User login
Grand Rounds: Boy, 10, With Knee Pain
A 10-year-old boy first complained of right knee pain two months prior to presentation. There was no traumatic event to explain the pain and no prior viral or bacterial illness. Radiographs taken earlier at another facility were initially pronounced normal. One month later, repeat x-rays showed a possible hairline fracture, and MRI was ordered. MRI documented a destructive lesion in the right distal femur with a soft-tissue mass that was worrisome for primary bone malignancy.
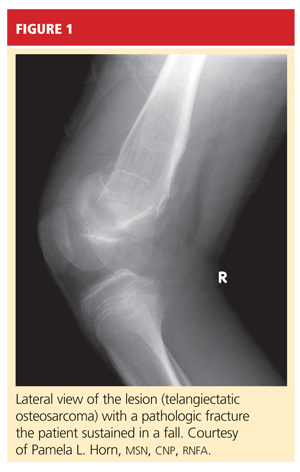
The boy was placed on weight-bearing restrictions and was given a wheelchair. Unfortunately, he fell from the wheelchair and sustained a pathologic fracture through the lesion (see Figure 1). He was transported to the hospital and admitted. A biopsy was performed with a closed reduction, as the fracture was maligned. The patient was placed in a long leg cast with a pelvic band.
His history was previously unremarkable. He was taking no medications and had experienced no recent illnesses. His surgical/medical history was positive for a tonsillectomy at an early age and a fracture of the right proximal femur at age 2. On examination, he was noted to be talkative with his family but guarded during conversations with staff.
His physical exam was positive for pain at the right distal femur and knee with palpation; otherwise, all other systems were unremarkable. The patient was in too much pain to range the knee and had been placed in a long posterior leg splint (prior to surgery and application of the cast). Distally, his right lower extremity motor and sensory function were intact.
The patient’s vital signs were within normal limits, and results from his blood chemistries and alkaline phosphatase and C-reactive protein levels were unremarkable. Findings on the complete blood cell count were slightly abnormal: Hemoglobin was 11 g and the hematocrit, 33% (both within normal limits); however, in the differential there was an elevation in segmented neutrophils (72%, compared with a reference range of 31% to 61%), with Döhle bodies present—possibly signifying acute and/or chronic systemic infection or malignancy. The lymphocyte count represented 11% of the total white blood cell count (range, 28% to 48%), and platelets were 82 x 103/mL (normal range, 150 to 350 x 103/mL). The patient’s erythrocyte sedimentation rate was 44 mm/h (normal range, 0 to 20).
Result from pathology were positive for osteosarcoma, telangiectatic type. The patient underwent a nuclear medicine bone scan that showed no metastases, and chest CT was negative for pulmonary lesions as well. After a psychology consult, the boy was gently told about his condition.
Treatment then proceeded, including surgical placement of a double-lumen chest catheter for delivery of neoadjuvant and adjuvant chemotherapy. Doxorubicin, cisplatin, and methotrexate were used because the boy was enrolled in an international cooperative trial through the Children’s Oncology Group for treatment of localized osteosarcoma.
Discussion
Osteosarcoma (OS) is the most common primary bone malignancy.1,2 Approximately 5% of all pediatric patients with tumors present with this diagnosis, and about 400 new cases are diagnosed in the United States each year.1 Most osteosarcomas develop in the bones of the lower extremities and in the humerus, affecting males more often than females.1-3 This kind of malignancy is frequently seen during the adolescent growth spurt, but it can affect patients of any age.1,2 Patients usually present with pain or functional limitation in gait or daily activities or both.1-3
The telangiectatic subtype of OS is a rare, aggressive variant that represents 2% to 12% of all cases of OS.4-6 Telangiectatic OS (TOS) is characterized by multiple aneurysmally dilated, blood-filled cavities with high-grade sarcomatous cells seen in the peripheral rim and septae.3,7,8 This process can cause the lesion to resemble an aneurysmal bone cyst, explaining why some cases of TOS are misdiagnosed—with delayed time to treatment and increased morbidity and mortality.3,5 Generally, TOS patients are more likely than other OS patients to have tumors of femoral location, larger lesions, and normal alkaline phosphatase values. Many have pathologic fractures on presentation.7
The medical literature chronicles a long debate regarding the difference in mortality between patients with OS and those with TOS. It was once believed that patients with TOS were at higher risk for recurrence (especially those with a pathologic fracture) and mortality. However, in recent studies examining newer neoadjuvant and adjuvant chemotherapies, mortality rates for the two conditions are similar and certainly lower than they were many years ago.7,8 In one study, a better histologic response was reported to neoadjuvant chemotherapy in patients with TOS than with OS.7
Diagnosis
The first diagnostic tool used for patients with suspected OS or TOS is a plain radiographic film. A TOS lesion is lytic, with no areas of sclerosis, and almost always involves the long bones. It is poorly defined, destroying the cortex with formation of periosteal bone and invading the soft tissue. An initial pattern of parallel striations is highly suggestive of TOS.5
MRI and CT often reveal thick nodular tissue in a largely hemorrhagic and/or necrotic osseous lesion, with an associated soft-tissue mass that allows distinction from an aneurysmal bone cyst.3 Next, patients generally undergo a nuclear medicine bone scan and CT of the chest to observe for signs of metastases. Chest CT is commonly repeated on a regular basis during and after treatment.9
Pathologic evaluation, the final step to diagnosis, is very important, especially in the effort to differentiate TOS from an aneurysmal bone cyst. The typical gross findings for a TOS tumor include a dominant cystic cavity–like architecture, with a pushing peripheral margin that frequently expands and erodes the adjacent cortex and extends into the surrounding tissue. There is usually no area of intramural bone tissue.
