User login
Actinic Keratosis Treatment With Diclofenac Gel 1%
To the Editor:
Actinic keratoses (AKs) are keratinocyte neoplasms that manifest as rough, scaly, erythematous papules with ill-defined borders (commonly known as precancers) and develop due to long-term UV light exposure.1 They must be treated promptly due to the risk for progression to squamous cell carcinoma (SCC). One US Department of Veterans Affairs study reported that 0.6% of AKs progress to SCC in 1 year and 2.6% progressed to SCC in 4 years.2 In 10% of AKs that will progress to SCC, one study reported progression in approximately 2 years.3
The risk for progression also increases in patients with multiple AKs; the risk is 4-fold higher in patients with 6 to 20 AKs and 11-fold higher in patients with more than 20 AKs.4 Common treatment options include lesion-directed therapies such as cryotherapy, laser therapy, surgery, and curettage, as well as field-directed therapies such as topical 5-fluorouracil (5-FU), diclofenac gel 3%, chemical peeling, topical imiquimod, and photodynamic therapy (PDT).4 When diclofenac gel is chosen as a treatment modality, it is commonly prescribed in the 3% formulation. Diclofenac gel 3% has been shown to be effective in the treatment of AKs,5,6 but diclofenac gel 1% has not been well described in the literature. We report the case of a patient with AKs on the lower legs who was treated with diclofenac gel after other therapies failed.
A 55-year-old woman presented for a routine skin check due to a history of nonmelanoma skin cancer. Her medical history also included palmar hyperhidrosis, disseminated superficial actinic porokeratosis, and extensive actinic damage, as well as numerous biopsy-proven AKs. She had been evaluated every 3 months up to presentation due to the frequency of AK development over the past 5 years. The lesions were mainly localized to both lower legs, where the patient had acquired considerable lifetime sun exposure from tanning beds and sunbathing while boating. She also noted exposure to well water as a child, but none of her family members had a similar issue with AKs.
Prior to this visit, the patient had undergone 5 years of therapy for AKs. She initially was treated with multiple courses of topical 5-FU, but she consequently developed severe allergic contact dermatitis. Subsequent treatments included cryotherapy as well as application of tretinoin cream nightly for 2 weeks followed by PDT. She was unable to tolerate the tretinoin, which she reported led to dryness and irritation. She reported mild improvement after her first session of PDT but only minimal improvement after the next session. Ingenol mebutate was then prescribed for topical use on the legs for 2 days, which did not result in improvement. The patient continued to follow up for unresolved AKs on the legs and was prescribed acitretin to help reduce the risk for progression to SCC. At follow-up 3 months later, she reported decreased soreness from AKs after starting the acitretin and, aside from mild dryness, she tolerated the medication well; however, with continued use of acitretin, she began to experience adverse effects 6 months later, including thyroid suppression and hair loss, leading to discontinuation. Instead, 3 months later, she was recommended to start nicotinamide supplementation for prevention of SCC.
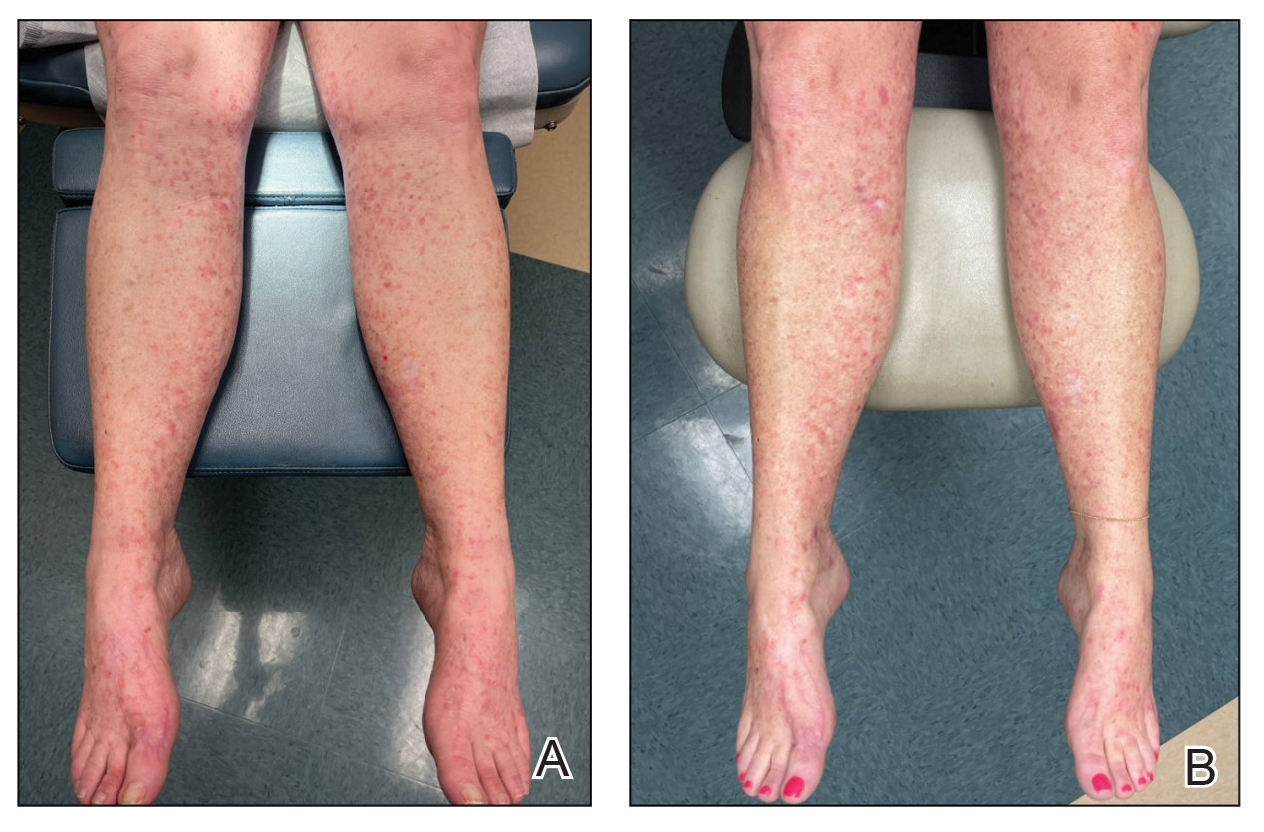
