User login
Elliptical excision
Shave biopsy



Punch biopsy
Elbow nodules
A 42-year-old woman came to our clinic to have lumps on her right elbow removed. She said the lumps did not bother her, but on further questioning, admitted that her fingers turned white when they were exposed to the cold. She had frequent heartburn, but denied fatigue, weight loss, dysphagia, diarrhea, dyspnea, chest pain, palpitations, muscle weakness, or digital pain.
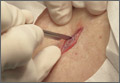
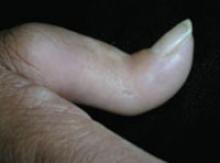
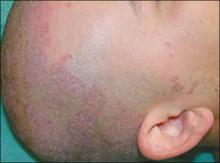
On physical exam, there were multiple small, firm subcutaneous nodules—some with a white surface protruding through the skin of her right elbow (FIGURE 1A). The nodules were slightly tender to palpation. On further examination we noted tight, smooth skin on her fingers (FIGURE 1B). Her right thumb was fixed in an extended position (FIGURE 2). There were also small blood vessels on her hands and pitted scars on her fingertips.
FIGURE 1
Elbow nodules and clubbing of the fingers
The 42-year-old patient sought care at the clinic to have slightly tender nodules removed from her right elbow. The patient also had tight skin and clubbing of the fingers.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
FIGURE 2
Thumb fixed in an extended position
The patient had tight skin on her fingers and a pitted scar on her thumb, which was fixed in an extended position.
Diagnosis: CREST syndrome
Our patient had CREST syndrome, a variant of limited systemic scleroderma. CREST syndrome is characterized by Calcinosis cutis, Raynaud’s phenomenon, Esophageal dysmotility, Sclerodactyly, and Telangiectasias.
Systemic scleroderma is a chronic auto-immune disease involving sclerotic, vascular, and inflammatory changes of the skin and internal organs. There are 19 new cases per million adults per year, with an estimated annual prevalence of 276 cases per million adults in the United States.1 Scleroderma occurs in women 4.6 times more often than in men; the mean age at diagnosis is 45 years.1 Although the pathogenesis of scleroderma remains unclear, interactions among leukocytes, endothelial cells, and fibroblasts are likely to be central in this disease.2
According to the 1980 American College of Rheumatology (ACR) guidelines,3 a diagnosis of systemic scleroderma can be made with either 1 major criterion or 2 minor criteria present. The major criterion is symmetric thickening, tightening, and induration of the skin proximal to the metatarsal-phalangeal or metacarpal-phalangeal joints. This may affect the whole extremity, trunk, neck, and face. The minor criteria include sclerodactyly, digital pitting scars or a loss of substance from the finger pads, and bibasilar pulmonary fibrosis.
Two forms of scleroderma. To improve sensitivity for milder forms of disease, the condition is often divided into diffuse systemic scleroderma (dSSc) or limited systemic scleroderma (lSSc) (TABLE 1), with CREST syndrome being a variant of the limited form.4 Patients with dSSc usually have a rapid diffuse involvement of the trunk, hands, feet, and face with early internal organ involvement. Patients with lSSc, however, usually have slow skin involvement limited to hands, feet, and face, and delayed systemic involvement, if any.
If CREST syndrome is suspected, it is important to look for its cardinal features. Cutaneous calcinosis usually presents over the bony prominences of knees, elbows, spine, and iliac crests, and may be painful. Patients may complain of Raynaud’s phenomenon with triphasic color changes, ie, pallor, cyanosis, and rubor, occurring months to years before sclerosis. Ulcerations at fingertips from Raynaud’s may be evident as pitted scars on physical exam. There is also a nonpitting edema of hands and feet that later progresses into sclerodactyly with tapering of fingers (our patient actually had clubbing). Patients may complain of stiffness of the hands and feet as the sclerosis progresses.
As a result of the edema and fibrosis of the face, patients may lose facial lines and comment that they look younger. Often, they will indicate that they have noticed the appearance of small blood vessels on their face, mouth, or hands. Patients may also complain of gastrointestinal problems such as esophageal reflux, diarrhea, or dysphagia.
TABLE 1
Characteristics of systemic scleroderma4-6
| Diffuse systemic scleroderma | Limited systemic scleroderma (Includes CREST syndrome) | |
|---|---|---|
| Constitutional | Fatigue and weight loss or gain | None |
| Vascular | Mild to moderate Raynaud’s phenomenon | Moderate to severe Raynaud’s phenomenon |
| Cutaneous | Sclerosis to trunk, arms, and face; rapid progression | Sclerosis to hands or toes and face; slow progression; calcinosis is prominent |
| Musculoskeletal | Arthralgias and deformities, muscle weakness, and tendon friction rubs | Arthralgias |
| Gastrointestinal | GERD, esophageal dysmotility, and malabsorption are common; all may be severe | Mild to moderate GERD and esophageal dysmotility are common; malabsorption is less common |
| Renal | Severe hypertension, and renal infarcts in renal crisis are common | Rare |
| Pulmonary | Pulmonary hypertension and interstitial lung disease are common | Uncommon |
| Cardiac | Cardiomyopathy, heart failure, and arrhythmias are common | Uncommon |
| GERD, gastroesophageal reflux disease. | ||
Differential includes morphea and scleromyxedema
A thorough history, physical exam, lab work, and possibly biopsy will help differentiate systemic scleroderma from the possible diagnoses with sclerosis listed below:
- Morphea features a localized, patchy distribution of skin fibrosis. There is no systemic involvement or Raynaud’s phenomenon.
- Mixed connective tissue disease has features of other autoimmune diseases, along with those of scleroderma.
- Eosinophilic fasciitis involves the fascia and muscle on biopsy. There is sparing of the hands.
- Scleromyxedema represents the skin thickening seen in patients with a gammopathy. Raynaud’s phenomenon may also be present.
- Scleredema is associated with diabetes. Skin changes are found mostly on the neck, shoulders, and upper arms. On rare occasions, there is visceral involvement. Raynaud’s phenomenon is not present.
In addition, the differential includes chronic graft-vs-host disease; lichen sclerosis et atrophicus; amyloidosis; porphyria cutanea et tardia; primary Raynaud’s phenomenon; and polyvinyl chloride, bleomycin, or pentazocyine exposure.
Nailfold capillary abnormalities help with the diagnosis
As noted earlier, diagnosing systemic scleroderma hinges on taking a good history, doing a thorough physical exam, applying the ACR diagnostic criteria, and ordering lab work. The sensitivity of the ACR criteria increases from 67% to 99% with the addition of nailfold capillary abnormalities (telangiectasias), identified using a dermatoscope.5
The initial lab work that you should consider includes an antinuclear antibody (ANA) test, complete blood count, and erythrocyte sedimentation rate. If the ANA is positive, anticentromere antibody (ACA) and DNA topoisomerase I (Scl-70) antibody tests should be ordered to see if scleroderma is likely limited or diffuse. ACA will be present in 21% of dSSc and 71% of lSSc cases, whereas Scl-70 will be present in 33% of dSSc and 18% of lSSc cases.6
If the diagnosis is still unclear, do a punch biopsy. Histology in the early phase will show mild cellular infiltrates around dermal blood vessels and at the dermal subcutaneous inter-phase, while the later phase will show thickening of dermis with broadening of collagen fibers and hyalinization of blood vessel walls.6 If lung, kidney, heart, or gastrointestinal involvement is suspected based on symptoms or physical exam, you’ll need to do an organ function work-up.
Treatment focuses on organ-specific problems
Currently, there are no guidelines for the treatment of scleroderma, given that the exact pathogenesis remains unclear and the disease course varies from patient to patient. Pharmacologic therapy is focused on symptoms and organ-specific problems (TABLE 2). Prazosin 1 to 3 mg TID is moderately effective in treating Raynaud’s phenomenon secondary to scleroderma7 (SOR: A). Losartan has been reported to reduce the frequency and severity of Raynaud’s phenomenon compared with a low dose of nifedepine,8 in a nonblinded randomized controlled trial (SOR: B). For interstitial lung disease, cyclophosphamide has been found to modestly reduce dyspnea while improving lung function, but it requires close monitoring9 (SOR: A). Bosentan is approved for symptomatic pulmonary hypertension and has been shown to decrease the occurrence of digital ulcers secondary to Raynaud’s phenomenon10 (SOR: A). (Bosentan is available in the United States under a special restricted distribution program called the Tracleer Access Program.)
Nonpharmacologic treatments should also be considered in the management of scleroderma. Advise patients that Raynaud’s phenomenon may be improved by avoiding exposure to the cold and by not smoking (SOR: C). Cutaneous ulcers can be protected with an occlusive dressing and treated with antibiotics if infected (SOR: C). To remove painful calcinotic nodules or release contractures secondary to sclerosis that may limit movement, you may want to consider surgery for your patient11 (SOR: C). If aesthetically unappealing, telangiectasias may be removed with electrosurgery or laser therapy (SOR: C).
Prognosis for scleroderma varies, depending on whether it is diffuse or limited. One large study found that patients with lSSc had a 10-year survival rate of 71%, compared with 21% for patients with dSSc (SOR: B).4 Patients with systemic sclerosis should be monitored for interstitial lung disease, pulmonary hypertension, renal failure, and cardiomyopathy (SOR: C). Prognosis worsens with renal, pulmonary, or cardiac involvement; pulmonary disease is the leading cause of death in dSSc.1
TABLE 2
Medical treatment of systemic scleroderma5-9
| Organ-specific problem/symptom | Treatment |
|---|---|
| Raynaud’s phenomenon | Nifedipine, verapamil, losartan, iloprost, prazosin, bosentan* |
| Pulmonary hypertension | Bosentan,* iloprost, captopril, enalapril, sildenafil |
| Interstitial lung disease | Cyclophosphamide, prednisone |
| Cardiomyopathy or arrhythmias | Antiarrhythmics, diuretics, digoxin, pacemaker, transplant |
| Renal failure or crisis | Captopril, kidney dialysis, transplant |
| Skin fibrosis | Methotrexate, cyclosporine, D-penicillamine |
| Arthralgias | Ibuprofen, acetaminophen |
| GERD, gastroparesis, diarrhea | H2 blockers, proton pump inhibitors, prokinetics, antibiotics |
| Pruritus | Antihistamines, low-dose topical steroids |
| GERD, gastroesophageal reflux disease. | |
| *Restricted access in the United States. | |
Our patient has a change of heart
Our patient had all the cardinal features of CREST syndrome on history and physical exam. She had an ANA of 1:640 with speckled pattern and ACA and Scl-70 were negative, demonstrating that diagnosis must be made in clinical context and not just based on lab markers. We treated her GERD with lifestyle changes and a proton pump inhibitor. We explained the risks and benefits of cutting out her calcinosis nodules and she chose not to have surgery.
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CORRESPONDENCE
Lucia Diaz, MD, 7703 Floyd Curl Drive, Mail #7816, San Antonio, TX 78229; Diazl3@uthscsa.edu
1. Mayes MD, Lacey JV, Jr, Beebe-Deemer J, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in large US population. Arthritis Rheum. 2003;48:2246-2255.
2. Hasegawa M, Sato S. The roles of chemokines in leukocyte recruitment in systemic sclerosis. Front Biosci. 2008;13:3637-3647.
3. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581-590.
4. Barnett AJ, Miller MH, Littlejohn GO. A survival study of patients with scleroderma diagnosed over 30 years (1953-1983): the value of a simple cutaneous classification in the early stages of disease. J Rheumatol. 1988;15:276-283.
5. Hudson M, Taillerfer S, Steele R, et al. Improving sensitivity of the American College of Rheumatology classification criteria for systemic sclerosis. Clin Exp Rheumatol. 2007;25:754-757.
6. Wolff K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 5th ed. New York, NY: McGraw-Hill; 2005.
7. Harding SE, Tingey PC, Pope J, et al. Prazosin for Raynaud’s phenomenon in progressive systemic sclerosis. Cochrane Database Syst Rev. 1998;(2):CD000956.-
8. Dziadzio M, Denton CP, Smith R, et al. Losartan therapy for Raynaud’s phenomenon and scleroderma. Arthritis Rheum. 1999;42:2646-2655.
9. Tashkin DP, Elashoff R, Clements P, et al. For the Scleroderma Lung Study Research Group. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354:2655-2666.
10. Korn JH, Mayes M, Matucci Cerinic M, et al. Digital ulcers in systemic sclerosis: prevention by treatment with bosentan, an oral endothelial receptor antagonist. Arthritis Rheum. 2004;50:3884-3995.
11. Melone CP, Jr, McLoughlin JC, Beldner S. Surgical management of the hand in scleroderma. Curr Opin Rheumatol. 1999;11:514-520.
A 42-year-old woman came to our clinic to have lumps on her right elbow removed. She said the lumps did not bother her, but on further questioning, admitted that her fingers turned white when they were exposed to the cold. She had frequent heartburn, but denied fatigue, weight loss, dysphagia, diarrhea, dyspnea, chest pain, palpitations, muscle weakness, or digital pain.
On physical exam, there were multiple small, firm subcutaneous nodules—some with a white surface protruding through the skin of her right elbow (FIGURE 1A). The nodules were slightly tender to palpation. On further examination we noted tight, smooth skin on her fingers (FIGURE 1B). Her right thumb was fixed in an extended position (FIGURE 2). There were also small blood vessels on her hands and pitted scars on her fingertips.
FIGURE 1
Elbow nodules and clubbing of the fingers
The 42-year-old patient sought care at the clinic to have slightly tender nodules removed from her right elbow. The patient also had tight skin and clubbing of the fingers.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
FIGURE 2
Thumb fixed in an extended position
The patient had tight skin on her fingers and a pitted scar on her thumb, which was fixed in an extended position.
Diagnosis: CREST syndrome
Our patient had CREST syndrome, a variant of limited systemic scleroderma. CREST syndrome is characterized by Calcinosis cutis, Raynaud’s phenomenon, Esophageal dysmotility, Sclerodactyly, and Telangiectasias.
Systemic scleroderma is a chronic auto-immune disease involving sclerotic, vascular, and inflammatory changes of the skin and internal organs. There are 19 new cases per million adults per year, with an estimated annual prevalence of 276 cases per million adults in the United States.1 Scleroderma occurs in women 4.6 times more often than in men; the mean age at diagnosis is 45 years.1 Although the pathogenesis of scleroderma remains unclear, interactions among leukocytes, endothelial cells, and fibroblasts are likely to be central in this disease.2
According to the 1980 American College of Rheumatology (ACR) guidelines,3 a diagnosis of systemic scleroderma can be made with either 1 major criterion or 2 minor criteria present. The major criterion is symmetric thickening, tightening, and induration of the skin proximal to the metatarsal-phalangeal or metacarpal-phalangeal joints. This may affect the whole extremity, trunk, neck, and face. The minor criteria include sclerodactyly, digital pitting scars or a loss of substance from the finger pads, and bibasilar pulmonary fibrosis.
Two forms of scleroderma. To improve sensitivity for milder forms of disease, the condition is often divided into diffuse systemic scleroderma (dSSc) or limited systemic scleroderma (lSSc) (TABLE 1), with CREST syndrome being a variant of the limited form.4 Patients with dSSc usually have a rapid diffuse involvement of the trunk, hands, feet, and face with early internal organ involvement. Patients with lSSc, however, usually have slow skin involvement limited to hands, feet, and face, and delayed systemic involvement, if any.
If CREST syndrome is suspected, it is important to look for its cardinal features. Cutaneous calcinosis usually presents over the bony prominences of knees, elbows, spine, and iliac crests, and may be painful. Patients may complain of Raynaud’s phenomenon with triphasic color changes, ie, pallor, cyanosis, and rubor, occurring months to years before sclerosis. Ulcerations at fingertips from Raynaud’s may be evident as pitted scars on physical exam. There is also a nonpitting edema of hands and feet that later progresses into sclerodactyly with tapering of fingers (our patient actually had clubbing). Patients may complain of stiffness of the hands and feet as the sclerosis progresses.
As a result of the edema and fibrosis of the face, patients may lose facial lines and comment that they look younger. Often, they will indicate that they have noticed the appearance of small blood vessels on their face, mouth, or hands. Patients may also complain of gastrointestinal problems such as esophageal reflux, diarrhea, or dysphagia.
TABLE 1
Characteristics of systemic scleroderma4-6
| Diffuse systemic scleroderma | Limited systemic scleroderma (Includes CREST syndrome) | |
|---|---|---|
| Constitutional | Fatigue and weight loss or gain | None |
| Vascular | Mild to moderate Raynaud’s phenomenon | Moderate to severe Raynaud’s phenomenon |
| Cutaneous | Sclerosis to trunk, arms, and face; rapid progression | Sclerosis to hands or toes and face; slow progression; calcinosis is prominent |
| Musculoskeletal | Arthralgias and deformities, muscle weakness, and tendon friction rubs | Arthralgias |
| Gastrointestinal | GERD, esophageal dysmotility, and malabsorption are common; all may be severe | Mild to moderate GERD and esophageal dysmotility are common; malabsorption is less common |
| Renal | Severe hypertension, and renal infarcts in renal crisis are common | Rare |
| Pulmonary | Pulmonary hypertension and interstitial lung disease are common | Uncommon |
| Cardiac | Cardiomyopathy, heart failure, and arrhythmias are common | Uncommon |
| GERD, gastroesophageal reflux disease. | ||
Differential includes morphea and scleromyxedema
A thorough history, physical exam, lab work, and possibly biopsy will help differentiate systemic scleroderma from the possible diagnoses with sclerosis listed below:
- Morphea features a localized, patchy distribution of skin fibrosis. There is no systemic involvement or Raynaud’s phenomenon.
- Mixed connective tissue disease has features of other autoimmune diseases, along with those of scleroderma.
- Eosinophilic fasciitis involves the fascia and muscle on biopsy. There is sparing of the hands.
- Scleromyxedema represents the skin thickening seen in patients with a gammopathy. Raynaud’s phenomenon may also be present.
- Scleredema is associated with diabetes. Skin changes are found mostly on the neck, shoulders, and upper arms. On rare occasions, there is visceral involvement. Raynaud’s phenomenon is not present.
In addition, the differential includes chronic graft-vs-host disease; lichen sclerosis et atrophicus; amyloidosis; porphyria cutanea et tardia; primary Raynaud’s phenomenon; and polyvinyl chloride, bleomycin, or pentazocyine exposure.
Nailfold capillary abnormalities help with the diagnosis
As noted earlier, diagnosing systemic scleroderma hinges on taking a good history, doing a thorough physical exam, applying the ACR diagnostic criteria, and ordering lab work. The sensitivity of the ACR criteria increases from 67% to 99% with the addition of nailfold capillary abnormalities (telangiectasias), identified using a dermatoscope.5
The initial lab work that you should consider includes an antinuclear antibody (ANA) test, complete blood count, and erythrocyte sedimentation rate. If the ANA is positive, anticentromere antibody (ACA) and DNA topoisomerase I (Scl-70) antibody tests should be ordered to see if scleroderma is likely limited or diffuse. ACA will be present in 21% of dSSc and 71% of lSSc cases, whereas Scl-70 will be present in 33% of dSSc and 18% of lSSc cases.6
If the diagnosis is still unclear, do a punch biopsy. Histology in the early phase will show mild cellular infiltrates around dermal blood vessels and at the dermal subcutaneous inter-phase, while the later phase will show thickening of dermis with broadening of collagen fibers and hyalinization of blood vessel walls.6 If lung, kidney, heart, or gastrointestinal involvement is suspected based on symptoms or physical exam, you’ll need to do an organ function work-up.
Treatment focuses on organ-specific problems
Currently, there are no guidelines for the treatment of scleroderma, given that the exact pathogenesis remains unclear and the disease course varies from patient to patient. Pharmacologic therapy is focused on symptoms and organ-specific problems (TABLE 2). Prazosin 1 to 3 mg TID is moderately effective in treating Raynaud’s phenomenon secondary to scleroderma7 (SOR: A). Losartan has been reported to reduce the frequency and severity of Raynaud’s phenomenon compared with a low dose of nifedepine,8 in a nonblinded randomized controlled trial (SOR: B). For interstitial lung disease, cyclophosphamide has been found to modestly reduce dyspnea while improving lung function, but it requires close monitoring9 (SOR: A). Bosentan is approved for symptomatic pulmonary hypertension and has been shown to decrease the occurrence of digital ulcers secondary to Raynaud’s phenomenon10 (SOR: A). (Bosentan is available in the United States under a special restricted distribution program called the Tracleer Access Program.)
Nonpharmacologic treatments should also be considered in the management of scleroderma. Advise patients that Raynaud’s phenomenon may be improved by avoiding exposure to the cold and by not smoking (SOR: C). Cutaneous ulcers can be protected with an occlusive dressing and treated with antibiotics if infected (SOR: C). To remove painful calcinotic nodules or release contractures secondary to sclerosis that may limit movement, you may want to consider surgery for your patient11 (SOR: C). If aesthetically unappealing, telangiectasias may be removed with electrosurgery or laser therapy (SOR: C).
Prognosis for scleroderma varies, depending on whether it is diffuse or limited. One large study found that patients with lSSc had a 10-year survival rate of 71%, compared with 21% for patients with dSSc (SOR: B).4 Patients with systemic sclerosis should be monitored for interstitial lung disease, pulmonary hypertension, renal failure, and cardiomyopathy (SOR: C). Prognosis worsens with renal, pulmonary, or cardiac involvement; pulmonary disease is the leading cause of death in dSSc.1
TABLE 2
Medical treatment of systemic scleroderma5-9
| Organ-specific problem/symptom | Treatment |
|---|---|
| Raynaud’s phenomenon | Nifedipine, verapamil, losartan, iloprost, prazosin, bosentan* |
| Pulmonary hypertension | Bosentan,* iloprost, captopril, enalapril, sildenafil |
| Interstitial lung disease | Cyclophosphamide, prednisone |
| Cardiomyopathy or arrhythmias | Antiarrhythmics, diuretics, digoxin, pacemaker, transplant |
| Renal failure or crisis | Captopril, kidney dialysis, transplant |
| Skin fibrosis | Methotrexate, cyclosporine, D-penicillamine |
| Arthralgias | Ibuprofen, acetaminophen |
| GERD, gastroparesis, diarrhea | H2 blockers, proton pump inhibitors, prokinetics, antibiotics |
| Pruritus | Antihistamines, low-dose topical steroids |
| GERD, gastroesophageal reflux disease. | |
| *Restricted access in the United States. | |
Our patient has a change of heart
Our patient had all the cardinal features of CREST syndrome on history and physical exam. She had an ANA of 1:640 with speckled pattern and ACA and Scl-70 were negative, demonstrating that diagnosis must be made in clinical context and not just based on lab markers. We treated her GERD with lifestyle changes and a proton pump inhibitor. We explained the risks and benefits of cutting out her calcinosis nodules and she chose not to have surgery.
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CORRESPONDENCE
Lucia Diaz, MD, 7703 Floyd Curl Drive, Mail #7816, San Antonio, TX 78229; Diazl3@uthscsa.edu
A 42-year-old woman came to our clinic to have lumps on her right elbow removed. She said the lumps did not bother her, but on further questioning, admitted that her fingers turned white when they were exposed to the cold. She had frequent heartburn, but denied fatigue, weight loss, dysphagia, diarrhea, dyspnea, chest pain, palpitations, muscle weakness, or digital pain.
On physical exam, there were multiple small, firm subcutaneous nodules—some with a white surface protruding through the skin of her right elbow (FIGURE 1A). The nodules were slightly tender to palpation. On further examination we noted tight, smooth skin on her fingers (FIGURE 1B). Her right thumb was fixed in an extended position (FIGURE 2). There were also small blood vessels on her hands and pitted scars on her fingertips.
FIGURE 1
Elbow nodules and clubbing of the fingers
The 42-year-old patient sought care at the clinic to have slightly tender nodules removed from her right elbow. The patient also had tight skin and clubbing of the fingers.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
FIGURE 2
Thumb fixed in an extended position
The patient had tight skin on her fingers and a pitted scar on her thumb, which was fixed in an extended position.
Diagnosis: CREST syndrome
Our patient had CREST syndrome, a variant of limited systemic scleroderma. CREST syndrome is characterized by Calcinosis cutis, Raynaud’s phenomenon, Esophageal dysmotility, Sclerodactyly, and Telangiectasias.
Systemic scleroderma is a chronic auto-immune disease involving sclerotic, vascular, and inflammatory changes of the skin and internal organs. There are 19 new cases per million adults per year, with an estimated annual prevalence of 276 cases per million adults in the United States.1 Scleroderma occurs in women 4.6 times more often than in men; the mean age at diagnosis is 45 years.1 Although the pathogenesis of scleroderma remains unclear, interactions among leukocytes, endothelial cells, and fibroblasts are likely to be central in this disease.2
According to the 1980 American College of Rheumatology (ACR) guidelines,3 a diagnosis of systemic scleroderma can be made with either 1 major criterion or 2 minor criteria present. The major criterion is symmetric thickening, tightening, and induration of the skin proximal to the metatarsal-phalangeal or metacarpal-phalangeal joints. This may affect the whole extremity, trunk, neck, and face. The minor criteria include sclerodactyly, digital pitting scars or a loss of substance from the finger pads, and bibasilar pulmonary fibrosis.
Two forms of scleroderma. To improve sensitivity for milder forms of disease, the condition is often divided into diffuse systemic scleroderma (dSSc) or limited systemic scleroderma (lSSc) (TABLE 1), with CREST syndrome being a variant of the limited form.4 Patients with dSSc usually have a rapid diffuse involvement of the trunk, hands, feet, and face with early internal organ involvement. Patients with lSSc, however, usually have slow skin involvement limited to hands, feet, and face, and delayed systemic involvement, if any.
If CREST syndrome is suspected, it is important to look for its cardinal features. Cutaneous calcinosis usually presents over the bony prominences of knees, elbows, spine, and iliac crests, and may be painful. Patients may complain of Raynaud’s phenomenon with triphasic color changes, ie, pallor, cyanosis, and rubor, occurring months to years before sclerosis. Ulcerations at fingertips from Raynaud’s may be evident as pitted scars on physical exam. There is also a nonpitting edema of hands and feet that later progresses into sclerodactyly with tapering of fingers (our patient actually had clubbing). Patients may complain of stiffness of the hands and feet as the sclerosis progresses.
As a result of the edema and fibrosis of the face, patients may lose facial lines and comment that they look younger. Often, they will indicate that they have noticed the appearance of small blood vessels on their face, mouth, or hands. Patients may also complain of gastrointestinal problems such as esophageal reflux, diarrhea, or dysphagia.
TABLE 1
Characteristics of systemic scleroderma4-6
| Diffuse systemic scleroderma | Limited systemic scleroderma (Includes CREST syndrome) | |
|---|---|---|
| Constitutional | Fatigue and weight loss or gain | None |
| Vascular | Mild to moderate Raynaud’s phenomenon | Moderate to severe Raynaud’s phenomenon |
| Cutaneous | Sclerosis to trunk, arms, and face; rapid progression | Sclerosis to hands or toes and face; slow progression; calcinosis is prominent |
| Musculoskeletal | Arthralgias and deformities, muscle weakness, and tendon friction rubs | Arthralgias |
| Gastrointestinal | GERD, esophageal dysmotility, and malabsorption are common; all may be severe | Mild to moderate GERD and esophageal dysmotility are common; malabsorption is less common |
| Renal | Severe hypertension, and renal infarcts in renal crisis are common | Rare |
| Pulmonary | Pulmonary hypertension and interstitial lung disease are common | Uncommon |
| Cardiac | Cardiomyopathy, heart failure, and arrhythmias are common | Uncommon |
| GERD, gastroesophageal reflux disease. | ||
Differential includes morphea and scleromyxedema
A thorough history, physical exam, lab work, and possibly biopsy will help differentiate systemic scleroderma from the possible diagnoses with sclerosis listed below:
- Morphea features a localized, patchy distribution of skin fibrosis. There is no systemic involvement or Raynaud’s phenomenon.