Microscopically, the cystic areas contain clots and fragments of tumor that are often lined with a layer of neoplasm. The blood-filled telangiectatic spaces form in these areas. The spaces are irregularly shaped and typically traversed by septae composed in part of neoplastic cells. Osteoid formation through these cells can appear as a fine, ice-like material between tumor cells.4,7
Treatment
The main goals of treatment are to limit the anatomical extent of the disease, decrease the possibility of recurrence, and restore the highest possible level of function.2 Initial treatment of any OS or TOS consists of aggressive, immediate chemotherapy prior to and after any surgical intervention.1 (Chemotherapy will not be discussed in further detail here.) Surgical treatments for patients younger than 14 include amputation (above the lesion with wide margins), an expanding prosthesis, or rotationplasty. The location and extent of the tumor, the patient’s age, and his or her desired lifestyle will all have an impact on the choice of surgery.10
Historic data demonstrate that patients who undergo amputation alone almost always develop metastatic disease.1 Other data show that only 10% of patients with OS have been cured by chemotherapy alone. Yet when medical treatment is combined with surgical treatment, the overall expected cure rate can be as high as 65%.2
Discussing amputation with a young patient and the family can be emotionally difficult. If functional levels are to be restored, above-knee amputation (AKA) is the least favored surgical method. Compared with healthy individuals, patients who undergo AKA will walk 43% less quickly and will expend much more energy. These patients frequently have an inefficient gait and, given their limited reserve, they may lose the ability to walk altogether.2
Reconstructive surgical options include limb-salvage procedures; since the late 1980s, these have become the standard of care for OS at all sites.11 One such option includes removal of the lesion (eg, a distal femoral or proximal tibial lesion) with acceptable margins and replacement of the lost bone with an allograft or with a metallic prosthesis and knee joint (called arthroplasty). This endoprosthesis expands as the child grows (by way of a minor surgical procedure or a magnetic spring) so there is no apparent discrepancy between limb lengths, and the patient’s appearance is as normal and socially acceptable as possible.1,2
Because the case patient developed a pathologic fracture through his TOS tumor, he was not a candidate for endoprosthesis. His options were AKA or rotationplasty.
This procedure was first described in 195012 for treatment of proximal focal femoral deficiency. It is considered an alternative for skeletally immature individuals for whom the goal is to preserve function.
When AKA is indicated, the lower limb can be salvaged to allow functioning similar to that of a patient with a below-knee amputation (BKA). During rotationplasty, all but the most proximal aspect of the femur is resected. The tibia is externally rotated on the axis of the neurovascular bundle, then an arthrodesis of the proximal portion of the femur and the tibial plateau is performed (see Figure 2).
