session included 3 simulation‐based exercises. Participants rotated roles in the scenarios (eg, patient, family member, physician) and facilitated debriefing was co‐led by a hospitalist leader (K.J.O.) and a patient‐experience administrative leader (either T.D. or J.R.). The third session involved direct observation of participants' clinical encounters and immediate feedback. This coaching session was performed for an initial group of 5 hospitalist‐unit medical directors by the manager of patient experience (T.D.) and subsequently by these medical directors for the remaining participants in the program. Each of the 3 sessions lasted 90 minutes. Instructional materials are available from the authors upon request.
The communication‐skills training program began in August 2011 and extended through January 2012. Participation was strongly encouraged but not mandatory. Sessions were offered multiple times to accommodate clinical schedules. One of the co‐investigators took attendance at each session to assess participation rates.
Survey Instruments and Data
During the study period, NMH used a third‐party vendor, Press Ganey Associates, Inc., to administer the HCAHPS survey to a random sample of 40% of hospitalized patients between 48 hours and 6 weeks after discharge. The HCAHPS survey has 27 total questions, including 3 questions assessing doctor communication as a domain.[3] In addition to the HCAHPS questions, the survey administered to NMH patients included questions developed by Press Ganey. Questions in the surveys used ordinal response scales. Specifically, response options for HCAHPS doctor‐communication questions were never, sometimes, usually, and always. Response options for Press Ganey doctor‐communication questions were very poor, poor, fair, good, and very good. Patients provided an overall hospital rating in the HCAHPS survey using a 010 scale, with 0=worst hospital possible and 10=best hospital possible.
We defined the preintervention period as the 26 weeks prior to implementation of the communication‐skills program (patients admitted on or between January 31, 2011, and July 31, 2011) and the postintervention period as the 22 weeks after implementation (patients admitted on or between January 31, 2012, and June 30, 2012). The postintervention period was 1 month shorter than the preintervention period in an effort to avoid confounding due to a number of new hospitalists starting in July 2012. We defined a discharge attending as highly trained if he/she attended all 3 sessions of the communication‐skills training program. The discharge attending was designated as no/low training if he/she attended fewer than the full 3 sessions.
Data Analysis
Data were obtained from the Northwestern Medicine Enterprise Data Warehouse, a single, integrated database of all clinical and research data from all patients receiving treatment through Northwestern University healthcare affiliates. We used 2 and Student t tests to compare patient demographic characteristics preintervention vs postintervention. We used 2 tests to compare the percentage of patients giving top‐box ratings to each doctor‐communication question (ie, always for HCAHPS and very good for Press Ganey) and giving an overall hospital rating of 9 or 10. We used top‐box comparisons, rather than comparison of mean or median scores, because patient‐satisfaction data are typically highly skewed toward favorable responses. This approach is consistent with prior HCAHPS research.[4, 5] We calculated composite doctor‐communication scores as the proportion of top‐box responses across items in each survey (ie, HCAHPS and Press Ganey). We first compared all patients during the preintervention and postintervention period. We then identified patients for whom the discharge attending worked as a hospitalist at NMH during both the preintervention and postintervention periods and compared satisfaction for patients discharged by hospitalists who had no/low training and for patients discharged by hospitalists who were highly trained. We performed multivariate logistic regression, using intervention period as the predictor variable and top‐box rating as the outcome variable for each doctor‐communication question and for overall hospital rating of 9 or 10. Covariates included patient age, sex, race, payer, self‐reported education level, and self‐reported health status. Models accounted for clustering of patients within discharge physicians. Similarly, we conducted multivariate logistic regression, using discharge attending category as the predictor variable (no/low training vs highly trained). The various comparisons described were intended to mimic intention to treat and treatment received analyses in light of incomplete participation in the communication‐skills program. All analyses were conducted using Stata version 11.2 (StataCorp, College Station, TX).
RESULTS
Overall, 61 (97%) of 63 hospitalists completed the first session, 44 (70%) completed the second session, and 25 (40%) completed the third session of program. Patient‐satisfaction data were available for 278 patients during the preintervention period and 186 patients during the postintervention period. Patient demographic characteristics were similar for the 2 periods (Table 2).
| Characteristic | Preintervention (n=278) | Postintervention (n=186) | P Value |
|---|---|---|---|
| |||
| Mean age, y (SD) | 62.8 (17.0) | 61.6 (17.6) | 0.45 |
| Female, no. (%) | 155 (55.8) | 114 (61.3) | 0.24 |
| Nonwhite race, no. (%) | 87 (32.2) | 53 (29.1) | 0.48 |
| Highest education completed, no. (%) | |||
| Did not complete high school | 12 (4.6) | 6 (3.3) | 0.45 |
| High school | 110 (41.7) | 81 (44.0) | |
| 4‐year college | 50 (18.9) | 43 (23.4) | |
| Advanced degree | 92 (34.9) | 54 (29.4) | |
| Payer, no. (%) | |||
| Medicare | 137 (49.3) | 89 (47.9) | 0.83 |
| Private | 113 (40.7) | 73 (39.3) | |
| Medicaid | 13 (4.7) | 11 (5.9) | |
| Self‐pay/other | 15 (5.4) | 13 (7.0) | |
| Self‐reported health status, no. (%) | |||
| Poor | 19 (7.1) | 18 (9.8) | 0.41 |
| Fair | 53 (19.7) | 43 (23.4) | |
| Good | 89 (33.1) | 57 (31.0) | |
| Very good | 89 (33.1) | 49 (26.6) | |
| Excellent | 19 (7.1) | 17 (9.2) | |
Patient Satisfaction With Hospitalist Communication
The HCAHPS and Press Ganey doctor communication domain scores were not significantly different between the preintervention and postintervention periods (75.8 vs 79.2, P=0.42 and 61.4 vs 65.9, P=0.39). Two of the 3 HCAHPS items assessing doctor communication were rated higher during the postintervention period, but no result was statistically significant (Table 3). Similarly, all 5 of the Press Ganey items assessing doctor communication were rated higher during the postintervention period, but no result was statistically significant. The HCAHPS overall rating of hospital care was also not significantly different between the preintervention and postintervention period. Results were similar in multivariate analyses, with no items showing statistically significant differences between the preintervention and postintervention periods.
| Unadjusted Analysisa | Adjusted Analysis | ||||
|---|---|---|---|---|---|
| Preintervention, No. (%) [n=270277] | Postintervention, No. (%) [n=183186] | P Value | OR (95% CI) | P Value | |
| |||||
| HCAHPS doctor‐communication domain | |||||
| How often did doctors treat you with courtesy and respect? | 224 (83) | 160 (86) | 0.31 | 1.23 (0.81‐2.44) | 0.22 |
| How often did doctors listen carefully to you? | 205 (75) | 145 (78) | 0.52 | 1.22 (0.74‐2.04) | 0.42 |
| How often did doctors explain things in a way you could understand? | 203 (75) | 137 (74) | 0.84 | 0.98 (0.59‐1.64) | 0.94 |
| Press Ganey physician‐communication domain | |||||
| Skill of physician | 189 (68) | 137 (74) | 0.19 | 1.38 (0.82‐2.31) | 0.22 |
| Physician's concern for your questions and worries | 157 (57) | 117 (64) | 0.14 | 1.30 (0.79‐2.12) | 0.30 |
| How well physician kept you informed | 158 (58) | 114 (62) | 0.36 | 1.15 (0.78‐1.72) | 0.71 |
| Time physician spent with you | 140 (51) | 101 (54) | 0.43 | 1.12 (0.66‐1.89) | 0.67 |
| Friendliness/courtesy of physician | 198 (71) | 136 (74) | 0.57 | 1.20 (0.74‐1.94) | 0.46 |
| HCAHPS global ratings | |||||
| Overall rating of hospital | 189 (70) [n=270] | 137 (74) [n=186] | 0.40 | 1.33 (0.82‐2.17) | 0.24 |
Pre‐post comparisons based on level of hospitalist participation in the training program are shown in Table 4. For patients discharged by no/low‐training hospitalists, 4 of the 8 total items assessing doctor communication were rated higher during the postintervention period, and 4 were rated lower, but no result was statistically significant. For patients discharged by highly trained hospitalists, all 8 items assessing doctor communication were rated higher during the postintervention period, but no result was statistically significant. Multivariate analyses were similar, with no items showing statistically significant differences between the preintervention and postintervention periods for either group.
| No/Low Training | Highly Trained | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted Analysisa | Adjusted Analysis | Unadjusted Analysisa | Adjusted Analysis | |||||||
| Preintervention, No. (%) [n=151156] | Postintervention, No. (%) [n=6770] | P Value | OR (95% CI) | P Value | Preintervention, No. (%) [n=119122] | Postintervention, No. (%) [n=115116] | P Value | OR (95% CI) | P Value | |
| ||||||||||
| HCAHPS doctor‐ communication domain | ||||||||||
| How often did doctors treat you with courtesy and respect? | 125 (83) | 61 (88) | 0.28 | 1.79 (0.82‐3.89) | 0.14 | 99 (83) | 99 (85) | 0.65 | 1.33 (0.62‐2.91) | 0.46 |
| How often did doctors listen carefully to you? | 116 (77) | 53 (76) | 0.86 | 1.08 (0.49‐2.38) | 0.19 | 89 (74) | 92 (79) | 0.30 | 1.43 (0.76‐2.69) | 0.27 |
| How often did doctors explain things in a way you could understand? | 115 (76) | 47 (68) | 0.24 | 0.59 (0.27‐1.28) | 0.18 | 88 (74) | 90 (78) | 0.52 | 1.31 (0.68‐2.50) | 0.42 |
| Press Ganey physician‐communication domain | ||||||||||
| Skill of physician | 110 (71) | 52 (74) | 0.56 | 1.32 (0.78‐2.22) | 0.31 | 79 (65) | 85 (73) | 0.16 | 1.45 (0.65‐3.27) | 0.37 |
| Physician's concern for your questions and worries | 92 (60) | 41 (61) | 0.88 | 1.00 (0.59‐1.77) | 0.99 | 65 (53) | 76 (66) | 0.06 | 1.71 (0.81‐3.60) | 0.16 |
| How well physician kept you informed | 89 (59) | 42 (61) | 0.75 | 1.16 (0.64‐2.08) | 0.62 | 69 (57) | 72 (63) | 0.34 | 1.29 (0.75‐2.20) | 0.35 |
| Time physician spent with you | 83 (54) | 37 (53) | 0.92 | 0.87 (0.47‐1.61) | 0.65 | 57 (47) | 64 (55) | 0.19 | 1.44 (0.64‐3.21) | 0.38 |
| Friendliness/courtesy of physician | 116 (75) | 45 (66) | 0.18 | 0.72 (0.37‐1.38) | 0.32 | 82 (67) | 91 (78) | 0.05 | 1.89 (0.97‐3.68) | 0.60 |
| HCAHPS global ratings | ||||||||||
| Overall rating of hospital | 109 (73) | 53 (75) | 0.63 | 1.37 (0.67‐2.81) | 0.39 | 86 (71) | 90 (78) | 0.21 | 1.60 (0.73‐3.53) | 0.24 |
DISCUSSION
We found no significant improvement in patient satisfaction with doctor communication or overall rating of hospital care after implementation of a communication‐skills training program for hospitalists. There are several potential explanations for our results. First, though we used sound educational methods and attempted to replicate common clinical scenarios during simulation exercises, our program may not have resulted in improved communication behaviors during actual clinical care. We attempted to balance instructional methods that would result in behavioral change with a feasible investment of time and effort on the part of our learners (ie, practicing hospitalists). It is possible that additional time, feedback, and practice of communication skills would be necessary to change behaviors in the clinical setting. However, prior communication‐skills interventions have similarly struggled to show an impact on patient satisfaction.[13, 14] Second, we had incomplete participation in the program, with only 40% of hospitalists completing all 3 planned sessions. We encouraged all hospitalists, regardless of job type, to participate in the program. Participation rates were lower for 1‐year hospitalists compared with career hospitalists. The results of our analyses based on level of hospitalist participation in the training program, although not achieving statistical significance, suggest a greater effect of the program with higher degrees of participation.
Most important, the study was likely underpowered to detect a statistically significant difference in satisfaction results. Leaders were committed to providing communication‐skills training throughout our organization. We did not know the magnitude of potential improvement in satisfaction scores that might arise from our efforts, and therefore we did not conduct power calculations before designing and implementing the training program. Our HCAHPS composite doctor‐communication domain performance was 76% during the preintervention period and 79% during the postintervention period. Assuming an absolute 3% improvement is indeed possible, we would have needed >3000 patients in each period to have 80% power to detect a significant difference. Similarly, we would have needed >2000 patients during each period to have 80% power to detect an absolute 4% improvement in global rating of hospital care.
In an attempt to discern whether our favorable results were due to secular trends, we conducted post hoc analyses of HCAHPS nurse‐communication and hospital‐environment domains for the preintervention vs postintervention periods. Two of the 3 nurse‐communication items were rated lower during the postintervention period, but no result was statistically significant. Both hospital‐environment domain items were rated lower during the postintervention period, and 1 result was statistically significant (quiet at night). This post hoc evaluation lends additional support to the potential benefit of the communication‐skills training program.
The findings from this study represent an important issue for leaders attempting to improve quality performance within their organizations. What level of proof is needed before investing time and effort in implementing an intervention? With mounting pressure to improve performance, leaders are often left to make informed decisions based on data that fall short of scientifically rigorous evidence. Importantly, an increase in composite doctor‐communication ratings from 76% to 79% would translate into an improvement from the 25th percentile to 50th‐percentile performance in the fiscal‐year 2011 Press Ganey University Healthcare Consortium benchmark comparison (based on surveys received from September 1, 2010, to August 31, 2011).[15]
Our study has several limitations. First, we assessed an intervention on a single service in a single hospital. Generalizability may be limited, as hospital medicine groups, hospitals, and the patients they serve vary. Second, our intervention was based on a framework (ie, AIDET) that has face validity but has not undergone extensive study to confirm that the underlying constructs, and the behaviors related to them, are tightly linked to patient satisfaction. Third, as previously mentioned, we were likely underpowered to detect a significant improvement in satisfaction resulting from our intervention. Incomplete participation in the training program may have also limited the effect of our intervention. Finally, our comparisons by hospitalist level of participation were based on the discharging physician. Attribution of a patient response to a single physician is problematic because many patients encounter more than 1 hospitalist and 1 or more specialist physicians during their stay.
CONCLUSION
In summary, we found improvements in patient satisfaction with doctor communication, which were not statistically significant, after implementation of a communication‐skills training program for hospitalists. Larger studies are needed to assess whether a communication‐skills training program can truly improve patient satisfaction with doctor communication and overall hospital care.
Acknowledgments
The authors express their gratitude to the hospitalists involved in this program, especially Eric Schaefer, Nita Kulkarni, Stevie Mazyck, Rachel Cyrus, and Hiren Shah. The authors also thank Nicholas Christensen for assistance in data acquisition.
Disclosures: Nothing to report.
- , , , , , . Four minutes for a patient, twenty seconds for a relative—an observational study at a university hospital. BMC Health Serv Res. 2010;10:94.
- , , . How hospitalists spend their time: insights on efficiency and safety. J Hosp Med. 2006;1(2):88–93.
- CAHPS: Surveys and Tools to Advance Patient‐Centered Care. Available at: http://cahps.ahrq.gov. Accessed July 12, 2012.
- , , , , . Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27–37.
- , , , , . Measuring hospital care from the patients' perspective: an overview of the CAHPS Hospital Survey development process. Health Serv Res. 2005;40(6 part 2):1977–1995.
- US Department of Health and Human Services. Hospital Compare. Available at: http://hospitalcompare.hhs.gov/. Accessed November 5, 2012.
- Center for Medicare and Medicaid Services. Hospital Value Based Purchasing Program. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/hospital‐value‐based‐purchasing/index.html?redirect=/hospital‐value‐based‐purchasing. Accessed August 1, 2012.
- , , , . Communication interventions make a difference in conversations between physicians and patients: a systematic review of the evidence. Med Care. 2007;45(4):340–349.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- Studer Group. Acknowledge, Introduce, Duration, Explanation and Thank You. Available at: http://www.studergroup.com/aidet. Accessed November 5, 2012.
- Kern DE, Thomas PA, Bass EB, Howard DM, eds. Curriculum Development for Medical Education: A Six‐Step Approach. Baltimore, MD: Johns Hopkins University Press; 1998.
- Vanderbilt University Medical Center and Studer Group. Building Patient Trust with AIDET®: Clinical Excellence with Patient Compliance Through Effective Communication. Gulf Breeze, FL: Fire Starter Publishing; 2008.
- , , , . Effect of clinician communication skills training on patient satisfaction: a randomized, controlled trial. Ann Intern Med. 1999;131(11):822–829.
- , , , , , . Effectiveness of a short course in clinical communication skills for hospital doctors: results of a crossover randomized controlled trial (ISRCTN22153332). Patient Educ Couns. 2010;84(2):163–169.
- Press Ganey HCAHPS Top Box and Rank Report, Fiscal Year 2011. Inpatient, University Healthcare Consortium Peer Group. South Bend, IN: Press Ganey Associates; 2011.
Hospital settings present unique challenges to patient‐clinician communication and collaboration. Patients frequently have multiple, active conditions. Interprofessional teams are large and care for multiple patients at the same time, and team membership is dynamic and dispersed. Moreover, physicians spend relatively little time with patients[1, 2] and seldom receive training in communication skills after medical school.
The Agency for Healthcare Research and Quality (AHRQ) has developed the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey to assess hospitalized patients' experiences with care.[3, 4, 5] Results are publicly reported on the US Department of Health and Human Services Hospital Compare Web site[6] and now affect hospital payment through the Center for Medicare and Medicaid Services Hospital Value‐Based Purchasing Program.[7]
Despite this increased transparency and accountability for performance related to the patient experience, little research has been conducted on how hospitals or clinicians might improve performance. Although interventions to enhance physician communication skills have shown improvements in observed behaviors, few studies have assessed benefit from the patient's perspective and few interventions have been integrated into practice.[8] We sought to assess the impact of a communication‐skills training program, based on a common framework used by hospitals, on patient satisfaction with doctor communication and overall hospital care.
METHODS
Setting and Study Design
The study was conducted at Northwestern Memorial Hospital (NMH), an 897‐bed tertiary‐care teaching hospital in Chicago, IL, and was approved by the institutional review board of Northwestern University. This study was a preintervention vs postintervention comparison of patient‐satisfaction scores. The intervention was a communication‐skills training program for all NMH hospitalists. We compared patient‐satisfaction survey data for patients admitted to the nonteaching hospitalist service during the 26 weeks prior to the intervention with data for patients admitted to the same service during the 22 weeks afterward. Hospitalists on this service worked 7 consecutive days, usually followed by 7 days free from clinical duty. Hospitalists cared for approximately 1014 patients per day without the assistance of resident physicians or midlevel providers (ie, physician assistants or nurse practitioners). Nighttime patient care was provided by in‐house hospitalists (ie, nocturnists). A majority of nighttime shifts were staffed by physicians who worked for the group for a single year. As a result of a prior intervention, hospitalists' patients were localized to specific units, each overseen by a hospitalist‐unit medical director.[9] We excluded all patients initially admitted to other services (eg, intensive care unit, surgical services) and patients discharged from other services.
Hospitalist Communication Skills Training Program
Northwestern Memorial Hospital implemented a communication‐skills training program in 2009 intended to enhance patient experience and improve patient‐satisfaction scores. All nonphysician staff were required to attend a 4‐hour training session based on the AIDET (Acknowledge, Introduce, Duration, Explanation, and Thank You) principles developed by the Studer Group.[10] The Studer Group is a well‐known healthcare consulting firm that aims to assist healthcare organizations to improve clinical, operational, and financial outcomes. The acronym AIDET provides a framework for communication‐skills behaviors (Table 1).
| AIDET Element | Explanation | Examples |
|---|---|---|
| ||
| Acknowledge | Use appropriate greeting, smile, and make eye contact. | Knock on patient's door. Hello, may I come in now? |
| Respect privacy: Knock and ask for permission before entering. Use curtains/doors appropriately. | Good morning. Is it a good time to talk? | |
| Position yourself on the same level as the patient. | Who do you have here with you today? | |
| Do not ignore others in the room (visitors or colleagues). | ||
| Introduce | Introduce yourself by name and role. | My name is Dr. Smith and I am your hospitalist physician. I'll be taking care of you while you are in the hospital. |
| Introduce any accompanying members of your team. | When on teaching service: I'm the supervising physician or I'm the physician in charge of your care. | |
| Address patients by title and last name (eg, Mrs. Smith) unless given permission to use first name. | ||
| Explain why you are there. | ||
| Do not assume patients remember your name or role. | ||
| Duration | Provide specific information on when you will be available, or when you will be back. | I'll be back between 2 and 3 pm, so if you think of any additional questions I can answer them then. |
| For tests/procedures: Explain how long it will take. Provide a time range for when it will happen. | In my experience, the test I am ordering for you will be done within the next 12 to 24 hours. | |
| Provide updates to the patient if the expected wait time has changed. | I should have the results for this test when I see you tomorrow morning. | |
| Do not blame another department or staff for delays. | ||
| Explanation | Explain your rationale for decisions. | I have ordered this test because |
| Use terms the patient can understand. | The possible side effects of this medication include | |
| Explain next steps/summarize plan for the day. | What questions do you have? | |
| Confirm understanding using teach back. | What are you most concerned about? | |
| Assume patients have questions and/or concerns. | I want to make sure you understood everything. Can you tell me in your own words what you will need to do once you are at home? | |
| Do not use acronyms that patients may not understand (eg, PRN, IR, ICU). | ||
| Thank you | Thank the patient and/or family. | I really appreciate you telling me about your symptoms. I know you told several people before. |
| Ask if there is anything else you can do for the patient. | Thank you for giving me the opportunity to care for you. What else can I do for you today? | |
| Explain when you will be back and how the patient can reach you if needed. | I'll see you again tomorrow morning. If you need me before then, just ask the nurse to page me. | |
| Do not appear rushed or distracted when ending your interaction. | ||
We adapted the AIDET framework and designed a communication‐skills training program, specifically for physicians, to emphasize reflection on current communication behaviors, deliberate practice of enhanced communication skills, and feedback based on performance during simulated and real clinical encounters. These educational methods are consistent with recommended strategies to improve behavioral performance.[11] During the first session, we discussed measurement of patient satisfaction, introduced AIDET principles, gave examples of specific behaviors for each principle, and had participants view 2 short videos displaying a range of communication skills followed by facilitated debriefing.[12] The second session included 3 simulation‐based exercises. Participants rotated roles in the scenarios (eg, patient, family member, physician) and facilitated debriefing was co‐led by a hospitalist leader (K.J.O.) and a patient‐experience administrative leader (either T.D. or J.R.). The third session involved direct observation of participants' clinical encounters and immediate feedback. This coaching session was performed for an initial group of 5 hospitalist‐unit medical directors by the manager of patient experience (T.D.) and subsequently by these medical directors for the remaining participants in the program. Each of the 3 sessions lasted 90 minutes. Instructional materials are available from the authors upon request.
The communication‐skills training program began in August 2011 and extended through January 2012. Participation was strongly encouraged but not mandatory. Sessions were offered multiple times to accommodate clinical schedules. One of the co‐investigators took attendance at each session to assess participation rates.
Survey Instruments and Data
During the study period, NMH used a third‐party vendor, Press Ganey Associates, Inc., to administer the HCAHPS survey to a random sample of 40% of hospitalized patients between 48 hours and 6 weeks after discharge. The HCAHPS survey has 27 total questions, including 3 questions assessing doctor communication as a domain.[3] In addition to the HCAHPS questions, the survey administered to NMH patients included questions developed by Press Ganey. Questions in the surveys used ordinal response scales. Specifically, response options for HCAHPS doctor‐communication questions were never, sometimes, usually, and always. Response options for Press Ganey doctor‐communication questions were very poor, poor, fair, good, and very good. Patients provided an overall hospital rating in the HCAHPS survey using a 010 scale, with 0=worst hospital possible and 10=best hospital possible.
We defined the preintervention period as the 26 weeks prior to implementation of the communication‐skills program (patients admitted on or between January 31, 2011, and July 31, 2011) and the postintervention period as the 22 weeks after implementation (patients admitted on or between January 31, 2012, and June 30, 2012). The postintervention period was 1 month shorter than the preintervention period in an effort to avoid confounding due to a number of new hospitalists starting in July 2012. We defined a discharge attending as highly trained if he/she attended all 3 sessions of the communication‐skills training program. The discharge attending was designated as no/low training if he/she attended fewer than the full 3 sessions.
Data Analysis
Data were obtained from the Northwestern Medicine Enterprise Data Warehouse, a single, integrated database of all clinical and research data from all patients receiving treatment through Northwestern University healthcare affiliates. We used 2 and Student t tests to compare patient demographic characteristics preintervention vs postintervention. We used 2 tests to compare the percentage of patients giving top‐box ratings to each doctor‐communication question (ie, always for HCAHPS and very good for Press Ganey) and giving an overall hospital rating of 9 or 10. We used top‐box comparisons, rather than comparison of mean or median scores, because patient‐satisfaction data are typically highly skewed toward favorable responses. This approach is consistent with prior HCAHPS research.[4, 5] We calculated composite doctor‐communication scores as the proportion of top‐box responses across items in each survey (ie, HCAHPS and Press Ganey). We first compared all patients during the preintervention and postintervention period. We then identified patients for whom the discharge attending worked as a hospitalist at NMH during both the preintervention and postintervention periods and compared satisfaction for patients discharged by hospitalists who had no/low training and for patients discharged by hospitalists who were highly trained. We performed multivariate logistic regression, using intervention period as the predictor variable and top‐box rating as the outcome variable for each doctor‐communication question and for overall hospital rating of 9 or 10. Covariates included patient age, sex, race, payer, self‐reported education level, and self‐reported health status. Models accounted for clustering of patients within discharge physicians. Similarly, we conducted multivariate logistic regression, using discharge attending category as the predictor variable (no/low training vs highly trained). The various comparisons described were intended to mimic intention to treat and treatment received analyses in light of incomplete participation in the communication‐skills program. All analyses were conducted using Stata version 11.2 (StataCorp, College Station, TX).
RESULTS
Overall, 61 (97%) of 63 hospitalists completed the first session, 44 (70%) completed the second session, and 25 (40%) completed the third session of program. Patient‐satisfaction data were available for 278 patients during the preintervention period and 186 patients during the postintervention period. Patient demographic characteristics were similar for the 2 periods (Table 2).
| Characteristic | Preintervention (n=278) | Postintervention (n=186) | P Value |
|---|---|---|---|
| |||
| Mean age, y (SD) | 62.8 (17.0) | 61.6 (17.6) | 0.45 |
| Female, no. (%) | 155 (55.8) | 114 (61.3) | 0.24 |
| Nonwhite race, no. (%) | 87 (32.2) | 53 (29.1) | 0.48 |
| Highest education completed, no. (%) | |||
| Did not complete high school | 12 (4.6) | 6 (3.3) | 0.45 |
| High school | 110 (41.7) | 81 (44.0) | |
| 4‐year college | 50 (18.9) | 43 (23.4) | |
| Advanced degree | 92 (34.9) | 54 (29.4) | |
| Payer, no. (%) | |||
| Medicare | 137 (49.3) | 89 (47.9) | 0.83 |
| Private | 113 (40.7) | 73 (39.3) | |
| Medicaid | 13 (4.7) | 11 (5.9) | |
| Self‐pay/other | 15 (5.4) | 13 (7.0) | |
| Self‐reported health status, no. (%) | |||
| Poor | 19 (7.1) | 18 (9.8) | 0.41 |
| Fair | 53 (19.7) | 43 (23.4) | |
| Good | 89 (33.1) | 57 (31.0) | |
| Very good | 89 (33.1) | 49 (26.6) | |
| Excellent | 19 (7.1) | 17 (9.2) | |
Patient Satisfaction With Hospitalist Communication
The HCAHPS and Press Ganey doctor communication domain scores were not significantly different between the preintervention and postintervention periods (75.8 vs 79.2, P=0.42 and 61.4 vs 65.9, P=0.39). Two of the 3 HCAHPS items assessing doctor communication were rated higher during the postintervention period, but no result was statistically significant (Table 3). Similarly, all 5 of the Press Ganey items assessing doctor communication were rated higher during the postintervention period, but no result was statistically significant. The HCAHPS overall rating of hospital care was also not significantly different between the preintervention and postintervention period. Results were similar in multivariate analyses, with no items showing statistically significant differences between the preintervention and postintervention periods.
| Unadjusted Analysisa | Adjusted Analysis | ||||
|---|---|---|---|---|---|
| Preintervention, No. (%) [n=270277] | Postintervention, No. (%) [n=183186] | P Value | OR (95% CI) | P Value | |
| |||||
| HCAHPS doctor‐communication domain | |||||
| How often did doctors treat you with courtesy and respect? | 224 (83) | 160 (86) | 0.31 | 1.23 (0.81‐2.44) | 0.22 |
| How often did doctors listen carefully to you? | 205 (75) | 145 (78) | 0.52 | 1.22 (0.74‐2.04) | 0.42 |
| How often did doctors explain things in a way you could understand? | 203 (75) | 137 (74) | 0.84 | 0.98 (0.59‐1.64) | 0.94 |
| Press Ganey physician‐communication domain | |||||
| Skill of physician | 189 (68) | 137 (74) | 0.19 | 1.38 (0.82‐2.31) | 0.22 |
| Physician's concern for your questions and worries | 157 (57) | 117 (64) | 0.14 | 1.30 (0.79‐2.12) | 0.30 |
| How well physician kept you informed | 158 (58) | 114 (62) | 0.36 | 1.15 (0.78‐1.72) | 0.71 |
| Time physician spent with you | 140 (51) | 101 (54) | 0.43 | 1.12 (0.66‐1.89) | 0.67 |
| Friendliness/courtesy of physician | 198 (71) | 136 (74) | 0.57 | 1.20 (0.74‐1.94) | 0.46 |
| HCAHPS global ratings | |||||
| Overall rating of hospital | 189 (70) [n=270] | 137 (74) [n=186] | 0.40 | 1.33 (0.82‐2.17) | 0.24 |
Pre‐post comparisons based on level of hospitalist participation in the training program are shown in Table 4. For patients discharged by no/low‐training hospitalists, 4 of the 8 total items assessing doctor communication were rated higher during the postintervention period, and 4 were rated lower, but no result was statistically significant. For patients discharged by highly trained hospitalists, all 8 items assessing doctor communication were rated higher during the postintervention period, but no result was statistically significant. Multivariate analyses were similar, with no items showing statistically significant differences between the preintervention and postintervention periods for either group.
| No/Low Training | Highly Trained | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted Analysisa | Adjusted Analysis | Unadjusted Analysisa | Adjusted Analysis | |||||||
| Preintervention, No. (%) [n=151156] | Postintervention, No. (%) [n=6770] | P Value | OR (95% CI) | P Value | Preintervention, No. (%) [n=119122] | Postintervention, No. (%) [n=115116] | P Value | OR (95% CI) | P Value | |
| ||||||||||
| HCAHPS doctor‐ communication domain | ||||||||||
| How often did doctors treat you with courtesy and respect? | 125 (83) | 61 (88) | 0.28 | 1.79 (0.82‐3.89) | 0.14 | 99 (83) | 99 (85) | 0.65 | 1.33 (0.62‐2.91) | 0.46 |
| How often did doctors listen carefully to you? | 116 (77) | 53 (76) | 0.86 | 1.08 (0.49‐2.38) | 0.19 | 89 (74) | 92 (79) | 0.30 | 1.43 (0.76‐2.69) | 0.27 |
| How often did doctors explain things in a way you could understand? | 115 (76) | 47 (68) | 0.24 | 0.59 (0.27‐1.28) | 0.18 | 88 (74) | 90 (78) | 0.52 | 1.31 (0.68‐2.50) | 0.42 |
| Press Ganey physician‐communication domain | ||||||||||
| Skill of physician | 110 (71) | 52 (74) | 0.56 | 1.32 (0.78‐2.22) | 0.31 | 79 (65) | 85 (73) | 0.16 | 1.45 (0.65‐3.27) | 0.37 |
| Physician's concern for your questions and worries | 92 (60) | 41 (61) | 0.88 | 1.00 (0.59‐1.77) | 0.99 | 65 (53) | 76 (66) | 0.06 | 1.71 (0.81‐3.60) | 0.16 |
| How well physician kept you informed | 89 (59) | 42 (61) | 0.75 | 1.16 (0.64‐2.08) | 0.62 | 69 (57) | 72 (63) | 0.34 | 1.29 (0.75‐2.20) | 0.35 |
| Time physician spent with you | 83 (54) | 37 (53) | 0.92 | 0.87 (0.47‐1.61) | 0.65 | 57 (47) | 64 (55) | 0.19 | 1.44 (0.64‐3.21) | 0.38 |
| Friendliness/courtesy of physician | 116 (75) | 45 (66) | 0.18 | 0.72 (0.37‐1.38) | 0.32 | 82 (67) | 91 (78) | 0.05 | 1.89 (0.97‐3.68) | 0.60 |
| HCAHPS global ratings | ||||||||||
| Overall rating of hospital | 109 (73) | 53 (75) | 0.63 | 1.37 (0.67‐2.81) | 0.39 | 86 (71) | 90 (78) | 0.21 | 1.60 (0.73‐3.53) | 0.24 |
DISCUSSION
We found no significant improvement in patient satisfaction with doctor communication or overall rating of hospital care after implementation of a communication‐skills training program for hospitalists. There are several potential explanations for our results. First, though we used sound educational methods and attempted to replicate common clinical scenarios during simulation exercises, our program may not have resulted in improved communication behaviors during actual clinical care. We attempted to balance instructional methods that would result in behavioral change with a feasible investment of time and effort on the part of our learners (ie, practicing hospitalists). It is possible that additional time, feedback, and practice of communication skills would be necessary to change behaviors in the clinical setting. However, prior communication‐skills interventions have similarly struggled to show an impact on patient satisfaction.[13, 14] Second, we had incomplete participation in the program, with only 40% of hospitalists completing all 3 planned sessions. We encouraged all hospitalists, regardless of job type, to participate in the program. Participation rates were lower for 1‐year hospitalists compared with career hospitalists. The results of our analyses based on level of hospitalist participation in the training program, although not achieving statistical significance, suggest a greater effect of the program with higher degrees of participation.
Most important, the study was likely underpowered to detect a statistically significant difference in satisfaction results. Leaders were committed to providing communication‐skills training throughout our organization. We did not know the magnitude of potential improvement in satisfaction scores that might arise from our efforts, and therefore we did not conduct power calculations before designing and implementing the training program. Our HCAHPS composite doctor‐communication domain performance was 76% during the preintervention period and 79% during the postintervention period. Assuming an absolute 3% improvement is indeed possible, we would have needed >3000 patients in each period to have 80% power to detect a significant difference. Similarly, we would have needed >2000 patients during each period to have 80% power to detect an absolute 4% improvement in global rating of hospital care.
In an attempt to discern whether our favorable results were due to secular trends, we conducted post hoc analyses of HCAHPS nurse‐communication and hospital‐environment domains for the preintervention vs postintervention periods. Two of the 3 nurse‐communication items were rated lower during the postintervention period, but no result was statistically significant. Both hospital‐environment domain items were rated lower during the postintervention period, and 1 result was statistically significant (quiet at night). This post hoc evaluation lends additional support to the potential benefit of the communication‐skills training program.
The findings from this study represent an important issue for leaders attempting to improve quality performance within their organizations. What level of proof is needed before investing time and effort in implementing an intervention? With mounting pressure to improve performance, leaders are often left to make informed decisions based on data that fall short of scientifically rigorous evidence. Importantly, an increase in composite doctor‐communication ratings from 76% to 79% would translate into an improvement from the 25th percentile to 50th‐percentile performance in the fiscal‐year 2011 Press Ganey University Healthcare Consortium benchmark comparison (based on surveys received from September 1, 2010, to August 31, 2011).[15]
Our study has several limitations. First, we assessed an intervention on a single service in a single hospital. Generalizability may be limited, as hospital medicine groups, hospitals, and the patients they serve vary. Second, our intervention was based on a framework (ie, AIDET) that has face validity but has not undergone extensive study to confirm that the underlying constructs, and the behaviors related to them, are tightly linked to patient satisfaction. Third, as previously mentioned, we were likely underpowered to detect a significant improvement in satisfaction resulting from our intervention. Incomplete participation in the training program may have also limited the effect of our intervention. Finally, our comparisons by hospitalist level of participation were based on the discharging physician. Attribution of a patient response to a single physician is problematic because many patients encounter more than 1 hospitalist and 1 or more specialist physicians during their stay.
CONCLUSION
In summary, we found improvements in patient satisfaction with doctor communication, which were not statistically significant, after implementation of a communication‐skills training program for hospitalists. Larger studies are needed to assess whether a communication‐skills training program can truly improve patient satisfaction with doctor communication and overall hospital care.
Acknowledgments
The authors express their gratitude to the hospitalists involved in this program, especially Eric Schaefer, Nita Kulkarni, Stevie Mazyck, Rachel Cyrus, and Hiren Shah. The authors also thank Nicholas Christensen for assistance in data acquisition.
Disclosures: Nothing to report.
Hospital settings present unique challenges to patient‐clinician communication and collaboration. Patients frequently have multiple, active conditions. Interprofessional teams are large and care for multiple patients at the same time, and team membership is dynamic and dispersed. Moreover, physicians spend relatively little time with patients[1, 2] and seldom receive training in communication skills after medical school.
The Agency for Healthcare Research and Quality (AHRQ) has developed the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey to assess hospitalized patients' experiences with care.[3, 4, 5] Results are publicly reported on the US Department of Health and Human Services Hospital Compare Web site[6] and now affect hospital payment through the Center for Medicare and Medicaid Services Hospital Value‐Based Purchasing Program.[7]
Despite this increased transparency and accountability for performance related to the patient experience, little research has been conducted on how hospitals or clinicians might improve performance. Although interventions to enhance physician communication skills have shown improvements in observed behaviors, few studies have assessed benefit from the patient's perspective and few interventions have been integrated into practice.[8] We sought to assess the impact of a communication‐skills training program, based on a common framework used by hospitals, on patient satisfaction with doctor communication and overall hospital care.
METHODS
Setting and Study Design
The study was conducted at Northwestern Memorial Hospital (NMH), an 897‐bed tertiary‐care teaching hospital in Chicago, IL, and was approved by the institutional review board of Northwestern University. This study was a preintervention vs postintervention comparison of patient‐satisfaction scores. The intervention was a communication‐skills training program for all NMH hospitalists. We compared patient‐satisfaction survey data for patients admitted to the nonteaching hospitalist service during the 26 weeks prior to the intervention with data for patients admitted to the same service during the 22 weeks afterward. Hospitalists on this service worked 7 consecutive days, usually followed by 7 days free from clinical duty. Hospitalists cared for approximately 1014 patients per day without the assistance of resident physicians or midlevel providers (ie, physician assistants or nurse practitioners). Nighttime patient care was provided by in‐house hospitalists (ie, nocturnists). A majority of nighttime shifts were staffed by physicians who worked for the group for a single year. As a result of a prior intervention, hospitalists' patients were localized to specific units, each overseen by a hospitalist‐unit medical director.[9] We excluded all patients initially admitted to other services (eg, intensive care unit, surgical services) and patients discharged from other services.
Hospitalist Communication Skills Training Program
Northwestern Memorial Hospital implemented a communication‐skills training program in 2009 intended to enhance patient experience and improve patient‐satisfaction scores. All nonphysician staff were required to attend a 4‐hour training session based on the AIDET (Acknowledge, Introduce, Duration, Explanation, and Thank You) principles developed by the Studer Group.[10] The Studer Group is a well‐known healthcare consulting firm that aims to assist healthcare organizations to improve clinical, operational, and financial outcomes. The acronym AIDET provides a framework for communication‐skills behaviors (Table 1).
| AIDET Element | Explanation | Examples |
|---|---|---|
| ||
| Acknowledge | Use appropriate greeting, smile, and make eye contact. | Knock on patient's door. Hello, may I come in now? |
| Respect privacy: Knock and ask for permission before entering. Use curtains/doors appropriately. | Good morning. Is it a good time to talk? | |
| Position yourself on the same level as the patient. | Who do you have here with you today? | |
| Do not ignore others in the room (visitors or colleagues). | ||
| Introduce | Introduce yourself by name and role. | My name is Dr. Smith and I am your hospitalist physician. I'll be taking care of you while you are in the hospital. |
| Introduce any accompanying members of your team. | When on teaching service: I'm the supervising physician or I'm the physician in charge of your care. | |
| Address patients by title and last name (eg, Mrs. Smith) unless given permission to use first name. | ||
| Explain why you are there. | ||
| Do not assume patients remember your name or role. | ||
| Duration | Provide specific information on when you will be available, or when you will be back. | I'll be back between 2 and 3 pm, so if you think of any additional questions I can answer them then. |
| For tests/procedures: Explain how long it will take. Provide a time range for when it will happen. | In my experience, the test I am ordering for you will be done within the next 12 to 24 hours. | |
| Provide updates to the patient if the expected wait time has changed. | I should have the results for this test when I see you tomorrow morning. | |
| Do not blame another department or staff for delays. | ||
| Explanation | Explain your rationale for decisions. | I have ordered this test because |
| Use terms the patient can understand. | The possible side effects of this medication include | |
| Explain next steps/summarize plan for the day. | What questions do you have? | |
| Confirm understanding using teach back. | What are you most concerned about? | |
| Assume patients have questions and/or concerns. | I want to make sure you understood everything. Can you tell me in your own words what you will need to do once you are at home? | |
| Do not use acronyms that patients may not understand (eg, PRN, IR, ICU). | ||
| Thank you | Thank the patient and/or family. | I really appreciate you telling me about your symptoms. I know you told several people before. |
| Ask if there is anything else you can do for the patient. | Thank you for giving me the opportunity to care for you. What else can I do for you today? | |
| Explain when you will be back and how the patient can reach you if needed. | I'll see you again tomorrow morning. If you need me before then, just ask the nurse to page me. | |
| Do not appear rushed or distracted when ending your interaction. | ||
We adapted the AIDET framework and designed a communication‐skills training program, specifically for physicians, to emphasize reflection on current communication behaviors, deliberate practice of enhanced communication skills, and feedback based on performance during simulated and real clinical encounters. These educational methods are consistent with recommended strategies to improve behavioral performance.[11] During the first session, we discussed measurement of patient satisfaction, introduced AIDET principles, gave examples of specific behaviors for each principle, and had participants view 2 short videos displaying a range of communication skills followed by facilitated debriefing.[12] The second session included 3 simulation‐based exercises. Participants rotated roles in the scenarios (eg, patient, family member, physician) and facilitated debriefing was co‐led by a hospitalist leader (K.J.O.) and a patient‐experience administrative leader (either T.D. or J.R.). The third session involved direct observation of participants' clinical encounters and immediate feedback. This coaching session was performed for an initial group of 5 hospitalist‐unit medical directors by the manager of patient experience (T.D.) and subsequently by these medical directors for the remaining participants in the program. Each of the 3 sessions lasted 90 minutes. Instructional materials are available from the authors upon request.
The communication‐skills training program began in August 2011 and extended through January 2012. Participation was strongly encouraged but not mandatory. Sessions were offered multiple times to accommodate clinical schedules. One of the co‐investigators took attendance at each session to assess participation rates.
Survey Instruments and Data
During the study period, NMH used a third‐party vendor, Press Ganey Associates, Inc., to administer the HCAHPS survey to a random sample of 40% of hospitalized patients between 48 hours and 6 weeks after discharge. The HCAHPS survey has 27 total questions, including 3 questions assessing doctor communication as a domain.[3] In addition to the HCAHPS questions, the survey administered to NMH patients included questions developed by Press Ganey. Questions in the surveys used ordinal response scales. Specifically, response options for HCAHPS doctor‐communication questions were never, sometimes, usually, and always. Response options for Press Ganey doctor‐communication questions were very poor, poor, fair, good, and very good. Patients provided an overall hospital rating in the HCAHPS survey using a 010 scale, with 0=worst hospital possible and 10=best hospital possible.
We defined the preintervention period as the 26 weeks prior to implementation of the communication‐skills program (patients admitted on or between January 31, 2011, and July 31, 2011) and the postintervention period as the 22 weeks after implementation (patients admitted on or between January 31, 2012, and June 30, 2012). The postintervention period was 1 month shorter than the preintervention period in an effort to avoid confounding due to a number of new hospitalists starting in July 2012. We defined a discharge attending as highly trained if he/she attended all 3 sessions of the communication‐skills training program. The discharge attending was designated as no/low training if he/she attended fewer than the full 3 sessions.
Data Analysis
Data were obtained from the Northwestern Medicine Enterprise Data Warehouse, a single, integrated database of all clinical and research data from all patients receiving treatment through Northwestern University healthcare affiliates. We used 2 and Student t tests to compare patient demographic characteristics preintervention vs postintervention. We used 2 tests to compare the percentage of patients giving top‐box ratings to each doctor‐communication question (ie, always for HCAHPS and very good for Press Ganey) and giving an overall hospital rating of 9 or 10. We used top‐box comparisons, rather than comparison of mean or median scores, because patient‐satisfaction data are typically highly skewed toward favorable responses. This approach is consistent with prior HCAHPS research.[4, 5] We calculated composite doctor‐communication scores as the proportion of top‐box responses across items in each survey (ie, HCAHPS and Press Ganey). We first compared all patients during the preintervention and postintervention period. We then identified patients for whom the discharge attending worked as a hospitalist at NMH during both the preintervention and postintervention periods and compared satisfaction for patients discharged by hospitalists who had no/low training and for patients discharged by hospitalists who were highly trained. We performed multivariate logistic regression, using intervention period as the predictor variable and top‐box rating as the outcome variable for each doctor‐communication question and for overall hospital rating of 9 or 10. Covariates included patient age, sex, race, payer, self‐reported education level, and self‐reported health status. Models accounted for clustering of patients within discharge physicians. Similarly, we conducted multivariate logistic regression, using discharge attending category as the predictor variable (no/low training vs highly trained). The various comparisons described were intended to mimic intention to treat and treatment received analyses in light of incomplete participation in the communication‐skills program. All analyses were conducted using Stata version 11.2 (StataCorp, College Station, TX).
RESULTS
Overall, 61 (97%) of 63 hospitalists completed the first session, 44 (70%) completed the second session, and 25 (40%) completed the third session of program. Patient‐satisfaction data were available for 278 patients during the preintervention period and 186 patients during the postintervention period. Patient demographic characteristics were similar for the 2 periods (Table 2).
| Characteristic | Preintervention (n=278) | Postintervention (n=186) | P Value |
|---|---|---|---|
| |||
| Mean age, y (SD) | 62.8 (17.0) | 61.6 (17.6) | 0.45 |
| Female, no. (%) | 155 (55.8) | 114 (61.3) | 0.24 |
| Nonwhite race, no. (%) | 87 (32.2) | 53 (29.1) | 0.48 |
| Highest education completed, no. (%) | |||
| Did not complete high school | 12 (4.6) | 6 (3.3) | 0.45 |
| High school | 110 (41.7) | 81 (44.0) | |
| 4‐year college | 50 (18.9) | 43 (23.4) | |
| Advanced degree | 92 (34.9) | 54 (29.4) | |
| Payer, no. (%) | |||
| Medicare | 137 (49.3) | 89 (47.9) | 0.83 |
| Private | 113 (40.7) | 73 (39.3) | |
| Medicaid | 13 (4.7) | 11 (5.9) | |
| Self‐pay/other | 15 (5.4) | 13 (7.0) | |
| Self‐reported health status, no. (%) | |||
| Poor | 19 (7.1) | 18 (9.8) | 0.41 |
| Fair | 53 (19.7) | 43 (23.4) | |
| Good | 89 (33.1) | 57 (31.0) | |
| Very good | 89 (33.1) | 49 (26.6) | |
| Excellent | 19 (7.1) | 17 (9.2) | |
Patient Satisfaction With Hospitalist Communication
The HCAHPS and Press Ganey doctor communication domain scores were not significantly different between the preintervention and postintervention periods (75.8 vs 79.2, P=0.42 and 61.4 vs 65.9, P=0.39). Two of the 3 HCAHPS items assessing doctor communication were rated higher during the postintervention period, but no result was statistically significant (Table 3). Similarly, all 5 of the Press Ganey items assessing doctor communication were rated higher during the postintervention period, but no result was statistically significant. The HCAHPS overall rating of hospital care was also not significantly different between the preintervention and postintervention period. Results were similar in multivariate analyses, with no items showing statistically significant differences between the preintervention and postintervention periods.
| Unadjusted Analysisa | Adjusted Analysis | ||||
|---|---|---|---|---|---|
| Preintervention, No. (%) [n=270277] | Postintervention, No. (%) [n=183186] | P Value | OR (95% CI) | P Value | |
| |||||
| HCAHPS doctor‐communication domain | |||||
| How often did doctors treat you with courtesy and respect? | 224 (83) | 160 (86) | 0.31 | 1.23 (0.81‐2.44) | 0.22 |
| How often did doctors listen carefully to you? | 205 (75) | 145 (78) | 0.52 | 1.22 (0.74‐2.04) | 0.42 |
| How often did doctors explain things in a way you could understand? | 203 (75) | 137 (74) | 0.84 | 0.98 (0.59‐1.64) | 0.94 |
| Press Ganey physician‐communication domain | |||||
| Skill of physician | 189 (68) | 137 (74) | 0.19 | 1.38 (0.82‐2.31) | 0.22 |
| Physician's concern for your questions and worries | 157 (57) | 117 (64) | 0.14 | 1.30 (0.79‐2.12) | 0.30 |
| How well physician kept you informed | 158 (58) | 114 (62) | 0.36 | 1.15 (0.78‐1.72) | 0.71 |
| Time physician spent with you | 140 (51) | 101 (54) | 0.43 | 1.12 (0.66‐1.89) | 0.67 |
| Friendliness/courtesy of physician | 198 (71) | 136 (74) | 0.57 | 1.20 (0.74‐1.94) | 0.46 |
| HCAHPS global ratings | |||||
| Overall rating of hospital | 189 (70) [n=270] | 137 (74) [n=186] | 0.40 | 1.33 (0.82‐2.17) | 0.24 |
Pre‐post comparisons based on level of hospitalist participation in the training program are shown in Table 4. For patients discharged by no/low‐training hospitalists, 4 of the 8 total items assessing doctor communication were rated higher during the postintervention period, and 4 were rated lower, but no result was statistically significant. For patients discharged by highly trained hospitalists, all 8 items assessing doctor communication were rated higher during the postintervention period, but no result was statistically significant. Multivariate analyses were similar, with no items showing statistically significant differences between the preintervention and postintervention periods for either group.
| No/Low Training | Highly Trained | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted Analysisa | Adjusted Analysis | Unadjusted Analysisa | Adjusted Analysis | |||||||
| Preintervention, No. (%) [n=151156] | Postintervention, No. (%) [n=6770] | P Value | OR (95% CI) | P Value | Preintervention, No. (%) [n=119122] | Postintervention, No. (%) [n=115116] | P Value | OR (95% CI) | P Value | |
| ||||||||||
| HCAHPS doctor‐ communication domain | ||||||||||
| How often did doctors treat you with courtesy and respect? | 125 (83) | 61 (88) | 0.28 | 1.79 (0.82‐3.89) | 0.14 | 99 (83) | 99 (85) | 0.65 | 1.33 (0.62‐2.91) | 0.46 |
| How often did doctors listen carefully to you? | 116 (77) | 53 (76) | 0.86 | 1.08 (0.49‐2.38) | 0.19 | 89 (74) | 92 (79) | 0.30 | 1.43 (0.76‐2.69) | 0.27 |
| How often did doctors explain things in a way you could understand? | 115 (76) | 47 (68) | 0.24 | 0.59 (0.27‐1.28) | 0.18 | 88 (74) | 90 (78) | 0.52 | 1.31 (0.68‐2.50) | 0.42 |
| Press Ganey physician‐communication domain | ||||||||||
| Skill of physician | 110 (71) | 52 (74) | 0.56 | 1.32 (0.78‐2.22) | 0.31 | 79 (65) | 85 (73) | 0.16 | 1.45 (0.65‐3.27) | 0.37 |
| Physician's concern for your questions and worries | 92 (60) | 41 (61) | 0.88 | 1.00 (0.59‐1.77) | 0.99 | 65 (53) | 76 (66) | 0.06 | 1.71 (0.81‐3.60) | 0.16 |
| How well physician kept you informed | 89 (59) | 42 (61) | 0.75 | 1.16 (0.64‐2.08) | 0.62 | 69 (57) | 72 (63) | 0.34 | 1.29 (0.75‐2.20) | 0.35 |
| Time physician spent with you | 83 (54) | 37 (53) | 0.92 | 0.87 (0.47‐1.61) | 0.65 | 57 (47) | 64 (55) | 0.19 | 1.44 (0.64‐3.21) | 0.38 |
| Friendliness/courtesy of physician | 116 (75) | 45 (66) | 0.18 | 0.72 (0.37‐1.38) | 0.32 | 82 (67) | 91 (78) | 0.05 | 1.89 (0.97‐3.68) | 0.60 |
| HCAHPS global ratings | ||||||||||
| Overall rating of hospital | 109 (73) | 53 (75) | 0.63 | 1.37 (0.67‐2.81) | 0.39 | 86 (71) | 90 (78) | 0.21 | 1.60 (0.73‐3.53) | 0.24 |
DISCUSSION
We found no significant improvement in patient satisfaction with doctor communication or overall rating of hospital care after implementation of a communication‐skills training program for hospitalists. There are several potential explanations for our results. First, though we used sound educational methods and attempted to replicate common clinical scenarios during simulation exercises, our program may not have resulted in improved communication behaviors during actual clinical care. We attempted to balance instructional methods that would result in behavioral change with a feasible investment of time and effort on the part of our learners (ie, practicing hospitalists). It is possible that additional time, feedback, and practice of communication skills would be necessary to change behaviors in the clinical setting. However, prior communication‐skills interventions have similarly struggled to show an impact on patient satisfaction.[13, 14] Second, we had incomplete participation in the program, with only 40% of hospitalists completing all 3 planned sessions. We encouraged all hospitalists, regardless of job type, to participate in the program. Participation rates were lower for 1‐year hospitalists compared with career hospitalists. The results of our analyses based on level of hospitalist participation in the training program, although not achieving statistical significance, suggest a greater effect of the program with higher degrees of participation.
Most important, the study was likely underpowered to detect a statistically significant difference in satisfaction results. Leaders were committed to providing communication‐skills training throughout our organization. We did not know the magnitude of potential improvement in satisfaction scores that might arise from our efforts, and therefore we did not conduct power calculations before designing and implementing the training program. Our HCAHPS composite doctor‐communication domain performance was 76% during the preintervention period and 79% during the postintervention period. Assuming an absolute 3% improvement is indeed possible, we would have needed >3000 patients in each period to have 80% power to detect a significant difference. Similarly, we would have needed >2000 patients during each period to have 80% power to detect an absolute 4% improvement in global rating of hospital care.
In an attempt to discern whether our favorable results were due to secular trends, we conducted post hoc analyses of HCAHPS nurse‐communication and hospital‐environment domains for the preintervention vs postintervention periods. Two of the 3 nurse‐communication items were rated lower during the postintervention period, but no result was statistically significant. Both hospital‐environment domain items were rated lower during the postintervention period, and 1 result was statistically significant (quiet at night). This post hoc evaluation lends additional support to the potential benefit of the communication‐skills training program.
The findings from this study represent an important issue for leaders attempting to improve quality performance within their organizations. What level of proof is needed before investing time and effort in implementing an intervention? With mounting pressure to improve performance, leaders are often left to make informed decisions based on data that fall short of scientifically rigorous evidence. Importantly, an increase in composite doctor‐communication ratings from 76% to 79% would translate into an improvement from the 25th percentile to 50th‐percentile performance in the fiscal‐year 2011 Press Ganey University Healthcare Consortium benchmark comparison (based on surveys received from September 1, 2010, to August 31, 2011).[15]
Our study has several limitations. First, we assessed an intervention on a single service in a single hospital. Generalizability may be limited, as hospital medicine groups, hospitals, and the patients they serve vary. Second, our intervention was based on a framework (ie, AIDET) that has face validity but has not undergone extensive study to confirm that the underlying constructs, and the behaviors related to them, are tightly linked to patient satisfaction. Third, as previously mentioned, we were likely underpowered to detect a significant improvement in satisfaction resulting from our intervention. Incomplete participation in the training program may have also limited the effect of our intervention. Finally, our comparisons by hospitalist level of participation were based on the discharging physician. Attribution of a patient response to a single physician is problematic because many patients encounter more than 1 hospitalist and 1 or more specialist physicians during their stay.
CONCLUSION
In summary, we found improvements in patient satisfaction with doctor communication, which were not statistically significant, after implementation of a communication‐skills training program for hospitalists. Larger studies are needed to assess whether a communication‐skills training program can truly improve patient satisfaction with doctor communication and overall hospital care.
Acknowledgments
The authors express their gratitude to the hospitalists involved in this program, especially Eric Schaefer, Nita Kulkarni, Stevie Mazyck, Rachel Cyrus, and Hiren Shah. The authors also thank Nicholas Christensen for assistance in data acquisition.
Disclosures: Nothing to report.
- , , , , , . Four minutes for a patient, twenty seconds for a relative—an observational study at a university hospital. BMC Health Serv Res. 2010;10:94.
- , , . How hospitalists spend their time: insights on efficiency and safety. J Hosp Med. 2006;1(2):88–93.
- CAHPS: Surveys and Tools to Advance Patient‐Centered Care. Available at: http://cahps.ahrq.gov. Accessed July 12, 2012.
- , , , , . Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27–37.
- , , , , . Measuring hospital care from the patients' perspective: an overview of the CAHPS Hospital Survey development process. Health Serv Res. 2005;40(6 part 2):1977–1995.
- US Department of Health and Human Services. Hospital Compare. Available at: http://hospitalcompare.hhs.gov/. Accessed November 5, 2012.
- Center for Medicare and Medicaid Services. Hospital Value Based Purchasing Program. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/hospital‐value‐based‐purchasing/index.html?redirect=/hospital‐value‐based‐purchasing. Accessed August 1, 2012.
- , , , . Communication interventions make a difference in conversations between physicians and patients: a systematic review of the evidence. Med Care. 2007;45(4):340–349.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- Studer Group. Acknowledge, Introduce, Duration, Explanation and Thank You. Available at: http://www.studergroup.com/aidet. Accessed November 5, 2012.
- Kern DE, Thomas PA, Bass EB, Howard DM, eds. Curriculum Development for Medical Education: A Six‐Step Approach. Baltimore, MD: Johns Hopkins University Press; 1998.
- Vanderbilt University Medical Center and Studer Group. Building Patient Trust with AIDET®: Clinical Excellence with Patient Compliance Through Effective Communication. Gulf Breeze, FL: Fire Starter Publishing; 2008.
- , , , . Effect of clinician communication skills training on patient satisfaction: a randomized, controlled trial. Ann Intern Med. 1999;131(11):822–829.
- , , , , , . Effectiveness of a short course in clinical communication skills for hospital doctors: results of a crossover randomized controlled trial (ISRCTN22153332). Patient Educ Couns. 2010;84(2):163–169.
- Press Ganey HCAHPS Top Box and Rank Report, Fiscal Year 2011. Inpatient, University Healthcare Consortium Peer Group. South Bend, IN: Press Ganey Associates; 2011.
- , , , , , . Four minutes for a patient, twenty seconds for a relative—an observational study at a university hospital. BMC Health Serv Res. 2010;10:94.
- , , . How hospitalists spend their time: insights on efficiency and safety. J Hosp Med. 2006;1(2):88–93.
- CAHPS: Surveys and Tools to Advance Patient‐Centered Care. Available at: http://cahps.ahrq.gov. Accessed July 12, 2012.
- , , , , . Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27–37.
- , , , , . Measuring hospital care from the patients' perspective: an overview of the CAHPS Hospital Survey development process. Health Serv Res. 2005;40(6 part 2):1977–1995.
- US Department of Health and Human Services. Hospital Compare. Available at: http://hospitalcompare.hhs.gov/. Accessed November 5, 2012.
- Center for Medicare and Medicaid Services. Hospital Value Based Purchasing Program. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/hospital‐value‐based‐purchasing/index.html?redirect=/hospital‐value‐based‐purchasing. Accessed August 1, 2012.
- , , , . Communication interventions make a difference in conversations between physicians and patients: a systematic review of the evidence. Med Care. 2007;45(4):340–349.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- Studer Group. Acknowledge, Introduce, Duration, Explanation and Thank You. Available at: http://www.studergroup.com/aidet. Accessed November 5, 2012.
- Kern DE, Thomas PA, Bass EB, Howard DM, eds. Curriculum Development for Medical Education: A Six‐Step Approach. Baltimore, MD: Johns Hopkins University Press; 1998.
- Vanderbilt University Medical Center and Studer Group. Building Patient Trust with AIDET®: Clinical Excellence with Patient Compliance Through Effective Communication. Gulf Breeze, FL: Fire Starter Publishing; 2008.
- , , , . Effect of clinician communication skills training on patient satisfaction: a randomized, controlled trial. Ann Intern Med. 1999;131(11):822–829.
- , , , , , . Effectiveness of a short course in clinical communication skills for hospital doctors: results of a crossover randomized controlled trial (ISRCTN22153332). Patient Educ Couns. 2010;84(2):163–169.
- Press Ganey HCAHPS Top Box and Rank Report, Fiscal Year 2011. Inpatient, University Healthcare Consortium Peer Group. South Bend, IN: Press Ganey Associates; 2011.
Copyright © 2013 Society of Hospital Medicine
Assessing Teamwork in SIDR
Teamwork is essential to delivering safe and effective hospital care,15 yet the fluidity and geographic dispersion of team members in the hospital setting presents a significant barrier to teamwork.6 Physicians, nurses, and other hospital professionals frequently lack convenient and reliable opportunities to interact, and may struggle in efforts to discuss the care of their patients in person. Research studies show that nurses and physicians on patient care units do not communicate consistently and frequently do not agree on key aspects of their patients' plans of care.7, 8
Interdisciplinary rounds (IDR), also known as multidisciplinary rounds, provide a means to assemble hospital care team members and improve collaboration.913 Prior research on the use of IDR has demonstrated improved ratings of collaboration,11, 12 but inconsistent effects on length of stay and cost.10, 12, 13 Notably, the format, frequency, and duration of IDR in prior studies has been variable and no studies, to our knowledge, have evaluated teamwork performance during IDR. Lamb and colleagues conducted observations of cancer teams during multidisciplinary meetings.14 Trained observers used a validated observation tool to rate teamwork and found significant variation in performance by subteams. However, the study focused mainly on discussion among physician team members during meetings to plan longitudinal care for oncology patients.
We recently reported on the use of structured interdisciplinary rounds (SIDR) on 2 medical units in our hospital.15, 16 SIDR combines a structured format for communication, similar to a goals‐of‐care form,17, 18 with a forum for daily interdisciplinary meetings. Though no effect was seen on length of stay or cost, SIDR was associated with significantly higher ratings of the quality of collaboration and teamwork climate, and a reduction in the rate of adverse events.19 In March 2010, we implemented SIDR across all medical units in our hospital. We subjectively noted variation in teamwork performance during SIDR after a modification of nurse manager roles. We sought to evaluate teamwork during SIDR and to determine whether variation in performance existed and, if present, to characterize it.
METHODS
Setting and Study Design
The study was conducted at Northwestern Memorial Hospital (NMH), a 920‐bed tertiary care teaching hospital in Chicago, IL, and was deemed exempt by the Institutional Review Board of Northwestern University. General medical patients were admitted to 1 of 6 units based on bed availability. Five of the medical units consisted of 30 beds, and 1 unit consisted of 23. Each unit was equipped with continuous cardiac telemetry monitoring. Three units were staffed by teaching service physician teams consisting of 1 attending, 1 resident, and 1 or 2 interns. The other 3 units were staffed by hospitalists without the assistance of resident or intern physicians. As a result of a prior intervention, physicians' patients were localized to specific units in an effort to improve communication practices among nurses and physicians.20
Beginning in March 2010, all general medical units held SIDR each weekday morning. SIDR took place in the unit conference room, was expected to last approximately 3040 minutes, and was co‐led by the unit nurse manager and a medical director. Unit nurse managers and medical directors received specific training for their roles, including 3 hours of simulation‐based exercises designed to enhance their skills in facilitating discussion during SIDR. All nurses and physicians caring for patients on the unit, as well as the pharmacist, social worker, and case manager assigned to the unit, attended SIDR. Attendees used a structured communication tool to review patients admitted in the previous 24 hours. The plan of care for other patients was also discussed in SIDR, but without the use of the structured communication tool.
Importantly, nurse management underwent restructuring in the summer of 2011. Nurse managers, who had previously been responsible for overseeing all nursing activities on a single unit, were redeployed to be responsible for specific activities across 34 units. This restructuring made it very difficult for nurse managers to colead SIDR. As a result, the unit nurse clinical coordinator assumed coleadership of SIDR with the unit medical director. Nurse clinical coordinators worked every weekday and did not have patient care responsibilities while on duty. In addition to their role in coleading SIDR, nurse clinical coordinators addressed daily staffing and scheduling challenges and other short‐term patient care needs.
Teamwork Assessment
We adapted the Observational Teamwork Assessment for Surgery (OTAS) tool, a behaviorally anchored rating scale shown to be reliable and valid in surgical settings.2123 The OTAS tool provides scores ranging from 0 to 6 (0 = problematic behavior; 3 = team function neither hindered nor enhanced by behavior; 6 = exemplary behavior) across 5 domains (communication, coordination, cooperation/backup behavior, leadership, and monitoring/situational awareness) and for prespecified subteams. We defined domains as described by the researchers who developed OTAS. Communication referred to the quality and the quantity of information exchanged by team members. Coordination referred to management and timing of activities and tasks. Cooperation and backup behavior referred to assistance provided among members of the team, supporting others and correcting errors. Leadership referred to provision of directions, assertiveness, and support among team members. Monitoring and situational awareness referred to team observation and awareness of ongoing processes. We defined subteams for each group of professionals expected to attend SIDR. Specifically, subteams included physicians, nurses, social work‐case management (SW‐CM), pharmacy, and coleaders. We combined social work and case management because these professionals have similar patient care activities. Similarly, we combined unit medical directors and nurse clinical coordinators as coleaders. By providing data on teamwork performance within specific domains and for specific subteams, the OTAS instrument helps identify factors influencing overall teamwork performance. We modified OTAS anchors to reflect behaviors during SIDR. Anchors assisted observers in their rating of teamwork behaviors during SIDR. For example, an anchor for exemplary physician communication behavior was listens actively to other team members (looks at other team members, nods, etc). An anchor for exemplary physician leadership was assigns responsibility for task completion when appropriate.
Two researchers conducted unannounced direct observations of SIDRs. One researcher (Y.N.B) was a medical librarian with previous experience conducting observational research. The other researcher (A.J.C.) had observed 170 prior SIDRs as part of a related study. Both researchers observed 10 SIDRs to practice data collection and to inform minor revisions of the anchors. We aimed to conduct 78 independent observations for each unit, and 20 joint observations to assess inter‐rater reliability. All subteams were scored for each domain. For example, all subteams received leadership domain scores because all team members exhibit leadership behaviors, depending on the situation. In addition to teamwork scores, observers recorded the number of patients on the unit, the number of patients discussed during SIDR, attendance by subteam members, and the duration of SIDR. For the SW‐CM and coleader subteams, we documented presence if one of the subteam members was present for each patients' discussion. For example, we recorded present for SW‐CM if the social worker was in attendance but the case manager was not.
Data Analysis
We calculated descriptive statistics to characterize SIDRs. We used Spearman's rank correlation coefficients to assess inter‐rater reliability for joint observations. Spearman's rank correlation is a nonparametric test of association and appropriate for assessing agreement between observers when using data that is not normally distributed. Spearman rho values range from 1 to 1, with 1 signifying perfect inverse correlation, 0 signifying no correlation, and 1 signifying perfect correlation. We used the MannWhitney U test to assess for differences in overall team scores between services (teaching vs nonteaching hospitalist service) and KruskalWallis tests to assess for differences across units, domains, and subteams. The Kruskal‐Wallis test is a nonparametric test appropriate for comparing three or more independent samples in which the outcome is not normally distributed. We used a t test to assess for difference in duration by service, and Spearman rank correlation to assess for correlation between time spent in discussion per patient and overall team score. All analyses were conducted using Stata version 11.0 (College Station, TX).
RESULTS
SIDR Characteristics
We performed 7 direct observations of SIDR for 4 units, and 8 observations for 2 units (44 total observations). Units were at 99% capacity, and SIDR attendees discussed 98% of patients on the unit. Attendance exceeded 98% for each subteam (physicians, nurses, SW‐CM, pharmacy, and coleaders). SIDR lasted a mean 41.4 11.1 minutes, with a mean 1.5 0.4 minutes spent in discussion per patient. SIDR was significantly longer in duration on teaching service units compared to the nonteaching hospitalist service units (1.7 0.3 vs 1.3 0.4 minutes per patient; P < 0.001).
Inter‐Rater Reliability
Inter‐rater reliability across unit level scores was excellent (rho = 0.75). As shown in Table 1, inter‐rater reliability across domains was good (rho = 0.530.68). Inter‐rater reliability across subteams was good to excellent (rho = 0.530.76) with the exception of the physician subteam, for which it was poor (rho = 0.35).
| Spearman's rho | P Value | |
|---|---|---|
| ||
| Domain (n = 20) | ||
| Communication | 0.62 | <0.01 |
| Coordination | 0.60 | <0.01 |
| Cooperation/backup behavior | 0.66 | <0.01 |
| Leadership | 0.68 | <0.01 |
| Monitoring/situational awareness | 0.53 | 0.02 |
| Subteam (n = 20) | ||
| Physicians | 0.35 | 0.14 |
| Nurses | 0.53 | 0.02 |
| SW‐CM | 0.60 | <0.01 |
| Pharmacy | 0.76 | <0.01 |
| Coleaders | 0.68 | <0.01 |
Assessment of Teamwork by Unit, Domain, and Subteams
Teaching and nonteaching hospitalist units had similar team scores (median [interquartile range (IQR)] = 5.2 [1.0] vs 5.2 [0.4]; P = 0.55). We found significant differences in teamwork scores across units and domains, and found differences of borderline statistical significance across subteams (see Table 2). For unit teamwork scores, the median (IQR) was 4.4 (3.94.9) for the lowest and 5.4 (5.35.5) for the highest performing unit (P = 0.008). Across domain scores, leadership received the lowest score (median [IQR] = 5.0 [4.65.3]), and cooperation/backup behavior and monitoring/situational awareness received the highest scores (median [IQR]) = 5.4 [5.05.5] and 5.4 [5.05.7]; P = 0.02). Subteam scores ranged from a median (IQR) 5.0 (4.45.8) for coleaders to 5.5 (5.05.8) for SW‐CM (P = 0.05). We found no relationship between unit teamwork score and time spent in discussion per patient (rho = 0.04; P = 0.79).
| Median (IQR) | P Value | |
|---|---|---|
| ||
| Unit (n = 44)* | ||
| A | 5.3 (5.15.4) | 0.008 |
| B | 5.4 (5.35.5) | |
| C | 5.1 (4.95.2) | |
| D | 5.4 (5.25.6) | |
| E | 4.4 (3.94.9) | |
| F | 5.3 (5.15.5) | |
| Domain (n = 44) | ||
| Communication | 5.2 (4.95.4) | 0.02 |
| Coordination | 5.2 (4.75.4) | |
| Cooperation/backup behavior | 5.4 (5.05.5) | |
| Leadership | 5.0 (4.65.3) | |
| Monitoring/situational awareness | 5.4 (5.05.7) | |
| Subteam (n = 44) | ||
| Physicians | 5.2 (4.95.4) | 0.05 |
| Nurses | 5.2 (5.05.4) | |
| SW‐CM | 5.5 (5.05.8) | |
| Pharmacy | 5.3 (4.85.8) | |
| Coleaders | 5.0 (4.45.8) | |
DISCUSSION
We found that the adapted OTAS instrument demonstrated acceptable reliability for assessing teamwork during SIDR across units, domains, and most subteams. Although teamwork scores during SIDR were generally high, we found variation in performance across units, domains, and subteams. Variation in performance is notable in light of our efforts to implement a consistent format for SIDR across units. Specifically, all units have similar timing, duration, frequency, and location of SIDR, use a structured communication tool for new patients, expect the same professions to be represented, and use coleaders to facilitate discussion. We believe teamwork within IDR likely varies across units in other hospitals, and perhaps to a larger degree, given the emphasis on purposeful design and implementation of SIDR in our hospital.
Our findings are important for several reasons. First, though commonly used in hospital settings, the effectiveness of IDR is seldom assessed. Hospitalists and other professionals may not be able to identify or characterize deficiencies in teamwork during IDR without objective assessment. The adapted OTAS instrument provides a useful tool to evaluate team performance during IDR. Second, professionals may conclude that the mere implementation of an intervention such as SIDR will improve teamwork ratings and improve patient safety. Importantly, published studies evaluating the benefits of SIDR reflected a pilot study occurring on 2 units.15, 16, 19 The current study emphasizes the need to ensure that interventions proven to be effective on a small scale are implemented consistently when put into place on a larger scale.
Despite good reliability for assessing teamwork during SIDR across units, domains, and most subteams, we found poor inter‐rater reliability for the physician subteam. The explanation for this finding is not entirely clear. We reviewed the anchors for the physician subteam behaviors and were unable to identify ambiguity in anchor definitions. An analysis of domain scores within the physician subteam did not reveal any specific pattern to explain the poor correlation.
We found that the leadership domain and coleader subteam received particularly low scores. The explanation for this finding likely relates to changes in the nurse management structure shortly before our study, which reduced attendance by nurse managers and created a need for clinical coordinators to take on a leadership role during SIDR. Although we provided simulation‐based training to unit medical directors and nurse managers prior to implementing SIDR in March 2010, clinical coordinators were not part of the initial training. Our study suggests a need to provide additional training to coleaders, including clinical coordinators, to enhance their ability to facilitate discussion in SIDR.
We found no difference in overall teamwork scores when comparing teaching service units to nonteaching hospitalist service units. Duration of SIDR was significantly longer on teaching service units, but there was no association between duration of discussion and overall team score. The difference in duration of SIDR is likely explained by less succinct discussions on the part of housestaff physicians compared to more experienced hospitalists. Importantly, the quality of input, and its impact on teamwork during SIDR, does not appear to suffer when physician discussion is less efficient.
Our study has several limitations. First, we evaluated IDR in a single, urban, academic institution, which may limit generalizability. Our version of IDR (ie, SIDR) was designed to improve teamwork and incorporate a structured communication tool with regularly held interdisciplinary meetings. Features of IDR may differ in other hospitals. Second, the high teamwork scores seen in our study may not be generalizable to hospitals which have used a less rigorous, less standardized approach to IDR. Third, SIDR did not include patients or caregivers. Research is needed to test strategies to include patients and caregivers as active team members and participants in clinical decisions during hospitalization. Finally, we used the term interdisciplinary rounds to be consistent with prior published research. The term interprofessional may be more appropriate, as it specifically describes interactions among members of different professions (eg, physicians, nurses, social workers) rather than among different disciplines within a profession (eg, cardiologists, hospitalists, surgeons).
In summary, we found that teamwork during IDR could be reliably assessed using an adapted OTAS instrument. Although scores were generally high, we found variation in performance across units and domains suggesting a need to improve consistency of teamwork performance across units, domains, and subteams. Our study fills an important gap in the literature. Although IDR is commonly used in hospitals, and research shows improvements in ratings of collaboration,11, 12 little if any research has evaluated teamwork during IDR. Beyond the mere implementation of IDR, our study suggests the need to confirm that teamwork is optimal and consistent. Furthermore, hospital leaders should consider specific training for clinicians leading discussion during IDR.
Acknowledgements
The authors express their gratitude to Nick Sevdalis, BSc, MSc, PhD for providing the OTAS instrument and detailed instructions on its use.
Disclosures: Dr O'Leary, Ms Creden, and Dr Williams received salary support from the Agency for Healthcare Research and Quality, grant R18 HS019630. All authors disclose no other relevant or financial conflicts of interest.
- Joint Commission on Accreditation of Healthcare Organizations. Sentinel Event Statistics. Available at: http://www.jointcommission.org/SentinelEvents/Statistics/. Accessed January 19,2012.
- ,,, et al.A look into the nature and causes of human errors in the intensive care unit.Crit Care Med.1995;23(2):294–300.
- ,,, et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324(6):377–384.
- ,,.Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79(2):186–194.
- ,,,,,.The Quality in Australian Health Care Study.Med J Aust.1995;163(9):458–471.
- ,,,,,.Teamwork on inpatient medical units: assessing attitudes and barriers.Qual Saf Health Care.2010;19(2):117–121.
- ,,,,,.Can we talk? Priorities for patient care differed among health care providers. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds.Advances in Patient Safety: From Research to Implementation. Vol1: Research Findings. AHRQ Publication No. 05–0021‐1.Rockville, MD:Agency for Healthcare Research and Quality;2005.
- ,,, et al.Patterns of nurse‐physician communication and agreement on the plan of care.Qual Saf Health Care.2010;19(3):195–199.
- ,,, et al.The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs.J Nurs Adm.2006;36(2):79–85.
- ,,.A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement.Med Care.1998;36(8 suppl):AS4–AS12.
- ,,,.Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses.Am J Crit Care.2005;14(1):71–77.
- ,,,,.Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay.J Gen Intern Med.2007;22(8):1073–1079.
- ,,,.Effects of interdisciplinary rounds on length of stay in a telemetry unit.J Public Health Manag Pract.2004;10(1):63–69.
- ,,,,.Teamwork and team performance in multidisciplinary cancer teams: development and evaluation of an observational assessment tool.BMJ Qual Saf.2011 [Epub ahead of print].
- ,,,,,.Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit.J Hosp Med.2011;6(2):88–93.
- ,,,,,.Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit.J Gen Intern Med.2010;25(8):826–832.
- ,,,,.Improving nurse‐physician communication and satisfaction in the intensive care unit with a daily goals worksheet.Am J Crit Care.2006;15(2):217–222.
- ,,,,,.Improving communication in the ICU using daily goals.J Crit Care.2003;18(2):71–75.
- ,,, et al.Structured interdisciplinary rounds in a medical teaching unit: improving patient safety.Arch Intern Med.2011;171(7):678–684.
- ,,, et al.Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care.J Gen Intern Med.2009;24(11):1223–1227.
- ,,,,.Observational teamwork assessment for surgery (OTAS): refinement and application in urological surgery.World J Surg.2007;31(7):1373–1381.
- ,,,,,.Observational teamwork assessment for surgery: construct validation with expert versus novice raters.Ann Surg.2009;249(6):1047–1051.
- ,,,,.Observational teamwork assessment for surgery: content validation and tool refinement.J Am Coll Surg.2011;212(2):234–243.e1–5.
Teamwork is essential to delivering safe and effective hospital care,15 yet the fluidity and geographic dispersion of team members in the hospital setting presents a significant barrier to teamwork.6 Physicians, nurses, and other hospital professionals frequently lack convenient and reliable opportunities to interact, and may struggle in efforts to discuss the care of their patients in person. Research studies show that nurses and physicians on patient care units do not communicate consistently and frequently do not agree on key aspects of their patients' plans of care.7, 8
Interdisciplinary rounds (IDR), also known as multidisciplinary rounds, provide a means to assemble hospital care team members and improve collaboration.913 Prior research on the use of IDR has demonstrated improved ratings of collaboration,11, 12 but inconsistent effects on length of stay and cost.10, 12, 13 Notably, the format, frequency, and duration of IDR in prior studies has been variable and no studies, to our knowledge, have evaluated teamwork performance during IDR. Lamb and colleagues conducted observations of cancer teams during multidisciplinary meetings.14 Trained observers used a validated observation tool to rate teamwork and found significant variation in performance by subteams. However, the study focused mainly on discussion among physician team members during meetings to plan longitudinal care for oncology patients.
We recently reported on the use of structured interdisciplinary rounds (SIDR) on 2 medical units in our hospital.15, 16 SIDR combines a structured format for communication, similar to a goals‐of‐care form,17, 18 with a forum for daily interdisciplinary meetings. Though no effect was seen on length of stay or cost, SIDR was associated with significantly higher ratings of the quality of collaboration and teamwork climate, and a reduction in the rate of adverse events.19 In March 2010, we implemented SIDR across all medical units in our hospital. We subjectively noted variation in teamwork performance during SIDR after a modification of nurse manager roles. We sought to evaluate teamwork during SIDR and to determine whether variation in performance existed and, if present, to characterize it.
METHODS
Setting and Study Design
The study was conducted at Northwestern Memorial Hospital (NMH), a 920‐bed tertiary care teaching hospital in Chicago, IL, and was deemed exempt by the Institutional Review Board of Northwestern University. General medical patients were admitted to 1 of 6 units based on bed availability. Five of the medical units consisted of 30 beds, and 1 unit consisted of 23. Each unit was equipped with continuous cardiac telemetry monitoring. Three units were staffed by teaching service physician teams consisting of 1 attending, 1 resident, and 1 or 2 interns. The other 3 units were staffed by hospitalists without the assistance of resident or intern physicians. As a result of a prior intervention, physicians' patients were localized to specific units in an effort to improve communication practices among nurses and physicians.20
Beginning in March 2010, all general medical units held SIDR each weekday morning. SIDR took place in the unit conference room, was expected to last approximately 3040 minutes, and was co‐led by the unit nurse manager and a medical director. Unit nurse managers and medical directors received specific training for their roles, including 3 hours of simulation‐based exercises designed to enhance their skills in facilitating discussion during SIDR. All nurses and physicians caring for patients on the unit, as well as the pharmacist, social worker, and case manager assigned to the unit, attended SIDR. Attendees used a structured communication tool to review patients admitted in the previous 24 hours. The plan of care for other patients was also discussed in SIDR, but without the use of the structured communication tool.
Importantly, nurse management underwent restructuring in the summer of 2011. Nurse managers, who had previously been responsible for overseeing all nursing activities on a single unit, were redeployed to be responsible for specific activities across 34 units. This restructuring made it very difficult for nurse managers to colead SIDR. As a result, the unit nurse clinical coordinator assumed coleadership of SIDR with the unit medical director. Nurse clinical coordinators worked every weekday and did not have patient care responsibilities while on duty. In addition to their role in coleading SIDR, nurse clinical coordinators addressed daily staffing and scheduling challenges and other short‐term patient care needs.
Teamwork Assessment
We adapted the Observational Teamwork Assessment for Surgery (OTAS) tool, a behaviorally anchored rating scale shown to be reliable and valid in surgical settings.2123 The OTAS tool provides scores ranging from 0 to 6 (0 = problematic behavior; 3 = team function neither hindered nor enhanced by behavior; 6 = exemplary behavior) across 5 domains (communication, coordination, cooperation/backup behavior, leadership, and monitoring/situational awareness) and for prespecified subteams. We defined domains as described by the researchers who developed OTAS. Communication referred to the quality and the quantity of information exchanged by team members. Coordination referred to management and timing of activities and tasks. Cooperation and backup behavior referred to assistance provided among members of the team, supporting others and correcting errors. Leadership referred to provision of directions, assertiveness, and support among team members. Monitoring and situational awareness referred to team observation and awareness of ongoing processes. We defined subteams for each group of professionals expected to attend SIDR. Specifically, subteams included physicians, nurses, social work‐case management (SW‐CM), pharmacy, and coleaders. We combined social work and case management because these professionals have similar patient care activities. Similarly, we combined unit medical directors and nurse clinical coordinators as coleaders. By providing data on teamwork performance within specific domains and for specific subteams, the OTAS instrument helps identify factors influencing overall teamwork performance. We modified OTAS anchors to reflect behaviors during SIDR. Anchors assisted observers in their rating of teamwork behaviors during SIDR. For example, an anchor for exemplary physician communication behavior was listens actively to other team members (looks at other team members, nods, etc). An anchor for exemplary physician leadership was assigns responsibility for task completion when appropriate.
Two researchers conducted unannounced direct observations of SIDRs. One researcher (Y.N.B) was a medical librarian with previous experience conducting observational research. The other researcher (A.J.C.) had observed 170 prior SIDRs as part of a related study. Both researchers observed 10 SIDRs to practice data collection and to inform minor revisions of the anchors. We aimed to conduct 78 independent observations for each unit, and 20 joint observations to assess inter‐rater reliability. All subteams were scored for each domain. For example, all subteams received leadership domain scores because all team members exhibit leadership behaviors, depending on the situation. In addition to teamwork scores, observers recorded the number of patients on the unit, the number of patients discussed during SIDR, attendance by subteam members, and the duration of SIDR. For the SW‐CM and coleader subteams, we documented presence if one of the subteam members was present for each patients' discussion. For example, we recorded present for SW‐CM if the social worker was in attendance but the case manager was not.
Data Analysis
We calculated descriptive statistics to characterize SIDRs. We used Spearman's rank correlation coefficients to assess inter‐rater reliability for joint observations. Spearman's rank correlation is a nonparametric test of association and appropriate for assessing agreement between observers when using data that is not normally distributed. Spearman rho values range from 1 to 1, with 1 signifying perfect inverse correlation, 0 signifying no correlation, and 1 signifying perfect correlation. We used the MannWhitney U test to assess for differences in overall team scores between services (teaching vs nonteaching hospitalist service) and KruskalWallis tests to assess for differences across units, domains, and subteams. The Kruskal‐Wallis test is a nonparametric test appropriate for comparing three or more independent samples in which the outcome is not normally distributed. We used a t test to assess for difference in duration by service, and Spearman rank correlation to assess for correlation between time spent in discussion per patient and overall team score. All analyses were conducted using Stata version 11.0 (College Station, TX).
RESULTS
SIDR Characteristics
We performed 7 direct observations of SIDR for 4 units, and 8 observations for 2 units (44 total observations). Units were at 99% capacity, and SIDR attendees discussed 98% of patients on the unit. Attendance exceeded 98% for each subteam (physicians, nurses, SW‐CM, pharmacy, and coleaders). SIDR lasted a mean 41.4 11.1 minutes, with a mean 1.5 0.4 minutes spent in discussion per patient. SIDR was significantly longer in duration on teaching service units compared to the nonteaching hospitalist service units (1.7 0.3 vs 1.3 0.4 minutes per patient; P < 0.001).
Inter‐Rater Reliability
Inter‐rater reliability across unit level scores was excellent (rho = 0.75). As shown in Table 1, inter‐rater reliability across domains was good (rho = 0.530.68). Inter‐rater reliability across subteams was good to excellent (rho = 0.530.76) with the exception of the physician subteam, for which it was poor (rho = 0.35).
| Spearman's rho | P Value | |
|---|---|---|
| ||
| Domain (n = 20) | ||
| Communication | 0.62 | <0.01 |
| Coordination | 0.60 | <0.01 |
| Cooperation/backup behavior | 0.66 | <0.01 |
| Leadership | 0.68 | <0.01 |
| Monitoring/situational awareness | 0.53 | 0.02 |
| Subteam (n = 20) | ||
| Physicians | 0.35 | 0.14 |
| Nurses | 0.53 | 0.02 |
| SW‐CM | 0.60 | <0.01 |
| Pharmacy | 0.76 | <0.01 |
| Coleaders | 0.68 | <0.01 |
Assessment of Teamwork by Unit, Domain, and Subteams
Teaching and nonteaching hospitalist units had similar team scores (median [interquartile range (IQR)] = 5.2 [1.0] vs 5.2 [0.4]; P = 0.55). We found significant differences in teamwork scores across units and domains, and found differences of borderline statistical significance across subteams (see Table 2). For unit teamwork scores, the median (IQR) was 4.4 (3.94.9) for the lowest and 5.4 (5.35.5) for the highest performing unit (P = 0.008). Across domain scores, leadership received the lowest score (median [IQR] = 5.0 [4.65.3]), and cooperation/backup behavior and monitoring/situational awareness received the highest scores (median [IQR]) = 5.4 [5.05.5] and 5.4 [5.05.7]; P = 0.02). Subteam scores ranged from a median (IQR) 5.0 (4.45.8) for coleaders to 5.5 (5.05.8) for SW‐CM (P = 0.05). We found no relationship between unit teamwork score and time spent in discussion per patient (rho = 0.04; P = 0.79).
| Median (IQR) | P Value | |
|---|---|---|
| ||
| Unit (n = 44)* | ||
| A | 5.3 (5.15.4) | 0.008 |
| B | 5.4 (5.35.5) | |
| C | 5.1 (4.95.2) | |
| D | 5.4 (5.25.6) | |
| E | 4.4 (3.94.9) | |
| F | 5.3 (5.15.5) | |
| Domain (n = 44) | ||
| Communication | 5.2 (4.95.4) | 0.02 |
| Coordination | 5.2 (4.75.4) | |
| Cooperation/backup behavior | 5.4 (5.05.5) | |
| Leadership | 5.0 (4.65.3) | |
| Monitoring/situational awareness | 5.4 (5.05.7) | |
| Subteam (n = 44) | ||
| Physicians | 5.2 (4.95.4) | 0.05 |
| Nurses | 5.2 (5.05.4) | |
| SW‐CM | 5.5 (5.05.8) | |
| Pharmacy | 5.3 (4.85.8) | |
| Coleaders | 5.0 (4.45.8) | |
DISCUSSION
We found that the adapted OTAS instrument demonstrated acceptable reliability for assessing teamwork during SIDR across units, domains, and most subteams. Although teamwork scores during SIDR were generally high, we found variation in performance across units, domains, and subteams. Variation in performance is notable in light of our efforts to implement a consistent format for SIDR across units. Specifically, all units have similar timing, duration, frequency, and location of SIDR, use a structured communication tool for new patients, expect the same professions to be represented, and use coleaders to facilitate discussion. We believe teamwork within IDR likely varies across units in other hospitals, and perhaps to a larger degree, given the emphasis on purposeful design and implementation of SIDR in our hospital.
Our findings are important for several reasons. First, though commonly used in hospital settings, the effectiveness of IDR is seldom assessed. Hospitalists and other professionals may not be able to identify or characterize deficiencies in teamwork during IDR without objective assessment. The adapted OTAS instrument provides a useful tool to evaluate team performance during IDR. Second, professionals may conclude that the mere implementation of an intervention such as SIDR will improve teamwork ratings and improve patient safety. Importantly, published studies evaluating the benefits of SIDR reflected a pilot study occurring on 2 units.15, 16, 19 The current study emphasizes the need to ensure that interventions proven to be effective on a small scale are implemented consistently when put into place on a larger scale.
Despite good reliability for assessing teamwork during SIDR across units, domains, and most subteams, we found poor inter‐rater reliability for the physician subteam. The explanation for this finding is not entirely clear. We reviewed the anchors for the physician subteam behaviors and were unable to identify ambiguity in anchor definitions. An analysis of domain scores within the physician subteam did not reveal any specific pattern to explain the poor correlation.
We found that the leadership domain and coleader subteam received particularly low scores. The explanation for this finding likely relates to changes in the nurse management structure shortly before our study, which reduced attendance by nurse managers and created a need for clinical coordinators to take on a leadership role during SIDR. Although we provided simulation‐based training to unit medical directors and nurse managers prior to implementing SIDR in March 2010, clinical coordinators were not part of the initial training. Our study suggests a need to provide additional training to coleaders, including clinical coordinators, to enhance their ability to facilitate discussion in SIDR.
We found no difference in overall teamwork scores when comparing teaching service units to nonteaching hospitalist service units. Duration of SIDR was significantly longer on teaching service units, but there was no association between duration of discussion and overall team score. The difference in duration of SIDR is likely explained by less succinct discussions on the part of housestaff physicians compared to more experienced hospitalists. Importantly, the quality of input, and its impact on teamwork during SIDR, does not appear to suffer when physician discussion is less efficient.
Our study has several limitations. First, we evaluated IDR in a single, urban, academic institution, which may limit generalizability. Our version of IDR (ie, SIDR) was designed to improve teamwork and incorporate a structured communication tool with regularly held interdisciplinary meetings. Features of IDR may differ in other hospitals. Second, the high teamwork scores seen in our study may not be generalizable to hospitals which have used a less rigorous, less standardized approach to IDR. Third, SIDR did not include patients or caregivers. Research is needed to test strategies to include patients and caregivers as active team members and participants in clinical decisions during hospitalization. Finally, we used the term interdisciplinary rounds to be consistent with prior published research. The term interprofessional may be more appropriate, as it specifically describes interactions among members of different professions (eg, physicians, nurses, social workers) rather than among different disciplines within a profession (eg, cardiologists, hospitalists, surgeons).
In summary, we found that teamwork during IDR could be reliably assessed using an adapted OTAS instrument. Although scores were generally high, we found variation in performance across units and domains suggesting a need to improve consistency of teamwork performance across units, domains, and subteams. Our study fills an important gap in the literature. Although IDR is commonly used in hospitals, and research shows improvements in ratings of collaboration,11, 12 little if any research has evaluated teamwork during IDR. Beyond the mere implementation of IDR, our study suggests the need to confirm that teamwork is optimal and consistent. Furthermore, hospital leaders should consider specific training for clinicians leading discussion during IDR.
Acknowledgements
The authors express their gratitude to Nick Sevdalis, BSc, MSc, PhD for providing the OTAS instrument and detailed instructions on its use.
Disclosures: Dr O'Leary, Ms Creden, and Dr Williams received salary support from the Agency for Healthcare Research and Quality, grant R18 HS019630. All authors disclose no other relevant or financial conflicts of interest.
Teamwork is essential to delivering safe and effective hospital care,15 yet the fluidity and geographic dispersion of team members in the hospital setting presents a significant barrier to teamwork.6 Physicians, nurses, and other hospital professionals frequently lack convenient and reliable opportunities to interact, and may struggle in efforts to discuss the care of their patients in person. Research studies show that nurses and physicians on patient care units do not communicate consistently and frequently do not agree on key aspects of their patients' plans of care.7, 8
Interdisciplinary rounds (IDR), also known as multidisciplinary rounds, provide a means to assemble hospital care team members and improve collaboration.913 Prior research on the use of IDR has demonstrated improved ratings of collaboration,11, 12 but inconsistent effects on length of stay and cost.10, 12, 13 Notably, the format, frequency, and duration of IDR in prior studies has been variable and no studies, to our knowledge, have evaluated teamwork performance during IDR. Lamb and colleagues conducted observations of cancer teams during multidisciplinary meetings.14 Trained observers used a validated observation tool to rate teamwork and found significant variation in performance by subteams. However, the study focused mainly on discussion among physician team members during meetings to plan longitudinal care for oncology patients.
We recently reported on the use of structured interdisciplinary rounds (SIDR) on 2 medical units in our hospital.15, 16 SIDR combines a structured format for communication, similar to a goals‐of‐care form,17, 18 with a forum for daily interdisciplinary meetings. Though no effect was seen on length of stay or cost, SIDR was associated with significantly higher ratings of the quality of collaboration and teamwork climate, and a reduction in the rate of adverse events.19 In March 2010, we implemented SIDR across all medical units in our hospital. We subjectively noted variation in teamwork performance during SIDR after a modification of nurse manager roles. We sought to evaluate teamwork during SIDR and to determine whether variation in performance existed and, if present, to characterize it.
METHODS
Setting and Study Design
The study was conducted at Northwestern Memorial Hospital (NMH), a 920‐bed tertiary care teaching hospital in Chicago, IL, and was deemed exempt by the Institutional Review Board of Northwestern University. General medical patients were admitted to 1 of 6 units based on bed availability. Five of the medical units consisted of 30 beds, and 1 unit consisted of 23. Each unit was equipped with continuous cardiac telemetry monitoring. Three units were staffed by teaching service physician teams consisting of 1 attending, 1 resident, and 1 or 2 interns. The other 3 units were staffed by hospitalists without the assistance of resident or intern physicians. As a result of a prior intervention, physicians' patients were localized to specific units in an effort to improve communication practices among nurses and physicians.20
Beginning in March 2010, all general medical units held SIDR each weekday morning. SIDR took place in the unit conference room, was expected to last approximately 3040 minutes, and was co‐led by the unit nurse manager and a medical director. Unit nurse managers and medical directors received specific training for their roles, including 3 hours of simulation‐based exercises designed to enhance their skills in facilitating discussion during SIDR. All nurses and physicians caring for patients on the unit, as well as the pharmacist, social worker, and case manager assigned to the unit, attended SIDR. Attendees used a structured communication tool to review patients admitted in the previous 24 hours. The plan of care for other patients was also discussed in SIDR, but without the use of the structured communication tool.
Importantly, nurse management underwent restructuring in the summer of 2011. Nurse managers, who had previously been responsible for overseeing all nursing activities on a single unit, were redeployed to be responsible for specific activities across 34 units. This restructuring made it very difficult for nurse managers to colead SIDR. As a result, the unit nurse clinical coordinator assumed coleadership of SIDR with the unit medical director. Nurse clinical coordinators worked every weekday and did not have patient care responsibilities while on duty. In addition to their role in coleading SIDR, nurse clinical coordinators addressed daily staffing and scheduling challenges and other short‐term patient care needs.
Teamwork Assessment
We adapted the Observational Teamwork Assessment for Surgery (OTAS) tool, a behaviorally anchored rating scale shown to be reliable and valid in surgical settings.2123 The OTAS tool provides scores ranging from 0 to 6 (0 = problematic behavior; 3 = team function neither hindered nor enhanced by behavior; 6 = exemplary behavior) across 5 domains (communication, coordination, cooperation/backup behavior, leadership, and monitoring/situational awareness) and for prespecified subteams. We defined domains as described by the researchers who developed OTAS. Communication referred to the quality and the quantity of information exchanged by team members. Coordination referred to management and timing of activities and tasks. Cooperation and backup behavior referred to assistance provided among members of the team, supporting others and correcting errors. Leadership referred to provision of directions, assertiveness, and support among team members. Monitoring and situational awareness referred to team observation and awareness of ongoing processes. We defined subteams for each group of professionals expected to attend SIDR. Specifically, subteams included physicians, nurses, social work‐case management (SW‐CM), pharmacy, and coleaders. We combined social work and case management because these professionals have similar patient care activities. Similarly, we combined unit medical directors and nurse clinical coordinators as coleaders. By providing data on teamwork performance within specific domains and for specific subteams, the OTAS instrument helps identify factors influencing overall teamwork performance. We modified OTAS anchors to reflect behaviors during SIDR. Anchors assisted observers in their rating of teamwork behaviors during SIDR. For example, an anchor for exemplary physician communication behavior was listens actively to other team members (looks at other team members, nods, etc). An anchor for exemplary physician leadership was assigns responsibility for task completion when appropriate.
Two researchers conducted unannounced direct observations of SIDRs. One researcher (Y.N.B) was a medical librarian with previous experience conducting observational research. The other researcher (A.J.C.) had observed 170 prior SIDRs as part of a related study. Both researchers observed 10 SIDRs to practice data collection and to inform minor revisions of the anchors. We aimed to conduct 78 independent observations for each unit, and 20 joint observations to assess inter‐rater reliability. All subteams were scored for each domain. For example, all subteams received leadership domain scores because all team members exhibit leadership behaviors, depending on the situation. In addition to teamwork scores, observers recorded the number of patients on the unit, the number of patients discussed during SIDR, attendance by subteam members, and the duration of SIDR. For the SW‐CM and coleader subteams, we documented presence if one of the subteam members was present for each patients' discussion. For example, we recorded present for SW‐CM if the social worker was in attendance but the case manager was not.
Data Analysis
We calculated descriptive statistics to characterize SIDRs. We used Spearman's rank correlation coefficients to assess inter‐rater reliability for joint observations. Spearman's rank correlation is a nonparametric test of association and appropriate for assessing agreement between observers when using data that is not normally distributed. Spearman rho values range from 1 to 1, with 1 signifying perfect inverse correlation, 0 signifying no correlation, and 1 signifying perfect correlation. We used the MannWhitney U test to assess for differences in overall team scores between services (teaching vs nonteaching hospitalist service) and KruskalWallis tests to assess for differences across units, domains, and subteams. The Kruskal‐Wallis test is a nonparametric test appropriate for comparing three or more independent samples in which the outcome is not normally distributed. We used a t test to assess for difference in duration by service, and Spearman rank correlation to assess for correlation between time spent in discussion per patient and overall team score. All analyses were conducted using Stata version 11.0 (College Station, TX).
RESULTS
SIDR Characteristics
We performed 7 direct observations of SIDR for 4 units, and 8 observations for 2 units (44 total observations). Units were at 99% capacity, and SIDR attendees discussed 98% of patients on the unit. Attendance exceeded 98% for each subteam (physicians, nurses, SW‐CM, pharmacy, and coleaders). SIDR lasted a mean 41.4 11.1 minutes, with a mean 1.5 0.4 minutes spent in discussion per patient. SIDR was significantly longer in duration on teaching service units compared to the nonteaching hospitalist service units (1.7 0.3 vs 1.3 0.4 minutes per patient; P < 0.001).
Inter‐Rater Reliability
Inter‐rater reliability across unit level scores was excellent (rho = 0.75). As shown in Table 1, inter‐rater reliability across domains was good (rho = 0.530.68). Inter‐rater reliability across subteams was good to excellent (rho = 0.530.76) with the exception of the physician subteam, for which it was poor (rho = 0.35).
| Spearman's rho | P Value | |
|---|---|---|
| ||
| Domain (n = 20) | ||
| Communication | 0.62 | <0.01 |
| Coordination | 0.60 | <0.01 |
| Cooperation/backup behavior | 0.66 | <0.01 |
| Leadership | 0.68 | <0.01 |
| Monitoring/situational awareness | 0.53 | 0.02 |
| Subteam (n = 20) | ||
| Physicians | 0.35 | 0.14 |
| Nurses | 0.53 | 0.02 |
| SW‐CM | 0.60 | <0.01 |
| Pharmacy | 0.76 | <0.01 |
| Coleaders | 0.68 | <0.01 |
Assessment of Teamwork by Unit, Domain, and Subteams
Teaching and nonteaching hospitalist units had similar team scores (median [interquartile range (IQR)] = 5.2 [1.0] vs 5.2 [0.4]; P = 0.55). We found significant differences in teamwork scores across units and domains, and found differences of borderline statistical significance across subteams (see Table 2). For unit teamwork scores, the median (IQR) was 4.4 (3.94.9) for the lowest and 5.4 (5.35.5) for the highest performing unit (P = 0.008). Across domain scores, leadership received the lowest score (median [IQR] = 5.0 [4.65.3]), and cooperation/backup behavior and monitoring/situational awareness received the highest scores (median [IQR]) = 5.4 [5.05.5] and 5.4 [5.05.7]; P = 0.02). Subteam scores ranged from a median (IQR) 5.0 (4.45.8) for coleaders to 5.5 (5.05.8) for SW‐CM (P = 0.05). We found no relationship between unit teamwork score and time spent in discussion per patient (rho = 0.04; P = 0.79).
| Median (IQR) | P Value | |
|---|---|---|
| ||
| Unit (n = 44)* | ||
| A | 5.3 (5.15.4) | 0.008 |
| B | 5.4 (5.35.5) | |
| C | 5.1 (4.95.2) | |
| D | 5.4 (5.25.6) | |
| E | 4.4 (3.94.9) | |
| F | 5.3 (5.15.5) | |
| Domain (n = 44) | ||
| Communication | 5.2 (4.95.4) | 0.02 |
| Coordination | 5.2 (4.75.4) | |
| Cooperation/backup behavior | 5.4 (5.05.5) | |
| Leadership | 5.0 (4.65.3) | |
| Monitoring/situational awareness | 5.4 (5.05.7) | |
| Subteam (n = 44) | ||
| Physicians | 5.2 (4.95.4) | 0.05 |
| Nurses | 5.2 (5.05.4) | |
| SW‐CM | 5.5 (5.05.8) | |
| Pharmacy | 5.3 (4.85.8) | |
| Coleaders | 5.0 (4.45.8) | |
DISCUSSION
We found that the adapted OTAS instrument demonstrated acceptable reliability for assessing teamwork during SIDR across units, domains, and most subteams. Although teamwork scores during SIDR were generally high, we found variation in performance across units, domains, and subteams. Variation in performance is notable in light of our efforts to implement a consistent format for SIDR across units. Specifically, all units have similar timing, duration, frequency, and location of SIDR, use a structured communication tool for new patients, expect the same professions to be represented, and use coleaders to facilitate discussion. We believe teamwork within IDR likely varies across units in other hospitals, and perhaps to a larger degree, given the emphasis on purposeful design and implementation of SIDR in our hospital.
Our findings are important for several reasons. First, though commonly used in hospital settings, the effectiveness of IDR is seldom assessed. Hospitalists and other professionals may not be able to identify or characterize deficiencies in teamwork during IDR without objective assessment. The adapted OTAS instrument provides a useful tool to evaluate team performance during IDR. Second, professionals may conclude that the mere implementation of an intervention such as SIDR will improve teamwork ratings and improve patient safety. Importantly, published studies evaluating the benefits of SIDR reflected a pilot study occurring on 2 units.15, 16, 19 The current study emphasizes the need to ensure that interventions proven to be effective on a small scale are implemented consistently when put into place on a larger scale.
Despite good reliability for assessing teamwork during SIDR across units, domains, and most subteams, we found poor inter‐rater reliability for the physician subteam. The explanation for this finding is not entirely clear. We reviewed the anchors for the physician subteam behaviors and were unable to identify ambiguity in anchor definitions. An analysis of domain scores within the physician subteam did not reveal any specific pattern to explain the poor correlation.
We found that the leadership domain and coleader subteam received particularly low scores. The explanation for this finding likely relates to changes in the nurse management structure shortly before our study, which reduced attendance by nurse managers and created a need for clinical coordinators to take on a leadership role during SIDR. Although we provided simulation‐based training to unit medical directors and nurse managers prior to implementing SIDR in March 2010, clinical coordinators were not part of the initial training. Our study suggests a need to provide additional training to coleaders, including clinical coordinators, to enhance their ability to facilitate discussion in SIDR.
We found no difference in overall teamwork scores when comparing teaching service units to nonteaching hospitalist service units. Duration of SIDR was significantly longer on teaching service units, but there was no association between duration of discussion and overall team score. The difference in duration of SIDR is likely explained by less succinct discussions on the part of housestaff physicians compared to more experienced hospitalists. Importantly, the quality of input, and its impact on teamwork during SIDR, does not appear to suffer when physician discussion is less efficient.
Our study has several limitations. First, we evaluated IDR in a single, urban, academic institution, which may limit generalizability. Our version of IDR (ie, SIDR) was designed to improve teamwork and incorporate a structured communication tool with regularly held interdisciplinary meetings. Features of IDR may differ in other hospitals. Second, the high teamwork scores seen in our study may not be generalizable to hospitals which have used a less rigorous, less standardized approach to IDR. Third, SIDR did not include patients or caregivers. Research is needed to test strategies to include patients and caregivers as active team members and participants in clinical decisions during hospitalization. Finally, we used the term interdisciplinary rounds to be consistent with prior published research. The term interprofessional may be more appropriate, as it specifically describes interactions among members of different professions (eg, physicians, nurses, social workers) rather than among different disciplines within a profession (eg, cardiologists, hospitalists, surgeons).
In summary, we found that teamwork during IDR could be reliably assessed using an adapted OTAS instrument. Although scores were generally high, we found variation in performance across units and domains suggesting a need to improve consistency of teamwork performance across units, domains, and subteams. Our study fills an important gap in the literature. Although IDR is commonly used in hospitals, and research shows improvements in ratings of collaboration,11, 12 little if any research has evaluated teamwork during IDR. Beyond the mere implementation of IDR, our study suggests the need to confirm that teamwork is optimal and consistent. Furthermore, hospital leaders should consider specific training for clinicians leading discussion during IDR.
Acknowledgements
The authors express their gratitude to Nick Sevdalis, BSc, MSc, PhD for providing the OTAS instrument and detailed instructions on its use.
Disclosures: Dr O'Leary, Ms Creden, and Dr Williams received salary support from the Agency for Healthcare Research and Quality, grant R18 HS019630. All authors disclose no other relevant or financial conflicts of interest.
- Joint Commission on Accreditation of Healthcare Organizations. Sentinel Event Statistics. Available at: http://www.jointcommission.org/SentinelEvents/Statistics/. Accessed January 19,2012.
- ,,, et al.A look into the nature and causes of human errors in the intensive care unit.Crit Care Med.1995;23(2):294–300.
- ,,, et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324(6):377–384.
- ,,.Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79(2):186–194.
- ,,,,,.The Quality in Australian Health Care Study.Med J Aust.1995;163(9):458–471.
- ,,,,,.Teamwork on inpatient medical units: assessing attitudes and barriers.Qual Saf Health Care.2010;19(2):117–121.
- ,,,,,.Can we talk? Priorities for patient care differed among health care providers. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds.Advances in Patient Safety: From Research to Implementation. Vol1: Research Findings. AHRQ Publication No. 05–0021‐1.Rockville, MD:Agency for Healthcare Research and Quality;2005.
- ,,, et al.Patterns of nurse‐physician communication and agreement on the plan of care.Qual Saf Health Care.2010;19(3):195–199.
- ,,, et al.The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs.J Nurs Adm.2006;36(2):79–85.
- ,,.A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement.Med Care.1998;36(8 suppl):AS4–AS12.
- ,,,.Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses.Am J Crit Care.2005;14(1):71–77.
- ,,,,.Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay.J Gen Intern Med.2007;22(8):1073–1079.
- ,,,.Effects of interdisciplinary rounds on length of stay in a telemetry unit.J Public Health Manag Pract.2004;10(1):63–69.
- ,,,,.Teamwork and team performance in multidisciplinary cancer teams: development and evaluation of an observational assessment tool.BMJ Qual Saf.2011 [Epub ahead of print].
- ,,,,,.Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit.J Hosp Med.2011;6(2):88–93.
- ,,,,,.Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit.J Gen Intern Med.2010;25(8):826–832.
- ,,,,.Improving nurse‐physician communication and satisfaction in the intensive care unit with a daily goals worksheet.Am J Crit Care.2006;15(2):217–222.
- ,,,,,.Improving communication in the ICU using daily goals.J Crit Care.2003;18(2):71–75.
- ,,, et al.Structured interdisciplinary rounds in a medical teaching unit: improving patient safety.Arch Intern Med.2011;171(7):678–684.
- ,,, et al.Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care.J Gen Intern Med.2009;24(11):1223–1227.
- ,,,,.Observational teamwork assessment for surgery (OTAS): refinement and application in urological surgery.World J Surg.2007;31(7):1373–1381.
- ,,,,,.Observational teamwork assessment for surgery: construct validation with expert versus novice raters.Ann Surg.2009;249(6):1047–1051.
- ,,,,.Observational teamwork assessment for surgery: content validation and tool refinement.J Am Coll Surg.2011;212(2):234–243.e1–5.
- Joint Commission on Accreditation of Healthcare Organizations. Sentinel Event Statistics. Available at: http://www.jointcommission.org/SentinelEvents/Statistics/. Accessed January 19,2012.
- ,,, et al.A look into the nature and causes of human errors in the intensive care unit.Crit Care Med.1995;23(2):294–300.
- ,,, et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324(6):377–384.
- ,,.Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79(2):186–194.
- ,,,,,.The Quality in Australian Health Care Study.Med J Aust.1995;163(9):458–471.
- ,,,,,.Teamwork on inpatient medical units: assessing attitudes and barriers.Qual Saf Health Care.2010;19(2):117–121.
- ,,,,,.Can we talk? Priorities for patient care differed among health care providers. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds.Advances in Patient Safety: From Research to Implementation. Vol1: Research Findings. AHRQ Publication No. 05–0021‐1.Rockville, MD:Agency for Healthcare Research and Quality;2005.
- ,,, et al.Patterns of nurse‐physician communication and agreement on the plan of care.Qual Saf Health Care.2010;19(3):195–199.
- ,,, et al.The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs.J Nurs Adm.2006;36(2):79–85.
- ,,.A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement.Med Care.1998;36(8 suppl):AS4–AS12.
- ,,,.Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses.Am J Crit Care.2005;14(1):71–77.
- ,,,,.Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay.J Gen Intern Med.2007;22(8):1073–1079.
- ,,,.Effects of interdisciplinary rounds on length of stay in a telemetry unit.J Public Health Manag Pract.2004;10(1):63–69.
- ,,,,.Teamwork and team performance in multidisciplinary cancer teams: development and evaluation of an observational assessment tool.BMJ Qual Saf.2011 [Epub ahead of print].
- ,,,,,.Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit.J Hosp Med.2011;6(2):88–93.
- ,,,,,.Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit.J Gen Intern Med.2010;25(8):826–832.
- ,,,,.Improving nurse‐physician communication and satisfaction in the intensive care unit with a daily goals worksheet.Am J Crit Care.2006;15(2):217–222.
- ,,,,,.Improving communication in the ICU using daily goals.J Crit Care.2003;18(2):71–75.
- ,,, et al.Structured interdisciplinary rounds in a medical teaching unit: improving patient safety.Arch Intern Med.2011;171(7):678–684.
- ,,, et al.Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care.J Gen Intern Med.2009;24(11):1223–1227.
- ,,,,.Observational teamwork assessment for surgery (OTAS): refinement and application in urological surgery.World J Surg.2007;31(7):1373–1381.
- ,,,,,.Observational teamwork assessment for surgery: construct validation with expert versus novice raters.Ann Surg.2009;249(6):1047–1051.
- ,,,,.Observational teamwork assessment for surgery: content validation and tool refinement.J Am Coll Surg.2011;212(2):234–243.e1–5.