- Mixed connective tissue disease has features of other autoimmune diseases, along with those of scleroderma.
- Eosinophilic fasciitis involves the fascia and muscle on biopsy. There is sparing of the hands.
- Scleromyxedema represents the skin thickening seen in patients with a gammopathy. Raynaud’s phenomenon may also be present.
- Scleredema is associated with diabetes. Skin changes are found mostly on the neck, shoulders, and upper arms. On rare occasions, there is visceral involvement. Raynaud’s phenomenon is not present.
In addition, the differential includes chronic graft-vs-host disease; lichen sclerosis et atrophicus; amyloidosis; porphyria cutanea et tardia; primary Raynaud’s phenomenon; and polyvinyl chloride, bleomycin, or pentazocyine exposure.
Nailfold capillary abnormalities help with the diagnosis
As noted earlier, diagnosing systemic scleroderma hinges on taking a good history, doing a thorough physical exam, applying the ACR diagnostic criteria, and ordering lab work. The sensitivity of the ACR criteria increases from 67% to 99% with the addition of nailfold capillary abnormalities (telangiectasias), identified using a dermatoscope.5
The initial lab work that you should consider includes an antinuclear antibody (ANA) test, complete blood count, and erythrocyte sedimentation rate. If the ANA is positive, anticentromere antibody (ACA) and DNA topoisomerase I (Scl-70) antibody tests should be ordered to see if scleroderma is likely limited or diffuse. ACA will be present in 21% of dSSc and 71% of lSSc cases, whereas Scl-70 will be present in 33% of dSSc and 18% of lSSc cases.6
If the diagnosis is still unclear, do a punch biopsy. Histology in the early phase will show mild cellular infiltrates around dermal blood vessels and at the dermal subcutaneous inter-phase, while the later phase will show thickening of dermis with broadening of collagen fibers and hyalinization of blood vessel walls.6 If lung, kidney, heart, or gastrointestinal involvement is suspected based on symptoms or physical exam, you’ll need to do an organ function work-up.
Treatment focuses on organ-specific problems
Currently, there are no guidelines for the treatment of scleroderma, given that the exact pathogenesis remains unclear and the disease course varies from patient to patient. Pharmacologic therapy is focused on symptoms and organ-specific problems (TABLE 2). Prazosin 1 to 3 mg TID is moderately effective in treating Raynaud’s phenomenon secondary to scleroderma7 (SOR: A). Losartan has been reported to reduce the frequency and severity of Raynaud’s phenomenon compared with a low dose of nifedepine,8 in a nonblinded randomized controlled trial (SOR: B). For interstitial lung disease, cyclophosphamide has been found to modestly reduce dyspnea while improving lung function, but it requires close monitoring9 (SOR: A). Bosentan is approved for symptomatic pulmonary hypertension and has been shown to decrease the occurrence of digital ulcers secondary to Raynaud’s phenomenon10 (SOR: A). (Bosentan is available in the United States under a special restricted distribution program called the Tracleer Access Program.)
Nonpharmacologic treatments should also be considered in the management of scleroderma. Advise patients that Raynaud’s phenomenon may be improved by avoiding exposure to the cold and by not smoking (SOR: C). Cutaneous ulcers can be protected with an occlusive dressing and treated with antibiotics if infected (SOR: C). To remove painful calcinotic nodules or release contractures secondary to sclerosis that may limit movement, you may want to consider surgery for your patient11 (SOR: C). If aesthetically unappealing, telangiectasias may be removed with electrosurgery or laser therapy (SOR: C).
Prognosis for scleroderma varies, depending on whether it is diffuse or limited. One large study found that patients with lSSc had a 10-year survival rate of 71%, compared with 21% for patients with dSSc (SOR: B).4 Patients with systemic sclerosis should be monitored for interstitial lung disease, pulmonary hypertension, renal failure, and cardiomyopathy (SOR: C). Prognosis worsens with renal, pulmonary, or cardiac involvement; pulmonary disease is the leading cause of death in dSSc.1
TABLE 2
Medical treatment of systemic scleroderma5-9
| Organ-specific problem/symptom | Treatment |
|---|---|
| Raynaud’s phenomenon | Nifedipine, verapamil, losartan, iloprost, prazosin, bosentan* |
| Pulmonary hypertension | Bosentan,* iloprost, captopril, enalapril, sildenafil |
| Interstitial lung disease | Cyclophosphamide, prednisone |
| Cardiomyopathy or arrhythmias | Antiarrhythmics, diuretics, digoxin, pacemaker, transplant |
| Renal failure or crisis | Captopril, kidney dialysis, transplant |
| Skin fibrosis | Methotrexate, cyclosporine, D-penicillamine |
| Arthralgias | Ibuprofen, acetaminophen |
| GERD, gastroparesis, diarrhea | H2 blockers, proton pump inhibitors, prokinetics, antibiotics |
| Pruritus | Antihistamines, low-dose topical steroids |
| GERD, gastroesophageal reflux disease. | |
| *Restricted access in the United States. | |
Our patient has a change of heart
Our patient had all the cardinal features of CREST syndrome on history and physical exam. She had an ANA of 1:640 with speckled pattern and ACA and Scl-70 were negative, demonstrating that diagnosis must be made in clinical context and not just based on lab markers. We treated her GERD with lifestyle changes and a proton pump inhibitor. We explained the risks and benefits of cutting out her calcinosis nodules and she chose not to have surgery.
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CORRESPONDENCE
Lucia Diaz, MD, 7703 Floyd Curl Drive, Mail #7816, San Antonio, TX 78229; Diazl3@uthscsa.edu
1. Mayes MD, Lacey JV, Jr, Beebe-Deemer J, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in large US population. Arthritis Rheum. 2003;48:2246-2255.
2. Hasegawa M, Sato S. The roles of chemokines in leukocyte recruitment in systemic sclerosis. Front Biosci. 2008;13:3637-3647.
3. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581-590.
4. Barnett AJ, Miller MH, Littlejohn GO. A survival study of patients with scleroderma diagnosed over 30 years (1953-1983): the value of a simple cutaneous classification in the early stages of disease. J Rheumatol. 1988;15:276-283.
5. Hudson M, Taillerfer S, Steele R, et al. Improving sensitivity of the American College of Rheumatology classification criteria for systemic sclerosis. Clin Exp Rheumatol. 2007;25:754-757.
6. Wolff K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 5th ed. New York, NY: McGraw-Hill; 2005.
7. Harding SE, Tingey PC, Pope J, et al. Prazosin for Raynaud’s phenomenon in progressive systemic sclerosis. Cochrane Database Syst Rev. 1998;(2):CD000956.-
8. Dziadzio M, Denton CP, Smith R, et al. Losartan therapy for Raynaud’s phenomenon and scleroderma. Arthritis Rheum. 1999;42:2646-2655.
9. Tashkin DP, Elashoff R, Clements P, et al. For the Scleroderma Lung Study Research Group. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354:2655-2666.
10. Korn JH, Mayes M, Matucci Cerinic M, et al. Digital ulcers in systemic sclerosis: prevention by treatment with bosentan, an oral endothelial receptor antagonist. Arthritis Rheum. 2004;50:3884-3995.
11. Melone CP, Jr, McLoughlin JC, Beldner S. Surgical management of the hand in scleroderma. Curr Opin Rheumatol. 1999;11:514-520.
1. Mayes MD, Lacey JV, Jr, Beebe-Deemer J, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in large US population. Arthritis Rheum. 2003;48:2246-2255.
2. Hasegawa M, Sato S. The roles of chemokines in leukocyte recruitment in systemic sclerosis. Front Biosci. 2008;13:3637-3647.
3. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581-590.
4. Barnett AJ, Miller MH, Littlejohn GO. A survival study of patients with scleroderma diagnosed over 30 years (1953-1983): the value of a simple cutaneous classification in the early stages of disease. J Rheumatol. 1988;15:276-283.
5. Hudson M, Taillerfer S, Steele R, et al. Improving sensitivity of the American College of Rheumatology classification criteria for systemic sclerosis. Clin Exp Rheumatol. 2007;25:754-757.
6. Wolff K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 5th ed. New York, NY: McGraw-Hill; 2005.
7. Harding SE, Tingey PC, Pope J, et al. Prazosin for Raynaud’s phenomenon in progressive systemic sclerosis. Cochrane Database Syst Rev. 1998;(2):CD000956.-
8. Dziadzio M, Denton CP, Smith R, et al. Losartan therapy for Raynaud’s phenomenon and scleroderma. Arthritis Rheum. 1999;42:2646-2655.
9. Tashkin DP, Elashoff R, Clements P, et al. For the Scleroderma Lung Study Research Group. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354:2655-2666.
10. Korn JH, Mayes M, Matucci Cerinic M, et al. Digital ulcers in systemic sclerosis: prevention by treatment with bosentan, an oral endothelial receptor antagonist. Arthritis Rheum. 2004;50:3884-3995.
11. Melone CP, Jr, McLoughlin JC, Beldner S. Surgical management of the hand in scleroderma. Curr Opin Rheumatol. 1999;11:514-520.
A white spot since birth
A 13-year-old Hispanic girl came into our skin clinic with her grandmother for evaluation of suspicious moles on her arms. The grandmother was also concerned about a hypopigmented lesion on the young woman’s chest.
The patient and her grandmother said that the chest lesion had been there since birth, but it had been slowly growing over the years. The lesion was asymptomatic—there was no pruritus, bleeding, or pain. The patient was otherwise healthy and was not taking any medications.
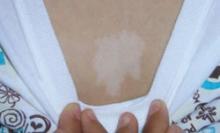
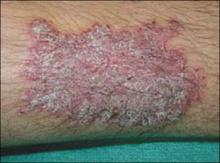
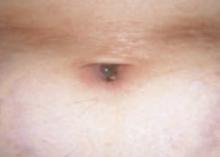
The patient and her grandmother indicated that no one in the family had a similar lesion. The patient had no fever or chills, nor any neurological, respiratory, cardiac, or gastrointestinal problems. The hypopigmented lesion on the patient’s chest had irregular borders and no scale (FIGURE 1).
There was no loss of sensation at the site and, upon applying pressure to the surrounding skin with a glass slide, the border between the lesion and normal skin disappeared.
FIGURE 1
Hypopigmented patch on chest
What is your diagnosis?
How would you manage this condition?
Diagnosis: Nevus anemicus
The patient had a nevus anemicus, which typically presents as an irregularly shaped hypopigmented patch on surrounding normal skin.1 Sometimes there are satellite macules, as well.2
Nevus anemicus is present at birth or appears shortly thereafter. It continues to grow with the child, and it remains asymptomatic. It is usually located on the trunk—primarily on the upper chest. However, there have been cases involving the extremities, head, and neck.3 The prevalence of this condition in the United States is unknown, but it is not considered rare. It is more common in females.4
Although nevus anemicus is an isolated finding in normal healthy individuals, it may occur in association with genodermatoses such as neurofibromatosis, and in conjunction with nevus flammeus and Mongolian spot in phakomatosis pigmentovascularis.1
Not a true nevus
This lesion is not a true nevus; rather, it is a congenital vascular anomaly with localized hypersensitivity to catecholamines. The vasoconstriction caused by this hypersensitivity results in skin pallor. When pressure is applied to the surrounding skin (diascopy), the border between the nevus and surrounding skin is lost due to blanching of surrounding skin.5
Intralesional injection of vasodilators, such as bradykinin, pilocarpine, acetylcholine, 5-hydroxytryptamine, nicotine, or histamine, fails to produce vasodilation in the affected areas.6 Axillary sympathetic block and intralesional injection of α-adrenergic blocking agents result in erythema.3 These findings support the conclusion that it is not a true nevus, but rather a vascular anomaly.
Differential Dx includes infectious diseases
The differential includes the following:
- Hansen’s disease (leprosy), caused by Mycobacterium leprae, presents with a loss of sensation at the site of hypopigmentation. This loss of sensation is due to nerve involvement. Histopathology yields a very specific pattern of epithelioid cell granulomas around the dermal nerves.7
- Tinea versicolor, caused by Malassezia furfur, has a fine scale on the hypopigmented patch. The lesion fluoresces under a Wood’s lamp, and a potassium hydroxide (KOH) preparation will be positive, revealing the well-known “spaghetti and meatballs” pattern. Hypopigmentation in tinea versicolor results from the inhibition of the enzyme tyrosinase in the melanocytes.7
- Vitiligo results from the complete absence of melanocytes. Vitiligo is rarely present at birth.
- Nevus depigmentosus, also known as nevus achromicus, is a well-demarcated patch of hypopigmentation that tends to occur on the trunk or proximal extremities in a dermatomal pattern (FIGURE 2). It is a true nevus and diascopy will not result in the loss of the border between the hypopigmented patch and surrounding skin.5
FIGURE 2
Don’t be fooled: This is not nevus anemicus
Make the diagnosis based on the exam
The diagnosis of nevus anemicus is made primarily based on the history and exam. A number of techniques aid in confirming the diagnosis and excluding some of the diagnoses mentioned above.
Diascopy results in the loss of the border between nevus anemicus and normal skin.4,5 Anatomic nevi do not demonstrate this loss in border. Shining a Wood’s lamp does not accentuate the lesion, helping to distinguish nevus anemicus from fungal infections that tend to fluoresce.
Neither friction (produced by scratching a line across both the lesion and normal surrounding skin), nor cold or heat application, induces erythema in the involved areas.2 And unlike leprosy, there is no loss of sensation at the site of the hypopigmentation. A biopsy of the lesion is not needed, but would reveal normal histology. Melanocytes are preserved and normally distributed. Electron microscopy, while not needed, would not detect any abnormalities in the vascular structure.4
Nothing to worry about for our patient
Our patient required no treatment. We simply provided her with some information on the benign nature of nevus anemicus. (In addition, we dealt with the moles on her arms that prompted her visit. They turned out to be normal melanocytic nevi.)
We told the patient that if the lesion on her chest bothered her, she could hide it with concealer make-up.1,2 Our patient and her grandmother were happy with the explanation and did not seek further care.
CORRESPONDENCE
Shehnaz Zaman, MD, 420 Elmington Avenue, #417, Nashville, TN 37205; shehnazzaman@gmail.com
1. Ahkami RN, Schwartz RA. Nevus anemicus. Dermatology. 1999;198:327-329.
2. Requena L, Sangueza OP. Cutaneous vascular anomalies. Part 1. Hamartomas, malformations, and dilation of preexisting vessels. J Am Acad Dermatol. 1997;37:523-549.
3. Mountcastle EA, Diestelmeier MR, Lupton GP. Nevus anemicus. J Am Acad Dermatol. 1986;14:628-632.
4. Knoepp TG, Davis L. Nevus anemicus. e Medicine Web site. Available at: http://www.emedicine.com/derm/topic292.htm. Accessed October 13, 2007.
5. Hsu S. Photo quiz: white patch on back. Am Fam Physician. 1999;60:1489-1490.
6. Greaves MW, Birkett D, Johnson C. Nevus anemicus: a unique catecholamine-dependent nevus. Arch Dermatol. 1970;102:172-176.
7. Wolff K, Johnson R, Suurmond R. Fitzpatrick’s Color Atlas & Synopsis of Clinical Dermatology. 5th ed. New York: McGraw Hill; 2005.
A 13-year-old Hispanic girl came into our skin clinic with her grandmother for evaluation of suspicious moles on her arms. The grandmother was also concerned about a hypopigmented lesion on the young woman’s chest.
The patient and her grandmother said that the chest lesion had been there since birth, but it had been slowly growing over the years. The lesion was asymptomatic—there was no pruritus, bleeding, or pain. The patient was otherwise healthy and was not taking any medications.
The patient and her grandmother indicated that no one in the family had a similar lesion. The patient had no fever or chills, nor any neurological, respiratory, cardiac, or gastrointestinal problems. The hypopigmented lesion on the patient’s chest had irregular borders and no scale (FIGURE 1).
There was no loss of sensation at the site and, upon applying pressure to the surrounding skin with a glass slide, the border between the lesion and normal skin disappeared.
FIGURE 1
Hypopigmented patch on chest
What is your diagnosis?
How would you manage this condition?
Diagnosis: Nevus anemicus
The patient had a nevus anemicus, which typically presents as an irregularly shaped hypopigmented patch on surrounding normal skin.1 Sometimes there are satellite macules, as well.2
Nevus anemicus is present at birth or appears shortly thereafter. It continues to grow with the child, and it remains asymptomatic. It is usually located on the trunk—primarily on the upper chest. However, there have been cases involving the extremities, head, and neck.3 The prevalence of this condition in the United States is unknown, but it is not considered rare. It is more common in females.4
Although nevus anemicus is an isolated finding in normal healthy individuals, it may occur in association with genodermatoses such as neurofibromatosis, and in conjunction with nevus flammeus and Mongolian spot in phakomatosis pigmentovascularis.1
Not a true nevus
This lesion is not a true nevus; rather, it is a congenital vascular anomaly with localized hypersensitivity to catecholamines. The vasoconstriction caused by this hypersensitivity results in skin pallor. When pressure is applied to the surrounding skin (diascopy), the border between the nevus and surrounding skin is lost due to blanching of surrounding skin.5
Intralesional injection of vasodilators, such as bradykinin, pilocarpine, acetylcholine, 5-hydroxytryptamine, nicotine, or histamine, fails to produce vasodilation in the affected areas.6 Axillary sympathetic block and intralesional injection of α-adrenergic blocking agents result in erythema.3 These findings support the conclusion that it is not a true nevus, but rather a vascular anomaly.
Differential Dx includes infectious diseases
The differential includes the following:
- Hansen’s disease (leprosy), caused by Mycobacterium leprae, presents with a loss of sensation at the site of hypopigmentation. This loss of sensation is due to nerve involvement. Histopathology yields a very specific pattern of epithelioid cell granulomas around the dermal nerves.7
- Tinea versicolor, caused by Malassezia furfur, has a fine scale on the hypopigmented patch. The lesion fluoresces under a Wood’s lamp, and a potassium hydroxide (KOH) preparation will be positive, revealing the well-known “spaghetti and meatballs” pattern. Hypopigmentation in tinea versicolor results from the inhibition of the enzyme tyrosinase in the melanocytes.7
- Vitiligo results from the complete absence of melanocytes. Vitiligo is rarely present at birth.
- Nevus depigmentosus, also known as nevus achromicus, is a well-demarcated patch of hypopigmentation that tends to occur on the trunk or proximal extremities in a dermatomal pattern (FIGURE 2). It is a true nevus and diascopy will not result in the loss of the border between the hypopigmented patch and surrounding skin.5
FIGURE 2
Don’t be fooled: This is not nevus anemicus
Make the diagnosis based on the exam
The diagnosis of nevus anemicus is made primarily based on the history and exam. A number of techniques aid in confirming the diagnosis and excluding some of the diagnoses mentioned above.
Diascopy results in the loss of the border between nevus anemicus and normal skin.4,5 Anatomic nevi do not demonstrate this loss in border. Shining a Wood’s lamp does not accentuate the lesion, helping to distinguish nevus anemicus from fungal infections that tend to fluoresce.
Neither friction (produced by scratching a line across both the lesion and normal surrounding skin), nor cold or heat application, induces erythema in the involved areas.2 And unlike leprosy, there is no loss of sensation at the site of the hypopigmentation. A biopsy of the lesion is not needed, but would reveal normal histology. Melanocytes are preserved and normally distributed. Electron microscopy, while not needed, would not detect any abnormalities in the vascular structure.4
Nothing to worry about for our patient
Our patient required no treatment. We simply provided her with some information on the benign nature of nevus anemicus. (In addition, we dealt with the moles on her arms that prompted her visit. They turned out to be normal melanocytic nevi.)
We told the patient that if the lesion on her chest bothered her, she could hide it with concealer make-up.1,2 Our patient and her grandmother were happy with the explanation and did not seek further care.
CORRESPONDENCE
Shehnaz Zaman, MD, 420 Elmington Avenue, #417, Nashville, TN 37205; shehnazzaman@gmail.com
A 13-year-old Hispanic girl came into our skin clinic with her grandmother for evaluation of suspicious moles on her arms. The grandmother was also concerned about a hypopigmented lesion on the young woman’s chest.
The patient and her grandmother said that the chest lesion had been there since birth, but it had been slowly growing over the years. The lesion was asymptomatic—there was no pruritus, bleeding, or pain. The patient was otherwise healthy and was not taking any medications.
The patient and her grandmother indicated that no one in the family had a similar lesion. The patient had no fever or chills, nor any neurological, respiratory, cardiac, or gastrointestinal problems. The hypopigmented lesion on the patient’s chest had irregular borders and no scale (FIGURE 1).
There was no loss of sensation at the site and, upon applying pressure to the surrounding skin with a glass slide, the border between the lesion and normal skin disappeared.
FIGURE 1
Hypopigmented patch on chest
What is your diagnosis?
How would you manage this condition?
Diagnosis: Nevus anemicus
The patient had a nevus anemicus, which typically presents as an irregularly shaped hypopigmented patch on surrounding normal skin.1 Sometimes there are satellite macules, as well.2
Nevus anemicus is present at birth or appears shortly thereafter. It continues to grow with the child, and it remains asymptomatic. It is usually located on the trunk—primarily on the upper chest. However, there have been cases involving the extremities, head, and neck.3 The prevalence of this condition in the United States is unknown, but it is not considered rare. It is more common in females.4
Although nevus anemicus is an isolated finding in normal healthy individuals, it may occur in association with genodermatoses such as neurofibromatosis, and in conjunction with nevus flammeus and Mongolian spot in phakomatosis pigmentovascularis.1
Not a true nevus
This lesion is not a true nevus; rather, it is a congenital vascular anomaly with localized hypersensitivity to catecholamines. The vasoconstriction caused by this hypersensitivity results in skin pallor. When pressure is applied to the surrounding skin (diascopy), the border between the nevus and surrounding skin is lost due to blanching of surrounding skin.5
Intralesional injection of vasodilators, such as bradykinin, pilocarpine, acetylcholine, 5-hydroxytryptamine, nicotine, or histamine, fails to produce vasodilation in the affected areas.6 Axillary sympathetic block and intralesional injection of α-adrenergic blocking agents result in erythema.3 These findings support the conclusion that it is not a true nevus, but rather a vascular anomaly.
Differential Dx includes infectious diseases
The differential includes the following:
- Hansen’s disease (leprosy), caused by Mycobacterium leprae, presents with a loss of sensation at the site of hypopigmentation. This loss of sensation is due to nerve involvement. Histopathology yields a very specific pattern of epithelioid cell granulomas around the dermal nerves.7
- Tinea versicolor, caused by Malassezia furfur, has a fine scale on the hypopigmented patch. The lesion fluoresces under a Wood’s lamp, and a potassium hydroxide (KOH) preparation will be positive, revealing the well-known “spaghetti and meatballs” pattern. Hypopigmentation in tinea versicolor results from the inhibition of the enzyme tyrosinase in the melanocytes.7
- Vitiligo results from the complete absence of melanocytes. Vitiligo is rarely present at birth.
- Nevus depigmentosus, also known as nevus achromicus, is a well-demarcated patch of hypopigmentation that tends to occur on the trunk or proximal extremities in a dermatomal pattern (FIGURE 2). It is a true nevus and diascopy will not result in the loss of the border between the hypopigmented patch and surrounding skin.5
FIGURE 2
Don’t be fooled: This is not nevus anemicus
Make the diagnosis based on the exam
The diagnosis of nevus anemicus is made primarily based on the history and exam. A number of techniques aid in confirming the diagnosis and excluding some of the diagnoses mentioned above.
Diascopy results in the loss of the border between nevus anemicus and normal skin.4,5 Anatomic nevi do not demonstrate this loss in border. Shining a Wood’s lamp does not accentuate the lesion, helping to distinguish nevus anemicus from fungal infections that tend to fluoresce.
Neither friction (produced by scratching a line across both the lesion and normal surrounding skin), nor cold or heat application, induces erythema in the involved areas.2 And unlike leprosy, there is no loss of sensation at the site of the hypopigmentation. A biopsy of the lesion is not needed, but would reveal normal histology. Melanocytes are preserved and normally distributed. Electron microscopy, while not needed, would not detect any abnormalities in the vascular structure.4
Nothing to worry about for our patient
Our patient required no treatment. We simply provided her with some information on the benign nature of nevus anemicus. (In addition, we dealt with the moles on her arms that prompted her visit. They turned out to be normal melanocytic nevi.)
We told the patient that if the lesion on her chest bothered her, she could hide it with concealer make-up.1,2 Our patient and her grandmother were happy with the explanation and did not seek further care.
CORRESPONDENCE
Shehnaz Zaman, MD, 420 Elmington Avenue, #417, Nashville, TN 37205; shehnazzaman@gmail.com
1. Ahkami RN, Schwartz RA. Nevus anemicus. Dermatology. 1999;198:327-329.
2. Requena L, Sangueza OP. Cutaneous vascular anomalies. Part 1. Hamartomas, malformations, and dilation of preexisting vessels. J Am Acad Dermatol. 1997;37:523-549.
3. Mountcastle EA, Diestelmeier MR, Lupton GP. Nevus anemicus. J Am Acad Dermatol. 1986;14:628-632.
4. Knoepp TG, Davis L. Nevus anemicus. e Medicine Web site. Available at: http://www.emedicine.com/derm/topic292.htm. Accessed October 13, 2007.
5. Hsu S. Photo quiz: white patch on back. Am Fam Physician. 1999;60:1489-1490.
6. Greaves MW, Birkett D, Johnson C. Nevus anemicus: a unique catecholamine-dependent nevus. Arch Dermatol. 1970;102:172-176.
7. Wolff K, Johnson R, Suurmond R. Fitzpatrick’s Color Atlas & Synopsis of Clinical Dermatology. 5th ed. New York: McGraw Hill; 2005.
1. Ahkami RN, Schwartz RA. Nevus anemicus. Dermatology. 1999;198:327-329.
2. Requena L, Sangueza OP. Cutaneous vascular anomalies. Part 1. Hamartomas, malformations, and dilation of preexisting vessels. J Am Acad Dermatol. 1997;37:523-549.
3. Mountcastle EA, Diestelmeier MR, Lupton GP. Nevus anemicus. J Am Acad Dermatol. 1986;14:628-632.
4. Knoepp TG, Davis L. Nevus anemicus. e Medicine Web site. Available at: http://www.emedicine.com/derm/topic292.htm. Accessed October 13, 2007.
5. Hsu S. Photo quiz: white patch on back. Am Fam Physician. 1999;60:1489-1490.
6. Greaves MW, Birkett D, Johnson C. Nevus anemicus: a unique catecholamine-dependent nevus. Arch Dermatol. 1970;102:172-176.
7. Wolff K, Johnson R, Suurmond R. Fitzpatrick’s Color Atlas & Synopsis of Clinical Dermatology. 5th ed. New York: McGraw Hill; 2005.
Pruritus in pregnancy
A 32-year-old mother of 2 came into our facility during her 31st week of pregnancy and told us that she couldn’t stop itching. She said that her whole body was itchy and it got worse at night. She was unable to get a good night’s sleep. Up until this point, her pregnancy had been uncomplicated and she had no past history of medical problems.
