User login
Incident Database
Recognition that healthcare carries considerable risks of patient injury has focused efforts on identifying problems before they occur, and understanding the root causes of those problems that do occur to prevent them from happening again.1 To further these efforts, a Joint Commission (JC) standard requires hospitals to review sentinel events (SE).2 Reviews must develop a timely, thorough, and credible root cause analysis (RCA), implement action plans to reduce risk, and monitor the effectiveness of implemented improvements.3
Ideally, hospitals would summarize their experiences with SE reviews, identify high‐risk activities and patients, institute system changes to prevent SE recurrences, and share their findings with other healthcare organizations to help them avoid similar patient injuries.1 In support of this last goal, the JC maintains a voluntary database system that allows hospitals to report their SE analyses for other facilities to review and institute preventative actions.
Unfortunately, the reality of SE reviews does not match their ideals for improving patient safety.4 Healthcare organizations often describe their review process as less than credible and note a need for ongoing oversight to maintain the reviews' effectiveness.5 The JC voluntary reporting system captures less than 1% of the SEs that occur nationally,2 because hospitals perceive barriers to external reporting.1 If healthcare organizations decide against reporting externally, they can create their own internal systems to aggregate and summarize SEs, but few such systems exist. A major impediment to designing internal systems is the absence of universally endorsed nomenclature for safety‐related events.6, 7 Poorly aligned terminology and subjective conceptualizations for safety incidents impede the aggregation of SEs, comparisons between facilities, and trend analyses for tracking SE patterns.
In 2005, the World Health Organization (WHO) World Alliance for Patient Safety, in collaboration with the JC, began developing an International Classification for Patient Safety (ICPS) to provide healthcare organizations a consistent conceptual model for safety incidents and promote their classification by a standardized taxonomy.810 Although this system has promise for allowing standardization, data aggregation, analysis, and learning between institutions,11 integration of the ICPS conceptual model into an SE decision support tool with summarizing and reporting features has not been reported.
This report describes our development of an intranet‐based SE reporting system, called Incident Tracker (I‐Tracker), based on the ICPS model. For our SE review groups from the 4 Providence Health Systems (PHS) Portland Service Area (PSA) hospitals, the I‐Tracker system offers a tool to guide efforts in developing RCAs and action plans in alignment with the ICPS framework. The system includes scripts that automatically generate and distribute standardized reports of individual and aggregated SEs. The objectives of this project were to report our experience with developing a flexible and accessible intranet‐based system that assists RCA participants in conforming to the ICPS framework and oversight safety staff in summarizing and reporting root cause analyses.
METHODS
The 4 PSA hospitals have 1083 licensed beds and perform SE reviews with a centralized process that reports results to a Community Governing Board. An ad hoc team for each SE performs the RCAs. The SE groups report RCAs and action plans in an unstructured format that varies for each event. A paper file is maintained for each SE report, but a system for aggregating reports to track trends, disseminating SE trends, or monitoring the completion or effectiveness of action plans is not available.