Due to continued AK development (Figure, A), we eventually prescribed diclofenac gel 3% twice daily for both legs 9 months after prescribing nicotinamide. This regimen was cost prohibitive, as the medication was not covered by her insurance and the cost was $300 for one tube. We recommended the patient instead apply the 3% gel to the right leg only due to greater severity of AKs on this leg and over-the-counter diclofenac gel 1% twice daily to the left leg. Approximately 5 months later, she reported a reduction in the discomfort from AKs as well as a reduction in the total number of AKs. She applied the 2 different products as instructed for the first month but did not notice a difference between them. She then continued to apply only the 1% gel on both legs for a total of 8 months with excellent response (Figure, B). At subsequent follow-up visits over a 2-year period, she has only required cryotherapy as spot treatment for AKs.
For 1 to a few discrete AKs, liquid nitrogen cryotherapy is considered first-line therapy.7 However, if multiple AKs are present, surrounding photodamaged skin also should be treated with field-directed therapy due to surrounding keratinocytes bearing a high mutational burden and risk of cancerization.8 Common field-directed therapies include topical 5-FU, topical imiquimod, topical tirbanibulin, PDT, retinoids, and topical diclofenac 3%.
One challenge in field-directed treatment of AKs is the side-effect profile seen in some patients, causing them to prematurely discontinue treatment. In our patient, 5-FU cream, tretinoin cream, and oral acitretin were not well tolerated. Topical diclofenac generally is well tolerated, with mostly mild local skin reactions and low risk for systemic adverse events. Adverse effects mainly consist of mild local skin reactions including pruritus (reported in 31%-52% of patients who used topical diclofenac), dryness (25%-27%), and irritation (less than 1%).9,10 Although diclofenac carries a black-box warning for serious cardiovascular thrombotic events and serious gastrointestinal tract bleeding, systemic absorption of topical diclofenac has been proven to be substantially lower (5- to 17-fold) compared to the oral formulation, and resulting serious adverse effects have been found to be largely reduced compared to the oral formulation.11,12 If allergic contact dermatitis develops, diclofenac should be discontinued.9,13
Diclofenac’s antineoplastic mechanism of action of cyclooxygenase-2 inhibition involves induction of apoptosis as well as reduction in tumor cell proliferation and tumor angiogenesis.14,15 Topical diclofenac may result in decreased levels of lactate and amino acid in AK lesions, particularly in lesions responding to treatment.16 Topical diclofenac may alter immune infiltration by inducing infiltration of dermal CD8+ T cells along with high IFN-γ messenger RNA expression, suggesting improvement of T-cell function after topical diclofenac treatment.16
Although diclofenac gel 3% has been shown to be effective in treatment of AKs,5,6 diclofenac gel 1% has not yet been well studied. Use of the 1% gel is indicated for osteoarthritis and musculoskeletal pain by the US Food and Drug Administration.10,17 Efficacy of the 1% gel has been documented for these and other conditions including seborrheic keratoses.18-20
Because the 1% diclofenac formulation is available over-the-counter, it is more accessible to patients compared to the 3% formulation and often substantially decreases the cost of the medication for the patient. The cost of diclofenac gel 1% in the United States ranges from $0.04 to $0.31 per gram compared to $1.07 to $11.79 per gram for the 3% gel prescription formulation.17 Efficacy of the 1% formulation compared to the 3% formulation could represent an avenue to increase accessibility to field-directed therapy in the population for the treatment of AKs with a potentially well-tolerated, effective, and low-cost medication formulation.
