User login
Subclinical hypothyroidism and pregnancy: Public health problem or lab finding with minimal clinical significance?
In a US study of more than 17,000 people, overt hypothyroidism and hyperthyroidism were detected in about 4.6% and 1.3% of adults, respectively.1 In this population-based study, thyroid disease was 5 times more prevalent among women than among men. In our ObGyn practices, there are many women of reproductive age with thyroid disease who are considering pregnancy. Treatment of active hyperthyroidism in a woman planning pregnancy is complex and best managed by endocrinologists. Treatment of hypothyroidism is more straightforward, however, and typically managed by internists, family medicine clinicians, and obstetrician-gynecologists.
Clinical management of hypothyroidism and pregnancy
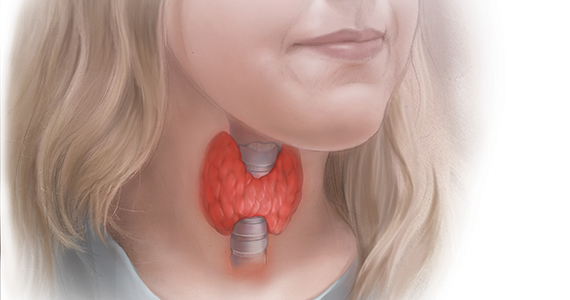
Pregnancy results in a doubling of thyroxine-binding globulin (TBG) levels and a 40% increase in plasma volume, resulting in a need for more thyroxine production.2 Of note, from conception to approximately 13 weeks’ gestation, the sole source of embryonic and fetal thyroid hormones is from the mother.2 Women who have been taking chronic thyroxine treatment may have suppressed thyroid gland activity and be unable to increase thyroxine production in response to pregnancy, necessitating a 30% to 50% increase in their thyroxine dose to maintain TSH levels in the normal range.
For hypothyroid women on long-term thyroxine treatment, recommend increasing the thyroxine dose when pregnancy is recognized. For your patients on chronic thyroxine treatment who are planning a pregnancy, a multiprong approach is helpful in preparing the patient for the increased thyroxine requirements of early pregnancy. First, it is important to counsel the woman that she should not stop the thyroxine medication because it may adversely affect the pregnancy. In my experience, most cases of overt hypothyroidism during pregnancy occur because the patient stopped taking her thyroxine therapy. Second, for hypothyroid women who are considering conception it is reasonable to adjust the thyroxine dose to keep the TSH concentration in the lower range of normal (0.5 to 2.5 mU/L). This will give the woman a “buffer,” reducing the risk that in early pregnancy she and her fetus will have a thyroxine deficit. Third, in early pregnancy, following detection of a positive pregnancy test, your patient can start to increase her thyroxine dose by about two tablets weekly (a 28% increase in the dose). Fourth, TSH levels can be measured every 4 weeks during the first trimester, with appropriate adjustment of the thyroxine dose to keep the TSH concentration below the trimester-specific upper limit of normal (< 4 mU/L).2
TSH and free thyroxine measurements identify women with overt hypothyroidism who need thyroxine treatment. Overt hypothyroidism is associated with adverse reproductive outcomes, including decreased fertility, increased spontaneous abortion, increased fetal loss, and preterm birth.2,3 Hence it is important to immediately initiate thyroxine treatment in pregnant women who have overt hypothyroidism. A diagnosis of overt hypothyroidism is indicated in women with an intact hypothalamic-pituitary axis and a TSH level ≥10 mU/L plus a low free thyroxine concentration. A TSH level of >4 to 10 mU/L, with normal free thyroxine concentration, is evidence of subclinical hypothyroidism (SCH). Among women, there are about 5 times more cases of SCH than overt hypothyroidism.
Continue to: The literature concerning SCH and pregnancy...
The literature concerning SCH and pregnancy is vast, and often contradictory, leading to confusion among clinicians. Contributing to the confusion is that some observational studies report a modest association between SCH and adverse pregnancy outcomes. To date, however, randomized clinical trials show no benefit of thyroxine treatment in these cases. I explore these contradictory pieces of evidence below.

Is SCH associated with adverse pregnancy outcomes due to low thyroxine levels?
There is conflicting literature about the association of SCH and adverse reproductive outcomes. A meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with SCH and euthyroid women (normal TSH and normal free thyroxine levels) was 6.1% and 5.0%, respectively (odds ratio [OR], 1.29; 95% CI, 1.01–1.64).4 Interestingly, pregnant women with normal TSH levels but a low free thyroxine level also had an increased rate of preterm birth (7.1% vs 5.0%; OR, 1.46; 95% CI, 1.12–1.90).
Although observational studies report an association between SCH and adverse reproductive outcomes, multiple randomized clinical trials conducted in women with SCH or hypothyroxinemia have failed to demonstrate that thyroxine replacement improves reproductive outcomes. For example, in a study of 794 pregnant women with elevated TSH and/or low free thyroxine levels randomly assigned to thyroxine treatment (0.15 mg daily) or no treatment, there was no difference in preterm birth rate (5.6% vs 7.9%, P = .2), mean birth weight (3.5 kg vs 3.3 kg, P = .15), gestational age at delivery (40.1 vs 40.2 weeks, P = .10), or the intelligence quotient of children at 3 years (99 vs 100, P = .40).5
In another study, 674 pregnant women with mild SCH (mean TSH, 4.4 mU/L) were randomly assigned to receive thyroxine (0.1 mg daily and dose adjusted to achieve a normal TSH level) or placebo. In this study there was no difference between the thyroxine treatment or placebo groups in preterm birth rate (9% vs 11%, P = .44), gestational age at delivery (39.1 vs 38.9 weeks, P = .57) or intelligence quotient of children at 5 years (97 and 94, P = .71).6
The same investigators also randomized 524 pregnant women with isolated hypothyroxinema (mean free thyroxine level, 0.83 ng/dL) and normal TSH level (mean, 1.5 mU/L) to thyroxine (0.05 mg daily and dose adjusted to achieve a normal free thyroxine level) or placebo.6 In this study there was no difference in preterm birth rate (12% vs 8%, P = .11), gestational age at delivery (39.0 vs 38.8 weeks, P = .46) or intelligence quotient of children at 5 years (94 and 91, P = .31).6
When large randomized clinical trials and observational studies report discrepant results, many authorities prioritize the findings from the randomized clinical trials because those results are less prone to being confounded by unrecognized factors. Randomized trials do not demonstrate that mild SCH or isolated hypothyroxinemia have a major impact on pregnancy outcomes.
Thyroid antibodies, fertility, miscarriage, and preterm birth
Some observational studies report that the presence of thyroid antibodies in a euthyroid woman reduces fecundity and increases the risk for miscarriage and preterm birth. For example, a meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with and without antithyroid antibodies was 6.9% and 4.9%, respectively (OR, 1.33; 95% CI, 1.15–1.56). However, in euthyroid women with antithyroid antibodies, low-dose thyroxine therapy has not been shown to improve fertility, or reduce miscarriages or preterm birth rate.
Continue to: In a large randomized clinical trial, 952 euthyroid women...
In a large randomized clinical trial, 952 euthyroid women (normal TSH level; range, 0.44 to 3.63 mIU/L and free thyroxine level; range, 10 to 21 pmol/L) who were planning on conceiving and had elevated thyroid peroxidase antibodies were randomized prior to conception to receive either thyroxine (50 µg) or placebo.7 After 12 months, outcomes were similar for women treated with thyroxine or placebo, including live birth rate (37.4% vs 37.9%), miscarriage rate for those who became pregnant (28.2% vs 29.6%), and preterm birth ≤ 34 weeks of gestation (3.8% vs 3.6%, respectively).7 The investigators concluded that the use of low-dose thyroxine in euthyroid women with thyroid peroxidase antibodies was not effective for increasing the rate of live birth or reducing the rate of miscarriage or early preterm birth.
Thyroid antibodies and the rate of IVF pregnancy and miscarriage
Some observational studies suggest that the presence of antithyroid antibodies may be associated with an increased rate of miscarriage.8 To test the effects of thyroxine treatment on the rate of miscarriage in euthyroid women with antithyroid antibodies, 600 euthyroid infertile women with antithyroid antibodies (antithyroid peroxidase levels ≥ 60 IU/mL) scheduled to have in vitro fertilization (IVF) were randomly assigned to receive thyroxine (dose adjustment to keep TSH levels in the range of 0.1 to 2.5 mIU/L) or no treatment.9 The thyroxine treatment was initiated 2 to 4 weeks before initiation of ovarian stimulation. In this study, treatment with thyroxine or no treatment resulted in similar rates of clinical pregnancy (35.7% vs 37.7%) and live birth (31.7% vs 32.3%).9 Among the women who achieved a clinical pregnancy, miscarriage rates were similar in the thyroxine and no treatment groups (10.3% vs 10.6%).9
Let’s focus on more serious problems that affect pregnancy
There is a clear consensus that women with overt hypothyroidism should be treated with thyroxine prior to attempting pregnancy.2,6 There is no clear consensus about how to treat women considering pregnancy who have one isolated laboratory finding, such as mild subclinical hypothyroidism, mild isolated hypothyroxinemia, or antithyroid antibodies. Given the lack of evidence from randomized trials that thyroxine improves pregnancy outcomes in these cases, obstetrician-gynecologists may want to either refer women with these problems to an endocrinologist for consultation or sequentially measure laboratory values to assess whether the patient’s laboratory abnormality is transient, stable, or worsening.
Obstetrician-gynecologists and their patients are confronted by many serious problems that adversely affect pregnancy and deserve priority attention, including iron deficiency anemia, excess gestational weight gain, peripartum depression, intimate partner violence, housing insecurity, cigarette smoking, substance misuse, chronic hypertension, morbid obesity, diabetes, gestational diabetes, preeclampsia, venous thromboembolism, obstetrical hemorrhage, sepsis, and infectious diseases. Given limited resources our expertise should be focused on these major obstetric public health problems rather than screening for mild subclinical hypothyroidism.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2017;27:315-389.
- Abalovich M, Gutierrez S, Alcaraz G, et al. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2012;12:63-68.
- Consortium on Thyroid and Pregnancy--Study Group on Preterm Birth. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA. 2019;322:632-641.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366:493-501.
- Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825.
- Dhillon-Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med. 2019;380:1316-1325.
- Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:513-519.
- Wang H, Gao H, Chi H, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA. 2017;318:2190-2198.
In a US study of more than 17,000 people, overt hypothyroidism and hyperthyroidism were detected in about 4.6% and 1.3% of adults, respectively.1 In this population-based study, thyroid disease was 5 times more prevalent among women than among men. In our ObGyn practices, there are many women of reproductive age with thyroid disease who are considering pregnancy. Treatment of active hyperthyroidism in a woman planning pregnancy is complex and best managed by endocrinologists. Treatment of hypothyroidism is more straightforward, however, and typically managed by internists, family medicine clinicians, and obstetrician-gynecologists.
Clinical management of hypothyroidism and pregnancy
Pregnancy results in a doubling of thyroxine-binding globulin (TBG) levels and a 40% increase in plasma volume, resulting in a need for more thyroxine production.2 Of note, from conception to approximately 13 weeks’ gestation, the sole source of embryonic and fetal thyroid hormones is from the mother.2 Women who have been taking chronic thyroxine treatment may have suppressed thyroid gland activity and be unable to increase thyroxine production in response to pregnancy, necessitating a 30% to 50% increase in their thyroxine dose to maintain TSH levels in the normal range.
For hypothyroid women on long-term thyroxine treatment, recommend increasing the thyroxine dose when pregnancy is recognized. For your patients on chronic thyroxine treatment who are planning a pregnancy, a multiprong approach is helpful in preparing the patient for the increased thyroxine requirements of early pregnancy. First, it is important to counsel the woman that she should not stop the thyroxine medication because it may adversely affect the pregnancy. In my experience, most cases of overt hypothyroidism during pregnancy occur because the patient stopped taking her thyroxine therapy. Second, for hypothyroid women who are considering conception it is reasonable to adjust the thyroxine dose to keep the TSH concentration in the lower range of normal (0.5 to 2.5 mU/L). This will give the woman a “buffer,” reducing the risk that in early pregnancy she and her fetus will have a thyroxine deficit. Third, in early pregnancy, following detection of a positive pregnancy test, your patient can start to increase her thyroxine dose by about two tablets weekly (a 28% increase in the dose). Fourth, TSH levels can be measured every 4 weeks during the first trimester, with appropriate adjustment of the thyroxine dose to keep the TSH concentration below the trimester-specific upper limit of normal (< 4 mU/L).2
TSH and free thyroxine measurements identify women with overt hypothyroidism who need thyroxine treatment. Overt hypothyroidism is associated with adverse reproductive outcomes, including decreased fertility, increased spontaneous abortion, increased fetal loss, and preterm birth.2,3 Hence it is important to immediately initiate thyroxine treatment in pregnant women who have overt hypothyroidism. A diagnosis of overt hypothyroidism is indicated in women with an intact hypothalamic-pituitary axis and a TSH level ≥10 mU/L plus a low free thyroxine concentration. A TSH level of >4 to 10 mU/L, with normal free thyroxine concentration, is evidence of subclinical hypothyroidism (SCH). Among women, there are about 5 times more cases of SCH than overt hypothyroidism.
Continue to: The literature concerning SCH and pregnancy...
The literature concerning SCH and pregnancy is vast, and often contradictory, leading to confusion among clinicians. Contributing to the confusion is that some observational studies report a modest association between SCH and adverse pregnancy outcomes. To date, however, randomized clinical trials show no benefit of thyroxine treatment in these cases. I explore these contradictory pieces of evidence below.

Is SCH associated with adverse pregnancy outcomes due to low thyroxine levels?
There is conflicting literature about the association of SCH and adverse reproductive outcomes. A meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with SCH and euthyroid women (normal TSH and normal free thyroxine levels) was 6.1% and 5.0%, respectively (odds ratio [OR], 1.29; 95% CI, 1.01–1.64).4 Interestingly, pregnant women with normal TSH levels but a low free thyroxine level also had an increased rate of preterm birth (7.1% vs 5.0%; OR, 1.46; 95% CI, 1.12–1.90).
Although observational studies report an association between SCH and adverse reproductive outcomes, multiple randomized clinical trials conducted in women with SCH or hypothyroxinemia have failed to demonstrate that thyroxine replacement improves reproductive outcomes. For example, in a study of 794 pregnant women with elevated TSH and/or low free thyroxine levels randomly assigned to thyroxine treatment (0.15 mg daily) or no treatment, there was no difference in preterm birth rate (5.6% vs 7.9%, P = .2), mean birth weight (3.5 kg vs 3.3 kg, P = .15), gestational age at delivery (40.1 vs 40.2 weeks, P = .10), or the intelligence quotient of children at 3 years (99 vs 100, P = .40).5
In another study, 674 pregnant women with mild SCH (mean TSH, 4.4 mU/L) were randomly assigned to receive thyroxine (0.1 mg daily and dose adjusted to achieve a normal TSH level) or placebo. In this study there was no difference between the thyroxine treatment or placebo groups in preterm birth rate (9% vs 11%, P = .44), gestational age at delivery (39.1 vs 38.9 weeks, P = .57) or intelligence quotient of children at 5 years (97 and 94, P = .71).6
The same investigators also randomized 524 pregnant women with isolated hypothyroxinema (mean free thyroxine level, 0.83 ng/dL) and normal TSH level (mean, 1.5 mU/L) to thyroxine (0.05 mg daily and dose adjusted to achieve a normal free thyroxine level) or placebo.6 In this study there was no difference in preterm birth rate (12% vs 8%, P = .11), gestational age at delivery (39.0 vs 38.8 weeks, P = .46) or intelligence quotient of children at 5 years (94 and 91, P = .31).6
When large randomized clinical trials and observational studies report discrepant results, many authorities prioritize the findings from the randomized clinical trials because those results are less prone to being confounded by unrecognized factors. Randomized trials do not demonstrate that mild SCH or isolated hypothyroxinemia have a major impact on pregnancy outcomes.
Thyroid antibodies, fertility, miscarriage, and preterm birth
Some observational studies report that the presence of thyroid antibodies in a euthyroid woman reduces fecundity and increases the risk for miscarriage and preterm birth. For example, a meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with and without antithyroid antibodies was 6.9% and 4.9%, respectively (OR, 1.33; 95% CI, 1.15–1.56). However, in euthyroid women with antithyroid antibodies, low-dose thyroxine therapy has not been shown to improve fertility, or reduce miscarriages or preterm birth rate.
Continue to: In a large randomized clinical trial, 952 euthyroid women...
In a large randomized clinical trial, 952 euthyroid women (normal TSH level; range, 0.44 to 3.63 mIU/L and free thyroxine level; range, 10 to 21 pmol/L) who were planning on conceiving and had elevated thyroid peroxidase antibodies were randomized prior to conception to receive either thyroxine (50 µg) or placebo.7 After 12 months, outcomes were similar for women treated with thyroxine or placebo, including live birth rate (37.4% vs 37.9%), miscarriage rate for those who became pregnant (28.2% vs 29.6%), and preterm birth ≤ 34 weeks of gestation (3.8% vs 3.6%, respectively).7 The investigators concluded that the use of low-dose thyroxine in euthyroid women with thyroid peroxidase antibodies was not effective for increasing the rate of live birth or reducing the rate of miscarriage or early preterm birth.
Thyroid antibodies and the rate of IVF pregnancy and miscarriage
Some observational studies suggest that the presence of antithyroid antibodies may be associated with an increased rate of miscarriage.8 To test the effects of thyroxine treatment on the rate of miscarriage in euthyroid women with antithyroid antibodies, 600 euthyroid infertile women with antithyroid antibodies (antithyroid peroxidase levels ≥ 60 IU/mL) scheduled to have in vitro fertilization (IVF) were randomly assigned to receive thyroxine (dose adjustment to keep TSH levels in the range of 0.1 to 2.5 mIU/L) or no treatment.9 The thyroxine treatment was initiated 2 to 4 weeks before initiation of ovarian stimulation. In this study, treatment with thyroxine or no treatment resulted in similar rates of clinical pregnancy (35.7% vs 37.7%) and live birth (31.7% vs 32.3%).9 Among the women who achieved a clinical pregnancy, miscarriage rates were similar in the thyroxine and no treatment groups (10.3% vs 10.6%).9
Let’s focus on more serious problems that affect pregnancy
There is a clear consensus that women with overt hypothyroidism should be treated with thyroxine prior to attempting pregnancy.2,6 There is no clear consensus about how to treat women considering pregnancy who have one isolated laboratory finding, such as mild subclinical hypothyroidism, mild isolated hypothyroxinemia, or antithyroid antibodies. Given the lack of evidence from randomized trials that thyroxine improves pregnancy outcomes in these cases, obstetrician-gynecologists may want to either refer women with these problems to an endocrinologist for consultation or sequentially measure laboratory values to assess whether the patient’s laboratory abnormality is transient, stable, or worsening.
Obstetrician-gynecologists and their patients are confronted by many serious problems that adversely affect pregnancy and deserve priority attention, including iron deficiency anemia, excess gestational weight gain, peripartum depression, intimate partner violence, housing insecurity, cigarette smoking, substance misuse, chronic hypertension, morbid obesity, diabetes, gestational diabetes, preeclampsia, venous thromboembolism, obstetrical hemorrhage, sepsis, and infectious diseases. Given limited resources our expertise should be focused on these major obstetric public health problems rather than screening for mild subclinical hypothyroidism.
In a US study of more than 17,000 people, overt hypothyroidism and hyperthyroidism were detected in about 4.6% and 1.3% of adults, respectively.1 In this population-based study, thyroid disease was 5 times more prevalent among women than among men. In our ObGyn practices, there are many women of reproductive age with thyroid disease who are considering pregnancy. Treatment of active hyperthyroidism in a woman planning pregnancy is complex and best managed by endocrinologists. Treatment of hypothyroidism is more straightforward, however, and typically managed by internists, family medicine clinicians, and obstetrician-gynecologists.
Clinical management of hypothyroidism and pregnancy
Pregnancy results in a doubling of thyroxine-binding globulin (TBG) levels and a 40% increase in plasma volume, resulting in a need for more thyroxine production.2 Of note, from conception to approximately 13 weeks’ gestation, the sole source of embryonic and fetal thyroid hormones is from the mother.2 Women who have been taking chronic thyroxine treatment may have suppressed thyroid gland activity and be unable to increase thyroxine production in response to pregnancy, necessitating a 30% to 50% increase in their thyroxine dose to maintain TSH levels in the normal range.
For hypothyroid women on long-term thyroxine treatment, recommend increasing the thyroxine dose when pregnancy is recognized. For your patients on chronic thyroxine treatment who are planning a pregnancy, a multiprong approach is helpful in preparing the patient for the increased thyroxine requirements of early pregnancy. First, it is important to counsel the woman that she should not stop the thyroxine medication because it may adversely affect the pregnancy. In my experience, most cases of overt hypothyroidism during pregnancy occur because the patient stopped taking her thyroxine therapy. Second, for hypothyroid women who are considering conception it is reasonable to adjust the thyroxine dose to keep the TSH concentration in the lower range of normal (0.5 to 2.5 mU/L). This will give the woman a “buffer,” reducing the risk that in early pregnancy she and her fetus will have a thyroxine deficit. Third, in early pregnancy, following detection of a positive pregnancy test, your patient can start to increase her thyroxine dose by about two tablets weekly (a 28% increase in the dose). Fourth, TSH levels can be measured every 4 weeks during the first trimester, with appropriate adjustment of the thyroxine dose to keep the TSH concentration below the trimester-specific upper limit of normal (< 4 mU/L).2
TSH and free thyroxine measurements identify women with overt hypothyroidism who need thyroxine treatment. Overt hypothyroidism is associated with adverse reproductive outcomes, including decreased fertility, increased spontaneous abortion, increased fetal loss, and preterm birth.2,3 Hence it is important to immediately initiate thyroxine treatment in pregnant women who have overt hypothyroidism. A diagnosis of overt hypothyroidism is indicated in women with an intact hypothalamic-pituitary axis and a TSH level ≥10 mU/L plus a low free thyroxine concentration. A TSH level of >4 to 10 mU/L, with normal free thyroxine concentration, is evidence of subclinical hypothyroidism (SCH). Among women, there are about 5 times more cases of SCH than overt hypothyroidism.
Continue to: The literature concerning SCH and pregnancy...
The literature concerning SCH and pregnancy is vast, and often contradictory, leading to confusion among clinicians. Contributing to the confusion is that some observational studies report a modest association between SCH and adverse pregnancy outcomes. To date, however, randomized clinical trials show no benefit of thyroxine treatment in these cases. I explore these contradictory pieces of evidence below.

Is SCH associated with adverse pregnancy outcomes due to low thyroxine levels?
There is conflicting literature about the association of SCH and adverse reproductive outcomes. A meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with SCH and euthyroid women (normal TSH and normal free thyroxine levels) was 6.1% and 5.0%, respectively (odds ratio [OR], 1.29; 95% CI, 1.01–1.64).4 Interestingly, pregnant women with normal TSH levels but a low free thyroxine level also had an increased rate of preterm birth (7.1% vs 5.0%; OR, 1.46; 95% CI, 1.12–1.90).
Although observational studies report an association between SCH and adverse reproductive outcomes, multiple randomized clinical trials conducted in women with SCH or hypothyroxinemia have failed to demonstrate that thyroxine replacement improves reproductive outcomes. For example, in a study of 794 pregnant women with elevated TSH and/or low free thyroxine levels randomly assigned to thyroxine treatment (0.15 mg daily) or no treatment, there was no difference in preterm birth rate (5.6% vs 7.9%, P = .2), mean birth weight (3.5 kg vs 3.3 kg, P = .15), gestational age at delivery (40.1 vs 40.2 weeks, P = .10), or the intelligence quotient of children at 3 years (99 vs 100, P = .40).5
In another study, 674 pregnant women with mild SCH (mean TSH, 4.4 mU/L) were randomly assigned to receive thyroxine (0.1 mg daily and dose adjusted to achieve a normal TSH level) or placebo. In this study there was no difference between the thyroxine treatment or placebo groups in preterm birth rate (9% vs 11%, P = .44), gestational age at delivery (39.1 vs 38.9 weeks, P = .57) or intelligence quotient of children at 5 years (97 and 94, P = .71).6
The same investigators also randomized 524 pregnant women with isolated hypothyroxinema (mean free thyroxine level, 0.83 ng/dL) and normal TSH level (mean, 1.5 mU/L) to thyroxine (0.05 mg daily and dose adjusted to achieve a normal free thyroxine level) or placebo.6 In this study there was no difference in preterm birth rate (12% vs 8%, P = .11), gestational age at delivery (39.0 vs 38.8 weeks, P = .46) or intelligence quotient of children at 5 years (94 and 91, P = .31).6
When large randomized clinical trials and observational studies report discrepant results, many authorities prioritize the findings from the randomized clinical trials because those results are less prone to being confounded by unrecognized factors. Randomized trials do not demonstrate that mild SCH or isolated hypothyroxinemia have a major impact on pregnancy outcomes.
Thyroid antibodies, fertility, miscarriage, and preterm birth
Some observational studies report that the presence of thyroid antibodies in a euthyroid woman reduces fecundity and increases the risk for miscarriage and preterm birth. For example, a meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with and without antithyroid antibodies was 6.9% and 4.9%, respectively (OR, 1.33; 95% CI, 1.15–1.56). However, in euthyroid women with antithyroid antibodies, low-dose thyroxine therapy has not been shown to improve fertility, or reduce miscarriages or preterm birth rate.
Continue to: In a large randomized clinical trial, 952 euthyroid women...
In a large randomized clinical trial, 952 euthyroid women (normal TSH level; range, 0.44 to 3.63 mIU/L and free thyroxine level; range, 10 to 21 pmol/L) who were planning on conceiving and had elevated thyroid peroxidase antibodies were randomized prior to conception to receive either thyroxine (50 µg) or placebo.7 After 12 months, outcomes were similar for women treated with thyroxine or placebo, including live birth rate (37.4% vs 37.9%), miscarriage rate for those who became pregnant (28.2% vs 29.6%), and preterm birth ≤ 34 weeks of gestation (3.8% vs 3.6%, respectively).7 The investigators concluded that the use of low-dose thyroxine in euthyroid women with thyroid peroxidase antibodies was not effective for increasing the rate of live birth or reducing the rate of miscarriage or early preterm birth.
Thyroid antibodies and the rate of IVF pregnancy and miscarriage
Some observational studies suggest that the presence of antithyroid antibodies may be associated with an increased rate of miscarriage.8 To test the effects of thyroxine treatment on the rate of miscarriage in euthyroid women with antithyroid antibodies, 600 euthyroid infertile women with antithyroid antibodies (antithyroid peroxidase levels ≥ 60 IU/mL) scheduled to have in vitro fertilization (IVF) were randomly assigned to receive thyroxine (dose adjustment to keep TSH levels in the range of 0.1 to 2.5 mIU/L) or no treatment.9 The thyroxine treatment was initiated 2 to 4 weeks before initiation of ovarian stimulation. In this study, treatment with thyroxine or no treatment resulted in similar rates of clinical pregnancy (35.7% vs 37.7%) and live birth (31.7% vs 32.3%).9 Among the women who achieved a clinical pregnancy, miscarriage rates were similar in the thyroxine and no treatment groups (10.3% vs 10.6%).9
Let’s focus on more serious problems that affect pregnancy
There is a clear consensus that women with overt hypothyroidism should be treated with thyroxine prior to attempting pregnancy.2,6 There is no clear consensus about how to treat women considering pregnancy who have one isolated laboratory finding, such as mild subclinical hypothyroidism, mild isolated hypothyroxinemia, or antithyroid antibodies. Given the lack of evidence from randomized trials that thyroxine improves pregnancy outcomes in these cases, obstetrician-gynecologists may want to either refer women with these problems to an endocrinologist for consultation or sequentially measure laboratory values to assess whether the patient’s laboratory abnormality is transient, stable, or worsening.
Obstetrician-gynecologists and their patients are confronted by many serious problems that adversely affect pregnancy and deserve priority attention, including iron deficiency anemia, excess gestational weight gain, peripartum depression, intimate partner violence, housing insecurity, cigarette smoking, substance misuse, chronic hypertension, morbid obesity, diabetes, gestational diabetes, preeclampsia, venous thromboembolism, obstetrical hemorrhage, sepsis, and infectious diseases. Given limited resources our expertise should be focused on these major obstetric public health problems rather than screening for mild subclinical hypothyroidism.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2017;27:315-389.
- Abalovich M, Gutierrez S, Alcaraz G, et al. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2012;12:63-68.
- Consortium on Thyroid and Pregnancy--Study Group on Preterm Birth. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA. 2019;322:632-641.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366:493-501.
- Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825.
- Dhillon-Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med. 2019;380:1316-1325.
- Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:513-519.
- Wang H, Gao H, Chi H, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA. 2017;318:2190-2198.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2017;27:315-389.
- Abalovich M, Gutierrez S, Alcaraz G, et al. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2012;12:63-68.
- Consortium on Thyroid and Pregnancy--Study Group on Preterm Birth. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA. 2019;322:632-641.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366:493-501.
- Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825.
- Dhillon-Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med. 2019;380:1316-1325.
- Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:513-519.
- Wang H, Gao H, Chi H, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA. 2017;318:2190-2198.
When providing contraceptive counseling to women with migraine headaches, how do you identify migraine with aura?
Most physicians know that migraine with aura is a risk factor for ischemic stroke and that the use of an estrogen-containing contraceptive further increases this risk.1-3 Additional important and prevalent risk factors for ischemic stroke include cigarette smoking, hypertension, diabetes, and ischemic heart disease.1 The American College of Obstetricians and Gynecologists (ACOG)2 and the Centers for Disease Control and Prevention (CDC)3 recommend against the use of estrogen-containing contraceptives for women with migraine with aura because of the increased risk of ischemic stroke (Medical Eligibility Criteria [MEC] category 4—unacceptable health risk, method not to be used).
However, those who have migraine with aura can use nonhormonal and progestin-only forms of contraception, including copper- and levonorgestrel-intrauterine devices, the etonogestrel subdermal implant, depot medroxyprogesterone acetate, and progestin-only pills (MEC category 1—no restriction).2,3 ACOG and the CDC advise that estrogen-containing contraceptives can be used for those with migraine without aura who have no other risk factors for stroke (MEC category 2—advantages generally outweigh theoretical or proven risks).2,3 Given the high prevalence of migraine in reproductive-age women, accurate diagnosis of aura is of paramount importance in order to provide appropriate contraceptive counseling.
When is migraine with aura the right diagnosis?
In clinical practice, there is a high level of confusion about the migraine symptoms that warrant a diagnosis of migraine with aura. One approach to improving the accuracy of such a diagnosis is to refer every woman seeking contraceptive counseling who has migraine headaches to a neurologist for expert adjudication of the presence or absence of aura. But in the clinical context of contraceptive counseling, neurology consultation is not always readily available, and requiring consultation increases barriers to care. However, there are tools—such as the Visual Aura Rating Scale (VARS), which is discussed below—that may help non-neurologists identify migraine with aura.4 First, let us review the data that links migraine with aura with increased risk of ischemic stroke.

Migraine with aura is a risk factor for stroke
Multiple case-control studies report that migraine with aura is a risk factor for ischemic stroke.1,5,6 Studies also report that women with migraine with aura who use estrogen-containing contraceptives have an even greater risk of ischemic stroke. For example, one recent case-control study used a commercial claims database of 1,884 cases of ischemic stroke among individuals who identify as women 15 to 49 years of age matched to 7,536 controls without ischemic stroke.1 In this study, the risk of ischemic stroke was increased more than 2.5-fold by cigarette smoking (adjusted odds ratio [aOR], 2.59), hypertension (aOR, 2.73), diabetes (aOR, 2.78), migraine with aura (aOR, 2.89), and ischemic heart disease (aOR, 5.49). For those with migraine with aura who also used an estrogen-containing contraceptive, the aOR for ischemic stroke was 6.08. By contrast, the risk for stroke among those with migraine with aura who were not using an estrogen-containing contraceptive was 2.65. Furthermore, among those with migraine without aura, the risk of ischemic stroke was only 1.77 with the use of an estrogen-containing contraceptive.
Continue to: Although women with migraine...
Although women with migraine with and without aura are at increased risk for stroke, the absolute risk is still very low. For example, one review reported that the incidence of ischemic stroke per 100,000 person-years among women 20 to 44 years of age was 2.5 for those without migraine not taking estrogen-containing contraceptives, 5.9 for those with migraine with aura not taking estrogen-containing contraceptives, and 14.5 among those with migraine with aura and taking estrogen-containing contraceptives.6 Another important observation is that the incidence of thrombotic stroke dramatically increases from adolescence (3.4 per 100,000 person-years) to 45-49 years of age (64.4 per 100,000 person-years).7 Therefore, older women with migraine are at greater risk for stroke than adolescents.
Diagnostic criteria for migraine with and without aura
In contraceptive counseling, if an estrogen-containing contraceptive is being considered, it is important to identify women with migraine headache, determine migraine subtype, assess the frequency of migraines and identify other cardiovascular risk factors, such as hypertension and cigarette smoking. The International Headache Society has evolved the diagnostic criteria for migraine with and without aura, and now endorses the criteria published in the 3rd edition of the International Classification of Headache Disorders (ICHD-3; TABLES 1 and 2).8 For non-neurologists, these criteria may be difficult to remember and impractical to utilize in daily contraceptive counseling. Two simplified tools, the ID Migraine Questionnaire9 and the Visual Aura Rating Scale (TABLE 3)4 may help identify women who have migraine headaches and assess for the presence of aura.


The ID Migraine Questionnaire
In a study of 563 people seeking primary care who had headaches in the past 3 months, 3 questions were identified as being helpful in identifying women with migraine. This 3-question screening tool had reasonable sensitivity (81%), specificity (75%), and positive predictive value (93%) compared with expert diagnosis using the ICHD-3.9 The 3 questions in this screening tool, which are answered “Yes” or “No,” are:
During the last 3 months did you have the following symptoms with your headaches:
- Feel nauseated or sick to your stomach?
- Light bothered you?
- Your headaches limited your ability to work, study or do what you needed to do for at least 1 day?
If two questions are answered “Yes” the patient may have migraine headaches.
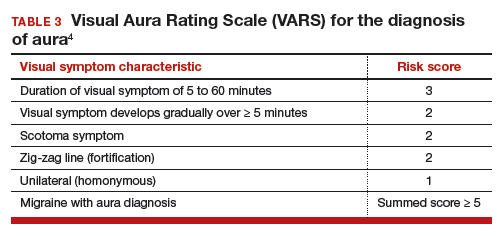
Visual Aura Rating Scale for the diagnosis of migraine with aura
More than 90% of women with migraine with aura have visual auras, leaving only a minority with non–visual aura, such as tingling or numbness in a limb, speech or language problems, or muscle weakness. Hence for non-neurologists, it is reasonable to focus on the accurate diagnosis of visual aura to identify those with migraine with aura.
In the clinical context of contraceptive counseling, the Visual Aura Rating Scale (VARS) is especially useful because it has good sensitivity and specificity, and it is easy to use in practice (TABLE 3).4 VARS assesses for 5 characteristics of a visual aura, and each characteristic is associated with a weighted risk score. The 5 symptoms assessed include:
- duration of visual symptom between 5 and 60 minutes (3 points)
- visual symptom develops gradually over 5 minutes (2 points)
- scotoma (2 points)
- zig-zag line (2 points)
- unilateral (1 point).
Continue to: Of note, visual aura is usually...
Of note, visual aura is usually slow-spreading and persists for more than 5 minutes but less than 60 minutes. If a visual symptom has a sudden onset and persists for much longer than 60 minutes, concern is heightened for a more serious neurologic diagnosis such as transient ischemic attack or stroke. A summed score of 5 or more points supports the diagnosis of migraine with aura. In one study, VARS had a sensitivity of 91% and specificity of 96% for identifying women with migraine with aura diagnosed by the ICHD-3 criteria.4
Consider using VARS to identify migraine with aura
Epidemiologic studies report that about 17% of adults have migraine, and about 5% have migraine with aura.10,11 Consequently, migraine with aura is one of the most common medical conditions encountered during contraceptive counseling. The CDC MEC recommend against the use of estrogen-containing contraceptives in women with migraine with aura (Category 4 rating). The VARS may help clinicians identify those who have migraine with aura who should not be offered estrogen-containing contraceptives. Equally important, the use of VARS could help reduce the number of women who are inappropriately diagnosed as having migraine with aura based on fleeting visual symptoms lasting far less than 5 minutes during a migraine headache.
- Champaloux SW, Tepper NK, Monsour M, et al. Use of combined hormonal contraceptives among women with migraine and risk of ischemic stroke. Am J Obstet Gynecol. 2017;216:489.e1-e7.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Eriksen MK, Thomsen LL, Olesen J. The Visual Aura Rating Scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914.
- Sacco S, Merki-Feld G, Aegidius KL, et al. Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain. 2017;18:108.
- Lidegaard Ø, Lokkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
- Headache Classification Committee of the International Headache Society. International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
- Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: the ID Migraine validation study. Neurology. 2003;12;61:375-382.
- Lipton RB, Scher AI, Kolodner K, et al. Migraine in the United States: epidemiology and patterns of health care use. Neurology. 2002;58:885-894.
- Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
Most physicians know that migraine with aura is a risk factor for ischemic stroke and that the use of an estrogen-containing contraceptive further increases this risk.1-3 Additional important and prevalent risk factors for ischemic stroke include cigarette smoking, hypertension, diabetes, and ischemic heart disease.1 The American College of Obstetricians and Gynecologists (ACOG)2 and the Centers for Disease Control and Prevention (CDC)3 recommend against the use of estrogen-containing contraceptives for women with migraine with aura because of the increased risk of ischemic stroke (Medical Eligibility Criteria [MEC] category 4—unacceptable health risk, method not to be used).
However, those who have migraine with aura can use nonhormonal and progestin-only forms of contraception, including copper- and levonorgestrel-intrauterine devices, the etonogestrel subdermal implant, depot medroxyprogesterone acetate, and progestin-only pills (MEC category 1—no restriction).2,3 ACOG and the CDC advise that estrogen-containing contraceptives can be used for those with migraine without aura who have no other risk factors for stroke (MEC category 2—advantages generally outweigh theoretical or proven risks).2,3 Given the high prevalence of migraine in reproductive-age women, accurate diagnosis of aura is of paramount importance in order to provide appropriate contraceptive counseling.
When is migraine with aura the right diagnosis?
In clinical practice, there is a high level of confusion about the migraine symptoms that warrant a diagnosis of migraine with aura. One approach to improving the accuracy of such a diagnosis is to refer every woman seeking contraceptive counseling who has migraine headaches to a neurologist for expert adjudication of the presence or absence of aura. But in the clinical context of contraceptive counseling, neurology consultation is not always readily available, and requiring consultation increases barriers to care. However, there are tools—such as the Visual Aura Rating Scale (VARS), which is discussed below—that may help non-neurologists identify migraine with aura.4 First, let us review the data that links migraine with aura with increased risk of ischemic stroke.