We designed a system to achieve the following objectives:
-
Apply the ICPS framework (Figure 1) and taxonomy of terms to SE analyses;
-
Provide a computer‐based tool to assist review groups and quality staff to perform their SE reviews and data collection in alignment with the ICPS framework;
-
Create an intranet‐based database that captures elements of the reviews, RCAs, and action plans with the use of drop‐down lists, help windows, windows with live access to Internet educational resources and tools, decision support tools, default entries, and audio prompts to streamline data entry;
-
Generate a suite of standardized reports customized for different audiences that can be accessed online and printed from the database with automated scripts;
-
Produce intranet‐based summaries of aggregated events to identify common causes and disseminate observed patterns and action plans to other PSA hospitals.
We selected FileMaker Pro 11 Advanced (FMP11) for authoring and maintaining the decision support tool and database, and FileMaker Pro Server 11 Advanced (FMPS11) (Filemaker, Inc, Santa Clara, CA) for hosting the system, because it provides intranet access and tools for updating the system by personnel with minimal programming experience. End users can view and enter data through layouts that display only the information allowed by the user's login password and access privileges, with external authentication by Active Directory and Open Directory technology. Staff who author and manage the database do so through client FMP11 software loaded on a computer that provides remote server access.
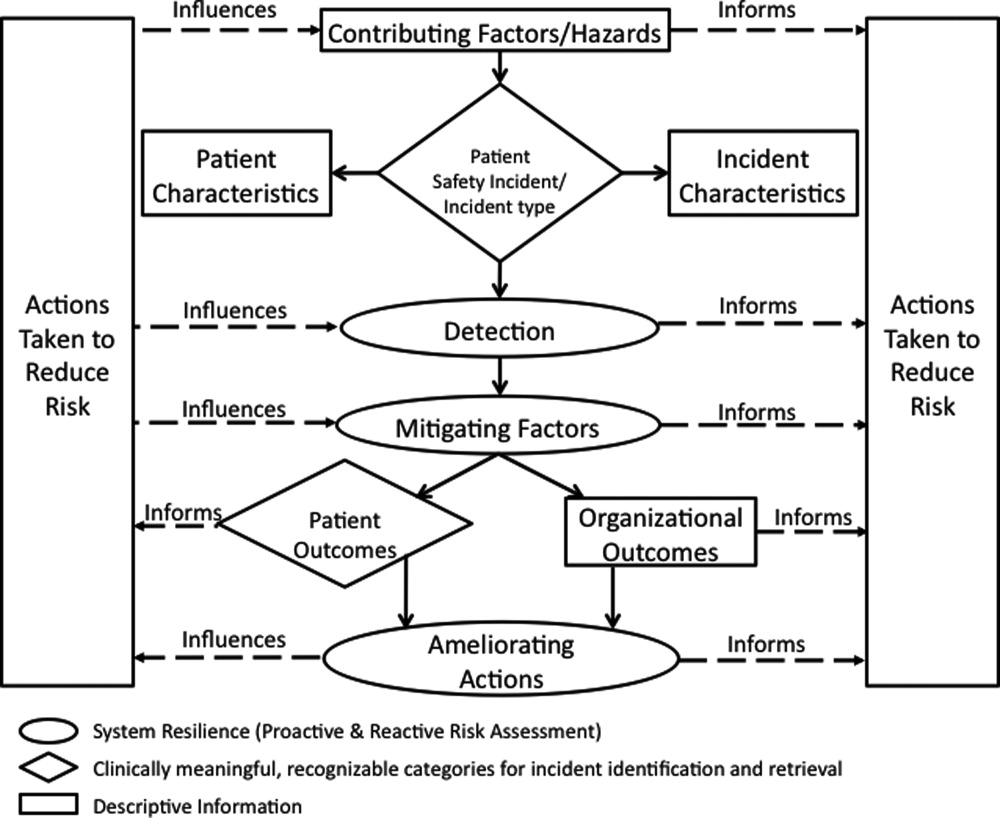
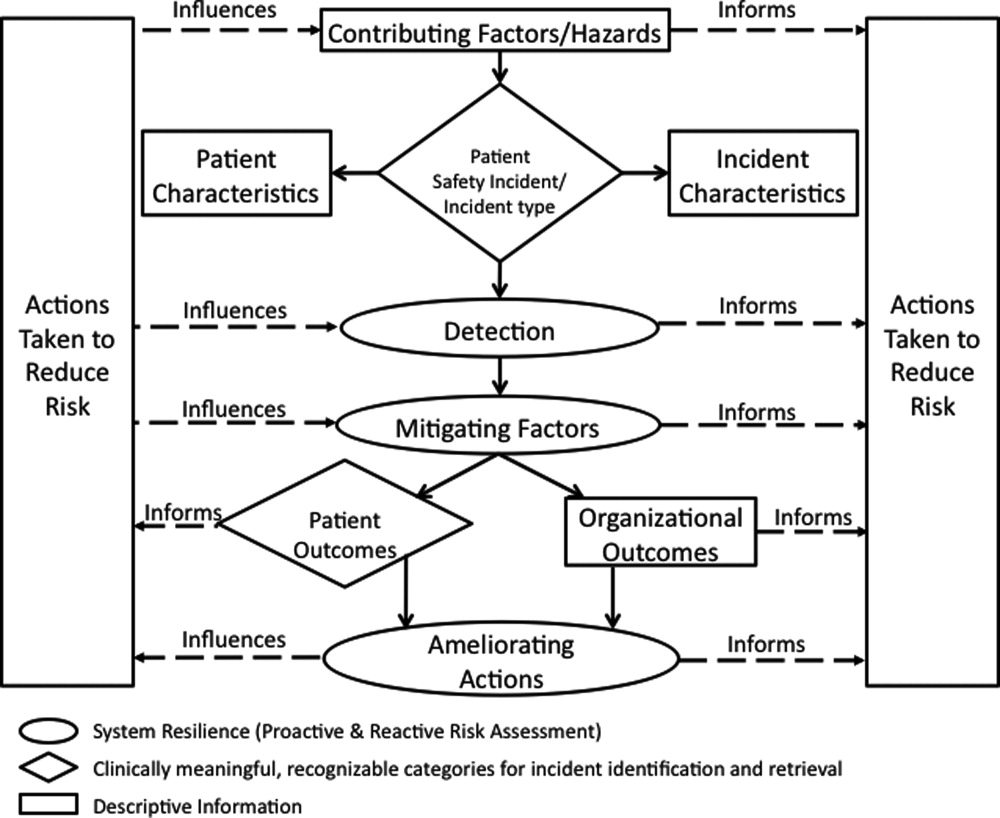
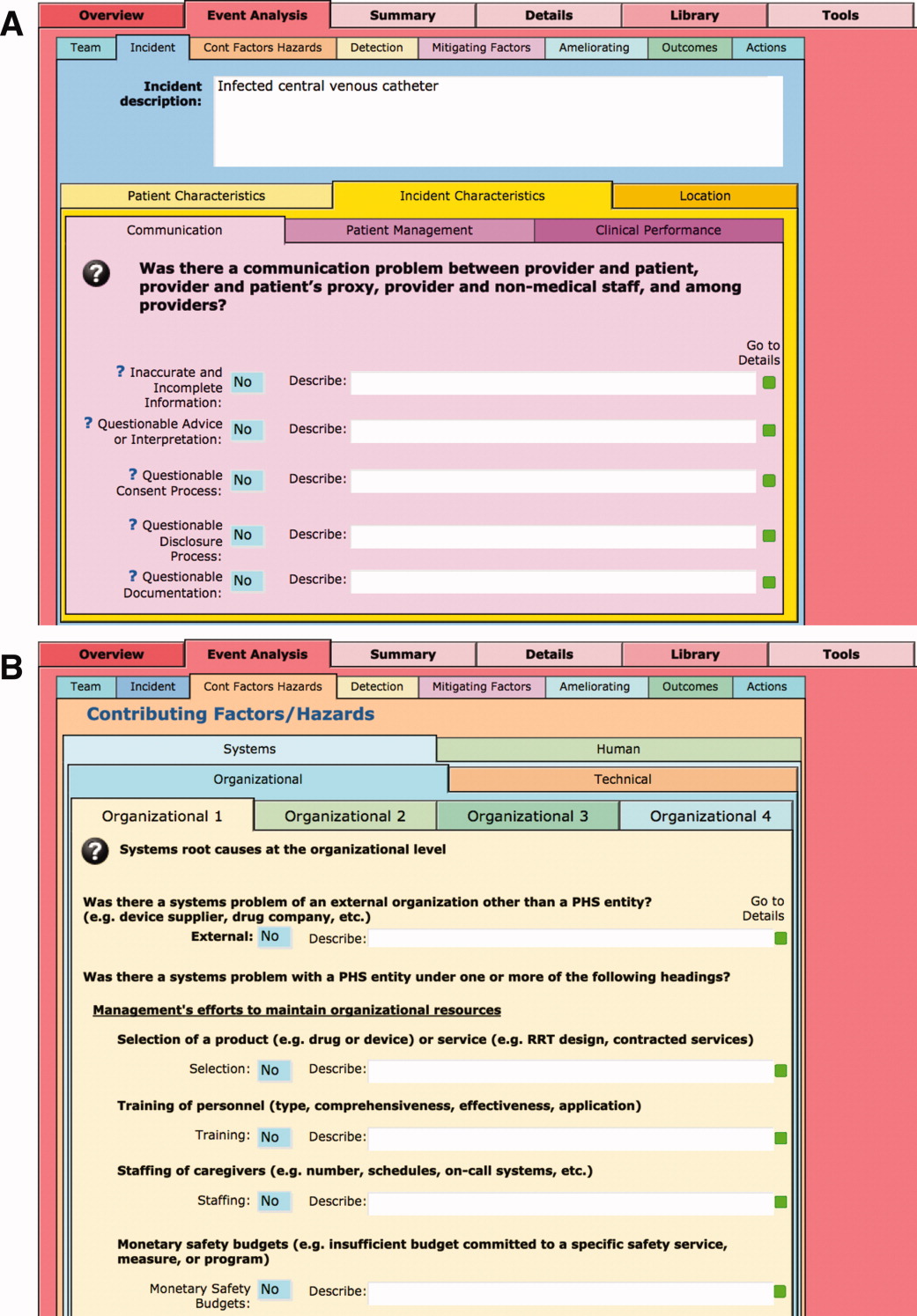
The I‐Tracker system was authored using the ICPS definitions for the 48 preferred terms for safety incidents and the ICPS conceptual framework.8 The conceptual framework consists of 10 major incident domains, that include incident type, patient outcomes, patient characteristics, incident characteristics, contributing factors and hazards, organizational outcomes, detection, mitigating factors, ameliorating actions, and actions taken to reduce risk (Figure 1).11 The framework is applicable to all hospital safety incidents, but we limited I‐Tracker to SEs because our hospitals had completed comprehensive reviews and action plans only for these more serious events. The literature on the ICPS framework812 was carefully reviewed to identify the specific data fields that were recommended by ICPS developers to be included under each of the 10 major classification domains. In most instances, data fields existed only in the body of these reports. Article texts, however, provided sufficient descriptions of these data fields to allow their translation into data entry fields in I‐Tracker with accompanying help windows and explanations to guide I‐Tracker users. Sixty ICPS data fields were programmed into I‐Tracker, with another 120 fields that allowed entry of descriptions and explanations of the ICPS data field entries. For instance, an entry of Yes into an ICPS data field that queried Was there a systems problem of an external organization other than a Providence entity opens a Describe field that allows a brief description of the problem, and an additional Details field that allows a longer explanation of the problem if necessary. The brief Describe field contents populate an automatically generated fishbone diagram.
