User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Patient Navigators for Serious Illnesses Can Now Bill Under New Medicare Codes
In a move that acknowledges the gauntlet the US health system poses for people facing serious and fatal illnesses, Medicare will pay for a new class of workers to help patients manage treatments for conditions like cancer and heart failure.
The 2024 Medicare physician fee schedule includes new billing codes, including G0023, to pay for 60 minutes a month of care coordination by certified or trained auxiliary personnel working under the direction of a clinician.
A diagnosis of cancer or another serious illness takes a toll beyond the physical effects of the disease. Patients often scramble to make adjustments in family and work schedules to manage treatment, said Samyukta Mullangi, MD, MBA, medical director of oncology at Thyme Care, a Nashville, Tennessee–based firm that provides navigation and coordination services to oncology practices and insurers.
“It just really does create a bit of a pressure cooker for patients,” Dr. Mullangi told this news organization.
Medicare has for many years paid for medical professionals to help patients cope with the complexities of disease, such as chronic care management (CCM) provided by physicians, nurses, and physician assistants.
The new principal illness navigation (PIN) payments are intended to pay for work that to date typically has been done by people without medical degrees, including those involved in peer support networks and community health programs. The US Centers for Medicare and Medicaid Services(CMS) expects these navigators will undergo training and work under the supervision of clinicians.
The new navigators may coordinate care transitions between medical settings, follow up with patients after emergency department (ED) visits, or communicate with skilled nursing facilities regarding the psychosocial needs and functional deficits of a patient, among other functions.
CMS expects the new navigators may:
- Conduct assessments to understand a patient’s life story, strengths, needs, goals, preferences, and desired outcomes, including understanding cultural and linguistic factors.
- Provide support to accomplish the clinician’s treatment plan.
- Coordinate the receipt of needed services from healthcare facilities, home- and community-based service providers, and caregivers.
Peers as Navigators
The new navigators can be former patients who have undergone similar treatments for serious diseases, CMS said. This approach sets the new program apart from other care management services Medicare already covers, program officials wrote in the 2024 physician fee schedule.
“For some conditions, patients are best able to engage with the healthcare system and access care if they have assistance from a single, dedicated individual who has ‘lived experience,’ ” according to the rule.
The agency has taken a broad initial approach in defining what kinds of illnesses a patient may have to qualify for services. Patients must have a serious condition that is expected to last at least 3 months, such as cancer, heart failure, or substance use disorder.
But those without a definitive diagnosis may also qualify to receive navigator services.
In the rule, CMS cited a case in which a CT scan identified a suspicious mass in a patient’s colon. A clinician might decide this person would benefit from navigation services due to the potential risks for an undiagnosed illness.
“Regardless of the definitive diagnosis of the mass, presence of a colonic mass for that patient may be a serious high-risk condition that could, for example, cause obstruction and lead the patient to present to the emergency department, as well as be potentially indicative of an underlying life-threatening illness such as colon cancer,” CMS wrote in the rule.
Navigators often start their work when cancer patients are screened and guide them through initial diagnosis, potential surgery, radiation, or chemotherapy, said Sharon Gentry, MSN, RN, a former nurse navigator who is now the editor in chief of the Journal of the Academy of Oncology Nurse & Patient Navigators.
The navigators are meant to be a trusted and continual presence for patients, who otherwise might be left to start anew in finding help at each phase of care.
The navigators “see the whole picture. They see the whole journey the patient takes, from pre-diagnosis all the way through diagnosis care out through survival,” Ms. Gentry said.
Gaining a special Medicare payment for these kinds of services will elevate this work, she said.
Many newer drugs can target specific mechanisms and proteins of cancer. Often, oncology treatment involves testing to find out if mutations are allowing the cancer cells to evade a patient’s immune system.
Checking these biomarkers takes time, however. Patients sometimes become frustrated because they are anxious to begin treatment. Patients may receive inaccurate information from friends or family who went through treatment previously. Navigators can provide knowledge on the current state of care for a patient’s disease, helping them better manage anxieties.
“You have to explain to them that things have changed since the guy you drink coffee with was diagnosed with cancer, and there may be a drug that could target that,” Ms. Gentry said.
Potential Challenges
Initial uptake of the new PIN codes may be slow going, however, as clinicians and health systems may already use well-established codes. These include CCM and principal care management services, which may pay higher rates, Mullangi said.
“There might be sensitivity around not wanting to cannibalize existing programs with a new program,” Dr. Mullangi said.
In addition, many patients will have a copay for the services of principal illness navigators, Dr. Mullangi said.
While many patients have additional insurance that would cover the service, not all do. People with traditional Medicare coverage can sometimes pay 20% of the cost of some medical services.
“I think that may give patients pause, particularly if they’re already feeling the financial burden of a cancer treatment journey,” Dr. Mullangi said.
Pay rates for PIN services involve calculations of regional price differences, which are posted publicly by CMS, and potential added fees for services provided by hospital-affiliated organizations.
Consider payments for code G0023, covering 60 minutes of principal navigation services provided in a single month.
A set reimbursement for patients cared for in independent medical practices exists, with variation for local costs. Medicare’s non-facility price for G0023 would be $102.41 in some parts of Silicon Valley in California, including San Jose. In Arkansas, where costs are lower, reimbursement would be $73.14 for this same service.
Patients who get services covered by code G0023 in independent medical practices would have monthly copays of about $15-$20, depending on where they live.
The tab for patients tends to be higher for these same services if delivered through a medical practice owned by a hospital, as this would trigger the addition of facility fees to the payments made to cover the services. Facility fees are difficult for the public to ascertain before getting a treatment or service.
Dr. Mullangi and Ms. Gentry reported no relevant financial disclosures outside of their employers.
A version of this article first appeared on Medscape.com.
In a move that acknowledges the gauntlet the US health system poses for people facing serious and fatal illnesses, Medicare will pay for a new class of workers to help patients manage treatments for conditions like cancer and heart failure.
The 2024 Medicare physician fee schedule includes new billing codes, including G0023, to pay for 60 minutes a month of care coordination by certified or trained auxiliary personnel working under the direction of a clinician.
A diagnosis of cancer or another serious illness takes a toll beyond the physical effects of the disease. Patients often scramble to make adjustments in family and work schedules to manage treatment, said Samyukta Mullangi, MD, MBA, medical director of oncology at Thyme Care, a Nashville, Tennessee–based firm that provides navigation and coordination services to oncology practices and insurers.
“It just really does create a bit of a pressure cooker for patients,” Dr. Mullangi told this news organization.
Medicare has for many years paid for medical professionals to help patients cope with the complexities of disease, such as chronic care management (CCM) provided by physicians, nurses, and physician assistants.
The new principal illness navigation (PIN) payments are intended to pay for work that to date typically has been done by people without medical degrees, including those involved in peer support networks and community health programs. The US Centers for Medicare and Medicaid Services(CMS) expects these navigators will undergo training and work under the supervision of clinicians.
The new navigators may coordinate care transitions between medical settings, follow up with patients after emergency department (ED) visits, or communicate with skilled nursing facilities regarding the psychosocial needs and functional deficits of a patient, among other functions.
CMS expects the new navigators may:
- Conduct assessments to understand a patient’s life story, strengths, needs, goals, preferences, and desired outcomes, including understanding cultural and linguistic factors.
- Provide support to accomplish the clinician’s treatment plan.
- Coordinate the receipt of needed services from healthcare facilities, home- and community-based service providers, and caregivers.
Peers as Navigators
The new navigators can be former patients who have undergone similar treatments for serious diseases, CMS said. This approach sets the new program apart from other care management services Medicare already covers, program officials wrote in the 2024 physician fee schedule.
“For some conditions, patients are best able to engage with the healthcare system and access care if they have assistance from a single, dedicated individual who has ‘lived experience,’ ” according to the rule.
The agency has taken a broad initial approach in defining what kinds of illnesses a patient may have to qualify for services. Patients must have a serious condition that is expected to last at least 3 months, such as cancer, heart failure, or substance use disorder.
But those without a definitive diagnosis may also qualify to receive navigator services.
In the rule, CMS cited a case in which a CT scan identified a suspicious mass in a patient’s colon. A clinician might decide this person would benefit from navigation services due to the potential risks for an undiagnosed illness.
“Regardless of the definitive diagnosis of the mass, presence of a colonic mass for that patient may be a serious high-risk condition that could, for example, cause obstruction and lead the patient to present to the emergency department, as well as be potentially indicative of an underlying life-threatening illness such as colon cancer,” CMS wrote in the rule.
Navigators often start their work when cancer patients are screened and guide them through initial diagnosis, potential surgery, radiation, or chemotherapy, said Sharon Gentry, MSN, RN, a former nurse navigator who is now the editor in chief of the Journal of the Academy of Oncology Nurse & Patient Navigators.
The navigators are meant to be a trusted and continual presence for patients, who otherwise might be left to start anew in finding help at each phase of care.
The navigators “see the whole picture. They see the whole journey the patient takes, from pre-diagnosis all the way through diagnosis care out through survival,” Ms. Gentry said.
Gaining a special Medicare payment for these kinds of services will elevate this work, she said.
Many newer drugs can target specific mechanisms and proteins of cancer. Often, oncology treatment involves testing to find out if mutations are allowing the cancer cells to evade a patient’s immune system.
Checking these biomarkers takes time, however. Patients sometimes become frustrated because they are anxious to begin treatment. Patients may receive inaccurate information from friends or family who went through treatment previously. Navigators can provide knowledge on the current state of care for a patient’s disease, helping them better manage anxieties.
“You have to explain to them that things have changed since the guy you drink coffee with was diagnosed with cancer, and there may be a drug that could target that,” Ms. Gentry said.
Potential Challenges
Initial uptake of the new PIN codes may be slow going, however, as clinicians and health systems may already use well-established codes. These include CCM and principal care management services, which may pay higher rates, Mullangi said.
“There might be sensitivity around not wanting to cannibalize existing programs with a new program,” Dr. Mullangi said.
In addition, many patients will have a copay for the services of principal illness navigators, Dr. Mullangi said.
While many patients have additional insurance that would cover the service, not all do. People with traditional Medicare coverage can sometimes pay 20% of the cost of some medical services.
“I think that may give patients pause, particularly if they’re already feeling the financial burden of a cancer treatment journey,” Dr. Mullangi said.
Pay rates for PIN services involve calculations of regional price differences, which are posted publicly by CMS, and potential added fees for services provided by hospital-affiliated organizations.
Consider payments for code G0023, covering 60 minutes of principal navigation services provided in a single month.
A set reimbursement for patients cared for in independent medical practices exists, with variation for local costs. Medicare’s non-facility price for G0023 would be $102.41 in some parts of Silicon Valley in California, including San Jose. In Arkansas, where costs are lower, reimbursement would be $73.14 for this same service.
Patients who get services covered by code G0023 in independent medical practices would have monthly copays of about $15-$20, depending on where they live.
The tab for patients tends to be higher for these same services if delivered through a medical practice owned by a hospital, as this would trigger the addition of facility fees to the payments made to cover the services. Facility fees are difficult for the public to ascertain before getting a treatment or service.
Dr. Mullangi and Ms. Gentry reported no relevant financial disclosures outside of their employers.
A version of this article first appeared on Medscape.com.
In a move that acknowledges the gauntlet the US health system poses for people facing serious and fatal illnesses, Medicare will pay for a new class of workers to help patients manage treatments for conditions like cancer and heart failure.
The 2024 Medicare physician fee schedule includes new billing codes, including G0023, to pay for 60 minutes a month of care coordination by certified or trained auxiliary personnel working under the direction of a clinician.
A diagnosis of cancer or another serious illness takes a toll beyond the physical effects of the disease. Patients often scramble to make adjustments in family and work schedules to manage treatment, said Samyukta Mullangi, MD, MBA, medical director of oncology at Thyme Care, a Nashville, Tennessee–based firm that provides navigation and coordination services to oncology practices and insurers.
“It just really does create a bit of a pressure cooker for patients,” Dr. Mullangi told this news organization.
Medicare has for many years paid for medical professionals to help patients cope with the complexities of disease, such as chronic care management (CCM) provided by physicians, nurses, and physician assistants.
The new principal illness navigation (PIN) payments are intended to pay for work that to date typically has been done by people without medical degrees, including those involved in peer support networks and community health programs. The US Centers for Medicare and Medicaid Services(CMS) expects these navigators will undergo training and work under the supervision of clinicians.
The new navigators may coordinate care transitions between medical settings, follow up with patients after emergency department (ED) visits, or communicate with skilled nursing facilities regarding the psychosocial needs and functional deficits of a patient, among other functions.
CMS expects the new navigators may:
- Conduct assessments to understand a patient’s life story, strengths, needs, goals, preferences, and desired outcomes, including understanding cultural and linguistic factors.
- Provide support to accomplish the clinician’s treatment plan.
- Coordinate the receipt of needed services from healthcare facilities, home- and community-based service providers, and caregivers.
Peers as Navigators
The new navigators can be former patients who have undergone similar treatments for serious diseases, CMS said. This approach sets the new program apart from other care management services Medicare already covers, program officials wrote in the 2024 physician fee schedule.
“For some conditions, patients are best able to engage with the healthcare system and access care if they have assistance from a single, dedicated individual who has ‘lived experience,’ ” according to the rule.
The agency has taken a broad initial approach in defining what kinds of illnesses a patient may have to qualify for services. Patients must have a serious condition that is expected to last at least 3 months, such as cancer, heart failure, or substance use disorder.
But those without a definitive diagnosis may also qualify to receive navigator services.
In the rule, CMS cited a case in which a CT scan identified a suspicious mass in a patient’s colon. A clinician might decide this person would benefit from navigation services due to the potential risks for an undiagnosed illness.
“Regardless of the definitive diagnosis of the mass, presence of a colonic mass for that patient may be a serious high-risk condition that could, for example, cause obstruction and lead the patient to present to the emergency department, as well as be potentially indicative of an underlying life-threatening illness such as colon cancer,” CMS wrote in the rule.
Navigators often start their work when cancer patients are screened and guide them through initial diagnosis, potential surgery, radiation, or chemotherapy, said Sharon Gentry, MSN, RN, a former nurse navigator who is now the editor in chief of the Journal of the Academy of Oncology Nurse & Patient Navigators.
The navigators are meant to be a trusted and continual presence for patients, who otherwise might be left to start anew in finding help at each phase of care.
The navigators “see the whole picture. They see the whole journey the patient takes, from pre-diagnosis all the way through diagnosis care out through survival,” Ms. Gentry said.
Gaining a special Medicare payment for these kinds of services will elevate this work, she said.
Many newer drugs can target specific mechanisms and proteins of cancer. Often, oncology treatment involves testing to find out if mutations are allowing the cancer cells to evade a patient’s immune system.
Checking these biomarkers takes time, however. Patients sometimes become frustrated because they are anxious to begin treatment. Patients may receive inaccurate information from friends or family who went through treatment previously. Navigators can provide knowledge on the current state of care for a patient’s disease, helping them better manage anxieties.
“You have to explain to them that things have changed since the guy you drink coffee with was diagnosed with cancer, and there may be a drug that could target that,” Ms. Gentry said.
Potential Challenges
Initial uptake of the new PIN codes may be slow going, however, as clinicians and health systems may already use well-established codes. These include CCM and principal care management services, which may pay higher rates, Mullangi said.
“There might be sensitivity around not wanting to cannibalize existing programs with a new program,” Dr. Mullangi said.
In addition, many patients will have a copay for the services of principal illness navigators, Dr. Mullangi said.
While many patients have additional insurance that would cover the service, not all do. People with traditional Medicare coverage can sometimes pay 20% of the cost of some medical services.
“I think that may give patients pause, particularly if they’re already feeling the financial burden of a cancer treatment journey,” Dr. Mullangi said.
Pay rates for PIN services involve calculations of regional price differences, which are posted publicly by CMS, and potential added fees for services provided by hospital-affiliated organizations.
Consider payments for code G0023, covering 60 minutes of principal navigation services provided in a single month.
A set reimbursement for patients cared for in independent medical practices exists, with variation for local costs. Medicare’s non-facility price for G0023 would be $102.41 in some parts of Silicon Valley in California, including San Jose. In Arkansas, where costs are lower, reimbursement would be $73.14 for this same service.
Patients who get services covered by code G0023 in independent medical practices would have monthly copays of about $15-$20, depending on where they live.
The tab for patients tends to be higher for these same services if delivered through a medical practice owned by a hospital, as this would trigger the addition of facility fees to the payments made to cover the services. Facility fees are difficult for the public to ascertain before getting a treatment or service.
Dr. Mullangi and Ms. Gentry reported no relevant financial disclosures outside of their employers.
A version of this article first appeared on Medscape.com.

How to explain physician compounding to legislators
In Ohio, new limits on drug compounding in physicians’ offices went into effect in April and have become a real hindrance to care for dermatology patients. The State of Ohio Board of Pharmacy has defined compounding as combining two or more prescription drugs and has required that physicians who perform this “compounding” must obtain a “Terminal Distributor of Dangerous Drugs” license. Ohio is the “test state,” and these rules, unless vigorously opposed, will be coming to your state.
[polldaddy:9779752]
The rules state that “compounded” drugs used within 6 hours of preparation must be prepared in a designated clean medication area with proper hand hygiene and the use of powder-free gloves. “Compounded” drugs that are used more than 6 hours after preparation, require a designated clean room with access limited to authorized personnel, environmental control devices such as a laminar flow hood, and additional equipment and training of personnel to maintain an aseptic environment. A separate license is required for each office location.
The state pharmacy boards are eager to restrict physicians – as well as dentists and veterinarians – and to collect annual licensing fees. Additionally, according to an article from the Ohio State Medical Association, noncompliant physicians can be fined by the pharmacy board.
We are talking big money, power, and dreams of clinical relevancy (and billable activities) here.
What can dermatologists do to prevent this regulatory overreach? I encourage you to plan a visit to your state representative, where you can demonstrate how these restrictions affect you and your patients – an exercise that should be both fun and compelling. All you need to illustrate your case is a simple kit that includes a syringe (but no needles in the statehouse!), a bottle of lidocaine with epinephrine, a bottle of 8.4% bicarbonate, alcohol pads, and gloves.
First, explain to your audience that there is a skin cancer epidemic with more than 5.4 million new cases a year and that, over the past 20 years, the incidence of skin cancer has doubled and is projected to double again over the next 20 years. Further, explain that dermatologists treat more than 70% of these cases in the office setting, under local anesthesia, at a huge cost savings to the public and government (it costs an average of 12 times as much to remove these cancers in the outpatient department at the hospital). Remember, states foot most of the bill for Medicaid and Medicare gap indigent coverage.
Take the bottle of lidocaine with epinephrine and open the syringe pack (Staffers love this demonstration; everyone is fascinated with shots.). Put on your gloves, wipe the top of the lidocaine bottle with an alcohol swab, and explain that this medicine is the anesthetic preferred for skin cancer surgery. Explain how it not only numbs the skin, but also causes vasoconstriction, so that the cancer can be easily and safely removed in the office.
Then explain that, in order for the epinephrine to be stable, the solution has to be very acidic (a pH of 4.2, in fact). Explain that this makes it burn like hell unless you add 0.1 cc per cc of 8.4% bicarbonate, in which case the perceived pain on a 10-point scale will drop from 8 to 2. Then pick up the bottle of bicarbonate and explain that you will no longer be able to mix these two components anymore without a “Terminal Distributor of Dangerous Drugs” license because your state pharmacy board considers this compounding. Your representative is likely to give you looks of astonishment, disbelief, and then a dawning realization of the absurdity of the situation.
Follow-up questions may include “Why can’t you buy buffered lidocaine with epinephrine from the compounding pharmacy?” Easy answer: because each patient needs an individual prescription, and you may not know in advance which patient will need it, and how much the patient will need, and it becomes unstable once it has been buffered. It also will cost the patient $45 per 5-cc syringe, and it will be degraded by the time the patient returns from the compounding pharmacy. Explain further that it costs you only 84 cents to make a 5-cc syringe of buffered lidocaine; that some patients may need as many as 10 syringes; and that these costs are all included in the surgery (free!) if the physician draws it up in the office.
A simple summary is – less pain, less cost – and no history of infections or complications.
It is an eye-opener when you demonstrate how ridiculous the compounding rules being imposed are for physicians and patients. I’ve used this demonstration at the state and federal legislative level, and more recently, at the Food and Drug Administration.
If you get the chance, when a state legislator is in your office, become an advocate for your patients and fellow physicians. Make sure physician offices are excluded from these definitions of com
This column was updated June 22, 2017.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@frontlinemedcom.com.
In Ohio, new limits on drug compounding in physicians’ offices went into effect in April and have become a real hindrance to care for dermatology patients. The State of Ohio Board of Pharmacy has defined compounding as combining two or more prescription drugs and has required that physicians who perform this “compounding” must obtain a “Terminal Distributor of Dangerous Drugs” license. Ohio is the “test state,” and these rules, unless vigorously opposed, will be coming to your state.
[polldaddy:9779752]
The rules state that “compounded” drugs used within 6 hours of preparation must be prepared in a designated clean medication area with proper hand hygiene and the use of powder-free gloves. “Compounded” drugs that are used more than 6 hours after preparation, require a designated clean room with access limited to authorized personnel, environmental control devices such as a laminar flow hood, and additional equipment and training of personnel to maintain an aseptic environment. A separate license is required for each office location.
The state pharmacy boards are eager to restrict physicians – as well as dentists and veterinarians – and to collect annual licensing fees. Additionally, according to an article from the Ohio State Medical Association, noncompliant physicians can be fined by the pharmacy board.
We are talking big money, power, and dreams of clinical relevancy (and billable activities) here.
What can dermatologists do to prevent this regulatory overreach? I encourage you to plan a visit to your state representative, where you can demonstrate how these restrictions affect you and your patients – an exercise that should be both fun and compelling. All you need to illustrate your case is a simple kit that includes a syringe (but no needles in the statehouse!), a bottle of lidocaine with epinephrine, a bottle of 8.4% bicarbonate, alcohol pads, and gloves.
First, explain to your audience that there is a skin cancer epidemic with more than 5.4 million new cases a year and that, over the past 20 years, the incidence of skin cancer has doubled and is projected to double again over the next 20 years. Further, explain that dermatologists treat more than 70% of these cases in the office setting, under local anesthesia, at a huge cost savings to the public and government (it costs an average of 12 times as much to remove these cancers in the outpatient department at the hospital). Remember, states foot most of the bill for Medicaid and Medicare gap indigent coverage.
Take the bottle of lidocaine with epinephrine and open the syringe pack (Staffers love this demonstration; everyone is fascinated with shots.). Put on your gloves, wipe the top of the lidocaine bottle with an alcohol swab, and explain that this medicine is the anesthetic preferred for skin cancer surgery. Explain how it not only numbs the skin, but also causes vasoconstriction, so that the cancer can be easily and safely removed in the office.
Then explain that, in order for the epinephrine to be stable, the solution has to be very acidic (a pH of 4.2, in fact). Explain that this makes it burn like hell unless you add 0.1 cc per cc of 8.4% bicarbonate, in which case the perceived pain on a 10-point scale will drop from 8 to 2. Then pick up the bottle of bicarbonate and explain that you will no longer be able to mix these two components anymore without a “Terminal Distributor of Dangerous Drugs” license because your state pharmacy board considers this compounding. Your representative is likely to give you looks of astonishment, disbelief, and then a dawning realization of the absurdity of the situation.
Follow-up questions may include “Why can’t you buy buffered lidocaine with epinephrine from the compounding pharmacy?” Easy answer: because each patient needs an individual prescription, and you may not know in advance which patient will need it, and how much the patient will need, and it becomes unstable once it has been buffered. It also will cost the patient $45 per 5-cc syringe, and it will be degraded by the time the patient returns from the compounding pharmacy. Explain further that it costs you only 84 cents to make a 5-cc syringe of buffered lidocaine; that some patients may need as many as 10 syringes; and that these costs are all included in the surgery (free!) if the physician draws it up in the office.
A simple summary is – less pain, less cost – and no history of infections or complications.
It is an eye-opener when you demonstrate how ridiculous the compounding rules being imposed are for physicians and patients. I’ve used this demonstration at the state and federal legislative level, and more recently, at the Food and Drug Administration.
If you get the chance, when a state legislator is in your office, become an advocate for your patients and fellow physicians. Make sure physician offices are excluded from these definitions of com
This column was updated June 22, 2017.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@frontlinemedcom.com.
In Ohio, new limits on drug compounding in physicians’ offices went into effect in April and have become a real hindrance to care for dermatology patients. The State of Ohio Board of Pharmacy has defined compounding as combining two or more prescription drugs and has required that physicians who perform this “compounding” must obtain a “Terminal Distributor of Dangerous Drugs” license. Ohio is the “test state,” and these rules, unless vigorously opposed, will be coming to your state.
[polldaddy:9779752]
The rules state that “compounded” drugs used within 6 hours of preparation must be prepared in a designated clean medication area with proper hand hygiene and the use of powder-free gloves. “Compounded” drugs that are used more than 6 hours after preparation, require a designated clean room with access limited to authorized personnel, environmental control devices such as a laminar flow hood, and additional equipment and training of personnel to maintain an aseptic environment. A separate license is required for each office location.
The state pharmacy boards are eager to restrict physicians – as well as dentists and veterinarians – and to collect annual licensing fees. Additionally, according to an article from the Ohio State Medical Association, noncompliant physicians can be fined by the pharmacy board.
We are talking big money, power, and dreams of clinical relevancy (and billable activities) here.
What can dermatologists do to prevent this regulatory overreach? I encourage you to plan a visit to your state representative, where you can demonstrate how these restrictions affect you and your patients – an exercise that should be both fun and compelling. All you need to illustrate your case is a simple kit that includes a syringe (but no needles in the statehouse!), a bottle of lidocaine with epinephrine, a bottle of 8.4% bicarbonate, alcohol pads, and gloves.
First, explain to your audience that there is a skin cancer epidemic with more than 5.4 million new cases a year and that, over the past 20 years, the incidence of skin cancer has doubled and is projected to double again over the next 20 years. Further, explain that dermatologists treat more than 70% of these cases in the office setting, under local anesthesia, at a huge cost savings to the public and government (it costs an average of 12 times as much to remove these cancers in the outpatient department at the hospital). Remember, states foot most of the bill for Medicaid and Medicare gap indigent coverage.
Take the bottle of lidocaine with epinephrine and open the syringe pack (Staffers love this demonstration; everyone is fascinated with shots.). Put on your gloves, wipe the top of the lidocaine bottle with an alcohol swab, and explain that this medicine is the anesthetic preferred for skin cancer surgery. Explain how it not only numbs the skin, but also causes vasoconstriction, so that the cancer can be easily and safely removed in the office.
Then explain that, in order for the epinephrine to be stable, the solution has to be very acidic (a pH of 4.2, in fact). Explain that this makes it burn like hell unless you add 0.1 cc per cc of 8.4% bicarbonate, in which case the perceived pain on a 10-point scale will drop from 8 to 2. Then pick up the bottle of bicarbonate and explain that you will no longer be able to mix these two components anymore without a “Terminal Distributor of Dangerous Drugs” license because your state pharmacy board considers this compounding. Your representative is likely to give you looks of astonishment, disbelief, and then a dawning realization of the absurdity of the situation.
Follow-up questions may include “Why can’t you buy buffered lidocaine with epinephrine from the compounding pharmacy?” Easy answer: because each patient needs an individual prescription, and you may not know in advance which patient will need it, and how much the patient will need, and it becomes unstable once it has been buffered. It also will cost the patient $45 per 5-cc syringe, and it will be degraded by the time the patient returns from the compounding pharmacy. Explain further that it costs you only 84 cents to make a 5-cc syringe of buffered lidocaine; that some patients may need as many as 10 syringes; and that these costs are all included in the surgery (free!) if the physician draws it up in the office.
A simple summary is – less pain, less cost – and no history of infections or complications.
It is an eye-opener when you demonstrate how ridiculous the compounding rules being imposed are for physicians and patients. I’ve used this demonstration at the state and federal legislative level, and more recently, at the Food and Drug Administration.
If you get the chance, when a state legislator is in your office, become an advocate for your patients and fellow physicians. Make sure physician offices are excluded from these definitions of com
This column was updated June 22, 2017.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@frontlinemedcom.com.
Best Practices: Protecting Dry Vulnerable Skin with CeraVe® Healing Ointment
A supplement to Dermatology News. This advertising supplement is sponsored by Valeant Pharmaceuticals.
- Reinforcing the Skin Barrier
- NEA Seal of Acceptance
- A Preventative Approach to Dry, Cracked Skin
- CeraVe Ointment in the Clinical Setting
Faculty/Faculty Disclosure
Sheila Fallon Friedlander, MD
Professor of Clinical Dermatology & Pediatrics
Director, Pediatric Dermatology Fellowship Training Program
University of California at San Diego School of Medicine
Rady Children’s Hospital,
San Diego, California
Dr. Friedlander was compensated for her participation in the development of this article.
CeraVe is a registered trademark of Valeant Pharmaceuticals International, Inc. or its affiliates.
A supplement to Dermatology News. This advertising supplement is sponsored by Valeant Pharmaceuticals.
- Reinforcing the Skin Barrier
- NEA Seal of Acceptance
- A Preventative Approach to Dry, Cracked Skin
- CeraVe Ointment in the Clinical Setting
Faculty/Faculty Disclosure
Sheila Fallon Friedlander, MD
Professor of Clinical Dermatology & Pediatrics
Director, Pediatric Dermatology Fellowship Training Program
University of California at San Diego School of Medicine
Rady Children’s Hospital,
San Diego, California
Dr. Friedlander was compensated for her participation in the development of this article.
CeraVe is a registered trademark of Valeant Pharmaceuticals International, Inc. or its affiliates.
A supplement to Dermatology News. This advertising supplement is sponsored by Valeant Pharmaceuticals.
- Reinforcing the Skin Barrier
- NEA Seal of Acceptance
- A Preventative Approach to Dry, Cracked Skin
- CeraVe Ointment in the Clinical Setting
Faculty/Faculty Disclosure
Sheila Fallon Friedlander, MD
Professor of Clinical Dermatology & Pediatrics
Director, Pediatric Dermatology Fellowship Training Program
University of California at San Diego School of Medicine
Rady Children’s Hospital,
San Diego, California
Dr. Friedlander was compensated for her participation in the development of this article.
CeraVe is a registered trademark of Valeant Pharmaceuticals International, Inc. or its affiliates.
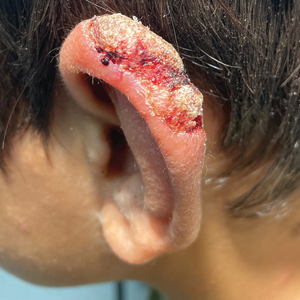
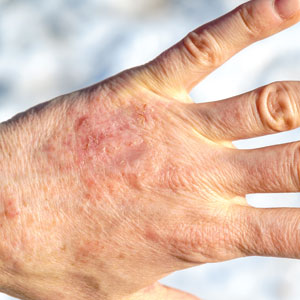
Nonhealing Lesion on the Ear in a Child
Nonhealing Lesion on the Ear in a Child
THE DIAGNOSIS: Cutaneous Leishmaniasis
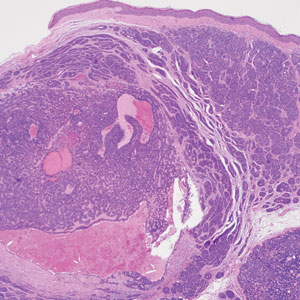
The biopsy results demonstrated a nonspecific chronic granulomatous inflammatory infiltrate, including few multinucleated histiocytes, a surrounding mixed inflammatory infiltrate, mostly mature lymphocytes, few plasma cells, and fragmented neutrophils. A special stain panel was negative for acid-fast bacilli (AFB), Fite, and periodic acid–Schiff for fungi. Bacterial cultures from biopsy tissue grew normal skin flora, and both fungal and AFB cultures were negative. A second punch biopsy was recommended by infectious disease due to clinical suspicion of cutaneous leishmaniasis (CL). Histopathology showed nonnecrotizing granulomas with dense lymphoplasmacytic inflammation and negative Giemsa staining for Leishmania amastigotes; however, it was concluded by pathology that the reason for the negative Leishmania staining was the late stage of the disease, indicated by the presence of granulomas, which can make visualization of organisms difficult. Nonetheless, universal polymerase chain reaction (PCR) testing was positive for Leishmania tropica. Thus, although microscopic analysis was negative for visualization of Leishmania amastigotes, molecular analysis via PCR ultimately demonstrated a positive result and confirmed the diagnosis of CL (Figure 1). The variance in diagnostic accuracy exemplified in our case reinforces the need for multimodal diagnosis.
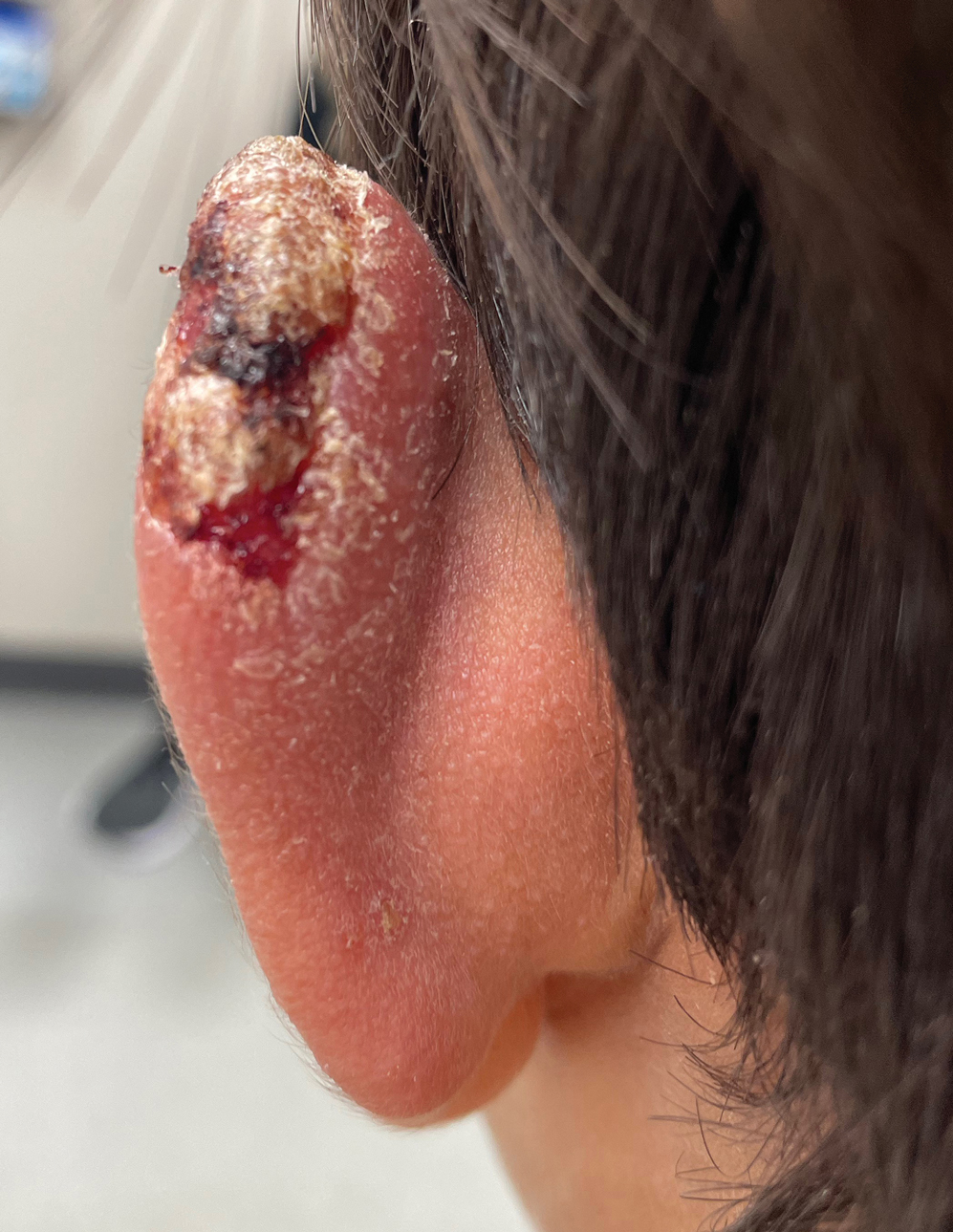
Multiple factors needed to be considered with regard to treatment in our patient, including but not limited to the location of the lesion on a slow-healing cartilaginous surface and the patient’s age. Considering the recalcitrant nature of the lesion and the L tropica strain exhibiting resistance to topical treatments, systemic therapies were the only option. Furthermore, parenteral routes of administration were confounded by the patient’s age, decreasing the likelihood of compliance with therapy. With these variables in mind and recommendations from the Infectious Diseases Society of America and the Centers for Disease Control and Prevention, the best treatment for our patient was deemed to be a 28-day course of oral miltefosine 50 mg twice daily. Compared to the initial presentation, a 1-month follow-up visit after completing the 28-day course of treatment demonstrated flattening of the lesion (Figure 2).
Leishmaniasis is a disease caused by a protozoan parasite of the Leishmania genus, spread via inoculation from the bite of sandfly vectors.1 Cutaneous leishmaniasis is the most common clinical manifestation of leishmaniasis. Other clinical manifestations include mucocutaneous leishmaniasis and visceral leishmaniasis.1,2 Cutaneous leishmaniasis typically manifests as open wounds on areas of skin that may have been exposed to sandfly bites.3 The lesion may not appear until weeks to months or even years after the initial inoculation.2 Initially, CL manifests as papules that may progress to nodular plaques, with eventual evolution to volcanic ulcerations with raised borders and central crateriform indentations covered by scabs or crusting.2,3 The infection may be localized or diffuse—in either case, development of satellite lesions, regional lymphadenopathy, and/or nodular lymphangitis is not uncommon. Generally, CL is not lethal, but the severity of the lesions may vary and can lead to permanent scarring and atrophy.2 Many cases of CL remain undiagnosed because of its appearance as a nonspecific ulcer that can mimic many other cutaneous lesions and because it generally heals spontaneously, leaving only scarring as an indicator of prior infection.4 Thus, CL requires a high diagnostic suspicion, as it can have a nonspecific presentation and is rare in nonendemic regions.
Diagnosis of CL is accomplished via microscopy, isoenzyme analysis, or serology or is made molecularly.3 Microscopic diagnosis includes visualization of Leishmania amastigotes, the stage of replication that occurs after the promastigote stage is phagocytosed by macrophages.3 Amastigote is the only stage that can be visualized in human tissue and is stained via Giemsa and/or hematoxylin and eosin.3 However, Leishmania amastigotes are morphologically indistinguishable from Trypanosoma cruzi amastigotes on microscopy, thus limiting diagnostic accuracy.3 Moreover, there is potential for missed diagnosis of persistent CL caused by L tropica due to fewer parasites being present, further complicating the diagnosis.5 In these cases, molecular diagnostics are helpful as they have higher sensitivity and quicker results. Additionally, DNA technologies can differentiate strains, which is beneficial for guiding treatment. Isoenzyme analysis also can help identify Leishmania species, although results can take weeks to return.3 Serologic testing is useful for suspected visceral leishmaniasis despite negative definitive diagnoses or conflicts with conducting definitive studies; however, there is not a strong antibody response in CL, thus serology is ineffective.3,5 Furthermore, serology can have cross-reactivity with T cruzi and cannot be used to assess for treatment response.3,5 The Infectious Diseases Society of America guidelines for diagnosis of leishmaniasis recommend using multiple methods to ensure a positive result, with molecular assays being the most sensitive.5
Differential diagnoses include any cause of cutaneous ulcerated lesions, including but not limited to mycobacterial or fungal infections. Leprosy often initially manifests with a hypopigmented macule with a raised border, although there often are associated neuropathic symptoms.6 Cutaneous tuberculosis is an extremely rare manifestation that occurs via direct inoculation of the mycobacterium, occurring primarily in children. Initially, it may manifest as a firm red papule that progresses to a painless shallow ulcer with a granular base.7 Cutaneous chromoblastomycosis is a fungal infection resulting from an initial cutaneous injury, similar to our patient, followed by a slow-developing warty lesion that may heal into ivory scars or spread as plaques on normal skin.8 The verrucous lesions seen in cutaneous chromoblastomycosis tend to manifest on the lower extremities and are unlikely to manifest on the head. Sarcoidosis is another granulomatous skin eruption that can be clinically nonspecific.9 Histologically, lesions may demonstrate noncaseating granulomatous inflammation, as seen with cutaneous leishmaniasis, with a broad presentation; for example, lupus pernio, a sarcoid variant, manifests as large blue-red dusky nodules/plaques on the face, ears, or digits.9 Other sarcoid lesions include red/brown, thickened, circular plaques; variably discolored papulonodular lesions; or mucosal involvement.9 Ultimately, it is important to differentiate these nonspecific and similarly appearing lesions through diagnostic techniques such as AFB culture and smear, fungal staining, tuberculosis testing, and PCR in more challenging cases.
Treatment of cutaneous leishmaniasis should be individualized to each case.5 A more than 50% reduction in lesion size within 4 to 6 weeks indicates successful treatment. Ulcerated lesions should be fully re-epithelialized and healed by 3 months posttreatment. Treatment failure is categorized by failure of reepithelization, incomplete healing by 3 months, or worsening of the lesion at any time, each necessitating additional treatment, such as a second course of miltefosine or a different medication regimen.5 Careful monitoring is required throughout treatment, assessing for treatment failure, adding to the challenges of leishmaniasis.
In conclusion, CL requires a high index of suspicion in nonendemic areas to ensure successful diagnosis and treatment. Our case highlights the importance of using multimodal diagnostic techniques for CL, as a single modality may not exhibit a positive result due to variations in diagnostic accuracy. Our case also exhibits the complex treatment of CL, and the considerations that should be made when choosing a treatment modality.
- Leishmaniasis. World Health Organization. Accessed September 14, 2024. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis
- Clinical overview of leishmaniasis. Centers for Disease Control and Prevention. Accessed September 14, 2024. https://www.cdc.gov/leishmaniasis/hcp/clinical-overview/index.html
- CDC DPDx Leishmaniasis. Centers for Disease Control and Prevention. Accessed September 15, 2024. https://www.cdc.gov/dpdx/leishmaniasis/index.html
- Stark CG. Leishmaniasis differential diagnoses. Medscape. March 21, 2023. Accessed October 12, 2024. https://emedicine.medscape.com/article/220298-differential
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63:E202-E264. doi:10.1093/cid/ciw670
- Lewis FS. Dermatologic manifestations of leprosy. Medscape. June 19, 2023. Accessed October 12, 2024. https://emedicine.medscape.com/article/1104977-overview
- Ngan V. Cutaneous tuberculosis. DermNet. Accessed October 12, 2024. https://dermnetnz.org/topics/cutaneous-tuberculosis
- Schwartz RA. Chromoblastomycosis. Medscape. May 13, 2023. Accessed October 12, 2024. https://emedicine.medscape.com/article/1092695-overview#a4
- Elyoussfi S, Coulson I. Sarcoidosis. DermNet. May 31, 2024. Accessed October 25, 2024. https://dermnetnz.org/topics/sarcoidosis
THE DIAGNOSIS: Cutaneous Leishmaniasis
The biopsy results demonstrated a nonspecific chronic granulomatous inflammatory infiltrate, including few multinucleated histiocytes, a surrounding mixed inflammatory infiltrate, mostly mature lymphocytes, few plasma cells, and fragmented neutrophils. A special stain panel was negative for acid-fast bacilli (AFB), Fite, and periodic acid–Schiff for fungi. Bacterial cultures from biopsy tissue grew normal skin flora, and both fungal and AFB cultures were negative. A second punch biopsy was recommended by infectious disease due to clinical suspicion of cutaneous leishmaniasis (CL). Histopathology showed nonnecrotizing granulomas with dense lymphoplasmacytic inflammation and negative Giemsa staining for Leishmania amastigotes; however, it was concluded by pathology that the reason for the negative Leishmania staining was the late stage of the disease, indicated by the presence of granulomas, which can make visualization of organisms difficult. Nonetheless, universal polymerase chain reaction (PCR) testing was positive for Leishmania tropica. Thus, although microscopic analysis was negative for visualization of Leishmania amastigotes, molecular analysis via PCR ultimately demonstrated a positive result and confirmed the diagnosis of CL (Figure 1). The variance in diagnostic accuracy exemplified in our case reinforces the need for multimodal diagnosis.

