User login
Radiographically Silent Loosening of the Acetabular Component in Hip Arthroplasty
Total hip arthroplasty (THA) is an excellent option for the treatment of osteoarthritis of the hip. In numerous studies, modern implants have shown survivorship of more than 90% at 10 years.1,2 Polyethylene wear and subsequent osteolysis are major obstacles to the long-term success of THA.3-5 Polyethylene wear particles are phagocytized by macrophages, inducing an inflammatory response that can ultimately lead to osteolysis of the bony architecture surrounding the bone–implant interface.6,7 As modern implants often rely on direct implant-to-bone ingrowth to maintain fixation, wear at this junction can lead to aseptic component loosening and ultimately require revision surgery.8-10 Osteolysis can be diagnosed with plain radiography or computed tomography (CT).11 CT is more sensitive than plain radiography for the diagnosis of osteolysis and is better able to determine the size and location of osteolytic lesions.12,13
Although diagnosis of osteolysis is well defined in the literature, what is more challenging is radiographic diagnosis of a loose acetabular component.11 The most commonly described criteria for loosening are presence of a complete radiolucent line of more than 2 mm in width at the bone–implant interface and any progressive tilting or migration of the component.14,15 CT-based criteria for component loosening remain largely undefined, though Egawa and colleagues16 showed that acetabular osteolysis involving less than 40% of the total cup surface is not associated with component loosening. Although a patient may show signs of osteolysis on postoperative imaging, this finding does not necessitate immediate revision surgery.17 Osteolysis may be monitored clinically and followed radiographically to determine when intervention is necessary.13
The goals of revision surgery are to eliminate the wear generator and bone-graft lytic lesions where needed to help maintain the bone–implant interface.17 The timing of such surgery is important, as the surgeon must balance the risk for gross component migration against the morbidity and mortality associated with acetabular component revision.18 This is in contrast to the settings of infection, periprosthetic fracture, recurrent instability, and component fracture/loosening, in which revision is urgently indicated and the case cannot be managed conservatively.
We conducted a study to determine the incidence of loose acetabular components without radiographic or clinical findings that would necessitate urgent revision THA. Radiographically silent loosening (RSL) was defined as an acetabular component that was loose at time of revision surgery but that did not show frank signs of loosening on either plain radiography or CT. Although these patients make up a small minority of the revision population, knowing the incidence of RSL can help raise surgeon awareness of this potentially dangerous situation. We further sought to determine whether patients with RSL present with different demographic characteristics or clinical symptoms than patients with stable acetabular components.
Materials and Methods
In this retrospective, case–control, institutional review board–approved study, we evaluated patients who had undergone revision THA and had preoperative plain radiographs and CT images. We identified patients by International Classification of Diseases, Ninth Revision (ICD‑9) codes (00.70, 00.71, 00.72, 00.73, 80.05, 81.53, 84.56, 84.57) and searched for those cases treated between 2000 and 2012.
Inclusion criteria were confirmed revision THA and confirmed plain radiography and CT of the THA performed before revision. When osteolysis was diagnosed by plain radiography, CT was ordered to determine the extent of bony lesions or to evaluate for eccentric head position or component malposition. Last, all patients included in the study had a detailed operative report clearly indicating acetabular component stability at time of revision. Acetabular component stability at time of surgery was determined according to the criteria defined by Berger and colleagues.19 Cups were evaluated for gross motion during both hip dislocation and during edge loading of the component after thorough scar and capsular débridement.
Patients who did not have CT performed before revision surgery were excluded from the study. These patients had been diagnosed by clinical history and/or plain radiography. Cases revised for periprosthetic infection or periprosthetic fracture were also excluded. Patients with metal-on-metal bearings were excluded, as were any cases revised from hemiarthroplasty to THA, as well as cases revised for recurrent dislocations or component malposition.
All plain radiographs and CT images were evaluated by the orthopedic surgeon who performed the revision and by a radiologist. Images were inspected for signs of gross component migration, tilting, and concentric lucency of the bone–implant interface. Patients with imaging that showed signs of component movement or migration (as seen by the attending surgeon or the radiologist) were excluded. Patients with radiographic evidence of femoral stem loosening were also excluded, as they had an indication to undergo revision arthroplasty. The remaining patients were then stratified into 2 groups: those with stable acetabular components at time of revision and those with loose acetabular components. Stable acetabular shells showed no gross motion of the implant with dislocation, edge loading with an impactor, or pulling with a Kocher clamp after débridement and screw removal.15,19 The 2 groups were then compared with respect to age, sex, and most common presenting symptoms and diagnoses. Fischer exact test and Student t test were used to statistically compare the groups.
Results
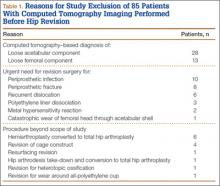
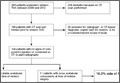
Overall, 393 patients underwent revision arthroplasty for the diagnoses (ICD-9 codes) indicated (Figure). One hundred eighty-nine patients (48.1%) had CT performed before revision. Of these 189 patients, 85 were excluded for diagnoses that were evident on either plain radiography or CT, that necessitated urgent revision, or for procedures beyond the scope of the study (Table 1). CT showed a loose cup in 28 patients; 6 of these cups were also seen on CT. Thirteen patients were diagnosed with a loose femoral stem, 10 with a periprosthetic infection, and 8 with a periprosthetic fracture.
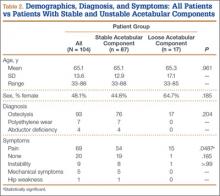
One hundred four patients (54 men, 50 women) met the study inclusion criteria. Mean age was 65.1 years. Of these 104 patients, 87 (83.7%) had a stable acetabular shell at time of revision surgery; the other 17 (16.3%) were diagnosed with RSL of the acetabular shell. Osteolysis was the most common diagnosis (89.4%) in the overall population, and pain was the most common complaint at time of presentation (66.6%). Lack of symptoms was the second most common presentation at time of revision (19.2%) (Table 2). Patients without symptoms underwent revision surgery because of concern about impending compromise of the bone–implant interface and progressive osteolysis.
