User login
Angioedema Following tPA Administration for Acute Cerebrovascular Accident
The use of thrombolytic medications for the treatment of acute ischemic cerebral infarctions has dynamically altered stroke care. However, there are both major and minor side effects associated with its use—most notably major bleeding, which led to strict inclusion and exclusion criteria governing the administration of this medication class. One less recognized but potentially serious complication is angioedema secondary to tissue plasminogen activator (tPA). Our case emphasizes the importance of early recognition of this clinical syndrome as it relates to airway compromise and potential respiratory failure in patients who are treated with tPA.
Case
A 70-year-old woman with a history of diabetes and hypertension and a remote history of breast cancer, nonhemiplegic migraines, and hypothyroidism presented to the ED with complaints of aphasia and right-sided paralysis, with onset 2 hours prior. Regarding the patient’s medication history, she had been taking lisinopril for hypertension.
Upon assessment, the patient was awake and alert and her vital signs were normal and stable, but she was aphasic, unable to accurately phonate, and was not able to move her right arm or leg against gravity. Her sensation appeared intact, and she had mild facial asymmetry with inability to raise the right corner of her mouth; her tongue had midline protrusion.
An emergent computed tomography (CT) scan of the head demonstrated mild brain atrophy and minimal low attenuation within the cerebral hemispheric white matter—most noticeably within the subcortical region of the left frontal lobe, consistent with small vessel ischemia. There was no evidence of acute intracranial hemorrhage, midline shift, or focal mass effect, and no convincing CT evidence for acute large vessel, cortical-based infarction.
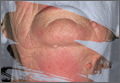
The patient was determined to be an appropriate candidate for tPA, and was consented in the usual fashion. Within 15 minutes of administration of intravenous (IV) tPA, her symptoms improved, the aphasia resolved, and she was able to lift her right arm and leg against gravity and verbally communicate. Approximately 30 minutes following the resolution of her neurological symptoms, however, the patient was noted to have bleeding around a tooth socket, which was controlled with gauze and pressure. She subsequently began to complain of swelling on her right inferior lip without acute airway compromise. Over the next 10 to 15 minutes, she began to develop tongue swelling and feelings of dyspnea without wheezing.
The patient’s airway was reassessed and was classified as a Mallampati class IV. Anesthesia services were consulted for an emergent, awake intubation for airway protection. She was medicated with midazolam IV, as well as atomized lidocaine and lidocaine gargle for local anesthesia. The patient was successfully intubated awake using a flexible fiber optic technique. She was admitted to the medical intensive care unit for further monitoring, where she was treated with IV methylprednisolone, famotidine, and diphenhydramine. She was extubated the following day, had a relatively uncomplicated hospital course, and was discharged on hospital day 5 with improvement in her speech and right-sided weakness.
Discussion
The risk of angioedema associated with tPA administration has been previously described, with an estimated rate of 1.3 to 5.1%.1-3 Studies have shown the risk of developing angioedema is significantly increased in the setting of concomitant use of an angiotensin converting enzyme inhibitor (ACE-I); CT studies have also shown evidence of frontal and insular ischemia, with an odds radio of 13.6 and 9.1, respectively.2 Our patient was on lisinopril and had early signs of ischemia in the frontal lobe on initial CT scan, which likely increased her risk for angioedema.
How tPA Can Trigger Angioedema
The development of angioedema after administration of tPA has a well-described biochemical basis. Angioedema has been linked to the local vasodilatory effects of bradykinin, mast cell degranulation, and histamine release from activation of the complement pathway.4 Tissue plasminogen activator may trigger both of these pathways. It is a serine protease that cleaves plasminogen to plasmin; the plasmin in turn cleaves fibrin, resulting in the desired thrombolytic effects.5 Plasmin can cause mast cell degranulation through conversion of C3 to C3a and through activation of the complement pathway through conversion of C1 to C1a.6
Studies have shown tPA to have low antigenicity, and activation of this pathway is most likely secondary to direct proteolytic effects as opposed to antibody complexes.7 In a study by Bennett et al,6 tPA was shown to significantly increase C3a, C4a, and C5a serum levels when given in the setting of myocardial infarction (MI). It has also been shown to activate and increase serum kallikrein, which cleaves high-molecular weight kininogen to bradykinin, a potent vasodilator.8,9
Since bradykinin is broken down by several enzymes, including ACE, degradation is therefore delayed in patients on ACE-I.10 The alternate pathway for bradykinin degradation in the absence of ACE may also result in formation of des-Arg bradykinin, another similar active metabolite that mimics the effects of bradykinins.9 The formation of bradykinin through the proteolytic effects of tPA, in combination with the delayed breakdown in patient’s taking an ACE-I, likely plays a significant role in the development of angioedema.
