User login
E-cigarettes and vapes: Do they work for smoking cessation and should we be recommending their use?
The popularity of electronic cigarettes (E-cigs) and “vapes” has grown dramatically, spawning a new industry of electronic nicotine delivery systems (ENDS). With the increasing use of E-cigs not only for smoking cessation, but also as a primary nicotine source, it is important for mental health professionals to be prepared to discuss use of these devices with patients. In this article, we will describe:
- the composition of E-cigs and their current use
- evidence for their use for smoking cessation
- adverse health effects
- recommendations of major regulatory agencies.
Finally, we will provide recommendations for E-cig use in clinical populations.
What is an electronic nicotine delivery system?
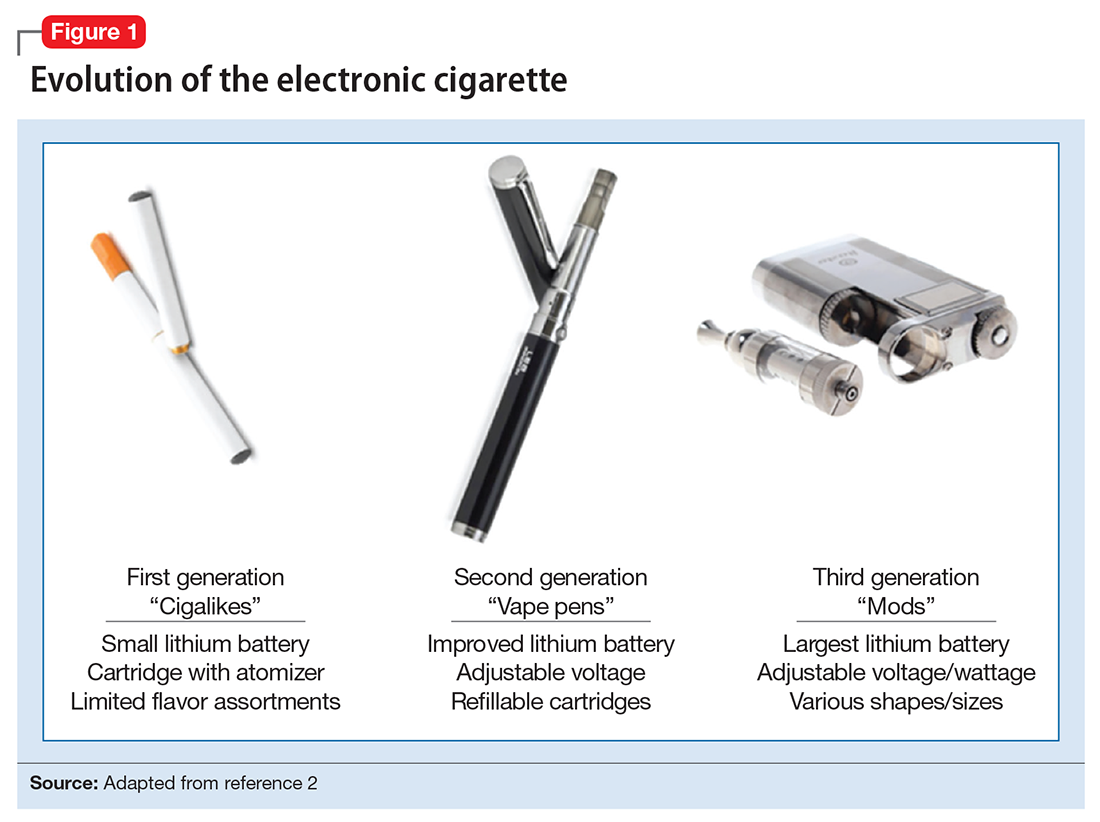
ENDS produce an aerosol with or without nicotine that is inhaled and is thought to mimic the use of combustible cigarettes. ENDS evolved from basic E-cigs into a less “cigarette-like” and more customizable product (Figure 1). ENDS include a range of designs and go by various names, including “personal vaporizers,” “e-cigars,” and “e-hookahs” (in this article, we will use the term “ENDS” to refer to these devices).
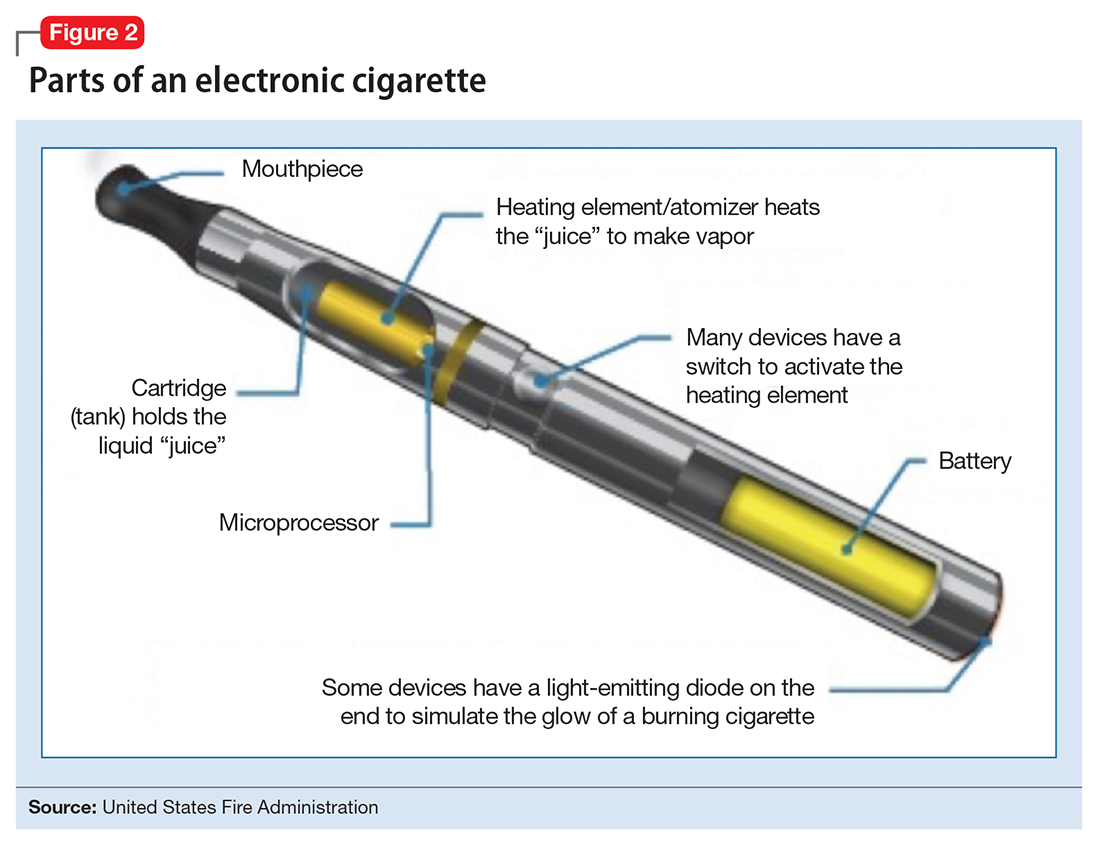
The general design of ENDS is a plastic tubing system that contains a mouthpiece, battery, electronic heating element (“vaporizer”), and a cartridge with liquid solvent with or without nicotine or flavoring (Figure 2). One draw on the mouthpiece or press of a button activates the device, heats the solution, and delivers a vapor in a similar manner to taking a puff of a cigarette. Although studies have shown that ENDS result in significant increases in plasma nicotine concentrations in 5 minutes,1 the plasma nicotine levels obtained with the first-generation “cigarette-like” ENDS are much lower than those caused by inhaling tobacco smoke.2 Over time nicotine delivery capability has improved as ENDS have evolved such that the rate of nicotine delivery and peak concentration obtained with newer models more closely mirror tobacco cigarettes.3 Whether the rapid delivery of larger amounts of nicotine helps or hinders one’s efforts to break nicotine addiction remains to be determined because of the reinforcing properties of the drug.
The liquid in the E-cig cartridge typically contains not only nicotine but a number of chemical compounds with potentially deleterious or unknown health risks. The 3 main ingredients include:
- a solvent of glycerin and/or propylene glycol
- nicotine in various concentrations
- flavorings.
The glycerin or propylene glycol forms the basis for the aerosol. Nicotine concentrations vary from 0 (denicotinized) to 35 mcg per puff.4 A study reported 7,700 unique flavors available for vaping liquid.5 The liquid also contains impurities, such as anabasine, which has effects on the α-7 nicotinic acetylcholine receptor and its principal use is as an insecticide and β-nicotyrine, which inhibits cytochrome P450 2A.
Epidemiology and end-user perspectives
In 2014, 12.4% of U.S. adults classified themselves as “ever users” of ENDS (used at least once) and 3.7% of adults classified themselves as current users, according to the National Health Interview Study.6 Importantly, among E-cig users who had not used combustible cigarettes, young adults (age 18 to 24) were more likely to have tried ENDS than older adults. ENDS are becoming more popular across the globe. A study in the European Union found that ever users of ENDS most commonly were current cigarette smokers (31%) followed by former (10.8%) and never smokers (2.3%).7
ENDS use is relevant for mental health professionals because of the high rate of comorbid tobacco use disorder in individuals with psychiatric conditions. For example, 2 U.S. population surveys8,9 revealed those with mental health conditions were 1.5 to 2 times more likely to have tried ENDS and 2 to 3 times more likely to be current users. Those with psychiatric illness reported similar reasons for ENDS use as other individuals, including “just because,” use as a smoking cessation aid, ease of use, and perceived safety vs combustible cigarettes.
A recent review that included 9 studies focusing on ENDS use in those with mental illness reported mixed findings on the utility of these devices to reduce or stop use of combustible cigarettes.10 Additionally, it is important to monitor the use of cigarettes and ENDS in patients with psychiatric illness because the byproducts of tobacco smoke can affect the metabolism of some psychotropic medications.11 Although reduced use of combustible cigarettes could lead to lower dosing of some psychotropics, an unreported decrease in combustible cigarette use could lead to supratherapeutic drug levels. There are no data on the effect of ENDS on the metabolism of psychotropics.
ENDS are increasingly popular among adolescents. In 2015, there were an estimated 4.6 million current tobacco users among middle/high school youths in the United States and 3 million current ENDS users, according to the National Youth Tobacco Surveys.12 The shift from combustible cigarettes to ENDS is notable, with an increase in the percentage of current E-cig users and a decrease in the percentage of exclusive combustible cigarette users. In addition, there has been no change in the prevalence of lifetime tobacco users.12 This is a global issue, as reports of ever use of ENDS by adolescents range from 6.5% to 31% in the United States, 14.6% in Canada, and 4.7% to 38.5% in Europe.13 Based on these trends, the U.S. Surgeon General released a statement warning against the use of ENDS in youth because of the lack of safety data and strong association with use of tobacco products.14
There are a number of possible reasons for the increasing popularity of ENDS, including the product’s novelty, lack of regulations regarding their sale, availability of flavorings, and the perception that ENDS are safe alternatives to cigarettes. E-cig–using youths have described ENDS as “not at all harmful” and “not at all addictive” and believe that ENDS with flavoring are less harmful than those without.15 Although studies in adults show some users reporting that ENDS are less satisfying, they are seen as useful in decreasing craving and a safer alternative to cigarettes.16,17
Are ENDS effective for smoking cessation?
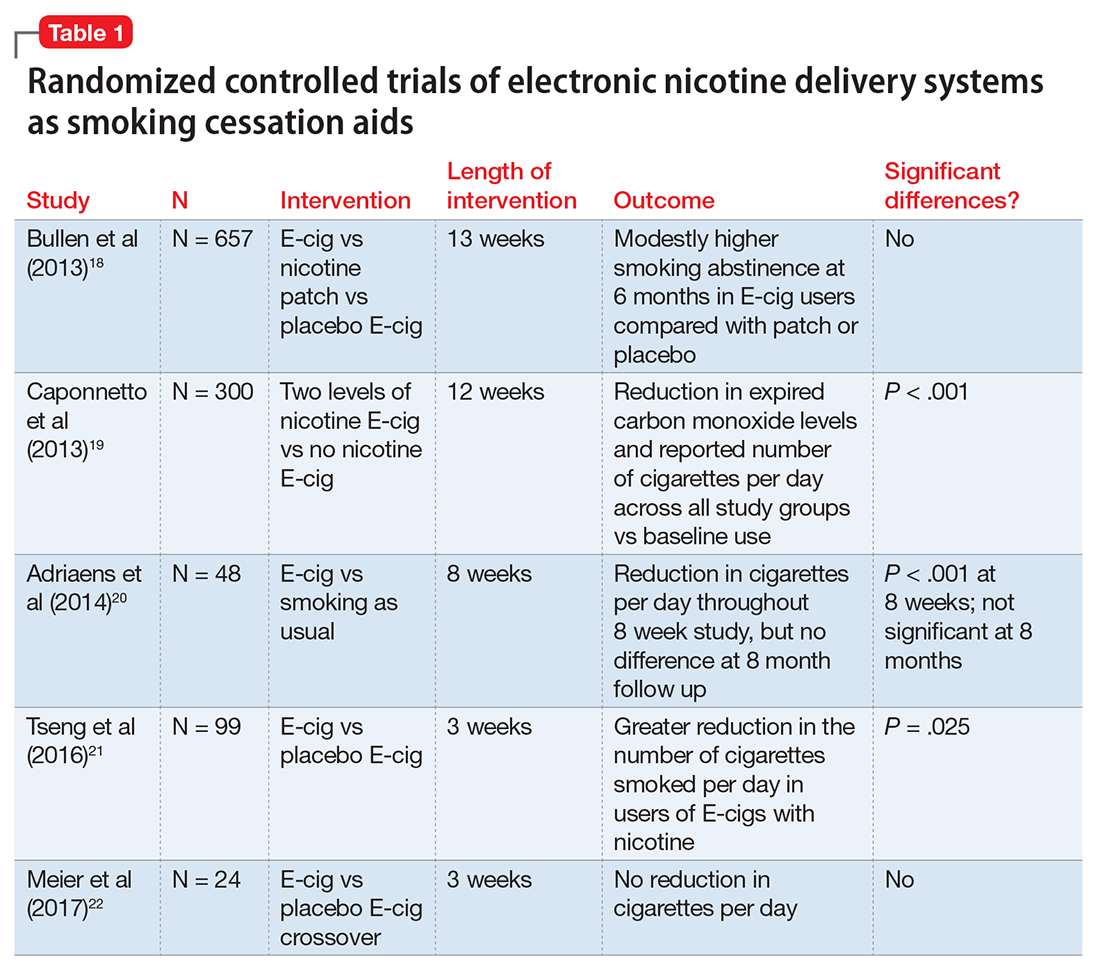
The evidence for ENDS as aids to smoking cessation remains murky (Table 118-22). There is a paucity of randomized controlled clinical trials (RCTs) investigating ENDS for smoking cessation or reduction, and it is difficult to quantify the amount of nicotine used in ENDS because of the variety of delivery systems and cartridges. In a recent Cochrane review, those using ENDS to quit smoking were more likely to be abstinent from combustible cigarettes at 6 months vs those using nicotine-free ENDS (relative risk = 2.29; 95% CI, 1.05 to 4.96), but there was no significant difference in quit rates compared with nicotine patches.23 However, the confidence in this finding was rated as low because of the limited number of RCTs. Of note, the authors found 15 ongoing RCTs at the time of publication that might be eligible for later evaluation.
Non-RCTs reveal mixed data. Positive results include 1 study with an odds ratio of 6.07 to quit for intensive ENDS users vs non-users,24 and another with dual users of combustible and electronic cigarettes having a 46% quit rate at 1 year.25 Additionally, in a pilot study providing ENDS to 14 patients with schizophrenia who had no previous desire to quit smoking, authors noted a reduction in the number of cigarettes smoked per day by 50% in one-half of participants and abstinence in 14% of participants at 52 weeks.26 Studies with neutral or negative results include those showing ENDS users to be current combustible tobacco smokers, and use of ENDS not predicting smoking cessation.4,27 Data also are mixed regarding the use of ENDS as a harm reduction strategy. One study found that ENDS decreased cigarette consumption, but did not increase the likelihood of quitting,28 while another reported that daily use of ENDS increased the odds of reducing smoking by as much as 2.5 times compared with non-use of such aids.29 In a 24-month prospective cohort study following tobacco users, there was no difference in the number of cigarettes smoked per day in those who started the trial as users of combustible cigarettes alone vs combustible cigarettes plus ENDS users.30 Interestingly, those who started the study as combustible cigarette users and switched to ENDS and those who had continued dual use throughout the 24 months smoked fewer combustible cigarettes per day than those who never tried ENDS or quit during the study period.
Health effects

To better understand the adverse health effects of ENDS, one must consider potential short- and long-term consequences (Table 2). In the short-term, ENDS have been found to increase markers of inflammation and oxidative stress acutely as evidenced by in vivo laboratory studies.31,32 ENDS also have been linked to upper respiratory irritation, in part, because of the transformation of glycerin in the nicotine cartridge to acrolein upon combustion.33 Even 5 minutes of ad lib E-cig use has been found to significantly increase airflow resistance during pulmonary function tests34—changes that have been shown to precede more persistent alterations in peak expiratory flow, such as those seen in chronic obstructive pulmonary disease. The more common patient-reported side effects include:
- daytime cough (27%)
- phlegm production (25%)
- headache (21%)
- dry mouth/throat (20%)
- vertigo, headache, or nausea (9%).35,36
A RCT investigating efficacy of E-cigs vs nicotine patches vs denicotinized E-cigs found no difference among the groups in the number of reported adverse events.18 Interestingly, another RCT found a decrease in adverse events, such as dry cough, mouth irritation, throat irritation, shortness of breath, and headache, compared with baseline in combustible cigarette smokers who used regular or denicotinized E-cigs.19
Although no studies have directly investigated long-term health consequences of ENDS because of their relative novelty, one can extrapolate potential harmful long-term effects based on knowledge of the products’ chemical constituents. For example, propylene glycol can degrade into propylene oxide, a class 2B carcinogen.37 Other potential carcinogens in the aerosol include formaldehyde and acetaldehyde. On a broader scale, many of the particulates have been shown to cause systemic inflammation, which is thought to increase cardiovascular and respiratory disease and death.38 Flavorings in ENDS include a variety of components including, but not limited to, aldehydes, which are irritants, and other additives that have been associated with respiratory disease.39
Second-hand exposure. There are no long-term studies of second-hand vapor exposure, but similar to long-term health on primary users, one can glean some observations from the literature. It is promising that compared with cigarettes, ENDS lack sidestream smoke and the vapor has not been found to contain carbon monoxide.40 Some research has demonstrated that the size and spray of fine particles in the aerosol is as large or larger than combustible cigarettes.41 Formaldehyde, acetaldehyde, isoprene, and acetic acid have been found in ENDS vapor.40 Interestingly, a simulated café study found elevated nicotine, glycerine, hydrocarbon, and other materials classified as carcinogens in the air.42
Although it is popularly thought that ENDS are less toxic than tobacco cigarettes, there is not enough evidence to estimate precisely as to how much less toxic or the consequences of use. ENDS are increasingly popular and are being used by never smokers who should be educated on the potential harm that ENDS pose.
Recommendations from agencies and medical organizations
The World Health Organization (WHO) recommended prohibiting the use of ENDS in indoor spaces to minimize potential health risks to users and non-users. The WHO also aims to prevent dissemination of unproven health claims, including claims that ENDS are effective—or not—or that the devices are innocuous.36 In the United States, the FDA has stated that ENDS are not recommended for safe quitting (2009). In August 2016, the FDA introduced regulations banning the sale of ENDS to individuals age <18 and required manufacturers to submit documents detailing all ingredients for review and possible approval.
The American Lung Association has stated its concerns about the use of ENDS but has not made any direct recommendations. The American Heart Association reports a potential negative public health impact and provides clinical guideline recommendations.43 Prominent psychiatric organizations such as the American Psychiatric Association, American Academy of Addiction Psychiatry (AAAP), the Substance Abuse and Mental Health Services Administration (SAMHSA), and the National Institute of Drug Abuse do not have official statements supporting or rejecting the use of ENDS. However, they do note the potential harm and lack of substantial evidence for efficacy of ENDS as a smoking cessation tool, and the AAAP and SAMHSA state that they will work with regulatory agencies to reduce the use of toxic products with addictive potential including ENDS.44-46
Clinical recommendations
We do not recommend ENDS as a first-line treatment for smoking cessation because there is no evidence they are superior to the FDA-approved nicotine replacement therapies (NRTs), the paucity of research into the potential short- and long-term health risks of ENDS, and the fact that these products are not regulated for use as smoking cessation aids. It is, however, advisable to discuss ENDS use with patients by:
- asking if they are using the products
- assessing whether the user also is a smoker
- advising the patient to quit.
It also is important to assess the patient’s knowledge and attitudes regarding ENDS use and provide education about the products. Some patients firmly believe that ENDS are the lesser of 2 evils, and they are decreasing the harms of smoking by using these devices. While the debate over a potential harm reduction strategy unfolds,47 we think that because of the state of the evidence it is prudent to adopt a more precautionary stance and recommend that patients work toward abstinence from nicotine in any form.
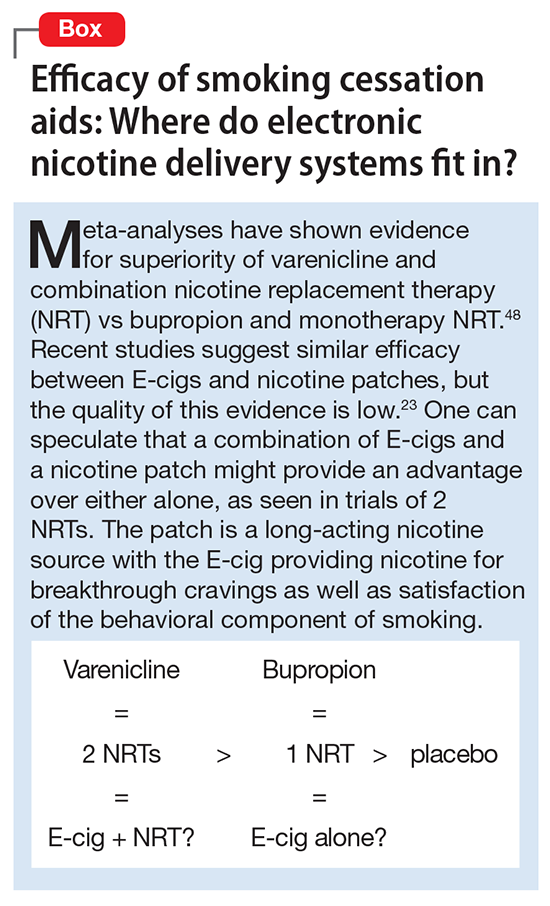
For dual tobacco/ENDS users and for patients using ENDS who want to quit smoking, we recommend treatment with an approved pharmacotherapy (ie, NRTs, bupropion, and varenicline) combined with counseling. A 2013 Cochrane Review found that all pharamacotherapy options are more effective than placebo, and combination NRT and varenicline are superior to single NRT or bupropion (Box).23,48
1. Hajek P, Goniewicz ML, Phillips A, et al. Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nicotine Tob Res. 2015;17(2):175-179.
2. Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf. 2014;5(2):67-86.
3. St Helen G, Havel C, Dempsey DA, et al. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction. 2016;111(3):535-544.
4. Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129(19):1972-1986.
5. Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23(suppl 3):iii3-iii9. doi: 10.1136/tobaccocontrol-2014-051670.
6. Schoenborn CA, Gindi RM. Electronic cigarette use among adults: United States, 2014. NCHS Data Brief. 2015;(217):1-8.
7. Farsalinos KE, Poulas K, Voudris V, et al. Electronic cigarette use in the European Union: analysis of a representative sample of 27 460 Europeans from 28 countries. Addiction. 2016;111(11):2032-2040.
8. Cummins SE, Zhu SH, Tedeschi GJ, et al. Use of e-cigarettes by individuals with mental health conditions. Tob Control. 2015;23(suppl 3):iii48-iii53. doi: 10.1136/tobaccocontrol-2013-051511.
9. Spears CA, Jones DM, Weaver SR, et al. Use of electronic nicotine delivery systems among adults with mental health conditions, 2015. Int J Environ Res Public Heal. 2017;14(1):10.
10. Hefner K, Valentine G, Sofuoglu M. Electronic cigarettes and mental illness: reviewing the evidence for help and harm among those with psychiatric and substance use disorders [published online February 2, 2017]. Am J Addict. doi: 10.1111/ajad.12504.
11. Anthenelli R. How—and why—to help psychiatric patients stop smoking. Current Psychiatry. 2005;4(1):77-87.
12. Singh T, Arrazola RA, Corey CG, et al. Tobacco use among middle and high school students—United States, 2011-2015. MMWR Morb Mortal Wkly Rep. 2016;65(14):361-367.
13. Greenhill R, Dawkins L, Notley C, et al. Adolescent awareness and use of electronic cigarettes: a review of emerging trends and findings. J Adolesc Heal. 2016;59(6):612-619.
14. U.S. Department of Health and Human Services. E-cigarette use among youth and young adults: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2016.
15. Cooper M, Harrell MB, Pérez A, et al. Flavorings and perceived harm and addictiveness of e-cigarettes among youth. Tob Regul Sci. 2016;2(3):278-289.
16. Kim H, Davis AH, Dohack JL, et al. E-cigarettes use behavior and experience of adults: qualitative research findings to inform e-cigarette use measure development. Nicotine Tob Res. 2017;19(2):190-196.
17. Czoli CD, Fong GT, Mays D, et al. How do consumers perceive differences in risk across nicotine products? A review of relative risk perceptions across smokeless tobacco, e-cigarettes, nicotine replacement therapy and combustible cigarettes. Tob Control. 2017;26(e1):e49-e58.
18. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382(9905):1629-1637.
19. Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8(6):e66317. doi: 10.1371/journal.pone.0066317.
20. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11(11):11220-11248.
21. Tseng TY, Ostroff JS, Campo A, et al. A randomized trial comparing the effect of nicotine versus placebo electronic cigarettes on smoking reduction among young adult smokers. Nicotine Tob Res. 2016;18(10):1937-1943.
22. Meier E, Wahlquist AE, Heckman BW, et al. A pilot randomized crossover trial of electronic cigarette sampling among smokers. Nicotine Tob Res. 2017;19(2):176-182.
23. Hartmann-Boyce J, McRobbie H, Bullen C, et al. Electronic cigarettes for smoking cessation [published online September 14, 2016]. Cochrane Database Syst Rev. 2016;9:CD010216.
24. Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2014;17(2):127-133.
25. Etter JF, Bullen C. A longitudinal study of electronic cigarette users. Addict Behav. 2014;39(2):491-494.
26. Caponnetto P, Auditore R, Russo C, et al. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot study. Int J Environ Res Public Health. 2013;10(2):446-461.
27. Popova L, Ling PM. Alternative tobacco product use and smoking cessation: a national study. Am J Public Health. 2013;103(5):923-930.
28. Adkison SE, O’Connor RJ, Bansal-Travers M, et al. Electronic nicotine delivery systems: International Tobacco Control Four-Country Survey. Am J Prev Med. 2013;44(3):207-215.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110(7):1160-1168.
30. Manzoli L, Flacco ME, Ferrante M, et al; ISLESE Working Group. Cohort study of electronic cigarette use: effectiveness and safety at 24 months [published online June 6, 2016]. Tob Control. doi: 10.1136/tobaccocontrol-2015-052822.
31. Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and E-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10(2):e0116732. doi: 10.1371/journal.pone.0116732.
32. Sussan TE, Gajghate S, Thimmulappa RK, et al. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One. 2015;10(2):e0116861. doi: 10.1371/journal.pone.0116861.
33. US Environmental Protection Agency. Acrolein. https://www.epa.gov/sites/production/files/2016-08/documents/acrolein.pdf. Updated September 2009. Accessed April 7, 2017.
34. Vardavas CI, Anagnostopoulos N, Kougias M, et al. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest. 2012;141(6):1400-1406.
35. Etter JF. Electronic cigarettes: a survey of users. BMC Public Health. 2010;10:231.
36. Goniewicz ML, Lingas EO, Hajek P. Patterns of electronic cigarette use and user beliefs about their safety and benefits: an internet survey. Drug Alcohol Rev. 2013;32(2):133-140.
37. Laino T, Tuma C, Moor P, et al. Mechanisms of propylene glycol and triacetin pyrolysis. J Phys Chem A. 2012;116(18):4602-4609.
38. Brook RD, Rajagopalan S, Pope CA 3rd, et al; American Heart Association Council on Epidemiology and Prevention; Council on the Kidney in Cardiovascular Disease; Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331-2378.
39. Barrington-Trimis JL, Samet JM, McConnell R. Flavorings in electronic cigarettes: an unrecognized respiratory health hazard? JAMA. 2014;312(23):2493-2494.
40. Schripp T, Markewitz D, Uhde E, et al. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23(1):25-31.
41. Fuoco FC, Buonanno G, Stabile L, et al. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ Pollut. 2014;184:523-529.
42. Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217(6):628-637.
43. Bhatnagar A, Whitsel L, Ribisl K, et al; American Heart Association Advocacy Coordinating Committee; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Quality of Care and Outcomes Research. Electronic cigarettes: a policy statement from the American Heart Association. Circulation. 2014;130(16):1418-1436.
44. E-cigarettes pose risks. SAMHSA News. https://www.samhsa.gov/samhsaNewsLetter/Volume_22_Number_3/e_cigarettes. Published 2014. Accessed April 7, 2017.
45. National Institute on Drug Abuse. Electronic cigarettes (e-cigarettes). https://www.drugabuse.gov/publications/drugfacts/electronic-cigarettes-e-cigarettes. Revised May 2016. Accessed April 7, 2017.
46. American Academy of Addiction Psychiatry. Nicotine dependence. East Providence, RI: American Academy of Addition Psychiatry; 2015.
47. Green SH, Bayer R, Fairchild AL. Evidence, policy, and e-cigarettes — will England reframe the debate. N Engl J Med. 2016;374(14):1301-1303.
48. Cahill K, Stevens S, Lancaster T. Pharmacological treatments for smoking cessation. JAMA. 2014;311(2):193-194.
The popularity of electronic cigarettes (E-cigs) and “vapes” has grown dramatically, spawning a new industry of electronic nicotine delivery systems (ENDS). With the increasing use of E-cigs not only for smoking cessation, but also as a primary nicotine source, it is important for mental health professionals to be prepared to discuss use of these devices with patients. In this article, we will describe:
- the composition of E-cigs and their current use
- evidence for their use for smoking cessation
- adverse health effects
- recommendations of major regulatory agencies.
Finally, we will provide recommendations for E-cig use in clinical populations.
What is an electronic nicotine delivery system?
ENDS produce an aerosol with or without nicotine that is inhaled and is thought to mimic the use of combustible cigarettes. ENDS evolved from basic E-cigs into a less “cigarette-like” and more customizable product (Figure 1). ENDS include a range of designs and go by various names, including “personal vaporizers,” “e-cigars,” and “e-hookahs” (in this article, we will use the term “ENDS” to refer to these devices).
The general design of ENDS is a plastic tubing system that contains a mouthpiece, battery, electronic heating element (“vaporizer”), and a cartridge with liquid solvent with or without nicotine or flavoring (Figure 2). One draw on the mouthpiece or press of a button activates the device, heats the solution, and delivers a vapor in a similar manner to taking a puff of a cigarette. Although studies have shown that ENDS result in significant increases in plasma nicotine concentrations in 5 minutes,1 the plasma nicotine levels obtained with the first-generation “cigarette-like” ENDS are much lower than those caused by inhaling tobacco smoke.2 Over time nicotine delivery capability has improved as ENDS have evolved such that the rate of nicotine delivery and peak concentration obtained with newer models more closely mirror tobacco cigarettes.3 Whether the rapid delivery of larger amounts of nicotine helps or hinders one’s efforts to break nicotine addiction remains to be determined because of the reinforcing properties of the drug.
The liquid in the E-cig cartridge typically contains not only nicotine but a number of chemical compounds with potentially deleterious or unknown health risks. The 3 main ingredients include:
- a solvent of glycerin and/or propylene glycol
- nicotine in various concentrations
- flavorings.
The glycerin or propylene glycol forms the basis for the aerosol. Nicotine concentrations vary from 0 (denicotinized) to 35 mcg per puff.4 A study reported 7,700 unique flavors available for vaping liquid.5 The liquid also contains impurities, such as anabasine, which has effects on the α-7 nicotinic acetylcholine receptor and its principal use is as an insecticide and β-nicotyrine, which inhibits cytochrome P450 2A.
Epidemiology and end-user perspectives
In 2014, 12.4% of U.S. adults classified themselves as “ever users” of ENDS (used at least once) and 3.7% of adults classified themselves as current users, according to the National Health Interview Study.6 Importantly, among E-cig users who had not used combustible cigarettes, young adults (age 18 to 24) were more likely to have tried ENDS than older adults. ENDS are becoming more popular across the globe. A study in the European Union found that ever users of ENDS most commonly were current cigarette smokers (31%) followed by former (10.8%) and never smokers (2.3%).7
ENDS use is relevant for mental health professionals because of the high rate of comorbid tobacco use disorder in individuals with psychiatric conditions. For example, 2 U.S. population surveys8,9 revealed those with mental health conditions were 1.5 to 2 times more likely to have tried ENDS and 2 to 3 times more likely to be current users. Those with psychiatric illness reported similar reasons for ENDS use as other individuals, including “just because,” use as a smoking cessation aid, ease of use, and perceived safety vs combustible cigarettes.
A recent review that included 9 studies focusing on ENDS use in those with mental illness reported mixed findings on the utility of these devices to reduce or stop use of combustible cigarettes.10 Additionally, it is important to monitor the use of cigarettes and ENDS in patients with psychiatric illness because the byproducts of tobacco smoke can affect the metabolism of some psychotropic medications.11 Although reduced use of combustible cigarettes could lead to lower dosing of some psychotropics, an unreported decrease in combustible cigarette use could lead to supratherapeutic drug levels. There are no data on the effect of ENDS on the metabolism of psychotropics.
ENDS are increasingly popular among adolescents. In 2015, there were an estimated 4.6 million current tobacco users among middle/high school youths in the United States and 3 million current ENDS users, according to the National Youth Tobacco Surveys.12 The shift from combustible cigarettes to ENDS is notable, with an increase in the percentage of current E-cig users and a decrease in the percentage of exclusive combustible cigarette users. In addition, there has been no change in the prevalence of lifetime tobacco users.12 This is a global issue, as reports of ever use of ENDS by adolescents range from 6.5% to 31% in the United States, 14.6% in Canada, and 4.7% to 38.5% in Europe.13 Based on these trends, the U.S. Surgeon General released a statement warning against the use of ENDS in youth because of the lack of safety data and strong association with use of tobacco products.14
There are a number of possible reasons for the increasing popularity of ENDS, including the product’s novelty, lack of regulations regarding their sale, availability of flavorings, and the perception that ENDS are safe alternatives to cigarettes. E-cig–using youths have described ENDS as “not at all harmful” and “not at all addictive” and believe that ENDS with flavoring are less harmful than those without.15 Although studies in adults show some users reporting that ENDS are less satisfying, they are seen as useful in decreasing craving and a safer alternative to cigarettes.16,17
Are ENDS effective for smoking cessation?
The evidence for ENDS as aids to smoking cessation remains murky (Table 118-22). There is a paucity of randomized controlled clinical trials (RCTs) investigating ENDS for smoking cessation or reduction, and it is difficult to quantify the amount of nicotine used in ENDS because of the variety of delivery systems and cartridges. In a recent Cochrane review, those using ENDS to quit smoking were more likely to be abstinent from combustible cigarettes at 6 months vs those using nicotine-free ENDS (relative risk = 2.29; 95% CI, 1.05 to 4.96), but there was no significant difference in quit rates compared with nicotine patches.23 However, the confidence in this finding was rated as low because of the limited number of RCTs. Of note, the authors found 15 ongoing RCTs at the time of publication that might be eligible for later evaluation.
Non-RCTs reveal mixed data. Positive results include 1 study with an odds ratio of 6.07 to quit for intensive ENDS users vs non-users,24 and another with dual users of combustible and electronic cigarettes having a 46% quit rate at 1 year.25 Additionally, in a pilot study providing ENDS to 14 patients with schizophrenia who had no previous desire to quit smoking, authors noted a reduction in the number of cigarettes smoked per day by 50% in one-half of participants and abstinence in 14% of participants at 52 weeks.26 Studies with neutral or negative results include those showing ENDS users to be current combustible tobacco smokers, and use of ENDS not predicting smoking cessation.4,27 Data also are mixed regarding the use of ENDS as a harm reduction strategy. One study found that ENDS decreased cigarette consumption, but did not increase the likelihood of quitting,28 while another reported that daily use of ENDS increased the odds of reducing smoking by as much as 2.5 times compared with non-use of such aids.29 In a 24-month prospective cohort study following tobacco users, there was no difference in the number of cigarettes smoked per day in those who started the trial as users of combustible cigarettes alone vs combustible cigarettes plus ENDS users.30 Interestingly, those who started the study as combustible cigarette users and switched to ENDS and those who had continued dual use throughout the 24 months smoked fewer combustible cigarettes per day than those who never tried ENDS or quit during the study period.
Health effects


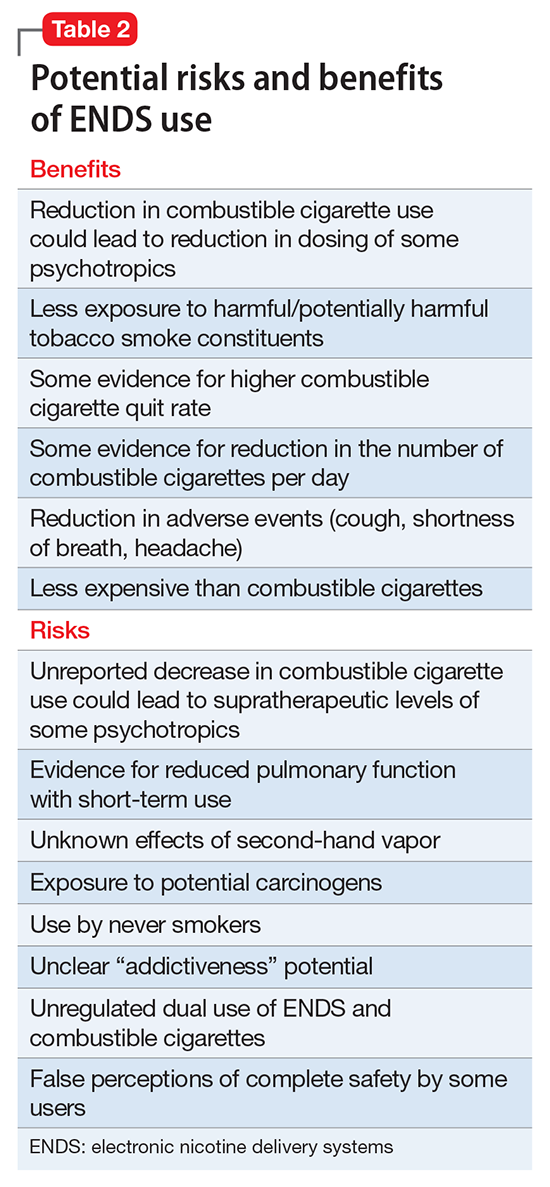
To better understand the adverse health effects of ENDS, one must consider potential short- and long-term consequences (Table 2). In the short-term, ENDS have been found to increase markers of inflammation and oxidative stress acutely as evidenced by in vivo laboratory studies.31,32 ENDS also have been linked to upper respiratory irritation, in part, because of the transformation of glycerin in the nicotine cartridge to acrolein upon combustion.33 Even 5 minutes of ad lib E-cig use has been found to significantly increase airflow resistance during pulmonary function tests34—changes that have been shown to precede more persistent alterations in peak expiratory flow, such as those seen in chronic obstructive pulmonary disease. The more common patient-reported side effects include:
- daytime cough (27%)
- phlegm production (25%)
- headache (21%)
- dry mouth/throat (20%)
- vertigo, headache, or nausea (9%).35,36
A RCT investigating efficacy of E-cigs vs nicotine patches vs denicotinized E-cigs found no difference among the groups in the number of reported adverse events.18 Interestingly, another RCT found a decrease in adverse events, such as dry cough, mouth irritation, throat irritation, shortness of breath, and headache, compared with baseline in combustible cigarette smokers who used regular or denicotinized E-cigs.19
Although no studies have directly investigated long-term health consequences of ENDS because of their relative novelty, one can extrapolate potential harmful long-term effects based on knowledge of the products’ chemical constituents. For example, propylene glycol can degrade into propylene oxide, a class 2B carcinogen.37 Other potential carcinogens in the aerosol include formaldehyde and acetaldehyde. On a broader scale, many of the particulates have been shown to cause systemic inflammation, which is thought to increase cardiovascular and respiratory disease and death.38 Flavorings in ENDS include a variety of components including, but not limited to, aldehydes, which are irritants, and other additives that have been associated with respiratory disease.39
Second-hand exposure. There are no long-term studies of second-hand vapor exposure, but similar to long-term health on primary users, one can glean some observations from the literature. It is promising that compared with cigarettes, ENDS lack sidestream smoke and the vapor has not been found to contain carbon monoxide.40 Some research has demonstrated that the size and spray of fine particles in the aerosol is as large or larger than combustible cigarettes.41 Formaldehyde, acetaldehyde, isoprene, and acetic acid have been found in ENDS vapor.40 Interestingly, a simulated café study found elevated nicotine, glycerine, hydrocarbon, and other materials classified as carcinogens in the air.42
Although it is popularly thought that ENDS are less toxic than tobacco cigarettes, there is not enough evidence to estimate precisely as to how much less toxic or the consequences of use. ENDS are increasingly popular and are being used by never smokers who should be educated on the potential harm that ENDS pose.
Recommendations from agencies and medical organizations
The World Health Organization (WHO) recommended prohibiting the use of ENDS in indoor spaces to minimize potential health risks to users and non-users. The WHO also aims to prevent dissemination of unproven health claims, including claims that ENDS are effective—or not—or that the devices are innocuous.36 In the United States, the FDA has stated that ENDS are not recommended for safe quitting (2009). In August 2016, the FDA introduced regulations banning the sale of ENDS to individuals age <18 and required manufacturers to submit documents detailing all ingredients for review and possible approval.
The American Lung Association has stated its concerns about the use of ENDS but has not made any direct recommendations. The American Heart Association reports a potential negative public health impact and provides clinical guideline recommendations.43 Prominent psychiatric organizations such as the American Psychiatric Association, American Academy of Addiction Psychiatry (AAAP), the Substance Abuse and Mental Health Services Administration (SAMHSA), and the National Institute of Drug Abuse do not have official statements supporting or rejecting the use of ENDS. However, they do note the potential harm and lack of substantial evidence for efficacy of ENDS as a smoking cessation tool, and the AAAP and SAMHSA state that they will work with regulatory agencies to reduce the use of toxic products with addictive potential including ENDS.44-46
Clinical recommendations
We do not recommend ENDS as a first-line treatment for smoking cessation because there is no evidence they are superior to the FDA-approved nicotine replacement therapies (NRTs), the paucity of research into the potential short- and long-term health risks of ENDS, and the fact that these products are not regulated for use as smoking cessation aids. It is, however, advisable to discuss ENDS use with patients by:
- asking if they are using the products
- assessing whether the user also is a smoker
- advising the patient to quit.
It also is important to assess the patient’s knowledge and attitudes regarding ENDS use and provide education about the products. Some patients firmly believe that ENDS are the lesser of 2 evils, and they are decreasing the harms of smoking by using these devices. While the debate over a potential harm reduction strategy unfolds,47 we think that because of the state of the evidence it is prudent to adopt a more precautionary stance and recommend that patients work toward abstinence from nicotine in any form.
For dual tobacco/ENDS users and for patients using ENDS who want to quit smoking, we recommend treatment with an approved pharmacotherapy (ie, NRTs, bupropion, and varenicline) combined with counseling. A 2013 Cochrane Review found that all pharamacotherapy options are more effective than placebo, and combination NRT and varenicline are superior to single NRT or bupropion (Box).23,48
The popularity of electronic cigarettes (E-cigs) and “vapes” has grown dramatically, spawning a new industry of electronic nicotine delivery systems (ENDS). With the increasing use of E-cigs not only for smoking cessation, but also as a primary nicotine source, it is important for mental health professionals to be prepared to discuss use of these devices with patients. In this article, we will describe:
- the composition of E-cigs and their current use
- evidence for their use for smoking cessation
- adverse health effects
- recommendations of major regulatory agencies.
Finally, we will provide recommendations for E-cig use in clinical populations.
What is an electronic nicotine delivery system?
ENDS produce an aerosol with or without nicotine that is inhaled and is thought to mimic the use of combustible cigarettes. ENDS evolved from basic E-cigs into a less “cigarette-like” and more customizable product (Figure 1). ENDS include a range of designs and go by various names, including “personal vaporizers,” “e-cigars,” and “e-hookahs” (in this article, we will use the term “ENDS” to refer to these devices).
The general design of ENDS is a plastic tubing system that contains a mouthpiece, battery, electronic heating element (“vaporizer”), and a cartridge with liquid solvent with or without nicotine or flavoring (Figure 2). One draw on the mouthpiece or press of a button activates the device, heats the solution, and delivers a vapor in a similar manner to taking a puff of a cigarette. Although studies have shown that ENDS result in significant increases in plasma nicotine concentrations in 5 minutes,1 the plasma nicotine levels obtained with the first-generation “cigarette-like” ENDS are much lower than those caused by inhaling tobacco smoke.2 Over time nicotine delivery capability has improved as ENDS have evolved such that the rate of nicotine delivery and peak concentration obtained with newer models more closely mirror tobacco cigarettes.3 Whether the rapid delivery of larger amounts of nicotine helps or hinders one’s efforts to break nicotine addiction remains to be determined because of the reinforcing properties of the drug.
The liquid in the E-cig cartridge typically contains not only nicotine but a number of chemical compounds with potentially deleterious or unknown health risks. The 3 main ingredients include:
- a solvent of glycerin and/or propylene glycol
- nicotine in various concentrations
- flavorings.
The glycerin or propylene glycol forms the basis for the aerosol. Nicotine concentrations vary from 0 (denicotinized) to 35 mcg per puff.4 A study reported 7,700 unique flavors available for vaping liquid.5 The liquid also contains impurities, such as anabasine, which has effects on the α-7 nicotinic acetylcholine receptor and its principal use is as an insecticide and β-nicotyrine, which inhibits cytochrome P450 2A.
Epidemiology and end-user perspectives
In 2014, 12.4% of U.S. adults classified themselves as “ever users” of ENDS (used at least once) and 3.7% of adults classified themselves as current users, according to the National Health Interview Study.6 Importantly, among E-cig users who had not used combustible cigarettes, young adults (age 18 to 24) were more likely to have tried ENDS than older adults. ENDS are becoming more popular across the globe. A study in the European Union found that ever users of ENDS most commonly were current cigarette smokers (31%) followed by former (10.8%) and never smokers (2.3%).7
ENDS use is relevant for mental health professionals because of the high rate of comorbid tobacco use disorder in individuals with psychiatric conditions. For example, 2 U.S. population surveys8,9 revealed those with mental health conditions were 1.5 to 2 times more likely to have tried ENDS and 2 to 3 times more likely to be current users. Those with psychiatric illness reported similar reasons for ENDS use as other individuals, including “just because,” use as a smoking cessation aid, ease of use, and perceived safety vs combustible cigarettes.
A recent review that included 9 studies focusing on ENDS use in those with mental illness reported mixed findings on the utility of these devices to reduce or stop use of combustible cigarettes.10 Additionally, it is important to monitor the use of cigarettes and ENDS in patients with psychiatric illness because the byproducts of tobacco smoke can affect the metabolism of some psychotropic medications.11 Although reduced use of combustible cigarettes could lead to lower dosing of some psychotropics, an unreported decrease in combustible cigarette use could lead to supratherapeutic drug levels. There are no data on the effect of ENDS on the metabolism of psychotropics.
ENDS are increasingly popular among adolescents. In 2015, there were an estimated 4.6 million current tobacco users among middle/high school youths in the United States and 3 million current ENDS users, according to the National Youth Tobacco Surveys.12 The shift from combustible cigarettes to ENDS is notable, with an increase in the percentage of current E-cig users and a decrease in the percentage of exclusive combustible cigarette users. In addition, there has been no change in the prevalence of lifetime tobacco users.12 This is a global issue, as reports of ever use of ENDS by adolescents range from 6.5% to 31% in the United States, 14.6% in Canada, and 4.7% to 38.5% in Europe.13 Based on these trends, the U.S. Surgeon General released a statement warning against the use of ENDS in youth because of the lack of safety data and strong association with use of tobacco products.14
There are a number of possible reasons for the increasing popularity of ENDS, including the product’s novelty, lack of regulations regarding their sale, availability of flavorings, and the perception that ENDS are safe alternatives to cigarettes. E-cig–using youths have described ENDS as “not at all harmful” and “not at all addictive” and believe that ENDS with flavoring are less harmful than those without.15 Although studies in adults show some users reporting that ENDS are less satisfying, they are seen as useful in decreasing craving and a safer alternative to cigarettes.16,17
Are ENDS effective for smoking cessation?
The evidence for ENDS as aids to smoking cessation remains murky (Table 118-22). There is a paucity of randomized controlled clinical trials (RCTs) investigating ENDS for smoking cessation or reduction, and it is difficult to quantify the amount of nicotine used in ENDS because of the variety of delivery systems and cartridges. In a recent Cochrane review, those using ENDS to quit smoking were more likely to be abstinent from combustible cigarettes at 6 months vs those using nicotine-free ENDS (relative risk = 2.29; 95% CI, 1.05 to 4.96), but there was no significant difference in quit rates compared with nicotine patches.23 However, the confidence in this finding was rated as low because of the limited number of RCTs. Of note, the authors found 15 ongoing RCTs at the time of publication that might be eligible for later evaluation.
Non-RCTs reveal mixed data. Positive results include 1 study with an odds ratio of 6.07 to quit for intensive ENDS users vs non-users,24 and another with dual users of combustible and electronic cigarettes having a 46% quit rate at 1 year.25 Additionally, in a pilot study providing ENDS to 14 patients with schizophrenia who had no previous desire to quit smoking, authors noted a reduction in the number of cigarettes smoked per day by 50% in one-half of participants and abstinence in 14% of participants at 52 weeks.26 Studies with neutral or negative results include those showing ENDS users to be current combustible tobacco smokers, and use of ENDS not predicting smoking cessation.4,27 Data also are mixed regarding the use of ENDS as a harm reduction strategy. One study found that ENDS decreased cigarette consumption, but did not increase the likelihood of quitting,28 while another reported that daily use of ENDS increased the odds of reducing smoking by as much as 2.5 times compared with non-use of such aids.29 In a 24-month prospective cohort study following tobacco users, there was no difference in the number of cigarettes smoked per day in those who started the trial as users of combustible cigarettes alone vs combustible cigarettes plus ENDS users.30 Interestingly, those who started the study as combustible cigarette users and switched to ENDS and those who had continued dual use throughout the 24 months smoked fewer combustible cigarettes per day than those who never tried ENDS or quit during the study period.
Health effects


To better understand the adverse health effects of ENDS, one must consider potential short- and long-term consequences (Table 2). In the short-term, ENDS have been found to increase markers of inflammation and oxidative stress acutely as evidenced by in vivo laboratory studies.31,32 ENDS also have been linked to upper respiratory irritation, in part, because of the transformation of glycerin in the nicotine cartridge to acrolein upon combustion.33 Even 5 minutes of ad lib E-cig use has been found to significantly increase airflow resistance during pulmonary function tests34—changes that have been shown to precede more persistent alterations in peak expiratory flow, such as those seen in chronic obstructive pulmonary disease. The more common patient-reported side effects include:
- daytime cough (27%)
- phlegm production (25%)
- headache (21%)
- dry mouth/throat (20%)
- vertigo, headache, or nausea (9%).35,36
A RCT investigating efficacy of E-cigs vs nicotine patches vs denicotinized E-cigs found no difference among the groups in the number of reported adverse events.18 Interestingly, another RCT found a decrease in adverse events, such as dry cough, mouth irritation, throat irritation, shortness of breath, and headache, compared with baseline in combustible cigarette smokers who used regular or denicotinized E-cigs.19
Although no studies have directly investigated long-term health consequences of ENDS because of their relative novelty, one can extrapolate potential harmful long-term effects based on knowledge of the products’ chemical constituents. For example, propylene glycol can degrade into propylene oxide, a class 2B carcinogen.37 Other potential carcinogens in the aerosol include formaldehyde and acetaldehyde. On a broader scale, many of the particulates have been shown to cause systemic inflammation, which is thought to increase cardiovascular and respiratory disease and death.38 Flavorings in ENDS include a variety of components including, but not limited to, aldehydes, which are irritants, and other additives that have been associated with respiratory disease.39
Second-hand exposure. There are no long-term studies of second-hand vapor exposure, but similar to long-term health on primary users, one can glean some observations from the literature. It is promising that compared with cigarettes, ENDS lack sidestream smoke and the vapor has not been found to contain carbon monoxide.40 Some research has demonstrated that the size and spray of fine particles in the aerosol is as large or larger than combustible cigarettes.41 Formaldehyde, acetaldehyde, isoprene, and acetic acid have been found in ENDS vapor.40 Interestingly, a simulated café study found elevated nicotine, glycerine, hydrocarbon, and other materials classified as carcinogens in the air.42
Although it is popularly thought that ENDS are less toxic than tobacco cigarettes, there is not enough evidence to estimate precisely as to how much less toxic or the consequences of use. ENDS are increasingly popular and are being used by never smokers who should be educated on the potential harm that ENDS pose.
