User login
Evaluating the Impact of a Urinalysis to Reflex Culture Process Change in the Emergency Department at a Veterans Affairs Hospital
Automated urine cultures (UCs) following urinalysis (UA) are often used in emergency departments (EDs) to identify urinary tract infections (UTIs). The fast-paced environment of the ED makes this method of proactive collection and facilitation of UC favorable. However, results are often reported as no organism growth or the growth of clinically insignificant organisms, leading to the overdetection and overtreatment of asymptomatic bacteriuria (ASB).1-3 An estimated 30 to 60% of patients with ASB receive unwarranted antibiotic treatment, which is associated with an increased risk of developing Clostridioides difficile infection and contributes to the development of antimicrobial resistance.4-10 The costs associated with UC are an important consideration given the use of resources, the time and effort required to collect and process large numbers of negative cultures, and further efforts devoted to the follow-up of ED culture results.
Changes in traditional testing involving testing of both a UA and UC to reflex testing where urine specimens undergo culture only if they meet certain criteria have been described.11-14 This change in traditional testing aims to reduce the number of potentially unnecessary cultures performed without compromising clinical care. Leukocyte quantity in the UA has been shown to be a reliable predictor of true infection.11,15 Fok and colleagues demonstrated that reflex urine testing in ambulatory male urology patients in which cultures were done on only urine specimens with > 5 white blood cells per high-power field (WBC/HPF) would have missed only 7% of positive UCs, while avoiding 69% of cultures.11
At the Edward Hines, Jr Veterans Affairs Hospital (Hines VA), inappropriate UC ordering and treatment for ASB has been identified as an area needing improvement. An evaluation was conducted at the facility to determine the population of inpatient veterans with a positive UC who were appropriately managed. Of the 113 study patients with a positive UC included in this review, 77 (68%) had a diagnosis of ASB, with > 80% of patients with ASB (and no other suspected infections) receiving antimicrobial therapy.8 A subsequent evaluation was conducted at the Hines VA ED to evaluate UTI treatment and follow-up. Of the 173 ED patients included, 23% received antibiotic therapy for an ASB and 60% had a UA and UC collected but did not report symptoms.9 Finally, a review by the Hines VA laboratory showed that in May 2017, of 359 UCs sent from various locations of the hospital, 38% were obtained in the setting of a negative UA.
A multidisciplinary group with representation from primary care, infectious diseases, pharmacy, nursing, laboratory, and informatics was created with a goal to improve the workup and management of UTIs. In addition to periodic education for the clinicians regarding appropriate use and interpretation of UA and UC along with judicious use of antimicrobials especially in the setting of ASB, a UA to reflex culture process change was implemented. This allowed for automatic cancellation of a UC in the setting of a negative UA, which was designed to help facilitate appropriate UC ordering.
Methods
The primary objective of this study was to compare the frequency of inappropriate UC use and inappropriate antibiotic prescribing pre- and postimplementation of this UA to reflex culture process change. An inappropriate UC was defined as a UC ordered despite a negative UA in asymptomatic patients. Inappropriate antibiotic prescribing was defined as treatment of patients with ASB. The secondary objective evaluated postintervention data to assess the frequency of outpatient, ED, and hospital visits for UTI-related symptoms in the group of patients that had a UC cancelled as a result of the new process change (within a 7-day period of the initial UA) to determine whether patients with true infections were missed due to the process change.
Study Design and Setting
This pre-post quality improvement (QI) study analyzed the UC-ordering practices for UTIs sent from the ED at the Hines VA. This VA is a 483-bed tertiary care hospital in Chicago, Illinois, and serves > 57,000 veterans and about 23,000 ED visits annually. This study was approved by the Edward Hines, Jr VA Institutional Review Board as a quality assurance/QI proposal prior to data collection.
Patient Selection
All patients who
When comparing postintervention data with preintervention data for the primary study objective, the same exclusion criteria from the 2015 study were applied to the present study, which excluded ED patients who were admitted for inpatient care, concurrent antibiotic therapy for a non-UTI indication, duplicate cultures, and use of chronic bladder management devices. All patients identified as receiving a UA during the specified postintervention study period were included for evaluation of the secondary study objective.
Interventions
After physician education, an ED process change was implemented on October 3, 2017. This process change involved the creation of new order sets in the EHR that allowed clinicians to order a UA only, a UA with culture that would be cancelled by laboratory personnel if the UA did not result in > 5 WBC/HPF, and a UA with culture designated as do not cancel, where the UC was processed regardless of the UA results. The scenarios in which the latter option was considered appropriate were listed on the ordering screen and included pregnancy, a genitourinary procedure with necessary preoperative culture, and neutropenia.
Measurements
Postimplementation, all UAs were reviewed and grouped as follows: (1) positive UA with subsequent UC; (2) negative UA, culture cancelled; (3) only UA ordered (no culture); or (4) do not cancel UC ordered. Of the UAs that were analyzed, the following data were collected: demographics, comorbidities, concurrent medications for benign prostatic hyperplasia (BPH) and/or overactive bladder (OAB), documented allergies/adverse drug reactions to antibiotics, date of ED visit, documented UTI signs/symptoms (defined as frequency, urgency, dysuria, fever, suprapubic pain, or altered mental status in patients unable to verbalize urinary symptoms), UC results and susceptibilities, number of UCs repeated within 7 days after initial UA, requirement of antibiotic for UTI within 7 days of initial UA, antibiotic prescribed, duration of antibiotic therapy, and outpatient visits, ED visits, or need for hospital admission within 7 days of the initial UA for UTI-related symptoms. Other relevant UA and UC data that could not be obtained from the EHR were collected by generating a report using the Veterans Information Systems and Technology Architecture (VistA).
Analysis
Statistical analysis was performed using SAS v9.4. Independent t tests and Fisher exact tests were used to describe difference pre- and postintervention. Statistical significance was considered for P < .05. Based on results from the previous study conducted at this facility in addition to a literature review, it was determined that 92 patients in each group (pre- and postintervention) would be necessary to detect a 15% increase in percentage of patients appropriately treated for a UTI.