Multiple factors needed to be considered with regard to treatment in our patient, including but not limited to the location of the lesion on a slow-healing cartilaginous surface and the patient’s age. Considering the recalcitrant nature of the lesion and the L tropica strain exhibiting resistance to topical treatments, systemic therapies were the only option. Furthermore, parenteral routes of administration were confounded by the patient’s age, decreasing the likelihood of compliance with therapy. With these variables in mind and recommendations from the Infectious Diseases Society of America and the Centers for Disease Control and Prevention, the best treatment for our patient was deemed to be a 28-day course of oral miltefosine 50 mg twice daily. Compared to the initial presentation, a 1-month follow-up visit after completing the 28-day course of treatment demonstrated flattening of the lesion (Figure 2).

Leishmaniasis is a disease caused by a protozoan parasite of the Leishmania genus, spread via inoculation from the bite of sandfly vectors.1 Cutaneous leishmaniasis is the most common clinical manifestation of leishmaniasis. Other clinical manifestations include mucocutaneous leishmaniasis and visceral leishmaniasis.1,2 Cutaneous leishmaniasis typically manifests as open wounds on areas of skin that may have been exposed to sandfly bites.3 The lesion may not appear until weeks to months or even years after the initial inoculation.2 Initially, CL manifests as papules that may progress to nodular plaques, with eventual evolution to volcanic ulcerations with raised borders and central crateriform indentations covered by scabs or crusting.2,3 The infection may be localized or diffuse—in either case, development of satellite lesions, regional lymphadenopathy, and/or nodular lymphangitis is not uncommon. Generally, CL is not lethal, but the severity of the lesions may vary and can lead to permanent scarring and atrophy.2 Many cases of CL remain undiagnosed because of its appearance as a nonspecific ulcer that can mimic many other cutaneous lesions and because it generally heals spontaneously, leaving only scarring as an indicator of prior infection.4 Thus, CL requires a high diagnostic suspicion, as it can have a nonspecific presentation and is rare in nonendemic regions.
Diagnosis of CL is accomplished via microscopy, isoenzyme analysis, or serology or is made molecularly.3 Microscopic diagnosis includes visualization of Leishmania amastigotes, the stage of replication that occurs after the promastigote stage is phagocytosed by macrophages.3 Amastigote is the only stage that can be visualized in human tissue and is stained via Giemsa and/or hematoxylin and eosin.3 However, Leishmania amastigotes are morphologically indistinguishable from Trypanosoma cruzi amastigotes on microscopy, thus limiting diagnostic accuracy.3 Moreover, there is potential for missed diagnosis of persistent CL caused by L tropica due to fewer parasites being present, further complicating the diagnosis.5 In these cases, molecular diagnostics are helpful as they have higher sensitivity and quicker results. Additionally, DNA technologies can differentiate strains, which is beneficial for guiding treatment. Isoenzyme analysis also can help identify Leishmania species, although results can take weeks to return.3 Serologic testing is useful for suspected visceral leishmaniasis despite negative definitive diagnoses or conflicts with conducting definitive studies; however, there is not a strong antibody response in CL, thus serology is ineffective.3,5 Furthermore, serology can have cross-reactivity with T cruzi and cannot be used to assess for treatment response.3,5 The Infectious Diseases Society of America guidelines for diagnosis of leishmaniasis recommend using multiple methods to ensure a positive result, with molecular assays being the most sensitive.5
Differential diagnoses include any cause of cutaneous ulcerated lesions, including but not limited to mycobacterial or fungal infections. Leprosy often initially manifests with a hypopigmented macule with a raised border, although there often are associated neuropathic symptoms.6 Cutaneous tuberculosis is an extremely rare manifestation that occurs via direct inoculation of the mycobacterium, occurring primarily in children. Initially, it may manifest as a firm red papule that progresses to a painless shallow ulcer with a granular base.7 Cutaneous chromoblastomycosis is a fungal infection resulting from an initial cutaneous injury, similar to our patient, followed by a slow-developing warty lesion that may heal into ivory scars or spread as plaques on normal skin.8 The verrucous lesions seen in cutaneous chromoblastomycosis tend to manifest on the lower extremities and are unlikely to manifest on the head. Sarcoidosis is another granulomatous skin eruption that can be clinically nonspecific.9 Histologically, lesions may demonstrate noncaseating granulomatous inflammation, as seen with cutaneous leishmaniasis, with a broad presentation; for example, lupus pernio, a sarcoid variant, manifests as large blue-red dusky nodules/plaques on the face, ears, or digits.9 Other sarcoid lesions include red/brown, thickened, circular plaques; variably discolored papulonodular lesions; or mucosal involvement.9 Ultimately, it is important to differentiate these nonspecific and similarly appearing lesions through diagnostic techniques such as AFB culture and smear, fungal staining, tuberculosis testing, and PCR in more challenging cases.
Treatment of cutaneous leishmaniasis should be individualized to each case.5 A more than 50% reduction in lesion size within 4 to 6 weeks indicates successful treatment. Ulcerated lesions should be fully re-epithelialized and healed by 3 months posttreatment. Treatment failure is categorized by failure of reepithelization, incomplete healing by 3 months, or worsening of the lesion at any time, each necessitating additional treatment, such as a second course of miltefosine or a different medication regimen.5 Careful monitoring is required throughout treatment, assessing for treatment failure, adding to the challenges of leishmaniasis.
In conclusion, CL requires a high index of suspicion in nonendemic areas to ensure successful diagnosis and treatment. Our case highlights the importance of using multimodal diagnostic techniques for CL, as a single modality may not exhibit a positive result due to variations in diagnostic accuracy. Our case also exhibits the complex treatment of CL, and the considerations that should be made when choosing a treatment modality.
THE DIAGNOSIS: Cutaneous Leishmaniasis
The biopsy results demonstrated a nonspecific chronic granulomatous inflammatory infiltrate, including few multinucleated histiocytes, a surrounding mixed inflammatory infiltrate, mostly mature lymphocytes, few plasma cells, and fragmented neutrophils. A special stain panel was negative for acid-fast bacilli (AFB), Fite, and periodic acid–Schiff for fungi. Bacterial cultures from biopsy tissue grew normal skin flora, and both fungal and AFB cultures were negative. A second punch biopsy was recommended by infectious disease due to clinical suspicion of cutaneous leishmaniasis (CL). Histopathology showed nonnecrotizing granulomas with dense lymphoplasmacytic inflammation and negative Giemsa staining for Leishmania amastigotes; however, it was concluded by pathology that the reason for the negative Leishmania staining was the late stage of the disease, indicated by the presence of granulomas, which can make visualization of organisms difficult. Nonetheless, universal polymerase chain reaction (PCR) testing was positive for Leishmania tropica. Thus, although microscopic analysis was negative for visualization of Leishmania amastigotes, molecular analysis via PCR ultimately demonstrated a positive result and confirmed the diagnosis of CL (Figure 1). The variance in diagnostic accuracy exemplified in our case reinforces the need for multimodal diagnosis.

Multiple factors needed to be considered with regard to treatment in our patient, including but not limited to the location of the lesion on a slow-healing cartilaginous surface and the patient’s age. Considering the recalcitrant nature of the lesion and the L tropica strain exhibiting resistance to topical treatments, systemic therapies were the only option. Furthermore, parenteral routes of administration were confounded by the patient’s age, decreasing the likelihood of compliance with therapy. With these variables in mind and recommendations from the Infectious Diseases Society of America and the Centers for Disease Control and Prevention, the best treatment for our patient was deemed to be a 28-day course of oral miltefosine 50 mg twice daily. Compared to the initial presentation, a 1-month follow-up visit after completing the 28-day course of treatment demonstrated flattening of the lesion (Figure 2).

Leishmaniasis is a disease caused by a protozoan parasite of the Leishmania genus, spread via inoculation from the bite of sandfly vectors.1 Cutaneous leishmaniasis is the most common clinical manifestation of leishmaniasis. Other clinical manifestations include mucocutaneous leishmaniasis and visceral leishmaniasis.1,2 Cutaneous leishmaniasis typically manifests as open wounds on areas of skin that may have been exposed to sandfly bites.3 The lesion may not appear until weeks to months or even years after the initial inoculation.2 Initially, CL manifests as papules that may progress to nodular plaques, with eventual evolution to volcanic ulcerations with raised borders and central crateriform indentations covered by scabs or crusting.2,3 The infection may be localized or diffuse—in either case, development of satellite lesions, regional lymphadenopathy, and/or nodular lymphangitis is not uncommon. Generally, CL is not lethal, but the severity of the lesions may vary and can lead to permanent scarring and atrophy.2 Many cases of CL remain undiagnosed because of its appearance as a nonspecific ulcer that can mimic many other cutaneous lesions and because it generally heals spontaneously, leaving only scarring as an indicator of prior infection.4 Thus, CL requires a high diagnostic suspicion, as it can have a nonspecific presentation and is rare in nonendemic regions.
Diagnosis of CL is accomplished via microscopy, isoenzyme analysis, or serology or is made molecularly.3 Microscopic diagnosis includes visualization of Leishmania amastigotes, the stage of replication that occurs after the promastigote stage is phagocytosed by macrophages.3 Amastigote is the only stage that can be visualized in human tissue and is stained via Giemsa and/or hematoxylin and eosin.3 However, Leishmania amastigotes are morphologically indistinguishable from Trypanosoma cruzi amastigotes on microscopy, thus limiting diagnostic accuracy.3 Moreover, there is potential for missed diagnosis of persistent CL caused by L tropica due to fewer parasites being present, further complicating the diagnosis.5 In these cases, molecular diagnostics are helpful as they have higher sensitivity and quicker results. Additionally, DNA technologies can differentiate strains, which is beneficial for guiding treatment. Isoenzyme analysis also can help identify Leishmania species, although results can take weeks to return.3 Serologic testing is useful for suspected visceral leishmaniasis despite negative definitive diagnoses or conflicts with conducting definitive studies; however, there is not a strong antibody response in CL, thus serology is ineffective.3,5 Furthermore, serology can have cross-reactivity with T cruzi and cannot be used to assess for treatment response.3,5 The Infectious Diseases Society of America guidelines for diagnosis of leishmaniasis recommend using multiple methods to ensure a positive result, with molecular assays being the most sensitive.5
Differential diagnoses include any cause of cutaneous ulcerated lesions, including but not limited to mycobacterial or fungal infections. Leprosy often initially manifests with a hypopigmented macule with a raised border, although there often are associated neuropathic symptoms.6 Cutaneous tuberculosis is an extremely rare manifestation that occurs via direct inoculation of the mycobacterium, occurring primarily in children. Initially, it may manifest as a firm red papule that progresses to a painless shallow ulcer with a granular base.7 Cutaneous chromoblastomycosis is a fungal infection resulting from an initial cutaneous injury, similar to our patient, followed by a slow-developing warty lesion that may heal into ivory scars or spread as plaques on normal skin.8 The verrucous lesions seen in cutaneous chromoblastomycosis tend to manifest on the lower extremities and are unlikely to manifest on the head. Sarcoidosis is another granulomatous skin eruption that can be clinically nonspecific.9 Histologically, lesions may demonstrate noncaseating granulomatous inflammation, as seen with cutaneous leishmaniasis, with a broad presentation; for example, lupus pernio, a sarcoid variant, manifests as large blue-red dusky nodules/plaques on the face, ears, or digits.9 Other sarcoid lesions include red/brown, thickened, circular plaques; variably discolored papulonodular lesions; or mucosal involvement.9 Ultimately, it is important to differentiate these nonspecific and similarly appearing lesions through diagnostic techniques such as AFB culture and smear, fungal staining, tuberculosis testing, and PCR in more challenging cases.
Treatment of cutaneous leishmaniasis should be individualized to each case.5 A more than 50% reduction in lesion size within 4 to 6 weeks indicates successful treatment. Ulcerated lesions should be fully re-epithelialized and healed by 3 months posttreatment. Treatment failure is categorized by failure of reepithelization, incomplete healing by 3 months, or worsening of the lesion at any time, each necessitating additional treatment, such as a second course of miltefosine or a different medication regimen.5 Careful monitoring is required throughout treatment, assessing for treatment failure, adding to the challenges of leishmaniasis.
In conclusion, CL requires a high index of suspicion in nonendemic areas to ensure successful diagnosis and treatment. Our case highlights the importance of using multimodal diagnostic techniques for CL, as a single modality may not exhibit a positive result due to variations in diagnostic accuracy. Our case also exhibits the complex treatment of CL, and the considerations that should be made when choosing a treatment modality.
- Leishmaniasis. World Health Organization. Accessed September 14, 2024. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis
- Clinical overview of leishmaniasis. Centers for Disease Control and Prevention. Accessed September 14, 2024. https://www.cdc.gov/leishmaniasis/hcp/clinical-overview/index.html
- CDC DPDx Leishmaniasis. Centers for Disease Control and Prevention. Accessed September 15, 2024. https://www.cdc.gov/dpdx/leishmaniasis/index.html
- Stark CG. Leishmaniasis differential diagnoses. Medscape. March 21, 2023. Accessed October 12, 2024. https://emedicine.medscape.com/article/220298-differential
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63:E202-E264. doi:10.1093/cid/ciw670
- Lewis FS. Dermatologic manifestations of leprosy. Medscape. June 19, 2023. Accessed October 12, 2024. https://emedicine.medscape.com/article/1104977-overview
- Ngan V. Cutaneous tuberculosis. DermNet. Accessed October 12, 2024. https://dermnetnz.org/topics/cutaneous-tuberculosis
- Schwartz RA. Chromoblastomycosis. Medscape. May 13, 2023. Accessed October 12, 2024. https://emedicine.medscape.com/article/1092695-overview#a4
- Elyoussfi S, Coulson I. Sarcoidosis. DermNet. May 31, 2024. Accessed October 25, 2024. https://dermnetnz.org/topics/sarcoidosis
- Leishmaniasis. World Health Organization. Accessed September 14, 2024. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis
- Clinical overview of leishmaniasis. Centers for Disease Control and Prevention. Accessed September 14, 2024. https://www.cdc.gov/leishmaniasis/hcp/clinical-overview/index.html
- CDC DPDx Leishmaniasis. Centers for Disease Control and Prevention. Accessed September 15, 2024. https://www.cdc.gov/dpdx/leishmaniasis/index.html
- Stark CG. Leishmaniasis differential diagnoses. Medscape. March 21, 2023. Accessed October 12, 2024. https://emedicine.medscape.com/article/220298-differential
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63:E202-E264. doi:10.1093/cid/ciw670
- Lewis FS. Dermatologic manifestations of leprosy. Medscape. June 19, 2023. Accessed October 12, 2024. https://emedicine.medscape.com/article/1104977-overview
- Ngan V. Cutaneous tuberculosis. DermNet. Accessed October 12, 2024. https://dermnetnz.org/topics/cutaneous-tuberculosis
- Schwartz RA. Chromoblastomycosis. Medscape. May 13, 2023. Accessed October 12, 2024. https://emedicine.medscape.com/article/1092695-overview#a4
- Elyoussfi S, Coulson I. Sarcoidosis. DermNet. May 31, 2024. Accessed October 25, 2024. https://dermnetnz.org/topics/sarcoidosis
Nonhealing Lesion on the Ear in a Child
Nonhealing Lesion on the Ear in a Child
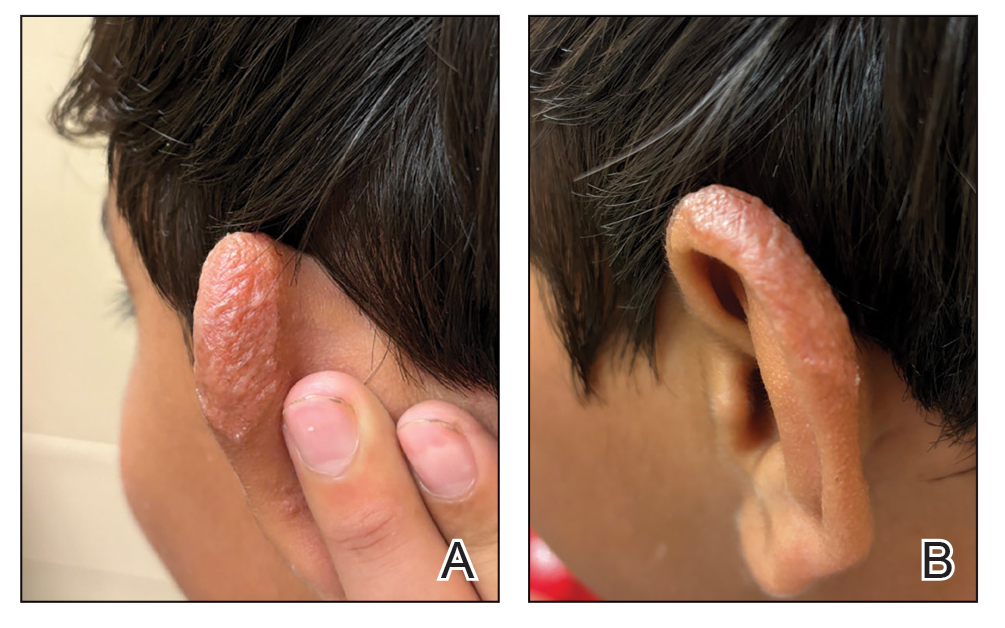
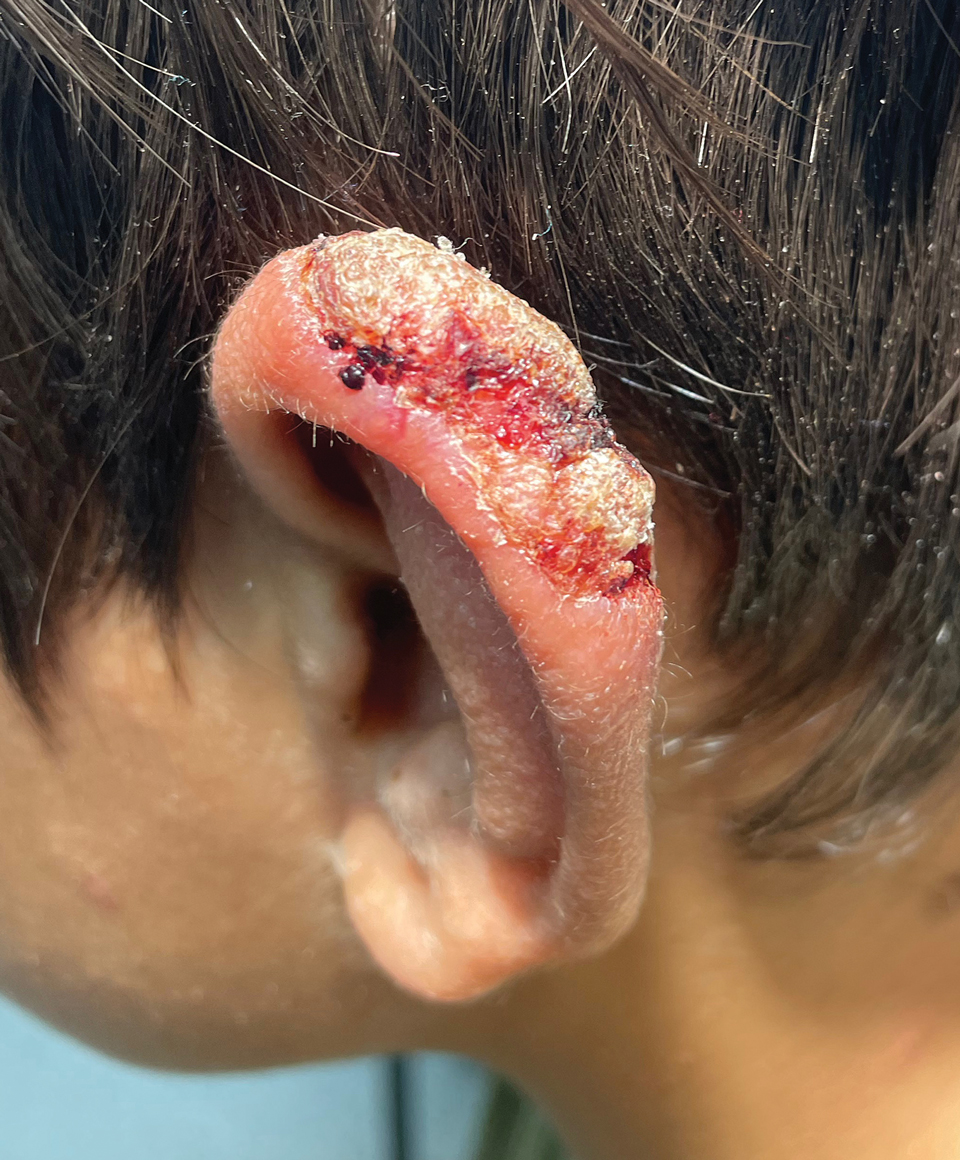
A 10-year-old boy who recently emigrated from Afghanistan presented to his pediatrician for evaluation of a painless nonhealing plaque on the posterior left pinna of more than 1 year's duration. The lesion reportedly started as a small scratch following an ear injury, initially improved with an unknown topical treatment administered in Afghanistan, and then recurred with no other associated lesions and no known insect bite. The lesion persisted for more than 1 year postemigration before the patient presented to his pediatrician, who noted signs of excoriation, which was confirmed by the patient's father. The patient was started on a 7-day course of cephalexin oral suspension and topical mupirocin 2%. After 2 months without improvement, a 2-week course of oral trimethoprim/sulfamethoxazole was initiated; however, the lesion continued to grow with no signs of healing, and he was referred to dermatology.
The patient presented to pediatric dermatology 3 months after the initial presentation to his pediatrician and 2 weeks after he completed the course of oral trimethoprim/sulfamethoxazole. Physical examination demonstrated a papulosquamous eruption with swelling and blistering on the helix of the left ear. Based on these findings, the patient was started on a 1-month trial of topical triamcinolone 1% followed by the addition of topical pimecrolimus 1%. Due to no improvement of the lesion and subsequent progression to ulceration, a punch biopsy was performed.
Impact of a Museum-Based Retreat on the Clinical Skills and Well-Being of Dermatology Residents and Faculty
Impact of a Museum-Based Retreat on the Clinical Skills and Well-Being of Dermatology Residents and Faculty
Prior research has demonstrated that museum-based programming decreases resident burnout and depersonalization.1 A partnership between the Museum of Fine Arts Boston and the Harvard Combined Dermatology Residency Program was well received by residents and resulted in improvement of their observational skills.2 The impact of museum-based programming on the clinical practice skills and well-being of Duke dermatology residents and faculty has not been previously assessed.
In this study, our objective was to evaluate the impact of a 3-part museum-based arts retreat on arts appreciation, clinical practice skills, and well-being among dermatology resident and faculty participants. Surveys administered before and after the retreat were used to assess the value that participants attributed to the arts in various areas of clinical practice.
Methods
A 3-part museum-based retreat held on February 7, 2024, was developed with a Nasher Museum of Art (Durham, North Carolina) curator (E.R.). Part 1 was a personal response tour in which 15 residents and 3 faculty members were given individualized prompts and asked to identify an art piece in the museum that encapsulated their response; they then were asked to explain to the group why they chose that particular piece. Participants were given 10 minutes to explore the museum galleries to choose their piece, followed by 15 minutes to share their selected work in groups of 3 to 4.
Part 2 encompassed visual-thinking strategies, a research-based method that uses art to teach visual literacy, thinking, and communication skills.2 Using this method, facilitators follow a specific protocol to guide participants in the exploration of an art piece through sharing observations and interpretations.4 Participants were divided into 2 groups led by trained museum educators (including E.R.) to analyze and ascribe meaning to a chosen art piece. Three questions were asked: What’s going on in this picture? What do you see that makes you say that? What else can we find?
Part 3 involved back-to-back drawing, in which participants were paired up and tasked with recreating an art piece in the museum based solely on their partner’s verbal description. In each pair, both participants took turns as the describer and the drawer.
After each part of the retreat, 5 to 10 minutes were dedicated to debriefing in small groups about how each activity may connect to the role of a clinician. A total of 15 participants completed pre- and post-retreat surveys to assess the value they attributed to the arts and identify in which aspects of clinical practice they believe the arts play a role.
Results
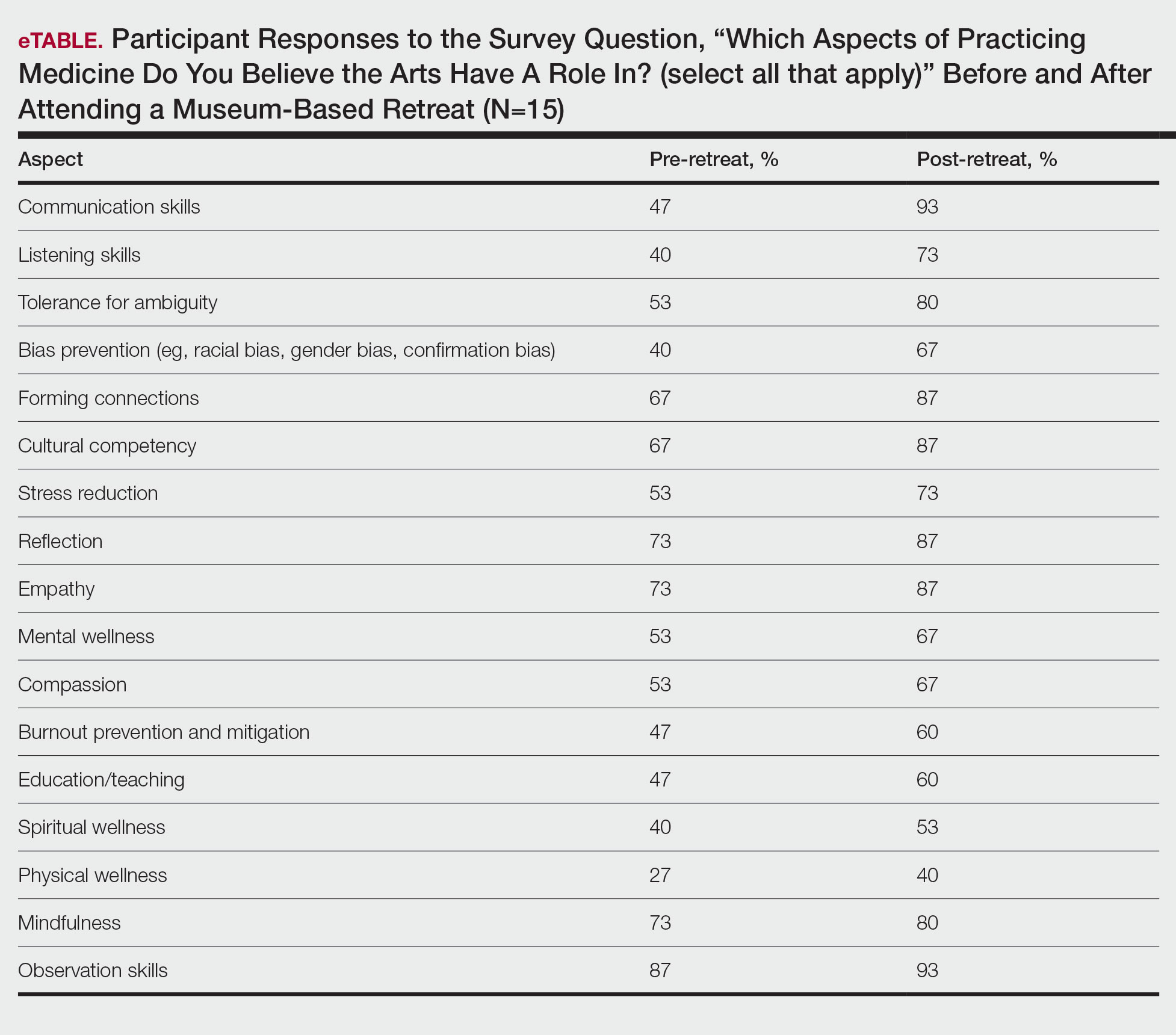
Seventy-three percent of participants (11/15) found the museum-based retreat “extremely useful” or “very useful.” There was a 20% increase in those who attributed at least moderate value to the arts as a clinician after compared to before the retreat (13/15 [87%] vs 8/15 [53%]), and 100% of the participants desired to participate in future arts-based programming. Following the retreat, a greater percentage of participants believed the arts have a role in the following aspects of clinical practice: education, observation, listening, communication, empathy, compassion, forming connections, cultural sensitivity, tolerance for ambiguity, reflection, mindfulness, stress reduction, preventing burnout, bias prevention, mental wellness, spiritual wellness, and physical wellness (eTable). Qualitative feedback compiled from the participants’ responses to survey questions following the retreat about their thoughts on each activity and overall feedback was used to create a word cloud (eFigure).