Recommendations from agencies and medical organizations
The World Health Organization (WHO) recommended prohibiting the use of ENDS in indoor spaces to minimize potential health risks to users and non-users. The WHO also aims to prevent dissemination of unproven health claims, including claims that ENDS are effective—or not—or that the devices are innocuous.36 In the United States, the FDA has stated that ENDS are not recommended for safe quitting (2009). In August 2016, the FDA introduced regulations banning the sale of ENDS to individuals age <18 and required manufacturers to submit documents detailing all ingredients for review and possible approval.
The American Lung Association has stated its concerns about the use of ENDS but has not made any direct recommendations. The American Heart Association reports a potential negative public health impact and provides clinical guideline recommendations.43 Prominent psychiatric organizations such as the American Psychiatric Association, American Academy of Addiction Psychiatry (AAAP), the Substance Abuse and Mental Health Services Administration (SAMHSA), and the National Institute of Drug Abuse do not have official statements supporting or rejecting the use of ENDS. However, they do note the potential harm and lack of substantial evidence for efficacy of ENDS as a smoking cessation tool, and the AAAP and SAMHSA state that they will work with regulatory agencies to reduce the use of toxic products with addictive potential including ENDS.44-46
Clinical recommendations
We do not recommend ENDS as a first-line treatment for smoking cessation because there is no evidence they are superior to the FDA-approved nicotine replacement therapies (NRTs), the paucity of research into the potential short- and long-term health risks of ENDS, and the fact that these products are not regulated for use as smoking cessation aids. It is, however, advisable to discuss ENDS use with patients by:
- asking if they are using the products
- assessing whether the user also is a smoker
- advising the patient to quit.
It also is important to assess the patient’s knowledge and attitudes regarding ENDS use and provide education about the products. Some patients firmly believe that ENDS are the lesser of 2 evils, and they are decreasing the harms of smoking by using these devices. While the debate over a potential harm reduction strategy unfolds,47 we think that because of the state of the evidence it is prudent to adopt a more precautionary stance and recommend that patients work toward abstinence from nicotine in any form.
For dual tobacco/ENDS users and for patients using ENDS who want to quit smoking, we recommend treatment with an approved pharmacotherapy (ie, NRTs, bupropion, and varenicline) combined with counseling. A 2013 Cochrane Review found that all pharamacotherapy options are more effective than placebo, and combination NRT and varenicline are superior to single NRT or bupropion (Box).23,48
1. Hajek P, Goniewicz ML, Phillips A, et al. Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nicotine Tob Res. 2015;17(2):175-179.
2. Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf. 2014;5(2):67-86.
3. St Helen G, Havel C, Dempsey DA, et al. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction. 2016;111(3):535-544.
4. Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129(19):1972-1986.
5. Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23(suppl 3):iii3-iii9. doi: 10.1136/tobaccocontrol-2014-051670.
6. Schoenborn CA, Gindi RM. Electronic cigarette use among adults: United States, 2014. NCHS Data Brief. 2015;(217):1-8.
7. Farsalinos KE, Poulas K, Voudris V, et al. Electronic cigarette use in the European Union: analysis of a representative sample of 27 460 Europeans from 28 countries. Addiction. 2016;111(11):2032-2040.
8. Cummins SE, Zhu SH, Tedeschi GJ, et al. Use of e-cigarettes by individuals with mental health conditions. Tob Control. 2015;23(suppl 3):iii48-iii53. doi: 10.1136/tobaccocontrol-2013-051511.
9. Spears CA, Jones DM, Weaver SR, et al. Use of electronic nicotine delivery systems among adults with mental health conditions, 2015. Int J Environ Res Public Heal. 2017;14(1):10.
10. Hefner K, Valentine G, Sofuoglu M. Electronic cigarettes and mental illness: reviewing the evidence for help and harm among those with psychiatric and substance use disorders [published online February 2, 2017]. Am J Addict. doi: 10.1111/ajad.12504.
11. Anthenelli R. How—and why—to help psychiatric patients stop smoking. Current Psychiatry. 2005;4(1):77-87.
12. Singh T, Arrazola RA, Corey CG, et al. Tobacco use among middle and high school students—United States, 2011-2015. MMWR Morb Mortal Wkly Rep. 2016;65(14):361-367.
13. Greenhill R, Dawkins L, Notley C, et al. Adolescent awareness and use of electronic cigarettes: a review of emerging trends and findings. J Adolesc Heal. 2016;59(6):612-619.
14. U.S. Department of Health and Human Services. E-cigarette use among youth and young adults: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2016.
15. Cooper M, Harrell MB, Pérez A, et al. Flavorings and perceived harm and addictiveness of e-cigarettes among youth. Tob Regul Sci. 2016;2(3):278-289.
16. Kim H, Davis AH, Dohack JL, et al. E-cigarettes use behavior and experience of adults: qualitative research findings to inform e-cigarette use measure development. Nicotine Tob Res. 2017;19(2):190-196.
17. Czoli CD, Fong GT, Mays D, et al. How do consumers perceive differences in risk across nicotine products? A review of relative risk perceptions across smokeless tobacco, e-cigarettes, nicotine replacement therapy and combustible cigarettes. Tob Control. 2017;26(e1):e49-e58.
18. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382(9905):1629-1637.
19. Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8(6):e66317. doi: 10.1371/journal.pone.0066317.
20. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11(11):11220-11248.
21. Tseng TY, Ostroff JS, Campo A, et al. A randomized trial comparing the effect of nicotine versus placebo electronic cigarettes on smoking reduction among young adult smokers. Nicotine Tob Res. 2016;18(10):1937-1943.
22. Meier E, Wahlquist AE, Heckman BW, et al. A pilot randomized crossover trial of electronic cigarette sampling among smokers. Nicotine Tob Res. 2017;19(2):176-182.
23. Hartmann-Boyce J, McRobbie H, Bullen C, et al. Electronic cigarettes for smoking cessation [published online September 14, 2016]. Cochrane Database Syst Rev. 2016;9:CD010216.
24. Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2014;17(2):127-133.
25. Etter JF, Bullen C. A longitudinal study of electronic cigarette users. Addict Behav. 2014;39(2):491-494.
26. Caponnetto P, Auditore R, Russo C, et al. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot study. Int J Environ Res Public Health. 2013;10(2):446-461.
27. Popova L, Ling PM. Alternative tobacco product use and smoking cessation: a national study. Am J Public Health. 2013;103(5):923-930.
28. Adkison SE, O’Connor RJ, Bansal-Travers M, et al. Electronic nicotine delivery systems: International Tobacco Control Four-Country Survey. Am J Prev Med. 2013;44(3):207-215.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110(7):1160-1168.
30. Manzoli L, Flacco ME, Ferrante M, et al; ISLESE Working Group. Cohort study of electronic cigarette use: effectiveness and safety at 24 months [published online June 6, 2016]. Tob Control. doi: 10.1136/tobaccocontrol-2015-052822.
31. Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and E-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10(2):e0116732. doi: 10.1371/journal.pone.0116732.
32. Sussan TE, Gajghate S, Thimmulappa RK, et al. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One. 2015;10(2):e0116861. doi: 10.1371/journal.pone.0116861.
33. US Environmental Protection Agency. Acrolein. https://www.epa.gov/sites/production/files/2016-08/documents/acrolein.pdf. Updated September 2009. Accessed April 7, 2017.
34. Vardavas CI, Anagnostopoulos N, Kougias M, et al. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest. 2012;141(6):1400-1406.
35. Etter JF. Electronic cigarettes: a survey of users. BMC Public Health. 2010;10:231.
36. Goniewicz ML, Lingas EO, Hajek P. Patterns of electronic cigarette use and user beliefs about their safety and benefits: an internet survey. Drug Alcohol Rev. 2013;32(2):133-140.
37. Laino T, Tuma C, Moor P, et al. Mechanisms of propylene glycol and triacetin pyrolysis. J Phys Chem A. 2012;116(18):4602-4609.
38. Brook RD, Rajagopalan S, Pope CA 3rd, et al; American Heart Association Council on Epidemiology and Prevention; Council on the Kidney in Cardiovascular Disease; Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331-2378.
39. Barrington-Trimis JL, Samet JM, McConnell R. Flavorings in electronic cigarettes: an unrecognized respiratory health hazard? JAMA. 2014;312(23):2493-2494.
40. Schripp T, Markewitz D, Uhde E, et al. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23(1):25-31.
41. Fuoco FC, Buonanno G, Stabile L, et al. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ Pollut. 2014;184:523-529.
42. Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217(6):628-637.
43. Bhatnagar A, Whitsel L, Ribisl K, et al; American Heart Association Advocacy Coordinating Committee; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Quality of Care and Outcomes Research. Electronic cigarettes: a policy statement from the American Heart Association. Circulation. 2014;130(16):1418-1436.
44. E-cigarettes pose risks. SAMHSA News. https://www.samhsa.gov/samhsaNewsLetter/Volume_22_Number_3/e_cigarettes. Published 2014. Accessed April 7, 2017.
45. National Institute on Drug Abuse. Electronic cigarettes (e-cigarettes). https://www.drugabuse.gov/publications/drugfacts/electronic-cigarettes-e-cigarettes. Revised May 2016. Accessed April 7, 2017.
46. American Academy of Addiction Psychiatry. Nicotine dependence. East Providence, RI: American Academy of Addition Psychiatry; 2015.
47. Green SH, Bayer R, Fairchild AL. Evidence, policy, and e-cigarettes — will England reframe the debate. N Engl J Med. 2016;374(14):1301-1303.
48. Cahill K, Stevens S, Lancaster T. Pharmacological treatments for smoking cessation. JAMA. 2014;311(2):193-194.
1. Hajek P, Goniewicz ML, Phillips A, et al. Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nicotine Tob Res. 2015;17(2):175-179.
2. Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf. 2014;5(2):67-86.
3. St Helen G, Havel C, Dempsey DA, et al. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction. 2016;111(3):535-544.
4. Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129(19):1972-1986.
5. Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23(suppl 3):iii3-iii9. doi: 10.1136/tobaccocontrol-2014-051670.
6. Schoenborn CA, Gindi RM. Electronic cigarette use among adults: United States, 2014. NCHS Data Brief. 2015;(217):1-8.
7. Farsalinos KE, Poulas K, Voudris V, et al. Electronic cigarette use in the European Union: analysis of a representative sample of 27 460 Europeans from 28 countries. Addiction. 2016;111(11):2032-2040.
8. Cummins SE, Zhu SH, Tedeschi GJ, et al. Use of e-cigarettes by individuals with mental health conditions. Tob Control. 2015;23(suppl 3):iii48-iii53. doi: 10.1136/tobaccocontrol-2013-051511.
9. Spears CA, Jones DM, Weaver SR, et al. Use of electronic nicotine delivery systems among adults with mental health conditions, 2015. Int J Environ Res Public Heal. 2017;14(1):10.
10. Hefner K, Valentine G, Sofuoglu M. Electronic cigarettes and mental illness: reviewing the evidence for help and harm among those with psychiatric and substance use disorders [published online February 2, 2017]. Am J Addict. doi: 10.1111/ajad.12504.
11. Anthenelli R. How—and why—to help psychiatric patients stop smoking. Current Psychiatry. 2005;4(1):77-87.
12. Singh T, Arrazola RA, Corey CG, et al. Tobacco use among middle and high school students—United States, 2011-2015. MMWR Morb Mortal Wkly Rep. 2016;65(14):361-367.
13. Greenhill R, Dawkins L, Notley C, et al. Adolescent awareness and use of electronic cigarettes: a review of emerging trends and findings. J Adolesc Heal. 2016;59(6):612-619.
14. U.S. Department of Health and Human Services. E-cigarette use among youth and young adults: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2016.
15. Cooper M, Harrell MB, Pérez A, et al. Flavorings and perceived harm and addictiveness of e-cigarettes among youth. Tob Regul Sci. 2016;2(3):278-289.
16. Kim H, Davis AH, Dohack JL, et al. E-cigarettes use behavior and experience of adults: qualitative research findings to inform e-cigarette use measure development. Nicotine Tob Res. 2017;19(2):190-196.
17. Czoli CD, Fong GT, Mays D, et al. How do consumers perceive differences in risk across nicotine products? A review of relative risk perceptions across smokeless tobacco, e-cigarettes, nicotine replacement therapy and combustible cigarettes. Tob Control. 2017;26(e1):e49-e58.
18. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382(9905):1629-1637.
19. Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8(6):e66317. doi: 10.1371/journal.pone.0066317.
20. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11(11):11220-11248.
21. Tseng TY, Ostroff JS, Campo A, et al. A randomized trial comparing the effect of nicotine versus placebo electronic cigarettes on smoking reduction among young adult smokers. Nicotine Tob Res. 2016;18(10):1937-1943.
22. Meier E, Wahlquist AE, Heckman BW, et al. A pilot randomized crossover trial of electronic cigarette sampling among smokers. Nicotine Tob Res. 2017;19(2):176-182.
23. Hartmann-Boyce J, McRobbie H, Bullen C, et al. Electronic cigarettes for smoking cessation [published online September 14, 2016]. Cochrane Database Syst Rev. 2016;9:CD010216.
24. Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2014;17(2):127-133.
25. Etter JF, Bullen C. A longitudinal study of electronic cigarette users. Addict Behav. 2014;39(2):491-494.
26. Caponnetto P, Auditore R, Russo C, et al. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot study. Int J Environ Res Public Health. 2013;10(2):446-461.
27. Popova L, Ling PM. Alternative tobacco product use and smoking cessation: a national study. Am J Public Health. 2013;103(5):923-930.
28. Adkison SE, O’Connor RJ, Bansal-Travers M, et al. Electronic nicotine delivery systems: International Tobacco Control Four-Country Survey. Am J Prev Med. 2013;44(3):207-215.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110(7):1160-1168.
30. Manzoli L, Flacco ME, Ferrante M, et al; ISLESE Working Group. Cohort study of electronic cigarette use: effectiveness and safety at 24 months [published online June 6, 2016]. Tob Control. doi: 10.1136/tobaccocontrol-2015-052822.
31. Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and E-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10(2):e0116732. doi: 10.1371/journal.pone.0116732.
32. Sussan TE, Gajghate S, Thimmulappa RK, et al. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One. 2015;10(2):e0116861. doi: 10.1371/journal.pone.0116861.
33. US Environmental Protection Agency. Acrolein. https://www.epa.gov/sites/production/files/2016-08/documents/acrolein.pdf. Updated September 2009. Accessed April 7, 2017.
34. Vardavas CI, Anagnostopoulos N, Kougias M, et al. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest. 2012;141(6):1400-1406.
35. Etter JF. Electronic cigarettes: a survey of users. BMC Public Health. 2010;10:231.
36. Goniewicz ML, Lingas EO, Hajek P. Patterns of electronic cigarette use and user beliefs about their safety and benefits: an internet survey. Drug Alcohol Rev. 2013;32(2):133-140.
37. Laino T, Tuma C, Moor P, et al. Mechanisms of propylene glycol and triacetin pyrolysis. J Phys Chem A. 2012;116(18):4602-4609.
38. Brook RD, Rajagopalan S, Pope CA 3rd, et al; American Heart Association Council on Epidemiology and Prevention; Council on the Kidney in Cardiovascular Disease; Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331-2378.
39. Barrington-Trimis JL, Samet JM, McConnell R. Flavorings in electronic cigarettes: an unrecognized respiratory health hazard? JAMA. 2014;312(23):2493-2494.
40. Schripp T, Markewitz D, Uhde E, et al. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23(1):25-31.
41. Fuoco FC, Buonanno G, Stabile L, et al. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ Pollut. 2014;184:523-529.
42. Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217(6):628-637.
43. Bhatnagar A, Whitsel L, Ribisl K, et al; American Heart Association Advocacy Coordinating Committee; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Quality of Care and Outcomes Research. Electronic cigarettes: a policy statement from the American Heart Association. Circulation. 2014;130(16):1418-1436.
44. E-cigarettes pose risks. SAMHSA News. https://www.samhsa.gov/samhsaNewsLetter/Volume_22_Number_3/e_cigarettes. Published 2014. Accessed April 7, 2017.
45. National Institute on Drug Abuse. Electronic cigarettes (e-cigarettes). https://www.drugabuse.gov/publications/drugfacts/electronic-cigarettes-e-cigarettes. Revised May 2016. Accessed April 7, 2017.
46. American Academy of Addiction Psychiatry. Nicotine dependence. East Providence, RI: American Academy of Addition Psychiatry; 2015.
47. Green SH, Bayer R, Fairchild AL. Evidence, policy, and e-cigarettes — will England reframe the debate. N Engl J Med. 2016;374(14):1301-1303.
48. Cahill K, Stevens S, Lancaster T. Pharmacological treatments for smoking cessation. JAMA. 2014;311(2):193-194.
Forget the myths and help your psychiatric patients quit smoking
The National Ambulatory Medical Care Survey1,2 (NAMCS) indicates that less than 1 out of 4 (23%) psychiatrists provide smoking cessation counseling to their patients, and even fewer prescribe medications.
What gives? How is it that so many psychiatrists endorse having recently helped a patient quit smoking when the data from large-scale surveys1,2 indicate they do not?
From the “glass is half-full” perspective, the discrepancy might indicate that psychiatrists finally have bought into the message put forth 20 years ago when the American Psychiatric Association first published its clinical practice guidelines for treating nicotine dependence.3 Because the figures I cited from NAMCS reflect data from 2006 to 2010, it is possible that in the last 5 years more psychiatrists have started to help their patients quit smoking. Such an hypothesis is further supported by the increasing number of research papers on smoking cessation in individuals with mental illness published over the past 8 years—a period that coincides with the release of the second edition of the Treating tobacco use and dependence clinical practice guideline from the U.S. Agency for Healthcare Research and Quality, which highlighted the need for more research in this population of smokers.4
Regardless of the reason, the fact that my informal surveys indicate a likely uptick in activity among psychiatrists to help their patients quit smoking is welcome news. With nearly 1 out of 2 cigarettes sold in the United States being smoked by individuals with psychiatric and substance use disorders,5 psychiatrists and other mental health professionals play a vital role in addressing this epidemic. That our patients smoke at rates 2- to 4-times that of the general population and die decades earlier than their non-smoking, non-mentally ill counterparts6 are compelling reasons urging us to end our complacency and help our patients quit smoking.
EAGLES trial results help debunk the latest myth about smoking cessation
In an article that I wrote for
In addition to applying the “black-box” warning, the FDA issued a post-marketing requirement to the manufacturers of bupropion and varenicline to conduct a large randomized controlled trial—Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES)—the top-line results of which were published in The Lancet this spring.12
Key results of the EAGLES trial
The researchers found no significant increase in serious neuropsychiatric AEs—a composite measure assessing depression, anxiety, suicidality, and 13 other symptom clusters—attributable to varenicline or bupropion compared with placebo or the nicotine patch in smokers with or without psychiatric disorders. The study did detect a significant difference—approximately 4% (2% in non-psychiatric cohort vs 6% in psychiatric cohort)—in the rate of serious neuropsychiatric AEs regardless of treatment condition. In both cohorts, varenicline was more effective than bupropion, which had similar efficacy to the nicotine patch; all interventions were superior to placebo. Importantly, all 3 medications significantly improved quit rates in smokers with and without psychiatric disorders. Although the efficacy of medications in smokers with or without psychiatric disorders was similar in terms of odds ratios, overall, those with psychiatric disorders had 20% to 30% lower quit rates compared with non-psychiatrically ill smokers.
The EAGLES study results, when viewed in the context of findings from other clinical trials and large-scale observational studies, provide further evidence that smokers with stable mental illness can use bupropion and varenicline safely. It also demonstrates that moderate to severe neuropsychiatric AEs occur during a smoking cessation attempt regardless of the medication used, therefore, monitoring smokers—especially those with psychiatric disorders—is important, a role that psychiatrists are uniquely poised to play.
That all 3 smoking cessation medications are effective in patients with mood, anxiety, and psychotic disorders is good news for our patients. Combined with the EAGLES safety findings, there is no better time to intervene in tobacco dependence
1. Rogers E, Sherman S. Tobacco use screening and treatment by outpatient psychiatrists before and after release of the American Psychiatric Association treatment guidelines for nicotine dependence. Am J Public Health. 2014;104(1):90-95.
2. Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling? Am J Psychiatry. 2003;160(12):2228-2230.
3. Practice guideline for the treatment of patients with nicotine dependence. American Psychiatric Association. Am J Psychiatry. 1996;53;153(suppl 10):1-31.
4. U.S. Department of Health and Human Services. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. http://www.ahrq.gov/sites/default/files/wysiwyg/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treating_tobacco_use08.pdf. Published May 2008. Accessed September 12, 2016.
5. Grant BF, Hasin DS, Chou SP, et al. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(11):1107-1115.
6. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
7. Anthenelli RM. How—and why—to help psychiatric patients stop smoking. Current Psychiatry. 2005;4(1):77-87.
8. Zyban [package insert]. Research Triangle Park, NC; GlaxoSmithKline; 2016.
9. Chantix [package insert]. New York, NY: Pfizer; 2016.
10. U.S. Department of Health and Human Services. The health consequences of smoking – 50 years of progress: a report of the surgeon general, 2014. Rockville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
11. World Health Organization. WHO report on the global tobacco epidemic, 2011: warning about the dangers of tobacco. http://www.who.int/tobacco/global_report/2011/en/index.html. Published 2011. Accessed December 1, 2015.
12. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomized, placebo-controlled clinical trial. Lancet. 2016;18;387(10037):2507-2520.
13. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
14. First MB, Spitzer RL, Gibbon M, et al. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/P). New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002.
15. First M, Gibbon M, Spitzer RL, et al. Structured clinical interview for DSM-IV axis II personality disorders (SCID-II). Washington, DC: American Psychiatric Press, Inc.; 1997.
16. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361-370.
17. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2001;168(12):1266-1277.
The National Ambulatory Medical Care Survey1,2 (NAMCS) indicates that less than 1 out of 4 (23%) psychiatrists provide smoking cessation counseling to their patients, and even fewer prescribe medications.
What gives? How is it that so many psychiatrists endorse having recently helped a patient quit smoking when the data from large-scale surveys1,2 indicate they do not?
From the “glass is half-full” perspective, the discrepancy might indicate that psychiatrists finally have bought into the message put forth 20 years ago when the American Psychiatric Association first published its clinical practice guidelines for treating nicotine dependence.3 Because the figures I cited from NAMCS reflect data from 2006 to 2010, it is possible that in the last 5 years more psychiatrists have started to help their patients quit smoking. Such an hypothesis is further supported by the increasing number of research papers on smoking cessation in individuals with mental illness published over the past 8 years—a period that coincides with the release of the second edition of the Treating tobacco use and dependence clinical practice guideline from the U.S. Agency for Healthcare Research and Quality, which highlighted the need for more research in this population of smokers.4
Regardless of the reason, the fact that my informal surveys indicate a likely uptick in activity among psychiatrists to help their patients quit smoking is welcome news. With nearly 1 out of 2 cigarettes sold in the United States being smoked by individuals with psychiatric and substance use disorders,5 psychiatrists and other mental health professionals play a vital role in addressing this epidemic. That our patients smoke at rates 2- to 4-times that of the general population and die decades earlier than their non-smoking, non-mentally ill counterparts6 are compelling reasons urging us to end our complacency and help our patients quit smoking.
EAGLES trial results help debunk the latest myth about smoking cessation
In an article that I wrote for
In addition to applying the “black-box” warning, the FDA issued a post-marketing requirement to the manufacturers of bupropion and varenicline to conduct a large randomized controlled trial—Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES)—the top-line results of which were published in The Lancet this spring.12
Key results of the EAGLES trial
The researchers found no significant increase in serious neuropsychiatric AEs—a composite measure assessing depression, anxiety, suicidality, and 13 other symptom clusters—attributable to varenicline or bupropion compared with placebo or the nicotine patch in smokers with or without psychiatric disorders. The study did detect a significant difference—approximately 4% (2% in non-psychiatric cohort vs 6% in psychiatric cohort)—in the rate of serious neuropsychiatric AEs regardless of treatment condition. In both cohorts, varenicline was more effective than bupropion, which had similar efficacy to the nicotine patch; all interventions were superior to placebo. Importantly, all 3 medications significantly improved quit rates in smokers with and without psychiatric disorders. Although the efficacy of medications in smokers with or without psychiatric disorders was similar in terms of odds ratios, overall, those with psychiatric disorders had 20% to 30% lower quit rates compared with non-psychiatrically ill smokers.
The EAGLES study results, when viewed in the context of findings from other clinical trials and large-scale observational studies, provide further evidence that smokers with stable mental illness can use bupropion and varenicline safely. It also demonstrates that moderate to severe neuropsychiatric AEs occur during a smoking cessation attempt regardless of the medication used, therefore, monitoring smokers—especially those with psychiatric disorders—is important, a role that psychiatrists are uniquely poised to play.
That all 3 smoking cessation medications are effective in patients with mood, anxiety, and psychotic disorders is good news for our patients. Combined with the EAGLES safety findings, there is no better time to intervene in tobacco dependence
The National Ambulatory Medical Care Survey1,2 (NAMCS) indicates that less than 1 out of 4 (23%) psychiatrists provide smoking cessation counseling to their patients, and even fewer prescribe medications.
What gives? How is it that so many psychiatrists endorse having recently helped a patient quit smoking when the data from large-scale surveys1,2 indicate they do not?