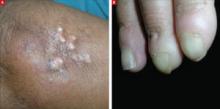
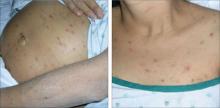
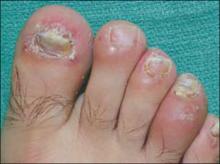
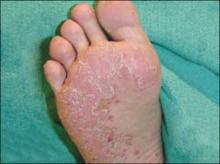
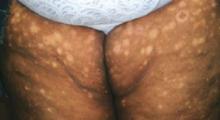
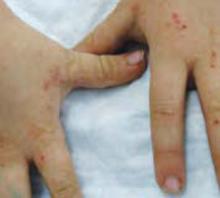
An examination revealed excoriations—but no blistering—on her abdomen, chest, arms, and legs (FIGURES 1 AND 2). She had no jaundice or scleral icterus. The fundal height was 31 mm and the fetal heart tones were 150 bpm.
FIGURE 1 & 2
Excoriations on the abdomen and chest
What is your diagnosis?
How would you manage this condition?
Diagnosis: Intrahepatic cholestasis of pregnancy
Intrahepatic cholestasis of pregnancy (ICP) is caused by maternal intrahepatic bile secretory dysfunction.1 The disorder, which is also referred to as obstetric cholestasis and pruritus gravidarum, has no primary skin lesions. Patients have generalized pruritus and secondary excoriations (FIGURE 3). In about 20% of cases, patients are also jaundiced.2
The sudden onset of generalized pruritus, which is the hallmark of ICP, starts during the late second (20%) or third (80%) trimester, followed by secondary skin lesions, namely linear excoriations and excoriated papules caused by scratching.3 These excoriations are typically localized on the extensor surfaces of the limbs, but may also be found on the abdomen and back. The itching may involve the palms and soles, as well. The severity of the skin lesions correlates with the duration and degree of pruritus.3
According to one study, ICP occurred in 0.5% of 3192 pregnancies. The disorder resolves after delivery, and recurs with subsequent pregnancies.4
ICP has been linked to fetal distress (20%–30%), stillbirths (1%–2%), and preterm delivery (20%–60%).3 Autopsies of the placenta have shown signs of acute anoxia. Fetal complications in ICP may be caused by decreased fetal elimination of toxic bile acids.3
Hormones, genes, and even the weather may play a role
Increased hormone production during pregnancy plays a role in ICP. Estrogen, which increases 100-fold during pregnancy, interferes with bile acid secretion across the basolateral and canalicular membrane of the hepatocyte. Particularly noteworthy is the fact that estriol-16 α-D-glucuronide, the estrogen metabolite that increases most during pregnancy, is cholestatic, according to animal studies.3 In addition, progesterone metabolites play an important part in the pathogenesis of ICP. Progesterone inhibits hepatic glucuronyl transferase, thereby reducing the clearance of estrogens and amplifying their effects.
Familial clustering and geographical variation indicate that there is a genetic predisposition for ICP. There is a high prevalence of ICP in Chile (14%), especially among Araucanian Indian women (24%), and in Bolivia.3 ICP patients may have a family history of cholelithiasis and a higher risk of gallstones. There is a family history of cholelithiasis in 50% of ICP cases.2
Exogenous factors have been implicated in the pathogenesis of ICP. There is a higher incidence of ICP during the winter. Low selenium levels have also been linked to ICP. This suggests that the environment and nutritional factors play a role.2
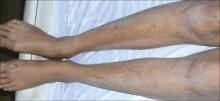
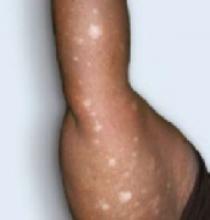
FIGURE 3
Excoriations down the legs
The differential Dx includes scabies
Itching has been reported to occur in 17% of pregnancies,2 so it is important to differentiate ICP from the conditions listed below.
- Pruritic urticarial papules and plaques of pregnancy—also known as polymorphic eruption of pregnancy—is a dermatosis of pregnancy. Unlike the excoriations of ICP, this condition involves papulovesicular or urticarial eruptions on the trunk and extremities. It is particularly pronounced around the abdominal striae, and is more common in nulliparous women.
- Pemphigoid gestationis, also known as herpes gestationis because of its appearance, is a bullous or blistering disease that is associated with pregnancy. It is often periumbilical and can also have target lesions, which are absent in ICP.
- Atopic eruption of pregnancy is a new term used to include previous non-specific diagnoses such as prurigo of pregnancy and pruritic folliculitis of pregnancy. Prurigo of pregnancy, which is also called Besnier’s prurigo gestationis, involves bite-like papules that resemble scabies. Pruritic folliculitis of pregnancy is characterized by red, follicle-based papules. These 2 conditions differ from ICP in that there is no cholestasis and liver studies are normal. (In ICP, there is an elevation in liver enzymes and serum bile acids.)
- Scabies infestation can occur during pregnancy. The mite burrows in the skin and produces severe itching between the fingers and in skin folds. Look for burrows and the typical distribution between the fingers, on the wrists, in the axilla, and around the waist. A positive scraping viewed under the microscope will show mites, eggs, and mite feces.
If you suspect ICP in a patient who is also jaundiced, you’ll also need to rule out several other conditions. These include:
- acute liver disease of pregnancy
- preeclampsia complicated by increased liver enzymes
- hyperemesis gravidarum
- viral hepatitis
- drug reaction
- obstructive biliary disease, such as a gallstone lodged in the common bile duct.
Order a blood chemistry or liver profile
If you suspect that your patient has ICP, start by ordering a blood chemistry or liver profile. If any of the liver tests are elevated, order a total bile acid level (which is the most sensitive indicator of ICP) and a hepatitis panel (or specific hepatitis tests based on the patient’s history of exposures and vaccinations). If there is laboratory evidence of cholestasis, a right upper quadrant ultrasound will help you to spot gallstones and evidence of obstruction.
In ICP, there will be mild abnormalities of the liver function tests, including transaminases, alkaline phosphatase, and bilirubin. Bilirubin may be mildly to moderately elevated (2–5 mg/dL). (Jaundice is seen only at the higher levels of bilirubin.) Our patient’s tests, for instance, revealed that her ALT and AST were both over 300; her total bilirubin was elevated at 2.1.
Serum levels of bile acid correlate with the severity of pruritus. Our patient’s bile acids were elevated and her hepatitis panel was negative. Her ultrasound showed gallstones, but we saw no obstruction. An ICP patient’s lipid profile may show mild elevations in total cholesterol and triglycerides, as was the case for our patient.
Malabsorption of fat may cause vitamin K deficiency resulting in a prolonged prothrombin time. Liver biopsy is unnecessary in suspected cases of ICP, but would show cholestatic changes such as dilated bile canaliculi, bile pigment in the parenchyma, and minimal inflammation.
Skin biopsy is not helpful in ICP
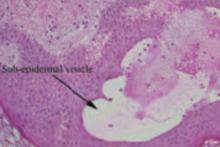
In suspected cases of ICP, skin biopsy will only reveal a spectrum of non-specific findings. It is, however, helpful if you suspect pemphigoid gestationis, since it will reveal subepidermal blisters. Similarly, biopsy for direct immunofluorescence is nonspecific in ICP, but helpful in pemphigoid gestationis.
Soothing baths can help, ursodiol is most effective
Mild cholestasis responds to symptomatic treatment with soothing baths, topical antipruritics, emollients, and primrose oil, among others. Antihistamines are rarely effective. Anion exchange resins, such as cholestyramine, can be helpful, too; they bind bile acids and decrease their enterohepatic circulation.2
Patients who do not respond to cholestyramine, or who cannot tolerate it, may be treated with ursodeoxycholic acid (ursodiol). The research indicates that ursodiol works faster than cholestyramine, has a more sustained effect on pruritus, and is more effective in improving the biochemical abnormalities of ICP (strength of recommendation [SOR]: A, based on good-quality patient-oriented evidence). Ursodiol is considered safe for both mother and fetus.5 For all of these reasons, ursodiol has replaced cholestyramine as the first-line agent for ICP.
Doses range from 1 g/day to high doses of 1.5 to 2.0 g/d.6 The dose is maintained until delivery. Davies et al5 suggest that the use of ursodiol can reduce fetal mortality associated with ICP (SOR: C, based on consensus, usual practice, opinion, disease-oriented evidence, case series).
Weekly non-stress tests beginning at the 34th week of gestation are advisable (SOR: C).2 Labor may need to be induced in the 38th week in mild cases of ICP, and in the 36th week in severe cases (SOR: C).2
Ursodiol for our patient, labor was induced
We treated our patient with oral ursodiol and topical 1% hydrocortisone cream. Her bile acids and transaminase levels dropped and her pruritus improved—though it did not completely resolve until after delivery. Our obstetrics department recommended weekly non-stress tests starting at the 34th week of gestation. The non-stress tests were reactive. Due to the severity of her condition, labor was induced at 36 weeks.
Our patient had a healthy baby girl without complications. After delivery, the itching went away completely and her skin began to heal from all of those excoriations. Our patient is planning an elective cholecystectomy in the coming months because she doesn’t want to take a chance that she might have problems with her gallstones in the future.
Disclosure
1. Galaria NA, Mercurio MG. Dermatoses of pregnancy. The Female Patient 2003. Available at: www.femalepatient.com/html/arc/sel/may03/028_05_024.asp. Accessed on October 8, 2007.
2. Kroumpouzos G. Intrahepatic cholestasis of pregnancy: what’s new. European Academy of Dermatology and Venereology 2002;16:316-318.
3. Ambros-Rudolph CM, Müllegger RR, Vaughan Jones SA, et al. The specific dermatoses of pregnancy revisited and reclassified: results of a retrospective two-center study on 505 pregnant patients. J Am Acad Dermatol 2006;54:395-404.
4. Odom R, James W. Andrews’Diseases of the Skin. 10th ed. Philadelphia, PA: WB Saunders Company; 2006.
5. Davies MH, da Silva RCMA, Jones SR, et al. Fetal mortality associated with cholestasis of pregnancy and the potential benefit of therapy with ursodeoxycholic acid. Gut 1995;37:580-584.
6. Mazzella G, Nicola R, Francesco A, et al. Ursodeoxycholic acid administration in patients with cholestasis of pregnancy: Effects on primary bile acids in babies and mothers. Hepatology 2001;33:504-508.
A 32-year-old mother of 2 came into our facility during her 31st week of pregnancy and told us that she couldn’t stop itching. She said that her whole body was itchy and it got worse at night. She was unable to get a good night’s sleep. Up until this point, her pregnancy had been uncomplicated and she had no past history of medical problems.
An examination revealed excoriations—but no blistering—on her abdomen, chest, arms, and legs (FIGURES 1 AND 2). She had no jaundice or scleral icterus. The fundal height was 31 mm and the fetal heart tones were 150 bpm.
FIGURE 1 & 2
Excoriations on the abdomen and chest
What is your diagnosis?
How would you manage this condition?
Diagnosis: Intrahepatic cholestasis of pregnancy
Intrahepatic cholestasis of pregnancy (ICP) is caused by maternal intrahepatic bile secretory dysfunction.1 The disorder, which is also referred to as obstetric cholestasis and pruritus gravidarum, has no primary skin lesions. Patients have generalized pruritus and secondary excoriations (FIGURE 3). In about 20% of cases, patients are also jaundiced.2
The sudden onset of generalized pruritus, which is the hallmark of ICP, starts during the late second (20%) or third (80%) trimester, followed by secondary skin lesions, namely linear excoriations and excoriated papules caused by scratching.3 These excoriations are typically localized on the extensor surfaces of the limbs, but may also be found on the abdomen and back. The itching may involve the palms and soles, as well. The severity of the skin lesions correlates with the duration and degree of pruritus.3
According to one study, ICP occurred in 0.5% of 3192 pregnancies. The disorder resolves after delivery, and recurs with subsequent pregnancies.4
ICP has been linked to fetal distress (20%–30%), stillbirths (1%–2%), and preterm delivery (20%–60%).3 Autopsies of the placenta have shown signs of acute anoxia. Fetal complications in ICP may be caused by decreased fetal elimination of toxic bile acids.3
Hormones, genes, and even the weather may play a role
Increased hormone production during pregnancy plays a role in ICP. Estrogen, which increases 100-fold during pregnancy, interferes with bile acid secretion across the basolateral and canalicular membrane of the hepatocyte. Particularly noteworthy is the fact that estriol-16 α-D-glucuronide, the estrogen metabolite that increases most during pregnancy, is cholestatic, according to animal studies.3 In addition, progesterone metabolites play an important part in the pathogenesis of ICP. Progesterone inhibits hepatic glucuronyl transferase, thereby reducing the clearance of estrogens and amplifying their effects.
Familial clustering and geographical variation indicate that there is a genetic predisposition for ICP. There is a high prevalence of ICP in Chile (14%), especially among Araucanian Indian women (24%), and in Bolivia.3 ICP patients may have a family history of cholelithiasis and a higher risk of gallstones. There is a family history of cholelithiasis in 50% of ICP cases.2
Exogenous factors have been implicated in the pathogenesis of ICP. There is a higher incidence of ICP during the winter. Low selenium levels have also been linked to ICP. This suggests that the environment and nutritional factors play a role.2
FIGURE 3
Excoriations down the legs
The differential Dx includes scabies
Itching has been reported to occur in 17% of pregnancies,2 so it is important to differentiate ICP from the conditions listed below.
- Pruritic urticarial papules and plaques of pregnancy—also known as polymorphic eruption of pregnancy—is a dermatosis of pregnancy. Unlike the excoriations of ICP, this condition involves papulovesicular or urticarial eruptions on the trunk and extremities. It is particularly pronounced around the abdominal striae, and is more common in nulliparous women.
- Pemphigoid gestationis, also known as herpes gestationis because of its appearance, is a bullous or blistering disease that is associated with pregnancy. It is often periumbilical and can also have target lesions, which are absent in ICP.
- Atopic eruption of pregnancy is a new term used to include previous non-specific diagnoses such as prurigo of pregnancy and pruritic folliculitis of pregnancy. Prurigo of pregnancy, which is also called Besnier’s prurigo gestationis, involves bite-like papules that resemble scabies. Pruritic folliculitis of pregnancy is characterized by red, follicle-based papules. These 2 conditions differ from ICP in that there is no cholestasis and liver studies are normal. (In ICP, there is an elevation in liver enzymes and serum bile acids.)
- Scabies infestation can occur during pregnancy. The mite burrows in the skin and produces severe itching between the fingers and in skin folds. Look for burrows and the typical distribution between the fingers, on the wrists, in the axilla, and around the waist. A positive scraping viewed under the microscope will show mites, eggs, and mite feces.
If you suspect ICP in a patient who is also jaundiced, you’ll also need to rule out several other conditions. These include:
- acute liver disease of pregnancy
- preeclampsia complicated by increased liver enzymes
- hyperemesis gravidarum
- viral hepatitis
- drug reaction
- obstructive biliary disease, such as a gallstone lodged in the common bile duct.
Order a blood chemistry or liver profile
If you suspect that your patient has ICP, start by ordering a blood chemistry or liver profile. If any of the liver tests are elevated, order a total bile acid level (which is the most sensitive indicator of ICP) and a hepatitis panel (or specific hepatitis tests based on the patient’s history of exposures and vaccinations). If there is laboratory evidence of cholestasis, a right upper quadrant ultrasound will help you to spot gallstones and evidence of obstruction.
In ICP, there will be mild abnormalities of the liver function tests, including transaminases, alkaline phosphatase, and bilirubin. Bilirubin may be mildly to moderately elevated (2–5 mg/dL). (Jaundice is seen only at the higher levels of bilirubin.) Our patient’s tests, for instance, revealed that her ALT and AST were both over 300; her total bilirubin was elevated at 2.1.
Serum levels of bile acid correlate with the severity of pruritus. Our patient’s bile acids were elevated and her hepatitis panel was negative. Her ultrasound showed gallstones, but we saw no obstruction. An ICP patient’s lipid profile may show mild elevations in total cholesterol and triglycerides, as was the case for our patient.
Malabsorption of fat may cause vitamin K deficiency resulting in a prolonged prothrombin time. Liver biopsy is unnecessary in suspected cases of ICP, but would show cholestatic changes such as dilated bile canaliculi, bile pigment in the parenchyma, and minimal inflammation.
Skin biopsy is not helpful in ICP
In suspected cases of ICP, skin biopsy will only reveal a spectrum of non-specific findings. It is, however, helpful if you suspect pemphigoid gestationis, since it will reveal subepidermal blisters. Similarly, biopsy for direct immunofluorescence is nonspecific in ICP, but helpful in pemphigoid gestationis.
Soothing baths can help, ursodiol is most effective
Mild cholestasis responds to symptomatic treatment with soothing baths, topical antipruritics, emollients, and primrose oil, among others. Antihistamines are rarely effective. Anion exchange resins, such as cholestyramine, can be helpful, too; they bind bile acids and decrease their enterohepatic circulation.2
Patients who do not respond to cholestyramine, or who cannot tolerate it, may be treated with ursodeoxycholic acid (ursodiol). The research indicates that ursodiol works faster than cholestyramine, has a more sustained effect on pruritus, and is more effective in improving the biochemical abnormalities of ICP (strength of recommendation [SOR]: A, based on good-quality patient-oriented evidence). Ursodiol is considered safe for both mother and fetus.5 For all of these reasons, ursodiol has replaced cholestyramine as the first-line agent for ICP.
Doses range from 1 g/day to high doses of 1.5 to 2.0 g/d.6 The dose is maintained until delivery. Davies et al5 suggest that the use of ursodiol can reduce fetal mortality associated with ICP (SOR: C, based on consensus, usual practice, opinion, disease-oriented evidence, case series).
Weekly non-stress tests beginning at the 34th week of gestation are advisable (SOR: C).2 Labor may need to be induced in the 38th week in mild cases of ICP, and in the 36th week in severe cases (SOR: C).2
Ursodiol for our patient, labor was induced
We treated our patient with oral ursodiol and topical 1% hydrocortisone cream. Her bile acids and transaminase levels dropped and her pruritus improved—though it did not completely resolve until after delivery. Our obstetrics department recommended weekly non-stress tests starting at the 34th week of gestation. The non-stress tests were reactive. Due to the severity of her condition, labor was induced at 36 weeks.
Our patient had a healthy baby girl without complications. After delivery, the itching went away completely and her skin began to heal from all of those excoriations. Our patient is planning an elective cholecystectomy in the coming months because she doesn’t want to take a chance that she might have problems with her gallstones in the future.
Disclosure
A 32-year-old mother of 2 came into our facility during her 31st week of pregnancy and told us that she couldn’t stop itching. She said that her whole body was itchy and it got worse at night. She was unable to get a good night’s sleep. Up until this point, her pregnancy had been uncomplicated and she had no past history of medical problems.
An examination revealed excoriations—but no blistering—on her abdomen, chest, arms, and legs (FIGURES 1 AND 2). She had no jaundice or scleral icterus. The fundal height was 31 mm and the fetal heart tones were 150 bpm.
FIGURE 1 & 2
Excoriations on the abdomen and chest
What is your diagnosis?
How would you manage this condition?
Diagnosis: Intrahepatic cholestasis of pregnancy
Intrahepatic cholestasis of pregnancy (ICP) is caused by maternal intrahepatic bile secretory dysfunction.1 The disorder, which is also referred to as obstetric cholestasis and pruritus gravidarum, has no primary skin lesions. Patients have generalized pruritus and secondary excoriations (FIGURE 3). In about 20% of cases, patients are also jaundiced.2
The sudden onset of generalized pruritus, which is the hallmark of ICP, starts during the late second (20%) or third (80%) trimester, followed by secondary skin lesions, namely linear excoriations and excoriated papules caused by scratching.3 These excoriations are typically localized on the extensor surfaces of the limbs, but may also be found on the abdomen and back. The itching may involve the palms and soles, as well. The severity of the skin lesions correlates with the duration and degree of pruritus.3
According to one study, ICP occurred in 0.5% of 3192 pregnancies. The disorder resolves after delivery, and recurs with subsequent pregnancies.4
ICP has been linked to fetal distress (20%–30%), stillbirths (1%–2%), and preterm delivery (20%–60%).3 Autopsies of the placenta have shown signs of acute anoxia. Fetal complications in ICP may be caused by decreased fetal elimination of toxic bile acids.3
Hormones, genes, and even the weather may play a role
Increased hormone production during pregnancy plays a role in ICP. Estrogen, which increases 100-fold during pregnancy, interferes with bile acid secretion across the basolateral and canalicular membrane of the hepatocyte. Particularly noteworthy is the fact that estriol-16 α-D-glucuronide, the estrogen metabolite that increases most during pregnancy, is cholestatic, according to animal studies.3 In addition, progesterone metabolites play an important part in the pathogenesis of ICP. Progesterone inhibits hepatic glucuronyl transferase, thereby reducing the clearance of estrogens and amplifying their effects.
Familial clustering and geographical variation indicate that there is a genetic predisposition for ICP. There is a high prevalence of ICP in Chile (14%), especially among Araucanian Indian women (24%), and in Bolivia.3 ICP patients may have a family history of cholelithiasis and a higher risk of gallstones. There is a family history of cholelithiasis in 50% of ICP cases.2
Exogenous factors have been implicated in the pathogenesis of ICP. There is a higher incidence of ICP during the winter. Low selenium levels have also been linked to ICP. This suggests that the environment and nutritional factors play a role.2
FIGURE 3
Excoriations down the legs
The differential Dx includes scabies
Itching has been reported to occur in 17% of pregnancies,2 so it is important to differentiate ICP from the conditions listed below.
- Pruritic urticarial papules and plaques of pregnancy—also known as polymorphic eruption of pregnancy—is a dermatosis of pregnancy. Unlike the excoriations of ICP, this condition involves papulovesicular or urticarial eruptions on the trunk and extremities. It is particularly pronounced around the abdominal striae, and is more common in nulliparous women.
- Pemphigoid gestationis, also known as herpes gestationis because of its appearance, is a bullous or blistering disease that is associated with pregnancy. It is often periumbilical and can also have target lesions, which are absent in ICP.
- Atopic eruption of pregnancy is a new term used to include previous non-specific diagnoses such as prurigo of pregnancy and pruritic folliculitis of pregnancy. Prurigo of pregnancy, which is also called Besnier’s prurigo gestationis, involves bite-like papules that resemble scabies. Pruritic folliculitis of pregnancy is characterized by red, follicle-based papules. These 2 conditions differ from ICP in that there is no cholestasis and liver studies are normal. (In ICP, there is an elevation in liver enzymes and serum bile acids.)
- Scabies infestation can occur during pregnancy. The mite burrows in the skin and produces severe itching between the fingers and in skin folds. Look for burrows and the typical distribution between the fingers, on the wrists, in the axilla, and around the waist. A positive scraping viewed under the microscope will show mites, eggs, and mite feces.
If you suspect ICP in a patient who is also jaundiced, you’ll also need to rule out several other conditions. These include:
- acute liver disease of pregnancy
- preeclampsia complicated by increased liver enzymes
- hyperemesis gravidarum
- viral hepatitis
- drug reaction
- obstructive biliary disease, such as a gallstone lodged in the common bile duct.
Order a blood chemistry or liver profile
If you suspect that your patient has ICP, start by ordering a blood chemistry or liver profile. If any of the liver tests are elevated, order a total bile acid level (which is the most sensitive indicator of ICP) and a hepatitis panel (or specific hepatitis tests based on the patient’s history of exposures and vaccinations). If there is laboratory evidence of cholestasis, a right upper quadrant ultrasound will help you to spot gallstones and evidence of obstruction.
In ICP, there will be mild abnormalities of the liver function tests, including transaminases, alkaline phosphatase, and bilirubin. Bilirubin may be mildly to moderately elevated (2–5 mg/dL). (Jaundice is seen only at the higher levels of bilirubin.) Our patient’s tests, for instance, revealed that her ALT and AST were both over 300; her total bilirubin was elevated at 2.1.
Serum levels of bile acid correlate with the severity of pruritus. Our patient’s bile acids were elevated and her hepatitis panel was negative. Her ultrasound showed gallstones, but we saw no obstruction. An ICP patient’s lipid profile may show mild elevations in total cholesterol and triglycerides, as was the case for our patient.
Malabsorption of fat may cause vitamin K deficiency resulting in a prolonged prothrombin time. Liver biopsy is unnecessary in suspected cases of ICP, but would show cholestatic changes such as dilated bile canaliculi, bile pigment in the parenchyma, and minimal inflammation.
Skin biopsy is not helpful in ICP
In suspected cases of ICP, skin biopsy will only reveal a spectrum of non-specific findings. It is, however, helpful if you suspect pemphigoid gestationis, since it will reveal subepidermal blisters. Similarly, biopsy for direct immunofluorescence is nonspecific in ICP, but helpful in pemphigoid gestationis.
Soothing baths can help, ursodiol is most effective
Mild cholestasis responds to symptomatic treatment with soothing baths, topical antipruritics, emollients, and primrose oil, among others. Antihistamines are rarely effective. Anion exchange resins, such as cholestyramine, can be helpful, too; they bind bile acids and decrease their enterohepatic circulation.2
Patients who do not respond to cholestyramine, or who cannot tolerate it, may be treated with ursodeoxycholic acid (ursodiol). The research indicates that ursodiol works faster than cholestyramine, has a more sustained effect on pruritus, and is more effective in improving the biochemical abnormalities of ICP (strength of recommendation [SOR]: A, based on good-quality patient-oriented evidence). Ursodiol is considered safe for both mother and fetus.5 For all of these reasons, ursodiol has replaced cholestyramine as the first-line agent for ICP.
Doses range from 1 g/day to high doses of 1.5 to 2.0 g/d.6 The dose is maintained until delivery. Davies et al5 suggest that the use of ursodiol can reduce fetal mortality associated with ICP (SOR: C, based on consensus, usual practice, opinion, disease-oriented evidence, case series).
Weekly non-stress tests beginning at the 34th week of gestation are advisable (SOR: C).2 Labor may need to be induced in the 38th week in mild cases of ICP, and in the 36th week in severe cases (SOR: C).2
Ursodiol for our patient, labor was induced
We treated our patient with oral ursodiol and topical 1% hydrocortisone cream. Her bile acids and transaminase levels dropped and her pruritus improved—though it did not completely resolve until after delivery. Our obstetrics department recommended weekly non-stress tests starting at the 34th week of gestation. The non-stress tests were reactive. Due to the severity of her condition, labor was induced at 36 weeks.
Our patient had a healthy baby girl without complications. After delivery, the itching went away completely and her skin began to heal from all of those excoriations. Our patient is planning an elective cholecystectomy in the coming months because she doesn’t want to take a chance that she might have problems with her gallstones in the future.
Disclosure
1. Galaria NA, Mercurio MG. Dermatoses of pregnancy. The Female Patient 2003. Available at: www.femalepatient.com/html/arc/sel/may03/028_05_024.asp. Accessed on October 8, 2007.
2. Kroumpouzos G. Intrahepatic cholestasis of pregnancy: what’s new. European Academy of Dermatology and Venereology 2002;16:316-318.
3. Ambros-Rudolph CM, Müllegger RR, Vaughan Jones SA, et al. The specific dermatoses of pregnancy revisited and reclassified: results of a retrospective two-center study on 505 pregnant patients. J Am Acad Dermatol 2006;54:395-404.
4. Odom R, James W. Andrews’Diseases of the Skin. 10th ed. Philadelphia, PA: WB Saunders Company; 2006.
5. Davies MH, da Silva RCMA, Jones SR, et al. Fetal mortality associated with cholestasis of pregnancy and the potential benefit of therapy with ursodeoxycholic acid. Gut 1995;37:580-584.