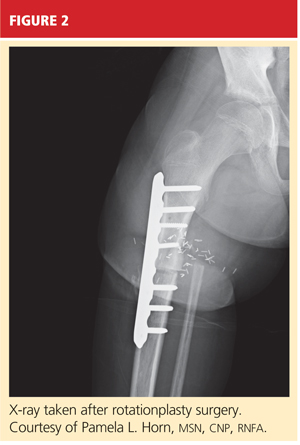
The end result is an extremity with the appearance, dimensions, and functional potential of a BKA. The ankle is rotated 180° so that it can serve as the new knee joint, and the attached foot, now pointing in the opposite direction, acts as the residual limb for fitting a prosthesis.2 This procedure is favored in patients with an extensive soft-tissue mass, intra-articular extension of the tumor, and/or pathologic fractures. It can also help prevent phantom pain.13
The Case Patient
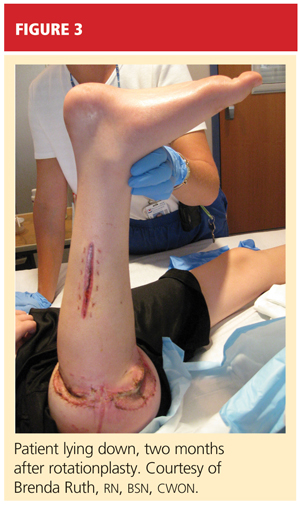
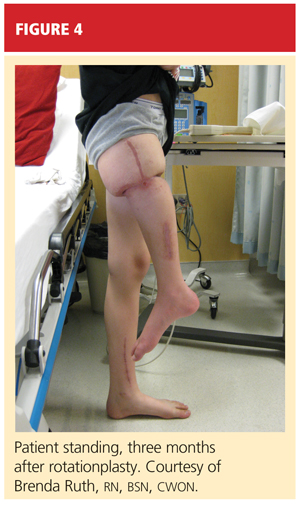
After psychological evaluation of the patient and extensive family discussion, he underwent successful rotationplasty. The day after his surgery, however, he developed compartment syndrome and was required to undergo fasciotomies of the calf and proximal thigh. His wounds were treated, a skin graft was performed to close the proximal thigh wound, and his calf wounds were sutured closed (see Figures 3 and 4). His hip range of motion is excellent, and his ankle range of motion continues to improve with physical therapy.
At this writing, the patient was scheduled for his first prosthetic fitting, and he had nearly completed his chemotherapy. His outlook is very promising.
Conclusion
TOS is a rare, aggressive subtype of OS but the most common primary malignant bone tumor of childhood. In the past, outcomes in patients treated with surgery alone were poor. With the advent of chemotherapy and the combination of medical and surgical treatment, TOS-associated mortality has continued to decline. There is no significant difference in outcomes among the available surgical options, but limb-salvage surgical procedures usually offer patients much better function and quality of life. The most important consideration is early diagnosis followed by immediate treatment.
1. Siegel HJ, Pressey JG. Current concepts on the surgical and medical management of osteosarcoma. Expert Rev Anticancer Ther. 2008;8(8):1257-1269.
2. Marulanda GA, Henderson ER, Johnson DA, et al. Orthopedic surgery options for the treatment of primary osteosarcoma. Cancer Control. 2008;15(1):13-20.
3. Murphey MD, wan Jaovisidha S, Temple HT, et al. Telangiectatic osteosarcoma: radiologic-pathologic comparison. Radiology. 2003;229(2):545-553.
4. Mervak TR, Unni KK, Pritchard DJ, McLeod RA. Telangiectatic osteosarcoma. Clin Orthop Relat Res. 1991 Sep;270:135-139.
5. Vanel D, Tcheng S, Contesso G, et al. The radiological appearances of telangiectatic osteosarcoma: a study of 14 cases. Skeletal Radiol. 1987;16(3):196-200.
6. Ferrari S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23(34):8845-8852.
7. Bacci G, Ferrari S, Ruggieri P, et al. Telangiectatic osteosarcoma of the extremity: neoadjuvant chemotherapy in 24 cases. Acta Orthop Scand. 2001;72(2):167-172.
8. Weiss A, Khoury JD, Hoffer FA, et al. Telangiectatic osteosarcoma: the St. Jude Children’s Research Hospital’s experience. Cancer. 2007;109(8):1627-1637.
9. Agarwal M, Anchan C, Shah M, et al. Limb salvage surgery for osteosarcoma: effective low-cost treatment. Clin Orthop Relat Res. 2007;459:82-91.
10. Bacci G, Ferrari S, Lari S, et al. Osteosarcoma of the limb: amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84(1):88-92.
11. Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68(9):1331-1337.
12. Van Nes CP. Rotation-plasty for congenital defects of the femur: making use of the shortened limb to control the knee joint of a prosthesis. J Bone Joint Surg. 1950;32B:12-16.
13. Sawamura C, Hornicek FJ, Gebhardt MC. Complications and risk factors for failure of rotationplasty: review of 25 patients. Clin Orthop Relat Res. 2008;466(6):1302-1308.