From the “glass is half-full” perspective, the discrepancy might indicate that psychiatrists finally have bought into the message put forth 20 years ago when the American Psychiatric Association first published its clinical practice guidelines for treating nicotine dependence.3 Because the figures I cited from NAMCS reflect data from 2006 to 2010, it is possible that in the last 5 years more psychiatrists have started to help their patients quit smoking. Such an hypothesis is further supported by the increasing number of research papers on smoking cessation in individuals with mental illness published over the past 8 years—a period that coincides with the release of the second edition of the Treating tobacco use and dependence clinical practice guideline from the U.S. Agency for Healthcare Research and Quality, which highlighted the need for more research in this population of smokers.4
Regardless of the reason, the fact that my informal surveys indicate a likely uptick in activity among psychiatrists to help their patients quit smoking is welcome news. With nearly 1 out of 2 cigarettes sold in the United States being smoked by individuals with psychiatric and substance use disorders,5 psychiatrists and other mental health professionals play a vital role in addressing this epidemic. That our patients smoke at rates 2- to 4-times that of the general population and die decades earlier than their non-smoking, non-mentally ill counterparts6 are compelling reasons urging us to end our complacency and help our patients quit smoking.
EAGLES trial results help debunk the latest myth about smoking cessation
In an article that I wrote for
In addition to applying the “black-box” warning, the FDA issued a post-marketing requirement to the manufacturers of bupropion and varenicline to conduct a large randomized controlled trial—Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES)—the top-line results of which were published in The Lancet this spring.12
Key results of the EAGLES trial
The researchers found no significant increase in serious neuropsychiatric AEs—a composite measure assessing depression, anxiety, suicidality, and 13 other symptom clusters—attributable to varenicline or bupropion compared with placebo or the nicotine patch in smokers with or without psychiatric disorders. The study did detect a significant difference—approximately 4% (2% in non-psychiatric cohort vs 6% in psychiatric cohort)—in the rate of serious neuropsychiatric AEs regardless of treatment condition. In both cohorts, varenicline was more effective than bupropion, which had similar efficacy to the nicotine patch; all interventions were superior to placebo. Importantly, all 3 medications significantly improved quit rates in smokers with and without psychiatric disorders. Although the efficacy of medications in smokers with or without psychiatric disorders was similar in terms of odds ratios, overall, those with psychiatric disorders had 20% to 30% lower quit rates compared with non-psychiatrically ill smokers.
The EAGLES study results, when viewed in the context of findings from other clinical trials and large-scale observational studies, provide further evidence that smokers with stable mental illness can use bupropion and varenicline safely. It also demonstrates that moderate to severe neuropsychiatric AEs occur during a smoking cessation attempt regardless of the medication used, therefore, monitoring smokers—especially those with psychiatric disorders—is important, a role that psychiatrists are uniquely poised to play.
That all 3 smoking cessation medications are effective in patients with mood, anxiety, and psychotic disorders is good news for our patients. Combined with the EAGLES safety findings, there is no better time to intervene in tobacco dependence
1. Rogers E, Sherman S. Tobacco use screening and treatment by outpatient psychiatrists before and after release of the American Psychiatric Association treatment guidelines for nicotine dependence. Am J Public Health. 2014;104(1):90-95.
2. Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling? Am J Psychiatry. 2003;160(12):2228-2230.
3. Practice guideline for the treatment of patients with nicotine dependence. American Psychiatric Association. Am J Psychiatry. 1996;53;153(suppl 10):1-31.
4. U.S. Department of Health and Human Services. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. http://www.ahrq.gov/sites/default/files/wysiwyg/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treating_tobacco_use08.pdf. Published May 2008. Accessed September 12, 2016.
5. Grant BF, Hasin DS, Chou SP, et al. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(11):1107-1115.
6. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
7. Anthenelli RM. How—and why—to help psychiatric patients stop smoking. Current Psychiatry. 2005;4(1):77-87.
8. Zyban [package insert]. Research Triangle Park, NC; GlaxoSmithKline; 2016.
9. Chantix [package insert]. New York, NY: Pfizer; 2016.
10. U.S. Department of Health and Human Services. The health consequences of smoking – 50 years of progress: a report of the surgeon general, 2014. Rockville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
11. World Health Organization. WHO report on the global tobacco epidemic, 2011: warning about the dangers of tobacco. http://www.who.int/tobacco/global_report/2011/en/index.html. Published 2011. Accessed December 1, 2015.
12. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomized, placebo-controlled clinical trial. Lancet. 2016;18;387(10037):2507-2520.
13. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
14. First MB, Spitzer RL, Gibbon M, et al. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/P). New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002.
15. First M, Gibbon M, Spitzer RL, et al. Structured clinical interview for DSM-IV axis II personality disorders (SCID-II). Washington, DC: American Psychiatric Press, Inc.; 1997.
16. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361-370.
17. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2001;168(12):1266-1277.
1. Rogers E, Sherman S. Tobacco use screening and treatment by outpatient psychiatrists before and after release of the American Psychiatric Association treatment guidelines for nicotine dependence. Am J Public Health. 2014;104(1):90-95.
2. Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling? Am J Psychiatry. 2003;160(12):2228-2230.
3. Practice guideline for the treatment of patients with nicotine dependence. American Psychiatric Association. Am J Psychiatry. 1996;53;153(suppl 10):1-31.
4. U.S. Department of Health and Human Services. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. http://www.ahrq.gov/sites/default/files/wysiwyg/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treating_tobacco_use08.pdf. Published May 2008. Accessed September 12, 2016.
5. Grant BF, Hasin DS, Chou SP, et al. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(11):1107-1115.
6. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
7. Anthenelli RM. How—and why—to help psychiatric patients stop smoking. Current Psychiatry. 2005;4(1):77-87.
8. Zyban [package insert]. Research Triangle Park, NC; GlaxoSmithKline; 2016.
9. Chantix [package insert]. New York, NY: Pfizer; 2016.
10. U.S. Department of Health and Human Services. The health consequences of smoking – 50 years of progress: a report of the surgeon general, 2014. Rockville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
11. World Health Organization. WHO report on the global tobacco epidemic, 2011: warning about the dangers of tobacco. http://www.who.int/tobacco/global_report/2011/en/index.html. Published 2011. Accessed December 1, 2015.
12. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomized, placebo-controlled clinical trial. Lancet. 2016;18;387(10037):2507-2520.
13. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
14. First MB, Spitzer RL, Gibbon M, et al. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/P). New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002.
15. First M, Gibbon M, Spitzer RL, et al. Structured clinical interview for DSM-IV axis II personality disorders (SCID-II). Washington, DC: American Psychiatric Press, Inc.; 1997.
16. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361-370.
17. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2001;168(12):1266-1277.
Adherence to the vaccine
Varenicline
Varenicline tartrate—the first nicotine-free medication FDA-approved for smoking cessation in nearly a decade (Table 1)—has helped patients stop smoking and remain smoke-free for up to 1 year in clinical trials. Its selective action on the receptor subtype that makes tobacco enjoyable offers a novel approach to antismoking therapy.
Table 1
Varenicline: Fast facts
| Brand name: Chantix |
| Class: Partial nicotinic acetylcholine receptor agonist |
| Indication: Tobacco dependence |
| Approval date: May 10, 2006 |
| Manufacturer: Pfizer |
| Dosing forms: 0.5- and 1-mg tablets |
| Recommended dosage: 0.5 mg/d for 3 days, 0.5 mg bid for next 4 days, then 1 mg bid for 11 weeks. Patients who quit successfully should receive an additional 12-week course to reduce relapse risk. |
How it works
Unlike other FDA-approved smoking cessation treatments such as nicotine replacement therapy and sustained-release bupropion, varenicline selectively targets the α4β2 nicotinic acetylcholine receptor (nAChR),1 which helps mediate nicotine’s reinforcing effects.2-4 By targeting this receptor subtype, varenicline ultimately diminishes these effects in the mesocorticolimbic dopamine system—the brain’s “reward center.”
As a partial α4β2 nAChR agonist, varenicline offers a two-pronged approach to smoking cessation:1
- During abstinence, varenicline stimulates low-level dopamine release by binding to α4β2 receptors located on dopamine neurons. This action, which compensates for loss of exogenous nicotine after quitting, can help counteract craving and other signs and symptoms of nicotine withdrawal caused by dopamine depletion.
- If the patient resumes smoking, varenicline makes tobacco less pleasurable by competitively binding at the α4β2 receptor.1,5
Pharmacokinetics
Varenicline is rapidly absorbed across the gut mucosa and reaches maximum concentration in approximately 4 hours. After repeated dosing, the drug reaches steady-state concentrations within 4 days, and its elimination half-life is 17 to 24 hours.
Because varenicline’s simple benzazepine structure lacks bulky moieties that would promote hepatic biotransformation,5 90% of the drug is excreted through the kidneys. To date, no clinically relevant drug-drug interactions have been reported.6
Efficacy
Varenicline showed a dose-dependent effect in phase-2 clinical trials,7,8 with 1 mg bid providing optimal efficacy and tolerability. Compared with placebo, varenicline was significantly more effective in initiating:
- continuous abstinence for ≥4 weeks during active treatment, confirmed by measuring carbon monoxide (CO) in exhaled breath
- long-term abstinence, evidenced by self-report and exhaled CO ≤10 ppm at 24 and 52 weeks.7,8
Odds ratios calculated for patients who stayed smoke-free for ≥4 weeks during 7 to 12 weeks of active treatment suggest that smokers who use varenicline, 1 mg bid, are approximately 4 to 8 times more likely to achieve short-term abstinence during this active treatment period than those who received placebo.7,8
In phase-3 trials,9-11 patients were also followed for up to 1 year and received brief, standardized counseling along with medication or placebo—as recommended in the U.S. Department of Health and Human Services Clinical Practice Guideline, Treating Tobacco Use and Dependence.12
12-week treatment trials.9,10 A total of 2,052 adults in two randomized, double-blind trials received varenicline, sustained-release (SR) bupropion, or placebo for 12 weeks. Based on phase-2 trial results—which showed that varenicline was better tolerated after a 1-week dosage titration period—varenicline was given at:
- 0.5 mg/d for days 1 through 3
- 0.5 mg bid for days 4 through 7
- 1 mg bid through week 12.
Bupropion SR was given at 150 mg/d for days 1 through 3, then 150 mg bid through week 12.
Patients were then followed for up to 40 weeks after drug discontinuation. Patients had been smoking ≥10 cigarettes/day at baseline and were motivated to stop smoking.
Overall, varenicline was associated with higher short- and long-term abstinence rates compared with bupropion SR or placebo (Table 2), although the comparison with bupropion SR was not statistically significant (P=0.057) for weeks 9 through 52 in one study.9 As in the phase-2 studies, abstinence was confirmed by measuring CO in exhaled breath.
Compared with placebo, varenicline also reduced cravings and other signs and symptoms of tobacco withdrawal as measured with the Brief Questionnaire of Smoking Urges and Minnesota Nicotine Withdrawal Scale.9,10
Relapse prevention study. Tonstad et al11 investigated whether extended varenicline treatment prolongs smoking abstinence. A total of 1,210 patients who quit smoking after 12 weeks of open-label varenicline treatment continued taking varenicline at 1 mg bid or were switched to placebo during a 3-month, double-blind phase.
Compared with placebo, rates of continuous smoking abstinence were significantly higher among the varenicline group during the double-blind active treatment phase (70.5% vs. 49.6%) and for 6 months after drug discontinuation (43.6% vs. 36.9%).11 These data suggest that an extended varenicline regimen might promote long-term abstinence.6,13
Table 2
Smoking abstinence* rates among patients
during varenicline phase-3 clinical trials
| 4 weeks of continued abstinence, weeks 9 through 12 | Continued abstinence, weeks 9 through 24 | Continued abstinence, weeks 9 through 52 | |
|---|---|---|---|
| Gonzales et al 20069 | |||
| Varenicline, 1 mg bid | 44% | 29.5% | 21.9% |
| Bupropion SR, 150 mg bid | 29.5% | 20.7% | 16.1% |
| Placebo | 17.7% | 10.5% | 8.4% |
| Jorenby et al 200610 | |||
| Varenicline, 1 mg bid | 43.9% | 29.7% | 23% |
| Bupropion SR, 150 mg bid | 29.8% | 20.2% | 14.6% |
| Placebo | 17.6% | 13.2% | 10.3% |
| * Confirmed by self-report and exhaled carbon monoxide ≤10 ppm. | |||
Tolerability
Overall, varenicline was safe and well tolerated in clinical trials.
Nausea was the most commonly reported adverse event in fixed-dose, placebo-controlled studies.6 Although approximately 3% of patients stopped varenicline prematurely because of upset stomach,6 most rated their nausea as mild to moderate and reported reduced nausea with continued varenicline use. For patients with intolerable nausea, consider reducing the dosage.
Sleep disturbance, constipation, flatulence, and vomiting were twice as prevalent among the varenicline groups compared with placebo.6 Overall treatment discontinuation rates were similar with varenicline, 1 mg bid, and placebo (12% vs. 10%) in 12-week phase-2 and phase-3 clinical trials.6
To improve tolerability, the FDA recommends splitting varenicline into twice-daily doses.6,13
Dosing
Start varenicline at 0.5 mg/d for 3 days, 0.5 mg bid for the next 4 days, then 1 mg bid through week 12. To improve tolerability, advise patients to take varenicline after eating and with a full glass of water.
Setting a target quit date (TQD) is a critical element of smoking cessation treatment. Schedule the TQD for the same day the patient begins 1 mg bid varenicline dosing so that the medication is approaching maximal steady-state concentrations during the quit attempt to help counter withdrawal. Allow patients to continue smoking during the 1-week titration period, but stress the importance of trying to quit on the TQD.
Because varenicline is primarily eliminated through the kidneys, limit dosages to 0.5 mg bid in patients with severe renal impairment (estimated creatinine clearance 6,13
Varenicline has not been studied in patients with substance use and other psychiatric disorders—patients who account for most of a psychiatrist’s caseload and whose nicotine dependence is difficult to treat. Even so, the medication’s lack of discernible drug-drug interactions and selectivity of α4β2 nAChR action make varenicline worth considering for these patients.
Varenicline also has not been tested or approved for use in adolescent or pregnant smokers; research is needed on how the medication works in these patients.
Role of behavioral treatment
The Clinical Practice Guideline, Treating Tobacco Use and Dependence12 suggests combining antismoking pharmacotherapy with counseling to maximize outcome. To that end, varenicline’s manufacturer has developed a personalized behavioral support program for patients taking the medication.14 Adjunctive therapy via the Internet, telephone, or direct mail can complement other extra-treatment supports—such as toll-free quit lines and classes offered through health organizations—and more-formal, intensive behavioral interventions.
Related resources
- Varenicline Web site. www.chantix.com.
- World Health Organization. Causes of death. In: Epidemiology and burden of disease. Geneva: World Health Organization, 2003.
- Fiore MC, Bailey WC, Cohen SJ, et al. Clinical practice guideline. Treating tobacco use and dependence. Rockville, MD: U.S. Department of Health and Human Services, 2000. www.surgeongeneral.gov/tobacco/treating_tobacco_use.pdf.
Drug brand names
- Bupropion SR • Wellbutrin, Zyban
- Varenicline • Chantix
Disclosures
Dr. Anthenelli is a consultant for Alkermes and Cephalon and a speaker and consultant for Pfizer and sanofi-aventis.
The Tri-State Tobacco and Alcohol Research Center receives research grants from Addex, Ortho-McNeil Neurologics, and sanofi-aventis.
Dr. Anthenelli and the Tri-State Tobacco and Alcohol Research Center were members of the Varenicline Study Group.
Acknowledgments
The writing of this article was supported in part by National Institutes of Health Grant Awards Nos. AA013957 and AA013307.
The author thanks Reene Cantwell for her technical assistance in preparing this article.
1. Coe JW, Brooks PR, Vetelino MG, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem 2005;48:3474-7.
2. Marubio LM, Gardier AM, Durier S, et al. Effects of nicotine in the dopaminergic system of mice lacking the alpha4 subunit of neuronal nicotinic acetylcholine receptors. Eur J Neurosci 2003;17:1329-37.
3. Picciotto MR, Zoli M, Rimondini R, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 1998;391:173-7.
4. Tapper AR, McKinney SL, Nashmi R, et al. Nicotine activation of α4* receptors: sufficient for reward, tolerance, and sensitization. Science 2004;306:1029-32.
5. Obach RS, Reed-Hagen AE, Krueger SS, et al. Metabolism and disposition of varenicline, a selective alpha4beta2 acetylcholine receptor partial agonist, in vivo and in vitro. Drug Metab Dispos 2006;34:121-30.
6. Chantix (varenicline) package insert. Available at: http://www.pfizer.com/pfizer/download/uspi_chantix.pdf. Accessed November 28, 2006.
7. Oncken C, Gonzales D, Nides M, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med 2006;166:1571-7.
8. Nides M, Oncken C, Gonzales D, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med 2006;166:1561-8.
9. Gonzales D, Rennard SI, Nides M, et al. Varenicline Phase 3 Study Group. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 2006;296:47-55.
10. Jorenby DE, Hays JT, Rigotti NA, et al. Varenicline Phase 3 Study Group. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 2006;296:56-63.
11. Tonstad S, Tonnesen P, Hajek P, et al. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA 2006;296:64-71.
12. Fiore MC, Bailey WC, Cohen SJ, et al. Clinical practice guideline. Treating tobacco use and dependence. Rockville, MD: U.S. Department of Health and Human Services, 2000. Available at: http://www.surgeongeneral.gov/tobacco/treating_tobacco_use.pdf. Accessed November 30, 2006.
13. Varenicline (Chantix) for tobacco dependence. Med Lett Drugs Ther 2006;48:66-8.
14. Varenicline. Web site. Available at: http://www.chantix.com. Accessed November 30, 2006.
Varenicline tartrate—the first nicotine-free medication FDA-approved for smoking cessation in nearly a decade (Table 1)—has helped patients stop smoking and remain smoke-free for up to 1 year in clinical trials. Its selective action on the receptor subtype that makes tobacco enjoyable offers a novel approach to antismoking therapy.
Table 1
Varenicline: Fast facts
| Brand name: Chantix |
| Class: Partial nicotinic acetylcholine receptor agonist |
| Indication: Tobacco dependence |
| Approval date: May 10, 2006 |
| Manufacturer: Pfizer |
| Dosing forms: 0.5- and 1-mg tablets |
| Recommended dosage: 0.5 mg/d for 3 days, 0.5 mg bid for next 4 days, then 1 mg bid for 11 weeks. Patients who quit successfully should receive an additional 12-week course to reduce relapse risk. |
How it works
Unlike other FDA-approved smoking cessation treatments such as nicotine replacement therapy and sustained-release bupropion, varenicline selectively targets the α4β2 nicotinic acetylcholine receptor (nAChR),1 which helps mediate nicotine’s reinforcing effects.2-4 By targeting this receptor subtype, varenicline ultimately diminishes these effects in the mesocorticolimbic dopamine system—the brain’s “reward center.”
As a partial α4β2 nAChR agonist, varenicline offers a two-pronged approach to smoking cessation:1
- During abstinence, varenicline stimulates low-level dopamine release by binding to α4β2 receptors located on dopamine neurons. This action, which compensates for loss of exogenous nicotine after quitting, can help counteract craving and other signs and symptoms of nicotine withdrawal caused by dopamine depletion.
- If the patient resumes smoking, varenicline makes tobacco less pleasurable by competitively binding at the α4β2 receptor.1,5
Pharmacokinetics
Varenicline is rapidly absorbed across the gut mucosa and reaches maximum concentration in approximately 4 hours. After repeated dosing, the drug reaches steady-state concentrations within 4 days, and its elimination half-life is 17 to 24 hours.
Because varenicline’s simple benzazepine structure lacks bulky moieties that would promote hepatic biotransformation,5 90% of the drug is excreted through the kidneys. To date, no clinically relevant drug-drug interactions have been reported.6
Efficacy
Varenicline showed a dose-dependent effect in phase-2 clinical trials,7,8 with 1 mg bid providing optimal efficacy and tolerability. Compared with placebo, varenicline was significantly more effective in initiating:
- continuous abstinence for ≥4 weeks during active treatment, confirmed by measuring carbon monoxide (CO) in exhaled breath
- long-term abstinence, evidenced by self-report and exhaled CO ≤10 ppm at 24 and 52 weeks.7,8
Odds ratios calculated for patients who stayed smoke-free for ≥4 weeks during 7 to 12 weeks of active treatment suggest that smokers who use varenicline, 1 mg bid, are approximately 4 to 8 times more likely to achieve short-term abstinence during this active treatment period than those who received placebo.7,8
In phase-3 trials,9-11 patients were also followed for up to 1 year and received brief, standardized counseling along with medication or placebo—as recommended in the U.S. Department of Health and Human Services Clinical Practice Guideline, Treating Tobacco Use and Dependence.12
12-week treatment trials.9,10 A total of 2,052 adults in two randomized, double-blind trials received varenicline, sustained-release (SR) bupropion, or placebo for 12 weeks. Based on phase-2 trial results—which showed that varenicline was better tolerated after a 1-week dosage titration period—varenicline was given at:
- 0.5 mg/d for days 1 through 3
- 0.5 mg bid for days 4 through 7
- 1 mg bid through week 12.
Bupropion SR was given at 150 mg/d for days 1 through 3, then 150 mg bid through week 12.
Patients were then followed for up to 40 weeks after drug discontinuation. Patients had been smoking ≥10 cigarettes/day at baseline and were motivated to stop smoking.
Overall, varenicline was associated with higher short- and long-term abstinence rates compared with bupropion SR or placebo (Table 2), although the comparison with bupropion SR was not statistically significant (P=0.057) for weeks 9 through 52 in one study.9 As in the phase-2 studies, abstinence was confirmed by measuring CO in exhaled breath.
Compared with placebo, varenicline also reduced cravings and other signs and symptoms of tobacco withdrawal as measured with the Brief Questionnaire of Smoking Urges and Minnesota Nicotine Withdrawal Scale.9,10
Relapse prevention study. Tonstad et al11 investigated whether extended varenicline treatment prolongs smoking abstinence. A total of 1,210 patients who quit smoking after 12 weeks of open-label varenicline treatment continued taking varenicline at 1 mg bid or were switched to placebo during a 3-month, double-blind phase.
Compared with placebo, rates of continuous smoking abstinence were significantly higher among the varenicline group during the double-blind active treatment phase (70.5% vs. 49.6%) and for 6 months after drug discontinuation (43.6% vs. 36.9%).11 These data suggest that an extended varenicline regimen might promote long-term abstinence.6,13
Table 2
Smoking abstinence* rates among patients
during varenicline phase-3 clinical trials
| 4 weeks of continued abstinence, weeks 9 through 12 | Continued abstinence, weeks 9 through 24 | Continued abstinence, weeks 9 through 52 | |
|---|---|---|---|
| Gonzales et al 20069 | |||
| Varenicline, 1 mg bid | 44% | 29.5% | 21.9% |
| Bupropion SR, 150 mg bid | 29.5% | 20.7% | 16.1% |
| Placebo | 17.7% | 10.5% | 8.4% |
| Jorenby et al 200610 | |||
| Varenicline, 1 mg bid | 43.9% | 29.7% | 23% |
| Bupropion SR, 150 mg bid | 29.8% | 20.2% | 14.6% |
| Placebo | 17.6% | 13.2% | 10.3% |
| * Confirmed by self-report and exhaled carbon monoxide ≤10 ppm. | |||
Tolerability
Overall, varenicline was safe and well tolerated in clinical trials.
Nausea was the most commonly reported adverse event in fixed-dose, placebo-controlled studies.6 Although approximately 3% of patients stopped varenicline prematurely because of upset stomach,6 most rated their nausea as mild to moderate and reported reduced nausea with continued varenicline use. For patients with intolerable nausea, consider reducing the dosage.
Sleep disturbance, constipation, flatulence, and vomiting were twice as prevalent among the varenicline groups compared with placebo.6 Overall treatment discontinuation rates were similar with varenicline, 1 mg bid, and placebo (12% vs. 10%) in 12-week phase-2 and phase-3 clinical trials.6
To improve tolerability, the FDA recommends splitting varenicline into twice-daily doses.6,13
Dosing
Start varenicline at 0.5 mg/d for 3 days, 0.5 mg bid for the next 4 days, then 1 mg bid through week 12. To improve tolerability, advise patients to take varenicline after eating and with a full glass of water.
Setting a target quit date (TQD) is a critical element of smoking cessation treatment. Schedule the TQD for the same day the patient begins 1 mg bid varenicline dosing so that the medication is approaching maximal steady-state concentrations during the quit attempt to help counter withdrawal. Allow patients to continue smoking during the 1-week titration period, but stress the importance of trying to quit on the TQD.
Because varenicline is primarily eliminated through the kidneys, limit dosages to 0.5 mg bid in patients with severe renal impairment (estimated creatinine clearance 6,13
Varenicline has not been studied in patients with substance use and other psychiatric disorders—patients who account for most of a psychiatrist’s caseload and whose nicotine dependence is difficult to treat. Even so, the medication’s lack of discernible drug-drug interactions and selectivity of α4β2 nAChR action make varenicline worth considering for these patients.
Varenicline also has not been tested or approved for use in adolescent or pregnant smokers; research is needed on how the medication works in these patients.
Role of behavioral treatment
The Clinical Practice Guideline, Treating Tobacco Use and Dependence12 suggests combining antismoking pharmacotherapy with counseling to maximize outcome. To that end, varenicline’s manufacturer has developed a personalized behavioral support program for patients taking the medication.14 Adjunctive therapy via the Internet, telephone, or direct mail can complement other extra-treatment supports—such as toll-free quit lines and classes offered through health organizations—and more-formal, intensive behavioral interventions.
Related resources
- Varenicline Web site. www.chantix.com.
- World Health Organization. Causes of death. In: Epidemiology and burden of disease. Geneva: World Health Organization, 2003.
- Fiore MC, Bailey WC, Cohen SJ, et al. Clinical practice guideline. Treating tobacco use and dependence. Rockville, MD: U.S. Department of Health and Human Services, 2000. www.surgeongeneral.gov/tobacco/treating_tobacco_use.pdf.
Drug brand names
- Bupropion SR • Wellbutrin, Zyban
- Varenicline • Chantix
Disclosures
Dr. Anthenelli is a consultant for Alkermes and Cephalon and a speaker and consultant for Pfizer and sanofi-aventis.
The Tri-State Tobacco and Alcohol Research Center receives research grants from Addex, Ortho-McNeil Neurologics, and sanofi-aventis.
Dr. Anthenelli and the Tri-State Tobacco and Alcohol Research Center were members of the Varenicline Study Group.
Acknowledgments
The writing of this article was supported in part by National Institutes of Health Grant Awards Nos. AA013957 and AA013307.
The author thanks Reene Cantwell for her technical assistance in preparing this article.
