User login
Shoulder Arthroplasty: Disposition and Perioperative Outcomes in Patients With and Without Rheumatoid Arthritis
Shoulder arthroplasty (SA), including total SA (TSA) and reverse TSA, is an effective surgical treatment for fracture and primary or secondary degenerative disease of the shoulder.1 Over the past few decades, use of SA has increased dramatically, from about 5000 cases in 1990 to 7000 in 2000 and more than 26,000 in 2008.1,2
Complications associated with SA generally are classified as perioperative (occurring during the operative index) or long-term (postdischarge).3 Long-term complications include implant loosening, instability, revision, infection, rotator cuff tear, neural injury, and deltoid detachment.1,4,5 Perioperative complications, which are less commonly reported, include intraoperative fracture, infection, neural injury, venous thromboembolic events (VTEs, including pulmonary embolism [PE] and deep vein thrombosis [DVT]), transfusion, and death.3,6-10
SA is an attractive treatment option for patients with rheumatoid arthritis (RA), as the effects of pain on these patients are greater in the shoulder joint than in any other joint.11 Patients with RA pose unique orthopedic surgical challenges, including any combination of decreased bone mineralization, poor capsular tissue integrity, and osteonecrosis.3,12 In addition, RA patients may be taking immunosuppressive medications that have severe side effects, and they may require multiple surgeries.12,13 These factors predispose patients with RA to complications that include infection and wound dehiscence.3,5,12-14
The complex nature of RA has prompted investigators to examine outcome measures in this patient group. Hambright and colleagues3 used the Nationwide Inpatient Sample (NIS) to examine perioperative outcomes in RA patients who underwent TSA between 1988 and 2005.3 They found that TSA patients with RA had shorter and less costly hospital stays and were more likely to have a routine discharge.3 Using the same patient population drawn from the period 2006–2011, we conducted a study to determine if this unexpected trend persists as the number of TSAs and quality of postoperative care continue to increase. Given the potential for anemia of chronic disease and the systemic inflammatory nature of RA, we hypothesized that the perioperative complication profile of RA patients would be worse than that of non-RA patients.
Materials and Methods
NIS data were acquired for the period 2006–2011. The NIS is the largest publicly available all-payer inpatient database, with a random 20% sample of about 1000 US hospitals accounting for 7 to 8 million inpatient stays. The database supplies weights used to estimate national totals, at about 35 million inpatient visits per year. NIS inpatient data are limited to the operative index. Postdischarge information is not available. The NIS is managed by the Healthcare Cost and Utilization Project, which is sponsored by the Agency for Healthcare Research and Quality. The quality of NIS data is assessed and validated by an independent contractor. NIS data have been widely used to examine perioperative outcomes.15-17
NIS data cover patient and hospital demographics, hospital length of stay (LOS), discharge status, payer information, charges, and perioperative outcomes and procedure/diagnosis codes (ICD-9; International Classification of Diseases, Ninth Revision18).
As our Institutional Review Board (IRB) reviewed the database and determined the project was not human subject research, IRB involvement was not required. This study paralleled successful efforts with similar RA and non-RA patients who had shoulder and elbow surgery.3,19 SA patients were identified by ICD-9 procedure code 81.80, but this code does not specify whether the prosthesis was unconstrained, semiconstrained, or constrained. ICD-9 coding also does not specify whether the TSA was traditional or reverse. Patients with RA were identified by ICD-9 diagnosis codes 714.0, 714.1, and 714.2. Patients without one of these codes were placed in the non-RA cohort. Patients with codes associated with pathologic fractures secondary to metastatic cancer or bone malignant neoplasm as a secondary or primary diagnosis and patients who had revision surgery indicated by code 81.83 were excluded, as they have a disproportionately higher comorbidity burden.
After each cohort was defined, demographic data (age, sex, race, income quartile based on ZIP postal code) were compared, as were data on primary payer, hospital demographics, LOS (≤5 days, defined as perioperative index), discharge type, inflation-adjusted charges in 2014 dollars based on the Consumer Price Indexes (http://www.bls.gov/cpi/), and mortality. Perioperative complications—respiratory, gastrointestinal, genitourinary, accidental puncture/laceration, central nervous system, wound dehiscence, device-related (including embolism, fibrosis, hemorrhage, pain, stenosis, or thrombus caused by any device, implant, or graft), cardiac, hematoma/seroma, acute respiratory distress syndrome, postoperative shock, VTE, postoperative infection complications, and intraoperative transfusions—were considered using ICD-9 codes (996.X-999.X and 99.X, respectively).20 Although commonly used to determine perioperative comorbidity burden using ICD-9 coding, the modified Charlson index was not considered because RA is a component of the index and would therefore bias the variable.3,21
Statistical analyses, including χ2 tests and 2-sample t tests, were performed for categorical and continuous variables, respectively. P < .05 was considered significant. Fisher exact test was used for cohorts with fewer than 5 occurrences. Multivariate logistic regression models were then calculated to determine the effect of RA on different outcomes and complications, with age, race, sex, hospital region, hospital type, number of hospital beds, primary payer, and hospital ownership as covariates. Statistical analyses were performed using the R statistical programming language.22
Results
Of the 34,970 patients who underwent SA between 2006 and 2011, 1674 (4.8%) had a diagnosis of RA and 33,296 (95.2%) did not. On average, patients with RA tended to be younger than patients without RA (66.4 vs 69.1 years; P < .001), and a larger percentage of RA patients were female (75.5% vs 54.4%; P < .001). Compared with non-RA patients, RA patients comprised a different ethnic group and had a different expected primary payer (P < .001). SA patients with and without RA did not differ in income quartile based on ZIP code, total number of hospital beds, hospital region, or hospital teaching status (P = .34, .78, .59, and .82, respectively) (Table 1).
LOS was significantly (P < .001) statistically longer for RA patients (2.196 days) than for non-RA patients (2.085 days). RA patients were significantly less likely to be discharged home (63.0% vs 67.6%; P < .001). (Routine discharge was defined as discharge home, whereas nonroutine discharge was defined as discharge to a short-term hospital, skilled nursing facility, intermediate care, another type of facility, home health care, against medical advice, or death.) In addition, inflation-adjusted charges associated with SA were significantly higher (P = .018) for RA patients ($54,284) than for non-RA patients ($52,663) (Table 1).
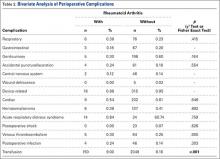
Regarding the rates of complications that occurred during the perioperative index, there were no significant differences between RA and non-RA cohorts. These complications included respiratory, gastrointestinal, genitourinary, accidental puncture/laceration, central nervous system, wound dehiscence, device-related, cardiac, hematoma/seroma, acute respiratory distress syndrome, postoperative shock, VTE, and postoperative infection (Table 2). In addition, there was no significant difference in mortality between the groups (P = .48).
In TSA, blood transfusions were more likely (P < .001) to be given to RA patients (9.00%) than to non-RA patients (6.16%). Multivariate regression analyses were performed with age, race, sex, hospital region, hospital type, number of hospital beds, primary payer, and hospital ownership as covariates. These analyses revealed that transfusion (P < .001), discharge type (P = .002), total inflation-adjusted charges (P < .001), and LOS (P = .047) remained significant (Table 3).
Discussion
Large national databases like NIS allow study of uncommon medical occurrences and help delineate risks and trends that otherwise might be indeterminable. Although it has been suggested that patients with RA may have poorer long-term outcomes after SA, the perioperative risk profile indicates that TSA is well tolerated in RA patients during the operative index.3,23-25
The data on this study’s 34,970 patients, drawn from the period 2006–2011, demonstrated no significant differences in safety profile with respect to the 14 perioperative complications and outcomes examined, except blood transfusion rate. Rates of postoperative infection (RA, 0.24%; non-RA, 0.14%; P = .303), VTE (RA, 0.30%; non-RA, 0.25%; P = .905), and transfusion (RA, 9.00%; non-RA, 6.16%; P < .001) are of particular interest because of the severity of these situations.