Comment
The importance of arts and humanities integration into medical education previously has been described.5 Our survey results suggest that museum-based programming increases dermatology resident and faculty appreciation for the arts and encourages participation in future arts-based programming. Our results also demonstrate that arts-based programming positively impacts important resident competencies in the practice of medicine including tolerance for ambiguity, bias prevention, and cultural competency, and that the incorporation of arts-based programming can enhance residents’ well-being (physical, mental, and spiritual) as well as their ability to be better clinicians by addressing skills in communication, listening, and observation. The structure of our 3-part museum-based retreat offers practical implementation strategies for integrating the humanities into dermatology residency curricula and easily can be modified to meet the needs of different dermatology residency programs.
Orr AR, Moghbeli N, Swain A, et al. The Fostering Resilience through Art in Medical Education (FRAME) workshop: a partnership with the Philadelphia Museum of Art. Adv Med Educ Pract. 2019;10:361-369. doi:10.2147/AMEP.S194575
Zimmermann C, Huang JT, Buzney EA. Refining the eye: dermatology and visual literacy. J Museum Ed. 2016;41:116-122.
Yenawine P. Visual Thinking Strategies: Using Art to Deepen Learning Across School Disciplines. Harvard Education Press; 2013.
Hailey D, Miller A, Yenawine P. Understanding visual literacy: the visual thinking strategies approach. In: Baylen DM, D’Alba A. Essentials of Teaching and Integrating Visual and Media Literacy: Visualizing Learning. Springer Cham; 2015:49-73. doi:10.1007/978-3-319-05837-5
Howley L, Gaufberg E, King BE. The Fundamental Role of the Arts and Humanities in Medical Education. Association of American Medical Colleges; 2020. Accessed December 18, 2025. https://store.aamc.org/the-fundamental-role-of-the-arts-and-humanities-in-medical-education.html
Prior research has demonstrated that museum-based programming decreases resident burnout and depersonalization.1 A partnership between the Museum of Fine Arts Boston and the Harvard Combined Dermatology Residency Program was well received by residents and resulted in improvement of their observational skills.2 The impact of museum-based programming on the clinical practice skills and well-being of Duke dermatology residents and faculty has not been previously assessed.
In this study, our objective was to evaluate the impact of a 3-part museum-based arts retreat on arts appreciation, clinical practice skills, and well-being among dermatology resident and faculty participants. Surveys administered before and after the retreat were used to assess the value that participants attributed to the arts in various areas of clinical practice.
Methods
A 3-part museum-based retreat held on February 7, 2024, was developed with a Nasher Museum of Art (Durham, North Carolina) curator (E.R.). Part 1 was a personal response tour in which 15 residents and 3 faculty members were given individualized prompts and asked to identify an art piece in the museum that encapsulated their response; they then were asked to explain to the group why they chose that particular piece. Participants were given 10 minutes to explore the museum galleries to choose their piece, followed by 15 minutes to share their selected work in groups of 3 to 4.
Part 2 encompassed visual-thinking strategies, a research-based method that uses art to teach visual literacy, thinking, and communication skills.2 Using this method, facilitators follow a specific protocol to guide participants in the exploration of an art piece through sharing observations and interpretations.4 Participants were divided into 2 groups led by trained museum educators (including E.R.) to analyze and ascribe meaning to a chosen art piece. Three questions were asked: What’s going on in this picture? What do you see that makes you say that? What else can we find?
Part 3 involved back-to-back drawing, in which participants were paired up and tasked with recreating an art piece in the museum based solely on their partner’s verbal description. In each pair, both participants took turns as the describer and the drawer.
After each part of the retreat, 5 to 10 minutes were dedicated to debriefing in small groups about how each activity may connect to the role of a clinician. A total of 15 participants completed pre- and post-retreat surveys to assess the value they attributed to the arts and identify in which aspects of clinical practice they believe the arts play a role.
Results
Seventy-three percent of participants (11/15) found the museum-based retreat “extremely useful” or “very useful.” There was a 20% increase in those who attributed at least moderate value to the arts as a clinician after compared to before the retreat (13/15 [87%] vs 8/15 [53%]), and 100% of the participants desired to participate in future arts-based programming. Following the retreat, a greater percentage of participants believed the arts have a role in the following aspects of clinical practice: education, observation, listening, communication, empathy, compassion, forming connections, cultural sensitivity, tolerance for ambiguity, reflection, mindfulness, stress reduction, preventing burnout, bias prevention, mental wellness, spiritual wellness, and physical wellness (eTable). Qualitative feedback compiled from the participants’ responses to survey questions following the retreat about their thoughts on each activity and overall feedback was used to create a word cloud (eFigure).


Comment
The importance of arts and humanities integration into medical education previously has been described.5 Our survey results suggest that museum-based programming increases dermatology resident and faculty appreciation for the arts and encourages participation in future arts-based programming. Our results also demonstrate that arts-based programming positively impacts important resident competencies in the practice of medicine including tolerance for ambiguity, bias prevention, and cultural competency, and that the incorporation of arts-based programming can enhance residents’ well-being (physical, mental, and spiritual) as well as their ability to be better clinicians by addressing skills in communication, listening, and observation. The structure of our 3-part museum-based retreat offers practical implementation strategies for integrating the humanities into dermatology residency curricula and easily can be modified to meet the needs of different dermatology residency programs.
Prior research has demonstrated that museum-based programming decreases resident burnout and depersonalization.1 A partnership between the Museum of Fine Arts Boston and the Harvard Combined Dermatology Residency Program was well received by residents and resulted in improvement of their observational skills.2 The impact of museum-based programming on the clinical practice skills and well-being of Duke dermatology residents and faculty has not been previously assessed.
In this study, our objective was to evaluate the impact of a 3-part museum-based arts retreat on arts appreciation, clinical practice skills, and well-being among dermatology resident and faculty participants. Surveys administered before and after the retreat were used to assess the value that participants attributed to the arts in various areas of clinical practice.
Methods
A 3-part museum-based retreat held on February 7, 2024, was developed with a Nasher Museum of Art (Durham, North Carolina) curator (E.R.). Part 1 was a personal response tour in which 15 residents and 3 faculty members were given individualized prompts and asked to identify an art piece in the museum that encapsulated their response; they then were asked to explain to the group why they chose that particular piece. Participants were given 10 minutes to explore the museum galleries to choose their piece, followed by 15 minutes to share their selected work in groups of 3 to 4.
Part 2 encompassed visual-thinking strategies, a research-based method that uses art to teach visual literacy, thinking, and communication skills.2 Using this method, facilitators follow a specific protocol to guide participants in the exploration of an art piece through sharing observations and interpretations.4 Participants were divided into 2 groups led by trained museum educators (including E.R.) to analyze and ascribe meaning to a chosen art piece. Three questions were asked: What’s going on in this picture? What do you see that makes you say that? What else can we find?
Part 3 involved back-to-back drawing, in which participants were paired up and tasked with recreating an art piece in the museum based solely on their partner’s verbal description. In each pair, both participants took turns as the describer and the drawer.
After each part of the retreat, 5 to 10 minutes were dedicated to debriefing in small groups about how each activity may connect to the role of a clinician. A total of 15 participants completed pre- and post-retreat surveys to assess the value they attributed to the arts and identify in which aspects of clinical practice they believe the arts play a role.
Results
Seventy-three percent of participants (11/15) found the museum-based retreat “extremely useful” or “very useful.” There was a 20% increase in those who attributed at least moderate value to the arts as a clinician after compared to before the retreat (13/15 [87%] vs 8/15 [53%]), and 100% of the participants desired to participate in future arts-based programming. Following the retreat, a greater percentage of participants believed the arts have a role in the following aspects of clinical practice: education, observation, listening, communication, empathy, compassion, forming connections, cultural sensitivity, tolerance for ambiguity, reflection, mindfulness, stress reduction, preventing burnout, bias prevention, mental wellness, spiritual wellness, and physical wellness (eTable). Qualitative feedback compiled from the participants’ responses to survey questions following the retreat about their thoughts on each activity and overall feedback was used to create a word cloud (eFigure).


Comment
The importance of arts and humanities integration into medical education previously has been described.5 Our survey results suggest that museum-based programming increases dermatology resident and faculty appreciation for the arts and encourages participation in future arts-based programming. Our results also demonstrate that arts-based programming positively impacts important resident competencies in the practice of medicine including tolerance for ambiguity, bias prevention, and cultural competency, and that the incorporation of arts-based programming can enhance residents’ well-being (physical, mental, and spiritual) as well as their ability to be better clinicians by addressing skills in communication, listening, and observation. The structure of our 3-part museum-based retreat offers practical implementation strategies for integrating the humanities into dermatology residency curricula and easily can be modified to meet the needs of different dermatology residency programs.
Orr AR, Moghbeli N, Swain A, et al. The Fostering Resilience through Art in Medical Education (FRAME) workshop: a partnership with the Philadelphia Museum of Art. Adv Med Educ Pract. 2019;10:361-369. doi:10.2147/AMEP.S194575
Zimmermann C, Huang JT, Buzney EA. Refining the eye: dermatology and visual literacy. J Museum Ed. 2016;41:116-122.
Yenawine P. Visual Thinking Strategies: Using Art to Deepen Learning Across School Disciplines. Harvard Education Press; 2013.
Hailey D, Miller A, Yenawine P. Understanding visual literacy: the visual thinking strategies approach. In: Baylen DM, D’Alba A. Essentials of Teaching and Integrating Visual and Media Literacy: Visualizing Learning. Springer Cham; 2015:49-73. doi:10.1007/978-3-319-05837-5
Howley L, Gaufberg E, King BE. The Fundamental Role of the Arts and Humanities in Medical Education. Association of American Medical Colleges; 2020. Accessed December 18, 2025. https://store.aamc.org/the-fundamental-role-of-the-arts-and-humanities-in-medical-education.html
Orr AR, Moghbeli N, Swain A, et al. The Fostering Resilience through Art in Medical Education (FRAME) workshop: a partnership with the Philadelphia Museum of Art. Adv Med Educ Pract. 2019;10:361-369. doi:10.2147/AMEP.S194575
Zimmermann C, Huang JT, Buzney EA. Refining the eye: dermatology and visual literacy. J Museum Ed. 2016;41:116-122.
Yenawine P. Visual Thinking Strategies: Using Art to Deepen Learning Across School Disciplines. Harvard Education Press; 2013.
Hailey D, Miller A, Yenawine P. Understanding visual literacy: the visual thinking strategies approach. In: Baylen DM, D’Alba A. Essentials of Teaching and Integrating Visual and Media Literacy: Visualizing Learning. Springer Cham; 2015:49-73. doi:10.1007/978-3-319-05837-5
Howley L, Gaufberg E, King BE. The Fundamental Role of the Arts and Humanities in Medical Education. Association of American Medical Colleges; 2020. Accessed December 18, 2025. https://store.aamc.org/the-fundamental-role-of-the-arts-and-humanities-in-medical-education.html
Impact of a Museum-Based Retreat on the Clinical Skills and Well-Being of Dermatology Residents and Faculty
Impact of a Museum-Based Retreat on the Clinical Skills and Well-Being of Dermatology Residents and Faculty
Practice Points
- Arts-based programming positively impacts resident competencies that are important to the practice of medicine.
- Incorporating arts-based programming in the dermatology residency curriculum can enhance resident well-being and the ability to be better clinicians.
Interactive Approach to Teaching Mohs Micrographic Surgery to Dermatology Residents
Interactive Approach to Teaching Mohs Micrographic Surgery to Dermatology Residents
Practice Gap
Tissue processing and complete margin assessment in Mohs micrographic surgery (MMS) are challenging concepts for residents, yet they are essential components of the dermatology residency curriculum. We propose a hands-on active teaching method using craft foam blocks to help residents master these techniques. Prior educational tools have included instructional videos1 as well as the peanut butter–cup and cantaloupe analogies.2,3 Specifically, our method utilizes inexpensive, readily available supplies that allow for repeated practice in a low-stakes environment without limitation of resources. This method provides an immersive, hands-on experience that allows residents to perform multiple practice excisions and simulate positive peripheral or deep margins, unlike tools that offer only fixed-depth or purely visual representations. Additionally, our learning model uniquely enables residents to flatten the simulated tissue, providing a clearer understanding of how a 3-dimensional specimen is transformed on a slide during histologic preparation. This step is particularly important, as tissue architecture can shift during processing, making it one of the most difficult concepts to grasp without hands-on experience. Having a multitude of teaching methods is crucial to accommodate various learning styles, and active learning has been shown to enhance retention for dermatology residents.4
The Technique
Residents use simple art supplies (including craft foam blocks and ink) and inexpensive, readily available surgical tools to simulate MMS (Table)(Figure 1). If desired, the resident can follow along with the comprehensive, stepwise textbook description of MMS, outlined by Benedetto et al5 to contextualize this hands-on exercise within a standardized didactic framework.


The foam block, which represents patient tissue, serves as the specimen. The resident begins by freehand drawing a simulated cutaneous tumor directly onto the foam using a surgical marking pen. At this point, the instructor discusses the advantages and limitations of tumor debulking with a sharp blade or curette. Residents then mark appropriate margins (1-3 mm) of normal-appearing “epidermis” on the foam block and add hash marks for orientation. This is another opportunity for the instructor to discuss common methods for marking tissue in vivo and to review situations when larger or smaller margins might be appropriate.
Next, the resident removes the first layer of simulated tissue using a disposable #15 blade scalpel at a 45° angle circumferentially and deep around the representative tumor. The resident also may use scissors and tissue forceps to remove the representative tumor. Next, the excised foam layer (the simulated “specimen”) is transferred to gauze. To demonstrate a positive margin, the resident or instructor marks the deep or peripheral foam block with a surgical marking pen, indicating residual tumor (Figure 2). This allows for multiple sequential layers of foam to be removed, demonstrating successive stages of MMS.

An inkwell holds different colors of washable paint to simulate tissue inking. After excision, the resident uses cotton-tipped applicators to apply different paint colors to the edges of the excised foam specimen at designated orientation points (eg, 3 o'clock and 12 o'clock). The resident then records the location of the excised sample by hand-drawing it on a printable Mohs map, labeling the corresponding paint colors to indicate orientation (Figure 3).
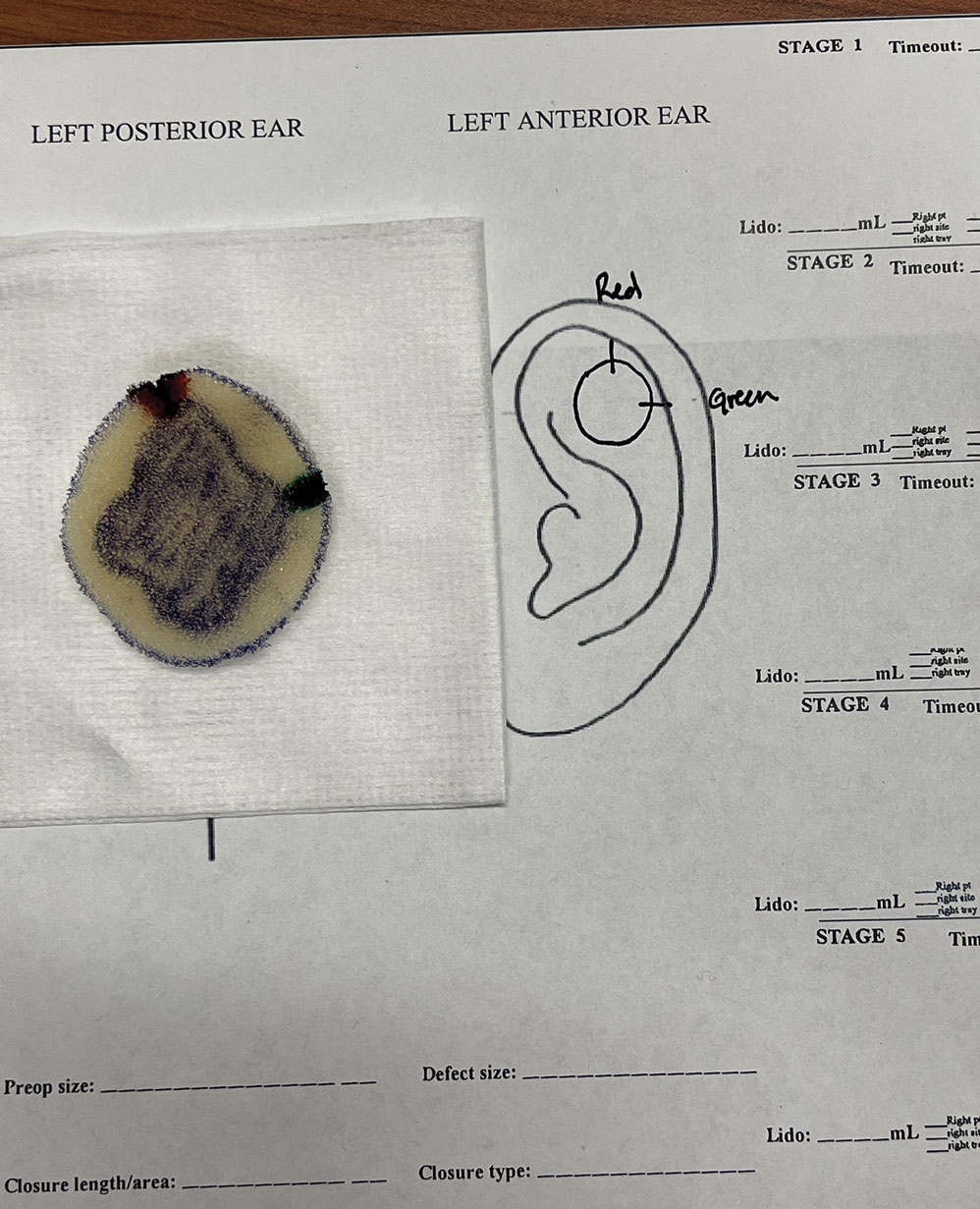
The resident then places the specimen between 2 plastic page protectors mimicking a glass slide and cover slip. Clear tape can be used to help flatten the specimen (Figure 4). The tissue is compressed between the page protector so that the simulated epidermis, dermis, and subcutaneous fat are all in the same plane. At this stage, the instructor may discuss the use of relaxing incisions, especially for deeper tissue specimens or when excision at a 45° bevel is not achieved.5 The view from the underside of the page protector reveals 100% of the specimen’s margin and mimics the first cut off the tissue block. The resident can visualize the complete circumferential, peripheral, and deep margins and can easily identify any positive margins. At this point, the exercise can conclude, or the resident can explore further stages for positive margins, bisected specimens, or other tissue preparation variations.

Practice Implications
By individually designing and removing a representative tumor with margins, creating hash marks, and preparing a tissue specimen for histologic analysis, our interactive teaching method provides dermatology residents with a relatively simple, effective, and active learning experience for MMS outside the surgical setting. Using a piece of craft foam allows the representative tissue to be manipulated and flattened, similar to cutaneous tissue. This method was implemented and refined across 3 separate teaching sessions held by teaching faculty (E.I.P and E.B.W.) at the San Antonio Uniformed Services Health Education Consortium Dermatology Residency Program (San Antonio, Texas). This method has consistently generated strong resident engagement and prompted insightful questions and discussions. Program directors at other residency programs can readily incorporate this method in their surgical curriculum by allocating a brief didactic period to the exercise and facilitating the discussion with a dermatologic surgeon. Its simplicity, low cost, and effectiveness make the foam block model an easily adoptable teaching tool for dermatology residency programs seeking to provide a comprehensive, hands-on understanding of MMS.
- McNeil E, Reich H, Hurliman E. Educational video improves dermatology residents’ understanding of Mohs micrographic surgery: a surveybased matched cohort study. J Am Acad Dermatol. 2020;83:926-927. doi:10.1016/j.jaad.2020.01.013
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744. doi:10.1001 /jamadermatol.2017.0614
- Vassantachart JM, Guccione J, Seeburger J. Clinical pearl: Mohs cantaloupe analogy for the dermatology resident. Cutis. 2018; 102:65-66.
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425. doi:10.1080/01421590801946988
- Benedetto PX, Poblete-Lopez C. Mohs micrographic surgery technique. Dermatol Clinics. 2011;29:141-151. doi:10.1016/j.det.2011.02.002
Practice Gap
Tissue processing and complete margin assessment in Mohs micrographic surgery (MMS) are challenging concepts for residents, yet they are essential components of the dermatology residency curriculum. We propose a hands-on active teaching method using craft foam blocks to help residents master these techniques. Prior educational tools have included instructional videos1 as well as the peanut butter–cup and cantaloupe analogies.2,3 Specifically, our method utilizes inexpensive, readily available supplies that allow for repeated practice in a low-stakes environment without limitation of resources. This method provides an immersive, hands-on experience that allows residents to perform multiple practice excisions and simulate positive peripheral or deep margins, unlike tools that offer only fixed-depth or purely visual representations. Additionally, our learning model uniquely enables residents to flatten the simulated tissue, providing a clearer understanding of how a 3-dimensional specimen is transformed on a slide during histologic preparation. This step is particularly important, as tissue architecture can shift during processing, making it one of the most difficult concepts to grasp without hands-on experience. Having a multitude of teaching methods is crucial to accommodate various learning styles, and active learning has been shown to enhance retention for dermatology residents.4
The Technique
Residents use simple art supplies (including craft foam blocks and ink) and inexpensive, readily available surgical tools to simulate MMS (Table)(Figure 1). If desired, the resident can follow along with the comprehensive, stepwise textbook description of MMS, outlined by Benedetto et al5 to contextualize this hands-on exercise within a standardized didactic framework.


The foam block, which represents patient tissue, serves as the specimen. The resident begins by freehand drawing a simulated cutaneous tumor directly onto the foam using a surgical marking pen. At this point, the instructor discusses the advantages and limitations of tumor debulking with a sharp blade or curette. Residents then mark appropriate margins (1-3 mm) of normal-appearing “epidermis” on the foam block and add hash marks for orientation. This is another opportunity for the instructor to discuss common methods for marking tissue in vivo and to review situations when larger or smaller margins might be appropriate.
Next, the resident removes the first layer of simulated tissue using a disposable #15 blade scalpel at a 45° angle circumferentially and deep around the representative tumor. The resident also may use scissors and tissue forceps to remove the representative tumor. Next, the excised foam layer (the simulated “specimen”) is transferred to gauze. To demonstrate a positive margin, the resident or instructor marks the deep or peripheral foam block with a surgical marking pen, indicating residual tumor (Figure 2). This allows for multiple sequential layers of foam to be removed, demonstrating successive stages of MMS.

An inkwell holds different colors of washable paint to simulate tissue inking. After excision, the resident uses cotton-tipped applicators to apply different paint colors to the edges of the excised foam specimen at designated orientation points (eg, 3 o'clock and 12 o'clock). The resident then records the location of the excised sample by hand-drawing it on a printable Mohs map, labeling the corresponding paint colors to indicate orientation (Figure 3).

The resident then places the specimen between 2 plastic page protectors mimicking a glass slide and cover slip. Clear tape can be used to help flatten the specimen (Figure 4). The tissue is compressed between the page protector so that the simulated epidermis, dermis, and subcutaneous fat are all in the same plane. At this stage, the instructor may discuss the use of relaxing incisions, especially for deeper tissue specimens or when excision at a 45° bevel is not achieved.5 The view from the underside of the page protector reveals 100% of the specimen’s margin and mimics the first cut off the tissue block. The resident can visualize the complete circumferential, peripheral, and deep margins and can easily identify any positive margins. At this point, the exercise can conclude, or the resident can explore further stages for positive margins, bisected specimens, or other tissue preparation variations.

Practice Implications
By individually designing and removing a representative tumor with margins, creating hash marks, and preparing a tissue specimen for histologic analysis, our interactive teaching method provides dermatology residents with a relatively simple, effective, and active learning experience for MMS outside the surgical setting. Using a piece of craft foam allows the representative tissue to be manipulated and flattened, similar to cutaneous tissue. This method was implemented and refined across 3 separate teaching sessions held by teaching faculty (E.I.P and E.B.W.) at the San Antonio Uniformed Services Health Education Consortium Dermatology Residency Program (San Antonio, Texas). This method has consistently generated strong resident engagement and prompted insightful questions and discussions. Program directors at other residency programs can readily incorporate this method in their surgical curriculum by allocating a brief didactic period to the exercise and facilitating the discussion with a dermatologic surgeon. Its simplicity, low cost, and effectiveness make the foam block model an easily adoptable teaching tool for dermatology residency programs seeking to provide a comprehensive, hands-on understanding of MMS.
Practice Gap
Tissue processing and complete margin assessment in Mohs micrographic surgery (MMS) are challenging concepts for residents, yet they are essential components of the dermatology residency curriculum. We propose a hands-on active teaching method using craft foam blocks to help residents master these techniques. Prior educational tools have included instructional videos1 as well as the peanut butter–cup and cantaloupe analogies.2,3 Specifically, our method utilizes inexpensive, readily available supplies that allow for repeated practice in a low-stakes environment without limitation of resources. This method provides an immersive, hands-on experience that allows residents to perform multiple practice excisions and simulate positive peripheral or deep margins, unlike tools that offer only fixed-depth or purely visual representations. Additionally, our learning model uniquely enables residents to flatten the simulated tissue, providing a clearer understanding of how a 3-dimensional specimen is transformed on a slide during histologic preparation. This step is particularly important, as tissue architecture can shift during processing, making it one of the most difficult concepts to grasp without hands-on experience. Having a multitude of teaching methods is crucial to accommodate various learning styles, and active learning has been shown to enhance retention for dermatology residents.4
The Technique
Residents use simple art supplies (including craft foam blocks and ink) and inexpensive, readily available surgical tools to simulate MMS (Table)(Figure 1). If desired, the resident can follow along with the comprehensive, stepwise textbook description of MMS, outlined by Benedetto et al5 to contextualize this hands-on exercise within a standardized didactic framework.


The foam block, which represents patient tissue, serves as the specimen. The resident begins by freehand drawing a simulated cutaneous tumor directly onto the foam using a surgical marking pen. At this point, the instructor discusses the advantages and limitations of tumor debulking with a sharp blade or curette. Residents then mark appropriate margins (1-3 mm) of normal-appearing “epidermis” on the foam block and add hash marks for orientation. This is another opportunity for the instructor to discuss common methods for marking tissue in vivo and to review situations when larger or smaller margins might be appropriate.
Next, the resident removes the first layer of simulated tissue using a disposable #15 blade scalpel at a 45° angle circumferentially and deep around the representative tumor. The resident also may use scissors and tissue forceps to remove the representative tumor. Next, the excised foam layer (the simulated “specimen”) is transferred to gauze. To demonstrate a positive margin, the resident or instructor marks the deep or peripheral foam block with a surgical marking pen, indicating residual tumor (Figure 2). This allows for multiple sequential layers of foam to be removed, demonstrating successive stages of MMS.

An inkwell holds different colors of washable paint to simulate tissue inking. After excision, the resident uses cotton-tipped applicators to apply different paint colors to the edges of the excised foam specimen at designated orientation points (eg, 3 o'clock and 12 o'clock). The resident then records the location of the excised sample by hand-drawing it on a printable Mohs map, labeling the corresponding paint colors to indicate orientation (Figure 3).

The resident then places the specimen between 2 plastic page protectors mimicking a glass slide and cover slip. Clear tape can be used to help flatten the specimen (Figure 4). The tissue is compressed between the page protector so that the simulated epidermis, dermis, and subcutaneous fat are all in the same plane. At this stage, the instructor may discuss the use of relaxing incisions, especially for deeper tissue specimens or when excision at a 45° bevel is not achieved.5 The view from the underside of the page protector reveals 100% of the specimen’s margin and mimics the first cut off the tissue block. The resident can visualize the complete circumferential, peripheral, and deep margins and can easily identify any positive margins. At this point, the exercise can conclude, or the resident can explore further stages for positive margins, bisected specimens, or other tissue preparation variations.