In addition to the direct proteolytic effect of tPA resulting in angioedema, the underlying ischemic insult may also predispose patients to angioedema. As was the case with our patient, angioedema preferentially affects the ipsilateral side of the patient’s deficit.2,11,12 Theories suggest this is due to the lack of autonomic compensatory responses in the setting of ischemic insult.2 Interestingly, the development of angioedema in relation to the use of recombinant-tPA (eg, alteplase) in the setting of MI has not been as well described and may be related to the effect of central nervous system insult.3
Treatment
Although hemorrhagic complications of tPA therapy for cerebrovascular accident are well known, the risk for angioedema as a complication is less recognized. In most cases, angioedema is transient, and very few patients require aggressive support.3,12 Treatments that have previously been described include antihistamines and steroids.1,11,13 Epinephrine has been reported in one case study as an adjunct treatment of tPA-induced angioedema; however, it was given in combination with steroids and antihistimines.14 Therefore, caution should be taken regarding the use of epinephrine in this setting as there may be a theoretical precipitation of intracranial hypertension or hemorrhage.2
Given the likely significant role of the bradykinin-mediated pathway in tPA-induced angioedema, the true efficacy of these agents is unknown. Our patient had significant labial and lingual involvement, and given the concern for impending airway compromise, fiber optic intubation was performed. The decision to intubate and the technique employed must be carefully considered as a failed airway and need for a surgical airway is a concerning prospect in the setting of fibrinolytics. Successful cricothyroidotomy without significant complications has been described in the setting of streptokinase-induced angioedema when given for MI.15
Conclusion
The use of tPA for the treatment of ischemic stroke has been increasing over the last decade.16,17 Given the high prevalence of ACE-I use in patients who are also at risk for ischemic stroke, physicians administering tPA must be aware of the risk of tPA-associated angioedema. Patients with a known history of angioedema or anaphylaxis to tPA should be counseled on these risks and should not be given this medication, but rather considered for potential endovascular or mechanical clot retrieval therapy if they meet inclusion criteria for its use.
1. Hill MD, Barber PA, Takahashi J, Demchuk AM, Feasby TE, Buchan AM. Anaphylactoid reactions and angioedema during alteplase treatment of acute ischemic stroke. CMAJ. 2000;162(9):1281-1284.
2. Hill MD, Lye T, Moss H, et al. Hemi-orolingual angioedema and ACE inhibition after alteplase treatment of stroke. Neurology. 2003;60(9):1525-1527.
3. Hill MD, Buchan AM; Canadian Alteplase for Stroke Effectiveness Study (CASES) Investigators. Thrombolysis for acute ischemic stroke: results of the Canadian Alteplase for Stroke Effectiveness Study. CMAJ. 2005;172(10):1307-1312.
4. Lewis LM. Angioedema: etiology, pathophysiology, current and emerging therapies. J Emerg Med. 2013;45(5):789-796.
5. Loscalzo J, Braunwald E. Tissue plasminogen activator. N Engl J Med. 1988;319(14):935-931.
6. Bennett WR, Yawn DH, Migliore PJ, et al. Activation of the complement system by recombinant tissue plasminogen activator. J Am Coll Cardiol. 1987;10(3):627-632.
7. Reed BR, Chen AB, Tanswell P, et al. Low incidence of antibodies to recombinant human tissue-type plasminogen activator in treated patients. Thromb Haemost. 1990;64(2):276-280.
8. Hoffmeister HM, Szabo S, Kastner C, et al. Thrombolytic therapy in acute myocardial infarction: comparison of procoagulant effects of streptokinase and alteplase regimens with focus on the kallikrein system and plasmin. Circulation. 1998;98(23):2527-2533.
9. Molinaro G, Gervais N, Adam A. Biochemical basis of angioedema associated with recombinant tissue plasminogen activator treatment: an in vitro experimental approach. Stroke. 2002;33(6):1712-1716.