Results
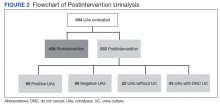
There were 684 UAs evaluated from ED visits, 429 preintervention and 255 postintervention. The 255 patients were evaluated for the secondary objective of the study. Of the 255 patients with UAs identified postintervention, 150 were excluded based on the predefined exclusion criteria, and the remaining 105 were compared with the 173 patients from the preintervention group and were included in the analysis for the primary objective (Figure 1).
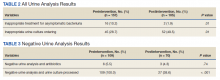
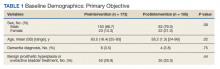
Patients in the postintervention group were younger than those in the preintervention group (P < .02): otherwise the groups were similar (Table 1). Inappropriate antibiotics for ASB decreased from 10.2% preintervention to 1.9% postintervention (odds ratio, 0.17; P = .01) (Table 2). UC processing despite a negative UA significantly decreased from 100% preintervention to 38.6% postintervention (P < .001) (Table 3). In patients with a negative UA, antibiotic prescribing decreased by 25.3% postintervention, but this difference was not statistically significant.
Postintervention, of 255 UAs evaluated, 95 (37.3%) were positive with a processed UC and 95 (37.3%) were negative with UC cancelled, 43 (16.9%) were ordered as DNC, and 22 (8.6%) were ordered without a UC (Figure 2). Twenty-eight of the 95 (29.5%) UAs with processed UCs did not meet the criteria for a positive UA and were not designated as DNC. When the UCs of this subgroup of patients were further analyzed, we found that 2 of the cultures were positive of which 1 patient was symptomatic and required antibiotic therapy.
Of the 95 patients with a negative UA, 69 (72.6%) presented without any UTI-related symptoms. In this group, there were no reports of outpatient visits, ED visits, or hospital admissions within 7 days of initial UA for UTI-related symptoms. None of the UCs ordered as DNC had a supporting reason identified. Nonetheless, the UC results from this patient subgroup also were analyzed further and resulted in 4 patients with negative UA and positive subsequent UC, 1 was symptomatic and required antibiotic therapy.
Discussion
A simple process change at the Hines VA resulted in benefits related to antimicrobial stewardship without conferring adverse outcomes on patient safety. Both UC processing despite a negative UA and inappropriate antibiotic prescribing for ASB were reduced significantly postintervention. This process change was piloted in the ED where UCs are often included as part of the initial diagnostic testing in patients who may not report UTI-related symptoms but for whom a UC is often bundled with other infectious workup, depending on the patient presentation.
Reflex testing of urine specimens has been described in the literature, both in an exploratory nature where impact of a reflex UC cancellation protocol based on certain UA criteria is measured by percent reduction of UCs processed as well as results of such interventions implemented into clinical practice.11-13 A retrospective study performed at the University of North Carolina Medical Center evaluated patients who presented to the ED during a 6-month period and had both an automated UA and UC collected. UC processing was restricted to UA that was positive for nitrites, leukocyte esterase, bacteria, or > 10 WBC/HPF. Use of this reflex culture cancellation protocol could have eliminated 604 of the 1546 (39.1%) cultures processed. However, 11 of the 314 (3.5%) positive cultures could have been missed.13 This same protocol was externally validated at another large academic ED setting, where similar results were found.14
In clinical practice, there is a natural tendency to reflexively prescribe antibiotics based on the results of a positive UC due to the hesitancy in ignoring these results, despite lack of a suspicion for a true infection. Leis and colleagues explored this in a proof-of-concept study evaluating the impact of discontinuing the routine reporting of positive UC results from noncatheterized inpatients and requesting clinicians to call the laboratory for results if a UTI was suspected.16 This intervention resulted in a statistically significant reduction in treatment of ASB in noncatheterized patients from 48 to 12% pre- and postintervention. Clinicians requested culture results only 14% of the time, and there were no adverse outcomes among untreated noncatheterized patients. More recently, a QI study conducted at a large community hospital in Toronto, Ontario, Canada, implemented a 2-step model of care for urine collection.17 UC was collected but only processed by the microbiology laboratory if the ED physicians deemed it necessary after clinical assessment.
After implementation, there was a decrease in the proportion of ED visits associated with processed UC (from 6.0% to 4.7% of visits per week; P < .001), ED visits associated with callbacks for processing UC (1.8% to 1.1% of visits per month; P < .001), and antimicrobial prescriptions for urinary symptoms among hospitalized patients (from 20.6% to 10.9%; P < .001). Equally important, despite the 937 cases in which urine was collected but cultures were not processed, no evidence of untreated UTIs was identified.17
The results from the present study similarly demonstrate minimal concern for potentially undertreating these patients. As seen in the subgroup of patients included in the positive UA group, which did not meet criteria for positive UA per protocol (n = 29), only 2 of the subsequent cultures were positive, of which only 1 patient required antibiotic therapy based on the clinical presentation. In addition, in the group of negative UAs with subsequent cancellation of the UC, there were no found reports of outpatient visits, ED visits, or hospital admissions within 7 days of the initial UA for UTI-related symptoms.
Limitations
This single-center, pre-post QI study was not without limitations. Manual chart reviews were required, and accuracy of information was dependent on clinician documentation and assessment of UTI-related symptoms. The population studied was predominately older males; thus, results may not be applicable to females or young adults. Additionally, recognition of a negative UA and subsequent cancellation of the UC was dependent on laboratory personnel. As noted in the patient group with a positive UA, some of these UAs were negative and may have been overlooked; therefore, subsequent UCs were inappropriately processed. However, this occurred infrequently and confirmed the low probability of true UTI in the setting of a negative UA. Follow-up for UTI-related symptoms may not have been captured if a patient had presented to an outside facility. Last, definitions of a positive UA differed slightly between the pre- and postintervention groups. The preintervention study defined a positive UA as a WBC count > 5 WBC/HPF and positive leukocyte esterase, whereas the present study defined a positive UA with a WBC count > 5. This may have resulted in an overestimation of positive UA in the postintervention group.