Copyright © 2012 Society of Hospital Medicine
Sleep and Circadian Misalignment
For hospitalists, patient care is 24 hours a day. To provide continual patient care, shift work has become a way of life for hospitalists, similar to hospital nurses, residents in training, and emergency medicine physicians. Notably, they belong to a substantial minority of the workforce as shift workers, starting after 6 PM or before 6 AM, approximately one‐fifth of the total work force in industrialized nations.1, 2 Unfortunately, shift workers suffer from misalignment of their endogenous circadian system, which regulates daily sleep and alertness patterns, and work obligations beyond daylight hours. Such a misalignment can lead to fatigue, sleep loss, and excessive sleepiness, which can adversely affect personal health and safety, as well as the quality of medical care delivered.3
The relationship between shift work, extended work hours, and medical safety is a topic currently under intense scrutiny, as reviewed in the Institute of Medicine's (IOM) controversial report on residents and sleep.4 This publication led the Accreditation Council of Graduate Medical Education (ACGME) to mandate more changes to residents' work hours,5 adding to those first implemented in 2003.6 These restrictions forbid residents from working more than 30 consecutive hours, and required at least 10 hours off between shifts and an average of 1 day off in 7. Subsequent studies suggested that the reduction in resident work hours led to greater resident well‐being, fewer attention failures. and fewer medical errors.3, 7
In 2007, amid growing public concern over sleep‐deprived residents and patient safety, Congress requested the IOM investigate additional safeguards for residents.8 In 2008, the IOM published a report calling for more protection against resident fatigue.4 They recommended integrating a protected sleep period into any 24‐hour shift. If residents cannot get protected sleep time, then the maximal shift duration should not exceed 16 hoursreduced from the previous ACGME recommendation of 30. Further provisions to allow adequate sleep include capping the number of consecutive night shifts at 4, and extending the time off after a night shift. In response, the ACGME recently updated their recommendations effective July 1, 2011,5 though not following all the IOM's recommendations (Table 1).
| 2003 ACGME Limits | 2008 IOM Recommendation | 2010 ACGME Limits | |
|---|---|---|---|
| |||
| Maximum work hours per week | 80 hr, averaged over 4 wk | No change | No change |
| Maximum shift length | 30 hr (admitting patients up to 24 hr, with 6 hr of transition activities) | 30 hr (admitting patients up to 16 hr, with 5 hr protected sleep between 10 PM to 8 AM, and remaining hours for transition activities) | PGY‐1: 16 hr; PGY‐2 and above: 28 hr (admitting patients up to 24 hr, with 4 hr of transition activities) |
| Minimum time off between shifts | 10 hr after shift | 10 hr after day shift; 12 hr after night shift; 14 hr after any extended shift of 30 hr and not return until 6 AM the next day | 10 hr after shift; 14 hr free after 24‐hr shift for intermediate level residents |
| Maximum frequency of in‐hospital night shifts | No limits | 4 night maximum, with 48 hr off after 3 or 4 consecutive shifts | 6 consecutive night maximum |
The growing nationwide emphasis on fatigue prevention within healthcare settings now clearly impacts residents and their training schedule. But why focus only on residents? Why not other physicians, such as hospitalists, who work shifts to cover 24 hours each day? Are they any less prone to making medical errors when fatigued? Given that hospitalists' represent the fastest growing specialty in the history of American medicine,9 we sought to inform decisions about their scheduling by reviewing normal regulation of sleep and wake patterns, addressing the problems associated with misalignment between sleep and work, and identifying strategies to realign circadian schedules.
NORMAL SLEEP AND CIRCADIAN RHYTHMS
An understanding of sleep physiology begins with the endogenous circadian timekeeping system. At the center of this timekeeping system is a master circadian clock, located in the suprachiasmatic nucleus (SCN) of the hypothalamus. Cells within the SCN generate a near 24‐hour rhythm, transmitted through neural connections, to rhythmically influence the entire central nervous system and other bodily systems.10
The SCN and the circadian rhythm interact with the need to sleep (sleep homeostasis) to form the 2‐process model of sleepwakefulness.11 In this model, progression of biological day (a time when wakefulness and its associated functions are promoted) coincides with a rise in homeostatic pressure to sleep (see Figure 1). Daytime alertness is maintained by increasing SCN neuronal activity to counterbalance rising sleep pressure. After peaking in the early evening, SCN activity falls to begin biological night (a time when sleep and its associated functions are promoted). To facilitate the onset of biological night, the SCN coordinates the activity of sleep‐promoting centers and the release of melatonin from the pineal gland which promotes sleep.
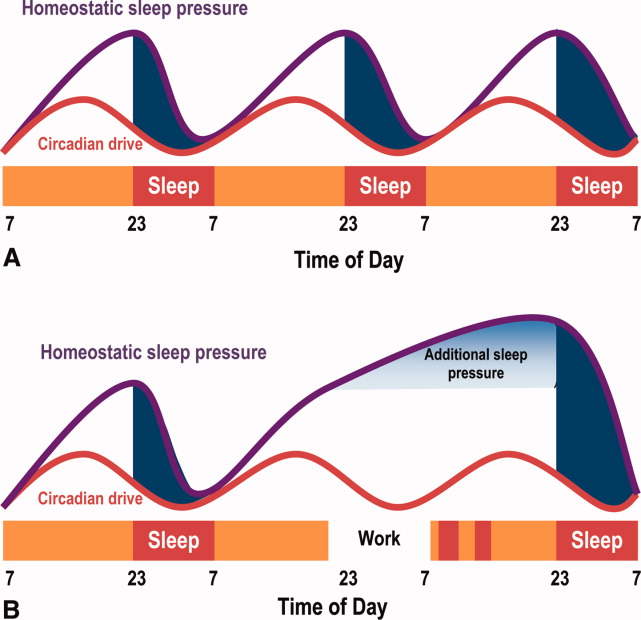
This endogenous circadian clock runs slightly longer than 24 hours and must be resynchronized daily to the 24‐hour day, a process known as entrainment. This occurs primarily through environmental exposure of retinalhypothalamic links to the lightdark cycle. The intensity, duration, and wave length of light all influence the circadian system,12 but perhaps most importantly is the timing. In general, light exposure in the evening will shift the circadian clock later (phase delay shift), whereas light exposure in the morning will shift the clock earlier (phase advance shift). Exogenous melatonin can also shift the circadian system. However, when endogenous levels of melatonin are high, ingested melatonin has little influence on sleep.13
Balancing sleep and wakefulness requires an interweaving of endogenous and exogenous factors. This balance is disturbed if we try to sleep or be wakeful during incorrect endogenous biological times, a process called circadian misalignment.
DELETERIOUS EFFECTS OF CIRCADIAN MISALIGNMENT
Hospitalists and other shift workers required to work during the biological night risk circadian misalignment and, consequently, poor sleep, shift work disorder, errors on the job, and possibly long‐term health consequences.
Chronic Sleep Loss
When working at night or in the early morning, nearly 75% of shift workers encounter some amount of at‐work fatigue and sleepiness.14 After the shift is over, objective assessments among rotating shift workers15, 16 and interns7 demonstrated that day sleep is 1 to 4 hours shorter than night sleep. Chronic or recurring night shifts can therefore lead to chronic sleep loss. While it seems reasonable that permanent night shift workers have greater circadian adjustment to suit their work schedule, little evidence supports this argument.17 Permanent night shift workers may sleep a little longer during the day than rotating shift workers. Yet, the sleep quality does not match night sleep, presumably from conflict between external factors, such as light and activity, and the scheduled sleep period.
Shift Work Disorder
If severe and chronic, sleepiness and impaired performance during work hours and poor sleep during the day can be enough to warrant a diagnosis of shift work disorder (SWD), one of the several circadian rhythm sleep disorders (CRSD). The prevalence of SWD among rotating and night workers is estimated to be 10%25%.18 Patients with SWD can experience similar levels of nighttime sleepiness as patients with narcolepsy and sleep apnea.19 These patients experience reduced satisfaction with the work schedule, and suffer higher rates of depression, ulcers, and sleepiness‐related accidents, compared to other shift workers.18 What distinguishes those shift workers who suffer from normal fatigue and those with SWD is not easily identified. The International Classification of Sleep Disorders‐2 (ICSD‐2) lists the diagnostic criteria for SWD20:
-
Symptoms of insomnia associated with a work schedule that overlaps the usual time for sleep.
-
Symptoms are directly associated with shift work schedule over the course of at least 1 month.
-
Sleep log monitoring for at least 7 days demonstrates circadian and sleep‐time misalignment.
-
Sleep disturbance is not better explained by another sleep disorder or by a medical, neurological, or mental disorder; medication use; or substance‐use disorder.
Symptoms must be present for at least 1 month, and comorbid mood or sleep/wake disorders (commonly found in this disorder) need to be treated. SWD is more common among night shift workers, although those workers starting shifts between 4 AM and 7 AM (early morning shift) are also subject to SWD.21 Type of work schedule, along with physical or mental disorders, domestic responsibilities, and commute times are examples of factors that may increase vulnerability for SWD.18 In addition, genetic factors may explain the considerable inter‐individual differences in susceptibility to SWD. For example, a polymorphism in the circadian gene, PER 3, present in 10%15% of the population, is believed to decrease tolerability to acute sleep loss,22 while genetic variation in the adenosine A2A receptor may be associated with resistance to the effects of sleep loss.23 If a hospitalist suspects a diagnosis SWD, they should seek evaluation by a physician specializing in sleep medicine.
Errors
Disruption of the circadian rhythm influences neurocognitive and psychomotor function, and can lead to human error. Human errors that result in serious accidents or injuries typically result from interaction of circadian rhythm misalignment with multiple other factors, including task duration and complexity, motivation and proficiency, and level of sleep deprivation.24 Though difficult to isolate from the environmental and work experience factors, consistent evidence identifies circadian misalignment as a cause of errors and serious accidents. Most evidence comes from night shift workers trying to remain awake when the circadian signal for alertness is low, or attempting sleep when the circadian alerting signal is high. Compared to day workers, night shift workers are 1.63 times more likely to suffer a fatal accident.25 A study of critical care nurses revealed a prominent circadian pattern of inadvertent sleep episodes during work with the highest peak between 2 AM and 6 AM.26 In addition, nurses working the night shift have been shown to commit more medication administration errors than day workers.27
Medical errors among resident physicians during extended shift durations is well documented.28 On the other hand, not much research has examined error rates among attending physicians. In 1 small study, attending surgeons made more cognitive errors using a simulated laparoscopic exercise as the amount of on‐call overnight sleep decreased.29 A large, single‐center review reported an increased rate of complications among post‐nighttime surgical procedures performed by attendings who slept 6 hours or less the preceding night.30 Notably, proposed legislation would require physicians who have been awake 22 of the preceding 24 hours to inform patients of the potential safety impact of their sleep deprivation prior to providing clinical care.31
Chronic Health Morbidity
Several studies reveal the effect of shift work on chronic health conditions among healthcare workers, such as obesity, cardiovascular disease, and certain cancers (eg, breast, colorectal). These results are summarized in Table 2, with the largest evaluation of healthcare shift workers coming from the Nurses' Health Study.3234
| Disease | Study Design | Population | Comparison | Health Risk | Adjusted Risk Factors |
|---|---|---|---|---|---|
| |||||
| Acute myocardial infarction | Prospective cohort32 | 79,109 US nurses | Working 3 night shifts/mo for 6 yr | RR 1.51 | CAD risk factors, aspirin use, hormone replacement therapy |
| 95% CI (1.12‐2.03) | |||||
| Obesity (BMI 30) | Cross‐sectional72 | 27,485 Swedish workers | Shift‐workers vs day workers | OR 1.41 | Age, socioeconomic status |
| 95% CI (1.25‐1.59) | |||||
| Breast cancer | Prospective cohort33 | 116,087 US nurses | Working 3 night shifts/mo for 20 yr | RR 1.79 | Breast cancer risk factors |
| 95% CI (1.06‐3.01) | |||||
| Colon cancer | Prospective cohort34 | 78,586 US nurses | Working 3 night shifts/mo for 15 yr | RR 1.35 | Family history of colon cancer, dietary intake, activity |
| 95% CI (1.03‐1.77) | |||||
Some believe that adverse health outcomes in shift workers derive from circadian stressan alteration of psychosocial and physiological homeostasis (eg, increased cortisol and catecholamine output) resulting from circadian misalignment.35 Based on data suggesting an increased risk for certain cancers among shift workers, the International Agency for Research of Cancer, a unit of the World Health Organization, announced that shift work resulting in circadian misalignment is probably carcinogenic.36 Researchers propose several biologic mechanisms to explain the increased cancer riskmost revolve around the alteration of the melatonin circadian cycle, found in night shift workers,37 and subsequent disruption of its believed cancer‐protective biologic pathways.
Overall, however, the heterogeneous nature of shift work limits conclusions regarding the long‐term health of shift workers. That is, as the shift work intensity and composition varies, and as the number and timing of these shifts change, so too can the adverse health consequences.
HOSPITALISTS AND NIGHT SHIFTS
Hospital medicine is the fastest growing specialty in the history of medicine, with an estimated 30,000 practicing hospitalists in 2010.38 Survey results from 2009 indicate that hospitalists staff 58% of hospitals; 89% of hospitals with more than 200 beds (J. Miller, Society of Hospital Medicine, personal communication). One reason for the growth in the number of hospitalists at academic medical centers has been the imposed work‐hour restrictions for residents.39
Across the county, hospitalist programs use a variety of shift work systems to ensure 24‐hour patient care. Among those programs that provide continuous on‐site coverage, many staff 3 shiftsday, late afternoon/evening (swing), and night shifts. Some permanently partition the scheduling, with dedicated night hospitalists or nocturnists.40
Hospitalists do not have mandated work‐hour restrictions and, in general, are older than resident physicians. Whether or not hospitalists who trained before the era of work‐hour regulations are better prepared for practicing in a real‐world, after‐hours scenario than hospitalists with previous work‐hour restrictions is a matter of debate. That said, hospitalists who are fatigued, just like residents, may be at increased risk for committing medical errors, particularly when the fatigue is unrecognized. Yet, limiting hospitalists' work hours would have obvious financial implications, likely similar those from resident work‐hour reductions.41 As part of the ACGME 2011 recommendations, faculty and residents now must be trained to recognize signs of fatigue and sleep deprivation, and adopt management strategies such as naps or backup call schedules. Fatigue that results in excessive sleepiness while at work may manifest as weariness, difficulty concentrating, headache, irritability or depressed mood, and feeling unrefreshed after sleeping.42
STRATEGIES TO IMPROVE CIRCADIAN ADAPTATION
Hospitalists can help limit fatigue and improve performance and safety through circadian adaptation: a multimodal approach to realign work and circadian schedules. Depending on whether the shift starts at night or in the early morning (4 AM to 7 AM), circadian adaptation aims may differ. For night shift workers, the overall aim is to delay the timing of circadian rhythms such that the highest propensity of wakefulness occurs during the night work period, while the highest propensity for sleep occurs during the day.17, 43 For early morning shift workers, circadian rhythms for wakefulness and sleep propensity should be shifted earlier. Circadian adaptation involves not only sleeping well before work, but also preventing dips in wakefulness during work. Adaptation strategies are listed in Table 3.
| Night Shift60 | Early Day Shift (Starting at 4 AM‐7 AM) | |
|---|---|---|
| Prior to shift | Avoid sleep debt | Avoid sleep debt |
| Proper sleep hygiene | Proper sleep hygiene | |
| Planned napping | Bright light exposure | |
| Caffeine use | ||
| During the shift | Bright light exposure | Caffeine use |
| Planned napping | ||
| After the shift | Avoid bright light | Avoid late evening bright light (when applicable) |
| Melatonin prior to sleep | ||
| Careful use of other hypnotics | Initiate sleep early |
Improved Sleep Before Work
As an essential first step, hospitalists must get a full night's rest before starting a night shift, as sleep debt will worsen fatigue while at work. Tips for proper sleep hygiene are listed in Table 4. Some shift workers stay up late the night before a scheduled night shift, in order to sleep during the day and awaken shortly before their scheduled night shift, to combat fatigue at work. Such an approach to shift work is typically met with 3 barriers. First, environmental factors often prevent 6 hours of uninterrupted day sleep. Second, 6 hours of continual day sleep is typically difficult because rising circadian activity often limits the sleep period to just a few hours. Third, an adequate amount of sleep prior to a night shift will itself not be enough to prevent sleepiness from occurring after midnightreducing the fall in circadian activity is also essential to maintaining alertness and performance.
| Physical | Adhere to regular wake and sleep schedule |
| activities73 | Engage in regular exercise early in the day |
| Avoid caffeine, nicotine, and alcohol use 6 hr prior to sleeping | |
| Avoid stimulating or stressful activities 30 min prior to sleeping | |
| Proper sleep | Well ventilated, temperature‐controlled bedroom |
| environment | Use heavy curtains to provide as much darkness as possible |
| Comfortable mattress and pillow | |
| Remove television and pets from the bedroom | |
| Housemates should help provide quiet sleep environment |
Napping
Napping prior to a night shift, or during the work shift, can improve alertness and performance and decrease accident rates.44, 45 During shift work, naps of 20 to 50 minutes in duration have demonstrated improvements in reaction time, and restoration of performance to that seen at the start of the shift. Napping early in the night shift can improve objective measures of alertness.44 To avoid increased drowsiness that sometimes occurs when waking from a nap, naps should not be longer than 50 minutes, and can be as short as 10 to 15 minutes.44, 46 Although effective, napping may be impractical for many workers due to time or space constraints. To facilitate brief naps, hospitalist practices should ensure they have a dark, quiet call room for use by overnight hospitalists.
Bright Light Exposure
Studies demonstrate that light exposure during the night shift improves circadian alignment, mood, and performance during the night shift.47, 48 Light exposure ranged from 6 hours to 5 light treatments of 15 minutes each, with brightness ranging from 2,500 to 10,000 lux (approximating outdoor daylight; typical office lighting provides 200‐500 lux).47, 49 Results demonstrate that bright light exposure during the night shift acutely improves alertness and performance, though not to daytime levels.50 The greatest circadian adjustments occur in groups using both bright light during the night shift and light avoidance the following morning.51 Dark sunglasses and a dark home environment can decrease bright light exposure during the day. Though little evidence exists to support widespread application of bright light devices in hospitalists' call‐rooms, a hospitalist practice should consider installing one to promote circadian adaptation if physicians working overnight have multiple consecutive shifts. Likewise, these physicians should be vigilant and wear dark sunglasses during the day after their night shifteven a few minutes of light exposure at the wrong time of the day may disrupt the intended circadian adaptation.
Wake‐Promoting Agents
Numerous studies demonstrate that 150 mg to 400 mg of caffeine (a 16 oz grande cup of coffee from Starbucks contains between 200 to 500 mg of caffeine52; a Diet Coke contains 46 mg/12 oz53) reduces sleepiness, increases alertness, and improves performance during the night shift.54, 55 Thus, judicious use of caffeine may be recommended in hospital practices during extended work hours. Other wake‐promoting agents, such as modafinil and armodafinil, are US Food and Drug Administration (FDA)‐approved in the treatment of excessive sleepiness associated with SWD. Typically taken 3060 minutes before the start of the night shift, these medications have been shown in trials, enrolling mostly permanent night shift workers, to reduce excessive nighttime sleepiness and improve performance.19, 56 Armodafinil used to treat SWD‐associated excessive sleepiness, has been safely tolerated for durations of 1 year or more.57 However, these agents are not approved for use in patients without a diagnosed sleep disorder.
Melatonin
Exogenous melatonin has been used to reset circadian rhythms in patients with CRSDs.58 Melatonin administered in the late afternoon to early evening directs the largest phase advance. In contrast, melatonin given in the morning produces the largest phase delays.59 When taken after a night shift, melatonin (at a dose 1.8 to 3.0 mg) can improve day sleep quality and duration.60 Despite this result, melatonin's effectiveness in improving circadian adaptation has been mixed.61 For example, improvements in nighttime alertness during the night shift were not seen, despite the use of melatonin to facilitate daytime sleep beforehand.62 Hospitalists may consider a trial of melatonin to improve circadian alignment and facilitate daytime sleep, but its chronic use and long‐term safety has not been adequately studied.
Hypnotics After Work
Hypnotics such as temazepam 20 mg,63 triazolam,64 and zolpidem65 taken after night shift work have been shown to improve day sleep quality under simulated conditions, but do not improve shift work performance. These medications should be reserved for judicious short‐term use in patients with insomnia associated with SWD.
NIGHT SHIFT SCHEDULING TO REDUCE CIRCADIAN MISALIGNMENT
When providing 24‐hour, on‐site medical care, questions may arise about how to incorporate circadian adaptation into the daily schedule.
How Should Shifts Be Rotated?
When scheduling shifts with different start times, evidence suggests that sleep disturbance is reduced with a clockwise progression in shifts (eg, day shift to evening shift to night shift). This reduction in sleep disturbance is thought due to increased time between shifts and the circadian timekeeping tendency to extend beyond 24 hours.66
When Should the Night Shift Start?
Those hospitalist programs using an evening swing shift from afternoon to late evening may have the option of using a 12‐hour night shift starting around 7 PM, or a shorter night shift beginning later at night. Though there are no data among hospitalists to suggest which night shift start time and duration would lead to the least amount of fatigue and errors, healthcare providers working a 12‐hour night shift may have increased morale due to fewer shifts, but may suffer a reduction in the quality of care provided compared with working an 8‐hour night shift.67 In either case, shift workers given flexibility in scheduling have been shown to have positive effects on sleep.68
Should Night Shifts Be Bunched?
The decision to bunch night shifts together depends on how many night shifts are required, and how quickly circadian adaptation can occur. Under simulated conditions, circadian adaptation can yield significant changes in sleep/wake cycles in as little as 4 days.48 In real‐world settings, more time may be required to achieve significant shifts in the circadian cycle. Therefore, hospitalists who have 7 or fewer night shifts during the academic year may want to space the shifts out to prevent sleep debt on consecutive shifts, since significant circadian adaptation would be difficult to achieve in less than a week. In this situation, after a night shift, the hospitalist should have at least one 9‐hour sleep period at night to relieve their sleep debt before staffing another night shift.69 Consecutive night shifts require at least 2 nighttime sleep periods of 9 hours to recover from sleep debt.70 The IOM recommends setting a limit of 4 consecutive night float shifts for resident physicians, however, a recent systematic review of resident night float models did not find data supporting use of a 4‐night‐maximum model.28
If more than 7 night shifts are required, then scheduling the shifts close together with use of circadian adaptation techniques may result in increased nighttime alertness, less fatigue, and fewer errors while at work than widely spacing out the shifts. For example, 1 recent study simulated 8 night shifts over a 10‐day period, and compared circadian schedules and work performance between those subjects who used circadian adaptation strategies and those that did not.71 Circadian adaptation techniques included: four 15‐minute bright light pulses during each night shift, dark sunglasses when outside, dark bedrooms and delayed sleeping until 3 AM on the nights off in between the night shift blocks. The group who shifted their circadian schedule improved night shift alertness and performance as measured by neurophysiological testing, while permitting sufficient daytime sleep after work, as well as late‐night sleep on days off. The group without circadian interventions did not shift their circadian schedule as significantly as the intervention group, and performed worse on the performance testing.
CONCLUSIONS
The nationwide use of hospitalists to provide 24‐hour patient care continues to expand, thus subjecting more hospitalists to work hours asynchronous with the lightdark cycle. Resultant circadian misalignment can result in fatigue while at work, shift work disorder, and, potentially, an increased rate of medical errors. Recognition of these dangers among resident physicians has prompted the ACGME to intensify their regulations on work hours, shift schedules, and time off between shifts. However, no such recommendations exist for hospitalists or emergency physicians and nurses.
Given the potential risk to both physicians and patients, we recommend more research examining the effects of circadian misalignment within the hospitalist community. Sample research questions are offered in Table 5. More information is urgently needed to provide evidence‐based practice guidelines to ensure the safety of this growing workforce and the patients they treat.
| Are hospitalists more immune to fatigue than resident physicians? |
| Are hospitalists better able to recognize fatigue while at work than resident physicians? |
| Does 1 shift work schedule promote better circadian alignment than other shift schedules? |
| Do consistent nighttime hours of nocturnists make them more prone to commit medical errors than hospitalists rotating their shifts? |
- .Job, family, and gender: determinants of nonstandard work schedules among employed Americans in 1991.Demography.1995;32:577–598.
- ,.Shift work among dual‐earner couples with children.Science.1983;219:876–879.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units.N Engl J Med.2004;351:1838–1848.
- Institute of Medicine. Resident Duty Hours: Enhancing Sleep, Supervision and Safety. Released December 15,2008. Available at: http://www.iom.edu/Reports/2008/Resident‐Duty‐Hours‐Enhancing‐Sleep‐Supervision‐and‐Safety.aspx. Accessed on October 20, 2010.
- ACGME. ACGME Approved Standards. Effective July2011. Available at: http://acgme‐2010standards.org/. Accessed on January 6, 2011
- ACGME. Common Program Requirements: Resident Duty Hours in the Learning and Work Environment. Effective July 1, 2007. Available at: http://www.acgme.org/acWebsite/dutyHours/dh_ComProgrRequirmentsDutyHours0707.pdf. Accessed October 20,2010.
- ,,, et al.Effect of reducing interns' weekly work hours on sleep and attentional failures.N Engl J Med.2004;351:1829–1837.
- Letter written to William Munier, Agency for Healthcare Research and Quality.Washington, DC:US House of Representatives, Committee on Energy and Commerce, March 29,2007. Available at: http://energycommerce.house.gov/Press_110/110‐ltr.032907.HHS.Munier.pdf. Accessed on October 24, 2010.
- ,.The evolution and future of hospital medicine.Mt Sinai J Med.2008;75:418–423.
- .Brain structures and receptors involved in alertness.Sleep Med.2005;6(suppl 1):S3–S7.
- .A two process model of sleep regulation.Hum Neurobiol.1982;1:195–204.
- ,.Entrainment of the human circadian system by light.J Biol Rhythms.2005;20:326–338.
- ,.Sleep‐promoting and hypothermic effects of daytime melatonin administration in humans.Sleep.1997;20:124–131.
- .Sleepiness as a consequence of shift work.Sleep.1988;11:17–34.
- ,,,,.The sleep of long‐haul truck drivers.N Engl J Med.1997;337:755–761.
- ,,,.Sleep on the night shift: 24‐hour EEG monitoring of spontaneous sleep/wake behavior.Psychophysiology.1989;26:352–358.
- .Do permanent night workers show circadian adjustment? A review based on the endogenous melatonin rhythm.Chronobiol Int.2008;25:215–224.
- ,,,,.Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers.Sleep.2004;27:1453–1462.
- ,,, et al.Modafinil for excessive sleepiness associated with shift‐work sleep disorder.N Engl J Med.2005;353:476–486.
- American Academy of Sleep Medicine.The International Classification of Sleep Disorders (ICSD).2nd ed.Chicago, IL:American Academy of Sleep Medicine;2005.
- ,.Circadian rhythm sleep disorders.Chest.2006;130:1915–1923.
- ,,,,,.Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism.Sleep.2008;31:1159–1167.
- ,,,,,.Genetic variation in the adenosine A2A receptor gene modulates performance on the psychomotor vigilance task.Sleep Biol Rhythms.2007;5:A47.
- ,.Circadian rhythms in cognitive performance: methodological constraints, protocols, theoretical underpinnings.Physiol Behav.2007;90:196–208.
- ,,,.A prospective study of fatal occupational accidents—relationship to sleeping difficulties and occupational factors.J Sleep Res.2002;11:69–71.
- ,,,.Effects of critical care nurses' work hours on vigilance and patients' safety.Am J Crit Care.2006;15:30–37.
- ,,,,.Daytime sleepiness, sleep habits and occupational accidents among hospital nurses.J Adv Nurs.2005;52:445–453.
- ,,.Systematic review: association of shift length, protected sleep time, and night float with patient care, residents' health, and education.Ann Intern Med.2010;153:829–842.
- ,,,,.Jack Barney Award: the effect of fatigue on cognitive and psychomotor skills of trauma residents and attending surgeons.Am J Surg.2008;196:813–819; discussion 9–20.
- ,,, et al.Risks of complications by attending physicians after performing nighttime procedures.JAMA.2009;302:1565–1572.
- ,,.Sleep deprivation, elective surgical procedures, and informed consent.N Engl J Med.2010;363:2577–2579.
- ,,,,.A prospective study of anger and coronary heart disease. The Normative Aging Study.Circulation.1996;94:2090–2095.
- ,,,.Night work and risk of breast cancer.Epidemiology.2006;17:108–111.
- ,,, et al.Night‐shift work and risk of colorectal cancer in the nurses' health study.J Natl Cancer Inst.2003;95:825–828.
- ,,.Shift work and cardiovascular disease—pathways from circadian stress to morbidity.Scand J Work Environ Health.2010;36:96–108.
- IARC. IARC monographs on the evalutaion of carcinogenic risks to humans. Vol 98. Painting, firefighting, and shiftwork. 2007. Available at: monographs.iarc.fr/ENG/Monographs/vol98/mono98.pdf. Accessed January 16,2011.
- ,,,.Influence of melatonin on invasive and metastatic properties of MCF‐7 human breast cancer cells.Cancer Res.1998;58:4383–4390.
- Society of Hospital Medicine. Society of Hospital Medicine releases results of the 2007–2008 survey on the state of the hospital medicine movement. 2008. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Press_Releases3:247–255.
- ,,.Hospitalists: A Guide to Building and Sustaining a Successful Program.Chicago, IL:Health Administration Press;2008.
- ,,,,.The increased financial burden of further proposed orthopaedic resident work‐hour reductions.J Bone Joint Surg Am.2011;93:e31.
- ,,,,,.Fatigue and shift work.J Sleep Res.2006;15:1–5.
- ,,, et al.Rapid shift in peak melatonin secretion associated with improved performance in short shift work schedule.Sleep.1997;20:1145–1150.
- ,,.The impact of a nap opportunity during the night shift on the performance and alertness of 12‐h shift workers.J Sleep Res.2002;11:219–227.
- ,,, et al.Improving alertness and performance in emergency department physicians and nurses: the use of planned naps.Ann Emerg Med.2006;48:596–604, e1‐e3.
- ,,,,.Promoting alertness with a short nap during a night shift.J Sleep Res.1998;7:240–247.
- ,.Circadian adaptation to night‐shift work by judicious light and darkness exposure.J Biol Rhythms.2002;17:556–567.
- ,,,,,.Exposure to bright light and darkness to treat physiologic maladaptation to night work.N Engl J Med.1990;322:1253–1259.
- ,.Night shift performance is improved by a compromise circadian phase position: study 3. Circadian phase after 7 night shifts with an intervening weekend off.Sleep.2008;31:1639–1645.
- ,,,.Dose‐response relationship for light intensity and ocular and electroencephalographic correlates of human alertness.Behav Brain Res.2000;115:75–83.
- ,,,,.Dark goggles and bright light improve circadian rhythm adaptation to night‐shift work.Sleep.1994;17:535–543.
- ,,.Caffeine content of specialty coffees.J Anal Toxicol.2003;27:520–522.
- ,.Caffeine content of prepackaged national‐brand and private‐label carbonated beverages.J Food Sci.2007;72:C337–C342.
- ,,, et al.The effects of coffee and napping on nighttime highway driving: a randomized trial.Ann Intern Med.2006;144:785–791.
- ,,,,.Laboratory and field studies of naps and caffeine as practical countermeasures for sleep‐wake problems associated with night work.Sleep.2006;29:39–50.
- ,,,,.Armodafinil for treatment of excessive sleepiness associated with shift work disorder: a randomized controlled study.Mayo Clin Proc.2009;84:958–972.
- ,,,,.The long‐term tolerability and efficacy of armodafinil in patients with excessive sleepiness associated with treated obstructive sleep apnea, shift work disorder, or narcolepsy: an open‐label extension study.J Clin Sleep Med.2010;6:458–466.
- ,,.Circadian rhythm sleep disorders.Med Clin North Am.2004;88:631–651, viii.
- ,,.A three pulse phase response curve to three milligrams of melatonin in humans.J Physiol.2008;586:639–647.
- ,,, et al.Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report.Sleep.2007;30:1445–1459.
- ,,, et al.Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta‐analysis.BMJ.2006;332:385–393.
- ,,.Effects of melatonin administration on daytime sleep after simulated night shift work.J Sleep Res.2001;10:181–192.
- ,,,.Performance, ability to stay awake, and tendency to fall asleep during the night after a diurnal sleep with temazepam or placebo.Sleep.1997;20:535–541.
- ,,,,,.Sleepiness/alertness on a simulated night shift following sleep at home with triazolam.Sleep.1991;14:140–146.
- ,,,.Zolpidem‐related effects on performance and mood during simulated night‐shift work.Exp Clin Psychopharmacol.2003;11:259–268.
- ,,,,.Intrinsic near‐24‐h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans.Proc Natl Acad Sci USA.2001;98:14027–14032.
- ,,,.Work shift duration: a review comparing eight hour and 12 hour shift systems.Occup Environ Med.1998;55:217–229.
- ,,.Influence of flexibility and variability of working hours on health and well‐being.Chronobiol Int.2006;23:1125–1137.
- ,,.Impact of night‐float rotation on sleep, mood, and alertness: the resident's perception.Chronobiol Int.2002;19:893–902.
- ,,,,,.The characteristics of recovery sleep when recovery opportunity is restricted.Sleep.2007;30:353–360.
- ,,.Practical interventions to promote circadian adaptation to permanent night shift work: study 4.J Biol Rhythms.2009;24:161–172.
- ,,.Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people.Occup Environ Med.2001;58:747–752.
- ,.Use of sleep hygiene in the treatment of insomnia.Sleep Med Rev.2003;7:215–225.
For hospitalists, patient care is 24 hours a day. To provide continual patient care, shift work has become a way of life for hospitalists, similar to hospital nurses, residents in training, and emergency medicine physicians. Notably, they belong to a substantial minority of the workforce as shift workers, starting after 6 PM or before 6 AM, approximately one‐fifth of the total work force in industrialized nations.1, 2 Unfortunately, shift workers suffer from misalignment of their endogenous circadian system, which regulates daily sleep and alertness patterns, and work obligations beyond daylight hours. Such a misalignment can lead to fatigue, sleep loss, and excessive sleepiness, which can adversely affect personal health and safety, as well as the quality of medical care delivered.3
The relationship between shift work, extended work hours, and medical safety is a topic currently under intense scrutiny, as reviewed in the Institute of Medicine's (IOM) controversial report on residents and sleep.4 This publication led the Accreditation Council of Graduate Medical Education (ACGME) to mandate more changes to residents' work hours,5 adding to those first implemented in 2003.6 These restrictions forbid residents from working more than 30 consecutive hours, and required at least 10 hours off between shifts and an average of 1 day off in 7. Subsequent studies suggested that the reduction in resident work hours led to greater resident well‐being, fewer attention failures. and fewer medical errors.3, 7
In 2007, amid growing public concern over sleep‐deprived residents and patient safety, Congress requested the IOM investigate additional safeguards for residents.8 In 2008, the IOM published a report calling for more protection against resident fatigue.4 They recommended integrating a protected sleep period into any 24‐hour shift. If residents cannot get protected sleep time, then the maximal shift duration should not exceed 16 hoursreduced from the previous ACGME recommendation of 30. Further provisions to allow adequate sleep include capping the number of consecutive night shifts at 4, and extending the time off after a night shift. In response, the ACGME recently updated their recommendations effective July 1, 2011,5 though not following all the IOM's recommendations (Table 1).
| 2003 ACGME Limits | 2008 IOM Recommendation | 2010 ACGME Limits | |
|---|---|---|---|
| |||
| Maximum work hours per week | 80 hr, averaged over 4 wk | No change | No change |
| Maximum shift length | 30 hr (admitting patients up to 24 hr, with 6 hr of transition activities) | 30 hr (admitting patients up to 16 hr, with 5 hr protected sleep between 10 PM to 8 AM, and remaining hours for transition activities) | PGY‐1: 16 hr; PGY‐2 and above: 28 hr (admitting patients up to 24 hr, with 4 hr of transition activities) |
| Minimum time off between shifts | 10 hr after shift | 10 hr after day shift; 12 hr after night shift; 14 hr after any extended shift of 30 hr and not return until 6 AM the next day | 10 hr after shift; 14 hr free after 24‐hr shift for intermediate level residents |
| Maximum frequency of in‐hospital night shifts | No limits | 4 night maximum, with 48 hr off after 3 or 4 consecutive shifts | 6 consecutive night maximum |
The growing nationwide emphasis on fatigue prevention within healthcare settings now clearly impacts residents and their training schedule. But why focus only on residents? Why not other physicians, such as hospitalists, who work shifts to cover 24 hours each day? Are they any less prone to making medical errors when fatigued? Given that hospitalists' represent the fastest growing specialty in the history of American medicine,9 we sought to inform decisions about their scheduling by reviewing normal regulation of sleep and wake patterns, addressing the problems associated with misalignment between sleep and work, and identifying strategies to realign circadian schedules.
NORMAL SLEEP AND CIRCADIAN RHYTHMS
An understanding of sleep physiology begins with the endogenous circadian timekeeping system. At the center of this timekeeping system is a master circadian clock, located in the suprachiasmatic nucleus (SCN) of the hypothalamus. Cells within the SCN generate a near 24‐hour rhythm, transmitted through neural connections, to rhythmically influence the entire central nervous system and other bodily systems.10
The SCN and the circadian rhythm interact with the need to sleep (sleep homeostasis) to form the 2‐process model of sleepwakefulness.11 In this model, progression of biological day (a time when wakefulness and its associated functions are promoted) coincides with a rise in homeostatic pressure to sleep (see Figure 1). Daytime alertness is maintained by increasing SCN neuronal activity to counterbalance rising sleep pressure. After peaking in the early evening, SCN activity falls to begin biological night (a time when sleep and its associated functions are promoted). To facilitate the onset of biological night, the SCN coordinates the activity of sleep‐promoting centers and the release of melatonin from the pineal gland which promotes sleep.

This endogenous circadian clock runs slightly longer than 24 hours and must be resynchronized daily to the 24‐hour day, a process known as entrainment. This occurs primarily through environmental exposure of retinalhypothalamic links to the lightdark cycle. The intensity, duration, and wave length of light all influence the circadian system,12 but perhaps most importantly is the timing. In general, light exposure in the evening will shift the circadian clock later (phase delay shift), whereas light exposure in the morning will shift the clock earlier (phase advance shift). Exogenous melatonin can also shift the circadian system. However, when endogenous levels of melatonin are high, ingested melatonin has little influence on sleep.13
Balancing sleep and wakefulness requires an interweaving of endogenous and exogenous factors. This balance is disturbed if we try to sleep or be wakeful during incorrect endogenous biological times, a process called circadian misalignment.
DELETERIOUS EFFECTS OF CIRCADIAN MISALIGNMENT
Hospitalists and other shift workers required to work during the biological night risk circadian misalignment and, consequently, poor sleep, shift work disorder, errors on the job, and possibly long‐term health consequences.
Chronic Sleep Loss
When working at night or in the early morning, nearly 75% of shift workers encounter some amount of at‐work fatigue and sleepiness.14 After the shift is over, objective assessments among rotating shift workers15, 16 and interns7 demonstrated that day sleep is 1 to 4 hours shorter than night sleep. Chronic or recurring night shifts can therefore lead to chronic sleep loss. While it seems reasonable that permanent night shift workers have greater circadian adjustment to suit their work schedule, little evidence supports this argument.17 Permanent night shift workers may sleep a little longer during the day than rotating shift workers. Yet, the sleep quality does not match night sleep, presumably from conflict between external factors, such as light and activity, and the scheduled sleep period.
Shift Work Disorder
If severe and chronic, sleepiness and impaired performance during work hours and poor sleep during the day can be enough to warrant a diagnosis of shift work disorder (SWD), one of the several circadian rhythm sleep disorders (CRSD). The prevalence of SWD among rotating and night workers is estimated to be 10%25%.18 Patients with SWD can experience similar levels of nighttime sleepiness as patients with narcolepsy and sleep apnea.19 These patients experience reduced satisfaction with the work schedule, and suffer higher rates of depression, ulcers, and sleepiness‐related accidents, compared to other shift workers.18 What distinguishes those shift workers who suffer from normal fatigue and those with SWD is not easily identified. The International Classification of Sleep Disorders‐2 (ICSD‐2) lists the diagnostic criteria for SWD20:
-
Symptoms of insomnia associated with a work schedule that overlaps the usual time for sleep.
-
Symptoms are directly associated with shift work schedule over the course of at least 1 month.
-
Sleep log monitoring for at least 7 days demonstrates circadian and sleep‐time misalignment.
-
Sleep disturbance is not better explained by another sleep disorder or by a medical, neurological, or mental disorder; medication use; or substance‐use disorder.
Symptoms must be present for at least 1 month, and comorbid mood or sleep/wake disorders (commonly found in this disorder) need to be treated. SWD is more common among night shift workers, although those workers starting shifts between 4 AM and 7 AM (early morning shift) are also subject to SWD.21 Type of work schedule, along with physical or mental disorders, domestic responsibilities, and commute times are examples of factors that may increase vulnerability for SWD.18 In addition, genetic factors may explain the considerable inter‐individual differences in susceptibility to SWD. For example, a polymorphism in the circadian gene, PER 3, present in 10%15% of the population, is believed to decrease tolerability to acute sleep loss,22 while genetic variation in the adenosine A2A receptor may be associated with resistance to the effects of sleep loss.23 If a hospitalist suspects a diagnosis SWD, they should seek evaluation by a physician specializing in sleep medicine.
Errors
Disruption of the circadian rhythm influences neurocognitive and psychomotor function, and can lead to human error. Human errors that result in serious accidents or injuries typically result from interaction of circadian rhythm misalignment with multiple other factors, including task duration and complexity, motivation and proficiency, and level of sleep deprivation.24 Though difficult to isolate from the environmental and work experience factors, consistent evidence identifies circadian misalignment as a cause of errors and serious accidents. Most evidence comes from night shift workers trying to remain awake when the circadian signal for alertness is low, or attempting sleep when the circadian alerting signal is high. Compared to day workers, night shift workers are 1.63 times more likely to suffer a fatal accident.25 A study of critical care nurses revealed a prominent circadian pattern of inadvertent sleep episodes during work with the highest peak between 2 AM and 6 AM.26 In addition, nurses working the night shift have been shown to commit more medication administration errors than day workers.27
Medical errors among resident physicians during extended shift durations is well documented.28 On the other hand, not much research has examined error rates among attending physicians. In 1 small study, attending surgeons made more cognitive errors using a simulated laparoscopic exercise as the amount of on‐call overnight sleep decreased.29 A large, single‐center review reported an increased rate of complications among post‐nighttime surgical procedures performed by attendings who slept 6 hours or less the preceding night.30 Notably, proposed legislation would require physicians who have been awake 22 of the preceding 24 hours to inform patients of the potential safety impact of their sleep deprivation prior to providing clinical care.31
Chronic Health Morbidity
Several studies reveal the effect of shift work on chronic health conditions among healthcare workers, such as obesity, cardiovascular disease, and certain cancers (eg, breast, colorectal). These results are summarized in Table 2, with the largest evaluation of healthcare shift workers coming from the Nurses' Health Study.3234
| Disease | Study Design | Population | Comparison | Health Risk | Adjusted Risk Factors |
|---|---|---|---|---|---|
| |||||
| Acute myocardial infarction | Prospective cohort32 | 79,109 US nurses | Working 3 night shifts/mo for 6 yr | RR 1.51 | CAD risk factors, aspirin use, hormone replacement therapy |
| 95% CI (1.12‐2.03) | |||||
| Obesity (BMI 30) | Cross‐sectional72 | 27,485 Swedish workers | Shift‐workers vs day workers | OR 1.41 | Age, socioeconomic status |
| 95% CI (1.25‐1.59) | |||||
| Breast cancer | Prospective cohort33 | 116,087 US nurses | Working 3 night shifts/mo for 20 yr | RR 1.79 | Breast cancer risk factors |
| 95% CI (1.06‐3.01) | |||||
| Colon cancer | Prospective cohort34 | 78,586 US nurses | Working 3 night shifts/mo for 15 yr | RR 1.35 | Family history of colon cancer, dietary intake, activity |
| 95% CI (1.03‐1.77) | |||||
Some believe that adverse health outcomes in shift workers derive from circadian stressan alteration of psychosocial and physiological homeostasis (eg, increased cortisol and catecholamine output) resulting from circadian misalignment.35 Based on data suggesting an increased risk for certain cancers among shift workers, the International Agency for Research of Cancer, a unit of the World Health Organization, announced that shift work resulting in circadian misalignment is probably carcinogenic.36 Researchers propose several biologic mechanisms to explain the increased cancer riskmost revolve around the alteration of the melatonin circadian cycle, found in night shift workers,37 and subsequent disruption of its believed cancer‐protective biologic pathways.
Overall, however, the heterogeneous nature of shift work limits conclusions regarding the long‐term health of shift workers. That is, as the shift work intensity and composition varies, and as the number and timing of these shifts change, so too can the adverse health consequences.
HOSPITALISTS AND NIGHT SHIFTS
Hospital medicine is the fastest growing specialty in the history of medicine, with an estimated 30,000 practicing hospitalists in 2010.38 Survey results from 2009 indicate that hospitalists staff 58% of hospitals; 89% of hospitals with more than 200 beds (J. Miller, Society of Hospital Medicine, personal communication). One reason for the growth in the number of hospitalists at academic medical centers has been the imposed work‐hour restrictions for residents.39
Across the county, hospitalist programs use a variety of shift work systems to ensure 24‐hour patient care. Among those programs that provide continuous on‐site coverage, many staff 3 shiftsday, late afternoon/evening (swing), and night shifts. Some permanently partition the scheduling, with dedicated night hospitalists or nocturnists.40
Hospitalists do not have mandated work‐hour restrictions and, in general, are older than resident physicians. Whether or not hospitalists who trained before the era of work‐hour regulations are better prepared for practicing in a real‐world, after‐hours scenario than hospitalists with previous work‐hour restrictions is a matter of debate. That said, hospitalists who are fatigued, just like residents, may be at increased risk for committing medical errors, particularly when the fatigue is unrecognized. Yet, limiting hospitalists' work hours would have obvious financial implications, likely similar those from resident work‐hour reductions.41 As part of the ACGME 2011 recommendations, faculty and residents now must be trained to recognize signs of fatigue and sleep deprivation, and adopt management strategies such as naps or backup call schedules. Fatigue that results in excessive sleepiness while at work may manifest as weariness, difficulty concentrating, headache, irritability or depressed mood, and feeling unrefreshed after sleeping.42
STRATEGIES TO IMPROVE CIRCADIAN ADAPTATION
Hospitalists can help limit fatigue and improve performance and safety through circadian adaptation: a multimodal approach to realign work and circadian schedules. Depending on whether the shift starts at night or in the early morning (4 AM to 7 AM), circadian adaptation aims may differ. For night shift workers, the overall aim is to delay the timing of circadian rhythms such that the highest propensity of wakefulness occurs during the night work period, while the highest propensity for sleep occurs during the day.17, 43 For early morning shift workers, circadian rhythms for wakefulness and sleep propensity should be shifted earlier. Circadian adaptation involves not only sleeping well before work, but also preventing dips in wakefulness during work. Adaptation strategies are listed in Table 3.
| Night Shift60 | Early Day Shift (Starting at 4 AM‐7 AM) | |
|---|---|---|
| Prior to shift | Avoid sleep debt | Avoid sleep debt |
| Proper sleep hygiene | Proper sleep hygiene | |
| Planned napping | Bright light exposure | |
| Caffeine use | ||
| During the shift | Bright light exposure | Caffeine use |
| Planned napping | ||
| After the shift | Avoid bright light | Avoid late evening bright light (when applicable) |
| Melatonin prior to sleep | ||
| Careful use of other hypnotics | Initiate sleep early |
Improved Sleep Before Work
As an essential first step, hospitalists must get a full night's rest before starting a night shift, as sleep debt will worsen fatigue while at work. Tips for proper sleep hygiene are listed in Table 4. Some shift workers stay up late the night before a scheduled night shift, in order to sleep during the day and awaken shortly before their scheduled night shift, to combat fatigue at work. Such an approach to shift work is typically met with 3 barriers. First, environmental factors often prevent 6 hours of uninterrupted day sleep. Second, 6 hours of continual day sleep is typically difficult because rising circadian activity often limits the sleep period to just a few hours. Third, an adequate amount of sleep prior to a night shift will itself not be enough to prevent sleepiness from occurring after midnightreducing the fall in circadian activity is also essential to maintaining alertness and performance.
| Physical | Adhere to regular wake and sleep schedule |
| activities73 | Engage in regular exercise early in the day |
| Avoid caffeine, nicotine, and alcohol use 6 hr prior to sleeping | |
| Avoid stimulating or stressful activities 30 min prior to sleeping | |
| Proper sleep | Well ventilated, temperature‐controlled bedroom |
| environment | Use heavy curtains to provide as much darkness as possible |
| Comfortable mattress and pillow | |
| Remove television and pets from the bedroom | |
| Housemates should help provide quiet sleep environment |
Napping
Napping prior to a night shift, or during the work shift, can improve alertness and performance and decrease accident rates.44, 45 During shift work, naps of 20 to 50 minutes in duration have demonstrated improvements in reaction time, and restoration of performance to that seen at the start of the shift. Napping early in the night shift can improve objective measures of alertness.44 To avoid increased drowsiness that sometimes occurs when waking from a nap, naps should not be longer than 50 minutes, and can be as short as 10 to 15 minutes.44, 46 Although effective, napping may be impractical for many workers due to time or space constraints. To facilitate brief naps, hospitalist practices should ensure they have a dark, quiet call room for use by overnight hospitalists.
Bright Light Exposure
Studies demonstrate that light exposure during the night shift improves circadian alignment, mood, and performance during the night shift.47, 48 Light exposure ranged from 6 hours to 5 light treatments of 15 minutes each, with brightness ranging from 2,500 to 10,000 lux (approximating outdoor daylight; typical office lighting provides 200‐500 lux).47, 49 Results demonstrate that bright light exposure during the night shift acutely improves alertness and performance, though not to daytime levels.50 The greatest circadian adjustments occur in groups using both bright light during the night shift and light avoidance the following morning.51 Dark sunglasses and a dark home environment can decrease bright light exposure during the day. Though little evidence exists to support widespread application of bright light devices in hospitalists' call‐rooms, a hospitalist practice should consider installing one to promote circadian adaptation if physicians working overnight have multiple consecutive shifts. Likewise, these physicians should be vigilant and wear dark sunglasses during the day after their night shifteven a few minutes of light exposure at the wrong time of the day may disrupt the intended circadian adaptation.
Wake‐Promoting Agents
Numerous studies demonstrate that 150 mg to 400 mg of caffeine (a 16 oz grande cup of coffee from Starbucks contains between 200 to 500 mg of caffeine52; a Diet Coke contains 46 mg/12 oz53) reduces sleepiness, increases alertness, and improves performance during the night shift.54, 55 Thus, judicious use of caffeine may be recommended in hospital practices during extended work hours. Other wake‐promoting agents, such as modafinil and armodafinil, are US Food and Drug Administration (FDA)‐approved in the treatment of excessive sleepiness associated with SWD. Typically taken 3060 minutes before the start of the night shift, these medications have been shown in trials, enrolling mostly permanent night shift workers, to reduce excessive nighttime sleepiness and improve performance.19, 56 Armodafinil used to treat SWD‐associated excessive sleepiness, has been safely tolerated for durations of 1 year or more.57 However, these agents are not approved for use in patients without a diagnosed sleep disorder.
Melatonin
Exogenous melatonin has been used to reset circadian rhythms in patients with CRSDs.58 Melatonin administered in the late afternoon to early evening directs the largest phase advance. In contrast, melatonin given in the morning produces the largest phase delays.59 When taken after a night shift, melatonin (at a dose 1.8 to 3.0 mg) can improve day sleep quality and duration.60 Despite this result, melatonin's effectiveness in improving circadian adaptation has been mixed.61 For example, improvements in nighttime alertness during the night shift were not seen, despite the use of melatonin to facilitate daytime sleep beforehand.62 Hospitalists may consider a trial of melatonin to improve circadian alignment and facilitate daytime sleep, but its chronic use and long‐term safety has not been adequately studied.
Hypnotics After Work
Hypnotics such as temazepam 20 mg,63 triazolam,64 and zolpidem65 taken after night shift work have been shown to improve day sleep quality under simulated conditions, but do not improve shift work performance. These medications should be reserved for judicious short‐term use in patients with insomnia associated with SWD.
NIGHT SHIFT SCHEDULING TO REDUCE CIRCADIAN MISALIGNMENT
When providing 24‐hour, on‐site medical care, questions may arise about how to incorporate circadian adaptation into the daily schedule.
How Should Shifts Be Rotated?
When scheduling shifts with different start times, evidence suggests that sleep disturbance is reduced with a clockwise progression in shifts (eg, day shift to evening shift to night shift). This reduction in sleep disturbance is thought due to increased time between shifts and the circadian timekeeping tendency to extend beyond 24 hours.66
When Should the Night Shift Start?
Those hospitalist programs using an evening swing shift from afternoon to late evening may have the option of using a 12‐hour night shift starting around 7 PM, or a shorter night shift beginning later at night. Though there are no data among hospitalists to suggest which night shift start time and duration would lead to the least amount of fatigue and errors, healthcare providers working a 12‐hour night shift may have increased morale due to fewer shifts, but may suffer a reduction in the quality of care provided compared with working an 8‐hour night shift.67 In either case, shift workers given flexibility in scheduling have been shown to have positive effects on sleep.68
Should Night Shifts Be Bunched?
The decision to bunch night shifts together depends on how many night shifts are required, and how quickly circadian adaptation can occur. Under simulated conditions, circadian adaptation can yield significant changes in sleep/wake cycles in as little as 4 days.48 In real‐world settings, more time may be required to achieve significant shifts in the circadian cycle. Therefore, hospitalists who have 7 or fewer night shifts during the academic year may want to space the shifts out to prevent sleep debt on consecutive shifts, since significant circadian adaptation would be difficult to achieve in less than a week. In this situation, after a night shift, the hospitalist should have at least one 9‐hour sleep period at night to relieve their sleep debt before staffing another night shift.69 Consecutive night shifts require at least 2 nighttime sleep periods of 9 hours to recover from sleep debt.70 The IOM recommends setting a limit of 4 consecutive night float shifts for resident physicians, however, a recent systematic review of resident night float models did not find data supporting use of a 4‐night‐maximum model.28
If more than 7 night shifts are required, then scheduling the shifts close together with use of circadian adaptation techniques may result in increased nighttime alertness, less fatigue, and fewer errors while at work than widely spacing out the shifts. For example, 1 recent study simulated 8 night shifts over a 10‐day period, and compared circadian schedules and work performance between those subjects who used circadian adaptation strategies and those that did not.71 Circadian adaptation techniques included: four 15‐minute bright light pulses during each night shift, dark sunglasses when outside, dark bedrooms and delayed sleeping until 3 AM on the nights off in between the night shift blocks. The group who shifted their circadian schedule improved night shift alertness and performance as measured by neurophysiological testing, while permitting sufficient daytime sleep after work, as well as late‐night sleep on days off. The group without circadian interventions did not shift their circadian schedule as significantly as the intervention group, and performed worse on the performance testing.
CONCLUSIONS
The nationwide use of hospitalists to provide 24‐hour patient care continues to expand, thus subjecting more hospitalists to work hours asynchronous with the lightdark cycle. Resultant circadian misalignment can result in fatigue while at work, shift work disorder, and, potentially, an increased rate of medical errors. Recognition of these dangers among resident physicians has prompted the ACGME to intensify their regulations on work hours, shift schedules, and time off between shifts. However, no such recommendations exist for hospitalists or emergency physicians and nurses.
Given the potential risk to both physicians and patients, we recommend more research examining the effects of circadian misalignment within the hospitalist community. Sample research questions are offered in Table 5. More information is urgently needed to provide evidence‐based practice guidelines to ensure the safety of this growing workforce and the patients they treat.
| Are hospitalists more immune to fatigue than resident physicians? |
| Are hospitalists better able to recognize fatigue while at work than resident physicians? |
| Does 1 shift work schedule promote better circadian alignment than other shift schedules? |
| Do consistent nighttime hours of nocturnists make them more prone to commit medical errors than hospitalists rotating their shifts? |
For hospitalists, patient care is 24 hours a day. To provide continual patient care, shift work has become a way of life for hospitalists, similar to hospital nurses, residents in training, and emergency medicine physicians. Notably, they belong to a substantial minority of the workforce as shift workers, starting after 6 PM or before 6 AM, approximately one‐fifth of the total work force in industrialized nations.1, 2 Unfortunately, shift workers suffer from misalignment of their endogenous circadian system, which regulates daily sleep and alertness patterns, and work obligations beyond daylight hours. Such a misalignment can lead to fatigue, sleep loss, and excessive sleepiness, which can adversely affect personal health and safety, as well as the quality of medical care delivered.3
The relationship between shift work, extended work hours, and medical safety is a topic currently under intense scrutiny, as reviewed in the Institute of Medicine's (IOM) controversial report on residents and sleep.4 This publication led the Accreditation Council of Graduate Medical Education (ACGME) to mandate more changes to residents' work hours,5 adding to those first implemented in 2003.6 These restrictions forbid residents from working more than 30 consecutive hours, and required at least 10 hours off between shifts and an average of 1 day off in 7. Subsequent studies suggested that the reduction in resident work hours led to greater resident well‐being, fewer attention failures. and fewer medical errors.3, 7
In 2007, amid growing public concern over sleep‐deprived residents and patient safety, Congress requested the IOM investigate additional safeguards for residents.8 In 2008, the IOM published a report calling for more protection against resident fatigue.4 They recommended integrating a protected sleep period into any 24‐hour shift. If residents cannot get protected sleep time, then the maximal shift duration should not exceed 16 hoursreduced from the previous ACGME recommendation of 30. Further provisions to allow adequate sleep include capping the number of consecutive night shifts at 4, and extending the time off after a night shift. In response, the ACGME recently updated their recommendations effective July 1, 2011,5 though not following all the IOM's recommendations (Table 1).
| 2003 ACGME Limits | 2008 IOM Recommendation | 2010 ACGME Limits | |
|---|---|---|---|
| |||
| Maximum work hours per week | 80 hr, averaged over 4 wk | No change | No change |
| Maximum shift length | 30 hr (admitting patients up to 24 hr, with 6 hr of transition activities) | 30 hr (admitting patients up to 16 hr, with 5 hr protected sleep between 10 PM to 8 AM, and remaining hours for transition activities) | PGY‐1: 16 hr; PGY‐2 and above: 28 hr (admitting patients up to 24 hr, with 4 hr of transition activities) |
| Minimum time off between shifts | 10 hr after shift | 10 hr after day shift; 12 hr after night shift; 14 hr after any extended shift of 30 hr and not return until 6 AM the next day | 10 hr after shift; 14 hr free after 24‐hr shift for intermediate level residents |
| Maximum frequency of in‐hospital night shifts | No limits | 4 night maximum, with 48 hr off after 3 or 4 consecutive shifts | 6 consecutive night maximum |
The growing nationwide emphasis on fatigue prevention within healthcare settings now clearly impacts residents and their training schedule. But why focus only on residents? Why not other physicians, such as hospitalists, who work shifts to cover 24 hours each day? Are they any less prone to making medical errors when fatigued? Given that hospitalists' represent the fastest growing specialty in the history of American medicine,9 we sought to inform decisions about their scheduling by reviewing normal regulation of sleep and wake patterns, addressing the problems associated with misalignment between sleep and work, and identifying strategies to realign circadian schedules.