A 10-year-old boy first complained of right knee pain two months prior to presentation. There was no traumatic event to explain the pain and no prior viral or bacterial illness. Radiographs taken earlier at another facility were initially pronounced normal. One month later, repeat x-rays showed a possible hairline fracture, and MRI was ordered. MRI documented a destructive lesion in the right distal femur with a soft-tissue mass that was worrisome for primary bone malignancy.
The boy was placed on weight-bearing restrictions and was given a wheelchair. Unfortunately, he fell from the wheelchair and sustained a pathologic fracture through the lesion (see Figure 1). He was transported to the hospital and admitted. A biopsy was performed with a closed reduction, as the fracture was maligned. The patient was placed in a long leg cast with a pelvic band.

His history was previously unremarkable. He was taking no medications and had experienced no recent illnesses. His surgical/medical history was positive for a tonsillectomy at an early age and a fracture of the right proximal femur at age 2. On examination, he was noted to be talkative with his family but guarded during conversations with staff.
His physical exam was positive for pain at the right distal femur and knee with palpation; otherwise, all other systems were unremarkable. The patient was in too much pain to range the knee and had been placed in a long posterior leg splint (prior to surgery and application of the cast). Distally, his right lower extremity motor and sensory function were intact.
The patient’s vital signs were within normal limits, and results from his blood chemistries and alkaline phosphatase and C-reactive protein levels were unremarkable. Findings on the complete blood cell count were slightly abnormal: Hemoglobin was 11 g and the hematocrit, 33% (both within normal limits); however, in the differential there was an elevation in segmented neutrophils (72%, compared with a reference range of 31% to 61%), with Döhle bodies present—possibly signifying acute and/or chronic systemic infection or malignancy. The lymphocyte count represented 11% of the total white blood cell count (range, 28% to 48%), and platelets were 82 x 103/mL (normal range, 150 to 350 x 103/mL). The patient’s erythrocyte sedimentation rate was 44 mm/h (normal range, 0 to 20).
Result from pathology were positive for osteosarcoma, telangiectatic type. The patient underwent a nuclear medicine bone scan that showed no metastases, and chest CT was negative for pulmonary lesions as well. After a psychology consult, the boy was gently told about his condition.
Treatment then proceeded, including surgical placement of a double-lumen chest catheter for delivery of neoadjuvant and adjuvant chemotherapy. Doxorubicin, cisplatin, and methotrexate were used because the boy was enrolled in an international cooperative trial through the Children’s Oncology Group for treatment of localized osteosarcoma.
Discussion
Osteosarcoma (OS) is the most common primary bone malignancy.1,2 Approximately 5% of all pediatric patients with tumors present with this diagnosis, and about 400 new cases are diagnosed in the United States each year.1 Most osteosarcomas develop in the bones of the lower extremities and in the humerus, affecting males more often than females.1-3 This kind of malignancy is frequently seen during the adolescent growth spurt, but it can affect patients of any age.1,2 Patients usually present with pain or functional limitation in gait or daily activities or both.1-3
The telangiectatic subtype of OS is a rare, aggressive variant that represents 2% to 12% of all cases of OS.4-6 Telangiectatic OS (TOS) is characterized by multiple aneurysmally dilated, blood-filled cavities with high-grade sarcomatous cells seen in the peripheral rim and septae.3,7,8 This process can cause the lesion to resemble an aneurysmal bone cyst, explaining why some cases of TOS are misdiagnosed—with delayed time to treatment and increased morbidity and mortality.3,5 Generally, TOS patients are more likely than other OS patients to have tumors of femoral location, larger lesions, and normal alkaline phosphatase values. Many have pathologic fractures on presentation.7
The medical literature chronicles a long debate regarding the difference in mortality between patients with OS and those with TOS. It was once believed that patients with TOS were at higher risk for recurrence (especially those with a pathologic fracture) and mortality. However, in recent studies examining newer neoadjuvant and adjuvant chemotherapies, mortality rates for the two conditions are similar and certainly lower than they were many years ago.7,8 In one study, a better histologic response was reported to neoadjuvant chemotherapy in patients with TOS than with OS.7
Diagnosis
The first diagnostic tool used for patients with suspected OS or TOS is a plain radiographic film. A TOS lesion is lytic, with no areas of sclerosis, and almost always involves the long bones. It is poorly defined, destroying the cortex with formation of periosteal bone and invading the soft tissue. An initial pattern of parallel striations is highly suggestive of TOS.5
MRI and CT often reveal thick nodular tissue in a largely hemorrhagic and/or necrotic osseous lesion, with an associated soft-tissue mass that allows distinction from an aneurysmal bone cyst.3 Next, patients generally undergo a nuclear medicine bone scan and CT of the chest to observe for signs of metastases. Chest CT is commonly repeated on a regular basis during and after treatment.9
Pathologic evaluation, the final step to diagnosis, is very important, especially in the effort to differentiate TOS from an aneurysmal bone cyst. The typical gross findings for a TOS tumor include a dominant cystic cavity–like architecture, with a pushing peripheral margin that frequently expands and erodes the adjacent cortex and extends into the surrounding tissue. There is usually no area of intramural bone tissue.
