User login
Contributors to Patient Care Mistakes
Patient safety can be understood in terms of the Swiss cheese model of systems accidents. This model implies that many holes must align before an adverse event occurs.1 The limitations on work hours instituted by the Accreditation Council for Graduate Medical Education (ACGME)2 sought to close one hole by reducing fatigue in residents. As programs comply with these regulations, new interventions are being implemented to limit resident hours. This has resulted in more handoffs of care and therefore less continuity. The ultimate result may be to increase patient care errors by opening up new holes, the opposite of the stated goal of this reform.
Some residency programs have reported on their experience with hour reductions, giving insight into residents' perceptions on the benefits and drawbacks of such interventions. Residents have reported concern about continuity of care after such interventions.37 However, some residents believed they provided better patient care after the interventions to reduce hours.8, 9 Few studies have actually documented changes in the incidence of adverse events or errors as a result of work hour limitations.10 One study conducted prior to implementation of the ACGME work hour rules demonstrated more complications in internal medicine patients after New York's Code 405 (a state regulation that limited resident work hours, similar to the ACGME rules) was implemented.11 In contrast, another study showed that errors committed by interns were reduced with scheduling changes that resulted in shorter shifts and reduced hours.12
Because residents are on the front lines of patient care, they are uniquely positioned to provide insight into the impact of the work hour rules on patient safety. We conducted this study to more fully understand the effect of the ACGME work hour limitations and other possible factors on patient care errors from the perspectives of internal medicine residents.
METHODS
Participants and Sites
All internal medicine residents and interns from 3 residency programs were recruited to participate in focus groups. We purposely chose programs based at diverse health care organizations. The first program was based at a university and had approximately 160 residents, who rotated at both the university hospital and the affiliated Veterans Affairs Medical Center (VAMC). The second program was based at a community teaching hospital and had approximately 65 residents. The third program was affiliated with a freestanding medical college and had approximately 95 residents, who rotated at a large, private tertiary‐care hospital and also at the affiliated VAMC. Each program had a different call structure (Table 1).
| Site | Call system on general medicine services |
|---|---|
| Community | Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. |
| Teams take call every fourth day. Interns stay overnight and leave on the postcall day by 1 PM. Junior or senior resident on team admits patients until 9 PM on call and returns at 7 AM postcall. Night float resident admits patients with on‐call interns from 9 PM until 7 AM. | |
| On postcall day team resident stays the entire day, addressing all postcall clinical issues and follow‐up. | |
| University | At primary teaching hospital and VA: |
| Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. | |
| Teams take call every fourth day. Interns stay overnight, whereas residents leave at 9 PM on call and return at 7 AM postcall. Night‐float resident admits with interns from 9 PMto midnight, and then interns admit by themselves after midnight. | |
| Day‐float resident present on postcall days to help team's senior resident finish the work. | |
| Freestanding medical college | At primary teaching hospital: |
| Six teams, each with 1 attending, 1 junior or senior resident, and 1 or 2 interns. | |
| Call is not as a team and is approximately every fifth day. Two residents and 3 interns take call overnight together. At VA hospital: | |
| Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. | |
| Teams take call every fourth day. One intern leaves at 9 PM on call and returns at 7 AM postcall; stays until 4 PM to cover team. |
Potential participants were recruited via E‐mail, which explained that the study was about common scenarios for patient care errors and how the ACGME work hour rules affected patient care and errors.
Design
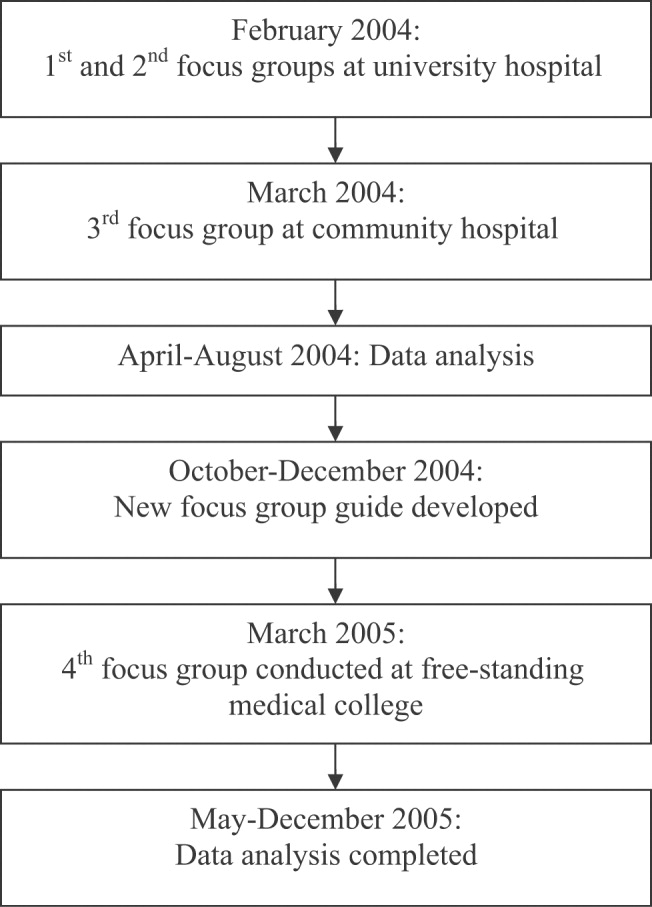
We conducted 4 focus groups in total (Appendix 1). The first 3 focus groups followed the same focus group guide, developed after a literature review. Focus groups 1 and 2 were conducted at the university‐based program. Focus group 3 was conducted at the community teaching hospitalaffiliated program. The first 3 focus groups were analyzed before the fourth focus group was conducted. A new focus group guide was developed for the fourth focus group to further explore themes identified in the first 3 focus groups (Fig. 1 and Appendix 2). The fourth focus group was conducted at the program affiliated with a freestanding medical college. All focus groups were audiotaped and transcribed verbatim. Each lasted approximately 90‐120 minutes.

Intervention
The focus group guide for the first 3 focus groups consisted of main questions and follow‐up prompts (Appendix 1). The focus group guide for the fourth focus group (Appendix 2) was developed based on themes from the first 3 focus groups, consistent with the iterative approach of grounded theory.13 Some of the questions were the same as in the first focus group guide; others were added to better understand the roles of faculty, teamwork, and inexperience in patient care errors.
Written informed consent was obtained before the focus groups began. Participants were paid $20 and given dinner. All internal medicine residents at the institutions included were eligible. The focus groups were held after work. Each focus group comprised participants from a single institution. The investigators who were the moderators were all junior faculty. They did not moderate the focus group at their own institution so as to minimize barriers to the residents' ability to speak freely about their experiences. The moderators prepared for their roles through discussion and assigned reading.14 The investigators used the focus group guide to ask questions of the group as a whole and facilitated the discussion that arose as a result. After each focus group, the moderator and assistant moderator debriefed each other about the important themes from the session.
Ethics
The institutional review boards at all sites approved this study.
Analysis
We used grounded theory to analyze the transcripts.15 Grounded theory is an iterative process that allows for themes to arise from the data.16 After the first 3 focus groups were completed, 5 of the investigators read all 3 transcripts at least twice and noted themes of interest in the text in a process of open coding.13 These investigators met in August 2004 to discuss the transcripts and the themes that had been identified by the individual investigators. A coding scheme of 33 codes was devised based on this meeting and the notes of individual investigators about the process of reading the transcripts. The need to conduct a fourth focus group to further explore certain issues was also identified. Two investigators (K.F., V.P.) independently coded the first 3 transcripts using the agreed‐on coding scheme. One investigator used NVivo (QSR International, Doncaster, Australia), an appropriate software package, and the other investigator coded by hand. During this process, 2 additional themes were identified. The 2 coders agreed on the need to add them, and they were incorporated into the coding scheme, yielding a total of 35 codes. Three of the investigators met again to begin constructing a model to represent the relationships among the themes. The model was developed iteratively over the following year by considering the most important themes, their relationships to one another, unifying concepts identified during the textual analysis, and team meetings. To provide additional validity, peer checking occurred. Specifically, iterations of the model were discussed by the team of investigators, in local research‐in‐progress sessions, with groups of residents at 2 of the participating institutions, and at national meetings. The fourth focus group was conducted at the third site in March 2005. The same 2 investigators applied the 35‐code scheme and determined that thematic saturation had occurred; that is, no new themes were identified.
Agreement between the 2 coders was evaluated by reviewing 15% of each transcript and dividing the number of agreed‐on codes by the total number of codes assigned to each section of text. The starting point of the text checked for agreement was chosen randomly. Agreement between the 2 coders for the first 3 focus groups was 43%, 48%, and 56%, respectively. The fourth focus group was analyzed a year later, and the initial agreement between the coders was 23%. After comparison and discussion, it was clear that 1 coder had coded many passages with more than 1 code, whereas the second coder had tried to choose the most pertinent code. The second coder recoded the transcript, and a new section was compared, resulting in agreement in 45% of that section. Discrepancies between the coders were resolved by consensus. None represented major differences of opinion; rather, they usually indicated the difficulty in choosing 1 primary code to fit an utterance that could be represented by several codes.
RESULTS
Twenty‐eight residents participated. Some of these residents had experience in the prework hour era, and some did not. Average age was 28 years (range 26‐33 years); 18 were women, and 11 were interns (Table 2). The focus groups ranged in size from 5 to 9. A sample of the codes and their definitions can be found in Table 3.
| Number of participants by site | |
| Community | 9 |
| University | 13 |
| Freestanding medical college | 6 |
| Age (years), mean | 28.5 |
| Sex (female), n (%) | 18 (64%) |
| Postgraduate year, n (%) | |
| Intern | 11 (39%) |
| Second year and above | 17 (61%) |
| Type of resident, n (%) | |
| Categorical | 23 (82%) |
| Codes | Definitions |
|---|---|
| Fatigue | How fatigue contributes to patient care problems. |
| How not being fatigued contributes to improved patient care. | |
| Workload | How workload issues (eg, patient complexity) may contribute to patient care problems. |
| Descriptions of times that workload was overwhelming: overextendedHave to be in 4 places at once. | |
| Entropy | Residents' descriptions of too much of everything (information, interruptions); house of cards. |
| How this chaos contributes to patient care problems. | |
| Being overwhelmed may be a facet. | |
| Not knowing own patients | Contributors to not knowing patients. |
| How not knowing patients affects patient care. | |
| Sign‐out/cross‐cover | Description of sign‐out practices, problems, and solutions. |
| Inexperience/lack of knowledge | How inexperience can contribute to patient care problems. |
| Challenges and attributes of delivering patient care in the setting of learning to deliver patient care. | |
| Personal well‐being | Discussions about residents lives, spouses, homes. |
| How this affects patient care. | |
| Continuity of doctor care | Examples of discontinuity. |
| How continuity and discontinuity contribute to patient care problems. | |
| Other aspects or attributes of continuity or discontinuity. | |
| Work hour rules as a goal | Examples of compliance with ACGME rules becoming a goal in itself and its impact on patient care |
The Model
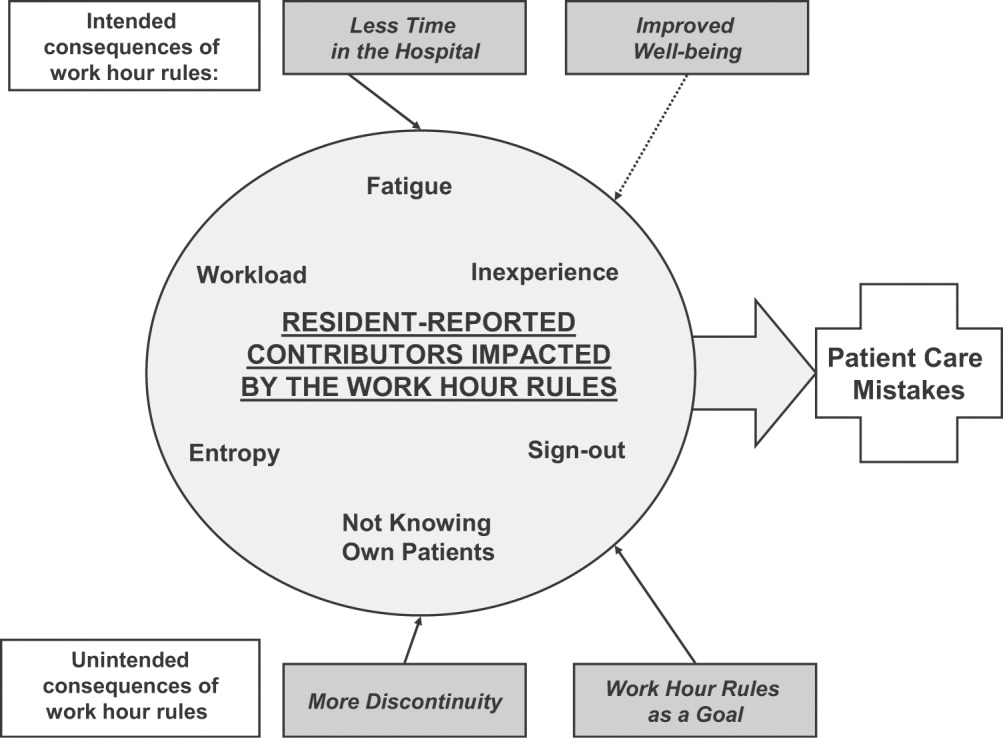
The model (Fig. 2) illustrates resident‐perceived contributors to patient care mistakes related to the ACGME work hour rules. These contributors are in the center circle. They include fatigue, inexperience, sign‐out, not knowing their own patients well enough, entropy (which we defined as the amount of chaos in the system), and workload. They are not listed in order of importance. The boxes outside the circle are consequences of the ACGME work hour rules and their perceived impact on the contributors to patient care mistakes. At the top are the intended consequences, that is the specific goals of the ACGME: less resident time in the hospital (ie, reduced hours) and improved well‐being.17 At the bottom are the unintended consequences: more patient care discontinuity and compliance with the work hour rules becoming a goal equally important to providing high‐quality patient care. Of these 4 consequences, only improved well‐being was viewed by the residents as decreasing patient care mistakes. The other consequences were cited by residents as sometimes increasing patient care errors. Because of the complexity of the model, several factors not directly related to resident work hours were identified in the analysis but are not shown in the model. They include faculty involvement and team work (usually positive influences), nurses and information technology (could be positive or negative), and late‐night/early‐morning hours (negative).
The quotations below illustrate the relationships between the consequences of the work hour rules, resident‐perceived contributors to patient care mistakes, and actual patient care.
Impact of Improved Well‐Being
Residents noted that improved well‐being resulting from the work hour rules could mitigate the impact of fatigue on patient care, as described by this resident who discussed late‐night admissions when on night float as opposed to on a regular call night. When I was night float, though, I was refreshed and more energized, and the patientI think got better care because I wasn't as tired andbasically could function better. So I think that's a good part about this year is that I'm not as toxic, and I think I can think betterand care more when I'm not so tired, and my own needs have been met, in terms of sleep and rest and being home and stuff
Residents often described tension between the benefits of being well rested and the benefits of continuity: I don't know how it affects patient care unless you sort of make a leap and say that people whohave better well‐being perform better. I don't know if that's true. Certainly, you could make the other argument and say if you're here all the time and miserable, and that's all you do, well, that's all you do. I'm not sure if maybe that's better. But I think for the physician when you compare them to lawyersany other field, engineers, architectsI think they sort of have a more well‐balanced life. So I think it is good for physician safety or their marriage safety. I'm not sure what it does with patient care.
Impact of Having Less Time in the Hospital
Having less time contributed to at least 2 factors, entropy and workload, as described in this passage: I think with the80‐hour system there is a total of at least 1 less senior in house, if not more at times, and I know that when I was doing the night float thing and then even when I was doing senior call once, all it takes is one sick patient that is too much for the intern alone to deal with,and it's all of a sudden 6 in the morning, and there are 3 other admissions that the other intern has done that the senior hasn't seen yet, and that happened to me more than once. One resident discussed the workload on inpatient services: I feel like I end up doing the same amount of work, but I have that much more pressure to do it all, and the notes are shorter, and you can't think through everything, and I actually find myself avoiding going in and talking to a family because I know that it is going to end up being a half‐hour conversation when all I really wanted to do was to communicate what the plan was, but I don't have a chance to because I know it is going to turn into a longer conversation, and I know I don't have time to do that and get out on time.
Impact of More Discontinuity
Discontinuity could also exacerbate contributors to patient care mistakes, especially through sign‐out/cross‐cover: I think continuity of care is very important, obviously, whenever there is transition of caring for a patient from one physician to another physicianthat information that gets transmitted from each other needs to be very well emphasized and clearly explained to the subsequent caretaker. And if that continuity of care is disrupted in some way, either through poor communication or lack of communication or a lot of different people having different responses to specific situations, that it can lead to [an] adverse event or medical errors like we just talked about.
Discontinuity also led to team members feeling they did not know their own patients well enough, which in turn could lead to mistakes in patient care. For example, residents described discharging patients on the wrong medications, overlooking important secondary problems, and failing to anticipate drug interactions. As a resident said: I feel you almost have to [do] another H and P [history and physical] on the people that came in overnight, especially if they're going to be in the hospital some time becausethe initial H and P and differentials oftentimes is going to change, and you have to be able to adjust to that.I would say there's definitely errors there, coming on and making decisions without knowing the nuances of the history and physical.So you essentially are making important decisions on patients you really don't know that well Another resident explained that the real problem with discontinuity was having inadequate time to get to know the patient: The thing I always think about as far as continuity isif you get a patient [transferred] to your care, how much time do you have which is allotted to you to get to know that patient? And actually, sometimes, I think that the continuity change in care is a good thing because you look at it through different eyes than the person before. So it really depends whether you have enough time to get to know them. On the other hand if you don't, then that's of course where errors I think occur.
Some also noted a sense of loss about not knowing their patients well: You have a sick patient at 1 o'clock, andyou have to turn their care over to your resident or the next intern who's on, and you know this patient best, they know you best, and you've got a relationship, and who knows? That patient might die in the next 12 hours, and you feel some sort of responsibility, but you're not allowed to stay and take care of them, and that kind of takes away a little bit of your autonomy and just like your spirit, I guess.
Impact of Having Compliance with Work Hour Rules Be a Goal
Some residents reported problems when the work hour rules became the primary goal of team members. I certainly have had some interns that I was supervising who made it clear that to them, the most important thing was getting out, and patient care maybe didn't even hit the list, explained one resident. That bothers me a lot because I think that then that focus has become too strict, and the rules have become too importantI mean, if patient care has to happen for whatever reasonthe patient's really sickthen there's enough flexibility to stay the half hour, hour; and I had an intern tell me that if she stayed the extra half hour that she would be over her 80 hours, and so she wasn't going to do it.
Having the rules as a goal affects the process of sign‐out, as explained by a resident, because they want us to track time in and time out and are really strict about sticking particularly to the 30‐hour portion of the rule, the 10 hours off between shifts, and I find that affecting patient care more than anything else because you feel like you can't stay that extra half an hour to wrap things up with a patient who you've been taking care of all night or to sit and talk with the family about something that came up overnight orto do accurate and adequate documentation of things in order to hand that off to the next team because you got to get out of there
DISCUSSION
We conducted this study to better understand why internal medicine residents thought patient care mistakes occurred; we were particularly interested in how they perceived the impact of certain aspects of the ACGME work hour rules on patient care mistakes. Designing systems that achieve compliance with the work hour rules while minimizing patient risk can best be accomplished by fully understanding why errors occur.
Our study revealed that in the opinion of some interns and residents, the work hour rules had consequences for patient care. Like any intervention, this one had both intended and unintended consequences.18 The ACGME has stated that improvement in residents' quality of life was an intended consequence,17 and the participants in our study reported that this had occurred. Despite uncertainty about the overall impact on patient outcomes, residents were glad to have more time away from the hospital.
Our respondents reported that not knowing patients well was a factor that contributed to patient care errors. It is intuitive that working fewer hours often results in more handoffs of care,19 a situation characterized by not knowing patients well. However, residents also identified not getting to know their own patients well as a factor that led to patient care mistakes because of (1) incomplete knowledge of a patient's status, (2) delays in diagnosis, and (3) errors in management. They also described feelings of professional disappointment and frustration at not being able to perform certain aspects of patient care (eg, family meetings) because of the hour limits and the inflexibility of the rules. As we strive to redefine professionalism in the setting of reduced work hours,20 this phenomenon should be addressed.
Sign‐out was identified as another contributor to patient care errors. The effectiveness of sign‐outs is a concern across medicine, and the Joint Commission on Accreditation of Healthcare Organizations made sign‐out procedures one of its priority areas in 2006.21 Much has been written about resident sign‐out, emphasizing the relationship between poor‐quality sign‐outs and patient safety.19, 22 However, barriers to effective sign‐out processes persist,23 even though standardized sign‐out strategies have been described.24, 25 Even in a rigorous study of work hours and patient safety, the computerized sign‐out template for the residents was rarely used.12 Cross‐coverage, or the patient care that occurs after sign‐out is complete, has also been linked to a greater likelihood of adverse events.26
Several factors not related to resident work hours were noted to often mitigate patient care mistakes. Physician teamwork, nursing, information technology (eg, computerized medical records), and faculty supervision were the most prominent. For example, the information technology available at the VA hospitals often helped to facilitate patient care, but it also provided an overwhelming amount of information to sift through. It was clear that the influence of some of these factors varied from institution to institution, reflecting the cultures of different programs.
Our results are consistent with those reported from previous studies. Striking a balance between preventing resident fatigue and preserving continuity of care has been debated since the ACGME announced changes to resident work hour limits.27 Resident quality of life generally improves and fatigue decreases with work hour limits in place,28 but patient safety remains a concern.10 Our findings corroborate the benefits of improved resident well‐being and the persistent concerns about patient safety, identified in a recently published study at a different institution.29 However, our findings expand on those reported in the literature by offering additional empirical evidence, albeit qualitative, about the way that residents see the relationships among the consequences of work hour rules, resident‐reported contributors to patient care mistakes, and the mistakes themselves.
Our study should be interpreted in the context of several limitations. First, the use of qualitative methods did not allow us to generalize or quantify our findings. However, we purposely included 3 diverse institutions with differing responses to the work hour rules to enhance the external validity of our findings. Second, the last focus group was conducted a year after the first 3; by that point, the work hour rules had been in place for 20 months. We believe that this was both a strength and a limitation because it allowed us to gain a perspective after some of the initial growing pains were over. This time lag also allowed for analysis of the first 3 transcripts so we could revise the focus group guide and ultimately determine that thematic saturation had occurred. In addition, few of our questions were phrased to evaluate the ACGME rules; instead, they asked about links among discontinuity, scheduling, fatigue, and patient care. We therefore believe that even residents who were not in the programs before the work hour rules began were still able to knowledgeably participate in the conversation. One question directly referable to the ACGME rules asked residents to reflect on problems arising from them. This could have led residents to only reflect on the problems associated with the rules. However, in all 4 focus groups, residents commented on the positive impact of improved well‐being resulting from the work hour rules. This led us to believe the respondents felt they could voice their favorable feelings as well as their unfavorable feelings about the rules. An additional limitation is that the agreement between coders was only 45%. It is important to realize that assessing coding agreement in qualitative work is quite difficult because it is often difficult to assign a single code to a section of text. When the coders discussed a disagreement, it was almost always the case that the difference was subtle and that the coding of either investigator would made sense for that text. Finally, our results are based on the participation of 28 residents. To be certain we were not representing the opinions of only a few people, we presented iterations of this model to faculty and resident groups for their feedback. Importantly, the residents offered no substantial changes or criticisms of the model.
Limitations notwithstanding, we believe our findings have important policy implications. First, despite work hours successfully being reduced, residents perceived no decrease in the amount of work they did. This resulted in higher workload and more entropy, suggesting that residency programs may need to carefully evaluate the patient care responsibility carried by residents. Second, continued effort to educate residents to provide effective sign‐out is needed. As one participant pointed out, residency offers a unique opportunity to learn to manage discontinuity in a controlled setting. Another educational opportunity is the chance to teach physician teamwork. Participants believed that effective teamwork could ameliorate some of the discontinuity in patient care. This teamwork training should include faculty as well, although further work is needed to define how faculty can best add to patient continuity while still fostering resident autonomy. Finally, the impact of work hour rules on the professional development of residents should be further explored.
In conclusion, we have proposed a model to explain the major resident‐reported contributors to patient care mistakes with respect to resident work hour rules. Our results help to clarify the next steps needed: testing the proposed relationships between the factors and patient care mistakes and rigorously evaluating solutions that minimize the impact of these factors. Returning to the Swiss cheese framework for describing systems accidents, our results suggest that although resident work hour reductions may have sufficiently filled the hole caused by resident fatigue, other gaps may have actually widened as a result of the systems put into place to achieve compliance. Continued vigilance is therefore necessary to both identify the additional holes likely to appear and, more importantly, effectively close those holes before patient harm occurs.
Appendix
APPENDIX 1.
INITIAL FOCUS GROUP GUIDE (FOCUS GROUPS 13)
How would you define the following:
A medical error?
An adverse patient event?
The IOM definition of a medical error is the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim (IOM report summary). From this point on, let us try to use this definition when we refer to errors.
What is the impact of continuity of care on medical errors, mistakes, or adverse outcomes?
Team versus individual continuity.
What are some settings at the hospitals where you work in which you have seen mistakes, errors, or bad outcomes in patient care?
Time of day?
Day in call cycle?
Other factors?
What types of mistakes, errors, or bad outcomes do you notice with patient care at the hospitals where you work? Please describe.
What are the things that contribute to patient‐related mistakes, errors, or bad outcomes at the hospitals where you work? (If needed, some prompts include)
How does fatigue contribute?
How do days off or lack of days off contribute?
What are the effects of nurses?
What types of mistakes, errors, or bad outcomes have you noticed with transitions in care (eg, sign‐outs, cross‐coverage) in your patients at the hospitals where you work? Please describe.
How has technology impacted errors, mistakes, and adverse outcomes?
PDA.
Computer access.
Computer‐order entry (if applicable).
What problems have you seen with the new ACGME regulations on work hours at the hospitals where you work?
What are some possible solutions?
Appendix
APPENDIX 2.
FOCUS GROUP GUIDE (4TH FOCUS GROUP)
The IOM definition of a medical error is the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim.
Please describe the call structure at each institution where you do ward months (eg, non‐ICU months).
What are some settings at the hospitals where you work where you have seen medical errors, mistakes, or adverse outcomes?
How do you think that other nurses influence the occurrence of medical errors, mistakes, or adverse outcomes?
Clerks?
Other ancillary staff?
How would you describe the responsibilities of a cross‐covering resident or intern?
How do you think continuity of care impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
What role do sign‐outs have?
How do you think that fatigue impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
How do you think that technology such as computerized physician order entry impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
Electronic medical records?
Palm pilots?
Is there such a thing as too much information?
How do you think that experience (or inexperience) impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
Please describe how attendings supervise you when you are on a ward team. How do you think that attending supervision impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
What about resident supervision of interns?
What is the ideal role of an attending on a team?
Can you think of a time when having attending input changed the plans or the course of a patient in a major way, good, bad, or neutral?
How do you think that time of day impacts patient care in terms of in terms of medical errors, mistakes, or adverse outcomes?
How comfortable do you feel calling for help at night? What makes you more or less likely to do it (personal attributes of person to be called, situation, etc.)?
What do you think is an ideal workload? (eg, How many complex patients are typical of your hospitals?) Does that vary from the VA to St. Joe's to Froedtert? How many patients should be admitted in 1 night by an intern? How many should an intern have ongoing responsibility for? Is there such a thing as too few patients?
If one of your family members were to admitted to your hospital at night with a life‐threatening condition, which situation would you prefer for their care (all other things being equal): admission by night float with handoff to a new but well‐rested resident or admission by a resident who then continues to care for that family member the next day but has been awake for 24 hours, admitting and cross‐covering other patients? Why?
What do you think was the intent of the ACGME rules? Do you think that those goals have been accomplished? Why or why not? How have they affected you as residents? How do you think that the ACGME work hour rules have influenced patient care?
- .Human error: Models and management.Br Med J.2000;320:768–770.
- ,,,ACGME Work Group on Resident Duty Hours,Accreditation Council for Graduate Medical Education.New requirements for resident duty hours.JAMA.2002;288:1112–1114.
- ,,,.The effect of the New York State restrictions on resident work hours.Obstet Gynecol.1991;78(3 Pt 1):468–473.
- ,,,.Impact of a night float system on internal medicine residency programs.Acad Med.1991;66:370.
- .Coping with pressures in acute medicine. The Royal College of Physicians Consultant Questionnaire Survey.J R Coll Physicians Lond.1998;32:211–218.
- ,,.New York regulation of residents' working conditions. 1 year's experience.Am J Dis Child.1990;144:799–802.
- ,,,,.Senior house officers in medicine: Postal survey of training and work experience.Br Med J.1997;314:740–743.
- ,,,.Resident and faculty evaluations of a psychiatry night‐float system.Acad Psychiatry.1996;20(1):26–34.
- ,,,.Doctors as workers: work‐hour regulations and interns' perceptions of responsibility, quality of care, and training.J Gen Intern Med.1993;8:429–435.
- ,,,,,.Systematic review: effects of resident work hours on patient safety [review] [39 refs].Ann Intern Med.2004;141:851–857.
- ,,,.The impact of a regulation restricting medical house staff working hours on the quality of patient care.JAMA.1993;269:374–378.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units [see comment].N Engl J Med.2004;351:1838–1848.
- .Qualitative Inquiry and Research Design: Choosing among Five Traditions.Thousand Oaks, CA:Sage Publications, Inc.;1998.
- .Moderating Focus Groups.Thousand Oaks, CA:Sage Publications;1998.
- ,.The Discovery of Grounded Theory: Strategies for Qualitative Research.Chicago, IL:Aldine Publishing Company;1967.
- ,. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Vol.2.Thousand Oaks, CA:Sage Publications;1998.
- ACGME. Statement of Justification/Impact for the Final Approval of Common Standards Related to Resident Duty Hours. Available at: http://www.acgme.org/DutyHours/impactStatement.pdf.Accessed February 21,2003.
- ,.Program Evaluation: Alternative Approaches and Practical Guidelines.New York, NY:Longman;1997.
- . Fumbled handoff. Web M117:846–850.
- Helpful solutions for meeting the 2006 national patient safety goals.Jt Comm Perspect Patient Saf.2005;5(8):1–20.
- .Fumbled handoffs: one dropped ball after another.Ann Intern Med.2005;142:352–358.
- ,,,.Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs.Acad Med.2005;80:1094–1099.
- .Handling handoffs safely.Am J Matern Child Nurs.2005;30(2):152.
- ,,,,.Handoff strategies in settings with high consequences for failure: lessons for health care operations.Int J Qual Health Care.2004;16(2):125–132.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121:866–872.
- ,,.Balancing continuity of care with residents' limited work hours: defining the implications.Acad Med.2005;80(1):39–43.
- ,,,,,.Effects of work hour reduction on residents' lives: a systematic review.JAMA.2005;294:1088–1100.
- ,,.Residents' perceptions of the effects of work hour limitations at a large teaching hospital.Acad Med.2006;81(1):63–67.
Patient safety can be understood in terms of the Swiss cheese model of systems accidents. This model implies that many holes must align before an adverse event occurs.1 The limitations on work hours instituted by the Accreditation Council for Graduate Medical Education (ACGME)2 sought to close one hole by reducing fatigue in residents. As programs comply with these regulations, new interventions are being implemented to limit resident hours. This has resulted in more handoffs of care and therefore less continuity. The ultimate result may be to increase patient care errors by opening up new holes, the opposite of the stated goal of this reform.
Some residency programs have reported on their experience with hour reductions, giving insight into residents' perceptions on the benefits and drawbacks of such interventions. Residents have reported concern about continuity of care after such interventions.37 However, some residents believed they provided better patient care after the interventions to reduce hours.8, 9 Few studies have actually documented changes in the incidence of adverse events or errors as a result of work hour limitations.10 One study conducted prior to implementation of the ACGME work hour rules demonstrated more complications in internal medicine patients after New York's Code 405 (a state regulation that limited resident work hours, similar to the ACGME rules) was implemented.11 In contrast, another study showed that errors committed by interns were reduced with scheduling changes that resulted in shorter shifts and reduced hours.12
Because residents are on the front lines of patient care, they are uniquely positioned to provide insight into the impact of the work hour rules on patient safety. We conducted this study to more fully understand the effect of the ACGME work hour limitations and other possible factors on patient care errors from the perspectives of internal medicine residents.
METHODS
Participants and Sites
All internal medicine residents and interns from 3 residency programs were recruited to participate in focus groups. We purposely chose programs based at diverse health care organizations. The first program was based at a university and had approximately 160 residents, who rotated at both the university hospital and the affiliated Veterans Affairs Medical Center (VAMC). The second program was based at a community teaching hospital and had approximately 65 residents. The third program was affiliated with a freestanding medical college and had approximately 95 residents, who rotated at a large, private tertiary‐care hospital and also at the affiliated VAMC. Each program had a different call structure (Table 1).
| Site | Call system on general medicine services |
|---|---|
| Community | Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. |
| Teams take call every fourth day. Interns stay overnight and leave on the postcall day by 1 PM. Junior or senior resident on team admits patients until 9 PM on call and returns at 7 AM postcall. Night float resident admits patients with on‐call interns from 9 PM until 7 AM. | |
| On postcall day team resident stays the entire day, addressing all postcall clinical issues and follow‐up. | |
| University | At primary teaching hospital and VA: |
| Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. | |
| Teams take call every fourth day. Interns stay overnight, whereas residents leave at 9 PM on call and return at 7 AM postcall. Night‐float resident admits with interns from 9 PMto midnight, and then interns admit by themselves after midnight. | |
| Day‐float resident present on postcall days to help team's senior resident finish the work. | |
| Freestanding medical college | At primary teaching hospital: |
| Six teams, each with 1 attending, 1 junior or senior resident, and 1 or 2 interns. | |
| Call is not as a team and is approximately every fifth day. Two residents and 3 interns take call overnight together. At VA hospital: | |
| Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. | |
| Teams take call every fourth day. One intern leaves at 9 PM on call and returns at 7 AM postcall; stays until 4 PM to cover team. |
Potential participants were recruited via E‐mail, which explained that the study was about common scenarios for patient care errors and how the ACGME work hour rules affected patient care and errors.
Design
We conducted 4 focus groups in total (Appendix 1). The first 3 focus groups followed the same focus group guide, developed after a literature review. Focus groups 1 and 2 were conducted at the university‐based program. Focus group 3 was conducted at the community teaching hospitalaffiliated program. The first 3 focus groups were analyzed before the fourth focus group was conducted. A new focus group guide was developed for the fourth focus group to further explore themes identified in the first 3 focus groups (Fig. 1 and Appendix 2). The fourth focus group was conducted at the program affiliated with a freestanding medical college. All focus groups were audiotaped and transcribed verbatim. Each lasted approximately 90‐120 minutes.

Intervention
The focus group guide for the first 3 focus groups consisted of main questions and follow‐up prompts (Appendix 1). The focus group guide for the fourth focus group (Appendix 2) was developed based on themes from the first 3 focus groups, consistent with the iterative approach of grounded theory.13 Some of the questions were the same as in the first focus group guide; others were added to better understand the roles of faculty, teamwork, and inexperience in patient care errors.
Written informed consent was obtained before the focus groups began. Participants were paid $20 and given dinner. All internal medicine residents at the institutions included were eligible. The focus groups were held after work. Each focus group comprised participants from a single institution. The investigators who were the moderators were all junior faculty. They did not moderate the focus group at their own institution so as to minimize barriers to the residents' ability to speak freely about their experiences. The moderators prepared for their roles through discussion and assigned reading.14 The investigators used the focus group guide to ask questions of the group as a whole and facilitated the discussion that arose as a result. After each focus group, the moderator and assistant moderator debriefed each other about the important themes from the session.
Ethics
The institutional review boards at all sites approved this study.
Analysis
We used grounded theory to analyze the transcripts.15 Grounded theory is an iterative process that allows for themes to arise from the data.16 After the first 3 focus groups were completed, 5 of the investigators read all 3 transcripts at least twice and noted themes of interest in the text in a process of open coding.13 These investigators met in August 2004 to discuss the transcripts and the themes that had been identified by the individual investigators. A coding scheme of 33 codes was devised based on this meeting and the notes of individual investigators about the process of reading the transcripts. The need to conduct a fourth focus group to further explore certain issues was also identified. Two investigators (K.F., V.P.) independently coded the first 3 transcripts using the agreed‐on coding scheme. One investigator used NVivo (QSR International, Doncaster, Australia), an appropriate software package, and the other investigator coded by hand. During this process, 2 additional themes were identified. The 2 coders agreed on the need to add them, and they were incorporated into the coding scheme, yielding a total of 35 codes. Three of the investigators met again to begin constructing a model to represent the relationships among the themes. The model was developed iteratively over the following year by considering the most important themes, their relationships to one another, unifying concepts identified during the textual analysis, and team meetings. To provide additional validity, peer checking occurred. Specifically, iterations of the model were discussed by the team of investigators, in local research‐in‐progress sessions, with groups of residents at 2 of the participating institutions, and at national meetings. The fourth focus group was conducted at the third site in March 2005. The same 2 investigators applied the 35‐code scheme and determined that thematic saturation had occurred; that is, no new themes were identified.
Agreement between the 2 coders was evaluated by reviewing 15% of each transcript and dividing the number of agreed‐on codes by the total number of codes assigned to each section of text. The starting point of the text checked for agreement was chosen randomly. Agreement between the 2 coders for the first 3 focus groups was 43%, 48%, and 56%, respectively. The fourth focus group was analyzed a year later, and the initial agreement between the coders was 23%. After comparison and discussion, it was clear that 1 coder had coded many passages with more than 1 code, whereas the second coder had tried to choose the most pertinent code. The second coder recoded the transcript, and a new section was compared, resulting in agreement in 45% of that section. Discrepancies between the coders were resolved by consensus. None represented major differences of opinion; rather, they usually indicated the difficulty in choosing 1 primary code to fit an utterance that could be represented by several codes.
RESULTS
Twenty‐eight residents participated. Some of these residents had experience in the prework hour era, and some did not. Average age was 28 years (range 26‐33 years); 18 were women, and 11 were interns (Table 2). The focus groups ranged in size from 5 to 9. A sample of the codes and their definitions can be found in Table 3.
| Number of participants by site | |
| Community | 9 |
| University | 13 |
| Freestanding medical college | 6 |
| Age (years), mean | 28.5 |
| Sex (female), n (%) | 18 (64%) |
| Postgraduate year, n (%) | |
| Intern | 11 (39%) |
| Second year and above | 17 (61%) |
| Type of resident, n (%) | |
| Categorical | 23 (82%) |
| Codes | Definitions |
|---|---|
| Fatigue | How fatigue contributes to patient care problems. |
| How not being fatigued contributes to improved patient care. | |
| Workload | How workload issues (eg, patient complexity) may contribute to patient care problems. |
| Descriptions of times that workload was overwhelming: overextendedHave to be in 4 places at once. | |
| Entropy | Residents' descriptions of too much of everything (information, interruptions); house of cards. |
| How this chaos contributes to patient care problems. | |
| Being overwhelmed may be a facet. | |
| Not knowing own patients | Contributors to not knowing patients. |
| How not knowing patients affects patient care. | |
| Sign‐out/cross‐cover | Description of sign‐out practices, problems, and solutions. |
| Inexperience/lack of knowledge | How inexperience can contribute to patient care problems. |
| Challenges and attributes of delivering patient care in the setting of learning to deliver patient care. | |
| Personal well‐being | Discussions about residents lives, spouses, homes. |
| How this affects patient care. | |
| Continuity of doctor care | Examples of discontinuity. |
| How continuity and discontinuity contribute to patient care problems. | |
| Other aspects or attributes of continuity or discontinuity. | |
| Work hour rules as a goal | Examples of compliance with ACGME rules becoming a goal in itself and its impact on patient care |
The Model
The model (Fig. 2) illustrates resident‐perceived contributors to patient care mistakes related to the ACGME work hour rules. These contributors are in the center circle. They include fatigue, inexperience, sign‐out, not knowing their own patients well enough, entropy (which we defined as the amount of chaos in the system), and workload. They are not listed in order of importance. The boxes outside the circle are consequences of the ACGME work hour rules and their perceived impact on the contributors to patient care mistakes. At the top are the intended consequences, that is the specific goals of the ACGME: less resident time in the hospital (ie, reduced hours) and improved well‐being.17 At the bottom are the unintended consequences: more patient care discontinuity and compliance with the work hour rules becoming a goal equally important to providing high‐quality patient care. Of these 4 consequences, only improved well‐being was viewed by the residents as decreasing patient care mistakes. The other consequences were cited by residents as sometimes increasing patient care errors. Because of the complexity of the model, several factors not directly related to resident work hours were identified in the analysis but are not shown in the model. They include faculty involvement and team work (usually positive influences), nurses and information technology (could be positive or negative), and late‐night/early‐morning hours (negative).

