User login
2017 Update on fertility
Zika virus is a serious problem. Education and infection prevention are critical to effective management, and why we chose to include Zika virus as a topic for this year’s Update. We also discuss obesity’s effects on reproduction—a very relevant concern for all ObGyns and patients alike as about half of reproductive-age women are obese. Finally, subclinical hypothyroidism can present unique management challenges, such as determining when it is present and when treatment is indicated.
Read about counseling patients about Zika virus
Managing attempted pregnancy in the era of Zika virus
Oduyebo T, Igbinosa I, Petersen EE, et al. Update: interim guidance for health care providers caring for pregnant women with possible Zika virus exposure--United States, July 2016. MMWR Morb Mortal Wkly Rep. 2016;65(29):739-744.
Petersen EE, Meaney-Delman D, Neblett-Fanfair R, et al. Update: interim guidance for preconception counseling and prevention of sexual transmission of Zika virus for persons with possible Zika virus exposure--United States, September 2016. MMWR Morb Mortal Wkly Rep. 2016;65(39):1077-1081.
US Food and Drug Administration. Donor Screening Recommendations to Reduce the Risk of Transmission of Zika Virus by Human Cells, Tissues, and Cellular and Tissue-Based Products. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/UCM488582.pdf. Published March 2016. Accessed January 12, 2017.
National Institutes of Health. Zika: Overview. https://www.nichd.nih.gov/health/topics/zika/Pages/default.aspx. Accessed January 12, 2017.
World Health Organization. Prevention of sexual transmission of Zika virus interim guidance. WHO reference number: WHO/ZIKV/MOC/16. 1 Rev. 3, September 6, 2016.
Zika Virus Guidance Task Force of the American Society for Reproductive Medicine. Rev. 13, September 2016.
Zika virus presents unique challenges to physicians managing the care of patients attempting pregnancy, with or without fertility treatment. Neonatal Zika virus infection sequelae only recently have been appreciated; microcephaly was associated with Zika virus in October 2015, followed by other neurologic conditions including brain abnormalities, neural tube defects, and eye abnormalities. Results of recent studies involving the US Zika Pregnancy Registry show that 6% of women with Zika at any time in pregnancy had affected babies, but 11% of those who contracted the disease in the first trimester were affected.
Diagnosis is difficult because symptoms are generally mild, with 80% of affected patients asymptomatic. Possible Zika virus exposure is defined as travel to or residence in an area of active Zika virus transmission, or sex without a condom with a partner who traveled to or lived in an area of active transmission. Much is unknown about the interval from exposure to symptoms. Testing availability is limited and variable, and much is unknown about sensitivity and specificity of direct viral RNA testing, appearance and disappearance of detectable immunoglobulin (Ig) M and IgG antibodies that affect false positive and false negative test results, duration of infectious phase, risk of transmission, and numerous other factors.
Positive serum viral testing likely indicates virus in semen or other bodily fluids, but a negative serum viral test cannot definitively preclude virus in other bodily fluids. Zika virus likely can be passed from any combination of semen and vaginal and cervical fluids, but validating tests for these fluids are not yet available. It is not known if sperm preparation and assisted reproductive technology (ART) procedures that minimize risk of HIV transmission are effective against Zika virus or whether or not cryopreservation can destroy the virus.
Pregnancy timing
The Centers for Disease Control and Prevention now recommends that all men with possible Zika virus exposure who are considering attempting pregnancy with their partner wait to get pregnant until at least 6 months after symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic). Women with possible Zika virus exposure are recommended to wait to get pregnant until at least 8 weeks after symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic).
Women and men with possible exposure to Zika virus but without clinical symptoms of illness should consider testing for Zika viral RNA within 2 weeks of suspected exposure and wait at least 8 weeks after the last date of exposure before being re-tested. If direct viral testing (using rRT-PCR) results initially are negative, ideally, antibody testing would be obtained, if available, at 8 weeks. However, no testing paradigm will absolutely guarantee lack of Zika virus infectivity.
Virus management problems are dramatically compounded in areas endemic for Zika. Women and men who have had Zika virus disease should wait at least 6 months after illness onset to attempt reproduction. The temporal relationship between the presence of viral RNA and infectivity is not known definitively, and so the absolute duration of time to wait before attempting pregnancy is unknown. Male and female partners who become infected should avoid all forms of intimate sexual conduct or use condoms for the same 6 months. There is no evidence Zika will cause congenital infection in pregnancies initiated after resolution of maternal Zika viremia. However, any testing performed at a time other than the time of treatment might not reflect true viral status, particularly in areas of active Zika virus transmission.
Prevention
Women and men, especially those residing in areas of active Zika virus transmission, should talk with their physicians regarding pregnancy plans and avoid mosquito bites using the usual precautions: avoid mosquito areas, drain standing water, use mosquito repellent containing DEET, and use mosquito netting. Some people have gone so far as to relocate to nonendemic areas.
Those contemplating pregnancy should be advised to consider what they would do if they become exposed to or have suspected or confirmed Zika virus during pregnancy. Additional considerations are gamete or embryo cryopreservation and quarantine until a subsequent rRT-PCR test result is negative in both the male and female and at least 8 weeks have passed from gamete collection.

Patient counseling essentials
Counsel patients considering reproduction about:
- Zika virus as a new reproductive hazard
- the significance of the hazard to the fetus if infected
- the areas of active transmission, and that they are constantly changing
- avoidance of Zika areas if possible
- methods of transmission through mosquito bites or sex
- avoidance of mosquito bites
- symptoms of Zika infection
- safe sex practices
- testing limitations and knowledge deficiency about Zika.
Not uncommonly, clinical situations require complex individualized management decisions regarding trade-offs of risks, especially in older patients with decreased ovarian reserve. Consultation with infectious disease and reproductive specialists should be obtained when complicated and consequential decisions have to be made.
All practitioners should inform their patients, especially those undergoing fertility treatments, about Zika, and develop language in their informed consent that conveys the gap in knowledge to these patients.
Read how obesity specifically affects reproduction in an adverse way
Obesity adversely affects reproduction, but how specifically?
Practice Committee of the American Society for Reproductive Medicine. Obesity and Reproduction: A committee opinion. Fertil Steril. 2015;104(5):1116-1126.
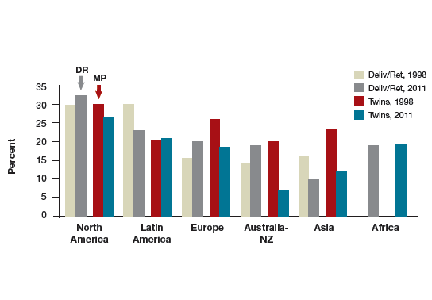
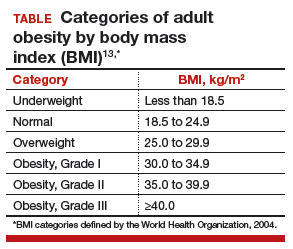
The prevalence of obesity has increased substantially over the past 2 decades. Almost two-thirds of women and three-fourths of men in the United States are overweight or obese (defined as a body mass index [BMI] ≥25 kg/m2 and BMI ≥30 kg/m2, respectively; TABLE). Nearly 50% of reproductive-age women are obese.
A disease of excess body fat and insulin resistance, obesity increases the risks of hypertension, diabetes, dyslipidemia, cardiovascular disease, sleep apnea, respiratory problems, and cancer as well as other serious health problems. While not all individuals with obesity will have infertility, obesity is associated with impaired reproduction in both women and men, adverse obstetric outcomes, and health problems in offspring. The American Society for Reproductive Medicine (ASRM) reviewed this important issue in a recent practice committee opinion.
Menstrual cycle and ovulatory dysfunction
Menstrual cycle abnormalities are more common in women with obesity. Elevated levels of insulin in obese women suppress sex hormone−binding globulin (SHBG) which in turn reduces gonadotropin secretion due to increased production of estrogen from conversion of androgens by adipose aromatase.1 Adipose tissue produces adipokines, which directly can suppress ovarian function.2
Ovulatory dysfunction is common among obese women; the relative risk of such dysfunction is 3.1 (95% confidence interval [CI], 2.2−4.4) among women with BMI levels >27 kg/m2 versus BMI levels 20.0 to 24.9 kg/m2.3,4 Obesity decreases fecundity even in women with normal menstrual cycles.5 This may in part be due to altered ovulatory dynamics with reduced early follicular luteinizing hormone pulse amplitude accompanied by prolonged folliculogenesis and reduced luteal progesterone levels.6
Compared with normal-weight women, obese women have a lower chance of conception within 1 year of stopping contraception; about 66% of obese women conceive within 1 year of stopping contraception, compared with about 81% of women with normal weight.7 Results of a Dutch study of 3,029 women with regular ovulation, at least one patent tube, and a partner with a normal semen analysis indicated a direct correlation between obesity and delayed conception, with a 4% lower spontaneous pregnancy rate per kg/m2 increase in women with a BMI >29 kg/m2 versus a BMI of 21 to 29 kg/m2 (hazard ratio, 0.96; 95% CI, 0.91−0.99).8
Assisted reproduction
Assisted reproduction in women with obesity is associated with lower success rates than in women with normal weight. A systematic review of 27 in vitro fertilization (IVF) studies (23 of which were retrospective) reveals 10% lower live-birth rate in overweight (BMI >25 kg/m2) versus normal-weight women (BMI <25 kg/m2) undergoing IVF (odds ratio [OR], 0.90; 95% CI, 0.82−1.0).9 Data from a meta-analysis of 33 IVF studies, including 47,967 cycles, show that, compared with women with a BMI <25 kg/m2, overweight or obese women have significantly reduced rates of clinical pregnancy (relative risk [RR], 0.90; P<.0001) and live birth (RR, 0.84; P = .0002).10
Results of a retrospective study of 4,609 women undergoing first IVF or IVF/intracytoplasmic sperm injection cycles revealed impaired embryo implantation (controlling for embryo quality and transfer day), reducing the age-adjusted odds of live birth in a BMI-dependent manner by 37% (BMI, 30.0−34.9 kg/m2), 61% (BMI, 35.0−39.9 kg/m2), and 68% (BMI, >40 kg/m2) compared with women with a BMI of 18.5 to 24.9 kg/m2.11 In a study of 12,566 Danish couples undergoing assisted reproduction, overweight and obese ovulatory women had a 12% (95% CI, 0.79−0.99) and 25% (95% CI, 0.63−0.90) reduction in IVF-related live birth rate, respectively (referent BMI, 18.5−24.9 kg/m2), with a 2% (95% CI, 0.97−0.99) decrease in live-birth rate for every one-unit increase in BMI.12 Putative mechanisms for these findings include altered oocyte morphology and reduced fertilization in eggs from obese women,13 and impaired embryo quality in women less than age 35.14 Oocytes from women with a BMI >25 kg/m2 are smaller and less likely to complete development postfertilization, with embryos arrested prior to blastulation containing more triglyceride than those forming blastocysts.15
Blastocysts developed from oocytes of high-BMI women are smaller, contain fewer cells and have a higher content of triglycerides, lower glucose consumption, and altered amino acid metabolism compared with embryos of normal-weight women (BMI <24.9 kg/m2).15 Obesity may alter endometrial receptivity during IVF given the finding that third-party surrogate women with a BMI >35 kg/m2 have a lower live-birth rate (25%) compared with women with a BMI <35 kg/m2 (49%; P<.05).16
Pregnancy outcomes
Obesity is linked to an increased risk of miscarriage. Results of a meta-analysis of 33 IVF studies including 47,967 cycles indicated that overweight or obese women have a higher rate of miscarriage (RR, 1.31; P<.0001) than normal-weight women (BMI <25 kg/m2).17 Maternal and perinatal morbid obesity are strongly associated with obstetric and perinatal complications, including gestational diabetes, hypertension, preeclampsia, preterm delivery, shoulder dystocia, fetal distress, early neonatal death, and small- as well as large-for-gestational age infants.
Obese women who conceive by IVF are at increased risk for preeclampsia, gestational diabetes, preterm delivery, and cesarean delivery.13 Authors of a meta-analysis of 18 observational studies concluded that obese mothers were at increased odds of pregnancies affected by such birth defects as neural tube defects, cardiovascular anomalies, and cleft lip and palate, among others.18
In addition to being the cause of these fetal abnormalities, maternal metabolic dysfunction is linked to promoting obesity in offspring, thereby perpetuating a cycle of obesity and adverse health outcomes that include an increased risk of premature death in adult offspring in subsequent generations.13
Treatment for obesity
Lifestyle modification is the first-line treatment for obesity.
Pre-fertility therapy and pregnancy goals. Targets for pregnancy should include:
- preconception weight loss to a BMI of 35 kg/m2
- prevention of excess weight gain in pregnancy
- long-term reduction in weight.
For all obese individuals, lifestyle modifications should include a weight loss of 7% of body weight and increased physical activity to at least 150 minutes of moderate activity, such as walking, per week. Calorie restriction should be emphasized. A 500 to 1,000 kcal/day decrease from usual dietary intake is expected to result in a 1- to 2-lb weight loss per week. A low-calorie diet of 1,000 to 1,200 kcal/day can lead to an average 10% decrease in total body weight over 6 months.
Adjunct supervised medical therapy or bariatric surgery can play an important role in successful weight loss prepregnancy but are not appropriate for women actively attempting conception. Importantly, pregnancy should be deferred for a minimum of 1 year after bariatric surgery. The decision to postpone pregnancy to achieve weight loss must be balanced against the risk of declining fertility with advancing age of the woman.
Read about when to treat subclinical hypothyroidism
Optimal management of subclinical hypothyroidism in women with infertility
Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545-553.
Thyroid disorders long have been associated with the potential for adverse reproductive outcomes. While overt hypothyroidism has been linked to infertility, increased miscarriage risk, and poor maternal and fetal outcomes, controversy has existed regarding the association between subclinical hypothyroidism (SCH) and reproductive problems. The ASRM recently published a guideline on the role of SCH in the infertile female population.
How is subclinical hypothyroidism defined?
SCH is classically defined as a thyrotropin (TSH) level above the upper limit of normal range (4.5−5.0 mIU/L) with normal free thyroxine (FT4) levels. The National Health and Nutrition Examination Survey (NHANES III) population has been used to establish normative data for TSH for a disease-free population. These include a median serum level for TSH of 1.5 mIU/L, with the corresponding 2.5 and 97.5 percentiles of 0.41 and 6.10, respectively.19 Data from the National Academy of Clinical Biochemistry, however, reveal that 95% of individuals without evidence of thyroid disease have a TSH level <2.5 mIU/L, and that the normal reference range is skewed to the right.20 Adjusting the upper limit of the normal range to 2.5 mIU/L would result in an additional 11.8% to 14.2% of the United States population (22 to 28 million individuals) being diagnosed with hypothyroidism.
This information raises several important questions.
1. Should nonpregnant women be treated for SCH?
No. There is no benefit from the standpoint of lipid profile or alteration of cardiovascular risk in the treatment of TSH levels between 5 and 10 mIU/L and, therefore, treatment of individuals with TSH <5 mIU/L is questionable. Furthermore, the risk of overtreatment resulting in bone loss is a concern. The Endocrine Society does not recommend changing the current normal TSH range for nonpregnant women.
2. What are normal TSH levels in pregnant women?
Because human chorionic gonadotropin (hCG) can bind to and affect the TSH receptor, thereby influencing TSH values, the normal range for TSH is modified in pregnancy. The Endocrine Society recommends the following pregnancy trimester guidelines for TSH levels: 2.5 mIU/L is the recommended upper limit of normal in the first trimester, 3.0 mIU/L in the second trimester, and 3.5 mIU/L in the third trimester.
3. Is untreated SCH associated with miscarriage?
There is fair evidence that SCH, defined as a TSH level >4 mIU/L during pregnancy, is associated with miscarriage, but there is insufficient evidence that TSH levels between 2.5 and 4 mIU/L are associated with miscarriage.
4. Is untreated SCH associated with infertility?
Limited data are available to assess the effect of SCH on infertility. While a few studies show an association between SCH on unexplained infertility and ovulatory disorders, SCH does not appear to be increased in other causes of infertility.
5. Is SCH associated with adverse obstetric outcomes?
Available data reveal that SCH with TSH levels outside the normal pregnancy range are associated with an increased risk of such obstetric complications as placental abruption, preterm birth, fetal death, and preterm premature rupture of membranes (PPROM). However, it is unclear if prepregnancy TSH levels between 2.5 and 4 mIU/L are associated with adverse obstetric outcomes.
6. Does untreated SCH affect developmental outcomes in children?
The fetus is solely dependent on maternal thyroid hormone in early pregnancy because the fetal thyroid does not produce thyroid hormone before 10 to 13 weeks of gestation. Significant evidence has associated untreated maternal hypothyroidism with delayed fetal neurologic development, impaired school performance, and lower intelligence quotient (IQ) among offspring.21 There is fair evidence that SCH diagnosed in pregnancy is associated with adverse neurologic development. There is no evidence that SCH prior to pregnancy is associated with adverse neurodevelopmental outcomes. It should be noted that only one study has examined whether treatment of SCH improves developmental outcomes (measured by IQ scored at age 3 years) and no significant differences were observed in women with SCH who were treated with levothyroxine versus those who were not.22
7. Does treatment of SCH improve miscarriage rates, live-birth rates, and/or clinical pregnancy rates?
Small randomized controlled studies of women undergoing infertility treatment and a few observational studies in the general population yield good evidence that levothyroxine treatment in women with SCH defined as TSH >4.0 mIU/L is associated with improvement in pregnancy, live birth, and miscarriage rates. There are no randomized trials assessing whether levothyroxine treatment in women with TSH levels between 2.5 and 4 mIU/L would yield similar benefits to those observed in women with TSH levels above 4 mIU/L.
8. Are thyroid antibodies associated with infertility or adverse reproductive outcomes?
There is good evidence that the thyroid autoimmunity, or the presence of TPO-Ab, is associated with miscarriage and fair evidence that it is associated with infertility. Treatment with levothyroxine may improve pregnancy outcomes especially if the TSH level is above 2.5 mIU/L.
9. Should there be universal screening for hypothyroidism in the first trimester of pregnancy?
Current evidence does not reveal a benefit of universal screening at this time. The American College of Obstetricians and Gynecologists does not recommend routine screening for hypothyroidism in pregnancy unless women have risk factors for thyroid disease, including a personal or family history of thyroid disease, physical findings or symptoms of goiter or hypothyroidism, type 1 diabetes mellitus, infertility, history of miscarriage or preterm delivery, and/or personal or family history of autoimmune disease.
The bottom line
SCH, defined as a TSH level greater than the upper limit of normal range (4.5−5.0 mIU/L)with normal FT4 levels, is associated with adverse reproductive outcomes including miscarriage, pregnancy complications, and delayed fetal neurodevelopment. Thyroid supplementation is beneficial; however, treatment has not been shown to improve long-term neurologic developmental outcomes in offspring. Data are limited on whether TSH values between 2.5 mIU/L and the upper range of normal are associated with adverse pregnancy outcomes and therefore treatment in this group remains controversial. Although available evidence is weak, there may be a benefit in some subgroups, and because risk is minimal, it may be reasonable to treat or to monitor levels and treat above nonpregnant and pregnancy ranges. There is fair evidence that thyroid autoimmunity (positive thyroid antibody) is associated with miscarriage and infertility. Levothyroxine therapy may improve pregnancy outcomes especially if the TSH level is above 2.5 mIU/L. While universal screening of thyroid function in pregnancy is not recommended, women at high risk for thyroid disease should be screened.23
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. Obesity and reproductive disorders in women. Hum Reprod Update. 2003;9(4):359-372.
- Greisen S, Ledet T, Møller N, et al. Effects of leptin on basal and FSH stimulated steroidogenesis in human granulosa luteal cells. Acta Obstet Gynecol Scand. 2000;79(11):931-935.
- Rich-Edwards JW, Goldman MB, Willett WC, et al. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171(1):171-177.
- Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994;5(2):247-250.
- Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22(2):414-420.
- Jain A, Polotsky AJ, Rochester D, et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92(7):2468-2473.
- Lake JK, Power C, Cole TJ. Women's reproductive health: the role of body mass index in early and adult life. Int J Obes Relat Metab Disord. 1997;21(6):432-438.
- van der Steeg JW, Steures P, Eijkemans MJ, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod. 2008;23(2):324-328.
- Koning AM, Mutsaerts MA, Kuchenbecker WK, et al. Complications and outcome of assisted reproduction technologies in overweight and obese women [Published correction appears in Hum Reprod. 2012;27(8):2570.] Hum Reprod. 2012;27(2):457-467.
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421-439.
- Moragianni VA, Jones SM, Ryley DA. The effect of body mass index on the outcomes of first assisted reproductive technology cycles. Fertil Steril. 2012;98(1):102-108.
- Petersen GL, Schmidt L, Pinborg A, Kamper-Jørgensen M. The influence of female and male body mass index on live births after assisted reproductive technology treatment: a nationwide register-based cohort study. Fertil Steril. 2013;99(6):1654-1662.
- Practice Committee of the American Society for Reproductive Medicine. Obesity and Reproduction: A committee opinion. Fertil Steril. 2015;104(5):1116-1126.
- Metwally M, Cutting R, Tipton A, Skull J, Ledger WL, Li TC. Effect of increased body mass index on oocyte and embryo quality in IVF patients. Reprod Biomed Online. 2007;15(5):532-538.
- Leary C, Leese HJ, Sturmey RG. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum Reprod. 2015;30(1):122-132.
- Deugarte D, Deugarte C, Sahakian V. Surrogate obesity negatively impacts pregnancy rates in third-party reproduction. Fertil Steril. 2010;93(3):1008-1010.
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421-439.
- Stothard KJ, Tennant PWG, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301(6):636-650.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499.
- Baloch Z, Carayon P, Conte-Devolx B, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13(1):3-126.
- Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf). 1999;50(2):149-155.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366(17):493-501.
- Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545-553.
Zika virus is a serious problem. Education and infection prevention are critical to effective management, and why we chose to include Zika virus as a topic for this year’s Update. We also discuss obesity’s effects on reproduction—a very relevant concern for all ObGyns and patients alike as about half of reproductive-age women are obese. Finally, subclinical hypothyroidism can present unique management challenges, such as determining when it is present and when treatment is indicated.
Read about counseling patients about Zika virus
Managing attempted pregnancy in the era of Zika virus
Oduyebo T, Igbinosa I, Petersen EE, et al. Update: interim guidance for health care providers caring for pregnant women with possible Zika virus exposure--United States, July 2016. MMWR Morb Mortal Wkly Rep. 2016;65(29):739-744.
Petersen EE, Meaney-Delman D, Neblett-Fanfair R, et al. Update: interim guidance for preconception counseling and prevention of sexual transmission of Zika virus for persons with possible Zika virus exposure--United States, September 2016. MMWR Morb Mortal Wkly Rep. 2016;65(39):1077-1081.
US Food and Drug Administration. Donor Screening Recommendations to Reduce the Risk of Transmission of Zika Virus by Human Cells, Tissues, and Cellular and Tissue-Based Products. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/UCM488582.pdf. Published March 2016. Accessed January 12, 2017.
National Institutes of Health. Zika: Overview. https://www.nichd.nih.gov/health/topics/zika/Pages/default.aspx. Accessed January 12, 2017.
World Health Organization. Prevention of sexual transmission of Zika virus interim guidance. WHO reference number: WHO/ZIKV/MOC/16. 1 Rev. 3, September 6, 2016.
Zika Virus Guidance Task Force of the American Society for Reproductive Medicine. Rev. 13, September 2016.
Zika virus presents unique challenges to physicians managing the care of patients attempting pregnancy, with or without fertility treatment. Neonatal Zika virus infection sequelae only recently have been appreciated; microcephaly was associated with Zika virus in October 2015, followed by other neurologic conditions including brain abnormalities, neural tube defects, and eye abnormalities. Results of recent studies involving the US Zika Pregnancy Registry show that 6% of women with Zika at any time in pregnancy had affected babies, but 11% of those who contracted the disease in the first trimester were affected.
Diagnosis is difficult because symptoms are generally mild, with 80% of affected patients asymptomatic. Possible Zika virus exposure is defined as travel to or residence in an area of active Zika virus transmission, or sex without a condom with a partner who traveled to or lived in an area of active transmission. Much is unknown about the interval from exposure to symptoms. Testing availability is limited and variable, and much is unknown about sensitivity and specificity of direct viral RNA testing, appearance and disappearance of detectable immunoglobulin (Ig) M and IgG antibodies that affect false positive and false negative test results, duration of infectious phase, risk of transmission, and numerous other factors.
Positive serum viral testing likely indicates virus in semen or other bodily fluids, but a negative serum viral test cannot definitively preclude virus in other bodily fluids. Zika virus likely can be passed from any combination of semen and vaginal and cervical fluids, but validating tests for these fluids are not yet available. It is not known if sperm preparation and assisted reproductive technology (ART) procedures that minimize risk of HIV transmission are effective against Zika virus or whether or not cryopreservation can destroy the virus.
Pregnancy timing
The Centers for Disease Control and Prevention now recommends that all men with possible Zika virus exposure who are considering attempting pregnancy with their partner wait to get pregnant until at least 6 months after symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic). Women with possible Zika virus exposure are recommended to wait to get pregnant until at least 8 weeks after symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic).
Women and men with possible exposure to Zika virus but without clinical symptoms of illness should consider testing for Zika viral RNA within 2 weeks of suspected exposure and wait at least 8 weeks after the last date of exposure before being re-tested. If direct viral testing (using rRT-PCR) results initially are negative, ideally, antibody testing would be obtained, if available, at 8 weeks. However, no testing paradigm will absolutely guarantee lack of Zika virus infectivity.
Virus management problems are dramatically compounded in areas endemic for Zika. Women and men who have had Zika virus disease should wait at least 6 months after illness onset to attempt reproduction. The temporal relationship between the presence of viral RNA and infectivity is not known definitively, and so the absolute duration of time to wait before attempting pregnancy is unknown. Male and female partners who become infected should avoid all forms of intimate sexual conduct or use condoms for the same 6 months. There is no evidence Zika will cause congenital infection in pregnancies initiated after resolution of maternal Zika viremia. However, any testing performed at a time other than the time of treatment might not reflect true viral status, particularly in areas of active Zika virus transmission.
Prevention
Women and men, especially those residing in areas of active Zika virus transmission, should talk with their physicians regarding pregnancy plans and avoid mosquito bites using the usual precautions: avoid mosquito areas, drain standing water, use mosquito repellent containing DEET, and use mosquito netting. Some people have gone so far as to relocate to nonendemic areas.
Those contemplating pregnancy should be advised to consider what they would do if they become exposed to or have suspected or confirmed Zika virus during pregnancy. Additional considerations are gamete or embryo cryopreservation and quarantine until a subsequent rRT-PCR test result is negative in both the male and female and at least 8 weeks have passed from gamete collection.

Patient counseling essentials
Counsel patients considering reproduction about:
- Zika virus as a new reproductive hazard
- the significance of the hazard to the fetus if infected
- the areas of active transmission, and that they are constantly changing
- avoidance of Zika areas if possible
- methods of transmission through mosquito bites or sex
- avoidance of mosquito bites
- symptoms of Zika infection
- safe sex practices
- testing limitations and knowledge deficiency about Zika.
Not uncommonly, clinical situations require complex individualized management decisions regarding trade-offs of risks, especially in older patients with decreased ovarian reserve. Consultation with infectious disease and reproductive specialists should be obtained when complicated and consequential decisions have to be made.
All practitioners should inform their patients, especially those undergoing fertility treatments, about Zika, and develop language in their informed consent that conveys the gap in knowledge to these patients.
Read how obesity specifically affects reproduction in an adverse way
Obesity adversely affects reproduction, but how specifically?
Practice Committee of the American Society for Reproductive Medicine. Obesity and Reproduction: A committee opinion. Fertil Steril. 2015;104(5):1116-1126.
The prevalence of obesity has increased substantially over the past 2 decades. Almost two-thirds of women and three-fourths of men in the United States are overweight or obese (defined as a body mass index [BMI] ≥25 kg/m2 and BMI ≥30 kg/m2, respectively; TABLE). Nearly 50% of reproductive-age women are obese.

A disease of excess body fat and insulin resistance, obesity increases the risks of hypertension, diabetes, dyslipidemia, cardiovascular disease, sleep apnea, respiratory problems, and cancer as well as other serious health problems. While not all individuals with obesity will have infertility, obesity is associated with impaired reproduction in both women and men, adverse obstetric outcomes, and health problems in offspring. The American Society for Reproductive Medicine (ASRM) reviewed this important issue in a recent practice committee opinion.
Menstrual cycle and ovulatory dysfunction
Menstrual cycle abnormalities are more common in women with obesity. Elevated levels of insulin in obese women suppress sex hormone−binding globulin (SHBG) which in turn reduces gonadotropin secretion due to increased production of estrogen from conversion of androgens by adipose aromatase.1 Adipose tissue produces adipokines, which directly can suppress ovarian function.2
Ovulatory dysfunction is common among obese women; the relative risk of such dysfunction is 3.1 (95% confidence interval [CI], 2.2−4.4) among women with BMI levels >27 kg/m2 versus BMI levels 20.0 to 24.9 kg/m2.3,4 Obesity decreases fecundity even in women with normal menstrual cycles.5 This may in part be due to altered ovulatory dynamics with reduced early follicular luteinizing hormone pulse amplitude accompanied by prolonged folliculogenesis and reduced luteal progesterone levels.6
Compared with normal-weight women, obese women have a lower chance of conception within 1 year of stopping contraception; about 66% of obese women conceive within 1 year of stopping contraception, compared with about 81% of women with normal weight.7 Results of a Dutch study of 3,029 women with regular ovulation, at least one patent tube, and a partner with a normal semen analysis indicated a direct correlation between obesity and delayed conception, with a 4% lower spontaneous pregnancy rate per kg/m2 increase in women with a BMI >29 kg/m2 versus a BMI of 21 to 29 kg/m2 (hazard ratio, 0.96; 95% CI, 0.91−0.99).8
Assisted reproduction
Assisted reproduction in women with obesity is associated with lower success rates than in women with normal weight. A systematic review of 27 in vitro fertilization (IVF) studies (23 of which were retrospective) reveals 10% lower live-birth rate in overweight (BMI >25 kg/m2) versus normal-weight women (BMI <25 kg/m2) undergoing IVF (odds ratio [OR], 0.90; 95% CI, 0.82−1.0).9 Data from a meta-analysis of 33 IVF studies, including 47,967 cycles, show that, compared with women with a BMI <25 kg/m2, overweight or obese women have significantly reduced rates of clinical pregnancy (relative risk [RR], 0.90; P<.0001) and live birth (RR, 0.84; P = .0002).10
Results of a retrospective study of 4,609 women undergoing first IVF or IVF/intracytoplasmic sperm injection cycles revealed impaired embryo implantation (controlling for embryo quality and transfer day), reducing the age-adjusted odds of live birth in a BMI-dependent manner by 37% (BMI, 30.0−34.9 kg/m2), 61% (BMI, 35.0−39.9 kg/m2), and 68% (BMI, >40 kg/m2) compared with women with a BMI of 18.5 to 24.9 kg/m2.11 In a study of 12,566 Danish couples undergoing assisted reproduction, overweight and obese ovulatory women had a 12% (95% CI, 0.79−0.99) and 25% (95% CI, 0.63−0.90) reduction in IVF-related live birth rate, respectively (referent BMI, 18.5−24.9 kg/m2), with a 2% (95% CI, 0.97−0.99) decrease in live-birth rate for every one-unit increase in BMI.12 Putative mechanisms for these findings include altered oocyte morphology and reduced fertilization in eggs from obese women,13 and impaired embryo quality in women less than age 35.14 Oocytes from women with a BMI >25 kg/m2 are smaller and less likely to complete development postfertilization, with embryos arrested prior to blastulation containing more triglyceride than those forming blastocysts.15
Blastocysts developed from oocytes of high-BMI women are smaller, contain fewer cells and have a higher content of triglycerides, lower glucose consumption, and altered amino acid metabolism compared with embryos of normal-weight women (BMI <24.9 kg/m2).15 Obesity may alter endometrial receptivity during IVF given the finding that third-party surrogate women with a BMI >35 kg/m2 have a lower live-birth rate (25%) compared with women with a BMI <35 kg/m2 (49%; P<.05).16
Pregnancy outcomes
Obesity is linked to an increased risk of miscarriage. Results of a meta-analysis of 33 IVF studies including 47,967 cycles indicated that overweight or obese women have a higher rate of miscarriage (RR, 1.31; P<.0001) than normal-weight women (BMI <25 kg/m2).17 Maternal and perinatal morbid obesity are strongly associated with obstetric and perinatal complications, including gestational diabetes, hypertension, preeclampsia, preterm delivery, shoulder dystocia, fetal distress, early neonatal death, and small- as well as large-for-gestational age infants.
Obese women who conceive by IVF are at increased risk for preeclampsia, gestational diabetes, preterm delivery, and cesarean delivery.13 Authors of a meta-analysis of 18 observational studies concluded that obese mothers were at increased odds of pregnancies affected by such birth defects as neural tube defects, cardiovascular anomalies, and cleft lip and palate, among others.18
In addition to being the cause of these fetal abnormalities, maternal metabolic dysfunction is linked to promoting obesity in offspring, thereby perpetuating a cycle of obesity and adverse health outcomes that include an increased risk of premature death in adult offspring in subsequent generations.13
Treatment for obesity
Lifestyle modification is the first-line treatment for obesity.
Pre-fertility therapy and pregnancy goals. Targets for pregnancy should include:
- preconception weight loss to a BMI of 35 kg/m2
- prevention of excess weight gain in pregnancy
- long-term reduction in weight.
For all obese individuals, lifestyle modifications should include a weight loss of 7% of body weight and increased physical activity to at least 150 minutes of moderate activity, such as walking, per week. Calorie restriction should be emphasized. A 500 to 1,000 kcal/day decrease from usual dietary intake is expected to result in a 1- to 2-lb weight loss per week. A low-calorie diet of 1,000 to 1,200 kcal/day can lead to an average 10% decrease in total body weight over 6 months.
Adjunct supervised medical therapy or bariatric surgery can play an important role in successful weight loss prepregnancy but are not appropriate for women actively attempting conception. Importantly, pregnancy should be deferred for a minimum of 1 year after bariatric surgery. The decision to postpone pregnancy to achieve weight loss must be balanced against the risk of declining fertility with advancing age of the woman.
Read about when to treat subclinical hypothyroidism
Optimal management of subclinical hypothyroidism in women with infertility
Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545-553.
Thyroid disorders long have been associated with the potential for adverse reproductive outcomes. While overt hypothyroidism has been linked to infertility, increased miscarriage risk, and poor maternal and fetal outcomes, controversy has existed regarding the association between subclinical hypothyroidism (SCH) and reproductive problems. The ASRM recently published a guideline on the role of SCH in the infertile female population.
How is subclinical hypothyroidism defined?
SCH is classically defined as a thyrotropin (TSH) level above the upper limit of normal range (4.5−5.0 mIU/L) with normal free thyroxine (FT4) levels. The National Health and Nutrition Examination Survey (NHANES III) population has been used to establish normative data for TSH for a disease-free population. These include a median serum level for TSH of 1.5 mIU/L, with the corresponding 2.5 and 97.5 percentiles of 0.41 and 6.10, respectively.19 Data from the National Academy of Clinical Biochemistry, however, reveal that 95% of individuals without evidence of thyroid disease have a TSH level <2.5 mIU/L, and that the normal reference range is skewed to the right.20 Adjusting the upper limit of the normal range to 2.5 mIU/L would result in an additional 11.8% to 14.2% of the United States population (22 to 28 million individuals) being diagnosed with hypothyroidism.
This information raises several important questions.
1. Should nonpregnant women be treated for SCH?
No. There is no benefit from the standpoint of lipid profile or alteration of cardiovascular risk in the treatment of TSH levels between 5 and 10 mIU/L and, therefore, treatment of individuals with TSH <5 mIU/L is questionable. Furthermore, the risk of overtreatment resulting in bone loss is a concern. The Endocrine Society does not recommend changing the current normal TSH range for nonpregnant women.
2. What are normal TSH levels in pregnant women?
Because human chorionic gonadotropin (hCG) can bind to and affect the TSH receptor, thereby influencing TSH values, the normal range for TSH is modified in pregnancy. The Endocrine Society recommends the following pregnancy trimester guidelines for TSH levels: 2.5 mIU/L is the recommended upper limit of normal in the first trimester, 3.0 mIU/L in the second trimester, and 3.5 mIU/L in the third trimester.
3. Is untreated SCH associated with miscarriage?
There is fair evidence that SCH, defined as a TSH level >4 mIU/L during pregnancy, is associated with miscarriage, but there is insufficient evidence that TSH levels between 2.5 and 4 mIU/L are associated with miscarriage.
4. Is untreated SCH associated with infertility?
Limited data are available to assess the effect of SCH on infertility. While a few studies show an association between SCH on unexplained infertility and ovulatory disorders, SCH does not appear to be increased in other causes of infertility.
5. Is SCH associated with adverse obstetric outcomes?
Available data reveal that SCH with TSH levels outside the normal pregnancy range are associated with an increased risk of such obstetric complications as placental abruption, preterm birth, fetal death, and preterm premature rupture of membranes (PPROM). However, it is unclear if prepregnancy TSH levels between 2.5 and 4 mIU/L are associated with adverse obstetric outcomes.
6. Does untreated SCH affect developmental outcomes in children?
The fetus is solely dependent on maternal thyroid hormone in early pregnancy because the fetal thyroid does not produce thyroid hormone before 10 to 13 weeks of gestation. Significant evidence has associated untreated maternal hypothyroidism with delayed fetal neurologic development, impaired school performance, and lower intelligence quotient (IQ) among offspring.21 There is fair evidence that SCH diagnosed in pregnancy is associated with adverse neurologic development. There is no evidence that SCH prior to pregnancy is associated with adverse neurodevelopmental outcomes. It should be noted that only one study has examined whether treatment of SCH improves developmental outcomes (measured by IQ scored at age 3 years) and no significant differences were observed in women with SCH who were treated with levothyroxine versus those who were not.22
7. Does treatment of SCH improve miscarriage rates, live-birth rates, and/or clinical pregnancy rates?
Small randomized controlled studies of women undergoing infertility treatment and a few observational studies in the general population yield good evidence that levothyroxine treatment in women with SCH defined as TSH >4.0 mIU/L is associated with improvement in pregnancy, live birth, and miscarriage rates. There are no randomized trials assessing whether levothyroxine treatment in women with TSH levels between 2.5 and 4 mIU/L would yield similar benefits to those observed in women with TSH levels above 4 mIU/L.
8. Are thyroid antibodies associated with infertility or adverse reproductive outcomes?
There is good evidence that the thyroid autoimmunity, or the presence of TPO-Ab, is associated with miscarriage and fair evidence that it is associated with infertility. Treatment with levothyroxine may improve pregnancy outcomes especially if the TSH level is above 2.5 mIU/L.
9. Should there be universal screening for hypothyroidism in the first trimester of pregnancy?
Current evidence does not reveal a benefit of universal screening at this time. The American College of Obstetricians and Gynecologists does not recommend routine screening for hypothyroidism in pregnancy unless women have risk factors for thyroid disease, including a personal or family history of thyroid disease, physical findings or symptoms of goiter or hypothyroidism, type 1 diabetes mellitus, infertility, history of miscarriage or preterm delivery, and/or personal or family history of autoimmune disease.
The bottom line
SCH, defined as a TSH level greater than the upper limit of normal range (4.5−5.0 mIU/L)with normal FT4 levels, is associated with adverse reproductive outcomes including miscarriage, pregnancy complications, and delayed fetal neurodevelopment. Thyroid supplementation is beneficial; however, treatment has not been shown to improve long-term neurologic developmental outcomes in offspring. Data are limited on whether TSH values between 2.5 mIU/L and the upper range of normal are associated with adverse pregnancy outcomes and therefore treatment in this group remains controversial. Although available evidence is weak, there may be a benefit in some subgroups, and because risk is minimal, it may be reasonable to treat or to monitor levels and treat above nonpregnant and pregnancy ranges. There is fair evidence that thyroid autoimmunity (positive thyroid antibody) is associated with miscarriage and infertility. Levothyroxine therapy may improve pregnancy outcomes especially if the TSH level is above 2.5 mIU/L. While universal screening of thyroid function in pregnancy is not recommended, women at high risk for thyroid disease should be screened.23
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Zika virus is a serious problem. Education and infection prevention are critical to effective management, and why we chose to include Zika virus as a topic for this year’s Update. We also discuss obesity’s effects on reproduction—a very relevant concern for all ObGyns and patients alike as about half of reproductive-age women are obese. Finally, subclinical hypothyroidism can present unique management challenges, such as determining when it is present and when treatment is indicated.
Read about counseling patients about Zika virus
Managing attempted pregnancy in the era of Zika virus
Oduyebo T, Igbinosa I, Petersen EE, et al. Update: interim guidance for health care providers caring for pregnant women with possible Zika virus exposure--United States, July 2016. MMWR Morb Mortal Wkly Rep. 2016;65(29):739-744.
Petersen EE, Meaney-Delman D, Neblett-Fanfair R, et al. Update: interim guidance for preconception counseling and prevention of sexual transmission of Zika virus for persons with possible Zika virus exposure--United States, September 2016. MMWR Morb Mortal Wkly Rep. 2016;65(39):1077-1081.
US Food and Drug Administration. Donor Screening Recommendations to Reduce the Risk of Transmission of Zika Virus by Human Cells, Tissues, and Cellular and Tissue-Based Products. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/UCM488582.pdf. Published March 2016. Accessed January 12, 2017.
National Institutes of Health. Zika: Overview. https://www.nichd.nih.gov/health/topics/zika/Pages/default.aspx. Accessed January 12, 2017.
World Health Organization. Prevention of sexual transmission of Zika virus interim guidance. WHO reference number: WHO/ZIKV/MOC/16. 1 Rev. 3, September 6, 2016.
Zika Virus Guidance Task Force of the American Society for Reproductive Medicine. Rev. 13, September 2016.
Zika virus presents unique challenges to physicians managing the care of patients attempting pregnancy, with or without fertility treatment. Neonatal Zika virus infection sequelae only recently have been appreciated; microcephaly was associated with Zika virus in October 2015, followed by other neurologic conditions including brain abnormalities, neural tube defects, and eye abnormalities. Results of recent studies involving the US Zika Pregnancy Registry show that 6% of women with Zika at any time in pregnancy had affected babies, but 11% of those who contracted the disease in the first trimester were affected.
Diagnosis is difficult because symptoms are generally mild, with 80% of affected patients asymptomatic. Possible Zika virus exposure is defined as travel to or residence in an area of active Zika virus transmission, or sex without a condom with a partner who traveled to or lived in an area of active transmission. Much is unknown about the interval from exposure to symptoms. Testing availability is limited and variable, and much is unknown about sensitivity and specificity of direct viral RNA testing, appearance and disappearance of detectable immunoglobulin (Ig) M and IgG antibodies that affect false positive and false negative test results, duration of infectious phase, risk of transmission, and numerous other factors.
Positive serum viral testing likely indicates virus in semen or other bodily fluids, but a negative serum viral test cannot definitively preclude virus in other bodily fluids. Zika virus likely can be passed from any combination of semen and vaginal and cervical fluids, but validating tests for these fluids are not yet available. It is not known if sperm preparation and assisted reproductive technology (ART) procedures that minimize risk of HIV transmission are effective against Zika virus or whether or not cryopreservation can destroy the virus.
Pregnancy timing
The Centers for Disease Control and Prevention now recommends that all men with possible Zika virus exposure who are considering attempting pregnancy with their partner wait to get pregnant until at least 6 months after symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic). Women with possible Zika virus exposure are recommended to wait to get pregnant until at least 8 weeks after symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic).
Women and men with possible exposure to Zika virus but without clinical symptoms of illness should consider testing for Zika viral RNA within 2 weeks of suspected exposure and wait at least 8 weeks after the last date of exposure before being re-tested. If direct viral testing (using rRT-PCR) results initially are negative, ideally, antibody testing would be obtained, if available, at 8 weeks. However, no testing paradigm will absolutely guarantee lack of Zika virus infectivity.
Virus management problems are dramatically compounded in areas endemic for Zika. Women and men who have had Zika virus disease should wait at least 6 months after illness onset to attempt reproduction. The temporal relationship between the presence of viral RNA and infectivity is not known definitively, and so the absolute duration of time to wait before attempting pregnancy is unknown. Male and female partners who become infected should avoid all forms of intimate sexual conduct or use condoms for the same 6 months. There is no evidence Zika will cause congenital infection in pregnancies initiated after resolution of maternal Zika viremia. However, any testing performed at a time other than the time of treatment might not reflect true viral status, particularly in areas of active Zika virus transmission.
Prevention
Women and men, especially those residing in areas of active Zika virus transmission, should talk with their physicians regarding pregnancy plans and avoid mosquito bites using the usual precautions: avoid mosquito areas, drain standing water, use mosquito repellent containing DEET, and use mosquito netting. Some people have gone so far as to relocate to nonendemic areas.
Those contemplating pregnancy should be advised to consider what they would do if they become exposed to or have suspected or confirmed Zika virus during pregnancy. Additional considerations are gamete or embryo cryopreservation and quarantine until a subsequent rRT-PCR test result is negative in both the male and female and at least 8 weeks have passed from gamete collection.