This case represents the effectiveness of diclofenac gel 1% in treating AKs. Several treatment modalities failed in our case, but she experienced improvement with use of over-the-counter diclofenac gel 1%. She also noted no difference in response between the prescription 3% diclofenac formulation and the over-the-counter 1% formulation. Diclofenac gel 1% may represent an excellent therapeutic option in treatment-refractory cases of AKs. Larger randomized trials should be considered to assess safety and efficacy.
- FEisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:e209-e233.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Fuchs A, Marmur E. The kinetics of skin cancer: progression of actinic keratosis to squamous cell carcinoma. Dermatol Surg. 2007;33: 1099-1101.
- Dianzani C, Conforti C, Giuffrida R, et al. Current therapies for actinic keratosis. Int J Dermatol. 2020;59:677-684.
- Javor S, Cozzani E, Parodi A. Topical treatment of actinic keratosis with 3.0% diclofenac in 2.5% hyaluronan gel: review of the literature about the cumulative evidence of its efficacy and safety. G Ital Dermatol Venereol. 2016;151:275-280.
- Martin GM, Stockfleth E. Diclofenac sodium 3% gel for the management of actinic keratosis: 10+ years of cumulative evidence of efficacy and safety. J Drugs Dermatol. 2012;11:600-608.
- Arisi M, Guasco Pisani E, et al. Cryotherapy for actinic keratosis: basic principles and literature review. Clin Cosmet Investig Dermatol. 2022;15:357-365.
- Calzavara-Pinton P, Calzavara-Pinton I, Rovati C, et al. Topical pharmacotherapy for actinic keratoses in older adults. Drugs Aging. 2022;39:143-152.
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance. Contact Dermatitis. 2022;86:41-43.
- Voltaren gel (diclofenac sodium topical gel). Prescribing information. Novartis Consumer Health, Inc; 2009. Accessed May 21, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2009/022122s006lbl.pdf
- Moreira SA, Liu DJ. Diclofenac systemic bioavailability of a topical 1% diclofenac + 3% menthol combination gel vs. an oral diclofenac tablet in healthy volunteers: a randomized, open-label, crossover study. Int J Clin Pharmacol Ther. 2017;55:368-372.
- Kienzler JL, Gold M, Nollevaux F. Systemic bioavailability of topical diclofenac sodium gel 1% versus oral diclofenac sodium in healthy volunteers. J Clin Pharmacol. 2010;50:50-61.
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15.
- Fecker LF, Stockfleth E, Nindl I, et al. The role of apoptosis in therapy and prophylaxis of epithelial tumours by nonsteroidal antiinflammatory drugs (NSAIDs). Br J Dermatol. 2007;156(Suppl 3):25-33.
- Thomas GJ, Herranz P, Cruz SB, et al. Treatment of actinic keratosis through inhibition of cyclooxygenase-2: potential mechanism of action of diclofenac sodium 3% in hyaluronic acid 2.5. Dermatol Ther. 2019;32:e12800.
- Singer K, Dettmer K, Unger P, et al. Topical diclofenac reprograms metabolism and immune cell infiltration in actinic keratosis. Front Oncol. 2019;9:605.
- Diclofenac (topical). Drug information. UpToDate. https://www-uptodate-com.libraryaccess.elpaso.ttuhsc.edu/contents/diclofenac-topical-drug-information?source=auto_suggest&selectedTitle=1~3---3~4---diclofenac&search=diclofenac%20topical#F8017265
- Afify AA, Hana MR. Comparative evaluation of topical diclofenac sodium versus topical ibuprofen in the treatment of seborrheic keratosis. Dermatol Ther. 2020;33:e14370.
