User login
The Fetal Pillow: A new option for delivering the deeply impacted fetal head
Obstetricians know that a cesarean delivery (CD) for a woman with a prolonged second stage and a fetal head deeply impacted in the pelvis is challenging. In this situation, extensions of the uterine incision commonly occur, resulting in prolonged operative time and increased blood loss. Even more harrowing is the inability to deliver the fetal head, necessitating emergency assistance from other clinicians. In this situation, interventions that may be helpful include:
- extend or T the uterine incision
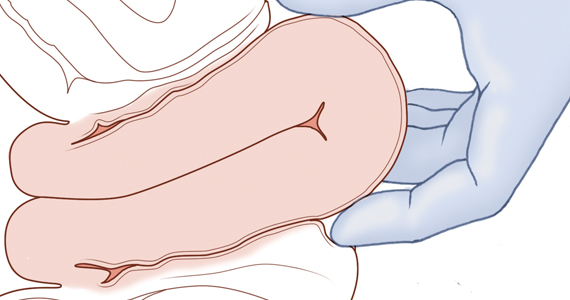
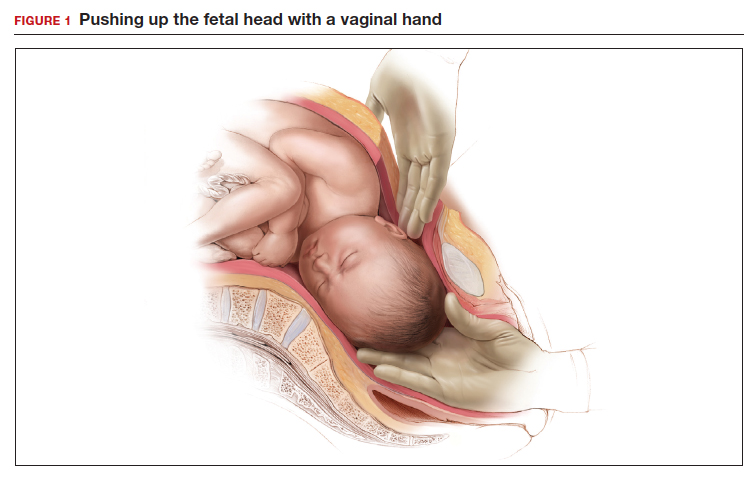
- enlist the aid of a clinician to push up on the fetal head with a vaginal hand (FIGURE 1)
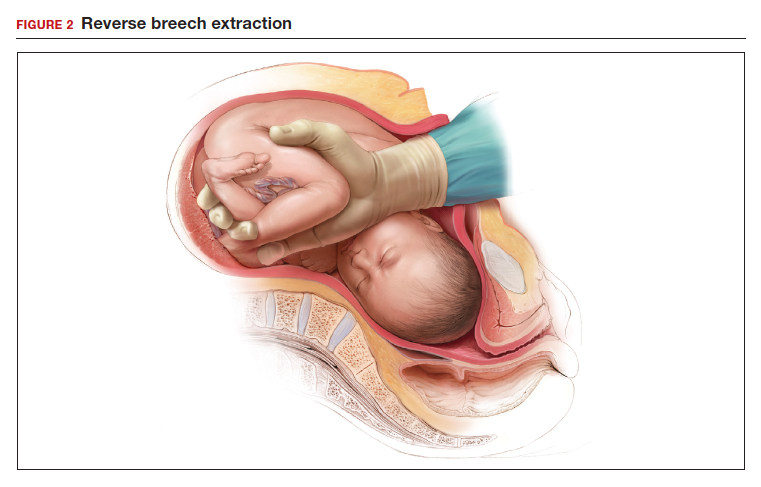
- reverse breech extraction (FIGURE 2), and
- vaginal insertion of a Fetal Pillow prior to starting the delivery.
Evidence from clinical trials indicates that reverse breech extraction or insertion of a Fetal Pillow result in the best clinical outcomes.
Reverse breech extraction vs the push technique
Although the data are limited, most studies report that compared with pushing up with a vaginal hand (as shown in Figure 1), the reverse breech extraction technique (as shown in Figure 2) is associated with a reduction in extensions of the uterine incision, reduced blood loss, and reduced operative time.1 In a randomized trial, 108 women with obstructed labor undergoing CD in the second stage were randomly assigned to reverse breech extraction or pushing up with a vaginal hand.2 Following the uterine incision, the reverse breech extraction technique is performed by immediately reaching into the upper uterus and grasping the lower portion of the fetal leg and applying gentle traction on the leg until the second leg appeared. The lower legs are then pulled out of the uterus. Standard breech delivery maneuvers are used to deliver the shoulders and head. In the trial, compared with the push technique, reverse breech extraction was associated with fewer extensions of the uterine incision (30% vs 11%; P<.05), less blood loss (899 mL vs 1,257 mL; P<.001), and shorter operative time (56 min vs 89 min, P<.001). Fetal injury was similar with the push and breech extraction techniques (6% and 7%).
In another randomized trial, 192 women undergoing CD for obstructed labor were randomly assigned to reverse breech extraction or pushing the head up with a hand in the vagina.3 Compared with the vaginal push technique, reverse breech extraction was associated with fewer extensions of the uterine incision (19% vs 48%; P = .003), fewer cases of wound infection (2% vs 13%; P = .007), and fewer blood transfusions (2 vs 11; P = .012).
Additional options and adjuvants for facilitating delivery of a fetal head deeply impacted in the pelvis include: using a Coyne spoon, using nitroglycerine or terbutaline to relax the myometrium, breaking the vaginal suction on the fetal head before attempting delivery, keeping the wrist of the delivering hand as straight as possible to reduce uterine incision extensions, and incising the ring (if a Bandl’s ring is detected).
Continue to: The Fetal Pillow...
The Fetal Pillow
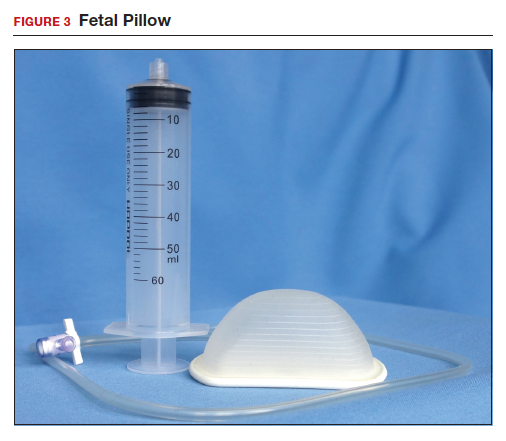
The Fetal Pillow (Safe Obstetric Systems, New York, New York) is a single-use fetal cephalic elevation device for managing the deeply impacted fetal head (FIGURE 3). The Fetal Pillow has a firm plastic base upon which is attached a soft silicon balloon. The Fetal Pillow is inserted into the vagina prior to initiating CD and the balloon is filled with 180 mL of saline, causing the fetal head to be pushed to a higher station (FIGURE 4). Use of the Fetal Pillow may be indicated prior to CD in the following situations:
- second stage labor with a deeply impacted head
- second stage labor and failed operative delivery
- occiput posterior position or deep transverse arrest
- absent progress in the first stage between 8 cm and 10 cm with a deeply impacted fetal head or excessive caput of the fetal head.
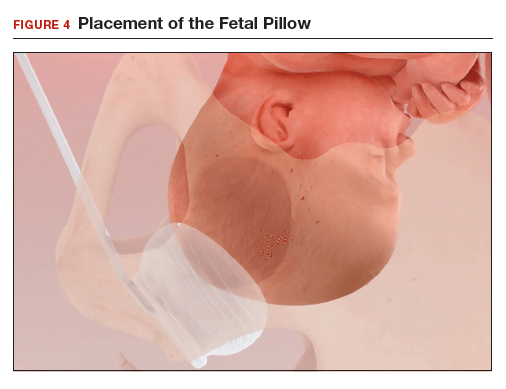
The Fetal Pillow is inserted after completing vaginal preparation for CD and before initiating skin preparation and abdominal draping. The steps for inserting the Fetal Pillow include:
- Use the 60 mL syringe to fully deflate the Fetal Pillow and leave the cock-stop open.
- Fold the Fetal Pillow by squeezing the firm plastic base, and with the patient’s legs in a frog-leg position, place the device in the vagina.
- Allow the firm plastic base to open to a flat position with the base against the posterior vaginal wall and the soft silicon balloon against the fetal head.
- Using pressure on the plastic base, gently push the Fetal Pillow posteriorly toward the sacrum of the mother.
- Use the 60 mL syringe to inflate the balloon with 180 mL of normal saline and close the valve.
- Straighten the patient’s legs and proceed with skin preparation and abdominal draping (FIGURE 4).
When the CD is completed, deflate the balloon by drawing out the saline with the 60 mL syringe and remove the device by hooking a finger around the firm plastic base. The Fetal Pillow is surprisingly easy to use.
Continue to: Effectiveness of the Fetal Pillow...
Effectiveness of the Fetal Pillow
In one randomized trial, 240 women undergoing CD were randomly allocated to a group in which the Fetal Pillow was placed in the vagina and inflated prior to the cesarean and a control group in which the Fetal Pillow was not used.
In another randomized trial, 60 nulliparous women undergoing CD in the second stage of labor had a Fetal Pillow inserted in the vagina and were randomly allocated to inflation of the pillow (Fetal Pillow group) or noninflation of the pillow (control group).5 In this study the mean length of the second stage was 4 hours. Compared with noninflation of the Fetal Pillow, use of the inflated Fetal Pillow was associated with a reduction in grade 3 extension of the uterine incision (extensions into the uterine artery, vagina, or bladder) (0% for inflation vs 13% for noninflation) and fewer difficult plus very difficult deliveries of the fetal head as reported by the surgeon (0% for inflation vs 37% for noninflation). There was no significant difference in blood loss between the two groups (800 mL vs 900 mL). These two randomized studies both reported that the use of the Fetal Pillow was associated with a reduction in grade 3 extensions of the uterine incision and a decrease in the difficulty of delivering the fetal head.
Consider trialing the Fetal Pillow
When a CD is performed after a prolonged second stage of labor, surgical complications are common, including extensions of the uterine incision and difficulty delivering the fetal head. When a grade 3 extension occurs—with tearing of a uterine artery, deep extension into the vagina, or damage to the bladder—the surgical repair can be extraordinarily challenging. Clinical trials report that both reverse breech extraction and the Fetal Pillow can facilitate CD in the setting of a prolonged second stage. For many obstetricians reverse breech extraction is a challenging obstetric maneuver. The insertion and inflation of a Fetal Pillow is a simple procedure. Obstetrician-gynecologists learn by doing. If you have never used the Fetal Pillow, I suggest you consider trialing it in your practice. ●
- Jeve YB, Navti OB, Konje JC. Comparison of techniques used to deliver a deeply impacted fetal head at full dilation: a systematic review and meta-analysis. BJOG. 2016;123:337-345.
- Fasubaa OB, Ezechi OC, Orji EO, et al. Delivery of the impacted head of the fetus at cesarean section after prolonged obstructed labor: a randomised comparative study of two methods. J Obstet Gynaecol. 2002;22:375-378.
- Nooh AM, Abdeldayem HM, Ben-Affan O. Reverse breech extraction versus the standard approach of pushing the impacted fetal head up through the vagina in caesarean section for obstructed labour: a randomised controlled trial. J Obstet Gynaecol. 2017;37:459-463.
- Seal SL, Dey A, Barman SC, et al. Randomized controlled trial of elevation of the fetal head with a fetal pillow during cesarean delivery at full cervical dilatation. Int J Gynaecol Obstet. 2016;133:178-182.
- Lassey SC, Little SE, Saadeh M,et al. Cephalic elevation device for second-stage cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2020;135:879-884.
Obstetricians know that a cesarean delivery (CD) for a woman with a prolonged second stage and a fetal head deeply impacted in the pelvis is challenging. In this situation, extensions of the uterine incision commonly occur, resulting in prolonged operative time and increased blood loss. Even more harrowing is the inability to deliver the fetal head, necessitating emergency assistance from other clinicians. In this situation, interventions that may be helpful include:
- extend or T the uterine incision
- enlist the aid of a clinician to push up on the fetal head with a vaginal hand (FIGURE 1)
- reverse breech extraction (FIGURE 2), and
- vaginal insertion of a Fetal Pillow prior to starting the delivery.


Evidence from clinical trials indicates that reverse breech extraction or insertion of a Fetal Pillow result in the best clinical outcomes.
Reverse breech extraction vs the push technique
Although the data are limited, most studies report that compared with pushing up with a vaginal hand (as shown in Figure 1), the reverse breech extraction technique (as shown in Figure 2) is associated with a reduction in extensions of the uterine incision, reduced blood loss, and reduced operative time.1 In a randomized trial, 108 women with obstructed labor undergoing CD in the second stage were randomly assigned to reverse breech extraction or pushing up with a vaginal hand.2 Following the uterine incision, the reverse breech extraction technique is performed by immediately reaching into the upper uterus and grasping the lower portion of the fetal leg and applying gentle traction on the leg until the second leg appeared. The lower legs are then pulled out of the uterus. Standard breech delivery maneuvers are used to deliver the shoulders and head. In the trial, compared with the push technique, reverse breech extraction was associated with fewer extensions of the uterine incision (30% vs 11%; P<.05), less blood loss (899 mL vs 1,257 mL; P<.001), and shorter operative time (56 min vs 89 min, P<.001). Fetal injury was similar with the push and breech extraction techniques (6% and 7%).
In another randomized trial, 192 women undergoing CD for obstructed labor were randomly assigned to reverse breech extraction or pushing the head up with a hand in the vagina.3 Compared with the vaginal push technique, reverse breech extraction was associated with fewer extensions of the uterine incision (19% vs 48%; P = .003), fewer cases of wound infection (2% vs 13%; P = .007), and fewer blood transfusions (2 vs 11; P = .012).
Additional options and adjuvants for facilitating delivery of a fetal head deeply impacted in the pelvis include: using a Coyne spoon, using nitroglycerine or terbutaline to relax the myometrium, breaking the vaginal suction on the fetal head before attempting delivery, keeping the wrist of the delivering hand as straight as possible to reduce uterine incision extensions, and incising the ring (if a Bandl’s ring is detected).
Continue to: The Fetal Pillow...
The Fetal Pillow
The Fetal Pillow (Safe Obstetric Systems, New York, New York) is a single-use fetal cephalic elevation device for managing the deeply impacted fetal head (FIGURE 3). The Fetal Pillow has a firm plastic base upon which is attached a soft silicon balloon. The Fetal Pillow is inserted into the vagina prior to initiating CD and the balloon is filled with 180 mL of saline, causing the fetal head to be pushed to a higher station (FIGURE 4). Use of the Fetal Pillow may be indicated prior to CD in the following situations:
- second stage labor with a deeply impacted head
- second stage labor and failed operative delivery
- occiput posterior position or deep transverse arrest
- absent progress in the first stage between 8 cm and 10 cm with a deeply impacted fetal head or excessive caput of the fetal head.


The Fetal Pillow is inserted after completing vaginal preparation for CD and before initiating skin preparation and abdominal draping. The steps for inserting the Fetal Pillow include:
- Use the 60 mL syringe to fully deflate the Fetal Pillow and leave the cock-stop open.
- Fold the Fetal Pillow by squeezing the firm plastic base, and with the patient’s legs in a frog-leg position, place the device in the vagina.
- Allow the firm plastic base to open to a flat position with the base against the posterior vaginal wall and the soft silicon balloon against the fetal head.
- Using pressure on the plastic base, gently push the Fetal Pillow posteriorly toward the sacrum of the mother.
- Use the 60 mL syringe to inflate the balloon with 180 mL of normal saline and close the valve.
- Straighten the patient’s legs and proceed with skin preparation and abdominal draping (FIGURE 4).
When the CD is completed, deflate the balloon by drawing out the saline with the 60 mL syringe and remove the device by hooking a finger around the firm plastic base. The Fetal Pillow is surprisingly easy to use.
Continue to: Effectiveness of the Fetal Pillow...
Effectiveness of the Fetal Pillow
In one randomized trial, 240 women undergoing CD were randomly allocated to a group in which the Fetal Pillow was placed in the vagina and inflated prior to the cesarean and a control group in which the Fetal Pillow was not used.
In another randomized trial, 60 nulliparous women undergoing CD in the second stage of labor had a Fetal Pillow inserted in the vagina and were randomly allocated to inflation of the pillow (Fetal Pillow group) or noninflation of the pillow (control group).5 In this study the mean length of the second stage was 4 hours. Compared with noninflation of the Fetal Pillow, use of the inflated Fetal Pillow was associated with a reduction in grade 3 extension of the uterine incision (extensions into the uterine artery, vagina, or bladder) (0% for inflation vs 13% for noninflation) and fewer difficult plus very difficult deliveries of the fetal head as reported by the surgeon (0% for inflation vs 37% for noninflation). There was no significant difference in blood loss between the two groups (800 mL vs 900 mL). These two randomized studies both reported that the use of the Fetal Pillow was associated with a reduction in grade 3 extensions of the uterine incision and a decrease in the difficulty of delivering the fetal head.
Consider trialing the Fetal Pillow
When a CD is performed after a prolonged second stage of labor, surgical complications are common, including extensions of the uterine incision and difficulty delivering the fetal head. When a grade 3 extension occurs—with tearing of a uterine artery, deep extension into the vagina, or damage to the bladder—the surgical repair can be extraordinarily challenging. Clinical trials report that both reverse breech extraction and the Fetal Pillow can facilitate CD in the setting of a prolonged second stage. For many obstetricians reverse breech extraction is a challenging obstetric maneuver. The insertion and inflation of a Fetal Pillow is a simple procedure. Obstetrician-gynecologists learn by doing. If you have never used the Fetal Pillow, I suggest you consider trialing it in your practice. ●
Obstetricians know that a cesarean delivery (CD) for a woman with a prolonged second stage and a fetal head deeply impacted in the pelvis is challenging. In this situation, extensions of the uterine incision commonly occur, resulting in prolonged operative time and increased blood loss. Even more harrowing is the inability to deliver the fetal head, necessitating emergency assistance from other clinicians. In this situation, interventions that may be helpful include:
- extend or T the uterine incision
- enlist the aid of a clinician to push up on the fetal head with a vaginal hand (FIGURE 1)
- reverse breech extraction (FIGURE 2), and
- vaginal insertion of a Fetal Pillow prior to starting the delivery.


Evidence from clinical trials indicates that reverse breech extraction or insertion of a Fetal Pillow result in the best clinical outcomes.
Reverse breech extraction vs the push technique
Although the data are limited, most studies report that compared with pushing up with a vaginal hand (as shown in Figure 1), the reverse breech extraction technique (as shown in Figure 2) is associated with a reduction in extensions of the uterine incision, reduced blood loss, and reduced operative time.1 In a randomized trial, 108 women with obstructed labor undergoing CD in the second stage were randomly assigned to reverse breech extraction or pushing up with a vaginal hand.2 Following the uterine incision, the reverse breech extraction technique is performed by immediately reaching into the upper uterus and grasping the lower portion of the fetal leg and applying gentle traction on the leg until the second leg appeared. The lower legs are then pulled out of the uterus. Standard breech delivery maneuvers are used to deliver the shoulders and head. In the trial, compared with the push technique, reverse breech extraction was associated with fewer extensions of the uterine incision (30% vs 11%; P<.05), less blood loss (899 mL vs 1,257 mL; P<.001), and shorter operative time (56 min vs 89 min, P<.001). Fetal injury was similar with the push and breech extraction techniques (6% and 7%).
In another randomized trial, 192 women undergoing CD for obstructed labor were randomly assigned to reverse breech extraction or pushing the head up with a hand in the vagina.3 Compared with the vaginal push technique, reverse breech extraction was associated with fewer extensions of the uterine incision (19% vs 48%; P = .003), fewer cases of wound infection (2% vs 13%; P = .007), and fewer blood transfusions (2 vs 11; P = .012).
Additional options and adjuvants for facilitating delivery of a fetal head deeply impacted in the pelvis include: using a Coyne spoon, using nitroglycerine or terbutaline to relax the myometrium, breaking the vaginal suction on the fetal head before attempting delivery, keeping the wrist of the delivering hand as straight as possible to reduce uterine incision extensions, and incising the ring (if a Bandl’s ring is detected).
Continue to: The Fetal Pillow...
The Fetal Pillow
The Fetal Pillow (Safe Obstetric Systems, New York, New York) is a single-use fetal cephalic elevation device for managing the deeply impacted fetal head (FIGURE 3). The Fetal Pillow has a firm plastic base upon which is attached a soft silicon balloon. The Fetal Pillow is inserted into the vagina prior to initiating CD and the balloon is filled with 180 mL of saline, causing the fetal head to be pushed to a higher station (FIGURE 4). Use of the Fetal Pillow may be indicated prior to CD in the following situations:
- second stage labor with a deeply impacted head
- second stage labor and failed operative delivery
- occiput posterior position or deep transverse arrest
- absent progress in the first stage between 8 cm and 10 cm with a deeply impacted fetal head or excessive caput of the fetal head.


