User login
The mammography controversy: When should you screen?
Breast cancer is the second most common cause of cancer death in US women,1,2 and screening mammography has been shown to decrease mortality.3,4 But the age at which to start screening, the intervals between mammograms, and the extent of the benefits (and harmful effects) of mammography are still hotly debated.
The clash between those who favor greater use of mammography and those who prefer less frequent and delayed screening heated up in July, when the American College of Obstetricians and Gynecologists (ACOG) released its new breast cancer screening guidelines.5 ACOG now recommends annual mammography starting at age 40; its previous guidelines called for mammograms every 1 to 2 years for women in their 40s and annual screening beginning at age 50.5
The US Preventive Services Task Force (USPSTF) issued updated breast cancer screening guidelines in November 2009 (TABLE 1).5,6 The new guidelines oppose routine screening for women ages 40 to 49 and recommend biennial, rather than annual, mammography for women ages 50 through 74. The decision to initiate screening before age 50 should be an individual one, based on the patient’s values as well as her individual risk factors, the USPSTF maintains. The Task Force, which previously recommended mammography every 1 to 2 years for all women ages 40 and older, does not recommend breast self-examination and finds insufficient evidence to assess the benefits of clinical breast exams.7
Both organizations have prominent medical groups in their camp: The American Cancer Society, National Comprehensive Cancer Network, American College of Surgeons, and American College of Radiology, among others, echo ACOG’s call for annual screening starting at age 40, while the American Academy of Family Physicians, American College of Physicians, National Breast Cancer Coalition, and World Health Organization (WHO) support the USPSTF’s position.8-10
Where does this leave you and your female patients? A look at the rationale behind these divergent recommendations and the latest evidence of the benefits and risks associated with screening mammography will help you cut through the controversy.
TABLE 1
Breast cancer screening: Divergent views5,6
| Organization | Age (years) | BSE | CBE | Mammography |
|---|---|---|---|---|
| ACOG | ≥40 | Encourages breast self-awareness | Annually | Annually |
| USPSTF | 40-49 50-74 | Recommends against teaching (D) | Insufficient evidence (I) | Not routinely recommended (C) Every 2 y (B) |
| USPSTF grades | ||||
| A: Recommended (high certainty of substantial benefit) | ||||
| B: Recommended (moderate or high certainty of moderate benefit or moderate certainty of substantial benefit) | ||||
| C: Not routinely recommended (at least moderate certainty that benefit is small) | ||||
| D: Not recommended (moderate or high certainty of no benefit or that harms outweigh benefits) | ||||
| I: Evidence is insufficient to assess benefits and harms | ||||
| ACOG, American College of Obstetricians and Gynecologists; BSE, breast self-examination; CBE, clinical breast examination; USPSTF, United States Preventive Services Task Force. Source: USPSTF. Grade definitions. May 2008. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm. Accessed August 19, 2011. | ||||
Same facts, different conclusions
The recommendations of the USPSTF are based on a systematic review of randomized clinical trials and data from the Cancer Intervention and Surveillance Modeling Network (CISNET) that allowed the researchers to assess various screening parameters.6,8,11 ACOG, too, based its guidelines on an evidence review,12 including the same data used by the USPSTF. Each organization interpreted the findings differently, however, particularly with regard to the benefits and potential harms associated with screening mammography.
The USPSTF points out that screening leads to the greatest absolute reduction in breast cancer mortality in women older than 50. For women ages 39 to 49, the USPSTF analysis revealed, it would take 1904 mammograms to prevent one breast cancer death. For women ages 50 to 59, the number of mammograms needed to prevent a single breast cancer death is 1339; and for women in their 60s, the number needed to screen is just 377.8
The USPSTF notes that false-positive results can lead to additional medical visits and unnecessary treatment, as well as potential psychological harm.7,8
ACOG focused more on cancer growth. Although women in their 40s have a lower probability of breast cancer (1 in 69) than their older counterparts (1 in 42 for women in their 50s and 1 in 29 for women in their 60s) (TABLE 2),2,5,8,12 tumors tend to grow faster in the younger women. That fact played a key role in shaping ACOG’s new guidelines. The average “sojourn time” (the interval between the time a breast tumor can be detected by mammogram and the time at which it has grown enough to become symptomatic) is 2 to 2.4 years for women in their 40s, compared with 4 to 4.1 years for women ages 70 to 74, ACOG estimates. Annual mammograms starting at age 40 provide a better chance of finding and treating breast cancer in an early stage.5,12
The reduction in breast cancer deaths associated with annual screening is about the same for both groups, according to ACOG—16% for women in their 40s, and 15% for women 50 and older.12 The 5-year survival rate for women whose breast tumors are discovered before they’re palpable and before the cancer has spread is 98%.13
ACOG also interpreted the potential harms associated with screening differently. The organization acknowledges that false-positive findings are a continuing concern, but has determined that the benefits of annual screening outweigh the risks.12
TABLE 2
Breast cancer and mammography: How age affects outcomes
| Breast cancer | |
|---|---|
| Age range (y) | Probability (%)2,12 |
| 40-49 | 1 in 69 (1.4) |
| 50-59 | 1 in 42 (2.4) |
| 60-69 | 1 in 29 (3.5) |
| Sojourn time*5,12 | |
| 40-49 | 2-2.4 y |
| ≥70 | 4-4.1 y |
| NNS to prevent 1 breast cancer death8 | |
| 40-49 | 1904 |
| 50-59 | 1339 |
| 60-69 | 377 |
| NNS, number needed to screen. *Interval between the time a breast tumor is detectable by mammography and it becomes symptomatic. | |
Recent studies hit the headlines, but fail to lend clarity
Norwegian cohort study. One study examining the effect of mammography on breast cancer mortality in a large cohort of Norwegian women found that patients ages 50 to 69 who were screened biennially had a 10% reduction in breast cancer death.14 However, further analysis suggested that screening in and of itself accounted for only about one-third of the reduction—an absolute risk reduction of 2.4 deaths per 100,000 person-years. (The rest was attributed to other factors, such as advances in breast cancer awareness and treatment.14) The study was published in the New England Journal of Medicine along with an editorial suggesting that it might be time to consider the rather small effects of screening mammography.15
Swedish cohort study. A study involving a large cohort of Swedish women found that mammography screening was associated with a 29% reduction in breast cancer mortality for women between the ages of 40 and 49.16 Notably, however, the difference in relative risk (RR) for women who were invited to be screened (0.74; 95% confidence interval [CI], 0.66-0.83) vs those who underwent regular screening (0.71; 95% CI, 0.62-0.80) was small.