Postoperative infection is a potentially serious complication and often occurs secondary to diabetes, RA, lupus erythematosus, prior surgery, or a nosocomial or remote source.1 The often costly treatment options include antibiotic suppression, irrigation and debridement with implant retention, 1-stage exchange with antibiotic-impregnated cement fixation, staged reimplantation, resection arthroplasty, arthrodesis, and amputation.1 The overall 0.14% infection rate determined in this study is lower than the 0.7% reported for SA patients in the literature.1 Given the nature of the NIS database, this rate underestimates the true postoperative infection rate, as any infection that occurred after the perioperative period is not captured.26 The present study’s perioperative infection rates (RA, 0.24%; non-RA, 0.14%) for the period 2006–2011 are comparable to the rates (RA, 0.17%; non-RA, 0.24%) reported by Hambright and colleagues3 for the same patient population over the preceding, 18-year period (1988–2005) and similarly do not significantly differ between groups. Although infection is uncommon in the immediate perioperative period, the ICD-9 codes used refer specifically to infection resulting from surgery and do not represent concomitant infection.
VTEs, which include PEs and DVTs, are rare but potentially life-threatening surgical complications.27,28 Mechanical prophylaxis and chemical prophylaxis have been recommended for major orthopedic surgery, particularly lower extremity surgery, such as total hip arthroplasty (THA) and total knee arthroplasty (TKA).28,29 In the present study, VTE rates were low, 0.30% (RA) and 0.25% (non-RA), and not significantly different in bivariate or multivariate analyses. These rates are comparable to those found in other national-database SA studies.28 VTEs that occur outside the index hospital admission are not captured in this database. Therefore, the rates in the present study may be lower than the true incidence after SA. Mortality secondary to VTE usually occurs within 24 hours but may occur up to 90 days after surgery. DVT rates, on the other hand, are difficult to evaluate because of differences in screening practices.27,28,30,31
That RA patients were more likely than non-RA patients to receive perioperative blood transfusions supports prior findings that SA patients with RA were more likely than SA patients with osteoarthritis (OA) to receive perioperative blood transfusions.8 RA patients have been shown to have high rates of anemia of chronic disease, ranging from 22% to 77%.32 During joint replacement, these patients often require transfusions.32,33 However, these findings differ from prior findings of no differences between RA and non-RA patients in the same patient population during the period 1988–2005.3 This difference may be a product of the constantly changing transfusion guidelines and increased use; transfusion rates increased 140% between 1997 and 2007, making transfusions the fastest growing common procedure in the United States during that time.34 There was no difference between RA and non-RA patients in household income (as determined by ZIP code analysis), number of hospital beds, hospital region, or hospital teaching status. Compared with non-RA patients, RA patients were more likely to be younger, female, and of a difference race and to have a different expected primary payer (P < .001).These findings are consistent with previous findings in the literature.3 In the present SA study, however, RA patients were more likely than non-RA patients to have longer LOS, higher inflation-adjusted hospital charges, and nonroutine discharge. These findings deviate from those of the study covering the preceding 18 years (1988–2005).3 Despite the findings of a changing environment of care for RA patients, by Hambright and colleagues3 and Weiss and colleagues,35 the trend appears to have shifted. Both groups had shorter average LOS than either group from the preceding 18 years.3 Although statistically significant in bivariate analysis, the difference in LOS between the 2 groups differed by an average of 0.11 day (2 hours 24 minutes) and was not clinically relevant.
In addition, the higher charges for patients with RA represent a deviation from the preceding 18 years.3 Other studies have also shown that RA is associated with increased cost in TSA.36 Patients with RA often have rotator cuff pathology, indicating reverse SA may be used more frequently.37,38 The increased implant cost associated with reverse SA may account for the increased costs in RA patients.39 As mentioned, TSA type is not captured in the NIS database. In addition, that RA patients were less likely than non-RA patients to have routine discharge may indicate RA cases are more complex because of their complications.1,5,14,40 A recent study of complications in RA patients (1163 who underwent THA, 2692 who underwent TKA) found that THA patients with RA were significantly more likely than THA patients with OA to dislocate, and TKA patients with RA were significantly more likely than TKA patients with OA to develop an infection after surgery.41 Postoperative dislocation has been shown to increase hospital costs in other orthopedic procedures.42 Also, during TSA, patients with RA are more likely than patients with OA to receive intraoperative blood transfusions.8 These complications—combined with the fact that RA is a chronic, progressive, systemic inflammatory disease that can affect soft tissue and blood vessel wall healing and is associated with medications having potential side effects—could contribute to the apparent increased hospital charges and LOS.3,12,13,43 Factors that include surgeon preference, impact of primary payer, and hospital practice may also affect final charges. Total charges in the NIS database include administrative fees, hospital costs, device-related costs, operating room costs, and ancillary staff costs. Total charges do not include professional fees and differ from the total cost that represents the amount reimbursed by the payer. Charges tend to correlate with but overestimate the total costs.44
This study had several important limitations. As mentioned, only events that occur during the operative admission are captured in the NIS database, and thus postoperative complications or serious adverse events that lead to readmission cannot be identified. In addition, outpatient TSAs are not captured in the NIS database, and thus inclusion of only inpatient procedures yields higher average LOS and total charges.45 Given the limited granularity of ICD-9 coding, this study could not determine RA severity, estimated blood loss, length of surgery, complication severity, type of TSA procedure/prosthesis, or cause of death. Although commonly used to determine comorbidity burden, the modified Charlson index could not be used, and therefore could not be entered as a covariate in multivariate analysis. Furthermore, the NIS database does not include imaging or patient-reported outcomes information, such as improvements in pain or function, which are of crucial importance in considering surgery.
Conclusion
Our findings corroborated findings that the demographics and the perioperative safety profile for TSA were similar for patients with and without RA. The risk for complications or death in the perioperative period was low. Compared with non-RA patients, RA patients had significantly higher charges and longer LOS and were less likely to be discharged home after surgery. The 0.11-day difference in LOS, though statistically significant, was not clinically relevant. These findings differ from those for the preceding, 18-year period (1988–2005). Future research should focus on the causes of these changes.
1. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
3. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
4. van de Sande MA, Brand R, Rozing PM. Indications, complications, and results of shoulder arthroplasty. Scand J Rheumatol. 2006;35(6):426-434.
5. Wirth MA, Rockwood CA Jr. Complications of shoulder arthroplasty. Clin Orthop Relat Res. 1994;(307):47-69.
6. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):
1915-1923.
7. Sperling JW, Kozak TK, Hanssen AD, Cofield RH. Infection after shoulder arthroplasty. Clin Orthop Relat Res. 2001;(382):206-216.
8. Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(6):599-601.
9. Kumar S, Sperling JW, Haidukewych GH, Cofield RH. Periprosthetic humeral fractures after shoulder arthroplasty. J Bone Joint Surg Am. 2004;86(4):680-689.
10. Sperling JW, Cofield RH. Pulmonary embolism following shoulder arthroplasty. J Bone Joint Surg Am. 2002;84(11):1939-1941.
11. Tanaka E, Saito A, Kamitsuji S, et al. Impact of shoulder, elbow, and knee joint involvement on assessment of rheumatoid arthritis using the American College of Rheumatology core data set. Arthritis Rheum. 2005;53(6):864-871.