- Yin F, Ma J, Xiao H, et al. Randomized, double-blind, noninferiority study of diclofenac diethylamine 2.32% gel applied twice daily versus diclofenac diethylamine 1.16% gel applied four times daily in patients with acute ankle sprain. BMC Musculoskelet Disord. 2022;23:1125.
- van Herwaarden N, van den Elsen GAH, de Jong ICA, et al. Topical NSAIDs: ineffective or undervalued? [in Dutch]. Ned Tijdschr Geneeskd. 2021;165:D5317.
To the Editor:
Actinic keratoses (AKs) are keratinocyte neoplasms that manifest as rough, scaly, erythematous papules with ill-defined borders (commonly known as precancers) and develop due to long-term UV light exposure.1 They must be treated promptly due to the risk for progression to squamous cell carcinoma (SCC). One US Department of Veterans Affairs study reported that 0.6% of AKs progress to SCC in 1 year and 2.6% progressed to SCC in 4 years.2 In 10% of AKs that will progress to SCC, one study reported progression in approximately 2 years.3
The risk for progression also increases in patients with multiple AKs; the risk is 4-fold higher in patients with 6 to 20 AKs and 11-fold higher in patients with more than 20 AKs.4 Common treatment options include lesion-directed therapies such as cryotherapy, laser therapy, surgery, and curettage, as well as field-directed therapies such as topical 5-fluorouracil (5-FU), diclofenac gel 3%, chemical peeling, topical imiquimod, and photodynamic therapy (PDT).4 When diclofenac gel is chosen as a treatment modality, it is commonly prescribed in the 3% formulation. Diclofenac gel 3% has been shown to be effective in the treatment of AKs,5,6 but diclofenac gel 1% has not been well described in the literature. We report the case of a patient with AKs on the lower legs who was treated with diclofenac gel after other therapies failed.
A 55-year-old woman presented for a routine skin check due to a history of nonmelanoma skin cancer. Her medical history also included palmar hyperhidrosis, disseminated superficial actinic porokeratosis, and extensive actinic damage, as well as numerous biopsy-proven AKs. She had been evaluated every 3 months up to presentation due to the frequency of AK development over the past 5 years. The lesions were mainly localized to both lower legs, where the patient had acquired considerable lifetime sun exposure from tanning beds and sunbathing while boating. She also noted exposure to well water as a child, but none of her family members had a similar issue with AKs.
Prior to this visit, the patient had undergone 5 years of therapy for AKs. She initially was treated with multiple courses of topical 5-FU, but she consequently developed severe allergic contact dermatitis. Subsequent treatments included cryotherapy as well as application of tretinoin cream nightly for 2 weeks followed by PDT. She was unable to tolerate the tretinoin, which she reported led to dryness and irritation. She reported mild improvement after her first session of PDT but only minimal improvement after the next session. Ingenol mebutate was then prescribed for topical use on the legs for 2 days, which did not result in improvement. The patient continued to follow up for unresolved AKs on the legs and was prescribed acitretin to help reduce the risk for progression to SCC. At follow-up 3 months later, she reported decreased soreness from AKs after starting the acitretin and, aside from mild dryness, she tolerated the medication well; however, with continued use of acitretin, she began to experience adverse effects 6 months later, including thyroid suppression and hair loss, leading to discontinuation. Instead, 3 months later, she was recommended to start nicotinamide supplementation for prevention of SCC.
Due to continued AK development (Figure, A), we eventually prescribed diclofenac gel 3% twice daily for both legs 9 months after prescribing nicotinamide. This regimen was cost prohibitive, as the medication was not covered by her insurance and the cost was $300 for one tube. We recommended the patient instead apply the 3% gel to the right leg only due to greater severity of AKs on this leg and over-the-counter diclofenac gel 1% twice daily to the left leg. Approximately 5 months later, she reported a reduction in the discomfort from AKs as well as a reduction in the total number of AKs. She applied the 2 different products as instructed for the first month but did not notice a difference between them. She then continued to apply only the 1% gel on both legs for a total of 8 months with excellent response (Figure, B). At subsequent follow-up visits over a 2-year period, she has only required cryotherapy as spot treatment for AKs.