The authors and quality staff translated the most recent 15 SE reviews into ICPS terms and classifications, and entered the results into I‐Tracker as it was being developed, to assist system design and programming of the system. The authors noted during data entry which of the 10 ICPS major domains had not been analyzed by the previous 15 reviews. Because existing reports were unstructured with considerable variation in style and usage of terms, the authors and quality staff made group decisions regarding how to cross‐walk existing information into the standardized ICPS data fields.
RESULTS
In developing I‐Tracker, the authors and quality staff observed that the ICPS framework and recommended data fields were logical and straightforward to learn. Although it was difficult to find the definitions of specific ICPS data fields within the 10 major domains in the text of retrieved articles, these fields could be readily cross‐walked into I‐Tracker data entry fields. Translating existing SE reports into I‐Tracker classifications, however, presented considerable challenges because of the unstructured, discursive, and variable nature of our SE review and reports. The authors and staff spent 1 to 2 hours conferring over each report to make judgments as to which elements of the review would be entered into which I‐Tracker data fields. Once the authors and staff translated existing reports into ICPS terms, actual data entry into I‐Tracker took typically less than 30 minutes for each review. We found that none of our 15 SE reviews included information on the following ICPS major domains: detection, mitigating factors, and ameliorating actions. We also observed that many ICPS data fields were not assessed, such as patient contributions to errors and external organization's contributions to a safety incident.
The latest version of I‐Tracker receives and displays information at the individual patient level. Records are shown onscreen with different screen layouts depending on the viewer's login security clearance. Hospital safety staff have full access to view and enter data on the initial layout, which displays patient demographic information and folder tabs that navigate when clicked to other database fields (Figure 2). Viewers with lower security clearance either view the same opening screen, but have limited access to other screens, or view a different opening screen designed to meet their specific needs. All screens provide definitions of terms and information to assist data entry, buttons that navigate to help pages, pop‐up windows that provide tips, and buttons that trigger brief audio explanations. Most fields use drop‐down lists to standardize data entry around the ICPS definitions, with default values entered into many fields to streamline data entry. A list view allows review of all patients and quick access to an individual patient's record. All fields and combinations of fields with Boolean rules are searchable within the database.
I‐Tracker has features that support SE review groups in beginning an SE review by providing them a paper form or electronic interface by way of a portable computer or tablet device, that guides their discussions and analyses toward providing conclusions that can be entered into the database fields, thereby aligning their deliberations with the ICPS conceptual framework. The same resource is available within the database online for those groups who would prefer to use computer prompts and enter data directly into the database as they proceed through their analyses. Some layouts contain windows that port live views from external Web sites (eg, JC RCA resources) that provide participants of RCA groups with tools to assist their work. FMP11 allows users to access the database by portable computers or handheld tablet devices using the hospitals' WiFi network.
A report screen allows automatic generation of different printouts of individual or aggregate summary reports. A Comprehensive Report includes all of the data fields included in the ICPS conceptual framework. Other reports present subsets of data depending on the user's needs and access privileges. The FMPS11 database allows printing the reports to paper or Portable Document Format (pdf), exporting data into an Excel spreadsheet, or e‐mailing reports to recipients from within I‐Tracker.
Additionally, I‐Tracker functionality facilitates follow‐up and monitoring of action items developed during the RCA process in a manner that conforms to the ICPS framework. We are now developing educational resources for RCA team members to investigate the implementation of I‐Tracker into future RCAs.
DISCUSSION
I‐Tracker provides an intranet‐based tool that met the objectives of the present project. The process of entering 15 existing SE reviews and action plans from our healthcare system into I‐Tracker allowed an incremental development of the database and identified gaps in our existing RCA process. For instance, none of the previous RCAs critically appraised detection, mitigating factors, or ameliorating actions; defined the specific nature or quantified severity of patient injuries using standardized terms; distinguished between human errors and negligence; or comprehensively reported the full spectrum of underlying causes of Tracker's use of standardized terms based on the ICPS conceptual framework provided a potential resource for focusing SE reviews and producing more comprehensive RCAs and action plans in the future. I‐Tracker has additional potential to facilitate dissemination of RCAs to other facilities, both as individual incident reports and aggregated summaries as recommended by experts in patient safety.13
The deficiencies in our existing RCA analyses, identified during data entry into I‐Tracker, represent common shortcomings experienced by other healthcare organizations and summarized in a report by the Agency for Healthcare Research and Quality.4 Considerable hindsight bias and prevailing concerns of the day taint the RCA process, which is time‐consuming and labor intensive, and thereby hinders comprehensive reviews. Also, our SE reviews, like others reviewed in the literature,14 focused on biologic injury to patients and omitted assessment of psychologic, organizational, social, and economic injury domains. Although SE review teams benefit from involvement of quality improvement staff who are trained in techniques and goals of RCA,15 many hospitals like ours have limited resources for fully staffing all SE reviews with trained facilitators. These SE reviews generate both quantitative and qualitative data, the latter of which hinders standardized data entry in the absence of a conceptual framework. A structured database with formative tools to guide RCAs in conformance with the ICPS framework in organizations without sufficient numbers of trained facilitators offers opportunities to produce more comprehensive, standardized, and actionable reports. To date, our quality staff and leadership have responded positively to presentations of the functional features of I‐Tracker (Table 1).
|
| Online availability of the system that allows access both from client database software loaded on Quality Office computers and through intranet browser software (Explorer, Safari, Firefox, etc) |
| Security features of encrypted software that allow full or limited views depending on the user's password security clearance and purpose for reviewing data |
| Software accessibility in authoring and managing the database, which do not require support from information technology data analysts |
| Decision support tools provided in the system to assist RCA analysis |
| System flexibility that allows scripted reporting of single SEs or multiple SE summaries within any selected timeframe |
Limitations of our report include its focus solely on the development and programming phase of I‐Tracker and the absence of information on its actual implementation. We believe, however, the development phase is important to report because it demonstrates that the ICPS framework and specific ICPS data fields are amenable to incorporation into a decision support and reporting tool, which to our knowledge has not been previously reported. We begin implementation of I‐Tracker within our organization this year and will have observations on its feasibility, acceptability, and staff training needs. As an additional limitation, we emphasize that we do not propose I‐Tracker as a solution for other organizations, because we have no plans for its commercial or public domain development. This report is intended to demonstrate, however, that commercially available software, such as FileMaker, can readily support the ICPS Framework and thereby has potential to assist RCAs and SE reporting. Other organizations may develop similar systems on other database platforms that incorporate the ICPS system into their reviews.