Migraine with aura is a risk factor for stroke
Multiple case-control studies report that migraine with aura is a risk factor for ischemic stroke.1,5,6 Studies also report that women with migraine with aura who use estrogen-containing contraceptives have an even greater risk of ischemic stroke. For example, one recent case-control study used a commercial claims database of 1,884 cases of ischemic stroke among individuals who identify as women 15 to 49 years of age matched to 7,536 controls without ischemic stroke.1 In this study, the risk of ischemic stroke was increased more than 2.5-fold by cigarette smoking (adjusted odds ratio [aOR], 2.59), hypertension (aOR, 2.73), diabetes (aOR, 2.78), migraine with aura (aOR, 2.89), and ischemic heart disease (aOR, 5.49). For those with migraine with aura who also used an estrogen-containing contraceptive, the aOR for ischemic stroke was 6.08. By contrast, the risk for stroke among those with migraine with aura who were not using an estrogen-containing contraceptive was 2.65. Furthermore, among those with migraine without aura, the risk of ischemic stroke was only 1.77 with the use of an estrogen-containing contraceptive.
Continue to: Although women with migraine...
Although women with migraine with and without aura are at increased risk for stroke, the absolute risk is still very low. For example, one review reported that the incidence of ischemic stroke per 100,000 person-years among women 20 to 44 years of age was 2.5 for those without migraine not taking estrogen-containing contraceptives, 5.9 for those with migraine with aura not taking estrogen-containing contraceptives, and 14.5 among those with migraine with aura and taking estrogen-containing contraceptives.6 Another important observation is that the incidence of thrombotic stroke dramatically increases from adolescence (3.4 per 100,000 person-years) to 45-49 years of age (64.4 per 100,000 person-years).7 Therefore, older women with migraine are at greater risk for stroke than adolescents.
Diagnostic criteria for migraine with and without aura
In contraceptive counseling, if an estrogen-containing contraceptive is being considered, it is important to identify women with migraine headache, determine migraine subtype, assess the frequency of migraines and identify other cardiovascular risk factors, such as hypertension and cigarette smoking. The International Headache Society has evolved the diagnostic criteria for migraine with and without aura, and now endorses the criteria published in the 3rd edition of the International Classification of Headache Disorders (ICHD-3; TABLES 1 and 2).8 For non-neurologists, these criteria may be difficult to remember and impractical to utilize in daily contraceptive counseling. Two simplified tools, the ID Migraine Questionnaire9 and the Visual Aura Rating Scale (TABLE 3)4 may help identify women who have migraine headaches and assess for the presence of aura.


The ID Migraine Questionnaire
In a study of 563 people seeking primary care who had headaches in the past 3 months, 3 questions were identified as being helpful in identifying women with migraine. This 3-question screening tool had reasonable sensitivity (81%), specificity (75%), and positive predictive value (93%) compared with expert diagnosis using the ICHD-3.9 The 3 questions in this screening tool, which are answered “Yes” or “No,” are:
During the last 3 months did you have the following symptoms with your headaches:
- Feel nauseated or sick to your stomach?
- Light bothered you?
- Your headaches limited your ability to work, study or do what you needed to do for at least 1 day?
If two questions are answered “Yes” the patient may have migraine headaches.
Visual Aura Rating Scale for the diagnosis of migraine with aura
More than 90% of women with migraine with aura have visual auras, leaving only a minority with non–visual aura, such as tingling or numbness in a limb, speech or language problems, or muscle weakness. Hence for non-neurologists, it is reasonable to focus on the accurate diagnosis of visual aura to identify those with migraine with aura.
In the clinical context of contraceptive counseling, the Visual Aura Rating Scale (VARS) is especially useful because it has good sensitivity and specificity, and it is easy to use in practice (TABLE 3).4 VARS assesses for 5 characteristics of a visual aura, and each characteristic is associated with a weighted risk score. The 5 symptoms assessed include:
- duration of visual symptom between 5 and 60 minutes (3 points)
- visual symptom develops gradually over 5 minutes (2 points)
- scotoma (2 points)
- zig-zag line (2 points)
- unilateral (1 point).
Continue to: Of note, visual aura is usually...
Of note, visual aura is usually slow-spreading and persists for more than 5 minutes but less than 60 minutes. If a visual symptom has a sudden onset and persists for much longer than 60 minutes, concern is heightened for a more serious neurologic diagnosis such as transient ischemic attack or stroke. A summed score of 5 or more points supports the diagnosis of migraine with aura. In one study, VARS had a sensitivity of 91% and specificity of 96% for identifying women with migraine with aura diagnosed by the ICHD-3 criteria.4

Consider using VARS to identify migraine with aura
Epidemiologic studies report that about 17% of adults have migraine, and about 5% have migraine with aura.10,11 Consequently, migraine with aura is one of the most common medical conditions encountered during contraceptive counseling. The CDC MEC recommend against the use of estrogen-containing contraceptives in women with migraine with aura (Category 4 rating). The VARS may help clinicians identify those who have migraine with aura who should not be offered estrogen-containing contraceptives. Equally important, the use of VARS could help reduce the number of women who are inappropriately diagnosed as having migraine with aura based on fleeting visual symptoms lasting far less than 5 minutes during a migraine headache.
Most physicians know that migraine with aura is a risk factor for ischemic stroke and that the use of an estrogen-containing contraceptive further increases this risk.1-3 Additional important and prevalent risk factors for ischemic stroke include cigarette smoking, hypertension, diabetes, and ischemic heart disease.1 The American College of Obstetricians and Gynecologists (ACOG)2 and the Centers for Disease Control and Prevention (CDC)3 recommend against the use of estrogen-containing contraceptives for women with migraine with aura because of the increased risk of ischemic stroke (Medical Eligibility Criteria [MEC] category 4—unacceptable health risk, method not to be used).
However, those who have migraine with aura can use nonhormonal and progestin-only forms of contraception, including copper- and levonorgestrel-intrauterine devices, the etonogestrel subdermal implant, depot medroxyprogesterone acetate, and progestin-only pills (MEC category 1—no restriction).2,3 ACOG and the CDC advise that estrogen-containing contraceptives can be used for those with migraine without aura who have no other risk factors for stroke (MEC category 2—advantages generally outweigh theoretical or proven risks).2,3 Given the high prevalence of migraine in reproductive-age women, accurate diagnosis of aura is of paramount importance in order to provide appropriate contraceptive counseling.
When is migraine with aura the right diagnosis?
In clinical practice, there is a high level of confusion about the migraine symptoms that warrant a diagnosis of migraine with aura. One approach to improving the accuracy of such a diagnosis is to refer every woman seeking contraceptive counseling who has migraine headaches to a neurologist for expert adjudication of the presence or absence of aura. But in the clinical context of contraceptive counseling, neurology consultation is not always readily available, and requiring consultation increases barriers to care. However, there are tools—such as the Visual Aura Rating Scale (VARS), which is discussed below—that may help non-neurologists identify migraine with aura.4 First, let us review the data that links migraine with aura with increased risk of ischemic stroke.

Migraine with aura is a risk factor for stroke
Multiple case-control studies report that migraine with aura is a risk factor for ischemic stroke.1,5,6 Studies also report that women with migraine with aura who use estrogen-containing contraceptives have an even greater risk of ischemic stroke. For example, one recent case-control study used a commercial claims database of 1,884 cases of ischemic stroke among individuals who identify as women 15 to 49 years of age matched to 7,536 controls without ischemic stroke.1 In this study, the risk of ischemic stroke was increased more than 2.5-fold by cigarette smoking (adjusted odds ratio [aOR], 2.59), hypertension (aOR, 2.73), diabetes (aOR, 2.78), migraine with aura (aOR, 2.89), and ischemic heart disease (aOR, 5.49). For those with migraine with aura who also used an estrogen-containing contraceptive, the aOR for ischemic stroke was 6.08. By contrast, the risk for stroke among those with migraine with aura who were not using an estrogen-containing contraceptive was 2.65. Furthermore, among those with migraine without aura, the risk of ischemic stroke was only 1.77 with the use of an estrogen-containing contraceptive.
Continue to: Although women with migraine...
Although women with migraine with and without aura are at increased risk for stroke, the absolute risk is still very low. For example, one review reported that the incidence of ischemic stroke per 100,000 person-years among women 20 to 44 years of age was 2.5 for those without migraine not taking estrogen-containing contraceptives, 5.9 for those with migraine with aura not taking estrogen-containing contraceptives, and 14.5 among those with migraine with aura and taking estrogen-containing contraceptives.6 Another important observation is that the incidence of thrombotic stroke dramatically increases from adolescence (3.4 per 100,000 person-years) to 45-49 years of age (64.4 per 100,000 person-years).7 Therefore, older women with migraine are at greater risk for stroke than adolescents.
Diagnostic criteria for migraine with and without aura
In contraceptive counseling, if an estrogen-containing contraceptive is being considered, it is important to identify women with migraine headache, determine migraine subtype, assess the frequency of migraines and identify other cardiovascular risk factors, such as hypertension and cigarette smoking. The International Headache Society has evolved the diagnostic criteria for migraine with and without aura, and now endorses the criteria published in the 3rd edition of the International Classification of Headache Disorders (ICHD-3; TABLES 1 and 2).8 For non-neurologists, these criteria may be difficult to remember and impractical to utilize in daily contraceptive counseling. Two simplified tools, the ID Migraine Questionnaire9 and the Visual Aura Rating Scale (TABLE 3)4 may help identify women who have migraine headaches and assess for the presence of aura.


The ID Migraine Questionnaire
In a study of 563 people seeking primary care who had headaches in the past 3 months, 3 questions were identified as being helpful in identifying women with migraine. This 3-question screening tool had reasonable sensitivity (81%), specificity (75%), and positive predictive value (93%) compared with expert diagnosis using the ICHD-3.9 The 3 questions in this screening tool, which are answered “Yes” or “No,” are:
During the last 3 months did you have the following symptoms with your headaches:
- Feel nauseated or sick to your stomach?
- Light bothered you?
- Your headaches limited your ability to work, study or do what you needed to do for at least 1 day?
If two questions are answered “Yes” the patient may have migraine headaches.
Visual Aura Rating Scale for the diagnosis of migraine with aura
More than 90% of women with migraine with aura have visual auras, leaving only a minority with non–visual aura, such as tingling or numbness in a limb, speech or language problems, or muscle weakness. Hence for non-neurologists, it is reasonable to focus on the accurate diagnosis of visual aura to identify those with migraine with aura.
In the clinical context of contraceptive counseling, the Visual Aura Rating Scale (VARS) is especially useful because it has good sensitivity and specificity, and it is easy to use in practice (TABLE 3).4 VARS assesses for 5 characteristics of a visual aura, and each characteristic is associated with a weighted risk score. The 5 symptoms assessed include:
- duration of visual symptom between 5 and 60 minutes (3 points)
- visual symptom develops gradually over 5 minutes (2 points)
- scotoma (2 points)
- zig-zag line (2 points)
- unilateral (1 point).
Continue to: Of note, visual aura is usually...
Of note, visual aura is usually slow-spreading and persists for more than 5 minutes but less than 60 minutes. If a visual symptom has a sudden onset and persists for much longer than 60 minutes, concern is heightened for a more serious neurologic diagnosis such as transient ischemic attack or stroke. A summed score of 5 or more points supports the diagnosis of migraine with aura. In one study, VARS had a sensitivity of 91% and specificity of 96% for identifying women with migraine with aura diagnosed by the ICHD-3 criteria.4

Consider using VARS to identify migraine with aura
Epidemiologic studies report that about 17% of adults have migraine, and about 5% have migraine with aura.10,11 Consequently, migraine with aura is one of the most common medical conditions encountered during contraceptive counseling. The CDC MEC recommend against the use of estrogen-containing contraceptives in women with migraine with aura (Category 4 rating). The VARS may help clinicians identify those who have migraine with aura who should not be offered estrogen-containing contraceptives. Equally important, the use of VARS could help reduce the number of women who are inappropriately diagnosed as having migraine with aura based on fleeting visual symptoms lasting far less than 5 minutes during a migraine headache.
- Champaloux SW, Tepper NK, Monsour M, et al. Use of combined hormonal contraceptives among women with migraine and risk of ischemic stroke. Am J Obstet Gynecol. 2017;216:489.e1-e7.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Eriksen MK, Thomsen LL, Olesen J. The Visual Aura Rating Scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914.
- Sacco S, Merki-Feld G, Aegidius KL, et al. Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain. 2017;18:108.
- Lidegaard Ø, Lokkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
- Headache Classification Committee of the International Headache Society. International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
- Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: the ID Migraine validation study. Neurology. 2003;12;61:375-382.
- Lipton RB, Scher AI, Kolodner K, et al. Migraine in the United States: epidemiology and patterns of health care use. Neurology. 2002;58:885-894.
- Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
- Champaloux SW, Tepper NK, Monsour M, et al. Use of combined hormonal contraceptives among women with migraine and risk of ischemic stroke. Am J Obstet Gynecol. 2017;216:489.e1-e7.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Eriksen MK, Thomsen LL, Olesen J. The Visual Aura Rating Scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914.
- Sacco S, Merki-Feld G, Aegidius KL, et al. Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain. 2017;18:108.
- Lidegaard Ø, Lokkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
- Headache Classification Committee of the International Headache Society. International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
- Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: the ID Migraine validation study. Neurology. 2003;12;61:375-382.
- Lipton RB, Scher AI, Kolodner K, et al. Migraine in the United States: epidemiology and patterns of health care use. Neurology. 2002;58:885-894.
- Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
Women with epilepsy: 5 clinical pearls for contraception and preconception counseling
In 2015, 1.2% of the US population was estimated to have active epilepsy.1 For neurologists, key goals in the treatment of epilepsy include: controlling seizures, minimizing adverse effects of antiepileptic drugs (AEDs) and optimizing quality of life. For obstetrician-gynecologists, women with epilepsy (WWE) have unique contraceptive, preconception, and obstetric needs that require highly specialized approaches to care. Here, I highlight 5 care points that are important to keep in mind when counseling WWE.
1. Enzyme-inducing AEDs reduce the effectiveness of estrogen-progestin and some progestin contraceptives.
AEDs can induce hepatic enzymes that accelerate steroid hormone metabolism, producing clinically important reductions in bioavailable steroid hormone concentration (TABLE 1). According to Lexicomp, AEDs that are inducers of hepatic enzymes that metabolize steroid hormones include: carbamazepine (Tegretol), eslicarbazepine (Aptiom), felbamate (Felbatol), oxcarbazepine (Trileptal), perampanel (Fycompa), phenobarbital, phenytoin (Dilantin), primidone (Mysoline), rufinamide (Banzel), and topiramate (Topamax) (at dosages >200 mg daily). According to Lexicomp, the following AEDs do not cause clinically significant changes in hepatic enzymes that metabolize steroid hormones: acetazolamide (Diamox), clonazepam (Klonopin), ethosuximide (Zarontin), gabapentin (Neurontin), lacosamide (Vimpat), levetiracetam (Keppra), pregabalin (Lyrica), tiagabine (Gabitril), vigabatrin (Vigadrone), and zonisamide (Zonegran).2,3 In addition, lamotrigine (Lamictal) and valproate (Depakote) do not significantly influence the metabolism of contraceptive steroids,4,5 but contraceptive steroids significantly influence their metabolism (TABLE 2).
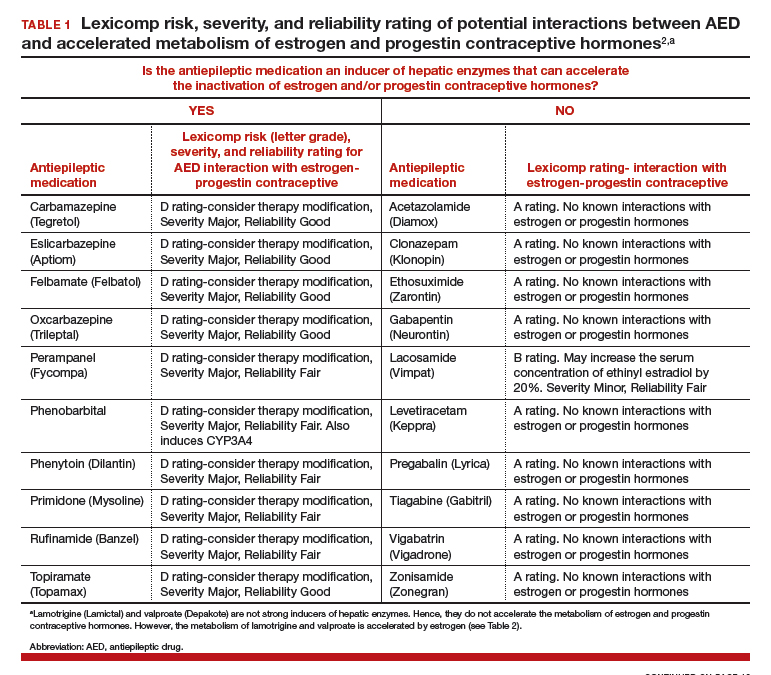

For WWE taking an AED that accelerates steroid hormone metabolism, estrogen-progestin contraceptive failure is common. In a survey of 111 WWE taking both an oral contraceptive and an AED, 27 reported becoming pregnant while taking the oral contraceptive.6 Carbamazepine, a strong inducer of hepatic enzymes, was the most frequently used AED in this sample.
Many studies report that carbamazepine accelerates the metabolisms of estrogen and progestins and reduces contraceptive efficacy. For example, in one study 20 healthy women were administered an ethinyl estradiol (20 µg)-levonorgestrel (100 µg) contraceptive, and randomly assigned to either receive carbamazepine 600 mg daily or a placebo pill.7 In this study, based on serum progesterone measurements, 5 of 10 women in the carbamazepine group ovulated, compared with 1 of 10 women in the placebo group. Women taking carbamazepine had integrated serum ethinyl estradiol and levonorgestrel concentrations approximately 45% lower than women taking placebo.7 Other studies also report that carbamazepine accelerates steroid hormone metabolism and reduces the circulating concentration of ethinyl estradiol, norethindrone, and levonorgestrel by about 50%.5,8
WWE taking an AED that induces hepatic enzymes should be counseled to use a copper or levonorgestrel (LNG) intrauterine device (IUD) or depot medroxyprogesterone acetate (DMPA) for contraception.9 WWE taking AEDs that do not induce hepatic enzymes can be offered the full array of contraceptive options, as outlined in Table 1. Occasionally, a WWE taking an AED that is an inducer of hepatic enzymes may strongly prefer to use an estrogen-progestin contraceptive and decline the preferred option of using an IUD or DMPA. If an estrogen-progestin contraceptive is to be prescribed, safeguards to reduce the risk of pregnancy include:
- prescribe a contraceptive with ≥35 µg of ethinyl estradiol
- prescribe a contraceptive with the highest dose of progestin with a long half-life (drospirenone, desogestrel, levonorgestrel)
- consider continuous hormonal contraception rather than 4 or 7 days off hormones and
- recommend use of a barrier contraceptive in addition to the hormonal contraceptive.
The effectiveness of levonorgestrel emergency contraception may also be reduced in WWE taking an enzyme-inducing AED. In these cases, some experts recommend a regimen of two doses of levonorgestrel 1.5 mg, separated by 12 hours.10 The effectiveness of progestin subdermal contraceptives may be reduced in women taking phenytoin. In one study of 9 WWE using a progestin subdermal implant, phenytoin reduced the circulating levonorgestrel level by approximately 40%.11
Continue to: 2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives...
2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives.
Estrogens, but not progestins, are known to reduce the serum concentration of lamotrigine by about 50%.12,13 This is a clinically significant pharmacologic interaction. Consequently, when a cyclic estrogen-progestin contraceptive is prescribed to a woman taking lamotrigine, oscillation in lamotrigine serum concentration can occur. When the woman is taking estrogen-containing pills, lamotrigine levels decrease, which increases the risk of seizure. When the woman is not taking the estrogen-containing pills, lamotrigine levels increase, possibly causing such adverse effects as nausea and vomiting. If a woman taking lamotrigine insists on using an estrogen-progestin contraceptive, the medication should be prescribed in a continuous regimen and the neurologist alerted so that they can increase the dose of lamotrigine and intensify their monitoring of lamotrigine levels. Lamotrigine does not change the metabolism of ethinyl estradiol and has minimal impact on the metabolism of levonorgestrel.4
3. Estrogen-progestin contraceptives require valproate dosage adjustment.
A few studies report that estrogen-progestin contraceptives accelerate the metabolism of valproate and reduce circulating valproate concentration,14,15 as noted in Table 2.In one study, estrogen-progestin contraceptive was associated with 18% and 29% decreases in total and unbound valproate concentrations, respectively.14 Valproate may induce polycystic ovary syndrome in women.16 Therefore, it is common that valproate and an estrogen-progestin contraceptive are co-prescribed. In these situations, the neurologist should be alerted prior to prescribing an estrogen-progestin contraceptive to WWE taking valproate so that dosage adjustment may occur, if indicated. Valproate does not appear to change the metabolism of ethinyl estradiol or levonorgestrel.5
4. Preconception counseling: Before conception consider using an AED with low teratogenicity.
Valproate is a potent teratogen, and consideration should be given to discontinuing valproate prior to conception. In a study of 1,788 pregnancies exposed to valproate, the risk of a major congenital malformation was 10% for valproate monotherapy, 11.3% for valproate combined with lamotrigine, and 11.7% for valproate combined with another AED, but not lamotrigine.17 At a valproate dose of ≥1,500 mg daily, the risk of major malformation was 24% for valproate monotherapy, 31% for valproate plus lamotrigine, and 19% for valproate plus another AED, but not lamotrigine.17 Valproate is reported to be associated with the following major congenital malformations: spina bifida, ventricular and atrial septal defects, pulmonary valve atresia, hypoplastic left heart syndrome, cleft palate, anorectal atresia, and hypospadias.18
In a study of 7,555 pregnancies in women using a single AED, the risk of major congenital anomalies varied greatly among the AEDs, including: valproate (10.3%), phenobarbital (6.5%), phenytoin (6.4%), carbamazepine (5.5%), topiramate (3.9%), oxcarbazepine (3.0%), lamotrigine (2.9%), and levetiracetam (2.8%).19 For WWE considering pregnancy, many experts recommend use of lamotrigine, levetiracetam, or oxcarbazepine to minimize the risk of fetal anomalies.
Continue to: 5. Folic acid...
5. Folic acid: Although the optimal dose for WWE taking an AED and planning to become pregnant is unknown, a high dose is reasonable.
The American College of Obstetricians and Gynecologists (ACOG) recommends that women planning pregnancy take 0.4 mg of folic acid daily, starting at least 1 month before pregnancy and continuing through at least the 12th week of gestation.20 ACOG also recommends that women at high risk of a neural tube defect should take 4 mg of folic acid daily. WWE taking a teratogenic AED are known to be at increased risk for fetal malformations, including neural tube defects. Should these women take 4 mg of folic acid daily? ACOG notes that, for women taking valproate, the benefit of high-dose folic acid (4 mg daily) has not been definitively proven,21 and guidelines from the American Academy of Neurology do not recommend high-dose folic acid for women receiving AEDs.22 Hence, ACOG does not recommend that WWE taking an AED take high-dose folic acid.
By contrast, the Royal College of Obstetricians and Gynecologists (RCOG) recommends that all WWE planning a pregnancy take folic acid 5 mg daily, initiated 3 months before conception and continued through the first trimester of pregnancy.23 The RCOG notes that among WWE taking an AED, intelligence quotient is greater in children whose mothers took folic acid during pregnancy.24 Given the potential benefit of folic acid on long-term outcomes and the known safety of folic acid, it is reasonable to recommend high-dose folic acid for WWE.
Final takeaways
Surveys consistently report that WWE have a low-level of awareness about the interaction between AEDs and hormonal contraceptives and the teratogenicity of AEDs. For example, in a survey of 2,000 WWE, 45% who were taking an enzyme-inducing AED and an estrogen-progestin oral contraceptive reported that they had not been warned about the potential interaction between the medications.25 Surprisingly, surveys of neurologists and obstetrician-gynecologists also report that there is a low level of awareness about the interaction between AEDs and hormonal contraceptives.26 When providing contraceptive counseling for WWE, prioritize the use of a copper or levonorgestrel IUD. When providing preconception counseling for WWE, educate the patient about the high teratogenicity of valproate and the lower risk of malformations associated with the use of lamotrigine, levetiracetam, and oxcarbazepine.
For most women with epilepsy, maintaining a valid driver's license is important for completion of daily life tasks. Most states require that a patient with seizures be seizure-free for 6 to 12 months to operate a motor vehicle. Estrogen-containing hormonal contraceptives can reduce the concentration of some AEDs, such as lamotrigine. Hence, it is important that the patient be aware of this interaction and that the primary neurologist be alerted if an estrogen-containing contraceptive is prescribed to a woman taking lamotrigine or valproate. Specific state laws related to epilepsy and driving are available at the Epilepsy Foundation website (https://www.epilepsy.com/driving-laws).
- Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States 2015. MMWR Morb Mortal Wkly Rep. 2017;66:821-825.
- Lexicomp. https://www.wolterskluwercdi.com/lexicomp-online/. Accessed August 16, 2019.
- Reimers A, Brodtkorb E, Sabers A. Interactions between hormonal contraception and antiepileptic drugs: clinical and mechanistic considerations. Seizure. 2015;28:66-70.
- Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61:191-199.
- Crawford P, Chadwick D, Cleland P, et al. The lack of effect of sodium valproate on the pharmacokinetics of oral contraceptive steroids. Contraception. 1986;33:23-29.
- Fairgrieve SD, Jackson M, Jonas P, et al. Population-based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321:674-675.
- Davis AR, Westhoff CL, Stanczyk FZ. Carbamazepine coadministration with an oral contraceptive: effects on steroid pharmacokinetics, ovulation, and bleeding. Epilepsia. 2011;52:243-247.
- Doose DR, Wang SS, Padmanabhan M, et al. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia. 2003;44:540-549.
- Vieira CS, Pack A, Roberts K, et al. A pilot study of levonorgestrel concentrations and bleeding patterns in women with epilepsy using a levonorgestrel IUD and treated with antiepileptic drugs. Contraception. 2019;99:251-255.
- O'Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
- Haukkamaa M. Contraception by Norplant subdermal capsules is not reliable in epileptic patients on anticonvulsant treatment. Contraception. 1986;33:559-565.
- Sabers A, Buchholt JM, Uldall P, et al. Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res. 2001;47:151-154.
- Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46:1414-1417.
- Galimberti CA, Mazzucchelli I, Arbasino C, et al. Increased apparent oral clearance of valproic acid during intake of combined contraceptive steroids in women with epilepsy. Epilepsia. 2006;47:1569-1572.
- Herzog AG, Farina EL, Blum AS. Serum valproate levels with oral contraceptive use. Epilepsia. 2005;46:970-971.
- Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol. 2008;64:200-211.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology. 2015;85:866-872.
- Blotière PO, Raguideau F, Weill A, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology. 2019;93:e167-e180.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530-538.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 187: neural tube defects. Obstet Gynecol. 2017;130:e279-e290.
- Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One. 2015;10:e0131130.
- Harden CL, Pennell PB, Koppel BS, et al; American Academy of Neurology and American Epilepsy Society. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:142-149.
- Royal College of Obstetricians and Gynecologists. Epilepsy in pregnancy. Green-top Guideline No. 68; June 2016. https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg68_epilepsy.pdf. Accessed August 16, 2019.
- Meador KJ, Baker GA, Browning N, et al; NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244-252.
- Crawford P, Hudson S. Understanding the information needs of women with epilepsy at different life stages: results of the 'Ideal World' survey. Seizure. 2003;12:502-507.
- Krauss GL, Brandt J, Campbell M, et al. Antiepileptic medication and oral contraceptive interactions: a national survey of neurologists and obstetricians. Neurology. 1996;46:1534-1539.
In 2015, 1.2% of the US population was estimated to have active epilepsy.1 For neurologists, key goals in the treatment of epilepsy include: controlling seizures, minimizing adverse effects of antiepileptic drugs (AEDs) and optimizing quality of life. For obstetrician-gynecologists, women with epilepsy (WWE) have unique contraceptive, preconception, and obstetric needs that require highly specialized approaches to care. Here, I highlight 5 care points that are important to keep in mind when counseling WWE.
1. Enzyme-inducing AEDs reduce the effectiveness of estrogen-progestin and some progestin contraceptives.
AEDs can induce hepatic enzymes that accelerate steroid hormone metabolism, producing clinically important reductions in bioavailable steroid hormone concentration (TABLE 1). According to Lexicomp, AEDs that are inducers of hepatic enzymes that metabolize steroid hormones include: carbamazepine (Tegretol), eslicarbazepine (Aptiom), felbamate (Felbatol), oxcarbazepine (Trileptal), perampanel (Fycompa), phenobarbital, phenytoin (Dilantin), primidone (Mysoline), rufinamide (Banzel), and topiramate (Topamax) (at dosages >200 mg daily). According to Lexicomp, the following AEDs do not cause clinically significant changes in hepatic enzymes that metabolize steroid hormones: acetazolamide (Diamox), clonazepam (Klonopin), ethosuximide (Zarontin), gabapentin (Neurontin), lacosamide (Vimpat), levetiracetam (Keppra), pregabalin (Lyrica), tiagabine (Gabitril), vigabatrin (Vigadrone), and zonisamide (Zonegran).2,3 In addition, lamotrigine (Lamictal) and valproate (Depakote) do not significantly influence the metabolism of contraceptive steroids,4,5 but contraceptive steroids significantly influence their metabolism (TABLE 2).


For WWE taking an AED that accelerates steroid hormone metabolism, estrogen-progestin contraceptive failure is common. In a survey of 111 WWE taking both an oral contraceptive and an AED, 27 reported becoming pregnant while taking the oral contraceptive.6 Carbamazepine, a strong inducer of hepatic enzymes, was the most frequently used AED in this sample.
Many studies report that carbamazepine accelerates the metabolisms of estrogen and progestins and reduces contraceptive efficacy. For example, in one study 20 healthy women were administered an ethinyl estradiol (20 µg)-levonorgestrel (100 µg) contraceptive, and randomly assigned to either receive carbamazepine 600 mg daily or a placebo pill.7 In this study, based on serum progesterone measurements, 5 of 10 women in the carbamazepine group ovulated, compared with 1 of 10 women in the placebo group. Women taking carbamazepine had integrated serum ethinyl estradiol and levonorgestrel concentrations approximately 45% lower than women taking placebo.7 Other studies also report that carbamazepine accelerates steroid hormone metabolism and reduces the circulating concentration of ethinyl estradiol, norethindrone, and levonorgestrel by about 50%.5,8
WWE taking an AED that induces hepatic enzymes should be counseled to use a copper or levonorgestrel (LNG) intrauterine device (IUD) or depot medroxyprogesterone acetate (DMPA) for contraception.9 WWE taking AEDs that do not induce hepatic enzymes can be offered the full array of contraceptive options, as outlined in Table 1. Occasionally, a WWE taking an AED that is an inducer of hepatic enzymes may strongly prefer to use an estrogen-progestin contraceptive and decline the preferred option of using an IUD or DMPA. If an estrogen-progestin contraceptive is to be prescribed, safeguards to reduce the risk of pregnancy include:
- prescribe a contraceptive with ≥35 µg of ethinyl estradiol
- prescribe a contraceptive with the highest dose of progestin with a long half-life (drospirenone, desogestrel, levonorgestrel)
- consider continuous hormonal contraception rather than 4 or 7 days off hormones and
- recommend use of a barrier contraceptive in addition to the hormonal contraceptive.
The effectiveness of levonorgestrel emergency contraception may also be reduced in WWE taking an enzyme-inducing AED. In these cases, some experts recommend a regimen of two doses of levonorgestrel 1.5 mg, separated by 12 hours.10 The effectiveness of progestin subdermal contraceptives may be reduced in women taking phenytoin. In one study of 9 WWE using a progestin subdermal implant, phenytoin reduced the circulating levonorgestrel level by approximately 40%.11
Continue to: 2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives...
2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives.
Estrogens, but not progestins, are known to reduce the serum concentration of lamotrigine by about 50%.12,13 This is a clinically significant pharmacologic interaction. Consequently, when a cyclic estrogen-progestin contraceptive is prescribed to a woman taking lamotrigine, oscillation in lamotrigine serum concentration can occur. When the woman is taking estrogen-containing pills, lamotrigine levels decrease, which increases the risk of seizure. When the woman is not taking the estrogen-containing pills, lamotrigine levels increase, possibly causing such adverse effects as nausea and vomiting. If a woman taking lamotrigine insists on using an estrogen-progestin contraceptive, the medication should be prescribed in a continuous regimen and the neurologist alerted so that they can increase the dose of lamotrigine and intensify their monitoring of lamotrigine levels. Lamotrigine does not change the metabolism of ethinyl estradiol and has minimal impact on the metabolism of levonorgestrel.4
3. Estrogen-progestin contraceptives require valproate dosage adjustment.
A few studies report that estrogen-progestin contraceptives accelerate the metabolism of valproate and reduce circulating valproate concentration,14,15 as noted in Table 2.In one study, estrogen-progestin contraceptive was associated with 18% and 29% decreases in total and unbound valproate concentrations, respectively.14 Valproate may induce polycystic ovary syndrome in women.16 Therefore, it is common that valproate and an estrogen-progestin contraceptive are co-prescribed. In these situations, the neurologist should be alerted prior to prescribing an estrogen-progestin contraceptive to WWE taking valproate so that dosage adjustment may occur, if indicated. Valproate does not appear to change the metabolism of ethinyl estradiol or levonorgestrel.5
4. Preconception counseling: Before conception consider using an AED with low teratogenicity.
Valproate is a potent teratogen, and consideration should be given to discontinuing valproate prior to conception. In a study of 1,788 pregnancies exposed to valproate, the risk of a major congenital malformation was 10% for valproate monotherapy, 11.3% for valproate combined with lamotrigine, and 11.7% for valproate combined with another AED, but not lamotrigine.17 At a valproate dose of ≥1,500 mg daily, the risk of major malformation was 24% for valproate monotherapy, 31% for valproate plus lamotrigine, and 19% for valproate plus another AED, but not lamotrigine.17 Valproate is reported to be associated with the following major congenital malformations: spina bifida, ventricular and atrial septal defects, pulmonary valve atresia, hypoplastic left heart syndrome, cleft palate, anorectal atresia, and hypospadias.18
In a study of 7,555 pregnancies in women using a single AED, the risk of major congenital anomalies varied greatly among the AEDs, including: valproate (10.3%), phenobarbital (6.5%), phenytoin (6.4%), carbamazepine (5.5%), topiramate (3.9%), oxcarbazepine (3.0%), lamotrigine (2.9%), and levetiracetam (2.8%).19 For WWE considering pregnancy, many experts recommend use of lamotrigine, levetiracetam, or oxcarbazepine to minimize the risk of fetal anomalies.
Continue to: 5. Folic acid...
5. Folic acid: Although the optimal dose for WWE taking an AED and planning to become pregnant is unknown, a high dose is reasonable.
The American College of Obstetricians and Gynecologists (ACOG) recommends that women planning pregnancy take 0.4 mg of folic acid daily, starting at least 1 month before pregnancy and continuing through at least the 12th week of gestation.20 ACOG also recommends that women at high risk of a neural tube defect should take 4 mg of folic acid daily. WWE taking a teratogenic AED are known to be at increased risk for fetal malformations, including neural tube defects. Should these women take 4 mg of folic acid daily? ACOG notes that, for women taking valproate, the benefit of high-dose folic acid (4 mg daily) has not been definitively proven,21 and guidelines from the American Academy of Neurology do not recommend high-dose folic acid for women receiving AEDs.22 Hence, ACOG does not recommend that WWE taking an AED take high-dose folic acid.
By contrast, the Royal College of Obstetricians and Gynecologists (RCOG) recommends that all WWE planning a pregnancy take folic acid 5 mg daily, initiated 3 months before conception and continued through the first trimester of pregnancy.23 The RCOG notes that among WWE taking an AED, intelligence quotient is greater in children whose mothers took folic acid during pregnancy.24 Given the potential benefit of folic acid on long-term outcomes and the known safety of folic acid, it is reasonable to recommend high-dose folic acid for WWE.
Final takeaways
Surveys consistently report that WWE have a low-level of awareness about the interaction between AEDs and hormonal contraceptives and the teratogenicity of AEDs. For example, in a survey of 2,000 WWE, 45% who were taking an enzyme-inducing AED and an estrogen-progestin oral contraceptive reported that they had not been warned about the potential interaction between the medications.25 Surprisingly, surveys of neurologists and obstetrician-gynecologists also report that there is a low level of awareness about the interaction between AEDs and hormonal contraceptives.26 When providing contraceptive counseling for WWE, prioritize the use of a copper or levonorgestrel IUD. When providing preconception counseling for WWE, educate the patient about the high teratogenicity of valproate and the lower risk of malformations associated with the use of lamotrigine, levetiracetam, and oxcarbazepine.
For most women with epilepsy, maintaining a valid driver's license is important for completion of daily life tasks. Most states require that a patient with seizures be seizure-free for 6 to 12 months to operate a motor vehicle. Estrogen-containing hormonal contraceptives can reduce the concentration of some AEDs, such as lamotrigine. Hence, it is important that the patient be aware of this interaction and that the primary neurologist be alerted if an estrogen-containing contraceptive is prescribed to a woman taking lamotrigine or valproate. Specific state laws related to epilepsy and driving are available at the Epilepsy Foundation website (https://www.epilepsy.com/driving-laws).
In 2015, 1.2% of the US population was estimated to have active epilepsy.1 For neurologists, key goals in the treatment of epilepsy include: controlling seizures, minimizing adverse effects of antiepileptic drugs (AEDs) and optimizing quality of life. For obstetrician-gynecologists, women with epilepsy (WWE) have unique contraceptive, preconception, and obstetric needs that require highly specialized approaches to care. Here, I highlight 5 care points that are important to keep in mind when counseling WWE.
1. Enzyme-inducing AEDs reduce the effectiveness of estrogen-progestin and some progestin contraceptives.
AEDs can induce hepatic enzymes that accelerate steroid hormone metabolism, producing clinically important reductions in bioavailable steroid hormone concentration (TABLE 1). According to Lexicomp, AEDs that are inducers of hepatic enzymes that metabolize steroid hormones include: carbamazepine (Tegretol), eslicarbazepine (Aptiom), felbamate (Felbatol), oxcarbazepine (Trileptal), perampanel (Fycompa), phenobarbital, phenytoin (Dilantin), primidone (Mysoline), rufinamide (Banzel), and topiramate (Topamax) (at dosages >200 mg daily). According to Lexicomp, the following AEDs do not cause clinically significant changes in hepatic enzymes that metabolize steroid hormones: acetazolamide (Diamox), clonazepam (Klonopin), ethosuximide (Zarontin), gabapentin (Neurontin), lacosamide (Vimpat), levetiracetam (Keppra), pregabalin (Lyrica), tiagabine (Gabitril), vigabatrin (Vigadrone), and zonisamide (Zonegran).2,3 In addition, lamotrigine (Lamictal) and valproate (Depakote) do not significantly influence the metabolism of contraceptive steroids,4,5 but contraceptive steroids significantly influence their metabolism (TABLE 2).