The quotations below illustrate the relationships between the consequences of the work hour rules, resident‐perceived contributors to patient care mistakes, and actual patient care.
Impact of Improved Well‐Being
Residents noted that improved well‐being resulting from the work hour rules could mitigate the impact of fatigue on patient care, as described by this resident who discussed late‐night admissions when on night float as opposed to on a regular call night. When I was night float, though, I was refreshed and more energized, and the patientI think got better care because I wasn't as tired andbasically could function better. So I think that's a good part about this year is that I'm not as toxic, and I think I can think betterand care more when I'm not so tired, and my own needs have been met, in terms of sleep and rest and being home and stuff
Residents often described tension between the benefits of being well rested and the benefits of continuity: I don't know how it affects patient care unless you sort of make a leap and say that people whohave better well‐being perform better. I don't know if that's true. Certainly, you could make the other argument and say if you're here all the time and miserable, and that's all you do, well, that's all you do. I'm not sure if maybe that's better. But I think for the physician when you compare them to lawyersany other field, engineers, architectsI think they sort of have a more well‐balanced life. So I think it is good for physician safety or their marriage safety. I'm not sure what it does with patient care.
Impact of Having Less Time in the Hospital
Having less time contributed to at least 2 factors, entropy and workload, as described in this passage: I think with the80‐hour system there is a total of at least 1 less senior in house, if not more at times, and I know that when I was doing the night float thing and then even when I was doing senior call once, all it takes is one sick patient that is too much for the intern alone to deal with,and it's all of a sudden 6 in the morning, and there are 3 other admissions that the other intern has done that the senior hasn't seen yet, and that happened to me more than once. One resident discussed the workload on inpatient services: I feel like I end up doing the same amount of work, but I have that much more pressure to do it all, and the notes are shorter, and you can't think through everything, and I actually find myself avoiding going in and talking to a family because I know that it is going to end up being a half‐hour conversation when all I really wanted to do was to communicate what the plan was, but I don't have a chance to because I know it is going to turn into a longer conversation, and I know I don't have time to do that and get out on time.
Impact of More Discontinuity
Discontinuity could also exacerbate contributors to patient care mistakes, especially through sign‐out/cross‐cover: I think continuity of care is very important, obviously, whenever there is transition of caring for a patient from one physician to another physicianthat information that gets transmitted from each other needs to be very well emphasized and clearly explained to the subsequent caretaker. And if that continuity of care is disrupted in some way, either through poor communication or lack of communication or a lot of different people having different responses to specific situations, that it can lead to [an] adverse event or medical errors like we just talked about.
Discontinuity also led to team members feeling they did not know their own patients well enough, which in turn could lead to mistakes in patient care. For example, residents described discharging patients on the wrong medications, overlooking important secondary problems, and failing to anticipate drug interactions. As a resident said: I feel you almost have to [do] another H and P [history and physical] on the people that came in overnight, especially if they're going to be in the hospital some time becausethe initial H and P and differentials oftentimes is going to change, and you have to be able to adjust to that.I would say there's definitely errors there, coming on and making decisions without knowing the nuances of the history and physical.So you essentially are making important decisions on patients you really don't know that well Another resident explained that the real problem with discontinuity was having inadequate time to get to know the patient: The thing I always think about as far as continuity isif you get a patient [transferred] to your care, how much time do you have which is allotted to you to get to know that patient? And actually, sometimes, I think that the continuity change in care is a good thing because you look at it through different eyes than the person before. So it really depends whether you have enough time to get to know them. On the other hand if you don't, then that's of course where errors I think occur.
Some also noted a sense of loss about not knowing their patients well: You have a sick patient at 1 o'clock, andyou have to turn their care over to your resident or the next intern who's on, and you know this patient best, they know you best, and you've got a relationship, and who knows? That patient might die in the next 12 hours, and you feel some sort of responsibility, but you're not allowed to stay and take care of them, and that kind of takes away a little bit of your autonomy and just like your spirit, I guess.
Impact of Having Compliance with Work Hour Rules Be a Goal
Some residents reported problems when the work hour rules became the primary goal of team members. I certainly have had some interns that I was supervising who made it clear that to them, the most important thing was getting out, and patient care maybe didn't even hit the list, explained one resident. That bothers me a lot because I think that then that focus has become too strict, and the rules have become too importantI mean, if patient care has to happen for whatever reasonthe patient's really sickthen there's enough flexibility to stay the half hour, hour; and I had an intern tell me that if she stayed the extra half hour that she would be over her 80 hours, and so she wasn't going to do it.
Having the rules as a goal affects the process of sign‐out, as explained by a resident, because they want us to track time in and time out and are really strict about sticking particularly to the 30‐hour portion of the rule, the 10 hours off between shifts, and I find that affecting patient care more than anything else because you feel like you can't stay that extra half an hour to wrap things up with a patient who you've been taking care of all night or to sit and talk with the family about something that came up overnight orto do accurate and adequate documentation of things in order to hand that off to the next team because you got to get out of there
DISCUSSION
We conducted this study to better understand why internal medicine residents thought patient care mistakes occurred; we were particularly interested in how they perceived the impact of certain aspects of the ACGME work hour rules on patient care mistakes. Designing systems that achieve compliance with the work hour rules while minimizing patient risk can best be accomplished by fully understanding why errors occur.
Our study revealed that in the opinion of some interns and residents, the work hour rules had consequences for patient care. Like any intervention, this one had both intended and unintended consequences.18 The ACGME has stated that improvement in residents' quality of life was an intended consequence,17 and the participants in our study reported that this had occurred. Despite uncertainty about the overall impact on patient outcomes, residents were glad to have more time away from the hospital.
Our respondents reported that not knowing patients well was a factor that contributed to patient care errors. It is intuitive that working fewer hours often results in more handoffs of care,19 a situation characterized by not knowing patients well. However, residents also identified not getting to know their own patients well as a factor that led to patient care mistakes because of (1) incomplete knowledge of a patient's status, (2) delays in diagnosis, and (3) errors in management. They also described feelings of professional disappointment and frustration at not being able to perform certain aspects of patient care (eg, family meetings) because of the hour limits and the inflexibility of the rules. As we strive to redefine professionalism in the setting of reduced work hours,20 this phenomenon should be addressed.
Sign‐out was identified as another contributor to patient care errors. The effectiveness of sign‐outs is a concern across medicine, and the Joint Commission on Accreditation of Healthcare Organizations made sign‐out procedures one of its priority areas in 2006.21 Much has been written about resident sign‐out, emphasizing the relationship between poor‐quality sign‐outs and patient safety.19, 22 However, barriers to effective sign‐out processes persist,23 even though standardized sign‐out strategies have been described.24, 25 Even in a rigorous study of work hours and patient safety, the computerized sign‐out template for the residents was rarely used.12 Cross‐coverage, or the patient care that occurs after sign‐out is complete, has also been linked to a greater likelihood of adverse events.26
Several factors not related to resident work hours were noted to often mitigate patient care mistakes. Physician teamwork, nursing, information technology (eg, computerized medical records), and faculty supervision were the most prominent. For example, the information technology available at the VA hospitals often helped to facilitate patient care, but it also provided an overwhelming amount of information to sift through. It was clear that the influence of some of these factors varied from institution to institution, reflecting the cultures of different programs.
Our results are consistent with those reported from previous studies. Striking a balance between preventing resident fatigue and preserving continuity of care has been debated since the ACGME announced changes to resident work hour limits.27 Resident quality of life generally improves and fatigue decreases with work hour limits in place,28 but patient safety remains a concern.10 Our findings corroborate the benefits of improved resident well‐being and the persistent concerns about patient safety, identified in a recently published study at a different institution.29 However, our findings expand on those reported in the literature by offering additional empirical evidence, albeit qualitative, about the way that residents see the relationships among the consequences of work hour rules, resident‐reported contributors to patient care mistakes, and the mistakes themselves.
Our study should be interpreted in the context of several limitations. First, the use of qualitative methods did not allow us to generalize or quantify our findings. However, we purposely included 3 diverse institutions with differing responses to the work hour rules to enhance the external validity of our findings. Second, the last focus group was conducted a year after the first 3; by that point, the work hour rules had been in place for 20 months. We believe that this was both a strength and a limitation because it allowed us to gain a perspective after some of the initial growing pains were over. This time lag also allowed for analysis of the first 3 transcripts so we could revise the focus group guide and ultimately determine that thematic saturation had occurred. In addition, few of our questions were phrased to evaluate the ACGME rules; instead, they asked about links among discontinuity, scheduling, fatigue, and patient care. We therefore believe that even residents who were not in the programs before the work hour rules began were still able to knowledgeably participate in the conversation. One question directly referable to the ACGME rules asked residents to reflect on problems arising from them. This could have led residents to only reflect on the problems associated with the rules. However, in all 4 focus groups, residents commented on the positive impact of improved well‐being resulting from the work hour rules. This led us to believe the respondents felt they could voice their favorable feelings as well as their unfavorable feelings about the rules. An additional limitation is that the agreement between coders was only 45%. It is important to realize that assessing coding agreement in qualitative work is quite difficult because it is often difficult to assign a single code to a section of text. When the coders discussed a disagreement, it was almost always the case that the difference was subtle and that the coding of either investigator would made sense for that text. Finally, our results are based on the participation of 28 residents. To be certain we were not representing the opinions of only a few people, we presented iterations of this model to faculty and resident groups for their feedback. Importantly, the residents offered no substantial changes or criticisms of the model.
Limitations notwithstanding, we believe our findings have important policy implications. First, despite work hours successfully being reduced, residents perceived no decrease in the amount of work they did. This resulted in higher workload and more entropy, suggesting that residency programs may need to carefully evaluate the patient care responsibility carried by residents. Second, continued effort to educate residents to provide effective sign‐out is needed. As one participant pointed out, residency offers a unique opportunity to learn to manage discontinuity in a controlled setting. Another educational opportunity is the chance to teach physician teamwork. Participants believed that effective teamwork could ameliorate some of the discontinuity in patient care. This teamwork training should include faculty as well, although further work is needed to define how faculty can best add to patient continuity while still fostering resident autonomy. Finally, the impact of work hour rules on the professional development of residents should be further explored.
In conclusion, we have proposed a model to explain the major resident‐reported contributors to patient care mistakes with respect to resident work hour rules. Our results help to clarify the next steps needed: testing the proposed relationships between the factors and patient care mistakes and rigorously evaluating solutions that minimize the impact of these factors. Returning to the Swiss cheese framework for describing systems accidents, our results suggest that although resident work hour reductions may have sufficiently filled the hole caused by resident fatigue, other gaps may have actually widened as a result of the systems put into place to achieve compliance. Continued vigilance is therefore necessary to both identify the additional holes likely to appear and, more importantly, effectively close those holes before patient harm occurs.
Appendix
APPENDIX 1.
INITIAL FOCUS GROUP GUIDE (FOCUS GROUPS 13)
How would you define the following:
A medical error?
An adverse patient event?
The IOM definition of a medical error is the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim (IOM report summary). From this point on, let us try to use this definition when we refer to errors.
What is the impact of continuity of care on medical errors, mistakes, or adverse outcomes?
Team versus individual continuity.
What are some settings at the hospitals where you work in which you have seen mistakes, errors, or bad outcomes in patient care?
Time of day?
Day in call cycle?
Other factors?
What types of mistakes, errors, or bad outcomes do you notice with patient care at the hospitals where you work? Please describe.
What are the things that contribute to patient‐related mistakes, errors, or bad outcomes at the hospitals where you work? (If needed, some prompts include)
How does fatigue contribute?
How do days off or lack of days off contribute?
What are the effects of nurses?
What types of mistakes, errors, or bad outcomes have you noticed with transitions in care (eg, sign‐outs, cross‐coverage) in your patients at the hospitals where you work? Please describe.
How has technology impacted errors, mistakes, and adverse outcomes?
PDA.
Computer access.
Computer‐order entry (if applicable).
What problems have you seen with the new ACGME regulations on work hours at the hospitals where you work?
What are some possible solutions?
Appendix
APPENDIX 2.
FOCUS GROUP GUIDE (4TH FOCUS GROUP)
The IOM definition of a medical error is the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim.
Please describe the call structure at each institution where you do ward months (eg, non‐ICU months).
What are some settings at the hospitals where you work where you have seen medical errors, mistakes, or adverse outcomes?
How do you think that other nurses influence the occurrence of medical errors, mistakes, or adverse outcomes?
Clerks?
Other ancillary staff?
How would you describe the responsibilities of a cross‐covering resident or intern?
How do you think continuity of care impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
What role do sign‐outs have?
How do you think that fatigue impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
How do you think that technology such as computerized physician order entry impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
Electronic medical records?
Palm pilots?
Is there such a thing as too much information?
How do you think that experience (or inexperience) impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
Please describe how attendings supervise you when you are on a ward team. How do you think that attending supervision impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
What about resident supervision of interns?
What is the ideal role of an attending on a team?
Can you think of a time when having attending input changed the plans or the course of a patient in a major way, good, bad, or neutral?
How do you think that time of day impacts patient care in terms of in terms of medical errors, mistakes, or adverse outcomes?
How comfortable do you feel calling for help at night? What makes you more or less likely to do it (personal attributes of person to be called, situation, etc.)?
What do you think is an ideal workload? (eg, How many complex patients are typical of your hospitals?) Does that vary from the VA to St. Joe's to Froedtert? How many patients should be admitted in 1 night by an intern? How many should an intern have ongoing responsibility for? Is there such a thing as too few patients?
If one of your family members were to admitted to your hospital at night with a life‐threatening condition, which situation would you prefer for their care (all other things being equal): admission by night float with handoff to a new but well‐rested resident or admission by a resident who then continues to care for that family member the next day but has been awake for 24 hours, admitting and cross‐covering other patients? Why?
What do you think was the intent of the ACGME rules? Do you think that those goals have been accomplished? Why or why not? How have they affected you as residents? How do you think that the ACGME work hour rules have influenced patient care?
Patient safety can be understood in terms of the Swiss cheese model of systems accidents. This model implies that many holes must align before an adverse event occurs.1 The limitations on work hours instituted by the Accreditation Council for Graduate Medical Education (ACGME)2 sought to close one hole by reducing fatigue in residents. As programs comply with these regulations, new interventions are being implemented to limit resident hours. This has resulted in more handoffs of care and therefore less continuity. The ultimate result may be to increase patient care errors by opening up new holes, the opposite of the stated goal of this reform.
Some residency programs have reported on their experience with hour reductions, giving insight into residents' perceptions on the benefits and drawbacks of such interventions. Residents have reported concern about continuity of care after such interventions.37 However, some residents believed they provided better patient care after the interventions to reduce hours.8, 9 Few studies have actually documented changes in the incidence of adverse events or errors as a result of work hour limitations.10 One study conducted prior to implementation of the ACGME work hour rules demonstrated more complications in internal medicine patients after New York's Code 405 (a state regulation that limited resident work hours, similar to the ACGME rules) was implemented.11 In contrast, another study showed that errors committed by interns were reduced with scheduling changes that resulted in shorter shifts and reduced hours.12
Because residents are on the front lines of patient care, they are uniquely positioned to provide insight into the impact of the work hour rules on patient safety. We conducted this study to more fully understand the effect of the ACGME work hour limitations and other possible factors on patient care errors from the perspectives of internal medicine residents.
METHODS
Participants and Sites
All internal medicine residents and interns from 3 residency programs were recruited to participate in focus groups. We purposely chose programs based at diverse health care organizations. The first program was based at a university and had approximately 160 residents, who rotated at both the university hospital and the affiliated Veterans Affairs Medical Center (VAMC). The second program was based at a community teaching hospital and had approximately 65 residents. The third program was affiliated with a freestanding medical college and had approximately 95 residents, who rotated at a large, private tertiary‐care hospital and also at the affiliated VAMC. Each program had a different call structure (Table 1).
| Site | Call system on general medicine services |
|---|---|
| Community | Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. |
| Teams take call every fourth day. Interns stay overnight and leave on the postcall day by 1 PM. Junior or senior resident on team admits patients until 9 PM on call and returns at 7 AM postcall. Night float resident admits patients with on‐call interns from 9 PM until 7 AM. | |
| On postcall day team resident stays the entire day, addressing all postcall clinical issues and follow‐up. | |
| University | At primary teaching hospital and VA: |
| Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. | |
| Teams take call every fourth day. Interns stay overnight, whereas residents leave at 9 PM on call and return at 7 AM postcall. Night‐float resident admits with interns from 9 PMto midnight, and then interns admit by themselves after midnight. | |
| Day‐float resident present on postcall days to help team's senior resident finish the work. | |
| Freestanding medical college | At primary teaching hospital: |
| Six teams, each with 1 attending, 1 junior or senior resident, and 1 or 2 interns. | |
| Call is not as a team and is approximately every fifth day. Two residents and 3 interns take call overnight together. At VA hospital: | |
| Four teams, each with 1 attending, 1 junior or senior resident, 2 interns. | |
| Teams take call every fourth day. One intern leaves at 9 PM on call and returns at 7 AM postcall; stays until 4 PM to cover team. |
Potential participants were recruited via E‐mail, which explained that the study was about common scenarios for patient care errors and how the ACGME work hour rules affected patient care and errors.
Design
We conducted 4 focus groups in total (Appendix 1). The first 3 focus groups followed the same focus group guide, developed after a literature review. Focus groups 1 and 2 were conducted at the university‐based program. Focus group 3 was conducted at the community teaching hospitalaffiliated program. The first 3 focus groups were analyzed before the fourth focus group was conducted. A new focus group guide was developed for the fourth focus group to further explore themes identified in the first 3 focus groups (Fig. 1 and Appendix 2). The fourth focus group was conducted at the program affiliated with a freestanding medical college. All focus groups were audiotaped and transcribed verbatim. Each lasted approximately 90‐120 minutes.

Intervention
The focus group guide for the first 3 focus groups consisted of main questions and follow‐up prompts (Appendix 1). The focus group guide for the fourth focus group (Appendix 2) was developed based on themes from the first 3 focus groups, consistent with the iterative approach of grounded theory.13 Some of the questions were the same as in the first focus group guide; others were added to better understand the roles of faculty, teamwork, and inexperience in patient care errors.
Written informed consent was obtained before the focus groups began. Participants were paid $20 and given dinner. All internal medicine residents at the institutions included were eligible. The focus groups were held after work. Each focus group comprised participants from a single institution. The investigators who were the moderators were all junior faculty. They did not moderate the focus group at their own institution so as to minimize barriers to the residents' ability to speak freely about their experiences. The moderators prepared for their roles through discussion and assigned reading.14 The investigators used the focus group guide to ask questions of the group as a whole and facilitated the discussion that arose as a result. After each focus group, the moderator and assistant moderator debriefed each other about the important themes from the session.
Ethics
The institutional review boards at all sites approved this study.
Analysis
We used grounded theory to analyze the transcripts.15 Grounded theory is an iterative process that allows for themes to arise from the data.16 After the first 3 focus groups were completed, 5 of the investigators read all 3 transcripts at least twice and noted themes of interest in the text in a process of open coding.13 These investigators met in August 2004 to discuss the transcripts and the themes that had been identified by the individual investigators. A coding scheme of 33 codes was devised based on this meeting and the notes of individual investigators about the process of reading the transcripts. The need to conduct a fourth focus group to further explore certain issues was also identified. Two investigators (K.F., V.P.) independently coded the first 3 transcripts using the agreed‐on coding scheme. One investigator used NVivo (QSR International, Doncaster, Australia), an appropriate software package, and the other investigator coded by hand. During this process, 2 additional themes were identified. The 2 coders agreed on the need to add them, and they were incorporated into the coding scheme, yielding a total of 35 codes. Three of the investigators met again to begin constructing a model to represent the relationships among the themes. The model was developed iteratively over the following year by considering the most important themes, their relationships to one another, unifying concepts identified during the textual analysis, and team meetings. To provide additional validity, peer checking occurred. Specifically, iterations of the model were discussed by the team of investigators, in local research‐in‐progress sessions, with groups of residents at 2 of the participating institutions, and at national meetings. The fourth focus group was conducted at the third site in March 2005. The same 2 investigators applied the 35‐code scheme and determined that thematic saturation had occurred; that is, no new themes were identified.
Agreement between the 2 coders was evaluated by reviewing 15% of each transcript and dividing the number of agreed‐on codes by the total number of codes assigned to each section of text. The starting point of the text checked for agreement was chosen randomly. Agreement between the 2 coders for the first 3 focus groups was 43%, 48%, and 56%, respectively. The fourth focus group was analyzed a year later, and the initial agreement between the coders was 23%. After comparison and discussion, it was clear that 1 coder had coded many passages with more than 1 code, whereas the second coder had tried to choose the most pertinent code. The second coder recoded the transcript, and a new section was compared, resulting in agreement in 45% of that section. Discrepancies between the coders were resolved by consensus. None represented major differences of opinion; rather, they usually indicated the difficulty in choosing 1 primary code to fit an utterance that could be represented by several codes.
RESULTS
Twenty‐eight residents participated. Some of these residents had experience in the prework hour era, and some did not. Average age was 28 years (range 26‐33 years); 18 were women, and 11 were interns (Table 2). The focus groups ranged in size from 5 to 9. A sample of the codes and their definitions can be found in Table 3.
| Number of participants by site | |
| Community | 9 |
| University | 13 |
| Freestanding medical college | 6 |
| Age (years), mean | 28.5 |
| Sex (female), n (%) | 18 (64%) |
| Postgraduate year, n (%) | |
| Intern | 11 (39%) |
| Second year and above | 17 (61%) |
| Type of resident, n (%) | |
| Categorical | 23 (82%) |
| Codes | Definitions |
|---|---|
| Fatigue | How fatigue contributes to patient care problems. |
| How not being fatigued contributes to improved patient care. | |
| Workload | How workload issues (eg, patient complexity) may contribute to patient care problems. |
| Descriptions of times that workload was overwhelming: overextendedHave to be in 4 places at once. | |
| Entropy | Residents' descriptions of too much of everything (information, interruptions); house of cards. |
| How this chaos contributes to patient care problems. | |
| Being overwhelmed may be a facet. | |
| Not knowing own patients | Contributors to not knowing patients. |
| How not knowing patients affects patient care. | |
| Sign‐out/cross‐cover | Description of sign‐out practices, problems, and solutions. |
| Inexperience/lack of knowledge | How inexperience can contribute to patient care problems. |
| Challenges and attributes of delivering patient care in the setting of learning to deliver patient care. | |
| Personal well‐being | Discussions about residents lives, spouses, homes. |
| How this affects patient care. | |
| Continuity of doctor care | Examples of discontinuity. |
| How continuity and discontinuity contribute to patient care problems. | |
| Other aspects or attributes of continuity or discontinuity. | |
| Work hour rules as a goal | Examples of compliance with ACGME rules becoming a goal in itself and its impact on patient care |
The Model
The model (Fig. 2) illustrates resident‐perceived contributors to patient care mistakes related to the ACGME work hour rules. These contributors are in the center circle. They include fatigue, inexperience, sign‐out, not knowing their own patients well enough, entropy (which we defined as the amount of chaos in the system), and workload. They are not listed in order of importance. The boxes outside the circle are consequences of the ACGME work hour rules and their perceived impact on the contributors to patient care mistakes. At the top are the intended consequences, that is the specific goals of the ACGME: less resident time in the hospital (ie, reduced hours) and improved well‐being.17 At the bottom are the unintended consequences: more patient care discontinuity and compliance with the work hour rules becoming a goal equally important to providing high‐quality patient care. Of these 4 consequences, only improved well‐being was viewed by the residents as decreasing patient care mistakes. The other consequences were cited by residents as sometimes increasing patient care errors. Because of the complexity of the model, several factors not directly related to resident work hours were identified in the analysis but are not shown in the model. They include faculty involvement and team work (usually positive influences), nurses and information technology (could be positive or negative), and late‐night/early‐morning hours (negative).

The quotations below illustrate the relationships between the consequences of the work hour rules, resident‐perceived contributors to patient care mistakes, and actual patient care.
Impact of Improved Well‐Being
Residents noted that improved well‐being resulting from the work hour rules could mitigate the impact of fatigue on patient care, as described by this resident who discussed late‐night admissions when on night float as opposed to on a regular call night. When I was night float, though, I was refreshed and more energized, and the patientI think got better care because I wasn't as tired andbasically could function better. So I think that's a good part about this year is that I'm not as toxic, and I think I can think betterand care more when I'm not so tired, and my own needs have been met, in terms of sleep and rest and being home and stuff
Residents often described tension between the benefits of being well rested and the benefits of continuity: I don't know how it affects patient care unless you sort of make a leap and say that people whohave better well‐being perform better. I don't know if that's true. Certainly, you could make the other argument and say if you're here all the time and miserable, and that's all you do, well, that's all you do. I'm not sure if maybe that's better. But I think for the physician when you compare them to lawyersany other field, engineers, architectsI think they sort of have a more well‐balanced life. So I think it is good for physician safety or their marriage safety. I'm not sure what it does with patient care.
Impact of Having Less Time in the Hospital
Having less time contributed to at least 2 factors, entropy and workload, as described in this passage: I think with the80‐hour system there is a total of at least 1 less senior in house, if not more at times, and I know that when I was doing the night float thing and then even when I was doing senior call once, all it takes is one sick patient that is too much for the intern alone to deal with,and it's all of a sudden 6 in the morning, and there are 3 other admissions that the other intern has done that the senior hasn't seen yet, and that happened to me more than once. One resident discussed the workload on inpatient services: I feel like I end up doing the same amount of work, but I have that much more pressure to do it all, and the notes are shorter, and you can't think through everything, and I actually find myself avoiding going in and talking to a family because I know that it is going to end up being a half‐hour conversation when all I really wanted to do was to communicate what the plan was, but I don't have a chance to because I know it is going to turn into a longer conversation, and I know I don't have time to do that and get out on time.
Impact of More Discontinuity
Discontinuity could also exacerbate contributors to patient care mistakes, especially through sign‐out/cross‐cover: I think continuity of care is very important, obviously, whenever there is transition of caring for a patient from one physician to another physicianthat information that gets transmitted from each other needs to be very well emphasized and clearly explained to the subsequent caretaker. And if that continuity of care is disrupted in some way, either through poor communication or lack of communication or a lot of different people having different responses to specific situations, that it can lead to [an] adverse event or medical errors like we just talked about.
Discontinuity also led to team members feeling they did not know their own patients well enough, which in turn could lead to mistakes in patient care. For example, residents described discharging patients on the wrong medications, overlooking important secondary problems, and failing to anticipate drug interactions. As a resident said: I feel you almost have to [do] another H and P [history and physical] on the people that came in overnight, especially if they're going to be in the hospital some time becausethe initial H and P and differentials oftentimes is going to change, and you have to be able to adjust to that.I would say there's definitely errors there, coming on and making decisions without knowing the nuances of the history and physical.So you essentially are making important decisions on patients you really don't know that well Another resident explained that the real problem with discontinuity was having inadequate time to get to know the patient: The thing I always think about as far as continuity isif you get a patient [transferred] to your care, how much time do you have which is allotted to you to get to know that patient? And actually, sometimes, I think that the continuity change in care is a good thing because you look at it through different eyes than the person before. So it really depends whether you have enough time to get to know them. On the other hand if you don't, then that's of course where errors I think occur.
Some also noted a sense of loss about not knowing their patients well: You have a sick patient at 1 o'clock, andyou have to turn their care over to your resident or the next intern who's on, and you know this patient best, they know you best, and you've got a relationship, and who knows? That patient might die in the next 12 hours, and you feel some sort of responsibility, but you're not allowed to stay and take care of them, and that kind of takes away a little bit of your autonomy and just like your spirit, I guess.
Impact of Having Compliance with Work Hour Rules Be a Goal
Some residents reported problems when the work hour rules became the primary goal of team members. I certainly have had some interns that I was supervising who made it clear that to them, the most important thing was getting out, and patient care maybe didn't even hit the list, explained one resident. That bothers me a lot because I think that then that focus has become too strict, and the rules have become too importantI mean, if patient care has to happen for whatever reasonthe patient's really sickthen there's enough flexibility to stay the half hour, hour; and I had an intern tell me that if she stayed the extra half hour that she would be over her 80 hours, and so she wasn't going to do it.
Having the rules as a goal affects the process of sign‐out, as explained by a resident, because they want us to track time in and time out and are really strict about sticking particularly to the 30‐hour portion of the rule, the 10 hours off between shifts, and I find that affecting patient care more than anything else because you feel like you can't stay that extra half an hour to wrap things up with a patient who you've been taking care of all night or to sit and talk with the family about something that came up overnight orto do accurate and adequate documentation of things in order to hand that off to the next team because you got to get out of there
DISCUSSION
We conducted this study to better understand why internal medicine residents thought patient care mistakes occurred; we were particularly interested in how they perceived the impact of certain aspects of the ACGME work hour rules on patient care mistakes. Designing systems that achieve compliance with the work hour rules while minimizing patient risk can best be accomplished by fully understanding why errors occur.
Our study revealed that in the opinion of some interns and residents, the work hour rules had consequences for patient care. Like any intervention, this one had both intended and unintended consequences.18 The ACGME has stated that improvement in residents' quality of life was an intended consequence,17 and the participants in our study reported that this had occurred. Despite uncertainty about the overall impact on patient outcomes, residents were glad to have more time away from the hospital.
Our respondents reported that not knowing patients well was a factor that contributed to patient care errors. It is intuitive that working fewer hours often results in more handoffs of care,19 a situation characterized by not knowing patients well. However, residents also identified not getting to know their own patients well as a factor that led to patient care mistakes because of (1) incomplete knowledge of a patient's status, (2) delays in diagnosis, and (3) errors in management. They also described feelings of professional disappointment and frustration at not being able to perform certain aspects of patient care (eg, family meetings) because of the hour limits and the inflexibility of the rules. As we strive to redefine professionalism in the setting of reduced work hours,20 this phenomenon should be addressed.
Sign‐out was identified as another contributor to patient care errors. The effectiveness of sign‐outs is a concern across medicine, and the Joint Commission on Accreditation of Healthcare Organizations made sign‐out procedures one of its priority areas in 2006.21 Much has been written about resident sign‐out, emphasizing the relationship between poor‐quality sign‐outs and patient safety.19, 22 However, barriers to effective sign‐out processes persist,23 even though standardized sign‐out strategies have been described.24, 25 Even in a rigorous study of work hours and patient safety, the computerized sign‐out template for the residents was rarely used.12 Cross‐coverage, or the patient care that occurs after sign‐out is complete, has also been linked to a greater likelihood of adverse events.26
Several factors not related to resident work hours were noted to often mitigate patient care mistakes. Physician teamwork, nursing, information technology (eg, computerized medical records), and faculty supervision were the most prominent. For example, the information technology available at the VA hospitals often helped to facilitate patient care, but it also provided an overwhelming amount of information to sift through. It was clear that the influence of some of these factors varied from institution to institution, reflecting the cultures of different programs.
Our results are consistent with those reported from previous studies. Striking a balance between preventing resident fatigue and preserving continuity of care has been debated since the ACGME announced changes to resident work hour limits.27 Resident quality of life generally improves and fatigue decreases with work hour limits in place,28 but patient safety remains a concern.10 Our findings corroborate the benefits of improved resident well‐being and the persistent concerns about patient safety, identified in a recently published study at a different institution.29 However, our findings expand on those reported in the literature by offering additional empirical evidence, albeit qualitative, about the way that residents see the relationships among the consequences of work hour rules, resident‐reported contributors to patient care mistakes, and the mistakes themselves.
Our study should be interpreted in the context of several limitations. First, the use of qualitative methods did not allow us to generalize or quantify our findings. However, we purposely included 3 diverse institutions with differing responses to the work hour rules to enhance the external validity of our findings. Second, the last focus group was conducted a year after the first 3; by that point, the work hour rules had been in place for 20 months. We believe that this was both a strength and a limitation because it allowed us to gain a perspective after some of the initial growing pains were over. This time lag also allowed for analysis of the first 3 transcripts so we could revise the focus group guide and ultimately determine that thematic saturation had occurred. In addition, few of our questions were phrased to evaluate the ACGME rules; instead, they asked about links among discontinuity, scheduling, fatigue, and patient care. We therefore believe that even residents who were not in the programs before the work hour rules began were still able to knowledgeably participate in the conversation. One question directly referable to the ACGME rules asked residents to reflect on problems arising from them. This could have led residents to only reflect on the problems associated with the rules. However, in all 4 focus groups, residents commented on the positive impact of improved well‐being resulting from the work hour rules. This led us to believe the respondents felt they could voice their favorable feelings as well as their unfavorable feelings about the rules. An additional limitation is that the agreement between coders was only 45%. It is important to realize that assessing coding agreement in qualitative work is quite difficult because it is often difficult to assign a single code to a section of text. When the coders discussed a disagreement, it was almost always the case that the difference was subtle and that the coding of either investigator would made sense for that text. Finally, our results are based on the participation of 28 residents. To be certain we were not representing the opinions of only a few people, we presented iterations of this model to faculty and resident groups for their feedback. Importantly, the residents offered no substantial changes or criticisms of the model.
Limitations notwithstanding, we believe our findings have important policy implications. First, despite work hours successfully being reduced, residents perceived no decrease in the amount of work they did. This resulted in higher workload and more entropy, suggesting that residency programs may need to carefully evaluate the patient care responsibility carried by residents. Second, continued effort to educate residents to provide effective sign‐out is needed. As one participant pointed out, residency offers a unique opportunity to learn to manage discontinuity in a controlled setting. Another educational opportunity is the chance to teach physician teamwork. Participants believed that effective teamwork could ameliorate some of the discontinuity in patient care. This teamwork training should include faculty as well, although further work is needed to define how faculty can best add to patient continuity while still fostering resident autonomy. Finally, the impact of work hour rules on the professional development of residents should be further explored.
In conclusion, we have proposed a model to explain the major resident‐reported contributors to patient care mistakes with respect to resident work hour rules. Our results help to clarify the next steps needed: testing the proposed relationships between the factors and patient care mistakes and rigorously evaluating solutions that minimize the impact of these factors. Returning to the Swiss cheese framework for describing systems accidents, our results suggest that although resident work hour reductions may have sufficiently filled the hole caused by resident fatigue, other gaps may have actually widened as a result of the systems put into place to achieve compliance. Continued vigilance is therefore necessary to both identify the additional holes likely to appear and, more importantly, effectively close those holes before patient harm occurs.
Appendix
APPENDIX 1.
INITIAL FOCUS GROUP GUIDE (FOCUS GROUPS 13)
How would you define the following:
A medical error?
An adverse patient event?
The IOM definition of a medical error is the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim (IOM report summary). From this point on, let us try to use this definition when we refer to errors.
What is the impact of continuity of care on medical errors, mistakes, or adverse outcomes?
Team versus individual continuity.
What are some settings at the hospitals where you work in which you have seen mistakes, errors, or bad outcomes in patient care?
Time of day?
Day in call cycle?
Other factors?
What types of mistakes, errors, or bad outcomes do you notice with patient care at the hospitals where you work? Please describe.
What are the things that contribute to patient‐related mistakes, errors, or bad outcomes at the hospitals where you work? (If needed, some prompts include)
How does fatigue contribute?
How do days off or lack of days off contribute?
What are the effects of nurses?
What types of mistakes, errors, or bad outcomes have you noticed with transitions in care (eg, sign‐outs, cross‐coverage) in your patients at the hospitals where you work? Please describe.
How has technology impacted errors, mistakes, and adverse outcomes?
PDA.
Computer access.
Computer‐order entry (if applicable).
What problems have you seen with the new ACGME regulations on work hours at the hospitals where you work?
What are some possible solutions?
Appendix
APPENDIX 2.
FOCUS GROUP GUIDE (4TH FOCUS GROUP)
The IOM definition of a medical error is the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim.
Please describe the call structure at each institution where you do ward months (eg, non‐ICU months).
What are some settings at the hospitals where you work where you have seen medical errors, mistakes, or adverse outcomes?
How do you think that other nurses influence the occurrence of medical errors, mistakes, or adverse outcomes?
Clerks?
Other ancillary staff?
How would you describe the responsibilities of a cross‐covering resident or intern?
How do you think continuity of care impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
What role do sign‐outs have?
How do you think that fatigue impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
How do you think that technology such as computerized physician order entry impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
Electronic medical records?
Palm pilots?
Is there such a thing as too much information?
How do you think that experience (or inexperience) impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
Please describe how attendings supervise you when you are on a ward team. How do you think that attending supervision impacts patient care in terms of medical errors, mistakes, or adverse outcomes?
What about resident supervision of interns?
What is the ideal role of an attending on a team?
Can you think of a time when having attending input changed the plans or the course of a patient in a major way, good, bad, or neutral?
How do you think that time of day impacts patient care in terms of in terms of medical errors, mistakes, or adverse outcomes?
How comfortable do you feel calling for help at night? What makes you more or less likely to do it (personal attributes of person to be called, situation, etc.)?
What do you think is an ideal workload? (eg, How many complex patients are typical of your hospitals?) Does that vary from the VA to St. Joe's to Froedtert? How many patients should be admitted in 1 night by an intern? How many should an intern have ongoing responsibility for? Is there such a thing as too few patients?
If one of your family members were to admitted to your hospital at night with a life‐threatening condition, which situation would you prefer for their care (all other things being equal): admission by night float with handoff to a new but well‐rested resident or admission by a resident who then continues to care for that family member the next day but has been awake for 24 hours, admitting and cross‐covering other patients? Why?
What do you think was the intent of the ACGME rules? Do you think that those goals have been accomplished? Why or why not? How have they affected you as residents? How do you think that the ACGME work hour rules have influenced patient care?
- .Human error: Models and management.Br Med J.2000;320:768–770.
- ,,,ACGME Work Group on Resident Duty Hours,Accreditation Council for Graduate Medical Education.New requirements for resident duty hours.JAMA.2002;288:1112–1114.
- ,,,.The effect of the New York State restrictions on resident work hours.Obstet Gynecol.1991;78(3 Pt 1):468–473.
- ,,,.Impact of a night float system on internal medicine residency programs.Acad Med.1991;66:370.
- .Coping with pressures in acute medicine. The Royal College of Physicians Consultant Questionnaire Survey.J R Coll Physicians Lond.1998;32:211–218.
- ,,.New York regulation of residents' working conditions. 1 year's experience.Am J Dis Child.1990;144:799–802.
- ,,,,.Senior house officers in medicine: Postal survey of training and work experience.Br Med J.1997;314:740–743.
- ,,,.Resident and faculty evaluations of a psychiatry night‐float system.Acad Psychiatry.1996;20(1):26–34.
- ,,,.Doctors as workers: work‐hour regulations and interns' perceptions of responsibility, quality of care, and training.J Gen Intern Med.1993;8:429–435.
- ,,,,,.Systematic review: effects of resident work hours on patient safety [review] [39 refs].Ann Intern Med.2004;141:851–857.
- ,,,.The impact of a regulation restricting medical house staff working hours on the quality of patient care.JAMA.1993;269:374–378.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units [see comment].N Engl J Med.2004;351:1838–1848.
- .Qualitative Inquiry and Research Design: Choosing among Five Traditions.Thousand Oaks, CA:Sage Publications, Inc.;1998.
- .Moderating Focus Groups.Thousand Oaks, CA:Sage Publications;1998.
- ,.The Discovery of Grounded Theory: Strategies for Qualitative Research.Chicago, IL:Aldine Publishing Company;1967.
- ,. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Vol.2.Thousand Oaks, CA:Sage Publications;1998.
- ACGME. Statement of Justification/Impact for the Final Approval of Common Standards Related to Resident Duty Hours. Available at: http://www.acgme.org/DutyHours/impactStatement.pdf.Accessed February 21,2003.
- ,.Program Evaluation: Alternative Approaches and Practical Guidelines.New York, NY:Longman;1997.
- . Fumbled handoff. Web M117:846–850.
- Helpful solutions for meeting the 2006 national patient safety goals.Jt Comm Perspect Patient Saf.2005;5(8):1–20.
- .Fumbled handoffs: one dropped ball after another.Ann Intern Med.2005;142:352–358.
- ,,,.Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs.Acad Med.2005;80:1094–1099.
- .Handling handoffs safely.Am J Matern Child Nurs.2005;30(2):152.
- ,,,,.Handoff strategies in settings with high consequences for failure: lessons for health care operations.Int J Qual Health Care.2004;16(2):125–132.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121:866–872.
- ,,.Balancing continuity of care with residents' limited work hours: defining the implications.Acad Med.2005;80(1):39–43.
- ,,,,,.Effects of work hour reduction on residents' lives: a systematic review.JAMA.2005;294:1088–1100.
- ,,.Residents' perceptions of the effects of work hour limitations at a large teaching hospital.Acad Med.2006;81(1):63–67.
- .Human error: Models and management.Br Med J.2000;320:768–770.
- ,,,ACGME Work Group on Resident Duty Hours,Accreditation Council for Graduate Medical Education.New requirements for resident duty hours.JAMA.2002;288:1112–1114.
- ,,,.The effect of the New York State restrictions on resident work hours.Obstet Gynecol.1991;78(3 Pt 1):468–473.
- ,,,.Impact of a night float system on internal medicine residency programs.Acad Med.1991;66:370.
- .Coping with pressures in acute medicine. The Royal College of Physicians Consultant Questionnaire Survey.J R Coll Physicians Lond.1998;32:211–218.
- ,,.New York regulation of residents' working conditions. 1 year's experience.Am J Dis Child.1990;144:799–802.
- ,,,,.Senior house officers in medicine: Postal survey of training and work experience.Br Med J.1997;314:740–743.
- ,,,.Resident and faculty evaluations of a psychiatry night‐float system.Acad Psychiatry.1996;20(1):26–34.
- ,,,.Doctors as workers: work‐hour regulations and interns' perceptions of responsibility, quality of care, and training.J Gen Intern Med.1993;8:429–435.
- ,,,,,.Systematic review: effects of resident work hours on patient safety [review] [39 refs].Ann Intern Med.2004;141:851–857.
- ,,,.The impact of a regulation restricting medical house staff working hours on the quality of patient care.JAMA.1993;269:374–378.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units [see comment].N Engl J Med.2004;351:1838–1848.
- .Qualitative Inquiry and Research Design: Choosing among Five Traditions.Thousand Oaks, CA:Sage Publications, Inc.;1998.
- .Moderating Focus Groups.Thousand Oaks, CA:Sage Publications;1998.
- ,.The Discovery of Grounded Theory: Strategies for Qualitative Research.Chicago, IL:Aldine Publishing Company;1967.
- ,. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Vol.2.Thousand Oaks, CA:Sage Publications;1998.
- ACGME. Statement of Justification/Impact for the Final Approval of Common Standards Related to Resident Duty Hours. Available at: http://www.acgme.org/DutyHours/impactStatement.pdf.Accessed February 21,2003.
- ,.Program Evaluation: Alternative Approaches and Practical Guidelines.New York, NY:Longman;1997.
- . Fumbled handoff. Web M117:846–850.
- Helpful solutions for meeting the 2006 national patient safety goals.Jt Comm Perspect Patient Saf.2005;5(8):1–20.
- .Fumbled handoffs: one dropped ball after another.Ann Intern Med.2005;142:352–358.
- ,,,.Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs.Acad Med.2005;80:1094–1099.
- .Handling handoffs safely.Am J Matern Child Nurs.2005;30(2):152.
- ,,,,.Handoff strategies in settings with high consequences for failure: lessons for health care operations.Int J Qual Health Care.2004;16(2):125–132.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121:866–872.
- ,,.Balancing continuity of care with residents' limited work hours: defining the implications.Acad Med.2005;80(1):39–43.
- ,,,,,.Effects of work hour reduction on residents' lives: a systematic review.JAMA.2005;294:1088–1100.
- ,,.Residents' perceptions of the effects of work hour limitations at a large teaching hospital.Acad Med.2006;81(1):63–67.
Copyright © 2008 Society of Hospital Medicine
Clinical Conundrum
A 62‐year‐old man with psoriasis for more than 30 years presented to the emergency department with a scaly, pruritic rash involving his face, trunk, and extremities that he had had for the past 10 days. The rash was spreading and not responding to application of clobetasol ointment, which had helped his psoriasis in the past. He also reported mild pharyngitis, headache, and myalgias.
A patient with a chronic skin condition presenting with a new rash means the clinician must consider whether it is an alternative manifestation of the chronic disorder or a new illness. Psoriasis takes many forms including guttate psoriasis, which presents with small, droplike plaques and frequently follows respiratory infections (particularly those caused by Streptococcus). Well‐controlled psoriasis rarely transforms after 3 decades, so I would consider other conditions. The tempo of illness makes certain life‐threatening syndromes, including Stevens‐Johnson, toxic shock, and purpura fulminans, unlikely. An allergic reaction, atopic dermatitis, or medication reaction is possible. Infections, either systemic (eg, syphilis) or dermatologic (eg, scabies), should be considered. Photosensitivity could involve the sun‐exposed areas, such as the extremities and face. Seborrheic dermatitis can cause scaling lesions of the face and trunk but not the extremities. Vasculitis merits consideration, but dependent regions are typically affected more than the head. Mycosis fungoides or a paraneoplastic phenomenon could cause a diffuse rash in this age group.
The patient had diabetes mellitus, hypertension, diverticulosis, and depression. Three months earlier he had undergone surgical drainage of a perirectal abscess. His usual medications were lovastatin, paroxetine, insulin, hydrochlorothiazide, and lisinopril. Three weeks previously he had completed a 10‐day course of trimethoprim/sulfamethoxazole for an upper respiratory infection. Otherwise, he was taking no new medications. He was allergic to penicillin. He denied substance abuse, recent travel, or risk factors for human immunodeficiency virus (HIV) infection. He worked as an automobile painter, lived with his wife, and had a pet dog.
Physical examination revealed a well‐appearing man with normal vital signs. His skin had well‐defined circumscribed pink plaques, mostly 1‐2 cm in size, with thick, silvery scales in the ears and on the dorsal and ventral arms and legs, chest, back, face, and scalp. There were no pustules or other signs of infection (Figs. 1and 2). The nails exhibited distal onycholysis, oil spots, and rare pits. His posterior pharynx was mildly erythematous. The results of cardiovascular, pulmonary, and abdominal examinations were normal.