To implement I‐Tracker, we are now working with nursing and pharmacy leadership focus groups to develop formative tools, data collection forms, and other resources to assist their RCA efforts and data entry into the database. We also plan to apply the database tool to our residency training program to promote resident involvement in SE reviews by providing standardized, reproducible, and structured processes.16 Our 5‐state healthcare system has funded an evaluation of the implementation phase of I‐Tracker to other Providence facilities. Because the ICPS framework applies to all safety incidents beyond SEs (Table 2), a successful implementation of I‐Tracker for SEs will allow its eventual application to other types of critical incidents.
|
| Clinical administration |
| Clinical process/procedure |
| Documentation |
| Healthcare‐associated infection |
| Medication/IV fluids |
| Blood/blood products |
| Nutrition |
| Oxygen/gas/vapor |
| Medical device/equipment |
| Behavior |
| Patient accidents |
| Infrastructure/building/fixtures |
| Resources/organizational management |
The strength of this project derives from its innovative development of an intranet‐based tool that allows groups to conform their RCAs to the ICPS framework. Because the absence of a standardized classification for patient safety concepts has hindered advances in patient safety,11 we believe I‐Tracker, or decision support tools like it that use the ICPS framework, can standardize RCAs and promote dissemination and adoption of action plans.
Acknowledgements
We appreciate the support of Judy Stenstrom, Lynette Savage, and the Portland Service Area Quality Improvement Office.
- .Reporting of adverse events.N Engl J Med.2002;347:1633–1638.
- The Joint Commission's Sentinel Event Policy: ten years of improving the quality and safety of health care.Jt Comm Perspect.2005;25(1):3–5.
- ,.Root cause analysis and nursing management responsibilities in wrong‐site surgery.Dimens Crit Care Nurs.2006;25,221–225.
- ,.Root Cause Analysis.Making Health Care Safer. Available at: http://archive.ahrq.gov/clinic/ptsafety/chap5.htm. Accessed May 21,2010.
- Oversight group holds RCA teams accountable.Healthcare Benchmarks Qual Improv.2008;15:117–118.
- .Shared meanings: preferred terms and definitions for safety and quality concepts.Med J Aust.2006;184:S41–S43.
- ,,.What do family physicians consider an error? A comparison of definitions and physician perception.BMC Fam Pract.2006;7:73.
- ,,,,,.Towards an International Classification for Patient Safety: key concepts and terms.Int J Qual Health Care.2009;21:18–26.
- ,,,,.The JCAHO patient safety event taxonomy: a standardized terminology and classification schema for near misses and adverse events.Int J Qual Health Care.2005;17:95–105.
- World Health Organization. 2009 Conceptual Framework for the International Classification for Patient Safety. Final Technical Report Version 1.1. Available at: http://www.who.int/patientsafety/taxonomy/icps_full_report.pdf. Accessed April 25,2011.
- ,,, et al.Towards an International Classification for Patient Safety: the conceptual framework.Int J Qual Health Care.2009;21:2–8.
- ,,,,,.Towards an International Classification for Patient Safety: a Delphi survey.Int J Qual Health Care.2009;21:9–17.
- ,,.Effectiveness and efficiency of root cause analysis in medicine.JAMA.2008;299:685–687.
- ,,,,.How can clinicians measure safety and quality in acute care?Lancet.2004;363:1061–1067.
- ,,,,.Systematic root cause analysis of adverse drug events in a tertiary referral hospital.Jt Comm J Qual Improv.2000;26:563–575.
- ,,,,,.Educational quality improvement report: outcomes from a revised morbidity and mortality format that emphasised patient safety.Postgrad Med J.2008;84:211–216.
Recognition that healthcare carries considerable risks of patient injury has focused efforts on identifying problems before they occur, and understanding the root causes of those problems that do occur to prevent them from happening again.1 To further these efforts, a Joint Commission (JC) standard requires hospitals to review sentinel events (SE).2 Reviews must develop a timely, thorough, and credible root cause analysis (RCA), implement action plans to reduce risk, and monitor the effectiveness of implemented improvements.3
Ideally, hospitals would summarize their experiences with SE reviews, identify high‐risk activities and patients, institute system changes to prevent SE recurrences, and share their findings with other healthcare organizations to help them avoid similar patient injuries.1 In support of this last goal, the JC maintains a voluntary database system that allows hospitals to report their SE analyses for other facilities to review and institute preventative actions.
Unfortunately, the reality of SE reviews does not match their ideals for improving patient safety.4 Healthcare organizations often describe their review process as less than credible and note a need for ongoing oversight to maintain the reviews' effectiveness.5 The JC voluntary reporting system captures less than 1% of the SEs that occur nationally,2 because hospitals perceive barriers to external reporting.1 If healthcare organizations decide against reporting externally, they can create their own internal systems to aggregate and summarize SEs, but few such systems exist. A major impediment to designing internal systems is the absence of universally endorsed nomenclature for safety‐related events.6, 7 Poorly aligned terminology and subjective conceptualizations for safety incidents impede the aggregation of SEs, comparisons between facilities, and trend analyses for tracking SE patterns.
In 2005, the World Health Organization (WHO) World Alliance for Patient Safety, in collaboration with the JC, began developing an International Classification for Patient Safety (ICPS) to provide healthcare organizations a consistent conceptual model for safety incidents and promote their classification by a standardized taxonomy.810 Although this system has promise for allowing standardization, data aggregation, analysis, and learning between institutions,11 integration of the ICPS conceptual model into an SE decision support tool with summarizing and reporting features has not been reported.
This report describes our development of an intranet‐based SE reporting system, called Incident Tracker (I‐Tracker), based on the ICPS model. For our SE review groups from the 4 Providence Health Systems (PHS) Portland Service Area (PSA) hospitals, the I‐Tracker system offers a tool to guide efforts in developing RCAs and action plans in alignment with the ICPS framework. The system includes scripts that automatically generate and distribute standardized reports of individual and aggregated SEs. The objectives of this project were to report our experience with developing a flexible and accessible intranet‐based system that assists RCA participants in conforming to the ICPS framework and oversight safety staff in summarizing and reporting root cause analyses.
METHODS
The 4 PSA hospitals have 1083 licensed beds and perform SE reviews with a centralized process that reports results to a Community Governing Board. An ad hoc team for each SE performs the RCAs. The SE groups report RCAs and action plans in an unstructured format that varies for each event. A paper file is maintained for each SE report, but a system for aggregating reports to track trends, disseminating SE trends, or monitoring the completion or effectiveness of action plans is not available.