For WWE taking an AED that accelerates steroid hormone metabolism, estrogen-progestin contraceptive failure is common. In a survey of 111 WWE taking both an oral contraceptive and an AED, 27 reported becoming pregnant while taking the oral contraceptive.6 Carbamazepine, a strong inducer of hepatic enzymes, was the most frequently used AED in this sample.
Many studies report that carbamazepine accelerates the metabolisms of estrogen and progestins and reduces contraceptive efficacy. For example, in one study 20 healthy women were administered an ethinyl estradiol (20 µg)-levonorgestrel (100 µg) contraceptive, and randomly assigned to either receive carbamazepine 600 mg daily or a placebo pill.7 In this study, based on serum progesterone measurements, 5 of 10 women in the carbamazepine group ovulated, compared with 1 of 10 women in the placebo group. Women taking carbamazepine had integrated serum ethinyl estradiol and levonorgestrel concentrations approximately 45% lower than women taking placebo.7 Other studies also report that carbamazepine accelerates steroid hormone metabolism and reduces the circulating concentration of ethinyl estradiol, norethindrone, and levonorgestrel by about 50%.5,8
WWE taking an AED that induces hepatic enzymes should be counseled to use a copper or levonorgestrel (LNG) intrauterine device (IUD) or depot medroxyprogesterone acetate (DMPA) for contraception.9 WWE taking AEDs that do not induce hepatic enzymes can be offered the full array of contraceptive options, as outlined in Table 1. Occasionally, a WWE taking an AED that is an inducer of hepatic enzymes may strongly prefer to use an estrogen-progestin contraceptive and decline the preferred option of using an IUD or DMPA. If an estrogen-progestin contraceptive is to be prescribed, safeguards to reduce the risk of pregnancy include:
- prescribe a contraceptive with ≥35 µg of ethinyl estradiol
- prescribe a contraceptive with the highest dose of progestin with a long half-life (drospirenone, desogestrel, levonorgestrel)
- consider continuous hormonal contraception rather than 4 or 7 days off hormones and
- recommend use of a barrier contraceptive in addition to the hormonal contraceptive.
The effectiveness of levonorgestrel emergency contraception may also be reduced in WWE taking an enzyme-inducing AED. In these cases, some experts recommend a regimen of two doses of levonorgestrel 1.5 mg, separated by 12 hours.10 The effectiveness of progestin subdermal contraceptives may be reduced in women taking phenytoin. In one study of 9 WWE using a progestin subdermal implant, phenytoin reduced the circulating levonorgestrel level by approximately 40%.11
Continue to: 2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives...
2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives.
Estrogens, but not progestins, are known to reduce the serum concentration of lamotrigine by about 50%.12,13 This is a clinically significant pharmacologic interaction. Consequently, when a cyclic estrogen-progestin contraceptive is prescribed to a woman taking lamotrigine, oscillation in lamotrigine serum concentration can occur. When the woman is taking estrogen-containing pills, lamotrigine levels decrease, which increases the risk of seizure. When the woman is not taking the estrogen-containing pills, lamotrigine levels increase, possibly causing such adverse effects as nausea and vomiting. If a woman taking lamotrigine insists on using an estrogen-progestin contraceptive, the medication should be prescribed in a continuous regimen and the neurologist alerted so that they can increase the dose of lamotrigine and intensify their monitoring of lamotrigine levels. Lamotrigine does not change the metabolism of ethinyl estradiol and has minimal impact on the metabolism of levonorgestrel.4
3. Estrogen-progestin contraceptives require valproate dosage adjustment.
A few studies report that estrogen-progestin contraceptives accelerate the metabolism of valproate and reduce circulating valproate concentration,14,15 as noted in Table 2.In one study, estrogen-progestin contraceptive was associated with 18% and 29% decreases in total and unbound valproate concentrations, respectively.14 Valproate may induce polycystic ovary syndrome in women.16 Therefore, it is common that valproate and an estrogen-progestin contraceptive are co-prescribed. In these situations, the neurologist should be alerted prior to prescribing an estrogen-progestin contraceptive to WWE taking valproate so that dosage adjustment may occur, if indicated. Valproate does not appear to change the metabolism of ethinyl estradiol or levonorgestrel.5
4. Preconception counseling: Before conception consider using an AED with low teratogenicity.
Valproate is a potent teratogen, and consideration should be given to discontinuing valproate prior to conception. In a study of 1,788 pregnancies exposed to valproate, the risk of a major congenital malformation was 10% for valproate monotherapy, 11.3% for valproate combined with lamotrigine, and 11.7% for valproate combined with another AED, but not lamotrigine.17 At a valproate dose of ≥1,500 mg daily, the risk of major malformation was 24% for valproate monotherapy, 31% for valproate plus lamotrigine, and 19% for valproate plus another AED, but not lamotrigine.17 Valproate is reported to be associated with the following major congenital malformations: spina bifida, ventricular and atrial septal defects, pulmonary valve atresia, hypoplastic left heart syndrome, cleft palate, anorectal atresia, and hypospadias.18
In a study of 7,555 pregnancies in women using a single AED, the risk of major congenital anomalies varied greatly among the AEDs, including: valproate (10.3%), phenobarbital (6.5%), phenytoin (6.4%), carbamazepine (5.5%), topiramate (3.9%), oxcarbazepine (3.0%), lamotrigine (2.9%), and levetiracetam (2.8%).19 For WWE considering pregnancy, many experts recommend use of lamotrigine, levetiracetam, or oxcarbazepine to minimize the risk of fetal anomalies.
Continue to: 5. Folic acid...
5. Folic acid: Although the optimal dose for WWE taking an AED and planning to become pregnant is unknown, a high dose is reasonable.
The American College of Obstetricians and Gynecologists (ACOG) recommends that women planning pregnancy take 0.4 mg of folic acid daily, starting at least 1 month before pregnancy and continuing through at least the 12th week of gestation.20 ACOG also recommends that women at high risk of a neural tube defect should take 4 mg of folic acid daily. WWE taking a teratogenic AED are known to be at increased risk for fetal malformations, including neural tube defects. Should these women take 4 mg of folic acid daily? ACOG notes that, for women taking valproate, the benefit of high-dose folic acid (4 mg daily) has not been definitively proven,21 and guidelines from the American Academy of Neurology do not recommend high-dose folic acid for women receiving AEDs.22 Hence, ACOG does not recommend that WWE taking an AED take high-dose folic acid.
By contrast, the Royal College of Obstetricians and Gynecologists (RCOG) recommends that all WWE planning a pregnancy take folic acid 5 mg daily, initiated 3 months before conception and continued through the first trimester of pregnancy.23 The RCOG notes that among WWE taking an AED, intelligence quotient is greater in children whose mothers took folic acid during pregnancy.24 Given the potential benefit of folic acid on long-term outcomes and the known safety of folic acid, it is reasonable to recommend high-dose folic acid for WWE.
Final takeaways
Surveys consistently report that WWE have a low-level of awareness about the interaction between AEDs and hormonal contraceptives and the teratogenicity of AEDs. For example, in a survey of 2,000 WWE, 45% who were taking an enzyme-inducing AED and an estrogen-progestin oral contraceptive reported that they had not been warned about the potential interaction between the medications.25 Surprisingly, surveys of neurologists and obstetrician-gynecologists also report that there is a low level of awareness about the interaction between AEDs and hormonal contraceptives.26 When providing contraceptive counseling for WWE, prioritize the use of a copper or levonorgestrel IUD. When providing preconception counseling for WWE, educate the patient about the high teratogenicity of valproate and the lower risk of malformations associated with the use of lamotrigine, levetiracetam, and oxcarbazepine.
For most women with epilepsy, maintaining a valid driver's license is important for completion of daily life tasks. Most states require that a patient with seizures be seizure-free for 6 to 12 months to operate a motor vehicle. Estrogen-containing hormonal contraceptives can reduce the concentration of some AEDs, such as lamotrigine. Hence, it is important that the patient be aware of this interaction and that the primary neurologist be alerted if an estrogen-containing contraceptive is prescribed to a woman taking lamotrigine or valproate. Specific state laws related to epilepsy and driving are available at the Epilepsy Foundation website (https://www.epilepsy.com/driving-laws).
- Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States 2015. MMWR Morb Mortal Wkly Rep. 2017;66:821-825.
- Lexicomp. https://www.wolterskluwercdi.com/lexicomp-online/. Accessed August 16, 2019.
- Reimers A, Brodtkorb E, Sabers A. Interactions between hormonal contraception and antiepileptic drugs: clinical and mechanistic considerations. Seizure. 2015;28:66-70.
- Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61:191-199.
- Crawford P, Chadwick D, Cleland P, et al. The lack of effect of sodium valproate on the pharmacokinetics of oral contraceptive steroids. Contraception. 1986;33:23-29.
- Fairgrieve SD, Jackson M, Jonas P, et al. Population-based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321:674-675.
- Davis AR, Westhoff CL, Stanczyk FZ. Carbamazepine coadministration with an oral contraceptive: effects on steroid pharmacokinetics, ovulation, and bleeding. Epilepsia. 2011;52:243-247.
- Doose DR, Wang SS, Padmanabhan M, et al. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia. 2003;44:540-549.
- Vieira CS, Pack A, Roberts K, et al. A pilot study of levonorgestrel concentrations and bleeding patterns in women with epilepsy using a levonorgestrel IUD and treated with antiepileptic drugs. Contraception. 2019;99:251-255.
- O'Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
- Haukkamaa M. Contraception by Norplant subdermal capsules is not reliable in epileptic patients on anticonvulsant treatment. Contraception. 1986;33:559-565.
- Sabers A, Buchholt JM, Uldall P, et al. Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res. 2001;47:151-154.
- Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46:1414-1417.
- Galimberti CA, Mazzucchelli I, Arbasino C, et al. Increased apparent oral clearance of valproic acid during intake of combined contraceptive steroids in women with epilepsy. Epilepsia. 2006;47:1569-1572.
- Herzog AG, Farina EL, Blum AS. Serum valproate levels with oral contraceptive use. Epilepsia. 2005;46:970-971.
- Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol. 2008;64:200-211.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology. 2015;85:866-872.
- Blotière PO, Raguideau F, Weill A, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology. 2019;93:e167-e180.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530-538.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 187: neural tube defects. Obstet Gynecol. 2017;130:e279-e290.
- Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One. 2015;10:e0131130.
- Harden CL, Pennell PB, Koppel BS, et al; American Academy of Neurology and American Epilepsy Society. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:142-149.
- Royal College of Obstetricians and Gynecologists. Epilepsy in pregnancy. Green-top Guideline No. 68; June 2016. https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg68_epilepsy.pdf. Accessed August 16, 2019.
- Meador KJ, Baker GA, Browning N, et al; NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244-252.
- Crawford P, Hudson S. Understanding the information needs of women with epilepsy at different life stages: results of the 'Ideal World' survey. Seizure. 2003;12:502-507.
- Krauss GL, Brandt J, Campbell M, et al. Antiepileptic medication and oral contraceptive interactions: a national survey of neurologists and obstetricians. Neurology. 1996;46:1534-1539.
- Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States 2015. MMWR Morb Mortal Wkly Rep. 2017;66:821-825.
- Lexicomp. https://www.wolterskluwercdi.com/lexicomp-online/. Accessed August 16, 2019.
- Reimers A, Brodtkorb E, Sabers A. Interactions between hormonal contraception and antiepileptic drugs: clinical and mechanistic considerations. Seizure. 2015;28:66-70.
- Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61:191-199.
- Crawford P, Chadwick D, Cleland P, et al. The lack of effect of sodium valproate on the pharmacokinetics of oral contraceptive steroids. Contraception. 1986;33:23-29.
- Fairgrieve SD, Jackson M, Jonas P, et al. Population-based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321:674-675.
- Davis AR, Westhoff CL, Stanczyk FZ. Carbamazepine coadministration with an oral contraceptive: effects on steroid pharmacokinetics, ovulation, and bleeding. Epilepsia. 2011;52:243-247.
- Doose DR, Wang SS, Padmanabhan M, et al. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia. 2003;44:540-549.
- Vieira CS, Pack A, Roberts K, et al. A pilot study of levonorgestrel concentrations and bleeding patterns in women with epilepsy using a levonorgestrel IUD and treated with antiepileptic drugs. Contraception. 2019;99:251-255.
- O'Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
- Haukkamaa M. Contraception by Norplant subdermal capsules is not reliable in epileptic patients on anticonvulsant treatment. Contraception. 1986;33:559-565.
- Sabers A, Buchholt JM, Uldall P, et al. Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res. 2001;47:151-154.
- Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46:1414-1417.
- Galimberti CA, Mazzucchelli I, Arbasino C, et al. Increased apparent oral clearance of valproic acid during intake of combined contraceptive steroids in women with epilepsy. Epilepsia. 2006;47:1569-1572.
- Herzog AG, Farina EL, Blum AS. Serum valproate levels with oral contraceptive use. Epilepsia. 2005;46:970-971.
- Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol. 2008;64:200-211.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology. 2015;85:866-872.
- Blotière PO, Raguideau F, Weill A, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology. 2019;93:e167-e180.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530-538.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 187: neural tube defects. Obstet Gynecol. 2017;130:e279-e290.
- Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One. 2015;10:e0131130.
- Harden CL, Pennell PB, Koppel BS, et al; American Academy of Neurology and American Epilepsy Society. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:142-149.
- Royal College of Obstetricians and Gynecologists. Epilepsy in pregnancy. Green-top Guideline No. 68; June 2016. https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg68_epilepsy.pdf. Accessed August 16, 2019.
- Meador KJ, Baker GA, Browning N, et al; NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244-252.
- Crawford P, Hudson S. Understanding the information needs of women with epilepsy at different life stages: results of the 'Ideal World' survey. Seizure. 2003;12:502-507.
- Krauss GL, Brandt J, Campbell M, et al. Antiepileptic medication and oral contraceptive interactions: a national survey of neurologists and obstetricians. Neurology. 1996;46:1534-1539.
Why do so many women aged 65 years and older die of cervical cancer?
Surprisingly, the cervical cancer death rate is greater among women aged >65 years than among younger women1,2 (FIGURE). Paradoxically, most of our screening programs focus on women <65 years of age. A nationwide study from Denmark estimated that the cervical cancer death rate per 100,000 women at ages 40 to 44 and 65 to 69 was 3.8 and 9.0, respectively.1 In other words, the cervical cancer death rate at age 65 to 69 years was 2.36 times higher than at age 40 to 44 years.1
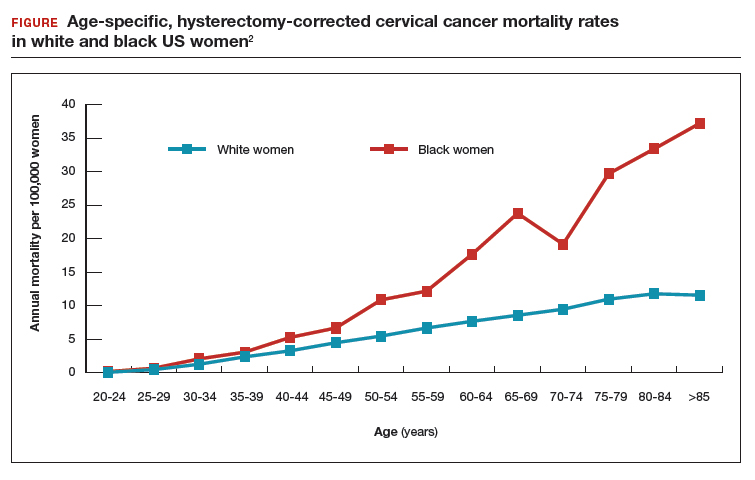
A study from the United States estimated that the cervical cancer death rate per 100,000 white women at ages 40 to 44 and 65 to 69 was 3.3 and 8.6, respectively,2 very similar to the findings from Denmark. The same US study estimated that the cervical cancer death rate per 100,000 black women at ages 40 to 44 and 65 to 69 was 5.3 and 23.8, highlighting the fact that, in the United States, cervical cancer disease burden is disproportionately greater among black than among white women.2 In addition, the cervical cancer death rate among black women at age 65 to 69 was 4.49 times higher than at age 40 to 44 years.2
Given the high death rate from cervical cancer in women >65 years of age, it is paradoxical that most professional society guidelines recommend discontinuing cervical cancer screening at 65 years of age, if previous cervical cancer screening is normal.3,4 Is the problem due to an inability to implement the current guidelines? Or is the problem that the guidelines are not optimally designed to reduce cervical cancer risk in women >65 years of age?
The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) recommend against cervical cancer screening in women >65 years of age who have had adequate prior screening and are not otherwise at high risk for cervical cancer. However, ACOG and the USPSTF caution that there are many groups of women that may benefit from continued screening after 65 years of age, including women with HIV infection, a compromised immune system, or previous high-grade precancerous lesion or cervicalcancer; women with limited access to care; women from racial/ethnic minority groups; and migrant women.4 Many clinicians remember the guidance, “discontinue cervical cancer screening at 65 years” but do not recall all the clinical factors that might warrant continued screening past age 65. Of special concern is that black,2 Hispanic,5 and migrant women6 are at much higher risk for invasive cervical cancer than white or US-born women.
The optimal implementation of the ACOG and USPSTF guidelines are undermined by a fractured health care system, where key pieces of information may be unavailable to the clinician tasked with making a decision about discontinuing cervical cancer screening. Imagine the case in which a 65-year-old woman pre‑sents to her primary care physician for cervical cancer screening. The clinician performs a cervical cytology test and obtains a report of “no intraepithelial lesion or malignancy.” The clinician then recommends that the patient discontinue cervical cancer screening. Unbeknownst to the clinician, the patient had a positive HPV 16/18/45 test within the past 10 years in another health system. In this case, it would be inappropriate to terminate the patient from cervical cancer screening.
Continue to: Testing for hrHPV is superior to cervical cytology in women >65 years...
Testing for hrHPV is superior to cervical cytology in women >65 years
In Sweden, about 30% of cervical cancer cases occur in women aged >60 years.7 To assess the prevalence of oncogenic high-risk HPV (hrHPV), women at ages 60, 65, 70, and 75 years were invited to send sequential self-collected vaginal samples for nucleic acid testing for hrHPV. The prevalence of hrHPV was found to be 4.4%. Women with a second positive, self-collected, hrHPV test were invited for colposcopy, cervical biopsy, and cytology testing. Among the women with two positive hrHPV tests, cervical biopsy revealed 7 cases of cervical intraepithelial neoplasia grade 2 (CIN2), 6 cases of CIN1, and 4 biopsies without CIN. In these women 94% of the cervical cytology samples returned, “no intraepithelial lesion or malignancy” and 6% revealed atypical squamous cells of undetermined significance. This study suggests that, in women aged >65 years, cervical cytology may have a high rate of false-negative results, possibly due to epithelial atrophy. An evolving clinical pearl is that, when using the current cervical cancer screening guidelines, the final screen for cervical cancer must include a nucleic acid test for hrHPV.
In women 65 to 90 years, the prevalence of hrHPV is approximately 5%
In a study of 40,382 women aged 14 to 95 years, the prevalence of hrHPV was 46% in 20- to 23-year-old women and 5.7% in women older than 65 years of age.8 In a study of more than 108,000 women aged 69 to >89 years the prevalence of hrHPV was 4.3%, and similar prevalence rates were seen across all ages from 69 to >89 years.9 The carcinogenic role of persistent hrHPV infection in women >65 years is an important area for future research.
Latent HPV virus infection
Following a primary varicella-zoster infection (chickenpox), the virus may remain in a latent state in sensory ganglia, reactivating later in life to cause shingles. Thirty percent of people who have a primary chickenpox infection eventually will develop a case of shingles. Immunocompromised populations are at an increased risk of developing shingles because of reduced T-cell mediated immunity.
A recent hypothesis is that in immunocompromised and older women, latent HPV can reactivate and cause clinically significant infection.10 Following renal transplantation investigators have reported a significant increase in the prevalence of genital HPV, without a change in sexual behavior.11 In cervical tissue from women with no evidence of active HPV infection, highly sensitive PCR-based assays detected HPV16 virus in a latent state in some women, possibly due to disruption of the viral E2 gene.12 If latent HPV infection is a valid biological concept, it suggests that there is no “safe age” at which to discontinue screening for HPV infection because the virus cannot be detected in screening samples while it is latent.
Options for cervical cancer screening in women >65 years
Three options might reduce the morbidity and mortality associated with cervical cancer in women >65 years.
Option 1: Double-down on trying to effectively implement current guidelines. The high rate of cervical cancer mortality in women >65 years of age indicates that the current guidelines, as implemented in real clinical practice, are not working. A problem with the current screening guidelines is that clinicians are expected to be capable of finding all relevant cervical cancer test results and properly interpreting the results. Clinicians are over-taxed and fallible, and the current approach is not likely to be successful unless additional information technology solutions are implemented.
Continue to: Health systems could use information...
Health systems could use information technology to mitigate these problems. For example, health systems could deploy software to assemble every cervical screening result on each woman and pre‑sent those results to clinicians in a single integrated view in the electronic record. Additionally, once all lifetime screening results are consolidated in one view, artificial intelligence systems could be used to analyze the totality of results and identify women who would benefit by continued screening past age 65 and women who could safely discontinue screening.
Option 2: Adopt the Australian approach to cervical cancer screening. The current Australian approach to cervical cancer screening is built on 3 pillars: 1) school-based vaccination of all children against hrHPV, 2) screening all women from 25 to 74 years of age every 5 years using nucleic acid testing for hrHPV, and 3) providing a system for the testing of samples self-collected by women who are reluctant to visit a clinician for screening.13 Australia has one of the lowest cervical cancer death rates in the world.
Option 3: Continue screening most women past age 65. Women >65 years of age are known to be infected with hrHPV genotypes. hrHPV infection causes cervical cancer. Cervical cancer causes many deaths in women aged >65 years. There is no strong rationale for ignoring these three facts. hrHPV screening every 5 years as long as the woman is healthy and has a reasonable life expectancy is an option that could be evaluated in randomized studies.
Given the high rate of cervical cancer death in women >65 years of age, I plan to be very cautious about discontinuing cervical cancer screening until I can personally ensure that my patient has no evidence of hrHPV infection.
In 2008, Harald zur Hausen, MD, received the Nobel Prize in Physiology or Medicine for discovering that human papilloma virus (HPV) caused cervical cancer. In a recent study, 74% of cervical cancers were associated with HPV 16 or 18 infections. A total of 89% of the cancers were associated with one of the high-risk HPV genotypes, including HPV 16/18/31/33/45/52/58.1
Recently, HPV has been shown to be a major cause of oropharyngeal cancer. The Centers for Disease Control and Prevention calculated that in CY2015 in the United States there were 18,917 cases of HPV-associated oropharyngeal squamous cell cancer and 11,788 cases of cervical cancer.2 Most cases of HPV-associated oropharyngeal cancer occur in men, and HPV vaccination of boys may help to prevent this cancer type. Oncogenic HPV produce two proteins (E6 and E7) that promote viral replication and squamous cell growth by inhibiting the function of p53 and retinoblastoma protein. The immortalized HeLa cell line, derived from Ms. Henrietta Lack's cervical cancer, contains integrated HPV18 nucleic acid sequences.3,4
The discovery that HPV causes cancer catalyzed the development of nucleic acid tests to identify high-risk oncogenic HPV and vaccines against high-risk oncogenic HPV genotypes that prevent cervical cancer. From a public health perspective, it is more effective to vaccinate the population against oncogenic HPV genotypes than to screen and treat cancer. In the United States, vaccination rates range from a high of 92% (District of Columbia) and 89% (Rhode Island) to a low of 47% (Wyoming) and 50% (Kentucky and Mississippi).5 To reduce HPV-associated cancer mortality, the gap in vaccination compliance must be closed.
References
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers - United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018;67:918-924.
- Rosl F, Westphal EM, zur Hausen H. Chromatin structure and transcriptional regulation of human papillomavirus type 18 DNA in HeLa cells. Mol Carcinog. 1989;2:72-80.
- Adey A, Burton JN, Kitzman, et al. The haplotype-resolved genome and epigenome of the aneuploid HeLa cancer cell line. Nature. 2013;500:207-211.
- Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
- Hammer A, Kahlert J, Gravitt PE, et al. Hysterectomy-corrected cervical cancer mortality rates in Denmark during 2002-2015: a registry-based cohort study. Acta Obstet Gynecol Scand. 2019;98:1063-1069.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128:e111-30.
- Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Stang A, Hawk H, Knowlton R, et al. Hysterectomy-corrected incidence rates of cervical and uterine cancers in Massachusetts, 1995-2010. Ann Epidemiol. 2014;24:849-854.
- Hallowell BD, Endeshaw M, McKenna MT, et al. Cervical cancer death rates among U.S.- and foreign-born women: U.S., 2005-2014. Am J Prev Med. 2019;56:869-874.
- Lindström AK, Hermansson RS, Gustavsson I, et al. Cervical dysplasia in elderly women performing repeated self-sampling for HPV testing. PLoS One. 2018;13:e0207714.
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Andersen B, Christensen BS, Christensen J, et al. HPV-prevalence in elderly women in Denmark. Gynecol Oncol. 2019;154:118-123.
- Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: role of viral latency. Viruses. 2017;9:E267.
- Hinten F, Hilbrands LB, Meeuwis KAP, et al. Reactivation of latent HPV infections after renal transplantation. Am J Transplant. 2017;17:1563-1573.
- Leonard SM, Pereira M, Roberts S, et al. Evidence of disrupted high-risk human papillomavirus DNA in morphologically normal cervices of older women. Sci Rep. 2016;6:20847.
- Cervical cancer screening. Cancer Council website. https://www.cancer.org.au/about-cancer/early-detection/screening-programs/cervical-cancer-screening.html. Updated March 15, 2019. Accessed July 23, 2019.
Surprisingly, the cervical cancer death rate is greater among women aged >65 years than among younger women1,2 (FIGURE). Paradoxically, most of our screening programs focus on women <65 years of age. A nationwide study from Denmark estimated that the cervical cancer death rate per 100,000 women at ages 40 to 44 and 65 to 69 was 3.8 and 9.0, respectively.1 In other words, the cervical cancer death rate at age 65 to 69 years was 2.36 times higher than at age 40 to 44 years.1

A study from the United States estimated that the cervical cancer death rate per 100,000 white women at ages 40 to 44 and 65 to 69 was 3.3 and 8.6, respectively,2 very similar to the findings from Denmark. The same US study estimated that the cervical cancer death rate per 100,000 black women at ages 40 to 44 and 65 to 69 was 5.3 and 23.8, highlighting the fact that, in the United States, cervical cancer disease burden is disproportionately greater among black than among white women.2 In addition, the cervical cancer death rate among black women at age 65 to 69 was 4.49 times higher than at age 40 to 44 years.2
Given the high death rate from cervical cancer in women >65 years of age, it is paradoxical that most professional society guidelines recommend discontinuing cervical cancer screening at 65 years of age, if previous cervical cancer screening is normal.3,4 Is the problem due to an inability to implement the current guidelines? Or is the problem that the guidelines are not optimally designed to reduce cervical cancer risk in women >65 years of age?
The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) recommend against cervical cancer screening in women >65 years of age who have had adequate prior screening and are not otherwise at high risk for cervical cancer. However, ACOG and the USPSTF caution that there are many groups of women that may benefit from continued screening after 65 years of age, including women with HIV infection, a compromised immune system, or previous high-grade precancerous lesion or cervicalcancer; women with limited access to care; women from racial/ethnic minority groups; and migrant women.4 Many clinicians remember the guidance, “discontinue cervical cancer screening at 65 years” but do not recall all the clinical factors that might warrant continued screening past age 65. Of special concern is that black,2 Hispanic,5 and migrant women6 are at much higher risk for invasive cervical cancer than white or US-born women.
The optimal implementation of the ACOG and USPSTF guidelines are undermined by a fractured health care system, where key pieces of information may be unavailable to the clinician tasked with making a decision about discontinuing cervical cancer screening. Imagine the case in which a 65-year-old woman pre‑sents to her primary care physician for cervical cancer screening. The clinician performs a cervical cytology test and obtains a report of “no intraepithelial lesion or malignancy.” The clinician then recommends that the patient discontinue cervical cancer screening. Unbeknownst to the clinician, the patient had a positive HPV 16/18/45 test within the past 10 years in another health system. In this case, it would be inappropriate to terminate the patient from cervical cancer screening.
Continue to: Testing for hrHPV is superior to cervical cytology in women >65 years...
Testing for hrHPV is superior to cervical cytology in women >65 years
In Sweden, about 30% of cervical cancer cases occur in women aged >60 years.7 To assess the prevalence of oncogenic high-risk HPV (hrHPV), women at ages 60, 65, 70, and 75 years were invited to send sequential self-collected vaginal samples for nucleic acid testing for hrHPV. The prevalence of hrHPV was found to be 4.4%. Women with a second positive, self-collected, hrHPV test were invited for colposcopy, cervical biopsy, and cytology testing. Among the women with two positive hrHPV tests, cervical biopsy revealed 7 cases of cervical intraepithelial neoplasia grade 2 (CIN2), 6 cases of CIN1, and 4 biopsies without CIN. In these women 94% of the cervical cytology samples returned, “no intraepithelial lesion or malignancy” and 6% revealed atypical squamous cells of undetermined significance. This study suggests that, in women aged >65 years, cervical cytology may have a high rate of false-negative results, possibly due to epithelial atrophy. An evolving clinical pearl is that, when using the current cervical cancer screening guidelines, the final screen for cervical cancer must include a nucleic acid test for hrHPV.
In women 65 to 90 years, the prevalence of hrHPV is approximately 5%
In a study of 40,382 women aged 14 to 95 years, the prevalence of hrHPV was 46% in 20- to 23-year-old women and 5.7% in women older than 65 years of age.8 In a study of more than 108,000 women aged 69 to >89 years the prevalence of hrHPV was 4.3%, and similar prevalence rates were seen across all ages from 69 to >89 years.9 The carcinogenic role of persistent hrHPV infection in women >65 years is an important area for future research.
Latent HPV virus infection
Following a primary varicella-zoster infection (chickenpox), the virus may remain in a latent state in sensory ganglia, reactivating later in life to cause shingles. Thirty percent of people who have a primary chickenpox infection eventually will develop a case of shingles. Immunocompromised populations are at an increased risk of developing shingles because of reduced T-cell mediated immunity.
A recent hypothesis is that in immunocompromised and older women, latent HPV can reactivate and cause clinically significant infection.10 Following renal transplantation investigators have reported a significant increase in the prevalence of genital HPV, without a change in sexual behavior.11 In cervical tissue from women with no evidence of active HPV infection, highly sensitive PCR-based assays detected HPV16 virus in a latent state in some women, possibly due to disruption of the viral E2 gene.12 If latent HPV infection is a valid biological concept, it suggests that there is no “safe age” at which to discontinue screening for HPV infection because the virus cannot be detected in screening samples while it is latent.
Options for cervical cancer screening in women >65 years
Three options might reduce the morbidity and mortality associated with cervical cancer in women >65 years.
Option 1: Double-down on trying to effectively implement current guidelines. The high rate of cervical cancer mortality in women >65 years of age indicates that the current guidelines, as implemented in real clinical practice, are not working. A problem with the current screening guidelines is that clinicians are expected to be capable of finding all relevant cervical cancer test results and properly interpreting the results. Clinicians are over-taxed and fallible, and the current approach is not likely to be successful unless additional information technology solutions are implemented.
Continue to: Health systems could use information...
Health systems could use information technology to mitigate these problems. For example, health systems could deploy software to assemble every cervical screening result on each woman and pre‑sent those results to clinicians in a single integrated view in the electronic record. Additionally, once all lifetime screening results are consolidated in one view, artificial intelligence systems could be used to analyze the totality of results and identify women who would benefit by continued screening past age 65 and women who could safely discontinue screening.
Option 2: Adopt the Australian approach to cervical cancer screening. The current Australian approach to cervical cancer screening is built on 3 pillars: 1) school-based vaccination of all children against hrHPV, 2) screening all women from 25 to 74 years of age every 5 years using nucleic acid testing for hrHPV, and 3) providing a system for the testing of samples self-collected by women who are reluctant to visit a clinician for screening.13 Australia has one of the lowest cervical cancer death rates in the world.
Option 3: Continue screening most women past age 65. Women >65 years of age are known to be infected with hrHPV genotypes. hrHPV infection causes cervical cancer. Cervical cancer causes many deaths in women aged >65 years. There is no strong rationale for ignoring these three facts. hrHPV screening every 5 years as long as the woman is healthy and has a reasonable life expectancy is an option that could be evaluated in randomized studies.
Given the high rate of cervical cancer death in women >65 years of age, I plan to be very cautious about discontinuing cervical cancer screening until I can personally ensure that my patient has no evidence of hrHPV infection.
In 2008, Harald zur Hausen, MD, received the Nobel Prize in Physiology or Medicine for discovering that human papilloma virus (HPV) caused cervical cancer. In a recent study, 74% of cervical cancers were associated with HPV 16 or 18 infections. A total of 89% of the cancers were associated with one of the high-risk HPV genotypes, including HPV 16/18/31/33/45/52/58.1
Recently, HPV has been shown to be a major cause of oropharyngeal cancer. The Centers for Disease Control and Prevention calculated that in CY2015 in the United States there were 18,917 cases of HPV-associated oropharyngeal squamous cell cancer and 11,788 cases of cervical cancer.2 Most cases of HPV-associated oropharyngeal cancer occur in men, and HPV vaccination of boys may help to prevent this cancer type. Oncogenic HPV produce two proteins (E6 and E7) that promote viral replication and squamous cell growth by inhibiting the function of p53 and retinoblastoma protein. The immortalized HeLa cell line, derived from Ms. Henrietta Lack's cervical cancer, contains integrated HPV18 nucleic acid sequences.3,4
The discovery that HPV causes cancer catalyzed the development of nucleic acid tests to identify high-risk oncogenic HPV and vaccines against high-risk oncogenic HPV genotypes that prevent cervical cancer. From a public health perspective, it is more effective to vaccinate the population against oncogenic HPV genotypes than to screen and treat cancer. In the United States, vaccination rates range from a high of 92% (District of Columbia) and 89% (Rhode Island) to a low of 47% (Wyoming) and 50% (Kentucky and Mississippi).5 To reduce HPV-associated cancer mortality, the gap in vaccination compliance must be closed.
References
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers - United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018;67:918-924.
- Rosl F, Westphal EM, zur Hausen H. Chromatin structure and transcriptional regulation of human papillomavirus type 18 DNA in HeLa cells. Mol Carcinog. 1989;2:72-80.
- Adey A, Burton JN, Kitzman, et al. The haplotype-resolved genome and epigenome of the aneuploid HeLa cancer cell line. Nature. 2013;500:207-211.
- Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
Surprisingly, the cervical cancer death rate is greater among women aged >65 years than among younger women1,2 (FIGURE). Paradoxically, most of our screening programs focus on women <65 years of age. A nationwide study from Denmark estimated that the cervical cancer death rate per 100,000 women at ages 40 to 44 and 65 to 69 was 3.8 and 9.0, respectively.1 In other words, the cervical cancer death rate at age 65 to 69 years was 2.36 times higher than at age 40 to 44 years.1