NORMAL SLEEP AND CIRCADIAN RHYTHMS
An understanding of sleep physiology begins with the endogenous circadian timekeeping system. At the center of this timekeeping system is a master circadian clock, located in the suprachiasmatic nucleus (SCN) of the hypothalamus. Cells within the SCN generate a near 24‐hour rhythm, transmitted through neural connections, to rhythmically influence the entire central nervous system and other bodily systems.10
The SCN and the circadian rhythm interact with the need to sleep (sleep homeostasis) to form the 2‐process model of sleepwakefulness.11 In this model, progression of biological day (a time when wakefulness and its associated functions are promoted) coincides with a rise in homeostatic pressure to sleep (see Figure 1). Daytime alertness is maintained by increasing SCN neuronal activity to counterbalance rising sleep pressure. After peaking in the early evening, SCN activity falls to begin biological night (a time when sleep and its associated functions are promoted). To facilitate the onset of biological night, the SCN coordinates the activity of sleep‐promoting centers and the release of melatonin from the pineal gland which promotes sleep.

This endogenous circadian clock runs slightly longer than 24 hours and must be resynchronized daily to the 24‐hour day, a process known as entrainment. This occurs primarily through environmental exposure of retinalhypothalamic links to the lightdark cycle. The intensity, duration, and wave length of light all influence the circadian system,12 but perhaps most importantly is the timing. In general, light exposure in the evening will shift the circadian clock later (phase delay shift), whereas light exposure in the morning will shift the clock earlier (phase advance shift). Exogenous melatonin can also shift the circadian system. However, when endogenous levels of melatonin are high, ingested melatonin has little influence on sleep.13
Balancing sleep and wakefulness requires an interweaving of endogenous and exogenous factors. This balance is disturbed if we try to sleep or be wakeful during incorrect endogenous biological times, a process called circadian misalignment.
DELETERIOUS EFFECTS OF CIRCADIAN MISALIGNMENT
Hospitalists and other shift workers required to work during the biological night risk circadian misalignment and, consequently, poor sleep, shift work disorder, errors on the job, and possibly long‐term health consequences.
Chronic Sleep Loss
When working at night or in the early morning, nearly 75% of shift workers encounter some amount of at‐work fatigue and sleepiness.14 After the shift is over, objective assessments among rotating shift workers15, 16 and interns7 demonstrated that day sleep is 1 to 4 hours shorter than night sleep. Chronic or recurring night shifts can therefore lead to chronic sleep loss. While it seems reasonable that permanent night shift workers have greater circadian adjustment to suit their work schedule, little evidence supports this argument.17 Permanent night shift workers may sleep a little longer during the day than rotating shift workers. Yet, the sleep quality does not match night sleep, presumably from conflict between external factors, such as light and activity, and the scheduled sleep period.
Shift Work Disorder
If severe and chronic, sleepiness and impaired performance during work hours and poor sleep during the day can be enough to warrant a diagnosis of shift work disorder (SWD), one of the several circadian rhythm sleep disorders (CRSD). The prevalence of SWD among rotating and night workers is estimated to be 10%25%.18 Patients with SWD can experience similar levels of nighttime sleepiness as patients with narcolepsy and sleep apnea.19 These patients experience reduced satisfaction with the work schedule, and suffer higher rates of depression, ulcers, and sleepiness‐related accidents, compared to other shift workers.18 What distinguishes those shift workers who suffer from normal fatigue and those with SWD is not easily identified. The International Classification of Sleep Disorders‐2 (ICSD‐2) lists the diagnostic criteria for SWD20:
-
Symptoms of insomnia associated with a work schedule that overlaps the usual time for sleep.
-
Symptoms are directly associated with shift work schedule over the course of at least 1 month.
-
Sleep log monitoring for at least 7 days demonstrates circadian and sleep‐time misalignment.
-
Sleep disturbance is not better explained by another sleep disorder or by a medical, neurological, or mental disorder; medication use; or substance‐use disorder.
Symptoms must be present for at least 1 month, and comorbid mood or sleep/wake disorders (commonly found in this disorder) need to be treated. SWD is more common among night shift workers, although those workers starting shifts between 4 AM and 7 AM (early morning shift) are also subject to SWD.21 Type of work schedule, along with physical or mental disorders, domestic responsibilities, and commute times are examples of factors that may increase vulnerability for SWD.18 In addition, genetic factors may explain the considerable inter‐individual differences in susceptibility to SWD. For example, a polymorphism in the circadian gene, PER 3, present in 10%15% of the population, is believed to decrease tolerability to acute sleep loss,22 while genetic variation in the adenosine A2A receptor may be associated with resistance to the effects of sleep loss.23 If a hospitalist suspects a diagnosis SWD, they should seek evaluation by a physician specializing in sleep medicine.
Errors
Disruption of the circadian rhythm influences neurocognitive and psychomotor function, and can lead to human error. Human errors that result in serious accidents or injuries typically result from interaction of circadian rhythm misalignment with multiple other factors, including task duration and complexity, motivation and proficiency, and level of sleep deprivation.24 Though difficult to isolate from the environmental and work experience factors, consistent evidence identifies circadian misalignment as a cause of errors and serious accidents. Most evidence comes from night shift workers trying to remain awake when the circadian signal for alertness is low, or attempting sleep when the circadian alerting signal is high. Compared to day workers, night shift workers are 1.63 times more likely to suffer a fatal accident.25 A study of critical care nurses revealed a prominent circadian pattern of inadvertent sleep episodes during work with the highest peak between 2 AM and 6 AM.26 In addition, nurses working the night shift have been shown to commit more medication administration errors than day workers.27
Medical errors among resident physicians during extended shift durations is well documented.28 On the other hand, not much research has examined error rates among attending physicians. In 1 small study, attending surgeons made more cognitive errors using a simulated laparoscopic exercise as the amount of on‐call overnight sleep decreased.29 A large, single‐center review reported an increased rate of complications among post‐nighttime surgical procedures performed by attendings who slept 6 hours or less the preceding night.30 Notably, proposed legislation would require physicians who have been awake 22 of the preceding 24 hours to inform patients of the potential safety impact of their sleep deprivation prior to providing clinical care.31
Chronic Health Morbidity
Several studies reveal the effect of shift work on chronic health conditions among healthcare workers, such as obesity, cardiovascular disease, and certain cancers (eg, breast, colorectal). These results are summarized in Table 2, with the largest evaluation of healthcare shift workers coming from the Nurses' Health Study.3234
| Disease | Study Design | Population | Comparison | Health Risk | Adjusted Risk Factors |
|---|---|---|---|---|---|
| |||||
| Acute myocardial infarction | Prospective cohort32 | 79,109 US nurses | Working 3 night shifts/mo for 6 yr | RR 1.51 | CAD risk factors, aspirin use, hormone replacement therapy |
| 95% CI (1.12‐2.03) | |||||
| Obesity (BMI 30) | Cross‐sectional72 | 27,485 Swedish workers | Shift‐workers vs day workers | OR 1.41 | Age, socioeconomic status |
| 95% CI (1.25‐1.59) | |||||
| Breast cancer | Prospective cohort33 | 116,087 US nurses | Working 3 night shifts/mo for 20 yr | RR 1.79 | Breast cancer risk factors |
| 95% CI (1.06‐3.01) | |||||
| Colon cancer | Prospective cohort34 | 78,586 US nurses | Working 3 night shifts/mo for 15 yr | RR 1.35 | Family history of colon cancer, dietary intake, activity |
| 95% CI (1.03‐1.77) | |||||
Some believe that adverse health outcomes in shift workers derive from circadian stressan alteration of psychosocial and physiological homeostasis (eg, increased cortisol and catecholamine output) resulting from circadian misalignment.35 Based on data suggesting an increased risk for certain cancers among shift workers, the International Agency for Research of Cancer, a unit of the World Health Organization, announced that shift work resulting in circadian misalignment is probably carcinogenic.36 Researchers propose several biologic mechanisms to explain the increased cancer riskmost revolve around the alteration of the melatonin circadian cycle, found in night shift workers,37 and subsequent disruption of its believed cancer‐protective biologic pathways.
Overall, however, the heterogeneous nature of shift work limits conclusions regarding the long‐term health of shift workers. That is, as the shift work intensity and composition varies, and as the number and timing of these shifts change, so too can the adverse health consequences.
HOSPITALISTS AND NIGHT SHIFTS
Hospital medicine is the fastest growing specialty in the history of medicine, with an estimated 30,000 practicing hospitalists in 2010.38 Survey results from 2009 indicate that hospitalists staff 58% of hospitals; 89% of hospitals with more than 200 beds (J. Miller, Society of Hospital Medicine, personal communication). One reason for the growth in the number of hospitalists at academic medical centers has been the imposed work‐hour restrictions for residents.39
Across the county, hospitalist programs use a variety of shift work systems to ensure 24‐hour patient care. Among those programs that provide continuous on‐site coverage, many staff 3 shiftsday, late afternoon/evening (swing), and night shifts. Some permanently partition the scheduling, with dedicated night hospitalists or nocturnists.40
Hospitalists do not have mandated work‐hour restrictions and, in general, are older than resident physicians. Whether or not hospitalists who trained before the era of work‐hour regulations are better prepared for practicing in a real‐world, after‐hours scenario than hospitalists with previous work‐hour restrictions is a matter of debate. That said, hospitalists who are fatigued, just like residents, may be at increased risk for committing medical errors, particularly when the fatigue is unrecognized. Yet, limiting hospitalists' work hours would have obvious financial implications, likely similar those from resident work‐hour reductions.41 As part of the ACGME 2011 recommendations, faculty and residents now must be trained to recognize signs of fatigue and sleep deprivation, and adopt management strategies such as naps or backup call schedules. Fatigue that results in excessive sleepiness while at work may manifest as weariness, difficulty concentrating, headache, irritability or depressed mood, and feeling unrefreshed after sleeping.42
STRATEGIES TO IMPROVE CIRCADIAN ADAPTATION
Hospitalists can help limit fatigue and improve performance and safety through circadian adaptation: a multimodal approach to realign work and circadian schedules. Depending on whether the shift starts at night or in the early morning (4 AM to 7 AM), circadian adaptation aims may differ. For night shift workers, the overall aim is to delay the timing of circadian rhythms such that the highest propensity of wakefulness occurs during the night work period, while the highest propensity for sleep occurs during the day.17, 43 For early morning shift workers, circadian rhythms for wakefulness and sleep propensity should be shifted earlier. Circadian adaptation involves not only sleeping well before work, but also preventing dips in wakefulness during work. Adaptation strategies are listed in Table 3.
| Night Shift60 | Early Day Shift (Starting at 4 AM‐7 AM) | |
|---|---|---|
| Prior to shift | Avoid sleep debt | Avoid sleep debt |
| Proper sleep hygiene | Proper sleep hygiene | |
| Planned napping | Bright light exposure | |
| Caffeine use | ||
| During the shift | Bright light exposure | Caffeine use |
| Planned napping | ||
| After the shift | Avoid bright light | Avoid late evening bright light (when applicable) |
| Melatonin prior to sleep | ||
| Careful use of other hypnotics | Initiate sleep early |
Improved Sleep Before Work
As an essential first step, hospitalists must get a full night's rest before starting a night shift, as sleep debt will worsen fatigue while at work. Tips for proper sleep hygiene are listed in Table 4. Some shift workers stay up late the night before a scheduled night shift, in order to sleep during the day and awaken shortly before their scheduled night shift, to combat fatigue at work. Such an approach to shift work is typically met with 3 barriers. First, environmental factors often prevent 6 hours of uninterrupted day sleep. Second, 6 hours of continual day sleep is typically difficult because rising circadian activity often limits the sleep period to just a few hours. Third, an adequate amount of sleep prior to a night shift will itself not be enough to prevent sleepiness from occurring after midnightreducing the fall in circadian activity is also essential to maintaining alertness and performance.
| Physical | Adhere to regular wake and sleep schedule |
| activities73 | Engage in regular exercise early in the day |
| Avoid caffeine, nicotine, and alcohol use 6 hr prior to sleeping | |
| Avoid stimulating or stressful activities 30 min prior to sleeping | |
| Proper sleep | Well ventilated, temperature‐controlled bedroom |
| environment | Use heavy curtains to provide as much darkness as possible |
| Comfortable mattress and pillow | |
| Remove television and pets from the bedroom | |
| Housemates should help provide quiet sleep environment |
Napping
Napping prior to a night shift, or during the work shift, can improve alertness and performance and decrease accident rates.44, 45 During shift work, naps of 20 to 50 minutes in duration have demonstrated improvements in reaction time, and restoration of performance to that seen at the start of the shift. Napping early in the night shift can improve objective measures of alertness.44 To avoid increased drowsiness that sometimes occurs when waking from a nap, naps should not be longer than 50 minutes, and can be as short as 10 to 15 minutes.44, 46 Although effective, napping may be impractical for many workers due to time or space constraints. To facilitate brief naps, hospitalist practices should ensure they have a dark, quiet call room for use by overnight hospitalists.
Bright Light Exposure
Studies demonstrate that light exposure during the night shift improves circadian alignment, mood, and performance during the night shift.47, 48 Light exposure ranged from 6 hours to 5 light treatments of 15 minutes each, with brightness ranging from 2,500 to 10,000 lux (approximating outdoor daylight; typical office lighting provides 200‐500 lux).47, 49 Results demonstrate that bright light exposure during the night shift acutely improves alertness and performance, though not to daytime levels.50 The greatest circadian adjustments occur in groups using both bright light during the night shift and light avoidance the following morning.51 Dark sunglasses and a dark home environment can decrease bright light exposure during the day. Though little evidence exists to support widespread application of bright light devices in hospitalists' call‐rooms, a hospitalist practice should consider installing one to promote circadian adaptation if physicians working overnight have multiple consecutive shifts. Likewise, these physicians should be vigilant and wear dark sunglasses during the day after their night shifteven a few minutes of light exposure at the wrong time of the day may disrupt the intended circadian adaptation.
Wake‐Promoting Agents
Numerous studies demonstrate that 150 mg to 400 mg of caffeine (a 16 oz grande cup of coffee from Starbucks contains between 200 to 500 mg of caffeine52; a Diet Coke contains 46 mg/12 oz53) reduces sleepiness, increases alertness, and improves performance during the night shift.54, 55 Thus, judicious use of caffeine may be recommended in hospital practices during extended work hours. Other wake‐promoting agents, such as modafinil and armodafinil, are US Food and Drug Administration (FDA)‐approved in the treatment of excessive sleepiness associated with SWD. Typically taken 3060 minutes before the start of the night shift, these medications have been shown in trials, enrolling mostly permanent night shift workers, to reduce excessive nighttime sleepiness and improve performance.19, 56 Armodafinil used to treat SWD‐associated excessive sleepiness, has been safely tolerated for durations of 1 year or more.57 However, these agents are not approved for use in patients without a diagnosed sleep disorder.
Melatonin
Exogenous melatonin has been used to reset circadian rhythms in patients with CRSDs.58 Melatonin administered in the late afternoon to early evening directs the largest phase advance. In contrast, melatonin given in the morning produces the largest phase delays.59 When taken after a night shift, melatonin (at a dose 1.8 to 3.0 mg) can improve day sleep quality and duration.60 Despite this result, melatonin's effectiveness in improving circadian adaptation has been mixed.61 For example, improvements in nighttime alertness during the night shift were not seen, despite the use of melatonin to facilitate daytime sleep beforehand.62 Hospitalists may consider a trial of melatonin to improve circadian alignment and facilitate daytime sleep, but its chronic use and long‐term safety has not been adequately studied.
Hypnotics After Work
Hypnotics such as temazepam 20 mg,63 triazolam,64 and zolpidem65 taken after night shift work have been shown to improve day sleep quality under simulated conditions, but do not improve shift work performance. These medications should be reserved for judicious short‐term use in patients with insomnia associated with SWD.
NIGHT SHIFT SCHEDULING TO REDUCE CIRCADIAN MISALIGNMENT
When providing 24‐hour, on‐site medical care, questions may arise about how to incorporate circadian adaptation into the daily schedule.
How Should Shifts Be Rotated?
When scheduling shifts with different start times, evidence suggests that sleep disturbance is reduced with a clockwise progression in shifts (eg, day shift to evening shift to night shift). This reduction in sleep disturbance is thought due to increased time between shifts and the circadian timekeeping tendency to extend beyond 24 hours.66
When Should the Night Shift Start?
Those hospitalist programs using an evening swing shift from afternoon to late evening may have the option of using a 12‐hour night shift starting around 7 PM, or a shorter night shift beginning later at night. Though there are no data among hospitalists to suggest which night shift start time and duration would lead to the least amount of fatigue and errors, healthcare providers working a 12‐hour night shift may have increased morale due to fewer shifts, but may suffer a reduction in the quality of care provided compared with working an 8‐hour night shift.67 In either case, shift workers given flexibility in scheduling have been shown to have positive effects on sleep.68
Should Night Shifts Be Bunched?
The decision to bunch night shifts together depends on how many night shifts are required, and how quickly circadian adaptation can occur. Under simulated conditions, circadian adaptation can yield significant changes in sleep/wake cycles in as little as 4 days.48 In real‐world settings, more time may be required to achieve significant shifts in the circadian cycle. Therefore, hospitalists who have 7 or fewer night shifts during the academic year may want to space the shifts out to prevent sleep debt on consecutive shifts, since significant circadian adaptation would be difficult to achieve in less than a week. In this situation, after a night shift, the hospitalist should have at least one 9‐hour sleep period at night to relieve their sleep debt before staffing another night shift.69 Consecutive night shifts require at least 2 nighttime sleep periods of 9 hours to recover from sleep debt.70 The IOM recommends setting a limit of 4 consecutive night float shifts for resident physicians, however, a recent systematic review of resident night float models did not find data supporting use of a 4‐night‐maximum model.28
If more than 7 night shifts are required, then scheduling the shifts close together with use of circadian adaptation techniques may result in increased nighttime alertness, less fatigue, and fewer errors while at work than widely spacing out the shifts. For example, 1 recent study simulated 8 night shifts over a 10‐day period, and compared circadian schedules and work performance between those subjects who used circadian adaptation strategies and those that did not.71 Circadian adaptation techniques included: four 15‐minute bright light pulses during each night shift, dark sunglasses when outside, dark bedrooms and delayed sleeping until 3 AM on the nights off in between the night shift blocks. The group who shifted their circadian schedule improved night shift alertness and performance as measured by neurophysiological testing, while permitting sufficient daytime sleep after work, as well as late‐night sleep on days off. The group without circadian interventions did not shift their circadian schedule as significantly as the intervention group, and performed worse on the performance testing.
CONCLUSIONS
The nationwide use of hospitalists to provide 24‐hour patient care continues to expand, thus subjecting more hospitalists to work hours asynchronous with the lightdark cycle. Resultant circadian misalignment can result in fatigue while at work, shift work disorder, and, potentially, an increased rate of medical errors. Recognition of these dangers among resident physicians has prompted the ACGME to intensify their regulations on work hours, shift schedules, and time off between shifts. However, no such recommendations exist for hospitalists or emergency physicians and nurses.
Given the potential risk to both physicians and patients, we recommend more research examining the effects of circadian misalignment within the hospitalist community. Sample research questions are offered in Table 5. More information is urgently needed to provide evidence‐based practice guidelines to ensure the safety of this growing workforce and the patients they treat.
| Are hospitalists more immune to fatigue than resident physicians? |
| Are hospitalists better able to recognize fatigue while at work than resident physicians? |
| Does 1 shift work schedule promote better circadian alignment than other shift schedules? |
| Do consistent nighttime hours of nocturnists make them more prone to commit medical errors than hospitalists rotating their shifts? |
- .Job, family, and gender: determinants of nonstandard work schedules among employed Americans in 1991.Demography.1995;32:577–598.
- ,.Shift work among dual‐earner couples with children.Science.1983;219:876–879.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units.N Engl J Med.2004;351:1838–1848.
- Institute of Medicine. Resident Duty Hours: Enhancing Sleep, Supervision and Safety. Released December 15,2008. Available at: http://www.iom.edu/Reports/2008/Resident‐Duty‐Hours‐Enhancing‐Sleep‐Supervision‐and‐Safety.aspx. Accessed on October 20, 2010.
- ACGME. ACGME Approved Standards. Effective July2011. Available at: http://acgme‐2010standards.org/. Accessed on January 6, 2011
- ACGME. Common Program Requirements: Resident Duty Hours in the Learning and Work Environment. Effective July 1, 2007. Available at: http://www.acgme.org/acWebsite/dutyHours/dh_ComProgrRequirmentsDutyHours0707.pdf. Accessed October 20,2010.
- ,,, et al.Effect of reducing interns' weekly work hours on sleep and attentional failures.N Engl J Med.2004;351:1829–1837.
- Letter written to William Munier, Agency for Healthcare Research and Quality.Washington, DC:US House of Representatives, Committee on Energy and Commerce, March 29,2007. Available at: http://energycommerce.house.gov/Press_110/110‐ltr.032907.HHS.Munier.pdf. Accessed on October 24, 2010.
- ,.The evolution and future of hospital medicine.Mt Sinai J Med.2008;75:418–423.
- .Brain structures and receptors involved in alertness.Sleep Med.2005;6(suppl 1):S3–S7.
- .A two process model of sleep regulation.Hum Neurobiol.1982;1:195–204.
- ,.Entrainment of the human circadian system by light.J Biol Rhythms.2005;20:326–338.
- ,.Sleep‐promoting and hypothermic effects of daytime melatonin administration in humans.Sleep.1997;20:124–131.
- .Sleepiness as a consequence of shift work.Sleep.1988;11:17–34.
- ,,,,.The sleep of long‐haul truck drivers.N Engl J Med.1997;337:755–761.
- ,,,.Sleep on the night shift: 24‐hour EEG monitoring of spontaneous sleep/wake behavior.Psychophysiology.1989;26:352–358.
- .Do permanent night workers show circadian adjustment? A review based on the endogenous melatonin rhythm.Chronobiol Int.2008;25:215–224.
- ,,,,.Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers.Sleep.2004;27:1453–1462.
- ,,, et al.Modafinil for excessive sleepiness associated with shift‐work sleep disorder.N Engl J Med.2005;353:476–486.
- American Academy of Sleep Medicine.The International Classification of Sleep Disorders (ICSD).2nd ed.Chicago, IL:American Academy of Sleep Medicine;2005.
- ,.Circadian rhythm sleep disorders.Chest.2006;130:1915–1923.
- ,,,,,.Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism.Sleep.2008;31:1159–1167.
- ,,,,,.Genetic variation in the adenosine A2A receptor gene modulates performance on the psychomotor vigilance task.Sleep Biol Rhythms.2007;5:A47.
- ,.Circadian rhythms in cognitive performance: methodological constraints, protocols, theoretical underpinnings.Physiol Behav.2007;90:196–208.
- ,,,.A prospective study of fatal occupational accidents—relationship to sleeping difficulties and occupational factors.J Sleep Res.2002;11:69–71.
- ,,,.Effects of critical care nurses' work hours on vigilance and patients' safety.Am J Crit Care.2006;15:30–37.
- ,,,,.Daytime sleepiness, sleep habits and occupational accidents among hospital nurses.J Adv Nurs.2005;52:445–453.
- ,,.Systematic review: association of shift length, protected sleep time, and night float with patient care, residents' health, and education.Ann Intern Med.2010;153:829–842.
- ,,,,.Jack Barney Award: the effect of fatigue on cognitive and psychomotor skills of trauma residents and attending surgeons.Am J Surg.2008;196:813–819; discussion 9–20.
- ,,, et al.Risks of complications by attending physicians after performing nighttime procedures.JAMA.2009;302:1565–1572.
- ,,.Sleep deprivation, elective surgical procedures, and informed consent.N Engl J Med.2010;363:2577–2579.
- ,,,,.A prospective study of anger and coronary heart disease. The Normative Aging Study.Circulation.1996;94:2090–2095.
- ,,,.Night work and risk of breast cancer.Epidemiology.2006;17:108–111.
- ,,, et al.Night‐shift work and risk of colorectal cancer in the nurses' health study.J Natl Cancer Inst.2003;95:825–828.
- ,,.Shift work and cardiovascular disease—pathways from circadian stress to morbidity.Scand J Work Environ Health.2010;36:96–108.
- IARC. IARC monographs on the evalutaion of carcinogenic risks to humans. Vol 98. Painting, firefighting, and shiftwork. 2007. Available at: monographs.iarc.fr/ENG/Monographs/vol98/mono98.pdf. Accessed January 16,2011.
- ,,,.Influence of melatonin on invasive and metastatic properties of MCF‐7 human breast cancer cells.Cancer Res.1998;58:4383–4390.
- Society of Hospital Medicine. Society of Hospital Medicine releases results of the 2007–2008 survey on the state of the hospital medicine movement. 2008. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Press_Releases3:247–255.
- ,,.Hospitalists: A Guide to Building and Sustaining a Successful Program.Chicago, IL:Health Administration Press;2008.
- ,,,,.The increased financial burden of further proposed orthopaedic resident work‐hour reductions.J Bone Joint Surg Am.2011;93:e31.
- ,,,,,.Fatigue and shift work.J Sleep Res.2006;15:1–5.
- ,,, et al.Rapid shift in peak melatonin secretion associated with improved performance in short shift work schedule.Sleep.1997;20:1145–1150.
- ,,.The impact of a nap opportunity during the night shift on the performance and alertness of 12‐h shift workers.J Sleep Res.2002;11:219–227.
- ,,, et al.Improving alertness and performance in emergency department physicians and nurses: the use of planned naps.Ann Emerg Med.2006;48:596–604, e1‐e3.
- ,,,,.Promoting alertness with a short nap during a night shift.J Sleep Res.1998;7:240–247.
- ,.Circadian adaptation to night‐shift work by judicious light and darkness exposure.J Biol Rhythms.2002;17:556–567.
- ,,,,,.Exposure to bright light and darkness to treat physiologic maladaptation to night work.N Engl J Med.1990;322:1253–1259.
- ,.Night shift performance is improved by a compromise circadian phase position: study 3. Circadian phase after 7 night shifts with an intervening weekend off.Sleep.2008;31:1639–1645.
- ,,,.Dose‐response relationship for light intensity and ocular and electroencephalographic correlates of human alertness.Behav Brain Res.2000;115:75–83.
- ,,,,.Dark goggles and bright light improve circadian rhythm adaptation to night‐shift work.Sleep.1994;17:535–543.
- ,,.Caffeine content of specialty coffees.J Anal Toxicol.2003;27:520–522.
- ,.Caffeine content of prepackaged national‐brand and private‐label carbonated beverages.J Food Sci.2007;72:C337–C342.
- ,,, et al.The effects of coffee and napping on nighttime highway driving: a randomized trial.Ann Intern Med.2006;144:785–791.
- ,,,,.Laboratory and field studies of naps and caffeine as practical countermeasures for sleep‐wake problems associated with night work.Sleep.2006;29:39–50.
- ,,,,.Armodafinil for treatment of excessive sleepiness associated with shift work disorder: a randomized controlled study.Mayo Clin Proc.2009;84:958–972.
- ,,,,.The long‐term tolerability and efficacy of armodafinil in patients with excessive sleepiness associated with treated obstructive sleep apnea, shift work disorder, or narcolepsy: an open‐label extension study.J Clin Sleep Med.2010;6:458–466.
- ,,.Circadian rhythm sleep disorders.Med Clin North Am.2004;88:631–651, viii.
- ,,.A three pulse phase response curve to three milligrams of melatonin in humans.J Physiol.2008;586:639–647.
- ,,, et al.Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report.Sleep.2007;30:1445–1459.
- ,,, et al.Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta‐analysis.BMJ.2006;332:385–393.
- ,,.Effects of melatonin administration on daytime sleep after simulated night shift work.J Sleep Res.2001;10:181–192.
- ,,,.Performance, ability to stay awake, and tendency to fall asleep during the night after a diurnal sleep with temazepam or placebo.Sleep.1997;20:535–541.
- ,,,,,.Sleepiness/alertness on a simulated night shift following sleep at home with triazolam.Sleep.1991;14:140–146.
- ,,,.Zolpidem‐related effects on performance and mood during simulated night‐shift work.Exp Clin Psychopharmacol.2003;11:259–268.
- ,,,,.Intrinsic near‐24‐h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans.Proc Natl Acad Sci USA.2001;98:14027–14032.
- ,,,.Work shift duration: a review comparing eight hour and 12 hour shift systems.Occup Environ Med.1998;55:217–229.
- ,,.Influence of flexibility and variability of working hours on health and well‐being.Chronobiol Int.2006;23:1125–1137.
- ,,.Impact of night‐float rotation on sleep, mood, and alertness: the resident's perception.Chronobiol Int.2002;19:893–902.
- ,,,,,.The characteristics of recovery sleep when recovery opportunity is restricted.Sleep.2007;30:353–360.
- ,,.Practical interventions to promote circadian adaptation to permanent night shift work: study 4.J Biol Rhythms.2009;24:161–172.
- ,,.Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people.Occup Environ Med.2001;58:747–752.
- ,.Use of sleep hygiene in the treatment of insomnia.Sleep Med Rev.2003;7:215–225.
- .Job, family, and gender: determinants of nonstandard work schedules among employed Americans in 1991.Demography.1995;32:577–598.
- ,.Shift work among dual‐earner couples with children.Science.1983;219:876–879.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units.N Engl J Med.2004;351:1838–1848.
- Institute of Medicine. Resident Duty Hours: Enhancing Sleep, Supervision and Safety. Released December 15,2008. Available at: http://www.iom.edu/Reports/2008/Resident‐Duty‐Hours‐Enhancing‐Sleep‐Supervision‐and‐Safety.aspx. Accessed on October 20, 2010.
- ACGME. ACGME Approved Standards. Effective July2011. Available at: http://acgme‐2010standards.org/. Accessed on January 6, 2011
- ACGME. Common Program Requirements: Resident Duty Hours in the Learning and Work Environment. Effective July 1, 2007. Available at: http://www.acgme.org/acWebsite/dutyHours/dh_ComProgrRequirmentsDutyHours0707.pdf. Accessed October 20,2010.
- ,,, et al.Effect of reducing interns' weekly work hours on sleep and attentional failures.N Engl J Med.2004;351:1829–1837.
- Letter written to William Munier, Agency for Healthcare Research and Quality.Washington, DC:US House of Representatives, Committee on Energy and Commerce, March 29,2007. Available at: http://energycommerce.house.gov/Press_110/110‐ltr.032907.HHS.Munier.pdf. Accessed on October 24, 2010.
- ,.The evolution and future of hospital medicine.Mt Sinai J Med.2008;75:418–423.
- .Brain structures and receptors involved in alertness.Sleep Med.2005;6(suppl 1):S3–S7.
- .A two process model of sleep regulation.Hum Neurobiol.1982;1:195–204.
- ,.Entrainment of the human circadian system by light.J Biol Rhythms.2005;20:326–338.
- ,.Sleep‐promoting and hypothermic effects of daytime melatonin administration in humans.Sleep.1997;20:124–131.
- .Sleepiness as a consequence of shift work.Sleep.1988;11:17–34.
- ,,,,.The sleep of long‐haul truck drivers.N Engl J Med.1997;337:755–761.
- ,,,.Sleep on the night shift: 24‐hour EEG monitoring of spontaneous sleep/wake behavior.Psychophysiology.1989;26:352–358.
- .Do permanent night workers show circadian adjustment? A review based on the endogenous melatonin rhythm.Chronobiol Int.2008;25:215–224.
- ,,,,.Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers.Sleep.2004;27:1453–1462.
- ,,, et al.Modafinil for excessive sleepiness associated with shift‐work sleep disorder.N Engl J Med.2005;353:476–486.
- American Academy of Sleep Medicine.The International Classification of Sleep Disorders (ICSD).2nd ed.Chicago, IL:American Academy of Sleep Medicine;2005.
- ,.Circadian rhythm sleep disorders.Chest.2006;130:1915–1923.
- ,,,,,.Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism.Sleep.2008;31:1159–1167.
- ,,,,,.Genetic variation in the adenosine A2A receptor gene modulates performance on the psychomotor vigilance task.Sleep Biol Rhythms.2007;5:A47.
- ,.Circadian rhythms in cognitive performance: methodological constraints, protocols, theoretical underpinnings.Physiol Behav.2007;90:196–208.
- ,,,.A prospective study of fatal occupational accidents—relationship to sleeping difficulties and occupational factors.J Sleep Res.2002;11:69–71.
- ,,,.Effects of critical care nurses' work hours on vigilance and patients' safety.Am J Crit Care.2006;15:30–37.
- ,,,,.Daytime sleepiness, sleep habits and occupational accidents among hospital nurses.J Adv Nurs.2005;52:445–453.
- ,,.Systematic review: association of shift length, protected sleep time, and night float with patient care, residents' health, and education.Ann Intern Med.2010;153:829–842.
- ,,,,.Jack Barney Award: the effect of fatigue on cognitive and psychomotor skills of trauma residents and attending surgeons.Am J Surg.2008;196:813–819; discussion 9–20.
- ,,, et al.Risks of complications by attending physicians after performing nighttime procedures.JAMA.2009;302:1565–1572.
- ,,.Sleep deprivation, elective surgical procedures, and informed consent.N Engl J Med.2010;363:2577–2579.
- ,,,,.A prospective study of anger and coronary heart disease. The Normative Aging Study.Circulation.1996;94:2090–2095.
- ,,,.Night work and risk of breast cancer.Epidemiology.2006;17:108–111.
- ,,, et al.Night‐shift work and risk of colorectal cancer in the nurses' health study.J Natl Cancer Inst.2003;95:825–828.
- ,,.Shift work and cardiovascular disease—pathways from circadian stress to morbidity.Scand J Work Environ Health.2010;36:96–108.
- IARC. IARC monographs on the evalutaion of carcinogenic risks to humans. Vol 98. Painting, firefighting, and shiftwork. 2007. Available at: monographs.iarc.fr/ENG/Monographs/vol98/mono98.pdf. Accessed January 16,2011.
- ,,,.Influence of melatonin on invasive and metastatic properties of MCF‐7 human breast cancer cells.Cancer Res.1998;58:4383–4390.
- Society of Hospital Medicine. Society of Hospital Medicine releases results of the 2007–2008 survey on the state of the hospital medicine movement. 2008. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Press_Releases3:247–255.
- ,,.Hospitalists: A Guide to Building and Sustaining a Successful Program.Chicago, IL:Health Administration Press;2008.
- ,,,,.The increased financial burden of further proposed orthopaedic resident work‐hour reductions.J Bone Joint Surg Am.2011;93:e31.
- ,,,,,.Fatigue and shift work.J Sleep Res.2006;15:1–5.
- ,,, et al.Rapid shift in peak melatonin secretion associated with improved performance in short shift work schedule.Sleep.1997;20:1145–1150.
- ,,.The impact of a nap opportunity during the night shift on the performance and alertness of 12‐h shift workers.J Sleep Res.2002;11:219–227.
- ,,, et al.Improving alertness and performance in emergency department physicians and nurses: the use of planned naps.Ann Emerg Med.2006;48:596–604, e1‐e3.
- ,,,,.Promoting alertness with a short nap during a night shift.J Sleep Res.1998;7:240–247.
- ,.Circadian adaptation to night‐shift work by judicious light and darkness exposure.J Biol Rhythms.2002;17:556–567.
- ,,,,,.Exposure to bright light and darkness to treat physiologic maladaptation to night work.N Engl J Med.1990;322:1253–1259.
- ,.Night shift performance is improved by a compromise circadian phase position: study 3. Circadian phase after 7 night shifts with an intervening weekend off.Sleep.2008;31:1639–1645.
- ,,,.Dose‐response relationship for light intensity and ocular and electroencephalographic correlates of human alertness.Behav Brain Res.2000;115:75–83.
- ,,,,.Dark goggles and bright light improve circadian rhythm adaptation to night‐shift work.Sleep.1994;17:535–543.
- ,,.Caffeine content of specialty coffees.J Anal Toxicol.2003;27:520–522.
- ,.Caffeine content of prepackaged national‐brand and private‐label carbonated beverages.J Food Sci.2007;72:C337–C342.
- ,,, et al.The effects of coffee and napping on nighttime highway driving: a randomized trial.Ann Intern Med.2006;144:785–791.
- ,,,,.Laboratory and field studies of naps and caffeine as practical countermeasures for sleep‐wake problems associated with night work.Sleep.2006;29:39–50.
- ,,,,.Armodafinil for treatment of excessive sleepiness associated with shift work disorder: a randomized controlled study.Mayo Clin Proc.2009;84:958–972.
- ,,,,.The long‐term tolerability and efficacy of armodafinil in patients with excessive sleepiness associated with treated obstructive sleep apnea, shift work disorder, or narcolepsy: an open‐label extension study.J Clin Sleep Med.2010;6:458–466.
- ,,.Circadian rhythm sleep disorders.Med Clin North Am.2004;88:631–651, viii.
- ,,.A three pulse phase response curve to three milligrams of melatonin in humans.J Physiol.2008;586:639–647.
- ,,, et al.Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report.Sleep.2007;30:1445–1459.
- ,,, et al.Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta‐analysis.BMJ.2006;332:385–393.
- ,,.Effects of melatonin administration on daytime sleep after simulated night shift work.J Sleep Res.2001;10:181–192.
- ,,,.Performance, ability to stay awake, and tendency to fall asleep during the night after a diurnal sleep with temazepam or placebo.Sleep.1997;20:535–541.
- ,,,,,.Sleepiness/alertness on a simulated night shift following sleep at home with triazolam.Sleep.1991;14:140–146.
- ,,,.Zolpidem‐related effects on performance and mood during simulated night‐shift work.Exp Clin Psychopharmacol.2003;11:259–268.
- ,,,,.Intrinsic near‐24‐h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans.Proc Natl Acad Sci USA.2001;98:14027–14032.
- ,,,.Work shift duration: a review comparing eight hour and 12 hour shift systems.Occup Environ Med.1998;55:217–229.
- ,,.Influence of flexibility and variability of working hours on health and well‐being.Chronobiol Int.2006;23:1125–1137.
- ,,.Impact of night‐float rotation on sleep, mood, and alertness: the resident's perception.Chronobiol Int.2002;19:893–902.
- ,,,,,.The characteristics of recovery sleep when recovery opportunity is restricted.Sleep.2007;30:353–360.
- ,,.Practical interventions to promote circadian adaptation to permanent night shift work: study 4.J Biol Rhythms.2009;24:161–172.
- ,,.Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people.Occup Environ Med.2001;58:747–752.
- ,.Use of sleep hygiene in the treatment of insomnia.Sleep Med Rev.2003;7:215–225.
Teamwork in Hospitals
Teamwork is important in providing high‐quality hospital care. Despite tremendous efforts in the 10 years since publication of the Institute of Medicine's To Err is Human report,1 hospitalized patients remain at risk for adverse events (AEs).2 Although many AEs are not preventable, a large portion of those which are identified as preventable can be attributed to communication and teamwork failures.35 A Joint Commission study indicated that communication failures were the root cause for two‐thirds of the 3548 sentinel events reported from 1995 to 2005.6 Another study, involving interviews of resident physicians about recent medical mishaps, found that communication failures contributed to 91% of the AEs they reported.5
Teamwork also plays an important role in other aspects of hospital care delivery. Patients' ratings of nurse‐physician coordination correlate with their overall perception of the quality of care received.7, 8 A study of Veterans Health Administration (VHA) hospitals found that teamwork culture was significantly and positively associated with overall patient satisfaction.9 Another VHA study found that hospitals with higher teamwork culture ratings had lower nurse resignations rates.10 Furthermore, poor teamwork within hospitals may have an adverse effect on financial performance, as a result of inefficiencies in physician and nurse workflow.11
Some organizations are capable of operating in complex, hazardous environments while maintaining exceptional performance over long periods of time. These high reliability organizations (HRO) include aircraft carriers, air traffic control systems, and nuclear power plants, and are characterized by their preoccupation with failure, reluctance to simplify interpretations, sensitivity to operations, commitment to resilience, and deference to expertise.12, 13 Preoccupation with failure is manifested by an organization's efforts to avoid complacency and persist in the search for additional risks. Reluctance to simplify interpretations is exemplified by an interest in pursuing a deep understanding of the issues that arise. Sensitivity to operations is the close attention paid to input from front‐line personnel and processes. Commitment to resilience relates to an organization's ability to contain errors once they occur and mitigate harm. Deference to expertise describes the practice of having authority migrate to the people with the most expertise, regardless of rank. Collectively, these qualities produce a state of mindfulness, allowing teams to anticipate and become aware of unexpected events, yet also quickly contain and learn from them. Recent publications have highlighted the need for hospitals to learn from HROs and the teams within them.14, 15
Recognizing the importance of teamwork in hospitals, senior leadership from the American College of Physician Executives (ACPE), the American Hospital Association (AHA), the American Organization of Nurse Executives (AONE), and the Society of Hospital Medicine (SHM) established the High Performance Teams and the Hospital of the Future project. This collaborative learning effort aims to redesign care delivery to provide optimal value to hospitalized patients. As an initial step, the High Performance Teams and the Hospital of the Future project team completed a literature review related to teamwork in hospitals. The purpose of this report is to summarize the current understanding of teamwork, describe interventions designed to improve teamwork, and make practical recommendations for hospitals to assess and improve teamwork‐related performance. We approach teamwork from the hospitalized patient's perspective, and restrict our discussion to interactions occurring among healthcare professionals within the hospital. We recognize the importance of teamwork at all points in the continuum of patient care. Highly functional inpatient teams should be integrated into an overall system of coordinated and collaborative care.
TEAMWORK: DEFINITION AND CONSTRUCTS
Physicians, nurses, and other healthcare professionals spend a great deal of their time on communication and coordination of care activities.1618 In spite of this and the patient safety concerns previously noted, interpersonal communication skills and teamwork have been historically underemphasized in professional training.1922 A team is defined as 2 or more individuals with specified roles interacting adaptively, interdependently, and dynamically toward a shared and common goal.23 Elements of effective teamwork have been identified through research conducted in aviation, the military, and more recently, healthcare. Salas and colleagues have synthesized this research into 5 core components: team leadership, mutual performance monitoring, backup behavior, adaptability, and team orientation (see Table 1).23 Additionally, 3 supporting and coordinating mechanisms are essential for effective teamwork: shared mental model, closed‐loop communication, and mutual trust (see Table 1).23 High‐performing teams use these elements to develop a culture for speaking up, and situational awareness among team members. Situational awareness refers to a person's perception and understanding of their dynamic environment, and human errors often result from a lack of such awareness.24 These teamwork constructs provide the foundational basis for understanding how hospitals can identify teamwork challenges, assess team performance, and design effective interventions.
| Teamwork | Definition | Behavioral Examples |
|---|---|---|
| ||
| Component | ||
| Team leadership | The leader directs and coordinates team members activities | Facilitate team problem solving; |
| Provide performance expectations; | ||
| Clarify team member roles; | ||
| Assist in conflict resolution | ||
| Mutual performance monitoring | Team members are able to monitor one another's performance | Identify mistakes and lapses in other team member actions; |
| Provide feedback to fellow team members to facilitate self‐correction | ||
| Backup behavior | Team members anticipate and respond to one another's needs | Recognize workload distribution problem; |
| Shift work responsibilities to underutilized members | ||
| Adaptability | The team adjusts strategies based on new information | Identify cues that change has occurred and develop plan to deal with changes; |
| Remain vigilant to change in internal and external environment | ||
| Team orientation | Team members prioritize team goals above individual goals | Take into account alternate solutions by teammates; |
| Increased task involvement, information sharing, and participatory goal setting | ||
| Coordinating mechanism | ||
| Shared mental model | An organizing knowledge of the task of the team and how members will interact to achieve their goal | Anticipate and predict each other's needs; |
| Identify changes in team, task, or teammates | ||
| Closed‐loop communication | Acknowledgement and confirmation of information received | Follow up with team members to ensure message received; |
| Acknowledge that message was received; | ||
| Clarify information received | ||
| Mutual trust | Shared belief that team members will perform their roles | Share information; |
| Willingly admit mistakes and accept feedback | ||
CHALLENGES TO EFFECTIVE TEAMWORK
Several important and unique barriers to teamwork exist in hospitals. Teams are large and formed in an ad hoc fashion. On a given day, a patient's hospital team might include a hospitalist, a nurse, a case manager, a pharmacist, and 1 or more consulting physicians and therapists. Team members in each respective discipline care for multiple patients at the same time, yet few hospitals align team membership (ie, patient assignment). Therefore, a nurse caring for 4 patients may interact with 4 different hospitalists. Similarly, a hospitalist caring for 14 patients may interact with multiple nurses in a given day. Team membership is ever changing because hospital professionals work in shifts and rotations. Finally, team members are seldom in the same place at the same time because physicians often care for patients on multiple units and floors, while nurses and other team members are often unit‐based. Salas and others have noted that team size, instability, and geographic dispersion of membership serve as important barriers to improving teamwork.25, 26 As a result of these barriers, nurses and physicians do not communicate consistently, and often disagree on the daily plan of care for their patients.27, 28 When communication does occur, clinicians may overestimate how well their messages are understood by other team members, reflecting a phenomenon well known in communication psychology related to egocentric thought processes.29, 30
The traditionally steep hierarchy within medicine may also serve as a barrier to teamwork. Studies in intensive care units (ICUs), operating rooms, and general medical units reveal widely discrepant views on the quality of collaboration and communication between healthcare professionals.3133 Although physicians generally give high ratings to the quality of collaboration with nurses, nurses consistently rate the quality of collaboration with physicians as poor. Similarly, specialist physicians rate collaboration with hospitalists higher than hospitalists rate collaboration with specialists.33 Effective teams in other high‐risk industries, like aviation, strive to flatten hierarchy so that team members feel comfortable raising concerns and engaging in open and respectful communications.34
The effect of technology on communication practices and teamwork is complex and incompletely understood. The implementation of electronic heath records and computerized provider order entry systems fundamentally changes work‐flow, and may result in less synchronization and feedback during nurse‐physician collaboration.35 Similarly, the expanded use of text messages delivered via alphanumeric paging or mobile phone results in a transition toward asynchronous modes of communication. These asynchronous modes allow healthcare professionals to review and respond to messages at their convenience, and may reduce unnecessary interruptions. Research shows that these systems are popular among clinicians.3638 However, receipt and understanding of the intended message may not be confirmed with the use of asynchronous modes of communication. Moreover, important face‐to‐face communication elements (tone of voice, expression, gesture, eye contract)39, 40 are lacking. One promising approach is a system which sends low‐priority messages to a Web‐based task list for periodic review, while allowing higher priority messages to pass through to an alphanumeric pager and interrupt the intended recipient.41 Another common frustration in hospitals, despite advancing technology, is difficulty identifying the correct physician(s) and nurse(s) caring for a particular patient at a given point in time.33 Wong and colleagues found that 14% of pages in their hospital were initially sent to the wrong physician.42
ASSESSMENT OF TEAMWORK
One of the challenges in improving teamwork is the difficulty in measuring it. Teamwork assessment entails measuring the performance of teams composed of multiple individuals. Methods of teamwork assessment can be broadly categorized as self assessment, peer assessment, direct observation, survey of team climate or culture, and measurement of the outcome of effective teamwork. While self‐report tools are easy to administer and can capture affective components influencing team performance, they may not reflect actual skills on the part of individuals or teams. Peer assessment includes the use of 360‐degree evaluations or multisource feedback, and provides an evaluation of individual performance.4347
Direct observation provides a more accurate assessment of team‐related behaviors using trained observers. Observers use checklists and/or behaviorally anchored rating scales (BARS) to evaluate individual and team performance. A number of BARS have been developed and validated for the evaluation of team performance.4852 Of note, direct observation may be difficult in settings in which team members are not in the same place at the same time. An alternative method, which may be better suited for general medical units, is the use of survey instruments designed to assess attitudes and teamwork climate.5355 Importantly, higher survey ratings of collaboration and teamwork have been associated with better patient outcomes in observational studies.5658
The ultimate goal of teamwork efforts is to improve patient outcomes. Because patient outcomes are affected by a number of factors and because hospitals frequently engage in multiple, simultaneous efforts to improve care, it is often difficult to clearly link improved outcomes with teamwork interventions. Continued efforts to rigorously evaluate teamwork interventions should remain a priority, particularly as the cost of these interventions must be weighed against other interventions and investments.
EXAMPLES OF SUCCESSFUL INTERVENTIONS
A number of interventions have been used to improve teamwork in hospitals (see Table 2).
| Intervention | Advantages | Disadvantages |
|---|---|---|
| Localization of physicians | Increases frequency of nurse‐physician communication; provides foundation for additional interventions | Insufficient in creating a shared mental model; does not specifically enhance communication skills |
| Daily goals‐of‐care forms and checklists | Provides structure to interdisciplinary discussions and ensures input from all team members | May be completed in a perfunctory manner and may not be updated as plans of care evolve |
| Teamwork training | Emphasizes improved communication behaviors relevant across a range of team member interactions | Requires time and deliberate practice of new skills; effect may be attenuated if members are dispersed. |
| Interdisciplinary rounds | Provides a forum for regular interdisciplinary communication | Requires leadership to organize discussion and does not address need for updates as plans of care evolve |
Geographic Localization of Physicians
As mentioned earlier, physicians in large hospitals may care for patients on multiple units or floors. Designating certain physicians to care for patients admitted to specific units may improve efficiency and communication among healthcare professionals. One study recently reported on the effect of localization of hospital physicians to specific patient care units. Localization resulted in an increase in the rate of nurse‐physician communication, but did not improve providers' shared understanding of the plan of care.56 Notably, localizing physicians may improve the feasibility of additional interventions, like teamwork training and interdisciplinary rounds.
Daily Goals of Care and Surgery Safety Checklists
In ICU and operating room settings, physicians and nurses work in proximity, allowing interdisciplinary discussions to occur at the bedside. The finding that professionals in ICUs and operating rooms have widely discrepant views on the quality of collaboration31, 32 indicates that proximity, alone, is not sufficient for effective communication. Pronovost et al. used a daily goals form for bedside ICU rounds in an effort to standardize communication about the daily plan of care.57 The form defined essential goals of care for patients, and its use resulted in a significant improvement in the team's understanding of the daily goals. Narasimhan et al. performed a similar study using a daily goals worksheet during ICU rounds,58 and also found a significant improvement in physicians' and nurses' ratings of their understanding of the goals of care. The forms used in these studies provided structure to the interdisciplinary conversations during rounds to create a shared understanding of patients' plans of care.
Haynes and colleagues recently reported on the use of a surgical safety checklist in a large, multicenter pre‐post study.59 The checklist consisted of verbal confirmation of the completion of basic steps essential to safe care in the operating room, and provided structure to communication among surgical team members to ensure a shared understanding of the operative plan. The intervention resulted in a significant reduction in inpatient complications and mortality.
Team Training
Formalized team training, based on crew resource management, has been studied as a potential method to improve teamwork in a variety of medical settings.6062 Training emphasizes the core components of successful teamwork and essential coordinating mechanisms previously mentioned.23 Team training appears to positively influence culture, as assessed by teamwork and patient safety climate survey instruments.60 Based on these findings and extensive research demonstrating the success of teamwork training in aviation,63 the Agency for Healthcare Research and Quality (AHRQ) and the Department of Defense (DoD) have partnered in offering the Team Strategies and Tools to Enhance Performance and Patient Safety (TeamSTEPPS) program, designed to improve teamwork skills for healthcare professionals.64, 65
Only a handful of studies have evaluated the effectiveness of teamwork training programs on patient outcomes, and the results are mixed.66 Morey et al. found a reduction in the rate of observed errors as a result of teamwork training in emergency departments, but observers in the study were not blinded with regard to whether teams had undergone training.61 A research group in the United Kingdom evaluated the benefit of simulation‐based team training on outcomes in an obstetrical setting.67, 68 Training included management of specific complications, including shoulder dystocia and uterine cord prolapse. Using retrospective chart review, the investigators found a significant reduction in the proportion of babies born with an obstetric brachial palsy injury and a reduction in the time from diagnosis of uterine cord prolapse to infant delivery. Nielsen and colleagues also evaluated the use of teamwork training in an obstetric setting.62 In a cluster randomized controlled trial, the investigators found no reduction in the rate of adverse outcomes. Differences in the duration of teamwork training and the degree of emphasis on deliberate practice of new skills (eg, with the use of simulation‐based training) likely explains the lack of consistent results.
Very little research has evaluated teamwork training in the general medical environment.69, 70 Sehgal and colleagues recently published an evaluation of the effect of teamwork training delivered to internal medicine residents, hospitalists, nurses, pharmacists, case managers, and social workers on medical services in 3 Northern California hospitals.69 The 4‐hour training sessions covered topical areas of safety culture, teamwork, and communication through didactics, videos, facilitated discussions, and small group role plays to practice new skills and behaviors. The intervention was rated highly among participants,69 and the training along with subsequent follow‐up interventions resulted in improved patient perceptions of teamwork and communication but had no impact on key patient outcomes.71
Interdisciplinary Rounds
Interdisciplinary rounds (IDR) have been used for many years as a means to assemble team members in a single location,7275 and the use of IDR has been associated with lower mortality among ICU patients.76 Interdisciplinary rounds may be particularly useful for clinical settings in which team members are traditionally dispersed in time and place, such as medical‐surgical units. Recent studies have evaluated the effect of structured inter‐disciplinary rounds (SIDR),77, 78 which combine a structured format for communication, similar to a daily goals‐of‐care form, with a forum for daily interdisciplinary meetings. Though no effect was seen on length of stay or cost, SIDR resulted in significantly higher ratings of the quality of collaboration and teamwork climate, and a reduction in the rate of AEs.79 Importantly, the majority of clinicians in the studies agreed that SIDR improved the efficiency of their work day, and expressed a desire that SIDR continue indefinitely. Many investigators have emphasized the importance of leadership during IDR, often by a medical director, nurse manager, or both.74, 77, 78
Summary of Interventions to Improve Teamwork
Localization of physicians increases the frequency of nurse‐physician communication, but is insufficient in creating a shared understanding of patients' plans of care. Providing structure for the discussion among team members (eg, daily goals of care forms and checklists) ensures that critical elements of the plan of care are communicated. Teamwork training is based upon a strong foundation of research both inside and outside of healthcare, and has demonstrated improved knowledge of teamwork principles, attitudes about the importance of teamwork, and overall safety climate. Creating a forum for team members to assemble and discuss their patients (eg, IDR) can overcome some of the unique barriers to collaboration in settings where members are dispersed in time and space. Leaders wishing to improve interdisciplinary teamwork should consider implementing a combination of complementary interventions. For example, localization may increase the frequency of team member interactions, the quality of which may be enhanced with teamwork training and reinforced with the use of structured communication tools and IDR. Future research should evaluate the effect of these combined interventions.
CONCLUSIONS
In summary, teamwork is critically important to provide safe and effective care. Important and unique barriers to teamwork exist in hospitals. We recommend the use of survey instruments, such as those mentioned earlier, as the most feasible method to assess teamwork in the general medical setting. Because each intervention addresses only a portion of the barriers to optimal teamwork, we encourage leaders to use a multifaceted approach. We recommend the implementation of a combination of interventions with adaptations to fit unique clinical settings and local culture.
Acknowledgements
This manuscript was prepared as part of the High Performance Teams and the Hospital of the Future project, a collaborative effort including senior leadership from the American College of Physician Executives, the American Hospital Association, the American Organization of Nurse Executives, and the Society of Hospital Medicine. The authors thank Taylor Marsh for her administrative support and help in coordinating project meetings.
- To Err Is Human: Building a Safer Health System.Washington, DC:Institute of Medicine;1999.
- ,,,,,.Temporal trends in rates of patient harm resulting from medical care.N Engl J Med.2010;363(22):2124–2134.
- ,,.Exploring the causes of adverse events in NHS hospital practice.J R Soc Med.2001;94(7):322–330.
- ,,,,,.The Quality in Australian Health Care Study.Med J Aust.1995;163(9):458–471.
- ,,.Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79(2):186–194.
- Improving America's Hospitals: The Joint Commission's Annual Report on Quality and Safety 2007. Available at: http://www.jointcommissionreport.org. Accessed November2007.
- ,,.Patient perceptions of coordinated care: the importance of organized communication in hospitals.J Healthc Qual.1999;21(5):18–23.
- ,,.Am I safe here? Improving patients' perceptions of safety in hospitals.J Nurs Care Qual.2006;21(1):30–40.
- ,,.Teamwork culture and patient satisfaction in hospitals.Med Care.2004;42(5):492–498.
- ,,.The influence of teamwork culture on physician and nurse resignation rates in hospitals.Health Serv Manage Res.2008;21(1):23–31.
- ,,.Quantifying the economic impact of communication inefficiencies in U.S. hospitals.J Healthc Manag.2010;55(4):265–282.
- ,.Managing the Unexpected: Assuring High Performance in an Age of Complexity.San Francisco, CA:Jossey‐Bass;2001.
- .Some characteristics of high reliability organizations.Organization Science.1990;1(2):160–177.
- ,,.Teamwork as an essential component of high‐reliability organizations.Health Serv Res.2006;41(4 pt 2):1576–1598.
- ,,,.Promoting health care safety through training high reliability teams.Qual Saf Health Care.2005;14(4):303–309.
- ,,,,,.Analyzing the time and value of housestaff inpatient work.J Gen Intern Med.1998;13(8):534–540.
- ,,, et al.Quantifying nursing workflow in medication administration.J Nurs Adm.2008;38(1):19–26.