Although other scaling skin conditions such as eczema, irritant dermatitis, or malignancy remain possible, his rash is most consistent with widespread psoriasis. I would consider immunological changes that may have caused a remarkably altered and more severe expression of his chronic disease, for example, recent steroid therapy or HIV infection. The company a rash keeps helps frame the differential diagnosis. Based on the patient's well appearance, the time course, his minimal systemic symptoms, and the appearance of the rash, my leading considerations are psoriasis or an allergic dermatitis. Cutaneous T‐cell malignancy, with its indolent and sometimes protean manifestations, remains possible in a patient of his age. I would now consult a dermatologist for 3 reasons: this patient has a chronic disease that I do not manage beyond basic treatments (eg, topical steroids), he has an undiagnosed illness with substantial dermatologic manifestations, and he may need a skin biopsy for definitive diagnosis.
The dermatology team diagnosed a guttate psoriasis flare, possibly associated with streptococcal pharyngitis. The differential diagnosis included secondary syphilis, although the team believed this was less likely. The dermatology team recommended obtaining a throat culture, streptozyme assay, and rapid plasma reagin and prescribed oral erythromycin and topical steroid ointment under a sauna suit.
I would follow his response to the prescribed steroid treatments. If the patient's course deviates from the dermatologists' expectations, I would request a skin biopsy and undertake further evaluations in search of an underlying systemic disease.
The patient followed up in the dermatology clinic 3 weeks later. His rash had worsened, and he had developed patchy alopecia and progressive edema of the face, ears, and eyes. He denied mouth or tongue swelling, difficulty breathing, or hives. The streptozyme assay was positive, but the other laboratory test results were negative.
The dermatology team diagnosed a severely inflammatory psoriasis flare and prescribed an oral retinoid, acitretin, and referred him for ultraviolet light therapy. He was unable to travel for phototherapy, and acitretin was discontinued after 1 week because of elevated serum transaminase levels. The dermatologists then prescribed oral cyclosporine.
The progression of disease despite standard treatment suggests a nonpsoriatic condition. Although medications could cause the abnormal liver tests, so could another underlying illness that involves the liver. An infiltrative disorder of the skin with hair follicle destruction and local lymphedema could explain both alopecia and facial edema.
I am unable account for his clinical features with a single disease, so the differential remains broad, including severe psoriasis, an infiltrating cutaneous malignancy, or a toxic exposure. Arsenic poisoning causes hyperkeratotic skin lesions, although he lacks the associated gastrointestinal and neurological symptoms. I would not have added the potentially toxic cyclosporine.
When he returned to dermatology clinic 1 week later, his rash and facial swelling had worsened. He also reported muscle and joint aches, fatigue, lightheadedness, anorexia, nausea, abdominal pain, diarrhea, and dyspnea on exertion. He denied fever, chills, and night sweats.
He appeared ill and used a cane to arise and walk. His vital signs and oxygen saturation were normal. He had marked swelling of his face, diffuse erythema and swelling on the chest, and widespread scaly, erythematous plaques (Fig. 3). The proximal nail folds of his fingers were erythematous, with ragged cuticles. His abdomen was mildly distended, but the rest of the physical examination was normal.
He has become too systemically ill to attribute his condition to psoriasis. The nail findings suggest dermatomyositis, which could explain many of his findings. The diffuse erythema and his difficulty walking are consistent with its skin and muscle involvement. Dyspnea could be explained by dermatomyositis‐associated interstitial lung disease. A dermatomyositis‐associated hematological or solid malignancy could account for his multisystem ailments and functional decline. A point against dermatomyositis is the relatively explosive onset of his disease. He should be carefully examined for any motor weakness. With his progressive erythroderma, I am also concerned about an advancing cutaneous T‐cell lymphoma (with leukemic transformation).
Blood tests revealed the following values: white‐blood‐cell count, 8700/L; hematocrit, 46%; platelet count, 172,000/L; blood urea nitrogen, 26 mg/dL; creatinine, 1.0 mg/dL; glucose, 199 mg/dL; albumin, 3.1 g/dL; alkaline phosphatase, 172 U/L (normal range 45‐129); alanine aminotransferase, 75 U/L (normal range 0‐39 U/L); aspartate aminotransferase, 263 U/L (normal range 0‐37 U/L); total bilirubin, 1.1 mg/dL; prothrombin time, 16 seconds (normal range 11.7‐14.3 seconds), and serum creatinine, kinase, 4253 U/L (normal range 0‐194 U/L). HIV serology was negative. Urinalysis revealed trace protein. The results of chest radiographs and an electrocardiogram were normal.
The liver function tests results are consistent with medication effects or liver involvement in a systemic disease. The creatinine kinase elevation is consistent with a myopathy such as dermatomyositis. A skin biopsy would still be useful. Depending on those results, he may need a muscle biopsy, urine heavy metal testing, and computed tomography body imaging. Considering his transaminase and creatinine kinase elevations, I would discontinue lovastatin.
The patient was hospitalized. Further questioning revealed that he had typical Raynaud's phenomenon and odynophagia. A detailed neurological examination showed weakness (3/5) of the triceps and iliopsoas muscles and difficulty rising from a chair without using his arms. Dermatoscopic examination of the proximal nail folds showed dilated capillary loops and foci of hemorrhage.
Blood tests showed a lactate dehydrogenase level of 456 U/L (normal range 0‐249 U/L) and an aldolase of 38 U/L (normal range 1.2‐7.6 U/L). Tests for antinuclear antibodies, anti‐Jo antibody, and antimyeloperoxidase antibodies were negative. Two skin biopsies were interpreted by general pathology as consistent with partially treated psoriasis, whereas another showed nonspecific changes with minimal superficial perivascular lymphohistiocytic inflammation (Fig. 4). Lisinopril was discontinued because of its possible contribution to the facial edema.
Dermatomyositis is now the leading diagnosis. Characteristic features include his proximal muscle weakness, Raynaud's phenomenon, and dilated nailfold capillary loops. I am not overly dissuaded by the negative antinuclear antibodies, but because of additional atypical features (ie, extensive cutaneous edema, rapid onset, illness severity, prominent gastrointestinal symptoms), a confirmatory muscle biopsy is needed. Endoscopy of the proximal aerodigestive tract would help evaluate the odynophagia. There is little to suggest infection, malignancy, or metabolic derangement.
The inpatient medical team considered myositis related to retinoid or cyclosporine therapy. They discontinued cyclosporine and began systemic corticosteroid therapy. Within a few days, the patient's rash, muscle pain, and weakness improved, and the elevated transaminase and creatinine kinase levels decreased.
Dermatology recommended an evaluation for dermatomyositis‐associated malignancy, but the medicine team and rheumatology consultants, noting the lack of classic skin findings (heliotrope rash and Gottron's papules) and the uncharacteristically rapid onset and improvement of myositis, suggested delaying the evaluation until dermatomyositis was proven.
An immediate improvement in symptoms with steroids is nonspecific, often occurring in autoimmune, infectious, and neoplastic diseases. This juncture in the case is common in complex multisystem illnesses, where various consultants may arrive at differing conclusions. With both typical and atypical features of dermatomyositis, where should one set the therapeutic threshold, that is, the point where one ends testing, accepts a diagnosis, and initiates treatment? Several factors raise the level of certainty I would require. First, dermatomyositis is quite rare. Adding atypical features further increases the burden of proof for that illness. Second, the existence of alternative possibilities (admittedly of equal uncertainty) gives me some pause. Finally, the toxicity of the proposed treatments raises the therapeutic threshold. Acknowledging that empiric treatment may be indicated for a severely ill patient at a lower level of certainty, I would hesitate to commit a patient to long‐term steroids without being confident of the diagnosis. I would therefore require a muscle biopsy, or at least electromyography to support or exclude dermatomyositis.
The patient was discharged from the hospital on high‐dose prednisone. He underwent electromyography, which revealed inflammatory myopathic changes more apparent in the proximal than distal muscles. These findings were thought to be compatible with dermatomyositis, although the fibrillations and positive sharp waves characteristic of acute inflammation were absent, perhaps because of corticosteroid therapy.
The patient mistakenly stopped taking his prednisone. Within days, his weakness and skin rash worsened, and he developed nausea with vomiting. He returned to clinic, where his creatinine kinase level was again found to be elevated, and he was rehospitalized. Oral corticosteroid therapy was restarted with prompt improvement. On review of the original skin biopsies, a dermatopathologist observed areas of thickened dermal collagen and a superficial and deep perivascular lymphocytic infiltrate, both consistent with connective tissue disease.
These 3 additional findings (ie, electromyography results, temporally established steroid responsiveness, and the new skin biopsy interpretation) in aggregate support the diagnosis of dermatomyositis, but the nausea and vomiting are unusual. I would discuss these results with a rheumatologist and still request a confirmatory muscle biopsy. Because diagnosing dermatomyositis should prompt consideration of seeking an underlying malignancy in a patient of this age group, I would repeat a targeted history and physical examination along with age‐ and risk‐factor‐appropriate screening. If muscle biopsy results are not definitive, finding an underlying malignancy would lend support to dermatomyositis.
While hospitalized, the patient complained of continued odynophagia and was noted to have oral candidiasis. Upper endoscopy, undertaken to evaluate for esophageal candidiasis, revealed a mass at the gastroesophageal junction. Biopsy revealed gastric‐type adenocarcinoma. An abdominal computed tomography scan demonstrated 3 hypodense hepatic lesions, evidence of cirrhosis, and ascites. Cytology of paracentesis fluid revealed cells compatible with adenocarcinoma. The patient died in hospice care 2 weeks later.
At autopsy, he had metastatic gastric‐type adenocarcinoma. A muscle biopsy (Fig. 5) revealed muscle atrophy with small foci of lymphocytic infiltrates, most compatible with dermatomyositis. Another dermatopathologist reviewed the skin biopsies and noted interface dermatitis, which is typical of connective tissue diseases like dermatomyositis (Fig. 6A,B).
COMMENTARY
Dermatomyositis is an idiopathic inflammatory myopathy characterized by endomysial inflammation and muscle weakness and differentiated from other myopathies by the presence of a rash.1 Muscle disease may manifest with or precede the rash, but up to 40% of patients present with skin manifestations alone, an entity called amyopathic dermatomyositis.2 When present, the myositis generally develops over months, but the onset can be acute.1 The weakness is typically symmetrical and proximal,1 and many patients have oropharyngeal dysphagia.3
The characteristic rash is erythematous, symmetrical, and photodistributed.4 Classic cutaneous findings are the heliotrope rash (violaceous eyelid erythema), which is pathognomonic but uncommon, and the more common Gottron's papules (violaceous, slightly elevated papules and plaques on bony prominences and extensor surfaces, especially the knuckles).4 Other findings include periorbital edema, scalp dermatitis, poikiloderma (ie, hyperpigmentation, hypopigmentation, atrophy, and telangiectasia), periungual erythema, and dystrophic cuticles.2 The cutaneous manifestations of dermatomyositis may be similar to those of psoriasis, systemic lupus erythematosus, lichen planus, rosacea, polymorphous light eruption, drug eruption, atopic dermatitis, seborrheic dermatitis, or allergic contact dermatitis.4
Diagnosing dermatomyositis requires considering clinical, laboratory, electromyographical, and histological evidence, as there are no widely accepted, validated diagnostic criteria.1, 5 The diagnosis is usually suspected if there is a characteristic rash and symptoms of myositis (eg, proximal muscle weakness, myalgias, fatigue, or an inability to swallow). When the patient has an atypical rash, skin biopsy can differentiate dermatomyositis from other conditions, except lupus, which shares the key finding of interface dermatitis.2 The histological findings can be variable and subtle,6 so consultation with a dermatopathologist may be helpful.
Myositis may be confirmed by various studies. Most patients have elevated muscle enzymes (ie, creatinine kinase, aldolase, lactate dehydrogenase, or transaminases)1; for those who do not, magnetic resonance imaging can be helpful in detecting muscle involvement and locating the best site for muscle biopsy.7 Electromyography reveals nonspecific muscle membrane instability.8 Muscle biopsy shows muscle fiber necrosis, perifascicular atrophy, and perivascular and perifascicular lymphocytic infiltrates. These can be patchy, diminished by steroid use, and occasionally seen in noninflammatory muscular dystrophies.8 For a patient with typical myositis and a characteristic rash, muscle biopsy may be unnecessary.1
The clinical utility of serologic testing for diagnosing dermatomyositis is controversial.2 Myositis‐specific antibody testing is insensitive but specific; these antibodies include Jo‐1, an antisynthetase antibody that predicts incomplete response to therapy and lung involvement, and Mi‐2, which is associated with better response to therapy.2, 9, 10 The sensitivity and specificity of antinuclear antibodies are both approximately 60%.10
Patients with dermatomyositis have higher rates of cancers than age‐matched controls, and nearly 25% of patients are diagnosed with a malignancy at some point during the course of the disease.11 Malignancies are typically solid tumors that manifest within 3 years of the diagnosis,1214 although the increased risk may exist for at least 5 years.14 There is a 10‐fold higher risk of ovarian cancer in women with dermatomyositis.12, 15 Other associated malignancies include lung, gastric, colorectal, pancreatic, and breast carcinomas and non‐Hodgkin's lymphoma.14
Recommendations for screening affected patients for cancer have changed over the years, with increasing evidence of an association between dermatomyositis and malignancy and evolving improvements in diagnostic techniques.16 Many authorities recommend that all adult patients with dermatomyositis be evaluated for cancer, including a complete physical examination, basic hematological tests, age‐ and sex‐appropriate screening (eg, mammography, pap smear, and colonoscopy), and chest x‐ray.16 Some would add upper endoscopy; imaging of the chest, abdomen, and pelvis; gynecological examination; and serum CA‐125 level to better evaluate for the most common malignancies (ie, ovarian, gastric, lung, and pancreatic carcinomas and non‐Hodgkins lymphoma).12, 1720
In 19% of adults, dermatomyositis overlaps with other autoimmune disorders, usually systemic lupus erythematosus and systemic sclerosis.21 These manifest as Raynaud's phenomenon, arthritis, esophageal dysmotility, renal disease, or neuropathy.21 Other potentially serious systemic manifestations of dermatomyositis include proximal dysphagia from pharyngeal myopathy; distal dysphagia from esophageal dysmotility in systemic sclerosis overlap; pulmonary disease from autoimmune interstitial lung disease or aspiration; cardiac disease from conduction abnormalities, myocarditis, pericarditis, and valvular disease; and rhabdomyolysis.2
Treatment of dermatomyositis requires systemic immunosuppression with 1 or more agents. The prognosis of dermatomyositis is variable. Mortality at 5 years ranges from 23% to 73%. At least a third of patients are left with mild to severe disability.1 In addition to older age, predictors of poor outcome include male sex, dysphagia, longstanding symptoms before treatment, pulmonary or cardiac involvement, and presence of antisynthetase antibodies.22
Dermatomyositis is often treated in the outpatient setting, but there are many reasons for hospitalization. Complications of treatment, like infection or adverse effects of medications, could result in hospitalization. Treatment with intravenous pulse corticosteroids or IVIG may require inpatient administration if no infusion center is available. Other indications for inpatient evaluation include the consequences of various malignancies and the more severe expression of systemic complications of dermatomyositis (eg, dysphagia and pulmonary, cardiac, or renal disease).
Every parent knows the plaintive backseat whine, Are we there, yet? Clinicians may also experience this feeling when attempting to diagnose a perplexing illness, especially one that lacks a definitive diagnostic test. It was easy for this patient's doctors to assume initially that his new rash was a manifestation of his long‐standing psoriasis. Having done so, they could understandably attribute the subsequent findings to either evolution of this disease or to consequences of the prescribed treatments, rather than considering a novel diagnosis. Only when faced with new (or newly appreciated) findings suggesting myopathy did the clinicians (and our discussant) consider the diagnosis of dermatomyositis. Even then, the primary inpatient medical team and their consultants were unsure when they had sufficient evidence to be certain.
Several factors compounded the difficulty of making a diagnosis in this case: the clinicians were dealing with a rare disease; they were considering alternative diagnoses (ie, psoriasis or a toxic effect of medication); and the disease presented somewhat atypically. The clinicians initially failed to consider and then accept the correct diagnosis because the patient's rash was not classic, his biopsy was interpreted as nonspecific, and he lacked myositis at presentation. Furthermore, when the generalists sought expert assistance, they encountered a difference of opinion among the consultants. These complex situations should goad the clinician into carefully considering the therapeutic threshold, that is, the transition point from diagnostic testing to therapeutic intervention.23 With complex cases like this, it may be difficult to know when one has reached a strongly supported diagnosis, and frequently asking whether we are there yet may be appropriate.
Take‐Home Points for the Hospitalist
-
A skin rash, which may have typical or atypical features, distinguishes dermatomyositis from other acquired myopathies.
-
Consider consultation with pathology specialists for skin and muscle biopsies.
-
Ovarian, lung, gastric, colorectal, pancreatic, and breast carcinomas and non‐Hodgkin's lymphoma are the most common cancers associated with dermatomyositis.
-
In addition to age‐appropriate cancer screening, consider obtaining upper endoscopy, imaging of the chest/abdomen/pelvis, and CA‐125.
-
Patients with dermatomyositis and no obvious concurrent malignancy need long‐term outpatient follow‐up for repeated malignancy screening.
- ,.Polymyositis and dermatomyositis.Lancet.2003;362:971–982.
- .Dermatomyositis.Lancet.2000;355:53–47.
- ,,,.Oropharyngeal dysphagia in polymyositis/dermatomyositis.Clin Neurol Neurosurg.2004;107(1):32–37.
- ,.Skin involvement in dermatomyositis.Curr Opin Rheumatol.2003;15:714–22.
- ,,,,,.Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: analysis of 100 French Canadian patients.Medicine (Baltimore).2005;84:231–249.
- .Skin Pathology.2nd ed.New York:Churchill Livingstone;2002.
- ,.Utility of magnetic resonance imaging in the evaluation of patients with inflammatory myopathies.Curr Rheumatol Rep.2001;3:334–245.
- ,,.Is it really myositis? A consideration of the differential diagnosis.Curr Opin Rheumatol2004;16:684–691.
- .Idiopathic inflammatory myopathy: autoantibody update.Curr Rheumatol Rep.2002;4:434–441.
- ,,.Laboratory assessment in musculoskeletal disorders.Best Pract Res Clin Rheumatol.2003;17:475–494.
- ,.Dermatomyositis.Clin Dermatol.2006;24:363–373.
- ,,, et al.Frequency of specific cancer types in dermatomyositis and polymyositis: a population‐based study.Lancet.2001;357:96–100.
- ,,, et al.Cancer‐associated myositis: clinical features and prognostic signs.Ann N Y Acad Sci.2005;1051:64–71.
- ,,,,.Incidence of malignant disease in biopsy‐proven inflammatory myopathy. A population‐based cohort study.Ann Intern Med.2001;134:1087–1095.
- ,,.Risk of cancer in patients with dermatomyositis or polymyositis, and follow‐up implications: a Scottish population‐based cohort study.Br J Cancer.2001;85 (1):41–45.
- .When and how should the patient with dermatomyositis or amyopathic dermatomyositis be assessed for possible cancer?Arch Dermatol.2002;138:969–971.
- ,,.Ovarian cancer in patients with dermatomyositis.Medicine (Baltimore).1994;73(3):153–160.
- ,,,.Dermatomyositis sine myositis: association with malignancy.J Rheumatol.1996;23 (1):101–105.
- ,,, et al.Tumor antigen markers for the detection of solid cancers in inflammatory myopathies.Cancer Epidemiol Biomarkers Prev.2005;14:1279–1282.
- ,,, et al.Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: a study of 40 patients.Arch Dermatol.2002;138:885–890.
- ,,,,,.Dermatomyositis: a dermatology‐based case series.J Am Acad Dermatol.1998;38:397–404.
- ,,, et al.Long‐term outcome in polymyositis and dermatomyositis.Ann Rheum Dis.2006;65:1456–1461.
- .Our stubborn quest for diagnostic certainty. A cause of excessive testing.N Engl J Med.1989;320:1489–1491.
A 62‐year‐old man with psoriasis for more than 30 years presented to the emergency department with a scaly, pruritic rash involving his face, trunk, and extremities that he had had for the past 10 days. The rash was spreading and not responding to application of clobetasol ointment, which had helped his psoriasis in the past. He also reported mild pharyngitis, headache, and myalgias.
A patient with a chronic skin condition presenting with a new rash means the clinician must consider whether it is an alternative manifestation of the chronic disorder or a new illness. Psoriasis takes many forms including guttate psoriasis, which presents with small, droplike plaques and frequently follows respiratory infections (particularly those caused by Streptococcus). Well‐controlled psoriasis rarely transforms after 3 decades, so I would consider other conditions. The tempo of illness makes certain life‐threatening syndromes, including Stevens‐Johnson, toxic shock, and purpura fulminans, unlikely. An allergic reaction, atopic dermatitis, or medication reaction is possible. Infections, either systemic (eg, syphilis) or dermatologic (eg, scabies), should be considered. Photosensitivity could involve the sun‐exposed areas, such as the extremities and face. Seborrheic dermatitis can cause scaling lesions of the face and trunk but not the extremities. Vasculitis merits consideration, but dependent regions are typically affected more than the head. Mycosis fungoides or a paraneoplastic phenomenon could cause a diffuse rash in this age group.
The patient had diabetes mellitus, hypertension, diverticulosis, and depression. Three months earlier he had undergone surgical drainage of a perirectal abscess. His usual medications were lovastatin, paroxetine, insulin, hydrochlorothiazide, and lisinopril. Three weeks previously he had completed a 10‐day course of trimethoprim/sulfamethoxazole for an upper respiratory infection. Otherwise, he was taking no new medications. He was allergic to penicillin. He denied substance abuse, recent travel, or risk factors for human immunodeficiency virus (HIV) infection. He worked as an automobile painter, lived with his wife, and had a pet dog.
Physical examination revealed a well‐appearing man with normal vital signs. His skin had well‐defined circumscribed pink plaques, mostly 1‐2 cm in size, with thick, silvery scales in the ears and on the dorsal and ventral arms and legs, chest, back, face, and scalp. There were no pustules or other signs of infection (Figs. 1and 2). The nails exhibited distal onycholysis, oil spots, and rare pits. His posterior pharynx was mildly erythematous. The results of cardiovascular, pulmonary, and abdominal examinations were normal.


Although other scaling skin conditions such as eczema, irritant dermatitis, or malignancy remain possible, his rash is most consistent with widespread psoriasis. I would consider immunological changes that may have caused a remarkably altered and more severe expression of his chronic disease, for example, recent steroid therapy or HIV infection. The company a rash keeps helps frame the differential diagnosis. Based on the patient's well appearance, the time course, his minimal systemic symptoms, and the appearance of the rash, my leading considerations are psoriasis or an allergic dermatitis. Cutaneous T‐cell malignancy, with its indolent and sometimes protean manifestations, remains possible in a patient of his age. I would now consult a dermatologist for 3 reasons: this patient has a chronic disease that I do not manage beyond basic treatments (eg, topical steroids), he has an undiagnosed illness with substantial dermatologic manifestations, and he may need a skin biopsy for definitive diagnosis.
The dermatology team diagnosed a guttate psoriasis flare, possibly associated with streptococcal pharyngitis. The differential diagnosis included secondary syphilis, although the team believed this was less likely. The dermatology team recommended obtaining a throat culture, streptozyme assay, and rapid plasma reagin and prescribed oral erythromycin and topical steroid ointment under a sauna suit.
I would follow his response to the prescribed steroid treatments. If the patient's course deviates from the dermatologists' expectations, I would request a skin biopsy and undertake further evaluations in search of an underlying systemic disease.
The patient followed up in the dermatology clinic 3 weeks later. His rash had worsened, and he had developed patchy alopecia and progressive edema of the face, ears, and eyes. He denied mouth or tongue swelling, difficulty breathing, or hives. The streptozyme assay was positive, but the other laboratory test results were negative.
The dermatology team diagnosed a severely inflammatory psoriasis flare and prescribed an oral retinoid, acitretin, and referred him for ultraviolet light therapy. He was unable to travel for phototherapy, and acitretin was discontinued after 1 week because of elevated serum transaminase levels. The dermatologists then prescribed oral cyclosporine.
The progression of disease despite standard treatment suggests a nonpsoriatic condition. Although medications could cause the abnormal liver tests, so could another underlying illness that involves the liver. An infiltrative disorder of the skin with hair follicle destruction and local lymphedema could explain both alopecia and facial edema.
I am unable account for his clinical features with a single disease, so the differential remains broad, including severe psoriasis, an infiltrating cutaneous malignancy, or a toxic exposure. Arsenic poisoning causes hyperkeratotic skin lesions, although he lacks the associated gastrointestinal and neurological symptoms. I would not have added the potentially toxic cyclosporine.
When he returned to dermatology clinic 1 week later, his rash and facial swelling had worsened. He also reported muscle and joint aches, fatigue, lightheadedness, anorexia, nausea, abdominal pain, diarrhea, and dyspnea on exertion. He denied fever, chills, and night sweats.
He appeared ill and used a cane to arise and walk. His vital signs and oxygen saturation were normal. He had marked swelling of his face, diffuse erythema and swelling on the chest, and widespread scaly, erythematous plaques (Fig. 3). The proximal nail folds of his fingers were erythematous, with ragged cuticles. His abdomen was mildly distended, but the rest of the physical examination was normal.

He has become too systemically ill to attribute his condition to psoriasis. The nail findings suggest dermatomyositis, which could explain many of his findings. The diffuse erythema and his difficulty walking are consistent with its skin and muscle involvement. Dyspnea could be explained by dermatomyositis‐associated interstitial lung disease. A dermatomyositis‐associated hematological or solid malignancy could account for his multisystem ailments and functional decline. A point against dermatomyositis is the relatively explosive onset of his disease. He should be carefully examined for any motor weakness. With his progressive erythroderma, I am also concerned about an advancing cutaneous T‐cell lymphoma (with leukemic transformation).
Blood tests revealed the following values: white‐blood‐cell count, 8700/L; hematocrit, 46%; platelet count, 172,000/L; blood urea nitrogen, 26 mg/dL; creatinine, 1.0 mg/dL; glucose, 199 mg/dL; albumin, 3.1 g/dL; alkaline phosphatase, 172 U/L (normal range 45‐129); alanine aminotransferase, 75 U/L (normal range 0‐39 U/L); aspartate aminotransferase, 263 U/L (normal range 0‐37 U/L); total bilirubin, 1.1 mg/dL; prothrombin time, 16 seconds (normal range 11.7‐14.3 seconds), and serum creatinine, kinase, 4253 U/L (normal range 0‐194 U/L). HIV serology was negative. Urinalysis revealed trace protein. The results of chest radiographs and an electrocardiogram were normal.
The liver function tests results are consistent with medication effects or liver involvement in a systemic disease. The creatinine kinase elevation is consistent with a myopathy such as dermatomyositis. A skin biopsy would still be useful. Depending on those results, he may need a muscle biopsy, urine heavy metal testing, and computed tomography body imaging. Considering his transaminase and creatinine kinase elevations, I would discontinue lovastatin.
The patient was hospitalized. Further questioning revealed that he had typical Raynaud's phenomenon and odynophagia. A detailed neurological examination showed weakness (3/5) of the triceps and iliopsoas muscles and difficulty rising from a chair without using his arms. Dermatoscopic examination of the proximal nail folds showed dilated capillary loops and foci of hemorrhage.
Blood tests showed a lactate dehydrogenase level of 456 U/L (normal range 0‐249 U/L) and an aldolase of 38 U/L (normal range 1.2‐7.6 U/L). Tests for antinuclear antibodies, anti‐Jo antibody, and antimyeloperoxidase antibodies were negative. Two skin biopsies were interpreted by general pathology as consistent with partially treated psoriasis, whereas another showed nonspecific changes with minimal superficial perivascular lymphohistiocytic inflammation (Fig. 4). Lisinopril was discontinued because of its possible contribution to the facial edema.

Dermatomyositis is now the leading diagnosis. Characteristic features include his proximal muscle weakness, Raynaud's phenomenon, and dilated nailfold capillary loops. I am not overly dissuaded by the negative antinuclear antibodies, but because of additional atypical features (ie, extensive cutaneous edema, rapid onset, illness severity, prominent gastrointestinal symptoms), a confirmatory muscle biopsy is needed. Endoscopy of the proximal aerodigestive tract would help evaluate the odynophagia. There is little to suggest infection, malignancy, or metabolic derangement.
The inpatient medical team considered myositis related to retinoid or cyclosporine therapy. They discontinued cyclosporine and began systemic corticosteroid therapy. Within a few days, the patient's rash, muscle pain, and weakness improved, and the elevated transaminase and creatinine kinase levels decreased.
Dermatology recommended an evaluation for dermatomyositis‐associated malignancy, but the medicine team and rheumatology consultants, noting the lack of classic skin findings (heliotrope rash and Gottron's papules) and the uncharacteristically rapid onset and improvement of myositis, suggested delaying the evaluation until dermatomyositis was proven.
An immediate improvement in symptoms with steroids is nonspecific, often occurring in autoimmune, infectious, and neoplastic diseases. This juncture in the case is common in complex multisystem illnesses, where various consultants may arrive at differing conclusions. With both typical and atypical features of dermatomyositis, where should one set the therapeutic threshold, that is, the point where one ends testing, accepts a diagnosis, and initiates treatment? Several factors raise the level of certainty I would require. First, dermatomyositis is quite rare. Adding atypical features further increases the burden of proof for that illness. Second, the existence of alternative possibilities (admittedly of equal uncertainty) gives me some pause. Finally, the toxicity of the proposed treatments raises the therapeutic threshold. Acknowledging that empiric treatment may be indicated for a severely ill patient at a lower level of certainty, I would hesitate to commit a patient to long‐term steroids without being confident of the diagnosis. I would therefore require a muscle biopsy, or at least electromyography to support or exclude dermatomyositis.
The patient was discharged from the hospital on high‐dose prednisone. He underwent electromyography, which revealed inflammatory myopathic changes more apparent in the proximal than distal muscles. These findings were thought to be compatible with dermatomyositis, although the fibrillations and positive sharp waves characteristic of acute inflammation were absent, perhaps because of corticosteroid therapy.
The patient mistakenly stopped taking his prednisone. Within days, his weakness and skin rash worsened, and he developed nausea with vomiting. He returned to clinic, where his creatinine kinase level was again found to be elevated, and he was rehospitalized. Oral corticosteroid therapy was restarted with prompt improvement. On review of the original skin biopsies, a dermatopathologist observed areas of thickened dermal collagen and a superficial and deep perivascular lymphocytic infiltrate, both consistent with connective tissue disease.
These 3 additional findings (ie, electromyography results, temporally established steroid responsiveness, and the new skin biopsy interpretation) in aggregate support the diagnosis of dermatomyositis, but the nausea and vomiting are unusual. I would discuss these results with a rheumatologist and still request a confirmatory muscle biopsy. Because diagnosing dermatomyositis should prompt consideration of seeking an underlying malignancy in a patient of this age group, I would repeat a targeted history and physical examination along with age‐ and risk‐factor‐appropriate screening. If muscle biopsy results are not definitive, finding an underlying malignancy would lend support to dermatomyositis.
While hospitalized, the patient complained of continued odynophagia and was noted to have oral candidiasis. Upper endoscopy, undertaken to evaluate for esophageal candidiasis, revealed a mass at the gastroesophageal junction. Biopsy revealed gastric‐type adenocarcinoma. An abdominal computed tomography scan demonstrated 3 hypodense hepatic lesions, evidence of cirrhosis, and ascites. Cytology of paracentesis fluid revealed cells compatible with adenocarcinoma. The patient died in hospice care 2 weeks later.
At autopsy, he had metastatic gastric‐type adenocarcinoma. A muscle biopsy (Fig. 5) revealed muscle atrophy with small foci of lymphocytic infiltrates, most compatible with dermatomyositis. Another dermatopathologist reviewed the skin biopsies and noted interface dermatitis, which is typical of connective tissue diseases like dermatomyositis (Fig. 6A,B).


COMMENTARY
Dermatomyositis is an idiopathic inflammatory myopathy characterized by endomysial inflammation and muscle weakness and differentiated from other myopathies by the presence of a rash.1 Muscle disease may manifest with or precede the rash, but up to 40% of patients present with skin manifestations alone, an entity called amyopathic dermatomyositis.2 When present, the myositis generally develops over months, but the onset can be acute.1 The weakness is typically symmetrical and proximal,1 and many patients have oropharyngeal dysphagia.3
The characteristic rash is erythematous, symmetrical, and photodistributed.4 Classic cutaneous findings are the heliotrope rash (violaceous eyelid erythema), which is pathognomonic but uncommon, and the more common Gottron's papules (violaceous, slightly elevated papules and plaques on bony prominences and extensor surfaces, especially the knuckles).4 Other findings include periorbital edema, scalp dermatitis, poikiloderma (ie, hyperpigmentation, hypopigmentation, atrophy, and telangiectasia), periungual erythema, and dystrophic cuticles.2 The cutaneous manifestations of dermatomyositis may be similar to those of psoriasis, systemic lupus erythematosus, lichen planus, rosacea, polymorphous light eruption, drug eruption, atopic dermatitis, seborrheic dermatitis, or allergic contact dermatitis.4
Diagnosing dermatomyositis requires considering clinical, laboratory, electromyographical, and histological evidence, as there are no widely accepted, validated diagnostic criteria.1, 5 The diagnosis is usually suspected if there is a characteristic rash and symptoms of myositis (eg, proximal muscle weakness, myalgias, fatigue, or an inability to swallow). When the patient has an atypical rash, skin biopsy can differentiate dermatomyositis from other conditions, except lupus, which shares the key finding of interface dermatitis.2 The histological findings can be variable and subtle,6 so consultation with a dermatopathologist may be helpful.
Myositis may be confirmed by various studies. Most patients have elevated muscle enzymes (ie, creatinine kinase, aldolase, lactate dehydrogenase, or transaminases)1; for those who do not, magnetic resonance imaging can be helpful in detecting muscle involvement and locating the best site for muscle biopsy.7 Electromyography reveals nonspecific muscle membrane instability.8 Muscle biopsy shows muscle fiber necrosis, perifascicular atrophy, and perivascular and perifascicular lymphocytic infiltrates. These can be patchy, diminished by steroid use, and occasionally seen in noninflammatory muscular dystrophies.8 For a patient with typical myositis and a characteristic rash, muscle biopsy may be unnecessary.1
The clinical utility of serologic testing for diagnosing dermatomyositis is controversial.2 Myositis‐specific antibody testing is insensitive but specific; these antibodies include Jo‐1, an antisynthetase antibody that predicts incomplete response to therapy and lung involvement, and Mi‐2, which is associated with better response to therapy.2, 9, 10 The sensitivity and specificity of antinuclear antibodies are both approximately 60%.10
Patients with dermatomyositis have higher rates of cancers than age‐matched controls, and nearly 25% of patients are diagnosed with a malignancy at some point during the course of the disease.11 Malignancies are typically solid tumors that manifest within 3 years of the diagnosis,1214 although the increased risk may exist for at least 5 years.14 There is a 10‐fold higher risk of ovarian cancer in women with dermatomyositis.12, 15 Other associated malignancies include lung, gastric, colorectal, pancreatic, and breast carcinomas and non‐Hodgkin's lymphoma.14
Recommendations for screening affected patients for cancer have changed over the years, with increasing evidence of an association between dermatomyositis and malignancy and evolving improvements in diagnostic techniques.16 Many authorities recommend that all adult patients with dermatomyositis be evaluated for cancer, including a complete physical examination, basic hematological tests, age‐ and sex‐appropriate screening (eg, mammography, pap smear, and colonoscopy), and chest x‐ray.16 Some would add upper endoscopy; imaging of the chest, abdomen, and pelvis; gynecological examination; and serum CA‐125 level to better evaluate for the most common malignancies (ie, ovarian, gastric, lung, and pancreatic carcinomas and non‐Hodgkins lymphoma).12, 1720
In 19% of adults, dermatomyositis overlaps with other autoimmune disorders, usually systemic lupus erythematosus and systemic sclerosis.21 These manifest as Raynaud's phenomenon, arthritis, esophageal dysmotility, renal disease, or neuropathy.21 Other potentially serious systemic manifestations of dermatomyositis include proximal dysphagia from pharyngeal myopathy; distal dysphagia from esophageal dysmotility in systemic sclerosis overlap; pulmonary disease from autoimmune interstitial lung disease or aspiration; cardiac disease from conduction abnormalities, myocarditis, pericarditis, and valvular disease; and rhabdomyolysis.2
Treatment of dermatomyositis requires systemic immunosuppression with 1 or more agents. The prognosis of dermatomyositis is variable. Mortality at 5 years ranges from 23% to 73%. At least a third of patients are left with mild to severe disability.1 In addition to older age, predictors of poor outcome include male sex, dysphagia, longstanding symptoms before treatment, pulmonary or cardiac involvement, and presence of antisynthetase antibodies.22
Dermatomyositis is often treated in the outpatient setting, but there are many reasons for hospitalization. Complications of treatment, like infection or adverse effects of medications, could result in hospitalization. Treatment with intravenous pulse corticosteroids or IVIG may require inpatient administration if no infusion center is available. Other indications for inpatient evaluation include the consequences of various malignancies and the more severe expression of systemic complications of dermatomyositis (eg, dysphagia and pulmonary, cardiac, or renal disease).
Every parent knows the plaintive backseat whine, Are we there, yet? Clinicians may also experience this feeling when attempting to diagnose a perplexing illness, especially one that lacks a definitive diagnostic test. It was easy for this patient's doctors to assume initially that his new rash was a manifestation of his long‐standing psoriasis. Having done so, they could understandably attribute the subsequent findings to either evolution of this disease or to consequences of the prescribed treatments, rather than considering a novel diagnosis. Only when faced with new (or newly appreciated) findings suggesting myopathy did the clinicians (and our discussant) consider the diagnosis of dermatomyositis. Even then, the primary inpatient medical team and their consultants were unsure when they had sufficient evidence to be certain.
Several factors compounded the difficulty of making a diagnosis in this case: the clinicians were dealing with a rare disease; they were considering alternative diagnoses (ie, psoriasis or a toxic effect of medication); and the disease presented somewhat atypically. The clinicians initially failed to consider and then accept the correct diagnosis because the patient's rash was not classic, his biopsy was interpreted as nonspecific, and he lacked myositis at presentation. Furthermore, when the generalists sought expert assistance, they encountered a difference of opinion among the consultants. These complex situations should goad the clinician into carefully considering the therapeutic threshold, that is, the transition point from diagnostic testing to therapeutic intervention.23 With complex cases like this, it may be difficult to know when one has reached a strongly supported diagnosis, and frequently asking whether we are there yet may be appropriate.
Take‐Home Points for the Hospitalist
-
A skin rash, which may have typical or atypical features, distinguishes dermatomyositis from other acquired myopathies.
-
Consider consultation with pathology specialists for skin and muscle biopsies.
-
Ovarian, lung, gastric, colorectal, pancreatic, and breast carcinomas and non‐Hodgkin's lymphoma are the most common cancers associated with dermatomyositis.
-
In addition to age‐appropriate cancer screening, consider obtaining upper endoscopy, imaging of the chest/abdomen/pelvis, and CA‐125.
-
Patients with dermatomyositis and no obvious concurrent malignancy need long‐term outpatient follow‐up for repeated malignancy screening.
A 62‐year‐old man with psoriasis for more than 30 years presented to the emergency department with a scaly, pruritic rash involving his face, trunk, and extremities that he had had for the past 10 days. The rash was spreading and not responding to application of clobetasol ointment, which had helped his psoriasis in the past. He also reported mild pharyngitis, headache, and myalgias.
A patient with a chronic skin condition presenting with a new rash means the clinician must consider whether it is an alternative manifestation of the chronic disorder or a new illness. Psoriasis takes many forms including guttate psoriasis, which presents with small, droplike plaques and frequently follows respiratory infections (particularly those caused by Streptococcus). Well‐controlled psoriasis rarely transforms after 3 decades, so I would consider other conditions. The tempo of illness makes certain life‐threatening syndromes, including Stevens‐Johnson, toxic shock, and purpura fulminans, unlikely. An allergic reaction, atopic dermatitis, or medication reaction is possible. Infections, either systemic (eg, syphilis) or dermatologic (eg, scabies), should be considered. Photosensitivity could involve the sun‐exposed areas, such as the extremities and face. Seborrheic dermatitis can cause scaling lesions of the face and trunk but not the extremities. Vasculitis merits consideration, but dependent regions are typically affected more than the head. Mycosis fungoides or a paraneoplastic phenomenon could cause a diffuse rash in this age group.
The patient had diabetes mellitus, hypertension, diverticulosis, and depression. Three months earlier he had undergone surgical drainage of a perirectal abscess. His usual medications were lovastatin, paroxetine, insulin, hydrochlorothiazide, and lisinopril. Three weeks previously he had completed a 10‐day course of trimethoprim/sulfamethoxazole for an upper respiratory infection. Otherwise, he was taking no new medications. He was allergic to penicillin. He denied substance abuse, recent travel, or risk factors for human immunodeficiency virus (HIV) infection. He worked as an automobile painter, lived with his wife, and had a pet dog.
Physical examination revealed a well‐appearing man with normal vital signs. His skin had well‐defined circumscribed pink plaques, mostly 1‐2 cm in size, with thick, silvery scales in the ears and on the dorsal and ventral arms and legs, chest, back, face, and scalp. There were no pustules or other signs of infection (Figs. 1and 2). The nails exhibited distal onycholysis, oil spots, and rare pits. His posterior pharynx was mildly erythematous. The results of cardiovascular, pulmonary, and abdominal examinations were normal.