We designed a system to achieve the following objectives:
-
Apply the ICPS framework (Figure 1) and taxonomy of terms to SE analyses;
-
Provide a computer‐based tool to assist review groups and quality staff to perform their SE reviews and data collection in alignment with the ICPS framework;
-
Create an intranet‐based database that captures elements of the reviews, RCAs, and action plans with the use of drop‐down lists, help windows, windows with live access to Internet educational resources and tools, decision support tools, default entries, and audio prompts to streamline data entry;
-
Generate a suite of standardized reports customized for different audiences that can be accessed online and printed from the database with automated scripts;
-
Produce intranet‐based summaries of aggregated events to identify common causes and disseminate observed patterns and action plans to other PSA hospitals.

We selected FileMaker Pro 11 Advanced (FMP11) for authoring and maintaining the decision support tool and database, and FileMaker Pro Server 11 Advanced (FMPS11) (Filemaker, Inc, Santa Clara, CA) for hosting the system, because it provides intranet access and tools for updating the system by personnel with minimal programming experience. End users can view and enter data through layouts that display only the information allowed by the user's login password and access privileges, with external authentication by Active Directory and Open Directory technology. Staff who author and manage the database do so through client FMP11 software loaded on a computer that provides remote server access.
The I‐Tracker system was authored using the ICPS definitions for the 48 preferred terms for safety incidents and the ICPS conceptual framework.8 The conceptual framework consists of 10 major incident domains, that include incident type, patient outcomes, patient characteristics, incident characteristics, contributing factors and hazards, organizational outcomes, detection, mitigating factors, ameliorating actions, and actions taken to reduce risk (Figure 1).11 The framework is applicable to all hospital safety incidents, but we limited I‐Tracker to SEs because our hospitals had completed comprehensive reviews and action plans only for these more serious events. The literature on the ICPS framework812 was carefully reviewed to identify the specific data fields that were recommended by ICPS developers to be included under each of the 10 major classification domains. In most instances, data fields existed only in the body of these reports. Article texts, however, provided sufficient descriptions of these data fields to allow their translation into data entry fields in I‐Tracker with accompanying help windows and explanations to guide I‐Tracker users. Sixty ICPS data fields were programmed into I‐Tracker, with another 120 fields that allowed entry of descriptions and explanations of the ICPS data field entries. For instance, an entry of Yes into an ICPS data field that queried Was there a systems problem of an external organization other than a Providence entity opens a Describe field that allows a brief description of the problem, and an additional Details field that allows a longer explanation of the problem if necessary. The brief Describe field contents populate an automatically generated fishbone diagram.
The authors and quality staff translated the most recent 15 SE reviews into ICPS terms and classifications, and entered the results into I‐Tracker as it was being developed, to assist system design and programming of the system. The authors noted during data entry which of the 10 ICPS major domains had not been analyzed by the previous 15 reviews. Because existing reports were unstructured with considerable variation in style and usage of terms, the authors and quality staff made group decisions regarding how to cross‐walk existing information into the standardized ICPS data fields.
RESULTS
In developing I‐Tracker, the authors and quality staff observed that the ICPS framework and recommended data fields were logical and straightforward to learn. Although it was difficult to find the definitions of specific ICPS data fields within the 10 major domains in the text of retrieved articles, these fields could be readily cross‐walked into I‐Tracker data entry fields. Translating existing SE reports into I‐Tracker classifications, however, presented considerable challenges because of the unstructured, discursive, and variable nature of our SE review and reports. The authors and staff spent 1 to 2 hours conferring over each report to make judgments as to which elements of the review would be entered into which I‐Tracker data fields. Once the authors and staff translated existing reports into ICPS terms, actual data entry into I‐Tracker took typically less than 30 minutes for each review. We found that none of our 15 SE reviews included information on the following ICPS major domains: detection, mitigating factors, and ameliorating actions. We also observed that many ICPS data fields were not assessed, such as patient contributions to errors and external organization's contributions to a safety incident.
The latest version of I‐Tracker receives and displays information at the individual patient level. Records are shown onscreen with different screen layouts depending on the viewer's login security clearance. Hospital safety staff have full access to view and enter data on the initial layout, which displays patient demographic information and folder tabs that navigate when clicked to other database fields (Figure 2). Viewers with lower security clearance either view the same opening screen, but have limited access to other screens, or view a different opening screen designed to meet their specific needs. All screens provide definitions of terms and information to assist data entry, buttons that navigate to help pages, pop‐up windows that provide tips, and buttons that trigger brief audio explanations. Most fields use drop‐down lists to standardize data entry around the ICPS definitions, with default values entered into many fields to streamline data entry. A list view allows review of all patients and quick access to an individual patient's record. All fields and combinations of fields with Boolean rules are searchable within the database.

I‐Tracker has features that support SE review groups in beginning an SE review by providing them a paper form or electronic interface by way of a portable computer or tablet device, that guides their discussions and analyses toward providing conclusions that can be entered into the database fields, thereby aligning their deliberations with the ICPS conceptual framework. The same resource is available within the database online for those groups who would prefer to use computer prompts and enter data directly into the database as they proceed through their analyses. Some layouts contain windows that port live views from external Web sites (eg, JC RCA resources) that provide participants of RCA groups with tools to assist their work. FMP11 allows users to access the database by portable computers or handheld tablet devices using the hospitals' WiFi network.
A report screen allows automatic generation of different printouts of individual or aggregate summary reports. A Comprehensive Report includes all of the data fields included in the ICPS conceptual framework. Other reports present subsets of data depending on the user's needs and access privileges. The FMPS11 database allows printing the reports to paper or Portable Document Format (pdf), exporting data into an Excel spreadsheet, or e‐mailing reports to recipients from within I‐Tracker.
Additionally, I‐Tracker functionality facilitates follow‐up and monitoring of action items developed during the RCA process in a manner that conforms to the ICPS framework. We are now developing educational resources for RCA team members to investigate the implementation of I‐Tracker into future RCAs.
DISCUSSION
I‐Tracker provides an intranet‐based tool that met the objectives of the present project. The process of entering 15 existing SE reviews and action plans from our healthcare system into I‐Tracker allowed an incremental development of the database and identified gaps in our existing RCA process. For instance, none of the previous RCAs critically appraised detection, mitigating factors, or ameliorating actions; defined the specific nature or quantified severity of patient injuries using standardized terms; distinguished between human errors and negligence; or comprehensively reported the full spectrum of underlying causes of Tracker's use of standardized terms based on the ICPS conceptual framework provided a potential resource for focusing SE reviews and producing more comprehensive RCAs and action plans in the future. I‐Tracker has additional potential to facilitate dissemination of RCAs to other facilities, both as individual incident reports and aggregated summaries as recommended by experts in patient safety.13
The deficiencies in our existing RCA analyses, identified during data entry into I‐Tracker, represent common shortcomings experienced by other healthcare organizations and summarized in a report by the Agency for Healthcare Research and Quality.4 Considerable hindsight bias and prevailing concerns of the day taint the RCA process, which is time‐consuming and labor intensive, and thereby hinders comprehensive reviews. Also, our SE reviews, like others reviewed in the literature,14 focused on biologic injury to patients and omitted assessment of psychologic, organizational, social, and economic injury domains. Although SE review teams benefit from involvement of quality improvement staff who are trained in techniques and goals of RCA,15 many hospitals like ours have limited resources for fully staffing all SE reviews with trained facilitators. These SE reviews generate both quantitative and qualitative data, the latter of which hinders standardized data entry in the absence of a conceptual framework. A structured database with formative tools to guide RCAs in conformance with the ICPS framework in organizations without sufficient numbers of trained facilitators offers opportunities to produce more comprehensive, standardized, and actionable reports. To date, our quality staff and leadership have responded positively to presentations of the functional features of I‐Tracker (Table 1).