The 2 groups (stable vs unstable acetabular shells) were not significantly different with respect to age (P = .961) or sex distribution (P = .185). All patients in the RSL group were diagnosed with osteolysis radiographically, and 15 (88%) of 17 patients presented with pain as the primary complaint, compared with only 54 (62%) of 87 patients in the group with stable shells. Patients with RSL were significantly more likely to present with pain as the primary complaint (P = .0487). Nineteen patients in the stable implant group and only 1 patient in the RSL group were asymptomatic, but this was not statistically significant (P = .185) when compared against all other diagnoses.
Discussion
We defined RSL as an acetabular component that was loose at time of revision surgery but that did not show frank signs of loosening on either plain radiography or CT. Patients with RSL and the surgeons who treat them are in a difficult position. In the setting of osteolysis, patients can be treated with serial radiographic imaging and clinical monitoring to determine if and when revision arthroplasty should be performed.17 However, given the complexity and risks associated with revision THA, surgeons should be aware that the acetabular shell may necessitate revision even if it does not appear to be frankly unstable on radiographic imaging.18
Of the 393 patients who underwent revision THA at our institution, 48.1% were evaluated with CT. Eighty-five of the 189 patients who underwent CT were diagnosed with radiographic loosening, or were diagnosed as needing urgent revision THA in the setting of loose components, periprosthetic infection, periprosthetic fracture, or catastrophic implant failure. Of the remaining 104 patients, 17 (16.3%) met the diagnosis of RSL of the acetabular component. The most common complaint was pain, and the most common diagnoses were osteolysis and polyethylene wear. Age and sex were not associated with increased likelihood of RSL.
Our study has several limitations. We defined the radiographic diagnosis of loose acetabular components as components showing frank migration, tilting, or a 2-mm concentric lucency on plain radiography or CT. Although these are common definitions of loose acetabular components, more sensitive radiographic measures have been described.16 We also excluded patients with recurrent dislocations and metal-on-metal prostheses, as these cases increase the metal artifact on CT and limit the ability to evaluate the bone–implant interface. Metal artifact remains an ongoing challenge to use of CT for post-THA imaging. However, tailored imaging protocols are helping to eliminate metal artifact. Bone scan was not used to evaluate for possible component loosening. Although sensitivity and specificity are about 67% and 76%, respectively,20 Temmerman and colleagues21 also found poor intraobserver reliability (0.53) for bone scans in the setting of uncemented acetabular components. Last, our study did not evaluate the bony ingrowth patterns that corresponded to stable or unstable fixation and did not evaluate the volumetric size or anatomical location of the osteolytic lesions on CT. Careful assessment of these variables is clinically relevant when trying to determine if revision arthroplasty is warranted.
Although we used relatively simple radiographic criteria to define loose components, more sensitive and specific techniques have been described for both plain radiography and CT. Moore and colleagues22 described 5 radiographic signs of bony ingrowth; when 3 or more were present, sensitivity was 89.6% and specificity 76.9%. Mehin and colleagues23 suggested that osteolysis involving more than 50% of the circumference of the shell on a standard pelvic radiograph might require revision arthroplasty. However, more recent studies have found that anteroposterior and lateral radiographs are less able to evaluate the anterior and posterior rims of the bone–implant interface, and it is this ingrowth area that may be the most crucial for stable osseointegration.12,16
CT has expanded our ability to evaluate the bone–implant interface in 3 dimensions. Egawa and colleagues16 described using CT to evaluate the surface area involved with osteolysis and found that less than 40% involvement of the surface area generally corresponded to well-fixed components. Furthermore, they found that osteolysis generally creates lesions inferior and superior to the acetabular component and less often involves the anterior and posterior rims, which may be more important for stable fixation. The authors noted that volumetric analysis and CT were not as cost-effective as plain radiography and were more time- and skill-intensive.
Osteolysis itself remains a common indication for revision THA. However, the most appropriate procedure remains controversial. Mallory and colleagues24 recommended explanting all acetabular shells in the setting of revision arthroplasty. They indicated that full assessment of the bony pelvis and any lytic defects was possible only with the wide exposure gained by acetabular component removal. More recent studies have begun to justify isolated component revision in the setting of well-fixed acetabular shells. Studies by Maloney and colleagues,10 Park and colleagues,15 and Beaulé and colleagues25 have shown excellent outcomes with retention of well-fixed acetabular shells and removal of the wear generator in the setting of osteolysis. Haidukewych17 wrote that the goals in addressing osteolysis in revision THA are to eliminate the wear generator, débride osteolytic lesions, and restore bone stock. Surgeons should weigh the benefits of component retention and isolated liner exchange against the morbidity associated with explantation and preparation for implanting a new component. Good outcomes have been achieved with isolated component exchange, but surgeons should be aware that instability remains the most common complication after isolated liner exchange.8
The majority of our patients with RSL presented with complaints of pain and the diagnosis of osteolysis. One patient who had the diagnosis but was clinically asymptomatic was found to have a loose acetabular shell at time of revision surgery. Given the increased morbidity associated with acetabular component revision, this patient’s condition represents a dangerous combination of RSL and clinically asymptomatic component loosening. By raising awareness about RSL and its incidence, we should be able to increase our ability to detect RSL. A surgeon who detects RSL before gross migration or movement of the acetabular component may be better able to plan for revision arthroplasty before a catastrophic event that may necessitate a larger, more complex procedure. With the number of patients who require revision THA continuing to rise, surgeons should be aware of the incidence of RSL and the potential of RSL to affect patient care and potential surgical options.
1. Milošev I, Kovač S, Trebše R, Levašič V, Pišot V. Comparison of ten-year survivorship of hip prostheses with use of conventional polyethylene, metal-on-metal, or ceramic-on-ceramic bearings. J Bone Joint Surg Am. 2012;94(19):1756-1763.
2. D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop Relat Res. 2012;470(2):373-381.
3. Dowd JE, Sychterz CJ, Young AM, Engh CA. Characterization of long-term femoral-head-penetration rates. Association with and prediction of osteolysis. J Bone Joint Surg Am. 2000;82(8):1102-1107.
4. Orishimo KF, Claus AM, Sychterz CJ, Engh CA. Relationship between polyethylene wear and osteolysis in hips with a second-generation porous-coated cementless cup after seven years of follow-up. J Bone Joint Surg Am. 2003;85(6):1095-1099.