The Fetal Pillow is inserted after completing vaginal preparation for CD and before initiating skin preparation and abdominal draping. The steps for inserting the Fetal Pillow include:
- Use the 60 mL syringe to fully deflate the Fetal Pillow and leave the cock-stop open.
- Fold the Fetal Pillow by squeezing the firm plastic base, and with the patient’s legs in a frog-leg position, place the device in the vagina.
- Allow the firm plastic base to open to a flat position with the base against the posterior vaginal wall and the soft silicon balloon against the fetal head.
- Using pressure on the plastic base, gently push the Fetal Pillow posteriorly toward the sacrum of the mother.
- Use the 60 mL syringe to inflate the balloon with 180 mL of normal saline and close the valve.
- Straighten the patient’s legs and proceed with skin preparation and abdominal draping (FIGURE 4).
When the CD is completed, deflate the balloon by drawing out the saline with the 60 mL syringe and remove the device by hooking a finger around the firm plastic base. The Fetal Pillow is surprisingly easy to use.
Continue to: Effectiveness of the Fetal Pillow...
Effectiveness of the Fetal Pillow
In one randomized trial, 240 women undergoing CD were randomly allocated to a group in which the Fetal Pillow was placed in the vagina and inflated prior to the cesarean and a control group in which the Fetal Pillow was not used.
In another randomized trial, 60 nulliparous women undergoing CD in the second stage of labor had a Fetal Pillow inserted in the vagina and were randomly allocated to inflation of the pillow (Fetal Pillow group) or noninflation of the pillow (control group).5 In this study the mean length of the second stage was 4 hours. Compared with noninflation of the Fetal Pillow, use of the inflated Fetal Pillow was associated with a reduction in grade 3 extension of the uterine incision (extensions into the uterine artery, vagina, or bladder) (0% for inflation vs 13% for noninflation) and fewer difficult plus very difficult deliveries of the fetal head as reported by the surgeon (0% for inflation vs 37% for noninflation). There was no significant difference in blood loss between the two groups (800 mL vs 900 mL). These two randomized studies both reported that the use of the Fetal Pillow was associated with a reduction in grade 3 extensions of the uterine incision and a decrease in the difficulty of delivering the fetal head.
Consider trialing the Fetal Pillow
When a CD is performed after a prolonged second stage of labor, surgical complications are common, including extensions of the uterine incision and difficulty delivering the fetal head. When a grade 3 extension occurs—with tearing of a uterine artery, deep extension into the vagina, or damage to the bladder—the surgical repair can be extraordinarily challenging. Clinical trials report that both reverse breech extraction and the Fetal Pillow can facilitate CD in the setting of a prolonged second stage. For many obstetricians reverse breech extraction is a challenging obstetric maneuver. The insertion and inflation of a Fetal Pillow is a simple procedure. Obstetrician-gynecologists learn by doing. If you have never used the Fetal Pillow, I suggest you consider trialing it in your practice. ●
- Jeve YB, Navti OB, Konje JC. Comparison of techniques used to deliver a deeply impacted fetal head at full dilation: a systematic review and meta-analysis. BJOG. 2016;123:337-345.
- Fasubaa OB, Ezechi OC, Orji EO, et al. Delivery of the impacted head of the fetus at cesarean section after prolonged obstructed labor: a randomised comparative study of two methods. J Obstet Gynaecol. 2002;22:375-378.
- Nooh AM, Abdeldayem HM, Ben-Affan O. Reverse breech extraction versus the standard approach of pushing the impacted fetal head up through the vagina in caesarean section for obstructed labour: a randomised controlled trial. J Obstet Gynaecol. 2017;37:459-463.
- Seal SL, Dey A, Barman SC, et al. Randomized controlled trial of elevation of the fetal head with a fetal pillow during cesarean delivery at full cervical dilatation. Int J Gynaecol Obstet. 2016;133:178-182.
- Lassey SC, Little SE, Saadeh M,et al. Cephalic elevation device for second-stage cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2020;135:879-884.
- Jeve YB, Navti OB, Konje JC. Comparison of techniques used to deliver a deeply impacted fetal head at full dilation: a systematic review and meta-analysis. BJOG. 2016;123:337-345.
- Fasubaa OB, Ezechi OC, Orji EO, et al. Delivery of the impacted head of the fetus at cesarean section after prolonged obstructed labor: a randomised comparative study of two methods. J Obstet Gynaecol. 2002;22:375-378.
- Nooh AM, Abdeldayem HM, Ben-Affan O. Reverse breech extraction versus the standard approach of pushing the impacted fetal head up through the vagina in caesarean section for obstructed labour: a randomised controlled trial. J Obstet Gynaecol. 2017;37:459-463.
- Seal SL, Dey A, Barman SC, et al. Randomized controlled trial of elevation of the fetal head with a fetal pillow during cesarean delivery at full cervical dilatation. Int J Gynaecol Obstet. 2016;133:178-182.
- Lassey SC, Little SE, Saadeh M,et al. Cephalic elevation device for second-stage cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2020;135:879-884.
In your practice, are you planning to have a chaperone present for all intimate examinations?
Although pelvic examinations may only last a few minutes, the examination is scary and uncomfortable for many patients. To help minimize fear and discomfort, the exam should take place in a comfortable and professional environment. The clinician should provide appropriate gowns, private facilities for undressing, sensitively use draping, and clearly explain the components of the examination. Trained professional chaperones play an important role in intimate physical examinations, including:
- providing reassurance to the patient of the professional integrity of the intimate examination
- supporting and educating the patient during the examination
- increasing the efficiency of the clinician during a procedure
- acting as a witness should a misunderstanding with the patient arise.
Major medical professional societies have issued guidance to clinicians on the use of a chaperone during intimate physical examinations. Professional society guidance ranges from endorsing joint decision-making between physician and patient on the presence of a chaperone to more proscriptive guidance that emphasizes the importance of a chaperone at every intimate physical examination.
Examples of professional societies’ guidance that supports joint decision-making between physician and patient about the presence of a chaperone include:
- American Medical Association: “Adopt a policy that patients are free to request a chaperone and ensure that the policy is communicated to patients. Always honor a patient’s request to have a chaperone.”1
- Society of Obstetricians and Gynaecologists of Canada: “It is a reasonable and acceptable practice to perform a physical examination, including breast and pelvic examination without the presence of a third person in the room unless the woman or health care provider indicates a desire for a third party to be present.” “If the health care provider chooses to have a third person present during all examinations, the health care provider should explain this policy to the woman.”2
- American College of Physicians: “Care and respect should guide the performance of the physical examination. The location and degree of privacy should be appropriate for the examination being performed, with chaperone services as an option. An appropriate setting and sufficient time should be allocated to encourage exploration of aspects of the patient’s life pertinent to health, including habits, relationships, sexuality, vocation, culture, religion, and spirituality.”3
By contrast, the following professional society guidance strongly recommends the presence of a chaperone for every intimate physical examination:
- United States Veterans Administration: “A female chaperone must be in the examination room during breast and pelvic exams…this includes procedures such as urodynamic testing or treatments such as pelvic floor physical therapy.”4
- Royal College of Obstetricians and Gynaecologists: “The presence of a chaperone is considered essential for every pelvic examination. Verbal consent should be obtained in the presence of the chaperone who is to be present during the examination and recorded in the notes. If the patient declines the presence of a chaperone, the doctor should explain that a chaperone is also required to help in many cases and then attempt to arrange for the chaperone to be standing nearby within earshot. The reasons for declining a chaperone and alternative arrangements offered should be documented. Consent should also be specific to whether the intended examination is vaginal, rectal or both. Communication skills are essential in conducting intimate examinations.”5
- American College Health Association (ACHA): “It is ACHA’s recommendation that, as part of institutional policy, a chaperone be provided for every sensitive medical examination and procedure.”6
Continue to: New guidance from ACOG on trained chaperones...
New guidance from ACOG on trained chaperones
The American College of Obstetricians and Gynecologists (ACOG) recently issued a committee opinion recommending “that a chaperone be present for all breast, genital, and rectal examinations. The need for a chaperone is irrespective of the sex or gender of the person performing the examination and applies to examinations performed in the outpatient and inpatient settings, including labor and delivery, as well as during diagnostic studies such as transvaginal ultrasonography and urodynamic testing.”7
This new proscriptive guidance will significantly change practice for the many obstetrician-gynecologists who do not routinely have a chaperone present during intimate examinations. The policy provides exceptions to the presence of a chaperone in cases of medical emergencies and if the patient declines a chaperone. ACOG recommends that when a patient declines a chaperone the clinician should educate the patient that a “chaperone is an integral part of the clinical team whose role includes assisting with the examination and protecting the patient and the physician. Any concerns the patient has regarding the presence of a chaperone should be elicited and addressed if feasible. If, after counseling, the patient refuses the chaperone, this decision should be respected and documented in the medical record.”7 ACOG discourages the use of family members, medical students, and residents as chaperones.
Sexual trauma is common and may cause lasting adverse effects, including poor health.1 When sexual trauma is reported, the experience may not be believed or taken seriously, compounding the injury. Sometimes sexual trauma contributes to risky behaviors including smoking cigarettes, excessive alcohol consumption, drug misuse, and risky sex as a means to cope with the mental distress of the trauma.
Trauma-informed medical care has four pillars:
1. Recognize that many people have experienced significant trauma(s), which adversely impacts their health.
2. Be aware of the signs and symptoms of trauma.
3. Integrate knowledge about trauma into medical encounters.
4. Avoid re-traumatizing the person.
Symptoms of psychological distress caused by past trauma include anxiety, fear, anger, irritability, mood swings, feeling disconnected, numbness, sadness, or hopelessness. Clinical actions that help to reduce distress among trauma survivors include:
• sensitively ask patients to share their traumatic experiences
• empower the patient by explicitly giving her control over all aspects of the examination, indicating that the exam will stop if the patient feels uncomfortable
• explain the steps in the exam and educate about the purpose of each step
• keep the patient’s body covered as much as possible
• use the smallest speculum that permits an adequate exam
• utilize a chaperone to help support the patient.
Clinicians can strengthen their empathic skills by reflecting on how their own personal experiences, traumas, cultural-biases, and gender influence their ap-proach to the care of patients.
Reference
1. Hall KS, Moreau C, Trussell J. Young women’s perceived health and lifetime sexual experience: results from the national survey of family growth. J Sex Med. 2012;9:1382-1391. doi: 10.1111/j.1743-6109.2012.02686.x.
Training of chaperones
Chaperones are health care professionals who should be trained for their specific role. Chaperones need to protect patient privacy and the confidentiality of health information. Chaperones should be trained to recognize the components of a professional intimate examination and to identify variances from standard practice. In many ambulatory practices, medical assistants perform the role of chaperone. The American Association of Medical Assistants (AAMA) offers national certification for medical assistants through an examination developed by the National Board of Medical Examiners. To be eligible for AAMA certification an individual must complete at least two semesters of medical assisting education that includes courses in anatomy, physiology, pharmacology, and relevant mathematics.
Reporting variances that occur during an intimate examination
Best practices are evolving on how to deal with the rare event of a chaperone witnessing a physician perform an intimate examination that is outside of standard professional practice. Chaperones may be reluctant to report a variance because physicians are in a powerful position, and the accuracy of their report will be challenged, threatening the chaperone’s employment. Processes for encouraging all team members to report concerns must be clearly explained to the chaperone and other members of the health care team. Clinicians should be aware that deviations from standard practice will be reported and investigated. Medical practices must develop a reporting system that ensures the reporting individual will be protected from retaliation.
In addition, the chaperone needs to know to whom they should report a variance. In large multispecialty medical practices, chaperones often can report concerns to nursing leaders or human resources. In small ambulatory practices, chaperones may be advised to report concerns about a physician to the practice manager or medical director. Regardless, every practice should have the best process for reporting a concern. In turn, the practice leaders who are responsible for investigating reports of concerning behavior should have a defined process for confidentially interviewing the chaperone, clinician, and patient.
Even when a chaperone is present for intimate examinations, problems can arise if the chaperone is not trained to recognize variances from standard practice or does not have a clear means for reporting variances and when the practice does not have a process for investigating reported variances.
Sadly, misconduct has been documented among priests, ministers, sports coaches, professors, scout masters, and clinicians. Trusted professionals are in positions of power in relation to their clients, patients, and students. Physicians and nurses are held in high esteem and trust by patients. To preserve the trust of the public we must treat all people with dignity and respect their autonomy. The presence of a chaperone during intimate examinations may help us fulfill Hippocrates’ edict, “First, do no harm.” ●
Ronee A. Skornik, MSW, MD
As a female obstetrician-gynecologist trained in psychiatric social work, I have found that some of my patients who have known me over a long period of time find the presence of a chaperone not only unnecessary but also uncomfortable both in terms of physical exposure and in what they may want to tell me during the examination. Personally, I strongly favor a chaperone for all intimate examinations, to safeguard both the patient and the clinician. However, I do understand why some patients prefer to see me without the presence of a chaperone, and I want to honor their wishes. If a chaperone is responsive to the patient’s requests, including where the chaperone stands and his or her role during the exam, the reluctant patient may be more willing to have a chaperone. A chaperone who develops a relationship with the patient and honors the patient’s preferences is a valuable member of the care team.
- American Medical Association. Code of Medical Ethics Opinion 1.2.4. https://www.ama-assn.org/delivering-care/ethics/use-chaperones. Accessed May 26, 2020.
- Society of Obstetricians and Gynaecologists of Canada. No. 266—The presence of a third party during breast and pelvic examinations. J Obstet Gynaecol Can. 2017;39:e496-e497. doi: 10.1016/j.jogc.2017.09.005.
- American College of Physicians. ACP Policy Com-pendium Summer 2016. https://www.acponline.org/system/files/documents/advocacy/acp_policy_compendium_summer_2016.pdf. Accessed May 26, 2020.
- Department of Veterans Affairs. VHA Directive 1330.01(2). Healthcare Services for Women Veterans. February 15, 2017. Amended July 24, 2018. http://www.va.gov/ vhapublications/ viewpublication.asp?pub_id=5332. Accessed May 26, 2020.
- Royal College of Obstetricians and Gynaecologists. Obtaining valid consent: clinical governance advice no. 6. January 2015. https://www.rcog.org.uk/globalassets/documents/guidelines/clinical-governance-advice/cga6.pdf. Accessed May 26, 2020.
- American College Health Association Guidelines. Best practices for sensitive exams. October 2019. https://www.acha.org/documents/resources/guidelines/ACHA_Best_Practices_for_Sensitive_Exams_October2019.pdf. Accessed May 26, 2020.
- American College of Obstetricians and Gynecologists Committee on Ethics. Sexual misconduct: ACOG Committee Opinion No. 796. Obstet Gynecol. 2020;135:e43-e50
Although pelvic examinations may only last a few minutes, the examination is scary and uncomfortable for many patients. To help minimize fear and discomfort, the exam should take place in a comfortable and professional environment. The clinician should provide appropriate gowns, private facilities for undressing, sensitively use draping, and clearly explain the components of the examination. Trained professional chaperones play an important role in intimate physical examinations, including:
- providing reassurance to the patient of the professional integrity of the intimate examination
- supporting and educating the patient during the examination
- increasing the efficiency of the clinician during a procedure
- acting as a witness should a misunderstanding with the patient arise.
Major medical professional societies have issued guidance to clinicians on the use of a chaperone during intimate physical examinations. Professional society guidance ranges from endorsing joint decision-making between physician and patient on the presence of a chaperone to more proscriptive guidance that emphasizes the importance of a chaperone at every intimate physical examination.
Examples of professional societies’ guidance that supports joint decision-making between physician and patient about the presence of a chaperone include:
- American Medical Association: “Adopt a policy that patients are free to request a chaperone and ensure that the policy is communicated to patients. Always honor a patient’s request to have a chaperone.”1
- Society of Obstetricians and Gynaecologists of Canada: “It is a reasonable and acceptable practice to perform a physical examination, including breast and pelvic examination without the presence of a third person in the room unless the woman or health care provider indicates a desire for a third party to be present.” “If the health care provider chooses to have a third person present during all examinations, the health care provider should explain this policy to the woman.”2
- American College of Physicians: “Care and respect should guide the performance of the physical examination. The location and degree of privacy should be appropriate for the examination being performed, with chaperone services as an option. An appropriate setting and sufficient time should be allocated to encourage exploration of aspects of the patient’s life pertinent to health, including habits, relationships, sexuality, vocation, culture, religion, and spirituality.”3
By contrast, the following professional society guidance strongly recommends the presence of a chaperone for every intimate physical examination:
- United States Veterans Administration: “A female chaperone must be in the examination room during breast and pelvic exams…this includes procedures such as urodynamic testing or treatments such as pelvic floor physical therapy.”4
- Royal College of Obstetricians and Gynaecologists: “The presence of a chaperone is considered essential for every pelvic examination. Verbal consent should be obtained in the presence of the chaperone who is to be present during the examination and recorded in the notes. If the patient declines the presence of a chaperone, the doctor should explain that a chaperone is also required to help in many cases and then attempt to arrange for the chaperone to be standing nearby within earshot. The reasons for declining a chaperone and alternative arrangements offered should be documented. Consent should also be specific to whether the intended examination is vaginal, rectal or both. Communication skills are essential in conducting intimate examinations.”5
- American College Health Association (ACHA): “It is ACHA’s recommendation that, as part of institutional policy, a chaperone be provided for every sensitive medical examination and procedure.”6
Continue to: New guidance from ACOG on trained chaperones...
New guidance from ACOG on trained chaperones
The American College of Obstetricians and Gynecologists (ACOG) recently issued a committee opinion recommending “that a chaperone be present for all breast, genital, and rectal examinations. The need for a chaperone is irrespective of the sex or gender of the person performing the examination and applies to examinations performed in the outpatient and inpatient settings, including labor and delivery, as well as during diagnostic studies such as transvaginal ultrasonography and urodynamic testing.”7
This new proscriptive guidance will significantly change practice for the many obstetrician-gynecologists who do not routinely have a chaperone present during intimate examinations. The policy provides exceptions to the presence of a chaperone in cases of medical emergencies and if the patient declines a chaperone. ACOG recommends that when a patient declines a chaperone the clinician should educate the patient that a “chaperone is an integral part of the clinical team whose role includes assisting with the examination and protecting the patient and the physician. Any concerns the patient has regarding the presence of a chaperone should be elicited and addressed if feasible. If, after counseling, the patient refuses the chaperone, this decision should be respected and documented in the medical record.”7 ACOG discourages the use of family members, medical students, and residents as chaperones.
Sexual trauma is common and may cause lasting adverse effects, including poor health.1 When sexual trauma is reported, the experience may not be believed or taken seriously, compounding the injury. Sometimes sexual trauma contributes to risky behaviors including smoking cigarettes, excessive alcohol consumption, drug misuse, and risky sex as a means to cope with the mental distress of the trauma.
Trauma-informed medical care has four pillars:
1. Recognize that many people have experienced significant trauma(s), which adversely impacts their health.
2. Be aware of the signs and symptoms of trauma.
3. Integrate knowledge about trauma into medical encounters.
4. Avoid re-traumatizing the person.
Symptoms of psychological distress caused by past trauma include anxiety, fear, anger, irritability, mood swings, feeling disconnected, numbness, sadness, or hopelessness. Clinical actions that help to reduce distress among trauma survivors include:
• sensitively ask patients to share their traumatic experiences
• empower the patient by explicitly giving her control over all aspects of the examination, indicating that the exam will stop if the patient feels uncomfortable
• explain the steps in the exam and educate about the purpose of each step
• keep the patient’s body covered as much as possible
• use the smallest speculum that permits an adequate exam
• utilize a chaperone to help support the patient.
Clinicians can strengthen their empathic skills by reflecting on how their own personal experiences, traumas, cultural-biases, and gender influence their ap-proach to the care of patients.
Reference
1. Hall KS, Moreau C, Trussell J. Young women’s perceived health and lifetime sexual experience: results from the national survey of family growth. J Sex Med. 2012;9:1382-1391. doi: 10.1111/j.1743-6109.2012.02686.x.
Training of chaperones
Chaperones are health care professionals who should be trained for their specific role. Chaperones need to protect patient privacy and the confidentiality of health information. Chaperones should be trained to recognize the components of a professional intimate examination and to identify variances from standard practice. In many ambulatory practices, medical assistants perform the role of chaperone. The American Association of Medical Assistants (AAMA) offers national certification for medical assistants through an examination developed by the National Board of Medical Examiners. To be eligible for AAMA certification an individual must complete at least two semesters of medical assisting education that includes courses in anatomy, physiology, pharmacology, and relevant mathematics.
Reporting variances that occur during an intimate examination
Best practices are evolving on how to deal with the rare event of a chaperone witnessing a physician perform an intimate examination that is outside of standard professional practice. Chaperones may be reluctant to report a variance because physicians are in a powerful position, and the accuracy of their report will be challenged, threatening the chaperone’s employment. Processes for encouraging all team members to report concerns must be clearly explained to the chaperone and other members of the health care team. Clinicians should be aware that deviations from standard practice will be reported and investigated. Medical practices must develop a reporting system that ensures the reporting individual will be protected from retaliation.
In addition, the chaperone needs to know to whom they should report a variance. In large multispecialty medical practices, chaperones often can report concerns to nursing leaders or human resources. In small ambulatory practices, chaperones may be advised to report concerns about a physician to the practice manager or medical director. Regardless, every practice should have the best process for reporting a concern. In turn, the practice leaders who are responsible for investigating reports of concerning behavior should have a defined process for confidentially interviewing the chaperone, clinician, and patient.
Even when a chaperone is present for intimate examinations, problems can arise if the chaperone is not trained to recognize variances from standard practice or does not have a clear means for reporting variances and when the practice does not have a process for investigating reported variances.
Sadly, misconduct has been documented among priests, ministers, sports coaches, professors, scout masters, and clinicians. Trusted professionals are in positions of power in relation to their clients, patients, and students. Physicians and nurses are held in high esteem and trust by patients. To preserve the trust of the public we must treat all people with dignity and respect their autonomy. The presence of a chaperone during intimate examinations may help us fulfill Hippocrates’ edict, “First, do no harm.” ●
Ronee A. Skornik, MSW, MD
As a female obstetrician-gynecologist trained in psychiatric social work, I have found that some of my patients who have known me over a long period of time find the presence of a chaperone not only unnecessary but also uncomfortable both in terms of physical exposure and in what they may want to tell me during the examination. Personally, I strongly favor a chaperone for all intimate examinations, to safeguard both the patient and the clinician. However, I do understand why some patients prefer to see me without the presence of a chaperone, and I want to honor their wishes. If a chaperone is responsive to the patient’s requests, including where the chaperone stands and his or her role during the exam, the reluctant patient may be more willing to have a chaperone. A chaperone who develops a relationship with the patient and honors the patient’s preferences is a valuable member of the care team.
Although pelvic examinations may only last a few minutes, the examination is scary and uncomfortable for many patients. To help minimize fear and discomfort, the exam should take place in a comfortable and professional environment. The clinician should provide appropriate gowns, private facilities for undressing, sensitively use draping, and clearly explain the components of the examination. Trained professional chaperones play an important role in intimate physical examinations, including:
- providing reassurance to the patient of the professional integrity of the intimate examination
- supporting and educating the patient during the examination
- increasing the efficiency of the clinician during a procedure
- acting as a witness should a misunderstanding with the patient arise.
Major medical professional societies have issued guidance to clinicians on the use of a chaperone during intimate physical examinations. Professional society guidance ranges from endorsing joint decision-making between physician and patient on the presence of a chaperone to more proscriptive guidance that emphasizes the importance of a chaperone at every intimate physical examination.
Examples of professional societies’ guidance that supports joint decision-making between physician and patient about the presence of a chaperone include:
- American Medical Association: “Adopt a policy that patients are free to request a chaperone and ensure that the policy is communicated to patients. Always honor a patient’s request to have a chaperone.”1
- Society of Obstetricians and Gynaecologists of Canada: “It is a reasonable and acceptable practice to perform a physical examination, including breast and pelvic examination without the presence of a third person in the room unless the woman or health care provider indicates a desire for a third party to be present.” “If the health care provider chooses to have a third person present during all examinations, the health care provider should explain this policy to the woman.”2
- American College of Physicians: “Care and respect should guide the performance of the physical examination. The location and degree of privacy should be appropriate for the examination being performed, with chaperone services as an option. An appropriate setting and sufficient time should be allocated to encourage exploration of aspects of the patient’s life pertinent to health, including habits, relationships, sexuality, vocation, culture, religion, and spirituality.”3
By contrast, the following professional society guidance strongly recommends the presence of a chaperone for every intimate physical examination:
- United States Veterans Administration: “A female chaperone must be in the examination room during breast and pelvic exams…this includes procedures such as urodynamic testing or treatments such as pelvic floor physical therapy.”4
- Royal College of Obstetricians and Gynaecologists: “The presence of a chaperone is considered essential for every pelvic examination. Verbal consent should be obtained in the presence of the chaperone who is to be present during the examination and recorded in the notes. If the patient declines the presence of a chaperone, the doctor should explain that a chaperone is also required to help in many cases and then attempt to arrange for the chaperone to be standing nearby within earshot. The reasons for declining a chaperone and alternative arrangements offered should be documented. Consent should also be specific to whether the intended examination is vaginal, rectal or both. Communication skills are essential in conducting intimate examinations.”5
- American College Health Association (ACHA): “It is ACHA’s recommendation that, as part of institutional policy, a chaperone be provided for every sensitive medical examination and procedure.”6
Continue to: New guidance from ACOG on trained chaperones...
New guidance from ACOG on trained chaperones
The American College of Obstetricians and Gynecologists (ACOG) recently issued a committee opinion recommending “that a chaperone be present for all breast, genital, and rectal examinations. The need for a chaperone is irrespective of the sex or gender of the person performing the examination and applies to examinations performed in the outpatient and inpatient settings, including labor and delivery, as well as during diagnostic studies such as transvaginal ultrasonography and urodynamic testing.”7
This new proscriptive guidance will significantly change practice for the many obstetrician-gynecologists who do not routinely have a chaperone present during intimate examinations. The policy provides exceptions to the presence of a chaperone in cases of medical emergencies and if the patient declines a chaperone. ACOG recommends that when a patient declines a chaperone the clinician should educate the patient that a “chaperone is an integral part of the clinical team whose role includes assisting with the examination and protecting the patient and the physician. Any concerns the patient has regarding the presence of a chaperone should be elicited and addressed if feasible. If, after counseling, the patient refuses the chaperone, this decision should be respected and documented in the medical record.”7 ACOG discourages the use of family members, medical students, and residents as chaperones.
Sexual trauma is common and may cause lasting adverse effects, including poor health.1 When sexual trauma is reported, the experience may not be believed or taken seriously, compounding the injury. Sometimes sexual trauma contributes to risky behaviors including smoking cigarettes, excessive alcohol consumption, drug misuse, and risky sex as a means to cope with the mental distress of the trauma.
Trauma-informed medical care has four pillars:
1. Recognize that many people have experienced significant trauma(s), which adversely impacts their health.
2. Be aware of the signs and symptoms of trauma.
3. Integrate knowledge about trauma into medical encounters.
4. Avoid re-traumatizing the person.
Symptoms of psychological distress caused by past trauma include anxiety, fear, anger, irritability, mood swings, feeling disconnected, numbness, sadness, or hopelessness. Clinical actions that help to reduce distress among trauma survivors include:
• sensitively ask patients to share their traumatic experiences
• empower the patient by explicitly giving her control over all aspects of the examination, indicating that the exam will stop if the patient feels uncomfortable
• explain the steps in the exam and educate about the purpose of each step
• keep the patient’s body covered as much as possible
• use the smallest speculum that permits an adequate exam
• utilize a chaperone to help support the patient.
Clinicians can strengthen their empathic skills by reflecting on how their own personal experiences, traumas, cultural-biases, and gender influence their ap-proach to the care of patients.
Reference
1. Hall KS, Moreau C, Trussell J. Young women’s perceived health and lifetime sexual experience: results from the national survey of family growth. J Sex Med. 2012;9:1382-1391. doi: 10.1111/j.1743-6109.2012.02686.x.
Training of chaperones
Chaperones are health care professionals who should be trained for their specific role. Chaperones need to protect patient privacy and the confidentiality of health information. Chaperones should be trained to recognize the components of a professional intimate examination and to identify variances from standard practice. In many ambulatory practices, medical assistants perform the role of chaperone. The American Association of Medical Assistants (AAMA) offers national certification for medical assistants through an examination developed by the National Board of Medical Examiners. To be eligible for AAMA certification an individual must complete at least two semesters of medical assisting education that includes courses in anatomy, physiology, pharmacology, and relevant mathematics.
Reporting variances that occur during an intimate examination
Best practices are evolving on how to deal with the rare event of a chaperone witnessing a physician perform an intimate examination that is outside of standard professional practice. Chaperones may be reluctant to report a variance because physicians are in a powerful position, and the accuracy of their report will be challenged, threatening the chaperone’s employment. Processes for encouraging all team members to report concerns must be clearly explained to the chaperone and other members of the health care team. Clinicians should be aware that deviations from standard practice will be reported and investigated. Medical practices must develop a reporting system that ensures the reporting individual will be protected from retaliation.
In addition, the chaperone needs to know to whom they should report a variance. In large multispecialty medical practices, chaperones often can report concerns to nursing leaders or human resources. In small ambulatory practices, chaperones may be advised to report concerns about a physician to the practice manager or medical director. Regardless, every practice should have the best process for reporting a concern. In turn, the practice leaders who are responsible for investigating reports of concerning behavior should have a defined process for confidentially interviewing the chaperone, clinician, and patient.
Even when a chaperone is present for intimate examinations, problems can arise if the chaperone is not trained to recognize variances from standard practice or does not have a clear means for reporting variances and when the practice does not have a process for investigating reported variances.
Sadly, misconduct has been documented among priests, ministers, sports coaches, professors, scout masters, and clinicians. Trusted professionals are in positions of power in relation to their clients, patients, and students. Physicians and nurses are held in high esteem and trust by patients. To preserve the trust of the public we must treat all people with dignity and respect their autonomy. The presence of a chaperone during intimate examinations may help us fulfill Hippocrates’ edict, “First, do no harm.” ●
Ronee A. Skornik, MSW, MD
As a female obstetrician-gynecologist trained in psychiatric social work, I have found that some of my patients who have known me over a long period of time find the presence of a chaperone not only unnecessary but also uncomfortable both in terms of physical exposure and in what they may want to tell me during the examination. Personally, I strongly favor a chaperone for all intimate examinations, to safeguard both the patient and the clinician. However, I do understand why some patients prefer to see me without the presence of a chaperone, and I want to honor their wishes. If a chaperone is responsive to the patient’s requests, including where the chaperone stands and his or her role during the exam, the reluctant patient may be more willing to have a chaperone. A chaperone who develops a relationship with the patient and honors the patient’s preferences is a valuable member of the care team.
- American Medical Association. Code of Medical Ethics Opinion 1.2.4. https://www.ama-assn.org/delivering-care/ethics/use-chaperones. Accessed May 26, 2020.
- Society of Obstetricians and Gynaecologists of Canada. No. 266—The presence of a third party during breast and pelvic examinations. J Obstet Gynaecol Can. 2017;39:e496-e497. doi: 10.1016/j.jogc.2017.09.005.
- American College of Physicians. ACP Policy Com-pendium Summer 2016. https://www.acponline.org/system/files/documents/advocacy/acp_policy_compendium_summer_2016.pdf. Accessed May 26, 2020.
- Department of Veterans Affairs. VHA Directive 1330.01(2). Healthcare Services for Women Veterans. February 15, 2017. Amended July 24, 2018. http://www.va.gov/ vhapublications/ viewpublication.asp?pub_id=5332. Accessed May 26, 2020.
- Royal College of Obstetricians and Gynaecologists. Obtaining valid consent: clinical governance advice no. 6. January 2015. https://www.rcog.org.uk/globalassets/documents/guidelines/clinical-governance-advice/cga6.pdf. Accessed May 26, 2020.
- American College Health Association Guidelines. Best practices for sensitive exams. October 2019. https://www.acha.org/documents/resources/guidelines/ACHA_Best_Practices_for_Sensitive_Exams_October2019.pdf. Accessed May 26, 2020.
- American College of Obstetricians and Gynecologists Committee on Ethics. Sexual misconduct: ACOG Committee Opinion No. 796. Obstet Gynecol. 2020;135:e43-e50
- American Medical Association. Code of Medical Ethics Opinion 1.2.4. https://www.ama-assn.org/delivering-care/ethics/use-chaperones. Accessed May 26, 2020.
- Society of Obstetricians and Gynaecologists of Canada. No. 266—The presence of a third party during breast and pelvic examinations. J Obstet Gynaecol Can. 2017;39:e496-e497. doi: 10.1016/j.jogc.2017.09.005.
- American College of Physicians. ACP Policy Com-pendium Summer 2016. https://www.acponline.org/system/files/documents/advocacy/acp_policy_compendium_summer_2016.pdf. Accessed May 26, 2020.
- Department of Veterans Affairs. VHA Directive 1330.01(2). Healthcare Services for Women Veterans. February 15, 2017. Amended July 24, 2018. http://www.va.gov/ vhapublications/ viewpublication.asp?pub_id=5332. Accessed May 26, 2020.
- Royal College of Obstetricians and Gynaecologists. Obtaining valid consent: clinical governance advice no. 6. January 2015. https://www.rcog.org.uk/globalassets/documents/guidelines/clinical-governance-advice/cga6.pdf. Accessed May 26, 2020.
- American College Health Association Guidelines. Best practices for sensitive exams. October 2019. https://www.acha.org/documents/resources/guidelines/ACHA_Best_Practices_for_Sensitive_Exams_October2019.pdf. Accessed May 26, 2020.
- American College of Obstetricians and Gynecologists Committee on Ethics. Sexual misconduct: ACOG Committee Opinion No. 796. Obstet Gynecol. 2020;135:e43-e50
Out of the pipeline: Remdesivir
Although the US Food and Drug Administration (FDA) has granted emergency use authorization of remdesivir (Gilead Sciences, Inc., Foster City, California) to treat COVID-19, the disease caused by SARS-CoV-2, the drug is considered an investigational agent, not yet formally approved by the FDA and whose efficacy and safety has not yet been fully characterized. Remdesivir has been shown to be effective in reducing the time to recovery of people with COVID-19 disease. It has not been tested in a large controlled clinical trial of pregnant women with COVID-19; however, remdesivir has been given to pregnant women infected with COVID-19 in a compassionate use protocol. For pregnant women, the drug should only be used if the potential benefit justifies the potential risk to the mother and fetus.1
Pharmacology. Remdesivir is a nucleoside RNA polymerase inhibitor. It has a molecular formula of
C27H35N6O8P and a molecular weight of 602.6 g/mol.1
Mechanism of action. From FDA’s fact sheet: “Remdesivir is an adenosine nucleotide prodrug that distributes into cells where it is metabolized to form the pharmacologically active nucleoside triphosphate metabolite. Metabolism of remdesivir to remdesivir triphosphate has been demonstrated in multiple cell types. Remdesivir triphosphate acts as an analog of adenosine triphosphate (ATP) and competes with the natural ATP substrate for incorporation into nascent RNA chains by the SARS-CoV-2 RNA-dependent RNA polymerase, which results in chain termination during replication of the viral RNA. Remdesivir triphosphate is a weak inhibitor of mammalian DNA and RNA polymerases with low potential for mitochondrial toxicity.”1
Treatment protocols
Remdesivir is authorized for treatment of hospitalized patients with severe COVID-19 disease, defined as patients with an oxygen saturation ≤ 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO). The optimal dose and duration of treatment of COVID-19 with remdesivir is unknown.1
Prior to initiating treatment, the estimated glomerular filtration rate should be documented to be ≥ 30 mL/min. An excipient used in the remdesivir formulation—sulfobutylether-β-cylcodextrin sodium salt—is renally cleared and accumulates in patients with decreased renal function.
Baseline liver function tests should be performed prior to treatment and daily during the course of treatment. Remdesivir should not be initiated in patients with an alanine aminotransferase (ALT) level ≥ 5 times the upper limit of normal at baseline. Remdesivir should be discontinued in patients who develop an ALT level ≥ 5 times the upper limit of normal or in patients who develop elevated ALT levels and have increased bilirubin, alkaline phosphatase, or international normalized ratio.1
In one open-label study (GS-US-540-5773), remdesivir treatment was discontinued due to an adverse event in 5% of patients on a 5-day regimen and in 10% of patients on a 10-day regimen.1
Under the emergency use authorization, two treatment protocols have been proposed depending on the clinical severity of the COVID-19 infection1:
- Protocol 1: For people with COVID-19 requiring mechanical ventilation and/or ECMO, the duration of therapy is 10 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 9 days.
- Protocol 2: For people with COVID-19 disease not requiring mechanical ventilation and/or ECMO, the duration of therapy is 5 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 4 days. If the patient does not show clinical improvement, treatment may be extended for an additional 5 days.
Continue to: Randomized placebo-controlled trial results...
Randomized placebo-controlled trial results
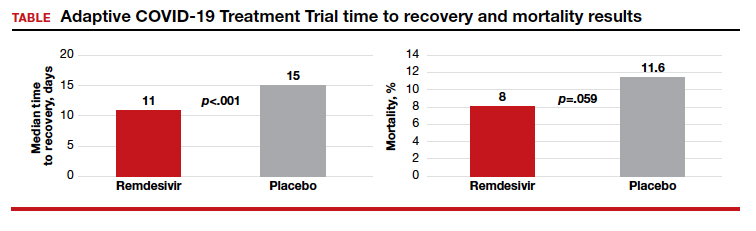
The Adaptive COVID-19 Treatment Trial (ACTT), sponsored by the National Institute of Allergy and Infectious Diseases, is a randomized, double-blind, placebo-controlled trial conducted by Gilead Sciences. The study began in February and evaluated up to 10 days of remdesivir treatment—200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days in hospitalized adult patients with COVID-19. Patients were enrolled in a 1:1 manner to remdesivir or placebo, and time to recovery within 28 days after randomization was the trial’s endpoint. According to preliminary analysis of 606 recovered patients, recovery took a median of 11 days in the remdesivir group and 15 days in the placebo group (hazard ratio, 1.31; 95% confidence interval (CI), 1.12‒1.54; P<.001). Mortality rates were 8.0% and 11.6% in the remdesivir and placebo groups, respectively (P=.059).1
5 vs 10 days of remdesivir treatment
The Gilead Sciences‒sponsored study GS-US-540-5773 was a randomized, open-label multicenter trial of patients with severe COVID-19. A total of 197 adult patients received 10-day remdesivir treatment (200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days). An additional 200 adult patients received 5-day remdesivir treatment (200 mg IV once daily followed by 100 mg IV for 4 days). Both groups also received standard of care. Results suggested that patients receiving 10 days of remdesivir had similar improvement in clinical status compared with those receiving a 5-day treatment course (10-to-5 day odds ratio, 0.76; 95% CI, 0.51‒1.13] on day 14).1 Improvement in clinical status was defined as an improvement of 2 or more points from baseline on a predefined 7-point scale that ranged from hospital discharge to increasing levels of oxygen support to death. Clinical recovery was achieved if patients ceased the need for oxygen support or were discharged.1
The time to clinical improvement for 50% of patients was similar in each treatment group (10 days in the 5-day group versus 11 days in the 10-day group). By day 14, observed clinical improvement rates were 65% and 54% in the 5- and 10-day treatment groups, respectively. Clinical recovery rates were 70% and 59% in the 5- and 10-day treatment groups and mortality rates were 8% and 11%.1
Adverse events
The use of remdesivir is contraindicated in patients who are hypersensitive to the drug. Its infusion may cause hypotension, nausea, vomiting, diaphoresis, and shivering. If signs of a clinically significant infusion reaction are observed the infusion should be discontinued. As noted above, elevation in ALT levels occurs with remdesivir treatment.1
Reporting serious adverse events. If a serious and unexpected adverse event occurs and appears to be associated with the use of remdesivir, the prescribing health care provider and/or the provider’s designee should complete and submit a MedWatch form to the FDA using one of the following methods1:
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Return form FDA 3500 (available at http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/UCM163919.pdf) to the FDA by mail (MedWatch, 5600 Fishers Lane, Rockville, MD 20852-9787) or fax (1-800-FDA-0178)
- Gilead requests that all FDA MedWatch forms also be returned to Gilead Pharmacovigilance and Epidemiology: fax: 1-650-522-5477 726; e-mail: Safety_fc@gilead.com
Continue to: Drug interactions...
Drug interactions
Remdesivir has not been evaluated for drug-drug interactions in humans. The clinical relevance of in vitro drug interactions also has not been established. According to the FDA, remdesivir is a substrate for the drug metabolizing enzymes CYP2C8, CYP2D6, and CYP3A4, and is a substrate for organic anion transporting polypeptides 1B1 (OAPT1B1) and P-glycoprotein (P-gp) transporters. In vitro, remdesivir inhibits CYP3A4, OATP1B1, OATP1B3, BSEP, MRP4, and NTCP.1
Pregnancy risk summary
Remdesivir has not been studied adequately in pregnant women and only should be used during pregnancy if the potential benefit of the drug justifies the potential risk to both mother and fetus.
Nonclinical animal studies that included systemic exposure of the predominant circulating metabolite of remdesivir in pregnant rats and rabbits (at 4 times the recommended dose of human exposure) demonstrated no adverse effect on embryofetal development.1
Breastfeeding
The only information regarding breastfeeding and remdesivir comes from animal studies. The drug and its metabolites were detected in the plasma of nursing rat pups whose mothers given intravenous remdesivir daily from gestation day 6 to lactation day 20. Measured on lactation day 10, remdesivir exposure in the pups was about 1% that of maternal exposure.1
“Because of the potential for viral transmission to SARS-CoV-2-negative infants and adverse reactions from the drug in breastfeeding infants, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for remdesivir and any potential adverse effects on the breastfed child from remdesivir or from the underlying maternal condition.”1
- US Food and Drug Administration. Fact Sheet for Health Care Providers Emergency Use Authorization (UA) of Remdesivir (GS-5734)TM. https://www.fda.gov/media/137566/download. Accessed May 19, 2020.
Although the US Food and Drug Administration (FDA) has granted emergency use authorization of remdesivir (Gilead Sciences, Inc., Foster City, California) to treat COVID-19, the disease caused by SARS-CoV-2, the drug is considered an investigational agent, not yet formally approved by the FDA and whose efficacy and safety has not yet been fully characterized. Remdesivir has been shown to be effective in reducing the time to recovery of people with COVID-19 disease. It has not been tested in a large controlled clinical trial of pregnant women with COVID-19; however, remdesivir has been given to pregnant women infected with COVID-19 in a compassionate use protocol. For pregnant women, the drug should only be used if the potential benefit justifies the potential risk to the mother and fetus.1
Pharmacology. Remdesivir is a nucleoside RNA polymerase inhibitor. It has a molecular formula of
C27H35N6O8P and a molecular weight of 602.6 g/mol.1
Mechanism of action. From FDA’s fact sheet: “Remdesivir is an adenosine nucleotide prodrug that distributes into cells where it is metabolized to form the pharmacologically active nucleoside triphosphate metabolite. Metabolism of remdesivir to remdesivir triphosphate has been demonstrated in multiple cell types. Remdesivir triphosphate acts as an analog of adenosine triphosphate (ATP) and competes with the natural ATP substrate for incorporation into nascent RNA chains by the SARS-CoV-2 RNA-dependent RNA polymerase, which results in chain termination during replication of the viral RNA. Remdesivir triphosphate is a weak inhibitor of mammalian DNA and RNA polymerases with low potential for mitochondrial toxicity.”1
Treatment protocols
Remdesivir is authorized for treatment of hospitalized patients with severe COVID-19 disease, defined as patients with an oxygen saturation ≤ 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO). The optimal dose and duration of treatment of COVID-19 with remdesivir is unknown.1
Prior to initiating treatment, the estimated glomerular filtration rate should be documented to be ≥ 30 mL/min. An excipient used in the remdesivir formulation—sulfobutylether-β-cylcodextrin sodium salt—is renally cleared and accumulates in patients with decreased renal function.
Baseline liver function tests should be performed prior to treatment and daily during the course of treatment. Remdesivir should not be initiated in patients with an alanine aminotransferase (ALT) level ≥ 5 times the upper limit of normal at baseline. Remdesivir should be discontinued in patients who develop an ALT level ≥ 5 times the upper limit of normal or in patients who develop elevated ALT levels and have increased bilirubin, alkaline phosphatase, or international normalized ratio.1
In one open-label study (GS-US-540-5773), remdesivir treatment was discontinued due to an adverse event in 5% of patients on a 5-day regimen and in 10% of patients on a 10-day regimen.1
Under the emergency use authorization, two treatment protocols have been proposed depending on the clinical severity of the COVID-19 infection1:
- Protocol 1: For people with COVID-19 requiring mechanical ventilation and/or ECMO, the duration of therapy is 10 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 9 days.
- Protocol 2: For people with COVID-19 disease not requiring mechanical ventilation and/or ECMO, the duration of therapy is 5 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 4 days. If the patient does not show clinical improvement, treatment may be extended for an additional 5 days.
Continue to: Randomized placebo-controlled trial results...
Randomized placebo-controlled trial results
The Adaptive COVID-19 Treatment Trial (ACTT), sponsored by the National Institute of Allergy and Infectious Diseases, is a randomized, double-blind, placebo-controlled trial conducted by Gilead Sciences. The study began in February and evaluated up to 10 days of remdesivir treatment—200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days in hospitalized adult patients with COVID-19. Patients were enrolled in a 1:1 manner to remdesivir or placebo, and time to recovery within 28 days after randomization was the trial’s endpoint. According to preliminary analysis of 606 recovered patients, recovery took a median of 11 days in the remdesivir group and 15 days in the placebo group (hazard ratio, 1.31; 95% confidence interval (CI), 1.12‒1.54; P<.001). Mortality rates were 8.0% and 11.6% in the remdesivir and placebo groups, respectively (P=.059).1

5 vs 10 days of remdesivir treatment
The Gilead Sciences‒sponsored study GS-US-540-5773 was a randomized, open-label multicenter trial of patients with severe COVID-19. A total of 197 adult patients received 10-day remdesivir treatment (200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days). An additional 200 adult patients received 5-day remdesivir treatment (200 mg IV once daily followed by 100 mg IV for 4 days). Both groups also received standard of care. Results suggested that patients receiving 10 days of remdesivir had similar improvement in clinical status compared with those receiving a 5-day treatment course (10-to-5 day odds ratio, 0.76; 95% CI, 0.51‒1.13] on day 14).1 Improvement in clinical status was defined as an improvement of 2 or more points from baseline on a predefined 7-point scale that ranged from hospital discharge to increasing levels of oxygen support to death. Clinical recovery was achieved if patients ceased the need for oxygen support or were discharged.1
The time to clinical improvement for 50% of patients was similar in each treatment group (10 days in the 5-day group versus 11 days in the 10-day group). By day 14, observed clinical improvement rates were 65% and 54% in the 5- and 10-day treatment groups, respectively. Clinical recovery rates were 70% and 59% in the 5- and 10-day treatment groups and mortality rates were 8% and 11%.1
Adverse events
The use of remdesivir is contraindicated in patients who are hypersensitive to the drug. Its infusion may cause hypotension, nausea, vomiting, diaphoresis, and shivering. If signs of a clinically significant infusion reaction are observed the infusion should be discontinued. As noted above, elevation in ALT levels occurs with remdesivir treatment.1
Reporting serious adverse events. If a serious and unexpected adverse event occurs and appears to be associated with the use of remdesivir, the prescribing health care provider and/or the provider’s designee should complete and submit a MedWatch form to the FDA using one of the following methods1:
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Return form FDA 3500 (available at http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/UCM163919.pdf) to the FDA by mail (MedWatch, 5600 Fishers Lane, Rockville, MD 20852-9787) or fax (1-800-FDA-0178)
- Gilead requests that all FDA MedWatch forms also be returned to Gilead Pharmacovigilance and Epidemiology: fax: 1-650-522-5477 726; e-mail: Safety_fc@gilead.com
Continue to: Drug interactions...
Drug interactions
Remdesivir has not been evaluated for drug-drug interactions in humans. The clinical relevance of in vitro drug interactions also has not been established. According to the FDA, remdesivir is a substrate for the drug metabolizing enzymes CYP2C8, CYP2D6, and CYP3A4, and is a substrate for organic anion transporting polypeptides 1B1 (OAPT1B1) and P-glycoprotein (P-gp) transporters. In vitro, remdesivir inhibits CYP3A4, OATP1B1, OATP1B3, BSEP, MRP4, and NTCP.1
Pregnancy risk summary
Remdesivir has not been studied adequately in pregnant women and only should be used during pregnancy if the potential benefit of the drug justifies the potential risk to both mother and fetus.
Nonclinical animal studies that included systemic exposure of the predominant circulating metabolite of remdesivir in pregnant rats and rabbits (at 4 times the recommended dose of human exposure) demonstrated no adverse effect on embryofetal development.1
Breastfeeding
The only information regarding breastfeeding and remdesivir comes from animal studies. The drug and its metabolites were detected in the plasma of nursing rat pups whose mothers given intravenous remdesivir daily from gestation day 6 to lactation day 20. Measured on lactation day 10, remdesivir exposure in the pups was about 1% that of maternal exposure.1
“Because of the potential for viral transmission to SARS-CoV-2-negative infants and adverse reactions from the drug in breastfeeding infants, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for remdesivir and any potential adverse effects on the breastfed child from remdesivir or from the underlying maternal condition.”1
Although the US Food and Drug Administration (FDA) has granted emergency use authorization of remdesivir (Gilead Sciences, Inc., Foster City, California) to treat COVID-19, the disease caused by SARS-CoV-2, the drug is considered an investigational agent, not yet formally approved by the FDA and whose efficacy and safety has not yet been fully characterized. Remdesivir has been shown to be effective in reducing the time to recovery of people with COVID-19 disease. It has not been tested in a large controlled clinical trial of pregnant women with COVID-19; however, remdesivir has been given to pregnant women infected with COVID-19 in a compassionate use protocol. For pregnant women, the drug should only be used if the potential benefit justifies the potential risk to the mother and fetus.1
Pharmacology. Remdesivir is a nucleoside RNA polymerase inhibitor. It has a molecular formula of
C27H35N6O8P and a molecular weight of 602.6 g/mol.1
Mechanism of action. From FDA’s fact sheet: “Remdesivir is an adenosine nucleotide prodrug that distributes into cells where it is metabolized to form the pharmacologically active nucleoside triphosphate metabolite. Metabolism of remdesivir to remdesivir triphosphate has been demonstrated in multiple cell types. Remdesivir triphosphate acts as an analog of adenosine triphosphate (ATP) and competes with the natural ATP substrate for incorporation into nascent RNA chains by the SARS-CoV-2 RNA-dependent RNA polymerase, which results in chain termination during replication of the viral RNA. Remdesivir triphosphate is a weak inhibitor of mammalian DNA and RNA polymerases with low potential for mitochondrial toxicity.”1
Treatment protocols
Remdesivir is authorized for treatment of hospitalized patients with severe COVID-19 disease, defined as patients with an oxygen saturation ≤ 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO). The optimal dose and duration of treatment of COVID-19 with remdesivir is unknown.1
Prior to initiating treatment, the estimated glomerular filtration rate should be documented to be ≥ 30 mL/min. An excipient used in the remdesivir formulation—sulfobutylether-β-cylcodextrin sodium salt—is renally cleared and accumulates in patients with decreased renal function.
Baseline liver function tests should be performed prior to treatment and daily during the course of treatment. Remdesivir should not be initiated in patients with an alanine aminotransferase (ALT) level ≥ 5 times the upper limit of normal at baseline. Remdesivir should be discontinued in patients who develop an ALT level ≥ 5 times the upper limit of normal or in patients who develop elevated ALT levels and have increased bilirubin, alkaline phosphatase, or international normalized ratio.1
In one open-label study (GS-US-540-5773), remdesivir treatment was discontinued due to an adverse event in 5% of patients on a 5-day regimen and in 10% of patients on a 10-day regimen.1
Under the emergency use authorization, two treatment protocols have been proposed depending on the clinical severity of the COVID-19 infection1:
- Protocol 1: For people with COVID-19 requiring mechanical ventilation and/or ECMO, the duration of therapy is 10 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 9 days.
- Protocol 2: For people with COVID-19 disease not requiring mechanical ventilation and/or ECMO, the duration of therapy is 5 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 4 days. If the patient does not show clinical improvement, treatment may be extended for an additional 5 days.
Continue to: Randomized placebo-controlled trial results...
Randomized placebo-controlled trial results
The Adaptive COVID-19 Treatment Trial (ACTT), sponsored by the National Institute of Allergy and Infectious Diseases, is a randomized, double-blind, placebo-controlled trial conducted by Gilead Sciences. The study began in February and evaluated up to 10 days of remdesivir treatment—200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days in hospitalized adult patients with COVID-19. Patients were enrolled in a 1:1 manner to remdesivir or placebo, and time to recovery within 28 days after randomization was the trial’s endpoint. According to preliminary analysis of 606 recovered patients, recovery took a median of 11 days in the remdesivir group and 15 days in the placebo group (hazard ratio, 1.31; 95% confidence interval (CI), 1.12‒1.54; P<.001). Mortality rates were 8.0% and 11.6% in the remdesivir and placebo groups, respectively (P=.059).1

