User login
Hospitalist Experiences With PICCs
Peripherally inserted central catheters (PICCs) are central venous catheters that are inserted through peripheral veins of the upper extremities in adults. Because they are safer to insert than central venous catheters (CVCs) and have become increasingly available at the bedside through the advent of specially trained vascular access nurses,[1] the use of PICCs in hospitalized patients has risen across the United States.[2] As the largest group of inpatient providers, hospitalists play a key role in the decision to insert and subsequently manage PICCs in hospitalized patients. Unfortunately, little is known about national hospitalist experiences, practice patterns, or knowledge when it comes to these commonly used devices. Therefore, we designed a 10‐question survey to investigate PICC‐related practices and knowledge among adult hospitalists practicing throughout the United States.
PATIENTS AND METHODS
Questions for this survey were derived from a previously published study conducted across 10 hospitals in the state of Michigan.[3] To assess external validity and test specific hypotheses formulated from the Michigan study, those questions with the greatest variation in response or those most amenable to interventions were chosen for inclusion in this survey.
To reach a national audience of practicing adult hospitalists, we submitted a survey proposal to the Society of Hospital Medicine's (SHM) Research Committee. The SHM Research Committee reviews such proposals using a peer‐review process to ensure both scientific integrity and validity of the survey instrument. Because the survey was already distributed to many hospitalists in Michigan, we requested that only hospitalists outside of Michigan be invited to participate in the national survey. All responses were collected anonymously, and no identifiable data were collected from respondents. Between February 1, 2013 and March 15, 2013, data were collected via an e‐mail sent directly from the SHM to members that contained a link to the study survey administered using SurveyMonkey. To augment data collection, nonresponders to the original e‐mail invitation were sent a second reminder e‐mail midway through the study. Descriptive statistics (percentages) were used to tabulate responses. The institutional review board at the University of Michigan Health System provided ethical and regulatory approval for this study.
RESULTS
A total of 2112 electronic survey invitations were sent to non‐Michigan adult hospitalists, with 381 completing the online survey (response rate 18%). Among respondents to the national survey, 86% reported having placed a PICC solely to obtain venous access in a hospitalized patient (rather than for specific indications such as long‐term intravenous antibiotics, chemotherapy, or parenteral nutrition), whereas 82% reported having cared for a patient who specifically requested a PICC (Table 1). PICC‐related deep vein thrombosis (DVT) and bloodstream infections were reported as being the most frequent PICC complications encountered by hospitalists, followed by superficial thrombophlebitis and mechanical complications such as coiling, kinking, and migration of the PICC tip.
| Total (N=381) | |
|---|---|
| |
| Hospitalist experiences related to PICCs | |
| Among hospitalized patients you have cared for, have any of your patients ever had a PICC placed solely to obtain venous access (eg, not for an indication such as long‐term IV antibiotics, chemotherapy, or TPN)? | |
| Yes | 328 (86.1%) |
| No | 53 (13.9%) |
| Have you ever cared for a patient who specifically requested a PICC because of prior experience with this device? | |
| Yes | 311 (81.6%) |
| No | 70 (18.4%) |
| Most frequently encountered PICC complications | |
| Upper‐extremity DVT or PE | 48 (12.6%) |
| Bloodstream infection | 41 (10.8%) |
| Superficial thrombophlebitis | 34 (8.9%) |
| Cellulitis/exit site erythema | 26 (6.8%) |
| Coiling, kinking of the PICC | 14 (3.7%) |
| Migration of the PICC tip | 9 (2.4%) |
| Breakage of PICC (anywhere) | 6 (1.6%) |
| Hospitalist practice related to PICCs | |
| During patient rounds, do you routinely examine PICCs for external problems (eg, cracks, breaks, leaks, or redness at the insertion site)? | |
| Yes, daily | 97 (25.5%) |
| Yes, but only if the nurse or patient alerts me to a problem with the PICC | 190 (49.9%) |
| No, I don't routinely examine the PICC for external problems | 94 (24.7%) |
| Have you ever forgotten or been unaware of the presence of a PICC? | |
| Yes | 216 (56.7%) |
| No | 165 (43.3%) |
| Assuming no contraindications exist, do you anticoagulate patients who develop a PICC‐associated DVT? | |
| Yes, for at least 1 month | 41(10.8%) |
| Yes, for at least 3 months* | 198 (52.0%) |
| Yes, for at least 6 months | 11 (2.9%) |
| Yes, I anticoagulate for as long as the line remains in place. Once the line is removed, I stop anticoagulation | 30 (7.9%) |
| Yes, I anticoagulate for as long as the line remains in place followed by another 4 weeks of therapy | 72 (18.9%) |
| I don't usually anticoagulate patients who develop a PICC‐related DVT | 29 (7.6%) |
| When a hospitalized patient develops a PICC‐related DVT, do you routinely remove the PICC? | |
| Yes | 271 (71.1%) |
| No | 110 (28.9%) |
| Hospitalist opinions related to PICCs | |
| Thinking about your hospital and your experiences, what percentage of PICC insertions may represent inappropriate use (eg, PICC placed for short‐term venous access for a presumed infection that could be treated with oral antibiotic or PICCs that were promptly removed as the patient no longer needed it for clinical management)? | |
| <10% | 192 (50.4%) |
| 10%25% | 160 (42.0%) |
| 26%50% | 22 (5.8%) |
| >50% | 7 (1.8%) |
| Do you think hospitalists should be trained to insert PICCs? | |
| Yes | 162 (42.5%) |
| No | 219 (57.5%) |
| Hospitalist knowledge related to PICCs | |
| Why is the position of the PICC‐tip checked following bedside PICC insertion? | |
| To decrease the risk of arrhythmia from tip placement in the right atrial | 267 (70.1%) |
| To ensure it is not accidentally placed into an artery | 44 (11.5%) |
| To minimize the risk of venous thrombosis* | 33 (8.7%) |
| For documentation purposes (to reduce the risk of lawsuits related tocomplications) | 16 (4.2%) |
| I don't know | 21 (5.5%) |
Several potentially important safety concerns regarding hospitalist PICC practices were observed in this survey. For instance, only 25% of hospitalists reported examining PICCs on daily rounds for external problems. When alerted by nurses or patients about problems with the device, this number doubled to 50%. In addition, 57% of respondents admitted to having at least once forgotten about the presence of a PICC in their hospitalized patient.
Participants also reported significant variation in duration of anticoagulation therapy for PICC‐related DVT, with only half of all respondents selecting the guideline‐recommended 3 months of anticoagulation.[4, 5] With respect to knowledge regarding PICCs, only 9% of respondents recognized that tip verification performed after PICC insertion was conducted to lower risk of venous thromboembolism, not that of arrhythmia.[6] Hospitalists were ambivalent about being trained on how to place PICCs, with only 43% indicating this skill was necessary. Finally, as many as 10% to 25% of PICCs inserted in their hospitals were felt to be inappropriately placed and/or avoidable by 42% of those surveyed.
DISCUSSION
As the use of PICCs rises in hospitalized patients, variability in practices associated with the use of these indwelling vascular catheters is being increasingly recognized. For instance, Tejedor and colleagues reported that PICCs placed in hospitalized patients at their academic medical center were often idle or inserted in patients who simultaneously have peripheral intravenous catheters.[7] Recent data from a tertiary care pediatric center found significantly greater PICC utilization rates over the past decade in association with shorter dwell times, suggesting important and dynamic changes in patterns of use of these devices.[2] Our prior survey of hospitalists in 10 Michigan hospitals also found variations in reported hospitalist practices, knowledge, and experiences related to PICCs.[3] However, the extent to which the Michigan experience portrayed a national trend remained unclear and was the impetus behind this survey. Results from this study appear to support findings from Michigan and highlight several potential opportunities to improve hospitalist PICC practices on a national scale.
In particular, 57% of respondents in this study (compared to 51% of Michigan hospitalists) stated they had at least once forgotten that their patient had a PICC. As early removal of PICCs that are clinically no longer necessary is a cornerstone to preventing thrombosis and infection,[4, 5, 6, 8] the potential impact of such forgetfulness on clinical outcomes and patient safety is of concern. Notably, PICC‐related DVT and bloodstream infection remained the 2 most commonly encountered complications in this survey, just as in the Michigan study.
Reported variations in treatment duration for PICC‐related DVT were also common in this study, with only half of all respondents in both surveys selecting the guideline‐recommended minimum of 3 months of anticoagulation. Finally, a substantial proportion (42%) of participants felt that 10% to 25% of PICCs placed in their hospitals might be inappropriately placed and avoidable, again echoing the sentiments of 51% of the participants in the Michigan survey. These findings strengthen the call to develop a research agenda focused on PICC use in hospitalized patients across the United States.
Why may hospitalists across the country demonstrate such variability when it comes to these indwelling vascular devices? PICCs have historically been viewed as safer with respect to complications such as infection and thrombosis than other central venous catheters, a viewpoint that has likely promulgated their use in the inpatient setting. However, as we and others have shown,[8, 9, 10, 11, 12] this notion is rapidly vanishing and being replaced by the recognition that severity of illness and patient comorbidities are more important determinants of complications than the device itself. Additionally, important knowledge gaps exist when it comes to the safe use of PICCs in hospitalized patients, contributing to variation in indications for insertion, removal, and treatment of complications related to these devices.
Our study is notably limited by a low response rate. Because the survey was administered directly by SHM without collection of respondent data (eg, practice location, years in practice), we are unable to adjust or weight these data to represent a national cohort of adult hospitalists. However, as responses to questions are consistent with our findings from Michigan, and the response rates of this survey are comparable to observed response rates from prior SHM‐administered nationwide surveys (10%40%),[13, 14, 15] we do not believe our findings necessarily represent systematic deviations from the truth and assumed that these responses were missing at random. In addition, owing to use of a survey‐based design, our study is inherently limited by a number of biases, including the use of a convenience sample of SHM members, nonresponse bias, and recall bias. Given these limitations, the association between the available responses and real‐world clinical practice is unclear and deserving of further investigation.
These limitations notwithstanding, our study has several strengths. We found important national variations in reported practices and knowledge related to PICCs, affirming the need to develop a research agenda to improve practice. Further, because a significant proportion of hospitalists may forget their patients have PICCs, our study supports the role of technologies such as catheter reminder systems, computerized decision aids, and automatic stop orders to improve PICC use. These technologies, if utilized in a workflow‐sensitive fashion, could improve PICC safety in hospitalized settings and merit exploration. In addition, our study highlights the growing need for criteria to guide the use of PICCs in hospital settings. Although the Infusion Nursing Society of America has published indications and guidelines for use of vascular devices,[6] these do not always incorporate clinical nuances such as necessity of intravenous therapy or duration of treatment in decision making. The development of evidence‐based appropriateness criteria to guide clinical decision making is thus critical to improving use of PICCs in inpatient settings.[16]
With growing recognition of PICC‐related complications in hospitalized patients, an urgent need to improve practice related to these devices exists. This study begins to define the scope of such work across the United States. Until more rigorous evidence becomes available to guide clinical practice, hospitals and hospitalists should begin to carefully monitor PICC use to safeguard and improve patient safety.
Disclosures
The Blue Cross/Blue Shield of Michigan Foundation funded this study through an investigator‐initiated research proposal (1931‐PIRAP to Dr. Chopra). The funding source played no role in study design, acquisition of data, data analysis, or reporting of these results. The authors report no conflicts of interest.
- , . Peripherally inserted central catheter: compliance with evidence‐based indications for insertion in an inpatient setting. J Infus Nurs. 2013;36(4):291–296.
- , , , , , . Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323–1331.
- , , , et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. J Hosp Med. 2013;8(6):309–314.
- , , , et al. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , , , et al. Quality improvement guidelines for central venous access. J Vasc Interv Radiol. 2010;21(7):976–981.
- , , , et al. Infusion nursing standards of practice. J Infus Nurs. 2011;34(1S):1–115.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , , , . Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429–435.
- , , , et al. Patient‐ and device‐specific risk factors for peripherally inserted central venous catheter‐related bloodstream infections. Infect Control Hosp Epidemiol. 2013;34(2):184–189.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , . Risk of catheter‐related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495.
- , , , , ; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402–410.
- , . Clinical hospital medicine fellowships: perspectives of employers, hospitalists, and medicine residents. J Hosp Med. 2008;3(1):28–34.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5–9.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
Peripherally inserted central catheters (PICCs) are central venous catheters that are inserted through peripheral veins of the upper extremities in adults. Because they are safer to insert than central venous catheters (CVCs) and have become increasingly available at the bedside through the advent of specially trained vascular access nurses,[1] the use of PICCs in hospitalized patients has risen across the United States.[2] As the largest group of inpatient providers, hospitalists play a key role in the decision to insert and subsequently manage PICCs in hospitalized patients. Unfortunately, little is known about national hospitalist experiences, practice patterns, or knowledge when it comes to these commonly used devices. Therefore, we designed a 10‐question survey to investigate PICC‐related practices and knowledge among adult hospitalists practicing throughout the United States.
PATIENTS AND METHODS
Questions for this survey were derived from a previously published study conducted across 10 hospitals in the state of Michigan.[3] To assess external validity and test specific hypotheses formulated from the Michigan study, those questions with the greatest variation in response or those most amenable to interventions were chosen for inclusion in this survey.
To reach a national audience of practicing adult hospitalists, we submitted a survey proposal to the Society of Hospital Medicine's (SHM) Research Committee. The SHM Research Committee reviews such proposals using a peer‐review process to ensure both scientific integrity and validity of the survey instrument. Because the survey was already distributed to many hospitalists in Michigan, we requested that only hospitalists outside of Michigan be invited to participate in the national survey. All responses were collected anonymously, and no identifiable data were collected from respondents. Between February 1, 2013 and March 15, 2013, data were collected via an e‐mail sent directly from the SHM to members that contained a link to the study survey administered using SurveyMonkey. To augment data collection, nonresponders to the original e‐mail invitation were sent a second reminder e‐mail midway through the study. Descriptive statistics (percentages) were used to tabulate responses. The institutional review board at the University of Michigan Health System provided ethical and regulatory approval for this study.
RESULTS
A total of 2112 electronic survey invitations were sent to non‐Michigan adult hospitalists, with 381 completing the online survey (response rate 18%). Among respondents to the national survey, 86% reported having placed a PICC solely to obtain venous access in a hospitalized patient (rather than for specific indications such as long‐term intravenous antibiotics, chemotherapy, or parenteral nutrition), whereas 82% reported having cared for a patient who specifically requested a PICC (Table 1). PICC‐related deep vein thrombosis (DVT) and bloodstream infections were reported as being the most frequent PICC complications encountered by hospitalists, followed by superficial thrombophlebitis and mechanical complications such as coiling, kinking, and migration of the PICC tip.
| Total (N=381) | |
|---|---|
| |
| Hospitalist experiences related to PICCs | |
| Among hospitalized patients you have cared for, have any of your patients ever had a PICC placed solely to obtain venous access (eg, not for an indication such as long‐term IV antibiotics, chemotherapy, or TPN)? | |
| Yes | 328 (86.1%) |
| No | 53 (13.9%) |
| Have you ever cared for a patient who specifically requested a PICC because of prior experience with this device? | |
| Yes | 311 (81.6%) |
| No | 70 (18.4%) |
| Most frequently encountered PICC complications | |
| Upper‐extremity DVT or PE | 48 (12.6%) |
| Bloodstream infection | 41 (10.8%) |
| Superficial thrombophlebitis | 34 (8.9%) |
| Cellulitis/exit site erythema | 26 (6.8%) |
| Coiling, kinking of the PICC | 14 (3.7%) |
| Migration of the PICC tip | 9 (2.4%) |
| Breakage of PICC (anywhere) | 6 (1.6%) |
| Hospitalist practice related to PICCs | |
| During patient rounds, do you routinely examine PICCs for external problems (eg, cracks, breaks, leaks, or redness at the insertion site)? | |
| Yes, daily | 97 (25.5%) |
| Yes, but only if the nurse or patient alerts me to a problem with the PICC | 190 (49.9%) |
| No, I don't routinely examine the PICC for external problems | 94 (24.7%) |
| Have you ever forgotten or been unaware of the presence of a PICC? | |
| Yes | 216 (56.7%) |
| No | 165 (43.3%) |
| Assuming no contraindications exist, do you anticoagulate patients who develop a PICC‐associated DVT? | |
| Yes, for at least 1 month | 41(10.8%) |
| Yes, for at least 3 months* | 198 (52.0%) |
| Yes, for at least 6 months | 11 (2.9%) |
| Yes, I anticoagulate for as long as the line remains in place. Once the line is removed, I stop anticoagulation | 30 (7.9%) |
| Yes, I anticoagulate for as long as the line remains in place followed by another 4 weeks of therapy | 72 (18.9%) |
| I don't usually anticoagulate patients who develop a PICC‐related DVT | 29 (7.6%) |
| When a hospitalized patient develops a PICC‐related DVT, do you routinely remove the PICC? | |
| Yes | 271 (71.1%) |
| No | 110 (28.9%) |
| Hospitalist opinions related to PICCs | |
| Thinking about your hospital and your experiences, what percentage of PICC insertions may represent inappropriate use (eg, PICC placed for short‐term venous access for a presumed infection that could be treated with oral antibiotic or PICCs that were promptly removed as the patient no longer needed it for clinical management)? | |
| <10% | 192 (50.4%) |
| 10%25% | 160 (42.0%) |
| 26%50% | 22 (5.8%) |
| >50% | 7 (1.8%) |
| Do you think hospitalists should be trained to insert PICCs? | |
| Yes | 162 (42.5%) |
| No | 219 (57.5%) |
| Hospitalist knowledge related to PICCs | |
| Why is the position of the PICC‐tip checked following bedside PICC insertion? | |
| To decrease the risk of arrhythmia from tip placement in the right atrial | 267 (70.1%) |
| To ensure it is not accidentally placed into an artery | 44 (11.5%) |
| To minimize the risk of venous thrombosis* | 33 (8.7%) |
| For documentation purposes (to reduce the risk of lawsuits related tocomplications) | 16 (4.2%) |
| I don't know | 21 (5.5%) |
Several potentially important safety concerns regarding hospitalist PICC practices were observed in this survey. For instance, only 25% of hospitalists reported examining PICCs on daily rounds for external problems. When alerted by nurses or patients about problems with the device, this number doubled to 50%. In addition, 57% of respondents admitted to having at least once forgotten about the presence of a PICC in their hospitalized patient.
Participants also reported significant variation in duration of anticoagulation therapy for PICC‐related DVT, with only half of all respondents selecting the guideline‐recommended 3 months of anticoagulation.[4, 5] With respect to knowledge regarding PICCs, only 9% of respondents recognized that tip verification performed after PICC insertion was conducted to lower risk of venous thromboembolism, not that of arrhythmia.[6] Hospitalists were ambivalent about being trained on how to place PICCs, with only 43% indicating this skill was necessary. Finally, as many as 10% to 25% of PICCs inserted in their hospitals were felt to be inappropriately placed and/or avoidable by 42% of those surveyed.
DISCUSSION
As the use of PICCs rises in hospitalized patients, variability in practices associated with the use of these indwelling vascular catheters is being increasingly recognized. For instance, Tejedor and colleagues reported that PICCs placed in hospitalized patients at their academic medical center were often idle or inserted in patients who simultaneously have peripheral intravenous catheters.[7] Recent data from a tertiary care pediatric center found significantly greater PICC utilization rates over the past decade in association with shorter dwell times, suggesting important and dynamic changes in patterns of use of these devices.[2] Our prior survey of hospitalists in 10 Michigan hospitals also found variations in reported hospitalist practices, knowledge, and experiences related to PICCs.[3] However, the extent to which the Michigan experience portrayed a national trend remained unclear and was the impetus behind this survey. Results from this study appear to support findings from Michigan and highlight several potential opportunities to improve hospitalist PICC practices on a national scale.
In particular, 57% of respondents in this study (compared to 51% of Michigan hospitalists) stated they had at least once forgotten that their patient had a PICC. As early removal of PICCs that are clinically no longer necessary is a cornerstone to preventing thrombosis and infection,[4, 5, 6, 8] the potential impact of such forgetfulness on clinical outcomes and patient safety is of concern. Notably, PICC‐related DVT and bloodstream infection remained the 2 most commonly encountered complications in this survey, just as in the Michigan study.
Reported variations in treatment duration for PICC‐related DVT were also common in this study, with only half of all respondents in both surveys selecting the guideline‐recommended minimum of 3 months of anticoagulation. Finally, a substantial proportion (42%) of participants felt that 10% to 25% of PICCs placed in their hospitals might be inappropriately placed and avoidable, again echoing the sentiments of 51% of the participants in the Michigan survey. These findings strengthen the call to develop a research agenda focused on PICC use in hospitalized patients across the United States.
Why may hospitalists across the country demonstrate such variability when it comes to these indwelling vascular devices? PICCs have historically been viewed as safer with respect to complications such as infection and thrombosis than other central venous catheters, a viewpoint that has likely promulgated their use in the inpatient setting. However, as we and others have shown,[8, 9, 10, 11, 12] this notion is rapidly vanishing and being replaced by the recognition that severity of illness and patient comorbidities are more important determinants of complications than the device itself. Additionally, important knowledge gaps exist when it comes to the safe use of PICCs in hospitalized patients, contributing to variation in indications for insertion, removal, and treatment of complications related to these devices.
Our study is notably limited by a low response rate. Because the survey was administered directly by SHM without collection of respondent data (eg, practice location, years in practice), we are unable to adjust or weight these data to represent a national cohort of adult hospitalists. However, as responses to questions are consistent with our findings from Michigan, and the response rates of this survey are comparable to observed response rates from prior SHM‐administered nationwide surveys (10%40%),[13, 14, 15] we do not believe our findings necessarily represent systematic deviations from the truth and assumed that these responses were missing at random. In addition, owing to use of a survey‐based design, our study is inherently limited by a number of biases, including the use of a convenience sample of SHM members, nonresponse bias, and recall bias. Given these limitations, the association between the available responses and real‐world clinical practice is unclear and deserving of further investigation.
These limitations notwithstanding, our study has several strengths. We found important national variations in reported practices and knowledge related to PICCs, affirming the need to develop a research agenda to improve practice. Further, because a significant proportion of hospitalists may forget their patients have PICCs, our study supports the role of technologies such as catheter reminder systems, computerized decision aids, and automatic stop orders to improve PICC use. These technologies, if utilized in a workflow‐sensitive fashion, could improve PICC safety in hospitalized settings and merit exploration. In addition, our study highlights the growing need for criteria to guide the use of PICCs in hospital settings. Although the Infusion Nursing Society of America has published indications and guidelines for use of vascular devices,[6] these do not always incorporate clinical nuances such as necessity of intravenous therapy or duration of treatment in decision making. The development of evidence‐based appropriateness criteria to guide clinical decision making is thus critical to improving use of PICCs in inpatient settings.[16]
With growing recognition of PICC‐related complications in hospitalized patients, an urgent need to improve practice related to these devices exists. This study begins to define the scope of such work across the United States. Until more rigorous evidence becomes available to guide clinical practice, hospitals and hospitalists should begin to carefully monitor PICC use to safeguard and improve patient safety.
Disclosures
The Blue Cross/Blue Shield of Michigan Foundation funded this study through an investigator‐initiated research proposal (1931‐PIRAP to Dr. Chopra). The funding source played no role in study design, acquisition of data, data analysis, or reporting of these results. The authors report no conflicts of interest.
Peripherally inserted central catheters (PICCs) are central venous catheters that are inserted through peripheral veins of the upper extremities in adults. Because they are safer to insert than central venous catheters (CVCs) and have become increasingly available at the bedside through the advent of specially trained vascular access nurses,[1] the use of PICCs in hospitalized patients has risen across the United States.[2] As the largest group of inpatient providers, hospitalists play a key role in the decision to insert and subsequently manage PICCs in hospitalized patients. Unfortunately, little is known about national hospitalist experiences, practice patterns, or knowledge when it comes to these commonly used devices. Therefore, we designed a 10‐question survey to investigate PICC‐related practices and knowledge among adult hospitalists practicing throughout the United States.
PATIENTS AND METHODS
Questions for this survey were derived from a previously published study conducted across 10 hospitals in the state of Michigan.[3] To assess external validity and test specific hypotheses formulated from the Michigan study, those questions with the greatest variation in response or those most amenable to interventions were chosen for inclusion in this survey.
To reach a national audience of practicing adult hospitalists, we submitted a survey proposal to the Society of Hospital Medicine's (SHM) Research Committee. The SHM Research Committee reviews such proposals using a peer‐review process to ensure both scientific integrity and validity of the survey instrument. Because the survey was already distributed to many hospitalists in Michigan, we requested that only hospitalists outside of Michigan be invited to participate in the national survey. All responses were collected anonymously, and no identifiable data were collected from respondents. Between February 1, 2013 and March 15, 2013, data were collected via an e‐mail sent directly from the SHM to members that contained a link to the study survey administered using SurveyMonkey. To augment data collection, nonresponders to the original e‐mail invitation were sent a second reminder e‐mail midway through the study. Descriptive statistics (percentages) were used to tabulate responses. The institutional review board at the University of Michigan Health System provided ethical and regulatory approval for this study.
RESULTS
A total of 2112 electronic survey invitations were sent to non‐Michigan adult hospitalists, with 381 completing the online survey (response rate 18%). Among respondents to the national survey, 86% reported having placed a PICC solely to obtain venous access in a hospitalized patient (rather than for specific indications such as long‐term intravenous antibiotics, chemotherapy, or parenteral nutrition), whereas 82% reported having cared for a patient who specifically requested a PICC (Table 1). PICC‐related deep vein thrombosis (DVT) and bloodstream infections were reported as being the most frequent PICC complications encountered by hospitalists, followed by superficial thrombophlebitis and mechanical complications such as coiling, kinking, and migration of the PICC tip.
| Total (N=381) | |
|---|---|
| |
| Hospitalist experiences related to PICCs | |
| Among hospitalized patients you have cared for, have any of your patients ever had a PICC placed solely to obtain venous access (eg, not for an indication such as long‐term IV antibiotics, chemotherapy, or TPN)? | |
| Yes | 328 (86.1%) |
| No | 53 (13.9%) |
| Have you ever cared for a patient who specifically requested a PICC because of prior experience with this device? | |
| Yes | 311 (81.6%) |
| No | 70 (18.4%) |
| Most frequently encountered PICC complications | |
| Upper‐extremity DVT or PE | 48 (12.6%) |
| Bloodstream infection | 41 (10.8%) |
| Superficial thrombophlebitis | 34 (8.9%) |
| Cellulitis/exit site erythema | 26 (6.8%) |
| Coiling, kinking of the PICC | 14 (3.7%) |
| Migration of the PICC tip | 9 (2.4%) |
| Breakage of PICC (anywhere) | 6 (1.6%) |
| Hospitalist practice related to PICCs | |
| During patient rounds, do you routinely examine PICCs for external problems (eg, cracks, breaks, leaks, or redness at the insertion site)? | |
| Yes, daily | 97 (25.5%) |
| Yes, but only if the nurse or patient alerts me to a problem with the PICC | 190 (49.9%) |
| No, I don't routinely examine the PICC for external problems | 94 (24.7%) |
| Have you ever forgotten or been unaware of the presence of a PICC? | |
| Yes | 216 (56.7%) |
| No | 165 (43.3%) |
| Assuming no contraindications exist, do you anticoagulate patients who develop a PICC‐associated DVT? | |
| Yes, for at least 1 month | 41(10.8%) |
| Yes, for at least 3 months* | 198 (52.0%) |
| Yes, for at least 6 months | 11 (2.9%) |
| Yes, I anticoagulate for as long as the line remains in place. Once the line is removed, I stop anticoagulation | 30 (7.9%) |
| Yes, I anticoagulate for as long as the line remains in place followed by another 4 weeks of therapy | 72 (18.9%) |
| I don't usually anticoagulate patients who develop a PICC‐related DVT | 29 (7.6%) |
| When a hospitalized patient develops a PICC‐related DVT, do you routinely remove the PICC? | |
| Yes | 271 (71.1%) |
| No | 110 (28.9%) |
| Hospitalist opinions related to PICCs | |
| Thinking about your hospital and your experiences, what percentage of PICC insertions may represent inappropriate use (eg, PICC placed for short‐term venous access for a presumed infection that could be treated with oral antibiotic or PICCs that were promptly removed as the patient no longer needed it for clinical management)? | |
| <10% | 192 (50.4%) |
| 10%25% | 160 (42.0%) |
| 26%50% | 22 (5.8%) |
| >50% | 7 (1.8%) |
| Do you think hospitalists should be trained to insert PICCs? | |
| Yes | 162 (42.5%) |
| No | 219 (57.5%) |
| Hospitalist knowledge related to PICCs | |
| Why is the position of the PICC‐tip checked following bedside PICC insertion? | |
| To decrease the risk of arrhythmia from tip placement in the right atrial | 267 (70.1%) |
| To ensure it is not accidentally placed into an artery | 44 (11.5%) |
| To minimize the risk of venous thrombosis* | 33 (8.7%) |
| For documentation purposes (to reduce the risk of lawsuits related tocomplications) | 16 (4.2%) |
| I don't know | 21 (5.5%) |
Several potentially important safety concerns regarding hospitalist PICC practices were observed in this survey. For instance, only 25% of hospitalists reported examining PICCs on daily rounds for external problems. When alerted by nurses or patients about problems with the device, this number doubled to 50%. In addition, 57% of respondents admitted to having at least once forgotten about the presence of a PICC in their hospitalized patient.
Participants also reported significant variation in duration of anticoagulation therapy for PICC‐related DVT, with only half of all respondents selecting the guideline‐recommended 3 months of anticoagulation.[4, 5] With respect to knowledge regarding PICCs, only 9% of respondents recognized that tip verification performed after PICC insertion was conducted to lower risk of venous thromboembolism, not that of arrhythmia.[6] Hospitalists were ambivalent about being trained on how to place PICCs, with only 43% indicating this skill was necessary. Finally, as many as 10% to 25% of PICCs inserted in their hospitals were felt to be inappropriately placed and/or avoidable by 42% of those surveyed.
DISCUSSION
As the use of PICCs rises in hospitalized patients, variability in practices associated with the use of these indwelling vascular catheters is being increasingly recognized. For instance, Tejedor and colleagues reported that PICCs placed in hospitalized patients at their academic medical center were often idle or inserted in patients who simultaneously have peripheral intravenous catheters.[7] Recent data from a tertiary care pediatric center found significantly greater PICC utilization rates over the past decade in association with shorter dwell times, suggesting important and dynamic changes in patterns of use of these devices.[2] Our prior survey of hospitalists in 10 Michigan hospitals also found variations in reported hospitalist practices, knowledge, and experiences related to PICCs.[3] However, the extent to which the Michigan experience portrayed a national trend remained unclear and was the impetus behind this survey. Results from this study appear to support findings from Michigan and highlight several potential opportunities to improve hospitalist PICC practices on a national scale.
In particular, 57% of respondents in this study (compared to 51% of Michigan hospitalists) stated they had at least once forgotten that their patient had a PICC. As early removal of PICCs that are clinically no longer necessary is a cornerstone to preventing thrombosis and infection,[4, 5, 6, 8] the potential impact of such forgetfulness on clinical outcomes and patient safety is of concern. Notably, PICC‐related DVT and bloodstream infection remained the 2 most commonly encountered complications in this survey, just as in the Michigan study.
Reported variations in treatment duration for PICC‐related DVT were also common in this study, with only half of all respondents in both surveys selecting the guideline‐recommended minimum of 3 months of anticoagulation. Finally, a substantial proportion (42%) of participants felt that 10% to 25% of PICCs placed in their hospitals might be inappropriately placed and avoidable, again echoing the sentiments of 51% of the participants in the Michigan survey. These findings strengthen the call to develop a research agenda focused on PICC use in hospitalized patients across the United States.
Why may hospitalists across the country demonstrate such variability when it comes to these indwelling vascular devices? PICCs have historically been viewed as safer with respect to complications such as infection and thrombosis than other central venous catheters, a viewpoint that has likely promulgated their use in the inpatient setting. However, as we and others have shown,[8, 9, 10, 11, 12] this notion is rapidly vanishing and being replaced by the recognition that severity of illness and patient comorbidities are more important determinants of complications than the device itself. Additionally, important knowledge gaps exist when it comes to the safe use of PICCs in hospitalized patients, contributing to variation in indications for insertion, removal, and treatment of complications related to these devices.
Our study is notably limited by a low response rate. Because the survey was administered directly by SHM without collection of respondent data (eg, practice location, years in practice), we are unable to adjust or weight these data to represent a national cohort of adult hospitalists. However, as responses to questions are consistent with our findings from Michigan, and the response rates of this survey are comparable to observed response rates from prior SHM‐administered nationwide surveys (10%40%),[13, 14, 15] we do not believe our findings necessarily represent systematic deviations from the truth and assumed that these responses were missing at random. In addition, owing to use of a survey‐based design, our study is inherently limited by a number of biases, including the use of a convenience sample of SHM members, nonresponse bias, and recall bias. Given these limitations, the association between the available responses and real‐world clinical practice is unclear and deserving of further investigation.
These limitations notwithstanding, our study has several strengths. We found important national variations in reported practices and knowledge related to PICCs, affirming the need to develop a research agenda to improve practice. Further, because a significant proportion of hospitalists may forget their patients have PICCs, our study supports the role of technologies such as catheter reminder systems, computerized decision aids, and automatic stop orders to improve PICC use. These technologies, if utilized in a workflow‐sensitive fashion, could improve PICC safety in hospitalized settings and merit exploration. In addition, our study highlights the growing need for criteria to guide the use of PICCs in hospital settings. Although the Infusion Nursing Society of America has published indications and guidelines for use of vascular devices,[6] these do not always incorporate clinical nuances such as necessity of intravenous therapy or duration of treatment in decision making. The development of evidence‐based appropriateness criteria to guide clinical decision making is thus critical to improving use of PICCs in inpatient settings.[16]
With growing recognition of PICC‐related complications in hospitalized patients, an urgent need to improve practice related to these devices exists. This study begins to define the scope of such work across the United States. Until more rigorous evidence becomes available to guide clinical practice, hospitals and hospitalists should begin to carefully monitor PICC use to safeguard and improve patient safety.
Disclosures
The Blue Cross/Blue Shield of Michigan Foundation funded this study through an investigator‐initiated research proposal (1931‐PIRAP to Dr. Chopra). The funding source played no role in study design, acquisition of data, data analysis, or reporting of these results. The authors report no conflicts of interest.
- , . Peripherally inserted central catheter: compliance with evidence‐based indications for insertion in an inpatient setting. J Infus Nurs. 2013;36(4):291–296.
- , , , , , . Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323–1331.
- , , , et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. J Hosp Med. 2013;8(6):309–314.
- , , , et al. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , , , et al. Quality improvement guidelines for central venous access. J Vasc Interv Radiol. 2010;21(7):976–981.
- , , , et al. Infusion nursing standards of practice. J Infus Nurs. 2011;34(1S):1–115.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , , , . Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429–435.
- , , , et al. Patient‐ and device‐specific risk factors for peripherally inserted central venous catheter‐related bloodstream infections. Infect Control Hosp Epidemiol. 2013;34(2):184–189.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , . Risk of catheter‐related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495.
- , , , , ; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402–410.
- , . Clinical hospital medicine fellowships: perspectives of employers, hospitalists, and medicine residents. J Hosp Med. 2008;3(1):28–34.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5–9.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , . Peripherally inserted central catheter: compliance with evidence‐based indications for insertion in an inpatient setting. J Infus Nurs. 2013;36(4):291–296.
- , , , , , . Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323–1331.
- , , , et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. J Hosp Med. 2013;8(6):309–314.
- , , , et al. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , , , et al. Quality improvement guidelines for central venous access. J Vasc Interv Radiol. 2010;21(7):976–981.
- , , , et al. Infusion nursing standards of practice. J Infus Nurs. 2011;34(1S):1–115.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , , , . Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429–435.
- , , , et al. Patient‐ and device‐specific risk factors for peripherally inserted central venous catheter‐related bloodstream infections. Infect Control Hosp Epidemiol. 2013;34(2):184–189.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , . Risk of catheter‐related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495.
- , , , , ; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402–410.
- , . Clinical hospital medicine fellowships: perspectives of employers, hospitalists, and medicine residents. J Hosp Med. 2008;3(1):28–34.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5–9.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
Estimating Hospital Costs of CAUTI
Healthcare‐associated infections affect 5% to 10% of all hospitalized patients each year in the United States, account for nearly $45 billion in direct hospital costs, and cause nearly 100,000 deaths annually.[1, 2] Because catheter‐associated urinary tract infection (CAUTI) is one of the most common healthcare‐associated infections in the United States and is reasonably preventable, the Centers for Medicare and Medicaid Services stopped reimbursing hospitals in 2008 for the additional costs of caring for patients who develop CAUTI during hospitalization.[3] Still, strategies for reducing inappropriate urinary catheterization are infrequently implemented in practice; this is despite a consensus that such strategies are effective.[4]
To help motivate hospitals to reduce inappropriate urinary catheter use, we present a tool for estimating costs of CAUTI for individual hospitals. Although other tools for estimating the excess costs of healthcare‐associated infections are available (eg, the APIC Cost of Healthcare‐Associated Infections Model available at
METHODS
General Setup
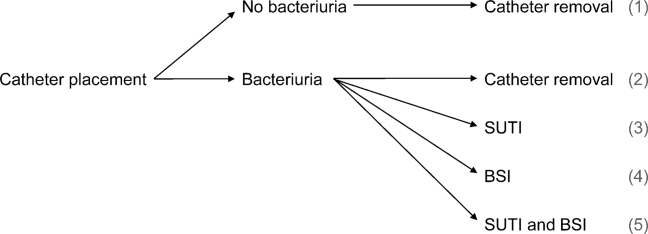
We consider 4 possible events after urinary catheter placement: bacteriuria, symptomatic urinary tract infection (SUTI), bloodstream infection (BSI), and catheter removal. Conservatively, assuming that bacteriuria must precede SUTI and BSI, there are 5 possible trajectories for any hospitalized patient (Figure 1): (1) no infection, (2) only bacteriuria, (3) bacteriuria and SUTI, (4) bacteriuria and BSI, or (5) bacteriuria, SUTI, and BSI. The cost of CAUTI for a particular hospital is therefore the per‐patient cost of each trajectory multiplied by the number of patients experiencing each trajectory. Our approach for estimating hospital costs is based on factorizing the number of patients experiencing each trajectory into a product of terms for which estimates are available from the literature (see the Supporting Information, Appendix, in the online version of this article for all technical details).
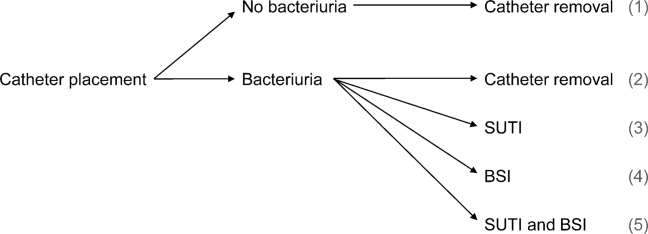
Deriving Estimates of Current Costs
We start with 2 minor simplifying assumptions. First, because the presence of asymptomatic bacteriuria is typically unknown, we only consider costs to the hospital due to SUTI and BSI[6]; in other words, we assume hospitals do not incur costs for patients with trajectories 1 or 2. This assumption should only bias cost estimates conservatively. Second, we assume that patients with both SUTI and BSI (trajectory 5) incur costs equal to those for patients with only BSI (trajectory 4). Further, because the joint risk of SUTI and BSI is unknown, we conservatively assume SUTI must precede BSI. Under these assumptions we can write: (total CAUTI costs)=(per‐patient SUTI cost) (number with SUTI but no BSI)+(perpatient BSI cost) (number with BSI).
We use per‐patient hospital costs of SUTI and BSI of $911 and $3824, respectively, which were determined using a microcosting approach[6] and adjusted for inflation using the general Consumer Price Index.[7] Although an alternative strategy for estimating costs would be to enter the hospital‐specific, per‐patient costs of SUTI and BSI into the above equation, these quantities are often difficult to measure or otherwise unavailable. Thus, it remains to factorize the number of hospitalized patients who develop SUTI and BSI into component terms for which we have accessible estimates. First note that the number with only SUTI (or any BSI) equals the total number of patients hospitalized times the proportion of hospitalizations with only SUTI (or any BSI). The former quantity depends on the particular hospital and so is specified as an input by the user. The latter quantity can be factorized further under our aforementioned conservative assumption that bacteriuria must precede SUTI and BSI.
Specifically, for SUTI:
(Proportion SUTI but no BSI)={(SUTI risk among those catheterized with bacteriuria)(BSI risk among those catheterized with bacteriuria)} (bacteriuria risk among those catheterized) (proportion catheterized).
And for BSI:
(Proportion BSI)=(BSI risk among those catheterized with bacteriuria) (bacteriuria risk among those catheterized) (proportion catheterized).
The risks of SUTI and BSI among those catheterized with bacteriuria, along with the risk of bacteriuria among those catheterized, have been estimated previously via a meta‐analytic approach.[6] The proportion catheterized depends on the particular hospital, such as the total number of patients hospitalized, and so is also specified as a user input. Therefore, we have now factorized the total hospital costs due to CAUTI as a product of either user‐specified terms or terms for which we have estimates from the literature. All estimates and corresponding standard errors derived from the literature are listed together in Table 1 (see the Supporting Information, Appendix Section 1, for further details in the online version of this article).
| Quantity | Estimate (SE) |
|---|---|
| |
| Overall risk of bacteriuria among those catheterized | 26.0% (1.53%) |
| Per‐day risk of bacteriuria among those catheterized | 5.0% |
| days | 6.68 |
| Risk of SUTI among those catheterized with bacteriuria | 24.0% (4.08%) |
| Risk of BSI among those catheterized with bacteriuria | 3.6% (0.10%) |
| Per‐patient SUTI cost | $911 ($911) |
| Per‐patient BSI cost | $3824 ($3824) |
Deriving Projected Costs After Intervention
The approach described above permits estimation of current costs for managing patients with CAUTI for a particular hospital. To estimate projected costs after participation in an intervention to reduce infection risk, we characterize interventions of interest and introduce additional factorization. Specifically, following previous work,[8] we consider interventions that reduce (1) placement (ie, the proportion catheterized) and (2) duration (ie, the mean duration of catheterization). Incorporating reductions in placement is straightforward, because our above expression for costs already contains a term for the proportion catheterized. However, incorporating reductions in duration requires further factorization. Under the assumptions of constant per‐day risks of bacteriuria and of catheter removal, we can write the postintervention risk of bacteriuria among the catheterized as a function of (1) the percent decrease in mean duration of catheterization due to intervention, and (2) the preintervention risk of bacteriuria among the catheterized (see the Supporting Information, Appendix Section 2, for further details in the online version of this article). This means we can fully characterize postintervention costs as a function of user‐specified quantities, quantities specific to the intervention (which are varied across plausible ranges), and quantities for which we have estimates from the literature. Therefore, we can estimate savings by subtracting postintervention costs from current costs.
Because our estimators of current costs, projected costs, and savings are all formulated as functions of other estimators, we use the standard delta method approach[9] to derive appropriate variance estimates (see the Supporting Information, Appendix Section 3, for further details in the online version of this article).
Online Implementation
Customized results (based on annual admissions, urinary catheter prevalence, and other inputs) can be computed using online implementation of our proposed method at
RESULTS
Figure 2 shows the projected savings in hospital costs due to CAUTI across a range of interventions defined by percent decreases in placement and duration, for a hypothetical hospital with 3000 total patients, 15% with urinary catheters preintervention, and with all other default values listed in Table 1. The current costs for this hospital (ie, the costs when the percent reduction in placement and duration is zero) are estimated to be $37,868 (95% confidence interval [CI]: $9159‐$156,564). After an intervention resulting in 40% reductions in both urinary catheter placement and duration, this hospital would be expected to save $22,653 (95% CI: $5479‐$93,656). A less effective intervention yielding a 10% reduction in both urinary catheter placement and duration would result in more modest savings of $6376 (95% CI: $1542‐$26,360).
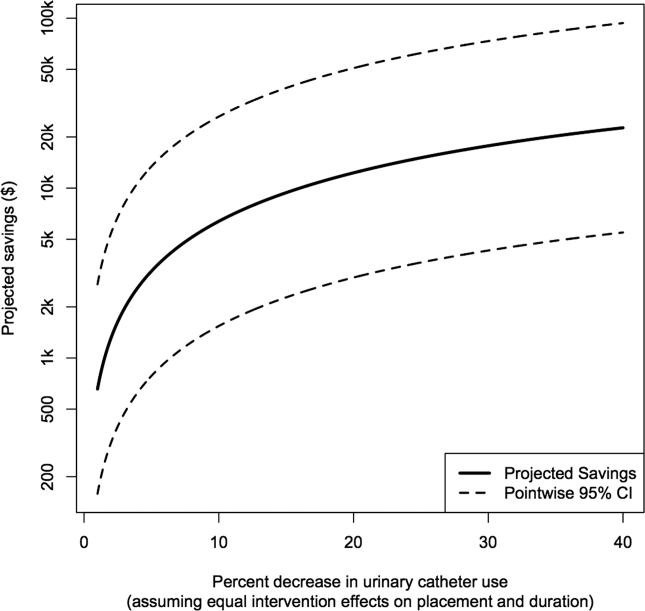
After an intervention resulting in 29% and 37% reductions in placement and duration, respectively, reflecting reductions seen in practice,[10, 11] our hypothetical hospital is estimated to save $19,126 (95% CI: $4626‐$79,074). This reflects an estimated savings of nearly 50%.
DISCUSSION
We have presented a tool for estimating customized hospital costs of CAUTI, both before and after a hypothetical intervention to reduce risk of infection. Our approach relies on mostly conservative assumptions, incorporates published risk estimates (properly accounting for their associated variability), and has easy‐to‐use online implementation. We believe this can play an important role in motivating hospitals to reduce inappropriate urinary catheter use.
The methodology employed here does have a few limitations. First and foremost, our results depend on the reliability of the input values, which are either provided by users or are based on estimates from the literature (see Table 1 for a complete list of suggested defaults). New information could potentially be incorporated if and when available. For example, substitution of more precise risk estimates could help reduce confidence interval length. Second, our approach essentially averages over hospital quality; we do not directly take into account quality of care or variation in underlying infection risk across hospitals in computing estimated costs. Finally, we only compute direct costs due to infection; other costs (eg, intervention costs) would typically also need to be considered for decision making.
Despite these limitations, we believe that our tool can help infection control professionals demonstrate the values of CAUTI prevention efforts to key administrators, particularly at a time where it has become increasingly necessary to develop a business case to initiate new interventions or justify the continued support for ongoing programs.[12] Additionally, we believe the proposed approach can be an important supplement to initiatives like the Society of Hospital Medicine's Choosing Wisely campaign, which aims to help reduce inappropriate urinary catheter use. Reducing catheter utilization has the potential to reduce costs associated with caring for CAUTI patients, but more importantly would help reduce CAUTI incidence as well as catheter‐related, noninfectious complications.[13, 14] We hope that our tool will greatly assist hospitals in promoting their CAUTI prevention efforts and improve the overall safety of hospitalized patients.
Disclosures
This project was supported by the Ann Arbor VA Medical Center/University of Michigan Patient Safety Enhancement Program (PSEP) and a subcontract to implement multistate CAUTI prevention with the Agency for Healthcare Research and Quality/Health Educational and Research Trust. Mr. Kennedy has no conflicts of interest to report. Drs. Saint and Greene are subcontracted to implement multistate CAUTI prevention with the Agency for Healthcare Research and Quality/Health Educational and Research Trust. Dr. Saint has received numerous honoraria and speaking fees for lectures on healthcare‐associated infection prevention, implementation science, and patient safety from hospitals, academic medical centers, professional societies, and nonprofit foundations. None of these activities are related to speaker's bureaus. Dr. Saint is also on the medical advisory board of Doximity, a new social networking site for physicians. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
- , , , et al. Estimating health care‐associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166.
- The direct medical costs of healthcare‐associated infections in US hospitals and the benefits of prevention. US Centers for Disease Control and Prevention Web site. Published 2009. Available at: http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf. Accessed March 24, 2013.
- , , , , . Catheter‐associated urinary tract infection and the Medicare rule changes. Ann Intern Med. 2009;150(12):877–884.
- , . Improving use of the other catheter. Arch Intern Med. 2012;172(3):260–261.
- Choosing Wisely: five things patients and physicians should question. Society of Hospital Medicine. Published 2012. Available at: http://www.hospitalmedicine.org/AM/pdf/SHM‐Adult_5things_List_Web.pdf. Accessed March 24, 2013.
- . Clinical and economic consequences of nosocomial catheter‐related bacteriuria. Am J Infect Control. 2000;28(1):68–75.
- CPI Inflation Calculator. United States Department of Labor, Bureau of Labor Statistics Web site. Published 2013. Available at: http://www.bls.gov/data/inflation_calculator.htm. Accessed March 24, 2013.
- , , , et al. Introducing a population‐based outcome measure to evaluate the effect of interventions to reduce catheter‐associated urinary tract infection. Am J Infect Control. 2012;40(4):359–364.
- . Asymptotic Statistics. Cambridge, UK: Cambridge University Press; 2000.
- , , , et al. Effect of establishing guidelines on appropriate urinary catheter placement. Acad Emerg Med. 2010;17:337–340.
- , , , . Systematic review and meta‐analysis: reminder systems to reduce catheter‐associated urinary tract infections and urinary catheter use in hospitalized patients. Clin Infect Dis. 2010;51(5):550–560.
- , , , et al. Raising standards while watching the bottom line: making a business case for infection control. Infect Control Hosp Epidemiol. 2007;28:1121–1133.
- , , , , . Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453–1457.
- , , . Indwelling urinary catheters: a one‐point restraint? Ann Intern Med. 2002;137(2):125–127.
Healthcare‐associated infections affect 5% to 10% of all hospitalized patients each year in the United States, account for nearly $45 billion in direct hospital costs, and cause nearly 100,000 deaths annually.[1, 2] Because catheter‐associated urinary tract infection (CAUTI) is one of the most common healthcare‐associated infections in the United States and is reasonably preventable, the Centers for Medicare and Medicaid Services stopped reimbursing hospitals in 2008 for the additional costs of caring for patients who develop CAUTI during hospitalization.[3] Still, strategies for reducing inappropriate urinary catheterization are infrequently implemented in practice; this is despite a consensus that such strategies are effective.[4]
To help motivate hospitals to reduce inappropriate urinary catheter use, we present a tool for estimating costs of CAUTI for individual hospitals. Although other tools for estimating the excess costs of healthcare‐associated infections are available (eg, the APIC Cost of Healthcare‐Associated Infections Model available at
METHODS
General Setup
We consider 4 possible events after urinary catheter placement: bacteriuria, symptomatic urinary tract infection (SUTI), bloodstream infection (BSI), and catheter removal. Conservatively, assuming that bacteriuria must precede SUTI and BSI, there are 5 possible trajectories for any hospitalized patient (Figure 1): (1) no infection, (2) only bacteriuria, (3) bacteriuria and SUTI, (4) bacteriuria and BSI, or (5) bacteriuria, SUTI, and BSI. The cost of CAUTI for a particular hospital is therefore the per‐patient cost of each trajectory multiplied by the number of patients experiencing each trajectory. Our approach for estimating hospital costs is based on factorizing the number of patients experiencing each trajectory into a product of terms for which estimates are available from the literature (see the Supporting Information, Appendix, in the online version of this article for all technical details).

Deriving Estimates of Current Costs
We start with 2 minor simplifying assumptions. First, because the presence of asymptomatic bacteriuria is typically unknown, we only consider costs to the hospital due to SUTI and BSI[6]; in other words, we assume hospitals do not incur costs for patients with trajectories 1 or 2. This assumption should only bias cost estimates conservatively. Second, we assume that patients with both SUTI and BSI (trajectory 5) incur costs equal to those for patients with only BSI (trajectory 4). Further, because the joint risk of SUTI and BSI is unknown, we conservatively assume SUTI must precede BSI. Under these assumptions we can write: (total CAUTI costs)=(per‐patient SUTI cost) (number with SUTI but no BSI)+(perpatient BSI cost) (number with BSI).
We use per‐patient hospital costs of SUTI and BSI of $911 and $3824, respectively, which were determined using a microcosting approach[6] and adjusted for inflation using the general Consumer Price Index.[7] Although an alternative strategy for estimating costs would be to enter the hospital‐specific, per‐patient costs of SUTI and BSI into the above equation, these quantities are often difficult to measure or otherwise unavailable. Thus, it remains to factorize the number of hospitalized patients who develop SUTI and BSI into component terms for which we have accessible estimates. First note that the number with only SUTI (or any BSI) equals the total number of patients hospitalized times the proportion of hospitalizations with only SUTI (or any BSI). The former quantity depends on the particular hospital and so is specified as an input by the user. The latter quantity can be factorized further under our aforementioned conservative assumption that bacteriuria must precede SUTI and BSI.
Specifically, for SUTI:
(Proportion SUTI but no BSI)={(SUTI risk among those catheterized with bacteriuria)(BSI risk among those catheterized with bacteriuria)} (bacteriuria risk among those catheterized) (proportion catheterized).
And for BSI:
(Proportion BSI)=(BSI risk among those catheterized with bacteriuria) (bacteriuria risk among those catheterized) (proportion catheterized).
The risks of SUTI and BSI among those catheterized with bacteriuria, along with the risk of bacteriuria among those catheterized, have been estimated previously via a meta‐analytic approach.[6] The proportion catheterized depends on the particular hospital, such as the total number of patients hospitalized, and so is also specified as a user input. Therefore, we have now factorized the total hospital costs due to CAUTI as a product of either user‐specified terms or terms for which we have estimates from the literature. All estimates and corresponding standard errors derived from the literature are listed together in Table 1 (see the Supporting Information, Appendix Section 1, for further details in the online version of this article).
| Quantity | Estimate (SE) |
|---|---|
| |
| Overall risk of bacteriuria among those catheterized | 26.0% (1.53%) |
| Per‐day risk of bacteriuria among those catheterized | 5.0% |
| days | 6.68 |
| Risk of SUTI among those catheterized with bacteriuria | 24.0% (4.08%) |
| Risk of BSI among those catheterized with bacteriuria | 3.6% (0.10%) |
| Per‐patient SUTI cost | $911 ($911) |
| Per‐patient BSI cost | $3824 ($3824) |
Deriving Projected Costs After Intervention
The approach described above permits estimation of current costs for managing patients with CAUTI for a particular hospital. To estimate projected costs after participation in an intervention to reduce infection risk, we characterize interventions of interest and introduce additional factorization. Specifically, following previous work,[8] we consider interventions that reduce (1) placement (ie, the proportion catheterized) and (2) duration (ie, the mean duration of catheterization). Incorporating reductions in placement is straightforward, because our above expression for costs already contains a term for the proportion catheterized. However, incorporating reductions in duration requires further factorization. Under the assumptions of constant per‐day risks of bacteriuria and of catheter removal, we can write the postintervention risk of bacteriuria among the catheterized as a function of (1) the percent decrease in mean duration of catheterization due to intervention, and (2) the preintervention risk of bacteriuria among the catheterized (see the Supporting Information, Appendix Section 2, for further details in the online version of this article). This means we can fully characterize postintervention costs as a function of user‐specified quantities, quantities specific to the intervention (which are varied across plausible ranges), and quantities for which we have estimates from the literature. Therefore, we can estimate savings by subtracting postintervention costs from current costs.
Because our estimators of current costs, projected costs, and savings are all formulated as functions of other estimators, we use the standard delta method approach[9] to derive appropriate variance estimates (see the Supporting Information, Appendix Section 3, for further details in the online version of this article).
Online Implementation
Customized results (based on annual admissions, urinary catheter prevalence, and other inputs) can be computed using online implementation of our proposed method at
RESULTS
Figure 2 shows the projected savings in hospital costs due to CAUTI across a range of interventions defined by percent decreases in placement and duration, for a hypothetical hospital with 3000 total patients, 15% with urinary catheters preintervention, and with all other default values listed in Table 1. The current costs for this hospital (ie, the costs when the percent reduction in placement and duration is zero) are estimated to be $37,868 (95% confidence interval [CI]: $9159‐$156,564). After an intervention resulting in 40% reductions in both urinary catheter placement and duration, this hospital would be expected to save $22,653 (95% CI: $5479‐$93,656). A less effective intervention yielding a 10% reduction in both urinary catheter placement and duration would result in more modest savings of $6376 (95% CI: $1542‐$26,360).

After an intervention resulting in 29% and 37% reductions in placement and duration, respectively, reflecting reductions seen in practice,[10, 11] our hypothetical hospital is estimated to save $19,126 (95% CI: $4626‐$79,074). This reflects an estimated savings of nearly 50%.
DISCUSSION
We have presented a tool for estimating customized hospital costs of CAUTI, both before and after a hypothetical intervention to reduce risk of infection. Our approach relies on mostly conservative assumptions, incorporates published risk estimates (properly accounting for their associated variability), and has easy‐to‐use online implementation. We believe this can play an important role in motivating hospitals to reduce inappropriate urinary catheter use.
The methodology employed here does have a few limitations. First and foremost, our results depend on the reliability of the input values, which are either provided by users or are based on estimates from the literature (see Table 1 for a complete list of suggested defaults). New information could potentially be incorporated if and when available. For example, substitution of more precise risk estimates could help reduce confidence interval length. Second, our approach essentially averages over hospital quality; we do not directly take into account quality of care or variation in underlying infection risk across hospitals in computing estimated costs. Finally, we only compute direct costs due to infection; other costs (eg, intervention costs) would typically also need to be considered for decision making.
Despite these limitations, we believe that our tool can help infection control professionals demonstrate the values of CAUTI prevention efforts to key administrators, particularly at a time where it has become increasingly necessary to develop a business case to initiate new interventions or justify the continued support for ongoing programs.[12] Additionally, we believe the proposed approach can be an important supplement to initiatives like the Society of Hospital Medicine's Choosing Wisely campaign, which aims to help reduce inappropriate urinary catheter use. Reducing catheter utilization has the potential to reduce costs associated with caring for CAUTI patients, but more importantly would help reduce CAUTI incidence as well as catheter‐related, noninfectious complications.[13, 14] We hope that our tool will greatly assist hospitals in promoting their CAUTI prevention efforts and improve the overall safety of hospitalized patients.
Disclosures
This project was supported by the Ann Arbor VA Medical Center/University of Michigan Patient Safety Enhancement Program (PSEP) and a subcontract to implement multistate CAUTI prevention with the Agency for Healthcare Research and Quality/Health Educational and Research Trust. Mr. Kennedy has no conflicts of interest to report. Drs. Saint and Greene are subcontracted to implement multistate CAUTI prevention with the Agency for Healthcare Research and Quality/Health Educational and Research Trust. Dr. Saint has received numerous honoraria and speaking fees for lectures on healthcare‐associated infection prevention, implementation science, and patient safety from hospitals, academic medical centers, professional societies, and nonprofit foundations. None of these activities are related to speaker's bureaus. Dr. Saint is also on the medical advisory board of Doximity, a new social networking site for physicians. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Healthcare‐associated infections affect 5% to 10% of all hospitalized patients each year in the United States, account for nearly $45 billion in direct hospital costs, and cause nearly 100,000 deaths annually.[1, 2] Because catheter‐associated urinary tract infection (CAUTI) is one of the most common healthcare‐associated infections in the United States and is reasonably preventable, the Centers for Medicare and Medicaid Services stopped reimbursing hospitals in 2008 for the additional costs of caring for patients who develop CAUTI during hospitalization.[3] Still, strategies for reducing inappropriate urinary catheterization are infrequently implemented in practice; this is despite a consensus that such strategies are effective.[4]
To help motivate hospitals to reduce inappropriate urinary catheter use, we present a tool for estimating costs of CAUTI for individual hospitals. Although other tools for estimating the excess costs of healthcare‐associated infections are available (eg, the APIC Cost of Healthcare‐Associated Infections Model available at
METHODS
General Setup
We consider 4 possible events after urinary catheter placement: bacteriuria, symptomatic urinary tract infection (SUTI), bloodstream infection (BSI), and catheter removal. Conservatively, assuming that bacteriuria must precede SUTI and BSI, there are 5 possible trajectories for any hospitalized patient (Figure 1): (1) no infection, (2) only bacteriuria, (3) bacteriuria and SUTI, (4) bacteriuria and BSI, or (5) bacteriuria, SUTI, and BSI. The cost of CAUTI for a particular hospital is therefore the per‐patient cost of each trajectory multiplied by the number of patients experiencing each trajectory. Our approach for estimating hospital costs is based on factorizing the number of patients experiencing each trajectory into a product of terms for which estimates are available from the literature (see the Supporting Information, Appendix, in the online version of this article for all technical details).