CISNET modeling study. In a study in the American Journal of Roentgenology, researchers used the same data and CISNET modeling as the USPSTF, but compared lives saved with biennial screening mammography starting at age 50 vs annual screening starting at 40. The researchers reported that for women ages 40 to 84 years, approximately 12 lives per 1000 women screened annually would be saved; for women between the ages of 50 to 74 years screened biennially, 7 lives per 1000 people screened would be saved. That translates into 71% more lives saved with annual, rather than biennial, screening—a reduction of approximately 23%.17
There was a downside, however: The researchers estimated that, on average, women who initiated annual mammography at age 40 would receive a false-positive result every 10 years, and be recalled for imaging every 12 years. Other potential (albeit rare) harms identified by the researchers: one false-positive biopsy (every 149 years), one missed case of breast cancer (every 1000 years), and one fatal radiation-induced breast cancer (every 76,000-79,000 years). 17
2011 Cochrane review. In an update of a 2006 meta-analysis, Cochrane reviewers estimated that screening mammography results in a 15% decrease in breast cancer deaths (an absolute risk reduction of 0.05%).18 But screening also led to a 30% increase in overdiagnosis and overtreatment (an increase in absolute risk of 0.5%). That finding, which prompted the reviewers to conclude that it is not clear whether screening mammography does more good than harm, means that over the course of 10 years, for every 2000 women screened, 10 healthy women can expect to undergo unnecessary diagnostic procedures and receive unnecessary treatment.18
European trend analysis. A retrospective trend analysis published in the British Medical Journal in July 2011 is the latest assessment of the benefits of screening mammography.19 The researchers used WHO data to evaluate breast cancer mortality in several European countries, comparing nations with similar demographics and access to care but different levels of breast cancer screening. Their findings? From 1989 to 2006, reductions in breast cancer mortality were about the same in countries with similarities in levels of health care and demographics, regardless of mammography screening.19
How best to meet your patient’s needs
Where does this leave you? Supporters of the USPSTF’s recommendations have argued that they offer an evidence-based approach to mammography screening for women at average risk, and will help decrease excessive screening and the overdiagnosis, overtreatment, and psychological stress that often result. Critics maintain that trying to fit all women into a single model of breast cancer screening continues to be a problem—one that neither the USPSTF or ACOG has adequately addressed. The risks of breast cancer among various minority groups, for example, have not been taken into account.
Poll finds that patients and providers don’t see eye to eye
In February 2010, Annals of Internal Medicine conducted a Web-based survey relating to the USPSTF’s new screening guidelines. Of the 651 respondents, more than half (54%) were physicians, 9% were nonphysician health care providers, and 37% were potential patients. The findings suggest that health care providers and those they treat do not always see eye-to-eye when it comes to breast cancer screening.20
Two-thirds of the health care professionals surveyed said they would stop offering routine mammograms to women ages 40 to 49, in accordance with the USPSTF’s recommendation, and 62% would advise women ages 50 to 74 to have biennial, rather than annual, mammograms. In addition, 54% of clinicians indicated that they would stop recommending routine screening mammography to women who are 75 or older—a group for whom the USPSTF has stated that evidence is insufficient to assess the benefits and harms of screening. In contrast, 71% of the women said they were unlikely to forego routine mammography in their 40s—and less than 20% said they would wait until age 50 to begin screening or opt for biennial, rather than annual, screenings.20 Although the women’s views may be similar to those held by many of your female patients, the American Cancer Society estimates that about half of US women who are eligible for screening do not get mammograms.21
FIGURE
A digital mammogram showing normal but dense breast tissue
What is your patient’s level of risk?
Individual risk assessment, as stated earlier, is a key factor in determining whether to initiate screening for women younger than 50. It’s important to keep in mind, however, that only half of all breast cancers occur in women with well-established risk factors, including family history, a variety of reproductive risk factors, a high body mass index, and exposure to exogenous estrogen. Fully 50% of women who develop breast cancer are not at elevated risk.13
New models to aid in the shared decision-making process and risk assessment are being developed. One example is a Web-based interactive tool developed by researchers at the University of Sydney to give women in their 40s the information they need to make an informed decision about whether to start screening before age 50 (http://www.mammogram.med.usyd.edu.au/).22 This decision tool answers 2 key questions for women who are not at elevated risk for breast cancer:
| Q: | How many 40-year-old women who start having screening mammograms every 2 years will die from breast cancer in the next 10 years? |
| A: | Out of 1000 40-year-old women who start having screening mammograms every 2 years for the next 10 years, 2 women will die of breast cancer. |
| Q: | How many 40-year-old women who do not have screening mammograms will die from breast cancer in the next 10 years? |
| A: | Out of 1000 40-year-old women who do not have screening mammograms every 2 years for the next 10 years, 2.5 women will die of breast cancer.22,23 |
To our knowledge, there is no such patient-focused decision aid intended for use in the United States. There are assessment tools recommended for use by health care professionals, however. The interactive Breast Cancer Risk Assessment Tool, also known as the Gail Model (http://www.cancer.gov/bcrisktool), provides a population-based, rather than an individualized, estimate of a woman’s risk of developing invasive breast cancer in the next 5 years, as well as her lifetime risk. It incorporates current age, age at menarche, age at parity, number of first-degree maternal relatives with breast cancer, number of breast biopsies, and history of atypical hyperplasia. However, the Gail Model has a C-statistic (a measure of how well a clinical prediction tool correctly ranks patient risk) of just 0.5 to 0.6, which is slightly better than chance. The addition of breast density as a risk criterion in an attempt to boost the tool’s predictive value resulted in minimal improvement. 24,25
A novel approach. In the absence of ideal screening methodology or risk assessment tools, the authors of a recent cost-effectiveness analysis suggest a novel approach: They recommend that all women have a screening mammogram at the age of 40. The primary purpose is to assess breast density.26 That assessment should be key in making decisions about future screenings, as increased breast density is associated with a 4-fold increase in breast cancer risk.27,28
Faced with 2 very different recommendations for breast cancer screening from 2 very reputable organizations, JFP asked these physicians how they handle the mammography controversy, and what they recommend that primary care physicians do.
 |  |  |
| Andrew M. Kaunitz, MD | Jane L. Murray, MD | Cheryl Iglesia, MD, FACOG |
Andrew M. Kaunitz, MD, a professor of obstetrics and gynecology at the University of Florida College of Medicine and a member of the editorial board of OBG Management, says he continues to recommend mammography to all women ages 50 and older, regardless of risk. He has stopped “nagging” women to get screened, however, and—in the absence of elevated risk—has become more flexible about the frequency of mammograms and the age at which to initiate screening.
Dr. Kaunitz encourages women in their 40s to be screened if they have a history of breast cancer, a high body mass index, or other risk factors. If a woman in her 40s is not at elevated risk but is more comfortable being screened, he says, “I’ll order a mammogram for her, too. I’m certainly not going to stand in the way.”
Most women in their 50s prefer annual mammography, Dr. Kaunitz has found, although some appreciate his flexibility. “We recently moved to an office with imaging facilities and I often tell women they can wait until their next visit to be screened—which may be 3 months, 6 months, 9 months, or more.” Others are “aghast” if their physician does not recommend an annual mammogram.
Jane L. Murray, MD, founder of the Sastun Center of Integrative Health Care in Overland Park, Kan, and a member of the editorial board of The Journal of Family Practice, maintains a similar approach.
“I tell patients that the latest guidelines from an unbiased group [USPSTF] state that low-risk women—women who have no family history of breast cancer and are not taking hormones—can begin screening at age 50 and have mammograms every other year,” Dr. Murray says. “I recommend imaging if there is any suspicion at all.”
About two-thirds of her patients are happy to hear that an annual mammogram is no longer necessary. Some patients insist on annual screening—”‘My best friend got breast cancer,’ they often say.”
Dr. Murray’s approach to screening for patients at low risk for breast cancer is to explain that mammograms aren’t perfect and can miss some tumors and overdiagnose others. “Nonetheless, they’re the best we’ve got,” she tells patients, adding, “I recommend screening, but you decide for yourself. “If I thought mammography was a perfect test, I’d be a lot more adamant,” she says.
Cheryl Iglesia, MD, FACOG, is director, section of female pelvic medicine and reconstructive surgery at Washington (DC) Hospital Center, and a member of the board of OBG Management. Dr. Iglesia was chair of ACOG’s gynecologic practice committee and helped to develop the organization’s new guidelines, and has a different view.