5 vs 10 days of remdesivir treatment
The Gilead Sciences‒sponsored study GS-US-540-5773 was a randomized, open-label multicenter trial of patients with severe COVID-19. A total of 197 adult patients received 10-day remdesivir treatment (200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days). An additional 200 adult patients received 5-day remdesivir treatment (200 mg IV once daily followed by 100 mg IV for 4 days). Both groups also received standard of care. Results suggested that patients receiving 10 days of remdesivir had similar improvement in clinical status compared with those receiving a 5-day treatment course (10-to-5 day odds ratio, 0.76; 95% CI, 0.51‒1.13] on day 14).1 Improvement in clinical status was defined as an improvement of 2 or more points from baseline on a predefined 7-point scale that ranged from hospital discharge to increasing levels of oxygen support to death. Clinical recovery was achieved if patients ceased the need for oxygen support or were discharged.1
The time to clinical improvement for 50% of patients was similar in each treatment group (10 days in the 5-day group versus 11 days in the 10-day group). By day 14, observed clinical improvement rates were 65% and 54% in the 5- and 10-day treatment groups, respectively. Clinical recovery rates were 70% and 59% in the 5- and 10-day treatment groups and mortality rates were 8% and 11%.1
Adverse events
The use of remdesivir is contraindicated in patients who are hypersensitive to the drug. Its infusion may cause hypotension, nausea, vomiting, diaphoresis, and shivering. If signs of a clinically significant infusion reaction are observed the infusion should be discontinued. As noted above, elevation in ALT levels occurs with remdesivir treatment.1
Reporting serious adverse events. If a serious and unexpected adverse event occurs and appears to be associated with the use of remdesivir, the prescribing health care provider and/or the provider’s designee should complete and submit a MedWatch form to the FDA using one of the following methods1:
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Return form FDA 3500 (available at http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/UCM163919.pdf) to the FDA by mail (MedWatch, 5600 Fishers Lane, Rockville, MD 20852-9787) or fax (1-800-FDA-0178)
- Gilead requests that all FDA MedWatch forms also be returned to Gilead Pharmacovigilance and Epidemiology: fax: 1-650-522-5477 726; e-mail: Safety_fc@gilead.com
Continue to: Drug interactions...
Drug interactions
Remdesivir has not been evaluated for drug-drug interactions in humans. The clinical relevance of in vitro drug interactions also has not been established. According to the FDA, remdesivir is a substrate for the drug metabolizing enzymes CYP2C8, CYP2D6, and CYP3A4, and is a substrate for organic anion transporting polypeptides 1B1 (OAPT1B1) and P-glycoprotein (P-gp) transporters. In vitro, remdesivir inhibits CYP3A4, OATP1B1, OATP1B3, BSEP, MRP4, and NTCP.1
Pregnancy risk summary
Remdesivir has not been studied adequately in pregnant women and only should be used during pregnancy if the potential benefit of the drug justifies the potential risk to both mother and fetus.
Nonclinical animal studies that included systemic exposure of the predominant circulating metabolite of remdesivir in pregnant rats and rabbits (at 4 times the recommended dose of human exposure) demonstrated no adverse effect on embryofetal development.1
Breastfeeding
The only information regarding breastfeeding and remdesivir comes from animal studies. The drug and its metabolites were detected in the plasma of nursing rat pups whose mothers given intravenous remdesivir daily from gestation day 6 to lactation day 20. Measured on lactation day 10, remdesivir exposure in the pups was about 1% that of maternal exposure.1
“Because of the potential for viral transmission to SARS-CoV-2-negative infants and adverse reactions from the drug in breastfeeding infants, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for remdesivir and any potential adverse effects on the breastfed child from remdesivir or from the underlying maternal condition.”1
- US Food and Drug Administration. Fact Sheet for Health Care Providers Emergency Use Authorization (UA) of Remdesivir (GS-5734)TM. https://www.fda.gov/media/137566/download. Accessed May 19, 2020.
- US Food and Drug Administration. Fact Sheet for Health Care Providers Emergency Use Authorization (UA) of Remdesivir (GS-5734)TM. https://www.fda.gov/media/137566/download. Accessed May 19, 2020.
Respiratory particles generated by speech can remain airborne for up to 14 minutes
Stadnytskyi and colleagues explored the size of droplets created by speech using a highly sensitive laser system. They reported in PNAS that speaking resulted in the generation of a high number of medium-sized droplets (10- to 100-µm in diameter). Under the conditions of their experiment (27% humidity and 23° C) they reported that speech probably generates droplets that originate at a size of 12 to 21 µm in diameter and quickly dehydrate to an estimated diameter of 4 µm. The 4 µm-sized particles had a falling rate of only 0.06 cm·s−1 and remained airborne for 8 to 14 minutes.1
As reported by Hamner and colleagues, on March 10, 2020, 61 persons attended a 2.5-hour choir practice. One choir member had symptoms of an upper respiratory infection that began on March 7. Eventually that choir member tested positive for SARS-CoV-2. Of the 60 remaining persons, 52 (86.7%) eventually developed an upper respiratory illness. In total, 33 cases of SARS-CoV-2 were confirmed by nucleic acid testing and 20 probable cases were diagnosed (these individuals declined testing). The choir attendees developed symptoms at a median of 3 days following the practice, with a range of 1 to 12 days. Three of the 53 ill people were hospitalized, and two died.2
The Stadnytskyi study suggests that speech generates large respiratory droplets that dehydrate into very small droplets that may remain in the air for an extended period of time. If the SARS-CoV-2 virus were in the original large droplet, the rapid dehydration of the droplet would result in prolonged airborne presence of the virus and enhance its infectivity.
The Hamner study highlights the importance of vocalization and respiratory particles in transmitting the SARS-CoV-2 virus. For clinicians and patients, both studies support many recommendations to reduce viral transmission, including:
- all clinicians and patients need to wear face masks
- all clinicians and patients should avoid face-to-face contact if alternative approaches to communication are possible
- all clinicians and patients should avoid gathering in large groups or crowded public spaces and need to maintain physical distancing.
The COVID pandemic has dramatically changed how we practice medicine and socialize.
- Stadnytskyi V, Bax CE, Bax A, et al. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. PNAS. May 13, 2020. https://doi.org/10.1073/pnas.2006874117.
- Hamner L, Dubbel P, Capron I, et al. High SARS-CoV-2 attack rate following exposure at choir practice—Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:606-610. Early release, May 12, 2020.
Stadnytskyi and colleagues explored the size of droplets created by speech using a highly sensitive laser system. They reported in PNAS that speaking resulted in the generation of a high number of medium-sized droplets (10- to 100-µm in diameter). Under the conditions of their experiment (27% humidity and 23° C) they reported that speech probably generates droplets that originate at a size of 12 to 21 µm in diameter and quickly dehydrate to an estimated diameter of 4 µm. The 4 µm-sized particles had a falling rate of only 0.06 cm·s−1 and remained airborne for 8 to 14 minutes.1
As reported by Hamner and colleagues, on March 10, 2020, 61 persons attended a 2.5-hour choir practice. One choir member had symptoms of an upper respiratory infection that began on March 7. Eventually that choir member tested positive for SARS-CoV-2. Of the 60 remaining persons, 52 (86.7%) eventually developed an upper respiratory illness. In total, 33 cases of SARS-CoV-2 were confirmed by nucleic acid testing and 20 probable cases were diagnosed (these individuals declined testing). The choir attendees developed symptoms at a median of 3 days following the practice, with a range of 1 to 12 days. Three of the 53 ill people were hospitalized, and two died.2
The Stadnytskyi study suggests that speech generates large respiratory droplets that dehydrate into very small droplets that may remain in the air for an extended period of time. If the SARS-CoV-2 virus were in the original large droplet, the rapid dehydration of the droplet would result in prolonged airborne presence of the virus and enhance its infectivity.
The Hamner study highlights the importance of vocalization and respiratory particles in transmitting the SARS-CoV-2 virus. For clinicians and patients, both studies support many recommendations to reduce viral transmission, including:
- all clinicians and patients need to wear face masks
- all clinicians and patients should avoid face-to-face contact if alternative approaches to communication are possible
- all clinicians and patients should avoid gathering in large groups or crowded public spaces and need to maintain physical distancing.
The COVID pandemic has dramatically changed how we practice medicine and socialize.
Stadnytskyi and colleagues explored the size of droplets created by speech using a highly sensitive laser system. They reported in PNAS that speaking resulted in the generation of a high number of medium-sized droplets (10- to 100-µm in diameter). Under the conditions of their experiment (27% humidity and 23° C) they reported that speech probably generates droplets that originate at a size of 12 to 21 µm in diameter and quickly dehydrate to an estimated diameter of 4 µm. The 4 µm-sized particles had a falling rate of only 0.06 cm·s−1 and remained airborne for 8 to 14 minutes.1
As reported by Hamner and colleagues, on March 10, 2020, 61 persons attended a 2.5-hour choir practice. One choir member had symptoms of an upper respiratory infection that began on March 7. Eventually that choir member tested positive for SARS-CoV-2. Of the 60 remaining persons, 52 (86.7%) eventually developed an upper respiratory illness. In total, 33 cases of SARS-CoV-2 were confirmed by nucleic acid testing and 20 probable cases were diagnosed (these individuals declined testing). The choir attendees developed symptoms at a median of 3 days following the practice, with a range of 1 to 12 days. Three of the 53 ill people were hospitalized, and two died.2
The Stadnytskyi study suggests that speech generates large respiratory droplets that dehydrate into very small droplets that may remain in the air for an extended period of time. If the SARS-CoV-2 virus were in the original large droplet, the rapid dehydration of the droplet would result in prolonged airborne presence of the virus and enhance its infectivity.
The Hamner study highlights the importance of vocalization and respiratory particles in transmitting the SARS-CoV-2 virus. For clinicians and patients, both studies support many recommendations to reduce viral transmission, including:
- all clinicians and patients need to wear face masks
- all clinicians and patients should avoid face-to-face contact if alternative approaches to communication are possible
- all clinicians and patients should avoid gathering in large groups or crowded public spaces and need to maintain physical distancing.
The COVID pandemic has dramatically changed how we practice medicine and socialize.
- Stadnytskyi V, Bax CE, Bax A, et al. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. PNAS. May 13, 2020. https://doi.org/10.1073/pnas.2006874117.
- Hamner L, Dubbel P, Capron I, et al. High SARS-CoV-2 attack rate following exposure at choir practice—Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:606-610. Early release, May 12, 2020.
- Stadnytskyi V, Bax CE, Bax A, et al. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. PNAS. May 13, 2020. https://doi.org/10.1073/pnas.2006874117.
- Hamner L, Dubbel P, Capron I, et al. High SARS-CoV-2 attack rate following exposure at choir practice—Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:606-610. Early release, May 12, 2020.
COVID-19: We are in a war, without the most effective weapons to fight a novel viral pathogen
On June 17, 1775, American colonists, defending a forward redoubt on Breed’s Hill, ran out of gunpowder, and their position was overrun by British troops. The Battle of Bunker Hill resulted in the death of 140 colonists and 226 British soldiers, setting the stage for major combat throughout the colonies. American colonists lacked many necessary weapons. They had almost no gunpowder, few field cannons, and no warships. Yet, they fought on with the weapons at hand for 6 long years.
In the spring of 2020, American society has been shaken by the COVID-19 pandemic. Hospitals have been overrun with thousands of people infected with the disease. Some hospitals are breaking under the crush of intensely ill people filling up and spilling out of intensive care units. We are in a war, fighting a viral disease with a limited supply of weapons. We do not have access to the most powerful medical munitions: easily available rapid testing, proven antiviral medications, and an effective vaccine. Nevertheless, clinicians and patients are courageous, and we will continue the fight with the limited weapons we have until the pandemic is brought to an end.

The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19). The virus is aptly named because it is usually transmitted through close contact with respiratory droplets. The disease can progress acutely, and some people experience a remarkably severe respiratory syndrome, including tachypnea, hypoxia, and interstitial and alveolar opacities on chest x-ray, necessitating ventilatory support. The virus is an encapsulated single-stranded RNA virus. When viewed by electron microscopy, the virus appears to have a halo or crown, hence it is named “coronavirus.” Among infected individuals, the virus is present in the upper respiratory system and in feces but not in urine.1 The World Health Organization (WHO) believes that respiratory droplets and contaminated surfaces are the major routes of transmission.2 The highest risk of developing severe COVID-19 disease occurs in people with one or more of the following characteristics: age greater than 70 years, hypertension, diabetes, respiratory disease, heart disease, and immunosuppression.3,4 Pregnant women do not appear to be at increased risk for severe COVID-19 disease.4 The case fatality rate is highest in people 80 years of age or older.5
Who is infected with SARS-CoV-2?
Rapid high-fidelity testing for SARS-CoV-2 nucleic acid sequences would be the best approach to identifying people with COVID-19 disease. At the beginning of the pandemic, testing was strictly rationed because of lack of reagents and test swabs. Clinicians were permitted to test only a minority of people who had symptoms. Asymptomatic individuals were not eligible to be tested. This terribly flawed approach to screening permitted a vast army of SARS-CoV-2–positive asymptomatic and mildly symptomatic people to circulate unchecked in the general population, infecting dozens of other people, some of whom developed moderate or severe disease. The Centers for Disease Control and Prevention (CDC) has reported on 7 independent clusters of COVID-19 disease, each of which appear to have been caused by one asymptomatic infected individual.6 Another cluster of COVID-19 disease from China appears to have been caused by one asymptomatic infected individual.7 Based on limited data, it appears that there may be a 1- to 3-day window where an individual with COVID-19 may be asymptomatic and able to infect others. I suspect that we will soon discover, based on testing for the presence of high-titre anti SARS-CoV-2 antibodies, that many people with no history of illness and people with mild respiratory symptoms had an undiagnosed COVID-19 infection.
As testing capacity expands we likely will be testing all women, including asymptomatic women, before they arrive at the hospital for childbirth or gynecologic surgery, as well as all inpatients and women with respiratory symptoms having an ambulatory encounter.
With expanded testing capability, some pregnant women who were symptomatic and tested positive for SARS-CoV-2 have had sequential long-term follow-up testing. A frequent observation is that over one to two weeks the viral symptoms resolve and the nasopharyngeal test becomes negative for SARS-CoV-2 on multiple sequential tests, only to become positive at a later date. The cause of the positive-negative-negative-positive test results is unknown, but it raises the possibility that once a person tests positive for SARS-CoV-2, they may be able to transmit the infection over many weeks, even after viral symptoms resolve.
Continue to: COVID-19: Respiratory droplet or aerosol transmission?
COVID-19: Respiratory droplet or aerosol transmission?
Respiratory droplets are large particles (> 5 µm in diameter) that tend to be pulled to the ground or furniture surfaces by gravity. Respiratory droplets do not circulate in the air for an extended period of time. Droplet nuclei are small particles less than 5 µm in diameter. These small particles may become aerosolized and float through the air for an extended period of time. The CDC and WHO believe that under ordinary conditions, SARS-CoV-2 is transmitted through respiratory droplets and contact routes.2 In an analysis of more than 75,000 COVID-19 cases in China there were no reports of transmission by aerosolized airborne virus. Therefore, under ordinary conditions, surgical masks, face shields, gowns, and gloves provide a high level of protection from infection.8
In contrast to the WHO’s perspective, Dr. Harvey Fineberg, Chair of the National Academies of Sciences, Engineering, and Medicine’s Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats, wrote a letter to the federal Office of Science and Technology Policy warning that normal breathing might generate aerosolization of the SARS-CoV-2 virus and result in airborne transmission.9 A report from the University of Nebraska Medical Center supports the concept of airborne transmission of SARS-CoV-2. In a study of 13 patients with COVID-19, room surfaces, toilet facilities, and air had evidence of viral contamination.10 The investigators concluded that disease spreads through respiratory droplets, person-to-person touch, contaminated surfaces, and airborne routes. Other investigators also have reported that aersolization of SARS-CoV-2 may occur.11 Professional societies recommend that all medical staff caring for potential or confirmed COVID-19 patients should use personal protective equipment (PPE), including respirators (N95 respirators) when available. Importantly, all medical staff should be trained in and adhere to proper donning and doffing of PPE. The controversy about the modes of transmission of SARS-CoV-2 will continue, but as clinicians we need to work within the constraints of the equipment we have.
Certain medical procedures and devices are known to generate aerosolization of respiratory secretions. These procedures and devices include: bronchoscopy, intubation, extubation, cardiopulmonary resuscitation, nebulization, high-flow oxygen masks, and continuous- and bilevel-positive airway pressure devices. When aerosols are generated during the care of a patient with COVID-19, surgical masks are not sufficient protection against infection. When an aerosol is generated maximal protection of health care workers from viral transmission requires use of a negative-pressure room and an N95 respirator or powered air-purifying respirator (PAPR) device. However, negative-pressure rooms, N95 masks, and PAPRs are in very short supply or are unavailable in some health systems. We are lucky at our hospital that all of the labor rooms can be configured to operate in a negative-pressure mode, limiting potential airborne spread of the virus on the unit. Many hospitals restrict the use of N95 masks to anesthesiologists, leaving nurses, ObGyns, and surgical technicians without the best protective equipment, risking their health. As one action to reduce aerosolization of virus, obstetricians can markedly reduce the use of oxygen masks and nasal cannulas by laboring women.
Universal use of surgical masks and mouth-nose coverings
During the entire COVID-19 pandemic, PPE has been in short supply, including severe shortages of N95 masks, PAPRs, and in some health systems, surgical masks, gowns, eye protection, and face shields. Given the severe shortages, some clinicians have needed to conserve PPE, using the same PPE across multiple patient encounters and across multiple work shifts.
Given that the virus is transmitted by respiratory droplets and contaminated surfaces, use of face coverings, including surgical masks, face shields, and gloves is critically important. Scrupulous hand hygiene is a simple approach to reducing infection risk. In my health system, all employees are required to wear a surgical mask, all day every day, requiring distribution of 35,000 masks daily.12 We also require every patient and visitor to our health care facilities to use a face mask. The purpose of the procedure or surgical mask is to prevent presymptomatic spread of COVID-19 from an asymptomatic health care worker to an uninfected patient or a colleague by reducing the transmission of respiratory droplets. Another benefit is to protect the uninfected health care worker from patients and colleagues who are infected and not yet diagnosed with COVID-19. The CDC now recommends that all people wear a mouth and nose covering when they are outside of their residence. America may become a nation where wearing masks in public becomes a routine practice. Since SARS-CoV-2 is transmitted by respiratory droplets, social distancing is an important preventive measure.
Continue to: Obstetric care...
Obstetric care
Can it be repeated too often? No. Containing COVID-19 disease requires social distancing, fastidious hand hygiene, and using a mask that covers the mouth and nose.
Pregnant women should be advised to assiduously practice social distancing and to wear a face covering or mask in public. Hand hygiene should be emphasized. Pregnant women with children should be advised to not allow their children to play with non‒cohabiting children because children may be asymptomatic vectors for COVID-19.
Pregnant health care workers should stop face-to-face contact with patients after 36 weeks’ gestation to avoid a late pregnancy infection that might cause the mother to be separated from her newborn. Based on data currently available, pregnancy in the absence of another risk factor is not a major risk factor for developing severe COVID-19 disease.13
Hyperthermia is a common feature of COVID-19. Acetaminophen is recommended treatment to suppress pyrexia during pregnancy.
The COVID-19 pandemic has transformed prenatal care from a series of face-to-face encounters at a health care facility to telemedicine either by telephone or a videoconferencing portal. Many factors contributed to the rapid switch to telemedicine, including orders by governors to restrict unnecessary travel, patients’ fear of contracting COVID-19 at their clinicians’ offices, clinicians’ fear of contracting COVID-19 from patients, and insurers’ rapid implementation of policies to pay for telemedicine visits. Most prenatal visits can be provided through telemedicine as long as the patient has a home blood pressure cuff and can reliably use the instrument. In-person visits may be required for blood testing, ultrasound assessment, anti-Rh immunoglobulin administration, and group B streptococcal infection screening. One regimen is to limit in-person prenatal visits to encounters at 12, 20, 28, and 36 weeks’ gestation when blood testing and ultrasound examinations are needed. The postpartum visit also may be conducted using telemedicine.
Pregnant women with COVID-19 and pneumonia are reported to have high rates of preterm birth less than 37 weeks (41%) and preterm prelabor rupture of membranes (19%).14
The rate of vertical transmission from mother to fetus is probably very low (<1%).15 However, based on serological studies, an occasional newborn has been reported to have IgM and IgG antibodies to the SARS-CoV-2 nucleoprotein at birth.16,17
Pregnant women should be consistently and regularly screened for symptoms of an upper respiratory infection, including: fever, new cough, new runny nose or nasal congestion, new sore throat, shortness of breath, muscle aches, and anosmia. A report of any of these symptoms should result in nucleic acid testing of a nasal swab for SARS-CoV-2 of all pregnant women. Given limited testing resources, however, symptomatic pregnant women with the following characteristics should be prioritized for testing: if the woman is more than 36 weeks pregnant, intrapartum, or in the hospital after delivery. Ambulatory pregnant women with symptoms who do not need medical care should quarantine themselves at home, if possible, or at another secure location away from their families. In some regions, testing of ambulatory patients with upper respiratory symptoms is limited.
All women scheduled for induction or cesarean delivery (CD) and their support person should have a symptom screen 24 to 48 hours before arrival to the hospital and should be rescreened prior to entry to labor and delivery. In this situation if the pregnant woman screens positive, she should be tested for SARS-CoV-2, and if the test result is positive, the scheduled induction and CD should be rescheduled, if possible. All hospitalized women and their support persons should be screened for symptoms daily. If the pregnant woman screens positive she should have a nucleic acid test for SARS-CoV-2. If the support person screens positive, he or she should be sent home.
Systemic glucocorticoids may worsen the course of COVID-19. For pregnant women with COVID-19 disease, betamethasone administration should be limited to women at high risk for preterm delivery within 7 days and only given to women between 23 weeks to 33 weeks 6 days of gestation. Women at risk for preterm delivery at 34 weeks to 36 weeks and 6 days of gestation should not be given betamethasone.
If cervical ripening is required, outpatient regimens should be prioritized.
One support person plays an important role in optimal labor outcome and should be permitted at the hospital. All support persons should wear a surgical or procedure mask.
Nitrous oxide for labor anesthesia should not be used during the pandemic because it might cause aerosolization of respiratory secretions, endangering health care workers. Neuraxial anesthesia is an optimal approach to labor anesthesia.
Labor management and timing of delivery does not need to be altered during the COVID-19 pandemic. However, pregnant women with moderate or severe COVID-19 disease who are not improving may have a modest improvement in respiratory function if they are delivered preterm.
At the beginning of the COVID pandemic, the CDC recommended separation of a COVID-positive mother and her newborn until the mother’s respiratory symptoms resolved. However, the CDC now recommends that, for a COVID-positive mother, joint decision-making should be used to decide whether to support the baby rooming-in with the mother or to practice separation of mother and baby at birth to reduce the risk for postnatal infection from mother to newborn. There is no evidence that breast milk contains virus that can cause an infection. One option is for the mother who recently tested positive for SARS-CoV-2 to provide newborn nutrition with expressed breast milk.
Pregnant women with COVID-19 may be at increased risk for venous thromboembolism. Some experts recommend that hospitalized pregnant women and postpartum women with COVID-19 receive thromboembolism prophylaxis.
The Chinese Centers for Disease Control and Prevention described a classification system for COVID-19 disease, including 3 categories18:
- mild: no dyspnea, no pneumonia, or mild pneumonia
- severe: dyspnea, respiratory frequency ≥ 30 breaths per minute, blood oxygen saturation ≤ 93%, lung infiltrates > 50% within 48 hours of onset of symptoms
- critical: respiratory failure, septic shock, or multiple organ dysfunction or failure.
Among 72,314 cases in China, 81% had mild disease, 14% had severe disease, and 5% had critical disease. In a report of 118 pregnant women in China, 92% of the women had mild disease; 8% had severe disease (hypoxemia), one of whom developed critical disease requiring mechanical ventilation.19 In this cohort, the most common presenting symptoms were fever (75%), cough (73%), chest tightness (18%), fatigue (17%), shortness of breath (7%), diarrhea (7%), and headache (6%). Lymphopenia was present in 44% of the women.
Severe and critical COVID-19 disease are associated with elevations in D-dimer, C-reactive protein, troponin, ferritin, and creatine phosphokinase levels. These markers return to the normal range with resolution of disease.
Continue to: Gynecologic care...
Gynecologic care
Gynecologists are highly impacted by the COVID-19 pandemic. Most state governments have requested that all elective surgery be suspended for the duration of the pandemic in order to redeploy health resources to the care of COVID-19 patients. Except for high-priority gynecologic surgery, including cancer surgery, treatment of heavy vaginal bleeding, and surgical care of ectopic pregnancy and miscarriage, most gynecologic surgery has ceased.
All office visits for routine gynecologic care have been suspended. Video and telephone visits can be used for contraceptive counseling and prescribing and for managing problems associated with the menopause, endometriosis, and vaginitis. Cervical cancer screening can be deferred for 3 to 6 months, depending on patient risk factors.
Medicines to treat COVID-19 infections
There are many highly effective medicines to manage HIV infection and medicines that cure hepatitis C. There is an urgent need to develop precision medicines to treat this disease. Early in the pandemic some experts thought that hydroxychloroquine might be helpful in the treatment of COVID-19 disease. But recent evidence suggests that hydroxychloroquine is probably not an effective treatment. As the pandemic has evolved, there is evidence that remdesivir may have modest efficacy in treating COVID-19 disease.20 Remdesivir has received emergency-use authorization by the FDA to treat COVID-19 infection.
Remdesivir
Based on expert opinion, in the absence of high-quality clinical trial evidence, our current practice is to offer pregnant women with severe or critical COVID-19 disease treatment with remdesivir.
Remdesivir (Gilead Sciences, Inc) is a nucleoside analog that inhibits RNA synthesis. A dose regimen for remdesivir is a 200-mg loading dose given intravenously, followed by 100 mg daily given intravenously for 5 to 10 days. Remdesivir may cause elevation of hepatic enzymes. Remdesivir has been administered to a few pregnant women to treat Ebola and Marburg virus disease.21
Experts in infectious disease are important resources for determining optimal medication regimens for the treatment of COVID-19 disease in pregnant women.
Continue to: Convalescent serum...
Convalescent serum
There are no high-quality studies demonstrating the efficacy of convalescent serum for treatment of COVID-19. A small case series suggests that there may be modest benefit to treatment of people with severe COVID-19 disease with convalescent serum.22
Testing for anti-SARS-CoV-2 IgM and IgG antibodies
We may have a serious problem in our current approach to detecting COVID-19 disease. Based on measurement of IgM and IgG antibodies to SARS-CoV-2 nucleocapsid protein, our current nucleic acid tests for SARS-CoV-2 may detect less than 80% of infections early in the course of disease. In two studies of IgM and IgG antibodies to the SARS-CoV-2 nucleocapsid protein, a single polymerase chain reaction test for SARS-CoV-2 had less than a 60% sensitivity for detecting the virus.23,24 During the second week of COVID-19 illness, IgM or IgG antibodies were detected in greater than 89% of infected patients.23 Severe disease resulted in high concentrations of antibody.
When testing for IgM and IgG antibodies is widely available, it may become an option to test all health care workers. This will permit the assignment of those health care workers with the highest levels of antibody to frontline duties with COVID-19 patients during the next disease outbreak, likely to occur at some point during the next 12 months.
A COVID-19 vaccine
Dozens of research teams, including pharmaceutical and biotechnology companies and many academic laboratories, are working on developing and testing vaccines to prevent COVID-19 disease. An effective vaccine would reduce the number of people who develop severe disease during the next outbreak, reducing deaths, avoiding a shutdown of the country, and allowing the health systems to function normally. A vaccine is unlikely to be widely available until sometime early in 2021.
Facing COVID-19 well-being and mental health
SARS-CoV-2, like all viral particles, is incredibly small. Remarkably, it has changed permanently life on earth. COVID-19 is affecting our physical health, psychological well-being, economics, and patterns of social interaction. As clinicians it is difficult to face a viral enemy that cannot be stopped from causing the death of more than 100,000 people, including some of our clinical colleagues, within a short period of time.
- F—focus on what is in your control
- A—acknowledge your thoughts and feelings
- C—come back to a focus on your body
- E—engage in what you are doing
- C—commit to acting effectively based on your core values
- O—opening up to difficult feelings and being kind to yourself and others
- V—values should guide your actions
- I—identify resources for help, assistance, support, and advice
- D—disinfect and practice social distancing.
This war will come to an end
During the American Revolution, colonists faced housing and food insecurity, epidemics of typhus and smallpox, traumatic injury including amputation of limbs, and a complete disruption of normal life activities. They persevered and, against the odds, successfully concluded the war. Unlike the colonists, who did not know if their conflict would end with success or failure, we clinicians know that the COVID-19 pandemic will end. We also know that eventually the global community of clinicians will develop and deploy the effective weapons we need to prevent a recurrence of this traumatic pandemic: population-wide testing for both the SARS-CoV-2 virus and serologic testing for IgG and IgM antibodies to the virus, effective antiviral medications, and a potent vaccine. ●
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens [published online March 11, 2020]. JAMA . doi: 10.1001/ jama . 2020 .3786.
- World Health Organization. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. March 29, 2020. https://www.who.int/publications-detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations. Accessed April 16, 2020.
- Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State [published online March 19, 2020]. JAMA . doi: 10.1001/ jama . 2020 .4326.
- Guan WJ, Liang WH, Zhao Y, et al; China Medical Treatment Expert Group for Covid-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis [published online March 26, 2020]. Eur Respir J . doi: 10.1183/13993003.00547- 2020 .
- Onder G, Rezza G, Brusaferro S. Case fatality rate and characteristics of patients dying in relation to COVID-19 in Italy [published online March 23, 2020]. JAMA. doi: 10.1001/ jama . 2020 .4683.
- Wei WE, Li Z, Chiew CJ, et al. Presymptomatic transmission of SARS-CoV-2 - Singapore, January 23 to March 16, 2020. MMWR Morb Mortal Wkly Rep . 2020;69:411-415.
- Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19 [published online February 21, 2020]. JAMA. doi: 10.1001/ jama . 2020 .2565.
- Ong SW, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient [published online March 4, 2020]. JAMA . doi: 10.1001/ jama .2020.3227.
- Fineberg HV. Rapid expert consultation on the possibility of bioaerosol spread of SARS-CoV-2 for the COVID-19 pandemic. April 1, 2020. https://www.nap.edu/read/25769/chapter/1. Accessed April 16, 2020.
- Santarpia JL, River DN, Herrera V, et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. MedRxiv. March 26, 2020. doi.org10.1101/2020.03.23.20039466.
- Liu Y, Ning Z, Chen Y, et al. Aerodynamic characteristics and RNA concentration of SARS-CoV-2 aerosol in Wuhan Hospitals during COVID-19 outbreak. BioRxiv. March 10, 2020. doi.org/10.1101/2020.03.08.982637.
- Klompas M, Morris CA, Sinclair J, et al. Universal masking in hospitals in the COVID-19 era [published online April 1, 2020]. N Engl J Med. doi: 10.1056/NEJMp2006372.
- Liu D, Li L, Wu X, et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. 2020:1-6. doi: 10.2214/AJR.20.23072.
- Di Mascio D, Khalik A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol. doi:10.1016/j.ajogmf.2020.
100107. - Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens [published online March 11, 2020]. JAMA. doi: 10.1001/jama.2020.3786.
- Dong L, Tian J, He S, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn [published online March 26, 2020]. JAMA. doi: 10.1001/ jama .2020.4621.
- Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID-19 pneumonia [published online March 26, 2020]. JAMA. doi: 10.1001/ jama .2020.4861.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Diease 2019 (COVID-19) outbreak in China. Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention [published online February 24, 2020]. JAMA . doi: 10.1001/jama.2020.2648.
- Chen L, Li Q, Zheng D, et al. Clinical characteristics of pregnant women with COVID-19 in Wuhan, China [published online April 17, 2020]. N Engl J Med. doi 10.1056/NEJMc2009226.
- Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. MedRxiv. April 10, 2020. https://doi.org/10.1101/2020.03.22.20040758.
- Maulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381:2293-2303.
- Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma [published online March 27, 2020]. JAMA. doi: 10.1001/ jama . 2020 .4783.
- Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019 [published online March 29, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa344.
- Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) [published online March 21, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa310.
On June 17, 1775, American colonists, defending a forward redoubt on Breed’s Hill, ran out of gunpowder, and their position was overrun by British troops. The Battle of Bunker Hill resulted in the death of 140 colonists and 226 British soldiers, setting the stage for major combat throughout the colonies. American colonists lacked many necessary weapons. They had almost no gunpowder, few field cannons, and no warships. Yet, they fought on with the weapons at hand for 6 long years.
In the spring of 2020, American society has been shaken by the COVID-19 pandemic. Hospitals have been overrun with thousands of people infected with the disease. Some hospitals are breaking under the crush of intensely ill people filling up and spilling out of intensive care units. We are in a war, fighting a viral disease with a limited supply of weapons. We do not have access to the most powerful medical munitions: easily available rapid testing, proven antiviral medications, and an effective vaccine. Nevertheless, clinicians and patients are courageous, and we will continue the fight with the limited weapons we have until the pandemic is brought to an end.

The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19). The virus is aptly named because it is usually transmitted through close contact with respiratory droplets. The disease can progress acutely, and some people experience a remarkably severe respiratory syndrome, including tachypnea, hypoxia, and interstitial and alveolar opacities on chest x-ray, necessitating ventilatory support. The virus is an encapsulated single-stranded RNA virus. When viewed by electron microscopy, the virus appears to have a halo or crown, hence it is named “coronavirus.” Among infected individuals, the virus is present in the upper respiratory system and in feces but not in urine.1 The World Health Organization (WHO) believes that respiratory droplets and contaminated surfaces are the major routes of transmission.2 The highest risk of developing severe COVID-19 disease occurs in people with one or more of the following characteristics: age greater than 70 years, hypertension, diabetes, respiratory disease, heart disease, and immunosuppression.3,4 Pregnant women do not appear to be at increased risk for severe COVID-19 disease.4 The case fatality rate is highest in people 80 years of age or older.5
Who is infected with SARS-CoV-2?
Rapid high-fidelity testing for SARS-CoV-2 nucleic acid sequences would be the best approach to identifying people with COVID-19 disease. At the beginning of the pandemic, testing was strictly rationed because of lack of reagents and test swabs. Clinicians were permitted to test only a minority of people who had symptoms. Asymptomatic individuals were not eligible to be tested. This terribly flawed approach to screening permitted a vast army of SARS-CoV-2–positive asymptomatic and mildly symptomatic people to circulate unchecked in the general population, infecting dozens of other people, some of whom developed moderate or severe disease. The Centers for Disease Control and Prevention (CDC) has reported on 7 independent clusters of COVID-19 disease, each of which appear to have been caused by one asymptomatic infected individual.6 Another cluster of COVID-19 disease from China appears to have been caused by one asymptomatic infected individual.7 Based on limited data, it appears that there may be a 1- to 3-day window where an individual with COVID-19 may be asymptomatic and able to infect others. I suspect that we will soon discover, based on testing for the presence of high-titre anti SARS-CoV-2 antibodies, that many people with no history of illness and people with mild respiratory symptoms had an undiagnosed COVID-19 infection.
As testing capacity expands we likely will be testing all women, including asymptomatic women, before they arrive at the hospital for childbirth or gynecologic surgery, as well as all inpatients and women with respiratory symptoms having an ambulatory encounter.
With expanded testing capability, some pregnant women who were symptomatic and tested positive for SARS-CoV-2 have had sequential long-term follow-up testing. A frequent observation is that over one to two weeks the viral symptoms resolve and the nasopharyngeal test becomes negative for SARS-CoV-2 on multiple sequential tests, only to become positive at a later date. The cause of the positive-negative-negative-positive test results is unknown, but it raises the possibility that once a person tests positive for SARS-CoV-2, they may be able to transmit the infection over many weeks, even after viral symptoms resolve.
Continue to: COVID-19: Respiratory droplet or aerosol transmission?
COVID-19: Respiratory droplet or aerosol transmission?
Respiratory droplets are large particles (> 5 µm in diameter) that tend to be pulled to the ground or furniture surfaces by gravity. Respiratory droplets do not circulate in the air for an extended period of time. Droplet nuclei are small particles less than 5 µm in diameter. These small particles may become aerosolized and float through the air for an extended period of time. The CDC and WHO believe that under ordinary conditions, SARS-CoV-2 is transmitted through respiratory droplets and contact routes.2 In an analysis of more than 75,000 COVID-19 cases in China there were no reports of transmission by aerosolized airborne virus. Therefore, under ordinary conditions, surgical masks, face shields, gowns, and gloves provide a high level of protection from infection.8
In contrast to the WHO’s perspective, Dr. Harvey Fineberg, Chair of the National Academies of Sciences, Engineering, and Medicine’s Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats, wrote a letter to the federal Office of Science and Technology Policy warning that normal breathing might generate aerosolization of the SARS-CoV-2 virus and result in airborne transmission.9 A report from the University of Nebraska Medical Center supports the concept of airborne transmission of SARS-CoV-2. In a study of 13 patients with COVID-19, room surfaces, toilet facilities, and air had evidence of viral contamination.10 The investigators concluded that disease spreads through respiratory droplets, person-to-person touch, contaminated surfaces, and airborne routes. Other investigators also have reported that aersolization of SARS-CoV-2 may occur.11 Professional societies recommend that all medical staff caring for potential or confirmed COVID-19 patients should use personal protective equipment (PPE), including respirators (N95 respirators) when available. Importantly, all medical staff should be trained in and adhere to proper donning and doffing of PPE. The controversy about the modes of transmission of SARS-CoV-2 will continue, but as clinicians we need to work within the constraints of the equipment we have.
Certain medical procedures and devices are known to generate aerosolization of respiratory secretions. These procedures and devices include: bronchoscopy, intubation, extubation, cardiopulmonary resuscitation, nebulization, high-flow oxygen masks, and continuous- and bilevel-positive airway pressure devices. When aerosols are generated during the care of a patient with COVID-19, surgical masks are not sufficient protection against infection. When an aerosol is generated maximal protection of health care workers from viral transmission requires use of a negative-pressure room and an N95 respirator or powered air-purifying respirator (PAPR) device. However, negative-pressure rooms, N95 masks, and PAPRs are in very short supply or are unavailable in some health systems. We are lucky at our hospital that all of the labor rooms can be configured to operate in a negative-pressure mode, limiting potential airborne spread of the virus on the unit. Many hospitals restrict the use of N95 masks to anesthesiologists, leaving nurses, ObGyns, and surgical technicians without the best protective equipment, risking their health. As one action to reduce aerosolization of virus, obstetricians can markedly reduce the use of oxygen masks and nasal cannulas by laboring women.
Universal use of surgical masks and mouth-nose coverings
During the entire COVID-19 pandemic, PPE has been in short supply, including severe shortages of N95 masks, PAPRs, and in some health systems, surgical masks, gowns, eye protection, and face shields. Given the severe shortages, some clinicians have needed to conserve PPE, using the same PPE across multiple patient encounters and across multiple work shifts.
Given that the virus is transmitted by respiratory droplets and contaminated surfaces, use of face coverings, including surgical masks, face shields, and gloves is critically important. Scrupulous hand hygiene is a simple approach to reducing infection risk. In my health system, all employees are required to wear a surgical mask, all day every day, requiring distribution of 35,000 masks daily.12 We also require every patient and visitor to our health care facilities to use a face mask. The purpose of the procedure or surgical mask is to prevent presymptomatic spread of COVID-19 from an asymptomatic health care worker to an uninfected patient or a colleague by reducing the transmission of respiratory droplets. Another benefit is to protect the uninfected health care worker from patients and colleagues who are infected and not yet diagnosed with COVID-19. The CDC now recommends that all people wear a mouth and nose covering when they are outside of their residence. America may become a nation where wearing masks in public becomes a routine practice. Since SARS-CoV-2 is transmitted by respiratory droplets, social distancing is an important preventive measure.
Continue to: Obstetric care...
Obstetric care
Can it be repeated too often? No. Containing COVID-19 disease requires social distancing, fastidious hand hygiene, and using a mask that covers the mouth and nose.
Pregnant women should be advised to assiduously practice social distancing and to wear a face covering or mask in public. Hand hygiene should be emphasized. Pregnant women with children should be advised to not allow their children to play with non‒cohabiting children because children may be asymptomatic vectors for COVID-19.
Pregnant health care workers should stop face-to-face contact with patients after 36 weeks’ gestation to avoid a late pregnancy infection that might cause the mother to be separated from her newborn. Based on data currently available, pregnancy in the absence of another risk factor is not a major risk factor for developing severe COVID-19 disease.13
Hyperthermia is a common feature of COVID-19. Acetaminophen is recommended treatment to suppress pyrexia during pregnancy.
The COVID-19 pandemic has transformed prenatal care from a series of face-to-face encounters at a health care facility to telemedicine either by telephone or a videoconferencing portal. Many factors contributed to the rapid switch to telemedicine, including orders by governors to restrict unnecessary travel, patients’ fear of contracting COVID-19 at their clinicians’ offices, clinicians’ fear of contracting COVID-19 from patients, and insurers’ rapid implementation of policies to pay for telemedicine visits. Most prenatal visits can be provided through telemedicine as long as the patient has a home blood pressure cuff and can reliably use the instrument. In-person visits may be required for blood testing, ultrasound assessment, anti-Rh immunoglobulin administration, and group B streptococcal infection screening. One regimen is to limit in-person prenatal visits to encounters at 12, 20, 28, and 36 weeks’ gestation when blood testing and ultrasound examinations are needed. The postpartum visit also may be conducted using telemedicine.
Pregnant women with COVID-19 and pneumonia are reported to have high rates of preterm birth less than 37 weeks (41%) and preterm prelabor rupture of membranes (19%).14
The rate of vertical transmission from mother to fetus is probably very low (<1%).15 However, based on serological studies, an occasional newborn has been reported to have IgM and IgG antibodies to the SARS-CoV-2 nucleoprotein at birth.16,17
Pregnant women should be consistently and regularly screened for symptoms of an upper respiratory infection, including: fever, new cough, new runny nose or nasal congestion, new sore throat, shortness of breath, muscle aches, and anosmia. A report of any of these symptoms should result in nucleic acid testing of a nasal swab for SARS-CoV-2 of all pregnant women. Given limited testing resources, however, symptomatic pregnant women with the following characteristics should be prioritized for testing: if the woman is more than 36 weeks pregnant, intrapartum, or in the hospital after delivery. Ambulatory pregnant women with symptoms who do not need medical care should quarantine themselves at home, if possible, or at another secure location away from their families. In some regions, testing of ambulatory patients with upper respiratory symptoms is limited.
All women scheduled for induction or cesarean delivery (CD) and their support person should have a symptom screen 24 to 48 hours before arrival to the hospital and should be rescreened prior to entry to labor and delivery. In this situation if the pregnant woman screens positive, she should be tested for SARS-CoV-2, and if the test result is positive, the scheduled induction and CD should be rescheduled, if possible. All hospitalized women and their support persons should be screened for symptoms daily. If the pregnant woman screens positive she should have a nucleic acid test for SARS-CoV-2. If the support person screens positive, he or she should be sent home.
Systemic glucocorticoids may worsen the course of COVID-19. For pregnant women with COVID-19 disease, betamethasone administration should be limited to women at high risk for preterm delivery within 7 days and only given to women between 23 weeks to 33 weeks 6 days of gestation. Women at risk for preterm delivery at 34 weeks to 36 weeks and 6 days of gestation should not be given betamethasone.
If cervical ripening is required, outpatient regimens should be prioritized.
One support person plays an important role in optimal labor outcome and should be permitted at the hospital. All support persons should wear a surgical or procedure mask.
Nitrous oxide for labor anesthesia should not be used during the pandemic because it might cause aerosolization of respiratory secretions, endangering health care workers. Neuraxial anesthesia is an optimal approach to labor anesthesia.
Labor management and timing of delivery does not need to be altered during the COVID-19 pandemic. However, pregnant women with moderate or severe COVID-19 disease who are not improving may have a modest improvement in respiratory function if they are delivered preterm.
At the beginning of the COVID pandemic, the CDC recommended separation of a COVID-positive mother and her newborn until the mother’s respiratory symptoms resolved. However, the CDC now recommends that, for a COVID-positive mother, joint decision-making should be used to decide whether to support the baby rooming-in with the mother or to practice separation of mother and baby at birth to reduce the risk for postnatal infection from mother to newborn. There is no evidence that breast milk contains virus that can cause an infection. One option is for the mother who recently tested positive for SARS-CoV-2 to provide newborn nutrition with expressed breast milk.
Pregnant women with COVID-19 may be at increased risk for venous thromboembolism. Some experts recommend that hospitalized pregnant women and postpartum women with COVID-19 receive thromboembolism prophylaxis.
The Chinese Centers for Disease Control and Prevention described a classification system for COVID-19 disease, including 3 categories18:
- mild: no dyspnea, no pneumonia, or mild pneumonia
- severe: dyspnea, respiratory frequency ≥ 30 breaths per minute, blood oxygen saturation ≤ 93%, lung infiltrates > 50% within 48 hours of onset of symptoms
- critical: respiratory failure, septic shock, or multiple organ dysfunction or failure.
Among 72,314 cases in China, 81% had mild disease, 14% had severe disease, and 5% had critical disease. In a report of 118 pregnant women in China, 92% of the women had mild disease; 8% had severe disease (hypoxemia), one of whom developed critical disease requiring mechanical ventilation.19 In this cohort, the most common presenting symptoms were fever (75%), cough (73%), chest tightness (18%), fatigue (17%), shortness of breath (7%), diarrhea (7%), and headache (6%). Lymphopenia was present in 44% of the women.
Severe and critical COVID-19 disease are associated with elevations in D-dimer, C-reactive protein, troponin, ferritin, and creatine phosphokinase levels. These markers return to the normal range with resolution of disease.
Continue to: Gynecologic care...
Gynecologic care
Gynecologists are highly impacted by the COVID-19 pandemic. Most state governments have requested that all elective surgery be suspended for the duration of the pandemic in order to redeploy health resources to the care of COVID-19 patients. Except for high-priority gynecologic surgery, including cancer surgery, treatment of heavy vaginal bleeding, and surgical care of ectopic pregnancy and miscarriage, most gynecologic surgery has ceased.
All office visits for routine gynecologic care have been suspended. Video and telephone visits can be used for contraceptive counseling and prescribing and for managing problems associated with the menopause, endometriosis, and vaginitis. Cervical cancer screening can be deferred for 3 to 6 months, depending on patient risk factors.
Medicines to treat COVID-19 infections
There are many highly effective medicines to manage HIV infection and medicines that cure hepatitis C. There is an urgent need to develop precision medicines to treat this disease. Early in the pandemic some experts thought that hydroxychloroquine might be helpful in the treatment of COVID-19 disease. But recent evidence suggests that hydroxychloroquine is probably not an effective treatment. As the pandemic has evolved, there is evidence that remdesivir may have modest efficacy in treating COVID-19 disease.20 Remdesivir has received emergency-use authorization by the FDA to treat COVID-19 infection.
Remdesivir
Based on expert opinion, in the absence of high-quality clinical trial evidence, our current practice is to offer pregnant women with severe or critical COVID-19 disease treatment with remdesivir.
Remdesivir (Gilead Sciences, Inc) is a nucleoside analog that inhibits RNA synthesis. A dose regimen for remdesivir is a 200-mg loading dose given intravenously, followed by 100 mg daily given intravenously for 5 to 10 days. Remdesivir may cause elevation of hepatic enzymes. Remdesivir has been administered to a few pregnant women to treat Ebola and Marburg virus disease.21
Experts in infectious disease are important resources for determining optimal medication regimens for the treatment of COVID-19 disease in pregnant women.
Continue to: Convalescent serum...
Convalescent serum
There are no high-quality studies demonstrating the efficacy of convalescent serum for treatment of COVID-19. A small case series suggests that there may be modest benefit to treatment of people with severe COVID-19 disease with convalescent serum.22
Testing for anti-SARS-CoV-2 IgM and IgG antibodies
We may have a serious problem in our current approach to detecting COVID-19 disease. Based on measurement of IgM and IgG antibodies to SARS-CoV-2 nucleocapsid protein, our current nucleic acid tests for SARS-CoV-2 may detect less than 80% of infections early in the course of disease. In two studies of IgM and IgG antibodies to the SARS-CoV-2 nucleocapsid protein, a single polymerase chain reaction test for SARS-CoV-2 had less than a 60% sensitivity for detecting the virus.23,24 During the second week of COVID-19 illness, IgM or IgG antibodies were detected in greater than 89% of infected patients.23 Severe disease resulted in high concentrations of antibody.
When testing for IgM and IgG antibodies is widely available, it may become an option to test all health care workers. This will permit the assignment of those health care workers with the highest levels of antibody to frontline duties with COVID-19 patients during the next disease outbreak, likely to occur at some point during the next 12 months.
A COVID-19 vaccine
Dozens of research teams, including pharmaceutical and biotechnology companies and many academic laboratories, are working on developing and testing vaccines to prevent COVID-19 disease. An effective vaccine would reduce the number of people who develop severe disease during the next outbreak, reducing deaths, avoiding a shutdown of the country, and allowing the health systems to function normally. A vaccine is unlikely to be widely available until sometime early in 2021.
Facing COVID-19 well-being and mental health
SARS-CoV-2, like all viral particles, is incredibly small. Remarkably, it has changed permanently life on earth. COVID-19 is affecting our physical health, psychological well-being, economics, and patterns of social interaction. As clinicians it is difficult to face a viral enemy that cannot be stopped from causing the death of more than 100,000 people, including some of our clinical colleagues, within a short period of time.
- F—focus on what is in your control
- A—acknowledge your thoughts and feelings
- C—come back to a focus on your body
- E—engage in what you are doing
- C—commit to acting effectively based on your core values
- O—opening up to difficult feelings and being kind to yourself and others
- V—values should guide your actions
- I—identify resources for help, assistance, support, and advice
- D—disinfect and practice social distancing.
This war will come to an end
During the American Revolution, colonists faced housing and food insecurity, epidemics of typhus and smallpox, traumatic injury including amputation of limbs, and a complete disruption of normal life activities. They persevered and, against the odds, successfully concluded the war. Unlike the colonists, who did not know if their conflict would end with success or failure, we clinicians know that the COVID-19 pandemic will end. We also know that eventually the global community of clinicians will develop and deploy the effective weapons we need to prevent a recurrence of this traumatic pandemic: population-wide testing for both the SARS-CoV-2 virus and serologic testing for IgG and IgM antibodies to the virus, effective antiviral medications, and a potent vaccine. ●
On June 17, 1775, American colonists, defending a forward redoubt on Breed’s Hill, ran out of gunpowder, and their position was overrun by British troops. The Battle of Bunker Hill resulted in the death of 140 colonists and 226 British soldiers, setting the stage for major combat throughout the colonies. American colonists lacked many necessary weapons. They had almost no gunpowder, few field cannons, and no warships. Yet, they fought on with the weapons at hand for 6 long years.
In the spring of 2020, American society has been shaken by the COVID-19 pandemic. Hospitals have been overrun with thousands of people infected with the disease. Some hospitals are breaking under the crush of intensely ill people filling up and spilling out of intensive care units. We are in a war, fighting a viral disease with a limited supply of weapons. We do not have access to the most powerful medical munitions: easily available rapid testing, proven antiviral medications, and an effective vaccine. Nevertheless, clinicians and patients are courageous, and we will continue the fight with the limited weapons we have until the pandemic is brought to an end.