12. Nassar J, Cracchiolo A 3rd. Complications in surgery of the foot and ankle in patients with rheumatoid arthritis. Clin Orthop Relat Res. 2001;(391):140-152.
13. den Broeder AA, Creemers MC, Fransen J, et al. Risk factors for surgical site infections and other complications in elective surgery in patients with rheumatoid arthritis with special attention for anti-tumor necrosis factor: a large retrospective study. J Rheumatol. 2007;34(4):689-695.
14. Sanchez-Sotelo J. (i) Shoulder arthroplasty for osteoarthritis and rheumatoid arthritis. Curr Orthop. 2007;21(6):405-414.
15. Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project (HCUP). Overview of the National (Nationwide) Inpatient Sample (NIS). 2012. http://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed February 3, 2015.
16. Hervey SL, Purves HR, Guller U, Toth AP, Vail TP, Pietrobon R. Provider volume of total knee arthroplasties and patient outcomes in the HCUP-Nationwide Inpatient Sample. J Bone Joint Surg Am. 2003;85(9):1775-1783.
17. Noskin GA, Rubin RJ, Schentag JJ, et al. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample database. Arch Intern Med. 2005;165(15):1756-1761.
18. World Health Organization. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Geneva, Switzerland: World Health Organization; 2008.
19. Cook C, Hawkins R, Aldridge JM 3rd, Tolan S, Krupp R, Bolognesi M. Comparison of perioperative complications in patients with and without rheumatoid arthritis who receive total elbow replacement. J Shoulder Elbow Surg. 2009;18(1):21-26.
20. Goz V, Weinreb JH, McCarthy I, Schwab F, Lafage V, Errico TJ. Perioperative complications and mortality after spinal fusions: analysis of trends and risk factors. Spine. 2013;38(22):1970-1976.
21. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
22. R: a language and environment for statistical computing [computer program]. Vienna, Austria: Foundation for Statistical Computing; 2012.
23. Cuomo F, Greller MJ, Zuckerman JD. The rheumatoid shoulder. Rheum Dis Clin North Am. 1998;24(1):67-82.
24. Kelly IG, Foster RS, Fisher WD. Neer total shoulder replacement in rheumatoid arthritis. J Bone Joint Surg Br. 1987;69(5):723-726.
25. Donigan JA, Frisella WA, Haase D, Dolan L, Wolf B. Pre-operative and intra-operative factors related to shoulder arthroplasty outcomes. Iowa Orthop J. 2009;29:60-66.
26. Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479.
27. Willis AA, Warren RF, Craig EV, et al. Deep vein thrombosis after reconstructive shoulder arthroplasty: a prospective observational study. J Shoulder Elbow Surg. 2009;18(1):100-106.
28. Jameson SS, James P, Howcroft DW, et al. Venous thromboembolic events are rare after shoulder surgery: analysis of a national database. J Shoulder Elbow Surg. 2011;20(5):
764-770.
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest J. 2012;141(2 suppl):e278S-e325S.
30. White CB, Sperling JW, Cofield RH, Rowland CM. Ninety-day mortality after shoulder arthroplasty. J Arthroplasty. 2003;18(7):886-888.
31. Lussana F, Squizzato A, Permunian ET, Cattaneo M. A systematic review on the effect of aspirin in the prevention of post-operative arterial thrombosis in patients undergoing total hip and total knee arthroplasty. Thromb Res. 2014;134(3):599-603.
32. Wilson A, Yu H, Goodnough LT, Nissenson AR. Prevalence and outcomes of anemia in rheumatoid arthritis: a systematic review of the literature. Am J Med. 2004;116(7):50-57.
33. Mercuriali F, Gualtieri G, Sinigaglia L, et al. Use of recombinant human erythropoietin to assist autologous blood donation by anemic rheumatoid arthritis patients undergoing major orthopedic surgery. Transfusion. 1994;34(6):501-506.
34. Shander A, Gross I, Hill S, et al. A new perspective on best transfusion practices. Blood Transfus. 2013;11(2):193-202.
35. Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatology. 2008;47(4):491-494.
36. Davis DE, Paxton ES, Maltenfort M, Abboud J. Factors affecting hospital charges after total shoulder arthroplasty: an evaluation of the national inpatient sample database.
J Shoulder Elbow Surg. 2014;23(12):1860-1866.
37. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
38. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
39. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288.
40. Garner RW, Mowat AG, Hazleman BL. Wound healing after operations of patients with rheumatoid arthritis. J Bone Joint Surg Br. 1973;55(1):134-144.
41. Ravi B, Croxford R, Hollands S, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263.
42. Sanchez-Sotelo J, Haidukewych GJ, Boberg CJ. Hospital cost of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2006;88(2):290-294.
43. Ward MM. Decreases in rates of hospitalizations for manifestations of severe rheumatoid arthritis, 1983-2001. Arthritis Rheum. 2004;50(4):1122-1131.
44. Goz V, Weinreb JH, Schwab F, Lafage V, Errico TJ. Comparison of complications, costs, and length of stay of three different lumbar interbody fusion techniques: an analysis of the Nationwide Inpatient Sample database. Spine J. 2014;14(9):2019-2027.
45. Goz V, Errico TJ, Weinreb JH, et al. Vertebroplasty and kyphoplasty: national outcomes and trends in utilization from 2005 through 2010. Spine J. 2015;15(5):959-965.
Shoulder arthroplasty (SA), including total SA (TSA) and reverse TSA, is an effective surgical treatment for fracture and primary or secondary degenerative disease of the shoulder.1 Over the past few decades, use of SA has increased dramatically, from about 5000 cases in 1990 to 7000 in 2000 and more than 26,000 in 2008.1,2
Complications associated with SA generally are classified as perioperative (occurring during the operative index) or long-term (postdischarge).3 Long-term complications include implant loosening, instability, revision, infection, rotator cuff tear, neural injury, and deltoid detachment.1,4,5 Perioperative complications, which are less commonly reported, include intraoperative fracture, infection, neural injury, venous thromboembolic events (VTEs, including pulmonary embolism [PE] and deep vein thrombosis [DVT]), transfusion, and death.3,6-10
SA is an attractive treatment option for patients with rheumatoid arthritis (RA), as the effects of pain on these patients are greater in the shoulder joint than in any other joint.11 Patients with RA pose unique orthopedic surgical challenges, including any combination of decreased bone mineralization, poor capsular tissue integrity, and osteonecrosis.3,12 In addition, RA patients may be taking immunosuppressive medications that have severe side effects, and they may require multiple surgeries.12,13 These factors predispose patients with RA to complications that include infection and wound dehiscence.3,5,12-14
The complex nature of RA has prompted investigators to examine outcome measures in this patient group. Hambright and colleagues3 used the Nationwide Inpatient Sample (NIS) to examine perioperative outcomes in RA patients who underwent TSA between 1988 and 2005.3 They found that TSA patients with RA had shorter and less costly hospital stays and were more likely to have a routine discharge.3 Using the same patient population drawn from the period 2006–2011, we conducted a study to determine if this unexpected trend persists as the number of TSAs and quality of postoperative care continue to increase. Given the potential for anemia of chronic disease and the systemic inflammatory nature of RA, we hypothesized that the perioperative complication profile of RA patients would be worse than that of non-RA patients.
Materials and Methods
NIS data were acquired for the period 2006–2011. The NIS is the largest publicly available all-payer inpatient database, with a random 20% sample of about 1000 US hospitals accounting for 7 to 8 million inpatient stays. The database supplies weights used to estimate national totals, at about 35 million inpatient visits per year. NIS inpatient data are limited to the operative index. Postdischarge information is not available. The NIS is managed by the Healthcare Cost and Utilization Project, which is sponsored by the Agency for Healthcare Research and Quality. The quality of NIS data is assessed and validated by an independent contractor. NIS data have been widely used to examine perioperative outcomes.15-17
NIS data cover patient and hospital demographics, hospital length of stay (LOS), discharge status, payer information, charges, and perioperative outcomes and procedure/diagnosis codes (ICD-9; International Classification of Diseases, Ninth Revision18).