Deriving Estimates of Current Costs
We start with 2 minor simplifying assumptions. First, because the presence of asymptomatic bacteriuria is typically unknown, we only consider costs to the hospital due to SUTI and BSI[6]; in other words, we assume hospitals do not incur costs for patients with trajectories 1 or 2. This assumption should only bias cost estimates conservatively. Second, we assume that patients with both SUTI and BSI (trajectory 5) incur costs equal to those for patients with only BSI (trajectory 4). Further, because the joint risk of SUTI and BSI is unknown, we conservatively assume SUTI must precede BSI. Under these assumptions we can write: (total CAUTI costs)=(per‐patient SUTI cost) (number with SUTI but no BSI)+(perpatient BSI cost) (number with BSI).
We use per‐patient hospital costs of SUTI and BSI of $911 and $3824, respectively, which were determined using a microcosting approach[6] and adjusted for inflation using the general Consumer Price Index.[7] Although an alternative strategy for estimating costs would be to enter the hospital‐specific, per‐patient costs of SUTI and BSI into the above equation, these quantities are often difficult to measure or otherwise unavailable. Thus, it remains to factorize the number of hospitalized patients who develop SUTI and BSI into component terms for which we have accessible estimates. First note that the number with only SUTI (or any BSI) equals the total number of patients hospitalized times the proportion of hospitalizations with only SUTI (or any BSI). The former quantity depends on the particular hospital and so is specified as an input by the user. The latter quantity can be factorized further under our aforementioned conservative assumption that bacteriuria must precede SUTI and BSI.
Specifically, for SUTI:
(Proportion SUTI but no BSI)={(SUTI risk among those catheterized with bacteriuria)(BSI risk among those catheterized with bacteriuria)} (bacteriuria risk among those catheterized) (proportion catheterized).
And for BSI:
(Proportion BSI)=(BSI risk among those catheterized with bacteriuria) (bacteriuria risk among those catheterized) (proportion catheterized).
The risks of SUTI and BSI among those catheterized with bacteriuria, along with the risk of bacteriuria among those catheterized, have been estimated previously via a meta‐analytic approach.[6] The proportion catheterized depends on the particular hospital, such as the total number of patients hospitalized, and so is also specified as a user input. Therefore, we have now factorized the total hospital costs due to CAUTI as a product of either user‐specified terms or terms for which we have estimates from the literature. All estimates and corresponding standard errors derived from the literature are listed together in Table 1 (see the Supporting Information, Appendix Section 1, for further details in the online version of this article).
| Quantity | Estimate (SE) |
|---|---|
| |
| Overall risk of bacteriuria among those catheterized | 26.0% (1.53%) |
| Per‐day risk of bacteriuria among those catheterized | 5.0% |
| days | 6.68 |
| Risk of SUTI among those catheterized with bacteriuria | 24.0% (4.08%) |
| Risk of BSI among those catheterized with bacteriuria | 3.6% (0.10%) |
| Per‐patient SUTI cost | $911 ($911) |
| Per‐patient BSI cost | $3824 ($3824) |
Deriving Projected Costs After Intervention
The approach described above permits estimation of current costs for managing patients with CAUTI for a particular hospital. To estimate projected costs after participation in an intervention to reduce infection risk, we characterize interventions of interest and introduce additional factorization. Specifically, following previous work,[8] we consider interventions that reduce (1) placement (ie, the proportion catheterized) and (2) duration (ie, the mean duration of catheterization). Incorporating reductions in placement is straightforward, because our above expression for costs already contains a term for the proportion catheterized. However, incorporating reductions in duration requires further factorization. Under the assumptions of constant per‐day risks of bacteriuria and of catheter removal, we can write the postintervention risk of bacteriuria among the catheterized as a function of (1) the percent decrease in mean duration of catheterization due to intervention, and (2) the preintervention risk of bacteriuria among the catheterized (see the Supporting Information, Appendix Section 2, for further details in the online version of this article). This means we can fully characterize postintervention costs as a function of user‐specified quantities, quantities specific to the intervention (which are varied across plausible ranges), and quantities for which we have estimates from the literature. Therefore, we can estimate savings by subtracting postintervention costs from current costs.
Because our estimators of current costs, projected costs, and savings are all formulated as functions of other estimators, we use the standard delta method approach[9] to derive appropriate variance estimates (see the Supporting Information, Appendix Section 3, for further details in the online version of this article).
Online Implementation
Customized results (based on annual admissions, urinary catheter prevalence, and other inputs) can be computed using online implementation of our proposed method at
RESULTS
Figure 2 shows the projected savings in hospital costs due to CAUTI across a range of interventions defined by percent decreases in placement and duration, for a hypothetical hospital with 3000 total patients, 15% with urinary catheters preintervention, and with all other default values listed in Table 1. The current costs for this hospital (ie, the costs when the percent reduction in placement and duration is zero) are estimated to be $37,868 (95% confidence interval [CI]: $9159‐$156,564). After an intervention resulting in 40% reductions in both urinary catheter placement and duration, this hospital would be expected to save $22,653 (95% CI: $5479‐$93,656). A less effective intervention yielding a 10% reduction in both urinary catheter placement and duration would result in more modest savings of $6376 (95% CI: $1542‐$26,360).

After an intervention resulting in 29% and 37% reductions in placement and duration, respectively, reflecting reductions seen in practice,[10, 11] our hypothetical hospital is estimated to save $19,126 (95% CI: $4626‐$79,074). This reflects an estimated savings of nearly 50%.
DISCUSSION
We have presented a tool for estimating customized hospital costs of CAUTI, both before and after a hypothetical intervention to reduce risk of infection. Our approach relies on mostly conservative assumptions, incorporates published risk estimates (properly accounting for their associated variability), and has easy‐to‐use online implementation. We believe this can play an important role in motivating hospitals to reduce inappropriate urinary catheter use.
The methodology employed here does have a few limitations. First and foremost, our results depend on the reliability of the input values, which are either provided by users or are based on estimates from the literature (see Table 1 for a complete list of suggested defaults). New information could potentially be incorporated if and when available. For example, substitution of more precise risk estimates could help reduce confidence interval length. Second, our approach essentially averages over hospital quality; we do not directly take into account quality of care or variation in underlying infection risk across hospitals in computing estimated costs. Finally, we only compute direct costs due to infection; other costs (eg, intervention costs) would typically also need to be considered for decision making.
Despite these limitations, we believe that our tool can help infection control professionals demonstrate the values of CAUTI prevention efforts to key administrators, particularly at a time where it has become increasingly necessary to develop a business case to initiate new interventions or justify the continued support for ongoing programs.[12] Additionally, we believe the proposed approach can be an important supplement to initiatives like the Society of Hospital Medicine's Choosing Wisely campaign, which aims to help reduce inappropriate urinary catheter use. Reducing catheter utilization has the potential to reduce costs associated with caring for CAUTI patients, but more importantly would help reduce CAUTI incidence as well as catheter‐related, noninfectious complications.[13, 14] We hope that our tool will greatly assist hospitals in promoting their CAUTI prevention efforts and improve the overall safety of hospitalized patients.
Disclosures
This project was supported by the Ann Arbor VA Medical Center/University of Michigan Patient Safety Enhancement Program (PSEP) and a subcontract to implement multistate CAUTI prevention with the Agency for Healthcare Research and Quality/Health Educational and Research Trust. Mr. Kennedy has no conflicts of interest to report. Drs. Saint and Greene are subcontracted to implement multistate CAUTI prevention with the Agency for Healthcare Research and Quality/Health Educational and Research Trust. Dr. Saint has received numerous honoraria and speaking fees for lectures on healthcare‐associated infection prevention, implementation science, and patient safety from hospitals, academic medical centers, professional societies, and nonprofit foundations. None of these activities are related to speaker's bureaus. Dr. Saint is also on the medical advisory board of Doximity, a new social networking site for physicians. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
- , , , et al. Estimating health care‐associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166.
- The direct medical costs of healthcare‐associated infections in US hospitals and the benefits of prevention. US Centers for Disease Control and Prevention Web site. Published 2009. Available at: http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf. Accessed March 24, 2013.
- , , , , . Catheter‐associated urinary tract infection and the Medicare rule changes. Ann Intern Med. 2009;150(12):877–884.
- , . Improving use of the other catheter. Arch Intern Med. 2012;172(3):260–261.
- Choosing Wisely: five things patients and physicians should question. Society of Hospital Medicine. Published 2012. Available at: http://www.hospitalmedicine.org/AM/pdf/SHM‐Adult_5things_List_Web.pdf. Accessed March 24, 2013.
- . Clinical and economic consequences of nosocomial catheter‐related bacteriuria. Am J Infect Control. 2000;28(1):68–75.
- CPI Inflation Calculator. United States Department of Labor, Bureau of Labor Statistics Web site. Published 2013. Available at: http://www.bls.gov/data/inflation_calculator.htm. Accessed March 24, 2013.
- , , , et al. Introducing a population‐based outcome measure to evaluate the effect of interventions to reduce catheter‐associated urinary tract infection. Am J Infect Control. 2012;40(4):359–364.
- . Asymptotic Statistics. Cambridge, UK: Cambridge University Press; 2000.
- , , , et al. Effect of establishing guidelines on appropriate urinary catheter placement. Acad Emerg Med. 2010;17:337–340.
- , , , . Systematic review and meta‐analysis: reminder systems to reduce catheter‐associated urinary tract infections and urinary catheter use in hospitalized patients. Clin Infect Dis. 2010;51(5):550–560.
- , , , et al. Raising standards while watching the bottom line: making a business case for infection control. Infect Control Hosp Epidemiol. 2007;28:1121–1133.
- , , , , . Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453–1457.
- , , . Indwelling urinary catheters: a one‐point restraint? Ann Intern Med. 2002;137(2):125–127.
- , , , et al. Estimating health care‐associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166.
- The direct medical costs of healthcare‐associated infections in US hospitals and the benefits of prevention. US Centers for Disease Control and Prevention Web site. Published 2009. Available at: http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf. Accessed March 24, 2013.
- , , , , . Catheter‐associated urinary tract infection and the Medicare rule changes. Ann Intern Med. 2009;150(12):877–884.
- , . Improving use of the other catheter. Arch Intern Med. 2012;172(3):260–261.
- Choosing Wisely: five things patients and physicians should question. Society of Hospital Medicine. Published 2012. Available at: http://www.hospitalmedicine.org/AM/pdf/SHM‐Adult_5things_List_Web.pdf. Accessed March 24, 2013.
- . Clinical and economic consequences of nosocomial catheter‐related bacteriuria. Am J Infect Control. 2000;28(1):68–75.
- CPI Inflation Calculator. United States Department of Labor, Bureau of Labor Statistics Web site. Published 2013. Available at: http://www.bls.gov/data/inflation_calculator.htm. Accessed March 24, 2013.
- , , , et al. Introducing a population‐based outcome measure to evaluate the effect of interventions to reduce catheter‐associated urinary tract infection. Am J Infect Control. 2012;40(4):359–364.
- . Asymptotic Statistics. Cambridge, UK: Cambridge University Press; 2000.
- , , , et al. Effect of establishing guidelines on appropriate urinary catheter placement. Acad Emerg Med. 2010;17:337–340.
- , , , . Systematic review and meta‐analysis: reminder systems to reduce catheter‐associated urinary tract infections and urinary catheter use in hospitalized patients. Clin Infect Dis. 2010;51(5):550–560.
- , , , et al. Raising standards while watching the bottom line: making a business case for infection control. Infect Control Hosp Epidemiol. 2007;28:1121–1133.
- , , , , . Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453–1457.
- , , . Indwelling urinary catheters: a one‐point restraint? Ann Intern Med. 2002;137(2):125–127.
Hospitalist Experiences Regarding PICCs
Peripherally inserted central catheters (PICCs) have become among the most common central venous catheters (CVCs) used in contemporary medical practice.[1] Although they were originally developed for delivery of parenteral nutrition, the use of PICCs has expanded to include chemotherapy administration, long‐term intravenous (IV) antibiotic treatment, and venous access when obtaining peripheral veins is difficult (eg, occluded peripheral veins, unusual venous anatomies).[2] Despite these roles, little is known about PICC use in hospitalized patients. This knowledge gap is important, as PICCs are placed in inpatient settings for a variety of reasons. Some of these reasons may not be appropriate, and inappropriate PICC use may worsen outcomes and increase healthcare costs.[3] In addition, PICCs are not innocuous and are frequently associated with important complications including thrombophlebitis, central‐lineassociated bloodstream infection and venous thromboembolism.[4, 5, 6] Therefore, understanding patterns and knowledge associated with PICC use is also an important patient safety concern.
As the main providers of inpatient care, hospitalists frequently order the insertion of PICCs and treat PICC‐related complications. Unfortunately, to date, no study has surveyed hospitalists regarding management or use of PICCs. Understanding hospitalist experiences, practice, opinions, and knowledge related to PICCs is therefore of significant interest when examining present‐day PICC use. To bridge this important knowledge gap and better understand these practices, we conducted a Web‐based survey of hospitalists in 5 healthcare systems in the state of Michigan.
METHODS
A convenience sample of hospitalists (N=227) was assembled from 5 large healthcare systems (representing 10 hospitals) that participate in the Hospital Medicine Safety (HMS) Consortium, a Blue Cross/Blue Shield of Michiganfunded statewide collaborative quality initiative. Individuals engaged in research, quality improvement, or leadership at HMS sites were invited to serve as site principal investigators (site PIs). Site PIs were responsible for obtaining regulatory approval at their parent facilities and disseminating the survey to providers in their group. Participation in the survey was solicited via e‐mail invitations from site PIs to hospitalists within their provider group. To encourage participation, a $10 electronic gift card was offered to respondents who successfully completed the survey. Reminder e‐mails were also sent each week by site PIs to augment participation. To enhance study recruitment, all responses were collected anonymously. The survey was administered between August 2012 and September 2012; data collection occurred for 5 weeks during this interval.
Survey questions were derived from our published, evidence‐based conceptual framework of PICC‐related complications. Briefly, this model identifies complications related to PICCs as arising from domains related to patient‐, provider‐, and device‐related characteristics based on existing evidence.[2] For our survey, questions were sourced from each of these domains so as to improve understanding of hospitalist experience, practice, opinions, and knowledge regarding PICC use. To ensure clarity of the survey questions, all questions were first pilot‐tested with a group of randomly selected hospitalist respondents at the University of Michigan Health System. Direct feedback obtained from these respondents was then used to iteratively improve each question. In order to generate holistic responses, questions were designed to generate a response reflective of the participants typical PICC use/subenario. We used SurveyMonkey to collect and manage survey data.
Statistical Analyses
Variation in hospitalist experience, reported practice, opinions, and knowledge regarding PICCs was assessed by hospitalist type (full time vs part time), years of practice (<1, 15, >5), and care‐delivery model (direct care vs learner‐based care). Bivariate comparisons were made using the 2 or Fisher exact tests as appropriate; 2‐sided with a P value <0.05 was considered statistically significant. All analyses were conducted using Stata version 11 (StataCorp, College Station, TX). Local institutional review board approval was obtained at each site participating in the survey.
RESULTS
A total of 227 surveys were administered and 144 responses collected, for a survey response rate of 63%. Each participating site had unique characteristics including size, number of hospitalists, and modality of PICC insertion (Table 1). Of the hospitalists who completed the survey, 81% held full‐time clinical positions and had been in practice an average of 5.6 years. Surveyed hospitalists reported caring for an average of 40.6 patients per week and ordering a mean of 2.9 (range, 015) PICCs per week of clinical service. Among survey respondents, 36% provided direct patient care, 34% provided care either directly or through mid‐level providers and housestaff, and 9% delivered care exclusively through mid‐level providers or housestaff (Table 2). As our survey was conducted anonymously, potential identifying information such as age, race, and sex of those responding was not collected.
| Survey Site | No. of Hospitals | No. of Inpatient Beds | No. of Annual Inpatient Encounters | No. of Hospitalists | Full‐Time Hospitalists, % | Avg. No. Weeks/Year on Service | Avg. Years of Experience | No. PICCs/Week, 2012 | Modality of PICC Insertion Available |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| University of Michigan Health System | 1 | 900+ | 5,775 | 46 | 100 | 25 | 6 | 42 | Vascular access nurse |
| Ann Arbor VA Medical Center | 1 | 135 | 825 | 16 | 50 | 17.6 | 5.1 | 12 | Vascular access nurse |
| Spectrum Health System | 2 | 800 | 14,000 | 47 | 80 | 34 | 3.75 | 56 | Interventional radiology |
| Trinity Health System | 3 | 634 | 2,300 | 67 | 80 | 24 | 4 | 31 | Interventional radiology and hospitalists |
| Henry Ford Health System | 3 | 1,150 | 1,450 | 51 | 100 | 20.4 | 5.6 | 15 | Vascular access nurse |
| Characteristic | Total (N=144) |
|---|---|
| |
| Hospitalist type, n (%) | |
| Full time | 117 (81) |
| Part time | 19 (13) |
| Unknown | 8 (6) |
| Weeks/year on a clinical service, n (%) | |
| <20 | 24 (17) |
| 20 | 107 (74) |
| Unknown | 13 (9) |
| Mean (SD) | 25.5 (10.7) |
| Median | 26 |
| Type of patients treated, n (%) | |
| Adults only | 129 (90) |
| Adults and children | 7 (5) |
| Unknown | 8 (6) |
| Years in practice as a hospitalist, n (%) | |
| 5 | 81 (56) |
| >5 | 54 (38) |
| Unknown | 9 (6) |
| Model of care delivery, n (%) | |
| Direct | 52 (36) |
| Some midlevel or housestaff providers (<50% of all encounters) | 49 (34) |
| Mostly midlevel or housestaff providers (>50% of all encounters) | 22 (15) |
| Only midlevel or housestaff providers | 13 (9) |
| Unknown | 8 (6) |
| Location of practice | |
| Trinity Health System | 39 (27) |
| University of Michigan Health System | 37 (26) |
| Henry Ford Health System | 28 (19) |
| Spectrum Health System | 21 (15) |
| Ann Arbor VA Medical Center | 11 (8) |
| Unknown | 8 (6) |
Hospitalist Experiences and Practice Related to Peripherally Inserted Central Catheters
According to responding hospitalists, the most common indications for PICC placement were long‐term IV antibiotic treatment (64%), followed by inability to obtain peripheral venous access (24%). Hospitalists reported an average duration of PICC placement of 17 days (range, 342 days). A significant percentage of hospitalists (93%) stated that they had cared for patients where a PICC was placed only for use during hospitalization, with the most common reason for such insertion being difficulty in otherwise securing venous access (67%). Respondents also reported caring for patients who had both PICCs and peripheral IV catheters in place at the same time; 49% stated that they had experienced this <5 times, whereas 33% stated they had experienced this 510 times. Furthermore, 87% of respondents indicated having admitted a patient who specifically requested a PICC due to prior difficulties with venous access. More than half of surveyed hospitalists (63%) admitted to having been contacted by a PICC nurse enquiring as to whether their patient might benefit from PICC insertion.
The majority of hospitalists (66%) reported that they specified the number of lumens when ordering PICCs. Thirty‐eight percent indicated that this decision was based on type of medication, whereas 35% selected the lowest number of lumens possible. A power PICC (specialized PICCs that are designed to withstand high‐pressure contrast injections), was specifically requested for radiographic studies (56%), infusion of large volume of fluids (10%), or was the default PICC type at their facility (34%).
A majority (74%) of survey respondents also reported that once inserted, PICCs were always used to obtain blood for routine laboratory testing. Moreover, 41% indicated that PICCs were also always used to obtain blood for microbiological cultures. The 3 most frequently encountered PICC‐related complications reported by hospitalists in our survey were blockage of a PICC lumen, bloodstream infection, and venous thromboembolism (VTE; Table 3).
| Hospitalist Experiences With PICCs | Total (N=144) |
|---|---|
| |
| Primary indication for PICC placement* | |
| Long‐term IV antibiotics | 64 |
| Venous access in a patient with poor peripheral veins | 24 |
| Parenteral nutrition | 5 |
| Chemotherapy | 4 |
| Patient specifically requested a PICC | 1 |
| Unknown/other | 2 |
| PICC placed only for venous access, n (%) | |
| Yes | 135 (94) |
| No | 9 (6) |
| PICC placed only during hospitalization, n (%) | |
| Yes | 134 (93) |
| No | 10 (7) |
| Notified by a PICC nurse (or other provider) that patient may need or benefit from a PICC, n (%) | |
| Yes | 91 (63) |
| No | 53 (37) |
| How frequently PICCs are used to obtain blood for routine laboratory testing, n (%) | |
| Always | 106 (74) |
| Unknown/other | 38 (26) |
| How frequently PICCs are used to obtain blood for blood cultures, n (%) | |
| Always | 59 (41) |
| Unknown/other | 85 (59) |
| Hospitalist Opinions on PICCs | Total (N=144) |
| In your opinion, is it appropriate to place a vascular in a hospitalized patient if other forms of peripheral access cannot be obtained? n (%) | |
| Yes | 121 (84) |
| No | 21 (15) |
| Unknown | 2 (1) |
| In your opinion, should hospitalists be trained to insert PICCs? n (%) | |
| No | 57 (40) |
| Yes, this is an important skill set for hospitalists | 46 (32) |
| Unsure | 39 (27) |
| Unknown/other | 2 (1) |
| Do you think the increasing number of vascular nurses and PICC nursing teams has influenced the use of PICCs in hospitalized patients? n (%) | |
| Yes | 112 (78) |
| No | 30 (21) |
| Unknown | 2 (1) |
| What % of PICC insertions do you think may represent inappropriate use in your hospital? n (%) | |
| <10 | 53 (37) |
| 1025 | 68 (47) |
| 2550 | 18 (13) |
| >50 | 3 (2) |
| Unknown/other | 2 (1) |
Hospitalist Opinions Regarding Peripherally Inserted Central Catheters
Compared with CVCs, 69% of hospitalists felt that PICCs were safer and more efficient because they could stay in place longer and were less likely to cause infection. Most (65%) also agreed that PICCs were more convenient than CVCs because they were inserted by PICC teams. Additionally, 74% of hospitalists felt that their patients preferred PICCs because they minimize pain from routine peripheral IV changes and phlebotomy. A majority of respondents (84%) indicated that it was appropriate to place a PICC if other forms of peripheral venous access could not be obtained. However, when specifically questioned, 47% of hospitalists indicated that at least 10%25% of PICCs placed in their hospitals might represent inappropriate use. A majority (78%) agreed with the statement that the increase in numbers of vascular nurses had influenced use of PICCs in hospitalized patients, but most (45%) were neutral when asked if PICCs were more cost‐effective than traditional CVCs.
Hospitalist Knowledge Regarding Risk of Peripherally Inserted Central CatheterRelated Venous Thromboembolism and Bloodstream Infection
Although 65% of responding hospitalists disagreed with the statement that PICCs were less likely to lead to VTE, important knowledge gaps regarding PICCs and VTE were identified (Table 4). For instance, only 4% of hospitalists were correctly aware that the PICC‐tip position is checked to reduce risk of PICC‐related VTE, and only 12% knew that the site of PICC insertion has also been associated with VTE risk. Although 85% of respondents stated they would prescribe a therapeutic dose of an anticoagulant in the case of PICC‐associated VTE, deviations from the guideline‐recommended 3‐month treatment period were noted. For example, 6% of hospitalists reported treating with anticoagulation for 6 months, and 19% stated they would treat as long as the PICC remained in place, plus an additional period of time (eg, 24 weeks) after removal. With respect to bloodstream infection, 92% of responding hospitalists correctly identified PICC duration and prompt removal as factors promoting PICC‐related bloodstream infection and 78% accurately identified components of the catheter‐associated bloodstream infection bundle. When specifically asked about factors associated with risk of PICC‐related bloodstream infection, only half of respondents recognized the number of PICC lumens as being associated with this outcome.
| Total (N=144) | |
|---|---|
| |
| Why is the position of the PICC tip checked after bedside PICC insertion? n (%) | |
| To decrease the risk of arrhythmia related to right‐atrial positioning | 108 (75) |
| To minimize the risk of VTEa | 6 (4) |
| To ensure it is not accidentally placed into an artery | 16 (11) |
| For documentation purposes (to reduce the risk of lawsuits related to line‐insertion complications) | 6 (4) |
| Unsure/Unknown | 8 (6) |
| According to the 2012 ACCP Guidelines on VTE prevention, is pharmacologic prophylaxis for DVT recommended in patients who receive long‐term PICCs? n (%) | |
| No; no anticoagulant prophylaxis is recommended for patients who receive long‐term PICCsa | 107 (74) |
| Yes, but the choice and duration of anticoagulant is at the discretion of the provider | 23 (16) |
| Yes; aspirin is recommended for 3 months | 4 (3) |
| Yes; anticoagulation with warfarin or enoxaparin is recommended for 3 months | 3 (2) |
| Yes; anticoagulation with warfarin or enoxaparin is recommended for 6 months | 2 (1) |
| Unknown | 5 (4) |
| Assuming no contraindications exist, do you anticoagulate patients who develop a PICC‐associated DVT (with any therapeutic anticoagulant)? n (%) | |
| Yesa | 122 (85) |
| No | 16 (11) |
| Unknown | 6 (4) |
| How long do you usually prescribe anticoagulation for patients who develop PICC‐associated DVT? n (%) | |
| I don't prescribe anticoagulation | 12 (8) |
| 1 month | 4 (3) |
| 3 monthsa | 84 (58) |
| 6 months | 8 (6) |
| As long as the line remains in place; I stop anticoagulation once the PICC comes out | 3 (2) |
| As long as the line remains in place and for an additional specified period of time after line removal, such as 2 or 4 weeks | 27 (19) |
| Unknown | 6 (4) |
| As part of the treatment of PICC‐related DVT, do you routinely remove the PICC?b n (%) | |
| Yes | 102 (71) |
| No | 36 (25) |
| Unknown | 6 (4) |
Variation in Hospitalist Knowledge, Experience, or Opinions
We assessed whether any of our findings varied according to hospitalist type (full time versus part time), years of practice (<1, 15, >5), and model of care delivery (direct care vs learner‐based care). Our analyses suggested that part‐time hospitalists were more likely to select rarely when it came to finding patients with a PICC and a working peripheral IV at the same time (74% vs 45%, P=0.02). Interestingly, a higher percentage of those in practice <5 years indicated that 10%25% of PICCs represented inappropriate placement (58% vs 33%, P<0.01) and that vascular nurses had influenced the use of PICCs in hospitalized patients (88% vs 69%, P=0.01). Lastly, a higher percentage of hospitalists who provided direct patient care reported that PICCs were always used to obtain blood for microbiological culture (54% vs 37%, P=0.05).
DISCUSSION
In this survey of hospitalists practicing at 5 large healthcare systems in Michigan, we observed significant variation in experience, reported practice, opinions, and knowledge related to PICCs. Our findings highlight important concerns related to inpatient PICC use and suggest a need for greater scrutiny related to these devices in these settings.
The use of PICCs in hospitalized patients has risen dramatically over the past decade. Though such growth is multifactorial and relates in part to increasing inpatient volume and complexity, hospitalists have increasingly turned to PICCs as a convenient and reliable tool to obtain venous access.[7] Indeed, in our survey, PICCs that were only used during hospitalization were most likely to be placed for this very reason. Because PICCs are safer to insert than CVCs and the original evidence regarding PICC‐related VTE or bloodstream infection suggested low rates of these events,[8, 9, 10, 11, 12, 13, 14] many hospitalists may not perceive these devices as being associated with significant risks. In fact, some have suggested that hospitalists be specifically trained to insert these devices, given their safety compared with traditional CVCs.[7]
However, accumulating evidence suggests that PICCs are associated with important complications.[5, 15, 16] In studies examining risk of bloodstream infection, PICCs were associated with significant risk of this outcome.[6, 17, 18] Recently, the presence of a PICC was identified as an independent predictor of VTE in hospitalized patients.[19] Several studies and systematic reviews have repeatedly demonstrated these findings.[19, 20, 21, 22] A recent systematic review examining nonpharmacologic methods to prevent catheter‐related thrombosis specifically called for avoidance of PICC insertion to prevent thrombosis in hospitalized patients.[23] Despite this growing evidence base, the use of PICCs in the inpatient setting is likely to rise, and our survey highlights several practices that may contribute to adverse outcomes. For instance, hospitalists in our survey were unlikely to remove a PICC until a patient was discharged, irrespective of the need for this device. As each day with a PICC increases the risk of complications, such practice poses potential patient safety concerns. Similarly, many hospitalists believe that PICCs are safer than CVCs, a viewpoint that does not stand up to increasing scrutiny and highlights important knowledge gaps. The risk of PICC‐related complications appears not to be a stationary target, but rather a dynamic balance that is influenced by patient‐, provider‐, and device‐specific characteristics.[2] Increasing discretionary use (especially for patients with poor peripheral venous access), forgetting at times that a patient has a PICC, and the finding that up to 25% of PICCs placed in their hospitals may be unnecessary underscore concerns regarding the safety of current practice trends. Interestingly, the viewpoints of hospitalists in practice <5 years and those providing direct patient care were more likely to reflect concerns regarding inappropriate placement, influence of vascular nurses, and use of PICCs for blood culture. This finding may reflect that these nuances are more recent phenomena or perhaps most apparent when care is delivered directly.
Our study must be interpreted in the context of several limitations. First, as this was a survey‐based study of a small, convenience sample of hospitalists in a single state, recall, respondent, and systematic biases remain threats to our findings. However, all site PIs encouraged survey participation and (through local dialogue) none were aware of material differences between those who did or did not participate in the study. Similarly, Michigan is a diverse and relatively large state, and our results should be generalizable to other settings; however, national studies are necessary to confirm our findings. Second, our response rate may be perceived as low; however, our rates are in accordance with, and, in fact, superior to those of many existing physician surveys.[24] Finally, only 1 federal facility was included in this study; thus, this care‐delivery model is underrepresented, limiting generalization of findings to other such sites.
However, our study also has important strengths. First, this is the only survey that specifically examines hospitalist viewpoints when it comes to PICCs. As hospitalists frequently order and/or insert these devices, their perspectives are highly pertinent to discussions regarding current PICC use. Second, our survey highlights several instances that may be associated with preventable patient harm and identifies areas where interventions may be valuable. For example, forgetting the presence of a device, keeping PICCs in place throughout hospitalization, and rendering treatment for PICC‐related VTE not in accordance with accepted guidelines are remediable practices that may lead to poor outcomes. Interventions such as device‐reminder alerts, provider education regarding complications from PICCs, and systematic efforts to identify and remove unnecessary PICCs may mitigate these problems. Finally, our findings highlight the need for data repositories that track PICC use and hospitalist practice on a national scale. Given the risk and significance of the complications associated with these devices, understanding the epidemiology, use, and potential misuse of PICCs are important areas for hospitalist research.
In conclusion, our study of hospitalist experience, practice, opinions, and knowledge related to PICCs suggests important gaps between available evidence and current practice. There is growing need for the development of appropriateness criteria to guide vascular access in inpatient settings.[25, 26] Such criteria should consider not only type of venous access device, but granular details including rationale for venous access, nature of the infusate, optimal number of lumens, and safest gauge when recommending devices. Until such criteria and comparative studies become available, hospitals should consider instituting policies to monitor PICC use with specific attention to indication for insertion, duration of placement, and complications. These interventions represent a first and necessary step in improving patient safety when it comes to preventing PICC‐related complications.
Disclosures
The Blue Cross/Blue Shield of Michigan Foundation in Detroit funded this study through an investigator‐initiated research proposal (1931‐PIRAP). The funding source, however, played no role in study design, acquisition of data, data analysis, or reporting of these results. The authors report no conflicts of interest.
- , , , et al. Hospital‐wide survey of the use of central venous catheters. J Hosp Infect. 2011;77(4):304–308.
- , , , , . Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733–741.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , , , et al. Reduction of peripherally inserted central catheter associated deep venous thrombosis [published online ahead of print August 1, 2012]. Chest. doi: 10.1378/chest.12–0923.
- , , . Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65–71.
- , , , et al. Patient‐ and device‐specific risk factors for peripherally inserted central venous catheter‐related bloodstream infections. Infect Control Hosp Epidemiol. 2013;34(2):184–189.
- , . Peripherally inserted central catheter use in the hospitalized patient: is there a role for the hospitalist? J Hosp Med. 2009;4(6):E1–E4.
- , , , , , . Peripherally inserted central catheter (PICC)‐associated upper‐extremity deep venous thrombosis (UEDVT) in critical‐care setting. Chest. 2005;128(4 suppl S):193S–194S.
- , , , , , . Complications and cost associated with parenteral nutrition delivered to hospitalized patients through either subclavian or peripherally inserted central catheters. Clin Nutr. 2000;19(4):237–243.
- , . Risk of catheter‐related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495.
- , , , . Long‐term intravenous therapy with peripherally inserted silicone elastomer central venous catheters in patients with malignant diseases. Cancer. 1979;43(5):1937–1943.
- , , , , . Central vs peripheral venous catheters in critically ill patients. Chest. 1986;90(6):806–809.
- , , , , . Infectious complications among patients receiving home intravenous therapy with peripheral, central, or peripherally placed central venous catheters. Am J Med. 1991;91(3B):95S–100S.
- , , , , . Upper‐extremity deep venous thrombosis and pulmonary embolism: a prospective study. Chest. 1991;99(2):280–283.
- , , , et al. Risk factors for catheter‐related thrombosis (CRT) in cancer patients: a patient‐level data (IPD) meta‐analysis of clinical trials and prospective studies. J Thromb Haemost. 2011;9(2):312–319.
- , , , et al. Venous thrombosis associated with peripherally inserted central catheters: a retrospective analysis of the Cleveland Clinic experience. Clin Infect Dis. 2002;34(9):1179–1183.
- , , , et al. Peripherally inserted central venous catheter–associated bloodstream infections in hospitalized adult patients. Infect Control Hosp Epidemiol. 2011;32(2):125–130.
- , , . Peripherally inserted central catheter bloodstream infection surveillance rates in an acute care setting in Saudi Arabia. Ann Saudi Med. 2012;32(2):169–173.
- , , , et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947.e942–954.e942.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810.
- , , . The clinical significance of peripherally inserted central venous catheter‐related deep vein thrombosis. Neurocrit Care. 2011;15(3):454–460.
- , , , et al. Catheter‐associated bloodstream infection incidence and risk factors in adults with cancer: a prospective cohort study. J Hosp Infect. 2011;78(1):26–30.
- , , , . Nonpharmacologic interventions for prevention of catheter‐related thrombosis: a systematic review [published online ahead of print September 13, 2012]. J Crit Care. doi: 10.1016/j.jcrc.2012.07.007.
- , , . Why are response rates in clinician surveys declining? Can Fam Physician. 2012;58(4):e225–e228.
- , , , , , . Sensitivity and specificity of the RAND/UCLA Appropriateness Method to identify the overuse and underuse of coronary revascularization and hysterectomy. J Clin Epidemiol. 2001;54(10):1004–1010.
- , , , et al. Variations by specialty in physician ratings of the appropriateness and necessity of indications for procedures. Med Care. 1996;34(6):512–523.
Peripherally inserted central catheters (PICCs) have become among the most common central venous catheters (CVCs) used in contemporary medical practice.[1] Although they were originally developed for delivery of parenteral nutrition, the use of PICCs has expanded to include chemotherapy administration, long‐term intravenous (IV) antibiotic treatment, and venous access when obtaining peripheral veins is difficult (eg, occluded peripheral veins, unusual venous anatomies).[2] Despite these roles, little is known about PICC use in hospitalized patients. This knowledge gap is important, as PICCs are placed in inpatient settings for a variety of reasons. Some of these reasons may not be appropriate, and inappropriate PICC use may worsen outcomes and increase healthcare costs.[3] In addition, PICCs are not innocuous and are frequently associated with important complications including thrombophlebitis, central‐lineassociated bloodstream infection and venous thromboembolism.[4, 5, 6] Therefore, understanding patterns and knowledge associated with PICC use is also an important patient safety concern.
As the main providers of inpatient care, hospitalists frequently order the insertion of PICCs and treat PICC‐related complications. Unfortunately, to date, no study has surveyed hospitalists regarding management or use of PICCs. Understanding hospitalist experiences, practice, opinions, and knowledge related to PICCs is therefore of significant interest when examining present‐day PICC use. To bridge this important knowledge gap and better understand these practices, we conducted a Web‐based survey of hospitalists in 5 healthcare systems in the state of Michigan.
METHODS
A convenience sample of hospitalists (N=227) was assembled from 5 large healthcare systems (representing 10 hospitals) that participate in the Hospital Medicine Safety (HMS) Consortium, a Blue Cross/Blue Shield of Michiganfunded statewide collaborative quality initiative. Individuals engaged in research, quality improvement, or leadership at HMS sites were invited to serve as site principal investigators (site PIs). Site PIs were responsible for obtaining regulatory approval at their parent facilities and disseminating the survey to providers in their group. Participation in the survey was solicited via e‐mail invitations from site PIs to hospitalists within their provider group. To encourage participation, a $10 electronic gift card was offered to respondents who successfully completed the survey. Reminder e‐mails were also sent each week by site PIs to augment participation. To enhance study recruitment, all responses were collected anonymously. The survey was administered between August 2012 and September 2012; data collection occurred for 5 weeks during this interval.
Survey questions were derived from our published, evidence‐based conceptual framework of PICC‐related complications. Briefly, this model identifies complications related to PICCs as arising from domains related to patient‐, provider‐, and device‐related characteristics based on existing evidence.[2] For our survey, questions were sourced from each of these domains so as to improve understanding of hospitalist experience, practice, opinions, and knowledge regarding PICC use. To ensure clarity of the survey questions, all questions were first pilot‐tested with a group of randomly selected hospitalist respondents at the University of Michigan Health System. Direct feedback obtained from these respondents was then used to iteratively improve each question. In order to generate holistic responses, questions were designed to generate a response reflective of the participants typical PICC use/subenario. We used SurveyMonkey to collect and manage survey data.
Statistical Analyses
Variation in hospitalist experience, reported practice, opinions, and knowledge regarding PICCs was assessed by hospitalist type (full time vs part time), years of practice (<1, 15, >5), and care‐delivery model (direct care vs learner‐based care). Bivariate comparisons were made using the 2 or Fisher exact tests as appropriate; 2‐sided with a P value <0.05 was considered statistically significant. All analyses were conducted using Stata version 11 (StataCorp, College Station, TX). Local institutional review board approval was obtained at each site participating in the survey.
RESULTS
A total of 227 surveys were administered and 144 responses collected, for a survey response rate of 63%. Each participating site had unique characteristics including size, number of hospitalists, and modality of PICC insertion (Table 1). Of the hospitalists who completed the survey, 81% held full‐time clinical positions and had been in practice an average of 5.6 years. Surveyed hospitalists reported caring for an average of 40.6 patients per week and ordering a mean of 2.9 (range, 015) PICCs per week of clinical service. Among survey respondents, 36% provided direct patient care, 34% provided care either directly or through mid‐level providers and housestaff, and 9% delivered care exclusively through mid‐level providers or housestaff (Table 2). As our survey was conducted anonymously, potential identifying information such as age, race, and sex of those responding was not collected.
| Survey Site | No. of Hospitals | No. of Inpatient Beds | No. of Annual Inpatient Encounters | No. of Hospitalists | Full‐Time Hospitalists, % | Avg. No. Weeks/Year on Service | Avg. Years of Experience | No. PICCs/Week, 2012 | Modality of PICC Insertion Available |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| University of Michigan Health System | 1 | 900+ | 5,775 | 46 | 100 | 25 | 6 | 42 | Vascular access nurse |
| Ann Arbor VA Medical Center | 1 | 135 | 825 | 16 | 50 | 17.6 | 5.1 | 12 | Vascular access nurse |
| Spectrum Health System | 2 | 800 | 14,000 | 47 | 80 | 34 | 3.75 | 56 | Interventional radiology |
| Trinity Health System | 3 | 634 | 2,300 | 67 | 80 | 24 | 4 | 31 | Interventional radiology and hospitalists |
| Henry Ford Health System | 3 | 1,150 | 1,450 | 51 | 100 | 20.4 | 5.6 | 15 | Vascular access nurse |
| Characteristic | Total (N=144) |
|---|---|
| |
| Hospitalist type, n (%) | |
| Full time | 117 (81) |
| Part time | 19 (13) |
| Unknown | 8 (6) |
| Weeks/year on a clinical service, n (%) | |
| <20 | 24 (17) |
| 20 | 107 (74) |
| Unknown | 13 (9) |
| Mean (SD) | 25.5 (10.7) |
| Median | 26 |
| Type of patients treated, n (%) | |
| Adults only | 129 (90) |
| Adults and children | 7 (5) |
| Unknown | 8 (6) |
| Years in practice as a hospitalist, n (%) | |
| 5 | 81 (56) |
| >5 | 54 (38) |
| Unknown | 9 (6) |
| Model of care delivery, n (%) | |
| Direct | 52 (36) |
| Some midlevel or housestaff providers (<50% of all encounters) | 49 (34) |
| Mostly midlevel or housestaff providers (>50% of all encounters) | 22 (15) |
| Only midlevel or housestaff providers | 13 (9) |
| Unknown | 8 (6) |
| Location of practice | |
| Trinity Health System | 39 (27) |
| University of Michigan Health System | 37 (26) |
| Henry Ford Health System | 28 (19) |
| Spectrum Health System | 21 (15) |
| Ann Arbor VA Medical Center | 11 (8) |
| Unknown | 8 (6) |
Hospitalist Experiences and Practice Related to Peripherally Inserted Central Catheters
According to responding hospitalists, the most common indications for PICC placement were long‐term IV antibiotic treatment (64%), followed by inability to obtain peripheral venous access (24%). Hospitalists reported an average duration of PICC placement of 17 days (range, 342 days). A significant percentage of hospitalists (93%) stated that they had cared for patients where a PICC was placed only for use during hospitalization, with the most common reason for such insertion being difficulty in otherwise securing venous access (67%). Respondents also reported caring for patients who had both PICCs and peripheral IV catheters in place at the same time; 49% stated that they had experienced this <5 times, whereas 33% stated they had experienced this 510 times. Furthermore, 87% of respondents indicated having admitted a patient who specifically requested a PICC due to prior difficulties with venous access. More than half of surveyed hospitalists (63%) admitted to having been contacted by a PICC nurse enquiring as to whether their patient might benefit from PICC insertion.
The majority of hospitalists (66%) reported that they specified the number of lumens when ordering PICCs. Thirty‐eight percent indicated that this decision was based on type of medication, whereas 35% selected the lowest number of lumens possible. A power PICC (specialized PICCs that are designed to withstand high‐pressure contrast injections), was specifically requested for radiographic studies (56%), infusion of large volume of fluids (10%), or was the default PICC type at their facility (34%).
A majority (74%) of survey respondents also reported that once inserted, PICCs were always used to obtain blood for routine laboratory testing. Moreover, 41% indicated that PICCs were also always used to obtain blood for microbiological cultures. The 3 most frequently encountered PICC‐related complications reported by hospitalists in our survey were blockage of a PICC lumen, bloodstream infection, and venous thromboembolism (VTE; Table 3).
| Hospitalist Experiences With PICCs | Total (N=144) |
|---|---|
| |
| Primary indication for PICC placement* | |
| Long‐term IV antibiotics | 64 |
| Venous access in a patient with poor peripheral veins | 24 |
| Parenteral nutrition | 5 |
| Chemotherapy | 4 |
| Patient specifically requested a PICC | 1 |
| Unknown/other | 2 |
| PICC placed only for venous access, n (%) | |
| Yes | 135 (94) |
| No | 9 (6) |
| PICC placed only during hospitalization, n (%) | |
| Yes | 134 (93) |
| No | 10 (7) |
| Notified by a PICC nurse (or other provider) that patient may need or benefit from a PICC, n (%) | |
| Yes | 91 (63) |
| No | 53 (37) |
| How frequently PICCs are used to obtain blood for routine laboratory testing, n (%) | |
| Always | 106 (74) |
| Unknown/other | 38 (26) |
| How frequently PICCs are used to obtain blood for blood cultures, n (%) | |
| Always | 59 (41) |
| Unknown/other | 85 (59) |
| Hospitalist Opinions on PICCs | Total (N=144) |
| In your opinion, is it appropriate to place a vascular in a hospitalized patient if other forms of peripheral access cannot be obtained? n (%) | |
| Yes | 121 (84) |
| No | 21 (15) |
| Unknown | 2 (1) |
| In your opinion, should hospitalists be trained to insert PICCs? n (%) | |
| No | 57 (40) |
| Yes, this is an important skill set for hospitalists | 46 (32) |
| Unsure | 39 (27) |
| Unknown/other | 2 (1) |
| Do you think the increasing number of vascular nurses and PICC nursing teams has influenced the use of PICCs in hospitalized patients? n (%) | |
| Yes | 112 (78) |
| No | 30 (21) |
| Unknown | 2 (1) |
| What % of PICC insertions do you think may represent inappropriate use in your hospital? n (%) | |
| <10 | 53 (37) |
| 1025 | 68 (47) |
| 2550 | 18 (13) |
| >50 | 3 (2) |
| Unknown/other | 2 (1) |
Hospitalist Opinions Regarding Peripherally Inserted Central Catheters
Compared with CVCs, 69% of hospitalists felt that PICCs were safer and more efficient because they could stay in place longer and were less likely to cause infection. Most (65%) also agreed that PICCs were more convenient than CVCs because they were inserted by PICC teams. Additionally, 74% of hospitalists felt that their patients preferred PICCs because they minimize pain from routine peripheral IV changes and phlebotomy. A majority of respondents (84%) indicated that it was appropriate to place a PICC if other forms of peripheral venous access could not be obtained. However, when specifically questioned, 47% of hospitalists indicated that at least 10%25% of PICCs placed in their hospitals might represent inappropriate use. A majority (78%) agreed with the statement that the increase in numbers of vascular nurses had influenced use of PICCs in hospitalized patients, but most (45%) were neutral when asked if PICCs were more cost‐effective than traditional CVCs.
Hospitalist Knowledge Regarding Risk of Peripherally Inserted Central CatheterRelated Venous Thromboembolism and Bloodstream Infection
Although 65% of responding hospitalists disagreed with the statement that PICCs were less likely to lead to VTE, important knowledge gaps regarding PICCs and VTE were identified (Table 4). For instance, only 4% of hospitalists were correctly aware that the PICC‐tip position is checked to reduce risk of PICC‐related VTE, and only 12% knew that the site of PICC insertion has also been associated with VTE risk. Although 85% of respondents stated they would prescribe a therapeutic dose of an anticoagulant in the case of PICC‐associated VTE, deviations from the guideline‐recommended 3‐month treatment period were noted. For example, 6% of hospitalists reported treating with anticoagulation for 6 months, and 19% stated they would treat as long as the PICC remained in place, plus an additional period of time (eg, 24 weeks) after removal. With respect to bloodstream infection, 92% of responding hospitalists correctly identified PICC duration and prompt removal as factors promoting PICC‐related bloodstream infection and 78% accurately identified components of the catheter‐associated bloodstream infection bundle. When specifically asked about factors associated with risk of PICC‐related bloodstream infection, only half of respondents recognized the number of PICC lumens as being associated with this outcome.
| Total (N=144) | |
|---|---|
| |
| Why is the position of the PICC tip checked after bedside PICC insertion? n (%) | |
| To decrease the risk of arrhythmia related to right‐atrial positioning | 108 (75) |
| To minimize the risk of VTEa | 6 (4) |
| To ensure it is not accidentally placed into an artery | 16 (11) |
| For documentation purposes (to reduce the risk of lawsuits related to line‐insertion complications) | 6 (4) |
| Unsure/Unknown | 8 (6) |
| According to the 2012 ACCP Guidelines on VTE prevention, is pharmacologic prophylaxis for DVT recommended in patients who receive long‐term PICCs? n (%) | |
| No; no anticoagulant prophylaxis is recommended for patients who receive long‐term PICCsa | 107 (74) |
| Yes, but the choice and duration of anticoagulant is at the discretion of the provider | 23 (16) |
| Yes; aspirin is recommended for 3 months | 4 (3) |
| Yes; anticoagulation with warfarin or enoxaparin is recommended for 3 months | 3 (2) |
| Yes; anticoagulation with warfarin or enoxaparin is recommended for 6 months | 2 (1) |
| Unknown | 5 (4) |
| Assuming no contraindications exist, do you anticoagulate patients who develop a PICC‐associated DVT (with any therapeutic anticoagulant)? n (%) | |
| Yesa | 122 (85) |
| No | 16 (11) |
| Unknown | 6 (4) |
| How long do you usually prescribe anticoagulation for patients who develop PICC‐associated DVT? n (%) | |
| I don't prescribe anticoagulation | 12 (8) |
| 1 month | 4 (3) |
| 3 monthsa | 84 (58) |
| 6 months | 8 (6) |
| As long as the line remains in place; I stop anticoagulation once the PICC comes out | 3 (2) |
| As long as the line remains in place and for an additional specified period of time after line removal, such as 2 or 4 weeks | 27 (19) |
| Unknown | 6 (4) |
| As part of the treatment of PICC‐related DVT, do you routinely remove the PICC?b n (%) | |
| Yes | 102 (71) |
| No | 36 (25) |
| Unknown | 6 (4) |
Variation in Hospitalist Knowledge, Experience, or Opinions
We assessed whether any of our findings varied according to hospitalist type (full time versus part time), years of practice (<1, 15, >5), and model of care delivery (direct care vs learner‐based care). Our analyses suggested that part‐time hospitalists were more likely to select rarely when it came to finding patients with a PICC and a working peripheral IV at the same time (74% vs 45%, P=0.02). Interestingly, a higher percentage of those in practice <5 years indicated that 10%25% of PICCs represented inappropriate placement (58% vs 33%, P<0.01) and that vascular nurses had influenced the use of PICCs in hospitalized patients (88% vs 69%, P=0.01). Lastly, a higher percentage of hospitalists who provided direct patient care reported that PICCs were always used to obtain blood for microbiological culture (54% vs 37%, P=0.05).
DISCUSSION
In this survey of hospitalists practicing at 5 large healthcare systems in Michigan, we observed significant variation in experience, reported practice, opinions, and knowledge related to PICCs. Our findings highlight important concerns related to inpatient PICC use and suggest a need for greater scrutiny related to these devices in these settings.
The use of PICCs in hospitalized patients has risen dramatically over the past decade. Though such growth is multifactorial and relates in part to increasing inpatient volume and complexity, hospitalists have increasingly turned to PICCs as a convenient and reliable tool to obtain venous access.[7] Indeed, in our survey, PICCs that were only used during hospitalization were most likely to be placed for this very reason. Because PICCs are safer to insert than CVCs and the original evidence regarding PICC‐related VTE or bloodstream infection suggested low rates of these events,[8, 9, 10, 11, 12, 13, 14] many hospitalists may not perceive these devices as being associated with significant risks. In fact, some have suggested that hospitalists be specifically trained to insert these devices, given their safety compared with traditional CVCs.[7]
However, accumulating evidence suggests that PICCs are associated with important complications.[5, 15, 16] In studies examining risk of bloodstream infection, PICCs were associated with significant risk of this outcome.[6, 17, 18] Recently, the presence of a PICC was identified as an independent predictor of VTE in hospitalized patients.[19] Several studies and systematic reviews have repeatedly demonstrated these findings.[19, 20, 21, 22] A recent systematic review examining nonpharmacologic methods to prevent catheter‐related thrombosis specifically called for avoidance of PICC insertion to prevent thrombosis in hospitalized patients.[23] Despite this growing evidence base, the use of PICCs in the inpatient setting is likely to rise, and our survey highlights several practices that may contribute to adverse outcomes. For instance, hospitalists in our survey were unlikely to remove a PICC until a patient was discharged, irrespective of the need for this device. As each day with a PICC increases the risk of complications, such practice poses potential patient safety concerns. Similarly, many hospitalists believe that PICCs are safer than CVCs, a viewpoint that does not stand up to increasing scrutiny and highlights important knowledge gaps. The risk of PICC‐related complications appears not to be a stationary target, but rather a dynamic balance that is influenced by patient‐, provider‐, and device‐specific characteristics.[2] Increasing discretionary use (especially for patients with poor peripheral venous access), forgetting at times that a patient has a PICC, and the finding that up to 25% of PICCs placed in their hospitals may be unnecessary underscore concerns regarding the safety of current practice trends. Interestingly, the viewpoints of hospitalists in practice <5 years and those providing direct patient care were more likely to reflect concerns regarding inappropriate placement, influence of vascular nurses, and use of PICCs for blood culture. This finding may reflect that these nuances are more recent phenomena or perhaps most apparent when care is delivered directly.
Our study must be interpreted in the context of several limitations. First, as this was a survey‐based study of a small, convenience sample of hospitalists in a single state, recall, respondent, and systematic biases remain threats to our findings. However, all site PIs encouraged survey participation and (through local dialogue) none were aware of material differences between those who did or did not participate in the study. Similarly, Michigan is a diverse and relatively large state, and our results should be generalizable to other settings; however, national studies are necessary to confirm our findings. Second, our response rate may be perceived as low; however, our rates are in accordance with, and, in fact, superior to those of many existing physician surveys.[24] Finally, only 1 federal facility was included in this study; thus, this care‐delivery model is underrepresented, limiting generalization of findings to other such sites.
However, our study also has important strengths. First, this is the only survey that specifically examines hospitalist viewpoints when it comes to PICCs. As hospitalists frequently order and/or insert these devices, their perspectives are highly pertinent to discussions regarding current PICC use. Second, our survey highlights several instances that may be associated with preventable patient harm and identifies areas where interventions may be valuable. For example, forgetting the presence of a device, keeping PICCs in place throughout hospitalization, and rendering treatment for PICC‐related VTE not in accordance with accepted guidelines are remediable practices that may lead to poor outcomes. Interventions such as device‐reminder alerts, provider education regarding complications from PICCs, and systematic efforts to identify and remove unnecessary PICCs may mitigate these problems. Finally, our findings highlight the need for data repositories that track PICC use and hospitalist practice on a national scale. Given the risk and significance of the complications associated with these devices, understanding the epidemiology, use, and potential misuse of PICCs are important areas for hospitalist research.
In conclusion, our study of hospitalist experience, practice, opinions, and knowledge related to PICCs suggests important gaps between available evidence and current practice. There is growing need for the development of appropriateness criteria to guide vascular access in inpatient settings.[25, 26] Such criteria should consider not only type of venous access device, but granular details including rationale for venous access, nature of the infusate, optimal number of lumens, and safest gauge when recommending devices. Until such criteria and comparative studies become available, hospitals should consider instituting policies to monitor PICC use with specific attention to indication for insertion, duration of placement, and complications. These interventions represent a first and necessary step in improving patient safety when it comes to preventing PICC‐related complications.
Disclosures
The Blue Cross/Blue Shield of Michigan Foundation in Detroit funded this study through an investigator‐initiated research proposal (1931‐PIRAP). The funding source, however, played no role in study design, acquisition of data, data analysis, or reporting of these results. The authors report no conflicts of interest.
Peripherally inserted central catheters (PICCs) have become among the most common central venous catheters (CVCs) used in contemporary medical practice.[1] Although they were originally developed for delivery of parenteral nutrition, the use of PICCs has expanded to include chemotherapy administration, long‐term intravenous (IV) antibiotic treatment, and venous access when obtaining peripheral veins is difficult (eg, occluded peripheral veins, unusual venous anatomies).[2] Despite these roles, little is known about PICC use in hospitalized patients. This knowledge gap is important, as PICCs are placed in inpatient settings for a variety of reasons. Some of these reasons may not be appropriate, and inappropriate PICC use may worsen outcomes and increase healthcare costs.[3] In addition, PICCs are not innocuous and are frequently associated with important complications including thrombophlebitis, central‐lineassociated bloodstream infection and venous thromboembolism.[4, 5, 6] Therefore, understanding patterns and knowledge associated with PICC use is also an important patient safety concern.
As the main providers of inpatient care, hospitalists frequently order the insertion of PICCs and treat PICC‐related complications. Unfortunately, to date, no study has surveyed hospitalists regarding management or use of PICCs. Understanding hospitalist experiences, practice, opinions, and knowledge related to PICCs is therefore of significant interest when examining present‐day PICC use. To bridge this important knowledge gap and better understand these practices, we conducted a Web‐based survey of hospitalists in 5 healthcare systems in the state of Michigan.
METHODS
A convenience sample of hospitalists (N=227) was assembled from 5 large healthcare systems (representing 10 hospitals) that participate in the Hospital Medicine Safety (HMS) Consortium, a Blue Cross/Blue Shield of Michiganfunded statewide collaborative quality initiative. Individuals engaged in research, quality improvement, or leadership at HMS sites were invited to serve as site principal investigators (site PIs). Site PIs were responsible for obtaining regulatory approval at their parent facilities and disseminating the survey to providers in their group. Participation in the survey was solicited via e‐mail invitations from site PIs to hospitalists within their provider group. To encourage participation, a $10 electronic gift card was offered to respondents who successfully completed the survey. Reminder e‐mails were also sent each week by site PIs to augment participation. To enhance study recruitment, all responses were collected anonymously. The survey was administered between August 2012 and September 2012; data collection occurred for 5 weeks during this interval.
Survey questions were derived from our published, evidence‐based conceptual framework of PICC‐related complications. Briefly, this model identifies complications related to PICCs as arising from domains related to patient‐, provider‐, and device‐related characteristics based on existing evidence.[2] For our survey, questions were sourced from each of these domains so as to improve understanding of hospitalist experience, practice, opinions, and knowledge regarding PICC use. To ensure clarity of the survey questions, all questions were first pilot‐tested with a group of randomly selected hospitalist respondents at the University of Michigan Health System. Direct feedback obtained from these respondents was then used to iteratively improve each question. In order to generate holistic responses, questions were designed to generate a response reflective of the participants typical PICC use/subenario. We used SurveyMonkey to collect and manage survey data.
Statistical Analyses
Variation in hospitalist experience, reported practice, opinions, and knowledge regarding PICCs was assessed by hospitalist type (full time vs part time), years of practice (<1, 15, >5), and care‐delivery model (direct care vs learner‐based care). Bivariate comparisons were made using the 2 or Fisher exact tests as appropriate; 2‐sided with a P value <0.05 was considered statistically significant. All analyses were conducted using Stata version 11 (StataCorp, College Station, TX). Local institutional review board approval was obtained at each site participating in the survey.
RESULTS
A total of 227 surveys were administered and 144 responses collected, for a survey response rate of 63%. Each participating site had unique characteristics including size, number of hospitalists, and modality of PICC insertion (Table 1). Of the hospitalists who completed the survey, 81% held full‐time clinical positions and had been in practice an average of 5.6 years. Surveyed hospitalists reported caring for an average of 40.6 patients per week and ordering a mean of 2.9 (range, 015) PICCs per week of clinical service. Among survey respondents, 36% provided direct patient care, 34% provided care either directly or through mid‐level providers and housestaff, and 9% delivered care exclusively through mid‐level providers or housestaff (Table 2). As our survey was conducted anonymously, potential identifying information such as age, race, and sex of those responding was not collected.
| Survey Site | No. of Hospitals | No. of Inpatient Beds | No. of Annual Inpatient Encounters | No. of Hospitalists | Full‐Time Hospitalists, % | Avg. No. Weeks/Year on Service | Avg. Years of Experience | No. PICCs/Week, 2012 | Modality of PICC Insertion Available |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| University of Michigan Health System | 1 | 900+ | 5,775 | 46 | 100 | 25 | 6 | 42 | Vascular access nurse |
| Ann Arbor VA Medical Center | 1 | 135 | 825 | 16 | 50 | 17.6 | 5.1 | 12 | Vascular access nurse |
| Spectrum Health System | 2 | 800 | 14,000 | 47 | 80 | 34 | 3.75 | 56 | Interventional radiology |
| Trinity Health System | 3 | 634 | 2,300 | 67 | 80 | 24 | 4 | 31 | Interventional radiology and hospitalists |
| Henry Ford Health System | 3 | 1,150 | 1,450 | 51 | 100 | 20.4 | 5.6 | 15 | Vascular access nurse |
| Characteristic | Total (N=144) |
|---|---|
| |
| Hospitalist type, n (%) | |
| Full time | 117 (81) |
| Part time | 19 (13) |
| Unknown | 8 (6) |
| Weeks/year on a clinical service, n (%) | |
| <20 | 24 (17) |
| 20 | 107 (74) |
| Unknown | 13 (9) |
| Mean (SD) | 25.5 (10.7) |
| Median | 26 |
| Type of patients treated, n (%) | |
| Adults only | 129 (90) |
| Adults and children | 7 (5) |
| Unknown | 8 (6) |
| Years in practice as a hospitalist, n (%) | |
| 5 | 81 (56) |
| >5 | 54 (38) |
| Unknown | 9 (6) |
| Model of care delivery, n (%) | |
| Direct | 52 (36) |
| Some midlevel or housestaff providers (<50% of all encounters) | 49 (34) |
| Mostly midlevel or housestaff providers (>50% of all encounters) | 22 (15) |
| Only midlevel or housestaff providers | 13 (9) |
| Unknown | 8 (6) |
| Location of practice | |
| Trinity Health System | 39 (27) |
| University of Michigan Health System | 37 (26) |
| Henry Ford Health System | 28 (19) |
| Spectrum Health System | 21 (15) |
| Ann Arbor VA Medical Center | 11 (8) |
| Unknown | 8 (6) |
Hospitalist Experiences and Practice Related to Peripherally Inserted Central Catheters
According to responding hospitalists, the most common indications for PICC placement were long‐term IV antibiotic treatment (64%), followed by inability to obtain peripheral venous access (24%). Hospitalists reported an average duration of PICC placement of 17 days (range, 342 days). A significant percentage of hospitalists (93%) stated that they had cared for patients where a PICC was placed only for use during hospitalization, with the most common reason for such insertion being difficulty in otherwise securing venous access (67%). Respondents also reported caring for patients who had both PICCs and peripheral IV catheters in place at the same time; 49% stated that they had experienced this <5 times, whereas 33% stated they had experienced this 510 times. Furthermore, 87% of respondents indicated having admitted a patient who specifically requested a PICC due to prior difficulties with venous access. More than half of surveyed hospitalists (63%) admitted to having been contacted by a PICC nurse enquiring as to whether their patient might benefit from PICC insertion.
The majority of hospitalists (66%) reported that they specified the number of lumens when ordering PICCs. Thirty‐eight percent indicated that this decision was based on type of medication, whereas 35% selected the lowest number of lumens possible. A power PICC (specialized PICCs that are designed to withstand high‐pressure contrast injections), was specifically requested for radiographic studies (56%), infusion of large volume of fluids (10%), or was the default PICC type at their facility (34%).
A majority (74%) of survey respondents also reported that once inserted, PICCs were always used to obtain blood for routine laboratory testing. Moreover, 41% indicated that PICCs were also always used to obtain blood for microbiological cultures. The 3 most frequently encountered PICC‐related complications reported by hospitalists in our survey were blockage of a PICC lumen, bloodstream infection, and venous thromboembolism (VTE; Table 3).
| Hospitalist Experiences With PICCs | Total (N=144) |
|---|---|
| |
| Primary indication for PICC placement* | |
| Long‐term IV antibiotics | 64 |
| Venous access in a patient with poor peripheral veins | 24 |
| Parenteral nutrition | 5 |
| Chemotherapy | 4 |
| Patient specifically requested a PICC | 1 |
| Unknown/other | 2 |
| PICC placed only for venous access, n (%) | |
| Yes | 135 (94) |
| No | 9 (6) |
| PICC placed only during hospitalization, n (%) | |
| Yes | 134 (93) |
| No | 10 (7) |
| Notified by a PICC nurse (or other provider) that patient may need or benefit from a PICC, n (%) | |
| Yes | 91 (63) |
| No | 53 (37) |
| How frequently PICCs are used to obtain blood for routine laboratory testing, n (%) | |
| Always | 106 (74) |
| Unknown/other | 38 (26) |
| How frequently PICCs are used to obtain blood for blood cultures, n (%) | |
| Always | 59 (41) |
| Unknown/other | 85 (59) |
| Hospitalist Opinions on PICCs | Total (N=144) |
| In your opinion, is it appropriate to place a vascular in a hospitalized patient if other forms of peripheral access cannot be obtained? n (%) | |
| Yes | 121 (84) |
| No | 21 (15) |
| Unknown | 2 (1) |
| In your opinion, should hospitalists be trained to insert PICCs? n (%) | |
| No | 57 (40) |
| Yes, this is an important skill set for hospitalists | 46 (32) |
| Unsure | 39 (27) |
| Unknown/other | 2 (1) |
| Do you think the increasing number of vascular nurses and PICC nursing teams has influenced the use of PICCs in hospitalized patients? n (%) | |
| Yes | 112 (78) |
| No | 30 (21) |
| Unknown | 2 (1) |
| What % of PICC insertions do you think may represent inappropriate use in your hospital? n (%) | |
| <10 | 53 (37) |
| 1025 | 68 (47) |
| 2550 | 18 (13) |
| >50 | 3 (2) |
| Unknown/other | 2 (1) |
Hospitalist Opinions Regarding Peripherally Inserted Central Catheters
Compared with CVCs, 69% of hospitalists felt that PICCs were safer and more efficient because they could stay in place longer and were less likely to cause infection. Most (65%) also agreed that PICCs were more convenient than CVCs because they were inserted by PICC teams. Additionally, 74% of hospitalists felt that their patients preferred PICCs because they minimize pain from routine peripheral IV changes and phlebotomy. A majority of respondents (84%) indicated that it was appropriate to place a PICC if other forms of peripheral venous access could not be obtained. However, when specifically questioned, 47% of hospitalists indicated that at least 10%25% of PICCs placed in their hospitals might represent inappropriate use. A majority (78%) agreed with the statement that the increase in numbers of vascular nurses had influenced use of PICCs in hospitalized patients, but most (45%) were neutral when asked if PICCs were more cost‐effective than traditional CVCs.
Hospitalist Knowledge Regarding Risk of Peripherally Inserted Central CatheterRelated Venous Thromboembolism and Bloodstream Infection
Although 65% of responding hospitalists disagreed with the statement that PICCs were less likely to lead to VTE, important knowledge gaps regarding PICCs and VTE were identified (Table 4). For instance, only 4% of hospitalists were correctly aware that the PICC‐tip position is checked to reduce risk of PICC‐related VTE, and only 12% knew that the site of PICC insertion has also been associated with VTE risk. Although 85% of respondents stated they would prescribe a therapeutic dose of an anticoagulant in the case of PICC‐associated VTE, deviations from the guideline‐recommended 3‐month treatment period were noted. For example, 6% of hospitalists reported treating with anticoagulation for 6 months, and 19% stated they would treat as long as the PICC remained in place, plus an additional period of time (eg, 24 weeks) after removal. With respect to bloodstream infection, 92% of responding hospitalists correctly identified PICC duration and prompt removal as factors promoting PICC‐related bloodstream infection and 78% accurately identified components of the catheter‐associated bloodstream infection bundle. When specifically asked about factors associated with risk of PICC‐related bloodstream infection, only half of respondents recognized the number of PICC lumens as being associated with this outcome.
| Total (N=144) | |
|---|---|
| |
| Why is the position of the PICC tip checked after bedside PICC insertion? n (%) | |
| To decrease the risk of arrhythmia related to right‐atrial positioning | 108 (75) |
| To minimize the risk of VTEa | 6 (4) |
| To ensure it is not accidentally placed into an artery | 16 (11) |
| For documentation purposes (to reduce the risk of lawsuits related to line‐insertion complications) | 6 (4) |
| Unsure/Unknown | 8 (6) |
| According to the 2012 ACCP Guidelines on VTE prevention, is pharmacologic prophylaxis for DVT recommended in patients who receive long‐term PICCs? n (%) | |
| No; no anticoagulant prophylaxis is recommended for patients who receive long‐term PICCsa | 107 (74) |
| Yes, but the choice and duration of anticoagulant is at the discretion of the provider | 23 (16) |
| Yes; aspirin is recommended for 3 months | 4 (3) |
| Yes; anticoagulation with warfarin or enoxaparin is recommended for 3 months | 3 (2) |
| Yes; anticoagulation with warfarin or enoxaparin is recommended for 6 months | 2 (1) |
| Unknown | 5 (4) |
| Assuming no contraindications exist, do you anticoagulate patients who develop a PICC‐associated DVT (with any therapeutic anticoagulant)? n (%) | |
| Yesa | 122 (85) |
| No | 16 (11) |
| Unknown | 6 (4) |
| How long do you usually prescribe anticoagulation for patients who develop PICC‐associated DVT? n (%) | |
| I don't prescribe anticoagulation | 12 (8) |
| 1 month | 4 (3) |
| 3 monthsa | 84 (58) |
| 6 months | 8 (6) |
| As long as the line remains in place; I stop anticoagulation once the PICC comes out | 3 (2) |
| As long as the line remains in place and for an additional specified period of time after line removal, such as 2 or 4 weeks | 27 (19) |
| Unknown | 6 (4) |
| As part of the treatment of PICC‐related DVT, do you routinely remove the PICC?b n (%) | |
| Yes | 102 (71) |
| No | 36 (25) |
| Unknown | 6 (4) |
Variation in Hospitalist Knowledge, Experience, or Opinions
We assessed whether any of our findings varied according to hospitalist type (full time versus part time), years of practice (<1, 15, >5), and model of care delivery (direct care vs learner‐based care). Our analyses suggested that part‐time hospitalists were more likely to select rarely when it came to finding patients with a PICC and a working peripheral IV at the same time (74% vs 45%, P=0.02). Interestingly, a higher percentage of those in practice <5 years indicated that 10%25% of PICCs represented inappropriate placement (58% vs 33%, P<0.01) and that vascular nurses had influenced the use of PICCs in hospitalized patients (88% vs 69%, P=0.01). Lastly, a higher percentage of hospitalists who provided direct patient care reported that PICCs were always used to obtain blood for microbiological culture (54% vs 37%, P=0.05).
DISCUSSION
In this survey of hospitalists practicing at 5 large healthcare systems in Michigan, we observed significant variation in experience, reported practice, opinions, and knowledge related to PICCs. Our findings highlight important concerns related to inpatient PICC use and suggest a need for greater scrutiny related to these devices in these settings.
The use of PICCs in hospitalized patients has risen dramatically over the past decade. Though such growth is multifactorial and relates in part to increasing inpatient volume and complexity, hospitalists have increasingly turned to PICCs as a convenient and reliable tool to obtain venous access.[7] Indeed, in our survey, PICCs that were only used during hospitalization were most likely to be placed for this very reason. Because PICCs are safer to insert than CVCs and the original evidence regarding PICC‐related VTE or bloodstream infection suggested low rates of these events,[8, 9, 10, 11, 12, 13, 14] many hospitalists may not perceive these devices as being associated with significant risks. In fact, some have suggested that hospitalists be specifically trained to insert these devices, given their safety compared with traditional CVCs.[7]
However, accumulating evidence suggests that PICCs are associated with important complications.[5, 15, 16] In studies examining risk of bloodstream infection, PICCs were associated with significant risk of this outcome.[6, 17, 18] Recently, the presence of a PICC was identified as an independent predictor of VTE in hospitalized patients.[19] Several studies and systematic reviews have repeatedly demonstrated these findings.[19, 20, 21, 22] A recent systematic review examining nonpharmacologic methods to prevent catheter‐related thrombosis specifically called for avoidance of PICC insertion to prevent thrombosis in hospitalized patients.[23] Despite this growing evidence base, the use of PICCs in the inpatient setting is likely to rise, and our survey highlights several practices that may contribute to adverse outcomes. For instance, hospitalists in our survey were unlikely to remove a PICC until a patient was discharged, irrespective of the need for this device. As each day with a PICC increases the risk of complications, such practice poses potential patient safety concerns. Similarly, many hospitalists believe that PICCs are safer than CVCs, a viewpoint that does not stand up to increasing scrutiny and highlights important knowledge gaps. The risk of PICC‐related complications appears not to be a stationary target, but rather a dynamic balance that is influenced by patient‐, provider‐, and device‐specific characteristics.[2] Increasing discretionary use (especially for patients with poor peripheral venous access), forgetting at times that a patient has a PICC, and the finding that up to 25% of PICCs placed in their hospitals may be unnecessary underscore concerns regarding the safety of current practice trends. Interestingly, the viewpoints of hospitalists in practice <5 years and those providing direct patient care were more likely to reflect concerns regarding inappropriate placement, influence of vascular nurses, and use of PICCs for blood culture. This finding may reflect that these nuances are more recent phenomena or perhaps most apparent when care is delivered directly.
Our study must be interpreted in the context of several limitations. First, as this was a survey‐based study of a small, convenience sample of hospitalists in a single state, recall, respondent, and systematic biases remain threats to our findings. However, all site PIs encouraged survey participation and (through local dialogue) none were aware of material differences between those who did or did not participate in the study. Similarly, Michigan is a diverse and relatively large state, and our results should be generalizable to other settings; however, national studies are necessary to confirm our findings. Second, our response rate may be perceived as low; however, our rates are in accordance with, and, in fact, superior to those of many existing physician surveys.[24] Finally, only 1 federal facility was included in this study; thus, this care‐delivery model is underrepresented, limiting generalization of findings to other such sites.
However, our study also has important strengths. First, this is the only survey that specifically examines hospitalist viewpoints when it comes to PICCs. As hospitalists frequently order and/or insert these devices, their perspectives are highly pertinent to discussions regarding current PICC use. Second, our survey highlights several instances that may be associated with preventable patient harm and identifies areas where interventions may be valuable. For example, forgetting the presence of a device, keeping PICCs in place throughout hospitalization, and rendering treatment for PICC‐related VTE not in accordance with accepted guidelines are remediable practices that may lead to poor outcomes. Interventions such as device‐reminder alerts, provider education regarding complications from PICCs, and systematic efforts to identify and remove unnecessary PICCs may mitigate these problems. Finally, our findings highlight the need for data repositories that track PICC use and hospitalist practice on a national scale. Given the risk and significance of the complications associated with these devices, understanding the epidemiology, use, and potential misuse of PICCs are important areas for hospitalist research.
In conclusion, our study of hospitalist experience, practice, opinions, and knowledge related to PICCs suggests important gaps between available evidence and current practice. There is growing need for the development of appropriateness criteria to guide vascular access in inpatient settings.[25, 26] Such criteria should consider not only type of venous access device, but granular details including rationale for venous access, nature of the infusate, optimal number of lumens, and safest gauge when recommending devices. Until such criteria and comparative studies become available, hospitals should consider instituting policies to monitor PICC use with specific attention to indication for insertion, duration of placement, and complications. These interventions represent a first and necessary step in improving patient safety when it comes to preventing PICC‐related complications.
Disclosures
The Blue Cross/Blue Shield of Michigan Foundation in Detroit funded this study through an investigator‐initiated research proposal (1931‐PIRAP). The funding source, however, played no role in study design, acquisition of data, data analysis, or reporting of these results. The authors report no conflicts of interest.
- , , , et al. Hospital‐wide survey of the use of central venous catheters. J Hosp Infect. 2011;77(4):304–308.
- , , , , . Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733–741.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , , , et al. Reduction of peripherally inserted central catheter associated deep venous thrombosis [published online ahead of print August 1, 2012]. Chest. doi: 10.1378/chest.12–0923.
- , , . Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65–71.
- , , , et al. Patient‐ and device‐specific risk factors for peripherally inserted central venous catheter‐related bloodstream infections. Infect Control Hosp Epidemiol. 2013;34(2):184–189.
- , . Peripherally inserted central catheter use in the hospitalized patient: is there a role for the hospitalist? J Hosp Med. 2009;4(6):E1–E4.
- , , , , , . Peripherally inserted central catheter (PICC)‐associated upper‐extremity deep venous thrombosis (UEDVT) in critical‐care setting. Chest. 2005;128(4 suppl S):193S–194S.
- , , , , , . Complications and cost associated with parenteral nutrition delivered to hospitalized patients through either subclavian or peripherally inserted central catheters. Clin Nutr. 2000;19(4):237–243.
- , . Risk of catheter‐related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495.
- , , , . Long‐term intravenous therapy with peripherally inserted silicone elastomer central venous catheters in patients with malignant diseases. Cancer. 1979;43(5):1937–1943.
- , , , , . Central vs peripheral venous catheters in critically ill patients. Chest. 1986;90(6):806–809.
- , , , , . Infectious complications among patients receiving home intravenous therapy with peripheral, central, or peripherally placed central venous catheters. Am J Med. 1991;91(3B):95S–100S.
- , , , , . Upper‐extremity deep venous thrombosis and pulmonary embolism: a prospective study. Chest. 1991;99(2):280–283.
- , , , et al. Risk factors for catheter‐related thrombosis (CRT) in cancer patients: a patient‐level data (IPD) meta‐analysis of clinical trials and prospective studies. J Thromb Haemost. 2011;9(2):312–319.
- , , , et al. Venous thrombosis associated with peripherally inserted central catheters: a retrospective analysis of the Cleveland Clinic experience. Clin Infect Dis. 2002;34(9):1179–1183.
- , , , et al. Peripherally inserted central venous catheter–associated bloodstream infections in hospitalized adult patients. Infect Control Hosp Epidemiol. 2011;32(2):125–130.
- , , . Peripherally inserted central catheter bloodstream infection surveillance rates in an acute care setting in Saudi Arabia. Ann Saudi Med. 2012;32(2):169–173.
- , , , et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947.e942–954.e942.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810.
- , , . The clinical significance of peripherally inserted central venous catheter‐related deep vein thrombosis. Neurocrit Care. 2011;15(3):454–460.
- , , , et al. Catheter‐associated bloodstream infection incidence and risk factors in adults with cancer: a prospective cohort study. J Hosp Infect. 2011;78(1):26–30.
- , , , . Nonpharmacologic interventions for prevention of catheter‐related thrombosis: a systematic review [published online ahead of print September 13, 2012]. J Crit Care. doi: 10.1016/j.jcrc.2012.07.007.
- , , . Why are response rates in clinician surveys declining? Can Fam Physician. 2012;58(4):e225–e228.
- , , , , , . Sensitivity and specificity of the RAND/UCLA Appropriateness Method to identify the overuse and underuse of coronary revascularization and hysterectomy. J Clin Epidemiol. 2001;54(10):1004–1010.
- , , , et al. Variations by specialty in physician ratings of the appropriateness and necessity of indications for procedures. Med Care. 1996;34(6):512–523.
- , , , et al. Hospital‐wide survey of the use of central venous catheters. J Hosp Infect. 2011;77(4):304–308.
- , , , , . Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733–741.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , , , et al. Reduction of peripherally inserted central catheter associated deep venous thrombosis [published online ahead of print August 1, 2012]. Chest. doi: 10.1378/chest.12–0923.
- , , . Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65–71.
- , , , et al. Patient‐ and device‐specific risk factors for peripherally inserted central venous catheter‐related bloodstream infections. Infect Control Hosp Epidemiol. 2013;34(2):184–189.
- , . Peripherally inserted central catheter use in the hospitalized patient: is there a role for the hospitalist? J Hosp Med. 2009;4(6):E1–E4.
- , , , , , . Peripherally inserted central catheter (PICC)‐associated upper‐extremity deep venous thrombosis (UEDVT) in critical‐care setting. Chest. 2005;128(4 suppl S):193S–194S.
- , , , , , . Complications and cost associated with parenteral nutrition delivered to hospitalized patients through either subclavian or peripherally inserted central catheters. Clin Nutr. 2000;19(4):237–243.
- , . Risk of catheter‐related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495.
- , , , . Long‐term intravenous therapy with peripherally inserted silicone elastomer central venous catheters in patients with malignant diseases. Cancer. 1979;43(5):1937–1943.
- , , , , . Central vs peripheral venous catheters in critically ill patients. Chest. 1986;90(6):806–809.
- , , , , . Infectious complications among patients receiving home intravenous therapy with peripheral, central, or peripherally placed central venous catheters. Am J Med. 1991;91(3B):95S–100S.
- , , , , . Upper‐extremity deep venous thrombosis and pulmonary embolism: a prospective study. Chest. 1991;99(2):280–283.
- , , , et al. Risk factors for catheter‐related thrombosis (CRT) in cancer patients: a patient‐level data (IPD) meta‐analysis of clinical trials and prospective studies. J Thromb Haemost. 2011;9(2):312–319.
- , , , et al. Venous thrombosis associated with peripherally inserted central catheters: a retrospective analysis of the Cleveland Clinic experience. Clin Infect Dis. 2002;34(9):1179–1183.
- , , , et al. Peripherally inserted central venous catheter–associated bloodstream infections in hospitalized adult patients. Infect Control Hosp Epidemiol. 2011;32(2):125–130.
- , , . Peripherally inserted central catheter bloodstream infection surveillance rates in an acute care setting in Saudi Arabia. Ann Saudi Med. 2012;32(2):169–173.
- , , , et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947.e942–954.e942.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810.
- , , . The clinical significance of peripherally inserted central venous catheter‐related deep vein thrombosis. Neurocrit Care. 2011;15(3):454–460.
- , , , et al. Catheter‐associated bloodstream infection incidence and risk factors in adults with cancer: a prospective cohort study. J Hosp Infect. 2011;78(1):26–30.
- , , , . Nonpharmacologic interventions for prevention of catheter‐related thrombosis: a systematic review [published online ahead of print September 13, 2012]. J Crit Care. doi: 10.1016/j.jcrc.2012.07.007.
- , , . Why are response rates in clinician surveys declining? Can Fam Physician. 2012;58(4):e225–e228.
- , , , , , . Sensitivity and specificity of the RAND/UCLA Appropriateness Method to identify the overuse and underuse of coronary revascularization and hysterectomy. J Clin Epidemiol. 2001;54(10):1004–1010.
- , , , et al. Variations by specialty in physician ratings of the appropriateness and necessity of indications for procedures. Med Care. 1996;34(6):512–523.
Copyright © 2013 Society of Hospital Medicine
Hospitalist Care and Patient Satisfaction
Payers and policymakers are increasingly holding hospitals accountable for patients' experiences with their care. Since 2006, the Centers for Medicare and Medicaid Services (CMS) have collected data on patients' experiences with inpatient care using the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey, a well‐validated and widely used tool. In 2008, these data on patient experience began to be publicly reported, and CMS now plans to base part of its payments to hospitals on HCAHPS performance scores. In this context, hospitals are looking for ways to improve patient satisfaction.
The effort to hold hospitals accountable for patient experience may conflict with another major trend in US hospitals: the increasing use of hospitalists.[1] Although hospitalists may have greater expertise in the day‐to‐day care of the hospitalized patient, they generally do not know the patient and cannot cater to patients' preferences in ways that the primary‐care provider might. Therefore, given that patients may prefer to be seen by their primary‐care provider,[2] greater use of hospitalists may actually lead to a decrease in patient satisfaction. Unfortunately, we are unaware of any national examination of the relationship between hospitalist use in an institution and that entity's performance on patient‐experience scores.
To better understand the relationship between greater hospitalist staffing and patient‐centered care, we examined the association between hospitalist staffing and patient satisfaction with both overall care and specific domains of patient‐centered care. We hypothesized that hospitals that used a high proportion of hospitalists would generally have lower patient‐experience scores. Further, we expected that the relationship would be monotonic (greater use of hospitalists associated with lower scores) and particularly pronounced in 2 domains: patient experience with discharge planning and patient experience with physician communication.
METHODS
Data
We sought to identify acute‐care hospitals with elderly medical patients cared for by hospitalists, non‐hospitalists, or some combination of the 2. To construct this cohort, we used 3 2009 Medicare files. The Beneficiary Summary File contains demographic information on Medicare beneficiaries and data on enrollment in managed‐care plans. To identify medical hospitalizations, we used the Medicare Provider Analysis and Review (MedPAR) 100% Files, which contain the clinical diagnoses and payments for all fee‐for‐service Medicare beneficiaries discharged from acute‐care hospitals. To identify hospitalists and non‐hospitalists, we used the 5% Carrier File, which contains physician billing data for a 5% random sample of fee‐for‐service Medicare beneficiaries. We also obtained information on hospital characteristics from the American Hospital Association (AHA) Annual Survey. We supplemented this with hospital‐level data on patient satisfaction from the HCAHPS survey conducted in 2009. The HCAHPS is a standard survey developed by the Agency for Healthcare Research and Quality (AHRQ) and administered by hospitals to a random sample of adult patients 48 hours to 6 weeks after discharge. The HCAHPS results are adjusted for patient mix and have been tested for nonresponse bias.[3] Details about the development and design of HCAHPS have been described previously.[4]
Patient and Hospital Sample
We started with 48,861,000 Medicare beneficiaries in the Beneficiary Summary File and excluded 38% either because their age was <65 years or they were members of an HMO. At the same time, from the 1,850,000 patients in the 5% Carrier File, we excluded 55% who had not been cared for by a general internist. Finally, we used the MedPAR File to identify 17,387,000 hospital admissions by fee‐for‐service Medicare beneficiaries. From MedPAR, we excluded admissions to a facility other than an acute‐care hospital (24%), surgical admissions identified by diagnosis‐related group (DRG) (29%), and admissions to hospitals with <5 medicine admissions in 2009 (<0.1%). After merging these 3 files (Beneficiary Summary, MedPAR, and 5% Carrier), we were left with 229,496 admissions among 180,399 patients at 3365 hospitals. We subsequently excluded readmissions and were left with 156,333 admissions at 3244 hospitals. Finally, we excluded those patients cared for by both hospitalists and non‐hospitalists during the same hospitalization, and those hospitals missing AHA or HCAHPS data, leaving us with 132,814 patients at 2843 hospitals.
Definition of Hospitalist
We used the claims‐based definition developed and validated by Kuo and Goodwin in earlier work.[1] Hospitalists are defined as those general internists (providers in general practice or internal medicine) who had 5 evaluation and management (E&M) billings (in a 5% sample of Medicare beneficiaries) in 2009 and generated >90% of their claims from the care of hospitalized patients in 2009.
Measures of Patient Satisfaction
There are 2 HCAHPS questions about overall satisfaction, one that asks patients to rate their experience on a scale of 0 to 10 and another that asks whether they would recommend the hospital. Not surprisingly, hospitals' performance on these 2 questions is highly correlated.[5] We measured overall patient experience using commonly used approaches: the proportion of patients who gave the hospital a 9 or 10 (on the 10‐point scale) or the proportion of patients who reported that they would definitely recommend the hospital. The HCAHPS also contains 24 questions, which are reported by CMS in 8 domains: communication with nurse, communication with physician, responsiveness of the staff, pain control, communication about medications, adequacy of discharge planning, cleanliness of the room, and quietness of the room. The patient‐satisfaction score for each of these domains represents the proportion of patients who answered always to each of the questions, or who answered yes to the question about discharge.
Potentially Confounding Variables
Because we were worried that hospitals with hospitalists would be different from hospitals without hospitalists, we identified a series of covariates for adjustment in a multivariable model. We extracted data from the AHA on hospitals' structural characteristics that we assumed might be associated both with having a hospitalist and with patient experience. These variables were size (number of beds), teaching status (membership in the Council of Teaching Hospitals vs no membership), location (urban vs rural), region (the 4 census regions), ownership (for profit, private nonprofit, or public), and presence of advanced clinical capabilities (as measured by having a medical, surgical, and/or cardiac intensive care unit [ICU]). We also used information about the patient population (proportion of patients with Medicare or with Medicaid) as well as nurse‐staffing level (ratio of full‐time equivalent registered nurses to total hospital beds).
Statistical Analyses
We first quantified hospital variation in the proportion of general‐medicine patients cared for by hospitalists, using basic descriptive statistics. Based on these analyses, we categorized hospitals into 3 groups: non‐hospitalist, mixed, and hospitalist (corresponding to lowest, middle, and highest tertile of hospitalist use respectively). We used bivariate techniques to describe the patient and hospital characteristics of hospitals in each group. Patient characteristics included number of comorbidities, which were calculated using software from the Healthcare Cost and Utilization Project (HCUP),[6] based on methods developed by Elixhauser et al.[7] We used the ‐square test to assess whether hospital or patient characteristics differed between hospitalist, mixed, and non‐hospitalist hospitals.
To examine the association between hospitalist care and patient satisfaction, we first constructed bivariate models for each measure of patient satisfaction. In these models, hospital type (hospitalist, mixed, and non‐hospitalist) was our predictor. We next constructed multivariable models, which adjusted for each of the hospital characteristics described above in order to assess the independent relationship between hospitalist care and HCAHPS performance.
In sensitivity analyses, we first examined hospitalist use as a continuous variable and had qualitatively very similar results. Those data are not presented. Additionally, we conducted a propensity score analysis, with results presented in the Appendix (see Supporting Information, Appendix 1, in the online version of this article). In our first‐stage logistic regression model, being a hospitalist hospital (defined as being in the top tertile of hospitalist use vs bottom 2 tertiles) was the outcome. Hospital structural factors were covariates. Based on this first‐stage model, each hospital was assigned a propensity of being a hospitalist hospital. We divided the hospitals into 3 groups (highest propensity tertile, middle propensity tertile, and lowest propensity tertile). In a second‐stage linear regression model, patient satisfaction score was the outcome. The predictors were hospital type (dichotomized, and defined as being in the top tertile of hospitalist use vs bottom 2 tertiles), and propensity of being a hospitalist hospital (3 categories, with low propensity as the reference).
All analyses were performed using SAS version 9.2. The project was reviewed by the Institutional Review Board at the University of Michigan and determined to be not regulated given our use of publicly available datasets.
RESULTS
Among all hospitals, the median proportion of general‐medicine admissions cared for by hospitalists was 41.2% (interquartile range [IQR], 11.5%67.4%). However, US hospitals varied widely in the proportion of general‐medicine patients cared for by hospitalists (Figure 1). Whereas 3.5% of hospitals had all of their general‐medicine patients cared for by hospitalists, 16.6% had none of their general‐medicine patients seen by hospitalists. For hospitals with at least some hospitalist care, the proportion of patients cared for by hospitalists was distributed fairly evenly across the range of possibilities (Figure 1).
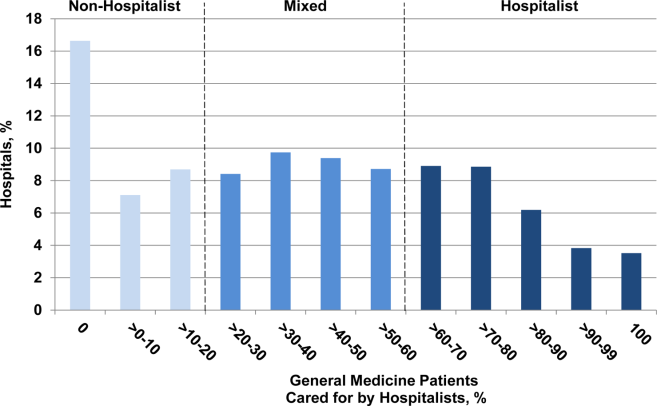
Because hospitalist care varied widely among hospitals, we categorized hospitals into 3 groups (non‐hospitalist, mixed, and hospitalist). The median proportion of patients cared for by hospitalists in the 3 groups was 0%, 39.5%, and 76.5%, respectively (Table 1). The non‐hospitalist hospitals, when compared with mixed and hospitalist hospitals, were more likely to be small, nonteaching, for‐profit institutions located in the Midwestern United States. They also were less likely to have an ICU and had lower nurse‐to‐bed ratios.
| Hospital Characteristics | Hospital Type | P Value | ||
|---|---|---|---|---|
| Non‐Hospitalist (N = 943) | Mixed (N = 948) | Hospitalist (N = 952) | ||
| ||||
| GM admissions cared for by hospitalists, median (range), % | 0 (021) | 40 (2158) | 77 (58100) | <0.001 |
| Nurse‐to‐bed ratio | 1 | 1 | 2 | <0.001 |
| Presence of MICU, % | 79 | 84 | 85 | 0.001 |
| Medicaid patients, % | 19 | 18 | 18 | 0.06 |
| Hospital beds, % | <0.001 | |||
| Small (99) | 36 | 16 | 24 | |
| Medium (100399) | 59 | 64 | 58 | |
| Large (400) | 6 | 21 | 18 | |
| COTH membership, % | <0.001 | |||
| Yes | 3 | 13 | 11 | |
| No | 97 | 87 | 89 | |
| Urban, % | 0.10 | |||
| Yes | 88 | 89 | 91 | |
| No | 12 | 11 | 9 | |
| Profit status, % | <0.001 | |||
| For profit | 21 | 17 | 18 | |
| Not for profit, private | 62 | 71 | 67 | |
| Other | 18 | 12 | 15 | |
| Region, % | <0.001 | |||
| South | 41 | 42 | 42 | |
| Northeast | 14 | 21 | 16 | |
| Midwest | 30 | 22 | 18 | |
| West | 15 | 15 | 24 | |
The types of patients cared for at all 3 hospital types (non‐hospitalist, mixed, and hospitalist) were similar in age and day of admission (Table 2). Patients cared for at non‐hospitalist hospitals were slightly more likely to be female and non‐White, and less likely to be admitted from the emergency department or another hospital or healthcare facility.
| Patient Characteristics | Hospital Type | P Value | ||
|---|---|---|---|---|
| Non‐Hospitalist (N = 33,265) | Mixed (N = 52,844) | Hospitalist (N = 46,705) | ||
| ||||
| Age, y | 0.51 | |||
| 6574 | 27 | 27 | 27 | |
| 7584 | 39 | 39 | 39 | |
| 85 | 34 | 34 | 34 | |
| Sex | <0.001 | |||
| M | 35 | 35 | 36 | |
| F | 65 | 65 | 64 | |
| Race/ethnicity | <0.001 | |||
| White | 85 | 85 | 87 | |
| Black | 10 | 11 | 9 | |
| Other | 5 | 4 | 4 | |
| Unknown | 0 | 0 | 0 | |
| Comorbidities, % | <0.001 | |||
| 0 | 8 | 8 | 7 | |
| 1 | 23 | 23 | 22 | |
| 2+ | 69 | 69 | 71 | |
| Day of admission | 0.08 | |||
| Weekday | 73 | 73 | 73 | |
| Weekend | 27 | 27 | 27 | |
| Admission source | <0.001 | |||
| ED | 75 | 78 | 80 | |
| Another ACH | 1 | 2 | 3 | |
| Other healthcare facility | 4 | 4 | 4 | |
| Other | 20 | 17 | 13 | |
| ICU stay | <0.001 | |||
| Yes | 13 | 12 | 12 | |
| No | 87 | 88 | 88 | |
| Length of stay, d | <0.001 | |||
| Median (Q1, Q3) | 4 (3, 6) | 4 (2, 6) | 3 (2, 5) | |
| DRG | <0.001 | |||
| Septicemia or severe sepsis | 3 | 4 | 4 | |
| Esophagitis, gastroenteritis | 3 | 3 | 3 | |
| Kidney and urinary tract infections | 3 | 3 | 3 | |
| Syncope | 3 | 3 | 3 | |
| Pneumonia | 3 | 3 | 3 | |
When we examined unadjusted relationships between type of hospital and patient experience, we found that patients at hospitalist vs non‐hospitalist hospitals were more likely to recommend the hospital (69.4% vs 65.1%: P < 0.001), and report higher overall satisfaction (65.9% vs 63.6%: P < 0.001) ((see Supporting Information, Appendix, Table A1, in the online version of this article)). Care at hospitalist hospitals was associated with higher satisfaction with discharge, but lower satisfaction with room cleanliness and communication with doctors. These differences were statistically significant at the P < 0.05 level.
When we examined the relationship between having more hospitalists and patient experience using multivariable models that accounted for differences in hospital characteristics, we found largely similar results: The proportion of patients who were satisfied with their overall care was still higher at hospitalist compared with non‐hospitalist hospitals (65.6% vs 63.9%: P < 0.001) (Figure 2). Similarly, patients were more likely to definitely recommend their hospital if they had been cared for at a hospitalist vs non‐hospitalist hospital (66.0% vs 63.4%: P < 0.001).
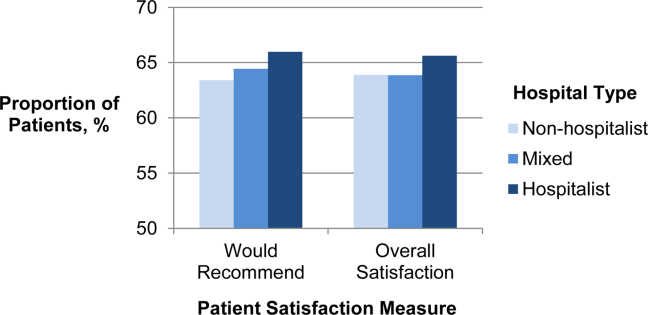
To better understand which domains of care might be contributing to greater overall satisfaction, we also examined patient satisfaction with specific domains of care at hospitalist vs non‐hospitalist hospitals (Table 3) in our adjusted analyses. Among 8 domains, the largest difference in satisfaction between patients cared for at hospitalist vs non‐hospitalist hospitals occurred with discharge. At hospitalist hospitals, 80.3% of patients said they were satisfied with the quality of the discharge planning compared with 78.1% at non‐hospitalist hospitals (P < 0.001). Patients at hospitalist hospitals were more satisfied with most other domains of care as well. Patients cared for at hospitalist hospitals were slightly less likely to be satisfied with communication with doctors, but this difference was not statistically significant (P = 0.45). Results were qualitatively similar in propensity‐score analyses (see Supporting Information, Appendix, Table A2, in the online version of this article).
| Specific Domains of Care | Hospital Type, % Satisfied | Hospitalist vs Non‐Hospitalist | |||
|---|---|---|---|---|---|
| Non‐Hospitalist | Mixed | Hospitalist | Difference in % Satisfied | P Value | |
| |||||
| Discharge | 78.1 | 79.1 | 80.3 | 2.1 | <0.001 |
| Nursing services | 66.0 | 65.8 | 67.1 | 1.1 | <0.001 |
| Quiet | 63.3 | 63.1 | 64.4 | 1.1 | 0.001 |
| Communication, nurse | 76.7 | 76.7 | 77.7 | 1.0 | <0.001 |
| Pain control | 69.7 | 69.7 | 70.4 | 0.7 | 0.001 |
| Medications | 60.5 | 60.5 | 61.2 | 0.7 | 0.002 |
| Cleanliness | 72.7 | 72.1 | 72.9 | 0.2 | 0.56 |
| Communication, physician | 83.6 | 83.1 | 83.5 | 0.2 | 0.45 |
DISCUSSION
We found that in 2009, US hospitals varied widely in the proportion of general medicine patients cared for by hospitalists. Hospitals with higher levels of hospitalist care did better on most measures of patient satisfaction. Differences were largest in overall satisfaction and for discharge planning. In 5 other domains of care, differences were smaller, but hospitals with more hospitalist care consistently performed better than non‐hospitalist hospitals. Hospitalist care was not associated with patient satisfaction in 2 domains: communication with doctors and cleanliness of room.
Our findings of modestly higher patient satisfaction at hospitalist hospitals along most dimensions of care are surprising and reassuring. Indeed, when hospitalists first began caring for inpatients, some expressed concerns that hospitalist care would decrease patient satisfaction.[8, 9] Though this has been an ongoing concern, we found no evidence to support this contention. It may be that as a response to the concern, hospitals with hospitalists have paid particular attention to issues such as effective handoffs to primary‐care providers.[10, 11, 12, 13] Whether due to these efforts or other factors such as the 24/7 inpatient presence of hospitalists, we found that patients at hospitalist hospitals were more likely to be satisfied with their inpatient care, including their experience at discharge. In contrast, one area that may offer room for improvement for hospitalist hospitals is communication with physicians. It may be that patients cared for by hospitalists do not know their physicians as well as patients whose care is being orchestrated by their primary‐care provider, and thus the benefits of having an ever‐present hospitalist are diminished.
The magnitude of the associations that we found should also be placed in the context of existing research on patient satisfaction. Prior work has described baseline hospital performance, changes over time, and factors associated with greater inpatient satisfaction.[5, 14, 15] The associations that we found between hospitalist care and satisfaction with care at discharge were larger than those found for teaching (vs non‐teaching) hospitals.[5] However, compared with other hospital characteristics such as nurse staffing or profit status, hospitalist care was associated with smaller differences in patient satisfaction. In one study, hospitals in the highest quartile of nurse staffing had HCAHPS scores (ie, willingness to recommend measure) that were 6.7 points higher than those in the lowest quartile of nurse staffing, and similar differences existed between not‐for‐profit, public hospitals vs for‐profit hospitals.[5]
Taken together, our findings address an important gap in knowledge about hospitalist care. Prior research has documented growth in the use of hospitalist care[1] and described the association of hospitalist care with outcomes such as mortality and resource use, and receipt of recommended care.[16, 17, 18, 19] However, we are unaware of any national study that has examined the association of hospitalist care with patient satisfaction. One study surveyed patients in a single health system and found that patients were similarly satisfied with care provided by hospitalists and primary‐care physicians.[20] Our findings should be reassuring to clinical leaders and policymakers who have advocated greater use of hospitalists: the results suggest that there need be no tradeoff between greater use of hospitalist services and patient satisfaction. Indeed, patients appear to be even more satisfied in hospitals that have greater use of hospitalist physicians.
Our study has several limitations. First, it was a cross‐sectional study, and thus we cannot make any conclusions about causality. Although we adjusted for several potential confounders (eg, teaching status, advanced care capabilities, nurse staffing), it is possible that hospitalist care is a surrogate marker for features of hospitals that we could not measure but that directly influence patient experience. In addition, it is possible that patients cared for at hospitalist hospitals differ in unmeasured ways from patients cared for at other types of hospitals. Second, we constructed our primary predictor and outcome from different cohorts. Our primary predictor was derived from the proportion of general‐medicine patients cared for by hospitalists in Medicare claims data. In contrast, our primary outcome was based on HCAHPS responses from a random sampling of all hospital admissions. This misclassification likely would have biased us towards finding small or no associations. Therefore, we are likely underestimating the true association between hospitalist use and patient experience. Third, our findings may not be generalizable to hospitals that serve younger patients or have a large number of specialist hospitalists (who were not included in our definition of hospitalists). For example, compared with older patients with multiple comorbidities, relatively healthy younger patients may derive less benefit from an ever‐present hospitalist who can explain discharge plans or an attentive nurse.
In summary, we found that US hospitals varied widely in their use of hospitalist physicians, and those which a greater proportion of care was delivered by hospitalists generally had better scores on patient experience, especially on the global assessment of satisfaction and in discharge care. Our findings suggest that adoption of the hospitalist modelone of the strategies employed by US hospitals in the past 2 decades to provide efficient careshould not detract from achieving the goal of more patient‐centered care.
Disclosures
Dr. Chen's work is supported in part by the National Institutes of Health/National Institute on Aging (AG024824, University of Michigan Claude D. Pepper Older Americans Independence Center), and the National Institutes of Health/National Center for Research Resources (UL1‐RR024986, Michigan Institute for Clinical and Health Research). Dr. Chen is also supported by a Career Development Grant Award (K08HS020671) from the Agency for Healthcare Research and Quality.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , , . How do patients view the role of the primary care physician in inpatient care? Dis Mon. 2002;48(4):230–238.
- , , , et al. Effects of survey mode, patient mix, and nonresponse on CAHPS hospital survey scores. Health Serv Res. 2009;44(2 pt 1):501–518.
- , , , , . Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27–37.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921–1931.
- Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP). HCUP Comorbidity Software. http://www.hcup‐us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp Accessed November 12, 2012.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- , , . Physician views on caring for hospitalized patients and the hospitalist model of inpatient care. J Gen Intern Med. 2001;16(2):116–119.
- , , , . How physicians perceive hospitalist services after implementation: anticipation vs reality. Arch Intern Med. 2003;163(19):2330–2336.
- , , , et al. Association of communication between hospital‐based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381–386.
- , , . Passing the clinical baton: 6 principles to guide the hospitalist. Dis Mon. 2002;48(4):260–266.
- , , , . Primary care physician attitudes regarding communication with hospitalists. Am J Med. 2001;111(9B):15S–20S.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364–370.
- , , , et al. Hospital survey shows improvements in patient experience. Health Aff (Millwood). 2010;29(11):2061–2067.
- , , , , , . Characteristics of hospitals demonstrating superior performance in patient experience and clinical process measures of care. Med Care Res Rev. 2010;67(1):38–55.
- , . The impact of hospitalists on the cost and quality of inpatient care in the United States: a research synthesis. Med Care Res Rev. 2005;62(4):379–406.
- , , , , . Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists. Arch Intern Med. 2002;162(11):1251–1256.
- , , , , . Hospitalists and the quality of care in hospitals. Arch Intern Med. 2009;169(15):1389–1394.
- , , , et al. Quality of care for decompensated heart failure: comparable performance between academic hospitalists and non‐hospitalists. J Gen Intern Med. 2008;23(9):1399–1406.
- , , , et al. Patient satisfaction with hospital care provided by hospitalists and primary care physicians. J Hosp Med. 2012;7(2):131–136.
Payers and policymakers are increasingly holding hospitals accountable for patients' experiences with their care. Since 2006, the Centers for Medicare and Medicaid Services (CMS) have collected data on patients' experiences with inpatient care using the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey, a well‐validated and widely used tool. In 2008, these data on patient experience began to be publicly reported, and CMS now plans to base part of its payments to hospitals on HCAHPS performance scores. In this context, hospitals are looking for ways to improve patient satisfaction.
The effort to hold hospitals accountable for patient experience may conflict with another major trend in US hospitals: the increasing use of hospitalists.[1] Although hospitalists may have greater expertise in the day‐to‐day care of the hospitalized patient, they generally do not know the patient and cannot cater to patients' preferences in ways that the primary‐care provider might. Therefore, given that patients may prefer to be seen by their primary‐care provider,[2] greater use of hospitalists may actually lead to a decrease in patient satisfaction. Unfortunately, we are unaware of any national examination of the relationship between hospitalist use in an institution and that entity's performance on patient‐experience scores.
To better understand the relationship between greater hospitalist staffing and patient‐centered care, we examined the association between hospitalist staffing and patient satisfaction with both overall care and specific domains of patient‐centered care. We hypothesized that hospitals that used a high proportion of hospitalists would generally have lower patient‐experience scores. Further, we expected that the relationship would be monotonic (greater use of hospitalists associated with lower scores) and particularly pronounced in 2 domains: patient experience with discharge planning and patient experience with physician communication.
METHODS
Data
We sought to identify acute‐care hospitals with elderly medical patients cared for by hospitalists, non‐hospitalists, or some combination of the 2. To construct this cohort, we used 3 2009 Medicare files. The Beneficiary Summary File contains demographic information on Medicare beneficiaries and data on enrollment in managed‐care plans. To identify medical hospitalizations, we used the Medicare Provider Analysis and Review (MedPAR) 100% Files, which contain the clinical diagnoses and payments for all fee‐for‐service Medicare beneficiaries discharged from acute‐care hospitals. To identify hospitalists and non‐hospitalists, we used the 5% Carrier File, which contains physician billing data for a 5% random sample of fee‐for‐service Medicare beneficiaries. We also obtained information on hospital characteristics from the American Hospital Association (AHA) Annual Survey. We supplemented this with hospital‐level data on patient satisfaction from the HCAHPS survey conducted in 2009. The HCAHPS is a standard survey developed by the Agency for Healthcare Research and Quality (AHRQ) and administered by hospitals to a random sample of adult patients 48 hours to 6 weeks after discharge. The HCAHPS results are adjusted for patient mix and have been tested for nonresponse bias.[3] Details about the development and design of HCAHPS have been described previously.[4]
Patient and Hospital Sample
We started with 48,861,000 Medicare beneficiaries in the Beneficiary Summary File and excluded 38% either because their age was <65 years or they were members of an HMO. At the same time, from the 1,850,000 patients in the 5% Carrier File, we excluded 55% who had not been cared for by a general internist. Finally, we used the MedPAR File to identify 17,387,000 hospital admissions by fee‐for‐service Medicare beneficiaries. From MedPAR, we excluded admissions to a facility other than an acute‐care hospital (24%), surgical admissions identified by diagnosis‐related group (DRG) (29%), and admissions to hospitals with <5 medicine admissions in 2009 (<0.1%). After merging these 3 files (Beneficiary Summary, MedPAR, and 5% Carrier), we were left with 229,496 admissions among 180,399 patients at 3365 hospitals. We subsequently excluded readmissions and were left with 156,333 admissions at 3244 hospitals. Finally, we excluded those patients cared for by both hospitalists and non‐hospitalists during the same hospitalization, and those hospitals missing AHA or HCAHPS data, leaving us with 132,814 patients at 2843 hospitals.
Definition of Hospitalist
We used the claims‐based definition developed and validated by Kuo and Goodwin in earlier work.[1] Hospitalists are defined as those general internists (providers in general practice or internal medicine) who had 5 evaluation and management (E&M) billings (in a 5% sample of Medicare beneficiaries) in 2009 and generated >90% of their claims from the care of hospitalized patients in 2009.
Measures of Patient Satisfaction
There are 2 HCAHPS questions about overall satisfaction, one that asks patients to rate their experience on a scale of 0 to 10 and another that asks whether they would recommend the hospital. Not surprisingly, hospitals' performance on these 2 questions is highly correlated.[5] We measured overall patient experience using commonly used approaches: the proportion of patients who gave the hospital a 9 or 10 (on the 10‐point scale) or the proportion of patients who reported that they would definitely recommend the hospital. The HCAHPS also contains 24 questions, which are reported by CMS in 8 domains: communication with nurse, communication with physician, responsiveness of the staff, pain control, communication about medications, adequacy of discharge planning, cleanliness of the room, and quietness of the room. The patient‐satisfaction score for each of these domains represents the proportion of patients who answered always to each of the questions, or who answered yes to the question about discharge.
Potentially Confounding Variables
Because we were worried that hospitals with hospitalists would be different from hospitals without hospitalists, we identified a series of covariates for adjustment in a multivariable model. We extracted data from the AHA on hospitals' structural characteristics that we assumed might be associated both with having a hospitalist and with patient experience. These variables were size (number of beds), teaching status (membership in the Council of Teaching Hospitals vs no membership), location (urban vs rural), region (the 4 census regions), ownership (for profit, private nonprofit, or public), and presence of advanced clinical capabilities (as measured by having a medical, surgical, and/or cardiac intensive care unit [ICU]). We also used information about the patient population (proportion of patients with Medicare or with Medicaid) as well as nurse‐staffing level (ratio of full‐time equivalent registered nurses to total hospital beds).
Statistical Analyses
We first quantified hospital variation in the proportion of general‐medicine patients cared for by hospitalists, using basic descriptive statistics. Based on these analyses, we categorized hospitals into 3 groups: non‐hospitalist, mixed, and hospitalist (corresponding to lowest, middle, and highest tertile of hospitalist use respectively). We used bivariate techniques to describe the patient and hospital characteristics of hospitals in each group. Patient characteristics included number of comorbidities, which were calculated using software from the Healthcare Cost and Utilization Project (HCUP),[6] based on methods developed by Elixhauser et al.[7] We used the ‐square test to assess whether hospital or patient characteristics differed between hospitalist, mixed, and non‐hospitalist hospitals.
To examine the association between hospitalist care and patient satisfaction, we first constructed bivariate models for each measure of patient satisfaction. In these models, hospital type (hospitalist, mixed, and non‐hospitalist) was our predictor. We next constructed multivariable models, which adjusted for each of the hospital characteristics described above in order to assess the independent relationship between hospitalist care and HCAHPS performance.
In sensitivity analyses, we first examined hospitalist use as a continuous variable and had qualitatively very similar results. Those data are not presented. Additionally, we conducted a propensity score analysis, with results presented in the Appendix (see Supporting Information, Appendix 1, in the online version of this article). In our first‐stage logistic regression model, being a hospitalist hospital (defined as being in the top tertile of hospitalist use vs bottom 2 tertiles) was the outcome. Hospital structural factors were covariates. Based on this first‐stage model, each hospital was assigned a propensity of being a hospitalist hospital. We divided the hospitals into 3 groups (highest propensity tertile, middle propensity tertile, and lowest propensity tertile). In a second‐stage linear regression model, patient satisfaction score was the outcome. The predictors were hospital type (dichotomized, and defined as being in the top tertile of hospitalist use vs bottom 2 tertiles), and propensity of being a hospitalist hospital (3 categories, with low propensity as the reference).
All analyses were performed using SAS version 9.2. The project was reviewed by the Institutional Review Board at the University of Michigan and determined to be not regulated given our use of publicly available datasets.
RESULTS
Among all hospitals, the median proportion of general‐medicine admissions cared for by hospitalists was 41.2% (interquartile range [IQR], 11.5%67.4%). However, US hospitals varied widely in the proportion of general‐medicine patients cared for by hospitalists (Figure 1). Whereas 3.5% of hospitals had all of their general‐medicine patients cared for by hospitalists, 16.6% had none of their general‐medicine patients seen by hospitalists. For hospitals with at least some hospitalist care, the proportion of patients cared for by hospitalists was distributed fairly evenly across the range of possibilities (Figure 1).