|
| Online availability of the system that allows access both from client database software loaded on Quality Office computers and through intranet browser software (Explorer, Safari, Firefox, etc) |
| Security features of encrypted software that allow full or limited views depending on the user's password security clearance and purpose for reviewing data |
| Software accessibility in authoring and managing the database, which do not require support from information technology data analysts |
| Decision support tools provided in the system to assist RCA analysis |
| System flexibility that allows scripted reporting of single SEs or multiple SE summaries within any selected timeframe |
Limitations of our report include its focus solely on the development and programming phase of I‐Tracker and the absence of information on its actual implementation. We believe, however, the development phase is important to report because it demonstrates that the ICPS framework and specific ICPS data fields are amenable to incorporation into a decision support and reporting tool, which to our knowledge has not been previously reported. We begin implementation of I‐Tracker within our organization this year and will have observations on its feasibility, acceptability, and staff training needs. As an additional limitation, we emphasize that we do not propose I‐Tracker as a solution for other organizations, because we have no plans for its commercial or public domain development. This report is intended to demonstrate, however, that commercially available software, such as FileMaker, can readily support the ICPS Framework and thereby has potential to assist RCAs and SE reporting. Other organizations may develop similar systems on other database platforms that incorporate the ICPS system into their reviews.
To implement I‐Tracker, we are now working with nursing and pharmacy leadership focus groups to develop formative tools, data collection forms, and other resources to assist their RCA efforts and data entry into the database. We also plan to apply the database tool to our residency training program to promote resident involvement in SE reviews by providing standardized, reproducible, and structured processes.16 Our 5‐state healthcare system has funded an evaluation of the implementation phase of I‐Tracker to other Providence facilities. Because the ICPS framework applies to all safety incidents beyond SEs (Table 2), a successful implementation of I‐Tracker for SEs will allow its eventual application to other types of critical incidents.
|
| Clinical administration |
| Clinical process/procedure |
| Documentation |
| Healthcare‐associated infection |
| Medication/IV fluids |
| Blood/blood products |
| Nutrition |
| Oxygen/gas/vapor |
| Medical device/equipment |
| Behavior |
| Patient accidents |
| Infrastructure/building/fixtures |
| Resources/organizational management |
The strength of this project derives from its innovative development of an intranet‐based tool that allows groups to conform their RCAs to the ICPS framework. Because the absence of a standardized classification for patient safety concepts has hindered advances in patient safety,11 we believe I‐Tracker, or decision support tools like it that use the ICPS framework, can standardize RCAs and promote dissemination and adoption of action plans.
Acknowledgements
We appreciate the support of Judy Stenstrom, Lynette Savage, and the Portland Service Area Quality Improvement Office.
Recognition that healthcare carries considerable risks of patient injury has focused efforts on identifying problems before they occur, and understanding the root causes of those problems that do occur to prevent them from happening again.1 To further these efforts, a Joint Commission (JC) standard requires hospitals to review sentinel events (SE).2 Reviews must develop a timely, thorough, and credible root cause analysis (RCA), implement action plans to reduce risk, and monitor the effectiveness of implemented improvements.3
Ideally, hospitals would summarize their experiences with SE reviews, identify high‐risk activities and patients, institute system changes to prevent SE recurrences, and share their findings with other healthcare organizations to help them avoid similar patient injuries.1 In support of this last goal, the JC maintains a voluntary database system that allows hospitals to report their SE analyses for other facilities to review and institute preventative actions.
Unfortunately, the reality of SE reviews does not match their ideals for improving patient safety.4 Healthcare organizations often describe their review process as less than credible and note a need for ongoing oversight to maintain the reviews' effectiveness.5 The JC voluntary reporting system captures less than 1% of the SEs that occur nationally,2 because hospitals perceive barriers to external reporting.1 If healthcare organizations decide against reporting externally, they can create their own internal systems to aggregate and summarize SEs, but few such systems exist. A major impediment to designing internal systems is the absence of universally endorsed nomenclature for safety‐related events.6, 7 Poorly aligned terminology and subjective conceptualizations for safety incidents impede the aggregation of SEs, comparisons between facilities, and trend analyses for tracking SE patterns.
In 2005, the World Health Organization (WHO) World Alliance for Patient Safety, in collaboration with the JC, began developing an International Classification for Patient Safety (ICPS) to provide healthcare organizations a consistent conceptual model for safety incidents and promote their classification by a standardized taxonomy.810 Although this system has promise for allowing standardization, data aggregation, analysis, and learning between institutions,11 integration of the ICPS conceptual model into an SE decision support tool with summarizing and reporting features has not been reported.
This report describes our development of an intranet‐based SE reporting system, called Incident Tracker (I‐Tracker), based on the ICPS model. For our SE review groups from the 4 Providence Health Systems (PHS) Portland Service Area (PSA) hospitals, the I‐Tracker system offers a tool to guide efforts in developing RCAs and action plans in alignment with the ICPS framework. The system includes scripts that automatically generate and distribute standardized reports of individual and aggregated SEs. The objectives of this project were to report our experience with developing a flexible and accessible intranet‐based system that assists RCA participants in conforming to the ICPS framework and oversight safety staff in summarizing and reporting root cause analyses.
METHODS
The 4 PSA hospitals have 1083 licensed beds and perform SE reviews with a centralized process that reports results to a Community Governing Board. An ad hoc team for each SE performs the RCAs. The SE groups report RCAs and action plans in an unstructured format that varies for each event. A paper file is maintained for each SE report, but a system for aggregating reports to track trends, disseminating SE trends, or monitoring the completion or effectiveness of action plans is not available.
We designed a system to achieve the following objectives:
-
Apply the ICPS framework (Figure 1) and taxonomy of terms to SE analyses;
-
Provide a computer‐based tool to assist review groups and quality staff to perform their SE reviews and data collection in alignment with the ICPS framework;
-
Create an intranet‐based database that captures elements of the reviews, RCAs, and action plans with the use of drop‐down lists, help windows, windows with live access to Internet educational resources and tools, decision support tools, default entries, and audio prompts to streamline data entry;
-
Generate a suite of standardized reports customized for different audiences that can be accessed online and printed from the database with automated scripts;
-
Produce intranet‐based summaries of aggregated events to identify common causes and disseminate observed patterns and action plans to other PSA hospitals.