- ,,.How hospitalists spend their time: insights on efficiency and safety.J Hosp Med.2006;1(2):88–93.
- ,,,,.Redesigning residency education in internal medicine: a position paper from the Association of Program Directors in Internal Medicine.Ann Intern Med.2006;144(12):920–926.
- ,,,.Hospitalists' perceptions of their residency training needs: results of a national survey.Am J Med.2001;111(3):247–254.
- ,,.Redesigning training for internal medicine.Ann Intern Med.2006;144(12):927–932.
- ,,,,.The role of teamwork in the professional education of physicians: current status and assessment recommendations.Jt Comm J Qual Patient Saf.2005;31(4):185–202.
- ,,.Is there a “big five” in teamwork?Small Group Research.2005;36:555–599.
- ,,.Objective measures of situation awareness in a simulated medical environment.Qual Saf Health Care.2004;13(suppl 1):i65–i71.
- ,.What do we know about health care team effectiveness? A review of the literature.Med Care Res Rev.2006;63(3):263–300.
- ,,, et al.Does team training improve team performance? A meta‐analysis.Hum Factors.2008;50(6):903–933.
- ,,,,,.Can we talk? Priorities for patient care differed among health care providers. AHRQ Publication No. 05–0021‐1.Rockville, MD:Agency for Healthcare Research and Quality;2005.
- ,,, et al.Patterns of nurse–physicians communication and agreement on the plan of care.Qual Saf Health Care.2010;19:195–199.
- ,,,,.Interns overestimate the effectiveness of their hand‐off communication.Pediatrics.2010;125(3):491–496.
- ,.Speakers' overestimation of their effectiveness.Psychol Sci.2002;13(3):207–212.
- ,,, et al.Operating room teamwork among physicians and nurses: teamwork in the eye of the beholder.J Am Coll Surg.2006;202(5):746–752.
- ,,.Discrepant attitudes about teamwork among critical care nurses and physicians.Crit Care Med.2003;31(3):956–959.
- ,,,,,.Teamwork on inpatient medical units: assessing attitudes and barriers.Qual Saf Health Care.2010;19(2):117–121.
- ,,.Error, stress, and teamwork in medicine and aviation: cross sectional surveys.BMJ.2000;320(7237):745–749.
- ,,,,.Impact of a computerized physician order entry system on nurse‐physician collaboration in the medication process.Int J Med Inform.2008;77(11):735–744.
- ,,,,.Alphanumeric paging in an academic hospital setting.Am J Surg.2006;191(4):561–565.
- ,,,.Implementation and evaluation of an alpha‐numeric paging system on a resident inpatient teaching service.J Hosp Med.2009;4(8):E34–E40.
- ,,, et al.The use of smartphones for clinical communication on internal medicine wards.J Hosp Med.2010;5(9):553–559.
- ,.Organizational information requirements, media richness, and structural design.Management Science.1986;32(5):554–571.
- ,.Decoding of inconsistent communications of personality and social psychology.J Pers Soc Psychol.1967;6(1):109–114.
- ,,,,.Beyond paging: building a Web‐based communication tool for nurses and physicians.J Gen Intern Med.2009;24(1):105–110.
- ,,, et al.Frequency and clinical importance of pages sent to the wrong physician.Arch Intern Med.2009;169(11):1072–1073.
- ,,, et al.Evaluation of resident communication skills and professionalism: a matter of perspective?Pediatrics.2006;118(4):1371–1379.
- ,,, et al.Effect of multisource feedback on resident communication skills and professionalism: a randomized controlled trial.Arch Pediatr Adolesc Med.2007;161(1):44–49.
- .Multisource feedback in the assessment of physician competencies.J Contin Educ Health Prof.2003;23(1):4–12.
- ,.Reliability of a 360‐degree evaluation to assess resident competence.Am J Phys Med Rehabil.2007;86(10):845–852.
- ,,,.Pilot study of a 360‐degree assessment instrument for physical medicine 82(5):394–402.
- ,,,,,.Anaesthetists' Non‐Technical Skills (ANTS): evaluation of a behavioural marker system.Br J Anaesth.2003;90(5):580–588.
- ,,,.Using the Communication and Teamwork Skills (CATS) Assessment to measure health care team performance.Jt Comm J Qual Patient Saf.2007;33(9):549–558.
- ,,, et al.The Mayo High Performance Teamwork Scale: reliability and validity for evaluating key crew resource management skills.Simul Healthc.2007;2(1):4–10.
- ,,,,,.Reliability of a revised NOTECHS scale for use in surgical teams.Am J Surg.2008;196(2):184–190.
- ,,,,,.Observational teamwork assessment for surgery: construct validation with expert versus novice raters.Ann Surg.2009;249(6):1047–1051.
- ,,, et al.The Safety Attitudes Questionnaire: psychometric properties, benchmarking data, and emerging research.BMC Health Serv Res.2006;6:44.
- .Development of an instrument to measure collaboration and satisfaction about care decisions.J Adv Nurs.1994;20(1):176–182.
- ,,,,,.Psychometric properties of an attitude scale measuring physician‐nurse collaboration.Eval Health Prof.1999;22(2):208–220.
- ,,, et al.Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care.J Gen Intern Med.2009;24(11):1223–1227.
- ,,,,,.Improving communication in the ICU using daily goals.J Crit Care.2003;18(2):71–75.
- ,,,,.Improving nurse‐physician communication and satisfaction in the intensive care unit with a daily goals worksheet.Am J Crit Care.2006;15(2):217–222.
- ,,, et al.A surgical safety checklist to reduce morbidity and mortality in a global population.N Engl J Med.2009;360(5):491–499.
- ,,, et al.Effect of crew resource management training in a multidisciplinary obstetrical setting.Int J Qual Health Care.2008;20(4):254–263.
- ,,, et al.Error reduction and performance improvement in the emergency department through formal teamwork training: evaluation results of the MedTeams project.Health Serv Res.2002;37(6):1553–1581.
- ,,, et al.Effects of teamwork training on adverse outcomes and process of care in labor and delivery: a randomized controlled trial.Obstet Gynecol.2007;109(1):48–55.
- ,,,,.Medical Teamwork and Patient Safety: The Evidence‐Based Relation.Rockville, MD:Agency for Healthcare Research and Quality;2005.
- Agency for Healthcare Research and Quality. TeamSTEPPS Home. Available at: http://teamstepps.ahrq.gov/index.htm. Accessed January 18,2010.
- ,.TeamSTEPPS: assuring optimal teamwork in clinical settings.Am J Med Qual.2007;22(3):214–217.
- ,,,.Does crew resource management training work? An update, an extension, and some critical needs.Hum Factors.2006;48(2):392–412.
- ,,, et al.Improving neonatal outcome through practical shoulder dystocia training.Obstet Gynecol.2008;112(1):14–20.
- ,,, et al.Retrospective cohort study of diagnosis‐delivery interval with umbilical cord prolapse: the effect of team training.Br J Obstet Gynaecol.2009;116(8):1089–1096.
- ,,, et al.A multidisciplinary teamwork training program: the Triad for Optimal Patient Safety (TOPS) experience.J Gen Intern Med.2008;23(12):2053–2057.
- ,,,,.Teambuilding and leadership training in an internal medicine residency training program.J Gen Intern Med.2004;19(6):692–697.
- ,,, et al. Effects of a multicenter teamwork and communication program on patient outcomes: results from the Triad for Optimal Patient Safety (TOPS) project. In press.
- ,,, et al.The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs.J Nurs Adm.2006;36(2):79–85.
- ,,.A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement.Med Care.1998;36(8 suppl):AS4–A12.
- ,,,,.Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay.J Gen Intern Med.2007;22(8):1073–1079.
- ,,,.Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses.Am J Crit Care.2005;14(1):71–77.
- ,,,,.The effect of multidisciplinary care teams on intensive care unit mortality.Arch Intern Med.2010;170(4):369–376.
- ,,,,,.Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit.J Hosp Med.2011;6(2):88–93.
- ,,,,,.Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit.J Gen Intern Med.2010;25(8):826–832.
- ,,, et al.Structured interdisciplinary rounds in a medical teaching unit: improving patient safety.Arch Intern Med.2011;171(7):678–684.
Teamwork is important in providing high‐quality hospital care. Despite tremendous efforts in the 10 years since publication of the Institute of Medicine's To Err is Human report,1 hospitalized patients remain at risk for adverse events (AEs).2 Although many AEs are not preventable, a large portion of those which are identified as preventable can be attributed to communication and teamwork failures.35 A Joint Commission study indicated that communication failures were the root cause for two‐thirds of the 3548 sentinel events reported from 1995 to 2005.6 Another study, involving interviews of resident physicians about recent medical mishaps, found that communication failures contributed to 91% of the AEs they reported.5
Teamwork also plays an important role in other aspects of hospital care delivery. Patients' ratings of nurse‐physician coordination correlate with their overall perception of the quality of care received.7, 8 A study of Veterans Health Administration (VHA) hospitals found that teamwork culture was significantly and positively associated with overall patient satisfaction.9 Another VHA study found that hospitals with higher teamwork culture ratings had lower nurse resignations rates.10 Furthermore, poor teamwork within hospitals may have an adverse effect on financial performance, as a result of inefficiencies in physician and nurse workflow.11
Some organizations are capable of operating in complex, hazardous environments while maintaining exceptional performance over long periods of time. These high reliability organizations (HRO) include aircraft carriers, air traffic control systems, and nuclear power plants, and are characterized by their preoccupation with failure, reluctance to simplify interpretations, sensitivity to operations, commitment to resilience, and deference to expertise.12, 13 Preoccupation with failure is manifested by an organization's efforts to avoid complacency and persist in the search for additional risks. Reluctance to simplify interpretations is exemplified by an interest in pursuing a deep understanding of the issues that arise. Sensitivity to operations is the close attention paid to input from front‐line personnel and processes. Commitment to resilience relates to an organization's ability to contain errors once they occur and mitigate harm. Deference to expertise describes the practice of having authority migrate to the people with the most expertise, regardless of rank. Collectively, these qualities produce a state of mindfulness, allowing teams to anticipate and become aware of unexpected events, yet also quickly contain and learn from them. Recent publications have highlighted the need for hospitals to learn from HROs and the teams within them.14, 15
Recognizing the importance of teamwork in hospitals, senior leadership from the American College of Physician Executives (ACPE), the American Hospital Association (AHA), the American Organization of Nurse Executives (AONE), and the Society of Hospital Medicine (SHM) established the High Performance Teams and the Hospital of the Future project. This collaborative learning effort aims to redesign care delivery to provide optimal value to hospitalized patients. As an initial step, the High Performance Teams and the Hospital of the Future project team completed a literature review related to teamwork in hospitals. The purpose of this report is to summarize the current understanding of teamwork, describe interventions designed to improve teamwork, and make practical recommendations for hospitals to assess and improve teamwork‐related performance. We approach teamwork from the hospitalized patient's perspective, and restrict our discussion to interactions occurring among healthcare professionals within the hospital. We recognize the importance of teamwork at all points in the continuum of patient care. Highly functional inpatient teams should be integrated into an overall system of coordinated and collaborative care.
TEAMWORK: DEFINITION AND CONSTRUCTS
Physicians, nurses, and other healthcare professionals spend a great deal of their time on communication and coordination of care activities.1618 In spite of this and the patient safety concerns previously noted, interpersonal communication skills and teamwork have been historically underemphasized in professional training.1922 A team is defined as 2 or more individuals with specified roles interacting adaptively, interdependently, and dynamically toward a shared and common goal.23 Elements of effective teamwork have been identified through research conducted in aviation, the military, and more recently, healthcare. Salas and colleagues have synthesized this research into 5 core components: team leadership, mutual performance monitoring, backup behavior, adaptability, and team orientation (see Table 1).23 Additionally, 3 supporting and coordinating mechanisms are essential for effective teamwork: shared mental model, closed‐loop communication, and mutual trust (see Table 1).23 High‐performing teams use these elements to develop a culture for speaking up, and situational awareness among team members. Situational awareness refers to a person's perception and understanding of their dynamic environment, and human errors often result from a lack of such awareness.24 These teamwork constructs provide the foundational basis for understanding how hospitals can identify teamwork challenges, assess team performance, and design effective interventions.
| Teamwork | Definition | Behavioral Examples |
|---|---|---|
| ||
| Component | ||
| Team leadership | The leader directs and coordinates team members activities | Facilitate team problem solving; |
| Provide performance expectations; | ||
| Clarify team member roles; | ||
| Assist in conflict resolution | ||
| Mutual performance monitoring | Team members are able to monitor one another's performance | Identify mistakes and lapses in other team member actions; |
| Provide feedback to fellow team members to facilitate self‐correction | ||
| Backup behavior | Team members anticipate and respond to one another's needs | Recognize workload distribution problem; |
| Shift work responsibilities to underutilized members | ||
| Adaptability | The team adjusts strategies based on new information | Identify cues that change has occurred and develop plan to deal with changes; |
| Remain vigilant to change in internal and external environment | ||
| Team orientation | Team members prioritize team goals above individual goals | Take into account alternate solutions by teammates; |
| Increased task involvement, information sharing, and participatory goal setting | ||
| Coordinating mechanism | ||
| Shared mental model | An organizing knowledge of the task of the team and how members will interact to achieve their goal | Anticipate and predict each other's needs; |
| Identify changes in team, task, or teammates | ||
| Closed‐loop communication | Acknowledgement and confirmation of information received | Follow up with team members to ensure message received; |
| Acknowledge that message was received; | ||
| Clarify information received | ||
| Mutual trust | Shared belief that team members will perform their roles | Share information; |
| Willingly admit mistakes and accept feedback | ||
CHALLENGES TO EFFECTIVE TEAMWORK
Several important and unique barriers to teamwork exist in hospitals. Teams are large and formed in an ad hoc fashion. On a given day, a patient's hospital team might include a hospitalist, a nurse, a case manager, a pharmacist, and 1 or more consulting physicians and therapists. Team members in each respective discipline care for multiple patients at the same time, yet few hospitals align team membership (ie, patient assignment). Therefore, a nurse caring for 4 patients may interact with 4 different hospitalists. Similarly, a hospitalist caring for 14 patients may interact with multiple nurses in a given day. Team membership is ever changing because hospital professionals work in shifts and rotations. Finally, team members are seldom in the same place at the same time because physicians often care for patients on multiple units and floors, while nurses and other team members are often unit‐based. Salas and others have noted that team size, instability, and geographic dispersion of membership serve as important barriers to improving teamwork.25, 26 As a result of these barriers, nurses and physicians do not communicate consistently, and often disagree on the daily plan of care for their patients.27, 28 When communication does occur, clinicians may overestimate how well their messages are understood by other team members, reflecting a phenomenon well known in communication psychology related to egocentric thought processes.29, 30
The traditionally steep hierarchy within medicine may also serve as a barrier to teamwork. Studies in intensive care units (ICUs), operating rooms, and general medical units reveal widely discrepant views on the quality of collaboration and communication between healthcare professionals.3133 Although physicians generally give high ratings to the quality of collaboration with nurses, nurses consistently rate the quality of collaboration with physicians as poor. Similarly, specialist physicians rate collaboration with hospitalists higher than hospitalists rate collaboration with specialists.33 Effective teams in other high‐risk industries, like aviation, strive to flatten hierarchy so that team members feel comfortable raising concerns and engaging in open and respectful communications.34
The effect of technology on communication practices and teamwork is complex and incompletely understood. The implementation of electronic heath records and computerized provider order entry systems fundamentally changes work‐flow, and may result in less synchronization and feedback during nurse‐physician collaboration.35 Similarly, the expanded use of text messages delivered via alphanumeric paging or mobile phone results in a transition toward asynchronous modes of communication. These asynchronous modes allow healthcare professionals to review and respond to messages at their convenience, and may reduce unnecessary interruptions. Research shows that these systems are popular among clinicians.3638 However, receipt and understanding of the intended message may not be confirmed with the use of asynchronous modes of communication. Moreover, important face‐to‐face communication elements (tone of voice, expression, gesture, eye contract)39, 40 are lacking. One promising approach is a system which sends low‐priority messages to a Web‐based task list for periodic review, while allowing higher priority messages to pass through to an alphanumeric pager and interrupt the intended recipient.41 Another common frustration in hospitals, despite advancing technology, is difficulty identifying the correct physician(s) and nurse(s) caring for a particular patient at a given point in time.33 Wong and colleagues found that 14% of pages in their hospital were initially sent to the wrong physician.42
ASSESSMENT OF TEAMWORK
One of the challenges in improving teamwork is the difficulty in measuring it. Teamwork assessment entails measuring the performance of teams composed of multiple individuals. Methods of teamwork assessment can be broadly categorized as self assessment, peer assessment, direct observation, survey of team climate or culture, and measurement of the outcome of effective teamwork. While self‐report tools are easy to administer and can capture affective components influencing team performance, they may not reflect actual skills on the part of individuals or teams. Peer assessment includes the use of 360‐degree evaluations or multisource feedback, and provides an evaluation of individual performance.4347
Direct observation provides a more accurate assessment of team‐related behaviors using trained observers. Observers use checklists and/or behaviorally anchored rating scales (BARS) to evaluate individual and team performance. A number of BARS have been developed and validated for the evaluation of team performance.4852 Of note, direct observation may be difficult in settings in which team members are not in the same place at the same time. An alternative method, which may be better suited for general medical units, is the use of survey instruments designed to assess attitudes and teamwork climate.5355 Importantly, higher survey ratings of collaboration and teamwork have been associated with better patient outcomes in observational studies.5658
The ultimate goal of teamwork efforts is to improve patient outcomes. Because patient outcomes are affected by a number of factors and because hospitals frequently engage in multiple, simultaneous efforts to improve care, it is often difficult to clearly link improved outcomes with teamwork interventions. Continued efforts to rigorously evaluate teamwork interventions should remain a priority, particularly as the cost of these interventions must be weighed against other interventions and investments.
EXAMPLES OF SUCCESSFUL INTERVENTIONS
A number of interventions have been used to improve teamwork in hospitals (see Table 2).
| Intervention | Advantages | Disadvantages |
|---|---|---|
| Localization of physicians | Increases frequency of nurse‐physician communication; provides foundation for additional interventions | Insufficient in creating a shared mental model; does not specifically enhance communication skills |
| Daily goals‐of‐care forms and checklists | Provides structure to interdisciplinary discussions and ensures input from all team members | May be completed in a perfunctory manner and may not be updated as plans of care evolve |
| Teamwork training | Emphasizes improved communication behaviors relevant across a range of team member interactions | Requires time and deliberate practice of new skills; effect may be attenuated if members are dispersed. |
| Interdisciplinary rounds | Provides a forum for regular interdisciplinary communication | Requires leadership to organize discussion and does not address need for updates as plans of care evolve |
Geographic Localization of Physicians
As mentioned earlier, physicians in large hospitals may care for patients on multiple units or floors. Designating certain physicians to care for patients admitted to specific units may improve efficiency and communication among healthcare professionals. One study recently reported on the effect of localization of hospital physicians to specific patient care units. Localization resulted in an increase in the rate of nurse‐physician communication, but did not improve providers' shared understanding of the plan of care.56 Notably, localizing physicians may improve the feasibility of additional interventions, like teamwork training and interdisciplinary rounds.
Daily Goals of Care and Surgery Safety Checklists
In ICU and operating room settings, physicians and nurses work in proximity, allowing interdisciplinary discussions to occur at the bedside. The finding that professionals in ICUs and operating rooms have widely discrepant views on the quality of collaboration31, 32 indicates that proximity, alone, is not sufficient for effective communication. Pronovost et al. used a daily goals form for bedside ICU rounds in an effort to standardize communication about the daily plan of care.57 The form defined essential goals of care for patients, and its use resulted in a significant improvement in the team's understanding of the daily goals. Narasimhan et al. performed a similar study using a daily goals worksheet during ICU rounds,58 and also found a significant improvement in physicians' and nurses' ratings of their understanding of the goals of care. The forms used in these studies provided structure to the interdisciplinary conversations during rounds to create a shared understanding of patients' plans of care.
Haynes and colleagues recently reported on the use of a surgical safety checklist in a large, multicenter pre‐post study.59 The checklist consisted of verbal confirmation of the completion of basic steps essential to safe care in the operating room, and provided structure to communication among surgical team members to ensure a shared understanding of the operative plan. The intervention resulted in a significant reduction in inpatient complications and mortality.
Team Training
Formalized team training, based on crew resource management, has been studied as a potential method to improve teamwork in a variety of medical settings.6062 Training emphasizes the core components of successful teamwork and essential coordinating mechanisms previously mentioned.23 Team training appears to positively influence culture, as assessed by teamwork and patient safety climate survey instruments.60 Based on these findings and extensive research demonstrating the success of teamwork training in aviation,63 the Agency for Healthcare Research and Quality (AHRQ) and the Department of Defense (DoD) have partnered in offering the Team Strategies and Tools to Enhance Performance and Patient Safety (TeamSTEPPS) program, designed to improve teamwork skills for healthcare professionals.64, 65
Only a handful of studies have evaluated the effectiveness of teamwork training programs on patient outcomes, and the results are mixed.66 Morey et al. found a reduction in the rate of observed errors as a result of teamwork training in emergency departments, but observers in the study were not blinded with regard to whether teams had undergone training.61 A research group in the United Kingdom evaluated the benefit of simulation‐based team training on outcomes in an obstetrical setting.67, 68 Training included management of specific complications, including shoulder dystocia and uterine cord prolapse. Using retrospective chart review, the investigators found a significant reduction in the proportion of babies born with an obstetric brachial palsy injury and a reduction in the time from diagnosis of uterine cord prolapse to infant delivery. Nielsen and colleagues also evaluated the use of teamwork training in an obstetric setting.62 In a cluster randomized controlled trial, the investigators found no reduction in the rate of adverse outcomes. Differences in the duration of teamwork training and the degree of emphasis on deliberate practice of new skills (eg, with the use of simulation‐based training) likely explains the lack of consistent results.
Very little research has evaluated teamwork training in the general medical environment.69, 70 Sehgal and colleagues recently published an evaluation of the effect of teamwork training delivered to internal medicine residents, hospitalists, nurses, pharmacists, case managers, and social workers on medical services in 3 Northern California hospitals.69 The 4‐hour training sessions covered topical areas of safety culture, teamwork, and communication through didactics, videos, facilitated discussions, and small group role plays to practice new skills and behaviors. The intervention was rated highly among participants,69 and the training along with subsequent follow‐up interventions resulted in improved patient perceptions of teamwork and communication but had no impact on key patient outcomes.71
Interdisciplinary Rounds
Interdisciplinary rounds (IDR) have been used for many years as a means to assemble team members in a single location,7275 and the use of IDR has been associated with lower mortality among ICU patients.76 Interdisciplinary rounds may be particularly useful for clinical settings in which team members are traditionally dispersed in time and place, such as medical‐surgical units. Recent studies have evaluated the effect of structured inter‐disciplinary rounds (SIDR),77, 78 which combine a structured format for communication, similar to a daily goals‐of‐care form, with a forum for daily interdisciplinary meetings. Though no effect was seen on length of stay or cost, SIDR resulted in significantly higher ratings of the quality of collaboration and teamwork climate, and a reduction in the rate of AEs.79 Importantly, the majority of clinicians in the studies agreed that SIDR improved the efficiency of their work day, and expressed a desire that SIDR continue indefinitely. Many investigators have emphasized the importance of leadership during IDR, often by a medical director, nurse manager, or both.74, 77, 78
Summary of Interventions to Improve Teamwork
Localization of physicians increases the frequency of nurse‐physician communication, but is insufficient in creating a shared understanding of patients' plans of care. Providing structure for the discussion among team members (eg, daily goals of care forms and checklists) ensures that critical elements of the plan of care are communicated. Teamwork training is based upon a strong foundation of research both inside and outside of healthcare, and has demonstrated improved knowledge of teamwork principles, attitudes about the importance of teamwork, and overall safety climate. Creating a forum for team members to assemble and discuss their patients (eg, IDR) can overcome some of the unique barriers to collaboration in settings where members are dispersed in time and space. Leaders wishing to improve interdisciplinary teamwork should consider implementing a combination of complementary interventions. For example, localization may increase the frequency of team member interactions, the quality of which may be enhanced with teamwork training and reinforced with the use of structured communication tools and IDR. Future research should evaluate the effect of these combined interventions.
CONCLUSIONS
In summary, teamwork is critically important to provide safe and effective care. Important and unique barriers to teamwork exist in hospitals. We recommend the use of survey instruments, such as those mentioned earlier, as the most feasible method to assess teamwork in the general medical setting. Because each intervention addresses only a portion of the barriers to optimal teamwork, we encourage leaders to use a multifaceted approach. We recommend the implementation of a combination of interventions with adaptations to fit unique clinical settings and local culture.
Acknowledgements
This manuscript was prepared as part of the High Performance Teams and the Hospital of the Future project, a collaborative effort including senior leadership from the American College of Physician Executives, the American Hospital Association, the American Organization of Nurse Executives, and the Society of Hospital Medicine. The authors thank Taylor Marsh for her administrative support and help in coordinating project meetings.
Teamwork is important in providing high‐quality hospital care. Despite tremendous efforts in the 10 years since publication of the Institute of Medicine's To Err is Human report,1 hospitalized patients remain at risk for adverse events (AEs).2 Although many AEs are not preventable, a large portion of those which are identified as preventable can be attributed to communication and teamwork failures.35 A Joint Commission study indicated that communication failures were the root cause for two‐thirds of the 3548 sentinel events reported from 1995 to 2005.6 Another study, involving interviews of resident physicians about recent medical mishaps, found that communication failures contributed to 91% of the AEs they reported.5
Teamwork also plays an important role in other aspects of hospital care delivery. Patients' ratings of nurse‐physician coordination correlate with their overall perception of the quality of care received.7, 8 A study of Veterans Health Administration (VHA) hospitals found that teamwork culture was significantly and positively associated with overall patient satisfaction.9 Another VHA study found that hospitals with higher teamwork culture ratings had lower nurse resignations rates.10 Furthermore, poor teamwork within hospitals may have an adverse effect on financial performance, as a result of inefficiencies in physician and nurse workflow.11
Some organizations are capable of operating in complex, hazardous environments while maintaining exceptional performance over long periods of time. These high reliability organizations (HRO) include aircraft carriers, air traffic control systems, and nuclear power plants, and are characterized by their preoccupation with failure, reluctance to simplify interpretations, sensitivity to operations, commitment to resilience, and deference to expertise.12, 13 Preoccupation with failure is manifested by an organization's efforts to avoid complacency and persist in the search for additional risks. Reluctance to simplify interpretations is exemplified by an interest in pursuing a deep understanding of the issues that arise. Sensitivity to operations is the close attention paid to input from front‐line personnel and processes. Commitment to resilience relates to an organization's ability to contain errors once they occur and mitigate harm. Deference to expertise describes the practice of having authority migrate to the people with the most expertise, regardless of rank. Collectively, these qualities produce a state of mindfulness, allowing teams to anticipate and become aware of unexpected events, yet also quickly contain and learn from them. Recent publications have highlighted the need for hospitals to learn from HROs and the teams within them.14, 15
Recognizing the importance of teamwork in hospitals, senior leadership from the American College of Physician Executives (ACPE), the American Hospital Association (AHA), the American Organization of Nurse Executives (AONE), and the Society of Hospital Medicine (SHM) established the High Performance Teams and the Hospital of the Future project. This collaborative learning effort aims to redesign care delivery to provide optimal value to hospitalized patients. As an initial step, the High Performance Teams and the Hospital of the Future project team completed a literature review related to teamwork in hospitals. The purpose of this report is to summarize the current understanding of teamwork, describe interventions designed to improve teamwork, and make practical recommendations for hospitals to assess and improve teamwork‐related performance. We approach teamwork from the hospitalized patient's perspective, and restrict our discussion to interactions occurring among healthcare professionals within the hospital. We recognize the importance of teamwork at all points in the continuum of patient care. Highly functional inpatient teams should be integrated into an overall system of coordinated and collaborative care.
TEAMWORK: DEFINITION AND CONSTRUCTS
Physicians, nurses, and other healthcare professionals spend a great deal of their time on communication and coordination of care activities.1618 In spite of this and the patient safety concerns previously noted, interpersonal communication skills and teamwork have been historically underemphasized in professional training.1922 A team is defined as 2 or more individuals with specified roles interacting adaptively, interdependently, and dynamically toward a shared and common goal.23 Elements of effective teamwork have been identified through research conducted in aviation, the military, and more recently, healthcare. Salas and colleagues have synthesized this research into 5 core components: team leadership, mutual performance monitoring, backup behavior, adaptability, and team orientation (see Table 1).23 Additionally, 3 supporting and coordinating mechanisms are essential for effective teamwork: shared mental model, closed‐loop communication, and mutual trust (see Table 1).23 High‐performing teams use these elements to develop a culture for speaking up, and situational awareness among team members. Situational awareness refers to a person's perception and understanding of their dynamic environment, and human errors often result from a lack of such awareness.24 These teamwork constructs provide the foundational basis for understanding how hospitals can identify teamwork challenges, assess team performance, and design effective interventions.
| Teamwork | Definition | Behavioral Examples |
|---|---|---|
| ||
| Component | ||
| Team leadership | The leader directs and coordinates team members activities | Facilitate team problem solving; |
| Provide performance expectations; | ||
| Clarify team member roles; | ||
| Assist in conflict resolution | ||
| Mutual performance monitoring | Team members are able to monitor one another's performance | Identify mistakes and lapses in other team member actions; |
| Provide feedback to fellow team members to facilitate self‐correction | ||
| Backup behavior | Team members anticipate and respond to one another's needs | Recognize workload distribution problem; |
| Shift work responsibilities to underutilized members | ||
| Adaptability | The team adjusts strategies based on new information | Identify cues that change has occurred and develop plan to deal with changes; |
| Remain vigilant to change in internal and external environment | ||
| Team orientation | Team members prioritize team goals above individual goals | Take into account alternate solutions by teammates; |
| Increased task involvement, information sharing, and participatory goal setting | ||
| Coordinating mechanism | ||
| Shared mental model | An organizing knowledge of the task of the team and how members will interact to achieve their goal | Anticipate and predict each other's needs; |
| Identify changes in team, task, or teammates | ||
| Closed‐loop communication | Acknowledgement and confirmation of information received | Follow up with team members to ensure message received; |
| Acknowledge that message was received; | ||
| Clarify information received | ||
| Mutual trust | Shared belief that team members will perform their roles | Share information; |
| Willingly admit mistakes and accept feedback | ||
CHALLENGES TO EFFECTIVE TEAMWORK
Several important and unique barriers to teamwork exist in hospitals. Teams are large and formed in an ad hoc fashion. On a given day, a patient's hospital team might include a hospitalist, a nurse, a case manager, a pharmacist, and 1 or more consulting physicians and therapists. Team members in each respective discipline care for multiple patients at the same time, yet few hospitals align team membership (ie, patient assignment). Therefore, a nurse caring for 4 patients may interact with 4 different hospitalists. Similarly, a hospitalist caring for 14 patients may interact with multiple nurses in a given day. Team membership is ever changing because hospital professionals work in shifts and rotations. Finally, team members are seldom in the same place at the same time because physicians often care for patients on multiple units and floors, while nurses and other team members are often unit‐based. Salas and others have noted that team size, instability, and geographic dispersion of membership serve as important barriers to improving teamwork.25, 26 As a result of these barriers, nurses and physicians do not communicate consistently, and often disagree on the daily plan of care for their patients.27, 28 When communication does occur, clinicians may overestimate how well their messages are understood by other team members, reflecting a phenomenon well known in communication psychology related to egocentric thought processes.29, 30
The traditionally steep hierarchy within medicine may also serve as a barrier to teamwork. Studies in intensive care units (ICUs), operating rooms, and general medical units reveal widely discrepant views on the quality of collaboration and communication between healthcare professionals.3133 Although physicians generally give high ratings to the quality of collaboration with nurses, nurses consistently rate the quality of collaboration with physicians as poor. Similarly, specialist physicians rate collaboration with hospitalists higher than hospitalists rate collaboration with specialists.33 Effective teams in other high‐risk industries, like aviation, strive to flatten hierarchy so that team members feel comfortable raising concerns and engaging in open and respectful communications.34
The effect of technology on communication practices and teamwork is complex and incompletely understood. The implementation of electronic heath records and computerized provider order entry systems fundamentally changes work‐flow, and may result in less synchronization and feedback during nurse‐physician collaboration.35 Similarly, the expanded use of text messages delivered via alphanumeric paging or mobile phone results in a transition toward asynchronous modes of communication. These asynchronous modes allow healthcare professionals to review and respond to messages at their convenience, and may reduce unnecessary interruptions. Research shows that these systems are popular among clinicians.3638 However, receipt and understanding of the intended message may not be confirmed with the use of asynchronous modes of communication. Moreover, important face‐to‐face communication elements (tone of voice, expression, gesture, eye contract)39, 40 are lacking. One promising approach is a system which sends low‐priority messages to a Web‐based task list for periodic review, while allowing higher priority messages to pass through to an alphanumeric pager and interrupt the intended recipient.41 Another common frustration in hospitals, despite advancing technology, is difficulty identifying the correct physician(s) and nurse(s) caring for a particular patient at a given point in time.33 Wong and colleagues found that 14% of pages in their hospital were initially sent to the wrong physician.42
ASSESSMENT OF TEAMWORK
One of the challenges in improving teamwork is the difficulty in measuring it. Teamwork assessment entails measuring the performance of teams composed of multiple individuals. Methods of teamwork assessment can be broadly categorized as self assessment, peer assessment, direct observation, survey of team climate or culture, and measurement of the outcome of effective teamwork. While self‐report tools are easy to administer and can capture affective components influencing team performance, they may not reflect actual skills on the part of individuals or teams. Peer assessment includes the use of 360‐degree evaluations or multisource feedback, and provides an evaluation of individual performance.4347
Direct observation provides a more accurate assessment of team‐related behaviors using trained observers. Observers use checklists and/or behaviorally anchored rating scales (BARS) to evaluate individual and team performance. A number of BARS have been developed and validated for the evaluation of team performance.4852 Of note, direct observation may be difficult in settings in which team members are not in the same place at the same time. An alternative method, which may be better suited for general medical units, is the use of survey instruments designed to assess attitudes and teamwork climate.5355 Importantly, higher survey ratings of collaboration and teamwork have been associated with better patient outcomes in observational studies.5658
The ultimate goal of teamwork efforts is to improve patient outcomes. Because patient outcomes are affected by a number of factors and because hospitals frequently engage in multiple, simultaneous efforts to improve care, it is often difficult to clearly link improved outcomes with teamwork interventions. Continued efforts to rigorously evaluate teamwork interventions should remain a priority, particularly as the cost of these interventions must be weighed against other interventions and investments.
EXAMPLES OF SUCCESSFUL INTERVENTIONS
A number of interventions have been used to improve teamwork in hospitals (see Table 2).
| Intervention | Advantages | Disadvantages |
|---|---|---|
| Localization of physicians | Increases frequency of nurse‐physician communication; provides foundation for additional interventions | Insufficient in creating a shared mental model; does not specifically enhance communication skills |
| Daily goals‐of‐care forms and checklists | Provides structure to interdisciplinary discussions and ensures input from all team members | May be completed in a perfunctory manner and may not be updated as plans of care evolve |
| Teamwork training | Emphasizes improved communication behaviors relevant across a range of team member interactions | Requires time and deliberate practice of new skills; effect may be attenuated if members are dispersed. |
| Interdisciplinary rounds | Provides a forum for regular interdisciplinary communication | Requires leadership to organize discussion and does not address need for updates as plans of care evolve |
Geographic Localization of Physicians
As mentioned earlier, physicians in large hospitals may care for patients on multiple units or floors. Designating certain physicians to care for patients admitted to specific units may improve efficiency and communication among healthcare professionals. One study recently reported on the effect of localization of hospital physicians to specific patient care units. Localization resulted in an increase in the rate of nurse‐physician communication, but did not improve providers' shared understanding of the plan of care.56 Notably, localizing physicians may improve the feasibility of additional interventions, like teamwork training and interdisciplinary rounds.
Daily Goals of Care and Surgery Safety Checklists
In ICU and operating room settings, physicians and nurses work in proximity, allowing interdisciplinary discussions to occur at the bedside. The finding that professionals in ICUs and operating rooms have widely discrepant views on the quality of collaboration31, 32 indicates that proximity, alone, is not sufficient for effective communication. Pronovost et al. used a daily goals form for bedside ICU rounds in an effort to standardize communication about the daily plan of care.57 The form defined essential goals of care for patients, and its use resulted in a significant improvement in the team's understanding of the daily goals. Narasimhan et al. performed a similar study using a daily goals worksheet during ICU rounds,58 and also found a significant improvement in physicians' and nurses' ratings of their understanding of the goals of care. The forms used in these studies provided structure to the interdisciplinary conversations during rounds to create a shared understanding of patients' plans of care.
Haynes and colleagues recently reported on the use of a surgical safety checklist in a large, multicenter pre‐post study.59 The checklist consisted of verbal confirmation of the completion of basic steps essential to safe care in the operating room, and provided structure to communication among surgical team members to ensure a shared understanding of the operative plan. The intervention resulted in a significant reduction in inpatient complications and mortality.
Team Training
Formalized team training, based on crew resource management, has been studied as a potential method to improve teamwork in a variety of medical settings.6062 Training emphasizes the core components of successful teamwork and essential coordinating mechanisms previously mentioned.23 Team training appears to positively influence culture, as assessed by teamwork and patient safety climate survey instruments.60 Based on these findings and extensive research demonstrating the success of teamwork training in aviation,63 the Agency for Healthcare Research and Quality (AHRQ) and the Department of Defense (DoD) have partnered in offering the Team Strategies and Tools to Enhance Performance and Patient Safety (TeamSTEPPS) program, designed to improve teamwork skills for healthcare professionals.64, 65
Only a handful of studies have evaluated the effectiveness of teamwork training programs on patient outcomes, and the results are mixed.66 Morey et al. found a reduction in the rate of observed errors as a result of teamwork training in emergency departments, but observers in the study were not blinded with regard to whether teams had undergone training.61 A research group in the United Kingdom evaluated the benefit of simulation‐based team training on outcomes in an obstetrical setting.67, 68 Training included management of specific complications, including shoulder dystocia and uterine cord prolapse. Using retrospective chart review, the investigators found a significant reduction in the proportion of babies born with an obstetric brachial palsy injury and a reduction in the time from diagnosis of uterine cord prolapse to infant delivery. Nielsen and colleagues also evaluated the use of teamwork training in an obstetric setting.62 In a cluster randomized controlled trial, the investigators found no reduction in the rate of adverse outcomes. Differences in the duration of teamwork training and the degree of emphasis on deliberate practice of new skills (eg, with the use of simulation‐based training) likely explains the lack of consistent results.
Very little research has evaluated teamwork training in the general medical environment.69, 70 Sehgal and colleagues recently published an evaluation of the effect of teamwork training delivered to internal medicine residents, hospitalists, nurses, pharmacists, case managers, and social workers on medical services in 3 Northern California hospitals.69 The 4‐hour training sessions covered topical areas of safety culture, teamwork, and communication through didactics, videos, facilitated discussions, and small group role plays to practice new skills and behaviors. The intervention was rated highly among participants,69 and the training along with subsequent follow‐up interventions resulted in improved patient perceptions of teamwork and communication but had no impact on key patient outcomes.71
Interdisciplinary Rounds
Interdisciplinary rounds (IDR) have been used for many years as a means to assemble team members in a single location,7275 and the use of IDR has been associated with lower mortality among ICU patients.76 Interdisciplinary rounds may be particularly useful for clinical settings in which team members are traditionally dispersed in time and place, such as medical‐surgical units. Recent studies have evaluated the effect of structured inter‐disciplinary rounds (SIDR),77, 78 which combine a structured format for communication, similar to a daily goals‐of‐care form, with a forum for daily interdisciplinary meetings. Though no effect was seen on length of stay or cost, SIDR resulted in significantly higher ratings of the quality of collaboration and teamwork climate, and a reduction in the rate of AEs.79 Importantly, the majority of clinicians in the studies agreed that SIDR improved the efficiency of their work day, and expressed a desire that SIDR continue indefinitely. Many investigators have emphasized the importance of leadership during IDR, often by a medical director, nurse manager, or both.74, 77, 78
Summary of Interventions to Improve Teamwork
Localization of physicians increases the frequency of nurse‐physician communication, but is insufficient in creating a shared understanding of patients' plans of care. Providing structure for the discussion among team members (eg, daily goals of care forms and checklists) ensures that critical elements of the plan of care are communicated. Teamwork training is based upon a strong foundation of research both inside and outside of healthcare, and has demonstrated improved knowledge of teamwork principles, attitudes about the importance of teamwork, and overall safety climate. Creating a forum for team members to assemble and discuss their patients (eg, IDR) can overcome some of the unique barriers to collaboration in settings where members are dispersed in time and space. Leaders wishing to improve interdisciplinary teamwork should consider implementing a combination of complementary interventions. For example, localization may increase the frequency of team member interactions, the quality of which may be enhanced with teamwork training and reinforced with the use of structured communication tools and IDR. Future research should evaluate the effect of these combined interventions.
CONCLUSIONS
In summary, teamwork is critically important to provide safe and effective care. Important and unique barriers to teamwork exist in hospitals. We recommend the use of survey instruments, such as those mentioned earlier, as the most feasible method to assess teamwork in the general medical setting. Because each intervention addresses only a portion of the barriers to optimal teamwork, we encourage leaders to use a multifaceted approach. We recommend the implementation of a combination of interventions with adaptations to fit unique clinical settings and local culture.
Acknowledgements
This manuscript was prepared as part of the High Performance Teams and the Hospital of the Future project, a collaborative effort including senior leadership from the American College of Physician Executives, the American Hospital Association, the American Organization of Nurse Executives, and the Society of Hospital Medicine. The authors thank Taylor Marsh for her administrative support and help in coordinating project meetings.
- To Err Is Human: Building a Safer Health System.Washington, DC:Institute of Medicine;1999.
- ,,,,,.Temporal trends in rates of patient harm resulting from medical care.N Engl J Med.2010;363(22):2124–2134.
- ,,.Exploring the causes of adverse events in NHS hospital practice.J R Soc Med.2001;94(7):322–330.
- ,,,,,.The Quality in Australian Health Care Study.Med J Aust.1995;163(9):458–471.
- ,,.Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79(2):186–194.
- Improving America's Hospitals: The Joint Commission's Annual Report on Quality and Safety 2007. Available at: http://www.jointcommissionreport.org. Accessed November2007.
- ,,.Patient perceptions of coordinated care: the importance of organized communication in hospitals.J Healthc Qual.1999;21(5):18–23.
- ,,.Am I safe here? Improving patients' perceptions of safety in hospitals.J Nurs Care Qual.2006;21(1):30–40.
- ,,.Teamwork culture and patient satisfaction in hospitals.Med Care.2004;42(5):492–498.
- ,,.The influence of teamwork culture on physician and nurse resignation rates in hospitals.Health Serv Manage Res.2008;21(1):23–31.
- ,,.Quantifying the economic impact of communication inefficiencies in U.S. hospitals.J Healthc Manag.2010;55(4):265–282.
- ,.Managing the Unexpected: Assuring High Performance in an Age of Complexity.San Francisco, CA:Jossey‐Bass;2001.
- .Some characteristics of high reliability organizations.Organization Science.1990;1(2):160–177.
- ,,.Teamwork as an essential component of high‐reliability organizations.Health Serv Res.2006;41(4 pt 2):1576–1598.
- ,,,.Promoting health care safety through training high reliability teams.Qual Saf Health Care.2005;14(4):303–309.
- ,,,,,.Analyzing the time and value of housestaff inpatient work.J Gen Intern Med.1998;13(8):534–540.
- ,,, et al.Quantifying nursing workflow in medication administration.J Nurs Adm.2008;38(1):19–26.
- ,,.How hospitalists spend their time: insights on efficiency and safety.J Hosp Med.2006;1(2):88–93.
- ,,,,.Redesigning residency education in internal medicine: a position paper from the Association of Program Directors in Internal Medicine.Ann Intern Med.2006;144(12):920–926.
- ,,,.Hospitalists' perceptions of their residency training needs: results of a national survey.Am J Med.2001;111(3):247–254.
- ,,.Redesigning training for internal medicine.Ann Intern Med.2006;144(12):927–932.
- ,,,,.The role of teamwork in the professional education of physicians: current status and assessment recommendations.Jt Comm J Qual Patient Saf.2005;31(4):185–202.
- ,,.Is there a “big five” in teamwork?Small Group Research.2005;36:555–599.
- ,,.Objective measures of situation awareness in a simulated medical environment.Qual Saf Health Care.2004;13(suppl 1):i65–i71.
- ,.What do we know about health care team effectiveness? A review of the literature.Med Care Res Rev.2006;63(3):263–300.
- ,,, et al.Does team training improve team performance? A meta‐analysis.Hum Factors.2008;50(6):903–933.
- ,,,,,.Can we talk? Priorities for patient care differed among health care providers. AHRQ Publication No. 05–0021‐1.Rockville, MD:Agency for Healthcare Research and Quality;2005.
- ,,, et al.Patterns of nurse–physicians communication and agreement on the plan of care.Qual Saf Health Care.2010;19:195–199.
- ,,,,.Interns overestimate the effectiveness of their hand‐off communication.Pediatrics.2010;125(3):491–496.
- ,.Speakers' overestimation of their effectiveness.Psychol Sci.2002;13(3):207–212.
- ,,, et al.Operating room teamwork among physicians and nurses: teamwork in the eye of the beholder.J Am Coll Surg.2006;202(5):746–752.
- ,,.Discrepant attitudes about teamwork among critical care nurses and physicians.Crit Care Med.2003;31(3):956–959.
- ,,,,,.Teamwork on inpatient medical units: assessing attitudes and barriers.Qual Saf Health Care.2010;19(2):117–121.
- ,,.Error, stress, and teamwork in medicine and aviation: cross sectional surveys.BMJ.2000;320(7237):745–749.
- ,,,,.Impact of a computerized physician order entry system on nurse‐physician collaboration in the medication process.Int J Med Inform.2008;77(11):735–744.
- ,,,,.Alphanumeric paging in an academic hospital setting.Am J Surg.2006;191(4):561–565.
- ,,,.Implementation and evaluation of an alpha‐numeric paging system on a resident inpatient teaching service.J Hosp Med.2009;4(8):E34–E40.
- ,,, et al.The use of smartphones for clinical communication on internal medicine wards.J Hosp Med.2010;5(9):553–559.
- ,.Organizational information requirements, media richness, and structural design.Management Science.1986;32(5):554–571.
- ,.Decoding of inconsistent communications of personality and social psychology.J Pers Soc Psychol.1967;6(1):109–114.
- ,,,,.Beyond paging: building a Web‐based communication tool for nurses and physicians.J Gen Intern Med.2009;24(1):105–110.
- ,,, et al.Frequency and clinical importance of pages sent to the wrong physician.Arch Intern Med.2009;169(11):1072–1073.
- ,,, et al.Evaluation of resident communication skills and professionalism: a matter of perspective?Pediatrics.2006;118(4):1371–1379.
- ,,, et al.Effect of multisource feedback on resident communication skills and professionalism: a randomized controlled trial.Arch Pediatr Adolesc Med.2007;161(1):44–49.
- .Multisource feedback in the assessment of physician competencies.J Contin Educ Health Prof.2003;23(1):4–12.
- ,.Reliability of a 360‐degree evaluation to assess resident competence.Am J Phys Med Rehabil.2007;86(10):845–852.
- ,,,.Pilot study of a 360‐degree assessment instrument for physical medicine 82(5):394–402.
- ,,,,,.Anaesthetists' Non‐Technical Skills (ANTS): evaluation of a behavioural marker system.Br J Anaesth.2003;90(5):580–588.
- ,,,.Using the Communication and Teamwork Skills (CATS) Assessment to measure health care team performance.Jt Comm J Qual Patient Saf.2007;33(9):549–558.
- ,,, et al.The Mayo High Performance Teamwork Scale: reliability and validity for evaluating key crew resource management skills.Simul Healthc.2007;2(1):4–10.
- ,,,,,.Reliability of a revised NOTECHS scale for use in surgical teams.Am J Surg.2008;196(2):184–190.
- ,,,,,.Observational teamwork assessment for surgery: construct validation with expert versus novice raters.Ann Surg.2009;249(6):1047–1051.
- ,,, et al.The Safety Attitudes Questionnaire: psychometric properties, benchmarking data, and emerging research.BMC Health Serv Res.2006;6:44.
- .Development of an instrument to measure collaboration and satisfaction about care decisions.J Adv Nurs.1994;20(1):176–182.
- ,,,,,.Psychometric properties of an attitude scale measuring physician‐nurse collaboration.Eval Health Prof.1999;22(2):208–220.
- ,,, et al.Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care.J Gen Intern Med.2009;24(11):1223–1227.
- ,,,,,.Improving communication in the ICU using daily goals.J Crit Care.2003;18(2):71–75.
- ,,,,.Improving nurse‐physician communication and satisfaction in the intensive care unit with a daily goals worksheet.Am J Crit Care.2006;15(2):217–222.
- ,,, et al.A surgical safety checklist to reduce morbidity and mortality in a global population.N Engl J Med.2009;360(5):491–499.
- ,,, et al.Effect of crew resource management training in a multidisciplinary obstetrical setting.Int J Qual Health Care.2008;20(4):254–263.
- ,,, et al.Error reduction and performance improvement in the emergency department through formal teamwork training: evaluation results of the MedTeams project.Health Serv Res.2002;37(6):1553–1581.
- ,,, et al.Effects of teamwork training on adverse outcomes and process of care in labor and delivery: a randomized controlled trial.Obstet Gynecol.2007;109(1):48–55.
- ,,,,.Medical Teamwork and Patient Safety: The Evidence‐Based Relation.Rockville, MD:Agency for Healthcare Research and Quality;2005.
- Agency for Healthcare Research and Quality. TeamSTEPPS Home. Available at: http://teamstepps.ahrq.gov/index.htm. Accessed January 18,2010.
- ,.TeamSTEPPS: assuring optimal teamwork in clinical settings.Am J Med Qual.2007;22(3):214–217.
- ,,,.Does crew resource management training work? An update, an extension, and some critical needs.Hum Factors.2006;48(2):392–412.
- ,,, et al.Improving neonatal outcome through practical shoulder dystocia training.Obstet Gynecol.2008;112(1):14–20.
- ,,, et al.Retrospective cohort study of diagnosis‐delivery interval with umbilical cord prolapse: the effect of team training.Br J Obstet Gynaecol.2009;116(8):1089–1096.
- ,,, et al.A multidisciplinary teamwork training program: the Triad for Optimal Patient Safety (TOPS) experience.J Gen Intern Med.2008;23(12):2053–2057.
- ,,,,.Teambuilding and leadership training in an internal medicine residency training program.J Gen Intern Med.2004;19(6):692–697.
- ,,, et al. Effects of a multicenter teamwork and communication program on patient outcomes: results from the Triad for Optimal Patient Safety (TOPS) project. In press.
- ,,, et al.The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs.J Nurs Adm.2006;36(2):79–85.
- ,,.A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement.Med Care.1998;36(8 suppl):AS4–A12.
- ,,,,.Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay.J Gen Intern Med.2007;22(8):1073–1079.
- ,,,.Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses.Am J Crit Care.2005;14(1):71–77.
- ,,,,.The effect of multidisciplinary care teams on intensive care unit mortality.Arch Intern Med.2010;170(4):369–376.
- ,,,,,.Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit.J Hosp Med.2011;6(2):88–93.
- ,,,,,.Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit.J Gen Intern Med.2010;25(8):826–832.
- ,,, et al.Structured interdisciplinary rounds in a medical teaching unit: improving patient safety.Arch Intern Med.2011;171(7):678–684.
- To Err Is Human: Building a Safer Health System.Washington, DC:Institute of Medicine;1999.
- ,,,,,.Temporal trends in rates of patient harm resulting from medical care.N Engl J Med.2010;363(22):2124–2134.
- ,,.Exploring the causes of adverse events in NHS hospital practice.J R Soc Med.2001;94(7):322–330.
- ,,,,,.The Quality in Australian Health Care Study.Med J Aust.1995;163(9):458–471.
- ,,.Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79(2):186–194.
- Improving America's Hospitals: The Joint Commission's Annual Report on Quality and Safety 2007. Available at: http://www.jointcommissionreport.org. Accessed November2007.
- ,,.Patient perceptions of coordinated care: the importance of organized communication in hospitals.J Healthc Qual.1999;21(5):18–23.
- ,,.Am I safe here? Improving patients' perceptions of safety in hospitals.J Nurs Care Qual.2006;21(1):30–40.
- ,,.Teamwork culture and patient satisfaction in hospitals.Med Care.2004;42(5):492–498.
- ,,.The influence of teamwork culture on physician and nurse resignation rates in hospitals.Health Serv Manage Res.2008;21(1):23–31.
- ,,.Quantifying the economic impact of communication inefficiencies in U.S. hospitals.J Healthc Manag.2010;55(4):265–282.
- ,.Managing the Unexpected: Assuring High Performance in an Age of Complexity.San Francisco, CA:Jossey‐Bass;2001.
- .Some characteristics of high reliability organizations.Organization Science.1990;1(2):160–177.
- ,,.Teamwork as an essential component of high‐reliability organizations.Health Serv Res.2006;41(4 pt 2):1576–1598.
- ,,,.Promoting health care safety through training high reliability teams.Qual Saf Health Care.2005;14(4):303–309.
- ,,,,,.Analyzing the time and value of housestaff inpatient work.J Gen Intern Med.1998;13(8):534–540.
- ,,, et al.Quantifying nursing workflow in medication administration.J Nurs Adm.2008;38(1):19–26.
- ,,.How hospitalists spend their time: insights on efficiency and safety.J Hosp Med.2006;1(2):88–93.
- ,,,,.Redesigning residency education in internal medicine: a position paper from the Association of Program Directors in Internal Medicine.Ann Intern Med.2006;144(12):920–926.
- ,,,.Hospitalists' perceptions of their residency training needs: results of a national survey.Am J Med.2001;111(3):247–254.
- ,,.Redesigning training for internal medicine.Ann Intern Med.2006;144(12):927–932.
- ,,,,.The role of teamwork in the professional education of physicians: current status and assessment recommendations.Jt Comm J Qual Patient Saf.2005;31(4):185–202.
- ,,.Is there a “big five” in teamwork?Small Group Research.2005;36:555–599.
- ,,.Objective measures of situation awareness in a simulated medical environment.Qual Saf Health Care.2004;13(suppl 1):i65–i71.
- ,.What do we know about health care team effectiveness? A review of the literature.Med Care Res Rev.2006;63(3):263–300.
- ,,, et al.Does team training improve team performance? A meta‐analysis.Hum Factors.2008;50(6):903–933.
- ,,,,,.Can we talk? Priorities for patient care differed among health care providers. AHRQ Publication No. 05–0021‐1.Rockville, MD:Agency for Healthcare Research and Quality;2005.
- ,,, et al.Patterns of nurse–physicians communication and agreement on the plan of care.Qual Saf Health Care.2010;19:195–199.
- ,,,,.Interns overestimate the effectiveness of their hand‐off communication.Pediatrics.2010;125(3):491–496.
- ,.Speakers' overestimation of their effectiveness.Psychol Sci.2002;13(3):207–212.
- ,,, et al.Operating room teamwork among physicians and nurses: teamwork in the eye of the beholder.J Am Coll Surg.2006;202(5):746–752.
- ,,.Discrepant attitudes about teamwork among critical care nurses and physicians.Crit Care Med.2003;31(3):956–959.
- ,,,,,.Teamwork on inpatient medical units: assessing attitudes and barriers.Qual Saf Health Care.2010;19(2):117–121.
- ,,.Error, stress, and teamwork in medicine and aviation: cross sectional surveys.BMJ.2000;320(7237):745–749.
- ,,,,.Impact of a computerized physician order entry system on nurse‐physician collaboration in the medication process.Int J Med Inform.2008;77(11):735–744.
- ,,,,.Alphanumeric paging in an academic hospital setting.Am J Surg.2006;191(4):561–565.
- ,,,.Implementation and evaluation of an alpha‐numeric paging system on a resident inpatient teaching service.J Hosp Med.2009;4(8):E34–E40.
- ,,, et al.The use of smartphones for clinical communication on internal medicine wards.J Hosp Med.2010;5(9):553–559.
- ,.Organizational information requirements, media richness, and structural design.Management Science.1986;32(5):554–571.
- ,.Decoding of inconsistent communications of personality and social psychology.J Pers Soc Psychol.1967;6(1):109–114.
- ,,,,.Beyond paging: building a Web‐based communication tool for nurses and physicians.J Gen Intern Med.2009;24(1):105–110.
- ,,, et al.Frequency and clinical importance of pages sent to the wrong physician.Arch Intern Med.2009;169(11):1072–1073.
- ,,, et al.Evaluation of resident communication skills and professionalism: a matter of perspective?Pediatrics.2006;118(4):1371–1379.
- ,,, et al.Effect of multisource feedback on resident communication skills and professionalism: a randomized controlled trial.Arch Pediatr Adolesc Med.2007;161(1):44–49.
- .Multisource feedback in the assessment of physician competencies.J Contin Educ Health Prof.2003;23(1):4–12.
- ,.Reliability of a 360‐degree evaluation to assess resident competence.Am J Phys Med Rehabil.2007;86(10):845–852.
- ,,,.Pilot study of a 360‐degree assessment instrument for physical medicine 82(5):394–402.
- ,,,,,.Anaesthetists' Non‐Technical Skills (ANTS): evaluation of a behavioural marker system.Br J Anaesth.2003;90(5):580–588.