For 1 to a few discrete AKs, liquid nitrogen cryotherapy is considered first-line therapy.7 However, if multiple AKs are present, surrounding photodamaged skin also should be treated with field-directed therapy due to surrounding keratinocytes bearing a high mutational burden and risk of cancerization.8 Common field-directed therapies include topical 5-FU, topical imiquimod, topical tirbanibulin, PDT, retinoids, and topical diclofenac 3%.
One challenge in field-directed treatment of AKs is the side-effect profile seen in some patients, causing them to prematurely discontinue treatment. In our patient, 5-FU cream, tretinoin cream, and oral acitretin were not well tolerated. Topical diclofenac generally is well tolerated, with mostly mild local skin reactions and low risk for systemic adverse events. Adverse effects mainly consist of mild local skin reactions including pruritus (reported in 31%-52% of patients who used topical diclofenac), dryness (25%-27%), and irritation (less than 1%).9,10 Although diclofenac carries a black-box warning for serious cardiovascular thrombotic events and serious gastrointestinal tract bleeding, systemic absorption of topical diclofenac has been proven to be substantially lower (5- to 17-fold) compared to the oral formulation, and resulting serious adverse effects have been found to be largely reduced compared to the oral formulation.11,12 If allergic contact dermatitis develops, diclofenac should be discontinued.9,13
Diclofenac’s antineoplastic mechanism of action of cyclooxygenase-2 inhibition involves induction of apoptosis as well as reduction in tumor cell proliferation and tumor angiogenesis.14,15 Topical diclofenac may result in decreased levels of lactate and amino acid in AK lesions, particularly in lesions responding to treatment.16 Topical diclofenac may alter immune infiltration by inducing infiltration of dermal CD8+ T cells along with high IFN-γ messenger RNA expression, suggesting improvement of T-cell function after topical diclofenac treatment.16
Although diclofenac gel 3% has been shown to be effective in treatment of AKs,5,6 diclofenac gel 1% has not yet been well studied. Use of the 1% gel is indicated for osteoarthritis and musculoskeletal pain by the US Food and Drug Administration.10,17 Efficacy of the 1% gel has been documented for these and other conditions including seborrheic keratoses.18-20
Because the 1% diclofenac formulation is available over-the-counter, it is more accessible to patients compared to the 3% formulation and often substantially decreases the cost of the medication for the patient. The cost of diclofenac gel 1% in the United States ranges from $0.04 to $0.31 per gram compared to $1.07 to $11.79 per gram for the 3% gel prescription formulation.17 Efficacy of the 1% formulation compared to the 3% formulation could represent an avenue to increase accessibility to field-directed therapy in the population for the treatment of AKs with a potentially well-tolerated, effective, and low-cost medication formulation.
This case represents the effectiveness of diclofenac gel 1% in treating AKs. Several treatment modalities failed in our case, but she experienced improvement with use of over-the-counter diclofenac gel 1%. She also noted no difference in response between the prescription 3% diclofenac formulation and the over-the-counter 1% formulation. Diclofenac gel 1% may represent an excellent therapeutic option in treatment-refractory cases of AKs. Larger randomized trials should be considered to assess safety and efficacy.
To the Editor:
Actinic keratoses (AKs) are keratinocyte neoplasms that manifest as rough, scaly, erythematous papules with ill-defined borders (commonly known as precancers) and develop due to long-term UV light exposure.1 They must be treated promptly due to the risk for progression to squamous cell carcinoma (SCC). One US Department of Veterans Affairs study reported that 0.6% of AKs progress to SCC in 1 year and 2.6% progressed to SCC in 4 years.2 In 10% of AKs that will progress to SCC, one study reported progression in approximately 2 years.3
The risk for progression also increases in patients with multiple AKs; the risk is 4-fold higher in patients with 6 to 20 AKs and 11-fold higher in patients with more than 20 AKs.4 Common treatment options include lesion-directed therapies such as cryotherapy, laser therapy, surgery, and curettage, as well as field-directed therapies such as topical 5-fluorouracil (5-FU), diclofenac gel 3%, chemical peeling, topical imiquimod, and photodynamic therapy (PDT).4 When diclofenac gel is chosen as a treatment modality, it is commonly prescribed in the 3% formulation. Diclofenac gel 3% has been shown to be effective in the treatment of AKs,5,6 but diclofenac gel 1% has not been well described in the literature. We report the case of a patient with AKs on the lower legs who was treated with diclofenac gel after other therapies failed.