The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19). The virus is aptly named because it is usually transmitted through close contact with respiratory droplets. The disease can progress acutely, and some people experience a remarkably severe respiratory syndrome, including tachypnea, hypoxia, and interstitial and alveolar opacities on chest x-ray, necessitating ventilatory support. The virus is an encapsulated single-stranded RNA virus. When viewed by electron microscopy, the virus appears to have a halo or crown, hence it is named “coronavirus.” Among infected individuals, the virus is present in the upper respiratory system and in feces but not in urine.1 The World Health Organization (WHO) believes that respiratory droplets and contaminated surfaces are the major routes of transmission.2 The highest risk of developing severe COVID-19 disease occurs in people with one or more of the following characteristics: age greater than 70 years, hypertension, diabetes, respiratory disease, heart disease, and immunosuppression.3,4 Pregnant women do not appear to be at increased risk for severe COVID-19 disease.4 The case fatality rate is highest in people 80 years of age or older.5
Who is infected with SARS-CoV-2?
Rapid high-fidelity testing for SARS-CoV-2 nucleic acid sequences would be the best approach to identifying people with COVID-19 disease. At the beginning of the pandemic, testing was strictly rationed because of lack of reagents and test swabs. Clinicians were permitted to test only a minority of people who had symptoms. Asymptomatic individuals were not eligible to be tested. This terribly flawed approach to screening permitted a vast army of SARS-CoV-2–positive asymptomatic and mildly symptomatic people to circulate unchecked in the general population, infecting dozens of other people, some of whom developed moderate or severe disease. The Centers for Disease Control and Prevention (CDC) has reported on 7 independent clusters of COVID-19 disease, each of which appear to have been caused by one asymptomatic infected individual.6 Another cluster of COVID-19 disease from China appears to have been caused by one asymptomatic infected individual.7 Based on limited data, it appears that there may be a 1- to 3-day window where an individual with COVID-19 may be asymptomatic and able to infect others. I suspect that we will soon discover, based on testing for the presence of high-titre anti SARS-CoV-2 antibodies, that many people with no history of illness and people with mild respiratory symptoms had an undiagnosed COVID-19 infection.
As testing capacity expands we likely will be testing all women, including asymptomatic women, before they arrive at the hospital for childbirth or gynecologic surgery, as well as all inpatients and women with respiratory symptoms having an ambulatory encounter.
With expanded testing capability, some pregnant women who were symptomatic and tested positive for SARS-CoV-2 have had sequential long-term follow-up testing. A frequent observation is that over one to two weeks the viral symptoms resolve and the nasopharyngeal test becomes negative for SARS-CoV-2 on multiple sequential tests, only to become positive at a later date. The cause of the positive-negative-negative-positive test results is unknown, but it raises the possibility that once a person tests positive for SARS-CoV-2, they may be able to transmit the infection over many weeks, even after viral symptoms resolve.
Continue to: COVID-19: Respiratory droplet or aerosol transmission?
COVID-19: Respiratory droplet or aerosol transmission?
Respiratory droplets are large particles (> 5 µm in diameter) that tend to be pulled to the ground or furniture surfaces by gravity. Respiratory droplets do not circulate in the air for an extended period of time. Droplet nuclei are small particles less than 5 µm in diameter. These small particles may become aerosolized and float through the air for an extended period of time. The CDC and WHO believe that under ordinary conditions, SARS-CoV-2 is transmitted through respiratory droplets and contact routes.2 In an analysis of more than 75,000 COVID-19 cases in China there were no reports of transmission by aerosolized airborne virus. Therefore, under ordinary conditions, surgical masks, face shields, gowns, and gloves provide a high level of protection from infection.8
In contrast to the WHO’s perspective, Dr. Harvey Fineberg, Chair of the National Academies of Sciences, Engineering, and Medicine’s Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats, wrote a letter to the federal Office of Science and Technology Policy warning that normal breathing might generate aerosolization of the SARS-CoV-2 virus and result in airborne transmission.9 A report from the University of Nebraska Medical Center supports the concept of airborne transmission of SARS-CoV-2. In a study of 13 patients with COVID-19, room surfaces, toilet facilities, and air had evidence of viral contamination.10 The investigators concluded that disease spreads through respiratory droplets, person-to-person touch, contaminated surfaces, and airborne routes. Other investigators also have reported that aersolization of SARS-CoV-2 may occur.11 Professional societies recommend that all medical staff caring for potential or confirmed COVID-19 patients should use personal protective equipment (PPE), including respirators (N95 respirators) when available. Importantly, all medical staff should be trained in and adhere to proper donning and doffing of PPE. The controversy about the modes of transmission of SARS-CoV-2 will continue, but as clinicians we need to work within the constraints of the equipment we have.
Certain medical procedures and devices are known to generate aerosolization of respiratory secretions. These procedures and devices include: bronchoscopy, intubation, extubation, cardiopulmonary resuscitation, nebulization, high-flow oxygen masks, and continuous- and bilevel-positive airway pressure devices. When aerosols are generated during the care of a patient with COVID-19, surgical masks are not sufficient protection against infection. When an aerosol is generated maximal protection of health care workers from viral transmission requires use of a negative-pressure room and an N95 respirator or powered air-purifying respirator (PAPR) device. However, negative-pressure rooms, N95 masks, and PAPRs are in very short supply or are unavailable in some health systems. We are lucky at our hospital that all of the labor rooms can be configured to operate in a negative-pressure mode, limiting potential airborne spread of the virus on the unit. Many hospitals restrict the use of N95 masks to anesthesiologists, leaving nurses, ObGyns, and surgical technicians without the best protective equipment, risking their health. As one action to reduce aerosolization of virus, obstetricians can markedly reduce the use of oxygen masks and nasal cannulas by laboring women.
Universal use of surgical masks and mouth-nose coverings
During the entire COVID-19 pandemic, PPE has been in short supply, including severe shortages of N95 masks, PAPRs, and in some health systems, surgical masks, gowns, eye protection, and face shields. Given the severe shortages, some clinicians have needed to conserve PPE, using the same PPE across multiple patient encounters and across multiple work shifts.
Given that the virus is transmitted by respiratory droplets and contaminated surfaces, use of face coverings, including surgical masks, face shields, and gloves is critically important. Scrupulous hand hygiene is a simple approach to reducing infection risk. In my health system, all employees are required to wear a surgical mask, all day every day, requiring distribution of 35,000 masks daily.12 We also require every patient and visitor to our health care facilities to use a face mask. The purpose of the procedure or surgical mask is to prevent presymptomatic spread of COVID-19 from an asymptomatic health care worker to an uninfected patient or a colleague by reducing the transmission of respiratory droplets. Another benefit is to protect the uninfected health care worker from patients and colleagues who are infected and not yet diagnosed with COVID-19. The CDC now recommends that all people wear a mouth and nose covering when they are outside of their residence. America may become a nation where wearing masks in public becomes a routine practice. Since SARS-CoV-2 is transmitted by respiratory droplets, social distancing is an important preventive measure.
Continue to: Obstetric care...
Obstetric care
Can it be repeated too often? No. Containing COVID-19 disease requires social distancing, fastidious hand hygiene, and using a mask that covers the mouth and nose.
Pregnant women should be advised to assiduously practice social distancing and to wear a face covering or mask in public. Hand hygiene should be emphasized. Pregnant women with children should be advised to not allow their children to play with non‒cohabiting children because children may be asymptomatic vectors for COVID-19.
Pregnant health care workers should stop face-to-face contact with patients after 36 weeks’ gestation to avoid a late pregnancy infection that might cause the mother to be separated from her newborn. Based on data currently available, pregnancy in the absence of another risk factor is not a major risk factor for developing severe COVID-19 disease.13
Hyperthermia is a common feature of COVID-19. Acetaminophen is recommended treatment to suppress pyrexia during pregnancy.
The COVID-19 pandemic has transformed prenatal care from a series of face-to-face encounters at a health care facility to telemedicine either by telephone or a videoconferencing portal. Many factors contributed to the rapid switch to telemedicine, including orders by governors to restrict unnecessary travel, patients’ fear of contracting COVID-19 at their clinicians’ offices, clinicians’ fear of contracting COVID-19 from patients, and insurers’ rapid implementation of policies to pay for telemedicine visits. Most prenatal visits can be provided through telemedicine as long as the patient has a home blood pressure cuff and can reliably use the instrument. In-person visits may be required for blood testing, ultrasound assessment, anti-Rh immunoglobulin administration, and group B streptococcal infection screening. One regimen is to limit in-person prenatal visits to encounters at 12, 20, 28, and 36 weeks’ gestation when blood testing and ultrasound examinations are needed. The postpartum visit also may be conducted using telemedicine.
Pregnant women with COVID-19 and pneumonia are reported to have high rates of preterm birth less than 37 weeks (41%) and preterm prelabor rupture of membranes (19%).14
The rate of vertical transmission from mother to fetus is probably very low (<1%).15 However, based on serological studies, an occasional newborn has been reported to have IgM and IgG antibodies to the SARS-CoV-2 nucleoprotein at birth.16,17
Pregnant women should be consistently and regularly screened for symptoms of an upper respiratory infection, including: fever, new cough, new runny nose or nasal congestion, new sore throat, shortness of breath, muscle aches, and anosmia. A report of any of these symptoms should result in nucleic acid testing of a nasal swab for SARS-CoV-2 of all pregnant women. Given limited testing resources, however, symptomatic pregnant women with the following characteristics should be prioritized for testing: if the woman is more than 36 weeks pregnant, intrapartum, or in the hospital after delivery. Ambulatory pregnant women with symptoms who do not need medical care should quarantine themselves at home, if possible, or at another secure location away from their families. In some regions, testing of ambulatory patients with upper respiratory symptoms is limited.
All women scheduled for induction or cesarean delivery (CD) and their support person should have a symptom screen 24 to 48 hours before arrival to the hospital and should be rescreened prior to entry to labor and delivery. In this situation if the pregnant woman screens positive, she should be tested for SARS-CoV-2, and if the test result is positive, the scheduled induction and CD should be rescheduled, if possible. All hospitalized women and their support persons should be screened for symptoms daily. If the pregnant woman screens positive she should have a nucleic acid test for SARS-CoV-2. If the support person screens positive, he or she should be sent home.
Systemic glucocorticoids may worsen the course of COVID-19. For pregnant women with COVID-19 disease, betamethasone administration should be limited to women at high risk for preterm delivery within 7 days and only given to women between 23 weeks to 33 weeks 6 days of gestation. Women at risk for preterm delivery at 34 weeks to 36 weeks and 6 days of gestation should not be given betamethasone.
If cervical ripening is required, outpatient regimens should be prioritized.
One support person plays an important role in optimal labor outcome and should be permitted at the hospital. All support persons should wear a surgical or procedure mask.
Nitrous oxide for labor anesthesia should not be used during the pandemic because it might cause aerosolization of respiratory secretions, endangering health care workers. Neuraxial anesthesia is an optimal approach to labor anesthesia.
Labor management and timing of delivery does not need to be altered during the COVID-19 pandemic. However, pregnant women with moderate or severe COVID-19 disease who are not improving may have a modest improvement in respiratory function if they are delivered preterm.
At the beginning of the COVID pandemic, the CDC recommended separation of a COVID-positive mother and her newborn until the mother’s respiratory symptoms resolved. However, the CDC now recommends that, for a COVID-positive mother, joint decision-making should be used to decide whether to support the baby rooming-in with the mother or to practice separation of mother and baby at birth to reduce the risk for postnatal infection from mother to newborn. There is no evidence that breast milk contains virus that can cause an infection. One option is for the mother who recently tested positive for SARS-CoV-2 to provide newborn nutrition with expressed breast milk.
Pregnant women with COVID-19 may be at increased risk for venous thromboembolism. Some experts recommend that hospitalized pregnant women and postpartum women with COVID-19 receive thromboembolism prophylaxis.
The Chinese Centers for Disease Control and Prevention described a classification system for COVID-19 disease, including 3 categories18:
- mild: no dyspnea, no pneumonia, or mild pneumonia
- severe: dyspnea, respiratory frequency ≥ 30 breaths per minute, blood oxygen saturation ≤ 93%, lung infiltrates > 50% within 48 hours of onset of symptoms
- critical: respiratory failure, septic shock, or multiple organ dysfunction or failure.
Among 72,314 cases in China, 81% had mild disease, 14% had severe disease, and 5% had critical disease. In a report of 118 pregnant women in China, 92% of the women had mild disease; 8% had severe disease (hypoxemia), one of whom developed critical disease requiring mechanical ventilation.19 In this cohort, the most common presenting symptoms were fever (75%), cough (73%), chest tightness (18%), fatigue (17%), shortness of breath (7%), diarrhea (7%), and headache (6%). Lymphopenia was present in 44% of the women.
Severe and critical COVID-19 disease are associated with elevations in D-dimer, C-reactive protein, troponin, ferritin, and creatine phosphokinase levels. These markers return to the normal range with resolution of disease.
Continue to: Gynecologic care...
Gynecologic care
Gynecologists are highly impacted by the COVID-19 pandemic. Most state governments have requested that all elective surgery be suspended for the duration of the pandemic in order to redeploy health resources to the care of COVID-19 patients. Except for high-priority gynecologic surgery, including cancer surgery, treatment of heavy vaginal bleeding, and surgical care of ectopic pregnancy and miscarriage, most gynecologic surgery has ceased.
All office visits for routine gynecologic care have been suspended. Video and telephone visits can be used for contraceptive counseling and prescribing and for managing problems associated with the menopause, endometriosis, and vaginitis. Cervical cancer screening can be deferred for 3 to 6 months, depending on patient risk factors.
Medicines to treat COVID-19 infections
There are many highly effective medicines to manage HIV infection and medicines that cure hepatitis C. There is an urgent need to develop precision medicines to treat this disease. Early in the pandemic some experts thought that hydroxychloroquine might be helpful in the treatment of COVID-19 disease. But recent evidence suggests that hydroxychloroquine is probably not an effective treatment. As the pandemic has evolved, there is evidence that remdesivir may have modest efficacy in treating COVID-19 disease.20 Remdesivir has received emergency-use authorization by the FDA to treat COVID-19 infection.
Remdesivir
Based on expert opinion, in the absence of high-quality clinical trial evidence, our current practice is to offer pregnant women with severe or critical COVID-19 disease treatment with remdesivir.
Remdesivir (Gilead Sciences, Inc) is a nucleoside analog that inhibits RNA synthesis. A dose regimen for remdesivir is a 200-mg loading dose given intravenously, followed by 100 mg daily given intravenously for 5 to 10 days. Remdesivir may cause elevation of hepatic enzymes. Remdesivir has been administered to a few pregnant women to treat Ebola and Marburg virus disease.21
Experts in infectious disease are important resources for determining optimal medication regimens for the treatment of COVID-19 disease in pregnant women.
Continue to: Convalescent serum...
Convalescent serum
There are no high-quality studies demonstrating the efficacy of convalescent serum for treatment of COVID-19. A small case series suggests that there may be modest benefit to treatment of people with severe COVID-19 disease with convalescent serum.22
Testing for anti-SARS-CoV-2 IgM and IgG antibodies
We may have a serious problem in our current approach to detecting COVID-19 disease. Based on measurement of IgM and IgG antibodies to SARS-CoV-2 nucleocapsid protein, our current nucleic acid tests for SARS-CoV-2 may detect less than 80% of infections early in the course of disease. In two studies of IgM and IgG antibodies to the SARS-CoV-2 nucleocapsid protein, a single polymerase chain reaction test for SARS-CoV-2 had less than a 60% sensitivity for detecting the virus.23,24 During the second week of COVID-19 illness, IgM or IgG antibodies were detected in greater than 89% of infected patients.23 Severe disease resulted in high concentrations of antibody.
When testing for IgM and IgG antibodies is widely available, it may become an option to test all health care workers. This will permit the assignment of those health care workers with the highest levels of antibody to frontline duties with COVID-19 patients during the next disease outbreak, likely to occur at some point during the next 12 months.
A COVID-19 vaccine
Dozens of research teams, including pharmaceutical and biotechnology companies and many academic laboratories, are working on developing and testing vaccines to prevent COVID-19 disease. An effective vaccine would reduce the number of people who develop severe disease during the next outbreak, reducing deaths, avoiding a shutdown of the country, and allowing the health systems to function normally. A vaccine is unlikely to be widely available until sometime early in 2021.
Facing COVID-19 well-being and mental health
SARS-CoV-2, like all viral particles, is incredibly small. Remarkably, it has changed permanently life on earth. COVID-19 is affecting our physical health, psychological well-being, economics, and patterns of social interaction. As clinicians it is difficult to face a viral enemy that cannot be stopped from causing the death of more than 100,000 people, including some of our clinical colleagues, within a short period of time.
- F—focus on what is in your control
- A—acknowledge your thoughts and feelings
- C—come back to a focus on your body
- E—engage in what you are doing
- C—commit to acting effectively based on your core values
- O—opening up to difficult feelings and being kind to yourself and others
- V—values should guide your actions
- I—identify resources for help, assistance, support, and advice
- D—disinfect and practice social distancing.
This war will come to an end
During the American Revolution, colonists faced housing and food insecurity, epidemics of typhus and smallpox, traumatic injury including amputation of limbs, and a complete disruption of normal life activities. They persevered and, against the odds, successfully concluded the war. Unlike the colonists, who did not know if their conflict would end with success or failure, we clinicians know that the COVID-19 pandemic will end. We also know that eventually the global community of clinicians will develop and deploy the effective weapons we need to prevent a recurrence of this traumatic pandemic: population-wide testing for both the SARS-CoV-2 virus and serologic testing for IgG and IgM antibodies to the virus, effective antiviral medications, and a potent vaccine. ●
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens [published online March 11, 2020]. JAMA . doi: 10.1001/ jama . 2020 .3786.
- World Health Organization. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. March 29, 2020. https://www.who.int/publications-detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations. Accessed April 16, 2020.
- Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State [published online March 19, 2020]. JAMA . doi: 10.1001/ jama . 2020 .4326.
- Guan WJ, Liang WH, Zhao Y, et al; China Medical Treatment Expert Group for Covid-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis [published online March 26, 2020]. Eur Respir J . doi: 10.1183/13993003.00547- 2020 .
- Onder G, Rezza G, Brusaferro S. Case fatality rate and characteristics of patients dying in relation to COVID-19 in Italy [published online March 23, 2020]. JAMA. doi: 10.1001/ jama . 2020 .4683.
- Wei WE, Li Z, Chiew CJ, et al. Presymptomatic transmission of SARS-CoV-2 - Singapore, January 23 to March 16, 2020. MMWR Morb Mortal Wkly Rep . 2020;69:411-415.
- Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19 [published online February 21, 2020]. JAMA. doi: 10.1001/ jama . 2020 .2565.
- Ong SW, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient [published online March 4, 2020]. JAMA . doi: 10.1001/ jama .2020.3227.
- Fineberg HV. Rapid expert consultation on the possibility of bioaerosol spread of SARS-CoV-2 for the COVID-19 pandemic. April 1, 2020. https://www.nap.edu/read/25769/chapter/1. Accessed April 16, 2020.
- Santarpia JL, River DN, Herrera V, et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. MedRxiv. March 26, 2020. doi.org10.1101/2020.03.23.20039466.
- Liu Y, Ning Z, Chen Y, et al. Aerodynamic characteristics and RNA concentration of SARS-CoV-2 aerosol in Wuhan Hospitals during COVID-19 outbreak. BioRxiv. March 10, 2020. doi.org/10.1101/2020.03.08.982637.
- Klompas M, Morris CA, Sinclair J, et al. Universal masking in hospitals in the COVID-19 era [published online April 1, 2020]. N Engl J Med. doi: 10.1056/NEJMp2006372.
- Liu D, Li L, Wu X, et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. 2020:1-6. doi: 10.2214/AJR.20.23072.
- Di Mascio D, Khalik A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol. doi:10.1016/j.ajogmf.2020.
100107. - Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens [published online March 11, 2020]. JAMA. doi: 10.1001/jama.2020.3786.
- Dong L, Tian J, He S, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn [published online March 26, 2020]. JAMA. doi: 10.1001/ jama .2020.4621.
- Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID-19 pneumonia [published online March 26, 2020]. JAMA. doi: 10.1001/ jama .2020.4861.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Diease 2019 (COVID-19) outbreak in China. Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention [published online February 24, 2020]. JAMA . doi: 10.1001/jama.2020.2648.
- Chen L, Li Q, Zheng D, et al. Clinical characteristics of pregnant women with COVID-19 in Wuhan, China [published online April 17, 2020]. N Engl J Med. doi 10.1056/NEJMc2009226.
- Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. MedRxiv. April 10, 2020. https://doi.org/10.1101/2020.03.22.20040758.
- Maulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381:2293-2303.
- Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma [published online March 27, 2020]. JAMA. doi: 10.1001/ jama . 2020 .4783.
- Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019 [published online March 29, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa344.
- Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) [published online March 21, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa310.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens [published online March 11, 2020]. JAMA . doi: 10.1001/ jama . 2020 .3786.
- World Health Organization. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. March 29, 2020. https://www.who.int/publications-detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations. Accessed April 16, 2020.
- Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State [published online March 19, 2020]. JAMA . doi: 10.1001/ jama . 2020 .4326.
- Guan WJ, Liang WH, Zhao Y, et al; China Medical Treatment Expert Group for Covid-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis [published online March 26, 2020]. Eur Respir J . doi: 10.1183/13993003.00547- 2020 .
- Onder G, Rezza G, Brusaferro S. Case fatality rate and characteristics of patients dying in relation to COVID-19 in Italy [published online March 23, 2020]. JAMA. doi: 10.1001/ jama . 2020 .4683.
- Wei WE, Li Z, Chiew CJ, et al. Presymptomatic transmission of SARS-CoV-2 - Singapore, January 23 to March 16, 2020. MMWR Morb Mortal Wkly Rep . 2020;69:411-415.
- Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19 [published online February 21, 2020]. JAMA. doi: 10.1001/ jama . 2020 .2565.
- Ong SW, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient [published online March 4, 2020]. JAMA . doi: 10.1001/ jama .2020.3227.
- Fineberg HV. Rapid expert consultation on the possibility of bioaerosol spread of SARS-CoV-2 for the COVID-19 pandemic. April 1, 2020. https://www.nap.edu/read/25769/chapter/1. Accessed April 16, 2020.
- Santarpia JL, River DN, Herrera V, et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. MedRxiv. March 26, 2020. doi.org10.1101/2020.03.23.20039466.
- Liu Y, Ning Z, Chen Y, et al. Aerodynamic characteristics and RNA concentration of SARS-CoV-2 aerosol in Wuhan Hospitals during COVID-19 outbreak. BioRxiv. March 10, 2020. doi.org/10.1101/2020.03.08.982637.
- Klompas M, Morris CA, Sinclair J, et al. Universal masking in hospitals in the COVID-19 era [published online April 1, 2020]. N Engl J Med. doi: 10.1056/NEJMp2006372.
- Liu D, Li L, Wu X, et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. 2020:1-6. doi: 10.2214/AJR.20.23072.
- Di Mascio D, Khalik A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol. doi:10.1016/j.ajogmf.2020.
100107. - Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens [published online March 11, 2020]. JAMA. doi: 10.1001/jama.2020.3786.
- Dong L, Tian J, He S, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn [published online March 26, 2020]. JAMA. doi: 10.1001/ jama .2020.4621.
- Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID-19 pneumonia [published online March 26, 2020]. JAMA. doi: 10.1001/ jama .2020.4861.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Diease 2019 (COVID-19) outbreak in China. Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention [published online February 24, 2020]. JAMA . doi: 10.1001/jama.2020.2648.
- Chen L, Li Q, Zheng D, et al. Clinical characteristics of pregnant women with COVID-19 in Wuhan, China [published online April 17, 2020]. N Engl J Med. doi 10.1056/NEJMc2009226.
- Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. MedRxiv. April 10, 2020. https://doi.org/10.1101/2020.03.22.20040758.
- Maulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381:2293-2303.
- Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma [published online March 27, 2020]. JAMA. doi: 10.1001/ jama . 2020 .4783.
- Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019 [published online March 29, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa344.
- Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) [published online March 21, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa310.
Prescribing aspirin to improve pregnancy outcomes: Expand the indications? Increase the dose?
Authors of a recent Cochrane review concluded that low-dose aspirin treatment of 1,000 pregnant women at risk of developing preeclampsia resulted in 16 fewer cases of preeclampsia, 16 fewer preterm births, 7 fewer cases of small-for-gestational age newborns, and 5 fewer fetal or neonatal deaths.1
The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) recommend treatment with 81 mg of aspirin daily, initiated before 16 weeks of pregnancy to prevent preeclampsia in women with one major risk factor (personal history of preeclampsia, multifetal gestation, chronic hypertension, type 1 or 2 diabetes, renal or autoimmune disease) or at least two moderate risk factors (nulliparity; obesity; mother or sister with preeclampsia; a sociodemographic characteristic such as African American race or low socioeconomic status; age ≥35 years; personal history factors such as prior low birth weight infant, previous adverse pregnancy outcome, or >10-year interpregnancy interval).2,3 Healthy pregnant women with a previous uncomplicated full-term delivery do not need treatment with low-dose aspirin.2,3
However, evolving data and expert opinion suggest that expanding the indications for aspirin treatment and increasing the recommended dose of aspirin may be warranted.
Nulliparity
Nulliparity is the single clinical characteristic that is associated with the greatest number of cases of preeclampsia.4 Hence, from a public health perspective, reducing the rate of preeclampsia among nulliparous women is a top priority.
ACOG and USPSTF do not recommend aspirin treatment for all nulliparous women because risk factors help to identify those nulliparous women who benefit from aspirin treatment.
However, a recent cost-effectiveness analysis compared the health care costs and rates of preeclampsia for 4 prevention strategies among all pregnant women in the United States (nulliparous and parous)5:
- no aspirin use
- use of aspirin based on biomarker and ultrasound measurements
- use of aspirin based on USPSTF guidelines for identifying women at risk
- prescription of aspirin to all pregnant women.
Health care costs and rates of preeclampsia were lowest with the universal prescription of aspirin to all pregnant women in the United States. Compared with universal prescription of aspirin, the USPSTF approach, the biomarker-ultrasound approach, and the no aspirin approach were associated with 346, 308, and 762 additional cases of preeclampsia per 100,000 women. In sensitivity analyses, universal aspirin was the optimal strategy under most assumptions.
Another cost effectiveness analysis concluded that among nulliparous pregnant women, universal aspirin treatment was superior to aspirin treatment based on biomarker-ultrasound identification of women at high risk.6
In a recent clinical trial performed in India, Guatemala, Pakistan, Democratic Republic of Congo, Kenya, and Zambia, 14,361 nulliparous women were randomly assigned to placebo or 81 mg of aspirin daily between 6 and 14 weeks of gestation.7 Preterm birth (<37 weeks’ gestation) occurred in 13.1% and 11.6% of women treated with placebo or aspirin (relative risk [RR], 0.89; 95% confidence interval [CI], 0.81 to 0.98, P = .012). Most of the decrease in preterm birth appeared to be due to a decrease in the rate of preeclampsia in the aspirin-treated nulliparous women. The investigators also noted that aspirin treatment of nulliparous women resulted in a statistically significant decrease in perinatal mortality (RR, 0.86) and early preterm delivery, <34 weeks’ gestation (RR, 0.75).
Universal prescription of low-dose aspirin to nulliparous women in order to prevent preeclampsia and preterm birth may become recognized as an optimal public health strategy. As a step toward universal prescription of aspirin to nulliparous women, an opt-out rather than a screen-in strategy might be considered.8

Continue to: Booking systolic blood pressure, 120 to 134 mm Hg...
Booking systolic blood pressure, 120 to 134 mm Hg
All obstetricians recognize that women with chronic hypertension should be treated with low-dose aspirin because they are at high risk for preeclampsia. However, there is evidence that nulliparous women with a booking systolic pressure ≥120 mm Hg might also benefit from low-dose aspirin treatment. In one US trial, 3,135 nulliparous normotensive women (booking blood pressure [BP] <135/85 mm Hg) were randomly assigned to treatment with aspirin (60 mg daily) or placebo initiated between 13 and 26 weeks’ gestation. Preeclampsia occurred in 6.3% and 4.6% of the women treated with placebo or aspirin, respectively (RR, 0.7; 95% CI, 0.6–1.0; P = .05).9 A secondary analysis showed that, among 519 nulliparous women with a booking systolic BP from 120 to 134 mm Hg, compared with placebo, low-dose aspirin treatment reduced the rate of preeclampsia from 11.9% to 5.6%.9 Aspirin did not reduce the rate of preeclampsia among nulliparous women with a booking systolic BP <120 mm Hg.9 A systematic review of risk factors for developing preeclampsia reported that a booking diastolic BP of ≥80 mm Hg was associated with an increased risk of developing preeclampsia (RR, 1.38).10
The American Heart Association (AHA) and the American College of Cardiology (ACC) recently updated the definition of hypertension.11 Normal BP is now defined as a systolic pressure <120 mm Hg and diastolic pressure <80 mm Hg. Elevated BP is a systolic pressure of 120 to 129 mm Hg and diastolic pressure of <80 mm Hg. Stage I hypertension is a systolic BP from 130 to 139 mm Hg or diastolic blood pressure from 80 to 89 mm Hg. Stage II hypertension is a systolic BP of ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg.11
A recent study reported that 90% of women at 12 weeks’ gestation have a BP of ≤130 mm Hg systolic and ≤80 mm Hg diastolic, suggesting that the AHA-ACC criteria for stage I hypertension are reasonable.12 Obstetricians have not yet fully adopted the AHA-ACC criteria for defining stage I hypertension in pregnant women. Future research may demonstrate that a booking systolic BP
≥130 mm Hg or a diastolic BP ≥80 mm Hg are major risk factors for developing preeclampsia and warrant treatment with low-dose aspirin.