As our Institutional Review Board (IRB) reviewed the database and determined the project was not human subject research, IRB involvement was not required. This study paralleled successful efforts with similar RA and non-RA patients who had shoulder and elbow surgery.3,19 SA patients were identified by ICD-9 procedure code 81.80, but this code does not specify whether the prosthesis was unconstrained, semiconstrained, or constrained. ICD-9 coding also does not specify whether the TSA was traditional or reverse. Patients with RA were identified by ICD-9 diagnosis codes 714.0, 714.1, and 714.2. Patients without one of these codes were placed in the non-RA cohort. Patients with codes associated with pathologic fractures secondary to metastatic cancer or bone malignant neoplasm as a secondary or primary diagnosis and patients who had revision surgery indicated by code 81.83 were excluded, as they have a disproportionately higher comorbidity burden.
After each cohort was defined, demographic data (age, sex, race, income quartile based on ZIP postal code) were compared, as were data on primary payer, hospital demographics, LOS (≤5 days, defined as perioperative index), discharge type, inflation-adjusted charges in 2014 dollars based on the Consumer Price Indexes (http://www.bls.gov/cpi/), and mortality. Perioperative complications—respiratory, gastrointestinal, genitourinary, accidental puncture/laceration, central nervous system, wound dehiscence, device-related (including embolism, fibrosis, hemorrhage, pain, stenosis, or thrombus caused by any device, implant, or graft), cardiac, hematoma/seroma, acute respiratory distress syndrome, postoperative shock, VTE, postoperative infection complications, and intraoperative transfusions—were considered using ICD-9 codes (996.X-999.X and 99.X, respectively).20 Although commonly used to determine perioperative comorbidity burden using ICD-9 coding, the modified Charlson index was not considered because RA is a component of the index and would therefore bias the variable.3,21
Statistical analyses, including χ2 tests and 2-sample t tests, were performed for categorical and continuous variables, respectively. P < .05 was considered significant. Fisher exact test was used for cohorts with fewer than 5 occurrences. Multivariate logistic regression models were then calculated to determine the effect of RA on different outcomes and complications, with age, race, sex, hospital region, hospital type, number of hospital beds, primary payer, and hospital ownership as covariates. Statistical analyses were performed using the R statistical programming language.22
Results
Of the 34,970 patients who underwent SA between 2006 and 2011, 1674 (4.8%) had a diagnosis of RA and 33,296 (95.2%) did not. On average, patients with RA tended to be younger than patients without RA (66.4 vs 69.1 years; P < .001), and a larger percentage of RA patients were female (75.5% vs 54.4%; P < .001). Compared with non-RA patients, RA patients comprised a different ethnic group and had a different expected primary payer (P < .001). SA patients with and without RA did not differ in income quartile based on ZIP code, total number of hospital beds, hospital region, or hospital teaching status (P = .34, .78, .59, and .82, respectively) (Table 1).
LOS was significantly (P < .001) statistically longer for RA patients (2.196 days) than for non-RA patients (2.085 days). RA patients were significantly less likely to be discharged home (63.0% vs 67.6%; P < .001). (Routine discharge was defined as discharge home, whereas nonroutine discharge was defined as discharge to a short-term hospital, skilled nursing facility, intermediate care, another type of facility, home health care, against medical advice, or death.) In addition, inflation-adjusted charges associated with SA were significantly higher (P = .018) for RA patients ($54,284) than for non-RA patients ($52,663) (Table 1).
Regarding the rates of complications that occurred during the perioperative index, there were no significant differences between RA and non-RA cohorts. These complications included respiratory, gastrointestinal, genitourinary, accidental puncture/laceration, central nervous system, wound dehiscence, device-related, cardiac, hematoma/seroma, acute respiratory distress syndrome, postoperative shock, VTE, and postoperative infection (Table 2). In addition, there was no significant difference in mortality between the groups (P = .48).
In TSA, blood transfusions were more likely (P < .001) to be given to RA patients (9.00%) than to non-RA patients (6.16%). Multivariate regression analyses were performed with age, race, sex, hospital region, hospital type, number of hospital beds, primary payer, and hospital ownership as covariates. These analyses revealed that transfusion (P < .001), discharge type (P = .002), total inflation-adjusted charges (P < .001), and LOS (P = .047) remained significant (Table 3).
Discussion
Large national databases like NIS allow study of uncommon medical occurrences and help delineate risks and trends that otherwise might be indeterminable. Although it has been suggested that patients with RA may have poorer long-term outcomes after SA, the perioperative risk profile indicates that TSA is well tolerated in RA patients during the operative index.3,23-25
The data on this study’s 34,970 patients, drawn from the period 2006–2011, demonstrated no significant differences in safety profile with respect to the 14 perioperative complications and outcomes examined, except blood transfusion rate. Rates of postoperative infection (RA, 0.24%; non-RA, 0.14%; P = .303), VTE (RA, 0.30%; non-RA, 0.25%; P = .905), and transfusion (RA, 9.00%; non-RA, 6.16%; P < .001) are of particular interest because of the severity of these situations.
Postoperative infection is a potentially serious complication and often occurs secondary to diabetes, RA, lupus erythematosus, prior surgery, or a nosocomial or remote source.1 The often costly treatment options include antibiotic suppression, irrigation and debridement with implant retention, 1-stage exchange with antibiotic-impregnated cement fixation, staged reimplantation, resection arthroplasty, arthrodesis, and amputation.1 The overall 0.14% infection rate determined in this study is lower than the 0.7% reported for SA patients in the literature.1 Given the nature of the NIS database, this rate underestimates the true postoperative infection rate, as any infection that occurred after the perioperative period is not captured.26 The present study’s perioperative infection rates (RA, 0.24%; non-RA, 0.14%) for the period 2006–2011 are comparable to the rates (RA, 0.17%; non-RA, 0.24%) reported by Hambright and colleagues3 for the same patient population over the preceding, 18-year period (1988–2005) and similarly do not significantly differ between groups. Although infection is uncommon in the immediate perioperative period, the ICD-9 codes used refer specifically to infection resulting from surgery and do not represent concomitant infection.
VTEs, which include PEs and DVTs, are rare but potentially life-threatening surgical complications.27,28 Mechanical prophylaxis and chemical prophylaxis have been recommended for major orthopedic surgery, particularly lower extremity surgery, such as total hip arthroplasty (THA) and total knee arthroplasty (TKA).28,29 In the present study, VTE rates were low, 0.30% (RA) and 0.25% (non-RA), and not significantly different in bivariate or multivariate analyses. These rates are comparable to those found in other national-database SA studies.28 VTEs that occur outside the index hospital admission are not captured in this database. Therefore, the rates in the present study may be lower than the true incidence after SA. Mortality secondary to VTE usually occurs within 24 hours but may occur up to 90 days after surgery. DVT rates, on the other hand, are difficult to evaluate because of differences in screening practices.27,28,30,31
That RA patients were more likely than non-RA patients to receive perioperative blood transfusions supports prior findings that SA patients with RA were more likely than SA patients with osteoarthritis (OA) to receive perioperative blood transfusions.8 RA patients have been shown to have high rates of anemia of chronic disease, ranging from 22% to 77%.32 During joint replacement, these patients often require transfusions.32,33 However, these findings differ from prior findings of no differences between RA and non-RA patients in the same patient population during the period 1988–2005.3 This difference may be a product of the constantly changing transfusion guidelines and increased use; transfusion rates increased 140% between 1997 and 2007, making transfusions the fastest growing common procedure in the United States during that time.34 There was no difference between RA and non-RA patients in household income (as determined by ZIP code analysis), number of hospital beds, hospital region, or hospital teaching status. Compared with non-RA patients, RA patients were more likely to be younger, female, and of a difference race and to have a different expected primary payer (P < .001).These findings are consistent with previous findings in the literature.3 In the present SA study, however, RA patients were more likely than non-RA patients to have longer LOS, higher inflation-adjusted hospital charges, and nonroutine discharge. These findings deviate from those of the study covering the preceding 18 years (1988–2005).3 Despite the findings of a changing environment of care for RA patients, by Hambright and colleagues3 and Weiss and colleagues,35 the trend appears to have shifted. Both groups had shorter average LOS than either group from the preceding 18 years.3 Although statistically significant in bivariate analysis, the difference in LOS between the 2 groups differed by an average of 0.11 day (2 hours 24 minutes) and was not clinically relevant.