5. Harris WH. Wear and periprosthetic osteolysis: the problem. Clin Orthop Relat Res. 2001;(393):66-70.
6. Holt G, Murnaghan C, Reilly J, Meek RM. The biology of aseptic osteolysis. Clin Orthop Relat Res. 2007;(460):240-252.
7. Catelas I, Jacobs JJ. Biologic activity of wear particles. Instr Course Lect. 2010;59:3-16.
8. Paprosky WG, Nourbash P, Gill P. Treatment of progressive periacetabular osteolysis: cup revision versus liner exchange and bone grafting. Paper presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; February 4-8, 1999; Anaheim, CA.
9. Engh CA Jr, Claus AM, Hopper RH Jr, Engh CA. Long-term results using the anatomic medullary locking hip prosthesis. Clin Orthop Relat Res. 2001;(393):137-146.
10. Maloney WJ, Peters P, Engh CA, Chandler H. Severe osteolysis of the pelvic in association with acetabular replacement without cement. J Bone Joint Surg Am. 1993;75(11):1627-1635.
11. Claus AM, Engh CA Jr, Sychterz CJ, Xenos JS, Orishimo KF, Engh CA Sr. Radiographic definition of pelvic osteolysis following total hip arthroplasty. J Bone Joint Surg Am. 2003;85(8):1519-1526.
12. Puri L, Wixson RL, Stern SH, Kohli J, Hendrix RW, Stulberg SD. Use of helical computed tomography for the assessment of acetabular osteolysis after total hip arthroplasty. J Bone Joint Surg Am. 2002;84(4):609-614.
13. Stulberg SD, Wixson RL, Adams AD, Hendrix RW, Bernfield JB. Monitoring pelvic osteolysis following total hip replacement surgery: an algorithm for surveillance. J Bone Joint Surg Am. 2002;84(suppl 2):116-122.
14. Massin P, Schmidt L, Engh CA. Evaluation of cementless acetabular component migration. An experimental study. J Arthroplasty. 1989;4(3):245-251.
15. Park KS, Yoon TR, Song EK, Lee KB. Results of isolated femoral component revision with well-fixed acetabular implant retention. J Arthroplasty. 2010;25(8):1188-1195.
16. Egawa H, Ho H, Hopper RH Jr, Engh CA Jr, Engh CA. Computed tomography assessment of pelvic osteolysis and cup–lesion interface involvement with a press-fit porous-coated acetabular cup. J Arthroplasty. 2009;24(2):233-239.
17. Haidukewych GJ. Osteolysis in the well-fixed socket: cup retention or revision? J Bone Joint Surg Br. 2012;94(12):65-69.
18. Stulberg BN, Della Valle AG. What are the guidelines for the surgical and nonsurgical treatment of periprosthetic osteolysis? J Am Acad Orthop Surg. 2008;16(suppl 1):S20-S25.
19. Berger RA, Quigley LR, Jacobs JJ, Sheinkop MB, Rosenberg AG, Galante JO. The fate of stable cemented acetabular components retained during revision of a femoral component of a total hip arthroplasty. J Bone Joint Surg Am. 1999;81(12):1682-1691.
20. Temmerman OP, Raijmakers PG, Deville WL, Berkhof J, Hooft L, Heyligers IC. The use of plain radiography, subtraction arthrography, nuclear arthrography, and bone scintigraphy in the diagnosis of a loose acetabular component of a total hip prosthesis: a systematic review. J Arthroplasty. 2007;22(6):818-827.
21. Temmerman OP, Raijmakers PG, David EF, et al. A comparison of radiographic and scintigraphic techniques to assess aseptic loosening of the acetabular component in a total hip replacement. J Bone Joint Surg Am. 2004;86(11):2456-2463.
22. Moore MS, McAuley JP, Young AM, Engh CA Sr. Radiographic signs of osseointegration in porous-coated acetabular components. Clin Orthop Relat Res. 2006;(444):176-183.
23. Mehin R, Yuan X, Haydon C, et al. Retroacetabular osteolysis: when to operate? Clin Orthop Relat Res. 2004;(428):247-255.
24. Mallory TH, Lombardi AV Jr, Fada RA, Adams JB, Kefauver CA, Eberle RW. Noncemented acetabular component removal in the presence of osteolysis: the affirmative. Clin Orthop Relat Res. 2000;(381):120-128.
25. Beaulé PE, Le Duff MJ, Dorey FJ, Amstutz HC. Fate of cementless acetabular components retained during revision total hip arthroplasty. J Bone Joint Surg Am. 2003;85(12):2288-2293.
Total hip arthroplasty (THA) is an excellent option for the treatment of osteoarthritis of the hip. In numerous studies, modern implants have shown survivorship of more than 90% at 10 years.1,2 Polyethylene wear and subsequent osteolysis are major obstacles to the long-term success of THA.3-5 Polyethylene wear particles are phagocytized by macrophages, inducing an inflammatory response that can ultimately lead to osteolysis of the bony architecture surrounding the bone–implant interface.6,7 As modern implants often rely on direct implant-to-bone ingrowth to maintain fixation, wear at this junction can lead to aseptic component loosening and ultimately require revision surgery.8-10 Osteolysis can be diagnosed with plain radiography or computed tomography (CT).11 CT is more sensitive than plain radiography for the diagnosis of osteolysis and is better able to determine the size and location of osteolytic lesions.12,13
Although diagnosis of osteolysis is well defined in the literature, what is more challenging is radiographic diagnosis of a loose acetabular component.11 The most commonly described criteria for loosening are presence of a complete radiolucent line of more than 2 mm in width at the bone–implant interface and any progressive tilting or migration of the component.14,15 CT-based criteria for component loosening remain largely undefined, though Egawa and colleagues16 showed that acetabular osteolysis involving less than 40% of the total cup surface is not associated with component loosening. Although a patient may show signs of osteolysis on postoperative imaging, this finding does not necessitate immediate revision surgery.17 Osteolysis may be monitored clinically and followed radiographically to determine when intervention is necessary.13
The goals of revision surgery are to eliminate the wear generator and bone-graft lytic lesions where needed to help maintain the bone–implant interface.17 The timing of such surgery is important, as the surgeon must balance the risk for gross component migration against the morbidity and mortality associated with acetabular component revision.18 This is in contrast to the settings of infection, periprosthetic fracture, recurrent instability, and component fracture/loosening, in which revision is urgently indicated and the case cannot be managed conservatively.