Patient counseling essentials
Counsel patients considering reproduction about:
- Zika virus as a new reproductive hazard
- the significance of the hazard to the fetus if infected
- the areas of active transmission, and that they are constantly changing
- avoidance of Zika areas if possible
- methods of transmission through mosquito bites or sex
- avoidance of mosquito bites
- symptoms of Zika infection
- safe sex practices
- testing limitations and knowledge deficiency about Zika.
Not uncommonly, clinical situations require complex individualized management decisions regarding trade-offs of risks, especially in older patients with decreased ovarian reserve. Consultation with infectious disease and reproductive specialists should be obtained when complicated and consequential decisions have to be made.
All practitioners should inform their patients, especially those undergoing fertility treatments, about Zika, and develop language in their informed consent that conveys the gap in knowledge to these patients.
Read how obesity specifically affects reproduction in an adverse way
Obesity adversely affects reproduction, but how specifically?
Practice Committee of the American Society for Reproductive Medicine. Obesity and Reproduction: A committee opinion. Fertil Steril. 2015;104(5):1116-1126.
The prevalence of obesity has increased substantially over the past 2 decades. Almost two-thirds of women and three-fourths of men in the United States are overweight or obese (defined as a body mass index [BMI] ≥25 kg/m2 and BMI ≥30 kg/m2, respectively; TABLE). Nearly 50% of reproductive-age women are obese.