A study from the United States estimated that the cervical cancer death rate per 100,000 white women at ages 40 to 44 and 65 to 69 was 3.3 and 8.6, respectively,2 very similar to the findings from Denmark. The same US study estimated that the cervical cancer death rate per 100,000 black women at ages 40 to 44 and 65 to 69 was 5.3 and 23.8, highlighting the fact that, in the United States, cervical cancer disease burden is disproportionately greater among black than among white women.2 In addition, the cervical cancer death rate among black women at age 65 to 69 was 4.49 times higher than at age 40 to 44 years.2
Given the high death rate from cervical cancer in women >65 years of age, it is paradoxical that most professional society guidelines recommend discontinuing cervical cancer screening at 65 years of age, if previous cervical cancer screening is normal.3,4 Is the problem due to an inability to implement the current guidelines? Or is the problem that the guidelines are not optimally designed to reduce cervical cancer risk in women >65 years of age?
The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) recommend against cervical cancer screening in women >65 years of age who have had adequate prior screening and are not otherwise at high risk for cervical cancer. However, ACOG and the USPSTF caution that there are many groups of women that may benefit from continued screening after 65 years of age, including women with HIV infection, a compromised immune system, or previous high-grade precancerous lesion or cervicalcancer; women with limited access to care; women from racial/ethnic minority groups; and migrant women.4 Many clinicians remember the guidance, “discontinue cervical cancer screening at 65 years” but do not recall all the clinical factors that might warrant continued screening past age 65. Of special concern is that black,2 Hispanic,5 and migrant women6 are at much higher risk for invasive cervical cancer than white or US-born women.
The optimal implementation of the ACOG and USPSTF guidelines are undermined by a fractured health care system, where key pieces of information may be unavailable to the clinician tasked with making a decision about discontinuing cervical cancer screening. Imagine the case in which a 65-year-old woman pre‑sents to her primary care physician for cervical cancer screening. The clinician performs a cervical cytology test and obtains a report of “no intraepithelial lesion or malignancy.” The clinician then recommends that the patient discontinue cervical cancer screening. Unbeknownst to the clinician, the patient had a positive HPV 16/18/45 test within the past 10 years in another health system. In this case, it would be inappropriate to terminate the patient from cervical cancer screening.
Continue to: Testing for hrHPV is superior to cervical cytology in women >65 years...
Testing for hrHPV is superior to cervical cytology in women >65 years
In Sweden, about 30% of cervical cancer cases occur in women aged >60 years.7 To assess the prevalence of oncogenic high-risk HPV (hrHPV), women at ages 60, 65, 70, and 75 years were invited to send sequential self-collected vaginal samples for nucleic acid testing for hrHPV. The prevalence of hrHPV was found to be 4.4%. Women with a second positive, self-collected, hrHPV test were invited for colposcopy, cervical biopsy, and cytology testing. Among the women with two positive hrHPV tests, cervical biopsy revealed 7 cases of cervical intraepithelial neoplasia grade 2 (CIN2), 6 cases of CIN1, and 4 biopsies without CIN. In these women 94% of the cervical cytology samples returned, “no intraepithelial lesion or malignancy” and 6% revealed atypical squamous cells of undetermined significance. This study suggests that, in women aged >65 years, cervical cytology may have a high rate of false-negative results, possibly due to epithelial atrophy. An evolving clinical pearl is that, when using the current cervical cancer screening guidelines, the final screen for cervical cancer must include a nucleic acid test for hrHPV.
In women 65 to 90 years, the prevalence of hrHPV is approximately 5%
In a study of 40,382 women aged 14 to 95 years, the prevalence of hrHPV was 46% in 20- to 23-year-old women and 5.7% in women older than 65 years of age.8 In a study of more than 108,000 women aged 69 to >89 years the prevalence of hrHPV was 4.3%, and similar prevalence rates were seen across all ages from 69 to >89 years.9 The carcinogenic role of persistent hrHPV infection in women >65 years is an important area for future research.
Latent HPV virus infection
Following a primary varicella-zoster infection (chickenpox), the virus may remain in a latent state in sensory ganglia, reactivating later in life to cause shingles. Thirty percent of people who have a primary chickenpox infection eventually will develop a case of shingles. Immunocompromised populations are at an increased risk of developing shingles because of reduced T-cell mediated immunity.
A recent hypothesis is that in immunocompromised and older women, latent HPV can reactivate and cause clinically significant infection.10 Following renal transplantation investigators have reported a significant increase in the prevalence of genital HPV, without a change in sexual behavior.11 In cervical tissue from women with no evidence of active HPV infection, highly sensitive PCR-based assays detected HPV16 virus in a latent state in some women, possibly due to disruption of the viral E2 gene.12 If latent HPV infection is a valid biological concept, it suggests that there is no “safe age” at which to discontinue screening for HPV infection because the virus cannot be detected in screening samples while it is latent.
Options for cervical cancer screening in women >65 years
Three options might reduce the morbidity and mortality associated with cervical cancer in women >65 years.
Option 1: Double-down on trying to effectively implement current guidelines. The high rate of cervical cancer mortality in women >65 years of age indicates that the current guidelines, as implemented in real clinical practice, are not working. A problem with the current screening guidelines is that clinicians are expected to be capable of finding all relevant cervical cancer test results and properly interpreting the results. Clinicians are over-taxed and fallible, and the current approach is not likely to be successful unless additional information technology solutions are implemented.
Continue to: Health systems could use information...
Health systems could use information technology to mitigate these problems. For example, health systems could deploy software to assemble every cervical screening result on each woman and pre‑sent those results to clinicians in a single integrated view in the electronic record. Additionally, once all lifetime screening results are consolidated in one view, artificial intelligence systems could be used to analyze the totality of results and identify women who would benefit by continued screening past age 65 and women who could safely discontinue screening.
Option 2: Adopt the Australian approach to cervical cancer screening. The current Australian approach to cervical cancer screening is built on 3 pillars: 1) school-based vaccination of all children against hrHPV, 2) screening all women from 25 to 74 years of age every 5 years using nucleic acid testing for hrHPV, and 3) providing a system for the testing of samples self-collected by women who are reluctant to visit a clinician for screening.13 Australia has one of the lowest cervical cancer death rates in the world.
Option 3: Continue screening most women past age 65. Women >65 years of age are known to be infected with hrHPV genotypes. hrHPV infection causes cervical cancer. Cervical cancer causes many deaths in women aged >65 years. There is no strong rationale for ignoring these three facts. hrHPV screening every 5 years as long as the woman is healthy and has a reasonable life expectancy is an option that could be evaluated in randomized studies.
Given the high rate of cervical cancer death in women >65 years of age, I plan to be very cautious about discontinuing cervical cancer screening until I can personally ensure that my patient has no evidence of hrHPV infection.
In 2008, Harald zur Hausen, MD, received the Nobel Prize in Physiology or Medicine for discovering that human papilloma virus (HPV) caused cervical cancer. In a recent study, 74% of cervical cancers were associated with HPV 16 or 18 infections. A total of 89% of the cancers were associated with one of the high-risk HPV genotypes, including HPV 16/18/31/33/45/52/58.1
Recently, HPV has been shown to be a major cause of oropharyngeal cancer. The Centers for Disease Control and Prevention calculated that in CY2015 in the United States there were 18,917 cases of HPV-associated oropharyngeal squamous cell cancer and 11,788 cases of cervical cancer.2 Most cases of HPV-associated oropharyngeal cancer occur in men, and HPV vaccination of boys may help to prevent this cancer type. Oncogenic HPV produce two proteins (E6 and E7) that promote viral replication and squamous cell growth by inhibiting the function of p53 and retinoblastoma protein. The immortalized HeLa cell line, derived from Ms. Henrietta Lack's cervical cancer, contains integrated HPV18 nucleic acid sequences.3,4
The discovery that HPV causes cancer catalyzed the development of nucleic acid tests to identify high-risk oncogenic HPV and vaccines against high-risk oncogenic HPV genotypes that prevent cervical cancer. From a public health perspective, it is more effective to vaccinate the population against oncogenic HPV genotypes than to screen and treat cancer. In the United States, vaccination rates range from a high of 92% (District of Columbia) and 89% (Rhode Island) to a low of 47% (Wyoming) and 50% (Kentucky and Mississippi).5 To reduce HPV-associated cancer mortality, the gap in vaccination compliance must be closed.
References
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers - United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018;67:918-924.
- Rosl F, Westphal EM, zur Hausen H. Chromatin structure and transcriptional regulation of human papillomavirus type 18 DNA in HeLa cells. Mol Carcinog. 1989;2:72-80.
- Adey A, Burton JN, Kitzman, et al. The haplotype-resolved genome and epigenome of the aneuploid HeLa cancer cell line. Nature. 2013;500:207-211.
- Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
- Hammer A, Kahlert J, Gravitt PE, et al. Hysterectomy-corrected cervical cancer mortality rates in Denmark during 2002-2015: a registry-based cohort study. Acta Obstet Gynecol Scand. 2019;98:1063-1069.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128:e111-30.
- Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Stang A, Hawk H, Knowlton R, et al. Hysterectomy-corrected incidence rates of cervical and uterine cancers in Massachusetts, 1995-2010. Ann Epidemiol. 2014;24:849-854.
- Hallowell BD, Endeshaw M, McKenna MT, et al. Cervical cancer death rates among U.S.- and foreign-born women: U.S., 2005-2014. Am J Prev Med. 2019;56:869-874.
- Lindström AK, Hermansson RS, Gustavsson I, et al. Cervical dysplasia in elderly women performing repeated self-sampling for HPV testing. PLoS One. 2018;13:e0207714.
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Andersen B, Christensen BS, Christensen J, et al. HPV-prevalence in elderly women in Denmark. Gynecol Oncol. 2019;154:118-123.
- Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: role of viral latency. Viruses. 2017;9:E267.
- Hinten F, Hilbrands LB, Meeuwis KAP, et al. Reactivation of latent HPV infections after renal transplantation. Am J Transplant. 2017;17:1563-1573.
- Leonard SM, Pereira M, Roberts S, et al. Evidence of disrupted high-risk human papillomavirus DNA in morphologically normal cervices of older women. Sci Rep. 2016;6:20847.
- Cervical cancer screening. Cancer Council website. https://www.cancer.org.au/about-cancer/early-detection/screening-programs/cervical-cancer-screening.html. Updated March 15, 2019. Accessed July 23, 2019.
- Hammer A, Kahlert J, Gravitt PE, et al. Hysterectomy-corrected cervical cancer mortality rates in Denmark during 2002-2015: a registry-based cohort study. Acta Obstet Gynecol Scand. 2019;98:1063-1069.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128:e111-30.
- Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Stang A, Hawk H, Knowlton R, et al. Hysterectomy-corrected incidence rates of cervical and uterine cancers in Massachusetts, 1995-2010. Ann Epidemiol. 2014;24:849-854.
- Hallowell BD, Endeshaw M, McKenna MT, et al. Cervical cancer death rates among U.S.- and foreign-born women: U.S., 2005-2014. Am J Prev Med. 2019;56:869-874.
- Lindström AK, Hermansson RS, Gustavsson I, et al. Cervical dysplasia in elderly women performing repeated self-sampling for HPV testing. PLoS One. 2018;13:e0207714.
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Andersen B, Christensen BS, Christensen J, et al. HPV-prevalence in elderly women in Denmark. Gynecol Oncol. 2019;154:118-123.
- Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: role of viral latency. Viruses. 2017;9:E267.
- Hinten F, Hilbrands LB, Meeuwis KAP, et al. Reactivation of latent HPV infections after renal transplantation. Am J Transplant. 2017;17:1563-1573.
- Leonard SM, Pereira M, Roberts S, et al. Evidence of disrupted high-risk human papillomavirus DNA in morphologically normal cervices of older women. Sci Rep. 2016;6:20847.
- Cervical cancer screening. Cancer Council website. https://www.cancer.org.au/about-cancer/early-detection/screening-programs/cervical-cancer-screening.html. Updated March 15, 2019. Accessed July 23, 2019.
Uterus-sparing interventions to treat postpartum hemorrhage during cesarean delivery surgery
Postpartum blood loss greater than 1,000 mL occurs in approximately 7% of cesarean delivery (CD) procedures with the administration of oxytocin alone or oxytocin plus misoprostol.1 Rapid identification and control of hemorrhage is essential to avoid escalating coagulopathy and maternal instability. In cases of excess blood loss, clinicians request assistance from colleagues, endeavor to identify the cause of the bleeding, utilize additional uterotonics (methylergonovine, carboprost, misoprostol), perform uterine massage, warm the uterus, repair lacerations and replace blood products. If blood loss continues after these initial measures, obstetricians may consider uterine artery embolization (UAE) or hysterectomy. While UAE is a highly effective measure to control postpartum hemorrhage, it is not available at all obstetric hospitals. Even when available, there may be a significant time delay from the decision to consult an interventional radiologist to completion of the embolization procedure.
To avoid the permanent sterilization of a hysterectomy, or to obtain time for UAE or correction of coagulopathy, additional uterus-sparing surgical interventions should be considered. These include: 1) progressive uterine devascularization, 2) uterine compression sutures, and 3) intrauterine balloon tamponade. One caveat is that there is very little high-quality evidence from randomized trials to compare the efficacy or outcome of these uterine-sparing surgical interventions. Most of our evidence is based on limited case series and expert recommendations.
Uterine devascularization
Many techniques have been described for performing progressive uterine devascularization. Most experts recommend first performing an O’Leary suture, ligating both ascending uterine arteries and accompanying veins at a point approximately 2 cm closer to the cervix than the uterine incision (FIGURE 1). An absorbable suture is passed through the myometrium, being sure to remain medial to the ascending uterine vessels. Clear visualization of the vessels posteriorly is essential, usually necessitating exteriorization of the uterus. The needle is then driven through an avascular space in the broad ligament close to the uterine vessels, and the suture is tied down. Ureteral injury can be avoided by extending the bladder flap laterally to the level of the round ligament and mobilizing the vesicouterine peritoneum inferiorly, with the suture placed directly on endopelvic fascia. If necessary, the utero-ovarian ligament can be ligated in a second step, just below the uterine-tubal junction. The progressive devascularization intervention can be limited to the first or second steps if bleeding is well controlled.

In our experience, bilateral O’Leary sutures are highly effective at controlling ongoing uterine bleeding, particularly from the lower uterine segment. In the event that they are not successful, placement does not preclude later use of UAE.
Uterine compression sutures
Compression sutures are most often used in the setting of refractory uterine atony. They also may be helpful for controlling focal atony or bleeding from a placental implantation site. More than a dozen different types of uterine compression sutures have been reported in the literature; the B-Lynch, Hyman, and Pereira sutures are most commonly performed.2
Continue to: The B-Lynch suture3 is performed with...
The B-Lynch suture3 is performed with a long, rapidly absorbable suture on a large needle (FIGURE 2). We use a 60-inch #1 or #2 chromic suture on a TP-1 needle in the following steps:
- Take bites on either side of the right edge of the hysterotomy incision (A and B). Place these bites approximately 3 cm from the edge of the hysterotomy incision.
- Loop the suture around the fundus and reenter the uterus through the posterior uterine wall at point C, which is directly posterior to point B.
- Exit the posterior wall of the uterus through point D.
- Loop the suture over the uterine fundus.
- Anchor the suture in the lower uterine segment by taking bites on either side of the left edge of the uterine hysterotomy incision (points E and F).
- Pull the two ends of the suture tight while an assistant squeezes the uterus to aid compression.
- Place a surgical knot to secure the suture.
- Close the hysterotomy incision.
The B-Lynch suture was described with an open hysterotomy incision,3 which avoids closing off the lower uterine segment. We have successfully performed a modific tion on a closed uterus, taking care to not drive the lower uterine sutures through both the anterior and posterior walls.

The Hayman suture4 was proposed with two important modifications: The suture is placed through-and-through the lower uterine segment with a closed hysterotomy, and the suture can be fixed to the uterine fundus to avoid slippage. This vertical compression suture (FIGURE 3) is performed by placing two to four vertical #2 chromic sutures directly through the anterior to posterior uterine wall, tying the suture on the fundus using a 3-throw technique to minimize slippage of the first knot. In the original description, Hayman also described injecting carboprost into the uterine fundus to stimulate uterine contraction and regularly inspecting the vagina to evaluate the extent of continued bleeding.4

The Pereira sutures,5 also described on a closed uterus, combine vertical and horizontal sutures placed as a series of bites into the submucosal myometrium using #1 polyglactin 910 (Vicryl) sutures (FIGURE 4). The sutures do not enter the uterine cavity. Two to three transverse sutures are initially placed followed by two vertical sutures. When placing the transverse sutures, it is important to cross the broad ligament in an avascular area and avoid trauma to blood vessels, ureters, gonadal vessels and fallopian tubes. The vertical sutures begin and end at the level of the transverse suture closest to the cervix.

Intrauterine balloon tamponade
Many types of balloon tamponade devices have been developed, ranging from the humble condom tied to a Foley urinary catheter to the sophisticated Bakri6,7 and Belfort-Dildy8 balloon tamponade devices. Intrauterine balloon tamponade is highly effective in controlling excess bleeding following vaginal delivery and less effective when used following a CD. In one study of 226 women with postpartum hemorrhage treated with a Bakri balloon the success rate was 89% and 66% following vaginal delivery and CD, respectively.9
Continue to: When using balloon tamponade during a CD...
When using balloon tamponade during a CD, some experts recommend partially closing the transverse hysterotomy incision by placing sutures to close edges of the hysterotomy, followed by insertion of the balloon into the uterus and the stem through the cervix into the vagina. Attachment of the stem to a collection bag should help to quickly assess the rate of blood loss. The balloon is inflated after the hysterotomy is closed. Following inflation of an intrauterine balloon, blood loss should decrease almost immediately.10 If excessive blood loss continues for more than 10 minutes, additional uterus-sparing interventions or hysterectomy may be required. Following successful balloon tamponade, the balloon may be deflated 12 to 24 hours postpartum when maternal stabilization and normal coagulation have been achieved. If bleeding resumes, the balloon may be reinflated and UAE should be considered.
Combined interventions: Uterine devascularization plus uterine compression sutures
There are no high-quality randomized trials comparing the devascularization plus compression sutures versus a single intervention alone, and case series and case reports on this topic are lacking. If uterine devascularization alone does not sufficiently control bleeding, adding a uterine compression stitch might resolve the hemorrhage. Both procedures require only suture material, which is immediately available in all operating rooms. Hence, this combination of interventions can be executed quickly.
Uterine sandwich: Intrauterine balloon tamponade plus uterine compression sutures
CD for placenta previa is associated with an increased risk of postpartum hemorrhage, with bleeding from the lower uterine segment greatly contributing to total blood loss. While O’Leary sutures can stem the flow of bleeding in this area, the use of both an intrauterine balloon tamponade plus uterine compression sutures—a so-called uterine sandwich—may result in maximal reduction in blood loss.11,12
In one randomized trial, 106 women undergoing CD for a placenta previa were randomly assigned to uterine devascularization alone or double transverse compression suture at the lower uterine segment plus intrauterine Foley catheter balloon. Compared with women receiving devascularization alone, the combination of compression suture plus intrauterine balloon significantly reduced blood loss (1,350 mL vs 750 mL, respectively; P = .0001).13
Underutilization of uterine-sparing interventions
In a nationwide study of 50 consecutive Danish peripartum hysterectomy cases, an audit committee concluded that 24% of the hysterectomies could have been avoided, and an additional 30% of hysterectomies might have been avoided, if uterine-sparing surgical interventions had been utilized.14 In a recent survey of senior ObGyn residents in France, greater than 70% of respondents reported that they had not mastered uterine-sparing techniques of uterine devascularization and compression sutures, nor peripartum hysterectomy.15 Together, these studies suggest that uterine-sparing interventions are underutilized and that with more training and practice clinicians would become facile with these interventions.
The cornerstones of uterine-sparing surgical interventions are simplicity, safety, and efficacy. If a combination of pharmacologic and multiple uterine-sparing surgical interventions do not control the bleeding, the patient may need an emergency hysterectomy or, if stable, a UAE. While devascularization and compression sutures are described during CD, it is reasonable to use them after vaginal delivery if the next reasonable step would be a laparotomy. When you next face the clinical challenge of a postpartum hemorrhage, rapid recognition of excess blood loss, early identification of the cause, swift pharmacologic treatment, and timely escalation of surgical interventions will help you reduce the risk of hysterectomy and severe maternal morbidity.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database of Syst Rev. 2018;12:CD011689.
- Li GT, Li XF, Wu BP, et al. Three cornerstones of uterine compression sutures: simplicity, safety, and efficacy. Arch Gynecol Obstet. 2015;292:949-952.
- B-Lynch C, Coker A, Lawal AH, et al. The B-Lynch surgical technique for the control of massive postpartum hemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104:372-375.
- Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstet Gynecol. 2002;99:502-506.
- Pereira A, Nunes F, Pedroso S, et al. Compressive sutures to treat postpartum bleeding secondary to uterine atony. Obstet Gynecol. 2005;106:569-572.
- Bakri YN. Uterine tamponade-drain for hemorrhage secondary to placenta previa-accreta. Int J Gynaecol Obstet. 1992;37:302-303.
- Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139-142.
- Dildy GA, Belfort MA, Adair CD, et al; ebb Surveillance Study Team. Initial experience with a dual-balloon catheter for the management of postpartum hemorrhage. Am J Obstet Gynecol. 2014;210:136.e1-e6.
- Revert M, Cottenet J, Raynal P, et al. Intrauterine balloon tamponade for management of severe postpartum hemorrhage in a perinatal network: a prospective cohort study. BJOG. 2017;124:1255-1262.
- Condous GS, Arulkumaran S, Symonds I, et al. The “tamponade test” in the management of massive postpartum hemorrhage. Obstet Gynecol. 2003;101:767-772.
- Nelson WL, O’Brien JM. The uterine sandwich for persistent uterine atony: combining the B-Lynch compression suture and an intrauterine Bakri balloon. Am J Obstet Gynecol. 2007;196:e9-e10.
- Matsubara S, Kuwata T, Baba Y, et al. A novel “uterine sandwich” for haemorrhage at cesarean section for placenta praevia. Aust N Z J Obstet Gynaecol. 2014;54:283-286.
- Sallam HF, Shady NW. A sandwich technique (N&H variation technique) to reduce blood loss during cesarean delivery for complete placenta previa: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-8.
- Colmorn LB, Krebs L, Langhoff-Roos J; NOSS study group. Potentially avoidable peripartum hysterectomies in Denmark: a population based clinical audit. PLoS One. 2016;11:e0161302.
- Bouet PE, Madar H, Froeliger A, et al. Surgical treatment of postpartum haemorrhage: national survey of French residents in obstetrics and gynecology. BMC Pregnancy Childbirth. 2019;19:91.
Postpartum blood loss greater than 1,000 mL occurs in approximately 7% of cesarean delivery (CD) procedures with the administration of oxytocin alone or oxytocin plus misoprostol.1 Rapid identification and control of hemorrhage is essential to avoid escalating coagulopathy and maternal instability. In cases of excess blood loss, clinicians request assistance from colleagues, endeavor to identify the cause of the bleeding, utilize additional uterotonics (methylergonovine, carboprost, misoprostol), perform uterine massage, warm the uterus, repair lacerations and replace blood products. If blood loss continues after these initial measures, obstetricians may consider uterine artery embolization (UAE) or hysterectomy. While UAE is a highly effective measure to control postpartum hemorrhage, it is not available at all obstetric hospitals. Even when available, there may be a significant time delay from the decision to consult an interventional radiologist to completion of the embolization procedure.
To avoid the permanent sterilization of a hysterectomy, or to obtain time for UAE or correction of coagulopathy, additional uterus-sparing surgical interventions should be considered. These include: 1) progressive uterine devascularization, 2) uterine compression sutures, and 3) intrauterine balloon tamponade. One caveat is that there is very little high-quality evidence from randomized trials to compare the efficacy or outcome of these uterine-sparing surgical interventions. Most of our evidence is based on limited case series and expert recommendations.
Uterine devascularization
Many techniques have been described for performing progressive uterine devascularization. Most experts recommend first performing an O’Leary suture, ligating both ascending uterine arteries and accompanying veins at a point approximately 2 cm closer to the cervix than the uterine incision (FIGURE 1). An absorbable suture is passed through the myometrium, being sure to remain medial to the ascending uterine vessels. Clear visualization of the vessels posteriorly is essential, usually necessitating exteriorization of the uterus. The needle is then driven through an avascular space in the broad ligament close to the uterine vessels, and the suture is tied down. Ureteral injury can be avoided by extending the bladder flap laterally to the level of the round ligament and mobilizing the vesicouterine peritoneum inferiorly, with the suture placed directly on endopelvic fascia. If necessary, the utero-ovarian ligament can be ligated in a second step, just below the uterine-tubal junction. The progressive devascularization intervention can be limited to the first or second steps if bleeding is well controlled.

In our experience, bilateral O’Leary sutures are highly effective at controlling ongoing uterine bleeding, particularly from the lower uterine segment. In the event that they are not successful, placement does not preclude later use of UAE.
Uterine compression sutures
Compression sutures are most often used in the setting of refractory uterine atony. They also may be helpful for controlling focal atony or bleeding from a placental implantation site. More than a dozen different types of uterine compression sutures have been reported in the literature; the B-Lynch, Hyman, and Pereira sutures are most commonly performed.2
Continue to: The B-Lynch suture3 is performed with...
The B-Lynch suture3 is performed with a long, rapidly absorbable suture on a large needle (FIGURE 2). We use a 60-inch #1 or #2 chromic suture on a TP-1 needle in the following steps:
- Take bites on either side of the right edge of the hysterotomy incision (A and B). Place these bites approximately 3 cm from the edge of the hysterotomy incision.
- Loop the suture around the fundus and reenter the uterus through the posterior uterine wall at point C, which is directly posterior to point B.
- Exit the posterior wall of the uterus through point D.
- Loop the suture over the uterine fundus.
- Anchor the suture in the lower uterine segment by taking bites on either side of the left edge of the uterine hysterotomy incision (points E and F).
- Pull the two ends of the suture tight while an assistant squeezes the uterus to aid compression.
- Place a surgical knot to secure the suture.
- Close the hysterotomy incision.
The B-Lynch suture was described with an open hysterotomy incision,3 which avoids closing off the lower uterine segment. We have successfully performed a modific tion on a closed uterus, taking care to not drive the lower uterine sutures through both the anterior and posterior walls.

The Hayman suture4 was proposed with two important modifications: The suture is placed through-and-through the lower uterine segment with a closed hysterotomy, and the suture can be fixed to the uterine fundus to avoid slippage. This vertical compression suture (FIGURE 3) is performed by placing two to four vertical #2 chromic sutures directly through the anterior to posterior uterine wall, tying the suture on the fundus using a 3-throw technique to minimize slippage of the first knot. In the original description, Hayman also described injecting carboprost into the uterine fundus to stimulate uterine contraction and regularly inspecting the vagina to evaluate the extent of continued bleeding.4

The Pereira sutures,5 also described on a closed uterus, combine vertical and horizontal sutures placed as a series of bites into the submucosal myometrium using #1 polyglactin 910 (Vicryl) sutures (FIGURE 4). The sutures do not enter the uterine cavity. Two to three transverse sutures are initially placed followed by two vertical sutures. When placing the transverse sutures, it is important to cross the broad ligament in an avascular area and avoid trauma to blood vessels, ureters, gonadal vessels and fallopian tubes. The vertical sutures begin and end at the level of the transverse suture closest to the cervix.

Intrauterine balloon tamponade
Many types of balloon tamponade devices have been developed, ranging from the humble condom tied to a Foley urinary catheter to the sophisticated Bakri6,7 and Belfort-Dildy8 balloon tamponade devices. Intrauterine balloon tamponade is highly effective in controlling excess bleeding following vaginal delivery and less effective when used following a CD. In one study of 226 women with postpartum hemorrhage treated with a Bakri balloon the success rate was 89% and 66% following vaginal delivery and CD, respectively.9
Continue to: When using balloon tamponade during a CD...
When using balloon tamponade during a CD, some experts recommend partially closing the transverse hysterotomy incision by placing sutures to close edges of the hysterotomy, followed by insertion of the balloon into the uterus and the stem through the cervix into the vagina. Attachment of the stem to a collection bag should help to quickly assess the rate of blood loss. The balloon is inflated after the hysterotomy is closed. Following inflation of an intrauterine balloon, blood loss should decrease almost immediately.10 If excessive blood loss continues for more than 10 minutes, additional uterus-sparing interventions or hysterectomy may be required. Following successful balloon tamponade, the balloon may be deflated 12 to 24 hours postpartum when maternal stabilization and normal coagulation have been achieved. If bleeding resumes, the balloon may be reinflated and UAE should be considered.
Combined interventions: Uterine devascularization plus uterine compression sutures
There are no high-quality randomized trials comparing the devascularization plus compression sutures versus a single intervention alone, and case series and case reports on this topic are lacking. If uterine devascularization alone does not sufficiently control bleeding, adding a uterine compression stitch might resolve the hemorrhage. Both procedures require only suture material, which is immediately available in all operating rooms. Hence, this combination of interventions can be executed quickly.
Uterine sandwich: Intrauterine balloon tamponade plus uterine compression sutures
CD for placenta previa is associated with an increased risk of postpartum hemorrhage, with bleeding from the lower uterine segment greatly contributing to total blood loss. While O’Leary sutures can stem the flow of bleeding in this area, the use of both an intrauterine balloon tamponade plus uterine compression sutures—a so-called uterine sandwich—may result in maximal reduction in blood loss.11,12
In one randomized trial, 106 women undergoing CD for a placenta previa were randomly assigned to uterine devascularization alone or double transverse compression suture at the lower uterine segment plus intrauterine Foley catheter balloon. Compared with women receiving devascularization alone, the combination of compression suture plus intrauterine balloon significantly reduced blood loss (1,350 mL vs 750 mL, respectively; P = .0001).13
Underutilization of uterine-sparing interventions
In a nationwide study of 50 consecutive Danish peripartum hysterectomy cases, an audit committee concluded that 24% of the hysterectomies could have been avoided, and an additional 30% of hysterectomies might have been avoided, if uterine-sparing surgical interventions had been utilized.14 In a recent survey of senior ObGyn residents in France, greater than 70% of respondents reported that they had not mastered uterine-sparing techniques of uterine devascularization and compression sutures, nor peripartum hysterectomy.15 Together, these studies suggest that uterine-sparing interventions are underutilized and that with more training and practice clinicians would become facile with these interventions.
The cornerstones of uterine-sparing surgical interventions are simplicity, safety, and efficacy. If a combination of pharmacologic and multiple uterine-sparing surgical interventions do not control the bleeding, the patient may need an emergency hysterectomy or, if stable, a UAE. While devascularization and compression sutures are described during CD, it is reasonable to use them after vaginal delivery if the next reasonable step would be a laparotomy. When you next face the clinical challenge of a postpartum hemorrhage, rapid recognition of excess blood loss, early identification of the cause, swift pharmacologic treatment, and timely escalation of surgical interventions will help you reduce the risk of hysterectomy and severe maternal morbidity.
Postpartum blood loss greater than 1,000 mL occurs in approximately 7% of cesarean delivery (CD) procedures with the administration of oxytocin alone or oxytocin plus misoprostol.1 Rapid identification and control of hemorrhage is essential to avoid escalating coagulopathy and maternal instability. In cases of excess blood loss, clinicians request assistance from colleagues, endeavor to identify the cause of the bleeding, utilize additional uterotonics (methylergonovine, carboprost, misoprostol), perform uterine massage, warm the uterus, repair lacerations and replace blood products. If blood loss continues after these initial measures, obstetricians may consider uterine artery embolization (UAE) or hysterectomy. While UAE is a highly effective measure to control postpartum hemorrhage, it is not available at all obstetric hospitals. Even when available, there may be a significant time delay from the decision to consult an interventional radiologist to completion of the embolization procedure.
To avoid the permanent sterilization of a hysterectomy, or to obtain time for UAE or correction of coagulopathy, additional uterus-sparing surgical interventions should be considered. These include: 1) progressive uterine devascularization, 2) uterine compression sutures, and 3) intrauterine balloon tamponade. One caveat is that there is very little high-quality evidence from randomized trials to compare the efficacy or outcome of these uterine-sparing surgical interventions. Most of our evidence is based on limited case series and expert recommendations.
Uterine devascularization
Many techniques have been described for performing progressive uterine devascularization. Most experts recommend first performing an O’Leary suture, ligating both ascending uterine arteries and accompanying veins at a point approximately 2 cm closer to the cervix than the uterine incision (FIGURE 1). An absorbable suture is passed through the myometrium, being sure to remain medial to the ascending uterine vessels. Clear visualization of the vessels posteriorly is essential, usually necessitating exteriorization of the uterus. The needle is then driven through an avascular space in the broad ligament close to the uterine vessels, and the suture is tied down. Ureteral injury can be avoided by extending the bladder flap laterally to the level of the round ligament and mobilizing the vesicouterine peritoneum inferiorly, with the suture placed directly on endopelvic fascia. If necessary, the utero-ovarian ligament can be ligated in a second step, just below the uterine-tubal junction. The progressive devascularization intervention can be limited to the first or second steps if bleeding is well controlled.

In our experience, bilateral O’Leary sutures are highly effective at controlling ongoing uterine bleeding, particularly from the lower uterine segment. In the event that they are not successful, placement does not preclude later use of UAE.
Uterine compression sutures
Compression sutures are most often used in the setting of refractory uterine atony. They also may be helpful for controlling focal atony or bleeding from a placental implantation site. More than a dozen different types of uterine compression sutures have been reported in the literature; the B-Lynch, Hyman, and Pereira sutures are most commonly performed.2
Continue to: The B-Lynch suture3 is performed with...
The B-Lynch suture3 is performed with a long, rapidly absorbable suture on a large needle (FIGURE 2). We use a 60-inch #1 or #2 chromic suture on a TP-1 needle in the following steps:
- Take bites on either side of the right edge of the hysterotomy incision (A and B). Place these bites approximately 3 cm from the edge of the hysterotomy incision.
- Loop the suture around the fundus and reenter the uterus through the posterior uterine wall at point C, which is directly posterior to point B.
- Exit the posterior wall of the uterus through point D.
- Loop the suture over the uterine fundus.
- Anchor the suture in the lower uterine segment by taking bites on either side of the left edge of the uterine hysterotomy incision (points E and F).
- Pull the two ends of the suture tight while an assistant squeezes the uterus to aid compression.
- Place a surgical knot to secure the suture.
- Close the hysterotomy incision.
The B-Lynch suture was described with an open hysterotomy incision,3 which avoids closing off the lower uterine segment. We have successfully performed a modific tion on a closed uterus, taking care to not drive the lower uterine sutures through both the anterior and posterior walls.

The Hayman suture4 was proposed with two important modifications: The suture is placed through-and-through the lower uterine segment with a closed hysterotomy, and the suture can be fixed to the uterine fundus to avoid slippage. This vertical compression suture (FIGURE 3) is performed by placing two to four vertical #2 chromic sutures directly through the anterior to posterior uterine wall, tying the suture on the fundus using a 3-throw technique to minimize slippage of the first knot. In the original description, Hayman also described injecting carboprost into the uterine fundus to stimulate uterine contraction and regularly inspecting the vagina to evaluate the extent of continued bleeding.4

The Pereira sutures,5 also described on a closed uterus, combine vertical and horizontal sutures placed as a series of bites into the submucosal myometrium using #1 polyglactin 910 (Vicryl) sutures (FIGURE 4). The sutures do not enter the uterine cavity. Two to three transverse sutures are initially placed followed by two vertical sutures. When placing the transverse sutures, it is important to cross the broad ligament in an avascular area and avoid trauma to blood vessels, ureters, gonadal vessels and fallopian tubes. The vertical sutures begin and end at the level of the transverse suture closest to the cervix.