- ,,,.Using the Communication and Teamwork Skills (CATS) Assessment to measure health care team performance.Jt Comm J Qual Patient Saf.2007;33(9):549–558.
- ,,, et al.The Mayo High Performance Teamwork Scale: reliability and validity for evaluating key crew resource management skills.Simul Healthc.2007;2(1):4–10.
- ,,,,,.Reliability of a revised NOTECHS scale for use in surgical teams.Am J Surg.2008;196(2):184–190.
- ,,,,,.Observational teamwork assessment for surgery: construct validation with expert versus novice raters.Ann Surg.2009;249(6):1047–1051.
- ,,, et al.The Safety Attitudes Questionnaire: psychometric properties, benchmarking data, and emerging research.BMC Health Serv Res.2006;6:44.
- .Development of an instrument to measure collaboration and satisfaction about care decisions.J Adv Nurs.1994;20(1):176–182.
- ,,,,,.Psychometric properties of an attitude scale measuring physician‐nurse collaboration.Eval Health Prof.1999;22(2):208–220.
- ,,, et al.Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care.J Gen Intern Med.2009;24(11):1223–1227.
- ,,,,,.Improving communication in the ICU using daily goals.J Crit Care.2003;18(2):71–75.
- ,,,,.Improving nurse‐physician communication and satisfaction in the intensive care unit with a daily goals worksheet.Am J Crit Care.2006;15(2):217–222.
- ,,, et al.A surgical safety checklist to reduce morbidity and mortality in a global population.N Engl J Med.2009;360(5):491–499.
- ,,, et al.Effect of crew resource management training in a multidisciplinary obstetrical setting.Int J Qual Health Care.2008;20(4):254–263.
- ,,, et al.Error reduction and performance improvement in the emergency department through formal teamwork training: evaluation results of the MedTeams project.Health Serv Res.2002;37(6):1553–1581.
- ,,, et al.Effects of teamwork training on adverse outcomes and process of care in labor and delivery: a randomized controlled trial.Obstet Gynecol.2007;109(1):48–55.
- ,,,,.Medical Teamwork and Patient Safety: The Evidence‐Based Relation.Rockville, MD:Agency for Healthcare Research and Quality;2005.
- Agency for Healthcare Research and Quality. TeamSTEPPS Home. Available at: http://teamstepps.ahrq.gov/index.htm. Accessed January 18,2010.
- ,.TeamSTEPPS: assuring optimal teamwork in clinical settings.Am J Med Qual.2007;22(3):214–217.
- ,,,.Does crew resource management training work? An update, an extension, and some critical needs.Hum Factors.2006;48(2):392–412.
- ,,, et al.Improving neonatal outcome through practical shoulder dystocia training.Obstet Gynecol.2008;112(1):14–20.
- ,,, et al.Retrospective cohort study of diagnosis‐delivery interval with umbilical cord prolapse: the effect of team training.Br J Obstet Gynaecol.2009;116(8):1089–1096.
- ,,, et al.A multidisciplinary teamwork training program: the Triad for Optimal Patient Safety (TOPS) experience.J Gen Intern Med.2008;23(12):2053–2057.
- ,,,,.Teambuilding and leadership training in an internal medicine residency training program.J Gen Intern Med.2004;19(6):692–697.
- ,,, et al. Effects of a multicenter teamwork and communication program on patient outcomes: results from the Triad for Optimal Patient Safety (TOPS) project. In press.
- ,,, et al.The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs.J Nurs Adm.2006;36(2):79–85.
- ,,.A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement.Med Care.1998;36(8 suppl):AS4–A12.
- ,,,,.Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay.J Gen Intern Med.2007;22(8):1073–1079.
- ,,,.Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses.Am J Crit Care.2005;14(1):71–77.
- ,,,,.The effect of multidisciplinary care teams on intensive care unit mortality.Arch Intern Med.2010;170(4):369–376.
- ,,,,,.Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit.J Hosp Med.2011;6(2):88–93.
- ,,,,,.Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit.J Gen Intern Med.2010;25(8):826–832.
- ,,, et al.Structured interdisciplinary rounds in a medical teaching unit: improving patient safety.Arch Intern Med.2011;171(7):678–684.
Night or Weekend Admission and Outcomes
The hospitalist movement and increasingly stringent resident work hour restrictions have led to the utilization of shift work in many hospitals.1 Use of nocturnist and night float systems, while often necessary, results in increased patient hand‐offs. Research suggests that hand‐offs in the inpatient setting can adversely affect patient outcomes as lack of continuity may increase the possibility of medical error.2, 3 In 2001, Bell et al.4 found that mortality was higher among patients admitted on weekends as compared to weekdays. Uneven staffing, lack of supervision, and fragmented care were cited as potential contributing factors.4 Similarly, Peberdy et al.5 in 2008 revealed that patients were less likely to survive a cardiac arrest if it occurred at night or on weekends, again attributed in part to fragmented patient care and understaffing.
The results of these studies raise concerns as to whether increased reliance on shift work and resulting handoffs compromises patient care.6, 7 The aim of this study was to evaluate the potential association between night admission and hospitalization‐relevant outcomes (length of stay [LOS], hospital charges, intensive care unit [ICU] transfer during hospitalization, repeat emergency department [ED] visit within 30 days of discharge, readmission within 30 days of discharge, and poor outcome [transfer to the ICU, cardiac arrest, or death] within the first 24 hours of admission) at an institution that exclusively uses nocturnists (night‐shift based hospitalists) and a resident night float system for patients admitted at night to the general medicine service. A secondary aim was to determine the potential association between weekend admission and hospitalization‐relevant outcomes.
Methods
Study Sample and Selection
We conducted a retrospective medical record review at a large urban academic hospital. Using an administrative hospital data set, we assembled a list of approximately 9000 admissions to the general medicine service from the ED between January 2008 and October 2008. We sampled consecutive admissions from 3 distinct periods beginning in January, April, and July to capture outcomes at various points in the academic year. We attempted to review approximately 10% of all charts equally distributed among the 3 sampling periods (ie, 900 charts total with one‐third from each period) based on time available to the reviewers. We excluded patients not admitted to the general medicine service and patients without complete demographic or outcome information. We also excluded patients not admitted from the ED given that the vast majority of admissions to our hospital during the night (96%) or weekend (93%) are from the ED. Patients admitted to the general medicine service are cared for either by a hospitalist or by a teaching team comprised of 1 attending (about 40% of whom are hospitalists), 1 resident, 1 to 2 interns, and 1 to 3 medical students. From 7 am to 6:59 pm patients are admitted to the care of 1 of the primary daytime admitting teams. From 7 pm to 6:59 am patients are admitted by nocturnists (hospitalist service) or night float residents (teaching service). These patients are handed off to day teams at 7 am. Hospitalist teams change service on a weekly to biweekly basis and resident teams switch on a monthly basis; there is no difference in physician staffing between the weekend and weekdays. The Northwestern University Institutional Review Board approved this study.
Data Acquisition and Medical Records Reviews
We obtained demographic data including gender, age, race and ethnicity, patient insurance, admission day (weekday vs. weekend), admission time (defined as the time that a patient receives a hospital bed, which at our institution is also the time that admitting teams receive report and assume care for the patient), and the International Classification of Disease codes required to determine the Major Diagnostic Category (MDC) and calculate the Charlson Comorbidity Index8, 9 as part of an administrative data set. We divided the admission time into night admission (defined as 7 pm to 6:59 am) and day admission (defined as 7:00 am to 6:59 pm). We created a chart abstraction tool to allow manual recording of the additional fields of admitting team (hospitalist vs. resident), 30 day repeat ED visit, 30 day readmission, and poor outcomes within the first 24 hours of admission, directly from the electronic record.
Study Outcomes
We evaluated each admission for the following 6 primary outcomes which were specified a priori: LOS (defined as discharge date and time minus admission date and time), hospital charges (defined as charges billed as recorded in the administrative data set), ICU transfer during hospitalization (defined as 1 ICU day in the administrative data set), 30 day repeat ED visit (defined as a visit to our ED within 30 days of discharge as assessed by chart abstraction), 30 day readmission (defined as any planned or unplanned admission to any inpatient service at our institution within 30 days of discharge as assessed by chart abstraction), and poor outcome within 24 hours of admission (defined as transfer to the ICU, cardiac arrest, or death as assessed by chart abstraction). Each of these outcomes has been used in prior work to assess the quality of inpatient care.10, 11
Statistical Analysis
Interrater reliability between the 3 physician reviewers was assessed for 20 randomly selected admissions across the 4 separate review measures using interclass correlation coefficients. Comparisons between night admissions and day admissions, and between weekend and weekday admissions, for the continuous primary outcomes (LOS, hospital charges) were assessed using 2‐tailed t‐tests as well as Wilcoxon rank sum test. In the multivariable modeling, these outcomes were assessed by linear regression controlling for age, gender, race and ethnicity, Medicaid or self‐pay insurance, admission to the hospitalist or teaching service, most common MDC categories, and Charlson Comorbidity Index. Because both outcomes were right‐skewed, we separately assessed each after log‐transformation controlling for the same variables.
All comparisons of the dichotomous primary outcomes (ICU transfer during hospitalization, 30 day repeat ED visit, 30 day readmission, and poor outcome within the first 24 hours after admission) were assessed at the univariate level by chi‐squared test, and in the multivariable models using logistic regression, controlling for the same variables as the linear models above. All adjustments were specified a priori. All data analyses were conducted using Stata (College Station, TX; Version 11).
Results
We reviewed 857 records. After excluding 33 records lacking administrative data regarding gender, race and ethnicity, and other demographic variables, there were 824 medical records available for analysis. We reviewed a similar number of records from each time period: 274 from January 2008, 265 from April 2008, and 285 from July 2008. A total of 345 (42%) patients were admitted during the day, and 479 (58%) at night; 641 (78%) were admitted on weekdays, and 183 (22%) on weekends. The 33 excluded charts were similar to the included charts for both time of admission and outcomes. Results for parametric testing and nonparametric testing, as well as for log‐transformation and non‐log‐transformation of the continuous outcomes were similar in both magnitude and statistical significance, so we present the parametric and nonlog‐transformed results below for ease of interpretation.
Interrater reliability among the 3 reviewers was very high. There were no disagreements among the 20 multiple reviews for either poor outcomes within 24 hours of admission or admitting service; the interclass correlation coefficients for 30 day repeat ED visit and 30 day readmission were 0.97 and 0.87, respectively.
Patients admitted at night or on the weekend were similar to patients admitted during the day and week across age, gender, insurance class, MDC, and Charlson Comorbidity Index (Table 1). For unadjusted outcomes, patients admitted at night has a similar LOS, hospital charges, 30 day repeat ED visits, 30 day readmissions, and poor outcome within 24 hours of admission as those patients admitted during the day. They had a potentially lower chance of any ICU transfer during hospitalization though this did not reach statistical significance at P < 0.05 (night admission 6%, day admission 3%, P = 0.06) (Table 2).
| Characteristics | Time of Day | Day of the Week | ||
|---|---|---|---|---|
| Day Admission (n = 345) | Night Admission (n = 479) | Weekday Admission (n = 641) | Weekend Admission (n = 183) | |
| ||||
| Age (years) | 60.8 | 59.7 | 60.6 | 58.7 |
| Gender (% male) | 47 | 43 | 45 | 46 |
| Race/Ethnicity (%) | ||||
| White, Asian, other | 61 | 54 | 57 | 55 |
| Black | 34 | 38 | 37 | 34 |
| Hispanic | 5 | 8 | 6 | 10 |
| Medicaid or self pay (%) | 9 | 10 | 10 | 11 |
| Major diagnostic category (%) | ||||
| Respiratory disease | 14 | 13 | 14 | 13 |
| Circulatory disease | 28 | 23 | 26 | 24 |
| Digestive disease | 12 | 12 | 12 | 12 |
| Other | 45 | 52 | 48 | 51 |
| Charlson Comorbidity Index | 3.71 | 3.60 | 3.66 | 3.60 |
| Outcomes | Time of Day | Day of the Week | ||
|---|---|---|---|---|
| Day Admission (n = 345) | Night Admission (n = 479) | Weekday Admission (n = 641) | Weekend Admission (n = 183) | |
| ||||
| Length of stay | 4.3 | 4.1 | 4.3 | 3.8 |
| Hospital charges | $27,500 | $25,200 | $27,200* | $22,700* |
| ICU transfer during hospitalization (%) | 6 | 3 | 5* | 1* |
| Repeat ED visit at 30 days (%) | 20 | 22 | 22 | 21 |
| Readmission at 30 days (%) | 17 | 20 | 20 | 17 |
| Poor outcome at 24 hours (ICU transfer, cardiac arrest, or death)(%) | 2 | 1 | 2 | 1 |
Patients admitted to the hospital during the weekend were similar to patients admitted during the week for unadjusted LOS, 30 day repeat ED visit or readmission rate, and poor outcomes within 24 hours of admission as those admitted during the week; however, they had lower hospital charges (weekend admission $22,700, weekday admission $27,200; P = 0.02), and a lower chance of ICU transfer during hospitalization (weekend admission 1%, weekday admission 5%; P = 0.02) (Table 2).
In the multivariable linear and logistic regression models (Tables 3 and 4), we assessed the independent association between night admission or weekend admission and each hospitalization‐relevant outcome except for poor outcome within 24 hours of admission (poor outcome within 24 hours of admission was not modeled to avoid the risk of overfitting because there were only 13 total events). After adjustment for age, gender, race and ethnicity, admitting service (hospitalist or teaching), Medicaid or self‐pay insurance, MDC, and Charlson Comorbidity Index, there was no statistically significant association between night admission and worse outcomes for LOS, hospital charges, 30 day repeat ED visit, or 30 day readmission. Night admission was associated with a decreased chance of ICU transfer during hospitalization, but the difference was not statistically significant (odds ratio, 0.54; 95% confidence interval [CI], 0.26‐1.11, P = 0.09). Weekend admission was not associated with worse outcomes for LOS or 30 day repeat ED visit or readmission; however, weekend admission was associated with a decrease in overall charges ($4400; 95% CI, $8300 to $600) and a decreased chance of ICU transfer during hospitalization (odds ratio, 0.20; 95% CI, 0.050.88).
| Predictors | Length of Stay (days), Coefficient (95% CI) | Hospital Charges (dollars), Coefficient (95% CI) |
|---|---|---|
| ||
| Night admission | 0.23 (0.77 to 0.32) | 2100 (5400 to 1100) |
| Weekend admission | 0.42 (1.07 to 0.23) | 4400 (8300 to 600)* |
| Age | 0.01 (0.01 to 0.03) | 0 (100 to 100) |
| Male gender | 0.15 (0.70 to 0.39) | 400 (3700 to 2800) |
| Race, Black | 0.18 (0.41 to 0.78) | 200 (3700 to 3400) |
| Ethnicity, Hispanic | 0.62 (1.73 to 0.49) | 2300 (8900 to 4300) |
| Medicaid or self‐pay insurance | 1.87 (0.93 to 2.82)* | 8900 (3300 to 14600)* |
| Hospitalist service | 0.26 (0.29 to 0.81) | 600 (3900 to 2700) |
| MDC: respiratory | 0.36 (1.18 to 0.46) | 700 (4200 to 5600) |
| MDC: circulatory | 1.36 (2.04 to 0.68)* | 600 (4600 to 3400) |
| MDC: digestive | 1.22 (2.08 to 0.35)* | 6800 (12000 to 1700)* |
| Charlson Comorbidity Index | 0.35 (0.22 to 0.49)* | 2200 (1400 to 3000)* |
| Predictors | ICU Transfer during Hospitalization, Odds Ratio (95% CI) | Repeat ED Visit at 30 days, Odds Ratio (95% CI) | Readmission at 30 days, Odds Ratio (95% CI) |
|---|---|---|---|
| |||
| Night admission | 0.53 (0.26 to 1.11) | 1.13 (0.80 to 1.60) | 1.23 (0.86 to 1.78) |
| Weekend admission | 0.20 (0.05 to 0.88)* | 0.95 (0.63 to 1.44) | 0.80 (0.51 to 1.25) |
| Age | 1.00 (0.98 to 1.02) | 0.99 (0.98 to 1.002) | 1.00 (0.99 to 1.01) |
| Male gender | 0.98 (0.47 to 2.02) | 1.09 (0.78 to 1.54) | 0.91 (0.64 to 1.31) |
| Race, Black | 0.75 (0.33 to 1.70) | 1.48 (1.02 to 2.14)* | 1.12 (0.76 to 1.65) |
| Ethnicity, Hispanic | 0.76 (0.16 to 3.73) | 1.09 (0.55 to 2.17) | 1.11 (0.55 to 2.22) |
| Medicaid or self‐pay insurance | 0.75 (0.16 to 3.49) | 1.61 (0.95 to 2.72) | 2.14 (1.24 to 3.67)* |
| Hospitalist service | 0.68 (0.33 to 1.44) | 1.15 (0.81 to 1.63) | 0.99 (0.69 to 1.43) |
| MDC: respiratory | 1.18 (0.41 to 3.38) | 1.02 (0.61 to 1.69) | 1.16 (0.69 to 1.95) |
| MDC: circulatory | 1.22 (0.52 to 2.87) | 0.79 (0.51 to 1.22) | 0.80 (0.51 to 1.27) |
| MDC: digestive | 0.51 (0.11 to 2.32) | 0.83 (0.47 to 1.46) | 1.08 (0.62 to 1.91) |
| Charlson Comobrbidity Index | 1.25 (1.09 to 1.45)* | 1.09 (1.01 to 1.19)* | 1.11 (1.02 to 1.21)* |
Our multivariate models explained very little of the variance in patient outcomes. For LOS and hospital charges, adjusted R2 values were 0.06 and 0.05, respectively. For ICU transfer during hospitalization, 30 day repeat ED visit, and 30 day readmission, the areas under the receiver operator curves were 0.75, 0.51, and 0.61 respectively.
To assess the robustness of our conclusions regarding night admission, we redefined night to include only patients admitted between the hours of 8 pm and 5:59 am. This did not change our conclusions. We also tested for interaction between night admission and weekend admission for all outcomes to assess whether night admissions on the weekend were in fact at increased risk of worse outcomes; we found no evidence of interaction (P > 0.3 for the interaction terms in each model).
Discussion
Among patients admitted to the medicine services at our academic medical center, night or weekend admission was not associated with worse hospitalization‐relevant outcomes. In some cases, night or weekend admission was associated with better outcomes, particularly in terms of ICU transfer during hospitalization and hospital charges. Prior research indicates worse outcomes during off‐hours,5 but we did not replicate this finding in our study.
The finding that admission at night was not associated with worse outcomes, particularly proximal outcomes such as LOS or ICU transfer during hospitalization, was surprising, though reassuring in view of the fact that more than half of our patients are admitted at night. We believe a few factors may be responsible. First, our general medicine service is staffed during the night (7 pm to 7 am) by in‐house nocturnists and night float residents. Second, our staffing ratio, while lower at night than during the day, remains the same on weekends and may be higher than in other settings. In continuously well‐staffed settings such as the ED12 and ICU,13 night and weekend admissions are only inconsistently associated with worse outcomes, which may be the same phenomena we observed in the current study. Third, the hospital used as the site of this study has received Nursing Magnet recognition and numerous quality awards such as the National Research Corporation's Consumer Choice Award and recognition as a Distinguished Hospital for Clinical Excellence by HealthGrades. Fourth, our integrated electronic medical record, computerized physician order entry system, and automatically generated sign out serve as complements to the morning hand off. Fifth, hospitalists and teaching teams rotate on a weekly, biweekly, or every 4 week basis, which may protect against discontinuity associated with the weekend. We believe that all of these factors may facilitate alert, comprehensive care during the night and weekend as well as safe and efficient transfer of patients from the night to the day providers.
We were also surprised by the association between weekend admission and lower charges and a lower chance of ICU transfer during hospitalization. We believe many of the same factors noted above may have played a role in these findings. In terms of hospital charges, it is possible that some workups were completed outside of the hospital rather than during the hospitalization, and that some tests were not ordered at all due to unavailability on weekends. The decreased chance of ICU transfer is unexplained. We hypothesize that there may have been a more conservative admission strategy within the ED, such that patients with high baseline severity were admitted directly to the ICU on the weekend rather than being admitted first to the general medicine floor. This hypothesis requires further study.
Our study had important limitations. It was a retrospective study from a single academic hospital. The sample size lacked sufficient power to detect differences in the low frequency of certain outcomes such as poor outcomes within 24 hours of admission (2% vs. 1%), and also for more frequent outcomes such as 30 day readmission; it is possible that with a larger sample there would have been statistically significant differences. Further, we recognize that the Charlson Comorbidity Index, which was developed to predict 1‐year mortality for medicine service patients, does not adjust for severity of illness at presentation, particularly for outcomes such as readmission. If patients admitted at night and during the weekend were less acutely ill despite having similar comorbidities and MDCs at admission, true associations between time of admission and worse outcomes could have been masked. Furthermore, the multivariable modeling explained very little of the variance in patient outcomes such that significant unmeasured confounding may still be present, and consequently our results cannot be interpreted in a causal way. Data was collected from electronic records, so it is possible that some adverse events were not recorded. However, it seems unlikely that major events such as death and transfer to an ICU would have been missed.
Several aspects of the study strengthen our confidence in the findings, including a large sample size, relevance of the outcomes, the adjustment for confounders, and an assessment for robustness of the conclusions based on restricting the definition of night and also testing for interaction between night and weekend admission. Our patient demographics and insurance mix resemble that of other academic hospitals,10 and perhaps our results may be generalizable to these settings, if not to non‐urban or community hospitals. Furthermore, the Charlson Comorbidity Index was associated with all 5 of the modeled outcomes we chose for our study, reaffirming their utility in assessing the quality of hospital care. Future directions for investigation may include examining the association of night admission with hospitalization‐relevant outcomes in nonacademic, nonurban settings, and examining whether the lack of association between night and weekend admission and worse outcomes persists with adjustment for initial severity of illness.
In summary, at a large, well‐staffed urban academic hospital, day or time of admission were not associated with worse hospitalization‐relevant outcomes. The use of nocturnists and night float teams for night admissions and continuity across weekends appears to be a safe approach to handling the increased volume of patients admitted at night, and a viable alternative to overnight call in the era of work hour restrictions.
- ,,, et al.Three‐year results of mandated work hour restrictions: attending and resident perspectives and effects in a community hospital.Am Surg.2008;74(6):542–546; discussion 546–547.
- ,,, et al.Handoffs causing patient harm: a survey of medical and surgical house staff.Jt Comm J Qual Patient Saf.2008;34(10):563–570.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121(11):866–872.
- ,.Mortality among patients admitted to hospitals on weekends as compared with weekdays.N Engl J Med.2001;345(9):663–668.
- ,,, et al.Survival from in‐hospital cardiac arrest during nights and weekends.JAMA.2008;299(7):785–792.
- ,,,.Continuity of care and intensive care unit use at the end of life.Arch Intern Med.2009;169(1):81–86.
- ,,,,,.Continuity of outpatient and inpatient care by primary care physicians for hospitalized older adults.JAMA.2009;301(16):1671–1680.
- ,,,.Why predictive indexes perform less well in validation studies: is it magic or methods?Arch Intern Med.1987;147:2155–2161.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45(6):613–619.
- ,,, et al.Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes.J Hosp Med.2008;3(5):361–368.
- ,,, et al.Use of an admission early warning score to predict patient morbidity and mortality and treatment success.Emerg Med J.2008;25(12):803–806.
- ,,.The impact of weekends on outcome for emergency patients.Clin Med.2005;5(6):621–625.
- ,,,,,.Off hour admission to an intensivist‐led ICU is not associated with increased mortality.Crit Care.2009;13(3):R84.
The hospitalist movement and increasingly stringent resident work hour restrictions have led to the utilization of shift work in many hospitals.1 Use of nocturnist and night float systems, while often necessary, results in increased patient hand‐offs. Research suggests that hand‐offs in the inpatient setting can adversely affect patient outcomes as lack of continuity may increase the possibility of medical error.2, 3 In 2001, Bell et al.4 found that mortality was higher among patients admitted on weekends as compared to weekdays. Uneven staffing, lack of supervision, and fragmented care were cited as potential contributing factors.4 Similarly, Peberdy et al.5 in 2008 revealed that patients were less likely to survive a cardiac arrest if it occurred at night or on weekends, again attributed in part to fragmented patient care and understaffing.
The results of these studies raise concerns as to whether increased reliance on shift work and resulting handoffs compromises patient care.6, 7 The aim of this study was to evaluate the potential association between night admission and hospitalization‐relevant outcomes (length of stay [LOS], hospital charges, intensive care unit [ICU] transfer during hospitalization, repeat emergency department [ED] visit within 30 days of discharge, readmission within 30 days of discharge, and poor outcome [transfer to the ICU, cardiac arrest, or death] within the first 24 hours of admission) at an institution that exclusively uses nocturnists (night‐shift based hospitalists) and a resident night float system for patients admitted at night to the general medicine service. A secondary aim was to determine the potential association between weekend admission and hospitalization‐relevant outcomes.
Methods
Study Sample and Selection
We conducted a retrospective medical record review at a large urban academic hospital. Using an administrative hospital data set, we assembled a list of approximately 9000 admissions to the general medicine service from the ED between January 2008 and October 2008. We sampled consecutive admissions from 3 distinct periods beginning in January, April, and July to capture outcomes at various points in the academic year. We attempted to review approximately 10% of all charts equally distributed among the 3 sampling periods (ie, 900 charts total with one‐third from each period) based on time available to the reviewers. We excluded patients not admitted to the general medicine service and patients without complete demographic or outcome information. We also excluded patients not admitted from the ED given that the vast majority of admissions to our hospital during the night (96%) or weekend (93%) are from the ED. Patients admitted to the general medicine service are cared for either by a hospitalist or by a teaching team comprised of 1 attending (about 40% of whom are hospitalists), 1 resident, 1 to 2 interns, and 1 to 3 medical students. From 7 am to 6:59 pm patients are admitted to the care of 1 of the primary daytime admitting teams. From 7 pm to 6:59 am patients are admitted by nocturnists (hospitalist service) or night float residents (teaching service). These patients are handed off to day teams at 7 am. Hospitalist teams change service on a weekly to biweekly basis and resident teams switch on a monthly basis; there is no difference in physician staffing between the weekend and weekdays. The Northwestern University Institutional Review Board approved this study.
Data Acquisition and Medical Records Reviews
We obtained demographic data including gender, age, race and ethnicity, patient insurance, admission day (weekday vs. weekend), admission time (defined as the time that a patient receives a hospital bed, which at our institution is also the time that admitting teams receive report and assume care for the patient), and the International Classification of Disease codes required to determine the Major Diagnostic Category (MDC) and calculate the Charlson Comorbidity Index8, 9 as part of an administrative data set. We divided the admission time into night admission (defined as 7 pm to 6:59 am) and day admission (defined as 7:00 am to 6:59 pm). We created a chart abstraction tool to allow manual recording of the additional fields of admitting team (hospitalist vs. resident), 30 day repeat ED visit, 30 day readmission, and poor outcomes within the first 24 hours of admission, directly from the electronic record.
Study Outcomes
We evaluated each admission for the following 6 primary outcomes which were specified a priori: LOS (defined as discharge date and time minus admission date and time), hospital charges (defined as charges billed as recorded in the administrative data set), ICU transfer during hospitalization (defined as 1 ICU day in the administrative data set), 30 day repeat ED visit (defined as a visit to our ED within 30 days of discharge as assessed by chart abstraction), 30 day readmission (defined as any planned or unplanned admission to any inpatient service at our institution within 30 days of discharge as assessed by chart abstraction), and poor outcome within 24 hours of admission (defined as transfer to the ICU, cardiac arrest, or death as assessed by chart abstraction). Each of these outcomes has been used in prior work to assess the quality of inpatient care.10, 11
Statistical Analysis
Interrater reliability between the 3 physician reviewers was assessed for 20 randomly selected admissions across the 4 separate review measures using interclass correlation coefficients. Comparisons between night admissions and day admissions, and between weekend and weekday admissions, for the continuous primary outcomes (LOS, hospital charges) were assessed using 2‐tailed t‐tests as well as Wilcoxon rank sum test. In the multivariable modeling, these outcomes were assessed by linear regression controlling for age, gender, race and ethnicity, Medicaid or self‐pay insurance, admission to the hospitalist or teaching service, most common MDC categories, and Charlson Comorbidity Index. Because both outcomes were right‐skewed, we separately assessed each after log‐transformation controlling for the same variables.
All comparisons of the dichotomous primary outcomes (ICU transfer during hospitalization, 30 day repeat ED visit, 30 day readmission, and poor outcome within the first 24 hours after admission) were assessed at the univariate level by chi‐squared test, and in the multivariable models using logistic regression, controlling for the same variables as the linear models above. All adjustments were specified a priori. All data analyses were conducted using Stata (College Station, TX; Version 11).
Results
We reviewed 857 records. After excluding 33 records lacking administrative data regarding gender, race and ethnicity, and other demographic variables, there were 824 medical records available for analysis. We reviewed a similar number of records from each time period: 274 from January 2008, 265 from April 2008, and 285 from July 2008. A total of 345 (42%) patients were admitted during the day, and 479 (58%) at night; 641 (78%) were admitted on weekdays, and 183 (22%) on weekends. The 33 excluded charts were similar to the included charts for both time of admission and outcomes. Results for parametric testing and nonparametric testing, as well as for log‐transformation and non‐log‐transformation of the continuous outcomes were similar in both magnitude and statistical significance, so we present the parametric and nonlog‐transformed results below for ease of interpretation.
Interrater reliability among the 3 reviewers was very high. There were no disagreements among the 20 multiple reviews for either poor outcomes within 24 hours of admission or admitting service; the interclass correlation coefficients for 30 day repeat ED visit and 30 day readmission were 0.97 and 0.87, respectively.
Patients admitted at night or on the weekend were similar to patients admitted during the day and week across age, gender, insurance class, MDC, and Charlson Comorbidity Index (Table 1). For unadjusted outcomes, patients admitted at night has a similar LOS, hospital charges, 30 day repeat ED visits, 30 day readmissions, and poor outcome within 24 hours of admission as those patients admitted during the day. They had a potentially lower chance of any ICU transfer during hospitalization though this did not reach statistical significance at P < 0.05 (night admission 6%, day admission 3%, P = 0.06) (Table 2).
| Characteristics | Time of Day | Day of the Week | ||
|---|---|---|---|---|
| Day Admission (n = 345) | Night Admission (n = 479) | Weekday Admission (n = 641) | Weekend Admission (n = 183) | |
| ||||
| Age (years) | 60.8 | 59.7 | 60.6 | 58.7 |
| Gender (% male) | 47 | 43 | 45 | 46 |
| Race/Ethnicity (%) | ||||
| White, Asian, other | 61 | 54 | 57 | 55 |
| Black | 34 | 38 | 37 | 34 |
| Hispanic | 5 | 8 | 6 | 10 |
| Medicaid or self pay (%) | 9 | 10 | 10 | 11 |
| Major diagnostic category (%) | ||||
| Respiratory disease | 14 | 13 | 14 | 13 |
| Circulatory disease | 28 | 23 | 26 | 24 |
| Digestive disease | 12 | 12 | 12 | 12 |
| Other | 45 | 52 | 48 | 51 |
| Charlson Comorbidity Index | 3.71 | 3.60 | 3.66 | 3.60 |
| Outcomes | Time of Day | Day of the Week | ||
|---|---|---|---|---|
| Day Admission (n = 345) | Night Admission (n = 479) | Weekday Admission (n = 641) | Weekend Admission (n = 183) | |
| ||||
| Length of stay | 4.3 | 4.1 | 4.3 | 3.8 |
| Hospital charges | $27,500 | $25,200 | $27,200* | $22,700* |
| ICU transfer during hospitalization (%) | 6 | 3 | 5* | 1* |
| Repeat ED visit at 30 days (%) | 20 | 22 | 22 | 21 |
| Readmission at 30 days (%) | 17 | 20 | 20 | 17 |
| Poor outcome at 24 hours (ICU transfer, cardiac arrest, or death)(%) | 2 | 1 | 2 | 1 |
Patients admitted to the hospital during the weekend were similar to patients admitted during the week for unadjusted LOS, 30 day repeat ED visit or readmission rate, and poor outcomes within 24 hours of admission as those admitted during the week; however, they had lower hospital charges (weekend admission $22,700, weekday admission $27,200; P = 0.02), and a lower chance of ICU transfer during hospitalization (weekend admission 1%, weekday admission 5%; P = 0.02) (Table 2).
In the multivariable linear and logistic regression models (Tables 3 and 4), we assessed the independent association between night admission or weekend admission and each hospitalization‐relevant outcome except for poor outcome within 24 hours of admission (poor outcome within 24 hours of admission was not modeled to avoid the risk of overfitting because there were only 13 total events). After adjustment for age, gender, race and ethnicity, admitting service (hospitalist or teaching), Medicaid or self‐pay insurance, MDC, and Charlson Comorbidity Index, there was no statistically significant association between night admission and worse outcomes for LOS, hospital charges, 30 day repeat ED visit, or 30 day readmission. Night admission was associated with a decreased chance of ICU transfer during hospitalization, but the difference was not statistically significant (odds ratio, 0.54; 95% confidence interval [CI], 0.26‐1.11, P = 0.09). Weekend admission was not associated with worse outcomes for LOS or 30 day repeat ED visit or readmission; however, weekend admission was associated with a decrease in overall charges ($4400; 95% CI, $8300 to $600) and a decreased chance of ICU transfer during hospitalization (odds ratio, 0.20; 95% CI, 0.050.88).
| Predictors | Length of Stay (days), Coefficient (95% CI) | Hospital Charges (dollars), Coefficient (95% CI) |
|---|---|---|
| ||
| Night admission | 0.23 (0.77 to 0.32) | 2100 (5400 to 1100) |
| Weekend admission | 0.42 (1.07 to 0.23) | 4400 (8300 to 600)* |
| Age | 0.01 (0.01 to 0.03) | 0 (100 to 100) |
| Male gender | 0.15 (0.70 to 0.39) | 400 (3700 to 2800) |
| Race, Black | 0.18 (0.41 to 0.78) | 200 (3700 to 3400) |
| Ethnicity, Hispanic | 0.62 (1.73 to 0.49) | 2300 (8900 to 4300) |
| Medicaid or self‐pay insurance | 1.87 (0.93 to 2.82)* | 8900 (3300 to 14600)* |
| Hospitalist service | 0.26 (0.29 to 0.81) | 600 (3900 to 2700) |
| MDC: respiratory | 0.36 (1.18 to 0.46) | 700 (4200 to 5600) |
| MDC: circulatory | 1.36 (2.04 to 0.68)* | 600 (4600 to 3400) |
| MDC: digestive | 1.22 (2.08 to 0.35)* | 6800 (12000 to 1700)* |
| Charlson Comorbidity Index | 0.35 (0.22 to 0.49)* | 2200 (1400 to 3000)* |
| Predictors | ICU Transfer during Hospitalization, Odds Ratio (95% CI) | Repeat ED Visit at 30 days, Odds Ratio (95% CI) | Readmission at 30 days, Odds Ratio (95% CI) |
|---|---|---|---|
| |||
| Night admission | 0.53 (0.26 to 1.11) | 1.13 (0.80 to 1.60) | 1.23 (0.86 to 1.78) |
| Weekend admission | 0.20 (0.05 to 0.88)* | 0.95 (0.63 to 1.44) | 0.80 (0.51 to 1.25) |
| Age | 1.00 (0.98 to 1.02) | 0.99 (0.98 to 1.002) | 1.00 (0.99 to 1.01) |
| Male gender | 0.98 (0.47 to 2.02) | 1.09 (0.78 to 1.54) | 0.91 (0.64 to 1.31) |
| Race, Black | 0.75 (0.33 to 1.70) | 1.48 (1.02 to 2.14)* | 1.12 (0.76 to 1.65) |
| Ethnicity, Hispanic | 0.76 (0.16 to 3.73) | 1.09 (0.55 to 2.17) | 1.11 (0.55 to 2.22) |
| Medicaid or self‐pay insurance | 0.75 (0.16 to 3.49) | 1.61 (0.95 to 2.72) | 2.14 (1.24 to 3.67)* |
| Hospitalist service | 0.68 (0.33 to 1.44) | 1.15 (0.81 to 1.63) | 0.99 (0.69 to 1.43) |
| MDC: respiratory | 1.18 (0.41 to 3.38) | 1.02 (0.61 to 1.69) | 1.16 (0.69 to 1.95) |
| MDC: circulatory | 1.22 (0.52 to 2.87) | 0.79 (0.51 to 1.22) | 0.80 (0.51 to 1.27) |
| MDC: digestive | 0.51 (0.11 to 2.32) | 0.83 (0.47 to 1.46) | 1.08 (0.62 to 1.91) |
| Charlson Comobrbidity Index | 1.25 (1.09 to 1.45)* | 1.09 (1.01 to 1.19)* | 1.11 (1.02 to 1.21)* |
Our multivariate models explained very little of the variance in patient outcomes. For LOS and hospital charges, adjusted R2 values were 0.06 and 0.05, respectively. For ICU transfer during hospitalization, 30 day repeat ED visit, and 30 day readmission, the areas under the receiver operator curves were 0.75, 0.51, and 0.61 respectively.
To assess the robustness of our conclusions regarding night admission, we redefined night to include only patients admitted between the hours of 8 pm and 5:59 am. This did not change our conclusions. We also tested for interaction between night admission and weekend admission for all outcomes to assess whether night admissions on the weekend were in fact at increased risk of worse outcomes; we found no evidence of interaction (P > 0.3 for the interaction terms in each model).
Discussion
Among patients admitted to the medicine services at our academic medical center, night or weekend admission was not associated with worse hospitalization‐relevant outcomes. In some cases, night or weekend admission was associated with better outcomes, particularly in terms of ICU transfer during hospitalization and hospital charges. Prior research indicates worse outcomes during off‐hours,5 but we did not replicate this finding in our study.
The finding that admission at night was not associated with worse outcomes, particularly proximal outcomes such as LOS or ICU transfer during hospitalization, was surprising, though reassuring in view of the fact that more than half of our patients are admitted at night. We believe a few factors may be responsible. First, our general medicine service is staffed during the night (7 pm to 7 am) by in‐house nocturnists and night float residents. Second, our staffing ratio, while lower at night than during the day, remains the same on weekends and may be higher than in other settings. In continuously well‐staffed settings such as the ED12 and ICU,13 night and weekend admissions are only inconsistently associated with worse outcomes, which may be the same phenomena we observed in the current study. Third, the hospital used as the site of this study has received Nursing Magnet recognition and numerous quality awards such as the National Research Corporation's Consumer Choice Award and recognition as a Distinguished Hospital for Clinical Excellence by HealthGrades. Fourth, our integrated electronic medical record, computerized physician order entry system, and automatically generated sign out serve as complements to the morning hand off. Fifth, hospitalists and teaching teams rotate on a weekly, biweekly, or every 4 week basis, which may protect against discontinuity associated with the weekend. We believe that all of these factors may facilitate alert, comprehensive care during the night and weekend as well as safe and efficient transfer of patients from the night to the day providers.
We were also surprised by the association between weekend admission and lower charges and a lower chance of ICU transfer during hospitalization. We believe many of the same factors noted above may have played a role in these findings. In terms of hospital charges, it is possible that some workups were completed outside of the hospital rather than during the hospitalization, and that some tests were not ordered at all due to unavailability on weekends. The decreased chance of ICU transfer is unexplained. We hypothesize that there may have been a more conservative admission strategy within the ED, such that patients with high baseline severity were admitted directly to the ICU on the weekend rather than being admitted first to the general medicine floor. This hypothesis requires further study.
Our study had important limitations. It was a retrospective study from a single academic hospital. The sample size lacked sufficient power to detect differences in the low frequency of certain outcomes such as poor outcomes within 24 hours of admission (2% vs. 1%), and also for more frequent outcomes such as 30 day readmission; it is possible that with a larger sample there would have been statistically significant differences. Further, we recognize that the Charlson Comorbidity Index, which was developed to predict 1‐year mortality for medicine service patients, does not adjust for severity of illness at presentation, particularly for outcomes such as readmission. If patients admitted at night and during the weekend were less acutely ill despite having similar comorbidities and MDCs at admission, true associations between time of admission and worse outcomes could have been masked. Furthermore, the multivariable modeling explained very little of the variance in patient outcomes such that significant unmeasured confounding may still be present, and consequently our results cannot be interpreted in a causal way. Data was collected from electronic records, so it is possible that some adverse events were not recorded. However, it seems unlikely that major events such as death and transfer to an ICU would have been missed.
Several aspects of the study strengthen our confidence in the findings, including a large sample size, relevance of the outcomes, the adjustment for confounders, and an assessment for robustness of the conclusions based on restricting the definition of night and also testing for interaction between night and weekend admission. Our patient demographics and insurance mix resemble that of other academic hospitals,10 and perhaps our results may be generalizable to these settings, if not to non‐urban or community hospitals. Furthermore, the Charlson Comorbidity Index was associated with all 5 of the modeled outcomes we chose for our study, reaffirming their utility in assessing the quality of hospital care. Future directions for investigation may include examining the association of night admission with hospitalization‐relevant outcomes in nonacademic, nonurban settings, and examining whether the lack of association between night and weekend admission and worse outcomes persists with adjustment for initial severity of illness.
In summary, at a large, well‐staffed urban academic hospital, day or time of admission were not associated with worse hospitalization‐relevant outcomes. The use of nocturnists and night float teams for night admissions and continuity across weekends appears to be a safe approach to handling the increased volume of patients admitted at night, and a viable alternative to overnight call in the era of work hour restrictions.
The hospitalist movement and increasingly stringent resident work hour restrictions have led to the utilization of shift work in many hospitals.1 Use of nocturnist and night float systems, while often necessary, results in increased patient hand‐offs. Research suggests that hand‐offs in the inpatient setting can adversely affect patient outcomes as lack of continuity may increase the possibility of medical error.2, 3 In 2001, Bell et al.4 found that mortality was higher among patients admitted on weekends as compared to weekdays. Uneven staffing, lack of supervision, and fragmented care were cited as potential contributing factors.4 Similarly, Peberdy et al.5 in 2008 revealed that patients were less likely to survive a cardiac arrest if it occurred at night or on weekends, again attributed in part to fragmented patient care and understaffing.
The results of these studies raise concerns as to whether increased reliance on shift work and resulting handoffs compromises patient care.6, 7 The aim of this study was to evaluate the potential association between night admission and hospitalization‐relevant outcomes (length of stay [LOS], hospital charges, intensive care unit [ICU] transfer during hospitalization, repeat emergency department [ED] visit within 30 days of discharge, readmission within 30 days of discharge, and poor outcome [transfer to the ICU, cardiac arrest, or death] within the first 24 hours of admission) at an institution that exclusively uses nocturnists (night‐shift based hospitalists) and a resident night float system for patients admitted at night to the general medicine service. A secondary aim was to determine the potential association between weekend admission and hospitalization‐relevant outcomes.
Methods
Study Sample and Selection
We conducted a retrospective medical record review at a large urban academic hospital. Using an administrative hospital data set, we assembled a list of approximately 9000 admissions to the general medicine service from the ED between January 2008 and October 2008. We sampled consecutive admissions from 3 distinct periods beginning in January, April, and July to capture outcomes at various points in the academic year. We attempted to review approximately 10% of all charts equally distributed among the 3 sampling periods (ie, 900 charts total with one‐third from each period) based on time available to the reviewers. We excluded patients not admitted to the general medicine service and patients without complete demographic or outcome information. We also excluded patients not admitted from the ED given that the vast majority of admissions to our hospital during the night (96%) or weekend (93%) are from the ED. Patients admitted to the general medicine service are cared for either by a hospitalist or by a teaching team comprised of 1 attending (about 40% of whom are hospitalists), 1 resident, 1 to 2 interns, and 1 to 3 medical students. From 7 am to 6:59 pm patients are admitted to the care of 1 of the primary daytime admitting teams. From 7 pm to 6:59 am patients are admitted by nocturnists (hospitalist service) or night float residents (teaching service). These patients are handed off to day teams at 7 am. Hospitalist teams change service on a weekly to biweekly basis and resident teams switch on a monthly basis; there is no difference in physician staffing between the weekend and weekdays. The Northwestern University Institutional Review Board approved this study.
Data Acquisition and Medical Records Reviews
We obtained demographic data including gender, age, race and ethnicity, patient insurance, admission day (weekday vs. weekend), admission time (defined as the time that a patient receives a hospital bed, which at our institution is also the time that admitting teams receive report and assume care for the patient), and the International Classification of Disease codes required to determine the Major Diagnostic Category (MDC) and calculate the Charlson Comorbidity Index8, 9 as part of an administrative data set. We divided the admission time into night admission (defined as 7 pm to 6:59 am) and day admission (defined as 7:00 am to 6:59 pm). We created a chart abstraction tool to allow manual recording of the additional fields of admitting team (hospitalist vs. resident), 30 day repeat ED visit, 30 day readmission, and poor outcomes within the first 24 hours of admission, directly from the electronic record.
Study Outcomes
We evaluated each admission for the following 6 primary outcomes which were specified a priori: LOS (defined as discharge date and time minus admission date and time), hospital charges (defined as charges billed as recorded in the administrative data set), ICU transfer during hospitalization (defined as 1 ICU day in the administrative data set), 30 day repeat ED visit (defined as a visit to our ED within 30 days of discharge as assessed by chart abstraction), 30 day readmission (defined as any planned or unplanned admission to any inpatient service at our institution within 30 days of discharge as assessed by chart abstraction), and poor outcome within 24 hours of admission (defined as transfer to the ICU, cardiac arrest, or death as assessed by chart abstraction). Each of these outcomes has been used in prior work to assess the quality of inpatient care.10, 11
Statistical Analysis
Interrater reliability between the 3 physician reviewers was assessed for 20 randomly selected admissions across the 4 separate review measures using interclass correlation coefficients. Comparisons between night admissions and day admissions, and between weekend and weekday admissions, for the continuous primary outcomes (LOS, hospital charges) were assessed using 2‐tailed t‐tests as well as Wilcoxon rank sum test. In the multivariable modeling, these outcomes were assessed by linear regression controlling for age, gender, race and ethnicity, Medicaid or self‐pay insurance, admission to the hospitalist or teaching service, most common MDC categories, and Charlson Comorbidity Index. Because both outcomes were right‐skewed, we separately assessed each after log‐transformation controlling for the same variables.
All comparisons of the dichotomous primary outcomes (ICU transfer during hospitalization, 30 day repeat ED visit, 30 day readmission, and poor outcome within the first 24 hours after admission) were assessed at the univariate level by chi‐squared test, and in the multivariable models using logistic regression, controlling for the same variables as the linear models above. All adjustments were specified a priori. All data analyses were conducted using Stata (College Station, TX; Version 11).
Results
We reviewed 857 records. After excluding 33 records lacking administrative data regarding gender, race and ethnicity, and other demographic variables, there were 824 medical records available for analysis. We reviewed a similar number of records from each time period: 274 from January 2008, 265 from April 2008, and 285 from July 2008. A total of 345 (42%) patients were admitted during the day, and 479 (58%) at night; 641 (78%) were admitted on weekdays, and 183 (22%) on weekends. The 33 excluded charts were similar to the included charts for both time of admission and outcomes. Results for parametric testing and nonparametric testing, as well as for log‐transformation and non‐log‐transformation of the continuous outcomes were similar in both magnitude and statistical significance, so we present the parametric and nonlog‐transformed results below for ease of interpretation.
Interrater reliability among the 3 reviewers was very high. There were no disagreements among the 20 multiple reviews for either poor outcomes within 24 hours of admission or admitting service; the interclass correlation coefficients for 30 day repeat ED visit and 30 day readmission were 0.97 and 0.87, respectively.
Patients admitted at night or on the weekend were similar to patients admitted during the day and week across age, gender, insurance class, MDC, and Charlson Comorbidity Index (Table 1). For unadjusted outcomes, patients admitted at night has a similar LOS, hospital charges, 30 day repeat ED visits, 30 day readmissions, and poor outcome within 24 hours of admission as those patients admitted during the day. They had a potentially lower chance of any ICU transfer during hospitalization though this did not reach statistical significance at P < 0.05 (night admission 6%, day admission 3%, P = 0.06) (Table 2).
| Characteristics | Time of Day | Day of the Week | ||
|---|---|---|---|---|
| Day Admission (n = 345) | Night Admission (n = 479) | Weekday Admission (n = 641) | Weekend Admission (n = 183) | |
| ||||
| Age (years) | 60.8 | 59.7 | 60.6 | 58.7 |
| Gender (% male) | 47 | 43 | 45 | 46 |
| Race/Ethnicity (%) | ||||
| White, Asian, other | 61 | 54 | 57 | 55 |
| Black | 34 | 38 | 37 | 34 |
| Hispanic | 5 | 8 | 6 | 10 |
| Medicaid or self pay (%) | 9 | 10 | 10 | 11 |
| Major diagnostic category (%) | ||||
| Respiratory disease | 14 | 13 | 14 | 13 |
| Circulatory disease | 28 | 23 | 26 | 24 |
| Digestive disease | 12 | 12 | 12 | 12 |
| Other | 45 | 52 | 48 | 51 |
| Charlson Comorbidity Index | 3.71 | 3.60 | 3.66 | 3.60 |
| Outcomes | Time of Day | Day of the Week | ||
|---|---|---|---|---|
| Day Admission (n = 345) | Night Admission (n = 479) | Weekday Admission (n = 641) | Weekend Admission (n = 183) | |
| ||||
| Length of stay | 4.3 | 4.1 | 4.3 | 3.8 |
| Hospital charges | $27,500 | $25,200 | $27,200* | $22,700* |
| ICU transfer during hospitalization (%) | 6 | 3 | 5* | 1* |
| Repeat ED visit at 30 days (%) | 20 | 22 | 22 | 21 |
| Readmission at 30 days (%) | 17 | 20 | 20 | 17 |
| Poor outcome at 24 hours (ICU transfer, cardiac arrest, or death)(%) | 2 | 1 | 2 | 1 |
Patients admitted to the hospital during the weekend were similar to patients admitted during the week for unadjusted LOS, 30 day repeat ED visit or readmission rate, and poor outcomes within 24 hours of admission as those admitted during the week; however, they had lower hospital charges (weekend admission $22,700, weekday admission $27,200; P = 0.02), and a lower chance of ICU transfer during hospitalization (weekend admission 1%, weekday admission 5%; P = 0.02) (Table 2).
In the multivariable linear and logistic regression models (Tables 3 and 4), we assessed the independent association between night admission or weekend admission and each hospitalization‐relevant outcome except for poor outcome within 24 hours of admission (poor outcome within 24 hours of admission was not modeled to avoid the risk of overfitting because there were only 13 total events). After adjustment for age, gender, race and ethnicity, admitting service (hospitalist or teaching), Medicaid or self‐pay insurance, MDC, and Charlson Comorbidity Index, there was no statistically significant association between night admission and worse outcomes for LOS, hospital charges, 30 day repeat ED visit, or 30 day readmission. Night admission was associated with a decreased chance of ICU transfer during hospitalization, but the difference was not statistically significant (odds ratio, 0.54; 95% confidence interval [CI], 0.26‐1.11, P = 0.09). Weekend admission was not associated with worse outcomes for LOS or 30 day repeat ED visit or readmission; however, weekend admission was associated with a decrease in overall charges ($4400; 95% CI, $8300 to $600) and a decreased chance of ICU transfer during hospitalization (odds ratio, 0.20; 95% CI, 0.050.88).
| Predictors | Length of Stay (days), Coefficient (95% CI) | Hospital Charges (dollars), Coefficient (95% CI) |
|---|---|---|
| ||
| Night admission | 0.23 (0.77 to 0.32) | 2100 (5400 to 1100) |
| Weekend admission | 0.42 (1.07 to 0.23) | 4400 (8300 to 600)* |
| Age | 0.01 (0.01 to 0.03) | 0 (100 to 100) |
| Male gender | 0.15 (0.70 to 0.39) | 400 (3700 to 2800) |
| Race, Black | 0.18 (0.41 to 0.78) | 200 (3700 to 3400) |
| Ethnicity, Hispanic | 0.62 (1.73 to 0.49) | 2300 (8900 to 4300) |
| Medicaid or self‐pay insurance | 1.87 (0.93 to 2.82)* | 8900 (3300 to 14600)* |
| Hospitalist service | 0.26 (0.29 to 0.81) | 600 (3900 to 2700) |
| MDC: respiratory | 0.36 (1.18 to 0.46) | 700 (4200 to 5600) |
| MDC: circulatory | 1.36 (2.04 to 0.68)* | 600 (4600 to 3400) |
| MDC: digestive | 1.22 (2.08 to 0.35)* | 6800 (12000 to 1700)* |
| Charlson Comorbidity Index | 0.35 (0.22 to 0.49)* | 2200 (1400 to 3000)* |
| Predictors | ICU Transfer during Hospitalization, Odds Ratio (95% CI) | Repeat ED Visit at 30 days, Odds Ratio (95% CI) | Readmission at 30 days, Odds Ratio (95% CI) |
|---|---|---|---|
| |||
| Night admission | 0.53 (0.26 to 1.11) | 1.13 (0.80 to 1.60) | 1.23 (0.86 to 1.78) |
| Weekend admission | 0.20 (0.05 to 0.88)* | 0.95 (0.63 to 1.44) | 0.80 (0.51 to 1.25) |
| Age | 1.00 (0.98 to 1.02) | 0.99 (0.98 to 1.002) | 1.00 (0.99 to 1.01) |
| Male gender | 0.98 (0.47 to 2.02) | 1.09 (0.78 to 1.54) | 0.91 (0.64 to 1.31) |
| Race, Black | 0.75 (0.33 to 1.70) | 1.48 (1.02 to 2.14)* | 1.12 (0.76 to 1.65) |
| Ethnicity, Hispanic | 0.76 (0.16 to 3.73) | 1.09 (0.55 to 2.17) | 1.11 (0.55 to 2.22) |
| Medicaid or self‐pay insurance | 0.75 (0.16 to 3.49) | 1.61 (0.95 to 2.72) | 2.14 (1.24 to 3.67)* |
| Hospitalist service | 0.68 (0.33 to 1.44) | 1.15 (0.81 to 1.63) | 0.99 (0.69 to 1.43) |
| MDC: respiratory | 1.18 (0.41 to 3.38) | 1.02 (0.61 to 1.69) | 1.16 (0.69 to 1.95) |
| MDC: circulatory | 1.22 (0.52 to 2.87) | 0.79 (0.51 to 1.22) | 0.80 (0.51 to 1.27) |
| MDC: digestive | 0.51 (0.11 to 2.32) | 0.83 (0.47 to 1.46) | 1.08 (0.62 to 1.91) |
| Charlson Comobrbidity Index | 1.25 (1.09 to 1.45)* | 1.09 (1.01 to 1.19)* | 1.11 (1.02 to 1.21)* |
Our multivariate models explained very little of the variance in patient outcomes. For LOS and hospital charges, adjusted R2 values were 0.06 and 0.05, respectively. For ICU transfer during hospitalization, 30 day repeat ED visit, and 30 day readmission, the areas under the receiver operator curves were 0.75, 0.51, and 0.61 respectively.
To assess the robustness of our conclusions regarding night admission, we redefined night to include only patients admitted between the hours of 8 pm and 5:59 am. This did not change our conclusions. We also tested for interaction between night admission and weekend admission for all outcomes to assess whether night admissions on the weekend were in fact at increased risk of worse outcomes; we found no evidence of interaction (P > 0.3 for the interaction terms in each model).
Discussion
Among patients admitted to the medicine services at our academic medical center, night or weekend admission was not associated with worse hospitalization‐relevant outcomes. In some cases, night or weekend admission was associated with better outcomes, particularly in terms of ICU transfer during hospitalization and hospital charges. Prior research indicates worse outcomes during off‐hours,5 but we did not replicate this finding in our study.
The finding that admission at night was not associated with worse outcomes, particularly proximal outcomes such as LOS or ICU transfer during hospitalization, was surprising, though reassuring in view of the fact that more than half of our patients are admitted at night. We believe a few factors may be responsible. First, our general medicine service is staffed during the night (7 pm to 7 am) by in‐house nocturnists and night float residents. Second, our staffing ratio, while lower at night than during the day, remains the same on weekends and may be higher than in other settings. In continuously well‐staffed settings such as the ED12 and ICU,13 night and weekend admissions are only inconsistently associated with worse outcomes, which may be the same phenomena we observed in the current study. Third, the hospital used as the site of this study has received Nursing Magnet recognition and numerous quality awards such as the National Research Corporation's Consumer Choice Award and recognition as a Distinguished Hospital for Clinical Excellence by HealthGrades. Fourth, our integrated electronic medical record, computerized physician order entry system, and automatically generated sign out serve as complements to the morning hand off. Fifth, hospitalists and teaching teams rotate on a weekly, biweekly, or every 4 week basis, which may protect against discontinuity associated with the weekend. We believe that all of these factors may facilitate alert, comprehensive care during the night and weekend as well as safe and efficient transfer of patients from the night to the day providers.
We were also surprised by the association between weekend admission and lower charges and a lower chance of ICU transfer during hospitalization. We believe many of the same factors noted above may have played a role in these findings. In terms of hospital charges, it is possible that some workups were completed outside of the hospital rather than during the hospitalization, and that some tests were not ordered at all due to unavailability on weekends. The decreased chance of ICU transfer is unexplained. We hypothesize that there may have been a more conservative admission strategy within the ED, such that patients with high baseline severity were admitted directly to the ICU on the weekend rather than being admitted first to the general medicine floor. This hypothesis requires further study.