Although other scaling skin conditions such as eczema, irritant dermatitis, or malignancy remain possible, his rash is most consistent with widespread psoriasis. I would consider immunological changes that may have caused a remarkably altered and more severe expression of his chronic disease, for example, recent steroid therapy or HIV infection. The company a rash keeps helps frame the differential diagnosis. Based on the patient's well appearance, the time course, his minimal systemic symptoms, and the appearance of the rash, my leading considerations are psoriasis or an allergic dermatitis. Cutaneous T‐cell malignancy, with its indolent and sometimes protean manifestations, remains possible in a patient of his age. I would now consult a dermatologist for 3 reasons: this patient has a chronic disease that I do not manage beyond basic treatments (eg, topical steroids), he has an undiagnosed illness with substantial dermatologic manifestations, and he may need a skin biopsy for definitive diagnosis.
The dermatology team diagnosed a guttate psoriasis flare, possibly associated with streptococcal pharyngitis. The differential diagnosis included secondary syphilis, although the team believed this was less likely. The dermatology team recommended obtaining a throat culture, streptozyme assay, and rapid plasma reagin and prescribed oral erythromycin and topical steroid ointment under a sauna suit.
I would follow his response to the prescribed steroid treatments. If the patient's course deviates from the dermatologists' expectations, I would request a skin biopsy and undertake further evaluations in search of an underlying systemic disease.
The patient followed up in the dermatology clinic 3 weeks later. His rash had worsened, and he had developed patchy alopecia and progressive edema of the face, ears, and eyes. He denied mouth or tongue swelling, difficulty breathing, or hives. The streptozyme assay was positive, but the other laboratory test results were negative.
The dermatology team diagnosed a severely inflammatory psoriasis flare and prescribed an oral retinoid, acitretin, and referred him for ultraviolet light therapy. He was unable to travel for phototherapy, and acitretin was discontinued after 1 week because of elevated serum transaminase levels. The dermatologists then prescribed oral cyclosporine.
The progression of disease despite standard treatment suggests a nonpsoriatic condition. Although medications could cause the abnormal liver tests, so could another underlying illness that involves the liver. An infiltrative disorder of the skin with hair follicle destruction and local lymphedema could explain both alopecia and facial edema.
I am unable account for his clinical features with a single disease, so the differential remains broad, including severe psoriasis, an infiltrating cutaneous malignancy, or a toxic exposure. Arsenic poisoning causes hyperkeratotic skin lesions, although he lacks the associated gastrointestinal and neurological symptoms. I would not have added the potentially toxic cyclosporine.
When he returned to dermatology clinic 1 week later, his rash and facial swelling had worsened. He also reported muscle and joint aches, fatigue, lightheadedness, anorexia, nausea, abdominal pain, diarrhea, and dyspnea on exertion. He denied fever, chills, and night sweats.
He appeared ill and used a cane to arise and walk. His vital signs and oxygen saturation were normal. He had marked swelling of his face, diffuse erythema and swelling on the chest, and widespread scaly, erythematous plaques (Fig. 3). The proximal nail folds of his fingers were erythematous, with ragged cuticles. His abdomen was mildly distended, but the rest of the physical examination was normal.

He has become too systemically ill to attribute his condition to psoriasis. The nail findings suggest dermatomyositis, which could explain many of his findings. The diffuse erythema and his difficulty walking are consistent with its skin and muscle involvement. Dyspnea could be explained by dermatomyositis‐associated interstitial lung disease. A dermatomyositis‐associated hematological or solid malignancy could account for his multisystem ailments and functional decline. A point against dermatomyositis is the relatively explosive onset of his disease. He should be carefully examined for any motor weakness. With his progressive erythroderma, I am also concerned about an advancing cutaneous T‐cell lymphoma (with leukemic transformation).
Blood tests revealed the following values: white‐blood‐cell count, 8700/L; hematocrit, 46%; platelet count, 172,000/L; blood urea nitrogen, 26 mg/dL; creatinine, 1.0 mg/dL; glucose, 199 mg/dL; albumin, 3.1 g/dL; alkaline phosphatase, 172 U/L (normal range 45‐129); alanine aminotransferase, 75 U/L (normal range 0‐39 U/L); aspartate aminotransferase, 263 U/L (normal range 0‐37 U/L); total bilirubin, 1.1 mg/dL; prothrombin time, 16 seconds (normal range 11.7‐14.3 seconds), and serum creatinine, kinase, 4253 U/L (normal range 0‐194 U/L). HIV serology was negative. Urinalysis revealed trace protein. The results of chest radiographs and an electrocardiogram were normal.
The liver function tests results are consistent with medication effects or liver involvement in a systemic disease. The creatinine kinase elevation is consistent with a myopathy such as dermatomyositis. A skin biopsy would still be useful. Depending on those results, he may need a muscle biopsy, urine heavy metal testing, and computed tomography body imaging. Considering his transaminase and creatinine kinase elevations, I would discontinue lovastatin.
The patient was hospitalized. Further questioning revealed that he had typical Raynaud's phenomenon and odynophagia. A detailed neurological examination showed weakness (3/5) of the triceps and iliopsoas muscles and difficulty rising from a chair without using his arms. Dermatoscopic examination of the proximal nail folds showed dilated capillary loops and foci of hemorrhage.
Blood tests showed a lactate dehydrogenase level of 456 U/L (normal range 0‐249 U/L) and an aldolase of 38 U/L (normal range 1.2‐7.6 U/L). Tests for antinuclear antibodies, anti‐Jo antibody, and antimyeloperoxidase antibodies were negative. Two skin biopsies were interpreted by general pathology as consistent with partially treated psoriasis, whereas another showed nonspecific changes with minimal superficial perivascular lymphohistiocytic inflammation (Fig. 4). Lisinopril was discontinued because of its possible contribution to the facial edema.

Dermatomyositis is now the leading diagnosis. Characteristic features include his proximal muscle weakness, Raynaud's phenomenon, and dilated nailfold capillary loops. I am not overly dissuaded by the negative antinuclear antibodies, but because of additional atypical features (ie, extensive cutaneous edema, rapid onset, illness severity, prominent gastrointestinal symptoms), a confirmatory muscle biopsy is needed. Endoscopy of the proximal aerodigestive tract would help evaluate the odynophagia. There is little to suggest infection, malignancy, or metabolic derangement.
The inpatient medical team considered myositis related to retinoid or cyclosporine therapy. They discontinued cyclosporine and began systemic corticosteroid therapy. Within a few days, the patient's rash, muscle pain, and weakness improved, and the elevated transaminase and creatinine kinase levels decreased.
Dermatology recommended an evaluation for dermatomyositis‐associated malignancy, but the medicine team and rheumatology consultants, noting the lack of classic skin findings (heliotrope rash and Gottron's papules) and the uncharacteristically rapid onset and improvement of myositis, suggested delaying the evaluation until dermatomyositis was proven.
An immediate improvement in symptoms with steroids is nonspecific, often occurring in autoimmune, infectious, and neoplastic diseases. This juncture in the case is common in complex multisystem illnesses, where various consultants may arrive at differing conclusions. With both typical and atypical features of dermatomyositis, where should one set the therapeutic threshold, that is, the point where one ends testing, accepts a diagnosis, and initiates treatment? Several factors raise the level of certainty I would require. First, dermatomyositis is quite rare. Adding atypical features further increases the burden of proof for that illness. Second, the existence of alternative possibilities (admittedly of equal uncertainty) gives me some pause. Finally, the toxicity of the proposed treatments raises the therapeutic threshold. Acknowledging that empiric treatment may be indicated for a severely ill patient at a lower level of certainty, I would hesitate to commit a patient to long‐term steroids without being confident of the diagnosis. I would therefore require a muscle biopsy, or at least electromyography to support or exclude dermatomyositis.
The patient was discharged from the hospital on high‐dose prednisone. He underwent electromyography, which revealed inflammatory myopathic changes more apparent in the proximal than distal muscles. These findings were thought to be compatible with dermatomyositis, although the fibrillations and positive sharp waves characteristic of acute inflammation were absent, perhaps because of corticosteroid therapy.
The patient mistakenly stopped taking his prednisone. Within days, his weakness and skin rash worsened, and he developed nausea with vomiting. He returned to clinic, where his creatinine kinase level was again found to be elevated, and he was rehospitalized. Oral corticosteroid therapy was restarted with prompt improvement. On review of the original skin biopsies, a dermatopathologist observed areas of thickened dermal collagen and a superficial and deep perivascular lymphocytic infiltrate, both consistent with connective tissue disease.
These 3 additional findings (ie, electromyography results, temporally established steroid responsiveness, and the new skin biopsy interpretation) in aggregate support the diagnosis of dermatomyositis, but the nausea and vomiting are unusual. I would discuss these results with a rheumatologist and still request a confirmatory muscle biopsy. Because diagnosing dermatomyositis should prompt consideration of seeking an underlying malignancy in a patient of this age group, I would repeat a targeted history and physical examination along with age‐ and risk‐factor‐appropriate screening. If muscle biopsy results are not definitive, finding an underlying malignancy would lend support to dermatomyositis.
While hospitalized, the patient complained of continued odynophagia and was noted to have oral candidiasis. Upper endoscopy, undertaken to evaluate for esophageal candidiasis, revealed a mass at the gastroesophageal junction. Biopsy revealed gastric‐type adenocarcinoma. An abdominal computed tomography scan demonstrated 3 hypodense hepatic lesions, evidence of cirrhosis, and ascites. Cytology of paracentesis fluid revealed cells compatible with adenocarcinoma. The patient died in hospice care 2 weeks later.
At autopsy, he had metastatic gastric‐type adenocarcinoma. A muscle biopsy (Fig. 5) revealed muscle atrophy with small foci of lymphocytic infiltrates, most compatible with dermatomyositis. Another dermatopathologist reviewed the skin biopsies and noted interface dermatitis, which is typical of connective tissue diseases like dermatomyositis (Fig. 6A,B).


COMMENTARY
Dermatomyositis is an idiopathic inflammatory myopathy characterized by endomysial inflammation and muscle weakness and differentiated from other myopathies by the presence of a rash.1 Muscle disease may manifest with or precede the rash, but up to 40% of patients present with skin manifestations alone, an entity called amyopathic dermatomyositis.2 When present, the myositis generally develops over months, but the onset can be acute.1 The weakness is typically symmetrical and proximal,1 and many patients have oropharyngeal dysphagia.3
The characteristic rash is erythematous, symmetrical, and photodistributed.4 Classic cutaneous findings are the heliotrope rash (violaceous eyelid erythema), which is pathognomonic but uncommon, and the more common Gottron's papules (violaceous, slightly elevated papules and plaques on bony prominences and extensor surfaces, especially the knuckles).4 Other findings include periorbital edema, scalp dermatitis, poikiloderma (ie, hyperpigmentation, hypopigmentation, atrophy, and telangiectasia), periungual erythema, and dystrophic cuticles.2 The cutaneous manifestations of dermatomyositis may be similar to those of psoriasis, systemic lupus erythematosus, lichen planus, rosacea, polymorphous light eruption, drug eruption, atopic dermatitis, seborrheic dermatitis, or allergic contact dermatitis.4
Diagnosing dermatomyositis requires considering clinical, laboratory, electromyographical, and histological evidence, as there are no widely accepted, validated diagnostic criteria.1, 5 The diagnosis is usually suspected if there is a characteristic rash and symptoms of myositis (eg, proximal muscle weakness, myalgias, fatigue, or an inability to swallow). When the patient has an atypical rash, skin biopsy can differentiate dermatomyositis from other conditions, except lupus, which shares the key finding of interface dermatitis.2 The histological findings can be variable and subtle,6 so consultation with a dermatopathologist may be helpful.
Myositis may be confirmed by various studies. Most patients have elevated muscle enzymes (ie, creatinine kinase, aldolase, lactate dehydrogenase, or transaminases)1; for those who do not, magnetic resonance imaging can be helpful in detecting muscle involvement and locating the best site for muscle biopsy.7 Electromyography reveals nonspecific muscle membrane instability.8 Muscle biopsy shows muscle fiber necrosis, perifascicular atrophy, and perivascular and perifascicular lymphocytic infiltrates. These can be patchy, diminished by steroid use, and occasionally seen in noninflammatory muscular dystrophies.8 For a patient with typical myositis and a characteristic rash, muscle biopsy may be unnecessary.1
The clinical utility of serologic testing for diagnosing dermatomyositis is controversial.2 Myositis‐specific antibody testing is insensitive but specific; these antibodies include Jo‐1, an antisynthetase antibody that predicts incomplete response to therapy and lung involvement, and Mi‐2, which is associated with better response to therapy.2, 9, 10 The sensitivity and specificity of antinuclear antibodies are both approximately 60%.10
Patients with dermatomyositis have higher rates of cancers than age‐matched controls, and nearly 25% of patients are diagnosed with a malignancy at some point during the course of the disease.11 Malignancies are typically solid tumors that manifest within 3 years of the diagnosis,1214 although the increased risk may exist for at least 5 years.14 There is a 10‐fold higher risk of ovarian cancer in women with dermatomyositis.12, 15 Other associated malignancies include lung, gastric, colorectal, pancreatic, and breast carcinomas and non‐Hodgkin's lymphoma.14
Recommendations for screening affected patients for cancer have changed over the years, with increasing evidence of an association between dermatomyositis and malignancy and evolving improvements in diagnostic techniques.16 Many authorities recommend that all adult patients with dermatomyositis be evaluated for cancer, including a complete physical examination, basic hematological tests, age‐ and sex‐appropriate screening (eg, mammography, pap smear, and colonoscopy), and chest x‐ray.16 Some would add upper endoscopy; imaging of the chest, abdomen, and pelvis; gynecological examination; and serum CA‐125 level to better evaluate for the most common malignancies (ie, ovarian, gastric, lung, and pancreatic carcinomas and non‐Hodgkins lymphoma).12, 1720
In 19% of adults, dermatomyositis overlaps with other autoimmune disorders, usually systemic lupus erythematosus and systemic sclerosis.21 These manifest as Raynaud's phenomenon, arthritis, esophageal dysmotility, renal disease, or neuropathy.21 Other potentially serious systemic manifestations of dermatomyositis include proximal dysphagia from pharyngeal myopathy; distal dysphagia from esophageal dysmotility in systemic sclerosis overlap; pulmonary disease from autoimmune interstitial lung disease or aspiration; cardiac disease from conduction abnormalities, myocarditis, pericarditis, and valvular disease; and rhabdomyolysis.2
Treatment of dermatomyositis requires systemic immunosuppression with 1 or more agents. The prognosis of dermatomyositis is variable. Mortality at 5 years ranges from 23% to 73%. At least a third of patients are left with mild to severe disability.1 In addition to older age, predictors of poor outcome include male sex, dysphagia, longstanding symptoms before treatment, pulmonary or cardiac involvement, and presence of antisynthetase antibodies.22
Dermatomyositis is often treated in the outpatient setting, but there are many reasons for hospitalization. Complications of treatment, like infection or adverse effects of medications, could result in hospitalization. Treatment with intravenous pulse corticosteroids or IVIG may require inpatient administration if no infusion center is available. Other indications for inpatient evaluation include the consequences of various malignancies and the more severe expression of systemic complications of dermatomyositis (eg, dysphagia and pulmonary, cardiac, or renal disease).
Every parent knows the plaintive backseat whine, Are we there, yet? Clinicians may also experience this feeling when attempting to diagnose a perplexing illness, especially one that lacks a definitive diagnostic test. It was easy for this patient's doctors to assume initially that his new rash was a manifestation of his long‐standing psoriasis. Having done so, they could understandably attribute the subsequent findings to either evolution of this disease or to consequences of the prescribed treatments, rather than considering a novel diagnosis. Only when faced with new (or newly appreciated) findings suggesting myopathy did the clinicians (and our discussant) consider the diagnosis of dermatomyositis. Even then, the primary inpatient medical team and their consultants were unsure when they had sufficient evidence to be certain.
Several factors compounded the difficulty of making a diagnosis in this case: the clinicians were dealing with a rare disease; they were considering alternative diagnoses (ie, psoriasis or a toxic effect of medication); and the disease presented somewhat atypically. The clinicians initially failed to consider and then accept the correct diagnosis because the patient's rash was not classic, his biopsy was interpreted as nonspecific, and he lacked myositis at presentation. Furthermore, when the generalists sought expert assistance, they encountered a difference of opinion among the consultants. These complex situations should goad the clinician into carefully considering the therapeutic threshold, that is, the transition point from diagnostic testing to therapeutic intervention.23 With complex cases like this, it may be difficult to know when one has reached a strongly supported diagnosis, and frequently asking whether we are there yet may be appropriate.
Take‐Home Points for the Hospitalist
-
A skin rash, which may have typical or atypical features, distinguishes dermatomyositis from other acquired myopathies.
-
Consider consultation with pathology specialists for skin and muscle biopsies.
-
Ovarian, lung, gastric, colorectal, pancreatic, and breast carcinomas and non‐Hodgkin's lymphoma are the most common cancers associated with dermatomyositis.
-
In addition to age‐appropriate cancer screening, consider obtaining upper endoscopy, imaging of the chest/abdomen/pelvis, and CA‐125.
-
Patients with dermatomyositis and no obvious concurrent malignancy need long‐term outpatient follow‐up for repeated malignancy screening.
- ,.Polymyositis and dermatomyositis.Lancet.2003;362:971–982.
- .Dermatomyositis.Lancet.2000;355:53–47.
- ,,,.Oropharyngeal dysphagia in polymyositis/dermatomyositis.Clin Neurol Neurosurg.2004;107(1):32–37.
- ,.Skin involvement in dermatomyositis.Curr Opin Rheumatol.2003;15:714–22.
- ,,,,,.Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: analysis of 100 French Canadian patients.Medicine (Baltimore).2005;84:231–249.
- .Skin Pathology.2nd ed.New York:Churchill Livingstone;2002.
- ,.Utility of magnetic resonance imaging in the evaluation of patients with inflammatory myopathies.Curr Rheumatol Rep.2001;3:334–245.
- ,,.Is it really myositis? A consideration of the differential diagnosis.Curr Opin Rheumatol2004;16:684–691.
- .Idiopathic inflammatory myopathy: autoantibody update.Curr Rheumatol Rep.2002;4:434–441.
- ,,.Laboratory assessment in musculoskeletal disorders.Best Pract Res Clin Rheumatol.2003;17:475–494.
- ,.Dermatomyositis.Clin Dermatol.2006;24:363–373.
- ,,, et al.Frequency of specific cancer types in dermatomyositis and polymyositis: a population‐based study.Lancet.2001;357:96–100.
- ,,, et al.Cancer‐associated myositis: clinical features and prognostic signs.Ann N Y Acad Sci.2005;1051:64–71.
- ,,,,.Incidence of malignant disease in biopsy‐proven inflammatory myopathy. A population‐based cohort study.Ann Intern Med.2001;134:1087–1095.
- ,,.Risk of cancer in patients with dermatomyositis or polymyositis, and follow‐up implications: a Scottish population‐based cohort study.Br J Cancer.2001;85 (1):41–45.
- .When and how should the patient with dermatomyositis or amyopathic dermatomyositis be assessed for possible cancer?Arch Dermatol.2002;138:969–971.
- ,,.Ovarian cancer in patients with dermatomyositis.Medicine (Baltimore).1994;73(3):153–160.
- ,,,.Dermatomyositis sine myositis: association with malignancy.J Rheumatol.1996;23 (1):101–105.
- ,,, et al.Tumor antigen markers for the detection of solid cancers in inflammatory myopathies.Cancer Epidemiol Biomarkers Prev.2005;14:1279–1282.
- ,,, et al.Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: a study of 40 patients.Arch Dermatol.2002;138:885–890.
- ,,,,,.Dermatomyositis: a dermatology‐based case series.J Am Acad Dermatol.1998;38:397–404.
- ,,, et al.Long‐term outcome in polymyositis and dermatomyositis.Ann Rheum Dis.2006;65:1456–1461.
- .Our stubborn quest for diagnostic certainty. A cause of excessive testing.N Engl J Med.1989;320:1489–1491.
- ,.Polymyositis and dermatomyositis.Lancet.2003;362:971–982.
- .Dermatomyositis.Lancet.2000;355:53–47.
- ,,,.Oropharyngeal dysphagia in polymyositis/dermatomyositis.Clin Neurol Neurosurg.2004;107(1):32–37.
- ,.Skin involvement in dermatomyositis.Curr Opin Rheumatol.2003;15:714–22.
- ,,,,,.Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: analysis of 100 French Canadian patients.Medicine (Baltimore).2005;84:231–249.
- .Skin Pathology.2nd ed.New York:Churchill Livingstone;2002.
- ,.Utility of magnetic resonance imaging in the evaluation of patients with inflammatory myopathies.Curr Rheumatol Rep.2001;3:334–245.
- ,,.Is it really myositis? A consideration of the differential diagnosis.Curr Opin Rheumatol2004;16:684–691.
- .Idiopathic inflammatory myopathy: autoantibody update.Curr Rheumatol Rep.2002;4:434–441.
- ,,.Laboratory assessment in musculoskeletal disorders.Best Pract Res Clin Rheumatol.2003;17:475–494.
- ,.Dermatomyositis.Clin Dermatol.2006;24:363–373.
- ,,, et al.Frequency of specific cancer types in dermatomyositis and polymyositis: a population‐based study.Lancet.2001;357:96–100.
- ,,, et al.Cancer‐associated myositis: clinical features and prognostic signs.Ann N Y Acad Sci.2005;1051:64–71.
- ,,,,.Incidence of malignant disease in biopsy‐proven inflammatory myopathy. A population‐based cohort study.Ann Intern Med.2001;134:1087–1095.
- ,,.Risk of cancer in patients with dermatomyositis or polymyositis, and follow‐up implications: a Scottish population‐based cohort study.Br J Cancer.2001;85 (1):41–45.
- .When and how should the patient with dermatomyositis or amyopathic dermatomyositis be assessed for possible cancer?Arch Dermatol.2002;138:969–971.
- ,,.Ovarian cancer in patients with dermatomyositis.Medicine (Baltimore).1994;73(3):153–160.
- ,,,.Dermatomyositis sine myositis: association with malignancy.J Rheumatol.1996;23 (1):101–105.
- ,,, et al.Tumor antigen markers for the detection of solid cancers in inflammatory myopathies.Cancer Epidemiol Biomarkers Prev.2005;14:1279–1282.
- ,,, et al.Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: a study of 40 patients.Arch Dermatol.2002;138:885–890.
- ,,,,,.Dermatomyositis: a dermatology‐based case series.J Am Acad Dermatol.1998;38:397–404.
- ,,, et al.Long‐term outcome in polymyositis and dermatomyositis.Ann Rheum Dis.2006;65:1456–1461.
- .Our stubborn quest for diagnostic certainty. A cause of excessive testing.N Engl J Med.1989;320:1489–1491.
Effect of Educational Intervention on Intern Confidence
Cross‐cover is defined as an on‐call physician managing acute problems such as chest pain, dyspnea, and hypoxemia for patients primarily cared for by another physician. Cross‐cover problems are commonly encountered with hospitalized patients, and inappropriate evaluation and management can result in misdiagnosis. Residents in many internal medicine residency programs receive only informal instruction about how to manage cross‐cover problems, usually from senior medical residents. Unfortunately, instruction is often provided while a patient is experiencing a problem, a frequent occurrence in the chaotic circumstances of a stressful learning environment. Furthermore, the knowledge base, experience, and teaching skills of senior residents vary substantially, and typically senior residents receive no formal instruction to guide them in how or what to teach more junior residents. If formal instruction is provided to residents, it is typically through often poorly attended didactic lectures that have been shown to be an ineffective forum for acquiring skills or changing physician behavior.15
Although previous studies did find that educational interventions can improve confidence and increase knowledge about various aspects of residency training, many of these studies were not randomized,68 or they involved complex interventions requiring a significant amount of resident and teaching staff time.911 The few randomized studies that used simple educational interventions focused on outpatient education, but most of a resident's time is spent in an inpatient setting.1213
Therefore, we designed a simple, randomized educational intervention consisting of 2 formal small‐group, case‐based discussion sessions addressing 1 cross‐cover situation: a hospitalized patient with acute dyspnea. We hypothesized that the addition of small‐group sessions would improve intern knowledge about and confidence in managing acute dyspnea above that gained from a combination of informal education and formal but lecture‐based education.
METHODS
Thirty‐eight internal medicine residents in their first year of postgraduate training (interns) at the University of Michigan were approached to participate in the study. Twenty‐six interns signed informed consent forms and were randomized using a random number generator to receive either the standard education (the control group) or the standard education plus the educational intervention (the intervention group). The standard education was informal teaching by senior medical residents on the wards and a 1‐hour lecture on Approach to the Patient with Acute Dyspnea, taught by an attending physician from the Department of Pulmonary and Critical Care Medicine. The educational intervention included the standard education as well as 2 small‐group, case‐based interactive sessions on acute dyspnea management. Both sessions were developed and taught by the first author (T.M.R.), a third‐year resident in internal medicine. A senior resident taught the sessions to try to make the information more relevant and practical and to make asking questions less intimidating. The first session, which lasted 50 minutes, discussed cases of bronchospasm, pulmonary edema, and pulmonary embolism as causes of acute dyspnea. It addressed several concepts: knowing when and how quickly to evaluate a dyspneic patient, formulating a differential diagnosis, appropriately evaluating acute dyspnea, providing empiric therapy, and recognizing indications for intubation. The second small‐group session occurred approximately 1 month after the first session and lasted 30 minutes. In this session key concepts learned during the first session were reviewed, and a case of ventricular tachycardia presenting as acute dyspnea was discussed. In an effort to increase attendance, free food and drink were provided at each session, and participants were sent reminders via e‐mail and the paging system prior to each session.
All study participants completed pre‐ and postintervention surveys that assessed their knowledge of acute dyspnea management and their confidence in managing patients with this condition. The pretests were conducted just before the first small‐group session was held. The post‐tests were conducted 4 months later. Knowledge was assessed by the score on the 45‐point test, which contained both open‐ and closed‐ended questions derived from 10 case‐based items. The number of points that a question was worth varied depending on how many elements made up a correct answer. For example, one question asked, What tests (if any) do you plan to order immediately after you examine the patient? As 3 tests should have been obtained (EKG, CXR, and ABG), this item had a maximum score of 3 points. Confidence was assessed by averaging 17 items scored on a 5‐point Likert scale (from strongly agree to strongly disagree). The items measured the physician's confidence in managing various aspects of the dyspneic patient (eg, confidence in knowing when to intubate a patient, when to obtain an ABG/CXR/EKG, and when to transfer a patient to the ICU). Data were analyzed using repeated‐measures analysis of variance. Primary analysis was based on the intention‐to‐treat principle, with alpha set to .05 (2‐sided). A secondary, per‐protocol analysis was also performed. In this analysis, study participants who attended both small‐group sessions (ie, completed the entire intervention) were compared with the control group. The protocol was approved by the institutional review board at the University of Michigan Health System.
RESULTS
All participants completed the study. Overall, only 3 of the 26 interns attended the lecture on Approach to the Patient with Acute Dyspnea. Fourteen of the 16 interns assigned to the intervention group attended 1 of the 2 small‐group sessions (11 attended the first session, and 10 attended the second session). Seven interns attended both sessions. The study period was 4 months. Both the intervention and control groups reported managing a similar number of patients with acute dyspnea, both prior to the study (mean of 5.9 in the intervention group and 7.4 in the control group, P = .51) and at the end of study (mean 10.6 in the intervention group and 10.2 in the control group, P = .91). There was no significant difference in the total number of completed inpatient months (mean of 4.9 in the intervention group and 4.7 in the control group, P =. 32) or in the number of inpatient months completed prior to the start of the study (mean of 2 in the intervention group and 2.4 in the control group, P = .15).
Confidence
Subjects in both the intervention and control groups showed increased confidence over time. The mean score of the intervention group increased from 3.77 to 4.57 (a 21.2% increase) and that of the control group increased from 3.74 to 4.28 (a 14.4% increase). Although the trend over time was highly significant for both groups (P < .001), the effect of the intervention was not significant (P = .19). However, the power to detect a difference between the groups was low (0.25). In the per‐protocol analysis, there was no significant difference between the groups (P = .26; see Fig. 1).
Knowledge
In the primary analysis, results for knowledge were similar to those obtained for the confidence outcome. In the intervention group, the mean score increased from 35.6 to 38.3 (a 7.6% increase); in the control group, the mean increased from 36.2 to 38.2 (a 5.5% increase). Scores ranged from 31 to 42. Again, the trend for both groups was significant (P < .01), but the effect of the intervention was not significant (P = .65). The power to detect a difference between groups was again low (0.07). In the per‐protocol analysis a trend toward significance was seen, with mean scores increasing from 34.6 to 40.0, a 15.6% increase (P = .067; see Fig. 2).
DISCUSSION
Our randomized controlled trial found that intern confidence and knowledge about acute dyspnea management both increased significantly over time; however, no significant differences between the intervention and control groups were observed. The complete intervention was not administered to the vast majority of those in the intervention group, however, likely skewing results toward the null. As suggested by the per‐protocol analysis, there was a trend toward a significant increase in the knowledge of the interns who had received the entire intervention. This is similar to results found in a randomized study by Schroy et al., which demonstrated a significant increase in resident knowledge of colorectal cancer screening after an educational intervention that used an interactive, case‐based seminar.13
Our study had several strengths. First, we employed the most robust design to detect efficacy, a randomized controlled study design. Second, we had complete follow‐up because all participants finished the study. Finally, our intervention is easily reproducible.
Our findings should also be considered within the context of several limitations. Despite the use of a random number generator, the control and intervention groups were unequal in number, which may have affected the results, particularly with such a small sample size.
Second, the intervention did not occur until 3 months after the start of each participant's internship. The intention was to implement the intervention at the start of internship, but institutional review board approval did not occur for an additional 3 months. This late timing might have been unfortunate because interns may already have had an established management plan for acute dyspnea, making their behavior more difficult to alter, even with additional education.
Third, because we were unaware of available test instruments to assess resident knowledge of acute dyspnea in the hospitalized patient, we needed to create our own. Unfortunately, the instrument yielded only a small variance in test scores, which may have made it difficult to detect an effect on scores if present.
Fourth, attendance at each session was suboptimal, and thus the complete intervention was not administered to the vast majority of those in the intervention group. Because the first small‐group session was the main teaching session, interns who only attended the second session were exposed to just one case discussion and only a review, rather than a full formal discussion, of the material presented during the first session. Therefore, it is not known if the intervention really had no effect or if no differences were detected simply because the complete intervention was not received. The trend toward significance observed in the per‐protocol analysis suggests that compliance with the intervention may be the key to improving knowledge.
Given the small differences observed in this study, future interventions ideally should use a more sensitive testing instrument, a larger sample, and a more powerful intervention that occurs early in training. Future efforts should also be designed to improve attendance at educational interventions. In the setting of reduced resident work hours and increased demands on resident time, this will prove to be a true challenge for all educators and residency programs.
- ,,,.A standardized‐patient assessment of a continuing medical education program to improve physicians' cancer‐control clinical skills.Acad Med.1995;70(1):52–58.
- ,,.Teaching smoking cessation skills to senior medical students: a block‐randomized controlled trial of four different approaches.Prev Med.1996;25:251–258.
- ,,,,,.Impact of formal continuing medical education: Do conferences, workshops, rounds, and other traditional continuing education activities change physician behavior or health care outcomes?JAMA.1999;282:867–874.
- ,,,,,.Problem‐based learning versus lecture‐based learning in postgraduate medical education.Scand J Work Environ Health.2003;29:280–287.
- ,,,,,,,.Better Prescribing Project: A randomized controlled trial of the impact of case‐based educational modules and personal prescribing feedback on prescribing for hypertension in primary care.Family Pract.2004;21:575–581.
- ,.Effect of an educational intervention about breastfeeding on the knowledge, confidence, and behaviors of pediatric resident physicians.Pediatrics.2002;110(5):e59.
- ,,,,,.Effects of a depression education program on residents' knowledge, attitudes, and clinical skills.Obstet Gynecol.2003;101(1):167–174.
- ,,,.Implementation of a web‐ and simulation‐based curriculum to ease the transition from medical school to surgical internship.Am J Surg.2005;190(1):137–140.
- ,,, et al.The effectiveness of intensive training for residents in interviewing.Ann Intern Med.1998;128(2):118–126.
- ,.Structured teaching methods enhance skill acquisition but not problem‐solving abilities: an evaluation of the “silent run through.”Med Educ.1999;33:019–023.
- ,,,,,.An educational intervention to improve physician violence screening skills.Pediatrics.2001;107(5):e68.
- ,,,,,,.Improving emergency medicine residents' approach to patients with alcohol problems: a controlled educational trial.Ann Emerg Med.2002;40(1):50–62.
- ,,,,,.A novel educational strategy to enhance internal medicine residents' familial colorectal cancer knowledge and risk assessment skills.Am J Gastroenterol.2005;100:677–684.
Cross‐cover is defined as an on‐call physician managing acute problems such as chest pain, dyspnea, and hypoxemia for patients primarily cared for by another physician. Cross‐cover problems are commonly encountered with hospitalized patients, and inappropriate evaluation and management can result in misdiagnosis. Residents in many internal medicine residency programs receive only informal instruction about how to manage cross‐cover problems, usually from senior medical residents. Unfortunately, instruction is often provided while a patient is experiencing a problem, a frequent occurrence in the chaotic circumstances of a stressful learning environment. Furthermore, the knowledge base, experience, and teaching skills of senior residents vary substantially, and typically senior residents receive no formal instruction to guide them in how or what to teach more junior residents. If formal instruction is provided to residents, it is typically through often poorly attended didactic lectures that have been shown to be an ineffective forum for acquiring skills or changing physician behavior.15
Although previous studies did find that educational interventions can improve confidence and increase knowledge about various aspects of residency training, many of these studies were not randomized,68 or they involved complex interventions requiring a significant amount of resident and teaching staff time.911 The few randomized studies that used simple educational interventions focused on outpatient education, but most of a resident's time is spent in an inpatient setting.1213
Therefore, we designed a simple, randomized educational intervention consisting of 2 formal small‐group, case‐based discussion sessions addressing 1 cross‐cover situation: a hospitalized patient with acute dyspnea. We hypothesized that the addition of small‐group sessions would improve intern knowledge about and confidence in managing acute dyspnea above that gained from a combination of informal education and formal but lecture‐based education.
METHODS
Thirty‐eight internal medicine residents in their first year of postgraduate training (interns) at the University of Michigan were approached to participate in the study. Twenty‐six interns signed informed consent forms and were randomized using a random number generator to receive either the standard education (the control group) or the standard education plus the educational intervention (the intervention group). The standard education was informal teaching by senior medical residents on the wards and a 1‐hour lecture on Approach to the Patient with Acute Dyspnea, taught by an attending physician from the Department of Pulmonary and Critical Care Medicine. The educational intervention included the standard education as well as 2 small‐group, case‐based interactive sessions on acute dyspnea management. Both sessions were developed and taught by the first author (T.M.R.), a third‐year resident in internal medicine. A senior resident taught the sessions to try to make the information more relevant and practical and to make asking questions less intimidating. The first session, which lasted 50 minutes, discussed cases of bronchospasm, pulmonary edema, and pulmonary embolism as causes of acute dyspnea. It addressed several concepts: knowing when and how quickly to evaluate a dyspneic patient, formulating a differential diagnosis, appropriately evaluating acute dyspnea, providing empiric therapy, and recognizing indications for intubation. The second small‐group session occurred approximately 1 month after the first session and lasted 30 minutes. In this session key concepts learned during the first session were reviewed, and a case of ventricular tachycardia presenting as acute dyspnea was discussed. In an effort to increase attendance, free food and drink were provided at each session, and participants were sent reminders via e‐mail and the paging system prior to each session.
All study participants completed pre‐ and postintervention surveys that assessed their knowledge of acute dyspnea management and their confidence in managing patients with this condition. The pretests were conducted just before the first small‐group session was held. The post‐tests were conducted 4 months later. Knowledge was assessed by the score on the 45‐point test, which contained both open‐ and closed‐ended questions derived from 10 case‐based items. The number of points that a question was worth varied depending on how many elements made up a correct answer. For example, one question asked, What tests (if any) do you plan to order immediately after you examine the patient? As 3 tests should have been obtained (EKG, CXR, and ABG), this item had a maximum score of 3 points. Confidence was assessed by averaging 17 items scored on a 5‐point Likert scale (from strongly agree to strongly disagree). The items measured the physician's confidence in managing various aspects of the dyspneic patient (eg, confidence in knowing when to intubate a patient, when to obtain an ABG/CXR/EKG, and when to transfer a patient to the ICU). Data were analyzed using repeated‐measures analysis of variance. Primary analysis was based on the intention‐to‐treat principle, with alpha set to .05 (2‐sided). A secondary, per‐protocol analysis was also performed. In this analysis, study participants who attended both small‐group sessions (ie, completed the entire intervention) were compared with the control group. The protocol was approved by the institutional review board at the University of Michigan Health System.
RESULTS
All participants completed the study. Overall, only 3 of the 26 interns attended the lecture on Approach to the Patient with Acute Dyspnea. Fourteen of the 16 interns assigned to the intervention group attended 1 of the 2 small‐group sessions (11 attended the first session, and 10 attended the second session). Seven interns attended both sessions. The study period was 4 months. Both the intervention and control groups reported managing a similar number of patients with acute dyspnea, both prior to the study (mean of 5.9 in the intervention group and 7.4 in the control group, P = .51) and at the end of study (mean 10.6 in the intervention group and 10.2 in the control group, P = .91). There was no significant difference in the total number of completed inpatient months (mean of 4.9 in the intervention group and 4.7 in the control group, P =. 32) or in the number of inpatient months completed prior to the start of the study (mean of 2 in the intervention group and 2.4 in the control group, P = .15).
Confidence
Subjects in both the intervention and control groups showed increased confidence over time. The mean score of the intervention group increased from 3.77 to 4.57 (a 21.2% increase) and that of the control group increased from 3.74 to 4.28 (a 14.4% increase). Although the trend over time was highly significant for both groups (P < .001), the effect of the intervention was not significant (P = .19). However, the power to detect a difference between the groups was low (0.25). In the per‐protocol analysis, there was no significant difference between the groups (P = .26; see Fig. 1).