Intrauterine balloon tamponade
Many types of balloon tamponade devices have been developed, ranging from the humble condom tied to a Foley urinary catheter to the sophisticated Bakri6,7 and Belfort-Dildy8 balloon tamponade devices. Intrauterine balloon tamponade is highly effective in controlling excess bleeding following vaginal delivery and less effective when used following a CD. In one study of 226 women with postpartum hemorrhage treated with a Bakri balloon the success rate was 89% and 66% following vaginal delivery and CD, respectively.9
Continue to: When using balloon tamponade during a CD...
When using balloon tamponade during a CD, some experts recommend partially closing the transverse hysterotomy incision by placing sutures to close edges of the hysterotomy, followed by insertion of the balloon into the uterus and the stem through the cervix into the vagina. Attachment of the stem to a collection bag should help to quickly assess the rate of blood loss. The balloon is inflated after the hysterotomy is closed. Following inflation of an intrauterine balloon, blood loss should decrease almost immediately.10 If excessive blood loss continues for more than 10 minutes, additional uterus-sparing interventions or hysterectomy may be required. Following successful balloon tamponade, the balloon may be deflated 12 to 24 hours postpartum when maternal stabilization and normal coagulation have been achieved. If bleeding resumes, the balloon may be reinflated and UAE should be considered.
Combined interventions: Uterine devascularization plus uterine compression sutures
There are no high-quality randomized trials comparing the devascularization plus compression sutures versus a single intervention alone, and case series and case reports on this topic are lacking. If uterine devascularization alone does not sufficiently control bleeding, adding a uterine compression stitch might resolve the hemorrhage. Both procedures require only suture material, which is immediately available in all operating rooms. Hence, this combination of interventions can be executed quickly.
Uterine sandwich: Intrauterine balloon tamponade plus uterine compression sutures
CD for placenta previa is associated with an increased risk of postpartum hemorrhage, with bleeding from the lower uterine segment greatly contributing to total blood loss. While O’Leary sutures can stem the flow of bleeding in this area, the use of both an intrauterine balloon tamponade plus uterine compression sutures—a so-called uterine sandwich—may result in maximal reduction in blood loss.11,12
In one randomized trial, 106 women undergoing CD for a placenta previa were randomly assigned to uterine devascularization alone or double transverse compression suture at the lower uterine segment plus intrauterine Foley catheter balloon. Compared with women receiving devascularization alone, the combination of compression suture plus intrauterine balloon significantly reduced blood loss (1,350 mL vs 750 mL, respectively; P = .0001).13
Underutilization of uterine-sparing interventions
In a nationwide study of 50 consecutive Danish peripartum hysterectomy cases, an audit committee concluded that 24% of the hysterectomies could have been avoided, and an additional 30% of hysterectomies might have been avoided, if uterine-sparing surgical interventions had been utilized.14 In a recent survey of senior ObGyn residents in France, greater than 70% of respondents reported that they had not mastered uterine-sparing techniques of uterine devascularization and compression sutures, nor peripartum hysterectomy.15 Together, these studies suggest that uterine-sparing interventions are underutilized and that with more training and practice clinicians would become facile with these interventions.
The cornerstones of uterine-sparing surgical interventions are simplicity, safety, and efficacy. If a combination of pharmacologic and multiple uterine-sparing surgical interventions do not control the bleeding, the patient may need an emergency hysterectomy or, if stable, a UAE. While devascularization and compression sutures are described during CD, it is reasonable to use them after vaginal delivery if the next reasonable step would be a laparotomy. When you next face the clinical challenge of a postpartum hemorrhage, rapid recognition of excess blood loss, early identification of the cause, swift pharmacologic treatment, and timely escalation of surgical interventions will help you reduce the risk of hysterectomy and severe maternal morbidity.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database of Syst Rev. 2018;12:CD011689.
- Li GT, Li XF, Wu BP, et al. Three cornerstones of uterine compression sutures: simplicity, safety, and efficacy. Arch Gynecol Obstet. 2015;292:949-952.
- B-Lynch C, Coker A, Lawal AH, et al. The B-Lynch surgical technique for the control of massive postpartum hemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104:372-375.
- Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstet Gynecol. 2002;99:502-506.
- Pereira A, Nunes F, Pedroso S, et al. Compressive sutures to treat postpartum bleeding secondary to uterine atony. Obstet Gynecol. 2005;106:569-572.
- Bakri YN. Uterine tamponade-drain for hemorrhage secondary to placenta previa-accreta. Int J Gynaecol Obstet. 1992;37:302-303.
- Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139-142.
- Dildy GA, Belfort MA, Adair CD, et al; ebb Surveillance Study Team. Initial experience with a dual-balloon catheter for the management of postpartum hemorrhage. Am J Obstet Gynecol. 2014;210:136.e1-e6.
- Revert M, Cottenet J, Raynal P, et al. Intrauterine balloon tamponade for management of severe postpartum hemorrhage in a perinatal network: a prospective cohort study. BJOG. 2017;124:1255-1262.
- Condous GS, Arulkumaran S, Symonds I, et al. The “tamponade test” in the management of massive postpartum hemorrhage. Obstet Gynecol. 2003;101:767-772.
- Nelson WL, O’Brien JM. The uterine sandwich for persistent uterine atony: combining the B-Lynch compression suture and an intrauterine Bakri balloon. Am J Obstet Gynecol. 2007;196:e9-e10.
- Matsubara S, Kuwata T, Baba Y, et al. A novel “uterine sandwich” for haemorrhage at cesarean section for placenta praevia. Aust N Z J Obstet Gynaecol. 2014;54:283-286.
- Sallam HF, Shady NW. A sandwich technique (N&H variation technique) to reduce blood loss during cesarean delivery for complete placenta previa: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-8.
- Colmorn LB, Krebs L, Langhoff-Roos J; NOSS study group. Potentially avoidable peripartum hysterectomies in Denmark: a population based clinical audit. PLoS One. 2016;11:e0161302.
- Bouet PE, Madar H, Froeliger A, et al. Surgical treatment of postpartum haemorrhage: national survey of French residents in obstetrics and gynecology. BMC Pregnancy Childbirth. 2019;19:91.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database of Syst Rev. 2018;12:CD011689.
- Li GT, Li XF, Wu BP, et al. Three cornerstones of uterine compression sutures: simplicity, safety, and efficacy. Arch Gynecol Obstet. 2015;292:949-952.
- B-Lynch C, Coker A, Lawal AH, et al. The B-Lynch surgical technique for the control of massive postpartum hemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104:372-375.
- Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstet Gynecol. 2002;99:502-506.
- Pereira A, Nunes F, Pedroso S, et al. Compressive sutures to treat postpartum bleeding secondary to uterine atony. Obstet Gynecol. 2005;106:569-572.
- Bakri YN. Uterine tamponade-drain for hemorrhage secondary to placenta previa-accreta. Int J Gynaecol Obstet. 1992;37:302-303.
- Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139-142.
- Dildy GA, Belfort MA, Adair CD, et al; ebb Surveillance Study Team. Initial experience with a dual-balloon catheter for the management of postpartum hemorrhage. Am J Obstet Gynecol. 2014;210:136.e1-e6.
- Revert M, Cottenet J, Raynal P, et al. Intrauterine balloon tamponade for management of severe postpartum hemorrhage in a perinatal network: a prospective cohort study. BJOG. 2017;124:1255-1262.
- Condous GS, Arulkumaran S, Symonds I, et al. The “tamponade test” in the management of massive postpartum hemorrhage. Obstet Gynecol. 2003;101:767-772.
- Nelson WL, O’Brien JM. The uterine sandwich for persistent uterine atony: combining the B-Lynch compression suture and an intrauterine Bakri balloon. Am J Obstet Gynecol. 2007;196:e9-e10.
- Matsubara S, Kuwata T, Baba Y, et al. A novel “uterine sandwich” for haemorrhage at cesarean section for placenta praevia. Aust N Z J Obstet Gynaecol. 2014;54:283-286.
- Sallam HF, Shady NW. A sandwich technique (N&H variation technique) to reduce blood loss during cesarean delivery for complete placenta previa: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-8.
- Colmorn LB, Krebs L, Langhoff-Roos J; NOSS study group. Potentially avoidable peripartum hysterectomies in Denmark: a population based clinical audit. PLoS One. 2016;11:e0161302.
- Bouet PE, Madar H, Froeliger A, et al. Surgical treatment of postpartum haemorrhage: national survey of French residents in obstetrics and gynecology. BMC Pregnancy Childbirth. 2019;19:91.
One versus two uterotonics: Which is better for minimizing postpartum blood loss?
Excessive postpartum bleeding is a major cause of maternal morbidity and mortality. Worldwide, obstetric hemorrhage is the most common cause of maternal death.1,2 Medications reported to reduce postpartum bleeding include oxytocin, misoprostol, ergonovine, methylergonovine, carboprost, and tranexamic acid. A recent Cochrane network meta-analysis of 196 trials, including 135,559 women, distilled in 1,361 pages of analysis, reported on the medications associated with the greatest reduction in postpartum bleeding.3 Surprisingly, for preventing blood loss ≥ 500 mL, misoprostol plus oxytocin and ergonovine plus oxytocin were the highest ranked interventions. This evidence is summarized here.
Misoprostol plus oxytocin
After newborn delivery, active management of the third stage of labor, including uterotonic administration, is strongly recommended because it will reduce postpartum blood loss, decreasing the rate of postpartum hemorrhage (PPH).4 Both oxytocin and misoprostol are effective uterotonics. However, the combination of oxytocin plus misoprostol appears to be more effective than oxytocin alone in reducing the frequency of postpartum blood loss greater than 500 mL.3 To understand the clinical efficacy and adverse effects (AEs) of combined oxytocin plus misoprostol a meta-analysis was performed for both vaginal and cesarean deliveries (CDs).
Efficacy and AEs during vaginal delivery. In the meta-analysis, about 6,000 vaginal deliveries were analyzed, with no significant differences for misoprostol plus oxytocin versus oxytocin alone found for the following outcomes: maternal death, intensive care unit admissions, and rate of blood loss ≥ 1,000 mL (1.7% for both uterotonics vs 2.2% for oxytocin alone).3 Misoprostol plus oxytocin was significantly superior to oxytocin alone for the following outcomes: reduced risk of blood transfusion (0.95% vs 2.5%), reduced risk of blood loss ≥ 500 mL (5.9% vs 8.0%), reduced risk of requiring an additional uterotonic (3.6% vs 5.8%), and a smaller decrease in hemoglobin concentration from pre- to postdelivery (-0.89 g/L).3
In my opinion, the difference in hemoglobin concentration, although statistically significant, is not of clinical significance. However, compared with oxytocin alone, misoprostol plus oxytocin caused significantly more nausea (2.4% vs 0.66%), vomiting (3.1% vs 0.86%), and fever (21% vs 3.9%).3 A weakness of this meta-analysis is that the trials used a wide range of misoprostol dosages (200 to 600 µg) and multiple routes of administration, including sublingual (under the tongue), buccal, and rectal. This makes it impossible to identify a best misoprostol dosage and administration route.
Efficacy and AEs during CD. In the same meta-analysis about 2,000 CDs were analyzed, with no significant difference for misoprostol plus oxytocin versus oxytocin alone for the following outcomes: maternal death, intensive care unit admissions, and PPH ≥ 1,000 mL blood loss (6.2% vs 6.5%).3 Misoprostol plus oxytocin was significantly superior to oxytocin alone for the following outcomes: reduced risk of blood transfusion (2.6% vs 5.4%), reduced risk of blood loss ≥ 500 mL (32% vs 47%), reduced risk of requiring an additional uterotonic (14% vs 28%), and a smaller decrease in hemoglobin concentration from before to after delivery (-4.0 g/L).3 In my opinion, the statistically significant difference in hemoglobin concentration is not clinically significant. However, compared with oxytocin alone, misoprostol plus oxytocin caused significantly more nausea (12% vs 6.1%), vomiting (8.1% vs 5.4%), shivering (13% vs 7%), and fever (7.7% vs 4.0%).3
Continue to: Ergonovine plus oxytocin...
Ergonovine plus oxytocin
Ergonovine is an ergot derivative that causes uterine contractions and has been shown to effectively reduce blood loss at delivery. In the United States a methyl-derivative of ergonovine, methylergonovine, is widely available. In a meta-analysis with mostly vaginal deliveries, there were no significant differences for ergonovine plus oxytocin versus oxytocin alone for the following outcomes: death, intensive care unit admission, rate of blood loss ≥ 1,000 mL(2.0% vs 2.7%), blood transfusion, administration of an additional uterotonic, change in hemoglobin from pre- to postdelivery, nausea, hypertension, shivering, and fever.3 However, ergonovine plus oxytocin, compared with oxytocin alone, resulted in a significantly reduced rate of blood loss ≥ 500 mL (8.3% vs 10.2%) and an increased rate of vomiting (8.1% vs 1.6%).3 In these trials women with a blood pressure ≥ 150/100 mm Hg were generally excluded from receiving ergonovine because of its hypertensive effect.
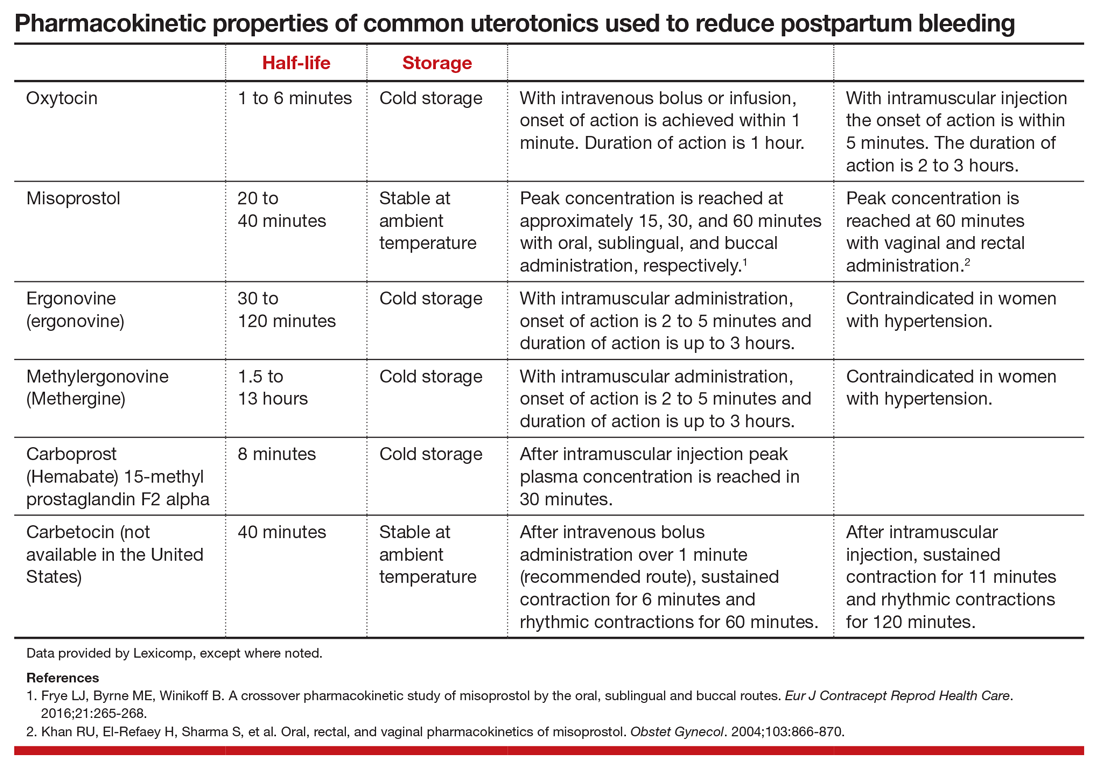
Clinical practice options
Given the Cochrane meta-analysis results, ObGyns have two approaches for optimizing PPH reduction.
Option 1: Use a single uterotonic to reduce postpartum blood loss. If excess bleeding occurs, rapidly administer a second uterotonic agent. Currently, monotherapy with intravenous or intramuscular oxytocin is the standard for reducing postpartum blood loss.5,6 Advantages of this approach compared with dual agent therapy include simplification of care and minimization of AEs. However, oxytocin monotherapy for minimizing postpartum bleeding may be suboptimal. In the largest trial ever performed (involving 29,645 women) when oxytocin was administered postpartum, the rates of estimated blood loss ≥ 500 mL and ≥ 1,000 mL were 9.1% and 1.45%, respectively.5 Is 9% an optimal rate for blood loss ≥ 500 mL following a vaginal delivery? Or should we try to achieve a lower rate?
Given the “high” rate of blood loss ≥ 500 mL with oxytocin alone, it is important for clinicians using the one-uterotonic approach to promptly recognize patients who have excessive bleeding and transition rapidly from prevention to treatment. When PPH cases are reviewed, a common finding is that the clinicians did not timely recognize excess bleeding, delaying transition to treatment with additional uterotonics and other interventions. When routinely using oxytocin monotherapy, lowering the threshold for administering a second uterotonic (methylergonovine, carboprost, misoprostol, or tranexamic acid) may help decrease the frequency of excess postpartum blood loss.
Option 2: Administer two uterotonics to reduce postpartum blood loss at all deliveries. Given the “high” rate of excess postpartum blood loss with oxytocin monotherapy, an alternative is to administer two uterotonics at all births or at births with a high risk of excess blood loss. As discussed, administering two uterotonics, oxytocin plus misoprostol or oxytocin plus ergonovine, has been reported to be more effective than oxytocin alone for reducing postpartum bleeding ≥ 500 mL.3 In the Cochrane meta-analysis, per 1,000 women given oxytocin following a vaginal birth, 122 would have blood loss ≥ 500 mL, compared with 85 given oxytocin plus misoprostol or oxytocin plus ergonovine.3
Misoprostol is administered sublingually, buccally, or rectally, and methylergonovine is administered by intramuscular injection. Although dual uterotonic therapy is more effective than monotherapy, dual therapy is associated with more AEs. As noted, compared with oxytocin monotherapy, the combination of oxytocin plus misoprostol is associated with more nausea, vomiting, shivering, and fever. Oxytocin plus ergonovine is associated with a higher rate of vomiting than oxytocin monotherapy. In my practice I prefer using intramuscular methylergonovine as the second agent to avoid the high rate of fever associated with misoprostol.
For dual agent therapy, one approach is to administer misoprostol 200 µg or 400 µg through the buccal7,8 or sublingual9,10 routes. Higher dosages of misoprostol (600 µg to 800 µg) have been used11,12 but are likely associated with higher rates of nausea, vomiting,shivering, and fever than the lower dosages. Methylergonovine 0.2 mg is administered intramuscularly.
Continue to: The bottom line...
The bottom line
PPH is a major cause of maternal morbidity, and in low-resource settings, mortality. Oxytocin is the standard for reducing postpartum blood loss, but rates of blood loss ≥ 500 mL are high following this monotherapy. To reduce postpartum blood loss beyond what is possible with oxytocin alone, clinicians can more rapidly transition to administering a second uterotonic when they suspect blood loss is becoming excessive or they can use two uterotonic agents with all births or in those at high risk for excess bleeding. If blood loss does become excessive, clinicians need to pivot rapidly from prevention with oxytocin to treatment with our entire therapeutic armamentarium.
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323-e333.
- Slomski A. Why do hundreds of US women die annually in childbirth? JAMA. 2019;321:1239-1241.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database Syst Rev. 2018;12:CD011689.
- American College of Obstetricians and Gynecologists. Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 183: postpartum hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
- Widmer M, Piaggio G, Nguyen TM, et al; WHO Champion Trial Group. Heat-stable carbetocin versus oxytocin to prevent hemorrhage after vaginal birth. N Engl J Med. 2018;379:743-752.
- Adnan N, Conlan-Trant R, McCormick C, et al. Intramuscular versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery: randomised controlled trial. BMJ. 2018;362:k3546.
- Hamm J, Russell Z, Botha T, et al. Buccal misoprostol to prevent hemorrhage at cesarean delivery: a randomized study. Am J Obstet Gynecol. 2005;192:1404-1406.
- Bhullar A, Carlan SJ, Hamm J, et al. Buccal misoprostol to decrease blood loss after vaginal delivery: a randomized trial. Obstet Gynecol. 2004;104:1282-1288.
- Hofmeyr GJ, Fawole B, Mugerwa K, et al. Administration of 400 µg of misoprostol to augment routine active management of the third stage of labor. Int J Gynaecol Obstet. 2011;112:98-102.
- Chaudhuri P, Majumdar A. A randomized trial of sublingual misoprostol to augment routine third-stage management among women at risk of postpartum hemorrhage. Int J Gynaecol Obstet. 2016;132:191-195.
- Winikoff B, Dabash R, Durocher J, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women not exposed to oxytocin during labor: a double-blind, randomised, non-inferiority trial. Lancet. 2010;375:210-216.
- Blum J, Winikoff B, Raghavan S, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double-blind, randomised, non-inferiority trial. Lancet. 2010;375:217-223.
Excessive postpartum bleeding is a major cause of maternal morbidity and mortality. Worldwide, obstetric hemorrhage is the most common cause of maternal death.1,2 Medications reported to reduce postpartum bleeding include oxytocin, misoprostol, ergonovine, methylergonovine, carboprost, and tranexamic acid. A recent Cochrane network meta-analysis of 196 trials, including 135,559 women, distilled in 1,361 pages of analysis, reported on the medications associated with the greatest reduction in postpartum bleeding.3 Surprisingly, for preventing blood loss ≥ 500 mL, misoprostol plus oxytocin and ergonovine plus oxytocin were the highest ranked interventions. This evidence is summarized here.
Misoprostol plus oxytocin
After newborn delivery, active management of the third stage of labor, including uterotonic administration, is strongly recommended because it will reduce postpartum blood loss, decreasing the rate of postpartum hemorrhage (PPH).4 Both oxytocin and misoprostol are effective uterotonics. However, the combination of oxytocin plus misoprostol appears to be more effective than oxytocin alone in reducing the frequency of postpartum blood loss greater than 500 mL.3 To understand the clinical efficacy and adverse effects (AEs) of combined oxytocin plus misoprostol a meta-analysis was performed for both vaginal and cesarean deliveries (CDs).
Efficacy and AEs during vaginal delivery. In the meta-analysis, about 6,000 vaginal deliveries were analyzed, with no significant differences for misoprostol plus oxytocin versus oxytocin alone found for the following outcomes: maternal death, intensive care unit admissions, and rate of blood loss ≥ 1,000 mL (1.7% for both uterotonics vs 2.2% for oxytocin alone).3 Misoprostol plus oxytocin was significantly superior to oxytocin alone for the following outcomes: reduced risk of blood transfusion (0.95% vs 2.5%), reduced risk of blood loss ≥ 500 mL (5.9% vs 8.0%), reduced risk of requiring an additional uterotonic (3.6% vs 5.8%), and a smaller decrease in hemoglobin concentration from pre- to postdelivery (-0.89 g/L).3
In my opinion, the difference in hemoglobin concentration, although statistically significant, is not of clinical significance. However, compared with oxytocin alone, misoprostol plus oxytocin caused significantly more nausea (2.4% vs 0.66%), vomiting (3.1% vs 0.86%), and fever (21% vs 3.9%).3 A weakness of this meta-analysis is that the trials used a wide range of misoprostol dosages (200 to 600 µg) and multiple routes of administration, including sublingual (under the tongue), buccal, and rectal. This makes it impossible to identify a best misoprostol dosage and administration route.
Efficacy and AEs during CD. In the same meta-analysis about 2,000 CDs were analyzed, with no significant difference for misoprostol plus oxytocin versus oxytocin alone for the following outcomes: maternal death, intensive care unit admissions, and PPH ≥ 1,000 mL blood loss (6.2% vs 6.5%).3 Misoprostol plus oxytocin was significantly superior to oxytocin alone for the following outcomes: reduced risk of blood transfusion (2.6% vs 5.4%), reduced risk of blood loss ≥ 500 mL (32% vs 47%), reduced risk of requiring an additional uterotonic (14% vs 28%), and a smaller decrease in hemoglobin concentration from before to after delivery (-4.0 g/L).3 In my opinion, the statistically significant difference in hemoglobin concentration is not clinically significant. However, compared with oxytocin alone, misoprostol plus oxytocin caused significantly more nausea (12% vs 6.1%), vomiting (8.1% vs 5.4%), shivering (13% vs 7%), and fever (7.7% vs 4.0%).3
Continue to: Ergonovine plus oxytocin...
Ergonovine plus oxytocin
Ergonovine is an ergot derivative that causes uterine contractions and has been shown to effectively reduce blood loss at delivery. In the United States a methyl-derivative of ergonovine, methylergonovine, is widely available. In a meta-analysis with mostly vaginal deliveries, there were no significant differences for ergonovine plus oxytocin versus oxytocin alone for the following outcomes: death, intensive care unit admission, rate of blood loss ≥ 1,000 mL(2.0% vs 2.7%), blood transfusion, administration of an additional uterotonic, change in hemoglobin from pre- to postdelivery, nausea, hypertension, shivering, and fever.3 However, ergonovine plus oxytocin, compared with oxytocin alone, resulted in a significantly reduced rate of blood loss ≥ 500 mL (8.3% vs 10.2%) and an increased rate of vomiting (8.1% vs 1.6%).3 In these trials women with a blood pressure ≥ 150/100 mm Hg were generally excluded from receiving ergonovine because of its hypertensive effect.

Clinical practice options
Given the Cochrane meta-analysis results, ObGyns have two approaches for optimizing PPH reduction.
Option 1: Use a single uterotonic to reduce postpartum blood loss. If excess bleeding occurs, rapidly administer a second uterotonic agent. Currently, monotherapy with intravenous or intramuscular oxytocin is the standard for reducing postpartum blood loss.5,6 Advantages of this approach compared with dual agent therapy include simplification of care and minimization of AEs. However, oxytocin monotherapy for minimizing postpartum bleeding may be suboptimal. In the largest trial ever performed (involving 29,645 women) when oxytocin was administered postpartum, the rates of estimated blood loss ≥ 500 mL and ≥ 1,000 mL were 9.1% and 1.45%, respectively.5 Is 9% an optimal rate for blood loss ≥ 500 mL following a vaginal delivery? Or should we try to achieve a lower rate?
Given the “high” rate of blood loss ≥ 500 mL with oxytocin alone, it is important for clinicians using the one-uterotonic approach to promptly recognize patients who have excessive bleeding and transition rapidly from prevention to treatment. When PPH cases are reviewed, a common finding is that the clinicians did not timely recognize excess bleeding, delaying transition to treatment with additional uterotonics and other interventions. When routinely using oxytocin monotherapy, lowering the threshold for administering a second uterotonic (methylergonovine, carboprost, misoprostol, or tranexamic acid) may help decrease the frequency of excess postpartum blood loss.
Option 2: Administer two uterotonics to reduce postpartum blood loss at all deliveries. Given the “high” rate of excess postpartum blood loss with oxytocin monotherapy, an alternative is to administer two uterotonics at all births or at births with a high risk of excess blood loss. As discussed, administering two uterotonics, oxytocin plus misoprostol or oxytocin plus ergonovine, has been reported to be more effective than oxytocin alone for reducing postpartum bleeding ≥ 500 mL.3 In the Cochrane meta-analysis, per 1,000 women given oxytocin following a vaginal birth, 122 would have blood loss ≥ 500 mL, compared with 85 given oxytocin plus misoprostol or oxytocin plus ergonovine.3
Misoprostol is administered sublingually, buccally, or rectally, and methylergonovine is administered by intramuscular injection. Although dual uterotonic therapy is more effective than monotherapy, dual therapy is associated with more AEs. As noted, compared with oxytocin monotherapy, the combination of oxytocin plus misoprostol is associated with more nausea, vomiting, shivering, and fever. Oxytocin plus ergonovine is associated with a higher rate of vomiting than oxytocin monotherapy. In my practice I prefer using intramuscular methylergonovine as the second agent to avoid the high rate of fever associated with misoprostol.
For dual agent therapy, one approach is to administer misoprostol 200 µg or 400 µg through the buccal7,8 or sublingual9,10 routes. Higher dosages of misoprostol (600 µg to 800 µg) have been used11,12 but are likely associated with higher rates of nausea, vomiting,shivering, and fever than the lower dosages. Methylergonovine 0.2 mg is administered intramuscularly.
Continue to: The bottom line...
The bottom line
PPH is a major cause of maternal morbidity, and in low-resource settings, mortality. Oxytocin is the standard for reducing postpartum blood loss, but rates of blood loss ≥ 500 mL are high following this monotherapy. To reduce postpartum blood loss beyond what is possible with oxytocin alone, clinicians can more rapidly transition to administering a second uterotonic when they suspect blood loss is becoming excessive or they can use two uterotonic agents with all births or in those at high risk for excess bleeding. If blood loss does become excessive, clinicians need to pivot rapidly from prevention with oxytocin to treatment with our entire therapeutic armamentarium.
Excessive postpartum bleeding is a major cause of maternal morbidity and mortality. Worldwide, obstetric hemorrhage is the most common cause of maternal death.1,2 Medications reported to reduce postpartum bleeding include oxytocin, misoprostol, ergonovine, methylergonovine, carboprost, and tranexamic acid. A recent Cochrane network meta-analysis of 196 trials, including 135,559 women, distilled in 1,361 pages of analysis, reported on the medications associated with the greatest reduction in postpartum bleeding.3 Surprisingly, for preventing blood loss ≥ 500 mL, misoprostol plus oxytocin and ergonovine plus oxytocin were the highest ranked interventions. This evidence is summarized here.
Misoprostol plus oxytocin
After newborn delivery, active management of the third stage of labor, including uterotonic administration, is strongly recommended because it will reduce postpartum blood loss, decreasing the rate of postpartum hemorrhage (PPH).4 Both oxytocin and misoprostol are effective uterotonics. However, the combination of oxytocin plus misoprostol appears to be more effective than oxytocin alone in reducing the frequency of postpartum blood loss greater than 500 mL.3 To understand the clinical efficacy and adverse effects (AEs) of combined oxytocin plus misoprostol a meta-analysis was performed for both vaginal and cesarean deliveries (CDs).
Efficacy and AEs during vaginal delivery. In the meta-analysis, about 6,000 vaginal deliveries were analyzed, with no significant differences for misoprostol plus oxytocin versus oxytocin alone found for the following outcomes: maternal death, intensive care unit admissions, and rate of blood loss ≥ 1,000 mL (1.7% for both uterotonics vs 2.2% for oxytocin alone).3 Misoprostol plus oxytocin was significantly superior to oxytocin alone for the following outcomes: reduced risk of blood transfusion (0.95% vs 2.5%), reduced risk of blood loss ≥ 500 mL (5.9% vs 8.0%), reduced risk of requiring an additional uterotonic (3.6% vs 5.8%), and a smaller decrease in hemoglobin concentration from pre- to postdelivery (-0.89 g/L).3
In my opinion, the difference in hemoglobin concentration, although statistically significant, is not of clinical significance. However, compared with oxytocin alone, misoprostol plus oxytocin caused significantly more nausea (2.4% vs 0.66%), vomiting (3.1% vs 0.86%), and fever (21% vs 3.9%).3 A weakness of this meta-analysis is that the trials used a wide range of misoprostol dosages (200 to 600 µg) and multiple routes of administration, including sublingual (under the tongue), buccal, and rectal. This makes it impossible to identify a best misoprostol dosage and administration route.
Efficacy and AEs during CD. In the same meta-analysis about 2,000 CDs were analyzed, with no significant difference for misoprostol plus oxytocin versus oxytocin alone for the following outcomes: maternal death, intensive care unit admissions, and PPH ≥ 1,000 mL blood loss (6.2% vs 6.5%).3 Misoprostol plus oxytocin was significantly superior to oxytocin alone for the following outcomes: reduced risk of blood transfusion (2.6% vs 5.4%), reduced risk of blood loss ≥ 500 mL (32% vs 47%), reduced risk of requiring an additional uterotonic (14% vs 28%), and a smaller decrease in hemoglobin concentration from before to after delivery (-4.0 g/L).3 In my opinion, the statistically significant difference in hemoglobin concentration is not clinically significant. However, compared with oxytocin alone, misoprostol plus oxytocin caused significantly more nausea (12% vs 6.1%), vomiting (8.1% vs 5.4%), shivering (13% vs 7%), and fever (7.7% vs 4.0%).3
Continue to: Ergonovine plus oxytocin...
Ergonovine plus oxytocin
Ergonovine is an ergot derivative that causes uterine contractions and has been shown to effectively reduce blood loss at delivery. In the United States a methyl-derivative of ergonovine, methylergonovine, is widely available. In a meta-analysis with mostly vaginal deliveries, there were no significant differences for ergonovine plus oxytocin versus oxytocin alone for the following outcomes: death, intensive care unit admission, rate of blood loss ≥ 1,000 mL(2.0% vs 2.7%), blood transfusion, administration of an additional uterotonic, change in hemoglobin from pre- to postdelivery, nausea, hypertension, shivering, and fever.3 However, ergonovine plus oxytocin, compared with oxytocin alone, resulted in a significantly reduced rate of blood loss ≥ 500 mL (8.3% vs 10.2%) and an increased rate of vomiting (8.1% vs 1.6%).3 In these trials women with a blood pressure ≥ 150/100 mm Hg were generally excluded from receiving ergonovine because of its hypertensive effect.