We conducted a study to determine the incidence of loose acetabular components without radiographic or clinical findings that would necessitate urgent revision THA. Radiographically silent loosening (RSL) was defined as an acetabular component that was loose at time of revision surgery but that did not show frank signs of loosening on either plain radiography or CT. Although these patients make up a small minority of the revision population, knowing the incidence of RSL can help raise surgeon awareness of this potentially dangerous situation. We further sought to determine whether patients with RSL present with different demographic characteristics or clinical symptoms than patients with stable acetabular components.
Materials and Methods
In this retrospective, case–control, institutional review board–approved study, we evaluated patients who had undergone revision THA and had preoperative plain radiographs and CT images. We identified patients by International Classification of Diseases, Ninth Revision (ICD‑9) codes (00.70, 00.71, 00.72, 00.73, 80.05, 81.53, 84.56, 84.57) and searched for those cases treated between 2000 and 2012.
Inclusion criteria were confirmed revision THA and confirmed plain radiography and CT of the THA performed before revision. When osteolysis was diagnosed by plain radiography, CT was ordered to determine the extent of bony lesions or to evaluate for eccentric head position or component malposition. Last, all patients included in the study had a detailed operative report clearly indicating acetabular component stability at time of revision. Acetabular component stability at time of surgery was determined according to the criteria defined by Berger and colleagues.19 Cups were evaluated for gross motion during both hip dislocation and during edge loading of the component after thorough scar and capsular débridement.
Patients who did not have CT performed before revision surgery were excluded from the study. These patients had been diagnosed by clinical history and/or plain radiography. Cases revised for periprosthetic infection or periprosthetic fracture were also excluded. Patients with metal-on-metal bearings were excluded, as were any cases revised from hemiarthroplasty to THA, as well as cases revised for recurrent dislocations or component malposition.
All plain radiographs and CT images were evaluated by the orthopedic surgeon who performed the revision and by a radiologist. Images were inspected for signs of gross component migration, tilting, and concentric lucency of the bone–implant interface. Patients with imaging that showed signs of component movement or migration (as seen by the attending surgeon or the radiologist) were excluded. Patients with radiographic evidence of femoral stem loosening were also excluded, as they had an indication to undergo revision arthroplasty. The remaining patients were then stratified into 2 groups: those with stable acetabular components at time of revision and those with loose acetabular components. Stable acetabular shells showed no gross motion of the implant with dislocation, edge loading with an impactor, or pulling with a Kocher clamp after débridement and screw removal.15,19 The 2 groups were then compared with respect to age, sex, and most common presenting symptoms and diagnoses. Fischer exact test and Student t test were used to statistically compare the groups.
Results
Overall, 393 patients underwent revision arthroplasty for the diagnoses (ICD-9 codes) indicated (Figure). One hundred eighty-nine patients (48.1%) had CT performed before revision. Of these 189 patients, 85 were excluded for diagnoses that were evident on either plain radiography or CT, that necessitated urgent revision, or for procedures beyond the scope of the study (Table 1). CT showed a loose cup in 28 patients; 6 of these cups were also seen on CT. Thirteen patients were diagnosed with a loose femoral stem, 10 with a periprosthetic infection, and 8 with a periprosthetic fracture.
One hundred four patients (54 men, 50 women) met the study inclusion criteria. Mean age was 65.1 years. Of these 104 patients, 87 (83.7%) had a stable acetabular shell at time of revision surgery; the other 17 (16.3%) were diagnosed with RSL of the acetabular shell. Osteolysis was the most common diagnosis (89.4%) in the overall population, and pain was the most common complaint at time of presentation (66.6%). Lack of symptoms was the second most common presentation at time of revision (19.2%) (Table 2). Patients without symptoms underwent revision surgery because of concern about impending compromise of the bone–implant interface and progressive osteolysis.
The 2 groups (stable vs unstable acetabular shells) were not significantly different with respect to age (P = .961) or sex distribution (P = .185). All patients in the RSL group were diagnosed with osteolysis radiographically, and 15 (88%) of 17 patients presented with pain as the primary complaint, compared with only 54 (62%) of 87 patients in the group with stable shells. Patients with RSL were significantly more likely to present with pain as the primary complaint (P = .0487). Nineteen patients in the stable implant group and only 1 patient in the RSL group were asymptomatic, but this was not statistically significant (P = .185) when compared against all other diagnoses.
Discussion
We defined RSL as an acetabular component that was loose at time of revision surgery but that did not show frank signs of loosening on either plain radiography or CT. Patients with RSL and the surgeons who treat them are in a difficult position. In the setting of osteolysis, patients can be treated with serial radiographic imaging and clinical monitoring to determine if and when revision arthroplasty should be performed.17 However, given the complexity and risks associated with revision THA, surgeons should be aware that the acetabular shell may necessitate revision even if it does not appear to be frankly unstable on radiographic imaging.18
Of the 393 patients who underwent revision THA at our institution, 48.1% were evaluated with CT. Eighty-five of the 189 patients who underwent CT were diagnosed with radiographic loosening, or were diagnosed as needing urgent revision THA in the setting of loose components, periprosthetic infection, periprosthetic fracture, or catastrophic implant failure. Of the remaining 104 patients, 17 (16.3%) met the diagnosis of RSL of the acetabular component. The most common complaint was pain, and the most common diagnoses were osteolysis and polyethylene wear. Age and sex were not associated with increased likelihood of RSL.
Our study has several limitations. We defined the radiographic diagnosis of loose acetabular components as components showing frank migration, tilting, or a 2-mm concentric lucency on plain radiography or CT. Although these are common definitions of loose acetabular components, more sensitive radiographic measures have been described.16 We also excluded patients with recurrent dislocations and metal-on-metal prostheses, as these cases increase the metal artifact on CT and limit the ability to evaluate the bone–implant interface. Metal artifact remains an ongoing challenge to use of CT for post-THA imaging. However, tailored imaging protocols are helping to eliminate metal artifact. Bone scan was not used to evaluate for possible component loosening. Although sensitivity and specificity are about 67% and 76%, respectively,20 Temmerman and colleagues21 also found poor intraobserver reliability (0.53) for bone scans in the setting of uncemented acetabular components. Last, our study did not evaluate the bony ingrowth patterns that corresponded to stable or unstable fixation and did not evaluate the volumetric size or anatomical location of the osteolytic lesions on CT. Careful assessment of these variables is clinically relevant when trying to determine if revision arthroplasty is warranted.