Conclusions
Better selective use of UC testing may improve stewardship resources and reduce costs impacting both ED and clinical laboratories. Furthermore, benefits can include a reduction in the use of time and resources required to collect samples for culture, use of test supplies, the time and effort required to process the large number of negative cultures, and resources devoted to the follow-up of these ED culture results. The described UA to reflex culture process change demonstrated a significant reduction in the processing of inappropriate UC and unnecessary antibiotics for ASB. There were no missed UTIs or other adverse patient outcomes noted. This process change has been implemented in all departments at the Hines VA and additional data will be collected to ensure consistent outcomes.
1. Chironda B, Clancy S, Powis JE. Optimizing urine culture collection in the emergency department using frontline ownership interventions. Clin Infect Dis. 2014;59(7):1038-1039. doi:10.1093/cid/ciu412
2. Nagurney JT, Brown DF, Chang Y, Sane S, Wang AC, Weiner JB. Use of diagnostic testing in the emergency department for patients presenting with non-traumatic abdominal pain. J Emerg Med. 2003;25(4):363-371. doi:10.1016/s0736-4679(03)00237-3
3. Lammers RL, Gibson S, Kovacs D, Sears W, Strachan G. Comparison of test characteristics of urine dipstick and urinalysis at various test cutoff points. Ann Emerg Med. 2001;38(5):505-512. doi:10.1067/mem.2001.119427
4. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68(10):1611-1615. doi:10.1093/cid/ciy1121
5. Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med. 2015;175(7):1120-1127. doi:10.1001/jamainternmed.2015.1878
6. Hartley S, Valley S, Kuhn L, et al. Overtreatment of asymptomatic bacteriuria: identifying targets for improvement. Infect Control Hosp Epidemiol. 2015;36(4):470-473. doi:10.1017/ice.2014.73
7. Bader MS, Loeb M, Brooks AA. An update on the management of urinary tract infections in the era of antimicrobial resistance. Postgrad Med. 2017;129(2):242-258. doi:10.1080/00325481.2017.1246055
8. Spivak ES, Burk M, Zhang R, et al. Management of bacteriuria in Veterans Affairs hospitals. Clin Infect Dis. 2017;65(6):910-917. doi:10.1093/cid/cix474
9. Kim EY, Patel U, Patel B, Suda KJ. Evaluation of bacteriuria treatment and follow-up initiated in the emergency department at a Veterans Affairs hospital. J Pharm Technol. 2017;33(5):183-188. doi:10.1177/8755122517718214
10. Brown E, Talbot GH, Axelrod P, Provencher M, Hoegg C. Risk factors for Clostridium difficile toxin-associated diarrhea. Infect Control Hosp Epidemiol. 1990;11(6):283-290. doi:10.1086/646173
11. Fok C, Fitzgerald MP, Turk T, Mueller E, Dalaza L, Schreckenberger P. Reflex testing of male urine specimens misses few positive cultures may reduce unnecessary testing of normal specimens. Urology. 2010;75(1):74-76. doi:10.1016/j.urology.2009.08.071
12. Munigala S, Jackups RR Jr, Poirier RF, et al. Impact of order set design on urine culturing practices at an academic medical centre emergency department. BMJ Qual Saf. 2018;27(8):587-592. doi:10.1136/bmjqs-2017-006899
13. Jones CW, Culbreath KD, Mehrotra A, Gilligan PH. Reflect urine culture cancellation in the emergency department. J Emerg Med. 2014;46(1):71-76. doi:10.1016/j.jemermed.2013.08.042
14. Hertz JT, Lescallette RD, Barrett TW, Ward MJ, Self WH. External validation of an ED protocol for reflex urine culture cancelation. Am J Emerg Med. 2015;33(12):1838-1839. doi:10.1016/j.ajem.2015.09.026
15. Stamm WE. Measurement of pyuria and its relation to bacteriuria. Am J Med. 1983;75(1B):53-58. doi:10.1016/0002-9343(83)90073-6
16. Leis JA, Rebick GW, Daneman N, et al. Reducing antimicrobial therapy for asymptomatic bacteriuria among noncatheterized inpatients: a proof-of-concept study. Clin Infect Dis. 2014;58(7):980-983. doi:10.1093/cid/ciu010
17. Stagg A, Lutz H, Kirpalaney S, et al. Impact of two-step urine culture ordering in the emergency department: a time series analysis. BMJ Qual Saf. 2017;27:140-147. doi:10.1136/bmjqs-2016-006250
Automated urine cultures (UCs) following urinalysis (UA) are often used in emergency departments (EDs) to identify urinary tract infections (UTIs). The fast-paced environment of the ED makes this method of proactive collection and facilitation of UC favorable. However, results are often reported as no organism growth or the growth of clinically insignificant organisms, leading to the overdetection and overtreatment of asymptomatic bacteriuria (ASB).1-3 An estimated 30 to 60% of patients with ASB receive unwarranted antibiotic treatment, which is associated with an increased risk of developing Clostridioides difficile infection and contributes to the development of antimicrobial resistance.4-10 The costs associated with UC are an important consideration given the use of resources, the time and effort required to collect and process large numbers of negative cultures, and further efforts devoted to the follow-up of ED culture results.
Changes in traditional testing involving testing of both a UA and UC to reflex testing where urine specimens undergo culture only if they meet certain criteria have been described.11-14 This change in traditional testing aims to reduce the number of potentially unnecessary cultures performed without compromising clinical care. Leukocyte quantity in the UA has been shown to be a reliable predictor of true infection.11,15 Fok and colleagues demonstrated that reflex urine testing in ambulatory male urology patients in which cultures were done on only urine specimens with > 5 white blood cells per high-power field (WBC/HPF) would have missed only 7% of positive UCs, while avoiding 69% of cultures.11
At the Edward Hines, Jr Veterans Affairs Hospital (Hines VA), inappropriate UC ordering and treatment for ASB has been identified as an area needing improvement. An evaluation was conducted at the facility to determine the population of inpatient veterans with a positive UC who were appropriately managed. Of the 113 study patients with a positive UC included in this review, 77 (68%) had a diagnosis of ASB, with > 80% of patients with ASB (and no other suspected infections) receiving antimicrobial therapy.8 A subsequent evaluation was conducted at the Hines VA ED to evaluate UTI treatment and follow-up. Of the 173 ED patients included, 23% received antibiotic therapy for an ASB and 60% had a UA and UC collected but did not report symptoms.9 Finally, a review by the Hines VA laboratory showed that in May 2017, of 359 UCs sent from various locations of the hospital, 38% were obtained in the setting of a negative UA.