10. Bezalel S, Mahlab-Guri K, Asher I, Werner B, Sthoeger ZM. Angiotensin-converting enzyme inhibitor-induced angioedema. Am J Med. 2015;128(2):120-125.
11. Pancioli A, Brott T, Donaldson V, Miller R. Asymmetric angioneurotic edema associated with thrombolysis for acute stroke. Ann Emerg Med. 1997;30(2):227-229.
12. Correia AS, Matias G, Calado S, Lourenço A, Viana-Baptista M. Orolingual angiodema associated with alteplase treatment of acute stroke: a reappraisal. J Stroke Cerebrovasc Dis. 2015;24(1):31-40.
13. Maertins M, Wol R, Swider M. Angioedema after administration of tPA for ischemic stroke: case report. Air Med J. 2011;30(5):276-278.
14. Fugate JE, Kalimullah EA, Wijdicks EF. Angioedema after tPA: what neurointensivists should know. Neurocrit Care. 2012;16(3):440-443.
15. Walls RM, Pollack CV Jr. Successful cricothyrotomy after thrombolytic therapy for acute myocardial infarction: a report of two cases. Ann Emerg Med. 2000;35(2):188-191.
16. Lichtman JH, Watanabe E, Allen NB, Jones SB, Dostal J, Goldstein LB. Hospital arrival time and intravenous t-PA use in US Academic Medical Centers, 2001-2004. Stroke. 2009;40(12):3845-3850.
17. Schwamm LH, Ali SF, Reeves MJ, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With the Guidelines-Stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6(5):543-549.
The use of thrombolytic medications for the treatment of acute ischemic cerebral infarctions has dynamically altered stroke care. However, there are both major and minor side effects associated with its use—most notably major bleeding, which led to strict inclusion and exclusion criteria governing the administration of this medication class. One less recognized but potentially serious complication is angioedema secondary to tissue plasminogen activator (tPA). Our case emphasizes the importance of early recognition of this clinical syndrome as it relates to airway compromise and potential respiratory failure in patients who are treated with tPA.
Case
A 70-year-old woman with a history of diabetes and hypertension and a remote history of breast cancer, nonhemiplegic migraines, and hypothyroidism presented to the ED with complaints of aphasia and right-sided paralysis, with onset 2 hours prior. Regarding the patient’s medication history, she had been taking lisinopril for hypertension.
Upon assessment, the patient was awake and alert and her vital signs were normal and stable, but she was aphasic, unable to accurately phonate, and was not able to move her right arm or leg against gravity. Her sensation appeared intact, and she had mild facial asymmetry with inability to raise the right corner of her mouth; her tongue had midline protrusion.
An emergent computed tomography (CT) scan of the head demonstrated mild brain atrophy and minimal low attenuation within the cerebral hemispheric white matter—most noticeably within the subcortical region of the left frontal lobe, consistent with small vessel ischemia. There was no evidence of acute intracranial hemorrhage, midline shift, or focal mass effect, and no convincing CT evidence for acute large vessel, cortical-based infarction.
The patient was determined to be an appropriate candidate for tPA, and was consented in the usual fashion. Within 15 minutes of administration of intravenous (IV) tPA, her symptoms improved, the aphasia resolved, and she was able to lift her right arm and leg against gravity and verbally communicate. Approximately 30 minutes following the resolution of her neurological symptoms, however, the patient was noted to have bleeding around a tooth socket, which was controlled with gauze and pressure. She subsequently began to complain of swelling on her right inferior lip without acute airway compromise. Over the next 10 to 15 minutes, she began to develop tongue swelling and feelings of dyspnea without wheezing.
The patient’s airway was reassessed and was classified as a Mallampati class IV. Anesthesia services were consulted for an emergent, awake intubation for airway protection. She was medicated with midazolam IV, as well as atomized lidocaine and lidocaine gargle for local anesthesia. The patient was successfully intubated awake using a flexible fiber optic technique. She was admitted to the medical intensive care unit for further monitoring, where she was treated with IV methylprednisolone, famotidine, and diphenhydramine. She was extubated the following day, had a relatively uncomplicated hospital course, and was discharged on hospital day 5 with improvement in her speech and right-sided weakness.