“After reviewing all the data, I think that the most important thing that came out of it is that in women ages 40 through 49, breast cancers are more aggressive than they are in older, post-menopausal women.” Thus, she recommends routine screening for women in this age group. “A practice that delays screening until age 50,” she observes, “may be missing the boat.”
Dr. Iglesia also recommends that women in their 40s receive annual mammograms—a practice that’s in line with the recommendations of the American Cancer Society and one that she herself adheres to. The interval between when a cancer is detectable on mammography and the time it becomes symptomatic—known as the “sojourn time”—is about 2 years for women ages 40 through 49, she explains, and more frequent screening would be more likely to catch breast cancer in the preclinical phase. “That’s what a screening test is supposed to do.”
Helen Lippman, Managing Editor
CORRESPONDENCE
Sandhya Pruthi, MD, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; pruthi.sandhya@mayo.edu
1. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA-Cancer J Clin. 2009;59:225-249.
2. National Cancer Institute. Fact sheet. Probability of breast cancer in American women. Available at: http://www.cancer.gov/cancertopics/factsheet/detection/probability-breast-cancer. Accessed August 23, 2011.
3. Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784-1792.
4. Humphrey LL, Helfand M, Chan BK, et al. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137(5 part 1):347-360.
5. American College of Obstetricians and Gynecologists. Annual mammograms now recommended for women beginning at age 40. July 20, 2011. Available at: http://www.acog.org/from_home/publications/press_releases/nr07-20-11-2.cfm. Accessed August 15, 2011.
6. US Preventive Services Task Force. Screening for breast cancer. July 2010. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrca.htm. Accessed August 15, 2011.
7. Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer:. an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727–737, W237–742.
8. US Preventive Services Task Force. Screening for breast cancer. US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
9. Smith RA, Cokkinides V, Brooks D, et al. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA-Cancer J Clin. 2010;60:99-119.
10. Lee CH, Dershaw DD, Kopans D, et al. Breast cancer screening with imaging: recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J Am Coll Radiol. 2010;7:18-27.
11. Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151:738-747.
12. American College of Obstetricians and Gynecologists. Practice bulletin no. 122: breast cancer screening. Obstet Gynecol. 2011;118:372-382.
13. Madigan MP, Ziegler RG, Benichou J, et al. Proportion of breast cancer cases in the United States explained by well-established risk factors. J Natl Cancer Inst. 1995;87:1681-1685.
14. Kalager M, Zelen M, Langmark F, et al. Effect of screening mammography on breast-cancer mortality in Norway. N Engl J Med. 2010;363:1203-1210.
15. Welch HG. Screening mammography—a long run for a short slide? N Engl J Med. 2010;363:1276-1278.
16. Hellquist BN, Duffy SW, Abdsaleh S, et al. Effectiveness of population-based service screening with mammography for women ages 40 to 49 years: evaluation of the Swedish Mammography Screening in Young Women (SCRY) cohort. Cancer. 2010;117:714-722.
17. Hendrick RE, Helvie MA. United States Preventive Services Task Force screening mammography recommendations: science ignored. AJR Am J Roentgenol. 2011;196:W112-W116.
18. Gotzche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2011;(1):CD001877.-
19. Autier P, Boniol M, Gavin A, et al. Breast cancer mortality in neighbouring European countries with different levels of screening but similar access to treatment: trend analysis of WHO mortality database. BMJ. 2011;343:d4411.-
20. When evidence collides with anecdote, politics, and emotion: breast cancer screening [editorial]. Ann Intern Med. 2010;152:531-532.
21. American Cancer Society. Cancer prevention and early detection facts and figures 2009. Available at: http://www.cancer.org/Research/CancerFactsFigures/CancerPreventionEarlyDetectionFactsFigures/index. Accessed August 15, 2011.
22. Australian Screening Mammography Decision Aid Trial. Available at: http://www.mammogram.med.usyd.edu.au/. Accessed August 15, 2011.
23. Barratt A, Howard K, Irwig L, et al. Model of outcomes of screening mammography: information to support informed choices. BMJ. 2005;330:936.-
24. Chen J, Pee D, Ayyagari R, et al. Projecting absolute invasive breast cancer risk in women with a model that includes mammographic density. J Natl Cancer Inst. 2006;98:1215-1226.
25. Tice JA, Cummings SR, Ziv E, et al. Mammographic breast density and the Gail Model for breast cancer risk prediction in a screening population. Breast Cancer Res Treat. 2005;94:115-122.
26. Schousboe JT, Kerlikowske K, Loh A, et al. Personalizing mammography by breast density and other risk factors for breast cancer: analysis of health benefits and cost-effectiveness. Ann Intern Med. 2011;155:10-20.
27. Boyd NF, Byng JW, Jong RA, et al. Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst. 1995;87:670-675.
28. Tamimi R, Byrne C, Colditz EG, et al. Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2007;99:1178-1187.
Breast cancer is the second most common cause of cancer death in US women,1,2 and screening mammography has been shown to decrease mortality.3,4 But the age at which to start screening, the intervals between mammograms, and the extent of the benefits (and harmful effects) of mammography are still hotly debated.
The clash between those who favor greater use of mammography and those who prefer less frequent and delayed screening heated up in July, when the American College of Obstetricians and Gynecologists (ACOG) released its new breast cancer screening guidelines.5 ACOG now recommends annual mammography starting at age 40; its previous guidelines called for mammograms every 1 to 2 years for women in their 40s and annual screening beginning at age 50.5
The US Preventive Services Task Force (USPSTF) issued updated breast cancer screening guidelines in November 2009 (TABLE 1).5,6 The new guidelines oppose routine screening for women ages 40 to 49 and recommend biennial, rather than annual, mammography for women ages 50 through 74. The decision to initiate screening before age 50 should be an individual one, based on the patient’s values as well as her individual risk factors, the USPSTF maintains. The Task Force, which previously recommended mammography every 1 to 2 years for all women ages 40 and older, does not recommend breast self-examination and finds insufficient evidence to assess the benefits of clinical breast exams.7
Both organizations have prominent medical groups in their camp: The American Cancer Society, National Comprehensive Cancer Network, American College of Surgeons, and American College of Radiology, among others, echo ACOG’s call for annual screening starting at age 40, while the American Academy of Family Physicians, American College of Physicians, National Breast Cancer Coalition, and World Health Organization (WHO) support the USPSTF’s position.8-10
Where does this leave you and your female patients? A look at the rationale behind these divergent recommendations and the latest evidence of the benefits and risks associated with screening mammography will help you cut through the controversy.
TABLE 1
Breast cancer screening: Divergent views5,6
| Organization | Age (years) | BSE | CBE | Mammography |
|---|---|---|---|---|
| ACOG | ≥40 | Encourages breast self-awareness | Annually | Annually |
| USPSTF | 40-49 50-74 | Recommends against teaching (D) | Insufficient evidence (I) | Not routinely recommended (C) Every 2 y (B) |
| USPSTF grades | ||||
| A: Recommended (high certainty of substantial benefit) | ||||
| B: Recommended (moderate or high certainty of moderate benefit or moderate certainty of substantial benefit) | ||||
| C: Not routinely recommended (at least moderate certainty that benefit is small) | ||||
| D: Not recommended (moderate or high certainty of no benefit or that harms outweigh benefits) | ||||
| I: Evidence is insufficient to assess benefits and harms | ||||
| ACOG, American College of Obstetricians and Gynecologists; BSE, breast self-examination; CBE, clinical breast examination; USPSTF, United States Preventive Services Task Force. Source: USPSTF. Grade definitions. May 2008. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm. Accessed August 19, 2011. | ||||
Same facts, different conclusions
The recommendations of the USPSTF are based on a systematic review of randomized clinical trials and data from the Cancer Intervention and Surveillance Modeling Network (CISNET) that allowed the researchers to assess various screening parameters.6,8,11 ACOG, too, based its guidelines on an evidence review,12 including the same data used by the USPSTF. Each organization interpreted the findings differently, however, particularly with regard to the benefits and potential harms associated with screening mammography.