Microscopically, the cystic areas contain clots and fragments of tumor that are often lined with a layer of neoplasm. The blood-filled telangiectatic spaces form in these areas. The spaces are irregularly shaped and typically traversed by septae composed in part of neoplastic cells. Osteoid formation through these cells can appear as a fine, ice-like material between tumor cells.4,7
Treatment
The main goals of treatment are to limit the anatomical extent of the disease, decrease the possibility of recurrence, and restore the highest possible level of function.2 Initial treatment of any OS or TOS consists of aggressive, immediate chemotherapy prior to and after any surgical intervention.1 (Chemotherapy will not be discussed in further detail here.) Surgical treatments for patients younger than 14 include amputation (above the lesion with wide margins), an expanding prosthesis, or rotationplasty. The location and extent of the tumor, the patient’s age, and his or her desired lifestyle will all have an impact on the choice of surgery.10
Historic data demonstrate that patients who undergo amputation alone almost always develop metastatic disease.1 Other data show that only 10% of patients with OS have been cured by chemotherapy alone. Yet when medical treatment is combined with surgical treatment, the overall expected cure rate can be as high as 65%.2
Discussing amputation with a young patient and the family can be emotionally difficult. If functional levels are to be restored, above-knee amputation (AKA) is the least favored surgical method. Compared with healthy individuals, patients who undergo AKA will walk 43% less quickly and will expend much more energy. These patients frequently have an inefficient gait and, given their limited reserve, they may lose the ability to walk altogether.2
Reconstructive surgical options include limb-salvage procedures; since the late 1980s, these have become the standard of care for OS at all sites.11 One such option includes removal of the lesion (eg, a distal femoral or proximal tibial lesion) with acceptable margins and replacement of the lost bone with an allograft or with a metallic prosthesis and knee joint (called arthroplasty). This endoprosthesis expands as the child grows (by way of a minor surgical procedure or a magnetic spring) so there is no apparent discrepancy between limb lengths, and the patient’s appearance is as normal and socially acceptable as possible.1,2
Because the case patient developed a pathologic fracture through his TOS tumor, he was not a candidate for endoprosthesis. His options were AKA or rotationplasty.
This procedure was first described in 195012 for treatment of proximal focal femoral deficiency. It is considered an alternative for skeletally immature individuals for whom the goal is to preserve function.
When AKA is indicated, the lower limb can be salvaged to allow functioning similar to that of a patient with a below-knee amputation (BKA). During rotationplasty, all but the most proximal aspect of the femur is resected. The tibia is externally rotated on the axis of the neurovascular bundle, then an arthrodesis of the proximal portion of the femur and the tibial plateau is performed (see Figure 2).

The end result is an extremity with the appearance, dimensions, and functional potential of a BKA. The ankle is rotated 180° so that it can serve as the new knee joint, and the attached foot, now pointing in the opposite direction, acts as the residual limb for fitting a prosthesis.2 This procedure is favored in patients with an extensive soft-tissue mass, intra-articular extension of the tumor, and/or pathologic fractures. It can also help prevent phantom pain.13

The Case Patient
After psychological evaluation of the patient and extensive family discussion, he underwent successful rotationplasty. The day after his surgery, however, he developed compartment syndrome and was required to undergo fasciotomies of the calf and proximal thigh. His wounds were treated, a skin graft was performed to close the proximal thigh wound, and his calf wounds were sutured closed (see Figures 3 and 4). His hip range of motion is excellent, and his ankle range of motion continues to improve with physical therapy.

At this writing, the patient was scheduled for his first prosthetic fitting, and he had nearly completed his chemotherapy. His outlook is very promising.
Conclusion
TOS is a rare, aggressive subtype of OS but the most common primary malignant bone tumor of childhood. In the past, outcomes in patients treated with surgery alone were poor. With the advent of chemotherapy and the combination of medical and surgical treatment, TOS-associated mortality has continued to decline. There is no significant difference in outcomes among the available surgical options, but limb-salvage surgical procedures usually offer patients much better function and quality of life. The most important consideration is early diagnosis followed by immediate treatment.