Clinical practice options
Given the Cochrane meta-analysis results, ObGyns have two approaches for optimizing PPH reduction.
Option 1: Use a single uterotonic to reduce postpartum blood loss. If excess bleeding occurs, rapidly administer a second uterotonic agent. Currently, monotherapy with intravenous or intramuscular oxytocin is the standard for reducing postpartum blood loss.5,6 Advantages of this approach compared with dual agent therapy include simplification of care and minimization of AEs. However, oxytocin monotherapy for minimizing postpartum bleeding may be suboptimal. In the largest trial ever performed (involving 29,645 women) when oxytocin was administered postpartum, the rates of estimated blood loss ≥ 500 mL and ≥ 1,000 mL were 9.1% and 1.45%, respectively.5 Is 9% an optimal rate for blood loss ≥ 500 mL following a vaginal delivery? Or should we try to achieve a lower rate?
Given the “high” rate of blood loss ≥ 500 mL with oxytocin alone, it is important for clinicians using the one-uterotonic approach to promptly recognize patients who have excessive bleeding and transition rapidly from prevention to treatment. When PPH cases are reviewed, a common finding is that the clinicians did not timely recognize excess bleeding, delaying transition to treatment with additional uterotonics and other interventions. When routinely using oxytocin monotherapy, lowering the threshold for administering a second uterotonic (methylergonovine, carboprost, misoprostol, or tranexamic acid) may help decrease the frequency of excess postpartum blood loss.
Option 2: Administer two uterotonics to reduce postpartum blood loss at all deliveries. Given the “high” rate of excess postpartum blood loss with oxytocin monotherapy, an alternative is to administer two uterotonics at all births or at births with a high risk of excess blood loss. As discussed, administering two uterotonics, oxytocin plus misoprostol or oxytocin plus ergonovine, has been reported to be more effective than oxytocin alone for reducing postpartum bleeding ≥ 500 mL.3 In the Cochrane meta-analysis, per 1,000 women given oxytocin following a vaginal birth, 122 would have blood loss ≥ 500 mL, compared with 85 given oxytocin plus misoprostol or oxytocin plus ergonovine.3
Misoprostol is administered sublingually, buccally, or rectally, and methylergonovine is administered by intramuscular injection. Although dual uterotonic therapy is more effective than monotherapy, dual therapy is associated with more AEs. As noted, compared with oxytocin monotherapy, the combination of oxytocin plus misoprostol is associated with more nausea, vomiting, shivering, and fever. Oxytocin plus ergonovine is associated with a higher rate of vomiting than oxytocin monotherapy. In my practice I prefer using intramuscular methylergonovine as the second agent to avoid the high rate of fever associated with misoprostol.
For dual agent therapy, one approach is to administer misoprostol 200 µg or 400 µg through the buccal7,8 or sublingual9,10 routes. Higher dosages of misoprostol (600 µg to 800 µg) have been used11,12 but are likely associated with higher rates of nausea, vomiting,shivering, and fever than the lower dosages. Methylergonovine 0.2 mg is administered intramuscularly.
Continue to: The bottom line...
The bottom line
PPH is a major cause of maternal morbidity, and in low-resource settings, mortality. Oxytocin is the standard for reducing postpartum blood loss, but rates of blood loss ≥ 500 mL are high following this monotherapy. To reduce postpartum blood loss beyond what is possible with oxytocin alone, clinicians can more rapidly transition to administering a second uterotonic when they suspect blood loss is becoming excessive or they can use two uterotonic agents with all births or in those at high risk for excess bleeding. If blood loss does become excessive, clinicians need to pivot rapidly from prevention with oxytocin to treatment with our entire therapeutic armamentarium.
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323-e333.
- Slomski A. Why do hundreds of US women die annually in childbirth? JAMA. 2019;321:1239-1241.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database Syst Rev. 2018;12:CD011689.
- American College of Obstetricians and Gynecologists. Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 183: postpartum hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
- Widmer M, Piaggio G, Nguyen TM, et al; WHO Champion Trial Group. Heat-stable carbetocin versus oxytocin to prevent hemorrhage after vaginal birth. N Engl J Med. 2018;379:743-752.
- Adnan N, Conlan-Trant R, McCormick C, et al. Intramuscular versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery: randomised controlled trial. BMJ. 2018;362:k3546.
- Hamm J, Russell Z, Botha T, et al. Buccal misoprostol to prevent hemorrhage at cesarean delivery: a randomized study. Am J Obstet Gynecol. 2005;192:1404-1406.
- Bhullar A, Carlan SJ, Hamm J, et al. Buccal misoprostol to decrease blood loss after vaginal delivery: a randomized trial. Obstet Gynecol. 2004;104:1282-1288.
- Hofmeyr GJ, Fawole B, Mugerwa K, et al. Administration of 400 µg of misoprostol to augment routine active management of the third stage of labor. Int J Gynaecol Obstet. 2011;112:98-102.
- Chaudhuri P, Majumdar A. A randomized trial of sublingual misoprostol to augment routine third-stage management among women at risk of postpartum hemorrhage. Int J Gynaecol Obstet. 2016;132:191-195.
- Winikoff B, Dabash R, Durocher J, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women not exposed to oxytocin during labor: a double-blind, randomised, non-inferiority trial. Lancet. 2010;375:210-216.
- Blum J, Winikoff B, Raghavan S, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double-blind, randomised, non-inferiority trial. Lancet. 2010;375:217-223.
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323-e333.
- Slomski A. Why do hundreds of US women die annually in childbirth? JAMA. 2019;321:1239-1241.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database Syst Rev. 2018;12:CD011689.
- American College of Obstetricians and Gynecologists. Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 183: postpartum hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
- Widmer M, Piaggio G, Nguyen TM, et al; WHO Champion Trial Group. Heat-stable carbetocin versus oxytocin to prevent hemorrhage after vaginal birth. N Engl J Med. 2018;379:743-752.
- Adnan N, Conlan-Trant R, McCormick C, et al. Intramuscular versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery: randomised controlled trial. BMJ. 2018;362:k3546.
- Hamm J, Russell Z, Botha T, et al. Buccal misoprostol to prevent hemorrhage at cesarean delivery: a randomized study. Am J Obstet Gynecol. 2005;192:1404-1406.
- Bhullar A, Carlan SJ, Hamm J, et al. Buccal misoprostol to decrease blood loss after vaginal delivery: a randomized trial. Obstet Gynecol. 2004;104:1282-1288.
- Hofmeyr GJ, Fawole B, Mugerwa K, et al. Administration of 400 µg of misoprostol to augment routine active management of the third stage of labor. Int J Gynaecol Obstet. 2011;112:98-102.
- Chaudhuri P, Majumdar A. A randomized trial of sublingual misoprostol to augment routine third-stage management among women at risk of postpartum hemorrhage. Int J Gynaecol Obstet. 2016;132:191-195.
- Winikoff B, Dabash R, Durocher J, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women not exposed to oxytocin during labor: a double-blind, randomised, non-inferiority trial. Lancet. 2010;375:210-216.
- Blum J, Winikoff B, Raghavan S, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double-blind, randomised, non-inferiority trial. Lancet. 2010;375:217-223.
Good news for ObGyns: Medical liability claims resulting in payment are decreasing!
Medical professional liability claims (claims) are a major cause of worry and agony for physicians who are dedicated to optimizing the health of all their patients. Among physicians, those who practice neurosurgery, thoracic surgery, plastic surgery, and obstetrics and gynecology have the greatest rate of making a payment on a claim per year of practice.1 Physicians who practice psychiatry, pediatrics, pathology, and internal medicine have the lowest rate of making a payment on a claim. Among the physicians in high-risk specialties, greater than 90% will have a claim filed against them during their career.2 Although professional liability exposure reached a crisis during the 1980s and 1990s, recent data have shown a decrease in overall professional liability risk.
The good news: Paid claims per 1,000 ObGyns have decreased greatly
In a review of all paid claims reported to the National Practitioner Data Bank from 1992 to 2014, the annual rate of paid claims per 1,000 ObGyn physician-years was determined.1 For the time periods 1992–1996, 1997–2002, 2003–2008,and 2009–2014, the annual rate of paid claims per 1,000 ObGyn physician-years was 57.6, 51.5, 40.0, and 25.9, representing an astounding 55% decrease in paid claims from 1992 to 2014 (FIGURE).1
The majority of claims result in no payment
In a review of the experience of a nationwide professional liability insurer from 1991 to 2005, only 22% of claims resulted in a payment.2 In this study, for obstetrics and gynecology and gynecologic surgery, only 11% and 8% of claims, respectively, resulted in a payment.2 However, being named in a malpractice claim results in significant stress for a physician and requires a great deal of work and time to defend.
In another study using data from the Physician Insurer’s Association of America, among 10,915 claims closed from 2005 to 2014, 59.5% were dropped, withdrawn, or dismissed; 27.7% were settled; 2.5% were resolved using an alternative dispute resolution process; 1.8% were uncategorized; and 8.6% went to trial.3 Of the cases that went to trial, 87% resulted in a verdict for the physician and 13% resulted in a verdict for the plaintiff.3
Not as good news: Payments per claim and claims settling for a payment > $1 million are increasing
In the period 1992–1996, the average payment per paid claim in the field of obstetrics and gynecology was $387,186, rising to $447,034 in 2009–2014—a 16% increase.1 From 2004 to 2010, million dollar payments occurred in about 8% of cases of paid claims, but they represent 36% of the total of all paid claims.4 In the time periods 1992–1996 and 2009–2014, payments greater than $1 million occurred in 6% and 8% of paid claims, respectively.1
Claims settled for much more than $1 million are of great concern to physicians because the payment may exceed their policy limit, creating a complex legal problem that may take time to resolve. In some cases, where the award is greater than the insurance policy limit, aggressive plaintiff attorneys have obtained a lien on the defendant physician’s home pending settlement of the case. When a multimillion dollar payment is made to settle a professional liability claim, it can greatly influence physician practice and change hospital policies. Frequently, following a multimillion dollar payment a physician may decide to limit their practice to low-risk cases or retire from the practice of medicine.
Liability premiums are stable or decreasing
From 2014 to 2019, my ObGyn professional liability insurance premiums decreased by 18%. During the same time period, my colleagues who practice surgical gynecology (no obstetrics) had a premium decrease of 22%. Insurers use a complex algorithm to determine annual liability insurance premiums, and premiums for ObGyns may not have stabilized or decreased in all regions. Take this Instant Poll:
Create your own user feedback survey
Reform of the liability tort system
Litigation policies and practices that reduce liability risk reduce total medical liability losses. Policies that have helped to constrain medical liability risk include state constitutional amendments limiting payments for pain and suffering, caps on compensation to plaintiff attorneys, increased early resolution programs that compensate patients who experience an adverse event and no-fault conflict resolution programs.5 In 2003, Texas implemented a comprehensive package of tort reform laws. Experts believe the reforms decreased the financial burden of professional liability insurance6 and led to less defensive medical practices, reducing excessive use of imaging and laboratory tests.
Medical factors contributing to a decrease in claims
In 1999, the Institute of Medicine released the report, “To Err is Human,” which galvanized health care systems to deploy systems of care that reduce the rate of adverse patient outcomes.7 Over the past 20 years, health systems have implemented quality improvement programs in obstetrics and gynecology that have contributed to a reduction in the rate of adverse patient outcomes. This may have contributed to the decrease in the rate of paid claims.
In a quasi-experimental study performed in 13 health systems, 7 interventions were implemented with the goal of improving outcomes and reducing medical liability. The 7 interventions included8:
- an elective induction bundle focused on the safe use of oxytocin
- an augmentation bundle focused on early intervention for possible fetal metabolic acidosis
- an operative vaginal delivery bundle
- TeamSTEPPS teamwork training to improve the quality of communication
- best practices education with a focus on electronic fetal monitoring
- regular performance feedback to hospitals and clinicians
- implementation of a quality improvement collaboration to support implementation of the interventions.
During the two-year baseline period prior to the intervention there were 185,373 deliveries with 6.7 perinatal claims made per 10,000 deliveries and 1.3 claims paid per 10,000 deliveries. Following the intervention, the rate of claims made and claims paid per 10,000 deliveries decreased by 22% and 37%, respectively. In addition there was a marked decrease in claims over $1 million paid, greatly limiting total financial liability losses.
Experts with vast experience in obstetrics and obstetric liability litigation have identified 4 priority interventions that may improve outcomes and mitigate liability risk, including: 1) 24-hour in-house physician coverage of an obstetrics service, 2) a conservative approach to trial of labor after a prior cesarean delivery, 3) utilization of a comprehensive, standardized event note in cases of a shoulder dystocia, and 4) judicious use of oxytocin, misoprostol, and magnesium sulfate.9
Other health system interventions that may contribute to a reduction in claims include:
- systematic improvement in the quality of communication among physicians and nurses through the use of team training, preprocedure huddles, and time-out processes10
- rapid response systems to rescue hospital patients with worrisome vital signs11
- standardized responses to a worrisome category 2 or 3 fetal heart-rate tracing12
- rapid recognition, evaluation, and treatment of women with hemorrhage, severe hypertension, sepsis, and venous thromboembolism13
- identification and referral of high-risk patients to tertiary centers14
- closed loop communication of critical imaging and laboratory results15
- universal insurance coverage for health care including contraception, obstetrics, and pediatric care.
Medical liability risk is an important practice issue because it causes excessive use of imaging and laboratory tests and often traumatizes clinicians, which can result in burnout. In the 1980s and 1990s, medical liability litigation reached a crescendo and was a prominent concern among obstetrician-gynecologists. The good news is that, for ObGyns, liability risk has stabilized. Hopefully our resolute efforts to continuously improve the quality of care will result in a long-term reduction in medical liability risk.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992–2014. JAMA Intern Med. 2017;177:710-718.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-e6.
- Bixenstine PJ, Shore AD, Mehtsun WT, et al. Catastrophic medical malpractice payouts in the United States. J Healthc Quality. 2014;36:43-53.
- Cardoso R, Zarin W, Nincic V, et al. Evaluative reports on medical malpractice policies in obstetrics: a rapid scoping review. Syst Rev. 2017;6:181.
- Stewart RM, Geoghegan K, Myers JG, et al. Malpractice risk and costs are significantly reduced after tort reform. J Am Coll Surg. 2011;212:463-467.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- Riley W, Meredith LW, Price R, et al. Decreasing malpractice claims by reducing preventable perinatal harm. Health Serv Res. 2016;51(suppl 3):2453-2471.
- Clark SL, Belfort MA, Dildy GA, et al. Reducing obstetric litigation through alterations in practice patterns. Obstet Gynecol. 2008;112:1279-1283.
- Haynes AB, Weiser TG, Berry WR, et al; Safe Surgery Saves Lives Study Group. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491-499.
- Patel S, Gillon SA, Jones DA. Rapid response systems: recognition and rescue of the deteriorating hospital patient. Br J Hosp Med (Lond). 2017;78:143-148.
- Clark SL, Hamilton EF, Garite TJ, et al. The limits of electronic fetal heart rate monitoring in the prevention of neonatal metabolic acidemia. Am J Obstet Gynecol. 2017;216:163.e1-163.e6.
- The Council on Patient Safety in Women’s Healthcare website. www.safehealthcareforeverywoman.org. Accessed April 12, 2019.
- Zahn CM, Remick A, Catalano A, et al. Levels of maternal care verification pilot: translating guidance into practice. Obstet Gynecol. 2018;132:1401-1406.
- Zuccotti G, Maloney FL, Feblowitz J, et al. Reducing risk with clinical decision support: a study of closed malpractice claims. Appl Clin Inform. 2014;5:746-756.
Medical professional liability claims (claims) are a major cause of worry and agony for physicians who are dedicated to optimizing the health of all their patients. Among physicians, those who practice neurosurgery, thoracic surgery, plastic surgery, and obstetrics and gynecology have the greatest rate of making a payment on a claim per year of practice.1 Physicians who practice psychiatry, pediatrics, pathology, and internal medicine have the lowest rate of making a payment on a claim. Among the physicians in high-risk specialties, greater than 90% will have a claim filed against them during their career.2 Although professional liability exposure reached a crisis during the 1980s and 1990s, recent data have shown a decrease in overall professional liability risk.
The good news: Paid claims per 1,000 ObGyns have decreased greatly
In a review of all paid claims reported to the National Practitioner Data Bank from 1992 to 2014, the annual rate of paid claims per 1,000 ObGyn physician-years was determined.1 For the time periods 1992–1996, 1997–2002, 2003–2008,and 2009–2014, the annual rate of paid claims per 1,000 ObGyn physician-years was 57.6, 51.5, 40.0, and 25.9, representing an astounding 55% decrease in paid claims from 1992 to 2014 (FIGURE).1
The majority of claims result in no payment
In a review of the experience of a nationwide professional liability insurer from 1991 to 2005, only 22% of claims resulted in a payment.2 In this study, for obstetrics and gynecology and gynecologic surgery, only 11% and 8% of claims, respectively, resulted in a payment.2 However, being named in a malpractice claim results in significant stress for a physician and requires a great deal of work and time to defend.
In another study using data from the Physician Insurer’s Association of America, among 10,915 claims closed from 2005 to 2014, 59.5% were dropped, withdrawn, or dismissed; 27.7% were settled; 2.5% were resolved using an alternative dispute resolution process; 1.8% were uncategorized; and 8.6% went to trial.3 Of the cases that went to trial, 87% resulted in a verdict for the physician and 13% resulted in a verdict for the plaintiff.3
Not as good news: Payments per claim and claims settling for a payment > $1 million are increasing
In the period 1992–1996, the average payment per paid claim in the field of obstetrics and gynecology was $387,186, rising to $447,034 in 2009–2014—a 16% increase.1 From 2004 to 2010, million dollar payments occurred in about 8% of cases of paid claims, but they represent 36% of the total of all paid claims.4 In the time periods 1992–1996 and 2009–2014, payments greater than $1 million occurred in 6% and 8% of paid claims, respectively.1
Claims settled for much more than $1 million are of great concern to physicians because the payment may exceed their policy limit, creating a complex legal problem that may take time to resolve. In some cases, where the award is greater than the insurance policy limit, aggressive plaintiff attorneys have obtained a lien on the defendant physician’s home pending settlement of the case. When a multimillion dollar payment is made to settle a professional liability claim, it can greatly influence physician practice and change hospital policies. Frequently, following a multimillion dollar payment a physician may decide to limit their practice to low-risk cases or retire from the practice of medicine.
Liability premiums are stable or decreasing
From 2014 to 2019, my ObGyn professional liability insurance premiums decreased by 18%. During the same time period, my colleagues who practice surgical gynecology (no obstetrics) had a premium decrease of 22%. Insurers use a complex algorithm to determine annual liability insurance premiums, and premiums for ObGyns may not have stabilized or decreased in all regions. Take this Instant Poll:
Create your own user feedback survey
Reform of the liability tort system
Litigation policies and practices that reduce liability risk reduce total medical liability losses. Policies that have helped to constrain medical liability risk include state constitutional amendments limiting payments for pain and suffering, caps on compensation to plaintiff attorneys, increased early resolution programs that compensate patients who experience an adverse event and no-fault conflict resolution programs.5 In 2003, Texas implemented a comprehensive package of tort reform laws. Experts believe the reforms decreased the financial burden of professional liability insurance6 and led to less defensive medical practices, reducing excessive use of imaging and laboratory tests.
Medical factors contributing to a decrease in claims
In 1999, the Institute of Medicine released the report, “To Err is Human,” which galvanized health care systems to deploy systems of care that reduce the rate of adverse patient outcomes.7 Over the past 20 years, health systems have implemented quality improvement programs in obstetrics and gynecology that have contributed to a reduction in the rate of adverse patient outcomes. This may have contributed to the decrease in the rate of paid claims.
In a quasi-experimental study performed in 13 health systems, 7 interventions were implemented with the goal of improving outcomes and reducing medical liability. The 7 interventions included8:
- an elective induction bundle focused on the safe use of oxytocin
- an augmentation bundle focused on early intervention for possible fetal metabolic acidosis
- an operative vaginal delivery bundle
- TeamSTEPPS teamwork training to improve the quality of communication
- best practices education with a focus on electronic fetal monitoring
- regular performance feedback to hospitals and clinicians
- implementation of a quality improvement collaboration to support implementation of the interventions.
During the two-year baseline period prior to the intervention there were 185,373 deliveries with 6.7 perinatal claims made per 10,000 deliveries and 1.3 claims paid per 10,000 deliveries. Following the intervention, the rate of claims made and claims paid per 10,000 deliveries decreased by 22% and 37%, respectively. In addition there was a marked decrease in claims over $1 million paid, greatly limiting total financial liability losses.
Experts with vast experience in obstetrics and obstetric liability litigation have identified 4 priority interventions that may improve outcomes and mitigate liability risk, including: 1) 24-hour in-house physician coverage of an obstetrics service, 2) a conservative approach to trial of labor after a prior cesarean delivery, 3) utilization of a comprehensive, standardized event note in cases of a shoulder dystocia, and 4) judicious use of oxytocin, misoprostol, and magnesium sulfate.9
Other health system interventions that may contribute to a reduction in claims include:
- systematic improvement in the quality of communication among physicians and nurses through the use of team training, preprocedure huddles, and time-out processes10
- rapid response systems to rescue hospital patients with worrisome vital signs11
- standardized responses to a worrisome category 2 or 3 fetal heart-rate tracing12
- rapid recognition, evaluation, and treatment of women with hemorrhage, severe hypertension, sepsis, and venous thromboembolism13
- identification and referral of high-risk patients to tertiary centers14
- closed loop communication of critical imaging and laboratory results15
- universal insurance coverage for health care including contraception, obstetrics, and pediatric care.
Medical liability risk is an important practice issue because it causes excessive use of imaging and laboratory tests and often traumatizes clinicians, which can result in burnout. In the 1980s and 1990s, medical liability litigation reached a crescendo and was a prominent concern among obstetrician-gynecologists. The good news is that, for ObGyns, liability risk has stabilized. Hopefully our resolute efforts to continuously improve the quality of care will result in a long-term reduction in medical liability risk.
Medical professional liability claims (claims) are a major cause of worry and agony for physicians who are dedicated to optimizing the health of all their patients. Among physicians, those who practice neurosurgery, thoracic surgery, plastic surgery, and obstetrics and gynecology have the greatest rate of making a payment on a claim per year of practice.1 Physicians who practice psychiatry, pediatrics, pathology, and internal medicine have the lowest rate of making a payment on a claim. Among the physicians in high-risk specialties, greater than 90% will have a claim filed against them during their career.2 Although professional liability exposure reached a crisis during the 1980s and 1990s, recent data have shown a decrease in overall professional liability risk.
The good news: Paid claims per 1,000 ObGyns have decreased greatly
In a review of all paid claims reported to the National Practitioner Data Bank from 1992 to 2014, the annual rate of paid claims per 1,000 ObGyn physician-years was determined.1 For the time periods 1992–1996, 1997–2002, 2003–2008,and 2009–2014, the annual rate of paid claims per 1,000 ObGyn physician-years was 57.6, 51.5, 40.0, and 25.9, representing an astounding 55% decrease in paid claims from 1992 to 2014 (FIGURE).1
The majority of claims result in no payment
In a review of the experience of a nationwide professional liability insurer from 1991 to 2005, only 22% of claims resulted in a payment.2 In this study, for obstetrics and gynecology and gynecologic surgery, only 11% and 8% of claims, respectively, resulted in a payment.2 However, being named in a malpractice claim results in significant stress for a physician and requires a great deal of work and time to defend.
In another study using data from the Physician Insurer’s Association of America, among 10,915 claims closed from 2005 to 2014, 59.5% were dropped, withdrawn, or dismissed; 27.7% were settled; 2.5% were resolved using an alternative dispute resolution process; 1.8% were uncategorized; and 8.6% went to trial.3 Of the cases that went to trial, 87% resulted in a verdict for the physician and 13% resulted in a verdict for the plaintiff.3
Not as good news: Payments per claim and claims settling for a payment > $1 million are increasing
In the period 1992–1996, the average payment per paid claim in the field of obstetrics and gynecology was $387,186, rising to $447,034 in 2009–2014—a 16% increase.1 From 2004 to 2010, million dollar payments occurred in about 8% of cases of paid claims, but they represent 36% of the total of all paid claims.4 In the time periods 1992–1996 and 2009–2014, payments greater than $1 million occurred in 6% and 8% of paid claims, respectively.1
Claims settled for much more than $1 million are of great concern to physicians because the payment may exceed their policy limit, creating a complex legal problem that may take time to resolve. In some cases, where the award is greater than the insurance policy limit, aggressive plaintiff attorneys have obtained a lien on the defendant physician’s home pending settlement of the case. When a multimillion dollar payment is made to settle a professional liability claim, it can greatly influence physician practice and change hospital policies. Frequently, following a multimillion dollar payment a physician may decide to limit their practice to low-risk cases or retire from the practice of medicine.
Liability premiums are stable or decreasing
From 2014 to 2019, my ObGyn professional liability insurance premiums decreased by 18%. During the same time period, my colleagues who practice surgical gynecology (no obstetrics) had a premium decrease of 22%. Insurers use a complex algorithm to determine annual liability insurance premiums, and premiums for ObGyns may not have stabilized or decreased in all regions. Take this Instant Poll:
Create your own user feedback survey
Reform of the liability tort system
Litigation policies and practices that reduce liability risk reduce total medical liability losses. Policies that have helped to constrain medical liability risk include state constitutional amendments limiting payments for pain and suffering, caps on compensation to plaintiff attorneys, increased early resolution programs that compensate patients who experience an adverse event and no-fault conflict resolution programs.5 In 2003, Texas implemented a comprehensive package of tort reform laws. Experts believe the reforms decreased the financial burden of professional liability insurance6 and led to less defensive medical practices, reducing excessive use of imaging and laboratory tests.
Medical factors contributing to a decrease in claims
In 1999, the Institute of Medicine released the report, “To Err is Human,” which galvanized health care systems to deploy systems of care that reduce the rate of adverse patient outcomes.7 Over the past 20 years, health systems have implemented quality improvement programs in obstetrics and gynecology that have contributed to a reduction in the rate of adverse patient outcomes. This may have contributed to the decrease in the rate of paid claims.
In a quasi-experimental study performed in 13 health systems, 7 interventions were implemented with the goal of improving outcomes and reducing medical liability. The 7 interventions included8:
- an elective induction bundle focused on the safe use of oxytocin
- an augmentation bundle focused on early intervention for possible fetal metabolic acidosis
- an operative vaginal delivery bundle
- TeamSTEPPS teamwork training to improve the quality of communication
- best practices education with a focus on electronic fetal monitoring
- regular performance feedback to hospitals and clinicians
- implementation of a quality improvement collaboration to support implementation of the interventions.
During the two-year baseline period prior to the intervention there were 185,373 deliveries with 6.7 perinatal claims made per 10,000 deliveries and 1.3 claims paid per 10,000 deliveries. Following the intervention, the rate of claims made and claims paid per 10,000 deliveries decreased by 22% and 37%, respectively. In addition there was a marked decrease in claims over $1 million paid, greatly limiting total financial liability losses.
Experts with vast experience in obstetrics and obstetric liability litigation have identified 4 priority interventions that may improve outcomes and mitigate liability risk, including: 1) 24-hour in-house physician coverage of an obstetrics service, 2) a conservative approach to trial of labor after a prior cesarean delivery, 3) utilization of a comprehensive, standardized event note in cases of a shoulder dystocia, and 4) judicious use of oxytocin, misoprostol, and magnesium sulfate.9
Other health system interventions that may contribute to a reduction in claims include:
- systematic improvement in the quality of communication among physicians and nurses through the use of team training, preprocedure huddles, and time-out processes10
- rapid response systems to rescue hospital patients with worrisome vital signs11
- standardized responses to a worrisome category 2 or 3 fetal heart-rate tracing12
- rapid recognition, evaluation, and treatment of women with hemorrhage, severe hypertension, sepsis, and venous thromboembolism13
- identification and referral of high-risk patients to tertiary centers14
- closed loop communication of critical imaging and laboratory results15
- universal insurance coverage for health care including contraception, obstetrics, and pediatric care.
Medical liability risk is an important practice issue because it causes excessive use of imaging and laboratory tests and often traumatizes clinicians, which can result in burnout. In the 1980s and 1990s, medical liability litigation reached a crescendo and was a prominent concern among obstetrician-gynecologists. The good news is that, for ObGyns, liability risk has stabilized. Hopefully our resolute efforts to continuously improve the quality of care will result in a long-term reduction in medical liability risk.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992–2014. JAMA Intern Med. 2017;177:710-718.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-e6.
- Bixenstine PJ, Shore AD, Mehtsun WT, et al. Catastrophic medical malpractice payouts in the United States. J Healthc Quality. 2014;36:43-53.
- Cardoso R, Zarin W, Nincic V, et al. Evaluative reports on medical malpractice policies in obstetrics: a rapid scoping review. Syst Rev. 2017;6:181.
- Stewart RM, Geoghegan K, Myers JG, et al. Malpractice risk and costs are significantly reduced after tort reform. J Am Coll Surg. 2011;212:463-467.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- Riley W, Meredith LW, Price R, et al. Decreasing malpractice claims by reducing preventable perinatal harm. Health Serv Res. 2016;51(suppl 3):2453-2471.
- Clark SL, Belfort MA, Dildy GA, et al. Reducing obstetric litigation through alterations in practice patterns. Obstet Gynecol. 2008;112:1279-1283.
- Haynes AB, Weiser TG, Berry WR, et al; Safe Surgery Saves Lives Study Group. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491-499.
- Patel S, Gillon SA, Jones DA. Rapid response systems: recognition and rescue of the deteriorating hospital patient. Br J Hosp Med (Lond). 2017;78:143-148.
- Clark SL, Hamilton EF, Garite TJ, et al. The limits of electronic fetal heart rate monitoring in the prevention of neonatal metabolic acidemia. Am J Obstet Gynecol. 2017;216:163.e1-163.e6.
- The Council on Patient Safety in Women’s Healthcare website. www.safehealthcareforeverywoman.org. Accessed April 12, 2019.
- Zahn CM, Remick A, Catalano A, et al. Levels of maternal care verification pilot: translating guidance into practice. Obstet Gynecol. 2018;132:1401-1406.
- Zuccotti G, Maloney FL, Feblowitz J, et al. Reducing risk with clinical decision support: a study of closed malpractice claims. Appl Clin Inform. 2014;5:746-756.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992–2014. JAMA Intern Med. 2017;177:710-718.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-e6.
- Bixenstine PJ, Shore AD, Mehtsun WT, et al. Catastrophic medical malpractice payouts in the United States. J Healthc Quality. 2014;36:43-53.
- Cardoso R, Zarin W, Nincic V, et al. Evaluative reports on medical malpractice policies in obstetrics: a rapid scoping review. Syst Rev. 2017;6:181.
- Stewart RM, Geoghegan K, Myers JG, et al. Malpractice risk and costs are significantly reduced after tort reform. J Am Coll Surg. 2011;212:463-467.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- Riley W, Meredith LW, Price R, et al. Decreasing malpractice claims by reducing preventable perinatal harm. Health Serv Res. 2016;51(suppl 3):2453-2471.
- Clark SL, Belfort MA, Dildy GA, et al. Reducing obstetric litigation through alterations in practice patterns. Obstet Gynecol. 2008;112:1279-1283.
- Haynes AB, Weiser TG, Berry WR, et al; Safe Surgery Saves Lives Study Group. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491-499.
- Patel S, Gillon SA, Jones DA. Rapid response systems: recognition and rescue of the deteriorating hospital patient. Br J Hosp Med (Lond). 2017;78:143-148.
- Clark SL, Hamilton EF, Garite TJ, et al. The limits of electronic fetal heart rate monitoring in the prevention of neonatal metabolic acidemia. Am J Obstet Gynecol. 2017;216:163.e1-163.e6.
- The Council on Patient Safety in Women’s Healthcare website. www.safehealthcareforeverywoman.org. Accessed April 12, 2019.
- Zahn CM, Remick A, Catalano A, et al. Levels of maternal care verification pilot: translating guidance into practice. Obstet Gynecol. 2018;132:1401-1406.
- Zuccotti G, Maloney FL, Feblowitz J, et al. Reducing risk with clinical decision support: a study of closed malpractice claims. Appl Clin Inform. 2014;5:746-756.
Screening and counseling interventions to prevent peripartum depression: A practical approach
Perinatal depression is an episode of major or minor depression that occurs during pregnancy or in the 12 months after birth; it affects about 10% of new mothers.1 Perinatal depression adversely impacts mothers, children, and their families. Pregnant women with depression are at increased risk for preterm birth and low birth weight.2 Infants of mothers with postpartum depression have reduced bonding, lower rates of breastfeeding, delayed cognitive and social development, and an increased risk of future mental health issues.3 Timely treatment of perinatal depression can improve health outcomes for the woman, her children, and their family.
Clinicians follow current screening recommendations
The American College of Obstetricians and Gynecologists (ACOG) currently recommends that ObGynsscreen all pregnant women for depression and anxiety symptoms at least once during the perinatal period.1 Many practices use the Edinburgh Postnatal Depression Scale (EPDS) during pregnancy and postpartum. Women who screen positive are referred to mental health clinicians or have treatment initiated by their primary obstetrician.
Clinicians have been phenomenally successful in screening for perinatal depression. In a recent study from Kaiser Permanente Northern California, 98% of pregnant women were screened for perinatal depression, and a diagnosis of depression was made in 12%.4 Of note, only 47% of women who screened positive for depression initiated treatment, although 82% of women with the most severe symptoms initiated treatment. These data demonstrate that ObGyns consistently screen pregnant women for depression but, due to patient and system issues, treatment of all screen-positive women remains a yet unattained goal.5,6
New USPSTF guideline: Identify women at risk for perinatal depression and refer for counseling
In 2016 the United States Preventive Services Task Force (USPSTF) recommended that pregnant and postpartum women be screened for depression with adequate systems in place to ensure diagnosis, effective treatment, and follow-up.7 The 2016 USPSTF recommendation was consistent with prior guidelines from both the American Academy of Pediatrics in 20108 and ACOG in 2015.9
Now, the USPSTF is making a bold new recommendation, jumping ahead of professional societies: screen pregnant women to identify those at risk for perinatal depression and refer them for counseling (B recommendation; net benefit is moderate).10,11 The USPSTF recommendation is based on growing literature that shows counseling women at risk for perinatal depression reduces the risk of having an episode of major depression by 40%.11 Both interpersonal psychotherapy and cognitive behavioral therapy have been reported to be effective for preventing perinatal depression.12,13
As an example of the relevant literature, in one trial performed in Rhode Island, women who were 20 to 35 weeks pregnant with a high score (≥27) on the Cooper Survey Questionnaire and on public assistance were randomized to counseling or usual care. The counseling intervention involved 4 small group (2 to 5 women) sessions of 90 minutes and one individual session of 50 minutes.14 The treatment focused on managing the transition to motherhood, developing a support system, improving communication skills to manage conflict, goal setting, and identifying psychosocial supports for new mothers. At 6 months after birth, a depressive episode had occurred in 31% of the control women and 16% of the women who had experienced the intervention (P = .041). At 12 months after birth, a depressive episode had occurred in 40% of control women and 26% of women in the intervention group (P = .052).
Of note, most cases of postpartum depression were diagnosed more than 3 months after birth, a time when new mothers generally no longer are receiving regular postpartum care by an obstetrician. The timing of the diagnosis of perinatal depression indicates that an effective handoff between the obstetrician and primary care and/or mental health clinicians is of great importance. The investigators concluded that pregnant women at very high risk for perinatal depression who receive interpersonal therapy have a lower rate of a postpartum depressive episode than women receiving usual care.14
Pregnancy, delivery, and the first year following birth are stressful for many women and their families. Women who are young, poor, and with minimal social supports are at especially high risk for developing perinatal depression. However, it will be challenging for obstetric practices to rapidly implement the new USPSTF recommendations because there is no professional consensus on how to screen women to identify those at high risk for perinatal depression, and mental health resources to care for the screen-positive women are not sufficient.

Continue to: Challenges to implementing new USPSTF guideline...
Challenges to implementing new USPSTF guideline
Obstetricians have had great success in screening for perinatal depression because validated screening tools are available. Professional societies need to reach a consensus on recommending a specific screening tool for perinatal depression risk that can be used in all obstetric practices.
- personal history of depression
- current depressive symptoms that do not reach a diagnostic threshold
- low income
- all adolescents
- all single mothers
- recent exposure to intimate partner violence
- elevated anxiety symptoms
- a history of significant negative life events.
For many obstetricians, most of their pregnant patients meet the USPSTF criteria for being at high risk for perinatal depression and, per the guideline, these women should have a counseling intervention.
For many health systems, the resources available to provide mental health services are very limited. If most pregnant women need a counseling intervention, the health system must evolve to meet this need. In addition, risk factors for perinatal depression are also risk factors for having difficulty in participating in mental health interventions due to limitations, such as lack of transportation, social support, and money.4
Fortunately, clinicians from many backgrounds, including psychologists, social workers, nurse practitioners, and public health workers have the experience and/or training to provide the counseling interventions that have been shown to reduce the risk of perinatal depression. Health systems will need to tap all these resources to accommodate the large numbers of pregnant women who will be referred for counseling interventions. Pilot projects using electronic interventions, including telephone counseling, smartphone apps, and internet programs show promise.15,16 Electronic interventions have the potential to reach many pregnant women without over-taxing limited mental health resources.
A practical approach
Identify women at the greatest risk for perinatal depression and focus counseling interventions on this group. In my opinion, implementation of the USPSTF recommendation will take time. A practical approach would be to implement them in a staged sequence, focusing first on the women at highest risk, later extending the program to women at lesser risk. The two factors that confer the greatest risk of perinatal depression are a personal history of depression and high depression symptoms that do not meet criteria for depression.17 Many women with depression who take antidepressants discontinue their medications during pregnancy. These women are at very high risk for perinatal depression and deserve extra attention.18
Continue to: To identify women with a prior personal history of depression...
To identify women with a prior personal history of depression, it may be helpful to ask open-ended questions about a past diagnosis of depression or a mood disorder or use of antidepressant medications. To identify women with the greatest depression symptoms, utilize a lower cut-off for screening positive in the Edinburgh questionnaire. Practices that use an EPDS screen-positive score of 13 or greater could reduce the cut-off to 10 or 11, which would increase the number of women referred for evaluation and treatment.19
Clinical judgment and screening
Screening for prevalent depression and screening for women at increased risk for perinatal depression is challenging. ACOG highlights two important clinical issues1:
“Women with current depression or anxiety, a history of perinatal mood disorders, risk factors for perinatal mood disorders or suicidal thoughts warrant particularly close monitoring, evaluation and assessment.”
When screening for perinatal depression, screening test results should be interpreted within the clinical context. “A normal score for a tearful patient with a flat affect does not exclude depression; an elevated score in the context of an acute stressful event may resolve with close follow-up.”
In addition, women who screen-positive for prevalent depression and are subsequently evaluated by a mental health specialist may be identified as having mental health problems such as an anxiety disorder, substance misuse, or borderline personality disorder.20
Policy changes that support pregnant women and mothers could help to reduce the stress of pregnancy, birth, and childrearing, thereby reducing the risk of perinatal depression. The United States stands alone among rich nations in not providing paid parental leave. Paid maternity and parental leave would help many families respond more effectively to the initial stresses of parenthood.21 For women and families living in poverty, improved social support, including secure housing, protection from abusive partners, transportation resources, and access to healthy foods likely will reduce both stress and the risk of depression.
The ultimate goal: A healthy pregnancy
Clinicians have been phenomenally successful in screening for perinatal depression. The new USPSTF recommendation adds the prevention of perinatal depression to the goals of a healthy pregnancy. This recommendation builds upon the foundation of screening for acute illness (depression), pivoting to the public health perspective of disease prevention.
- American College of Obstetricians and Gynecologists. Screening for perinatal depression. ACOG Committee Opinion No 757. Obstet Gynecol. 2018;132:e208-e212.
- Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012-1024.
- Pearlstein T, Howard M, Salisbury A, et al. Postpartum depression. Am J Obstet Gynecol. 2009;200:357-364.
- Avalos LA, Raine-Bennett T, Chen H, et al. Improved perinatal depression screening, treatment and outcomes with a universal obstetric program. Obstet Gynecol. 2016;127:917-925.
- Cox EQ, Sowa NA, Meltzer-Brody SE, et al. The perinatal depression treatment cascade: baby steps toward improving outcomes. J Clin Psychiatry. 2016;77:1189-1200.
- Byatt N, Simas TA, Lundquist RS, et al. Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. J Psychosom Obstet Gynaecol. 2012;33:143-161.
- Siu AL, Bibbins-Domingo K, Grossman DC, et al. US Preventive Services Task Force (USPSTF). Screening for depression in adults. JAMA. 2016;315:380-387.
- Earls MF. Committee on Psychological Aspects of Child and Family Health. American Academy of Pediatrics. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics. 2010;126:1032-1039.
- The American College of Obstetricians and Gynecologists. Committee on Obstetric Practice. Committee Opinion No 630. Screening for perinatal depression. Obstet Gynecol. 2015;125:1268-1271.
- US Preventive Services Task Force. Interventions to prevent perinatal depression: US Preventive Services Task Force recommendations statement. JAMA. 2019;321:580-587.
- O’Connor E, Senger CA, Henninger ML, et al. Interventions to prevent perinatal depression: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321:588-601.
- Sockol LE. A systematic review and meta-analysis of interpersonal psychotherapy for perinatal women. J Affective Disorders. 2018;232:316-328.
- Sockol LE. A systematic review of the efficacy of cognitive behavioral therapy for treating and preventing perinatal depression. J Affective Disorders. 2015;177:7-21.
- Zlotnick C, Tzilos G, Miller I, et al. Randomized controlled trial to prevent postpartum depression in mothers on public assistance. J Affective Disorders. 2016;189:263-268.
- Haga SM, Drozd F, Lisoy C, et al. Mamma Mia—a randomized controlled trial of an internet-based intervention for perinatal depression. Psycholog Med. 2018;1-9.
- Shorey S, Ng YM, Ng ED, et al. Effectiveness of a technology-based supportive educational parenting program on parent outcomes (Part 1): Randomized controlled trial. J Med Internet Res. 2019;21:e10816.
- Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295:499-507.
- Goodman JH. Women’s attitudes, preferences and perceived barriers to treatment for perinatal depression. Birth. 2009;36:60-69.
- Smith-Nielsen J, Matthey S, Lange T, Vaever MS. Validation of the Edinburgh Postnatal Depression Scale against both DSM-5 and ICD-10 diagnostic criteria for depression. BMC Psychiatry. 2018;18:393.
- Judd F, Lorimer S, Thomson RH, et al. Screening for depression with the Edinburgh Postnatal Depression Scale and finding borderline personality disorder. Aust N Z J Psychiatry. 2018;Epub Oct 12. doi: 10.1177/0004867418804067.
- Diamond R. Promoting sensible parenting policies. Leading by example. JAMA. 2019;321:645- 646.
Perinatal depression is an episode of major or minor depression that occurs during pregnancy or in the 12 months after birth; it affects about 10% of new mothers.1 Perinatal depression adversely impacts mothers, children, and their families. Pregnant women with depression are at increased risk for preterm birth and low birth weight.2 Infants of mothers with postpartum depression have reduced bonding, lower rates of breastfeeding, delayed cognitive and social development, and an increased risk of future mental health issues.3 Timely treatment of perinatal depression can improve health outcomes for the woman, her children, and their family.
Clinicians follow current screening recommendations
The American College of Obstetricians and Gynecologists (ACOG) currently recommends that ObGynsscreen all pregnant women for depression and anxiety symptoms at least once during the perinatal period.1 Many practices use the Edinburgh Postnatal Depression Scale (EPDS) during pregnancy and postpartum. Women who screen positive are referred to mental health clinicians or have treatment initiated by their primary obstetrician.
Clinicians have been phenomenally successful in screening for perinatal depression. In a recent study from Kaiser Permanente Northern California, 98% of pregnant women were screened for perinatal depression, and a diagnosis of depression was made in 12%.4 Of note, only 47% of women who screened positive for depression initiated treatment, although 82% of women with the most severe symptoms initiated treatment. These data demonstrate that ObGyns consistently screen pregnant women for depression but, due to patient and system issues, treatment of all screen-positive women remains a yet unattained goal.5,6
New USPSTF guideline: Identify women at risk for perinatal depression and refer for counseling
In 2016 the United States Preventive Services Task Force (USPSTF) recommended that pregnant and postpartum women be screened for depression with adequate systems in place to ensure diagnosis, effective treatment, and follow-up.7 The 2016 USPSTF recommendation was consistent with prior guidelines from both the American Academy of Pediatrics in 20108 and ACOG in 2015.9
Now, the USPSTF is making a bold new recommendation, jumping ahead of professional societies: screen pregnant women to identify those at risk for perinatal depression and refer them for counseling (B recommendation; net benefit is moderate).10,11 The USPSTF recommendation is based on growing literature that shows counseling women at risk for perinatal depression reduces the risk of having an episode of major depression by 40%.11 Both interpersonal psychotherapy and cognitive behavioral therapy have been reported to be effective for preventing perinatal depression.12,13
As an example of the relevant literature, in one trial performed in Rhode Island, women who were 20 to 35 weeks pregnant with a high score (≥27) on the Cooper Survey Questionnaire and on public assistance were randomized to counseling or usual care. The counseling intervention involved 4 small group (2 to 5 women) sessions of 90 minutes and one individual session of 50 minutes.14 The treatment focused on managing the transition to motherhood, developing a support system, improving communication skills to manage conflict, goal setting, and identifying psychosocial supports for new mothers. At 6 months after birth, a depressive episode had occurred in 31% of the control women and 16% of the women who had experienced the intervention (P = .041). At 12 months after birth, a depressive episode had occurred in 40% of control women and 26% of women in the intervention group (P = .052).
Of note, most cases of postpartum depression were diagnosed more than 3 months after birth, a time when new mothers generally no longer are receiving regular postpartum care by an obstetrician. The timing of the diagnosis of perinatal depression indicates that an effective handoff between the obstetrician and primary care and/or mental health clinicians is of great importance. The investigators concluded that pregnant women at very high risk for perinatal depression who receive interpersonal therapy have a lower rate of a postpartum depressive episode than women receiving usual care.14
Pregnancy, delivery, and the first year following birth are stressful for many women and their families. Women who are young, poor, and with minimal social supports are at especially high risk for developing perinatal depression. However, it will be challenging for obstetric practices to rapidly implement the new USPSTF recommendations because there is no professional consensus on how to screen women to identify those at high risk for perinatal depression, and mental health resources to care for the screen-positive women are not sufficient.

Continue to: Challenges to implementing new USPSTF guideline...
Challenges to implementing new USPSTF guideline
Obstetricians have had great success in screening for perinatal depression because validated screening tools are available. Professional societies need to reach a consensus on recommending a specific screening tool for perinatal depression risk that can be used in all obstetric practices.
- personal history of depression
- current depressive symptoms that do not reach a diagnostic threshold
- low income
- all adolescents
- all single mothers
- recent exposure to intimate partner violence
- elevated anxiety symptoms
- a history of significant negative life events.
For many obstetricians, most of their pregnant patients meet the USPSTF criteria for being at high risk for perinatal depression and, per the guideline, these women should have a counseling intervention.
For many health systems, the resources available to provide mental health services are very limited. If most pregnant women need a counseling intervention, the health system must evolve to meet this need. In addition, risk factors for perinatal depression are also risk factors for having difficulty in participating in mental health interventions due to limitations, such as lack of transportation, social support, and money.4
Fortunately, clinicians from many backgrounds, including psychologists, social workers, nurse practitioners, and public health workers have the experience and/or training to provide the counseling interventions that have been shown to reduce the risk of perinatal depression. Health systems will need to tap all these resources to accommodate the large numbers of pregnant women who will be referred for counseling interventions. Pilot projects using electronic interventions, including telephone counseling, smartphone apps, and internet programs show promise.15,16 Electronic interventions have the potential to reach many pregnant women without over-taxing limited mental health resources.
A practical approach
Identify women at the greatest risk for perinatal depression and focus counseling interventions on this group. In my opinion, implementation of the USPSTF recommendation will take time. A practical approach would be to implement them in a staged sequence, focusing first on the women at highest risk, later extending the program to women at lesser risk. The two factors that confer the greatest risk of perinatal depression are a personal history of depression and high depression symptoms that do not meet criteria for depression.17 Many women with depression who take antidepressants discontinue their medications during pregnancy. These women are at very high risk for perinatal depression and deserve extra attention.18
Continue to: To identify women with a prior personal history of depression...
To identify women with a prior personal history of depression, it may be helpful to ask open-ended questions about a past diagnosis of depression or a mood disorder or use of antidepressant medications. To identify women with the greatest depression symptoms, utilize a lower cut-off for screening positive in the Edinburgh questionnaire. Practices that use an EPDS screen-positive score of 13 or greater could reduce the cut-off to 10 or 11, which would increase the number of women referred for evaluation and treatment.19
Clinical judgment and screening
Screening for prevalent depression and screening for women at increased risk for perinatal depression is challenging. ACOG highlights two important clinical issues1:
“Women with current depression or anxiety, a history of perinatal mood disorders, risk factors for perinatal mood disorders or suicidal thoughts warrant particularly close monitoring, evaluation and assessment.”
When screening for perinatal depression, screening test results should be interpreted within the clinical context. “A normal score for a tearful patient with a flat affect does not exclude depression; an elevated score in the context of an acute stressful event may resolve with close follow-up.”
In addition, women who screen-positive for prevalent depression and are subsequently evaluated by a mental health specialist may be identified as having mental health problems such as an anxiety disorder, substance misuse, or borderline personality disorder.20
Policy changes that support pregnant women and mothers could help to reduce the stress of pregnancy, birth, and childrearing, thereby reducing the risk of perinatal depression. The United States stands alone among rich nations in not providing paid parental leave. Paid maternity and parental leave would help many families respond more effectively to the initial stresses of parenthood.21 For women and families living in poverty, improved social support, including secure housing, protection from abusive partners, transportation resources, and access to healthy foods likely will reduce both stress and the risk of depression.
The ultimate goal: A healthy pregnancy
Clinicians have been phenomenally successful in screening for perinatal depression. The new USPSTF recommendation adds the prevention of perinatal depression to the goals of a healthy pregnancy. This recommendation builds upon the foundation of screening for acute illness (depression), pivoting to the public health perspective of disease prevention.
Perinatal depression is an episode of major or minor depression that occurs during pregnancy or in the 12 months after birth; it affects about 10% of new mothers.1 Perinatal depression adversely impacts mothers, children, and their families. Pregnant women with depression are at increased risk for preterm birth and low birth weight.2 Infants of mothers with postpartum depression have reduced bonding, lower rates of breastfeeding, delayed cognitive and social development, and an increased risk of future mental health issues.3 Timely treatment of perinatal depression can improve health outcomes for the woman, her children, and their family.
Clinicians follow current screening recommendations
The American College of Obstetricians and Gynecologists (ACOG) currently recommends that ObGynsscreen all pregnant women for depression and anxiety symptoms at least once during the perinatal period.1 Many practices use the Edinburgh Postnatal Depression Scale (EPDS) during pregnancy and postpartum. Women who screen positive are referred to mental health clinicians or have treatment initiated by their primary obstetrician.
Clinicians have been phenomenally successful in screening for perinatal depression. In a recent study from Kaiser Permanente Northern California, 98% of pregnant women were screened for perinatal depression, and a diagnosis of depression was made in 12%.4 Of note, only 47% of women who screened positive for depression initiated treatment, although 82% of women with the most severe symptoms initiated treatment. These data demonstrate that ObGyns consistently screen pregnant women for depression but, due to patient and system issues, treatment of all screen-positive women remains a yet unattained goal.5,6
New USPSTF guideline: Identify women at risk for perinatal depression and refer for counseling
In 2016 the United States Preventive Services Task Force (USPSTF) recommended that pregnant and postpartum women be screened for depression with adequate systems in place to ensure diagnosis, effective treatment, and follow-up.7 The 2016 USPSTF recommendation was consistent with prior guidelines from both the American Academy of Pediatrics in 20108 and ACOG in 2015.9
Now, the USPSTF is making a bold new recommendation, jumping ahead of professional societies: screen pregnant women to identify those at risk for perinatal depression and refer them for counseling (B recommendation; net benefit is moderate).10,11 The USPSTF recommendation is based on growing literature that shows counseling women at risk for perinatal depression reduces the risk of having an episode of major depression by 40%.11 Both interpersonal psychotherapy and cognitive behavioral therapy have been reported to be effective for preventing perinatal depression.12,13
As an example of the relevant literature, in one trial performed in Rhode Island, women who were 20 to 35 weeks pregnant with a high score (≥27) on the Cooper Survey Questionnaire and on public assistance were randomized to counseling or usual care. The counseling intervention involved 4 small group (2 to 5 women) sessions of 90 minutes and one individual session of 50 minutes.14 The treatment focused on managing the transition to motherhood, developing a support system, improving communication skills to manage conflict, goal setting, and identifying psychosocial supports for new mothers. At 6 months after birth, a depressive episode had occurred in 31% of the control women and 16% of the women who had experienced the intervention (P = .041). At 12 months after birth, a depressive episode had occurred in 40% of control women and 26% of women in the intervention group (P = .052).
Of note, most cases of postpartum depression were diagnosed more than 3 months after birth, a time when new mothers generally no longer are receiving regular postpartum care by an obstetrician. The timing of the diagnosis of perinatal depression indicates that an effective handoff between the obstetrician and primary care and/or mental health clinicians is of great importance. The investigators concluded that pregnant women at very high risk for perinatal depression who receive interpersonal therapy have a lower rate of a postpartum depressive episode than women receiving usual care.14
Pregnancy, delivery, and the first year following birth are stressful for many women and their families. Women who are young, poor, and with minimal social supports are at especially high risk for developing perinatal depression. However, it will be challenging for obstetric practices to rapidly implement the new USPSTF recommendations because there is no professional consensus on how to screen women to identify those at high risk for perinatal depression, and mental health resources to care for the screen-positive women are not sufficient.