The USPSTF points out that screening leads to the greatest absolute reduction in breast cancer mortality in women older than 50. For women ages 39 to 49, the USPSTF analysis revealed, it would take 1904 mammograms to prevent one breast cancer death. For women ages 50 to 59, the number of mammograms needed to prevent a single breast cancer death is 1339; and for women in their 60s, the number needed to screen is just 377.8
The USPSTF notes that false-positive results can lead to additional medical visits and unnecessary treatment, as well as potential psychological harm.7,8
ACOG focused more on cancer growth. Although women in their 40s have a lower probability of breast cancer (1 in 69) than their older counterparts (1 in 42 for women in their 50s and 1 in 29 for women in their 60s) (TABLE 2),2,5,8,12 tumors tend to grow faster in the younger women. That fact played a key role in shaping ACOG’s new guidelines. The average “sojourn time” (the interval between the time a breast tumor can be detected by mammogram and the time at which it has grown enough to become symptomatic) is 2 to 2.4 years for women in their 40s, compared with 4 to 4.1 years for women ages 70 to 74, ACOG estimates. Annual mammograms starting at age 40 provide a better chance of finding and treating breast cancer in an early stage.5,12
The reduction in breast cancer deaths associated with annual screening is about the same for both groups, according to ACOG—16% for women in their 40s, and 15% for women 50 and older.12 The 5-year survival rate for women whose breast tumors are discovered before they’re palpable and before the cancer has spread is 98%.13
ACOG also interpreted the potential harms associated with screening differently. The organization acknowledges that false-positive findings are a continuing concern, but has determined that the benefits of annual screening outweigh the risks.12
TABLE 2
Breast cancer and mammography: How age affects outcomes
| Breast cancer | |
|---|---|
| Age range (y) | Probability (%)2,12 |
| 40-49 | 1 in 69 (1.4) |
| 50-59 | 1 in 42 (2.4) |
| 60-69 | 1 in 29 (3.5) |
| Sojourn time*5,12 | |
| 40-49 | 2-2.4 y |
| ≥70 | 4-4.1 y |
| NNS to prevent 1 breast cancer death8 | |
| 40-49 | 1904 |
| 50-59 | 1339 |
| 60-69 | 377 |
| NNS, number needed to screen. *Interval between the time a breast tumor is detectable by mammography and it becomes symptomatic. | |
Recent studies hit the headlines, but fail to lend clarity
Norwegian cohort study. One study examining the effect of mammography on breast cancer mortality in a large cohort of Norwegian women found that patients ages 50 to 69 who were screened biennially had a 10% reduction in breast cancer death.14 However, further analysis suggested that screening in and of itself accounted for only about one-third of the reduction—an absolute risk reduction of 2.4 deaths per 100,000 person-years. (The rest was attributed to other factors, such as advances in breast cancer awareness and treatment.14) The study was published in the New England Journal of Medicine along with an editorial suggesting that it might be time to consider the rather small effects of screening mammography.15
Swedish cohort study. A study involving a large cohort of Swedish women found that mammography screening was associated with a 29% reduction in breast cancer mortality for women between the ages of 40 and 49.16 Notably, however, the difference in relative risk (RR) for women who were invited to be screened (0.74; 95% confidence interval [CI], 0.66-0.83) vs those who underwent regular screening (0.71; 95% CI, 0.62-0.80) was small.
CISNET modeling study. In a study in the American Journal of Roentgenology, researchers used the same data and CISNET modeling as the USPSTF, but compared lives saved with biennial screening mammography starting at age 50 vs annual screening starting at 40. The researchers reported that for women ages 40 to 84 years, approximately 12 lives per 1000 women screened annually would be saved; for women between the ages of 50 to 74 years screened biennially, 7 lives per 1000 people screened would be saved. That translates into 71% more lives saved with annual, rather than biennial, screening—a reduction of approximately 23%.17
There was a downside, however: The researchers estimated that, on average, women who initiated annual mammography at age 40 would receive a false-positive result every 10 years, and be recalled for imaging every 12 years. Other potential (albeit rare) harms identified by the researchers: one false-positive biopsy (every 149 years), one missed case of breast cancer (every 1000 years), and one fatal radiation-induced breast cancer (every 76,000-79,000 years). 17
2011 Cochrane review. In an update of a 2006 meta-analysis, Cochrane reviewers estimated that screening mammography results in a 15% decrease in breast cancer deaths (an absolute risk reduction of 0.05%).18 But screening also led to a 30% increase in overdiagnosis and overtreatment (an increase in absolute risk of 0.5%). That finding, which prompted the reviewers to conclude that it is not clear whether screening mammography does more good than harm, means that over the course of 10 years, for every 2000 women screened, 10 healthy women can expect to undergo unnecessary diagnostic procedures and receive unnecessary treatment.18
European trend analysis. A retrospective trend analysis published in the British Medical Journal in July 2011 is the latest assessment of the benefits of screening mammography.19 The researchers used WHO data to evaluate breast cancer mortality in several European countries, comparing nations with similar demographics and access to care but different levels of breast cancer screening. Their findings? From 1989 to 2006, reductions in breast cancer mortality were about the same in countries with similarities in levels of health care and demographics, regardless of mammography screening.19
How best to meet your patient’s needs
Where does this leave you? Supporters of the USPSTF’s recommendations have argued that they offer an evidence-based approach to mammography screening for women at average risk, and will help decrease excessive screening and the overdiagnosis, overtreatment, and psychological stress that often result. Critics maintain that trying to fit all women into a single model of breast cancer screening continues to be a problem—one that neither the USPSTF or ACOG has adequately addressed. The risks of breast cancer among various minority groups, for example, have not been taken into account.
Poll finds that patients and providers don’t see eye to eye
In February 2010, Annals of Internal Medicine conducted a Web-based survey relating to the USPSTF’s new screening guidelines. Of the 651 respondents, more than half (54%) were physicians, 9% were nonphysician health care providers, and 37% were potential patients. The findings suggest that health care providers and those they treat do not always see eye-to-eye when it comes to breast cancer screening.20
Two-thirds of the health care professionals surveyed said they would stop offering routine mammograms to women ages 40 to 49, in accordance with the USPSTF’s recommendation, and 62% would advise women ages 50 to 74 to have biennial, rather than annual, mammograms. In addition, 54% of clinicians indicated that they would stop recommending routine screening mammography to women who are 75 or older—a group for whom the USPSTF has stated that evidence is insufficient to assess the benefits and harms of screening. In contrast, 71% of the women said they were unlikely to forego routine mammography in their 40s—and less than 20% said they would wait until age 50 to begin screening or opt for biennial, rather than annual, screenings.20 Although the women’s views may be similar to those held by many of your female patients, the American Cancer Society estimates that about half of US women who are eligible for screening do not get mammograms.21
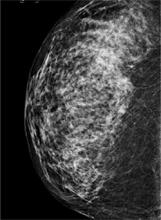
FIGURE
A digital mammogram showing normal but dense breast tissue
What is your patient’s level of risk?