A 10-year-old boy first complained of right knee pain two months prior to presentation. There was no traumatic event to explain the pain and no prior viral or bacterial illness. Radiographs taken earlier at another facility were initially pronounced normal. One month later, repeat x-rays showed a possible hairline fracture, and MRI was ordered. MRI documented a destructive lesion in the right distal femur with a soft-tissue mass that was worrisome for primary bone malignancy.
The boy was placed on weight-bearing restrictions and was given a wheelchair. Unfortunately, he fell from the wheelchair and sustained a pathologic fracture through the lesion (see Figure 1). He was transported to the hospital and admitted. A biopsy was performed with a closed reduction, as the fracture was maligned. The patient was placed in a long leg cast with a pelvic band.

His history was previously unremarkable. He was taking no medications and had experienced no recent illnesses. His surgical/medical history was positive for a tonsillectomy at an early age and a fracture of the right proximal femur at age 2. On examination, he was noted to be talkative with his family but guarded during conversations with staff.
His physical exam was positive for pain at the right distal femur and knee with palpation; otherwise, all other systems were unremarkable. The patient was in too much pain to range the knee and had been placed in a long posterior leg splint (prior to surgery and application of the cast). Distally, his right lower extremity motor and sensory function were intact.
The patient’s vital signs were within normal limits, and results from his blood chemistries and alkaline phosphatase and C-reactive protein levels were unremarkable. Findings on the complete blood cell count were slightly abnormal: Hemoglobin was 11 g and the hematocrit, 33% (both within normal limits); however, in the differential there was an elevation in segmented neutrophils (72%, compared with a reference range of 31% to 61%), with Döhle bodies present—possibly signifying acute and/or chronic systemic infection or malignancy. The lymphocyte count represented 11% of the total white blood cell count (range, 28% to 48%), and platelets were 82 x 103/mL (normal range, 150 to 350 x 103/mL). The patient’s erythrocyte sedimentation rate was 44 mm/h (normal range, 0 to 20).
Result from pathology were positive for osteosarcoma, telangiectatic type. The patient underwent a nuclear medicine bone scan that showed no metastases, and chest CT was negative for pulmonary lesions as well. After a psychology consult, the boy was gently told about his condition.
Treatment then proceeded, including surgical placement of a double-lumen chest catheter for delivery of neoadjuvant and adjuvant chemotherapy. Doxorubicin, cisplatin, and methotrexate were used because the boy was enrolled in an international cooperative trial through the Children’s Oncology Group for treatment of localized osteosarcoma.
Discussion
Osteosarcoma (OS) is the most common primary bone malignancy.1,2 Approximately 5% of all pediatric patients with tumors present with this diagnosis, and about 400 new cases are diagnosed in the United States each year.1 Most osteosarcomas develop in the bones of the lower extremities and in the humerus, affecting males more often than females.1-3 This kind of malignancy is frequently seen during the adolescent growth spurt, but it can affect patients of any age.1,2 Patients usually present with pain or functional limitation in gait or daily activities or both.1-3
The telangiectatic subtype of OS is a rare, aggressive variant that represents 2% to 12% of all cases of OS.4-6 Telangiectatic OS (TOS) is characterized by multiple aneurysmally dilated, blood-filled cavities with high-grade sarcomatous cells seen in the peripheral rim and septae.3,7,8 This process can cause the lesion to resemble an aneurysmal bone cyst, explaining why some cases of TOS are misdiagnosed—with delayed time to treatment and increased morbidity and mortality.3,5 Generally, TOS patients are more likely than other OS patients to have tumors of femoral location, larger lesions, and normal alkaline phosphatase values. Many have pathologic fractures on presentation.7
The medical literature chronicles a long debate regarding the difference in mortality between patients with OS and those with TOS. It was once believed that patients with TOS were at higher risk for recurrence (especially those with a pathologic fracture) and mortality. However, in recent studies examining newer neoadjuvant and adjuvant chemotherapies, mortality rates for the two conditions are similar and certainly lower than they were many years ago.7,8 In one study, a better histologic response was reported to neoadjuvant chemotherapy in patients with TOS than with OS.7
Diagnosis
The first diagnostic tool used for patients with suspected OS or TOS is a plain radiographic film. A TOS lesion is lytic, with no areas of sclerosis, and almost always involves the long bones. It is poorly defined, destroying the cortex with formation of periosteal bone and invading the soft tissue. An initial pattern of parallel striations is highly suggestive of TOS.5
MRI and CT often reveal thick nodular tissue in a largely hemorrhagic and/or necrotic osseous lesion, with an associated soft-tissue mass that allows distinction from an aneurysmal bone cyst.3 Next, patients generally undergo a nuclear medicine bone scan and CT of the chest to observe for signs of metastases. Chest CT is commonly repeated on a regular basis during and after treatment.9
Pathologic evaluation, the final step to diagnosis, is very important, especially in the effort to differentiate TOS from an aneurysmal bone cyst. The typical gross findings for a TOS tumor include a dominant cystic cavity–like architecture, with a pushing peripheral margin that frequently expands and erodes the adjacent cortex and extends into the surrounding tissue. There is usually no area of intramural bone tissue.