A disease of excess body fat and insulin resistance, obesity increases the risks of hypertension, diabetes, dyslipidemia, cardiovascular disease, sleep apnea, respiratory problems, and cancer as well as other serious health problems. While not all individuals with obesity will have infertility, obesity is associated with impaired reproduction in both women and men, adverse obstetric outcomes, and health problems in offspring. The American Society for Reproductive Medicine (ASRM) reviewed this important issue in a recent practice committee opinion.
Menstrual cycle and ovulatory dysfunction
Menstrual cycle abnormalities are more common in women with obesity. Elevated levels of insulin in obese women suppress sex hormone−binding globulin (SHBG) which in turn reduces gonadotropin secretion due to increased production of estrogen from conversion of androgens by adipose aromatase.1 Adipose tissue produces adipokines, which directly can suppress ovarian function.2
Ovulatory dysfunction is common among obese women; the relative risk of such dysfunction is 3.1 (95% confidence interval [CI], 2.2−4.4) among women with BMI levels >27 kg/m2 versus BMI levels 20.0 to 24.9 kg/m2.3,4 Obesity decreases fecundity even in women with normal menstrual cycles.5 This may in part be due to altered ovulatory dynamics with reduced early follicular luteinizing hormone pulse amplitude accompanied by prolonged folliculogenesis and reduced luteal progesterone levels.6
Compared with normal-weight women, obese women have a lower chance of conception within 1 year of stopping contraception; about 66% of obese women conceive within 1 year of stopping contraception, compared with about 81% of women with normal weight.7 Results of a Dutch study of 3,029 women with regular ovulation, at least one patent tube, and a partner with a normal semen analysis indicated a direct correlation between obesity and delayed conception, with a 4% lower spontaneous pregnancy rate per kg/m2 increase in women with a BMI >29 kg/m2 versus a BMI of 21 to 29 kg/m2 (hazard ratio, 0.96; 95% CI, 0.91−0.99).8
Assisted reproduction
Assisted reproduction in women with obesity is associated with lower success rates than in women with normal weight. A systematic review of 27 in vitro fertilization (IVF) studies (23 of which were retrospective) reveals 10% lower live-birth rate in overweight (BMI >25 kg/m2) versus normal-weight women (BMI <25 kg/m2) undergoing IVF (odds ratio [OR], 0.90; 95% CI, 0.82−1.0).9 Data from a meta-analysis of 33 IVF studies, including 47,967 cycles, show that, compared with women with a BMI <25 kg/m2, overweight or obese women have significantly reduced rates of clinical pregnancy (relative risk [RR], 0.90; P<.0001) and live birth (RR, 0.84; P = .0002).10
Results of a retrospective study of 4,609 women undergoing first IVF or IVF/intracytoplasmic sperm injection cycles revealed impaired embryo implantation (controlling for embryo quality and transfer day), reducing the age-adjusted odds of live birth in a BMI-dependent manner by 37% (BMI, 30.0−34.9 kg/m2), 61% (BMI, 35.0−39.9 kg/m2), and 68% (BMI, >40 kg/m2) compared with women with a BMI of 18.5 to 24.9 kg/m2.11 In a study of 12,566 Danish couples undergoing assisted reproduction, overweight and obese ovulatory women had a 12% (95% CI, 0.79−0.99) and 25% (95% CI, 0.63−0.90) reduction in IVF-related live birth rate, respectively (referent BMI, 18.5−24.9 kg/m2), with a 2% (95% CI, 0.97−0.99) decrease in live-birth rate for every one-unit increase in BMI.12 Putative mechanisms for these findings include altered oocyte morphology and reduced fertilization in eggs from obese women,13 and impaired embryo quality in women less than age 35.14 Oocytes from women with a BMI >25 kg/m2 are smaller and less likely to complete development postfertilization, with embryos arrested prior to blastulation containing more triglyceride than those forming blastocysts.15
Blastocysts developed from oocytes of high-BMI women are smaller, contain fewer cells and have a higher content of triglycerides, lower glucose consumption, and altered amino acid metabolism compared with embryos of normal-weight women (BMI <24.9 kg/m2).15 Obesity may alter endometrial receptivity during IVF given the finding that third-party surrogate women with a BMI >35 kg/m2 have a lower live-birth rate (25%) compared with women with a BMI <35 kg/m2 (49%; P<.05).16
Pregnancy outcomes
Obesity is linked to an increased risk of miscarriage. Results of a meta-analysis of 33 IVF studies including 47,967 cycles indicated that overweight or obese women have a higher rate of miscarriage (RR, 1.31; P<.0001) than normal-weight women (BMI <25 kg/m2).17 Maternal and perinatal morbid obesity are strongly associated with obstetric and perinatal complications, including gestational diabetes, hypertension, preeclampsia, preterm delivery, shoulder dystocia, fetal distress, early neonatal death, and small- as well as large-for-gestational age infants.
Obese women who conceive by IVF are at increased risk for preeclampsia, gestational diabetes, preterm delivery, and cesarean delivery.13 Authors of a meta-analysis of 18 observational studies concluded that obese mothers were at increased odds of pregnancies affected by such birth defects as neural tube defects, cardiovascular anomalies, and cleft lip and palate, among others.18
In addition to being the cause of these fetal abnormalities, maternal metabolic dysfunction is linked to promoting obesity in offspring, thereby perpetuating a cycle of obesity and adverse health outcomes that include an increased risk of premature death in adult offspring in subsequent generations.13
Treatment for obesity
Lifestyle modification is the first-line treatment for obesity.
Pre-fertility therapy and pregnancy goals. Targets for pregnancy should include:
- preconception weight loss to a BMI of 35 kg/m2
- prevention of excess weight gain in pregnancy
- long-term reduction in weight.
For all obese individuals, lifestyle modifications should include a weight loss of 7% of body weight and increased physical activity to at least 150 minutes of moderate activity, such as walking, per week. Calorie restriction should be emphasized. A 500 to 1,000 kcal/day decrease from usual dietary intake is expected to result in a 1- to 2-lb weight loss per week. A low-calorie diet of 1,000 to 1,200 kcal/day can lead to an average 10% decrease in total body weight over 6 months.
Adjunct supervised medical therapy or bariatric surgery can play an important role in successful weight loss prepregnancy but are not appropriate for women actively attempting conception. Importantly, pregnancy should be deferred for a minimum of 1 year after bariatric surgery. The decision to postpone pregnancy to achieve weight loss must be balanced against the risk of declining fertility with advancing age of the woman.
Read about when to treat subclinical hypothyroidism
Optimal management of subclinical hypothyroidism in women with infertility
Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545-553.
Thyroid disorders long have been associated with the potential for adverse reproductive outcomes. While overt hypothyroidism has been linked to infertility, increased miscarriage risk, and poor maternal and fetal outcomes, controversy has existed regarding the association between subclinical hypothyroidism (SCH) and reproductive problems. The ASRM recently published a guideline on the role of SCH in the infertile female population.
How is subclinical hypothyroidism defined?
SCH is classically defined as a thyrotropin (TSH) level above the upper limit of normal range (4.5−5.0 mIU/L) with normal free thyroxine (FT4) levels. The National Health and Nutrition Examination Survey (NHANES III) population has been used to establish normative data for TSH for a disease-free population. These include a median serum level for TSH of 1.5 mIU/L, with the corresponding 2.5 and 97.5 percentiles of 0.41 and 6.10, respectively.19 Data from the National Academy of Clinical Biochemistry, however, reveal that 95% of individuals without evidence of thyroid disease have a TSH level <2.5 mIU/L, and that the normal reference range is skewed to the right.20 Adjusting the upper limit of the normal range to 2.5 mIU/L would result in an additional 11.8% to 14.2% of the United States population (22 to 28 million individuals) being diagnosed with hypothyroidism.
This information raises several important questions.
1. Should nonpregnant women be treated for SCH?
No. There is no benefit from the standpoint of lipid profile or alteration of cardiovascular risk in the treatment of TSH levels between 5 and 10 mIU/L and, therefore, treatment of individuals with TSH <5 mIU/L is questionable. Furthermore, the risk of overtreatment resulting in bone loss is a concern. The Endocrine Society does not recommend changing the current normal TSH range for nonpregnant women.
2. What are normal TSH levels in pregnant women?
Because human chorionic gonadotropin (hCG) can bind to and affect the TSH receptor, thereby influencing TSH values, the normal range for TSH is modified in pregnancy. The Endocrine Society recommends the following pregnancy trimester guidelines for TSH levels: 2.5 mIU/L is the recommended upper limit of normal in the first trimester, 3.0 mIU/L in the second trimester, and 3.5 mIU/L in the third trimester.
3. Is untreated SCH associated with miscarriage?
There is fair evidence that SCH, defined as a TSH level >4 mIU/L during pregnancy, is associated with miscarriage, but there is insufficient evidence that TSH levels between 2.5 and 4 mIU/L are associated with miscarriage.
4. Is untreated SCH associated with infertility?
Limited data are available to assess the effect of SCH on infertility. While a few studies show an association between SCH on unexplained infertility and ovulatory disorders, SCH does not appear to be increased in other causes of infertility.
5. Is SCH associated with adverse obstetric outcomes?
Available data reveal that SCH with TSH levels outside the normal pregnancy range are associated with an increased risk of such obstetric complications as placental abruption, preterm birth, fetal death, and preterm premature rupture of membranes (PPROM). However, it is unclear if prepregnancy TSH levels between 2.5 and 4 mIU/L are associated with adverse obstetric outcomes.
6. Does untreated SCH affect developmental outcomes in children?
The fetus is solely dependent on maternal thyroid hormone in early pregnancy because the fetal thyroid does not produce thyroid hormone before 10 to 13 weeks of gestation. Significant evidence has associated untreated maternal hypothyroidism with delayed fetal neurologic development, impaired school performance, and lower intelligence quotient (IQ) among offspring.21 There is fair evidence that SCH diagnosed in pregnancy is associated with adverse neurologic development. There is no evidence that SCH prior to pregnancy is associated with adverse neurodevelopmental outcomes. It should be noted that only one study has examined whether treatment of SCH improves developmental outcomes (measured by IQ scored at age 3 years) and no significant differences were observed in women with SCH who were treated with levothyroxine versus those who were not.22
7. Does treatment of SCH improve miscarriage rates, live-birth rates, and/or clinical pregnancy rates?
Small randomized controlled studies of women undergoing infertility treatment and a few observational studies in the general population yield good evidence that levothyroxine treatment in women with SCH defined as TSH >4.0 mIU/L is associated with improvement in pregnancy, live birth, and miscarriage rates. There are no randomized trials assessing whether levothyroxine treatment in women with TSH levels between 2.5 and 4 mIU/L would yield similar benefits to those observed in women with TSH levels above 4 mIU/L.
8. Are thyroid antibodies associated with infertility or adverse reproductive outcomes?
There is good evidence that the thyroid autoimmunity, or the presence of TPO-Ab, is associated with miscarriage and fair evidence that it is associated with infertility. Treatment with levothyroxine may improve pregnancy outcomes especially if the TSH level is above 2.5 mIU/L.
9. Should there be universal screening for hypothyroidism in the first trimester of pregnancy?
Current evidence does not reveal a benefit of universal screening at this time. The American College of Obstetricians and Gynecologists does not recommend routine screening for hypothyroidism in pregnancy unless women have risk factors for thyroid disease, including a personal or family history of thyroid disease, physical findings or symptoms of goiter or hypothyroidism, type 1 diabetes mellitus, infertility, history of miscarriage or preterm delivery, and/or personal or family history of autoimmune disease.
The bottom line
SCH, defined as a TSH level greater than the upper limit of normal range (4.5−5.0 mIU/L)with normal FT4 levels, is associated with adverse reproductive outcomes including miscarriage, pregnancy complications, and delayed fetal neurodevelopment. Thyroid supplementation is beneficial; however, treatment has not been shown to improve long-term neurologic developmental outcomes in offspring. Data are limited on whether TSH values between 2.5 mIU/L and the upper range of normal are associated with adverse pregnancy outcomes and therefore treatment in this group remains controversial. Although available evidence is weak, there may be a benefit in some subgroups, and because risk is minimal, it may be reasonable to treat or to monitor levels and treat above nonpregnant and pregnancy ranges. There is fair evidence that thyroid autoimmunity (positive thyroid antibody) is associated with miscarriage and infertility. Levothyroxine therapy may improve pregnancy outcomes especially if the TSH level is above 2.5 mIU/L. While universal screening of thyroid function in pregnancy is not recommended, women at high risk for thyroid disease should be screened.23
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. Obesity and reproductive disorders in women. Hum Reprod Update. 2003;9(4):359-372.
- Greisen S, Ledet T, Møller N, et al. Effects of leptin on basal and FSH stimulated steroidogenesis in human granulosa luteal cells. Acta Obstet Gynecol Scand. 2000;79(11):931-935.
- Rich-Edwards JW, Goldman MB, Willett WC, et al. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171(1):171-177.
- Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994;5(2):247-250.
- Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22(2):414-420.
- Jain A, Polotsky AJ, Rochester D, et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92(7):2468-2473.
- Lake JK, Power C, Cole TJ. Women's reproductive health: the role of body mass index in early and adult life. Int J Obes Relat Metab Disord. 1997;21(6):432-438.
- van der Steeg JW, Steures P, Eijkemans MJ, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod. 2008;23(2):324-328.
- Koning AM, Mutsaerts MA, Kuchenbecker WK, et al. Complications and outcome of assisted reproduction technologies in overweight and obese women [Published correction appears in Hum Reprod. 2012;27(8):2570.] Hum Reprod. 2012;27(2):457-467.
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421-439.
- Moragianni VA, Jones SM, Ryley DA. The effect of body mass index on the outcomes of first assisted reproductive technology cycles. Fertil Steril. 2012;98(1):102-108.
- Petersen GL, Schmidt L, Pinborg A, Kamper-Jørgensen M. The influence of female and male body mass index on live births after assisted reproductive technology treatment: a nationwide register-based cohort study. Fertil Steril. 2013;99(6):1654-1662.
- Practice Committee of the American Society for Reproductive Medicine. Obesity and Reproduction: A committee opinion. Fertil Steril. 2015;104(5):1116-1126.
- Metwally M, Cutting R, Tipton A, Skull J, Ledger WL, Li TC. Effect of increased body mass index on oocyte and embryo quality in IVF patients. Reprod Biomed Online. 2007;15(5):532-538.
- Leary C, Leese HJ, Sturmey RG. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum Reprod. 2015;30(1):122-132.
- Deugarte D, Deugarte C, Sahakian V. Surrogate obesity negatively impacts pregnancy rates in third-party reproduction. Fertil Steril. 2010;93(3):1008-1010.
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421-439.
- Stothard KJ, Tennant PWG, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301(6):636-650.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499.
- Baloch Z, Carayon P, Conte-Devolx B, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13(1):3-126.
- Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf). 1999;50(2):149-155.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366(17):493-501.
- Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545-553.
- Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. Obesity and reproductive disorders in women. Hum Reprod Update. 2003;9(4):359-372.
- Greisen S, Ledet T, Møller N, et al. Effects of leptin on basal and FSH stimulated steroidogenesis in human granulosa luteal cells. Acta Obstet Gynecol Scand. 2000;79(11):931-935.
- Rich-Edwards JW, Goldman MB, Willett WC, et al. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171(1):171-177.
- Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994;5(2):247-250.
- Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22(2):414-420.
- Jain A, Polotsky AJ, Rochester D, et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92(7):2468-2473.
- Lake JK, Power C, Cole TJ. Women's reproductive health: the role of body mass index in early and adult life. Int J Obes Relat Metab Disord. 1997;21(6):432-438.
- van der Steeg JW, Steures P, Eijkemans MJ, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod. 2008;23(2):324-328.
- Koning AM, Mutsaerts MA, Kuchenbecker WK, et al. Complications and outcome of assisted reproduction technologies in overweight and obese women [Published correction appears in Hum Reprod. 2012;27(8):2570.] Hum Reprod. 2012;27(2):457-467.
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421-439.
- Moragianni VA, Jones SM, Ryley DA. The effect of body mass index on the outcomes of first assisted reproductive technology cycles. Fertil Steril. 2012;98(1):102-108.
- Petersen GL, Schmidt L, Pinborg A, Kamper-Jørgensen M. The influence of female and male body mass index on live births after assisted reproductive technology treatment: a nationwide register-based cohort study. Fertil Steril. 2013;99(6):1654-1662.
- Practice Committee of the American Society for Reproductive Medicine. Obesity and Reproduction: A committee opinion. Fertil Steril. 2015;104(5):1116-1126.
- Metwally M, Cutting R, Tipton A, Skull J, Ledger WL, Li TC. Effect of increased body mass index on oocyte and embryo quality in IVF patients. Reprod Biomed Online. 2007;15(5):532-538.
- Leary C, Leese HJ, Sturmey RG. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum Reprod. 2015;30(1):122-132.
- Deugarte D, Deugarte C, Sahakian V. Surrogate obesity negatively impacts pregnancy rates in third-party reproduction. Fertil Steril. 2010;93(3):1008-1010.
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421-439.
- Stothard KJ, Tennant PWG, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301(6):636-650.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499.
- Baloch Z, Carayon P, Conte-Devolx B, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13(1):3-126.
- Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf). 1999;50(2):149-155.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366(17):493-501.
- Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545-553.
2016 Update on fertility
Patients seeking fertility care commonly ask the physician for advice regarding ways to optimize their conception attempts. While evidence from randomized controlled trials is not available, data from observational studies provide parameters that can inform patient decision making. Knowledge about the fertility window, the decline in fecundability with age, and lifestyle practices that promote conception may be helpful to clinicians and aid in their ability to guide patients.
For those patients who will not achieve conception naturally, assisted reproductive technologies (ART) offer a promising alternative. ART options have improved greatly in effectiveness and safety since Louise Brown was born in 1978. More than 5 million babies have been born globally.1 However, even though the United States is wealthy, access to in vitro fertilization (IVF) is poor relative to many other countries, with not more than 1 in 3 people needing IVF actually receiving the treatment. Understanding the international experience enables physicians to take actions that help increase access for their patients who need IVF.
In this article we not only address ways in which your patients can optimize their natural fertility but also examine this country’s ability to offer ART options when they are needed. Without such examination, fundamental changes in societal attitudes toward infertility and payor attitudes toward reproductive care will not occur, and it is these changes, among others, that can move this country to more equitable ART access.
- Adamson GD, Tabangin M, Macaluso M, de Mouzon J. The number of babies born globally after treatment with the Assisted Reproductive Technologies (ART). Paper presented at International Federation of Fertility Societies/American Society for Reproductive Medicine Conjoint Meeting; October 12–17, 2013; Boston, Massachusetts.
- Dunson DB, Baird DD, Wilcox AJ, Weinberg CR. Day-specific probabilities of clinical pregnancy based on two studies with imperfect measures of ovulation. Hum Reprod. 1999;14(7):1835–1839.
- Keulers MJ, Hamilton CJ, Franx A, et al. The length of the fertile window is associated with the chance of spontaneously conceiving an ongoing pregnancy in subfertile couples. Hum Reprod. 2007;22(6):1652–1656.
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med. 1995;333(23):1517–1521.
- Levitas E, Lunenfeld E, Weiss N, et al. Relationship between the duration of sexual abstinence and semen quality: analysis of 9,489 semen samples. Fertil Steril. 2005;83(6):1680–1686.
- Elzanaty S, Malm J, Giwercman A. Duration of sexual abstinence: epididymal and accessory sex gland secretions and their relationship to sperm motility. Hum Reprod. 2005;20(1):221–225.
- Check JH, Epstein R, Long R. Effect of time interval between ejaculations on semen parameters. Arch Androl. 1991;27(2):93–95.
- Practice Committee of American Society for Reproductive Medicine in collaboration with Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility: a committee opinion. Fertil Steril. 2013;100(3):631–637.
- Gnoth C, Godehardt E, Frank-Herrmann P, Friol K, Tigges J, Freundi G. Definition and prevalence of subfertility and infertility. Hum Reprod. 2005;20(5):1144–1447.
- Howe G, Westhoff C, Vessey M, Yeates D. Effects of age, cigarette smoking, and other factors on fertility: findings in a large prospective study. BMJ (Clin Res Ed). 1985;290(6483):1697–700.
- Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol. 2004;103(1):51–56.
- Dunson DB, Colombo B, Baird DD. Changes with age in the level and duration of fertility in the menstrual cycle. Hum Reprod. 2002;17(5):1399–1403.
- Lumley J, Watson L, Watson M, Bower C. Periconceptional supplementation with folate and/or multivitamins for preventing neural tube defects. Cochrane Database Syst Rev. 2001;(3):CD001056.
- Augood C, Duckitt K, Templeton AA. Smoking and female infertility: a systematic review and meta-analysis. Hum Reprod. 1998;13(6):1532–1539.
- Winter E, Wang J, Davies MJ, Norman R. Early pregnancy loss following assisted reproductive technology treatment. Hum Reprod. 2002;17(12):3220–3223.
- Ness RB, Grisso JA, Hirschinger N, et al. Cocaine and tobacco use and the risk of spontaneous abortion. New Engl J Med. 1999;340(5):333–339.
- Mattison DR, Plowchalk DR, Meadows MJ, Miller MM, Malek A, London S. The effect of smoking on oogenesis, fertilization and implantation. Semin Reprod Med. 1989;7(4):291–304.
- Adena MA, Gallagher HG. Cigarette smoking and the age at menopause. Ann Hum Biol. 1982;9(2):121–130.
- Bolumar F, Olsen J, Rebagliato M, Bisanti L. Caffeine intake and delayed conception: a European multicenter study on infertility and subfecundity. European Study Group on Infertility Subfecundity. Am J Epidemiol. 1997;145(4):324–334.
- Wilcox A, Weinberg C, Baird D. Caffeinated beverages and decreased fertility. Lancet. 1988;2(8626–8627):1453–1456.
- Signorello LB, McLaughlin JK. Maternal caffeine consumption and spontaneous abortion: a review of the epidemiologic evidence. Epidemiology. 2004;15(2):229–239.
- Kesmodel U, Wisborg K, Olsen SF, Henriksen TB, Secher NJ. Moderate alcohol intake in pregnancy and the risk of spontaneous abortion. Alcohol. 2002;37(1):87–92.
- Adamson GD; International Council of Medical Acupuncture and Related Techniques (ICMART). ICMART World Report 2011. Webcast presented at: Annual Meeting European Society of Human Reproduction and Embryology (ESHRE); June 16, 2015; Lisbon, Portugal.
- Chambers G, Phuong Hoang V, et al. The impact of consumer affordability on access to assisted reproductive technologies and embryo transfer practices: an international analysis. Fertil Steril. 2014;101(1):191–198.
- Stovall DW, Allen BD, Sparks AE, Syrop CH, Saunders RG, VanVoorhis BJ. The cost of infertility evaluation and therapy: findings of a self-insured university healthcare plan. Fertil Steril. 1999;72(5):778–784.
- Chambers GM, Sullivan E, Ishihara O, Chapman MG, Adamson GD. The economic impact of assisted reproductive technology: a review of selected developed countries. Fertil Steril. 2009;91(6):2281–2294.
- Hamilton BH, McManus B. The effects of insurance mandates on choices and outcomes in infertility treatment markets. Health Econ. 2012;21(8):994–1016.
- Chambers GM, Adamson GD, Eijkemans MJC. Acceptable cost for the patient and society. Fertil Steril. 2013;100(2):319–327.
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al; ICMART, WHO. International Committee for Monitoring Assisted Reproductive Technology (ICMART); World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92(5):1520–1524.
Patients seeking fertility care commonly ask the physician for advice regarding ways to optimize their conception attempts. While evidence from randomized controlled trials is not available, data from observational studies provide parameters that can inform patient decision making. Knowledge about the fertility window, the decline in fecundability with age, and lifestyle practices that promote conception may be helpful to clinicians and aid in their ability to guide patients.
For those patients who will not achieve conception naturally, assisted reproductive technologies (ART) offer a promising alternative. ART options have improved greatly in effectiveness and safety since Louise Brown was born in 1978. More than 5 million babies have been born globally.1 However, even though the United States is wealthy, access to in vitro fertilization (IVF) is poor relative to many other countries, with not more than 1 in 3 people needing IVF actually receiving the treatment. Understanding the international experience enables physicians to take actions that help increase access for their patients who need IVF.
In this article we not only address ways in which your patients can optimize their natural fertility but also examine this country’s ability to offer ART options when they are needed. Without such examination, fundamental changes in societal attitudes toward infertility and payor attitudes toward reproductive care will not occur, and it is these changes, among others, that can move this country to more equitable ART access.
Patients seeking fertility care commonly ask the physician for advice regarding ways to optimize their conception attempts. While evidence from randomized controlled trials is not available, data from observational studies provide parameters that can inform patient decision making. Knowledge about the fertility window, the decline in fecundability with age, and lifestyle practices that promote conception may be helpful to clinicians and aid in their ability to guide patients.
For those patients who will not achieve conception naturally, assisted reproductive technologies (ART) offer a promising alternative. ART options have improved greatly in effectiveness and safety since Louise Brown was born in 1978. More than 5 million babies have been born globally.1 However, even though the United States is wealthy, access to in vitro fertilization (IVF) is poor relative to many other countries, with not more than 1 in 3 people needing IVF actually receiving the treatment. Understanding the international experience enables physicians to take actions that help increase access for their patients who need IVF.
In this article we not only address ways in which your patients can optimize their natural fertility but also examine this country’s ability to offer ART options when they are needed. Without such examination, fundamental changes in societal attitudes toward infertility and payor attitudes toward reproductive care will not occur, and it is these changes, among others, that can move this country to more equitable ART access.
- Adamson GD, Tabangin M, Macaluso M, de Mouzon J. The number of babies born globally after treatment with the Assisted Reproductive Technologies (ART). Paper presented at International Federation of Fertility Societies/American Society for Reproductive Medicine Conjoint Meeting; October 12–17, 2013; Boston, Massachusetts.
- Dunson DB, Baird DD, Wilcox AJ, Weinberg CR. Day-specific probabilities of clinical pregnancy based on two studies with imperfect measures of ovulation. Hum Reprod. 1999;14(7):1835–1839.
- Keulers MJ, Hamilton CJ, Franx A, et al. The length of the fertile window is associated with the chance of spontaneously conceiving an ongoing pregnancy in subfertile couples. Hum Reprod. 2007;22(6):1652–1656.
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med. 1995;333(23):1517–1521.
- Levitas E, Lunenfeld E, Weiss N, et al. Relationship between the duration of sexual abstinence and semen quality: analysis of 9,489 semen samples. Fertil Steril. 2005;83(6):1680–1686.
- Elzanaty S, Malm J, Giwercman A. Duration of sexual abstinence: epididymal and accessory sex gland secretions and their relationship to sperm motility. Hum Reprod. 2005;20(1):221–225.
- Check JH, Epstein R, Long R. Effect of time interval between ejaculations on semen parameters. Arch Androl. 1991;27(2):93–95.
- Practice Committee of American Society for Reproductive Medicine in collaboration with Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility: a committee opinion. Fertil Steril. 2013;100(3):631–637.
- Gnoth C, Godehardt E, Frank-Herrmann P, Friol K, Tigges J, Freundi G. Definition and prevalence of subfertility and infertility. Hum Reprod. 2005;20(5):1144–1447.
- Howe G, Westhoff C, Vessey M, Yeates D. Effects of age, cigarette smoking, and other factors on fertility: findings in a large prospective study. BMJ (Clin Res Ed). 1985;290(6483):1697–700.
- Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol. 2004;103(1):51–56.
- Dunson DB, Colombo B, Baird DD. Changes with age in the level and duration of fertility in the menstrual cycle. Hum Reprod. 2002;17(5):1399–1403.
- Lumley J, Watson L, Watson M, Bower C. Periconceptional supplementation with folate and/or multivitamins for preventing neural tube defects. Cochrane Database Syst Rev. 2001;(3):CD001056.
- Augood C, Duckitt K, Templeton AA. Smoking and female infertility: a systematic review and meta-analysis. Hum Reprod. 1998;13(6):1532–1539.
- Winter E, Wang J, Davies MJ, Norman R. Early pregnancy loss following assisted reproductive technology treatment. Hum Reprod. 2002;17(12):3220–3223.
- Ness RB, Grisso JA, Hirschinger N, et al. Cocaine and tobacco use and the risk of spontaneous abortion. New Engl J Med. 1999;340(5):333–339.
- Mattison DR, Plowchalk DR, Meadows MJ, Miller MM, Malek A, London S. The effect of smoking on oogenesis, fertilization and implantation. Semin Reprod Med. 1989;7(4):291–304.
- Adena MA, Gallagher HG. Cigarette smoking and the age at menopause. Ann Hum Biol. 1982;9(2):121–130.
- Bolumar F, Olsen J, Rebagliato M, Bisanti L. Caffeine intake and delayed conception: a European multicenter study on infertility and subfecundity. European Study Group on Infertility Subfecundity. Am J Epidemiol. 1997;145(4):324–334.
- Wilcox A, Weinberg C, Baird D. Caffeinated beverages and decreased fertility. Lancet. 1988;2(8626–8627):1453–1456.
- Signorello LB, McLaughlin JK. Maternal caffeine consumption and spontaneous abortion: a review of the epidemiologic evidence. Epidemiology. 2004;15(2):229–239.
- Kesmodel U, Wisborg K, Olsen SF, Henriksen TB, Secher NJ. Moderate alcohol intake in pregnancy and the risk of spontaneous abortion. Alcohol. 2002;37(1):87–92.
- Adamson GD; International Council of Medical Acupuncture and Related Techniques (ICMART). ICMART World Report 2011. Webcast presented at: Annual Meeting European Society of Human Reproduction and Embryology (ESHRE); June 16, 2015; Lisbon, Portugal.
- Chambers G, Phuong Hoang V, et al. The impact of consumer affordability on access to assisted reproductive technologies and embryo transfer practices: an international analysis. Fertil Steril. 2014;101(1):191–198.
- Stovall DW, Allen BD, Sparks AE, Syrop CH, Saunders RG, VanVoorhis BJ. The cost of infertility evaluation and therapy: findings of a self-insured university healthcare plan. Fertil Steril. 1999;72(5):778–784.
- Chambers GM, Sullivan E, Ishihara O, Chapman MG, Adamson GD. The economic impact of assisted reproductive technology: a review of selected developed countries. Fertil Steril. 2009;91(6):2281–2294.
- Hamilton BH, McManus B. The effects of insurance mandates on choices and outcomes in infertility treatment markets. Health Econ. 2012;21(8):994–1016.
- Chambers GM, Adamson GD, Eijkemans MJC. Acceptable cost for the patient and society. Fertil Steril. 2013;100(2):319–327.
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al; ICMART, WHO. International Committee for Monitoring Assisted Reproductive Technology (ICMART); World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92(5):1520–1524.
- Adamson GD, Tabangin M, Macaluso M, de Mouzon J. The number of babies born globally after treatment with the Assisted Reproductive Technologies (ART). Paper presented at International Federation of Fertility Societies/American Society for Reproductive Medicine Conjoint Meeting; October 12–17, 2013; Boston, Massachusetts.
- Dunson DB, Baird DD, Wilcox AJ, Weinberg CR. Day-specific probabilities of clinical pregnancy based on two studies with imperfect measures of ovulation. Hum Reprod. 1999;14(7):1835–1839.
- Keulers MJ, Hamilton CJ, Franx A, et al. The length of the fertile window is associated with the chance of spontaneously conceiving an ongoing pregnancy in subfertile couples. Hum Reprod. 2007;22(6):1652–1656.
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med. 1995;333(23):1517–1521.
- Levitas E, Lunenfeld E, Weiss N, et al. Relationship between the duration of sexual abstinence and semen quality: analysis of 9,489 semen samples. Fertil Steril. 2005;83(6):1680–1686.
- Elzanaty S, Malm J, Giwercman A. Duration of sexual abstinence: epididymal and accessory sex gland secretions and their relationship to sperm motility. Hum Reprod. 2005;20(1):221–225.
- Check JH, Epstein R, Long R. Effect of time interval between ejaculations on semen parameters. Arch Androl. 1991;27(2):93–95.
- Practice Committee of American Society for Reproductive Medicine in collaboration with Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility: a committee opinion. Fertil Steril. 2013;100(3):631–637.
- Gnoth C, Godehardt E, Frank-Herrmann P, Friol K, Tigges J, Freundi G. Definition and prevalence of subfertility and infertility. Hum Reprod. 2005;20(5):1144–1447.
- Howe G, Westhoff C, Vessey M, Yeates D. Effects of age, cigarette smoking, and other factors on fertility: findings in a large prospective study. BMJ (Clin Res Ed). 1985;290(6483):1697–700.
- Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol. 2004;103(1):51–56.
- Dunson DB, Colombo B, Baird DD. Changes with age in the level and duration of fertility in the menstrual cycle. Hum Reprod. 2002;17(5):1399–1403.
- Lumley J, Watson L, Watson M, Bower C. Periconceptional supplementation with folate and/or multivitamins for preventing neural tube defects. Cochrane Database Syst Rev. 2001;(3):CD001056.
- Augood C, Duckitt K, Templeton AA. Smoking and female infertility: a systematic review and meta-analysis. Hum Reprod. 1998;13(6):1532–1539.
- Winter E, Wang J, Davies MJ, Norman R. Early pregnancy loss following assisted reproductive technology treatment. Hum Reprod. 2002;17(12):3220–3223.
- Ness RB, Grisso JA, Hirschinger N, et al. Cocaine and tobacco use and the risk of spontaneous abortion. New Engl J Med. 1999;340(5):333–339.
- Mattison DR, Plowchalk DR, Meadows MJ, Miller MM, Malek A, London S. The effect of smoking on oogenesis, fertilization and implantation. Semin Reprod Med. 1989;7(4):291–304.
- Adena MA, Gallagher HG. Cigarette smoking and the age at menopause. Ann Hum Biol. 1982;9(2):121–130.
- Bolumar F, Olsen J, Rebagliato M, Bisanti L. Caffeine intake and delayed conception: a European multicenter study on infertility and subfecundity. European Study Group on Infertility Subfecundity. Am J Epidemiol. 1997;145(4):324–334.
- Wilcox A, Weinberg C, Baird D. Caffeinated beverages and decreased fertility. Lancet. 1988;2(8626–8627):1453–1456.
- Signorello LB, McLaughlin JK. Maternal caffeine consumption and spontaneous abortion: a review of the epidemiologic evidence. Epidemiology. 2004;15(2):229–239.
- Kesmodel U, Wisborg K, Olsen SF, Henriksen TB, Secher NJ. Moderate alcohol intake in pregnancy and the risk of spontaneous abortion. Alcohol. 2002;37(1):87–92.
- Adamson GD; International Council of Medical Acupuncture and Related Techniques (ICMART). ICMART World Report 2011. Webcast presented at: Annual Meeting European Society of Human Reproduction and Embryology (ESHRE); June 16, 2015; Lisbon, Portugal.
- Chambers G, Phuong Hoang V, et al. The impact of consumer affordability on access to assisted reproductive technologies and embryo transfer practices: an international analysis. Fertil Steril. 2014;101(1):191–198.
- Stovall DW, Allen BD, Sparks AE, Syrop CH, Saunders RG, VanVoorhis BJ. The cost of infertility evaluation and therapy: findings of a self-insured university healthcare plan. Fertil Steril. 1999;72(5):778–784.
- Chambers GM, Sullivan E, Ishihara O, Chapman MG, Adamson GD. The economic impact of assisted reproductive technology: a review of selected developed countries. Fertil Steril. 2009;91(6):2281–2294.
- Hamilton BH, McManus B. The effects of insurance mandates on choices and outcomes in infertility treatment markets. Health Econ. 2012;21(8):994–1016.
- Chambers GM, Adamson GD, Eijkemans MJC. Acceptable cost for the patient and society. Fertil Steril. 2013;100(2):319–327.
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al; ICMART, WHO. International Committee for Monitoring Assisted Reproductive Technology (ICMART); World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92(5):1520–1524.
In this Article
- Factors affecting the probability of conception
- Barriers to ART access
- Ways to increase ART funding
2015 Update on fertility
The first human birth from a frozen oocyte was reported in 1986.1 Nearly 3 decades later, mature oocyte cryopreservation has emerged as a meaningful technology to preserve reproductive potential in women of reproductive age. In 2013, the American Society for Reproductive Medicine (ASRM) removed the “experimental” label from egg freezing but cautioned that more data on safety and efficacy were needed prior to widespread adoption of the technique.2
In this Update, we present the current protocols for oocyte cryopreservation, how we arrived at them, and the questions regarding outcomes that still remain. In addition, we discuss the ethical dilemmas egg freezing presents, according to the varying rhetoric within the media and our own profession. Finally, we consider what preliminary data suggest as to the live-birth rate using frozen eggs from women of varying ages and what the costs are associated with using oocyte cryopreservation as the approach to fertility treatment.
Vitrification and slow freezing: How did we get here and how effective are they?
Fertility preservation is a rapidly advancing area of reproductive medicine. Cryopreservation is the cooling of cells to subzero temperatures to halt biologic activity and preserve the cells for future use. Clinically, oocyte cryopreservation requires a patient to undergo in vitro fertilization (IVF). After egg retrieval, the oocytes are cryopreserved for use at a later time.
The prefix “cryo” originated from the Greek word “kryos,” meaning icy cold or frost. Cryopreservation is not a new science. In 1776, the Italian priest and scientist Lazzaro Spallanzani reported that sperm became motionless when cooled by snow. A pivotal discovery in the field came in 1949, when Christopher Polge, an English scientist, showed that glycerol, a permeating solute, could provide protection to cells at low temperatures.3 Progress in sperm cryopreservation advanced quickly, partly due to the ease of observing sperm motility as a marker of postthaw function.4
The ongoing evolution of cryopreservation science led to landmark achievements, including the first birth using human cryopreserved sperm in the 1950s, and the first human birth after embryo thaw in 1983. Since that time cryopreservation has become a cornerstone in the field of reproductive medicine.
Initial problems encountered with egg freezing
Although the first birth after thaw of a human oocyte occurred in 1986, oocyte cryopreservation was fraught with technical difficulties. Oocytes (vs sperm and embryos) proved challenging to successfully cryopreserve. The problem lay in the damage caused by water crystals forming ice and rising concentrations of intracellular solutes as cells were cooled to freezing temperatures.5 The large size and high water content of the human oocyte made it particularly vulnerable to the detrimental effects of freezing. In addition, freeze−thaw hardening of the zone pellucida led to decreased postthaw fertilization. The delicate meiotic spindle within the oocyte was prone to injury from ice crystals.6
Use of cryoprotectants, such as ethylene glycol, glycerol, and dimethylsulfoxide (DMSO), can prevent ice crystal formation, but high concentrations are theoretically toxic. The fine balance between protection and toxicity led to the development of diverse egg freezing protocols using various types and concentrations of cryoprotectants. Inconsistent results and lack of reproducibility among labs, together with concerns about postthaw oocyte function and safety, slowed the progression of oocyte freezing. By the end of the 1980s, clinical oocyte cryopreservation had been effectively halted and the field was confined to small groups of researchers who continued laboratory experiments with limited success.5
In 1997, clinical work with frozen oocytes resumed with a Bologna team reporting postthawing oocyte survival rates of up to 80% using propanediol as the primary cryoprotectant, and viable pregnancies with the use of intracytoplasmic sperm injection (ICSI) for fertilization.7,8 Since the late 1990s, further modifications in freezing technologies have resulted in greater success. And currently, both slow freezing and vitrification methods are used to preserve oocytes.
Slow freezing
Slow freezing involves a low rate of oocyte temperature decline with a simultaneous gradual increase in the concentration of cryoprotectants. As the metabolic activity of the oocyte decreases, the concentration of cryoprotectant can be increased to prevent ice crystal formation. Once solidification of the oocyte is achieved, the oocyte can be exposed to freezing at colder temperatures. Results of a meta-analysis of 26 studies revealed that, compared with using fresh oocytes, eggs thawed after slow-freezing yielded significantly lower rates of fertilization (61.0% [1,346/2,217] vs 76.7% [2,788/3,637]), clinical pregnancy rate per transfer (27.1% [95/351] vs 68.5% [272/397]), and live birth per transfer (21.6% [76/351] vs 32.4% [24/72]).9
Vitrification
Vitrification involves the rapid cooling of cells to extremely low temperatures. During vitrification, oocytes are exposed to high concentrations of cryoprotectants and, after a short equilibration time, rapidly cooled. The rate of cooling is dramatic, up to 20,000°C per minute—so fast that ice does not have time to form and a glass-like state is achieved within the oocyte. Studies suggest that the use of vitrification improves oocyte survival and function compared with slow freezing.9-11 A prospective randomized controlled trial comparing frozen/thawed with vitrified/warmed oocytes demonstrated superior oocyte function in the vitrification group, with higher oocyte survival (81% for vitrification/warming vs 67% for slow freezing/thawing); higher rates of fertilization, cleavage, and embryo morphology; as well as higher clinical pregnancy rates (38% for vitrified/warmed vs 13% for frozen/thawed).10
The Practice Committee of ASRM published a guideline for mature cryopreservation in 2013.2 The committee reviewed the literature on efficacy and safety of mature oocyte cryopreservation. Although data are limited, studies comparing outcomes of IVF using cryopreserved versus fresh oocytes, including four randomized controlled trials and a meta-analysis, provide evidence that previously vitrified/thawed eggs result in similar fertilization and pregnancy rates as IVF/ICSI with fresh oocytes. Similar to data from fresh IVF cycles, decreased success with oocyte vitrification is seen in women with advanced age, and delivery rates, not unexpectedly, are inversely correlated with maternal age.12
Safety outcomes data are limited but reassuring
Two major factors limit our current understanding of egg cryopreservation outcomes. First, many women who have cryopreserved their eggs have not yet used them and, second, babies born after use of cryopreserved oocytes have not reached ages in which safety of the technique can be fully evaluated. Despite this important gap in our knowledge, to date, results of studies examining safety outcomes of the procedure have been reassuring.
For instance, chromosomal analysis via fluorescence in-situ hybridization of embryos created with thawed oocytes versus controls revealed no difference in the incidence of chromosomal abnormalities, decreasing concerns about damage to the oocyte spindle secondary to freezing.13
Data from a review of 900 live births resulting from embryos created from thawed oocytes frozen via the slow freeze technique revealed no increase in the risk of congenital anomalies.14 Similarly, no increased risk of congenital anomalies or difference in birth weights was noted in a study of 200 live births after transfers with embryos derived from vitrified oocytes compared with fresh oocytes.15
In a study of 954 clinical pregnancies occurring in 855 couples with cryopreserved oocytes after assisted reproductive technology, the outcomes of 197 pregnancies from frozen/thawed oocytes were compared with 757 obtained from fresh sibling oocyte cycles. A significantly higher rate of spontaneous abortions at 12 weeks or less was observed in the frozen/thawed oocyte group. No statistically significant differences were noted in gestational age at delivery or in the incidence of major congenital anomalies at birth, but mean birth weights were significantly lower in fresh oocyte pregnancies. Interestingly, in the group of 63 women who had pregnancies derived from both fresh and thawed oocytes, no differences were noted in the abortion rate or mean birth weight.16
We can freeze eggs, but when should we?
Based on media presentations and professional perspectives, it appears that many people differentiate between “medical” and “social” egg freezing.
Medical versus social freezing
Medical egg freezing is done when there is an immediate medical need to preserve fertility, such as before cancer treatment when the woman can’t reproduce now and will have reduced or no capacity later. Social freezing, on the other hand, occurs when there is no immediate need, such as when there is a desire to delay parenthood so that educational, professional, or other goals can be met. The difference is important because medical freezing is usually seen as a “need” and is therefore acceptable, whereas social freezing is elective or a “wish” and therefore is questionable.17
The labels are important for both ethical and political reasons because most people would consider medical freezing to be ethically acceptable and also worthy of societal support, perhaps even financial coverage, while some might consider social freezing to be neither ethically acceptable nor worthy of coverage.
What’s the difference?
But is the difference really all that clear? If a woman has a mother and a sister who have undergone premature menopause in their 30s and she now has signs of diminishing ovarian reserve in her late 20s, would a desire to freeze eggs be medical or social? She has no immediate need for treatment but a reasonable expectation of need later. One could argue that she should go to a sperm bank now if she has no partner, or change her life plans—but is this a reasonable expectation? If a woman is perfectly healthy but her husband has severe sperm problems and she elects IVF to treat male-factor infertility, is it medical or social? There are many situations in which it is unclear whether the reason for egg freezing would be medical or social.
Does it matter?
In any event, are social reasons to freeze eggs not legitimate? Many would argue that medical services should be used to treat diseases, not social causes. Yet we use medicine all the time to treat problems caused by social factors (obesity, depression, anxiety).
Some would argue that it is a personal decision to delay reproduction, and that health problems caused by personal decisions do not merit medical intervention. However, it is common to provide medical services to people who require the services only because of personal decisions—for instance, professional and amateur athletes who injure themselves pursuing activities for compensation or pleasure, or smokers or persons with alcoholism.
Others have argued that social freezing is inappropriate because it is only being done to avoid the consequences of aging, and that its need could be avoided by not waiting too long to get pregnant. But we treat many conditions that occur primarily as a result of aging (hypertension, dementia, poor eyesight).
Because it has become generally accepted to treat older women with diminished ovarian reserve and infertility, why is it inappropriate to treat women—when they are younger—with egg freezing to mitigate the impact of aging on reproductive performance that we know will occur later? If we could prevent or limit the impact of aging on the cardiovascular or neurologic systems by interventions earlier in a person’s life, would we not provide that medical service? Do we not provide statins and other medications to delay or limit the sequelae of aging? What is the difference with egg freezing?
Therefore, could it be discriminatory not to consider egg freezing ethical and acceptable, even if the reason for the procedure is considered social? Why should egg freezing for social reasons not be acceptable and widely available?
Who should pay for egg freezing?
Even if egg freezing performed because of social reasons is considered ethical and is supported by society, it does not necessarily follow that it will or should be paid for by society. The creation of policies determining coverage for health-care services is a complex process and is based on overall societal needs, economic capabilities, and relative social value of the services. Because infertility carries such a large personal burden and childbearing is so essential to any society, one can argue that infertility, per se, should be covered by society and, in the United States, its surrogate employers and insurance companies. This is often not the case, however. So, while it can be argued that egg freezing should be covered by insurance for both medical and social reasons, even the success of that argument does not mean it will be so in the current US health-care system.
Because egg freezing involves two major steps: (1) ovarian stimulation, egg retrieval, egg freezing, and egg storage followed at a later date by (2) egg thawing, fertilization, embryo culture, and embryo replacement in the uterus, what would be socially justified coverage of egg freezing? Society could cover just the first step or just the second, or both. Such decisions would depend on an assessment of the social benefit from coverage of these services. Such analysis is not yet available because of limited experience.
Is the cost worth it?
A major issue for women considering egg freezing for social reasons is whether a sufficient number of eggs will be retrieved to provide a reasonable chance for pregnancy later when they are used. The FIGURE illustrates the probability of a live birth after egg freezing. It should be noted that while most, but not all, eggs survive thawing after vitrification, not all eggs will become fertilized. Only about half of the fertilized eggs will grow to a day 3 embryo, and not all of those embryos will be viable. Therefore, constant reproductive loss occurs after the eggs are retrieved.
Furthermore, even after embryo replacement, pregnancy does not occur in every case, and some pregnancies are lost to miscarriage as well as other complications of pregnancy and childbirth. The FIGURE shows that a 25-year-old woman with 12 eggs frozen would have an estimated pregnancy rate much greater than 50%. However, the numbers also indicate that egg freezing is not very successful for older women who, at this time, constitute many of those considering the procedure.18
Another consideration is that a significant, but currently unknown, number of women who freeze their eggs will never use them for a variety of reasons. This is especially true of younger women, for whom many of the factors determining their eventual reproductive life might well change. They may eventually decide not to have children or they might become pregnant naturally or after fertility treatments that are cheaper than using the frozen eggs.
A $200,000 price tag?
Let’s consider the near 20% estimated pregnancy rate for age 35 in the FIGURE. If only half of the women aged 35 who freeze 6 eggs eventually use them (but, again, only about 20% have a baby), it means that only one of every 10 women who freeze their eggs eventually will have a baby as a result of the procedure. The number needed to treat (NNT) is therefore 10, and if the cost is $20,000 for the egg freezing procedure and storage over 5 to 10 years, the overall cost per baby born is about $200,000. If 12 eggs are frozen, the cost is $100,000. This clearly is a significant cost, and a greater cost than most other fertility treatments to achieve a baby, even in the older population. Therefore, the cost-effectiveness of social egg freezing is yet to be determined.
What should we do as we move forward?
Abandon the medical versus social rhetoric
It is difficult to argue against egg freezing for medical reasons, and the distinction between medical and social freezing is largely an artificial construct. In general, therefore, the differentiation between medical and social egg freezing should be abandoned, and egg freezing to preserve future fertility should be considered ethical for whatever reasons.
Consider the ideal time frame for health insurance coverage of egg freezing
That does not mean that egg freezing should always be reimbursed. The decision for coverage by employers, insurers, and other payers should be based on a cost–benefit analysis of the social benefit, individual benefit, biological chances of success, probability that the frozen eggs will be used, medical risks/sequelae, and the financial costs. Therefore, whether or not egg freezing for fertility preservation is covered will vary among countries and even within countries and among different individuals. Such an approach to coverage should apply to all medical interventions, including both medical and social egg freezing.
This approach could possibly result in findings and resulting policies that do not cover egg freezing before age 30 because too few women will return to use their eggs, or after age 38 because the chances of success are too low. Other instances of freezing should not be forbidden but would not be reimbursed by public or payer money.17
Many considerations must go into the development of social, professional, and payment policies. Policies that are seen as family-friendly that promote childbearing, especially at an earlier age, can be seen as limiting women’s academic and career opportunities and therefore women-unfriendly. Policies supporting women’s reproductive autonomy and ability to delay childbearing can be seen as women- but not family-friendly. Therefore, reproductive policies affect not only the individual woman but also society, its demographics, politics, and economics.17
The future
The new technology of egg freezing is a wonderful advance for many people. We are learning innovative ways to apply this technology for both infertile and noninfertile people. Research, better evidence, public education, informed consent, ethical practice of medicine, societal support for reproductive rights, and consideration of patient autonomy and social justice will enable us to optimize egg freezing as a treatment intervention.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Chen C. Pregnancy after human oocyte cryopreservation. Lancet. 1986;1(8486):884–886.
2. Practice Committees of the American Society of Reproductive Medicine; Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99(1):37–43.
3. Polge C, Smith AU, Parkes AS. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature. 1949;164(4172):666.
4. Gook D. History of oocyte cryopreservation. Reprod Biomed Online. 2011;23(3):281−289.
5. Gosden R. Cryopreservation: a cold look at technology for fertility preservation. Fertil Steril. 2011;96(2):264−268.
6. Van der Elst J. Oocyte freezing: here to stay? Hum Reprod Update. 2003;9(5):463–470.
7. Porcu E, Fabbri R, Seracchioli R, Ciotti PM, Magrini O, Flamigni C. Birth of a healthy female after intracytoplasmic sperm injection of cryopreserved human oocytes. Fertil Steril. 1997;68(4):724–726.
8. Fabbri R, Porcu E, Marsella T, Rocchetta G, Venturoli S, Flamigni C. Human oocyte cryopreservation: new perspectives regarding oocyte survival. Hum Reprod. 2001;16(3):411–416.
9. Oktay K, Cil AP, Bang H. Efficiency of oocyte cryopreservation: a meta-analysis. Fertil Steril. 2006;86(1):70–80.
10. Smith GD, Serafini PC, Fioravanti J, et al. Prospective randomized comparison of human oocyte cryopreservationwith slow-rate freezing or vitrification. Fertil Steril. 2010;94(6):2088–2095.
11. Gook DA, Edgar DH. Human oocyte cryopreservation. Hum Reprod Update. 2007;13(6):591–605.
12. Rienzi L, Cobo A, Paffoni A, et al. Consistent and predictable delivery rates after oocyte vitrification: an observational longitudinal cohort multicentric study. Hum Reprod. 2012;27(6):1606–1612.
13. Cobo A, Rubio C, Gerli S, Ruiz A, Pellicer A, Remohi J. Use of fluorescence in situ hybridization to assess the chromosomal status of embryos obtained from cryopreserved oocytes. Fertil Steril. 2001;75(2):354–360.
14. Noyes N, Porcu E, Borini A. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod Biomed Online. 2009;18(6):769–776.
15. Chian RC, Huang JY, Tan SL, et al. Obstetric and perinatal outcome in 200 infants conceived from vitrified oocytes. Reprod Biomed Online. 2008;16(5):608–610.
16. Levi Setti P, Albani E, Morenghi E, et al. Comparative analysis of fetal and neonatal outcomes of pregnancies from fresh and cryopreserved/thawed oocytes in the same group of patients. Fertil Steril. 2013;100(2):396–401.
17. Pennings G. Ethical aspects of social freezing. Gynecol Obstet Fertil. 2013;41(9):521–523.
18. Cil AP, Bang H, Oktay K. Age-specific probability of live birth with oocyte cryopreservation: an individual patient data meta-analysis. Fertil Steril. 2013;100(2):492–499.
The first human birth from a frozen oocyte was reported in 1986.1 Nearly 3 decades later, mature oocyte cryopreservation has emerged as a meaningful technology to preserve reproductive potential in women of reproductive age. In 2013, the American Society for Reproductive Medicine (ASRM) removed the “experimental” label from egg freezing but cautioned that more data on safety and efficacy were needed prior to widespread adoption of the technique.2
In this Update, we present the current protocols for oocyte cryopreservation, how we arrived at them, and the questions regarding outcomes that still remain. In addition, we discuss the ethical dilemmas egg freezing presents, according to the varying rhetoric within the media and our own profession. Finally, we consider what preliminary data suggest as to the live-birth rate using frozen eggs from women of varying ages and what the costs are associated with using oocyte cryopreservation as the approach to fertility treatment.
Vitrification and slow freezing: How did we get here and how effective are they?
Fertility preservation is a rapidly advancing area of reproductive medicine. Cryopreservation is the cooling of cells to subzero temperatures to halt biologic activity and preserve the cells for future use. Clinically, oocyte cryopreservation requires a patient to undergo in vitro fertilization (IVF). After egg retrieval, the oocytes are cryopreserved for use at a later time.
The prefix “cryo” originated from the Greek word “kryos,” meaning icy cold or frost. Cryopreservation is not a new science. In 1776, the Italian priest and scientist Lazzaro Spallanzani reported that sperm became motionless when cooled by snow. A pivotal discovery in the field came in 1949, when Christopher Polge, an English scientist, showed that glycerol, a permeating solute, could provide protection to cells at low temperatures.3 Progress in sperm cryopreservation advanced quickly, partly due to the ease of observing sperm motility as a marker of postthaw function.4
The ongoing evolution of cryopreservation science led to landmark achievements, including the first birth using human cryopreserved sperm in the 1950s, and the first human birth after embryo thaw in 1983. Since that time cryopreservation has become a cornerstone in the field of reproductive medicine.
Initial problems encountered with egg freezing
Although the first birth after thaw of a human oocyte occurred in 1986, oocyte cryopreservation was fraught with technical difficulties. Oocytes (vs sperm and embryos) proved challenging to successfully cryopreserve. The problem lay in the damage caused by water crystals forming ice and rising concentrations of intracellular solutes as cells were cooled to freezing temperatures.5 The large size and high water content of the human oocyte made it particularly vulnerable to the detrimental effects of freezing. In addition, freeze−thaw hardening of the zone pellucida led to decreased postthaw fertilization. The delicate meiotic spindle within the oocyte was prone to injury from ice crystals.6
Use of cryoprotectants, such as ethylene glycol, glycerol, and dimethylsulfoxide (DMSO), can prevent ice crystal formation, but high concentrations are theoretically toxic. The fine balance between protection and toxicity led to the development of diverse egg freezing protocols using various types and concentrations of cryoprotectants. Inconsistent results and lack of reproducibility among labs, together with concerns about postthaw oocyte function and safety, slowed the progression of oocyte freezing. By the end of the 1980s, clinical oocyte cryopreservation had been effectively halted and the field was confined to small groups of researchers who continued laboratory experiments with limited success.5
In 1997, clinical work with frozen oocytes resumed with a Bologna team reporting postthawing oocyte survival rates of up to 80% using propanediol as the primary cryoprotectant, and viable pregnancies with the use of intracytoplasmic sperm injection (ICSI) for fertilization.7,8 Since the late 1990s, further modifications in freezing technologies have resulted in greater success. And currently, both slow freezing and vitrification methods are used to preserve oocytes.
Slow freezing
Slow freezing involves a low rate of oocyte temperature decline with a simultaneous gradual increase in the concentration of cryoprotectants. As the metabolic activity of the oocyte decreases, the concentration of cryoprotectant can be increased to prevent ice crystal formation. Once solidification of the oocyte is achieved, the oocyte can be exposed to freezing at colder temperatures. Results of a meta-analysis of 26 studies revealed that, compared with using fresh oocytes, eggs thawed after slow-freezing yielded significantly lower rates of fertilization (61.0% [1,346/2,217] vs 76.7% [2,788/3,637]), clinical pregnancy rate per transfer (27.1% [95/351] vs 68.5% [272/397]), and live birth per transfer (21.6% [76/351] vs 32.4% [24/72]).9
Vitrification
Vitrification involves the rapid cooling of cells to extremely low temperatures. During vitrification, oocytes are exposed to high concentrations of cryoprotectants and, after a short equilibration time, rapidly cooled. The rate of cooling is dramatic, up to 20,000°C per minute—so fast that ice does not have time to form and a glass-like state is achieved within the oocyte. Studies suggest that the use of vitrification improves oocyte survival and function compared with slow freezing.9-11 A prospective randomized controlled trial comparing frozen/thawed with vitrified/warmed oocytes demonstrated superior oocyte function in the vitrification group, with higher oocyte survival (81% for vitrification/warming vs 67% for slow freezing/thawing); higher rates of fertilization, cleavage, and embryo morphology; as well as higher clinical pregnancy rates (38% for vitrified/warmed vs 13% for frozen/thawed).10
The Practice Committee of ASRM published a guideline for mature cryopreservation in 2013.2 The committee reviewed the literature on efficacy and safety of mature oocyte cryopreservation. Although data are limited, studies comparing outcomes of IVF using cryopreserved versus fresh oocytes, including four randomized controlled trials and a meta-analysis, provide evidence that previously vitrified/thawed eggs result in similar fertilization and pregnancy rates as IVF/ICSI with fresh oocytes. Similar to data from fresh IVF cycles, decreased success with oocyte vitrification is seen in women with advanced age, and delivery rates, not unexpectedly, are inversely correlated with maternal age.12
Safety outcomes data are limited but reassuring
Two major factors limit our current understanding of egg cryopreservation outcomes. First, many women who have cryopreserved their eggs have not yet used them and, second, babies born after use of cryopreserved oocytes have not reached ages in which safety of the technique can be fully evaluated. Despite this important gap in our knowledge, to date, results of studies examining safety outcomes of the procedure have been reassuring.
For instance, chromosomal analysis via fluorescence in-situ hybridization of embryos created with thawed oocytes versus controls revealed no difference in the incidence of chromosomal abnormalities, decreasing concerns about damage to the oocyte spindle secondary to freezing.13
Data from a review of 900 live births resulting from embryos created from thawed oocytes frozen via the slow freeze technique revealed no increase in the risk of congenital anomalies.14 Similarly, no increased risk of congenital anomalies or difference in birth weights was noted in a study of 200 live births after transfers with embryos derived from vitrified oocytes compared with fresh oocytes.15
In a study of 954 clinical pregnancies occurring in 855 couples with cryopreserved oocytes after assisted reproductive technology, the outcomes of 197 pregnancies from frozen/thawed oocytes were compared with 757 obtained from fresh sibling oocyte cycles. A significantly higher rate of spontaneous abortions at 12 weeks or less was observed in the frozen/thawed oocyte group. No statistically significant differences were noted in gestational age at delivery or in the incidence of major congenital anomalies at birth, but mean birth weights were significantly lower in fresh oocyte pregnancies. Interestingly, in the group of 63 women who had pregnancies derived from both fresh and thawed oocytes, no differences were noted in the abortion rate or mean birth weight.16
We can freeze eggs, but when should we?
Based on media presentations and professional perspectives, it appears that many people differentiate between “medical” and “social” egg freezing.
Medical versus social freezing
Medical egg freezing is done when there is an immediate medical need to preserve fertility, such as before cancer treatment when the woman can’t reproduce now and will have reduced or no capacity later. Social freezing, on the other hand, occurs when there is no immediate need, such as when there is a desire to delay parenthood so that educational, professional, or other goals can be met. The difference is important because medical freezing is usually seen as a “need” and is therefore acceptable, whereas social freezing is elective or a “wish” and therefore is questionable.17
The labels are important for both ethical and political reasons because most people would consider medical freezing to be ethically acceptable and also worthy of societal support, perhaps even financial coverage, while some might consider social freezing to be neither ethically acceptable nor worthy of coverage.
What’s the difference?
But is the difference really all that clear? If a woman has a mother and a sister who have undergone premature menopause in their 30s and she now has signs of diminishing ovarian reserve in her late 20s, would a desire to freeze eggs be medical or social? She has no immediate need for treatment but a reasonable expectation of need later. One could argue that she should go to a sperm bank now if she has no partner, or change her life plans—but is this a reasonable expectation? If a woman is perfectly healthy but her husband has severe sperm problems and she elects IVF to treat male-factor infertility, is it medical or social? There are many situations in which it is unclear whether the reason for egg freezing would be medical or social.
Does it matter?
In any event, are social reasons to freeze eggs not legitimate? Many would argue that medical services should be used to treat diseases, not social causes. Yet we use medicine all the time to treat problems caused by social factors (obesity, depression, anxiety).
Some would argue that it is a personal decision to delay reproduction, and that health problems caused by personal decisions do not merit medical intervention. However, it is common to provide medical services to people who require the services only because of personal decisions—for instance, professional and amateur athletes who injure themselves pursuing activities for compensation or pleasure, or smokers or persons with alcoholism.
Others have argued that social freezing is inappropriate because it is only being done to avoid the consequences of aging, and that its need could be avoided by not waiting too long to get pregnant. But we treat many conditions that occur primarily as a result of aging (hypertension, dementia, poor eyesight).
Because it has become generally accepted to treat older women with diminished ovarian reserve and infertility, why is it inappropriate to treat women—when they are younger—with egg freezing to mitigate the impact of aging on reproductive performance that we know will occur later? If we could prevent or limit the impact of aging on the cardiovascular or neurologic systems by interventions earlier in a person’s life, would we not provide that medical service? Do we not provide statins and other medications to delay or limit the sequelae of aging? What is the difference with egg freezing?
Therefore, could it be discriminatory not to consider egg freezing ethical and acceptable, even if the reason for the procedure is considered social? Why should egg freezing for social reasons not be acceptable and widely available?
Who should pay for egg freezing?
Even if egg freezing performed because of social reasons is considered ethical and is supported by society, it does not necessarily follow that it will or should be paid for by society. The creation of policies determining coverage for health-care services is a complex process and is based on overall societal needs, economic capabilities, and relative social value of the services. Because infertility carries such a large personal burden and childbearing is so essential to any society, one can argue that infertility, per se, should be covered by society and, in the United States, its surrogate employers and insurance companies. This is often not the case, however. So, while it can be argued that egg freezing should be covered by insurance for both medical and social reasons, even the success of that argument does not mean it will be so in the current US health-care system.
Because egg freezing involves two major steps: (1) ovarian stimulation, egg retrieval, egg freezing, and egg storage followed at a later date by (2) egg thawing, fertilization, embryo culture, and embryo replacement in the uterus, what would be socially justified coverage of egg freezing? Society could cover just the first step or just the second, or both. Such decisions would depend on an assessment of the social benefit from coverage of these services. Such analysis is not yet available because of limited experience.
Is the cost worth it?
A major issue for women considering egg freezing for social reasons is whether a sufficient number of eggs will be retrieved to provide a reasonable chance for pregnancy later when they are used. The FIGURE illustrates the probability of a live birth after egg freezing. It should be noted that while most, but not all, eggs survive thawing after vitrification, not all eggs will become fertilized. Only about half of the fertilized eggs will grow to a day 3 embryo, and not all of those embryos will be viable. Therefore, constant reproductive loss occurs after the eggs are retrieved.
Furthermore, even after embryo replacement, pregnancy does not occur in every case, and some pregnancies are lost to miscarriage as well as other complications of pregnancy and childbirth. The FIGURE shows that a 25-year-old woman with 12 eggs frozen would have an estimated pregnancy rate much greater than 50%. However, the numbers also indicate that egg freezing is not very successful for older women who, at this time, constitute many of those considering the procedure.18
Another consideration is that a significant, but currently unknown, number of women who freeze their eggs will never use them for a variety of reasons. This is especially true of younger women, for whom many of the factors determining their eventual reproductive life might well change. They may eventually decide not to have children or they might become pregnant naturally or after fertility treatments that are cheaper than using the frozen eggs.
A $200,000 price tag?
Let’s consider the near 20% estimated pregnancy rate for age 35 in the FIGURE. If only half of the women aged 35 who freeze 6 eggs eventually use them (but, again, only about 20% have a baby), it means that only one of every 10 women who freeze their eggs eventually will have a baby as a result of the procedure. The number needed to treat (NNT) is therefore 10, and if the cost is $20,000 for the egg freezing procedure and storage over 5 to 10 years, the overall cost per baby born is about $200,000. If 12 eggs are frozen, the cost is $100,000. This clearly is a significant cost, and a greater cost than most other fertility treatments to achieve a baby, even in the older population. Therefore, the cost-effectiveness of social egg freezing is yet to be determined.
What should we do as we move forward?
Abandon the medical versus social rhetoric
It is difficult to argue against egg freezing for medical reasons, and the distinction between medical and social freezing is largely an artificial construct. In general, therefore, the differentiation between medical and social egg freezing should be abandoned, and egg freezing to preserve future fertility should be considered ethical for whatever reasons.
Consider the ideal time frame for health insurance coverage of egg freezing
That does not mean that egg freezing should always be reimbursed. The decision for coverage by employers, insurers, and other payers should be based on a cost–benefit analysis of the social benefit, individual benefit, biological chances of success, probability that the frozen eggs will be used, medical risks/sequelae, and the financial costs. Therefore, whether or not egg freezing for fertility preservation is covered will vary among countries and even within countries and among different individuals. Such an approach to coverage should apply to all medical interventions, including both medical and social egg freezing.
This approach could possibly result in findings and resulting policies that do not cover egg freezing before age 30 because too few women will return to use their eggs, or after age 38 because the chances of success are too low. Other instances of freezing should not be forbidden but would not be reimbursed by public or payer money.17
Many considerations must go into the development of social, professional, and payment policies. Policies that are seen as family-friendly that promote childbearing, especially at an earlier age, can be seen as limiting women’s academic and career opportunities and therefore women-unfriendly. Policies supporting women’s reproductive autonomy and ability to delay childbearing can be seen as women- but not family-friendly. Therefore, reproductive policies affect not only the individual woman but also society, its demographics, politics, and economics.17
The future
The new technology of egg freezing is a wonderful advance for many people. We are learning innovative ways to apply this technology for both infertile and noninfertile people. Research, better evidence, public education, informed consent, ethical practice of medicine, societal support for reproductive rights, and consideration of patient autonomy and social justice will enable us to optimize egg freezing as a treatment intervention.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The first human birth from a frozen oocyte was reported in 1986.1 Nearly 3 decades later, mature oocyte cryopreservation has emerged as a meaningful technology to preserve reproductive potential in women of reproductive age. In 2013, the American Society for Reproductive Medicine (ASRM) removed the “experimental” label from egg freezing but cautioned that more data on safety and efficacy were needed prior to widespread adoption of the technique.2
In this Update, we present the current protocols for oocyte cryopreservation, how we arrived at them, and the questions regarding outcomes that still remain. In addition, we discuss the ethical dilemmas egg freezing presents, according to the varying rhetoric within the media and our own profession. Finally, we consider what preliminary data suggest as to the live-birth rate using frozen eggs from women of varying ages and what the costs are associated with using oocyte cryopreservation as the approach to fertility treatment.
Vitrification and slow freezing: How did we get here and how effective are they?
Fertility preservation is a rapidly advancing area of reproductive medicine. Cryopreservation is the cooling of cells to subzero temperatures to halt biologic activity and preserve the cells for future use. Clinically, oocyte cryopreservation requires a patient to undergo in vitro fertilization (IVF). After egg retrieval, the oocytes are cryopreserved for use at a later time.
The prefix “cryo” originated from the Greek word “kryos,” meaning icy cold or frost. Cryopreservation is not a new science. In 1776, the Italian priest and scientist Lazzaro Spallanzani reported that sperm became motionless when cooled by snow. A pivotal discovery in the field came in 1949, when Christopher Polge, an English scientist, showed that glycerol, a permeating solute, could provide protection to cells at low temperatures.3 Progress in sperm cryopreservation advanced quickly, partly due to the ease of observing sperm motility as a marker of postthaw function.4
The ongoing evolution of cryopreservation science led to landmark achievements, including the first birth using human cryopreserved sperm in the 1950s, and the first human birth after embryo thaw in 1983. Since that time cryopreservation has become a cornerstone in the field of reproductive medicine.
Initial problems encountered with egg freezing
Although the first birth after thaw of a human oocyte occurred in 1986, oocyte cryopreservation was fraught with technical difficulties. Oocytes (vs sperm and embryos) proved challenging to successfully cryopreserve. The problem lay in the damage caused by water crystals forming ice and rising concentrations of intracellular solutes as cells were cooled to freezing temperatures.5 The large size and high water content of the human oocyte made it particularly vulnerable to the detrimental effects of freezing. In addition, freeze−thaw hardening of the zone pellucida led to decreased postthaw fertilization. The delicate meiotic spindle within the oocyte was prone to injury from ice crystals.6
Use of cryoprotectants, such as ethylene glycol, glycerol, and dimethylsulfoxide (DMSO), can prevent ice crystal formation, but high concentrations are theoretically toxic. The fine balance between protection and toxicity led to the development of diverse egg freezing protocols using various types and concentrations of cryoprotectants. Inconsistent results and lack of reproducibility among labs, together with concerns about postthaw oocyte function and safety, slowed the progression of oocyte freezing. By the end of the 1980s, clinical oocyte cryopreservation had been effectively halted and the field was confined to small groups of researchers who continued laboratory experiments with limited success.5
In 1997, clinical work with frozen oocytes resumed with a Bologna team reporting postthawing oocyte survival rates of up to 80% using propanediol as the primary cryoprotectant, and viable pregnancies with the use of intracytoplasmic sperm injection (ICSI) for fertilization.7,8 Since the late 1990s, further modifications in freezing technologies have resulted in greater success. And currently, both slow freezing and vitrification methods are used to preserve oocytes.
Slow freezing
Slow freezing involves a low rate of oocyte temperature decline with a simultaneous gradual increase in the concentration of cryoprotectants. As the metabolic activity of the oocyte decreases, the concentration of cryoprotectant can be increased to prevent ice crystal formation. Once solidification of the oocyte is achieved, the oocyte can be exposed to freezing at colder temperatures. Results of a meta-analysis of 26 studies revealed that, compared with using fresh oocytes, eggs thawed after slow-freezing yielded significantly lower rates of fertilization (61.0% [1,346/2,217] vs 76.7% [2,788/3,637]), clinical pregnancy rate per transfer (27.1% [95/351] vs 68.5% [272/397]), and live birth per transfer (21.6% [76/351] vs 32.4% [24/72]).9
Vitrification
Vitrification involves the rapid cooling of cells to extremely low temperatures. During vitrification, oocytes are exposed to high concentrations of cryoprotectants and, after a short equilibration time, rapidly cooled. The rate of cooling is dramatic, up to 20,000°C per minute—so fast that ice does not have time to form and a glass-like state is achieved within the oocyte. Studies suggest that the use of vitrification improves oocyte survival and function compared with slow freezing.9-11 A prospective randomized controlled trial comparing frozen/thawed with vitrified/warmed oocytes demonstrated superior oocyte function in the vitrification group, with higher oocyte survival (81% for vitrification/warming vs 67% for slow freezing/thawing); higher rates of fertilization, cleavage, and embryo morphology; as well as higher clinical pregnancy rates (38% for vitrified/warmed vs 13% for frozen/thawed).10
The Practice Committee of ASRM published a guideline for mature cryopreservation in 2013.2 The committee reviewed the literature on efficacy and safety of mature oocyte cryopreservation. Although data are limited, studies comparing outcomes of IVF using cryopreserved versus fresh oocytes, including four randomized controlled trials and a meta-analysis, provide evidence that previously vitrified/thawed eggs result in similar fertilization and pregnancy rates as IVF/ICSI with fresh oocytes. Similar to data from fresh IVF cycles, decreased success with oocyte vitrification is seen in women with advanced age, and delivery rates, not unexpectedly, are inversely correlated with maternal age.12
Safety outcomes data are limited but reassuring
Two major factors limit our current understanding of egg cryopreservation outcomes. First, many women who have cryopreserved their eggs have not yet used them and, second, babies born after use of cryopreserved oocytes have not reached ages in which safety of the technique can be fully evaluated. Despite this important gap in our knowledge, to date, results of studies examining safety outcomes of the procedure have been reassuring.
For instance, chromosomal analysis via fluorescence in-situ hybridization of embryos created with thawed oocytes versus controls revealed no difference in the incidence of chromosomal abnormalities, decreasing concerns about damage to the oocyte spindle secondary to freezing.13
Data from a review of 900 live births resulting from embryos created from thawed oocytes frozen via the slow freeze technique revealed no increase in the risk of congenital anomalies.14 Similarly, no increased risk of congenital anomalies or difference in birth weights was noted in a study of 200 live births after transfers with embryos derived from vitrified oocytes compared with fresh oocytes.15
In a study of 954 clinical pregnancies occurring in 855 couples with cryopreserved oocytes after assisted reproductive technology, the outcomes of 197 pregnancies from frozen/thawed oocytes were compared with 757 obtained from fresh sibling oocyte cycles. A significantly higher rate of spontaneous abortions at 12 weeks or less was observed in the frozen/thawed oocyte group. No statistically significant differences were noted in gestational age at delivery or in the incidence of major congenital anomalies at birth, but mean birth weights were significantly lower in fresh oocyte pregnancies. Interestingly, in the group of 63 women who had pregnancies derived from both fresh and thawed oocytes, no differences were noted in the abortion rate or mean birth weight.16
We can freeze eggs, but when should we?
Based on media presentations and professional perspectives, it appears that many people differentiate between “medical” and “social” egg freezing.
Medical versus social freezing
Medical egg freezing is done when there is an immediate medical need to preserve fertility, such as before cancer treatment when the woman can’t reproduce now and will have reduced or no capacity later. Social freezing, on the other hand, occurs when there is no immediate need, such as when there is a desire to delay parenthood so that educational, professional, or other goals can be met. The difference is important because medical freezing is usually seen as a “need” and is therefore acceptable, whereas social freezing is elective or a “wish” and therefore is questionable.17
The labels are important for both ethical and political reasons because most people would consider medical freezing to be ethically acceptable and also worthy of societal support, perhaps even financial coverage, while some might consider social freezing to be neither ethically acceptable nor worthy of coverage.
What’s the difference?
But is the difference really all that clear? If a woman has a mother and a sister who have undergone premature menopause in their 30s and she now has signs of diminishing ovarian reserve in her late 20s, would a desire to freeze eggs be medical or social? She has no immediate need for treatment but a reasonable expectation of need later. One could argue that she should go to a sperm bank now if she has no partner, or change her life plans—but is this a reasonable expectation? If a woman is perfectly healthy but her husband has severe sperm problems and she elects IVF to treat male-factor infertility, is it medical or social? There are many situations in which it is unclear whether the reason for egg freezing would be medical or social.
Does it matter?
In any event, are social reasons to freeze eggs not legitimate? Many would argue that medical services should be used to treat diseases, not social causes. Yet we use medicine all the time to treat problems caused by social factors (obesity, depression, anxiety).
Some would argue that it is a personal decision to delay reproduction, and that health problems caused by personal decisions do not merit medical intervention. However, it is common to provide medical services to people who require the services only because of personal decisions—for instance, professional and amateur athletes who injure themselves pursuing activities for compensation or pleasure, or smokers or persons with alcoholism.
Others have argued that social freezing is inappropriate because it is only being done to avoid the consequences of aging, and that its need could be avoided by not waiting too long to get pregnant. But we treat many conditions that occur primarily as a result of aging (hypertension, dementia, poor eyesight).
Because it has become generally accepted to treat older women with diminished ovarian reserve and infertility, why is it inappropriate to treat women—when they are younger—with egg freezing to mitigate the impact of aging on reproductive performance that we know will occur later? If we could prevent or limit the impact of aging on the cardiovascular or neurologic systems by interventions earlier in a person’s life, would we not provide that medical service? Do we not provide statins and other medications to delay or limit the sequelae of aging? What is the difference with egg freezing?
Therefore, could it be discriminatory not to consider egg freezing ethical and acceptable, even if the reason for the procedure is considered social? Why should egg freezing for social reasons not be acceptable and widely available?
Who should pay for egg freezing?
Even if egg freezing performed because of social reasons is considered ethical and is supported by society, it does not necessarily follow that it will or should be paid for by society. The creation of policies determining coverage for health-care services is a complex process and is based on overall societal needs, economic capabilities, and relative social value of the services. Because infertility carries such a large personal burden and childbearing is so essential to any society, one can argue that infertility, per se, should be covered by society and, in the United States, its surrogate employers and insurance companies. This is often not the case, however. So, while it can be argued that egg freezing should be covered by insurance for both medical and social reasons, even the success of that argument does not mean it will be so in the current US health-care system.
Because egg freezing involves two major steps: (1) ovarian stimulation, egg retrieval, egg freezing, and egg storage followed at a later date by (2) egg thawing, fertilization, embryo culture, and embryo replacement in the uterus, what would be socially justified coverage of egg freezing? Society could cover just the first step or just the second, or both. Such decisions would depend on an assessment of the social benefit from coverage of these services. Such analysis is not yet available because of limited experience.
Is the cost worth it?
A major issue for women considering egg freezing for social reasons is whether a sufficient number of eggs will be retrieved to provide a reasonable chance for pregnancy later when they are used. The FIGURE illustrates the probability of a live birth after egg freezing. It should be noted that while most, but not all, eggs survive thawing after vitrification, not all eggs will become fertilized. Only about half of the fertilized eggs will grow to a day 3 embryo, and not all of those embryos will be viable. Therefore, constant reproductive loss occurs after the eggs are retrieved.
Furthermore, even after embryo replacement, pregnancy does not occur in every case, and some pregnancies are lost to miscarriage as well as other complications of pregnancy and childbirth. The FIGURE shows that a 25-year-old woman with 12 eggs frozen would have an estimated pregnancy rate much greater than 50%. However, the numbers also indicate that egg freezing is not very successful for older women who, at this time, constitute many of those considering the procedure.18
Another consideration is that a significant, but currently unknown, number of women who freeze their eggs will never use them for a variety of reasons. This is especially true of younger women, for whom many of the factors determining their eventual reproductive life might well change. They may eventually decide not to have children or they might become pregnant naturally or after fertility treatments that are cheaper than using the frozen eggs.
A $200,000 price tag?
Let’s consider the near 20% estimated pregnancy rate for age 35 in the FIGURE. If only half of the women aged 35 who freeze 6 eggs eventually use them (but, again, only about 20% have a baby), it means that only one of every 10 women who freeze their eggs eventually will have a baby as a result of the procedure. The number needed to treat (NNT) is therefore 10, and if the cost is $20,000 for the egg freezing procedure and storage over 5 to 10 years, the overall cost per baby born is about $200,000. If 12 eggs are frozen, the cost is $100,000. This clearly is a significant cost, and a greater cost than most other fertility treatments to achieve a baby, even in the older population. Therefore, the cost-effectiveness of social egg freezing is yet to be determined.
What should we do as we move forward?
Abandon the medical versus social rhetoric
It is difficult to argue against egg freezing for medical reasons, and the distinction between medical and social freezing is largely an artificial construct. In general, therefore, the differentiation between medical and social egg freezing should be abandoned, and egg freezing to preserve future fertility should be considered ethical for whatever reasons.
Consider the ideal time frame for health insurance coverage of egg freezing
That does not mean that egg freezing should always be reimbursed. The decision for coverage by employers, insurers, and other payers should be based on a cost–benefit analysis of the social benefit, individual benefit, biological chances of success, probability that the frozen eggs will be used, medical risks/sequelae, and the financial costs. Therefore, whether or not egg freezing for fertility preservation is covered will vary among countries and even within countries and among different individuals. Such an approach to coverage should apply to all medical interventions, including both medical and social egg freezing.
This approach could possibly result in findings and resulting policies that do not cover egg freezing before age 30 because too few women will return to use their eggs, or after age 38 because the chances of success are too low. Other instances of freezing should not be forbidden but would not be reimbursed by public or payer money.17
Many considerations must go into the development of social, professional, and payment policies. Policies that are seen as family-friendly that promote childbearing, especially at an earlier age, can be seen as limiting women’s academic and career opportunities and therefore women-unfriendly. Policies supporting women’s reproductive autonomy and ability to delay childbearing can be seen as women- but not family-friendly. Therefore, reproductive policies affect not only the individual woman but also society, its demographics, politics, and economics.17
The future
The new technology of egg freezing is a wonderful advance for many people. We are learning innovative ways to apply this technology for both infertile and noninfertile people. Research, better evidence, public education, informed consent, ethical practice of medicine, societal support for reproductive rights, and consideration of patient autonomy and social justice will enable us to optimize egg freezing as a treatment intervention.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Chen C. Pregnancy after human oocyte cryopreservation. Lancet. 1986;1(8486):884–886.
2. Practice Committees of the American Society of Reproductive Medicine; Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99(1):37–43.
3. Polge C, Smith AU, Parkes AS. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature. 1949;164(4172):666.
4. Gook D. History of oocyte cryopreservation. Reprod Biomed Online. 2011;23(3):281−289.
5. Gosden R. Cryopreservation: a cold look at technology for fertility preservation. Fertil Steril. 2011;96(2):264−268.
6. Van der Elst J. Oocyte freezing: here to stay? Hum Reprod Update. 2003;9(5):463–470.
7. Porcu E, Fabbri R, Seracchioli R, Ciotti PM, Magrini O, Flamigni C. Birth of a healthy female after intracytoplasmic sperm injection of cryopreserved human oocytes. Fertil Steril. 1997;68(4):724–726.
8. Fabbri R, Porcu E, Marsella T, Rocchetta G, Venturoli S, Flamigni C. Human oocyte cryopreservation: new perspectives regarding oocyte survival. Hum Reprod. 2001;16(3):411–416.
9. Oktay K, Cil AP, Bang H. Efficiency of oocyte cryopreservation: a meta-analysis. Fertil Steril. 2006;86(1):70–80.
10. Smith GD, Serafini PC, Fioravanti J, et al. Prospective randomized comparison of human oocyte cryopreservationwith slow-rate freezing or vitrification. Fertil Steril. 2010;94(6):2088–2095.
11. Gook DA, Edgar DH. Human oocyte cryopreservation. Hum Reprod Update. 2007;13(6):591–605.
12. Rienzi L, Cobo A, Paffoni A, et al. Consistent and predictable delivery rates after oocyte vitrification: an observational longitudinal cohort multicentric study. Hum Reprod. 2012;27(6):1606–1612.
13. Cobo A, Rubio C, Gerli S, Ruiz A, Pellicer A, Remohi J. Use of fluorescence in situ hybridization to assess the chromosomal status of embryos obtained from cryopreserved oocytes. Fertil Steril. 2001;75(2):354–360.
14. Noyes N, Porcu E, Borini A. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod Biomed Online. 2009;18(6):769–776.
15. Chian RC, Huang JY, Tan SL, et al. Obstetric and perinatal outcome in 200 infants conceived from vitrified oocytes. Reprod Biomed Online. 2008;16(5):608–610.
16. Levi Setti P, Albani E, Morenghi E, et al. Comparative analysis of fetal and neonatal outcomes of pregnancies from fresh and cryopreserved/thawed oocytes in the same group of patients. Fertil Steril. 2013;100(2):396–401.
17. Pennings G. Ethical aspects of social freezing. Gynecol Obstet Fertil. 2013;41(9):521–523.
18. Cil AP, Bang H, Oktay K. Age-specific probability of live birth with oocyte cryopreservation: an individual patient data meta-analysis. Fertil Steril. 2013;100(2):492–499.
1. Chen C. Pregnancy after human oocyte cryopreservation. Lancet. 1986;1(8486):884–886.
2. Practice Committees of the American Society of Reproductive Medicine; Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99(1):37–43.
3. Polge C, Smith AU, Parkes AS. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature. 1949;164(4172):666.
4. Gook D. History of oocyte cryopreservation. Reprod Biomed Online. 2011;23(3):281−289.
5. Gosden R. Cryopreservation: a cold look at technology for fertility preservation. Fertil Steril. 2011;96(2):264−268.
6. Van der Elst J. Oocyte freezing: here to stay? Hum Reprod Update. 2003;9(5):463–470.
7. Porcu E, Fabbri R, Seracchioli R, Ciotti PM, Magrini O, Flamigni C. Birth of a healthy female after intracytoplasmic sperm injection of cryopreserved human oocytes. Fertil Steril. 1997;68(4):724–726.
8. Fabbri R, Porcu E, Marsella T, Rocchetta G, Venturoli S, Flamigni C. Human oocyte cryopreservation: new perspectives regarding oocyte survival. Hum Reprod. 2001;16(3):411–416.
9. Oktay K, Cil AP, Bang H. Efficiency of oocyte cryopreservation: a meta-analysis. Fertil Steril. 2006;86(1):70–80.
10. Smith GD, Serafini PC, Fioravanti J, et al. Prospective randomized comparison of human oocyte cryopreservationwith slow-rate freezing or vitrification. Fertil Steril. 2010;94(6):2088–2095.
11. Gook DA, Edgar DH. Human oocyte cryopreservation. Hum Reprod Update. 2007;13(6):591–605.
12. Rienzi L, Cobo A, Paffoni A, et al. Consistent and predictable delivery rates after oocyte vitrification: an observational longitudinal cohort multicentric study. Hum Reprod. 2012;27(6):1606–1612.
13. Cobo A, Rubio C, Gerli S, Ruiz A, Pellicer A, Remohi J. Use of fluorescence in situ hybridization to assess the chromosomal status of embryos obtained from cryopreserved oocytes. Fertil Steril. 2001;75(2):354–360.
14. Noyes N, Porcu E, Borini A. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod Biomed Online. 2009;18(6):769–776.
15. Chian RC, Huang JY, Tan SL, et al. Obstetric and perinatal outcome in 200 infants conceived from vitrified oocytes. Reprod Biomed Online. 2008;16(5):608–610.
16. Levi Setti P, Albani E, Morenghi E, et al. Comparative analysis of fetal and neonatal outcomes of pregnancies from fresh and cryopreserved/thawed oocytes in the same group of patients. Fertil Steril. 2013;100(2):396–401.
17. Pennings G. Ethical aspects of social freezing. Gynecol Obstet Fertil. 2013;41(9):521–523.