Practice Implications
By individually designing and removing a representative tumor with margins, creating hash marks, and preparing a tissue specimen for histologic analysis, our interactive teaching method provides dermatology residents with a relatively simple, effective, and active learning experience for MMS outside the surgical setting. Using a piece of craft foam allows the representative tissue to be manipulated and flattened, similar to cutaneous tissue. This method was implemented and refined across 3 separate teaching sessions held by teaching faculty (E.I.P and E.B.W.) at the San Antonio Uniformed Services Health Education Consortium Dermatology Residency Program (San Antonio, Texas). This method has consistently generated strong resident engagement and prompted insightful questions and discussions. Program directors at other residency programs can readily incorporate this method in their surgical curriculum by allocating a brief didactic period to the exercise and facilitating the discussion with a dermatologic surgeon. Its simplicity, low cost, and effectiveness make the foam block model an easily adoptable teaching tool for dermatology residency programs seeking to provide a comprehensive, hands-on understanding of MMS.
- McNeil E, Reich H, Hurliman E. Educational video improves dermatology residents’ understanding of Mohs micrographic surgery: a surveybased matched cohort study. J Am Acad Dermatol. 2020;83:926-927. doi:10.1016/j.jaad.2020.01.013
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744. doi:10.1001 /jamadermatol.2017.0614
- Vassantachart JM, Guccione J, Seeburger J. Clinical pearl: Mohs cantaloupe analogy for the dermatology resident. Cutis. 2018; 102:65-66.
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425. doi:10.1080/01421590801946988
- Benedetto PX, Poblete-Lopez C. Mohs micrographic surgery technique. Dermatol Clinics. 2011;29:141-151. doi:10.1016/j.det.2011.02.002
- McNeil E, Reich H, Hurliman E. Educational video improves dermatology residents’ understanding of Mohs micrographic surgery: a surveybased matched cohort study. J Am Acad Dermatol. 2020;83:926-927. doi:10.1016/j.jaad.2020.01.013
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744. doi:10.1001 /jamadermatol.2017.0614
- Vassantachart JM, Guccione J, Seeburger J. Clinical pearl: Mohs cantaloupe analogy for the dermatology resident. Cutis. 2018; 102:65-66.
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425. doi:10.1080/01421590801946988
- Benedetto PX, Poblete-Lopez C. Mohs micrographic surgery technique. Dermatol Clinics. 2011;29:141-151. doi:10.1016/j.det.2011.02.002
Interactive Approach to Teaching Mohs Micrographic Surgery to Dermatology Residents
Interactive Approach to Teaching Mohs Micrographic Surgery to Dermatology Residents
Mobile Tender Papule on the Scalp
Mobile Tender Papule on the Scalp
The Diagnosis: Spiradenocylindroma
T he biopsy results confirmed the diagnosis of spiradenocylindroma with negative margins. At 6-week follow-up, the patient had no signs of recurrence. Spiradenocylindroma is a benign hybrid neoplasm consisting of histologically intermixed areas representing the spectrum of morphology between spiradenoma and cylindromas.1,2 Both spiradenoma and cylindroma comprise 2 distinct populations of dark and pale basaloid cells.2,3 The spiradenomatous areas of the spiradenocylindroma are arranged in large, well-circumscribed collections of small, darkly staining cells with interspersed lymphocytes and a thin basement membrane surrounding spiradenocylindroma component.2,3 The spiradenocylindroma regions also may contain tubular structures dilated by hemorrhage.2 In contrast, the cylindromatous regions have a jigsaw-puzzle configuration of polygonal tumor nests containing peripherally palisading dark cells and central pale cells, surrounded by a thick basement membrane (top quiz image).2,3
Clinically, sporadic spiradenocylindromas may resemble other lesions, manifesting as a papule or nodule with coloration ranging from gray-blue to salmon pink along with arborizing telangiectasias.4,5 Although spiradenocylindromas typically are found on the head, neck, and trunk, they also have been reported in the kidney, vulva, anus, and rectum.2,6,7 Not only are spiradenocylindromas clinically indistinct from other adnexal growths, but they also share some features with basal cell carcinomas (BCCs) and amelanotic melanomas.8 Features of arborizing telangiectasias on a papule may resemble BCC, requiring histopathology for a definitive diagnosis.
Spiradenocylindromas classically are associated with Brooke-Spiegler syndrome, a rare, autosomal-dominant genodermatosis caused by a germline mutation in the cylindromatosis lysine 63 deubiquitinase tumor-suppressor gene.5 Patients develop adnexal neoplasms of the folliculosebaceous-apocrine unit, including spiradenomas, cylindromas, and trichoepitheliomas.5 Rarely, malignant transformation to spiradenocylindrocarcinoma has been reported.9 Features of malignant transformation include loss of the 2-cell population, cytologic atypia, increased mitotic activity, and loss of intratumoral lymphocytes.10
Trichoepitheliomas are benign, firm, flesh-colored papules to nodules that commonly are found on the mid face but may appear on the scalp, neck, and upper trunk.5-11 Trichoepitheliomas are closely related to spiradenomas and cylindromas; the familial form, multiple familial trichoepitheliomas, exists on a spectrum with Brooke-Spiegler syndrome.3,11 Multiple familial trichoepithelioma is characterized by multiple trichoepitheliomas without accompanying spiradenomas, cylindromas, or spiradenocylindromas.3 On histopathology, trichoepitheliomas are distinguished by cribriform clusters or nests of basaloid follicular germinative cells with bulbar differentiation, known as papillary mesenchymal bodies, surrounded by an adherent stroma (eFigure 1).3,5,11 In addition to follicular bulbar differentiation, trichoepitheliomas are surrounded by an adherent cellular stroma without the retraction artifact around tumor islands seen in BCC, although artifactual clefts may occur within the stroma.11 In contrast, spiradenocylindromas do not demonstrate keratin cysts or artifactual clefts within the stroma.
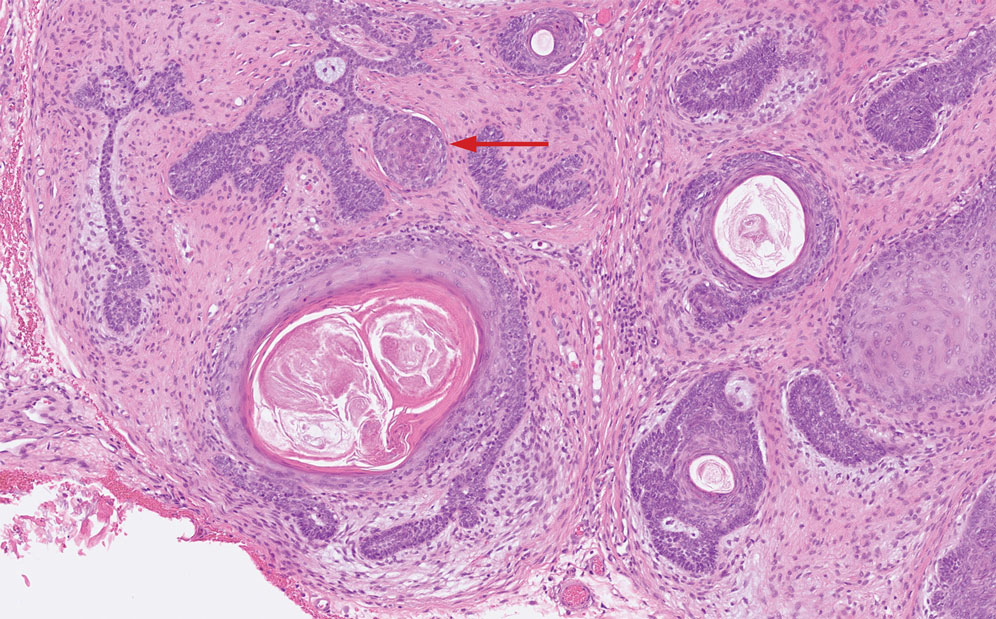
Trichilemmal cysts, or pilar cysts, are benign adnexal neoplasms derived from the outer root sheath at the isthmus.12-14 Approximately 90% of pilar cysts are found on the scalp and 2% of trichilemmal cysts may progress to a proliferating trichilemmal cyst, which is locally aggressive and contains an expanding buckled epithelium within the cyst space.12,14 Clinically, trichilemmal cysts are slow-growing, smooth, round, mobile nodules without a central punctum.12,13 On histopathology, the cyst wall contains peripherally palisading basal cells and maturing cells showing no intercellular bridging (eFigure 2). As the cells mature, they swell with pale cytoplasm and abruptly keratinize without a granular layer, a process known as trichilemmal keratinization.12-14 Additionally, cholesterol clefts are common in the keratinous lumen, and about 25% of cysts contain calcifications.13,14 The broadly basophilic spiradenocylindromas sharply contrast the abundant eosinophilic keratin of trichilemmal cysts.
Basal cell carcinoma is a slow-growing, locally destructive neoplasm that develops due to chronic sun exposure; thus, BCCs commonly arise on exposed areas of the face, head, neck, arms, and legs.15 Nodular BCC is the most common subtype and typically manifests as a shiny pearly papule or nodule with a smooth surface, rolled borders, and arborizing telangiectasias.16 On histopathology, nodular BCCs demonstrate nests or nodules of basaloid keratinocytes with peripheral palisading and retraction artifact between the tumor and stroma (eFigure 3).15,16 A lack of retraction artifact, cystic dilation of tubular structures, jigsaw molding of nests, and a distinct 2-cell population distinguish spiradenocylindroma from BCC. Of note, in rare instances BCCs also may display a thick fibrous stroma, similar to the stroma of cylindromas.15
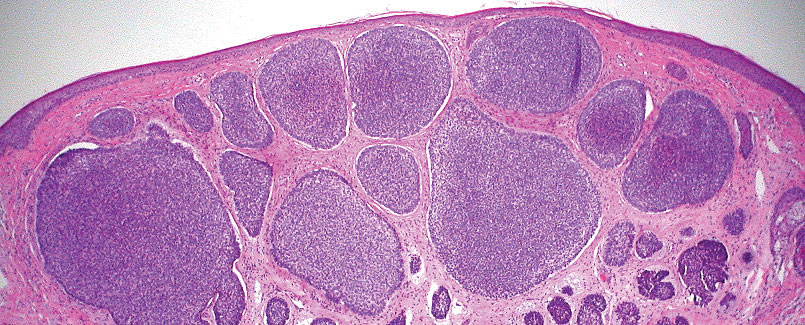
Amelanotic melanoma is a variant of melanoma characterized by little to no pigment. Any of the 4 classic subtypes of melanoma (nodular, superficial spreading, lentigo maligna, acral lentiginous) can be amelanotic.17 Clinically, amelanotic melanomas can vary greatly, manifesting as erythematous macules, dermal plaques, or papulonodular lesions, often with scaling.18 On histopathology, findings common to all melanomas include cellular atypia, mitoses, pagetoid spread, and pleomorphism (eFigure 4).18,19 Immunohistochemistry is an important method to distinguish melanoma from other melanocytic proliferations and to aid in the assessment of Breslow depth. Markers include SOX10 (sex-determining region Y-box transcription factor 10), S100, and MART-1 (melanoma antigen recognized by T cells 1/melan-A).19,20 Expression of PRAME (preferentially expressed antigen in melanoma) often is positive but is not necessary for diagnosis.21 Histologically, the atypical pleomorphic cells of melanoma are markedly distinct from both spiradenomas and cylindromas.
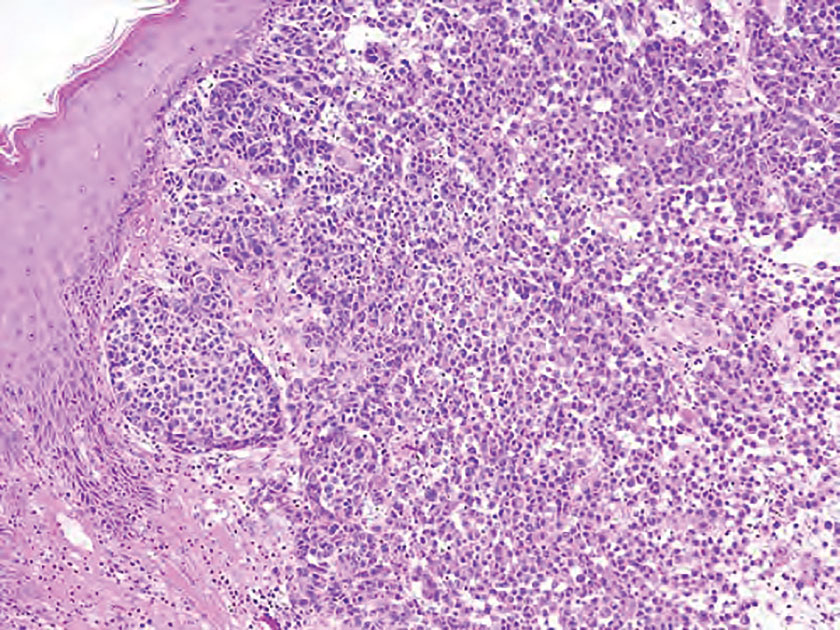
- Soyer HP, Kerl H, Ott A. Spiradenocylindroma—more than a coincidence? Am J Dermatopathol. 1998;20:315-317.
- Michal M, Lamovec J, Mukenˇ snabl P, et al. Spiradenocylindromas of the skin: tumors with morphological features of spiradenoma and cylindroma in the same lesion: report of 12 cases. Pathol Int. 1999;49:419-425.
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update. Head Neck Pathol. 2016;10:125-130.
- Bostan E, Boynuyogun E, Gokoz O, et al. Hybrid tumor “spiradenocylindroma” with unusual dermoscopic features. An Bras Dermatol. 2023;98:382-384.
- Pinho AC, Gouveia MJ, Gameiro AR, et al. Brooke-Spiegler syndrome—an underrecognized cause of multiple familial scalp tumors: report of a new germline mutation. J Dermatol Case Rep. 2015;9:67-70.
- Ströbel P, Zettl A, Ren Z, et al. Spiradenocylindroma of the kidney: clinical and genetic findings suggesting a role of somatic mutation of the CYLD1 gene in the oncogenesis of an unusual renal neoplasm. Am J Surg Pathol. 2002;26:119-124.
- Kacerovska D, Szepe P, Vanecek T, et al. Spiradenocylindroma-like basaloid carcinoma of the anus and rectum: case report, including HPV studies and analysis of the CYLD gene mutations. Am J Dermatopathol. 2008;30:472-476.
- Silvestri F, Maida P, Venturi F, et al. Scalp spiradenocylindroma: a challenging dermoscopic diagnosis. Dermatol Ther. 2020;33:E14307.
- Held L, Ruetten A, Saggini A, et al. Metaplastic spiradenocarcinoma: report of two cases with sarcomatous differentiation. J Cutan Pathol. 2021;48:384-389.
- Płachta I, Kleibert M, Czarnecka AM, et al. Current diagnosis and treatment options for cutaneous adnexal neoplasms with apocrine and eccrine differentiation. Int J Mol Sci. 2021;22:5077.
- Johnson H, Robles M, Kamino H, et al. Trichoepithelioma. Dermatol Online J. 2008;14:5.
- He P, Cui LG, Wang JR, et al. Trichilemmal cyst: clinical and sonographic feature. J Ultrasound Med. 2019;38:91-96.
- Liu M, Han H, Zheng Y, et al. Pilar cyst on the dorsum of hand: a case report and review of literature. Medicine (United States). 2020;99:E21519.
- Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128.
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. In: Archives of Pathology and Laboratory Medicine. Vol 141. College of American Pathologists; 2017:1490-1502.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Kaizer-Salk KA, Herten RJ, Ragsdale BD, et al. Amelanotic melanoma: a unique case study and review of the literature. BMJ Case Rep. 2018:bcr2017222751.
- Silva TS, de Araujo LR, Faro GB de A, et al. Nodular amelanotic melanoma. An Bras Dermatol. 2019;94:497-498.
- Bobos M. Histopathologic classification and prognostic factors of melanoma: a 2021 update. Ital J Dermatol Venereol. 2021;156:300-321.
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-444.
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465.
The Diagnosis: Spiradenocylindroma
T he biopsy results confirmed the diagnosis of spiradenocylindroma with negative margins. At 6-week follow-up, the patient had no signs of recurrence. Spiradenocylindroma is a benign hybrid neoplasm consisting of histologically intermixed areas representing the spectrum of morphology between spiradenoma and cylindromas.1,2 Both spiradenoma and cylindroma comprise 2 distinct populations of dark and pale basaloid cells.2,3 The spiradenomatous areas of the spiradenocylindroma are arranged in large, well-circumscribed collections of small, darkly staining cells with interspersed lymphocytes and a thin basement membrane surrounding spiradenocylindroma component.2,3 The spiradenocylindroma regions also may contain tubular structures dilated by hemorrhage.2 In contrast, the cylindromatous regions have a jigsaw-puzzle configuration of polygonal tumor nests containing peripherally palisading dark cells and central pale cells, surrounded by a thick basement membrane (top quiz image).2,3
Clinically, sporadic spiradenocylindromas may resemble other lesions, manifesting as a papule or nodule with coloration ranging from gray-blue to salmon pink along with arborizing telangiectasias.4,5 Although spiradenocylindromas typically are found on the head, neck, and trunk, they also have been reported in the kidney, vulva, anus, and rectum.2,6,7 Not only are spiradenocylindromas clinically indistinct from other adnexal growths, but they also share some features with basal cell carcinomas (BCCs) and amelanotic melanomas.8 Features of arborizing telangiectasias on a papule may resemble BCC, requiring histopathology for a definitive diagnosis.
Spiradenocylindromas classically are associated with Brooke-Spiegler syndrome, a rare, autosomal-dominant genodermatosis caused by a germline mutation in the cylindromatosis lysine 63 deubiquitinase tumor-suppressor gene.5 Patients develop adnexal neoplasms of the folliculosebaceous-apocrine unit, including spiradenomas, cylindromas, and trichoepitheliomas.5 Rarely, malignant transformation to spiradenocylindrocarcinoma has been reported.9 Features of malignant transformation include loss of the 2-cell population, cytologic atypia, increased mitotic activity, and loss of intratumoral lymphocytes.10
Trichoepitheliomas are benign, firm, flesh-colored papules to nodules that commonly are found on the mid face but may appear on the scalp, neck, and upper trunk.5-11 Trichoepitheliomas are closely related to spiradenomas and cylindromas; the familial form, multiple familial trichoepitheliomas, exists on a spectrum with Brooke-Spiegler syndrome.3,11 Multiple familial trichoepithelioma is characterized by multiple trichoepitheliomas without accompanying spiradenomas, cylindromas, or spiradenocylindromas.3 On histopathology, trichoepitheliomas are distinguished by cribriform clusters or nests of basaloid follicular germinative cells with bulbar differentiation, known as papillary mesenchymal bodies, surrounded by an adherent stroma (eFigure 1).3,5,11 In addition to follicular bulbar differentiation, trichoepitheliomas are surrounded by an adherent cellular stroma without the retraction artifact around tumor islands seen in BCC, although artifactual clefts may occur within the stroma.11 In contrast, spiradenocylindromas do not demonstrate keratin cysts or artifactual clefts within the stroma.

Trichilemmal cysts, or pilar cysts, are benign adnexal neoplasms derived from the outer root sheath at the isthmus.12-14 Approximately 90% of pilar cysts are found on the scalp and 2% of trichilemmal cysts may progress to a proliferating trichilemmal cyst, which is locally aggressive and contains an expanding buckled epithelium within the cyst space.12,14 Clinically, trichilemmal cysts are slow-growing, smooth, round, mobile nodules without a central punctum.12,13 On histopathology, the cyst wall contains peripherally palisading basal cells and maturing cells showing no intercellular bridging (eFigure 2). As the cells mature, they swell with pale cytoplasm and abruptly keratinize without a granular layer, a process known as trichilemmal keratinization.12-14 Additionally, cholesterol clefts are common in the keratinous lumen, and about 25% of cysts contain calcifications.13,14 The broadly basophilic spiradenocylindromas sharply contrast the abundant eosinophilic keratin of trichilemmal cysts.

Basal cell carcinoma is a slow-growing, locally destructive neoplasm that develops due to chronic sun exposure; thus, BCCs commonly arise on exposed areas of the face, head, neck, arms, and legs.15 Nodular BCC is the most common subtype and typically manifests as a shiny pearly papule or nodule with a smooth surface, rolled borders, and arborizing telangiectasias.16 On histopathology, nodular BCCs demonstrate nests or nodules of basaloid keratinocytes with peripheral palisading and retraction artifact between the tumor and stroma (eFigure 3).15,16 A lack of retraction artifact, cystic dilation of tubular structures, jigsaw molding of nests, and a distinct 2-cell population distinguish spiradenocylindroma from BCC. Of note, in rare instances BCCs also may display a thick fibrous stroma, similar to the stroma of cylindromas.15

Amelanotic melanoma is a variant of melanoma characterized by little to no pigment. Any of the 4 classic subtypes of melanoma (nodular, superficial spreading, lentigo maligna, acral lentiginous) can be amelanotic.17 Clinically, amelanotic melanomas can vary greatly, manifesting as erythematous macules, dermal plaques, or papulonodular lesions, often with scaling.18 On histopathology, findings common to all melanomas include cellular atypia, mitoses, pagetoid spread, and pleomorphism (eFigure 4).18,19 Immunohistochemistry is an important method to distinguish melanoma from other melanocytic proliferations and to aid in the assessment of Breslow depth. Markers include SOX10 (sex-determining region Y-box transcription factor 10), S100, and MART-1 (melanoma antigen recognized by T cells 1/melan-A).19,20 Expression of PRAME (preferentially expressed antigen in melanoma) often is positive but is not necessary for diagnosis.21 Histologically, the atypical pleomorphic cells of melanoma are markedly distinct from both spiradenomas and cylindromas.

The Diagnosis: Spiradenocylindroma
T he biopsy results confirmed the diagnosis of spiradenocylindroma with negative margins. At 6-week follow-up, the patient had no signs of recurrence. Spiradenocylindroma is a benign hybrid neoplasm consisting of histologically intermixed areas representing the spectrum of morphology between spiradenoma and cylindromas.1,2 Both spiradenoma and cylindroma comprise 2 distinct populations of dark and pale basaloid cells.2,3 The spiradenomatous areas of the spiradenocylindroma are arranged in large, well-circumscribed collections of small, darkly staining cells with interspersed lymphocytes and a thin basement membrane surrounding spiradenocylindroma component.2,3 The spiradenocylindroma regions also may contain tubular structures dilated by hemorrhage.2 In contrast, the cylindromatous regions have a jigsaw-puzzle configuration of polygonal tumor nests containing peripherally palisading dark cells and central pale cells, surrounded by a thick basement membrane (top quiz image).2,3
Clinically, sporadic spiradenocylindromas may resemble other lesions, manifesting as a papule or nodule with coloration ranging from gray-blue to salmon pink along with arborizing telangiectasias.4,5 Although spiradenocylindromas typically are found on the head, neck, and trunk, they also have been reported in the kidney, vulva, anus, and rectum.2,6,7 Not only are spiradenocylindromas clinically indistinct from other adnexal growths, but they also share some features with basal cell carcinomas (BCCs) and amelanotic melanomas.8 Features of arborizing telangiectasias on a papule may resemble BCC, requiring histopathology for a definitive diagnosis.
Spiradenocylindromas classically are associated with Brooke-Spiegler syndrome, a rare, autosomal-dominant genodermatosis caused by a germline mutation in the cylindromatosis lysine 63 deubiquitinase tumor-suppressor gene.5 Patients develop adnexal neoplasms of the folliculosebaceous-apocrine unit, including spiradenomas, cylindromas, and trichoepitheliomas.5 Rarely, malignant transformation to spiradenocylindrocarcinoma has been reported.9 Features of malignant transformation include loss of the 2-cell population, cytologic atypia, increased mitotic activity, and loss of intratumoral lymphocytes.10
Trichoepitheliomas are benign, firm, flesh-colored papules to nodules that commonly are found on the mid face but may appear on the scalp, neck, and upper trunk.5-11 Trichoepitheliomas are closely related to spiradenomas and cylindromas; the familial form, multiple familial trichoepitheliomas, exists on a spectrum with Brooke-Spiegler syndrome.3,11 Multiple familial trichoepithelioma is characterized by multiple trichoepitheliomas without accompanying spiradenomas, cylindromas, or spiradenocylindromas.3 On histopathology, trichoepitheliomas are distinguished by cribriform clusters or nests of basaloid follicular germinative cells with bulbar differentiation, known as papillary mesenchymal bodies, surrounded by an adherent stroma (eFigure 1).3,5,11 In addition to follicular bulbar differentiation, trichoepitheliomas are surrounded by an adherent cellular stroma without the retraction artifact around tumor islands seen in BCC, although artifactual clefts may occur within the stroma.11 In contrast, spiradenocylindromas do not demonstrate keratin cysts or artifactual clefts within the stroma.

Trichilemmal cysts, or pilar cysts, are benign adnexal neoplasms derived from the outer root sheath at the isthmus.12-14 Approximately 90% of pilar cysts are found on the scalp and 2% of trichilemmal cysts may progress to a proliferating trichilemmal cyst, which is locally aggressive and contains an expanding buckled epithelium within the cyst space.12,14 Clinically, trichilemmal cysts are slow-growing, smooth, round, mobile nodules without a central punctum.12,13 On histopathology, the cyst wall contains peripherally palisading basal cells and maturing cells showing no intercellular bridging (eFigure 2). As the cells mature, they swell with pale cytoplasm and abruptly keratinize without a granular layer, a process known as trichilemmal keratinization.12-14 Additionally, cholesterol clefts are common in the keratinous lumen, and about 25% of cysts contain calcifications.13,14 The broadly basophilic spiradenocylindromas sharply contrast the abundant eosinophilic keratin of trichilemmal cysts.

Basal cell carcinoma is a slow-growing, locally destructive neoplasm that develops due to chronic sun exposure; thus, BCCs commonly arise on exposed areas of the face, head, neck, arms, and legs.15 Nodular BCC is the most common subtype and typically manifests as a shiny pearly papule or nodule with a smooth surface, rolled borders, and arborizing telangiectasias.16 On histopathology, nodular BCCs demonstrate nests or nodules of basaloid keratinocytes with peripheral palisading and retraction artifact between the tumor and stroma (eFigure 3).15,16 A lack of retraction artifact, cystic dilation of tubular structures, jigsaw molding of nests, and a distinct 2-cell population distinguish spiradenocylindroma from BCC. Of note, in rare instances BCCs also may display a thick fibrous stroma, similar to the stroma of cylindromas.15

Amelanotic melanoma is a variant of melanoma characterized by little to no pigment. Any of the 4 classic subtypes of melanoma (nodular, superficial spreading, lentigo maligna, acral lentiginous) can be amelanotic.17 Clinically, amelanotic melanomas can vary greatly, manifesting as erythematous macules, dermal plaques, or papulonodular lesions, often with scaling.18 On histopathology, findings common to all melanomas include cellular atypia, mitoses, pagetoid spread, and pleomorphism (eFigure 4).18,19 Immunohistochemistry is an important method to distinguish melanoma from other melanocytic proliferations and to aid in the assessment of Breslow depth. Markers include SOX10 (sex-determining region Y-box transcription factor 10), S100, and MART-1 (melanoma antigen recognized by T cells 1/melan-A).19,20 Expression of PRAME (preferentially expressed antigen in melanoma) often is positive but is not necessary for diagnosis.21 Histologically, the atypical pleomorphic cells of melanoma are markedly distinct from both spiradenomas and cylindromas.

- Soyer HP, Kerl H, Ott A. Spiradenocylindroma—more than a coincidence? Am J Dermatopathol. 1998;20:315-317.
- Michal M, Lamovec J, Mukenˇ snabl P, et al. Spiradenocylindromas of the skin: tumors with morphological features of spiradenoma and cylindroma in the same lesion: report of 12 cases. Pathol Int. 1999;49:419-425.
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update. Head Neck Pathol. 2016;10:125-130.
- Bostan E, Boynuyogun E, Gokoz O, et al. Hybrid tumor “spiradenocylindroma” with unusual dermoscopic features. An Bras Dermatol. 2023;98:382-384.
- Pinho AC, Gouveia MJ, Gameiro AR, et al. Brooke-Spiegler syndrome—an underrecognized cause of multiple familial scalp tumors: report of a new germline mutation. J Dermatol Case Rep. 2015;9:67-70.
- Ströbel P, Zettl A, Ren Z, et al. Spiradenocylindroma of the kidney: clinical and genetic findings suggesting a role of somatic mutation of the CYLD1 gene in the oncogenesis of an unusual renal neoplasm. Am J Surg Pathol. 2002;26:119-124.
- Kacerovska D, Szepe P, Vanecek T, et al. Spiradenocylindroma-like basaloid carcinoma of the anus and rectum: case report, including HPV studies and analysis of the CYLD gene mutations. Am J Dermatopathol. 2008;30:472-476.
- Silvestri F, Maida P, Venturi F, et al. Scalp spiradenocylindroma: a challenging dermoscopic diagnosis. Dermatol Ther. 2020;33:E14307.
- Held L, Ruetten A, Saggini A, et al. Metaplastic spiradenocarcinoma: report of two cases with sarcomatous differentiation. J Cutan Pathol. 2021;48:384-389.
- Płachta I, Kleibert M, Czarnecka AM, et al. Current diagnosis and treatment options for cutaneous adnexal neoplasms with apocrine and eccrine differentiation. Int J Mol Sci. 2021;22:5077.
- Johnson H, Robles M, Kamino H, et al. Trichoepithelioma. Dermatol Online J. 2008;14:5.
- He P, Cui LG, Wang JR, et al. Trichilemmal cyst: clinical and sonographic feature. J Ultrasound Med. 2019;38:91-96.
- Liu M, Han H, Zheng Y, et al. Pilar cyst on the dorsum of hand: a case report and review of literature. Medicine (United States). 2020;99:E21519.
- Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128.
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. In: Archives of Pathology and Laboratory Medicine. Vol 141. College of American Pathologists; 2017:1490-1502.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Kaizer-Salk KA, Herten RJ, Ragsdale BD, et al. Amelanotic melanoma: a unique case study and review of the literature. BMJ Case Rep. 2018:bcr2017222751.
- Silva TS, de Araujo LR, Faro GB de A, et al. Nodular amelanotic melanoma. An Bras Dermatol. 2019;94:497-498.
- Bobos M. Histopathologic classification and prognostic factors of melanoma: a 2021 update. Ital J Dermatol Venereol. 2021;156:300-321.
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-444.
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465.
- Soyer HP, Kerl H, Ott A. Spiradenocylindroma—more than a coincidence? Am J Dermatopathol. 1998;20:315-317.
- Michal M, Lamovec J, Mukenˇ snabl P, et al. Spiradenocylindromas of the skin: tumors with morphological features of spiradenoma and cylindroma in the same lesion: report of 12 cases. Pathol Int. 1999;49:419-425.
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update. Head Neck Pathol. 2016;10:125-130.
- Bostan E, Boynuyogun E, Gokoz O, et al. Hybrid tumor “spiradenocylindroma” with unusual dermoscopic features. An Bras Dermatol. 2023;98:382-384.
- Pinho AC, Gouveia MJ, Gameiro AR, et al. Brooke-Spiegler syndrome—an underrecognized cause of multiple familial scalp tumors: report of a new germline mutation. J Dermatol Case Rep. 2015;9:67-70.
- Ströbel P, Zettl A, Ren Z, et al. Spiradenocylindroma of the kidney: clinical and genetic findings suggesting a role of somatic mutation of the CYLD1 gene in the oncogenesis of an unusual renal neoplasm. Am J Surg Pathol. 2002;26:119-124.
- Kacerovska D, Szepe P, Vanecek T, et al. Spiradenocylindroma-like basaloid carcinoma of the anus and rectum: case report, including HPV studies and analysis of the CYLD gene mutations. Am J Dermatopathol. 2008;30:472-476.
- Silvestri F, Maida P, Venturi F, et al. Scalp spiradenocylindroma: a challenging dermoscopic diagnosis. Dermatol Ther. 2020;33:E14307.
- Held L, Ruetten A, Saggini A, et al. Metaplastic spiradenocarcinoma: report of two cases with sarcomatous differentiation. J Cutan Pathol. 2021;48:384-389.
- Płachta I, Kleibert M, Czarnecka AM, et al. Current diagnosis and treatment options for cutaneous adnexal neoplasms with apocrine and eccrine differentiation. Int J Mol Sci. 2021;22:5077.
- Johnson H, Robles M, Kamino H, et al. Trichoepithelioma. Dermatol Online J. 2008;14:5.
- He P, Cui LG, Wang JR, et al. Trichilemmal cyst: clinical and sonographic feature. J Ultrasound Med. 2019;38:91-96.
- Liu M, Han H, Zheng Y, et al. Pilar cyst on the dorsum of hand: a case report and review of literature. Medicine (United States). 2020;99:E21519.
- Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128.
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. In: Archives of Pathology and Laboratory Medicine. Vol 141. College of American Pathologists; 2017:1490-1502.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Kaizer-Salk KA, Herten RJ, Ragsdale BD, et al. Amelanotic melanoma: a unique case study and review of the literature. BMJ Case Rep. 2018:bcr2017222751.
- Silva TS, de Araujo LR, Faro GB de A, et al. Nodular amelanotic melanoma. An Bras Dermatol. 2019;94:497-498.
- Bobos M. Histopathologic classification and prognostic factors of melanoma: a 2021 update. Ital J Dermatol Venereol. 2021;156:300-321.
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-444.
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465.
Mobile Tender Papule on the Scalp
Mobile Tender Papule on the Scalp
A 73-year-old man presented to the plastic surgery department with a single, progressively enlarging nodule on the scalp of 1 year’s duration. Dermatologic examination revealed a 0.8-cm, soft, mobile, gray-blue, dome-shaped papule on the left postauricular scalp that was tender to palpation. There was no central punctum, and the patient denied any history of drainage or odor. He had no personal or family history of similar lesions. An excisional biopsy of the papule was performed.
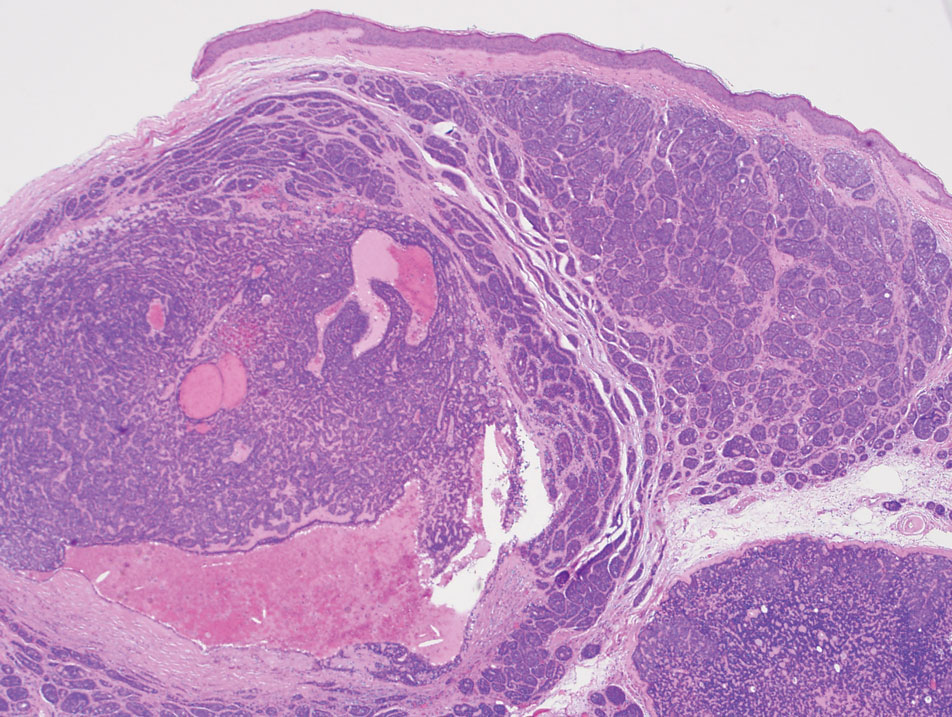
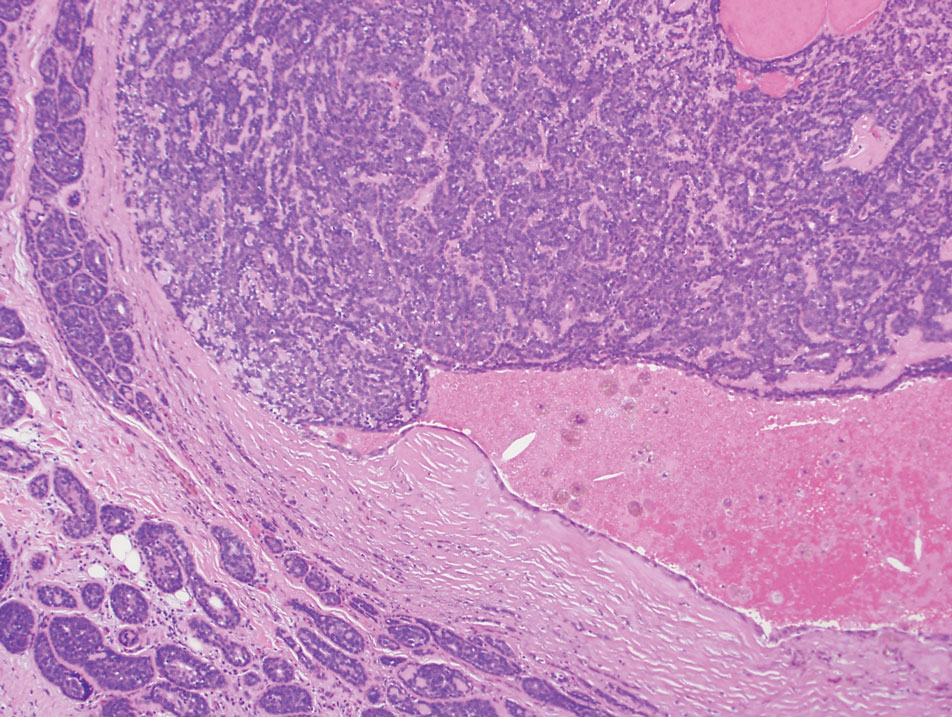
Rupioid Id Reaction With Peripheral Eosinophilia
Rupioid Id Reaction With Peripheral Eosinophilia
To the Editor:
In dermatology, rupioid describes dirty-appearing scale. The term is derived from the Greek word rhupos, which translates to “dirty” or “filthy.” This type of scale also is called ostraceous, owing to its resemblance to an oyster shell. Histopathologically, rupioid or ostraceous scale corresponds to epidermal hyperplasia and hyperkeratosis. Therefore, the presence of rupioid scale is believed to reflect an exuberant inflammatory response. Several dermatologic conditions have been associated with rupioid scale, including psoriasis, secondary syphilis, reactive arthritis, histoplasmosis, and Norwegian scabies.1-4 Peripheral eosinophilia has been reported in eczematous dermatoses such as atopic dermatitis and contact dermatitis,5,6 but our review of the literature did not find it described in the context of id reactions. We report the case of a patient who developed a rupioid id reaction with peripheral eosinophilia.
An otherwise healthy 40-year-old woman presented with a generalized pruritic eruption of 1 month’s duration. Prior to onset, she was bitten by a bug on the left arm and covered the site with a bandage. She subsequently noticed an erythematous papulopustular rash corresponding to the shape of the bandage adhesive. Shortly thereafter, a generalized eruption developed, prompting the patient to present for evaluation 1 month later. A review of systems was negative for fevers, chills, headaches, vision changes, and joint symptoms. She denied having a history of atopy.
Physical examination revealed numerous pink papules and plaques with rupioid scale scattered over the trunk and extremities (Figure). The palms, soles, and mucous membranes were spared. Laboratory studies revealed peripheral eosinophilia (9% eosinophils [reference range, 1%-6%] and an absolute eosinophil count of 600/µL [reference range, 0-400/µL]). A 3-mm punch biopsy of a representative lesion revealed a superficial perivascular infiltrate of lymphocytes, histiocytes, and eosinophils along with epidermal hyperplasia, spongiosis, and mounds of parakeratosis. Clinicopathologic correlation led to the diagnosis of a rupioid id reaction secondary to an arthropod assault and/or a reaction to the bandage adhesive.
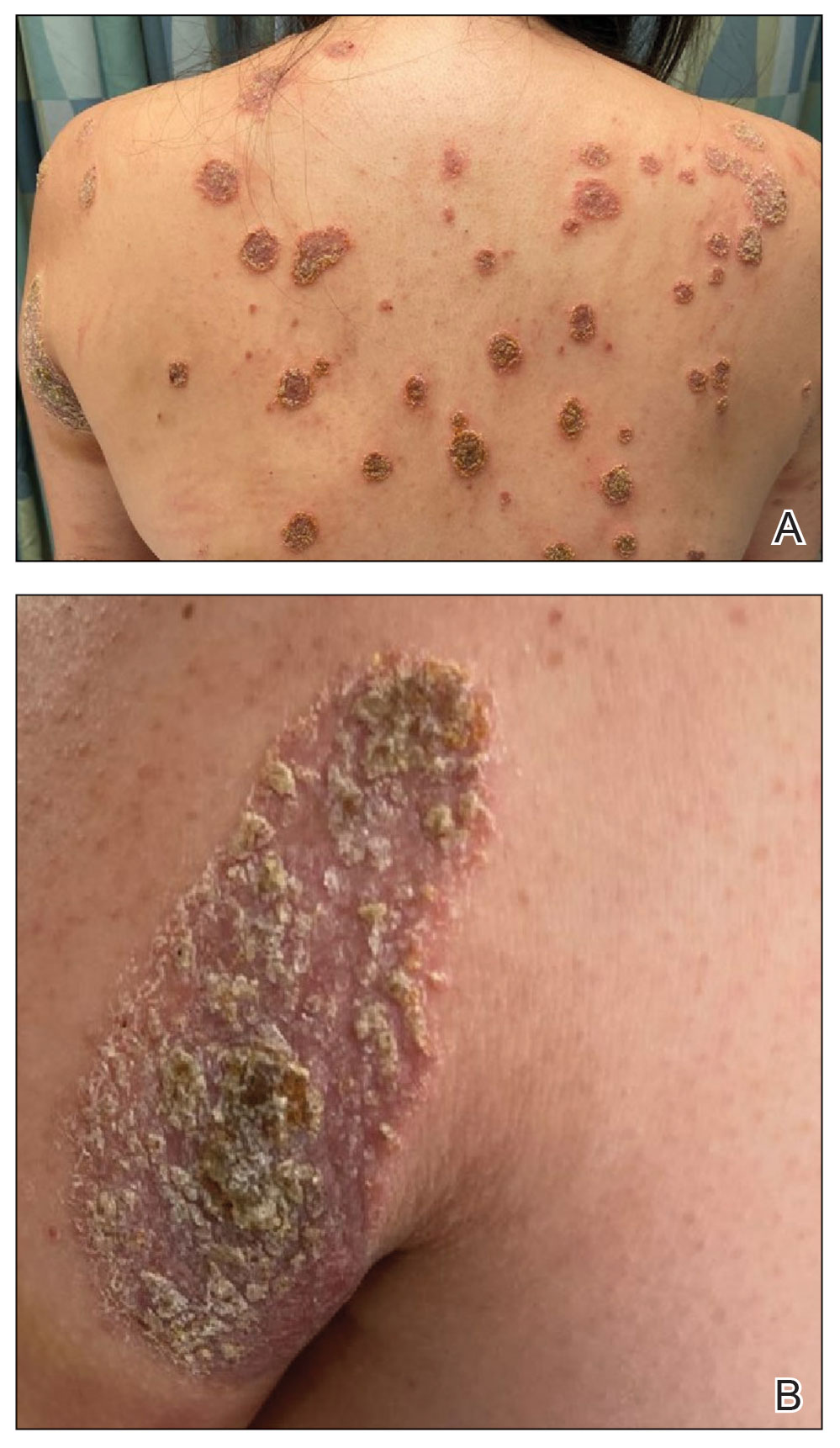
Treatment with topical corticosteroids was avoided at the patient’s request. Instead, a ceramide-based emollient and oral antihistamines (fexofenadine 180 mg in the morning and cetirizine 10 mg in the evening) were recommended and resulted in resolution of the eruption with postinflammatory hyperpigmentation at 2-week follow-up. The patient was advised to avoid further exposure to bandage adhesives.
An id reaction, or autoeczematization, is a cutaneous immunologic response to antigen(s) released from an initial, often distant site of inflammation.7,8 Clinically, it typically manifests as a pruritic, symmetrically distributed papulovesicular eruption. Although the pathogenesis of id reactions is uncertain, overactivation of T lymphocytes responding to the initial inflammatory insult has been implicated.7 A variety of noninfectious (eg, stasis dermatitis, contact dermatitis) and infectious dermatoses (eg, fungal, bacterial, viral, parasitic) may trigger id reactions.7,9-13 In this case, we believe an arthropod assault and/or reaction to the bandage adhesive was the primary insult, and the id reaction that ensued was so exuberant that it resulted not only in rupioid scale but also in peripheral eosinophilia—similar to how more severe forms of atopic dermatitis have been associated with peripheral eosinophilia.5 As such presentations of id reactions not have been widely described in the literature, this report expands our understanding of this condition to include rupioid scale and peripheral eosinophilia.
- Chung HJ, Marley-Kemp D, Keller M. Rupioid psoriasis and other skin diseases with rupioid manifestations. Cutis. 2014;94:119-121.
- Costa JB, de Sousa VLLR, da Trindade Neto PB, et al. Norwegian scabies mimicking rupioid psoriasis. An Bras Dermatol. 2012;87:910-913. doi:10.1590/S0365-05962012000600016
- Ip KH-K, Cheng HS, Oliver FG. Rupioid psoriasis. JAMA Dermatol. 2021;157:859. doi:10.1001/jamadermatol.2021.0451
- Wang Y, Wen Y. An AIDS patient with recurrent multiple skin crusted ulcerations. AIDS Res Hum Retroviruses. 2021;37:1-3. doi:10.1089/aid.2020.0212
- Staumont-Sallé D, Barbarot S, Bouaziz JD, et al. Effect of abrocitinib and dupilumab on eosinophil levels in patients with moderate-to-severe atopic dermatitis. JEADV Clin Pract. 2023;2:518-530. doi:10.1002/jvc2.192
- Savjani P. An unusual cause of eosinophilia—hypereosinophilia due to contact dermatitis. J Allergy Clin Immunol. 2016;137:AB168. doi:10.1016/j.jaci.2015.12.685
- Bertoli M, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi:10.12788/cutis.0342
- Ilkit M, Durdu M, Karakas¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202. doi:10.3109/1040841X.2011.645520
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129. doi:10.1111/j.1365-4362.1993.tb01454.x
- Jordan L, Jackson NAM, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;10:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6. doi:10.1067/mjd.2003.110
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:e1072-e1075. doi:10.1542/peds.2011-1054
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
To the Editor:
In dermatology, rupioid describes dirty-appearing scale. The term is derived from the Greek word rhupos, which translates to “dirty” or “filthy.” This type of scale also is called ostraceous, owing to its resemblance to an oyster shell. Histopathologically, rupioid or ostraceous scale corresponds to epidermal hyperplasia and hyperkeratosis. Therefore, the presence of rupioid scale is believed to reflect an exuberant inflammatory response. Several dermatologic conditions have been associated with rupioid scale, including psoriasis, secondary syphilis, reactive arthritis, histoplasmosis, and Norwegian scabies.1-4 Peripheral eosinophilia has been reported in eczematous dermatoses such as atopic dermatitis and contact dermatitis,5,6 but our review of the literature did not find it described in the context of id reactions. We report the case of a patient who developed a rupioid id reaction with peripheral eosinophilia.
An otherwise healthy 40-year-old woman presented with a generalized pruritic eruption of 1 month’s duration. Prior to onset, she was bitten by a bug on the left arm and covered the site with a bandage. She subsequently noticed an erythematous papulopustular rash corresponding to the shape of the bandage adhesive. Shortly thereafter, a generalized eruption developed, prompting the patient to present for evaluation 1 month later. A review of systems was negative for fevers, chills, headaches, vision changes, and joint symptoms. She denied having a history of atopy.
Physical examination revealed numerous pink papules and plaques with rupioid scale scattered over the trunk and extremities (Figure). The palms, soles, and mucous membranes were spared. Laboratory studies revealed peripheral eosinophilia (9% eosinophils [reference range, 1%-6%] and an absolute eosinophil count of 600/µL [reference range, 0-400/µL]). A 3-mm punch biopsy of a representative lesion revealed a superficial perivascular infiltrate of lymphocytes, histiocytes, and eosinophils along with epidermal hyperplasia, spongiosis, and mounds of parakeratosis. Clinicopathologic correlation led to the diagnosis of a rupioid id reaction secondary to an arthropod assault and/or a reaction to the bandage adhesive.