In addition, the higher charges for patients with RA represent a deviation from the preceding 18 years.3 Other studies have also shown that RA is associated with increased cost in TSA.36 Patients with RA often have rotator cuff pathology, indicating reverse SA may be used more frequently.37,38 The increased implant cost associated with reverse SA may account for the increased costs in RA patients.39 As mentioned, TSA type is not captured in the NIS database. In addition, that RA patients were less likely than non-RA patients to have routine discharge may indicate RA cases are more complex because of their complications.1,5,14,40 A recent study of complications in RA patients (1163 who underwent THA, 2692 who underwent TKA) found that THA patients with RA were significantly more likely than THA patients with OA to dislocate, and TKA patients with RA were significantly more likely than TKA patients with OA to develop an infection after surgery.41 Postoperative dislocation has been shown to increase hospital costs in other orthopedic procedures.42 Also, during TSA, patients with RA are more likely than patients with OA to receive intraoperative blood transfusions.8 These complications—combined with the fact that RA is a chronic, progressive, systemic inflammatory disease that can affect soft tissue and blood vessel wall healing and is associated with medications having potential side effects—could contribute to the apparent increased hospital charges and LOS.3,12,13,43 Factors that include surgeon preference, impact of primary payer, and hospital practice may also affect final charges. Total charges in the NIS database include administrative fees, hospital costs, device-related costs, operating room costs, and ancillary staff costs. Total charges do not include professional fees and differ from the total cost that represents the amount reimbursed by the payer. Charges tend to correlate with but overestimate the total costs.44
This study had several important limitations. As mentioned, only events that occur during the operative admission are captured in the NIS database, and thus postoperative complications or serious adverse events that lead to readmission cannot be identified. In addition, outpatient TSAs are not captured in the NIS database, and thus inclusion of only inpatient procedures yields higher average LOS and total charges.45 Given the limited granularity of ICD-9 coding, this study could not determine RA severity, estimated blood loss, length of surgery, complication severity, type of TSA procedure/prosthesis, or cause of death. Although commonly used to determine comorbidity burden, the modified Charlson index could not be used, and therefore could not be entered as a covariate in multivariate analysis. Furthermore, the NIS database does not include imaging or patient-reported outcomes information, such as improvements in pain or function, which are of crucial importance in considering surgery.
Conclusion
Our findings corroborated findings that the demographics and the perioperative safety profile for TSA were similar for patients with and without RA. The risk for complications or death in the perioperative period was low. Compared with non-RA patients, RA patients had significantly higher charges and longer LOS and were less likely to be discharged home after surgery. The 0.11-day difference in LOS, though statistically significant, was not clinically relevant. These findings differ from those for the preceding, 18-year period (1988–2005). Future research should focus on the causes of these changes.
Shoulder arthroplasty (SA), including total SA (TSA) and reverse TSA, is an effective surgical treatment for fracture and primary or secondary degenerative disease of the shoulder.1 Over the past few decades, use of SA has increased dramatically, from about 5000 cases in 1990 to 7000 in 2000 and more than 26,000 in 2008.1,2
Complications associated with SA generally are classified as perioperative (occurring during the operative index) or long-term (postdischarge).3 Long-term complications include implant loosening, instability, revision, infection, rotator cuff tear, neural injury, and deltoid detachment.1,4,5 Perioperative complications, which are less commonly reported, include intraoperative fracture, infection, neural injury, venous thromboembolic events (VTEs, including pulmonary embolism [PE] and deep vein thrombosis [DVT]), transfusion, and death.3,6-10
SA is an attractive treatment option for patients with rheumatoid arthritis (RA), as the effects of pain on these patients are greater in the shoulder joint than in any other joint.11 Patients with RA pose unique orthopedic surgical challenges, including any combination of decreased bone mineralization, poor capsular tissue integrity, and osteonecrosis.3,12 In addition, RA patients may be taking immunosuppressive medications that have severe side effects, and they may require multiple surgeries.12,13 These factors predispose patients with RA to complications that include infection and wound dehiscence.3,5,12-14
The complex nature of RA has prompted investigators to examine outcome measures in this patient group. Hambright and colleagues3 used the Nationwide Inpatient Sample (NIS) to examine perioperative outcomes in RA patients who underwent TSA between 1988 and 2005.3 They found that TSA patients with RA had shorter and less costly hospital stays and were more likely to have a routine discharge.3 Using the same patient population drawn from the period 2006–2011, we conducted a study to determine if this unexpected trend persists as the number of TSAs and quality of postoperative care continue to increase. Given the potential for anemia of chronic disease and the systemic inflammatory nature of RA, we hypothesized that the perioperative complication profile of RA patients would be worse than that of non-RA patients.
Materials and Methods
NIS data were acquired for the period 2006–2011. The NIS is the largest publicly available all-payer inpatient database, with a random 20% sample of about 1000 US hospitals accounting for 7 to 8 million inpatient stays. The database supplies weights used to estimate national totals, at about 35 million inpatient visits per year. NIS inpatient data are limited to the operative index. Postdischarge information is not available. The NIS is managed by the Healthcare Cost and Utilization Project, which is sponsored by the Agency for Healthcare Research and Quality. The quality of NIS data is assessed and validated by an independent contractor. NIS data have been widely used to examine perioperative outcomes.15-17
NIS data cover patient and hospital demographics, hospital length of stay (LOS), discharge status, payer information, charges, and perioperative outcomes and procedure/diagnosis codes (ICD-9; International Classification of Diseases, Ninth Revision18).
As our Institutional Review Board (IRB) reviewed the database and determined the project was not human subject research, IRB involvement was not required. This study paralleled successful efforts with similar RA and non-RA patients who had shoulder and elbow surgery.3,19 SA patients were identified by ICD-9 procedure code 81.80, but this code does not specify whether the prosthesis was unconstrained, semiconstrained, or constrained. ICD-9 coding also does not specify whether the TSA was traditional or reverse. Patients with RA were identified by ICD-9 diagnosis codes 714.0, 714.1, and 714.2. Patients without one of these codes were placed in the non-RA cohort. Patients with codes associated with pathologic fractures secondary to metastatic cancer or bone malignant neoplasm as a secondary or primary diagnosis and patients who had revision surgery indicated by code 81.83 were excluded, as they have a disproportionately higher comorbidity burden.