Our study had important limitations. It was a retrospective study from a single academic hospital. The sample size lacked sufficient power to detect differences in the low frequency of certain outcomes such as poor outcomes within 24 hours of admission (2% vs. 1%), and also for more frequent outcomes such as 30 day readmission; it is possible that with a larger sample there would have been statistically significant differences. Further, we recognize that the Charlson Comorbidity Index, which was developed to predict 1‐year mortality for medicine service patients, does not adjust for severity of illness at presentation, particularly for outcomes such as readmission. If patients admitted at night and during the weekend were less acutely ill despite having similar comorbidities and MDCs at admission, true associations between time of admission and worse outcomes could have been masked. Furthermore, the multivariable modeling explained very little of the variance in patient outcomes such that significant unmeasured confounding may still be present, and consequently our results cannot be interpreted in a causal way. Data was collected from electronic records, so it is possible that some adverse events were not recorded. However, it seems unlikely that major events such as death and transfer to an ICU would have been missed.
Several aspects of the study strengthen our confidence in the findings, including a large sample size, relevance of the outcomes, the adjustment for confounders, and an assessment for robustness of the conclusions based on restricting the definition of night and also testing for interaction between night and weekend admission. Our patient demographics and insurance mix resemble that of other academic hospitals,10 and perhaps our results may be generalizable to these settings, if not to non‐urban or community hospitals. Furthermore, the Charlson Comorbidity Index was associated with all 5 of the modeled outcomes we chose for our study, reaffirming their utility in assessing the quality of hospital care. Future directions for investigation may include examining the association of night admission with hospitalization‐relevant outcomes in nonacademic, nonurban settings, and examining whether the lack of association between night and weekend admission and worse outcomes persists with adjustment for initial severity of illness.
In summary, at a large, well‐staffed urban academic hospital, day or time of admission were not associated with worse hospitalization‐relevant outcomes. The use of nocturnists and night float teams for night admissions and continuity across weekends appears to be a safe approach to handling the increased volume of patients admitted at night, and a viable alternative to overnight call in the era of work hour restrictions.
- ,,, et al.Three‐year results of mandated work hour restrictions: attending and resident perspectives and effects in a community hospital.Am Surg.2008;74(6):542–546; discussion 546–547.
- ,,, et al.Handoffs causing patient harm: a survey of medical and surgical house staff.Jt Comm J Qual Patient Saf.2008;34(10):563–570.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121(11):866–872.
- ,.Mortality among patients admitted to hospitals on weekends as compared with weekdays.N Engl J Med.2001;345(9):663–668.
- ,,, et al.Survival from in‐hospital cardiac arrest during nights and weekends.JAMA.2008;299(7):785–792.
- ,,,.Continuity of care and intensive care unit use at the end of life.Arch Intern Med.2009;169(1):81–86.
- ,,,,,.Continuity of outpatient and inpatient care by primary care physicians for hospitalized older adults.JAMA.2009;301(16):1671–1680.
- ,,,.Why predictive indexes perform less well in validation studies: is it magic or methods?Arch Intern Med.1987;147:2155–2161.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45(6):613–619.
- ,,, et al.Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes.J Hosp Med.2008;3(5):361–368.
- ,,, et al.Use of an admission early warning score to predict patient morbidity and mortality and treatment success.Emerg Med J.2008;25(12):803–806.
- ,,.The impact of weekends on outcome for emergency patients.Clin Med.2005;5(6):621–625.
- ,,,,,.Off hour admission to an intensivist‐led ICU is not associated with increased mortality.Crit Care.2009;13(3):R84.
- ,,, et al.Three‐year results of mandated work hour restrictions: attending and resident perspectives and effects in a community hospital.Am Surg.2008;74(6):542–546; discussion 546–547.
- ,,, et al.Handoffs causing patient harm: a survey of medical and surgical house staff.Jt Comm J Qual Patient Saf.2008;34(10):563–570.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121(11):866–872.
- ,.Mortality among patients admitted to hospitals on weekends as compared with weekdays.N Engl J Med.2001;345(9):663–668.
- ,,, et al.Survival from in‐hospital cardiac arrest during nights and weekends.JAMA.2008;299(7):785–792.
- ,,,.Continuity of care and intensive care unit use at the end of life.Arch Intern Med.2009;169(1):81–86.
- ,,,,,.Continuity of outpatient and inpatient care by primary care physicians for hospitalized older adults.JAMA.2009;301(16):1671–1680.
- ,,,.Why predictive indexes perform less well in validation studies: is it magic or methods?Arch Intern Med.1987;147:2155–2161.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45(6):613–619.
- ,,, et al.Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes.J Hosp Med.2008;3(5):361–368.
- ,,, et al.Use of an admission early warning score to predict patient morbidity and mortality and treatment success.Emerg Med J.2008;25(12):803–806.
- ,,.The impact of weekends on outcome for emergency patients.Clin Med.2005;5(6):621–625.
- ,,,,,.Off hour admission to an intensivist‐led ICU is not associated with increased mortality.Crit Care.2009;13(3):R84.
Copyright © 2010 Society of Hospital Medicine
Medication Reconciliation: A Consensus Statement From Stakeholders
Medication reconciliation is integral to reducing medication errors surrounding hospitalizations.1, 2 The practice of medication reconciliation requires a systematic and comprehensive review of all the medications a patient is currently taking to ensure that medications being added, changed, or discontinued are carefully evaluated with the goal of maintaining an accurate list; that this process is undertaken at every transition along the continuum of care; and that an accurate list of medications is available to the patient or family/caregiver and all providers involved in the patient's care, especially when a care handoff takes place. With regulators, payers and the public increasingly demanding action to reduce medication errors in hospitals, all health care providers must support efforts to achieve accurate medication reconciliation.1, 3
The Joint Commission's Definition of Medication
Any prescription medications, sample medications, herbal remedies, vitamins, nutraceuticals, vaccines, or over‐the‐counter drugs; diagnostic and contrast agents used on or administered to persons to diagnose, treat, or prevent disease or other abnormal conditions; radioactive medications, respiratory therapy treatments, parenteral nutrition, blood derivatives, and intravenous solutions (plain, with electrolytes and/or drugs); and any product designated by the Food and Drug Administration (FDA) as a drug. This definition of medication does not include enteral nutrition solutions (which are considered food products), oxygen, and other medical gases.
2010 Hospital Accreditation Standards,
The Joint Commission, 2010, p. GL19.
While conceptually straightforward, implementing medication reconciliation has proved to be very difficult in the myriad healthcare settings that exist. The disjointed nature of the American health care system and a conglomeration of paper and electronic systems for tracking medications synergize to thwart efforts to maintain an accurate, up‐to‐date medication list at every step along the care continuum. Although The Joint Commission defines medication for the purpose of its accreditation standards (see box), the healthcare community lacks a common understanding or agreement regarding what constitutes a medication. There is also confusion about who should ultimately be responsible for obtaining the patient's medication information, for performing the various steps in the reconciliation process, and for managing the multiple providers who alter the medication list but may not feel competent to perform reconciliation of medications outside their area of expertise safely. Importantly, there is also a lack of clarity around how patients and family/caregivers should be involved in the process.
Despite these challenges, medication reconciliation remains a critical patient safety activity that is supported by the organizations signing this consensus statement, (Table 1). Although medication reconciliation has an impact on medication safety in all care settings, this paper focuses on issues most germane to the continuum of care involving the hospital setting. The themes and issues discussed will likely apply to other care settings as well. In this paper, we also recommend several concrete steps that we believe should be initiated immediately to begin to reach the goal of optimizing the medication safety achievable through effective medication reconciliation.
Background
Medication reconciliation is intended to be a systematic extension of the medication history‐taking process that has been used by health care providers for decades. Its recent iteration was developed to ensure that medications were not added, omitted, or changed inadvertently during care transitions. It became codified, refined, and tested over the past decade through the efforts of a number of groups focused on medication safety including the Institute for Healthcare Improvement (IHI) and the Institute for Safe Medication Practices (ISMP). With the reinforcing adoption of medication reconciliation as National Patient Safety Goal (NPSG) No. 8 in 2005 by The Joint Commission, efforts to implement it became widespread in both hospital‐based and ambulatory settings.
Medication reconciliation has three steps, as described by IHI4:
-
Verification (collection of the patient's medication history);
-
Clarification (ensuring that the medications and doses are appropriate); and
-
Reconciliation (documentation of changes in the orders).
The details of the process vary by setting and by the availability of paper or electronic medical records. However, the essential steps remain the same, as does the need to perform reconciliation each time the patient transfers to a new setting or level of care. Table 2 lists the most common points at which medication reconciliation occurs in hospitalized patients.
|
| American Academy of Pediatrics |
| American Association of Critical‐Care Nurses |
| Consumers Advancing Patient Safety |
| Institute for Healthcare Improvement |
| Institute for Safe Medication Practices |
| The Joint Commission |
| Massachusetts Coalition for Prevention of Medical Errors |
| Microsoft Corporation |
| Northwestern Memorial Hospital and Northwestern University School of Medicine |
| Society of General Internal Medicine |
| Society of Hospital Medicine |
| University of California San Diego Medical Center |
Because of their complexity, organizations must take care to design their medication reconciliation processes systematically. IHI lists elements of a well‐designed medication reconciliation process as part of its 5 Million Lives Campaign How‐to Guide.4 Such a process:
-
Uses a patient centered approach.
-
Makes it easy to complete the process for all involved. Staff members recognize the what's‐in‐it‐for‐me aspect of the change.
-
Minimizes the opportunity for drug interactions and therapeutic duplications by making the patient's list of current medications available when clinicians prescribe new medications.
-
Provides the patient with an up‐to‐date list of medications.
-
Ensures that other providers who need to know have information about changes in a patient's medication plan.
Research on how adverse drug events (ADE) occur supports the need for tight control of medication orders at transitions in care. For instance:
-
In a study conducted at Mayo Health System in Wisconsin, poor communication of medical information at transition points was responsible for as many as 50% of all medication errors in the hospital and up to 20% of ADEs.5
-
Variances between the medications patients were taking prior to admission and their admission orders ranged from 30% to 70% in 2 literature reviews.1, 6
-
The largest study of medication reconciliation errors and risk factors at hospital admission documented that 36% of patients had errors in their admission orders.7
When The Joint Commission adopted medication reconciliation as NPSG No. 8 in 2005 it had 2 parts: Requirement 8Aa process must exist for comparing the patient's current medications with those ordered for the patient while under the care of the organization; and requirement 8Ba complete list of the patient's medications must be communicated to the next provider of service on transfer within or outside the organization and a complete list of medications must be provided to the patient on discharge.8
However, many hospitals found it difficult to implement medication reconciliation in a systematic way. There was also confusion among hospital staff and administration about the exact definition of medication reconciliation in terms of what it should entail.9 Given these difficulties, The Joint Commission announced that effective January 1, 2009, medication reconciliation would no longer be factored into an organization's accreditation decision or be considered for Requirements for Improvement. Additionally, The Joint Commission stated it is reviewing and revising the NPSG so that it will be ready to be released in January 2011 for implementation later that year.10
Recognizing the difficulty hospitals were having with meaningfully implementing medication reconciliation, the Society of Hospital Medicine convened a 1‐day conference on March 6, 2009, to obtain input from key stakeholders and focus on several critical domains relevant to the success of hospital‐based medication reconciliation. The Agency for Healthcare Research and Quality provided funding support for this conference through grant 1R13HS017520‐01.
An overarching theme emerged from the meeting: the need to reorient the focus of medication reconciliation away from that of an accreditation mandate and toward a broader view of patient safety. Forcing medication reconciliation via a requirement for accreditation tended to limit an organization's efforts to specific process measures. Addressing it as a more global patient safety issue takes into account the entire patient care experience and then opens the door to leverage nonclinical venues (e.g., medical home, family home, community, religious, and other social organizations, as well as social networking platforms) and engage the patient and family/caregivers to reinforce the importance of medication safety.
This white paper evolved from discussions at the March 2009 conference,11 and subsequent structured communication among attendees. Formal endorsement of this document was obtained from the organizations listed in Table 1. In this document, we explore several key issues in implementing clinically meaningful and patient‐centered medication reconciliation. We focus on building common language and understanding of the processes of and participants in medication reconciliation; consider issues of implementation and risk stratification; emphasize the need for research to identify best practices and discusses how to disseminate the findings; promote health information technology platforms that will support interoperable medication information exchange; support the formation of partnerships between patient care sites and nonclinical sites as well as utilizing social marketing opportunities to enhance opportunities for transmitting messages about medication safety; and reinforce the ongoing healthcare reform discussion which aims to align financial incentives with patient safety efforts. After each section, we offer concrete first steps to address the issues discussed.
| Admission: When clinicians reconcile the patient's medications taken at home or at a prior care setting with any new prescription orders to be prescribed by an admitting clinician. |
| Transfer (intra‐ or inter‐facility; with change of clinician or site of care): When clinicians review previous medication orders in light of the patient's clinical status, along with new orders or plans of care. |
| Discharge: When clinicians review all medications the patient was taking prior to being hospitalized, incorporating new prescriptions from the hospitalization and determining whether any medication should be added, discontinued, or modified while being mindful of therapeutic interchanges needed for formulary purposes. |
Methods
The invitation‐only meeting held on the Northwestern Medical Campus in Chicago, IL, brought together stakeholders representing professional, clinical, health care quality, consumer, and regulatory organizations (Table 3). The conference convened these participants with the goals of identifying barriers to meaningful implementation of medication reconciliation and developing a feasible plan toward its effective implementation in the hospital setting. At the meeting, all participants were divided into 1 of 4 groups, which held a facilitated discussion around 1 of 4 key relevant domains: (1) how to measure success in medication reconciliation; (2) key elements of successful strategies; (3) leveraging partnerships outside the hospital setting to support medication reconciliation; and (4) the roles of the patient and family/caregivers and health literacy. Individual group discussions were cofacilitated by experts in the content area. After each discussion, the small group then rotated to a different discussion. Ultimately, each group participated in all four discussions, which built iteratively on the content derived from the prior groups' insights. Key comments were then shared with the large group for further discussion. To help build consensus, these large group discussions were directed by professional facilitators.
| AACN American Association of Critical Care Nurses |
| AAFP American Academy of Family Physicians |
| AAP American Academy of Pediatrics |
| ACEP American College of Emergency Physicians |
| ACP American College of Physicians |
| AMA American Medical Association |
| AMSN Academy of Medical Surgical Nurses |
| ASHP American Society of Health‐System Pharmacists |
| ASHP Foundation American Society of Health‐System Pharmacists Foundation |
| CAPS Consumers Advancing Patient Safety |
| CMS Centers for Medicare and Medicaid Services |
| CMSA Case Management Society of America |
| HCI Hospitalist Consultants, Inc |
| IHI Institute for Healthcare Improvement |
| InCompass Health |
| ISMP Institute For Safe Medication Practice |
| JCR Joint Commission Resources |
| Massachusetts Coalition for Prevention of Medical Errors |
| Microsoft Corporation |
| Northwestern Memorial Hospital MATCH Program |
| NQF National Quality Forum |
| SGIM Society of General Internal Medicine |
| SHM Society of Hospital Medicine |
| The Joint Commission |
| UCSD Hospital Medicine |
| University of Oklahoma College of Pharmacy Tulsa |
After the meeting, attendees participated in 2 follow‐up conference calls to discuss issues raised at the conference and responses obtained from host organizations. They also subsequently participated in two focus groups with The Joint Commission, giving input on the revision of the medication reconciliation NPSG.
Results
Addressing Barriers to Medication Reconciliation
In order to implement successful medication reconciliation processes, one must build the steps with the patient and family/caregiver as the focus and demonstrate an understanding of the intent of these processes. At its roots, medication reconciliation was developed to ensure that clinicians do not inadvertently add, change, or omit medications and that changes made are communicated to all relevant caregivers.
A number of key issues with respect to successful medication reconciliation processes surfaced in discussions with stakeholders. We believe addressing these issues is necessary before meaningful and standardized implementation can be achieved. After each discussion below, we provide suggested first steps to address these issues.
1. Achieve Consensus on the Definition of Medication and Reconciliation
Despite proposed definitions of these terms by various organizations, there was little agreement about them in the healthcare community. This ambiguity contributed to general confusion about what actually constitutes medication reconciliation. There needs to be a single, clear, and broadly accepted definition of what constitutes a medication. For the purposes of medication reconciliation, the term medication should be broadly inclusive of substances that may have an impact on the patient's care and treatments as well as those substances that may interact with other therapies potentially used during the medical care episode. Illicit or recreational substances may also have impact on therapies considered and therefore may influence this definition.12 Concretely, this definition should encompass prescription and over‐the‐counter medications as well as herbal and dietary supplements.
The term reconciliation in its simplest form implies the process of verifying that a patient's current list of medications (including dose, route, and frequency) are correct and that the medications are currently medically necessary and safe. Reconciliation suggests a process which, by necessity, will vary based on clinical context and setting. Further defining this termand the process of reconciliation itselfshould be carried out using patient safety principles with a focus on patient‐ and family‐centeredness.
Designing hospital‐based medication reconciliation processes should:
-
Employ a multidisciplinary approach that involves nurses, pharmacists, and other appropriate personnel from the inpatient setting as well as ambulatory and community/retail areas, both ambulatory and inpatient physicians, and a patient/family representative;
-
Involve hospital leaders who support, provide guidance, and remove barriers for the multidisciplinary team working to implement the processes;
-
Clearly define the roles of each participant in the processes developed;
-
Include methods to assess and address any special needs due to the developmental stage, age, dependency, language or literacy levels of patients and their family/caregiver;
-
Use clinically relevant process measures (e.g., adherence to procedural steps) and outcome measures (e.g., change in the number of ADEs, unnecessary hospitalizations, or emergency department visits) where appropriate to assess the impact of the process;
-
Include feedback systems to allow for clinically significant process improvement.
Once a common understanding of the terms and intent of medication reconciliation is achieved, it will be important for accrediting organizations, medical societies, quality improvement organizations, and other interested parties to adopt the same language.
First Step
A consortium of clinical, quality, and regulatory stakeholders should work to achieve consensus on the definition for medication and the intent and expectations for the reconciliation process.
2. Clarify Roles and Responsibilities
Given the differences in organizational and practice structures in hospitals and the varying numbers of health professionals involved in a patient's care, no one process design will meet the needs of all sites. As it is clear that interdisciplinary teams are best suited to develop, implement, and carry out complex patient‐centered processes like medication reconciliation, it is crucial that all involved parties have clearly defined roles and responsibilities, including patients and their families/caregivers. It is also important to recognize that these responsibilities may change depending on the dependency or vulnerability of the patient (e.g., children or geriatric patients) or the transition of care being undertaken by the patient (i.e., admission, transfer, or discharge), thus requiring sites to develop clear policies about these roles and responsibilities and how they may change in various situations.
First Step
Individual sites must clearly define the roles and responsibilities of all parties directly involved in medication reconciliation as a part of designing local medication reconciliation processes.
3. Develop Measurement Tools
Ensuring that medication reconciliation processes result in clinically meaningful outcomes requires the development and standardization of a limited number of metrics that may be used by organizations and reported centrally for benchmarking. This core set of measures should be developed by clinical, quality, accreditation, and regulatory organizations (see #10 below) through a consensus building process utilizing multi‐stakeholder input. The set should be supplemented by additional site‐specific measures determined locally that focus on steps in the process itself and allow sites to perform continuous quality improvement. Sites should be encouraged to develop tools locally to support and facilitate organizational and professional adherence to medication reconciliation processes.
First Steps
Clinical, quality, accreditation, and regulatory organizations should develop reliable metrics to be assessed and reported.
The principles of patient‐centeredness and family/caregiver‐centeredness, the medical home, and clinical relevance must be central to the metrics chosen for quality and regulatory purposes.
4. Phased Implementation
Ultimately, comprehensive medication reconciliation processes need to be implemented in hospitals. However, to succeed in integrating complex processes like medication reconciliation into routine hospital practices, implementation may be facilitated by using a phased approach to allow for participants to adapt new processes and procedures to the local environment iteratively. While the most appropriate phased approach to implementation will vary by site and setting, options for phasing might include:
-
Starting with one clinical area or service.
-
Starting with either the admission or discharge reconciliation process.
-
Starting with a patient population at high risk for adverse events.
Irrespective of the phasing strategy employed, development of a clear and pragmatic schedule for the entire implementation process should be established. Phasing decisions should be made based on organizational resources and the clinical needs of the patient population within each clinical setting. As noted, the ultimate goal is to develop comprehensive reconciliation processes occurring during all significant care transitions (i.e., admission, service or site‐of‐care transfers, and discharge) for all hospitalized patients and involving all of their medications. Flexibility in design should be encouraged to ensure the processes can work within local workflow as long as progress toward this primary goal is made.
First Steps
Clinical sites should establish local, pragmatic priorities for a phased approach to implementation.
Tie the phased approach to a timeline or blueprint for programmatic expansion with ultimate plans for comprehensive implementation.
5. Develop Risk Stratification Systems
Medication‐related adverse events related to inadequate reconciliation are more likely to occur in hospitalized patients with certain identifiable risk factors. For example, the MATCH study documented that polypharmacy and age over 65 years were independently associated with increased risk for errors at the time of hospital admission.7 Other factors that may increase the likelihood of medication‐related adverse events at care transitions in the hospital might include: patients with multiple providers, developmental/cognitive impairment, dependency/vulnerability, multiple or high‐risk medications, or poor health literacy or limited English proficiency. Research is needed to elucidate these risk factors further.
An alert system for key risk factors for complications related to incompletely, inappropriately, or inaccurately completed medication reconciliation due to patient, clinician, or system factors should be developed, tested, and broadly implemented. Additionally, an alert system would help maintain vigilance toward this patient safety issue and, potentially, help focus additional resources on high‐risk patients. Such a tool has been tested in ambulatory settings.15
First Step
Additional research on inpatient predictors of failed medication reconciliation and ADE should be prioritized (see #6 below).
6. Study Interventions and Processes
Despite having been an NPSG since 2005, there is still a relative paucity of literature about broadly applicable and effective implementation strategies and demonstrated interventions that improve medication safety related to medication reconciliation. Some strategies that have shown to reduce medication errors at transitions include the involvement of pharmacist medication review on discharge16, 17 and the usefulness of planning by multidisciplinary groups.18 Other studies have outlined the continuing barriers to successful implementation of reconciliation, including the difficulty patients have in accurately recalling their current medications19 and the high cost in nurse and pharmacist time of tracking down a patient's ongoing prescriptions.20, 21 Studies evaluating potential solutions to overcome these and other common barriers are still needed.
Future research should focus on a comprehensive review of implementation strategies, (specifically including the role of health information technology‐based innovations) clinically relevant outcomes, and best practices, while being sensitive to the different needs of varying care settings (e.g., pediatric vs. adult centers, emergency departments vs. inpatient units, community hospital vs. academic medical center, etc.) as well as the resource requirements engendered in the interventions.
First Step
Funding agencies should explicitly prioritize outcomes‐focused medication reconciliation‐related projects (e.g., those which demonstrate a reduction in postdischarge ADE or reduced medication‐related emergency department visits). Previously identified successful strategies should be further investigated. Funded projects should explicitly partner with patients and family/caregivers and also include pediatric and adult patients, rural and urban locations of care, as well as academic and nonacademic hospital settings, to promote more broadly applicable results.
7. Disseminate Success
Best practices and lessons learned, especially those rigorously tested and driven by data, stratified by patient type, care setting (emergency department, intensive care, surgical ward, etc.) and institutional type (community, teaching, safety net, critical access, etc.) need to be disseminated so others can adopt and adapt them effectively. High‐quality case studies with clear explanations of successes, failures, and lessons learned may prove valuable sources of information. This knowledge should foster a learning community approach and accelerate implementation at new sites.
First Step
Hospitals, healthcare systems, as well as quality and regulatory agencies should develop mechanisms within reporting systems to track performance, identify notably successful sites, and publicly report and share methods and lessons learned from them.
8. Promote the Personal Health Record
A fully integrated and transferable personal health record should be accepted as the standard for health information storage and interoperability, giving both the patient (or family/caregiver) and clinical providers access and ownership. Both the HL7 Continuity of Care Document (CCD) and the Continuity of Care Record (CCR) meet these criteria. The CCR was endorsed by the American Society for Testing and Materials22 and a coalition of other medical societies.23 Notably, CCR and CCD were recently adopted as standards for structured electronic health record (EHR) exchange through the July 2010 publication of the Final Rule of the Health Information Technology for Economic and Clinical Health Act provision of the American Recovery and Reinvestment Act of 2009 (ARRA/HITECH) and is now part of the formal US Department of Health and Human Services certification criteria for EHR technologies.24
Mandating a content exchange standard such as the CCR or the CCD should also have the desired effect of ensuring that patients (and their caregivers) become increasingly involved in maintaining an accurate list of the medications they take. Additionally, systems must be sufficiently flexible to address the unique medication management needs of children and geriatric patients. An electronic version of a personal health record is a promising method for improving consistency across care platforms, but to be implemented effectively the record must be compatible across all settings, including, where possible, the patient's home. All health care organizations, pharmacy systems, and insurers, must make medication reconciliation‐related interoperability and accessibility a priority as they pursue information technology strategies.
First Step
Stakeholder organizations must send a clear and convincing message to legislators under the current atmosphere of health care reform, urging them to mandate that health information technology standards include interoperability and support platforms that are consistent with standards put forth in the 2009 HITECH Act Interim Final Rule for EHR certification.
9. Promote Partnerships
At a broader health care system level, leveraging existing partnerships and creating new ones among health care, public/private sector‐affiliated organizations (e.g., community and mail order pharmacies, pharmaceutical organizations and manufacturers, and insurers), and public health organizations are extremely important mechanisms for broader scale impact. This view recognizes the numerous opportunities to educate and influence patients about medication safety outside the dyadic relationship of the clinician and patient in traditional clinical settings. Partnerships between health care and public entities may capitalize on these opportunities to foster adoption of healthy medication practices (e.g., maintaining an accurate and updated medication list), thereby supporting medication reconciliation efforts when individuals encounter health care settings. Partnership and information sharing could be enhanced through the use of a central coordinating body or coalition. This body could generate a shared common vision and contribute expertise to the myriad issues in medication reconciliation.
Partnerships should utilize the following:
-
Social marketing techniques to engage the community. Included within this strategy must be a clear and compelling message that transmits the importance of safe medication practices. Current messages such as keep a list while important, do not offer enough of a sense of urgency or importance. A more powerful message could involve highly publicized medication errors or close calls that would resonate with a broad audience.
-
Local and national champions. Such individuals should be trusted for their health knowledge (e.g., television health care reporters) or be prominent, influential, and trusted figures in other circles (e.g., clergy, politicians, movie celebrities). Indeed, taking advantage of popular media by weaving a theme into a movie or television program about medication safety may prove effective.
Relevant partnerships would include:
-
Quality organizations partnering with other stakeholders to establish unambiguous and unified medication reconciliation standards across the care continuum.
-
Health systems partnering with community pharmacy providers to ensure an uninterrupted communication link in both the inpatient and outpatient settings.
-
Manufacturers and distributors of medications partnering with health care and public health organizations, the media, insurers and other constituents to promote the importance of maintaining and sharing an accurate list of medications.
-
Public health systems partnering with community‐based organizations to encourage and promote the established standards for medication safety through messaging and educational campaigns.
All partnerships must consider issues of patient language and literacy as well as the needs of vulnerable populations in the scope of their activities.
First Step
Public health agencies should partner with health care quality organizations and others to begin a national public campaign to increase the awareness of medication safety (the broader public health concept under which medication reconciliation would fall) and support the importance of the patient's role in maintaining an updated medication list at all times.
10. Align Financial Incentives With Newly Developed Regulatory and Accreditation Requirements
Implementing and performing medication reconciliation takes time, particularly at the outset of a new program. Time requirements and associated costs are major barriers to undertaking comprehensive medication reconciliation, despite its recognized importance for reducing avoidable injury to patients. At present, systems that impede efficiency and slow hospital throughput may be discouraged due to their potential for having an adverse impact on access, finances, and other aspects of care delivery. Moreover, the changed economic climate with reduced hospital fiscal margins limits resources for new initiatives. Currently, failed medication reconciliationand the related avoidable adverse events, culminating in readmission to the hospital or emergency departmentyields additional revenue for hospitals and other providers in some reimbursement models.
Alignment of financial incentives that ensured adequate time and resources for appropriate medication reconciliation processes would facilitate implementation. Additionally, start‐up funding to create and implement these processes needs to be made available.
One example illustrating efforts to align payment policy with medication safety efforts occurred when the Office of the National Coordinator (ONC), in publishing its Final Rule under the 2009 HITECH Act,24 endorsed the importance of financially supporting proper medication reconciliation, particularly at first encounter and transitions in care, by requiring EHR systems seeking certification under the rule to support the care team in the task of reconciliation. For example, vendors will have to support the ability to compare 2 or more medication lists electronically, create medication lists, drug allergy lists, perform drug formulary look‐ups, drug‐drug and drug‐allergy checks, and support creating patient summaries after each visit or post discharge that include medication lists. The ONC, in defining Meaningful Use for eligible health care organizations, included in that definition the goal of exchanging meaningful clinical information among the professional health care teams. This goal is demonstrated through organizations reporting that they performed medication reconciliation for at least 50% of transitions of care in which the patient is transitioned into the care of the eligible professional or admitted to the eligible hospital's or Critical Access Hospital's inpatient or emergency department. Organizations able to demonstrate this level of compliance, along with other Meaningful Use requirements, will be eligible to receive stimulus funds through 2015 and avoid financial penalties that begin after that period.
First Step
Future health care reform must address the misalignment of financial policies and structures, and provide financial incentives to support the development and implementation of better medication management systems and prevent avoidable rehospitalizations and emergency department visits resulting from medication‐related adverse events.
Conclusion
Medication reconciliation involves highly complex processes and is hampered by the disjointed nature of the American health care system. It is, however, a vital part of reducing ADE. If employed more broadly, it has the added benefits of enhancing communication among all providers of care and engaging patients and families/caregivers more consistently and meaningfully in their overall care.
Despite the difficulty of maintaining an accurate medication record in real time across disparate settings, reconciliation is a goal to which our organizations are committed. Given the wide range of healthcare organizations involved in providing medications to patients and the many agencies evaluating those efforts, we believed it would be helpful to provide an overarching set of goals to move medication reconciliation forward.
Our main message is this: Patient safety and patient/family‐centered care must be the principal drivers in the development and implementation of medication reconciliation systems. Ultimately this process is about ensuring that patients are receiving the most appropriate medications no matter where they are treated. With this document, we hope to bring to light the importance of creating and implementing a medication reconciliation program, addressing some barriers to success, and identifying potential solutions that will ensure utility and sustainability of this critical patient safety issue.
- ,,, et al.Unintended medication discrepancies at the time of hospital admission.Arch Intern Med.2005;165(4):424–429.
- .Prevention of medication errors in the pediatric inpatient setting. The American Academy of Pediatrics Policy Statement.Pediatrics.2003;112(2):431–436.
- ,,, et al.Medication reconciliation: a practical tool to reduce the risk of medication errors.J Crit Care.2003;18(4):201–205.
- Institute for Healthcare Improvement. 5 million lives getting started kit: preventing adverse drug events (medication reconciliation), how‐to guide. Available at: http://www.ihi.org/IHI/Programs/Campaign/ADEsMedReconciliation.htm. Published Oct. 1, 2008. Accessed September2010.
- ,.Medication safety: one organization's approach to the challenge.J Clin Outcomes Mana.2001;8(10):27–34.
- ,,,,,.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health Syst Pharm.2004;61(16):1689–1695.
- ,,, et al.Results of the Medications At Transitions and Clinical Handoffs (MATCH) Study: an analysis of medication reconciliation errors and risk factors at hospital admission.J Gen Intern Med.2010;25(5):441–447.
- Joint Commission on Accreditation of Healthcare Organizations.2005 Hospital Accreditation Standards, p.NPSG‐4.
- ,,,,.Brief communication: Results of a medication reconciliation survey from the 2006 Society of Hospital Medicine national meeting.J Hosp Med.2008;3(6):465–472.
- The Joint Commission.Approved: will not score medication reconciliation in 2009.Jt Comm Perspect.2009;29(3):1,3.
- Society of Hospital Medicine. Medication reconciliation: a team approach, conference summary. December 2009. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/QualityImprovement/QICurrentInitiativesandTrainingOpportunities/QI_Current_Initiativ.htm. Accessed September2010.
- The American Medical Association. The physician's role in medication reconciliation: issues, strategies and safety principles. 2007. Available at: http://www.ama‐assn.org/ama1/pub/upload/mm/370/med‐rec‐monograph.pdf. Accessed September2010.
- Institute of Safe Medication Practices. ISMP's list of high alert medications. 2008. Available at: http://www.ismp.org/Tools/highalertmedications.pdf. Accessed September2010.
- ,,,.Medication use leading to emergency department visits for adverse drug events in older adults.Ann Intern Med.2007;147(11):755–765
- ,,, et al.Experience with a trigger tool for identifying adverse drug events among older adults in ambulatory primary care.Qual Saf Health Care.2009;18(3):199–204.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166(5):565–571.
- ,,,,.Medication reconciliation at an academic medical center: implementation of a comprehensive program from admission to discharge.Am J Health Syst Pharm.2009;66(23):2126–2131.
- ,,,,,.Multidisciplinary approach to inpatient medication reconciliation in an academic setting.Am J Health Syst Pharm.2007;64(8):850–854.
- ,,.Lack of patient knowledge regarding hospital medications.J Hosp Med.2010;5(2):83–86.
- .The unexpected challenges of accurate medication reconciliation.Ann Emerg Med.2008;52(5):493–495.
- ,,,.Medication reconciliation in a rural trauma population.Ann Emerg Med.2008;52(5):483–491.
- ASTM International. ASTM E2369 ‐ 05e1 standard specification for continuity of care record (CCR). Available at: http://www.astm.org/Standards/E2369.htm. Accessed September2010.
- ,,.The continuity of care record.Am Fam Physician.2004;70(7):1220,1222–1223.
- Department of Health and Human Services. Health information technology: initial set of standards, implementation specifications, and certification criteria for electronic health record technology; final rule. Available at: http://edocket.access.gpo.gov/2010/pdf/2010–17210.pdf. Accessed September2010.
Medication reconciliation is integral to reducing medication errors surrounding hospitalizations.1, 2 The practice of medication reconciliation requires a systematic and comprehensive review of all the medications a patient is currently taking to ensure that medications being added, changed, or discontinued are carefully evaluated with the goal of maintaining an accurate list; that this process is undertaken at every transition along the continuum of care; and that an accurate list of medications is available to the patient or family/caregiver and all providers involved in the patient's care, especially when a care handoff takes place. With regulators, payers and the public increasingly demanding action to reduce medication errors in hospitals, all health care providers must support efforts to achieve accurate medication reconciliation.1, 3
The Joint Commission's Definition of Medication
Any prescription medications, sample medications, herbal remedies, vitamins, nutraceuticals, vaccines, or over‐the‐counter drugs; diagnostic and contrast agents used on or administered to persons to diagnose, treat, or prevent disease or other abnormal conditions; radioactive medications, respiratory therapy treatments, parenteral nutrition, blood derivatives, and intravenous solutions (plain, with electrolytes and/or drugs); and any product designated by the Food and Drug Administration (FDA) as a drug. This definition of medication does not include enteral nutrition solutions (which are considered food products), oxygen, and other medical gases.
2010 Hospital Accreditation Standards,
The Joint Commission, 2010, p. GL19.
While conceptually straightforward, implementing medication reconciliation has proved to be very difficult in the myriad healthcare settings that exist. The disjointed nature of the American health care system and a conglomeration of paper and electronic systems for tracking medications synergize to thwart efforts to maintain an accurate, up‐to‐date medication list at every step along the care continuum. Although The Joint Commission defines medication for the purpose of its accreditation standards (see box), the healthcare community lacks a common understanding or agreement regarding what constitutes a medication. There is also confusion about who should ultimately be responsible for obtaining the patient's medication information, for performing the various steps in the reconciliation process, and for managing the multiple providers who alter the medication list but may not feel competent to perform reconciliation of medications outside their area of expertise safely. Importantly, there is also a lack of clarity around how patients and family/caregivers should be involved in the process.
Despite these challenges, medication reconciliation remains a critical patient safety activity that is supported by the organizations signing this consensus statement, (Table 1). Although medication reconciliation has an impact on medication safety in all care settings, this paper focuses on issues most germane to the continuum of care involving the hospital setting. The themes and issues discussed will likely apply to other care settings as well. In this paper, we also recommend several concrete steps that we believe should be initiated immediately to begin to reach the goal of optimizing the medication safety achievable through effective medication reconciliation.
Background
Medication reconciliation is intended to be a systematic extension of the medication history‐taking process that has been used by health care providers for decades. Its recent iteration was developed to ensure that medications were not added, omitted, or changed inadvertently during care transitions. It became codified, refined, and tested over the past decade through the efforts of a number of groups focused on medication safety including the Institute for Healthcare Improvement (IHI) and the Institute for Safe Medication Practices (ISMP). With the reinforcing adoption of medication reconciliation as National Patient Safety Goal (NPSG) No. 8 in 2005 by The Joint Commission, efforts to implement it became widespread in both hospital‐based and ambulatory settings.
Medication reconciliation has three steps, as described by IHI4:
-
Verification (collection of the patient's medication history);
-
Clarification (ensuring that the medications and doses are appropriate); and
-
Reconciliation (documentation of changes in the orders).
The details of the process vary by setting and by the availability of paper or electronic medical records. However, the essential steps remain the same, as does the need to perform reconciliation each time the patient transfers to a new setting or level of care. Table 2 lists the most common points at which medication reconciliation occurs in hospitalized patients.
|
| American Academy of Pediatrics |
| American Association of Critical‐Care Nurses |
| Consumers Advancing Patient Safety |
| Institute for Healthcare Improvement |
| Institute for Safe Medication Practices |
| The Joint Commission |
| Massachusetts Coalition for Prevention of Medical Errors |
| Microsoft Corporation |
| Northwestern Memorial Hospital and Northwestern University School of Medicine |
| Society of General Internal Medicine |
| Society of Hospital Medicine |
| University of California San Diego Medical Center |
Because of their complexity, organizations must take care to design their medication reconciliation processes systematically. IHI lists elements of a well‐designed medication reconciliation process as part of its 5 Million Lives Campaign How‐to Guide.4 Such a process:
-
Uses a patient centered approach.
-
Makes it easy to complete the process for all involved. Staff members recognize the what's‐in‐it‐for‐me aspect of the change.
-
Minimizes the opportunity for drug interactions and therapeutic duplications by making the patient's list of current medications available when clinicians prescribe new medications.
-
Provides the patient with an up‐to‐date list of medications.
-
Ensures that other providers who need to know have information about changes in a patient's medication plan.
Research on how adverse drug events (ADE) occur supports the need for tight control of medication orders at transitions in care. For instance:
-
In a study conducted at Mayo Health System in Wisconsin, poor communication of medical information at transition points was responsible for as many as 50% of all medication errors in the hospital and up to 20% of ADEs.5
-
Variances between the medications patients were taking prior to admission and their admission orders ranged from 30% to 70% in 2 literature reviews.1, 6
-
The largest study of medication reconciliation errors and risk factors at hospital admission documented that 36% of patients had errors in their admission orders.7
When The Joint Commission adopted medication reconciliation as NPSG No. 8 in 2005 it had 2 parts: Requirement 8Aa process must exist for comparing the patient's current medications with those ordered for the patient while under the care of the organization; and requirement 8Ba complete list of the patient's medications must be communicated to the next provider of service on transfer within or outside the organization and a complete list of medications must be provided to the patient on discharge.8
However, many hospitals found it difficult to implement medication reconciliation in a systematic way. There was also confusion among hospital staff and administration about the exact definition of medication reconciliation in terms of what it should entail.9 Given these difficulties, The Joint Commission announced that effective January 1, 2009, medication reconciliation would no longer be factored into an organization's accreditation decision or be considered for Requirements for Improvement. Additionally, The Joint Commission stated it is reviewing and revising the NPSG so that it will be ready to be released in January 2011 for implementation later that year.10
Recognizing the difficulty hospitals were having with meaningfully implementing medication reconciliation, the Society of Hospital Medicine convened a 1‐day conference on March 6, 2009, to obtain input from key stakeholders and focus on several critical domains relevant to the success of hospital‐based medication reconciliation. The Agency for Healthcare Research and Quality provided funding support for this conference through grant 1R13HS017520‐01.
An overarching theme emerged from the meeting: the need to reorient the focus of medication reconciliation away from that of an accreditation mandate and toward a broader view of patient safety. Forcing medication reconciliation via a requirement for accreditation tended to limit an organization's efforts to specific process measures. Addressing it as a more global patient safety issue takes into account the entire patient care experience and then opens the door to leverage nonclinical venues (e.g., medical home, family home, community, religious, and other social organizations, as well as social networking platforms) and engage the patient and family/caregivers to reinforce the importance of medication safety.
This white paper evolved from discussions at the March 2009 conference,11 and subsequent structured communication among attendees. Formal endorsement of this document was obtained from the organizations listed in Table 1. In this document, we explore several key issues in implementing clinically meaningful and patient‐centered medication reconciliation. We focus on building common language and understanding of the processes of and participants in medication reconciliation; consider issues of implementation and risk stratification; emphasize the need for research to identify best practices and discusses how to disseminate the findings; promote health information technology platforms that will support interoperable medication information exchange; support the formation of partnerships between patient care sites and nonclinical sites as well as utilizing social marketing opportunities to enhance opportunities for transmitting messages about medication safety; and reinforce the ongoing healthcare reform discussion which aims to align financial incentives with patient safety efforts. After each section, we offer concrete first steps to address the issues discussed.
| Admission: When clinicians reconcile the patient's medications taken at home or at a prior care setting with any new prescription orders to be prescribed by an admitting clinician. |
| Transfer (intra‐ or inter‐facility; with change of clinician or site of care): When clinicians review previous medication orders in light of the patient's clinical status, along with new orders or plans of care. |
| Discharge: When clinicians review all medications the patient was taking prior to being hospitalized, incorporating new prescriptions from the hospitalization and determining whether any medication should be added, discontinued, or modified while being mindful of therapeutic interchanges needed for formulary purposes. |
Methods
The invitation‐only meeting held on the Northwestern Medical Campus in Chicago, IL, brought together stakeholders representing professional, clinical, health care quality, consumer, and regulatory organizations (Table 3). The conference convened these participants with the goals of identifying barriers to meaningful implementation of medication reconciliation and developing a feasible plan toward its effective implementation in the hospital setting. At the meeting, all participants were divided into 1 of 4 groups, which held a facilitated discussion around 1 of 4 key relevant domains: (1) how to measure success in medication reconciliation; (2) key elements of successful strategies; (3) leveraging partnerships outside the hospital setting to support medication reconciliation; and (4) the roles of the patient and family/caregivers and health literacy. Individual group discussions were cofacilitated by experts in the content area. After each discussion, the small group then rotated to a different discussion. Ultimately, each group participated in all four discussions, which built iteratively on the content derived from the prior groups' insights. Key comments were then shared with the large group for further discussion. To help build consensus, these large group discussions were directed by professional facilitators.
| AACN American Association of Critical Care Nurses |
| AAFP American Academy of Family Physicians |
| AAP American Academy of Pediatrics |
| ACEP American College of Emergency Physicians |
| ACP American College of Physicians |
| AMA American Medical Association |
| AMSN Academy of Medical Surgical Nurses |
| ASHP American Society of Health‐System Pharmacists |
| ASHP Foundation American Society of Health‐System Pharmacists Foundation |
| CAPS Consumers Advancing Patient Safety |
| CMS Centers for Medicare and Medicaid Services |
| CMSA Case Management Society of America |
| HCI Hospitalist Consultants, Inc |
| IHI Institute for Healthcare Improvement |
| InCompass Health |
| ISMP Institute For Safe Medication Practice |
| JCR Joint Commission Resources |
| Massachusetts Coalition for Prevention of Medical Errors |
| Microsoft Corporation |
| Northwestern Memorial Hospital MATCH Program |
| NQF National Quality Forum |
| SGIM Society of General Internal Medicine |
| SHM Society of Hospital Medicine |
| The Joint Commission |
| UCSD Hospital Medicine |
| University of Oklahoma College of Pharmacy Tulsa |
After the meeting, attendees participated in 2 follow‐up conference calls to discuss issues raised at the conference and responses obtained from host organizations. They also subsequently participated in two focus groups with The Joint Commission, giving input on the revision of the medication reconciliation NPSG.
Results
Addressing Barriers to Medication Reconciliation
In order to implement successful medication reconciliation processes, one must build the steps with the patient and family/caregiver as the focus and demonstrate an understanding of the intent of these processes. At its roots, medication reconciliation was developed to ensure that clinicians do not inadvertently add, change, or omit medications and that changes made are communicated to all relevant caregivers.
A number of key issues with respect to successful medication reconciliation processes surfaced in discussions with stakeholders. We believe addressing these issues is necessary before meaningful and standardized implementation can be achieved. After each discussion below, we provide suggested first steps to address these issues.
1. Achieve Consensus on the Definition of Medication and Reconciliation
Despite proposed definitions of these terms by various organizations, there was little agreement about them in the healthcare community. This ambiguity contributed to general confusion about what actually constitutes medication reconciliation. There needs to be a single, clear, and broadly accepted definition of what constitutes a medication. For the purposes of medication reconciliation, the term medication should be broadly inclusive of substances that may have an impact on the patient's care and treatments as well as those substances that may interact with other therapies potentially used during the medical care episode. Illicit or recreational substances may also have impact on therapies considered and therefore may influence this definition.12 Concretely, this definition should encompass prescription and over‐the‐counter medications as well as herbal and dietary supplements.
The term reconciliation in its simplest form implies the process of verifying that a patient's current list of medications (including dose, route, and frequency) are correct and that the medications are currently medically necessary and safe. Reconciliation suggests a process which, by necessity, will vary based on clinical context and setting. Further defining this termand the process of reconciliation itselfshould be carried out using patient safety principles with a focus on patient‐ and family‐centeredness.
Designing hospital‐based medication reconciliation processes should:
-
Employ a multidisciplinary approach that involves nurses, pharmacists, and other appropriate personnel from the inpatient setting as well as ambulatory and community/retail areas, both ambulatory and inpatient physicians, and a patient/family representative;
-
Involve hospital leaders who support, provide guidance, and remove barriers for the multidisciplinary team working to implement the processes;
-
Clearly define the roles of each participant in the processes developed;
-
Include methods to assess and address any special needs due to the developmental stage, age, dependency, language or literacy levels of patients and their family/caregiver;
-
Use clinically relevant process measures (e.g., adherence to procedural steps) and outcome measures (e.g., change in the number of ADEs, unnecessary hospitalizations, or emergency department visits) where appropriate to assess the impact of the process;
-
Include feedback systems to allow for clinically significant process improvement.
Once a common understanding of the terms and intent of medication reconciliation is achieved, it will be important for accrediting organizations, medical societies, quality improvement organizations, and other interested parties to adopt the same language.
First Step
A consortium of clinical, quality, and regulatory stakeholders should work to achieve consensus on the definition for medication and the intent and expectations for the reconciliation process.
2. Clarify Roles and Responsibilities
Given the differences in organizational and practice structures in hospitals and the varying numbers of health professionals involved in a patient's care, no one process design will meet the needs of all sites. As it is clear that interdisciplinary teams are best suited to develop, implement, and carry out complex patient‐centered processes like medication reconciliation, it is crucial that all involved parties have clearly defined roles and responsibilities, including patients and their families/caregivers. It is also important to recognize that these responsibilities may change depending on the dependency or vulnerability of the patient (e.g., children or geriatric patients) or the transition of care being undertaken by the patient (i.e., admission, transfer, or discharge), thus requiring sites to develop clear policies about these roles and responsibilities and how they may change in various situations.
First Step
Individual sites must clearly define the roles and responsibilities of all parties directly involved in medication reconciliation as a part of designing local medication reconciliation processes.
3. Develop Measurement Tools
Ensuring that medication reconciliation processes result in clinically meaningful outcomes requires the development and standardization of a limited number of metrics that may be used by organizations and reported centrally for benchmarking. This core set of measures should be developed by clinical, quality, accreditation, and regulatory organizations (see #10 below) through a consensus building process utilizing multi‐stakeholder input. The set should be supplemented by additional site‐specific measures determined locally that focus on steps in the process itself and allow sites to perform continuous quality improvement. Sites should be encouraged to develop tools locally to support and facilitate organizational and professional adherence to medication reconciliation processes.
First Steps
Clinical, quality, accreditation, and regulatory organizations should develop reliable metrics to be assessed and reported.
The principles of patient‐centeredness and family/caregiver‐centeredness, the medical home, and clinical relevance must be central to the metrics chosen for quality and regulatory purposes.
4. Phased Implementation
Ultimately, comprehensive medication reconciliation processes need to be implemented in hospitals. However, to succeed in integrating complex processes like medication reconciliation into routine hospital practices, implementation may be facilitated by using a phased approach to allow for participants to adapt new processes and procedures to the local environment iteratively. While the most appropriate phased approach to implementation will vary by site and setting, options for phasing might include:
-
Starting with one clinical area or service.
-
Starting with either the admission or discharge reconciliation process.
-
Starting with a patient population at high risk for adverse events.
Irrespective of the phasing strategy employed, development of a clear and pragmatic schedule for the entire implementation process should be established. Phasing decisions should be made based on organizational resources and the clinical needs of the patient population within each clinical setting. As noted, the ultimate goal is to develop comprehensive reconciliation processes occurring during all significant care transitions (i.e., admission, service or site‐of‐care transfers, and discharge) for all hospitalized patients and involving all of their medications. Flexibility in design should be encouraged to ensure the processes can work within local workflow as long as progress toward this primary goal is made.
First Steps
Clinical sites should establish local, pragmatic priorities for a phased approach to implementation.
Tie the phased approach to a timeline or blueprint for programmatic expansion with ultimate plans for comprehensive implementation.
5. Develop Risk Stratification Systems
Medication‐related adverse events related to inadequate reconciliation are more likely to occur in hospitalized patients with certain identifiable risk factors. For example, the MATCH study documented that polypharmacy and age over 65 years were independently associated with increased risk for errors at the time of hospital admission.7 Other factors that may increase the likelihood of medication‐related adverse events at care transitions in the hospital might include: patients with multiple providers, developmental/cognitive impairment, dependency/vulnerability, multiple or high‐risk medications, or poor health literacy or limited English proficiency. Research is needed to elucidate these risk factors further.
An alert system for key risk factors for complications related to incompletely, inappropriately, or inaccurately completed medication reconciliation due to patient, clinician, or system factors should be developed, tested, and broadly implemented. Additionally, an alert system would help maintain vigilance toward this patient safety issue and, potentially, help focus additional resources on high‐risk patients. Such a tool has been tested in ambulatory settings.15
First Step
Additional research on inpatient predictors of failed medication reconciliation and ADE should be prioritized (see #6 below).
6. Study Interventions and Processes
Despite having been an NPSG since 2005, there is still a relative paucity of literature about broadly applicable and effective implementation strategies and demonstrated interventions that improve medication safety related to medication reconciliation. Some strategies that have shown to reduce medication errors at transitions include the involvement of pharmacist medication review on discharge16, 17 and the usefulness of planning by multidisciplinary groups.18 Other studies have outlined the continuing barriers to successful implementation of reconciliation, including the difficulty patients have in accurately recalling their current medications19 and the high cost in nurse and pharmacist time of tracking down a patient's ongoing prescriptions.20, 21 Studies evaluating potential solutions to overcome these and other common barriers are still needed.
Future research should focus on a comprehensive review of implementation strategies, (specifically including the role of health information technology‐based innovations) clinically relevant outcomes, and best practices, while being sensitive to the different needs of varying care settings (e.g., pediatric vs. adult centers, emergency departments vs. inpatient units, community hospital vs. academic medical center, etc.) as well as the resource requirements engendered in the interventions.
First Step
Funding agencies should explicitly prioritize outcomes‐focused medication reconciliation‐related projects (e.g., those which demonstrate a reduction in postdischarge ADE or reduced medication‐related emergency department visits). Previously identified successful strategies should be further investigated. Funded projects should explicitly partner with patients and family/caregivers and also include pediatric and adult patients, rural and urban locations of care, as well as academic and nonacademic hospital settings, to promote more broadly applicable results.
7. Disseminate Success
Best practices and lessons learned, especially those rigorously tested and driven by data, stratified by patient type, care setting (emergency department, intensive care, surgical ward, etc.) and institutional type (community, teaching, safety net, critical access, etc.) need to be disseminated so others can adopt and adapt them effectively. High‐quality case studies with clear explanations of successes, failures, and lessons learned may prove valuable sources of information. This knowledge should foster a learning community approach and accelerate implementation at new sites.
First Step
Hospitals, healthcare systems, as well as quality and regulatory agencies should develop mechanisms within reporting systems to track performance, identify notably successful sites, and publicly report and share methods and lessons learned from them.
8. Promote the Personal Health Record
A fully integrated and transferable personal health record should be accepted as the standard for health information storage and interoperability, giving both the patient (or family/caregiver) and clinical providers access and ownership. Both the HL7 Continuity of Care Document (CCD) and the Continuity of Care Record (CCR) meet these criteria. The CCR was endorsed by the American Society for Testing and Materials22 and a coalition of other medical societies.23 Notably, CCR and CCD were recently adopted as standards for structured electronic health record (EHR) exchange through the July 2010 publication of the Final Rule of the Health Information Technology for Economic and Clinical Health Act provision of the American Recovery and Reinvestment Act of 2009 (ARRA/HITECH) and is now part of the formal US Department of Health and Human Services certification criteria for EHR technologies.24
Mandating a content exchange standard such as the CCR or the CCD should also have the desired effect of ensuring that patients (and their caregivers) become increasingly involved in maintaining an accurate list of the medications they take. Additionally, systems must be sufficiently flexible to address the unique medication management needs of children and geriatric patients. An electronic version of a personal health record is a promising method for improving consistency across care platforms, but to be implemented effectively the record must be compatible across all settings, including, where possible, the patient's home. All health care organizations, pharmacy systems, and insurers, must make medication reconciliation‐related interoperability and accessibility a priority as they pursue information technology strategies.
First Step
Stakeholder organizations must send a clear and convincing message to legislators under the current atmosphere of health care reform, urging them to mandate that health information technology standards include interoperability and support platforms that are consistent with standards put forth in the 2009 HITECH Act Interim Final Rule for EHR certification.
9. Promote Partnerships
At a broader health care system level, leveraging existing partnerships and creating new ones among health care, public/private sector‐affiliated organizations (e.g., community and mail order pharmacies, pharmaceutical organizations and manufacturers, and insurers), and public health organizations are extremely important mechanisms for broader scale impact. This view recognizes the numerous opportunities to educate and influence patients about medication safety outside the dyadic relationship of the clinician and patient in traditional clinical settings. Partnerships between health care and public entities may capitalize on these opportunities to foster adoption of healthy medication practices (e.g., maintaining an accurate and updated medication list), thereby supporting medication reconciliation efforts when individuals encounter health care settings. Partnership and information sharing could be enhanced through the use of a central coordinating body or coalition. This body could generate a shared common vision and contribute expertise to the myriad issues in medication reconciliation.
Partnerships should utilize the following:
-
Social marketing techniques to engage the community. Included within this strategy must be a clear and compelling message that transmits the importance of safe medication practices. Current messages such as keep a list while important, do not offer enough of a sense of urgency or importance. A more powerful message could involve highly publicized medication errors or close calls that would resonate with a broad audience.
-
Local and national champions. Such individuals should be trusted for their health knowledge (e.g., television health care reporters) or be prominent, influential, and trusted figures in other circles (e.g., clergy, politicians, movie celebrities). Indeed, taking advantage of popular media by weaving a theme into a movie or television program about medication safety may prove effective.
Relevant partnerships would include:
-
Quality organizations partnering with other stakeholders to establish unambiguous and unified medication reconciliation standards across the care continuum.
-
Health systems partnering with community pharmacy providers to ensure an uninterrupted communication link in both the inpatient and outpatient settings.
-
Manufacturers and distributors of medications partnering with health care and public health organizations, the media, insurers and other constituents to promote the importance of maintaining and sharing an accurate list of medications.
-
Public health systems partnering with community‐based organizations to encourage and promote the established standards for medication safety through messaging and educational campaigns.
All partnerships must consider issues of patient language and literacy as well as the needs of vulnerable populations in the scope of their activities.
First Step
Public health agencies should partner with health care quality organizations and others to begin a national public campaign to increase the awareness of medication safety (the broader public health concept under which medication reconciliation would fall) and support the importance of the patient's role in maintaining an updated medication list at all times.
10. Align Financial Incentives With Newly Developed Regulatory and Accreditation Requirements
Implementing and performing medication reconciliation takes time, particularly at the outset of a new program. Time requirements and associated costs are major barriers to undertaking comprehensive medication reconciliation, despite its recognized importance for reducing avoidable injury to patients. At present, systems that impede efficiency and slow hospital throughput may be discouraged due to their potential for having an adverse impact on access, finances, and other aspects of care delivery. Moreover, the changed economic climate with reduced hospital fiscal margins limits resources for new initiatives. Currently, failed medication reconciliationand the related avoidable adverse events, culminating in readmission to the hospital or emergency departmentyields additional revenue for hospitals and other providers in some reimbursement models.
Alignment of financial incentives that ensured adequate time and resources for appropriate medication reconciliation processes would facilitate implementation. Additionally, start‐up funding to create and implement these processes needs to be made available.