Individual risk assessment, as stated earlier, is a key factor in determining whether to initiate screening for women younger than 50. It’s important to keep in mind, however, that only half of all breast cancers occur in women with well-established risk factors, including family history, a variety of reproductive risk factors, a high body mass index, and exposure to exogenous estrogen. Fully 50% of women who develop breast cancer are not at elevated risk.13
New models to aid in the shared decision-making process and risk assessment are being developed. One example is a Web-based interactive tool developed by researchers at the University of Sydney to give women in their 40s the information they need to make an informed decision about whether to start screening before age 50 (http://www.mammogram.med.usyd.edu.au/).22 This decision tool answers 2 key questions for women who are not at elevated risk for breast cancer:
| Q: | How many 40-year-old women who start having screening mammograms every 2 years will die from breast cancer in the next 10 years? |
| A: | Out of 1000 40-year-old women who start having screening mammograms every 2 years for the next 10 years, 2 women will die of breast cancer. |
| Q: | How many 40-year-old women who do not have screening mammograms will die from breast cancer in the next 10 years? |
| A: | Out of 1000 40-year-old women who do not have screening mammograms every 2 years for the next 10 years, 2.5 women will die of breast cancer.22,23 |
To our knowledge, there is no such patient-focused decision aid intended for use in the United States. There are assessment tools recommended for use by health care professionals, however. The interactive Breast Cancer Risk Assessment Tool, also known as the Gail Model (http://www.cancer.gov/bcrisktool), provides a population-based, rather than an individualized, estimate of a woman’s risk of developing invasive breast cancer in the next 5 years, as well as her lifetime risk. It incorporates current age, age at menarche, age at parity, number of first-degree maternal relatives with breast cancer, number of breast biopsies, and history of atypical hyperplasia. However, the Gail Model has a C-statistic (a measure of how well a clinical prediction tool correctly ranks patient risk) of just 0.5 to 0.6, which is slightly better than chance. The addition of breast density as a risk criterion in an attempt to boost the tool’s predictive value resulted in minimal improvement. 24,25
A novel approach. In the absence of ideal screening methodology or risk assessment tools, the authors of a recent cost-effectiveness analysis suggest a novel approach: They recommend that all women have a screening mammogram at the age of 40. The primary purpose is to assess breast density.26 That assessment should be key in making decisions about future screenings, as increased breast density is associated with a 4-fold increase in breast cancer risk.27,28
Faced with 2 very different recommendations for breast cancer screening from 2 very reputable organizations, JFP asked these physicians how they handle the mammography controversy, and what they recommend that primary care physicians do.
 |  |  |
| Andrew M. Kaunitz, MD | Jane L. Murray, MD | Cheryl Iglesia, MD, FACOG |
Andrew M. Kaunitz, MD, a professor of obstetrics and gynecology at the University of Florida College of Medicine and a member of the editorial board of OBG Management, says he continues to recommend mammography to all women ages 50 and older, regardless of risk. He has stopped “nagging” women to get screened, however, and—in the absence of elevated risk—has become more flexible about the frequency of mammograms and the age at which to initiate screening.
Dr. Kaunitz encourages women in their 40s to be screened if they have a history of breast cancer, a high body mass index, or other risk factors. If a woman in her 40s is not at elevated risk but is more comfortable being screened, he says, “I’ll order a mammogram for her, too. I’m certainly not going to stand in the way.”
Most women in their 50s prefer annual mammography, Dr. Kaunitz has found, although some appreciate his flexibility. “We recently moved to an office with imaging facilities and I often tell women they can wait until their next visit to be screened—which may be 3 months, 6 months, 9 months, or more.” Others are “aghast” if their physician does not recommend an annual mammogram.
Jane L. Murray, MD, founder of the Sastun Center of Integrative Health Care in Overland Park, Kan, and a member of the editorial board of The Journal of Family Practice, maintains a similar approach.
“I tell patients that the latest guidelines from an unbiased group [USPSTF] state that low-risk women—women who have no family history of breast cancer and are not taking hormones—can begin screening at age 50 and have mammograms every other year,” Dr. Murray says. “I recommend imaging if there is any suspicion at all.”
About two-thirds of her patients are happy to hear that an annual mammogram is no longer necessary. Some patients insist on annual screening—”‘My best friend got breast cancer,’ they often say.”
Dr. Murray’s approach to screening for patients at low risk for breast cancer is to explain that mammograms aren’t perfect and can miss some tumors and overdiagnose others. “Nonetheless, they’re the best we’ve got,” she tells patients, adding, “I recommend screening, but you decide for yourself. “If I thought mammography was a perfect test, I’d be a lot more adamant,” she says.
Cheryl Iglesia, MD, FACOG, is director, section of female pelvic medicine and reconstructive surgery at Washington (DC) Hospital Center, and a member of the board of OBG Management. Dr. Iglesia was chair of ACOG’s gynecologic practice committee and helped to develop the organization’s new guidelines, and has a different view.
“After reviewing all the data, I think that the most important thing that came out of it is that in women ages 40 through 49, breast cancers are more aggressive than they are in older, post-menopausal women.” Thus, she recommends routine screening for women in this age group. “A practice that delays screening until age 50,” she observes, “may be missing the boat.”
Dr. Iglesia also recommends that women in their 40s receive annual mammograms—a practice that’s in line with the recommendations of the American Cancer Society and one that she herself adheres to. The interval between when a cancer is detectable on mammography and the time it becomes symptomatic—known as the “sojourn time”—is about 2 years for women ages 40 through 49, she explains, and more frequent screening would be more likely to catch breast cancer in the preclinical phase. “That’s what a screening test is supposed to do.”
Helen Lippman, Managing Editor
CORRESPONDENCE
Sandhya Pruthi, MD, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; pruthi.sandhya@mayo.edu
Breast cancer is the second most common cause of cancer death in US women,1,2 and screening mammography has been shown to decrease mortality.3,4 But the age at which to start screening, the intervals between mammograms, and the extent of the benefits (and harmful effects) of mammography are still hotly debated.
The clash between those who favor greater use of mammography and those who prefer less frequent and delayed screening heated up in July, when the American College of Obstetricians and Gynecologists (ACOG) released its new breast cancer screening guidelines.5 ACOG now recommends annual mammography starting at age 40; its previous guidelines called for mammograms every 1 to 2 years for women in their 40s and annual screening beginning at age 50.5
The US Preventive Services Task Force (USPSTF) issued updated breast cancer screening guidelines in November 2009 (TABLE 1).5,6 The new guidelines oppose routine screening for women ages 40 to 49 and recommend biennial, rather than annual, mammography for women ages 50 through 74. The decision to initiate screening before age 50 should be an individual one, based on the patient’s values as well as her individual risk factors, the USPSTF maintains. The Task Force, which previously recommended mammography every 1 to 2 years for all women ages 40 and older, does not recommend breast self-examination and finds insufficient evidence to assess the benefits of clinical breast exams.7
Both organizations have prominent medical groups in their camp: The American Cancer Society, National Comprehensive Cancer Network, American College of Surgeons, and American College of Radiology, among others, echo ACOG’s call for annual screening starting at age 40, while the American Academy of Family Physicians, American College of Physicians, National Breast Cancer Coalition, and World Health Organization (WHO) support the USPSTF’s position.8-10
Where does this leave you and your female patients? A look at the rationale behind these divergent recommendations and the latest evidence of the benefits and risks associated with screening mammography will help you cut through the controversy.