Continue to: Pregnancy resulting from fertility therapy...
Pregnancy resulting from fertility therapy
Current ACOG and USPSTF guidelines do not specifically identify pregnancies resulting from assisted reproductive technology as a major or moderate risk factor for preeclampsia.2,3 In a study comparing 83,582 births resulting from in vitro fertilization (IVF) and 1,382,311 births to fertile women, treatment with autologous cryopreserved embryos (adjusted odds ratio [aOR], 1.30), fresh donor embryos (aOR, 1.92), and cryopreserved donor embryos (aOR, 1.70) significantly increased the risk of preeclampsia.13 However, use of fresh autologous embryos did not increase the risk of preeclampsia (aOR, 1.04). These associations persisted after controlling for diabetes, hypertension, body mass index, and cause of infertility.13
Other studies also have reported that use of cryopreserved embryos is associated with a higher rate of preeclampsia than use of fresh autologous embryos. In a study of 825 infertile women undergoing IVF and randomly assigned to single embryo cryopreserved or fresh cycles, the rate of preeclampsia was 3.1% and 1.0% in the pregnancies that resulted from cryopreserved versus fresh cycles.14
What is the optimal dose of aspirin?
ACOG and the USPSTF recommend aspirin 81 mg daily for the prevention of preeclampsia.2,3 The International Federation of Gynecology and Obstetrics (FIGO) recommends aspirin 150 mg daily for the prevention of preeclampsia.15 The FIGO recommendation is based, in part, on the results of a large international clinical trial that randomly assigned 1,776 women at high risk for preeclampsia as determined by clinical factors plus biomarker and ultrasound screening to receive aspirin 150 mg daily or placebo daily initiated at 11 to 14 weeks’ gestation and continued until 36 weeks’ gestation.16 Preeclampsia before 37 weeks’ gestation occurred in 4.3% and 1.6% of women in the placebo and aspirin groups (OR, 0.38; 95% CI, 0.20–0.74; P = .004).16 FIGO recommends that women at risk for preeclampsia with a body mass <40 kg take aspirin 100 mg daily and women with a body mass ≥40 kg take aspirin at a dose of 150 mg daily. For women who live in a country where aspirin is not available in a pill containing 150 mg, FIGO recommends taking two 81 mg tablets.15 FIGO recommends initiating aspirin between 11 and 14 weeks and 6 days of gestation and continuing aspirin therapy until 36 weeks of gestation.15
Aspirin is an inexpensive intervention with many possible benefits
For many nulliparous women and some parous women aspirin treatment initiated early in pregnancy will improve maternal and newborn outcomes, including reducing the risk of preeclampsia, preterm birth, and intrauterine growth restriction.1 Obstetricians may want to begin to expand the indications for offering aspirin to prevent preeclampsia from those recommended by ACOG and the USPSTF to include nulliparous women with a booking systolic pressure of 120 to 134 mm Hg and women whose pregnancy was the result of an assisted reproduction treatment that used cryopreserved embryos. In addition, obstetricians who currently prescribe 81 mg of aspirin daily might want to consider increasing the prescribed dose to 162 mg of aspirin daily (two 81 mg tablets daily or one-half of a 325 mg tablet). Aspirin costs about less than 5 cents per 81 mg tablet (according to GoodRx website). It is an inexpensive intervention that could benefit many mothers and newborns. ●
- Duley L, Meher S, Hunter KE, et al. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2019;CD004659.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44-e52.
- LeFevre ML; U.S. Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force Recommendation Statement. Ann Int Med. 2014;161: 819-826.
- Bartsch E, Medcalf KE, Park AL, et al. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ. 2016;353:i1753.
- Mallampati D, Grobman W, Rouse DJ, et al. Strategies for prescribing aspirin to prevent preeclampsia: a cost-effectiveness analysis. Obstet Gynecol. 2019;134:537-544.
- Mone F, O’Mahony JF, Tyrrell E, et al. Preeclampsia prevention using routine versus screening test-indicated aspirin in low-risk women. Hypertension. 2018;72:1391-1396.
- Hoffman MK, Goudar SS, Kodkany BS, et al. Low-dose aspirin for the prevention of preterm delivery in nulliparous women with a singleton pregnancy (ASPIRIN): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;395:285-293.
- Ayala NK, Rouse DJ. A nudge toward universal aspirin for preeclampsia prevention. Obstet Gynecol. 2019;133:725-728.
- Sibai BM, Caritis SN, Thom E, et al. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1993;329:1213-1218.
- Duckitt K, Harrington D. Risk factors for preeclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330:565.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:2199-2269.
- Green LJ, Mackillop LH, Salvi D, et al. Gestation-specific vital sign reference ranges in pregnancy. Obstet Gynecol. 2020;135:653-664.
- Luke B, Brown MB, Eisenberg ML, et al. In vitro fertilization and risk for hypertensive disorders of pregnancy: associations with treatment parameters. Am J Obstet Gynecol. October 17, 2019. doi:10.1016/j.ajog.2019.10.003.
- Wei D, Liu JY, Sun Y, et al. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet. 2019;393:1310-1318.
- Poon LC, Shennan A, Hyett JA, et al. International Federation of Gynecology and Obstetrics (FIGO) initiative on preeclampsia: A pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet. 2019;145(suppl 1):1-33.
- Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377:613-622.
Authors of a recent Cochrane review concluded that low-dose aspirin treatment of 1,000 pregnant women at risk of developing preeclampsia resulted in 16 fewer cases of preeclampsia, 16 fewer preterm births, 7 fewer cases of small-for-gestational age newborns, and 5 fewer fetal or neonatal deaths.1
The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) recommend treatment with 81 mg of aspirin daily, initiated before 16 weeks of pregnancy to prevent preeclampsia in women with one major risk factor (personal history of preeclampsia, multifetal gestation, chronic hypertension, type 1 or 2 diabetes, renal or autoimmune disease) or at least two moderate risk factors (nulliparity; obesity; mother or sister with preeclampsia; a sociodemographic characteristic such as African American race or low socioeconomic status; age ≥35 years; personal history factors such as prior low birth weight infant, previous adverse pregnancy outcome, or >10-year interpregnancy interval).2,3 Healthy pregnant women with a previous uncomplicated full-term delivery do not need treatment with low-dose aspirin.2,3
However, evolving data and expert opinion suggest that expanding the indications for aspirin treatment and increasing the recommended dose of aspirin may be warranted.
Nulliparity
Nulliparity is the single clinical characteristic that is associated with the greatest number of cases of preeclampsia.4 Hence, from a public health perspective, reducing the rate of preeclampsia among nulliparous women is a top priority.
ACOG and USPSTF do not recommend aspirin treatment for all nulliparous women because risk factors help to identify those nulliparous women who benefit from aspirin treatment.
However, a recent cost-effectiveness analysis compared the health care costs and rates of preeclampsia for 4 prevention strategies among all pregnant women in the United States (nulliparous and parous)5:
- no aspirin use
- use of aspirin based on biomarker and ultrasound measurements
- use of aspirin based on USPSTF guidelines for identifying women at risk
- prescription of aspirin to all pregnant women.
Health care costs and rates of preeclampsia were lowest with the universal prescription of aspirin to all pregnant women in the United States. Compared with universal prescription of aspirin, the USPSTF approach, the biomarker-ultrasound approach, and the no aspirin approach were associated with 346, 308, and 762 additional cases of preeclampsia per 100,000 women. In sensitivity analyses, universal aspirin was the optimal strategy under most assumptions.
Another cost effectiveness analysis concluded that among nulliparous pregnant women, universal aspirin treatment was superior to aspirin treatment based on biomarker-ultrasound identification of women at high risk.6
In a recent clinical trial performed in India, Guatemala, Pakistan, Democratic Republic of Congo, Kenya, and Zambia, 14,361 nulliparous women were randomly assigned to placebo or 81 mg of aspirin daily between 6 and 14 weeks of gestation.7 Preterm birth (<37 weeks’ gestation) occurred in 13.1% and 11.6% of women treated with placebo or aspirin (relative risk [RR], 0.89; 95% confidence interval [CI], 0.81 to 0.98, P = .012). Most of the decrease in preterm birth appeared to be due to a decrease in the rate of preeclampsia in the aspirin-treated nulliparous women. The investigators also noted that aspirin treatment of nulliparous women resulted in a statistically significant decrease in perinatal mortality (RR, 0.86) and early preterm delivery, <34 weeks’ gestation (RR, 0.75).
Universal prescription of low-dose aspirin to nulliparous women in order to prevent preeclampsia and preterm birth may become recognized as an optimal public health strategy. As a step toward universal prescription of aspirin to nulliparous women, an opt-out rather than a screen-in strategy might be considered.8

Continue to: Booking systolic blood pressure, 120 to 134 mm Hg...
Booking systolic blood pressure, 120 to 134 mm Hg
All obstetricians recognize that women with chronic hypertension should be treated with low-dose aspirin because they are at high risk for preeclampsia. However, there is evidence that nulliparous women with a booking systolic pressure ≥120 mm Hg might also benefit from low-dose aspirin treatment. In one US trial, 3,135 nulliparous normotensive women (booking blood pressure [BP] <135/85 mm Hg) were randomly assigned to treatment with aspirin (60 mg daily) or placebo initiated between 13 and 26 weeks’ gestation. Preeclampsia occurred in 6.3% and 4.6% of the women treated with placebo or aspirin, respectively (RR, 0.7; 95% CI, 0.6–1.0; P = .05).9 A secondary analysis showed that, among 519 nulliparous women with a booking systolic BP from 120 to 134 mm Hg, compared with placebo, low-dose aspirin treatment reduced the rate of preeclampsia from 11.9% to 5.6%.9 Aspirin did not reduce the rate of preeclampsia among nulliparous women with a booking systolic BP <120 mm Hg.9 A systematic review of risk factors for developing preeclampsia reported that a booking diastolic BP of ≥80 mm Hg was associated with an increased risk of developing preeclampsia (RR, 1.38).10
The American Heart Association (AHA) and the American College of Cardiology (ACC) recently updated the definition of hypertension.11 Normal BP is now defined as a systolic pressure <120 mm Hg and diastolic pressure <80 mm Hg. Elevated BP is a systolic pressure of 120 to 129 mm Hg and diastolic pressure of <80 mm Hg. Stage I hypertension is a systolic BP from 130 to 139 mm Hg or diastolic blood pressure from 80 to 89 mm Hg. Stage II hypertension is a systolic BP of ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg.11
A recent study reported that 90% of women at 12 weeks’ gestation have a BP of ≤130 mm Hg systolic and ≤80 mm Hg diastolic, suggesting that the AHA-ACC criteria for stage I hypertension are reasonable.12 Obstetricians have not yet fully adopted the AHA-ACC criteria for defining stage I hypertension in pregnant women. Future research may demonstrate that a booking systolic BP
≥130 mm Hg or a diastolic BP ≥80 mm Hg are major risk factors for developing preeclampsia and warrant treatment with low-dose aspirin.

Continue to: Pregnancy resulting from fertility therapy...
Pregnancy resulting from fertility therapy
Current ACOG and USPSTF guidelines do not specifically identify pregnancies resulting from assisted reproductive technology as a major or moderate risk factor for preeclampsia.2,3 In a study comparing 83,582 births resulting from in vitro fertilization (IVF) and 1,382,311 births to fertile women, treatment with autologous cryopreserved embryos (adjusted odds ratio [aOR], 1.30), fresh donor embryos (aOR, 1.92), and cryopreserved donor embryos (aOR, 1.70) significantly increased the risk of preeclampsia.13 However, use of fresh autologous embryos did not increase the risk of preeclampsia (aOR, 1.04). These associations persisted after controlling for diabetes, hypertension, body mass index, and cause of infertility.13
Other studies also have reported that use of cryopreserved embryos is associated with a higher rate of preeclampsia than use of fresh autologous embryos. In a study of 825 infertile women undergoing IVF and randomly assigned to single embryo cryopreserved or fresh cycles, the rate of preeclampsia was 3.1% and 1.0% in the pregnancies that resulted from cryopreserved versus fresh cycles.14
What is the optimal dose of aspirin?
ACOG and the USPSTF recommend aspirin 81 mg daily for the prevention of preeclampsia.2,3 The International Federation of Gynecology and Obstetrics (FIGO) recommends aspirin 150 mg daily for the prevention of preeclampsia.15 The FIGO recommendation is based, in part, on the results of a large international clinical trial that randomly assigned 1,776 women at high risk for preeclampsia as determined by clinical factors plus biomarker and ultrasound screening to receive aspirin 150 mg daily or placebo daily initiated at 11 to 14 weeks’ gestation and continued until 36 weeks’ gestation.16 Preeclampsia before 37 weeks’ gestation occurred in 4.3% and 1.6% of women in the placebo and aspirin groups (OR, 0.38; 95% CI, 0.20–0.74; P = .004).16 FIGO recommends that women at risk for preeclampsia with a body mass <40 kg take aspirin 100 mg daily and women with a body mass ≥40 kg take aspirin at a dose of 150 mg daily. For women who live in a country where aspirin is not available in a pill containing 150 mg, FIGO recommends taking two 81 mg tablets.15 FIGO recommends initiating aspirin between 11 and 14 weeks and 6 days of gestation and continuing aspirin therapy until 36 weeks of gestation.15
Aspirin is an inexpensive intervention with many possible benefits
For many nulliparous women and some parous women aspirin treatment initiated early in pregnancy will improve maternal and newborn outcomes, including reducing the risk of preeclampsia, preterm birth, and intrauterine growth restriction.1 Obstetricians may want to begin to expand the indications for offering aspirin to prevent preeclampsia from those recommended by ACOG and the USPSTF to include nulliparous women with a booking systolic pressure of 120 to 134 mm Hg and women whose pregnancy was the result of an assisted reproduction treatment that used cryopreserved embryos. In addition, obstetricians who currently prescribe 81 mg of aspirin daily might want to consider increasing the prescribed dose to 162 mg of aspirin daily (two 81 mg tablets daily or one-half of a 325 mg tablet). Aspirin costs about less than 5 cents per 81 mg tablet (according to GoodRx website). It is an inexpensive intervention that could benefit many mothers and newborns. ●
Authors of a recent Cochrane review concluded that low-dose aspirin treatment of 1,000 pregnant women at risk of developing preeclampsia resulted in 16 fewer cases of preeclampsia, 16 fewer preterm births, 7 fewer cases of small-for-gestational age newborns, and 5 fewer fetal or neonatal deaths.1
The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) recommend treatment with 81 mg of aspirin daily, initiated before 16 weeks of pregnancy to prevent preeclampsia in women with one major risk factor (personal history of preeclampsia, multifetal gestation, chronic hypertension, type 1 or 2 diabetes, renal or autoimmune disease) or at least two moderate risk factors (nulliparity; obesity; mother or sister with preeclampsia; a sociodemographic characteristic such as African American race or low socioeconomic status; age ≥35 years; personal history factors such as prior low birth weight infant, previous adverse pregnancy outcome, or >10-year interpregnancy interval).2,3 Healthy pregnant women with a previous uncomplicated full-term delivery do not need treatment with low-dose aspirin.2,3
However, evolving data and expert opinion suggest that expanding the indications for aspirin treatment and increasing the recommended dose of aspirin may be warranted.
Nulliparity
Nulliparity is the single clinical characteristic that is associated with the greatest number of cases of preeclampsia.4 Hence, from a public health perspective, reducing the rate of preeclampsia among nulliparous women is a top priority.
ACOG and USPSTF do not recommend aspirin treatment for all nulliparous women because risk factors help to identify those nulliparous women who benefit from aspirin treatment.
However, a recent cost-effectiveness analysis compared the health care costs and rates of preeclampsia for 4 prevention strategies among all pregnant women in the United States (nulliparous and parous)5:
- no aspirin use
- use of aspirin based on biomarker and ultrasound measurements
- use of aspirin based on USPSTF guidelines for identifying women at risk
- prescription of aspirin to all pregnant women.
Health care costs and rates of preeclampsia were lowest with the universal prescription of aspirin to all pregnant women in the United States. Compared with universal prescription of aspirin, the USPSTF approach, the biomarker-ultrasound approach, and the no aspirin approach were associated with 346, 308, and 762 additional cases of preeclampsia per 100,000 women. In sensitivity analyses, universal aspirin was the optimal strategy under most assumptions.
Another cost effectiveness analysis concluded that among nulliparous pregnant women, universal aspirin treatment was superior to aspirin treatment based on biomarker-ultrasound identification of women at high risk.6
In a recent clinical trial performed in India, Guatemala, Pakistan, Democratic Republic of Congo, Kenya, and Zambia, 14,361 nulliparous women were randomly assigned to placebo or 81 mg of aspirin daily between 6 and 14 weeks of gestation.7 Preterm birth (<37 weeks’ gestation) occurred in 13.1% and 11.6% of women treated with placebo or aspirin (relative risk [RR], 0.89; 95% confidence interval [CI], 0.81 to 0.98, P = .012). Most of the decrease in preterm birth appeared to be due to a decrease in the rate of preeclampsia in the aspirin-treated nulliparous women. The investigators also noted that aspirin treatment of nulliparous women resulted in a statistically significant decrease in perinatal mortality (RR, 0.86) and early preterm delivery, <34 weeks’ gestation (RR, 0.75).
Universal prescription of low-dose aspirin to nulliparous women in order to prevent preeclampsia and preterm birth may become recognized as an optimal public health strategy. As a step toward universal prescription of aspirin to nulliparous women, an opt-out rather than a screen-in strategy might be considered.8

Continue to: Booking systolic blood pressure, 120 to 134 mm Hg...
Booking systolic blood pressure, 120 to 134 mm Hg
All obstetricians recognize that women with chronic hypertension should be treated with low-dose aspirin because they are at high risk for preeclampsia. However, there is evidence that nulliparous women with a booking systolic pressure ≥120 mm Hg might also benefit from low-dose aspirin treatment. In one US trial, 3,135 nulliparous normotensive women (booking blood pressure [BP] <135/85 mm Hg) were randomly assigned to treatment with aspirin (60 mg daily) or placebo initiated between 13 and 26 weeks’ gestation. Preeclampsia occurred in 6.3% and 4.6% of the women treated with placebo or aspirin, respectively (RR, 0.7; 95% CI, 0.6–1.0; P = .05).9 A secondary analysis showed that, among 519 nulliparous women with a booking systolic BP from 120 to 134 mm Hg, compared with placebo, low-dose aspirin treatment reduced the rate of preeclampsia from 11.9% to 5.6%.9 Aspirin did not reduce the rate of preeclampsia among nulliparous women with a booking systolic BP <120 mm Hg.9 A systematic review of risk factors for developing preeclampsia reported that a booking diastolic BP of ≥80 mm Hg was associated with an increased risk of developing preeclampsia (RR, 1.38).10
The American Heart Association (AHA) and the American College of Cardiology (ACC) recently updated the definition of hypertension.11 Normal BP is now defined as a systolic pressure <120 mm Hg and diastolic pressure <80 mm Hg. Elevated BP is a systolic pressure of 120 to 129 mm Hg and diastolic pressure of <80 mm Hg. Stage I hypertension is a systolic BP from 130 to 139 mm Hg or diastolic blood pressure from 80 to 89 mm Hg. Stage II hypertension is a systolic BP of ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg.11
A recent study reported that 90% of women at 12 weeks’ gestation have a BP of ≤130 mm Hg systolic and ≤80 mm Hg diastolic, suggesting that the AHA-ACC criteria for stage I hypertension are reasonable.12 Obstetricians have not yet fully adopted the AHA-ACC criteria for defining stage I hypertension in pregnant women. Future research may demonstrate that a booking systolic BP
≥130 mm Hg or a diastolic BP ≥80 mm Hg are major risk factors for developing preeclampsia and warrant treatment with low-dose aspirin.

Continue to: Pregnancy resulting from fertility therapy...
Pregnancy resulting from fertility therapy
Current ACOG and USPSTF guidelines do not specifically identify pregnancies resulting from assisted reproductive technology as a major or moderate risk factor for preeclampsia.2,3 In a study comparing 83,582 births resulting from in vitro fertilization (IVF) and 1,382,311 births to fertile women, treatment with autologous cryopreserved embryos (adjusted odds ratio [aOR], 1.30), fresh donor embryos (aOR, 1.92), and cryopreserved donor embryos (aOR, 1.70) significantly increased the risk of preeclampsia.13 However, use of fresh autologous embryos did not increase the risk of preeclampsia (aOR, 1.04). These associations persisted after controlling for diabetes, hypertension, body mass index, and cause of infertility.13
Other studies also have reported that use of cryopreserved embryos is associated with a higher rate of preeclampsia than use of fresh autologous embryos. In a study of 825 infertile women undergoing IVF and randomly assigned to single embryo cryopreserved or fresh cycles, the rate of preeclampsia was 3.1% and 1.0% in the pregnancies that resulted from cryopreserved versus fresh cycles.14
What is the optimal dose of aspirin?
ACOG and the USPSTF recommend aspirin 81 mg daily for the prevention of preeclampsia.2,3 The International Federation of Gynecology and Obstetrics (FIGO) recommends aspirin 150 mg daily for the prevention of preeclampsia.15 The FIGO recommendation is based, in part, on the results of a large international clinical trial that randomly assigned 1,776 women at high risk for preeclampsia as determined by clinical factors plus biomarker and ultrasound screening to receive aspirin 150 mg daily or placebo daily initiated at 11 to 14 weeks’ gestation and continued until 36 weeks’ gestation.16 Preeclampsia before 37 weeks’ gestation occurred in 4.3% and 1.6% of women in the placebo and aspirin groups (OR, 0.38; 95% CI, 0.20–0.74; P = .004).16 FIGO recommends that women at risk for preeclampsia with a body mass <40 kg take aspirin 100 mg daily and women with a body mass ≥40 kg take aspirin at a dose of 150 mg daily. For women who live in a country where aspirin is not available in a pill containing 150 mg, FIGO recommends taking two 81 mg tablets.15 FIGO recommends initiating aspirin between 11 and 14 weeks and 6 days of gestation and continuing aspirin therapy until 36 weeks of gestation.15
Aspirin is an inexpensive intervention with many possible benefits
For many nulliparous women and some parous women aspirin treatment initiated early in pregnancy will improve maternal and newborn outcomes, including reducing the risk of preeclampsia, preterm birth, and intrauterine growth restriction.1 Obstetricians may want to begin to expand the indications for offering aspirin to prevent preeclampsia from those recommended by ACOG and the USPSTF to include nulliparous women with a booking systolic pressure of 120 to 134 mm Hg and women whose pregnancy was the result of an assisted reproduction treatment that used cryopreserved embryos. In addition, obstetricians who currently prescribe 81 mg of aspirin daily might want to consider increasing the prescribed dose to 162 mg of aspirin daily (two 81 mg tablets daily or one-half of a 325 mg tablet). Aspirin costs about less than 5 cents per 81 mg tablet (according to GoodRx website). It is an inexpensive intervention that could benefit many mothers and newborns. ●
- Duley L, Meher S, Hunter KE, et al. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2019;CD004659.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44-e52.
- LeFevre ML; U.S. Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force Recommendation Statement. Ann Int Med. 2014;161: 819-826.
- Bartsch E, Medcalf KE, Park AL, et al. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ. 2016;353:i1753.
- Mallampati D, Grobman W, Rouse DJ, et al. Strategies for prescribing aspirin to prevent preeclampsia: a cost-effectiveness analysis. Obstet Gynecol. 2019;134:537-544.
- Mone F, O’Mahony JF, Tyrrell E, et al. Preeclampsia prevention using routine versus screening test-indicated aspirin in low-risk women. Hypertension. 2018;72:1391-1396.
- Hoffman MK, Goudar SS, Kodkany BS, et al. Low-dose aspirin for the prevention of preterm delivery in nulliparous women with a singleton pregnancy (ASPIRIN): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;395:285-293.
- Ayala NK, Rouse DJ. A nudge toward universal aspirin for preeclampsia prevention. Obstet Gynecol. 2019;133:725-728.
- Sibai BM, Caritis SN, Thom E, et al. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1993;329:1213-1218.
- Duckitt K, Harrington D. Risk factors for preeclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330:565.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:2199-2269.
- Green LJ, Mackillop LH, Salvi D, et al. Gestation-specific vital sign reference ranges in pregnancy. Obstet Gynecol. 2020;135:653-664.
- Luke B, Brown MB, Eisenberg ML, et al. In vitro fertilization and risk for hypertensive disorders of pregnancy: associations with treatment parameters. Am J Obstet Gynecol. October 17, 2019. doi:10.1016/j.ajog.2019.10.003.
- Wei D, Liu JY, Sun Y, et al. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet. 2019;393:1310-1318.
- Poon LC, Shennan A, Hyett JA, et al. International Federation of Gynecology and Obstetrics (FIGO) initiative on preeclampsia: A pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet. 2019;145(suppl 1):1-33.
- Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377:613-622.
- Duley L, Meher S, Hunter KE, et al. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2019;CD004659.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44-e52.
- LeFevre ML; U.S. Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force Recommendation Statement. Ann Int Med. 2014;161: 819-826.
- Bartsch E, Medcalf KE, Park AL, et al. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ. 2016;353:i1753.
- Mallampati D, Grobman W, Rouse DJ, et al. Strategies for prescribing aspirin to prevent preeclampsia: a cost-effectiveness analysis. Obstet Gynecol. 2019;134:537-544.
- Mone F, O’Mahony JF, Tyrrell E, et al. Preeclampsia prevention using routine versus screening test-indicated aspirin in low-risk women. Hypertension. 2018;72:1391-1396.
- Hoffman MK, Goudar SS, Kodkany BS, et al. Low-dose aspirin for the prevention of preterm delivery in nulliparous women with a singleton pregnancy (ASPIRIN): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;395:285-293.
- Ayala NK, Rouse DJ. A nudge toward universal aspirin for preeclampsia prevention. Obstet Gynecol. 2019;133:725-728.
- Sibai BM, Caritis SN, Thom E, et al. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1993;329:1213-1218.
- Duckitt K, Harrington D. Risk factors for preeclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330:565.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:2199-2269.
- Green LJ, Mackillop LH, Salvi D, et al. Gestation-specific vital sign reference ranges in pregnancy. Obstet Gynecol. 2020;135:653-664.
- Luke B, Brown MB, Eisenberg ML, et al. In vitro fertilization and risk for hypertensive disorders of pregnancy: associations with treatment parameters. Am J Obstet Gynecol. October 17, 2019. doi:10.1016/j.ajog.2019.10.003.
- Wei D, Liu JY, Sun Y, et al. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet. 2019;393:1310-1318.
- Poon LC, Shennan A, Hyett JA, et al. International Federation of Gynecology and Obstetrics (FIGO) initiative on preeclampsia: A pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet. 2019;145(suppl 1):1-33.
- Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377:613-622.
What is the role of the ObGyn in preventing and treating obesity?
Obesity is a disease causing a public health crisis. In the United States, tobacco use and obesity are the two most important causes of preventable premature death. They result in an estimated 480,0001 and 300,0002 premature deaths per year, respectively. Obesity is a major contributor to diabetes mellitus, hypertension, dyslipidemia, and coronary heart disease. Obesity is also associated with increased rates of colon, breast, and endometrial cancer. Experts predict that in 2030, 50% of adults in the United States will have a body mass index (BMI) ≥ 30 kg/m2, and 25% will have a BMI ≥ 35 kg/m2.3 More women than men are predicted to be severely obese (FIGURE).3
As clinicians we need to increase our efforts to reduce the epidemic of obesity. ObGyns can play an important role in preventing and managing obesity, by recommending primary-care weight management practices, prescribing medications that influence central metabolism, and referring appropriate patients to bariatric surgery centers of excellence.
Primary-care weight management
Measuring BMI and recommending interventions to prevent and treat obesity are important components of a health maintenance encounter. For women who are overweight or obese, dietary changes and exercise are important recommendations. The American Heart Association recommends the following lifestyle interventions4:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Consider using an activity tracker to monitor activity level.
Clinicians should consider referring overweight and obese patients to a nutritionist for a consultation to plan how to consume a high-quality, low-calorie diet. A nutritionist can spend time with patients explaining options for implementing a calorie-restricted diet. In addition, some health insurers will require patients to participate in a supervised calorie-restricted diet plan for at least 6 months before authorizing coverage of expensive weight loss medications or bariatric surgery. In addition to recommending diet and exercise, ObGyns may consider prescribing metformin for their obese patients.
Continue to: Metformin...
Metformin
Metformin is approved for the treatment of type 2 diabetes mellitus. Unlike insulin therapy, which is associated with weight gain, metformin is associated with modest weight loss. The Diabetes Prevention Program (DPP) randomly assigned 3,234 nondiabetic participants with a fasting glucose level between 95 and 125 mg/dL and impaired glucose tolerance (140 to 199 mg/dL) after a 75-g oral glucose load to intensive lifestyle changes (calorie-restricted diet to achieve 7% weight loss plus 150 minutes of exercise weekly), metformin (850 mg twice daily), or placebo.5,6 The mean age of the participants was 51 years, with a mean BMI of 34 kg/m2. Most (68%) of the participants were women.
After 12 months of follow-up, mean weight loss in the intensive lifestyle change, metformin, and placebo groups was 6.5%, 2.7%, and 0.4%, respectively. After 2 years of treatment, weight loss among those who reliably took their metformin pills was approximately 4%, while participants in the placebo group had a 1% weight gain. Among those who continued to reliably take their metformin pills, the weight loss persisted through 9 years of follow up.
The mechanisms by which metformin causes weight loss are not clear. Metformin stimulates phosphorylation of adenosine monophosphate (AMP)-activated protein kinase, which regulates mitochondrial function, hepatic and muscle fatty acid oxidation, glucose transport, insulin secretion, and lipogenesis.7
Many ObGyns have experience in using metformin for the treatment of polycystic ovary syndrome or gestational diabetes. Hence, the dosing and adverse effects of metformin are familiar to many obstetricians-gynecologists. Metformin is contraindicated in individuals with creatinine clearance less than 30 mL/min. Rarely, metformin can cause lactic acidosis. According to Lexicomp,8 the most common adverse effects of metformin extended release (metformin ER) are diarrhea (17%), nausea and vomiting (7%), and decreased vitamin B12 concentration (7%) due to malabsorption in the terminal ileum. Of note, in the DPP study, hemoglobin concentration was slightly lower over time in the metformin compared with the placebo group (13.6 mg/dL vs 13.8 mg/dL, respectively; P<.001).6 Some experts recommend annual vitamin B12 measurement in individuals taking metformin.
In my practice, I only prescribe metformin ER. I usually start metformin treatment with one 750 mg ER tablet with dinner. If the patient tolerates that dose, I increase the dose to two 750 mg ER tablets with dinner. Metformin-induced adverse effects include diarrhea (17%) and nausea and vomiting (7%). Metformin ER is inexpensive. A one-month supply of metformin (sixty 750 mg tablets) costs between $4 and $21 at major pharmacies.9 Health insurance companies generally do not require preauthorization to cover metformin prescriptions.
Weight loss medications
US Food and Drug Administration (FDA)-approved weight loss medications include: liraglutide (Victoza), orlistat (Xenical, Alli), combination phentermine-extended release topiramate (Qsymia), and combination extended release naltrexone-bupropion (Contrave). All FDA-approved weight loss medications result in mean weight loss in the range of 6% to 10%. Many of these medications are very expensive (more than $200 per month).10 Insurance preauthorization is commonly required for these medications. For ObGyns, it may be best to refer patients who would like to use a weight loss medication to a specialist or specialty center with expertise in using these medications.
Sustainable weight loss is very difficult to achieve through dieting alone. A multitude of dietary interventions have been presented as “revolutionary approaches” to the challenging problem of sustainable weight loss, including the Paleo diet, the Vegan diet, the low-carb diet, the Dukan diet, the ultra-lowfat diet, the Atkins diet, the HCG diet, the Zone diet, the South Beach diet, the plant-based diet, the Mediterranean diet, the Asian diet, and intermittent fasting. Recently, intermittent fasting has been presented as the latest and greatest approach to dieting, with the dual goals of achieving weight loss and improved health.1 In some animal models, intermittent dieting has been shown to increase life-span, a finding that has attracted great interest. A major goal of intermittent fasting is to promote “metabolic switching” with increased reliance on ketones to fuel cellular energy needs.
Two approaches to “prescribing” an intermittent fasting diet are to limit food intake to a period of 6 to 10 hours each day or to markedly reduce caloric intake one or two days per week, for example to 750 calories in a 24-hour period. There are no long-term studies of the health outcomes associated with intermittent fasting. In head-to-head clinical trials of intermittent fasting and daily calorie restriction (classic dieting), both diets result in similar weight loss. For example, in one clinical trial 100 obese participants, with a mean body mass index (BMI) of 34 kg/m2 , including 86 women, were randomly assigned to2:
1. intermittent fasting (25% of energy needs every other day)
2. daily calorie restriction (75% of energy needs every day), or
3. no intervention.
After 12 months of follow up, the participants in the no intervention group had gained 0.5% of their starting weight. The intermittent fasting and the daily calorie restriction groups had similar amounts of weight loss, approximately 5% of their starting weight. More individuals dropped out of the study from the intermittent fasting group than the daily calorie restriction group (38% vs 29%, respectively).
In another clinical trial, 107 overweight or obese premenopausal women, average age 40 years and mean BMI 31 kg/m2 , were randomly assigned to intermittent fasting (25% of energy needs 2 days per week) or daily calorie restriction (75% of energy needs daily) for 6 months. The mean weight of the participants at baseline was 83 kg. Weight loss was similar in the intermittent fasting and daily calorie restriction groups, 6.4 kg (-7.7%) and 5.6 kg (-6.7%), respectively (P=.4).3
The investigators concluded that intermittent fasting and daily calorie restriction could both be offered as effective approaches to weight loss. My conclusion is that intermittent fasting is not a miracle dietary intervention, but it is another important option in the armamentarium of weight loss interventions.
References
1. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging and disease. N Engl J Med. 2019;381:2541-2551.
2. Trepanowski JF, Kroeger CM, Barnosky A, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177:930-938.
3. Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disc disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35:714-727.
Sleeve gastrectomy
Two children are playing in a school yard. One child proudly states, “My mother is an endocrinologist. She treats diabetes.” Not to be outdone, the other child replies, “My mother is a bariatric surgeon. She cures diabetes.”
The dialogue reflects the reality that bariatric surgery results in more reliable and significant weight loss than diet, exercise, or weight loss medications. Diet, exercise, and weight loss medications often result in a 5% to 10% decrease in weight, but bariatric surgery typically results in a 25% decrease in weight. Until recently, 3 bariatric surgical procedures were commonly performed: Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), and adjustable gastric banding (AGB). AGB is now seldom performed because it is less effective than RYGB and SG. Two recently published randomized trials compared the long-term outcomes associated with RYGB and SG. The studies found that SG and RYGB result in a similar degree of weight loss. RYGB resulted in slightly more weight loss than SG, but SG was associated with a lower rate of major complications, such as internal hernias. SG takes much less time to perform than RYGB. SG has become the most commonly performed bariatric surgery in premenopausal women considering pregnancy because of the low risk of internal hernias.
In the Swiss Multicenter Bypass or Sleeve Study (SM-BOSS), 217 participants with a mean BMI of 44 kg/m2 and mean age of 45.5 years were randomly assigned to RYGB or SG and followed for 5 years.11 The majority (72%) of the participants were women. At 5 years of follow-up, in the RYGB and SG groups, mean weight loss was 37 kg and 33 kg, respectively (P=.19). In both groups, weight loss nadir was reached 12 to 24 months after surgery. Expressed as a percentage of original weight, weight loss in the RYGB and SG groups was -29% and -25%, respectively (P=.02). Gastric reflux worsened in both the RYGB and SG groups (6% vs 32%, respectively). The number of reoperations in the RYGB and SG groups was 22% and 16%. Of note, among individuals with prevalent diabetes, RYGB and SG resulted in remission of the diabetes in 68% and 62% of participants, respectively.
In the Sleeve vs Bypass study (SLEEVEPASS), 240 participants, with mean BMI of 46 kg/m2 and mean age of 48 years, were randomly assigned to RYGB or SG and followed for 5 years.12 Most (70%) of the participants were women. Following bariatric surgery, BMI decreased significantly in both groups. In the RYGB group, BMI decreased from 48 kg/m2 preoperatively to 35.4 kg/m2 at 5 years of follow up. In the SG group, BMI decreased from 47 kg/m2 preoperatively to 36.5 kg/m2 at 5 years of follow up. Late major complications (defined as complications occurring from 30 days to 5 years postoperatively) occurred more frequently in the RYGB group (15%) versus the SG group (8%). All the late major complications required reoperation. In the SG group, 7 of 10 reoperations were for severe gastric reflux disease. In the RYGB group 17 of 18 reoperations were for suspected internal hernia, requiring closure of a mesenteric defect at reoperation. There was no treatment-related mortality during the 5-year follow up.
Guidelines for bariatric surgery are BMI ≥ 40 kg/m2 without a comorbid illness or BMI ≥ 35 kg/m2 with at least one serious comorbid disease, such as diabetes.13 ObGyns can build a synergistic relationship with bariatric surgeons by referring eligible patients for surgical consultation and, in return, accepting referrals. A paradox and challenge is that many health insurers require patients to complete a supervised medical weight loss management program prior to being approved for bariatric surgery. However, the medical weight loss program might result in the patient no longer being eligible for insurance coverage of their surgery. For example, a patient who had a BMI of 42 kg/m2 prior to a medical weight loss management program who then lost enough weight to achieve a BMI of 38 kg/m2 might no longer be eligible for insurance coverage of a bariatric operation.14
Continue to: ObGyns need to prioritize treatment for obesity...
ObGyns need to prioritize treatment for obesity
Between 1959 and 2014, US life expectancy increased from 69.9 years to 79.1 years. However, in 2015 and 2016 life expectancy in the United States decreased slightly to 78.9 years, while continuing to improve in other countries.15 What could cause such an unexpected trend? Some experts believe that excess overweight and obesity in the US population, resulting in increased rates of diabetes, hypertension, and heart disease, accounts for a significant proportion of the life expectancy gap between US citizens and those who reside in Australia, Finland, Japan, and Sweden.16,17 All frontline clinicians play an important role in reversing the decades-long trend of increasing rates of overweight and obesity. Interventions that ObGyns could prioritize in their practices for treating overweight and obese patients include: a calorie-restricted diet, exercise, metformin, and SG.
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
- Allison DB, Fontaine KR, Manson JE, et al. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530-1538.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440-2450.
- American Heart Association. My life check | Life’s simple 7. https://www.heart.org/en/healthyliving/healthy-lifestyle/my-life-check--lifessimple-7. Reviewed May 2, 2018. Accessed February 10, 2020.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
- Diabetes Prevention Program Research Group. Long-term safety, tolerability and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731-737.
- Winder WW, Hardie DG. Inactivation of acetylCoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270(2 pt 1):E299-E304.
- Lexicomp. https://online.lexi.com/lco/action/ home. Accessed February 13, 2020.
- Metformin ER (Glucophage XR). GoodRX website. https://www.goodrx.com/metformin-erglucophage-xr?dosage=750mg&form=tablet&la bel_override=metformin+ER+%28Glucophage+X R%29&quantity=60. Accessed February 13, 2020.
- GoodRX website. www.goodrx.com. Accessed February 10, 2020.
- Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319:255-265.
- Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy versus laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: The SLEEVEPASS randomized clinical trial. JAMA. 2018;319:241-254.
- Rubino F, Nathan DM, Eckel RH, et al; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Obes Surg. 2017;27:2-21.
- Gebran SG, Knighton B, Ngaage LM, et al. Insurance coverage criteria for bariatric surgery: a survey of policies. Obes Surg. 2020;30:707-713.
- Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
- Preston SH, Vierboom YC, Stokes A. The role of obesity in exceptionally slow US mortality improvement. Proc Natl Acad Sci U S A. 2019;115:957-961.
- Xu H, Cupples LA, Stokes A, et al. Association of obesity with mortality over 24 years of weight history: findings from the Framingham Heart Study. JAMA Network Open. 2018;1:e184587.
Obesity is a disease causing a public health crisis. In the United States, tobacco use and obesity are the two most important causes of preventable premature death. They result in an estimated 480,0001 and 300,0002 premature deaths per year, respectively. Obesity is a major contributor to diabetes mellitus, hypertension, dyslipidemia, and coronary heart disease. Obesity is also associated with increased rates of colon, breast, and endometrial cancer. Experts predict that in 2030, 50% of adults in the United States will have a body mass index (BMI) ≥ 30 kg/m2, and 25% will have a BMI ≥ 35 kg/m2.3 More women than men are predicted to be severely obese (FIGURE).3