Discussion
The risk of angioedema associated with tPA administration has been previously described, with an estimated rate of 1.3 to 5.1%.1-3 Studies have shown the risk of developing angioedema is significantly increased in the setting of concomitant use of an angiotensin converting enzyme inhibitor (ACE-I); CT studies have also shown evidence of frontal and insular ischemia, with an odds radio of 13.6 and 9.1, respectively.2 Our patient was on lisinopril and had early signs of ischemia in the frontal lobe on initial CT scan, which likely increased her risk for angioedema.
How tPA Can Trigger Angioedema
The development of angioedema after administration of tPA has a well-described biochemical basis. Angioedema has been linked to the local vasodilatory effects of bradykinin, mast cell degranulation, and histamine release from activation of the complement pathway.4 Tissue plasminogen activator may trigger both of these pathways. It is a serine protease that cleaves plasminogen to plasmin; the plasmin in turn cleaves fibrin, resulting in the desired thrombolytic effects.5 Plasmin can cause mast cell degranulation through conversion of C3 to C3a and through activation of the complement pathway through conversion of C1 to C1a.6
Studies have shown tPA to have low antigenicity, and activation of this pathway is most likely secondary to direct proteolytic effects as opposed to antibody complexes.7 In a study by Bennett et al,6 tPA was shown to significantly increase C3a, C4a, and C5a serum levels when given in the setting of myocardial infarction (MI). It has also been shown to activate and increase serum kallikrein, which cleaves high-molecular weight kininogen to bradykinin, a potent vasodilator.8,9
Since bradykinin is broken down by several enzymes, including ACE, degradation is therefore delayed in patients on ACE-I.10 The alternate pathway for bradykinin degradation in the absence of ACE may also result in formation of des-Arg bradykinin, another similar active metabolite that mimics the effects of bradykinins.9 The formation of bradykinin through the proteolytic effects of tPA, in combination with the delayed breakdown in patient’s taking an ACE-I, likely plays a significant role in the development of angioedema.
In addition to the direct proteolytic effect of tPA resulting in angioedema, the underlying ischemic insult may also predispose patients to angioedema. As was the case with our patient, angioedema preferentially affects the ipsilateral side of the patient’s deficit.2,11,12 Theories suggest this is due to the lack of autonomic compensatory responses in the setting of ischemic insult.2 Interestingly, the development of angioedema in relation to the use of recombinant-tPA (eg, alteplase) in the setting of MI has not been as well described and may be related to the effect of central nervous system insult.3
Treatment
Although hemorrhagic complications of tPA therapy for cerebrovascular accident are well known, the risk for angioedema as a complication is less recognized. In most cases, angioedema is transient, and very few patients require aggressive support.3,12 Treatments that have previously been described include antihistamines and steroids.1,11,13 Epinephrine has been reported in one case study as an adjunct treatment of tPA-induced angioedema; however, it was given in combination with steroids and antihistimines.14 Therefore, caution should be taken regarding the use of epinephrine in this setting as there may be a theoretical precipitation of intracranial hypertension or hemorrhage.2
Given the likely significant role of the bradykinin-mediated pathway in tPA-induced angioedema, the true efficacy of these agents is unknown. Our patient had significant labial and lingual involvement, and given the concern for impending airway compromise, fiber optic intubation was performed. The decision to intubate and the technique employed must be carefully considered as a failed airway and need for a surgical airway is a concerning prospect in the setting of fibrinolytics. Successful cricothyroidotomy without significant complications has been described in the setting of streptokinase-induced angioedema when given for MI.15
Conclusion
The use of tPA for the treatment of ischemic stroke has been increasing over the last decade.16,17 Given the high prevalence of ACE-I use in patients who are also at risk for ischemic stroke, physicians administering tPA must be aware of the risk of tPA-associated angioedema. Patients with a known history of angioedema or anaphylaxis to tPA should be counseled on these risks and should not be given this medication, but rather considered for potential endovascular or mechanical clot retrieval therapy if they meet inclusion criteria for its use.
The use of thrombolytic medications for the treatment of acute ischemic cerebral infarctions has dynamically altered stroke care. However, there are both major and minor side effects associated with its use—most notably major bleeding, which led to strict inclusion and exclusion criteria governing the administration of this medication class. One less recognized but potentially serious complication is angioedema secondary to tissue plasminogen activator (tPA). Our case emphasizes the importance of early recognition of this clinical syndrome as it relates to airway compromise and potential respiratory failure in patients who are treated with tPA.