6. Mazzella G, Nicola R, Francesco A, et al. Ursodeoxycholic acid administration in patients with cholestasis of pregnancy: Effects on primary bile acids in babies and mothers. Hepatology 2001;33:504-508.
1. Galaria NA, Mercurio MG. Dermatoses of pregnancy. The Female Patient 2003. Available at: www.femalepatient.com/html/arc/sel/may03/028_05_024.asp. Accessed on October 8, 2007.
2. Kroumpouzos G. Intrahepatic cholestasis of pregnancy: what’s new. European Academy of Dermatology and Venereology 2002;16:316-318.
3. Ambros-Rudolph CM, Müllegger RR, Vaughan Jones SA, et al. The specific dermatoses of pregnancy revisited and reclassified: results of a retrospective two-center study on 505 pregnant patients. J Am Acad Dermatol 2006;54:395-404.
4. Odom R, James W. Andrews’Diseases of the Skin. 10th ed. Philadelphia, PA: WB Saunders Company; 2006.
5. Davies MH, da Silva RCMA, Jones SR, et al. Fetal mortality associated with cholestasis of pregnancy and the potential benefit of therapy with ursodeoxycholic acid. Gut 1995;37:580-584.
6. Mazzella G, Nicola R, Francesco A, et al. Ursodeoxycholic acid administration in patients with cholestasis of pregnancy: Effects on primary bile acids in babies and mothers. Hepatology 2001;33:504-508.
Acute onset of rash and oligoarthritis
A 29-year-old man sought treatment at our clinic for an extensive rash he’d developed the month before. The rash was on his scalp, umbilicus, glans penis, palms, and soles of his feet. He reported swelling in his left knee and his fourth toes bilaterally that was exacerbated by weight bearing. During the 2 days prior to his visit to the clinic, the patient said he’d had a fever and night sweats; he denied ocular symptoms, GI complaints, dysuria, or penile discharge.
When asked about his sexual history, the patient noted that he’d had unprotected intercourse with a woman a year earlier that resulted in pain on urination and resolved on its own. Other than a resolved case of oral thrush, the patient had a noncontributory past medical history, took no medications, and had no family history of psoriasis.
A physical exam revealed circinate, scaly, and erythematous plaques covering his entire scalp ( FIGURE 1 ). The patient’s conjunctiva and oropharynx were clear. His fingernails showed hyperkeratosis, subungual debris, and nail fold erythema, without pitting. He also had bilateral swelling of the distal interphalangeal joints of his index fingers.
The patient’s umbilicus had a scaly erythematous plaque, while there were confluent erythematous plaques in the groin area, and on the glans penis. There were also similar erythematous plaques in the axilla and inguinal folds; plaques on the lower extremities had a thicker layer of scale. The patient’s feet had crusted plaques on the plantar surface, hyperkeratotic nails with thick subungual debris, and swelling and tenderness of the fourth toes bilaterally ( FIGURE 2 ).
FIGURE 1
Circinate plaques
FIGURE 2
Hyperkeratotic nails, swollen toe
What is your diagnosis?
Diagnosis: Reiter’s syndrome
This young man had Reiter’s syndrome (RS), a form of reactive arthritis that comprises a small subset of cases within the larger family of rheumatoid factor- seronegative spondyloarthritides—conditions noted primarily for inflammation of the axial skeleton.1
Of historical interest is the fact that this diagnosis shares its name with the man who first described it, Hans Reiter, a Nazi physician who tested unapproved vaccines and performed experimental procedures on victims in concentration camps. The infamous legacy of Reiter’s name has led to the proposal that the syndrome be referred to by another, more descriptive name.2 For the sake of simplicity, we’ll refer to the syndrome by the abbreviation RS.
Look for elements of the classic triad
RS is notoriously inconsistent in its presentation. Only a third of patients will develop the “classic triad”—that is: peripheral arthritis lasting at least 1 month, urethritis (or cervicitis), and conjunctivitis. Nearly half of patients will have only a single element of the triad.3
Patients with RS will complain of generalized malaise and fever and will often describe dysuria with concomitant urethral discharge. If conjunctivitis is present, the patient will report reddened, sensitive eyes. Pain will often originate from axial bones, lower extremities (in an oligoarticular asymmetrical pattern), swollen digits, and the heels (from enthesopathy).
Skin manifestations are often very noticeable and include psoriasiform lesions ( FIGURE 3 ) on the palms, soles, and glans penis. Specifically, you’ll see keratoderma blenorrhagicum ( FIGURE 4 ), brown and red macules/papules with pustular or hyperkeratotic features, on the palmar and plantar surfaces. Erythematous and scaly lesions resembling psoriatic plaques often appear elsewhere on the body. On the uncircumcised penis, these shallow ulcerations have a micropustular, serpiginous border and are referred to as balanitis circinata. However, they may also appear psoriasiform in nature on circumcised men, as was the case with our patient.
Coincident findings include onycholysis and subungual hyperkeratosis, lesions mimicking migratory glossitis, and anterior uveitis.
FIGURE 3
Psoriasiform plaque
FIGURE 4
Keratoderma blenorrhagicum
The typical patient? A young, white man
Patients with RS are almost always Caucasian males in their early twenties and are typically HLA-B27 positive. Seronegativity for this HLA factor may portend a less severe version of the syndrome. Individuals infected with HIV show increased incidence of developing RS.3
A microbial antigen is likely responsible for the initial activation of RS. This is followed by an immune reaction involving the joints, skin, and eyes. This theory is supported by the absence of auto- antibodies, the frequent association with HLA-B27, and the fact that patients with advanced AIDS experience the same severity of RS symptoms, despite depressed CD4+ T cell function.1
Bacteria trigger syndrome via 1 of 2 pathways
The bacteria that trigger RS typically enter the body through one of 2 pathways: the genitourinary tract or the gastrointestinal tract.
- The sexual transmission pathway involves infection with Chlamydia trachomatis or Ureaplasma urealyticum 1 to 4 weeks prior to development of urethritis and possibly conjunctivitis. The arthritic component follows later.
- The gastrointestinal pathway involves an enteric pathogen, such as Salmonella enteritidis, Yersinia enterocolitica, Campylobacter fetus, or Shigella flexneri that infects the host and follows the same time frame as noted earlier, though diarrhea rather than urethritis emerges as a chief complaint.4
Various forms of arthritis comprise the differential
A number of conditions must be ruled out before the RS diagnosis is considered definitive. The most likely imposters include:
- Gonococcal arthritis
- Rheumatoid arthritis
- Ankylosing spondylitis
- Psoriatic arthritis.
In addition, an attack of gouty arthritis, systemic lupus erythematosus, serum sickness, Behçet’s syndrome, rheumatic fever, Still’s disease, and HIV could also present in a similar fashion.
The lab work, detailed below, separates RS from the imposters.
Test blood and urine; check the ankles
Although there is no specific test for RS, several laboratory procedures are essential to honing in on the diagnosis. Hematological inquiry will confirm anemia, leukocytosis, thrombocytosis, and an elevated erythrocyte sedimentation rate (ESR). Though the urethral test may not be positive for a suspected organism, this procedure must be done to rule out gonococcal or chlamydial infection. This can now be done on a urine specimen rather than inserting a swab into the urethra. The urine is sent for a polymerase chain reaction (PCR) test rather than a culture. If enteritis was the preceding infection, a stool culture to elucidate potential pathogens is warranted.
You’ll also need to order serological tests for antinuclear antibodies (ANAs), rheumatoid factor (RF), and HIV. As you would expect, these tests will be abnormal for systemic lupus erythematosus, rheumatoid arthritis, and HIV respectively. Though these tests are often negative in RS patients, a strong association with HIV infection does exist.
Keep in mind, too, that you can differentiate gonococcal arthritis from RS based on historical features, as well as clinical features, including migratory polyarthritis with necrotic and pustular skin lesions. Patients with gonococcal arthritis will also have a positive gonococcal culture and rapid improvement with antibiotics.
If you order a biopsy, pathology is likely to find a variety of features in an RS patient, such as spongiform pustules, neutrophilic infiltrate in a perivascular pattern, and an epidermal hyperplasia that resembles psoriasis.3
Radiographic imaging for a suspected case of RS may reveal a number of signs that resemble psoriatic arthritis (pencilin-cup deformity, syndesmophytes, sacroiliitis), but enthesitis, particularly in the ankle joint region, should raise your index of suspicion for RS.6
Tx: Antibiotics, NSAIDs, and steroids
Antibiotic therapy for 3 months is indicated if a patient’s case of RS can be traced back to an infection. If a Chlamydia species is the offending organism, then doxycycline or tetracycline can be used7 (strength of recommendation [SOR]: B). If the infectious agent is unknown, then ciprofloxacin can offer broad-spectrum coverage8 (SOR: B).
Though few studies have evaluated the long-term effects of NSAID treatment on RS, a regular schedule of high doses for several weeks is appropriate for inflammation and pain management. It’s most effective when given early in the disease course5 (SOR: B).
Topical corticosteroids can be used on mucosal and skin lesions. For refractory disease, immunosuppressive agents such as sulfasalazine at 2000 mg/day9 (SOR: B) or a subcutaneous injection of etanercept at 25 mg twice weekly10 (SOR: B) offer relief.
Our patient’s treatment included an NSAID and corticosteroids
Because our patient’s syndrome involved a variety of systemic manifestations, we used several medications to cover all of his symptoms. We prescribed piroxicam 20 mg daily, clobetasol 0.05% ointment applied daily to legs and feet, triamcinolone 0.1% cream applied to scalp twice daily and genitals and armpits once daily, and acitretin 25 mg daily. We consulted Rheumatology to assess and treat his joint disease. We also consulted Ophthalmology to assess for potential ocular manifestations.
Though the patient did report a history of a sexually transmitted infection, it occurred long before his visit, and we were unable to identify an infectious agent. As a result, we did not start him on any antibiotics.
We instructed the patient to return in 2 weeks. Unfortunately, he was lost to follow-up. Patients with RS, though, typically make a full recovery from their symptoms. Some patients, however—10% to 20%—may go on to have a chronic, deforming arthritis.3
1. Winchester R. Reiter’s syndrome. In Freedberg IM, Eisen AZ, Wolf K, Austen KF, Goldsmith LA, Katz SI. Fitzpatrick’s Dermatology in General Medicine. 6th ed. New York, NY: McGraw-Hill; 2003:1769-1776.
2. James WD, Berger TG, Elston DM. Andrew’s Diseases of the Skin: Clinical Dermatology. 10th ed. Philadelphia, Pa: saunders elsevier; 2006.
3. Wolff K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 5th ed. New York, NY: McGraw-Hill, 2005.
4. Colmegna I, Cuchacovich R, Espinoza LR. HLA-B27-associated reactive arthritis: pathogenetic and clinical considerations. Clin Microbiol Rev 2004;17:348-369.
5. Schachner LA, Hansen RC. Pediatric Dermatology. 2nd ed. New York, NY: churchill livingstone, Inc; 1995.
6. Gladman DD. Clinical aspects of the spondyloarthropathies. Am J Med Sci 1998;316:234-238.
7. Lauhio A, Leirisalo-Repo M, Lähdevirta J, Saikku P, Repo H. Double-blind, placebo-controlled study of three-month treatment with lymecycline in reactive arthritis, with special reference to chlamydia arthritis. Arthritis Rheum 1991;34:6-14.
8. Yli-Kerttula T, Luukkainen R, Yli-Kerttula U, et al. Effect of a three month course of ciprofloxacin on the late prognosis of reactive arthritis. Ann Rheum Dis 2003;62:880-884.
9. Clegg DO, Reda DJ, Weisman MH, et al. Comparison of sulfasalazine and placebo in the treatment of reactive arthritis (Reiter’s syndrome). A Department of Veterans Affairs Cooperative Study. Arthritis Rheum 1996;39:2021-2027.
10. Flagg SD, Meador R, Hsia E, Kitumnuaypong T, Schumacher HR, Jr. Decreased pain and synovial inflammation after etanercept therapy in patients with reactive and undifferentiated arthritis: an open-label trial. Arthritis Rheum 2005;53:613-617.
A 29-year-old man sought treatment at our clinic for an extensive rash he’d developed the month before. The rash was on his scalp, umbilicus, glans penis, palms, and soles of his feet. He reported swelling in his left knee and his fourth toes bilaterally that was exacerbated by weight bearing. During the 2 days prior to his visit to the clinic, the patient said he’d had a fever and night sweats; he denied ocular symptoms, GI complaints, dysuria, or penile discharge.
When asked about his sexual history, the patient noted that he’d had unprotected intercourse with a woman a year earlier that resulted in pain on urination and resolved on its own. Other than a resolved case of oral thrush, the patient had a noncontributory past medical history, took no medications, and had no family history of psoriasis.
A physical exam revealed circinate, scaly, and erythematous plaques covering his entire scalp ( FIGURE 1 ). The patient’s conjunctiva and oropharynx were clear. His fingernails showed hyperkeratosis, subungual debris, and nail fold erythema, without pitting. He also had bilateral swelling of the distal interphalangeal joints of his index fingers.
The patient’s umbilicus had a scaly erythematous plaque, while there were confluent erythematous plaques in the groin area, and on the glans penis. There were also similar erythematous plaques in the axilla and inguinal folds; plaques on the lower extremities had a thicker layer of scale. The patient’s feet had crusted plaques on the plantar surface, hyperkeratotic nails with thick subungual debris, and swelling and tenderness of the fourth toes bilaterally ( FIGURE 2 ).
FIGURE 1
Circinate plaques
FIGURE 2
Hyperkeratotic nails, swollen toe
What is your diagnosis?
Diagnosis: Reiter’s syndrome
This young man had Reiter’s syndrome (RS), a form of reactive arthritis that comprises a small subset of cases within the larger family of rheumatoid factor- seronegative spondyloarthritides—conditions noted primarily for inflammation of the axial skeleton.1
Of historical interest is the fact that this diagnosis shares its name with the man who first described it, Hans Reiter, a Nazi physician who tested unapproved vaccines and performed experimental procedures on victims in concentration camps. The infamous legacy of Reiter’s name has led to the proposal that the syndrome be referred to by another, more descriptive name.2 For the sake of simplicity, we’ll refer to the syndrome by the abbreviation RS.
Look for elements of the classic triad
RS is notoriously inconsistent in its presentation. Only a third of patients will develop the “classic triad”—that is: peripheral arthritis lasting at least 1 month, urethritis (or cervicitis), and conjunctivitis. Nearly half of patients will have only a single element of the triad.3
Patients with RS will complain of generalized malaise and fever and will often describe dysuria with concomitant urethral discharge. If conjunctivitis is present, the patient will report reddened, sensitive eyes. Pain will often originate from axial bones, lower extremities (in an oligoarticular asymmetrical pattern), swollen digits, and the heels (from enthesopathy).
Skin manifestations are often very noticeable and include psoriasiform lesions ( FIGURE 3 ) on the palms, soles, and glans penis. Specifically, you’ll see keratoderma blenorrhagicum ( FIGURE 4 ), brown and red macules/papules with pustular or hyperkeratotic features, on the palmar and plantar surfaces. Erythematous and scaly lesions resembling psoriatic plaques often appear elsewhere on the body. On the uncircumcised penis, these shallow ulcerations have a micropustular, serpiginous border and are referred to as balanitis circinata. However, they may also appear psoriasiform in nature on circumcised men, as was the case with our patient.
Coincident findings include onycholysis and subungual hyperkeratosis, lesions mimicking migratory glossitis, and anterior uveitis.
FIGURE 3
Psoriasiform plaque
FIGURE 4
Keratoderma blenorrhagicum
The typical patient? A young, white man
Patients with RS are almost always Caucasian males in their early twenties and are typically HLA-B27 positive. Seronegativity for this HLA factor may portend a less severe version of the syndrome. Individuals infected with HIV show increased incidence of developing RS.3
A microbial antigen is likely responsible for the initial activation of RS. This is followed by an immune reaction involving the joints, skin, and eyes. This theory is supported by the absence of auto- antibodies, the frequent association with HLA-B27, and the fact that patients with advanced AIDS experience the same severity of RS symptoms, despite depressed CD4+ T cell function.1
Bacteria trigger syndrome via 1 of 2 pathways
The bacteria that trigger RS typically enter the body through one of 2 pathways: the genitourinary tract or the gastrointestinal tract.
- The sexual transmission pathway involves infection with Chlamydia trachomatis or Ureaplasma urealyticum 1 to 4 weeks prior to development of urethritis and possibly conjunctivitis. The arthritic component follows later.
- The gastrointestinal pathway involves an enteric pathogen, such as Salmonella enteritidis, Yersinia enterocolitica, Campylobacter fetus, or Shigella flexneri that infects the host and follows the same time frame as noted earlier, though diarrhea rather than urethritis emerges as a chief complaint.4
Various forms of arthritis comprise the differential
A number of conditions must be ruled out before the RS diagnosis is considered definitive. The most likely imposters include:
- Gonococcal arthritis
- Rheumatoid arthritis
- Ankylosing spondylitis
- Psoriatic arthritis.
In addition, an attack of gouty arthritis, systemic lupus erythematosus, serum sickness, Behçet’s syndrome, rheumatic fever, Still’s disease, and HIV could also present in a similar fashion.
The lab work, detailed below, separates RS from the imposters.
Test blood and urine; check the ankles
Although there is no specific test for RS, several laboratory procedures are essential to honing in on the diagnosis. Hematological inquiry will confirm anemia, leukocytosis, thrombocytosis, and an elevated erythrocyte sedimentation rate (ESR). Though the urethral test may not be positive for a suspected organism, this procedure must be done to rule out gonococcal or chlamydial infection. This can now be done on a urine specimen rather than inserting a swab into the urethra. The urine is sent for a polymerase chain reaction (PCR) test rather than a culture. If enteritis was the preceding infection, a stool culture to elucidate potential pathogens is warranted.
You’ll also need to order serological tests for antinuclear antibodies (ANAs), rheumatoid factor (RF), and HIV. As you would expect, these tests will be abnormal for systemic lupus erythematosus, rheumatoid arthritis, and HIV respectively. Though these tests are often negative in RS patients, a strong association with HIV infection does exist.
Keep in mind, too, that you can differentiate gonococcal arthritis from RS based on historical features, as well as clinical features, including migratory polyarthritis with necrotic and pustular skin lesions. Patients with gonococcal arthritis will also have a positive gonococcal culture and rapid improvement with antibiotics.
If you order a biopsy, pathology is likely to find a variety of features in an RS patient, such as spongiform pustules, neutrophilic infiltrate in a perivascular pattern, and an epidermal hyperplasia that resembles psoriasis.3
Radiographic imaging for a suspected case of RS may reveal a number of signs that resemble psoriatic arthritis (pencilin-cup deformity, syndesmophytes, sacroiliitis), but enthesitis, particularly in the ankle joint region, should raise your index of suspicion for RS.6
Tx: Antibiotics, NSAIDs, and steroids
Antibiotic therapy for 3 months is indicated if a patient’s case of RS can be traced back to an infection. If a Chlamydia species is the offending organism, then doxycycline or tetracycline can be used7 (strength of recommendation [SOR]: B). If the infectious agent is unknown, then ciprofloxacin can offer broad-spectrum coverage8 (SOR: B).
Though few studies have evaluated the long-term effects of NSAID treatment on RS, a regular schedule of high doses for several weeks is appropriate for inflammation and pain management. It’s most effective when given early in the disease course5 (SOR: B).
Topical corticosteroids can be used on mucosal and skin lesions. For refractory disease, immunosuppressive agents such as sulfasalazine at 2000 mg/day9 (SOR: B) or a subcutaneous injection of etanercept at 25 mg twice weekly10 (SOR: B) offer relief.
Our patient’s treatment included an NSAID and corticosteroids
Because our patient’s syndrome involved a variety of systemic manifestations, we used several medications to cover all of his symptoms. We prescribed piroxicam 20 mg daily, clobetasol 0.05% ointment applied daily to legs and feet, triamcinolone 0.1% cream applied to scalp twice daily and genitals and armpits once daily, and acitretin 25 mg daily. We consulted Rheumatology to assess and treat his joint disease. We also consulted Ophthalmology to assess for potential ocular manifestations.
Though the patient did report a history of a sexually transmitted infection, it occurred long before his visit, and we were unable to identify an infectious agent. As a result, we did not start him on any antibiotics.
We instructed the patient to return in 2 weeks. Unfortunately, he was lost to follow-up. Patients with RS, though, typically make a full recovery from their symptoms. Some patients, however—10% to 20%—may go on to have a chronic, deforming arthritis.3
A 29-year-old man sought treatment at our clinic for an extensive rash he’d developed the month before. The rash was on his scalp, umbilicus, glans penis, palms, and soles of his feet. He reported swelling in his left knee and his fourth toes bilaterally that was exacerbated by weight bearing. During the 2 days prior to his visit to the clinic, the patient said he’d had a fever and night sweats; he denied ocular symptoms, GI complaints, dysuria, or penile discharge.
When asked about his sexual history, the patient noted that he’d had unprotected intercourse with a woman a year earlier that resulted in pain on urination and resolved on its own. Other than a resolved case of oral thrush, the patient had a noncontributory past medical history, took no medications, and had no family history of psoriasis.
A physical exam revealed circinate, scaly, and erythematous plaques covering his entire scalp ( FIGURE 1 ). The patient’s conjunctiva and oropharynx were clear. His fingernails showed hyperkeratosis, subungual debris, and nail fold erythema, without pitting. He also had bilateral swelling of the distal interphalangeal joints of his index fingers.
The patient’s umbilicus had a scaly erythematous plaque, while there were confluent erythematous plaques in the groin area, and on the glans penis. There were also similar erythematous plaques in the axilla and inguinal folds; plaques on the lower extremities had a thicker layer of scale. The patient’s feet had crusted plaques on the plantar surface, hyperkeratotic nails with thick subungual debris, and swelling and tenderness of the fourth toes bilaterally ( FIGURE 2 ).
FIGURE 1
Circinate plaques
FIGURE 2
Hyperkeratotic nails, swollen toe
What is your diagnosis?
Diagnosis: Reiter’s syndrome
This young man had Reiter’s syndrome (RS), a form of reactive arthritis that comprises a small subset of cases within the larger family of rheumatoid factor- seronegative spondyloarthritides—conditions noted primarily for inflammation of the axial skeleton.1
Of historical interest is the fact that this diagnosis shares its name with the man who first described it, Hans Reiter, a Nazi physician who tested unapproved vaccines and performed experimental procedures on victims in concentration camps. The infamous legacy of Reiter’s name has led to the proposal that the syndrome be referred to by another, more descriptive name.2 For the sake of simplicity, we’ll refer to the syndrome by the abbreviation RS.
Look for elements of the classic triad
RS is notoriously inconsistent in its presentation. Only a third of patients will develop the “classic triad”—that is: peripheral arthritis lasting at least 1 month, urethritis (or cervicitis), and conjunctivitis. Nearly half of patients will have only a single element of the triad.3
Patients with RS will complain of generalized malaise and fever and will often describe dysuria with concomitant urethral discharge. If conjunctivitis is present, the patient will report reddened, sensitive eyes. Pain will often originate from axial bones, lower extremities (in an oligoarticular asymmetrical pattern), swollen digits, and the heels (from enthesopathy).
Skin manifestations are often very noticeable and include psoriasiform lesions ( FIGURE 3 ) on the palms, soles, and glans penis. Specifically, you’ll see keratoderma blenorrhagicum ( FIGURE 4 ), brown and red macules/papules with pustular or hyperkeratotic features, on the palmar and plantar surfaces. Erythematous and scaly lesions resembling psoriatic plaques often appear elsewhere on the body. On the uncircumcised penis, these shallow ulcerations have a micropustular, serpiginous border and are referred to as balanitis circinata. However, they may also appear psoriasiform in nature on circumcised men, as was the case with our patient.
Coincident findings include onycholysis and subungual hyperkeratosis, lesions mimicking migratory glossitis, and anterior uveitis.
FIGURE 3
Psoriasiform plaque
FIGURE 4
Keratoderma blenorrhagicum
The typical patient? A young, white man
Patients with RS are almost always Caucasian males in their early twenties and are typically HLA-B27 positive. Seronegativity for this HLA factor may portend a less severe version of the syndrome. Individuals infected with HIV show increased incidence of developing RS.3
A microbial antigen is likely responsible for the initial activation of RS. This is followed by an immune reaction involving the joints, skin, and eyes. This theory is supported by the absence of auto- antibodies, the frequent association with HLA-B27, and the fact that patients with advanced AIDS experience the same severity of RS symptoms, despite depressed CD4+ T cell function.1
Bacteria trigger syndrome via 1 of 2 pathways
The bacteria that trigger RS typically enter the body through one of 2 pathways: the genitourinary tract or the gastrointestinal tract.
- The sexual transmission pathway involves infection with Chlamydia trachomatis or Ureaplasma urealyticum 1 to 4 weeks prior to development of urethritis and possibly conjunctivitis. The arthritic component follows later.
- The gastrointestinal pathway involves an enteric pathogen, such as Salmonella enteritidis, Yersinia enterocolitica, Campylobacter fetus, or Shigella flexneri that infects the host and follows the same time frame as noted earlier, though diarrhea rather than urethritis emerges as a chief complaint.4
Various forms of arthritis comprise the differential
A number of conditions must be ruled out before the RS diagnosis is considered definitive. The most likely imposters include:
- Gonococcal arthritis
- Rheumatoid arthritis
- Ankylosing spondylitis
- Psoriatic arthritis.
In addition, an attack of gouty arthritis, systemic lupus erythematosus, serum sickness, Behçet’s syndrome, rheumatic fever, Still’s disease, and HIV could also present in a similar fashion.
The lab work, detailed below, separates RS from the imposters.
Test blood and urine; check the ankles
Although there is no specific test for RS, several laboratory procedures are essential to honing in on the diagnosis. Hematological inquiry will confirm anemia, leukocytosis, thrombocytosis, and an elevated erythrocyte sedimentation rate (ESR). Though the urethral test may not be positive for a suspected organism, this procedure must be done to rule out gonococcal or chlamydial infection. This can now be done on a urine specimen rather than inserting a swab into the urethra. The urine is sent for a polymerase chain reaction (PCR) test rather than a culture. If enteritis was the preceding infection, a stool culture to elucidate potential pathogens is warranted.
You’ll also need to order serological tests for antinuclear antibodies (ANAs), rheumatoid factor (RF), and HIV. As you would expect, these tests will be abnormal for systemic lupus erythematosus, rheumatoid arthritis, and HIV respectively. Though these tests are often negative in RS patients, a strong association with HIV infection does exist.
Keep in mind, too, that you can differentiate gonococcal arthritis from RS based on historical features, as well as clinical features, including migratory polyarthritis with necrotic and pustular skin lesions. Patients with gonococcal arthritis will also have a positive gonococcal culture and rapid improvement with antibiotics.
If you order a biopsy, pathology is likely to find a variety of features in an RS patient, such as spongiform pustules, neutrophilic infiltrate in a perivascular pattern, and an epidermal hyperplasia that resembles psoriasis.3
Radiographic imaging for a suspected case of RS may reveal a number of signs that resemble psoriatic arthritis (pencilin-cup deformity, syndesmophytes, sacroiliitis), but enthesitis, particularly in the ankle joint region, should raise your index of suspicion for RS.6
Tx: Antibiotics, NSAIDs, and steroids
Antibiotic therapy for 3 months is indicated if a patient’s case of RS can be traced back to an infection. If a Chlamydia species is the offending organism, then doxycycline or tetracycline can be used7 (strength of recommendation [SOR]: B). If the infectious agent is unknown, then ciprofloxacin can offer broad-spectrum coverage8 (SOR: B).