Treatment with topical corticosteroids was avoided at the patient’s request. Instead, a ceramide-based emollient and oral antihistamines (fexofenadine 180 mg in the morning and cetirizine 10 mg in the evening) were recommended and resulted in resolution of the eruption with postinflammatory hyperpigmentation at 2-week follow-up. The patient was advised to avoid further exposure to bandage adhesives.
An id reaction, or autoeczematization, is a cutaneous immunologic response to antigen(s) released from an initial, often distant site of inflammation.7,8 Clinically, it typically manifests as a pruritic, symmetrically distributed papulovesicular eruption. Although the pathogenesis of id reactions is uncertain, overactivation of T lymphocytes responding to the initial inflammatory insult has been implicated.7 A variety of noninfectious (eg, stasis dermatitis, contact dermatitis) and infectious dermatoses (eg, fungal, bacterial, viral, parasitic) may trigger id reactions.7,9-13 In this case, we believe an arthropod assault and/or reaction to the bandage adhesive was the primary insult, and the id reaction that ensued was so exuberant that it resulted not only in rupioid scale but also in peripheral eosinophilia—similar to how more severe forms of atopic dermatitis have been associated with peripheral eosinophilia.5 As such presentations of id reactions not have been widely described in the literature, this report expands our understanding of this condition to include rupioid scale and peripheral eosinophilia.
To the Editor:
In dermatology, rupioid describes dirty-appearing scale. The term is derived from the Greek word rhupos, which translates to “dirty” or “filthy.” This type of scale also is called ostraceous, owing to its resemblance to an oyster shell. Histopathologically, rupioid or ostraceous scale corresponds to epidermal hyperplasia and hyperkeratosis. Therefore, the presence of rupioid scale is believed to reflect an exuberant inflammatory response. Several dermatologic conditions have been associated with rupioid scale, including psoriasis, secondary syphilis, reactive arthritis, histoplasmosis, and Norwegian scabies.1-4 Peripheral eosinophilia has been reported in eczematous dermatoses such as atopic dermatitis and contact dermatitis,5,6 but our review of the literature did not find it described in the context of id reactions. We report the case of a patient who developed a rupioid id reaction with peripheral eosinophilia.
An otherwise healthy 40-year-old woman presented with a generalized pruritic eruption of 1 month’s duration. Prior to onset, she was bitten by a bug on the left arm and covered the site with a bandage. She subsequently noticed an erythematous papulopustular rash corresponding to the shape of the bandage adhesive. Shortly thereafter, a generalized eruption developed, prompting the patient to present for evaluation 1 month later. A review of systems was negative for fevers, chills, headaches, vision changes, and joint symptoms. She denied having a history of atopy.
Physical examination revealed numerous pink papules and plaques with rupioid scale scattered over the trunk and extremities (Figure). The palms, soles, and mucous membranes were spared. Laboratory studies revealed peripheral eosinophilia (9% eosinophils [reference range, 1%-6%] and an absolute eosinophil count of 600/µL [reference range, 0-400/µL]). A 3-mm punch biopsy of a representative lesion revealed a superficial perivascular infiltrate of lymphocytes, histiocytes, and eosinophils along with epidermal hyperplasia, spongiosis, and mounds of parakeratosis. Clinicopathologic correlation led to the diagnosis of a rupioid id reaction secondary to an arthropod assault and/or a reaction to the bandage adhesive.

Treatment with topical corticosteroids was avoided at the patient’s request. Instead, a ceramide-based emollient and oral antihistamines (fexofenadine 180 mg in the morning and cetirizine 10 mg in the evening) were recommended and resulted in resolution of the eruption with postinflammatory hyperpigmentation at 2-week follow-up. The patient was advised to avoid further exposure to bandage adhesives.
An id reaction, or autoeczematization, is a cutaneous immunologic response to antigen(s) released from an initial, often distant site of inflammation.7,8 Clinically, it typically manifests as a pruritic, symmetrically distributed papulovesicular eruption. Although the pathogenesis of id reactions is uncertain, overactivation of T lymphocytes responding to the initial inflammatory insult has been implicated.7 A variety of noninfectious (eg, stasis dermatitis, contact dermatitis) and infectious dermatoses (eg, fungal, bacterial, viral, parasitic) may trigger id reactions.7,9-13 In this case, we believe an arthropod assault and/or reaction to the bandage adhesive was the primary insult, and the id reaction that ensued was so exuberant that it resulted not only in rupioid scale but also in peripheral eosinophilia—similar to how more severe forms of atopic dermatitis have been associated with peripheral eosinophilia.5 As such presentations of id reactions not have been widely described in the literature, this report expands our understanding of this condition to include rupioid scale and peripheral eosinophilia.
- Chung HJ, Marley-Kemp D, Keller M. Rupioid psoriasis and other skin diseases with rupioid manifestations. Cutis. 2014;94:119-121.
- Costa JB, de Sousa VLLR, da Trindade Neto PB, et al. Norwegian scabies mimicking rupioid psoriasis. An Bras Dermatol. 2012;87:910-913. doi:10.1590/S0365-05962012000600016
- Ip KH-K, Cheng HS, Oliver FG. Rupioid psoriasis. JAMA Dermatol. 2021;157:859. doi:10.1001/jamadermatol.2021.0451
- Wang Y, Wen Y. An AIDS patient with recurrent multiple skin crusted ulcerations. AIDS Res Hum Retroviruses. 2021;37:1-3. doi:10.1089/aid.2020.0212
- Staumont-Sallé D, Barbarot S, Bouaziz JD, et al. Effect of abrocitinib and dupilumab on eosinophil levels in patients with moderate-to-severe atopic dermatitis. JEADV Clin Pract. 2023;2:518-530. doi:10.1002/jvc2.192
- Savjani P. An unusual cause of eosinophilia—hypereosinophilia due to contact dermatitis. J Allergy Clin Immunol. 2016;137:AB168. doi:10.1016/j.jaci.2015.12.685
- Bertoli M, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi:10.12788/cutis.0342
- Ilkit M, Durdu M, Karakas¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202. doi:10.3109/1040841X.2011.645520
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129. doi:10.1111/j.1365-4362.1993.tb01454.x
- Jordan L, Jackson NAM, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;10:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6. doi:10.1067/mjd.2003.110
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:e1072-e1075. doi:10.1542/peds.2011-1054
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
- Chung HJ, Marley-Kemp D, Keller M. Rupioid psoriasis and other skin diseases with rupioid manifestations. Cutis. 2014;94:119-121.
- Costa JB, de Sousa VLLR, da Trindade Neto PB, et al. Norwegian scabies mimicking rupioid psoriasis. An Bras Dermatol. 2012;87:910-913. doi:10.1590/S0365-05962012000600016
- Ip KH-K, Cheng HS, Oliver FG. Rupioid psoriasis. JAMA Dermatol. 2021;157:859. doi:10.1001/jamadermatol.2021.0451
- Wang Y, Wen Y. An AIDS patient with recurrent multiple skin crusted ulcerations. AIDS Res Hum Retroviruses. 2021;37:1-3. doi:10.1089/aid.2020.0212
- Staumont-Sallé D, Barbarot S, Bouaziz JD, et al. Effect of abrocitinib and dupilumab on eosinophil levels in patients with moderate-to-severe atopic dermatitis. JEADV Clin Pract. 2023;2:518-530. doi:10.1002/jvc2.192
- Savjani P. An unusual cause of eosinophilia—hypereosinophilia due to contact dermatitis. J Allergy Clin Immunol. 2016;137:AB168. doi:10.1016/j.jaci.2015.12.685
- Bertoli M, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi:10.12788/cutis.0342
- Ilkit M, Durdu M, Karakas¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202. doi:10.3109/1040841X.2011.645520
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129. doi:10.1111/j.1365-4362.1993.tb01454.x
- Jordan L, Jackson NAM, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;10:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6. doi:10.1067/mjd.2003.110
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:e1072-e1075. doi:10.1542/peds.2011-1054
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
Rupioid Id Reaction With Peripheral Eosinophilia
Rupioid Id Reaction With Peripheral Eosinophilia
Practice Points
- Consider a rupioid id reaction when a patient presents with lesions featuring scale that is dirty appearing and resembles an oyster shell.
- Recognize that exuberant id reactions can manifest with peripheral eosinophilia; its presence should not lead you to automatically rule out an id reaction in favor of other eosinophilic eruptions.
- Focus on uncovering the source of an id reaction (eg, contactants, infections, bites); resolving the primary insult is essential for rapid clearance of even dramatic rupioid eruptions.
Safety and Effectiveness of Nonsteroidal Tapinarof Cream 1% Added to Ongoing Biologic Therapy for Treatment of Moderate to Severe Plaque Psoriasis
Safety and Effectiveness of Nonsteroidal Tapinarof Cream 1% Added to Ongoing Biologic Therapy for Treatment of Moderate to Severe Plaque Psoriasis
The estimated prevalence of psoriasis in individuals older than 20 years in the United States has been reported at approximately 3%, or more than 7.5 million people.1 There currently is no cure for psoriasis, and available therapeutics, including phototherapy,2 topical therapies,3 systemic medications,4 and biologic agents,5 are focused only on controlling symptoms. The National Psoriasis Foundation defines an acceptable treatment response for plaque psoriasis as 3% or lower body surface area (BSA) involvement after 3 months of therapy, with a treat-to-target (TTT) goal of 1% or less BSA involvement.6
Cytokines are known to mediate psoriasis pathology, and biologic therapies target the signaling cascade of various cytokines. Biologics approved to treat moderate to severe plaque psoriasis include IgG monoclonal antibodies binding and inhibiting the activity of interleukin (IL)-17 (ixekizumab,7 secukinumab8), IL-23 (guselkumab,9 risankizumab,10 tildrakizumab11), and IL-12/23 (ustekinumab12). Despite targeting these cytokines, biologics may not sufficiently suppress the symptoms of psoriatic disease and their severity in all patients. Adding a topical treatment to biologic therapy can augment clinical response without increasing the incidence of adverse effects13-15 and may reduce the need to switch biologics due to ineffectiveness. Switching biologics likely would increase cost burden to the health care system and/or patient depending on their insurance plan and possibly introduce new safety and/or tolerability issues.16,17
In patients who do not adequately respond to biologics, better responses were reported when topical medications including halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17,18 were administered. In randomized or open-label, real-world studies, patients with psoriasis responded well when topical medications were added to a biologic, such as tildrakizumab combined with halcinonide ointment 0.1%,19 etanercept combined with topical clobetasol propionate foam,20 or adalimumab combined with calcipotriene/betamethasone dipropionate foam.21 No additional safety concerns were observed with the topical add-ons in any of these studies.
Tapinarof is an aryl hydrocarbon receptor agonist approved by the US Food and Drug Administration for topical treatment of plaque psoriasis in adults.22 It is a first-in-class small molecule with a novel mechanism of action that downregulates IL-17A and IL-17F and normalizes the skin barrier through expression of filaggrin, loricrin, and involucrin; it also has antioxidant activity.23 In the phase 3 PSOARING 1 and 2 trials, daily application of tapinarof cream was safe and efficacious in patients with plaque psoriasis,24,25 with a remittive (maintenance) effect of a median of approximately 4 months after discontinuation.25 In these 2 phase 3 studies, tapinarof significantly (P<0.01 at week 12) relieved itch, which was seen rapidly (P<0.05 at week 2),26 improved quality of life,27 and led to high patient satisfaction.27 When tapinarof cream was combined with deucravacitinib in a patient with severe plaque psoriasis, symptoms rapidly cleared, with a 75% decrease in disease severity after 4 weeks.28
The objective of this prospective, open-label, real-world, single-center study was to assess the effectiveness, safety, and remittive (or maintenance) effect of nonsteroidal tapinarof cream 1% added to ongoing biologic therapy in patients with plaque psoriasis who were not adequately responding to a biologic alone.
Methods
Study Design and Participants—This prospective, open-label, real-world, single-center study assessed the safety and effectiveness of
Eligible participants were otherwise healthy males and females aged 18 years and older with moderate to severe plaque psoriasis (BSA involvement ≥3%) who had been treated with a biologic for 24 weeks or more. Patients were recruited from the Psoriasis Treatment Center of New Jersey (East Windsor, New Jersey). Exclusion criteria were recent use of oral systemic therapies (within 4 weeks of baseline) or topical therapies (within 2 weeks) to treat psoriasis, recent use of UVB (within 2 weeks) or psoralen plus UVA (within 4 weeks) phototherapy, or use of any investigational drug within 4 weeks of baseline (or within 5 pharmacokinetic/pharmacodynamic half-lives, whichever was longer). Patients who were pregnant or breastfeeding or who had any known hypersensitivity to the excipients of tapinarof cream also were excluded from the study.
Eligible participants received tapinarof cream 1% once daily plus their ongoing biologic for 12 weeks, after which tapinarof was discontinued and the biologic was continued for an additional 4 weeks. A remittive (maintenance) effect was assessed at week 16.
Study Outcomes—Safety and efficacy were evaluated at baseline and weeks 2, 4, 8, 12, and 16. The primary end point was the proportion of patients who reached the TTT goal of 1% or less BSA involvement at week 12. Secondary end points included the proportion of patients with 1% or less BSA involvement at weeks 2, 4, 8, and 16; and PGA scores, composite PGA multiplied by mean percentage of BSA involvement (PGA×BSA), and PASI scores at baseline and weeks 2, 4, 8, 12, and 16. The patient-reported outcomes of Dermatology Life Quality Index (DLQI) and Worst Itch Numeric Rating Scale (WI-NRS) scores also were evaluated at baseline and weeks 2, 4, 8, 12, and 16. In patients who had disease involvement on the scalp or genital region at baseline, Psoriasis Scalp Severity Index (PSSI) and Static Physician’s Global Assessment of Genitalia scores, respectively, were assessed at baseline and weeks 2, 4, 8, 12, and 16. Safety was determined by the incidence, severity, and relatedness of adverse events (AEs) and serious AEs.
Statistical Analysis—Approximately 30 participants were planned for enrollment and recruited consecutively as they were identified during screening against inclusion and exclusion criteria. Changes from baseline in all outcomes were summarized descriptively. Missing data were not imputed. Given the sample size, no formal statistical analyses were conducted. Safety was summarized by descriptively collating AEs and serious AEs, including their frequency, severity, and treatment relatedness.
Results
Thirty participants were enrolled in the study, and 20 fully completed the study. Nine discontinued treatment before week 12 (6 were lost to follow-up, 2 were terminated early by the investigators, and 1 voluntarily withdrew); 1 additional participant was lost to follow-up after week 12. Patients were predominantly male (20/30 [66.7%]) and White (21/30 [70.0%]); the mean age of all participants was 55.4 years, and the mean (SD) duration of psoriasis was 21.4 (15.0) years (Table 1). The mean baseline percentage of BSA involvement and mean baseline PGA, PASI, and DLQI scores are shown in Table 1. Most (19/30 [63.3%]) patients received biologics that inhibited IL-23 activity (guselkumab, risankizumab, tildrakizumab), approximately one-third (9/30 [30.0%]) received biologics that inhibited IL-17 activity (ixekizumab, secukinumab), and 2 (6.7%) received biologics that inhibited IL-12/IL-23 activity (ustekinumab)(Table 1).

For the primary end point, 52.4% (11/21) of patients reached the TTT goal (BSA involvement ≤1% after 12 weeks of treatment with tapinarof cream added to a prescribed biologic). The proportion of patients reaching the TTT goal increased over time with the combined treatment (eFigure 1). Additionally, the mean percentage of BSA involvement (eFigure 2) as well as the mean values for PGA (eFigure 3) and PGA×BSA decreased over time. The mean percentage of BSA involvement was 5.0% at baseline and dropped to 2.0% by week 12. Similar reductions were observed for PGA and PGA×BSA scores at week 12.
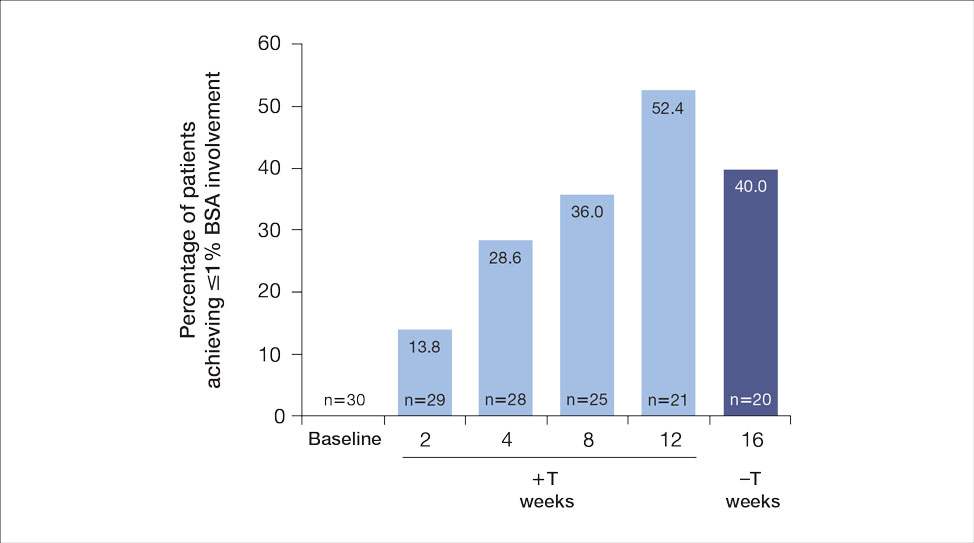
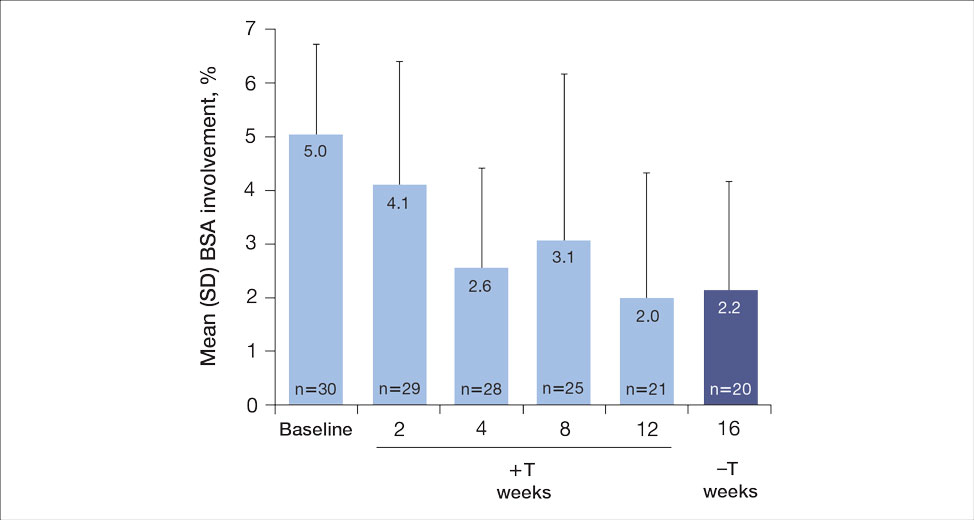
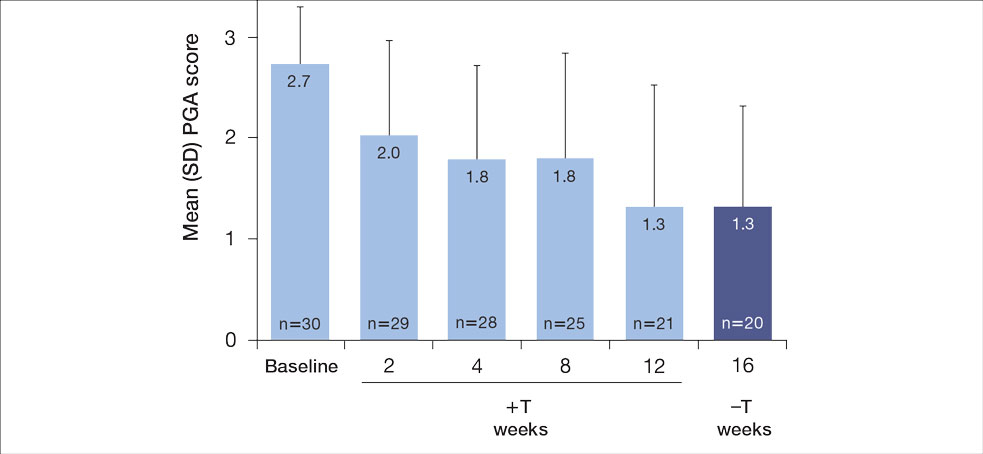
After discontinuing tapinarof cream at week 12 and receiving only the biologic for 4 weeks, the proportion of patients maintaining 1% or less BSA involvement fell to 40.0% (8/20) at week 16, which was closer to that observed at week 8 (36% [9/25]) than at week 12 (52.4% [11/21])(eFigure 1).
The mean PASI score was 5.5 at baseline, then decreased over time when tapinarof cream was combined with a biologic (eFigure 4), falling to 3.1 by week 2 and 1.6 by week 12; it was maintained at 1.7 at week 16. Nine (30.0%) participants had psoriasis on the scalp at baseline with a mean PSSI score of 2.6, which decreased to 0.83 by week 2. By week 12, the mean PSSI score remained stable at 0.95 in the 2 (9.5%) participants who still had scalp involvement. The mean PSSI score increased slightly to 1.45 after patients received only the biologic for 4 weeks. At baseline, 3 (10.0%) patients had genital involvement (mean Static Physician’s Global Assessment of Genitalia score, 0.27). Symptoms resolved in 2 (66.7%) of these patients at week 2 and stayed consistent until week 16; the third patient withdrew at week 2.
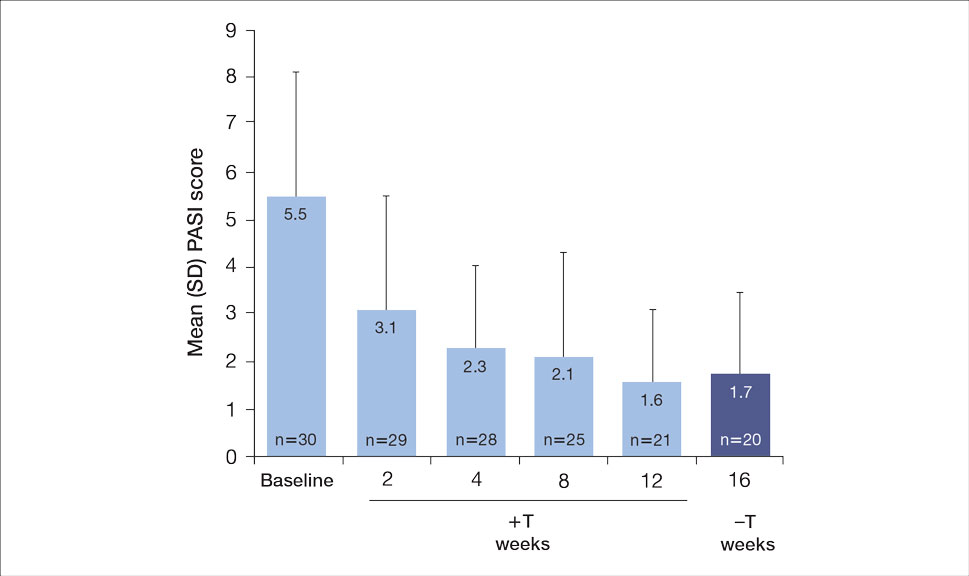
Both DLQI and WI-NRS scores decreased with use of tapinarof cream added to a biologic up to week 12 (eFigures 5 and 6). Mean DLQI scores were 5.3 at baseline and 3.1 at week 12. At week 16, the mean DLQI score remained stable at 2.8. Mean WI-NRS scores decreased from 4.0 at baseline to 2.7 at week 12 with the therapy combination; at week 16, the mean WI-NRS score fell further to 1.8.
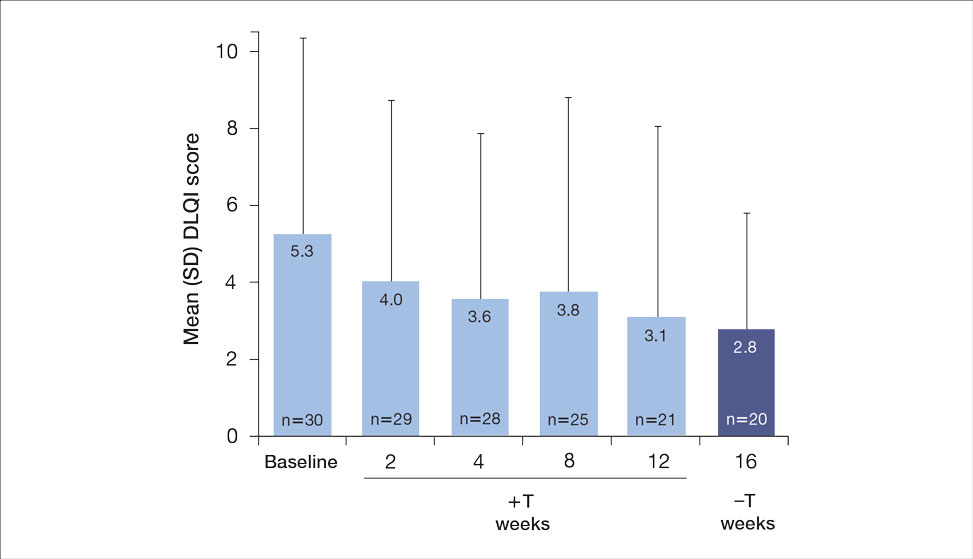