A multidisciplinary group with representation from primary care, infectious diseases, pharmacy, nursing, laboratory, and informatics was created with a goal to improve the workup and management of UTIs. In addition to periodic education for the clinicians regarding appropriate use and interpretation of UA and UC along with judicious use of antimicrobials especially in the setting of ASB, a UA to reflex culture process change was implemented. This allowed for automatic cancellation of a UC in the setting of a negative UA, which was designed to help facilitate appropriate UC ordering.
Methods
The primary objective of this study was to compare the frequency of inappropriate UC use and inappropriate antibiotic prescribing pre- and postimplementation of this UA to reflex culture process change. An inappropriate UC was defined as a UC ordered despite a negative UA in asymptomatic patients. Inappropriate antibiotic prescribing was defined as treatment of patients with ASB. The secondary objective evaluated postintervention data to assess the frequency of outpatient, ED, and hospital visits for UTI-related symptoms in the group of patients that had a UC cancelled as a result of the new process change (within a 7-day period of the initial UA) to determine whether patients with true infections were missed due to the process change.
Study Design and Setting
This pre-post quality improvement (QI) study analyzed the UC-ordering practices for UTIs sent from the ED at the Hines VA. This VA is a 483-bed tertiary care hospital in Chicago, Illinois, and serves > 57,000 veterans and about 23,000 ED visits annually. This study was approved by the Edward Hines, Jr VA Institutional Review Board as a quality assurance/QI proposal prior to data collection.
Patient Selection
All patients who
When comparing postintervention data with preintervention data for the primary study objective, the same exclusion criteria from the 2015 study were applied to the present study, which excluded ED patients who were admitted for inpatient care, concurrent antibiotic therapy for a non-UTI indication, duplicate cultures, and use of chronic bladder management devices. All patients identified as receiving a UA during the specified postintervention study period were included for evaluation of the secondary study objective.
Interventions
After physician education, an ED process change was implemented on October 3, 2017. This process change involved the creation of new order sets in the EHR that allowed clinicians to order a UA only, a UA with culture that would be cancelled by laboratory personnel if the UA did not result in > 5 WBC/HPF, and a UA with culture designated as do not cancel, where the UC was processed regardless of the UA results. The scenarios in which the latter option was considered appropriate were listed on the ordering screen and included pregnancy, a genitourinary procedure with necessary preoperative culture, and neutropenia.
Measurements
Postimplementation, all UAs were reviewed and grouped as follows: (1) positive UA with subsequent UC; (2) negative UA, culture cancelled; (3) only UA ordered (no culture); or (4) do not cancel UC ordered. Of the UAs that were analyzed, the following data were collected: demographics, comorbidities, concurrent medications for benign prostatic hyperplasia (BPH) and/or overactive bladder (OAB), documented allergies/adverse drug reactions to antibiotics, date of ED visit, documented UTI signs/symptoms (defined as frequency, urgency, dysuria, fever, suprapubic pain, or altered mental status in patients unable to verbalize urinary symptoms), UC results and susceptibilities, number of UCs repeated within 7 days after initial UA, requirement of antibiotic for UTI within 7 days of initial UA, antibiotic prescribed, duration of antibiotic therapy, and outpatient visits, ED visits, or need for hospital admission within 7 days of the initial UA for UTI-related symptoms. Other relevant UA and UC data that could not be obtained from the EHR were collected by generating a report using the Veterans Information Systems and Technology Architecture (VistA).
Analysis
Statistical analysis was performed using SAS v9.4. Independent t tests and Fisher exact tests were used to describe difference pre- and postintervention. Statistical significance was considered for P < .05. Based on results from the previous study conducted at this facility in addition to a literature review, it was determined that 92 patients in each group (pre- and postintervention) would be necessary to detect a 15% increase in percentage of patients appropriately treated for a UTI.
Results
There were 684 UAs evaluated from ED visits, 429 preintervention and 255 postintervention. The 255 patients were evaluated for the secondary objective of the study. Of the 255 patients with UAs identified postintervention, 150 were excluded based on the predefined exclusion criteria, and the remaining 105 were compared with the 173 patients from the preintervention group and were included in the analysis for the primary objective (Figure 1).
Patients in the postintervention group were younger than those in the preintervention group (P < .02): otherwise the groups were similar (Table 1). Inappropriate antibiotics for ASB decreased from 10.2% preintervention to 1.9% postintervention (odds ratio, 0.17; P = .01) (Table 2). UC processing despite a negative UA significantly decreased from 100% preintervention to 38.6% postintervention (P < .001) (Table 3). In patients with a negative UA, antibiotic prescribing decreased by 25.3% postintervention, but this difference was not statistically significant.
Postintervention, of 255 UAs evaluated, 95 (37.3%) were positive with a processed UC and 95 (37.3%) were negative with UC cancelled, 43 (16.9%) were ordered as DNC, and 22 (8.6%) were ordered without a UC (Figure 2). Twenty-eight of the 95 (29.5%) UAs with processed UCs did not meet the criteria for a positive UA and were not designated as DNC. When the UCs of this subgroup of patients were further analyzed, we found that 2 of the cultures were positive of which 1 patient was symptomatic and required antibiotic therapy.
Of the 95 patients with a negative UA, 69 (72.6%) presented without any UTI-related symptoms. In this group, there were no reports of outpatient visits, ED visits, or hospital admissions within 7 days of initial UA for UTI-related symptoms. None of the UCs ordered as DNC had a supporting reason identified. Nonetheless, the UC results from this patient subgroup also were analyzed further and resulted in 4 patients with negative UA and positive subsequent UC, 1 was symptomatic and required antibiotic therapy.