18. Cil AP, Bang H, Oktay K. Age-specific probability of live birth with oocyte cryopreservation: an individual patient data meta-analysis. Fertil Steril. 2013;100(2):492–499.
IN THIS ARTICLE
-Vitrification and slow freezing: How did we get here and how effective are they?
-Safety outcomes data are limited but reassuring
-We can freeze eggs, but when should we?
-Who should pay for egg freezing?
-What should we do as we move forward?
2014 Update on Fertility
These experts discuss three recent American Society for Reproductive Medicine Committee Opinions. The first is on the optimal use of the most widely prescribed medication for fertility, clomiphene citrate. The second highlights the currently recommended vaccinations for women who are of reproductive age. And the third is on the current evidence for prevention of postsurgical adhesions, which have the potential to cause infertility. Their discussions could affect how you approach your infertile patients.
SAFE, EFFECTIVE USE OF CLOMIPHENE
Practice Committee of the American Society for Reproductive Medicine. Use of clomiphene citrate in infertile women: A committee opinion. Fertil Steril. 2013;100(2):341–348.
Clomiphene citrate (CC) is the fertility medication most commonly used by gynecologists. However, important principles in its use often are not followed, resulting in suboptimal patient care. The American Society for Reproductive Medicine published a recent Committee Opinion on CC’s indications, use, and alternative treatments. We summarize the essential aspects of CC use.
Who should be treated?
CC can be used to treat both anovulation/oligo-ovulation and unexplained infertility, but it is not effective in hypothalamic amenorrhea or hypergonadotropic hypogonadism (usually premature ovarian insufficiency). Anovulation/oligo-ovulation may be due to polycystic ovary syndrome (PCOS), obesity, hypothalamic dysfunction related to eating disorders, weight, exercise, stress, hyperprolactinemia, pituitary tumors, or thyroid disease. The exact cause is often indeterminable, however.
Related Article: Polycystic ovary syndrome: Where we stand with diagnosis and treatment and where we're going Steven R. Lindheim, MD, MMM, and Leah Whigham, PhD (First of a 4-part series, September 2012)
There is no evidence CC is effective treatment for “luteal phase defect.” Unexplained infertility also can be treated with CC with intrauterine insemination (IUI).1
Pretreatment evaluation
Diagnosis of ovulatory dysfunction is usually made by menstrual history alone (normal menses, ≥24 and ≥35 days). Testing with luteal phase serum progesterone or serial transvaginal ultrasound generally is unnecessary.
Use the history, physical examination, and other testing, as necessary, to rule out other endocrinopathies, including diabetes mellitus (screening for impaired glucose tolerance), thyroid disorders (measurement of thyroid-stimulating hormone, or TSH), hyperprolactinemia (prolactin assessment), congenital adrenal hyperplasia (measurement of 17-alpha hydroxyprogesterone acetate), and virilization (assessment of testosterone and dehydroepiandrosterone sulfate, or DHEA-S).
If disease-specific treatment does not result in normal ovulation, then CC can be used. Although it may be difficult for them, obese women should be encouraged to lose weight. In infertile couples with a normal menstrual cycle and no other identifiable infertility factors, if hysterosalpingogram and semen analysis are normal, treatment of their unexplained infertility with CC and IUI may be effective. Ovulation induction or ovarian stimulation has little benefit when severe male, uterine, or tubal factors are present.
Treatment regimens
CC is usually given 50 mg/day orally for 5 days starting on the second to fifth spontaneous or progestin-induced menstrual cycle day, with equivalent treatment outcomes regardless of start day 2, 3, 4, or 5. If the patient’s response to this dose is inadequate, treatment can be increased 50 mg/day in each subsequent cycle, to a maximum of 250 mg/day. However, the maximum FDA-approved dose is 100 mg/day, and only 20% of patients respond when given doses higher than this. Obese patients may respond at the higher doses.
The luteinizing hormone (LH) surge occurs 5 to 12 days after the last CC dose is taken. There is no benefit to giving human chorionic gonadotropin (hCG) if the patient has a spontaneous LH surge. The pregnancy rate might actually be reduced by 25% when hCG is given unnecessarily.2
In anovulatory/oligo-ovulatory women, there is no benefit of IUI over timed intercourse for achieving pregnancy. For unexplained infertility, however, CC with timed intercourse does not appear effective, but CC combined with IUI is effective.3 Timed intercourse should occur approximately every 2 days (1–3 days) starting about 3 to 4 days before expected ovulation.
Treatment should continue 3 to 4 months. Younger patients (<35 years) with a short duration of infertility (<2 years) who respond to CC can receive up to 6 months of treatment. Treatment beyond 6 months is not recommended.
Ovulation and pregnancy rates
Half of anovulatory/oligo-ovulatory women will ovulate with a 50-mg dose of CC and half of the remaining will ovulate with a 100-mg dose. Among women who ovulate with CC, cumulative pregnancy rates for 50 mg/day, 100 mg/day, or 150 mg/day at 3 months are 50%, 45%, and 33%, respectively, and at 6 months are 62%, 66%, and 38%, respectively. In general, a 55% to 73% pregnancy rate can be expected.4 Increasing age, duration of infertility, and obesity are associated with lower pregnancy rates and treatment failure.
Alternative and adjunctive regimens
For patients who are not using progestin to induce menses and who have not responded with ovulation by day 14 to 21, longer courses of CC treatment (7 to 8 days) and a step-up protocol to the next highest CC dose are alternative regimens that may work in some cases.
Some anovulatory or oligo-ovulatory women with PCOS who do not respond to CC alone may respond to CC combined with metformin at 1,500 to 1,700 mg/day. Metformin combined with diet and exercise for weight loss is recommended. Metformin is associated with gastrointestinal side effects and rare hepatic toxicity or lactic acidosis; therefore, liver and renal functions should be assessed prior to treatment and monitored afterward.
Women with DHEA-S serum concentrations of 200 µg/dL or greater, and even some women with normal DHEA-S levels, may be more responsive to CC and achieve higher pregnancy rates when given dexamethasone 0.5 mg/daily on cycle days 3 to 12. Glucocorticoids have significant side effects and should be discontinued if treatment is unsuccessful or when pregnancy occurs.
Related Article: Clomiphene failure? Try adding dexamethasone to your clomiphene infertility regimen Robert L. Barbieri, MD (Editorial, May 2012)
Some CC-resistant anovulatory women and women with unexplained infertility may benefit from a trial of sequential CC/gonadotropin treatment consisting of standard CC treatment followed by human menopausal gonadotropins (hMG) or follicle-stimulating hormone (FSH) 75 to 150 IU/day for 3 days. Some, but not all, studies show pregnancy rates in these patients equivalent to those undergoing gonadotropin treatment alone (at a reduced cost). There are no studies directly comparing the treatment regimens, however, and risks of multiple pregnancy might be increased for patients taking both CC and gonadotropin, so this treatment should only be provided by clinicians with requisite training and experience.
Other alternatives to CC therapy in CC-resistant patients include aromatase inhibitors, tamoxifen, insulin-sensitizing agents, ovarian drilling, gonadotropins, and in vitro fertilization.
Monitoring of CC cycles
Objective evidence of ovulation is key to successful treatment. Ovulation predictor kits are more than 90% successful, if used properly, in identifying the LH surge 5 to 12 days after CC is finished (usually around cycle day 16 or 17). Ovulation occurs about one-half day to 2 days after the LH surge. Serum progesterone is the most certain test of prior ovulation (other than pregnancy) but cannot predict time of ovulation. Serial ultrasound shows the size and number of follicles and presumptive ovulation with follicle collapse, as well as echogenic corpus luteum and cul de sac fluid, but it is expensive and often not cost-effective.
It is prudent to postpone further treatment if the patient has large ovaries or a cyst, but routine baseline ultrasound monitoring is no longer considered necessary. However, regular contact with the patient should be maintained to review response to treatment and to ensure that any additional or alternative treatments are not delayed.
Side effects of CC treatment
Mood swings, visual disturbances, breast tenderness, pelvic discomfort, and nausea are reported in less than 10% of patients. Mild ovarian hyperstimulation syndrome (OHSS) is not uncommon, but severe OHSS is rare.
Related Article: Avoiding ovarian hyperstimulation syndrome G. David Adamson, MD (Audiocast, February 2011)
The major risk to CC treatment is twin (8% risk) and triplet (0.5% risk) pregnancies. There is no evidence of increased risk of congenital anomalies, miscarriage, or ovarian cancer.1,5,6
WHAT THIS EVIDENCE MEANS FOR PRACTICE
All gynecologists should be able to diagnose and treat infertility with clomiphene. It is effective for many patients with anovulatory/oligo-ovulatory infertility, and also for unexplained infertility when combined with IUI. Careful evaluation of fertility and endocrinologic status is necessary before treatment, as is monitoring during treatment. Although this treatment may appear to be simple, there are many important principles that need to be followed if treatment is to be effective and safe, and if the patient is to receive quality infertility care. Treatment is safe, (the major risk is multiple pregnancy) but should not be continued for more than 3 to 6 months.
STRIVE FOR PREPREGNANCY VACCINATION
Practice Committee of American Society for Reproductive Medicine. Vaccination guidelines for female infertility patients: A committee opinion. Fertil Steril. 2013;99(2):337–339.
Patients presenting for fertility treatment may have incomplete or unknown immunization status. Encounters with women who desire conception offer an opportunity for providers to optimize their patients’ health prior to pregnancy. Vaccination before or, when appropriate, during pregnancy protects women from preventable disease, decreases the risk for vertical fetal transmission, and enables the passage of maternal immunoglobulins to the fetus, conferring passive immunity to the newborn.
National standards for vaccination have been established by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC). This yearly updated vaccination schedule is available at the CDC’s Web site (http://www.cdc.gov/vaccines/schedules/hcp/adult.html).7 Ideally, a woman’s immunization status should be evaluated and made complete prior to pregnancy. Some vaccines are safe and appropriate for administration during pregnancy, provided the benefits clearly outweigh the risks. The recommended vaccines during pregnancy include inactivated influenza (seasonal and H1N1) and the combined tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap).
Related Article: CDC urges flu vaccination for all, especially pregnant women (News for Your Practice, October 2013)
Many physicians avoid giving vaccinations during pregnancy because of the concern that a spontaneous abortion or congenital anomaly might be incorrectly attributed to vaccine administration, but few vaccines are contradicted during pregnancy. Those that are contraindicated are those containing live virus, including measles, mumps, and rubella (MMR); varicella; and herpes zoster. Concerns also have been raised regarding the safety of administering influenza vaccines containing the mercury-based preservative thimerosol. However, no scientific evidence has conclusively linked adverse effects on offspring with thimerosol-containing vaccines administered during pregnancy.
Immunizations recommended for women of reproductive age
Measles, mumps, rubella (MMR). This vaccine is recommended for all women lacking confirmed immunity to rubella. The vaccine contains live, attenuated virus and is given as a single dose. Women should avoid pregnancy for 1 month after vaccination.
Varicella. This vaccine is for all women lacking confirmed immunity to varicella. It also contains a live, attenuated virus. It is administered in two doses, 1 month apart, and women should avoid pregnancy for 1 month after vaccination.
Influenza. The flu vaccine is recommended annually for individuals 6 months of age and older. The injectable vaccine contains inactivated virus and may be administered during pregnancy—at any time but optimally in October or November because the flu season occurs January through March. (The intranasal influenza vaccine contains live, attenuated virus and should be avoided in pregnancy.) Either method is administered as a single dose.
Thimerosal is a mercury-based preservative used in vaccines, including the influenza vaccine, and is appropriate for use in pregnant women; studies have not shown an association between vaccines containing thimerosal and adverse effects in pregnant women or their offspring.
Tetanus-diptheria-pertussis (Tdap) and tetanus-diphtheria (Td). Tdap or Td is recommended for adults aged 19 to 64 years who have or anticipate having close contact with an infant less than 12 months of age. Due to the recent increase in pertussis infection, Tdap should be given to all women who have not previously received the vaccine and who are pregnant or might become pregnant. It can be given anytime during pregnancy, but optimal administration is during the third trimester or late second trimester (after 20 weeks’ gestation) to confer the greatest amount of fetal protection.
If the vaccine is not being administered during pregnancy, it should be given in the immediate postpartum period to ensure pertussis immunity and to reduce transmission to the newborn. Tdap is administered as a single dose of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis.
Non-routine vaccines include pneumococcus, hepatitis A, hepatitis B, and meningococcus (TABLE). These vaccines should be administered as indicated in high-risk patients.
Health-care providers caring for women with infertility are urged to assess patients’ immunization status prior to attempting pregnancy, to counsel patients about the importance of protecting them and their potential offspring from preventable disease, and to facilitate vaccination prior to conception attempts.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Vaccination is a very important aspect of pre-pregnancy care but is especially important for infertile women who desire pregnancy. Planning of infertility treatment should include assessment of the patient’s vaccination status and completion of appropriate vaccinations before infertility treatment is initiated.
DO CURRENT OPTIONS EFFECTIVELY PREVENT POSTSURGICAL ADHESIONS?
Practice Committee of American Society for Reproductive Medicine in collaboration with Society of Reproductive Surgeons. Pathogenesis, consequences, and control of peritoneal adhesions in gynecologic surgery: A committee opinion. Fertil Steril. 2013;99(6):1550–1555.
Postoperative adhesions are a natural consequence of surgery and a major problem in gynecology. They may cause postsurgical infertility, abdominal/pelvic pain, or bowel obstruction as well as complicate subsequent surgeries by increasing operative times and the risk of bowel injury. The American Society for Reproductive Medicine (ASRM) and the Society of Reproductive Surgeons (SRS) recently evaluated the epidemiology, pathogenesis, and clinical consequences of adhesion formation and the evidence behind strategies for reducing adhesion formation.
In their joint Committee Opinion, they noted that open and laparoscopic approaches to surgery carry comparable levels of risk for adhesion-related hospital readmission. Ovarian surgery has the highest risk for adhesion-related readmission, at 7.5 per 100 initial operations, and the incidence of small bowel obstruction after hysterectomy was found to be 1.6 per 100 procedures. Adhesion-related US health-care costs are estimated at approximately $1 billion annually.
The Societies noted that more severe adnexal adhesions are associated with lower pregnancy rates, and treatment of adnexal adhesions appears to improve pregnancy rates. Investigators found adhesions to cause about three-quarters of postoperative small bowel obstructions; however, the relationship between adhesions and pelvic pain remains unclear. It is thought that adhesions may cause visceral pain by impairing organ mobility, but there is no relationship between the extent of adhesions and the severity of pain. It appears that only dense adhesions involving the bowel are associated with chronic pelvic pain. Predicting the outcome of lysis of adnexal or bowel adhesions is difficult.
Reduction of adhesion formation
Theoretically, adhesions may be reduced by minimizing peritoneal injury during surgery, avoiding intraoperative reactive foreign bodies, reducing local inflammatory response, inhibiting the coagulation cascade and promoting fibrinolysis, or by placing barriers between damaged tissues.
Related Article: Update on Fertility G. David Adamson, MD (February 2008)
Careful surgical technique includes gentle tissue handling, meticulous hemostasis, excision of necrotic tissue, minimizing ischemia and desiccation, using fine and nonreactive suture, and preventing foreign-body reaction and infection, all “microsurgical principles.”
ASRM and SRS reported that the surgical approach (laparoscopy vs laparotomy) is much less important than the extent of tissue injury. However, laparoscopy may result in less tissue and organ handling and trauma, avoid contamination with foreign bodies, enable more precise tissue handling, and result in less postoperative infection. The pneumoperitoneum has a tamponade effect that facilitates hemostasis during laparoscopy, but the process also can be associated with peritoneal desiccation and reduced temperatures that can increase injury.
Laparoscopic myomectomy was found to have a 70% risk of postoperative adhesions, compared with a 90% risk after laparotomy. It is unclear whether peritoneal closure at laparotomy reduces or increases adhesions, but parietal peritoneal closure at primary cesarean delivery results in fewer dense and filmy adhesions.
Related Article: How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy Michael Baggish, MD (Second of a 2-part series on laparoscopic complications, October 2012)
Adjuncts to surgical technique
SRM and SRS reported on three adjuncts to surgical technique that have been proposed to reduce the risk of postoperative adhesions: anti-inflammatory agents, peritoneal instillates, and adhesion barriers.
Dexamethasone, promethazine, and other local and systemic anti-inflammatory drugs and adhesion-reducing substances have not been found effective for reducing postoperative adhesions.
Peritoneal instillates—which create “hydroflotation” and include antibiotic solutions, 32% dextran 70, and crystalloid solutions such as normal saline and Ringer’s lactate with or without heparin or corticosteroids—have not been found effective.8 Icodextrin 4% (Adept Adhesion Reduction Solution, Baxter Healthcare) is FDA approved as an adjunct to good surgical technique for the reduction of postoperative adhesions in patients undergoing gynecologic laparoscopic adhesiolysis. However, a systematic review concluded that there is insufficient evidence for its use as an adhesion-preventing agent.8
Adhesion barriers may help reduce postoperative adhesions but cannot compensate for poor surgical technique. Although the bioresorbable membrane sodium hyaluronic acid and carboxymethyl cellulose (Seprafilm, Genzyme Corp) is FDA-approved, there is limited evidence that it prevents adhesions after myomectomy.9 Because it fragments easily, it is mostly used at laparotomy.
Oxidized regenerated cellulose (Interceed, Ethicon Women’s Health and Urology) is an FDA-approved absorbable adhesion barrier for use at laparotomy that requires no suturing and has been shown to reduce the incidence and extent of new and recurrent adhesions at both laparoscopy and laparotomy by 40% to 50%, although there is little evidence that this improves fertility.9 Complete hemostasis must be achieved to use Interceed, and the addition of heparin confers no benefit.
Another product is expanded polytetrafluoroethylene (ePTFE, Gore-Tex Surgical Membrane, WL Gore and Associates), a nonabsorbable adhesion barrier produced in thin sheets and approved by the FDA for peritoneal repair. ePTFE must be sutured to tissue and helps prevent adhesion formation and reformation regardless of the type of injury or whether complete hemostasis has been achieved. In a small trial, it decreased postmyomectomy adhesions.10 ePTFE also was more effective than oxidized regenerated cellulose in preventing adhesions after adnexal surgery.11 Its use has been limited by the need for suturing and later reoperation for removal, although it probably does not have to be removed if it will not interfere with normal organ function since it has been used as a pericardial graft for many years.12
Hyaluronic acid (HA) solution (Sepracoat, Genzyme) is a natural bioabsorbable component of the extracellular matrix. Women undergoing laparotomy have fewer new adhesions with HA solution, but it is not approved for use in the United States.13 Polyethylene glycol (PEG; SprayGel, Confluent Surgical) was effective in early clinical trials but is not FDA-approved.12 Fibrin sealant (Tisseel VH, Baxter Healthcare) has been reported to decrease the formation of adhesions after salpingostomy, salpingolysis, and ovariolysis. Because it is a biologic product derived from human blood donors, it poses a risk for transmission of infectious agents. It is FDA-approved for use in cardiothoracic surgery, splenic injuries, and colostomy closure for hemostasis.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Adhesions are the most common complication following gynecologic surgery, and they pose potential longstanding consequences to patients. There is no evidence that anti-inflammatory agents reduce postoperative adhesions and insufficient evidence to recommend peritoneal instillates. FDA-approved surgical barriers reduce postoperative adhesions but there is not substantial evidence that their use improves fertility, decreases pain, or reduces the incidence of postoperative bowel obstruction. All gynecologists need to understand the importance of using microsurgical principles rather than relying on adhesion barriers to reduce postoperative adhesions.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: obg@frontlinemedcom.com
- Practice Committee of the American Society for Reproductive Medicine. Use of clomiphene citrate in infertile women: A committee opinion. Fertil Steril. 2013;100(2):341–348.
- George K, Nair R, Tharyan P. Ovulation triggers in anovulatory women undergoing ovulation induction. Cochrane Database Syst Rev. 2008;(3):CD006900.
- Deaton JL, Gibson M, Blackmer KM, Nakajima ST, Badger GJ, Brumsted JR. A randomized, controlled trial of clomiphene citrate and intrauterine insemination in couples with unexplained infertility or surgically corrected endometriosis. Fertil Steril. 1990;54(6):1083–1088.
- Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril. 2008;89(3):505–522.
- Reefhuis J, Honein MA, Schieve LA, Rasmussen SA; National Birth Defects Prevention Study. Use of clomiphene citrate and birth defects, National Birth Defects Prevention Study, 1997-2005. Hum Reprod. 2011;26(2):451–457.
- Silva Idos S, Wark PA, McCormack VA, et al. Ovulation-stimulation drugs and cancer risks: a long-term follow-up of a British cohort. Br J Cancer. 2009;100(11):1824–1831.
- Adult immunization schedules. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vaccines/schedules/hcp/adult.html. Updated October 19, 2013. Accessed January 16, 2014.
- Metwally M, Watson A, Lilford R, Vandekerckhove P. Fluid and pharmacological agents for adhesion prevention after gynaecological surgery. Cochrane Database Syst Rev. 2006;(2):CD001298.
- Farquhar C, Vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility. Cochrane Database Syst Rev. 2000;(2):CD000475.
- The Myomectomy Adhesion Multicenter Study Group. An expanded polytetrafluoroethylene barrier (Gore-Tex Surgical Membrane) reduces post-myomectomy adhesion formation. Fertil Steril. 1995;63(3):491–493.
- Haney AF, Hesla J, Hurst BS, et al. Expanded polytetrafluoroethylene (Gore-Tex Surgical Membrane) is superior to oxidized regenerated cellulose (Interceed TC7+) in preventing adhesions. Fertil Steril. 1995;63(5):1021–1026.
- Alejandro G, Flores RM. Surgical management of tumors invading the superior vena cava. Ann Thorac Surg 2008;85(6):2144−2146.
- Diamond MP; The Sepracoat Adhesion Study Group. Reduction of de novo postsurgical adhesions by intraoperative precoating with Sepracoat (HAL-C) solution: A prospective, randomized blinded, placebo-controlled multicenter study. Fertil Steril. 1998;69(6):1067–1074.
These experts discuss three recent American Society for Reproductive Medicine Committee Opinions. The first is on the optimal use of the most widely prescribed medication for fertility, clomiphene citrate. The second highlights the currently recommended vaccinations for women who are of reproductive age. And the third is on the current evidence for prevention of postsurgical adhesions, which have the potential to cause infertility. Their discussions could affect how you approach your infertile patients.
SAFE, EFFECTIVE USE OF CLOMIPHENE
Practice Committee of the American Society for Reproductive Medicine. Use of clomiphene citrate in infertile women: A committee opinion. Fertil Steril. 2013;100(2):341–348.
Clomiphene citrate (CC) is the fertility medication most commonly used by gynecologists. However, important principles in its use often are not followed, resulting in suboptimal patient care. The American Society for Reproductive Medicine published a recent Committee Opinion on CC’s indications, use, and alternative treatments. We summarize the essential aspects of CC use.
Who should be treated?
CC can be used to treat both anovulation/oligo-ovulation and unexplained infertility, but it is not effective in hypothalamic amenorrhea or hypergonadotropic hypogonadism (usually premature ovarian insufficiency). Anovulation/oligo-ovulation may be due to polycystic ovary syndrome (PCOS), obesity, hypothalamic dysfunction related to eating disorders, weight, exercise, stress, hyperprolactinemia, pituitary tumors, or thyroid disease. The exact cause is often indeterminable, however.
Related Article: Polycystic ovary syndrome: Where we stand with diagnosis and treatment and where we're going Steven R. Lindheim, MD, MMM, and Leah Whigham, PhD (First of a 4-part series, September 2012)
There is no evidence CC is effective treatment for “luteal phase defect.” Unexplained infertility also can be treated with CC with intrauterine insemination (IUI).1
Pretreatment evaluation
Diagnosis of ovulatory dysfunction is usually made by menstrual history alone (normal menses, ≥24 and ≥35 days). Testing with luteal phase serum progesterone or serial transvaginal ultrasound generally is unnecessary.
Use the history, physical examination, and other testing, as necessary, to rule out other endocrinopathies, including diabetes mellitus (screening for impaired glucose tolerance), thyroid disorders (measurement of thyroid-stimulating hormone, or TSH), hyperprolactinemia (prolactin assessment), congenital adrenal hyperplasia (measurement of 17-alpha hydroxyprogesterone acetate), and virilization (assessment of testosterone and dehydroepiandrosterone sulfate, or DHEA-S).
If disease-specific treatment does not result in normal ovulation, then CC can be used. Although it may be difficult for them, obese women should be encouraged to lose weight. In infertile couples with a normal menstrual cycle and no other identifiable infertility factors, if hysterosalpingogram and semen analysis are normal, treatment of their unexplained infertility with CC and IUI may be effective. Ovulation induction or ovarian stimulation has little benefit when severe male, uterine, or tubal factors are present.
Treatment regimens
CC is usually given 50 mg/day orally for 5 days starting on the second to fifth spontaneous or progestin-induced menstrual cycle day, with equivalent treatment outcomes regardless of start day 2, 3, 4, or 5. If the patient’s response to this dose is inadequate, treatment can be increased 50 mg/day in each subsequent cycle, to a maximum of 250 mg/day. However, the maximum FDA-approved dose is 100 mg/day, and only 20% of patients respond when given doses higher than this. Obese patients may respond at the higher doses.
The luteinizing hormone (LH) surge occurs 5 to 12 days after the last CC dose is taken. There is no benefit to giving human chorionic gonadotropin (hCG) if the patient has a spontaneous LH surge. The pregnancy rate might actually be reduced by 25% when hCG is given unnecessarily.2
In anovulatory/oligo-ovulatory women, there is no benefit of IUI over timed intercourse for achieving pregnancy. For unexplained infertility, however, CC with timed intercourse does not appear effective, but CC combined with IUI is effective.3 Timed intercourse should occur approximately every 2 days (1–3 days) starting about 3 to 4 days before expected ovulation.
Treatment should continue 3 to 4 months. Younger patients (<35 years) with a short duration of infertility (<2 years) who respond to CC can receive up to 6 months of treatment. Treatment beyond 6 months is not recommended.
Ovulation and pregnancy rates
Half of anovulatory/oligo-ovulatory women will ovulate with a 50-mg dose of CC and half of the remaining will ovulate with a 100-mg dose. Among women who ovulate with CC, cumulative pregnancy rates for 50 mg/day, 100 mg/day, or 150 mg/day at 3 months are 50%, 45%, and 33%, respectively, and at 6 months are 62%, 66%, and 38%, respectively. In general, a 55% to 73% pregnancy rate can be expected.4 Increasing age, duration of infertility, and obesity are associated with lower pregnancy rates and treatment failure.
Alternative and adjunctive regimens
For patients who are not using progestin to induce menses and who have not responded with ovulation by day 14 to 21, longer courses of CC treatment (7 to 8 days) and a step-up protocol to the next highest CC dose are alternative regimens that may work in some cases.
Some anovulatory or oligo-ovulatory women with PCOS who do not respond to CC alone may respond to CC combined with metformin at 1,500 to 1,700 mg/day. Metformin combined with diet and exercise for weight loss is recommended. Metformin is associated with gastrointestinal side effects and rare hepatic toxicity or lactic acidosis; therefore, liver and renal functions should be assessed prior to treatment and monitored afterward.
Women with DHEA-S serum concentrations of 200 µg/dL or greater, and even some women with normal DHEA-S levels, may be more responsive to CC and achieve higher pregnancy rates when given dexamethasone 0.5 mg/daily on cycle days 3 to 12. Glucocorticoids have significant side effects and should be discontinued if treatment is unsuccessful or when pregnancy occurs.
Related Article: Clomiphene failure? Try adding dexamethasone to your clomiphene infertility regimen Robert L. Barbieri, MD (Editorial, May 2012)
Some CC-resistant anovulatory women and women with unexplained infertility may benefit from a trial of sequential CC/gonadotropin treatment consisting of standard CC treatment followed by human menopausal gonadotropins (hMG) or follicle-stimulating hormone (FSH) 75 to 150 IU/day for 3 days. Some, but not all, studies show pregnancy rates in these patients equivalent to those undergoing gonadotropin treatment alone (at a reduced cost). There are no studies directly comparing the treatment regimens, however, and risks of multiple pregnancy might be increased for patients taking both CC and gonadotropin, so this treatment should only be provided by clinicians with requisite training and experience.
Other alternatives to CC therapy in CC-resistant patients include aromatase inhibitors, tamoxifen, insulin-sensitizing agents, ovarian drilling, gonadotropins, and in vitro fertilization.
Monitoring of CC cycles
Objective evidence of ovulation is key to successful treatment. Ovulation predictor kits are more than 90% successful, if used properly, in identifying the LH surge 5 to 12 days after CC is finished (usually around cycle day 16 or 17). Ovulation occurs about one-half day to 2 days after the LH surge. Serum progesterone is the most certain test of prior ovulation (other than pregnancy) but cannot predict time of ovulation. Serial ultrasound shows the size and number of follicles and presumptive ovulation with follicle collapse, as well as echogenic corpus luteum and cul de sac fluid, but it is expensive and often not cost-effective.
It is prudent to postpone further treatment if the patient has large ovaries or a cyst, but routine baseline ultrasound monitoring is no longer considered necessary. However, regular contact with the patient should be maintained to review response to treatment and to ensure that any additional or alternative treatments are not delayed.
Side effects of CC treatment
Mood swings, visual disturbances, breast tenderness, pelvic discomfort, and nausea are reported in less than 10% of patients. Mild ovarian hyperstimulation syndrome (OHSS) is not uncommon, but severe OHSS is rare.
Related Article: Avoiding ovarian hyperstimulation syndrome G. David Adamson, MD (Audiocast, February 2011)
The major risk to CC treatment is twin (8% risk) and triplet (0.5% risk) pregnancies. There is no evidence of increased risk of congenital anomalies, miscarriage, or ovarian cancer.1,5,6
WHAT THIS EVIDENCE MEANS FOR PRACTICE
All gynecologists should be able to diagnose and treat infertility with clomiphene. It is effective for many patients with anovulatory/oligo-ovulatory infertility, and also for unexplained infertility when combined with IUI. Careful evaluation of fertility and endocrinologic status is necessary before treatment, as is monitoring during treatment. Although this treatment may appear to be simple, there are many important principles that need to be followed if treatment is to be effective and safe, and if the patient is to receive quality infertility care. Treatment is safe, (the major risk is multiple pregnancy) but should not be continued for more than 3 to 6 months.
STRIVE FOR PREPREGNANCY VACCINATION
Practice Committee of American Society for Reproductive Medicine. Vaccination guidelines for female infertility patients: A committee opinion. Fertil Steril. 2013;99(2):337–339.
Patients presenting for fertility treatment may have incomplete or unknown immunization status. Encounters with women who desire conception offer an opportunity for providers to optimize their patients’ health prior to pregnancy. Vaccination before or, when appropriate, during pregnancy protects women from preventable disease, decreases the risk for vertical fetal transmission, and enables the passage of maternal immunoglobulins to the fetus, conferring passive immunity to the newborn.
National standards for vaccination have been established by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC). This yearly updated vaccination schedule is available at the CDC’s Web site (http://www.cdc.gov/vaccines/schedules/hcp/adult.html).7 Ideally, a woman’s immunization status should be evaluated and made complete prior to pregnancy. Some vaccines are safe and appropriate for administration during pregnancy, provided the benefits clearly outweigh the risks. The recommended vaccines during pregnancy include inactivated influenza (seasonal and H1N1) and the combined tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap).
Related Article: CDC urges flu vaccination for all, especially pregnant women (News for Your Practice, October 2013)
Many physicians avoid giving vaccinations during pregnancy because of the concern that a spontaneous abortion or congenital anomaly might be incorrectly attributed to vaccine administration, but few vaccines are contradicted during pregnancy. Those that are contraindicated are those containing live virus, including measles, mumps, and rubella (MMR); varicella; and herpes zoster. Concerns also have been raised regarding the safety of administering influenza vaccines containing the mercury-based preservative thimerosol. However, no scientific evidence has conclusively linked adverse effects on offspring with thimerosol-containing vaccines administered during pregnancy.
Immunizations recommended for women of reproductive age
Measles, mumps, rubella (MMR). This vaccine is recommended for all women lacking confirmed immunity to rubella. The vaccine contains live, attenuated virus and is given as a single dose. Women should avoid pregnancy for 1 month after vaccination.
Varicella. This vaccine is for all women lacking confirmed immunity to varicella. It also contains a live, attenuated virus. It is administered in two doses, 1 month apart, and women should avoid pregnancy for 1 month after vaccination.
Influenza. The flu vaccine is recommended annually for individuals 6 months of age and older. The injectable vaccine contains inactivated virus and may be administered during pregnancy—at any time but optimally in October or November because the flu season occurs January through March. (The intranasal influenza vaccine contains live, attenuated virus and should be avoided in pregnancy.) Either method is administered as a single dose.
Thimerosal is a mercury-based preservative used in vaccines, including the influenza vaccine, and is appropriate for use in pregnant women; studies have not shown an association between vaccines containing thimerosal and adverse effects in pregnant women or their offspring.
Tetanus-diptheria-pertussis (Tdap) and tetanus-diphtheria (Td). Tdap or Td is recommended for adults aged 19 to 64 years who have or anticipate having close contact with an infant less than 12 months of age. Due to the recent increase in pertussis infection, Tdap should be given to all women who have not previously received the vaccine and who are pregnant or might become pregnant. It can be given anytime during pregnancy, but optimal administration is during the third trimester or late second trimester (after 20 weeks’ gestation) to confer the greatest amount of fetal protection.
If the vaccine is not being administered during pregnancy, it should be given in the immediate postpartum period to ensure pertussis immunity and to reduce transmission to the newborn. Tdap is administered as a single dose of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis.
Non-routine vaccines include pneumococcus, hepatitis A, hepatitis B, and meningococcus (TABLE). These vaccines should be administered as indicated in high-risk patients.
Health-care providers caring for women with infertility are urged to assess patients’ immunization status prior to attempting pregnancy, to counsel patients about the importance of protecting them and their potential offspring from preventable disease, and to facilitate vaccination prior to conception attempts.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Vaccination is a very important aspect of pre-pregnancy care but is especially important for infertile women who desire pregnancy. Planning of infertility treatment should include assessment of the patient’s vaccination status and completion of appropriate vaccinations before infertility treatment is initiated.
DO CURRENT OPTIONS EFFECTIVELY PREVENT POSTSURGICAL ADHESIONS?
Practice Committee of American Society for Reproductive Medicine in collaboration with Society of Reproductive Surgeons. Pathogenesis, consequences, and control of peritoneal adhesions in gynecologic surgery: A committee opinion. Fertil Steril. 2013;99(6):1550–1555.
Postoperative adhesions are a natural consequence of surgery and a major problem in gynecology. They may cause postsurgical infertility, abdominal/pelvic pain, or bowel obstruction as well as complicate subsequent surgeries by increasing operative times and the risk of bowel injury. The American Society for Reproductive Medicine (ASRM) and the Society of Reproductive Surgeons (SRS) recently evaluated the epidemiology, pathogenesis, and clinical consequences of adhesion formation and the evidence behind strategies for reducing adhesion formation.
In their joint Committee Opinion, they noted that open and laparoscopic approaches to surgery carry comparable levels of risk for adhesion-related hospital readmission. Ovarian surgery has the highest risk for adhesion-related readmission, at 7.5 per 100 initial operations, and the incidence of small bowel obstruction after hysterectomy was found to be 1.6 per 100 procedures. Adhesion-related US health-care costs are estimated at approximately $1 billion annually.
The Societies noted that more severe adnexal adhesions are associated with lower pregnancy rates, and treatment of adnexal adhesions appears to improve pregnancy rates. Investigators found adhesions to cause about three-quarters of postoperative small bowel obstructions; however, the relationship between adhesions and pelvic pain remains unclear. It is thought that adhesions may cause visceral pain by impairing organ mobility, but there is no relationship between the extent of adhesions and the severity of pain. It appears that only dense adhesions involving the bowel are associated with chronic pelvic pain. Predicting the outcome of lysis of adnexal or bowel adhesions is difficult.
Reduction of adhesion formation
Theoretically, adhesions may be reduced by minimizing peritoneal injury during surgery, avoiding intraoperative reactive foreign bodies, reducing local inflammatory response, inhibiting the coagulation cascade and promoting fibrinolysis, or by placing barriers between damaged tissues.
Related Article: Update on Fertility G. David Adamson, MD (February 2008)
Careful surgical technique includes gentle tissue handling, meticulous hemostasis, excision of necrotic tissue, minimizing ischemia and desiccation, using fine and nonreactive suture, and preventing foreign-body reaction and infection, all “microsurgical principles.”
ASRM and SRS reported that the surgical approach (laparoscopy vs laparotomy) is much less important than the extent of tissue injury. However, laparoscopy may result in less tissue and organ handling and trauma, avoid contamination with foreign bodies, enable more precise tissue handling, and result in less postoperative infection. The pneumoperitoneum has a tamponade effect that facilitates hemostasis during laparoscopy, but the process also can be associated with peritoneal desiccation and reduced temperatures that can increase injury.
Laparoscopic myomectomy was found to have a 70% risk of postoperative adhesions, compared with a 90% risk after laparotomy. It is unclear whether peritoneal closure at laparotomy reduces or increases adhesions, but parietal peritoneal closure at primary cesarean delivery results in fewer dense and filmy adhesions.
Related Article: How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy Michael Baggish, MD (Second of a 2-part series on laparoscopic complications, October 2012)
Adjuncts to surgical technique
SRM and SRS reported on three adjuncts to surgical technique that have been proposed to reduce the risk of postoperative adhesions: anti-inflammatory agents, peritoneal instillates, and adhesion barriers.
Dexamethasone, promethazine, and other local and systemic anti-inflammatory drugs and adhesion-reducing substances have not been found effective for reducing postoperative adhesions.
Peritoneal instillates—which create “hydroflotation” and include antibiotic solutions, 32% dextran 70, and crystalloid solutions such as normal saline and Ringer’s lactate with or without heparin or corticosteroids—have not been found effective.8 Icodextrin 4% (Adept Adhesion Reduction Solution, Baxter Healthcare) is FDA approved as an adjunct to good surgical technique for the reduction of postoperative adhesions in patients undergoing gynecologic laparoscopic adhesiolysis. However, a systematic review concluded that there is insufficient evidence for its use as an adhesion-preventing agent.8
Adhesion barriers may help reduce postoperative adhesions but cannot compensate for poor surgical technique. Although the bioresorbable membrane sodium hyaluronic acid and carboxymethyl cellulose (Seprafilm, Genzyme Corp) is FDA-approved, there is limited evidence that it prevents adhesions after myomectomy.9 Because it fragments easily, it is mostly used at laparotomy.
Oxidized regenerated cellulose (Interceed, Ethicon Women’s Health and Urology) is an FDA-approved absorbable adhesion barrier for use at laparotomy that requires no suturing and has been shown to reduce the incidence and extent of new and recurrent adhesions at both laparoscopy and laparotomy by 40% to 50%, although there is little evidence that this improves fertility.9 Complete hemostasis must be achieved to use Interceed, and the addition of heparin confers no benefit.
Another product is expanded polytetrafluoroethylene (ePTFE, Gore-Tex Surgical Membrane, WL Gore and Associates), a nonabsorbable adhesion barrier produced in thin sheets and approved by the FDA for peritoneal repair. ePTFE must be sutured to tissue and helps prevent adhesion formation and reformation regardless of the type of injury or whether complete hemostasis has been achieved. In a small trial, it decreased postmyomectomy adhesions.10 ePTFE also was more effective than oxidized regenerated cellulose in preventing adhesions after adnexal surgery.11 Its use has been limited by the need for suturing and later reoperation for removal, although it probably does not have to be removed if it will not interfere with normal organ function since it has been used as a pericardial graft for many years.12
Hyaluronic acid (HA) solution (Sepracoat, Genzyme) is a natural bioabsorbable component of the extracellular matrix. Women undergoing laparotomy have fewer new adhesions with HA solution, but it is not approved for use in the United States.13 Polyethylene glycol (PEG; SprayGel, Confluent Surgical) was effective in early clinical trials but is not FDA-approved.12 Fibrin sealant (Tisseel VH, Baxter Healthcare) has been reported to decrease the formation of adhesions after salpingostomy, salpingolysis, and ovariolysis. Because it is a biologic product derived from human blood donors, it poses a risk for transmission of infectious agents. It is FDA-approved for use in cardiothoracic surgery, splenic injuries, and colostomy closure for hemostasis.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Adhesions are the most common complication following gynecologic surgery, and they pose potential longstanding consequences to patients. There is no evidence that anti-inflammatory agents reduce postoperative adhesions and insufficient evidence to recommend peritoneal instillates. FDA-approved surgical barriers reduce postoperative adhesions but there is not substantial evidence that their use improves fertility, decreases pain, or reduces the incidence of postoperative bowel obstruction. All gynecologists need to understand the importance of using microsurgical principles rather than relying on adhesion barriers to reduce postoperative adhesions.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: obg@frontlinemedcom.com
These experts discuss three recent American Society for Reproductive Medicine Committee Opinions. The first is on the optimal use of the most widely prescribed medication for fertility, clomiphene citrate. The second highlights the currently recommended vaccinations for women who are of reproductive age. And the third is on the current evidence for prevention of postsurgical adhesions, which have the potential to cause infertility. Their discussions could affect how you approach your infertile patients.
SAFE, EFFECTIVE USE OF CLOMIPHENE
Practice Committee of the American Society for Reproductive Medicine. Use of clomiphene citrate in infertile women: A committee opinion. Fertil Steril. 2013;100(2):341–348.
Clomiphene citrate (CC) is the fertility medication most commonly used by gynecologists. However, important principles in its use often are not followed, resulting in suboptimal patient care. The American Society for Reproductive Medicine published a recent Committee Opinion on CC’s indications, use, and alternative treatments. We summarize the essential aspects of CC use.
Who should be treated?
CC can be used to treat both anovulation/oligo-ovulation and unexplained infertility, but it is not effective in hypothalamic amenorrhea or hypergonadotropic hypogonadism (usually premature ovarian insufficiency). Anovulation/oligo-ovulation may be due to polycystic ovary syndrome (PCOS), obesity, hypothalamic dysfunction related to eating disorders, weight, exercise, stress, hyperprolactinemia, pituitary tumors, or thyroid disease. The exact cause is often indeterminable, however.
Related Article: Polycystic ovary syndrome: Where we stand with diagnosis and treatment and where we're going Steven R. Lindheim, MD, MMM, and Leah Whigham, PhD (First of a 4-part series, September 2012)
There is no evidence CC is effective treatment for “luteal phase defect.” Unexplained infertility also can be treated with CC with intrauterine insemination (IUI).1
Pretreatment evaluation
Diagnosis of ovulatory dysfunction is usually made by menstrual history alone (normal menses, ≥24 and ≥35 days). Testing with luteal phase serum progesterone or serial transvaginal ultrasound generally is unnecessary.
Use the history, physical examination, and other testing, as necessary, to rule out other endocrinopathies, including diabetes mellitus (screening for impaired glucose tolerance), thyroid disorders (measurement of thyroid-stimulating hormone, or TSH), hyperprolactinemia (prolactin assessment), congenital adrenal hyperplasia (measurement of 17-alpha hydroxyprogesterone acetate), and virilization (assessment of testosterone and dehydroepiandrosterone sulfate, or DHEA-S).
If disease-specific treatment does not result in normal ovulation, then CC can be used. Although it may be difficult for them, obese women should be encouraged to lose weight. In infertile couples with a normal menstrual cycle and no other identifiable infertility factors, if hysterosalpingogram and semen analysis are normal, treatment of their unexplained infertility with CC and IUI may be effective. Ovulation induction or ovarian stimulation has little benefit when severe male, uterine, or tubal factors are present.
Treatment regimens
CC is usually given 50 mg/day orally for 5 days starting on the second to fifth spontaneous or progestin-induced menstrual cycle day, with equivalent treatment outcomes regardless of start day 2, 3, 4, or 5. If the patient’s response to this dose is inadequate, treatment can be increased 50 mg/day in each subsequent cycle, to a maximum of 250 mg/day. However, the maximum FDA-approved dose is 100 mg/day, and only 20% of patients respond when given doses higher than this. Obese patients may respond at the higher doses.
The luteinizing hormone (LH) surge occurs 5 to 12 days after the last CC dose is taken. There is no benefit to giving human chorionic gonadotropin (hCG) if the patient has a spontaneous LH surge. The pregnancy rate might actually be reduced by 25% when hCG is given unnecessarily.2
In anovulatory/oligo-ovulatory women, there is no benefit of IUI over timed intercourse for achieving pregnancy. For unexplained infertility, however, CC with timed intercourse does not appear effective, but CC combined with IUI is effective.3 Timed intercourse should occur approximately every 2 days (1–3 days) starting about 3 to 4 days before expected ovulation.
Treatment should continue 3 to 4 months. Younger patients (<35 years) with a short duration of infertility (<2 years) who respond to CC can receive up to 6 months of treatment. Treatment beyond 6 months is not recommended.
Ovulation and pregnancy rates
Half of anovulatory/oligo-ovulatory women will ovulate with a 50-mg dose of CC and half of the remaining will ovulate with a 100-mg dose. Among women who ovulate with CC, cumulative pregnancy rates for 50 mg/day, 100 mg/day, or 150 mg/day at 3 months are 50%, 45%, and 33%, respectively, and at 6 months are 62%, 66%, and 38%, respectively. In general, a 55% to 73% pregnancy rate can be expected.4 Increasing age, duration of infertility, and obesity are associated with lower pregnancy rates and treatment failure.
Alternative and adjunctive regimens
For patients who are not using progestin to induce menses and who have not responded with ovulation by day 14 to 21, longer courses of CC treatment (7 to 8 days) and a step-up protocol to the next highest CC dose are alternative regimens that may work in some cases.
Some anovulatory or oligo-ovulatory women with PCOS who do not respond to CC alone may respond to CC combined with metformin at 1,500 to 1,700 mg/day. Metformin combined with diet and exercise for weight loss is recommended. Metformin is associated with gastrointestinal side effects and rare hepatic toxicity or lactic acidosis; therefore, liver and renal functions should be assessed prior to treatment and monitored afterward.
Women with DHEA-S serum concentrations of 200 µg/dL or greater, and even some women with normal DHEA-S levels, may be more responsive to CC and achieve higher pregnancy rates when given dexamethasone 0.5 mg/daily on cycle days 3 to 12. Glucocorticoids have significant side effects and should be discontinued if treatment is unsuccessful or when pregnancy occurs.
Related Article: Clomiphene failure? Try adding dexamethasone to your clomiphene infertility regimen Robert L. Barbieri, MD (Editorial, May 2012)
Some CC-resistant anovulatory women and women with unexplained infertility may benefit from a trial of sequential CC/gonadotropin treatment consisting of standard CC treatment followed by human menopausal gonadotropins (hMG) or follicle-stimulating hormone (FSH) 75 to 150 IU/day for 3 days. Some, but not all, studies show pregnancy rates in these patients equivalent to those undergoing gonadotropin treatment alone (at a reduced cost). There are no studies directly comparing the treatment regimens, however, and risks of multiple pregnancy might be increased for patients taking both CC and gonadotropin, so this treatment should only be provided by clinicians with requisite training and experience.
Other alternatives to CC therapy in CC-resistant patients include aromatase inhibitors, tamoxifen, insulin-sensitizing agents, ovarian drilling, gonadotropins, and in vitro fertilization.
Monitoring of CC cycles
Objective evidence of ovulation is key to successful treatment. Ovulation predictor kits are more than 90% successful, if used properly, in identifying the LH surge 5 to 12 days after CC is finished (usually around cycle day 16 or 17). Ovulation occurs about one-half day to 2 days after the LH surge. Serum progesterone is the most certain test of prior ovulation (other than pregnancy) but cannot predict time of ovulation. Serial ultrasound shows the size and number of follicles and presumptive ovulation with follicle collapse, as well as echogenic corpus luteum and cul de sac fluid, but it is expensive and often not cost-effective.
It is prudent to postpone further treatment if the patient has large ovaries or a cyst, but routine baseline ultrasound monitoring is no longer considered necessary. However, regular contact with the patient should be maintained to review response to treatment and to ensure that any additional or alternative treatments are not delayed.
Side effects of CC treatment
Mood swings, visual disturbances, breast tenderness, pelvic discomfort, and nausea are reported in less than 10% of patients. Mild ovarian hyperstimulation syndrome (OHSS) is not uncommon, but severe OHSS is rare.
Related Article: Avoiding ovarian hyperstimulation syndrome G. David Adamson, MD (Audiocast, February 2011)
The major risk to CC treatment is twin (8% risk) and triplet (0.5% risk) pregnancies. There is no evidence of increased risk of congenital anomalies, miscarriage, or ovarian cancer.1,5,6
WHAT THIS EVIDENCE MEANS FOR PRACTICE
All gynecologists should be able to diagnose and treat infertility with clomiphene. It is effective for many patients with anovulatory/oligo-ovulatory infertility, and also for unexplained infertility when combined with IUI. Careful evaluation of fertility and endocrinologic status is necessary before treatment, as is monitoring during treatment. Although this treatment may appear to be simple, there are many important principles that need to be followed if treatment is to be effective and safe, and if the patient is to receive quality infertility care. Treatment is safe, (the major risk is multiple pregnancy) but should not be continued for more than 3 to 6 months.
STRIVE FOR PREPREGNANCY VACCINATION
Practice Committee of American Society for Reproductive Medicine. Vaccination guidelines for female infertility patients: A committee opinion. Fertil Steril. 2013;99(2):337–339.
Patients presenting for fertility treatment may have incomplete or unknown immunization status. Encounters with women who desire conception offer an opportunity for providers to optimize their patients’ health prior to pregnancy. Vaccination before or, when appropriate, during pregnancy protects women from preventable disease, decreases the risk for vertical fetal transmission, and enables the passage of maternal immunoglobulins to the fetus, conferring passive immunity to the newborn.
National standards for vaccination have been established by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC). This yearly updated vaccination schedule is available at the CDC’s Web site (http://www.cdc.gov/vaccines/schedules/hcp/adult.html).7 Ideally, a woman’s immunization status should be evaluated and made complete prior to pregnancy. Some vaccines are safe and appropriate for administration during pregnancy, provided the benefits clearly outweigh the risks. The recommended vaccines during pregnancy include inactivated influenza (seasonal and H1N1) and the combined tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap).
Related Article: CDC urges flu vaccination for all, especially pregnant women (News for Your Practice, October 2013)
Many physicians avoid giving vaccinations during pregnancy because of the concern that a spontaneous abortion or congenital anomaly might be incorrectly attributed to vaccine administration, but few vaccines are contradicted during pregnancy. Those that are contraindicated are those containing live virus, including measles, mumps, and rubella (MMR); varicella; and herpes zoster. Concerns also have been raised regarding the safety of administering influenza vaccines containing the mercury-based preservative thimerosol. However, no scientific evidence has conclusively linked adverse effects on offspring with thimerosol-containing vaccines administered during pregnancy.
Immunizations recommended for women of reproductive age
Measles, mumps, rubella (MMR). This vaccine is recommended for all women lacking confirmed immunity to rubella. The vaccine contains live, attenuated virus and is given as a single dose. Women should avoid pregnancy for 1 month after vaccination.
Varicella. This vaccine is for all women lacking confirmed immunity to varicella. It also contains a live, attenuated virus. It is administered in two doses, 1 month apart, and women should avoid pregnancy for 1 month after vaccination.
Influenza. The flu vaccine is recommended annually for individuals 6 months of age and older. The injectable vaccine contains inactivated virus and may be administered during pregnancy—at any time but optimally in October or November because the flu season occurs January through March. (The intranasal influenza vaccine contains live, attenuated virus and should be avoided in pregnancy.) Either method is administered as a single dose.
Thimerosal is a mercury-based preservative used in vaccines, including the influenza vaccine, and is appropriate for use in pregnant women; studies have not shown an association between vaccines containing thimerosal and adverse effects in pregnant women or their offspring.
Tetanus-diptheria-pertussis (Tdap) and tetanus-diphtheria (Td). Tdap or Td is recommended for adults aged 19 to 64 years who have or anticipate having close contact with an infant less than 12 months of age. Due to the recent increase in pertussis infection, Tdap should be given to all women who have not previously received the vaccine and who are pregnant or might become pregnant. It can be given anytime during pregnancy, but optimal administration is during the third trimester or late second trimester (after 20 weeks’ gestation) to confer the greatest amount of fetal protection.
If the vaccine is not being administered during pregnancy, it should be given in the immediate postpartum period to ensure pertussis immunity and to reduce transmission to the newborn. Tdap is administered as a single dose of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis.
Non-routine vaccines include pneumococcus, hepatitis A, hepatitis B, and meningococcus (TABLE). These vaccines should be administered as indicated in high-risk patients.
Health-care providers caring for women with infertility are urged to assess patients’ immunization status prior to attempting pregnancy, to counsel patients about the importance of protecting them and their potential offspring from preventable disease, and to facilitate vaccination prior to conception attempts.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Vaccination is a very important aspect of pre-pregnancy care but is especially important for infertile women who desire pregnancy. Planning of infertility treatment should include assessment of the patient’s vaccination status and completion of appropriate vaccinations before infertility treatment is initiated.
DO CURRENT OPTIONS EFFECTIVELY PREVENT POSTSURGICAL ADHESIONS?
Practice Committee of American Society for Reproductive Medicine in collaboration with Society of Reproductive Surgeons. Pathogenesis, consequences, and control of peritoneal adhesions in gynecologic surgery: A committee opinion. Fertil Steril. 2013;99(6):1550–1555.
Postoperative adhesions are a natural consequence of surgery and a major problem in gynecology. They may cause postsurgical infertility, abdominal/pelvic pain, or bowel obstruction as well as complicate subsequent surgeries by increasing operative times and the risk of bowel injury. The American Society for Reproductive Medicine (ASRM) and the Society of Reproductive Surgeons (SRS) recently evaluated the epidemiology, pathogenesis, and clinical consequences of adhesion formation and the evidence behind strategies for reducing adhesion formation.
In their joint Committee Opinion, they noted that open and laparoscopic approaches to surgery carry comparable levels of risk for adhesion-related hospital readmission. Ovarian surgery has the highest risk for adhesion-related readmission, at 7.5 per 100 initial operations, and the incidence of small bowel obstruction after hysterectomy was found to be 1.6 per 100 procedures. Adhesion-related US health-care costs are estimated at approximately $1 billion annually.
The Societies noted that more severe adnexal adhesions are associated with lower pregnancy rates, and treatment of adnexal adhesions appears to improve pregnancy rates. Investigators found adhesions to cause about three-quarters of postoperative small bowel obstructions; however, the relationship between adhesions and pelvic pain remains unclear. It is thought that adhesions may cause visceral pain by impairing organ mobility, but there is no relationship between the extent of adhesions and the severity of pain. It appears that only dense adhesions involving the bowel are associated with chronic pelvic pain. Predicting the outcome of lysis of adnexal or bowel adhesions is difficult.
Reduction of adhesion formation
Theoretically, adhesions may be reduced by minimizing peritoneal injury during surgery, avoiding intraoperative reactive foreign bodies, reducing local inflammatory response, inhibiting the coagulation cascade and promoting fibrinolysis, or by placing barriers between damaged tissues.
Related Article: Update on Fertility G. David Adamson, MD (February 2008)
Careful surgical technique includes gentle tissue handling, meticulous hemostasis, excision of necrotic tissue, minimizing ischemia and desiccation, using fine and nonreactive suture, and preventing foreign-body reaction and infection, all “microsurgical principles.”
ASRM and SRS reported that the surgical approach (laparoscopy vs laparotomy) is much less important than the extent of tissue injury. However, laparoscopy may result in less tissue and organ handling and trauma, avoid contamination with foreign bodies, enable more precise tissue handling, and result in less postoperative infection. The pneumoperitoneum has a tamponade effect that facilitates hemostasis during laparoscopy, but the process also can be associated with peritoneal desiccation and reduced temperatures that can increase injury.
Laparoscopic myomectomy was found to have a 70% risk of postoperative adhesions, compared with a 90% risk after laparotomy. It is unclear whether peritoneal closure at laparotomy reduces or increases adhesions, but parietal peritoneal closure at primary cesarean delivery results in fewer dense and filmy adhesions.
Related Article: How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy Michael Baggish, MD (Second of a 2-part series on laparoscopic complications, October 2012)
Adjuncts to surgical technique
SRM and SRS reported on three adjuncts to surgical technique that have been proposed to reduce the risk of postoperative adhesions: anti-inflammatory agents, peritoneal instillates, and adhesion barriers.
Dexamethasone, promethazine, and other local and systemic anti-inflammatory drugs and adhesion-reducing substances have not been found effective for reducing postoperative adhesions.
Peritoneal instillates—which create “hydroflotation” and include antibiotic solutions, 32% dextran 70, and crystalloid solutions such as normal saline and Ringer’s lactate with or without heparin or corticosteroids—have not been found effective.8 Icodextrin 4% (Adept Adhesion Reduction Solution, Baxter Healthcare) is FDA approved as an adjunct to good surgical technique for the reduction of postoperative adhesions in patients undergoing gynecologic laparoscopic adhesiolysis. However, a systematic review concluded that there is insufficient evidence for its use as an adhesion-preventing agent.8
Adhesion barriers may help reduce postoperative adhesions but cannot compensate for poor surgical technique. Although the bioresorbable membrane sodium hyaluronic acid and carboxymethyl cellulose (Seprafilm, Genzyme Corp) is FDA-approved, there is limited evidence that it prevents adhesions after myomectomy.9 Because it fragments easily, it is mostly used at laparotomy.
Oxidized regenerated cellulose (Interceed, Ethicon Women’s Health and Urology) is an FDA-approved absorbable adhesion barrier for use at laparotomy that requires no suturing and has been shown to reduce the incidence and extent of new and recurrent adhesions at both laparoscopy and laparotomy by 40% to 50%, although there is little evidence that this improves fertility.9 Complete hemostasis must be achieved to use Interceed, and the addition of heparin confers no benefit.
Another product is expanded polytetrafluoroethylene (ePTFE, Gore-Tex Surgical Membrane, WL Gore and Associates), a nonabsorbable adhesion barrier produced in thin sheets and approved by the FDA for peritoneal repair. ePTFE must be sutured to tissue and helps prevent adhesion formation and reformation regardless of the type of injury or whether complete hemostasis has been achieved. In a small trial, it decreased postmyomectomy adhesions.10 ePTFE also was more effective than oxidized regenerated cellulose in preventing adhesions after adnexal surgery.11 Its use has been limited by the need for suturing and later reoperation for removal, although it probably does not have to be removed if it will not interfere with normal organ function since it has been used as a pericardial graft for many years.12
Hyaluronic acid (HA) solution (Sepracoat, Genzyme) is a natural bioabsorbable component of the extracellular matrix. Women undergoing laparotomy have fewer new adhesions with HA solution, but it is not approved for use in the United States.13 Polyethylene glycol (PEG; SprayGel, Confluent Surgical) was effective in early clinical trials but is not FDA-approved.12 Fibrin sealant (Tisseel VH, Baxter Healthcare) has been reported to decrease the formation of adhesions after salpingostomy, salpingolysis, and ovariolysis. Because it is a biologic product derived from human blood donors, it poses a risk for transmission of infectious agents. It is FDA-approved for use in cardiothoracic surgery, splenic injuries, and colostomy closure for hemostasis.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Adhesions are the most common complication following gynecologic surgery, and they pose potential longstanding consequences to patients. There is no evidence that anti-inflammatory agents reduce postoperative adhesions and insufficient evidence to recommend peritoneal instillates. FDA-approved surgical barriers reduce postoperative adhesions but there is not substantial evidence that their use improves fertility, decreases pain, or reduces the incidence of postoperative bowel obstruction. All gynecologists need to understand the importance of using microsurgical principles rather than relying on adhesion barriers to reduce postoperative adhesions.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: obg@frontlinemedcom.com
- Practice Committee of the American Society for Reproductive Medicine. Use of clomiphene citrate in infertile women: A committee opinion. Fertil Steril. 2013;100(2):341–348.
- George K, Nair R, Tharyan P. Ovulation triggers in anovulatory women undergoing ovulation induction. Cochrane Database Syst Rev. 2008;(3):CD006900.
- Deaton JL, Gibson M, Blackmer KM, Nakajima ST, Badger GJ, Brumsted JR. A randomized, controlled trial of clomiphene citrate and intrauterine insemination in couples with unexplained infertility or surgically corrected endometriosis. Fertil Steril. 1990;54(6):1083–1088.
- Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril. 2008;89(3):505–522.
- Reefhuis J, Honein MA, Schieve LA, Rasmussen SA; National Birth Defects Prevention Study. Use of clomiphene citrate and birth defects, National Birth Defects Prevention Study, 1997-2005. Hum Reprod. 2011;26(2):451–457.
- Silva Idos S, Wark PA, McCormack VA, et al. Ovulation-stimulation drugs and cancer risks: a long-term follow-up of a British cohort. Br J Cancer. 2009;100(11):1824–1831.
- Adult immunization schedules. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vaccines/schedules/hcp/adult.html. Updated October 19, 2013. Accessed January 16, 2014.
- Metwally M, Watson A, Lilford R, Vandekerckhove P. Fluid and pharmacological agents for adhesion prevention after gynaecological surgery. Cochrane Database Syst Rev. 2006;(2):CD001298.
- Farquhar C, Vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility. Cochrane Database Syst Rev. 2000;(2):CD000475.
- The Myomectomy Adhesion Multicenter Study Group. An expanded polytetrafluoroethylene barrier (Gore-Tex Surgical Membrane) reduces post-myomectomy adhesion formation. Fertil Steril. 1995;63(3):491–493.
- Haney AF, Hesla J, Hurst BS, et al. Expanded polytetrafluoroethylene (Gore-Tex Surgical Membrane) is superior to oxidized regenerated cellulose (Interceed TC7+) in preventing adhesions. Fertil Steril. 1995;63(5):1021–1026.
- Alejandro G, Flores RM. Surgical management of tumors invading the superior vena cava. Ann Thorac Surg 2008;85(6):2144−2146.
- Diamond MP; The Sepracoat Adhesion Study Group. Reduction of de novo postsurgical adhesions by intraoperative precoating with Sepracoat (HAL-C) solution: A prospective, randomized blinded, placebo-controlled multicenter study. Fertil Steril. 1998;69(6):1067–1074.
- Practice Committee of the American Society for Reproductive Medicine. Use of clomiphene citrate in infertile women: A committee opinion. Fertil Steril. 2013;100(2):341–348.
- George K, Nair R, Tharyan P. Ovulation triggers in anovulatory women undergoing ovulation induction. Cochrane Database Syst Rev. 2008;(3):CD006900.
- Deaton JL, Gibson M, Blackmer KM, Nakajima ST, Badger GJ, Brumsted JR. A randomized, controlled trial of clomiphene citrate and intrauterine insemination in couples with unexplained infertility or surgically corrected endometriosis. Fertil Steril. 1990;54(6):1083–1088.
- Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril. 2008;89(3):505–522.
- Reefhuis J, Honein MA, Schieve LA, Rasmussen SA; National Birth Defects Prevention Study. Use of clomiphene citrate and birth defects, National Birth Defects Prevention Study, 1997-2005. Hum Reprod. 2011;26(2):451–457.
- Silva Idos S, Wark PA, McCormack VA, et al. Ovulation-stimulation drugs and cancer risks: a long-term follow-up of a British cohort. Br J Cancer. 2009;100(11):1824–1831.
- Adult immunization schedules. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vaccines/schedules/hcp/adult.html. Updated October 19, 2013. Accessed January 16, 2014.
- Metwally M, Watson A, Lilford R, Vandekerckhove P. Fluid and pharmacological agents for adhesion prevention after gynaecological surgery. Cochrane Database Syst Rev. 2006;(2):CD001298.
- Farquhar C, Vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility. Cochrane Database Syst Rev. 2000;(2):CD000475.
- The Myomectomy Adhesion Multicenter Study Group. An expanded polytetrafluoroethylene barrier (Gore-Tex Surgical Membrane) reduces post-myomectomy adhesion formation. Fertil Steril. 1995;63(3):491–493.
- Haney AF, Hesla J, Hurst BS, et al. Expanded polytetrafluoroethylene (Gore-Tex Surgical Membrane) is superior to oxidized regenerated cellulose (Interceed TC7+) in preventing adhesions. Fertil Steril. 1995;63(5):1021–1026.
- Alejandro G, Flores RM. Surgical management of tumors invading the superior vena cava. Ann Thorac Surg 2008;85(6):2144−2146.
- Diamond MP; The Sepracoat Adhesion Study Group. Reduction of de novo postsurgical adhesions by intraoperative precoating with Sepracoat (HAL-C) solution: A prospective, randomized blinded, placebo-controlled multicenter study. Fertil Steril. 1998;69(6):1067–1074.
2013 Update on fertility
CLICK HERE to access several articles on treating fertility issues.
Dr. Abusief reports no financial relationships relevant to this article. Dr. Adamson reports that he receives research grants from LabCorp and Auxogyn, and is the founder and CEO of Advanced Reproductive Care.
Infertility is not just a woman’s issue; it is a couple’s issue. According to the Centers for Disease Control and Prevention, one-third of cases of infertility are caused by a reproductive problem for the woman, one-third are caused by a problem for the man, and one-third are due to problems for both partners or to unknown causes.1
Here, we discuss three developments within the past 12 months related to the treatment of infertility:
- The International Federation of Gynecology and Obstetrics (FIGO) Committee on Reproductive Medicine—charged with developing evidence-based, cost-effective guidelines that would be accepted as standards for increasing access to quality reproductive medical care in all countries of the world—has developed The FIGO Fertility Tool Box™.
- Smoking cigarettes negatively affects a man’s and woman’s fertility, yet smoking’s contribution to infertility is under-recognized. The Practice Committee of the American Society for Reproductive Medicine culled the evidence, and published its review on the effects of smoking on fertility.
- Results of a large, population-wide cohort study shed light on the association of in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) with birth defects and whether underlying factors present in patients with infertility also may play a role.
FIGO offers Tools for managing infertility
Adamson GD. A quick guide to the FIGO Fertility Tool Box. The FIGO Fertility Tool Box. The International Federation of Gynecology and Obstetrics Web site. http://www.arcfertility.com/figo. Published 2013. Accessed January 21, 2013.
FIGO has as members 125 national ObGyn societies. The FIGO Committee on Reproductive Medicine’s mission is to create access to quality reproductive medical care and is focused on helping infertile women become pregnant and/or on alleviating the burden of infertility. The Committee has just released The FIGO Fertility Tool Box™ to further this goal.
Who should use the Tool Box?
Anybody who wants to help infertile people! It is designed for health-care workers and others who want to make a difference in the lives of infertile people. The Tool Box can be accessed electronically on both computers and cell phones at http://www.figo.org/news/resources/FIGO_Fertility_Tool_Box.
What’s in the Tool Box?
Seven Tools help you tackle the disease/ disability of infertility. Each Tool provides information on how to manage a particular aspect of infertility:
- Tool 1: The FIGO Fertility Daisy—why we should care about infertility
- Tool 2: Overcome personal barriers
- Tool 3: Overcome societal barriers
- Tool 4: Diagnose infertility
- Tool 5: Treat infertility
- Tool 6: Refer/resolve infertility
- Tool 7: Prevent infertility.
The Tools Pyramid ( FIGURE 1 ) contains these seven Tools.
FIGURE 1 The Tools Pyramid™
How do the Tools work?
Each of the seven Tools consists of three levels:
Level 1: Basic Tools. The first level consists of 7 Basic Tools™, which contain information that is brief and succinct—just a simple statement or summary of the Daisy and each of the six Pyramids of Action. The Basic Tools are colored orange.
Level 2: Support Tools. The second level is Support Tools™, which provide more information and detail—enough so that you know what to do to take action. Support Tools are colored green.
Level 3: Reference Tools. The third level is Reference Tools™, which are lists of references that provide evidence for the information and recommended actions in the Basic and Support Tools. Reference Tools are colored white.
The Glossary provides definitions and explanations of abbreviations and acronyms and is colored white like the References. By coloring the levels icons this way, you can always tell whether you are using a Basic Tool, Support Tool, or Reference Tool.
The Levels Pyramid (FIGURE 2 ) shows how the Basic Tools, Support Tools, and Reference Tools relate to each other. You can choose your Tool and level by clicking on the icons from your computer or cellphone.
FIGURE 2 The Levels Pyramid™
How do I know what to do?—The Actions Pyramids™
With the exception of the Daisy (Tool 1), all Tools have the shape of a pyramid ( FIGURE 3 ). At the base of each pyramid are actions that can be taken in low-resource settings; that is, they are often simpler, elementary, involve fewer people and are low-cost interventions or opportunities. Overall, there are 64 total actions described in the seven Tools.
As you move higher in the pyramid, generally more resources are required to take actions that are usually more complex. You can think of it as a kind of ladder—as you climb higher it usually gets a bit more complex or complicated. Sometimes, however, it might also be easier higher up on the ladder, and the elementary aspects might be those most challenging.
If you are interested in helping a patient with infertility, you are encouraged to do whatever you can do at any level in any of the Tools in The Actions Pyramid. In the online version, you can click on the actions arrow or icons to click immediately to the action you wish to learn about and do.
FIGURE 3 The Actions Pyramid™
Which Tool should I use?
The one you think will work best for you and will give you some results quickly. Doing something is better than doing nothing. There is no right or wrong way to make a start. Then, if you want to do more you can choose other Tools or individual aspects of other Tools to build on what you have already achieved. Or, if you want to be very systematic and are very committed you can start with Tool 1 and work your way through the entire Tool Box.
What if I can’t implement some of the recommendations?
Then drop it and move onto something that you can do or implement. No single component of the Tool Box is so essential to helping infertile couples that your efforts will fail if you can’t apply it. Using even one or two actions in one or two Tools will empower you to help many infertile individuals.
What if I want to change a Tool?
Just do it. The Tool Box is made to be changed so that it can be adapted to work in any type of health-care setting anywhere in the world. You know what works best in your situation. Just never stop caring and trying to help infertile people.
Does the Tool Box have a compliments and complaints section?
Yes, it is called the FIGO Committee on Reproductive Medicine. E-mail us at fertilitytool box@figo.org. We would love to hear from you about what you like and what works in the Tool Box and what doesn’t. We hope to constantly improve The FIGO Fertility Tool Box to make it a better Tool to help you tackle the disease/disability of infertility.
This Tool Box now gives providers at any level of women’s health care anywhere in the world easy electronic access to comprehensive evidence-based actions that can be used to help those with infertility
Smoking, by either partner, active or passive, negatively affects reproductive health
Pfeifer S, Fritz M, Goldberg J, et al; the Practice Committee of the American Society for Reproductive Medicine. Smoking and infertility: a committee opinion. Fertil Steril. 2012;98(6):1400–1406.
Approximately 30% of reproductive-age women and 35% of reproductive-age men smoke cigarettes. Although smoking has been linked to many adverse health effects, the substantial detrimental effects of cigarette smoking on fecundity and reproduction are under-recognized. In a recent publication, the Practice Committee of the American Society for Reproductive Medicine reviewed the effects of smoking on fertility.
Smoking’s ill effects on fertility
Conception delay
Smokers are at an increased risk for infertility and conception delay. Independent of other factors, smoking has a negative impact on fecundity, with a trend toward increased time to conception with increased number of cigarettes smoked.2,3 The percentage of women experiencing conception delay for more than 12 months was shown to be 54% higher in smoking versus nonsmoking women in one study.3 These authors found that active smoking by either partner had an adverse effect on conception. Furthermore, the impact of passive smoking by either partner was found to be only slightly less than the impact found for active smoking by either partner.3
Ovarian follicular depletion
Basal levels of follicle-stimulating hormone (FSH) are significantly higher in smokers, with one study demonstrating a 66% increase in smoking versus nonsmoking women and a 39% increase in passive versus nonsmoking women.4 Chemicals in cigarette smoke appear to accelerate follicular depletion and loss of reproductive function, and menopause has been found to occur 1 to 4 years earlier in smoking versus nonsmoking women.2
Effects on sperm parameters
Poor function. Smoking has been found to reduce sperm density, motility, and possibly morphology. Sperm function tests appear to be 22% poorer in smokers versus nonsmokers, and the effects are dose-dependent.
No link to male infertility, yet. While evidence suggests an adverse effect on sperm function from smoking, available data do not conclusively demonstrate a reduction in male fertility due to smoking. This could be due to secondary confounding effects of partner status.2
Maternal smoking may decrease sperm counts in offspring, according to Storgaard and colleagues, who found that men whose mothers had smoked more than 10 cigarettes per day had lower sperm densities than men with nonsmoking mothers.5
Mutagenic potential
Tobacco smoke exposure may harm gametogenesis by adversely affecting chromosomes and damaging the meiotic spindle and has been associated with an increased risk of trisomy 21 offspring resulting from maternal nondisjunction.6,7 Gene damage in sperm may be secondary to direct binding of tobacco smoke constituents or chemical byproducts to DNA, creating premutational lesions or “adducts.” These mutagenic adducts have been found in greater numbers in embryos from smokers versus nonsmokers, suggesting a mechanism for the transmission of adversely modified DNA from parental smoking.2
Early pregnancy effects
Smoking increases the risk of spontaneous miscarriage in both natural and assisted conceptions and has been linked to an increased risk for bacterial vaginosis, which in turn increases the risk for second trimester miscarriage and preterm labor.2 Studies also have identified an increased risk of ectopic pregnancy in smokers, including one study demonstrating an odds ratio (OR) for ectopic pregnancy of 3.5 (95% confidence interval [CI], 1.4–8.6) in women who smoked more than 20 cigarettes per day versus nonsmokers.6
Assisted reproductive therapies rendered less effective
Studies of IVF have demonstrated that smokers versus nonsmokers have an increased gonadotropin requirement for ovarian stimulation, lower peak estradiol levels, elevated testosterone levels, fewer oocytes retrieved, higher numbers of cancelled cycles, thicker zone pellucida, lower implantation rates, and an increased rate of failed fertilization.2 In order to achieve conception, smokers require nearly twice the number of IVF cycles versus nonsmokers.2 Authors of a 5-year, prospective study controlling for potential confounders found that if a woman ever smoked in her lifetime, her risk of failing to conceive with assisted reproductive technologies (ART) more than doubled (relative risk, 2.5; 95% CI, 1.38–4.55). Each year of smoking was associated with a 9% increase in the risk of unsuccessful ART cycles (95% CI, 1.02–1.15;
P <.01).8
We have an important role in helping patients quit
A study involving smoking cessation in infertile women found that simple interventions, such as counseling, education, and encouragement during each clinic visit, were more successful than merely providing educational materials and Web site addresses. The rates of smoking cessation increased from 4% at baseline to 24% after 12 months.9
The Public Health Service and National Cancer Institute offer validated, office-based intervention guidelines for smoking cessation, including a five-step approach2 :
- Ask about smoking at every opportunity
- Advise all smokers to stop
- Assist willingness to stop
- Assist patients in stopping (including through the use of pharmaceuticals and carbon monoxide handheld monitors)
- Arrange follow-up visits.
The use of adjunctive medical therapies, including nicotine replacement therapy and/or buproprion, has resulted in a twofold increase in the proportion of nonpregnant women who quit smoking.2 These medical therapies may be useful if behavioral approaches alone fail—although their use has not been studied in infertile women. Smoking cessation rates appear to be higher in infertile versus pregnant women, yet only 18% of women referred for infertility care have received advice on smoking cessation from their referring provider.9
The detrimental effects of smoking on reproductive health are substantial. Nonsmokers with excessive exposure to tobacco smoke have adverse reproductive effects that may be as great as those observed in smokers.
Studies suggest that much of the reduced fecundity observed in smokers may be reversed within 1 year of smoking cessation.2 Clinicians who care for smokers with infertility have a tremendous opportunity to facilitate smoking cessation in their patients and their partners. Smoking-cessation intervention should be a key component of effective treatment of infertility.
The safety of assisted reproductive technologies
Davies ML, Moore VM, Willson KJ, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366(19):1803–1813.
Since the birth of Louise Brown, the first baby born after being conceived with in vitro fertilization (IVF) in 1978, IVF has become a pillar in the treatment of infertility. Although recognized as a highly effective treatment, the safety of IVF and its related technologies, such as intracytoplasmic sperm injection (ICSI), has been questioned. Studies have linked the use of assisted reproduction, including IVF and ICSI, with an increased risk of birth defects.10-15 Findings, however, were limited by small sample sizes and lack of appropriate controls. Furthermore, it has been unclear if this increased risk is due to factors related to treatment or to an underlying factor present in patients with infertility. It also has been unclear whether there is a differential in risk according to the type of ART used. In a large population-wide cohort study, Davies and colleagues linked a census of treatment with ART in South Australia to a registry of births and terminations with a gestation period of at least 20 weeks or a birth weight of 400 g and registries of birth defects.
The authors compared the risk of birth defects in pregnancies among women who had conceived with the use of ART, women with spontaneous pregnancies who had had a previous birth after ART treatment, women with a diagnosis of infertility who had conceived without ART, and pregnancies in women without infertility. Births and pregnancy terminations secondary to birth defects were studied to assess the birth defect risk from pregnancy to a child’s fifth birthday.
Details of the trial
A total of 308,974 births were included in the analysis. Births in women who conceived with the use of ART were associated with a significant increase in risk of birth defects (8.3%) compared with births conceived spontaneously in fertile women (8.3% vs 5.8%, respectively; unadjusted OR, 1.47; 95% CI, 1.33-1.62). This effect remained significant after multivariate adjustment (adjusted OR, 1.28; 95% CI, 1.16-1.41).
While there was no significant association between ART and the risk of specific syndromes such as Down’s, Turner’s, Edward’s, and others, there was a significantly increased adjusted OR for any defect and multiple defects in births conceived with ART versus those conceived spontaneously in fertile women.
The OR for birth defects associated with IVF was 1.26 (95% CI, 1.07-1.48) in unadjusted analyses and 1.07 (95% CI, 0.9-1.26) after multivariate adjustment. The OR for birth defects associated with IVF with ICSI were 1.77 (95% CI, 1.47-2.12) in unadjusted and 1.57 (95% CI, 1.30-1.90) after multivariate analysis. Compared with ICSI, IVF was associated with a reduced risk of any birth defect (OR, 0.68; 95% CI, 0.53-0.87).
Births after gamete intrafallopian transfer, intrauterine insemination, or the use of clomiphene citrate at home were associated with significantly increased risks of any birth defect in adjusted analyses. Births after conception with donor insemination and clinically supervised ovulation induction were not associated with an increased risk of birth defects. Births occurring after spontaneous conception in women with a history of a previous birth with ART were also associated with an increased risk of birth defects, even after adjustment for confounders (adjusted OR, 1.25; 95% CI, 1.01-1.56). Births occurring after spontaneous conception in women with a history of infertility without previous ART treatment were also significantly associated with a small increased risk in birth defects (OR, 1.29; 95% CI, 0.99-1.68).
ICSI and birth-defect association persisted
In this large observational study, the authors confirmed findings from previous studies11,12,16-18 that the number of birth defects found in pregnancies conceived with ART are higher than the number found in pregnancies conceived spontaneously. In this study, after multivariate adjustment, the association between IVF and an increased risk of birth defects was found to be no longer significant, but the risk remained elevated after ART with ICSI. These findings are similar to results in previous studies.18,19 The increased risk may be secondary to the ICSI procedure itself19,20 or to underlying male infertility factors leading to the use of ICSI.14
Birth defects appeared to be highest in fresh embryo cycles of ICSI versus IVF and lowest in frozen-embryo cycles. A reduction in birth defects with cryopreservation may be secondary to a reduced likelihood that cryopreserved embryos would survive the thawing process as well as the temporal separation of the developing embryo from a hormonally stimulated cycle.21-23 Treatment with ART was associated with an increased risk of cardiovascular, musculoskeletal, urogenital, and gastrointestinal defects, as well as cerebral palsy. The observation of an increased risk of cerebral palsy with ART treatment is consistent with findings from a previous study. Strömberg and colleagues found that the risk of cerebral palsy was increased by a factor of 3.7 among multiples conceived with IVF and 2.8 among singletons conceived with IVF.24
Davies and colleagues also observed that the risk of a birth defect was increased among women with a history of infertility who were able to conceive without ART,25 a finding observed in a previous large Danish registry.15
Although the vast majority of births resulting from assisted reproduction were free of birth defects, treatment with ART was associated with an increased risk of birth defects, compared with spontaneous conception. After adjustment for potential confounders, including maternal age, the risk persisted for conceptions associated with ICSI but not IVF.
While the exact mechanisms responsible for this increased risk remain unknown, the finding of an increased risk of birth defects among women with infertility who conceived without ART indicates that inherent patient factors, rather than assisted reproductive technologies alone, contribute to the risk. These findings can help to guide couples considering assisted reproduction for the treatment of infertility.
We want to hear from you! Tell us what you think.
1. Infertility FAQs. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/reproductivehealth/infertility. Updated April 19 2012. Accessed January 20, 2013.
2. Pfeifer S, Fritz M, Goldberg J, et al. the Practice Committee of the American Society for Reproductive Medicine. Smoking and infertility: a committee opinion. Fertil Steril. 2012;98(6):1400-1406.
3. Hull MG, North K, Taylor H, Farrow A, Ford WC. Delayed conception and active and passive smoking: The Avon Longitudinal Study of Pregnancy and Childhood Study Team. Fertil Steril. 2000;74(4):725-733.
4. Cooper GS, Baird DD, Hulka BS, Weinberg CR, Savitz DA, Hughes CL, Jr. Follicle-stimulating hormone concentrations in relation to active and passive smoking. Obstet Gynecol. 1995;85(3):407-411.
5. Storgaard L, Bonde JP, Ernst E, et al. Does smoking during pregnancy affect sons’ sperm counts? Epidemiology. 2003;14(3):278-286.
6. Yang Q, Sherman SL, Hassold TJ, et al. Risk factors for trisomy 21: maternal cigarette smoking and oral contraceptive use in a population-based case-control study. Genet Med. 1999;1(3):80-88.
7. Zenzes MT, Wang P, Casper RF. Cigarette smoking may affect meiotic maturation of human oocytes. Hum Reprod. 1995;10(12):3213-3217.
8. Klonoff-Cohen H, Natarajan L, Marrs R, Yee B. Effects of female and male smoking on success rates of IVF and gamete intra-Fallopian transfer. Hum Reprod. 2001;16(7):1382-1390.
9. Hughes EG, Lamont DA, Beecroft ML, Wilson DM, Brennan BG, Rice SC. Randomized trial of a “stage of change” oriented smoking cessation intervention in infertile and pregnant women. Fertil Steril. 2000;74(3):498-503.
10. Rimm AA, Katayama AC, Diaz M, Katayama KP. A meta-analysis of controlled studies comparing major malformation rates in IVF and ICSI infants with naturally conceived children. J Assist Reprod Genet. 2004;21(12):437-443.
11. Hansen M, Kurinczuk JJ, Bower C, Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002;346(10):725-730.
12. Hansen M, Bower C, Milne E, de Klerk N, Kurinczuk JJ. Assisted reproductive technologies and the risk of birth defects—a systematic review. Hum Reprod. 2005;20(2):328-338.
13. Schieve LA, Rasmussen SA, Reefhuis J. Risk of birth defects among children conceived with assisted reproductive technology: providing an epidemiologic context to the data. Fertil Steril. 2005;84(5):1320-1324.
14. Lie RT, Lyngstadaas A, Ørstavik KH, Bakketeig LS, Jacobsen G, Tanbo T. Birth defects in children conceived by ICSI compared with children conceived by other IVF-methods; a meta-analysis. Int J Epidemiol. 2005;34(3):696-701.
15. Zhu JL, Basso O, Obel C, Bille C, Olsen J. Infertility infertility treatment, and congenital malformations: Danish national birth cohort. BMJ. 2006;333(7570):679.-
16. Reefhuis J, Honein MA, Schieve LA, Correa A, Hobbs CA, Rasmussen SA. National Birth Defects Prevention Study. Assisted reproductive technology and major structural birth defects in the United States. Hum Reprod. 2009;24(2):360-366.
17. El-Chaar D, Yang Q, Gao J, et al. Risk of birth defects increased in pregnancies conceived by assisted human reproduction. Fertil Steril. 2009;92(5):1557-1561.
18. Källén B, Finnström O, Nygren KG, Olausson PO. In vitro fertilization (IVF) in Sweden: infant outcome after different IVF fertilization methods. Fertil Steril. 2005;84(3):611-617.
19. Bonduelle M, Wennerholm U, Loft A, et al. A multi-centre cohort study of the physical health of 5-year-old children conceived after intracytoplasmic sperm injection, in vitro fertilization and natural conception. Hum Reprod. 2005;20(2):413-419.
20. Kurinczuk JJ. Safety issues in assisted reproduction technology: from theory to reality — just what are the data telling us about ICSI offspring health and future fertility and should we be concerned? Hum Reprod. 2003;18(5):925-931.
21. Pinborg A, Loft A, Aaris Henningsen AK, Rasmussen S, Andersen AN. Infant outcome of 957 singletons born after frozen embryo replacement: the Danish National Cohort Study 1995-2006. Fertil Steril. 2010;94(4):1320-1327.
22. Halliday JL, Ukoumunne OC, Baker HW, et al. Increased risk of blastogenesis birth defects, arising in the first 4 weeks of pregnancy, after assisted reproductive technologies. Hum Reprod. 2010;25(1):59-65.
23. Wennerholm U, Söderström-Anttila V, Bergh C, et al. Children born after cryopreservation of embryos or oocytes: a systematic review of outcome data. Hum Reprod. 2009;24(9):2158-2172.
24. Strömberg B, Dahlquist G, Ericson A, Finnström O, Köster M, Stjernqvist K. Neurological sequelae in children born after in-vitro fertilisation: a population based study. Lancet. 2002;359(9305):461-465.
25. Davies MJ, Moore VM, Willson KJ, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366(19):1803-1813.
CLICK HERE to access several articles on treating fertility issues.
Dr. Abusief reports no financial relationships relevant to this article. Dr. Adamson reports that he receives research grants from LabCorp and Auxogyn, and is the founder and CEO of Advanced Reproductive Care.
Infertility is not just a woman’s issue; it is a couple’s issue. According to the Centers for Disease Control and Prevention, one-third of cases of infertility are caused by a reproductive problem for the woman, one-third are caused by a problem for the man, and one-third are due to problems for both partners or to unknown causes.1
Here, we discuss three developments within the past 12 months related to the treatment of infertility:
- The International Federation of Gynecology and Obstetrics (FIGO) Committee on Reproductive Medicine—charged with developing evidence-based, cost-effective guidelines that would be accepted as standards for increasing access to quality reproductive medical care in all countries of the world—has developed The FIGO Fertility Tool Box™.
- Smoking cigarettes negatively affects a man’s and woman’s fertility, yet smoking’s contribution to infertility is under-recognized. The Practice Committee of the American Society for Reproductive Medicine culled the evidence, and published its review on the effects of smoking on fertility.
- Results of a large, population-wide cohort study shed light on the association of in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) with birth defects and whether underlying factors present in patients with infertility also may play a role.
FIGO offers Tools for managing infertility
Adamson GD. A quick guide to the FIGO Fertility Tool Box. The FIGO Fertility Tool Box. The International Federation of Gynecology and Obstetrics Web site. http://www.arcfertility.com/figo. Published 2013. Accessed January 21, 2013.
FIGO has as members 125 national ObGyn societies. The FIGO Committee on Reproductive Medicine’s mission is to create access to quality reproductive medical care and is focused on helping infertile women become pregnant and/or on alleviating the burden of infertility. The Committee has just released The FIGO Fertility Tool Box™ to further this goal.
Who should use the Tool Box?
Anybody who wants to help infertile people! It is designed for health-care workers and others who want to make a difference in the lives of infertile people. The Tool Box can be accessed electronically on both computers and cell phones at http://www.figo.org/news/resources/FIGO_Fertility_Tool_Box.
What’s in the Tool Box?
Seven Tools help you tackle the disease/ disability of infertility. Each Tool provides information on how to manage a particular aspect of infertility:
- Tool 1: The FIGO Fertility Daisy—why we should care about infertility
- Tool 2: Overcome personal barriers
- Tool 3: Overcome societal barriers
- Tool 4: Diagnose infertility
- Tool 5: Treat infertility
- Tool 6: Refer/resolve infertility
- Tool 7: Prevent infertility.
The Tools Pyramid ( FIGURE 1 ) contains these seven Tools.