A 55-year-old woman presented for a routine skin check due to a history of nonmelanoma skin cancer. Her medical history also included palmar hyperhidrosis, disseminated superficial actinic porokeratosis, and extensive actinic damage, as well as numerous biopsy-proven AKs. She had been evaluated every 3 months up to presentation due to the frequency of AK development over the past 5 years. The lesions were mainly localized to both lower legs, where the patient had acquired considerable lifetime sun exposure from tanning beds and sunbathing while boating. She also noted exposure to well water as a child, but none of her family members had a similar issue with AKs.
Prior to this visit, the patient had undergone 5 years of therapy for AKs. She initially was treated with multiple courses of topical 5-FU, but she consequently developed severe allergic contact dermatitis. Subsequent treatments included cryotherapy as well as application of tretinoin cream nightly for 2 weeks followed by PDT. She was unable to tolerate the tretinoin, which she reported led to dryness and irritation. She reported mild improvement after her first session of PDT but only minimal improvement after the next session. Ingenol mebutate was then prescribed for topical use on the legs for 2 days, which did not result in improvement. The patient continued to follow up for unresolved AKs on the legs and was prescribed acitretin to help reduce the risk for progression to SCC. At follow-up 3 months later, she reported decreased soreness from AKs after starting the acitretin and, aside from mild dryness, she tolerated the medication well; however, with continued use of acitretin, she began to experience adverse effects 6 months later, including thyroid suppression and hair loss, leading to discontinuation. Instead, 3 months later, she was recommended to start nicotinamide supplementation for prevention of SCC.
Due to continued AK development (Figure, A), we eventually prescribed diclofenac gel 3% twice daily for both legs 9 months after prescribing nicotinamide. This regimen was cost prohibitive, as the medication was not covered by her insurance and the cost was $300 for one tube. We recommended the patient instead apply the 3% gel to the right leg only due to greater severity of AKs on this leg and over-the-counter diclofenac gel 1% twice daily to the left leg. Approximately 5 months later, she reported a reduction in the discomfort from AKs as well as a reduction in the total number of AKs. She applied the 2 different products as instructed for the first month but did not notice a difference between them. She then continued to apply only the 1% gel on both legs for a total of 8 months with excellent response (Figure, B). At subsequent follow-up visits over a 2-year period, she has only required cryotherapy as spot treatment for AKs.

For 1 to a few discrete AKs, liquid nitrogen cryotherapy is considered first-line therapy.7 However, if multiple AKs are present, surrounding photodamaged skin also should be treated with field-directed therapy due to surrounding keratinocytes bearing a high mutational burden and risk of cancerization.8 Common field-directed therapies include topical 5-FU, topical imiquimod, topical tirbanibulin, PDT, retinoids, and topical diclofenac 3%.
One challenge in field-directed treatment of AKs is the side-effect profile seen in some patients, causing them to prematurely discontinue treatment. In our patient, 5-FU cream, tretinoin cream, and oral acitretin were not well tolerated. Topical diclofenac generally is well tolerated, with mostly mild local skin reactions and low risk for systemic adverse events. Adverse effects mainly consist of mild local skin reactions including pruritus (reported in 31%-52% of patients who used topical diclofenac), dryness (25%-27%), and irritation (less than 1%).9,10 Although diclofenac carries a black-box warning for serious cardiovascular thrombotic events and serious gastrointestinal tract bleeding, systemic absorption of topical diclofenac has been proven to be substantially lower (5- to 17-fold) compared to the oral formulation, and resulting serious adverse effects have been found to be largely reduced compared to the oral formulation.11,12 If allergic contact dermatitis develops, diclofenac should be discontinued.9,13
Diclofenac’s antineoplastic mechanism of action of cyclooxygenase-2 inhibition involves induction of apoptosis as well as reduction in tumor cell proliferation and tumor angiogenesis.14,15 Topical diclofenac may result in decreased levels of lactate and amino acid in AK lesions, particularly in lesions responding to treatment.16 Topical diclofenac may alter immune infiltration by inducing infiltration of dermal CD8+ T cells along with high IFN-γ messenger RNA expression, suggesting improvement of T-cell function after topical diclofenac treatment.16
Although diclofenac gel 3% has been shown to be effective in treatment of AKs,5,6 diclofenac gel 1% has not yet been well studied. Use of the 1% gel is indicated for osteoarthritis and musculoskeletal pain by the US Food and Drug Administration.10,17 Efficacy of the 1% gel has been documented for these and other conditions including seborrheic keratoses.18-20
Because the 1% diclofenac formulation is available over-the-counter, it is more accessible to patients compared to the 3% formulation and often substantially decreases the cost of the medication for the patient. The cost of diclofenac gel 1% in the United States ranges from $0.04 to $0.31 per gram compared to $1.07 to $11.79 per gram for the 3% gel prescription formulation.17 Efficacy of the 1% formulation compared to the 3% formulation could represent an avenue to increase accessibility to field-directed therapy in the population for the treatment of AKs with a potentially well-tolerated, effective, and low-cost medication formulation.