Because hospitalist care varied widely among hospitals, we categorized hospitals into 3 groups (non‐hospitalist, mixed, and hospitalist). The median proportion of patients cared for by hospitalists in the 3 groups was 0%, 39.5%, and 76.5%, respectively (Table 1). The non‐hospitalist hospitals, when compared with mixed and hospitalist hospitals, were more likely to be small, nonteaching, for‐profit institutions located in the Midwestern United States. They also were less likely to have an ICU and had lower nurse‐to‐bed ratios.
| Hospital Characteristics | Hospital Type | P Value | ||
|---|---|---|---|---|
| Non‐Hospitalist (N = 943) | Mixed (N = 948) | Hospitalist (N = 952) | ||
| ||||
| GM admissions cared for by hospitalists, median (range), % | 0 (021) | 40 (2158) | 77 (58100) | <0.001 |
| Nurse‐to‐bed ratio | 1 | 1 | 2 | <0.001 |
| Presence of MICU, % | 79 | 84 | 85 | 0.001 |
| Medicaid patients, % | 19 | 18 | 18 | 0.06 |
| Hospital beds, % | <0.001 | |||
| Small (99) | 36 | 16 | 24 | |
| Medium (100399) | 59 | 64 | 58 | |
| Large (400) | 6 | 21 | 18 | |
| COTH membership, % | <0.001 | |||
| Yes | 3 | 13 | 11 | |
| No | 97 | 87 | 89 | |
| Urban, % | 0.10 | |||
| Yes | 88 | 89 | 91 | |
| No | 12 | 11 | 9 | |
| Profit status, % | <0.001 | |||
| For profit | 21 | 17 | 18 | |
| Not for profit, private | 62 | 71 | 67 | |
| Other | 18 | 12 | 15 | |
| Region, % | <0.001 | |||
| South | 41 | 42 | 42 | |
| Northeast | 14 | 21 | 16 | |
| Midwest | 30 | 22 | 18 | |
| West | 15 | 15 | 24 | |
The types of patients cared for at all 3 hospital types (non‐hospitalist, mixed, and hospitalist) were similar in age and day of admission (Table 2). Patients cared for at non‐hospitalist hospitals were slightly more likely to be female and non‐White, and less likely to be admitted from the emergency department or another hospital or healthcare facility.
| Patient Characteristics | Hospital Type | P Value | ||
|---|---|---|---|---|
| Non‐Hospitalist (N = 33,265) | Mixed (N = 52,844) | Hospitalist (N = 46,705) | ||
| ||||
| Age, y | 0.51 | |||
| 6574 | 27 | 27 | 27 | |
| 7584 | 39 | 39 | 39 | |
| 85 | 34 | 34 | 34 | |
| Sex | <0.001 | |||
| M | 35 | 35 | 36 | |
| F | 65 | 65 | 64 | |
| Race/ethnicity | <0.001 | |||
| White | 85 | 85 | 87 | |
| Black | 10 | 11 | 9 | |
| Other | 5 | 4 | 4 | |
| Unknown | 0 | 0 | 0 | |
| Comorbidities, % | <0.001 | |||
| 0 | 8 | 8 | 7 | |
| 1 | 23 | 23 | 22 | |
| 2+ | 69 | 69 | 71 | |
| Day of admission | 0.08 | |||
| Weekday | 73 | 73 | 73 | |
| Weekend | 27 | 27 | 27 | |
| Admission source | <0.001 | |||
| ED | 75 | 78 | 80 | |
| Another ACH | 1 | 2 | 3 | |
| Other healthcare facility | 4 | 4 | 4 | |
| Other | 20 | 17 | 13 | |
| ICU stay | <0.001 | |||
| Yes | 13 | 12 | 12 | |
| No | 87 | 88 | 88 | |
| Length of stay, d | <0.001 | |||
| Median (Q1, Q3) | 4 (3, 6) | 4 (2, 6) | 3 (2, 5) | |
| DRG | <0.001 | |||
| Septicemia or severe sepsis | 3 | 4 | 4 | |
| Esophagitis, gastroenteritis | 3 | 3 | 3 | |
| Kidney and urinary tract infections | 3 | 3 | 3 | |
| Syncope | 3 | 3 | 3 | |
| Pneumonia | 3 | 3 | 3 | |
When we examined unadjusted relationships between type of hospital and patient experience, we found that patients at hospitalist vs non‐hospitalist hospitals were more likely to recommend the hospital (69.4% vs 65.1%: P < 0.001), and report higher overall satisfaction (65.9% vs 63.6%: P < 0.001) ((see Supporting Information, Appendix, Table A1, in the online version of this article)). Care at hospitalist hospitals was associated with higher satisfaction with discharge, but lower satisfaction with room cleanliness and communication with doctors. These differences were statistically significant at the P < 0.05 level.
When we examined the relationship between having more hospitalists and patient experience using multivariable models that accounted for differences in hospital characteristics, we found largely similar results: The proportion of patients who were satisfied with their overall care was still higher at hospitalist compared with non‐hospitalist hospitals (65.6% vs 63.9%: P < 0.001) (Figure 2). Similarly, patients were more likely to definitely recommend their hospital if they had been cared for at a hospitalist vs non‐hospitalist hospital (66.0% vs 63.4%: P < 0.001).

To better understand which domains of care might be contributing to greater overall satisfaction, we also examined patient satisfaction with specific domains of care at hospitalist vs non‐hospitalist hospitals (Table 3) in our adjusted analyses. Among 8 domains, the largest difference in satisfaction between patients cared for at hospitalist vs non‐hospitalist hospitals occurred with discharge. At hospitalist hospitals, 80.3% of patients said they were satisfied with the quality of the discharge planning compared with 78.1% at non‐hospitalist hospitals (P < 0.001). Patients at hospitalist hospitals were more satisfied with most other domains of care as well. Patients cared for at hospitalist hospitals were slightly less likely to be satisfied with communication with doctors, but this difference was not statistically significant (P = 0.45). Results were qualitatively similar in propensity‐score analyses (see Supporting Information, Appendix, Table A2, in the online version of this article).
| Specific Domains of Care | Hospital Type, % Satisfied | Hospitalist vs Non‐Hospitalist | |||
|---|---|---|---|---|---|
| Non‐Hospitalist | Mixed | Hospitalist | Difference in % Satisfied | P Value | |
| |||||
| Discharge | 78.1 | 79.1 | 80.3 | 2.1 | <0.001 |
| Nursing services | 66.0 | 65.8 | 67.1 | 1.1 | <0.001 |
| Quiet | 63.3 | 63.1 | 64.4 | 1.1 | 0.001 |
| Communication, nurse | 76.7 | 76.7 | 77.7 | 1.0 | <0.001 |
| Pain control | 69.7 | 69.7 | 70.4 | 0.7 | 0.001 |
| Medications | 60.5 | 60.5 | 61.2 | 0.7 | 0.002 |
| Cleanliness | 72.7 | 72.1 | 72.9 | 0.2 | 0.56 |
| Communication, physician | 83.6 | 83.1 | 83.5 | 0.2 | 0.45 |
DISCUSSION
We found that in 2009, US hospitals varied widely in the proportion of general medicine patients cared for by hospitalists. Hospitals with higher levels of hospitalist care did better on most measures of patient satisfaction. Differences were largest in overall satisfaction and for discharge planning. In 5 other domains of care, differences were smaller, but hospitals with more hospitalist care consistently performed better than non‐hospitalist hospitals. Hospitalist care was not associated with patient satisfaction in 2 domains: communication with doctors and cleanliness of room.
Our findings of modestly higher patient satisfaction at hospitalist hospitals along most dimensions of care are surprising and reassuring. Indeed, when hospitalists first began caring for inpatients, some expressed concerns that hospitalist care would decrease patient satisfaction.[8, 9] Though this has been an ongoing concern, we found no evidence to support this contention. It may be that as a response to the concern, hospitals with hospitalists have paid particular attention to issues such as effective handoffs to primary‐care providers.[10, 11, 12, 13] Whether due to these efforts or other factors such as the 24/7 inpatient presence of hospitalists, we found that patients at hospitalist hospitals were more likely to be satisfied with their inpatient care, including their experience at discharge. In contrast, one area that may offer room for improvement for hospitalist hospitals is communication with physicians. It may be that patients cared for by hospitalists do not know their physicians as well as patients whose care is being orchestrated by their primary‐care provider, and thus the benefits of having an ever‐present hospitalist are diminished.
The magnitude of the associations that we found should also be placed in the context of existing research on patient satisfaction. Prior work has described baseline hospital performance, changes over time, and factors associated with greater inpatient satisfaction.[5, 14, 15] The associations that we found between hospitalist care and satisfaction with care at discharge were larger than those found for teaching (vs non‐teaching) hospitals.[5] However, compared with other hospital characteristics such as nurse staffing or profit status, hospitalist care was associated with smaller differences in patient satisfaction. In one study, hospitals in the highest quartile of nurse staffing had HCAHPS scores (ie, willingness to recommend measure) that were 6.7 points higher than those in the lowest quartile of nurse staffing, and similar differences existed between not‐for‐profit, public hospitals vs for‐profit hospitals.[5]
Taken together, our findings address an important gap in knowledge about hospitalist care. Prior research has documented growth in the use of hospitalist care[1] and described the association of hospitalist care with outcomes such as mortality and resource use, and receipt of recommended care.[16, 17, 18, 19] However, we are unaware of any national study that has examined the association of hospitalist care with patient satisfaction. One study surveyed patients in a single health system and found that patients were similarly satisfied with care provided by hospitalists and primary‐care physicians.[20] Our findings should be reassuring to clinical leaders and policymakers who have advocated greater use of hospitalists: the results suggest that there need be no tradeoff between greater use of hospitalist services and patient satisfaction. Indeed, patients appear to be even more satisfied in hospitals that have greater use of hospitalist physicians.
Our study has several limitations. First, it was a cross‐sectional study, and thus we cannot make any conclusions about causality. Although we adjusted for several potential confounders (eg, teaching status, advanced care capabilities, nurse staffing), it is possible that hospitalist care is a surrogate marker for features of hospitals that we could not measure but that directly influence patient experience. In addition, it is possible that patients cared for at hospitalist hospitals differ in unmeasured ways from patients cared for at other types of hospitals. Second, we constructed our primary predictor and outcome from different cohorts. Our primary predictor was derived from the proportion of general‐medicine patients cared for by hospitalists in Medicare claims data. In contrast, our primary outcome was based on HCAHPS responses from a random sampling of all hospital admissions. This misclassification likely would have biased us towards finding small or no associations. Therefore, we are likely underestimating the true association between hospitalist use and patient experience. Third, our findings may not be generalizable to hospitals that serve younger patients or have a large number of specialist hospitalists (who were not included in our definition of hospitalists). For example, compared with older patients with multiple comorbidities, relatively healthy younger patients may derive less benefit from an ever‐present hospitalist who can explain discharge plans or an attentive nurse.
In summary, we found that US hospitals varied widely in their use of hospitalist physicians, and those which a greater proportion of care was delivered by hospitalists generally had better scores on patient experience, especially on the global assessment of satisfaction and in discharge care. Our findings suggest that adoption of the hospitalist modelone of the strategies employed by US hospitals in the past 2 decades to provide efficient careshould not detract from achieving the goal of more patient‐centered care.
Disclosures
Dr. Chen's work is supported in part by the National Institutes of Health/National Institute on Aging (AG024824, University of Michigan Claude D. Pepper Older Americans Independence Center), and the National Institutes of Health/National Center for Research Resources (UL1‐RR024986, Michigan Institute for Clinical and Health Research). Dr. Chen is also supported by a Career Development Grant Award (K08HS020671) from the Agency for Healthcare Research and Quality.
Payers and policymakers are increasingly holding hospitals accountable for patients' experiences with their care. Since 2006, the Centers for Medicare and Medicaid Services (CMS) have collected data on patients' experiences with inpatient care using the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey, a well‐validated and widely used tool. In 2008, these data on patient experience began to be publicly reported, and CMS now plans to base part of its payments to hospitals on HCAHPS performance scores. In this context, hospitals are looking for ways to improve patient satisfaction.
The effort to hold hospitals accountable for patient experience may conflict with another major trend in US hospitals: the increasing use of hospitalists.[1] Although hospitalists may have greater expertise in the day‐to‐day care of the hospitalized patient, they generally do not know the patient and cannot cater to patients' preferences in ways that the primary‐care provider might. Therefore, given that patients may prefer to be seen by their primary‐care provider,[2] greater use of hospitalists may actually lead to a decrease in patient satisfaction. Unfortunately, we are unaware of any national examination of the relationship between hospitalist use in an institution and that entity's performance on patient‐experience scores.
To better understand the relationship between greater hospitalist staffing and patient‐centered care, we examined the association between hospitalist staffing and patient satisfaction with both overall care and specific domains of patient‐centered care. We hypothesized that hospitals that used a high proportion of hospitalists would generally have lower patient‐experience scores. Further, we expected that the relationship would be monotonic (greater use of hospitalists associated with lower scores) and particularly pronounced in 2 domains: patient experience with discharge planning and patient experience with physician communication.
METHODS
Data
We sought to identify acute‐care hospitals with elderly medical patients cared for by hospitalists, non‐hospitalists, or some combination of the 2. To construct this cohort, we used 3 2009 Medicare files. The Beneficiary Summary File contains demographic information on Medicare beneficiaries and data on enrollment in managed‐care plans. To identify medical hospitalizations, we used the Medicare Provider Analysis and Review (MedPAR) 100% Files, which contain the clinical diagnoses and payments for all fee‐for‐service Medicare beneficiaries discharged from acute‐care hospitals. To identify hospitalists and non‐hospitalists, we used the 5% Carrier File, which contains physician billing data for a 5% random sample of fee‐for‐service Medicare beneficiaries. We also obtained information on hospital characteristics from the American Hospital Association (AHA) Annual Survey. We supplemented this with hospital‐level data on patient satisfaction from the HCAHPS survey conducted in 2009. The HCAHPS is a standard survey developed by the Agency for Healthcare Research and Quality (AHRQ) and administered by hospitals to a random sample of adult patients 48 hours to 6 weeks after discharge. The HCAHPS results are adjusted for patient mix and have been tested for nonresponse bias.[3] Details about the development and design of HCAHPS have been described previously.[4]
Patient and Hospital Sample
We started with 48,861,000 Medicare beneficiaries in the Beneficiary Summary File and excluded 38% either because their age was <65 years or they were members of an HMO. At the same time, from the 1,850,000 patients in the 5% Carrier File, we excluded 55% who had not been cared for by a general internist. Finally, we used the MedPAR File to identify 17,387,000 hospital admissions by fee‐for‐service Medicare beneficiaries. From MedPAR, we excluded admissions to a facility other than an acute‐care hospital (24%), surgical admissions identified by diagnosis‐related group (DRG) (29%), and admissions to hospitals with <5 medicine admissions in 2009 (<0.1%). After merging these 3 files (Beneficiary Summary, MedPAR, and 5% Carrier), we were left with 229,496 admissions among 180,399 patients at 3365 hospitals. We subsequently excluded readmissions and were left with 156,333 admissions at 3244 hospitals. Finally, we excluded those patients cared for by both hospitalists and non‐hospitalists during the same hospitalization, and those hospitals missing AHA or HCAHPS data, leaving us with 132,814 patients at 2843 hospitals.
Definition of Hospitalist
We used the claims‐based definition developed and validated by Kuo and Goodwin in earlier work.[1] Hospitalists are defined as those general internists (providers in general practice or internal medicine) who had 5 evaluation and management (E&M) billings (in a 5% sample of Medicare beneficiaries) in 2009 and generated >90% of their claims from the care of hospitalized patients in 2009.
Measures of Patient Satisfaction
There are 2 HCAHPS questions about overall satisfaction, one that asks patients to rate their experience on a scale of 0 to 10 and another that asks whether they would recommend the hospital. Not surprisingly, hospitals' performance on these 2 questions is highly correlated.[5] We measured overall patient experience using commonly used approaches: the proportion of patients who gave the hospital a 9 or 10 (on the 10‐point scale) or the proportion of patients who reported that they would definitely recommend the hospital. The HCAHPS also contains 24 questions, which are reported by CMS in 8 domains: communication with nurse, communication with physician, responsiveness of the staff, pain control, communication about medications, adequacy of discharge planning, cleanliness of the room, and quietness of the room. The patient‐satisfaction score for each of these domains represents the proportion of patients who answered always to each of the questions, or who answered yes to the question about discharge.
Potentially Confounding Variables
Because we were worried that hospitals with hospitalists would be different from hospitals without hospitalists, we identified a series of covariates for adjustment in a multivariable model. We extracted data from the AHA on hospitals' structural characteristics that we assumed might be associated both with having a hospitalist and with patient experience. These variables were size (number of beds), teaching status (membership in the Council of Teaching Hospitals vs no membership), location (urban vs rural), region (the 4 census regions), ownership (for profit, private nonprofit, or public), and presence of advanced clinical capabilities (as measured by having a medical, surgical, and/or cardiac intensive care unit [ICU]). We also used information about the patient population (proportion of patients with Medicare or with Medicaid) as well as nurse‐staffing level (ratio of full‐time equivalent registered nurses to total hospital beds).
Statistical Analyses
We first quantified hospital variation in the proportion of general‐medicine patients cared for by hospitalists, using basic descriptive statistics. Based on these analyses, we categorized hospitals into 3 groups: non‐hospitalist, mixed, and hospitalist (corresponding to lowest, middle, and highest tertile of hospitalist use respectively). We used bivariate techniques to describe the patient and hospital characteristics of hospitals in each group. Patient characteristics included number of comorbidities, which were calculated using software from the Healthcare Cost and Utilization Project (HCUP),[6] based on methods developed by Elixhauser et al.[7] We used the ‐square test to assess whether hospital or patient characteristics differed between hospitalist, mixed, and non‐hospitalist hospitals.
To examine the association between hospitalist care and patient satisfaction, we first constructed bivariate models for each measure of patient satisfaction. In these models, hospital type (hospitalist, mixed, and non‐hospitalist) was our predictor. We next constructed multivariable models, which adjusted for each of the hospital characteristics described above in order to assess the independent relationship between hospitalist care and HCAHPS performance.
In sensitivity analyses, we first examined hospitalist use as a continuous variable and had qualitatively very similar results. Those data are not presented. Additionally, we conducted a propensity score analysis, with results presented in the Appendix (see Supporting Information, Appendix 1, in the online version of this article). In our first‐stage logistic regression model, being a hospitalist hospital (defined as being in the top tertile of hospitalist use vs bottom 2 tertiles) was the outcome. Hospital structural factors were covariates. Based on this first‐stage model, each hospital was assigned a propensity of being a hospitalist hospital. We divided the hospitals into 3 groups (highest propensity tertile, middle propensity tertile, and lowest propensity tertile). In a second‐stage linear regression model, patient satisfaction score was the outcome. The predictors were hospital type (dichotomized, and defined as being in the top tertile of hospitalist use vs bottom 2 tertiles), and propensity of being a hospitalist hospital (3 categories, with low propensity as the reference).
All analyses were performed using SAS version 9.2. The project was reviewed by the Institutional Review Board at the University of Michigan and determined to be not regulated given our use of publicly available datasets.
RESULTS
Among all hospitals, the median proportion of general‐medicine admissions cared for by hospitalists was 41.2% (interquartile range [IQR], 11.5%67.4%). However, US hospitals varied widely in the proportion of general‐medicine patients cared for by hospitalists (Figure 1). Whereas 3.5% of hospitals had all of their general‐medicine patients cared for by hospitalists, 16.6% had none of their general‐medicine patients seen by hospitalists. For hospitals with at least some hospitalist care, the proportion of patients cared for by hospitalists was distributed fairly evenly across the range of possibilities (Figure 1).