Discussion
A simple process change at the Hines VA resulted in benefits related to antimicrobial stewardship without conferring adverse outcomes on patient safety. Both UC processing despite a negative UA and inappropriate antibiotic prescribing for ASB were reduced significantly postintervention. This process change was piloted in the ED where UCs are often included as part of the initial diagnostic testing in patients who may not report UTI-related symptoms but for whom a UC is often bundled with other infectious workup, depending on the patient presentation.
Reflex testing of urine specimens has been described in the literature, both in an exploratory nature where impact of a reflex UC cancellation protocol based on certain UA criteria is measured by percent reduction of UCs processed as well as results of such interventions implemented into clinical practice.11-13 A retrospective study performed at the University of North Carolina Medical Center evaluated patients who presented to the ED during a 6-month period and had both an automated UA and UC collected. UC processing was restricted to UA that was positive for nitrites, leukocyte esterase, bacteria, or > 10 WBC/HPF. Use of this reflex culture cancellation protocol could have eliminated 604 of the 1546 (39.1%) cultures processed. However, 11 of the 314 (3.5%) positive cultures could have been missed.13 This same protocol was externally validated at another large academic ED setting, where similar results were found.14
In clinical practice, there is a natural tendency to reflexively prescribe antibiotics based on the results of a positive UC due to the hesitancy in ignoring these results, despite lack of a suspicion for a true infection. Leis and colleagues explored this in a proof-of-concept study evaluating the impact of discontinuing the routine reporting of positive UC results from noncatheterized inpatients and requesting clinicians to call the laboratory for results if a UTI was suspected.16 This intervention resulted in a statistically significant reduction in treatment of ASB in noncatheterized patients from 48 to 12% pre- and postintervention. Clinicians requested culture results only 14% of the time, and there were no adverse outcomes among untreated noncatheterized patients. More recently, a QI study conducted at a large community hospital in Toronto, Ontario, Canada, implemented a 2-step model of care for urine collection.17 UC was collected but only processed by the microbiology laboratory if the ED physicians deemed it necessary after clinical assessment.
After implementation, there was a decrease in the proportion of ED visits associated with processed UC (from 6.0% to 4.7% of visits per week; P < .001), ED visits associated with callbacks for processing UC (1.8% to 1.1% of visits per month; P < .001), and antimicrobial prescriptions for urinary symptoms among hospitalized patients (from 20.6% to 10.9%; P < .001). Equally important, despite the 937 cases in which urine was collected but cultures were not processed, no evidence of untreated UTIs was identified.17
The results from the present study similarly demonstrate minimal concern for potentially undertreating these patients. As seen in the subgroup of patients included in the positive UA group, which did not meet criteria for positive UA per protocol (n = 29), only 2 of the subsequent cultures were positive, of which only 1 patient required antibiotic therapy based on the clinical presentation. In addition, in the group of negative UAs with subsequent cancellation of the UC, there were no found reports of outpatient visits, ED visits, or hospital admissions within 7 days of the initial UA for UTI-related symptoms.
Limitations
This single-center, pre-post QI study was not without limitations. Manual chart reviews were required, and accuracy of information was dependent on clinician documentation and assessment of UTI-related symptoms. The population studied was predominately older males; thus, results may not be applicable to females or young adults. Additionally, recognition of a negative UA and subsequent cancellation of the UC was dependent on laboratory personnel. As noted in the patient group with a positive UA, some of these UAs were negative and may have been overlooked; therefore, subsequent UCs were inappropriately processed. However, this occurred infrequently and confirmed the low probability of true UTI in the setting of a negative UA. Follow-up for UTI-related symptoms may not have been captured if a patient had presented to an outside facility. Last, definitions of a positive UA differed slightly between the pre- and postintervention groups. The preintervention study defined a positive UA as a WBC count > 5 WBC/HPF and positive leukocyte esterase, whereas the present study defined a positive UA with a WBC count > 5. This may have resulted in an overestimation of positive UA in the postintervention group.
Conclusions
Better selective use of UC testing may improve stewardship resources and reduce costs impacting both ED and clinical laboratories. Furthermore, benefits can include a reduction in the use of time and resources required to collect samples for culture, use of test supplies, the time and effort required to process the large number of negative cultures, and resources devoted to the follow-up of these ED culture results. The described UA to reflex culture process change demonstrated a significant reduction in the processing of inappropriate UC and unnecessary antibiotics for ASB. There were no missed UTIs or other adverse patient outcomes noted. This process change has been implemented in all departments at the Hines VA and additional data will be collected to ensure consistent outcomes.
Automated urine cultures (UCs) following urinalysis (UA) are often used in emergency departments (EDs) to identify urinary tract infections (UTIs). The fast-paced environment of the ED makes this method of proactive collection and facilitation of UC favorable. However, results are often reported as no organism growth or the growth of clinically insignificant organisms, leading to the overdetection and overtreatment of asymptomatic bacteriuria (ASB).1-3 An estimated 30 to 60% of patients with ASB receive unwarranted antibiotic treatment, which is associated with an increased risk of developing Clostridioides difficile infection and contributes to the development of antimicrobial resistance.4-10 The costs associated with UC are an important consideration given the use of resources, the time and effort required to collect and process large numbers of negative cultures, and further efforts devoted to the follow-up of ED culture results.
Changes in traditional testing involving testing of both a UA and UC to reflex testing where urine specimens undergo culture only if they meet certain criteria have been described.11-14 This change in traditional testing aims to reduce the number of potentially unnecessary cultures performed without compromising clinical care. Leukocyte quantity in the UA has been shown to be a reliable predictor of true infection.11,15 Fok and colleagues demonstrated that reflex urine testing in ambulatory male urology patients in which cultures were done on only urine specimens with > 5 white blood cells per high-power field (WBC/HPF) would have missed only 7% of positive UCs, while avoiding 69% of cultures.11
At the Edward Hines, Jr Veterans Affairs Hospital (Hines VA), inappropriate UC ordering and treatment for ASB has been identified as an area needing improvement. An evaluation was conducted at the facility to determine the population of inpatient veterans with a positive UC who were appropriately managed. Of the 113 study patients with a positive UC included in this review, 77 (68%) had a diagnosis of ASB, with > 80% of patients with ASB (and no other suspected infections) receiving antimicrobial therapy.8 A subsequent evaluation was conducted at the Hines VA ED to evaluate UTI treatment and follow-up. Of the 173 ED patients included, 23% received antibiotic therapy for an ASB and 60% had a UA and UC collected but did not report symptoms.9 Finally, a review by the Hines VA laboratory showed that in May 2017, of 359 UCs sent from various locations of the hospital, 38% were obtained in the setting of a negative UA.