One example illustrating efforts to align payment policy with medication safety efforts occurred when the Office of the National Coordinator (ONC), in publishing its Final Rule under the 2009 HITECH Act,24 endorsed the importance of financially supporting proper medication reconciliation, particularly at first encounter and transitions in care, by requiring EHR systems seeking certification under the rule to support the care team in the task of reconciliation. For example, vendors will have to support the ability to compare 2 or more medication lists electronically, create medication lists, drug allergy lists, perform drug formulary look‐ups, drug‐drug and drug‐allergy checks, and support creating patient summaries after each visit or post discharge that include medication lists. The ONC, in defining Meaningful Use for eligible health care organizations, included in that definition the goal of exchanging meaningful clinical information among the professional health care teams. This goal is demonstrated through organizations reporting that they performed medication reconciliation for at least 50% of transitions of care in which the patient is transitioned into the care of the eligible professional or admitted to the eligible hospital's or Critical Access Hospital's inpatient or emergency department. Organizations able to demonstrate this level of compliance, along with other Meaningful Use requirements, will be eligible to receive stimulus funds through 2015 and avoid financial penalties that begin after that period.
First Step
Future health care reform must address the misalignment of financial policies and structures, and provide financial incentives to support the development and implementation of better medication management systems and prevent avoidable rehospitalizations and emergency department visits resulting from medication‐related adverse events.
Conclusion
Medication reconciliation involves highly complex processes and is hampered by the disjointed nature of the American health care system. It is, however, a vital part of reducing ADE. If employed more broadly, it has the added benefits of enhancing communication among all providers of care and engaging patients and families/caregivers more consistently and meaningfully in their overall care.
Despite the difficulty of maintaining an accurate medication record in real time across disparate settings, reconciliation is a goal to which our organizations are committed. Given the wide range of healthcare organizations involved in providing medications to patients and the many agencies evaluating those efforts, we believed it would be helpful to provide an overarching set of goals to move medication reconciliation forward.
Our main message is this: Patient safety and patient/family‐centered care must be the principal drivers in the development and implementation of medication reconciliation systems. Ultimately this process is about ensuring that patients are receiving the most appropriate medications no matter where they are treated. With this document, we hope to bring to light the importance of creating and implementing a medication reconciliation program, addressing some barriers to success, and identifying potential solutions that will ensure utility and sustainability of this critical patient safety issue.
Medication reconciliation is integral to reducing medication errors surrounding hospitalizations.1, 2 The practice of medication reconciliation requires a systematic and comprehensive review of all the medications a patient is currently taking to ensure that medications being added, changed, or discontinued are carefully evaluated with the goal of maintaining an accurate list; that this process is undertaken at every transition along the continuum of care; and that an accurate list of medications is available to the patient or family/caregiver and all providers involved in the patient's care, especially when a care handoff takes place. With regulators, payers and the public increasingly demanding action to reduce medication errors in hospitals, all health care providers must support efforts to achieve accurate medication reconciliation.1, 3
The Joint Commission's Definition of Medication
Any prescription medications, sample medications, herbal remedies, vitamins, nutraceuticals, vaccines, or over‐the‐counter drugs; diagnostic and contrast agents used on or administered to persons to diagnose, treat, or prevent disease or other abnormal conditions; radioactive medications, respiratory therapy treatments, parenteral nutrition, blood derivatives, and intravenous solutions (plain, with electrolytes and/or drugs); and any product designated by the Food and Drug Administration (FDA) as a drug. This definition of medication does not include enteral nutrition solutions (which are considered food products), oxygen, and other medical gases.
2010 Hospital Accreditation Standards,
The Joint Commission, 2010, p. GL19.
While conceptually straightforward, implementing medication reconciliation has proved to be very difficult in the myriad healthcare settings that exist. The disjointed nature of the American health care system and a conglomeration of paper and electronic systems for tracking medications synergize to thwart efforts to maintain an accurate, up‐to‐date medication list at every step along the care continuum. Although The Joint Commission defines medication for the purpose of its accreditation standards (see box), the healthcare community lacks a common understanding or agreement regarding what constitutes a medication. There is also confusion about who should ultimately be responsible for obtaining the patient's medication information, for performing the various steps in the reconciliation process, and for managing the multiple providers who alter the medication list but may not feel competent to perform reconciliation of medications outside their area of expertise safely. Importantly, there is also a lack of clarity around how patients and family/caregivers should be involved in the process.
Despite these challenges, medication reconciliation remains a critical patient safety activity that is supported by the organizations signing this consensus statement, (Table 1). Although medication reconciliation has an impact on medication safety in all care settings, this paper focuses on issues most germane to the continuum of care involving the hospital setting. The themes and issues discussed will likely apply to other care settings as well. In this paper, we also recommend several concrete steps that we believe should be initiated immediately to begin to reach the goal of optimizing the medication safety achievable through effective medication reconciliation.
Background
Medication reconciliation is intended to be a systematic extension of the medication history‐taking process that has been used by health care providers for decades. Its recent iteration was developed to ensure that medications were not added, omitted, or changed inadvertently during care transitions. It became codified, refined, and tested over the past decade through the efforts of a number of groups focused on medication safety including the Institute for Healthcare Improvement (IHI) and the Institute for Safe Medication Practices (ISMP). With the reinforcing adoption of medication reconciliation as National Patient Safety Goal (NPSG) No. 8 in 2005 by The Joint Commission, efforts to implement it became widespread in both hospital‐based and ambulatory settings.
Medication reconciliation has three steps, as described by IHI4:
-
Verification (collection of the patient's medication history);
-
Clarification (ensuring that the medications and doses are appropriate); and
-
Reconciliation (documentation of changes in the orders).
The details of the process vary by setting and by the availability of paper or electronic medical records. However, the essential steps remain the same, as does the need to perform reconciliation each time the patient transfers to a new setting or level of care. Table 2 lists the most common points at which medication reconciliation occurs in hospitalized patients.
|
| American Academy of Pediatrics |
| American Association of Critical‐Care Nurses |
| Consumers Advancing Patient Safety |
| Institute for Healthcare Improvement |
| Institute for Safe Medication Practices |
| The Joint Commission |
| Massachusetts Coalition for Prevention of Medical Errors |
| Microsoft Corporation |
| Northwestern Memorial Hospital and Northwestern University School of Medicine |
| Society of General Internal Medicine |
| Society of Hospital Medicine |
| University of California San Diego Medical Center |
Because of their complexity, organizations must take care to design their medication reconciliation processes systematically. IHI lists elements of a well‐designed medication reconciliation process as part of its 5 Million Lives Campaign How‐to Guide.4 Such a process:
-
Uses a patient centered approach.
-
Makes it easy to complete the process for all involved. Staff members recognize the what's‐in‐it‐for‐me aspect of the change.
-
Minimizes the opportunity for drug interactions and therapeutic duplications by making the patient's list of current medications available when clinicians prescribe new medications.
-
Provides the patient with an up‐to‐date list of medications.
-
Ensures that other providers who need to know have information about changes in a patient's medication plan.
Research on how adverse drug events (ADE) occur supports the need for tight control of medication orders at transitions in care. For instance:
-
In a study conducted at Mayo Health System in Wisconsin, poor communication of medical information at transition points was responsible for as many as 50% of all medication errors in the hospital and up to 20% of ADEs.5
-
Variances between the medications patients were taking prior to admission and their admission orders ranged from 30% to 70% in 2 literature reviews.1, 6
-
The largest study of medication reconciliation errors and risk factors at hospital admission documented that 36% of patients had errors in their admission orders.7
When The Joint Commission adopted medication reconciliation as NPSG No. 8 in 2005 it had 2 parts: Requirement 8Aa process must exist for comparing the patient's current medications with those ordered for the patient while under the care of the organization; and requirement 8Ba complete list of the patient's medications must be communicated to the next provider of service on transfer within or outside the organization and a complete list of medications must be provided to the patient on discharge.8
However, many hospitals found it difficult to implement medication reconciliation in a systematic way. There was also confusion among hospital staff and administration about the exact definition of medication reconciliation in terms of what it should entail.9 Given these difficulties, The Joint Commission announced that effective January 1, 2009, medication reconciliation would no longer be factored into an organization's accreditation decision or be considered for Requirements for Improvement. Additionally, The Joint Commission stated it is reviewing and revising the NPSG so that it will be ready to be released in January 2011 for implementation later that year.10
Recognizing the difficulty hospitals were having with meaningfully implementing medication reconciliation, the Society of Hospital Medicine convened a 1‐day conference on March 6, 2009, to obtain input from key stakeholders and focus on several critical domains relevant to the success of hospital‐based medication reconciliation. The Agency for Healthcare Research and Quality provided funding support for this conference through grant 1R13HS017520‐01.
An overarching theme emerged from the meeting: the need to reorient the focus of medication reconciliation away from that of an accreditation mandate and toward a broader view of patient safety. Forcing medication reconciliation via a requirement for accreditation tended to limit an organization's efforts to specific process measures. Addressing it as a more global patient safety issue takes into account the entire patient care experience and then opens the door to leverage nonclinical venues (e.g., medical home, family home, community, religious, and other social organizations, as well as social networking platforms) and engage the patient and family/caregivers to reinforce the importance of medication safety.
This white paper evolved from discussions at the March 2009 conference,11 and subsequent structured communication among attendees. Formal endorsement of this document was obtained from the organizations listed in Table 1. In this document, we explore several key issues in implementing clinically meaningful and patient‐centered medication reconciliation. We focus on building common language and understanding of the processes of and participants in medication reconciliation; consider issues of implementation and risk stratification; emphasize the need for research to identify best practices and discusses how to disseminate the findings; promote health information technology platforms that will support interoperable medication information exchange; support the formation of partnerships between patient care sites and nonclinical sites as well as utilizing social marketing opportunities to enhance opportunities for transmitting messages about medication safety; and reinforce the ongoing healthcare reform discussion which aims to align financial incentives with patient safety efforts. After each section, we offer concrete first steps to address the issues discussed.
| Admission: When clinicians reconcile the patient's medications taken at home or at a prior care setting with any new prescription orders to be prescribed by an admitting clinician. |
| Transfer (intra‐ or inter‐facility; with change of clinician or site of care): When clinicians review previous medication orders in light of the patient's clinical status, along with new orders or plans of care. |
| Discharge: When clinicians review all medications the patient was taking prior to being hospitalized, incorporating new prescriptions from the hospitalization and determining whether any medication should be added, discontinued, or modified while being mindful of therapeutic interchanges needed for formulary purposes. |
Methods
The invitation‐only meeting held on the Northwestern Medical Campus in Chicago, IL, brought together stakeholders representing professional, clinical, health care quality, consumer, and regulatory organizations (Table 3). The conference convened these participants with the goals of identifying barriers to meaningful implementation of medication reconciliation and developing a feasible plan toward its effective implementation in the hospital setting. At the meeting, all participants were divided into 1 of 4 groups, which held a facilitated discussion around 1 of 4 key relevant domains: (1) how to measure success in medication reconciliation; (2) key elements of successful strategies; (3) leveraging partnerships outside the hospital setting to support medication reconciliation; and (4) the roles of the patient and family/caregivers and health literacy. Individual group discussions were cofacilitated by experts in the content area. After each discussion, the small group then rotated to a different discussion. Ultimately, each group participated in all four discussions, which built iteratively on the content derived from the prior groups' insights. Key comments were then shared with the large group for further discussion. To help build consensus, these large group discussions were directed by professional facilitators.
| AACN American Association of Critical Care Nurses |
| AAFP American Academy of Family Physicians |
| AAP American Academy of Pediatrics |
| ACEP American College of Emergency Physicians |
| ACP American College of Physicians |
| AMA American Medical Association |
| AMSN Academy of Medical Surgical Nurses |
| ASHP American Society of Health‐System Pharmacists |
| ASHP Foundation American Society of Health‐System Pharmacists Foundation |
| CAPS Consumers Advancing Patient Safety |
| CMS Centers for Medicare and Medicaid Services |
| CMSA Case Management Society of America |
| HCI Hospitalist Consultants, Inc |
| IHI Institute for Healthcare Improvement |
| InCompass Health |
| ISMP Institute For Safe Medication Practice |
| JCR Joint Commission Resources |
| Massachusetts Coalition for Prevention of Medical Errors |
| Microsoft Corporation |
| Northwestern Memorial Hospital MATCH Program |
| NQF National Quality Forum |
| SGIM Society of General Internal Medicine |
| SHM Society of Hospital Medicine |
| The Joint Commission |
| UCSD Hospital Medicine |
| University of Oklahoma College of Pharmacy Tulsa |
After the meeting, attendees participated in 2 follow‐up conference calls to discuss issues raised at the conference and responses obtained from host organizations. They also subsequently participated in two focus groups with The Joint Commission, giving input on the revision of the medication reconciliation NPSG.
Results
Addressing Barriers to Medication Reconciliation
In order to implement successful medication reconciliation processes, one must build the steps with the patient and family/caregiver as the focus and demonstrate an understanding of the intent of these processes. At its roots, medication reconciliation was developed to ensure that clinicians do not inadvertently add, change, or omit medications and that changes made are communicated to all relevant caregivers.
A number of key issues with respect to successful medication reconciliation processes surfaced in discussions with stakeholders. We believe addressing these issues is necessary before meaningful and standardized implementation can be achieved. After each discussion below, we provide suggested first steps to address these issues.
1. Achieve Consensus on the Definition of Medication and Reconciliation
Despite proposed definitions of these terms by various organizations, there was little agreement about them in the healthcare community. This ambiguity contributed to general confusion about what actually constitutes medication reconciliation. There needs to be a single, clear, and broadly accepted definition of what constitutes a medication. For the purposes of medication reconciliation, the term medication should be broadly inclusive of substances that may have an impact on the patient's care and treatments as well as those substances that may interact with other therapies potentially used during the medical care episode. Illicit or recreational substances may also have impact on therapies considered and therefore may influence this definition.12 Concretely, this definition should encompass prescription and over‐the‐counter medications as well as herbal and dietary supplements.
The term reconciliation in its simplest form implies the process of verifying that a patient's current list of medications (including dose, route, and frequency) are correct and that the medications are currently medically necessary and safe. Reconciliation suggests a process which, by necessity, will vary based on clinical context and setting. Further defining this termand the process of reconciliation itselfshould be carried out using patient safety principles with a focus on patient‐ and family‐centeredness.
Designing hospital‐based medication reconciliation processes should:
-
Employ a multidisciplinary approach that involves nurses, pharmacists, and other appropriate personnel from the inpatient setting as well as ambulatory and community/retail areas, both ambulatory and inpatient physicians, and a patient/family representative;
-
Involve hospital leaders who support, provide guidance, and remove barriers for the multidisciplinary team working to implement the processes;
-
Clearly define the roles of each participant in the processes developed;
-
Include methods to assess and address any special needs due to the developmental stage, age, dependency, language or literacy levels of patients and their family/caregiver;
-
Use clinically relevant process measures (e.g., adherence to procedural steps) and outcome measures (e.g., change in the number of ADEs, unnecessary hospitalizations, or emergency department visits) where appropriate to assess the impact of the process;
-
Include feedback systems to allow for clinically significant process improvement.
Once a common understanding of the terms and intent of medication reconciliation is achieved, it will be important for accrediting organizations, medical societies, quality improvement organizations, and other interested parties to adopt the same language.
First Step
A consortium of clinical, quality, and regulatory stakeholders should work to achieve consensus on the definition for medication and the intent and expectations for the reconciliation process.
2. Clarify Roles and Responsibilities
Given the differences in organizational and practice structures in hospitals and the varying numbers of health professionals involved in a patient's care, no one process design will meet the needs of all sites. As it is clear that interdisciplinary teams are best suited to develop, implement, and carry out complex patient‐centered processes like medication reconciliation, it is crucial that all involved parties have clearly defined roles and responsibilities, including patients and their families/caregivers. It is also important to recognize that these responsibilities may change depending on the dependency or vulnerability of the patient (e.g., children or geriatric patients) or the transition of care being undertaken by the patient (i.e., admission, transfer, or discharge), thus requiring sites to develop clear policies about these roles and responsibilities and how they may change in various situations.
First Step
Individual sites must clearly define the roles and responsibilities of all parties directly involved in medication reconciliation as a part of designing local medication reconciliation processes.
3. Develop Measurement Tools
Ensuring that medication reconciliation processes result in clinically meaningful outcomes requires the development and standardization of a limited number of metrics that may be used by organizations and reported centrally for benchmarking. This core set of measures should be developed by clinical, quality, accreditation, and regulatory organizations (see #10 below) through a consensus building process utilizing multi‐stakeholder input. The set should be supplemented by additional site‐specific measures determined locally that focus on steps in the process itself and allow sites to perform continuous quality improvement. Sites should be encouraged to develop tools locally to support and facilitate organizational and professional adherence to medication reconciliation processes.
First Steps
Clinical, quality, accreditation, and regulatory organizations should develop reliable metrics to be assessed and reported.
The principles of patient‐centeredness and family/caregiver‐centeredness, the medical home, and clinical relevance must be central to the metrics chosen for quality and regulatory purposes.
4. Phased Implementation
Ultimately, comprehensive medication reconciliation processes need to be implemented in hospitals. However, to succeed in integrating complex processes like medication reconciliation into routine hospital practices, implementation may be facilitated by using a phased approach to allow for participants to adapt new processes and procedures to the local environment iteratively. While the most appropriate phased approach to implementation will vary by site and setting, options for phasing might include:
-
Starting with one clinical area or service.
-
Starting with either the admission or discharge reconciliation process.
-
Starting with a patient population at high risk for adverse events.
Irrespective of the phasing strategy employed, development of a clear and pragmatic schedule for the entire implementation process should be established. Phasing decisions should be made based on organizational resources and the clinical needs of the patient population within each clinical setting. As noted, the ultimate goal is to develop comprehensive reconciliation processes occurring during all significant care transitions (i.e., admission, service or site‐of‐care transfers, and discharge) for all hospitalized patients and involving all of their medications. Flexibility in design should be encouraged to ensure the processes can work within local workflow as long as progress toward this primary goal is made.
First Steps
Clinical sites should establish local, pragmatic priorities for a phased approach to implementation.
Tie the phased approach to a timeline or blueprint for programmatic expansion with ultimate plans for comprehensive implementation.
5. Develop Risk Stratification Systems
Medication‐related adverse events related to inadequate reconciliation are more likely to occur in hospitalized patients with certain identifiable risk factors. For example, the MATCH study documented that polypharmacy and age over 65 years were independently associated with increased risk for errors at the time of hospital admission.7 Other factors that may increase the likelihood of medication‐related adverse events at care transitions in the hospital might include: patients with multiple providers, developmental/cognitive impairment, dependency/vulnerability, multiple or high‐risk medications, or poor health literacy or limited English proficiency. Research is needed to elucidate these risk factors further.
An alert system for key risk factors for complications related to incompletely, inappropriately, or inaccurately completed medication reconciliation due to patient, clinician, or system factors should be developed, tested, and broadly implemented. Additionally, an alert system would help maintain vigilance toward this patient safety issue and, potentially, help focus additional resources on high‐risk patients. Such a tool has been tested in ambulatory settings.15
First Step
Additional research on inpatient predictors of failed medication reconciliation and ADE should be prioritized (see #6 below).
6. Study Interventions and Processes
Despite having been an NPSG since 2005, there is still a relative paucity of literature about broadly applicable and effective implementation strategies and demonstrated interventions that improve medication safety related to medication reconciliation. Some strategies that have shown to reduce medication errors at transitions include the involvement of pharmacist medication review on discharge16, 17 and the usefulness of planning by multidisciplinary groups.18 Other studies have outlined the continuing barriers to successful implementation of reconciliation, including the difficulty patients have in accurately recalling their current medications19 and the high cost in nurse and pharmacist time of tracking down a patient's ongoing prescriptions.20, 21 Studies evaluating potential solutions to overcome these and other common barriers are still needed.
Future research should focus on a comprehensive review of implementation strategies, (specifically including the role of health information technology‐based innovations) clinically relevant outcomes, and best practices, while being sensitive to the different needs of varying care settings (e.g., pediatric vs. adult centers, emergency departments vs. inpatient units, community hospital vs. academic medical center, etc.) as well as the resource requirements engendered in the interventions.
First Step
Funding agencies should explicitly prioritize outcomes‐focused medication reconciliation‐related projects (e.g., those which demonstrate a reduction in postdischarge ADE or reduced medication‐related emergency department visits). Previously identified successful strategies should be further investigated. Funded projects should explicitly partner with patients and family/caregivers and also include pediatric and adult patients, rural and urban locations of care, as well as academic and nonacademic hospital settings, to promote more broadly applicable results.
7. Disseminate Success
Best practices and lessons learned, especially those rigorously tested and driven by data, stratified by patient type, care setting (emergency department, intensive care, surgical ward, etc.) and institutional type (community, teaching, safety net, critical access, etc.) need to be disseminated so others can adopt and adapt them effectively. High‐quality case studies with clear explanations of successes, failures, and lessons learned may prove valuable sources of information. This knowledge should foster a learning community approach and accelerate implementation at new sites.
First Step
Hospitals, healthcare systems, as well as quality and regulatory agencies should develop mechanisms within reporting systems to track performance, identify notably successful sites, and publicly report and share methods and lessons learned from them.
8. Promote the Personal Health Record
A fully integrated and transferable personal health record should be accepted as the standard for health information storage and interoperability, giving both the patient (or family/caregiver) and clinical providers access and ownership. Both the HL7 Continuity of Care Document (CCD) and the Continuity of Care Record (CCR) meet these criteria. The CCR was endorsed by the American Society for Testing and Materials22 and a coalition of other medical societies.23 Notably, CCR and CCD were recently adopted as standards for structured electronic health record (EHR) exchange through the July 2010 publication of the Final Rule of the Health Information Technology for Economic and Clinical Health Act provision of the American Recovery and Reinvestment Act of 2009 (ARRA/HITECH) and is now part of the formal US Department of Health and Human Services certification criteria for EHR technologies.24
Mandating a content exchange standard such as the CCR or the CCD should also have the desired effect of ensuring that patients (and their caregivers) become increasingly involved in maintaining an accurate list of the medications they take. Additionally, systems must be sufficiently flexible to address the unique medication management needs of children and geriatric patients. An electronic version of a personal health record is a promising method for improving consistency across care platforms, but to be implemented effectively the record must be compatible across all settings, including, where possible, the patient's home. All health care organizations, pharmacy systems, and insurers, must make medication reconciliation‐related interoperability and accessibility a priority as they pursue information technology strategies.
First Step
Stakeholder organizations must send a clear and convincing message to legislators under the current atmosphere of health care reform, urging them to mandate that health information technology standards include interoperability and support platforms that are consistent with standards put forth in the 2009 HITECH Act Interim Final Rule for EHR certification.
9. Promote Partnerships
At a broader health care system level, leveraging existing partnerships and creating new ones among health care, public/private sector‐affiliated organizations (e.g., community and mail order pharmacies, pharmaceutical organizations and manufacturers, and insurers), and public health organizations are extremely important mechanisms for broader scale impact. This view recognizes the numerous opportunities to educate and influence patients about medication safety outside the dyadic relationship of the clinician and patient in traditional clinical settings. Partnerships between health care and public entities may capitalize on these opportunities to foster adoption of healthy medication practices (e.g., maintaining an accurate and updated medication list), thereby supporting medication reconciliation efforts when individuals encounter health care settings. Partnership and information sharing could be enhanced through the use of a central coordinating body or coalition. This body could generate a shared common vision and contribute expertise to the myriad issues in medication reconciliation.
Partnerships should utilize the following:
-
Social marketing techniques to engage the community. Included within this strategy must be a clear and compelling message that transmits the importance of safe medication practices. Current messages such as keep a list while important, do not offer enough of a sense of urgency or importance. A more powerful message could involve highly publicized medication errors or close calls that would resonate with a broad audience.
-
Local and national champions. Such individuals should be trusted for their health knowledge (e.g., television health care reporters) or be prominent, influential, and trusted figures in other circles (e.g., clergy, politicians, movie celebrities). Indeed, taking advantage of popular media by weaving a theme into a movie or television program about medication safety may prove effective.
Relevant partnerships would include:
-
Quality organizations partnering with other stakeholders to establish unambiguous and unified medication reconciliation standards across the care continuum.
-
Health systems partnering with community pharmacy providers to ensure an uninterrupted communication link in both the inpatient and outpatient settings.
-
Manufacturers and distributors of medications partnering with health care and public health organizations, the media, insurers and other constituents to promote the importance of maintaining and sharing an accurate list of medications.
-
Public health systems partnering with community‐based organizations to encourage and promote the established standards for medication safety through messaging and educational campaigns.
All partnerships must consider issues of patient language and literacy as well as the needs of vulnerable populations in the scope of their activities.
First Step
Public health agencies should partner with health care quality organizations and others to begin a national public campaign to increase the awareness of medication safety (the broader public health concept under which medication reconciliation would fall) and support the importance of the patient's role in maintaining an updated medication list at all times.
10. Align Financial Incentives With Newly Developed Regulatory and Accreditation Requirements
Implementing and performing medication reconciliation takes time, particularly at the outset of a new program. Time requirements and associated costs are major barriers to undertaking comprehensive medication reconciliation, despite its recognized importance for reducing avoidable injury to patients. At present, systems that impede efficiency and slow hospital throughput may be discouraged due to their potential for having an adverse impact on access, finances, and other aspects of care delivery. Moreover, the changed economic climate with reduced hospital fiscal margins limits resources for new initiatives. Currently, failed medication reconciliationand the related avoidable adverse events, culminating in readmission to the hospital or emergency departmentyields additional revenue for hospitals and other providers in some reimbursement models.
Alignment of financial incentives that ensured adequate time and resources for appropriate medication reconciliation processes would facilitate implementation. Additionally, start‐up funding to create and implement these processes needs to be made available.
One example illustrating efforts to align payment policy with medication safety efforts occurred when the Office of the National Coordinator (ONC), in publishing its Final Rule under the 2009 HITECH Act,24 endorsed the importance of financially supporting proper medication reconciliation, particularly at first encounter and transitions in care, by requiring EHR systems seeking certification under the rule to support the care team in the task of reconciliation. For example, vendors will have to support the ability to compare 2 or more medication lists electronically, create medication lists, drug allergy lists, perform drug formulary look‐ups, drug‐drug and drug‐allergy checks, and support creating patient summaries after each visit or post discharge that include medication lists. The ONC, in defining Meaningful Use for eligible health care organizations, included in that definition the goal of exchanging meaningful clinical information among the professional health care teams. This goal is demonstrated through organizations reporting that they performed medication reconciliation for at least 50% of transitions of care in which the patient is transitioned into the care of the eligible professional or admitted to the eligible hospital's or Critical Access Hospital's inpatient or emergency department. Organizations able to demonstrate this level of compliance, along with other Meaningful Use requirements, will be eligible to receive stimulus funds through 2015 and avoid financial penalties that begin after that period.
First Step
Future health care reform must address the misalignment of financial policies and structures, and provide financial incentives to support the development and implementation of better medication management systems and prevent avoidable rehospitalizations and emergency department visits resulting from medication‐related adverse events.
Conclusion
Medication reconciliation involves highly complex processes and is hampered by the disjointed nature of the American health care system. It is, however, a vital part of reducing ADE. If employed more broadly, it has the added benefits of enhancing communication among all providers of care and engaging patients and families/caregivers more consistently and meaningfully in their overall care.
Despite the difficulty of maintaining an accurate medication record in real time across disparate settings, reconciliation is a goal to which our organizations are committed. Given the wide range of healthcare organizations involved in providing medications to patients and the many agencies evaluating those efforts, we believed it would be helpful to provide an overarching set of goals to move medication reconciliation forward.
Our main message is this: Patient safety and patient/family‐centered care must be the principal drivers in the development and implementation of medication reconciliation systems. Ultimately this process is about ensuring that patients are receiving the most appropriate medications no matter where they are treated. With this document, we hope to bring to light the importance of creating and implementing a medication reconciliation program, addressing some barriers to success, and identifying potential solutions that will ensure utility and sustainability of this critical patient safety issue.
- ,,, et al.Unintended medication discrepancies at the time of hospital admission.Arch Intern Med.2005;165(4):424–429.
- .Prevention of medication errors in the pediatric inpatient setting. The American Academy of Pediatrics Policy Statement.Pediatrics.2003;112(2):431–436.
- ,,, et al.Medication reconciliation: a practical tool to reduce the risk of medication errors.J Crit Care.2003;18(4):201–205.
- Institute for Healthcare Improvement. 5 million lives getting started kit: preventing adverse drug events (medication reconciliation), how‐to guide. Available at: http://www.ihi.org/IHI/Programs/Campaign/ADEsMedReconciliation.htm. Published Oct. 1, 2008. Accessed September2010.
- ,.Medication safety: one organization's approach to the challenge.J Clin Outcomes Mana.2001;8(10):27–34.
- ,,,,,.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health Syst Pharm.2004;61(16):1689–1695.
- ,,, et al.Results of the Medications At Transitions and Clinical Handoffs (MATCH) Study: an analysis of medication reconciliation errors and risk factors at hospital admission.J Gen Intern Med.2010;25(5):441–447.
- Joint Commission on Accreditation of Healthcare Organizations.2005 Hospital Accreditation Standards, p.NPSG‐4.
- ,,,,.Brief communication: Results of a medication reconciliation survey from the 2006 Society of Hospital Medicine national meeting.J Hosp Med.2008;3(6):465–472.
- The Joint Commission.Approved: will not score medication reconciliation in 2009.Jt Comm Perspect.2009;29(3):1,3.
- Society of Hospital Medicine. Medication reconciliation: a team approach, conference summary. December 2009. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/QualityImprovement/QICurrentInitiativesandTrainingOpportunities/QI_Current_Initiativ.htm. Accessed September2010.
- The American Medical Association. The physician's role in medication reconciliation: issues, strategies and safety principles. 2007. Available at: http://www.ama‐assn.org/ama1/pub/upload/mm/370/med‐rec‐monograph.pdf. Accessed September2010.
- Institute of Safe Medication Practices. ISMP's list of high alert medications. 2008. Available at: http://www.ismp.org/Tools/highalertmedications.pdf. Accessed September2010.
- ,,,.Medication use leading to emergency department visits for adverse drug events in older adults.Ann Intern Med.2007;147(11):755–765
- ,,, et al.Experience with a trigger tool for identifying adverse drug events among older adults in ambulatory primary care.Qual Saf Health Care.2009;18(3):199–204.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166(5):565–571.
- ,,,,.Medication reconciliation at an academic medical center: implementation of a comprehensive program from admission to discharge.Am J Health Syst Pharm.2009;66(23):2126–2131.
- ,,,,,.Multidisciplinary approach to inpatient medication reconciliation in an academic setting.Am J Health Syst Pharm.2007;64(8):850–854.
- ,,.Lack of patient knowledge regarding hospital medications.J Hosp Med.2010;5(2):83–86.
- .The unexpected challenges of accurate medication reconciliation.Ann Emerg Med.2008;52(5):493–495.
- ,,,.Medication reconciliation in a rural trauma population.Ann Emerg Med.2008;52(5):483–491.
- ASTM International. ASTM E2369 ‐ 05e1 standard specification for continuity of care record (CCR). Available at: http://www.astm.org/Standards/E2369.htm. Accessed September2010.
- ,,.The continuity of care record.Am Fam Physician.2004;70(7):1220,1222–1223.
- Department of Health and Human Services. Health information technology: initial set of standards, implementation specifications, and certification criteria for electronic health record technology; final rule. Available at: http://edocket.access.gpo.gov/2010/pdf/2010–17210.pdf. Accessed September2010.
- ,,, et al.Unintended medication discrepancies at the time of hospital admission.Arch Intern Med.2005;165(4):424–429.
- .Prevention of medication errors in the pediatric inpatient setting. The American Academy of Pediatrics Policy Statement.Pediatrics.2003;112(2):431–436.
- ,,, et al.Medication reconciliation: a practical tool to reduce the risk of medication errors.J Crit Care.2003;18(4):201–205.
- Institute for Healthcare Improvement. 5 million lives getting started kit: preventing adverse drug events (medication reconciliation), how‐to guide. Available at: http://www.ihi.org/IHI/Programs/Campaign/ADEsMedReconciliation.htm. Published Oct. 1, 2008. Accessed September2010.
- ,.Medication safety: one organization's approach to the challenge.J Clin Outcomes Mana.2001;8(10):27–34.
- ,,,,,.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health Syst Pharm.2004;61(16):1689–1695.
- ,,, et al.Results of the Medications At Transitions and Clinical Handoffs (MATCH) Study: an analysis of medication reconciliation errors and risk factors at hospital admission.J Gen Intern Med.2010;25(5):441–447.
- Joint Commission on Accreditation of Healthcare Organizations.2005 Hospital Accreditation Standards, p.NPSG‐4.
- ,,,,.Brief communication: Results of a medication reconciliation survey from the 2006 Society of Hospital Medicine national meeting.J Hosp Med.2008;3(6):465–472.
- The Joint Commission.Approved: will not score medication reconciliation in 2009.Jt Comm Perspect.2009;29(3):1,3.
- Society of Hospital Medicine. Medication reconciliation: a team approach, conference summary. December 2009. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/QualityImprovement/QICurrentInitiativesandTrainingOpportunities/QI_Current_Initiativ.htm. Accessed September2010.
- The American Medical Association. The physician's role in medication reconciliation: issues, strategies and safety principles. 2007. Available at: http://www.ama‐assn.org/ama1/pub/upload/mm/370/med‐rec‐monograph.pdf. Accessed September2010.
- Institute of Safe Medication Practices. ISMP's list of high alert medications. 2008. Available at: http://www.ismp.org/Tools/highalertmedications.pdf. Accessed September2010.
- ,,,.Medication use leading to emergency department visits for adverse drug events in older adults.Ann Intern Med.2007;147(11):755–765
- ,,, et al.Experience with a trigger tool for identifying adverse drug events among older adults in ambulatory primary care.Qual Saf Health Care.2009;18(3):199–204.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166(5):565–571.
- ,,,,.Medication reconciliation at an academic medical center: implementation of a comprehensive program from admission to discharge.Am J Health Syst Pharm.2009;66(23):2126–2131.
- ,,,,,.Multidisciplinary approach to inpatient medication reconciliation in an academic setting.Am J Health Syst Pharm.2007;64(8):850–854.
- ,,.Lack of patient knowledge regarding hospital medications.J Hosp Med.2010;5(2):83–86.
- .The unexpected challenges of accurate medication reconciliation.Ann Emerg Med.2008;52(5):493–495.
- ,,,.Medication reconciliation in a rural trauma population.Ann Emerg Med.2008;52(5):483–491.
- ASTM International. ASTM E2369 ‐ 05e1 standard specification for continuity of care record (CCR). Available at: http://www.astm.org/Standards/E2369.htm. Accessed September2010.
- ,,.The continuity of care record.Am Fam Physician.2004;70(7):1220,1222–1223.
- Department of Health and Human Services. Health information technology: initial set of standards, implementation specifications, and certification criteria for electronic health record technology; final rule. Available at: http://edocket.access.gpo.gov/2010/pdf/2010–17210.pdf. Accessed September2010.
Continuing Medical Education Program in
If you wish to receive credit for this activity, which begins on the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to
www.blackwellpublishing.com/cme . -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which begins on the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to
www.blackwellpublishing.com/cme . -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which begins on the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to
www.blackwellpublishing.com/cme . -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
Finger Points to the Diagnosis
A 24‐year‐old man presented to the emergency room with a 3‐month history of bright red blood per rectum and increasing fatigue. Review of systems was significant for intermittent hematuria, swelling, and pain in his lower extremities. He denied abdominal pain, nausea, or vomiting, and was otherwise asymptomatic. He was not taking any medicines. He said that he has had this bleeding problem on and off since he was a child. The chronic intermittent rectal bleeding usually resolved spontaneously. Previous treatments have consisted of blood transfusions, small bowel resections, and a partial colectomy.
Physical exam demonstrates a thin and well‐nourished African American male in no distress. Temperature 36.7C, blood pressure while sitting was 111/65 mmHg, with a pulse of 117 beats per minute; on standing his blood pressure was 103/54 mmHg, with a pulse of 137 beats per minute, and respirations were 18 breaths per minute. Abdominal examination revealed splenomegaly. Rectal exam revealed the presence of bright red blood. Other significant findings include unilateral limb skeletal asymmetry with the right upper and lower extremity being longer than the left side. There was significant hypertrophy of several digits of the hands and feet bilaterally (Figure 1). Notable was the presence of raised, hyperpigmented irregular linear plaques, extending from his right medial forearm to his chest and also from his abdomen to right medial thigh. Additional skin examination was remarkable for well‐demarcated, raised vascular areas on the lateral thighs and knees bilaterally (Figure 2), as well as the dorsum of both the feet. Laboratory workup was notable for hemoglobin of 2.7gm/dl, a hematocrit of 9%, and mean corpuscular volume (MCV) of 58 fl. Normal coagulation parameters, and profound iron deficiency (iron level 16 mcg/dl and ferritin <20 ng/ml).

Other routine laboratory results including coagulation parameters were unremarkable.
Discussion
Based on the classic examination findings and history of gastrointestinal bleeding, this patient has Klippel‐Trenaunay‐Weber syndrome (KTWS), which is characterized by cutaneous malformations of the capillary and venous systems, bony and soft tissue hypertrophy, and arteriovenous malformations (AVMs).1 Many patients with KTWS suffer recurrent bleeding from gastrointestinal AVMs.
Although involvement is usually unilateral, this patient had bilateral limb hypertrophy and hemangiomas. His nevus flammeus was unilateral and incidentally was present over the lower abdomen and posterior thigh and buttock, with significant underlying varices in the pelvis and rectum. His hematuria was secondary to AVMs in the bladder and resolved by itself. The size and extent of his pelvic and rectal varices presented a therapeutic challenge. With blood transfusions and a conservative approach, his bleeding diminished spontaneously. A rectal artery was thought to be contributing to the problem, so a prophylactic embolization was performed by interventional radiology. Follow‐up at 2 months revealed no further bleeding.
Hospitalists treat common causes of gastrointestinal (GI) bleeding such as ulcers, polyps, malignancies, varices, inflammatory bowel disease, AVMs, and, rarely, mucosal Kaposi sarcoma. However, they may occasionally encounter an adult with skin manifestations of a congenital cause of GI bleeding. The 4 most common congenital disorders with primary cutaneous manifestations that also involve the GI tract are reviewed below (also see Table 1).
| Vascular Malformation Syndromes | Characteristics |
|---|---|
| Klippel‐Trenaunay‐Weber | Soft tissue; bony, vascular lesions; and varices |
| Mafucci | Enchondromas, subcutaneous visceral lesions |
| Blue rubber bleb nevus | Bluish black sessile venous malformations |
| Osler‐Maffuci‐Weber‐Rendu | Mucocutaneous telangiectasias |
Blue rubber bleb nevus syndrome, also known as Bean syndrome, is the rarest of these disorders, characterized by cutaneous and intestinal cavernous hemangiomas that may occasionally be painful and tender.2 Hemangiomas may measure from a few millimeters to approximately 5 cm and are raised, blue‐purple, and rubbery in consistency, with a wrinkled surface. They are usually located on the trunk, extremities, face, and any part of the GI tract, with the small intestine and distal colon being the most common sites involved. Given that the lesions may involve the full thickness of the bowel wall, surgery is often required, as less invasive measures such as endoscopic laser coagulation may be inadequate. Orthopedic problems such as scoliosis arise from pressure exerted by large vascular malformations.
Maffucci syndrome is characterized by skeletal and vascular malformations manifested as enchondromas in the metaphyseal and diaphyseal portion of long bones. The vascular lesions, which may involve mucous membranes or viscera, are compressible blue‐purple hemangiomas that follow the rate of the growth of the child. Limb deformities, pathological fractures, and malignant transformation into chondrosarcomas are common complications.3
Osler‐Weber‐Rendu syndrome is also known as hereditary hemorrhagic telangiectasia. In this disorder, mucocutaneous telangiectatic lesions usually develop by puberty and may involve the conjunctiva, respiratory tract, brain, liver, GI tract, and genitourinary (GU) tract. Most patients exhibit only epistaxis, yet massive hemorrhage may occur in the lung, GI tract, and GU tract. These hemorrhages can usually be managed by cautery or electrocoagulation but pulmonary and GI lesions may need excision.4
KTWS consists of the triad of cutaneous vascular malformations of the capillary, venous and lymphatic systems, bony and soft tissue hypertrophy, and venous varicosities in association with AVMs. The name Weber is added when patients have AVMs that are clinically significant; otherwise, it is simply known as Klippel‐Trenaunay syndrome. The most common cutaneous vascular lesion is a capillary hemangioma known as a nevus flammeus. The distribution of the nevus flammeus usually indicates underlying vascular malformations that may extend as deep as the bone, causing limb or digit hypertrophy, as seen in this patient.5 Delineation of the extent of vascular abnormalities is accomplished by noninvasive methods such as color ultrasonography, magnetic resonance imaging, and computer‐aided angiography. Symptomatic GI or GU involvement is rare (1%), but can cause significant hemorrhage.6 Surgical correction is often difficult and the lesions tend to recur.
In the largest published series of Klippel‐Trenaunay patients, followed over 30 years at the Mayo Clinic, most patients were treated conservatively, with surgery limited to epiphysiodesis to prevent excessive leg length in the affected limbs and selected superficial vein stripping in patients with large venous varicosities with preserved deep venous systems.7, 8 For the treatment of AVMs, nonsurgical measures such as foam embolization and radiotherapy are increasingly being used due to their safety and precise application.9, 10
- ,,, et al.Klippel‐Trenaunay syndrome.Am J Med Genet.1998;79(4):319–326.
- .Blue rubber bleb nevus syndrome.Curr Treat Options Gastroenterol.2001;4(5):433–440.
- ,.Maffucci's syndrome, functional and neoplastic significance. Case report and review of the literature.J Bone Joint Surg Am.1973;55:1465–1479.
- ,,.Hereditary hemorrhagic telangiectasia (Osler‐Weber‐Rendu syndrome): a view from the 21st century.Postgrad Med J.2003;79:18–24.
- ,.Klippel Trenaunay syndrome: the importance of “geographic stains” in identifying lymphatic disease and risk of complications.J Am Acad Dermatol.2004;51(3):391–398.
- ,,,.Klippel‐Trenaunay syndrome with involvement of cecum and rectum: a rare cause of lower gastrointestinal bleeding.Eur J Med Res.2004;9(11):515–517.
- ,,.Klippel‐Trenaunay syndrome: spectrum and management.Mayo Clinic Proc.1998;73:28–36.
- ,,,,,.Surgical treatment of venous malformations in Klippel‐Trenaunay syndrome.J Vasc Surg.2000;32:840–847.
- .Radiotherapy in the management of Klippel‐Trenaunay‐Weber syndrome: report of two cases.Ann Vasc Surg.2005;19(4):566–571.
- ,,.Venous angiomata: treatment with sclerosant foam.Ann Vasc Surg.2005;19:457–464.
A 24‐year‐old man presented to the emergency room with a 3‐month history of bright red blood per rectum and increasing fatigue. Review of systems was significant for intermittent hematuria, swelling, and pain in his lower extremities. He denied abdominal pain, nausea, or vomiting, and was otherwise asymptomatic. He was not taking any medicines. He said that he has had this bleeding problem on and off since he was a child. The chronic intermittent rectal bleeding usually resolved spontaneously. Previous treatments have consisted of blood transfusions, small bowel resections, and a partial colectomy.
Physical exam demonstrates a thin and well‐nourished African American male in no distress. Temperature 36.7C, blood pressure while sitting was 111/65 mmHg, with a pulse of 117 beats per minute; on standing his blood pressure was 103/54 mmHg, with a pulse of 137 beats per minute, and respirations were 18 breaths per minute. Abdominal examination revealed splenomegaly. Rectal exam revealed the presence of bright red blood. Other significant findings include unilateral limb skeletal asymmetry with the right upper and lower extremity being longer than the left side. There was significant hypertrophy of several digits of the hands and feet bilaterally (Figure 1). Notable was the presence of raised, hyperpigmented irregular linear plaques, extending from his right medial forearm to his chest and also from his abdomen to right medial thigh. Additional skin examination was remarkable for well‐demarcated, raised vascular areas on the lateral thighs and knees bilaterally (Figure 2), as well as the dorsum of both the feet. Laboratory workup was notable for hemoglobin of 2.7gm/dl, a hematocrit of 9%, and mean corpuscular volume (MCV) of 58 fl. Normal coagulation parameters, and profound iron deficiency (iron level 16 mcg/dl and ferritin <20 ng/ml).


Other routine laboratory results including coagulation parameters were unremarkable.
Discussion
Based on the classic examination findings and history of gastrointestinal bleeding, this patient has Klippel‐Trenaunay‐Weber syndrome (KTWS), which is characterized by cutaneous malformations of the capillary and venous systems, bony and soft tissue hypertrophy, and arteriovenous malformations (AVMs).1 Many patients with KTWS suffer recurrent bleeding from gastrointestinal AVMs.
Although involvement is usually unilateral, this patient had bilateral limb hypertrophy and hemangiomas. His nevus flammeus was unilateral and incidentally was present over the lower abdomen and posterior thigh and buttock, with significant underlying varices in the pelvis and rectum. His hematuria was secondary to AVMs in the bladder and resolved by itself. The size and extent of his pelvic and rectal varices presented a therapeutic challenge. With blood transfusions and a conservative approach, his bleeding diminished spontaneously. A rectal artery was thought to be contributing to the problem, so a prophylactic embolization was performed by interventional radiology. Follow‐up at 2 months revealed no further bleeding.
Hospitalists treat common causes of gastrointestinal (GI) bleeding such as ulcers, polyps, malignancies, varices, inflammatory bowel disease, AVMs, and, rarely, mucosal Kaposi sarcoma. However, they may occasionally encounter an adult with skin manifestations of a congenital cause of GI bleeding. The 4 most common congenital disorders with primary cutaneous manifestations that also involve the GI tract are reviewed below (also see Table 1).
| Vascular Malformation Syndromes | Characteristics |
|---|---|
| Klippel‐Trenaunay‐Weber | Soft tissue; bony, vascular lesions; and varices |
| Mafucci | Enchondromas, subcutaneous visceral lesions |
| Blue rubber bleb nevus | Bluish black sessile venous malformations |
| Osler‐Maffuci‐Weber‐Rendu | Mucocutaneous telangiectasias |
Blue rubber bleb nevus syndrome, also known as Bean syndrome, is the rarest of these disorders, characterized by cutaneous and intestinal cavernous hemangiomas that may occasionally be painful and tender.2 Hemangiomas may measure from a few millimeters to approximately 5 cm and are raised, blue‐purple, and rubbery in consistency, with a wrinkled surface. They are usually located on the trunk, extremities, face, and any part of the GI tract, with the small intestine and distal colon being the most common sites involved. Given that the lesions may involve the full thickness of the bowel wall, surgery is often required, as less invasive measures such as endoscopic laser coagulation may be inadequate. Orthopedic problems such as scoliosis arise from pressure exerted by large vascular malformations.
Maffucci syndrome is characterized by skeletal and vascular malformations manifested as enchondromas in the metaphyseal and diaphyseal portion of long bones. The vascular lesions, which may involve mucous membranes or viscera, are compressible blue‐purple hemangiomas that follow the rate of the growth of the child. Limb deformities, pathological fractures, and malignant transformation into chondrosarcomas are common complications.3
Osler‐Weber‐Rendu syndrome is also known as hereditary hemorrhagic telangiectasia. In this disorder, mucocutaneous telangiectatic lesions usually develop by puberty and may involve the conjunctiva, respiratory tract, brain, liver, GI tract, and genitourinary (GU) tract. Most patients exhibit only epistaxis, yet massive hemorrhage may occur in the lung, GI tract, and GU tract. These hemorrhages can usually be managed by cautery or electrocoagulation but pulmonary and GI lesions may need excision.4
KTWS consists of the triad of cutaneous vascular malformations of the capillary, venous and lymphatic systems, bony and soft tissue hypertrophy, and venous varicosities in association with AVMs. The name Weber is added when patients have AVMs that are clinically significant; otherwise, it is simply known as Klippel‐Trenaunay syndrome. The most common cutaneous vascular lesion is a capillary hemangioma known as a nevus flammeus. The distribution of the nevus flammeus usually indicates underlying vascular malformations that may extend as deep as the bone, causing limb or digit hypertrophy, as seen in this patient.5 Delineation of the extent of vascular abnormalities is accomplished by noninvasive methods such as color ultrasonography, magnetic resonance imaging, and computer‐aided angiography. Symptomatic GI or GU involvement is rare (1%), but can cause significant hemorrhage.6 Surgical correction is often difficult and the lesions tend to recur.
In the largest published series of Klippel‐Trenaunay patients, followed over 30 years at the Mayo Clinic, most patients were treated conservatively, with surgery limited to epiphysiodesis to prevent excessive leg length in the affected limbs and selected superficial vein stripping in patients with large venous varicosities with preserved deep venous systems.7, 8 For the treatment of AVMs, nonsurgical measures such as foam embolization and radiotherapy are increasingly being used due to their safety and precise application.9, 10
A 24‐year‐old man presented to the emergency room with a 3‐month history of bright red blood per rectum and increasing fatigue. Review of systems was significant for intermittent hematuria, swelling, and pain in his lower extremities. He denied abdominal pain, nausea, or vomiting, and was otherwise asymptomatic. He was not taking any medicines. He said that he has had this bleeding problem on and off since he was a child. The chronic intermittent rectal bleeding usually resolved spontaneously. Previous treatments have consisted of blood transfusions, small bowel resections, and a partial colectomy.
Physical exam demonstrates a thin and well‐nourished African American male in no distress. Temperature 36.7C, blood pressure while sitting was 111/65 mmHg, with a pulse of 117 beats per minute; on standing his blood pressure was 103/54 mmHg, with a pulse of 137 beats per minute, and respirations were 18 breaths per minute. Abdominal examination revealed splenomegaly. Rectal exam revealed the presence of bright red blood. Other significant findings include unilateral limb skeletal asymmetry with the right upper and lower extremity being longer than the left side. There was significant hypertrophy of several digits of the hands and feet bilaterally (Figure 1). Notable was the presence of raised, hyperpigmented irregular linear plaques, extending from his right medial forearm to his chest and also from his abdomen to right medial thigh. Additional skin examination was remarkable for well‐demarcated, raised vascular areas on the lateral thighs and knees bilaterally (Figure 2), as well as the dorsum of both the feet. Laboratory workup was notable for hemoglobin of 2.7gm/dl, a hematocrit of 9%, and mean corpuscular volume (MCV) of 58 fl. Normal coagulation parameters, and profound iron deficiency (iron level 16 mcg/dl and ferritin <20 ng/ml).


Other routine laboratory results including coagulation parameters were unremarkable.
Discussion
Based on the classic examination findings and history of gastrointestinal bleeding, this patient has Klippel‐Trenaunay‐Weber syndrome (KTWS), which is characterized by cutaneous malformations of the capillary and venous systems, bony and soft tissue hypertrophy, and arteriovenous malformations (AVMs).1 Many patients with KTWS suffer recurrent bleeding from gastrointestinal AVMs.
Although involvement is usually unilateral, this patient had bilateral limb hypertrophy and hemangiomas. His nevus flammeus was unilateral and incidentally was present over the lower abdomen and posterior thigh and buttock, with significant underlying varices in the pelvis and rectum. His hematuria was secondary to AVMs in the bladder and resolved by itself. The size and extent of his pelvic and rectal varices presented a therapeutic challenge. With blood transfusions and a conservative approach, his bleeding diminished spontaneously. A rectal artery was thought to be contributing to the problem, so a prophylactic embolization was performed by interventional radiology. Follow‐up at 2 months revealed no further bleeding.
Hospitalists treat common causes of gastrointestinal (GI) bleeding such as ulcers, polyps, malignancies, varices, inflammatory bowel disease, AVMs, and, rarely, mucosal Kaposi sarcoma. However, they may occasionally encounter an adult with skin manifestations of a congenital cause of GI bleeding. The 4 most common congenital disorders with primary cutaneous manifestations that also involve the GI tract are reviewed below (also see Table 1).
| Vascular Malformation Syndromes | Characteristics |
|---|---|
| Klippel‐Trenaunay‐Weber | Soft tissue; bony, vascular lesions; and varices |
| Mafucci | Enchondromas, subcutaneous visceral lesions |
| Blue rubber bleb nevus | Bluish black sessile venous malformations |
| Osler‐Maffuci‐Weber‐Rendu | Mucocutaneous telangiectasias |
Blue rubber bleb nevus syndrome, also known as Bean syndrome, is the rarest of these disorders, characterized by cutaneous and intestinal cavernous hemangiomas that may occasionally be painful and tender.2 Hemangiomas may measure from a few millimeters to approximately 5 cm and are raised, blue‐purple, and rubbery in consistency, with a wrinkled surface. They are usually located on the trunk, extremities, face, and any part of the GI tract, with the small intestine and distal colon being the most common sites involved. Given that the lesions may involve the full thickness of the bowel wall, surgery is often required, as less invasive measures such as endoscopic laser coagulation may be inadequate. Orthopedic problems such as scoliosis arise from pressure exerted by large vascular malformations.
Maffucci syndrome is characterized by skeletal and vascular malformations manifested as enchondromas in the metaphyseal and diaphyseal portion of long bones. The vascular lesions, which may involve mucous membranes or viscera, are compressible blue‐purple hemangiomas that follow the rate of the growth of the child. Limb deformities, pathological fractures, and malignant transformation into chondrosarcomas are common complications.3
Osler‐Weber‐Rendu syndrome is also known as hereditary hemorrhagic telangiectasia. In this disorder, mucocutaneous telangiectatic lesions usually develop by puberty and may involve the conjunctiva, respiratory tract, brain, liver, GI tract, and genitourinary (GU) tract. Most patients exhibit only epistaxis, yet massive hemorrhage may occur in the lung, GI tract, and GU tract. These hemorrhages can usually be managed by cautery or electrocoagulation but pulmonary and GI lesions may need excision.4
KTWS consists of the triad of cutaneous vascular malformations of the capillary, venous and lymphatic systems, bony and soft tissue hypertrophy, and venous varicosities in association with AVMs. The name Weber is added when patients have AVMs that are clinically significant; otherwise, it is simply known as Klippel‐Trenaunay syndrome. The most common cutaneous vascular lesion is a capillary hemangioma known as a nevus flammeus. The distribution of the nevus flammeus usually indicates underlying vascular malformations that may extend as deep as the bone, causing limb or digit hypertrophy, as seen in this patient.5 Delineation of the extent of vascular abnormalities is accomplished by noninvasive methods such as color ultrasonography, magnetic resonance imaging, and computer‐aided angiography. Symptomatic GI or GU involvement is rare (1%), but can cause significant hemorrhage.6 Surgical correction is often difficult and the lesions tend to recur.
In the largest published series of Klippel‐Trenaunay patients, followed over 30 years at the Mayo Clinic, most patients were treated conservatively, with surgery limited to epiphysiodesis to prevent excessive leg length in the affected limbs and selected superficial vein stripping in patients with large venous varicosities with preserved deep venous systems.7, 8 For the treatment of AVMs, nonsurgical measures such as foam embolization and radiotherapy are increasingly being used due to their safety and precise application.9, 10
- ,,, et al.Klippel‐Trenaunay syndrome.Am J Med Genet.1998;79(4):319–326.
- .Blue rubber bleb nevus syndrome.Curr Treat Options Gastroenterol.2001;4(5):433–440.
- ,.Maffucci's syndrome, functional and neoplastic significance. Case report and review of the literature.J Bone Joint Surg Am.1973;55:1465–1479.
- ,,.Hereditary hemorrhagic telangiectasia (Osler‐Weber‐Rendu syndrome): a view from the 21st century.Postgrad Med J.2003;79:18–24.
- ,.Klippel Trenaunay syndrome: the importance of “geographic stains” in identifying lymphatic disease and risk of complications.J Am Acad Dermatol.2004;51(3):391–398.
- ,,,.Klippel‐Trenaunay syndrome with involvement of cecum and rectum: a rare cause of lower gastrointestinal bleeding.Eur J Med Res.2004;9(11):515–517.
- ,,.Klippel‐Trenaunay syndrome: spectrum and management.Mayo Clinic Proc.1998;73:28–36.
- ,,,,,.Surgical treatment of venous malformations in Klippel‐Trenaunay syndrome.J Vasc Surg.2000;32:840–847.
- .Radiotherapy in the management of Klippel‐Trenaunay‐Weber syndrome: report of two cases.Ann Vasc Surg.2005;19(4):566–571.
- ,,.Venous angiomata: treatment with sclerosant foam.Ann Vasc Surg.2005;19:457–464.
- ,,, et al.Klippel‐Trenaunay syndrome.Am J Med Genet.1998;79(4):319–326.
- .Blue rubber bleb nevus syndrome.Curr Treat Options Gastroenterol.2001;4(5):433–440.
- ,.Maffucci's syndrome, functional and neoplastic significance. Case report and review of the literature.J Bone Joint Surg Am.1973;55:1465–1479.
- ,,.Hereditary hemorrhagic telangiectasia (Osler‐Weber‐Rendu syndrome): a view from the 21st century.Postgrad Med J.2003;79:18–24.
- ,.Klippel Trenaunay syndrome: the importance of “geographic stains” in identifying lymphatic disease and risk of complications.J Am Acad Dermatol.2004;51(3):391–398.
- ,,,.Klippel‐Trenaunay syndrome with involvement of cecum and rectum: a rare cause of lower gastrointestinal bleeding.Eur J Med Res.2004;9(11):515–517.
- ,,.Klippel‐Trenaunay syndrome: spectrum and management.Mayo Clinic Proc.1998;73:28–36.
- ,,,,,.Surgical treatment of venous malformations in Klippel‐Trenaunay syndrome.J Vasc Surg.2000;32:840–847.
- .Radiotherapy in the management of Klippel‐Trenaunay‐Weber syndrome: report of two cases.Ann Vasc Surg.2005;19(4):566–571.
- ,,.Venous angiomata: treatment with sclerosant foam.Ann Vasc Surg.2005;19:457–464.