This case represents the effectiveness of diclofenac gel 1% in treating AKs. Several treatment modalities failed in our case, but she experienced improvement with use of over-the-counter diclofenac gel 1%. She also noted no difference in response between the prescription 3% diclofenac formulation and the over-the-counter 1% formulation. Diclofenac gel 1% may represent an excellent therapeutic option in treatment-refractory cases of AKs. Larger randomized trials should be considered to assess safety and efficacy.
- FEisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:e209-e233.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Fuchs A, Marmur E. The kinetics of skin cancer: progression of actinic keratosis to squamous cell carcinoma. Dermatol Surg. 2007;33: 1099-1101.
- Dianzani C, Conforti C, Giuffrida R, et al. Current therapies for actinic keratosis. Int J Dermatol. 2020;59:677-684.
- Javor S, Cozzani E, Parodi A. Topical treatment of actinic keratosis with 3.0% diclofenac in 2.5% hyaluronan gel: review of the literature about the cumulative evidence of its efficacy and safety. G Ital Dermatol Venereol. 2016;151:275-280.
- Martin GM, Stockfleth E. Diclofenac sodium 3% gel for the management of actinic keratosis: 10+ years of cumulative evidence of efficacy and safety. J Drugs Dermatol. 2012;11:600-608.
- Arisi M, Guasco Pisani E, et al. Cryotherapy for actinic keratosis: basic principles and literature review. Clin Cosmet Investig Dermatol. 2022;15:357-365.
- Calzavara-Pinton P, Calzavara-Pinton I, Rovati C, et al. Topical pharmacotherapy for actinic keratoses in older adults. Drugs Aging. 2022;39:143-152.
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance. Contact Dermatitis. 2022;86:41-43.
- Voltaren gel (diclofenac sodium topical gel). Prescribing information. Novartis Consumer Health, Inc; 2009. Accessed May 21, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2009/022122s006lbl.pdf
- Moreira SA, Liu DJ. Diclofenac systemic bioavailability of a topical 1% diclofenac + 3% menthol combination gel vs. an oral diclofenac tablet in healthy volunteers: a randomized, open-label, crossover study. Int J Clin Pharmacol Ther. 2017;55:368-372.
- Kienzler JL, Gold M, Nollevaux F. Systemic bioavailability of topical diclofenac sodium gel 1% versus oral diclofenac sodium in healthy volunteers. J Clin Pharmacol. 2010;50:50-61.
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15.
- Fecker LF, Stockfleth E, Nindl I, et al. The role of apoptosis in therapy and prophylaxis of epithelial tumours by nonsteroidal antiinflammatory drugs (NSAIDs). Br J Dermatol. 2007;156(Suppl 3):25-33.
- Thomas GJ, Herranz P, Cruz SB, et al. Treatment of actinic keratosis through inhibition of cyclooxygenase-2: potential mechanism of action of diclofenac sodium 3% in hyaluronic acid 2.5. Dermatol Ther. 2019;32:e12800.
- Singer K, Dettmer K, Unger P, et al. Topical diclofenac reprograms metabolism and immune cell infiltration in actinic keratosis. Front Oncol. 2019;9:605.
- Diclofenac (topical). Drug information. UpToDate. https://www-uptodate-com.libraryaccess.elpaso.ttuhsc.edu/contents/diclofenac-topical-drug-information?source=auto_suggest&selectedTitle=1~3---3~4---diclofenac&search=diclofenac%20topical#F8017265
- Afify AA, Hana MR. Comparative evaluation of topical diclofenac sodium versus topical ibuprofen in the treatment of seborrheic keratosis. Dermatol Ther. 2020;33:e14370.
- Yin F, Ma J, Xiao H, et al. Randomized, double-blind, noninferiority study of diclofenac diethylamine 2.32% gel applied twice daily versus diclofenac diethylamine 1.16% gel applied four times daily in patients with acute ankle sprain. BMC Musculoskelet Disord. 2022;23:1125.