A total of 6 AEs were reported in 5 (16.7%) patients (Table 2). The majority (4/6 [67.0%]) of AEs were considered mild. Two reported cases of COVID-19 were both considered mild and unrelated. Mild folliculitis and moderate worsening of psoriasis in 2 (6.7%) different patients were the only AEs considered related to treatment. No serious AEs were reported, and no patient withdrew from the study due to an AE.
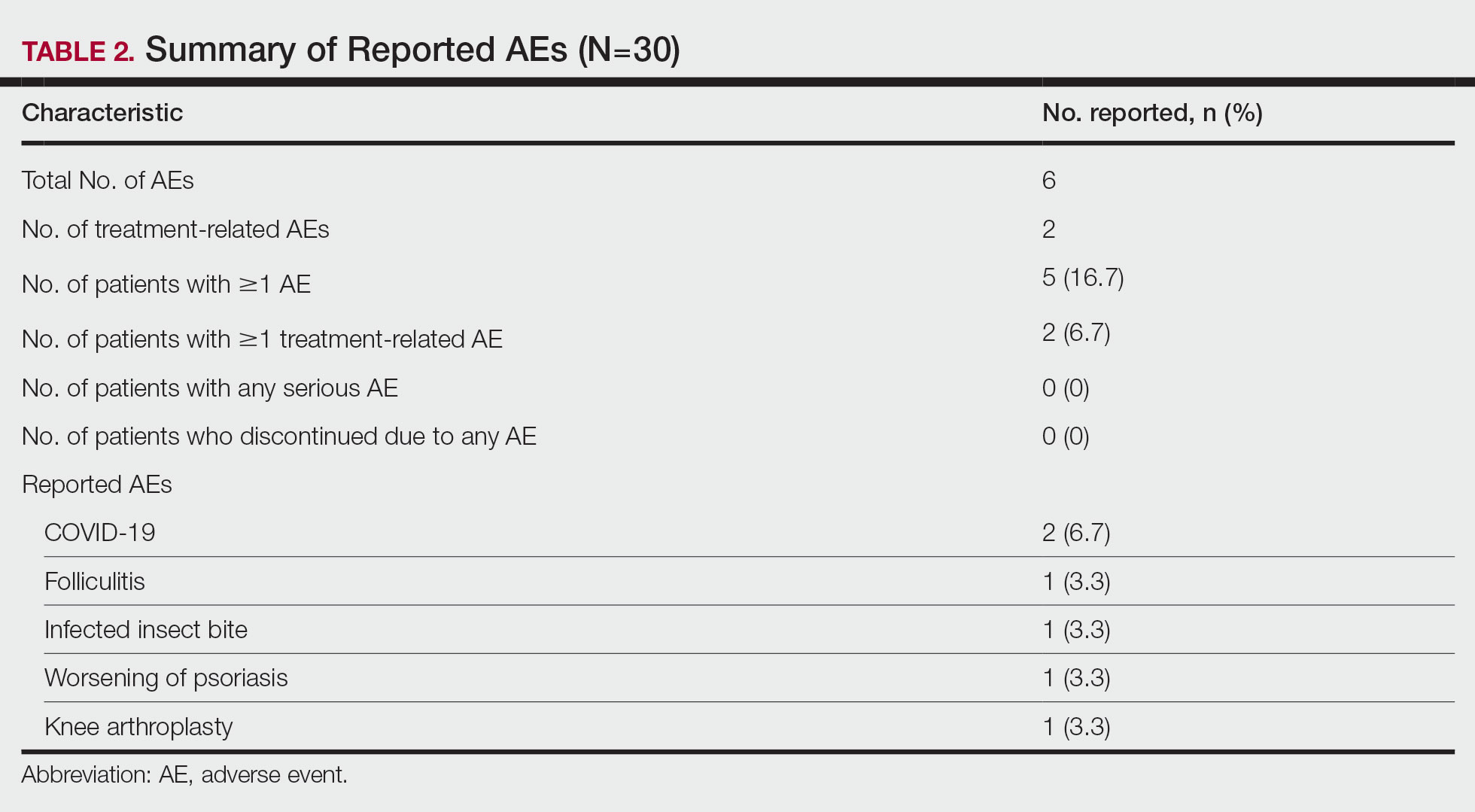
Comment
Disease activity improvements we observed with the nonsteroidal tapinarof cream were consistent with those reported when topical steroidal therapies were given to patients responding poorly to their current biologic. Our primary end point (proportion of patients with BSA involvement ≤1% after 12 weeks) showed that half (52% [11/21]) of patients whose BSA involvement was 3% or greater with a biologic for 24 weeks or more reached the TTT goal after 12 weeks of tapinarof-biologic treatment. Other studies of halobetasol propionate–tazarotene lotion16 and calcipotriene/betamethasone dipropionate foam17,18 added to the current biologic of poor responders found 60% to 68% of patients had reductions in their percentage BSA to 1% or lower at 12 to 16 weeks of treatment. Randomized studies showed etanercept plus topical clobetasol propionate foam20 or adalimumab plus calcipotriene/betamethasone dipropionate foam21 similarly enhanced treatment effects vs biologic alone.
A phase 3 PSOARING trial demonstrated benefit from treatment with tapinarof alone, with a remittive effect of approximately 4 months after discontinuation.25 Our data are consistent with these findings, with 40% (8/20) of patients demonstrating a remittive effect 4 weeks after discontinuing tapinarof while receiving a biologic. A similar maintenance effect was reported in another study in 50% (9/18) of patients treated with a biologic plus halobetasol propionate–tazarotene lotion.16 Additionally, when halcinonide ointment was given to patients receiving tildrakizumab, mean percentage of BSA involvement, PGA scores, PGA×BSA, and DLQI scores improved and were maintained 4 weeks after halcinonide ointment was stopped.19 Thus, topical therapy can augment and extend a biologic’s effect for up to 4 weeks.
In our study, tapinarof cream added to a biologic had a good safety and tolerability profile. Few AEs were recorded, with most being mild in nature, and no serious AEs or discontinuations due to AEs were reported. Only 1 case of mild folliculitis and 1 case of moderate worsening of psoriasis were considered treatment related. Further, no unexpected or new safety signals with the tapinarof-biologic combination were observed compared with tapinarof alone.27Prior studies have found that supplementing a biologic with topical therapy can reduce the probability of patients switching to another biologic.16,19 We previously found that adding halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17 to a biologic helped reduce the probability of switching biologics from 88% to 90% at baseline to 12% to 24% after 12 weeks of combined therapy. Such combinations also could prevent a less responsive patient from being prescribed a higher biologic dose.19 These are important research findings, as patients—even when not responding well to their current biologic—are more likely to be tolerating that biologic well, and switching to a new biologic may introduce new safety or tolerability concerns. Thus, by enhancing the effect of a biologic with a topical therapy, one can avoid increasing the dose of the current biologic or switching to a new biologic, either of which may increase safety and/or tolerability risks. Switching biologics also has increased cost implications to the health care system and/or the patient. When comparing the cost of adding halobetasol propionate–tazarotene lotion to a biologic compared with switching to another biologic, the cost was 1.2 to 2.9 times higher to switch, depending on the biologic, compared with a smaller incremental cost increase to add a topical to the current biologic.16 Similar observations were reported with calcipotriene/betamethasone dipropionate foam plus a biologic.17 Although we did not evaluate biologic switching here, we anticipate a similar clinical scenario with a tapinarof-biologic combination.
Limitations of our study included the open-label design, lack of a control arm, and the relatively small study population; however, for studies investigating the safety and effectiveness of a treatment in a real-world setting, these limitations are common and are not unexpected. Our results also are consistent with the overall improvement seen in other studies16-21 examining the effects of adding a topical to a biologic. Future research is warranted to investigate a longer remittive effect and potential health care system and patient cost savings without having to switch biologics due to lack of effectiveness.
Conclusion
This study demonstrated that adjunctive use of nonsteroidal tapinarof cream 1% may enhance a biologic treatment effect in patients with moderate to severe plaque psoriasis, providing an adequate response for many patients who were not responding well to a biologic alone. Clinical outcomes improved with the tapinarof-biologic combination, and a remittive effect was noted 4 weeks after tapinarof discontinuation without any new safety signals. Adding tapinarof cream to a biologic also may prevent the need to switch biologics when patients do not sufficiently respond, preserving the safety and cost associated with a patient’s current biologic.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804. doi:10.1016/j.jaad.2019.04.042
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi:10.1016/j.jaad.2020.07.087
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiological therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072. doi:10.1016/j.jaad.2018.11.057
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis.J Am Acad Dermatol. 2017;76:290-298. doi:10.1016/j.jaad.2016.10.017
- Taltz. Prescribing information. Eli Lilly and Company; 2024.
- Cosentyx. Prescribing information. Novartis Pharmaceuticals Corporation; 2023.
- Tremfya. Prescribing information. Janssen Biotech, Inc; 2023.
- Skyrizi. Prescribing information. AbbVie Inc; 2024.
- Ilumya. Prescribing information. Sun Pharmaceutical Industries, Inc; 2020.
- Stelara. Prescribing information. Janssen Biotech, Inc; 2022.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Jensen JD, Delcambre MR, Nguyen G, et al. Biologic therapy with or without topical treatment in psoriasis: what does the current evidence say? Am J Clin Dermatol. 2014;15:379-385. doi:10.1007/s40257-014-0089-1
- Gustafson CJ, Watkins C, Hix E, et al. Combination therapy in psoriasis: an evidence-based review. Am J Clin Dermatol. 2013;14:9-25. doi:10.1007/s40257-012-0003-7
- Bagel J, Novak K, Nelson E. Adjunctive use of halobetasol propionate-tazarotene in biologic-experienced patients with psoriasis. Cutis. 2022;109:103-109. doi:10.12788/cutis.0451
- Bagel J, Nelson E, Zapata J, et al. Adjunctive use of calcipotriene/betamethasone dipropionate foam in a real-world setting curtails the cost of biologics without reducing efficacy in psoriasis. Dermatol Ther (Heidelb). 2020;10:1383-1396. doi:10.1007/s13555-020-00454-z
- Bagel J, Zapata J, Nelson E. A prospective, open-label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018;17:611-616.
- Bagel J, Novak K, Nelson E. Tildrakizumab in combination with topical halcinonide 0.1% ointment for treating moderate to severe plaque psoriasis. J Drugs Dermatol. 2023;22:766-772. doi:10.36849/jdd.6830
- Lebwohl MG, Kircik L, Callis Duffin K, et al. A randomized study to evaluate the efficacy and safety of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2013;69:385-392. doi:10.1016/j.jaad.2013.03.031
- Thaci D, Ortonne JP, Chimenti S, et al. A phase IIIb, multicentre, randomized, double-blind, vehicle-controlled study of the efficacy and safety of adalimumab with and without calcipotriol/betamethasone topical treatment in patients with moderate to severe psoriasis: the BELIEVE study. Br J Dermatol. 2010;163:402-411. doi:10.1111/j.1365-2133.2010.09791.x
- Vtama. Prescribing information. Dermavant Sciences, Inc; 2022.
- Bobonich M, Gorelick J, Aldredge L, et al. Tapinarof, a novel, first-in-class, topical therapeutic aryl hydrocarbon receptor agonist for the management of psoriasis. J Drugs Dermatol. 2023;22:779-784. doi:10.36849/jdd.7317
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Kircik L, Zirwas M, Kwatra SG, et al. Rapid improvements in itch with tapinarof cream 1% once daily in two phase 3 trials in adults with mild to severe plaque psoriasis. Dermatol Ther (Heidelb). 2024;14:201-211. doi:10.1007/s13555-023-01068-x
- Bagel J, Gold LS, Del Rosso J, et al. Tapinarof cream 1% once daily for the treatment of plaque psoriasis: patient-reported outcomes from the PSOARING 3 trial. J Am Acad Dermatol. 2023;89:936-944. doi:10.1016/j.jaad.2023.04.061
- Abdin R, Kircik L, Issa NT. First use of combination oral deucravacitinib with tapinarof cream for treatment of severe plaque psoriasis. J Drugs Dermatol. 2024;23:192-194. doi:10.36849/jdd.8091
The estimated prevalence of psoriasis in individuals older than 20 years in the United States has been reported at approximately 3%, or more than 7.5 million people.1 There currently is no cure for psoriasis, and available therapeutics, including phototherapy,2 topical therapies,3 systemic medications,4 and biologic agents,5 are focused only on controlling symptoms. The National Psoriasis Foundation defines an acceptable treatment response for plaque psoriasis as 3% or lower body surface area (BSA) involvement after 3 months of therapy, with a treat-to-target (TTT) goal of 1% or less BSA involvement.6
Cytokines are known to mediate psoriasis pathology, and biologic therapies target the signaling cascade of various cytokines. Biologics approved to treat moderate to severe plaque psoriasis include IgG monoclonal antibodies binding and inhibiting the activity of interleukin (IL)-17 (ixekizumab,7 secukinumab8), IL-23 (guselkumab,9 risankizumab,10 tildrakizumab11), and IL-12/23 (ustekinumab12). Despite targeting these cytokines, biologics may not sufficiently suppress the symptoms of psoriatic disease and their severity in all patients. Adding a topical treatment to biologic therapy can augment clinical response without increasing the incidence of adverse effects13-15 and may reduce the need to switch biologics due to ineffectiveness. Switching biologics likely would increase cost burden to the health care system and/or patient depending on their insurance plan and possibly introduce new safety and/or tolerability issues.16,17
In patients who do not adequately respond to biologics, better responses were reported when topical medications including halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17,18 were administered. In randomized or open-label, real-world studies, patients with psoriasis responded well when topical medications were added to a biologic, such as tildrakizumab combined with halcinonide ointment 0.1%,19 etanercept combined with topical clobetasol propionate foam,20 or adalimumab combined with calcipotriene/betamethasone dipropionate foam.21 No additional safety concerns were observed with the topical add-ons in any of these studies.
Tapinarof is an aryl hydrocarbon receptor agonist approved by the US Food and Drug Administration for topical treatment of plaque psoriasis in adults.22 It is a first-in-class small molecule with a novel mechanism of action that downregulates IL-17A and IL-17F and normalizes the skin barrier through expression of filaggrin, loricrin, and involucrin; it also has antioxidant activity.23 In the phase 3 PSOARING 1 and 2 trials, daily application of tapinarof cream was safe and efficacious in patients with plaque psoriasis,24,25 with a remittive (maintenance) effect of a median of approximately 4 months after discontinuation.25 In these 2 phase 3 studies, tapinarof significantly (P<0.01 at week 12) relieved itch, which was seen rapidly (P<0.05 at week 2),26 improved quality of life,27 and led to high patient satisfaction.27 When tapinarof cream was combined with deucravacitinib in a patient with severe plaque psoriasis, symptoms rapidly cleared, with a 75% decrease in disease severity after 4 weeks.28
The objective of this prospective, open-label, real-world, single-center study was to assess the effectiveness, safety, and remittive (or maintenance) effect of nonsteroidal tapinarof cream 1% added to ongoing biologic therapy in patients with plaque psoriasis who were not adequately responding to a biologic alone.
Methods
Study Design and Participants—This prospective, open-label, real-world, single-center study assessed the safety and effectiveness of
Eligible participants were otherwise healthy males and females aged 18 years and older with moderate to severe plaque psoriasis (BSA involvement ≥3%) who had been treated with a biologic for 24 weeks or more. Patients were recruited from the Psoriasis Treatment Center of New Jersey (East Windsor, New Jersey). Exclusion criteria were recent use of oral systemic therapies (within 4 weeks of baseline) or topical therapies (within 2 weeks) to treat psoriasis, recent use of UVB (within 2 weeks) or psoralen plus UVA (within 4 weeks) phototherapy, or use of any investigational drug within 4 weeks of baseline (or within 5 pharmacokinetic/pharmacodynamic half-lives, whichever was longer). Patients who were pregnant or breastfeeding or who had any known hypersensitivity to the excipients of tapinarof cream also were excluded from the study.
Eligible participants received tapinarof cream 1% once daily plus their ongoing biologic for 12 weeks, after which tapinarof was discontinued and the biologic was continued for an additional 4 weeks. A remittive (maintenance) effect was assessed at week 16.
Study Outcomes—Safety and efficacy were evaluated at baseline and weeks 2, 4, 8, 12, and 16. The primary end point was the proportion of patients who reached the TTT goal of 1% or less BSA involvement at week 12. Secondary end points included the proportion of patients with 1% or less BSA involvement at weeks 2, 4, 8, and 16; and PGA scores, composite PGA multiplied by mean percentage of BSA involvement (PGA×BSA), and PASI scores at baseline and weeks 2, 4, 8, 12, and 16. The patient-reported outcomes of Dermatology Life Quality Index (DLQI) and Worst Itch Numeric Rating Scale (WI-NRS) scores also were evaluated at baseline and weeks 2, 4, 8, 12, and 16. In patients who had disease involvement on the scalp or genital region at baseline, Psoriasis Scalp Severity Index (PSSI) and Static Physician’s Global Assessment of Genitalia scores, respectively, were assessed at baseline and weeks 2, 4, 8, 12, and 16. Safety was determined by the incidence, severity, and relatedness of adverse events (AEs) and serious AEs.
Statistical Analysis—Approximately 30 participants were planned for enrollment and recruited consecutively as they were identified during screening against inclusion and exclusion criteria. Changes from baseline in all outcomes were summarized descriptively. Missing data were not imputed. Given the sample size, no formal statistical analyses were conducted. Safety was summarized by descriptively collating AEs and serious AEs, including their frequency, severity, and treatment relatedness.
Results
Thirty participants were enrolled in the study, and 20 fully completed the study. Nine discontinued treatment before week 12 (6 were lost to follow-up, 2 were terminated early by the investigators, and 1 voluntarily withdrew); 1 additional participant was lost to follow-up after week 12. Patients were predominantly male (20/30 [66.7%]) and White (21/30 [70.0%]); the mean age of all participants was 55.4 years, and the mean (SD) duration of psoriasis was 21.4 (15.0) years (Table 1). The mean baseline percentage of BSA involvement and mean baseline PGA, PASI, and DLQI scores are shown in Table 1. Most (19/30 [63.3%]) patients received biologics that inhibited IL-23 activity (guselkumab, risankizumab, tildrakizumab), approximately one-third (9/30 [30.0%]) received biologics that inhibited IL-17 activity (ixekizumab, secukinumab), and 2 (6.7%) received biologics that inhibited IL-12/IL-23 activity (ustekinumab)(Table 1).

For the primary end point, 52.4% (11/21) of patients reached the TTT goal (BSA involvement ≤1% after 12 weeks of treatment with tapinarof cream added to a prescribed biologic). The proportion of patients reaching the TTT goal increased over time with the combined treatment (eFigure 1). Additionally, the mean percentage of BSA involvement (eFigure 2) as well as the mean values for PGA (eFigure 3) and PGA×BSA decreased over time. The mean percentage of BSA involvement was 5.0% at baseline and dropped to 2.0% by week 12. Similar reductions were observed for PGA and PGA×BSA scores at week 12.



After discontinuing tapinarof cream at week 12 and receiving only the biologic for 4 weeks, the proportion of patients maintaining 1% or less BSA involvement fell to 40.0% (8/20) at week 16, which was closer to that observed at week 8 (36% [9/25]) than at week 12 (52.4% [11/21])(eFigure 1).
The mean PASI score was 5.5 at baseline, then decreased over time when tapinarof cream was combined with a biologic (eFigure 4), falling to 3.1 by week 2 and 1.6 by week 12; it was maintained at 1.7 at week 16. Nine (30.0%) participants had psoriasis on the scalp at baseline with a mean PSSI score of 2.6, which decreased to 0.83 by week 2. By week 12, the mean PSSI score remained stable at 0.95 in the 2 (9.5%) participants who still had scalp involvement. The mean PSSI score increased slightly to 1.45 after patients received only the biologic for 4 weeks. At baseline, 3 (10.0%) patients had genital involvement (mean Static Physician’s Global Assessment of Genitalia score, 0.27). Symptoms resolved in 2 (66.7%) of these patients at week 2 and stayed consistent until week 16; the third patient withdrew at week 2.

Both DLQI and WI-NRS scores decreased with use of tapinarof cream added to a biologic up to week 12 (eFigures 5 and 6). Mean DLQI scores were 5.3 at baseline and 3.1 at week 12. At week 16, the mean DLQI score remained stable at 2.8. Mean WI-NRS scores decreased from 4.0 at baseline to 2.7 at week 12 with the therapy combination; at week 16, the mean WI-NRS score fell further to 1.8.


A total of 6 AEs were reported in 5 (16.7%) patients (Table 2). The majority (4/6 [67.0%]) of AEs were considered mild. Two reported cases of COVID-19 were both considered mild and unrelated. Mild folliculitis and moderate worsening of psoriasis in 2 (6.7%) different patients were the only AEs considered related to treatment. No serious AEs were reported, and no patient withdrew from the study due to an AE.

Comment
Disease activity improvements we observed with the nonsteroidal tapinarof cream were consistent with those reported when topical steroidal therapies were given to patients responding poorly to their current biologic. Our primary end point (proportion of patients with BSA involvement ≤1% after 12 weeks) showed that half (52% [11/21]) of patients whose BSA involvement was 3% or greater with a biologic for 24 weeks or more reached the TTT goal after 12 weeks of tapinarof-biologic treatment. Other studies of halobetasol propionate–tazarotene lotion16 and calcipotriene/betamethasone dipropionate foam17,18 added to the current biologic of poor responders found 60% to 68% of patients had reductions in their percentage BSA to 1% or lower at 12 to 16 weeks of treatment. Randomized studies showed etanercept plus topical clobetasol propionate foam20 or adalimumab plus calcipotriene/betamethasone dipropionate foam21 similarly enhanced treatment effects vs biologic alone.
A phase 3 PSOARING trial demonstrated benefit from treatment with tapinarof alone, with a remittive effect of approximately 4 months after discontinuation.25 Our data are consistent with these findings, with 40% (8/20) of patients demonstrating a remittive effect 4 weeks after discontinuing tapinarof while receiving a biologic. A similar maintenance effect was reported in another study in 50% (9/18) of patients treated with a biologic plus halobetasol propionate–tazarotene lotion.16 Additionally, when halcinonide ointment was given to patients receiving tildrakizumab, mean percentage of BSA involvement, PGA scores, PGA×BSA, and DLQI scores improved and were maintained 4 weeks after halcinonide ointment was stopped.19 Thus, topical therapy can augment and extend a biologic’s effect for up to 4 weeks.
In our study, tapinarof cream added to a biologic had a good safety and tolerability profile. Few AEs were recorded, with most being mild in nature, and no serious AEs or discontinuations due to AEs were reported. Only 1 case of mild folliculitis and 1 case of moderate worsening of psoriasis were considered treatment related. Further, no unexpected or new safety signals with the tapinarof-biologic combination were observed compared with tapinarof alone.27Prior studies have found that supplementing a biologic with topical therapy can reduce the probability of patients switching to another biologic.16,19 We previously found that adding halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17 to a biologic helped reduce the probability of switching biologics from 88% to 90% at baseline to 12% to 24% after 12 weeks of combined therapy. Such combinations also could prevent a less responsive patient from being prescribed a higher biologic dose.19 These are important research findings, as patients—even when not responding well to their current biologic—are more likely to be tolerating that biologic well, and switching to a new biologic may introduce new safety or tolerability concerns. Thus, by enhancing the effect of a biologic with a topical therapy, one can avoid increasing the dose of the current biologic or switching to a new biologic, either of which may increase safety and/or tolerability risks. Switching biologics also has increased cost implications to the health care system and/or the patient. When comparing the cost of adding halobetasol propionate–tazarotene lotion to a biologic compared with switching to another biologic, the cost was 1.2 to 2.9 times higher to switch, depending on the biologic, compared with a smaller incremental cost increase to add a topical to the current biologic.16 Similar observations were reported with calcipotriene/betamethasone dipropionate foam plus a biologic.17 Although we did not evaluate biologic switching here, we anticipate a similar clinical scenario with a tapinarof-biologic combination.
Limitations of our study included the open-label design, lack of a control arm, and the relatively small study population; however, for studies investigating the safety and effectiveness of a treatment in a real-world setting, these limitations are common and are not unexpected. Our results also are consistent with the overall improvement seen in other studies16-21 examining the effects of adding a topical to a biologic. Future research is warranted to investigate a longer remittive effect and potential health care system and patient cost savings without having to switch biologics due to lack of effectiveness.
Conclusion
This study demonstrated that adjunctive use of nonsteroidal tapinarof cream 1% may enhance a biologic treatment effect in patients with moderate to severe plaque psoriasis, providing an adequate response for many patients who were not responding well to a biologic alone. Clinical outcomes improved with the tapinarof-biologic combination, and a remittive effect was noted 4 weeks after tapinarof discontinuation without any new safety signals. Adding tapinarof cream to a biologic also may prevent the need to switch biologics when patients do not sufficiently respond, preserving the safety and cost associated with a patient’s current biologic.
The estimated prevalence of psoriasis in individuals older than 20 years in the United States has been reported at approximately 3%, or more than 7.5 million people.1 There currently is no cure for psoriasis, and available therapeutics, including phototherapy,2 topical therapies,3 systemic medications,4 and biologic agents,5 are focused only on controlling symptoms. The National Psoriasis Foundation defines an acceptable treatment response for plaque psoriasis as 3% or lower body surface area (BSA) involvement after 3 months of therapy, with a treat-to-target (TTT) goal of 1% or less BSA involvement.6
Cytokines are known to mediate psoriasis pathology, and biologic therapies target the signaling cascade of various cytokines. Biologics approved to treat moderate to severe plaque psoriasis include IgG monoclonal antibodies binding and inhibiting the activity of interleukin (IL)-17 (ixekizumab,7 secukinumab8), IL-23 (guselkumab,9 risankizumab,10 tildrakizumab11), and IL-12/23 (ustekinumab12). Despite targeting these cytokines, biologics may not sufficiently suppress the symptoms of psoriatic disease and their severity in all patients. Adding a topical treatment to biologic therapy can augment clinical response without increasing the incidence of adverse effects13-15 and may reduce the need to switch biologics due to ineffectiveness. Switching biologics likely would increase cost burden to the health care system and/or patient depending on their insurance plan and possibly introduce new safety and/or tolerability issues.16,17
In patients who do not adequately respond to biologics, better responses were reported when topical medications including halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17,18 were administered. In randomized or open-label, real-world studies, patients with psoriasis responded well when topical medications were added to a biologic, such as tildrakizumab combined with halcinonide ointment 0.1%,19 etanercept combined with topical clobetasol propionate foam,20 or adalimumab combined with calcipotriene/betamethasone dipropionate foam.21 No additional safety concerns were observed with the topical add-ons in any of these studies.
Tapinarof is an aryl hydrocarbon receptor agonist approved by the US Food and Drug Administration for topical treatment of plaque psoriasis in adults.22 It is a first-in-class small molecule with a novel mechanism of action that downregulates IL-17A and IL-17F and normalizes the skin barrier through expression of filaggrin, loricrin, and involucrin; it also has antioxidant activity.23 In the phase 3 PSOARING 1 and 2 trials, daily application of tapinarof cream was safe and efficacious in patients with plaque psoriasis,24,25 with a remittive (maintenance) effect of a median of approximately 4 months after discontinuation.25 In these 2 phase 3 studies, tapinarof significantly (P<0.01 at week 12) relieved itch, which was seen rapidly (P<0.05 at week 2),26 improved quality of life,27 and led to high patient satisfaction.27 When tapinarof cream was combined with deucravacitinib in a patient with severe plaque psoriasis, symptoms rapidly cleared, with a 75% decrease in disease severity after 4 weeks.28
The objective of this prospective, open-label, real-world, single-center study was to assess the effectiveness, safety, and remittive (or maintenance) effect of nonsteroidal tapinarof cream 1% added to ongoing biologic therapy in patients with plaque psoriasis who were not adequately responding to a biologic alone.
Methods
Study Design and Participants—This prospective, open-label, real-world, single-center study assessed the safety and effectiveness of
Eligible participants were otherwise healthy males and females aged 18 years and older with moderate to severe plaque psoriasis (BSA involvement ≥3%) who had been treated with a biologic for 24 weeks or more. Patients were recruited from the Psoriasis Treatment Center of New Jersey (East Windsor, New Jersey). Exclusion criteria were recent use of oral systemic therapies (within 4 weeks of baseline) or topical therapies (within 2 weeks) to treat psoriasis, recent use of UVB (within 2 weeks) or psoralen plus UVA (within 4 weeks) phototherapy, or use of any investigational drug within 4 weeks of baseline (or within 5 pharmacokinetic/pharmacodynamic half-lives, whichever was longer). Patients who were pregnant or breastfeeding or who had any known hypersensitivity to the excipients of tapinarof cream also were excluded from the study.
Eligible participants received tapinarof cream 1% once daily plus their ongoing biologic for 12 weeks, after which tapinarof was discontinued and the biologic was continued for an additional 4 weeks. A remittive (maintenance) effect was assessed at week 16.
Study Outcomes—Safety and efficacy were evaluated at baseline and weeks 2, 4, 8, 12, and 16. The primary end point was the proportion of patients who reached the TTT goal of 1% or less BSA involvement at week 12. Secondary end points included the proportion of patients with 1% or less BSA involvement at weeks 2, 4, 8, and 16; and PGA scores, composite PGA multiplied by mean percentage of BSA involvement (PGA×BSA), and PASI scores at baseline and weeks 2, 4, 8, 12, and 16. The patient-reported outcomes of Dermatology Life Quality Index (DLQI) and Worst Itch Numeric Rating Scale (WI-NRS) scores also were evaluated at baseline and weeks 2, 4, 8, 12, and 16. In patients who had disease involvement on the scalp or genital region at baseline, Psoriasis Scalp Severity Index (PSSI) and Static Physician’s Global Assessment of Genitalia scores, respectively, were assessed at baseline and weeks 2, 4, 8, 12, and 16. Safety was determined by the incidence, severity, and relatedness of adverse events (AEs) and serious AEs.
Statistical Analysis—Approximately 30 participants were planned for enrollment and recruited consecutively as they were identified during screening against inclusion and exclusion criteria. Changes from baseline in all outcomes were summarized descriptively. Missing data were not imputed. Given the sample size, no formal statistical analyses were conducted. Safety was summarized by descriptively collating AEs and serious AEs, including their frequency, severity, and treatment relatedness.
Results
Thirty participants were enrolled in the study, and 20 fully completed the study. Nine discontinued treatment before week 12 (6 were lost to follow-up, 2 were terminated early by the investigators, and 1 voluntarily withdrew); 1 additional participant was lost to follow-up after week 12. Patients were predominantly male (20/30 [66.7%]) and White (21/30 [70.0%]); the mean age of all participants was 55.4 years, and the mean (SD) duration of psoriasis was 21.4 (15.0) years (Table 1). The mean baseline percentage of BSA involvement and mean baseline PGA, PASI, and DLQI scores are shown in Table 1. Most (19/30 [63.3%]) patients received biologics that inhibited IL-23 activity (guselkumab, risankizumab, tildrakizumab), approximately one-third (9/30 [30.0%]) received biologics that inhibited IL-17 activity (ixekizumab, secukinumab), and 2 (6.7%) received biologics that inhibited IL-12/IL-23 activity (ustekinumab)(Table 1).

For the primary end point, 52.4% (11/21) of patients reached the TTT goal (BSA involvement ≤1% after 12 weeks of treatment with tapinarof cream added to a prescribed biologic). The proportion of patients reaching the TTT goal increased over time with the combined treatment (eFigure 1). Additionally, the mean percentage of BSA involvement (eFigure 2) as well as the mean values for PGA (eFigure 3) and PGA×BSA decreased over time. The mean percentage of BSA involvement was 5.0% at baseline and dropped to 2.0% by week 12. Similar reductions were observed for PGA and PGA×BSA scores at week 12.



After discontinuing tapinarof cream at week 12 and receiving only the biologic for 4 weeks, the proportion of patients maintaining 1% or less BSA involvement fell to 40.0% (8/20) at week 16, which was closer to that observed at week 8 (36% [9/25]) than at week 12 (52.4% [11/21])(eFigure 1).
The mean PASI score was 5.5 at baseline, then decreased over time when tapinarof cream was combined with a biologic (eFigure 4), falling to 3.1 by week 2 and 1.6 by week 12; it was maintained at 1.7 at week 16. Nine (30.0%) participants had psoriasis on the scalp at baseline with a mean PSSI score of 2.6, which decreased to 0.83 by week 2. By week 12, the mean PSSI score remained stable at 0.95 in the 2 (9.5%) participants who still had scalp involvement. The mean PSSI score increased slightly to 1.45 after patients received only the biologic for 4 weeks. At baseline, 3 (10.0%) patients had genital involvement (mean Static Physician’s Global Assessment of Genitalia score, 0.27). Symptoms resolved in 2 (66.7%) of these patients at week 2 and stayed consistent until week 16; the third patient withdrew at week 2.

Both DLQI and WI-NRS scores decreased with use of tapinarof cream added to a biologic up to week 12 (eFigures 5 and 6). Mean DLQI scores were 5.3 at baseline and 3.1 at week 12. At week 16, the mean DLQI score remained stable at 2.8. Mean WI-NRS scores decreased from 4.0 at baseline to 2.7 at week 12 with the therapy combination; at week 16, the mean WI-NRS score fell further to 1.8.


A total of 6 AEs were reported in 5 (16.7%) patients (Table 2). The majority (4/6 [67.0%]) of AEs were considered mild. Two reported cases of COVID-19 were both considered mild and unrelated. Mild folliculitis and moderate worsening of psoriasis in 2 (6.7%) different patients were the only AEs considered related to treatment. No serious AEs were reported, and no patient withdrew from the study due to an AE.