We selected FileMaker Pro 11 Advanced (FMP11) for authoring and maintaining the decision support tool and database, and FileMaker Pro Server 11 Advanced (FMPS11) (Filemaker, Inc, Santa Clara, CA) for hosting the system, because it provides intranet access and tools for updating the system by personnel with minimal programming experience. End users can view and enter data through layouts that display only the information allowed by the user's login password and access privileges, with external authentication by Active Directory and Open Directory technology. Staff who author and manage the database do so through client FMP11 software loaded on a computer that provides remote server access.
The I‐Tracker system was authored using the ICPS definitions for the 48 preferred terms for safety incidents and the ICPS conceptual framework.8 The conceptual framework consists of 10 major incident domains, that include incident type, patient outcomes, patient characteristics, incident characteristics, contributing factors and hazards, organizational outcomes, detection, mitigating factors, ameliorating actions, and actions taken to reduce risk (Figure 1).11 The framework is applicable to all hospital safety incidents, but we limited I‐Tracker to SEs because our hospitals had completed comprehensive reviews and action plans only for these more serious events. The literature on the ICPS framework812 was carefully reviewed to identify the specific data fields that were recommended by ICPS developers to be included under each of the 10 major classification domains. In most instances, data fields existed only in the body of these reports. Article texts, however, provided sufficient descriptions of these data fields to allow their translation into data entry fields in I‐Tracker with accompanying help windows and explanations to guide I‐Tracker users. Sixty ICPS data fields were programmed into I‐Tracker, with another 120 fields that allowed entry of descriptions and explanations of the ICPS data field entries. For instance, an entry of Yes into an ICPS data field that queried Was there a systems problem of an external organization other than a Providence entity opens a Describe field that allows a brief description of the problem, and an additional Details field that allows a longer explanation of the problem if necessary. The brief Describe field contents populate an automatically generated fishbone diagram.
The authors and quality staff translated the most recent 15 SE reviews into ICPS terms and classifications, and entered the results into I‐Tracker as it was being developed, to assist system design and programming of the system. The authors noted during data entry which of the 10 ICPS major domains had not been analyzed by the previous 15 reviews. Because existing reports were unstructured with considerable variation in style and usage of terms, the authors and quality staff made group decisions regarding how to cross‐walk existing information into the standardized ICPS data fields.
RESULTS
In developing I‐Tracker, the authors and quality staff observed that the ICPS framework and recommended data fields were logical and straightforward to learn. Although it was difficult to find the definitions of specific ICPS data fields within the 10 major domains in the text of retrieved articles, these fields could be readily cross‐walked into I‐Tracker data entry fields. Translating existing SE reports into I‐Tracker classifications, however, presented considerable challenges because of the unstructured, discursive, and variable nature of our SE review and reports. The authors and staff spent 1 to 2 hours conferring over each report to make judgments as to which elements of the review would be entered into which I‐Tracker data fields. Once the authors and staff translated existing reports into ICPS terms, actual data entry into I‐Tracker took typically less than 30 minutes for each review. We found that none of our 15 SE reviews included information on the following ICPS major domains: detection, mitigating factors, and ameliorating actions. We also observed that many ICPS data fields were not assessed, such as patient contributions to errors and external organization's contributions to a safety incident.
The latest version of I‐Tracker receives and displays information at the individual patient level. Records are shown onscreen with different screen layouts depending on the viewer's login security clearance. Hospital safety staff have full access to view and enter data on the initial layout, which displays patient demographic information and folder tabs that navigate when clicked to other database fields (Figure 2). Viewers with lower security clearance either view the same opening screen, but have limited access to other screens, or view a different opening screen designed to meet their specific needs. All screens provide definitions of terms and information to assist data entry, buttons that navigate to help pages, pop‐up windows that provide tips, and buttons that trigger brief audio explanations. Most fields use drop‐down lists to standardize data entry around the ICPS definitions, with default values entered into many fields to streamline data entry. A list view allows review of all patients and quick access to an individual patient's record. All fields and combinations of fields with Boolean rules are searchable within the database.

I‐Tracker has features that support SE review groups in beginning an SE review by providing them a paper form or electronic interface by way of a portable computer or tablet device, that guides their discussions and analyses toward providing conclusions that can be entered into the database fields, thereby aligning their deliberations with the ICPS conceptual framework. The same resource is available within the database online for those groups who would prefer to use computer prompts and enter data directly into the database as they proceed through their analyses. Some layouts contain windows that port live views from external Web sites (eg, JC RCA resources) that provide participants of RCA groups with tools to assist their work. FMP11 allows users to access the database by portable computers or handheld tablet devices using the hospitals' WiFi network.
A report screen allows automatic generation of different printouts of individual or aggregate summary reports. A Comprehensive Report includes all of the data fields included in the ICPS conceptual framework. Other reports present subsets of data depending on the user's needs and access privileges. The FMPS11 database allows printing the reports to paper or Portable Document Format (pdf), exporting data into an Excel spreadsheet, or e‐mailing reports to recipients from within I‐Tracker.
Additionally, I‐Tracker functionality facilitates follow‐up and monitoring of action items developed during the RCA process in a manner that conforms to the ICPS framework. We are now developing educational resources for RCA team members to investigate the implementation of I‐Tracker into future RCAs.
DISCUSSION
I‐Tracker provides an intranet‐based tool that met the objectives of the present project. The process of entering 15 existing SE reviews and action plans from our healthcare system into I‐Tracker allowed an incremental development of the database and identified gaps in our existing RCA process. For instance, none of the previous RCAs critically appraised detection, mitigating factors, or ameliorating actions; defined the specific nature or quantified severity of patient injuries using standardized terms; distinguished between human errors and negligence; or comprehensively reported the full spectrum of underlying causes of Tracker's use of standardized terms based on the ICPS conceptual framework provided a potential resource for focusing SE reviews and producing more comprehensive RCAs and action plans in the future. I‐Tracker has additional potential to facilitate dissemination of RCAs to other facilities, both as individual incident reports and aggregated summaries as recommended by experts in patient safety.13
The deficiencies in our existing RCA analyses, identified during data entry into I‐Tracker, represent common shortcomings experienced by other healthcare organizations and summarized in a report by the Agency for Healthcare Research and Quality.4 Considerable hindsight bias and prevailing concerns of the day taint the RCA process, which is time‐consuming and labor intensive, and thereby hinders comprehensive reviews. Also, our SE reviews, like others reviewed in the literature,14 focused on biologic injury to patients and omitted assessment of psychologic, organizational, social, and economic injury domains. Although SE review teams benefit from involvement of quality improvement staff who are trained in techniques and goals of RCA,15 many hospitals like ours have limited resources for fully staffing all SE reviews with trained facilitators. These SE reviews generate both quantitative and qualitative data, the latter of which hinders standardized data entry in the absence of a conceptual framework. A structured database with formative tools to guide RCAs in conformance with the ICPS framework in organizations without sufficient numbers of trained facilitators offers opportunities to produce more comprehensive, standardized, and actionable reports. To date, our quality staff and leadership have responded positively to presentations of the functional features of I‐Tracker (Table 1).