Because hospitalist care varied widely among hospitals, we categorized hospitals into 3 groups (non‐hospitalist, mixed, and hospitalist). The median proportion of patients cared for by hospitalists in the 3 groups was 0%, 39.5%, and 76.5%, respectively (Table 1). The non‐hospitalist hospitals, when compared with mixed and hospitalist hospitals, were more likely to be small, nonteaching, for‐profit institutions located in the Midwestern United States. They also were less likely to have an ICU and had lower nurse‐to‐bed ratios.
| Hospital Characteristics | Hospital Type | P Value | ||
|---|---|---|---|---|
| Non‐Hospitalist (N = 943) | Mixed (N = 948) | Hospitalist (N = 952) | ||
| ||||
| GM admissions cared for by hospitalists, median (range), % | 0 (021) | 40 (2158) | 77 (58100) | <0.001 |
| Nurse‐to‐bed ratio | 1 | 1 | 2 | <0.001 |
| Presence of MICU, % | 79 | 84 | 85 | 0.001 |
| Medicaid patients, % | 19 | 18 | 18 | 0.06 |
| Hospital beds, % | <0.001 | |||
| Small (99) | 36 | 16 | 24 | |
| Medium (100399) | 59 | 64 | 58 | |
| Large (400) | 6 | 21 | 18 | |
| COTH membership, % | <0.001 | |||
| Yes | 3 | 13 | 11 | |
| No | 97 | 87 | 89 | |
| Urban, % | 0.10 | |||
| Yes | 88 | 89 | 91 | |
| No | 12 | 11 | 9 | |
| Profit status, % | <0.001 | |||
| For profit | 21 | 17 | 18 | |
| Not for profit, private | 62 | 71 | 67 | |
| Other | 18 | 12 | 15 | |
| Region, % | <0.001 | |||
| South | 41 | 42 | 42 | |
| Northeast | 14 | 21 | 16 | |
| Midwest | 30 | 22 | 18 | |
| West | 15 | 15 | 24 | |
The types of patients cared for at all 3 hospital types (non‐hospitalist, mixed, and hospitalist) were similar in age and day of admission (Table 2). Patients cared for at non‐hospitalist hospitals were slightly more likely to be female and non‐White, and less likely to be admitted from the emergency department or another hospital or healthcare facility.
| Patient Characteristics | Hospital Type | P Value | ||
|---|---|---|---|---|
| Non‐Hospitalist (N = 33,265) | Mixed (N = 52,844) | Hospitalist (N = 46,705) | ||
| ||||
| Age, y | 0.51 | |||
| 6574 | 27 | 27 | 27 | |
| 7584 | 39 | 39 | 39 | |
| 85 | 34 | 34 | 34 | |
| Sex | <0.001 | |||
| M | 35 | 35 | 36 | |
| F | 65 | 65 | 64 | |
| Race/ethnicity | <0.001 | |||
| White | 85 | 85 | 87 | |
| Black | 10 | 11 | 9 | |
| Other | 5 | 4 | 4 | |
| Unknown | 0 | 0 | 0 | |
| Comorbidities, % | <0.001 | |||
| 0 | 8 | 8 | 7 | |
| 1 | 23 | 23 | 22 | |
| 2+ | 69 | 69 | 71 | |
| Day of admission | 0.08 | |||
| Weekday | 73 | 73 | 73 | |
| Weekend | 27 | 27 | 27 | |
| Admission source | <0.001 | |||
| ED | 75 | 78 | 80 | |
| Another ACH | 1 | 2 | 3 | |
| Other healthcare facility | 4 | 4 | 4 | |
| Other | 20 | 17 | 13 | |
| ICU stay | <0.001 | |||
| Yes | 13 | 12 | 12 | |
| No | 87 | 88 | 88 | |
| Length of stay, d | <0.001 | |||
| Median (Q1, Q3) | 4 (3, 6) | 4 (2, 6) | 3 (2, 5) | |
| DRG | <0.001 | |||
| Septicemia or severe sepsis | 3 | 4 | 4 | |
| Esophagitis, gastroenteritis | 3 | 3 | 3 | |
| Kidney and urinary tract infections | 3 | 3 | 3 | |
| Syncope | 3 | 3 | 3 | |
| Pneumonia | 3 | 3 | 3 | |
When we examined unadjusted relationships between type of hospital and patient experience, we found that patients at hospitalist vs non‐hospitalist hospitals were more likely to recommend the hospital (69.4% vs 65.1%: P < 0.001), and report higher overall satisfaction (65.9% vs 63.6%: P < 0.001) ((see Supporting Information, Appendix, Table A1, in the online version of this article)). Care at hospitalist hospitals was associated with higher satisfaction with discharge, but lower satisfaction with room cleanliness and communication with doctors. These differences were statistically significant at the P < 0.05 level.
When we examined the relationship between having more hospitalists and patient experience using multivariable models that accounted for differences in hospital characteristics, we found largely similar results: The proportion of patients who were satisfied with their overall care was still higher at hospitalist compared with non‐hospitalist hospitals (65.6% vs 63.9%: P < 0.001) (Figure 2). Similarly, patients were more likely to definitely recommend their hospital if they had been cared for at a hospitalist vs non‐hospitalist hospital (66.0% vs 63.4%: P < 0.001).

To better understand which domains of care might be contributing to greater overall satisfaction, we also examined patient satisfaction with specific domains of care at hospitalist vs non‐hospitalist hospitals (Table 3) in our adjusted analyses. Among 8 domains, the largest difference in satisfaction between patients cared for at hospitalist vs non‐hospitalist hospitals occurred with discharge. At hospitalist hospitals, 80.3% of patients said they were satisfied with the quality of the discharge planning compared with 78.1% at non‐hospitalist hospitals (P < 0.001). Patients at hospitalist hospitals were more satisfied with most other domains of care as well. Patients cared for at hospitalist hospitals were slightly less likely to be satisfied with communication with doctors, but this difference was not statistically significant (P = 0.45). Results were qualitatively similar in propensity‐score analyses (see Supporting Information, Appendix, Table A2, in the online version of this article).
| Specific Domains of Care | Hospital Type, % Satisfied | Hospitalist vs Non‐Hospitalist | |||
|---|---|---|---|---|---|
| Non‐Hospitalist | Mixed | Hospitalist | Difference in % Satisfied | P Value | |
| |||||
| Discharge | 78.1 | 79.1 | 80.3 | 2.1 | <0.001 |
| Nursing services | 66.0 | 65.8 | 67.1 | 1.1 | <0.001 |
| Quiet | 63.3 | 63.1 | 64.4 | 1.1 | 0.001 |
| Communication, nurse | 76.7 | 76.7 | 77.7 | 1.0 | <0.001 |
| Pain control | 69.7 | 69.7 | 70.4 | 0.7 | 0.001 |
| Medications | 60.5 | 60.5 | 61.2 | 0.7 | 0.002 |
| Cleanliness | 72.7 | 72.1 | 72.9 | 0.2 | 0.56 |
| Communication, physician | 83.6 | 83.1 | 83.5 | 0.2 | 0.45 |
DISCUSSION
We found that in 2009, US hospitals varied widely in the proportion of general medicine patients cared for by hospitalists. Hospitals with higher levels of hospitalist care did better on most measures of patient satisfaction. Differences were largest in overall satisfaction and for discharge planning. In 5 other domains of care, differences were smaller, but hospitals with more hospitalist care consistently performed better than non‐hospitalist hospitals. Hospitalist care was not associated with patient satisfaction in 2 domains: communication with doctors and cleanliness of room.
Our findings of modestly higher patient satisfaction at hospitalist hospitals along most dimensions of care are surprising and reassuring. Indeed, when hospitalists first began caring for inpatients, some expressed concerns that hospitalist care would decrease patient satisfaction.[8, 9] Though this has been an ongoing concern, we found no evidence to support this contention. It may be that as a response to the concern, hospitals with hospitalists have paid particular attention to issues such as effective handoffs to primary‐care providers.[10, 11, 12, 13] Whether due to these efforts or other factors such as the 24/7 inpatient presence of hospitalists, we found that patients at hospitalist hospitals were more likely to be satisfied with their inpatient care, including their experience at discharge. In contrast, one area that may offer room for improvement for hospitalist hospitals is communication with physicians. It may be that patients cared for by hospitalists do not know their physicians as well as patients whose care is being orchestrated by their primary‐care provider, and thus the benefits of having an ever‐present hospitalist are diminished.
The magnitude of the associations that we found should also be placed in the context of existing research on patient satisfaction. Prior work has described baseline hospital performance, changes over time, and factors associated with greater inpatient satisfaction.[5, 14, 15] The associations that we found between hospitalist care and satisfaction with care at discharge were larger than those found for teaching (vs non‐teaching) hospitals.[5] However, compared with other hospital characteristics such as nurse staffing or profit status, hospitalist care was associated with smaller differences in patient satisfaction. In one study, hospitals in the highest quartile of nurse staffing had HCAHPS scores (ie, willingness to recommend measure) that were 6.7 points higher than those in the lowest quartile of nurse staffing, and similar differences existed between not‐for‐profit, public hospitals vs for‐profit hospitals.[5]
Taken together, our findings address an important gap in knowledge about hospitalist care. Prior research has documented growth in the use of hospitalist care[1] and described the association of hospitalist care with outcomes such as mortality and resource use, and receipt of recommended care.[16, 17, 18, 19] However, we are unaware of any national study that has examined the association of hospitalist care with patient satisfaction. One study surveyed patients in a single health system and found that patients were similarly satisfied with care provided by hospitalists and primary‐care physicians.[20] Our findings should be reassuring to clinical leaders and policymakers who have advocated greater use of hospitalists: the results suggest that there need be no tradeoff between greater use of hospitalist services and patient satisfaction. Indeed, patients appear to be even more satisfied in hospitals that have greater use of hospitalist physicians.
Our study has several limitations. First, it was a cross‐sectional study, and thus we cannot make any conclusions about causality. Although we adjusted for several potential confounders (eg, teaching status, advanced care capabilities, nurse staffing), it is possible that hospitalist care is a surrogate marker for features of hospitals that we could not measure but that directly influence patient experience. In addition, it is possible that patients cared for at hospitalist hospitals differ in unmeasured ways from patients cared for at other types of hospitals. Second, we constructed our primary predictor and outcome from different cohorts. Our primary predictor was derived from the proportion of general‐medicine patients cared for by hospitalists in Medicare claims data. In contrast, our primary outcome was based on HCAHPS responses from a random sampling of all hospital admissions. This misclassification likely would have biased us towards finding small or no associations. Therefore, we are likely underestimating the true association between hospitalist use and patient experience. Third, our findings may not be generalizable to hospitals that serve younger patients or have a large number of specialist hospitalists (who were not included in our definition of hospitalists). For example, compared with older patients with multiple comorbidities, relatively healthy younger patients may derive less benefit from an ever‐present hospitalist who can explain discharge plans or an attentive nurse.
In summary, we found that US hospitals varied widely in their use of hospitalist physicians, and those which a greater proportion of care was delivered by hospitalists generally had better scores on patient experience, especially on the global assessment of satisfaction and in discharge care. Our findings suggest that adoption of the hospitalist modelone of the strategies employed by US hospitals in the past 2 decades to provide efficient careshould not detract from achieving the goal of more patient‐centered care.
Disclosures
Dr. Chen's work is supported in part by the National Institutes of Health/National Institute on Aging (AG024824, University of Michigan Claude D. Pepper Older Americans Independence Center), and the National Institutes of Health/National Center for Research Resources (UL1‐RR024986, Michigan Institute for Clinical and Health Research). Dr. Chen is also supported by a Career Development Grant Award (K08HS020671) from the Agency for Healthcare Research and Quality.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , , . How do patients view the role of the primary care physician in inpatient care? Dis Mon. 2002;48(4):230–238.
- , , , et al. Effects of survey mode, patient mix, and nonresponse on CAHPS hospital survey scores. Health Serv Res. 2009;44(2 pt 1):501–518.
- , , , , . Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27–37.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921–1931.
- Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP). HCUP Comorbidity Software. http://www.hcup‐us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp Accessed November 12, 2012.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- , , . Physician views on caring for hospitalized patients and the hospitalist model of inpatient care. J Gen Intern Med. 2001;16(2):116–119.
- , , , . How physicians perceive hospitalist services after implementation: anticipation vs reality. Arch Intern Med. 2003;163(19):2330–2336.
- , , , et al. Association of communication between hospital‐based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381–386.
- , , . Passing the clinical baton: 6 principles to guide the hospitalist. Dis Mon. 2002;48(4):260–266.
- , , , . Primary care physician attitudes regarding communication with hospitalists. Am J Med. 2001;111(9B):15S–20S.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364–370.
- , , , et al. Hospital survey shows improvements in patient experience. Health Aff (Millwood). 2010;29(11):2061–2067.
- , , , , , . Characteristics of hospitals demonstrating superior performance in patient experience and clinical process measures of care. Med Care Res Rev. 2010;67(1):38–55.
- , . The impact of hospitalists on the cost and quality of inpatient care in the United States: a research synthesis. Med Care Res Rev. 2005;62(4):379–406.
- , , , , . Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists. Arch Intern Med. 2002;162(11):1251–1256.
- , , , , . Hospitalists and the quality of care in hospitals. Arch Intern Med. 2009;169(15):1389–1394.
- , , , et al. Quality of care for decompensated heart failure: comparable performance between academic hospitalists and non‐hospitalists. J Gen Intern Med. 2008;23(9):1399–1406.
- , , , et al. Patient satisfaction with hospital care provided by hospitalists and primary care physicians. J Hosp Med. 2012;7(2):131–136.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , , . How do patients view the role of the primary care physician in inpatient care? Dis Mon. 2002;48(4):230–238.
- , , , et al. Effects of survey mode, patient mix, and nonresponse on CAHPS hospital survey scores. Health Serv Res. 2009;44(2 pt 1):501–518.
- , , , , . Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27–37.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921–1931.
- Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP). HCUP Comorbidity Software. http://www.hcup‐us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp Accessed November 12, 2012.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- , , . Physician views on caring for hospitalized patients and the hospitalist model of inpatient care. J Gen Intern Med. 2001;16(2):116–119.
- , , , . How physicians perceive hospitalist services after implementation: anticipation vs reality. Arch Intern Med. 2003;163(19):2330–2336.
- , , , et al. Association of communication between hospital‐based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381–386.
- , , . Passing the clinical baton: 6 principles to guide the hospitalist. Dis Mon. 2002;48(4):260–266.
- , , , . Primary care physician attitudes regarding communication with hospitalists. Am J Med. 2001;111(9B):15S–20S.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364–370.
- , , , et al. Hospital survey shows improvements in patient experience. Health Aff (Millwood). 2010;29(11):2061–2067.
- , , , , , . Characteristics of hospitals demonstrating superior performance in patient experience and clinical process measures of care. Med Care Res Rev. 2010;67(1):38–55.
- , . The impact of hospitalists on the cost and quality of inpatient care in the United States: a research synthesis. Med Care Res Rev. 2005;62(4):379–406.
- , , , , . Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists. Arch Intern Med. 2002;162(11):1251–1256.
- , , , , . Hospitalists and the quality of care in hospitals. Arch Intern Med. 2009;169(15):1389–1394.
- , , , et al. Quality of care for decompensated heart failure: comparable performance between academic hospitalists and non‐hospitalists. J Gen Intern Med. 2008;23(9):1399–1406.
- , , , et al. Patient satisfaction with hospital care provided by hospitalists and primary care physicians. J Hosp Med. 2012;7(2):131–136.
Copyright © 2012 Society of Hospital Medicine
Different Strokes for Different Folks
A 35‐year‐old woman presented to her primary care physician complaining of left post‐auricular pain, swelling, and redness. She described the pain as 8 out of 10, constant, sharp, and nonradiating. She denied fever or chills. A presumptive diagnosis of cellulitis led to a prescription for oral trimethoprim‐sulfamethoxazole. Left facial swelling worsened despite 4 days of antibiotics, so she came to the emergency department.
Noninfectious causes of this woman's symptoms include trauma, or an inflammatory condition such as polychondritis. Key infectious considerations are mastoiditis or a mastoid abscess. Herpes zoster with involvement of the pinna and auditory canal may also present with pain and redness. In the absence of findings suggestive of an infection arising from the auditory canal, cellulitis is a reasonable consideration. With the growing incidence of community‐acquired methicillin‐resistant Staphylococcus aureus infections, an agent effective against this pathogen such as trimethoprim‐sulfamethoxazole may be used, usually in combination with an antibiotic that provides more reliable coverage for group A streptococcus.
Her past medical history included poorly controlled type II diabetes mellitus and asthma. She reported no previous surgical history. Her current medications were insulin, albuterol inhaler, and trimethoprim‐sulfamethoxazole, although she had a history of noncompliance with her insulin. She was married with 1 child and was unemployed. She smoked 1 pack of cigarettes daily, drank up to 6 beers daily, and denied use of illicit drugs.
Her history of diabetes increases her risk of malignant otitis externa. Both diabetes and excess alcohol consumption are risk factors for herpes zoster. Smoking has been shown to increase the risk of otitis media and carriage by S. pneumoniae, a common pathogen in ear infections.
She was ill‐appearing and in moderate respiratory distress. Her temperature was 39C, blood pressure 149/93 mmHg, pulse 95 beats per minute, respiratory rate of 26 times per minute, with an oxygen saturation of 96% while breathing ambient air. She had swelling of the left side of the face extending to the left forehead and lateral neck. Examination of the external ear and auditory canal were unremarkable. The swelling had no associated erythema, tenderness, or lymphadenopathy. She had no oropharyngeal or nasal ulcers present. Her pupils were equal, round, and reactive to light and accommodation with normal sclera. Her trachea was midline; thyroid exam was normal. The heart sounds included normal S1 and S2 without murmurs, rubs, or gallops. Her lung exam was remarkable for inspiratory stridor. The abdominal examination revealed no distention, tenderness, organomegaly, or masses. Cranial nerve testing revealed a left‐sided central seventh nerve palsy along with decreased visual acuity of the left eye. Strength, sensation, and deep tendon reflexes were normal.
While there are many causes of facial nerve palsy, distinguishing between a peripheral palsy (which causes paralysis of the entire ipsilateral side of the face) and a central palsy (which spares the musculature of the forehead) is important. The most common type of peripheral facial nerve palsy is Bell's palsy. Infections such as meningitis or tumors of the central nervous system can cause central facial nerve or other cranial nerve palsy. Important infections to consider in this case would be viral such as herpes zoster or simplex, or atypical bacteria such as Mycoplasma and Rickettsia, which may explain the neurologic but not all of the other clinical findings in this case. It is also critical to determine whether she has an isolated seventh cranial nerve palsy or if other cranial nerves are involved such as may occur with basilar meningitis, which has a myriad of infectious and noninfectious causes. The decreased visual acuity may be a result of corneal dryness and abrasions from inability to close the eye but may also represent optic nerve problems, so detailed ophthalmologic exam is essential. Her ill appearance coupled with facial and neck swelling leads me to at least consider Lemierre's syndrome with central nervous system involvement. Finally, facial swelling and the inspiratory stridor may represent angioedema, although one‐sided involvement of the face would be unusual.
The results of initial laboratory testing were as follows: sodium, 138 mmol/L; potassium, 3.4 mmol/L; chloride, 109 mmol/L; bicarbonate, 14 mmol/L; blood urea nitrogen level, 19 mg/dL; creatinine, 1.1 mg/dL; white cell count, 23,510/mm3; differential, 90% neutrophils, 1% bands, 7% lymphocytes, 2% monocytes; hemoglobin level, 12.5 g/dL; platelet count, 566,000/mm3; hemoglobin A1c, 11%; albumin, 1.6 g/dL; total protein, 6.2 g/dL; total bilirubin, 0.8 mg/dL; alkaline phosphatase, 103 U/L; alanine aminotransferase level, 14 U/L; international normalized ratio of 1.2; partial thromboplastin time, 29 seconds (normal value, 2434 seconds); erythrocyte sedimentation rate, 121 mm/hr; creatine kinase, 561 U/L (normal value 25190). Arterial blood gas measurements with the patient breathing 50% oxygen revealed a pH of 7.34, a partial pressure of carbon dioxide of 28 mmHg, and a partial pressure of oxygen of 228 mmHg.
I am concerned that this patient has sepsis, likely due to an infectious trigger. With her clinical presentation localized to the head and neck, her history of diabetes, and the accelerated sedimentation rate, malignant otitis externa would explain many of her findings. Empiric anti‐infective therapy directed toward Pseudomonas aeruginosa should be initiated, and imaging of the head and ear should be undertaken.
The patient required intubation due to increased respiratory distress and stridor. Her physicians used intravenous vancomycin, clindamycin, and piperacillin/tazobactam to treat presumed cellulitis. Her abnormal neurologic exam led to magnetic resonance (MR) imaging and MR angiography of her neck and brain, which showed evidence of multiple regions of ischemia in the left occipital and inferior parietal distributions, as well as bilateral cerebellar distributions and enhancement of the parotid gland and mastoid air cells (Figure 1). A cerebral angiogram revealed irregularity and caliber reduction in multiple cervical and intracranial arteries, associated with intraluminal thrombi within the left intracranial vertebral artery, consistent with either vasculitis or infectious angioinvasion (Figure 2).

The angioinvasive nature of the findings on imaging leads me to suspect fungal infection. The patient's history of diabetes mellitus and acidosis are risk factors for mucormycosis. Aspergillus and Fusarium may also be angioinvasive but would be much more likely in neutropenic or severely immunocompromised patients. S. aureus may cause septic emboli mimicking angioinvasion but should be readily detected in conventional blood cultures. At this point, I would empirically begin amphotericin B; tissue, however, is needed for definitive diagnosis and a surgical consult should be requested.
After reviewing her imaging studies, an investigation for vasculitis and hypercoagulable states including antinuclear antibody, anti‐deoxyribonucleic acid, anti‐Smith antibody, anti‐SSA antibody level, anti‐SSB level, antineutrophil cytoplasmic antibody, activated protein C resistance level, factor VIII level, human immunodeficiency virus antibody, homocysteine level, cardiolipin antibody testing, lupus anticoagulant, prothrombin 20210 mutation, and protein C level was done, and all tests were normal. Protein S level was slightly low at 64% (normal value 65%140%). Given the enlarged parotid gland and the enhancement of the left parotid bed on magnetic resonance imaging, she underwent a parotid biopsy that revealed sialadenitis.
Systemic vasculitides can result in tissue damage, mediated by the release of endogenous cellular contents from dying cells, known as damage‐associated molecular patterns, sufficient to cause systemic inflammatory response syndrome (SIRS). This patient presented with acute symptoms but has negative laboratory studies for autoantibodies. The parotid biopsy also did not reveal evidence of vasculitis. All these findings make the diagnosis of vasculitis much less likely.
She remained in the medical intensive care unit on mechanical ventilation, with minimal symptomatic improvement. On hospital day 10, the patient developed necrosis of the left external ear. A punch biopsy of the necrotic area of her left pinna was performed; the pathology report read: Sections of punch biopsy of skin show an unremarkable epidermis. There is dermal necrosis involving the stroma and adnexal structures. Intravascular thrombi within the deep dermis are seen. Within superficial dermis there are broad, elongated, nonseptated hyaline structures reminiscent of Mucor. Special stains (periodic acid‐Schiff stain and Grocott Gomori methenamine silver stain [GMS]) performed with appropriately reactive controls fail to highlight these structures (Figure 3). The infectious disease team reviewed the pathology slides with the pathologist. As there was inconclusive evidence for zygomycosis, ie, only a few hyaline structures which failed to stain with GMS stain, the consultants recommended no change in the patient's management.
The gross and microscopic evidence of necrosis and areas of intravascular thrombi are nonspecific but compatible with a fungal infection in a patient with risk factors for zygomycosis. The GMS stain is a very sensitive stain for fungal structures, so a negative stain in this case is surprising, but additional testing such as immunohistochemistry should be pursued to confirm or refute this diagnosis. While Rhizopus species can be contaminants, the laboratory finding of these organisms in specimens from patients with risk factors for zygomycosis should not be ignored.
On hospital day 12, the patient was noted to have increased facial swelling. A computed tomographic (CT) angiogram of the neck revealed necrosis of the anterior and posterior paraspinal muscles from the skull base to C34, marked swelling of the left parotid gland, and left inferior parieto‐occipital enhancing lesion. An incisional parotid biopsy was performed. Special stains were positive for broad‐based fungal hyphae consistent with mucormycosis (Figure 4).
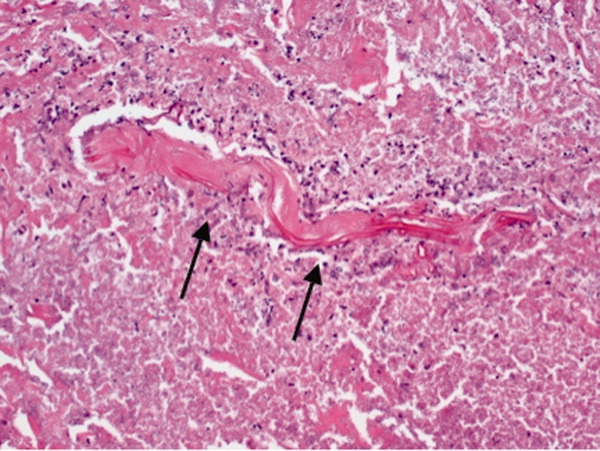
Given these findings, the patient should be started on amphotericin B immediately. Medical therapy alone generally does not suffice, and aggressive surgical debridement combined with intravenous antifungal therapy results in better outcomes. The longer the duration of symptoms and the greater the progression of disease, the less favorable the prognosis.
The patient was started on amphotericin B lipid complex and micafungin. However, after 16 days of therapy, repeat imaging of the neck showed worsening necrosis of the neck muscles. At this time, she underwent extensive debridement of face and neck, and posaconazole was added. After prolonged hospitalization, she was discharged to a rehabilitation facility on posaconazole. She resided in a nursing facility for 6 months. One year after her hospitalization, she is living at home and is able to ambulate independently, but requires feeding through a percutaneous endoscopic gastrostomy (PEG) tube because she remains dysphagic.
COMMENTARY
Infections caused by the ubiquitous fungi of the class Zygomycetes typically take 1 of 5 forms: rhinocerebral, pulmonary, gastrointestinal, disseminated, and cutaneous. The presentation varies widely, ranging from plaques, skin swelling, pustules, cellulitis, blisters, nodules, ulcerations, and ecthyma gangrenosum‐like lesions to deeper infections such as necrotizing fasciitis, osteomyelitis, and disseminated infection.1 Infections typically occur in immunocompromised hosts, including transplant recipients and patients with hematologic malignancy, but also occur in patients with diabetes mellitus, intravenous drug users, and patients on deferoxamine therapy.2 Deferoxamine and other iron‐binding therapy is thought to predispose to zygomycetes infections because of improved iron uptake of the fungal species and, thus, stimulation of growth.3 Pulmonary and rhinocerebral infections are the most common clinically encountered forms, and 44% of cutaneous infections are complicated by deep extension or dissemination.4
The articles cited above describe the more typical presentations of this rare disease. However, this patient had an unusual presentation, as parotid involvement due to zygomycosis has only been described once previously.5 Her inflammatory vasculitis and ensuing strokes from involvement of the carotid artery are recognized complications of zygomycosis, and in 1 case series of 41 patients with rhinocerebral mucormycosis, carotid involvement was seen in 31% of patients.6 After the punch biopsy of the patient's pinna showing nonseptated hyphae reminiscent of Mucor, why did her physicians delay administering amphotericin?
There are 2 likely possibilities: anchoring bias or error in medical decision‐making due to inaccurate probability estimates. Anchoring bias describes a heuristic where the initial diagnosis or gestalt biases the physician's process for assigning a final diagnosis.7, 8 This bias creates cognitive errors by limiting creativity in diagnosis. In this case, the infectious disease team carefully weighed the information obtained from the first biopsy. Given their low pretest estimate of this virtually unreported presentation of a rare disease, they decided to evaluate further without beginning antifungal therapy. Of note, there were few hyaline structures, and those structures lacked uptake of GMS. Since they considered the diagnosis yet rejected the diagnosis due to insufficient evidence, it is unlikely that anchoring bias played a role.
Was there an error in medical decision‐making? The physicians in this case faced a very common medical dilemma: whether or not to start a toxic medication empirically or wait for diagnostic confirmation prior to treatment.9 To solve this dilemma, one can apply decision analysis. Moskowitz et al described 5 phases of medical decision analysis by which a probabilistic right answer to clinical scenarios can be deduced mathematically.10 To solve this problem, probabilities must be assigned to the risk of giving a drug to a patient without the disease versus the risk of not giving a drug to a patient with the disease. For example, amphotericin deoxycholate causes acute renal failure in 30% to 47% of patients. Newer formulations of amphotericin, such as liposomal amphotericin and lipid complex, result in lower rates of nephrotoxicity (27% vs 47%). The risk of not giving amphotericin to a patient with zygomycosis is death. Even in patients treated with amphotericin, the mortality rate has been shown to be 66%, and up to 100% in those with strokes related to zygomycosis.2, 6, 11 Simply looking at these probabilities, decision analysis would favor empiric treatment.
The physicians caring for this patient did not have the luxury of retrospective speculation. After looking at all of the data, the equivocal skin biopsy and rare clinical presentation, the question to ask would change: What is the risk of giving amphotericin empirically to someone who, based on available information, has a very low probability of having zygomycosis? When phrased in this manner, there is a 47% chance of nephrotoxicity with amphotericin versus the very small probability that you have diagnosed a case of zygomycosis that has only been described once in the literature. Mathematically andmore importantlyclinically, this question becomes more difficult to answer. However, no value can be placed on the possibility of death in suspected zygomycosis, and the risk of short‐term amphotericin use is much less than that of a course of treatment. As such, empiric therapy should always be given.
Physicians are not mathematicians, and dynamic clinical scenarios are not so easily made into static math problems. Disease presentations evolve over time towards a diagnosable clinical pattern, as was the case with this patient. Two days after the aforementioned biopsy, she worsened and in less time than it would have taken to isolate zygomycosis from the first biopsy, a second biopsy revealed the typical nonseptated hyphae demarcated with the GMS stain. Even appropriate diagnostic testing, thoughtful interpretation, and avoidance of certain cognitive errors can result in incorrect diagnoses and delayed treatment. It is monitoring the progression of disease and collecting additional data that allows physicians to mold a diagnosis and create a treatment plan.
The primary treatment of zygomycosis should include amphotericin. However, there are limited data to support combination therapy with an echinocandin in severe cases, as in this patient.12 Posaconazole is not recommended for monotherapy as an initial therapy, but there is data for its use as salvage therapy in zygomycosis.13 This case highlights the difficulties that physicians face in the diagnosis and treatment of rare diseases. Cerebral infarction in a hematologic malignancy, uncontrolled diabetes, or iron chelation therapy could be the initial presentation of rhinocerebral zygomycosis. There truly are different strokes for different folks. Recognizing this and similar presentations may lead to a more rapid diagnosis and treatment of zygomycosis.
TEACHING POINTS
-
Zygomycosis has a wide range of clinical presentations ranging from skin lesions to deep tissue infections. As it is an angioinvasive organism, it can also present as cerebral infarcts and brain abscesses.
-
Zygomycosis infections should be suspected in patients with uncontrolled diabetes, hematologic or oncologic malignancies, and patients on iron chelation therapy with a potentially compatible clinical picture.
-
If zygomycosis infection is suspected, rapid histologic diagnosis should be attempted. However, as histologic diagnosis can take time, empiric therapy with amphotericin should always be administered.
-
Amphotericin remains the primary medical therapy for this disease; however, there is limited emerging evidence to suggest that echinocandins can be used in combination with amphotericin for improved treatment of severe rhinocerebral zygomyocosis. Posaconazole has a role as salvage therapy in zygomycosis, but should not be used as the sole primary treatment.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
This icon represents the patient's case. Each paragraph that follows represents the discussant's thoughts.
Acknowledgements
The authors are indebted to Dr Glenn Roberson at the Department of Radiology, University of Alabama at Birmingham, for providing the radiographic images; to Dr Aleodor Andea at the Department of Pathology, University of Alabama at Birmingham, for providing the pathology images; and to Dr. Crysten Brinkley at the Department of Neurology at the University of Alabama at Birmingham for her assistance with this case presentation.
Disclosure: Nothing to report.
- ,,,.Mucormycosis: emerging prominence of cutaneous infections.Clin Infect Dis.1994;19:67–76.
- ,,,.Zygomycosis in the 1990s in a tertiary‐care cancer center.Clin Infect Dis.2000;30:851–856.
- ,,, et al.Mucormycosis during deferoxamine therapy is a siderophore‐mediated infection. In vitro and in vivo animal studies.J Clin Invest.1993;91:1979–1986.
- ,,, et al.Epidemiology and outcome of zygomycosis: a review of 929 reported cases.Clin Infect Dis.2005;41:634–653.
- ,,,,,.Cutaneous mucormycosis of the head and neck with parotid gland involvement: first report of a case.Ear Nose Throat J.2004;83:282–286.
- ,,,,,.A successful combined endovascular and surgical treatment of a cranial base mucormycosis with an associated internal carotid artery pseudoaneurysm.Neurosurgery.2009;65:733–740.
- ,.Judgment under uncertainty: heuristics and biases.Science.1974;185:1124–1131.
- ,,,,.Clinical problem‐solving. Anchors away.N Engl J Med.2007;356:504–509.
- ,,,.Clinical problem‐solving. Empirically incorrect.N Engl J Med.2006;354:509–514.
- ,,.Dealing with uncertainty, risks, and tradeoffs in clinical decisions. A cognitive science approach.Ann Intern Med.1988;108:435–449.
- ,,.Fatal strokes in patients with rhino‐orbito‐cerebral mucormycosis and associated vasculopathy.Scand J Infect Dis.2004;36:643–648.
- ,,, et al.Combination polyene‐caspofungin treatment of rhino‐orbital‐cerebral mucormycosis.Clin Infect Dis.2008;47:364–371.
- ,,,,.Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases.Clin Infect Dis.2006;42:e61–e65.
A 35‐year‐old woman presented to her primary care physician complaining of left post‐auricular pain, swelling, and redness. She described the pain as 8 out of 10, constant, sharp, and nonradiating. She denied fever or chills. A presumptive diagnosis of cellulitis led to a prescription for oral trimethoprim‐sulfamethoxazole. Left facial swelling worsened despite 4 days of antibiotics, so she came to the emergency department.
Noninfectious causes of this woman's symptoms include trauma, or an inflammatory condition such as polychondritis. Key infectious considerations are mastoiditis or a mastoid abscess. Herpes zoster with involvement of the pinna and auditory canal may also present with pain and redness. In the absence of findings suggestive of an infection arising from the auditory canal, cellulitis is a reasonable consideration. With the growing incidence of community‐acquired methicillin‐resistant Staphylococcus aureus infections, an agent effective against this pathogen such as trimethoprim‐sulfamethoxazole may be used, usually in combination with an antibiotic that provides more reliable coverage for group A streptococcus.
Her past medical history included poorly controlled type II diabetes mellitus and asthma. She reported no previous surgical history. Her current medications were insulin, albuterol inhaler, and trimethoprim‐sulfamethoxazole, although she had a history of noncompliance with her insulin. She was married with 1 child and was unemployed. She smoked 1 pack of cigarettes daily, drank up to 6 beers daily, and denied use of illicit drugs.
Her history of diabetes increases her risk of malignant otitis externa. Both diabetes and excess alcohol consumption are risk factors for herpes zoster. Smoking has been shown to increase the risk of otitis media and carriage by S. pneumoniae, a common pathogen in ear infections.
She was ill‐appearing and in moderate respiratory distress. Her temperature was 39C, blood pressure 149/93 mmHg, pulse 95 beats per minute, respiratory rate of 26 times per minute, with an oxygen saturation of 96% while breathing ambient air. She had swelling of the left side of the face extending to the left forehead and lateral neck. Examination of the external ear and auditory canal were unremarkable. The swelling had no associated erythema, tenderness, or lymphadenopathy. She had no oropharyngeal or nasal ulcers present. Her pupils were equal, round, and reactive to light and accommodation with normal sclera. Her trachea was midline; thyroid exam was normal. The heart sounds included normal S1 and S2 without murmurs, rubs, or gallops. Her lung exam was remarkable for inspiratory stridor. The abdominal examination revealed no distention, tenderness, organomegaly, or masses. Cranial nerve testing revealed a left‐sided central seventh nerve palsy along with decreased visual acuity of the left eye. Strength, sensation, and deep tendon reflexes were normal.
While there are many causes of facial nerve palsy, distinguishing between a peripheral palsy (which causes paralysis of the entire ipsilateral side of the face) and a central palsy (which spares the musculature of the forehead) is important. The most common type of peripheral facial nerve palsy is Bell's palsy. Infections such as meningitis or tumors of the central nervous system can cause central facial nerve or other cranial nerve palsy. Important infections to consider in this case would be viral such as herpes zoster or simplex, or atypical bacteria such as Mycoplasma and Rickettsia, which may explain the neurologic but not all of the other clinical findings in this case. It is also critical to determine whether she has an isolated seventh cranial nerve palsy or if other cranial nerves are involved such as may occur with basilar meningitis, which has a myriad of infectious and noninfectious causes. The decreased visual acuity may be a result of corneal dryness and abrasions from inability to close the eye but may also represent optic nerve problems, so detailed ophthalmologic exam is essential. Her ill appearance coupled with facial and neck swelling leads me to at least consider Lemierre's syndrome with central nervous system involvement. Finally, facial swelling and the inspiratory stridor may represent angioedema, although one‐sided involvement of the face would be unusual.
The results of initial laboratory testing were as follows: sodium, 138 mmol/L; potassium, 3.4 mmol/L; chloride, 109 mmol/L; bicarbonate, 14 mmol/L; blood urea nitrogen level, 19 mg/dL; creatinine, 1.1 mg/dL; white cell count, 23,510/mm3; differential, 90% neutrophils, 1% bands, 7% lymphocytes, 2% monocytes; hemoglobin level, 12.5 g/dL; platelet count, 566,000/mm3; hemoglobin A1c, 11%; albumin, 1.6 g/dL; total protein, 6.2 g/dL; total bilirubin, 0.8 mg/dL; alkaline phosphatase, 103 U/L; alanine aminotransferase level, 14 U/L; international normalized ratio of 1.2; partial thromboplastin time, 29 seconds (normal value, 2434 seconds); erythrocyte sedimentation rate, 121 mm/hr; creatine kinase, 561 U/L (normal value 25190). Arterial blood gas measurements with the patient breathing 50% oxygen revealed a pH of 7.34, a partial pressure of carbon dioxide of 28 mmHg, and a partial pressure of oxygen of 228 mmHg.
I am concerned that this patient has sepsis, likely due to an infectious trigger. With her clinical presentation localized to the head and neck, her history of diabetes, and the accelerated sedimentation rate, malignant otitis externa would explain many of her findings. Empiric anti‐infective therapy directed toward Pseudomonas aeruginosa should be initiated, and imaging of the head and ear should be undertaken.
The patient required intubation due to increased respiratory distress and stridor. Her physicians used intravenous vancomycin, clindamycin, and piperacillin/tazobactam to treat presumed cellulitis. Her abnormal neurologic exam led to magnetic resonance (MR) imaging and MR angiography of her neck and brain, which showed evidence of multiple regions of ischemia in the left occipital and inferior parietal distributions, as well as bilateral cerebellar distributions and enhancement of the parotid gland and mastoid air cells (Figure 1). A cerebral angiogram revealed irregularity and caliber reduction in multiple cervical and intracranial arteries, associated with intraluminal thrombi within the left intracranial vertebral artery, consistent with either vasculitis or infectious angioinvasion (Figure 2).


The angioinvasive nature of the findings on imaging leads me to suspect fungal infection. The patient's history of diabetes mellitus and acidosis are risk factors for mucormycosis. Aspergillus and Fusarium may also be angioinvasive but would be much more likely in neutropenic or severely immunocompromised patients. S. aureus may cause septic emboli mimicking angioinvasion but should be readily detected in conventional blood cultures. At this point, I would empirically begin amphotericin B; tissue, however, is needed for definitive diagnosis and a surgical consult should be requested.
After reviewing her imaging studies, an investigation for vasculitis and hypercoagulable states including antinuclear antibody, anti‐deoxyribonucleic acid, anti‐Smith antibody, anti‐SSA antibody level, anti‐SSB level, antineutrophil cytoplasmic antibody, activated protein C resistance level, factor VIII level, human immunodeficiency virus antibody, homocysteine level, cardiolipin antibody testing, lupus anticoagulant, prothrombin 20210 mutation, and protein C level was done, and all tests were normal. Protein S level was slightly low at 64% (normal value 65%140%). Given the enlarged parotid gland and the enhancement of the left parotid bed on magnetic resonance imaging, she underwent a parotid biopsy that revealed sialadenitis.
Systemic vasculitides can result in tissue damage, mediated by the release of endogenous cellular contents from dying cells, known as damage‐associated molecular patterns, sufficient to cause systemic inflammatory response syndrome (SIRS). This patient presented with acute symptoms but has negative laboratory studies for autoantibodies. The parotid biopsy also did not reveal evidence of vasculitis. All these findings make the diagnosis of vasculitis much less likely.
She remained in the medical intensive care unit on mechanical ventilation, with minimal symptomatic improvement. On hospital day 10, the patient developed necrosis of the left external ear. A punch biopsy of the necrotic area of her left pinna was performed; the pathology report read: Sections of punch biopsy of skin show an unremarkable epidermis. There is dermal necrosis involving the stroma and adnexal structures. Intravascular thrombi within the deep dermis are seen. Within superficial dermis there are broad, elongated, nonseptated hyaline structures reminiscent of Mucor. Special stains (periodic acid‐Schiff stain and Grocott Gomori methenamine silver stain [GMS]) performed with appropriately reactive controls fail to highlight these structures (Figure 3). The infectious disease team reviewed the pathology slides with the pathologist. As there was inconclusive evidence for zygomycosis, ie, only a few hyaline structures which failed to stain with GMS stain, the consultants recommended no change in the patient's management.

The gross and microscopic evidence of necrosis and areas of intravascular thrombi are nonspecific but compatible with a fungal infection in a patient with risk factors for zygomycosis. The GMS stain is a very sensitive stain for fungal structures, so a negative stain in this case is surprising, but additional testing such as immunohistochemistry should be pursued to confirm or refute this diagnosis. While Rhizopus species can be contaminants, the laboratory finding of these organisms in specimens from patients with risk factors for zygomycosis should not be ignored.
On hospital day 12, the patient was noted to have increased facial swelling. A computed tomographic (CT) angiogram of the neck revealed necrosis of the anterior and posterior paraspinal muscles from the skull base to C34, marked swelling of the left parotid gland, and left inferior parieto‐occipital enhancing lesion. An incisional parotid biopsy was performed. Special stains were positive for broad‐based fungal hyphae consistent with mucormycosis (Figure 4).

Given these findings, the patient should be started on amphotericin B immediately. Medical therapy alone generally does not suffice, and aggressive surgical debridement combined with intravenous antifungal therapy results in better outcomes. The longer the duration of symptoms and the greater the progression of disease, the less favorable the prognosis.
The patient was started on amphotericin B lipid complex and micafungin. However, after 16 days of therapy, repeat imaging of the neck showed worsening necrosis of the neck muscles. At this time, she underwent extensive debridement of face and neck, and posaconazole was added. After prolonged hospitalization, she was discharged to a rehabilitation facility on posaconazole. She resided in a nursing facility for 6 months. One year after her hospitalization, she is living at home and is able to ambulate independently, but requires feeding through a percutaneous endoscopic gastrostomy (PEG) tube because she remains dysphagic.
COMMENTARY
Infections caused by the ubiquitous fungi of the class Zygomycetes typically take 1 of 5 forms: rhinocerebral, pulmonary, gastrointestinal, disseminated, and cutaneous. The presentation varies widely, ranging from plaques, skin swelling, pustules, cellulitis, blisters, nodules, ulcerations, and ecthyma gangrenosum‐like lesions to deeper infections such as necrotizing fasciitis, osteomyelitis, and disseminated infection.1 Infections typically occur in immunocompromised hosts, including transplant recipients and patients with hematologic malignancy, but also occur in patients with diabetes mellitus, intravenous drug users, and patients on deferoxamine therapy.2 Deferoxamine and other iron‐binding therapy is thought to predispose to zygomycetes infections because of improved iron uptake of the fungal species and, thus, stimulation of growth.3 Pulmonary and rhinocerebral infections are the most common clinically encountered forms, and 44% of cutaneous infections are complicated by deep extension or dissemination.4
The articles cited above describe the more typical presentations of this rare disease. However, this patient had an unusual presentation, as parotid involvement due to zygomycosis has only been described once previously.5 Her inflammatory vasculitis and ensuing strokes from involvement of the carotid artery are recognized complications of zygomycosis, and in 1 case series of 41 patients with rhinocerebral mucormycosis, carotid involvement was seen in 31% of patients.6 After the punch biopsy of the patient's pinna showing nonseptated hyphae reminiscent of Mucor, why did her physicians delay administering amphotericin?
There are 2 likely possibilities: anchoring bias or error in medical decision‐making due to inaccurate probability estimates. Anchoring bias describes a heuristic where the initial diagnosis or gestalt biases the physician's process for assigning a final diagnosis.7, 8 This bias creates cognitive errors by limiting creativity in diagnosis. In this case, the infectious disease team carefully weighed the information obtained from the first biopsy. Given their low pretest estimate of this virtually unreported presentation of a rare disease, they decided to evaluate further without beginning antifungal therapy. Of note, there were few hyaline structures, and those structures lacked uptake of GMS. Since they considered the diagnosis yet rejected the diagnosis due to insufficient evidence, it is unlikely that anchoring bias played a role.
Was there an error in medical decision‐making? The physicians in this case faced a very common medical dilemma: whether or not to start a toxic medication empirically or wait for diagnostic confirmation prior to treatment.9 To solve this dilemma, one can apply decision analysis. Moskowitz et al described 5 phases of medical decision analysis by which a probabilistic right answer to clinical scenarios can be deduced mathematically.10 To solve this problem, probabilities must be assigned to the risk of giving a drug to a patient without the disease versus the risk of not giving a drug to a patient with the disease. For example, amphotericin deoxycholate causes acute renal failure in 30% to 47% of patients. Newer formulations of amphotericin, such as liposomal amphotericin and lipid complex, result in lower rates of nephrotoxicity (27% vs 47%). The risk of not giving amphotericin to a patient with zygomycosis is death. Even in patients treated with amphotericin, the mortality rate has been shown to be 66%, and up to 100% in those with strokes related to zygomycosis.2, 6, 11 Simply looking at these probabilities, decision analysis would favor empiric treatment.
The physicians caring for this patient did not have the luxury of retrospective speculation. After looking at all of the data, the equivocal skin biopsy and rare clinical presentation, the question to ask would change: What is the risk of giving amphotericin empirically to someone who, based on available information, has a very low probability of having zygomycosis? When phrased in this manner, there is a 47% chance of nephrotoxicity with amphotericin versus the very small probability that you have diagnosed a case of zygomycosis that has only been described once in the literature. Mathematically andmore importantlyclinically, this question becomes more difficult to answer. However, no value can be placed on the possibility of death in suspected zygomycosis, and the risk of short‐term amphotericin use is much less than that of a course of treatment. As such, empiric therapy should always be given.
Physicians are not mathematicians, and dynamic clinical scenarios are not so easily made into static math problems. Disease presentations evolve over time towards a diagnosable clinical pattern, as was the case with this patient. Two days after the aforementioned biopsy, she worsened and in less time than it would have taken to isolate zygomycosis from the first biopsy, a second biopsy revealed the typical nonseptated hyphae demarcated with the GMS stain. Even appropriate diagnostic testing, thoughtful interpretation, and avoidance of certain cognitive errors can result in incorrect diagnoses and delayed treatment. It is monitoring the progression of disease and collecting additional data that allows physicians to mold a diagnosis and create a treatment plan.
The primary treatment of zygomycosis should include amphotericin. However, there are limited data to support combination therapy with an echinocandin in severe cases, as in this patient.12 Posaconazole is not recommended for monotherapy as an initial therapy, but there is data for its use as salvage therapy in zygomycosis.13 This case highlights the difficulties that physicians face in the diagnosis and treatment of rare diseases. Cerebral infarction in a hematologic malignancy, uncontrolled diabetes, or iron chelation therapy could be the initial presentation of rhinocerebral zygomycosis. There truly are different strokes for different folks. Recognizing this and similar presentations may lead to a more rapid diagnosis and treatment of zygomycosis.
TEACHING POINTS
-
Zygomycosis has a wide range of clinical presentations ranging from skin lesions to deep tissue infections. As it is an angioinvasive organism, it can also present as cerebral infarcts and brain abscesses.
-
Zygomycosis infections should be suspected in patients with uncontrolled diabetes, hematologic or oncologic malignancies, and patients on iron chelation therapy with a potentially compatible clinical picture.
-
If zygomycosis infection is suspected, rapid histologic diagnosis should be attempted. However, as histologic diagnosis can take time, empiric therapy with amphotericin should always be administered.
-
Amphotericin remains the primary medical therapy for this disease; however, there is limited emerging evidence to suggest that echinocandins can be used in combination with amphotericin for improved treatment of severe rhinocerebral zygomyocosis. Posaconazole has a role as salvage therapy in zygomycosis, but should not be used as the sole primary treatment.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
This icon represents the patient's case. Each paragraph that follows represents the discussant's thoughts.
Acknowledgements
The authors are indebted to Dr Glenn Roberson at the Department of Radiology, University of Alabama at Birmingham, for providing the radiographic images; to Dr Aleodor Andea at the Department of Pathology, University of Alabama at Birmingham, for providing the pathology images; and to Dr. Crysten Brinkley at the Department of Neurology at the University of Alabama at Birmingham for her assistance with this case presentation.
Disclosure: Nothing to report.
A 35‐year‐old woman presented to her primary care physician complaining of left post‐auricular pain, swelling, and redness. She described the pain as 8 out of 10, constant, sharp, and nonradiating. She denied fever or chills. A presumptive diagnosis of cellulitis led to a prescription for oral trimethoprim‐sulfamethoxazole. Left facial swelling worsened despite 4 days of antibiotics, so she came to the emergency department.
Noninfectious causes of this woman's symptoms include trauma, or an inflammatory condition such as polychondritis. Key infectious considerations are mastoiditis or a mastoid abscess. Herpes zoster with involvement of the pinna and auditory canal may also present with pain and redness. In the absence of findings suggestive of an infection arising from the auditory canal, cellulitis is a reasonable consideration. With the growing incidence of community‐acquired methicillin‐resistant Staphylococcus aureus infections, an agent effective against this pathogen such as trimethoprim‐sulfamethoxazole may be used, usually in combination with an antibiotic that provides more reliable coverage for group A streptococcus.
Her past medical history included poorly controlled type II diabetes mellitus and asthma. She reported no previous surgical history. Her current medications were insulin, albuterol inhaler, and trimethoprim‐sulfamethoxazole, although she had a history of noncompliance with her insulin. She was married with 1 child and was unemployed. She smoked 1 pack of cigarettes daily, drank up to 6 beers daily, and denied use of illicit drugs.
Her history of diabetes increases her risk of malignant otitis externa. Both diabetes and excess alcohol consumption are risk factors for herpes zoster. Smoking has been shown to increase the risk of otitis media and carriage by S. pneumoniae, a common pathogen in ear infections.
She was ill‐appearing and in moderate respiratory distress. Her temperature was 39C, blood pressure 149/93 mmHg, pulse 95 beats per minute, respiratory rate of 26 times per minute, with an oxygen saturation of 96% while breathing ambient air. She had swelling of the left side of the face extending to the left forehead and lateral neck. Examination of the external ear and auditory canal were unremarkable. The swelling had no associated erythema, tenderness, or lymphadenopathy. She had no oropharyngeal or nasal ulcers present. Her pupils were equal, round, and reactive to light and accommodation with normal sclera. Her trachea was midline; thyroid exam was normal. The heart sounds included normal S1 and S2 without murmurs, rubs, or gallops. Her lung exam was remarkable for inspiratory stridor. The abdominal examination revealed no distention, tenderness, organomegaly, or masses. Cranial nerve testing revealed a left‐sided central seventh nerve palsy along with decreased visual acuity of the left eye. Strength, sensation, and deep tendon reflexes were normal.
While there are many causes of facial nerve palsy, distinguishing between a peripheral palsy (which causes paralysis of the entire ipsilateral side of the face) and a central palsy (which spares the musculature of the forehead) is important. The most common type of peripheral facial nerve palsy is Bell's palsy. Infections such as meningitis or tumors of the central nervous system can cause central facial nerve or other cranial nerve palsy. Important infections to consider in this case would be viral such as herpes zoster or simplex, or atypical bacteria such as Mycoplasma and Rickettsia, which may explain the neurologic but not all of the other clinical findings in this case. It is also critical to determine whether she has an isolated seventh cranial nerve palsy or if other cranial nerves are involved such as may occur with basilar meningitis, which has a myriad of infectious and noninfectious causes. The decreased visual acuity may be a result of corneal dryness and abrasions from inability to close the eye but may also represent optic nerve problems, so detailed ophthalmologic exam is essential. Her ill appearance coupled with facial and neck swelling leads me to at least consider Lemierre's syndrome with central nervous system involvement. Finally, facial swelling and the inspiratory stridor may represent angioedema, although one‐sided involvement of the face would be unusual.
The results of initial laboratory testing were as follows: sodium, 138 mmol/L; potassium, 3.4 mmol/L; chloride, 109 mmol/L; bicarbonate, 14 mmol/L; blood urea nitrogen level, 19 mg/dL; creatinine, 1.1 mg/dL; white cell count, 23,510/mm3; differential, 90% neutrophils, 1% bands, 7% lymphocytes, 2% monocytes; hemoglobin level, 12.5 g/dL; platelet count, 566,000/mm3; hemoglobin A1c, 11%; albumin, 1.6 g/dL; total protein, 6.2 g/dL; total bilirubin, 0.8 mg/dL; alkaline phosphatase, 103 U/L; alanine aminotransferase level, 14 U/L; international normalized ratio of 1.2; partial thromboplastin time, 29 seconds (normal value, 2434 seconds); erythrocyte sedimentation rate, 121 mm/hr; creatine kinase, 561 U/L (normal value 25190). Arterial blood gas measurements with the patient breathing 50% oxygen revealed a pH of 7.34, a partial pressure of carbon dioxide of 28 mmHg, and a partial pressure of oxygen of 228 mmHg.
I am concerned that this patient has sepsis, likely due to an infectious trigger. With her clinical presentation localized to the head and neck, her history of diabetes, and the accelerated sedimentation rate, malignant otitis externa would explain many of her findings. Empiric anti‐infective therapy directed toward Pseudomonas aeruginosa should be initiated, and imaging of the head and ear should be undertaken.
The patient required intubation due to increased respiratory distress and stridor. Her physicians used intravenous vancomycin, clindamycin, and piperacillin/tazobactam to treat presumed cellulitis. Her abnormal neurologic exam led to magnetic resonance (MR) imaging and MR angiography of her neck and brain, which showed evidence of multiple regions of ischemia in the left occipital and inferior parietal distributions, as well as bilateral cerebellar distributions and enhancement of the parotid gland and mastoid air cells (Figure 1). A cerebral angiogram revealed irregularity and caliber reduction in multiple cervical and intracranial arteries, associated with intraluminal thrombi within the left intracranial vertebral artery, consistent with either vasculitis or infectious angioinvasion (Figure 2).