Varenicline tartrate—the first nicotine-free medication FDA-approved for smoking cessation in nearly a decade (Table 1)—has helped patients stop smoking and remain smoke-free for up to 1 year in clinical trials. Its selective action on the receptor subtype that makes tobacco enjoyable offers a novel approach to antismoking therapy.
Table 1
Varenicline: Fast facts
| Brand name: Chantix |
| Class: Partial nicotinic acetylcholine receptor agonist |
| Indication: Tobacco dependence |
| Approval date: May 10, 2006 |
| Manufacturer: Pfizer |
| Dosing forms: 0.5- and 1-mg tablets |
| Recommended dosage: 0.5 mg/d for 3 days, 0.5 mg bid for next 4 days, then 1 mg bid for 11 weeks. Patients who quit successfully should receive an additional 12-week course to reduce relapse risk. |
How it works
Unlike other FDA-approved smoking cessation treatments such as nicotine replacement therapy and sustained-release bupropion, varenicline selectively targets the α4β2 nicotinic acetylcholine receptor (nAChR),1 which helps mediate nicotine’s reinforcing effects.2-4 By targeting this receptor subtype, varenicline ultimately diminishes these effects in the mesocorticolimbic dopamine system—the brain’s “reward center.”
As a partial α4β2 nAChR agonist, varenicline offers a two-pronged approach to smoking cessation:1
- During abstinence, varenicline stimulates low-level dopamine release by binding to α4β2 receptors located on dopamine neurons. This action, which compensates for loss of exogenous nicotine after quitting, can help counteract craving and other signs and symptoms of nicotine withdrawal caused by dopamine depletion.
- If the patient resumes smoking, varenicline makes tobacco less pleasurable by competitively binding at the α4β2 receptor.1,5
Pharmacokinetics
Varenicline is rapidly absorbed across the gut mucosa and reaches maximum concentration in approximately 4 hours. After repeated dosing, the drug reaches steady-state concentrations within 4 days, and its elimination half-life is 17 to 24 hours.
Because varenicline’s simple benzazepine structure lacks bulky moieties that would promote hepatic biotransformation,5 90% of the drug is excreted through the kidneys. To date, no clinically relevant drug-drug interactions have been reported.6
Efficacy
Varenicline showed a dose-dependent effect in phase-2 clinical trials,7,8 with 1 mg bid providing optimal efficacy and tolerability. Compared with placebo, varenicline was significantly more effective in initiating:
- continuous abstinence for ≥4 weeks during active treatment, confirmed by measuring carbon monoxide (CO) in exhaled breath
- long-term abstinence, evidenced by self-report and exhaled CO ≤10 ppm at 24 and 52 weeks.7,8
Odds ratios calculated for patients who stayed smoke-free for ≥4 weeks during 7 to 12 weeks of active treatment suggest that smokers who use varenicline, 1 mg bid, are approximately 4 to 8 times more likely to achieve short-term abstinence during this active treatment period than those who received placebo.7,8
In phase-3 trials,9-11 patients were also followed for up to 1 year and received brief, standardized counseling along with medication or placebo—as recommended in the U.S. Department of Health and Human Services Clinical Practice Guideline, Treating Tobacco Use and Dependence.12
12-week treatment trials.9,10 A total of 2,052 adults in two randomized, double-blind trials received varenicline, sustained-release (SR) bupropion, or placebo for 12 weeks. Based on phase-2 trial results—which showed that varenicline was better tolerated after a 1-week dosage titration period—varenicline was given at:
- 0.5 mg/d for days 1 through 3
- 0.5 mg bid for days 4 through 7
- 1 mg bid through week 12.
Bupropion SR was given at 150 mg/d for days 1 through 3, then 150 mg bid through week 12.
Patients were then followed for up to 40 weeks after drug discontinuation. Patients had been smoking ≥10 cigarettes/day at baseline and were motivated to stop smoking.
Overall, varenicline was associated with higher short- and long-term abstinence rates compared with bupropion SR or placebo (Table 2), although the comparison with bupropion SR was not statistically significant (P=0.057) for weeks 9 through 52 in one study.9 As in the phase-2 studies, abstinence was confirmed by measuring CO in exhaled breath.
Compared with placebo, varenicline also reduced cravings and other signs and symptoms of tobacco withdrawal as measured with the Brief Questionnaire of Smoking Urges and Minnesota Nicotine Withdrawal Scale.9,10
Relapse prevention study. Tonstad et al11 investigated whether extended varenicline treatment prolongs smoking abstinence. A total of 1,210 patients who quit smoking after 12 weeks of open-label varenicline treatment continued taking varenicline at 1 mg bid or were switched to placebo during a 3-month, double-blind phase.
Compared with placebo, rates of continuous smoking abstinence were significantly higher among the varenicline group during the double-blind active treatment phase (70.5% vs. 49.6%) and for 6 months after drug discontinuation (43.6% vs. 36.9%).11 These data suggest that an extended varenicline regimen might promote long-term abstinence.6,13
Table 2
Smoking abstinence* rates among patients
during varenicline phase-3 clinical trials
| 4 weeks of continued abstinence, weeks 9 through 12 | Continued abstinence, weeks 9 through 24 | Continued abstinence, weeks 9 through 52 | |
|---|---|---|---|
| Gonzales et al 20069 | |||
| Varenicline, 1 mg bid | 44% | 29.5% | 21.9% |
| Bupropion SR, 150 mg bid | 29.5% | 20.7% | 16.1% |
| Placebo | 17.7% | 10.5% | 8.4% |
| Jorenby et al 200610 | |||
| Varenicline, 1 mg bid | 43.9% | 29.7% | 23% |
| Bupropion SR, 150 mg bid | 29.8% | 20.2% | 14.6% |
| Placebo | 17.6% | 13.2% | 10.3% |
| * Confirmed by self-report and exhaled carbon monoxide ≤10 ppm. | |||
Tolerability
Overall, varenicline was safe and well tolerated in clinical trials.
Nausea was the most commonly reported adverse event in fixed-dose, placebo-controlled studies.6 Although approximately 3% of patients stopped varenicline prematurely because of upset stomach,6 most rated their nausea as mild to moderate and reported reduced nausea with continued varenicline use. For patients with intolerable nausea, consider reducing the dosage.
Sleep disturbance, constipation, flatulence, and vomiting were twice as prevalent among the varenicline groups compared with placebo.6 Overall treatment discontinuation rates were similar with varenicline, 1 mg bid, and placebo (12% vs. 10%) in 12-week phase-2 and phase-3 clinical trials.6
To improve tolerability, the FDA recommends splitting varenicline into twice-daily doses.6,13
Dosing
Start varenicline at 0.5 mg/d for 3 days, 0.5 mg bid for the next 4 days, then 1 mg bid through week 12. To improve tolerability, advise patients to take varenicline after eating and with a full glass of water.
Setting a target quit date (TQD) is a critical element of smoking cessation treatment. Schedule the TQD for the same day the patient begins 1 mg bid varenicline dosing so that the medication is approaching maximal steady-state concentrations during the quit attempt to help counter withdrawal. Allow patients to continue smoking during the 1-week titration period, but stress the importance of trying to quit on the TQD.
Because varenicline is primarily eliminated through the kidneys, limit dosages to 0.5 mg bid in patients with severe renal impairment (estimated creatinine clearance 6,13
Varenicline has not been studied in patients with substance use and other psychiatric disorders—patients who account for most of a psychiatrist’s caseload and whose nicotine dependence is difficult to treat. Even so, the medication’s lack of discernible drug-drug interactions and selectivity of α4β2 nAChR action make varenicline worth considering for these patients.
Varenicline also has not been tested or approved for use in adolescent or pregnant smokers; research is needed on how the medication works in these patients.
Role of behavioral treatment
The Clinical Practice Guideline, Treating Tobacco Use and Dependence12 suggests combining antismoking pharmacotherapy with counseling to maximize outcome. To that end, varenicline’s manufacturer has developed a personalized behavioral support program for patients taking the medication.14 Adjunctive therapy via the Internet, telephone, or direct mail can complement other extra-treatment supports—such as toll-free quit lines and classes offered through health organizations—and more-formal, intensive behavioral interventions.
Related resources
- Varenicline Web site. www.chantix.com.
- World Health Organization. Causes of death. In: Epidemiology and burden of disease. Geneva: World Health Organization, 2003.
- Fiore MC, Bailey WC, Cohen SJ, et al. Clinical practice guideline. Treating tobacco use and dependence. Rockville, MD: U.S. Department of Health and Human Services, 2000. www.surgeongeneral.gov/tobacco/treating_tobacco_use.pdf.
Drug brand names
- Bupropion SR • Wellbutrin, Zyban
- Varenicline • Chantix
Disclosures
Dr. Anthenelli is a consultant for Alkermes and Cephalon and a speaker and consultant for Pfizer and sanofi-aventis.
The Tri-State Tobacco and Alcohol Research Center receives research grants from Addex, Ortho-McNeil Neurologics, and sanofi-aventis.
Dr. Anthenelli and the Tri-State Tobacco and Alcohol Research Center were members of the Varenicline Study Group.
Acknowledgments
The writing of this article was supported in part by National Institutes of Health Grant Awards Nos. AA013957 and AA013307.
The author thanks Reene Cantwell for her technical assistance in preparing this article.
1. Coe JW, Brooks PR, Vetelino MG, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem 2005;48:3474-7.
2. Marubio LM, Gardier AM, Durier S, et al. Effects of nicotine in the dopaminergic system of mice lacking the alpha4 subunit of neuronal nicotinic acetylcholine receptors. Eur J Neurosci 2003;17:1329-37.
3. Picciotto MR, Zoli M, Rimondini R, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 1998;391:173-7.
4. Tapper AR, McKinney SL, Nashmi R, et al. Nicotine activation of α4* receptors: sufficient for reward, tolerance, and sensitization. Science 2004;306:1029-32.
5. Obach RS, Reed-Hagen AE, Krueger SS, et al. Metabolism and disposition of varenicline, a selective alpha4beta2 acetylcholine receptor partial agonist, in vivo and in vitro. Drug Metab Dispos 2006;34:121-30.
6. Chantix (varenicline) package insert. Available at: http://www.pfizer.com/pfizer/download/uspi_chantix.pdf. Accessed November 28, 2006.
7. Oncken C, Gonzales D, Nides M, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med 2006;166:1571-7.
8. Nides M, Oncken C, Gonzales D, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med 2006;166:1561-8.
9. Gonzales D, Rennard SI, Nides M, et al. Varenicline Phase 3 Study Group. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 2006;296:47-55.
10. Jorenby DE, Hays JT, Rigotti NA, et al. Varenicline Phase 3 Study Group. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 2006;296:56-63.
11. Tonstad S, Tonnesen P, Hajek P, et al. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA 2006;296:64-71.
12. Fiore MC, Bailey WC, Cohen SJ, et al. Clinical practice guideline. Treating tobacco use and dependence. Rockville, MD: U.S. Department of Health and Human Services, 2000. Available at: http://www.surgeongeneral.gov/tobacco/treating_tobacco_use.pdf. Accessed November 30, 2006.
13. Varenicline (Chantix) for tobacco dependence. Med Lett Drugs Ther 2006;48:66-8.
14. Varenicline. Web site. Available at: http://www.chantix.com. Accessed November 30, 2006.
1. Coe JW, Brooks PR, Vetelino MG, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem 2005;48:3474-7.
2. Marubio LM, Gardier AM, Durier S, et al. Effects of nicotine in the dopaminergic system of mice lacking the alpha4 subunit of neuronal nicotinic acetylcholine receptors. Eur J Neurosci 2003;17:1329-37.
3. Picciotto MR, Zoli M, Rimondini R, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 1998;391:173-7.
4. Tapper AR, McKinney SL, Nashmi R, et al. Nicotine activation of α4* receptors: sufficient for reward, tolerance, and sensitization. Science 2004;306:1029-32.
5. Obach RS, Reed-Hagen AE, Krueger SS, et al. Metabolism and disposition of varenicline, a selective alpha4beta2 acetylcholine receptor partial agonist, in vivo and in vitro. Drug Metab Dispos 2006;34:121-30.
6. Chantix (varenicline) package insert. Available at: http://www.pfizer.com/pfizer/download/uspi_chantix.pdf. Accessed November 28, 2006.
7. Oncken C, Gonzales D, Nides M, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med 2006;166:1571-7.
8. Nides M, Oncken C, Gonzales D, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med 2006;166:1561-8.
9. Gonzales D, Rennard SI, Nides M, et al. Varenicline Phase 3 Study Group. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 2006;296:47-55.
10. Jorenby DE, Hays JT, Rigotti NA, et al. Varenicline Phase 3 Study Group. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 2006;296:56-63.
11. Tonstad S, Tonnesen P, Hajek P, et al. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA 2006;296:64-71.
12. Fiore MC, Bailey WC, Cohen SJ, et al. Clinical practice guideline. Treating tobacco use and dependence. Rockville, MD: U.S. Department of Health and Human Services, 2000. Available at: http://www.surgeongeneral.gov/tobacco/treating_tobacco_use.pdf. Accessed November 30, 2006.
13. Varenicline (Chantix) for tobacco dependence. Med Lett Drugs Ther 2006;48:66-8.
14. Varenicline. Web site. Available at: http://www.chantix.com. Accessed November 30, 2006.
How—and why—to help psychiatric patients stop smoking
Three myths about cigarette smoking may explain why psychiatrists rarely intervene in their patients’ tobacco dependence:
- Cigarette smoking is an incurable habit in psychiatric patients and thus not worth the effort of intervening.
- Cigarette smoking is an acceptable form of self-medication in persons with psychiatric illness.
- Quitting smoking will worsen psychiatric symptoms.
Smoking by psychiatric patients is treatable, however, and evidence proves that many can quit.1 This article rebuts the “why-bother?” myths and provides practical tips on how to more effectively help psychiatric patients stop smoking.
DEBUNKING THREE MYTHS
Mentally ill women and men consume nearly one-half (44%) of the cigarettes smoked in the United States (Table 1)1-3 and thus are at high risk for tobacco-related premature death, cancer, cardiovascular disease, and respiratory disorders. Although recognized as a leading cause of death, cigarette smoking by psychiatric patients frequently goes unaddressed, contributing to excess mortality in this population.4
Table 1
Cigarette smoking: An epidemic among psychiatric patients
|
| Source: References 1-3 |
- psychiatrists seldom (6,7
- when counseling did occur, nicotine replacement therapy was not prescribed.6
Tobacco dependence is a syndrome with strong genetic and biologic roots. Family, twin, and adoption studies show consistently that tobacco dependence is genetically mediated.8 Genetic polymorphisms are being identified that may modify an individual’s risk for developing nicotine dependence—such as the gene encoding the cytochrome P-450 2A6 isoenzyme (CYP 2A6) that metabolizes nicotine to cotinine.9 Disturbed nicotinic receptor functioning has been shown in persons with schizophrenia, mood disorders, anxiety disorders, and attention-deficit/hyperactivity disorders.3,10,11
Tobacco dependence is a chronic, relapsing condition that usually requires repeated intervention to motivate patients to try to quit and to help those who are willing to quit to succeed. Effective smoking cessation aids include:
- behavioral therapy (brief physician advice, problem-solving skills/skills training)
- pharmacologic therapy (nicotine replacement, sustained-release bupropion).12
Is smoking ‘self-medication’? Compelling evidence indicates that cholinergic mechanisms and nicotinic receptors (nAChRs) are involved in the pathophysiology of schizophrenia and other neuropsychiatric disorders.3,10 Nicotine administration appears to improve sensory-processing and cognitive deficits observed in schizophrenia.2,3 Moreover, the association between depression and smoking13 —and tobacco smoke’s monoamine oxidase-inhibiting and other psychoactive properties14 —have led some to posit that cigarette smoking may have antidepressant actions.10
For all these reasons, some authors have speculated that tobacco use may be a form of self-medication among the psychiatrically ill.3 The problem with this hypothesis, however, is that tobacco smoke is—at best—an untested and potentially lethal cognitive enhancer, antidepressant, or anxiolytic. Animal and human studies may find therapeutic effects of acute nicotine administration, but the cognitive effects of chronic tobacco smoking are not known.
Table 2
5 ‘A’s of brief clinical intervention for tobacco dependence
|
| Source: References 5 and 12 |
Adverse effects from quitting? Smokers with a history of major depressive disorder have been shown to be at risk to:
- develop another depressive episode after they quit smoking15
- experience more severe withdrawal symptoms during abstinence, compared with smokers with no history of depression.13,16
Scant data support the myth that smoking cessation worsens psychiatric symptoms. For example, in a review on tobacco dependence and schizophrenia, George et al2 concluded that the effects of smoking cessation on schizophrenia symptoms are not clear. Two smoking cessation trials in schizophrenic patients treated with nicotine patches found no significant changes in postcessation psychotic symptoms.17,18
Concerns that substance-abusing patients should not attempt to quit smoking during alcohol and other drug dependence treatment are also unsubstantiated. Rather than exacerbating drug addiction, smoking cessation has been found to improve addicts’ abstinence rates.19
USING AVAILABLE THERAPIES
Evidence is insufficient so far to show whether psychiatrically ill smokers would benefit more from specially tailored cessation treatments than from standard treatments, according to the 2000 U.S. Public Health Service clinical practice guide.12 Thus, while researchers try to resolve this issue, psychiatrists are left to use medications found to be effective in smokers overall.
Clinical vignette. Mr. J, age 45, has paranoid-type schizophrenia and has been smoking at least two packs of cigarettes daily for 25 years. He complains of a productive cough and expresses interest in quitting smoking when his psychiatrist raises this topic.
His persecutory delusions are well-controlled on olanzapine, 10 mg/d. He is adhering with his medications and participating in weekly group counseling that provides supportive therapy for patients with serious mental illness.
In this schizophrenic smoker who is willing to try to quit, the psychiatrist performed the first three of “5 ‘A’s” (Table 2) of brief clinical intervention for tobacco dependence.5,12 The next steps are to assist the patient’s effort to quit and arrange follow-up.
When to quit. The best time for a smoker with psychiatric illness to try to quit is when he or she:
- is psychiatrically stable
- is not in crisis
- has no recent or planned psychiatric drug changes.
Smoking cessation may increase blood levels of these psychotropics
| Antipsychotics | Antidepressants | Mood stabilizers | Anxiolytics |
|---|---|---|---|
| Haloperidol | Clomipramine | Carbamazepine | Desmethyldiazepam |
| Chlorpromazine | Desipramine | Oxazepam | |
| Fluphenazine | Doxepin | ||
| Olanzapine | Imipramine | ||
| Clozapine | Nortriptyline | ||
| Source: References 2, 5, and 20 | |||
Olanzapine’s clearance is approximately 40% higher in smokers than in nonsmokers. The psychiatrist discussed this with Mr. J and:
- asked him to call if side effects develop during the quit attempt
- scheduled more-frequent appointments to monitor side effects.
Mr. J’s schizophrenia is stable on maintenance therapy with an atypical antipsychotic. Schizophrenic smokers taking atypicals may be more able to quit smoking with NRT or sustained-release bupropion, compared with those taking conventional antipsychotics.2
The psychiatrist also determined that Mr. J had tried to quit smoking three times. Two of these attempts were done “cold turkey,” without pharmacotherapy, and one involved using nicotine gum. Mr. J said that although the gum “worked well at first,” he stopped using it because it was expensive and made his mouth sore. This information helped the psychiatrist choose medication for this quit attempt.
Most smoking cessation guidelines rely on a stepped-care approach, progressing from minimal to more-intensive interventions as needed.5 Mr. J’s psychiatrist devised an intensive treatment plan because:
- Mr. J has tried to quit before
- schizophrenic patients generally have more difficulty quitting and are more nicotine-dependent than other smokers.
Table 4
Nicotine replacement and other options for smoking cessation
| Drug | Daily dosage | Treatment duration* | Common side effects |
|---|---|---|---|
| Nicotine replacement therapy† | |||
| Transdermal | Skin irritation, insomnia | ||
| 24-hr patch | Starting dose is 21 mg/d; also in 7- and 14-mg patches for tapering dosage | 8 wk | |
| 16-hr patch | 15 mg | 8 wk | |
| Polacrilex (gum) 2- or 4-mg piece | 1 piece/hr ( | 8 to 12 wk | Mouth irritation, sore jaw, dyspepsia, hiccups |
| Vapor inhaler | 6 to 16 cartridges/day (delivers 4/mg/cartridge) | 3 to 6 mo | Mouth and throat irritation, cough |
| Nasal spray | 1 to 2 doses/hr; dose = 1 mg (0.5 mg per nostril); maximum dosage 40 mg/d | 3 to 6 mo | Nasal irritation, sneezing, cough, tearing eyes |
| Lozenge | 2- or 4-mg dose; see dosage formula, titration schedule in over-the-counter package | 12 wk | Hiccups, nausea, heartburn |
| Non-nicotine replacement therapy | |||
| Sustained-release bupropion† | 150 mg/d for 3 days, then 150 mg bid; start 1 week before quit date | 7 to 12 wk; up to 6 mo. to maintain abstinence | Insomnia, dry mouth, agitation |
| Nortriptyline | 75 to 100 mg/d; start 10 to 28 days before quit date at 25 mg/d and increase as tolerated | 12 wk | Dry mouth, sedation, dizziness |
| Clonidine | 0.1 to 0.3 mg bid | 3 to 10 wk | Dry mouth, sedation, dizziness |
| * Treatment duration varies and may be longer in patients with psychiatric disorders. | |||
| † FDA-approved as a smoking cessation aid and recommended as a first-line drug by Public Health Service clinical guidelines. | |||
| Source: Adapted from reference 21. | |||
On the morning of his TQD, Mr. J is to apply the first 21-mg transdermal nicotine patch. He is told not to smoke that day and to apply a new patch daily. The psychiatrist also tells him he will most likely remain on that dosage for 4 weeks. Then the patch strength will be reduced in 7-mg aliquots every 2 to 4 weeks, depending on his progress. The psychiatrist also provides him with educational materials on how to quit successfully.
Follow-up. Recognizing that most relapses occur in the first few days of quitting, the psychiatrist sets Mr. J’s first follow-up appointment for the day after his TQD to assess:
- whether he has smoked and number of cigarettes smoked per day
- presence and severity of withdrawal symptoms
- onset of psychiatric symptoms
- treatment adherence
- how he is handling high-risk situations and urges to smoke
- medication side effects.6
- American Psychiatric Association. Practice guideline for the treatment of patients with nicotine dependence. Am J Psychiatry 1996; 53(153[suppl]): 1-31.
- Fiore MC, Bailey WC, Cohen SJ, et.al. Treating tobacco use and dependence. Clinical practice guideline. Rockville, MD: U.S. Public Health Service, 2000. http://www.ahcpr.gov/path/tobacco.htm. Accessed Dec. 13, 2004.
- Amantadine • Symmetrel
- Bupropion • Wellbutrin SR, Zyban
- Clonidine • Catapres
- Nicotine nasal spray • Nicotrol NS
- Nicotine polacrilex • Nicorette
- Nicotine replacement patch • Nicoderm CQ, Nicotrol, others
- Nicotine vapor inhaler • Nicotrol Inhaler
- Nortriptyline •Aventyl, Pamelor
Dr. Anthenelli receives grant/research support from Sanofi-Aventis and Ortho-McNeil Pharmaceuticals and is a consultant and speaker for Sanofi-Aventis.
Acknowledgments
The author would like to thank Reene Cantwell for technical assistance in preparing this manuscript. This work was supported by grants R01 AA13307 and R01 AA13957 from the National Institute on Alcohol Abuse and Alcoholism and by the Department of Veterans Affairs.
1. Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA 2000;284(20):2606-10.
2. George TP, Vessicchio JC, Termine A. Nicotine and tobacco use in schizophrenia. In: Meyer JM, Nasrallah HR (eds). Medical illness and schizophrenia. Washington, DC: American Psychiatric Publishing, 2003:81-98.
3. Leonard S, Adler LE, Benhammou K, et al. Smoking and mental illness. Pharmacol Biochem Behav 2001;70(4):561-70.
4. Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry 2000;177:212-17.
5. American Psychiatric Association. Practice guideline for the treatment of patients with nicotine dependence. Am J Psychiatry 1996;53[153(suppl)]:1-31.
6. Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling? Am J Psychiatry 2003;160(12):2228-30.
7. Thorndike AN, Stafford RS, Rigotti NA. US physicians’ treatment of smoking in outpatients with psychiatric diagnoses. Nicotine Tob Res 2001;3(1):85-91.
8. Lin SW, Anthenelli RM. Genetic factors in the risk for substance use disorders. In: Lowinson J, Ruiz P, Millman RB, Langrod JC (eds). Substance abuse: a comprehensive textbook (4th ed). Philadelphia: Lippincott Williams and Wilkins, 2004.
9. Tyndale RF, Sellers EM. Genetic variation in CYP2A6-mediated nicotine metabolism alters smoking behavior. Ther Drug Monit 2002;24(1):163-71.
10. Newhouse P, Singh A, Potter A. Nicotine and nicotinic receptor involvement in neuropsychiatric disorders. Curr Top Med Chem 2004;4(3):267-82.
11. McEvoy JP, Allen TB. The importance of nicotinic acetylcholine receptors in schizophrenia, bipolar disorder and Tourette’s syndrome. Curr Drug Target CNS Neurol Disord 2002;1(4):433-42.
12. Fiore MC, Bailey WC, Cohen SJ, et al. Treating tobacco use and dependence. Clinical practice guideline. U.S. Public Health Service. Rockville, MD: Department of Health and Human Services, 2000. Available at http:www.ahcpr.gov/path/tobacco.htm.
13. Covey LS, Glassman AH, Stetner F. Cigarette smoking and major depression. J Addict Disord 1998;17(1):35-46.
14. Berlin I, Anthenelli RM. Monoamine oxidases and tobacco smoking. Intl J Neuropsychopharmacol 2001;4(1):33-42.
15. Killen JD, Fortmann SP, Schatzberg A, et al. Onset of major depression during treatment for nicotine dependence. Addict Behav 2003;28(3):461-70.
16. Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behav Genet 1995;25:95-101.
17. Addington J, el Guebaly N, Campbell W, et al. Smoking cessation treatment for patients with schizophrenia. Am J Psychiatry 1998;155(7):974-6.
18. George TP, Ziedonis DM, Feingold A, et al. Nicotine transdermal patch and atypical antipsychotic medications for smoking cessation in schizophrenia. Am J Psychiatry 2000;157(11):1835-42.
19. Lemon SC, Friedmann PD, Stein MD. The impact of smoking cessation on drug abuse treatment outcome. Addict Behav 2003;28(7):1323-31.