As clinicians we need to increase our efforts to reduce the epidemic of obesity. ObGyns can play an important role in preventing and managing obesity, by recommending primary-care weight management practices, prescribing medications that influence central metabolism, and referring appropriate patients to bariatric surgery centers of excellence.
Primary-care weight management
Measuring BMI and recommending interventions to prevent and treat obesity are important components of a health maintenance encounter. For women who are overweight or obese, dietary changes and exercise are important recommendations. The American Heart Association recommends the following lifestyle interventions4:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Consider using an activity tracker to monitor activity level.
Clinicians should consider referring overweight and obese patients to a nutritionist for a consultation to plan how to consume a high-quality, low-calorie diet. A nutritionist can spend time with patients explaining options for implementing a calorie-restricted diet. In addition, some health insurers will require patients to participate in a supervised calorie-restricted diet plan for at least 6 months before authorizing coverage of expensive weight loss medications or bariatric surgery. In addition to recommending diet and exercise, ObGyns may consider prescribing metformin for their obese patients.
Continue to: Metformin...
Metformin
Metformin is approved for the treatment of type 2 diabetes mellitus. Unlike insulin therapy, which is associated with weight gain, metformin is associated with modest weight loss. The Diabetes Prevention Program (DPP) randomly assigned 3,234 nondiabetic participants with a fasting glucose level between 95 and 125 mg/dL and impaired glucose tolerance (140 to 199 mg/dL) after a 75-g oral glucose load to intensive lifestyle changes (calorie-restricted diet to achieve 7% weight loss plus 150 minutes of exercise weekly), metformin (850 mg twice daily), or placebo.5,6 The mean age of the participants was 51 years, with a mean BMI of 34 kg/m2. Most (68%) of the participants were women.
After 12 months of follow-up, mean weight loss in the intensive lifestyle change, metformin, and placebo groups was 6.5%, 2.7%, and 0.4%, respectively. After 2 years of treatment, weight loss among those who reliably took their metformin pills was approximately 4%, while participants in the placebo group had a 1% weight gain. Among those who continued to reliably take their metformin pills, the weight loss persisted through 9 years of follow up.
The mechanisms by which metformin causes weight loss are not clear. Metformin stimulates phosphorylation of adenosine monophosphate (AMP)-activated protein kinase, which regulates mitochondrial function, hepatic and muscle fatty acid oxidation, glucose transport, insulin secretion, and lipogenesis.7
Many ObGyns have experience in using metformin for the treatment of polycystic ovary syndrome or gestational diabetes. Hence, the dosing and adverse effects of metformin are familiar to many obstetricians-gynecologists. Metformin is contraindicated in individuals with creatinine clearance less than 30 mL/min. Rarely, metformin can cause lactic acidosis. According to Lexicomp,8 the most common adverse effects of metformin extended release (metformin ER) are diarrhea (17%), nausea and vomiting (7%), and decreased vitamin B12 concentration (7%) due to malabsorption in the terminal ileum. Of note, in the DPP study, hemoglobin concentration was slightly lower over time in the metformin compared with the placebo group (13.6 mg/dL vs 13.8 mg/dL, respectively; P<.001).6 Some experts recommend annual vitamin B12 measurement in individuals taking metformin.
In my practice, I only prescribe metformin ER. I usually start metformin treatment with one 750 mg ER tablet with dinner. If the patient tolerates that dose, I increase the dose to two 750 mg ER tablets with dinner. Metformin-induced adverse effects include diarrhea (17%) and nausea and vomiting (7%). Metformin ER is inexpensive. A one-month supply of metformin (sixty 750 mg tablets) costs between $4 and $21 at major pharmacies.9 Health insurance companies generally do not require preauthorization to cover metformin prescriptions.
Weight loss medications
US Food and Drug Administration (FDA)-approved weight loss medications include: liraglutide (Victoza), orlistat (Xenical, Alli), combination phentermine-extended release topiramate (Qsymia), and combination extended release naltrexone-bupropion (Contrave). All FDA-approved weight loss medications result in mean weight loss in the range of 6% to 10%. Many of these medications are very expensive (more than $200 per month).10 Insurance preauthorization is commonly required for these medications. For ObGyns, it may be best to refer patients who would like to use a weight loss medication to a specialist or specialty center with expertise in using these medications.
Sustainable weight loss is very difficult to achieve through dieting alone. A multitude of dietary interventions have been presented as “revolutionary approaches” to the challenging problem of sustainable weight loss, including the Paleo diet, the Vegan diet, the low-carb diet, the Dukan diet, the ultra-lowfat diet, the Atkins diet, the HCG diet, the Zone diet, the South Beach diet, the plant-based diet, the Mediterranean diet, the Asian diet, and intermittent fasting. Recently, intermittent fasting has been presented as the latest and greatest approach to dieting, with the dual goals of achieving weight loss and improved health.1 In some animal models, intermittent dieting has been shown to increase life-span, a finding that has attracted great interest. A major goal of intermittent fasting is to promote “metabolic switching” with increased reliance on ketones to fuel cellular energy needs.
Two approaches to “prescribing” an intermittent fasting diet are to limit food intake to a period of 6 to 10 hours each day or to markedly reduce caloric intake one or two days per week, for example to 750 calories in a 24-hour period. There are no long-term studies of the health outcomes associated with intermittent fasting. In head-to-head clinical trials of intermittent fasting and daily calorie restriction (classic dieting), both diets result in similar weight loss. For example, in one clinical trial 100 obese participants, with a mean body mass index (BMI) of 34 kg/m2 , including 86 women, were randomly assigned to2:
1. intermittent fasting (25% of energy needs every other day)
2. daily calorie restriction (75% of energy needs every day), or
3. no intervention.
After 12 months of follow up, the participants in the no intervention group had gained 0.5% of their starting weight. The intermittent fasting and the daily calorie restriction groups had similar amounts of weight loss, approximately 5% of their starting weight. More individuals dropped out of the study from the intermittent fasting group than the daily calorie restriction group (38% vs 29%, respectively).
In another clinical trial, 107 overweight or obese premenopausal women, average age 40 years and mean BMI 31 kg/m2 , were randomly assigned to intermittent fasting (25% of energy needs 2 days per week) or daily calorie restriction (75% of energy needs daily) for 6 months. The mean weight of the participants at baseline was 83 kg. Weight loss was similar in the intermittent fasting and daily calorie restriction groups, 6.4 kg (-7.7%) and 5.6 kg (-6.7%), respectively (P=.4).3
The investigators concluded that intermittent fasting and daily calorie restriction could both be offered as effective approaches to weight loss. My conclusion is that intermittent fasting is not a miracle dietary intervention, but it is another important option in the armamentarium of weight loss interventions.
References
1. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging and disease. N Engl J Med. 2019;381:2541-2551.
2. Trepanowski JF, Kroeger CM, Barnosky A, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177:930-938.
3. Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disc disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35:714-727.
Sleeve gastrectomy
Two children are playing in a school yard. One child proudly states, “My mother is an endocrinologist. She treats diabetes.” Not to be outdone, the other child replies, “My mother is a bariatric surgeon. She cures diabetes.”
The dialogue reflects the reality that bariatric surgery results in more reliable and significant weight loss than diet, exercise, or weight loss medications. Diet, exercise, and weight loss medications often result in a 5% to 10% decrease in weight, but bariatric surgery typically results in a 25% decrease in weight. Until recently, 3 bariatric surgical procedures were commonly performed: Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), and adjustable gastric banding (AGB). AGB is now seldom performed because it is less effective than RYGB and SG. Two recently published randomized trials compared the long-term outcomes associated with RYGB and SG. The studies found that SG and RYGB result in a similar degree of weight loss. RYGB resulted in slightly more weight loss than SG, but SG was associated with a lower rate of major complications, such as internal hernias. SG takes much less time to perform than RYGB. SG has become the most commonly performed bariatric surgery in premenopausal women considering pregnancy because of the low risk of internal hernias.
In the Swiss Multicenter Bypass or Sleeve Study (SM-BOSS), 217 participants with a mean BMI of 44 kg/m2 and mean age of 45.5 years were randomly assigned to RYGB or SG and followed for 5 years.11 The majority (72%) of the participants were women. At 5 years of follow-up, in the RYGB and SG groups, mean weight loss was 37 kg and 33 kg, respectively (P=.19). In both groups, weight loss nadir was reached 12 to 24 months after surgery. Expressed as a percentage of original weight, weight loss in the RYGB and SG groups was -29% and -25%, respectively (P=.02). Gastric reflux worsened in both the RYGB and SG groups (6% vs 32%, respectively). The number of reoperations in the RYGB and SG groups was 22% and 16%. Of note, among individuals with prevalent diabetes, RYGB and SG resulted in remission of the diabetes in 68% and 62% of participants, respectively.
In the Sleeve vs Bypass study (SLEEVEPASS), 240 participants, with mean BMI of 46 kg/m2 and mean age of 48 years, were randomly assigned to RYGB or SG and followed for 5 years.12 Most (70%) of the participants were women. Following bariatric surgery, BMI decreased significantly in both groups. In the RYGB group, BMI decreased from 48 kg/m2 preoperatively to 35.4 kg/m2 at 5 years of follow up. In the SG group, BMI decreased from 47 kg/m2 preoperatively to 36.5 kg/m2 at 5 years of follow up. Late major complications (defined as complications occurring from 30 days to 5 years postoperatively) occurred more frequently in the RYGB group (15%) versus the SG group (8%). All the late major complications required reoperation. In the SG group, 7 of 10 reoperations were for severe gastric reflux disease. In the RYGB group 17 of 18 reoperations were for suspected internal hernia, requiring closure of a mesenteric defect at reoperation. There was no treatment-related mortality during the 5-year follow up.
Guidelines for bariatric surgery are BMI ≥ 40 kg/m2 without a comorbid illness or BMI ≥ 35 kg/m2 with at least one serious comorbid disease, such as diabetes.13 ObGyns can build a synergistic relationship with bariatric surgeons by referring eligible patients for surgical consultation and, in return, accepting referrals. A paradox and challenge is that many health insurers require patients to complete a supervised medical weight loss management program prior to being approved for bariatric surgery. However, the medical weight loss program might result in the patient no longer being eligible for insurance coverage of their surgery. For example, a patient who had a BMI of 42 kg/m2 prior to a medical weight loss management program who then lost enough weight to achieve a BMI of 38 kg/m2 might no longer be eligible for insurance coverage of a bariatric operation.14
Continue to: ObGyns need to prioritize treatment for obesity...
ObGyns need to prioritize treatment for obesity
Between 1959 and 2014, US life expectancy increased from 69.9 years to 79.1 years. However, in 2015 and 2016 life expectancy in the United States decreased slightly to 78.9 years, while continuing to improve in other countries.15 What could cause such an unexpected trend? Some experts believe that excess overweight and obesity in the US population, resulting in increased rates of diabetes, hypertension, and heart disease, accounts for a significant proportion of the life expectancy gap between US citizens and those who reside in Australia, Finland, Japan, and Sweden.16,17 All frontline clinicians play an important role in reversing the decades-long trend of increasing rates of overweight and obesity. Interventions that ObGyns could prioritize in their practices for treating overweight and obese patients include: a calorie-restricted diet, exercise, metformin, and SG.
Obesity is a disease causing a public health crisis. In the United States, tobacco use and obesity are the two most important causes of preventable premature death. They result in an estimated 480,0001 and 300,0002 premature deaths per year, respectively. Obesity is a major contributor to diabetes mellitus, hypertension, dyslipidemia, and coronary heart disease. Obesity is also associated with increased rates of colon, breast, and endometrial cancer. Experts predict that in 2030, 50% of adults in the United States will have a body mass index (BMI) ≥ 30 kg/m2, and 25% will have a BMI ≥ 35 kg/m2.3 More women than men are predicted to be severely obese (FIGURE).3

As clinicians we need to increase our efforts to reduce the epidemic of obesity. ObGyns can play an important role in preventing and managing obesity, by recommending primary-care weight management practices, prescribing medications that influence central metabolism, and referring appropriate patients to bariatric surgery centers of excellence.
Primary-care weight management
Measuring BMI and recommending interventions to prevent and treat obesity are important components of a health maintenance encounter. For women who are overweight or obese, dietary changes and exercise are important recommendations. The American Heart Association recommends the following lifestyle interventions4:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Consider using an activity tracker to monitor activity level.
Clinicians should consider referring overweight and obese patients to a nutritionist for a consultation to plan how to consume a high-quality, low-calorie diet. A nutritionist can spend time with patients explaining options for implementing a calorie-restricted diet. In addition, some health insurers will require patients to participate in a supervised calorie-restricted diet plan for at least 6 months before authorizing coverage of expensive weight loss medications or bariatric surgery. In addition to recommending diet and exercise, ObGyns may consider prescribing metformin for their obese patients.
Continue to: Metformin...
Metformin
Metformin is approved for the treatment of type 2 diabetes mellitus. Unlike insulin therapy, which is associated with weight gain, metformin is associated with modest weight loss. The Diabetes Prevention Program (DPP) randomly assigned 3,234 nondiabetic participants with a fasting glucose level between 95 and 125 mg/dL and impaired glucose tolerance (140 to 199 mg/dL) after a 75-g oral glucose load to intensive lifestyle changes (calorie-restricted diet to achieve 7% weight loss plus 150 minutes of exercise weekly), metformin (850 mg twice daily), or placebo.5,6 The mean age of the participants was 51 years, with a mean BMI of 34 kg/m2. Most (68%) of the participants were women.
After 12 months of follow-up, mean weight loss in the intensive lifestyle change, metformin, and placebo groups was 6.5%, 2.7%, and 0.4%, respectively. After 2 years of treatment, weight loss among those who reliably took their metformin pills was approximately 4%, while participants in the placebo group had a 1% weight gain. Among those who continued to reliably take their metformin pills, the weight loss persisted through 9 years of follow up.
The mechanisms by which metformin causes weight loss are not clear. Metformin stimulates phosphorylation of adenosine monophosphate (AMP)-activated protein kinase, which regulates mitochondrial function, hepatic and muscle fatty acid oxidation, glucose transport, insulin secretion, and lipogenesis.7
Many ObGyns have experience in using metformin for the treatment of polycystic ovary syndrome or gestational diabetes. Hence, the dosing and adverse effects of metformin are familiar to many obstetricians-gynecologists. Metformin is contraindicated in individuals with creatinine clearance less than 30 mL/min. Rarely, metformin can cause lactic acidosis. According to Lexicomp,8 the most common adverse effects of metformin extended release (metformin ER) are diarrhea (17%), nausea and vomiting (7%), and decreased vitamin B12 concentration (7%) due to malabsorption in the terminal ileum. Of note, in the DPP study, hemoglobin concentration was slightly lower over time in the metformin compared with the placebo group (13.6 mg/dL vs 13.8 mg/dL, respectively; P<.001).6 Some experts recommend annual vitamin B12 measurement in individuals taking metformin.
In my practice, I only prescribe metformin ER. I usually start metformin treatment with one 750 mg ER tablet with dinner. If the patient tolerates that dose, I increase the dose to two 750 mg ER tablets with dinner. Metformin-induced adverse effects include diarrhea (17%) and nausea and vomiting (7%). Metformin ER is inexpensive. A one-month supply of metformin (sixty 750 mg tablets) costs between $4 and $21 at major pharmacies.9 Health insurance companies generally do not require preauthorization to cover metformin prescriptions.
Weight loss medications
US Food and Drug Administration (FDA)-approved weight loss medications include: liraglutide (Victoza), orlistat (Xenical, Alli), combination phentermine-extended release topiramate (Qsymia), and combination extended release naltrexone-bupropion (Contrave). All FDA-approved weight loss medications result in mean weight loss in the range of 6% to 10%. Many of these medications are very expensive (more than $200 per month).10 Insurance preauthorization is commonly required for these medications. For ObGyns, it may be best to refer patients who would like to use a weight loss medication to a specialist or specialty center with expertise in using these medications.
Sustainable weight loss is very difficult to achieve through dieting alone. A multitude of dietary interventions have been presented as “revolutionary approaches” to the challenging problem of sustainable weight loss, including the Paleo diet, the Vegan diet, the low-carb diet, the Dukan diet, the ultra-lowfat diet, the Atkins diet, the HCG diet, the Zone diet, the South Beach diet, the plant-based diet, the Mediterranean diet, the Asian diet, and intermittent fasting. Recently, intermittent fasting has been presented as the latest and greatest approach to dieting, with the dual goals of achieving weight loss and improved health.1 In some animal models, intermittent dieting has been shown to increase life-span, a finding that has attracted great interest. A major goal of intermittent fasting is to promote “metabolic switching” with increased reliance on ketones to fuel cellular energy needs.
Two approaches to “prescribing” an intermittent fasting diet are to limit food intake to a period of 6 to 10 hours each day or to markedly reduce caloric intake one or two days per week, for example to 750 calories in a 24-hour period. There are no long-term studies of the health outcomes associated with intermittent fasting. In head-to-head clinical trials of intermittent fasting and daily calorie restriction (classic dieting), both diets result in similar weight loss. For example, in one clinical trial 100 obese participants, with a mean body mass index (BMI) of 34 kg/m2 , including 86 women, were randomly assigned to2:
1. intermittent fasting (25% of energy needs every other day)
2. daily calorie restriction (75% of energy needs every day), or
3. no intervention.
After 12 months of follow up, the participants in the no intervention group had gained 0.5% of their starting weight. The intermittent fasting and the daily calorie restriction groups had similar amounts of weight loss, approximately 5% of their starting weight. More individuals dropped out of the study from the intermittent fasting group than the daily calorie restriction group (38% vs 29%, respectively).
In another clinical trial, 107 overweight or obese premenopausal women, average age 40 years and mean BMI 31 kg/m2 , were randomly assigned to intermittent fasting (25% of energy needs 2 days per week) or daily calorie restriction (75% of energy needs daily) for 6 months. The mean weight of the participants at baseline was 83 kg. Weight loss was similar in the intermittent fasting and daily calorie restriction groups, 6.4 kg (-7.7%) and 5.6 kg (-6.7%), respectively (P=.4).3
The investigators concluded that intermittent fasting and daily calorie restriction could both be offered as effective approaches to weight loss. My conclusion is that intermittent fasting is not a miracle dietary intervention, but it is another important option in the armamentarium of weight loss interventions.
References
1. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging and disease. N Engl J Med. 2019;381:2541-2551.
2. Trepanowski JF, Kroeger CM, Barnosky A, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177:930-938.
3. Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disc disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35:714-727.
Sleeve gastrectomy
Two children are playing in a school yard. One child proudly states, “My mother is an endocrinologist. She treats diabetes.” Not to be outdone, the other child replies, “My mother is a bariatric surgeon. She cures diabetes.”
The dialogue reflects the reality that bariatric surgery results in more reliable and significant weight loss than diet, exercise, or weight loss medications. Diet, exercise, and weight loss medications often result in a 5% to 10% decrease in weight, but bariatric surgery typically results in a 25% decrease in weight. Until recently, 3 bariatric surgical procedures were commonly performed: Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), and adjustable gastric banding (AGB). AGB is now seldom performed because it is less effective than RYGB and SG. Two recently published randomized trials compared the long-term outcomes associated with RYGB and SG. The studies found that SG and RYGB result in a similar degree of weight loss. RYGB resulted in slightly more weight loss than SG, but SG was associated with a lower rate of major complications, such as internal hernias. SG takes much less time to perform than RYGB. SG has become the most commonly performed bariatric surgery in premenopausal women considering pregnancy because of the low risk of internal hernias.
In the Swiss Multicenter Bypass or Sleeve Study (SM-BOSS), 217 participants with a mean BMI of 44 kg/m2 and mean age of 45.5 years were randomly assigned to RYGB or SG and followed for 5 years.11 The majority (72%) of the participants were women. At 5 years of follow-up, in the RYGB and SG groups, mean weight loss was 37 kg and 33 kg, respectively (P=.19). In both groups, weight loss nadir was reached 12 to 24 months after surgery. Expressed as a percentage of original weight, weight loss in the RYGB and SG groups was -29% and -25%, respectively (P=.02). Gastric reflux worsened in both the RYGB and SG groups (6% vs 32%, respectively). The number of reoperations in the RYGB and SG groups was 22% and 16%. Of note, among individuals with prevalent diabetes, RYGB and SG resulted in remission of the diabetes in 68% and 62% of participants, respectively.
In the Sleeve vs Bypass study (SLEEVEPASS), 240 participants, with mean BMI of 46 kg/m2 and mean age of 48 years, were randomly assigned to RYGB or SG and followed for 5 years.12 Most (70%) of the participants were women. Following bariatric surgery, BMI decreased significantly in both groups. In the RYGB group, BMI decreased from 48 kg/m2 preoperatively to 35.4 kg/m2 at 5 years of follow up. In the SG group, BMI decreased from 47 kg/m2 preoperatively to 36.5 kg/m2 at 5 years of follow up. Late major complications (defined as complications occurring from 30 days to 5 years postoperatively) occurred more frequently in the RYGB group (15%) versus the SG group (8%). All the late major complications required reoperation. In the SG group, 7 of 10 reoperations were for severe gastric reflux disease. In the RYGB group 17 of 18 reoperations were for suspected internal hernia, requiring closure of a mesenteric defect at reoperation. There was no treatment-related mortality during the 5-year follow up.
Guidelines for bariatric surgery are BMI ≥ 40 kg/m2 without a comorbid illness or BMI ≥ 35 kg/m2 with at least one serious comorbid disease, such as diabetes.13 ObGyns can build a synergistic relationship with bariatric surgeons by referring eligible patients for surgical consultation and, in return, accepting referrals. A paradox and challenge is that many health insurers require patients to complete a supervised medical weight loss management program prior to being approved for bariatric surgery. However, the medical weight loss program might result in the patient no longer being eligible for insurance coverage of their surgery. For example, a patient who had a BMI of 42 kg/m2 prior to a medical weight loss management program who then lost enough weight to achieve a BMI of 38 kg/m2 might no longer be eligible for insurance coverage of a bariatric operation.14
Continue to: ObGyns need to prioritize treatment for obesity...
ObGyns need to prioritize treatment for obesity
Between 1959 and 2014, US life expectancy increased from 69.9 years to 79.1 years. However, in 2015 and 2016 life expectancy in the United States decreased slightly to 78.9 years, while continuing to improve in other countries.15 What could cause such an unexpected trend? Some experts believe that excess overweight and obesity in the US population, resulting in increased rates of diabetes, hypertension, and heart disease, accounts for a significant proportion of the life expectancy gap between US citizens and those who reside in Australia, Finland, Japan, and Sweden.16,17 All frontline clinicians play an important role in reversing the decades-long trend of increasing rates of overweight and obesity. Interventions that ObGyns could prioritize in their practices for treating overweight and obese patients include: a calorie-restricted diet, exercise, metformin, and SG.
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
- Allison DB, Fontaine KR, Manson JE, et al. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530-1538.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440-2450.
- American Heart Association. My life check | Life’s simple 7. https://www.heart.org/en/healthyliving/healthy-lifestyle/my-life-check--lifessimple-7. Reviewed May 2, 2018. Accessed February 10, 2020.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
- Diabetes Prevention Program Research Group. Long-term safety, tolerability and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731-737.
- Winder WW, Hardie DG. Inactivation of acetylCoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270(2 pt 1):E299-E304.
- Lexicomp. https://online.lexi.com/lco/action/ home. Accessed February 13, 2020.
- Metformin ER (Glucophage XR). GoodRX website. https://www.goodrx.com/metformin-erglucophage-xr?dosage=750mg&form=tablet&la bel_override=metformin+ER+%28Glucophage+X R%29&quantity=60. Accessed February 13, 2020.
- GoodRX website. www.goodrx.com. Accessed February 10, 2020.
- Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319:255-265.
- Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy versus laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: The SLEEVEPASS randomized clinical trial. JAMA. 2018;319:241-254.
- Rubino F, Nathan DM, Eckel RH, et al; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Obes Surg. 2017;27:2-21.
- Gebran SG, Knighton B, Ngaage LM, et al. Insurance coverage criteria for bariatric surgery: a survey of policies. Obes Surg. 2020;30:707-713.
- Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
- Preston SH, Vierboom YC, Stokes A. The role of obesity in exceptionally slow US mortality improvement. Proc Natl Acad Sci U S A. 2019;115:957-961.
- Xu H, Cupples LA, Stokes A, et al. Association of obesity with mortality over 24 years of weight history: findings from the Framingham Heart Study. JAMA Network Open. 2018;1:e184587.
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
- Allison DB, Fontaine KR, Manson JE, et al. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530-1538.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440-2450.
- American Heart Association. My life check | Life’s simple 7. https://www.heart.org/en/healthyliving/healthy-lifestyle/my-life-check--lifessimple-7. Reviewed May 2, 2018. Accessed February 10, 2020.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
- Diabetes Prevention Program Research Group. Long-term safety, tolerability and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731-737.
- Winder WW, Hardie DG. Inactivation of acetylCoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270(2 pt 1):E299-E304.
- Lexicomp. https://online.lexi.com/lco/action/ home. Accessed February 13, 2020.
- Metformin ER (Glucophage XR). GoodRX website. https://www.goodrx.com/metformin-erglucophage-xr?dosage=750mg&form=tablet&la bel_override=metformin+ER+%28Glucophage+X R%29&quantity=60. Accessed February 13, 2020.
- GoodRX website. www.goodrx.com. Accessed February 10, 2020.
- Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319:255-265.
- Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy versus laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: The SLEEVEPASS randomized clinical trial. JAMA. 2018;319:241-254.
- Rubino F, Nathan DM, Eckel RH, et al; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Obes Surg. 2017;27:2-21.
- Gebran SG, Knighton B, Ngaage LM, et al. Insurance coverage criteria for bariatric surgery: a survey of policies. Obes Surg. 2020;30:707-713.
- Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
- Preston SH, Vierboom YC, Stokes A. The role of obesity in exceptionally slow US mortality improvement. Proc Natl Acad Sci U S A. 2019;115:957-961.
- Xu H, Cupples LA, Stokes A, et al. Association of obesity with mortality over 24 years of weight history: findings from the Framingham Heart Study. JAMA Network Open. 2018;1:e184587.
Progestin-only systemic hormone therapy for menopausal hot flashes
The field of menopause medicine is dominated by studies documenting the effectiveness of systemic estrogen or estrogen-progestin hormone therapy for the treatment of hot flashes caused by hypoestrogenism. The effectiveness of progestin-only systemic hormone therapy for the treatment of hot flashes is much less studied and seldom is utilized in clinical practice. A small number of studies have reported that progestins, including micronized progesterone, medroxyprogesterone acetate, and norethindrone acetate, are effective treatment for hot flashes. Progestin-only systemic hormone therapy might be especially helpful for postmenopausal women with moderate to severe hot flashes who have a contraindication to estrogen treatment.
Micronized progesterone
Micronized progesterone (Prometrium) 300 mg daily taken at bedtime has been reported to effectively treat hot flashes in postmenopausal women. In one study, 133 postmenopausal women with an average age of 55 years and approximately 3 years from their last menstrual period were randomly assigned to 12 weeks of treatment with placebo or micronized progesterone 300 mg daily taken at bedtime.1 Mean serum progesterone levels were 0.28 ng/mL (0.89 nM) and 27 ng/mL (86 nM) in the women taking placebo and micronized progesterone, respectively. Compared with placebo, micronized progesterone reduced daytime and nighttime hot flash frequency and severity. In addition, compared with placebo, micronized progesterone improved the quality of sleep.1
Most reviews conclude that micronized progesterone has minimal cardiovascular risk.2 Micronized progesterone therapy might be especially helpful for postmenopausal women with moderate to severe hot flashes who have a contraindication to estrogen treatment such as those at increased risk for cardiovascular disease or women with a thrombophilia. Many experts believe that systemic estrogen therapy is contraindicated in postmenopausal women with an American Heart Association risk score greater than 10% over 10 years.3 Additional contraindications to systemic estrogen include women with cardiac disease who have a thrombophilia, such as the Factor V Leiden mutation.4
For women who are at high risk for estrogen-induced cardiovascular events, micronized progesterone may be a better option than systemic estrogen for treating hot flashes. Alternatively, in these women at risk of cardiovascular disease a selective serotonin reuptake inhibitor, such as escitalopram, 10 mg to 20 mg daily, may be a good option for treating postmenopausal hot flashes.5
Medroxyprogesterone acetate
Medroxyprogesterone acetate, at a dosage of 20 mg daily, is an effective treatment for hot flashes. In a randomized clinical trial 27 postmenopausal women with hot flashes were randomly assigned to treatment with placebo or medroxyprogesterone acetate 20 mg daily for 4 weeks. Vasomotor flushes were decreased by 26% and 74% in the placebo and medroxyprogesterone groups, respectively.6
Depot medroxyprogesterone acetate injections at dosages from 150 mg to 400 mg also have been reported to effectively treat hot flashes.7,8 In a trial comparing the effectiveness of estrogen monotherapy (conjugated equine estrogen 0.6 mg daily) with progestin monotherapy (medroxyprogesterone acetate 10 mg daily), both treatments were equally effective in reducing hot flashes.9
Continue to: Micronized progesterone vs medroxyprogesterone acetate...
Micronized progesterone vs medroxyprogesterone acetate
Experts in menopause medicine have suggested that in postmenopausal women micronized progesterone has a better pattern of benefits and fewer risks than medroxyprogesterone acetate.10,11 For example, in the E3N observational study of hormones and breast cancer risk, among 80,377 French postmenopausal women followed for a mean of 8 years, the combination of transdermal estradiol plus oral micronized progesterone was associated with no significantly increased risk of breast cancer (relative risk [RR], 1.08, 95% confidence interval [CI], 0.89–1.31) compared with never users of postmenopausal hormone therapy.12 By contrast, the combination of oral estrogen plus medroxyprogesterone acetate was associated with an increased risk of breast cancer (RR, 1.48; 95% CI, 1.02–2.16) compared with never users of postmenopausal hormone therapy. The E3N study indicates that micronized progesterone may have a more favorable breast health profile than medroxyprogesterone acetate.12

Norethindrone acetate
Norethindrone acetate monotherapy is not commonly prescribed for the treatment of menopausal hot flashes. However, a large clinical trial has demonstrated that norethindrone acetate effectively suppresses hot flashes in women with endometriosis treated with depot leuprolide acetate (LA). In one trial 201 women with endometriosis were randomly assigned to 12 months of treatment with13:
- LA plus placebo pills
- LA plus norethindrone acetate (NEA) 5 mg daily
- LA plus NEA 5 mg daily plus conjugated equine estrogen (CEE) 0.625 mg daily, or
- LA plus NEA 5 mg daily plus CEE 1.25 mg daily.
The median number of hot flashes in 24 hours was 6 in the LA plus placebo group and 0 in both the LA plus NEA 5 mg daily group and the LA plus NEA 5 mg plus CEE 1.25 mg daily group. This study demonstrates that NEA 5 mg daily is an effective treatment for hot flashes.
In the same study, LA plus placebo was associated with a significant decrease in lumbar spine bone mineral density. No significant decrease in bone mineral density was observed in the women who received LA plus NEA 5 mg daily. This finding indicates that NEA 5 mg reduces bone absorption caused by hypoestrogenism. In humans, norethindrone is a substrate for the aromatase enzyme system.14 Small quantities of ethinyl estradiol may be formed by aromatization of norethindrone in vivo,15,16 contributing to the effectiveness of NEA in suppressing hot flashes and preserving bone density.
Progestin: The estrogen alternative to hot flashes
For postmenopausal women with moderate to severe hot flashes, estrogen treatment reliably suppresses hot flashes and often improves sleep quality and mood. For postmenopausal women with a contraindication to estrogen treatment, progestin-only treatment with micronized progesterone or norethindrone acetate may be an effective option.
- Hitchcock CL, Prior JC. Oral micronized progesterone for vasomotor symptoms—a placebo-controlled randomized trial in healthy postmenopausal women. Menopause. 2012;19:886-893.
- Spark MJ, Willis J. Systematic review of progesterone use by midlife menopausal women. Maturitas 2012; 72: 192-202.
- Manson JE, Ames JM, Shapiro M, et al. Algorithm and mobile app for menopausal symptom management and hormonal/nonhormonal therapy decision making: a clinical decision-suport tool from The North American Menopause Society. Menopause. 2015;22:247-253.
- Herrington DM, Vittinghoff E, Howard TD, et al. Factor V Leiden, hormone replacement therapy, and risk of venous thromboembolic events in women with coronary disease. Arterioscler Thromb Vasc Biol. 2002;22:1012-1017.
- Ensrud KE, Joffe H, Guthrie KA, et al. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy perimenopausal and postmenopausal women with hot flashes: a randomized controlled trial. Menopause. 2012;19:848-855.
- Schiff I, Tulchinsky D, Cramer D, et al. Oral medroxyprogesterone in the treatment of postmenopausal symptoms. JAMA. 1980;244:1443-1445.
- Bullock JL, Massey FM, Gambrell RD Jr. Use of medroxyprogesterone acetate to prevent menopausal symptoms. Obstet Gynecol. 1975;46:165-168.
- Loprinzi CL, Levitt R, Barton D, et al. Phase III comparison of depot medroxyprogesterone acetate to venlafaxine for managing hot flashes: North Central Cancer Treatment Group Trial N99C7. J Clin Oncol. 2006;24:1409-1414.
- Prior JC, Nielsen JD, Hitchcock CL, et al. Medroxyprogesterone and conjugated oestrogen are equivalent for hot flushes: 1-year randomized double-blind trial following premenopausal ovariectomy. Clin Sci (Lond). 2007;112:517-525.
- L’hermite M, Simoncini T, Fuller S, et al. Could transdermal estradiol + progesterone be a safer postmenopausal HRT? A review. Maturitas. 2008;60:185-201.
- Simon JA. What if the Women’s Health Initiative had used transdermal estradiol and oral progesterone instead? Menopause. 2014;21:769-783.
- Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103-111.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Barbieri RL, Canick JA, Ryan KJ. High-affinity steroid binding to rat testis 17 alpha-hydroxylase and human placental aromatase. J Steroid Biochem. 1981;14:387-393.
- Chu MC, Zhang X, Gentzschein E, et al. Formation of ethinyl estradiol in women during treatment with norethindrone acetate. J Clin Endocrinol Metab. 2007;92:2205-2207.
- Chwalisz K, Surrey E, Stanczyk FZ. The hormonal profile of norethindrone acetate: rationale for add-back therapy with gonadotropin-releasing hormone agonists in women with endometriosis. Reprod Sci. 2012;19:563-571.
The field of menopause medicine is dominated by studies documenting the effectiveness of systemic estrogen or estrogen-progestin hormone therapy for the treatment of hot flashes caused by hypoestrogenism. The effectiveness of progestin-only systemic hormone therapy for the treatment of hot flashes is much less studied and seldom is utilized in clinical practice. A small number of studies have reported that progestins, including micronized progesterone, medroxyprogesterone acetate, and norethindrone acetate, are effective treatment for hot flashes. Progestin-only systemic hormone therapy might be especially helpful for postmenopausal women with moderate to severe hot flashes who have a contraindication to estrogen treatment.
Micronized progesterone
Micronized progesterone (Prometrium) 300 mg daily taken at bedtime has been reported to effectively treat hot flashes in postmenopausal women. In one study, 133 postmenopausal women with an average age of 55 years and approximately 3 years from their last menstrual period were randomly assigned to 12 weeks of treatment with placebo or micronized progesterone 300 mg daily taken at bedtime.1 Mean serum progesterone levels were 0.28 ng/mL (0.89 nM) and 27 ng/mL (86 nM) in the women taking placebo and micronized progesterone, respectively. Compared with placebo, micronized progesterone reduced daytime and nighttime hot flash frequency and severity. In addition, compared with placebo, micronized progesterone improved the quality of sleep.1
Most reviews conclude that micronized progesterone has minimal cardiovascular risk.2 Micronized progesterone therapy might be especially helpful for postmenopausal women with moderate to severe hot flashes who have a contraindication to estrogen treatment such as those at increased risk for cardiovascular disease or women with a thrombophilia. Many experts believe that systemic estrogen therapy is contraindicated in postmenopausal women with an American Heart Association risk score greater than 10% over 10 years.3 Additional contraindications to systemic estrogen include women with cardiac disease who have a thrombophilia, such as the Factor V Leiden mutation.4
For women who are at high risk for estrogen-induced cardiovascular events, micronized progesterone may be a better option than systemic estrogen for treating hot flashes. Alternatively, in these women at risk of cardiovascular disease a selective serotonin reuptake inhibitor, such as escitalopram, 10 mg to 20 mg daily, may be a good option for treating postmenopausal hot flashes.5
Medroxyprogesterone acetate
Medroxyprogesterone acetate, at a dosage of 20 mg daily, is an effective treatment for hot flashes. In a randomized clinical trial 27 postmenopausal women with hot flashes were randomly assigned to treatment with placebo or medroxyprogesterone acetate 20 mg daily for 4 weeks. Vasomotor flushes were decreased by 26% and 74% in the placebo and medroxyprogesterone groups, respectively.6
Depot medroxyprogesterone acetate injections at dosages from 150 mg to 400 mg also have been reported to effectively treat hot flashes.7,8 In a trial comparing the effectiveness of estrogen monotherapy (conjugated equine estrogen 0.6 mg daily) with progestin monotherapy (medroxyprogesterone acetate 10 mg daily), both treatments were equally effective in reducing hot flashes.9
Continue to: Micronized progesterone vs medroxyprogesterone acetate...
Micronized progesterone vs medroxyprogesterone acetate
Experts in menopause medicine have suggested that in postmenopausal women micronized progesterone has a better pattern of benefits and fewer risks than medroxyprogesterone acetate.10,11 For example, in the E3N observational study of hormones and breast cancer risk, among 80,377 French postmenopausal women followed for a mean of 8 years, the combination of transdermal estradiol plus oral micronized progesterone was associated with no significantly increased risk of breast cancer (relative risk [RR], 1.08, 95% confidence interval [CI], 0.89–1.31) compared with never users of postmenopausal hormone therapy.12 By contrast, the combination of oral estrogen plus medroxyprogesterone acetate was associated with an increased risk of breast cancer (RR, 1.48; 95% CI, 1.02–2.16) compared with never users of postmenopausal hormone therapy. The E3N study indicates that micronized progesterone may have a more favorable breast health profile than medroxyprogesterone acetate.12