The angioinvasive nature of the findings on imaging leads me to suspect fungal infection. The patient's history of diabetes mellitus and acidosis are risk factors for mucormycosis. Aspergillus and Fusarium may also be angioinvasive but would be much more likely in neutropenic or severely immunocompromised patients. S. aureus may cause septic emboli mimicking angioinvasion but should be readily detected in conventional blood cultures. At this point, I would empirically begin amphotericin B; tissue, however, is needed for definitive diagnosis and a surgical consult should be requested.
After reviewing her imaging studies, an investigation for vasculitis and hypercoagulable states including antinuclear antibody, anti‐deoxyribonucleic acid, anti‐Smith antibody, anti‐SSA antibody level, anti‐SSB level, antineutrophil cytoplasmic antibody, activated protein C resistance level, factor VIII level, human immunodeficiency virus antibody, homocysteine level, cardiolipin antibody testing, lupus anticoagulant, prothrombin 20210 mutation, and protein C level was done, and all tests were normal. Protein S level was slightly low at 64% (normal value 65%140%). Given the enlarged parotid gland and the enhancement of the left parotid bed on magnetic resonance imaging, she underwent a parotid biopsy that revealed sialadenitis.
Systemic vasculitides can result in tissue damage, mediated by the release of endogenous cellular contents from dying cells, known as damage‐associated molecular patterns, sufficient to cause systemic inflammatory response syndrome (SIRS). This patient presented with acute symptoms but has negative laboratory studies for autoantibodies. The parotid biopsy also did not reveal evidence of vasculitis. All these findings make the diagnosis of vasculitis much less likely.
She remained in the medical intensive care unit on mechanical ventilation, with minimal symptomatic improvement. On hospital day 10, the patient developed necrosis of the left external ear. A punch biopsy of the necrotic area of her left pinna was performed; the pathology report read: Sections of punch biopsy of skin show an unremarkable epidermis. There is dermal necrosis involving the stroma and adnexal structures. Intravascular thrombi within the deep dermis are seen. Within superficial dermis there are broad, elongated, nonseptated hyaline structures reminiscent of Mucor. Special stains (periodic acid‐Schiff stain and Grocott Gomori methenamine silver stain [GMS]) performed with appropriately reactive controls fail to highlight these structures (Figure 3). The infectious disease team reviewed the pathology slides with the pathologist. As there was inconclusive evidence for zygomycosis, ie, only a few hyaline structures which failed to stain with GMS stain, the consultants recommended no change in the patient's management.

The gross and microscopic evidence of necrosis and areas of intravascular thrombi are nonspecific but compatible with a fungal infection in a patient with risk factors for zygomycosis. The GMS stain is a very sensitive stain for fungal structures, so a negative stain in this case is surprising, but additional testing such as immunohistochemistry should be pursued to confirm or refute this diagnosis. While Rhizopus species can be contaminants, the laboratory finding of these organisms in specimens from patients with risk factors for zygomycosis should not be ignored.
On hospital day 12, the patient was noted to have increased facial swelling. A computed tomographic (CT) angiogram of the neck revealed necrosis of the anterior and posterior paraspinal muscles from the skull base to C34, marked swelling of the left parotid gland, and left inferior parieto‐occipital enhancing lesion. An incisional parotid biopsy was performed. Special stains were positive for broad‐based fungal hyphae consistent with mucormycosis (Figure 4).

Given these findings, the patient should be started on amphotericin B immediately. Medical therapy alone generally does not suffice, and aggressive surgical debridement combined with intravenous antifungal therapy results in better outcomes. The longer the duration of symptoms and the greater the progression of disease, the less favorable the prognosis.
The patient was started on amphotericin B lipid complex and micafungin. However, after 16 days of therapy, repeat imaging of the neck showed worsening necrosis of the neck muscles. At this time, she underwent extensive debridement of face and neck, and posaconazole was added. After prolonged hospitalization, she was discharged to a rehabilitation facility on posaconazole. She resided in a nursing facility for 6 months. One year after her hospitalization, she is living at home and is able to ambulate independently, but requires feeding through a percutaneous endoscopic gastrostomy (PEG) tube because she remains dysphagic.
COMMENTARY
Infections caused by the ubiquitous fungi of the class Zygomycetes typically take 1 of 5 forms: rhinocerebral, pulmonary, gastrointestinal, disseminated, and cutaneous. The presentation varies widely, ranging from plaques, skin swelling, pustules, cellulitis, blisters, nodules, ulcerations, and ecthyma gangrenosum‐like lesions to deeper infections such as necrotizing fasciitis, osteomyelitis, and disseminated infection.1 Infections typically occur in immunocompromised hosts, including transplant recipients and patients with hematologic malignancy, but also occur in patients with diabetes mellitus, intravenous drug users, and patients on deferoxamine therapy.2 Deferoxamine and other iron‐binding therapy is thought to predispose to zygomycetes infections because of improved iron uptake of the fungal species and, thus, stimulation of growth.3 Pulmonary and rhinocerebral infections are the most common clinically encountered forms, and 44% of cutaneous infections are complicated by deep extension or dissemination.4
The articles cited above describe the more typical presentations of this rare disease. However, this patient had an unusual presentation, as parotid involvement due to zygomycosis has only been described once previously.5 Her inflammatory vasculitis and ensuing strokes from involvement of the carotid artery are recognized complications of zygomycosis, and in 1 case series of 41 patients with rhinocerebral mucormycosis, carotid involvement was seen in 31% of patients.6 After the punch biopsy of the patient's pinna showing nonseptated hyphae reminiscent of Mucor, why did her physicians delay administering amphotericin?
There are 2 likely possibilities: anchoring bias or error in medical decision‐making due to inaccurate probability estimates. Anchoring bias describes a heuristic where the initial diagnosis or gestalt biases the physician's process for assigning a final diagnosis.7, 8 This bias creates cognitive errors by limiting creativity in diagnosis. In this case, the infectious disease team carefully weighed the information obtained from the first biopsy. Given their low pretest estimate of this virtually unreported presentation of a rare disease, they decided to evaluate further without beginning antifungal therapy. Of note, there were few hyaline structures, and those structures lacked uptake of GMS. Since they considered the diagnosis yet rejected the diagnosis due to insufficient evidence, it is unlikely that anchoring bias played a role.
Was there an error in medical decision‐making? The physicians in this case faced a very common medical dilemma: whether or not to start a toxic medication empirically or wait for diagnostic confirmation prior to treatment.9 To solve this dilemma, one can apply decision analysis. Moskowitz et al described 5 phases of medical decision analysis by which a probabilistic right answer to clinical scenarios can be deduced mathematically.10 To solve this problem, probabilities must be assigned to the risk of giving a drug to a patient without the disease versus the risk of not giving a drug to a patient with the disease. For example, amphotericin deoxycholate causes acute renal failure in 30% to 47% of patients. Newer formulations of amphotericin, such as liposomal amphotericin and lipid complex, result in lower rates of nephrotoxicity (27% vs 47%). The risk of not giving amphotericin to a patient with zygomycosis is death. Even in patients treated with amphotericin, the mortality rate has been shown to be 66%, and up to 100% in those with strokes related to zygomycosis.2, 6, 11 Simply looking at these probabilities, decision analysis would favor empiric treatment.
The physicians caring for this patient did not have the luxury of retrospective speculation. After looking at all of the data, the equivocal skin biopsy and rare clinical presentation, the question to ask would change: What is the risk of giving amphotericin empirically to someone who, based on available information, has a very low probability of having zygomycosis? When phrased in this manner, there is a 47% chance of nephrotoxicity with amphotericin versus the very small probability that you have diagnosed a case of zygomycosis that has only been described once in the literature. Mathematically andmore importantlyclinically, this question becomes more difficult to answer. However, no value can be placed on the possibility of death in suspected zygomycosis, and the risk of short‐term amphotericin use is much less than that of a course of treatment. As such, empiric therapy should always be given.
Physicians are not mathematicians, and dynamic clinical scenarios are not so easily made into static math problems. Disease presentations evolve over time towards a diagnosable clinical pattern, as was the case with this patient. Two days after the aforementioned biopsy, she worsened and in less time than it would have taken to isolate zygomycosis from the first biopsy, a second biopsy revealed the typical nonseptated hyphae demarcated with the GMS stain. Even appropriate diagnostic testing, thoughtful interpretation, and avoidance of certain cognitive errors can result in incorrect diagnoses and delayed treatment. It is monitoring the progression of disease and collecting additional data that allows physicians to mold a diagnosis and create a treatment plan.
The primary treatment of zygomycosis should include amphotericin. However, there are limited data to support combination therapy with an echinocandin in severe cases, as in this patient.12 Posaconazole is not recommended for monotherapy as an initial therapy, but there is data for its use as salvage therapy in zygomycosis.13 This case highlights the difficulties that physicians face in the diagnosis and treatment of rare diseases. Cerebral infarction in a hematologic malignancy, uncontrolled diabetes, or iron chelation therapy could be the initial presentation of rhinocerebral zygomycosis. There truly are different strokes for different folks. Recognizing this and similar presentations may lead to a more rapid diagnosis and treatment of zygomycosis.
TEACHING POINTS
-
Zygomycosis has a wide range of clinical presentations ranging from skin lesions to deep tissue infections. As it is an angioinvasive organism, it can also present as cerebral infarcts and brain abscesses.
-
Zygomycosis infections should be suspected in patients with uncontrolled diabetes, hematologic or oncologic malignancies, and patients on iron chelation therapy with a potentially compatible clinical picture.
-
If zygomycosis infection is suspected, rapid histologic diagnosis should be attempted. However, as histologic diagnosis can take time, empiric therapy with amphotericin should always be administered.
-
Amphotericin remains the primary medical therapy for this disease; however, there is limited emerging evidence to suggest that echinocandins can be used in combination with amphotericin for improved treatment of severe rhinocerebral zygomyocosis. Posaconazole has a role as salvage therapy in zygomycosis, but should not be used as the sole primary treatment.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
This icon represents the patient's case. Each paragraph that follows represents the discussant's thoughts.
Acknowledgements
The authors are indebted to Dr Glenn Roberson at the Department of Radiology, University of Alabama at Birmingham, for providing the radiographic images; to Dr Aleodor Andea at the Department of Pathology, University of Alabama at Birmingham, for providing the pathology images; and to Dr. Crysten Brinkley at the Department of Neurology at the University of Alabama at Birmingham for her assistance with this case presentation.
Disclosure: Nothing to report.
- ,,,.Mucormycosis: emerging prominence of cutaneous infections.Clin Infect Dis.1994;19:67–76.
- ,,,.Zygomycosis in the 1990s in a tertiary‐care cancer center.Clin Infect Dis.2000;30:851–856.
- ,,, et al.Mucormycosis during deferoxamine therapy is a siderophore‐mediated infection. In vitro and in vivo animal studies.J Clin Invest.1993;91:1979–1986.
- ,,, et al.Epidemiology and outcome of zygomycosis: a review of 929 reported cases.Clin Infect Dis.2005;41:634–653.
- ,,,,,.Cutaneous mucormycosis of the head and neck with parotid gland involvement: first report of a case.Ear Nose Throat J.2004;83:282–286.
- ,,,,,.A successful combined endovascular and surgical treatment of a cranial base mucormycosis with an associated internal carotid artery pseudoaneurysm.Neurosurgery.2009;65:733–740.
- ,.Judgment under uncertainty: heuristics and biases.Science.1974;185:1124–1131.
- ,,,,.Clinical problem‐solving. Anchors away.N Engl J Med.2007;356:504–509.
- ,,,.Clinical problem‐solving. Empirically incorrect.N Engl J Med.2006;354:509–514.
- ,,.Dealing with uncertainty, risks, and tradeoffs in clinical decisions. A cognitive science approach.Ann Intern Med.1988;108:435–449.
- ,,.Fatal strokes in patients with rhino‐orbito‐cerebral mucormycosis and associated vasculopathy.Scand J Infect Dis.2004;36:643–648.
- ,,, et al.Combination polyene‐caspofungin treatment of rhino‐orbital‐cerebral mucormycosis.Clin Infect Dis.2008;47:364–371.
- ,,,,.Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases.Clin Infect Dis.2006;42:e61–e65.
- ,,,.Mucormycosis: emerging prominence of cutaneous infections.Clin Infect Dis.1994;19:67–76.
- ,,,.Zygomycosis in the 1990s in a tertiary‐care cancer center.Clin Infect Dis.2000;30:851–856.
- ,,, et al.Mucormycosis during deferoxamine therapy is a siderophore‐mediated infection. In vitro and in vivo animal studies.J Clin Invest.1993;91:1979–1986.
- ,,, et al.Epidemiology and outcome of zygomycosis: a review of 929 reported cases.Clin Infect Dis.2005;41:634–653.
- ,,,,,.Cutaneous mucormycosis of the head and neck with parotid gland involvement: first report of a case.Ear Nose Throat J.2004;83:282–286.
- ,,,,,.A successful combined endovascular and surgical treatment of a cranial base mucormycosis with an associated internal carotid artery pseudoaneurysm.Neurosurgery.2009;65:733–740.
- ,.Judgment under uncertainty: heuristics and biases.Science.1974;185:1124–1131.
- ,,,,.Clinical problem‐solving. Anchors away.N Engl J Med.2007;356:504–509.
- ,,,.Clinical problem‐solving. Empirically incorrect.N Engl J Med.2006;354:509–514.
- ,,.Dealing with uncertainty, risks, and tradeoffs in clinical decisions. A cognitive science approach.Ann Intern Med.1988;108:435–449.
- ,,.Fatal strokes in patients with rhino‐orbito‐cerebral mucormycosis and associated vasculopathy.Scand J Infect Dis.2004;36:643–648.
- ,,, et al.Combination polyene‐caspofungin treatment of rhino‐orbital‐cerebral mucormycosis.Clin Infect Dis.2008;47:364–371.
- ,,,,.Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases.Clin Infect Dis.2006;42:e61–e65.
A Lifetime in the Making
A 66‐year‐old man presented to the emergency department with 3 weeks of progressive exertional dyspnea. He also reported a single episode of chest pain 1 day prior to admission.
Cardiac and pulmonary causes of dyspnea are the most common. Other causes include anemia or a neuromuscular process. Given the recent episode of chest pain, coronary ischemia, congestive heart failure, chronic obstructive pulmonary disease (COPD), pulmonary embolism, and pericardial effusion must be considered.
Up until 3 weeks ago, he had no exercise intolerance, and had been relatively active. He began noticing progressive dyspnea to the point where he had considerable difficulty walking up stairs, and performing minor household chores. He also complained of orthopnea and paroxysmal nocturnal dyspnea for the last 3 weeks.He denied chest pain at presentation, but 24 hours prior, he experienced one episode of sharp, left‐sided, nonradiating, nonpositional chest pain that occurred at rest. It lasted approximately 20 minutes and was not associated with diaphoresis, nausea, vomiting, or palpitations. He had never experienced chest discomfort prior to this episode. He denied fever, chills, cough, or wheezing.
Progressive dyspnea on exertion with associated orthopnea and paroxysmal nocturnal dyspnea is classically seen in patients with heart failure and is typically associated with left ventricular failure. However, paroxysmal nocturnal dyspnea and orthopnea are only moderately specific for heart failure. Orthopnea can also be seen in pericardial disease, and in numerous pulmonary diseases, including asthma, COPD, pulmonary hypertension, diaphragmatic weakness, pleural effusion, pulmonary embolism, and any apical lung process including lung cancer or pneumonia. Paroxysmal nocturnal dyspnea can be seen in many of the same disorders and can also be reported in obstructive sleep apnea.
His past medical history was remarkable for two episodes of syncope, occurring 5 and 3 years ago, both while working outside in warm weather. Neither was associated with chest pain, diaphoresis, palpitations, or post‐ictal symptoms. He was diagnosed with prostate cancer 8 years ago, and underwent 2 years of androgen‐deprivation therapy with goserelin along with local radiation therapy. Medications included subcutaneous goserelin every 3 months and daily omeprazole. He denied any other prescription, over‐the‐counter, or herbal medications. He reported a 50‐pack‐year history of smoking, but denied alcohol or illicit drug abuse. He denied any travel history or recent immobilization. He had no children, and there was no known history of heart disease in his family.
The past medical history of two episodes of likely exertional syncope is interesting, but the episodes were sporadic and in the distant past, arguing against a serious and ongoing process. Nonetheless, this history still raises the possibility of cardiac causes of syncope, especially causes such as hypertrophic obstructive cardiomyopathy or aortic stenosis which are classically associated with exertional syncope. Either of these two conditions can result in heart failure if untreated. The history of goserelin therapy does make the possibility of heart failure higher, as there has been an association reported between use of this drug and heart failure. His history of tobacco use is a risk factor for coronary artery disease (CAD) and COPD. An active cancer history is also a risk factor for thromboembolic disease, which remains a consideration.
On admission, his temperature was 36.9C, heart rate 94 bpm, respiratory rate 22 breaths per minute, blood pressure 200/108 mmHg, and oxygen saturation 93% breathing ambient air. He was a thin man in no acute distress. Cardiovascular examination was significant for normal first and second heart sounds, with a soft left‐sided S3; the point of maximal impulse was diffuse, but displaced laterally. His jugular venous pressure was estimated at 9 cm of H2O while positioned at a 45‐degree angle. Rales were heard at the lung bases bilaterally. Abdominal exam was normal. His lower extremities were without edema. There were no focal neurological deficits appreciated. Skin examination was unremarkable.
His combination of physical exam findings strongly suggests heart failure, most likely related to a dilated cardiomyopathy and left ventricular dysfunction. The presence of a left‐sided S3 and rales, and the lack of markedly elevated central venous pressure and peripheral edema, suggest heart failure predominantly due to left ventricular dysfunction. Of note, he is very hypertensive. This would not be the typical finding with severely decompensated heart failure. It would be important to determine whether his elevated blood pressure is due to an acute, reversible cause (e.g., pain, dyspnea, anxiety) or whether cocaine use, psychotropic agents, rare causes such as catecholamine‐producing tumors, other neuroendocrine tumors or thyroid toxic states are at play. In addition, one might see hypertension early in the course of heart failure, from a left ventricular outflow obstructive etiology such as severe aortic stenosis or hypertrophic obstructive cardiomyopathy.
Laboratory evaluation revealed a white blood cell count of 8900/mm3, with a normal differential; hemoglobin was 13.9 g/dL; platelet count was 264,000/mm3. Serum electrolytes and liver enzymes were unremarkable, with serum creatinine 1.1 mg/dL and blood urea nitrogen 7 mg/dL. Serial cardiac troponin‐I levels drawn 8 hours apart were 0.04, 0.07, 0.08, and 0.04 ng/mL (normal <0.04). Brain natriuretic peptide was 1420 pg/mL (normal <100). Thyroid stimulating hormone was 1.19 uIU/mL (normal 0.34‐5.60). Chest radiography revealed mild cardiomegaly, with peripheral interstitial opacities in the mid and lower lobes bilaterally, with fluid within the minor fissure. A 12‐lead electrocardiogram (ECG) revealed normal sinus rhythm at 95 bpm with left anterior fascicular block; intraventricular conduction delay was present (QRS width 106 ms) and QS complexes were present in V1‐V3. In addition, there was a left atrial abnormality and voltage criteria for left ventricular hypertrophy with secondary T‐wave inversions laterally (Figure 1). No previous ECGs were available for comparison. A chest computed tomography scan with contrast showed no evidence of pulmonary embolus. It did show interlobular septal thickening and small bilateral pleural effusions, consistent with left ventricular dysfunction.
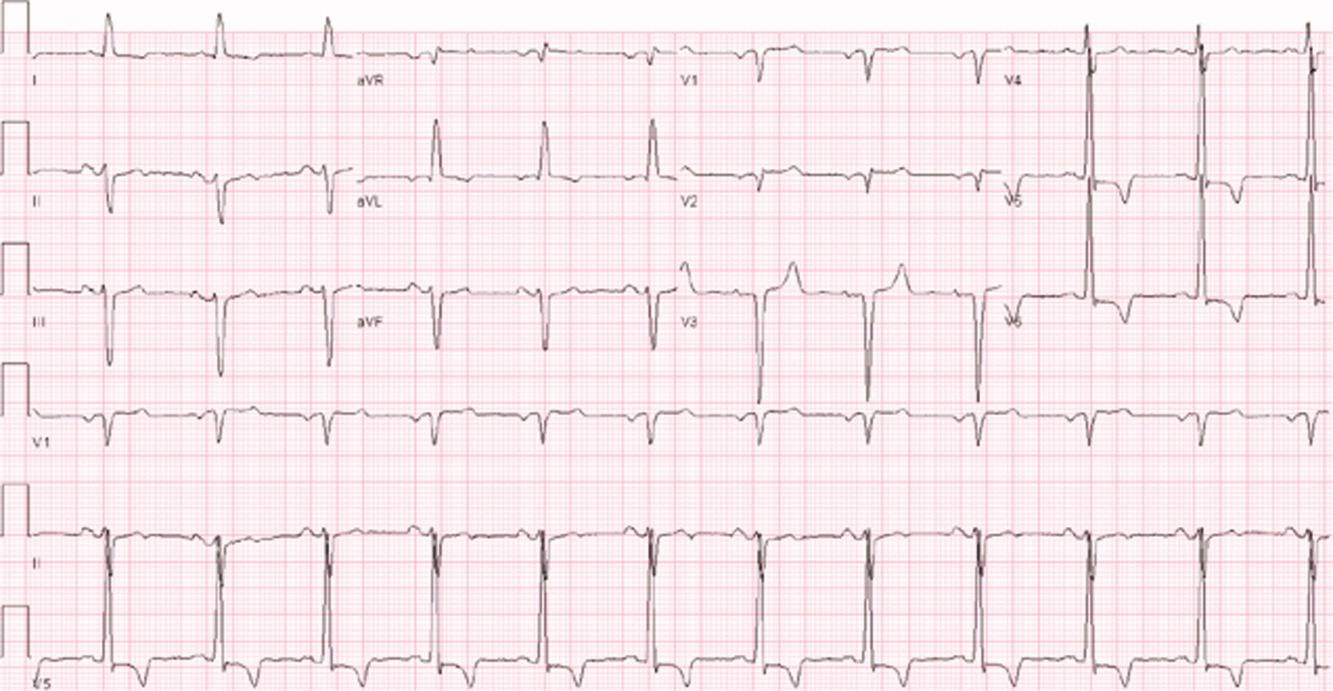
The patient's initial lab, imaging, and diagnostic work‐up continues to be consistent with the diagnosis of heart failure. The patient appears to have cardiomegaly and mild pulmonary edema by imaging. The etiology of heart failure remains unknown, but ischemia remains in the differential, given the mildly elevated troponins initially and the ECG findings of left anterior fascicular block and T‐wave inversions in the lateral leads. Left anterior fascicular block can be seen with ischemic heart disease (especially involving the left anterior descending coronary artery), hypertensive heart disease, valvular disease, and some infiltrative cardiac processes. The lateral T‐wave inversions are likely secondary to left ventricular hypertrophy (a so‐called strain pattern), rather than ischemia. Left ventricular hypertrophy is consistent with his hypertension, suggesting that it is chronic; his presentation may be due to hypertensive heart disease with new onset heart failure.
He was admitted to the hospital, and metoprolol, lisinopril, and intravenous furosemide were given. Transthoracic echocardiography demonstrated severe global hypokinesis with a left ventricular ejection fraction of 10%. There was no evidence of ventricular thrombus or valvular disease; however, prominent left ventricular trabeculation with deep recesses was noted (see Figure 2).
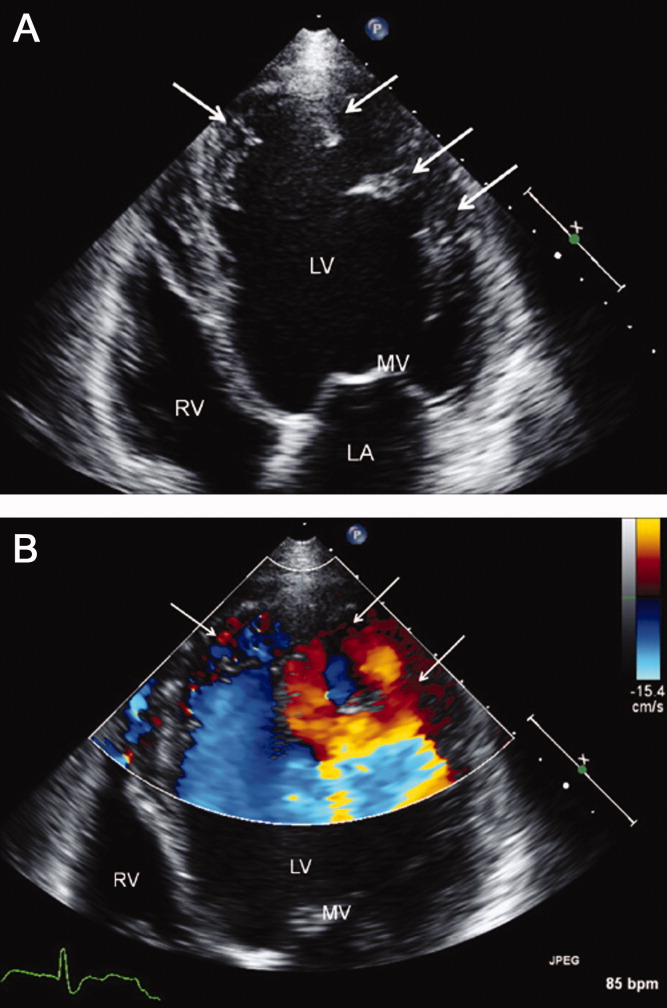
The echocardiographic findings of deep recesses and prominent left ventricular trabeculation are seen in only a few disorders. Sometimes these findings are thought to be due to hypertrophic obstructive cardiomyopathy. The deep trabeculations can be seen in patients with some forms of congenital heart disease associated with ventricular pressure overload during fetal development. The other cause is left ventricular noncompaction, a genetic cardiomyopathy which is becoming increasingly recognized. The disorder, along with causing heart failure, is associated with a high risk of ventricular thrombus and thromboembolic events, and a high risk of arrhythmias and sudden death. The overall prognosis appears to be poor, compared to some other cardiomyopathies. The imaging findings of left ventricular noncompaction are nearly pathognomonic, and experienced echocardiographers can usually make the diagnosis. Finally, left heart catheterization or noninvasive stress testing should be part of the workup to definitively exclude an ischemic cardiomyopathy, even in the setting of noncompaction, and especially given his recent history of chest pain.
A left heart catheterization with coronary arteriography demonstrated no angiographic evidence of obstructive coronary disease. Left ventriculography revealed severe global hypokinesis. The patient was diagnosed with left ventricular noncompaction.
The initial medical management centers upon the treatment of heart failure with a beta‐blocker, ACE‐inhibitor, and diuretics for fluid management. Patients with left ventricular noncompaction are at particularly high risk of both embolic events (thought due to propensity to develop left ventricular clots within the deep recesses of the endocardium) and sudden death from arrhythmias. Thus, anticoagulation with warfarin is often indicated and would be reasonable in this patient, given the extremely low ejection fraction. The patient does meet established criteria for primary prophylaxis of sudden death with an implantable cardioverter‐defibrillator in nonischemic cardiomyopathy (left ventricular ejection fraction <35% and New York Heart Association class II failure), and this would also be appropriate therapy as well, given the high‐risk profile of this patient population.
He was discharged in stable condition with a medical regimen consisting of diuretics, metoprolol, and lisinopril. Given the risk for thromboembolism, he was started on warfarin. On subsequent follow‐up, repeat echocardiogram revealed a persistently low left ventricular ejection fraction at 10%. Despite his marked improvement in exercise tolerance and overall well‐being after 4 months of treatment, his ejection fraction did not improve. As a result, he was evaluated and counseled for placement of an implantable cardioverter‐defibrillator, and received a dual‐chamber device shortly afterward.
COMMENTARY
Left ventricular noncompaction is a form of cardiomyopathy increasingly recognized in both pediatric and adult populations. The hallmark features are a pattern of prominent trabeculations and deep recesses in the left ventricular wall. During normal gestation, the myocardium compacts and matures while deep recesses evolve into capillary precursors of the coronary circulation. Left ventricular noncompaction may result from an arrest in this process, with cardiac myofibers failing to compact from their initial spongiform architecture into a developed endocardium.1 Restrictive relaxation from persistent trabeculae predisposes to diastolic dysfunction, while systolic dysfunction may be related to subendocardial hypoperfusion and mechanical dyssynchrony between compacted and noncompacted myocardium.2
Differentiation of left ventricular noncompaction from other cardiomyopathies, based on history and physical examination alone, is essentially impossible. There is high variability and lack of specificity in both clinical profile and onset of symptoms. Electrocardiographic findings are also nonspecific, and the diagnosis typically becomes evident only with transthoracic echocardiography. Current diagnostic criteria include: 1) absence of coexisting cardiac abnormalities; 2) a two‐layer structure with >2:1 ratio of noncompacted to compacted myocardium; 3) predominant involvement of the apical segment of myocardium; and 4) deep intertrabecular recesses demonstrated on Doppler imaging.2, 3 Although echocardiography remains the standard in clinical practice, cardiac magnetic resonance imaging is being increasingly employed as well.4
With more awareness of the disease and the development of higher resolution imaging, the reported incidence has risen. In one single‐center study performed at a heart failure/transplant clinic, 3% of 960 patients referred to heart failure clinic were diagnosed with left ventricular noncompaction, a prevalence similar to hypertensive disease and hypertrophic cardiomyopathy.5 In another community‐hospitalbased study of 4929 adult patients referred for echocardiography, 3.7% of those with systolic dysfunction were diagnosed with noncompaction.6
Left ventricular noncompaction is considered a genetic cardiomyopathy; a family history of heart failure is often present.7 Despite its congenital origin and genetic involvement,2 it is unclear why symptoms may first present at an advanced age. Chest pain and shortness of breath are common complaints, and approximately 62% of patients will have congestive heart failure at presentation.8
Tachyarrhythmia and ventricular tachycardia are commonly seen, as are systemic embolic events and pulmonary embolism. Significant predictors of death include New York Heart Association class III‐IV, sustained ventricular arrhythmias, and increased left atrial size.9
Management is focused on the treatment of arrhythmias, heart failure, and thromboembolic events. The use of standard medical therapy for heart failure (including ACE‐inhibitors and beta‐blockers) is not based on large‐scale studies, yet remains the cornerstone of therapy. An implantable cardioverter‐defibrillator is indicated after hemodynamically compromising sustained ventricular tachycardia or aborted sudden cardiac death, but there are no guidelines for primary prophylaxis outside of patients with heart failure and a depressed ejection fraction.10 Cardiac resynchronization therapy has been successful in some patients with isolated left ventricular noncompaction. Long‐term oral anticoagulation is recommended, especially when impaired left ventricular function, thrombi, or atrial fibrillation have been documented. Patients with left ventricular dysfunction in concert with left ventricular noncompaction are at 10% higher risk for embolic complications when compared to those without noncompaction.11 Familial screening with echocardiography is indicated once the diagnosis has been made.2
In this Clinical Care Conundrum, we describe a rare but increasingly recognized condition, and highlight the importance of delineating the underlying cause of cardiomyopathy when possible. Treatment of heart failure in the hospital setting is sometimes more focused on initiation of diuresis and further stabilization of the patient, and less focused on elucidation of the etiology. While recognition of left ventricular failure led to early treatment with standard therapy in this case, identification of the underlying cause allowed for targeted interventions directed at cardiac arrhythmias, embolic events, and familial screening. Of note, the discussant was careful not to let the prior history of syncopal events distract him from the central issues in this case.
This case also serves as a reminder that congenital anomalies should remain on the differential diagnosis when evaluating new complaints in adult patients. The discussant approached the presentation of new‐onset left ventricular dysfunction in a thorough manner, weighing the likelihood of ischemic and nonischemic causes in the context of the history and physical examination. Careful consideration of the patient's new clinical manifestationscoupled with characteristic echocardiographic findings and normal coronary anatomysolidified the diagnosis. By developing a broad differential, the discussant and clinical team arrived at a diagnosis that for this 66‐year‐old gentleman was a lifetime in the making.
Teaching Points
-
Left ventricular noncompaction is characterized by a pattern of prominent trabecular meshwork and deep intertrabecular recesses communicating with the left ventricular cavity. Heightened awareness among clinicians and echocardiographers has led to increased detection of this condition.
-
This disease needs to be considered in patients of all ages presenting with heart failure, especially in cases characterized by ventricular arrhythmias, thromboembolism, and a family history of similar events.
-
Left ventricular noncompaction management is mainly focused on the treatment of arrhythmias, heart failure, and thromboembolic events.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
- ,,.Isolated ventricular non‐compaction of the myocardium in adults.Heart.2006;93:11–15.
- .Left ventricular noncompaction.Circ J.2009;73:19–26.
- ,,,,.Echocardiographic and pathoanatomical characteristics of isolated left ventricular non‐compaction: a step towards classification as a distinct cardiomyopathy.Heart.2001;86:666–671.
- ,,, et al.Left ventricular non‐compaction: insights from cardiovascular magnetic resonance imaging.J Am Coll Cardiol.2005;46:101–105.
- ,,,,,.Isolated left ventricular noncompaction as a cause for heart failure and heart transplantation: a single center experience.Cardiology.2009;112:158–164.
- ,,,.Prevalence and characteristics of left ventricular noncompaction in a community hospital cohort of patients with systolic dysfunction.Echocardiography.2008;25(1):8–12.
- ,,, et al.Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention.Circulation.2006;113:1801–1816.
- ,,,,.Long‐term follow‐up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis.J Am Coll Cardiol.2000;36:493–500.
- ,,, et al.Wide spectrum of presentation and variable outcomes of isolated left ventricular non‐compaction.Heart.2007;93(1):65–71.
- ,,, et al.Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy.N Engl J Med.2004;350:2151–2159.
- ,.Left ventricular hypertrabeculation/noncompaction and stroke or embolism.Cardiology.2005;103:68–72.
A 66‐year‐old man presented to the emergency department with 3 weeks of progressive exertional dyspnea. He also reported a single episode of chest pain 1 day prior to admission.
Cardiac and pulmonary causes of dyspnea are the most common. Other causes include anemia or a neuromuscular process. Given the recent episode of chest pain, coronary ischemia, congestive heart failure, chronic obstructive pulmonary disease (COPD), pulmonary embolism, and pericardial effusion must be considered.
Up until 3 weeks ago, he had no exercise intolerance, and had been relatively active. He began noticing progressive dyspnea to the point where he had considerable difficulty walking up stairs, and performing minor household chores. He also complained of orthopnea and paroxysmal nocturnal dyspnea for the last 3 weeks.He denied chest pain at presentation, but 24 hours prior, he experienced one episode of sharp, left‐sided, nonradiating, nonpositional chest pain that occurred at rest. It lasted approximately 20 minutes and was not associated with diaphoresis, nausea, vomiting, or palpitations. He had never experienced chest discomfort prior to this episode. He denied fever, chills, cough, or wheezing.
Progressive dyspnea on exertion with associated orthopnea and paroxysmal nocturnal dyspnea is classically seen in patients with heart failure and is typically associated with left ventricular failure. However, paroxysmal nocturnal dyspnea and orthopnea are only moderately specific for heart failure. Orthopnea can also be seen in pericardial disease, and in numerous pulmonary diseases, including asthma, COPD, pulmonary hypertension, diaphragmatic weakness, pleural effusion, pulmonary embolism, and any apical lung process including lung cancer or pneumonia. Paroxysmal nocturnal dyspnea can be seen in many of the same disorders and can also be reported in obstructive sleep apnea.
His past medical history was remarkable for two episodes of syncope, occurring 5 and 3 years ago, both while working outside in warm weather. Neither was associated with chest pain, diaphoresis, palpitations, or post‐ictal symptoms. He was diagnosed with prostate cancer 8 years ago, and underwent 2 years of androgen‐deprivation therapy with goserelin along with local radiation therapy. Medications included subcutaneous goserelin every 3 months and daily omeprazole. He denied any other prescription, over‐the‐counter, or herbal medications. He reported a 50‐pack‐year history of smoking, but denied alcohol or illicit drug abuse. He denied any travel history or recent immobilization. He had no children, and there was no known history of heart disease in his family.
The past medical history of two episodes of likely exertional syncope is interesting, but the episodes were sporadic and in the distant past, arguing against a serious and ongoing process. Nonetheless, this history still raises the possibility of cardiac causes of syncope, especially causes such as hypertrophic obstructive cardiomyopathy or aortic stenosis which are classically associated with exertional syncope. Either of these two conditions can result in heart failure if untreated. The history of goserelin therapy does make the possibility of heart failure higher, as there has been an association reported between use of this drug and heart failure. His history of tobacco use is a risk factor for coronary artery disease (CAD) and COPD. An active cancer history is also a risk factor for thromboembolic disease, which remains a consideration.
On admission, his temperature was 36.9C, heart rate 94 bpm, respiratory rate 22 breaths per minute, blood pressure 200/108 mmHg, and oxygen saturation 93% breathing ambient air. He was a thin man in no acute distress. Cardiovascular examination was significant for normal first and second heart sounds, with a soft left‐sided S3; the point of maximal impulse was diffuse, but displaced laterally. His jugular venous pressure was estimated at 9 cm of H2O while positioned at a 45‐degree angle. Rales were heard at the lung bases bilaterally. Abdominal exam was normal. His lower extremities were without edema. There were no focal neurological deficits appreciated. Skin examination was unremarkable.
His combination of physical exam findings strongly suggests heart failure, most likely related to a dilated cardiomyopathy and left ventricular dysfunction. The presence of a left‐sided S3 and rales, and the lack of markedly elevated central venous pressure and peripheral edema, suggest heart failure predominantly due to left ventricular dysfunction. Of note, he is very hypertensive. This would not be the typical finding with severely decompensated heart failure. It would be important to determine whether his elevated blood pressure is due to an acute, reversible cause (e.g., pain, dyspnea, anxiety) or whether cocaine use, psychotropic agents, rare causes such as catecholamine‐producing tumors, other neuroendocrine tumors or thyroid toxic states are at play. In addition, one might see hypertension early in the course of heart failure, from a left ventricular outflow obstructive etiology such as severe aortic stenosis or hypertrophic obstructive cardiomyopathy.
Laboratory evaluation revealed a white blood cell count of 8900/mm3, with a normal differential; hemoglobin was 13.9 g/dL; platelet count was 264,000/mm3. Serum electrolytes and liver enzymes were unremarkable, with serum creatinine 1.1 mg/dL and blood urea nitrogen 7 mg/dL. Serial cardiac troponin‐I levels drawn 8 hours apart were 0.04, 0.07, 0.08, and 0.04 ng/mL (normal <0.04). Brain natriuretic peptide was 1420 pg/mL (normal <100). Thyroid stimulating hormone was 1.19 uIU/mL (normal 0.34‐5.60). Chest radiography revealed mild cardiomegaly, with peripheral interstitial opacities in the mid and lower lobes bilaterally, with fluid within the minor fissure. A 12‐lead electrocardiogram (ECG) revealed normal sinus rhythm at 95 bpm with left anterior fascicular block; intraventricular conduction delay was present (QRS width 106 ms) and QS complexes were present in V1‐V3. In addition, there was a left atrial abnormality and voltage criteria for left ventricular hypertrophy with secondary T‐wave inversions laterally (Figure 1). No previous ECGs were available for comparison. A chest computed tomography scan with contrast showed no evidence of pulmonary embolus. It did show interlobular septal thickening and small bilateral pleural effusions, consistent with left ventricular dysfunction.

The patient's initial lab, imaging, and diagnostic work‐up continues to be consistent with the diagnosis of heart failure. The patient appears to have cardiomegaly and mild pulmonary edema by imaging. The etiology of heart failure remains unknown, but ischemia remains in the differential, given the mildly elevated troponins initially and the ECG findings of left anterior fascicular block and T‐wave inversions in the lateral leads. Left anterior fascicular block can be seen with ischemic heart disease (especially involving the left anterior descending coronary artery), hypertensive heart disease, valvular disease, and some infiltrative cardiac processes. The lateral T‐wave inversions are likely secondary to left ventricular hypertrophy (a so‐called strain pattern), rather than ischemia. Left ventricular hypertrophy is consistent with his hypertension, suggesting that it is chronic; his presentation may be due to hypertensive heart disease with new onset heart failure.
He was admitted to the hospital, and metoprolol, lisinopril, and intravenous furosemide were given. Transthoracic echocardiography demonstrated severe global hypokinesis with a left ventricular ejection fraction of 10%. There was no evidence of ventricular thrombus or valvular disease; however, prominent left ventricular trabeculation with deep recesses was noted (see Figure 2).

The echocardiographic findings of deep recesses and prominent left ventricular trabeculation are seen in only a few disorders. Sometimes these findings are thought to be due to hypertrophic obstructive cardiomyopathy. The deep trabeculations can be seen in patients with some forms of congenital heart disease associated with ventricular pressure overload during fetal development. The other cause is left ventricular noncompaction, a genetic cardiomyopathy which is becoming increasingly recognized. The disorder, along with causing heart failure, is associated with a high risk of ventricular thrombus and thromboembolic events, and a high risk of arrhythmias and sudden death. The overall prognosis appears to be poor, compared to some other cardiomyopathies. The imaging findings of left ventricular noncompaction are nearly pathognomonic, and experienced echocardiographers can usually make the diagnosis. Finally, left heart catheterization or noninvasive stress testing should be part of the workup to definitively exclude an ischemic cardiomyopathy, even in the setting of noncompaction, and especially given his recent history of chest pain.
A left heart catheterization with coronary arteriography demonstrated no angiographic evidence of obstructive coronary disease. Left ventriculography revealed severe global hypokinesis. The patient was diagnosed with left ventricular noncompaction.
The initial medical management centers upon the treatment of heart failure with a beta‐blocker, ACE‐inhibitor, and diuretics for fluid management. Patients with left ventricular noncompaction are at particularly high risk of both embolic events (thought due to propensity to develop left ventricular clots within the deep recesses of the endocardium) and sudden death from arrhythmias. Thus, anticoagulation with warfarin is often indicated and would be reasonable in this patient, given the extremely low ejection fraction. The patient does meet established criteria for primary prophylaxis of sudden death with an implantable cardioverter‐defibrillator in nonischemic cardiomyopathy (left ventricular ejection fraction <35% and New York Heart Association class II failure), and this would also be appropriate therapy as well, given the high‐risk profile of this patient population.
He was discharged in stable condition with a medical regimen consisting of diuretics, metoprolol, and lisinopril. Given the risk for thromboembolism, he was started on warfarin. On subsequent follow‐up, repeat echocardiogram revealed a persistently low left ventricular ejection fraction at 10%. Despite his marked improvement in exercise tolerance and overall well‐being after 4 months of treatment, his ejection fraction did not improve. As a result, he was evaluated and counseled for placement of an implantable cardioverter‐defibrillator, and received a dual‐chamber device shortly afterward.
COMMENTARY
Left ventricular noncompaction is a form of cardiomyopathy increasingly recognized in both pediatric and adult populations. The hallmark features are a pattern of prominent trabeculations and deep recesses in the left ventricular wall. During normal gestation, the myocardium compacts and matures while deep recesses evolve into capillary precursors of the coronary circulation. Left ventricular noncompaction may result from an arrest in this process, with cardiac myofibers failing to compact from their initial spongiform architecture into a developed endocardium.1 Restrictive relaxation from persistent trabeculae predisposes to diastolic dysfunction, while systolic dysfunction may be related to subendocardial hypoperfusion and mechanical dyssynchrony between compacted and noncompacted myocardium.2
Differentiation of left ventricular noncompaction from other cardiomyopathies, based on history and physical examination alone, is essentially impossible. There is high variability and lack of specificity in both clinical profile and onset of symptoms. Electrocardiographic findings are also nonspecific, and the diagnosis typically becomes evident only with transthoracic echocardiography. Current diagnostic criteria include: 1) absence of coexisting cardiac abnormalities; 2) a two‐layer structure with >2:1 ratio of noncompacted to compacted myocardium; 3) predominant involvement of the apical segment of myocardium; and 4) deep intertrabecular recesses demonstrated on Doppler imaging.2, 3 Although echocardiography remains the standard in clinical practice, cardiac magnetic resonance imaging is being increasingly employed as well.4
With more awareness of the disease and the development of higher resolution imaging, the reported incidence has risen. In one single‐center study performed at a heart failure/transplant clinic, 3% of 960 patients referred to heart failure clinic were diagnosed with left ventricular noncompaction, a prevalence similar to hypertensive disease and hypertrophic cardiomyopathy.5 In another community‐hospitalbased study of 4929 adult patients referred for echocardiography, 3.7% of those with systolic dysfunction were diagnosed with noncompaction.6
Left ventricular noncompaction is considered a genetic cardiomyopathy; a family history of heart failure is often present.7 Despite its congenital origin and genetic involvement,2 it is unclear why symptoms may first present at an advanced age. Chest pain and shortness of breath are common complaints, and approximately 62% of patients will have congestive heart failure at presentation.8
Tachyarrhythmia and ventricular tachycardia are commonly seen, as are systemic embolic events and pulmonary embolism. Significant predictors of death include New York Heart Association class III‐IV, sustained ventricular arrhythmias, and increased left atrial size.9
Management is focused on the treatment of arrhythmias, heart failure, and thromboembolic events. The use of standard medical therapy for heart failure (including ACE‐inhibitors and beta‐blockers) is not based on large‐scale studies, yet remains the cornerstone of therapy. An implantable cardioverter‐defibrillator is indicated after hemodynamically compromising sustained ventricular tachycardia or aborted sudden cardiac death, but there are no guidelines for primary prophylaxis outside of patients with heart failure and a depressed ejection fraction.10 Cardiac resynchronization therapy has been successful in some patients with isolated left ventricular noncompaction. Long‐term oral anticoagulation is recommended, especially when impaired left ventricular function, thrombi, or atrial fibrillation have been documented. Patients with left ventricular dysfunction in concert with left ventricular noncompaction are at 10% higher risk for embolic complications when compared to those without noncompaction.11 Familial screening with echocardiography is indicated once the diagnosis has been made.2
In this Clinical Care Conundrum, we describe a rare but increasingly recognized condition, and highlight the importance of delineating the underlying cause of cardiomyopathy when possible. Treatment of heart failure in the hospital setting is sometimes more focused on initiation of diuresis and further stabilization of the patient, and less focused on elucidation of the etiology. While recognition of left ventricular failure led to early treatment with standard therapy in this case, identification of the underlying cause allowed for targeted interventions directed at cardiac arrhythmias, embolic events, and familial screening. Of note, the discussant was careful not to let the prior history of syncopal events distract him from the central issues in this case.
This case also serves as a reminder that congenital anomalies should remain on the differential diagnosis when evaluating new complaints in adult patients. The discussant approached the presentation of new‐onset left ventricular dysfunction in a thorough manner, weighing the likelihood of ischemic and nonischemic causes in the context of the history and physical examination. Careful consideration of the patient's new clinical manifestationscoupled with characteristic echocardiographic findings and normal coronary anatomysolidified the diagnosis. By developing a broad differential, the discussant and clinical team arrived at a diagnosis that for this 66‐year‐old gentleman was a lifetime in the making.
Teaching Points
-
Left ventricular noncompaction is characterized by a pattern of prominent trabecular meshwork and deep intertrabecular recesses communicating with the left ventricular cavity. Heightened awareness among clinicians and echocardiographers has led to increased detection of this condition.
-
This disease needs to be considered in patients of all ages presenting with heart failure, especially in cases characterized by ventricular arrhythmias, thromboembolism, and a family history of similar events.
-
Left ventricular noncompaction management is mainly focused on the treatment of arrhythmias, heart failure, and thromboembolic events.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 66‐year‐old man presented to the emergency department with 3 weeks of progressive exertional dyspnea. He also reported a single episode of chest pain 1 day prior to admission.
Cardiac and pulmonary causes of dyspnea are the most common. Other causes include anemia or a neuromuscular process. Given the recent episode of chest pain, coronary ischemia, congestive heart failure, chronic obstructive pulmonary disease (COPD), pulmonary embolism, and pericardial effusion must be considered.
Up until 3 weeks ago, he had no exercise intolerance, and had been relatively active. He began noticing progressive dyspnea to the point where he had considerable difficulty walking up stairs, and performing minor household chores. He also complained of orthopnea and paroxysmal nocturnal dyspnea for the last 3 weeks.He denied chest pain at presentation, but 24 hours prior, he experienced one episode of sharp, left‐sided, nonradiating, nonpositional chest pain that occurred at rest. It lasted approximately 20 minutes and was not associated with diaphoresis, nausea, vomiting, or palpitations. He had never experienced chest discomfort prior to this episode. He denied fever, chills, cough, or wheezing.
Progressive dyspnea on exertion with associated orthopnea and paroxysmal nocturnal dyspnea is classically seen in patients with heart failure and is typically associated with left ventricular failure. However, paroxysmal nocturnal dyspnea and orthopnea are only moderately specific for heart failure. Orthopnea can also be seen in pericardial disease, and in numerous pulmonary diseases, including asthma, COPD, pulmonary hypertension, diaphragmatic weakness, pleural effusion, pulmonary embolism, and any apical lung process including lung cancer or pneumonia. Paroxysmal nocturnal dyspnea can be seen in many of the same disorders and can also be reported in obstructive sleep apnea.
His past medical history was remarkable for two episodes of syncope, occurring 5 and 3 years ago, both while working outside in warm weather. Neither was associated with chest pain, diaphoresis, palpitations, or post‐ictal symptoms. He was diagnosed with prostate cancer 8 years ago, and underwent 2 years of androgen‐deprivation therapy with goserelin along with local radiation therapy. Medications included subcutaneous goserelin every 3 months and daily omeprazole. He denied any other prescription, over‐the‐counter, or herbal medications. He reported a 50‐pack‐year history of smoking, but denied alcohol or illicit drug abuse. He denied any travel history or recent immobilization. He had no children, and there was no known history of heart disease in his family.
The past medical history of two episodes of likely exertional syncope is interesting, but the episodes were sporadic and in the distant past, arguing against a serious and ongoing process. Nonetheless, this history still raises the possibility of cardiac causes of syncope, especially causes such as hypertrophic obstructive cardiomyopathy or aortic stenosis which are classically associated with exertional syncope. Either of these two conditions can result in heart failure if untreated. The history of goserelin therapy does make the possibility of heart failure higher, as there has been an association reported between use of this drug and heart failure. His history of tobacco use is a risk factor for coronary artery disease (CAD) and COPD. An active cancer history is also a risk factor for thromboembolic disease, which remains a consideration.
On admission, his temperature was 36.9C, heart rate 94 bpm, respiratory rate 22 breaths per minute, blood pressure 200/108 mmHg, and oxygen saturation 93% breathing ambient air. He was a thin man in no acute distress. Cardiovascular examination was significant for normal first and second heart sounds, with a soft left‐sided S3; the point of maximal impulse was diffuse, but displaced laterally. His jugular venous pressure was estimated at 9 cm of H2O while positioned at a 45‐degree angle. Rales were heard at the lung bases bilaterally. Abdominal exam was normal. His lower extremities were without edema. There were no focal neurological deficits appreciated. Skin examination was unremarkable.
His combination of physical exam findings strongly suggests heart failure, most likely related to a dilated cardiomyopathy and left ventricular dysfunction. The presence of a left‐sided S3 and rales, and the lack of markedly elevated central venous pressure and peripheral edema, suggest heart failure predominantly due to left ventricular dysfunction. Of note, he is very hypertensive. This would not be the typical finding with severely decompensated heart failure. It would be important to determine whether his elevated blood pressure is due to an acute, reversible cause (e.g., pain, dyspnea, anxiety) or whether cocaine use, psychotropic agents, rare causes such as catecholamine‐producing tumors, other neuroendocrine tumors or thyroid toxic states are at play. In addition, one might see hypertension early in the course of heart failure, from a left ventricular outflow obstructive etiology such as severe aortic stenosis or hypertrophic obstructive cardiomyopathy.
Laboratory evaluation revealed a white blood cell count of 8900/mm3, with a normal differential; hemoglobin was 13.9 g/dL; platelet count was 264,000/mm3. Serum electrolytes and liver enzymes were unremarkable, with serum creatinine 1.1 mg/dL and blood urea nitrogen 7 mg/dL. Serial cardiac troponin‐I levels drawn 8 hours apart were 0.04, 0.07, 0.08, and 0.04 ng/mL (normal <0.04). Brain natriuretic peptide was 1420 pg/mL (normal <100). Thyroid stimulating hormone was 1.19 uIU/mL (normal 0.34‐5.60). Chest radiography revealed mild cardiomegaly, with peripheral interstitial opacities in the mid and lower lobes bilaterally, with fluid within the minor fissure. A 12‐lead electrocardiogram (ECG) revealed normal sinus rhythm at 95 bpm with left anterior fascicular block; intraventricular conduction delay was present (QRS width 106 ms) and QS complexes were present in V1‐V3. In addition, there was a left atrial abnormality and voltage criteria for left ventricular hypertrophy with secondary T‐wave inversions laterally (Figure 1). No previous ECGs were available for comparison. A chest computed tomography scan with contrast showed no evidence of pulmonary embolus. It did show interlobular septal thickening and small bilateral pleural effusions, consistent with left ventricular dysfunction.

The patient's initial lab, imaging, and diagnostic work‐up continues to be consistent with the diagnosis of heart failure. The patient appears to have cardiomegaly and mild pulmonary edema by imaging. The etiology of heart failure remains unknown, but ischemia remains in the differential, given the mildly elevated troponins initially and the ECG findings of left anterior fascicular block and T‐wave inversions in the lateral leads. Left anterior fascicular block can be seen with ischemic heart disease (especially involving the left anterior descending coronary artery), hypertensive heart disease, valvular disease, and some infiltrative cardiac processes. The lateral T‐wave inversions are likely secondary to left ventricular hypertrophy (a so‐called strain pattern), rather than ischemia. Left ventricular hypertrophy is consistent with his hypertension, suggesting that it is chronic; his presentation may be due to hypertensive heart disease with new onset heart failure.
He was admitted to the hospital, and metoprolol, lisinopril, and intravenous furosemide were given. Transthoracic echocardiography demonstrated severe global hypokinesis with a left ventricular ejection fraction of 10%. There was no evidence of ventricular thrombus or valvular disease; however, prominent left ventricular trabeculation with deep recesses was noted (see Figure 2).