20. Prior TI, Baker GB. Interactions between the cytochrome P450 system and the second-generation antipsychotics. J Psychiatry Neurosci 2003;28(2):99-112.
21. Rogotti NA. Clinical practice: treatment of tobacco use and dependence. N Engl J Med 2002;346(7):506-12.
22. Ziedonis D, Williams JM, Smelson D. Serious mental illness and tobacco addiction: a model program to address this common but neglected issue. Am J Med Sci 2003;326(4):223-30.
Three myths about cigarette smoking may explain why psychiatrists rarely intervene in their patients’ tobacco dependence:
- Cigarette smoking is an incurable habit in psychiatric patients and thus not worth the effort of intervening.
- Cigarette smoking is an acceptable form of self-medication in persons with psychiatric illness.
- Quitting smoking will worsen psychiatric symptoms.
Smoking by psychiatric patients is treatable, however, and evidence proves that many can quit.1 This article rebuts the “why-bother?” myths and provides practical tips on how to more effectively help psychiatric patients stop smoking.
DEBUNKING THREE MYTHS
Mentally ill women and men consume nearly one-half (44%) of the cigarettes smoked in the United States (Table 1)1-3 and thus are at high risk for tobacco-related premature death, cancer, cardiovascular disease, and respiratory disorders. Although recognized as a leading cause of death, cigarette smoking by psychiatric patients frequently goes unaddressed, contributing to excess mortality in this population.4
Table 1
Cigarette smoking: An epidemic among psychiatric patients
|
| Source: References 1-3 |
- psychiatrists seldom (6,7
- when counseling did occur, nicotine replacement therapy was not prescribed.6
Tobacco dependence is a syndrome with strong genetic and biologic roots. Family, twin, and adoption studies show consistently that tobacco dependence is genetically mediated.8 Genetic polymorphisms are being identified that may modify an individual’s risk for developing nicotine dependence—such as the gene encoding the cytochrome P-450 2A6 isoenzyme (CYP 2A6) that metabolizes nicotine to cotinine.9 Disturbed nicotinic receptor functioning has been shown in persons with schizophrenia, mood disorders, anxiety disorders, and attention-deficit/hyperactivity disorders.3,10,11
Tobacco dependence is a chronic, relapsing condition that usually requires repeated intervention to motivate patients to try to quit and to help those who are willing to quit to succeed. Effective smoking cessation aids include:
- behavioral therapy (brief physician advice, problem-solving skills/skills training)
- pharmacologic therapy (nicotine replacement, sustained-release bupropion).12
Is smoking ‘self-medication’? Compelling evidence indicates that cholinergic mechanisms and nicotinic receptors (nAChRs) are involved in the pathophysiology of schizophrenia and other neuropsychiatric disorders.3,10 Nicotine administration appears to improve sensory-processing and cognitive deficits observed in schizophrenia.2,3 Moreover, the association between depression and smoking13 —and tobacco smoke’s monoamine oxidase-inhibiting and other psychoactive properties14 —have led some to posit that cigarette smoking may have antidepressant actions.10
For all these reasons, some authors have speculated that tobacco use may be a form of self-medication among the psychiatrically ill.3 The problem with this hypothesis, however, is that tobacco smoke is—at best—an untested and potentially lethal cognitive enhancer, antidepressant, or anxiolytic. Animal and human studies may find therapeutic effects of acute nicotine administration, but the cognitive effects of chronic tobacco smoking are not known.
Table 2
5 ‘A’s of brief clinical intervention for tobacco dependence
|
| Source: References 5 and 12 |
Adverse effects from quitting? Smokers with a history of major depressive disorder have been shown to be at risk to:
- develop another depressive episode after they quit smoking15
- experience more severe withdrawal symptoms during abstinence, compared with smokers with no history of depression.13,16
Scant data support the myth that smoking cessation worsens psychiatric symptoms. For example, in a review on tobacco dependence and schizophrenia, George et al2 concluded that the effects of smoking cessation on schizophrenia symptoms are not clear. Two smoking cessation trials in schizophrenic patients treated with nicotine patches found no significant changes in postcessation psychotic symptoms.17,18
Concerns that substance-abusing patients should not attempt to quit smoking during alcohol and other drug dependence treatment are also unsubstantiated. Rather than exacerbating drug addiction, smoking cessation has been found to improve addicts’ abstinence rates.19
USING AVAILABLE THERAPIES
Evidence is insufficient so far to show whether psychiatrically ill smokers would benefit more from specially tailored cessation treatments than from standard treatments, according to the 2000 U.S. Public Health Service clinical practice guide.12 Thus, while researchers try to resolve this issue, psychiatrists are left to use medications found to be effective in smokers overall.
Clinical vignette. Mr. J, age 45, has paranoid-type schizophrenia and has been smoking at least two packs of cigarettes daily for 25 years. He complains of a productive cough and expresses interest in quitting smoking when his psychiatrist raises this topic.
His persecutory delusions are well-controlled on olanzapine, 10 mg/d. He is adhering with his medications and participating in weekly group counseling that provides supportive therapy for patients with serious mental illness.
In this schizophrenic smoker who is willing to try to quit, the psychiatrist performed the first three of “5 ‘A’s” (Table 2) of brief clinical intervention for tobacco dependence.5,12 The next steps are to assist the patient’s effort to quit and arrange follow-up.
When to quit. The best time for a smoker with psychiatric illness to try to quit is when he or she:
- is psychiatrically stable
- is not in crisis
- has no recent or planned psychiatric drug changes.
Smoking cessation may increase blood levels of these psychotropics
| Antipsychotics | Antidepressants | Mood stabilizers | Anxiolytics |
|---|---|---|---|
| Haloperidol | Clomipramine | Carbamazepine | Desmethyldiazepam |
| Chlorpromazine | Desipramine | Oxazepam | |
| Fluphenazine | Doxepin | ||
| Olanzapine | Imipramine | ||
| Clozapine | Nortriptyline | ||
| Source: References 2, 5, and 20 | |||
Olanzapine’s clearance is approximately 40% higher in smokers than in nonsmokers. The psychiatrist discussed this with Mr. J and:
- asked him to call if side effects develop during the quit attempt
- scheduled more-frequent appointments to monitor side effects.
Mr. J’s schizophrenia is stable on maintenance therapy with an atypical antipsychotic. Schizophrenic smokers taking atypicals may be more able to quit smoking with NRT or sustained-release bupropion, compared with those taking conventional antipsychotics.2
The psychiatrist also determined that Mr. J had tried to quit smoking three times. Two of these attempts were done “cold turkey,” without pharmacotherapy, and one involved using nicotine gum. Mr. J said that although the gum “worked well at first,” he stopped using it because it was expensive and made his mouth sore. This information helped the psychiatrist choose medication for this quit attempt.
Most smoking cessation guidelines rely on a stepped-care approach, progressing from minimal to more-intensive interventions as needed.5 Mr. J’s psychiatrist devised an intensive treatment plan because:
- Mr. J has tried to quit before
- schizophrenic patients generally have more difficulty quitting and are more nicotine-dependent than other smokers.
Table 4
Nicotine replacement and other options for smoking cessation
| Drug | Daily dosage | Treatment duration* | Common side effects |
|---|---|---|---|
| Nicotine replacement therapy† | |||
| Transdermal | Skin irritation, insomnia | ||
| 24-hr patch | Starting dose is 21 mg/d; also in 7- and 14-mg patches for tapering dosage | 8 wk | |
| 16-hr patch | 15 mg | 8 wk | |
| Polacrilex (gum) 2- or 4-mg piece | 1 piece/hr ( | 8 to 12 wk | Mouth irritation, sore jaw, dyspepsia, hiccups |
| Vapor inhaler | 6 to 16 cartridges/day (delivers 4/mg/cartridge) | 3 to 6 mo | Mouth and throat irritation, cough |
| Nasal spray | 1 to 2 doses/hr; dose = 1 mg (0.5 mg per nostril); maximum dosage 40 mg/d | 3 to 6 mo | Nasal irritation, sneezing, cough, tearing eyes |
| Lozenge | 2- or 4-mg dose; see dosage formula, titration schedule in over-the-counter package | 12 wk | Hiccups, nausea, heartburn |
| Non-nicotine replacement therapy | |||
| Sustained-release bupropion† | 150 mg/d for 3 days, then 150 mg bid; start 1 week before quit date | 7 to 12 wk; up to 6 mo. to maintain abstinence | Insomnia, dry mouth, agitation |
| Nortriptyline | 75 to 100 mg/d; start 10 to 28 days before quit date at 25 mg/d and increase as tolerated | 12 wk | Dry mouth, sedation, dizziness |
| Clonidine | 0.1 to 0.3 mg bid | 3 to 10 wk | Dry mouth, sedation, dizziness |
| * Treatment duration varies and may be longer in patients with psychiatric disorders. | |||
| † FDA-approved as a smoking cessation aid and recommended as a first-line drug by Public Health Service clinical guidelines. | |||
| Source: Adapted from reference 21. | |||
On the morning of his TQD, Mr. J is to apply the first 21-mg transdermal nicotine patch. He is told not to smoke that day and to apply a new patch daily. The psychiatrist also tells him he will most likely remain on that dosage for 4 weeks. Then the patch strength will be reduced in 7-mg aliquots every 2 to 4 weeks, depending on his progress. The psychiatrist also provides him with educational materials on how to quit successfully.
Follow-up. Recognizing that most relapses occur in the first few days of quitting, the psychiatrist sets Mr. J’s first follow-up appointment for the day after his TQD to assess:
- whether he has smoked and number of cigarettes smoked per day
- presence and severity of withdrawal symptoms
- onset of psychiatric symptoms
- treatment adherence
- how he is handling high-risk situations and urges to smoke
- medication side effects.6
- American Psychiatric Association. Practice guideline for the treatment of patients with nicotine dependence. Am J Psychiatry 1996; 53(153[suppl]): 1-31.
- Fiore MC, Bailey WC, Cohen SJ, et.al. Treating tobacco use and dependence. Clinical practice guideline. Rockville, MD: U.S. Public Health Service, 2000. http://www.ahcpr.gov/path/tobacco.htm. Accessed Dec. 13, 2004.
- Amantadine • Symmetrel
- Bupropion • Wellbutrin SR, Zyban
- Clonidine • Catapres
- Nicotine nasal spray • Nicotrol NS
- Nicotine polacrilex • Nicorette
- Nicotine replacement patch • Nicoderm CQ, Nicotrol, others
- Nicotine vapor inhaler • Nicotrol Inhaler
- Nortriptyline •Aventyl, Pamelor
Dr. Anthenelli receives grant/research support from Sanofi-Aventis and Ortho-McNeil Pharmaceuticals and is a consultant and speaker for Sanofi-Aventis.
Acknowledgments
The author would like to thank Reene Cantwell for technical assistance in preparing this manuscript. This work was supported by grants R01 AA13307 and R01 AA13957 from the National Institute on Alcohol Abuse and Alcoholism and by the Department of Veterans Affairs.
Three myths about cigarette smoking may explain why psychiatrists rarely intervene in their patients’ tobacco dependence:
- Cigarette smoking is an incurable habit in psychiatric patients and thus not worth the effort of intervening.
- Cigarette smoking is an acceptable form of self-medication in persons with psychiatric illness.
- Quitting smoking will worsen psychiatric symptoms.
Smoking by psychiatric patients is treatable, however, and evidence proves that many can quit.1 This article rebuts the “why-bother?” myths and provides practical tips on how to more effectively help psychiatric patients stop smoking.
DEBUNKING THREE MYTHS
Mentally ill women and men consume nearly one-half (44%) of the cigarettes smoked in the United States (Table 1)1-3 and thus are at high risk for tobacco-related premature death, cancer, cardiovascular disease, and respiratory disorders. Although recognized as a leading cause of death, cigarette smoking by psychiatric patients frequently goes unaddressed, contributing to excess mortality in this population.4
Table 1
Cigarette smoking: An epidemic among psychiatric patients
|
| Source: References 1-3 |
- psychiatrists seldom (6,7
- when counseling did occur, nicotine replacement therapy was not prescribed.6
Tobacco dependence is a syndrome with strong genetic and biologic roots. Family, twin, and adoption studies show consistently that tobacco dependence is genetically mediated.8 Genetic polymorphisms are being identified that may modify an individual’s risk for developing nicotine dependence—such as the gene encoding the cytochrome P-450 2A6 isoenzyme (CYP 2A6) that metabolizes nicotine to cotinine.9 Disturbed nicotinic receptor functioning has been shown in persons with schizophrenia, mood disorders, anxiety disorders, and attention-deficit/hyperactivity disorders.3,10,11
Tobacco dependence is a chronic, relapsing condition that usually requires repeated intervention to motivate patients to try to quit and to help those who are willing to quit to succeed. Effective smoking cessation aids include:
- behavioral therapy (brief physician advice, problem-solving skills/skills training)
- pharmacologic therapy (nicotine replacement, sustained-release bupropion).12
Is smoking ‘self-medication’? Compelling evidence indicates that cholinergic mechanisms and nicotinic receptors (nAChRs) are involved in the pathophysiology of schizophrenia and other neuropsychiatric disorders.3,10 Nicotine administration appears to improve sensory-processing and cognitive deficits observed in schizophrenia.2,3 Moreover, the association between depression and smoking13 —and tobacco smoke’s monoamine oxidase-inhibiting and other psychoactive properties14 —have led some to posit that cigarette smoking may have antidepressant actions.10
For all these reasons, some authors have speculated that tobacco use may be a form of self-medication among the psychiatrically ill.3 The problem with this hypothesis, however, is that tobacco smoke is—at best—an untested and potentially lethal cognitive enhancer, antidepressant, or anxiolytic. Animal and human studies may find therapeutic effects of acute nicotine administration, but the cognitive effects of chronic tobacco smoking are not known.
Table 2
5 ‘A’s of brief clinical intervention for tobacco dependence
|
| Source: References 5 and 12 |
Adverse effects from quitting? Smokers with a history of major depressive disorder have been shown to be at risk to:
- develop another depressive episode after they quit smoking15
- experience more severe withdrawal symptoms during abstinence, compared with smokers with no history of depression.13,16
Scant data support the myth that smoking cessation worsens psychiatric symptoms. For example, in a review on tobacco dependence and schizophrenia, George et al2 concluded that the effects of smoking cessation on schizophrenia symptoms are not clear. Two smoking cessation trials in schizophrenic patients treated with nicotine patches found no significant changes in postcessation psychotic symptoms.17,18
Concerns that substance-abusing patients should not attempt to quit smoking during alcohol and other drug dependence treatment are also unsubstantiated. Rather than exacerbating drug addiction, smoking cessation has been found to improve addicts’ abstinence rates.19
USING AVAILABLE THERAPIES
Evidence is insufficient so far to show whether psychiatrically ill smokers would benefit more from specially tailored cessation treatments than from standard treatments, according to the 2000 U.S. Public Health Service clinical practice guide.12 Thus, while researchers try to resolve this issue, psychiatrists are left to use medications found to be effective in smokers overall.
Clinical vignette. Mr. J, age 45, has paranoid-type schizophrenia and has been smoking at least two packs of cigarettes daily for 25 years. He complains of a productive cough and expresses interest in quitting smoking when his psychiatrist raises this topic.
His persecutory delusions are well-controlled on olanzapine, 10 mg/d. He is adhering with his medications and participating in weekly group counseling that provides supportive therapy for patients with serious mental illness.
In this schizophrenic smoker who is willing to try to quit, the psychiatrist performed the first three of “5 ‘A’s” (Table 2) of brief clinical intervention for tobacco dependence.5,12 The next steps are to assist the patient’s effort to quit and arrange follow-up.
When to quit. The best time for a smoker with psychiatric illness to try to quit is when he or she:
- is psychiatrically stable
- is not in crisis
- has no recent or planned psychiatric drug changes.
Smoking cessation may increase blood levels of these psychotropics
| Antipsychotics | Antidepressants | Mood stabilizers | Anxiolytics |
|---|---|---|---|
| Haloperidol | Clomipramine | Carbamazepine | Desmethyldiazepam |
| Chlorpromazine | Desipramine | Oxazepam | |
| Fluphenazine | Doxepin | ||
| Olanzapine | Imipramine | ||
| Clozapine | Nortriptyline | ||
| Source: References 2, 5, and 20 | |||
Olanzapine’s clearance is approximately 40% higher in smokers than in nonsmokers. The psychiatrist discussed this with Mr. J and:
- asked him to call if side effects develop during the quit attempt
- scheduled more-frequent appointments to monitor side effects.
Mr. J’s schizophrenia is stable on maintenance therapy with an atypical antipsychotic. Schizophrenic smokers taking atypicals may be more able to quit smoking with NRT or sustained-release bupropion, compared with those taking conventional antipsychotics.2
The psychiatrist also determined that Mr. J had tried to quit smoking three times. Two of these attempts were done “cold turkey,” without pharmacotherapy, and one involved using nicotine gum. Mr. J said that although the gum “worked well at first,” he stopped using it because it was expensive and made his mouth sore. This information helped the psychiatrist choose medication for this quit attempt.
Most smoking cessation guidelines rely on a stepped-care approach, progressing from minimal to more-intensive interventions as needed.5 Mr. J’s psychiatrist devised an intensive treatment plan because:
- Mr. J has tried to quit before
- schizophrenic patients generally have more difficulty quitting and are more nicotine-dependent than other smokers.
Table 4
Nicotine replacement and other options for smoking cessation
| Drug | Daily dosage | Treatment duration* | Common side effects |
|---|---|---|---|
| Nicotine replacement therapy† | |||
| Transdermal | Skin irritation, insomnia | ||
| 24-hr patch | Starting dose is 21 mg/d; also in 7- and 14-mg patches for tapering dosage | 8 wk | |
| 16-hr patch | 15 mg | 8 wk | |
| Polacrilex (gum) 2- or 4-mg piece | 1 piece/hr ( | 8 to 12 wk | Mouth irritation, sore jaw, dyspepsia, hiccups |
| Vapor inhaler | 6 to 16 cartridges/day (delivers 4/mg/cartridge) | 3 to 6 mo | Mouth and throat irritation, cough |
| Nasal spray | 1 to 2 doses/hr; dose = 1 mg (0.5 mg per nostril); maximum dosage 40 mg/d | 3 to 6 mo | Nasal irritation, sneezing, cough, tearing eyes |
| Lozenge | 2- or 4-mg dose; see dosage formula, titration schedule in over-the-counter package | 12 wk | Hiccups, nausea, heartburn |
| Non-nicotine replacement therapy | |||
| Sustained-release bupropion† | 150 mg/d for 3 days, then 150 mg bid; start 1 week before quit date | 7 to 12 wk; up to 6 mo. to maintain abstinence | Insomnia, dry mouth, agitation |
| Nortriptyline | 75 to 100 mg/d; start 10 to 28 days before quit date at 25 mg/d and increase as tolerated | 12 wk | Dry mouth, sedation, dizziness |
| Clonidine | 0.1 to 0.3 mg bid | 3 to 10 wk | Dry mouth, sedation, dizziness |
| * Treatment duration varies and may be longer in patients with psychiatric disorders. | |||
| † FDA-approved as a smoking cessation aid and recommended as a first-line drug by Public Health Service clinical guidelines. | |||
| Source: Adapted from reference 21. | |||
On the morning of his TQD, Mr. J is to apply the first 21-mg transdermal nicotine patch. He is told not to smoke that day and to apply a new patch daily. The psychiatrist also tells him he will most likely remain on that dosage for 4 weeks. Then the patch strength will be reduced in 7-mg aliquots every 2 to 4 weeks, depending on his progress. The psychiatrist also provides him with educational materials on how to quit successfully.
Follow-up. Recognizing that most relapses occur in the first few days of quitting, the psychiatrist sets Mr. J’s first follow-up appointment for the day after his TQD to assess:
- whether he has smoked and number of cigarettes smoked per day
- presence and severity of withdrawal symptoms
- onset of psychiatric symptoms
- treatment adherence
- how he is handling high-risk situations and urges to smoke
- medication side effects.6
- American Psychiatric Association. Practice guideline for the treatment of patients with nicotine dependence. Am J Psychiatry 1996; 53(153[suppl]): 1-31.
- Fiore MC, Bailey WC, Cohen SJ, et.al. Treating tobacco use and dependence. Clinical practice guideline. Rockville, MD: U.S. Public Health Service, 2000. http://www.ahcpr.gov/path/tobacco.htm. Accessed Dec. 13, 2004.
- Amantadine • Symmetrel
- Bupropion • Wellbutrin SR, Zyban
- Clonidine • Catapres
- Nicotine nasal spray • Nicotrol NS
- Nicotine polacrilex • Nicorette
- Nicotine replacement patch • Nicoderm CQ, Nicotrol, others
- Nicotine vapor inhaler • Nicotrol Inhaler
- Nortriptyline •Aventyl, Pamelor
Dr. Anthenelli receives grant/research support from Sanofi-Aventis and Ortho-McNeil Pharmaceuticals and is a consultant and speaker for Sanofi-Aventis.
Acknowledgments
The author would like to thank Reene Cantwell for technical assistance in preparing this manuscript. This work was supported by grants R01 AA13307 and R01 AA13957 from the National Institute on Alcohol Abuse and Alcoholism and by the Department of Veterans Affairs.
1. Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA 2000;284(20):2606-10.
2. George TP, Vessicchio JC, Termine A. Nicotine and tobacco use in schizophrenia. In: Meyer JM, Nasrallah HR (eds). Medical illness and schizophrenia. Washington, DC: American Psychiatric Publishing, 2003:81-98.
3. Leonard S, Adler LE, Benhammou K, et al. Smoking and mental illness. Pharmacol Biochem Behav 2001;70(4):561-70.
4. Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry 2000;177:212-17.
5. American Psychiatric Association. Practice guideline for the treatment of patients with nicotine dependence. Am J Psychiatry 1996;53[153(suppl)]:1-31.
6. Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling? Am J Psychiatry 2003;160(12):2228-30.
7. Thorndike AN, Stafford RS, Rigotti NA. US physicians’ treatment of smoking in outpatients with psychiatric diagnoses. Nicotine Tob Res 2001;3(1):85-91.
8. Lin SW, Anthenelli RM. Genetic factors in the risk for substance use disorders. In: Lowinson J, Ruiz P, Millman RB, Langrod JC (eds). Substance abuse: a comprehensive textbook (4th ed). Philadelphia: Lippincott Williams and Wilkins, 2004.
9. Tyndale RF, Sellers EM. Genetic variation in CYP2A6-mediated nicotine metabolism alters smoking behavior. Ther Drug Monit 2002;24(1):163-71.
10. Newhouse P, Singh A, Potter A. Nicotine and nicotinic receptor involvement in neuropsychiatric disorders. Curr Top Med Chem 2004;4(3):267-82.
11. McEvoy JP, Allen TB. The importance of nicotinic acetylcholine receptors in schizophrenia, bipolar disorder and Tourette’s syndrome. Curr Drug Target CNS Neurol Disord 2002;1(4):433-42.
12. Fiore MC, Bailey WC, Cohen SJ, et al. Treating tobacco use and dependence. Clinical practice guideline. U.S. Public Health Service. Rockville, MD: Department of Health and Human Services, 2000. Available at http:www.ahcpr.gov/path/tobacco.htm.
13. Covey LS, Glassman AH, Stetner F. Cigarette smoking and major depression. J Addict Disord 1998;17(1):35-46.
14. Berlin I, Anthenelli RM. Monoamine oxidases and tobacco smoking. Intl J Neuropsychopharmacol 2001;4(1):33-42.
15. Killen JD, Fortmann SP, Schatzberg A, et al. Onset of major depression during treatment for nicotine dependence. Addict Behav 2003;28(3):461-70.
16. Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behav Genet 1995;25:95-101.
17. Addington J, el Guebaly N, Campbell W, et al. Smoking cessation treatment for patients with schizophrenia. Am J Psychiatry 1998;155(7):974-6.
18. George TP, Ziedonis DM, Feingold A, et al. Nicotine transdermal patch and atypical antipsychotic medications for smoking cessation in schizophrenia. Am J Psychiatry 2000;157(11):1835-42.
19. Lemon SC, Friedmann PD, Stein MD. The impact of smoking cessation on drug abuse treatment outcome. Addict Behav 2003;28(7):1323-31.
20. Prior TI, Baker GB. Interactions between the cytochrome P450 system and the second-generation antipsychotics. J Psychiatry Neurosci 2003;28(2):99-112.
21. Rogotti NA. Clinical practice: treatment of tobacco use and dependence. N Engl J Med 2002;346(7):506-12.
22. Ziedonis D, Williams JM, Smelson D. Serious mental illness and tobacco addiction: a model program to address this common but neglected issue. Am J Med Sci 2003;326(4):223-30.
1. Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA 2000;284(20):2606-10.
2. George TP, Vessicchio JC, Termine A. Nicotine and tobacco use in schizophrenia. In: Meyer JM, Nasrallah HR (eds). Medical illness and schizophrenia. Washington, DC: American Psychiatric Publishing, 2003:81-98.
3. Leonard S, Adler LE, Benhammou K, et al. Smoking and mental illness. Pharmacol Biochem Behav 2001;70(4):561-70.
4. Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry 2000;177:212-17.
5. American Psychiatric Association. Practice guideline for the treatment of patients with nicotine dependence. Am J Psychiatry 1996;53[153(suppl)]:1-31.
6. Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling? Am J Psychiatry 2003;160(12):2228-30.
7. Thorndike AN, Stafford RS, Rigotti NA. US physicians’ treatment of smoking in outpatients with psychiatric diagnoses. Nicotine Tob Res 2001;3(1):85-91.
8. Lin SW, Anthenelli RM. Genetic factors in the risk for substance use disorders. In: Lowinson J, Ruiz P, Millman RB, Langrod JC (eds). Substance abuse: a comprehensive textbook (4th ed). Philadelphia: Lippincott Williams and Wilkins, 2004.
9. Tyndale RF, Sellers EM. Genetic variation in CYP2A6-mediated nicotine metabolism alters smoking behavior. Ther Drug Monit 2002;24(1):163-71.
10. Newhouse P, Singh A, Potter A. Nicotine and nicotinic receptor involvement in neuropsychiatric disorders. Curr Top Med Chem 2004;4(3):267-82.
11. McEvoy JP, Allen TB. The importance of nicotinic acetylcholine receptors in schizophrenia, bipolar disorder and Tourette’s syndrome. Curr Drug Target CNS Neurol Disord 2002;1(4):433-42.