Although we used relatively simple radiographic criteria to define loose components, more sensitive and specific techniques have been described for both plain radiography and CT. Moore and colleagues22 described 5 radiographic signs of bony ingrowth; when 3 or more were present, sensitivity was 89.6% and specificity 76.9%. Mehin and colleagues23 suggested that osteolysis involving more than 50% of the circumference of the shell on a standard pelvic radiograph might require revision arthroplasty. However, more recent studies have found that anteroposterior and lateral radiographs are less able to evaluate the anterior and posterior rims of the bone–implant interface, and it is this ingrowth area that may be the most crucial for stable osseointegration.12,16
CT has expanded our ability to evaluate the bone–implant interface in 3 dimensions. Egawa and colleagues16 described using CT to evaluate the surface area involved with osteolysis and found that less than 40% involvement of the surface area generally corresponded to well-fixed components. Furthermore, they found that osteolysis generally creates lesions inferior and superior to the acetabular component and less often involves the anterior and posterior rims, which may be more important for stable fixation. The authors noted that volumetric analysis and CT were not as cost-effective as plain radiography and were more time- and skill-intensive.
Osteolysis itself remains a common indication for revision THA. However, the most appropriate procedure remains controversial. Mallory and colleagues24 recommended explanting all acetabular shells in the setting of revision arthroplasty. They indicated that full assessment of the bony pelvis and any lytic defects was possible only with the wide exposure gained by acetabular component removal. More recent studies have begun to justify isolated component revision in the setting of well-fixed acetabular shells. Studies by Maloney and colleagues,10 Park and colleagues,15 and Beaulé and colleagues25 have shown excellent outcomes with retention of well-fixed acetabular shells and removal of the wear generator in the setting of osteolysis. Haidukewych17 wrote that the goals in addressing osteolysis in revision THA are to eliminate the wear generator, débride osteolytic lesions, and restore bone stock. Surgeons should weigh the benefits of component retention and isolated liner exchange against the morbidity associated with explantation and preparation for implanting a new component. Good outcomes have been achieved with isolated component exchange, but surgeons should be aware that instability remains the most common complication after isolated liner exchange.8
The majority of our patients with RSL presented with complaints of pain and the diagnosis of osteolysis. One patient who had the diagnosis but was clinically asymptomatic was found to have a loose acetabular shell at time of revision surgery. Given the increased morbidity associated with acetabular component revision, this patient’s condition represents a dangerous combination of RSL and clinically asymptomatic component loosening. By raising awareness about RSL and its incidence, we should be able to increase our ability to detect RSL. A surgeon who detects RSL before gross migration or movement of the acetabular component may be better able to plan for revision arthroplasty before a catastrophic event that may necessitate a larger, more complex procedure. With the number of patients who require revision THA continuing to rise, surgeons should be aware of the incidence of RSL and the potential of RSL to affect patient care and potential surgical options.
Total hip arthroplasty (THA) is an excellent option for the treatment of osteoarthritis of the hip. In numerous studies, modern implants have shown survivorship of more than 90% at 10 years.1,2 Polyethylene wear and subsequent osteolysis are major obstacles to the long-term success of THA.3-5 Polyethylene wear particles are phagocytized by macrophages, inducing an inflammatory response that can ultimately lead to osteolysis of the bony architecture surrounding the bone–implant interface.6,7 As modern implants often rely on direct implant-to-bone ingrowth to maintain fixation, wear at this junction can lead to aseptic component loosening and ultimately require revision surgery.8-10 Osteolysis can be diagnosed with plain radiography or computed tomography (CT).11 CT is more sensitive than plain radiography for the diagnosis of osteolysis and is better able to determine the size and location of osteolytic lesions.12,13
Although diagnosis of osteolysis is well defined in the literature, what is more challenging is radiographic diagnosis of a loose acetabular component.11 The most commonly described criteria for loosening are presence of a complete radiolucent line of more than 2 mm in width at the bone–implant interface and any progressive tilting or migration of the component.14,15 CT-based criteria for component loosening remain largely undefined, though Egawa and colleagues16 showed that acetabular osteolysis involving less than 40% of the total cup surface is not associated with component loosening. Although a patient may show signs of osteolysis on postoperative imaging, this finding does not necessitate immediate revision surgery.17 Osteolysis may be monitored clinically and followed radiographically to determine when intervention is necessary.13
The goals of revision surgery are to eliminate the wear generator and bone-graft lytic lesions where needed to help maintain the bone–implant interface.17 The timing of such surgery is important, as the surgeon must balance the risk for gross component migration against the morbidity and mortality associated with acetabular component revision.18 This is in contrast to the settings of infection, periprosthetic fracture, recurrent instability, and component fracture/loosening, in which revision is urgently indicated and the case cannot be managed conservatively.
We conducted a study to determine the incidence of loose acetabular components without radiographic or clinical findings that would necessitate urgent revision THA. Radiographically silent loosening (RSL) was defined as an acetabular component that was loose at time of revision surgery but that did not show frank signs of loosening on either plain radiography or CT. Although these patients make up a small minority of the revision population, knowing the incidence of RSL can help raise surgeon awareness of this potentially dangerous situation. We further sought to determine whether patients with RSL present with different demographic characteristics or clinical symptoms than patients with stable acetabular components.
Materials and Methods
In this retrospective, case–control, institutional review board–approved study, we evaluated patients who had undergone revision THA and had preoperative plain radiographs and CT images. We identified patients by International Classification of Diseases, Ninth Revision (ICD‑9) codes (00.70, 00.71, 00.72, 00.73, 80.05, 81.53, 84.56, 84.57) and searched for those cases treated between 2000 and 2012.
Inclusion criteria were confirmed revision THA and confirmed plain radiography and CT of the THA performed before revision. When osteolysis was diagnosed by plain radiography, CT was ordered to determine the extent of bony lesions or to evaluate for eccentric head position or component malposition. Last, all patients included in the study had a detailed operative report clearly indicating acetabular component stability at time of revision. Acetabular component stability at time of surgery was determined according to the criteria defined by Berger and colleagues.19 Cups were evaluated for gross motion during both hip dislocation and during edge loading of the component after thorough scar and capsular débridement.