TABLE 1
Breast cancer screening: Divergent views5,6
| Organization | Age (years) | BSE | CBE | Mammography |
|---|---|---|---|---|
| ACOG | ≥40 | Encourages breast self-awareness | Annually | Annually |
| USPSTF | 40-49 50-74 | Recommends against teaching (D) | Insufficient evidence (I) | Not routinely recommended (C) Every 2 y (B) |
| USPSTF grades | ||||
| A: Recommended (high certainty of substantial benefit) | ||||
| B: Recommended (moderate or high certainty of moderate benefit or moderate certainty of substantial benefit) | ||||
| C: Not routinely recommended (at least moderate certainty that benefit is small) | ||||
| D: Not recommended (moderate or high certainty of no benefit or that harms outweigh benefits) | ||||
| I: Evidence is insufficient to assess benefits and harms | ||||
| ACOG, American College of Obstetricians and Gynecologists; BSE, breast self-examination; CBE, clinical breast examination; USPSTF, United States Preventive Services Task Force. Source: USPSTF. Grade definitions. May 2008. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm. Accessed August 19, 2011. | ||||
Same facts, different conclusions
The recommendations of the USPSTF are based on a systematic review of randomized clinical trials and data from the Cancer Intervention and Surveillance Modeling Network (CISNET) that allowed the researchers to assess various screening parameters.6,8,11 ACOG, too, based its guidelines on an evidence review,12 including the same data used by the USPSTF. Each organization interpreted the findings differently, however, particularly with regard to the benefits and potential harms associated with screening mammography.
The USPSTF points out that screening leads to the greatest absolute reduction in breast cancer mortality in women older than 50. For women ages 39 to 49, the USPSTF analysis revealed, it would take 1904 mammograms to prevent one breast cancer death. For women ages 50 to 59, the number of mammograms needed to prevent a single breast cancer death is 1339; and for women in their 60s, the number needed to screen is just 377.8
The USPSTF notes that false-positive results can lead to additional medical visits and unnecessary treatment, as well as potential psychological harm.7,8
ACOG focused more on cancer growth. Although women in their 40s have a lower probability of breast cancer (1 in 69) than their older counterparts (1 in 42 for women in their 50s and 1 in 29 for women in their 60s) (TABLE 2),2,5,8,12 tumors tend to grow faster in the younger women. That fact played a key role in shaping ACOG’s new guidelines. The average “sojourn time” (the interval between the time a breast tumor can be detected by mammogram and the time at which it has grown enough to become symptomatic) is 2 to 2.4 years for women in their 40s, compared with 4 to 4.1 years for women ages 70 to 74, ACOG estimates. Annual mammograms starting at age 40 provide a better chance of finding and treating breast cancer in an early stage.5,12
The reduction in breast cancer deaths associated with annual screening is about the same for both groups, according to ACOG—16% for women in their 40s, and 15% for women 50 and older.12 The 5-year survival rate for women whose breast tumors are discovered before they’re palpable and before the cancer has spread is 98%.13
ACOG also interpreted the potential harms associated with screening differently. The organization acknowledges that false-positive findings are a continuing concern, but has determined that the benefits of annual screening outweigh the risks.12
TABLE 2
Breast cancer and mammography: How age affects outcomes
| Breast cancer | |
|---|---|
| Age range (y) | Probability (%)2,12 |
| 40-49 | 1 in 69 (1.4) |
| 50-59 | 1 in 42 (2.4) |
| 60-69 | 1 in 29 (3.5) |
| Sojourn time*5,12 | |
| 40-49 | 2-2.4 y |
| ≥70 | 4-4.1 y |
| NNS to prevent 1 breast cancer death8 | |
| 40-49 | 1904 |
| 50-59 | 1339 |
| 60-69 | 377 |
| NNS, number needed to screen. *Interval between the time a breast tumor is detectable by mammography and it becomes symptomatic. | |
Recent studies hit the headlines, but fail to lend clarity
Norwegian cohort study. One study examining the effect of mammography on breast cancer mortality in a large cohort of Norwegian women found that patients ages 50 to 69 who were screened biennially had a 10% reduction in breast cancer death.14 However, further analysis suggested that screening in and of itself accounted for only about one-third of the reduction—an absolute risk reduction of 2.4 deaths per 100,000 person-years. (The rest was attributed to other factors, such as advances in breast cancer awareness and treatment.14) The study was published in the New England Journal of Medicine along with an editorial suggesting that it might be time to consider the rather small effects of screening mammography.15
Swedish cohort study. A study involving a large cohort of Swedish women found that mammography screening was associated with a 29% reduction in breast cancer mortality for women between the ages of 40 and 49.16 Notably, however, the difference in relative risk (RR) for women who were invited to be screened (0.74; 95% confidence interval [CI], 0.66-0.83) vs those who underwent regular screening (0.71; 95% CI, 0.62-0.80) was small.
CISNET modeling study. In a study in the American Journal of Roentgenology, researchers used the same data and CISNET modeling as the USPSTF, but compared lives saved with biennial screening mammography starting at age 50 vs annual screening starting at 40. The researchers reported that for women ages 40 to 84 years, approximately 12 lives per 1000 women screened annually would be saved; for women between the ages of 50 to 74 years screened biennially, 7 lives per 1000 people screened would be saved. That translates into 71% more lives saved with annual, rather than biennial, screening—a reduction of approximately 23%.17
There was a downside, however: The researchers estimated that, on average, women who initiated annual mammography at age 40 would receive a false-positive result every 10 years, and be recalled for imaging every 12 years. Other potential (albeit rare) harms identified by the researchers: one false-positive biopsy (every 149 years), one missed case of breast cancer (every 1000 years), and one fatal radiation-induced breast cancer (every 76,000-79,000 years). 17
2011 Cochrane review. In an update of a 2006 meta-analysis, Cochrane reviewers estimated that screening mammography results in a 15% decrease in breast cancer deaths (an absolute risk reduction of 0.05%).18 But screening also led to a 30% increase in overdiagnosis and overtreatment (an increase in absolute risk of 0.5%). That finding, which prompted the reviewers to conclude that it is not clear whether screening mammography does more good than harm, means that over the course of 10 years, for every 2000 women screened, 10 healthy women can expect to undergo unnecessary diagnostic procedures and receive unnecessary treatment.18
European trend analysis. A retrospective trend analysis published in the British Medical Journal in July 2011 is the latest assessment of the benefits of screening mammography.19 The researchers used WHO data to evaluate breast cancer mortality in several European countries, comparing nations with similar demographics and access to care but different levels of breast cancer screening. Their findings? From 1989 to 2006, reductions in breast cancer mortality were about the same in countries with similarities in levels of health care and demographics, regardless of mammography screening.19
How best to meet your patient’s needs
Where does this leave you? Supporters of the USPSTF’s recommendations have argued that they offer an evidence-based approach to mammography screening for women at average risk, and will help decrease excessive screening and the overdiagnosis, overtreatment, and psychological stress that often result. Critics maintain that trying to fit all women into a single model of breast cancer screening continues to be a problem—one that neither the USPSTF or ACOG has adequately addressed. The risks of breast cancer among various minority groups, for example, have not been taken into account.