Microscopically, the cystic areas contain clots and fragments of tumor that are often lined with a layer of neoplasm. The blood-filled telangiectatic spaces form in these areas. The spaces are irregularly shaped and typically traversed by septae composed in part of neoplastic cells. Osteoid formation through these cells can appear as a fine, ice-like material between tumor cells.4,7
Treatment
The main goals of treatment are to limit the anatomical extent of the disease, decrease the possibility of recurrence, and restore the highest possible level of function.2 Initial treatment of any OS or TOS consists of aggressive, immediate chemotherapy prior to and after any surgical intervention.1 (Chemotherapy will not be discussed in further detail here.) Surgical treatments for patients younger than 14 include amputation (above the lesion with wide margins), an expanding prosthesis, or rotationplasty. The location and extent of the tumor, the patient’s age, and his or her desired lifestyle will all have an impact on the choice of surgery.10
Historic data demonstrate that patients who undergo amputation alone almost always develop metastatic disease.1 Other data show that only 10% of patients with OS have been cured by chemotherapy alone. Yet when medical treatment is combined with surgical treatment, the overall expected cure rate can be as high as 65%.2
Discussing amputation with a young patient and the family can be emotionally difficult. If functional levels are to be restored, above-knee amputation (AKA) is the least favored surgical method. Compared with healthy individuals, patients who undergo AKA will walk 43% less quickly and will expend much more energy. These patients frequently have an inefficient gait and, given their limited reserve, they may lose the ability to walk altogether.2
Reconstructive surgical options include limb-salvage procedures; since the late 1980s, these have become the standard of care for OS at all sites.11 One such option includes removal of the lesion (eg, a distal femoral or proximal tibial lesion) with acceptable margins and replacement of the lost bone with an allograft or with a metallic prosthesis and knee joint (called arthroplasty). This endoprosthesis expands as the child grows (by way of a minor surgical procedure or a magnetic spring) so there is no apparent discrepancy between limb lengths, and the patient’s appearance is as normal and socially acceptable as possible.1,2
Because the case patient developed a pathologic fracture through his TOS tumor, he was not a candidate for endoprosthesis. His options were AKA or rotationplasty.
This procedure was first described in 195012 for treatment of proximal focal femoral deficiency. It is considered an alternative for skeletally immature individuals for whom the goal is to preserve function.
When AKA is indicated, the lower limb can be salvaged to allow functioning similar to that of a patient with a below-knee amputation (BKA). During rotationplasty, all but the most proximal aspect of the femur is resected. The tibia is externally rotated on the axis of the neurovascular bundle, then an arthrodesis of the proximal portion of the femur and the tibial plateau is performed (see Figure 2).

The end result is an extremity with the appearance, dimensions, and functional potential of a BKA. The ankle is rotated 180° so that it can serve as the new knee joint, and the attached foot, now pointing in the opposite direction, acts as the residual limb for fitting a prosthesis.2 This procedure is favored in patients with an extensive soft-tissue mass, intra-articular extension of the tumor, and/or pathologic fractures. It can also help prevent phantom pain.13

The Case Patient
After psychological evaluation of the patient and extensive family discussion, he underwent successful rotationplasty. The day after his surgery, however, he developed compartment syndrome and was required to undergo fasciotomies of the calf and proximal thigh. His wounds were treated, a skin graft was performed to close the proximal thigh wound, and his calf wounds were sutured closed (see Figures 3 and 4). His hip range of motion is excellent, and his ankle range of motion continues to improve with physical therapy.

At this writing, the patient was scheduled for his first prosthetic fitting, and he had nearly completed his chemotherapy. His outlook is very promising.