Patients who did not have CT performed before revision surgery were excluded from the study. These patients had been diagnosed by clinical history and/or plain radiography. Cases revised for periprosthetic infection or periprosthetic fracture were also excluded. Patients with metal-on-metal bearings were excluded, as were any cases revised from hemiarthroplasty to THA, as well as cases revised for recurrent dislocations or component malposition.
All plain radiographs and CT images were evaluated by the orthopedic surgeon who performed the revision and by a radiologist. Images were inspected for signs of gross component migration, tilting, and concentric lucency of the bone–implant interface. Patients with imaging that showed signs of component movement or migration (as seen by the attending surgeon or the radiologist) were excluded. Patients with radiographic evidence of femoral stem loosening were also excluded, as they had an indication to undergo revision arthroplasty. The remaining patients were then stratified into 2 groups: those with stable acetabular components at time of revision and those with loose acetabular components. Stable acetabular shells showed no gross motion of the implant with dislocation, edge loading with an impactor, or pulling with a Kocher clamp after débridement and screw removal.15,19 The 2 groups were then compared with respect to age, sex, and most common presenting symptoms and diagnoses. Fischer exact test and Student t test were used to statistically compare the groups.
Results
Overall, 393 patients underwent revision arthroplasty for the diagnoses (ICD-9 codes) indicated (Figure). One hundred eighty-nine patients (48.1%) had CT performed before revision. Of these 189 patients, 85 were excluded for diagnoses that were evident on either plain radiography or CT, that necessitated urgent revision, or for procedures beyond the scope of the study (Table 1). CT showed a loose cup in 28 patients; 6 of these cups were also seen on CT. Thirteen patients were diagnosed with a loose femoral stem, 10 with a periprosthetic infection, and 8 with a periprosthetic fracture.
One hundred four patients (54 men, 50 women) met the study inclusion criteria. Mean age was 65.1 years. Of these 104 patients, 87 (83.7%) had a stable acetabular shell at time of revision surgery; the other 17 (16.3%) were diagnosed with RSL of the acetabular shell. Osteolysis was the most common diagnosis (89.4%) in the overall population, and pain was the most common complaint at time of presentation (66.6%). Lack of symptoms was the second most common presentation at time of revision (19.2%) (Table 2). Patients without symptoms underwent revision surgery because of concern about impending compromise of the bone–implant interface and progressive osteolysis.
The 2 groups (stable vs unstable acetabular shells) were not significantly different with respect to age (P = .961) or sex distribution (P = .185). All patients in the RSL group were diagnosed with osteolysis radiographically, and 15 (88%) of 17 patients presented with pain as the primary complaint, compared with only 54 (62%) of 87 patients in the group with stable shells. Patients with RSL were significantly more likely to present with pain as the primary complaint (P = .0487). Nineteen patients in the stable implant group and only 1 patient in the RSL group were asymptomatic, but this was not statistically significant (P = .185) when compared against all other diagnoses.
Discussion
We defined RSL as an acetabular component that was loose at time of revision surgery but that did not show frank signs of loosening on either plain radiography or CT. Patients with RSL and the surgeons who treat them are in a difficult position. In the setting of osteolysis, patients can be treated with serial radiographic imaging and clinical monitoring to determine if and when revision arthroplasty should be performed.17 However, given the complexity and risks associated with revision THA, surgeons should be aware that the acetabular shell may necessitate revision even if it does not appear to be frankly unstable on radiographic imaging.18
Of the 393 patients who underwent revision THA at our institution, 48.1% were evaluated with CT. Eighty-five of the 189 patients who underwent CT were diagnosed with radiographic loosening, or were diagnosed as needing urgent revision THA in the setting of loose components, periprosthetic infection, periprosthetic fracture, or catastrophic implant failure. Of the remaining 104 patients, 17 (16.3%) met the diagnosis of RSL of the acetabular component. The most common complaint was pain, and the most common diagnoses were osteolysis and polyethylene wear. Age and sex were not associated with increased likelihood of RSL.
Our study has several limitations. We defined the radiographic diagnosis of loose acetabular components as components showing frank migration, tilting, or a 2-mm concentric lucency on plain radiography or CT. Although these are common definitions of loose acetabular components, more sensitive radiographic measures have been described.16 We also excluded patients with recurrent dislocations and metal-on-metal prostheses, as these cases increase the metal artifact on CT and limit the ability to evaluate the bone–implant interface. Metal artifact remains an ongoing challenge to use of CT for post-THA imaging. However, tailored imaging protocols are helping to eliminate metal artifact. Bone scan was not used to evaluate for possible component loosening. Although sensitivity and specificity are about 67% and 76%, respectively,20 Temmerman and colleagues21 also found poor intraobserver reliability (0.53) for bone scans in the setting of uncemented acetabular components. Last, our study did not evaluate the bony ingrowth patterns that corresponded to stable or unstable fixation and did not evaluate the volumetric size or anatomical location of the osteolytic lesions on CT. Careful assessment of these variables is clinically relevant when trying to determine if revision arthroplasty is warranted.
Although we used relatively simple radiographic criteria to define loose components, more sensitive and specific techniques have been described for both plain radiography and CT. Moore and colleagues22 described 5 radiographic signs of bony ingrowth; when 3 or more were present, sensitivity was 89.6% and specificity 76.9%. Mehin and colleagues23 suggested that osteolysis involving more than 50% of the circumference of the shell on a standard pelvic radiograph might require revision arthroplasty. However, more recent studies have found that anteroposterior and lateral radiographs are less able to evaluate the anterior and posterior rims of the bone–implant interface, and it is this ingrowth area that may be the most crucial for stable osseointegration.12,16
CT has expanded our ability to evaluate the bone–implant interface in 3 dimensions. Egawa and colleagues16 described using CT to evaluate the surface area involved with osteolysis and found that less than 40% involvement of the surface area generally corresponded to well-fixed components. Furthermore, they found that osteolysis generally creates lesions inferior and superior to the acetabular component and less often involves the anterior and posterior rims, which may be more important for stable fixation. The authors noted that volumetric analysis and CT were not as cost-effective as plain radiography and were more time- and skill-intensive.