Knowledge
In the primary analysis, results for knowledge were similar to those obtained for the confidence outcome. In the intervention group, the mean score increased from 35.6 to 38.3 (a 7.6% increase); in the control group, the mean increased from 36.2 to 38.2 (a 5.5% increase). Scores ranged from 31 to 42. Again, the trend for both groups was significant (P < .01), but the effect of the intervention was not significant (P = .65). The power to detect a difference between groups was again low (0.07). In the per‐protocol analysis a trend toward significance was seen, with mean scores increasing from 34.6 to 40.0, a 15.6% increase (P = .067; see Fig. 2).

DISCUSSION
Our randomized controlled trial found that intern confidence and knowledge about acute dyspnea management both increased significantly over time; however, no significant differences between the intervention and control groups were observed. The complete intervention was not administered to the vast majority of those in the intervention group, however, likely skewing results toward the null. As suggested by the per‐protocol analysis, there was a trend toward a significant increase in the knowledge of the interns who had received the entire intervention. This is similar to results found in a randomized study by Schroy et al., which demonstrated a significant increase in resident knowledge of colorectal cancer screening after an educational intervention that used an interactive, case‐based seminar.13
Our study had several strengths. First, we employed the most robust design to detect efficacy, a randomized controlled study design. Second, we had complete follow‐up because all participants finished the study. Finally, our intervention is easily reproducible.
Our findings should also be considered within the context of several limitations. Despite the use of a random number generator, the control and intervention groups were unequal in number, which may have affected the results, particularly with such a small sample size.
Second, the intervention did not occur until 3 months after the start of each participant's internship. The intention was to implement the intervention at the start of internship, but institutional review board approval did not occur for an additional 3 months. This late timing might have been unfortunate because interns may already have had an established management plan for acute dyspnea, making their behavior more difficult to alter, even with additional education.
Third, because we were unaware of available test instruments to assess resident knowledge of acute dyspnea in the hospitalized patient, we needed to create our own. Unfortunately, the instrument yielded only a small variance in test scores, which may have made it difficult to detect an effect on scores if present.
Fourth, attendance at each session was suboptimal, and thus the complete intervention was not administered to the vast majority of those in the intervention group. Because the first small‐group session was the main teaching session, interns who only attended the second session were exposed to just one case discussion and only a review, rather than a full formal discussion, of the material presented during the first session. Therefore, it is not known if the intervention really had no effect or if no differences were detected simply because the complete intervention was not received. The trend toward significance observed in the per‐protocol analysis suggests that compliance with the intervention may be the key to improving knowledge.
Given the small differences observed in this study, future interventions ideally should use a more sensitive testing instrument, a larger sample, and a more powerful intervention that occurs early in training. Future efforts should also be designed to improve attendance at educational interventions. In the setting of reduced resident work hours and increased demands on resident time, this will prove to be a true challenge for all educators and residency programs.
Cross‐cover is defined as an on‐call physician managing acute problems such as chest pain, dyspnea, and hypoxemia for patients primarily cared for by another physician. Cross‐cover problems are commonly encountered with hospitalized patients, and inappropriate evaluation and management can result in misdiagnosis. Residents in many internal medicine residency programs receive only informal instruction about how to manage cross‐cover problems, usually from senior medical residents. Unfortunately, instruction is often provided while a patient is experiencing a problem, a frequent occurrence in the chaotic circumstances of a stressful learning environment. Furthermore, the knowledge base, experience, and teaching skills of senior residents vary substantially, and typically senior residents receive no formal instruction to guide them in how or what to teach more junior residents. If formal instruction is provided to residents, it is typically through often poorly attended didactic lectures that have been shown to be an ineffective forum for acquiring skills or changing physician behavior.15
Although previous studies did find that educational interventions can improve confidence and increase knowledge about various aspects of residency training, many of these studies were not randomized,68 or they involved complex interventions requiring a significant amount of resident and teaching staff time.911 The few randomized studies that used simple educational interventions focused on outpatient education, but most of a resident's time is spent in an inpatient setting.1213
Therefore, we designed a simple, randomized educational intervention consisting of 2 formal small‐group, case‐based discussion sessions addressing 1 cross‐cover situation: a hospitalized patient with acute dyspnea. We hypothesized that the addition of small‐group sessions would improve intern knowledge about and confidence in managing acute dyspnea above that gained from a combination of informal education and formal but lecture‐based education.
METHODS
Thirty‐eight internal medicine residents in their first year of postgraduate training (interns) at the University of Michigan were approached to participate in the study. Twenty‐six interns signed informed consent forms and were randomized using a random number generator to receive either the standard education (the control group) or the standard education plus the educational intervention (the intervention group). The standard education was informal teaching by senior medical residents on the wards and a 1‐hour lecture on Approach to the Patient with Acute Dyspnea, taught by an attending physician from the Department of Pulmonary and Critical Care Medicine. The educational intervention included the standard education as well as 2 small‐group, case‐based interactive sessions on acute dyspnea management. Both sessions were developed and taught by the first author (T.M.R.), a third‐year resident in internal medicine. A senior resident taught the sessions to try to make the information more relevant and practical and to make asking questions less intimidating. The first session, which lasted 50 minutes, discussed cases of bronchospasm, pulmonary edema, and pulmonary embolism as causes of acute dyspnea. It addressed several concepts: knowing when and how quickly to evaluate a dyspneic patient, formulating a differential diagnosis, appropriately evaluating acute dyspnea, providing empiric therapy, and recognizing indications for intubation. The second small‐group session occurred approximately 1 month after the first session and lasted 30 minutes. In this session key concepts learned during the first session were reviewed, and a case of ventricular tachycardia presenting as acute dyspnea was discussed. In an effort to increase attendance, free food and drink were provided at each session, and participants were sent reminders via e‐mail and the paging system prior to each session.
All study participants completed pre‐ and postintervention surveys that assessed their knowledge of acute dyspnea management and their confidence in managing patients with this condition. The pretests were conducted just before the first small‐group session was held. The post‐tests were conducted 4 months later. Knowledge was assessed by the score on the 45‐point test, which contained both open‐ and closed‐ended questions derived from 10 case‐based items. The number of points that a question was worth varied depending on how many elements made up a correct answer. For example, one question asked, What tests (if any) do you plan to order immediately after you examine the patient? As 3 tests should have been obtained (EKG, CXR, and ABG), this item had a maximum score of 3 points. Confidence was assessed by averaging 17 items scored on a 5‐point Likert scale (from strongly agree to strongly disagree). The items measured the physician's confidence in managing various aspects of the dyspneic patient (eg, confidence in knowing when to intubate a patient, when to obtain an ABG/CXR/EKG, and when to transfer a patient to the ICU). Data were analyzed using repeated‐measures analysis of variance. Primary analysis was based on the intention‐to‐treat principle, with alpha set to .05 (2‐sided). A secondary, per‐protocol analysis was also performed. In this analysis, study participants who attended both small‐group sessions (ie, completed the entire intervention) were compared with the control group. The protocol was approved by the institutional review board at the University of Michigan Health System.
RESULTS
All participants completed the study. Overall, only 3 of the 26 interns attended the lecture on Approach to the Patient with Acute Dyspnea. Fourteen of the 16 interns assigned to the intervention group attended 1 of the 2 small‐group sessions (11 attended the first session, and 10 attended the second session). Seven interns attended both sessions. The study period was 4 months. Both the intervention and control groups reported managing a similar number of patients with acute dyspnea, both prior to the study (mean of 5.9 in the intervention group and 7.4 in the control group, P = .51) and at the end of study (mean 10.6 in the intervention group and 10.2 in the control group, P = .91). There was no significant difference in the total number of completed inpatient months (mean of 4.9 in the intervention group and 4.7 in the control group, P =. 32) or in the number of inpatient months completed prior to the start of the study (mean of 2 in the intervention group and 2.4 in the control group, P = .15).
Confidence
Subjects in both the intervention and control groups showed increased confidence over time. The mean score of the intervention group increased from 3.77 to 4.57 (a 21.2% increase) and that of the control group increased from 3.74 to 4.28 (a 14.4% increase). Although the trend over time was highly significant for both groups (P < .001), the effect of the intervention was not significant (P = .19). However, the power to detect a difference between the groups was low (0.25). In the per‐protocol analysis, there was no significant difference between the groups (P = .26; see Fig. 1).

Knowledge
In the primary analysis, results for knowledge were similar to those obtained for the confidence outcome. In the intervention group, the mean score increased from 35.6 to 38.3 (a 7.6% increase); in the control group, the mean increased from 36.2 to 38.2 (a 5.5% increase). Scores ranged from 31 to 42. Again, the trend for both groups was significant (P < .01), but the effect of the intervention was not significant (P = .65). The power to detect a difference between groups was again low (0.07). In the per‐protocol analysis a trend toward significance was seen, with mean scores increasing from 34.6 to 40.0, a 15.6% increase (P = .067; see Fig. 2).

DISCUSSION
Our randomized controlled trial found that intern confidence and knowledge about acute dyspnea management both increased significantly over time; however, no significant differences between the intervention and control groups were observed. The complete intervention was not administered to the vast majority of those in the intervention group, however, likely skewing results toward the null. As suggested by the per‐protocol analysis, there was a trend toward a significant increase in the knowledge of the interns who had received the entire intervention. This is similar to results found in a randomized study by Schroy et al., which demonstrated a significant increase in resident knowledge of colorectal cancer screening after an educational intervention that used an interactive, case‐based seminar.13
Our study had several strengths. First, we employed the most robust design to detect efficacy, a randomized controlled study design. Second, we had complete follow‐up because all participants finished the study. Finally, our intervention is easily reproducible.
Our findings should also be considered within the context of several limitations. Despite the use of a random number generator, the control and intervention groups were unequal in number, which may have affected the results, particularly with such a small sample size.
Second, the intervention did not occur until 3 months after the start of each participant's internship. The intention was to implement the intervention at the start of internship, but institutional review board approval did not occur for an additional 3 months. This late timing might have been unfortunate because interns may already have had an established management plan for acute dyspnea, making their behavior more difficult to alter, even with additional education.
Third, because we were unaware of available test instruments to assess resident knowledge of acute dyspnea in the hospitalized patient, we needed to create our own. Unfortunately, the instrument yielded only a small variance in test scores, which may have made it difficult to detect an effect on scores if present.
Fourth, attendance at each session was suboptimal, and thus the complete intervention was not administered to the vast majority of those in the intervention group. Because the first small‐group session was the main teaching session, interns who only attended the second session were exposed to just one case discussion and only a review, rather than a full formal discussion, of the material presented during the first session. Therefore, it is not known if the intervention really had no effect or if no differences were detected simply because the complete intervention was not received. The trend toward significance observed in the per‐protocol analysis suggests that compliance with the intervention may be the key to improving knowledge.
Given the small differences observed in this study, future interventions ideally should use a more sensitive testing instrument, a larger sample, and a more powerful intervention that occurs early in training. Future efforts should also be designed to improve attendance at educational interventions. In the setting of reduced resident work hours and increased demands on resident time, this will prove to be a true challenge for all educators and residency programs.
- ,,,.A standardized‐patient assessment of a continuing medical education program to improve physicians' cancer‐control clinical skills.Acad Med.1995;70(1):52–58.
- ,,.Teaching smoking cessation skills to senior medical students: a block‐randomized controlled trial of four different approaches.Prev Med.1996;25:251–258.
- ,,,,,.Impact of formal continuing medical education: Do conferences, workshops, rounds, and other traditional continuing education activities change physician behavior or health care outcomes?JAMA.1999;282:867–874.
- ,,,,,.Problem‐based learning versus lecture‐based learning in postgraduate medical education.Scand J Work Environ Health.2003;29:280–287.
- ,,,,,,,.Better Prescribing Project: A randomized controlled trial of the impact of case‐based educational modules and personal prescribing feedback on prescribing for hypertension in primary care.Family Pract.2004;21:575–581.
- ,.Effect of an educational intervention about breastfeeding on the knowledge, confidence, and behaviors of pediatric resident physicians.Pediatrics.2002;110(5):e59.
- ,,,,,.Effects of a depression education program on residents' knowledge, attitudes, and clinical skills.Obstet Gynecol.2003;101(1):167–174.
- ,,,.Implementation of a web‐ and simulation‐based curriculum to ease the transition from medical school to surgical internship.Am J Surg.2005;190(1):137–140.
- ,,, et al.The effectiveness of intensive training for residents in interviewing.Ann Intern Med.1998;128(2):118–126.
- ,.Structured teaching methods enhance skill acquisition but not problem‐solving abilities: an evaluation of the “silent run through.”Med Educ.1999;33:019–023.
- ,,,,,.An educational intervention to improve physician violence screening skills.Pediatrics.2001;107(5):e68.
- ,,,,,,.Improving emergency medicine residents' approach to patients with alcohol problems: a controlled educational trial.Ann Emerg Med.2002;40(1):50–62.
- ,,,,,.A novel educational strategy to enhance internal medicine residents' familial colorectal cancer knowledge and risk assessment skills.Am J Gastroenterol.2005;100:677–684.
- ,,,.A standardized‐patient assessment of a continuing medical education program to improve physicians' cancer‐control clinical skills.Acad Med.1995;70(1):52–58.
- ,,.Teaching smoking cessation skills to senior medical students: a block‐randomized controlled trial of four different approaches.Prev Med.1996;25:251–258.
- ,,,,,.Impact of formal continuing medical education: Do conferences, workshops, rounds, and other traditional continuing education activities change physician behavior or health care outcomes?JAMA.1999;282:867–874.
- ,,,,,.Problem‐based learning versus lecture‐based learning in postgraduate medical education.Scand J Work Environ Health.2003;29:280–287.
- ,,,,,,,.Better Prescribing Project: A randomized controlled trial of the impact of case‐based educational modules and personal prescribing feedback on prescribing for hypertension in primary care.Family Pract.2004;21:575–581.
- ,.Effect of an educational intervention about breastfeeding on the knowledge, confidence, and behaviors of pediatric resident physicians.Pediatrics.2002;110(5):e59.
- ,,,,,.Effects of a depression education program on residents' knowledge, attitudes, and clinical skills.Obstet Gynecol.2003;101(1):167–174.
- ,,,.Implementation of a web‐ and simulation‐based curriculum to ease the transition from medical school to surgical internship.Am J Surg.2005;190(1):137–140.
- ,,, et al.The effectiveness of intensive training for residents in interviewing.Ann Intern Med.1998;128(2):118–126.
- ,.Structured teaching methods enhance skill acquisition but not problem‐solving abilities: an evaluation of the “silent run through.”Med Educ.1999;33:019–023.
- ,,,,,.An educational intervention to improve physician violence screening skills.Pediatrics.2001;107(5):e68.
- ,,,,,,.Improving emergency medicine residents' approach to patients with alcohol problems: a controlled educational trial.Ann Emerg Med.2002;40(1):50–62.
- ,,,,,.A novel educational strategy to enhance internal medicine residents' familial colorectal cancer knowledge and risk assessment skills.Am J Gastroenterol.2005;100:677–684.
Copyright © 2006 Society of Hospital Medicine
Clinical Conundrum
A 47‐year‐old woman was brought to the emergency department by her family because of 1 week of abdominal pain. The pain had begun in the epigastrium but had spread across the abdomen. She described it as constant and 10 of 10 in intensity but could not identify aggravating or alleviating factors. She also complained of nausea and vomiting, beginning 4 days prior to presentation, occurring 25 times per day. She noted poor oral intake and mild diarrhea. She denied melena or hematochezia. She reported no recent fever, dysuria, chills, or night sweats; however, she reported upper respiratory symptoms 2 weeks prior to presentation. On the day of presentation, her family felt she was becoming increasingly lethargic.
Epigastric pain in a middle‐aged woman suggests several possible diagnoses. Conditions such as acute cholecystitis begin abruptly, whereas small bowel obstruction, appendicitis, and diverticulitis start gradually. Nausea and vomiting are common concomitants of abdominal pain and are nonspecific. The absence of fever and chills is reassuring. Of greatest concern is the mental status. Initially, I think of enterohemorrhagic E. coli syndromes with associated glomerulonephritis. With a more systemic metabolic abnormality such as this, the rapid development of the disease tends to exaggerate symptoms.
The patient had a history of nephrolithiasis and underwent total abdominal hysterectomy and bilateral salpingo‐oopherectomy secondary to uterine fibroids in the past. She took occasional acetaminophen, smoked two cigarettes per day, and rarely consumed alcohol. Temperature was 38.5C, heart rate was 160 beats/minute, respiratory rate was 28/minute, and blood pressure was 92/52 mm Hg; oxygen saturation was 100% breathing 2 L of oxygen by nasal cannula. She was a moderately obese African American woman in moderate distress, lying in bed moaning. Mucous membranes were dry. There was no lymphadenopathy or thyromegaly. Heart rate was regular without appreciable murmur, rub, or gallop. Lungs were clear. Abdomen was soft and nondistended, with diffuse tenderness to palpation; bowel sounds were present; there was no rebound or guarding. She had normal rectal tone with brown, guaiac‐negative stool. There was no costovertebral angle tenderness. She was oriented to person, place, and time but lethargic; deep tendon reflexes were 3+ bilaterally, and no focal signs were elicited.
Renal stones certainly produce abdominal pain, and the rare patient undergoes laparotomy for this reason. The hysterectomy tells us that small bowel obstruction could be a reason for her symptoms, although abnormal mental status would not be expected without additional problems such as infection. The tachycardia seems out of proportion to her temperature. Hyperpnea and absent respiratory symptoms, along with hypotension and tachycardia, suggest a sepsis syndrome. Her physical exam confirms dehydration. Examination of the abdomen makes me speculate about whether she has a nonsurgical cause of acute abdomen. The lethargy remains unexplained. Sepsis syndrome, possibly from a perinephric abscess, is my leading diagnosis.
White blood cell count was 15.9/mm3 with 78% neutrophils, a hemoglobin of 14.3 g/dL with a MCV of 76 and a platelet count of 320/mm3. Sodium was 159 mmol/L, chloride 128 mmol/L, bicarbonate 19 mmol/L, blood urea nitrogen 120 mmol/L, creatinine 3.1 mg/dL, calcium 11.7 mg/dL, albumin 3.3 g/dL, serum aspartate aminotransferase 65 U/L, serum alanine aminotransferase 72 U/L, total bilirubin 0.7 mg/dL, amylase 137 U/L (normal 30100), and lipase 92 IU/dL (normal 424). Urine obtained from a Foley catheter revealed negative nitrite and leukocyte esterase, 5075 red blood cells, and 1025 white blood cells per high‐powered field.
The elevated serum sodium is likely contributing to her abnormal mental status. It is unusual for a previously healthy and conscious woman to become this hypernatremic because persons with a normal mental status will defend their sodium balance strenuously, assuming regulatory mechanisms are intact. Generally, this level of hypernatremia indicates 2 things. One, a patient was not allowed, or did not seek access to, free water. The other is the presence of diabetes insipidus. It is unlikely she became this dehydrated from the initial gastrointestinal episode as described. The low MCV suggests she may be a thalassemia carrier, as microcytosis with iron deficiency typically does not occur until the patient is anemic, although she may be when rehydrated. Serum calcium, while elevated, also will likely return to the normal range with hydration. The metabolic abnormalities strongly suggest a problem in the central nervous system. The hematuria in the urinalysis continues to raise the possibility of nephrolithiasis as a cause of abdominal pain, though it does not fit well with the rest of the patient's clinical picture. The hematuria and pyuria both could still indicate a urinary tract infection such as pyelonephritis or perinephric abscess causing a sepsis syndrome.
An acute abdominal series and chest radiograph revealed a paucity of gas in the abdomen but no free air under the diaphragm or active cardiopulmonary disease. Abdominal ultrasound showed cholelithiasis without biliary dilation. There was no evidence of hydronephrosis, hydroureter, or perinephric abscess. A noncontrast abdominal‐pelvic computed tomography (CT) scan demonstrated no peripancreatic stranding or fluid collection and no nephrolithiasis or fluid collection suggestive of abscess. The admission electrocardiogram, read as sinus tachycardia with a rate of 160, is displayed in Figure 1.
I have long believed that unexplained sinus tachycardia is one of the most ominous rhythms in clinical medicine; it is expected after vigorous exercise, among other situations, but not in the condition in which this woman finds herself. The nature of the tracing does not indicate the likelihood of a supraventricular arrhythmia, particularly atrial flutter, which should be considered given the rate. The absence of free air under the diaphragm on chest radiography is reassuring. Though the pancreatic enzymes are mildly elevated, they are usually far more striking in gallstone pancreatitis. Hypercalcemia may result in abdominal pain by several mechanisms. I remain concerned about her central nervous system.
The patient was admitted to the intensive care unit (ICU), where she received intravenous antibiotics and aggressive rehydration. The following morning, she continued to complain of abdominal pain. Her systolic blood pressure was 115 mmHg, and her heart rate ranged between 140 and 150 beats/minute. The remainder of her physical exam was unchanged. Repeat laboratory tests revealed a white blood cell count of 14.7/mm3, a blood urea nitrogen of 66 mg/dL, a creatinine of 1.3 mg.dL, amylase of 67 IU/L, and lipase of 70 IU/dL. A contrast‐enhanced abdominal‐pelvic CT scan did not reveal intra‐abdominal pathology. Blood and urine cultures obtained at admission were negative for any growth.
The patient was appropriately admitted to the ICU. When caring for a critically ill patient, establishing a diagnosis is less important initially than addressing treatable conditions with dispatch. The negative CT scans rule out previously entertained diagnoses like nephrolithiasis and perinephric abscess. It is possible that the initially positive urinalysis was a result of urinary catheter placement trauma. Given the course to date, I believe this patient likely has a nonsurgical cause of abdominal pain. I am considering entities such as lead intoxication, hypercalcemia, a tear of the rectus abdominus caused by vomiting, systemic vasculitis, or a hypercoagulable state leading to intra‐abdominal venous thrombosis.
By hospital day 3 her sodium decreased to 149 mmol/L and her creatinine was 1.0 mg/dL. Abdominal pain persisted, unchanged from admission. Her systolic blood pressure had stabilized at 120 mmHg, but the heart rate remained near 150 beats/minute. Her abdomen remained soft and nondistended on exam but diffusely tender to palpation. Her amylase and lipase continued to decrease, and her repeat electrocardiogram demonstrated tachycardia with a rate of 144.
We are gratified to see that her serum sodium has waned but not with the persistence of the tachycardia. It must be assumed that this patient has an infectious disease that we are not clever enough to diagnose at this time. I am also considering an autoimmune process, such as systemic lupus erythematosus. It is difficult to envision a neoplastic disorder causing these problems. The differential remains broad, however, because we have not ruled out metabolic or endocrine causes. It is difficult to imagine she could have Addison's diseasea common cause of severe abdominal pain, tachycardia, and hypotensiongiven her serum sodium level. Hyperthyroidism has been known to produce mild hypercalcemia and abdominal complaints and is an intriguing possibility. The striking elevation of her serum sodium makes me consider the possibility of a problem in the posterior pituitary gland such as sarcoidosis. I cannot explain how sarcoidosis would cause her abdominal pain, unless the hypercalcemia were related. The tachycardia remains of concern, especially if she is otherwise improving. Thus, I would likely administer a small dose of adenosine to ascertain that this is not a different supraventricular tachycardia. In sinus tachycardia, the rate is usually attendant to the clinical picture and thus begs explanation given her clinical improvement.
After receiving 6 mg of intravenous adenosine, the patient's heart rate declined; atrial flutter waves were observed.
This case nicely demonstrates a key teaching point: a fast regular heart rate of about 150, irrespective of the electrocardiogram, suggests atrial flutter. Who gets atrial flutter? Patients with chronic lung disease, myocardial ischemia (albeit rarely), alcohol‐induced cardiomyopathy, and infiltrative cardiac disorders do. Additionally, we also have to consider thyroid dysfunction.
If forced to come up with a single unifying diagnosis at this point, I would have to say this patient most likely has sarcoidosis because this entity would account for modest hypercalcemia, the myocardial conduction disturbance, and hypernatremia because of diabetes insipidus; furthermore, it would fit the patient's demographic profile. However, I am also concerned about hyperthyroidism and would not proceed until thyroid function studies were obtained.
Thyroid studies revealed thyroid stimulating hormone of less than 0.01 mU/L (normal range, 0.305.50), free thyroxine (T4) of 5.81 ng.dL (normal range, 0.731.79), free triiodothyronine (T3) of 15.7 pg/mL (normal range, 2.85.3), and total triiodothyronine (T3) of 218 ng/dL (normal range, 95170). The patient was diagnosed with thyroid crisis and was started on propranolol, propylthiouracil, hydrocortisone, and a saturated solution of potassium iodine. Thyroid stimulating immunoglobulins were obtained and found to be markedly elevated (3.4 TSI index; normal < 1.3), suggestive of Grave's disease. Over the next several days, the patient's abdominal pain and tachycardia resolved. Her mental status returned to normal. A workup for her microcytic anemia revealed beta thalassemia trait. The patient was discharged home on hospital day 9 and has done well as an outpatient.
COMMENTARY
As Sir Zachary Cope stated in his classic text Cope's Early Diagnosis of the Acute Abdomen, [I]t is only by thorough history taking and physical examination that one can propound a diagnosis.1 When first presented with a patient whose chief complaint is abdominal pain, physicians tend to focus on the disorders of both the hollow and solid organs of the abdomen as potential sources of the pain. The differential diagnosis traditionally includes disorders such as cholecystitis, peptic ulcer disease, pancreatitis, small bowel obstruction, bowel ischemia or perforation, splenic abscess and infarct, nephrolithiasis, diverticulitis, and appendicitis, all of which were initially considered by the clinicians involved in this case. But as our discussant pointed out, in this case the differential needed to be broadened to include less common disorders, particularly given the patient's altered mental status, numerous electrolyte abnormalities, and lethargy and the lack of explanation provided by the physical examination and sophisticated imaging studies.
Specifically, a myriad of systemic diseases and metabolic derangements can cause abdominal complaints and mimic surgical abdominal disease, including hypercalcemia, acute intermittent porphyria, diabetic ketoacidosis, lead intoxication, familial Mediterranean fever, vasculopathies, adrenal insufficiency, and hyperthyroidism. Unfortunately, the frequency with which abdominal pain occurs in many of these less common disease processes and the pathophysiology that underlies its occurrence are not well defined. For example, abdominal pain is well described as a typical manifestation of both diabetic ketoacidosis and lead poisoning, but the pathophysiology behind its occurrence is poorly understood in both. Further, as a manifestation of thyrotoxicosis and as one of the diagnostic criteria for thyroid storm, the reported prevalence of abdominal pain in this condition is variable, ranging from rare to 20%47%.24 Also, although other gastrointestinal manifestations of hyperthyroidism (such as nausea, vomiting, and hyperdefecation) are thought to be the result of the effect of excess thyroid hormone on gastrointestinal motility, it is unclear whether this similar mechanism is responsible for the perception of abdominal pain.4
An important clue to the underlying diagnosis in this case was the patient's marked tachycardia. Classically, a persistent heart rate of 150 should raise suspicion of atrial flutter with a 2:1 conduction block, as was eventually discovered in this case. Adenosine, in addition to other vagal maneuvers such as carotid massage or Valsalva that also block atrioventricular (AV) node conduction, has been recognized as a safe and effective means of establishing a diagnosis in tachyarrhythmias.5 In AV nodal‐dependent tachycardias, such as AV node reentrant tachycardia or AV reentrant tachycardia, adenosine will often terminate the tachyarrhythmia by blocking the anterograde limb of the reentrant circuit. In AV nodeindependent tachyarrhythmias, such as atrial flutter or atrial fibrillation, adenosine will not terminate the rhythm. However, in the case of flutter, blocking the AV node will usually transiently unmask the underlying P waves, thereby facilitating the diagnosis.5, 6
In this patient, the discovery of atrial flutter was the main clue that thyrotoxicosis may provide the unifying diagnosis. Thyroid hormone has a direct positive cardiac chronotropic effect, resulting in the increased resting heart characteristic of thyrotoxicosis. Specifically, this hormone increases sinoatrial‐node firing, shortens the refractory period of conduction tissue within the heart, and decreases the electrical threshold for atrial excitation. In addition to predisposing to sinus tachycardia (the most common rhythm associated with this disorder), thyrotoxicosis is also associated with atrial tachycardias such as atrial flutter and, more classically, atrial fibrillation.7, 8 Though no studies have specifically evaluated the incidence of atrial flutter in thyrotoxicosis, atrial fibrillation has been found in 9%22% of these patients.7
Finally, several of the patient's electrolyte derangements could explain some of her clinical findings and are clues to the underlying diagnosis. She initially presented with a mild hypercalcemia that persisted even after hydration. Potential explanations include her severe dehydration or her underlying thyrotoxicosis because hypercalcemia is present in up to 20% of patients with hyperthyroidism.9, 10 However, the presence of significant hypercalcemia in the setting of thyrotoxicosis may actually make the diagnosis of thyrotoxicosis more difficult, masking the hypermetabolic signs and symptoms of the hyperthyroid state.11 Interestingly, coexistent primary hyperparathyroidism does occur in a few of these patients, but it likely was not an underlying cause in our patient given that her calcium normalized after receipt of propylthiouracil therapy.12
The patient's marked hypernatremia is more difficult to explain. She may have developed nephrogenic diabetes insipidus secondary to hypercalcemia, explained by a renal concentrating defect that can become evident once the calcium is persistently above 11 mg/dL.13 Combined with her altered mental status, which likely limited her ability to access free water, this may be enough to explain her marked hypernatremia. Her rapid improvement with rehydration is also consistent with this explanation, mediated through the improvement of her serum free calcium.
This case highlights the importance of using all the clinical clues provided by the history, physical exam, and laboratory and imaging studies when generating an initial differential diagnosis, as well as the importance of being willing to appropriately broaden and narrow the list of possibilities as a case evolves. When this patient was initially evaluated by physicians in the emergency department, they believed her symptoms were most consistent with generalized peritonitis that was likely secondary to an infectious or inflammatory intra‐abdominal process such as pancreatitis (especially in light of her mildly elevated lipase and amylase), appendicitis, or diverticulitis. When the medical team in the intensive care unit assumed care of this patient, members of the team failed to recognize several of the early clues, including the patient's markedly abnormal mental status, electrolyte derangements, and persistent tachycardia despite aggressive rehydration, which suggested the possibility of alternative, and less common, etiologies of her abdominal pain. Instead, they continued to aggressively pursue the possibility of the initial differential diagnosis, even repeating some of the previously negative studies from the emergency department. This case illustrates the importance of constantly reevaluating the available information from physical examination and laboratory and imaging studies and not falling victim to intellectual blind spots created by suggested diagnoses by other care providers. Fortunately for this patient, her thyroid crisis was diagnosed, albeit with some delay, before any long‐term complications occurred.
- Silen W, ed.Cope's Early Diagnosis of the Acute Abdomen.19th ed.New York:Oxford University Press;1995:4.
- ,.An unusual cause of abdominal pain in young woman.Ann Emerg Med.1991;20:574–582.
- .Vomiting, nausea and abdominal pain: unrecognized symptoms of thyrotoxicosis.J Fam Prac.1989;24:382–386.
- Powell DW,Alpers DH,Yamada,Owyang C,Laine L, eds.Textbook of Gastroenterology.3rd ed.Philadelphia, Pa:Lippincott Williams 783,2516.
- ,,.Usefulness of adenosine in diagnosis of tachyarrhythmias.Am J Cardiol.1995;75:952–955.
- ,,,,.Supraventricular tachycardia.Med Clin North Am.2001;85:193–223.
- .Thyrotoxicosis and the heart.N Engl J Med.1992;327:94–8.
- ,.Thyrotoxicosis and the heart.Endocrinol Metab Clin North Am.1998;27:51–62.
- ,,,.Treatment of thyrotoxic hypercalcemia with propranolol.N Engl J Med.1976;294:431.
- ,,,.Ionized and total plasma calcium and parathyroid hormone in hyperthyroidism.Ann Intern Med.1976;84:668.
- ,.Hypercalcemic crisis.Med Clin North Am.1995;79:79–92.
- ,,.Thyrotoxicosis, hypercalcemia, and secondary hyperparathyroidism.Arch Intern Med.1979;139:661–663.
- ,.Clinical Physiology of Acid‐Base and Electrolyte Disorders.5th ed.New York:McGraw‐Hill;2001:754–758.
A 47‐year‐old woman was brought to the emergency department by her family because of 1 week of abdominal pain. The pain had begun in the epigastrium but had spread across the abdomen. She described it as constant and 10 of 10 in intensity but could not identify aggravating or alleviating factors. She also complained of nausea and vomiting, beginning 4 days prior to presentation, occurring 25 times per day. She noted poor oral intake and mild diarrhea. She denied melena or hematochezia. She reported no recent fever, dysuria, chills, or night sweats; however, she reported upper respiratory symptoms 2 weeks prior to presentation. On the day of presentation, her family felt she was becoming increasingly lethargic.
Epigastric pain in a middle‐aged woman suggests several possible diagnoses. Conditions such as acute cholecystitis begin abruptly, whereas small bowel obstruction, appendicitis, and diverticulitis start gradually. Nausea and vomiting are common concomitants of abdominal pain and are nonspecific. The absence of fever and chills is reassuring. Of greatest concern is the mental status. Initially, I think of enterohemorrhagic E. coli syndromes with associated glomerulonephritis. With a more systemic metabolic abnormality such as this, the rapid development of the disease tends to exaggerate symptoms.
The patient had a history of nephrolithiasis and underwent total abdominal hysterectomy and bilateral salpingo‐oopherectomy secondary to uterine fibroids in the past. She took occasional acetaminophen, smoked two cigarettes per day, and rarely consumed alcohol. Temperature was 38.5C, heart rate was 160 beats/minute, respiratory rate was 28/minute, and blood pressure was 92/52 mm Hg; oxygen saturation was 100% breathing 2 L of oxygen by nasal cannula. She was a moderately obese African American woman in moderate distress, lying in bed moaning. Mucous membranes were dry. There was no lymphadenopathy or thyromegaly. Heart rate was regular without appreciable murmur, rub, or gallop. Lungs were clear. Abdomen was soft and nondistended, with diffuse tenderness to palpation; bowel sounds were present; there was no rebound or guarding. She had normal rectal tone with brown, guaiac‐negative stool. There was no costovertebral angle tenderness. She was oriented to person, place, and time but lethargic; deep tendon reflexes were 3+ bilaterally, and no focal signs were elicited.
Renal stones certainly produce abdominal pain, and the rare patient undergoes laparotomy for this reason. The hysterectomy tells us that small bowel obstruction could be a reason for her symptoms, although abnormal mental status would not be expected without additional problems such as infection. The tachycardia seems out of proportion to her temperature. Hyperpnea and absent respiratory symptoms, along with hypotension and tachycardia, suggest a sepsis syndrome. Her physical exam confirms dehydration. Examination of the abdomen makes me speculate about whether she has a nonsurgical cause of acute abdomen. The lethargy remains unexplained. Sepsis syndrome, possibly from a perinephric abscess, is my leading diagnosis.
White blood cell count was 15.9/mm3 with 78% neutrophils, a hemoglobin of 14.3 g/dL with a MCV of 76 and a platelet count of 320/mm3. Sodium was 159 mmol/L, chloride 128 mmol/L, bicarbonate 19 mmol/L, blood urea nitrogen 120 mmol/L, creatinine 3.1 mg/dL, calcium 11.7 mg/dL, albumin 3.3 g/dL, serum aspartate aminotransferase 65 U/L, serum alanine aminotransferase 72 U/L, total bilirubin 0.7 mg/dL, amylase 137 U/L (normal 30100), and lipase 92 IU/dL (normal 424). Urine obtained from a Foley catheter revealed negative nitrite and leukocyte esterase, 5075 red blood cells, and 1025 white blood cells per high‐powered field.
The elevated serum sodium is likely contributing to her abnormal mental status. It is unusual for a previously healthy and conscious woman to become this hypernatremic because persons with a normal mental status will defend their sodium balance strenuously, assuming regulatory mechanisms are intact. Generally, this level of hypernatremia indicates 2 things. One, a patient was not allowed, or did not seek access to, free water. The other is the presence of diabetes insipidus. It is unlikely she became this dehydrated from the initial gastrointestinal episode as described. The low MCV suggests she may be a thalassemia carrier, as microcytosis with iron deficiency typically does not occur until the patient is anemic, although she may be when rehydrated. Serum calcium, while elevated, also will likely return to the normal range with hydration. The metabolic abnormalities strongly suggest a problem in the central nervous system. The hematuria in the urinalysis continues to raise the possibility of nephrolithiasis as a cause of abdominal pain, though it does not fit well with the rest of the patient's clinical picture. The hematuria and pyuria both could still indicate a urinary tract infection such as pyelonephritis or perinephric abscess causing a sepsis syndrome.
An acute abdominal series and chest radiograph revealed a paucity of gas in the abdomen but no free air under the diaphragm or active cardiopulmonary disease. Abdominal ultrasound showed cholelithiasis without biliary dilation. There was no evidence of hydronephrosis, hydroureter, or perinephric abscess. A noncontrast abdominal‐pelvic computed tomography (CT) scan demonstrated no peripancreatic stranding or fluid collection and no nephrolithiasis or fluid collection suggestive of abscess. The admission electrocardiogram, read as sinus tachycardia with a rate of 160, is displayed in Figure 1.