After each cohort was defined, demographic data (age, sex, race, income quartile based on ZIP postal code) were compared, as were data on primary payer, hospital demographics, LOS (≤5 days, defined as perioperative index), discharge type, inflation-adjusted charges in 2014 dollars based on the Consumer Price Indexes (http://www.bls.gov/cpi/), and mortality. Perioperative complications—respiratory, gastrointestinal, genitourinary, accidental puncture/laceration, central nervous system, wound dehiscence, device-related (including embolism, fibrosis, hemorrhage, pain, stenosis, or thrombus caused by any device, implant, or graft), cardiac, hematoma/seroma, acute respiratory distress syndrome, postoperative shock, VTE, postoperative infection complications, and intraoperative transfusions—were considered using ICD-9 codes (996.X-999.X and 99.X, respectively).20 Although commonly used to determine perioperative comorbidity burden using ICD-9 coding, the modified Charlson index was not considered because RA is a component of the index and would therefore bias the variable.3,21
Statistical analyses, including χ2 tests and 2-sample t tests, were performed for categorical and continuous variables, respectively. P < .05 was considered significant. Fisher exact test was used for cohorts with fewer than 5 occurrences. Multivariate logistic regression models were then calculated to determine the effect of RA on different outcomes and complications, with age, race, sex, hospital region, hospital type, number of hospital beds, primary payer, and hospital ownership as covariates. Statistical analyses were performed using the R statistical programming language.22
Results
Of the 34,970 patients who underwent SA between 2006 and 2011, 1674 (4.8%) had a diagnosis of RA and 33,296 (95.2%) did not. On average, patients with RA tended to be younger than patients without RA (66.4 vs 69.1 years; P < .001), and a larger percentage of RA patients were female (75.5% vs 54.4%; P < .001). Compared with non-RA patients, RA patients comprised a different ethnic group and had a different expected primary payer (P < .001). SA patients with and without RA did not differ in income quartile based on ZIP code, total number of hospital beds, hospital region, or hospital teaching status (P = .34, .78, .59, and .82, respectively) (Table 1).
LOS was significantly (P < .001) statistically longer for RA patients (2.196 days) than for non-RA patients (2.085 days). RA patients were significantly less likely to be discharged home (63.0% vs 67.6%; P < .001). (Routine discharge was defined as discharge home, whereas nonroutine discharge was defined as discharge to a short-term hospital, skilled nursing facility, intermediate care, another type of facility, home health care, against medical advice, or death.) In addition, inflation-adjusted charges associated with SA were significantly higher (P = .018) for RA patients ($54,284) than for non-RA patients ($52,663) (Table 1).
Regarding the rates of complications that occurred during the perioperative index, there were no significant differences between RA and non-RA cohorts. These complications included respiratory, gastrointestinal, genitourinary, accidental puncture/laceration, central nervous system, wound dehiscence, device-related, cardiac, hematoma/seroma, acute respiratory distress syndrome, postoperative shock, VTE, and postoperative infection (Table 2). In addition, there was no significant difference in mortality between the groups (P = .48).
In TSA, blood transfusions were more likely (P < .001) to be given to RA patients (9.00%) than to non-RA patients (6.16%). Multivariate regression analyses were performed with age, race, sex, hospital region, hospital type, number of hospital beds, primary payer, and hospital ownership as covariates. These analyses revealed that transfusion (P < .001), discharge type (P = .002), total inflation-adjusted charges (P < .001), and LOS (P = .047) remained significant (Table 3).
Discussion
Large national databases like NIS allow study of uncommon medical occurrences and help delineate risks and trends that otherwise might be indeterminable. Although it has been suggested that patients with RA may have poorer long-term outcomes after SA, the perioperative risk profile indicates that TSA is well tolerated in RA patients during the operative index.3,23-25
The data on this study’s 34,970 patients, drawn from the period 2006–2011, demonstrated no significant differences in safety profile with respect to the 14 perioperative complications and outcomes examined, except blood transfusion rate. Rates of postoperative infection (RA, 0.24%; non-RA, 0.14%; P = .303), VTE (RA, 0.30%; non-RA, 0.25%; P = .905), and transfusion (RA, 9.00%; non-RA, 6.16%; P < .001) are of particular interest because of the severity of these situations.
Postoperative infection is a potentially serious complication and often occurs secondary to diabetes, RA, lupus erythematosus, prior surgery, or a nosocomial or remote source.1 The often costly treatment options include antibiotic suppression, irrigation and debridement with implant retention, 1-stage exchange with antibiotic-impregnated cement fixation, staged reimplantation, resection arthroplasty, arthrodesis, and amputation.1 The overall 0.14% infection rate determined in this study is lower than the 0.7% reported for SA patients in the literature.1 Given the nature of the NIS database, this rate underestimates the true postoperative infection rate, as any infection that occurred after the perioperative period is not captured.26 The present study’s perioperative infection rates (RA, 0.24%; non-RA, 0.14%) for the period 2006–2011 are comparable to the rates (RA, 0.17%; non-RA, 0.24%) reported by Hambright and colleagues3 for the same patient population over the preceding, 18-year period (1988–2005) and similarly do not significantly differ between groups. Although infection is uncommon in the immediate perioperative period, the ICD-9 codes used refer specifically to infection resulting from surgery and do not represent concomitant infection.
VTEs, which include PEs and DVTs, are rare but potentially life-threatening surgical complications.27,28 Mechanical prophylaxis and chemical prophylaxis have been recommended for major orthopedic surgery, particularly lower extremity surgery, such as total hip arthroplasty (THA) and total knee arthroplasty (TKA).28,29 In the present study, VTE rates were low, 0.30% (RA) and 0.25% (non-RA), and not significantly different in bivariate or multivariate analyses. These rates are comparable to those found in other national-database SA studies.28 VTEs that occur outside the index hospital admission are not captured in this database. Therefore, the rates in the present study may be lower than the true incidence after SA. Mortality secondary to VTE usually occurs within 24 hours but may occur up to 90 days after surgery. DVT rates, on the other hand, are difficult to evaluate because of differences in screening practices.27,28,30,31
That RA patients were more likely than non-RA patients to receive perioperative blood transfusions supports prior findings that SA patients with RA were more likely than SA patients with osteoarthritis (OA) to receive perioperative blood transfusions.8 RA patients have been shown to have high rates of anemia of chronic disease, ranging from 22% to 77%.32 During joint replacement, these patients often require transfusions.32,33 However, these findings differ from prior findings of no differences between RA and non-RA patients in the same patient population during the period 1988–2005.3 This difference may be a product of the constantly changing transfusion guidelines and increased use; transfusion rates increased 140% between 1997 and 2007, making transfusions the fastest growing common procedure in the United States during that time.34 There was no difference between RA and non-RA patients in household income (as determined by ZIP code analysis), number of hospital beds, hospital region, or hospital teaching status. Compared with non-RA patients, RA patients were more likely to be younger, female, and of a difference race and to have a different expected primary payer (P < .001).These findings are consistent with previous findings in the literature.3 In the present SA study, however, RA patients were more likely than non-RA patients to have longer LOS, higher inflation-adjusted hospital charges, and nonroutine discharge. These findings deviate from those of the study covering the preceding 18 years (1988–2005).3 Despite the findings of a changing environment of care for RA patients, by Hambright and colleagues3 and Weiss and colleagues,35 the trend appears to have shifted. Both groups had shorter average LOS than either group from the preceding 18 years.3 Although statistically significant in bivariate analysis, the difference in LOS between the 2 groups differed by an average of 0.11 day (2 hours 24 minutes) and was not clinically relevant.