Continue to: Challenges to implementing new USPSTF guideline...
Challenges to implementing new USPSTF guideline
Obstetricians have had great success in screening for perinatal depression because validated screening tools are available. Professional societies need to reach a consensus on recommending a specific screening tool for perinatal depression risk that can be used in all obstetric practices.
- personal history of depression
- current depressive symptoms that do not reach a diagnostic threshold
- low income
- all adolescents
- all single mothers
- recent exposure to intimate partner violence
- elevated anxiety symptoms
- a history of significant negative life events.
For many obstetricians, most of their pregnant patients meet the USPSTF criteria for being at high risk for perinatal depression and, per the guideline, these women should have a counseling intervention.
For many health systems, the resources available to provide mental health services are very limited. If most pregnant women need a counseling intervention, the health system must evolve to meet this need. In addition, risk factors for perinatal depression are also risk factors for having difficulty in participating in mental health interventions due to limitations, such as lack of transportation, social support, and money.4
Fortunately, clinicians from many backgrounds, including psychologists, social workers, nurse practitioners, and public health workers have the experience and/or training to provide the counseling interventions that have been shown to reduce the risk of perinatal depression. Health systems will need to tap all these resources to accommodate the large numbers of pregnant women who will be referred for counseling interventions. Pilot projects using electronic interventions, including telephone counseling, smartphone apps, and internet programs show promise.15,16 Electronic interventions have the potential to reach many pregnant women without over-taxing limited mental health resources.
A practical approach
Identify women at the greatest risk for perinatal depression and focus counseling interventions on this group. In my opinion, implementation of the USPSTF recommendation will take time. A practical approach would be to implement them in a staged sequence, focusing first on the women at highest risk, later extending the program to women at lesser risk. The two factors that confer the greatest risk of perinatal depression are a personal history of depression and high depression symptoms that do not meet criteria for depression.17 Many women with depression who take antidepressants discontinue their medications during pregnancy. These women are at very high risk for perinatal depression and deserve extra attention.18
Continue to: To identify women with a prior personal history of depression...
To identify women with a prior personal history of depression, it may be helpful to ask open-ended questions about a past diagnosis of depression or a mood disorder or use of antidepressant medications. To identify women with the greatest depression symptoms, utilize a lower cut-off for screening positive in the Edinburgh questionnaire. Practices that use an EPDS screen-positive score of 13 or greater could reduce the cut-off to 10 or 11, which would increase the number of women referred for evaluation and treatment.19
Clinical judgment and screening
Screening for prevalent depression and screening for women at increased risk for perinatal depression is challenging. ACOG highlights two important clinical issues1:
“Women with current depression or anxiety, a history of perinatal mood disorders, risk factors for perinatal mood disorders or suicidal thoughts warrant particularly close monitoring, evaluation and assessment.”
When screening for perinatal depression, screening test results should be interpreted within the clinical context. “A normal score for a tearful patient with a flat affect does not exclude depression; an elevated score in the context of an acute stressful event may resolve with close follow-up.”
In addition, women who screen-positive for prevalent depression and are subsequently evaluated by a mental health specialist may be identified as having mental health problems such as an anxiety disorder, substance misuse, or borderline personality disorder.20
Policy changes that support pregnant women and mothers could help to reduce the stress of pregnancy, birth, and childrearing, thereby reducing the risk of perinatal depression. The United States stands alone among rich nations in not providing paid parental leave. Paid maternity and parental leave would help many families respond more effectively to the initial stresses of parenthood.21 For women and families living in poverty, improved social support, including secure housing, protection from abusive partners, transportation resources, and access to healthy foods likely will reduce both stress and the risk of depression.
The ultimate goal: A healthy pregnancy
Clinicians have been phenomenally successful in screening for perinatal depression. The new USPSTF recommendation adds the prevention of perinatal depression to the goals of a healthy pregnancy. This recommendation builds upon the foundation of screening for acute illness (depression), pivoting to the public health perspective of disease prevention.
- American College of Obstetricians and Gynecologists. Screening for perinatal depression. ACOG Committee Opinion No 757. Obstet Gynecol. 2018;132:e208-e212.
- Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012-1024.
- Pearlstein T, Howard M, Salisbury A, et al. Postpartum depression. Am J Obstet Gynecol. 2009;200:357-364.
- Avalos LA, Raine-Bennett T, Chen H, et al. Improved perinatal depression screening, treatment and outcomes with a universal obstetric program. Obstet Gynecol. 2016;127:917-925.
- Cox EQ, Sowa NA, Meltzer-Brody SE, et al. The perinatal depression treatment cascade: baby steps toward improving outcomes. J Clin Psychiatry. 2016;77:1189-1200.
- Byatt N, Simas TA, Lundquist RS, et al. Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. J Psychosom Obstet Gynaecol. 2012;33:143-161.
- Siu AL, Bibbins-Domingo K, Grossman DC, et al. US Preventive Services Task Force (USPSTF). Screening for depression in adults. JAMA. 2016;315:380-387.
- Earls MF. Committee on Psychological Aspects of Child and Family Health. American Academy of Pediatrics. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics. 2010;126:1032-1039.
- The American College of Obstetricians and Gynecologists. Committee on Obstetric Practice. Committee Opinion No 630. Screening for perinatal depression. Obstet Gynecol. 2015;125:1268-1271.
- US Preventive Services Task Force. Interventions to prevent perinatal depression: US Preventive Services Task Force recommendations statement. JAMA. 2019;321:580-587.
- O’Connor E, Senger CA, Henninger ML, et al. Interventions to prevent perinatal depression: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321:588-601.
- Sockol LE. A systematic review and meta-analysis of interpersonal psychotherapy for perinatal women. J Affective Disorders. 2018;232:316-328.
- Sockol LE. A systematic review of the efficacy of cognitive behavioral therapy for treating and preventing perinatal depression. J Affective Disorders. 2015;177:7-21.
- Zlotnick C, Tzilos G, Miller I, et al. Randomized controlled trial to prevent postpartum depression in mothers on public assistance. J Affective Disorders. 2016;189:263-268.
- Haga SM, Drozd F, Lisoy C, et al. Mamma Mia—a randomized controlled trial of an internet-based intervention for perinatal depression. Psycholog Med. 2018;1-9.
- Shorey S, Ng YM, Ng ED, et al. Effectiveness of a technology-based supportive educational parenting program on parent outcomes (Part 1): Randomized controlled trial. J Med Internet Res. 2019;21:e10816.
- Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295:499-507.
- Goodman JH. Women’s attitudes, preferences and perceived barriers to treatment for perinatal depression. Birth. 2009;36:60-69.
- Smith-Nielsen J, Matthey S, Lange T, Vaever MS. Validation of the Edinburgh Postnatal Depression Scale against both DSM-5 and ICD-10 diagnostic criteria for depression. BMC Psychiatry. 2018;18:393.
- Judd F, Lorimer S, Thomson RH, et al. Screening for depression with the Edinburgh Postnatal Depression Scale and finding borderline personality disorder. Aust N Z J Psychiatry. 2018;Epub Oct 12. doi: 10.1177/0004867418804067.
- Diamond R. Promoting sensible parenting policies. Leading by example. JAMA. 2019;321:645- 646.
- American College of Obstetricians and Gynecologists. Screening for perinatal depression. ACOG Committee Opinion No 757. Obstet Gynecol. 2018;132:e208-e212.
- Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012-1024.
- Pearlstein T, Howard M, Salisbury A, et al. Postpartum depression. Am J Obstet Gynecol. 2009;200:357-364.
- Avalos LA, Raine-Bennett T, Chen H, et al. Improved perinatal depression screening, treatment and outcomes with a universal obstetric program. Obstet Gynecol. 2016;127:917-925.
- Cox EQ, Sowa NA, Meltzer-Brody SE, et al. The perinatal depression treatment cascade: baby steps toward improving outcomes. J Clin Psychiatry. 2016;77:1189-1200.
- Byatt N, Simas TA, Lundquist RS, et al. Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. J Psychosom Obstet Gynaecol. 2012;33:143-161.
- Siu AL, Bibbins-Domingo K, Grossman DC, et al. US Preventive Services Task Force (USPSTF). Screening for depression in adults. JAMA. 2016;315:380-387.
- Earls MF. Committee on Psychological Aspects of Child and Family Health. American Academy of Pediatrics. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics. 2010;126:1032-1039.
- The American College of Obstetricians and Gynecologists. Committee on Obstetric Practice. Committee Opinion No 630. Screening for perinatal depression. Obstet Gynecol. 2015;125:1268-1271.
- US Preventive Services Task Force. Interventions to prevent perinatal depression: US Preventive Services Task Force recommendations statement. JAMA. 2019;321:580-587.
- O’Connor E, Senger CA, Henninger ML, et al. Interventions to prevent perinatal depression: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321:588-601.
- Sockol LE. A systematic review and meta-analysis of interpersonal psychotherapy for perinatal women. J Affective Disorders. 2018;232:316-328.
- Sockol LE. A systematic review of the efficacy of cognitive behavioral therapy for treating and preventing perinatal depression. J Affective Disorders. 2015;177:7-21.
- Zlotnick C, Tzilos G, Miller I, et al. Randomized controlled trial to prevent postpartum depression in mothers on public assistance. J Affective Disorders. 2016;189:263-268.
- Haga SM, Drozd F, Lisoy C, et al. Mamma Mia—a randomized controlled trial of an internet-based intervention for perinatal depression. Psycholog Med. 2018;1-9.
- Shorey S, Ng YM, Ng ED, et al. Effectiveness of a technology-based supportive educational parenting program on parent outcomes (Part 1): Randomized controlled trial. J Med Internet Res. 2019;21:e10816.
- Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295:499-507.
- Goodman JH. Women’s attitudes, preferences and perceived barriers to treatment for perinatal depression. Birth. 2009;36:60-69.
- Smith-Nielsen J, Matthey S, Lange T, Vaever MS. Validation of the Edinburgh Postnatal Depression Scale against both DSM-5 and ICD-10 diagnostic criteria for depression. BMC Psychiatry. 2018;18:393.
- Judd F, Lorimer S, Thomson RH, et al. Screening for depression with the Edinburgh Postnatal Depression Scale and finding borderline personality disorder. Aust N Z J Psychiatry. 2018;Epub Oct 12. doi: 10.1177/0004867418804067.
- Diamond R. Promoting sensible parenting policies. Leading by example. JAMA. 2019;321:645- 646.
What is your approach to the persistent occiput posterior malposition?
CASE 7- to 8-lb baby suspected to be in occiput posterior (OP) position
A certified nurse midwife (CNM) asks you to consult on a 37-year-old woman (G1P0) at 41 weeks’ gestation who was admitted to labor and delivery for a late-term induction. The patient had a normal first stage of labor with placement of a combined spinal-epidural anesthetic at a cervical dilation of 4 cm. She has been fully dilated for 3.5 hours and pushing for 2.5 hours with a Category 1 fetal heart rate tracing. The CNM reports that the estimated fetal weight is 7 to 8 lb and the station is +3/5. She suspects that the fetus is in the left OP position. She asks for your advice on how to best deliver the fetus. The patient strongly prefers not to have a cesarean delivery (CD).
What is your recommended approach?
The cardinal movements of labor include cephalic engagement, descent, flexion, internal rotation, extension and rotation of the head at delivery, internal rotation of the shoulders, and expulsion of the body. In the first stage of labor many fetuses are in the OP position. Flexion and internal rotation of the fetal head in a mother with a gynecoid pelvis results in most fetuses assuming an occiput anterior (OA) position with the presenting diameter of the head (occipitobregmatic) being optimal for spontaneous vaginal delivery. Late in the second stage of labor only about 5% of fetuses are in the OP position with the presenting diameter of the head being large (occipitofrontal) with an extended head attitude, thereby reducing the probability of a rapid spontaneous vaginal delivery.
Risk factors for OP position late in the second stage of labor include1,2:
- nulliparity
- body mass index > 29 kg/m2
- gestation age ≥ 41 weeks
- birth weight > 4 kg
- regional anesthesia.
Maternal outcomes associated with persistent OP position include protracted first and second stage of labor, arrest of second stage of labor, and increased rates of operative vaginal delivery, anal sphincter injury, CD, postpartum hemorrhage, chorioamnionitis, and endomyometritis.1,3,4 The neonatal complications of persistent OP position include increased rates of shoulder dystocia, low Apgar score, umbilical artery acidemia, meconium, and admission to a neonatal intensive care unit.1,5
Diagnosis
Many obstetricians report that they can reliably detect a fetus in the OP position based upon abdominal palpation of the fetal spine and digital vaginal examination of the fetal sutures, fontanels, and ears. Such self-confidence may not be wholly warranted, however. Most contemporary data indicate that digital vaginal examination has an error rate of approximately 20% for identifying the position of the cephalic fetus, especially in the presence of fetal caput succedaneum and asynclitism.6-10
The International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) recommends that cephalic position be determined by transabdominal imaging.11 By placing the ultrasound probe on the maternal abdomen, a view of the fetal body at the level of the chest helps determine the position of the fetal spine. When the probe is placed in a suprapubic position, the observation of the fetal orbits facing the probe indicates an OP position.
When the presenting part is at a very low station, a transperineal ultrasound may be helpful to determine the position of the occiput. The ISUOG recommends that position be defined using a clock face, with positions from 330 h to 830 h being indicative of OP and positions from 930 h to 230 h being indicative of OA.11 The small remaining slivers on the clock face indicate an occiput transverse position (FIGURE).11

Continue to: Approaches to managing the OP position
Approaches to managing the OP position
First stage of labor
Identification of a cephalic-presenting fetus in the OP position in the first stage of labor might warrant increased attention to fetal position in the second stage of labor, but does not usually alter management of the first stage.
Second stage of labor
If an OP position is identified in the second stage of labor, many obstetricians will consider manual rotation of the fetal occiput to an anterior pelvic quadrant to facilitate labor progress. Because a fetus in the OP position may spontaneously rotate to the OA position at any point during the second stage, a judicious interval of waiting is reasonable before attempting a manual rotation in the second stage. For example, allowing the second stage to progress for 60 to 90 min in a nulliparous woman or 30 to 60 min in a multiparous woman will permit some fetuses to rotate to the OA position without intervention.
If the OP position persists beyond these time points, a manual rotation could be considered. There are no high-quality clinical trials to support this maneuver,12 but observational reports suggest that this low-risk maneuver may help reduce the rate of CD and anal sphincter trauma.13-15
Manual rotation from OP to OA. Prior to performing the rotation, the maternal bladder should be emptied and an adequate anesthetic provided. One technique is to use the 4 fingers of the hand as a “spatula” to turn the head. If the fetus is in a left OP position, the operator’s right hand is pronated and inserted into the vagina, palm up. Four fingers are placed under the posterior parietal bone with the thumb over the anterior parietal bone (ILLUSTRATION).4 The operator uses the fingers and thumb to flex and rotate the head to the right, moving the fetal occiput into an anterior pelvic quadrant.4 If the head is in the right OP position, the left hand is used to rotate the head. The nonvaginal hand can be placed on the maternal abdominal wall to assess the fetal spine position as the fetal head is rotated. The fetal head may need to be held in the anterior pelvic quadrant during a few maternal pushes to prevent the head from rotating back into the OP position.
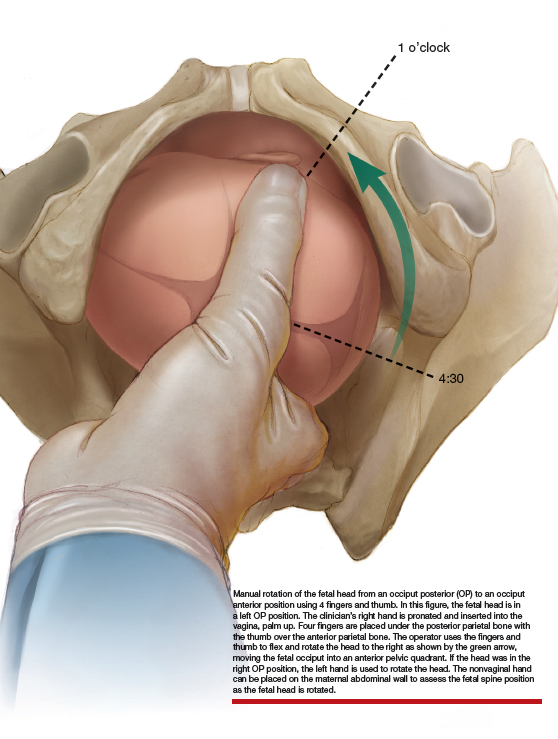
Approaching delivery late in the second stage
If the second stage has progressed for 3 or 4 hours, as in the case described above, and the fetus remains in the OP position, delivery may be indicated to avoid the maternal and fetal complications of an even more prolonged second stage. At some point in a prolonged second stage, expectant management carries more maternal and fetal risks than intervention.
Late in the second stage, options for delivery of the fetus in the OP include: CD, rotational forceps delivery, direct forceps delivery from the OP position, and vacuum delivery.
Cesarean delivery. CD of the fetus in the OP position may be indicated when the fetus is estimated to be macrosomic, the station is high (biparietal diameter palpable on abdominal examination), or when the parturient has an android pelvis (narrow fore-pelvis and anterior convergence of the pelvic bone structures in a wedge shape). During CD, if difficulty is encountered in delivering the fetal head, a hand from below, extension of the uterine incision, or reverse breech extraction may be necessary to complete the delivery. If the clinical situation is conducive to operative vaginal delivery, forceps or vacuum can be used.
Continue to: Rotational forceps delivery...
Rotational forceps delivery. During residency I was told to always use rotational forceps to deliver a fetus in the persistently OP position if the parturient had a gynecoid pelvis (wide oval shape of pelvic bones, wide subpubic arch). Dr. Frederick Irving wrote16:
“Although textbooks almost universally advocate the extraction of the occiput directly posterior without rotation we do not advise it.... Such an extraction maneuver is inartistic and show[s] a lack of regard for the mechanical factors involved in the mechanism of labor. The method used at the Boston Lying-In Hospital presupposes an accurate diagnosis of the primary position. If the fetal back is on the right the head should be rotated to the right; if on the left, toward the left. The head is always rotated in the direction in which the back lies. The forceps are applied as if the occiput was directly anterior. Carrying the forceps handles in a wide sweep the occiput is now rotated to the anterior quadrant of the pelvis or 135 degrees. It will be found that the head turns easily in the way it should go but that it is difficult or impossible to rotate it in the improper direction. The instrument is then reapplied as in the second part of the Scanzoni maneuver.”
Rotation of the fetus from the OP to the OA position may reduce the risk of sphincter injury with vaginal birth. With the waning of rotational forceps skills, many obstetricians prefer a nonrotational approach with direct forceps or vacuum delivery from the OP position.
Direct forceps delivery from the OP position. A fetus in the OP position for 3 to 4 hours of the second stage of labor will often have a significant degree of head molding. The Simpson forceps, with its shallow and longer cephalic curve, accommodates significant fetal head molding and is a good forceps choice in this situation.
Vacuum delivery. In the United States, approximately 5% of vaginal deliveries are performed with a vacuum device, and 1% with forceps.17 Consequently, many obstetricians frequently perform operative vaginal delivery with a vacuum device and infrequently or never perform operative vaginal delivery with forceps. Vacuum vaginal delivery may be the instrument of choice for many obstetricians performing an operative delivery of a fetus in the OP position. However, the vacuum has a higher rate of failure, especially if the OP fetus is at a higher station.18
In some centers, direct forceps delivery from the OP position is preferred over an attempt at vacuum delivery, because in contemporary obstetric practice most centers do not permit the sequential use of vacuum followed by forceps (due to the higher rate of fetal trauma of combination operative delivery). Since vacuum delivery of the fetus in the OP position has a greater rate of failure than forceps, it may be best to initiate operative vaginal delivery of the fetus in the OP position with forceps. If vacuum is used to attempt a vaginal delivery and fails due to too many pop-offs, a CD would be the next step.
Take action when needed to optimize outcomes
The persistent OP position is associated with a longer second stage of labor. It is common during a change of shift for an obstetrician to sign out to the on-coming clinician a case of a prolonged second stage with the fetus in the OP position. In this situation, the on-coming clinician cannot wait hour after hour after hour hoping for a spontaneous delivery. If the on-coming clinician has a clear plan of how to deal with the persistent OP position—including ultrasound confirmation of position and physical examination to determine station, fetal size and adequacy of the pelvis, and timely selection of a delivery technique—the adverse maternal and neonatal outcomes sometimes caused by the persistent OP position will be minimized.
Continue to: CASE Resolved...
CASE Resolved
The consulting obstetrician performed a transabdominal ultrasound and observed the fetal orbits were facing the transducer, confirming an OP position. On physical examination, the station was +3/5, and the fetal weight was confirmed to be approximately 8 lb. The obstetrician recommended a direct forceps delivery from the OP position. The patient and CNM agreed with the plan.
The obstetrician applied Simpson forceps and performed a mediolateral episiotomy just prior to delivery of the head. Following delivery, the rectal sphincter and anal mucosa were intact and the episiotomy was repaired. The newborn, safely delivered, and the mother, having avoided a CD, were transferred to the postpartum floor later in the day.
- Cheng YW, Hubbard A, Caughey AB, et al. The association between persistent fetal occiput posterior position and perinatal outcomes: An example of propensity score and covariate distance matching. Am J Epidemiol. 2010;171:656-663.
- Cheng YW, Shaffer BL, Caughey AB. Associated factors and outcomes of persistent occiput posterior position: a retrospective cohort study from 1976 to 2001. J Matern Fetal Neonatal Med. 2006;19:563-568.
- Ponkey SE, Cohen AP, Heffner LJ, et al. Persistent fetal occiput posterior position: obstetric outcomes. Obstet Gynecol. 2003;101:915-920.
- Barth WH Jr. Persistent occiput posterior. Obstet Gynecol. 2015;125:695-709.
- Cheng YW, Shaffer BL, Caughey AB. The association between persistent occiput posterior position and neonatal outcomes. Obstet Gynecol. 2006;107:837-844.
- Ghi T, Dall’Asta A, Masturzo B, et al. Randomised Italian sonography for occiput position trial ante vacuum. Ultrasound Obstet Gynecol. 2018;52:699-705.
- Bellussi F, Ghi T, Youssef A, et al. The use of intrapartum ultrasound to diagnose malpositions and cephalic malpresentations. Am J Obstet Gynecol. 2017;217:633-641.
- Ramphul M, Ooi PV, Burke G, et al. Instrumental delivery and ultrasound: a multicenter randomised controlled trial of ultrasound assessment of the fetal head position versus standard of care as an approach to prevent morbidity at instrumental delivery. BJOG. 2014;121:1029-1038.
- Malvasi A, Tinelli A, Barbera A, et al. Occiput posterior position diagnosis: vaginal examination or intrapartum sonography? A clinical review. J Matern Fetal Neonatal Med. 2014;27:520-526.
- Akmal S, Tsoi E, Kaemtas N, et al. Intrapartum sonography to determine fetal head position. J Matern Fetal Neonatal Med. 2002;12:172-177.
- Ghi T, Eggebo T, Lees C, et al. ISUOG practice guidelines: intrapartum ultrasound. Ultrasound Obstet Gynecol. 2018;52:128-139.
- Phipps H, de Vries B, Hyett J, et al. Prophylactic manual rotation for fetal malposition to reduce operative delivery. Cochrane Database Syst Rev. 2014;CD009298.
- Le Ray C, Serres P, Schmitz T, et al. Manual rotation in occiput posterior or transverse positions. Obstet Gynecol. 2007;110:873-879.
- Shaffer BL, Cheng YW, Vargas JE, et al. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24:65-72.
- Bertholdt C, Gauchotte E, Dap M, et al. Predictors of successful manual rotation for occiput posterior positions. Int J Gynaecol Obstet. 2019;144:210–215.
- Irving FC. A Textbook of Obstetrics. New York, NY: Macmillan, NY; 1936:426-428.
- Merriam AA, Ananth CV, Wright JD, et al. Trends in operative vaginal delivery, 2005–2013: a population-based study. BJOG. 2017;124:1365-1372.
- Verhoeven CJ, Nuij C, Janssen-Rolf CR, et al. Predictors of failure of vacuum-assisted vaginal delivery: a case-control study. Eur J Obstet Gynecol Reprod Biol. 2016;200:29-34.
CASE 7- to 8-lb baby suspected to be in occiput posterior (OP) position
A certified nurse midwife (CNM) asks you to consult on a 37-year-old woman (G1P0) at 41 weeks’ gestation who was admitted to labor and delivery for a late-term induction. The patient had a normal first stage of labor with placement of a combined spinal-epidural anesthetic at a cervical dilation of 4 cm. She has been fully dilated for 3.5 hours and pushing for 2.5 hours with a Category 1 fetal heart rate tracing. The CNM reports that the estimated fetal weight is 7 to 8 lb and the station is +3/5. She suspects that the fetus is in the left OP position. She asks for your advice on how to best deliver the fetus. The patient strongly prefers not to have a cesarean delivery (CD).
What is your recommended approach?
The cardinal movements of labor include cephalic engagement, descent, flexion, internal rotation, extension and rotation of the head at delivery, internal rotation of the shoulders, and expulsion of the body. In the first stage of labor many fetuses are in the OP position. Flexion and internal rotation of the fetal head in a mother with a gynecoid pelvis results in most fetuses assuming an occiput anterior (OA) position with the presenting diameter of the head (occipitobregmatic) being optimal for spontaneous vaginal delivery. Late in the second stage of labor only about 5% of fetuses are in the OP position with the presenting diameter of the head being large (occipitofrontal) with an extended head attitude, thereby reducing the probability of a rapid spontaneous vaginal delivery.
Risk factors for OP position late in the second stage of labor include1,2:
- nulliparity
- body mass index > 29 kg/m2
- gestation age ≥ 41 weeks
- birth weight > 4 kg
- regional anesthesia.
Maternal outcomes associated with persistent OP position include protracted first and second stage of labor, arrest of second stage of labor, and increased rates of operative vaginal delivery, anal sphincter injury, CD, postpartum hemorrhage, chorioamnionitis, and endomyometritis.1,3,4 The neonatal complications of persistent OP position include increased rates of shoulder dystocia, low Apgar score, umbilical artery acidemia, meconium, and admission to a neonatal intensive care unit.1,5
Diagnosis
Many obstetricians report that they can reliably detect a fetus in the OP position based upon abdominal palpation of the fetal spine and digital vaginal examination of the fetal sutures, fontanels, and ears. Such self-confidence may not be wholly warranted, however. Most contemporary data indicate that digital vaginal examination has an error rate of approximately 20% for identifying the position of the cephalic fetus, especially in the presence of fetal caput succedaneum and asynclitism.6-10
The International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) recommends that cephalic position be determined by transabdominal imaging.11 By placing the ultrasound probe on the maternal abdomen, a view of the fetal body at the level of the chest helps determine the position of the fetal spine. When the probe is placed in a suprapubic position, the observation of the fetal orbits facing the probe indicates an OP position.
When the presenting part is at a very low station, a transperineal ultrasound may be helpful to determine the position of the occiput. The ISUOG recommends that position be defined using a clock face, with positions from 330 h to 830 h being indicative of OP and positions from 930 h to 230 h being indicative of OA.11 The small remaining slivers on the clock face indicate an occiput transverse position (FIGURE).11

Continue to: Approaches to managing the OP position
Approaches to managing the OP position
First stage of labor
Identification of a cephalic-presenting fetus in the OP position in the first stage of labor might warrant increased attention to fetal position in the second stage of labor, but does not usually alter management of the first stage.
Second stage of labor
If an OP position is identified in the second stage of labor, many obstetricians will consider manual rotation of the fetal occiput to an anterior pelvic quadrant to facilitate labor progress. Because a fetus in the OP position may spontaneously rotate to the OA position at any point during the second stage, a judicious interval of waiting is reasonable before attempting a manual rotation in the second stage. For example, allowing the second stage to progress for 60 to 90 min in a nulliparous woman or 30 to 60 min in a multiparous woman will permit some fetuses to rotate to the OA position without intervention.
If the OP position persists beyond these time points, a manual rotation could be considered. There are no high-quality clinical trials to support this maneuver,12 but observational reports suggest that this low-risk maneuver may help reduce the rate of CD and anal sphincter trauma.13-15
Manual rotation from OP to OA. Prior to performing the rotation, the maternal bladder should be emptied and an adequate anesthetic provided. One technique is to use the 4 fingers of the hand as a “spatula” to turn the head. If the fetus is in a left OP position, the operator’s right hand is pronated and inserted into the vagina, palm up. Four fingers are placed under the posterior parietal bone with the thumb over the anterior parietal bone (ILLUSTRATION).4 The operator uses the fingers and thumb to flex and rotate the head to the right, moving the fetal occiput into an anterior pelvic quadrant.4 If the head is in the right OP position, the left hand is used to rotate the head. The nonvaginal hand can be placed on the maternal abdominal wall to assess the fetal spine position as the fetal head is rotated. The fetal head may need to be held in the anterior pelvic quadrant during a few maternal pushes to prevent the head from rotating back into the OP position.

Approaching delivery late in the second stage
If the second stage has progressed for 3 or 4 hours, as in the case described above, and the fetus remains in the OP position, delivery may be indicated to avoid the maternal and fetal complications of an even more prolonged second stage. At some point in a prolonged second stage, expectant management carries more maternal and fetal risks than intervention.
Late in the second stage, options for delivery of the fetus in the OP include: CD, rotational forceps delivery, direct forceps delivery from the OP position, and vacuum delivery.
Cesarean delivery. CD of the fetus in the OP position may be indicated when the fetus is estimated to be macrosomic, the station is high (biparietal diameter palpable on abdominal examination), or when the parturient has an android pelvis (narrow fore-pelvis and anterior convergence of the pelvic bone structures in a wedge shape). During CD, if difficulty is encountered in delivering the fetal head, a hand from below, extension of the uterine incision, or reverse breech extraction may be necessary to complete the delivery. If the clinical situation is conducive to operative vaginal delivery, forceps or vacuum can be used.
Continue to: Rotational forceps delivery...
Rotational forceps delivery. During residency I was told to always use rotational forceps to deliver a fetus in the persistently OP position if the parturient had a gynecoid pelvis (wide oval shape of pelvic bones, wide subpubic arch). Dr. Frederick Irving wrote16:
“Although textbooks almost universally advocate the extraction of the occiput directly posterior without rotation we do not advise it.... Such an extraction maneuver is inartistic and show[s] a lack of regard for the mechanical factors involved in the mechanism of labor. The method used at the Boston Lying-In Hospital presupposes an accurate diagnosis of the primary position. If the fetal back is on the right the head should be rotated to the right; if on the left, toward the left. The head is always rotated in the direction in which the back lies. The forceps are applied as if the occiput was directly anterior. Carrying the forceps handles in a wide sweep the occiput is now rotated to the anterior quadrant of the pelvis or 135 degrees. It will be found that the head turns easily in the way it should go but that it is difficult or impossible to rotate it in the improper direction. The instrument is then reapplied as in the second part of the Scanzoni maneuver.”
Rotation of the fetus from the OP to the OA position may reduce the risk of sphincter injury with vaginal birth. With the waning of rotational forceps skills, many obstetricians prefer a nonrotational approach with direct forceps or vacuum delivery from the OP position.
Direct forceps delivery from the OP position. A fetus in the OP position for 3 to 4 hours of the second stage of labor will often have a significant degree of head molding. The Simpson forceps, with its shallow and longer cephalic curve, accommodates significant fetal head molding and is a good forceps choice in this situation.
Vacuum delivery. In the United States, approximately 5% of vaginal deliveries are performed with a vacuum device, and 1% with forceps.17 Consequently, many obstetricians frequently perform operative vaginal delivery with a vacuum device and infrequently or never perform operative vaginal delivery with forceps. Vacuum vaginal delivery may be the instrument of choice for many obstetricians performing an operative delivery of a fetus in the OP position. However, the vacuum has a higher rate of failure, especially if the OP fetus is at a higher station.18
In some centers, direct forceps delivery from the OP position is preferred over an attempt at vacuum delivery, because in contemporary obstetric practice most centers do not permit the sequential use of vacuum followed by forceps (due to the higher rate of fetal trauma of combination operative delivery). Since vacuum delivery of the fetus in the OP position has a greater rate of failure than forceps, it may be best to initiate operative vaginal delivery of the fetus in the OP position with forceps. If vacuum is used to attempt a vaginal delivery and fails due to too many pop-offs, a CD would be the next step.
Take action when needed to optimize outcomes
The persistent OP position is associated with a longer second stage of labor. It is common during a change of shift for an obstetrician to sign out to the on-coming clinician a case of a prolonged second stage with the fetus in the OP position. In this situation, the on-coming clinician cannot wait hour after hour after hour hoping for a spontaneous delivery. If the on-coming clinician has a clear plan of how to deal with the persistent OP position—including ultrasound confirmation of position and physical examination to determine station, fetal size and adequacy of the pelvis, and timely selection of a delivery technique—the adverse maternal and neonatal outcomes sometimes caused by the persistent OP position will be minimized.
Continue to: CASE Resolved...
CASE Resolved
The consulting obstetrician performed a transabdominal ultrasound and observed the fetal orbits were facing the transducer, confirming an OP position. On physical examination, the station was +3/5, and the fetal weight was confirmed to be approximately 8 lb. The obstetrician recommended a direct forceps delivery from the OP position. The patient and CNM agreed with the plan.
The obstetrician applied Simpson forceps and performed a mediolateral episiotomy just prior to delivery of the head. Following delivery, the rectal sphincter and anal mucosa were intact and the episiotomy was repaired. The newborn, safely delivered, and the mother, having avoided a CD, were transferred to the postpartum floor later in the day.
CASE 7- to 8-lb baby suspected to be in occiput posterior (OP) position
A certified nurse midwife (CNM) asks you to consult on a 37-year-old woman (G1P0) at 41 weeks’ gestation who was admitted to labor and delivery for a late-term induction. The patient had a normal first stage of labor with placement of a combined spinal-epidural anesthetic at a cervical dilation of 4 cm. She has been fully dilated for 3.5 hours and pushing for 2.5 hours with a Category 1 fetal heart rate tracing. The CNM reports that the estimated fetal weight is 7 to 8 lb and the station is +3/5. She suspects that the fetus is in the left OP position. She asks for your advice on how to best deliver the fetus. The patient strongly prefers not to have a cesarean delivery (CD).
What is your recommended approach?
The cardinal movements of labor include cephalic engagement, descent, flexion, internal rotation, extension and rotation of the head at delivery, internal rotation of the shoulders, and expulsion of the body. In the first stage of labor many fetuses are in the OP position. Flexion and internal rotation of the fetal head in a mother with a gynecoid pelvis results in most fetuses assuming an occiput anterior (OA) position with the presenting diameter of the head (occipitobregmatic) being optimal for spontaneous vaginal delivery. Late in the second stage of labor only about 5% of fetuses are in the OP position with the presenting diameter of the head being large (occipitofrontal) with an extended head attitude, thereby reducing the probability of a rapid spontaneous vaginal delivery.
Risk factors for OP position late in the second stage of labor include1,2:
- nulliparity
- body mass index > 29 kg/m2
- gestation age ≥ 41 weeks
- birth weight > 4 kg
- regional anesthesia.
Maternal outcomes associated with persistent OP position include protracted first and second stage of labor, arrest of second stage of labor, and increased rates of operative vaginal delivery, anal sphincter injury, CD, postpartum hemorrhage, chorioamnionitis, and endomyometritis.1,3,4 The neonatal complications of persistent OP position include increased rates of shoulder dystocia, low Apgar score, umbilical artery acidemia, meconium, and admission to a neonatal intensive care unit.1,5
Diagnosis
Many obstetricians report that they can reliably detect a fetus in the OP position based upon abdominal palpation of the fetal spine and digital vaginal examination of the fetal sutures, fontanels, and ears. Such self-confidence may not be wholly warranted, however. Most contemporary data indicate that digital vaginal examination has an error rate of approximately 20% for identifying the position of the cephalic fetus, especially in the presence of fetal caput succedaneum and asynclitism.6-10
The International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) recommends that cephalic position be determined by transabdominal imaging.11 By placing the ultrasound probe on the maternal abdomen, a view of the fetal body at the level of the chest helps determine the position of the fetal spine. When the probe is placed in a suprapubic position, the observation of the fetal orbits facing the probe indicates an OP position.
When the presenting part is at a very low station, a transperineal ultrasound may be helpful to determine the position of the occiput. The ISUOG recommends that position be defined using a clock face, with positions from 330 h to 830 h being indicative of OP and positions from 930 h to 230 h being indicative of OA.11 The small remaining slivers on the clock face indicate an occiput transverse position (FIGURE).11

Continue to: Approaches to managing the OP position
Approaches to managing the OP position
First stage of labor
Identification of a cephalic-presenting fetus in the OP position in the first stage of labor might warrant increased attention to fetal position in the second stage of labor, but does not usually alter management of the first stage.
Second stage of labor
If an OP position is identified in the second stage of labor, many obstetricians will consider manual rotation of the fetal occiput to an anterior pelvic quadrant to facilitate labor progress. Because a fetus in the OP position may spontaneously rotate to the OA position at any point during the second stage, a judicious interval of waiting is reasonable before attempting a manual rotation in the second stage. For example, allowing the second stage to progress for 60 to 90 min in a nulliparous woman or 30 to 60 min in a multiparous woman will permit some fetuses to rotate to the OA position without intervention.
If the OP position persists beyond these time points, a manual rotation could be considered. There are no high-quality clinical trials to support this maneuver,12 but observational reports suggest that this low-risk maneuver may help reduce the rate of CD and anal sphincter trauma.13-15
Manual rotation from OP to OA. Prior to performing the rotation, the maternal bladder should be emptied and an adequate anesthetic provided. One technique is to use the 4 fingers of the hand as a “spatula” to turn the head. If the fetus is in a left OP position, the operator’s right hand is pronated and inserted into the vagina, palm up. Four fingers are placed under the posterior parietal bone with the thumb over the anterior parietal bone (ILLUSTRATION).4 The operator uses the fingers and thumb to flex and rotate the head to the right, moving the fetal occiput into an anterior pelvic quadrant.4 If the head is in the right OP position, the left hand is used to rotate the head. The nonvaginal hand can be placed on the maternal abdominal wall to assess the fetal spine position as the fetal head is rotated. The fetal head may need to be held in the anterior pelvic quadrant during a few maternal pushes to prevent the head from rotating back into the OP position.