A multidisciplinary group with representation from primary care, infectious diseases, pharmacy, nursing, laboratory, and informatics was created with a goal to improve the workup and management of UTIs. In addition to periodic education for the clinicians regarding appropriate use and interpretation of UA and UC along with judicious use of antimicrobials especially in the setting of ASB, a UA to reflex culture process change was implemented. This allowed for automatic cancellation of a UC in the setting of a negative UA, which was designed to help facilitate appropriate UC ordering.
Methods
The primary objective of this study was to compare the frequency of inappropriate UC use and inappropriate antibiotic prescribing pre- and postimplementation of this UA to reflex culture process change. An inappropriate UC was defined as a UC ordered despite a negative UA in asymptomatic patients. Inappropriate antibiotic prescribing was defined as treatment of patients with ASB. The secondary objective evaluated postintervention data to assess the frequency of outpatient, ED, and hospital visits for UTI-related symptoms in the group of patients that had a UC cancelled as a result of the new process change (within a 7-day period of the initial UA) to determine whether patients with true infections were missed due to the process change.
Study Design and Setting
This pre-post quality improvement (QI) study analyzed the UC-ordering practices for UTIs sent from the ED at the Hines VA. This VA is a 483-bed tertiary care hospital in Chicago, Illinois, and serves > 57,000 veterans and about 23,000 ED visits annually. This study was approved by the Edward Hines, Jr VA Institutional Review Board as a quality assurance/QI proposal prior to data collection.
Patient Selection
All patients who
When comparing postintervention data with preintervention data for the primary study objective, the same exclusion criteria from the 2015 study were applied to the present study, which excluded ED patients who were admitted for inpatient care, concurrent antibiotic therapy for a non-UTI indication, duplicate cultures, and use of chronic bladder management devices. All patients identified as receiving a UA during the specified postintervention study period were included for evaluation of the secondary study objective.
Interventions
After physician education, an ED process change was implemented on October 3, 2017. This process change involved the creation of new order sets in the EHR that allowed clinicians to order a UA only, a UA with culture that would be cancelled by laboratory personnel if the UA did not result in > 5 WBC/HPF, and a UA with culture designated as do not cancel, where the UC was processed regardless of the UA results. The scenarios in which the latter option was considered appropriate were listed on the ordering screen and included pregnancy, a genitourinary procedure with necessary preoperative culture, and neutropenia.
Measurements
Postimplementation, all UAs were reviewed and grouped as follows: (1) positive UA with subsequent UC; (2) negative UA, culture cancelled; (3) only UA ordered (no culture); or (4) do not cancel UC ordered. Of the UAs that were analyzed, the following data were collected: demographics, comorbidities, concurrent medications for benign prostatic hyperplasia (BPH) and/or overactive bladder (OAB), documented allergies/adverse drug reactions to antibiotics, date of ED visit, documented UTI signs/symptoms (defined as frequency, urgency, dysuria, fever, suprapubic pain, or altered mental status in patients unable to verbalize urinary symptoms), UC results and susceptibilities, number of UCs repeated within 7 days after initial UA, requirement of antibiotic for UTI within 7 days of initial UA, antibiotic prescribed, duration of antibiotic therapy, and outpatient visits, ED visits, or need for hospital admission within 7 days of the initial UA for UTI-related symptoms. Other relevant UA and UC data that could not be obtained from the EHR were collected by generating a report using the Veterans Information Systems and Technology Architecture (VistA).
Analysis
Statistical analysis was performed using SAS v9.4. Independent t tests and Fisher exact tests were used to describe difference pre- and postintervention. Statistical significance was considered for P < .05. Based on results from the previous study conducted at this facility in addition to a literature review, it was determined that 92 patients in each group (pre- and postintervention) would be necessary to detect a 15% increase in percentage of patients appropriately treated for a UTI.
Results
There were 684 UAs evaluated from ED visits, 429 preintervention and 255 postintervention. The 255 patients were evaluated for the secondary objective of the study. Of the 255 patients with UAs identified postintervention, 150 were excluded based on the predefined exclusion criteria, and the remaining 105 were compared with the 173 patients from the preintervention group and were included in the analysis for the primary objective (Figure 1).
Patients in the postintervention group were younger than those in the preintervention group (P < .02): otherwise the groups were similar (Table 1). Inappropriate antibiotics for ASB decreased from 10.2% preintervention to 1.9% postintervention (odds ratio, 0.17; P = .01) (Table 2). UC processing despite a negative UA significantly decreased from 100% preintervention to 38.6% postintervention (P < .001) (Table 3). In patients with a negative UA, antibiotic prescribing decreased by 25.3% postintervention, but this difference was not statistically significant.
Postintervention, of 255 UAs evaluated, 95 (37.3%) were positive with a processed UC and 95 (37.3%) were negative with UC cancelled, 43 (16.9%) were ordered as DNC, and 22 (8.6%) were ordered without a UC (Figure 2). Twenty-eight of the 95 (29.5%) UAs with processed UCs did not meet the criteria for a positive UA and were not designated as DNC. When the UCs of this subgroup of patients were further analyzed, we found that 2 of the cultures were positive of which 1 patient was symptomatic and required antibiotic therapy.
Of the 95 patients with a negative UA, 69 (72.6%) presented without any UTI-related symptoms. In this group, there were no reports of outpatient visits, ED visits, or hospital admissions within 7 days of initial UA for UTI-related symptoms. None of the UCs ordered as DNC had a supporting reason identified. Nonetheless, the UC results from this patient subgroup also were analyzed further and resulted in 4 patients with negative UA and positive subsequent UC, 1 was symptomatic and required antibiotic therapy.