The echocardiographic findings of deep recesses and prominent left ventricular trabeculation are seen in only a few disorders. Sometimes these findings are thought to be due to hypertrophic obstructive cardiomyopathy. The deep trabeculations can be seen in patients with some forms of congenital heart disease associated with ventricular pressure overload during fetal development. The other cause is left ventricular noncompaction, a genetic cardiomyopathy which is becoming increasingly recognized. The disorder, along with causing heart failure, is associated with a high risk of ventricular thrombus and thromboembolic events, and a high risk of arrhythmias and sudden death. The overall prognosis appears to be poor, compared to some other cardiomyopathies. The imaging findings of left ventricular noncompaction are nearly pathognomonic, and experienced echocardiographers can usually make the diagnosis. Finally, left heart catheterization or noninvasive stress testing should be part of the workup to definitively exclude an ischemic cardiomyopathy, even in the setting of noncompaction, and especially given his recent history of chest pain.
A left heart catheterization with coronary arteriography demonstrated no angiographic evidence of obstructive coronary disease. Left ventriculography revealed severe global hypokinesis. The patient was diagnosed with left ventricular noncompaction.
The initial medical management centers upon the treatment of heart failure with a beta‐blocker, ACE‐inhibitor, and diuretics for fluid management. Patients with left ventricular noncompaction are at particularly high risk of both embolic events (thought due to propensity to develop left ventricular clots within the deep recesses of the endocardium) and sudden death from arrhythmias. Thus, anticoagulation with warfarin is often indicated and would be reasonable in this patient, given the extremely low ejection fraction. The patient does meet established criteria for primary prophylaxis of sudden death with an implantable cardioverter‐defibrillator in nonischemic cardiomyopathy (left ventricular ejection fraction <35% and New York Heart Association class II failure), and this would also be appropriate therapy as well, given the high‐risk profile of this patient population.
He was discharged in stable condition with a medical regimen consisting of diuretics, metoprolol, and lisinopril. Given the risk for thromboembolism, he was started on warfarin. On subsequent follow‐up, repeat echocardiogram revealed a persistently low left ventricular ejection fraction at 10%. Despite his marked improvement in exercise tolerance and overall well‐being after 4 months of treatment, his ejection fraction did not improve. As a result, he was evaluated and counseled for placement of an implantable cardioverter‐defibrillator, and received a dual‐chamber device shortly afterward.
COMMENTARY
Left ventricular noncompaction is a form of cardiomyopathy increasingly recognized in both pediatric and adult populations. The hallmark features are a pattern of prominent trabeculations and deep recesses in the left ventricular wall. During normal gestation, the myocardium compacts and matures while deep recesses evolve into capillary precursors of the coronary circulation. Left ventricular noncompaction may result from an arrest in this process, with cardiac myofibers failing to compact from their initial spongiform architecture into a developed endocardium.1 Restrictive relaxation from persistent trabeculae predisposes to diastolic dysfunction, while systolic dysfunction may be related to subendocardial hypoperfusion and mechanical dyssynchrony between compacted and noncompacted myocardium.2
Differentiation of left ventricular noncompaction from other cardiomyopathies, based on history and physical examination alone, is essentially impossible. There is high variability and lack of specificity in both clinical profile and onset of symptoms. Electrocardiographic findings are also nonspecific, and the diagnosis typically becomes evident only with transthoracic echocardiography. Current diagnostic criteria include: 1) absence of coexisting cardiac abnormalities; 2) a two‐layer structure with >2:1 ratio of noncompacted to compacted myocardium; 3) predominant involvement of the apical segment of myocardium; and 4) deep intertrabecular recesses demonstrated on Doppler imaging.2, 3 Although echocardiography remains the standard in clinical practice, cardiac magnetic resonance imaging is being increasingly employed as well.4
With more awareness of the disease and the development of higher resolution imaging, the reported incidence has risen. In one single‐center study performed at a heart failure/transplant clinic, 3% of 960 patients referred to heart failure clinic were diagnosed with left ventricular noncompaction, a prevalence similar to hypertensive disease and hypertrophic cardiomyopathy.5 In another community‐hospitalbased study of 4929 adult patients referred for echocardiography, 3.7% of those with systolic dysfunction were diagnosed with noncompaction.6
Left ventricular noncompaction is considered a genetic cardiomyopathy; a family history of heart failure is often present.7 Despite its congenital origin and genetic involvement,2 it is unclear why symptoms may first present at an advanced age. Chest pain and shortness of breath are common complaints, and approximately 62% of patients will have congestive heart failure at presentation.8
Tachyarrhythmia and ventricular tachycardia are commonly seen, as are systemic embolic events and pulmonary embolism. Significant predictors of death include New York Heart Association class III‐IV, sustained ventricular arrhythmias, and increased left atrial size.9
Management is focused on the treatment of arrhythmias, heart failure, and thromboembolic events. The use of standard medical therapy for heart failure (including ACE‐inhibitors and beta‐blockers) is not based on large‐scale studies, yet remains the cornerstone of therapy. An implantable cardioverter‐defibrillator is indicated after hemodynamically compromising sustained ventricular tachycardia or aborted sudden cardiac death, but there are no guidelines for primary prophylaxis outside of patients with heart failure and a depressed ejection fraction.10 Cardiac resynchronization therapy has been successful in some patients with isolated left ventricular noncompaction. Long‐term oral anticoagulation is recommended, especially when impaired left ventricular function, thrombi, or atrial fibrillation have been documented. Patients with left ventricular dysfunction in concert with left ventricular noncompaction are at 10% higher risk for embolic complications when compared to those without noncompaction.11 Familial screening with echocardiography is indicated once the diagnosis has been made.2
In this Clinical Care Conundrum, we describe a rare but increasingly recognized condition, and highlight the importance of delineating the underlying cause of cardiomyopathy when possible. Treatment of heart failure in the hospital setting is sometimes more focused on initiation of diuresis and further stabilization of the patient, and less focused on elucidation of the etiology. While recognition of left ventricular failure led to early treatment with standard therapy in this case, identification of the underlying cause allowed for targeted interventions directed at cardiac arrhythmias, embolic events, and familial screening. Of note, the discussant was careful not to let the prior history of syncopal events distract him from the central issues in this case.
This case also serves as a reminder that congenital anomalies should remain on the differential diagnosis when evaluating new complaints in adult patients. The discussant approached the presentation of new‐onset left ventricular dysfunction in a thorough manner, weighing the likelihood of ischemic and nonischemic causes in the context of the history and physical examination. Careful consideration of the patient's new clinical manifestationscoupled with characteristic echocardiographic findings and normal coronary anatomysolidified the diagnosis. By developing a broad differential, the discussant and clinical team arrived at a diagnosis that for this 66‐year‐old gentleman was a lifetime in the making.
Teaching Points
-
Left ventricular noncompaction is characterized by a pattern of prominent trabecular meshwork and deep intertrabecular recesses communicating with the left ventricular cavity. Heightened awareness among clinicians and echocardiographers has led to increased detection of this condition.
-
This disease needs to be considered in patients of all ages presenting with heart failure, especially in cases characterized by ventricular arrhythmias, thromboembolism, and a family history of similar events.
-
Left ventricular noncompaction management is mainly focused on the treatment of arrhythmias, heart failure, and thromboembolic events.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
- ,,.Isolated ventricular non‐compaction of the myocardium in adults.Heart.2006;93:11–15.
- .Left ventricular noncompaction.Circ J.2009;73:19–26.
- ,,,,.Echocardiographic and pathoanatomical characteristics of isolated left ventricular non‐compaction: a step towards classification as a distinct cardiomyopathy.Heart.2001;86:666–671.
- ,,, et al.Left ventricular non‐compaction: insights from cardiovascular magnetic resonance imaging.J Am Coll Cardiol.2005;46:101–105.
- ,,,,,.Isolated left ventricular noncompaction as a cause for heart failure and heart transplantation: a single center experience.Cardiology.2009;112:158–164.
- ,,,.Prevalence and characteristics of left ventricular noncompaction in a community hospital cohort of patients with systolic dysfunction.Echocardiography.2008;25(1):8–12.
- ,,, et al.Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention.Circulation.2006;113:1801–1816.
- ,,,,.Long‐term follow‐up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis.J Am Coll Cardiol.2000;36:493–500.
- ,,, et al.Wide spectrum of presentation and variable outcomes of isolated left ventricular non‐compaction.Heart.2007;93(1):65–71.
- ,,, et al.Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy.N Engl J Med.2004;350:2151–2159.
- ,.Left ventricular hypertrabeculation/noncompaction and stroke or embolism.Cardiology.2005;103:68–72.
- ,,.Isolated ventricular non‐compaction of the myocardium in adults.Heart.2006;93:11–15.
- .Left ventricular noncompaction.Circ J.2009;73:19–26.
- ,,,,.Echocardiographic and pathoanatomical characteristics of isolated left ventricular non‐compaction: a step towards classification as a distinct cardiomyopathy.Heart.2001;86:666–671.
- ,,, et al.Left ventricular non‐compaction: insights from cardiovascular magnetic resonance imaging.J Am Coll Cardiol.2005;46:101–105.
- ,,,,,.Isolated left ventricular noncompaction as a cause for heart failure and heart transplantation: a single center experience.Cardiology.2009;112:158–164.
- ,,,.Prevalence and characteristics of left ventricular noncompaction in a community hospital cohort of patients with systolic dysfunction.Echocardiography.2008;25(1):8–12.
- ,,, et al.Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention.Circulation.2006;113:1801–1816.
- ,,,,.Long‐term follow‐up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis.J Am Coll Cardiol.2000;36:493–500.
- ,,, et al.Wide spectrum of presentation and variable outcomes of isolated left ventricular non‐compaction.Heart.2007;93(1):65–71.
- ,,, et al.Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy.N Engl J Med.2004;350:2151–2159.
- ,.Left ventricular hypertrabeculation/noncompaction and stroke or embolism.Cardiology.2005;103:68–72.
Mapping Out Diagnosis
A 19‐year‐old Japanese man was admitted to a hospital near Kyoto, Japan, because of fever and rash. Two weeks prior to admission, he developed mild headache and low‐grade fever; a rapid test for influenza was negative. His symptoms transiently improved with acetaminophen, but 8 days prior to admission, he developed fever to 38.5C and a pruritic maculopapular rash over his back that spread to his limbs. Six days prior to admission, a chest radiograph was clear; clarithromycin was prescribed for presumed upper respiratory infection. He visited the emergency department the day before admission because of continued fever of greater than 39C, fatigue, and headache. Because there was no jolt accentuation of the headache (ie, worsening with rapid horizontal rotation), or neck pain with extreme neck flexion, he was discharged on acetaminophen. He returned the next day with worsening fatigue and was admitted. He denied chills, rigor, weight loss, photosensitivity, sore throat, neck pain, cough, dyspnea, chest pain, nausea, vomiting, diarrhea, abdominal pain, back pain, and arthralgia.
Fever and diffuse rash are often due to infection, although drugs, autoimmune processes, and cancer must be considered. The presence of headache does not focus the differential diagnosis substantially, because many of the candidate diagnoses can be accompanied by meningitis or encephalitis, or even more frequently, nonspecific headaches. In one small study, jolt‐induced aggravation of headache was shown to be a sensitive indicator of cerebrospinal fluid pleocytosis. The absence of neck stiffness and the 2‐week duration makes bacterial meningitis unlikely, but a more indolent form of aseptic meningitis may need to be evaluated with a lumbar puncture.
The 2‐week illness without rapid deterioration makes some serious causes of fever and rash, such as toxic shock syndrome, disseminated meningococcal infection, or toxic epidermal necrolysis unlikely. A viral exanthema is possible, although the 2‐week duration is longer than usual. Given his youth, however, his immunization history should be queried, and acute infection with human immunodeficiency virus (HIV) should be considered. A more indolent infection, such as subacute bacterial endocarditis, disseminated gonococcal infection, or syphilis is plausible. Among autoimmune etiologies, systemic lupus erythematosus (SLE) and Behcet's disease (which is prevalent in Japan) can involve the central nervous system and cause fever. A careful inquiry directed at prescribed, complementary, and illicit drugs is required.
The patient's past medical history was notable only for mumps at the age of 10. His medications included acetaminophen, clarithromycin, and an herbal medicine, which he had been taking for the prior several days. He reported no tobacco or illicit drug use and rarely drank alcohol. He had never been sexually active. He worked in a factory and reported occasional contact with silver. He lived with his parents; there was no family history of tuberculosis or connective tissue diseases. His father was from Kyushu (the southernmost major island in Japan) and had chronic hepatitis C. The patient denied recent animal exposure or recent travel. His childhood vaccinations were said to be up to date.
Mumps at age 10 might signal general lack of immunization, in which case childhood viral exanthema‐like measles (characterized by fever, headache, and diffuse rash) would warrant consideration. The listed medications had been started after the onset of illness and therefore are unlikely to be causal. Silver causes at least 2 skin conditionscontact dermatitis and argyriabut not the systemic illness seen here. Human T lymphotropic virus‐1 (HTLV‐1) is endemic in southern Japan, but only a minority of infected humans are afflicted with associated adult T cell leukemia/lymphoma or myelopathy. Leukemia and lymphoma are the most likely cancers to cause fever, rash, and central nervous system involvement (with T cell disorders demonstrating a particular tropism for the skin). Overall, however, the differential has not changed substantially.
On physical examination, the patient was mildly overweight and appeared acutely ill. His blood pressure was 136/78 mm Hg, pulse rate was 76 and regular, temperature was 39.2C and respiratory rate was 20 with an oxygen saturation of 98% on room air. A diffuse but nonconfluent erythematous maculopapular rash was present over his chest wall, back, medial aspects of both thighs, and around the knees. There was no jolt‐induced headache. His eyes, nose, oral cavity, and throat were all clear. The neck was supple. There were palpable lymph nodes, each about 1 cm in size, which were firm and moderately tender, in his left neck and left axilla. Lungs and heart were normal. The abdomen was soft, nontender, with normal bowel sounds and no hepatosplenomegaly. His genitalia were normal. Rectal examination revealed no masses or tenderness and a scant amount of brown stool that was negative for occult blood. Neurologic examination was unremarkable.
The multifocal lymphadenopathy does not help distinguish among the categories of disease under consideration. The diffuse maculopapular rash is similarly nonspecific, occurring more frequently with infection and drug reaction than malignancy and autoimmunity. Acute HIV, Epstein‐Barr virus (EBV), syphilis, SLE, drug exposure, or a hematologic malignancy would all be suitable explanations for fever, headache, diffuse rash, and disseminated lymphadenopathy in a previously healthy young man.
Laboratory data obtained on admission was notable for a white blood cell (WBC) count of 2100/L with 72% neutrophils, 19% lymphocytes, and 9% monocytes. Hemoglobin was 13.5 mg/dL with a mean corpuscular volume of 85 fL. Platelet count was 136,000/L. Erythrocyte sedimentation rate was 26 mm/hour. Serum chemistries revealed a sodium level of 135 mEq/L, potassium level of 3.6 mEq/L, chloride level of 100 mEq/L, blood urea nitrogen of 9.8 mg/dL, creatinine level of 1.0 mg/dL, glucose level of 101 mg/dL, calcium level of 8.8 mg/dL, albumin of 4.6 mg/dL, total protein of 8.4 mg/dL, aspartate aminotransferase of 42 IU/L (normal < 35 IU/L), alanine aminotransferase of 27 IU/L, total bilirubin of 0.5 mg/dL, and lactate dehydrogenase (LDH) level of 463 IU/L (normal < 260 IU/L). Chest radiography and electrocardiogram were normal.
A mild elevation in LDH is nonspecific, but without hemolysis or infarction of the kidney, lung, or muscle, it suggests a lymphoproliferative process. Leukopenia with thrombocytopenia can be seen in a number of disorders, most commonly infections including viruses (e.g., EBV, HIV, dengue), malaria, Rocky Mountain spotted fever, or ehrlichiosis/anaplasmosis. Confirmation of his lack of travel could help prioritize those considerations. An invasive bone marrow disorder cannot be excluded, although the near‐normal hemoglobin argues against it. Autoimmune cytopenias are seen in SLE. Given his age, lymphadenopathy, LDH elevation, and absence of infectious exposures, lymphoma rises to the top of the list.
Noninvasive measures should include examination of the peripheral smear, HIV testing (including HIV RNA for acute infection), EBV serologies, and tests for syphilis and SLE. Lumbar puncture (for evaluation of aseptic meningitis) and lymph node biopsy would be informative. Skin biopsy may be helpful to evaluate for aggressive T cell lymphoproliferative disorder, but this can await the results of initial testing.
The patient was given intravenous fluids and acetaminophen as needed. Blood cultures, urine culture, cytomegalovirus and EBV serologies, hepatitis B surface antigen, hepatitis C virus antibody, HIV antibody, antinuclear antibody, complement and ferritin levels, and quantiferon‐TB were ordered. The urine was normal and a urinary antigen test for Legionella was negative. Contrast‐enhanced computed tomography scan of the chest and abdomen was normal except for mild splenomegaly and an enlarged left axillary lymph node.
The ferritin may have been ordered to help evaluate for Still's disease, which is characterized by sustained fever, lymphadenopathy, and transient rash; however, the characteristic leukocytosis and arthralgias are absent. The computed tomography findings are most notable for the absence of generalized lymphadenopathy or significant hepatosplenomegaly that is seen in lymphoma, leukemia, and lymphotropic processes such as acute EBV infection. The localization of disease to the skin (where the predominant lymphocytes are of T cell origin) with relatively modest lymphadenopathy suggests a T cell lymphoma, perhaps of an indolent variety. Vertical transmission of HTLV‐1 decades ago would make adult T cell leukemia or lymphoma a major consideration.
On the third hospital day, WBC count was 1800/L with 67% neutrophils, 22% lymphocytes, and 1% atypical lymphocytes; LDH rose to 623 IU/L. He had continued fatigue and high fever while the rash gradually faded with oral antihistamines and steroid ointment. On hospital day 4, bone marrow biopsy and skin biopsy of his left thigh were performed.
The further decline in WBC and rise in LDH are modest and therefore do not significantly modify the differential diagnosis. Likewise, 1% atypical lymphocytosis is too low to pinpoint an etiology. Because unremitting fevers start to extend into their third week without a clear source of infection, the probability of malignancy and autoimmunity rise. Improvement with oral antihistamines and topical steroids frequently suggests an underlying allergic process, but the remainder of the clinical picture is not in keeping with atopy or allergy. Cutaneous lymphomas (eg, mycosis fungoides) can have waxing and waning skin manifestations, and can be temporarily or definitively treated by topical steroids. The persistence of his fatigue is of concern given the absence of anemia, cardiopulmonary involvement, or motor weakness.
Bone marrow biopsy showed normocellular marrow with no abnormal cells and some activated macrophages with hemophagocytic activity. Skin biopsy failed to show specific pathology.
His left cervical lymph nodes gradually enlarged. Ultrasound of the neck showed multiple enlarged lymph nodes (left side dominant) with dimension of 17 mm 9 mm 31 mm. Blood and urine cultures returned negative, as did HIV antibody. cytomegalovirus and EBV serologies were consistent with previous infection and the ferritin level was 578 ng/mL (normal, 39‐340 ng/mL). Toxoplasma serology and HTLV‐1 antibody were ordered.
The absence of malignant cells on bone marrow biopsy does not exclude lymphoma, but makes a myelophthisic cause of the cytopenias less likely. The macrophage hemophagocytosis reflects immune activation, which in turn is usually caused by the same viral infections, autoimmune conditions, and lymphoproliferative disorders which constitute the current differential diagnosis.
Bone marrow and skin biopsies are both subject to sampling error, and detection of cutaneous T cell lymphoma is notoriously difficult. However, taken together, the absence of cancer on 2 specimens reduces that possibility.
Sustained unilateral cervical lymphadenopathy with fever in a young Japanese man without any histologic evidence of lymphoma points to Kikuchi's disease, ie, lymphadenitis of unknown etiology associated with varying degrees of systemic manifestations. Fever is a frequent feature, we believe, but diffuse sustained rash, cytopenias, and headache are less common or are seen in severe forms of the disease. The diagnosis of Kikuchi's requires the diligent exclusion of SLE and lymphoma. Examination of the peripheral smear and a lymph node biopsy are required.
Of note, there is also a localized form of Castleman's disease, a nonmalignant lymphoproliferative disorder, that similarly is characterized by focal lymphadenopathy. In distinction to Kikuchi's, however, localized Castleman's is largely asymptomatic and responds marvelously to excision.
On hospital day 9, an excisional biopsy of his left anterior cervical lymph nodes was performed, which revealed paracortical foci with necrosis and a histiocytic cellular infiltrate consistent with subacute necrotizing lymphadenitis (Kikuchi‐Fujimoto disease). Antinuclear antibody, Toxoplasma, and HTLV‐1 antibodies returned negative.
There is no treatment for Kikuchi's. It is usually self‐limited, but steroids are sometimes given for symptomatic control.
His condition began to improve after hospital day 9 without specific treatment, including his WBC count and LDH level. He was discharged home on hospital day 15. In the outpatient clinic 1 and 3 months later, he was well and active without recurrences of any symptoms or laboratory abnormalities. His WBC count was 6600/L and LDH was 268 IU/L.
Commentary
Kikuchi‐Fujimoto disease (KFD), also called Kikuchi's disease, is a benign histiocytic necrotizing lymphadenitis described by both Kikuchi and Fujimoto in 1972.1, 2 It is rare in the United States, but seems more common in Asia, especially Japan, where at least 143 cases have been reported since 1972. The etiology has not been determined, but a viral causeincluding EBV, and human herpesvirus 6 and 8has been suggested.3 An autoimmune etiology is also implicated because of infrequent association with SLE. In general, young women are most likely to be affected. In a review of 244 cases by Kucukardali and colleagues, 77% of patients were female and the mean age was 25; 70% were younger than 30 years of age.4
The common presentation is low‐grade fever with unilateral cervical lymphadenopathy.4 Although generalized lymphadenopathy can occur, it is rare. Other common clinical manifestations include malaise, joint pain, rash, arthritis, and hepatosplenomegaly. No specific laboratory tests for diagnosis are available, but leukopenia (seen in 43% of patients), increased erythrocyte sedimentation rate (40%), and anemia (23%) may be observed.4 In this case, atypical lymphocytes were seen, and are reported in one‐third of patients.5 KFD is generally diagnosed by lymph node biopsy, which typically shows irregular paracortical areas of coagulation necrosis that can distort the nodal architecture, while different types of histiocytes are observed at the margin of necrotic areas.
Other diseases in the differential diagnosisseveral of which were considered by the discussantinclude lymphoma, tuberculosis, SLE, and even metastatic adenocarcinoma. KFD is self‐limited; symptoms typically resolve within 1 to 4 months. Patients with severe manifestations have been treated with anti‐inflammatory drugs and glucocorticosteroids. A recurrence rate of 3% to 4% has been reported.6
The clinicians taking care of this patient initially focused on ruling out those infections occasionally resulting in prolonged fever in a previously healthy young man, such as viruses from the herpes family, HIV, viral hepatitis, tuberculosis, syphilis, infective endocarditis, and intra‐abdominal abscess. Physical examination, specifically lymphadenopathy and mild splenomegaly, made Herpesviridae infections, tuberculosis, syphilis, and lymphoma difficult to exclude. Once the initial evaluation ruled out common infections, attention focused on malignancy and histiocytic necrotizing lymphadenitis, given his ethnicity and geographic location.
The discussant was similarly concerned about infection, malignancy, and noninfectious inflammatory diseases, such as SLE, as possible causes. As evidence of these treatable diseases failed to accumulate, the discussant, an American physician with teaching and clinical experience in Japan, considered endemic diseases such as Behcet's, HTLV‐1, and KFD because they fit the unfolding pattern. Given our global society, clinicians will increasingly benefit from becoming familiar with the less common diseases that afflict the various populations around the world.
Teaching Points
-
The combination of fever, lymphadenopathy, and leukopenia in young adults suggests SLE, lymphoma, and HIV. Clinicians should also consider KFD in patients from Japan and neighboring countries.
-
Lymph node biopsy is usually diagnostic of KFD, although interpretation of histopathology can be difficult and sometimes leads to confusion with SLE and lymphoma.
-
KFD typically resolves without specific treatment.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
- .Lymphadenitis showing focal reticulum cell hyperplasia with nuclear debris and phagocytes: a clinicopathological study.Acta Hematol Jpn.1972;35:379–380.
- ,,.Cervical subacute necrotizing lymphadenitis: a new clinicopathological agent.Naika.1972;20:920–927.
- ,,,.Enigmatic Kikuchi‐Fujimoto disease: a comprehensive review.Am J Clin Pathol.2004;122:141–152.
- ,,,,,.Kikuchi‐Fujimoto Disease: analysis of 244 cases.Clin Rheumatol.2007;26:50–54.
- ,,,,.Kikuchi's disease: A review and analysis of 61 cases.Otolaryngol Head Neck Surg.2003;128:650–653.
- .Histiocytic necrotizing lymphadenitis of Kikuchi and Fujimoto.Arch Pathol Lab Med.1987;11:1026–1029.
A 19‐year‐old Japanese man was admitted to a hospital near Kyoto, Japan, because of fever and rash. Two weeks prior to admission, he developed mild headache and low‐grade fever; a rapid test for influenza was negative. His symptoms transiently improved with acetaminophen, but 8 days prior to admission, he developed fever to 38.5C and a pruritic maculopapular rash over his back that spread to his limbs. Six days prior to admission, a chest radiograph was clear; clarithromycin was prescribed for presumed upper respiratory infection. He visited the emergency department the day before admission because of continued fever of greater than 39C, fatigue, and headache. Because there was no jolt accentuation of the headache (ie, worsening with rapid horizontal rotation), or neck pain with extreme neck flexion, he was discharged on acetaminophen. He returned the next day with worsening fatigue and was admitted. He denied chills, rigor, weight loss, photosensitivity, sore throat, neck pain, cough, dyspnea, chest pain, nausea, vomiting, diarrhea, abdominal pain, back pain, and arthralgia.
Fever and diffuse rash are often due to infection, although drugs, autoimmune processes, and cancer must be considered. The presence of headache does not focus the differential diagnosis substantially, because many of the candidate diagnoses can be accompanied by meningitis or encephalitis, or even more frequently, nonspecific headaches. In one small study, jolt‐induced aggravation of headache was shown to be a sensitive indicator of cerebrospinal fluid pleocytosis. The absence of neck stiffness and the 2‐week duration makes bacterial meningitis unlikely, but a more indolent form of aseptic meningitis may need to be evaluated with a lumbar puncture.
The 2‐week illness without rapid deterioration makes some serious causes of fever and rash, such as toxic shock syndrome, disseminated meningococcal infection, or toxic epidermal necrolysis unlikely. A viral exanthema is possible, although the 2‐week duration is longer than usual. Given his youth, however, his immunization history should be queried, and acute infection with human immunodeficiency virus (HIV) should be considered. A more indolent infection, such as subacute bacterial endocarditis, disseminated gonococcal infection, or syphilis is plausible. Among autoimmune etiologies, systemic lupus erythematosus (SLE) and Behcet's disease (which is prevalent in Japan) can involve the central nervous system and cause fever. A careful inquiry directed at prescribed, complementary, and illicit drugs is required.
The patient's past medical history was notable only for mumps at the age of 10. His medications included acetaminophen, clarithromycin, and an herbal medicine, which he had been taking for the prior several days. He reported no tobacco or illicit drug use and rarely drank alcohol. He had never been sexually active. He worked in a factory and reported occasional contact with silver. He lived with his parents; there was no family history of tuberculosis or connective tissue diseases. His father was from Kyushu (the southernmost major island in Japan) and had chronic hepatitis C. The patient denied recent animal exposure or recent travel. His childhood vaccinations were said to be up to date.
Mumps at age 10 might signal general lack of immunization, in which case childhood viral exanthema‐like measles (characterized by fever, headache, and diffuse rash) would warrant consideration. The listed medications had been started after the onset of illness and therefore are unlikely to be causal. Silver causes at least 2 skin conditionscontact dermatitis and argyriabut not the systemic illness seen here. Human T lymphotropic virus‐1 (HTLV‐1) is endemic in southern Japan, but only a minority of infected humans are afflicted with associated adult T cell leukemia/lymphoma or myelopathy. Leukemia and lymphoma are the most likely cancers to cause fever, rash, and central nervous system involvement (with T cell disorders demonstrating a particular tropism for the skin). Overall, however, the differential has not changed substantially.
On physical examination, the patient was mildly overweight and appeared acutely ill. His blood pressure was 136/78 mm Hg, pulse rate was 76 and regular, temperature was 39.2C and respiratory rate was 20 with an oxygen saturation of 98% on room air. A diffuse but nonconfluent erythematous maculopapular rash was present over his chest wall, back, medial aspects of both thighs, and around the knees. There was no jolt‐induced headache. His eyes, nose, oral cavity, and throat were all clear. The neck was supple. There were palpable lymph nodes, each about 1 cm in size, which were firm and moderately tender, in his left neck and left axilla. Lungs and heart were normal. The abdomen was soft, nontender, with normal bowel sounds and no hepatosplenomegaly. His genitalia were normal. Rectal examination revealed no masses or tenderness and a scant amount of brown stool that was negative for occult blood. Neurologic examination was unremarkable.
The multifocal lymphadenopathy does not help distinguish among the categories of disease under consideration. The diffuse maculopapular rash is similarly nonspecific, occurring more frequently with infection and drug reaction than malignancy and autoimmunity. Acute HIV, Epstein‐Barr virus (EBV), syphilis, SLE, drug exposure, or a hematologic malignancy would all be suitable explanations for fever, headache, diffuse rash, and disseminated lymphadenopathy in a previously healthy young man.
Laboratory data obtained on admission was notable for a white blood cell (WBC) count of 2100/L with 72% neutrophils, 19% lymphocytes, and 9% monocytes. Hemoglobin was 13.5 mg/dL with a mean corpuscular volume of 85 fL. Platelet count was 136,000/L. Erythrocyte sedimentation rate was 26 mm/hour. Serum chemistries revealed a sodium level of 135 mEq/L, potassium level of 3.6 mEq/L, chloride level of 100 mEq/L, blood urea nitrogen of 9.8 mg/dL, creatinine level of 1.0 mg/dL, glucose level of 101 mg/dL, calcium level of 8.8 mg/dL, albumin of 4.6 mg/dL, total protein of 8.4 mg/dL, aspartate aminotransferase of 42 IU/L (normal < 35 IU/L), alanine aminotransferase of 27 IU/L, total bilirubin of 0.5 mg/dL, and lactate dehydrogenase (LDH) level of 463 IU/L (normal < 260 IU/L). Chest radiography and electrocardiogram were normal.
A mild elevation in LDH is nonspecific, but without hemolysis or infarction of the kidney, lung, or muscle, it suggests a lymphoproliferative process. Leukopenia with thrombocytopenia can be seen in a number of disorders, most commonly infections including viruses (e.g., EBV, HIV, dengue), malaria, Rocky Mountain spotted fever, or ehrlichiosis/anaplasmosis. Confirmation of his lack of travel could help prioritize those considerations. An invasive bone marrow disorder cannot be excluded, although the near‐normal hemoglobin argues against it. Autoimmune cytopenias are seen in SLE. Given his age, lymphadenopathy, LDH elevation, and absence of infectious exposures, lymphoma rises to the top of the list.
Noninvasive measures should include examination of the peripheral smear, HIV testing (including HIV RNA for acute infection), EBV serologies, and tests for syphilis and SLE. Lumbar puncture (for evaluation of aseptic meningitis) and lymph node biopsy would be informative. Skin biopsy may be helpful to evaluate for aggressive T cell lymphoproliferative disorder, but this can await the results of initial testing.
The patient was given intravenous fluids and acetaminophen as needed. Blood cultures, urine culture, cytomegalovirus and EBV serologies, hepatitis B surface antigen, hepatitis C virus antibody, HIV antibody, antinuclear antibody, complement and ferritin levels, and quantiferon‐TB were ordered. The urine was normal and a urinary antigen test for Legionella was negative. Contrast‐enhanced computed tomography scan of the chest and abdomen was normal except for mild splenomegaly and an enlarged left axillary lymph node.
The ferritin may have been ordered to help evaluate for Still's disease, which is characterized by sustained fever, lymphadenopathy, and transient rash; however, the characteristic leukocytosis and arthralgias are absent. The computed tomography findings are most notable for the absence of generalized lymphadenopathy or significant hepatosplenomegaly that is seen in lymphoma, leukemia, and lymphotropic processes such as acute EBV infection. The localization of disease to the skin (where the predominant lymphocytes are of T cell origin) with relatively modest lymphadenopathy suggests a T cell lymphoma, perhaps of an indolent variety. Vertical transmission of HTLV‐1 decades ago would make adult T cell leukemia or lymphoma a major consideration.
On the third hospital day, WBC count was 1800/L with 67% neutrophils, 22% lymphocytes, and 1% atypical lymphocytes; LDH rose to 623 IU/L. He had continued fatigue and high fever while the rash gradually faded with oral antihistamines and steroid ointment. On hospital day 4, bone marrow biopsy and skin biopsy of his left thigh were performed.
The further decline in WBC and rise in LDH are modest and therefore do not significantly modify the differential diagnosis. Likewise, 1% atypical lymphocytosis is too low to pinpoint an etiology. Because unremitting fevers start to extend into their third week without a clear source of infection, the probability of malignancy and autoimmunity rise. Improvement with oral antihistamines and topical steroids frequently suggests an underlying allergic process, but the remainder of the clinical picture is not in keeping with atopy or allergy. Cutaneous lymphomas (eg, mycosis fungoides) can have waxing and waning skin manifestations, and can be temporarily or definitively treated by topical steroids. The persistence of his fatigue is of concern given the absence of anemia, cardiopulmonary involvement, or motor weakness.
Bone marrow biopsy showed normocellular marrow with no abnormal cells and some activated macrophages with hemophagocytic activity. Skin biopsy failed to show specific pathology.
His left cervical lymph nodes gradually enlarged. Ultrasound of the neck showed multiple enlarged lymph nodes (left side dominant) with dimension of 17 mm 9 mm 31 mm. Blood and urine cultures returned negative, as did HIV antibody. cytomegalovirus and EBV serologies were consistent with previous infection and the ferritin level was 578 ng/mL (normal, 39‐340 ng/mL). Toxoplasma serology and HTLV‐1 antibody were ordered.
The absence of malignant cells on bone marrow biopsy does not exclude lymphoma, but makes a myelophthisic cause of the cytopenias less likely. The macrophage hemophagocytosis reflects immune activation, which in turn is usually caused by the same viral infections, autoimmune conditions, and lymphoproliferative disorders which constitute the current differential diagnosis.
Bone marrow and skin biopsies are both subject to sampling error, and detection of cutaneous T cell lymphoma is notoriously difficult. However, taken together, the absence of cancer on 2 specimens reduces that possibility.
Sustained unilateral cervical lymphadenopathy with fever in a young Japanese man without any histologic evidence of lymphoma points to Kikuchi's disease, ie, lymphadenitis of unknown etiology associated with varying degrees of systemic manifestations. Fever is a frequent feature, we believe, but diffuse sustained rash, cytopenias, and headache are less common or are seen in severe forms of the disease. The diagnosis of Kikuchi's requires the diligent exclusion of SLE and lymphoma. Examination of the peripheral smear and a lymph node biopsy are required.
Of note, there is also a localized form of Castleman's disease, a nonmalignant lymphoproliferative disorder, that similarly is characterized by focal lymphadenopathy. In distinction to Kikuchi's, however, localized Castleman's is largely asymptomatic and responds marvelously to excision.
On hospital day 9, an excisional biopsy of his left anterior cervical lymph nodes was performed, which revealed paracortical foci with necrosis and a histiocytic cellular infiltrate consistent with subacute necrotizing lymphadenitis (Kikuchi‐Fujimoto disease). Antinuclear antibody, Toxoplasma, and HTLV‐1 antibodies returned negative.
There is no treatment for Kikuchi's. It is usually self‐limited, but steroids are sometimes given for symptomatic control.
His condition began to improve after hospital day 9 without specific treatment, including his WBC count and LDH level. He was discharged home on hospital day 15. In the outpatient clinic 1 and 3 months later, he was well and active without recurrences of any symptoms or laboratory abnormalities. His WBC count was 6600/L and LDH was 268 IU/L.
Commentary
Kikuchi‐Fujimoto disease (KFD), also called Kikuchi's disease, is a benign histiocytic necrotizing lymphadenitis described by both Kikuchi and Fujimoto in 1972.1, 2 It is rare in the United States, but seems more common in Asia, especially Japan, where at least 143 cases have been reported since 1972. The etiology has not been determined, but a viral causeincluding EBV, and human herpesvirus 6 and 8has been suggested.3 An autoimmune etiology is also implicated because of infrequent association with SLE. In general, young women are most likely to be affected. In a review of 244 cases by Kucukardali and colleagues, 77% of patients were female and the mean age was 25; 70% were younger than 30 years of age.4
The common presentation is low‐grade fever with unilateral cervical lymphadenopathy.4 Although generalized lymphadenopathy can occur, it is rare. Other common clinical manifestations include malaise, joint pain, rash, arthritis, and hepatosplenomegaly. No specific laboratory tests for diagnosis are available, but leukopenia (seen in 43% of patients), increased erythrocyte sedimentation rate (40%), and anemia (23%) may be observed.4 In this case, atypical lymphocytes were seen, and are reported in one‐third of patients.5 KFD is generally diagnosed by lymph node biopsy, which typically shows irregular paracortical areas of coagulation necrosis that can distort the nodal architecture, while different types of histiocytes are observed at the margin of necrotic areas.
Other diseases in the differential diagnosisseveral of which were considered by the discussantinclude lymphoma, tuberculosis, SLE, and even metastatic adenocarcinoma. KFD is self‐limited; symptoms typically resolve within 1 to 4 months. Patients with severe manifestations have been treated with anti‐inflammatory drugs and glucocorticosteroids. A recurrence rate of 3% to 4% has been reported.6
The clinicians taking care of this patient initially focused on ruling out those infections occasionally resulting in prolonged fever in a previously healthy young man, such as viruses from the herpes family, HIV, viral hepatitis, tuberculosis, syphilis, infective endocarditis, and intra‐abdominal abscess. Physical examination, specifically lymphadenopathy and mild splenomegaly, made Herpesviridae infections, tuberculosis, syphilis, and lymphoma difficult to exclude. Once the initial evaluation ruled out common infections, attention focused on malignancy and histiocytic necrotizing lymphadenitis, given his ethnicity and geographic location.
The discussant was similarly concerned about infection, malignancy, and noninfectious inflammatory diseases, such as SLE, as possible causes. As evidence of these treatable diseases failed to accumulate, the discussant, an American physician with teaching and clinical experience in Japan, considered endemic diseases such as Behcet's, HTLV‐1, and KFD because they fit the unfolding pattern. Given our global society, clinicians will increasingly benefit from becoming familiar with the less common diseases that afflict the various populations around the world.
Teaching Points
-
The combination of fever, lymphadenopathy, and leukopenia in young adults suggests SLE, lymphoma, and HIV. Clinicians should also consider KFD in patients from Japan and neighboring countries.
-
Lymph node biopsy is usually diagnostic of KFD, although interpretation of histopathology can be difficult and sometimes leads to confusion with SLE and lymphoma.
-
KFD typically resolves without specific treatment.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 19‐year‐old Japanese man was admitted to a hospital near Kyoto, Japan, because of fever and rash. Two weeks prior to admission, he developed mild headache and low‐grade fever; a rapid test for influenza was negative. His symptoms transiently improved with acetaminophen, but 8 days prior to admission, he developed fever to 38.5C and a pruritic maculopapular rash over his back that spread to his limbs. Six days prior to admission, a chest radiograph was clear; clarithromycin was prescribed for presumed upper respiratory infection. He visited the emergency department the day before admission because of continued fever of greater than 39C, fatigue, and headache. Because there was no jolt accentuation of the headache (ie, worsening with rapid horizontal rotation), or neck pain with extreme neck flexion, he was discharged on acetaminophen. He returned the next day with worsening fatigue and was admitted. He denied chills, rigor, weight loss, photosensitivity, sore throat, neck pain, cough, dyspnea, chest pain, nausea, vomiting, diarrhea, abdominal pain, back pain, and arthralgia.
Fever and diffuse rash are often due to infection, although drugs, autoimmune processes, and cancer must be considered. The presence of headache does not focus the differential diagnosis substantially, because many of the candidate diagnoses can be accompanied by meningitis or encephalitis, or even more frequently, nonspecific headaches. In one small study, jolt‐induced aggravation of headache was shown to be a sensitive indicator of cerebrospinal fluid pleocytosis. The absence of neck stiffness and the 2‐week duration makes bacterial meningitis unlikely, but a more indolent form of aseptic meningitis may need to be evaluated with a lumbar puncture.
The 2‐week illness without rapid deterioration makes some serious causes of fever and rash, such as toxic shock syndrome, disseminated meningococcal infection, or toxic epidermal necrolysis unlikely. A viral exanthema is possible, although the 2‐week duration is longer than usual. Given his youth, however, his immunization history should be queried, and acute infection with human immunodeficiency virus (HIV) should be considered. A more indolent infection, such as subacute bacterial endocarditis, disseminated gonococcal infection, or syphilis is plausible. Among autoimmune etiologies, systemic lupus erythematosus (SLE) and Behcet's disease (which is prevalent in Japan) can involve the central nervous system and cause fever. A careful inquiry directed at prescribed, complementary, and illicit drugs is required.
The patient's past medical history was notable only for mumps at the age of 10. His medications included acetaminophen, clarithromycin, and an herbal medicine, which he had been taking for the prior several days. He reported no tobacco or illicit drug use and rarely drank alcohol. He had never been sexually active. He worked in a factory and reported occasional contact with silver. He lived with his parents; there was no family history of tuberculosis or connective tissue diseases. His father was from Kyushu (the southernmost major island in Japan) and had chronic hepatitis C. The patient denied recent animal exposure or recent travel. His childhood vaccinations were said to be up to date.
Mumps at age 10 might signal general lack of immunization, in which case childhood viral exanthema‐like measles (characterized by fever, headache, and diffuse rash) would warrant consideration. The listed medications had been started after the onset of illness and therefore are unlikely to be causal. Silver causes at least 2 skin conditionscontact dermatitis and argyriabut not the systemic illness seen here. Human T lymphotropic virus‐1 (HTLV‐1) is endemic in southern Japan, but only a minority of infected humans are afflicted with associated adult T cell leukemia/lymphoma or myelopathy. Leukemia and lymphoma are the most likely cancers to cause fever, rash, and central nervous system involvement (with T cell disorders demonstrating a particular tropism for the skin). Overall, however, the differential has not changed substantially.
On physical examination, the patient was mildly overweight and appeared acutely ill. His blood pressure was 136/78 mm Hg, pulse rate was 76 and regular, temperature was 39.2C and respiratory rate was 20 with an oxygen saturation of 98% on room air. A diffuse but nonconfluent erythematous maculopapular rash was present over his chest wall, back, medial aspects of both thighs, and around the knees. There was no jolt‐induced headache. His eyes, nose, oral cavity, and throat were all clear. The neck was supple. There were palpable lymph nodes, each about 1 cm in size, which were firm and moderately tender, in his left neck and left axilla. Lungs and heart were normal. The abdomen was soft, nontender, with normal bowel sounds and no hepatosplenomegaly. His genitalia were normal. Rectal examination revealed no masses or tenderness and a scant amount of brown stool that was negative for occult blood. Neurologic examination was unremarkable.
The multifocal lymphadenopathy does not help distinguish among the categories of disease under consideration. The diffuse maculopapular rash is similarly nonspecific, occurring more frequently with infection and drug reaction than malignancy and autoimmunity. Acute HIV, Epstein‐Barr virus (EBV), syphilis, SLE, drug exposure, or a hematologic malignancy would all be suitable explanations for fever, headache, diffuse rash, and disseminated lymphadenopathy in a previously healthy young man.
Laboratory data obtained on admission was notable for a white blood cell (WBC) count of 2100/L with 72% neutrophils, 19% lymphocytes, and 9% monocytes. Hemoglobin was 13.5 mg/dL with a mean corpuscular volume of 85 fL. Platelet count was 136,000/L. Erythrocyte sedimentation rate was 26 mm/hour. Serum chemistries revealed a sodium level of 135 mEq/L, potassium level of 3.6 mEq/L, chloride level of 100 mEq/L, blood urea nitrogen of 9.8 mg/dL, creatinine level of 1.0 mg/dL, glucose level of 101 mg/dL, calcium level of 8.8 mg/dL, albumin of 4.6 mg/dL, total protein of 8.4 mg/dL, aspartate aminotransferase of 42 IU/L (normal < 35 IU/L), alanine aminotransferase of 27 IU/L, total bilirubin of 0.5 mg/dL, and lactate dehydrogenase (LDH) level of 463 IU/L (normal < 260 IU/L). Chest radiography and electrocardiogram were normal.
A mild elevation in LDH is nonspecific, but without hemolysis or infarction of the kidney, lung, or muscle, it suggests a lymphoproliferative process. Leukopenia with thrombocytopenia can be seen in a number of disorders, most commonly infections including viruses (e.g., EBV, HIV, dengue), malaria, Rocky Mountain spotted fever, or ehrlichiosis/anaplasmosis. Confirmation of his lack of travel could help prioritize those considerations. An invasive bone marrow disorder cannot be excluded, although the near‐normal hemoglobin argues against it. Autoimmune cytopenias are seen in SLE. Given his age, lymphadenopathy, LDH elevation, and absence of infectious exposures, lymphoma rises to the top of the list.
Noninvasive measures should include examination of the peripheral smear, HIV testing (including HIV RNA for acute infection), EBV serologies, and tests for syphilis and SLE. Lumbar puncture (for evaluation of aseptic meningitis) and lymph node biopsy would be informative. Skin biopsy may be helpful to evaluate for aggressive T cell lymphoproliferative disorder, but this can await the results of initial testing.
The patient was given intravenous fluids and acetaminophen as needed. Blood cultures, urine culture, cytomegalovirus and EBV serologies, hepatitis B surface antigen, hepatitis C virus antibody, HIV antibody, antinuclear antibody, complement and ferritin levels, and quantiferon‐TB were ordered. The urine was normal and a urinary antigen test for Legionella was negative. Contrast‐enhanced computed tomography scan of the chest and abdomen was normal except for mild splenomegaly and an enlarged left axillary lymph node.
The ferritin may have been ordered to help evaluate for Still's disease, which is characterized by sustained fever, lymphadenopathy, and transient rash; however, the characteristic leukocytosis and arthralgias are absent. The computed tomography findings are most notable for the absence of generalized lymphadenopathy or significant hepatosplenomegaly that is seen in lymphoma, leukemia, and lymphotropic processes such as acute EBV infection. The localization of disease to the skin (where the predominant lymphocytes are of T cell origin) with relatively modest lymphadenopathy suggests a T cell lymphoma, perhaps of an indolent variety. Vertical transmission of HTLV‐1 decades ago would make adult T cell leukemia or lymphoma a major consideration.
On the third hospital day, WBC count was 1800/L with 67% neutrophils, 22% lymphocytes, and 1% atypical lymphocytes; LDH rose to 623 IU/L. He had continued fatigue and high fever while the rash gradually faded with oral antihistamines and steroid ointment. On hospital day 4, bone marrow biopsy and skin biopsy of his left thigh were performed.
The further decline in WBC and rise in LDH are modest and therefore do not significantly modify the differential diagnosis. Likewise, 1% atypical lymphocytosis is too low to pinpoint an etiology. Because unremitting fevers start to extend into their third week without a clear source of infection, the probability of malignancy and autoimmunity rise. Improvement with oral antihistamines and topical steroids frequently suggests an underlying allergic process, but the remainder of the clinical picture is not in keeping with atopy or allergy. Cutaneous lymphomas (eg, mycosis fungoides) can have waxing and waning skin manifestations, and can be temporarily or definitively treated by topical steroids. The persistence of his fatigue is of concern given the absence of anemia, cardiopulmonary involvement, or motor weakness.
Bone marrow biopsy showed normocellular marrow with no abnormal cells and some activated macrophages with hemophagocytic activity. Skin biopsy failed to show specific pathology.
His left cervical lymph nodes gradually enlarged. Ultrasound of the neck showed multiple enlarged lymph nodes (left side dominant) with dimension of 17 mm 9 mm 31 mm. Blood and urine cultures returned negative, as did HIV antibody. cytomegalovirus and EBV serologies were consistent with previous infection and the ferritin level was 578 ng/mL (normal, 39‐340 ng/mL). Toxoplasma serology and HTLV‐1 antibody were ordered.
The absence of malignant cells on bone marrow biopsy does not exclude lymphoma, but makes a myelophthisic cause of the cytopenias less likely. The macrophage hemophagocytosis reflects immune activation, which in turn is usually caused by the same viral infections, autoimmune conditions, and lymphoproliferative disorders which constitute the current differential diagnosis.
Bone marrow and skin biopsies are both subject to sampling error, and detection of cutaneous T cell lymphoma is notoriously difficult. However, taken together, the absence of cancer on 2 specimens reduces that possibility.
Sustained unilateral cervical lymphadenopathy with fever in a young Japanese man without any histologic evidence of lymphoma points to Kikuchi's disease, ie, lymphadenitis of unknown etiology associated with varying degrees of systemic manifestations. Fever is a frequent feature, we believe, but diffuse sustained rash, cytopenias, and headache are less common or are seen in severe forms of the disease. The diagnosis of Kikuchi's requires the diligent exclusion of SLE and lymphoma. Examination of the peripheral smear and a lymph node biopsy are required.
Of note, there is also a localized form of Castleman's disease, a nonmalignant lymphoproliferative disorder, that similarly is characterized by focal lymphadenopathy. In distinction to Kikuchi's, however, localized Castleman's is largely asymptomatic and responds marvelously to excision.
On hospital day 9, an excisional biopsy of his left anterior cervical lymph nodes was performed, which revealed paracortical foci with necrosis and a histiocytic cellular infiltrate consistent with subacute necrotizing lymphadenitis (Kikuchi‐Fujimoto disease). Antinuclear antibody, Toxoplasma, and HTLV‐1 antibodies returned negative.
There is no treatment for Kikuchi's. It is usually self‐limited, but steroids are sometimes given for symptomatic control.
His condition began to improve after hospital day 9 without specific treatment, including his WBC count and LDH level. He was discharged home on hospital day 15. In the outpatient clinic 1 and 3 months later, he was well and active without recurrences of any symptoms or laboratory abnormalities. His WBC count was 6600/L and LDH was 268 IU/L.
Commentary
Kikuchi‐Fujimoto disease (KFD), also called Kikuchi's disease, is a benign histiocytic necrotizing lymphadenitis described by both Kikuchi and Fujimoto in 1972.1, 2 It is rare in the United States, but seems more common in Asia, especially Japan, where at least 143 cases have been reported since 1972. The etiology has not been determined, but a viral causeincluding EBV, and human herpesvirus 6 and 8has been suggested.3 An autoimmune etiology is also implicated because of infrequent association with SLE. In general, young women are most likely to be affected. In a review of 244 cases by Kucukardali and colleagues, 77% of patients were female and the mean age was 25; 70% were younger than 30 years of age.4
The common presentation is low‐grade fever with unilateral cervical lymphadenopathy.4 Although generalized lymphadenopathy can occur, it is rare. Other common clinical manifestations include malaise, joint pain, rash, arthritis, and hepatosplenomegaly. No specific laboratory tests for diagnosis are available, but leukopenia (seen in 43% of patients), increased erythrocyte sedimentation rate (40%), and anemia (23%) may be observed.4 In this case, atypical lymphocytes were seen, and are reported in one‐third of patients.5 KFD is generally diagnosed by lymph node biopsy, which typically shows irregular paracortical areas of coagulation necrosis that can distort the nodal architecture, while different types of histiocytes are observed at the margin of necrotic areas.
Other diseases in the differential diagnosisseveral of which were considered by the discussantinclude lymphoma, tuberculosis, SLE, and even metastatic adenocarcinoma. KFD is self‐limited; symptoms typically resolve within 1 to 4 months. Patients with severe manifestations have been treated with anti‐inflammatory drugs and glucocorticosteroids. A recurrence rate of 3% to 4% has been reported.6
The clinicians taking care of this patient initially focused on ruling out those infections occasionally resulting in prolonged fever in a previously healthy young man, such as viruses from the herpes family, HIV, viral hepatitis, tuberculosis, syphilis, infective endocarditis, and intra‐abdominal abscess. Physical examination, specifically lymphadenopathy and mild splenomegaly, made Herpesviridae infections, tuberculosis, syphilis, and lymphoma difficult to exclude. Once the initial evaluation ruled out common infections, attention focused on malignancy and histiocytic necrotizing lymphadenitis, given his ethnicity and geographic location.
The discussant was similarly concerned about infection, malignancy, and noninfectious inflammatory diseases, such as SLE, as possible causes. As evidence of these treatable diseases failed to accumulate, the discussant, an American physician with teaching and clinical experience in Japan, considered endemic diseases such as Behcet's, HTLV‐1, and KFD because they fit the unfolding pattern. Given our global society, clinicians will increasingly benefit from becoming familiar with the less common diseases that afflict the various populations around the world.
Teaching Points
-
The combination of fever, lymphadenopathy, and leukopenia in young adults suggests SLE, lymphoma, and HIV. Clinicians should also consider KFD in patients from Japan and neighboring countries.
-
Lymph node biopsy is usually diagnostic of KFD, although interpretation of histopathology can be difficult and sometimes leads to confusion with SLE and lymphoma.
-
KFD typically resolves without specific treatment.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
- .Lymphadenitis showing focal reticulum cell hyperplasia with nuclear debris and phagocytes: a clinicopathological study.Acta Hematol Jpn.1972;35:379–380.
- ,,.Cervical subacute necrotizing lymphadenitis: a new clinicopathological agent.Naika.1972;20:920–927.
- ,,,.Enigmatic Kikuchi‐Fujimoto disease: a comprehensive review.Am J Clin Pathol.2004;122:141–152.
- ,,,,,.Kikuchi‐Fujimoto Disease: analysis of 244 cases.Clin Rheumatol.2007;26:50–54.
- ,,,,.Kikuchi's disease: A review and analysis of 61 cases.Otolaryngol Head Neck Surg.2003;128:650–653.
- .Histiocytic necrotizing lymphadenitis of Kikuchi and Fujimoto.Arch Pathol Lab Med.1987;11:1026–1029.
- .Lymphadenitis showing focal reticulum cell hyperplasia with nuclear debris and phagocytes: a clinicopathological study.Acta Hematol Jpn.1972;35:379–380.
- ,,.Cervical subacute necrotizing lymphadenitis: a new clinicopathological agent.Naika.1972;20:920–927.
- ,,,.Enigmatic Kikuchi‐Fujimoto disease: a comprehensive review.Am J Clin Pathol.2004;122:141–152.
- ,,,,,.Kikuchi‐Fujimoto Disease: analysis of 244 cases.Clin Rheumatol.2007;26:50–54.
- ,,,,.Kikuchi's disease: A review and analysis of 61 cases.Otolaryngol Head Neck Surg.2003;128:650–653.
- .Histiocytic necrotizing lymphadenitis of Kikuchi and Fujimoto.Arch Pathol Lab Med.1987;11:1026–1029.
In sight but out of mind
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 44‐year‐old woman was admitted to an Italian hospital with fever and chills that had started approximately 1 week earlier. A few days after onset of fever, she had noticed a red, nonpruritic, confluent, maculopapular rash which began on her face and descended to her body. She also complained of red eyes, photophobia, dyspnea, and watery diarrhea. She denied nausea, vomiting, headache, or neck stiffness. She had seen her primary care physician who had concomitantly prescribed amoxicillin, levofloxacin, and betamethasone. She took the medications for several days without symptomatic improvement.
The salient features of this acute illness include the maculopapular rash, fever, and red eyes with photophobia. The differential diagnosis includes infections, rheumatologic disorders, toxin exposure, and, less likely, hematologic malignancies. In the initial assessment it is crucial to rule out any life‐threatening etiologies of fever and rash such as septicemia from Neisseria meningitidis, bacterial endocarditis, toxic shock syndrome, typhoid fever, and rickettsial diseases. A number of critical components of the history would help narrow the diagnostic considerations, including any history of recent travel, animal or occupational exposure, sexual or medication history, and risk factors for immunosuppression.
The empiric use of antibiotics is indicated when a patient presents with symptoms that suggest life‐threatening illness. For nonemergent conditions, empiric antibiotics may be appropriate when a classic pattern for a given diagnosis is present. In this patient, however, the initial presentation does not appear to be life‐threatening, nor is it easily recognizable as a specific or classic diagnosis. Thus, I would not start antibiotics, because doing so may further disguise the diagnosis by interfering with culture results, or complicate the case by causing an adverse effect such as fever or rash.
One week before the onset of fever she went to the emergency department because of pain in both lower quadrants of her abdomen. The physician removed her intrauterine device (IUD), which appeared to be partially expelled. The patient returned the next day to the emergency department because of severe metrorrhagia.
Complications of IUDs include pelvic inflammatory disease, perforated uterus, myometrial abscess, partial or complete spontaneous abortion, and ectopic pregnancy. Toxic shock syndrome, pelvic inflammatory disease, and retained products from a partial spontaneous abortion can all lead to significant systemic disease and vaginal bleeding.
Her past medical history was unremarkable except for an episode of bacterial meningitis 20 years before. She lived in Florence, Italy, where she worked as a school teacher, and had not traveled outside of Italy in the last year. She was married with 2 children, and denied high‐risk sexual behavior. She did not own any animals.
The patient's lack of travel, high‐risk sexual behavior or animal exposure does not help to alter the differential diagnosis. The prior history of bacterial meningitis raises the question of an immunodeficiency syndrome. At this point, I remain concerned about toxic shock syndrome.
The patient's temperature was 38.2C, her blood pressure was 110/60 mm Hg, respiratory rate was 28 breaths per minute and her heart rate was 108 beats per minute. She was alert and oriented but appeared moderately ill. Her conjunctivae were hyperemic without any drainage, and her oropharynx was erythematous. Lung examination revealed diminished breath sounds in the lower right lung field and crackles bilaterally. Abdominal exam demonstrated mild hepatomegaly, but not splenomegaly. Skin exam showed an erythematous, confluent, maculopapular rash involving her face, torso, back, and extremities; no cutaneous abscesses were noted. Neurological and gynecological exams were both normal, as was the rectal examination.
Her vital signs suggest a progressive illness and possible sepsis. The conjunctival hyperemia could represent several pathologic findings including uveitis with ciliary flush, conjunctival hemorrhage, or hyperemia due to systemic illness. The pulmonary findings could be attributed to pulmonary edema, pneumonia, alveolar hemorrhage, or acute respiratory distress syndrome (ARDS) as a complication of sepsis and systemic inflammation. The hepatomegaly, while non‐specific, may be due to an inflammatory reaction to a systemic illness. If so, I would expect liver tests to be elevated as this can occur in a number of parasitic (eg, toxoplasmosis) and viral (eg, chickenpox, infectious mononucleosis, cytomegalovirus) infections. The lack of concurrent splenomegaly makes lymphoma or other hematologic malignancies less likely. Given the patient's constellation of symptoms, the progressive nature of her illness and the multiple organs involved, I continue to be most concerned about immediately life‐threatening diseases. Toxic shock syndrome secondary to staphylococcal infection can present with many of these signs and symptoms including conjunctival hyperemia, diffuse maculopapular erythema, pharyngitis and sepsis leading to pulmonary edema, pleural effusions and ARDS. Another possibility is leptospirosis, which can be associated with pharyngitis, hepatomegaly, diffuse rash, low‐grade fever, and frequently has conjunctival hyperemia. Moreover, leptospirosis has a markedly variable course and pulmonary hemorrhage and ARDS can occur in severe cases. However, the lack of clear exposure to an environmental source such as contaminated water or soil or animal tissue reduces my enthusiasm for it.
Routine laboratory studies demonstrated: white‐cell count 5210/mm3 (82% neutrophils, 10% lymphocytes, 7% monocytes, and 1% eosinophils); hematocrit 36.3%; platelet count 135,000/mm3; erythrocyte sedimentation rate 49 mm/hour; fibrinogen 591 mg/dL (normal range, 200 ‐ 450 mg/dL); C‐reactive protein 53 mg/L (normal range, <9 mg/L). Serum electrolyte levels were normal. Liver tests demonstrated: aspartate aminotransferase 75 U/L; alanine aminotransferase 135 U/L; total bilirubin within normal limits; gamma glutamyltransferase 86 U/L (normal range, 10‐40 U/L). The urea nitrogen and the creatinine were both normal. The creatine phosphokinase was 381 U/L. Urinalysis was normal. An arterial‐blood gas, obtained while the patient was breathing room air, revealed an oxygen saturation of 87%; pH of 7.45; pCO2 of 38 mm Hg; pO2 of 54 mm Hg; bicarbonate concentration of 27 mmol/L.
Her electrocardiogram was normal except for sinus tachycardia. Chest film revealed a right‐sided pleural effusion without evidence of parenchymal abnormalities (Figure 1).
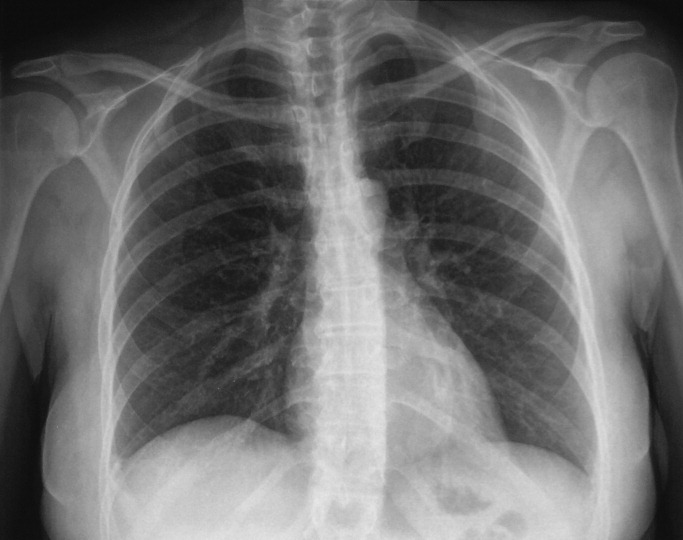
Despite the systemic illness, fever, and markedly abnormal inflammatory markers, the white blood cell count remains normal with a slight leftward shift. The most alarming finding is hypoxemia seen on the arterial blood gas. My leading diagnoses for this multisystemic febrile illness with a rash and hypoxia continue to be primarily infectious etiologies, including toxic shock syndrome with Staphylococcus species, leptospirosis, acute cytomegalovirus, and mycobacterial infections. Further diagnostic tests need to be performed but I would begin empiric antibiotics after appropriate cultures have been obtained. Rheumatologic etiologies such as systemic lupus erythematosus (SLE) and sarcoidosis seem less likely. SLE can present with a systemic illness, fever and rash, but the hepatitis, hepatomegaly and hyperemic conjunctivae are less common.
At the time of hospital admission, blood cultures were obtained before azithromycin, meropenem, and vancomycin were initiated for presumed toxic shock syndrome. Transvaginal and abdominal ultrasound studies revealed no abnormalities. She remained febrile but blood cultures returned negative. The results of the following investigations were also negative: immunoglobulin M (IgM) antibodies against Chlamydophila pneumoniae, cytomegalovirus, Epstein‐Barr virus, Legionella pneumophila, parvovirus B19, rubella virus, Coxiella burnetii, Mycoplasma pneumoniae, Chlamydophila psittaci, adenovirus, and coxsackieviruses. Antibodies against human immunodeficiency virus (HIV) 1 and 2 were negative. Tests for hepatitis B (HB surface antigen [HbsAg], HB core antibody [HbcAb] IgM) and C (HCV‐Ab) viruses were negative.
The lack of IgM antibodies for the infections listed markedly reduces their likelihood but does not exclude them. For example, given that the duration of symptoms is nearly 2 weeks at this point, it is possible that IgM has already decreased and IgG titers are now present. The lack of positive cultures does not exclude toxic shock, since in many severe cases the cultures remain negative. Thus, I remain concerned about toxic shock syndrome and would continue broad‐spectrum antibiotics.
After further investigating possible ill contacts to which the patient could have been exposed, it emerged that in the previous weeks there had been a case of measles in the kindergarten where she was working. The patient did not recall her vaccination history.
The recent exposure raises the risk of measles significantly, especially if she was not immunized as a child. Measles typically has an incubation period of 10 to 14 days, thus the prior exposure would fit the time course for the onset of this patient's symptoms. In retrospect, many of this patient's symptoms are classic for measles, including the maculopapular rash that begins on the face and extends downward, the conjunctival hyperemia, the persistent low‐grade fever, and the lack of clinical response to antibiotics.
In adults, measles can be complicated by inflammation in multiple organs resulting in myocarditis, pericarditis, hepatitis, encephalitis, and pneumonia. Thus, elevated transaminases would be consistent with the diagnosis as would a normal abdominal ultrasound. The pneumonia may be due to the measles infection itself or to coexisting viral or bacterial infections. The findings of a mild thrombocytopenia and a low normal leukocyte count can also be seen in measles infections. The diagnosis of measles is based on clinical presentation and by serologic confirmation: IgM antibodies are detectable within 1 or 2 days after the appearance of the rash, whereas the IgG titer rises significantly after 10 days.
I would continue the broad spectrum antibiotics until measles serologies could be confirmed. If the measles serologies are negative, I would continue the evaluation. If the serologies are positive, however, I would continue supportive care and review her pulmonary status to make sure she does not have a secondary bacterial infection. I strongly suspect that she has measles that is complicated by pneumonia and hepatitis.
The IgM antibody against measles virus returned positive and the patient was diagnosed with measles. By hospital day 5, her fever disappeared, her dyspnea resolved, and her rash had receded. Her oxygen saturation was 97% at the time of discharge.
Commentary
Measles is a highly contagious, acute‐onset, exanthematous disease that affects the respiratory tract and mucous membranes. Measles is clinically characterized by a prodromal stage of cough, conjunctivitis, coryza and high fever, typically lasting between 2 and 4 days.1, 2 The pathognomonic finding on the oral mucosa (Koplik spots) is usually followed by a generalized rash. The characteristic rash of measles is erythematous, nonpruritic, and maculopapular beginning at the hairline and behind the ears, and then spreads down the trunk and limbs and may include the palms and soles.1, 2 Often the patient has diarrhea, vomiting, lymphadenopathy, and splenomegaly; however, the clinical presentation can vary.1, 2 In partially immunized patients, symptoms are often atypical, whereas severe cases are characteristically seen in adults with the most frequent complication being pneumonia. About 3% of young adults with measles have a viral pneumonia that requires hospitalization.24 Adults are much more likely than children to develop hepatitis, bronchospasm and bacterial superinfection.2, 3, 5
The introduction of the measles vaccine initially led to a dramatic decrease in the incidence of measles. However, lack of adherence to vaccination campaigns among some families has been followed by small epidemics. Childhood vaccination rates against measles have recently been reported as 88% in Italy, and even higherover 90%in Tuscany. However, Italy has faced an upsurge of measles since September 2007, with almost 60% of cases occurring in the 15‐ to 44‐year‐old age group.6
Classic presentations of common diseases are easily recognized, but in those cases in which the clinical presentation of uncommon illnesseslike measles in adultsis atypical, the epidemiological data and the clinical history play key roles. In this patient, both the discussant and clinical team focused on the most alarming potential diagnosis: toxic shock syndrome related to the use of the IUD. While appropriate, there were historical clues that this patient had measles that were not specifically soughtthe immunization status and the workplace (school) exposure.
This case highlights 2 important aspects of making a difficult clinical diagnosis. First, the patient did not recall her immunization history, and the clinical team did not clarify it, and thus potential childhood illnesses such as measles and rubella did not remain on the differential diagnosis. Assuming that a patient has had the appropriate vaccinations is done at the clinician'sand the patient'speril. Second, many diseases that commonly afflict children can also occur in adult patients, albeit less frequently. Had this patient been a 5‐year‐old child with the same symptoms, the diagnosis would likely have been made with alacrity. However, maculopapular rashes that begin on the face and spread to the body are quite uncommon in adult medicine. For both discussant and the clinical team, the rash was clearly in sight but the correct diagnosis was out of mind given the rarity of this infection in adults. Fortunately, however, once it became clear that the patient was unlikely to have toxic shock syndrome, the epidemiological detail initially left behind became the sentinel clue necessary to solve the case.
Teaching Points
-
After nearly vanishing in the developed world, measles has shown sporadic signs of resurgence in recent years. The disease needs to be considered in patients presenting with a febrile illness accompanied by an exanthem that begins on the head and spreads inferiorly, especially when accompanied by cough, rhinorrhea, and conjunctival changes.
-
Measles tends to cause relatively severe illness and frequent complications in adults, the most common of which is pneumonia.
- .Measles Virus (Rubeola). In: Mandell GL, Bennett JE, Dolin R, eds.Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases.6th ed.Philadelphia, PA:Elsevier Churchill Livingstone;2005:2031–2038.
- ,.The clinical significance of measles: a review.J Infect Dis.2004;189(Suppl 1):S4–S16.
- ,.Measles in the United Kingdom: can we eradicate it by 2010?Br Med J.2006;333:890–895.
- ,,,,,.Familial cases of severe measles pneumonia.Intern Med.2000;39:670–674.
- ,,, et al.Measles encephalitis and acute pancreatitis in a young adult.Am J Med Sci.2004;327:299–303.
- ,,,, et al.Measles resurges in Italy: preliminary data from September 2007 to May 2008.Euro Surveill.2008;13(29):pii=18928.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 44‐year‐old woman was admitted to an Italian hospital with fever and chills that had started approximately 1 week earlier. A few days after onset of fever, she had noticed a red, nonpruritic, confluent, maculopapular rash which began on her face and descended to her body. She also complained of red eyes, photophobia, dyspnea, and watery diarrhea. She denied nausea, vomiting, headache, or neck stiffness. She had seen her primary care physician who had concomitantly prescribed amoxicillin, levofloxacin, and betamethasone. She took the medications for several days without symptomatic improvement.
The salient features of this acute illness include the maculopapular rash, fever, and red eyes with photophobia. The differential diagnosis includes infections, rheumatologic disorders, toxin exposure, and, less likely, hematologic malignancies. In the initial assessment it is crucial to rule out any life‐threatening etiologies of fever and rash such as septicemia from Neisseria meningitidis, bacterial endocarditis, toxic shock syndrome, typhoid fever, and rickettsial diseases. A number of critical components of the history would help narrow the diagnostic considerations, including any history of recent travel, animal or occupational exposure, sexual or medication history, and risk factors for immunosuppression.
The empiric use of antibiotics is indicated when a patient presents with symptoms that suggest life‐threatening illness. For nonemergent conditions, empiric antibiotics may be appropriate when a classic pattern for a given diagnosis is present. In this patient, however, the initial presentation does not appear to be life‐threatening, nor is it easily recognizable as a specific or classic diagnosis. Thus, I would not start antibiotics, because doing so may further disguise the diagnosis by interfering with culture results, or complicate the case by causing an adverse effect such as fever or rash.
One week before the onset of fever she went to the emergency department because of pain in both lower quadrants of her abdomen. The physician removed her intrauterine device (IUD), which appeared to be partially expelled. The patient returned the next day to the emergency department because of severe metrorrhagia.
Complications of IUDs include pelvic inflammatory disease, perforated uterus, myometrial abscess, partial or complete spontaneous abortion, and ectopic pregnancy. Toxic shock syndrome, pelvic inflammatory disease, and retained products from a partial spontaneous abortion can all lead to significant systemic disease and vaginal bleeding.
Her past medical history was unremarkable except for an episode of bacterial meningitis 20 years before. She lived in Florence, Italy, where she worked as a school teacher, and had not traveled outside of Italy in the last year. She was married with 2 children, and denied high‐risk sexual behavior. She did not own any animals.
The patient's lack of travel, high‐risk sexual behavior or animal exposure does not help to alter the differential diagnosis. The prior history of bacterial meningitis raises the question of an immunodeficiency syndrome. At this point, I remain concerned about toxic shock syndrome.
The patient's temperature was 38.2C, her blood pressure was 110/60 mm Hg, respiratory rate was 28 breaths per minute and her heart rate was 108 beats per minute. She was alert and oriented but appeared moderately ill. Her conjunctivae were hyperemic without any drainage, and her oropharynx was erythematous. Lung examination revealed diminished breath sounds in the lower right lung field and crackles bilaterally. Abdominal exam demonstrated mild hepatomegaly, but not splenomegaly. Skin exam showed an erythematous, confluent, maculopapular rash involving her face, torso, back, and extremities; no cutaneous abscesses were noted. Neurological and gynecological exams were both normal, as was the rectal examination.
Her vital signs suggest a progressive illness and possible sepsis. The conjunctival hyperemia could represent several pathologic findings including uveitis with ciliary flush, conjunctival hemorrhage, or hyperemia due to systemic illness. The pulmonary findings could be attributed to pulmonary edema, pneumonia, alveolar hemorrhage, or acute respiratory distress syndrome (ARDS) as a complication of sepsis and systemic inflammation. The hepatomegaly, while non‐specific, may be due to an inflammatory reaction to a systemic illness. If so, I would expect liver tests to be elevated as this can occur in a number of parasitic (eg, toxoplasmosis) and viral (eg, chickenpox, infectious mononucleosis, cytomegalovirus) infections. The lack of concurrent splenomegaly makes lymphoma or other hematologic malignancies less likely. Given the patient's constellation of symptoms, the progressive nature of her illness and the multiple organs involved, I continue to be most concerned about immediately life‐threatening diseases. Toxic shock syndrome secondary to staphylococcal infection can present with many of these signs and symptoms including conjunctival hyperemia, diffuse maculopapular erythema, pharyngitis and sepsis leading to pulmonary edema, pleural effusions and ARDS. Another possibility is leptospirosis, which can be associated with pharyngitis, hepatomegaly, diffuse rash, low‐grade fever, and frequently has conjunctival hyperemia. Moreover, leptospirosis has a markedly variable course and pulmonary hemorrhage and ARDS can occur in severe cases. However, the lack of clear exposure to an environmental source such as contaminated water or soil or animal tissue reduces my enthusiasm for it.
Routine laboratory studies demonstrated: white‐cell count 5210/mm3 (82% neutrophils, 10% lymphocytes, 7% monocytes, and 1% eosinophils); hematocrit 36.3%; platelet count 135,000/mm3; erythrocyte sedimentation rate 49 mm/hour; fibrinogen 591 mg/dL (normal range, 200 ‐ 450 mg/dL); C‐reactive protein 53 mg/L (normal range, <9 mg/L). Serum electrolyte levels were normal. Liver tests demonstrated: aspartate aminotransferase 75 U/L; alanine aminotransferase 135 U/L; total bilirubin within normal limits; gamma glutamyltransferase 86 U/L (normal range, 10‐40 U/L). The urea nitrogen and the creatinine were both normal. The creatine phosphokinase was 381 U/L. Urinalysis was normal. An arterial‐blood gas, obtained while the patient was breathing room air, revealed an oxygen saturation of 87%; pH of 7.45; pCO2 of 38 mm Hg; pO2 of 54 mm Hg; bicarbonate concentration of 27 mmol/L.
Her electrocardiogram was normal except for sinus tachycardia. Chest film revealed a right‐sided pleural effusion without evidence of parenchymal abnormalities (Figure 1).