Case
A 70-year-old woman with a history of diabetes and hypertension and a remote history of breast cancer, nonhemiplegic migraines, and hypothyroidism presented to the ED with complaints of aphasia and right-sided paralysis, with onset 2 hours prior. Regarding the patient’s medication history, she had been taking lisinopril for hypertension.
Upon assessment, the patient was awake and alert and her vital signs were normal and stable, but she was aphasic, unable to accurately phonate, and was not able to move her right arm or leg against gravity. Her sensation appeared intact, and she had mild facial asymmetry with inability to raise the right corner of her mouth; her tongue had midline protrusion.
An emergent computed tomography (CT) scan of the head demonstrated mild brain atrophy and minimal low attenuation within the cerebral hemispheric white matter—most noticeably within the subcortical region of the left frontal lobe, consistent with small vessel ischemia. There was no evidence of acute intracranial hemorrhage, midline shift, or focal mass effect, and no convincing CT evidence for acute large vessel, cortical-based infarction.
The patient was determined to be an appropriate candidate for tPA, and was consented in the usual fashion. Within 15 minutes of administration of intravenous (IV) tPA, her symptoms improved, the aphasia resolved, and she was able to lift her right arm and leg against gravity and verbally communicate. Approximately 30 minutes following the resolution of her neurological symptoms, however, the patient was noted to have bleeding around a tooth socket, which was controlled with gauze and pressure. She subsequently began to complain of swelling on her right inferior lip without acute airway compromise. Over the next 10 to 15 minutes, she began to develop tongue swelling and feelings of dyspnea without wheezing.
The patient’s airway was reassessed and was classified as a Mallampati class IV. Anesthesia services were consulted for an emergent, awake intubation for airway protection. She was medicated with midazolam IV, as well as atomized lidocaine and lidocaine gargle for local anesthesia. The patient was successfully intubated awake using a flexible fiber optic technique. She was admitted to the medical intensive care unit for further monitoring, where she was treated with IV methylprednisolone, famotidine, and diphenhydramine. She was extubated the following day, had a relatively uncomplicated hospital course, and was discharged on hospital day 5 with improvement in her speech and right-sided weakness.
Discussion
The risk of angioedema associated with tPA administration has been previously described, with an estimated rate of 1.3 to 5.1%.1-3 Studies have shown the risk of developing angioedema is significantly increased in the setting of concomitant use of an angiotensin converting enzyme inhibitor (ACE-I); CT studies have also shown evidence of frontal and insular ischemia, with an odds radio of 13.6 and 9.1, respectively.2 Our patient was on lisinopril and had early signs of ischemia in the frontal lobe on initial CT scan, which likely increased her risk for angioedema.
How tPA Can Trigger Angioedema
The development of angioedema after administration of tPA has a well-described biochemical basis. Angioedema has been linked to the local vasodilatory effects of bradykinin, mast cell degranulation, and histamine release from activation of the complement pathway.4 Tissue plasminogen activator may trigger both of these pathways. It is a serine protease that cleaves plasminogen to plasmin; the plasmin in turn cleaves fibrin, resulting in the desired thrombolytic effects.5 Plasmin can cause mast cell degranulation through conversion of C3 to C3a and through activation of the complement pathway through conversion of C1 to C1a.6
Studies have shown tPA to have low antigenicity, and activation of this pathway is most likely secondary to direct proteolytic effects as opposed to antibody complexes.7 In a study by Bennett et al,6 tPA was shown to significantly increase C3a, C4a, and C5a serum levels when given in the setting of myocardial infarction (MI). It has also been shown to activate and increase serum kallikrein, which cleaves high-molecular weight kininogen to bradykinin, a potent vasodilator.8,9
Since bradykinin is broken down by several enzymes, including ACE, degradation is therefore delayed in patients on ACE-I.10 The alternate pathway for bradykinin degradation in the absence of ACE may also result in formation of des-Arg bradykinin, another similar active metabolite that mimics the effects of bradykinins.9 The formation of bradykinin through the proteolytic effects of tPA, in combination with the delayed breakdown in patient’s taking an ACE-I, likely plays a significant role in the development of angioedema.