Discussion
A simple process change at the Hines VA resulted in benefits related to antimicrobial stewardship without conferring adverse outcomes on patient safety. Both UC processing despite a negative UA and inappropriate antibiotic prescribing for ASB were reduced significantly postintervention. This process change was piloted in the ED where UCs are often included as part of the initial diagnostic testing in patients who may not report UTI-related symptoms but for whom a UC is often bundled with other infectious workup, depending on the patient presentation.
Reflex testing of urine specimens has been described in the literature, both in an exploratory nature where impact of a reflex UC cancellation protocol based on certain UA criteria is measured by percent reduction of UCs processed as well as results of such interventions implemented into clinical practice.11-13 A retrospective study performed at the University of North Carolina Medical Center evaluated patients who presented to the ED during a 6-month period and had both an automated UA and UC collected. UC processing was restricted to UA that was positive for nitrites, leukocyte esterase, bacteria, or > 10 WBC/HPF. Use of this reflex culture cancellation protocol could have eliminated 604 of the 1546 (39.1%) cultures processed. However, 11 of the 314 (3.5%) positive cultures could have been missed.13 This same protocol was externally validated at another large academic ED setting, where similar results were found.14
In clinical practice, there is a natural tendency to reflexively prescribe antibiotics based on the results of a positive UC due to the hesitancy in ignoring these results, despite lack of a suspicion for a true infection. Leis and colleagues explored this in a proof-of-concept study evaluating the impact of discontinuing the routine reporting of positive UC results from noncatheterized inpatients and requesting clinicians to call the laboratory for results if a UTI was suspected.16 This intervention resulted in a statistically significant reduction in treatment of ASB in noncatheterized patients from 48 to 12% pre- and postintervention. Clinicians requested culture results only 14% of the time, and there were no adverse outcomes among untreated noncatheterized patients. More recently, a QI study conducted at a large community hospital in Toronto, Ontario, Canada, implemented a 2-step model of care for urine collection.17 UC was collected but only processed by the microbiology laboratory if the ED physicians deemed it necessary after clinical assessment.
After implementation, there was a decrease in the proportion of ED visits associated with processed UC (from 6.0% to 4.7% of visits per week; P < .001), ED visits associated with callbacks for processing UC (1.8% to 1.1% of visits per month; P < .001), and antimicrobial prescriptions for urinary symptoms among hospitalized patients (from 20.6% to 10.9%; P < .001). Equally important, despite the 937 cases in which urine was collected but cultures were not processed, no evidence of untreated UTIs was identified.17
The results from the present study similarly demonstrate minimal concern for potentially undertreating these patients. As seen in the subgroup of patients included in the positive UA group, which did not meet criteria for positive UA per protocol (n = 29), only 2 of the subsequent cultures were positive, of which only 1 patient required antibiotic therapy based on the clinical presentation. In addition, in the group of negative UAs with subsequent cancellation of the UC, there were no found reports of outpatient visits, ED visits, or hospital admissions within 7 days of the initial UA for UTI-related symptoms.
Limitations
This single-center, pre-post QI study was not without limitations. Manual chart reviews were required, and accuracy of information was dependent on clinician documentation and assessment of UTI-related symptoms. The population studied was predominately older males; thus, results may not be applicable to females or young adults. Additionally, recognition of a negative UA and subsequent cancellation of the UC was dependent on laboratory personnel. As noted in the patient group with a positive UA, some of these UAs were negative and may have been overlooked; therefore, subsequent UCs were inappropriately processed. However, this occurred infrequently and confirmed the low probability of true UTI in the setting of a negative UA. Follow-up for UTI-related symptoms may not have been captured if a patient had presented to an outside facility. Last, definitions of a positive UA differed slightly between the pre- and postintervention groups. The preintervention study defined a positive UA as a WBC count > 5 WBC/HPF and positive leukocyte esterase, whereas the present study defined a positive UA with a WBC count > 5. This may have resulted in an overestimation of positive UA in the postintervention group.
Conclusions
Better selective use of UC testing may improve stewardship resources and reduce costs impacting both ED and clinical laboratories. Furthermore, benefits can include a reduction in the use of time and resources required to collect samples for culture, use of test supplies, the time and effort required to process the large number of negative cultures, and resources devoted to the follow-up of these ED culture results. The described UA to reflex culture process change demonstrated a significant reduction in the processing of inappropriate UC and unnecessary antibiotics for ASB. There were no missed UTIs or other adverse patient outcomes noted. This process change has been implemented in all departments at the Hines VA and additional data will be collected to ensure consistent outcomes.