In addition, the higher charges for patients with RA represent a deviation from the preceding 18 years.3 Other studies have also shown that RA is associated with increased cost in TSA.36 Patients with RA often have rotator cuff pathology, indicating reverse SA may be used more frequently.37,38 The increased implant cost associated with reverse SA may account for the increased costs in RA patients.39 As mentioned, TSA type is not captured in the NIS database. In addition, that RA patients were less likely than non-RA patients to have routine discharge may indicate RA cases are more complex because of their complications.1,5,14,40 A recent study of complications in RA patients (1163 who underwent THA, 2692 who underwent TKA) found that THA patients with RA were significantly more likely than THA patients with OA to dislocate, and TKA patients with RA were significantly more likely than TKA patients with OA to develop an infection after surgery.41 Postoperative dislocation has been shown to increase hospital costs in other orthopedic procedures.42 Also, during TSA, patients with RA are more likely than patients with OA to receive intraoperative blood transfusions.8 These complications—combined with the fact that RA is a chronic, progressive, systemic inflammatory disease that can affect soft tissue and blood vessel wall healing and is associated with medications having potential side effects—could contribute to the apparent increased hospital charges and LOS.3,12,13,43 Factors that include surgeon preference, impact of primary payer, and hospital practice may also affect final charges. Total charges in the NIS database include administrative fees, hospital costs, device-related costs, operating room costs, and ancillary staff costs. Total charges do not include professional fees and differ from the total cost that represents the amount reimbursed by the payer. Charges tend to correlate with but overestimate the total costs.44
This study had several important limitations. As mentioned, only events that occur during the operative admission are captured in the NIS database, and thus postoperative complications or serious adverse events that lead to readmission cannot be identified. In addition, outpatient TSAs are not captured in the NIS database, and thus inclusion of only inpatient procedures yields higher average LOS and total charges.45 Given the limited granularity of ICD-9 coding, this study could not determine RA severity, estimated blood loss, length of surgery, complication severity, type of TSA procedure/prosthesis, or cause of death. Although commonly used to determine comorbidity burden, the modified Charlson index could not be used, and therefore could not be entered as a covariate in multivariate analysis. Furthermore, the NIS database does not include imaging or patient-reported outcomes information, such as improvements in pain or function, which are of crucial importance in considering surgery.
Conclusion
Our findings corroborated findings that the demographics and the perioperative safety profile for TSA were similar for patients with and without RA. The risk for complications or death in the perioperative period was low. Compared with non-RA patients, RA patients had significantly higher charges and longer LOS and were less likely to be discharged home after surgery. The 0.11-day difference in LOS, though statistically significant, was not clinically relevant. These findings differ from those for the preceding, 18-year period (1988–2005). Future research should focus on the causes of these changes.
1. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
3. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
4. van de Sande MA, Brand R, Rozing PM. Indications, complications, and results of shoulder arthroplasty. Scand J Rheumatol. 2006;35(6):426-434.
5. Wirth MA, Rockwood CA Jr. Complications of shoulder arthroplasty. Clin Orthop Relat Res. 1994;(307):47-69.
6. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):
1915-1923.
7. Sperling JW, Kozak TK, Hanssen AD, Cofield RH. Infection after shoulder arthroplasty. Clin Orthop Relat Res. 2001;(382):206-216.
8. Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(6):599-601.
9. Kumar S, Sperling JW, Haidukewych GH, Cofield RH. Periprosthetic humeral fractures after shoulder arthroplasty. J Bone Joint Surg Am. 2004;86(4):680-689.
10. Sperling JW, Cofield RH. Pulmonary embolism following shoulder arthroplasty. J Bone Joint Surg Am. 2002;84(11):1939-1941.
11. Tanaka E, Saito A, Kamitsuji S, et al. Impact of shoulder, elbow, and knee joint involvement on assessment of rheumatoid arthritis using the American College of Rheumatology core data set. Arthritis Rheum. 2005;53(6):864-871.
12. Nassar J, Cracchiolo A 3rd. Complications in surgery of the foot and ankle in patients with rheumatoid arthritis. Clin Orthop Relat Res. 2001;(391):140-152.
13. den Broeder AA, Creemers MC, Fransen J, et al. Risk factors for surgical site infections and other complications in elective surgery in patients with rheumatoid arthritis with special attention for anti-tumor necrosis factor: a large retrospective study. J Rheumatol. 2007;34(4):689-695.
14. Sanchez-Sotelo J. (i) Shoulder arthroplasty for osteoarthritis and rheumatoid arthritis. Curr Orthop. 2007;21(6):405-414.
15. Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project (HCUP). Overview of the National (Nationwide) Inpatient Sample (NIS). 2012. http://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed February 3, 2015.
16. Hervey SL, Purves HR, Guller U, Toth AP, Vail TP, Pietrobon R. Provider volume of total knee arthroplasties and patient outcomes in the HCUP-Nationwide Inpatient Sample. J Bone Joint Surg Am. 2003;85(9):1775-1783.
17. Noskin GA, Rubin RJ, Schentag JJ, et al. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample database. Arch Intern Med. 2005;165(15):1756-1761.
18. World Health Organization. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Geneva, Switzerland: World Health Organization; 2008.
19. Cook C, Hawkins R, Aldridge JM 3rd, Tolan S, Krupp R, Bolognesi M. Comparison of perioperative complications in patients with and without rheumatoid arthritis who receive total elbow replacement. J Shoulder Elbow Surg. 2009;18(1):21-26.
20. Goz V, Weinreb JH, McCarthy I, Schwab F, Lafage V, Errico TJ. Perioperative complications and mortality after spinal fusions: analysis of trends and risk factors. Spine. 2013;38(22):1970-1976.
21. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
22. R: a language and environment for statistical computing [computer program]. Vienna, Austria: Foundation for Statistical Computing; 2012.
23. Cuomo F, Greller MJ, Zuckerman JD. The rheumatoid shoulder. Rheum Dis Clin North Am. 1998;24(1):67-82.
24. Kelly IG, Foster RS, Fisher WD. Neer total shoulder replacement in rheumatoid arthritis. J Bone Joint Surg Br. 1987;69(5):723-726.
25. Donigan JA, Frisella WA, Haase D, Dolan L, Wolf B. Pre-operative and intra-operative factors related to shoulder arthroplasty outcomes. Iowa Orthop J. 2009;29:60-66.
26. Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479.
27. Willis AA, Warren RF, Craig EV, et al. Deep vein thrombosis after reconstructive shoulder arthroplasty: a prospective observational study. J Shoulder Elbow Surg. 2009;18(1):100-106.
28. Jameson SS, James P, Howcroft DW, et al. Venous thromboembolic events are rare after shoulder surgery: analysis of a national database. J Shoulder Elbow Surg. 2011;20(5):
764-770.
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest J. 2012;141(2 suppl):e278S-e325S.
30. White CB, Sperling JW, Cofield RH, Rowland CM. Ninety-day mortality after shoulder arthroplasty. J Arthroplasty. 2003;18(7):886-888.
31. Lussana F, Squizzato A, Permunian ET, Cattaneo M. A systematic review on the effect of aspirin in the prevention of post-operative arterial thrombosis in patients undergoing total hip and total knee arthroplasty. Thromb Res. 2014;134(3):599-603.
32. Wilson A, Yu H, Goodnough LT, Nissenson AR. Prevalence and outcomes of anemia in rheumatoid arthritis: a systematic review of the literature. Am J Med. 2004;116(7):50-57.
33. Mercuriali F, Gualtieri G, Sinigaglia L, et al. Use of recombinant human erythropoietin to assist autologous blood donation by anemic rheumatoid arthritis patients undergoing major orthopedic surgery. Transfusion. 1994;34(6):501-506.
34. Shander A, Gross I, Hill S, et al. A new perspective on best transfusion practices. Blood Transfus. 2013;11(2):193-202.
35. Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatology. 2008;47(4):491-494.
36. Davis DE, Paxton ES, Maltenfort M, Abboud J. Factors affecting hospital charges after total shoulder arthroplasty: an evaluation of the national inpatient sample database.