Though few studies have evaluated the long-term effects of NSAID treatment on RS, a regular schedule of high doses for several weeks is appropriate for inflammation and pain management. It’s most effective when given early in the disease course5 (SOR: B).
Topical corticosteroids can be used on mucosal and skin lesions. For refractory disease, immunosuppressive agents such as sulfasalazine at 2000 mg/day9 (SOR: B) or a subcutaneous injection of etanercept at 25 mg twice weekly10 (SOR: B) offer relief.
Our patient’s treatment included an NSAID and corticosteroids
Because our patient’s syndrome involved a variety of systemic manifestations, we used several medications to cover all of his symptoms. We prescribed piroxicam 20 mg daily, clobetasol 0.05% ointment applied daily to legs and feet, triamcinolone 0.1% cream applied to scalp twice daily and genitals and armpits once daily, and acitretin 25 mg daily. We consulted Rheumatology to assess and treat his joint disease. We also consulted Ophthalmology to assess for potential ocular manifestations.
Though the patient did report a history of a sexually transmitted infection, it occurred long before his visit, and we were unable to identify an infectious agent. As a result, we did not start him on any antibiotics.
We instructed the patient to return in 2 weeks. Unfortunately, he was lost to follow-up. Patients with RS, though, typically make a full recovery from their symptoms. Some patients, however—10% to 20%—may go on to have a chronic, deforming arthritis.3
1. Winchester R. Reiter’s syndrome. In Freedberg IM, Eisen AZ, Wolf K, Austen KF, Goldsmith LA, Katz SI. Fitzpatrick’s Dermatology in General Medicine. 6th ed. New York, NY: McGraw-Hill; 2003:1769-1776.
2. James WD, Berger TG, Elston DM. Andrew’s Diseases of the Skin: Clinical Dermatology. 10th ed. Philadelphia, Pa: saunders elsevier; 2006.
3. Wolff K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 5th ed. New York, NY: McGraw-Hill, 2005.
4. Colmegna I, Cuchacovich R, Espinoza LR. HLA-B27-associated reactive arthritis: pathogenetic and clinical considerations. Clin Microbiol Rev 2004;17:348-369.
5. Schachner LA, Hansen RC. Pediatric Dermatology. 2nd ed. New York, NY: churchill livingstone, Inc; 1995.
6. Gladman DD. Clinical aspects of the spondyloarthropathies. Am J Med Sci 1998;316:234-238.
7. Lauhio A, Leirisalo-Repo M, Lähdevirta J, Saikku P, Repo H. Double-blind, placebo-controlled study of three-month treatment with lymecycline in reactive arthritis, with special reference to chlamydia arthritis. Arthritis Rheum 1991;34:6-14.
8. Yli-Kerttula T, Luukkainen R, Yli-Kerttula U, et al. Effect of a three month course of ciprofloxacin on the late prognosis of reactive arthritis. Ann Rheum Dis 2003;62:880-884.
9. Clegg DO, Reda DJ, Weisman MH, et al. Comparison of sulfasalazine and placebo in the treatment of reactive arthritis (Reiter’s syndrome). A Department of Veterans Affairs Cooperative Study. Arthritis Rheum 1996;39:2021-2027.
10. Flagg SD, Meador R, Hsia E, Kitumnuaypong T, Schumacher HR, Jr. Decreased pain and synovial inflammation after etanercept therapy in patients with reactive and undifferentiated arthritis: an open-label trial. Arthritis Rheum 2005;53:613-617.
1. Winchester R. Reiter’s syndrome. In Freedberg IM, Eisen AZ, Wolf K, Austen KF, Goldsmith LA, Katz SI. Fitzpatrick’s Dermatology in General Medicine. 6th ed. New York, NY: McGraw-Hill; 2003:1769-1776.
2. James WD, Berger TG, Elston DM. Andrew’s Diseases of the Skin: Clinical Dermatology. 10th ed. Philadelphia, Pa: saunders elsevier; 2006.
3. Wolff K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 5th ed. New York, NY: McGraw-Hill, 2005.
4. Colmegna I, Cuchacovich R, Espinoza LR. HLA-B27-associated reactive arthritis: pathogenetic and clinical considerations. Clin Microbiol Rev 2004;17:348-369.
5. Schachner LA, Hansen RC. Pediatric Dermatology. 2nd ed. New York, NY: churchill livingstone, Inc; 1995.
6. Gladman DD. Clinical aspects of the spondyloarthropathies. Am J Med Sci 1998;316:234-238.
7. Lauhio A, Leirisalo-Repo M, Lähdevirta J, Saikku P, Repo H. Double-blind, placebo-controlled study of three-month treatment with lymecycline in reactive arthritis, with special reference to chlamydia arthritis. Arthritis Rheum 1991;34:6-14.
8. Yli-Kerttula T, Luukkainen R, Yli-Kerttula U, et al. Effect of a three month course of ciprofloxacin on the late prognosis of reactive arthritis. Ann Rheum Dis 2003;62:880-884.
9. Clegg DO, Reda DJ, Weisman MH, et al. Comparison of sulfasalazine and placebo in the treatment of reactive arthritis (Reiter’s syndrome). A Department of Veterans Affairs Cooperative Study. Arthritis Rheum 1996;39:2021-2027.
10. Flagg SD, Meador R, Hsia E, Kitumnuaypong T, Schumacher HR, Jr. Decreased pain and synovial inflammation after etanercept therapy in patients with reactive and undifferentiated arthritis: an open-label trial. Arthritis Rheum 2005;53:613-617.
Hurricane Katrina evacuee develops a persistent rash
Seven months after Hurricane Katrina, a 52-year-old African American woman who was evacuated from New Orleans came to our office with a hypopigmented rash on both her upper thighs and arms. Immediately following the 2005 hurricane, she was forced to wade through the polluted waters of New Orleans for many hours before being rescued by boat. Four days passed before she had access to a shower. It was after this shower that she first noticed a single erythematous spot the size of a silver dollar on her left thigh, that several weeks later faded to hypopigmented macules and plaques. Over time, the rash spread to both thighs and arms (FIGURE 1 AND 2. She did not have any itching, pain, bleeding, fever, chills, weight changes, or gastrointestinal symptoms after the rash appeared.
The patient reported that she was married and had worked as a chef for 20 years. She smoked cigarettes, drank alcohol occasionally, and was obese. She had synovitis of her left ankle, which led to surgery. She had no known drug allergies and was taking ibuprofen. Her mother, age 79, had glucose intolerance; her father, age 82, had a renal cell carcinoma removed.
Due to a confluence of situational, economic, and medical problems, she did not seek care for this rash until April 2006. At that time, a punch biopsy revealed the findings in FIGURE 3. Physical exam revealed her skin to have symmetrically distributed hypopigmented macules and plaques to all 4 extremities. She had no lymphadenopathy.
FIGURE 1
Rash on patients’ legs
FIGURE 2
Rash on right arm
FIGURE 3
Hematoxylin/eosin stain
What is the diagnosis?
Were Katrina’s flood waters to blame?
Dx: T-cell lymphoma—timing is coincidental
The biopsy revealed cutaneous T-cell lymphoma (CTCL)—specifically a type known as mycosis fungoides, named after the mushroom-like skin tumors seen in severe cases. CTCL is a malignant lymphoma of helper T-cells that have an affinity for the skin.
Normal life expectancy likely if diagnosed early
In about 10% of cases, the T-cells spread via the lymphatic system to metastasize to the liver, lung, and bone marrow, but more often remain confined to the skin and lymph nodes, and most patients diagnosed early have a normal life expectancy.1
The disease is rare, with about 1000 new cases per year in the US,1 and is more common in African Americans,2 although the photos in dermatological atlases overwhelmingly show Caucasians. CTCL presents in numerous ways, but patients usually have a long course of persistent rash that is often pruritic and usually erythematous or hyperpigmented.
No one knows what causes CTCL, but current theories point to exposure to environmental hazards such as Agent Orange.2 And while the number of environmental hazards in the floodwaters of Katrina were vast, we have no scientific evidence that an environmental exposure was to blame. In fact, while we cared for a number of Katrina evacuees—many of whom had skin infections—this was the only case of mycosis fungoides.
It’s likely that the start of the visible mycosis fungoides lesions after the flood was purely coincidental.
Diagnosis hinges on palpation and biopsy
To diagnose CTCL, you need to palpate for enlarged lymph nodes and perform a full-thickness punch biopsy of the lesion. If the biopsy is negative and the rash persists, take another biopsy—results can be falsely negative at the beginning stages of the disease.
There are no guidelines on which studies should be used for staging CTCL (TABLE), but some sources recommend that if lymph node involvement is suspected on physical exam, lymph node biopsies should be done in addition to a chest radiograph.3 In more advanced stages, consider a computed tomography (CT) scan of the abdomen and pelvis. A recent study concluded that CT and positron-emission tomography (PET) scans used together were more sensitive in staging, but this may not be cost-effective.4
Late-stage mycosis fungoides is usually associated with immunocompromise. Therefore, HIV testing should be performed in all patients with CTCL. Other important laboratory studies include complete blood count—with differential—as well as peripheral smear looking for Sézary cells, lactic dehydrogenase (LDH), liver function tests, and uric acid.
TABLE
Staging for cutaneous T-cell lymphoma based on the Tumor, Node, Metastasis (TNM) system
| STAGE | TNM STAGING | RECOMMENDED TREATMENTS |
|---|---|---|
| IA | T1, N0, M0: Patch or plaque <10% body surface area | Topical high-potency steroids, PUVA, topical nitrogen |
| IB | T2, N0, M0: Patch or plaque >10% body surface area | mustard, carmustine, or bexarotene |
| IIA | T1–2, N1, M0: Patch or plaque with palpable but pathologically normal lymph node | Same as Stage I; if refractory, use total skin electron beam therapy |
| IIB | T3, N0–1, M0: Tumor/nodule | |
| IIIA | T4, N0, M0:Generalized erythroderma | |
| IIIB | T4, N1, M0: Erythroderma and palpable but pathologically normal lymph node | Chemotherapy or photophoresis, refer to medical oncologist, radiation oncologist, and dermatologist |
| IVA | T1–4, N2–3, M0: Pathological lymph node | |
| IVB | T1–4, N0–3, M1: Visceral (M1) or blood involvement | |
| T: 0–4=indicates size or direct extent of the primary tumor | ||
| N: 0=tumor cells absent from regional lymph nodes; 1=tumor cells spread to closest or small number of regional lymph nodes; 3=tumor cells spread to most distant or numerous regional lymph nodes | ||
| M: 0=no distant metastasis; 1=metastasis to distant organs (beyond regional lymph nodes) | ||
A disease that’s easy to mistake for vitiligo
The hypopigmented spots of CTCL look so much like vitiligo, it is frightening to think how easy it would be to miss the diagnosis. Complicating matters: All vitiligo does not need a biopsy to confirm the clinical impression.
So what made this case suspicious enough to warrant a biopsy? First, the hypopigmented spots on our patient were not a typical distribution for vitiligo, which has a predilection for the hands and face. Second, our patient had a hypopigmented patch that had a dark, slightly raised plaque within it, which also would not be typical for vitiligo. (See the patient’s left upper thigh, just below the inguinal area, in FIGURE 1.) Finally, our patient had a rapid onset of multiple discrete macules that did not coalesce into larger hypopigmented areas; in vitiligo you would expect otherwise.
The differential diagnosis also includes idiopathic guttate hypomelanosis, a benign condition that can cause multiple small hypopigmented macules. The size of immunoglobulin H macules, however, is tiny compared with what you’ll see with CTCL. The absence of scales makes eczema, psoriasis, or tinea corporis very unlikely.
Treatment starts with topical steroids
This is a rare disease that lacks the data needed to support an evidence-based approach to treatment. Topical steroids are recommended for stage I when the disease is local to the skin (see TABLE for recommended treatments of other stages). Ultraviolet light (PUVA and UVB) is also used; a recent study suggests the PUVA is a good treatment alternative for stages IA and IB (SOR: C).1
Our patient’s course
Initially, we prescribed a high-potency generic steroid (clobetasol 0.05% cream) for this patient, to be used twice daily to the affected areas.
The patient reported no improvement with this approach, while she awaited her appointments with dermatology and oncology specialists.
Her blood tests were essentially normal, including a negative HIV test.
She is currently receiving narrowband UVB treatment twice weekly.
1. Pinter-Brown LC. Mycosis fungoides. Emedicine, 8 September 2006. Available at: www.emedicine.com/Med/topic1541.htm. Accessed on May 8, 2007.
2. James WD, Berger T, Elston T. Andrews’ Diseases of the Skin: Clinical Dermatology. 10th ed. Philadelphia, Pa: Saunders Elsevier; 2006:727–740.
3. Gettler SL, Fung MA. Efficacy of treatments for mycosis fungoides and sézary syndrome: nationwide survey responses. Dermatol Online J 2005;11:6.-
4. Tsai EY, Taur A, Espinosa L, et al. Staging accuracy in mycosis fungoides and sezary syndrome using integrated positron emission tomography and computed tomography. Arch Dermatol 2006;142:577-584.
Seven months after Hurricane Katrina, a 52-year-old African American woman who was evacuated from New Orleans came to our office with a hypopigmented rash on both her upper thighs and arms. Immediately following the 2005 hurricane, she was forced to wade through the polluted waters of New Orleans for many hours before being rescued by boat. Four days passed before she had access to a shower. It was after this shower that she first noticed a single erythematous spot the size of a silver dollar on her left thigh, that several weeks later faded to hypopigmented macules and plaques. Over time, the rash spread to both thighs and arms (FIGURE 1 AND 2. She did not have any itching, pain, bleeding, fever, chills, weight changes, or gastrointestinal symptoms after the rash appeared.
The patient reported that she was married and had worked as a chef for 20 years. She smoked cigarettes, drank alcohol occasionally, and was obese. She had synovitis of her left ankle, which led to surgery. She had no known drug allergies and was taking ibuprofen. Her mother, age 79, had glucose intolerance; her father, age 82, had a renal cell carcinoma removed.
Due to a confluence of situational, economic, and medical problems, she did not seek care for this rash until April 2006. At that time, a punch biopsy revealed the findings in FIGURE 3. Physical exam revealed her skin to have symmetrically distributed hypopigmented macules and plaques to all 4 extremities. She had no lymphadenopathy.
FIGURE 1
Rash on patients’ legs
FIGURE 2
Rash on right arm
FIGURE 3
Hematoxylin/eosin stain
What is the diagnosis?
Were Katrina’s flood waters to blame?
Dx: T-cell lymphoma—timing is coincidental
The biopsy revealed cutaneous T-cell lymphoma (CTCL)—specifically a type known as mycosis fungoides, named after the mushroom-like skin tumors seen in severe cases. CTCL is a malignant lymphoma of helper T-cells that have an affinity for the skin.
Normal life expectancy likely if diagnosed early
In about 10% of cases, the T-cells spread via the lymphatic system to metastasize to the liver, lung, and bone marrow, but more often remain confined to the skin and lymph nodes, and most patients diagnosed early have a normal life expectancy.1
The disease is rare, with about 1000 new cases per year in the US,1 and is more common in African Americans,2 although the photos in dermatological atlases overwhelmingly show Caucasians. CTCL presents in numerous ways, but patients usually have a long course of persistent rash that is often pruritic and usually erythematous or hyperpigmented.
No one knows what causes CTCL, but current theories point to exposure to environmental hazards such as Agent Orange.2 And while the number of environmental hazards in the floodwaters of Katrina were vast, we have no scientific evidence that an environmental exposure was to blame. In fact, while we cared for a number of Katrina evacuees—many of whom had skin infections—this was the only case of mycosis fungoides.
It’s likely that the start of the visible mycosis fungoides lesions after the flood was purely coincidental.
Diagnosis hinges on palpation and biopsy
To diagnose CTCL, you need to palpate for enlarged lymph nodes and perform a full-thickness punch biopsy of the lesion. If the biopsy is negative and the rash persists, take another biopsy—results can be falsely negative at the beginning stages of the disease.
There are no guidelines on which studies should be used for staging CTCL (TABLE), but some sources recommend that if lymph node involvement is suspected on physical exam, lymph node biopsies should be done in addition to a chest radiograph.3 In more advanced stages, consider a computed tomography (CT) scan of the abdomen and pelvis. A recent study concluded that CT and positron-emission tomography (PET) scans used together were more sensitive in staging, but this may not be cost-effective.4
Late-stage mycosis fungoides is usually associated with immunocompromise. Therefore, HIV testing should be performed in all patients with CTCL. Other important laboratory studies include complete blood count—with differential—as well as peripheral smear looking for Sézary cells, lactic dehydrogenase (LDH), liver function tests, and uric acid.
TABLE
Staging for cutaneous T-cell lymphoma based on the Tumor, Node, Metastasis (TNM) system
| STAGE | TNM STAGING | RECOMMENDED TREATMENTS |
|---|---|---|
| IA | T1, N0, M0: Patch or plaque <10% body surface area | Topical high-potency steroids, PUVA, topical nitrogen |
| IB | T2, N0, M0: Patch or plaque >10% body surface area | mustard, carmustine, or bexarotene |
| IIA | T1–2, N1, M0: Patch or plaque with palpable but pathologically normal lymph node | Same as Stage I; if refractory, use total skin electron beam therapy |
| IIB | T3, N0–1, M0: Tumor/nodule | |
| IIIA | T4, N0, M0:Generalized erythroderma | |
| IIIB | T4, N1, M0: Erythroderma and palpable but pathologically normal lymph node | Chemotherapy or photophoresis, refer to medical oncologist, radiation oncologist, and dermatologist |
| IVA | T1–4, N2–3, M0: Pathological lymph node | |
| IVB | T1–4, N0–3, M1: Visceral (M1) or blood involvement | |
| T: 0–4=indicates size or direct extent of the primary tumor | ||
| N: 0=tumor cells absent from regional lymph nodes; 1=tumor cells spread to closest or small number of regional lymph nodes; 3=tumor cells spread to most distant or numerous regional lymph nodes | ||
| M: 0=no distant metastasis; 1=metastasis to distant organs (beyond regional lymph nodes) | ||
A disease that’s easy to mistake for vitiligo
The hypopigmented spots of CTCL look so much like vitiligo, it is frightening to think how easy it would be to miss the diagnosis. Complicating matters: All vitiligo does not need a biopsy to confirm the clinical impression.
So what made this case suspicious enough to warrant a biopsy? First, the hypopigmented spots on our patient were not a typical distribution for vitiligo, which has a predilection for the hands and face. Second, our patient had a hypopigmented patch that had a dark, slightly raised plaque within it, which also would not be typical for vitiligo. (See the patient’s left upper thigh, just below the inguinal area, in FIGURE 1.) Finally, our patient had a rapid onset of multiple discrete macules that did not coalesce into larger hypopigmented areas; in vitiligo you would expect otherwise.
The differential diagnosis also includes idiopathic guttate hypomelanosis, a benign condition that can cause multiple small hypopigmented macules. The size of immunoglobulin H macules, however, is tiny compared with what you’ll see with CTCL. The absence of scales makes eczema, psoriasis, or tinea corporis very unlikely.
Treatment starts with topical steroids
This is a rare disease that lacks the data needed to support an evidence-based approach to treatment. Topical steroids are recommended for stage I when the disease is local to the skin (see TABLE for recommended treatments of other stages). Ultraviolet light (PUVA and UVB) is also used; a recent study suggests the PUVA is a good treatment alternative for stages IA and IB (SOR: C).1
Our patient’s course
Initially, we prescribed a high-potency generic steroid (clobetasol 0.05% cream) for this patient, to be used twice daily to the affected areas.
The patient reported no improvement with this approach, while she awaited her appointments with dermatology and oncology specialists.
Her blood tests were essentially normal, including a negative HIV test.
She is currently receiving narrowband UVB treatment twice weekly.
Seven months after Hurricane Katrina, a 52-year-old African American woman who was evacuated from New Orleans came to our office with a hypopigmented rash on both her upper thighs and arms. Immediately following the 2005 hurricane, she was forced to wade through the polluted waters of New Orleans for many hours before being rescued by boat. Four days passed before she had access to a shower. It was after this shower that she first noticed a single erythematous spot the size of a silver dollar on her left thigh, that several weeks later faded to hypopigmented macules and plaques. Over time, the rash spread to both thighs and arms (FIGURE 1 AND 2. She did not have any itching, pain, bleeding, fever, chills, weight changes, or gastrointestinal symptoms after the rash appeared.
The patient reported that she was married and had worked as a chef for 20 years. She smoked cigarettes, drank alcohol occasionally, and was obese. She had synovitis of her left ankle, which led to surgery. She had no known drug allergies and was taking ibuprofen. Her mother, age 79, had glucose intolerance; her father, age 82, had a renal cell carcinoma removed.
Due to a confluence of situational, economic, and medical problems, she did not seek care for this rash until April 2006. At that time, a punch biopsy revealed the findings in FIGURE 3. Physical exam revealed her skin to have symmetrically distributed hypopigmented macules and plaques to all 4 extremities. She had no lymphadenopathy.
FIGURE 1
Rash on patients’ legs
FIGURE 2
Rash on right arm
FIGURE 3
Hematoxylin/eosin stain
What is the diagnosis?
Were Katrina’s flood waters to blame?
Dx: T-cell lymphoma—timing is coincidental
The biopsy revealed cutaneous T-cell lymphoma (CTCL)—specifically a type known as mycosis fungoides, named after the mushroom-like skin tumors seen in severe cases. CTCL is a malignant lymphoma of helper T-cells that have an affinity for the skin.
Normal life expectancy likely if diagnosed early
In about 10% of cases, the T-cells spread via the lymphatic system to metastasize to the liver, lung, and bone marrow, but more often remain confined to the skin and lymph nodes, and most patients diagnosed early have a normal life expectancy.1
The disease is rare, with about 1000 new cases per year in the US,1 and is more common in African Americans,2 although the photos in dermatological atlases overwhelmingly show Caucasians. CTCL presents in numerous ways, but patients usually have a long course of persistent rash that is often pruritic and usually erythematous or hyperpigmented.
No one knows what causes CTCL, but current theories point to exposure to environmental hazards such as Agent Orange.2 And while the number of environmental hazards in the floodwaters of Katrina were vast, we have no scientific evidence that an environmental exposure was to blame. In fact, while we cared for a number of Katrina evacuees—many of whom had skin infections—this was the only case of mycosis fungoides.
It’s likely that the start of the visible mycosis fungoides lesions after the flood was purely coincidental.
Diagnosis hinges on palpation and biopsy
To diagnose CTCL, you need to palpate for enlarged lymph nodes and perform a full-thickness punch biopsy of the lesion. If the biopsy is negative and the rash persists, take another biopsy—results can be falsely negative at the beginning stages of the disease.
There are no guidelines on which studies should be used for staging CTCL (TABLE), but some sources recommend that if lymph node involvement is suspected on physical exam, lymph node biopsies should be done in addition to a chest radiograph.3 In more advanced stages, consider a computed tomography (CT) scan of the abdomen and pelvis. A recent study concluded that CT and positron-emission tomography (PET) scans used together were more sensitive in staging, but this may not be cost-effective.4
Late-stage mycosis fungoides is usually associated with immunocompromise. Therefore, HIV testing should be performed in all patients with CTCL. Other important laboratory studies include complete blood count—with differential—as well as peripheral smear looking for Sézary cells, lactic dehydrogenase (LDH), liver function tests, and uric acid.
TABLE
Staging for cutaneous T-cell lymphoma based on the Tumor, Node, Metastasis (TNM) system
| STAGE | TNM STAGING | RECOMMENDED TREATMENTS |
|---|---|---|
| IA | T1, N0, M0: Patch or plaque <10% body surface area | Topical high-potency steroids, PUVA, topical nitrogen |
| IB | T2, N0, M0: Patch or plaque >10% body surface area | mustard, carmustine, or bexarotene |
| IIA | T1–2, N1, M0: Patch or plaque with palpable but pathologically normal lymph node | Same as Stage I; if refractory, use total skin electron beam therapy |
| IIB | T3, N0–1, M0: Tumor/nodule | |
| IIIA | T4, N0, M0:Generalized erythroderma | |
| IIIB | T4, N1, M0: Erythroderma and palpable but pathologically normal lymph node | Chemotherapy or photophoresis, refer to medical oncologist, radiation oncologist, and dermatologist |
| IVA | T1–4, N2–3, M0: Pathological lymph node | |
| IVB | T1–4, N0–3, M1: Visceral (M1) or blood involvement | |
| T: 0–4=indicates size or direct extent of the primary tumor | ||
| N: 0=tumor cells absent from regional lymph nodes; 1=tumor cells spread to closest or small number of regional lymph nodes; 3=tumor cells spread to most distant or numerous regional lymph nodes | ||
| M: 0=no distant metastasis; 1=metastasis to distant organs (beyond regional lymph nodes) | ||
A disease that’s easy to mistake for vitiligo
The hypopigmented spots of CTCL look so much like vitiligo, it is frightening to think how easy it would be to miss the diagnosis. Complicating matters: All vitiligo does not need a biopsy to confirm the clinical impression.
So what made this case suspicious enough to warrant a biopsy? First, the hypopigmented spots on our patient were not a typical distribution for vitiligo, which has a predilection for the hands and face. Second, our patient had a hypopigmented patch that had a dark, slightly raised plaque within it, which also would not be typical for vitiligo. (See the patient’s left upper thigh, just below the inguinal area, in FIGURE 1.) Finally, our patient had a rapid onset of multiple discrete macules that did not coalesce into larger hypopigmented areas; in vitiligo you would expect otherwise.
The differential diagnosis also includes idiopathic guttate hypomelanosis, a benign condition that can cause multiple small hypopigmented macules. The size of immunoglobulin H macules, however, is tiny compared with what you’ll see with CTCL. The absence of scales makes eczema, psoriasis, or tinea corporis very unlikely.
Treatment starts with topical steroids
This is a rare disease that lacks the data needed to support an evidence-based approach to treatment. Topical steroids are recommended for stage I when the disease is local to the skin (see TABLE for recommended treatments of other stages). Ultraviolet light (PUVA and UVB) is also used; a recent study suggests the PUVA is a good treatment alternative for stages IA and IB (SOR: C).1
Our patient’s course
Initially, we prescribed a high-potency generic steroid (clobetasol 0.05% cream) for this patient, to be used twice daily to the affected areas.
The patient reported no improvement with this approach, while she awaited her appointments with dermatology and oncology specialists.
Her blood tests were essentially normal, including a negative HIV test.
She is currently receiving narrowband UVB treatment twice weekly.
1. Pinter-Brown LC. Mycosis fungoides. Emedicine, 8 September 2006. Available at: www.emedicine.com/Med/topic1541.htm. Accessed on May 8, 2007.
2. James WD, Berger T, Elston T. Andrews’ Diseases of the Skin: Clinical Dermatology. 10th ed. Philadelphia, Pa: Saunders Elsevier; 2006:727–740.
3. Gettler SL, Fung MA. Efficacy of treatments for mycosis fungoides and sézary syndrome: nationwide survey responses. Dermatol Online J 2005;11:6.-
4. Tsai EY, Taur A, Espinosa L, et al. Staging accuracy in mycosis fungoides and sezary syndrome using integrated positron emission tomography and computed tomography. Arch Dermatol 2006;142:577-584.
1. Pinter-Brown LC. Mycosis fungoides. Emedicine, 8 September 2006. Available at: www.emedicine.com/Med/topic1541.htm. Accessed on May 8, 2007.