Conclusion
TOS is a rare, aggressive subtype of OS but the most common primary malignant bone tumor of childhood. In the past, outcomes in patients treated with surgery alone were poor. With the advent of chemotherapy and the combination of medical and surgical treatment, TOS-associated mortality has continued to decline. There is no significant difference in outcomes among the available surgical options, but limb-salvage surgical procedures usually offer patients much better function and quality of life. The most important consideration is early diagnosis followed by immediate treatment.
1. Siegel HJ, Pressey JG. Current concepts on the surgical and medical management of osteosarcoma. Expert Rev Anticancer Ther. 2008;8(8):1257-1269.
2. Marulanda GA, Henderson ER, Johnson DA, et al. Orthopedic surgery options for the treatment of primary osteosarcoma. Cancer Control. 2008;15(1):13-20.
3. Murphey MD, wan Jaovisidha S, Temple HT, et al. Telangiectatic osteosarcoma: radiologic-pathologic comparison. Radiology. 2003;229(2):545-553.
4. Mervak TR, Unni KK, Pritchard DJ, McLeod RA. Telangiectatic osteosarcoma. Clin Orthop Relat Res. 1991 Sep;270:135-139.
5. Vanel D, Tcheng S, Contesso G, et al. The radiological appearances of telangiectatic osteosarcoma: a study of 14 cases. Skeletal Radiol. 1987;16(3):196-200.
6. Ferrari S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23(34):8845-8852.
7. Bacci G, Ferrari S, Ruggieri P, et al. Telangiectatic osteosarcoma of the extremity: neoadjuvant chemotherapy in 24 cases. Acta Orthop Scand. 2001;72(2):167-172.
8. Weiss A, Khoury JD, Hoffer FA, et al. Telangiectatic osteosarcoma: the St. Jude Children’s Research Hospital’s experience. Cancer. 2007;109(8):1627-1637.
9. Agarwal M, Anchan C, Shah M, et al. Limb salvage surgery for osteosarcoma: effective low-cost treatment. Clin Orthop Relat Res. 2007;459:82-91.
10. Bacci G, Ferrari S, Lari S, et al. Osteosarcoma of the limb: amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84(1):88-92.
11. Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68(9):1331-1337.
12. Van Nes CP. Rotation-plasty for congenital defects of the femur: making use of the shortened limb to control the knee joint of a prosthesis. J Bone Joint Surg. 1950;32B:12-16.
13. Sawamura C, Hornicek FJ, Gebhardt MC. Complications and risk factors for failure of rotationplasty: review of 25 patients. Clin Orthop Relat Res. 2008;466(6):1302-1308.
1. Siegel HJ, Pressey JG. Current concepts on the surgical and medical management of osteosarcoma. Expert Rev Anticancer Ther. 2008;8(8):1257-1269.
2. Marulanda GA, Henderson ER, Johnson DA, et al. Orthopedic surgery options for the treatment of primary osteosarcoma. Cancer Control. 2008;15(1):13-20.
3. Murphey MD, wan Jaovisidha S, Temple HT, et al. Telangiectatic osteosarcoma: radiologic-pathologic comparison. Radiology. 2003;229(2):545-553.
4. Mervak TR, Unni KK, Pritchard DJ, McLeod RA. Telangiectatic osteosarcoma. Clin Orthop Relat Res. 1991 Sep;270:135-139.
5. Vanel D, Tcheng S, Contesso G, et al. The radiological appearances of telangiectatic osteosarcoma: a study of 14 cases. Skeletal Radiol. 1987;16(3):196-200.
6. Ferrari S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23(34):8845-8852.
7. Bacci G, Ferrari S, Ruggieri P, et al. Telangiectatic osteosarcoma of the extremity: neoadjuvant chemotherapy in 24 cases. Acta Orthop Scand. 2001;72(2):167-172.
8. Weiss A, Khoury JD, Hoffer FA, et al. Telangiectatic osteosarcoma: the St. Jude Children’s Research Hospital’s experience. Cancer. 2007;109(8):1627-1637.
9. Agarwal M, Anchan C, Shah M, et al. Limb salvage surgery for osteosarcoma: effective low-cost treatment. Clin Orthop Relat Res. 2007;459:82-91.
10. Bacci G, Ferrari S, Lari S, et al. Osteosarcoma of the limb: amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84(1):88-92.
11. Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68(9):1331-1337.
12. Van Nes CP. Rotation-plasty for congenital defects of the femur: making use of the shortened limb to control the knee joint of a prosthesis. J Bone Joint Surg. 1950;32B:12-16.
13. Sawamura C, Hornicek FJ, Gebhardt MC. Complications and risk factors for failure of rotationplasty: review of 25 patients. Clin Orthop Relat Res. 2008;466(6):1302-1308.