Osteolysis itself remains a common indication for revision THA. However, the most appropriate procedure remains controversial. Mallory and colleagues24 recommended explanting all acetabular shells in the setting of revision arthroplasty. They indicated that full assessment of the bony pelvis and any lytic defects was possible only with the wide exposure gained by acetabular component removal. More recent studies have begun to justify isolated component revision in the setting of well-fixed acetabular shells. Studies by Maloney and colleagues,10 Park and colleagues,15 and Beaulé and colleagues25 have shown excellent outcomes with retention of well-fixed acetabular shells and removal of the wear generator in the setting of osteolysis. Haidukewych17 wrote that the goals in addressing osteolysis in revision THA are to eliminate the wear generator, débride osteolytic lesions, and restore bone stock. Surgeons should weigh the benefits of component retention and isolated liner exchange against the morbidity associated with explantation and preparation for implanting a new component. Good outcomes have been achieved with isolated component exchange, but surgeons should be aware that instability remains the most common complication after isolated liner exchange.8
The majority of our patients with RSL presented with complaints of pain and the diagnosis of osteolysis. One patient who had the diagnosis but was clinically asymptomatic was found to have a loose acetabular shell at time of revision surgery. Given the increased morbidity associated with acetabular component revision, this patient’s condition represents a dangerous combination of RSL and clinically asymptomatic component loosening. By raising awareness about RSL and its incidence, we should be able to increase our ability to detect RSL. A surgeon who detects RSL before gross migration or movement of the acetabular component may be better able to plan for revision arthroplasty before a catastrophic event that may necessitate a larger, more complex procedure. With the number of patients who require revision THA continuing to rise, surgeons should be aware of the incidence of RSL and the potential of RSL to affect patient care and potential surgical options.
1. Milošev I, Kovač S, Trebše R, Levašič V, Pišot V. Comparison of ten-year survivorship of hip prostheses with use of conventional polyethylene, metal-on-metal, or ceramic-on-ceramic bearings. J Bone Joint Surg Am. 2012;94(19):1756-1763.
2. D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop Relat Res. 2012;470(2):373-381.
3. Dowd JE, Sychterz CJ, Young AM, Engh CA. Characterization of long-term femoral-head-penetration rates. Association with and prediction of osteolysis. J Bone Joint Surg Am. 2000;82(8):1102-1107.
4. Orishimo KF, Claus AM, Sychterz CJ, Engh CA. Relationship between polyethylene wear and osteolysis in hips with a second-generation porous-coated cementless cup after seven years of follow-up. J Bone Joint Surg Am. 2003;85(6):1095-1099.
5. Harris WH. Wear and periprosthetic osteolysis: the problem. Clin Orthop Relat Res. 2001;(393):66-70.
6. Holt G, Murnaghan C, Reilly J, Meek RM. The biology of aseptic osteolysis. Clin Orthop Relat Res. 2007;(460):240-252.
7. Catelas I, Jacobs JJ. Biologic activity of wear particles. Instr Course Lect. 2010;59:3-16.
8. Paprosky WG, Nourbash P, Gill P. Treatment of progressive periacetabular osteolysis: cup revision versus liner exchange and bone grafting. Paper presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; February 4-8, 1999; Anaheim, CA.
9. Engh CA Jr, Claus AM, Hopper RH Jr, Engh CA. Long-term results using the anatomic medullary locking hip prosthesis. Clin Orthop Relat Res. 2001;(393):137-146.
10. Maloney WJ, Peters P, Engh CA, Chandler H. Severe osteolysis of the pelvic in association with acetabular replacement without cement. J Bone Joint Surg Am. 1993;75(11):1627-1635.
11. Claus AM, Engh CA Jr, Sychterz CJ, Xenos JS, Orishimo KF, Engh CA Sr. Radiographic definition of pelvic osteolysis following total hip arthroplasty. J Bone Joint Surg Am. 2003;85(8):1519-1526.
12. Puri L, Wixson RL, Stern SH, Kohli J, Hendrix RW, Stulberg SD. Use of helical computed tomography for the assessment of acetabular osteolysis after total hip arthroplasty. J Bone Joint Surg Am. 2002;84(4):609-614.
13. Stulberg SD, Wixson RL, Adams AD, Hendrix RW, Bernfield JB. Monitoring pelvic osteolysis following total hip replacement surgery: an algorithm for surveillance. J Bone Joint Surg Am. 2002;84(suppl 2):116-122.
14. Massin P, Schmidt L, Engh CA. Evaluation of cementless acetabular component migration. An experimental study. J Arthroplasty. 1989;4(3):245-251.
15. Park KS, Yoon TR, Song EK, Lee KB. Results of isolated femoral component revision with well-fixed acetabular implant retention. J Arthroplasty. 2010;25(8):1188-1195.
16. Egawa H, Ho H, Hopper RH Jr, Engh CA Jr, Engh CA. Computed tomography assessment of pelvic osteolysis and cup–lesion interface involvement with a press-fit porous-coated acetabular cup. J Arthroplasty. 2009;24(2):233-239.
17. Haidukewych GJ. Osteolysis in the well-fixed socket: cup retention or revision? J Bone Joint Surg Br. 2012;94(12):65-69.
18. Stulberg BN, Della Valle AG. What are the guidelines for the surgical and nonsurgical treatment of periprosthetic osteolysis? J Am Acad Orthop Surg. 2008;16(suppl 1):S20-S25.
19. Berger RA, Quigley LR, Jacobs JJ, Sheinkop MB, Rosenberg AG, Galante JO. The fate of stable cemented acetabular components retained during revision of a femoral component of a total hip arthroplasty. J Bone Joint Surg Am. 1999;81(12):1682-1691.
20. Temmerman OP, Raijmakers PG, Deville WL, Berkhof J, Hooft L, Heyligers IC. The use of plain radiography, subtraction arthrography, nuclear arthrography, and bone scintigraphy in the diagnosis of a loose acetabular component of a total hip prosthesis: a systematic review. J Arthroplasty. 2007;22(6):818-827.
21. Temmerman OP, Raijmakers PG, David EF, et al. A comparison of radiographic and scintigraphic techniques to assess aseptic loosening of the acetabular component in a total hip replacement. J Bone Joint Surg Am. 2004;86(11):2456-2463.