2. James WD, Berger T, Elston T. Andrews’ Diseases of the Skin: Clinical Dermatology. 10th ed. Philadelphia, Pa: Saunders Elsevier; 2006:727–740.
3. Gettler SL, Fung MA. Efficacy of treatments for mycosis fungoides and sézary syndrome: nationwide survey responses. Dermatol Online J 2005;11:6.-
4. Tsai EY, Taur A, Espinosa L, et al. Staging accuracy in mycosis fungoides and sezary syndrome using integrated positron emission tomography and computed tomography. Arch Dermatol 2006;142:577-584.
Blisters during pregnancy—just with the second husband
A 33-year-old Hispanic woman who was 5 months pregnant came to the hospital complaining of nausea and vomiting. She had a history of anticardiolipin antibody syndrome, diagnosed originally in 1993 after 2 spontaneous abortions. She had stopped taking warfarin (Coumadin) at the start of her pregnancy, and had been taking heparin for 3 months.
After 4 days of close monitoring, the patient had labor induced for severe life-threatening pre-eclampsia. One day after induction and delivery of a stillborn fetus, she began to develop painful swelling of both hands and feet along with targetoid, urticarial, edematous, deep pink, slightly dusky papules and plaques on her hands, abdomen, lower extremities, and proximal thighs. Some of the edematous sites began to form vesicles and bullae (FIGURE 1 AND 2). When asked about this eruption, the patient mentioned having a similar rash after delivery of one of her children about 10 years before.
Interestingly, she noted that she only experienced these cutaneous findings during pregnancies with her second husband and not with her first. Biopsies were performed and showed prominent eosinophils in the dermis and a subepidermal vesicle (FIGURE 3).
FIGURE 1
Blisters on the wrist…
FIGURE 2
…and the abdomen
FIGURE 3
Biopsy results
What is your diagnosis?
Diagnosis: Pemphigoid gestationis
The patient had pemphigoid gestationis, also known as herpes gestationis, a rare autoimmune bullous disease of pregnancy and the puerperium.1 Clinically and immunopathologically, pemphigoid gestationis is related to the pemphigoid group of disorders and is not virally mediated.2
In the United States, pemphigoid gestationis has an incidence of 1:10,000 to 1:50,000 pregnancies.3 Clinically, it manifests during the second or third trimester, with a sudden onset of extremely pruritic urticarial papules and plaques usually located around the umbilicus. These lesions often progress to tense vesicles and blisters and spread peripherally to the trunk, often sparing the face, palms, and soles.4 Worsening of the lesions at the time of delivery occurs in 75% of cases, and usually recurs with subsequent pregnancies.5 Occasionally, however, subsequent pregnancies are unaffected, so-called “skip pregnancies.”6 This occurs most often when there has been a change in paternity.7
The exact cause of pemphigoid gestationis is unknown. Investigative efforts lead to the identification of an immunoglobulin G (IgG) autoantibody, which binds to bullous pemphigoid (BP) antigen 2, also called BP180, which is a protein associated with hemidesmosomes of basal keratinocytes.8-10 These hemidesmosomes form the central portion of the dermalepidermal anchoring complex, whose function is to establish a connection between the basal keratinocytes and the upper dermis.11,12 This is critical for maintaining dermal-epidermal adhesion. It is hypothesized that binding of autoantibodies to BP180 initiates an inflammatory reaction, leading to blister formation at the dermal-epidermal junction.13
Pathology and immunology
Histopathologic findings demonstrate subepidermal vesicles, spongiosis, and perivascular lymphocyte, and histiocyte infiltrates with a preponderance of eosinophils.3 The sine qua non of the disease, though, is the demonstration through direct immunofluorescence of complement deposition and IgG in a linear band along the basement membrane.14
There appears to be a genetic predisposition toward the development of pemphigoid gestationis. Associations with human leukocyte antigens (HLAs) DR3 (61%–85%), DR4 (52%), or both (43%–50%) have been reported.3,15,16 Interestingly, 85% of persons with a history of pemphigoid gestationis were found to have anti-HLA antibodies, some of which were directed against paternal HLAs expressed in their placentae.17 These findings raised speculation about a possible immunologic insult against placental antigens during pregnancy. Evidence suggests that circulating autoantibodies in patients with pemphigoid gestationis bind to the dermal-epidermal junction of skin and amnion in which BP180 antigen is also present.18-20
It has been demonstrated that in patients with pemphigoid gestationis the cells of the placenta stroma express abnormal major histocompatibility complex (MHC) class II molecules.21,22 This lead to the proposition of 2 possible mechanisms for the initiation of an autoimmune response in pemphigoid gestationis. The first proposes that placental BP180 is presented to the maternal immune system in association with abnormal MHC molecules, which then trigger the production of autoantibodies that cross-react with the skin. Alternatively, the placental stromal cells may evoke an allogeneic reaction against the BP180 antigen presented by paternal MHC molecules of the placental stroma, which then cross-reacts with the skin.23 The latter theory supports the findings in this patient, who developed pemphigoid gestationis during the 2 pregnancies with her second husband and not during the pregnancies with her first husband.
Differential diagnosis
It is important to differentiate the prebullous stage of pemphigoid gestationis from other pregnancy-related dermatoses. These include polymorphic eruption of pregnancy (PEP), pruritic urticarial papules and plaques of pregnancy (PUPPP), erythema multiforme, prurigo annularis, intrahepatic cholestasis of pregnancy, and impetigo herpetiformis. Impetigo herpetiformis is not related to bacterial or viral causes, but is rather a manifestation of pustular psoriasis during pregnancy. The target lesions that form in pemphigoid gestationis look just like the target lesions of erythema multiforme.
When there is no blister formation, it is impossible to distinguish pemphigoid gestationis from many of the other cutaneous eruptions of pregnancy. If uncertain, the clinician should perform punch biopsies of the involved skin, with one specimen sent for immunofluoresence studies. The biopsy should not pass directly through a bullae, due to risk of losing the overlying epidermis in the specimen. Do the punch biopsy at the edge of the bulla including some normal skin. Other important laboratory exams to perform would include liver function tests to look for an upward trend associated with intrahepatic cholestasis, and herpes simplex virus antibody testing for the association with erythema multiforme. The cutaneous findings and pertinent tests are listed in the table below in order of increasing potential as a life-threatening dermatosis (TABLE).
TABLE
Differential diagnosis for blisters in pregnancy
| DISEASE | ASSOCIATIONS | DIAGNOSIS | TREATMENT |
|---|---|---|---|
| Polymorphous eruption of pregnancy | Nonspecific pruritic eruption of pregnancy | Biopsy to differentiate from prebullous stage of pemphigoid (herpes) gestationis | Mild to mid-potency topical steroids, oral antihistamines |
| Pruritic urticarial papules and plaques of pregnancy | Occur in stretch marks, spare umbilicus; more often in primigravidas | Unless history is very clear, biopsy to differentiate from prebullous stage of pemphigoid gestationis | Emollients, pulse-dye laser during violaceous stage of striae, topical steroids, oral antihistamines |
| Erythema multiforme | Can involve mucous membranes, targetoid lesions, absence of pruritus, centripetal spread, favors palms/soles | Viral, bacterial, or drug-related eruption. Most often with herpes simplex I or II virus. Biopsy to differentiate from pemphigoid gestationis | Acyclovir, valacyclovir if HSV-related, treatment of bacterial infection, or removal of offending drug |
| Pemphigoid gestationis | Blistering, urticarial papules/plaques, pruritus | Biopsy sent for histologic diagnosis and immunofluorescence | Prednisone for short course starting at 1 mg/kg, then tapering over 2–3 months, topical steroids |
| Intrahepatic cholestasis of pregnancy | +/- jaundice, otherwise no cutaneous findings other than generalized pruritus, risk of preterm birth | Elevation in liver function tests, cholesterol, triglycerides, dark urine, right upper quadrant pain, nausea, greasy stools | Ursodeoxycholic acid, S-adenosyl-L-methionine |
| Impetigo herpetiformis (pustular psoriasis of pregnancy) | Extremely ill with fever, chills, nausea, vascular instability, pustules rather than vesicles | Biopsy if uncertain, pustules sterile, risk of hypocalcemia, hypoparathyroidism | High dose oral steroids or cyclosporine |
Treatment
Pemphigoid gestationis should resolve spontaneously within 2 to 3 months of delivery. Treatment is aimed at preventing new blisters and relieving pruritus, with topical corticosteroids and oral antihistamines in mild cases.2,25 In advanced lesions as seen in this case, 0.3 to 0.5 mg/kg of prednisolone daily is usually sufficient.3,25 Alternative medications include sulfapyridine, dapsone, and cyclosporine, though disease response is variable and their safety is questionable.3
When the skin condition began, the patient was treated with oral antihistamines and topical steroids. On day 2, the diagnosis of pemphigoid gestationis was clear, and she was started on oral prednisone at 60 mg/d, which resulted in rapid symptom improvement in her lesions and swelling. New lesions stopped forming, and systemic steroids were tapered off over the 3 months after delivery. The skin lesions healed and she was given supportive counseling to help her cope with her pregnancy loss.
Conclusion
We have described a rare case of a patient with no cutaneous eruptions during her pregnancies with her first husband, who developed pemphigoid gestationis in 2 pregnancies with her second husband. While it is interesting that our patient also had the anticardiolipin syndrome, most patients do not have both conditions.
Our patient had the classic findings of pemphigoid gestationis with many characteristic lesions (including the umbilicus) making the diagnosis possible before biopsy confirmation. This was fortunate for her because her painful swelling responded quickly to the corticosteroids. When cases are less clinically obvious, biopsy for histopathology and immunofluorescence facilitates differentiation of pemphigoid gestationis from other dermatoses of pregnancy.
CORRESPONDENCE
Richard P. Usatine, MD, University of Texas Health Sciences Center at San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: usatine@uthscsa.edu
1. Coupe RL. Herpes gestationis. Arch Dermatol 1965;91:633-636.
2. Jenkins RE, Hern S, Black MM. Clinical features and management of 87 patients with pemphigoid gestationis. Clin Exp Dermatol 1999;24:255-259.
3. Al-Fouzan AW, Galadari I, Oumeish I, et al. Herpes gestationis (Pemphigoid gestationis). Clinics Dermatology 2006;24:109-112.
4. Shornick JK. Herpes gestationis. J Am Acad Dermatol 1987;17:539-556.
5. Holmes RC, Black MM, Dann J, et al. A comparative study of toxic erythema of pregnancy and herpes gestationis. Br J Dermatol 1982;106:499-510.
6. Cozzani E, Basso M, Parodi A, Rebora A. Pemphigoid gestationis post partum after changing husband. Intn J Dermatol 2005;44:1057-1058.
7. Shornick JK, Black MM. Fetal risks in herpes gestationis. J Am Acad Dermatol 1992;26:63-68.
8. Diaz LA, Ratrie H, III, Saunders WS, et al. Isolation of a human epidermal cDNA corresponding to the 180-kD autoantigen recognized by bullous pemphigoid and herpes gestationis sera. Immunolocalization of this protein to the hemidesmosome. J Clin Invest 1990;86:1088-1094.
9. Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol 1992;99:243-250.
10. Zillikens D, Giudice GJ. BP180/typeXVIII collagen: its role in acquired and inherited disorders of the dermal-epidermal junction. Arch Dermatol Res 1999;291:187-194.
11. Borradori L, Sonnenberg A. Hemidesmosomes: roles in adhesion, signaling and human diseases. Curr Opin Cell Biol 1996;8:647-656.
12. Zillikens D. Acquired skin disease of hemidesmosomes. J Dermatol Sci 1999;20:134-154.
13. Schmidt E, Zillikens D. Autoimmune and inherited subepidermal blistering diseases: advances in the clinic and the laboratory. Adv Dermatol 2000;16:113-157.
14. Shornick JD. Dermatoses of pregnancy. Semin Cutan Med Surg 1998;17:172-181.
15. Holmes RC, Black MM, Jurecka W, et al. Clues to the aetiology and pathogenesis of herpes gestationis. Br J Dermatol 1983;109:131-139.
16. Shornick JK, Stastny P, Gilliam JN. High frequency of histocompatibility antigens DR3 and DR4 in herpes gestationis. J Clin Invest 1981;68:553-555.
17. Shornick JK, Stastny P, Gilliam JN. Paternal histocompatibility (HLA) antigens and maternal anti-HLA antibodies in herpes gestationis. J Invest Dermatol 1983;81:407-409.
18. Ortonne JP, Hsi BL, Verrando P, et al. Herpes gestationis factor reacts with the amniotic epithelial basement membrane. Br J Dermatol 1987;117:147-154.
19. Kelly SE, Bhogal BS, Wojnarowska F, Black MM. Expression of a pemphigoid gestationis-related antigen by human placenta. Br J Dermatol 1988;118:605-611.
20. Fairley JA, Heintz PW, Neuburg M, et al. Expression pattern of the bullous pemphigoid-180 antigen in normal and neoplastic epithelia. Br J Dermatol 1995;133:385-391.
21. Kelly SE, Black MM, Fleming S. Antigen-presenting cells in the skin and placenta in pemphigoid gestationis. Br J Dermatol 1990;122:593-599.
22. Borthwick GM, Holmes RC, Stirrat GM. Abnormal expression of class II MHC antigens in placentae from patients with pemphigoid gestationis. Placenta 1988;9:81-94.
23. Kelly SE, Black MM, Fleming S. Pemphigoid gestationis: a unique mechanism of initiation of an autoimmune response by MHC class II molecules. J Pathol 1989;158:81-82.
24. Borradori L, Saurat JH. Specific dermatoses of pregnancy. Toward a comprehensive view. Arch Dermatol 1994;130:778-780.
25. Shimanovich I, Bröcker EB, Zillikens D. Pemphigoid gestationis: new insights into the pathogenesis lead to novel diagnostic tools. Br J Obstet Gynaecol 2002;109:970-976.
A 33-year-old Hispanic woman who was 5 months pregnant came to the hospital complaining of nausea and vomiting. She had a history of anticardiolipin antibody syndrome, diagnosed originally in 1993 after 2 spontaneous abortions. She had stopped taking warfarin (Coumadin) at the start of her pregnancy, and had been taking heparin for 3 months.
After 4 days of close monitoring, the patient had labor induced for severe life-threatening pre-eclampsia. One day after induction and delivery of a stillborn fetus, she began to develop painful swelling of both hands and feet along with targetoid, urticarial, edematous, deep pink, slightly dusky papules and plaques on her hands, abdomen, lower extremities, and proximal thighs. Some of the edematous sites began to form vesicles and bullae (FIGURE 1 AND 2). When asked about this eruption, the patient mentioned having a similar rash after delivery of one of her children about 10 years before.
Interestingly, she noted that she only experienced these cutaneous findings during pregnancies with her second husband and not with her first. Biopsies were performed and showed prominent eosinophils in the dermis and a subepidermal vesicle (FIGURE 3).
FIGURE 1
Blisters on the wrist…
FIGURE 2
…and the abdomen
FIGURE 3
Biopsy results
What is your diagnosis?
Diagnosis: Pemphigoid gestationis
The patient had pemphigoid gestationis, also known as herpes gestationis, a rare autoimmune bullous disease of pregnancy and the puerperium.1 Clinically and immunopathologically, pemphigoid gestationis is related to the pemphigoid group of disorders and is not virally mediated.2
In the United States, pemphigoid gestationis has an incidence of 1:10,000 to 1:50,000 pregnancies.3 Clinically, it manifests during the second or third trimester, with a sudden onset of extremely pruritic urticarial papules and plaques usually located around the umbilicus. These lesions often progress to tense vesicles and blisters and spread peripherally to the trunk, often sparing the face, palms, and soles.4 Worsening of the lesions at the time of delivery occurs in 75% of cases, and usually recurs with subsequent pregnancies.5 Occasionally, however, subsequent pregnancies are unaffected, so-called “skip pregnancies.”6 This occurs most often when there has been a change in paternity.7
The exact cause of pemphigoid gestationis is unknown. Investigative efforts lead to the identification of an immunoglobulin G (IgG) autoantibody, which binds to bullous pemphigoid (BP) antigen 2, also called BP180, which is a protein associated with hemidesmosomes of basal keratinocytes.8-10 These hemidesmosomes form the central portion of the dermalepidermal anchoring complex, whose function is to establish a connection between the basal keratinocytes and the upper dermis.11,12 This is critical for maintaining dermal-epidermal adhesion. It is hypothesized that binding of autoantibodies to BP180 initiates an inflammatory reaction, leading to blister formation at the dermal-epidermal junction.13
Pathology and immunology
Histopathologic findings demonstrate subepidermal vesicles, spongiosis, and perivascular lymphocyte, and histiocyte infiltrates with a preponderance of eosinophils.3 The sine qua non of the disease, though, is the demonstration through direct immunofluorescence of complement deposition and IgG in a linear band along the basement membrane.14
There appears to be a genetic predisposition toward the development of pemphigoid gestationis. Associations with human leukocyte antigens (HLAs) DR3 (61%–85%), DR4 (52%), or both (43%–50%) have been reported.3,15,16 Interestingly, 85% of persons with a history of pemphigoid gestationis were found to have anti-HLA antibodies, some of which were directed against paternal HLAs expressed in their placentae.17 These findings raised speculation about a possible immunologic insult against placental antigens during pregnancy. Evidence suggests that circulating autoantibodies in patients with pemphigoid gestationis bind to the dermal-epidermal junction of skin and amnion in which BP180 antigen is also present.18-20
It has been demonstrated that in patients with pemphigoid gestationis the cells of the placenta stroma express abnormal major histocompatibility complex (MHC) class II molecules.21,22 This lead to the proposition of 2 possible mechanisms for the initiation of an autoimmune response in pemphigoid gestationis. The first proposes that placental BP180 is presented to the maternal immune system in association with abnormal MHC molecules, which then trigger the production of autoantibodies that cross-react with the skin. Alternatively, the placental stromal cells may evoke an allogeneic reaction against the BP180 antigen presented by paternal MHC molecules of the placental stroma, which then cross-reacts with the skin.23 The latter theory supports the findings in this patient, who developed pemphigoid gestationis during the 2 pregnancies with her second husband and not during the pregnancies with her first husband.
Differential diagnosis
It is important to differentiate the prebullous stage of pemphigoid gestationis from other pregnancy-related dermatoses. These include polymorphic eruption of pregnancy (PEP), pruritic urticarial papules and plaques of pregnancy (PUPPP), erythema multiforme, prurigo annularis, intrahepatic cholestasis of pregnancy, and impetigo herpetiformis. Impetigo herpetiformis is not related to bacterial or viral causes, but is rather a manifestation of pustular psoriasis during pregnancy. The target lesions that form in pemphigoid gestationis look just like the target lesions of erythema multiforme.
When there is no blister formation, it is impossible to distinguish pemphigoid gestationis from many of the other cutaneous eruptions of pregnancy. If uncertain, the clinician should perform punch biopsies of the involved skin, with one specimen sent for immunofluoresence studies. The biopsy should not pass directly through a bullae, due to risk of losing the overlying epidermis in the specimen. Do the punch biopsy at the edge of the bulla including some normal skin. Other important laboratory exams to perform would include liver function tests to look for an upward trend associated with intrahepatic cholestasis, and herpes simplex virus antibody testing for the association with erythema multiforme. The cutaneous findings and pertinent tests are listed in the table below in order of increasing potential as a life-threatening dermatosis (TABLE).
TABLE
Differential diagnosis for blisters in pregnancy
| DISEASE | ASSOCIATIONS | DIAGNOSIS | TREATMENT |
|---|---|---|---|
| Polymorphous eruption of pregnancy | Nonspecific pruritic eruption of pregnancy | Biopsy to differentiate from prebullous stage of pemphigoid (herpes) gestationis | Mild to mid-potency topical steroids, oral antihistamines |
| Pruritic urticarial papules and plaques of pregnancy | Occur in stretch marks, spare umbilicus; more often in primigravidas | Unless history is very clear, biopsy to differentiate from prebullous stage of pemphigoid gestationis | Emollients, pulse-dye laser during violaceous stage of striae, topical steroids, oral antihistamines |
| Erythema multiforme | Can involve mucous membranes, targetoid lesions, absence of pruritus, centripetal spread, favors palms/soles | Viral, bacterial, or drug-related eruption. Most often with herpes simplex I or II virus. Biopsy to differentiate from pemphigoid gestationis | Acyclovir, valacyclovir if HSV-related, treatment of bacterial infection, or removal of offending drug |
| Pemphigoid gestationis | Blistering, urticarial papules/plaques, pruritus | Biopsy sent for histologic diagnosis and immunofluorescence | Prednisone for short course starting at 1 mg/kg, then tapering over 2–3 months, topical steroids |
| Intrahepatic cholestasis of pregnancy | +/- jaundice, otherwise no cutaneous findings other than generalized pruritus, risk of preterm birth | Elevation in liver function tests, cholesterol, triglycerides, dark urine, right upper quadrant pain, nausea, greasy stools | Ursodeoxycholic acid, S-adenosyl-L-methionine |
| Impetigo herpetiformis (pustular psoriasis of pregnancy) | Extremely ill with fever, chills, nausea, vascular instability, pustules rather than vesicles | Biopsy if uncertain, pustules sterile, risk of hypocalcemia, hypoparathyroidism | High dose oral steroids or cyclosporine |
Treatment
Pemphigoid gestationis should resolve spontaneously within 2 to 3 months of delivery. Treatment is aimed at preventing new blisters and relieving pruritus, with topical corticosteroids and oral antihistamines in mild cases.2,25 In advanced lesions as seen in this case, 0.3 to 0.5 mg/kg of prednisolone daily is usually sufficient.3,25 Alternative medications include sulfapyridine, dapsone, and cyclosporine, though disease response is variable and their safety is questionable.3
When the skin condition began, the patient was treated with oral antihistamines and topical steroids. On day 2, the diagnosis of pemphigoid gestationis was clear, and she was started on oral prednisone at 60 mg/d, which resulted in rapid symptom improvement in her lesions and swelling. New lesions stopped forming, and systemic steroids were tapered off over the 3 months after delivery. The skin lesions healed and she was given supportive counseling to help her cope with her pregnancy loss.
Conclusion
We have described a rare case of a patient with no cutaneous eruptions during her pregnancies with her first husband, who developed pemphigoid gestationis in 2 pregnancies with her second husband. While it is interesting that our patient also had the anticardiolipin syndrome, most patients do not have both conditions.
Our patient had the classic findings of pemphigoid gestationis with many characteristic lesions (including the umbilicus) making the diagnosis possible before biopsy confirmation. This was fortunate for her because her painful swelling responded quickly to the corticosteroids. When cases are less clinically obvious, biopsy for histopathology and immunofluorescence facilitates differentiation of pemphigoid gestationis from other dermatoses of pregnancy.
CORRESPONDENCE
Richard P. Usatine, MD, University of Texas Health Sciences Center at San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: usatine@uthscsa.edu
A 33-year-old Hispanic woman who was 5 months pregnant came to the hospital complaining of nausea and vomiting. She had a history of anticardiolipin antibody syndrome, diagnosed originally in 1993 after 2 spontaneous abortions. She had stopped taking warfarin (Coumadin) at the start of her pregnancy, and had been taking heparin for 3 months.
After 4 days of close monitoring, the patient had labor induced for severe life-threatening pre-eclampsia. One day after induction and delivery of a stillborn fetus, she began to develop painful swelling of both hands and feet along with targetoid, urticarial, edematous, deep pink, slightly dusky papules and plaques on her hands, abdomen, lower extremities, and proximal thighs. Some of the edematous sites began to form vesicles and bullae (FIGURE 1 AND 2). When asked about this eruption, the patient mentioned having a similar rash after delivery of one of her children about 10 years before.
Interestingly, she noted that she only experienced these cutaneous findings during pregnancies with her second husband and not with her first. Biopsies were performed and showed prominent eosinophils in the dermis and a subepidermal vesicle (FIGURE 3).
FIGURE 1
Blisters on the wrist…
FIGURE 2
…and the abdomen
FIGURE 3
Biopsy results
What is your diagnosis?
Diagnosis: Pemphigoid gestationis
The patient had pemphigoid gestationis, also known as herpes gestationis, a rare autoimmune bullous disease of pregnancy and the puerperium.1 Clinically and immunopathologically, pemphigoid gestationis is related to the pemphigoid group of disorders and is not virally mediated.2
In the United States, pemphigoid gestationis has an incidence of 1:10,000 to 1:50,000 pregnancies.3 Clinically, it manifests during the second or third trimester, with a sudden onset of extremely pruritic urticarial papules and plaques usually located around the umbilicus. These lesions often progress to tense vesicles and blisters and spread peripherally to the trunk, often sparing the face, palms, and soles.4 Worsening of the lesions at the time of delivery occurs in 75% of cases, and usually recurs with subsequent pregnancies.5 Occasionally, however, subsequent pregnancies are unaffected, so-called “skip pregnancies.”6 This occurs most often when there has been a change in paternity.7
The exact cause of pemphigoid gestationis is unknown. Investigative efforts lead to the identification of an immunoglobulin G (IgG) autoantibody, which binds to bullous pemphigoid (BP) antigen 2, also called BP180, which is a protein associated with hemidesmosomes of basal keratinocytes.8-10 These hemidesmosomes form the central portion of the dermalepidermal anchoring complex, whose function is to establish a connection between the basal keratinocytes and the upper dermis.11,12 This is critical for maintaining dermal-epidermal adhesion. It is hypothesized that binding of autoantibodies to BP180 initiates an inflammatory reaction, leading to blister formation at the dermal-epidermal junction.13
Pathology and immunology
Histopathologic findings demonstrate subepidermal vesicles, spongiosis, and perivascular lymphocyte, and histiocyte infiltrates with a preponderance of eosinophils.3 The sine qua non of the disease, though, is the demonstration through direct immunofluorescence of complement deposition and IgG in a linear band along the basement membrane.14
There appears to be a genetic predisposition toward the development of pemphigoid gestationis. Associations with human leukocyte antigens (HLAs) DR3 (61%–85%), DR4 (52%), or both (43%–50%) have been reported.3,15,16 Interestingly, 85% of persons with a history of pemphigoid gestationis were found to have anti-HLA antibodies, some of which were directed against paternal HLAs expressed in their placentae.17 These findings raised speculation about a possible immunologic insult against placental antigens during pregnancy. Evidence suggests that circulating autoantibodies in patients with pemphigoid gestationis bind to the dermal-epidermal junction of skin and amnion in which BP180 antigen is also present.18-20
It has been demonstrated that in patients with pemphigoid gestationis the cells of the placenta stroma express abnormal major histocompatibility complex (MHC) class II molecules.21,22 This lead to the proposition of 2 possible mechanisms for the initiation of an autoimmune response in pemphigoid gestationis. The first proposes that placental BP180 is presented to the maternal immune system in association with abnormal MHC molecules, which then trigger the production of autoantibodies that cross-react with the skin. Alternatively, the placental stromal cells may evoke an allogeneic reaction against the BP180 antigen presented by paternal MHC molecules of the placental stroma, which then cross-reacts with the skin.23 The latter theory supports the findings in this patient, who developed pemphigoid gestationis during the 2 pregnancies with her second husband and not during the pregnancies with her first husband.
Differential diagnosis
It is important to differentiate the prebullous stage of pemphigoid gestationis from other pregnancy-related dermatoses. These include polymorphic eruption of pregnancy (PEP), pruritic urticarial papules and plaques of pregnancy (PUPPP), erythema multiforme, prurigo annularis, intrahepatic cholestasis of pregnancy, and impetigo herpetiformis. Impetigo herpetiformis is not related to bacterial or viral causes, but is rather a manifestation of pustular psoriasis during pregnancy. The target lesions that form in pemphigoid gestationis look just like the target lesions of erythema multiforme.