I have long believed that unexplained sinus tachycardia is one of the most ominous rhythms in clinical medicine; it is expected after vigorous exercise, among other situations, but not in the condition in which this woman finds herself. The nature of the tracing does not indicate the likelihood of a supraventricular arrhythmia, particularly atrial flutter, which should be considered given the rate. The absence of free air under the diaphragm on chest radiography is reassuring. Though the pancreatic enzymes are mildly elevated, they are usually far more striking in gallstone pancreatitis. Hypercalcemia may result in abdominal pain by several mechanisms. I remain concerned about her central nervous system.
The patient was admitted to the intensive care unit (ICU), where she received intravenous antibiotics and aggressive rehydration. The following morning, she continued to complain of abdominal pain. Her systolic blood pressure was 115 mmHg, and her heart rate ranged between 140 and 150 beats/minute. The remainder of her physical exam was unchanged. Repeat laboratory tests revealed a white blood cell count of 14.7/mm3, a blood urea nitrogen of 66 mg/dL, a creatinine of 1.3 mg.dL, amylase of 67 IU/L, and lipase of 70 IU/dL. A contrast‐enhanced abdominal‐pelvic CT scan did not reveal intra‐abdominal pathology. Blood and urine cultures obtained at admission were negative for any growth.
The patient was appropriately admitted to the ICU. When caring for a critically ill patient, establishing a diagnosis is less important initially than addressing treatable conditions with dispatch. The negative CT scans rule out previously entertained diagnoses like nephrolithiasis and perinephric abscess. It is possible that the initially positive urinalysis was a result of urinary catheter placement trauma. Given the course to date, I believe this patient likely has a nonsurgical cause of abdominal pain. I am considering entities such as lead intoxication, hypercalcemia, a tear of the rectus abdominus caused by vomiting, systemic vasculitis, or a hypercoagulable state leading to intra‐abdominal venous thrombosis.
By hospital day 3 her sodium decreased to 149 mmol/L and her creatinine was 1.0 mg/dL. Abdominal pain persisted, unchanged from admission. Her systolic blood pressure had stabilized at 120 mmHg, but the heart rate remained near 150 beats/minute. Her abdomen remained soft and nondistended on exam but diffusely tender to palpation. Her amylase and lipase continued to decrease, and her repeat electrocardiogram demonstrated tachycardia with a rate of 144.
We are gratified to see that her serum sodium has waned but not with the persistence of the tachycardia. It must be assumed that this patient has an infectious disease that we are not clever enough to diagnose at this time. I am also considering an autoimmune process, such as systemic lupus erythematosus. It is difficult to envision a neoplastic disorder causing these problems. The differential remains broad, however, because we have not ruled out metabolic or endocrine causes. It is difficult to imagine she could have Addison's diseasea common cause of severe abdominal pain, tachycardia, and hypotensiongiven her serum sodium level. Hyperthyroidism has been known to produce mild hypercalcemia and abdominal complaints and is an intriguing possibility. The striking elevation of her serum sodium makes me consider the possibility of a problem in the posterior pituitary gland such as sarcoidosis. I cannot explain how sarcoidosis would cause her abdominal pain, unless the hypercalcemia were related. The tachycardia remains of concern, especially if she is otherwise improving. Thus, I would likely administer a small dose of adenosine to ascertain that this is not a different supraventricular tachycardia. In sinus tachycardia, the rate is usually attendant to the clinical picture and thus begs explanation given her clinical improvement.
After receiving 6 mg of intravenous adenosine, the patient's heart rate declined; atrial flutter waves were observed.
This case nicely demonstrates a key teaching point: a fast regular heart rate of about 150, irrespective of the electrocardiogram, suggests atrial flutter. Who gets atrial flutter? Patients with chronic lung disease, myocardial ischemia (albeit rarely), alcohol‐induced cardiomyopathy, and infiltrative cardiac disorders do. Additionally, we also have to consider thyroid dysfunction.
If forced to come up with a single unifying diagnosis at this point, I would have to say this patient most likely has sarcoidosis because this entity would account for modest hypercalcemia, the myocardial conduction disturbance, and hypernatremia because of diabetes insipidus; furthermore, it would fit the patient's demographic profile. However, I am also concerned about hyperthyroidism and would not proceed until thyroid function studies were obtained.
Thyroid studies revealed thyroid stimulating hormone of less than 0.01 mU/L (normal range, 0.305.50), free thyroxine (T4) of 5.81 ng.dL (normal range, 0.731.79), free triiodothyronine (T3) of 15.7 pg/mL (normal range, 2.85.3), and total triiodothyronine (T3) of 218 ng/dL (normal range, 95170). The patient was diagnosed with thyroid crisis and was started on propranolol, propylthiouracil, hydrocortisone, and a saturated solution of potassium iodine. Thyroid stimulating immunoglobulins were obtained and found to be markedly elevated (3.4 TSI index; normal < 1.3), suggestive of Grave's disease. Over the next several days, the patient's abdominal pain and tachycardia resolved. Her mental status returned to normal. A workup for her microcytic anemia revealed beta thalassemia trait. The patient was discharged home on hospital day 9 and has done well as an outpatient.
COMMENTARY
As Sir Zachary Cope stated in his classic text Cope's Early Diagnosis of the Acute Abdomen, [I]t is only by thorough history taking and physical examination that one can propound a diagnosis.1 When first presented with a patient whose chief complaint is abdominal pain, physicians tend to focus on the disorders of both the hollow and solid organs of the abdomen as potential sources of the pain. The differential diagnosis traditionally includes disorders such as cholecystitis, peptic ulcer disease, pancreatitis, small bowel obstruction, bowel ischemia or perforation, splenic abscess and infarct, nephrolithiasis, diverticulitis, and appendicitis, all of which were initially considered by the clinicians involved in this case. But as our discussant pointed out, in this case the differential needed to be broadened to include less common disorders, particularly given the patient's altered mental status, numerous electrolyte abnormalities, and lethargy and the lack of explanation provided by the physical examination and sophisticated imaging studies.
Specifically, a myriad of systemic diseases and metabolic derangements can cause abdominal complaints and mimic surgical abdominal disease, including hypercalcemia, acute intermittent porphyria, diabetic ketoacidosis, lead intoxication, familial Mediterranean fever, vasculopathies, adrenal insufficiency, and hyperthyroidism. Unfortunately, the frequency with which abdominal pain occurs in many of these less common disease processes and the pathophysiology that underlies its occurrence are not well defined. For example, abdominal pain is well described as a typical manifestation of both diabetic ketoacidosis and lead poisoning, but the pathophysiology behind its occurrence is poorly understood in both. Further, as a manifestation of thyrotoxicosis and as one of the diagnostic criteria for thyroid storm, the reported prevalence of abdominal pain in this condition is variable, ranging from rare to 20%47%.24 Also, although other gastrointestinal manifestations of hyperthyroidism (such as nausea, vomiting, and hyperdefecation) are thought to be the result of the effect of excess thyroid hormone on gastrointestinal motility, it is unclear whether this similar mechanism is responsible for the perception of abdominal pain.4
An important clue to the underlying diagnosis in this case was the patient's marked tachycardia. Classically, a persistent heart rate of 150 should raise suspicion of atrial flutter with a 2:1 conduction block, as was eventually discovered in this case. Adenosine, in addition to other vagal maneuvers such as carotid massage or Valsalva that also block atrioventricular (AV) node conduction, has been recognized as a safe and effective means of establishing a diagnosis in tachyarrhythmias.5 In AV nodal‐dependent tachycardias, such as AV node reentrant tachycardia or AV reentrant tachycardia, adenosine will often terminate the tachyarrhythmia by blocking the anterograde limb of the reentrant circuit. In AV nodeindependent tachyarrhythmias, such as atrial flutter or atrial fibrillation, adenosine will not terminate the rhythm. However, in the case of flutter, blocking the AV node will usually transiently unmask the underlying P waves, thereby facilitating the diagnosis.5, 6
In this patient, the discovery of atrial flutter was the main clue that thyrotoxicosis may provide the unifying diagnosis. Thyroid hormone has a direct positive cardiac chronotropic effect, resulting in the increased resting heart characteristic of thyrotoxicosis. Specifically, this hormone increases sinoatrial‐node firing, shortens the refractory period of conduction tissue within the heart, and decreases the electrical threshold for atrial excitation. In addition to predisposing to sinus tachycardia (the most common rhythm associated with this disorder), thyrotoxicosis is also associated with atrial tachycardias such as atrial flutter and, more classically, atrial fibrillation.7, 8 Though no studies have specifically evaluated the incidence of atrial flutter in thyrotoxicosis, atrial fibrillation has been found in 9%22% of these patients.7
Finally, several of the patient's electrolyte derangements could explain some of her clinical findings and are clues to the underlying diagnosis. She initially presented with a mild hypercalcemia that persisted even after hydration. Potential explanations include her severe dehydration or her underlying thyrotoxicosis because hypercalcemia is present in up to 20% of patients with hyperthyroidism.9, 10 However, the presence of significant hypercalcemia in the setting of thyrotoxicosis may actually make the diagnosis of thyrotoxicosis more difficult, masking the hypermetabolic signs and symptoms of the hyperthyroid state.11 Interestingly, coexistent primary hyperparathyroidism does occur in a few of these patients, but it likely was not an underlying cause in our patient given that her calcium normalized after receipt of propylthiouracil therapy.12
The patient's marked hypernatremia is more difficult to explain. She may have developed nephrogenic diabetes insipidus secondary to hypercalcemia, explained by a renal concentrating defect that can become evident once the calcium is persistently above 11 mg/dL.13 Combined with her altered mental status, which likely limited her ability to access free water, this may be enough to explain her marked hypernatremia. Her rapid improvement with rehydration is also consistent with this explanation, mediated through the improvement of her serum free calcium.
This case highlights the importance of using all the clinical clues provided by the history, physical exam, and laboratory and imaging studies when generating an initial differential diagnosis, as well as the importance of being willing to appropriately broaden and narrow the list of possibilities as a case evolves. When this patient was initially evaluated by physicians in the emergency department, they believed her symptoms were most consistent with generalized peritonitis that was likely secondary to an infectious or inflammatory intra‐abdominal process such as pancreatitis (especially in light of her mildly elevated lipase and amylase), appendicitis, or diverticulitis. When the medical team in the intensive care unit assumed care of this patient, members of the team failed to recognize several of the early clues, including the patient's markedly abnormal mental status, electrolyte derangements, and persistent tachycardia despite aggressive rehydration, which suggested the possibility of alternative, and less common, etiologies of her abdominal pain. Instead, they continued to aggressively pursue the possibility of the initial differential diagnosis, even repeating some of the previously negative studies from the emergency department. This case illustrates the importance of constantly reevaluating the available information from physical examination and laboratory and imaging studies and not falling victim to intellectual blind spots created by suggested diagnoses by other care providers. Fortunately for this patient, her thyroid crisis was diagnosed, albeit with some delay, before any long‐term complications occurred.
A 47‐year‐old woman was brought to the emergency department by her family because of 1 week of abdominal pain. The pain had begun in the epigastrium but had spread across the abdomen. She described it as constant and 10 of 10 in intensity but could not identify aggravating or alleviating factors. She also complained of nausea and vomiting, beginning 4 days prior to presentation, occurring 25 times per day. She noted poor oral intake and mild diarrhea. She denied melena or hematochezia. She reported no recent fever, dysuria, chills, or night sweats; however, she reported upper respiratory symptoms 2 weeks prior to presentation. On the day of presentation, her family felt she was becoming increasingly lethargic.
Epigastric pain in a middle‐aged woman suggests several possible diagnoses. Conditions such as acute cholecystitis begin abruptly, whereas small bowel obstruction, appendicitis, and diverticulitis start gradually. Nausea and vomiting are common concomitants of abdominal pain and are nonspecific. The absence of fever and chills is reassuring. Of greatest concern is the mental status. Initially, I think of enterohemorrhagic E. coli syndromes with associated glomerulonephritis. With a more systemic metabolic abnormality such as this, the rapid development of the disease tends to exaggerate symptoms.
The patient had a history of nephrolithiasis and underwent total abdominal hysterectomy and bilateral salpingo‐oopherectomy secondary to uterine fibroids in the past. She took occasional acetaminophen, smoked two cigarettes per day, and rarely consumed alcohol. Temperature was 38.5C, heart rate was 160 beats/minute, respiratory rate was 28/minute, and blood pressure was 92/52 mm Hg; oxygen saturation was 100% breathing 2 L of oxygen by nasal cannula. She was a moderately obese African American woman in moderate distress, lying in bed moaning. Mucous membranes were dry. There was no lymphadenopathy or thyromegaly. Heart rate was regular without appreciable murmur, rub, or gallop. Lungs were clear. Abdomen was soft and nondistended, with diffuse tenderness to palpation; bowel sounds were present; there was no rebound or guarding. She had normal rectal tone with brown, guaiac‐negative stool. There was no costovertebral angle tenderness. She was oriented to person, place, and time but lethargic; deep tendon reflexes were 3+ bilaterally, and no focal signs were elicited.
Renal stones certainly produce abdominal pain, and the rare patient undergoes laparotomy for this reason. The hysterectomy tells us that small bowel obstruction could be a reason for her symptoms, although abnormal mental status would not be expected without additional problems such as infection. The tachycardia seems out of proportion to her temperature. Hyperpnea and absent respiratory symptoms, along with hypotension and tachycardia, suggest a sepsis syndrome. Her physical exam confirms dehydration. Examination of the abdomen makes me speculate about whether she has a nonsurgical cause of acute abdomen. The lethargy remains unexplained. Sepsis syndrome, possibly from a perinephric abscess, is my leading diagnosis.
White blood cell count was 15.9/mm3 with 78% neutrophils, a hemoglobin of 14.3 g/dL with a MCV of 76 and a platelet count of 320/mm3. Sodium was 159 mmol/L, chloride 128 mmol/L, bicarbonate 19 mmol/L, blood urea nitrogen 120 mmol/L, creatinine 3.1 mg/dL, calcium 11.7 mg/dL, albumin 3.3 g/dL, serum aspartate aminotransferase 65 U/L, serum alanine aminotransferase 72 U/L, total bilirubin 0.7 mg/dL, amylase 137 U/L (normal 30100), and lipase 92 IU/dL (normal 424). Urine obtained from a Foley catheter revealed negative nitrite and leukocyte esterase, 5075 red blood cells, and 1025 white blood cells per high‐powered field.
The elevated serum sodium is likely contributing to her abnormal mental status. It is unusual for a previously healthy and conscious woman to become this hypernatremic because persons with a normal mental status will defend their sodium balance strenuously, assuming regulatory mechanisms are intact. Generally, this level of hypernatremia indicates 2 things. One, a patient was not allowed, or did not seek access to, free water. The other is the presence of diabetes insipidus. It is unlikely she became this dehydrated from the initial gastrointestinal episode as described. The low MCV suggests she may be a thalassemia carrier, as microcytosis with iron deficiency typically does not occur until the patient is anemic, although she may be when rehydrated. Serum calcium, while elevated, also will likely return to the normal range with hydration. The metabolic abnormalities strongly suggest a problem in the central nervous system. The hematuria in the urinalysis continues to raise the possibility of nephrolithiasis as a cause of abdominal pain, though it does not fit well with the rest of the patient's clinical picture. The hematuria and pyuria both could still indicate a urinary tract infection such as pyelonephritis or perinephric abscess causing a sepsis syndrome.
An acute abdominal series and chest radiograph revealed a paucity of gas in the abdomen but no free air under the diaphragm or active cardiopulmonary disease. Abdominal ultrasound showed cholelithiasis without biliary dilation. There was no evidence of hydronephrosis, hydroureter, or perinephric abscess. A noncontrast abdominal‐pelvic computed tomography (CT) scan demonstrated no peripancreatic stranding or fluid collection and no nephrolithiasis or fluid collection suggestive of abscess. The admission electrocardiogram, read as sinus tachycardia with a rate of 160, is displayed in Figure 1.

I have long believed that unexplained sinus tachycardia is one of the most ominous rhythms in clinical medicine; it is expected after vigorous exercise, among other situations, but not in the condition in which this woman finds herself. The nature of the tracing does not indicate the likelihood of a supraventricular arrhythmia, particularly atrial flutter, which should be considered given the rate. The absence of free air under the diaphragm on chest radiography is reassuring. Though the pancreatic enzymes are mildly elevated, they are usually far more striking in gallstone pancreatitis. Hypercalcemia may result in abdominal pain by several mechanisms. I remain concerned about her central nervous system.
The patient was admitted to the intensive care unit (ICU), where she received intravenous antibiotics and aggressive rehydration. The following morning, she continued to complain of abdominal pain. Her systolic blood pressure was 115 mmHg, and her heart rate ranged between 140 and 150 beats/minute. The remainder of her physical exam was unchanged. Repeat laboratory tests revealed a white blood cell count of 14.7/mm3, a blood urea nitrogen of 66 mg/dL, a creatinine of 1.3 mg.dL, amylase of 67 IU/L, and lipase of 70 IU/dL. A contrast‐enhanced abdominal‐pelvic CT scan did not reveal intra‐abdominal pathology. Blood and urine cultures obtained at admission were negative for any growth.
The patient was appropriately admitted to the ICU. When caring for a critically ill patient, establishing a diagnosis is less important initially than addressing treatable conditions with dispatch. The negative CT scans rule out previously entertained diagnoses like nephrolithiasis and perinephric abscess. It is possible that the initially positive urinalysis was a result of urinary catheter placement trauma. Given the course to date, I believe this patient likely has a nonsurgical cause of abdominal pain. I am considering entities such as lead intoxication, hypercalcemia, a tear of the rectus abdominus caused by vomiting, systemic vasculitis, or a hypercoagulable state leading to intra‐abdominal venous thrombosis.
By hospital day 3 her sodium decreased to 149 mmol/L and her creatinine was 1.0 mg/dL. Abdominal pain persisted, unchanged from admission. Her systolic blood pressure had stabilized at 120 mmHg, but the heart rate remained near 150 beats/minute. Her abdomen remained soft and nondistended on exam but diffusely tender to palpation. Her amylase and lipase continued to decrease, and her repeat electrocardiogram demonstrated tachycardia with a rate of 144.
We are gratified to see that her serum sodium has waned but not with the persistence of the tachycardia. It must be assumed that this patient has an infectious disease that we are not clever enough to diagnose at this time. I am also considering an autoimmune process, such as systemic lupus erythematosus. It is difficult to envision a neoplastic disorder causing these problems. The differential remains broad, however, because we have not ruled out metabolic or endocrine causes. It is difficult to imagine she could have Addison's diseasea common cause of severe abdominal pain, tachycardia, and hypotensiongiven her serum sodium level. Hyperthyroidism has been known to produce mild hypercalcemia and abdominal complaints and is an intriguing possibility. The striking elevation of her serum sodium makes me consider the possibility of a problem in the posterior pituitary gland such as sarcoidosis. I cannot explain how sarcoidosis would cause her abdominal pain, unless the hypercalcemia were related. The tachycardia remains of concern, especially if she is otherwise improving. Thus, I would likely administer a small dose of adenosine to ascertain that this is not a different supraventricular tachycardia. In sinus tachycardia, the rate is usually attendant to the clinical picture and thus begs explanation given her clinical improvement.
After receiving 6 mg of intravenous adenosine, the patient's heart rate declined; atrial flutter waves were observed.
This case nicely demonstrates a key teaching point: a fast regular heart rate of about 150, irrespective of the electrocardiogram, suggests atrial flutter. Who gets atrial flutter? Patients with chronic lung disease, myocardial ischemia (albeit rarely), alcohol‐induced cardiomyopathy, and infiltrative cardiac disorders do. Additionally, we also have to consider thyroid dysfunction.
If forced to come up with a single unifying diagnosis at this point, I would have to say this patient most likely has sarcoidosis because this entity would account for modest hypercalcemia, the myocardial conduction disturbance, and hypernatremia because of diabetes insipidus; furthermore, it would fit the patient's demographic profile. However, I am also concerned about hyperthyroidism and would not proceed until thyroid function studies were obtained.
Thyroid studies revealed thyroid stimulating hormone of less than 0.01 mU/L (normal range, 0.305.50), free thyroxine (T4) of 5.81 ng.dL (normal range, 0.731.79), free triiodothyronine (T3) of 15.7 pg/mL (normal range, 2.85.3), and total triiodothyronine (T3) of 218 ng/dL (normal range, 95170). The patient was diagnosed with thyroid crisis and was started on propranolol, propylthiouracil, hydrocortisone, and a saturated solution of potassium iodine. Thyroid stimulating immunoglobulins were obtained and found to be markedly elevated (3.4 TSI index; normal < 1.3), suggestive of Grave's disease. Over the next several days, the patient's abdominal pain and tachycardia resolved. Her mental status returned to normal. A workup for her microcytic anemia revealed beta thalassemia trait. The patient was discharged home on hospital day 9 and has done well as an outpatient.
COMMENTARY
As Sir Zachary Cope stated in his classic text Cope's Early Diagnosis of the Acute Abdomen, [I]t is only by thorough history taking and physical examination that one can propound a diagnosis.1 When first presented with a patient whose chief complaint is abdominal pain, physicians tend to focus on the disorders of both the hollow and solid organs of the abdomen as potential sources of the pain. The differential diagnosis traditionally includes disorders such as cholecystitis, peptic ulcer disease, pancreatitis, small bowel obstruction, bowel ischemia or perforation, splenic abscess and infarct, nephrolithiasis, diverticulitis, and appendicitis, all of which were initially considered by the clinicians involved in this case. But as our discussant pointed out, in this case the differential needed to be broadened to include less common disorders, particularly given the patient's altered mental status, numerous electrolyte abnormalities, and lethargy and the lack of explanation provided by the physical examination and sophisticated imaging studies.
Specifically, a myriad of systemic diseases and metabolic derangements can cause abdominal complaints and mimic surgical abdominal disease, including hypercalcemia, acute intermittent porphyria, diabetic ketoacidosis, lead intoxication, familial Mediterranean fever, vasculopathies, adrenal insufficiency, and hyperthyroidism. Unfortunately, the frequency with which abdominal pain occurs in many of these less common disease processes and the pathophysiology that underlies its occurrence are not well defined. For example, abdominal pain is well described as a typical manifestation of both diabetic ketoacidosis and lead poisoning, but the pathophysiology behind its occurrence is poorly understood in both. Further, as a manifestation of thyrotoxicosis and as one of the diagnostic criteria for thyroid storm, the reported prevalence of abdominal pain in this condition is variable, ranging from rare to 20%47%.24 Also, although other gastrointestinal manifestations of hyperthyroidism (such as nausea, vomiting, and hyperdefecation) are thought to be the result of the effect of excess thyroid hormone on gastrointestinal motility, it is unclear whether this similar mechanism is responsible for the perception of abdominal pain.4
An important clue to the underlying diagnosis in this case was the patient's marked tachycardia. Classically, a persistent heart rate of 150 should raise suspicion of atrial flutter with a 2:1 conduction block, as was eventually discovered in this case. Adenosine, in addition to other vagal maneuvers such as carotid massage or Valsalva that also block atrioventricular (AV) node conduction, has been recognized as a safe and effective means of establishing a diagnosis in tachyarrhythmias.5 In AV nodal‐dependent tachycardias, such as AV node reentrant tachycardia or AV reentrant tachycardia, adenosine will often terminate the tachyarrhythmia by blocking the anterograde limb of the reentrant circuit. In AV nodeindependent tachyarrhythmias, such as atrial flutter or atrial fibrillation, adenosine will not terminate the rhythm. However, in the case of flutter, blocking the AV node will usually transiently unmask the underlying P waves, thereby facilitating the diagnosis.5, 6
In this patient, the discovery of atrial flutter was the main clue that thyrotoxicosis may provide the unifying diagnosis. Thyroid hormone has a direct positive cardiac chronotropic effect, resulting in the increased resting heart characteristic of thyrotoxicosis. Specifically, this hormone increases sinoatrial‐node firing, shortens the refractory period of conduction tissue within the heart, and decreases the electrical threshold for atrial excitation. In addition to predisposing to sinus tachycardia (the most common rhythm associated with this disorder), thyrotoxicosis is also associated with atrial tachycardias such as atrial flutter and, more classically, atrial fibrillation.7, 8 Though no studies have specifically evaluated the incidence of atrial flutter in thyrotoxicosis, atrial fibrillation has been found in 9%22% of these patients.7
Finally, several of the patient's electrolyte derangements could explain some of her clinical findings and are clues to the underlying diagnosis. She initially presented with a mild hypercalcemia that persisted even after hydration. Potential explanations include her severe dehydration or her underlying thyrotoxicosis because hypercalcemia is present in up to 20% of patients with hyperthyroidism.9, 10 However, the presence of significant hypercalcemia in the setting of thyrotoxicosis may actually make the diagnosis of thyrotoxicosis more difficult, masking the hypermetabolic signs and symptoms of the hyperthyroid state.11 Interestingly, coexistent primary hyperparathyroidism does occur in a few of these patients, but it likely was not an underlying cause in our patient given that her calcium normalized after receipt of propylthiouracil therapy.12
The patient's marked hypernatremia is more difficult to explain. She may have developed nephrogenic diabetes insipidus secondary to hypercalcemia, explained by a renal concentrating defect that can become evident once the calcium is persistently above 11 mg/dL.13 Combined with her altered mental status, which likely limited her ability to access free water, this may be enough to explain her marked hypernatremia. Her rapid improvement with rehydration is also consistent with this explanation, mediated through the improvement of her serum free calcium.
This case highlights the importance of using all the clinical clues provided by the history, physical exam, and laboratory and imaging studies when generating an initial differential diagnosis, as well as the importance of being willing to appropriately broaden and narrow the list of possibilities as a case evolves. When this patient was initially evaluated by physicians in the emergency department, they believed her symptoms were most consistent with generalized peritonitis that was likely secondary to an infectious or inflammatory intra‐abdominal process such as pancreatitis (especially in light of her mildly elevated lipase and amylase), appendicitis, or diverticulitis. When the medical team in the intensive care unit assumed care of this patient, members of the team failed to recognize several of the early clues, including the patient's markedly abnormal mental status, electrolyte derangements, and persistent tachycardia despite aggressive rehydration, which suggested the possibility of alternative, and less common, etiologies of her abdominal pain. Instead, they continued to aggressively pursue the possibility of the initial differential diagnosis, even repeating some of the previously negative studies from the emergency department. This case illustrates the importance of constantly reevaluating the available information from physical examination and laboratory and imaging studies and not falling victim to intellectual blind spots created by suggested diagnoses by other care providers. Fortunately for this patient, her thyroid crisis was diagnosed, albeit with some delay, before any long‐term complications occurred.
- Silen W, ed.Cope's Early Diagnosis of the Acute Abdomen.19th ed.New York:Oxford University Press;1995:4.
- ,.An unusual cause of abdominal pain in young woman.Ann Emerg Med.1991;20:574–582.
- .Vomiting, nausea and abdominal pain: unrecognized symptoms of thyrotoxicosis.J Fam Prac.1989;24:382–386.
- Powell DW,Alpers DH,Yamada,Owyang C,Laine L, eds.Textbook of Gastroenterology.3rd ed.Philadelphia, Pa:Lippincott Williams 783,2516.
- ,,.Usefulness of adenosine in diagnosis of tachyarrhythmias.Am J Cardiol.1995;75:952–955.
- ,,,,.Supraventricular tachycardia.Med Clin North Am.2001;85:193–223.
- .Thyrotoxicosis and the heart.N Engl J Med.1992;327:94–8.
- ,.Thyrotoxicosis and the heart.Endocrinol Metab Clin North Am.1998;27:51–62.
- ,,,.Treatment of thyrotoxic hypercalcemia with propranolol.N Engl J Med.1976;294:431.
- ,,,.Ionized and total plasma calcium and parathyroid hormone in hyperthyroidism.Ann Intern Med.1976;84:668.
- ,.Hypercalcemic crisis.Med Clin North Am.1995;79:79–92.
- ,,.Thyrotoxicosis, hypercalcemia, and secondary hyperparathyroidism.Arch Intern Med.1979;139:661–663.
- ,.Clinical Physiology of Acid‐Base and Electrolyte Disorders.5th ed.New York:McGraw‐Hill;2001:754–758.
- Silen W, ed.Cope's Early Diagnosis of the Acute Abdomen.19th ed.New York:Oxford University Press;1995:4.
- ,.An unusual cause of abdominal pain in young woman.Ann Emerg Med.1991;20:574–582.
- .Vomiting, nausea and abdominal pain: unrecognized symptoms of thyrotoxicosis.J Fam Prac.1989;24:382–386.
- Powell DW,Alpers DH,Yamada,Owyang C,Laine L, eds.Textbook of Gastroenterology.3rd ed.Philadelphia, Pa:Lippincott Williams 783,2516.
- ,,.Usefulness of adenosine in diagnosis of tachyarrhythmias.Am J Cardiol.1995;75:952–955.
- ,,,,.Supraventricular tachycardia.Med Clin North Am.2001;85:193–223.
- .Thyrotoxicosis and the heart.N Engl J Med.1992;327:94–8.
- ,.Thyrotoxicosis and the heart.Endocrinol Metab Clin North Am.1998;27:51–62.
- ,,,.Treatment of thyrotoxic hypercalcemia with propranolol.N Engl J Med.1976;294:431.
- ,,,.Ionized and total plasma calcium and parathyroid hormone in hyperthyroidism.Ann Intern Med.1976;84:668.
- ,.Hypercalcemic crisis.Med Clin North Am.1995;79:79–92.
- ,,.Thyrotoxicosis, hypercalcemia, and secondary hyperparathyroidism.Arch Intern Med.1979;139:661–663.
- ,.Clinical Physiology of Acid‐Base and Electrolyte Disorders.5th ed.New York:McGraw‐Hill;2001:754–758.
Clinical Conundrum
A 46‐year‐old African American man presented to the emergency department with severe chest pain that awakened him from sleep. The pain was substernal, radiated to the neck and back, and was continuous, lasting approximately 1 hour. It was associated with nausea, diaphoresis, and dizziness. The patient denied dyspnea, orthopnea, paroxysmal nocturnal dyspnea, dysphagia, odynophagia, vomiting, fever, chills, or headache. He denied recent recreational drug use. He works as a landscaper.
Substernal chest pain radiating to the neck and back implicates structures in the middle mediastinum, chiefly the heart, aorta, esophagus, pulmonary arteries, and mediastinal pleura. The presence of nausea and diaphoresis suggests a vagal response to pain.
The sudden onset of symptoms and the lack of fevers and chills make infectious causes in the mediastinum such as mediastinitis and pneumonia less likely. Acute pulmonary embolism often presents with pleuritic pain and dyspnea, features not present in this patient.
The absence of odynophagia and dysphagia makes esophageal rupture or perforation of esophageal diverticula unlikely. Likewise, the absence of dyspnea, orthopnea, and paroxysmal nocturnal dyspnea makes it unlikely to be acute left ventricular failure from a sudden rise in left atrial pressure. Such a scenario may occur in the setting of a myocardial infarction or rupture of a papillary muscle, chordae tendineae, or sinus of Valsalva.
Dissection of the aorta with or without involvement of the aortic root merits strong consideration. Dissection involving the carotid or vertebral arteries could explain the patient's dizziness. Physical stresses in a landscaper may contribute to elevation in blood pressure and set the stage for an aortic dissection, especially with other risk factors.
The patient has a history of a positive PPD and was treated with isoniazid for 6 months. His mother had a history of hypertension and died from a myocardial infarction at age 64. His father's medical history is unknown. He has a history of alcohol abuse but has been abstinent for more than a year. He smoked marijuana and tobacco occasionally, with a 15 pack‐year cigarette history. He also stated that the last time he used cocaine was 1 year prior to admission.
That his mother succumbed to a myocardial infarction as well as having been hypertensive could be important family history risk factors given the patient's symptoms. Furthermore, the concurrent use of alcohol and tobacco by the patient increases his risk of developing severe hypertension. The use of cocaine is associated with sudden elevations in systemic blood pressure, which predispose to intimal damage in the aorta, especially if other risk factors are present. Marijuana smoking has been implicated in pulmonary aspergillosis, but this sudden presentation in the absence of pulmonary symptoms makes it most unlikely. Optimal therapy of a positive PPD should not predispose the patient to acute exudative pericarditis. Thus far the features suggest an acute vascular episode without significant compromise of cardiac output.
The patient was alert and in mild distress from chest discomfort. He was afebrile, with a blood pressure of 136/64 in the right arm and 139/63 in the left arm. Heart rate was 60 beats per minute, and the respiratory rate was 12 beats per minute, with an oxygen saturation of 99% while breathing room air. Examination of the head and neck revealed no signs of trauma. Jugular venous waveforms were normal, and carotid artery pulsations had normal strength and upstroke without audible bruits. The lungs were clear to auscultation. Heart sounds were notable for a 3/6 diastolic murmur heard best at the right sternal border. Rate and rhythm were regular, and the apical impulse was sustained but not displaced. The peripheral pulses were present and equal in quality throughout. The findings of the abdominal exam were normal, and the digital rectal examination was negative for occult blood. The findings of the neurologic, musculoskeletal, and dermatologic exams also were normal.
A slightly elevated pulse pressure without a significant difference in upper extremity blood pressure could be a result of aortic regurgitation, sinus of Valsalva rupture, or a high‐cardiac‐output state, as seen in thyrotoxicosis, anemia, or arteriovenous fistula.
The presence of a 3/6 diastolic murmur at the sternal border, however, favors conditions that cause aortic valve regurgitation and, less commonly, pulmonary valve regurgitation, or turbulent flow across the tricuspid valve. The murmur of pulmonary and aortic valve regurgitation can be difficult to distinguish by auscultation; however, the absence of other findings such as a right ventricular heave, elevated jugular venous pressure, or primary lung disease do not support a pulmonary valve etiology. Turbulent flow across the tricuspid valve can be seen in high‐output states, large atrial septal defects, and tricuspid stenosis. This type of murmur is heard best at the lower sternal border and tends to increase with inspiration. An early diastolic murmur would suggest aortic regurgitation, either from aortic valve or aortic root pathology. Aortic regurgitation would contribute to a sustained apical impulse.
Clear lungs and the absence of tachycardia suggest that left ventricle function is not severely compromised. These findings argue against acute rupture of the sinus of Valsalva, a condition that normally causes biventricular failure. The presence of equal peripheral pulses does not exclude the diagnosis of aortic dissection, as the dissection may have occurred proximal to the origins of the right innominate and the left subclavian arteries.
Initial laboratory studies revealed a hematocrit of 34.9, a leukocyte count of 6800/mm3, and a platelet count of 195 000/mm3. The levels of serum electrolytes, serum creatinine, blood urea nitrogen, and initial cardiac enzymes were normal. The urine drug screen was negative. The electrocardiogram showed evidence of left ventricular hypertrophy (Figure 1), and portable chest radiography revealed an enlarged cardiac silhouette and a widened mediastinum (Figure 2).
The presence of a widened mediastinum is consistent with aortic dissection but may also suggest a mass, aortic aneurysm, infiltrative disease, or a collection of fluid (eg, blood). A normal level of cardiac enzymes and the absence of ischemic findings on electrocardiography make myocardial infarction less likely. Given his hemodynamic stability, he is unlikely to have suffered cardiac rupture; however, he may still have a dissection of the aorta or a rupture of the sinus of Valsalva. The slight decline in hematocrit is compatible with either a mediastinal or pericardial collection of blood.
The patient was emergently sent for computerized axial tomography of the chest, which revealed a dilated aortic root at 6 cm but no evidence of aortic dissection (Figure 3). The lung fields were normal.
Despite a negative test, the presence of severe acute chest pain in the presence of a widened mediastinum is still concerning for aortic dissection. If the dissection involved the aortic valve annulus, the resulting acute regurgitation can not be severe, given the absence of left ventricular heart failure. Likewise, the presence of normal lung fields suggests the patient has no acute elevation of left ventricular end diastolic pressures such as is seen in ventricle septal defect, papillary muscle dysfunction, or acute mitral valvular lesion.
Cardiology consultants were emergently consulted and performed a transesophageal echocardiogram that confirmed a dilated aortic root (6.3 cm) without evidence of dissection. The patient was noted to have moderate to severe aortic regurgitation and a dilated left ventricle with moderate hypertrophy. A trace effusion was noted, and the left ventricular ejection fraction was estimated to be 50%.
The presence of moderate to severe aortic regurgitation with a dilated aortic root suggests 3 possibilities: undiagnosed dissection of the aortic root with preexisting aortic insufficiency (eg, bicuspid aortic valve, rheumatic valve disease, previous endocarditis); infectious (eg, syphilitic) or noninfectious (eg, ankylosing spondylitis, Takayasu's arteritis) meso‐aortitis causing aortic dilatation and subsequent regurgitation; or, finally, connective tissue diseases (eg, Marfan's syndrome, Ehlers‐Danlos), which can cause premature degeneration of the aortic media. The acuity of the patient's symptoms and the lack of systemic findings make a connective tissue or inflammatory disease unlikely. The clinical index of suspicion for aortic dissection needs to remain very high, as failure to make an expedient diagnosis may lead to complications and a deleterious outcome. Aortography may help to define this lesion.
The cardiothoracic surgical team was consulted and recommended aortic root and valve replacement. The patient was observed overnight and scheduled for preoperative cardiac catheterization the following morning. Aortogram revealed moderate to severe aortic insufficiency and a small dissection flap on the lesser curvature of the aorta, above the left coronary ostia (Figure 4). Coronary angiography revealed a 50% stenosis of the ostia of the first and second diagonal arteries, with no other flow‐limiting lesions.
The study has clearly demonstrated that the patient suffered an acute aortic dissection without involvement of the aortic annulus. Given the absence of left ventricular failure, it appears the aortic regurgitation was chronic and secondary to a previously existing aortic aneurysm. Asymptomatic congenital defects of the meso‐aorta such as cystic medical necrosis can predispose to aortic dissection at a relatively young age. However, this patient's condition may have been aggravated by drug abuse, paroxysmal elevation of blood pressure during landscaping, or other risk factors. Surgical correction of the dissection and aortic regurgitation is necessary.
The patient underwent aortic valve replacement with a 25‐mm St. Jude mechanical valve and an ascending aortic transection repair with a 32‐mm Dacron tube graft. On postoperative day 9, the patient was discharged home in stable condition.
COMMENTARY
Ensuring both accuracy and efficiency when making a diagnosis can be difficult, particularly when patients present in an atypical fashion or when diagnostic testing yields inconclusive results. Thus, a physician must sift through each clinical clue, remembering that although certain findings are pathognomonic for a disease process, a constellation of signs and symptoms when present can be equally diagnostic.
The initial likelihood of disease (ie, pretest probability) is generated by the history and physical and laboratory examinations. If the pretest probability is high and the subsequent diagnostic test is positive, the modified likelihood of disease (post‐test probability) is nearly 100%. If, however, the pretest probability is high and the diagnostic test is negative, the likelihood of disease is less clear. In these situations, the physician can either review the initial findings that generated the pretest probability or perform an additional diagnostic test of higher sensitivity.
In this exercise, a 46‐year‐old man presented to the emergency department with severe chest pain and findings characteristic of aortic dissection. The physicians appropriately sent him for chest computerized tomography (CT) because of a high pretest likelihood of aortic dissection. Because this test did not confirm the presence of aortic dissection, the patient underwent transesophageal echocardiography (TEE), a test of equal or greater sensitivity.1, 2 This test was also negative; however, a small dissection flap was subsequently found during cardiac catheterization. In this case, a test of lower sensitivity and specificity confirmed the diagnosis of dissection,3 demonstrating the possibility that either CT or TEE can be misinterpreted. Indeed, a final review of the TEE, showed the dissection flap, albeit small, had been missed. In this case, a diagnostic error was made that delayed the diagnosis when sufficient information was available earlier. Diagnostic errors are prevalent in medical practice and are commonly the result of numerous factors, though cognitive problems appear to be the largest contributor to this process.4 In particular, faulty synthesis of information, rather than inadequate medical knowledge, is the most common cause of cognitive medical errors. The error made in this case likely falls under the subcategory of faulty test detection or perception and subsequent premature closure (failure to consider other possibilities once an initial diagnosis of uncomplicated aortic aneurysm had been reached).4
Though medical errors cannot be completely eliminated, cases such as this should be reviewed to understand the cognitive processes that may lead to an erroneous diagnosis. In addition to the false‐negative TEE finding, this patient also had a coexisting condition that may have preoccupied the medical team. The thoracic aortic aneurysm seen by all imaging modalities was large and required intervention regardless of the presence of a dissection. However, this chronic condition became the focus of treatment, and the acute event that precipitated admission was missed. Perhaps if the primary team maintained a very high index of suspicion for dissection and conveyed this to the cardiology consultants, a meticulous review of the TEE would have then followed that may have uncovered the subtle findings of the dissection flap.
Fortunately, definitive treatment with surgical aortic root and valve replacement was performed in a timely manner, as the consequences of a delayed diagnosis in this situation could have been catastrophic. The mortality rate of a type A dissection is extremely high initially (1%‐2% per hour),5, 6 and thus surgical intervention is typically performed immediately after the diagnosis and the extent of this disease is established, rather than the following morning.7
This case highlights not only the problems resulting in diagnostic errors but also exemplifies the thought process required to make a challenging diagnosis. Our case discussant was able to avoid cognitive pitfalls by presenting a broad differential diagnosis and reevaluating the diagnosis with each additional piece of information provided.8 An experienced clinician should realize that patients with an extremely high pretest probability of disease and a negative diagnostic test should be further investigated, regardless of the test sensitivity. Furthermore, time‐honored methods such as history taking, physical examination. and thoughtful analyses should remain critical tools in the process of reaching an accurate diagnosis despite technological advances in diagnostic testing.
- ,,, et al.The diagnosis of thoracic aortic dissection by noninvasive imaging procedures.N Engl J Med.1993;328(1):1–9.
- ,,, et al.Accuracy of biplane and multiplane transesophageal echocardiography in diagnosis of typical acute aortic dissection and intramural hematoma.J Am Coll Cardiol.1996;28:627–636.
- ,.Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies.Circulation.2003;108:628–635.
- ,,.Diagnostic error in internal medicine.Arch Intern Med.2005;165:1493–1499.
- ,,.Dissecting aneurysm of the aorta: a review of 505 cases.Medicine (Baltimore).1958;37(3):217–279.
- ,,, et al.The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease.JAMA.2000;283:897–903.
- ,.Surgery of the thoracic aorta.N Engl J Med.1997;336:1876–1888.
- .Clinical reasoning in medicine. In:Higgs J,Jones MA, eds.Clinical Reasoning in the Health Professions.Woburn, Mass:Butterworth‐Heinemann;1995:49–59.
A 46‐year‐old African American man presented to the emergency department with severe chest pain that awakened him from sleep. The pain was substernal, radiated to the neck and back, and was continuous, lasting approximately 1 hour. It was associated with nausea, diaphoresis, and dizziness. The patient denied dyspnea, orthopnea, paroxysmal nocturnal dyspnea, dysphagia, odynophagia, vomiting, fever, chills, or headache. He denied recent recreational drug use. He works as a landscaper.
Substernal chest pain radiating to the neck and back implicates structures in the middle mediastinum, chiefly the heart, aorta, esophagus, pulmonary arteries, and mediastinal pleura. The presence of nausea and diaphoresis suggests a vagal response to pain.
The sudden onset of symptoms and the lack of fevers and chills make infectious causes in the mediastinum such as mediastinitis and pneumonia less likely. Acute pulmonary embolism often presents with pleuritic pain and dyspnea, features not present in this patient.
The absence of odynophagia and dysphagia makes esophageal rupture or perforation of esophageal diverticula unlikely. Likewise, the absence of dyspnea, orthopnea, and paroxysmal nocturnal dyspnea makes it unlikely to be acute left ventricular failure from a sudden rise in left atrial pressure. Such a scenario may occur in the setting of a myocardial infarction or rupture of a papillary muscle, chordae tendineae, or sinus of Valsalva.
Dissection of the aorta with or without involvement of the aortic root merits strong consideration. Dissection involving the carotid or vertebral arteries could explain the patient's dizziness. Physical stresses in a landscaper may contribute to elevation in blood pressure and set the stage for an aortic dissection, especially with other risk factors.
The patient has a history of a positive PPD and was treated with isoniazid for 6 months. His mother had a history of hypertension and died from a myocardial infarction at age 64. His father's medical history is unknown. He has a history of alcohol abuse but has been abstinent for more than a year. He smoked marijuana and tobacco occasionally, with a 15 pack‐year cigarette history. He also stated that the last time he used cocaine was 1 year prior to admission.
That his mother succumbed to a myocardial infarction as well as having been hypertensive could be important family history risk factors given the patient's symptoms. Furthermore, the concurrent use of alcohol and tobacco by the patient increases his risk of developing severe hypertension. The use of cocaine is associated with sudden elevations in systemic blood pressure, which predispose to intimal damage in the aorta, especially if other risk factors are present. Marijuana smoking has been implicated in pulmonary aspergillosis, but this sudden presentation in the absence of pulmonary symptoms makes it most unlikely. Optimal therapy of a positive PPD should not predispose the patient to acute exudative pericarditis. Thus far the features suggest an acute vascular episode without significant compromise of cardiac output.
The patient was alert and in mild distress from chest discomfort. He was afebrile, with a blood pressure of 136/64 in the right arm and 139/63 in the left arm. Heart rate was 60 beats per minute, and the respiratory rate was 12 beats per minute, with an oxygen saturation of 99% while breathing room air. Examination of the head and neck revealed no signs of trauma. Jugular venous waveforms were normal, and carotid artery pulsations had normal strength and upstroke without audible bruits. The lungs were clear to auscultation. Heart sounds were notable for a 3/6 diastolic murmur heard best at the right sternal border. Rate and rhythm were regular, and the apical impulse was sustained but not displaced. The peripheral pulses were present and equal in quality throughout. The findings of the abdominal exam were normal, and the digital rectal examination was negative for occult blood. The findings of the neurologic, musculoskeletal, and dermatologic exams also were normal.
A slightly elevated pulse pressure without a significant difference in upper extremity blood pressure could be a result of aortic regurgitation, sinus of Valsalva rupture, or a high‐cardiac‐output state, as seen in thyrotoxicosis, anemia, or arteriovenous fistula.
The presence of a 3/6 diastolic murmur at the sternal border, however, favors conditions that cause aortic valve regurgitation and, less commonly, pulmonary valve regurgitation, or turbulent flow across the tricuspid valve. The murmur of pulmonary and aortic valve regurgitation can be difficult to distinguish by auscultation; however, the absence of other findings such as a right ventricular heave, elevated jugular venous pressure, or primary lung disease do not support a pulmonary valve etiology. Turbulent flow across the tricuspid valve can be seen in high‐output states, large atrial septal defects, and tricuspid stenosis. This type of murmur is heard best at the lower sternal border and tends to increase with inspiration. An early diastolic murmur would suggest aortic regurgitation, either from aortic valve or aortic root pathology. Aortic regurgitation would contribute to a sustained apical impulse.
Clear lungs and the absence of tachycardia suggest that left ventricle function is not severely compromised. These findings argue against acute rupture of the sinus of Valsalva, a condition that normally causes biventricular failure. The presence of equal peripheral pulses does not exclude the diagnosis of aortic dissection, as the dissection may have occurred proximal to the origins of the right innominate and the left subclavian arteries.
Initial laboratory studies revealed a hematocrit of 34.9, a leukocyte count of 6800/mm3, and a platelet count of 195 000/mm3. The levels of serum electrolytes, serum creatinine, blood urea nitrogen, and initial cardiac enzymes were normal. The urine drug screen was negative. The electrocardiogram showed evidence of left ventricular hypertrophy (Figure 1), and portable chest radiography revealed an enlarged cardiac silhouette and a widened mediastinum (Figure 2).