Approaching delivery late in the second stage
If the second stage has progressed for 3 or 4 hours, as in the case described above, and the fetus remains in the OP position, delivery may be indicated to avoid the maternal and fetal complications of an even more prolonged second stage. At some point in a prolonged second stage, expectant management carries more maternal and fetal risks than intervention.
Late in the second stage, options for delivery of the fetus in the OP include: CD, rotational forceps delivery, direct forceps delivery from the OP position, and vacuum delivery.
Cesarean delivery. CD of the fetus in the OP position may be indicated when the fetus is estimated to be macrosomic, the station is high (biparietal diameter palpable on abdominal examination), or when the parturient has an android pelvis (narrow fore-pelvis and anterior convergence of the pelvic bone structures in a wedge shape). During CD, if difficulty is encountered in delivering the fetal head, a hand from below, extension of the uterine incision, or reverse breech extraction may be necessary to complete the delivery. If the clinical situation is conducive to operative vaginal delivery, forceps or vacuum can be used.
Continue to: Rotational forceps delivery...
Rotational forceps delivery. During residency I was told to always use rotational forceps to deliver a fetus in the persistently OP position if the parturient had a gynecoid pelvis (wide oval shape of pelvic bones, wide subpubic arch). Dr. Frederick Irving wrote16:
“Although textbooks almost universally advocate the extraction of the occiput directly posterior without rotation we do not advise it.... Such an extraction maneuver is inartistic and show[s] a lack of regard for the mechanical factors involved in the mechanism of labor. The method used at the Boston Lying-In Hospital presupposes an accurate diagnosis of the primary position. If the fetal back is on the right the head should be rotated to the right; if on the left, toward the left. The head is always rotated in the direction in which the back lies. The forceps are applied as if the occiput was directly anterior. Carrying the forceps handles in a wide sweep the occiput is now rotated to the anterior quadrant of the pelvis or 135 degrees. It will be found that the head turns easily in the way it should go but that it is difficult or impossible to rotate it in the improper direction. The instrument is then reapplied as in the second part of the Scanzoni maneuver.”
Rotation of the fetus from the OP to the OA position may reduce the risk of sphincter injury with vaginal birth. With the waning of rotational forceps skills, many obstetricians prefer a nonrotational approach with direct forceps or vacuum delivery from the OP position.
Direct forceps delivery from the OP position. A fetus in the OP position for 3 to 4 hours of the second stage of labor will often have a significant degree of head molding. The Simpson forceps, with its shallow and longer cephalic curve, accommodates significant fetal head molding and is a good forceps choice in this situation.
Vacuum delivery. In the United States, approximately 5% of vaginal deliveries are performed with a vacuum device, and 1% with forceps.17 Consequently, many obstetricians frequently perform operative vaginal delivery with a vacuum device and infrequently or never perform operative vaginal delivery with forceps. Vacuum vaginal delivery may be the instrument of choice for many obstetricians performing an operative delivery of a fetus in the OP position. However, the vacuum has a higher rate of failure, especially if the OP fetus is at a higher station.18
In some centers, direct forceps delivery from the OP position is preferred over an attempt at vacuum delivery, because in contemporary obstetric practice most centers do not permit the sequential use of vacuum followed by forceps (due to the higher rate of fetal trauma of combination operative delivery). Since vacuum delivery of the fetus in the OP position has a greater rate of failure than forceps, it may be best to initiate operative vaginal delivery of the fetus in the OP position with forceps. If vacuum is used to attempt a vaginal delivery and fails due to too many pop-offs, a CD would be the next step.
Take action when needed to optimize outcomes
The persistent OP position is associated with a longer second stage of labor. It is common during a change of shift for an obstetrician to sign out to the on-coming clinician a case of a prolonged second stage with the fetus in the OP position. In this situation, the on-coming clinician cannot wait hour after hour after hour hoping for a spontaneous delivery. If the on-coming clinician has a clear plan of how to deal with the persistent OP position—including ultrasound confirmation of position and physical examination to determine station, fetal size and adequacy of the pelvis, and timely selection of a delivery technique—the adverse maternal and neonatal outcomes sometimes caused by the persistent OP position will be minimized.
Continue to: CASE Resolved...
CASE Resolved
The consulting obstetrician performed a transabdominal ultrasound and observed the fetal orbits were facing the transducer, confirming an OP position. On physical examination, the station was +3/5, and the fetal weight was confirmed to be approximately 8 lb. The obstetrician recommended a direct forceps delivery from the OP position. The patient and CNM agreed with the plan.
The obstetrician applied Simpson forceps and performed a mediolateral episiotomy just prior to delivery of the head. Following delivery, the rectal sphincter and anal mucosa were intact and the episiotomy was repaired. The newborn, safely delivered, and the mother, having avoided a CD, were transferred to the postpartum floor later in the day.
- Cheng YW, Hubbard A, Caughey AB, et al. The association between persistent fetal occiput posterior position and perinatal outcomes: An example of propensity score and covariate distance matching. Am J Epidemiol. 2010;171:656-663.
- Cheng YW, Shaffer BL, Caughey AB. Associated factors and outcomes of persistent occiput posterior position: a retrospective cohort study from 1976 to 2001. J Matern Fetal Neonatal Med. 2006;19:563-568.
- Ponkey SE, Cohen AP, Heffner LJ, et al. Persistent fetal occiput posterior position: obstetric outcomes. Obstet Gynecol. 2003;101:915-920.
- Barth WH Jr. Persistent occiput posterior. Obstet Gynecol. 2015;125:695-709.
- Cheng YW, Shaffer BL, Caughey AB. The association between persistent occiput posterior position and neonatal outcomes. Obstet Gynecol. 2006;107:837-844.
- Ghi T, Dall’Asta A, Masturzo B, et al. Randomised Italian sonography for occiput position trial ante vacuum. Ultrasound Obstet Gynecol. 2018;52:699-705.
- Bellussi F, Ghi T, Youssef A, et al. The use of intrapartum ultrasound to diagnose malpositions and cephalic malpresentations. Am J Obstet Gynecol. 2017;217:633-641.
- Ramphul M, Ooi PV, Burke G, et al. Instrumental delivery and ultrasound: a multicenter randomised controlled trial of ultrasound assessment of the fetal head position versus standard of care as an approach to prevent morbidity at instrumental delivery. BJOG. 2014;121:1029-1038.
- Malvasi A, Tinelli A, Barbera A, et al. Occiput posterior position diagnosis: vaginal examination or intrapartum sonography? A clinical review. J Matern Fetal Neonatal Med. 2014;27:520-526.
- Akmal S, Tsoi E, Kaemtas N, et al. Intrapartum sonography to determine fetal head position. J Matern Fetal Neonatal Med. 2002;12:172-177.
- Ghi T, Eggebo T, Lees C, et al. ISUOG practice guidelines: intrapartum ultrasound. Ultrasound Obstet Gynecol. 2018;52:128-139.
- Phipps H, de Vries B, Hyett J, et al. Prophylactic manual rotation for fetal malposition to reduce operative delivery. Cochrane Database Syst Rev. 2014;CD009298.
- Le Ray C, Serres P, Schmitz T, et al. Manual rotation in occiput posterior or transverse positions. Obstet Gynecol. 2007;110:873-879.
- Shaffer BL, Cheng YW, Vargas JE, et al. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24:65-72.
- Bertholdt C, Gauchotte E, Dap M, et al. Predictors of successful manual rotation for occiput posterior positions. Int J Gynaecol Obstet. 2019;144:210–215.
- Irving FC. A Textbook of Obstetrics. New York, NY: Macmillan, NY; 1936:426-428.
- Merriam AA, Ananth CV, Wright JD, et al. Trends in operative vaginal delivery, 2005–2013: a population-based study. BJOG. 2017;124:1365-1372.
- Verhoeven CJ, Nuij C, Janssen-Rolf CR, et al. Predictors of failure of vacuum-assisted vaginal delivery: a case-control study. Eur J Obstet Gynecol Reprod Biol. 2016;200:29-34.
- Cheng YW, Hubbard A, Caughey AB, et al. The association between persistent fetal occiput posterior position and perinatal outcomes: An example of propensity score and covariate distance matching. Am J Epidemiol. 2010;171:656-663.
- Cheng YW, Shaffer BL, Caughey AB. Associated factors and outcomes of persistent occiput posterior position: a retrospective cohort study from 1976 to 2001. J Matern Fetal Neonatal Med. 2006;19:563-568.
- Ponkey SE, Cohen AP, Heffner LJ, et al. Persistent fetal occiput posterior position: obstetric outcomes. Obstet Gynecol. 2003;101:915-920.
- Barth WH Jr. Persistent occiput posterior. Obstet Gynecol. 2015;125:695-709.
- Cheng YW, Shaffer BL, Caughey AB. The association between persistent occiput posterior position and neonatal outcomes. Obstet Gynecol. 2006;107:837-844.
- Ghi T, Dall’Asta A, Masturzo B, et al. Randomised Italian sonography for occiput position trial ante vacuum. Ultrasound Obstet Gynecol. 2018;52:699-705.
- Bellussi F, Ghi T, Youssef A, et al. The use of intrapartum ultrasound to diagnose malpositions and cephalic malpresentations. Am J Obstet Gynecol. 2017;217:633-641.
- Ramphul M, Ooi PV, Burke G, et al. Instrumental delivery and ultrasound: a multicenter randomised controlled trial of ultrasound assessment of the fetal head position versus standard of care as an approach to prevent morbidity at instrumental delivery. BJOG. 2014;121:1029-1038.
- Malvasi A, Tinelli A, Barbera A, et al. Occiput posterior position diagnosis: vaginal examination or intrapartum sonography? A clinical review. J Matern Fetal Neonatal Med. 2014;27:520-526.
- Akmal S, Tsoi E, Kaemtas N, et al. Intrapartum sonography to determine fetal head position. J Matern Fetal Neonatal Med. 2002;12:172-177.
- Ghi T, Eggebo T, Lees C, et al. ISUOG practice guidelines: intrapartum ultrasound. Ultrasound Obstet Gynecol. 2018;52:128-139.
- Phipps H, de Vries B, Hyett J, et al. Prophylactic manual rotation for fetal malposition to reduce operative delivery. Cochrane Database Syst Rev. 2014;CD009298.
- Le Ray C, Serres P, Schmitz T, et al. Manual rotation in occiput posterior or transverse positions. Obstet Gynecol. 2007;110:873-879.
- Shaffer BL, Cheng YW, Vargas JE, et al. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24:65-72.
- Bertholdt C, Gauchotte E, Dap M, et al. Predictors of successful manual rotation for occiput posterior positions. Int J Gynaecol Obstet. 2019;144:210–215.
- Irving FC. A Textbook of Obstetrics. New York, NY: Macmillan, NY; 1936:426-428.
- Merriam AA, Ananth CV, Wright JD, et al. Trends in operative vaginal delivery, 2005–2013: a population-based study. BJOG. 2017;124:1365-1372.
- Verhoeven CJ, Nuij C, Janssen-Rolf CR, et al. Predictors of failure of vacuum-assisted vaginal delivery: a case-control study. Eur J Obstet Gynecol Reprod Biol. 2016;200:29-34.
How do you feel about expectantly managing a well-dated pregnancy past 41 weeks’ gestation?
Most people know that preterm birth is a major contributor to perinatal morbidity and mortality. Consequently, strict guidelines have been enforced to prevent non–medically indicated scheduled deliveries before 39 weeks’ gestation. Fewer people recognize that late-term birth is also an important and avoidable contributor to perinatal morbidity. To improve pregnancy outcomes, we may need enhanced guidelines about minimizing expectant management of pregnancy beyond 41 weeks’ gestation.
For the fetus, what is the optimal duration of a healthy pregnancy?
When pregnancy progresses past the date of the confinement, the risk of fetal or newborn injury or death increases, especially after 41 weeks’ gestation. Analysis of this risk, day by day, suggests that after 40 weeks’ and 3 days’ gestation there is no medical benefit to the fetus to remain in utero because, compared with induced delivery, expectant management of the pregnancy is associated with a greater rate of fetal and newborn morbidity and mortality.1
The fetal and newborn benefits of delivery, rather than expectant management, at term include: a decrease in stillbirth and perinatal death rates, a decrease in admissions to the neonatal intensive care unit (NICU), a decrease in meconium-stained amniotic fluid and meconium aspiration syndrome, a decrease in low Apgar scores, and a decrease in problems related to uteroplacental insufficiency, including oligohydramnios.2 In a comprehensive meta-analysis, induction of labor at or beyond term reduced the risk of perinatal death or stillbirth by 67%, the risk of a 5-minute Apgar score below 7 by 30%, and the risk of NICU admission by 12%.2 The number of women that would need to be induced to prevent 1 perinatal death was estimated to be 426.2
Maternal benefits of avoiding late-term pregnancy
The maternal benefits of avoiding continuing a pregnancy past 41 weeks’ gestation include a reduction in labor dystocia and the risk of cesarean delivery (CD).2,3 In one clinical trial, 3,407 women with low-risk pregnancy were randomly assigned to induction of labor at 41 weeks’ gestation or expectant management, awaiting the onset of labor with serial antenatal monitoring (nonstress tests and assessment of amniotic fluid volume).4 The CD rate was lower among the women randomized to induction of labor at 41 weeks’ (21.2% vs 24.5% in the expectant management group, P = .03). The rate of meconium-stained fluid was lower in the induction of labor group (25.0% vs 28.7%, P = .009). The rate of CD due to fetal distress also was lower in the induction of labor group (5.7% vs 8.3%, P = .003). The risks of maternal postpartum hemorrhage, sepsis, and endometritis did not differ between the groups. There were 2 stillbirths in the expectant management group (2/1,706) and none in the induction of labor group (0/1,701). There were no neonatal deaths in this study.4
Obstetric management, including accurate dating of pregnancy and membrane sweeping at term, can help to reduce the risk that a pregnancy will progress beyond 41 weeks’ gestation.5
Continue to: Routinely use ultrasound to accurately establish gestational age
Routinely use ultrasound to accurately establish gestational age
First trimester ultrasound should be offered to all pregnant women because it is a more accurate assessment of gestational age and will result in fewer pregnancies that are thought to be at or beyond 41 weeks’ gestation.5 In a meta-analysis of 8 studies, including 25,516 women, early ultrasonography reduced the rate of intervention for postterm pregnancy by 42% (31/1,000 to 18/1,000 pregnant women).6

Membrane sweeping (or stripping)
Membrane sweeping, which causes the release of prostaglandins, has been reported to reduce the risk of late-term and postterm induction of labor.7,8 In the most recent Cochrane review on the topic, sweeping membranes reduced the rate of induction of labor at 41 weeks by 41% and at 42 weeks by 72%.7 To avoid one induction of labor for late-term or postterm pregnancy, sweeping of membranes would need to be performed on 8 women. In a recent meta-analysis, membrane sweeping reduced the rate of induction of labor for postmaturity by 48%.9
Membrane sweeping is associated with pain and an increased rate of vaginal bleeding.10 It does not increase the rate of maternal or neonatal infection, however. It also does not reduce the CD rate. In the United Kingdom, the National Institute for Health and Clinical Excellence recommends that all clinicians have a discussion of membrane sweeping with their patients at 38 weeks’ gestation and offer membrane stripping at 40 weeks to increase the rate of timely spontaneous labor and to avoid the risks of prolonged pregnancy.11 Of note, in one randomized study of women planning a trial of labor after CD, membrane sweeping did not impact the duration of pregnancy, onset of spontaneous labor, or the CD rate.12
Steps from an expert. A skillfull midwife practicing in the United Kingdom provides the following guidance on how to perform membrane sweeping.13
- Prepare the patient. Explain the procedure, have the patient empty her bladder, and encourage relaxed breathing if the vaginal examination causes pain.
- Abdominal exam. Assess uterine size, fetal lie and presentation, and fetal heart tones.
- Vaginal exam. Ascertain cervical dilation, effacement, and position. If the cervix is closed a sweep may not be possible. In this case, massaging the vaginal fornices may help to release prostaglandins and stimulate uterine contractions. If the cervix is closed but soft, massage of the cervix may permit the insertion of a finger. If the cervix is favorable for sweeping, insert one finger in the cervix and rotate the finger in a circle to separate the amnion from the cervix.
- After the procedure. Provide the woman with a sanitary pad and recommend acetaminophen and a warm bath if she has discomfort or painful contractions. Advise her to come to the maternity unit in the following situations: severe pain, significant bleeding, or spontaneous rupture of the membranes.
Membrane sweeping can be performed as frequently as every 3 days. Formal cervical ripening and induction of labor may need to be planned if membrane sweeping does not result in the initiation of regular contractions.
Continue to: Collaborative decision making
Collaborative decision making
All clinicians recognize the primacy of patient autonomy.14 Competent patients have the right to select the course of care that they believe is optimal. When a patient decides to continue her pregnancy past 41 weeks, it is helpful to endorse respect for the decision and inquire about the patient’s reasons for continuing the pregnancy. Understanding the patient’s concerns may begin a conversation that will result in the patient accepting a plan for induction near 41 weeks’ gestation. If the patient insists on expectant management well beyond 41 weeks, the medical record should contain a summary of the clinician recommendation to induce labor at or before 41 weeks’ gestation and the patient’s preference for expectant management and her understanding of the decision’s risks.
Obstetricians and midwives constantly face the challenge of balancing the desire to avoid meddlesome interference in a pregnancy with the need to act to prevent adverse pregnancy outcomes. The challenge is daunting. A comprehensive meta-analysis of the benefit of induction of labor at or beyond term, estimated that 426 inductions would need to be initiated to prevent one perinatal death.2 From one perspective it is meddlesome to intervene on more than 400 women to prevent one perinatal death. However, substantial data indicate that expectant management of a well-dated pregnancy at 41 weeks’ gestation will result in adverse outcomes that likely could be prevented by induction of labor. If you ran an airline and could take an action to prevent one airplane crash for every 400 flights, you would likely move heaven and earth to try to prevent that disaster. Unless the patient strongly prefers expectant management, well-managed induction of labor at or before 41 weeks’ gestation is likely to reduce the rate of adverse pregnancy events and, hence, is warranted.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Divon MY, Ferber A, Sanderson M, et al. A functional definition of prolonged pregnancy based on daily fetal and neonatal mortality rates. Ultrasound Obstet Gynecol. 2004;23:423-426.
- Middleton P, Shepherd E, Crowther CA. Induction of labour for improving birth outcomes for women at or beyond term. Cochrane Database Syst Rev. 2018;5:CD004945.
- Caughey AB, Sundaram V, Kaimal AJ, et al. Systematic review: elective induction of labor versus expectant management of pregnancy. Ann Intern Med. 2009;151:252-263.
- Hannah ME, Hannah WJ, Hellmann J, et al; Canadian Multicenter Post-term Pregnancy Trial Group. Induction of labor as compared with serial antenatal monitoring in post-term pregnancy. N Engl J Med. 1992;326:1587-1592.
- Delaney M, Roggensack A. No. 214-Guidelines for the management of pregnancy at 41+0 to 42+0 weeks. J Obstet Gynaecol Can. 2017;39:e164-e174.
- Whitworth M, Bricker L, Mullan C. Ultrasound for fetal assessment in early pregnancy. Cochrane Database Syst Rev. 2015;7:CD007058.
- Boulvain M, Stan C, Irion O. Membrane sweeping for induction of labour. Cochrane Database Syst Rev. 2005;1:CD000451.
- Berghella V, Rogers RA, Lescale K. Stripping of membranes as a safe method to reduce prolonged pregnancies. Obstet Gynecol. 1996;87:927-931.
- Avdiyovski H, Haith-Cooper M, Scally A. Membrane sweeping at term to promote spontaneous labour and reduce the likelihood of a formal induction of labour for postmaturity: a systematic review and meta-analysis. J Obstet Gynaecol. 2018:1-9.
- de Miranda E, van der Bom JG, Bonsel G, et al. Membrane sweeping and prevention of post-term pregnancy in low-risk pregnancies: a randomised controlled trial. BJOG. 2006;113:402-408.
- National Collaborating Centre for Women's and Children's Health. NICE Guideline 70. Induction of labour; July 2008. https://www.nice.org.uk/guidance/cg70/evidence/cg70-induction-of-labour-full-guideline2. Accessed January 23, 2019.
- Hamdan M, Sidhu K, Sabir N, et al. Serial membrane sweeping at term in planned vaginal birth after cesarean: a randomized controlled trial. Obstet Gynecol. 2009;114:745-751.
- Gibbon K. How to perform a stretch and sweep. Midwives Magazine. 2012. https://www.rcm.org.uk/news-views-and-analysis/analysis/how-to%E2%80%A6-perform-a-stretch-and-sweep. Accessed January 23, 2019.
- Ryan KJ. Erosion of the rights of pregnant women: in the interest of fetal well-being. Womens Health Issues. 1990;1:21-24.
Most people know that preterm birth is a major contributor to perinatal morbidity and mortality. Consequently, strict guidelines have been enforced to prevent non–medically indicated scheduled deliveries before 39 weeks’ gestation. Fewer people recognize that late-term birth is also an important and avoidable contributor to perinatal morbidity. To improve pregnancy outcomes, we may need enhanced guidelines about minimizing expectant management of pregnancy beyond 41 weeks’ gestation.
For the fetus, what is the optimal duration of a healthy pregnancy?
When pregnancy progresses past the date of the confinement, the risk of fetal or newborn injury or death increases, especially after 41 weeks’ gestation. Analysis of this risk, day by day, suggests that after 40 weeks’ and 3 days’ gestation there is no medical benefit to the fetus to remain in utero because, compared with induced delivery, expectant management of the pregnancy is associated with a greater rate of fetal and newborn morbidity and mortality.1
The fetal and newborn benefits of delivery, rather than expectant management, at term include: a decrease in stillbirth and perinatal death rates, a decrease in admissions to the neonatal intensive care unit (NICU), a decrease in meconium-stained amniotic fluid and meconium aspiration syndrome, a decrease in low Apgar scores, and a decrease in problems related to uteroplacental insufficiency, including oligohydramnios.2 In a comprehensive meta-analysis, induction of labor at or beyond term reduced the risk of perinatal death or stillbirth by 67%, the risk of a 5-minute Apgar score below 7 by 30%, and the risk of NICU admission by 12%.2 The number of women that would need to be induced to prevent 1 perinatal death was estimated to be 426.2
Maternal benefits of avoiding late-term pregnancy
The maternal benefits of avoiding continuing a pregnancy past 41 weeks’ gestation include a reduction in labor dystocia and the risk of cesarean delivery (CD).2,3 In one clinical trial, 3,407 women with low-risk pregnancy were randomly assigned to induction of labor at 41 weeks’ gestation or expectant management, awaiting the onset of labor with serial antenatal monitoring (nonstress tests and assessment of amniotic fluid volume).4 The CD rate was lower among the women randomized to induction of labor at 41 weeks’ (21.2% vs 24.5% in the expectant management group, P = .03). The rate of meconium-stained fluid was lower in the induction of labor group (25.0% vs 28.7%, P = .009). The rate of CD due to fetal distress also was lower in the induction of labor group (5.7% vs 8.3%, P = .003). The risks of maternal postpartum hemorrhage, sepsis, and endometritis did not differ between the groups. There were 2 stillbirths in the expectant management group (2/1,706) and none in the induction of labor group (0/1,701). There were no neonatal deaths in this study.4
Obstetric management, including accurate dating of pregnancy and membrane sweeping at term, can help to reduce the risk that a pregnancy will progress beyond 41 weeks’ gestation.5
Continue to: Routinely use ultrasound to accurately establish gestational age
Routinely use ultrasound to accurately establish gestational age
First trimester ultrasound should be offered to all pregnant women because it is a more accurate assessment of gestational age and will result in fewer pregnancies that are thought to be at or beyond 41 weeks’ gestation.5 In a meta-analysis of 8 studies, including 25,516 women, early ultrasonography reduced the rate of intervention for postterm pregnancy by 42% (31/1,000 to 18/1,000 pregnant women).6

Membrane sweeping (or stripping)
Membrane sweeping, which causes the release of prostaglandins, has been reported to reduce the risk of late-term and postterm induction of labor.7,8 In the most recent Cochrane review on the topic, sweeping membranes reduced the rate of induction of labor at 41 weeks by 41% and at 42 weeks by 72%.7 To avoid one induction of labor for late-term or postterm pregnancy, sweeping of membranes would need to be performed on 8 women. In a recent meta-analysis, membrane sweeping reduced the rate of induction of labor for postmaturity by 48%.9
Membrane sweeping is associated with pain and an increased rate of vaginal bleeding.10 It does not increase the rate of maternal or neonatal infection, however. It also does not reduce the CD rate. In the United Kingdom, the National Institute for Health and Clinical Excellence recommends that all clinicians have a discussion of membrane sweeping with their patients at 38 weeks’ gestation and offer membrane stripping at 40 weeks to increase the rate of timely spontaneous labor and to avoid the risks of prolonged pregnancy.11 Of note, in one randomized study of women planning a trial of labor after CD, membrane sweeping did not impact the duration of pregnancy, onset of spontaneous labor, or the CD rate.12
Steps from an expert. A skillfull midwife practicing in the United Kingdom provides the following guidance on how to perform membrane sweeping.13
- Prepare the patient. Explain the procedure, have the patient empty her bladder, and encourage relaxed breathing if the vaginal examination causes pain.
- Abdominal exam. Assess uterine size, fetal lie and presentation, and fetal heart tones.
- Vaginal exam. Ascertain cervical dilation, effacement, and position. If the cervix is closed a sweep may not be possible. In this case, massaging the vaginal fornices may help to release prostaglandins and stimulate uterine contractions. If the cervix is closed but soft, massage of the cervix may permit the insertion of a finger. If the cervix is favorable for sweeping, insert one finger in the cervix and rotate the finger in a circle to separate the amnion from the cervix.
- After the procedure. Provide the woman with a sanitary pad and recommend acetaminophen and a warm bath if she has discomfort or painful contractions. Advise her to come to the maternity unit in the following situations: severe pain, significant bleeding, or spontaneous rupture of the membranes.
Membrane sweeping can be performed as frequently as every 3 days. Formal cervical ripening and induction of labor may need to be planned if membrane sweeping does not result in the initiation of regular contractions.
Continue to: Collaborative decision making
Collaborative decision making
All clinicians recognize the primacy of patient autonomy.14 Competent patients have the right to select the course of care that they believe is optimal. When a patient decides to continue her pregnancy past 41 weeks, it is helpful to endorse respect for the decision and inquire about the patient’s reasons for continuing the pregnancy. Understanding the patient’s concerns may begin a conversation that will result in the patient accepting a plan for induction near 41 weeks’ gestation. If the patient insists on expectant management well beyond 41 weeks, the medical record should contain a summary of the clinician recommendation to induce labor at or before 41 weeks’ gestation and the patient’s preference for expectant management and her understanding of the decision’s risks.
Obstetricians and midwives constantly face the challenge of balancing the desire to avoid meddlesome interference in a pregnancy with the need to act to prevent adverse pregnancy outcomes. The challenge is daunting. A comprehensive meta-analysis of the benefit of induction of labor at or beyond term, estimated that 426 inductions would need to be initiated to prevent one perinatal death.2 From one perspective it is meddlesome to intervene on more than 400 women to prevent one perinatal death. However, substantial data indicate that expectant management of a well-dated pregnancy at 41 weeks’ gestation will result in adverse outcomes that likely could be prevented by induction of labor. If you ran an airline and could take an action to prevent one airplane crash for every 400 flights, you would likely move heaven and earth to try to prevent that disaster. Unless the patient strongly prefers expectant management, well-managed induction of labor at or before 41 weeks’ gestation is likely to reduce the rate of adverse pregnancy events and, hence, is warranted.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Most people know that preterm birth is a major contributor to perinatal morbidity and mortality. Consequently, strict guidelines have been enforced to prevent non–medically indicated scheduled deliveries before 39 weeks’ gestation. Fewer people recognize that late-term birth is also an important and avoidable contributor to perinatal morbidity. To improve pregnancy outcomes, we may need enhanced guidelines about minimizing expectant management of pregnancy beyond 41 weeks’ gestation.
For the fetus, what is the optimal duration of a healthy pregnancy?
When pregnancy progresses past the date of the confinement, the risk of fetal or newborn injury or death increases, especially after 41 weeks’ gestation. Analysis of this risk, day by day, suggests that after 40 weeks’ and 3 days’ gestation there is no medical benefit to the fetus to remain in utero because, compared with induced delivery, expectant management of the pregnancy is associated with a greater rate of fetal and newborn morbidity and mortality.1
The fetal and newborn benefits of delivery, rather than expectant management, at term include: a decrease in stillbirth and perinatal death rates, a decrease in admissions to the neonatal intensive care unit (NICU), a decrease in meconium-stained amniotic fluid and meconium aspiration syndrome, a decrease in low Apgar scores, and a decrease in problems related to uteroplacental insufficiency, including oligohydramnios.2 In a comprehensive meta-analysis, induction of labor at or beyond term reduced the risk of perinatal death or stillbirth by 67%, the risk of a 5-minute Apgar score below 7 by 30%, and the risk of NICU admission by 12%.2 The number of women that would need to be induced to prevent 1 perinatal death was estimated to be 426.2
Maternal benefits of avoiding late-term pregnancy
The maternal benefits of avoiding continuing a pregnancy past 41 weeks’ gestation include a reduction in labor dystocia and the risk of cesarean delivery (CD).2,3 In one clinical trial, 3,407 women with low-risk pregnancy were randomly assigned to induction of labor at 41 weeks’ gestation or expectant management, awaiting the onset of labor with serial antenatal monitoring (nonstress tests and assessment of amniotic fluid volume).4 The CD rate was lower among the women randomized to induction of labor at 41 weeks’ (21.2% vs 24.5% in the expectant management group, P = .03). The rate of meconium-stained fluid was lower in the induction of labor group (25.0% vs 28.7%, P = .009). The rate of CD due to fetal distress also was lower in the induction of labor group (5.7% vs 8.3%, P = .003). The risks of maternal postpartum hemorrhage, sepsis, and endometritis did not differ between the groups. There were 2 stillbirths in the expectant management group (2/1,706) and none in the induction of labor group (0/1,701). There were no neonatal deaths in this study.4
Obstetric management, including accurate dating of pregnancy and membrane sweeping at term, can help to reduce the risk that a pregnancy will progress beyond 41 weeks’ gestation.5
Continue to: Routinely use ultrasound to accurately establish gestational age
Routinely use ultrasound to accurately establish gestational age
First trimester ultrasound should be offered to all pregnant women because it is a more accurate assessment of gestational age and will result in fewer pregnancies that are thought to be at or beyond 41 weeks’ gestation.5 In a meta-analysis of 8 studies, including 25,516 women, early ultrasonography reduced the rate of intervention for postterm pregnancy by 42% (31/1,000 to 18/1,000 pregnant women).6

Membrane sweeping (or stripping)
Membrane sweeping, which causes the release of prostaglandins, has been reported to reduce the risk of late-term and postterm induction of labor.7,8 In the most recent Cochrane review on the topic, sweeping membranes reduced the rate of induction of labor at 41 weeks by 41% and at 42 weeks by 72%.7 To avoid one induction of labor for late-term or postterm pregnancy, sweeping of membranes would need to be performed on 8 women. In a recent meta-analysis, membrane sweeping reduced the rate of induction of labor for postmaturity by 48%.9
Membrane sweeping is associated with pain and an increased rate of vaginal bleeding.10 It does not increase the rate of maternal or neonatal infection, however. It also does not reduce the CD rate. In the United Kingdom, the National Institute for Health and Clinical Excellence recommends that all clinicians have a discussion of membrane sweeping with their patients at 38 weeks’ gestation and offer membrane stripping at 40 weeks to increase the rate of timely spontaneous labor and to avoid the risks of prolonged pregnancy.11 Of note, in one randomized study of women planning a trial of labor after CD, membrane sweeping did not impact the duration of pregnancy, onset of spontaneous labor, or the CD rate.12
Steps from an expert. A skillfull midwife practicing in the United Kingdom provides the following guidance on how to perform membrane sweeping.13
- Prepare the patient. Explain the procedure, have the patient empty her bladder, and encourage relaxed breathing if the vaginal examination causes pain.
- Abdominal exam. Assess uterine size, fetal lie and presentation, and fetal heart tones.
- Vaginal exam. Ascertain cervical dilation, effacement, and position. If the cervix is closed a sweep may not be possible. In this case, massaging the vaginal fornices may help to release prostaglandins and stimulate uterine contractions. If the cervix is closed but soft, massage of the cervix may permit the insertion of a finger. If the cervix is favorable for sweeping, insert one finger in the cervix and rotate the finger in a circle to separate the amnion from the cervix.
- After the procedure. Provide the woman with a sanitary pad and recommend acetaminophen and a warm bath if she has discomfort or painful contractions. Advise her to come to the maternity unit in the following situations: severe pain, significant bleeding, or spontaneous rupture of the membranes.
Membrane sweeping can be performed as frequently as every 3 days. Formal cervical ripening and induction of labor may need to be planned if membrane sweeping does not result in the initiation of regular contractions.
Continue to: Collaborative decision making
Collaborative decision making
All clinicians recognize the primacy of patient autonomy.14 Competent patients have the right to select the course of care that they believe is optimal. When a patient decides to continue her pregnancy past 41 weeks, it is helpful to endorse respect for the decision and inquire about the patient’s reasons for continuing the pregnancy. Understanding the patient’s concerns may begin a conversation that will result in the patient accepting a plan for induction near 41 weeks’ gestation. If the patient insists on expectant management well beyond 41 weeks, the medical record should contain a summary of the clinician recommendation to induce labor at or before 41 weeks’ gestation and the patient’s preference for expectant management and her understanding of the decision’s risks.
Obstetricians and midwives constantly face the challenge of balancing the desire to avoid meddlesome interference in a pregnancy with the need to act to prevent adverse pregnancy outcomes. The challenge is daunting. A comprehensive meta-analysis of the benefit of induction of labor at or beyond term, estimated that 426 inductions would need to be initiated to prevent one perinatal death.2 From one perspective it is meddlesome to intervene on more than 400 women to prevent one perinatal death. However, substantial data indicate that expectant management of a well-dated pregnancy at 41 weeks’ gestation will result in adverse outcomes that likely could be prevented by induction of labor. If you ran an airline and could take an action to prevent one airplane crash for every 400 flights, you would likely move heaven and earth to try to prevent that disaster. Unless the patient strongly prefers expectant management, well-managed induction of labor at or before 41 weeks’ gestation is likely to reduce the rate of adverse pregnancy events and, hence, is warranted.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Divon MY, Ferber A, Sanderson M, et al. A functional definition of prolonged pregnancy based on daily fetal and neonatal mortality rates. Ultrasound Obstet Gynecol. 2004;23:423-426.
- Middleton P, Shepherd E, Crowther CA. Induction of labour for improving birth outcomes for women at or beyond term. Cochrane Database Syst Rev. 2018;5:CD004945.
- Caughey AB, Sundaram V, Kaimal AJ, et al. Systematic review: elective induction of labor versus expectant management of pregnancy. Ann Intern Med. 2009;151:252-263.
- Hannah ME, Hannah WJ, Hellmann J, et al; Canadian Multicenter Post-term Pregnancy Trial Group. Induction of labor as compared with serial antenatal monitoring in post-term pregnancy. N Engl J Med. 1992;326:1587-1592.
- Delaney M, Roggensack A. No. 214-Guidelines for the management of pregnancy at 41+0 to 42+0 weeks. J Obstet Gynaecol Can. 2017;39:e164-e174.
- Whitworth M, Bricker L, Mullan C. Ultrasound for fetal assessment in early pregnancy. Cochrane Database Syst Rev. 2015;7:CD007058.
- Boulvain M, Stan C, Irion O. Membrane sweeping for induction of labour. Cochrane Database Syst Rev. 2005;1:CD000451.
- Berghella V, Rogers RA, Lescale K. Stripping of membranes as a safe method to reduce prolonged pregnancies. Obstet Gynecol. 1996;87:927-931.
- Avdiyovski H, Haith-Cooper M, Scally A. Membrane sweeping at term to promote spontaneous labour and reduce the likelihood of a formal induction of labour for postmaturity: a systematic review and meta-analysis. J Obstet Gynaecol. 2018:1-9.
- de Miranda E, van der Bom JG, Bonsel G, et al. Membrane sweeping and prevention of post-term pregnancy in low-risk pregnancies: a randomised controlled trial. BJOG. 2006;113:402-408.
- National Collaborating Centre for Women's and Children's Health. NICE Guideline 70. Induction of labour; July 2008. https://www.nice.org.uk/guidance/cg70/evidence/cg70-induction-of-labour-full-guideline2. Accessed January 23, 2019.
- Hamdan M, Sidhu K, Sabir N, et al. Serial membrane sweeping at term in planned vaginal birth after cesarean: a randomized controlled trial. Obstet Gynecol. 2009;114:745-751.
- Gibbon K. How to perform a stretch and sweep. Midwives Magazine. 2012. https://www.rcm.org.uk/news-views-and-analysis/analysis/how-to%E2%80%A6-perform-a-stretch-and-sweep. Accessed January 23, 2019.
- Ryan KJ. Erosion of the rights of pregnant women: in the interest of fetal well-being. Womens Health Issues. 1990;1:21-24.
- Divon MY, Ferber A, Sanderson M, et al. A functional definition of prolonged pregnancy based on daily fetal and neonatal mortality rates. Ultrasound Obstet Gynecol. 2004;23:423-426.
- Middleton P, Shepherd E, Crowther CA. Induction of labour for improving birth outcomes for women at or beyond term. Cochrane Database Syst Rev. 2018;5:CD004945.
- Caughey AB, Sundaram V, Kaimal AJ, et al. Systematic review: elective induction of labor versus expectant management of pregnancy. Ann Intern Med. 2009;151:252-263.
- Hannah ME, Hannah WJ, Hellmann J, et al; Canadian Multicenter Post-term Pregnancy Trial Group. Induction of labor as compared with serial antenatal monitoring in post-term pregnancy. N Engl J Med. 1992;326:1587-1592.
- Delaney M, Roggensack A. No. 214-Guidelines for the management of pregnancy at 41+0 to 42+0 weeks. J Obstet Gynaecol Can. 2017;39:e164-e174.
- Whitworth M, Bricker L, Mullan C. Ultrasound for fetal assessment in early pregnancy. Cochrane Database Syst Rev. 2015;7:CD007058.
- Boulvain M, Stan C, Irion O. Membrane sweeping for induction of labour. Cochrane Database Syst Rev. 2005;1:CD000451.
- Berghella V, Rogers RA, Lescale K. Stripping of membranes as a safe method to reduce prolonged pregnancies. Obstet Gynecol. 1996;87:927-931.
- Avdiyovski H, Haith-Cooper M, Scally A. Membrane sweeping at term to promote spontaneous labour and reduce the likelihood of a formal induction of labour for postmaturity: a systematic review and meta-analysis. J Obstet Gynaecol. 2018:1-9.
- de Miranda E, van der Bom JG, Bonsel G, et al. Membrane sweeping and prevention of post-term pregnancy in low-risk pregnancies: a randomised controlled trial. BJOG. 2006;113:402-408.
- National Collaborating Centre for Women's and Children's Health. NICE Guideline 70. Induction of labour; July 2008. https://www.nice.org.uk/guidance/cg70/evidence/cg70-induction-of-labour-full-guideline2. Accessed January 23, 2019.
- Hamdan M, Sidhu K, Sabir N, et al. Serial membrane sweeping at term in planned vaginal birth after cesarean: a randomized controlled trial. Obstet Gynecol. 2009;114:745-751.
- Gibbon K. How to perform a stretch and sweep. Midwives Magazine. 2012. https://www.rcm.org.uk/news-views-and-analysis/analysis/how-to%E2%80%A6-perform-a-stretch-and-sweep. Accessed January 23, 2019.
- Ryan KJ. Erosion of the rights of pregnant women: in the interest of fetal well-being. Womens Health Issues. 1990;1:21-24.