Despite the systemic illness, fever, and markedly abnormal inflammatory markers, the white blood cell count remains normal with a slight leftward shift. The most alarming finding is hypoxemia seen on the arterial blood gas. My leading diagnoses for this multisystemic febrile illness with a rash and hypoxia continue to be primarily infectious etiologies, including toxic shock syndrome with Staphylococcus species, leptospirosis, acute cytomegalovirus, and mycobacterial infections. Further diagnostic tests need to be performed but I would begin empiric antibiotics after appropriate cultures have been obtained. Rheumatologic etiologies such as systemic lupus erythematosus (SLE) and sarcoidosis seem less likely. SLE can present with a systemic illness, fever and rash, but the hepatitis, hepatomegaly and hyperemic conjunctivae are less common.
At the time of hospital admission, blood cultures were obtained before azithromycin, meropenem, and vancomycin were initiated for presumed toxic shock syndrome. Transvaginal and abdominal ultrasound studies revealed no abnormalities. She remained febrile but blood cultures returned negative. The results of the following investigations were also negative: immunoglobulin M (IgM) antibodies against Chlamydophila pneumoniae, cytomegalovirus, Epstein‐Barr virus, Legionella pneumophila, parvovirus B19, rubella virus, Coxiella burnetii, Mycoplasma pneumoniae, Chlamydophila psittaci, adenovirus, and coxsackieviruses. Antibodies against human immunodeficiency virus (HIV) 1 and 2 were negative. Tests for hepatitis B (HB surface antigen [HbsAg], HB core antibody [HbcAb] IgM) and C (HCV‐Ab) viruses were negative.
The lack of IgM antibodies for the infections listed markedly reduces their likelihood but does not exclude them. For example, given that the duration of symptoms is nearly 2 weeks at this point, it is possible that IgM has already decreased and IgG titers are now present. The lack of positive cultures does not exclude toxic shock, since in many severe cases the cultures remain negative. Thus, I remain concerned about toxic shock syndrome and would continue broad‐spectrum antibiotics.
After further investigating possible ill contacts to which the patient could have been exposed, it emerged that in the previous weeks there had been a case of measles in the kindergarten where she was working. The patient did not recall her vaccination history.
The recent exposure raises the risk of measles significantly, especially if she was not immunized as a child. Measles typically has an incubation period of 10 to 14 days, thus the prior exposure would fit the time course for the onset of this patient's symptoms. In retrospect, many of this patient's symptoms are classic for measles, including the maculopapular rash that begins on the face and extends downward, the conjunctival hyperemia, the persistent low‐grade fever, and the lack of clinical response to antibiotics.
In adults, measles can be complicated by inflammation in multiple organs resulting in myocarditis, pericarditis, hepatitis, encephalitis, and pneumonia. Thus, elevated transaminases would be consistent with the diagnosis as would a normal abdominal ultrasound. The pneumonia may be due to the measles infection itself or to coexisting viral or bacterial infections. The findings of a mild thrombocytopenia and a low normal leukocyte count can also be seen in measles infections. The diagnosis of measles is based on clinical presentation and by serologic confirmation: IgM antibodies are detectable within 1 or 2 days after the appearance of the rash, whereas the IgG titer rises significantly after 10 days.
I would continue the broad spectrum antibiotics until measles serologies could be confirmed. If the measles serologies are negative, I would continue the evaluation. If the serologies are positive, however, I would continue supportive care and review her pulmonary status to make sure she does not have a secondary bacterial infection. I strongly suspect that she has measles that is complicated by pneumonia and hepatitis.
The IgM antibody against measles virus returned positive and the patient was diagnosed with measles. By hospital day 5, her fever disappeared, her dyspnea resolved, and her rash had receded. Her oxygen saturation was 97% at the time of discharge.
Commentary
Measles is a highly contagious, acute‐onset, exanthematous disease that affects the respiratory tract and mucous membranes. Measles is clinically characterized by a prodromal stage of cough, conjunctivitis, coryza and high fever, typically lasting between 2 and 4 days.1, 2 The pathognomonic finding on the oral mucosa (Koplik spots) is usually followed by a generalized rash. The characteristic rash of measles is erythematous, nonpruritic, and maculopapular beginning at the hairline and behind the ears, and then spreads down the trunk and limbs and may include the palms and soles.1, 2 Often the patient has diarrhea, vomiting, lymphadenopathy, and splenomegaly; however, the clinical presentation can vary.1, 2 In partially immunized patients, symptoms are often atypical, whereas severe cases are characteristically seen in adults with the most frequent complication being pneumonia. About 3% of young adults with measles have a viral pneumonia that requires hospitalization.24 Adults are much more likely than children to develop hepatitis, bronchospasm and bacterial superinfection.2, 3, 5
The introduction of the measles vaccine initially led to a dramatic decrease in the incidence of measles. However, lack of adherence to vaccination campaigns among some families has been followed by small epidemics. Childhood vaccination rates against measles have recently been reported as 88% in Italy, and even higherover 90%in Tuscany. However, Italy has faced an upsurge of measles since September 2007, with almost 60% of cases occurring in the 15‐ to 44‐year‐old age group.6
Classic presentations of common diseases are easily recognized, but in those cases in which the clinical presentation of uncommon illnesseslike measles in adultsis atypical, the epidemiological data and the clinical history play key roles. In this patient, both the discussant and clinical team focused on the most alarming potential diagnosis: toxic shock syndrome related to the use of the IUD. While appropriate, there were historical clues that this patient had measles that were not specifically soughtthe immunization status and the workplace (school) exposure.
This case highlights 2 important aspects of making a difficult clinical diagnosis. First, the patient did not recall her immunization history, and the clinical team did not clarify it, and thus potential childhood illnesses such as measles and rubella did not remain on the differential diagnosis. Assuming that a patient has had the appropriate vaccinations is done at the clinician'sand the patient'speril. Second, many diseases that commonly afflict children can also occur in adult patients, albeit less frequently. Had this patient been a 5‐year‐old child with the same symptoms, the diagnosis would likely have been made with alacrity. However, maculopapular rashes that begin on the face and spread to the body are quite uncommon in adult medicine. For both discussant and the clinical team, the rash was clearly in sight but the correct diagnosis was out of mind given the rarity of this infection in adults. Fortunately, however, once it became clear that the patient was unlikely to have toxic shock syndrome, the epidemiological detail initially left behind became the sentinel clue necessary to solve the case.
Teaching Points
-
After nearly vanishing in the developed world, measles has shown sporadic signs of resurgence in recent years. The disease needs to be considered in patients presenting with a febrile illness accompanied by an exanthem that begins on the head and spreads inferiorly, especially when accompanied by cough, rhinorrhea, and conjunctival changes.
-
Measles tends to cause relatively severe illness and frequent complications in adults, the most common of which is pneumonia.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 44‐year‐old woman was admitted to an Italian hospital with fever and chills that had started approximately 1 week earlier. A few days after onset of fever, she had noticed a red, nonpruritic, confluent, maculopapular rash which began on her face and descended to her body. She also complained of red eyes, photophobia, dyspnea, and watery diarrhea. She denied nausea, vomiting, headache, or neck stiffness. She had seen her primary care physician who had concomitantly prescribed amoxicillin, levofloxacin, and betamethasone. She took the medications for several days without symptomatic improvement.
The salient features of this acute illness include the maculopapular rash, fever, and red eyes with photophobia. The differential diagnosis includes infections, rheumatologic disorders, toxin exposure, and, less likely, hematologic malignancies. In the initial assessment it is crucial to rule out any life‐threatening etiologies of fever and rash such as septicemia from Neisseria meningitidis, bacterial endocarditis, toxic shock syndrome, typhoid fever, and rickettsial diseases. A number of critical components of the history would help narrow the diagnostic considerations, including any history of recent travel, animal or occupational exposure, sexual or medication history, and risk factors for immunosuppression.
The empiric use of antibiotics is indicated when a patient presents with symptoms that suggest life‐threatening illness. For nonemergent conditions, empiric antibiotics may be appropriate when a classic pattern for a given diagnosis is present. In this patient, however, the initial presentation does not appear to be life‐threatening, nor is it easily recognizable as a specific or classic diagnosis. Thus, I would not start antibiotics, because doing so may further disguise the diagnosis by interfering with culture results, or complicate the case by causing an adverse effect such as fever or rash.
One week before the onset of fever she went to the emergency department because of pain in both lower quadrants of her abdomen. The physician removed her intrauterine device (IUD), which appeared to be partially expelled. The patient returned the next day to the emergency department because of severe metrorrhagia.
Complications of IUDs include pelvic inflammatory disease, perforated uterus, myometrial abscess, partial or complete spontaneous abortion, and ectopic pregnancy. Toxic shock syndrome, pelvic inflammatory disease, and retained products from a partial spontaneous abortion can all lead to significant systemic disease and vaginal bleeding.
Her past medical history was unremarkable except for an episode of bacterial meningitis 20 years before. She lived in Florence, Italy, where she worked as a school teacher, and had not traveled outside of Italy in the last year. She was married with 2 children, and denied high‐risk sexual behavior. She did not own any animals.
The patient's lack of travel, high‐risk sexual behavior or animal exposure does not help to alter the differential diagnosis. The prior history of bacterial meningitis raises the question of an immunodeficiency syndrome. At this point, I remain concerned about toxic shock syndrome.
The patient's temperature was 38.2C, her blood pressure was 110/60 mm Hg, respiratory rate was 28 breaths per minute and her heart rate was 108 beats per minute. She was alert and oriented but appeared moderately ill. Her conjunctivae were hyperemic without any drainage, and her oropharynx was erythematous. Lung examination revealed diminished breath sounds in the lower right lung field and crackles bilaterally. Abdominal exam demonstrated mild hepatomegaly, but not splenomegaly. Skin exam showed an erythematous, confluent, maculopapular rash involving her face, torso, back, and extremities; no cutaneous abscesses were noted. Neurological and gynecological exams were both normal, as was the rectal examination.
Her vital signs suggest a progressive illness and possible sepsis. The conjunctival hyperemia could represent several pathologic findings including uveitis with ciliary flush, conjunctival hemorrhage, or hyperemia due to systemic illness. The pulmonary findings could be attributed to pulmonary edema, pneumonia, alveolar hemorrhage, or acute respiratory distress syndrome (ARDS) as a complication of sepsis and systemic inflammation. The hepatomegaly, while non‐specific, may be due to an inflammatory reaction to a systemic illness. If so, I would expect liver tests to be elevated as this can occur in a number of parasitic (eg, toxoplasmosis) and viral (eg, chickenpox, infectious mononucleosis, cytomegalovirus) infections. The lack of concurrent splenomegaly makes lymphoma or other hematologic malignancies less likely. Given the patient's constellation of symptoms, the progressive nature of her illness and the multiple organs involved, I continue to be most concerned about immediately life‐threatening diseases. Toxic shock syndrome secondary to staphylococcal infection can present with many of these signs and symptoms including conjunctival hyperemia, diffuse maculopapular erythema, pharyngitis and sepsis leading to pulmonary edema, pleural effusions and ARDS. Another possibility is leptospirosis, which can be associated with pharyngitis, hepatomegaly, diffuse rash, low‐grade fever, and frequently has conjunctival hyperemia. Moreover, leptospirosis has a markedly variable course and pulmonary hemorrhage and ARDS can occur in severe cases. However, the lack of clear exposure to an environmental source such as contaminated water or soil or animal tissue reduces my enthusiasm for it.
Routine laboratory studies demonstrated: white‐cell count 5210/mm3 (82% neutrophils, 10% lymphocytes, 7% monocytes, and 1% eosinophils); hematocrit 36.3%; platelet count 135,000/mm3; erythrocyte sedimentation rate 49 mm/hour; fibrinogen 591 mg/dL (normal range, 200 ‐ 450 mg/dL); C‐reactive protein 53 mg/L (normal range, <9 mg/L). Serum electrolyte levels were normal. Liver tests demonstrated: aspartate aminotransferase 75 U/L; alanine aminotransferase 135 U/L; total bilirubin within normal limits; gamma glutamyltransferase 86 U/L (normal range, 10‐40 U/L). The urea nitrogen and the creatinine were both normal. The creatine phosphokinase was 381 U/L. Urinalysis was normal. An arterial‐blood gas, obtained while the patient was breathing room air, revealed an oxygen saturation of 87%; pH of 7.45; pCO2 of 38 mm Hg; pO2 of 54 mm Hg; bicarbonate concentration of 27 mmol/L.
Her electrocardiogram was normal except for sinus tachycardia. Chest film revealed a right‐sided pleural effusion without evidence of parenchymal abnormalities (Figure 1).

Despite the systemic illness, fever, and markedly abnormal inflammatory markers, the white blood cell count remains normal with a slight leftward shift. The most alarming finding is hypoxemia seen on the arterial blood gas. My leading diagnoses for this multisystemic febrile illness with a rash and hypoxia continue to be primarily infectious etiologies, including toxic shock syndrome with Staphylococcus species, leptospirosis, acute cytomegalovirus, and mycobacterial infections. Further diagnostic tests need to be performed but I would begin empiric antibiotics after appropriate cultures have been obtained. Rheumatologic etiologies such as systemic lupus erythematosus (SLE) and sarcoidosis seem less likely. SLE can present with a systemic illness, fever and rash, but the hepatitis, hepatomegaly and hyperemic conjunctivae are less common.
At the time of hospital admission, blood cultures were obtained before azithromycin, meropenem, and vancomycin were initiated for presumed toxic shock syndrome. Transvaginal and abdominal ultrasound studies revealed no abnormalities. She remained febrile but blood cultures returned negative. The results of the following investigations were also negative: immunoglobulin M (IgM) antibodies against Chlamydophila pneumoniae, cytomegalovirus, Epstein‐Barr virus, Legionella pneumophila, parvovirus B19, rubella virus, Coxiella burnetii, Mycoplasma pneumoniae, Chlamydophila psittaci, adenovirus, and coxsackieviruses. Antibodies against human immunodeficiency virus (HIV) 1 and 2 were negative. Tests for hepatitis B (HB surface antigen [HbsAg], HB core antibody [HbcAb] IgM) and C (HCV‐Ab) viruses were negative.
The lack of IgM antibodies for the infections listed markedly reduces their likelihood but does not exclude them. For example, given that the duration of symptoms is nearly 2 weeks at this point, it is possible that IgM has already decreased and IgG titers are now present. The lack of positive cultures does not exclude toxic shock, since in many severe cases the cultures remain negative. Thus, I remain concerned about toxic shock syndrome and would continue broad‐spectrum antibiotics.
After further investigating possible ill contacts to which the patient could have been exposed, it emerged that in the previous weeks there had been a case of measles in the kindergarten where she was working. The patient did not recall her vaccination history.
The recent exposure raises the risk of measles significantly, especially if she was not immunized as a child. Measles typically has an incubation period of 10 to 14 days, thus the prior exposure would fit the time course for the onset of this patient's symptoms. In retrospect, many of this patient's symptoms are classic for measles, including the maculopapular rash that begins on the face and extends downward, the conjunctival hyperemia, the persistent low‐grade fever, and the lack of clinical response to antibiotics.
In adults, measles can be complicated by inflammation in multiple organs resulting in myocarditis, pericarditis, hepatitis, encephalitis, and pneumonia. Thus, elevated transaminases would be consistent with the diagnosis as would a normal abdominal ultrasound. The pneumonia may be due to the measles infection itself or to coexisting viral or bacterial infections. The findings of a mild thrombocytopenia and a low normal leukocyte count can also be seen in measles infections. The diagnosis of measles is based on clinical presentation and by serologic confirmation: IgM antibodies are detectable within 1 or 2 days after the appearance of the rash, whereas the IgG titer rises significantly after 10 days.
I would continue the broad spectrum antibiotics until measles serologies could be confirmed. If the measles serologies are negative, I would continue the evaluation. If the serologies are positive, however, I would continue supportive care and review her pulmonary status to make sure she does not have a secondary bacterial infection. I strongly suspect that she has measles that is complicated by pneumonia and hepatitis.
The IgM antibody against measles virus returned positive and the patient was diagnosed with measles. By hospital day 5, her fever disappeared, her dyspnea resolved, and her rash had receded. Her oxygen saturation was 97% at the time of discharge.
Commentary
Measles is a highly contagious, acute‐onset, exanthematous disease that affects the respiratory tract and mucous membranes. Measles is clinically characterized by a prodromal stage of cough, conjunctivitis, coryza and high fever, typically lasting between 2 and 4 days.1, 2 The pathognomonic finding on the oral mucosa (Koplik spots) is usually followed by a generalized rash. The characteristic rash of measles is erythematous, nonpruritic, and maculopapular beginning at the hairline and behind the ears, and then spreads down the trunk and limbs and may include the palms and soles.1, 2 Often the patient has diarrhea, vomiting, lymphadenopathy, and splenomegaly; however, the clinical presentation can vary.1, 2 In partially immunized patients, symptoms are often atypical, whereas severe cases are characteristically seen in adults with the most frequent complication being pneumonia. About 3% of young adults with measles have a viral pneumonia that requires hospitalization.24 Adults are much more likely than children to develop hepatitis, bronchospasm and bacterial superinfection.2, 3, 5
The introduction of the measles vaccine initially led to a dramatic decrease in the incidence of measles. However, lack of adherence to vaccination campaigns among some families has been followed by small epidemics. Childhood vaccination rates against measles have recently been reported as 88% in Italy, and even higherover 90%in Tuscany. However, Italy has faced an upsurge of measles since September 2007, with almost 60% of cases occurring in the 15‐ to 44‐year‐old age group.6
Classic presentations of common diseases are easily recognized, but in those cases in which the clinical presentation of uncommon illnesseslike measles in adultsis atypical, the epidemiological data and the clinical history play key roles. In this patient, both the discussant and clinical team focused on the most alarming potential diagnosis: toxic shock syndrome related to the use of the IUD. While appropriate, there were historical clues that this patient had measles that were not specifically soughtthe immunization status and the workplace (school) exposure.
This case highlights 2 important aspects of making a difficult clinical diagnosis. First, the patient did not recall her immunization history, and the clinical team did not clarify it, and thus potential childhood illnesses such as measles and rubella did not remain on the differential diagnosis. Assuming that a patient has had the appropriate vaccinations is done at the clinician'sand the patient'speril. Second, many diseases that commonly afflict children can also occur in adult patients, albeit less frequently. Had this patient been a 5‐year‐old child with the same symptoms, the diagnosis would likely have been made with alacrity. However, maculopapular rashes that begin on the face and spread to the body are quite uncommon in adult medicine. For both discussant and the clinical team, the rash was clearly in sight but the correct diagnosis was out of mind given the rarity of this infection in adults. Fortunately, however, once it became clear that the patient was unlikely to have toxic shock syndrome, the epidemiological detail initially left behind became the sentinel clue necessary to solve the case.
Teaching Points
-
After nearly vanishing in the developed world, measles has shown sporadic signs of resurgence in recent years. The disease needs to be considered in patients presenting with a febrile illness accompanied by an exanthem that begins on the head and spreads inferiorly, especially when accompanied by cough, rhinorrhea, and conjunctival changes.
-
Measles tends to cause relatively severe illness and frequent complications in adults, the most common of which is pneumonia.
- .Measles Virus (Rubeola). In: Mandell GL, Bennett JE, Dolin R, eds.Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases.6th ed.Philadelphia, PA:Elsevier Churchill Livingstone;2005:2031–2038.
- ,.The clinical significance of measles: a review.J Infect Dis.2004;189(Suppl 1):S4–S16.
- ,.Measles in the United Kingdom: can we eradicate it by 2010?Br Med J.2006;333:890–895.
- ,,,,,.Familial cases of severe measles pneumonia.Intern Med.2000;39:670–674.
- ,,, et al.Measles encephalitis and acute pancreatitis in a young adult.Am J Med Sci.2004;327:299–303.
- ,,,, et al.Measles resurges in Italy: preliminary data from September 2007 to May 2008.Euro Surveill.2008;13(29):pii=18928.
- .Measles Virus (Rubeola). In: Mandell GL, Bennett JE, Dolin R, eds.Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases.6th ed.Philadelphia, PA:Elsevier Churchill Livingstone;2005:2031–2038.
- ,.The clinical significance of measles: a review.J Infect Dis.2004;189(Suppl 1):S4–S16.
- ,.Measles in the United Kingdom: can we eradicate it by 2010?Br Med J.2006;333:890–895.
- ,,,,,.Familial cases of severe measles pneumonia.Intern Med.2000;39:670–674.
- ,,, et al.Measles encephalitis and acute pancreatitis in a young adult.Am J Med Sci.2004;327:299–303.
- ,,,, et al.Measles resurges in Italy: preliminary data from September 2007 to May 2008.Euro Surveill.2008;13(29):pii=18928.
The Tip of the Iceberg
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 33‐year‐old African American man was seen in the emergency department for bilateral wrist pain and forearm swelling. He was a professional mover and had transported a piano 3 days before. Several hours after the move, he noticed wrist pain and a few small red, slightly pruritic bumps on the palmar aspect of both wrists. The following day, he developed nausea, vomiting, and watery diarrhea. His wrists and forearms became more swollen and painful.
The most distinctive aspect of the patient's symptom complex is forearm swelling. I am not certain if the primary pathology lies in the wrist or in the forearm. Examination should focus on the presence or absence of arthritis and whether the forearm swelling is simply adjacent to the wrist and rash or extends the entire length from the wrist to the elbow.
Strenuous lifting can lead to rhabdomyolysis and forearm compartment syndrome. However, transporting a piano is not an unusual task for a professional mover. More common causes of arm swelling such as fracture, cellulitis, deep vein thrombosis, and lymphatic obstruction are possible but are unexpected in this patient because of the bilateral findings. An inflammatory myopathy would not present in the distal extremities, but an infectious myositis, such as trichinosis (which can have early gastrointestinal symptoms), could.
Given the patient's age and the temporal correlation, I am inclined to pursue a unifying diagnosis between his gastrointestinal and upper extremity symptoms. The gastrointestinal symptoms could reflect vasculitis (eg, polyarteritis nodosa) or a nonspecific manifestation of systemic illness (eg, sepsis), whereas the rash could be due to infection with petechiae (eg, meningococcemia, gonococcemia, or endocarditis) or an infection with a predilection for peripheral skin lesions that progress centripetally as the illness progresses, such as Rocky Mountain spotted fever.
Although the patient had not seen spiders, he was concerned that his skin lesions might have resulted from spider bites. He had a history of atopic dermatitis but took no medications, did not smoke, and drank alcohol rarely. He lived in Denver, CO, had not traveled outside of the region, and had not visited rural areas. He was monogamous with a female partner and had no known exposures to human immunodeficiency virus. He had no family history of rheumatological disorders.
Patients and physicians frequently attribute papules, ulcers, or necrotic skin lesions to spider bites far out of proportion to their true prevalence. Bites by more innocuous arthropods such as ticks, fleas, bedbugs, or mites are much more common. The symmetry of the lesions and extensive swelling, however, make such bites unlikely.
Although the patient hails from the southwestern United States, there is no compelling evidence for endemic illness. Hantavirus infection causes a viral prodrome, but a severe pulmonary syndrome is its primary manifestation. Plague presents in bubonic, pulmonary, or septicemic forms, but other than the gastrointestinal symptoms, there is nothing to suggest such a systemic illness. The initial pulmonary infection of coccidioidomycosis is often unnoticed, and patients may present with extrapulmonary manifestations, including skin lesions and skeletal disease.
More common ailments remain on the differential. Disseminated gonococcemia must be considered in a sexually active adult with skin lesions and what may be tenosynovitis or arthritis of the distal extremities. The history of atopic dermatitis supports a diagnosis of allergic or contact dermatitis on the wrists (perhaps inoculated during the move), explaining the rash and adjacent swelling (but not the gastrointestinal symptoms).
The patient's temperature was 36.5C with a pulse of 103 beats per minute, a blood pressure of 103/67 mm Hg, and a respiratory rate of 24 breaths per minute. He appeared uncomfortable and was in moderate distress. His sclerae were injected, and his mucous membranes were dry. He had diffusely swollen fingers and firm nonpitting edema in both hands and forearms. His wrists and hands were tender and warm but had no appreciable redness. Two 1‐mm ulcerated lesions were present on his right wrist, and one 2‐mm ulcerated lesion was present on his left wrist. No lymphadenopathy was present.
The patient meets the criteria for systemic inflammatory response syndrome (SIRS), and considering his ill appearance, I am concerned that he may have an infectious process that has evolved into sepsis. The absence of cutaneous erythema rules out cellulitis, and although skin findings in necrotizing fasciitis can be modest in comparison with the underlying infection, an examination with nothing more than punctate ulcerations would be atypical. Plague and tularemia cause ulcerative skin lesions and systemic illness but usually have prominent lymphadenopathy. Cutaneous anthrax can cause intense local edema but is usually accompanied by some degree of necrosis.
I am struck by the degree of local edema. It would be a remarkable coincidence for spider bites to occur on both wrists; however, given the size of the lesions, this remains a consideration. Spider bites can cause severe local swelling and occasionally even SIRS.
The white blood cellcount was 6000/mm3 with a normal differential. The hemoglobin level was 16.6 g/dL, and the platelet count was 227,000/mm3. The serum sodium level was measured to be 135 mmol/L, the potassium level was 3.6 mmol/L, the chloride level was 94 mmol/L, and the bicarbonate level was 9 mmol/L. The urea nitrogen level was measured to be 48 mg/dL (normal, 622), and the serum creatinine level was 5.6 mg/dL (normal, 0.41.2). An arterial blood gas test on room air revealed a pH of 7.22, a partial pressure of carbon dioxide of 24 mm Hg, and a partial pressure of oxygen of 128 mm Hg. Liver enzymes were normal. The serum creatine kinase level was measured to be 194 U/L (normal, 0250). A chest radiograph revealed clear lung fields.
The anion gap is elevated and could be explained in part by renal failure, but it is quite pronounced, and I suspect that there is lactic acidosis either from SIRS and systemic hypoperfusion or from local underperfusion of the distal upper extremities (eg, compartment syndrome). Rhabdomyolysis can cause an anion gap acidosis and acute renal failure but is ruled out by a normal creatine kinase level.
Rheumatological diseases (such as polyarteritis nodosa and systemic scleroderma) can cause renal failure, gastrointestinal symptoms, and cutaneous lesions with skin ulceration but are often accompanied by hypertension. The combination of SIRS and volume depletion from nausea, vomiting, and diarrhea seems more likely, although an etiology for SIRS remains elusive. I suspect infectionmost likely of the upper extremitiesis the underlying cause in this patient despite his normal body temperature and white cell count. I would therefore start with plain films of the arms and hands. If these are unrevealing, I would proceed with an ultrasound of the arms to evaluate the soft tissues and particularly the vessels. Although imaging studies are unlikely to provide a diagnosis, they will provide guidance in choosing the next step, such as biopsy or culture.
The patient wasvolume‐resuscitated with saline and treated with clindamycin, pipercillin‐tazobactam, and vancomycin. Radiographs of the wrists and hands demonstrated edema but no subcutaneous gas and no abnormalities of the bones or joints. An ultrasound of the upper extremities was negative for superficial or deep venous thrombosis. Renal ultrasonography revealed normal‐sized kidneys and no hydronephrosis.
Short of superior vena cava thrombosis, which the ultrasound could not visualize, deep venous thrombosis can be ruled out with confidence. Superior vena cava syndrome could account for the bilateral upper extremity symptoms, but complete sparing of the face would be unusual, and the chest radiograph was normal.
Despite the bilateral nature, I doubt that this patient has an arthritis of the wrists and hands (eg, rheumatoid or psoriatic arthritis). The plain films did not show evidence of joint destruction, although that would not be expected in the first few days of most noninfectious arthropathies. Many rheumatological diseases and vasculitides have renal manifestations, but I do not find convincing evidence of such diseases yet.
Despite intravenous fluidsover the next 4 hours, the patient remained anuric. His upper extremity edema worsened, and he became increasingly tachycardic and hypotensive. Vasopressor support was required. Repeat laboratory studies demonstrated a serum creatinine level of 7.0 mg/dL, a bicarbonate level of 6 mmol/L, and a creatine kinase level of 1183 U/L. The serum lactate level was measured to be 6.5. Blood cultures obtained on admission were negative. An echocardiogram demonstrated marked impairment of left ventricular systolic dysfunction with an estimated ejection fraction of 35%. Repeat chest radiography revealed only mild pulmonary vascular congestion.
The patient has become progressively ill despite initial resuscitation. Any infection (cellulitis, fasciitis, or myositis) may progress to such a point (although to do so without fever or leukocytosis with this degree of illness is unusual) and would prompt me to ask a surgeon to explore the upper extremities for diagnostic and possibly therapeutic purposes. Among the spectrum of possible soft tissue infections, this clinical presentation is most consistent with pyomyositis (caused by Staphylococcus aureus) because of the relatively modest creatine kinase elevations that accompany it and the overall absence of cutaneous findings (save the punctuate lesions), which I would expect to be present with cellulitis or necrotizing fasciitis. A deep forearm infection and perhaps compartment syndrome leading to sepsis could explain lactic acidosis, decreased cardiac function, hypotension, and acute renal failure.
Because of the unusual characteristics noted so farparticularly the bilateral diseaseI continue to also consider systemic diseases that can cause skin lesions, cardiomyopathy, renal disease, ocular involvement (eg, keratoconjunctivitis or uveitis), and myositis. Sarcoidosis is possible, although in most of the aforementioned organs, histological disease is far more common than clinical disease, and sarcoidosis typically does not cause this degree of illness. Furthermore, over 90% of patients with sarcoidosis have pulmonary involvement. Polyarteritis nodosa could explain the multiorgan involvement and the brisk pace. If no infection is present on exploration, I would ask the surgeon to biopsy the muscle, particularly looking for granulomas or vasculitis. A progressive soft tissue infection leading to sepsis remains my leading consideration at this point.
Surgical consultation was obtained. A muscle biopsy of the left biceps and left forearm revealed group A streptococcus within the muscle and evidence of necrosis (Figure 1). Debridement of both arms and wrists was performed. The patient subsequently developed erythema of the palms and soles followed by diffuse sloughing of the skin. Streptococcal toxic shock syndrome with necrotizing myositis was diagnosed, and the antimicrobial regimen was changed to intravenous penicillin, clindamycin, and intravenous immunoglobulin G. Repeat debridement of the arms was required.
Even when a soft tissue infection is suspected, it can be challenging to preoperatively localize or characterize with precision. In retrospect, the overall severity of his illness and his previously good health status perhaps favor necrotizing myositis (and fasciitis) over pyomyositis.
I may have put undue emphasis on the absence of skin findings. Although the symmetric nature of the disease is unusual, the small skin lesions may have been portals of entry, and in retrospect, they represent the tip of the iceberg. The absence of fever and leukocytosisor hypothermia and leukopeniain a young, previously healthy patient along with the bilateral and symmetric findings made me hesitant to definitely label his illness as a deep soft tissue infection early on, but the gravity of the illness, the lack of a plausible alternative explanation, and his precipitous decline all made surgical exploration imperative.
The patient's skin sloughing progressed, and he was transferred to a regional burn unit. Three additional operations were required for debridement of both upper extremities. Despite apparent control of the initial infection, the patient continued to require significant hemodynamic and ventilator support. He subsequently developed neutropenia, thrombocytopenia, and Escherichia coli urosepsis. Necrosis of the lower extremities developed, and additional surgical debridement was recommended. After extensive discussion regarding prognosis, the family decided against further surgery and withdrew life support. The patient died shortly after extubation. The family refused an autopsy.
Commentary
Expert clinicians employ a variety of approaches to solve complex clinical problems. One of the most effective strategies is pattern recognition, in which the clinician divides the case into recognizable portions and compares these to previous cases that he or she has encountered.1, 2 If patterns from the new case appear similar or identical to those of previous cases, a diagnosis can be made quickly and without the need for unnecessary testing. However, when features of the case are unusual or atypical, pattern recognition may be disrupted. For example, although the discussant suspected a soft tissue infection, a number of features (including a normal white blood cell count, minimal skin findings, and a bilateral and symmetric distribution of swelling) did not match his illness script (a mental representation of a disease) of necrotizing fasciitis.
When pattern recognition fails, other strategies are available.3 Hypothetico‐deductive reasoning is a data‐to‐diagnosis method whereby the clinician uses the presenting information to construct a list of diagnostic possibilities.4 Additional testing and gathering of information are then used to continuously revise the diagnostic possibilities until confirmatory information is obtained and the diagnosis is established. After a pattern failed to materialize, the discussant employed this analytical strategy by noting the unusual characteristics of the case and incorporating laboratory and physiological data to revise his differential diagnosis. As a result, he requested the appropriate diagnostic test: surgical exploration of the forearms.
Necrotizing soft tissue infections are characterized by fulminant tissue destruction, rapid spread along tissue planes, and local vascular thrombosis. Mixed aerobic and anaerobic infections typically occur after penetrating skin injury or following surgery in patients with diabetes mellitus or vascular disease. In contrast, monomicrobial infections with S. aureus or group A streptococcus generally occur in healthy individuals. The prevalence of necrotizing group A streptococcal infections has increased dramatically in the last 15 to 20 years.5 Over one‐third of these cases are complicated by toxic shock syndrome.57 Mortality rates for necrotizing fasciitis with toxic shock exceed 30%, and early surgical consultation is directly associated with a reduction in morbidity and mortality.5, 8
Toxic shock is an inflammatory response syndrome caused by release of exotoxins from group A streptococcus and S. aureus.9, 10 In streptococcal toxic shock, Streptococcus pyogenes exotoxin A and Streptococcus pyogenes exotoxin B are the major toxins produced.10 These toxins activate the systemic production of inflammatory cytokines such as interleukin‐1, gamma‐interferon, and tumor necrosis factor, resulting in capillary leak, systemic hypotension, tissue hypoperfusion, and organ failure. The most common initial symptom is diffuse or localized pain that is severe and abrupt in onset and often precedes or is out of proportion to other physical findings of soft tissue infection.8 Up to 20% of individuals may also develop a viral‐like syndrome with myalgias, fever, nausea, vomiting, and diarrhea.11 Erythroderma of the skin and mucous membranes can be another early finding. The rash is diffuse, erythematous, and macular, resembling a sunburn. It involves the palms and soles but can be subtle and fleeting. Erythroderma may be particularly difficult to detect in dark‐skinned individuals.12 It is also important to consider that the absence of fever, erythroderma, or leukocytosis does not necessarily rule out the possibility of serious infection in necrotizing fasciitis patients, as the development of these signs and symptoms may occur later in the disease process.
The most common portals of entry for group A streptococcus are the skin, vagina, and pharynx. Predisposing factors include varicella infection, penetrating injuries, minor cuts, burns, splinters, and surgery. Interestingly, a portal of entry cannot be identified in 45% of cases.8 These patients in particular are at risk for developing severe necrotizing myositis or fasciitis at the site of a minor injury such as a strained muscle. Hematogenous translocation from the pharynx to the site of injury is the probable mechanism13 and would provide one scenario by which our piano mover developed bilateral and symmetric disease. An alternative explanation would be direct extension of bacteria from small breaks in the skin to adjacent areas of muscle strain. Regardless of the portal of entry, as the small skin lesions demonstrate in this patient, the smallest physical finding can represent the tip of the iceberg.
- ,,,,,.Fast and frugal models of clinical judgment in novice and expert physicians.Med Decis Making.2003;23(4):293–300.
- .What is it to be an expert? In: Chi M, Farr MJ, Glaser R, eds.The Nature of Expertise.Hillsdale, NJ:Lawrence Erlbaum;1988.
- .Clinical decision‐making: understanding how clinicians make a diagnosis. In: Saint S, Drazen JM, Solomon CG, eds.Clinical Problem‐Solving.New York, NY:McGraw‐Hill;2006.
- ,,.Medical Problem Solving: An Analysis of Clinical Reasoning.Cambridge, MA:Harvard University Press;1978.
- ,,,,.Population‐based surveillance for group A streptococcal necrotizing fasciitis: clinical features, prognostic indicators, and microbiologic analysis of seventy‐seven cases. Ontario Group A Streptococcal Study.Am J Med.1997;103(1):18–24.
- ,,, et al.Invasive group A streptococcal infections in Sweden in 1994 and 1995: epidemiology and clinical spectrum.Scand J Infect Dis.2000;32(6):609–614.
- ,,,.Reemergence of emm1 and a changed superantigen profile for group A streptococci causing invasive infections: results from a nationwide study.J Clin Microbiol.2005;43(4):1789–1796.
- ,,, et al.Severe group A streptococcal infections associated with a toxic shock‐like syndrome and scarlet fever toxin A.N Engl J Med.1989;321(1):1–7.
- ,,,.Rapidly progressive necrotizing fasciitis caused by Staphylococcus aureus.J Microbiol Immunol Infect.2005;38(5):361–364.
- ,.Streptococcal infections of skin and soft tissues.N Engl J Med.1996;334(4):240–245.
- ,.Streptococcal toxic shock syndrome after breast reconstruction.Ann Plast Surg.2005;54(5):553–556.
- ,.The influence of pigmentation and illumination on the perception of erythema.Photodermatol Photoimmunol Photomed.1992;9(2):45–47.
- .Streptococcal toxic‐shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment.Emerg Infect Dis.1995;1(3):69–78.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 33‐year‐old African American man was seen in the emergency department for bilateral wrist pain and forearm swelling. He was a professional mover and had transported a piano 3 days before. Several hours after the move, he noticed wrist pain and a few small red, slightly pruritic bumps on the palmar aspect of both wrists. The following day, he developed nausea, vomiting, and watery diarrhea. His wrists and forearms became more swollen and painful.
The most distinctive aspect of the patient's symptom complex is forearm swelling. I am not certain if the primary pathology lies in the wrist or in the forearm. Examination should focus on the presence or absence of arthritis and whether the forearm swelling is simply adjacent to the wrist and rash or extends the entire length from the wrist to the elbow.
Strenuous lifting can lead to rhabdomyolysis and forearm compartment syndrome. However, transporting a piano is not an unusual task for a professional mover. More common causes of arm swelling such as fracture, cellulitis, deep vein thrombosis, and lymphatic obstruction are possible but are unexpected in this patient because of the bilateral findings. An inflammatory myopathy would not present in the distal extremities, but an infectious myositis, such as trichinosis (which can have early gastrointestinal symptoms), could.
Given the patient's age and the temporal correlation, I am inclined to pursue a unifying diagnosis between his gastrointestinal and upper extremity symptoms. The gastrointestinal symptoms could reflect vasculitis (eg, polyarteritis nodosa) or a nonspecific manifestation of systemic illness (eg, sepsis), whereas the rash could be due to infection with petechiae (eg, meningococcemia, gonococcemia, or endocarditis) or an infection with a predilection for peripheral skin lesions that progress centripetally as the illness progresses, such as Rocky Mountain spotted fever.
Although the patient had not seen spiders, he was concerned that his skin lesions might have resulted from spider bites. He had a history of atopic dermatitis but took no medications, did not smoke, and drank alcohol rarely. He lived in Denver, CO, had not traveled outside of the region, and had not visited rural areas. He was monogamous with a female partner and had no known exposures to human immunodeficiency virus. He had no family history of rheumatological disorders.
Patients and physicians frequently attribute papules, ulcers, or necrotic skin lesions to spider bites far out of proportion to their true prevalence. Bites by more innocuous arthropods such as ticks, fleas, bedbugs, or mites are much more common. The symmetry of the lesions and extensive swelling, however, make such bites unlikely.
Although the patient hails from the southwestern United States, there is no compelling evidence for endemic illness. Hantavirus infection causes a viral prodrome, but a severe pulmonary syndrome is its primary manifestation. Plague presents in bubonic, pulmonary, or septicemic forms, but other than the gastrointestinal symptoms, there is nothing to suggest such a systemic illness. The initial pulmonary infection of coccidioidomycosis is often unnoticed, and patients may present with extrapulmonary manifestations, including skin lesions and skeletal disease.
More common ailments remain on the differential. Disseminated gonococcemia must be considered in a sexually active adult with skin lesions and what may be tenosynovitis or arthritis of the distal extremities. The history of atopic dermatitis supports a diagnosis of allergic or contact dermatitis on the wrists (perhaps inoculated during the move), explaining the rash and adjacent swelling (but not the gastrointestinal symptoms).
The patient's temperature was 36.5C with a pulse of 103 beats per minute, a blood pressure of 103/67 mm Hg, and a respiratory rate of 24 breaths per minute. He appeared uncomfortable and was in moderate distress. His sclerae were injected, and his mucous membranes were dry. He had diffusely swollen fingers and firm nonpitting edema in both hands and forearms. His wrists and hands were tender and warm but had no appreciable redness. Two 1‐mm ulcerated lesions were present on his right wrist, and one 2‐mm ulcerated lesion was present on his left wrist. No lymphadenopathy was present.
The patient meets the criteria for systemic inflammatory response syndrome (SIRS), and considering his ill appearance, I am concerned that he may have an infectious process that has evolved into sepsis. The absence of cutaneous erythema rules out cellulitis, and although skin findings in necrotizing fasciitis can be modest in comparison with the underlying infection, an examination with nothing more than punctate ulcerations would be atypical. Plague and tularemia cause ulcerative skin lesions and systemic illness but usually have prominent lymphadenopathy. Cutaneous anthrax can cause intense local edema but is usually accompanied by some degree of necrosis.
I am struck by the degree of local edema. It would be a remarkable coincidence for spider bites to occur on both wrists; however, given the size of the lesions, this remains a consideration. Spider bites can cause severe local swelling and occasionally even SIRS.
The white blood cellcount was 6000/mm3 with a normal differential. The hemoglobin level was 16.6 g/dL, and the platelet count was 227,000/mm3. The serum sodium level was measured to be 135 mmol/L, the potassium level was 3.6 mmol/L, the chloride level was 94 mmol/L, and the bicarbonate level was 9 mmol/L. The urea nitrogen level was measured to be 48 mg/dL (normal, 622), and the serum creatinine level was 5.6 mg/dL (normal, 0.41.2). An arterial blood gas test on room air revealed a pH of 7.22, a partial pressure of carbon dioxide of 24 mm Hg, and a partial pressure of oxygen of 128 mm Hg. Liver enzymes were normal. The serum creatine kinase level was measured to be 194 U/L (normal, 0250). A chest radiograph revealed clear lung fields.
The anion gap is elevated and could be explained in part by renal failure, but it is quite pronounced, and I suspect that there is lactic acidosis either from SIRS and systemic hypoperfusion or from local underperfusion of the distal upper extremities (eg, compartment syndrome). Rhabdomyolysis can cause an anion gap acidosis and acute renal failure but is ruled out by a normal creatine kinase level.
Rheumatological diseases (such as polyarteritis nodosa and systemic scleroderma) can cause renal failure, gastrointestinal symptoms, and cutaneous lesions with skin ulceration but are often accompanied by hypertension. The combination of SIRS and volume depletion from nausea, vomiting, and diarrhea seems more likely, although an etiology for SIRS remains elusive. I suspect infectionmost likely of the upper extremitiesis the underlying cause in this patient despite his normal body temperature and white cell count. I would therefore start with plain films of the arms and hands. If these are unrevealing, I would proceed with an ultrasound of the arms to evaluate the soft tissues and particularly the vessels. Although imaging studies are unlikely to provide a diagnosis, they will provide guidance in choosing the next step, such as biopsy or culture.
The patient wasvolume‐resuscitated with saline and treated with clindamycin, pipercillin‐tazobactam, and vancomycin. Radiographs of the wrists and hands demonstrated edema but no subcutaneous gas and no abnormalities of the bones or joints. An ultrasound of the upper extremities was negative for superficial or deep venous thrombosis. Renal ultrasonography revealed normal‐sized kidneys and no hydronephrosis.
Short of superior vena cava thrombosis, which the ultrasound could not visualize, deep venous thrombosis can be ruled out with confidence. Superior vena cava syndrome could account for the bilateral upper extremity symptoms, but complete sparing of the face would be unusual, and the chest radiograph was normal.
Despite the bilateral nature, I doubt that this patient has an arthritis of the wrists and hands (eg, rheumatoid or psoriatic arthritis). The plain films did not show evidence of joint destruction, although that would not be expected in the first few days of most noninfectious arthropathies. Many rheumatological diseases and vasculitides have renal manifestations, but I do not find convincing evidence of such diseases yet.
Despite intravenous fluidsover the next 4 hours, the patient remained anuric. His upper extremity edema worsened, and he became increasingly tachycardic and hypotensive. Vasopressor support was required. Repeat laboratory studies demonstrated a serum creatinine level of 7.0 mg/dL, a bicarbonate level of 6 mmol/L, and a creatine kinase level of 1183 U/L. The serum lactate level was measured to be 6.5. Blood cultures obtained on admission were negative. An echocardiogram demonstrated marked impairment of left ventricular systolic dysfunction with an estimated ejection fraction of 35%. Repeat chest radiography revealed only mild pulmonary vascular congestion.
The patient has become progressively ill despite initial resuscitation. Any infection (cellulitis, fasciitis, or myositis) may progress to such a point (although to do so without fever or leukocytosis with this degree of illness is unusual) and would prompt me to ask a surgeon to explore the upper extremities for diagnostic and possibly therapeutic purposes. Among the spectrum of possible soft tissue infections, this clinical presentation is most consistent with pyomyositis (caused by Staphylococcus aureus) because of the relatively modest creatine kinase elevations that accompany it and the overall absence of cutaneous findings (save the punctuate lesions), which I would expect to be present with cellulitis or necrotizing fasciitis. A deep forearm infection and perhaps compartment syndrome leading to sepsis could explain lactic acidosis, decreased cardiac function, hypotension, and acute renal failure.
Because of the unusual characteristics noted so farparticularly the bilateral diseaseI continue to also consider systemic diseases that can cause skin lesions, cardiomyopathy, renal disease, ocular involvement (eg, keratoconjunctivitis or uveitis), and myositis. Sarcoidosis is possible, although in most of the aforementioned organs, histological disease is far more common than clinical disease, and sarcoidosis typically does not cause this degree of illness. Furthermore, over 90% of patients with sarcoidosis have pulmonary involvement. Polyarteritis nodosa could explain the multiorgan involvement and the brisk pace. If no infection is present on exploration, I would ask the surgeon to biopsy the muscle, particularly looking for granulomas or vasculitis. A progressive soft tissue infection leading to sepsis remains my leading consideration at this point.
Surgical consultation was obtained. A muscle biopsy of the left biceps and left forearm revealed group A streptococcus within the muscle and evidence of necrosis (Figure 1). Debridement of both arms and wrists was performed. The patient subsequently developed erythema of the palms and soles followed by diffuse sloughing of the skin. Streptococcal toxic shock syndrome with necrotizing myositis was diagnosed, and the antimicrobial regimen was changed to intravenous penicillin, clindamycin, and intravenous immunoglobulin G. Repeat debridement of the arms was required.

Even when a soft tissue infection is suspected, it can be challenging to preoperatively localize or characterize with precision. In retrospect, the overall severity of his illness and his previously good health status perhaps favor necrotizing myositis (and fasciitis) over pyomyositis.
I may have put undue emphasis on the absence of skin findings. Although the symmetric nature of the disease is unusual, the small skin lesions may have been portals of entry, and in retrospect, they represent the tip of the iceberg. The absence of fever and leukocytosisor hypothermia and leukopeniain a young, previously healthy patient along with the bilateral and symmetric findings made me hesitant to definitely label his illness as a deep soft tissue infection early on, but the gravity of the illness, the lack of a plausible alternative explanation, and his precipitous decline all made surgical exploration imperative.
The patient's skin sloughing progressed, and he was transferred to a regional burn unit. Three additional operations were required for debridement of both upper extremities. Despite apparent control of the initial infection, the patient continued to require significant hemodynamic and ventilator support. He subsequently developed neutropenia, thrombocytopenia, and Escherichia coli urosepsis. Necrosis of the lower extremities developed, and additional surgical debridement was recommended. After extensive discussion regarding prognosis, the family decided against further surgery and withdrew life support. The patient died shortly after extubation. The family refused an autopsy.
Commentary
Expert clinicians employ a variety of approaches to solve complex clinical problems. One of the most effective strategies is pattern recognition, in which the clinician divides the case into recognizable portions and compares these to previous cases that he or she has encountered.1, 2 If patterns from the new case appear similar or identical to those of previous cases, a diagnosis can be made quickly and without the need for unnecessary testing. However, when features of the case are unusual or atypical, pattern recognition may be disrupted. For example, although the discussant suspected a soft tissue infection, a number of features (including a normal white blood cell count, minimal skin findings, and a bilateral and symmetric distribution of swelling) did not match his illness script (a mental representation of a disease) of necrotizing fasciitis.
When pattern recognition fails, other strategies are available.3 Hypothetico‐deductive reasoning is a data‐to‐diagnosis method whereby the clinician uses the presenting information to construct a list of diagnostic possibilities.4 Additional testing and gathering of information are then used to continuously revise the diagnostic possibilities until confirmatory information is obtained and the diagnosis is established. After a pattern failed to materialize, the discussant employed this analytical strategy by noting the unusual characteristics of the case and incorporating laboratory and physiological data to revise his differential diagnosis. As a result, he requested the appropriate diagnostic test: surgical exploration of the forearms.
Necrotizing soft tissue infections are characterized by fulminant tissue destruction, rapid spread along tissue planes, and local vascular thrombosis. Mixed aerobic and anaerobic infections typically occur after penetrating skin injury or following surgery in patients with diabetes mellitus or vascular disease. In contrast, monomicrobial infections with S. aureus or group A streptococcus generally occur in healthy individuals. The prevalence of necrotizing group A streptococcal infections has increased dramatically in the last 15 to 20 years.5 Over one‐third of these cases are complicated by toxic shock syndrome.57 Mortality rates for necrotizing fasciitis with toxic shock exceed 30%, and early surgical consultation is directly associated with a reduction in morbidity and mortality.5, 8
Toxic shock is an inflammatory response syndrome caused by release of exotoxins from group A streptococcus and S. aureus.9, 10 In streptococcal toxic shock, Streptococcus pyogenes exotoxin A and Streptococcus pyogenes exotoxin B are the major toxins produced.10 These toxins activate the systemic production of inflammatory cytokines such as interleukin‐1, gamma‐interferon, and tumor necrosis factor, resulting in capillary leak, systemic hypotension, tissue hypoperfusion, and organ failure. The most common initial symptom is diffuse or localized pain that is severe and abrupt in onset and often precedes or is out of proportion to other physical findings of soft tissue infection.8 Up to 20% of individuals may also develop a viral‐like syndrome with myalgias, fever, nausea, vomiting, and diarrhea.11 Erythroderma of the skin and mucous membranes can be another early finding. The rash is diffuse, erythematous, and macular, resembling a sunburn. It involves the palms and soles but can be subtle and fleeting. Erythroderma may be particularly difficult to detect in dark‐skinned individuals.12 It is also important to consider that the absence of fever, erythroderma, or leukocytosis does not necessarily rule out the possibility of serious infection in necrotizing fasciitis patients, as the development of these signs and symptoms may occur later in the disease process.
The most common portals of entry for group A streptococcus are the skin, vagina, and pharynx. Predisposing factors include varicella infection, penetrating injuries, minor cuts, burns, splinters, and surgery. Interestingly, a portal of entry cannot be identified in 45% of cases.8 These patients in particular are at risk for developing severe necrotizing myositis or fasciitis at the site of a minor injury such as a strained muscle. Hematogenous translocation from the pharynx to the site of injury is the probable mechanism13 and would provide one scenario by which our piano mover developed bilateral and symmetric disease. An alternative explanation would be direct extension of bacteria from small breaks in the skin to adjacent areas of muscle strain. Regardless of the portal of entry, as the small skin lesions demonstrate in this patient, the smallest physical finding can represent the tip of the iceberg.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 33‐year‐old African American man was seen in the emergency department for bilateral wrist pain and forearm swelling. He was a professional mover and had transported a piano 3 days before. Several hours after the move, he noticed wrist pain and a few small red, slightly pruritic bumps on the palmar aspect of both wrists. The following day, he developed nausea, vomiting, and watery diarrhea. His wrists and forearms became more swollen and painful.
The most distinctive aspect of the patient's symptom complex is forearm swelling. I am not certain if the primary pathology lies in the wrist or in the forearm. Examination should focus on the presence or absence of arthritis and whether the forearm swelling is simply adjacent to the wrist and rash or extends the entire length from the wrist to the elbow.
Strenuous lifting can lead to rhabdomyolysis and forearm compartment syndrome. However, transporting a piano is not an unusual task for a professional mover. More common causes of arm swelling such as fracture, cellulitis, deep vein thrombosis, and lymphatic obstruction are possible but are unexpected in this patient because of the bilateral findings. An inflammatory myopathy would not present in the distal extremities, but an infectious myositis, such as trichinosis (which can have early gastrointestinal symptoms), could.
Given the patient's age and the temporal correlation, I am inclined to pursue a unifying diagnosis between his gastrointestinal and upper extremity symptoms. The gastrointestinal symptoms could reflect vasculitis (eg, polyarteritis nodosa) or a nonspecific manifestation of systemic illness (eg, sepsis), whereas the rash could be due to infection with petechiae (eg, meningococcemia, gonococcemia, or endocarditis) or an infection with a predilection for peripheral skin lesions that progress centripetally as the illness progresses, such as Rocky Mountain spotted fever.
Although the patient had not seen spiders, he was concerned that his skin lesions might have resulted from spider bites. He had a history of atopic dermatitis but took no medications, did not smoke, and drank alcohol rarely. He lived in Denver, CO, had not traveled outside of the region, and had not visited rural areas. He was monogamous with a female partner and had no known exposures to human immunodeficiency virus. He had no family history of rheumatological disorders.
Patients and physicians frequently attribute papules, ulcers, or necrotic skin lesions to spider bites far out of proportion to their true prevalence. Bites by more innocuous arthropods such as ticks, fleas, bedbugs, or mites are much more common. The symmetry of the lesions and extensive swelling, however, make such bites unlikely.
Although the patient hails from the southwestern United States, there is no compelling evidence for endemic illness. Hantavirus infection causes a viral prodrome, but a severe pulmonary syndrome is its primary manifestation. Plague presents in bubonic, pulmonary, or septicemic forms, but other than the gastrointestinal symptoms, there is nothing to suggest such a systemic illness. The initial pulmonary infection of coccidioidomycosis is often unnoticed, and patients may present with extrapulmonary manifestations, including skin lesions and skeletal disease.
More common ailments remain on the differential. Disseminated gonococcemia must be considered in a sexually active adult with skin lesions and what may be tenosynovitis or arthritis of the distal extremities. The history of atopic dermatitis supports a diagnosis of allergic or contact dermatitis on the wrists (perhaps inoculated during the move), explaining the rash and adjacent swelling (but not the gastrointestinal symptoms).
The patient's temperature was 36.5C with a pulse of 103 beats per minute, a blood pressure of 103/67 mm Hg, and a respiratory rate of 24 breaths per minute. He appeared uncomfortable and was in moderate distress. His sclerae were injected, and his mucous membranes were dry. He had diffusely swollen fingers and firm nonpitting edema in both hands and forearms. His wrists and hands were tender and warm but had no appreciable redness. Two 1‐mm ulcerated lesions were present on his right wrist, and one 2‐mm ulcerated lesion was present on his left wrist. No lymphadenopathy was present.
The patient meets the criteria for systemic inflammatory response syndrome (SIRS), and considering his ill appearance, I am concerned that he may have an infectious process that has evolved into sepsis. The absence of cutaneous erythema rules out cellulitis, and although skin findings in necrotizing fasciitis can be modest in comparison with the underlying infection, an examination with nothing more than punctate ulcerations would be atypical. Plague and tularemia cause ulcerative skin lesions and systemic illness but usually have prominent lymphadenopathy. Cutaneous anthrax can cause intense local edema but is usually accompanied by some degree of necrosis.
I am struck by the degree of local edema. It would be a remarkable coincidence for spider bites to occur on both wrists; however, given the size of the lesions, this remains a consideration. Spider bites can cause severe local swelling and occasionally even SIRS.
The white blood cellcount was 6000/mm3 with a normal differential. The hemoglobin level was 16.6 g/dL, and the platelet count was 227,000/mm3. The serum sodium level was measured to be 135 mmol/L, the potassium level was 3.6 mmol/L, the chloride level was 94 mmol/L, and the bicarbonate level was 9 mmol/L. The urea nitrogen level was measured to be 48 mg/dL (normal, 622), and the serum creatinine level was 5.6 mg/dL (normal, 0.41.2). An arterial blood gas test on room air revealed a pH of 7.22, a partial pressure of carbon dioxide of 24 mm Hg, and a partial pressure of oxygen of 128 mm Hg. Liver enzymes were normal. The serum creatine kinase level was measured to be 194 U/L (normal, 0250). A chest radiograph revealed clear lung fields.
The anion gap is elevated and could be explained in part by renal failure, but it is quite pronounced, and I suspect that there is lactic acidosis either from SIRS and systemic hypoperfusion or from local underperfusion of the distal upper extremities (eg, compartment syndrome). Rhabdomyolysis can cause an anion gap acidosis and acute renal failure but is ruled out by a normal creatine kinase level.
Rheumatological diseases (such as polyarteritis nodosa and systemic scleroderma) can cause renal failure, gastrointestinal symptoms, and cutaneous lesions with skin ulceration but are often accompanied by hypertension. The combination of SIRS and volume depletion from nausea, vomiting, and diarrhea seems more likely, although an etiology for SIRS remains elusive. I suspect infectionmost likely of the upper extremitiesis the underlying cause in this patient despite his normal body temperature and white cell count. I would therefore start with plain films of the arms and hands. If these are unrevealing, I would proceed with an ultrasound of the arms to evaluate the soft tissues and particularly the vessels. Although imaging studies are unlikely to provide a diagnosis, they will provide guidance in choosing the next step, such as biopsy or culture.
The patient wasvolume‐resuscitated with saline and treated with clindamycin, pipercillin‐tazobactam, and vancomycin. Radiographs of the wrists and hands demonstrated edema but no subcutaneous gas and no abnormalities of the bones or joints. An ultrasound of the upper extremities was negative for superficial or deep venous thrombosis. Renal ultrasonography revealed normal‐sized kidneys and no hydronephrosis.
Short of superior vena cava thrombosis, which the ultrasound could not visualize, deep venous thrombosis can be ruled out with confidence. Superior vena cava syndrome could account for the bilateral upper extremity symptoms, but complete sparing of the face would be unusual, and the chest radiograph was normal.
Despite the bilateral nature, I doubt that this patient has an arthritis of the wrists and hands (eg, rheumatoid or psoriatic arthritis). The plain films did not show evidence of joint destruction, although that would not be expected in the first few days of most noninfectious arthropathies. Many rheumatological diseases and vasculitides have renal manifestations, but I do not find convincing evidence of such diseases yet.
Despite intravenous fluidsover the next 4 hours, the patient remained anuric. His upper extremity edema worsened, and he became increasingly tachycardic and hypotensive. Vasopressor support was required. Repeat laboratory studies demonstrated a serum creatinine level of 7.0 mg/dL, a bicarbonate level of 6 mmol/L, and a creatine kinase level of 1183 U/L. The serum lactate level was measured to be 6.5. Blood cultures obtained on admission were negative. An echocardiogram demonstrated marked impairment of left ventricular systolic dysfunction with an estimated ejection fraction of 35%. Repeat chest radiography revealed only mild pulmonary vascular congestion.
The patient has become progressively ill despite initial resuscitation. Any infection (cellulitis, fasciitis, or myositis) may progress to such a point (although to do so without fever or leukocytosis with this degree of illness is unusual) and would prompt me to ask a surgeon to explore the upper extremities for diagnostic and possibly therapeutic purposes. Among the spectrum of possible soft tissue infections, this clinical presentation is most consistent with pyomyositis (caused by Staphylococcus aureus) because of the relatively modest creatine kinase elevations that accompany it and the overall absence of cutaneous findings (save the punctuate lesions), which I would expect to be present with cellulitis or necrotizing fasciitis. A deep forearm infection and perhaps compartment syndrome leading to sepsis could explain lactic acidosis, decreased cardiac function, hypotension, and acute renal failure.
Because of the unusual characteristics noted so farparticularly the bilateral diseaseI continue to also consider systemic diseases that can cause skin lesions, cardiomyopathy, renal disease, ocular involvement (eg, keratoconjunctivitis or uveitis), and myositis. Sarcoidosis is possible, although in most of the aforementioned organs, histological disease is far more common than clinical disease, and sarcoidosis typically does not cause this degree of illness. Furthermore, over 90% of patients with sarcoidosis have pulmonary involvement. Polyarteritis nodosa could explain the multiorgan involvement and the brisk pace. If no infection is present on exploration, I would ask the surgeon to biopsy the muscle, particularly looking for granulomas or vasculitis. A progressive soft tissue infection leading to sepsis remains my leading consideration at this point.
Surgical consultation was obtained. A muscle biopsy of the left biceps and left forearm revealed group A streptococcus within the muscle and evidence of necrosis (Figure 1). Debridement of both arms and wrists was performed. The patient subsequently developed erythema of the palms and soles followed by diffuse sloughing of the skin. Streptococcal toxic shock syndrome with necrotizing myositis was diagnosed, and the antimicrobial regimen was changed to intravenous penicillin, clindamycin, and intravenous immunoglobulin G. Repeat debridement of the arms was required.