The presence of a widened mediastinum is consistent with aortic dissection but may also suggest a mass, aortic aneurysm, infiltrative disease, or a collection of fluid (eg, blood). A normal level of cardiac enzymes and the absence of ischemic findings on electrocardiography make myocardial infarction less likely. Given his hemodynamic stability, he is unlikely to have suffered cardiac rupture; however, he may still have a dissection of the aorta or a rupture of the sinus of Valsalva. The slight decline in hematocrit is compatible with either a mediastinal or pericardial collection of blood.
The patient was emergently sent for computerized axial tomography of the chest, which revealed a dilated aortic root at 6 cm but no evidence of aortic dissection (Figure 3). The lung fields were normal.

Despite a negative test, the presence of severe acute chest pain in the presence of a widened mediastinum is still concerning for aortic dissection. If the dissection involved the aortic valve annulus, the resulting acute regurgitation can not be severe, given the absence of left ventricular heart failure. Likewise, the presence of normal lung fields suggests the patient has no acute elevation of left ventricular end diastolic pressures such as is seen in ventricle septal defect, papillary muscle dysfunction, or acute mitral valvular lesion.
Cardiology consultants were emergently consulted and performed a transesophageal echocardiogram that confirmed a dilated aortic root (6.3 cm) without evidence of dissection. The patient was noted to have moderate to severe aortic regurgitation and a dilated left ventricle with moderate hypertrophy. A trace effusion was noted, and the left ventricular ejection fraction was estimated to be 50%.
The presence of moderate to severe aortic regurgitation with a dilated aortic root suggests 3 possibilities: undiagnosed dissection of the aortic root with preexisting aortic insufficiency (eg, bicuspid aortic valve, rheumatic valve disease, previous endocarditis); infectious (eg, syphilitic) or noninfectious (eg, ankylosing spondylitis, Takayasu's arteritis) meso‐aortitis causing aortic dilatation and subsequent regurgitation; or, finally, connective tissue diseases (eg, Marfan's syndrome, Ehlers‐Danlos), which can cause premature degeneration of the aortic media. The acuity of the patient's symptoms and the lack of systemic findings make a connective tissue or inflammatory disease unlikely. The clinical index of suspicion for aortic dissection needs to remain very high, as failure to make an expedient diagnosis may lead to complications and a deleterious outcome. Aortography may help to define this lesion.
The cardiothoracic surgical team was consulted and recommended aortic root and valve replacement. The patient was observed overnight and scheduled for preoperative cardiac catheterization the following morning. Aortogram revealed moderate to severe aortic insufficiency and a small dissection flap on the lesser curvature of the aorta, above the left coronary ostia (Figure 4). Coronary angiography revealed a 50% stenosis of the ostia of the first and second diagonal arteries, with no other flow‐limiting lesions.

The study has clearly demonstrated that the patient suffered an acute aortic dissection without involvement of the aortic annulus. Given the absence of left ventricular failure, it appears the aortic regurgitation was chronic and secondary to a previously existing aortic aneurysm. Asymptomatic congenital defects of the meso‐aorta such as cystic medical necrosis can predispose to aortic dissection at a relatively young age. However, this patient's condition may have been aggravated by drug abuse, paroxysmal elevation of blood pressure during landscaping, or other risk factors. Surgical correction of the dissection and aortic regurgitation is necessary.
The patient underwent aortic valve replacement with a 25‐mm St. Jude mechanical valve and an ascending aortic transection repair with a 32‐mm Dacron tube graft. On postoperative day 9, the patient was discharged home in stable condition.
COMMENTARY
Ensuring both accuracy and efficiency when making a diagnosis can be difficult, particularly when patients present in an atypical fashion or when diagnostic testing yields inconclusive results. Thus, a physician must sift through each clinical clue, remembering that although certain findings are pathognomonic for a disease process, a constellation of signs and symptoms when present can be equally diagnostic.
The initial likelihood of disease (ie, pretest probability) is generated by the history and physical and laboratory examinations. If the pretest probability is high and the subsequent diagnostic test is positive, the modified likelihood of disease (post‐test probability) is nearly 100%. If, however, the pretest probability is high and the diagnostic test is negative, the likelihood of disease is less clear. In these situations, the physician can either review the initial findings that generated the pretest probability or perform an additional diagnostic test of higher sensitivity.
In this exercise, a 46‐year‐old man presented to the emergency department with severe chest pain and findings characteristic of aortic dissection. The physicians appropriately sent him for chest computerized tomography (CT) because of a high pretest likelihood of aortic dissection. Because this test did not confirm the presence of aortic dissection, the patient underwent transesophageal echocardiography (TEE), a test of equal or greater sensitivity.1, 2 This test was also negative; however, a small dissection flap was subsequently found during cardiac catheterization. In this case, a test of lower sensitivity and specificity confirmed the diagnosis of dissection,3 demonstrating the possibility that either CT or TEE can be misinterpreted. Indeed, a final review of the TEE, showed the dissection flap, albeit small, had been missed. In this case, a diagnostic error was made that delayed the diagnosis when sufficient information was available earlier. Diagnostic errors are prevalent in medical practice and are commonly the result of numerous factors, though cognitive problems appear to be the largest contributor to this process.4 In particular, faulty synthesis of information, rather than inadequate medical knowledge, is the most common cause of cognitive medical errors. The error made in this case likely falls under the subcategory of faulty test detection or perception and subsequent premature closure (failure to consider other possibilities once an initial diagnosis of uncomplicated aortic aneurysm had been reached).4
Though medical errors cannot be completely eliminated, cases such as this should be reviewed to understand the cognitive processes that may lead to an erroneous diagnosis. In addition to the false‐negative TEE finding, this patient also had a coexisting condition that may have preoccupied the medical team. The thoracic aortic aneurysm seen by all imaging modalities was large and required intervention regardless of the presence of a dissection. However, this chronic condition became the focus of treatment, and the acute event that precipitated admission was missed. Perhaps if the primary team maintained a very high index of suspicion for dissection and conveyed this to the cardiology consultants, a meticulous review of the TEE would have then followed that may have uncovered the subtle findings of the dissection flap.
Fortunately, definitive treatment with surgical aortic root and valve replacement was performed in a timely manner, as the consequences of a delayed diagnosis in this situation could have been catastrophic. The mortality rate of a type A dissection is extremely high initially (1%‐2% per hour),5, 6 and thus surgical intervention is typically performed immediately after the diagnosis and the extent of this disease is established, rather than the following morning.7
This case highlights not only the problems resulting in diagnostic errors but also exemplifies the thought process required to make a challenging diagnosis. Our case discussant was able to avoid cognitive pitfalls by presenting a broad differential diagnosis and reevaluating the diagnosis with each additional piece of information provided.8 An experienced clinician should realize that patients with an extremely high pretest probability of disease and a negative diagnostic test should be further investigated, regardless of the test sensitivity. Furthermore, time‐honored methods such as history taking, physical examination. and thoughtful analyses should remain critical tools in the process of reaching an accurate diagnosis despite technological advances in diagnostic testing.
A 46‐year‐old African American man presented to the emergency department with severe chest pain that awakened him from sleep. The pain was substernal, radiated to the neck and back, and was continuous, lasting approximately 1 hour. It was associated with nausea, diaphoresis, and dizziness. The patient denied dyspnea, orthopnea, paroxysmal nocturnal dyspnea, dysphagia, odynophagia, vomiting, fever, chills, or headache. He denied recent recreational drug use. He works as a landscaper.
Substernal chest pain radiating to the neck and back implicates structures in the middle mediastinum, chiefly the heart, aorta, esophagus, pulmonary arteries, and mediastinal pleura. The presence of nausea and diaphoresis suggests a vagal response to pain.
The sudden onset of symptoms and the lack of fevers and chills make infectious causes in the mediastinum such as mediastinitis and pneumonia less likely. Acute pulmonary embolism often presents with pleuritic pain and dyspnea, features not present in this patient.
The absence of odynophagia and dysphagia makes esophageal rupture or perforation of esophageal diverticula unlikely. Likewise, the absence of dyspnea, orthopnea, and paroxysmal nocturnal dyspnea makes it unlikely to be acute left ventricular failure from a sudden rise in left atrial pressure. Such a scenario may occur in the setting of a myocardial infarction or rupture of a papillary muscle, chordae tendineae, or sinus of Valsalva.
Dissection of the aorta with or without involvement of the aortic root merits strong consideration. Dissection involving the carotid or vertebral arteries could explain the patient's dizziness. Physical stresses in a landscaper may contribute to elevation in blood pressure and set the stage for an aortic dissection, especially with other risk factors.
The patient has a history of a positive PPD and was treated with isoniazid for 6 months. His mother had a history of hypertension and died from a myocardial infarction at age 64. His father's medical history is unknown. He has a history of alcohol abuse but has been abstinent for more than a year. He smoked marijuana and tobacco occasionally, with a 15 pack‐year cigarette history. He also stated that the last time he used cocaine was 1 year prior to admission.
That his mother succumbed to a myocardial infarction as well as having been hypertensive could be important family history risk factors given the patient's symptoms. Furthermore, the concurrent use of alcohol and tobacco by the patient increases his risk of developing severe hypertension. The use of cocaine is associated with sudden elevations in systemic blood pressure, which predispose to intimal damage in the aorta, especially if other risk factors are present. Marijuana smoking has been implicated in pulmonary aspergillosis, but this sudden presentation in the absence of pulmonary symptoms makes it most unlikely. Optimal therapy of a positive PPD should not predispose the patient to acute exudative pericarditis. Thus far the features suggest an acute vascular episode without significant compromise of cardiac output.
The patient was alert and in mild distress from chest discomfort. He was afebrile, with a blood pressure of 136/64 in the right arm and 139/63 in the left arm. Heart rate was 60 beats per minute, and the respiratory rate was 12 beats per minute, with an oxygen saturation of 99% while breathing room air. Examination of the head and neck revealed no signs of trauma. Jugular venous waveforms were normal, and carotid artery pulsations had normal strength and upstroke without audible bruits. The lungs were clear to auscultation. Heart sounds were notable for a 3/6 diastolic murmur heard best at the right sternal border. Rate and rhythm were regular, and the apical impulse was sustained but not displaced. The peripheral pulses were present and equal in quality throughout. The findings of the abdominal exam were normal, and the digital rectal examination was negative for occult blood. The findings of the neurologic, musculoskeletal, and dermatologic exams also were normal.
A slightly elevated pulse pressure without a significant difference in upper extremity blood pressure could be a result of aortic regurgitation, sinus of Valsalva rupture, or a high‐cardiac‐output state, as seen in thyrotoxicosis, anemia, or arteriovenous fistula.
The presence of a 3/6 diastolic murmur at the sternal border, however, favors conditions that cause aortic valve regurgitation and, less commonly, pulmonary valve regurgitation, or turbulent flow across the tricuspid valve. The murmur of pulmonary and aortic valve regurgitation can be difficult to distinguish by auscultation; however, the absence of other findings such as a right ventricular heave, elevated jugular venous pressure, or primary lung disease do not support a pulmonary valve etiology. Turbulent flow across the tricuspid valve can be seen in high‐output states, large atrial septal defects, and tricuspid stenosis. This type of murmur is heard best at the lower sternal border and tends to increase with inspiration. An early diastolic murmur would suggest aortic regurgitation, either from aortic valve or aortic root pathology. Aortic regurgitation would contribute to a sustained apical impulse.
Clear lungs and the absence of tachycardia suggest that left ventricle function is not severely compromised. These findings argue against acute rupture of the sinus of Valsalva, a condition that normally causes biventricular failure. The presence of equal peripheral pulses does not exclude the diagnosis of aortic dissection, as the dissection may have occurred proximal to the origins of the right innominate and the left subclavian arteries.
Initial laboratory studies revealed a hematocrit of 34.9, a leukocyte count of 6800/mm3, and a platelet count of 195 000/mm3. The levels of serum electrolytes, serum creatinine, blood urea nitrogen, and initial cardiac enzymes were normal. The urine drug screen was negative. The electrocardiogram showed evidence of left ventricular hypertrophy (Figure 1), and portable chest radiography revealed an enlarged cardiac silhouette and a widened mediastinum (Figure 2).


The presence of a widened mediastinum is consistent with aortic dissection but may also suggest a mass, aortic aneurysm, infiltrative disease, or a collection of fluid (eg, blood). A normal level of cardiac enzymes and the absence of ischemic findings on electrocardiography make myocardial infarction less likely. Given his hemodynamic stability, he is unlikely to have suffered cardiac rupture; however, he may still have a dissection of the aorta or a rupture of the sinus of Valsalva. The slight decline in hematocrit is compatible with either a mediastinal or pericardial collection of blood.
The patient was emergently sent for computerized axial tomography of the chest, which revealed a dilated aortic root at 6 cm but no evidence of aortic dissection (Figure 3). The lung fields were normal.

Despite a negative test, the presence of severe acute chest pain in the presence of a widened mediastinum is still concerning for aortic dissection. If the dissection involved the aortic valve annulus, the resulting acute regurgitation can not be severe, given the absence of left ventricular heart failure. Likewise, the presence of normal lung fields suggests the patient has no acute elevation of left ventricular end diastolic pressures such as is seen in ventricle septal defect, papillary muscle dysfunction, or acute mitral valvular lesion.
Cardiology consultants were emergently consulted and performed a transesophageal echocardiogram that confirmed a dilated aortic root (6.3 cm) without evidence of dissection. The patient was noted to have moderate to severe aortic regurgitation and a dilated left ventricle with moderate hypertrophy. A trace effusion was noted, and the left ventricular ejection fraction was estimated to be 50%.
The presence of moderate to severe aortic regurgitation with a dilated aortic root suggests 3 possibilities: undiagnosed dissection of the aortic root with preexisting aortic insufficiency (eg, bicuspid aortic valve, rheumatic valve disease, previous endocarditis); infectious (eg, syphilitic) or noninfectious (eg, ankylosing spondylitis, Takayasu's arteritis) meso‐aortitis causing aortic dilatation and subsequent regurgitation; or, finally, connective tissue diseases (eg, Marfan's syndrome, Ehlers‐Danlos), which can cause premature degeneration of the aortic media. The acuity of the patient's symptoms and the lack of systemic findings make a connective tissue or inflammatory disease unlikely. The clinical index of suspicion for aortic dissection needs to remain very high, as failure to make an expedient diagnosis may lead to complications and a deleterious outcome. Aortography may help to define this lesion.
The cardiothoracic surgical team was consulted and recommended aortic root and valve replacement. The patient was observed overnight and scheduled for preoperative cardiac catheterization the following morning. Aortogram revealed moderate to severe aortic insufficiency and a small dissection flap on the lesser curvature of the aorta, above the left coronary ostia (Figure 4). Coronary angiography revealed a 50% stenosis of the ostia of the first and second diagonal arteries, with no other flow‐limiting lesions.

The study has clearly demonstrated that the patient suffered an acute aortic dissection without involvement of the aortic annulus. Given the absence of left ventricular failure, it appears the aortic regurgitation was chronic and secondary to a previously existing aortic aneurysm. Asymptomatic congenital defects of the meso‐aorta such as cystic medical necrosis can predispose to aortic dissection at a relatively young age. However, this patient's condition may have been aggravated by drug abuse, paroxysmal elevation of blood pressure during landscaping, or other risk factors. Surgical correction of the dissection and aortic regurgitation is necessary.
The patient underwent aortic valve replacement with a 25‐mm St. Jude mechanical valve and an ascending aortic transection repair with a 32‐mm Dacron tube graft. On postoperative day 9, the patient was discharged home in stable condition.
COMMENTARY
Ensuring both accuracy and efficiency when making a diagnosis can be difficult, particularly when patients present in an atypical fashion or when diagnostic testing yields inconclusive results. Thus, a physician must sift through each clinical clue, remembering that although certain findings are pathognomonic for a disease process, a constellation of signs and symptoms when present can be equally diagnostic.
The initial likelihood of disease (ie, pretest probability) is generated by the history and physical and laboratory examinations. If the pretest probability is high and the subsequent diagnostic test is positive, the modified likelihood of disease (post‐test probability) is nearly 100%. If, however, the pretest probability is high and the diagnostic test is negative, the likelihood of disease is less clear. In these situations, the physician can either review the initial findings that generated the pretest probability or perform an additional diagnostic test of higher sensitivity.
In this exercise, a 46‐year‐old man presented to the emergency department with severe chest pain and findings characteristic of aortic dissection. The physicians appropriately sent him for chest computerized tomography (CT) because of a high pretest likelihood of aortic dissection. Because this test did not confirm the presence of aortic dissection, the patient underwent transesophageal echocardiography (TEE), a test of equal or greater sensitivity.1, 2 This test was also negative; however, a small dissection flap was subsequently found during cardiac catheterization. In this case, a test of lower sensitivity and specificity confirmed the diagnosis of dissection,3 demonstrating the possibility that either CT or TEE can be misinterpreted. Indeed, a final review of the TEE, showed the dissection flap, albeit small, had been missed. In this case, a diagnostic error was made that delayed the diagnosis when sufficient information was available earlier. Diagnostic errors are prevalent in medical practice and are commonly the result of numerous factors, though cognitive problems appear to be the largest contributor to this process.4 In particular, faulty synthesis of information, rather than inadequate medical knowledge, is the most common cause of cognitive medical errors. The error made in this case likely falls under the subcategory of faulty test detection or perception and subsequent premature closure (failure to consider other possibilities once an initial diagnosis of uncomplicated aortic aneurysm had been reached).4
Though medical errors cannot be completely eliminated, cases such as this should be reviewed to understand the cognitive processes that may lead to an erroneous diagnosis. In addition to the false‐negative TEE finding, this patient also had a coexisting condition that may have preoccupied the medical team. The thoracic aortic aneurysm seen by all imaging modalities was large and required intervention regardless of the presence of a dissection. However, this chronic condition became the focus of treatment, and the acute event that precipitated admission was missed. Perhaps if the primary team maintained a very high index of suspicion for dissection and conveyed this to the cardiology consultants, a meticulous review of the TEE would have then followed that may have uncovered the subtle findings of the dissection flap.
Fortunately, definitive treatment with surgical aortic root and valve replacement was performed in a timely manner, as the consequences of a delayed diagnosis in this situation could have been catastrophic. The mortality rate of a type A dissection is extremely high initially (1%‐2% per hour),5, 6 and thus surgical intervention is typically performed immediately after the diagnosis and the extent of this disease is established, rather than the following morning.7
This case highlights not only the problems resulting in diagnostic errors but also exemplifies the thought process required to make a challenging diagnosis. Our case discussant was able to avoid cognitive pitfalls by presenting a broad differential diagnosis and reevaluating the diagnosis with each additional piece of information provided.8 An experienced clinician should realize that patients with an extremely high pretest probability of disease and a negative diagnostic test should be further investigated, regardless of the test sensitivity. Furthermore, time‐honored methods such as history taking, physical examination. and thoughtful analyses should remain critical tools in the process of reaching an accurate diagnosis despite technological advances in diagnostic testing.
- ,,, et al.The diagnosis of thoracic aortic dissection by noninvasive imaging procedures.N Engl J Med.1993;328(1):1–9.
- ,,, et al.Accuracy of biplane and multiplane transesophageal echocardiography in diagnosis of typical acute aortic dissection and intramural hematoma.J Am Coll Cardiol.1996;28:627–636.
- ,.Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies.Circulation.2003;108:628–635.
- ,,.Diagnostic error in internal medicine.Arch Intern Med.2005;165:1493–1499.
- ,,.Dissecting aneurysm of the aorta: a review of 505 cases.Medicine (Baltimore).1958;37(3):217–279.
- ,,, et al.The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease.JAMA.2000;283:897–903.
- ,.Surgery of the thoracic aorta.N Engl J Med.1997;336:1876–1888.
- .Clinical reasoning in medicine. In:Higgs J,Jones MA, eds.Clinical Reasoning in the Health Professions.Woburn, Mass:Butterworth‐Heinemann;1995:49–59.
- ,,, et al.The diagnosis of thoracic aortic dissection by noninvasive imaging procedures.N Engl J Med.1993;328(1):1–9.
- ,,, et al.Accuracy of biplane and multiplane transesophageal echocardiography in diagnosis of typical acute aortic dissection and intramural hematoma.J Am Coll Cardiol.1996;28:627–636.
- ,.Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies.Circulation.2003;108:628–635.
- ,,.Diagnostic error in internal medicine.Arch Intern Med.2005;165:1493–1499.
- ,,.Dissecting aneurysm of the aorta: a review of 505 cases.Medicine (Baltimore).1958;37(3):217–279.
- ,,, et al.The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease.JAMA.2000;283:897–903.
- ,.Surgery of the thoracic aorta.N Engl J Med.1997;336:1876–1888.
- .Clinical reasoning in medicine. In:Higgs J,Jones MA, eds.Clinical Reasoning in the Health Professions.Woburn, Mass:Butterworth‐Heinemann;1995:49–59.
Clinical Conundrum
A 49‐year‐old man presented with 2 days of chills, fever, anorexia, and increased cough and dyspnea. The patient had a history of chronic obstructive pulmonary disease (COPD) and noted that his cough and dyspnea had increased above normal for several days. He was now dyspneic with minimal activity and had slept at a 45‐degree incline the night prior to evaluation due to dyspnea. He noted less improvement than usual with the use of his metered dose inhaler. His cough was occasionally productive of small amounts of white phlegm. He had vomited once. During a coughing episode the patient experienced a sudden onset of sharp right upper quadrant abdominal pain that worsened with coughing and sudden position changes. The patient denied a prior history of abdominal pain or surgery. The patient's last bowel movement was 2 days prior to admission. He denied melena or bright red blood per rectum.
My initial differential diagnosis for this patient's dyspnea and cough is pneumonia, acute exacerbation of COPD, or congestive heart failure. The presence of fever and anorexia increases the likelihood of infectious etiologies, whereas the presence of orthopnea points toward congestive heart failure. Noncardiac processessuch as a large pleural effusion or apical lung diseasecould also cause orthopnea. His abdominal pain could be a result of pneumonia alone (perhaps in the right lower lobe with diaphragmatic irritation), but I am also considering complications of pneumonia such as empyema. Although his abdominal pain, dyspnea, and cough could also be a result of hepatobiliary disease, a perforated viscus, or pancreatitis, we currently have little reason to suspect a direct abdominal etiology. My top diagnosis is community‐acquired pneumonia, perhaps accompanied by pleural effusion.
His medical history was significant for dilated cardiomyopathy and heavy alcohol use. His medications included various meter‐dosed inhalers, bupropion, digoxin, spironolactone, lisinopril, and metoprolol. He had never received corticosteroid therapy and had not previously been hospitalized for COPD‐related problems. He had smoked one pack of cigarettes daily for 40 years.
Heavy alcohol use is associated with an increased risk of several pulmonary infections such as gram‐negative necrotizing pneumonia (classically, Klebsiella pneumoniae), pneumococcal pneumonia, aspiration pneumonia, anaerobic lung abscesses, and tuberculosis. Given his right upper quadrant pain, acute alcoholic hepatitis and alcohol‐related pancreatitis enter the differential. His history of cardiomyopathy makes me consider congestive heart failure as more likely than before, and perhaps his abdominal pain is a result of hepatic congestion from right heart failure. His fever, however, cannot be attributed to cardiac failure. Less likely diagnoses include ischemic conditions related to his cardiomyopathy such as mesenteric ischemia from low perfusion or embolism from a cardiac thrombus. A pulmonary infection remains the most likely diagnosis.
He was an ill‐appearing man in moderate respiratory distress, looking older than his stated age. His temperature was 38.4C, heart rate 129 beats/minute, blood pressure 85/56 mm Hg, respiratory rate 24 breaths/minute, and oxygen saturation 92% on room air. A cardiovascular exam revealed no murmur, gallop, or rub. The jugular venous pulse was not elevated. His lungs were clear to auscultation. Abdominal exam revealed right‐sided abdominal tenderness that appeared to localize to the rectus sheath. Otherwise, the abdomen was soft, with normal bowel sounds and no organomegaly. Rectal examination revealed guaiac negative stool and no focal tenderness. His extremities were normal.
His vital signs are worrisome for impending cardiovascular collapse and shock, possibly due to sepsis. The relatively nonfocal cardiopulmonary exam is surprising given his initial symptoms and makes me wonder if his dyspnea is primarily related to an abdominal process leading to diaphragmatic irritation rather than to a thoracic process. Congestive heart failure seems unlikely given the lack of supportive physical examination findings. His abdominal exam findings are puzzling. Although his abdominal wall tenderness could be benignperhaps from muscular strain or a tear from coughingit could represent a more worrisome process such as infection or a hematoma in the abdominal wall muscles. Mesenteric ischemia is still possible, as the exam is often unimpressive. A hepatic abscess or subphrenic abscess should be considered, as physical exam findings in these conditions can be subtle.
My differential remains relatively unchanged, but I have now put consideration of a hepatic or subphrenic abscess higher on my list. Early empiric broad‐spectrum antibiotics seem necessary.
He had a white blood cell count of 26,700/mL with 92% neutrophils, a hemoglobin of 14.6 g/dL, and a platelet count of 312,000/mL. Sodium was 134 mmol/L, potassium was 4.3 mmol/L, chloride was 94 mmol/L, bicarbonate was 23 mmol/L, blood urea nitrogen was 23 mg/dL, and creatinine was 2.1 mg/dL. The results of the calcium, protein, albumin, and liver function tests were normal. Urinalysis was negative for protein and red blood cells. An electrocardiogram revealed sinus tachycardia. A chest radiograph at admission revealed mild opacities in both lower lobes and the right middle lobe consistent with either atelectasis or pneumonia (Fig. 1). A very small left effusion was also identified.
The additional data reinforce my clinical impression that this process is likely to be infectious. The chest radiograph is consistent with community‐acquired pneumonia, possibly from an atypical pathogen. Given his elevated creatinine, I am also considering a pulmonary‐renal syndrome such as vasculitis, though hematuria was not present. A subphrenic abscess, mesenteric ischemia, or an abdominal wall process (because his abdominal tenderness on exam still needs an explanation) remain possibilities; my suspicion would increase if he does not respond appropriately to therapy for community‐acquired pneumonia.
The clinical team's working diagnosis also was community‐acquired pneumonia. Blood and sputum cultures were obtained, and the patient was treated with intravenous ceftriaxone, azithromycin, and intravenous fluid. By the second day, his creatinine had normalized; however, his hypoxemia had worsened, and he now required supplemental oxygen. His temperature was 39.3C, and his heart rate was 150 beats/minute. The findings of an abdominal ultrasound of the kidneys, spleen, and right upper quadrant were normal.
It is too early to say the patient has failed therapy because a patient can get worse before getting better during the course of antibiotic therapy for community‐acquired pneumonia. Fever, for example, may take up to 7 days to resolve, depending on host factors and the pathogen. Though I typically wait about 72 hours before assuming a patient is not appropriately responding to therapy, the additional information has made me concerned. The degree of tachycardia is significant and warrants an EKG to exclude an arrthymia. I would also repeat the chest radiograph to evaluate for worsening infiltrates or increased pleural effusion.
On the third hospital day, the patient's abdominal pain had decreased with analgesia, but his fever, cough, and dyspnea remained largely unchanged. Antibiotics were changed to intravenous levofloxacin. A repeat chest radiograph revealed elevation of the right hemidiaphragm and bilateral effusions (Fig. 2). An electrocardiogram revealed sinus tachycardia. Blood cultures revealed no growth, and sputum cultures grew oral flora.
A significantly elevated right hemidiaphragm makes me reconsider the diagnosis of simple community‐acquired pneumonia. The differential diagnosis for an elevated hemidiaphragm is best considered by location in relation to the diaphragm. Causes above the diaphragm include rib fracture, atelectasis, pleural thickening, and volume loss of the lung for another reason (e.g., surgery, bronchial obstruction due to tumor or mucus plugging), as well as mimics such as a densely consolidated pneumonia, pulmonary infarction, or a subpulmonary effusion. Diaphragmatic causes include eventration, rupture, phrenic nerve weakness, and intrinsic weakness because of neuromuscular disease (usually bilateral). Causes below the diaphragm that must be considered are subphrenic or liver abscess, liver (and other abdominal) malignancy, pancreatic pseudocyst, and distended bowel. Given the clinical picture, I am focusing below the diaphragmespecially on a possible hepatic or subphrenic abscess (which could be missed on ultrasound) and mimics of it such as dense consolidation or a subpulmonary effusion. Given the lack of response to antibiotics, I need to consider an infection that is not being treated, either because of location (abscess, effusion) or microbiology (tuberculosis, a parasite, a fungus, resistant bacteria). After confirming that the patient has a substantive pleural effusion, he needs a thoracentesis.
On the fourth hospital day, his temperature was 38.8C, and his white blood cell count was 21,000/mL. A right‐sided thoracentesis was performed; approximately 250 cc of fluid was obtained. Pleural fluid analysis revealed bloody fluid, with a white blood cell count of 16,750/mL with 94% neutrophils, 40,000 red blood cells/mL, lactate dehydrogenase of 278 U/L (normal serum value 80200 U/L), protein of 3.7 g/dL, and glucose of 81 mg/dL. A pleural fluid pH was not obtained. A gram stain revealed many white blood cells with no organisms noted. Serum protein was 7.4 g/dL. These results were thought to represent an exudative parapneumonic effusion; levofloxacin and supplemental oxygen were continued.
The pleural fluid appears exudative, but I am not sure this man has a parapneumonic effusion because, despite clinical deterioration, an obvious infiltrate is not seen on interval chest radiography. We must look closely at the fluid because this is a bloody effusion and somewhat atypical for a parapneumonic effusion. Also, the effusion does not appear large enough to explain why he has not improved on the current antibiotics. We should thus reconsider our diagnosis and management. I would obtain additional imaging (such as an abdominal and chest computed tomography [CT]) and perhaps obtain a consultation from the pulmonary team regarding the postulated initial diagnosis of pneumonia with effusion.
On the fifth day of hospitalization, the patient's dyspnea and cough persisted but were improved. His abdominal pain was minimal and felt improved with flatus. Fever continued to 38.8C, and the white blood cell count was 20,000/mL. On examination the patient had decreased breath sounds at the right base and bibasilar crackles. His abdomen was soft, with tenderness in his right upper quadrant only with deep palpation; bowel sounds remained. An ultrasound of the chest was performed to look for a loculated effusion; however, no fluid was identified. The pulmonary consultant thought it likely that the patient had a subpulmonic effusion and recommended CT of the abdomen and chest.
His right upper quadrant tenderness is still unexplained. I would agree with the CT, primarily to evaluate other causes of his elevated diaphragm such as subphrenic or hepatic abscess. For now, I would make no change in antibiotic therapy.
On the sixth hospital day, the patient had an episode of bilious emesis. Chest and abdominal CT revealed collapse of the right middle and lower lobes with a small adjacent effusion, and a 6 6 16 cm abscess intimately opposed to the right lobe of the liver. Extending from the inferior extent of the abscess was a tubular thick‐walled structure connecting to the cecum that was suspicious of a thickened inflamed appendix. There was periappendiceal stranding suggesting inflammation. The small bowel was diffusely dilated up to 4.5 cm, suggesting a small bowel obstruction.
I suspect that his abscess is related to a perforated appendix and that the dilated small bowel is most likely a result of localized irritation of the bowel by the abscess and appendicitis. The collapsed lung is most likely due to local inflammation from the subdiaphragmatic abscess. Treatment should now be changed substantially. I would ask a surgeon to evaluate the patient because the most likely diagnosis is perforated appendicitis with abscess formation.
When the periappendiceal abscess was drained percutaneously, 190 mL of purulent fluid was removed. The cultures were positive for Klebsiella pneumonia, Enterococcus faecalis,and Streptococcus milleri. The patient was given 6 weeks of intravenous antibiotics with improvement in his clinical symptoms. During the interval the findings on his chest radiograph resolved completely. A laproscopic appendectomy 3 months later revealed significant right lower quadrant adhesions. The pathology specimen identified a distorted appendix with regeneration consistent with prior appendicitis. The patient was contacted 4 months after his surgery, and he reported that he was doing well, with no cardiopulmonary or gastrointestinal symptoms.
COMMENTARY
Community‐acquired pneumonia (CAP) is a common cause of acute illness and accounts for nearly 1 million admissions per year in the United States.1 The diagnosis of CAP is made when symptoms including dyspnea, fever, cough, or leukocytosis are present, with confirmation provided by a chest radiograph. Often the diagnosis is clear; however, there is no pathognomonic constellation of signs or symptoms that establish the diagnosis with certainty.2 Many physicians learn that pneumoniaespecially lower‐lobe pneumoniacan lead to abdominal findings such as upper quadrant pain, vomiting, and tenderness to palpation. Conversely, the patient discussed above illustrates that a primary abdominal process can also result in a symptom complex that mimics pneumonia.
The prevalence of CAP coupled with the inherent uncertainty of a clinical diagnosis of CAP leads to an important question: How long is too long before questioning the diagnosis? An analysis of the pneumonia Patient Outcomes Research Trial (PORT) limited to inpatients with CAP examined time to clinical stability. For the majority of patients, abnormal vital signs resolved within 23 days.3 In this study, 29% of patients had severe disease, and not surprisingly, these patients took longer to improve. Using the pneumonia severity index score, which accounts for age, comorbidity, abnormal vital signs, and laboratory data, the patient described in this article would be considered at high risk for death and complication with an estimated mortality of 9%.4 Using a combination of defervescence, resolution of tachycardia, tachypnea, and hypoxemia as markers of clinical stability, a patient like ours should respond within 4 days (with a range of 27 days). On the basis of these dataand the discrepancy between the patient's severe illness and relatively minor pulmonary infiltratesit seems reasonable to have considered this patient as failing CAP therapy as early as the fourth day of hospitalization.
In approximately 10% of hospitalized patients with CAP, the clinical course is protracted.5 When patients do not improve as quickly as expected, the reasons that could explain this should be investigated. In a cohort of 49 patients with CAP who failed therapy the most common reasons for failure to improve were severity of the pneumonia and drug resistance.6 A multicenter study found that the incidence of resistance to penicillin by Streptococcus pneumoniae, the most common bacterial pathogen in CAP, was 30%, with a 4% in vitro resistance rate to ceftriaxone.7 How well in vitro resistance predicts clinical response, however, is unclear. Risk factors for antibiotic resistance include close exposure to children, recent antibiotic use, and recent hospitalization. Immunosuppressive conditions should also be considered in patients who fail to improve. Suppurative complications of pneumoniasuch as empyema, parapneumonic effusion, and lung abscessalso delay recovery.
Another consideration in a patient with what appears to be a nonresolving pneumonia with pleural effusion is that the initial diagnosis is incorrect and the cause is extrathoracic. Pulmonary and cardiac diseases account for more than 90% of effusions, whereas less than 5% of pleural effusions result from intraabdominal causes.8 When should intraabdominal diseases be sought in patients with an effusion, fever, dyspnea, and cough? Light suggests that intraabdominal pathology should be investigated in patients who have pleural effusions without significant parenchymal disease.8 This point is underscored by the experience of our patient, whose chest radiographs showed, despite clinical decline, minimal airspace disease.
Several abdominal entities cause pleural effusion. Pancreatitis, either acute or chronic, with pseudocyst formation is the most common abdominal cause of exudative pleural effusions. Approximately 10% of patients with pancreatic disease will develop effusions, usually left‐sided.9 These left‐sided effusions are also seen in splenic abscesses, usually as a result of endocarditis. Intrahepatic abscess is associated with effusions in 20% of patients.10 A subphrenic abscess, as seen in our patient, is an uncommon cause of exudative pleural effusions. Historically, subphrenic abscesses resulted from a perforated viscus, with ruptured appendicitis the most common cause,11 followed by perforated peptic ulcers and biliary tract disease. With the advent of antibiotics, the causes of subphrenic abscess changed considerably, with the majority of current cases resulting from postsurgical complications.12 The findings of a chest radiograph are abnormal in 80% of patients with subphrenic abscess;1214 an elevated hemidiaphragm and pleural effusion are found in the majority of cases. The symptoms of a subphrenic abscess are nonspecific, and patient's complaints are equally split between predominantly thoracic and predomninantly abdominal complaints.15
Appendicitis, a common disease predominantly of the young, may lead to atypical presentations in older individuals. In a retrospective analysis of 113 patients older than 60 years with appendicitis, 70% presented in an atypical fashion.16 Typical symptoms include right lower quadrant pain, fever, anorexia and a white blood cell count greater than 10,000/mL. Fever was the most frequently absent symptom, seen in only 37% of older patients. In this cohort, approximately one third of older patients waited more than 48 hours prior to presentation. The time between symptom onset and clinical presentation is a strong predictor of perforation risk.17 As in this case, roughly 2% of patients with acute appendicitis will present with perforation and abscess formation.18 In such patients the management is initially conservative. Percutaneous drainage and broad spectrum antibiotics are the treatment of choice, followed by an interval appendectomy in 612 weeks.19 The rationale for delayed surgery is that earlier surgery may disseminate a localized inflammatory process.20
Community‐acquired pneumonia is a more frequent cause of hospital admission than is intraabdominal abscess. Physicians often face the dilemma of when to pursue alternative diagnoses after a patient who is thought to have an atypical presentation of a common disease (ie, CAP) fails to respond to conventional therapy. Although clinicians learn that right upper quadrant pain may be a symptom of pneumonia, our patient revealed that abdominal causes may mimic pneumonia and produce a pleural effusion. Determining whether the primary disease originates above or below the diaphragm is critical to guiding therapy. When patients fail to respond adequately to therapy, clinicians should set a low threshold for deciding to image the abdomen in a patient with modest pulmonary infiltrates, pleural effusion, and abdominal pain.
- ,,, et al.The cost of treating community‐acquired pneumonia.Clin Ther.1998;20:820–827.
- ,,.Does this patient have community‐acquired pneumonia? Diagnosing pneumonia by history and physical examination.JAMA.1997;278:1440–1445.
- ,,, et al.Time to clinical stability in patients hospitalized with community acquired pneumonia. Implications for practice guidelines.JAMA.1998;279:1452–1457.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336:243–250.
- ,,, et al.Utility of fiberoptic bronchoscopy in non resolving pneumonia.Chest.1990;98:1322–1326.
- ,,, et al.Antimicrobial treatment failures in patients with community acquired pneumonia. Causes and prognostic implications.Am J Respir Crit Care Med.2000;162:154–160.
- ,,, et al.Antimicrobial resistance with Streptococcus pneumoniae in the United States, 1997–98.Emerg Infect Dis.1999;5:757–765.
- ,.Pleural effusion. In:Murray JF,Nadel JA, eds.Textbook of respiratory medicine. 3rd ed.Philadelphia:WB Saunders,2000:2013–2041.
- ,,.Significance of pleural effusion in patients with acute pancreatitis.Am J Gastroenterol.1992;87:871–874.
- .Exudative pleural effusions secondary to gastrointestinal diseases.Clin Chest Med.1985;6(1):103–111.
- .Subphrenic abscess.Ann Surg.1963;158:240–248.
- ,,,.Upper abdominal abscess: a continuing and deadly problem.Am J Roentgenol.1980;134:759–765.
- .Subphrenic abscess. A clinical study of 101 cases.Acta Chir Scand.1959;117:388–408.
- ,,.Subphrenic abscess a continuing hazard.Am J Surg.1969:117–122.
- ,.Subphrenic abscess: a thoracoabdominal clinical complex. The changing picture with antibiotics.Am J Surg.1964;108:165–172.
- ,.What have we learned over the past 20 years about appendicitis in the elderly.Am J Surg.2003;185:198–201.
- ,,, et al.Appendicitis: why so complicated? Analysis of 5755 consecutive appendectomies.Am Surg.2000;66:548–554.
- ,,.Appendicitis with a palpable mass.Ann Surg.1981;193:227–229.
- ,,, et al.Nonoperative management of perforated appendicitis without periappendiceal mass.Am J Surg.2000;179:177–181.
- ,,.Appendix. In:Townsend CM, ed.Sabiston textbook of surgery. The biologic basis of modern surgical practice. 16th ed.Philadelphia:W. B. Saunders,2001:917–928.
A 49‐year‐old man presented with 2 days of chills, fever, anorexia, and increased cough and dyspnea. The patient had a history of chronic obstructive pulmonary disease (COPD) and noted that his cough and dyspnea had increased above normal for several days. He was now dyspneic with minimal activity and had slept at a 45‐degree incline the night prior to evaluation due to dyspnea. He noted less improvement than usual with the use of his metered dose inhaler. His cough was occasionally productive of small amounts of white phlegm. He had vomited once. During a coughing episode the patient experienced a sudden onset of sharp right upper quadrant abdominal pain that worsened with coughing and sudden position changes. The patient denied a prior history of abdominal pain or surgery. The patient's last bowel movement was 2 days prior to admission. He denied melena or bright red blood per rectum.
My initial differential diagnosis for this patient's dyspnea and cough is pneumonia, acute exacerbation of COPD, or congestive heart failure. The presence of fever and anorexia increases the likelihood of infectious etiologies, whereas the presence of orthopnea points toward congestive heart failure. Noncardiac processessuch as a large pleural effusion or apical lung diseasecould also cause orthopnea. His abdominal pain could be a result of pneumonia alone (perhaps in the right lower lobe with diaphragmatic irritation), but I am also considering complications of pneumonia such as empyema. Although his abdominal pain, dyspnea, and cough could also be a result of hepatobiliary disease, a perforated viscus, or pancreatitis, we currently have little reason to suspect a direct abdominal etiology. My top diagnosis is community‐acquired pneumonia, perhaps accompanied by pleural effusion.
His medical history was significant for dilated cardiomyopathy and heavy alcohol use. His medications included various meter‐dosed inhalers, bupropion, digoxin, spironolactone, lisinopril, and metoprolol. He had never received corticosteroid therapy and had not previously been hospitalized for COPD‐related problems. He had smoked one pack of cigarettes daily for 40 years.
Heavy alcohol use is associated with an increased risk of several pulmonary infections such as gram‐negative necrotizing pneumonia (classically, Klebsiella pneumoniae), pneumococcal pneumonia, aspiration pneumonia, anaerobic lung abscesses, and tuberculosis. Given his right upper quadrant pain, acute alcoholic hepatitis and alcohol‐related pancreatitis enter the differential. His history of cardiomyopathy makes me consider congestive heart failure as more likely than before, and perhaps his abdominal pain is a result of hepatic congestion from right heart failure. His fever, however, cannot be attributed to cardiac failure. Less likely diagnoses include ischemic conditions related to his cardiomyopathy such as mesenteric ischemia from low perfusion or embolism from a cardiac thrombus. A pulmonary infection remains the most likely diagnosis.
He was an ill‐appearing man in moderate respiratory distress, looking older than his stated age. His temperature was 38.4C, heart rate 129 beats/minute, blood pressure 85/56 mm Hg, respiratory rate 24 breaths/minute, and oxygen saturation 92% on room air. A cardiovascular exam revealed no murmur, gallop, or rub. The jugular venous pulse was not elevated. His lungs were clear to auscultation. Abdominal exam revealed right‐sided abdominal tenderness that appeared to localize to the rectus sheath. Otherwise, the abdomen was soft, with normal bowel sounds and no organomegaly. Rectal examination revealed guaiac negative stool and no focal tenderness. His extremities were normal.
His vital signs are worrisome for impending cardiovascular collapse and shock, possibly due to sepsis. The relatively nonfocal cardiopulmonary exam is surprising given his initial symptoms and makes me wonder if his dyspnea is primarily related to an abdominal process leading to diaphragmatic irritation rather than to a thoracic process. Congestive heart failure seems unlikely given the lack of supportive physical examination findings. His abdominal exam findings are puzzling. Although his abdominal wall tenderness could be benignperhaps from muscular strain or a tear from coughingit could represent a more worrisome process such as infection or a hematoma in the abdominal wall muscles. Mesenteric ischemia is still possible, as the exam is often unimpressive. A hepatic abscess or subphrenic abscess should be considered, as physical exam findings in these conditions can be subtle.
My differential remains relatively unchanged, but I have now put consideration of a hepatic or subphrenic abscess higher on my list. Early empiric broad‐spectrum antibiotics seem necessary.
He had a white blood cell count of 26,700/mL with 92% neutrophils, a hemoglobin of 14.6 g/dL, and a platelet count of 312,000/mL. Sodium was 134 mmol/L, potassium was 4.3 mmol/L, chloride was 94 mmol/L, bicarbonate was 23 mmol/L, blood urea nitrogen was 23 mg/dL, and creatinine was 2.1 mg/dL. The results of the calcium, protein, albumin, and liver function tests were normal. Urinalysis was negative for protein and red blood cells. An electrocardiogram revealed sinus tachycardia. A chest radiograph at admission revealed mild opacities in both lower lobes and the right middle lobe consistent with either atelectasis or pneumonia (Fig. 1). A very small left effusion was also identified.