In addition to the direct proteolytic effect of tPA resulting in angioedema, the underlying ischemic insult may also predispose patients to angioedema. As was the case with our patient, angioedema preferentially affects the ipsilateral side of the patient’s deficit.2,11,12 Theories suggest this is due to the lack of autonomic compensatory responses in the setting of ischemic insult.2 Interestingly, the development of angioedema in relation to the use of recombinant-tPA (eg, alteplase) in the setting of MI has not been as well described and may be related to the effect of central nervous system insult.3
Treatment
Although hemorrhagic complications of tPA therapy for cerebrovascular accident are well known, the risk for angioedema as a complication is less recognized. In most cases, angioedema is transient, and very few patients require aggressive support.3,12 Treatments that have previously been described include antihistamines and steroids.1,11,13 Epinephrine has been reported in one case study as an adjunct treatment of tPA-induced angioedema; however, it was given in combination with steroids and antihistimines.14 Therefore, caution should be taken regarding the use of epinephrine in this setting as there may be a theoretical precipitation of intracranial hypertension or hemorrhage.2
Given the likely significant role of the bradykinin-mediated pathway in tPA-induced angioedema, the true efficacy of these agents is unknown. Our patient had significant labial and lingual involvement, and given the concern for impending airway compromise, fiber optic intubation was performed. The decision to intubate and the technique employed must be carefully considered as a failed airway and need for a surgical airway is a concerning prospect in the setting of fibrinolytics. Successful cricothyroidotomy without significant complications has been described in the setting of streptokinase-induced angioedema when given for MI.15
Conclusion
The use of tPA for the treatment of ischemic stroke has been increasing over the last decade.16,17 Given the high prevalence of ACE-I use in patients who are also at risk for ischemic stroke, physicians administering tPA must be aware of the risk of tPA-associated angioedema. Patients with a known history of angioedema or anaphylaxis to tPA should be counseled on these risks and should not be given this medication, but rather considered for potential endovascular or mechanical clot retrieval therapy if they meet inclusion criteria for its use.
1. Hill MD, Barber PA, Takahashi J, Demchuk AM, Feasby TE, Buchan AM. Anaphylactoid reactions and angioedema during alteplase treatment of acute ischemic stroke. CMAJ. 2000;162(9):1281-1284.
2. Hill MD, Lye T, Moss H, et al. Hemi-orolingual angioedema and ACE inhibition after alteplase treatment of stroke. Neurology. 2003;60(9):1525-1527.
3. Hill MD, Buchan AM; Canadian Alteplase for Stroke Effectiveness Study (CASES) Investigators. Thrombolysis for acute ischemic stroke: results of the Canadian Alteplase for Stroke Effectiveness Study. CMAJ. 2005;172(10):1307-1312.
4. Lewis LM. Angioedema: etiology, pathophysiology, current and emerging therapies. J Emerg Med. 2013;45(5):789-796.
5. Loscalzo J, Braunwald E. Tissue plasminogen activator. N Engl J Med. 1988;319(14):935-931.
6. Bennett WR, Yawn DH, Migliore PJ, et al. Activation of the complement system by recombinant tissue plasminogen activator. J Am Coll Cardiol. 1987;10(3):627-632.
7. Reed BR, Chen AB, Tanswell P, et al. Low incidence of antibodies to recombinant human tissue-type plasminogen activator in treated patients. Thromb Haemost. 1990;64(2):276-280.
8. Hoffmeister HM, Szabo S, Kastner C, et al. Thrombolytic therapy in acute myocardial infarction: comparison of procoagulant effects of streptokinase and alteplase regimens with focus on the kallikrein system and plasmin. Circulation. 1998;98(23):2527-2533.
9. Molinaro G, Gervais N, Adam A. Biochemical basis of angioedema associated with recombinant tissue plasminogen activator treatment: an in vitro experimental approach. Stroke. 2002;33(6):1712-1716.
10. Bezalel S, Mahlab-Guri K, Asher I, Werner B, Sthoeger ZM. Angiotensin-converting enzyme inhibitor-induced angioedema. Am J Med. 2015;128(2):120-125.
11. Pancioli A, Brott T, Donaldson V, Miller R. Asymmetric angioneurotic edema associated with thrombolysis for acute stroke. Ann Emerg Med. 1997;30(2):227-229.
12. Correia AS, Matias G, Calado S, Lourenço A, Viana-Baptista M. Orolingual angiodema associated with alteplase treatment of acute stroke: a reappraisal. J Stroke Cerebrovasc Dis. 2015;24(1):31-40.