|
| Online availability of the system that allows access both from client database software loaded on Quality Office computers and through intranet browser software (Explorer, Safari, Firefox, etc) |
| Security features of encrypted software that allow full or limited views depending on the user's password security clearance and purpose for reviewing data |
| Software accessibility in authoring and managing the database, which do not require support from information technology data analysts |
| Decision support tools provided in the system to assist RCA analysis |
| System flexibility that allows scripted reporting of single SEs or multiple SE summaries within any selected timeframe |
Limitations of our report include its focus solely on the development and programming phase of I‐Tracker and the absence of information on its actual implementation. We believe, however, the development phase is important to report because it demonstrates that the ICPS framework and specific ICPS data fields are amenable to incorporation into a decision support and reporting tool, which to our knowledge has not been previously reported. We begin implementation of I‐Tracker within our organization this year and will have observations on its feasibility, acceptability, and staff training needs. As an additional limitation, we emphasize that we do not propose I‐Tracker as a solution for other organizations, because we have no plans for its commercial or public domain development. This report is intended to demonstrate, however, that commercially available software, such as FileMaker, can readily support the ICPS Framework and thereby has potential to assist RCAs and SE reporting. Other organizations may develop similar systems on other database platforms that incorporate the ICPS system into their reviews.
To implement I‐Tracker, we are now working with nursing and pharmacy leadership focus groups to develop formative tools, data collection forms, and other resources to assist their RCA efforts and data entry into the database. We also plan to apply the database tool to our residency training program to promote resident involvement in SE reviews by providing standardized, reproducible, and structured processes.16 Our 5‐state healthcare system has funded an evaluation of the implementation phase of I‐Tracker to other Providence facilities. Because the ICPS framework applies to all safety incidents beyond SEs (Table 2), a successful implementation of I‐Tracker for SEs will allow its eventual application to other types of critical incidents.
|
| Clinical administration |
| Clinical process/procedure |
| Documentation |
| Healthcare‐associated infection |
| Medication/IV fluids |
| Blood/blood products |
| Nutrition |
| Oxygen/gas/vapor |
| Medical device/equipment |
| Behavior |
| Patient accidents |
| Infrastructure/building/fixtures |
| Resources/organizational management |
The strength of this project derives from its innovative development of an intranet‐based tool that allows groups to conform their RCAs to the ICPS framework. Because the absence of a standardized classification for patient safety concepts has hindered advances in patient safety,11 we believe I‐Tracker, or decision support tools like it that use the ICPS framework, can standardize RCAs and promote dissemination and adoption of action plans.
Acknowledgements
We appreciate the support of Judy Stenstrom, Lynette Savage, and the Portland Service Area Quality Improvement Office.
- .Reporting of adverse events.N Engl J Med.2002;347:1633–1638.
- The Joint Commission's Sentinel Event Policy: ten years of improving the quality and safety of health care.Jt Comm Perspect.2005;25(1):3–5.
- ,.Root cause analysis and nursing management responsibilities in wrong‐site surgery.Dimens Crit Care Nurs.2006;25,221–225.
- ,.Root Cause Analysis.Making Health Care Safer. Available at: http://archive.ahrq.gov/clinic/ptsafety/chap5.htm. Accessed May 21,2010.
- Oversight group holds RCA teams accountable.Healthcare Benchmarks Qual Improv.2008;15:117–118.
- .Shared meanings: preferred terms and definitions for safety and quality concepts.Med J Aust.2006;184:S41–S43.
- ,,.What do family physicians consider an error? A comparison of definitions and physician perception.BMC Fam Pract.2006;7:73.
- ,,,,,.Towards an International Classification for Patient Safety: key concepts and terms.Int J Qual Health Care.2009;21:18–26.
- ,,,,.The JCAHO patient safety event taxonomy: a standardized terminology and classification schema for near misses and adverse events.Int J Qual Health Care.2005;17:95–105.
- World Health Organization. 2009 Conceptual Framework for the International Classification for Patient Safety. Final Technical Report Version 1.1. Available at: http://www.who.int/patientsafety/taxonomy/icps_full_report.pdf. Accessed April 25,2011.
- ,,, et al.Towards an International Classification for Patient Safety: the conceptual framework.Int J Qual Health Care.2009;21:2–8.
- ,,,,,.Towards an International Classification for Patient Safety: a Delphi survey.Int J Qual Health Care.2009;21:9–17.
- ,,.Effectiveness and efficiency of root cause analysis in medicine.JAMA.2008;299:685–687.
- ,,,,.How can clinicians measure safety and quality in acute care?Lancet.2004;363:1061–1067.
- ,,,,.Systematic root cause analysis of adverse drug events in a tertiary referral hospital.Jt Comm J Qual Improv.2000;26:563–575.
- ,,,,,.Educational quality improvement report: outcomes from a revised morbidity and mortality format that emphasised patient safety.Postgrad Med J.2008;84:211–216.
- .Reporting of adverse events.N Engl J Med.2002;347:1633–1638.
- The Joint Commission's Sentinel Event Policy: ten years of improving the quality and safety of health care.Jt Comm Perspect.2005;25(1):3–5.
- ,.Root cause analysis and nursing management responsibilities in wrong‐site surgery.Dimens Crit Care Nurs.2006;25,221–225.
- ,.Root Cause Analysis.Making Health Care Safer. Available at: http://archive.ahrq.gov/clinic/ptsafety/chap5.htm. Accessed May 21,2010.
- Oversight group holds RCA teams accountable.Healthcare Benchmarks Qual Improv.2008;15:117–118.
- .Shared meanings: preferred terms and definitions for safety and quality concepts.Med J Aust.2006;184:S41–S43.
- ,,.What do family physicians consider an error? A comparison of definitions and physician perception.BMC Fam Pract.2006;7:73.
- ,,,,,.Towards an International Classification for Patient Safety: key concepts and terms.Int J Qual Health Care.2009;21:18–26.
- ,,,,.The JCAHO patient safety event taxonomy: a standardized terminology and classification schema for near misses and adverse events.Int J Qual Health Care.2005;17:95–105.
- World Health Organization. 2009 Conceptual Framework for the International Classification for Patient Safety. Final Technical Report Version 1.1. Available at: http://www.who.int/patientsafety/taxonomy/icps_full_report.pdf. Accessed April 25,2011.
- ,,, et al.Towards an International Classification for Patient Safety: the conceptual framework.Int J Qual Health Care.2009;21:2–8.
- ,,,,,.Towards an International Classification for Patient Safety: a Delphi survey.Int J Qual Health Care.2009;21:9–17.
- ,,.Effectiveness and efficiency of root cause analysis in medicine.JAMA.2008;299:685–687.
- ,,,,.How can clinicians measure safety and quality in acute care?Lancet.2004;363:1061–1067.
- ,,,,.Systematic root cause analysis of adverse drug events in a tertiary referral hospital.Jt Comm J Qual Improv.2000;26:563–575.
- ,,,,,.Educational quality improvement report: outcomes from a revised morbidity and mortality format that emphasised patient safety.Postgrad Med J.2008;84:211–216.