J Shoulder Elbow Surg. 2014;23(12):1860-1866.
37. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
38. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
39. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288.
40. Garner RW, Mowat AG, Hazleman BL. Wound healing after operations of patients with rheumatoid arthritis. J Bone Joint Surg Br. 1973;55(1):134-144.
41. Ravi B, Croxford R, Hollands S, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263.
42. Sanchez-Sotelo J, Haidukewych GJ, Boberg CJ. Hospital cost of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2006;88(2):290-294.
43. Ward MM. Decreases in rates of hospitalizations for manifestations of severe rheumatoid arthritis, 1983-2001. Arthritis Rheum. 2004;50(4):1122-1131.
44. Goz V, Weinreb JH, Schwab F, Lafage V, Errico TJ. Comparison of complications, costs, and length of stay of three different lumbar interbody fusion techniques: an analysis of the Nationwide Inpatient Sample database. Spine J. 2014;14(9):2019-2027.
45. Goz V, Errico TJ, Weinreb JH, et al. Vertebroplasty and kyphoplasty: national outcomes and trends in utilization from 2005 through 2010. Spine J. 2015;15(5):959-965.
1. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
3. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
4. van de Sande MA, Brand R, Rozing PM. Indications, complications, and results of shoulder arthroplasty. Scand J Rheumatol. 2006;35(6):426-434.
5. Wirth MA, Rockwood CA Jr. Complications of shoulder arthroplasty. Clin Orthop Relat Res. 1994;(307):47-69.
6. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):
1915-1923.
7. Sperling JW, Kozak TK, Hanssen AD, Cofield RH. Infection after shoulder arthroplasty. Clin Orthop Relat Res. 2001;(382):206-216.
8. Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(6):599-601.
9. Kumar S, Sperling JW, Haidukewych GH, Cofield RH. Periprosthetic humeral fractures after shoulder arthroplasty. J Bone Joint Surg Am. 2004;86(4):680-689.
10. Sperling JW, Cofield RH. Pulmonary embolism following shoulder arthroplasty. J Bone Joint Surg Am. 2002;84(11):1939-1941.
11. Tanaka E, Saito A, Kamitsuji S, et al. Impact of shoulder, elbow, and knee joint involvement on assessment of rheumatoid arthritis using the American College of Rheumatology core data set. Arthritis Rheum. 2005;53(6):864-871.
12. Nassar J, Cracchiolo A 3rd. Complications in surgery of the foot and ankle in patients with rheumatoid arthritis. Clin Orthop Relat Res. 2001;(391):140-152.
13. den Broeder AA, Creemers MC, Fransen J, et al. Risk factors for surgical site infections and other complications in elective surgery in patients with rheumatoid arthritis with special attention for anti-tumor necrosis factor: a large retrospective study. J Rheumatol. 2007;34(4):689-695.
14. Sanchez-Sotelo J. (i) Shoulder arthroplasty for osteoarthritis and rheumatoid arthritis. Curr Orthop. 2007;21(6):405-414.
15. Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project (HCUP). Overview of the National (Nationwide) Inpatient Sample (NIS). 2012. http://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed February 3, 2015.
16. Hervey SL, Purves HR, Guller U, Toth AP, Vail TP, Pietrobon R. Provider volume of total knee arthroplasties and patient outcomes in the HCUP-Nationwide Inpatient Sample. J Bone Joint Surg Am. 2003;85(9):1775-1783.
17. Noskin GA, Rubin RJ, Schentag JJ, et al. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample database. Arch Intern Med. 2005;165(15):1756-1761.
18. World Health Organization. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Geneva, Switzerland: World Health Organization; 2008.
19. Cook C, Hawkins R, Aldridge JM 3rd, Tolan S, Krupp R, Bolognesi M. Comparison of perioperative complications in patients with and without rheumatoid arthritis who receive total elbow replacement. J Shoulder Elbow Surg. 2009;18(1):21-26.
20. Goz V, Weinreb JH, McCarthy I, Schwab F, Lafage V, Errico TJ. Perioperative complications and mortality after spinal fusions: analysis of trends and risk factors. Spine. 2013;38(22):1970-1976.
21. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
22. R: a language and environment for statistical computing [computer program]. Vienna, Austria: Foundation for Statistical Computing; 2012.
23. Cuomo F, Greller MJ, Zuckerman JD. The rheumatoid shoulder. Rheum Dis Clin North Am. 1998;24(1):67-82.
24. Kelly IG, Foster RS, Fisher WD. Neer total shoulder replacement in rheumatoid arthritis. J Bone Joint Surg Br. 1987;69(5):723-726.
25. Donigan JA, Frisella WA, Haase D, Dolan L, Wolf B. Pre-operative and intra-operative factors related to shoulder arthroplasty outcomes. Iowa Orthop J. 2009;29:60-66.
26. Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479.
27. Willis AA, Warren RF, Craig EV, et al. Deep vein thrombosis after reconstructive shoulder arthroplasty: a prospective observational study. J Shoulder Elbow Surg. 2009;18(1):100-106.
28. Jameson SS, James P, Howcroft DW, et al. Venous thromboembolic events are rare after shoulder surgery: analysis of a national database. J Shoulder Elbow Surg. 2011;20(5):
764-770.
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest J. 2012;141(2 suppl):e278S-e325S.
30. White CB, Sperling JW, Cofield RH, Rowland CM. Ninety-day mortality after shoulder arthroplasty. J Arthroplasty. 2003;18(7):886-888.
31. Lussana F, Squizzato A, Permunian ET, Cattaneo M. A systematic review on the effect of aspirin in the prevention of post-operative arterial thrombosis in patients undergoing total hip and total knee arthroplasty. Thromb Res. 2014;134(3):599-603.
32. Wilson A, Yu H, Goodnough LT, Nissenson AR. Prevalence and outcomes of anemia in rheumatoid arthritis: a systematic review of the literature. Am J Med. 2004;116(7):50-57.
33. Mercuriali F, Gualtieri G, Sinigaglia L, et al. Use of recombinant human erythropoietin to assist autologous blood donation by anemic rheumatoid arthritis patients undergoing major orthopedic surgery. Transfusion. 1994;34(6):501-506.
34. Shander A, Gross I, Hill S, et al. A new perspective on best transfusion practices. Blood Transfus. 2013;11(2):193-202.
35. Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatology. 2008;47(4):491-494.
36. Davis DE, Paxton ES, Maltenfort M, Abboud J. Factors affecting hospital charges after total shoulder arthroplasty: an evaluation of the national inpatient sample database.
J Shoulder Elbow Surg. 2014;23(12):1860-1866.
37. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
38. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
39. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288.
40. Garner RW, Mowat AG, Hazleman BL. Wound healing after operations of patients with rheumatoid arthritis. J Bone Joint Surg Br. 1973;55(1):134-144.
41. Ravi B, Croxford R, Hollands S, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263.
42. Sanchez-Sotelo J, Haidukewych GJ, Boberg CJ. Hospital cost of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2006;88(2):290-294.
43. Ward MM. Decreases in rates of hospitalizations for manifestations of severe rheumatoid arthritis, 1983-2001. Arthritis Rheum. 2004;50(4):1122-1131.
44. Goz V, Weinreb JH, Schwab F, Lafage V, Errico TJ. Comparison of complications, costs, and length of stay of three different lumbar interbody fusion techniques: an analysis of the Nationwide Inpatient Sample database. Spine J. 2014;14(9):2019-2027.
45. Goz V, Errico TJ, Weinreb JH, et al. Vertebroplasty and kyphoplasty: national outcomes and trends in utilization from 2005 through 2010. Spine J. 2015;15(5):959-965.