FIGURE 1 The Tools Pyramid™
How do the Tools work?
Each of the seven Tools consists of three levels:
Level 1: Basic Tools. The first level consists of 7 Basic Tools™, which contain information that is brief and succinct—just a simple statement or summary of the Daisy and each of the six Pyramids of Action. The Basic Tools are colored orange.
Level 2: Support Tools. The second level is Support Tools™, which provide more information and detail—enough so that you know what to do to take action. Support Tools are colored green.
Level 3: Reference Tools. The third level is Reference Tools™, which are lists of references that provide evidence for the information and recommended actions in the Basic and Support Tools. Reference Tools are colored white.
The Glossary provides definitions and explanations of abbreviations and acronyms and is colored white like the References. By coloring the levels icons this way, you can always tell whether you are using a Basic Tool, Support Tool, or Reference Tool.
The Levels Pyramid (FIGURE 2 ) shows how the Basic Tools, Support Tools, and Reference Tools relate to each other. You can choose your Tool and level by clicking on the icons from your computer or cellphone.

FIGURE 2 The Levels Pyramid™
How do I know what to do?—The Actions Pyramids™
With the exception of the Daisy (Tool 1), all Tools have the shape of a pyramid ( FIGURE 3 ). At the base of each pyramid are actions that can be taken in low-resource settings; that is, they are often simpler, elementary, involve fewer people and are low-cost interventions or opportunities. Overall, there are 64 total actions described in the seven Tools.
As you move higher in the pyramid, generally more resources are required to take actions that are usually more complex. You can think of it as a kind of ladder—as you climb higher it usually gets a bit more complex or complicated. Sometimes, however, it might also be easier higher up on the ladder, and the elementary aspects might be those most challenging.
If you are interested in helping a patient with infertility, you are encouraged to do whatever you can do at any level in any of the Tools in The Actions Pyramid. In the online version, you can click on the actions arrow or icons to click immediately to the action you wish to learn about and do.