Comment
Disease activity improvements we observed with the nonsteroidal tapinarof cream were consistent with those reported when topical steroidal therapies were given to patients responding poorly to their current biologic. Our primary end point (proportion of patients with BSA involvement ≤1% after 12 weeks) showed that half (52% [11/21]) of patients whose BSA involvement was 3% or greater with a biologic for 24 weeks or more reached the TTT goal after 12 weeks of tapinarof-biologic treatment. Other studies of halobetasol propionate–tazarotene lotion16 and calcipotriene/betamethasone dipropionate foam17,18 added to the current biologic of poor responders found 60% to 68% of patients had reductions in their percentage BSA to 1% or lower at 12 to 16 weeks of treatment. Randomized studies showed etanercept plus topical clobetasol propionate foam20 or adalimumab plus calcipotriene/betamethasone dipropionate foam21 similarly enhanced treatment effects vs biologic alone.
A phase 3 PSOARING trial demonstrated benefit from treatment with tapinarof alone, with a remittive effect of approximately 4 months after discontinuation.25 Our data are consistent with these findings, with 40% (8/20) of patients demonstrating a remittive effect 4 weeks after discontinuing tapinarof while receiving a biologic. A similar maintenance effect was reported in another study in 50% (9/18) of patients treated with a biologic plus halobetasol propionate–tazarotene lotion.16 Additionally, when halcinonide ointment was given to patients receiving tildrakizumab, mean percentage of BSA involvement, PGA scores, PGA×BSA, and DLQI scores improved and were maintained 4 weeks after halcinonide ointment was stopped.19 Thus, topical therapy can augment and extend a biologic’s effect for up to 4 weeks.
In our study, tapinarof cream added to a biologic had a good safety and tolerability profile. Few AEs were recorded, with most being mild in nature, and no serious AEs or discontinuations due to AEs were reported. Only 1 case of mild folliculitis and 1 case of moderate worsening of psoriasis were considered treatment related. Further, no unexpected or new safety signals with the tapinarof-biologic combination were observed compared with tapinarof alone.27Prior studies have found that supplementing a biologic with topical therapy can reduce the probability of patients switching to another biologic.16,19 We previously found that adding halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17 to a biologic helped reduce the probability of switching biologics from 88% to 90% at baseline to 12% to 24% after 12 weeks of combined therapy. Such combinations also could prevent a less responsive patient from being prescribed a higher biologic dose.19 These are important research findings, as patients—even when not responding well to their current biologic—are more likely to be tolerating that biologic well, and switching to a new biologic may introduce new safety or tolerability concerns. Thus, by enhancing the effect of a biologic with a topical therapy, one can avoid increasing the dose of the current biologic or switching to a new biologic, either of which may increase safety and/or tolerability risks. Switching biologics also has increased cost implications to the health care system and/or the patient. When comparing the cost of adding halobetasol propionate–tazarotene lotion to a biologic compared with switching to another biologic, the cost was 1.2 to 2.9 times higher to switch, depending on the biologic, compared with a smaller incremental cost increase to add a topical to the current biologic.16 Similar observations were reported with calcipotriene/betamethasone dipropionate foam plus a biologic.17 Although we did not evaluate biologic switching here, we anticipate a similar clinical scenario with a tapinarof-biologic combination.
Limitations of our study included the open-label design, lack of a control arm, and the relatively small study population; however, for studies investigating the safety and effectiveness of a treatment in a real-world setting, these limitations are common and are not unexpected. Our results also are consistent with the overall improvement seen in other studies16-21 examining the effects of adding a topical to a biologic. Future research is warranted to investigate a longer remittive effect and potential health care system and patient cost savings without having to switch biologics due to lack of effectiveness.
Conclusion
This study demonstrated that adjunctive use of nonsteroidal tapinarof cream 1% may enhance a biologic treatment effect in patients with moderate to severe plaque psoriasis, providing an adequate response for many patients who were not responding well to a biologic alone. Clinical outcomes improved with the tapinarof-biologic combination, and a remittive effect was noted 4 weeks after tapinarof discontinuation without any new safety signals. Adding tapinarof cream to a biologic also may prevent the need to switch biologics when patients do not sufficiently respond, preserving the safety and cost associated with a patient’s current biologic.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804. doi:10.1016/j.jaad.2019.04.042
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi:10.1016/j.jaad.2020.07.087
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiological therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072. doi:10.1016/j.jaad.2018.11.057
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis.J Am Acad Dermatol. 2017;76:290-298. doi:10.1016/j.jaad.2016.10.017
- Taltz. Prescribing information. Eli Lilly and Company; 2024.
- Cosentyx. Prescribing information. Novartis Pharmaceuticals Corporation; 2023.
- Tremfya. Prescribing information. Janssen Biotech, Inc; 2023.
- Skyrizi. Prescribing information. AbbVie Inc; 2024.
- Ilumya. Prescribing information. Sun Pharmaceutical Industries, Inc; 2020.
- Stelara. Prescribing information. Janssen Biotech, Inc; 2022.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Jensen JD, Delcambre MR, Nguyen G, et al. Biologic therapy with or without topical treatment in psoriasis: what does the current evidence say? Am J Clin Dermatol. 2014;15:379-385. doi:10.1007/s40257-014-0089-1
- Gustafson CJ, Watkins C, Hix E, et al. Combination therapy in psoriasis: an evidence-based review. Am J Clin Dermatol. 2013;14:9-25. doi:10.1007/s40257-012-0003-7
- Bagel J, Novak K, Nelson E. Adjunctive use of halobetasol propionate-tazarotene in biologic-experienced patients with psoriasis. Cutis. 2022;109:103-109. doi:10.12788/cutis.0451
- Bagel J, Nelson E, Zapata J, et al. Adjunctive use of calcipotriene/betamethasone dipropionate foam in a real-world setting curtails the cost of biologics without reducing efficacy in psoriasis. Dermatol Ther (Heidelb). 2020;10:1383-1396. doi:10.1007/s13555-020-00454-z
- Bagel J, Zapata J, Nelson E. A prospective, open-label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018;17:611-616.
- Bagel J, Novak K, Nelson E. Tildrakizumab in combination with topical halcinonide 0.1% ointment for treating moderate to severe plaque psoriasis. J Drugs Dermatol. 2023;22:766-772. doi:10.36849/jdd.6830
- Lebwohl MG, Kircik L, Callis Duffin K, et al. A randomized study to evaluate the efficacy and safety of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2013;69:385-392. doi:10.1016/j.jaad.2013.03.031
- Thaci D, Ortonne JP, Chimenti S, et al. A phase IIIb, multicentre, randomized, double-blind, vehicle-controlled study of the efficacy and safety of adalimumab with and without calcipotriol/betamethasone topical treatment in patients with moderate to severe psoriasis: the BELIEVE study. Br J Dermatol. 2010;163:402-411. doi:10.1111/j.1365-2133.2010.09791.x
- Vtama. Prescribing information. Dermavant Sciences, Inc; 2022.
- Bobonich M, Gorelick J, Aldredge L, et al. Tapinarof, a novel, first-in-class, topical therapeutic aryl hydrocarbon receptor agonist for the management of psoriasis. J Drugs Dermatol. 2023;22:779-784. doi:10.36849/jdd.7317
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Kircik L, Zirwas M, Kwatra SG, et al. Rapid improvements in itch with tapinarof cream 1% once daily in two phase 3 trials in adults with mild to severe plaque psoriasis. Dermatol Ther (Heidelb). 2024;14:201-211. doi:10.1007/s13555-023-01068-x
- Bagel J, Gold LS, Del Rosso J, et al. Tapinarof cream 1% once daily for the treatment of plaque psoriasis: patient-reported outcomes from the PSOARING 3 trial. J Am Acad Dermatol. 2023;89:936-944. doi:10.1016/j.jaad.2023.04.061
- Abdin R, Kircik L, Issa NT. First use of combination oral deucravacitinib with tapinarof cream for treatment of severe plaque psoriasis. J Drugs Dermatol. 2024;23:192-194. doi:10.36849/jdd.8091
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804. doi:10.1016/j.jaad.2019.04.042
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi:10.1016/j.jaad.2020.07.087
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiological therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072. doi:10.1016/j.jaad.2018.11.057
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis.J Am Acad Dermatol. 2017;76:290-298. doi:10.1016/j.jaad.2016.10.017
- Taltz. Prescribing information. Eli Lilly and Company; 2024.
- Cosentyx. Prescribing information. Novartis Pharmaceuticals Corporation; 2023.
- Tremfya. Prescribing information. Janssen Biotech, Inc; 2023.
- Skyrizi. Prescribing information. AbbVie Inc; 2024.
- Ilumya. Prescribing information. Sun Pharmaceutical Industries, Inc; 2020.
- Stelara. Prescribing information. Janssen Biotech, Inc; 2022.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Jensen JD, Delcambre MR, Nguyen G, et al. Biologic therapy with or without topical treatment in psoriasis: what does the current evidence say? Am J Clin Dermatol. 2014;15:379-385. doi:10.1007/s40257-014-0089-1
- Gustafson CJ, Watkins C, Hix E, et al. Combination therapy in psoriasis: an evidence-based review. Am J Clin Dermatol. 2013;14:9-25. doi:10.1007/s40257-012-0003-7
- Bagel J, Novak K, Nelson E. Adjunctive use of halobetasol propionate-tazarotene in biologic-experienced patients with psoriasis. Cutis. 2022;109:103-109. doi:10.12788/cutis.0451
- Bagel J, Nelson E, Zapata J, et al. Adjunctive use of calcipotriene/betamethasone dipropionate foam in a real-world setting curtails the cost of biologics without reducing efficacy in psoriasis. Dermatol Ther (Heidelb). 2020;10:1383-1396. doi:10.1007/s13555-020-00454-z
- Bagel J, Zapata J, Nelson E. A prospective, open-label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018;17:611-616.
- Bagel J, Novak K, Nelson E. Tildrakizumab in combination with topical halcinonide 0.1% ointment for treating moderate to severe plaque psoriasis. J Drugs Dermatol. 2023;22:766-772. doi:10.36849/jdd.6830
- Lebwohl MG, Kircik L, Callis Duffin K, et al. A randomized study to evaluate the efficacy and safety of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2013;69:385-392. doi:10.1016/j.jaad.2013.03.031
- Thaci D, Ortonne JP, Chimenti S, et al. A phase IIIb, multicentre, randomized, double-blind, vehicle-controlled study of the efficacy and safety of adalimumab with and without calcipotriol/betamethasone topical treatment in patients with moderate to severe psoriasis: the BELIEVE study. Br J Dermatol. 2010;163:402-411. doi:10.1111/j.1365-2133.2010.09791.x
- Vtama. Prescribing information. Dermavant Sciences, Inc; 2022.
- Bobonich M, Gorelick J, Aldredge L, et al. Tapinarof, a novel, first-in-class, topical therapeutic aryl hydrocarbon receptor agonist for the management of psoriasis. J Drugs Dermatol. 2023;22:779-784. doi:10.36849/jdd.7317
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Kircik L, Zirwas M, Kwatra SG, et al. Rapid improvements in itch with tapinarof cream 1% once daily in two phase 3 trials in adults with mild to severe plaque psoriasis. Dermatol Ther (Heidelb). 2024;14:201-211. doi:10.1007/s13555-023-01068-x
- Bagel J, Gold LS, Del Rosso J, et al. Tapinarof cream 1% once daily for the treatment of plaque psoriasis: patient-reported outcomes from the PSOARING 3 trial. J Am Acad Dermatol. 2023;89:936-944. doi:10.1016/j.jaad.2023.04.061
- Abdin R, Kircik L, Issa NT. First use of combination oral deucravacitinib with tapinarof cream for treatment of severe plaque psoriasis. J Drugs Dermatol. 2024;23:192-194. doi:10.36849/jdd.8091
Safety and Effectiveness of Nonsteroidal Tapinarof Cream 1% Added to Ongoing Biologic Therapy for Treatment of Moderate to Severe Plaque Psoriasis
Safety and Effectiveness of Nonsteroidal Tapinarof Cream 1% Added to Ongoing Biologic Therapy for Treatment of Moderate to Severe Plaque Psoriasis
Practice Points
- Patients with moderate to severe psoriasis do not always reach treatment goals with biologic therapy alone.
- Adjunctive use of nonsteroidal tapinarof cream 1% may enhance the effects of ongoing biologic therapy in patients with moderate to severe plaque psoriasis, possibly avoiding the need to switch to another biologic.
- Patients with moderate to severe plaque psoriasis who are not adequately responding to biologics may benefit from adding tapinarof cream 1% to their current regimen.
Pathogenic Significance of Serum Syndecan-1 and Syndecan-4 in Psoriasis
Pathogenic Significance of Serum Syndecan-1 and Syndecan-4 in Psoriasis
Psoriasis, one of the most researched diseases in dermatology, has a complex pathogenesis that is not yet fully understood. One of the most important stages of psoriasis pathogenesis is the proliferation of T helper (Th) 17 cells by IL-23 released from myeloid dendritic cells. Cytokines such as tumor necrosis factor (TNF) α released from Th1 cells and IL-17 and IL-22 released from Th17 cells are known to induce the proliferation of keratinocytes and the release of chemokines responsible for neutrophil chemotaxis.1
Although secondary messengers such as cytokines and chemokines, which provide cell interaction with the extracellular matrix (ECM), have their own specific receptors, it is known that syndecans (SDCs) play a role in ECM and cell interactions and have receptor or coreceptor functions.2 In humans, 4 types of SDCs have been identified (SDC1-SDC4), which are type I transmembrane proteoglycans found in all nucleated cells. Syndecans consist of heparan sulfate glycosaminoglycan chains that are structurally linked to a core protein sequence. The molecule has cytoplasmic, transmembrane, and extracellular domains.2,3 While SDCs often are described as coreceptors for integrins and growth factor and hormone receptors, they also are capable of acting as signaling receptors by engaging intracellular messengers, including actin-related proteins and protein kinases.4
Prior research has indicated that the release of heparanase from the lysosomes of leukocytes during infection, inflammation, and endothelial damage causes cleavage of heparan sulfate glycosaminoglycans from the extracellular domains of SDCs. The peptide chains at the SDC core then are separated by matrix metalloproteinases in a process known as shedding. The shed SDCs may have either a stimulating or a suppressive effect on their receptor activity. Several cytokines are known to cause SDC shedding.5,6 Many studies in recent years have reported that SDCs play a role in the pathogenesis of inflammatory diseases, for which serum levels of soluble SDCs can be biomarkers.7
In this study, we aimed to evaluate and compare serum SDC1, SDC4, TNF-α, and IL-17A levels in patients with psoriasis vs healthy controls. Additionally, by reviewing the literature data, we analyzed whether SDCs can be implicated in the pathogenesis of psoriasis and their potential role in this process.
Methods
The study population consisted of 40 patients with psoriasis and 40 healthy controls. Age and sex characteristics were similar between the 2 groups, but weight distribution was not. The psoriasis group included patients older than 18 years who had received a clinical and/or histologic diagnosis, had no systemic disease other than psoriasis in their medical history, and had not used any systemic treatment or phototherapy for the past 3 months. Healthy patients older than 18 years who had no medical history of inflammatory disease were included in the control group. Participants provided signed consent.
Data such as medical history, laboratory findings, and physical specifications were recorded. A Psoriasis Area and Severity Index (PASI) score of 10 or lower was considered mild disease, and a score higher than 10 was considered moderate to severe disease. An enzyme-linked immunosorbent assay was used to measure SDC1, SDC4, TNF-α, and IL-17A levels.
The data were evaluated using the IBM SPSS Statistics V22.0 statistical package program. A P value of <.05 was considered statistically significant. The conformity of the data to a normal distribution was examined using a Shapiro-Wilk test. Normally distributed variables were expressed as mean (SD) and nonnormally distributed variables were expressed as median (interquartile range [IQR]). Data were compared between the 2 study groups using either a student t test (normal distribution) or Mann-Whitney U test (nonnormal distribution). Categorical variables were expressed as numbers and percentages. Categorical data were compared using a χ2 test. Associations among SDC1, SDC4, TNF-α, IL-17A, and other variables were assessed using Spearman rank correlation. A binary logistic regression analysis was used to determine whether serum SDC1 and SDC4 levels were independent risk factors for psoriasis.
Results
The 2 study groups showed similar demographic characteristics in terms of sex (P=.67) and age (P=.22) distribution. The mean (SD) PASI score in the psoriasis group was 12.33 (7.62); the mean (SD) disease duration was 11.10 (8.00) years. Body weight and BMI were both significantly higher in the psoriasis group (P=.027 and P=.029, respectively) compared with the control group (eTable 1).
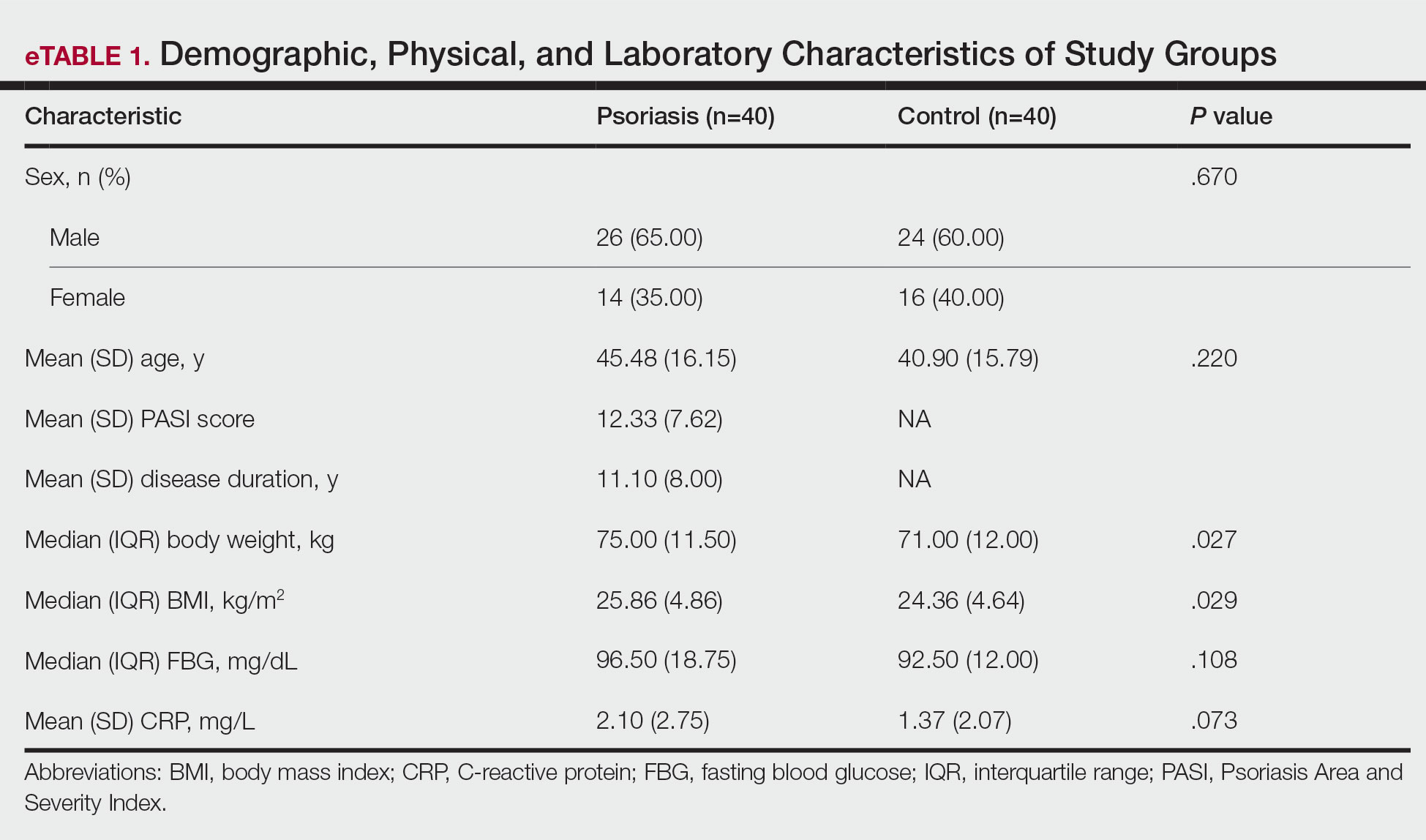
The mean (SD) serum SDC1 level was 119.52 ng/mL (69.53 ng/mL) in the psoriasis group, which was significantly higher than the control group (82.81 ng/mL [51.85 ng/mL])(P=.011)(eTable 2)(eFigure 1). The median (IQR) serum SDC4 level also was significantly higher in the psoriasis group compared with the control group (5.78 ng/mL [7.09 ng/mL] vs 3.92 ng/mL [2.88 ng/mL])(P=.030)(eTable 2)(eFigure 2). The median (IQR) IL-17A value was 59.94 pg/mL (12.97 pg/mL) in the psoriasis group, which was significantly higher than the control group (37.74 pg/mL [15.10 pg/mL])(P<.001)(eTable 2)(eFigure 3). The median (IQR) serum TNF-α level was 25.07 pg/mL (41.70 pg/mL) in the psoriasis group and 18.21 pg/mL (48.51 pg/mL) in the control group; however, the difference was not statistically significance (P=.444)(eTable 2)(eFigure 4).
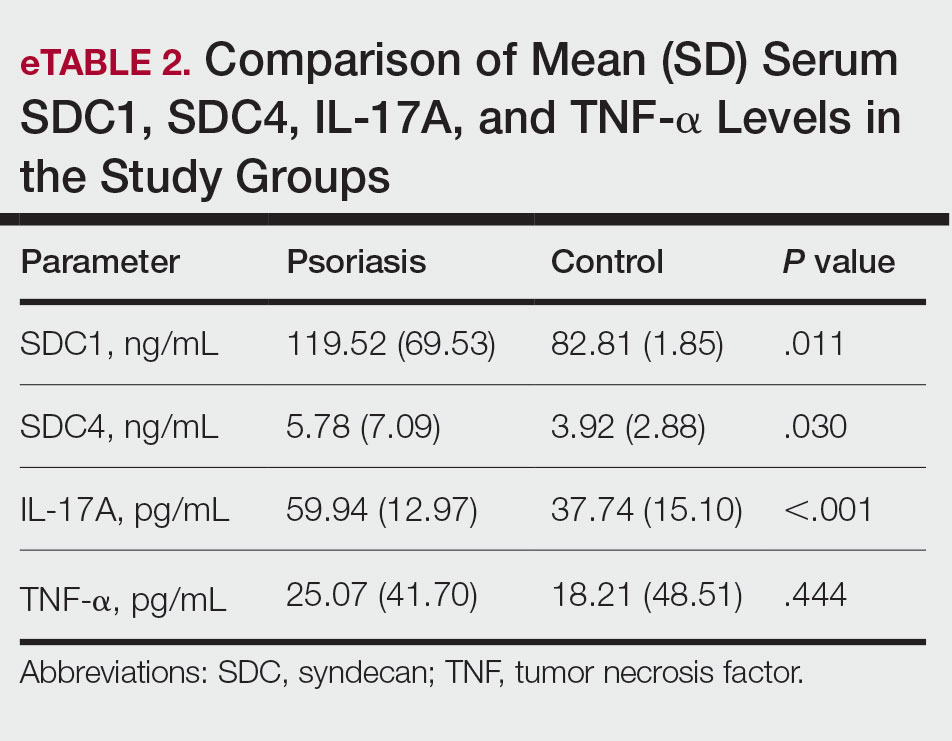
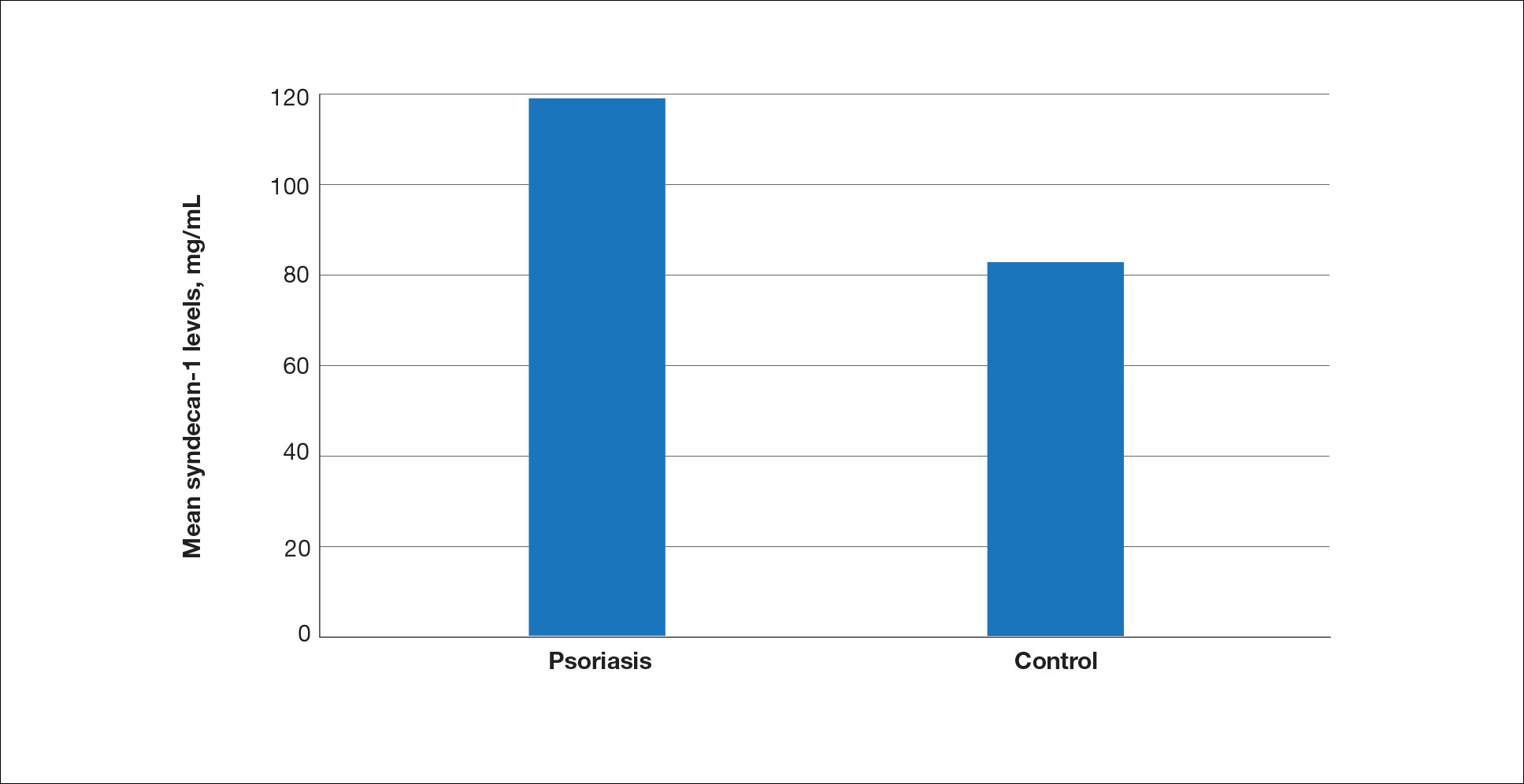
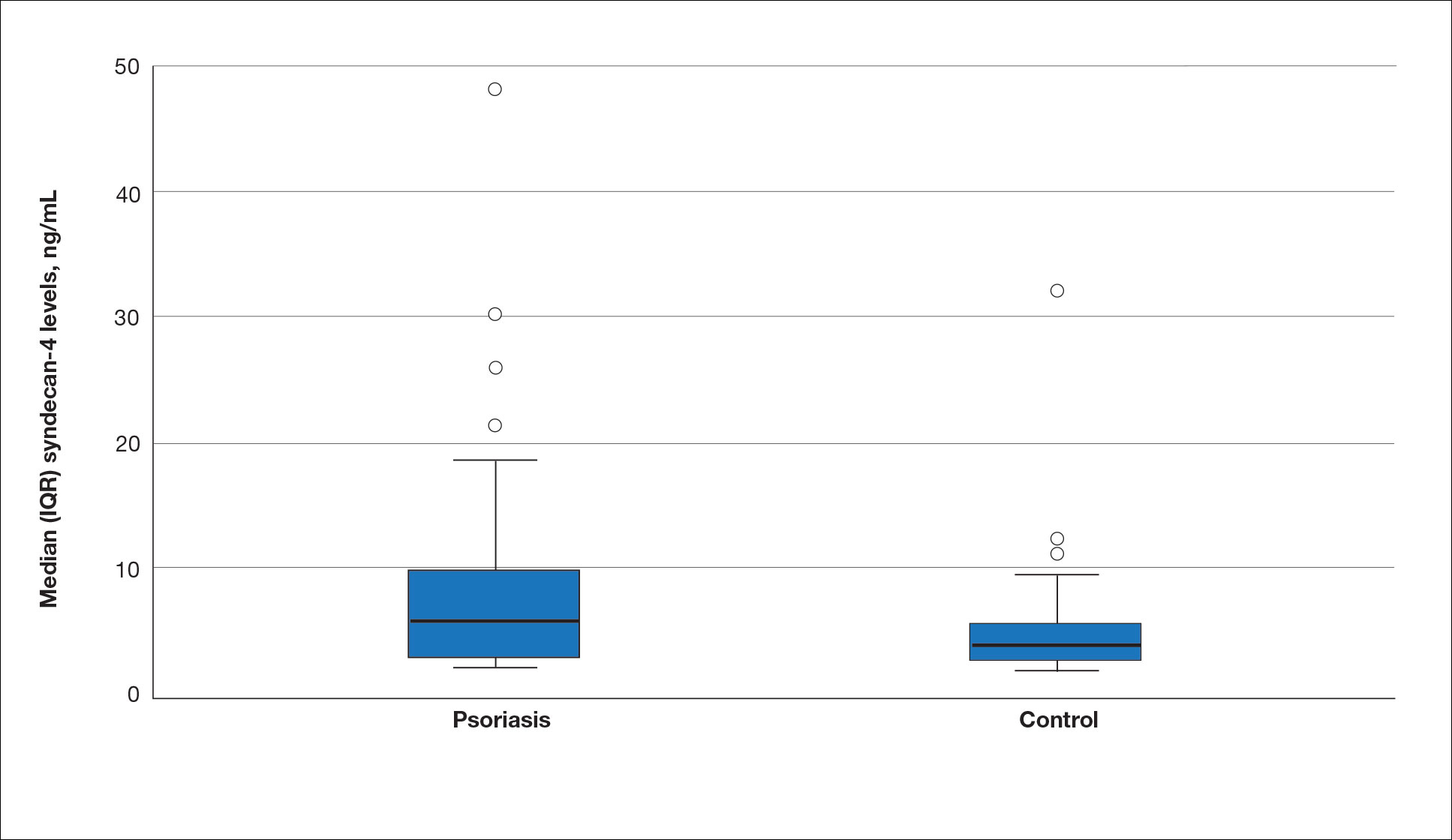
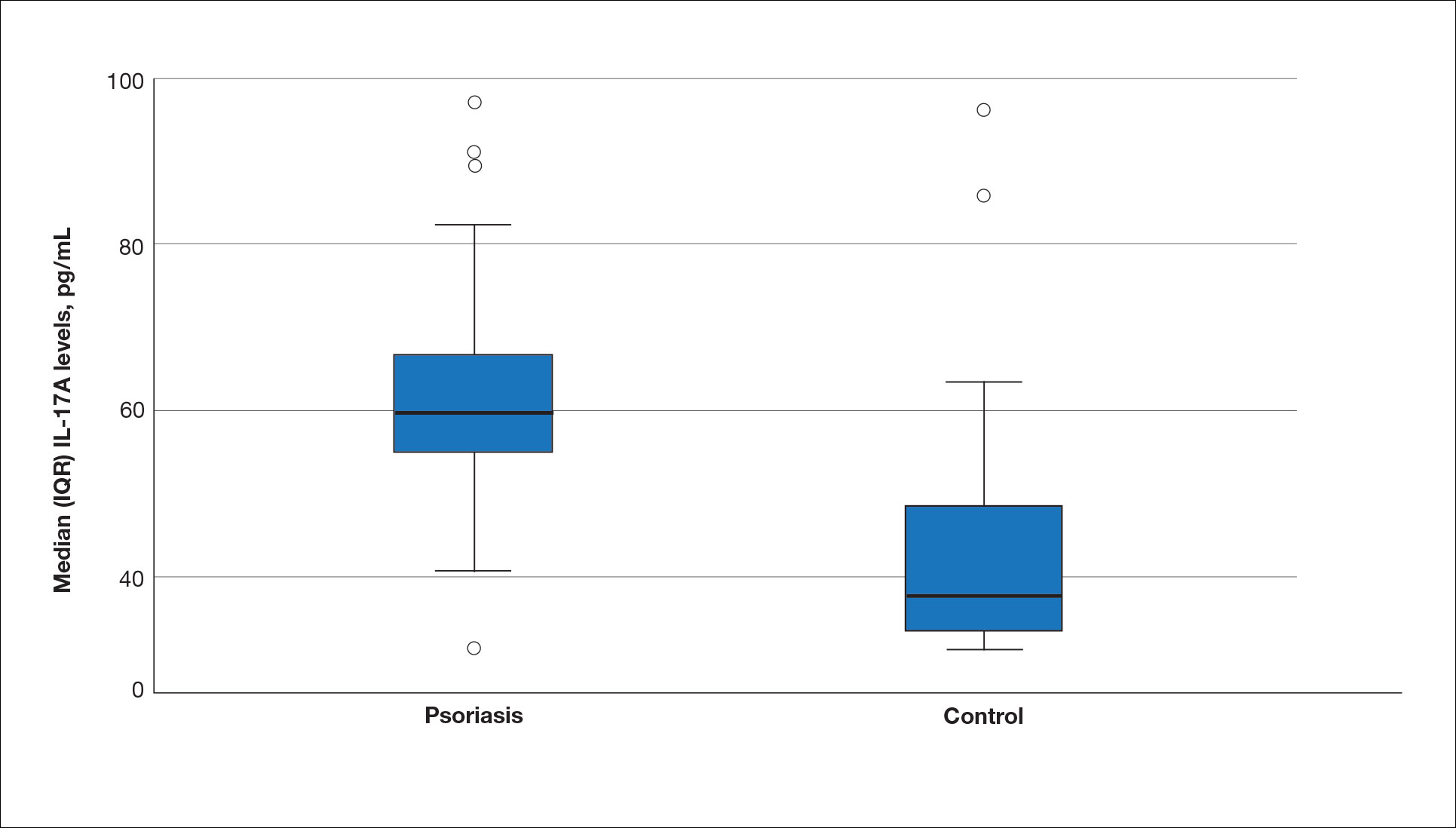
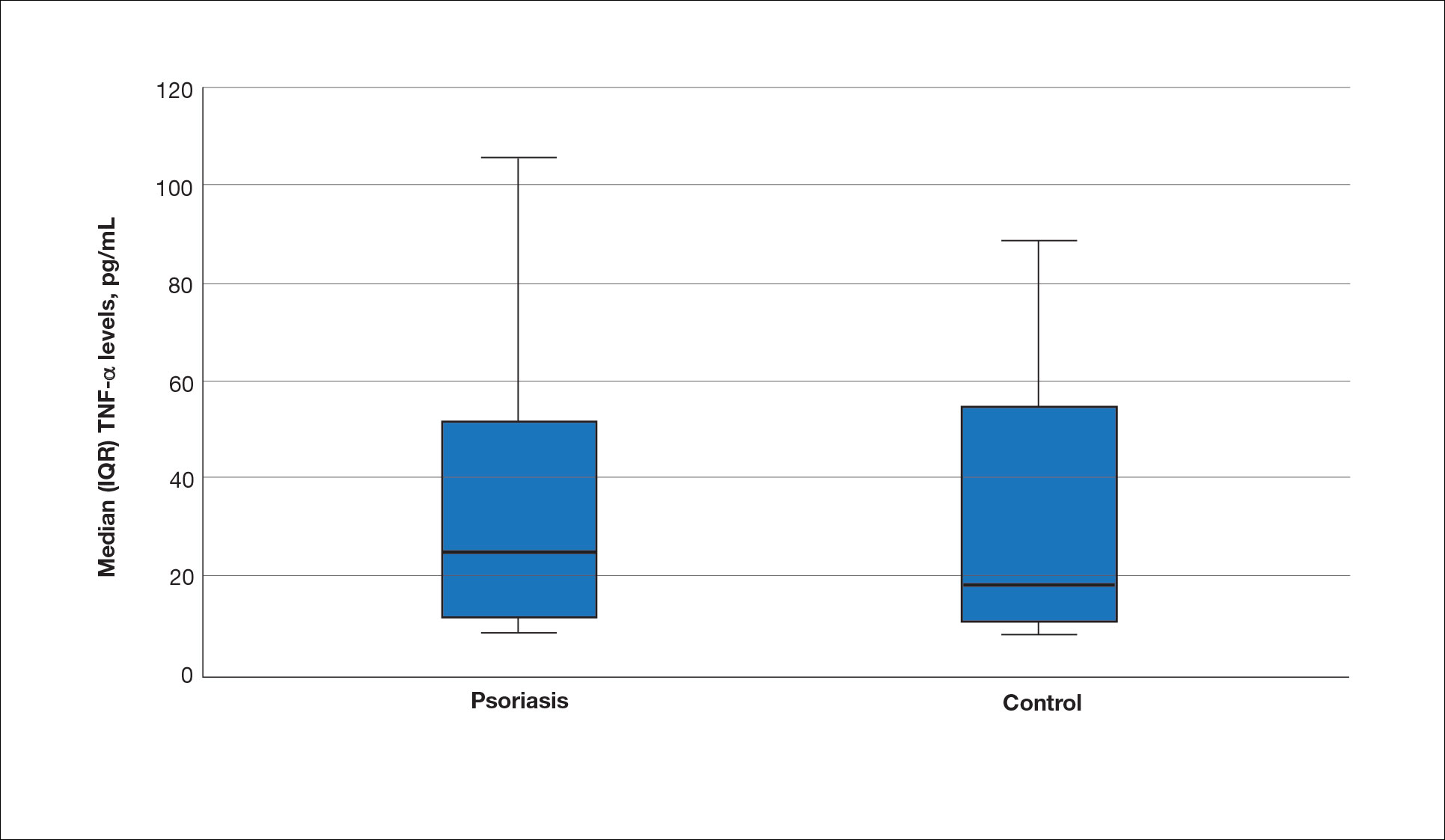
A significant positive correlation was found between serum SDC1 and PASI score (p=0.064; P=.03). Furthermore, significant positive correlations were identified between serum SDC1 and body weight (p=0.404; P<.001), disease duration (p=0.377; P=.008), and C-reactive protein (p=0.327; P=.002). A significant positive correlation also was identified between SDC4 and IL-17A (p=0.265; P=.009). Serum TNF-α was positively correlated with IL-17A (p=0.384; P<.001) and BMI (p=0.234; P=.020)(eTable 3).
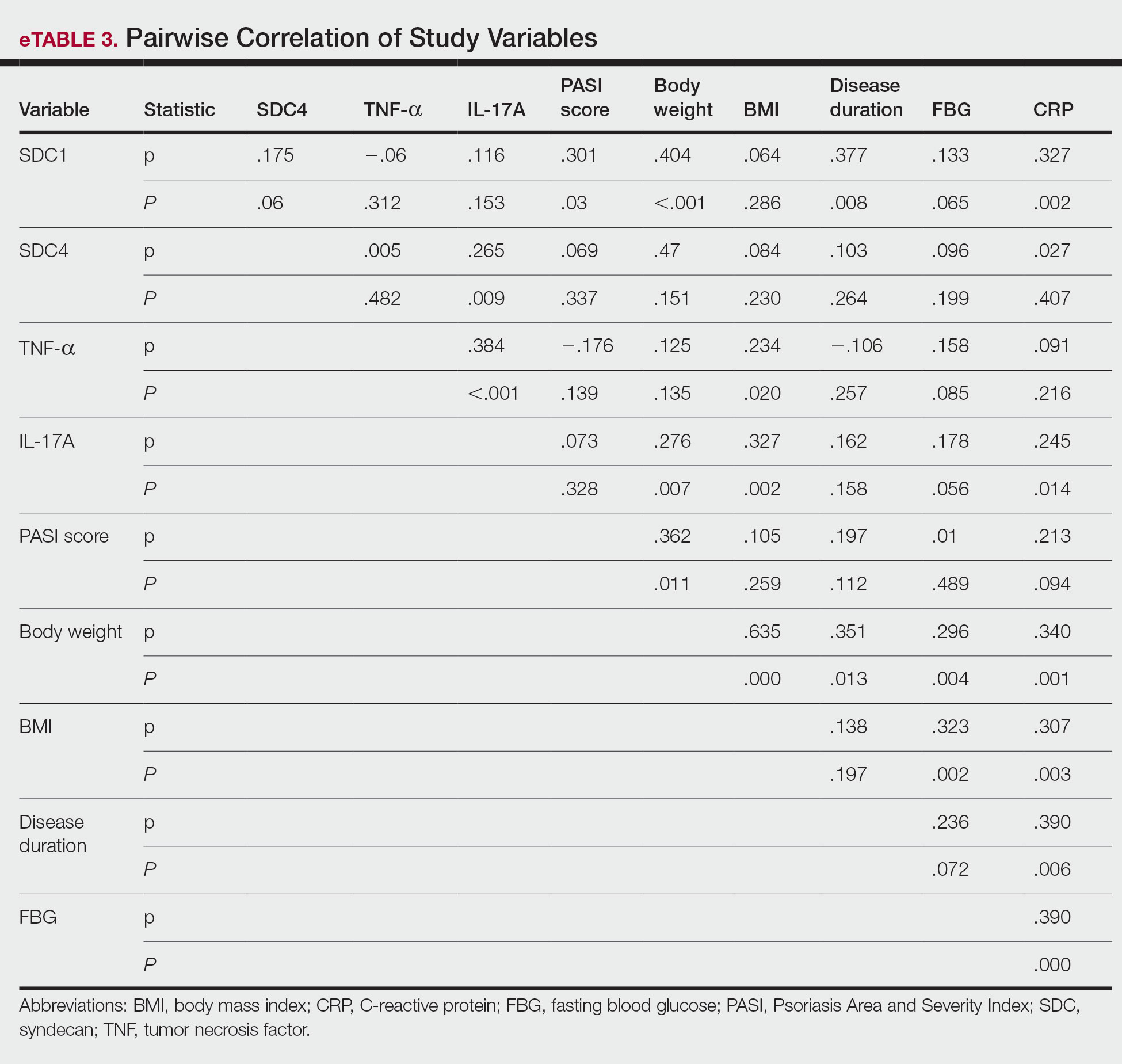
Logistic regression analysis showed that high SDC1 levels were independently associated with the development of psoriasis (odds ratio [OR], 1.009; 95% CI, 1.000-1.017; P=.049)(eTable 4).
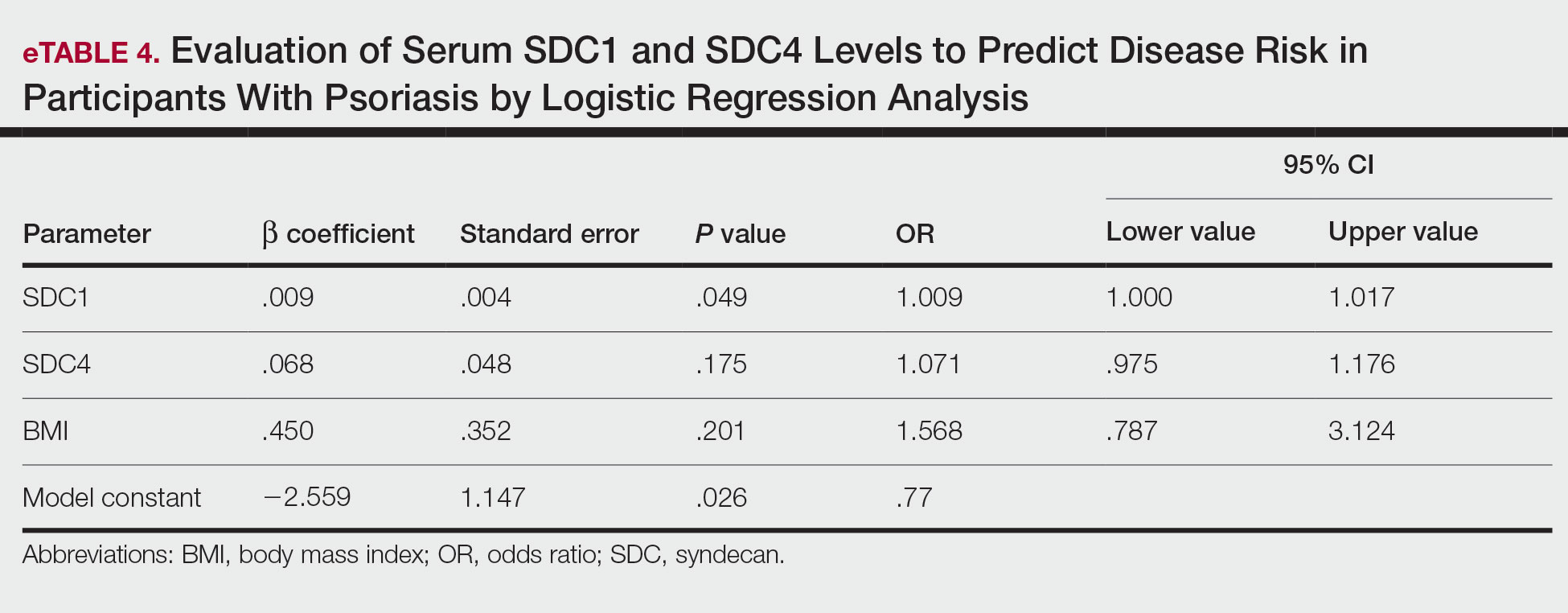
Comment
Tumor necrosis factor α and IL-17A are key cytokines whose roles in the pathogenesis of psoriasis are well established. Arican et al,8 Kyriakou et al,9 and Xuan et al10 previously reported a lack of any correlation between TNF-α and IL-17A in the pathogenesis of psoriasis; however, we observed a positive correlation between TNF-α and IL-17A in our study. This finding may be due to the abundant TNF-α production by myeloid dendritic cells involved in the transformation of naive T lymphocytes into IL-17A–secreting Th17 lymphocytes, which can also secrete TNF-α.
After the molecular cloning of SDCs by Saunders et al11 in 1989, SDCs gained attention and have been the focus of many studies for their part in the pathogenesis of conditions such as inflammatory diseases, carcinogenesis, infections, sepsis, and trauma.6,12 Among the inflammatory diseases sharing similar pathogenetic features to psoriasis, serum SDC4 levels are found to be elevated in rheumatoid arthritis and are correlated with disease activity.13 Cekic et al14 reported that serum SDC1 levels were significantly higher in patients with Crohn disease than controls (P=.03). Additionally, serum SDC1 levels were higher in patients with active disease compared with those who were in remission. Correlations between SDC1 and disease severity and C-reactive protein also have been found.14 Serum SDC-1 levels found to be elevated in patients with systemic lupus erythematosus were compared to the controls and were correlated with disease activity.15 Nakao et al16 reported that the serum SDC4 levels were significantly higher in patients with atopic dermatitis compared to controls (P<.01); further, SDC4 levels were correlated with severity of the disease.
Jaiswal et al17 reported that SDC1 is abundant on the surface of IL-17A–secreting γδ T lymphocytes (Tγδ17), whose contribution to psoriasis pathogenesis is known. When subjected to treatment with imiquimod, SDC1-suppressed mice displayed increased psoriasiform dermatitis compared with wild-type counterparts. The authors stated that SDC1 may play a role in controlling homeostasis of Tγδ17
In a study examining changes in the ECM in patients with psoriasis, it was observed that the expression of
A study conducted by Koliakou et al20 showed that, in healthy skin, SDC1 was expressed in almost the full thickness of the epidermis, but lowest expression was in the basal-layer keratinocytes. In a psoriatic epidermis, unlike the normal epidermis, SDC1 was found to be more intensely expressed in the keratinocytes of the basal layer, where keratinocyte proliferation occurs. In this study, SDC4 was expressed mainly at lower levels in a healthy epidermis, especially in the spinous and the basal layers. In a psoriatic epidermis, SDC4 was absent from all the layers. In the same study, gelatin-based carriers containing anti–TNF-α and anti–IL-17A were applied to a full-thickness epidermis with psoriatic lesions, after which SDC1 expression was observed to decrease almost completely in the psoriatic epidermis; there was no change in SDC4 expression, which also was not seen in the psoriatic epidermis. The authors claimed the application of these gelatin-based carriers could be a possible treatment modality for psoriasis, and the study provides evidence for the involvement of SDC1 and/or SDC4 in the pathogenesis of psoriasis
Limitations of the current study include small sample size, lack of longitudinal data, lack of tissue testing of these molecules, and lack of external validation.
Conclusion
Overall, research has shown that SDCs play important roles in inflammatory processes, and more widespread inflammation has been associated with increased shedding of these molecules into the ECM and higher serum levels. In our study, serum SDC1, SDC4, and IL-17A levels were increased in patients with psoriasis compared to the healthy controls. A logistic regression analysis indicated that high serum SDC1 levels may be an independent risk factor for development of psoriasis. The increase in serum SDC1 and SDC4 levels and the positive correlation between SDC1 levels and disease severity observed in our study strongly implicate SDCs in the inflammatory disease psoriasis. The precise role of SDCs in the pathogenesis of psoriasis and the implications of targeting these molecules are the subject of more in-depth studies in the future.
Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397:1301-1315.
Uings IJ, Farrow SN. Cell receptors and cell signaling. Mol Pathol. 2000;53:295-299.
Kirkpatrick CA, Selleck SB. Heparan sulfate proteoglycans at a glance.J Cell Sci. 2007;120:1829-1832.
Stepp MA, Pal-Ghosh S, Tadvalkar G, et al. Syndecan-1 and its expanding list of contacts. Adv Wound Care (New Rochelle). 2015;4:235-249.
Rangarajan S, Richter JR, Richter RP, et al. Heparanase-enhanced shedding of syndecan-1 and its role in driving disease pathogenesis and progression. J Histochem Cytochem. 2020;68:823-840.
Gopal S, Arokiasamy S, Pataki C, et al. Syndecan receptors: pericellular regulators in development and inflammatory disease. Open Biol. 2021;11:200377.
Bertrand J, Bollmann M. Soluble syndecans: biomarkers for diseases and therapeutic options. Br J Pharmacol. 2019;176:67-81.
Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273-279.
Kyriakou A, Patsatsi A, Vyzantiadis TA, et al. Serum levels of TNF-α, IL12/23 p40, and IL-17 in psoriatic patients with and without nail psoriasis: a cross-sectional study. ScientificWorldJournal. 2014;2014:508178.
Xuan ML, Lu CJ, Han L, et al. Circulating levels of inflammatory cytokines in patients with psoriasis vulgaris of different Chinese medicine syndromes. Chin J Integr Med. 2015;21:108-114.
Saunders S, Jalkanen M, O’Farrell S, et al. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989;108:1547-1556.
Manon-Jensen T, Itoh Y, Couchman JR. Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J. 2010;277:3876-3889.
Zhao J, Ye X, Zhang Z. Syndecan-4 is correlated with disease activity and serological characteristic of rheumatoid arthritis. Adv Rheumatol. 2022;62:21.
Cekic C, Kırcı A, Vatansever S, et al. Serum syndecan-1 levels and its relationship to disease activity in patients with Crohn’s disease. Gastroenterol Res Pract. 2015;2015:850351.
Minowa K, Amano H, Nakano S, et al. Elevated serum level of circulating syndecan-1 (CD138) in active systemic lupus erythematosus. Autoimmunity. 2011;44:357-362.
Nakao M, Sugaya M, Takahashi N, et al. Increased syndecan-4 expression in sera and skin of patients with atopic dermatitis. Arch Dermatol Res. 2016;308:655-660.
Jaiswal AK, Sadasivam M, Archer NK, et al. Syndecan-1 regulates psoriasiform dermatitis by controlling homeostasis of IL-17-producing γδ T cells. J Immunol. 2018;201:1651-1661
Wagner MFMG, Theodoro TR, Filho CASM, et al. Extracellular matrix alterations in the skin of patients affected by psoriasis. BMC Mol Cell Biol. 2021;22:55.
Peters F, Rahn S, Mengel M, et al. Syndecan-1 shedding by meprin β impairs keratinocyte adhesion and differentiation in hyperkeratosis. Matrix Biol. 2021;102:37-69.
Koliakou E, Eleni MM, Koumentakou I, et al. Altered distribution and expression of syndecan-1 and -4 as an additional hallmark in psoriasis. Int J Mol Sci. 2022;23:6511.
Doss RW, El-Rifaie AA, Said AN, et al. Cutaneous syndecan-1 expression before and after phototherapy in psoriasis. Indian J Dermatol Venereol Leprol. 2020;86:439-440.
Psoriasis, one of the most researched diseases in dermatology, has a complex pathogenesis that is not yet fully understood. One of the most important stages of psoriasis pathogenesis is the proliferation of T helper (Th) 17 cells by IL-23 released from myeloid dendritic cells. Cytokines such as tumor necrosis factor (TNF) α released from Th1 cells and IL-17 and IL-22 released from Th17 cells are known to induce the proliferation of keratinocytes and the release of chemokines responsible for neutrophil chemotaxis.1
Although secondary messengers such as cytokines and chemokines, which provide cell interaction with the extracellular matrix (ECM), have their own specific receptors, it is known that syndecans (SDCs) play a role in ECM and cell interactions and have receptor or coreceptor functions.2 In humans, 4 types of SDCs have been identified (SDC1-SDC4), which are type I transmembrane proteoglycans found in all nucleated cells. Syndecans consist of heparan sulfate glycosaminoglycan chains that are structurally linked to a core protein sequence. The molecule has cytoplasmic, transmembrane, and extracellular domains.2,3 While SDCs often are described as coreceptors for integrins and growth factor and hormone receptors, they also are capable of acting as signaling receptors by engaging intracellular messengers, including actin-related proteins and protein kinases.4
Prior research has indicated that the release of heparanase from the lysosomes of leukocytes during infection, inflammation, and endothelial damage causes cleavage of heparan sulfate glycosaminoglycans from the extracellular domains of SDCs. The peptide chains at the SDC core then are separated by matrix metalloproteinases in a process known as shedding. The shed SDCs may have either a stimulating or a suppressive effect on their receptor activity. Several cytokines are known to cause SDC shedding.5,6 Many studies in recent years have reported that SDCs play a role in the pathogenesis of inflammatory diseases, for which serum levels of soluble SDCs can be biomarkers.7
In this study, we aimed to evaluate and compare serum SDC1, SDC4, TNF-α, and IL-17A levels in patients with psoriasis vs healthy controls. Additionally, by reviewing the literature data, we analyzed whether SDCs can be implicated in the pathogenesis of psoriasis and their potential role in this process.
Methods
The study population consisted of 40 patients with psoriasis and 40 healthy controls. Age and sex characteristics were similar between the 2 groups, but weight distribution was not. The psoriasis group included patients older than 18 years who had received a clinical and/or histologic diagnosis, had no systemic disease other than psoriasis in their medical history, and had not used any systemic treatment or phototherapy for the past 3 months. Healthy patients older than 18 years who had no medical history of inflammatory disease were included in the control group. Participants provided signed consent.
Data such as medical history, laboratory findings, and physical specifications were recorded. A Psoriasis Area and Severity Index (PASI) score of 10 or lower was considered mild disease, and a score higher than 10 was considered moderate to severe disease. An enzyme-linked immunosorbent assay was used to measure SDC1, SDC4, TNF-α, and IL-17A levels.
The data were evaluated using the IBM SPSS Statistics V22.0 statistical package program. A P value of <.05 was considered statistically significant. The conformity of the data to a normal distribution was examined using a Shapiro-Wilk test. Normally distributed variables were expressed as mean (SD) and nonnormally distributed variables were expressed as median (interquartile range [IQR]). Data were compared between the 2 study groups using either a student t test (normal distribution) or Mann-Whitney U test (nonnormal distribution). Categorical variables were expressed as numbers and percentages. Categorical data were compared using a χ2 test. Associations among SDC1, SDC4, TNF-α, IL-17A, and other variables were assessed using Spearman rank correlation. A binary logistic regression analysis was used to determine whether serum SDC1 and SDC4 levels were independent risk factors for psoriasis.
Results
The 2 study groups showed similar demographic characteristics in terms of sex (P=.67) and age (P=.22) distribution. The mean (SD) PASI score in the psoriasis group was 12.33 (7.62); the mean (SD) disease duration was 11.10 (8.00) years. Body weight and BMI were both significantly higher in the psoriasis group (P=.027 and P=.029, respectively) compared with the control group (eTable 1).