When there is no blister formation, it is impossible to distinguish pemphigoid gestationis from many of the other cutaneous eruptions of pregnancy. If uncertain, the clinician should perform punch biopsies of the involved skin, with one specimen sent for immunofluoresence studies. The biopsy should not pass directly through a bullae, due to risk of losing the overlying epidermis in the specimen. Do the punch biopsy at the edge of the bulla including some normal skin. Other important laboratory exams to perform would include liver function tests to look for an upward trend associated with intrahepatic cholestasis, and herpes simplex virus antibody testing for the association with erythema multiforme. The cutaneous findings and pertinent tests are listed in the table below in order of increasing potential as a life-threatening dermatosis (TABLE).
TABLE
Differential diagnosis for blisters in pregnancy
| DISEASE | ASSOCIATIONS | DIAGNOSIS | TREATMENT |
|---|---|---|---|
| Polymorphous eruption of pregnancy | Nonspecific pruritic eruption of pregnancy | Biopsy to differentiate from prebullous stage of pemphigoid (herpes) gestationis | Mild to mid-potency topical steroids, oral antihistamines |
| Pruritic urticarial papules and plaques of pregnancy | Occur in stretch marks, spare umbilicus; more often in primigravidas | Unless history is very clear, biopsy to differentiate from prebullous stage of pemphigoid gestationis | Emollients, pulse-dye laser during violaceous stage of striae, topical steroids, oral antihistamines |
| Erythema multiforme | Can involve mucous membranes, targetoid lesions, absence of pruritus, centripetal spread, favors palms/soles | Viral, bacterial, or drug-related eruption. Most often with herpes simplex I or II virus. Biopsy to differentiate from pemphigoid gestationis | Acyclovir, valacyclovir if HSV-related, treatment of bacterial infection, or removal of offending drug |
| Pemphigoid gestationis | Blistering, urticarial papules/plaques, pruritus | Biopsy sent for histologic diagnosis and immunofluorescence | Prednisone for short course starting at 1 mg/kg, then tapering over 2–3 months, topical steroids |
| Intrahepatic cholestasis of pregnancy | +/- jaundice, otherwise no cutaneous findings other than generalized pruritus, risk of preterm birth | Elevation in liver function tests, cholesterol, triglycerides, dark urine, right upper quadrant pain, nausea, greasy stools | Ursodeoxycholic acid, S-adenosyl-L-methionine |
| Impetigo herpetiformis (pustular psoriasis of pregnancy) | Extremely ill with fever, chills, nausea, vascular instability, pustules rather than vesicles | Biopsy if uncertain, pustules sterile, risk of hypocalcemia, hypoparathyroidism | High dose oral steroids or cyclosporine |
Treatment
Pemphigoid gestationis should resolve spontaneously within 2 to 3 months of delivery. Treatment is aimed at preventing new blisters and relieving pruritus, with topical corticosteroids and oral antihistamines in mild cases.2,25 In advanced lesions as seen in this case, 0.3 to 0.5 mg/kg of prednisolone daily is usually sufficient.3,25 Alternative medications include sulfapyridine, dapsone, and cyclosporine, though disease response is variable and their safety is questionable.3
When the skin condition began, the patient was treated with oral antihistamines and topical steroids. On day 2, the diagnosis of pemphigoid gestationis was clear, and she was started on oral prednisone at 60 mg/d, which resulted in rapid symptom improvement in her lesions and swelling. New lesions stopped forming, and systemic steroids were tapered off over the 3 months after delivery. The skin lesions healed and she was given supportive counseling to help her cope with her pregnancy loss.
Conclusion
We have described a rare case of a patient with no cutaneous eruptions during her pregnancies with her first husband, who developed pemphigoid gestationis in 2 pregnancies with her second husband. While it is interesting that our patient also had the anticardiolipin syndrome, most patients do not have both conditions.
Our patient had the classic findings of pemphigoid gestationis with many characteristic lesions (including the umbilicus) making the diagnosis possible before biopsy confirmation. This was fortunate for her because her painful swelling responded quickly to the corticosteroids. When cases are less clinically obvious, biopsy for histopathology and immunofluorescence facilitates differentiation of pemphigoid gestationis from other dermatoses of pregnancy.
CORRESPONDENCE
Richard P. Usatine, MD, University of Texas Health Sciences Center at San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: usatine@uthscsa.edu
1. Coupe RL. Herpes gestationis. Arch Dermatol 1965;91:633-636.
2. Jenkins RE, Hern S, Black MM. Clinical features and management of 87 patients with pemphigoid gestationis. Clin Exp Dermatol 1999;24:255-259.
3. Al-Fouzan AW, Galadari I, Oumeish I, et al. Herpes gestationis (Pemphigoid gestationis). Clinics Dermatology 2006;24:109-112.
4. Shornick JK. Herpes gestationis. J Am Acad Dermatol 1987;17:539-556.
5. Holmes RC, Black MM, Dann J, et al. A comparative study of toxic erythema of pregnancy and herpes gestationis. Br J Dermatol 1982;106:499-510.
6. Cozzani E, Basso M, Parodi A, Rebora A. Pemphigoid gestationis post partum after changing husband. Intn J Dermatol 2005;44:1057-1058.
7. Shornick JK, Black MM. Fetal risks in herpes gestationis. J Am Acad Dermatol 1992;26:63-68.
8. Diaz LA, Ratrie H, III, Saunders WS, et al. Isolation of a human epidermal cDNA corresponding to the 180-kD autoantigen recognized by bullous pemphigoid and herpes gestationis sera. Immunolocalization of this protein to the hemidesmosome. J Clin Invest 1990;86:1088-1094.
9. Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol 1992;99:243-250.
10. Zillikens D, Giudice GJ. BP180/typeXVIII collagen: its role in acquired and inherited disorders of the dermal-epidermal junction. Arch Dermatol Res 1999;291:187-194.
11. Borradori L, Sonnenberg A. Hemidesmosomes: roles in adhesion, signaling and human diseases. Curr Opin Cell Biol 1996;8:647-656.
12. Zillikens D. Acquired skin disease of hemidesmosomes. J Dermatol Sci 1999;20:134-154.
13. Schmidt E, Zillikens D. Autoimmune and inherited subepidermal blistering diseases: advances in the clinic and the laboratory. Adv Dermatol 2000;16:113-157.
14. Shornick JD. Dermatoses of pregnancy. Semin Cutan Med Surg 1998;17:172-181.
15. Holmes RC, Black MM, Jurecka W, et al. Clues to the aetiology and pathogenesis of herpes gestationis. Br J Dermatol 1983;109:131-139.
16. Shornick JK, Stastny P, Gilliam JN. High frequency of histocompatibility antigens DR3 and DR4 in herpes gestationis. J Clin Invest 1981;68:553-555.
17. Shornick JK, Stastny P, Gilliam JN. Paternal histocompatibility (HLA) antigens and maternal anti-HLA antibodies in herpes gestationis. J Invest Dermatol 1983;81:407-409.
18. Ortonne JP, Hsi BL, Verrando P, et al. Herpes gestationis factor reacts with the amniotic epithelial basement membrane. Br J Dermatol 1987;117:147-154.
19. Kelly SE, Bhogal BS, Wojnarowska F, Black MM. Expression of a pemphigoid gestationis-related antigen by human placenta. Br J Dermatol 1988;118:605-611.
20. Fairley JA, Heintz PW, Neuburg M, et al. Expression pattern of the bullous pemphigoid-180 antigen in normal and neoplastic epithelia. Br J Dermatol 1995;133:385-391.
21. Kelly SE, Black MM, Fleming S. Antigen-presenting cells in the skin and placenta in pemphigoid gestationis. Br J Dermatol 1990;122:593-599.
22. Borthwick GM, Holmes RC, Stirrat GM. Abnormal expression of class II MHC antigens in placentae from patients with pemphigoid gestationis. Placenta 1988;9:81-94.
23. Kelly SE, Black MM, Fleming S. Pemphigoid gestationis: a unique mechanism of initiation of an autoimmune response by MHC class II molecules. J Pathol 1989;158:81-82.
24. Borradori L, Saurat JH. Specific dermatoses of pregnancy. Toward a comprehensive view. Arch Dermatol 1994;130:778-780.
25. Shimanovich I, Bröcker EB, Zillikens D. Pemphigoid gestationis: new insights into the pathogenesis lead to novel diagnostic tools. Br J Obstet Gynaecol 2002;109:970-976.
1. Coupe RL. Herpes gestationis. Arch Dermatol 1965;91:633-636.
2. Jenkins RE, Hern S, Black MM. Clinical features and management of 87 patients with pemphigoid gestationis. Clin Exp Dermatol 1999;24:255-259.
3. Al-Fouzan AW, Galadari I, Oumeish I, et al. Herpes gestationis (Pemphigoid gestationis). Clinics Dermatology 2006;24:109-112.
4. Shornick JK. Herpes gestationis. J Am Acad Dermatol 1987;17:539-556.
5. Holmes RC, Black MM, Dann J, et al. A comparative study of toxic erythema of pregnancy and herpes gestationis. Br J Dermatol 1982;106:499-510.
6. Cozzani E, Basso M, Parodi A, Rebora A. Pemphigoid gestationis post partum after changing husband. Intn J Dermatol 2005;44:1057-1058.
7. Shornick JK, Black MM. Fetal risks in herpes gestationis. J Am Acad Dermatol 1992;26:63-68.
8. Diaz LA, Ratrie H, III, Saunders WS, et al. Isolation of a human epidermal cDNA corresponding to the 180-kD autoantigen recognized by bullous pemphigoid and herpes gestationis sera. Immunolocalization of this protein to the hemidesmosome. J Clin Invest 1990;86:1088-1094.
9. Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol 1992;99:243-250.
10. Zillikens D, Giudice GJ. BP180/typeXVIII collagen: its role in acquired and inherited disorders of the dermal-epidermal junction. Arch Dermatol Res 1999;291:187-194.
11. Borradori L, Sonnenberg A. Hemidesmosomes: roles in adhesion, signaling and human diseases. Curr Opin Cell Biol 1996;8:647-656.
12. Zillikens D. Acquired skin disease of hemidesmosomes. J Dermatol Sci 1999;20:134-154.
13. Schmidt E, Zillikens D. Autoimmune and inherited subepidermal blistering diseases: advances in the clinic and the laboratory. Adv Dermatol 2000;16:113-157.
14. Shornick JD. Dermatoses of pregnancy. Semin Cutan Med Surg 1998;17:172-181.
15. Holmes RC, Black MM, Jurecka W, et al. Clues to the aetiology and pathogenesis of herpes gestationis. Br J Dermatol 1983;109:131-139.
16. Shornick JK, Stastny P, Gilliam JN. High frequency of histocompatibility antigens DR3 and DR4 in herpes gestationis. J Clin Invest 1981;68:553-555.
17. Shornick JK, Stastny P, Gilliam JN. Paternal histocompatibility (HLA) antigens and maternal anti-HLA antibodies in herpes gestationis. J Invest Dermatol 1983;81:407-409.
18. Ortonne JP, Hsi BL, Verrando P, et al. Herpes gestationis factor reacts with the amniotic epithelial basement membrane. Br J Dermatol 1987;117:147-154.
19. Kelly SE, Bhogal BS, Wojnarowska F, Black MM. Expression of a pemphigoid gestationis-related antigen by human placenta. Br J Dermatol 1988;118:605-611.
20. Fairley JA, Heintz PW, Neuburg M, et al. Expression pattern of the bullous pemphigoid-180 antigen in normal and neoplastic epithelia. Br J Dermatol 1995;133:385-391.
21. Kelly SE, Black MM, Fleming S. Antigen-presenting cells in the skin and placenta in pemphigoid gestationis. Br J Dermatol 1990;122:593-599.
22. Borthwick GM, Holmes RC, Stirrat GM. Abnormal expression of class II MHC antigens in placentae from patients with pemphigoid gestationis. Placenta 1988;9:81-94.
23. Kelly SE, Black MM, Fleming S. Pemphigoid gestationis: a unique mechanism of initiation of an autoimmune response by MHC class II molecules. J Pathol 1989;158:81-82.
24. Borradori L, Saurat JH. Specific dermatoses of pregnancy. Toward a comprehensive view. Arch Dermatol 1994;130:778-780.
25. Shimanovich I, Bröcker EB, Zillikens D. Pemphigoid gestationis: new insights into the pathogenesis lead to novel diagnostic tools. Br J Obstet Gynaecol 2002;109:970-976.
Itching and rash in a boy and his grandmother
A boy came to the office with a rash and progressively severe itching for approximately 2 months (FIGURE 1). Examination showed an excoriated generalized papular eruption, including some urticarial-type papules and chronic eczematoid changes near the waist, axillae, hands, and wrists.
His grandmother, with whom he spends most weekends and a lot of time after school, also has had a rash and progressive itch for approximately 3 weeks. One feature of the dermopathy observed clinically, first located by hand lens examination and then confirmed by dermoscopy, is depicted in FIGURE 2.
FIGURE 1
Excoriated eruptions
FIGURE 2
Dermoscopic photograph of the dermatosis
What is your diagnosis?
Diagnosis: Scabies
The boy and his grandmother both have scabies, an infectious disease—in fact, the first human disease proven to be caused by a specific agent.1Sarcoptes scabiei var hominis, or scabies, is a mite in the arachnid class.2 In some states and localities, scabies cases or scabies outbreaks are reportable to the public health department.
The cardinal symptom of scabies is pruritus. The itch, especially with initial scabietic infestation, may be gradual in onset.3 Physical examination findings can vary from subtle and nonspecific to overwhelming and distinctive. Scabies can also mimic other dermopathies, complicating diagnosis. Undiagnosed and untreated, scabies can last a protracted period.
The dermopathy may be characterized by urticarial-type papules, vesicles, eczematoid change, excoriation, and bacterial superinfection, especially in children. Nodules may be present, particularly on the penis and scrotum. These may last for months after the infestation has cleared.3 The most commonly involved areas include fingers and finger webs, wrist folds, elbows, knees, the lower abdomen, armpits, thighs, male genitals, nipples, breasts, buttocks, and shoulder blades.3,4 In young children, scabies may be found anywhere, including palms, soles, face and scalp.
Affliction of multiple family members and finding dermatitis in these distinctive locations is helpful in diagnosis. Finding the mites’ burrows is considered pathognomonic because other burrowing diseases (eg, cutaneous larva migrans) are easily distinguished clinically.4 Extensive excoriation is a clinical clue to look for burrows.3
Transmission usually skin-to-skin
Scabies is generally transmitted by prolonged skin-to-skin contact, such as occurs in families or during sexual contact. It is possible to acquire scabies infestation via contaminated items of clothing or bed linens, but this is not regarded as a significant route of transmission.3 Transmission by casual contact, such as a handshake or hug, is unlikely.
Infestation with the S scabiei mite, referred to as scabies in man, is termed “mange” in other mammals known to host the mite (dogs, cats, rabbits, cattle, pigs, and horses). Mites from one host species generally do not establish themselves on another species, and thus are referred to as varieties, variants, or forms. Humans develop a transient dermopathy from infestation by animal scabies, but such infestations are mild and disappear spontaneously unless the person is in frequent contact with the infested animal.3,4
Differential diagnosis
The differential diagnosis of scabies—a great masquerader—is extensive, and includes atopic dermatitis, contact dermatitis, impetigo, insect bites, vasculitis, neurodermatitis, folliculitis, prurigo nodularis, psoriasis (crusted scabies), and a host of other dermopathies.3,4
Confirming the diagnosis
Finding the causative mite, its ova (eggs), or scybala (feces), confirms the diagnosis, although failure to find these does not rule out scabies. Papules or burrows that have not been excoriated are best for obtaining preparations for microscopic examination.3 Burrows may be found with nakedeye inspection, although use of a hand-held magnifier and good illumination make finding burrows easier.
Dermoscopy
Dermoscopy, performed with an otoscope-like, illuminated magnifier designed for skin assessment, provides reliable confirmation of S- or Z-shaped burrows. During dermoscopy, carefully examining the distal end of the burrows in the skin may reveal the “triangular black dot” of the scabies mite (FIGURE 2, top right)—the head of the mite.5 The body of the mite—light in color and oval—is not visible even with the most careful dermoscopic examination. The “black dot” of the mite may be visible with careful inspection with a hand lens. In the appropriate clinical setting, dermoscopic identification of an unequivocal burrow with the dark “triangle sign” at one end is diagnostic for scabies. When a digital photograph obtained through the dermatoscope is magnified, the distal end of the burrow (FIGURE 3) reveals the triangular head parts of the mite and the body within the burrow. This body is not evident with dermoscopy alone; the additional magnification via photography allows its visualization.
FIGURE 3
Magnification
Scabies mount
In instances where the physician is going to make an institution-wide recommendation with major ramifications, it is wise to positively identify the mite. A scabies mount performed at the location of the triangular dot will readily provide a mite for identification. Scabies mounts are prepared via a very superficial shave technique without anesthesia. The skin flakes are transferred to a slide and a drop of mineral oil is added. Alternatively, a drop of mineral oil can be placed on the skin and a superficial sample obtained.
Note that this technique differs from that of potassium hydroxide (KOH) preparation for fungal identification. The scabies mount technique is more like a superficial shave biopsy (with the knife blade parallel to the skin) than a KOH preparation (blade dragged along the skin surface more perpendicular to the skin).Microscopic examination of each slide reveals a mite (FIGURES 4 AND 5).
In our patient, 3 burrows were identified with a hand lens and confirmed by dermoscopy. In 2 burrows, the triangular dots were transferred to slides by doing a very superficial shave (without anesthesia) of the stratum corneum with a number 15 blade and handle. The material was placed on a slide, a drop of mineral oil added, and the slide examined microscopically (FIGURES 4 AND 5). These dermoscopically guided preparations each yielded a mite and little other debris.
FIGURE 4
Scabies mite
FIGURE 5
Mite, ova, and feces
Ink test
The “ink test” is another adjunct to help identify burrows. A nontoxic, watersoluble felt-tip marker is rubbed over an area suspected of having burrows. After waiting a few moments for the ink to sink into the disrupted stratum corneum overlying burrows, the ink is washed off, leaving an ink-demarcated burrow to examine.4 This can be performed as an adjunct to dermoscopy.5
The course of scabies
The mite’s life cycle
There are 4 stages in the mite’s life cycle: egg, larva, nymph, and adult. Female mites deposit 2 to 3 eggs per day as they create their burrows. The eggs are oval, 0.1 to 0.15 mm in length, and hatch in 3 to 8 days. The resultant larvae migrate to the skin surface and burrow into the intact stratum corneum to construct almost invisible, short burrows called molting pouches.
The larvae progress through 2 nymphal stages before a final molt to the adult stage. Larvae and nymphs live in molting pouches or in hair follicles. They appear similar to adults except for smaller size and, during the larval stage, 3 pair of legs. Adult female mites are 0.3 to 0.4 mm long and about 0.25 to 0.35 mm wide, about twice the size of males. Mating occurs when a male penetrates the molting pouch of the adult female. Impregnated females then extend their molting pouches to form the characteristic serpentine burrows, laying eggs in the process. The total period to progress from egg to the gravid female stage takes 10 to 14 days. The impregnated females spend the remaining 2 months of their lives in burrows.3,4
The mites live in and on the stratum corneum, burrowing into but never below the stratum corneum. The burrows appear as raised, serpentine lines varying from a few millimeters up to several centimeters long. Transmission occurs by the transfer of ova-bearing females.3,4
Cause of the rash and itch
The mites do not “bite.” Instead, the hallmark of scabies, when found, are the burrows created by the mites. However, it is common to see a papular urticarial type response as an allergic reaction to antigens associated with the mite itself, its scybala, and eggs. In fact, after acquiring scabies for the first time, itch does not appear for 2 to 6 weeks (average, 3 to 4 weeks) because the host needs to be sensitized to these antigens.4
It is not until the immunologic reactivity or sensitization develops that the host becomes symptomatic and aware of a problem. This requirement for sensitization explains the often gradual onset of itch. The incubation period is important in transmission to other individuals during the asymptomatic phase.3 However, a previously sensitized host may experience itch within hours to days after reinfestation.4
Epidemiology of scabies
Scabies infestations occur in all geographic areas and climates, and affects people of all ages and socioeconomic strata.7 For unexplained reasons, those with African ancestry rarely acquire scabies.7
It is most common in those who have close physical contact with others and, therefore, disproportionately affects children, mothers of young children, sexually active young adults, nursing home populations, and those in crowded living situations. Scabies is commonplace in developing countries. It is possible to acquire scabies after sleeping in unsanitary bedding. The scabies mite does not carry other diseases.7
Crusted scabies
Crusted scabies, a rare form of scabies also known as Norwegian scabies, is an aggressive infestation that usually occurs in immunodeficient, debilitated, or malnourished persons. Crusted scabies, because of the huge mite burden, is associated with greater transmissibility than scabies.7 Interestingly, because of impaired allergic response or indifference to itch, some of these patients may exhibit little pruritus.7
Treatment of scabies
Perhaps the most difficult job in treatment of scabies is treating asymptomatic contacts. Physicians may be reluctant to prescribe, and contacts themselves may be reluctant to take, appropriate treatment. These individuals often spread the infection for 4 to 6 weeks before they develop sensitization and clinical symptoms. Thus, it is essential that these asymptomatic contacts be treated or a cycle of reinfestation will be created.3 All sexual contacts, close personal contacts, and household contacts from within the preceding month should be examined and treated.8
Permethrin cream. The recommended treatment by the Centers for Disease Control and Prevention (CDC) is permethrin cream (5%) applied to all areas of the body from the neck down and thoroughly washed off after 8 to 14 hours. This recommendation includes careful application under fingernails, between toes, and on palms and soles. Infants may need the face and scalp treated in addition. Treatment of the face beyond infancy frequently results in a contact irritant dermatitis. Permethrin is effective and safe but costs more than lindane.8
Lindane. The CDC guidelines offer 2 alternatives to permethrin. One alternative, lindane 1% cream or lotion, can be applied in a thin layer to all areas of the body from the neck down and thoroughly washed off after 8 hours.
Lindane should not be used immediately after a bath or shower, and should not be used by persons who have extensive dermatitis, pregnant or lactating women, or children aged less than 2 years. Lindane resistance has been reported, including in the United States. Seizures have occurred when lindane was applied after a bath or used by patients who had extensive dermatitis. Aplastic anemia following lindane use also has been reported. Infants, young children, and pregnant or lactating women should not be treated with lindane; they can be treated with permethrin.8
Applying topical treatments. Topical scabicides should be applied to all skin from neck down, including intertriginous areas and the gluteal fold. The medication needs to be reapplied to hands if the hands are washed after application. It is advisable to cut fingernails short before applying scabicides and to ensure that scabicide is applied under fingernails. A toothpick can be used if necessary to assist in application under nails. In infants and small children, medication should be applied to face and scalp, avoiding the periorbital area.6
Other treatments. Ivermectin, the third treatment recommended by the CDC, can be administered as a single dose of 200 mcg/kg orally, and repeated in 2 weeks. Ivermectin is not recommended for pregnant or lactating patients. The safety of ivermectin in children who weigh less than 15 kg has not been determined.8
Some specialists recommend retreatment after 1 to 2 weeks for patients who are still symptomatic. Patients who do not respond to the recommended treatment should be retreated with an alternative regimen.8
Patients who have uncomplicated scabies and also are infected with HIV should receive the same treatment regimens as those who are HIV-negative.8 For patients with crusted scabies, the optimal regimen is unknown because no controlled therapeutic trials have been conducted. Expert opinion suggests augmented and combined regimens should be used for this aggressive infestation. Lindane should be avoided because of risks of neurotoxicity with heavy applications.8 Control of scabies epidemics (eg, in nursing homes, hospitals, residential facilities) require treatment of the entire population at risk.
Ancillary measures
Scabies mites may survive for a few days after leaving human skin. Thus, frequent bed linen changes minimize transmission via bedding. Hot-water laundry in temperatures of 120°F (49°s mites in 10 minutes and is sufficient to disinfect all bedding, clothing, and washable items.3
Other methods of disinfection include placing items in a dryer on the hot cycle for 10 to 30 minutes, pressing them with a warm iron, dry-cleaning, or placing in a sealed plastic bag for 7 to 14 days. Carpets or upholstery should be vacuumed through the heavy traffic areas. Fumigation of living areas and furniture with insecticide is unnecessary.6-8 Pets do not need to be treated.6 Children may return to school and childcare immediately following initial treatment.6
Follow-up of scabies patient
The boy’s mother is allowed to view the mites through the microscope, fostering her accepting the diagnosis and enhancing the chance for compliance with treatment, which involves treating the entire family.
Patients should be informed that the rash and pruritus of scabies may persist for 4 weeks after treatment because scabietic antigenic material remains until natural epidermal sloughing and turnover occurs.3 When symptoms or signs persist, evaluation should ensue for faulty application of topical scabicides and for treatment failure.7
Acknowledgments
The author (GNF) wishes to acknowledge the assistance of Peggy Elston and Heather Martinez, without whose assistance photographs like these would never happen; and Lisa Nichols, without whose acquisitive skills it never would have occurred to me to mite-hunt with a dermatoscope.
CORRESPONDENCE
Gary N. Fox, MD, Defiance Clinic, 1400 East Second Street, Defiance, OH 43512. E-mail: foxgary@yahoo.com
1. Binder WD. Scabies. eMedicine [online database]. Available at: www.emedicine.com/emerg/topic517.htm. Accessed on July 6, 2006.
2. Arachnid. Wikipedia [online encyclopedia]. Available at: en.wikipedia.org/wiki/Arachnid. Accessed on July 6, 2006.
3. Scabies. DPDx—CDC Parasitology Diagnostic Website. Available at: www.dpd.cdc.gov/dpdx/HTML/Scabies.htm. Accessed on July 6, 2006.
4. Arya V, Molinaro MJ, Majewski SS, Schwartz RA. Pediatric scabies. Cutis 2003;71:193-196.
5. Vazquez-Lopez F, Kreusch JF, Marghoob AA. Other uses of dermoscopy. In: Marghoob AA, Braun RP, Kopf AW, eds. Atlas of Dermoscopy. New York: Taylor & Francis; 2005:301,305-306.
6. FAQs—scabies. Texas Department of Health Services, Infectious Disease Control Unit website. Available at: www.dshs.state.tx.us/idcu/disease/scabies/faqs/. Accessed on July 6, 2006.
7. Infestations and bites [chapter 15]. In: Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. New York: Mosby; 2004:497-503.
8. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines 2002. MMWR Recomm Rep 2002;51(RR-6):68-69.
A boy came to the office with a rash and progressively severe itching for approximately 2 months (FIGURE 1). Examination showed an excoriated generalized papular eruption, including some urticarial-type papules and chronic eczematoid changes near the waist, axillae, hands, and wrists.
His grandmother, with whom he spends most weekends and a lot of time after school, also has had a rash and progressive itch for approximately 3 weeks. One feature of the dermopathy observed clinically, first located by hand lens examination and then confirmed by dermoscopy, is depicted in FIGURE 2.
FIGURE 1
Excoriated eruptions
FIGURE 2
Dermoscopic photograph of the dermatosis
What is your diagnosis?
Diagnosis: Scabies
The boy and his grandmother both have scabies, an infectious disease—in fact, the first human disease proven to be caused by a specific agent.1Sarcoptes scabiei var hominis, or scabies, is a mite in the arachnid class.2 In some states and localities, scabies cases or scabies outbreaks are reportable to the public health department.