FIGURE 3 The Actions Pyramid™
Which Tool should I use?
The one you think will work best for you and will give you some results quickly. Doing something is better than doing nothing. There is no right or wrong way to make a start. Then, if you want to do more you can choose other Tools or individual aspects of other Tools to build on what you have already achieved. Or, if you want to be very systematic and are very committed you can start with Tool 1 and work your way through the entire Tool Box.
What if I can’t implement some of the recommendations?
Then drop it and move onto something that you can do or implement. No single component of the Tool Box is so essential to helping infertile couples that your efforts will fail if you can’t apply it. Using even one or two actions in one or two Tools will empower you to help many infertile individuals.
What if I want to change a Tool?
Just do it. The Tool Box is made to be changed so that it can be adapted to work in any type of health-care setting anywhere in the world. You know what works best in your situation. Just never stop caring and trying to help infertile people.
Does the Tool Box have a compliments and complaints section?
Yes, it is called the FIGO Committee on Reproductive Medicine. E-mail us at fertilitytool box@figo.org. We would love to hear from you about what you like and what works in the Tool Box and what doesn’t. We hope to constantly improve The FIGO Fertility Tool Box to make it a better Tool to help you tackle the disease/disability of infertility.
This Tool Box now gives providers at any level of women’s health care anywhere in the world easy electronic access to comprehensive evidence-based actions that can be used to help those with infertility
Smoking, by either partner, active or passive, negatively affects reproductive health
Pfeifer S, Fritz M, Goldberg J, et al; the Practice Committee of the American Society for Reproductive Medicine. Smoking and infertility: a committee opinion. Fertil Steril. 2012;98(6):1400–1406.
Approximately 30% of reproductive-age women and 35% of reproductive-age men smoke cigarettes. Although smoking has been linked to many adverse health effects, the substantial detrimental effects of cigarette smoking on fecundity and reproduction are under-recognized. In a recent publication, the Practice Committee of the American Society for Reproductive Medicine reviewed the effects of smoking on fertility.
Smoking’s ill effects on fertility
Conception delay
Smokers are at an increased risk for infertility and conception delay. Independent of other factors, smoking has a negative impact on fecundity, with a trend toward increased time to conception with increased number of cigarettes smoked.2,3 The percentage of women experiencing conception delay for more than 12 months was shown to be 54% higher in smoking versus nonsmoking women in one study.3 These authors found that active smoking by either partner had an adverse effect on conception. Furthermore, the impact of passive smoking by either partner was found to be only slightly less than the impact found for active smoking by either partner.3
Ovarian follicular depletion
Basal levels of follicle-stimulating hormone (FSH) are significantly higher in smokers, with one study demonstrating a 66% increase in smoking versus nonsmoking women and a 39% increase in passive versus nonsmoking women.4 Chemicals in cigarette smoke appear to accelerate follicular depletion and loss of reproductive function, and menopause has been found to occur 1 to 4 years earlier in smoking versus nonsmoking women.2
Effects on sperm parameters
Poor function. Smoking has been found to reduce sperm density, motility, and possibly morphology. Sperm function tests appear to be 22% poorer in smokers versus nonsmokers, and the effects are dose-dependent.
No link to male infertility, yet. While evidence suggests an adverse effect on sperm function from smoking, available data do not conclusively demonstrate a reduction in male fertility due to smoking. This could be due to secondary confounding effects of partner status.2
Maternal smoking may decrease sperm counts in offspring, according to Storgaard and colleagues, who found that men whose mothers had smoked more than 10 cigarettes per day had lower sperm densities than men with nonsmoking mothers.5
Mutagenic potential
Tobacco smoke exposure may harm gametogenesis by adversely affecting chromosomes and damaging the meiotic spindle and has been associated with an increased risk of trisomy 21 offspring resulting from maternal nondisjunction.6,7 Gene damage in sperm may be secondary to direct binding of tobacco smoke constituents or chemical byproducts to DNA, creating premutational lesions or “adducts.” These mutagenic adducts have been found in greater numbers in embryos from smokers versus nonsmokers, suggesting a mechanism for the transmission of adversely modified DNA from parental smoking.2
Early pregnancy effects
Smoking increases the risk of spontaneous miscarriage in both natural and assisted conceptions and has been linked to an increased risk for bacterial vaginosis, which in turn increases the risk for second trimester miscarriage and preterm labor.2 Studies also have identified an increased risk of ectopic pregnancy in smokers, including one study demonstrating an odds ratio (OR) for ectopic pregnancy of 3.5 (95% confidence interval [CI], 1.4–8.6) in women who smoked more than 20 cigarettes per day versus nonsmokers.6
Assisted reproductive therapies rendered less effective
Studies of IVF have demonstrated that smokers versus nonsmokers have an increased gonadotropin requirement for ovarian stimulation, lower peak estradiol levels, elevated testosterone levels, fewer oocytes retrieved, higher numbers of cancelled cycles, thicker zone pellucida, lower implantation rates, and an increased rate of failed fertilization.2 In order to achieve conception, smokers require nearly twice the number of IVF cycles versus nonsmokers.2 Authors of a 5-year, prospective study controlling for potential confounders found that if a woman ever smoked in her lifetime, her risk of failing to conceive with assisted reproductive technologies (ART) more than doubled (relative risk, 2.5; 95% CI, 1.38–4.55). Each year of smoking was associated with a 9% increase in the risk of unsuccessful ART cycles (95% CI, 1.02–1.15;
P <.01).8
We have an important role in helping patients quit
A study involving smoking cessation in infertile women found that simple interventions, such as counseling, education, and encouragement during each clinic visit, were more successful than merely providing educational materials and Web site addresses. The rates of smoking cessation increased from 4% at baseline to 24% after 12 months.9
The Public Health Service and National Cancer Institute offer validated, office-based intervention guidelines for smoking cessation, including a five-step approach2 :
- Ask about smoking at every opportunity
- Advise all smokers to stop
- Assist willingness to stop
- Assist patients in stopping (including through the use of pharmaceuticals and carbon monoxide handheld monitors)
- Arrange follow-up visits.
The use of adjunctive medical therapies, including nicotine replacement therapy and/or buproprion, has resulted in a twofold increase in the proportion of nonpregnant women who quit smoking.2 These medical therapies may be useful if behavioral approaches alone fail—although their use has not been studied in infertile women. Smoking cessation rates appear to be higher in infertile versus pregnant women, yet only 18% of women referred for infertility care have received advice on smoking cessation from their referring provider.9
The detrimental effects of smoking on reproductive health are substantial. Nonsmokers with excessive exposure to tobacco smoke have adverse reproductive effects that may be as great as those observed in smokers.
Studies suggest that much of the reduced fecundity observed in smokers may be reversed within 1 year of smoking cessation.2 Clinicians who care for smokers with infertility have a tremendous opportunity to facilitate smoking cessation in their patients and their partners. Smoking-cessation intervention should be a key component of effective treatment of infertility.
The safety of assisted reproductive technologies
Davies ML, Moore VM, Willson KJ, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366(19):1803–1813.
Since the birth of Louise Brown, the first baby born after being conceived with in vitro fertilization (IVF) in 1978, IVF has become a pillar in the treatment of infertility. Although recognized as a highly effective treatment, the safety of IVF and its related technologies, such as intracytoplasmic sperm injection (ICSI), has been questioned. Studies have linked the use of assisted reproduction, including IVF and ICSI, with an increased risk of birth defects.10-15 Findings, however, were limited by small sample sizes and lack of appropriate controls. Furthermore, it has been unclear if this increased risk is due to factors related to treatment or to an underlying factor present in patients with infertility. It also has been unclear whether there is a differential in risk according to the type of ART used. In a large population-wide cohort study, Davies and colleagues linked a census of treatment with ART in South Australia to a registry of births and terminations with a gestation period of at least 20 weeks or a birth weight of 400 g and registries of birth defects.
The authors compared the risk of birth defects in pregnancies among women who had conceived with the use of ART, women with spontaneous pregnancies who had had a previous birth after ART treatment, women with a diagnosis of infertility who had conceived without ART, and pregnancies in women without infertility. Births and pregnancy terminations secondary to birth defects were studied to assess the birth defect risk from pregnancy to a child’s fifth birthday.
Details of the trial
A total of 308,974 births were included in the analysis. Births in women who conceived with the use of ART were associated with a significant increase in risk of birth defects (8.3%) compared with births conceived spontaneously in fertile women (8.3% vs 5.8%, respectively; unadjusted OR, 1.47; 95% CI, 1.33-1.62). This effect remained significant after multivariate adjustment (adjusted OR, 1.28; 95% CI, 1.16-1.41).
While there was no significant association between ART and the risk of specific syndromes such as Down’s, Turner’s, Edward’s, and others, there was a significantly increased adjusted OR for any defect and multiple defects in births conceived with ART versus those conceived spontaneously in fertile women.
The OR for birth defects associated with IVF was 1.26 (95% CI, 1.07-1.48) in unadjusted analyses and 1.07 (95% CI, 0.9-1.26) after multivariate adjustment. The OR for birth defects associated with IVF with ICSI were 1.77 (95% CI, 1.47-2.12) in unadjusted and 1.57 (95% CI, 1.30-1.90) after multivariate analysis. Compared with ICSI, IVF was associated with a reduced risk of any birth defect (OR, 0.68; 95% CI, 0.53-0.87).
Births after gamete intrafallopian transfer, intrauterine insemination, or the use of clomiphene citrate at home were associated with significantly increased risks of any birth defect in adjusted analyses. Births after conception with donor insemination and clinically supervised ovulation induction were not associated with an increased risk of birth defects. Births occurring after spontaneous conception in women with a history of a previous birth with ART were also associated with an increased risk of birth defects, even after adjustment for confounders (adjusted OR, 1.25; 95% CI, 1.01-1.56). Births occurring after spontaneous conception in women with a history of infertility without previous ART treatment were also significantly associated with a small increased risk in birth defects (OR, 1.29; 95% CI, 0.99-1.68).
ICSI and birth-defect association persisted
In this large observational study, the authors confirmed findings from previous studies11,12,16-18 that the number of birth defects found in pregnancies conceived with ART are higher than the number found in pregnancies conceived spontaneously. In this study, after multivariate adjustment, the association between IVF and an increased risk of birth defects was found to be no longer significant, but the risk remained elevated after ART with ICSI. These findings are similar to results in previous studies.18,19 The increased risk may be secondary to the ICSI procedure itself19,20 or to underlying male infertility factors leading to the use of ICSI.14
Birth defects appeared to be highest in fresh embryo cycles of ICSI versus IVF and lowest in frozen-embryo cycles. A reduction in birth defects with cryopreservation may be secondary to a reduced likelihood that cryopreserved embryos would survive the thawing process as well as the temporal separation of the developing embryo from a hormonally stimulated cycle.21-23 Treatment with ART was associated with an increased risk of cardiovascular, musculoskeletal, urogenital, and gastrointestinal defects, as well as cerebral palsy. The observation of an increased risk of cerebral palsy with ART treatment is consistent with findings from a previous study. Strömberg and colleagues found that the risk of cerebral palsy was increased by a factor of 3.7 among multiples conceived with IVF and 2.8 among singletons conceived with IVF.24
Davies and colleagues also observed that the risk of a birth defect was increased among women with a history of infertility who were able to conceive without ART,25 a finding observed in a previous large Danish registry.15
Although the vast majority of births resulting from assisted reproduction were free of birth defects, treatment with ART was associated with an increased risk of birth defects, compared with spontaneous conception. After adjustment for potential confounders, including maternal age, the risk persisted for conceptions associated with ICSI but not IVF.
While the exact mechanisms responsible for this increased risk remain unknown, the finding of an increased risk of birth defects among women with infertility who conceived without ART indicates that inherent patient factors, rather than assisted reproductive technologies alone, contribute to the risk. These findings can help to guide couples considering assisted reproduction for the treatment of infertility.
We want to hear from you! Tell us what you think.
CLICK HERE to access several articles on treating fertility issues.
Dr. Abusief reports no financial relationships relevant to this article. Dr. Adamson reports that he receives research grants from LabCorp and Auxogyn, and is the founder and CEO of Advanced Reproductive Care.
Infertility is not just a woman’s issue; it is a couple’s issue. According to the Centers for Disease Control and Prevention, one-third of cases of infertility are caused by a reproductive problem for the woman, one-third are caused by a problem for the man, and one-third are due to problems for both partners or to unknown causes.1
Here, we discuss three developments within the past 12 months related to the treatment of infertility:
- The International Federation of Gynecology and Obstetrics (FIGO) Committee on Reproductive Medicine—charged with developing evidence-based, cost-effective guidelines that would be accepted as standards for increasing access to quality reproductive medical care in all countries of the world—has developed The FIGO Fertility Tool Box™.
- Smoking cigarettes negatively affects a man’s and woman’s fertility, yet smoking’s contribution to infertility is under-recognized. The Practice Committee of the American Society for Reproductive Medicine culled the evidence, and published its review on the effects of smoking on fertility.
- Results of a large, population-wide cohort study shed light on the association of in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) with birth defects and whether underlying factors present in patients with infertility also may play a role.
FIGO offers Tools for managing infertility
Adamson GD. A quick guide to the FIGO Fertility Tool Box. The FIGO Fertility Tool Box. The International Federation of Gynecology and Obstetrics Web site. http://www.arcfertility.com/figo. Published 2013. Accessed January 21, 2013.
FIGO has as members 125 national ObGyn societies. The FIGO Committee on Reproductive Medicine’s mission is to create access to quality reproductive medical care and is focused on helping infertile women become pregnant and/or on alleviating the burden of infertility. The Committee has just released The FIGO Fertility Tool Box™ to further this goal.
Who should use the Tool Box?
Anybody who wants to help infertile people! It is designed for health-care workers and others who want to make a difference in the lives of infertile people. The Tool Box can be accessed electronically on both computers and cell phones at http://www.figo.org/news/resources/FIGO_Fertility_Tool_Box.
What’s in the Tool Box?
Seven Tools help you tackle the disease/ disability of infertility. Each Tool provides information on how to manage a particular aspect of infertility:
- Tool 1: The FIGO Fertility Daisy—why we should care about infertility
- Tool 2: Overcome personal barriers
- Tool 3: Overcome societal barriers
- Tool 4: Diagnose infertility
- Tool 5: Treat infertility
- Tool 6: Refer/resolve infertility
- Tool 7: Prevent infertility.
The Tools Pyramid ( FIGURE 1 ) contains these seven Tools.

FIGURE 1 The Tools Pyramid™
How do the Tools work?
Each of the seven Tools consists of three levels:
Level 1: Basic Tools. The first level consists of 7 Basic Tools™, which contain information that is brief and succinct—just a simple statement or summary of the Daisy and each of the six Pyramids of Action. The Basic Tools are colored orange.
Level 2: Support Tools. The second level is Support Tools™, which provide more information and detail—enough so that you know what to do to take action. Support Tools are colored green.
Level 3: Reference Tools. The third level is Reference Tools™, which are lists of references that provide evidence for the information and recommended actions in the Basic and Support Tools. Reference Tools are colored white.
The Glossary provides definitions and explanations of abbreviations and acronyms and is colored white like the References. By coloring the levels icons this way, you can always tell whether you are using a Basic Tool, Support Tool, or Reference Tool.
The Levels Pyramid (FIGURE 2 ) shows how the Basic Tools, Support Tools, and Reference Tools relate to each other. You can choose your Tool and level by clicking on the icons from your computer or cellphone.

FIGURE 2 The Levels Pyramid™
How do I know what to do?—The Actions Pyramids™
With the exception of the Daisy (Tool 1), all Tools have the shape of a pyramid ( FIGURE 3 ). At the base of each pyramid are actions that can be taken in low-resource settings; that is, they are often simpler, elementary, involve fewer people and are low-cost interventions or opportunities. Overall, there are 64 total actions described in the seven Tools.
As you move higher in the pyramid, generally more resources are required to take actions that are usually more complex. You can think of it as a kind of ladder—as you climb higher it usually gets a bit more complex or complicated. Sometimes, however, it might also be easier higher up on the ladder, and the elementary aspects might be those most challenging.
If you are interested in helping a patient with infertility, you are encouraged to do whatever you can do at any level in any of the Tools in The Actions Pyramid. In the online version, you can click on the actions arrow or icons to click immediately to the action you wish to learn about and do.