13. Maertins M, Wol R, Swider M. Angioedema after administration of tPA for ischemic stroke: case report. Air Med J. 2011;30(5):276-278.
14. Fugate JE, Kalimullah EA, Wijdicks EF. Angioedema after tPA: what neurointensivists should know. Neurocrit Care. 2012;16(3):440-443.
15. Walls RM, Pollack CV Jr. Successful cricothyrotomy after thrombolytic therapy for acute myocardial infarction: a report of two cases. Ann Emerg Med. 2000;35(2):188-191.
16. Lichtman JH, Watanabe E, Allen NB, Jones SB, Dostal J, Goldstein LB. Hospital arrival time and intravenous t-PA use in US Academic Medical Centers, 2001-2004. Stroke. 2009;40(12):3845-3850.
17. Schwamm LH, Ali SF, Reeves MJ, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With the Guidelines-Stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6(5):543-549.
1. Hill MD, Barber PA, Takahashi J, Demchuk AM, Feasby TE, Buchan AM. Anaphylactoid reactions and angioedema during alteplase treatment of acute ischemic stroke. CMAJ. 2000;162(9):1281-1284.
2. Hill MD, Lye T, Moss H, et al. Hemi-orolingual angioedema and ACE inhibition after alteplase treatment of stroke. Neurology. 2003;60(9):1525-1527.
3. Hill MD, Buchan AM; Canadian Alteplase for Stroke Effectiveness Study (CASES) Investigators. Thrombolysis for acute ischemic stroke: results of the Canadian Alteplase for Stroke Effectiveness Study. CMAJ. 2005;172(10):1307-1312.
4. Lewis LM. Angioedema: etiology, pathophysiology, current and emerging therapies. J Emerg Med. 2013;45(5):789-796.
5. Loscalzo J, Braunwald E. Tissue plasminogen activator. N Engl J Med. 1988;319(14):935-931.
6. Bennett WR, Yawn DH, Migliore PJ, et al. Activation of the complement system by recombinant tissue plasminogen activator. J Am Coll Cardiol. 1987;10(3):627-632.
7. Reed BR, Chen AB, Tanswell P, et al. Low incidence of antibodies to recombinant human tissue-type plasminogen activator in treated patients. Thromb Haemost. 1990;64(2):276-280.
8. Hoffmeister HM, Szabo S, Kastner C, et al. Thrombolytic therapy in acute myocardial infarction: comparison of procoagulant effects of streptokinase and alteplase regimens with focus on the kallikrein system and plasmin. Circulation. 1998;98(23):2527-2533.
9. Molinaro G, Gervais N, Adam A. Biochemical basis of angioedema associated with recombinant tissue plasminogen activator treatment: an in vitro experimental approach. Stroke. 2002;33(6):1712-1716.
10. Bezalel S, Mahlab-Guri K, Asher I, Werner B, Sthoeger ZM. Angiotensin-converting enzyme inhibitor-induced angioedema. Am J Med. 2015;128(2):120-125.
11. Pancioli A, Brott T, Donaldson V, Miller R. Asymmetric angioneurotic edema associated with thrombolysis for acute stroke. Ann Emerg Med. 1997;30(2):227-229.
12. Correia AS, Matias G, Calado S, Lourenço A, Viana-Baptista M. Orolingual angiodema associated with alteplase treatment of acute stroke: a reappraisal. J Stroke Cerebrovasc Dis. 2015;24(1):31-40.
13. Maertins M, Wol R, Swider M. Angioedema after administration of tPA for ischemic stroke: case report. Air Med J. 2011;30(5):276-278.
14. Fugate JE, Kalimullah EA, Wijdicks EF. Angioedema after tPA: what neurointensivists should know. Neurocrit Care. 2012;16(3):440-443.
15. Walls RM, Pollack CV Jr. Successful cricothyrotomy after thrombolytic therapy for acute myocardial infarction: a report of two cases. Ann Emerg Med. 2000;35(2):188-191.
16. Lichtman JH, Watanabe E, Allen NB, Jones SB, Dostal J, Goldstein LB. Hospital arrival time and intravenous t-PA use in US Academic Medical Centers, 2001-2004. Stroke. 2009;40(12):3845-3850.
17. Schwamm LH, Ali SF, Reeves MJ, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With the Guidelines-Stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6(5):543-549.