Even when a soft tissue infection is suspected, it can be challenging to preoperatively localize or characterize with precision. In retrospect, the overall severity of his illness and his previously good health status perhaps favor necrotizing myositis (and fasciitis) over pyomyositis.
I may have put undue emphasis on the absence of skin findings. Although the symmetric nature of the disease is unusual, the small skin lesions may have been portals of entry, and in retrospect, they represent the tip of the iceberg. The absence of fever and leukocytosisor hypothermia and leukopeniain a young, previously healthy patient along with the bilateral and symmetric findings made me hesitant to definitely label his illness as a deep soft tissue infection early on, but the gravity of the illness, the lack of a plausible alternative explanation, and his precipitous decline all made surgical exploration imperative.
The patient's skin sloughing progressed, and he was transferred to a regional burn unit. Three additional operations were required for debridement of both upper extremities. Despite apparent control of the initial infection, the patient continued to require significant hemodynamic and ventilator support. He subsequently developed neutropenia, thrombocytopenia, and Escherichia coli urosepsis. Necrosis of the lower extremities developed, and additional surgical debridement was recommended. After extensive discussion regarding prognosis, the family decided against further surgery and withdrew life support. The patient died shortly after extubation. The family refused an autopsy.
Commentary
Expert clinicians employ a variety of approaches to solve complex clinical problems. One of the most effective strategies is pattern recognition, in which the clinician divides the case into recognizable portions and compares these to previous cases that he or she has encountered.1, 2 If patterns from the new case appear similar or identical to those of previous cases, a diagnosis can be made quickly and without the need for unnecessary testing. However, when features of the case are unusual or atypical, pattern recognition may be disrupted. For example, although the discussant suspected a soft tissue infection, a number of features (including a normal white blood cell count, minimal skin findings, and a bilateral and symmetric distribution of swelling) did not match his illness script (a mental representation of a disease) of necrotizing fasciitis.
When pattern recognition fails, other strategies are available.3 Hypothetico‐deductive reasoning is a data‐to‐diagnosis method whereby the clinician uses the presenting information to construct a list of diagnostic possibilities.4 Additional testing and gathering of information are then used to continuously revise the diagnostic possibilities until confirmatory information is obtained and the diagnosis is established. After a pattern failed to materialize, the discussant employed this analytical strategy by noting the unusual characteristics of the case and incorporating laboratory and physiological data to revise his differential diagnosis. As a result, he requested the appropriate diagnostic test: surgical exploration of the forearms.
Necrotizing soft tissue infections are characterized by fulminant tissue destruction, rapid spread along tissue planes, and local vascular thrombosis. Mixed aerobic and anaerobic infections typically occur after penetrating skin injury or following surgery in patients with diabetes mellitus or vascular disease. In contrast, monomicrobial infections with S. aureus or group A streptococcus generally occur in healthy individuals. The prevalence of necrotizing group A streptococcal infections has increased dramatically in the last 15 to 20 years.5 Over one‐third of these cases are complicated by toxic shock syndrome.57 Mortality rates for necrotizing fasciitis with toxic shock exceed 30%, and early surgical consultation is directly associated with a reduction in morbidity and mortality.5, 8
Toxic shock is an inflammatory response syndrome caused by release of exotoxins from group A streptococcus and S. aureus.9, 10 In streptococcal toxic shock, Streptococcus pyogenes exotoxin A and Streptococcus pyogenes exotoxin B are the major toxins produced.10 These toxins activate the systemic production of inflammatory cytokines such as interleukin‐1, gamma‐interferon, and tumor necrosis factor, resulting in capillary leak, systemic hypotension, tissue hypoperfusion, and organ failure. The most common initial symptom is diffuse or localized pain that is severe and abrupt in onset and often precedes or is out of proportion to other physical findings of soft tissue infection.8 Up to 20% of individuals may also develop a viral‐like syndrome with myalgias, fever, nausea, vomiting, and diarrhea.11 Erythroderma of the skin and mucous membranes can be another early finding. The rash is diffuse, erythematous, and macular, resembling a sunburn. It involves the palms and soles but can be subtle and fleeting. Erythroderma may be particularly difficult to detect in dark‐skinned individuals.12 It is also important to consider that the absence of fever, erythroderma, or leukocytosis does not necessarily rule out the possibility of serious infection in necrotizing fasciitis patients, as the development of these signs and symptoms may occur later in the disease process.
The most common portals of entry for group A streptococcus are the skin, vagina, and pharynx. Predisposing factors include varicella infection, penetrating injuries, minor cuts, burns, splinters, and surgery. Interestingly, a portal of entry cannot be identified in 45% of cases.8 These patients in particular are at risk for developing severe necrotizing myositis or fasciitis at the site of a minor injury such as a strained muscle. Hematogenous translocation from the pharynx to the site of injury is the probable mechanism13 and would provide one scenario by which our piano mover developed bilateral and symmetric disease. An alternative explanation would be direct extension of bacteria from small breaks in the skin to adjacent areas of muscle strain. Regardless of the portal of entry, as the small skin lesions demonstrate in this patient, the smallest physical finding can represent the tip of the iceberg.
- ,,,,,.Fast and frugal models of clinical judgment in novice and expert physicians.Med Decis Making.2003;23(4):293–300.
- .What is it to be an expert? In: Chi M, Farr MJ, Glaser R, eds.The Nature of Expertise.Hillsdale, NJ:Lawrence Erlbaum;1988.
- .Clinical decision‐making: understanding how clinicians make a diagnosis. In: Saint S, Drazen JM, Solomon CG, eds.Clinical Problem‐Solving.New York, NY:McGraw‐Hill;2006.
- ,,.Medical Problem Solving: An Analysis of Clinical Reasoning.Cambridge, MA:Harvard University Press;1978.
- ,,,,.Population‐based surveillance for group A streptococcal necrotizing fasciitis: clinical features, prognostic indicators, and microbiologic analysis of seventy‐seven cases. Ontario Group A Streptococcal Study.Am J Med.1997;103(1):18–24.
- ,,, et al.Invasive group A streptococcal infections in Sweden in 1994 and 1995: epidemiology and clinical spectrum.Scand J Infect Dis.2000;32(6):609–614.
- ,,,.Reemergence of emm1 and a changed superantigen profile for group A streptococci causing invasive infections: results from a nationwide study.J Clin Microbiol.2005;43(4):1789–1796.
- ,,, et al.Severe group A streptococcal infections associated with a toxic shock‐like syndrome and scarlet fever toxin A.N Engl J Med.1989;321(1):1–7.
- ,,,.Rapidly progressive necrotizing fasciitis caused by Staphylococcus aureus.J Microbiol Immunol Infect.2005;38(5):361–364.
- ,.Streptococcal infections of skin and soft tissues.N Engl J Med.1996;334(4):240–245.
- ,.Streptococcal toxic shock syndrome after breast reconstruction.Ann Plast Surg.2005;54(5):553–556.
- ,.The influence of pigmentation and illumination on the perception of erythema.Photodermatol Photoimmunol Photomed.1992;9(2):45–47.
- .Streptococcal toxic‐shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment.Emerg Infect Dis.1995;1(3):69–78.
- ,,,,,.Fast and frugal models of clinical judgment in novice and expert physicians.Med Decis Making.2003;23(4):293–300.
- .What is it to be an expert? In: Chi M, Farr MJ, Glaser R, eds.The Nature of Expertise.Hillsdale, NJ:Lawrence Erlbaum;1988.
- .Clinical decision‐making: understanding how clinicians make a diagnosis. In: Saint S, Drazen JM, Solomon CG, eds.Clinical Problem‐Solving.New York, NY:McGraw‐Hill;2006.
- ,,.Medical Problem Solving: An Analysis of Clinical Reasoning.Cambridge, MA:Harvard University Press;1978.
- ,,,,.Population‐based surveillance for group A streptococcal necrotizing fasciitis: clinical features, prognostic indicators, and microbiologic analysis of seventy‐seven cases. Ontario Group A Streptococcal Study.Am J Med.1997;103(1):18–24.
- ,,, et al.Invasive group A streptococcal infections in Sweden in 1994 and 1995: epidemiology and clinical spectrum.Scand J Infect Dis.2000;32(6):609–614.
- ,,,.Reemergence of emm1 and a changed superantigen profile for group A streptococci causing invasive infections: results from a nationwide study.J Clin Microbiol.2005;43(4):1789–1796.
- ,,, et al.Severe group A streptococcal infections associated with a toxic shock‐like syndrome and scarlet fever toxin A.N Engl J Med.1989;321(1):1–7.
- ,,,.Rapidly progressive necrotizing fasciitis caused by Staphylococcus aureus.J Microbiol Immunol Infect.2005;38(5):361–364.
- ,.Streptococcal infections of skin and soft tissues.N Engl J Med.1996;334(4):240–245.
- ,.Streptococcal toxic shock syndrome after breast reconstruction.Ann Plast Surg.2005;54(5):553–556.
- ,.The influence of pigmentation and illumination on the perception of erythema.Photodermatol Photoimmunol Photomed.1992;9(2):45–47.
- .Streptococcal toxic‐shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment.Emerg Infect Dis.1995;1(3):69–78.
Jumpstarting Hospital Medicine Research
Dramatic changes in the organization, financing, and delivery of hospital care that began a decade ago continue to accelerate. One of the most important changes has been the emergence of hospitalists as providers of inpatient care.1 Hospitalists are physicians, usually general internists, whose clinical focus is the hospitalized patient. As patient illnesses have become more severe and complex, physicians have found it difficult to balance inpatient and outpatient care and have focused on one of the two.25 It is estimated that there are currently 15,000 practicing hospitalists nationally, and projections suggest that this number may exceed 30,000 by 2010, which is equal to the number of cardiologists currently practicing in the United States.6 A 2003 survey from the American Hospital Association showed that more than 30% of the nation's 4900 community hospitals have hospital medicine groups.7 Furthermore, more than 70% of the nation's largest hospitals (>500 beds) and 66% of major teaching hospitals use hospitalists.7
The transition to a hospitalist model generates multiple new research questions about the best approach to caring for the hospitalized patient. Additionally, hospitalists may spawn new areas of clinical research by tackling clinical issues that formerly lacked a large number of specialist investigators. Examples include implementation‐based studies,8, 9 inpatient safety practices,1012 quasi‐experimental studies focusing on common inpatient issues,13, 14 and the evaluation of new methods for reducing resource utilization within various inpatient care delivery structures.15, 16
Similarly, if future clinical trials are to be carried out in real‐world settings, by necessity these will require the participation of hospitalists. Clinical research performed by hospitalists and hospital medicine programs, however, remains underdeveloped. Although this has been attributed to several variables, including the youth of the field, a paucity of fellowship‐trained hospitalist researchers, and a lack of a hospitalist‐oriented national funding source, we also believe that additional barriers exist which could be overcome if hospitalists actively partnered with specialists to perform hospital‐based clinical and translational research.
Hospitalists lack clinical expertise in many clinical issues. In both academic and nonacademic settings, the diagnostic approach, individual treatment decisions, and follow‐up of complex patients occur with frequent consultation of specialists. Specialists often provide a deeper understanding of both the pathophysiologic concepts and scientific principles underlying important clinical questions and are more likely to have had fellowship training that included clinical research experience. Specialists also have more access to extramural funding for disease‐based investigation, and thus their involvement in hospital‐based clinical research would likely enhance funding opportunities, improve project feasibility, and increase dissemination of the results. A successful clinical research program will therefore be one that combines specialists and hospitalists working collaboratively to determine the best way to care for inpatients. With that in mind, we created the University of Michigan SpecialistHospitalist Allied Research Program (SHARP).
METHODS
Setting
The University of Michigan Medical Center includes a 900‐bed teaching hospital with more than 44,000 yearly inpatient discharges, and the Department of Internal Medicine manages nearly 15,000 annual discharges. The University of Michigan Hospital Medicine Program has grown dramatically over the past few years and now includes more than 30 hospitalists. These hospitalists will manage nearly 8000 admissions in the upcoming year, which represent more than half of all the patients admitted to the Department of Internal Medicine. Five years ago, these 8000 admissions would have been cared for by 3 to 4 times as many providers, most of whom would have been specialists. Currently, specialists consult regularly on patients cared for by hospitalists, and as a result, a few loosely formed research collaborations developed spontaneously but lacked resources or infrastructure to facilitate their completion. SHARP was intended to organize these clinical research pilot studies and jumpstart hospital‐based clinical and translational research.
The SHARP Intervention
Objectives
In 2006, hospitalists and specialists with an interest in expanding clinical and translational research aimed at caring for inpatients were brought together for the SHARP intervention. This intervention had several objectives:
To develop a clinical research infrastructure within the University of Michigan Hospital Medicine Program to facilitate patient participation.
To foster increased specialisthospitalist collaboration for addressing common inpatient problems.
To facilitate pilot projects and preliminary data collection that enhance the ability to obtain subsequent extramural funding for collaborative research projects.
To facilitate multicenter investigation led by the University of Michigan by allowing the SHARP investigators to use an existing hospitalist consortium to expand the scope of research projects.
Ultimately, to develop the ability to perform multicenter intervention‐based clinical trials.
Structure
The key to SHARP's infrastructure is its personnel and governance structure. At the head of SHARP is an academic hospitalist as principal investigator (PI) and an academic cardiologist with health services research training serving as coprincipal investigator (Co‐PI). Key personnel also include a hospitalist investigator, a masters‐level research associate, a PhD clinical epidemiologist, and the hospitalists and subspecialists who serve as investigators. Although the program leadership has research experience, many of the hospitalist and specialist investigators are junior faculty without extensive prior research experience. Thus, SHARP was specifically designed to build the capacity to enhance inpatient clinical and translational research and to remove barriers for new investigators developing their academic careers.
It is critical that oversight provides direction for the research program, assists with project identification and selection, and facilitates collaborations that tie diverse projects together. We believe that this is best accomplished by the creation of a steering committee chaired by both the PI and Co‐PI. The steering committee also includes key individuals such as the Vice Chair of the Department of Medicine and the Associate Dean for Clinical and Translational Research at the University of Michigan. The 2 cochairs are responsible for overseeing the program and reporting the progress of SHARP to the University of Michigan Department of Internal Medicine. They will help identify and produce viable research proposals that can be brought to the full committee. To help the program understand and overcome bureaucratic obstacles, we have also included a former high‐level administrator on the steering committee as a consultant. Given the initial scope of the program, the SHARP steering committee has had a small number of key individuals. As the program grows and increases its number of ongoing collaborative projects, we will likely need to expand committee membership.
SHARP leadership meets regularly to plan projects, discuss grant ideas, make hiring decisions, and troubleshoot problems in existing projects. The entire steering committee meets quarterly to help chart the overall course of the program. A more thorough description of the program and its structure can be found on the SHARP Web site (
SHARP Funding
SHARP could not exist without resources. The funding for the program comes from the Department of Internal Medicine and uses revenue from the hospital medicine program that flows to the department. To garner support for the program, SHARP leadership sought buy‐in from the Chair of Medicine, all the division chiefs, and key faculty active in clinical research. The fact that the program has the potential to benefit not just hospitalists but also other department faculty such as specialists facilitated departmental funding. The program is funded for 3 years with an 18‐month program review to gauge progress. Funding is used to build clinical research infrastructure and facilitate collection of pilot data. SHARP resources support a portion of the salaries of key personnel for the 3‐year duration of the project (research associate, 50%; PI, 10%; Co‐PI, 5%; and epidemiologist, 5%), after which time intramural funding ends. Every SHARP project is, therefore, expected to apply for extramural funding with the goal of full extramural programmatic support after 3 years.
SHARP Performance Metrics
Measuring the accomplishments of SHARP is clearly important. As the program is intended to jumpstart collaborative inpatient clinical research, the number of such projects is important to track. An additional goal is to support work that leads to extramural funding. As the program started from scratch, it is unrealistic to have completed peer‐reviewed manuscripts or successful extramural grants as the sole metrics by which the program is judged, especially early in its initiation. In a yearly report to the department chair, we will report on primary and secondary outcomes (see Table 1).
| Primary outcomes |
|---|
|
| 1. Number of ongoing research projects involving SHARP support and a brief description of the aims and status of each |
| 2. Number of extramural grants submitted in which SHARP is mentioned or involved |
| 3. Extramural grants received (total and direct dollars) |
| 4. Peer‐reviewed publications authored by SHARP investigators |
| Secondary outcomes |
| 1. Abstracts accepted for presentation at national or international scientific meetings |
| 2. Non‐peer‐reviewed publications related to SHARP |
| 3. Invited presentations by SHARP investigators |
| 4. People who have visited the University of Michigan in conjunction with SHARP work (eg, visiting professors) |
Initial SHARP Projects
SHARP has a formal process for evaluating potential projects. A steering committee ultimately decides how best to use SHARP‐related resources. Key components in this decision are related to the proposal's innovation, feasibility, and importance as well as the extent of specialisthospitalist collaboration. The 2 projects described next are our initial areas of focus and exemplify these concepts. One project partners hospitalists with infectious disease specialists, whereas the second pairs hospitalists with geriatricians and clinical pharmacists.
Reducing False Positive Blood Cultures
The blood culture is an important tool for the diagnosis and management of bloodstream infections. As a result, physicians have a low threshold for obtaining blood cultures. Unfortunately, up to half of all positive blood cultures are positive because of contamination. These false positive cultures lead to additional diagnostic testing, unnecessary antibiotics, and increased healthcare costs.17 A variety of antiseptic agents and techniques are used to prevent falsely positive cultures. However, a recent evidence‐based systematic review performed by University of Michigan investigators found no clear evidence to suggest which antiseptic agent should be routinely used. They concluded that a randomized controlled trial was urgently needed.18
SHARP and its infrastructure have begun a cluster‐randomized crossover trial at the university hospital. The trial compares the effects of a variety of skin antiseptic agents on peripheral blood culture contamination rates. The study population includes hospitalized patients undergoing venipuncture for peripheral blood cultures on 3 general medicine and surgery floors. The trial will include over 12,000 blood culture sets and will have 85% power to detect a 0.5% difference in effectiveness between antiseptic agents. Key outcomes will be rates of positive blood cultures (true positive versus false positive), quantity of additional diagnostic testing generated by positive cultures, resource use (including antibiotics), and associated costs. Clinical outcomes such as length of stay and inpatient mortality will also be measured as secondary outcomes.
Pharmacist‐Facilitated Hospital Discharge
Hospital discharge is a complex process in which patients must be transferred from the care of an inpatient team to that of an outpatient provider. During most hospitalizations, a patient will have new medications added, a chronic medication stopped, or a change in medication dosage. Studies have revealed that the most common adverse events that have an impact on patients after discharge are related to medications.1921 In our experience at the University of Michigan, patients frequently have medication‐related adverse events after discharge because they do not understand what medications they should be taking, what they are used for, how to manage side effects, or whom to call with problems. In addition, predictable medication‐related issues (such as the ability to pay for a medicine or expected serum electrolyte changes with newly added medications) are not universally anticipated. The frail elderly are especially vulnerable to medication‐related adverse events.
Building on the work of others in the field, we proposed studying the impact of an inpatient clinical pharmacist to address medication misadventures related to hospital discharge in our elderly population.22 The study uses an interrupted time series design (the pharmacist will alternate months at a nonresident hospitalist service and a resident general medicine service) to measure the impact of the clinical pharmacist. The pharmacist will focus on patients over the age of 65 meeting criteria that identify them to be at high risk for an adverse medication event after discharge. These factors include any new medication started in the hospital, medication noncompliance or an adverse medication event that led to the admission, or use of a high‐risk medication (eg, anticoagulants, narcotics, diuretics, diabetic agents, and immunosuppressives). The pharmacist and inpatient physicians will identify high‐risk patients who will receive predischarge medication counseling. This process will identify problem medications and needed follow‐up (eg, laboratory testing) and assess compliance issues. After discharge, patients will be contacted by the pharmacist both within 72 hours and at 30 days. Standardized questions will be asked of patients to troubleshoot medication issues, assess them for problems with medications or follow‐up, and identify patients who may need more urgent access to a healthcare provider to address medication‐related problems.
Key outcomes will include the pharmacist's actions at discharge (eg, dose changes made, medication class switches, and side‐effect monitoring implemented). In addition, we will track types of medication issues identified after discharge and interventions made. Important clinical outcomes will include return to the emergency department after discharge, 30‐day readmission rates, and healthcare‐related costs.
DISCUSSION AND NEXT STEPS
SHARP is a novel clinical research program partnering hospitalists with specialists. Its current focus targets single‐institution studies that generate pilot data leading to larger projects. The ultimate goal is to develop the ability to do larger multicenter investigator‐initiated projects. The SHARP program will also have the ability to perform observational studies to identify predictors and risk factors and the ability to carry out implementation studies that show how best to translate results from published articles to direct patient care.
A specialisthospitalist collaboration overcomes barriers that we feel may impede hospital medicine research at an academic medical center. For a similar program to succeed at other institutions, key components from our program will have to be replicated. First, senior, fellowship‐trained researchers are required to mentor junior investigators (who may or may not have additional fellowship training), help guide project selection, oversee grant and manuscript submissions, and troubleshoot problems that arise in the course of any clinical research project. In our institution, this comes from within our hospitalist program and from our specialist collaborators. In institutions lacking hospitalists with research experience, this guidance could come from within a division of general medicine, internal medicine specialty divisions, internal medicine department leadership, or even noninternal medicine departments (eg, emergency medicine, neurology, and surgery) that have traditionally been involved in clinical research programs.
A second key component that must be considered is funding. An initial investment is necessary to fund key personnel dedicated to getting projects started on the right track, collecting pilot data, and ensuring project completion and dissemination of the results. The positive margin generated by our hospitalist program facilitated the initial investment. In the absence of a positive margin, resources could come directly from the hospital, the medical school, the department of internal medicine, or perhaps a foundation. The case would need to be made that an initial short‐term investment would enhance the academic standing of the institution, enhance the careers of young investigators, and over time lead to a self‐sustaining program through investigator‐initiated grants and extramural funding. In addition to experienced leadership and funding, we created an oversight committee, but we feel that this is not a critical component. A potential concern with a program that partners with specialists might be that research topics become too disease‐specific or specialty‐oriented. We specifically created the oversight committee to protect against this possibility, and other institutions might need similar safeguards.
Our next step includes leveraging existing hospitalist collaboratives that reach beyond academic medical centers to expand further the reach of SHARP. Ultimately, any new therapy, clinical tool, diagnostic paradigm, or implementation strategy that is developed or evaluated bythe SHARP program would need to be tested in a real‐world setting to assess external validity. With support from the Blue Cross Blue Shield of Michigan Foundation, we have created a multihospital patient safety consortium, the Hospitalists as Emerging Leaders in Patient Safety Consortium, which includes academic, government, urban, rural, teaching, and nonteaching hospitals.23 Although the initial focus is patient safety, our goal for the consortium is to develop it into a multihospital clinical research program that could take pilot projects developed by SHARP and test them in real‐world settings. We believe that full‐scale multihospital studies based on SHARP pilot data will be very attractive to external funding agencies and will help SHARP become financially self‐sufficient after the initial 3‐year start‐up.
Hospital medicine research is desperately needed.24, 25 Unfortunately, the clinical research capabilities of most hospital medicine programs are quite underdeveloped. We believe that partnering hospitalists with specialists can facilitate collaborative research to identify the best way to care for inpatients. If successful, we believe that variations of this model can be replicated at other institutions and will be a critical factor in jumpstarting hospital medicine clinical research.
Acknowledgements
The authors thank Dr. Marc E. Lippman, Dr. Robert F. Todd, Dr. Larry McMahon, Dr. Timothy J. Laing, and Mr. Lindsay J. Graham, whose support made this program possible.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- ,,,,,.What effect does increasing inpatient time have on outpatient‐oriented internist satisfaction?J Gen Intern Med.2003;18:725–729.
- ,,,,.Characteristics of general internists who practice only outpatient medicine: results from the physician worklife study.Semin Med Pract.2002;5:5–11.
- ,.Hospitalists in teaching hospitals: opportunities but not without danger.J Gen Intern Med.2004;19:392–393.
- ,.Hospitalists: the new model of inpatient medical care in the United States.Eur J Intern Med.2003;14:65–70.
- ,,,,.The potential size of the hospitalist workforce in the United States.Am J Med.1999;106:441–445.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,,, et al.Translating infection prevention evidence into practice using quantitative and qualitative research.Am J Infect Control.2006;34:507–512.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,,,.Making health care safer: a critical analysis of patient safety practices.Evid Rep Technol Assess (Summ).2001;(43):i–x,1–668.
- ,,,.Safe but sound: patient safety meets evidence‐based medicine.JAMA.2002;288:508–513.
- ,,,.Clinical pharmacists and inpatient medical care: a systematic review.Arch Intern Med.2006;166:955–964.
- ,,, et al.Are antiseptic‐coated central venous catheters effective in a real‐world setting?Am J Infect Control.2006;34:388–393.
- ,,,,.Effectiveness of ceftriaxone plus doxycycline in the treatment of patients hospitalized with community‐acquired pneumonia.J Hosp Med.2006;1:7–12.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,.What effect does physician “profiling” have on inpatient physician satisfaction and hospital length of stay?BMC Health Serv Res.2006;6:45.
- ,,.Contaminant blood cultures and resource utilization. The true consequences of false‐positive results.JAMA.1991;265:365–369.
- ,,,,,.Review of clinical trials of skin antiseptic agents used to reduce blood culture contamination.Infect Control Hosp Epidemiol.2007;28:892–895.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20:317–323.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170:345–349.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- ,,.Hospitalists as emerging leaders in patient safety: targeting a few to affect many.JPatient Saf.2005;1:78–82.
- .The future of hospital medicine: evolution or revolution?Am J Med.2004;117:446–450.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:72 e1–7.
Dramatic changes in the organization, financing, and delivery of hospital care that began a decade ago continue to accelerate. One of the most important changes has been the emergence of hospitalists as providers of inpatient care.1 Hospitalists are physicians, usually general internists, whose clinical focus is the hospitalized patient. As patient illnesses have become more severe and complex, physicians have found it difficult to balance inpatient and outpatient care and have focused on one of the two.25 It is estimated that there are currently 15,000 practicing hospitalists nationally, and projections suggest that this number may exceed 30,000 by 2010, which is equal to the number of cardiologists currently practicing in the United States.6 A 2003 survey from the American Hospital Association showed that more than 30% of the nation's 4900 community hospitals have hospital medicine groups.7 Furthermore, more than 70% of the nation's largest hospitals (>500 beds) and 66% of major teaching hospitals use hospitalists.7
The transition to a hospitalist model generates multiple new research questions about the best approach to caring for the hospitalized patient. Additionally, hospitalists may spawn new areas of clinical research by tackling clinical issues that formerly lacked a large number of specialist investigators. Examples include implementation‐based studies,8, 9 inpatient safety practices,1012 quasi‐experimental studies focusing on common inpatient issues,13, 14 and the evaluation of new methods for reducing resource utilization within various inpatient care delivery structures.15, 16
Similarly, if future clinical trials are to be carried out in real‐world settings, by necessity these will require the participation of hospitalists. Clinical research performed by hospitalists and hospital medicine programs, however, remains underdeveloped. Although this has been attributed to several variables, including the youth of the field, a paucity of fellowship‐trained hospitalist researchers, and a lack of a hospitalist‐oriented national funding source, we also believe that additional barriers exist which could be overcome if hospitalists actively partnered with specialists to perform hospital‐based clinical and translational research.
Hospitalists lack clinical expertise in many clinical issues. In both academic and nonacademic settings, the diagnostic approach, individual treatment decisions, and follow‐up of complex patients occur with frequent consultation of specialists. Specialists often provide a deeper understanding of both the pathophysiologic concepts and scientific principles underlying important clinical questions and are more likely to have had fellowship training that included clinical research experience. Specialists also have more access to extramural funding for disease‐based investigation, and thus their involvement in hospital‐based clinical research would likely enhance funding opportunities, improve project feasibility, and increase dissemination of the results. A successful clinical research program will therefore be one that combines specialists and hospitalists working collaboratively to determine the best way to care for inpatients. With that in mind, we created the University of Michigan SpecialistHospitalist Allied Research Program (SHARP).
METHODS
Setting
The University of Michigan Medical Center includes a 900‐bed teaching hospital with more than 44,000 yearly inpatient discharges, and the Department of Internal Medicine manages nearly 15,000 annual discharges. The University of Michigan Hospital Medicine Program has grown dramatically over the past few years and now includes more than 30 hospitalists. These hospitalists will manage nearly 8000 admissions in the upcoming year, which represent more than half of all the patients admitted to the Department of Internal Medicine. Five years ago, these 8000 admissions would have been cared for by 3 to 4 times as many providers, most of whom would have been specialists. Currently, specialists consult regularly on patients cared for by hospitalists, and as a result, a few loosely formed research collaborations developed spontaneously but lacked resources or infrastructure to facilitate their completion. SHARP was intended to organize these clinical research pilot studies and jumpstart hospital‐based clinical and translational research.
The SHARP Intervention
Objectives
In 2006, hospitalists and specialists with an interest in expanding clinical and translational research aimed at caring for inpatients were brought together for the SHARP intervention. This intervention had several objectives:
To develop a clinical research infrastructure within the University of Michigan Hospital Medicine Program to facilitate patient participation.
To foster increased specialisthospitalist collaboration for addressing common inpatient problems.
To facilitate pilot projects and preliminary data collection that enhance the ability to obtain subsequent extramural funding for collaborative research projects.
To facilitate multicenter investigation led by the University of Michigan by allowing the SHARP investigators to use an existing hospitalist consortium to expand the scope of research projects.
Ultimately, to develop the ability to perform multicenter intervention‐based clinical trials.
Structure
The key to SHARP's infrastructure is its personnel and governance structure. At the head of SHARP is an academic hospitalist as principal investigator (PI) and an academic cardiologist with health services research training serving as coprincipal investigator (Co‐PI). Key personnel also include a hospitalist investigator, a masters‐level research associate, a PhD clinical epidemiologist, and the hospitalists and subspecialists who serve as investigators. Although the program leadership has research experience, many of the hospitalist and specialist investigators are junior faculty without extensive prior research experience. Thus, SHARP was specifically designed to build the capacity to enhance inpatient clinical and translational research and to remove barriers for new investigators developing their academic careers.
It is critical that oversight provides direction for the research program, assists with project identification and selection, and facilitates collaborations that tie diverse projects together. We believe that this is best accomplished by the creation of a steering committee chaired by both the PI and Co‐PI. The steering committee also includes key individuals such as the Vice Chair of the Department of Medicine and the Associate Dean for Clinical and Translational Research at the University of Michigan. The 2 cochairs are responsible for overseeing the program and reporting the progress of SHARP to the University of Michigan Department of Internal Medicine. They will help identify and produce viable research proposals that can be brought to the full committee. To help the program understand and overcome bureaucratic obstacles, we have also included a former high‐level administrator on the steering committee as a consultant. Given the initial scope of the program, the SHARP steering committee has had a small number of key individuals. As the program grows and increases its number of ongoing collaborative projects, we will likely need to expand committee membership.
SHARP leadership meets regularly to plan projects, discuss grant ideas, make hiring decisions, and troubleshoot problems in existing projects. The entire steering committee meets quarterly to help chart the overall course of the program. A more thorough description of the program and its structure can be found on the SHARP Web site (
SHARP Funding
SHARP could not exist without resources. The funding for the program comes from the Department of Internal Medicine and uses revenue from the hospital medicine program that flows to the department. To garner support for the program, SHARP leadership sought buy‐in from the Chair of Medicine, all the division chiefs, and key faculty active in clinical research. The fact that the program has the potential to benefit not just hospitalists but also other department faculty such as specialists facilitated departmental funding. The program is funded for 3 years with an 18‐month program review to gauge progress. Funding is used to build clinical research infrastructure and facilitate collection of pilot data. SHARP resources support a portion of the salaries of key personnel for the 3‐year duration of the project (research associate, 50%; PI, 10%; Co‐PI, 5%; and epidemiologist, 5%), after which time intramural funding ends. Every SHARP project is, therefore, expected to apply for extramural funding with the goal of full extramural programmatic support after 3 years.
SHARP Performance Metrics
Measuring the accomplishments of SHARP is clearly important. As the program is intended to jumpstart collaborative inpatient clinical research, the number of such projects is important to track. An additional goal is to support work that leads to extramural funding. As the program started from scratch, it is unrealistic to have completed peer‐reviewed manuscripts or successful extramural grants as the sole metrics by which the program is judged, especially early in its initiation. In a yearly report to the department chair, we will report on primary and secondary outcomes (see Table 1).
| Primary outcomes |
|---|
|
| 1. Number of ongoing research projects involving SHARP support and a brief description of the aims and status of each |
| 2. Number of extramural grants submitted in which SHARP is mentioned or involved |
| 3. Extramural grants received (total and direct dollars) |
| 4. Peer‐reviewed publications authored by SHARP investigators |
| Secondary outcomes |
| 1. Abstracts accepted for presentation at national or international scientific meetings |
| 2. Non‐peer‐reviewed publications related to SHARP |
| 3. Invited presentations by SHARP investigators |
| 4. People who have visited the University of Michigan in conjunction with SHARP work (eg, visiting professors) |
Initial SHARP Projects
SHARP has a formal process for evaluating potential projects. A steering committee ultimately decides how best to use SHARP‐related resources. Key components in this decision are related to the proposal's innovation, feasibility, and importance as well as the extent of specialisthospitalist collaboration. The 2 projects described next are our initial areas of focus and exemplify these concepts. One project partners hospitalists with infectious disease specialists, whereas the second pairs hospitalists with geriatricians and clinical pharmacists.
Reducing False Positive Blood Cultures
The blood culture is an important tool for the diagnosis and management of bloodstream infections. As a result, physicians have a low threshold for obtaining blood cultures. Unfortunately, up to half of all positive blood cultures are positive because of contamination. These false positive cultures lead to additional diagnostic testing, unnecessary antibiotics, and increased healthcare costs.17 A variety of antiseptic agents and techniques are used to prevent falsely positive cultures. However, a recent evidence‐based systematic review performed by University of Michigan investigators found no clear evidence to suggest which antiseptic agent should be routinely used. They concluded that a randomized controlled trial was urgently needed.18
SHARP and its infrastructure have begun a cluster‐randomized crossover trial at the university hospital. The trial compares the effects of a variety of skin antiseptic agents on peripheral blood culture contamination rates. The study population includes hospitalized patients undergoing venipuncture for peripheral blood cultures on 3 general medicine and surgery floors. The trial will include over 12,000 blood culture sets and will have 85% power to detect a 0.5% difference in effectiveness between antiseptic agents. Key outcomes will be rates of positive blood cultures (true positive versus false positive), quantity of additional diagnostic testing generated by positive cultures, resource use (including antibiotics), and associated costs. Clinical outcomes such as length of stay and inpatient mortality will also be measured as secondary outcomes.
Pharmacist‐Facilitated Hospital Discharge
Hospital discharge is a complex process in which patients must be transferred from the care of an inpatient team to that of an outpatient provider. During most hospitalizations, a patient will have new medications added, a chronic medication stopped, or a change in medication dosage. Studies have revealed that the most common adverse events that have an impact on patients after discharge are related to medications.1921 In our experience at the University of Michigan, patients frequently have medication‐related adverse events after discharge because they do not understand what medications they should be taking, what they are used for, how to manage side effects, or whom to call with problems. In addition, predictable medication‐related issues (such as the ability to pay for a medicine or expected serum electrolyte changes with newly added medications) are not universally anticipated. The frail elderly are especially vulnerable to medication‐related adverse events.
Building on the work of others in the field, we proposed studying the impact of an inpatient clinical pharmacist to address medication misadventures related to hospital discharge in our elderly population.22 The study uses an interrupted time series design (the pharmacist will alternate months at a nonresident hospitalist service and a resident general medicine service) to measure the impact of the clinical pharmacist. The pharmacist will focus on patients over the age of 65 meeting criteria that identify them to be at high risk for an adverse medication event after discharge. These factors include any new medication started in the hospital, medication noncompliance or an adverse medication event that led to the admission, or use of a high‐risk medication (eg, anticoagulants, narcotics, diuretics, diabetic agents, and immunosuppressives). The pharmacist and inpatient physicians will identify high‐risk patients who will receive predischarge medication counseling. This process will identify problem medications and needed follow‐up (eg, laboratory testing) and assess compliance issues. After discharge, patients will be contacted by the pharmacist both within 72 hours and at 30 days. Standardized questions will be asked of patients to troubleshoot medication issues, assess them for problems with medications or follow‐up, and identify patients who may need more urgent access to a healthcare provider to address medication‐related problems.
Key outcomes will include the pharmacist's actions at discharge (eg, dose changes made, medication class switches, and side‐effect monitoring implemented). In addition, we will track types of medication issues identified after discharge and interventions made. Important clinical outcomes will include return to the emergency department after discharge, 30‐day readmission rates, and healthcare‐related costs.
DISCUSSION AND NEXT STEPS
SHARP is a novel clinical research program partnering hospitalists with specialists. Its current focus targets single‐institution studies that generate pilot data leading to larger projects. The ultimate goal is to develop the ability to do larger multicenter investigator‐initiated projects. The SHARP program will also have the ability to perform observational studies to identify predictors and risk factors and the ability to carry out implementation studies that show how best to translate results from published articles to direct patient care.
A specialisthospitalist collaboration overcomes barriers that we feel may impede hospital medicine research at an academic medical center. For a similar program to succeed at other institutions, key components from our program will have to be replicated. First, senior, fellowship‐trained researchers are required to mentor junior investigators (who may or may not have additional fellowship training), help guide project selection, oversee grant and manuscript submissions, and troubleshoot problems that arise in the course of any clinical research project. In our institution, this comes from within our hospitalist program and from our specialist collaborators. In institutions lacking hospitalists with research experience, this guidance could come from within a division of general medicine, internal medicine specialty divisions, internal medicine department leadership, or even noninternal medicine departments (eg, emergency medicine, neurology, and surgery) that have traditionally been involved in clinical research programs.
A second key component that must be considered is funding. An initial investment is necessary to fund key personnel dedicated to getting projects started on the right track, collecting pilot data, and ensuring project completion and dissemination of the results. The positive margin generated by our hospitalist program facilitated the initial investment. In the absence of a positive margin, resources could come directly from the hospital, the medical school, the department of internal medicine, or perhaps a foundation. The case would need to be made that an initial short‐term investment would enhance the academic standing of the institution, enhance the careers of young investigators, and over time lead to a self‐sustaining program through investigator‐initiated grants and extramural funding. In addition to experienced leadership and funding, we created an oversight committee, but we feel that this is not a critical component. A potential concern with a program that partners with specialists might be that research topics become too disease‐specific or specialty‐oriented. We specifically created the oversight committee to protect against this possibility, and other institutions might need similar safeguards.
Our next step includes leveraging existing hospitalist collaboratives that reach beyond academic medical centers to expand further the reach of SHARP. Ultimately, any new therapy, clinical tool, diagnostic paradigm, or implementation strategy that is developed or evaluated bythe SHARP program would need to be tested in a real‐world setting to assess external validity. With support from the Blue Cross Blue Shield of Michigan Foundation, we have created a multihospital patient safety consortium, the Hospitalists as Emerging Leaders in Patient Safety Consortium, which includes academic, government, urban, rural, teaching, and nonteaching hospitals.23 Although the initial focus is patient safety, our goal for the consortium is to develop it into a multihospital clinical research program that could take pilot projects developed by SHARP and test them in real‐world settings. We believe that full‐scale multihospital studies based on SHARP pilot data will be very attractive to external funding agencies and will help SHARP become financially self‐sufficient after the initial 3‐year start‐up.
Hospital medicine research is desperately needed.24, 25 Unfortunately, the clinical research capabilities of most hospital medicine programs are quite underdeveloped. We believe that partnering hospitalists with specialists can facilitate collaborative research to identify the best way to care for inpatients. If successful, we believe that variations of this model can be replicated at other institutions and will be a critical factor in jumpstarting hospital medicine clinical research.
Acknowledgements
The authors thank Dr. Marc E. Lippman, Dr. Robert F. Todd, Dr. Larry McMahon, Dr. Timothy J. Laing, and Mr. Lindsay J. Graham, whose support made this program possible.
Dramatic changes in the organization, financing, and delivery of hospital care that began a decade ago continue to accelerate. One of the most important changes has been the emergence of hospitalists as providers of inpatient care.1 Hospitalists are physicians, usually general internists, whose clinical focus is the hospitalized patient. As patient illnesses have become more severe and complex, physicians have found it difficult to balance inpatient and outpatient care and have focused on one of the two.25 It is estimated that there are currently 15,000 practicing hospitalists nationally, and projections suggest that this number may exceed 30,000 by 2010, which is equal to the number of cardiologists currently practicing in the United States.6 A 2003 survey from the American Hospital Association showed that more than 30% of the nation's 4900 community hospitals have hospital medicine groups.7 Furthermore, more than 70% of the nation's largest hospitals (>500 beds) and 66% of major teaching hospitals use hospitalists.7
The transition to a hospitalist model generates multiple new research questions about the best approach to caring for the hospitalized patient. Additionally, hospitalists may spawn new areas of clinical research by tackling clinical issues that formerly lacked a large number of specialist investigators. Examples include implementation‐based studies,8, 9 inpatient safety practices,1012 quasi‐experimental studies focusing on common inpatient issues,13, 14 and the evaluation of new methods for reducing resource utilization within various inpatient care delivery structures.15, 16
Similarly, if future clinical trials are to be carried out in real‐world settings, by necessity these will require the participation of hospitalists. Clinical research performed by hospitalists and hospital medicine programs, however, remains underdeveloped. Although this has been attributed to several variables, including the youth of the field, a paucity of fellowship‐trained hospitalist researchers, and a lack of a hospitalist‐oriented national funding source, we also believe that additional barriers exist which could be overcome if hospitalists actively partnered with specialists to perform hospital‐based clinical and translational research.
Hospitalists lack clinical expertise in many clinical issues. In both academic and nonacademic settings, the diagnostic approach, individual treatment decisions, and follow‐up of complex patients occur with frequent consultation of specialists. Specialists often provide a deeper understanding of both the pathophysiologic concepts and scientific principles underlying important clinical questions and are more likely to have had fellowship training that included clinical research experience. Specialists also have more access to extramural funding for disease‐based investigation, and thus their involvement in hospital‐based clinical research would likely enhance funding opportunities, improve project feasibility, and increase dissemination of the results. A successful clinical research program will therefore be one that combines specialists and hospitalists working collaboratively to determine the best way to care for inpatients. With that in mind, we created the University of Michigan SpecialistHospitalist Allied Research Program (SHARP).
METHODS
Setting
The University of Michigan Medical Center includes a 900‐bed teaching hospital with more than 44,000 yearly inpatient discharges, and the Department of Internal Medicine manages nearly 15,000 annual discharges. The University of Michigan Hospital Medicine Program has grown dramatically over the past few years and now includes more than 30 hospitalists. These hospitalists will manage nearly 8000 admissions in the upcoming year, which represent more than half of all the patients admitted to the Department of Internal Medicine. Five years ago, these 8000 admissions would have been cared for by 3 to 4 times as many providers, most of whom would have been specialists. Currently, specialists consult regularly on patients cared for by hospitalists, and as a result, a few loosely formed research collaborations developed spontaneously but lacked resources or infrastructure to facilitate their completion. SHARP was intended to organize these clinical research pilot studies and jumpstart hospital‐based clinical and translational research.
The SHARP Intervention
Objectives
In 2006, hospitalists and specialists with an interest in expanding clinical and translational research aimed at caring for inpatients were brought together for the SHARP intervention. This intervention had several objectives:
To develop a clinical research infrastructure within the University of Michigan Hospital Medicine Program to facilitate patient participation.
To foster increased specialisthospitalist collaboration for addressing common inpatient problems.
To facilitate pilot projects and preliminary data collection that enhance the ability to obtain subsequent extramural funding for collaborative research projects.
To facilitate multicenter investigation led by the University of Michigan by allowing the SHARP investigators to use an existing hospitalist consortium to expand the scope of research projects.
Ultimately, to develop the ability to perform multicenter intervention‐based clinical trials.
Structure
The key to SHARP's infrastructure is its personnel and governance structure. At the head of SHARP is an academic hospitalist as principal investigator (PI) and an academic cardiologist with health services research training serving as coprincipal investigator (Co‐PI). Key personnel also include a hospitalist investigator, a masters‐level research associate, a PhD clinical epidemiologist, and the hospitalists and subspecialists who serve as investigators. Although the program leadership has research experience, many of the hospitalist and specialist investigators are junior faculty without extensive prior research experience. Thus, SHARP was specifically designed to build the capacity to enhance inpatient clinical and translational research and to remove barriers for new investigators developing their academic careers.
It is critical that oversight provides direction for the research program, assists with project identification and selection, and facilitates collaborations that tie diverse projects together. We believe that this is best accomplished by the creation of a steering committee chaired by both the PI and Co‐PI. The steering committee also includes key individuals such as the Vice Chair of the Department of Medicine and the Associate Dean for Clinical and Translational Research at the University of Michigan. The 2 cochairs are responsible for overseeing the program and reporting the progress of SHARP to the University of Michigan Department of Internal Medicine. They will help identify and produce viable research proposals that can be brought to the full committee. To help the program understand and overcome bureaucratic obstacles, we have also included a former high‐level administrator on the steering committee as a consultant. Given the initial scope of the program, the SHARP steering committee has had a small number of key individuals. As the program grows and increases its number of ongoing collaborative projects, we will likely need to expand committee membership.
SHARP leadership meets regularly to plan projects, discuss grant ideas, make hiring decisions, and troubleshoot problems in existing projects. The entire steering committee meets quarterly to help chart the overall course of the program. A more thorough description of the program and its structure can be found on the SHARP Web site (
SHARP Funding
SHARP could not exist without resources. The funding for the program comes from the Department of Internal Medicine and uses revenue from the hospital medicine program that flows to the department. To garner support for the program, SHARP leadership sought buy‐in from the Chair of Medicine, all the division chiefs, and key faculty active in clinical research. The fact that the program has the potential to benefit not just hospitalists but also other department faculty such as specialists facilitated departmental funding. The program is funded for 3 years with an 18‐month program review to gauge progress. Funding is used to build clinical research infrastructure and facilitate collection of pilot data. SHARP resources support a portion of the salaries of key personnel for the 3‐year duration of the project (research associate, 50%; PI, 10%; Co‐PI, 5%; and epidemiologist, 5%), after which time intramural funding ends. Every SHARP project is, therefore, expected to apply for extramural funding with the goal of full extramural programmatic support after 3 years.
SHARP Performance Metrics
Measuring the accomplishments of SHARP is clearly important. As the program is intended to jumpstart collaborative inpatient clinical research, the number of such projects is important to track. An additional goal is to support work that leads to extramural funding. As the program started from scratch, it is unrealistic to have completed peer‐reviewed manuscripts or successful extramural grants as the sole metrics by which the program is judged, especially early in its initiation. In a yearly report to the department chair, we will report on primary and secondary outcomes (see Table 1).
| Primary outcomes |
|---|
|
| 1. Number of ongoing research projects involving SHARP support and a brief description of the aims and status of each |
| 2. Number of extramural grants submitted in which SHARP is mentioned or involved |
| 3. Extramural grants received (total and direct dollars) |
| 4. Peer‐reviewed publications authored by SHARP investigators |
| Secondary outcomes |
| 1. Abstracts accepted for presentation at national or international scientific meetings |
| 2. Non‐peer‐reviewed publications related to SHARP |
| 3. Invited presentations by SHARP investigators |
| 4. People who have visited the University of Michigan in conjunction with SHARP work (eg, visiting professors) |
Initial SHARP Projects
SHARP has a formal process for evaluating potential projects. A steering committee ultimately decides how best to use SHARP‐related resources. Key components in this decision are related to the proposal's innovation, feasibility, and importance as well as the extent of specialisthospitalist collaboration. The 2 projects described next are our initial areas of focus and exemplify these concepts. One project partners hospitalists with infectious disease specialists, whereas the second pairs hospitalists with geriatricians and clinical pharmacists.
Reducing False Positive Blood Cultures
The blood culture is an important tool for the diagnosis and management of bloodstream infections. As a result, physicians have a low threshold for obtaining blood cultures. Unfortunately, up to half of all positive blood cultures are positive because of contamination. These false positive cultures lead to additional diagnostic testing, unnecessary antibiotics, and increased healthcare costs.17 A variety of antiseptic agents and techniques are used to prevent falsely positive cultures. However, a recent evidence‐based systematic review performed by University of Michigan investigators found no clear evidence to suggest which antiseptic agent should be routinely used. They concluded that a randomized controlled trial was urgently needed.18
SHARP and its infrastructure have begun a cluster‐randomized crossover trial at the university hospital. The trial compares the effects of a variety of skin antiseptic agents on peripheral blood culture contamination rates. The study population includes hospitalized patients undergoing venipuncture for peripheral blood cultures on 3 general medicine and surgery floors. The trial will include over 12,000 blood culture sets and will have 85% power to detect a 0.5% difference in effectiveness between antiseptic agents. Key outcomes will be rates of positive blood cultures (true positive versus false positive), quantity of additional diagnostic testing generated by positive cultures, resource use (including antibiotics), and associated costs. Clinical outcomes such as length of stay and inpatient mortality will also be measured as secondary outcomes.
Pharmacist‐Facilitated Hospital Discharge
Hospital discharge is a complex process in which patients must be transferred from the care of an inpatient team to that of an outpatient provider. During most hospitalizations, a patient will have new medications added, a chronic medication stopped, or a change in medication dosage. Studies have revealed that the most common adverse events that have an impact on patients after discharge are related to medications.1921 In our experience at the University of Michigan, patients frequently have medication‐related adverse events after discharge because they do not understand what medications they should be taking, what they are used for, how to manage side effects, or whom to call with problems. In addition, predictable medication‐related issues (such as the ability to pay for a medicine or expected serum electrolyte changes with newly added medications) are not universally anticipated. The frail elderly are especially vulnerable to medication‐related adverse events.
Building on the work of others in the field, we proposed studying the impact of an inpatient clinical pharmacist to address medication misadventures related to hospital discharge in our elderly population.22 The study uses an interrupted time series design (the pharmacist will alternate months at a nonresident hospitalist service and a resident general medicine service) to measure the impact of the clinical pharmacist. The pharmacist will focus on patients over the age of 65 meeting criteria that identify them to be at high risk for an adverse medication event after discharge. These factors include any new medication started in the hospital, medication noncompliance or an adverse medication event that led to the admission, or use of a high‐risk medication (eg, anticoagulants, narcotics, diuretics, diabetic agents, and immunosuppressives). The pharmacist and inpatient physicians will identify high‐risk patients who will receive predischarge medication counseling. This process will identify problem medications and needed follow‐up (eg, laboratory testing) and assess compliance issues. After discharge, patients will be contacted by the pharmacist both within 72 hours and at 30 days. Standardized questions will be asked of patients to troubleshoot medication issues, assess them for problems with medications or follow‐up, and identify patients who may need more urgent access to a healthcare provider to address medication‐related problems.
Key outcomes will include the pharmacist's actions at discharge (eg, dose changes made, medication class switches, and side‐effect monitoring implemented). In addition, we will track types of medication issues identified after discharge and interventions made. Important clinical outcomes will include return to the emergency department after discharge, 30‐day readmission rates, and healthcare‐related costs.
DISCUSSION AND NEXT STEPS
SHARP is a novel clinical research program partnering hospitalists with specialists. Its current focus targets single‐institution studies that generate pilot data leading to larger projects. The ultimate goal is to develop the ability to do larger multicenter investigator‐initiated projects. The SHARP program will also have the ability to perform observational studies to identify predictors and risk factors and the ability to carry out implementation studies that show how best to translate results from published articles to direct patient care.
A specialisthospitalist collaboration overcomes barriers that we feel may impede hospital medicine research at an academic medical center. For a similar program to succeed at other institutions, key components from our program will have to be replicated. First, senior, fellowship‐trained researchers are required to mentor junior investigators (who may or may not have additional fellowship training), help guide project selection, oversee grant and manuscript submissions, and troubleshoot problems that arise in the course of any clinical research project. In our institution, this comes from within our hospitalist program and from our specialist collaborators. In institutions lacking hospitalists with research experience, this guidance could come from within a division of general medicine, internal medicine specialty divisions, internal medicine department leadership, or even noninternal medicine departments (eg, emergency medicine, neurology, and surgery) that have traditionally been involved in clinical research programs.
A second key component that must be considered is funding. An initial investment is necessary to fund key personnel dedicated to getting projects started on the right track, collecting pilot data, and ensuring project completion and dissemination of the results. The positive margin generated by our hospitalist program facilitated the initial investment. In the absence of a positive margin, resources could come directly from the hospital, the medical school, the department of internal medicine, or perhaps a foundation. The case would need to be made that an initial short‐term investment would enhance the academic standing of the institution, enhance the careers of young investigators, and over time lead to a self‐sustaining program through investigator‐initiated grants and extramural funding. In addition to experienced leadership and funding, we created an oversight committee, but we feel that this is not a critical component. A potential concern with a program that partners with specialists might be that research topics become too disease‐specific or specialty‐oriented. We specifically created the oversight committee to protect against this possibility, and other institutions might need similar safeguards.
Our next step includes leveraging existing hospitalist collaboratives that reach beyond academic medical centers to expand further the reach of SHARP. Ultimately, any new therapy, clinical tool, diagnostic paradigm, or implementation strategy that is developed or evaluated bythe SHARP program would need to be tested in a real‐world setting to assess external validity. With support from the Blue Cross Blue Shield of Michigan Foundation, we have created a multihospital patient safety consortium, the Hospitalists as Emerging Leaders in Patient Safety Consortium, which includes academic, government, urban, rural, teaching, and nonteaching hospitals.23 Although the initial focus is patient safety, our goal for the consortium is to develop it into a multihospital clinical research program that could take pilot projects developed by SHARP and test them in real‐world settings. We believe that full‐scale multihospital studies based on SHARP pilot data will be very attractive to external funding agencies and will help SHARP become financially self‐sufficient after the initial 3‐year start‐up.
Hospital medicine research is desperately needed.24, 25 Unfortunately, the clinical research capabilities of most hospital medicine programs are quite underdeveloped. We believe that partnering hospitalists with specialists can facilitate collaborative research to identify the best way to care for inpatients. If successful, we believe that variations of this model can be replicated at other institutions and will be a critical factor in jumpstarting hospital medicine clinical research.
Acknowledgements
The authors thank Dr. Marc E. Lippman, Dr. Robert F. Todd, Dr. Larry McMahon, Dr. Timothy J. Laing, and Mr. Lindsay J. Graham, whose support made this program possible.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- ,,,,,.What effect does increasing inpatient time have on outpatient‐oriented internist satisfaction?J Gen Intern Med.2003;18:725–729.
- ,,,,.Characteristics of general internists who practice only outpatient medicine: results from the physician worklife study.Semin Med Pract.2002;5:5–11.
- ,.Hospitalists in teaching hospitals: opportunities but not without danger.J Gen Intern Med.2004;19:392–393.
- ,.Hospitalists: the new model of inpatient medical care in the United States.Eur J Intern Med.2003;14:65–70.
- ,,,,.The potential size of the hospitalist workforce in the United States.Am J Med.1999;106:441–445.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,,, et al.Translating infection prevention evidence into practice using quantitative and qualitative research.Am J Infect Control.2006;34:507–512.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,,,.Making health care safer: a critical analysis of patient safety practices.Evid Rep Technol Assess (Summ).2001;(43):i–x,1–668.
- ,,,.Safe but sound: patient safety meets evidence‐based medicine.JAMA.2002;288:508–513.
- ,,,.Clinical pharmacists and inpatient medical care: a systematic review.Arch Intern Med.2006;166:955–964.
- ,,, et al.Are antiseptic‐coated central venous catheters effective in a real‐world setting?Am J Infect Control.2006;34:388–393.
- ,,,,.Effectiveness of ceftriaxone plus doxycycline in the treatment of patients hospitalized with community‐acquired pneumonia.J Hosp Med.2006;1:7–12.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,.What effect does physician “profiling” have on inpatient physician satisfaction and hospital length of stay?BMC Health Serv Res.2006;6:45.
- ,,.Contaminant blood cultures and resource utilization. The true consequences of false‐positive results.JAMA.1991;265:365–369.
- ,,,,,.Review of clinical trials of skin antiseptic agents used to reduce blood culture contamination.Infect Control Hosp Epidemiol.2007;28:892–895.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20:317–323.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170:345–349.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- ,,.Hospitalists as emerging leaders in patient safety: targeting a few to affect many.JPatient Saf.2005;1:78–82.
- .The future of hospital medicine: evolution or revolution?Am J Med.2004;117:446–450.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:72 e1–7.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- ,,,,,.What effect does increasing inpatient time have on outpatient‐oriented internist satisfaction?J Gen Intern Med.2003;18:725–729.
- ,,,,.Characteristics of general internists who practice only outpatient medicine: results from the physician worklife study.Semin Med Pract.2002;5:5–11.
- ,.Hospitalists in teaching hospitals: opportunities but not without danger.J Gen Intern Med.2004;19:392–393.
- ,.Hospitalists: the new model of inpatient medical care in the United States.Eur J Intern Med.2003;14:65–70.
- ,,,,.The potential size of the hospitalist workforce in the United States.Am J Med.1999;106:441–445.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,,, et al.Translating infection prevention evidence into practice using quantitative and qualitative research.Am J Infect Control.2006;34:507–512.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,,,.Making health care safer: a critical analysis of patient safety practices.Evid Rep Technol Assess (Summ).2001;(43):i–x,1–668.
- ,,,.Safe but sound: patient safety meets evidence‐based medicine.JAMA.2002;288:508–513.
- ,,,.Clinical pharmacists and inpatient medical care: a systematic review.Arch Intern Med.2006;166:955–964.
- ,,, et al.Are antiseptic‐coated central venous catheters effective in a real‐world setting?Am J Infect Control.2006;34:388–393.
- ,,,,.Effectiveness of ceftriaxone plus doxycycline in the treatment of patients hospitalized with community‐acquired pneumonia.J Hosp Med.2006;1:7–12.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,.What effect does physician “profiling” have on inpatient physician satisfaction and hospital length of stay?BMC Health Serv Res.2006;6:45.
- ,,.Contaminant blood cultures and resource utilization. The true consequences of false‐positive results.JAMA.1991;265:365–369.
- ,,,,,.Review of clinical trials of skin antiseptic agents used to reduce blood culture contamination.Infect Control Hosp Epidemiol.2007;28:892–895.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20:317–323.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170:345–349.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- ,,.Hospitalists as emerging leaders in patient safety: targeting a few to affect many.JPatient Saf.2005;1:78–82.
- .The future of hospital medicine: evolution or revolution?Am J Med.2004;117:446–450.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:72 e1–7.
Copyright © 2008 Society of Hospital Medicine