FIGURE 3 The Actions Pyramid™
Which Tool should I use?
The one you think will work best for you and will give you some results quickly. Doing something is better than doing nothing. There is no right or wrong way to make a start. Then, if you want to do more you can choose other Tools or individual aspects of other Tools to build on what you have already achieved. Or, if you want to be very systematic and are very committed you can start with Tool 1 and work your way through the entire Tool Box.
What if I can’t implement some of the recommendations?
Then drop it and move onto something that you can do or implement. No single component of the Tool Box is so essential to helping infertile couples that your efforts will fail if you can’t apply it. Using even one or two actions in one or two Tools will empower you to help many infertile individuals.
What if I want to change a Tool?
Just do it. The Tool Box is made to be changed so that it can be adapted to work in any type of health-care setting anywhere in the world. You know what works best in your situation. Just never stop caring and trying to help infertile people.
Does the Tool Box have a compliments and complaints section?
Yes, it is called the FIGO Committee on Reproductive Medicine. E-mail us at fertilitytool box@figo.org. We would love to hear from you about what you like and what works in the Tool Box and what doesn’t. We hope to constantly improve The FIGO Fertility Tool Box to make it a better Tool to help you tackle the disease/disability of infertility.
This Tool Box now gives providers at any level of women’s health care anywhere in the world easy electronic access to comprehensive evidence-based actions that can be used to help those with infertility
Smoking, by either partner, active or passive, negatively affects reproductive health
Pfeifer S, Fritz M, Goldberg J, et al; the Practice Committee of the American Society for Reproductive Medicine. Smoking and infertility: a committee opinion. Fertil Steril. 2012;98(6):1400–1406.
Approximately 30% of reproductive-age women and 35% of reproductive-age men smoke cigarettes. Although smoking has been linked to many adverse health effects, the substantial detrimental effects of cigarette smoking on fecundity and reproduction are under-recognized. In a recent publication, the Practice Committee of the American Society for Reproductive Medicine reviewed the effects of smoking on fertility.
Smoking’s ill effects on fertility
Conception delay
Smokers are at an increased risk for infertility and conception delay. Independent of other factors, smoking has a negative impact on fecundity, with a trend toward increased time to conception with increased number of cigarettes smoked.2,3 The percentage of women experiencing conception delay for more than 12 months was shown to be 54% higher in smoking versus nonsmoking women in one study.3 These authors found that active smoking by either partner had an adverse effect on conception. Furthermore, the impact of passive smoking by either partner was found to be only slightly less than the impact found for active smoking by either partner.3
Ovarian follicular depletion
Basal levels of follicle-stimulating hormone (FSH) are significantly higher in smokers, with one study demonstrating a 66% increase in smoking versus nonsmoking women and a 39% increase in passive versus nonsmoking women.4 Chemicals in cigarette smoke appear to accelerate follicular depletion and loss of reproductive function, and menopause has been found to occur 1 to 4 years earlier in smoking versus nonsmoking women.2
Effects on sperm parameters
Poor function. Smoking has been found to reduce sperm density, motility, and possibly morphology. Sperm function tests appear to be 22% poorer in smokers versus nonsmokers, and the effects are dose-dependent.
No link to male infertility, yet. While evidence suggests an adverse effect on sperm function from smoking, available data do not conclusively demonstrate a reduction in male fertility due to smoking. This could be due to secondary confounding effects of partner status.2
Maternal smoking may decrease sperm counts in offspring, according to Storgaard and colleagues, who found that men whose mothers had smoked more than 10 cigarettes per day had lower sperm densities than men with nonsmoking mothers.5
Mutagenic potential
Tobacco smoke exposure may harm gametogenesis by adversely affecting chromosomes and damaging the meiotic spindle and has been associated with an increased risk of trisomy 21 offspring resulting from maternal nondisjunction.6,7 Gene damage in sperm may be secondary to direct binding of tobacco smoke constituents or chemical byproducts to DNA, creating premutational lesions or “adducts.” These mutagenic adducts have been found in greater numbers in embryos from smokers versus nonsmokers, suggesting a mechanism for the transmission of adversely modified DNA from parental smoking.2
Early pregnancy effects
Smoking increases the risk of spontaneous miscarriage in both natural and assisted conceptions and has been linked to an increased risk for bacterial vaginosis, which in turn increases the risk for second trimester miscarriage and preterm labor.2 Studies also have identified an increased risk of ectopic pregnancy in smokers, including one study demonstrating an odds ratio (OR) for ectopic pregnancy of 3.5 (95% confidence interval [CI], 1.4–8.6) in women who smoked more than 20 cigarettes per day versus nonsmokers.6
Assisted reproductive therapies rendered less effective
Studies of IVF have demonstrated that smokers versus nonsmokers have an increased gonadotropin requirement for ovarian stimulation, lower peak estradiol levels, elevated testosterone levels, fewer oocytes retrieved, higher numbers of cancelled cycles, thicker zone pellucida, lower implantation rates, and an increased rate of failed fertilization.2 In order to achieve conception, smokers require nearly twice the number of IVF cycles versus nonsmokers.2 Authors of a 5-year, prospective study controlling for potential confounders found that if a woman ever smoked in her lifetime, her risk of failing to conceive with assisted reproductive technologies (ART) more than doubled (relative risk, 2.5; 95% CI, 1.38–4.55). Each year of smoking was associated with a 9% increase in the risk of unsuccessful ART cycles (95% CI, 1.02–1.15;
P <.01).8
We have an important role in helping patients quit
A study involving smoking cessation in infertile women found that simple interventions, such as counseling, education, and encouragement during each clinic visit, were more successful than merely providing educational materials and Web site addresses. The rates of smoking cessation increased from 4% at baseline to 24% after 12 months.9
The Public Health Service and National Cancer Institute offer validated, office-based intervention guidelines for smoking cessation, including a five-step approach2 :
- Ask about smoking at every opportunity
- Advise all smokers to stop
- Assist willingness to stop
- Assist patients in stopping (including through the use of pharmaceuticals and carbon monoxide handheld monitors)
- Arrange follow-up visits.
The use of adjunctive medical therapies, including nicotine replacement therapy and/or buproprion, has resulted in a twofold increase in the proportion of nonpregnant women who quit smoking.2 These medical therapies may be useful if behavioral approaches alone fail—although their use has not been studied in infertile women. Smoking cessation rates appear to be higher in infertile versus pregnant women, yet only 18% of women referred for infertility care have received advice on smoking cessation from their referring provider.9
The detrimental effects of smoking on reproductive health are substantial. Nonsmokers with excessive exposure to tobacco smoke have adverse reproductive effects that may be as great as those observed in smokers.
Studies suggest that much of the reduced fecundity observed in smokers may be reversed within 1 year of smoking cessation.2 Clinicians who care for smokers with infertility have a tremendous opportunity to facilitate smoking cessation in their patients and their partners. Smoking-cessation intervention should be a key component of effective treatment of infertility.
The safety of assisted reproductive technologies
Davies ML, Moore VM, Willson KJ, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366(19):1803–1813.
Since the birth of Louise Brown, the first baby born after being conceived with in vitro fertilization (IVF) in 1978, IVF has become a pillar in the treatment of infertility. Although recognized as a highly effective treatment, the safety of IVF and its related technologies, such as intracytoplasmic sperm injection (ICSI), has been questioned. Studies have linked the use of assisted reproduction, including IVF and ICSI, with an increased risk of birth defects.10-15 Findings, however, were limited by small sample sizes and lack of appropriate controls. Furthermore, it has been unclear if this increased risk is due to factors related to treatment or to an underlying factor present in patients with infertility. It also has been unclear whether there is a differential in risk according to the type of ART used. In a large population-wide cohort study, Davies and colleagues linked a census of treatment with ART in South Australia to a registry of births and terminations with a gestation period of at least 20 weeks or a birth weight of 400 g and registries of birth defects.
The authors compared the risk of birth defects in pregnancies among women who had conceived with the use of ART, women with spontaneous pregnancies who had had a previous birth after ART treatment, women with a diagnosis of infertility who had conceived without ART, and pregnancies in women without infertility. Births and pregnancy terminations secondary to birth defects were studied to assess the birth defect risk from pregnancy to a child’s fifth birthday.
Details of the trial
A total of 308,974 births were included in the analysis. Births in women who conceived with the use of ART were associated with a significant increase in risk of birth defects (8.3%) compared with births conceived spontaneously in fertile women (8.3% vs 5.8%, respectively; unadjusted OR, 1.47; 95% CI, 1.33-1.62). This effect remained significant after multivariate adjustment (adjusted OR, 1.28; 95% CI, 1.16-1.41).
While there was no significant association between ART and the risk of specific syndromes such as Down’s, Turner’s, Edward’s, and others, there was a significantly increased adjusted OR for any defect and multiple defects in births conceived with ART versus those conceived spontaneously in fertile women.
The OR for birth defects associated with IVF was 1.26 (95% CI, 1.07-1.48) in unadjusted analyses and 1.07 (95% CI, 0.9-1.26) after multivariate adjustment. The OR for birth defects associated with IVF with ICSI were 1.77 (95% CI, 1.47-2.12) in unadjusted and 1.57 (95% CI, 1.30-1.90) after multivariate analysis. Compared with ICSI, IVF was associated with a reduced risk of any birth defect (OR, 0.68; 95% CI, 0.53-0.87).
Births after gamete intrafallopian transfer, intrauterine insemination, or the use of clomiphene citrate at home were associated with significantly increased risks of any birth defect in adjusted analyses. Births after conception with donor insemination and clinically supervised ovulation induction were not associated with an increased risk of birth defects. Births occurring after spontaneous conception in women with a history of a previous birth with ART were also associated with an increased risk of birth defects, even after adjustment for confounders (adjusted OR, 1.25; 95% CI, 1.01-1.56). Births occurring after spontaneous conception in women with a history of infertility without previous ART treatment were also significantly associated with a small increased risk in birth defects (OR, 1.29; 95% CI, 0.99-1.68).
ICSI and birth-defect association persisted
In this large observational study, the authors confirmed findings from previous studies11,12,16-18 that the number of birth defects found in pregnancies conceived with ART are higher than the number found in pregnancies conceived spontaneously. In this study, after multivariate adjustment, the association between IVF and an increased risk of birth defects was found to be no longer significant, but the risk remained elevated after ART with ICSI. These findings are similar to results in previous studies.18,19 The increased risk may be secondary to the ICSI procedure itself19,20 or to underlying male infertility factors leading to the use of ICSI.14
Birth defects appeared to be highest in fresh embryo cycles of ICSI versus IVF and lowest in frozen-embryo cycles. A reduction in birth defects with cryopreservation may be secondary to a reduced likelihood that cryopreserved embryos would survive the thawing process as well as the temporal separation of the developing embryo from a hormonally stimulated cycle.21-23 Treatment with ART was associated with an increased risk of cardiovascular, musculoskeletal, urogenital, and gastrointestinal defects, as well as cerebral palsy. The observation of an increased risk of cerebral palsy with ART treatment is consistent with findings from a previous study. Strömberg and colleagues found that the risk of cerebral palsy was increased by a factor of 3.7 among multiples conceived with IVF and 2.8 among singletons conceived with IVF.24
Davies and colleagues also observed that the risk of a birth defect was increased among women with a history of infertility who were able to conceive without ART,25 a finding observed in a previous large Danish registry.15
Although the vast majority of births resulting from assisted reproduction were free of birth defects, treatment with ART was associated with an increased risk of birth defects, compared with spontaneous conception. After adjustment for potential confounders, including maternal age, the risk persisted for conceptions associated with ICSI but not IVF.
While the exact mechanisms responsible for this increased risk remain unknown, the finding of an increased risk of birth defects among women with infertility who conceived without ART indicates that inherent patient factors, rather than assisted reproductive technologies alone, contribute to the risk. These findings can help to guide couples considering assisted reproduction for the treatment of infertility.
We want to hear from you! Tell us what you think.
1. Infertility FAQs. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/reproductivehealth/infertility. Updated April 19 2012. Accessed January 20, 2013.
2. Pfeifer S, Fritz M, Goldberg J, et al. the Practice Committee of the American Society for Reproductive Medicine. Smoking and infertility: a committee opinion. Fertil Steril. 2012;98(6):1400-1406.
3. Hull MG, North K, Taylor H, Farrow A, Ford WC. Delayed conception and active and passive smoking: The Avon Longitudinal Study of Pregnancy and Childhood Study Team. Fertil Steril. 2000;74(4):725-733.
4. Cooper GS, Baird DD, Hulka BS, Weinberg CR, Savitz DA, Hughes CL, Jr. Follicle-stimulating hormone concentrations in relation to active and passive smoking. Obstet Gynecol. 1995;85(3):407-411.
5. Storgaard L, Bonde JP, Ernst E, et al. Does smoking during pregnancy affect sons’ sperm counts? Epidemiology. 2003;14(3):278-286.
6. Yang Q, Sherman SL, Hassold TJ, et al. Risk factors for trisomy 21: maternal cigarette smoking and oral contraceptive use in a population-based case-control study. Genet Med. 1999;1(3):80-88.
7. Zenzes MT, Wang P, Casper RF. Cigarette smoking may affect meiotic maturation of human oocytes. Hum Reprod. 1995;10(12):3213-3217.
8. Klonoff-Cohen H, Natarajan L, Marrs R, Yee B. Effects of female and male smoking on success rates of IVF and gamete intra-Fallopian transfer. Hum Reprod. 2001;16(7):1382-1390.
9. Hughes EG, Lamont DA, Beecroft ML, Wilson DM, Brennan BG, Rice SC. Randomized trial of a “stage of change” oriented smoking cessation intervention in infertile and pregnant women. Fertil Steril. 2000;74(3):498-503.
10. Rimm AA, Katayama AC, Diaz M, Katayama KP. A meta-analysis of controlled studies comparing major malformation rates in IVF and ICSI infants with naturally conceived children. J Assist Reprod Genet. 2004;21(12):437-443.
11. Hansen M, Kurinczuk JJ, Bower C, Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002;346(10):725-730.
12. Hansen M, Bower C, Milne E, de Klerk N, Kurinczuk JJ. Assisted reproductive technologies and the risk of birth defects—a systematic review. Hum Reprod. 2005;20(2):328-338.
13. Schieve LA, Rasmussen SA, Reefhuis J. Risk of birth defects among children conceived with assisted reproductive technology: providing an epidemiologic context to the data. Fertil Steril. 2005;84(5):1320-1324.
14. Lie RT, Lyngstadaas A, Ørstavik KH, Bakketeig LS, Jacobsen G, Tanbo T. Birth defects in children conceived by ICSI compared with children conceived by other IVF-methods; a meta-analysis. Int J Epidemiol. 2005;34(3):696-701.
15. Zhu JL, Basso O, Obel C, Bille C, Olsen J. Infertility infertility treatment, and congenital malformations: Danish national birth cohort. BMJ. 2006;333(7570):679.-
16. Reefhuis J, Honein MA, Schieve LA, Correa A, Hobbs CA, Rasmussen SA. National Birth Defects Prevention Study. Assisted reproductive technology and major structural birth defects in the United States. Hum Reprod. 2009;24(2):360-366.
17. El-Chaar D, Yang Q, Gao J, et al. Risk of birth defects increased in pregnancies conceived by assisted human reproduction. Fertil Steril. 2009;92(5):1557-1561.
18. Källén B, Finnström O, Nygren KG, Olausson PO. In vitro fertilization (IVF) in Sweden: infant outcome after different IVF fertilization methods. Fertil Steril. 2005;84(3):611-617.
19. Bonduelle M, Wennerholm U, Loft A, et al. A multi-centre cohort study of the physical health of 5-year-old children conceived after intracytoplasmic sperm injection, in vitro fertilization and natural conception. Hum Reprod. 2005;20(2):413-419.
20. Kurinczuk JJ. Safety issues in assisted reproduction technology: from theory to reality — just what are the data telling us about ICSI offspring health and future fertility and should we be concerned? Hum Reprod. 2003;18(5):925-931.
21. Pinborg A, Loft A, Aaris Henningsen AK, Rasmussen S, Andersen AN. Infant outcome of 957 singletons born after frozen embryo replacement: the Danish National Cohort Study 1995-2006. Fertil Steril. 2010;94(4):1320-1327.
22. Halliday JL, Ukoumunne OC, Baker HW, et al. Increased risk of blastogenesis birth defects, arising in the first 4 weeks of pregnancy, after assisted reproductive technologies. Hum Reprod. 2010;25(1):59-65.
23. Wennerholm U, Söderström-Anttila V, Bergh C, et al. Children born after cryopreservation of embryos or oocytes: a systematic review of outcome data. Hum Reprod. 2009;24(9):2158-2172.
24. Strömberg B, Dahlquist G, Ericson A, Finnström O, Köster M, Stjernqvist K. Neurological sequelae in children born after in-vitro fertilisation: a population based study. Lancet. 2002;359(9305):461-465.
25. Davies MJ, Moore VM, Willson KJ, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366(19):1803-1813.
1. Infertility FAQs. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/reproductivehealth/infertility. Updated April 19 2012. Accessed January 20, 2013.
2. Pfeifer S, Fritz M, Goldberg J, et al. the Practice Committee of the American Society for Reproductive Medicine. Smoking and infertility: a committee opinion. Fertil Steril. 2012;98(6):1400-1406.
3. Hull MG, North K, Taylor H, Farrow A, Ford WC. Delayed conception and active and passive smoking: The Avon Longitudinal Study of Pregnancy and Childhood Study Team. Fertil Steril. 2000;74(4):725-733.
4. Cooper GS, Baird DD, Hulka BS, Weinberg CR, Savitz DA, Hughes CL, Jr. Follicle-stimulating hormone concentrations in relation to active and passive smoking. Obstet Gynecol. 1995;85(3):407-411.
5. Storgaard L, Bonde JP, Ernst E, et al. Does smoking during pregnancy affect sons’ sperm counts? Epidemiology. 2003;14(3):278-286.
6. Yang Q, Sherman SL, Hassold TJ, et al. Risk factors for trisomy 21: maternal cigarette smoking and oral contraceptive use in a population-based case-control study. Genet Med. 1999;1(3):80-88.
7. Zenzes MT, Wang P, Casper RF. Cigarette smoking may affect meiotic maturation of human oocytes. Hum Reprod. 1995;10(12):3213-3217.
8. Klonoff-Cohen H, Natarajan L, Marrs R, Yee B. Effects of female and male smoking on success rates of IVF and gamete intra-Fallopian transfer. Hum Reprod. 2001;16(7):1382-1390.
9. Hughes EG, Lamont DA, Beecroft ML, Wilson DM, Brennan BG, Rice SC. Randomized trial of a “stage of change” oriented smoking cessation intervention in infertile and pregnant women. Fertil Steril. 2000;74(3):498-503.
10. Rimm AA, Katayama AC, Diaz M, Katayama KP. A meta-analysis of controlled studies comparing major malformation rates in IVF and ICSI infants with naturally conceived children. J Assist Reprod Genet. 2004;21(12):437-443.
11. Hansen M, Kurinczuk JJ, Bower C, Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002;346(10):725-730.
12. Hansen M, Bower C, Milne E, de Klerk N, Kurinczuk JJ. Assisted reproductive technologies and the risk of birth defects—a systematic review. Hum Reprod. 2005;20(2):328-338.
13. Schieve LA, Rasmussen SA, Reefhuis J. Risk of birth defects among children conceived with assisted reproductive technology: providing an epidemiologic context to the data. Fertil Steril. 2005;84(5):1320-1324.
14. Lie RT, Lyngstadaas A, Ørstavik KH, Bakketeig LS, Jacobsen G, Tanbo T. Birth defects in children conceived by ICSI compared with children conceived by other IVF-methods; a meta-analysis. Int J Epidemiol. 2005;34(3):696-701.
15. Zhu JL, Basso O, Obel C, Bille C, Olsen J. Infertility infertility treatment, and congenital malformations: Danish national birth cohort. BMJ. 2006;333(7570):679.-
16. Reefhuis J, Honein MA, Schieve LA, Correa A, Hobbs CA, Rasmussen SA. National Birth Defects Prevention Study. Assisted reproductive technology and major structural birth defects in the United States. Hum Reprod. 2009;24(2):360-366.
17. El-Chaar D, Yang Q, Gao J, et al. Risk of birth defects increased in pregnancies conceived by assisted human reproduction. Fertil Steril. 2009;92(5):1557-1561.
18. Källén B, Finnström O, Nygren KG, Olausson PO. In vitro fertilization (IVF) in Sweden: infant outcome after different IVF fertilization methods. Fertil Steril. 2005;84(3):611-617.
19. Bonduelle M, Wennerholm U, Loft A, et al. A multi-centre cohort study of the physical health of 5-year-old children conceived after intracytoplasmic sperm injection, in vitro fertilization and natural conception. Hum Reprod. 2005;20(2):413-419.
20. Kurinczuk JJ. Safety issues in assisted reproduction technology: from theory to reality — just what are the data telling us about ICSI offspring health and future fertility and should we be concerned? Hum Reprod. 2003;18(5):925-931.
21. Pinborg A, Loft A, Aaris Henningsen AK, Rasmussen S, Andersen AN. Infant outcome of 957 singletons born after frozen embryo replacement: the Danish National Cohort Study 1995-2006. Fertil Steril. 2010;94(4):1320-1327.
22. Halliday JL, Ukoumunne OC, Baker HW, et al. Increased risk of blastogenesis birth defects, arising in the first 4 weeks of pregnancy, after assisted reproductive technologies. Hum Reprod. 2010;25(1):59-65.
23. Wennerholm U, Söderström-Anttila V, Bergh C, et al. Children born after cryopreservation of embryos or oocytes: a systematic review of outcome data. Hum Reprod. 2009;24(9):2158-2172.
24. Strömberg B, Dahlquist G, Ericson A, Finnström O, Köster M, Stjernqvist K. Neurological sequelae in children born after in-vitro fertilisation: a population based study. Lancet. 2002;359(9305):461-465.
25. Davies MJ, Moore VM, Willson KJ, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366(19):1803-1813.
2011 Update on fertility
Impaired fertility is no small problem. According to the Centers for Disease Control and Prevention (CDC), it affects 7.3 million women 15 to 44 years old in the United States alone, or approximately 10% of the female population of reproductive age.1
Not long ago, there was little to be done about the problem. Today, however, we have many tools and tactics at our disposal, and another CDC statistic demonstrates their efficacy: Fewer than one third of women who have received medical intervention for impaired fertility in the past year continue to experience the problem.1
In this article, we highlight three recent developments in fertility:
- We know more about the effects of obesity on fecundity, and more about how to increase the likelihood of pregnancy and live birth in obese women
- The development of in vitro fertilization (IVF) more than 30 years ago represents a significant achievement and vast benefit to mankind, noted the Nobel Committee in awarding the 2010 prize for Physiology or Medicine to Robert Edwards, PhD
- Ovarian hyperstimulation syndrome after controlled ovarian stimulation cannot be avoided completely—but its likelihood can be reduced significantly through careful assessment of the patient and a cautious approach to ovarian stimulation.
Be mindful of the effects of obesity
on a woman’s reproductive function
Obese patients are almost three times as likely as women of normal weight to be infertile. Polycystic ovarian syndrome (PCOS) is generally unmasked or exacerbated, or both, by obesity, and the hyperandrogenicity associated with PCOS can cause ovulatory dysfunction. The hypothalamic-pituitary-ovarian (HPO) axis is also affected by overweight and obesity, resulting in oligo-ovulation in 30% to 47% of women.2 Some studies suggest that fecundity may be reduced in ovulatory obese women as well as those with ovulatory dysfunction.2 Most obese women are not infertile, however.
Once pregnancy is achieved, the risk of miscarriage is elevated in obese women (odds ratio [OR] ~1.67), and the live birth rate is lower (OR ~0.75), compared with women of normal weight.2–4 Obese women also have an elevated risk of miscarriage after egg donation (OR ~1.52) and ovulation induction (OR ~5.11). There is no evidence that the rate of miscarriage is increased after IVF, compared with other treatments.
The diagnosis of infertility is difficult in obese patients because the pelvic examination is less informative, although ultrasonography (US) is usually helpful.5 In addition, obesity can blur the distinction between PCOS and HPO axis-related oligo-ovulation. Laparoscopy and other diagnostic interventions are performed less frequently in obese women, and complications of diagnostic laparoscopy are higher in this population.3
Take the initiative in recommending weight loss
As health-care providers, we need to be more proactive in recommending lifestyle changes for obese women so that they lose weight before pregnancy. Women who have infertility are usually very motivated to conceive; as a result, they may also be motivated to lose weight. Caloric restriction, increased physical activity, behavioral modification, and professional expertise are all essential for successful weight loss.2 Even a reduction as small as 5% to 10% of body weight can have clinical benefit.2,4,5
Metformin is an additional option. When combined with a low-calorie diet, metformin may lead to weight loss, restore ovulation, and improve fecundity in women who have PCOS.2
Bariatric surgery is now commonly reserved for women whose body weight is 45 kg or more above normal. Bariatric surgery can improve the altered hormone profile, including elevated thyroid-stimulating hormone (TSH), of obese women. It also appears to improve fecundity and reduce pregnancy-associated complications. However, it is not always successful and can have complications of its own.
What can you offer to obese patients who experience infertility?
- Clomiphene citrate is the most commonly used ovarian-stimulation agent for oligo-ovulation that arises from PCOS or HPO-axis disruption; it is most effective in patients of normal weight.4,6 The protocols associated with clomiphene administration in obese patients are similar to those for women of normal weight; so are results, although the pregnancy rate is not as high in obese women.
- Gonadotropins are effective ovarian-stimulation drugs that are used in hypothalamic hypogonadal patients as well as after failed treatment with clomiphene citrate. Gonadotropins can be effective even in very obese patients; the dosage increases with body mass index (BMI).4,6
- Metformin reduces insulin resistance in women who have PCOS. By itself, metformin is ineffective at inducing ovulation and has not proved to increase the pregnancy rate when it is added to clomiphene.5 Nevertheless, it is commonly given at a daily dosage of 1,000 to 2,000 mg to women who have hyperinsulinemia, and it may reduce the miscarriage rate in women who have PCOS.
- Other medications that have been used to enhance ovulation in obese women include dexamethasone to reduce elevated androgen levels, bromocryptine for elevated prolactin levels, and thyroid hormone for hypothyroidism.
FOR WOMEN WHO HAVE PCOS
In laparoscopic ovarian drilling, an insulated needle unipolar electrode is inserted in the ovary perpendicular to the surface to create 6 to 12 evenly spaced punctures using 40 watts of coagulating current for 2 seconds at each point. The mechanism of action is unclear, but it is thought that the destruction of androgen-producing stroma is responsible for the reduction in testosterone level, increase in follicle-stimulating hormone (FSH), and return to FSH cyclicity in 80% of cases.
- Among obese women who have oligo-ovulation, ovarian drilling (FIGURE) is generally reserved for patients who have not responded to clomiphene or gonadotropins. This procedure has proved to be as effective as clomiphene administration, with the advantage that it does not increase the risk of multiple gestation and leads to longstanding improvement in one third of patients.5 Ovarian drilling is a modification of ovarian wedge resection for women who have PCOS. The mechanism of action is not clear, but it is thought that destruction of adrogen-producing stroma causes an immediate reduction in testosterone, an increase in follicle-stimulating hormone (FSH), and a return to FSH cyclicity in 80% of cases. These effects can persist for several years, and a pregnancy rate of approximately 60% can be attained in less than 6 months. Clomiphene-resistant women may be more responsive to the drug after ovarian drilling, and the risk of ovarian hyperstimulation appears to be reduced. Ovarian drilling is less effective in obese women than in women of normal weight. Complications include adhesions around the ovary and reduced ovarian reserve.
- Assisted reproductive technology (ART) is sometimes used in this population, but it is less likely to lead to pregnancy and live birth, for unknown reasons.2,3 We inform obese women that a BMI below 30 is desirable before ART.
Overall, the management of infertility in obese women is extremely challenging because of its multiple causes—many of which are still not well understood. However, the profound implications of obesity for all aspects of reproduction make it imperative that we pay more attention to identification and treatment of obesity in the infertile population.
IVF is cited by Nobel Committee for its “benefit to mankind”
On December 10, 2010, Robert Edwards, PhD, was awarded the Nobel Prize in Physiology or Medicine for his innovative and pioneering work to create IVF. In presenting the award, the Committee noted that Professor Edwards’ work “represents a monumental medical advance that can truly be said to confer the greatest benefit to mankind.”
Professor Edwards is the embryologist who performed the basic science and laboratory work, along with Dr. Patrick Steptoe, who provided clinical care, which led to the birth of the world’s first IVF baby, Louise Brown, on July 25, 1978. Since then, IVF has become the most successful treatment for infertility and is available in more than 100 countries. The delivery rate for each single IVF attempt is about 25% globally; it more than doubles in selected patients who have a good prognosis. In some countries, almost 5% of all births arise from IVF; in the United States, that figure is about 1%. The International Committee Monitoring ART (ICMART) estimates that more than 4 million babies have been born from IVF around the world.
Prof. Robert Edwards (left) holds the infant Louise Brown
Early road was rocky
Despite the broad and significant success of IVF, Professor Edwards struggled for years against scientific and social opposition. His research was opposed by some on ethical and religious grounds, and the United Kingdom refused to fund some of his early work. The initial lack of support for IVF has transformed into acceptance as infertility has been recognized as a disease by many governments and the World Health Organization (WHO). In addition, the Centers for Disease Control and Prevention (CDC) has recognized infertility as a public health issue.
Nevertheless, most insurers still provide inadequate IVF coverage because of misperceptions about infertility and lack of recognition that it is a disease that globally affects 9% of all women of reproductive age, with male-partner sperm problems contributing to the problem in about 50% of cases.
IVF technologies achieve many goals
Over the past 32 years, IVF has revolutionized reproductive medicine and the treatment of infertility and brought an entirely new science to human reproduction. Specific IVF technologies that have changed the face of medicine include:
- intracytoplasmic sperm injection (ICSI) to treat male factor infertility
- cryopreservation or vitrification of sperm, eggs, and embryos to allow optimal results from IVF and to help cancer patients have babies after treatment
- preimplantation genetic diagnosis (PGD) to prevent major genetic diseases. (For more on PGD, see the January 2009 “Update on Prenatal Counseling” in our archive at www.obgmanagement.com.)
Much scientific research is now directed toward assessing the quality of embryos so that the live birth rate can be increased at the same time that multiple births are reduced. Advances in PGD and stem cell research show great promise for the future of human reproduction and the management of diseases of all organ systems.
The Nobel Committee’s recognition of Professor Robert Edwards’s extraordinary and visionary accomplishments marks the highest global acknowledgement of the efficacy, safety, and applicability of IVF, as well as its great promise for the future. All physicians should be aware of how this powerful technology can be used in caring for their patients.
Ovarian hyperstimulation can be tempered
through strategic management
Controlled ovarian stimulation is pharmacotherapy of the ovaries to produce more than one oocyte in non-ART cycles or to produce multiple oocytes for retrieval at follicular aspiration.7 Ovarian hyperstimulation syndrome (OHSS) is an iatrogenic, and potentially serious, complication of controlled ovarian stimulation. With vigilant management, however, its risks and sequelae can be reduced.
Best approach: Prevent OHSS
To reduce a woman’s risk of OHSS, identify her risk factors and employ the appropriate prevention strategies. The list of potential risk factors includes:
- age <33 years
- PCOS or its features
- high antral follicle count
- history of OHSS
- high basal anti-müllerian hormone level
- robust response to ovarian stimulation (≥18 follicles or estrogen level of 5,000 ng/dL, or both).
Once that patient’s risk is established, steps can be taken to judiciously manage her cycle and reduce the likelihood that she will develop OHSS.
Prevention strategies include:
- lowering the dosage of gonadotropin (consider a gonadotropin-releasing hormone [GnRH] antagonist protocol)
- coasting cycles until the estradiol level plateaus or decreases (reduce the dosage of human chorionic gonadotropin [hCG], use a GnRH agonist trigger for antagonist cycles, and avoid using hCG for luteal support)
- using an insulin-sensitizing agent such as metformin
- cryopreserving embryos for transfer at a later date (consider in vitro maturation instead of standard IVF [experimental]).8
Proposed clinical grading system for OHSS
| Criteria | How would OHSS be graded? | ||
|---|---|---|---|
| Mild | Moderate | Severe | |
| Objective findings | |||
| Fluid in pouch of Douglas | |||
| Fluid around uterus (major pelvis) | |||
| Fluid around intestinal loops | |||
| Hematocrit >45% | |||
| White blood cells >15,000/mm3 | ±* | ||
| Low urine output <600 mL/24 h | ±* | ||
| Creatinine >1.5 mg/dL | ±* | ± | |
| Elevated transaminases | ±* | ± | |
| Clotting disorder | ±† | ||
| Pleural effusion | ±† | ||
| Subjective findings | |||
| Abdominal distention | |||
| Pelvic discomfort | |||
| Breathing disorder | ±** | ±** | |
| Acute pain | ±** | ±** | ±** |
| Nausea and vomiting | ± | ± | ± |
| Ovarian enlargement | |||
| Pregnancy occurrence | ± | ± | |
| Note: ± indicates that the finding may or may not be present. | |||
| * If two of these are present, consider hospitalization | |||
| † If present, consider intensive care | |||
| ** If present, consider hospitalization | |||
| SOURCE: Humaidan P, et al.8 | |||
OHSS has usually been classified according to the signs and symptoms present.9 However, Humaidan and colleagues recently presented a new classification system for OHSS that is also based on objective vaginal US and laboratory parameters, as well as volume of fluid shifts (TABLE).8
The most highly effective strategies for reducing OHSS include use of a GnRh antagonist protocol and use of a GnRH agonist as a trigger. Other prevention strategies, such as metformin administration and cryopreservation of embryos, can further reduce the risk of severe OHSS. Although absolute prevention is impossible, surveillance for risk factors and careful clinical management by all physicians, including, when appropriate, referral to specialists, can reduce the incidence and severity of this dangerous complication.
We want to hear from you! Tell us what you think.
1. Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, Family Planning, and Reproductive Health of US Women: Data from the 2002 National Survey of Family Growth. National Center for Health Statistics. Vital and Health Statistics. 2005;23:25. www.cdc.gov/nchs/data/series/sr_23/sr23_025.pdf. Published December 2005. Accessed January 5, 2011.
2. Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: an educational bulletin. Fertil Steril. 2008;90(suppl 5):S21-29.
3. Lash MM, Armstrong A. Impact of obesity on women’s health. Fertil Steril. 2009;91(5):1712-1716.
4. Davies M. Symposium: Diet, nutrition and exercise in reproduction. Evidence for effects of weight on reproduction in women. Reprod BioMed Online. 2006;12(5):552-561.
5. Loret de Mola, JR. Obesity and its relationship to infertility in men and women. Obstet Gynecol Clin N Am. 2009;36(2):333-346.
6. Parihar M. Obesity and infertility. Rev Gynaecol Practic. 2003;3:120-126.
7. Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. for ICMART and WHO. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART technology, 2009. Fertil Steril. 2009;92(5):1520-1524.
8. Humaidan P, Quartarolo J, Papanikolaou E. Preventing ovarian hyperstimulation syndrome: guidance for the clinician. Fertil Steril. 2010;94(2):389-400.
9. Practice Committee of the American Society for Reproductive Medicine. Ovarian hyperstimulation syndrome. Fertil Steril. 2008;90(suppl 5):S188-193.
Impaired fertility is no small problem. According to the Centers for Disease Control and Prevention (CDC), it affects 7.3 million women 15 to 44 years old in the United States alone, or approximately 10% of the female population of reproductive age.1
Not long ago, there was little to be done about the problem. Today, however, we have many tools and tactics at our disposal, and another CDC statistic demonstrates their efficacy: Fewer than one third of women who have received medical intervention for impaired fertility in the past year continue to experience the problem.1
In this article, we highlight three recent developments in fertility:
- We know more about the effects of obesity on fecundity, and more about how to increase the likelihood of pregnancy and live birth in obese women
- The development of in vitro fertilization (IVF) more than 30 years ago represents a significant achievement and vast benefit to mankind, noted the Nobel Committee in awarding the 2010 prize for Physiology or Medicine to Robert Edwards, PhD
- Ovarian hyperstimulation syndrome after controlled ovarian stimulation cannot be avoided completely—but its likelihood can be reduced significantly through careful assessment of the patient and a cautious approach to ovarian stimulation.
Be mindful of the effects of obesity
on a woman’s reproductive function
Obese patients are almost three times as likely as women of normal weight to be infertile. Polycystic ovarian syndrome (PCOS) is generally unmasked or exacerbated, or both, by obesity, and the hyperandrogenicity associated with PCOS can cause ovulatory dysfunction. The hypothalamic-pituitary-ovarian (HPO) axis is also affected by overweight and obesity, resulting in oligo-ovulation in 30% to 47% of women.2 Some studies suggest that fecundity may be reduced in ovulatory obese women as well as those with ovulatory dysfunction.2 Most obese women are not infertile, however.
Once pregnancy is achieved, the risk of miscarriage is elevated in obese women (odds ratio [OR] ~1.67), and the live birth rate is lower (OR ~0.75), compared with women of normal weight.2–4 Obese women also have an elevated risk of miscarriage after egg donation (OR ~1.52) and ovulation induction (OR ~5.11). There is no evidence that the rate of miscarriage is increased after IVF, compared with other treatments.
The diagnosis of infertility is difficult in obese patients because the pelvic examination is less informative, although ultrasonography (US) is usually helpful.5 In addition, obesity can blur the distinction between PCOS and HPO axis-related oligo-ovulation. Laparoscopy and other diagnostic interventions are performed less frequently in obese women, and complications of diagnostic laparoscopy are higher in this population.3
Take the initiative in recommending weight loss
As health-care providers, we need to be more proactive in recommending lifestyle changes for obese women so that they lose weight before pregnancy. Women who have infertility are usually very motivated to conceive; as a result, they may also be motivated to lose weight. Caloric restriction, increased physical activity, behavioral modification, and professional expertise are all essential for successful weight loss.2 Even a reduction as small as 5% to 10% of body weight can have clinical benefit.2,4,5
Metformin is an additional option. When combined with a low-calorie diet, metformin may lead to weight loss, restore ovulation, and improve fecundity in women who have PCOS.2
Bariatric surgery is now commonly reserved for women whose body weight is 45 kg or more above normal. Bariatric surgery can improve the altered hormone profile, including elevated thyroid-stimulating hormone (TSH), of obese women. It also appears to improve fecundity and reduce pregnancy-associated complications. However, it is not always successful and can have complications of its own.
What can you offer to obese patients who experience infertility?
- Clomiphene citrate is the most commonly used ovarian-stimulation agent for oligo-ovulation that arises from PCOS or HPO-axis disruption; it is most effective in patients of normal weight.4,6 The protocols associated with clomiphene administration in obese patients are similar to those for women of normal weight; so are results, although the pregnancy rate is not as high in obese women.
- Gonadotropins are effective ovarian-stimulation drugs that are used in hypothalamic hypogonadal patients as well as after failed treatment with clomiphene citrate. Gonadotropins can be effective even in very obese patients; the dosage increases with body mass index (BMI).4,6
- Metformin reduces insulin resistance in women who have PCOS. By itself, metformin is ineffective at inducing ovulation and has not proved to increase the pregnancy rate when it is added to clomiphene.5 Nevertheless, it is commonly given at a daily dosage of 1,000 to 2,000 mg to women who have hyperinsulinemia, and it may reduce the miscarriage rate in women who have PCOS.
- Other medications that have been used to enhance ovulation in obese women include dexamethasone to reduce elevated androgen levels, bromocryptine for elevated prolactin levels, and thyroid hormone for hypothyroidism.

FOR WOMEN WHO HAVE PCOS
In laparoscopic ovarian drilling, an insulated needle unipolar electrode is inserted in the ovary perpendicular to the surface to create 6 to 12 evenly spaced punctures using 40 watts of coagulating current for 2 seconds at each point. The mechanism of action is unclear, but it is thought that the destruction of androgen-producing stroma is responsible for the reduction in testosterone level, increase in follicle-stimulating hormone (FSH), and return to FSH cyclicity in 80% of cases.
- Among obese women who have oligo-ovulation, ovarian drilling (FIGURE) is generally reserved for patients who have not responded to clomiphene or gonadotropins. This procedure has proved to be as effective as clomiphene administration, with the advantage that it does not increase the risk of multiple gestation and leads to longstanding improvement in one third of patients.5 Ovarian drilling is a modification of ovarian wedge resection for women who have PCOS. The mechanism of action is not clear, but it is thought that destruction of adrogen-producing stroma causes an immediate reduction in testosterone, an increase in follicle-stimulating hormone (FSH), and a return to FSH cyclicity in 80% of cases. These effects can persist for several years, and a pregnancy rate of approximately 60% can be attained in less than 6 months. Clomiphene-resistant women may be more responsive to the drug after ovarian drilling, and the risk of ovarian hyperstimulation appears to be reduced. Ovarian drilling is less effective in obese women than in women of normal weight. Complications include adhesions around the ovary and reduced ovarian reserve.
- Assisted reproductive technology (ART) is sometimes used in this population, but it is less likely to lead to pregnancy and live birth, for unknown reasons.2,3 We inform obese women that a BMI below 30 is desirable before ART.
Overall, the management of infertility in obese women is extremely challenging because of its multiple causes—many of which are still not well understood. However, the profound implications of obesity for all aspects of reproduction make it imperative that we pay more attention to identification and treatment of obesity in the infertile population.
IVF is cited by Nobel Committee for its “benefit to mankind”
On December 10, 2010, Robert Edwards, PhD, was awarded the Nobel Prize in Physiology or Medicine for his innovative and pioneering work to create IVF. In presenting the award, the Committee noted that Professor Edwards’ work “represents a monumental medical advance that can truly be said to confer the greatest benefit to mankind.”
Professor Edwards is the embryologist who performed the basic science and laboratory work, along with Dr. Patrick Steptoe, who provided clinical care, which led to the birth of the world’s first IVF baby, Louise Brown, on July 25, 1978. Since then, IVF has become the most successful treatment for infertility and is available in more than 100 countries. The delivery rate for each single IVF attempt is about 25% globally; it more than doubles in selected patients who have a good prognosis. In some countries, almost 5% of all births arise from IVF; in the United States, that figure is about 1%. The International Committee Monitoring ART (ICMART) estimates that more than 4 million babies have been born from IVF around the world.

Prof. Robert Edwards (left) holds the infant Louise Brown
Early road was rocky
Despite the broad and significant success of IVF, Professor Edwards struggled for years against scientific and social opposition. His research was opposed by some on ethical and religious grounds, and the United Kingdom refused to fund some of his early work. The initial lack of support for IVF has transformed into acceptance as infertility has been recognized as a disease by many governments and the World Health Organization (WHO). In addition, the Centers for Disease Control and Prevention (CDC) has recognized infertility as a public health issue.
Nevertheless, most insurers still provide inadequate IVF coverage because of misperceptions about infertility and lack of recognition that it is a disease that globally affects 9% of all women of reproductive age, with male-partner sperm problems contributing to the problem in about 50% of cases.
IVF technologies achieve many goals
Over the past 32 years, IVF has revolutionized reproductive medicine and the treatment of infertility and brought an entirely new science to human reproduction. Specific IVF technologies that have changed the face of medicine include:
- intracytoplasmic sperm injection (ICSI) to treat male factor infertility
- cryopreservation or vitrification of sperm, eggs, and embryos to allow optimal results from IVF and to help cancer patients have babies after treatment
- preimplantation genetic diagnosis (PGD) to prevent major genetic diseases. (For more on PGD, see the January 2009 “Update on Prenatal Counseling” in our archive at www.obgmanagement.com.)
Much scientific research is now directed toward assessing the quality of embryos so that the live birth rate can be increased at the same time that multiple births are reduced. Advances in PGD and stem cell research show great promise for the future of human reproduction and the management of diseases of all organ systems.
The Nobel Committee’s recognition of Professor Robert Edwards’s extraordinary and visionary accomplishments marks the highest global acknowledgement of the efficacy, safety, and applicability of IVF, as well as its great promise for the future. All physicians should be aware of how this powerful technology can be used in caring for their patients.
Ovarian hyperstimulation can be tempered
through strategic management
Controlled ovarian stimulation is pharmacotherapy of the ovaries to produce more than one oocyte in non-ART cycles or to produce multiple oocytes for retrieval at follicular aspiration.7 Ovarian hyperstimulation syndrome (OHSS) is an iatrogenic, and potentially serious, complication of controlled ovarian stimulation. With vigilant management, however, its risks and sequelae can be reduced.
Best approach: Prevent OHSS
To reduce a woman’s risk of OHSS, identify her risk factors and employ the appropriate prevention strategies. The list of potential risk factors includes:
- age <33 years
- PCOS or its features
- high antral follicle count
- history of OHSS
- high basal anti-müllerian hormone level
- robust response to ovarian stimulation (≥18 follicles or estrogen level of 5,000 ng/dL, or both).
Once that patient’s risk is established, steps can be taken to judiciously manage her cycle and reduce the likelihood that she will develop OHSS.
Prevention strategies include:
- lowering the dosage of gonadotropin (consider a gonadotropin-releasing hormone [GnRH] antagonist protocol)
- coasting cycles until the estradiol level plateaus or decreases (reduce the dosage of human chorionic gonadotropin [hCG], use a GnRH agonist trigger for antagonist cycles, and avoid using hCG for luteal support)
- using an insulin-sensitizing agent such as metformin
- cryopreserving embryos for transfer at a later date (consider in vitro maturation instead of standard IVF [experimental]).8
Proposed clinical grading system for OHSS
| Criteria | How would OHSS be graded? | ||
|---|---|---|---|
| Mild | Moderate | Severe | |
| Objective findings | |||
| Fluid in pouch of Douglas | |||
| Fluid around uterus (major pelvis) | |||
| Fluid around intestinal loops | |||
| Hematocrit >45% | * | ||
| White blood cells >15,000/mm3 | ±* | ||
| Low urine output <600 mL/24 h | ±* | ||
| Creatinine >1.5 mg/dL | ±* | ± | |
| Elevated transaminases | ±* | ± | |
| Clotting disorder | ±† | ||
| Pleural effusion | ±† | ||
| Subjective findings | |||
| Abdominal distention | |||
| Pelvic discomfort | |||
| Breathing disorder | ±** | ±** | |
| Acute pain | ±** | ±** | ±** |
| Nausea and vomiting | ± | ± | ± |
| Ovarian enlargement | |||
| Pregnancy occurrence | ± | ± | |
| Note: ± indicates that the finding may or may not be present. | |||
| * If two of these are present, consider hospitalization | |||
| † If present, consider intensive care | |||
| ** If present, consider hospitalization | |||
| SOURCE: Humaidan P, et al.8 | |||
OHSS has usually been classified according to the signs and symptoms present.9 However, Humaidan and colleagues recently presented a new classification system for OHSS that is also based on objective vaginal US and laboratory parameters, as well as volume of fluid shifts (TABLE).8
The most highly effective strategies for reducing OHSS include use of a GnRh antagonist protocol and use of a GnRH agonist as a trigger. Other prevention strategies, such as metformin administration and cryopreservation of embryos, can further reduce the risk of severe OHSS. Although absolute prevention is impossible, surveillance for risk factors and careful clinical management by all physicians, including, when appropriate, referral to specialists, can reduce the incidence and severity of this dangerous complication.
We want to hear from you! Tell us what you think.
Impaired fertility is no small problem. According to the Centers for Disease Control and Prevention (CDC), it affects 7.3 million women 15 to 44 years old in the United States alone, or approximately 10% of the female population of reproductive age.1
Not long ago, there was little to be done about the problem. Today, however, we have many tools and tactics at our disposal, and another CDC statistic demonstrates their efficacy: Fewer than one third of women who have received medical intervention for impaired fertility in the past year continue to experience the problem.1
In this article, we highlight three recent developments in fertility:
- We know more about the effects of obesity on fecundity, and more about how to increase the likelihood of pregnancy and live birth in obese women
- The development of in vitro fertilization (IVF) more than 30 years ago represents a significant achievement and vast benefit to mankind, noted the Nobel Committee in awarding the 2010 prize for Physiology or Medicine to Robert Edwards, PhD
- Ovarian hyperstimulation syndrome after controlled ovarian stimulation cannot be avoided completely—but its likelihood can be reduced significantly through careful assessment of the patient and a cautious approach to ovarian stimulation.
Be mindful of the effects of obesity
on a woman’s reproductive function
Obese patients are almost three times as likely as women of normal weight to be infertile. Polycystic ovarian syndrome (PCOS) is generally unmasked or exacerbated, or both, by obesity, and the hyperandrogenicity associated with PCOS can cause ovulatory dysfunction. The hypothalamic-pituitary-ovarian (HPO) axis is also affected by overweight and obesity, resulting in oligo-ovulation in 30% to 47% of women.2 Some studies suggest that fecundity may be reduced in ovulatory obese women as well as those with ovulatory dysfunction.2 Most obese women are not infertile, however.
Once pregnancy is achieved, the risk of miscarriage is elevated in obese women (odds ratio [OR] ~1.67), and the live birth rate is lower (OR ~0.75), compared with women of normal weight.2–4 Obese women also have an elevated risk of miscarriage after egg donation (OR ~1.52) and ovulation induction (OR ~5.11). There is no evidence that the rate of miscarriage is increased after IVF, compared with other treatments.
The diagnosis of infertility is difficult in obese patients because the pelvic examination is less informative, although ultrasonography (US) is usually helpful.5 In addition, obesity can blur the distinction between PCOS and HPO axis-related oligo-ovulation. Laparoscopy and other diagnostic interventions are performed less frequently in obese women, and complications of diagnostic laparoscopy are higher in this population.3
Take the initiative in recommending weight loss
As health-care providers, we need to be more proactive in recommending lifestyle changes for obese women so that they lose weight before pregnancy. Women who have infertility are usually very motivated to conceive; as a result, they may also be motivated to lose weight. Caloric restriction, increased physical activity, behavioral modification, and professional expertise are all essential for successful weight loss.2 Even a reduction as small as 5% to 10% of body weight can have clinical benefit.2,4,5
Metformin is an additional option. When combined with a low-calorie diet, metformin may lead to weight loss, restore ovulation, and improve fecundity in women who have PCOS.2
Bariatric surgery is now commonly reserved for women whose body weight is 45 kg or more above normal. Bariatric surgery can improve the altered hormone profile, including elevated thyroid-stimulating hormone (TSH), of obese women. It also appears to improve fecundity and reduce pregnancy-associated complications. However, it is not always successful and can have complications of its own.
What can you offer to obese patients who experience infertility?
- Clomiphene citrate is the most commonly used ovarian-stimulation agent for oligo-ovulation that arises from PCOS or HPO-axis disruption; it is most effective in patients of normal weight.4,6 The protocols associated with clomiphene administration in obese patients are similar to those for women of normal weight; so are results, although the pregnancy rate is not as high in obese women.
- Gonadotropins are effective ovarian-stimulation drugs that are used in hypothalamic hypogonadal patients as well as after failed treatment with clomiphene citrate. Gonadotropins can be effective even in very obese patients; the dosage increases with body mass index (BMI).4,6
- Metformin reduces insulin resistance in women who have PCOS. By itself, metformin is ineffective at inducing ovulation and has not proved to increase the pregnancy rate when it is added to clomiphene.5 Nevertheless, it is commonly given at a daily dosage of 1,000 to 2,000 mg to women who have hyperinsulinemia, and it may reduce the miscarriage rate in women who have PCOS.
- Other medications that have been used to enhance ovulation in obese women include dexamethasone to reduce elevated androgen levels, bromocryptine for elevated prolactin levels, and thyroid hormone for hypothyroidism.

FOR WOMEN WHO HAVE PCOS
In laparoscopic ovarian drilling, an insulated needle unipolar electrode is inserted in the ovary perpendicular to the surface to create 6 to 12 evenly spaced punctures using 40 watts of coagulating current for 2 seconds at each point. The mechanism of action is unclear, but it is thought that the destruction of androgen-producing stroma is responsible for the reduction in testosterone level, increase in follicle-stimulating hormone (FSH), and return to FSH cyclicity in 80% of cases.
- Among obese women who have oligo-ovulation, ovarian drilling (FIGURE) is generally reserved for patients who have not responded to clomiphene or gonadotropins. This procedure has proved to be as effective as clomiphene administration, with the advantage that it does not increase the risk of multiple gestation and leads to longstanding improvement in one third of patients.5 Ovarian drilling is a modification of ovarian wedge resection for women who have PCOS. The mechanism of action is not clear, but it is thought that destruction of adrogen-producing stroma causes an immediate reduction in testosterone, an increase in follicle-stimulating hormone (FSH), and a return to FSH cyclicity in 80% of cases. These effects can persist for several years, and a pregnancy rate of approximately 60% can be attained in less than 6 months. Clomiphene-resistant women may be more responsive to the drug after ovarian drilling, and the risk of ovarian hyperstimulation appears to be reduced. Ovarian drilling is less effective in obese women than in women of normal weight. Complications include adhesions around the ovary and reduced ovarian reserve.
- Assisted reproductive technology (ART) is sometimes used in this population, but it is less likely to lead to pregnancy and live birth, for unknown reasons.2,3 We inform obese women that a BMI below 30 is desirable before ART.
Overall, the management of infertility in obese women is extremely challenging because of its multiple causes—many of which are still not well understood. However, the profound implications of obesity for all aspects of reproduction make it imperative that we pay more attention to identification and treatment of obesity in the infertile population.
IVF is cited by Nobel Committee for its “benefit to mankind”
On December 10, 2010, Robert Edwards, PhD, was awarded the Nobel Prize in Physiology or Medicine for his innovative and pioneering work to create IVF. In presenting the award, the Committee noted that Professor Edwards’ work “represents a monumental medical advance that can truly be said to confer the greatest benefit to mankind.”
Professor Edwards is the embryologist who performed the basic science and laboratory work, along with Dr. Patrick Steptoe, who provided clinical care, which led to the birth of the world’s first IVF baby, Louise Brown, on July 25, 1978. Since then, IVF has become the most successful treatment for infertility and is available in more than 100 countries. The delivery rate for each single IVF attempt is about 25% globally; it more than doubles in selected patients who have a good prognosis. In some countries, almost 5% of all births arise from IVF; in the United States, that figure is about 1%. The International Committee Monitoring ART (ICMART) estimates that more than 4 million babies have been born from IVF around the world.