Norethindrone acetate
Norethindrone acetate monotherapy is not commonly prescribed for the treatment of menopausal hot flashes. However, a large clinical trial has demonstrated that norethindrone acetate effectively suppresses hot flashes in women with endometriosis treated with depot leuprolide acetate (LA). In one trial 201 women with endometriosis were randomly assigned to 12 months of treatment with13:
- LA plus placebo pills
- LA plus norethindrone acetate (NEA) 5 mg daily
- LA plus NEA 5 mg daily plus conjugated equine estrogen (CEE) 0.625 mg daily, or
- LA plus NEA 5 mg daily plus CEE 1.25 mg daily.
The median number of hot flashes in 24 hours was 6 in the LA plus placebo group and 0 in both the LA plus NEA 5 mg daily group and the LA plus NEA 5 mg plus CEE 1.25 mg daily group. This study demonstrates that NEA 5 mg daily is an effective treatment for hot flashes.
In the same study, LA plus placebo was associated with a significant decrease in lumbar spine bone mineral density. No significant decrease in bone mineral density was observed in the women who received LA plus NEA 5 mg daily. This finding indicates that NEA 5 mg reduces bone absorption caused by hypoestrogenism. In humans, norethindrone is a substrate for the aromatase enzyme system.14 Small quantities of ethinyl estradiol may be formed by aromatization of norethindrone in vivo,15,16 contributing to the effectiveness of NEA in suppressing hot flashes and preserving bone density.
Progestin: The estrogen alternative to hot flashes
For postmenopausal women with moderate to severe hot flashes, estrogen treatment reliably suppresses hot flashes and often improves sleep quality and mood. For postmenopausal women with a contraindication to estrogen treatment, progestin-only treatment with micronized progesterone or norethindrone acetate may be an effective option.
The field of menopause medicine is dominated by studies documenting the effectiveness of systemic estrogen or estrogen-progestin hormone therapy for the treatment of hot flashes caused by hypoestrogenism. The effectiveness of progestin-only systemic hormone therapy for the treatment of hot flashes is much less studied and seldom is utilized in clinical practice. A small number of studies have reported that progestins, including micronized progesterone, medroxyprogesterone acetate, and norethindrone acetate, are effective treatment for hot flashes. Progestin-only systemic hormone therapy might be especially helpful for postmenopausal women with moderate to severe hot flashes who have a contraindication to estrogen treatment.
Micronized progesterone
Micronized progesterone (Prometrium) 300 mg daily taken at bedtime has been reported to effectively treat hot flashes in postmenopausal women. In one study, 133 postmenopausal women with an average age of 55 years and approximately 3 years from their last menstrual period were randomly assigned to 12 weeks of treatment with placebo or micronized progesterone 300 mg daily taken at bedtime.1 Mean serum progesterone levels were 0.28 ng/mL (0.89 nM) and 27 ng/mL (86 nM) in the women taking placebo and micronized progesterone, respectively. Compared with placebo, micronized progesterone reduced daytime and nighttime hot flash frequency and severity. In addition, compared with placebo, micronized progesterone improved the quality of sleep.1
Most reviews conclude that micronized progesterone has minimal cardiovascular risk.2 Micronized progesterone therapy might be especially helpful for postmenopausal women with moderate to severe hot flashes who have a contraindication to estrogen treatment such as those at increased risk for cardiovascular disease or women with a thrombophilia. Many experts believe that systemic estrogen therapy is contraindicated in postmenopausal women with an American Heart Association risk score greater than 10% over 10 years.3 Additional contraindications to systemic estrogen include women with cardiac disease who have a thrombophilia, such as the Factor V Leiden mutation.4
For women who are at high risk for estrogen-induced cardiovascular events, micronized progesterone may be a better option than systemic estrogen for treating hot flashes. Alternatively, in these women at risk of cardiovascular disease a selective serotonin reuptake inhibitor, such as escitalopram, 10 mg to 20 mg daily, may be a good option for treating postmenopausal hot flashes.5
Medroxyprogesterone acetate
Medroxyprogesterone acetate, at a dosage of 20 mg daily, is an effective treatment for hot flashes. In a randomized clinical trial 27 postmenopausal women with hot flashes were randomly assigned to treatment with placebo or medroxyprogesterone acetate 20 mg daily for 4 weeks. Vasomotor flushes were decreased by 26% and 74% in the placebo and medroxyprogesterone groups, respectively.6
Depot medroxyprogesterone acetate injections at dosages from 150 mg to 400 mg also have been reported to effectively treat hot flashes.7,8 In a trial comparing the effectiveness of estrogen monotherapy (conjugated equine estrogen 0.6 mg daily) with progestin monotherapy (medroxyprogesterone acetate 10 mg daily), both treatments were equally effective in reducing hot flashes.9
Continue to: Micronized progesterone vs medroxyprogesterone acetate...
Micronized progesterone vs medroxyprogesterone acetate
Experts in menopause medicine have suggested that in postmenopausal women micronized progesterone has a better pattern of benefits and fewer risks than medroxyprogesterone acetate.10,11 For example, in the E3N observational study of hormones and breast cancer risk, among 80,377 French postmenopausal women followed for a mean of 8 years, the combination of transdermal estradiol plus oral micronized progesterone was associated with no significantly increased risk of breast cancer (relative risk [RR], 1.08, 95% confidence interval [CI], 0.89–1.31) compared with never users of postmenopausal hormone therapy.12 By contrast, the combination of oral estrogen plus medroxyprogesterone acetate was associated with an increased risk of breast cancer (RR, 1.48; 95% CI, 1.02–2.16) compared with never users of postmenopausal hormone therapy. The E3N study indicates that micronized progesterone may have a more favorable breast health profile than medroxyprogesterone acetate.12

Norethindrone acetate
Norethindrone acetate monotherapy is not commonly prescribed for the treatment of menopausal hot flashes. However, a large clinical trial has demonstrated that norethindrone acetate effectively suppresses hot flashes in women with endometriosis treated with depot leuprolide acetate (LA). In one trial 201 women with endometriosis were randomly assigned to 12 months of treatment with13:
- LA plus placebo pills
- LA plus norethindrone acetate (NEA) 5 mg daily
- LA plus NEA 5 mg daily plus conjugated equine estrogen (CEE) 0.625 mg daily, or
- LA plus NEA 5 mg daily plus CEE 1.25 mg daily.
The median number of hot flashes in 24 hours was 6 in the LA plus placebo group and 0 in both the LA plus NEA 5 mg daily group and the LA plus NEA 5 mg plus CEE 1.25 mg daily group. This study demonstrates that NEA 5 mg daily is an effective treatment for hot flashes.
In the same study, LA plus placebo was associated with a significant decrease in lumbar spine bone mineral density. No significant decrease in bone mineral density was observed in the women who received LA plus NEA 5 mg daily. This finding indicates that NEA 5 mg reduces bone absorption caused by hypoestrogenism. In humans, norethindrone is a substrate for the aromatase enzyme system.14 Small quantities of ethinyl estradiol may be formed by aromatization of norethindrone in vivo,15,16 contributing to the effectiveness of NEA in suppressing hot flashes and preserving bone density.
Progestin: The estrogen alternative to hot flashes
For postmenopausal women with moderate to severe hot flashes, estrogen treatment reliably suppresses hot flashes and often improves sleep quality and mood. For postmenopausal women with a contraindication to estrogen treatment, progestin-only treatment with micronized progesterone or norethindrone acetate may be an effective option.
- Hitchcock CL, Prior JC. Oral micronized progesterone for vasomotor symptoms—a placebo-controlled randomized trial in healthy postmenopausal women. Menopause. 2012;19:886-893.
- Spark MJ, Willis J. Systematic review of progesterone use by midlife menopausal women. Maturitas 2012; 72: 192-202.
- Manson JE, Ames JM, Shapiro M, et al. Algorithm and mobile app for menopausal symptom management and hormonal/nonhormonal therapy decision making: a clinical decision-suport tool from The North American Menopause Society. Menopause. 2015;22:247-253.
- Herrington DM, Vittinghoff E, Howard TD, et al. Factor V Leiden, hormone replacement therapy, and risk of venous thromboembolic events in women with coronary disease. Arterioscler Thromb Vasc Biol. 2002;22:1012-1017.
- Ensrud KE, Joffe H, Guthrie KA, et al. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy perimenopausal and postmenopausal women with hot flashes: a randomized controlled trial. Menopause. 2012;19:848-855.
- Schiff I, Tulchinsky D, Cramer D, et al. Oral medroxyprogesterone in the treatment of postmenopausal symptoms. JAMA. 1980;244:1443-1445.
- Bullock JL, Massey FM, Gambrell RD Jr. Use of medroxyprogesterone acetate to prevent menopausal symptoms. Obstet Gynecol. 1975;46:165-168.
- Loprinzi CL, Levitt R, Barton D, et al. Phase III comparison of depot medroxyprogesterone acetate to venlafaxine for managing hot flashes: North Central Cancer Treatment Group Trial N99C7. J Clin Oncol. 2006;24:1409-1414.
- Prior JC, Nielsen JD, Hitchcock CL, et al. Medroxyprogesterone and conjugated oestrogen are equivalent for hot flushes: 1-year randomized double-blind trial following premenopausal ovariectomy. Clin Sci (Lond). 2007;112:517-525.
- L’hermite M, Simoncini T, Fuller S, et al. Could transdermal estradiol + progesterone be a safer postmenopausal HRT? A review. Maturitas. 2008;60:185-201.
- Simon JA. What if the Women’s Health Initiative had used transdermal estradiol and oral progesterone instead? Menopause. 2014;21:769-783.
- Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103-111.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Barbieri RL, Canick JA, Ryan KJ. High-affinity steroid binding to rat testis 17 alpha-hydroxylase and human placental aromatase. J Steroid Biochem. 1981;14:387-393.
- Chu MC, Zhang X, Gentzschein E, et al. Formation of ethinyl estradiol in women during treatment with norethindrone acetate. J Clin Endocrinol Metab. 2007;92:2205-2207.
- Chwalisz K, Surrey E, Stanczyk FZ. The hormonal profile of norethindrone acetate: rationale for add-back therapy with gonadotropin-releasing hormone agonists in women with endometriosis. Reprod Sci. 2012;19:563-571.
- Hitchcock CL, Prior JC. Oral micronized progesterone for vasomotor symptoms—a placebo-controlled randomized trial in healthy postmenopausal women. Menopause. 2012;19:886-893.
- Spark MJ, Willis J. Systematic review of progesterone use by midlife menopausal women. Maturitas 2012; 72: 192-202.
- Manson JE, Ames JM, Shapiro M, et al. Algorithm and mobile app for menopausal symptom management and hormonal/nonhormonal therapy decision making: a clinical decision-suport tool from The North American Menopause Society. Menopause. 2015;22:247-253.
- Herrington DM, Vittinghoff E, Howard TD, et al. Factor V Leiden, hormone replacement therapy, and risk of venous thromboembolic events in women with coronary disease. Arterioscler Thromb Vasc Biol. 2002;22:1012-1017.
- Ensrud KE, Joffe H, Guthrie KA, et al. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy perimenopausal and postmenopausal women with hot flashes: a randomized controlled trial. Menopause. 2012;19:848-855.
- Schiff I, Tulchinsky D, Cramer D, et al. Oral medroxyprogesterone in the treatment of postmenopausal symptoms. JAMA. 1980;244:1443-1445.
- Bullock JL, Massey FM, Gambrell RD Jr. Use of medroxyprogesterone acetate to prevent menopausal symptoms. Obstet Gynecol. 1975;46:165-168.
- Loprinzi CL, Levitt R, Barton D, et al. Phase III comparison of depot medroxyprogesterone acetate to venlafaxine for managing hot flashes: North Central Cancer Treatment Group Trial N99C7. J Clin Oncol. 2006;24:1409-1414.
- Prior JC, Nielsen JD, Hitchcock CL, et al. Medroxyprogesterone and conjugated oestrogen are equivalent for hot flushes: 1-year randomized double-blind trial following premenopausal ovariectomy. Clin Sci (Lond). 2007;112:517-525.
- L’hermite M, Simoncini T, Fuller S, et al. Could transdermal estradiol + progesterone be a safer postmenopausal HRT? A review. Maturitas. 2008;60:185-201.
- Simon JA. What if the Women’s Health Initiative had used transdermal estradiol and oral progesterone instead? Menopause. 2014;21:769-783.
- Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103-111.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Barbieri RL, Canick JA, Ryan KJ. High-affinity steroid binding to rat testis 17 alpha-hydroxylase and human placental aromatase. J Steroid Biochem. 1981;14:387-393.
- Chu MC, Zhang X, Gentzschein E, et al. Formation of ethinyl estradiol in women during treatment with norethindrone acetate. J Clin Endocrinol Metab. 2007;92:2205-2207.
- Chwalisz K, Surrey E, Stanczyk FZ. The hormonal profile of norethindrone acetate: rationale for add-back therapy with gonadotropin-releasing hormone agonists in women with endometriosis. Reprod Sci. 2012;19:563-571.
What is optimal hormonal treatment for women with polycystic ovary syndrome?
Polycystic ovary syndrome (PCOS) is the triad of oligo-ovulation resulting in oligomenorrhea, hyperandrogenism and, often, an excess number of small antral follicles on high-resolution pelvic ultrasound. One meta-analysis reported that, in women of reproductive age, the prevalence of PCOS was 10% using the Rotterdam-European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) criteria1 and 6% using the National Institutes of Health 1990 diagnostic criteria.2 (See “The PCOS trinity—3 findings in one syndrome: oligo-ovulation, hyperandrogenism, and a multifollicular ovary.”3)
PCOS is caused by abnormalities in 3 systems: reproductive, metabolic, and dermatologic. Reproductive abnormalities commonly observed in women with PCOS include4:
- an increase in pituitary secretion of luteinizing hormone (LH), resulting from both an increase in LH pulse amplitude and LH pulse frequency, suggesting a primary hypothalamic disorder
- an increase in ovarian secretion of androstenedione and testosterone due to stimulation by LH and possibly insulin
- oligo-ovulation with chronically low levels of progesterone that can result in endometrial hyperplasia
- ovulatory infertility.
Metabolic abnormalities commonly observed in women with PCOS include5,6:
- insulin resistance and hyperinsulinemia
- excess adipose tissue in the liver
- excess visceral fat
- elevated adipokines
- obesity
- an increased prevalence of glucose intolerance and frank diabetes.
Dermatologic abnormalities commonly observed in women with PCOS include7:
- facial hirsutism
- acne
- androgenetic alopecia.
Given that PCOS is caused by abnormalities in the reproductive, metabolic, and dermatologic systems, it is appropriate to consider multimodal hormonal therapy that addresses all 3 problems. In my practice, I believe that the best approach to the long-term hormonal treatment of PCOS for many women is to prescribe a combination of 3 medicines: a combination estrogen-progestin oral contraceptive (COC), an insulin sensitizer, and an antiandrogen.
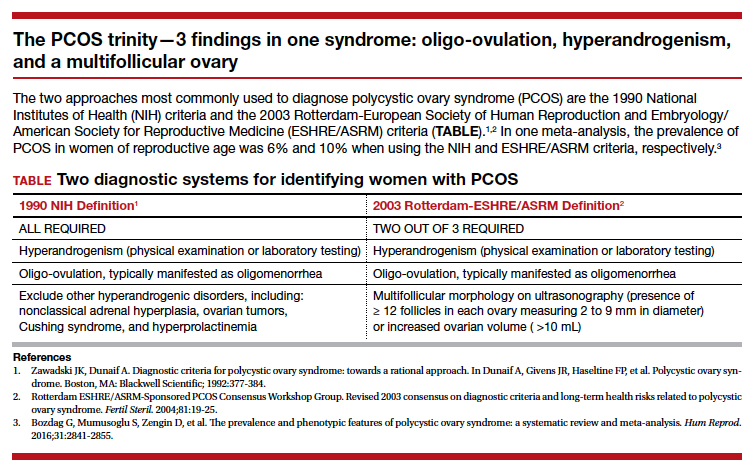
The COC reduces pituitary secretion of LH, decreases ovarian androgen production, and prevents the development of endometrial hyperplasia. When taken cyclically, the COC treatment also restores regular withdrawal uterine bleeding.
An insulin sensitizer, such as metformin or pioglitazone, helps to reduce insulin resistance, glucose intolerance, and hepatic adipose content, rebalancing central metabolism. It is important to include diet and exercise in the long-term treatment of PCOS, and I always encourage these lifestyle changes. However, my patients usually report that they have tried multiple times to restrict dietary caloric intake and increase exercise and have been unable to rebalance their metabolism with these interventions alone. Of note, in the women with PCOS and a body mass index >35 kg/m2, bariatric surgery, such as a sleeve gastrectomy, often results in marked improvement of their PCOS.8
The antiandrogen spironolactone provides effective treatment for the dermatologic problems of facial hirsutism and acne. Some COCs containing the progestins drospirenone, norgestimate, and norethindrone acetate are approved by the US Food and Drug Administration for the treatment of acne. A common approach I use in practice is to prescribe a COC, plus spironolactone 100 mg daily plus metformin extended-release 750 mg to 1,500 mg daily.
Continue to: Which COCs have low androgenicity?...
Which COCs have low androgenicity?
I believe that every COC is an effective treatment for PCOS, regardless of the androgenicity of the progestin in the contraceptive. However, some dermatologists believe that combination contraceptives containing progestins with low androgenicity, such as drospirenone, norgestimate, and desogestrel, are more likely to improve acne than contraceptives with an androgenic progestin such as levonorgestrel. In one study in which 2,147 women with acne were treated by one dermatologic practice, the percentage of women reporting that a birth control pill helped to improve their acne was 66% for pills containing drospirenone, 53% for pills containing norgestimate, 44% for pills containing desogestrel, 30% for pills containing norethindrone, and 25% for pills containing levonorgestrel. In the same study, the percent of women reporting that a birth control pill made their acne worse was 3% for pills containing drospirenone, 6% for pills containing norgestimate, 2% for pills containing desogestrel, 8% for pills containing norethindrone, and 10% for pills containing levonorgestrel.9 Given these findings, when treating a woman with PCOS, I generally prescribe a contraceptive that does not contain levonorgestrel.
Why is a spironolactone dose of 100 mg a good choice for PCOS treatment?
Spironolactone, an antiandrogen and inhibitor of 5-alpha-reductase, is commonly prescribed for the treatment of hirsutism and acne at doses ranging from 50 mg to 200 mg daily.10,11 In my clinical experience, spironolactone at a dose of 200 mg daily commonly causes irregular and bothersome uterine bleeding while spironolactone at a dose of 100 mg daily is seldom associated with irregular bleeding. I believe that spironolactone at a dose of 100 mg daily results in superior clinical efficacy than a 50-mg daily dose, although studies report that both doses are effective in the treatment of acne and hirsutism. Spironolactone should not be prescribed to women with renal failure because it can result in severe hyperkalemia. In a study of spironolactone safety in the treatment of acne, no adverse effects on the kidney, liver, or adrenal glands were reported over 8 years of use.12
What insulin sensitizers are useful in rebalancing the metabolic abnormalities observed with PCOS?
Diet and exercise are superb approaches to rebalancing metabolic abnormalities, but for many of my patients they are insufficient and treatment with an insulin sensitizer is warranted. The most commonly utilized insulin sensitizer for the treatment of PCOS is metformin because it is very inexpensive and has a low risk of serious adverse effects such as lactic acidosis. Metformin increases peripheral glucose uptake and reduces gastrointestinal glucose absorption. Insulin sensitizers also decrease visceral fat, a major source of adipokines. One major disadvantage of metformin is that at doses in the range of 1,500 mg to 2,250 mg it often causes gastrointestinal adverse effects such as borborygmi, nausea, abdominal discomfort, and loose stools.
Thiazolidinediones, including pioglitazone, have been reported to be effective in rebalancing central metabolism in women with PCOS. Pioglitazone carries a black box warning of an increased risk of congestive heart failure and nonfatal myocardial infarction. Pioglitazone is also associated with a risk of hepatotoxicity. However, at the pioglitazone dose commonly used in the treatment of PCOS (7.5 mg daily), these serious adverse effects are rare. In practice, I initiate metformin at a dose of 750 mg daily using the extended-release formulation. I increase the metformin dose to 1,500 mg daily if the patient has no bothersome gastrointestinal symptoms on the lower dose. If the patient cannot tolerate metformin treatment because of adverse effects, I will use pioglitazone 7.5 mg daily.
Continue to: Treatment of PCOS in women who are carriers of the Factor V Leiden mutation...
Treatment of PCOS in women who are carriers of the Factor V Leiden mutation
The Factor V Leiden allele is associated with an increased risk of venous thromboembolism. Estrogen-progestin contraception is contraindicated in women with the Factor V Leiden mutation. The prevalence of this mutation varies by race and ethnicity. It is present in about 5% of white, 2% of Hispanic, 1% of black, 1% of Native American, and 0.5% of Asian women. In women with PCOS who are known to be carriers of the mutation, dual therapy with metformin and spironolactone is highly effective.13-15 For these women I also offer a levonorgestrel IUD to provide contraception and reduce the risk of endometrial hyperplasia.
Combination triple medication treatment of PCOS
Optimal treatment of the reproductive, metabolic, and dermatologic problems associated with PCOS requires multimodal medications including an estrogen-progestin contraceptive, an antiandrogen, and an insulin sensitizer. In my practice, I initiate treatment of PCOS by offering patients 3 medications: a COC, spironolactone 100 mg daily, and metformin extended-release formulation 750 mg daily. Some patients elect dual medication therapy (COC plus spironolactone or COC plus metformin), but many patients select treatment with all 3 medications. Although triple medication treatment of PCOS has not been tested in large randomized clinical trials, small trials report that triple medication treatment produces optimal improvement in the reproductive, metabolic, and dermatologic problems associated with PCOS.16-18
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In Dunaif A, Givens JR, Haseltine FP, et al. Polycystic ovary syndrome. Boston, MA: Blackwell Scientific; 1992:377-384.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Baskind NE, Balen AH. Hypothalamic-pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:80-97.
- Gilbert EW, Tay CT, Hiam DS, et al. Comorbidities and complications of polycystic ovary syndrome: an overview of systematic reviews. Clin Endocrinol (Oxf). 2018;89:683-699.
- Harsha Varma S, Tirupati S, Pradeep TV, et al. Insulin resistance and hyperandrogenemia independently predict nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Diabetes Metab Syndr. 2019;13:1065-1069.
- Housman E, Reynolds RV. Polycystic ovary syndrome: a review for dermatologists: Part I. Diagnosis and manifestations. J Am Acad Dermatol. 2014;71:847.e1-e10.
- Dilday J, Derickson M, Kuckelman J, et al. Sleeve gastrectomy for obesity in polycystic ovarian syndrome: a pilot study evaluating weight loss and fertility outcomes. Obes Surg. 2019;29:93-98.
- Lortscher D, Admani S, Satur N, et al. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15:670-674.
- Brown J, Farquhar C, Lee O, et al. Spironolactone versus placebo or in combination with steroids for hirsutism and/or acne. Cochrane Database Syst Rev. 2009;CD000194.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
- Mazza A, Fruci B, Guzzi P, et al. In PCOS patients the addition of low-dose spironolactone induces a more marked reduction of clinical and biochemical hyperandrogenism than metformin alone. Nutr Metab Cardiovascular Dis. 2014;24:132-139.
- Ganie MA, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Ibanez L, de Zegher F. Low-dose combination flutamide, metformin and an oral contraceptive for non-obese, young women with polycystic ovary syndrome. Hum Reprod. 2003;18:57-60.
- Ibanez L, de Zegher F. Flutamide-metformin plus an oral contraceptive (OC) for young women with polycystic ovary syndrome: switch from third- to fourth-generation OC reduces body adiposity. Hum Reprod. 2004;19:1725-1727.
Polycystic ovary syndrome (PCOS) is the triad of oligo-ovulation resulting in oligomenorrhea, hyperandrogenism and, often, an excess number of small antral follicles on high-resolution pelvic ultrasound. One meta-analysis reported that, in women of reproductive age, the prevalence of PCOS was 10% using the Rotterdam-European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) criteria1 and 6% using the National Institutes of Health 1990 diagnostic criteria.2 (See “The PCOS trinity—3 findings in one syndrome: oligo-ovulation, hyperandrogenism, and a multifollicular ovary.”3)
PCOS is caused by abnormalities in 3 systems: reproductive, metabolic, and dermatologic. Reproductive abnormalities commonly observed in women with PCOS include4:
- an increase in pituitary secretion of luteinizing hormone (LH), resulting from both an increase in LH pulse amplitude and LH pulse frequency, suggesting a primary hypothalamic disorder
- an increase in ovarian secretion of androstenedione and testosterone due to stimulation by LH and possibly insulin
- oligo-ovulation with chronically low levels of progesterone that can result in endometrial hyperplasia
- ovulatory infertility.
Metabolic abnormalities commonly observed in women with PCOS include5,6:
- insulin resistance and hyperinsulinemia
- excess adipose tissue in the liver
- excess visceral fat
- elevated adipokines
- obesity
- an increased prevalence of glucose intolerance and frank diabetes.
Dermatologic abnormalities commonly observed in women with PCOS include7:
- facial hirsutism
- acne
- androgenetic alopecia.
Given that PCOS is caused by abnormalities in the reproductive, metabolic, and dermatologic systems, it is appropriate to consider multimodal hormonal therapy that addresses all 3 problems. In my practice, I believe that the best approach to the long-term hormonal treatment of PCOS for many women is to prescribe a combination of 3 medicines: a combination estrogen-progestin oral contraceptive (COC), an insulin sensitizer, and an antiandrogen.

The COC reduces pituitary secretion of LH, decreases ovarian androgen production, and prevents the development of endometrial hyperplasia. When taken cyclically, the COC treatment also restores regular withdrawal uterine bleeding.
An insulin sensitizer, such as metformin or pioglitazone, helps to reduce insulin resistance, glucose intolerance, and hepatic adipose content, rebalancing central metabolism. It is important to include diet and exercise in the long-term treatment of PCOS, and I always encourage these lifestyle changes. However, my patients usually report that they have tried multiple times to restrict dietary caloric intake and increase exercise and have been unable to rebalance their metabolism with these interventions alone. Of note, in the women with PCOS and a body mass index >35 kg/m2, bariatric surgery, such as a sleeve gastrectomy, often results in marked improvement of their PCOS.8
The antiandrogen spironolactone provides effective treatment for the dermatologic problems of facial hirsutism and acne. Some COCs containing the progestins drospirenone, norgestimate, and norethindrone acetate are approved by the US Food and Drug Administration for the treatment of acne. A common approach I use in practice is to prescribe a COC, plus spironolactone 100 mg daily plus metformin extended-release 750 mg to 1,500 mg daily.
Continue to: Which COCs have low androgenicity?...
Which COCs have low androgenicity?
I believe that every COC is an effective treatment for PCOS, regardless of the androgenicity of the progestin in the contraceptive. However, some dermatologists believe that combination contraceptives containing progestins with low androgenicity, such as drospirenone, norgestimate, and desogestrel, are more likely to improve acne than contraceptives with an androgenic progestin such as levonorgestrel. In one study in which 2,147 women with acne were treated by one dermatologic practice, the percentage of women reporting that a birth control pill helped to improve their acne was 66% for pills containing drospirenone, 53% for pills containing norgestimate, 44% for pills containing desogestrel, 30% for pills containing norethindrone, and 25% for pills containing levonorgestrel. In the same study, the percent of women reporting that a birth control pill made their acne worse was 3% for pills containing drospirenone, 6% for pills containing norgestimate, 2% for pills containing desogestrel, 8% for pills containing norethindrone, and 10% for pills containing levonorgestrel.9 Given these findings, when treating a woman with PCOS, I generally prescribe a contraceptive that does not contain levonorgestrel.

Why is a spironolactone dose of 100 mg a good choice for PCOS treatment?
Spironolactone, an antiandrogen and inhibitor of 5-alpha-reductase, is commonly prescribed for the treatment of hirsutism and acne at doses ranging from 50 mg to 200 mg daily.10,11 In my clinical experience, spironolactone at a dose of 200 mg daily commonly causes irregular and bothersome uterine bleeding while spironolactone at a dose of 100 mg daily is seldom associated with irregular bleeding. I believe that spironolactone at a dose of 100 mg daily results in superior clinical efficacy than a 50-mg daily dose, although studies report that both doses are effective in the treatment of acne and hirsutism. Spironolactone should not be prescribed to women with renal failure because it can result in severe hyperkalemia. In a study of spironolactone safety in the treatment of acne, no adverse effects on the kidney, liver, or adrenal glands were reported over 8 years of use.12
What insulin sensitizers are useful in rebalancing the metabolic abnormalities observed with PCOS?
Diet and exercise are superb approaches to rebalancing metabolic abnormalities, but for many of my patients they are insufficient and treatment with an insulin sensitizer is warranted. The most commonly utilized insulin sensitizer for the treatment of PCOS is metformin because it is very inexpensive and has a low risk of serious adverse effects such as lactic acidosis. Metformin increases peripheral glucose uptake and reduces gastrointestinal glucose absorption. Insulin sensitizers also decrease visceral fat, a major source of adipokines. One major disadvantage of metformin is that at doses in the range of 1,500 mg to 2,250 mg it often causes gastrointestinal adverse effects such as borborygmi, nausea, abdominal discomfort, and loose stools.
Thiazolidinediones, including pioglitazone, have been reported to be effective in rebalancing central metabolism in women with PCOS. Pioglitazone carries a black box warning of an increased risk of congestive heart failure and nonfatal myocardial infarction. Pioglitazone is also associated with a risk of hepatotoxicity. However, at the pioglitazone dose commonly used in the treatment of PCOS (7.5 mg daily), these serious adverse effects are rare. In practice, I initiate metformin at a dose of 750 mg daily using the extended-release formulation. I increase the metformin dose to 1,500 mg daily if the patient has no bothersome gastrointestinal symptoms on the lower dose. If the patient cannot tolerate metformin treatment because of adverse effects, I will use pioglitazone 7.5 mg daily.
Continue to: Treatment of PCOS in women who are carriers of the Factor V Leiden mutation...
Treatment of PCOS in women who are carriers of the Factor V Leiden mutation
The Factor V Leiden allele is associated with an increased risk of venous thromboembolism. Estrogen-progestin contraception is contraindicated in women with the Factor V Leiden mutation. The prevalence of this mutation varies by race and ethnicity. It is present in about 5% of white, 2% of Hispanic, 1% of black, 1% of Native American, and 0.5% of Asian women. In women with PCOS who are known to be carriers of the mutation, dual therapy with metformin and spironolactone is highly effective.13-15 For these women I also offer a levonorgestrel IUD to provide contraception and reduce the risk of endometrial hyperplasia.
Combination triple medication treatment of PCOS
Optimal treatment of the reproductive, metabolic, and dermatologic problems associated with PCOS requires multimodal medications including an estrogen-progestin contraceptive, an antiandrogen, and an insulin sensitizer. In my practice, I initiate treatment of PCOS by offering patients 3 medications: a COC, spironolactone 100 mg daily, and metformin extended-release formulation 750 mg daily. Some patients elect dual medication therapy (COC plus spironolactone or COC plus metformin), but many patients select treatment with all 3 medications. Although triple medication treatment of PCOS has not been tested in large randomized clinical trials, small trials report that triple medication treatment produces optimal improvement in the reproductive, metabolic, and dermatologic problems associated with PCOS.16-18
Polycystic ovary syndrome (PCOS) is the triad of oligo-ovulation resulting in oligomenorrhea, hyperandrogenism and, often, an excess number of small antral follicles on high-resolution pelvic ultrasound. One meta-analysis reported that, in women of reproductive age, the prevalence of PCOS was 10% using the Rotterdam-European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) criteria1 and 6% using the National Institutes of Health 1990 diagnostic criteria.2 (See “The PCOS trinity—3 findings in one syndrome: oligo-ovulation, hyperandrogenism, and a multifollicular ovary.”3)
PCOS is caused by abnormalities in 3 systems: reproductive, metabolic, and dermatologic. Reproductive abnormalities commonly observed in women with PCOS include4:
- an increase in pituitary secretion of luteinizing hormone (LH), resulting from both an increase in LH pulse amplitude and LH pulse frequency, suggesting a primary hypothalamic disorder
- an increase in ovarian secretion of androstenedione and testosterone due to stimulation by LH and possibly insulin
- oligo-ovulation with chronically low levels of progesterone that can result in endometrial hyperplasia
- ovulatory infertility.
Metabolic abnormalities commonly observed in women with PCOS include5,6:
- insulin resistance and hyperinsulinemia
- excess adipose tissue in the liver
- excess visceral fat
- elevated adipokines
- obesity
- an increased prevalence of glucose intolerance and frank diabetes.
Dermatologic abnormalities commonly observed in women with PCOS include7:
- facial hirsutism
- acne
- androgenetic alopecia.
Given that PCOS is caused by abnormalities in the reproductive, metabolic, and dermatologic systems, it is appropriate to consider multimodal hormonal therapy that addresses all 3 problems. In my practice, I believe that the best approach to the long-term hormonal treatment of PCOS for many women is to prescribe a combination of 3 medicines: a combination estrogen-progestin oral contraceptive (COC), an insulin sensitizer, and an antiandrogen.

The COC reduces pituitary secretion of LH, decreases ovarian androgen production, and prevents the development of endometrial hyperplasia. When taken cyclically, the COC treatment also restores regular withdrawal uterine bleeding.
An insulin sensitizer, such as metformin or pioglitazone, helps to reduce insulin resistance, glucose intolerance, and hepatic adipose content, rebalancing central metabolism. It is important to include diet and exercise in the long-term treatment of PCOS, and I always encourage these lifestyle changes. However, my patients usually report that they have tried multiple times to restrict dietary caloric intake and increase exercise and have been unable to rebalance their metabolism with these interventions alone. Of note, in the women with PCOS and a body mass index >35 kg/m2, bariatric surgery, such as a sleeve gastrectomy, often results in marked improvement of their PCOS.8
The antiandrogen spironolactone provides effective treatment for the dermatologic problems of facial hirsutism and acne. Some COCs containing the progestins drospirenone, norgestimate, and norethindrone acetate are approved by the US Food and Drug Administration for the treatment of acne. A common approach I use in practice is to prescribe a COC, plus spironolactone 100 mg daily plus metformin extended-release 750 mg to 1,500 mg daily.
Continue to: Which COCs have low androgenicity?...
Which COCs have low androgenicity?
I believe that every COC is an effective treatment for PCOS, regardless of the androgenicity of the progestin in the contraceptive. However, some dermatologists believe that combination contraceptives containing progestins with low androgenicity, such as drospirenone, norgestimate, and desogestrel, are more likely to improve acne than contraceptives with an androgenic progestin such as levonorgestrel. In one study in which 2,147 women with acne were treated by one dermatologic practice, the percentage of women reporting that a birth control pill helped to improve their acne was 66% for pills containing drospirenone, 53% for pills containing norgestimate, 44% for pills containing desogestrel, 30% for pills containing norethindrone, and 25% for pills containing levonorgestrel. In the same study, the percent of women reporting that a birth control pill made their acne worse was 3% for pills containing drospirenone, 6% for pills containing norgestimate, 2% for pills containing desogestrel, 8% for pills containing norethindrone, and 10% for pills containing levonorgestrel.9 Given these findings, when treating a woman with PCOS, I generally prescribe a contraceptive that does not contain levonorgestrel.