- van Herwaarden N, van den Elsen GAH, de Jong ICA, et al. Topical NSAIDs: ineffective or undervalued? [in Dutch]. Ned Tijdschr Geneeskd. 2021;165:D5317.
- FEisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:e209-e233.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Fuchs A, Marmur E. The kinetics of skin cancer: progression of actinic keratosis to squamous cell carcinoma. Dermatol Surg. 2007;33: 1099-1101.
- Dianzani C, Conforti C, Giuffrida R, et al. Current therapies for actinic keratosis. Int J Dermatol. 2020;59:677-684.
- Javor S, Cozzani E, Parodi A. Topical treatment of actinic keratosis with 3.0% diclofenac in 2.5% hyaluronan gel: review of the literature about the cumulative evidence of its efficacy and safety. G Ital Dermatol Venereol. 2016;151:275-280.
- Martin GM, Stockfleth E. Diclofenac sodium 3% gel for the management of actinic keratosis: 10+ years of cumulative evidence of efficacy and safety. J Drugs Dermatol. 2012;11:600-608.
- Arisi M, Guasco Pisani E, et al. Cryotherapy for actinic keratosis: basic principles and literature review. Clin Cosmet Investig Dermatol. 2022;15:357-365.
- Calzavara-Pinton P, Calzavara-Pinton I, Rovati C, et al. Topical pharmacotherapy for actinic keratoses in older adults. Drugs Aging. 2022;39:143-152.
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance. Contact Dermatitis. 2022;86:41-43.
- Voltaren gel (diclofenac sodium topical gel). Prescribing information. Novartis Consumer Health, Inc; 2009. Accessed May 21, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2009/022122s006lbl.pdf
- Moreira SA, Liu DJ. Diclofenac systemic bioavailability of a topical 1% diclofenac + 3% menthol combination gel vs. an oral diclofenac tablet in healthy volunteers: a randomized, open-label, crossover study. Int J Clin Pharmacol Ther. 2017;55:368-372.
- Kienzler JL, Gold M, Nollevaux F. Systemic bioavailability of topical diclofenac sodium gel 1% versus oral diclofenac sodium in healthy volunteers. J Clin Pharmacol. 2010;50:50-61.
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15.
- Fecker LF, Stockfleth E, Nindl I, et al. The role of apoptosis in therapy and prophylaxis of epithelial tumours by nonsteroidal antiinflammatory drugs (NSAIDs). Br J Dermatol. 2007;156(Suppl 3):25-33.
- Thomas GJ, Herranz P, Cruz SB, et al. Treatment of actinic keratosis through inhibition of cyclooxygenase-2: potential mechanism of action of diclofenac sodium 3% in hyaluronic acid 2.5. Dermatol Ther. 2019;32:e12800.
- Singer K, Dettmer K, Unger P, et al. Topical diclofenac reprograms metabolism and immune cell infiltration in actinic keratosis. Front Oncol. 2019;9:605.
- Diclofenac (topical). Drug information. UpToDate. https://www-uptodate-com.libraryaccess.elpaso.ttuhsc.edu/contents/diclofenac-topical-drug-information?source=auto_suggest&selectedTitle=1~3---3~4---diclofenac&search=diclofenac%20topical#F8017265
- Afify AA, Hana MR. Comparative evaluation of topical diclofenac sodium versus topical ibuprofen in the treatment of seborrheic keratosis. Dermatol Ther. 2020;33:e14370.
- Yin F, Ma J, Xiao H, et al. Randomized, double-blind, noninferiority study of diclofenac diethylamine 2.32% gel applied twice daily versus diclofenac diethylamine 1.16% gel applied four times daily in patients with acute ankle sprain. BMC Musculoskelet Disord. 2022;23:1125.
- van Herwaarden N, van den Elsen GAH, de Jong ICA, et al. Topical NSAIDs: ineffective or undervalued? [in Dutch]. Ned Tijdschr Geneeskd. 2021;165:D5317.
Actinic Keratosis Treatment With Diclofenac Gel 1%
Actinic Keratosis Treatment With Diclofenac Gel 1%
PRACTICE POINTS
- There are numerous field-directed therapies for actinic keratoses (AKs); however, efficacy and tolerability vary among the available treatments.
- Diclofenac gel 1% is an affordable option that could potentially increase accessibility and decrease cost of field therapy for the treatment of AKs, while maintaining therapeutic efficacy.