Poll finds that patients and providers don’t see eye to eye
In February 2010, Annals of Internal Medicine conducted a Web-based survey relating to the USPSTF’s new screening guidelines. Of the 651 respondents, more than half (54%) were physicians, 9% were nonphysician health care providers, and 37% were potential patients. The findings suggest that health care providers and those they treat do not always see eye-to-eye when it comes to breast cancer screening.20
Two-thirds of the health care professionals surveyed said they would stop offering routine mammograms to women ages 40 to 49, in accordance with the USPSTF’s recommendation, and 62% would advise women ages 50 to 74 to have biennial, rather than annual, mammograms. In addition, 54% of clinicians indicated that they would stop recommending routine screening mammography to women who are 75 or older—a group for whom the USPSTF has stated that evidence is insufficient to assess the benefits and harms of screening. In contrast, 71% of the women said they were unlikely to forego routine mammography in their 40s—and less than 20% said they would wait until age 50 to begin screening or opt for biennial, rather than annual, screenings.20 Although the women’s views may be similar to those held by many of your female patients, the American Cancer Society estimates that about half of US women who are eligible for screening do not get mammograms.21
FIGURE
A digital mammogram showing normal but dense breast tissue
What is your patient’s level of risk?
Individual risk assessment, as stated earlier, is a key factor in determining whether to initiate screening for women younger than 50. It’s important to keep in mind, however, that only half of all breast cancers occur in women with well-established risk factors, including family history, a variety of reproductive risk factors, a high body mass index, and exposure to exogenous estrogen. Fully 50% of women who develop breast cancer are not at elevated risk.13
New models to aid in the shared decision-making process and risk assessment are being developed. One example is a Web-based interactive tool developed by researchers at the University of Sydney to give women in their 40s the information they need to make an informed decision about whether to start screening before age 50 (http://www.mammogram.med.usyd.edu.au/).22 This decision tool answers 2 key questions for women who are not at elevated risk for breast cancer:
| Q: | How many 40-year-old women who start having screening mammograms every 2 years will die from breast cancer in the next 10 years? |
| A: | Out of 1000 40-year-old women who start having screening mammograms every 2 years for the next 10 years, 2 women will die of breast cancer. |
| Q: | How many 40-year-old women who do not have screening mammograms will die from breast cancer in the next 10 years? |
| A: | Out of 1000 40-year-old women who do not have screening mammograms every 2 years for the next 10 years, 2.5 women will die of breast cancer.22,23 |
To our knowledge, there is no such patient-focused decision aid intended for use in the United States. There are assessment tools recommended for use by health care professionals, however. The interactive Breast Cancer Risk Assessment Tool, also known as the Gail Model (http://www.cancer.gov/bcrisktool), provides a population-based, rather than an individualized, estimate of a woman’s risk of developing invasive breast cancer in the next 5 years, as well as her lifetime risk. It incorporates current age, age at menarche, age at parity, number of first-degree maternal relatives with breast cancer, number of breast biopsies, and history of atypical hyperplasia. However, the Gail Model has a C-statistic (a measure of how well a clinical prediction tool correctly ranks patient risk) of just 0.5 to 0.6, which is slightly better than chance. The addition of breast density as a risk criterion in an attempt to boost the tool’s predictive value resulted in minimal improvement. 24,25
A novel approach. In the absence of ideal screening methodology or risk assessment tools, the authors of a recent cost-effectiveness analysis suggest a novel approach: They recommend that all women have a screening mammogram at the age of 40. The primary purpose is to assess breast density.26 That assessment should be key in making decisions about future screenings, as increased breast density is associated with a 4-fold increase in breast cancer risk.27,28
Faced with 2 very different recommendations for breast cancer screening from 2 very reputable organizations, JFP asked these physicians how they handle the mammography controversy, and what they recommend that primary care physicians do.
 |  |  |
| Andrew M. Kaunitz, MD | Jane L. Murray, MD | Cheryl Iglesia, MD, FACOG |
Andrew M. Kaunitz, MD, a professor of obstetrics and gynecology at the University of Florida College of Medicine and a member of the editorial board of OBG Management, says he continues to recommend mammography to all women ages 50 and older, regardless of risk. He has stopped “nagging” women to get screened, however, and—in the absence of elevated risk—has become more flexible about the frequency of mammograms and the age at which to initiate screening.
Dr. Kaunitz encourages women in their 40s to be screened if they have a history of breast cancer, a high body mass index, or other risk factors. If a woman in her 40s is not at elevated risk but is more comfortable being screened, he says, “I’ll order a mammogram for her, too. I’m certainly not going to stand in the way.”
Most women in their 50s prefer annual mammography, Dr. Kaunitz has found, although some appreciate his flexibility. “We recently moved to an office with imaging facilities and I often tell women they can wait until their next visit to be screened—which may be 3 months, 6 months, 9 months, or more.” Others are “aghast” if their physician does not recommend an annual mammogram.
Jane L. Murray, MD, founder of the Sastun Center of Integrative Health Care in Overland Park, Kan, and a member of the editorial board of The Journal of Family Practice, maintains a similar approach.
“I tell patients that the latest guidelines from an unbiased group [USPSTF] state that low-risk women—women who have no family history of breast cancer and are not taking hormones—can begin screening at age 50 and have mammograms every other year,” Dr. Murray says. “I recommend imaging if there is any suspicion at all.”
About two-thirds of her patients are happy to hear that an annual mammogram is no longer necessary. Some patients insist on annual screening—”‘My best friend got breast cancer,’ they often say.”
Dr. Murray’s approach to screening for patients at low risk for breast cancer is to explain that mammograms aren’t perfect and can miss some tumors and overdiagnose others. “Nonetheless, they’re the best we’ve got,” she tells patients, adding, “I recommend screening, but you decide for yourself. “If I thought mammography was a perfect test, I’d be a lot more adamant,” she says.
Cheryl Iglesia, MD, FACOG, is director, section of female pelvic medicine and reconstructive surgery at Washington (DC) Hospital Center, and a member of the board of OBG Management. Dr. Iglesia was chair of ACOG’s gynecologic practice committee and helped to develop the organization’s new guidelines, and has a different view.
“After reviewing all the data, I think that the most important thing that came out of it is that in women ages 40 through 49, breast cancers are more aggressive than they are in older, post-menopausal women.” Thus, she recommends routine screening for women in this age group. “A practice that delays screening until age 50,” she observes, “may be missing the boat.”
Dr. Iglesia also recommends that women in their 40s receive annual mammograms—a practice that’s in line with the recommendations of the American Cancer Society and one that she herself adheres to. The interval between when a cancer is detectable on mammography and the time it becomes symptomatic—known as the “sojourn time”—is about 2 years for women ages 40 through 49, she explains, and more frequent screening would be more likely to catch breast cancer in the preclinical phase. “That’s what a screening test is supposed to do.”
Helen Lippman, Managing Editor
CORRESPONDENCE
Sandhya Pruthi, MD, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; pruthi.sandhya@mayo.edu
1. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA-Cancer J Clin. 2009;59:225-249.
2. National Cancer Institute. Fact sheet. Probability of breast cancer in American women. Available at: http://www.cancer.gov/cancertopics/factsheet/detection/probability-breast-cancer. Accessed August 23, 2011.
3. Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784-1792.
4. Humphrey LL, Helfand M, Chan BK, et al. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137(5 part 1):347-360.
5. American College of Obstetricians and Gynecologists. Annual mammograms now recommended for women beginning at age 40. July 20, 2011. Available at: http://www.acog.org/from_home/publications/press_releases/nr07-20-11-2.cfm. Accessed August 15, 2011.