12. Fiore MC, Bailey WC, Cohen SJ, et al. Treating tobacco use and dependence. Clinical practice guideline. U.S. Public Health Service. Rockville, MD: Department of Health and Human Services, 2000. Available at http:www.ahcpr.gov/path/tobacco.htm.
13. Covey LS, Glassman AH, Stetner F. Cigarette smoking and major depression. J Addict Disord 1998;17(1):35-46.
14. Berlin I, Anthenelli RM. Monoamine oxidases and tobacco smoking. Intl J Neuropsychopharmacol 2001;4(1):33-42.
15. Killen JD, Fortmann SP, Schatzberg A, et al. Onset of major depression during treatment for nicotine dependence. Addict Behav 2003;28(3):461-70.
16. Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behav Genet 1995;25:95-101.
17. Addington J, el Guebaly N, Campbell W, et al. Smoking cessation treatment for patients with schizophrenia. Am J Psychiatry 1998;155(7):974-6.
18. George TP, Ziedonis DM, Feingold A, et al. Nicotine transdermal patch and atypical antipsychotic medications for smoking cessation in schizophrenia. Am J Psychiatry 2000;157(11):1835-42.
19. Lemon SC, Friedmann PD, Stein MD. The impact of smoking cessation on drug abuse treatment outcome. Addict Behav 2003;28(7):1323-31.
20. Prior TI, Baker GB. Interactions between the cytochrome P450 system and the second-generation antipsychotics. J Psychiatry Neurosci 2003;28(2):99-112.
21. Rogotti NA. Clinical practice: treatment of tobacco use and dependence. N Engl J Med 2002;346(7):506-12.
22. Ziedonis D, Williams JM, Smelson D. Serious mental illness and tobacco addiction: a model program to address this common but neglected issue. Am J Med Sci 2003;326(4):223-30.
Getting to the bottom of problem drinking: The case for routine screening
Do you know which of your patients have alcohol problems? Though alcohol use disorders may be difficult to detect, self-report and biochemical measures followed by a thorough face-to-face assessment improve diagnostic accuracy. New tools—such as the serum carbohydrate-deficient transferrin (CDT) test—are changing how psychiatrists screen for alcohol problems, provide motivational feedback, and monitor patients for relapse.
FOUR REASONS TO SCREEN
Screening for excessive alcohol consumption is important in psychiatric practice because:
- Alcohol use disorders coexist with many psychiatric problems, most notably affective and anxiety disorders and—not surprisingly—other substance abuse disorders (Table 1).1,2
- Patients with psychiatric comorbidity who abuse alcohol have poorer prognoses, are less adherent to treatment, and are more likely to drop out of treatment than are psychiatric patients who do not have alcohol problems.3
- Alcohol interacts with many psychotropics, and chronic heavy drinking can cause pharmacokinetic changes that affect a patient’s response to medications.
- Alcohol-dependent patients are more likely than nondrinkers to become dependent on anxiolytics and sedative-hypnotics.
Table 1
Overlap of alcohol problems with common psychiatric disorders
| Disorder | Risk of alcohol use disorder (odds ratio) | Source of data (population survey) |
|---|---|---|
| Drug use disorder | 25.1 | NLAES |
| Mania | 5.6 | NCS |
| Major depression | 3.7 | NLAES |
| Obsessive-compulsive disorder | 3.4 | ECA |
| Generalized anxiety disorder | 2.7 | NCS |
| Phobia | 2.3 | NCS |
| Posttraumatic stress disorder | 2.2 | NCS |
| Panic disorder | 1.4 | NCS |
| NLAES: National Longitudinal Alcohol Epidemiological Survey | ||
| NCS: National Comorbidity Survey | ||
| ECA: Epidemiologic Catchment Area | ||
Because alcohol problems are common in psychiatric patients, routine screening for alcohol abuse and dependence at the onset of any treatment can be very useful. Thereafter, screening can be done periodically—perhaps annually or more often if the patient’s functioning declines.
CHOOSING A SELF-REPORT MEASURE
Many self-report alcohol screening scales are available,4 the most popular being the CAGE5 and the Michigan Alcoholism Screening Test (MAST).6 Though both instruments can help identify alcohol problems, each has shortcomings:
- The CAGE performs less reliably in women and adolescents than in men, and its validity depends on the patient’s sensitivity to the emotional impacts of alcohol dependence.
- The MAST is long (25 items), concentrates on late-stage alcoholism symptoms, and uses differential weighting—not validated in subsequent studies—of particular items in deriving the score.
Neither addresses drinking behavior or when symptoms occurred and thus may misclassify recovered alcoholics or former problem drinkers.
AUDIT. A more reliable choice is the Alcohol Use Disorders Identification Test (AUDIT).7 It was designed by the World Health Organization (WHO) to be valid across gender and culture and to identify even early stage problem drinking. The AUDIT’s 10 items deal with drinking behavior, dependence on alcohol, and adverse consequences of drinking during the past year (Box). The survey takes less than 5 minutes; can be administered orally, in writing, or online; and it retains its validity when given as part of a comprehensive health risk appraisal.8
The WHO offers an excellent manual detailing how to administer and interpret the AUDIT (see Related resources). A patient’s score is computed by summing the values associated with his or her responses to each item. A score of 8 or greater indicates excessive alcohol consumption, although some researchers have argued that for women a more accurate threshold might be 6 or 7 points.
Standardized for adults, the AUDIT also appears to accurately gauge drinking behavior in adolescents9 and in psychiatric patients, although only three studies have explored its use in the latter population.10-12 Abbreviated AUDIT versions have been found to be psychometrically sound8 and may be useful in an emergency room or busy primary care clinic. In comparisons with other screening tools, the AUDIT almost always has been found to be more valid.9,13
USING BIOCHEMICAL MEASURES
Self-report screens for alcohol problems, especially the AUDIT, are highly sensitive and specific, though their accuracy depends on the patient's memory, understanding of the questions, and candor. In chronic heavy drinkers, biochemical measures (Table 2) can augment self-reports.14
Self-report and biochemical screens have different strengths and weaknesses (Table 3). It is important to see them as complementary because each contributes to accurate screening.
CDT. Most biomarkers screen indirectly for alcohol problems by measuring damage to an end organ-typically the liver-caused by chronic excessive alcohol consumption. False positive results are common because of nonalcohol-related organ damage, medications, smoking, obesity, and other confounding factors. An exception appears to be the serum test for carbohydrate-deficient transferrin (CDT), a biomarker for heavy drinking approved in kit form 3 years ago by the Food and Drug Administration.
The value of measuring CDT levels is that few conditions other than excessive alcohol consumption elevate them. For unclear reasons,15 average daily consumption of >60 grams of alcohol (about five standard drinks) during the previous 2 weeks causes a higher percent of transferrin—a glycoenzyme that transports iron in the body—to lack its usual carbohydrate content.
Bio-Rad Laboratories (www.bio-rad.com) offers a reagent kit (%CDT Turbidimetric Immunoassay). It quantifies CDT as a percent of total serum transferrin, rather than total CDT, thus correcting for individual variations in transferrin levels. CDT values are obtained from a 100-microliter serum sample. The blood is clotted and the serum separated. The sample may be stored at 2 to 8 °C if the test is to be run within 1 week. Samples must be tested at a reference lab (Bio-Rad offers a list of labs). Results are available in a few days.
Patients who deny problem drinking may need convincing to submit to a blood draw. It may help to explain that alcohol use can exacerbate emotional problems and that the test can provide information on possible risky alcohol use.
GGT. Using a second biochemical marker may improve the sensitivity of CDT to detect heavy drinking.16-18
The most-researched choice for a second marker is gamma glutamyltransferase (GGT). Patients are considered to have tested positive for an alcohol problem if either CDT or GGT levels are elevated. Combining these tests may be especially useful in alcohol-dependent women, in whom the reliability of CDT testing alone has been questioned.
Recommendation. Start with a self-report screening measure. If the patient scores slightly below the threshold for an alcohol problem, follow up with the more costly CDT and GGT tests.
For example, biomarkers might be useful for follow-up in men with AUDIT screening scores of 6 or 7 or women with scores of 5 to 7. Biomarkers also are recommended when you suspect an alcohol problem for another reason or question whether the patient responded accurately to the self-report measure.
- How often do you have a drink containing alcohol?
- How many drinks containing alcohol do you have on a typical day when you are drinking?
- How often do you have 6 or more drinks on one occasion?
- How often during the last year have you found that you were not able to stop drinking once you had started?
- How often during the last year have you failed to do what was normally expected from you because of drinking?
- How often during the last year have you needed a first drink in the morning to get yourself going after a heavy drinking session?
- How often during the last year have you had a feeling of guilt or remorse after drinking?
- How often during the last year have you been unable to remember what happened the night before because you had been drinking?
- Have you or someone else been injured as a result of your drinking?
- Has a relative, friend, doctor, or other health worker been concerned about your drinking or suggested that you cut down?
The World Health Organization offers a manual on how to administer and interpret AUDIT (http://www.who.int/substance_abuse/pubs_alcohol.htm).
Source: World Health Organization
Table 2
Biochemical markers of heavy drinking
| Marker | Time needed for return to normal limits | Level of drinking characterized | Comments |
|---|---|---|---|
| Gamma glutamyltransferase (GGT) | 2 to 6 weeks of abstinence | ~70 drinks/wk for several weeks | Most common and reliable of the traditional markers of heavy drinking; many sources of false positives |
| Aspartate aminotransferase (AST) (formerly SGOT) | 7 days, but much variability in declines with abstinence | Unknown, but heavy | Present in many organs; many sources of false positives; moderate correlations with GGT |
| Alanine aminotransferase (ALT) (formerly SGPT) | Unknown | Unknown, but heavy | Many sources of false positives and less sensitive than AST; ratio of AST to ALT may be more accurate |
| Macrocytic volume (MCV) | Unknown; half-life ~40 days | Unknown, but regular and heavy | Poor sensitivity and specificity; even with abstinence, very slow return to normal limits and may increase at first; little, if any, gender effect |
| Carbohydrate-deficient transferrin (CDT) | 2 to 4 weeks of abstinence | >60 grams/day for approximately 2 weeks | Few sources of false positives; excellent indicator of relapse |
MONITORING PATIENTS IN TREATMENT
MET. Motivational enhancement therapy (MET) has gained popularity as a means of changing problematic drinking.19 Project MATCH—a multi-site trial on alcohol abuse treatment—studied MET and two other interventions. MET required fewer sessions but equaled the other interventions in reducing drinking days and aver-age amount of alcohol consumed.20
A key component of MET—and other brief interventions—is to provide patients with empathetic, nonjudgmental feedback.19-21 Responses to the first three AUDIT items can provide such feedback to patients with drinking problems. Amazingly, heavy drinkers and alcoholics often do not realize how much more they drink than other people. To help them develop this insight, show them their self-report responses in contrast with national normative data.
Biomarker results can be used similarly, in this case comparing the patient’s score with the test’s reference range values. Kristenson et al22 showed that giving men with elevated scores recurrent biomarker information and advice significantly reduced morbidity and mortality and improved their work performance.
Using visual aids can deepen patients’ under-standing of motivational feedback. For example, displaying sequential test results on a timeline can reinforce motivation by showing how their drinking behavior has improved with continuing treatment and sustained effort.
Table 3
Self-report and biochemical measures of drinking: Pros and cons
| Measure | Strengths | Weaknesses |
|---|---|---|
| Self-report | Noninvasive Inexpensive High validity Flexible window of assessment Immediate results | Easily feigned Accuracy depends on patient’s verbal skills and memory |
| Biochemical | Objective Results may be more compelling to patients than self-reports May reflect organ damage Useful in tracking treatment progress | Window of assessment is limited to recent past Results often not immediately available May be more costly than self-report measures |
DETECTING RELAPSE
Although treatment for alcohol problems is often successful,23 relapse to some level of drinking is not uncommon, especially during the first 3 or 4 months after patients complete treatment.20 Recognizing relapse quickly can help you:
- decrease risk of harm from resumed alcohol use
- reduce the potential for drinking to again become habitual
- identify circumstances and cues that may have triggered the drinking episode, for use in tailoring future interventions.
In clinical practice, relapse is most often revealed via comments from the family, direct observation by the clinician, or voluntary acknowledgment by the patient. Interestingly, CDT has demonstrated a relapse “heralding effect,”24 meaning that it tends to rise well before a patient will admit he or she resumed drinking.24-26 Although other markers may also rise following relapse, their elevation tends to be delayed and less dramatic.
The sensitivity and specificity of CDT alone and with GGT in identifying relapse have been evaluated. Across male-only studies, CDT’s median sensitivity (percent of relapsed patients with elevated scores) was 0.73, with a specificity (percent of those not relapsed who had low scores) of 0.91. In the two female-only studies, median sensitivity and specificity for CDT were 0.32 and 0.86, respectively. For women, using CDT and GGT in combination substantially raised median sensitivity to 0.62, although specificity fell slightly to 0.80.27
Recommendation. When using biomarkers to identify relapse, examine the temporal pattern of test results to date. Assume that an increase of 30% or more above the lowest observed lab value indicates a relapse.28
Frequent testing—probably biweekly—is recommended during the first 3 or 4 months after patients complete treatment. If there is no indication of relapse, testing frequency could be tapered down.
- AUDIT. The Alcohol Use Disorders Identification Test. Guidelines for primary care use. Available at: http://www.who.int/substance_abuse/pubs_alcohol.htm
- Allen JP, Litten RZ. Psychometric and laboratory measures to assist in the treatment of alcoholism. Clin Psychol Rev 1993;13(3):223-39.
- Salaspuro M. Carbohydrate-deficient transferrin as compared to other markers of alcoholism: a systematic review. Alcohol 1999; 19(3):261-71.
Disclosure
Dr. Allen reports that he serves as a consultant to Axis-Shield ASA and Bio-Rad Laboratories, patent holder and U.S. distributor, respectively, of the carbohydrate-deficient transferrin (%CDT) reagent kit.
Dr. Anthenelli reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Hall W. What have population surveys revealed about substance use disorders and their co-morbidity with other mental disorders? Drug Alcohol Rev 1996;15(2):157-70.
2. Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: Results of a national survey. Drug Alcohol Depend 1995;39(3):197-206.
3. Shivani R, Goldsmith RJ, Anthenelli RM. Alcoholism and psychiatric disorders: diagnostic challenges. Alcohol Res Health 2002;26(2):90-8.
4. Allen JP, Columbus M (eds). Assessing alcohol problems: a guide for clinicians and researchers. NIAAA Treatment Handbook, Series 4. Washington, DC: U.S. Department of Health and Human Services, 1995 [NIH publication no. 95-3745].
5. Mayfield D, McLeod G, Hall P. CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry 1974;131(10):1121-3.
6. Seltzer ML. The Michigan Alcoholism Screening Test: the quest for a new diagnostic instrument. Am J Psychiatry 1971;127:1653-8.
7. Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption—II. Addiction 1993;88(6):791-804.
8. Daeppen J, Yersin B, Landry U, et al. Reliability and validity of the Alcohol Use Disorders Identification Test (AUDIT) imbedded within a general health risk screening questionnaire: results of a survey in 332 primary care patients. Alcohol Clin Exp Res 2000;24:659-65.
9. Reinert DF, Allen JP. The Alcohol Use Disorders Identification Test: a review of recent research. Alcohol Clin Exp Res 2002;26(2):272-9.
10. Maisto SA, Carey MP, Carey KB, et al. Use of the AUDIT and the DAST-10 to identify alcohol and drug use disorders among adults with a severe and persistent mental illness. Psychol Assess 2002;12:186-92.
11. Hulse GK, Saunders JB, Roydhouse RM, et al. Screening for hazardous alcohol use and dependence in psychiatric inpatients using the AUDIT questionnaire. Drug Alcohol Rev 2000;19:291-8.
12. Dawe S, Seinen A, Kavanagh D. An examination of the utility of the AUDIT in people with schizophrenia. J Stud Alcohol 2000;61:744-75.
13. Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT). Alcohol Clin Exp Res 1997;21:613-19.
14. Hermansson U, Helander A, Huss A, et al. The Alcohol Use Disorders Identification Test (AUDIT) and carbohydrate-deficient transferrin (CDT) in a routine workplace health examination. Alcohol Clin Exp Res 2000;24:180-7.
15. Sillanaukee P, Strid N, Allen JP, Litten RZ. Possible reasons why heavy drinking increases carbohydrate-deficient transferrin. Alcohol Clin Exp Res 2001;25(1):34-40.
16. Litten RZ, Allen JP, Fertig JB. Gamma-glutamyltranspeptidase and carbohydrate deficient transferrin: alternative measures of excessive alcohol consumption. Alcohol Clin Exp Res 1995;19(6):1541-6.
17. Allen JP, Litten RZ, Fertig JB, Sillanaukee P. Carbohydrate-deficient transferrin, gamma glutamyl transferase and macrocytic volume as biomarkers of alcohol problems in women. Alcohol Clin Exp Res 2000;24(4):492-6.
18. Sillanaukee P, Strid N, Allen JP, Litten RZ. Combining biomarkers to screen for alcohol problems (manuscript submitted for publication).
19. Miller WR, Zweben A, DiClemente CC, et al. Motivational enhancement therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. Rockville, MD: U.S. Department of Health and Human Services, 1995.
20. Project MATCH research group. Matching alcoholism treatments to client heterogeneity: Project MATCH post-treatment drinking outcomes. J Stud Alcohol 1997;58(1):7-29.
21. Bien TH, Miller WR, Tonigan S. Brief intervention for alcohol problems: a review. Addiction 1993;88:315-36.
22. Kristenson H, Hood B, Peterson B, et al. Prevention of alcohol-related problems in urban middle-aged males. Alcohol 1985;2(3):545-9.
23. Miller WR, Walter ST, Bennett ME. How effective is alcoholism treatment in the United States? J Stud Alcohol. 2001;62:211-20.
24. Mitchell C, Simpson D, Chick J. Carbohydrate-deficient transferrin in detecting relapse in alcohol dependence. Drug Alcohol Depend 1997;48:97-103.
25. Borg S, Helander A, Voltaire Carlsson A, Hogstrom Brandt AM. Detection of relapses in alcohol-dependent patients using carbohydrate-deficient transferrin: improvement with individualized reference levels during long-term monitoring. Alcohol Clin Exp Res 1995;19(4):961-3.
26. Schmidt LG, Schmidt K, Dufeu P, et al. Superiority of carbohydrate-deficient transferrin to gamma-glutamyltransferase in detecting relapse in alcoholism. Am J Psychiatry 1997;154(1):75-80.
27. Allen JP, Anton R. Biomarkers as aids to identification of relapse in alcoholic patients. In: Galanter M (ed). Recent developments in alcoholism: research on alcoholism treatment (vol. XVI). New York: Plenum Press (in press).
28. Anton RF, Lieber C, Tabakoff B, et al. Carbohydrate-deficient transferrin and gamma-glutamyltransferase for the detection and monitoring of alcohol use: results from a multi-site study. Alcohol Clin Exp Res 2002;26(8):1215-22.
Do you know which of your patients have alcohol problems? Though alcohol use disorders may be difficult to detect, self-report and biochemical measures followed by a thorough face-to-face assessment improve diagnostic accuracy. New tools—such as the serum carbohydrate-deficient transferrin (CDT) test—are changing how psychiatrists screen for alcohol problems, provide motivational feedback, and monitor patients for relapse.
FOUR REASONS TO SCREEN
Screening for excessive alcohol consumption is important in psychiatric practice because:
- Alcohol use disorders coexist with many psychiatric problems, most notably affective and anxiety disorders and—not surprisingly—other substance abuse disorders (Table 1).1,2
- Patients with psychiatric comorbidity who abuse alcohol have poorer prognoses, are less adherent to treatment, and are more likely to drop out of treatment than are psychiatric patients who do not have alcohol problems.3
- Alcohol interacts with many psychotropics, and chronic heavy drinking can cause pharmacokinetic changes that affect a patient’s response to medications.
- Alcohol-dependent patients are more likely than nondrinkers to become dependent on anxiolytics and sedative-hypnotics.
Table 1
Overlap of alcohol problems with common psychiatric disorders
| Disorder | Risk of alcohol use disorder (odds ratio) | Source of data (population survey) |
|---|---|---|
| Drug use disorder | 25.1 | NLAES |
| Mania | 5.6 | NCS |
| Major depression | 3.7 | NLAES |
| Obsessive-compulsive disorder | 3.4 | ECA |
| Generalized anxiety disorder | 2.7 | NCS |
| Phobia | 2.3 | NCS |
| Posttraumatic stress disorder | 2.2 | NCS |
| Panic disorder | 1.4 | NCS |
| NLAES: National Longitudinal Alcohol Epidemiological Survey | ||
| NCS: National Comorbidity Survey | ||
| ECA: Epidemiologic Catchment Area | ||
Because alcohol problems are common in psychiatric patients, routine screening for alcohol abuse and dependence at the onset of any treatment can be very useful. Thereafter, screening can be done periodically—perhaps annually or more often if the patient’s functioning declines.
CHOOSING A SELF-REPORT MEASURE
Many self-report alcohol screening scales are available,4 the most popular being the CAGE5 and the Michigan Alcoholism Screening Test (MAST).6 Though both instruments can help identify alcohol problems, each has shortcomings:
- The CAGE performs less reliably in women and adolescents than in men, and its validity depends on the patient’s sensitivity to the emotional impacts of alcohol dependence.
- The MAST is long (25 items), concentrates on late-stage alcoholism symptoms, and uses differential weighting—not validated in subsequent studies—of particular items in deriving the score.
Neither addresses drinking behavior or when symptoms occurred and thus may misclassify recovered alcoholics or former problem drinkers.
AUDIT. A more reliable choice is the Alcohol Use Disorders Identification Test (AUDIT).7 It was designed by the World Health Organization (WHO) to be valid across gender and culture and to identify even early stage problem drinking. The AUDIT’s 10 items deal with drinking behavior, dependence on alcohol, and adverse consequences of drinking during the past year (Box). The survey takes less than 5 minutes; can be administered orally, in writing, or online; and it retains its validity when given as part of a comprehensive health risk appraisal.8
The WHO offers an excellent manual detailing how to administer and interpret the AUDIT (see Related resources). A patient’s score is computed by summing the values associated with his or her responses to each item. A score of 8 or greater indicates excessive alcohol consumption, although some researchers have argued that for women a more accurate threshold might be 6 or 7 points.
Standardized for adults, the AUDIT also appears to accurately gauge drinking behavior in adolescents9 and in psychiatric patients, although only three studies have explored its use in the latter population.10-12 Abbreviated AUDIT versions have been found to be psychometrically sound8 and may be useful in an emergency room or busy primary care clinic. In comparisons with other screening tools, the AUDIT almost always has been found to be more valid.9,13
USING BIOCHEMICAL MEASURES
Self-report screens for alcohol problems, especially the AUDIT, are highly sensitive and specific, though their accuracy depends on the patient's memory, understanding of the questions, and candor. In chronic heavy drinkers, biochemical measures (Table 2) can augment self-reports.14
Self-report and biochemical screens have different strengths and weaknesses (Table 3). It is important to see them as complementary because each contributes to accurate screening.
CDT. Most biomarkers screen indirectly for alcohol problems by measuring damage to an end organ-typically the liver-caused by chronic excessive alcohol consumption. False positive results are common because of nonalcohol-related organ damage, medications, smoking, obesity, and other confounding factors. An exception appears to be the serum test for carbohydrate-deficient transferrin (CDT), a biomarker for heavy drinking approved in kit form 3 years ago by the Food and Drug Administration.
The value of measuring CDT levels is that few conditions other than excessive alcohol consumption elevate them. For unclear reasons,15 average daily consumption of >60 grams of alcohol (about five standard drinks) during the previous 2 weeks causes a higher percent of transferrin—a glycoenzyme that transports iron in the body—to lack its usual carbohydrate content.
Bio-Rad Laboratories (www.bio-rad.com) offers a reagent kit (%CDT Turbidimetric Immunoassay). It quantifies CDT as a percent of total serum transferrin, rather than total CDT, thus correcting for individual variations in transferrin levels. CDT values are obtained from a 100-microliter serum sample. The blood is clotted and the serum separated. The sample may be stored at 2 to 8 °C if the test is to be run within 1 week. Samples must be tested at a reference lab (Bio-Rad offers a list of labs). Results are available in a few days.
Patients who deny problem drinking may need convincing to submit to a blood draw. It may help to explain that alcohol use can exacerbate emotional problems and that the test can provide information on possible risky alcohol use.
GGT. Using a second biochemical marker may improve the sensitivity of CDT to detect heavy drinking.16-18
The most-researched choice for a second marker is gamma glutamyltransferase (GGT). Patients are considered to have tested positive for an alcohol problem if either CDT or GGT levels are elevated. Combining these tests may be especially useful in alcohol-dependent women, in whom the reliability of CDT testing alone has been questioned.
Recommendation. Start with a self-report screening measure. If the patient scores slightly below the threshold for an alcohol problem, follow up with the more costly CDT and GGT tests.
For example, biomarkers might be useful for follow-up in men with AUDIT screening scores of 6 or 7 or women with scores of 5 to 7. Biomarkers also are recommended when you suspect an alcohol problem for another reason or question whether the patient responded accurately to the self-report measure.
- How often do you have a drink containing alcohol?
- How many drinks containing alcohol do you have on a typical day when you are drinking?
- How often do you have 6 or more drinks on one occasion?
- How often during the last year have you found that you were not able to stop drinking once you had started?
- How often during the last year have you failed to do what was normally expected from you because of drinking?
- How often during the last year have you needed a first drink in the morning to get yourself going after a heavy drinking session?
- How often during the last year have you had a feeling of guilt or remorse after drinking?
- How often during the last year have you been unable to remember what happened the night before because you had been drinking?
- Have you or someone else been injured as a result of your drinking?
- Has a relative, friend, doctor, or other health worker been concerned about your drinking or suggested that you cut down?
The World Health Organization offers a manual on how to administer and interpret AUDIT (http://www.who.int/substance_abuse/pubs_alcohol.htm).