22. Moore MS, McAuley JP, Young AM, Engh CA Sr. Radiographic signs of osseointegration in porous-coated acetabular components. Clin Orthop Relat Res. 2006;(444):176-183.
23. Mehin R, Yuan X, Haydon C, et al. Retroacetabular osteolysis: when to operate? Clin Orthop Relat Res. 2004;(428):247-255.
24. Mallory TH, Lombardi AV Jr, Fada RA, Adams JB, Kefauver CA, Eberle RW. Noncemented acetabular component removal in the presence of osteolysis: the affirmative. Clin Orthop Relat Res. 2000;(381):120-128.
25. Beaulé PE, Le Duff MJ, Dorey FJ, Amstutz HC. Fate of cementless acetabular components retained during revision total hip arthroplasty. J Bone Joint Surg Am. 2003;85(12):2288-2293.
1. Milošev I, Kovač S, Trebše R, Levašič V, Pišot V. Comparison of ten-year survivorship of hip prostheses with use of conventional polyethylene, metal-on-metal, or ceramic-on-ceramic bearings. J Bone Joint Surg Am. 2012;94(19):1756-1763.
2. D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop Relat Res. 2012;470(2):373-381.
3. Dowd JE, Sychterz CJ, Young AM, Engh CA. Characterization of long-term femoral-head-penetration rates. Association with and prediction of osteolysis. J Bone Joint Surg Am. 2000;82(8):1102-1107.
4. Orishimo KF, Claus AM, Sychterz CJ, Engh CA. Relationship between polyethylene wear and osteolysis in hips with a second-generation porous-coated cementless cup after seven years of follow-up. J Bone Joint Surg Am. 2003;85(6):1095-1099.
5. Harris WH. Wear and periprosthetic osteolysis: the problem. Clin Orthop Relat Res. 2001;(393):66-70.
6. Holt G, Murnaghan C, Reilly J, Meek RM. The biology of aseptic osteolysis. Clin Orthop Relat Res. 2007;(460):240-252.
7. Catelas I, Jacobs JJ. Biologic activity of wear particles. Instr Course Lect. 2010;59:3-16.
8. Paprosky WG, Nourbash P, Gill P. Treatment of progressive periacetabular osteolysis: cup revision versus liner exchange and bone grafting. Paper presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; February 4-8, 1999; Anaheim, CA.
9. Engh CA Jr, Claus AM, Hopper RH Jr, Engh CA. Long-term results using the anatomic medullary locking hip prosthesis. Clin Orthop Relat Res. 2001;(393):137-146.
10. Maloney WJ, Peters P, Engh CA, Chandler H. Severe osteolysis of the pelvic in association with acetabular replacement without cement. J Bone Joint Surg Am. 1993;75(11):1627-1635.
11. Claus AM, Engh CA Jr, Sychterz CJ, Xenos JS, Orishimo KF, Engh CA Sr. Radiographic definition of pelvic osteolysis following total hip arthroplasty. J Bone Joint Surg Am. 2003;85(8):1519-1526.
12. Puri L, Wixson RL, Stern SH, Kohli J, Hendrix RW, Stulberg SD. Use of helical computed tomography for the assessment of acetabular osteolysis after total hip arthroplasty. J Bone Joint Surg Am. 2002;84(4):609-614.
13. Stulberg SD, Wixson RL, Adams AD, Hendrix RW, Bernfield JB. Monitoring pelvic osteolysis following total hip replacement surgery: an algorithm for surveillance. J Bone Joint Surg Am. 2002;84(suppl 2):116-122.
14. Massin P, Schmidt L, Engh CA. Evaluation of cementless acetabular component migration. An experimental study. J Arthroplasty. 1989;4(3):245-251.
15. Park KS, Yoon TR, Song EK, Lee KB. Results of isolated femoral component revision with well-fixed acetabular implant retention. J Arthroplasty. 2010;25(8):1188-1195.
16. Egawa H, Ho H, Hopper RH Jr, Engh CA Jr, Engh CA. Computed tomography assessment of pelvic osteolysis and cup–lesion interface involvement with a press-fit porous-coated acetabular cup. J Arthroplasty. 2009;24(2):233-239.
17. Haidukewych GJ. Osteolysis in the well-fixed socket: cup retention or revision? J Bone Joint Surg Br. 2012;94(12):65-69.
18. Stulberg BN, Della Valle AG. What are the guidelines for the surgical and nonsurgical treatment of periprosthetic osteolysis? J Am Acad Orthop Surg. 2008;16(suppl 1):S20-S25.
19. Berger RA, Quigley LR, Jacobs JJ, Sheinkop MB, Rosenberg AG, Galante JO. The fate of stable cemented acetabular components retained during revision of a femoral component of a total hip arthroplasty. J Bone Joint Surg Am. 1999;81(12):1682-1691.
20. Temmerman OP, Raijmakers PG, Deville WL, Berkhof J, Hooft L, Heyligers IC. The use of plain radiography, subtraction arthrography, nuclear arthrography, and bone scintigraphy in the diagnosis of a loose acetabular component of a total hip prosthesis: a systematic review. J Arthroplasty. 2007;22(6):818-827.
21. Temmerman OP, Raijmakers PG, David EF, et al. A comparison of radiographic and scintigraphic techniques to assess aseptic loosening of the acetabular component in a total hip replacement. J Bone Joint Surg Am. 2004;86(11):2456-2463.
22. Moore MS, McAuley JP, Young AM, Engh CA Sr. Radiographic signs of osseointegration in porous-coated acetabular components. Clin Orthop Relat Res. 2006;(444):176-183.
23. Mehin R, Yuan X, Haydon C, et al. Retroacetabular osteolysis: when to operate? Clin Orthop Relat Res. 2004;(428):247-255.
24. Mallory TH, Lombardi AV Jr, Fada RA, Adams JB, Kefauver CA, Eberle RW. Noncemented acetabular component removal in the presence of osteolysis: the affirmative. Clin Orthop Relat Res. 2000;(381):120-128.
25. Beaulé PE, Le Duff MJ, Dorey FJ, Amstutz HC. Fate of cementless acetabular components retained during revision total hip arthroplasty. J Bone Joint Surg Am. 2003;85(12):2288-2293.