Why is a spironolactone dose of 100 mg a good choice for PCOS treatment?
Spironolactone, an antiandrogen and inhibitor of 5-alpha-reductase, is commonly prescribed for the treatment of hirsutism and acne at doses ranging from 50 mg to 200 mg daily.10,11 In my clinical experience, spironolactone at a dose of 200 mg daily commonly causes irregular and bothersome uterine bleeding while spironolactone at a dose of 100 mg daily is seldom associated with irregular bleeding. I believe that spironolactone at a dose of 100 mg daily results in superior clinical efficacy than a 50-mg daily dose, although studies report that both doses are effective in the treatment of acne and hirsutism. Spironolactone should not be prescribed to women with renal failure because it can result in severe hyperkalemia. In a study of spironolactone safety in the treatment of acne, no adverse effects on the kidney, liver, or adrenal glands were reported over 8 years of use.12
What insulin sensitizers are useful in rebalancing the metabolic abnormalities observed with PCOS?
Diet and exercise are superb approaches to rebalancing metabolic abnormalities, but for many of my patients they are insufficient and treatment with an insulin sensitizer is warranted. The most commonly utilized insulin sensitizer for the treatment of PCOS is metformin because it is very inexpensive and has a low risk of serious adverse effects such as lactic acidosis. Metformin increases peripheral glucose uptake and reduces gastrointestinal glucose absorption. Insulin sensitizers also decrease visceral fat, a major source of adipokines. One major disadvantage of metformin is that at doses in the range of 1,500 mg to 2,250 mg it often causes gastrointestinal adverse effects such as borborygmi, nausea, abdominal discomfort, and loose stools.
Thiazolidinediones, including pioglitazone, have been reported to be effective in rebalancing central metabolism in women with PCOS. Pioglitazone carries a black box warning of an increased risk of congestive heart failure and nonfatal myocardial infarction. Pioglitazone is also associated with a risk of hepatotoxicity. However, at the pioglitazone dose commonly used in the treatment of PCOS (7.5 mg daily), these serious adverse effects are rare. In practice, I initiate metformin at a dose of 750 mg daily using the extended-release formulation. I increase the metformin dose to 1,500 mg daily if the patient has no bothersome gastrointestinal symptoms on the lower dose. If the patient cannot tolerate metformin treatment because of adverse effects, I will use pioglitazone 7.5 mg daily.
Continue to: Treatment of PCOS in women who are carriers of the Factor V Leiden mutation...
Treatment of PCOS in women who are carriers of the Factor V Leiden mutation
The Factor V Leiden allele is associated with an increased risk of venous thromboembolism. Estrogen-progestin contraception is contraindicated in women with the Factor V Leiden mutation. The prevalence of this mutation varies by race and ethnicity. It is present in about 5% of white, 2% of Hispanic, 1% of black, 1% of Native American, and 0.5% of Asian women. In women with PCOS who are known to be carriers of the mutation, dual therapy with metformin and spironolactone is highly effective.13-15 For these women I also offer a levonorgestrel IUD to provide contraception and reduce the risk of endometrial hyperplasia.
Combination triple medication treatment of PCOS
Optimal treatment of the reproductive, metabolic, and dermatologic problems associated with PCOS requires multimodal medications including an estrogen-progestin contraceptive, an antiandrogen, and an insulin sensitizer. In my practice, I initiate treatment of PCOS by offering patients 3 medications: a COC, spironolactone 100 mg daily, and metformin extended-release formulation 750 mg daily. Some patients elect dual medication therapy (COC plus spironolactone or COC plus metformin), but many patients select treatment with all 3 medications. Although triple medication treatment of PCOS has not been tested in large randomized clinical trials, small trials report that triple medication treatment produces optimal improvement in the reproductive, metabolic, and dermatologic problems associated with PCOS.16-18
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In Dunaif A, Givens JR, Haseltine FP, et al. Polycystic ovary syndrome. Boston, MA: Blackwell Scientific; 1992:377-384.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Baskind NE, Balen AH. Hypothalamic-pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:80-97.
- Gilbert EW, Tay CT, Hiam DS, et al. Comorbidities and complications of polycystic ovary syndrome: an overview of systematic reviews. Clin Endocrinol (Oxf). 2018;89:683-699.
- Harsha Varma S, Tirupati S, Pradeep TV, et al. Insulin resistance and hyperandrogenemia independently predict nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Diabetes Metab Syndr. 2019;13:1065-1069.
- Housman E, Reynolds RV. Polycystic ovary syndrome: a review for dermatologists: Part I. Diagnosis and manifestations. J Am Acad Dermatol. 2014;71:847.e1-e10.
- Dilday J, Derickson M, Kuckelman J, et al. Sleeve gastrectomy for obesity in polycystic ovarian syndrome: a pilot study evaluating weight loss and fertility outcomes. Obes Surg. 2019;29:93-98.
- Lortscher D, Admani S, Satur N, et al. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15:670-674.
- Brown J, Farquhar C, Lee O, et al. Spironolactone versus placebo or in combination with steroids for hirsutism and/or acne. Cochrane Database Syst Rev. 2009;CD000194.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
- Mazza A, Fruci B, Guzzi P, et al. In PCOS patients the addition of low-dose spironolactone induces a more marked reduction of clinical and biochemical hyperandrogenism than metformin alone. Nutr Metab Cardiovascular Dis. 2014;24:132-139.
- Ganie MA, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Ibanez L, de Zegher F. Low-dose combination flutamide, metformin and an oral contraceptive for non-obese, young women with polycystic ovary syndrome. Hum Reprod. 2003;18:57-60.
- Ibanez L, de Zegher F. Flutamide-metformin plus an oral contraceptive (OC) for young women with polycystic ovary syndrome: switch from third- to fourth-generation OC reduces body adiposity. Hum Reprod. 2004;19:1725-1727.
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In Dunaif A, Givens JR, Haseltine FP, et al. Polycystic ovary syndrome. Boston, MA: Blackwell Scientific; 1992:377-384.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Baskind NE, Balen AH. Hypothalamic-pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:80-97.
- Gilbert EW, Tay CT, Hiam DS, et al. Comorbidities and complications of polycystic ovary syndrome: an overview of systematic reviews. Clin Endocrinol (Oxf). 2018;89:683-699.
- Harsha Varma S, Tirupati S, Pradeep TV, et al. Insulin resistance and hyperandrogenemia independently predict nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Diabetes Metab Syndr. 2019;13:1065-1069.
- Housman E, Reynolds RV. Polycystic ovary syndrome: a review for dermatologists: Part I. Diagnosis and manifestations. J Am Acad Dermatol. 2014;71:847.e1-e10.
- Dilday J, Derickson M, Kuckelman J, et al. Sleeve gastrectomy for obesity in polycystic ovarian syndrome: a pilot study evaluating weight loss and fertility outcomes. Obes Surg. 2019;29:93-98.
- Lortscher D, Admani S, Satur N, et al. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15:670-674.
- Brown J, Farquhar C, Lee O, et al. Spironolactone versus placebo or in combination with steroids for hirsutism and/or acne. Cochrane Database Syst Rev. 2009;CD000194.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
- Mazza A, Fruci B, Guzzi P, et al. In PCOS patients the addition of low-dose spironolactone induces a more marked reduction of clinical and biochemical hyperandrogenism than metformin alone. Nutr Metab Cardiovascular Dis. 2014;24:132-139.
- Ganie MA, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Ibanez L, de Zegher F. Low-dose combination flutamide, metformin and an oral contraceptive for non-obese, young women with polycystic ovary syndrome. Hum Reprod. 2003;18:57-60.
- Ibanez L, de Zegher F. Flutamide-metformin plus an oral contraceptive (OC) for young women with polycystic ovary syndrome: switch from third- to fourth-generation OC reduces body adiposity. Hum Reprod. 2004;19:1725-1727.
Retained placenta after vaginal birth: How long should you wait to manually remove the placenta?
You have just safely delivered the baby who is quietly resting on her mother’s chest. You begin active management of the third stage of labor, administering oxytocin, performing uterine massage and applying controlled tension on the umbilical cord. There is no evidence of excess postpartum bleeding.
How long will you wait to deliver the placenta?
Active management of the third stage of labor
Most authorities recommend active management of the third stage of labor because active management reduces the risk of maternal hemorrhage >1,000 mL (relative risk [RR], 0.34), postpartum hemoglobin levels < 9 g/dL (RR, 0.50), and maternal blood transfusion (RR, 0.35) compared with expectant management.1
The most important component of active management of the third stage of labor is the administration of a uterotonic after delivery of the newborn. In the United States, oxytocin is the uterotonic most often utilized for the active management of the third stage of labor. Authors of a recent randomized clinical trial reported that intravenous oxytocin is superior to intramuscular oxytocin for reducing postpartum blood loss (385 vs 445 mL), the frequency of blood loss greater than 1,000 mL (4.6% vs 8.1%), and the rate of maternal blood transfusion (1.5% vs 4.4%).2
In addition to administering oxytocin, the active management of the third stage often involves maneuvers to accelerate placental delivery, including the Crede and Brandt-Andrews maneuvers and controlled tension on the umbilical cord. The Crede maneuver, described in 1853, involves placing a hand on the abdominal wall near the uterine fundus and squeezing the uterine fundus between the thumb and fingers.3,4
The Brandt-Andrews maneuver, described in 1933, involves placing a clamp on the umbilical cord close to the vulva.5 The clamp is used to apply judicious tension on the cord with one hand, while the other hand is placed on the mother’s abdomen with the palm and fingers overlying the junction between the uterine corpus and the lower segment. With judicious tension on the cord, the abdominal hand pushes the uterus upward toward the umbilicus. Placental separation is indicated when lengthening of the umbilical cord occurs. The Brandt-Andrews maneuver may be associated with fewer cases of uterine inversion than the Crede maneuver.5-7
Of note, umbilical cord traction has not been demonstrated to reduce the need for blood transfusion or the incidence of postpartum hemorrhage (PPH) >1,000 mL, and it is commonly utilized by obstetricians and midwives.8,9 Hence, in the third stage, the delivering clinician should routinely administer a uterotonic, but use of judicious tension on the cord can be deferred if the woman prefers a noninterventional approach to delivery.
Following a vaginal birth, when should the diagnosis of retained placenta be made?
The historic definition of retained placenta is nonexpulsion of the placenta 30 minutes after delivery of the newborn. However, many observational studies report that, when active management of the third stage is utilized, 90%, 95%, and 99% of placentas deliver by 9 minutes, 13 minutes, and 28 minutes, respectively.10 In addition, many observational studies report that the incidence of PPH increases significantly with longer intervals between birth of the newborn and delivery of the placenta. In one study the rate of blood loss >500 mL was 8.5% when the placenta delivered between 5 and 9 minutes and 35.1% when the placenta delivered ≥30 minutes following birth of the baby.10 In another observational study, compared with women delivering the placenta < 10 minutes after birth, women delivering the placenta ≥30 minutes after birth had a 3-fold increased risk of PPH.11 Similar findings have been reported in other studies.12-14
Continue to: Based on the association between a delay in delivery...
Based on the association between a delay in delivery of the placenta and an increased risk of PPH, some authorities recommend that, in term pregnancy, the diagnosis of retained placenta should be made at 20 minutes following birth and consideration should be given to removing the placenta at this time. For women with effective neuraxial anesthesia, manual removal of the placenta 20 minutes following birth may be the best decision for balancing the benefit of preventing PPH with the risk of unnecessary intervention. For women with no anesthesia, delaying manual removal of the placenta to 30 minutes or more following birth may permit more time for the placenta to deliver prior to performing an intervention that might cause pain, but the delay increases the risk of PPH.
The retained placenta may prevent the uterine muscle from effectively contracting around penetrating veins and arteries, thereby increasing the risk of postpartum hemorrhage. The placenta that has separated from the uterine wall but is trapped inside the uterine cavity can be removed easily with manual extraction. If the placenta is physiologically adherent to the uterine wall, a gentle sweeping motion with an intrauterine hand usually can separate the placenta from the uterus in preparation for manual extraction. However, if a placenta accreta spectrum disorder is contributing to a retained placenta, it may be difficult to separate the densely adherent portion of the uterus from the uterine wall. In the presence of placenta accreta spectrum disorder, vigorous attempts to remove the placenta may precipitate massive bleeding. In some cases, the acchoucheur/midwife may recognize the presence of a focal accreta and cease attempts to remove the placenta in order to organize the personnel and equipment needed to effectively treat a potential case of placenta accreta. In one study, when a placenta accreta was recognized or suspected, immediately ceasing attempts at manually removing the placenta resulted in better case outcomes than continued attempts to remove the placenta.1
Uterine inversion may occur during an attempt to manually remove the placenta. There is universal agreement that once a uterine inversion is recognized it is critically important to immediately restore normal uterine anatomy to avoid massive hemorrhage and maternal shock. The initial management of uterine inversion includes:
- stopping oxytocin infusion
- initiating high volume fluid resuscitation
- considering a dose of a uterine relaxant, such as nitroglycerin or terbutaline
- preparing for blood product replacement.
In my experience, when uterine inversion is immediately recognized and successfully treated, blood product replacement is not usually necessary. However, if uterine inversion has not been immediately recognized or treated, massive hemorrhage and shock may occur.
Two approaches to the vaginal restoration of uterine anatomy involve using the tips of the fingers and palm of the hand to guide the wall of the uterus back to its normal position (FIGURE 1) or to forcefully use a fist to force the uterine wall back to its normal position (FIGURE 2). If these maneuvers are unsuccessful, a laparotomy may be necessary.
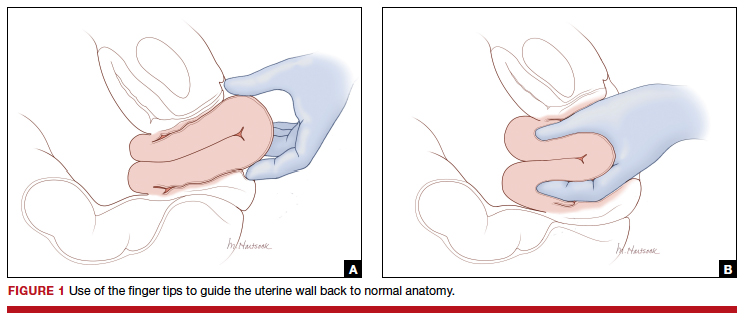
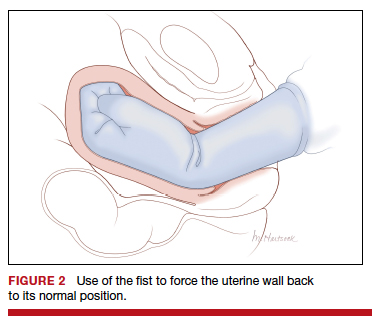
At laparotomy, the Huntington or Haultain procedures may help restore normal uterine anatomy. The Huntington procedure involves using clamps to apply symmetrical tension to the left and right round ligaments and/or uterine serosa to sequentially tease the uterus back to normal anatomy.2,3 The Haultain procedure involves a vertical incision on the posterior wall of the uterus to release the uterine constriction ring that is preventing the return of the uterine fundus to its normal position (FIGURE 3).4,5
References
- Kayem G, Anselem O, Schmitz T, et al. Conservative versus radical management in cases of placenta accreta: a historical study. J Gynecol Obstet Biol Reprod (Paris). 2007;36:680-687.
- Huntington JL. Acute inversion of the uterus. Boston Med Surg J. 1921;184:376-378.
- Huntington JL, Irving FC, Kellogg FS. Abdominal reposition in acute inversion of the puerperal uterus. Am J Obstet Gynecol. 1928;15:34-40.
- Haultain FW. Abdominal hysterotomy for chronic uterine inversion: a record of 3 cases. Proc Roy Soc Med. 1908;1:528-535.
- Easterday CL, Reid DE. Inversion of the puerperal uterus managed by the Haultain technique; A case report. Am J Obstet Gynecol. 1959;78:1224-1226.
Manual extraction of the placenta
Prior to performing manual extraction of the placenta, a decision should be made regarding the approach to anesthesia and perioperative antibiotics. Manual extraction of the placenta is performed by placing one hand on the uterine fundus to stabilize the uterus and using the other hand to follow the umbilical cord into the uterine cavity. The intrauterine hand is used to separate the uterine-placental interface with a gentle sweeping motion. The placental mass is grasped and gently teased through the cervix and vagina. Inspection of the placenta to ensure complete removal is necessary.
An alternative to manual extraction of the placenta is the use of Bierer forceps and ultrasound guidance to tease the placenta through the cervical os. This technique involves the following steps15:
1. use ultrasound to locate the placenta
2. place a ring forceps on the anterior lip of the cervix
3. introduce the Bierer forcep into the uterus
4. use the forceps to grasp the placenta and pull it toward the vagina
5. stop frequently to re-grasp placental tissue that is deeper in the uterine cavity
6. once the placenta is extracted, examine the placenta to ensure complete removal.
Of note when manual extraction is used to deliver a retained placenta, randomized clinical trials report no benefit for the following interventions:
- perioperative antibiotics16
- nitroglycerin to relax the uterus17
- ultrasound to detect retained placental tissue.18
Best timing for manual extraction of the placenta
The timing for the diagnosis of retained placenta, and the risks and benefits of manual extraction would be best evaluated in a large, randomized clinical trial. However, based on observational studies, in a term pregnancy, the diagnosis of retained placenta is best made using a 20-minute interval. In women with effective neuraxial anesthesia, consideration should be given to manual removal of the placenta at that time.
- Begley CM, Gyte GM, Devane D, et al. Active versus expectant management for women in the third stage of labor. Cochrane Database Syst Rev. 2019;2:CD007412.
- Adnan N, Conlan-Trant R, McCormick C, et al. Intramuscular versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery: randomized controlled trial. BMJ. 2018;362:k3546.
- Gülmezoglu AM, Souza JP. The evolving management of the third stage of labour. BJOG. 2009;116(suppl 1):26-28.
- Ebert AD, David M. Meilensteine der Praventionsmedizin. Carl Siegmund Franz Credé (1819-1882), der Credesche Handgriff und die Credesche Augenprophylaxe. Geburtshilfe Frauenheilkd. 2016;76:675-678.
- Brandt ML. The mechanism and management of the third stage of labor. Am J Obstet Gynecol. 1933;25:662-667.
- Kimbell N. Brandt-Andrews technique of delivery of the placenta. Br Med J. 1958;1:203-204.
- De Lee JB, Greenhill JP. Principles and Practice of Obstetrics. 9th ed. Philadelphia, PA: Saunders; 1947:275.
- Du Y, Ye M, Zheng F. Active management of the third stage of labor with and without controlled cord traction: a systematic review and meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand. 2014;93:626-633.
- Hofmeyr GJ, Mshweshwe NT, Gülmezoglu AM. Controlled cord traction for the third stage of labor. Cochrane Database Syst Rev. 2015;1:CD008020.
- Frolova AI, Stout MJ, Tuuli MG, et al. Duration of the third stage of labor and risk of postpartum hemorrhage. Obstet Gynecol. 2016;127:951-956.
- Shinar S, Schwartz A, Maslovitz S, et al. How long is safe? Setting the cutoff for uncomplicated third stage length: a retrospective case-control study. Birth. 2016;43:36-41.
- Magann EF, Evans S, Chauhan SP, et al. The length of the third stage of labor and the risk of postpartum hemorrhage. Obstet Gynecol. 2005;105:290-293.
- Cummings K, Doherty DA, Magann EF, et al. Timing of manual placenta removal to prevent postpartum hemorrhage: is it time to act? J Matern Fetal Neonatal Med. 2016;29:3930-3933.
- Rabie NZ, Ounpraseuth S, Hughes D, et al. Association of the length of the third stage of labor and blood loss following vaginal delivery. South Med J. 2018;111:178-182.
- Rosenstein MG, Vargas JE, Drey EA. Ultrasound-guided instrumental removal of the retained placenta after vaginal delivery. Am J Obstet Gynecol. 2014;211:180.e1-e3.
- Chibueze EC, Parsons AJ, Ota E, et al. Prophylactic antibiotics for manual removal of retained placenta during vaginal birth: a systematic review of observational studies and meta-analysis. BMC Pregnancy Childbirth. 2015;15:313.
- Abdel-Aleem H, Abdel-Aleem MA, Shaaban OM. Nitroglycerin for management of retained placenta. Cochrane Database Syst Rev. 2015;(11):CD007708.
- Weissback T, Haikin-Herzberger E, Bacci-Hugger K, et al. Immediate postpartum ultrasound evaluation for suspected retained placental tissue in patients undergoing manual removal of placenta. Eur J Obstet Gynecol Reprod Biol. 2015;192:37-40.
You have just safely delivered the baby who is quietly resting on her mother’s chest. You begin active management of the third stage of labor, administering oxytocin, performing uterine massage and applying controlled tension on the umbilical cord. There is no evidence of excess postpartum bleeding.
How long will you wait to deliver the placenta?
Active management of the third stage of labor
Most authorities recommend active management of the third stage of labor because active management reduces the risk of maternal hemorrhage >1,000 mL (relative risk [RR], 0.34), postpartum hemoglobin levels < 9 g/dL (RR, 0.50), and maternal blood transfusion (RR, 0.35) compared with expectant management.1
The most important component of active management of the third stage of labor is the administration of a uterotonic after delivery of the newborn. In the United States, oxytocin is the uterotonic most often utilized for the active management of the third stage of labor. Authors of a recent randomized clinical trial reported that intravenous oxytocin is superior to intramuscular oxytocin for reducing postpartum blood loss (385 vs 445 mL), the frequency of blood loss greater than 1,000 mL (4.6% vs 8.1%), and the rate of maternal blood transfusion (1.5% vs 4.4%).2
In addition to administering oxytocin, the active management of the third stage often involves maneuvers to accelerate placental delivery, including the Crede and Brandt-Andrews maneuvers and controlled tension on the umbilical cord. The Crede maneuver, described in 1853, involves placing a hand on the abdominal wall near the uterine fundus and squeezing the uterine fundus between the thumb and fingers.3,4
The Brandt-Andrews maneuver, described in 1933, involves placing a clamp on the umbilical cord close to the vulva.5 The clamp is used to apply judicious tension on the cord with one hand, while the other hand is placed on the mother’s abdomen with the palm and fingers overlying the junction between the uterine corpus and the lower segment. With judicious tension on the cord, the abdominal hand pushes the uterus upward toward the umbilicus. Placental separation is indicated when lengthening of the umbilical cord occurs. The Brandt-Andrews maneuver may be associated with fewer cases of uterine inversion than the Crede maneuver.5-7
Of note, umbilical cord traction has not been demonstrated to reduce the need for blood transfusion or the incidence of postpartum hemorrhage (PPH) >1,000 mL, and it is commonly utilized by obstetricians and midwives.8,9 Hence, in the third stage, the delivering clinician should routinely administer a uterotonic, but use of judicious tension on the cord can be deferred if the woman prefers a noninterventional approach to delivery.
Following a vaginal birth, when should the diagnosis of retained placenta be made?
The historic definition of retained placenta is nonexpulsion of the placenta 30 minutes after delivery of the newborn. However, many observational studies report that, when active management of the third stage is utilized, 90%, 95%, and 99% of placentas deliver by 9 minutes, 13 minutes, and 28 minutes, respectively.10 In addition, many observational studies report that the incidence of PPH increases significantly with longer intervals between birth of the newborn and delivery of the placenta. In one study the rate of blood loss >500 mL was 8.5% when the placenta delivered between 5 and 9 minutes and 35.1% when the placenta delivered ≥30 minutes following birth of the baby.10 In another observational study, compared with women delivering the placenta < 10 minutes after birth, women delivering the placenta ≥30 minutes after birth had a 3-fold increased risk of PPH.11 Similar findings have been reported in other studies.12-14
Continue to: Based on the association between a delay in delivery...
Based on the association between a delay in delivery of the placenta and an increased risk of PPH, some authorities recommend that, in term pregnancy, the diagnosis of retained placenta should be made at 20 minutes following birth and consideration should be given to removing the placenta at this time. For women with effective neuraxial anesthesia, manual removal of the placenta 20 minutes following birth may be the best decision for balancing the benefit of preventing PPH with the risk of unnecessary intervention. For women with no anesthesia, delaying manual removal of the placenta to 30 minutes or more following birth may permit more time for the placenta to deliver prior to performing an intervention that might cause pain, but the delay increases the risk of PPH.
The retained placenta may prevent the uterine muscle from effectively contracting around penetrating veins and arteries, thereby increasing the risk of postpartum hemorrhage. The placenta that has separated from the uterine wall but is trapped inside the uterine cavity can be removed easily with manual extraction. If the placenta is physiologically adherent to the uterine wall, a gentle sweeping motion with an intrauterine hand usually can separate the placenta from the uterus in preparation for manual extraction. However, if a placenta accreta spectrum disorder is contributing to a retained placenta, it may be difficult to separate the densely adherent portion of the uterus from the uterine wall. In the presence of placenta accreta spectrum disorder, vigorous attempts to remove the placenta may precipitate massive bleeding. In some cases, the acchoucheur/midwife may recognize the presence of a focal accreta and cease attempts to remove the placenta in order to organize the personnel and equipment needed to effectively treat a potential case of placenta accreta. In one study, when a placenta accreta was recognized or suspected, immediately ceasing attempts at manually removing the placenta resulted in better case outcomes than continued attempts to remove the placenta.1
Uterine inversion may occur during an attempt to manually remove the placenta. There is universal agreement that once a uterine inversion is recognized it is critically important to immediately restore normal uterine anatomy to avoid massive hemorrhage and maternal shock. The initial management of uterine inversion includes:
- stopping oxytocin infusion
- initiating high volume fluid resuscitation
- considering a dose of a uterine relaxant, such as nitroglycerin or terbutaline
- preparing for blood product replacement.
In my experience, when uterine inversion is immediately recognized and successfully treated, blood product replacement is not usually necessary. However, if uterine inversion has not been immediately recognized or treated, massive hemorrhage and shock may occur.
Two approaches to the vaginal restoration of uterine anatomy involve using the tips of the fingers and palm of the hand to guide the wall of the uterus back to its normal position (FIGURE 1) or to forcefully use a fist to force the uterine wall back to its normal position (FIGURE 2). If these maneuvers are unsuccessful, a laparotomy may be necessary.


At laparotomy, the Huntington or Haultain procedures may help restore normal uterine anatomy. The Huntington procedure involves using clamps to apply symmetrical tension to the left and right round ligaments and/or uterine serosa to sequentially tease the uterus back to normal anatomy.2,3 The Haultain procedure involves a vertical incision on the posterior wall of the uterus to release the uterine constriction ring that is preventing the return of the uterine fundus to its normal position (FIGURE 3).4,5

References
- Kayem G, Anselem O, Schmitz T, et al. Conservative versus radical management in cases of placenta accreta: a historical study. J Gynecol Obstet Biol Reprod (Paris). 2007;36:680-687.
- Huntington JL. Acute inversion of the uterus. Boston Med Surg J. 1921;184:376-378.
- Huntington JL, Irving FC, Kellogg FS. Abdominal reposition in acute inversion of the puerperal uterus. Am J Obstet Gynecol. 1928;15:34-40.
- Haultain FW. Abdominal hysterotomy for chronic uterine inversion: a record of 3 cases. Proc Roy Soc Med. 1908;1:528-535.
- Easterday CL, Reid DE. Inversion of the puerperal uterus managed by the Haultain technique; A case report. Am J Obstet Gynecol. 1959;78:1224-1226.
Manual extraction of the placenta
Prior to performing manual extraction of the placenta, a decision should be made regarding the approach to anesthesia and perioperative antibiotics. Manual extraction of the placenta is performed by placing one hand on the uterine fundus to stabilize the uterus and using the other hand to follow the umbilical cord into the uterine cavity. The intrauterine hand is used to separate the uterine-placental interface with a gentle sweeping motion. The placental mass is grasped and gently teased through the cervix and vagina. Inspection of the placenta to ensure complete removal is necessary.
An alternative to manual extraction of the placenta is the use of Bierer forceps and ultrasound guidance to tease the placenta through the cervical os. This technique involves the following steps15:
1. use ultrasound to locate the placenta
2. place a ring forceps on the anterior lip of the cervix
3. introduce the Bierer forcep into the uterus
4. use the forceps to grasp the placenta and pull it toward the vagina
5. stop frequently to re-grasp placental tissue that is deeper in the uterine cavity
6. once the placenta is extracted, examine the placenta to ensure complete removal.
Of note when manual extraction is used to deliver a retained placenta, randomized clinical trials report no benefit for the following interventions:
- perioperative antibiotics16
- nitroglycerin to relax the uterus17
- ultrasound to detect retained placental tissue.18
Best timing for manual extraction of the placenta
The timing for the diagnosis of retained placenta, and the risks and benefits of manual extraction would be best evaluated in a large, randomized clinical trial. However, based on observational studies, in a term pregnancy, the diagnosis of retained placenta is best made using a 20-minute interval. In women with effective neuraxial anesthesia, consideration should be given to manual removal of the placenta at that time.
You have just safely delivered the baby who is quietly resting on her mother’s chest. You begin active management of the third stage of labor, administering oxytocin, performing uterine massage and applying controlled tension on the umbilical cord. There is no evidence of excess postpartum bleeding.
How long will you wait to deliver the placenta?
Active management of the third stage of labor
Most authorities recommend active management of the third stage of labor because active management reduces the risk of maternal hemorrhage >1,000 mL (relative risk [RR], 0.34), postpartum hemoglobin levels < 9 g/dL (RR, 0.50), and maternal blood transfusion (RR, 0.35) compared with expectant management.1
The most important component of active management of the third stage of labor is the administration of a uterotonic after delivery of the newborn. In the United States, oxytocin is the uterotonic most often utilized for the active management of the third stage of labor. Authors of a recent randomized clinical trial reported that intravenous oxytocin is superior to intramuscular oxytocin for reducing postpartum blood loss (385 vs 445 mL), the frequency of blood loss greater than 1,000 mL (4.6% vs 8.1%), and the rate of maternal blood transfusion (1.5% vs 4.4%).2
In addition to administering oxytocin, the active management of the third stage often involves maneuvers to accelerate placental delivery, including the Crede and Brandt-Andrews maneuvers and controlled tension on the umbilical cord. The Crede maneuver, described in 1853, involves placing a hand on the abdominal wall near the uterine fundus and squeezing the uterine fundus between the thumb and fingers.3,4
The Brandt-Andrews maneuver, described in 1933, involves placing a clamp on the umbilical cord close to the vulva.5 The clamp is used to apply judicious tension on the cord with one hand, while the other hand is placed on the mother’s abdomen with the palm and fingers overlying the junction between the uterine corpus and the lower segment. With judicious tension on the cord, the abdominal hand pushes the uterus upward toward the umbilicus. Placental separation is indicated when lengthening of the umbilical cord occurs. The Brandt-Andrews maneuver may be associated with fewer cases of uterine inversion than the Crede maneuver.5-7
Of note, umbilical cord traction has not been demonstrated to reduce the need for blood transfusion or the incidence of postpartum hemorrhage (PPH) >1,000 mL, and it is commonly utilized by obstetricians and midwives.8,9 Hence, in the third stage, the delivering clinician should routinely administer a uterotonic, but use of judicious tension on the cord can be deferred if the woman prefers a noninterventional approach to delivery.
Following a vaginal birth, when should the diagnosis of retained placenta be made?
The historic definition of retained placenta is nonexpulsion of the placenta 30 minutes after delivery of the newborn. However, many observational studies report that, when active management of the third stage is utilized, 90%, 95%, and 99% of placentas deliver by 9 minutes, 13 minutes, and 28 minutes, respectively.10 In addition, many observational studies report that the incidence of PPH increases significantly with longer intervals between birth of the newborn and delivery of the placenta. In one study the rate of blood loss >500 mL was 8.5% when the placenta delivered between 5 and 9 minutes and 35.1% when the placenta delivered ≥30 minutes following birth of the baby.10 In another observational study, compared with women delivering the placenta < 10 minutes after birth, women delivering the placenta ≥30 minutes after birth had a 3-fold increased risk of PPH.11 Similar findings have been reported in other studies.12-14
Continue to: Based on the association between a delay in delivery...
Based on the association between a delay in delivery of the placenta and an increased risk of PPH, some authorities recommend that, in term pregnancy, the diagnosis of retained placenta should be made at 20 minutes following birth and consideration should be given to removing the placenta at this time. For women with effective neuraxial anesthesia, manual removal of the placenta 20 minutes following birth may be the best decision for balancing the benefit of preventing PPH with the risk of unnecessary intervention. For women with no anesthesia, delaying manual removal of the placenta to 30 minutes or more following birth may permit more time for the placenta to deliver prior to performing an intervention that might cause pain, but the delay increases the risk of PPH.
The retained placenta may prevent the uterine muscle from effectively contracting around penetrating veins and arteries, thereby increasing the risk of postpartum hemorrhage. The placenta that has separated from the uterine wall but is trapped inside the uterine cavity can be removed easily with manual extraction. If the placenta is physiologically adherent to the uterine wall, a gentle sweeping motion with an intrauterine hand usually can separate the placenta from the uterus in preparation for manual extraction. However, if a placenta accreta spectrum disorder is contributing to a retained placenta, it may be difficult to separate the densely adherent portion of the uterus from the uterine wall. In the presence of placenta accreta spectrum disorder, vigorous attempts to remove the placenta may precipitate massive bleeding. In some cases, the acchoucheur/midwife may recognize the presence of a focal accreta and cease attempts to remove the placenta in order to organize the personnel and equipment needed to effectively treat a potential case of placenta accreta. In one study, when a placenta accreta was recognized or suspected, immediately ceasing attempts at manually removing the placenta resulted in better case outcomes than continued attempts to remove the placenta.1
Uterine inversion may occur during an attempt to manually remove the placenta. There is universal agreement that once a uterine inversion is recognized it is critically important to immediately restore normal uterine anatomy to avoid massive hemorrhage and maternal shock. The initial management of uterine inversion includes:
- stopping oxytocin infusion
- initiating high volume fluid resuscitation
- considering a dose of a uterine relaxant, such as nitroglycerin or terbutaline
- preparing for blood product replacement.
In my experience, when uterine inversion is immediately recognized and successfully treated, blood product replacement is not usually necessary. However, if uterine inversion has not been immediately recognized or treated, massive hemorrhage and shock may occur.
Two approaches to the vaginal restoration of uterine anatomy involve using the tips of the fingers and palm of the hand to guide the wall of the uterus back to its normal position (FIGURE 1) or to forcefully use a fist to force the uterine wall back to its normal position (FIGURE 2). If these maneuvers are unsuccessful, a laparotomy may be necessary.


At laparotomy, the Huntington or Haultain procedures may help restore normal uterine anatomy. The Huntington procedure involves using clamps to apply symmetrical tension to the left and right round ligaments and/or uterine serosa to sequentially tease the uterus back to normal anatomy.2,3 The Haultain procedure involves a vertical incision on the posterior wall of the uterus to release the uterine constriction ring that is preventing the return of the uterine fundus to its normal position (FIGURE 3).4,5

References
- Kayem G, Anselem O, Schmitz T, et al. Conservative versus radical management in cases of placenta accreta: a historical study. J Gynecol Obstet Biol Reprod (Paris). 2007;36:680-687.
- Huntington JL. Acute inversion of the uterus. Boston Med Surg J. 1921;184:376-378.
- Huntington JL, Irving FC, Kellogg FS. Abdominal reposition in acute inversion of the puerperal uterus. Am J Obstet Gynecol. 1928;15:34-40.
- Haultain FW. Abdominal hysterotomy for chronic uterine inversion: a record of 3 cases. Proc Roy Soc Med. 1908;1:528-535.
- Easterday CL, Reid DE. Inversion of the puerperal uterus managed by the Haultain technique; A case report. Am J Obstet Gynecol. 1959;78:1224-1226.
Manual extraction of the placenta
Prior to performing manual extraction of the placenta, a decision should be made regarding the approach to anesthesia and perioperative antibiotics. Manual extraction of the placenta is performed by placing one hand on the uterine fundus to stabilize the uterus and using the other hand to follow the umbilical cord into the uterine cavity. The intrauterine hand is used to separate the uterine-placental interface with a gentle sweeping motion. The placental mass is grasped and gently teased through the cervix and vagina. Inspection of the placenta to ensure complete removal is necessary.
An alternative to manual extraction of the placenta is the use of Bierer forceps and ultrasound guidance to tease the placenta through the cervical os. This technique involves the following steps15:
1. use ultrasound to locate the placenta
2. place a ring forceps on the anterior lip of the cervix
3. introduce the Bierer forcep into the uterus
4. use the forceps to grasp the placenta and pull it toward the vagina
5. stop frequently to re-grasp placental tissue that is deeper in the uterine cavity
6. once the placenta is extracted, examine the placenta to ensure complete removal.
Of note when manual extraction is used to deliver a retained placenta, randomized clinical trials report no benefit for the following interventions:
- perioperative antibiotics16
- nitroglycerin to relax the uterus17
- ultrasound to detect retained placental tissue.18
Best timing for manual extraction of the placenta
The timing for the diagnosis of retained placenta, and the risks and benefits of manual extraction would be best evaluated in a large, randomized clinical trial. However, based on observational studies, in a term pregnancy, the diagnosis of retained placenta is best made using a 20-minute interval. In women with effective neuraxial anesthesia, consideration should be given to manual removal of the placenta at that time.
- Begley CM, Gyte GM, Devane D, et al. Active versus expectant management for women in the third stage of labor. Cochrane Database Syst Rev. 2019;2:CD007412.
- Adnan N, Conlan-Trant R, McCormick C, et al. Intramuscular versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery: randomized controlled trial. BMJ. 2018;362:k3546.
- Gülmezoglu AM, Souza JP. The evolving management of the third stage of labour. BJOG. 2009;116(suppl 1):26-28.
- Ebert AD, David M. Meilensteine der Praventionsmedizin. Carl Siegmund Franz Credé (1819-1882), der Credesche Handgriff und die Credesche Augenprophylaxe. Geburtshilfe Frauenheilkd. 2016;76:675-678.
- Brandt ML. The mechanism and management of the third stage of labor. Am J Obstet Gynecol. 1933;25:662-667.
- Kimbell N. Brandt-Andrews technique of delivery of the placenta. Br Med J. 1958;1:203-204.
- De Lee JB, Greenhill JP. Principles and Practice of Obstetrics. 9th ed. Philadelphia, PA: Saunders; 1947:275.
- Du Y, Ye M, Zheng F. Active management of the third stage of labor with and without controlled cord traction: a systematic review and meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand. 2014;93:626-633.
- Hofmeyr GJ, Mshweshwe NT, Gülmezoglu AM. Controlled cord traction for the third stage of labor. Cochrane Database Syst Rev. 2015;1:CD008020.
- Frolova AI, Stout MJ, Tuuli MG, et al. Duration of the third stage of labor and risk of postpartum hemorrhage. Obstet Gynecol. 2016;127:951-956.
- Shinar S, Schwartz A, Maslovitz S, et al. How long is safe? Setting the cutoff for uncomplicated third stage length: a retrospective case-control study. Birth. 2016;43:36-41.
- Magann EF, Evans S, Chauhan SP, et al. The length of the third stage of labor and the risk of postpartum hemorrhage. Obstet Gynecol. 2005;105:290-293.
- Cummings K, Doherty DA, Magann EF, et al. Timing of manual placenta removal to prevent postpartum hemorrhage: is it time to act? J Matern Fetal Neonatal Med. 2016;29:3930-3933.
- Rabie NZ, Ounpraseuth S, Hughes D, et al. Association of the length of the third stage of labor and blood loss following vaginal delivery. South Med J. 2018;111:178-182.
- Rosenstein MG, Vargas JE, Drey EA. Ultrasound-guided instrumental removal of the retained placenta after vaginal delivery. Am J Obstet Gynecol. 2014;211:180.e1-e3.
- Chibueze EC, Parsons AJ, Ota E, et al. Prophylactic antibiotics for manual removal of retained placenta during vaginal birth: a systematic review of observational studies and meta-analysis. BMC Pregnancy Childbirth. 2015;15:313.
- Abdel-Aleem H, Abdel-Aleem MA, Shaaban OM. Nitroglycerin for management of retained placenta. Cochrane Database Syst Rev. 2015;(11):CD007708.
- Weissback T, Haikin-Herzberger E, Bacci-Hugger K, et al. Immediate postpartum ultrasound evaluation for suspected retained placental tissue in patients undergoing manual removal of placenta. Eur J Obstet Gynecol Reprod Biol. 2015;192:37-40.
- Begley CM, Gyte GM, Devane D, et al. Active versus expectant management for women in the third stage of labor. Cochrane Database Syst Rev. 2019;2:CD007412.
- Adnan N, Conlan-Trant R, McCormick C, et al. Intramuscular versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery: randomized controlled trial. BMJ. 2018;362:k3546.
- Gülmezoglu AM, Souza JP. The evolving management of the third stage of labour. BJOG. 2009;116(suppl 1):26-28.
- Ebert AD, David M. Meilensteine der Praventionsmedizin. Carl Siegmund Franz Credé (1819-1882), der Credesche Handgriff und die Credesche Augenprophylaxe. Geburtshilfe Frauenheilkd. 2016;76:675-678.
- Brandt ML. The mechanism and management of the third stage of labor. Am J Obstet Gynecol. 1933;25:662-667.
- Kimbell N. Brandt-Andrews technique of delivery of the placenta. Br Med J. 1958;1:203-204.
- De Lee JB, Greenhill JP. Principles and Practice of Obstetrics. 9th ed. Philadelphia, PA: Saunders; 1947:275.
- Du Y, Ye M, Zheng F. Active management of the third stage of labor with and without controlled cord traction: a systematic review and meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand. 2014;93:626-633.
- Hofmeyr GJ, Mshweshwe NT, Gülmezoglu AM. Controlled cord traction for the third stage of labor. Cochrane Database Syst Rev. 2015;1:CD008020.
- Frolova AI, Stout MJ, Tuuli MG, et al. Duration of the third stage of labor and risk of postpartum hemorrhage. Obstet Gynecol. 2016;127:951-956.
- Shinar S, Schwartz A, Maslovitz S, et al. How long is safe? Setting the cutoff for uncomplicated third stage length: a retrospective case-control study. Birth. 2016;43:36-41.
- Magann EF, Evans S, Chauhan SP, et al. The length of the third stage of labor and the risk of postpartum hemorrhage. Obstet Gynecol. 2005;105:290-293.
- Cummings K, Doherty DA, Magann EF, et al. Timing of manual placenta removal to prevent postpartum hemorrhage: is it time to act? J Matern Fetal Neonatal Med. 2016;29:3930-3933.
- Rabie NZ, Ounpraseuth S, Hughes D, et al. Association of the length of the third stage of labor and blood loss following vaginal delivery. South Med J. 2018;111:178-182.
- Rosenstein MG, Vargas JE, Drey EA. Ultrasound-guided instrumental removal of the retained placenta after vaginal delivery. Am J Obstet Gynecol. 2014;211:180.e1-e3.
- Chibueze EC, Parsons AJ, Ota E, et al. Prophylactic antibiotics for manual removal of retained placenta during vaginal birth: a systematic review of observational studies and meta-analysis. BMC Pregnancy Childbirth. 2015;15:313.
- Abdel-Aleem H, Abdel-Aleem MA, Shaaban OM. Nitroglycerin for management of retained placenta. Cochrane Database Syst Rev. 2015;(11):CD007708.
- Weissback T, Haikin-Herzberger E, Bacci-Hugger K, et al. Immediate postpartum ultrasound evaluation for suspected retained placental tissue in patients undergoing manual removal of placenta. Eur J Obstet Gynecol Reprod Biol. 2015;192:37-40.