Source: World Health Organization
Table 2
Biochemical markers of heavy drinking
| Marker | Time needed for return to normal limits | Level of drinking characterized | Comments |
|---|---|---|---|
| Gamma glutamyltransferase (GGT) | 2 to 6 weeks of abstinence | ~70 drinks/wk for several weeks | Most common and reliable of the traditional markers of heavy drinking; many sources of false positives |
| Aspartate aminotransferase (AST) (formerly SGOT) | 7 days, but much variability in declines with abstinence | Unknown, but heavy | Present in many organs; many sources of false positives; moderate correlations with GGT |
| Alanine aminotransferase (ALT) (formerly SGPT) | Unknown | Unknown, but heavy | Many sources of false positives and less sensitive than AST; ratio of AST to ALT may be more accurate |
| Macrocytic volume (MCV) | Unknown; half-life ~40 days | Unknown, but regular and heavy | Poor sensitivity and specificity; even with abstinence, very slow return to normal limits and may increase at first; little, if any, gender effect |
| Carbohydrate-deficient transferrin (CDT) | 2 to 4 weeks of abstinence | >60 grams/day for approximately 2 weeks | Few sources of false positives; excellent indicator of relapse |
MONITORING PATIENTS IN TREATMENT
MET. Motivational enhancement therapy (MET) has gained popularity as a means of changing problematic drinking.19 Project MATCH—a multi-site trial on alcohol abuse treatment—studied MET and two other interventions. MET required fewer sessions but equaled the other interventions in reducing drinking days and aver-age amount of alcohol consumed.20
A key component of MET—and other brief interventions—is to provide patients with empathetic, nonjudgmental feedback.19-21 Responses to the first three AUDIT items can provide such feedback to patients with drinking problems. Amazingly, heavy drinkers and alcoholics often do not realize how much more they drink than other people. To help them develop this insight, show them their self-report responses in contrast with national normative data.
Biomarker results can be used similarly, in this case comparing the patient’s score with the test’s reference range values. Kristenson et al22 showed that giving men with elevated scores recurrent biomarker information and advice significantly reduced morbidity and mortality and improved their work performance.
Using visual aids can deepen patients’ under-standing of motivational feedback. For example, displaying sequential test results on a timeline can reinforce motivation by showing how their drinking behavior has improved with continuing treatment and sustained effort.
Table 3
Self-report and biochemical measures of drinking: Pros and cons
| Measure | Strengths | Weaknesses |
|---|---|---|
| Self-report | Noninvasive Inexpensive High validity Flexible window of assessment Immediate results | Easily feigned Accuracy depends on patient’s verbal skills and memory |
| Biochemical | Objective Results may be more compelling to patients than self-reports May reflect organ damage Useful in tracking treatment progress | Window of assessment is limited to recent past Results often not immediately available May be more costly than self-report measures |
DETECTING RELAPSE
Although treatment for alcohol problems is often successful,23 relapse to some level of drinking is not uncommon, especially during the first 3 or 4 months after patients complete treatment.20 Recognizing relapse quickly can help you:
- decrease risk of harm from resumed alcohol use
- reduce the potential for drinking to again become habitual
- identify circumstances and cues that may have triggered the drinking episode, for use in tailoring future interventions.
In clinical practice, relapse is most often revealed via comments from the family, direct observation by the clinician, or voluntary acknowledgment by the patient. Interestingly, CDT has demonstrated a relapse “heralding effect,”24 meaning that it tends to rise well before a patient will admit he or she resumed drinking.24-26 Although other markers may also rise following relapse, their elevation tends to be delayed and less dramatic.
The sensitivity and specificity of CDT alone and with GGT in identifying relapse have been evaluated. Across male-only studies, CDT’s median sensitivity (percent of relapsed patients with elevated scores) was 0.73, with a specificity (percent of those not relapsed who had low scores) of 0.91. In the two female-only studies, median sensitivity and specificity for CDT were 0.32 and 0.86, respectively. For women, using CDT and GGT in combination substantially raised median sensitivity to 0.62, although specificity fell slightly to 0.80.27
Recommendation. When using biomarkers to identify relapse, examine the temporal pattern of test results to date. Assume that an increase of 30% or more above the lowest observed lab value indicates a relapse.28
Frequent testing—probably biweekly—is recommended during the first 3 or 4 months after patients complete treatment. If there is no indication of relapse, testing frequency could be tapered down.
- AUDIT. The Alcohol Use Disorders Identification Test. Guidelines for primary care use. Available at: http://www.who.int/substance_abuse/pubs_alcohol.htm
- Allen JP, Litten RZ. Psychometric and laboratory measures to assist in the treatment of alcoholism. Clin Psychol Rev 1993;13(3):223-39.
- Salaspuro M. Carbohydrate-deficient transferrin as compared to other markers of alcoholism: a systematic review. Alcohol 1999; 19(3):261-71.
Disclosure
Dr. Allen reports that he serves as a consultant to Axis-Shield ASA and Bio-Rad Laboratories, patent holder and U.S. distributor, respectively, of the carbohydrate-deficient transferrin (%CDT) reagent kit.
Dr. Anthenelli reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Do you know which of your patients have alcohol problems? Though alcohol use disorders may be difficult to detect, self-report and biochemical measures followed by a thorough face-to-face assessment improve diagnostic accuracy. New tools—such as the serum carbohydrate-deficient transferrin (CDT) test—are changing how psychiatrists screen for alcohol problems, provide motivational feedback, and monitor patients for relapse.
FOUR REASONS TO SCREEN
Screening for excessive alcohol consumption is important in psychiatric practice because:
- Alcohol use disorders coexist with many psychiatric problems, most notably affective and anxiety disorders and—not surprisingly—other substance abuse disorders (Table 1).1,2
- Patients with psychiatric comorbidity who abuse alcohol have poorer prognoses, are less adherent to treatment, and are more likely to drop out of treatment than are psychiatric patients who do not have alcohol problems.3
- Alcohol interacts with many psychotropics, and chronic heavy drinking can cause pharmacokinetic changes that affect a patient’s response to medications.
- Alcohol-dependent patients are more likely than nondrinkers to become dependent on anxiolytics and sedative-hypnotics.
Table 1
Overlap of alcohol problems with common psychiatric disorders
| Disorder | Risk of alcohol use disorder (odds ratio) | Source of data (population survey) |
|---|---|---|
| Drug use disorder | 25.1 | NLAES |
| Mania | 5.6 | NCS |
| Major depression | 3.7 | NLAES |
| Obsessive-compulsive disorder | 3.4 | ECA |
| Generalized anxiety disorder | 2.7 | NCS |
| Phobia | 2.3 | NCS |
| Posttraumatic stress disorder | 2.2 | NCS |
| Panic disorder | 1.4 | NCS |
| NLAES: National Longitudinal Alcohol Epidemiological Survey | ||
| NCS: National Comorbidity Survey | ||
| ECA: Epidemiologic Catchment Area | ||
Because alcohol problems are common in psychiatric patients, routine screening for alcohol abuse and dependence at the onset of any treatment can be very useful. Thereafter, screening can be done periodically—perhaps annually or more often if the patient’s functioning declines.
CHOOSING A SELF-REPORT MEASURE
Many self-report alcohol screening scales are available,4 the most popular being the CAGE5 and the Michigan Alcoholism Screening Test (MAST).6 Though both instruments can help identify alcohol problems, each has shortcomings:
- The CAGE performs less reliably in women and adolescents than in men, and its validity depends on the patient’s sensitivity to the emotional impacts of alcohol dependence.
- The MAST is long (25 items), concentrates on late-stage alcoholism symptoms, and uses differential weighting—not validated in subsequent studies—of particular items in deriving the score.
Neither addresses drinking behavior or when symptoms occurred and thus may misclassify recovered alcoholics or former problem drinkers.
AUDIT. A more reliable choice is the Alcohol Use Disorders Identification Test (AUDIT).7 It was designed by the World Health Organization (WHO) to be valid across gender and culture and to identify even early stage problem drinking. The AUDIT’s 10 items deal with drinking behavior, dependence on alcohol, and adverse consequences of drinking during the past year (Box). The survey takes less than 5 minutes; can be administered orally, in writing, or online; and it retains its validity when given as part of a comprehensive health risk appraisal.8
The WHO offers an excellent manual detailing how to administer and interpret the AUDIT (see Related resources). A patient’s score is computed by summing the values associated with his or her responses to each item. A score of 8 or greater indicates excessive alcohol consumption, although some researchers have argued that for women a more accurate threshold might be 6 or 7 points.
Standardized for adults, the AUDIT also appears to accurately gauge drinking behavior in adolescents9 and in psychiatric patients, although only three studies have explored its use in the latter population.10-12 Abbreviated AUDIT versions have been found to be psychometrically sound8 and may be useful in an emergency room or busy primary care clinic. In comparisons with other screening tools, the AUDIT almost always has been found to be more valid.9,13
USING BIOCHEMICAL MEASURES
Self-report screens for alcohol problems, especially the AUDIT, are highly sensitive and specific, though their accuracy depends on the patient's memory, understanding of the questions, and candor. In chronic heavy drinkers, biochemical measures (Table 2) can augment self-reports.14
Self-report and biochemical screens have different strengths and weaknesses (Table 3). It is important to see them as complementary because each contributes to accurate screening.
CDT. Most biomarkers screen indirectly for alcohol problems by measuring damage to an end organ-typically the liver-caused by chronic excessive alcohol consumption. False positive results are common because of nonalcohol-related organ damage, medications, smoking, obesity, and other confounding factors. An exception appears to be the serum test for carbohydrate-deficient transferrin (CDT), a biomarker for heavy drinking approved in kit form 3 years ago by the Food and Drug Administration.
The value of measuring CDT levels is that few conditions other than excessive alcohol consumption elevate them. For unclear reasons,15 average daily consumption of >60 grams of alcohol (about five standard drinks) during the previous 2 weeks causes a higher percent of transferrin—a glycoenzyme that transports iron in the body—to lack its usual carbohydrate content.
Bio-Rad Laboratories (www.bio-rad.com) offers a reagent kit (%CDT Turbidimetric Immunoassay). It quantifies CDT as a percent of total serum transferrin, rather than total CDT, thus correcting for individual variations in transferrin levels. CDT values are obtained from a 100-microliter serum sample. The blood is clotted and the serum separated. The sample may be stored at 2 to 8 °C if the test is to be run within 1 week. Samples must be tested at a reference lab (Bio-Rad offers a list of labs). Results are available in a few days.
Patients who deny problem drinking may need convincing to submit to a blood draw. It may help to explain that alcohol use can exacerbate emotional problems and that the test can provide information on possible risky alcohol use.
GGT. Using a second biochemical marker may improve the sensitivity of CDT to detect heavy drinking.16-18
The most-researched choice for a second marker is gamma glutamyltransferase (GGT). Patients are considered to have tested positive for an alcohol problem if either CDT or GGT levels are elevated. Combining these tests may be especially useful in alcohol-dependent women, in whom the reliability of CDT testing alone has been questioned.
Recommendation. Start with a self-report screening measure. If the patient scores slightly below the threshold for an alcohol problem, follow up with the more costly CDT and GGT tests.
For example, biomarkers might be useful for follow-up in men with AUDIT screening scores of 6 or 7 or women with scores of 5 to 7. Biomarkers also are recommended when you suspect an alcohol problem for another reason or question whether the patient responded accurately to the self-report measure.
- How often do you have a drink containing alcohol?
- How many drinks containing alcohol do you have on a typical day when you are drinking?
- How often do you have 6 or more drinks on one occasion?
- How often during the last year have you found that you were not able to stop drinking once you had started?
- How often during the last year have you failed to do what was normally expected from you because of drinking?
- How often during the last year have you needed a first drink in the morning to get yourself going after a heavy drinking session?
- How often during the last year have you had a feeling of guilt or remorse after drinking?
- How often during the last year have you been unable to remember what happened the night before because you had been drinking?
- Have you or someone else been injured as a result of your drinking?
- Has a relative, friend, doctor, or other health worker been concerned about your drinking or suggested that you cut down?
The World Health Organization offers a manual on how to administer and interpret AUDIT (http://www.who.int/substance_abuse/pubs_alcohol.htm).
Source: World Health Organization
Table 2
Biochemical markers of heavy drinking
| Marker | Time needed for return to normal limits | Level of drinking characterized | Comments |
|---|---|---|---|
| Gamma glutamyltransferase (GGT) | 2 to 6 weeks of abstinence | ~70 drinks/wk for several weeks | Most common and reliable of the traditional markers of heavy drinking; many sources of false positives |
| Aspartate aminotransferase (AST) (formerly SGOT) | 7 days, but much variability in declines with abstinence | Unknown, but heavy | Present in many organs; many sources of false positives; moderate correlations with GGT |
| Alanine aminotransferase (ALT) (formerly SGPT) | Unknown | Unknown, but heavy | Many sources of false positives and less sensitive than AST; ratio of AST to ALT may be more accurate |
| Macrocytic volume (MCV) | Unknown; half-life ~40 days | Unknown, but regular and heavy | Poor sensitivity and specificity; even with abstinence, very slow return to normal limits and may increase at first; little, if any, gender effect |
| Carbohydrate-deficient transferrin (CDT) | 2 to 4 weeks of abstinence | >60 grams/day for approximately 2 weeks | Few sources of false positives; excellent indicator of relapse |
MONITORING PATIENTS IN TREATMENT
MET. Motivational enhancement therapy (MET) has gained popularity as a means of changing problematic drinking.19 Project MATCH—a multi-site trial on alcohol abuse treatment—studied MET and two other interventions. MET required fewer sessions but equaled the other interventions in reducing drinking days and aver-age amount of alcohol consumed.20
A key component of MET—and other brief interventions—is to provide patients with empathetic, nonjudgmental feedback.19-21 Responses to the first three AUDIT items can provide such feedback to patients with drinking problems. Amazingly, heavy drinkers and alcoholics often do not realize how much more they drink than other people. To help them develop this insight, show them their self-report responses in contrast with national normative data.
Biomarker results can be used similarly, in this case comparing the patient’s score with the test’s reference range values. Kristenson et al22 showed that giving men with elevated scores recurrent biomarker information and advice significantly reduced morbidity and mortality and improved their work performance.
Using visual aids can deepen patients’ under-standing of motivational feedback. For example, displaying sequential test results on a timeline can reinforce motivation by showing how their drinking behavior has improved with continuing treatment and sustained effort.
Table 3
Self-report and biochemical measures of drinking: Pros and cons
| Measure | Strengths | Weaknesses |
|---|---|---|
| Self-report | Noninvasive Inexpensive High validity Flexible window of assessment Immediate results | Easily feigned Accuracy depends on patient’s verbal skills and memory |
| Biochemical | Objective Results may be more compelling to patients than self-reports May reflect organ damage Useful in tracking treatment progress | Window of assessment is limited to recent past Results often not immediately available May be more costly than self-report measures |
DETECTING RELAPSE
Although treatment for alcohol problems is often successful,23 relapse to some level of drinking is not uncommon, especially during the first 3 or 4 months after patients complete treatment.20 Recognizing relapse quickly can help you:
- decrease risk of harm from resumed alcohol use
- reduce the potential for drinking to again become habitual
- identify circumstances and cues that may have triggered the drinking episode, for use in tailoring future interventions.
In clinical practice, relapse is most often revealed via comments from the family, direct observation by the clinician, or voluntary acknowledgment by the patient. Interestingly, CDT has demonstrated a relapse “heralding effect,”24 meaning that it tends to rise well before a patient will admit he or she resumed drinking.24-26 Although other markers may also rise following relapse, their elevation tends to be delayed and less dramatic.
The sensitivity and specificity of CDT alone and with GGT in identifying relapse have been evaluated. Across male-only studies, CDT’s median sensitivity (percent of relapsed patients with elevated scores) was 0.73, with a specificity (percent of those not relapsed who had low scores) of 0.91. In the two female-only studies, median sensitivity and specificity for CDT were 0.32 and 0.86, respectively. For women, using CDT and GGT in combination substantially raised median sensitivity to 0.62, although specificity fell slightly to 0.80.27
Recommendation. When using biomarkers to identify relapse, examine the temporal pattern of test results to date. Assume that an increase of 30% or more above the lowest observed lab value indicates a relapse.28
Frequent testing—probably biweekly—is recommended during the first 3 or 4 months after patients complete treatment. If there is no indication of relapse, testing frequency could be tapered down.
- AUDIT. The Alcohol Use Disorders Identification Test. Guidelines for primary care use. Available at: http://www.who.int/substance_abuse/pubs_alcohol.htm
- Allen JP, Litten RZ. Psychometric and laboratory measures to assist in the treatment of alcoholism. Clin Psychol Rev 1993;13(3):223-39.
- Salaspuro M. Carbohydrate-deficient transferrin as compared to other markers of alcoholism: a systematic review. Alcohol 1999; 19(3):261-71.
Disclosure
Dr. Allen reports that he serves as a consultant to Axis-Shield ASA and Bio-Rad Laboratories, patent holder and U.S. distributor, respectively, of the carbohydrate-deficient transferrin (%CDT) reagent kit.
Dr. Anthenelli reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Hall W. What have population surveys revealed about substance use disorders and their co-morbidity with other mental disorders? Drug Alcohol Rev 1996;15(2):157-70.
2. Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: Results of a national survey. Drug Alcohol Depend 1995;39(3):197-206.
3. Shivani R, Goldsmith RJ, Anthenelli RM. Alcoholism and psychiatric disorders: diagnostic challenges. Alcohol Res Health 2002;26(2):90-8.
4. Allen JP, Columbus M (eds). Assessing alcohol problems: a guide for clinicians and researchers. NIAAA Treatment Handbook, Series 4. Washington, DC: U.S. Department of Health and Human Services, 1995 [NIH publication no. 95-3745].
5. Mayfield D, McLeod G, Hall P. CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry 1974;131(10):1121-3.
6. Seltzer ML. The Michigan Alcoholism Screening Test: the quest for a new diagnostic instrument. Am J Psychiatry 1971;127:1653-8.
7. Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption—II. Addiction 1993;88(6):791-804.
8. Daeppen J, Yersin B, Landry U, et al. Reliability and validity of the Alcohol Use Disorders Identification Test (AUDIT) imbedded within a general health risk screening questionnaire: results of a survey in 332 primary care patients. Alcohol Clin Exp Res 2000;24:659-65.
9. Reinert DF, Allen JP. The Alcohol Use Disorders Identification Test: a review of recent research. Alcohol Clin Exp Res 2002;26(2):272-9.
10. Maisto SA, Carey MP, Carey KB, et al. Use of the AUDIT and the DAST-10 to identify alcohol and drug use disorders among adults with a severe and persistent mental illness. Psychol Assess 2002;12:186-92.
11. Hulse GK, Saunders JB, Roydhouse RM, et al. Screening for hazardous alcohol use and dependence in psychiatric inpatients using the AUDIT questionnaire. Drug Alcohol Rev 2000;19:291-8.
12. Dawe S, Seinen A, Kavanagh D. An examination of the utility of the AUDIT in people with schizophrenia. J Stud Alcohol 2000;61:744-75.
13. Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT). Alcohol Clin Exp Res 1997;21:613-19.
14. Hermansson U, Helander A, Huss A, et al. The Alcohol Use Disorders Identification Test (AUDIT) and carbohydrate-deficient transferrin (CDT) in a routine workplace health examination. Alcohol Clin Exp Res 2000;24:180-7.
15. Sillanaukee P, Strid N, Allen JP, Litten RZ. Possible reasons why heavy drinking increases carbohydrate-deficient transferrin. Alcohol Clin Exp Res 2001;25(1):34-40.
16. Litten RZ, Allen JP, Fertig JB. Gamma-glutamyltranspeptidase and carbohydrate deficient transferrin: alternative measures of excessive alcohol consumption. Alcohol Clin Exp Res 1995;19(6):1541-6.
17. Allen JP, Litten RZ, Fertig JB, Sillanaukee P. Carbohydrate-deficient transferrin, gamma glutamyl transferase and macrocytic volume as biomarkers of alcohol problems in women. Alcohol Clin Exp Res 2000;24(4):492-6.
18. Sillanaukee P, Strid N, Allen JP, Litten RZ. Combining biomarkers to screen for alcohol problems (manuscript submitted for publication).
19. Miller WR, Zweben A, DiClemente CC, et al. Motivational enhancement therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. Rockville, MD: U.S. Department of Health and Human Services, 1995.
20. Project MATCH research group. Matching alcoholism treatments to client heterogeneity: Project MATCH post-treatment drinking outcomes. J Stud Alcohol 1997;58(1):7-29.
21. Bien TH, Miller WR, Tonigan S. Brief intervention for alcohol problems: a review. Addiction 1993;88:315-36.
22. Kristenson H, Hood B, Peterson B, et al. Prevention of alcohol-related problems in urban middle-aged males. Alcohol 1985;2(3):545-9.
23. Miller WR, Walter ST, Bennett ME. How effective is alcoholism treatment in the United States? J Stud Alcohol. 2001;62:211-20.
24. Mitchell C, Simpson D, Chick J. Carbohydrate-deficient transferrin in detecting relapse in alcohol dependence. Drug Alcohol Depend 1997;48:97-103.
25. Borg S, Helander A, Voltaire Carlsson A, Hogstrom Brandt AM. Detection of relapses in alcohol-dependent patients using carbohydrate-deficient transferrin: improvement with individualized reference levels during long-term monitoring. Alcohol Clin Exp Res 1995;19(4):961-3.
26. Schmidt LG, Schmidt K, Dufeu P, et al. Superiority of carbohydrate-deficient transferrin to gamma-glutamyltransferase in detecting relapse in alcoholism. Am J Psychiatry 1997;154(1):75-80.
27. Allen JP, Anton R. Biomarkers as aids to identification of relapse in alcoholic patients. In: Galanter M (ed). Recent developments in alcoholism: research on alcoholism treatment (vol. XVI). New York: Plenum Press (in press).
28. Anton RF, Lieber C, Tabakoff B, et al. Carbohydrate-deficient transferrin and gamma-glutamyltransferase for the detection and monitoring of alcohol use: results from a multi-site study. Alcohol Clin Exp Res 2002;26(8):1215-22.
1. Hall W. What have population surveys revealed about substance use disorders and their co-morbidity with other mental disorders? Drug Alcohol Rev 1996;15(2):157-70.
2. Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: Results of a national survey. Drug Alcohol Depend 1995;39(3):197-206.
3. Shivani R, Goldsmith RJ, Anthenelli RM. Alcoholism and psychiatric disorders: diagnostic challenges. Alcohol Res Health 2002;26(2):90-8.
4. Allen JP, Columbus M (eds). Assessing alcohol problems: a guide for clinicians and researchers. NIAAA Treatment Handbook, Series 4. Washington, DC: U.S. Department of Health and Human Services, 1995 [NIH publication no. 95-3745].
5. Mayfield D, McLeod G, Hall P. CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry 1974;131(10):1121-3.
6. Seltzer ML. The Michigan Alcoholism Screening Test: the quest for a new diagnostic instrument. Am J Psychiatry 1971;127:1653-8.
7. Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption—II. Addiction 1993;88(6):791-804.
8. Daeppen J, Yersin B, Landry U, et al. Reliability and validity of the Alcohol Use Disorders Identification Test (AUDIT) imbedded within a general health risk screening questionnaire: results of a survey in 332 primary care patients. Alcohol Clin Exp Res 2000;24:659-65.
9. Reinert DF, Allen JP. The Alcohol Use Disorders Identification Test: a review of recent research. Alcohol Clin Exp Res 2002;26(2):272-9.
10. Maisto SA, Carey MP, Carey KB, et al. Use of the AUDIT and the DAST-10 to identify alcohol and drug use disorders among adults with a severe and persistent mental illness. Psychol Assess 2002;12:186-92.
11. Hulse GK, Saunders JB, Roydhouse RM, et al. Screening for hazardous alcohol use and dependence in psychiatric inpatients using the AUDIT questionnaire. Drug Alcohol Rev 2000;19:291-8.
12. Dawe S, Seinen A, Kavanagh D. An examination of the utility of the AUDIT in people with schizophrenia. J Stud Alcohol 2000;61:744-75.
13. Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT). Alcohol Clin Exp Res 1997;21:613-19.
14. Hermansson U, Helander A, Huss A, et al. The Alcohol Use Disorders Identification Test (AUDIT) and carbohydrate-deficient transferrin (CDT) in a routine workplace health examination. Alcohol Clin Exp Res 2000;24:180-7.
15. Sillanaukee P, Strid N, Allen JP, Litten RZ. Possible reasons why heavy drinking increases carbohydrate-deficient transferrin. Alcohol Clin Exp Res 2001;25(1):34-40.
16. Litten RZ, Allen JP, Fertig JB. Gamma-glutamyltranspeptidase and carbohydrate deficient transferrin: alternative measures of excessive alcohol consumption. Alcohol Clin Exp Res 1995;19(6):1541-6.
17. Allen JP, Litten RZ, Fertig JB, Sillanaukee P. Carbohydrate-deficient transferrin, gamma glutamyl transferase and macrocytic volume as biomarkers of alcohol problems in women. Alcohol Clin Exp Res 2000;24(4):492-6.
18. Sillanaukee P, Strid N, Allen JP, Litten RZ. Combining biomarkers to screen for alcohol problems (manuscript submitted for publication).
19. Miller WR, Zweben A, DiClemente CC, et al. Motivational enhancement therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. Rockville, MD: U.S. Department of Health and Human Services, 1995.
20. Project MATCH research group. Matching alcoholism treatments to client heterogeneity: Project MATCH post-treatment drinking outcomes. J Stud Alcohol 1997;58(1):7-29.
21. Bien TH, Miller WR, Tonigan S. Brief intervention for alcohol problems: a review. Addiction 1993;88:315-36.
22. Kristenson H, Hood B, Peterson B, et al. Prevention of alcohol-related problems in urban middle-aged males. Alcohol 1985;2(3):545-9.
23. Miller WR, Walter ST, Bennett ME. How effective is alcoholism treatment in the United States? J Stud Alcohol. 2001;62:211-20.
24. Mitchell C, Simpson D, Chick J. Carbohydrate-deficient transferrin in detecting relapse in alcohol dependence. Drug Alcohol Depend 1997;48:97-103.
25. Borg S, Helander A, Voltaire Carlsson A, Hogstrom Brandt AM. Detection of relapses in alcohol-dependent patients using carbohydrate-deficient transferrin: improvement with individualized reference levels during long-term monitoring. Alcohol Clin Exp Res 1995;19(4):961-3.
26. Schmidt LG, Schmidt K, Dufeu P, et al. Superiority of carbohydrate-deficient transferrin to gamma-glutamyltransferase in detecting relapse in alcoholism. Am J Psychiatry 1997;154(1):75-80.
27. Allen JP, Anton R. Biomarkers as aids to identification of relapse in alcoholic patients. In: Galanter M (ed). Recent developments in alcoholism: research on alcoholism treatment (vol. XVI). New York: Plenum Press (in press).
28. Anton RF, Lieber C, Tabakoff B, et al. Carbohydrate-deficient transferrin and gamma-glutamyltransferase for the detection and monitoring of alcohol use: results from a multi-site study. Alcohol Clin Exp Res 2002;26(8):1215-22.