The mean (SD) serum SDC1 level was 119.52 ng/mL (69.53 ng/mL) in the psoriasis group, which was significantly higher than the control group (82.81 ng/mL [51.85 ng/mL])(P=.011)(eTable 2)(eFigure 1). The median (IQR) serum SDC4 level also was significantly higher in the psoriasis group compared with the control group (5.78 ng/mL [7.09 ng/mL] vs 3.92 ng/mL [2.88 ng/mL])(P=.030)(eTable 2)(eFigure 2). The median (IQR) IL-17A value was 59.94 pg/mL (12.97 pg/mL) in the psoriasis group, which was significantly higher than the control group (37.74 pg/mL [15.10 pg/mL])(P<.001)(eTable 2)(eFigure 3). The median (IQR) serum TNF-α level was 25.07 pg/mL (41.70 pg/mL) in the psoriasis group and 18.21 pg/mL (48.51 pg/mL) in the control group; however, the difference was not statistically significance (P=.444)(eTable 2)(eFigure 4).





A significant positive correlation was found between serum SDC1 and PASI score (p=0.064; P=.03). Furthermore, significant positive correlations were identified between serum SDC1 and body weight (p=0.404; P<.001), disease duration (p=0.377; P=.008), and C-reactive protein (p=0.327; P=.002). A significant positive correlation also was identified between SDC4 and IL-17A (p=0.265; P=.009). Serum TNF-α was positively correlated with IL-17A (p=0.384; P<.001) and BMI (p=0.234; P=.020)(eTable 3).

Logistic regression analysis showed that high SDC1 levels were independently associated with the development of psoriasis (odds ratio [OR], 1.009; 95% CI, 1.000-1.017; P=.049)(eTable 4).

Comment
Tumor necrosis factor α and IL-17A are key cytokines whose roles in the pathogenesis of psoriasis are well established. Arican et al,8 Kyriakou et al,9 and Xuan et al10 previously reported a lack of any correlation between TNF-α and IL-17A in the pathogenesis of psoriasis; however, we observed a positive correlation between TNF-α and IL-17A in our study. This finding may be due to the abundant TNF-α production by myeloid dendritic cells involved in the transformation of naive T lymphocytes into IL-17A–secreting Th17 lymphocytes, which can also secrete TNF-α.
After the molecular cloning of SDCs by Saunders et al11 in 1989, SDCs gained attention and have been the focus of many studies for their part in the pathogenesis of conditions such as inflammatory diseases, carcinogenesis, infections, sepsis, and trauma.6,12 Among the inflammatory diseases sharing similar pathogenetic features to psoriasis, serum SDC4 levels are found to be elevated in rheumatoid arthritis and are correlated with disease activity.13 Cekic et al14 reported that serum SDC1 levels were significantly higher in patients with Crohn disease than controls (P=.03). Additionally, serum SDC1 levels were higher in patients with active disease compared with those who were in remission. Correlations between SDC1 and disease severity and C-reactive protein also have been found.14 Serum SDC-1 levels found to be elevated in patients with systemic lupus erythematosus were compared to the controls and were correlated with disease activity.15 Nakao et al16 reported that the serum SDC4 levels were significantly higher in patients with atopic dermatitis compared to controls (P<.01); further, SDC4 levels were correlated with severity of the disease.
Jaiswal et al17 reported that SDC1 is abundant on the surface of IL-17A–secreting γδ T lymphocytes (Tγδ17), whose contribution to psoriasis pathogenesis is known. When subjected to treatment with imiquimod, SDC1-suppressed mice displayed increased psoriasiform dermatitis compared with wild-type counterparts. The authors stated that SDC1 may play a role in controlling homeostasis of Tγδ17
In a study examining changes in the ECM in patients with psoriasis, it was observed that the expression of
A study conducted by Koliakou et al20 showed that, in healthy skin, SDC1 was expressed in almost the full thickness of the epidermis, but lowest expression was in the basal-layer keratinocytes. In a psoriatic epidermis, unlike the normal epidermis, SDC1 was found to be more intensely expressed in the keratinocytes of the basal layer, where keratinocyte proliferation occurs. In this study, SDC4 was expressed mainly at lower levels in a healthy epidermis, especially in the spinous and the basal layers. In a psoriatic epidermis, SDC4 was absent from all the layers. In the same study, gelatin-based carriers containing anti–TNF-α and anti–IL-17A were applied to a full-thickness epidermis with psoriatic lesions, after which SDC1 expression was observed to decrease almost completely in the psoriatic epidermis; there was no change in SDC4 expression, which also was not seen in the psoriatic epidermis. The authors claimed the application of these gelatin-based carriers could be a possible treatment modality for psoriasis, and the study provides evidence for the involvement of SDC1 and/or SDC4 in the pathogenesis of psoriasis
Limitations of the current study include small sample size, lack of longitudinal data, lack of tissue testing of these molecules, and lack of external validation.
Conclusion
Overall, research has shown that SDCs play important roles in inflammatory processes, and more widespread inflammation has been associated with increased shedding of these molecules into the ECM and higher serum levels. In our study, serum SDC1, SDC4, and IL-17A levels were increased in patients with psoriasis compared to the healthy controls. A logistic regression analysis indicated that high serum SDC1 levels may be an independent risk factor for development of psoriasis. The increase in serum SDC1 and SDC4 levels and the positive correlation between SDC1 levels and disease severity observed in our study strongly implicate SDCs in the inflammatory disease psoriasis. The precise role of SDCs in the pathogenesis of psoriasis and the implications of targeting these molecules are the subject of more in-depth studies in the future.
Psoriasis, one of the most researched diseases in dermatology, has a complex pathogenesis that is not yet fully understood. One of the most important stages of psoriasis pathogenesis is the proliferation of T helper (Th) 17 cells by IL-23 released from myeloid dendritic cells. Cytokines such as tumor necrosis factor (TNF) α released from Th1 cells and IL-17 and IL-22 released from Th17 cells are known to induce the proliferation of keratinocytes and the release of chemokines responsible for neutrophil chemotaxis.1
Although secondary messengers such as cytokines and chemokines, which provide cell interaction with the extracellular matrix (ECM), have their own specific receptors, it is known that syndecans (SDCs) play a role in ECM and cell interactions and have receptor or coreceptor functions.2 In humans, 4 types of SDCs have been identified (SDC1-SDC4), which are type I transmembrane proteoglycans found in all nucleated cells. Syndecans consist of heparan sulfate glycosaminoglycan chains that are structurally linked to a core protein sequence. The molecule has cytoplasmic, transmembrane, and extracellular domains.2,3 While SDCs often are described as coreceptors for integrins and growth factor and hormone receptors, they also are capable of acting as signaling receptors by engaging intracellular messengers, including actin-related proteins and protein kinases.4
Prior research has indicated that the release of heparanase from the lysosomes of leukocytes during infection, inflammation, and endothelial damage causes cleavage of heparan sulfate glycosaminoglycans from the extracellular domains of SDCs. The peptide chains at the SDC core then are separated by matrix metalloproteinases in a process known as shedding. The shed SDCs may have either a stimulating or a suppressive effect on their receptor activity. Several cytokines are known to cause SDC shedding.5,6 Many studies in recent years have reported that SDCs play a role in the pathogenesis of inflammatory diseases, for which serum levels of soluble SDCs can be biomarkers.7
In this study, we aimed to evaluate and compare serum SDC1, SDC4, TNF-α, and IL-17A levels in patients with psoriasis vs healthy controls. Additionally, by reviewing the literature data, we analyzed whether SDCs can be implicated in the pathogenesis of psoriasis and their potential role in this process.
Methods
The study population consisted of 40 patients with psoriasis and 40 healthy controls. Age and sex characteristics were similar between the 2 groups, but weight distribution was not. The psoriasis group included patients older than 18 years who had received a clinical and/or histologic diagnosis, had no systemic disease other than psoriasis in their medical history, and had not used any systemic treatment or phototherapy for the past 3 months. Healthy patients older than 18 years who had no medical history of inflammatory disease were included in the control group. Participants provided signed consent.
Data such as medical history, laboratory findings, and physical specifications were recorded. A Psoriasis Area and Severity Index (PASI) score of 10 or lower was considered mild disease, and a score higher than 10 was considered moderate to severe disease. An enzyme-linked immunosorbent assay was used to measure SDC1, SDC4, TNF-α, and IL-17A levels.
The data were evaluated using the IBM SPSS Statistics V22.0 statistical package program. A P value of <.05 was considered statistically significant. The conformity of the data to a normal distribution was examined using a Shapiro-Wilk test. Normally distributed variables were expressed as mean (SD) and nonnormally distributed variables were expressed as median (interquartile range [IQR]). Data were compared between the 2 study groups using either a student t test (normal distribution) or Mann-Whitney U test (nonnormal distribution). Categorical variables were expressed as numbers and percentages. Categorical data were compared using a χ2 test. Associations among SDC1, SDC4, TNF-α, IL-17A, and other variables were assessed using Spearman rank correlation. A binary logistic regression analysis was used to determine whether serum SDC1 and SDC4 levels were independent risk factors for psoriasis.
Results
The 2 study groups showed similar demographic characteristics in terms of sex (P=.67) and age (P=.22) distribution. The mean (SD) PASI score in the psoriasis group was 12.33 (7.62); the mean (SD) disease duration was 11.10 (8.00) years. Body weight and BMI were both significantly higher in the psoriasis group (P=.027 and P=.029, respectively) compared with the control group (eTable 1).

The mean (SD) serum SDC1 level was 119.52 ng/mL (69.53 ng/mL) in the psoriasis group, which was significantly higher than the control group (82.81 ng/mL [51.85 ng/mL])(P=.011)(eTable 2)(eFigure 1). The median (IQR) serum SDC4 level also was significantly higher in the psoriasis group compared with the control group (5.78 ng/mL [7.09 ng/mL] vs 3.92 ng/mL [2.88 ng/mL])(P=.030)(eTable 2)(eFigure 2). The median (IQR) IL-17A value was 59.94 pg/mL (12.97 pg/mL) in the psoriasis group, which was significantly higher than the control group (37.74 pg/mL [15.10 pg/mL])(P<.001)(eTable 2)(eFigure 3). The median (IQR) serum TNF-α level was 25.07 pg/mL (41.70 pg/mL) in the psoriasis group and 18.21 pg/mL (48.51 pg/mL) in the control group; however, the difference was not statistically significance (P=.444)(eTable 2)(eFigure 4).





A significant positive correlation was found between serum SDC1 and PASI score (p=0.064; P=.03). Furthermore, significant positive correlations were identified between serum SDC1 and body weight (p=0.404; P<.001), disease duration (p=0.377; P=.008), and C-reactive protein (p=0.327; P=.002). A significant positive correlation also was identified between SDC4 and IL-17A (p=0.265; P=.009). Serum TNF-α was positively correlated with IL-17A (p=0.384; P<.001) and BMI (p=0.234; P=.020)(eTable 3).

Logistic regression analysis showed that high SDC1 levels were independently associated with the development of psoriasis (odds ratio [OR], 1.009; 95% CI, 1.000-1.017; P=.049)(eTable 4).

Comment
Tumor necrosis factor α and IL-17A are key cytokines whose roles in the pathogenesis of psoriasis are well established. Arican et al,8 Kyriakou et al,9 and Xuan et al10 previously reported a lack of any correlation between TNF-α and IL-17A in the pathogenesis of psoriasis; however, we observed a positive correlation between TNF-α and IL-17A in our study. This finding may be due to the abundant TNF-α production by myeloid dendritic cells involved in the transformation of naive T lymphocytes into IL-17A–secreting Th17 lymphocytes, which can also secrete TNF-α.
After the molecular cloning of SDCs by Saunders et al11 in 1989, SDCs gained attention and have been the focus of many studies for their part in the pathogenesis of conditions such as inflammatory diseases, carcinogenesis, infections, sepsis, and trauma.6,12 Among the inflammatory diseases sharing similar pathogenetic features to psoriasis, serum SDC4 levels are found to be elevated in rheumatoid arthritis and are correlated with disease activity.13 Cekic et al14 reported that serum SDC1 levels were significantly higher in patients with Crohn disease than controls (P=.03). Additionally, serum SDC1 levels were higher in patients with active disease compared with those who were in remission. Correlations between SDC1 and disease severity and C-reactive protein also have been found.14 Serum SDC-1 levels found to be elevated in patients with systemic lupus erythematosus were compared to the controls and were correlated with disease activity.15 Nakao et al16 reported that the serum SDC4 levels were significantly higher in patients with atopic dermatitis compared to controls (P<.01); further, SDC4 levels were correlated with severity of the disease.
Jaiswal et al17 reported that SDC1 is abundant on the surface of IL-17A–secreting γδ T lymphocytes (Tγδ17), whose contribution to psoriasis pathogenesis is known. When subjected to treatment with imiquimod, SDC1-suppressed mice displayed increased psoriasiform dermatitis compared with wild-type counterparts. The authors stated that SDC1 may play a role in controlling homeostasis of Tγδ17
In a study examining changes in the ECM in patients with psoriasis, it was observed that the expression of
A study conducted by Koliakou et al20 showed that, in healthy skin, SDC1 was expressed in almost the full thickness of the epidermis, but lowest expression was in the basal-layer keratinocytes. In a psoriatic epidermis, unlike the normal epidermis, SDC1 was found to be more intensely expressed in the keratinocytes of the basal layer, where keratinocyte proliferation occurs. In this study, SDC4 was expressed mainly at lower levels in a healthy epidermis, especially in the spinous and the basal layers. In a psoriatic epidermis, SDC4 was absent from all the layers. In the same study, gelatin-based carriers containing anti–TNF-α and anti–IL-17A were applied to a full-thickness epidermis with psoriatic lesions, after which SDC1 expression was observed to decrease almost completely in the psoriatic epidermis; there was no change in SDC4 expression, which also was not seen in the psoriatic epidermis. The authors claimed the application of these gelatin-based carriers could be a possible treatment modality for psoriasis, and the study provides evidence for the involvement of SDC1 and/or SDC4 in the pathogenesis of psoriasis
Limitations of the current study include small sample size, lack of longitudinal data, lack of tissue testing of these molecules, and lack of external validation.
Conclusion
Overall, research has shown that SDCs play important roles in inflammatory processes, and more widespread inflammation has been associated with increased shedding of these molecules into the ECM and higher serum levels. In our study, serum SDC1, SDC4, and IL-17A levels were increased in patients with psoriasis compared to the healthy controls. A logistic regression analysis indicated that high serum SDC1 levels may be an independent risk factor for development of psoriasis. The increase in serum SDC1 and SDC4 levels and the positive correlation between SDC1 levels and disease severity observed in our study strongly implicate SDCs in the inflammatory disease psoriasis. The precise role of SDCs in the pathogenesis of psoriasis and the implications of targeting these molecules are the subject of more in-depth studies in the future.
Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397:1301-1315.
Uings IJ, Farrow SN. Cell receptors and cell signaling. Mol Pathol. 2000;53:295-299.
Kirkpatrick CA, Selleck SB. Heparan sulfate proteoglycans at a glance.J Cell Sci. 2007;120:1829-1832.
Stepp MA, Pal-Ghosh S, Tadvalkar G, et al. Syndecan-1 and its expanding list of contacts. Adv Wound Care (New Rochelle). 2015;4:235-249.
Rangarajan S, Richter JR, Richter RP, et al. Heparanase-enhanced shedding of syndecan-1 and its role in driving disease pathogenesis and progression. J Histochem Cytochem. 2020;68:823-840.
Gopal S, Arokiasamy S, Pataki C, et al. Syndecan receptors: pericellular regulators in development and inflammatory disease. Open Biol. 2021;11:200377.
Bertrand J, Bollmann M. Soluble syndecans: biomarkers for diseases and therapeutic options. Br J Pharmacol. 2019;176:67-81.
Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273-279.
Kyriakou A, Patsatsi A, Vyzantiadis TA, et al. Serum levels of TNF-α, IL12/23 p40, and IL-17 in psoriatic patients with and without nail psoriasis: a cross-sectional study. ScientificWorldJournal. 2014;2014:508178.
Xuan ML, Lu CJ, Han L, et al. Circulating levels of inflammatory cytokines in patients with psoriasis vulgaris of different Chinese medicine syndromes. Chin J Integr Med. 2015;21:108-114.
Saunders S, Jalkanen M, O’Farrell S, et al. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989;108:1547-1556.
Manon-Jensen T, Itoh Y, Couchman JR. Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J. 2010;277:3876-3889.
Zhao J, Ye X, Zhang Z. Syndecan-4 is correlated with disease activity and serological characteristic of rheumatoid arthritis. Adv Rheumatol. 2022;62:21.
Cekic C, Kırcı A, Vatansever S, et al. Serum syndecan-1 levels and its relationship to disease activity in patients with Crohn’s disease. Gastroenterol Res Pract. 2015;2015:850351.
Minowa K, Amano H, Nakano S, et al. Elevated serum level of circulating syndecan-1 (CD138) in active systemic lupus erythematosus. Autoimmunity. 2011;44:357-362.
Nakao M, Sugaya M, Takahashi N, et al. Increased syndecan-4 expression in sera and skin of patients with atopic dermatitis. Arch Dermatol Res. 2016;308:655-660.
Jaiswal AK, Sadasivam M, Archer NK, et al. Syndecan-1 regulates psoriasiform dermatitis by controlling homeostasis of IL-17-producing γδ T cells. J Immunol. 2018;201:1651-1661
Wagner MFMG, Theodoro TR, Filho CASM, et al. Extracellular matrix alterations in the skin of patients affected by psoriasis. BMC Mol Cell Biol. 2021;22:55.
Peters F, Rahn S, Mengel M, et al. Syndecan-1 shedding by meprin β impairs keratinocyte adhesion and differentiation in hyperkeratosis. Matrix Biol. 2021;102:37-69.
Koliakou E, Eleni MM, Koumentakou I, et al. Altered distribution and expression of syndecan-1 and -4 as an additional hallmark in psoriasis. Int J Mol Sci. 2022;23:6511.
Doss RW, El-Rifaie AA, Said AN, et al. Cutaneous syndecan-1 expression before and after phototherapy in psoriasis. Indian J Dermatol Venereol Leprol. 2020;86:439-440.
Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397:1301-1315.
Uings IJ, Farrow SN. Cell receptors and cell signaling. Mol Pathol. 2000;53:295-299.
Kirkpatrick CA, Selleck SB. Heparan sulfate proteoglycans at a glance.J Cell Sci. 2007;120:1829-1832.
Stepp MA, Pal-Ghosh S, Tadvalkar G, et al. Syndecan-1 and its expanding list of contacts. Adv Wound Care (New Rochelle). 2015;4:235-249.
Rangarajan S, Richter JR, Richter RP, et al. Heparanase-enhanced shedding of syndecan-1 and its role in driving disease pathogenesis and progression. J Histochem Cytochem. 2020;68:823-840.
Gopal S, Arokiasamy S, Pataki C, et al. Syndecan receptors: pericellular regulators in development and inflammatory disease. Open Biol. 2021;11:200377.
Bertrand J, Bollmann M. Soluble syndecans: biomarkers for diseases and therapeutic options. Br J Pharmacol. 2019;176:67-81.
Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273-279.
Kyriakou A, Patsatsi A, Vyzantiadis TA, et al. Serum levels of TNF-α, IL12/23 p40, and IL-17 in psoriatic patients with and without nail psoriasis: a cross-sectional study. ScientificWorldJournal. 2014;2014:508178.
Xuan ML, Lu CJ, Han L, et al. Circulating levels of inflammatory cytokines in patients with psoriasis vulgaris of different Chinese medicine syndromes. Chin J Integr Med. 2015;21:108-114.
Saunders S, Jalkanen M, O’Farrell S, et al. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989;108:1547-1556.
Manon-Jensen T, Itoh Y, Couchman JR. Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J. 2010;277:3876-3889.
Zhao J, Ye X, Zhang Z. Syndecan-4 is correlated with disease activity and serological characteristic of rheumatoid arthritis. Adv Rheumatol. 2022;62:21.
Cekic C, Kırcı A, Vatansever S, et al. Serum syndecan-1 levels and its relationship to disease activity in patients with Crohn’s disease. Gastroenterol Res Pract. 2015;2015:850351.
Minowa K, Amano H, Nakano S, et al. Elevated serum level of circulating syndecan-1 (CD138) in active systemic lupus erythematosus. Autoimmunity. 2011;44:357-362.
Nakao M, Sugaya M, Takahashi N, et al. Increased syndecan-4 expression in sera and skin of patients with atopic dermatitis. Arch Dermatol Res. 2016;308:655-660.
Jaiswal AK, Sadasivam M, Archer NK, et al. Syndecan-1 regulates psoriasiform dermatitis by controlling homeostasis of IL-17-producing γδ T cells. J Immunol. 2018;201:1651-1661
Wagner MFMG, Theodoro TR, Filho CASM, et al. Extracellular matrix alterations in the skin of patients affected by psoriasis. BMC Mol Cell Biol. 2021;22:55.
Peters F, Rahn S, Mengel M, et al. Syndecan-1 shedding by meprin β impairs keratinocyte adhesion and differentiation in hyperkeratosis. Matrix Biol. 2021;102:37-69.
Koliakou E, Eleni MM, Koumentakou I, et al. Altered distribution and expression of syndecan-1 and -4 as an additional hallmark in psoriasis. Int J Mol Sci. 2022;23:6511.
Doss RW, El-Rifaie AA, Said AN, et al. Cutaneous syndecan-1 expression before and after phototherapy in psoriasis. Indian J Dermatol Venereol Leprol. 2020;86:439-440.
Pathogenic Significance of Serum Syndecan-1 and Syndecan-4 in Psoriasis
Pathogenic Significance of Serum Syndecan-1 and Syndecan-4 in Psoriasis
PRACTICE POINTS
- Improved understanding of psoriasis pathogenesis has enabled the development of targeted treatments, although the mediators driving the disease have not yet been fully identified.
- Based on the findings of this study and existing literature, we suggest that syndecan-1 and syndecan-4 may play a role in the pathogenesis of psoriasis; however, further studies are needed to elucidate their precise mechanisms of action.