Prof. Robert Edwards (left) holds the infant Louise Brown
Early road was rocky
Despite the broad and significant success of IVF, Professor Edwards struggled for years against scientific and social opposition. His research was opposed by some on ethical and religious grounds, and the United Kingdom refused to fund some of his early work. The initial lack of support for IVF has transformed into acceptance as infertility has been recognized as a disease by many governments and the World Health Organization (WHO). In addition, the Centers for Disease Control and Prevention (CDC) has recognized infertility as a public health issue.
Nevertheless, most insurers still provide inadequate IVF coverage because of misperceptions about infertility and lack of recognition that it is a disease that globally affects 9% of all women of reproductive age, with male-partner sperm problems contributing to the problem in about 50% of cases.
IVF technologies achieve many goals
Over the past 32 years, IVF has revolutionized reproductive medicine and the treatment of infertility and brought an entirely new science to human reproduction. Specific IVF technologies that have changed the face of medicine include:
- intracytoplasmic sperm injection (ICSI) to treat male factor infertility
- cryopreservation or vitrification of sperm, eggs, and embryos to allow optimal results from IVF and to help cancer patients have babies after treatment
- preimplantation genetic diagnosis (PGD) to prevent major genetic diseases. (For more on PGD, see the January 2009 “Update on Prenatal Counseling” in our archive at www.obgmanagement.com.)
Much scientific research is now directed toward assessing the quality of embryos so that the live birth rate can be increased at the same time that multiple births are reduced. Advances in PGD and stem cell research show great promise for the future of human reproduction and the management of diseases of all organ systems.
The Nobel Committee’s recognition of Professor Robert Edwards’s extraordinary and visionary accomplishments marks the highest global acknowledgement of the efficacy, safety, and applicability of IVF, as well as its great promise for the future. All physicians should be aware of how this powerful technology can be used in caring for their patients.
Ovarian hyperstimulation can be tempered
through strategic management
Controlled ovarian stimulation is pharmacotherapy of the ovaries to produce more than one oocyte in non-ART cycles or to produce multiple oocytes for retrieval at follicular aspiration.7 Ovarian hyperstimulation syndrome (OHSS) is an iatrogenic, and potentially serious, complication of controlled ovarian stimulation. With vigilant management, however, its risks and sequelae can be reduced.
Best approach: Prevent OHSS
To reduce a woman’s risk of OHSS, identify her risk factors and employ the appropriate prevention strategies. The list of potential risk factors includes:
- age <33 years
- PCOS or its features
- high antral follicle count
- history of OHSS
- high basal anti-müllerian hormone level
- robust response to ovarian stimulation (≥18 follicles or estrogen level of 5,000 ng/dL, or both).
Once that patient’s risk is established, steps can be taken to judiciously manage her cycle and reduce the likelihood that she will develop OHSS.
Prevention strategies include:
- lowering the dosage of gonadotropin (consider a gonadotropin-releasing hormone [GnRH] antagonist protocol)
- coasting cycles until the estradiol level plateaus or decreases (reduce the dosage of human chorionic gonadotropin [hCG], use a GnRH agonist trigger for antagonist cycles, and avoid using hCG for luteal support)
- using an insulin-sensitizing agent such as metformin
- cryopreserving embryos for transfer at a later date (consider in vitro maturation instead of standard IVF [experimental]).8
Proposed clinical grading system for OHSS
| Criteria | How would OHSS be graded? | ||
|---|---|---|---|
| Mild | Moderate | Severe | |
| Objective findings | |||
| Fluid in pouch of Douglas | |||
| Fluid around uterus (major pelvis) | |||
| Fluid around intestinal loops | |||
| Hematocrit >45% | * | ||
| White blood cells >15,000/mm3 | ±* | ||
| Low urine output <600 mL/24 h | ±* | ||
| Creatinine >1.5 mg/dL | ±* | ± | |
| Elevated transaminases | ±* | ± | |
| Clotting disorder | ±† | ||
| Pleural effusion | ±† | ||
| Subjective findings | |||
| Abdominal distention | |||
| Pelvic discomfort | |||
| Breathing disorder | ±** | ±** | |
| Acute pain | ±** | ±** | ±** |
| Nausea and vomiting | ± | ± | ± |
| Ovarian enlargement | |||
| Pregnancy occurrence | ± | ± | |
| Note: ± indicates that the finding may or may not be present. | |||
| * If two of these are present, consider hospitalization | |||
| † If present, consider intensive care | |||
| ** If present, consider hospitalization | |||
| SOURCE: Humaidan P, et al.8 | |||
OHSS has usually been classified according to the signs and symptoms present.9 However, Humaidan and colleagues recently presented a new classification system for OHSS that is also based on objective vaginal US and laboratory parameters, as well as volume of fluid shifts (TABLE).8
The most highly effective strategies for reducing OHSS include use of a GnRh antagonist protocol and use of a GnRH agonist as a trigger. Other prevention strategies, such as metformin administration and cryopreservation of embryos, can further reduce the risk of severe OHSS. Although absolute prevention is impossible, surveillance for risk factors and careful clinical management by all physicians, including, when appropriate, referral to specialists, can reduce the incidence and severity of this dangerous complication.
We want to hear from you! Tell us what you think.
1. Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, Family Planning, and Reproductive Health of US Women: Data from the 2002 National Survey of Family Growth. National Center for Health Statistics. Vital and Health Statistics. 2005;23:25. www.cdc.gov/nchs/data/series/sr_23/sr23_025.pdf. Published December 2005. Accessed January 5, 2011.
2. Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: an educational bulletin. Fertil Steril. 2008;90(suppl 5):S21-29.
3. Lash MM, Armstrong A. Impact of obesity on women’s health. Fertil Steril. 2009;91(5):1712-1716.
4. Davies M. Symposium: Diet, nutrition and exercise in reproduction. Evidence for effects of weight on reproduction in women. Reprod BioMed Online. 2006;12(5):552-561.
5. Loret de Mola, JR. Obesity and its relationship to infertility in men and women. Obstet Gynecol Clin N Am. 2009;36(2):333-346.
6. Parihar M. Obesity and infertility. Rev Gynaecol Practic. 2003;3:120-126.
7. Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. for ICMART and WHO. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART technology, 2009. Fertil Steril. 2009;92(5):1520-1524.
8. Humaidan P, Quartarolo J, Papanikolaou E. Preventing ovarian hyperstimulation syndrome: guidance for the clinician. Fertil Steril. 2010;94(2):389-400.
9. Practice Committee of the American Society for Reproductive Medicine. Ovarian hyperstimulation syndrome. Fertil Steril. 2008;90(suppl 5):S188-193.
1. Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, Family Planning, and Reproductive Health of US Women: Data from the 2002 National Survey of Family Growth. National Center for Health Statistics. Vital and Health Statistics. 2005;23:25. www.cdc.gov/nchs/data/series/sr_23/sr23_025.pdf. Published December 2005. Accessed January 5, 2011.
2. Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: an educational bulletin. Fertil Steril. 2008;90(suppl 5):S21-29.
3. Lash MM, Armstrong A. Impact of obesity on women’s health. Fertil Steril. 2009;91(5):1712-1716.
4. Davies M. Symposium: Diet, nutrition and exercise in reproduction. Evidence for effects of weight on reproduction in women. Reprod BioMed Online. 2006;12(5):552-561.
5. Loret de Mola, JR. Obesity and its relationship to infertility in men and women. Obstet Gynecol Clin N Am. 2009;36(2):333-346.
6. Parihar M. Obesity and infertility. Rev Gynaecol Practic. 2003;3:120-126.
7. Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. for ICMART and WHO. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART technology, 2009. Fertil Steril. 2009;92(5):1520-1524.
8. Humaidan P, Quartarolo J, Papanikolaou E. Preventing ovarian hyperstimulation syndrome: guidance for the clinician. Fertil Steril. 2010;94(2):389-400.
9. Practice Committee of the American Society for Reproductive Medicine. Ovarian hyperstimulation syndrome. Fertil Steril. 2008;90(suppl 5):S188-193.
UPDATE: FERTILITY
Infertility and its treatment can be a roller-coaster ride for patient and physician. Amid the emotional stress that arises, the goal of treatment can inadvertently shift from achievement of a successful singleton pregnancy to pregnancy at any cost—even high-order multiple gestation.
Here’s an essential question: Can the rate of multiple gestation be reduced without seriously compromising the pregnancy rate? Several developments of the past year suggest that it can be. In this article, we discuss:
- new guidelines that limit the number of embryos to be transferred at in vitro fertilization (IVF)
- strategies to reduce the risk of multiple gestation after controlled ovarian stimulation or ovulation induction
- the need to address the patient’s emotional status during treatment
- a new index that helps predict the pregnancy rate after surgical staging of endometriosis.
Multiple gestation is known to have adverse effects on infants, including a significantly elevated risk of prematurity and related physical and developmental problems. It also greatly increases the need for resources. And the high cost of caring for infants affected by prematurity further burdens an already overwhelmed health-care system.
Not only is it essential that we reduce the rate of high-order multiple gestation (i.e., more than two fetuses), but we should also attempt to lower the rate of twin pregnancy. A healthy singleton pregnancy, with its diminished risks and more reasonable health-care cost, should be our goal.
New guidelines limit the number of embryos to be transferred at IVF
Practice Committee of the American Society for Reproductive Medicine, Practice Committee of the Society for Assisted Reproductive Technology. Guidelines on number of embryos transferred. Fertil Steril. 2009;92:1518–1519.
Since the birth of Louise Brown in 1978, assisted reproductive technology (ART) has enjoyed dramatic technological advances. Intracytoplasmic sperm injection (FIGURE 1), preimplantation genetic diagnosis, and improvements in cryopreservation have broadened the application of ART and increased the live birth rate to 30% for every cycle that is initiated. The cumulative live birth rate from additional fresh and frozen-thawed cycles can reach 50% to 80%.
These gains have not come without cost, however. Multiple emotional, financial, and other variables affecting the practice of IVF have produced a higher-than-natural rate of multiple gestation.
In November 2009, the Society for Assisted Reproductive Technology (SART) and the American Society for Reproductive Medicine (ASRM) issued new guidelines limiting the number of embryos that should be transferred in one IVF cycle. IVF clinics are required to report outcomes, and approximately 93% of US cycles are reported to SART. High-order multiple-pregnancy rates are audited by SART, and outlier clinics must implement remediation programs to lower their high rate or risk expulsion from SART.
The increasing emphasis on single-embryo transfer in young women who have a good prognosis reflects the societies’ commitment to help patients achieve a healthy singleton pregnancy and good birth outcome.
FIGURE 1 A wonder of technology
Intracytoplasmic sperm injection overcomes many barriers to fertilization, such as severe malefactor infertility. At some institutions, the technique yields a fertilization rate of 70% to 80%.
What makes a “good prognosis”?
Identification of patients who have a good prognosis is an essential component of these new guidelines. The patient is more likely to have a favorable outcome if one or more of the following is true:
- She is undergoing her first cycle of IVF
- The embryos have good morphology
- Excess embryos are available for cryopreservation
- She has had earlier success with IVF.
The TABLE details the recommended number of embryos to transfer, based on the age and prognosis of the patient. In cycles that involve a donor egg, base the number of embryos to be transferred on the age of the donor. In cycles that involve a frozen embryo, base the number of good-quality, thawed embryos to be transferred on the age of the patient at the time the embryos were created. One additional embryo may be transferred if the patient has a less favorable prognosis or a history of two failed, fresh IVF cycles.
Two important requisites: Careful counseling about the risk of high-order multiple gestation, and documentation of that counseling.
TABLE
SART and ASRM recommend limits on the number of embryos to be transferred at in vitro fertilization
| Prognosis | Age of patient (yr) | |||
|---|---|---|---|---|
| <35 | 35–37 | 38–40 | 41 and 42 | |
| CLEAVAGE-STAGE EMBRYOS* | ||||
| Favorable† | 1 or 2 | 2 | 3 | 5 |
| All others | 2 | 3 | 4 | 5 |
| BLASTOCYSTS* | ||||
| Favorable† | 1 | 2 | 2 | 3 |
| All others | 2 | 2 | 3 | 3 |
| * See text and guidelines for more complete explanations. Justification for transferring one additional embryo (above the recommended limit) should be clearly documented in the patient’s medical record. | ||||
| †Variables indicating favorable prognosis include first cycle of IVF, good embryo quality, availability of excess embryos for cryopreservation, and previous successful IVF cycle. | ||||
All IVF clinics must adhere to the new SART and ASRM guidelines limiting the number of embryos to transfer at in vitro fertilization. In addition, it is vital for you to counsel the patient about the risk of high-order multiple gestation, and to document that such counseling took place.
Judicious management can reduce the rate of multiple gestation in ovulation stimulation
Dickey RP. Strategies to reduce multiple pregnancies due to ovulation stimulation. Fertil Steril. 2009;91:1–17.
Efforts to reduce the rate of multiple gestation should focus not only on patients undergoing IVF but on those undergoing controlled ovarian stimulation (COS) or ovulation induction. In COS, pharmacologic treatment is used to stimulate the production of more than one oocyte. In ovulation induction, pharmacologic therapy is used to induce normal cycles in anovulatory or oligo-ovulatory women.1 A substantial majority of multiple gestations are conceived using ovarian stimulation and ovulation induction. These methods may be less difficult to manage than IVF because they are less dependent on technology. Like IVF, however, they carry a high risk of multiple gestation, especially high-order multiple gestation.2
Strategies to reduce multiple gestation
As Dickey points out in a comprehensive retrospective analysis, there are strategies that can help reduce multiple gestation during COS and ovulation induction. They include the following recommendations:
Be prepared to cancel a cycle. Initiate ovulation induction only if both patient and physician are prepared to cancel any cycle that involves an excessive number of preovulatory follicles. Singleton and twin births can be confidently expected only if the cycle is cancelled when there are more than two preovulatory follicles approximately 12 mm in diameter or larger. This may be psychologically difficult for some patients and doctors.
Preemptively identify risk factors for multiple gestation, including:
- seven or more preovulatory follicles
- an estradiol concentration of 1,000 pg/mL or higher
- early cycles of treatment (cycles 1–3)
- age younger than 32 years
- body mass index below 19 kg/m2
- use of donor sperm.
When any of these risk factors is present, consider starting the patient on a lower initial dosage of gonadotropin; perform more frequent monitoring; maintain a low threshold for cancellation; and consider performing IVF with single-embryo transfer rather than COS.
Use specific drugs. Increase the likelihood of monofollicular development and double-follicular recruitment and reduce the risk of high-order multiple gestation by using clomiphene citrate, a low dosage of gonadotropin, or pulsatile gonadotropin-releasing hormone (GnRH) in the initial three or four cycles.
Continue treatment for five or more cycles to achieve an overall pregnancy rate approaching 65% without high-order multiple gestation in patients younger than 38 years who develop one or two follicles in a cycle.
Don’t rely on multifetal pregnancy reduction
This strategy has been viewed by some as a way to control the outcome of multiple gestation. For example, this is a common approach in New York, New Jersey, and Connecticut. However, the procedure has pitfalls and should not be the primary means of reducing the rate of multiple gestation because:
- It is not an acceptable option for many patients
- All fetuses may be lost in some cases
- The risks associated with multiple gestation are not completely eliminated
- It may have adverse psychological consequences.3,4
A registry is needed
Although a registry exists for IVF cycles and their outcomes and complications, none exists for cycles involving COS or ovulation induction. Despite many challenges to its development, we support the creation of such a registry.
It is vital that you develop the expertise and adopt strategies to reduce the rate of multiple gestation associated with controlled ovarian stimulation and ovulation induction. If you chose not to do so, refer the patient to someone who has such expertise.
Consider the patient’s emotional status when determining treatment for infertility
Domar AD, Smith K, Conboy L, Iannone M, Alper M. A prospective investigation into the reasons why insured United States patients drop out of in vitro fertilization treatment. Fertil Steril. 2009 Jul 8 [Epub ahead of print].
Most physicians have been trained to concentrate on the physiologic diagnosis and management of disease. Many fertility specialists also pay attention to economic barriers to treatment, such as lack of insurance and high cost, and attempt to help their patients gain access to quality care. One aspect of infertility that might be overlooked, however, is the patient’s emotional health—but it may be as important to the success of treatment as physiologic and economic variables.
A recent prospective investigation into the reasons insured patients drop out of IVF in the United States clearly demonstrated the psychological toll infertility can take. The study found that emotional distress is the number one reason that patients discontinue treatment.
How to lower the patient’s stress level
It can be challenging to counsel the patient to set realistic expectations for success yet enable her to maintain a sense of optimism. Stress management may be a key to success.
Physicians who treat patients with fertility problems should consider offering an in-practice counseling service aimed at reducing stress and improving coping mechanisms. At the very least, physicians should refer patients to outside resources that may be able to provide these services in a way that is meaningful and accessible.
Caring for a patient’s emotional well-being takes both time and skill. Besides offering direct emotional support to your patients, you can be a bridge to mental health and support services.
Patients who participate in a stress-reduction program while undergoing fertility treatment are 1) less likely to experience harmful emotional side effects and 2) more likely to continue treatment. Physicians who make such “mind-body” programs available are likely to reduce treatment dropout, improve the pregnancy rate, and increase the number of patients who take home babies.
Pay attention to the patient’s emotional health during treatment for infertility. Offer her access to stress management and other resources.
New endometriosis fertility index predicts non-IVF success rate
Adamson GD, Pasta DJ. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil Steril. 2009 Nov 18 [Epub ahead of print].
Endometriosis remains a frustrating disease for patients who have infertility, in part because no staging system has made it possible for physicians to predict the pregnancy rate with fertility treatment other than IVF. The new, validated endometriosis fertility index (EFI) changes that. This simple, robust clinical tool predicts the pregnancy rate after surgical staging of endometriosis. Using it can provide reassurance to patients who have a good prognosis and avoid cost and distress of treatment in patients who have a poor prognosis.
Among the variables the index utilizes to predict the likelihood of pregnancy are:
- age of the patient
- duration of infertility
- gravidity
- total revised American Fertility Society (R-AFS) score
- R-AFS lesion score
- the new “least function score” (capability of the tubes, fimbria, and ovaries to effect their reproductive function, as determined by the surgeon after operative treatment) (FIGURES 2 AND 3).
FIGURE 2 Estimated pregnancy rate, by EFI score
FIGURE 3 A look at the endometriosis fertility index (EFI)
The least-function (LF) score (A) is determined at the conclusion of surgery using this form. The endometriosis fertility index (EFI) (B) incorporates the LF score and other variables to determine the overall score.
If you manage endometriosis patients who have infertility, use the new endometriosis fertility index to develop a realistic treatment plan in women who have a good prognosis—or to avert the need for treatment in patients who are unlikely to conceive.
1. Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. For ICMART and the World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92:1520-1524.
2. Reindollar RH, Regan MM, Neumann PJ, et al. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fasttrack and standard treatment (FASTT) trial. Fertil Steril. 2009 Jun 16 [Epub ahead of print].
3. Practice Committee of the American Society for Reproductive Medicine. Multiple pregnancy associated with infertility therapy. Fertil Steril. 2006;86(5 Suppl 1):S106-S110.
4. Stone J, Eddleman K, Lynch L, Berkowitz KL. A single center experience with 1,000 consecutive cases of multifetal pregnancy reduction. Am J Obstet Gynecol. 2002;187:1163-1167.
Infertility and its treatment can be a roller-coaster ride for patient and physician. Amid the emotional stress that arises, the goal of treatment can inadvertently shift from achievement of a successful singleton pregnancy to pregnancy at any cost—even high-order multiple gestation.
Here’s an essential question: Can the rate of multiple gestation be reduced without seriously compromising the pregnancy rate? Several developments of the past year suggest that it can be. In this article, we discuss:
- new guidelines that limit the number of embryos to be transferred at in vitro fertilization (IVF)
- strategies to reduce the risk of multiple gestation after controlled ovarian stimulation or ovulation induction
- the need to address the patient’s emotional status during treatment
- a new index that helps predict the pregnancy rate after surgical staging of endometriosis.
Multiple gestation is known to have adverse effects on infants, including a significantly elevated risk of prematurity and related physical and developmental problems. It also greatly increases the need for resources. And the high cost of caring for infants affected by prematurity further burdens an already overwhelmed health-care system.
Not only is it essential that we reduce the rate of high-order multiple gestation (i.e., more than two fetuses), but we should also attempt to lower the rate of twin pregnancy. A healthy singleton pregnancy, with its diminished risks and more reasonable health-care cost, should be our goal.
New guidelines limit the number of embryos to be transferred at IVF
Practice Committee of the American Society for Reproductive Medicine, Practice Committee of the Society for Assisted Reproductive Technology. Guidelines on number of embryos transferred. Fertil Steril. 2009;92:1518–1519.
Since the birth of Louise Brown in 1978, assisted reproductive technology (ART) has enjoyed dramatic technological advances. Intracytoplasmic sperm injection (FIGURE 1), preimplantation genetic diagnosis, and improvements in cryopreservation have broadened the application of ART and increased the live birth rate to 30% for every cycle that is initiated. The cumulative live birth rate from additional fresh and frozen-thawed cycles can reach 50% to 80%.
These gains have not come without cost, however. Multiple emotional, financial, and other variables affecting the practice of IVF have produced a higher-than-natural rate of multiple gestation.
In November 2009, the Society for Assisted Reproductive Technology (SART) and the American Society for Reproductive Medicine (ASRM) issued new guidelines limiting the number of embryos that should be transferred in one IVF cycle. IVF clinics are required to report outcomes, and approximately 93% of US cycles are reported to SART. High-order multiple-pregnancy rates are audited by SART, and outlier clinics must implement remediation programs to lower their high rate or risk expulsion from SART.
The increasing emphasis on single-embryo transfer in young women who have a good prognosis reflects the societies’ commitment to help patients achieve a healthy singleton pregnancy and good birth outcome.

FIGURE 1 A wonder of technology
Intracytoplasmic sperm injection overcomes many barriers to fertilization, such as severe malefactor infertility. At some institutions, the technique yields a fertilization rate of 70% to 80%.
What makes a “good prognosis”?
Identification of patients who have a good prognosis is an essential component of these new guidelines. The patient is more likely to have a favorable outcome if one or more of the following is true:
- She is undergoing her first cycle of IVF
- The embryos have good morphology
- Excess embryos are available for cryopreservation
- She has had earlier success with IVF.
The TABLE details the recommended number of embryos to transfer, based on the age and prognosis of the patient. In cycles that involve a donor egg, base the number of embryos to be transferred on the age of the donor. In cycles that involve a frozen embryo, base the number of good-quality, thawed embryos to be transferred on the age of the patient at the time the embryos were created. One additional embryo may be transferred if the patient has a less favorable prognosis or a history of two failed, fresh IVF cycles.
Two important requisites: Careful counseling about the risk of high-order multiple gestation, and documentation of that counseling.
TABLE
SART and ASRM recommend limits on the number of embryos to be transferred at in vitro fertilization
| Prognosis | Age of patient (yr) | |||
|---|---|---|---|---|
| <35 | 35–37 | 38–40 | 41 and 42 | |
| CLEAVAGE-STAGE EMBRYOS* | ||||
| Favorable† | 1 or 2 | 2 | 3 | 5 |
| All others | 2 | 3 | 4 | 5 |
| BLASTOCYSTS* | ||||
| Favorable† | 1 | 2 | 2 | 3 |
| All others | 2 | 2 | 3 | 3 |
| * See text and guidelines for more complete explanations. Justification for transferring one additional embryo (above the recommended limit) should be clearly documented in the patient’s medical record. | ||||
| †Variables indicating favorable prognosis include first cycle of IVF, good embryo quality, availability of excess embryos for cryopreservation, and previous successful IVF cycle. | ||||
All IVF clinics must adhere to the new SART and ASRM guidelines limiting the number of embryos to transfer at in vitro fertilization. In addition, it is vital for you to counsel the patient about the risk of high-order multiple gestation, and to document that such counseling took place.
Judicious management can reduce the rate of multiple gestation in ovulation stimulation
Dickey RP. Strategies to reduce multiple pregnancies due to ovulation stimulation. Fertil Steril. 2009;91:1–17.
Efforts to reduce the rate of multiple gestation should focus not only on patients undergoing IVF but on those undergoing controlled ovarian stimulation (COS) or ovulation induction. In COS, pharmacologic treatment is used to stimulate the production of more than one oocyte. In ovulation induction, pharmacologic therapy is used to induce normal cycles in anovulatory or oligo-ovulatory women.1 A substantial majority of multiple gestations are conceived using ovarian stimulation and ovulation induction. These methods may be less difficult to manage than IVF because they are less dependent on technology. Like IVF, however, they carry a high risk of multiple gestation, especially high-order multiple gestation.2
Strategies to reduce multiple gestation
As Dickey points out in a comprehensive retrospective analysis, there are strategies that can help reduce multiple gestation during COS and ovulation induction. They include the following recommendations:
Be prepared to cancel a cycle. Initiate ovulation induction only if both patient and physician are prepared to cancel any cycle that involves an excessive number of preovulatory follicles. Singleton and twin births can be confidently expected only if the cycle is cancelled when there are more than two preovulatory follicles approximately 12 mm in diameter or larger. This may be psychologically difficult for some patients and doctors.
Preemptively identify risk factors for multiple gestation, including:
- seven or more preovulatory follicles
- an estradiol concentration of 1,000 pg/mL or higher
- early cycles of treatment (cycles 1–3)
- age younger than 32 years
- body mass index below 19 kg/m2
- use of donor sperm.
When any of these risk factors is present, consider starting the patient on a lower initial dosage of gonadotropin; perform more frequent monitoring; maintain a low threshold for cancellation; and consider performing IVF with single-embryo transfer rather than COS.
Use specific drugs. Increase the likelihood of monofollicular development and double-follicular recruitment and reduce the risk of high-order multiple gestation by using clomiphene citrate, a low dosage of gonadotropin, or pulsatile gonadotropin-releasing hormone (GnRH) in the initial three or four cycles.
Continue treatment for five or more cycles to achieve an overall pregnancy rate approaching 65% without high-order multiple gestation in patients younger than 38 years who develop one or two follicles in a cycle.
Don’t rely on multifetal pregnancy reduction
This strategy has been viewed by some as a way to control the outcome of multiple gestation. For example, this is a common approach in New York, New Jersey, and Connecticut. However, the procedure has pitfalls and should not be the primary means of reducing the rate of multiple gestation because:
- It is not an acceptable option for many patients
- All fetuses may be lost in some cases
- The risks associated with multiple gestation are not completely eliminated
- It may have adverse psychological consequences.3,4
A registry is needed
Although a registry exists for IVF cycles and their outcomes and complications, none exists for cycles involving COS or ovulation induction. Despite many challenges to its development, we support the creation of such a registry.
It is vital that you develop the expertise and adopt strategies to reduce the rate of multiple gestation associated with controlled ovarian stimulation and ovulation induction. If you chose not to do so, refer the patient to someone who has such expertise.
Consider the patient’s emotional status when determining treatment for infertility
Domar AD, Smith K, Conboy L, Iannone M, Alper M. A prospective investigation into the reasons why insured United States patients drop out of in vitro fertilization treatment. Fertil Steril. 2009 Jul 8 [Epub ahead of print].
Most physicians have been trained to concentrate on the physiologic diagnosis and management of disease. Many fertility specialists also pay attention to economic barriers to treatment, such as lack of insurance and high cost, and attempt to help their patients gain access to quality care. One aspect of infertility that might be overlooked, however, is the patient’s emotional health—but it may be as important to the success of treatment as physiologic and economic variables.
A recent prospective investigation into the reasons insured patients drop out of IVF in the United States clearly demonstrated the psychological toll infertility can take. The study found that emotional distress is the number one reason that patients discontinue treatment.
How to lower the patient’s stress level
It can be challenging to counsel the patient to set realistic expectations for success yet enable her to maintain a sense of optimism. Stress management may be a key to success.
Physicians who treat patients with fertility problems should consider offering an in-practice counseling service aimed at reducing stress and improving coping mechanisms. At the very least, physicians should refer patients to outside resources that may be able to provide these services in a way that is meaningful and accessible.
Caring for a patient’s emotional well-being takes both time and skill. Besides offering direct emotional support to your patients, you can be a bridge to mental health and support services.
Patients who participate in a stress-reduction program while undergoing fertility treatment are 1) less likely to experience harmful emotional side effects and 2) more likely to continue treatment. Physicians who make such “mind-body” programs available are likely to reduce treatment dropout, improve the pregnancy rate, and increase the number of patients who take home babies.
Pay attention to the patient’s emotional health during treatment for infertility. Offer her access to stress management and other resources.
New endometriosis fertility index predicts non-IVF success rate
Adamson GD, Pasta DJ. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil Steril. 2009 Nov 18 [Epub ahead of print].
Endometriosis remains a frustrating disease for patients who have infertility, in part because no staging system has made it possible for physicians to predict the pregnancy rate with fertility treatment other than IVF. The new, validated endometriosis fertility index (EFI) changes that. This simple, robust clinical tool predicts the pregnancy rate after surgical staging of endometriosis. Using it can provide reassurance to patients who have a good prognosis and avoid cost and distress of treatment in patients who have a poor prognosis.
Among the variables the index utilizes to predict the likelihood of pregnancy are:
- age of the patient
- duration of infertility
- gravidity
- total revised American Fertility Society (R-AFS) score
- R-AFS lesion score
- the new “least function score” (capability of the tubes, fimbria, and ovaries to effect their reproductive function, as determined by the surgeon after operative treatment) (FIGURES 2 AND 3).

FIGURE 2 Estimated pregnancy rate, by EFI score
FIGURE 3 A look at the endometriosis fertility index (EFI)
The least-function (LF) score (A) is determined at the conclusion of surgery using this form. The endometriosis fertility index (EFI) (B) incorporates the LF score and other variables to determine the overall score.
If you manage endometriosis patients who have infertility, use the new endometriosis fertility index to develop a realistic treatment plan in women who have a good prognosis—or to avert the need for treatment in patients who are unlikely to conceive.
Infertility and its treatment can be a roller-coaster ride for patient and physician. Amid the emotional stress that arises, the goal of treatment can inadvertently shift from achievement of a successful singleton pregnancy to pregnancy at any cost—even high-order multiple gestation.
Here’s an essential question: Can the rate of multiple gestation be reduced without seriously compromising the pregnancy rate? Several developments of the past year suggest that it can be. In this article, we discuss:
- new guidelines that limit the number of embryos to be transferred at in vitro fertilization (IVF)
- strategies to reduce the risk of multiple gestation after controlled ovarian stimulation or ovulation induction
- the need to address the patient’s emotional status during treatment
- a new index that helps predict the pregnancy rate after surgical staging of endometriosis.
Multiple gestation is known to have adverse effects on infants, including a significantly elevated risk of prematurity and related physical and developmental problems. It also greatly increases the need for resources. And the high cost of caring for infants affected by prematurity further burdens an already overwhelmed health-care system.
Not only is it essential that we reduce the rate of high-order multiple gestation (i.e., more than two fetuses), but we should also attempt to lower the rate of twin pregnancy. A healthy singleton pregnancy, with its diminished risks and more reasonable health-care cost, should be our goal.
New guidelines limit the number of embryos to be transferred at IVF
Practice Committee of the American Society for Reproductive Medicine, Practice Committee of the Society for Assisted Reproductive Technology. Guidelines on number of embryos transferred. Fertil Steril. 2009;92:1518–1519.
Since the birth of Louise Brown in 1978, assisted reproductive technology (ART) has enjoyed dramatic technological advances. Intracytoplasmic sperm injection (FIGURE 1), preimplantation genetic diagnosis, and improvements in cryopreservation have broadened the application of ART and increased the live birth rate to 30% for every cycle that is initiated. The cumulative live birth rate from additional fresh and frozen-thawed cycles can reach 50% to 80%.
These gains have not come without cost, however. Multiple emotional, financial, and other variables affecting the practice of IVF have produced a higher-than-natural rate of multiple gestation.
In November 2009, the Society for Assisted Reproductive Technology (SART) and the American Society for Reproductive Medicine (ASRM) issued new guidelines limiting the number of embryos that should be transferred in one IVF cycle. IVF clinics are required to report outcomes, and approximately 93% of US cycles are reported to SART. High-order multiple-pregnancy rates are audited by SART, and outlier clinics must implement remediation programs to lower their high rate or risk expulsion from SART.
The increasing emphasis on single-embryo transfer in young women who have a good prognosis reflects the societies’ commitment to help patients achieve a healthy singleton pregnancy and good birth outcome.

FIGURE 1 A wonder of technology
Intracytoplasmic sperm injection overcomes many barriers to fertilization, such as severe malefactor infertility. At some institutions, the technique yields a fertilization rate of 70% to 80%.
What makes a “good prognosis”?
Identification of patients who have a good prognosis is an essential component of these new guidelines. The patient is more likely to have a favorable outcome if one or more of the following is true:
- She is undergoing her first cycle of IVF
- The embryos have good morphology
- Excess embryos are available for cryopreservation
- She has had earlier success with IVF.
The TABLE details the recommended number of embryos to transfer, based on the age and prognosis of the patient. In cycles that involve a donor egg, base the number of embryos to be transferred on the age of the donor. In cycles that involve a frozen embryo, base the number of good-quality, thawed embryos to be transferred on the age of the patient at the time the embryos were created. One additional embryo may be transferred if the patient has a less favorable prognosis or a history of two failed, fresh IVF cycles.
Two important requisites: Careful counseling about the risk of high-order multiple gestation, and documentation of that counseling.
TABLE
SART and ASRM recommend limits on the number of embryos to be transferred at in vitro fertilization
| Prognosis | Age of patient (yr) | |||
|---|---|---|---|---|
| <35 | 35–37 | 38–40 | 41 and 42 | |
| CLEAVAGE-STAGE EMBRYOS* | ||||
| Favorable† | 1 or 2 | 2 | 3 | 5 |
| All others | 2 | 3 | 4 | 5 |
| BLASTOCYSTS* | ||||
| Favorable† | 1 | 2 | 2 | 3 |
| All others | 2 | 2 | 3 | 3 |
| * See text and guidelines for more complete explanations. Justification for transferring one additional embryo (above the recommended limit) should be clearly documented in the patient’s medical record. | ||||
| †Variables indicating favorable prognosis include first cycle of IVF, good embryo quality, availability of excess embryos for cryopreservation, and previous successful IVF cycle. | ||||
All IVF clinics must adhere to the new SART and ASRM guidelines limiting the number of embryos to transfer at in vitro fertilization. In addition, it is vital for you to counsel the patient about the risk of high-order multiple gestation, and to document that such counseling took place.
Judicious management can reduce the rate of multiple gestation in ovulation stimulation
Dickey RP. Strategies to reduce multiple pregnancies due to ovulation stimulation. Fertil Steril. 2009;91:1–17.
Efforts to reduce the rate of multiple gestation should focus not only on patients undergoing IVF but on those undergoing controlled ovarian stimulation (COS) or ovulation induction. In COS, pharmacologic treatment is used to stimulate the production of more than one oocyte. In ovulation induction, pharmacologic therapy is used to induce normal cycles in anovulatory or oligo-ovulatory women.1 A substantial majority of multiple gestations are conceived using ovarian stimulation and ovulation induction. These methods may be less difficult to manage than IVF because they are less dependent on technology. Like IVF, however, they carry a high risk of multiple gestation, especially high-order multiple gestation.2
Strategies to reduce multiple gestation
As Dickey points out in a comprehensive retrospective analysis, there are strategies that can help reduce multiple gestation during COS and ovulation induction. They include the following recommendations:
Be prepared to cancel a cycle. Initiate ovulation induction only if both patient and physician are prepared to cancel any cycle that involves an excessive number of preovulatory follicles. Singleton and twin births can be confidently expected only if the cycle is cancelled when there are more than two preovulatory follicles approximately 12 mm in diameter or larger. This may be psychologically difficult for some patients and doctors.
Preemptively identify risk factors for multiple gestation, including:
- seven or more preovulatory follicles
- an estradiol concentration of 1,000 pg/mL or higher
- early cycles of treatment (cycles 1–3)
- age younger than 32 years
- body mass index below 19 kg/m2
- use of donor sperm.
When any of these risk factors is present, consider starting the patient on a lower initial dosage of gonadotropin; perform more frequent monitoring; maintain a low threshold for cancellation; and consider performing IVF with single-embryo transfer rather than COS.
Use specific drugs. Increase the likelihood of monofollicular development and double-follicular recruitment and reduce the risk of high-order multiple gestation by using clomiphene citrate, a low dosage of gonadotropin, or pulsatile gonadotropin-releasing hormone (GnRH) in the initial three or four cycles.
Continue treatment for five or more cycles to achieve an overall pregnancy rate approaching 65% without high-order multiple gestation in patients younger than 38 years who develop one or two follicles in a cycle.
Don’t rely on multifetal pregnancy reduction
This strategy has been viewed by some as a way to control the outcome of multiple gestation. For example, this is a common approach in New York, New Jersey, and Connecticut. However, the procedure has pitfalls and should not be the primary means of reducing the rate of multiple gestation because:
- It is not an acceptable option for many patients
- All fetuses may be lost in some cases
- The risks associated with multiple gestation are not completely eliminated
- It may have adverse psychological consequences.3,4
A registry is needed
Although a registry exists for IVF cycles and their outcomes and complications, none exists for cycles involving COS or ovulation induction. Despite many challenges to its development, we support the creation of such a registry.
It is vital that you develop the expertise and adopt strategies to reduce the rate of multiple gestation associated with controlled ovarian stimulation and ovulation induction. If you chose not to do so, refer the patient to someone who has such expertise.
Consider the patient’s emotional status when determining treatment for infertility
Domar AD, Smith K, Conboy L, Iannone M, Alper M. A prospective investigation into the reasons why insured United States patients drop out of in vitro fertilization treatment. Fertil Steril. 2009 Jul 8 [Epub ahead of print].
Most physicians have been trained to concentrate on the physiologic diagnosis and management of disease. Many fertility specialists also pay attention to economic barriers to treatment, such as lack of insurance and high cost, and attempt to help their patients gain access to quality care. One aspect of infertility that might be overlooked, however, is the patient’s emotional health—but it may be as important to the success of treatment as physiologic and economic variables.
A recent prospective investigation into the reasons insured patients drop out of IVF in the United States clearly demonstrated the psychological toll infertility can take. The study found that emotional distress is the number one reason that patients discontinue treatment.
How to lower the patient’s stress level
It can be challenging to counsel the patient to set realistic expectations for success yet enable her to maintain a sense of optimism. Stress management may be a key to success.
Physicians who treat patients with fertility problems should consider offering an in-practice counseling service aimed at reducing stress and improving coping mechanisms. At the very least, physicians should refer patients to outside resources that may be able to provide these services in a way that is meaningful and accessible.
Caring for a patient’s emotional well-being takes both time and skill. Besides offering direct emotional support to your patients, you can be a bridge to mental health and support services.
Patients who participate in a stress-reduction program while undergoing fertility treatment are 1) less likely to experience harmful emotional side effects and 2) more likely to continue treatment. Physicians who make such “mind-body” programs available are likely to reduce treatment dropout, improve the pregnancy rate, and increase the number of patients who take home babies.
Pay attention to the patient’s emotional health during treatment for infertility. Offer her access to stress management and other resources.
New endometriosis fertility index predicts non-IVF success rate
Adamson GD, Pasta DJ. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil Steril. 2009 Nov 18 [Epub ahead of print].
Endometriosis remains a frustrating disease for patients who have infertility, in part because no staging system has made it possible for physicians to predict the pregnancy rate with fertility treatment other than IVF. The new, validated endometriosis fertility index (EFI) changes that. This simple, robust clinical tool predicts the pregnancy rate after surgical staging of endometriosis. Using it can provide reassurance to patients who have a good prognosis and avoid cost and distress of treatment in patients who have a poor prognosis.
Among the variables the index utilizes to predict the likelihood of pregnancy are:
- age of the patient
- duration of infertility
- gravidity
- total revised American Fertility Society (R-AFS) score
- R-AFS lesion score
- the new “least function score” (capability of the tubes, fimbria, and ovaries to effect their reproductive function, as determined by the surgeon after operative treatment) (FIGURES 2 AND 3).

FIGURE 2 Estimated pregnancy rate, by EFI score
FIGURE 3 A look at the endometriosis fertility index (EFI)
The least-function (LF) score (A) is determined at the conclusion of surgery using this form. The endometriosis fertility index (EFI) (B) incorporates the LF score and other variables to determine the overall score.
If you manage endometriosis patients who have infertility, use the new endometriosis fertility index to develop a realistic treatment plan in women who have a good prognosis—or to avert the need for treatment in patients who are unlikely to conceive.
1. Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. For ICMART and the World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92:1520-1524.
2. Reindollar RH, Regan MM, Neumann PJ, et al. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fasttrack and standard treatment (FASTT) trial. Fertil Steril. 2009 Jun 16 [Epub ahead of print].
3. Practice Committee of the American Society for Reproductive Medicine. Multiple pregnancy associated with infertility therapy. Fertil Steril. 2006;86(5 Suppl 1):S106-S110.
4. Stone J, Eddleman K, Lynch L, Berkowitz KL. A single center experience with 1,000 consecutive cases of multifetal pregnancy reduction. Am J Obstet Gynecol. 2002;187:1163-1167.
1. Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. For ICMART and the World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92:1520-1524.
2. Reindollar RH, Regan MM, Neumann PJ, et al. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fasttrack and standard treatment (FASTT) trial. Fertil Steril. 2009 Jun 16 [Epub ahead of print].
3. Practice Committee of the American Society for Reproductive Medicine. Multiple pregnancy associated with infertility therapy. Fertil Steril. 2006;86(5 Suppl 1):S106-S110.
4. Stone J, Eddleman K, Lynch L, Berkowitz KL. A single center experience with 1,000 consecutive cases of multifetal pregnancy reduction. Am J Obstet Gynecol. 2002;187:1163-1167.