The cardinal symptom of scabies is pruritus. The itch, especially with initial scabietic infestation, may be gradual in onset.3 Physical examination findings can vary from subtle and nonspecific to overwhelming and distinctive. Scabies can also mimic other dermopathies, complicating diagnosis. Undiagnosed and untreated, scabies can last a protracted period.
The dermopathy may be characterized by urticarial-type papules, vesicles, eczematoid change, excoriation, and bacterial superinfection, especially in children. Nodules may be present, particularly on the penis and scrotum. These may last for months after the infestation has cleared.3 The most commonly involved areas include fingers and finger webs, wrist folds, elbows, knees, the lower abdomen, armpits, thighs, male genitals, nipples, breasts, buttocks, and shoulder blades.3,4 In young children, scabies may be found anywhere, including palms, soles, face and scalp.
Affliction of multiple family members and finding dermatitis in these distinctive locations is helpful in diagnosis. Finding the mites’ burrows is considered pathognomonic because other burrowing diseases (eg, cutaneous larva migrans) are easily distinguished clinically.4 Extensive excoriation is a clinical clue to look for burrows.3
Transmission usually skin-to-skin
Scabies is generally transmitted by prolonged skin-to-skin contact, such as occurs in families or during sexual contact. It is possible to acquire scabies infestation via contaminated items of clothing or bed linens, but this is not regarded as a significant route of transmission.3 Transmission by casual contact, such as a handshake or hug, is unlikely.
Infestation with the S scabiei mite, referred to as scabies in man, is termed “mange” in other mammals known to host the mite (dogs, cats, rabbits, cattle, pigs, and horses). Mites from one host species generally do not establish themselves on another species, and thus are referred to as varieties, variants, or forms. Humans develop a transient dermopathy from infestation by animal scabies, but such infestations are mild and disappear spontaneously unless the person is in frequent contact with the infested animal.3,4
Differential diagnosis
The differential diagnosis of scabies—a great masquerader—is extensive, and includes atopic dermatitis, contact dermatitis, impetigo, insect bites, vasculitis, neurodermatitis, folliculitis, prurigo nodularis, psoriasis (crusted scabies), and a host of other dermopathies.3,4
Confirming the diagnosis
Finding the causative mite, its ova (eggs), or scybala (feces), confirms the diagnosis, although failure to find these does not rule out scabies. Papules or burrows that have not been excoriated are best for obtaining preparations for microscopic examination.3 Burrows may be found with nakedeye inspection, although use of a hand-held magnifier and good illumination make finding burrows easier.
Dermoscopy
Dermoscopy, performed with an otoscope-like, illuminated magnifier designed for skin assessment, provides reliable confirmation of S- or Z-shaped burrows. During dermoscopy, carefully examining the distal end of the burrows in the skin may reveal the “triangular black dot” of the scabies mite (FIGURE 2, top right)—the head of the mite.5 The body of the mite—light in color and oval—is not visible even with the most careful dermoscopic examination. The “black dot” of the mite may be visible with careful inspection with a hand lens. In the appropriate clinical setting, dermoscopic identification of an unequivocal burrow with the dark “triangle sign” at one end is diagnostic for scabies. When a digital photograph obtained through the dermatoscope is magnified, the distal end of the burrow (FIGURE 3) reveals the triangular head parts of the mite and the body within the burrow. This body is not evident with dermoscopy alone; the additional magnification via photography allows its visualization.
FIGURE 3
Magnification
Scabies mount
In instances where the physician is going to make an institution-wide recommendation with major ramifications, it is wise to positively identify the mite. A scabies mount performed at the location of the triangular dot will readily provide a mite for identification. Scabies mounts are prepared via a very superficial shave technique without anesthesia. The skin flakes are transferred to a slide and a drop of mineral oil is added. Alternatively, a drop of mineral oil can be placed on the skin and a superficial sample obtained.
Note that this technique differs from that of potassium hydroxide (KOH) preparation for fungal identification. The scabies mount technique is more like a superficial shave biopsy (with the knife blade parallel to the skin) than a KOH preparation (blade dragged along the skin surface more perpendicular to the skin).Microscopic examination of each slide reveals a mite (FIGURES 4 AND 5).
In our patient, 3 burrows were identified with a hand lens and confirmed by dermoscopy. In 2 burrows, the triangular dots were transferred to slides by doing a very superficial shave (without anesthesia) of the stratum corneum with a number 15 blade and handle. The material was placed on a slide, a drop of mineral oil added, and the slide examined microscopically (FIGURES 4 AND 5). These dermoscopically guided preparations each yielded a mite and little other debris.
FIGURE 4
Scabies mite
FIGURE 5
Mite, ova, and feces
Ink test
The “ink test” is another adjunct to help identify burrows. A nontoxic, watersoluble felt-tip marker is rubbed over an area suspected of having burrows. After waiting a few moments for the ink to sink into the disrupted stratum corneum overlying burrows, the ink is washed off, leaving an ink-demarcated burrow to examine.4 This can be performed as an adjunct to dermoscopy.5
The course of scabies
The mite’s life cycle
There are 4 stages in the mite’s life cycle: egg, larva, nymph, and adult. Female mites deposit 2 to 3 eggs per day as they create their burrows. The eggs are oval, 0.1 to 0.15 mm in length, and hatch in 3 to 8 days. The resultant larvae migrate to the skin surface and burrow into the intact stratum corneum to construct almost invisible, short burrows called molting pouches.
The larvae progress through 2 nymphal stages before a final molt to the adult stage. Larvae and nymphs live in molting pouches or in hair follicles. They appear similar to adults except for smaller size and, during the larval stage, 3 pair of legs. Adult female mites are 0.3 to 0.4 mm long and about 0.25 to 0.35 mm wide, about twice the size of males. Mating occurs when a male penetrates the molting pouch of the adult female. Impregnated females then extend their molting pouches to form the characteristic serpentine burrows, laying eggs in the process. The total period to progress from egg to the gravid female stage takes 10 to 14 days. The impregnated females spend the remaining 2 months of their lives in burrows.3,4
The mites live in and on the stratum corneum, burrowing into but never below the stratum corneum. The burrows appear as raised, serpentine lines varying from a few millimeters up to several centimeters long. Transmission occurs by the transfer of ova-bearing females.3,4
Cause of the rash and itch
The mites do not “bite.” Instead, the hallmark of scabies, when found, are the burrows created by the mites. However, it is common to see a papular urticarial type response as an allergic reaction to antigens associated with the mite itself, its scybala, and eggs. In fact, after acquiring scabies for the first time, itch does not appear for 2 to 6 weeks (average, 3 to 4 weeks) because the host needs to be sensitized to these antigens.4
It is not until the immunologic reactivity or sensitization develops that the host becomes symptomatic and aware of a problem. This requirement for sensitization explains the often gradual onset of itch. The incubation period is important in transmission to other individuals during the asymptomatic phase.3 However, a previously sensitized host may experience itch within hours to days after reinfestation.4
Epidemiology of scabies
Scabies infestations occur in all geographic areas and climates, and affects people of all ages and socioeconomic strata.7 For unexplained reasons, those with African ancestry rarely acquire scabies.7
It is most common in those who have close physical contact with others and, therefore, disproportionately affects children, mothers of young children, sexually active young adults, nursing home populations, and those in crowded living situations. Scabies is commonplace in developing countries. It is possible to acquire scabies after sleeping in unsanitary bedding. The scabies mite does not carry other diseases.7
Crusted scabies
Crusted scabies, a rare form of scabies also known as Norwegian scabies, is an aggressive infestation that usually occurs in immunodeficient, debilitated, or malnourished persons. Crusted scabies, because of the huge mite burden, is associated with greater transmissibility than scabies.7 Interestingly, because of impaired allergic response or indifference to itch, some of these patients may exhibit little pruritus.7
Treatment of scabies
Perhaps the most difficult job in treatment of scabies is treating asymptomatic contacts. Physicians may be reluctant to prescribe, and contacts themselves may be reluctant to take, appropriate treatment. These individuals often spread the infection for 4 to 6 weeks before they develop sensitization and clinical symptoms. Thus, it is essential that these asymptomatic contacts be treated or a cycle of reinfestation will be created.3 All sexual contacts, close personal contacts, and household contacts from within the preceding month should be examined and treated.8
Permethrin cream. The recommended treatment by the Centers for Disease Control and Prevention (CDC) is permethrin cream (5%) applied to all areas of the body from the neck down and thoroughly washed off after 8 to 14 hours. This recommendation includes careful application under fingernails, between toes, and on palms and soles. Infants may need the face and scalp treated in addition. Treatment of the face beyond infancy frequently results in a contact irritant dermatitis. Permethrin is effective and safe but costs more than lindane.8
Lindane. The CDC guidelines offer 2 alternatives to permethrin. One alternative, lindane 1% cream or lotion, can be applied in a thin layer to all areas of the body from the neck down and thoroughly washed off after 8 hours.
Lindane should not be used immediately after a bath or shower, and should not be used by persons who have extensive dermatitis, pregnant or lactating women, or children aged less than 2 years. Lindane resistance has been reported, including in the United States. Seizures have occurred when lindane was applied after a bath or used by patients who had extensive dermatitis. Aplastic anemia following lindane use also has been reported. Infants, young children, and pregnant or lactating women should not be treated with lindane; they can be treated with permethrin.8
Applying topical treatments. Topical scabicides should be applied to all skin from neck down, including intertriginous areas and the gluteal fold. The medication needs to be reapplied to hands if the hands are washed after application. It is advisable to cut fingernails short before applying scabicides and to ensure that scabicide is applied under fingernails. A toothpick can be used if necessary to assist in application under nails. In infants and small children, medication should be applied to face and scalp, avoiding the periorbital area.6
Other treatments. Ivermectin, the third treatment recommended by the CDC, can be administered as a single dose of 200 mcg/kg orally, and repeated in 2 weeks. Ivermectin is not recommended for pregnant or lactating patients. The safety of ivermectin in children who weigh less than 15 kg has not been determined.8
Some specialists recommend retreatment after 1 to 2 weeks for patients who are still symptomatic. Patients who do not respond to the recommended treatment should be retreated with an alternative regimen.8
Patients who have uncomplicated scabies and also are infected with HIV should receive the same treatment regimens as those who are HIV-negative.8 For patients with crusted scabies, the optimal regimen is unknown because no controlled therapeutic trials have been conducted. Expert opinion suggests augmented and combined regimens should be used for this aggressive infestation. Lindane should be avoided because of risks of neurotoxicity with heavy applications.8 Control of scabies epidemics (eg, in nursing homes, hospitals, residential facilities) require treatment of the entire population at risk.
Ancillary measures
Scabies mites may survive for a few days after leaving human skin. Thus, frequent bed linen changes minimize transmission via bedding. Hot-water laundry in temperatures of 120°F (49°s mites in 10 minutes and is sufficient to disinfect all bedding, clothing, and washable items.3
Other methods of disinfection include placing items in a dryer on the hot cycle for 10 to 30 minutes, pressing them with a warm iron, dry-cleaning, or placing in a sealed plastic bag for 7 to 14 days. Carpets or upholstery should be vacuumed through the heavy traffic areas. Fumigation of living areas and furniture with insecticide is unnecessary.6-8 Pets do not need to be treated.6 Children may return to school and childcare immediately following initial treatment.6
Follow-up of scabies patient
The boy’s mother is allowed to view the mites through the microscope, fostering her accepting the diagnosis and enhancing the chance for compliance with treatment, which involves treating the entire family.
Patients should be informed that the rash and pruritus of scabies may persist for 4 weeks after treatment because scabietic antigenic material remains until natural epidermal sloughing and turnover occurs.3 When symptoms or signs persist, evaluation should ensue for faulty application of topical scabicides and for treatment failure.7
Acknowledgments
The author (GNF) wishes to acknowledge the assistance of Peggy Elston and Heather Martinez, without whose assistance photographs like these would never happen; and Lisa Nichols, without whose acquisitive skills it never would have occurred to me to mite-hunt with a dermatoscope.
CORRESPONDENCE
Gary N. Fox, MD, Defiance Clinic, 1400 East Second Street, Defiance, OH 43512. E-mail: foxgary@yahoo.com
A boy came to the office with a rash and progressively severe itching for approximately 2 months (FIGURE 1). Examination showed an excoriated generalized papular eruption, including some urticarial-type papules and chronic eczematoid changes near the waist, axillae, hands, and wrists.
His grandmother, with whom he spends most weekends and a lot of time after school, also has had a rash and progressive itch for approximately 3 weeks. One feature of the dermopathy observed clinically, first located by hand lens examination and then confirmed by dermoscopy, is depicted in FIGURE 2.
FIGURE 1
Excoriated eruptions
FIGURE 2
Dermoscopic photograph of the dermatosis
What is your diagnosis?
Diagnosis: Scabies
The boy and his grandmother both have scabies, an infectious disease—in fact, the first human disease proven to be caused by a specific agent.1Sarcoptes scabiei var hominis, or scabies, is a mite in the arachnid class.2 In some states and localities, scabies cases or scabies outbreaks are reportable to the public health department.
The cardinal symptom of scabies is pruritus. The itch, especially with initial scabietic infestation, may be gradual in onset.3 Physical examination findings can vary from subtle and nonspecific to overwhelming and distinctive. Scabies can also mimic other dermopathies, complicating diagnosis. Undiagnosed and untreated, scabies can last a protracted period.
The dermopathy may be characterized by urticarial-type papules, vesicles, eczematoid change, excoriation, and bacterial superinfection, especially in children. Nodules may be present, particularly on the penis and scrotum. These may last for months after the infestation has cleared.3 The most commonly involved areas include fingers and finger webs, wrist folds, elbows, knees, the lower abdomen, armpits, thighs, male genitals, nipples, breasts, buttocks, and shoulder blades.3,4 In young children, scabies may be found anywhere, including palms, soles, face and scalp.
Affliction of multiple family members and finding dermatitis in these distinctive locations is helpful in diagnosis. Finding the mites’ burrows is considered pathognomonic because other burrowing diseases (eg, cutaneous larva migrans) are easily distinguished clinically.4 Extensive excoriation is a clinical clue to look for burrows.3
Transmission usually skin-to-skin
Scabies is generally transmitted by prolonged skin-to-skin contact, such as occurs in families or during sexual contact. It is possible to acquire scabies infestation via contaminated items of clothing or bed linens, but this is not regarded as a significant route of transmission.3 Transmission by casual contact, such as a handshake or hug, is unlikely.
Infestation with the S scabiei mite, referred to as scabies in man, is termed “mange” in other mammals known to host the mite (dogs, cats, rabbits, cattle, pigs, and horses). Mites from one host species generally do not establish themselves on another species, and thus are referred to as varieties, variants, or forms. Humans develop a transient dermopathy from infestation by animal scabies, but such infestations are mild and disappear spontaneously unless the person is in frequent contact with the infested animal.3,4
Differential diagnosis
The differential diagnosis of scabies—a great masquerader—is extensive, and includes atopic dermatitis, contact dermatitis, impetigo, insect bites, vasculitis, neurodermatitis, folliculitis, prurigo nodularis, psoriasis (crusted scabies), and a host of other dermopathies.3,4
Confirming the diagnosis
Finding the causative mite, its ova (eggs), or scybala (feces), confirms the diagnosis, although failure to find these does not rule out scabies. Papules or burrows that have not been excoriated are best for obtaining preparations for microscopic examination.3 Burrows may be found with nakedeye inspection, although use of a hand-held magnifier and good illumination make finding burrows easier.
Dermoscopy
Dermoscopy, performed with an otoscope-like, illuminated magnifier designed for skin assessment, provides reliable confirmation of S- or Z-shaped burrows. During dermoscopy, carefully examining the distal end of the burrows in the skin may reveal the “triangular black dot” of the scabies mite (FIGURE 2, top right)—the head of the mite.5 The body of the mite—light in color and oval—is not visible even with the most careful dermoscopic examination. The “black dot” of the mite may be visible with careful inspection with a hand lens. In the appropriate clinical setting, dermoscopic identification of an unequivocal burrow with the dark “triangle sign” at one end is diagnostic for scabies. When a digital photograph obtained through the dermatoscope is magnified, the distal end of the burrow (FIGURE 3) reveals the triangular head parts of the mite and the body within the burrow. This body is not evident with dermoscopy alone; the additional magnification via photography allows its visualization.
FIGURE 3
Magnification
Scabies mount
In instances where the physician is going to make an institution-wide recommendation with major ramifications, it is wise to positively identify the mite. A scabies mount performed at the location of the triangular dot will readily provide a mite for identification. Scabies mounts are prepared via a very superficial shave technique without anesthesia. The skin flakes are transferred to a slide and a drop of mineral oil is added. Alternatively, a drop of mineral oil can be placed on the skin and a superficial sample obtained.
Note that this technique differs from that of potassium hydroxide (KOH) preparation for fungal identification. The scabies mount technique is more like a superficial shave biopsy (with the knife blade parallel to the skin) than a KOH preparation (blade dragged along the skin surface more perpendicular to the skin).Microscopic examination of each slide reveals a mite (FIGURES 4 AND 5).
In our patient, 3 burrows were identified with a hand lens and confirmed by dermoscopy. In 2 burrows, the triangular dots were transferred to slides by doing a very superficial shave (without anesthesia) of the stratum corneum with a number 15 blade and handle. The material was placed on a slide, a drop of mineral oil added, and the slide examined microscopically (FIGURES 4 AND 5). These dermoscopically guided preparations each yielded a mite and little other debris.
FIGURE 4
Scabies mite
FIGURE 5
Mite, ova, and feces
Ink test
The “ink test” is another adjunct to help identify burrows. A nontoxic, watersoluble felt-tip marker is rubbed over an area suspected of having burrows. After waiting a few moments for the ink to sink into the disrupted stratum corneum overlying burrows, the ink is washed off, leaving an ink-demarcated burrow to examine.4 This can be performed as an adjunct to dermoscopy.5
The course of scabies
The mite’s life cycle
There are 4 stages in the mite’s life cycle: egg, larva, nymph, and adult. Female mites deposit 2 to 3 eggs per day as they create their burrows. The eggs are oval, 0.1 to 0.15 mm in length, and hatch in 3 to 8 days. The resultant larvae migrate to the skin surface and burrow into the intact stratum corneum to construct almost invisible, short burrows called molting pouches.
The larvae progress through 2 nymphal stages before a final molt to the adult stage. Larvae and nymphs live in molting pouches or in hair follicles. They appear similar to adults except for smaller size and, during the larval stage, 3 pair of legs. Adult female mites are 0.3 to 0.4 mm long and about 0.25 to 0.35 mm wide, about twice the size of males. Mating occurs when a male penetrates the molting pouch of the adult female. Impregnated females then extend their molting pouches to form the characteristic serpentine burrows, laying eggs in the process. The total period to progress from egg to the gravid female stage takes 10 to 14 days. The impregnated females spend the remaining 2 months of their lives in burrows.3,4
The mites live in and on the stratum corneum, burrowing into but never below the stratum corneum. The burrows appear as raised, serpentine lines varying from a few millimeters up to several centimeters long. Transmission occurs by the transfer of ova-bearing females.3,4
Cause of the rash and itch
The mites do not “bite.” Instead, the hallmark of scabies, when found, are the burrows created by the mites. However, it is common to see a papular urticarial type response as an allergic reaction to antigens associated with the mite itself, its scybala, and eggs. In fact, after acquiring scabies for the first time, itch does not appear for 2 to 6 weeks (average, 3 to 4 weeks) because the host needs to be sensitized to these antigens.4
It is not until the immunologic reactivity or sensitization develops that the host becomes symptomatic and aware of a problem. This requirement for sensitization explains the often gradual onset of itch. The incubation period is important in transmission to other individuals during the asymptomatic phase.3 However, a previously sensitized host may experience itch within hours to days after reinfestation.4
Epidemiology of scabies
Scabies infestations occur in all geographic areas and climates, and affects people of all ages and socioeconomic strata.7 For unexplained reasons, those with African ancestry rarely acquire scabies.7
It is most common in those who have close physical contact with others and, therefore, disproportionately affects children, mothers of young children, sexually active young adults, nursing home populations, and those in crowded living situations. Scabies is commonplace in developing countries. It is possible to acquire scabies after sleeping in unsanitary bedding. The scabies mite does not carry other diseases.7
Crusted scabies
Crusted scabies, a rare form of scabies also known as Norwegian scabies, is an aggressive infestation that usually occurs in immunodeficient, debilitated, or malnourished persons. Crusted scabies, because of the huge mite burden, is associated with greater transmissibility than scabies.7 Interestingly, because of impaired allergic response or indifference to itch, some of these patients may exhibit little pruritus.7
Treatment of scabies
Perhaps the most difficult job in treatment of scabies is treating asymptomatic contacts. Physicians may be reluctant to prescribe, and contacts themselves may be reluctant to take, appropriate treatment. These individuals often spread the infection for 4 to 6 weeks before they develop sensitization and clinical symptoms. Thus, it is essential that these asymptomatic contacts be treated or a cycle of reinfestation will be created.3 All sexual contacts, close personal contacts, and household contacts from within the preceding month should be examined and treated.8
Permethrin cream. The recommended treatment by the Centers for Disease Control and Prevention (CDC) is permethrin cream (5%) applied to all areas of the body from the neck down and thoroughly washed off after 8 to 14 hours. This recommendation includes careful application under fingernails, between toes, and on palms and soles. Infants may need the face and scalp treated in addition. Treatment of the face beyond infancy frequently results in a contact irritant dermatitis. Permethrin is effective and safe but costs more than lindane.8
Lindane. The CDC guidelines offer 2 alternatives to permethrin. One alternative, lindane 1% cream or lotion, can be applied in a thin layer to all areas of the body from the neck down and thoroughly washed off after 8 hours.
Lindane should not be used immediately after a bath or shower, and should not be used by persons who have extensive dermatitis, pregnant or lactating women, or children aged less than 2 years. Lindane resistance has been reported, including in the United States. Seizures have occurred when lindane was applied after a bath or used by patients who had extensive dermatitis. Aplastic anemia following lindane use also has been reported. Infants, young children, and pregnant or lactating women should not be treated with lindane; they can be treated with permethrin.8
Applying topical treatments. Topical scabicides should be applied to all skin from neck down, including intertriginous areas and the gluteal fold. The medication needs to be reapplied to hands if the hands are washed after application. It is advisable to cut fingernails short before applying scabicides and to ensure that scabicide is applied under fingernails. A toothpick can be used if necessary to assist in application under nails. In infants and small children, medication should be applied to face and scalp, avoiding the periorbital area.6
Other treatments. Ivermectin, the third treatment recommended by the CDC, can be administered as a single dose of 200 mcg/kg orally, and repeated in 2 weeks. Ivermectin is not recommended for pregnant or lactating patients. The safety of ivermectin in children who weigh less than 15 kg has not been determined.8
Some specialists recommend retreatment after 1 to 2 weeks for patients who are still symptomatic. Patients who do not respond to the recommended treatment should be retreated with an alternative regimen.8
Patients who have uncomplicated scabies and also are infected with HIV should receive the same treatment regimens as those who are HIV-negative.8 For patients with crusted scabies, the optimal regimen is unknown because no controlled therapeutic trials have been conducted. Expert opinion suggests augmented and combined regimens should be used for this aggressive infestation. Lindane should be avoided because of risks of neurotoxicity with heavy applications.8 Control of scabies epidemics (eg, in nursing homes, hospitals, residential facilities) require treatment of the entire population at risk.
Ancillary measures
Scabies mites may survive for a few days after leaving human skin. Thus, frequent bed linen changes minimize transmission via bedding. Hot-water laundry in temperatures of 120°F (49°s mites in 10 minutes and is sufficient to disinfect all bedding, clothing, and washable items.3
Other methods of disinfection include placing items in a dryer on the hot cycle for 10 to 30 minutes, pressing them with a warm iron, dry-cleaning, or placing in a sealed plastic bag for 7 to 14 days. Carpets or upholstery should be vacuumed through the heavy traffic areas. Fumigation of living areas and furniture with insecticide is unnecessary.6-8 Pets do not need to be treated.6 Children may return to school and childcare immediately following initial treatment.6
Follow-up of scabies patient
The boy’s mother is allowed to view the mites through the microscope, fostering her accepting the diagnosis and enhancing the chance for compliance with treatment, which involves treating the entire family.
Patients should be informed that the rash and pruritus of scabies may persist for 4 weeks after treatment because scabietic antigenic material remains until natural epidermal sloughing and turnover occurs.3 When symptoms or signs persist, evaluation should ensue for faulty application of topical scabicides and for treatment failure.7
Acknowledgments
The author (GNF) wishes to acknowledge the assistance of Peggy Elston and Heather Martinez, without whose assistance photographs like these would never happen; and Lisa Nichols, without whose acquisitive skills it never would have occurred to me to mite-hunt with a dermatoscope.
CORRESPONDENCE
Gary N. Fox, MD, Defiance Clinic, 1400 East Second Street, Defiance, OH 43512. E-mail: foxgary@yahoo.com
1. Binder WD. Scabies. eMedicine [online database]. Available at: www.emedicine.com/emerg/topic517.htm. Accessed on July 6, 2006.
2. Arachnid. Wikipedia [online encyclopedia]. Available at: en.wikipedia.org/wiki/Arachnid. Accessed on July 6, 2006.
3. Scabies. DPDx—CDC Parasitology Diagnostic Website. Available at: www.dpd.cdc.gov/dpdx/HTML/Scabies.htm. Accessed on July 6, 2006.
4. Arya V, Molinaro MJ, Majewski SS, Schwartz RA. Pediatric scabies. Cutis 2003;71:193-196.
5. Vazquez-Lopez F, Kreusch JF, Marghoob AA. Other uses of dermoscopy. In: Marghoob AA, Braun RP, Kopf AW, eds. Atlas of Dermoscopy. New York: Taylor & Francis; 2005:301,305-306.
6. FAQs—scabies. Texas Department of Health Services, Infectious Disease Control Unit website. Available at: www.dshs.state.tx.us/idcu/disease/scabies/faqs/. Accessed on July 6, 2006.
7. Infestations and bites [chapter 15]. In: Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. New York: Mosby; 2004:497-503.
8. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines 2002. MMWR Recomm Rep 2002;51(RR-6):68-69.
1. Binder WD. Scabies. eMedicine [online database]. Available at: www.emedicine.com/emerg/topic517.htm. Accessed on July 6, 2006.
2. Arachnid. Wikipedia [online encyclopedia]. Available at: en.wikipedia.org/wiki/Arachnid. Accessed on July 6, 2006.
3. Scabies. DPDx—CDC Parasitology Diagnostic Website. Available at: www.dpd.cdc.gov/dpdx/HTML/Scabies.htm. Accessed on July 6, 2006.
4. Arya V, Molinaro MJ, Majewski SS, Schwartz RA. Pediatric scabies. Cutis 2003;71:193-196.
5. Vazquez-Lopez F, Kreusch JF, Marghoob AA. Other uses of dermoscopy. In: Marghoob AA, Braun RP, Kopf AW, eds. Atlas of Dermoscopy. New York: Taylor & Francis; 2005:301,305-306.
6. FAQs—scabies. Texas Department of Health Services, Infectious Disease Control Unit website. Available at: www.dshs.state.tx.us/idcu/disease/scabies/faqs/. Accessed on July 6, 2006.
7. Infestations and bites [chapter 15]. In: Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. New York: Mosby; 2004:497-503.
8. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines 2002. MMWR Recomm Rep 2002;51(RR-6):68-69.