1. Chironda B, Clancy S, Powis JE. Optimizing urine culture collection in the emergency department using frontline ownership interventions. Clin Infect Dis. 2014;59(7):1038-1039. doi:10.1093/cid/ciu412
2. Nagurney JT, Brown DF, Chang Y, Sane S, Wang AC, Weiner JB. Use of diagnostic testing in the emergency department for patients presenting with non-traumatic abdominal pain. J Emerg Med. 2003;25(4):363-371. doi:10.1016/s0736-4679(03)00237-3
3. Lammers RL, Gibson S, Kovacs D, Sears W, Strachan G. Comparison of test characteristics of urine dipstick and urinalysis at various test cutoff points. Ann Emerg Med. 2001;38(5):505-512. doi:10.1067/mem.2001.119427
4. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68(10):1611-1615. doi:10.1093/cid/ciy1121
5. Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med. 2015;175(7):1120-1127. doi:10.1001/jamainternmed.2015.1878
6. Hartley S, Valley S, Kuhn L, et al. Overtreatment of asymptomatic bacteriuria: identifying targets for improvement. Infect Control Hosp Epidemiol. 2015;36(4):470-473. doi:10.1017/ice.2014.73
7. Bader MS, Loeb M, Brooks AA. An update on the management of urinary tract infections in the era of antimicrobial resistance. Postgrad Med. 2017;129(2):242-258. doi:10.1080/00325481.2017.1246055
8. Spivak ES, Burk M, Zhang R, et al. Management of bacteriuria in Veterans Affairs hospitals. Clin Infect Dis. 2017;65(6):910-917. doi:10.1093/cid/cix474
9. Kim EY, Patel U, Patel B, Suda KJ. Evaluation of bacteriuria treatment and follow-up initiated in the emergency department at a Veterans Affairs hospital. J Pharm Technol. 2017;33(5):183-188. doi:10.1177/8755122517718214
10. Brown E, Talbot GH, Axelrod P, Provencher M, Hoegg C. Risk factors for Clostridium difficile toxin-associated diarrhea. Infect Control Hosp Epidemiol. 1990;11(6):283-290. doi:10.1086/646173
11. Fok C, Fitzgerald MP, Turk T, Mueller E, Dalaza L, Schreckenberger P. Reflex testing of male urine specimens misses few positive cultures may reduce unnecessary testing of normal specimens. Urology. 2010;75(1):74-76. doi:10.1016/j.urology.2009.08.071
12. Munigala S, Jackups RR Jr, Poirier RF, et al. Impact of order set design on urine culturing practices at an academic medical centre emergency department. BMJ Qual Saf. 2018;27(8):587-592. doi:10.1136/bmjqs-2017-006899
13. Jones CW, Culbreath KD, Mehrotra A, Gilligan PH. Reflect urine culture cancellation in the emergency department. J Emerg Med. 2014;46(1):71-76. doi:10.1016/j.jemermed.2013.08.042
14. Hertz JT, Lescallette RD, Barrett TW, Ward MJ, Self WH. External validation of an ED protocol for reflex urine culture cancelation. Am J Emerg Med. 2015;33(12):1838-1839. doi:10.1016/j.ajem.2015.09.026
15. Stamm WE. Measurement of pyuria and its relation to bacteriuria. Am J Med. 1983;75(1B):53-58. doi:10.1016/0002-9343(83)90073-6
16. Leis JA, Rebick GW, Daneman N, et al. Reducing antimicrobial therapy for asymptomatic bacteriuria among noncatheterized inpatients: a proof-of-concept study. Clin Infect Dis. 2014;58(7):980-983. doi:10.1093/cid/ciu010
17. Stagg A, Lutz H, Kirpalaney S, et al. Impact of two-step urine culture ordering in the emergency department: a time series analysis. BMJ Qual Saf. 2017;27:140-147. doi:10.1136/bmjqs-2016-006250
1. Chironda B, Clancy S, Powis JE. Optimizing urine culture collection in the emergency department using frontline ownership interventions. Clin Infect Dis. 2014;59(7):1038-1039. doi:10.1093/cid/ciu412
2. Nagurney JT, Brown DF, Chang Y, Sane S, Wang AC, Weiner JB. Use of diagnostic testing in the emergency department for patients presenting with non-traumatic abdominal pain. J Emerg Med. 2003;25(4):363-371. doi:10.1016/s0736-4679(03)00237-3
3. Lammers RL, Gibson S, Kovacs D, Sears W, Strachan G. Comparison of test characteristics of urine dipstick and urinalysis at various test cutoff points. Ann Emerg Med. 2001;38(5):505-512. doi:10.1067/mem.2001.119427
4. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68(10):1611-1615. doi:10.1093/cid/ciy1121
5. Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med. 2015;175(7):1120-1127. doi:10.1001/jamainternmed.2015.1878
6. Hartley S, Valley S, Kuhn L, et al. Overtreatment of asymptomatic bacteriuria: identifying targets for improvement. Infect Control Hosp Epidemiol. 2015;36(4):470-473. doi:10.1017/ice.2014.73
7. Bader MS, Loeb M, Brooks AA. An update on the management of urinary tract infections in the era of antimicrobial resistance. Postgrad Med. 2017;129(2):242-258. doi:10.1080/00325481.2017.1246055
8. Spivak ES, Burk M, Zhang R, et al. Management of bacteriuria in Veterans Affairs hospitals. Clin Infect Dis. 2017;65(6):910-917. doi:10.1093/cid/cix474
9. Kim EY, Patel U, Patel B, Suda KJ. Evaluation of bacteriuria treatment and follow-up initiated in the emergency department at a Veterans Affairs hospital. J Pharm Technol. 2017;33(5):183-188. doi:10.1177/8755122517718214
10. Brown E, Talbot GH, Axelrod P, Provencher M, Hoegg C. Risk factors for Clostridium difficile toxin-associated diarrhea. Infect Control Hosp Epidemiol. 1990;11(6):283-290. doi:10.1086/646173
11. Fok C, Fitzgerald MP, Turk T, Mueller E, Dalaza L, Schreckenberger P. Reflex testing of male urine specimens misses few positive cultures may reduce unnecessary testing of normal specimens. Urology. 2010;75(1):74-76. doi:10.1016/j.urology.2009.08.071
12. Munigala S, Jackups RR Jr, Poirier RF, et al. Impact of order set design on urine culturing practices at an academic medical centre emergency department. BMJ Qual Saf. 2018;27(8):587-592. doi:10.1136/bmjqs-2017-006899
13. Jones CW, Culbreath KD, Mehrotra A, Gilligan PH. Reflect urine culture cancellation in the emergency department. J Emerg Med. 2014;46(1):71-76. doi:10.1016/j.jemermed.2013.08.042
14. Hertz JT, Lescallette RD, Barrett TW, Ward MJ, Self WH. External validation of an ED protocol for reflex urine culture cancelation. Am J Emerg Med. 2015;33(12):1838-1839. doi:10.1016/j.ajem.2015.09.026
15. Stamm WE. Measurement of pyuria and its relation to bacteriuria. Am J Med. 1983;75(1B):53-58. doi:10.1016/0002-9343(83)90073-6
16. Leis JA, Rebick GW, Daneman N, et al. Reducing antimicrobial therapy for asymptomatic bacteriuria among noncatheterized inpatients: a proof-of-concept study. Clin Infect Dis. 2014;58(7):980-983. doi:10.1093/cid/ciu010
17. Stagg A, Lutz H, Kirpalaney S, et al. Impact of two-step urine culture ordering in the emergency department: a time series analysis. BMJ Qual Saf. 2017;27:140-147. doi:10.1136/bmjqs-2016-006250