The additional data reinforce my clinical impression that this process is likely to be infectious. The chest radiograph is consistent with community‐acquired pneumonia, possibly from an atypical pathogen. Given his elevated creatinine, I am also considering a pulmonary‐renal syndrome such as vasculitis, though hematuria was not present. A subphrenic abscess, mesenteric ischemia, or an abdominal wall process (because his abdominal tenderness on exam still needs an explanation) remain possibilities; my suspicion would increase if he does not respond appropriately to therapy for community‐acquired pneumonia.
The clinical team's working diagnosis also was community‐acquired pneumonia. Blood and sputum cultures were obtained, and the patient was treated with intravenous ceftriaxone, azithromycin, and intravenous fluid. By the second day, his creatinine had normalized; however, his hypoxemia had worsened, and he now required supplemental oxygen. His temperature was 39.3C, and his heart rate was 150 beats/minute. The findings of an abdominal ultrasound of the kidneys, spleen, and right upper quadrant were normal.
It is too early to say the patient has failed therapy because a patient can get worse before getting better during the course of antibiotic therapy for community‐acquired pneumonia. Fever, for example, may take up to 7 days to resolve, depending on host factors and the pathogen. Though I typically wait about 72 hours before assuming a patient is not appropriately responding to therapy, the additional information has made me concerned. The degree of tachycardia is significant and warrants an EKG to exclude an arrthymia. I would also repeat the chest radiograph to evaluate for worsening infiltrates or increased pleural effusion.
On the third hospital day, the patient's abdominal pain had decreased with analgesia, but his fever, cough, and dyspnea remained largely unchanged. Antibiotics were changed to intravenous levofloxacin. A repeat chest radiograph revealed elevation of the right hemidiaphragm and bilateral effusions (Fig. 2). An electrocardiogram revealed sinus tachycardia. Blood cultures revealed no growth, and sputum cultures grew oral flora.

A significantly elevated right hemidiaphragm makes me reconsider the diagnosis of simple community‐acquired pneumonia. The differential diagnosis for an elevated hemidiaphragm is best considered by location in relation to the diaphragm. Causes above the diaphragm include rib fracture, atelectasis, pleural thickening, and volume loss of the lung for another reason (e.g., surgery, bronchial obstruction due to tumor or mucus plugging), as well as mimics such as a densely consolidated pneumonia, pulmonary infarction, or a subpulmonary effusion. Diaphragmatic causes include eventration, rupture, phrenic nerve weakness, and intrinsic weakness because of neuromuscular disease (usually bilateral). Causes below the diaphragm that must be considered are subphrenic or liver abscess, liver (and other abdominal) malignancy, pancreatic pseudocyst, and distended bowel. Given the clinical picture, I am focusing below the diaphragmespecially on a possible hepatic or subphrenic abscess (which could be missed on ultrasound) and mimics of it such as dense consolidation or a subpulmonary effusion. Given the lack of response to antibiotics, I need to consider an infection that is not being treated, either because of location (abscess, effusion) or microbiology (tuberculosis, a parasite, a fungus, resistant bacteria). After confirming that the patient has a substantive pleural effusion, he needs a thoracentesis.
On the fourth hospital day, his temperature was 38.8C, and his white blood cell count was 21,000/mL. A right‐sided thoracentesis was performed; approximately 250 cc of fluid was obtained. Pleural fluid analysis revealed bloody fluid, with a white blood cell count of 16,750/mL with 94% neutrophils, 40,000 red blood cells/mL, lactate dehydrogenase of 278 U/L (normal serum value 80200 U/L), protein of 3.7 g/dL, and glucose of 81 mg/dL. A pleural fluid pH was not obtained. A gram stain revealed many white blood cells with no organisms noted. Serum protein was 7.4 g/dL. These results were thought to represent an exudative parapneumonic effusion; levofloxacin and supplemental oxygen were continued.
The pleural fluid appears exudative, but I am not sure this man has a parapneumonic effusion because, despite clinical deterioration, an obvious infiltrate is not seen on interval chest radiography. We must look closely at the fluid because this is a bloody effusion and somewhat atypical for a parapneumonic effusion. Also, the effusion does not appear large enough to explain why he has not improved on the current antibiotics. We should thus reconsider our diagnosis and management. I would obtain additional imaging (such as an abdominal and chest computed tomography [CT]) and perhaps obtain a consultation from the pulmonary team regarding the postulated initial diagnosis of pneumonia with effusion.
On the fifth day of hospitalization, the patient's dyspnea and cough persisted but were improved. His abdominal pain was minimal and felt improved with flatus. Fever continued to 38.8C, and the white blood cell count was 20,000/mL. On examination the patient had decreased breath sounds at the right base and bibasilar crackles. His abdomen was soft, with tenderness in his right upper quadrant only with deep palpation; bowel sounds remained. An ultrasound of the chest was performed to look for a loculated effusion; however, no fluid was identified. The pulmonary consultant thought it likely that the patient had a subpulmonic effusion and recommended CT of the abdomen and chest.
His right upper quadrant tenderness is still unexplained. I would agree with the CT, primarily to evaluate other causes of his elevated diaphragm such as subphrenic or hepatic abscess. For now, I would make no change in antibiotic therapy.
On the sixth hospital day, the patient had an episode of bilious emesis. Chest and abdominal CT revealed collapse of the right middle and lower lobes with a small adjacent effusion, and a 6 6 16 cm abscess intimately opposed to the right lobe of the liver. Extending from the inferior extent of the abscess was a tubular thick‐walled structure connecting to the cecum that was suspicious of a thickened inflamed appendix. There was periappendiceal stranding suggesting inflammation. The small bowel was diffusely dilated up to 4.5 cm, suggesting a small bowel obstruction.
I suspect that his abscess is related to a perforated appendix and that the dilated small bowel is most likely a result of localized irritation of the bowel by the abscess and appendicitis. The collapsed lung is most likely due to local inflammation from the subdiaphragmatic abscess. Treatment should now be changed substantially. I would ask a surgeon to evaluate the patient because the most likely diagnosis is perforated appendicitis with abscess formation.
When the periappendiceal abscess was drained percutaneously, 190 mL of purulent fluid was removed. The cultures were positive for Klebsiella pneumonia, Enterococcus faecalis,and Streptococcus milleri. The patient was given 6 weeks of intravenous antibiotics with improvement in his clinical symptoms. During the interval the findings on his chest radiograph resolved completely. A laproscopic appendectomy 3 months later revealed significant right lower quadrant adhesions. The pathology specimen identified a distorted appendix with regeneration consistent with prior appendicitis. The patient was contacted 4 months after his surgery, and he reported that he was doing well, with no cardiopulmonary or gastrointestinal symptoms.
COMMENTARY
Community‐acquired pneumonia (CAP) is a common cause of acute illness and accounts for nearly 1 million admissions per year in the United States.1 The diagnosis of CAP is made when symptoms including dyspnea, fever, cough, or leukocytosis are present, with confirmation provided by a chest radiograph. Often the diagnosis is clear; however, there is no pathognomonic constellation of signs or symptoms that establish the diagnosis with certainty.2 Many physicians learn that pneumoniaespecially lower‐lobe pneumoniacan lead to abdominal findings such as upper quadrant pain, vomiting, and tenderness to palpation. Conversely, the patient discussed above illustrates that a primary abdominal process can also result in a symptom complex that mimics pneumonia.
The prevalence of CAP coupled with the inherent uncertainty of a clinical diagnosis of CAP leads to an important question: How long is too long before questioning the diagnosis? An analysis of the pneumonia Patient Outcomes Research Trial (PORT) limited to inpatients with CAP examined time to clinical stability. For the majority of patients, abnormal vital signs resolved within 23 days.3 In this study, 29% of patients had severe disease, and not surprisingly, these patients took longer to improve. Using the pneumonia severity index score, which accounts for age, comorbidity, abnormal vital signs, and laboratory data, the patient described in this article would be considered at high risk for death and complication with an estimated mortality of 9%.4 Using a combination of defervescence, resolution of tachycardia, tachypnea, and hypoxemia as markers of clinical stability, a patient like ours should respond within 4 days (with a range of 27 days). On the basis of these dataand the discrepancy between the patient's severe illness and relatively minor pulmonary infiltratesit seems reasonable to have considered this patient as failing CAP therapy as early as the fourth day of hospitalization.
In approximately 10% of hospitalized patients with CAP, the clinical course is protracted.5 When patients do not improve as quickly as expected, the reasons that could explain this should be investigated. In a cohort of 49 patients with CAP who failed therapy the most common reasons for failure to improve were severity of the pneumonia and drug resistance.6 A multicenter study found that the incidence of resistance to penicillin by Streptococcus pneumoniae, the most common bacterial pathogen in CAP, was 30%, with a 4% in vitro resistance rate to ceftriaxone.7 How well in vitro resistance predicts clinical response, however, is unclear. Risk factors for antibiotic resistance include close exposure to children, recent antibiotic use, and recent hospitalization. Immunosuppressive conditions should also be considered in patients who fail to improve. Suppurative complications of pneumoniasuch as empyema, parapneumonic effusion, and lung abscessalso delay recovery.
Another consideration in a patient with what appears to be a nonresolving pneumonia with pleural effusion is that the initial diagnosis is incorrect and the cause is extrathoracic. Pulmonary and cardiac diseases account for more than 90% of effusions, whereas less than 5% of pleural effusions result from intraabdominal causes.8 When should intraabdominal diseases be sought in patients with an effusion, fever, dyspnea, and cough? Light suggests that intraabdominal pathology should be investigated in patients who have pleural effusions without significant parenchymal disease.8 This point is underscored by the experience of our patient, whose chest radiographs showed, despite clinical decline, minimal airspace disease.
Several abdominal entities cause pleural effusion. Pancreatitis, either acute or chronic, with pseudocyst formation is the most common abdominal cause of exudative pleural effusions. Approximately 10% of patients with pancreatic disease will develop effusions, usually left‐sided.9 These left‐sided effusions are also seen in splenic abscesses, usually as a result of endocarditis. Intrahepatic abscess is associated with effusions in 20% of patients.10 A subphrenic abscess, as seen in our patient, is an uncommon cause of exudative pleural effusions. Historically, subphrenic abscesses resulted from a perforated viscus, with ruptured appendicitis the most common cause,11 followed by perforated peptic ulcers and biliary tract disease. With the advent of antibiotics, the causes of subphrenic abscess changed considerably, with the majority of current cases resulting from postsurgical complications.12 The findings of a chest radiograph are abnormal in 80% of patients with subphrenic abscess;1214 an elevated hemidiaphragm and pleural effusion are found in the majority of cases. The symptoms of a subphrenic abscess are nonspecific, and patient's complaints are equally split between predominantly thoracic and predomninantly abdominal complaints.15
Appendicitis, a common disease predominantly of the young, may lead to atypical presentations in older individuals. In a retrospective analysis of 113 patients older than 60 years with appendicitis, 70% presented in an atypical fashion.16 Typical symptoms include right lower quadrant pain, fever, anorexia and a white blood cell count greater than 10,000/mL. Fever was the most frequently absent symptom, seen in only 37% of older patients. In this cohort, approximately one third of older patients waited more than 48 hours prior to presentation. The time between symptom onset and clinical presentation is a strong predictor of perforation risk.17 As in this case, roughly 2% of patients with acute appendicitis will present with perforation and abscess formation.18 In such patients the management is initially conservative. Percutaneous drainage and broad spectrum antibiotics are the treatment of choice, followed by an interval appendectomy in 612 weeks.19 The rationale for delayed surgery is that earlier surgery may disseminate a localized inflammatory process.20
Community‐acquired pneumonia is a more frequent cause of hospital admission than is intraabdominal abscess. Physicians often face the dilemma of when to pursue alternative diagnoses after a patient who is thought to have an atypical presentation of a common disease (ie, CAP) fails to respond to conventional therapy. Although clinicians learn that right upper quadrant pain may be a symptom of pneumonia, our patient revealed that abdominal causes may mimic pneumonia and produce a pleural effusion. Determining whether the primary disease originates above or below the diaphragm is critical to guiding therapy. When patients fail to respond adequately to therapy, clinicians should set a low threshold for deciding to image the abdomen in a patient with modest pulmonary infiltrates, pleural effusion, and abdominal pain.
A 49‐year‐old man presented with 2 days of chills, fever, anorexia, and increased cough and dyspnea. The patient had a history of chronic obstructive pulmonary disease (COPD) and noted that his cough and dyspnea had increased above normal for several days. He was now dyspneic with minimal activity and had slept at a 45‐degree incline the night prior to evaluation due to dyspnea. He noted less improvement than usual with the use of his metered dose inhaler. His cough was occasionally productive of small amounts of white phlegm. He had vomited once. During a coughing episode the patient experienced a sudden onset of sharp right upper quadrant abdominal pain that worsened with coughing and sudden position changes. The patient denied a prior history of abdominal pain or surgery. The patient's last bowel movement was 2 days prior to admission. He denied melena or bright red blood per rectum.
My initial differential diagnosis for this patient's dyspnea and cough is pneumonia, acute exacerbation of COPD, or congestive heart failure. The presence of fever and anorexia increases the likelihood of infectious etiologies, whereas the presence of orthopnea points toward congestive heart failure. Noncardiac processessuch as a large pleural effusion or apical lung diseasecould also cause orthopnea. His abdominal pain could be a result of pneumonia alone (perhaps in the right lower lobe with diaphragmatic irritation), but I am also considering complications of pneumonia such as empyema. Although his abdominal pain, dyspnea, and cough could also be a result of hepatobiliary disease, a perforated viscus, or pancreatitis, we currently have little reason to suspect a direct abdominal etiology. My top diagnosis is community‐acquired pneumonia, perhaps accompanied by pleural effusion.
His medical history was significant for dilated cardiomyopathy and heavy alcohol use. His medications included various meter‐dosed inhalers, bupropion, digoxin, spironolactone, lisinopril, and metoprolol. He had never received corticosteroid therapy and had not previously been hospitalized for COPD‐related problems. He had smoked one pack of cigarettes daily for 40 years.
Heavy alcohol use is associated with an increased risk of several pulmonary infections such as gram‐negative necrotizing pneumonia (classically, Klebsiella pneumoniae), pneumococcal pneumonia, aspiration pneumonia, anaerobic lung abscesses, and tuberculosis. Given his right upper quadrant pain, acute alcoholic hepatitis and alcohol‐related pancreatitis enter the differential. His history of cardiomyopathy makes me consider congestive heart failure as more likely than before, and perhaps his abdominal pain is a result of hepatic congestion from right heart failure. His fever, however, cannot be attributed to cardiac failure. Less likely diagnoses include ischemic conditions related to his cardiomyopathy such as mesenteric ischemia from low perfusion or embolism from a cardiac thrombus. A pulmonary infection remains the most likely diagnosis.
He was an ill‐appearing man in moderate respiratory distress, looking older than his stated age. His temperature was 38.4C, heart rate 129 beats/minute, blood pressure 85/56 mm Hg, respiratory rate 24 breaths/minute, and oxygen saturation 92% on room air. A cardiovascular exam revealed no murmur, gallop, or rub. The jugular venous pulse was not elevated. His lungs were clear to auscultation. Abdominal exam revealed right‐sided abdominal tenderness that appeared to localize to the rectus sheath. Otherwise, the abdomen was soft, with normal bowel sounds and no organomegaly. Rectal examination revealed guaiac negative stool and no focal tenderness. His extremities were normal.
His vital signs are worrisome for impending cardiovascular collapse and shock, possibly due to sepsis. The relatively nonfocal cardiopulmonary exam is surprising given his initial symptoms and makes me wonder if his dyspnea is primarily related to an abdominal process leading to diaphragmatic irritation rather than to a thoracic process. Congestive heart failure seems unlikely given the lack of supportive physical examination findings. His abdominal exam findings are puzzling. Although his abdominal wall tenderness could be benignperhaps from muscular strain or a tear from coughingit could represent a more worrisome process such as infection or a hematoma in the abdominal wall muscles. Mesenteric ischemia is still possible, as the exam is often unimpressive. A hepatic abscess or subphrenic abscess should be considered, as physical exam findings in these conditions can be subtle.
My differential remains relatively unchanged, but I have now put consideration of a hepatic or subphrenic abscess higher on my list. Early empiric broad‐spectrum antibiotics seem necessary.
He had a white blood cell count of 26,700/mL with 92% neutrophils, a hemoglobin of 14.6 g/dL, and a platelet count of 312,000/mL. Sodium was 134 mmol/L, potassium was 4.3 mmol/L, chloride was 94 mmol/L, bicarbonate was 23 mmol/L, blood urea nitrogen was 23 mg/dL, and creatinine was 2.1 mg/dL. The results of the calcium, protein, albumin, and liver function tests were normal. Urinalysis was negative for protein and red blood cells. An electrocardiogram revealed sinus tachycardia. A chest radiograph at admission revealed mild opacities in both lower lobes and the right middle lobe consistent with either atelectasis or pneumonia (Fig. 1). A very small left effusion was also identified.

The additional data reinforce my clinical impression that this process is likely to be infectious. The chest radiograph is consistent with community‐acquired pneumonia, possibly from an atypical pathogen. Given his elevated creatinine, I am also considering a pulmonary‐renal syndrome such as vasculitis, though hematuria was not present. A subphrenic abscess, mesenteric ischemia, or an abdominal wall process (because his abdominal tenderness on exam still needs an explanation) remain possibilities; my suspicion would increase if he does not respond appropriately to therapy for community‐acquired pneumonia.
The clinical team's working diagnosis also was community‐acquired pneumonia. Blood and sputum cultures were obtained, and the patient was treated with intravenous ceftriaxone, azithromycin, and intravenous fluid. By the second day, his creatinine had normalized; however, his hypoxemia had worsened, and he now required supplemental oxygen. His temperature was 39.3C, and his heart rate was 150 beats/minute. The findings of an abdominal ultrasound of the kidneys, spleen, and right upper quadrant were normal.
It is too early to say the patient has failed therapy because a patient can get worse before getting better during the course of antibiotic therapy for community‐acquired pneumonia. Fever, for example, may take up to 7 days to resolve, depending on host factors and the pathogen. Though I typically wait about 72 hours before assuming a patient is not appropriately responding to therapy, the additional information has made me concerned. The degree of tachycardia is significant and warrants an EKG to exclude an arrthymia. I would also repeat the chest radiograph to evaluate for worsening infiltrates or increased pleural effusion.
On the third hospital day, the patient's abdominal pain had decreased with analgesia, but his fever, cough, and dyspnea remained largely unchanged. Antibiotics were changed to intravenous levofloxacin. A repeat chest radiograph revealed elevation of the right hemidiaphragm and bilateral effusions (Fig. 2). An electrocardiogram revealed sinus tachycardia. Blood cultures revealed no growth, and sputum cultures grew oral flora.

A significantly elevated right hemidiaphragm makes me reconsider the diagnosis of simple community‐acquired pneumonia. The differential diagnosis for an elevated hemidiaphragm is best considered by location in relation to the diaphragm. Causes above the diaphragm include rib fracture, atelectasis, pleural thickening, and volume loss of the lung for another reason (e.g., surgery, bronchial obstruction due to tumor or mucus plugging), as well as mimics such as a densely consolidated pneumonia, pulmonary infarction, or a subpulmonary effusion. Diaphragmatic causes include eventration, rupture, phrenic nerve weakness, and intrinsic weakness because of neuromuscular disease (usually bilateral). Causes below the diaphragm that must be considered are subphrenic or liver abscess, liver (and other abdominal) malignancy, pancreatic pseudocyst, and distended bowel. Given the clinical picture, I am focusing below the diaphragmespecially on a possible hepatic or subphrenic abscess (which could be missed on ultrasound) and mimics of it such as dense consolidation or a subpulmonary effusion. Given the lack of response to antibiotics, I need to consider an infection that is not being treated, either because of location (abscess, effusion) or microbiology (tuberculosis, a parasite, a fungus, resistant bacteria). After confirming that the patient has a substantive pleural effusion, he needs a thoracentesis.
On the fourth hospital day, his temperature was 38.8C, and his white blood cell count was 21,000/mL. A right‐sided thoracentesis was performed; approximately 250 cc of fluid was obtained. Pleural fluid analysis revealed bloody fluid, with a white blood cell count of 16,750/mL with 94% neutrophils, 40,000 red blood cells/mL, lactate dehydrogenase of 278 U/L (normal serum value 80200 U/L), protein of 3.7 g/dL, and glucose of 81 mg/dL. A pleural fluid pH was not obtained. A gram stain revealed many white blood cells with no organisms noted. Serum protein was 7.4 g/dL. These results were thought to represent an exudative parapneumonic effusion; levofloxacin and supplemental oxygen were continued.
The pleural fluid appears exudative, but I am not sure this man has a parapneumonic effusion because, despite clinical deterioration, an obvious infiltrate is not seen on interval chest radiography. We must look closely at the fluid because this is a bloody effusion and somewhat atypical for a parapneumonic effusion. Also, the effusion does not appear large enough to explain why he has not improved on the current antibiotics. We should thus reconsider our diagnosis and management. I would obtain additional imaging (such as an abdominal and chest computed tomography [CT]) and perhaps obtain a consultation from the pulmonary team regarding the postulated initial diagnosis of pneumonia with effusion.
On the fifth day of hospitalization, the patient's dyspnea and cough persisted but were improved. His abdominal pain was minimal and felt improved with flatus. Fever continued to 38.8C, and the white blood cell count was 20,000/mL. On examination the patient had decreased breath sounds at the right base and bibasilar crackles. His abdomen was soft, with tenderness in his right upper quadrant only with deep palpation; bowel sounds remained. An ultrasound of the chest was performed to look for a loculated effusion; however, no fluid was identified. The pulmonary consultant thought it likely that the patient had a subpulmonic effusion and recommended CT of the abdomen and chest.
His right upper quadrant tenderness is still unexplained. I would agree with the CT, primarily to evaluate other causes of his elevated diaphragm such as subphrenic or hepatic abscess. For now, I would make no change in antibiotic therapy.
On the sixth hospital day, the patient had an episode of bilious emesis. Chest and abdominal CT revealed collapse of the right middle and lower lobes with a small adjacent effusion, and a 6 6 16 cm abscess intimately opposed to the right lobe of the liver. Extending from the inferior extent of the abscess was a tubular thick‐walled structure connecting to the cecum that was suspicious of a thickened inflamed appendix. There was periappendiceal stranding suggesting inflammation. The small bowel was diffusely dilated up to 4.5 cm, suggesting a small bowel obstruction.
I suspect that his abscess is related to a perforated appendix and that the dilated small bowel is most likely a result of localized irritation of the bowel by the abscess and appendicitis. The collapsed lung is most likely due to local inflammation from the subdiaphragmatic abscess. Treatment should now be changed substantially. I would ask a surgeon to evaluate the patient because the most likely diagnosis is perforated appendicitis with abscess formation.
When the periappendiceal abscess was drained percutaneously, 190 mL of purulent fluid was removed. The cultures were positive for Klebsiella pneumonia, Enterococcus faecalis,and Streptococcus milleri. The patient was given 6 weeks of intravenous antibiotics with improvement in his clinical symptoms. During the interval the findings on his chest radiograph resolved completely. A laproscopic appendectomy 3 months later revealed significant right lower quadrant adhesions. The pathology specimen identified a distorted appendix with regeneration consistent with prior appendicitis. The patient was contacted 4 months after his surgery, and he reported that he was doing well, with no cardiopulmonary or gastrointestinal symptoms.
COMMENTARY
Community‐acquired pneumonia (CAP) is a common cause of acute illness and accounts for nearly 1 million admissions per year in the United States.1 The diagnosis of CAP is made when symptoms including dyspnea, fever, cough, or leukocytosis are present, with confirmation provided by a chest radiograph. Often the diagnosis is clear; however, there is no pathognomonic constellation of signs or symptoms that establish the diagnosis with certainty.2 Many physicians learn that pneumoniaespecially lower‐lobe pneumoniacan lead to abdominal findings such as upper quadrant pain, vomiting, and tenderness to palpation. Conversely, the patient discussed above illustrates that a primary abdominal process can also result in a symptom complex that mimics pneumonia.
The prevalence of CAP coupled with the inherent uncertainty of a clinical diagnosis of CAP leads to an important question: How long is too long before questioning the diagnosis? An analysis of the pneumonia Patient Outcomes Research Trial (PORT) limited to inpatients with CAP examined time to clinical stability. For the majority of patients, abnormal vital signs resolved within 23 days.3 In this study, 29% of patients had severe disease, and not surprisingly, these patients took longer to improve. Using the pneumonia severity index score, which accounts for age, comorbidity, abnormal vital signs, and laboratory data, the patient described in this article would be considered at high risk for death and complication with an estimated mortality of 9%.4 Using a combination of defervescence, resolution of tachycardia, tachypnea, and hypoxemia as markers of clinical stability, a patient like ours should respond within 4 days (with a range of 27 days). On the basis of these dataand the discrepancy between the patient's severe illness and relatively minor pulmonary infiltratesit seems reasonable to have considered this patient as failing CAP therapy as early as the fourth day of hospitalization.
In approximately 10% of hospitalized patients with CAP, the clinical course is protracted.5 When patients do not improve as quickly as expected, the reasons that could explain this should be investigated. In a cohort of 49 patients with CAP who failed therapy the most common reasons for failure to improve were severity of the pneumonia and drug resistance.6 A multicenter study found that the incidence of resistance to penicillin by Streptococcus pneumoniae, the most common bacterial pathogen in CAP, was 30%, with a 4% in vitro resistance rate to ceftriaxone.7 How well in vitro resistance predicts clinical response, however, is unclear. Risk factors for antibiotic resistance include close exposure to children, recent antibiotic use, and recent hospitalization. Immunosuppressive conditions should also be considered in patients who fail to improve. Suppurative complications of pneumoniasuch as empyema, parapneumonic effusion, and lung abscessalso delay recovery.
Another consideration in a patient with what appears to be a nonresolving pneumonia with pleural effusion is that the initial diagnosis is incorrect and the cause is extrathoracic. Pulmonary and cardiac diseases account for more than 90% of effusions, whereas less than 5% of pleural effusions result from intraabdominal causes.8 When should intraabdominal diseases be sought in patients with an effusion, fever, dyspnea, and cough? Light suggests that intraabdominal pathology should be investigated in patients who have pleural effusions without significant parenchymal disease.8 This point is underscored by the experience of our patient, whose chest radiographs showed, despite clinical decline, minimal airspace disease.
Several abdominal entities cause pleural effusion. Pancreatitis, either acute or chronic, with pseudocyst formation is the most common abdominal cause of exudative pleural effusions. Approximately 10% of patients with pancreatic disease will develop effusions, usually left‐sided.9 These left‐sided effusions are also seen in splenic abscesses, usually as a result of endocarditis. Intrahepatic abscess is associated with effusions in 20% of patients.10 A subphrenic abscess, as seen in our patient, is an uncommon cause of exudative pleural effusions. Historically, subphrenic abscesses resulted from a perforated viscus, with ruptured appendicitis the most common cause,11 followed by perforated peptic ulcers and biliary tract disease. With the advent of antibiotics, the causes of subphrenic abscess changed considerably, with the majority of current cases resulting from postsurgical complications.12 The findings of a chest radiograph are abnormal in 80% of patients with subphrenic abscess;1214 an elevated hemidiaphragm and pleural effusion are found in the majority of cases. The symptoms of a subphrenic abscess are nonspecific, and patient's complaints are equally split between predominantly thoracic and predomninantly abdominal complaints.15
Appendicitis, a common disease predominantly of the young, may lead to atypical presentations in older individuals. In a retrospective analysis of 113 patients older than 60 years with appendicitis, 70% presented in an atypical fashion.16 Typical symptoms include right lower quadrant pain, fever, anorexia and a white blood cell count greater than 10,000/mL. Fever was the most frequently absent symptom, seen in only 37% of older patients. In this cohort, approximately one third of older patients waited more than 48 hours prior to presentation. The time between symptom onset and clinical presentation is a strong predictor of perforation risk.17 As in this case, roughly 2% of patients with acute appendicitis will present with perforation and abscess formation.18 In such patients the management is initially conservative. Percutaneous drainage and broad spectrum antibiotics are the treatment of choice, followed by an interval appendectomy in 612 weeks.19 The rationale for delayed surgery is that earlier surgery may disseminate a localized inflammatory process.20
Community‐acquired pneumonia is a more frequent cause of hospital admission than is intraabdominal abscess. Physicians often face the dilemma of when to pursue alternative diagnoses after a patient who is thought to have an atypical presentation of a common disease (ie, CAP) fails to respond to conventional therapy. Although clinicians learn that right upper quadrant pain may be a symptom of pneumonia, our patient revealed that abdominal causes may mimic pneumonia and produce a pleural effusion. Determining whether the primary disease originates above or below the diaphragm is critical to guiding therapy. When patients fail to respond adequately to therapy, clinicians should set a low threshold for deciding to image the abdomen in a patient with modest pulmonary infiltrates, pleural effusion, and abdominal pain.
- ,,, et al.The cost of treating community‐acquired pneumonia.Clin Ther.1998;20:820–827.
- ,,.Does this patient have community‐acquired pneumonia? Diagnosing pneumonia by history and physical examination.JAMA.1997;278:1440–1445.
- ,,, et al.Time to clinical stability in patients hospitalized with community acquired pneumonia. Implications for practice guidelines.JAMA.1998;279:1452–1457.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336:243–250.
- ,,, et al.Utility of fiberoptic bronchoscopy in non resolving pneumonia.Chest.1990;98:1322–1326.
- ,,, et al.Antimicrobial treatment failures in patients with community acquired pneumonia. Causes and prognostic implications.Am J Respir Crit Care Med.2000;162:154–160.
- ,,, et al.Antimicrobial resistance with Streptococcus pneumoniae in the United States, 1997–98.Emerg Infect Dis.1999;5:757–765.
- ,.Pleural effusion. In:Murray JF,Nadel JA, eds.Textbook of respiratory medicine. 3rd ed.Philadelphia:WB Saunders,2000:2013–2041.
- ,,.Significance of pleural effusion in patients with acute pancreatitis.Am J Gastroenterol.1992;87:871–874.
- .Exudative pleural effusions secondary to gastrointestinal diseases.Clin Chest Med.1985;6(1):103–111.
- .Subphrenic abscess.Ann Surg.1963;158:240–248.
- ,,,.Upper abdominal abscess: a continuing and deadly problem.Am J Roentgenol.1980;134:759–765.
- .Subphrenic abscess. A clinical study of 101 cases.Acta Chir Scand.1959;117:388–408.
- ,,.Subphrenic abscess a continuing hazard.Am J Surg.1969:117–122.
- ,.Subphrenic abscess: a thoracoabdominal clinical complex. The changing picture with antibiotics.Am J Surg.1964;108:165–172.
- ,.What have we learned over the past 20 years about appendicitis in the elderly.Am J Surg.2003;185:198–201.
- ,,, et al.Appendicitis: why so complicated? Analysis of 5755 consecutive appendectomies.Am Surg.2000;66:548–554.
- ,,.Appendicitis with a palpable mass.Ann Surg.1981;193:227–229.
- ,,, et al.Nonoperative management of perforated appendicitis without periappendiceal mass.Am J Surg.2000;179:177–181.
- ,,.Appendix. In:Townsend CM, ed.Sabiston textbook of surgery. The biologic basis of modern surgical practice. 16th ed.Philadelphia:W. B. Saunders,2001:917–928.
- ,,, et al.The cost of treating community‐acquired pneumonia.Clin Ther.1998;20:820–827.
- ,,.Does this patient have community‐acquired pneumonia? Diagnosing pneumonia by history and physical examination.JAMA.1997;278:1440–1445.
- ,,, et al.Time to clinical stability in patients hospitalized with community acquired pneumonia. Implications for practice guidelines.JAMA.1998;279:1452–1457.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336:243–250.
- ,,, et al.Utility of fiberoptic bronchoscopy in non resolving pneumonia.Chest.1990;98:1322–1326.
- ,,, et al.Antimicrobial treatment failures in patients with community acquired pneumonia. Causes and prognostic implications.Am J Respir Crit Care Med.2000;162:154–160.
- ,,, et al.Antimicrobial resistance with Streptococcus pneumoniae in the United States, 1997–98.Emerg Infect Dis.1999;5:757–765.
- ,.Pleural effusion. In:Murray JF,Nadel JA, eds.Textbook of respiratory medicine. 3rd ed.Philadelphia:WB Saunders,2000:2013–2041.
- ,,.Significance of pleural effusion in patients with acute pancreatitis.Am J Gastroenterol.1992;87:871–874.
- .Exudative pleural effusions secondary to gastrointestinal diseases.Clin Chest Med.1985;6(1):103–111.
- .Subphrenic abscess.Ann Surg.1963;158:240–248.
- ,,,.Upper abdominal abscess: a continuing and deadly problem.Am J Roentgenol.1980;134:759–765.
- .Subphrenic abscess. A clinical study of 101 cases.Acta Chir Scand.1959;117:388–408.
- ,,.Subphrenic abscess a continuing hazard.Am J Surg.1969:117–122.
- ,.Subphrenic abscess: a thoracoabdominal clinical complex. The changing picture with antibiotics.Am J Surg.1964;108:165–172.
- ,.What have we learned over the past 20 years about appendicitis in the elderly.Am J Surg.2003;185:198–201.
- ,,, et al.Appendicitis: why so complicated? Analysis of 5755 consecutive appendectomies.Am Surg.2000;66:548–554.
- ,,.Appendicitis with a palpable mass.Ann Surg.1981;193:227–229.
- ,,, et al.Nonoperative management of perforated appendicitis without periappendiceal mass.Am J Surg.2000;179:177–181.
- ,,.Appendix. In:Townsend CM, ed.Sabiston textbook of surgery. The biologic basis of modern surgical practice. 16th ed.Philadelphia:W. B. Saunders,2001:917–928.