6. US Preventive Services Task Force. Screening for breast cancer. July 2010. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrca.htm. Accessed August 15, 2011.
7. Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer:. an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727–737, W237–742.
8. US Preventive Services Task Force. Screening for breast cancer. US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
9. Smith RA, Cokkinides V, Brooks D, et al. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA-Cancer J Clin. 2010;60:99-119.
10. Lee CH, Dershaw DD, Kopans D, et al. Breast cancer screening with imaging: recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J Am Coll Radiol. 2010;7:18-27.
11. Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151:738-747.
12. American College of Obstetricians and Gynecologists. Practice bulletin no. 122: breast cancer screening. Obstet Gynecol. 2011;118:372-382.
13. Madigan MP, Ziegler RG, Benichou J, et al. Proportion of breast cancer cases in the United States explained by well-established risk factors. J Natl Cancer Inst. 1995;87:1681-1685.
14. Kalager M, Zelen M, Langmark F, et al. Effect of screening mammography on breast-cancer mortality in Norway. N Engl J Med. 2010;363:1203-1210.
15. Welch HG. Screening mammography—a long run for a short slide? N Engl J Med. 2010;363:1276-1278.
16. Hellquist BN, Duffy SW, Abdsaleh S, et al. Effectiveness of population-based service screening with mammography for women ages 40 to 49 years: evaluation of the Swedish Mammography Screening in Young Women (SCRY) cohort. Cancer. 2010;117:714-722.
17. Hendrick RE, Helvie MA. United States Preventive Services Task Force screening mammography recommendations: science ignored. AJR Am J Roentgenol. 2011;196:W112-W116.
18. Gotzche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2011;(1):CD001877.-
19. Autier P, Boniol M, Gavin A, et al. Breast cancer mortality in neighbouring European countries with different levels of screening but similar access to treatment: trend analysis of WHO mortality database. BMJ. 2011;343:d4411.-
20. When evidence collides with anecdote, politics, and emotion: breast cancer screening [editorial]. Ann Intern Med. 2010;152:531-532.
21. American Cancer Society. Cancer prevention and early detection facts and figures 2009. Available at: http://www.cancer.org/Research/CancerFactsFigures/CancerPreventionEarlyDetectionFactsFigures/index. Accessed August 15, 2011.
22. Australian Screening Mammography Decision Aid Trial. Available at: http://www.mammogram.med.usyd.edu.au/. Accessed August 15, 2011.
23. Barratt A, Howard K, Irwig L, et al. Model of outcomes of screening mammography: information to support informed choices. BMJ. 2005;330:936.-
24. Chen J, Pee D, Ayyagari R, et al. Projecting absolute invasive breast cancer risk in women with a model that includes mammographic density. J Natl Cancer Inst. 2006;98:1215-1226.
25. Tice JA, Cummings SR, Ziv E, et al. Mammographic breast density and the Gail Model for breast cancer risk prediction in a screening population. Breast Cancer Res Treat. 2005;94:115-122.
26. Schousboe JT, Kerlikowske K, Loh A, et al. Personalizing mammography by breast density and other risk factors for breast cancer: analysis of health benefits and cost-effectiveness. Ann Intern Med. 2011;155:10-20.
27. Boyd NF, Byng JW, Jong RA, et al. Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst. 1995;87:670-675.
28. Tamimi R, Byrne C, Colditz EG, et al. Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2007;99:1178-1187.
1. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA-Cancer J Clin. 2009;59:225-249.
2. National Cancer Institute. Fact sheet. Probability of breast cancer in American women. Available at: http://www.cancer.gov/cancertopics/factsheet/detection/probability-breast-cancer. Accessed August 23, 2011.
3. Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784-1792.
4. Humphrey LL, Helfand M, Chan BK, et al. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137(5 part 1):347-360.
5. American College of Obstetricians and Gynecologists. Annual mammograms now recommended for women beginning at age 40. July 20, 2011. Available at: http://www.acog.org/from_home/publications/press_releases/nr07-20-11-2.cfm. Accessed August 15, 2011.
6. US Preventive Services Task Force. Screening for breast cancer. July 2010. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrca.htm. Accessed August 15, 2011.
7. Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer:. an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727–737, W237–742.
8. US Preventive Services Task Force. Screening for breast cancer. US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
9. Smith RA, Cokkinides V, Brooks D, et al. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA-Cancer J Clin. 2010;60:99-119.
10. Lee CH, Dershaw DD, Kopans D, et al. Breast cancer screening with imaging: recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J Am Coll Radiol. 2010;7:18-27.
11. Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151:738-747.
12. American College of Obstetricians and Gynecologists. Practice bulletin no. 122: breast cancer screening. Obstet Gynecol. 2011;118:372-382.
13. Madigan MP, Ziegler RG, Benichou J, et al. Proportion of breast cancer cases in the United States explained by well-established risk factors. J Natl Cancer Inst. 1995;87:1681-1685.
14. Kalager M, Zelen M, Langmark F, et al. Effect of screening mammography on breast-cancer mortality in Norway. N Engl J Med. 2010;363:1203-1210.
15. Welch HG. Screening mammography—a long run for a short slide? N Engl J Med. 2010;363:1276-1278.
16. Hellquist BN, Duffy SW, Abdsaleh S, et al. Effectiveness of population-based service screening with mammography for women ages 40 to 49 years: evaluation of the Swedish Mammography Screening in Young Women (SCRY) cohort. Cancer. 2010;117:714-722.
17. Hendrick RE, Helvie MA. United States Preventive Services Task Force screening mammography recommendations: science ignored. AJR Am J Roentgenol. 2011;196:W112-W116.
18. Gotzche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2011;(1):CD001877.-
19. Autier P, Boniol M, Gavin A, et al. Breast cancer mortality in neighbouring European countries with different levels of screening but similar access to treatment: trend analysis of WHO mortality database. BMJ. 2011;343:d4411.-
20. When evidence collides with anecdote, politics, and emotion: breast cancer screening [editorial]. Ann Intern Med. 2010;152:531-532.
21. American Cancer Society. Cancer prevention and early detection facts and figures 2009. Available at: http://www.cancer.org/Research/CancerFactsFigures/CancerPreventionEarlyDetectionFactsFigures/index. Accessed August 15, 2011.
22. Australian Screening Mammography Decision Aid Trial. Available at: http://www.mammogram.med.usyd.edu.au/. Accessed August 15, 2011.
23. Barratt A, Howard K, Irwig L, et al. Model of outcomes of screening mammography: information to support informed choices. BMJ. 2005;330:936.-
24. Chen J, Pee D, Ayyagari R, et al. Projecting absolute invasive breast cancer risk in women with a model that includes mammographic density. J Natl Cancer Inst. 2006;98:1215-1226.
25. Tice JA, Cummings SR, Ziv E, et al. Mammographic breast density and the Gail Model for breast cancer risk prediction in a screening population. Breast Cancer Res Treat. 2005;94:115-122.
26. Schousboe JT, Kerlikowske K, Loh A, et al. Personalizing mammography by breast density and other risk factors for breast cancer: analysis of health benefits and cost-effectiveness. Ann Intern Med. 2011;155:10-20.
27. Boyd NF, Byng JW, Jong RA, et al. Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst. 1995;87:670-675.
28. Tamimi R, Byrne C, Colditz EG, et al. Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2007;99:1178-1187.