User login
Our understanding of pain mechanisms continues to evolve and, accordingly, so do our treatment strategies. The fundamental differences between acute and chronic pain were only recently recognized; this lack of recognition led to the application of acute pain treatments to chronic pain, contributing to the opioid epidemic in the United States.
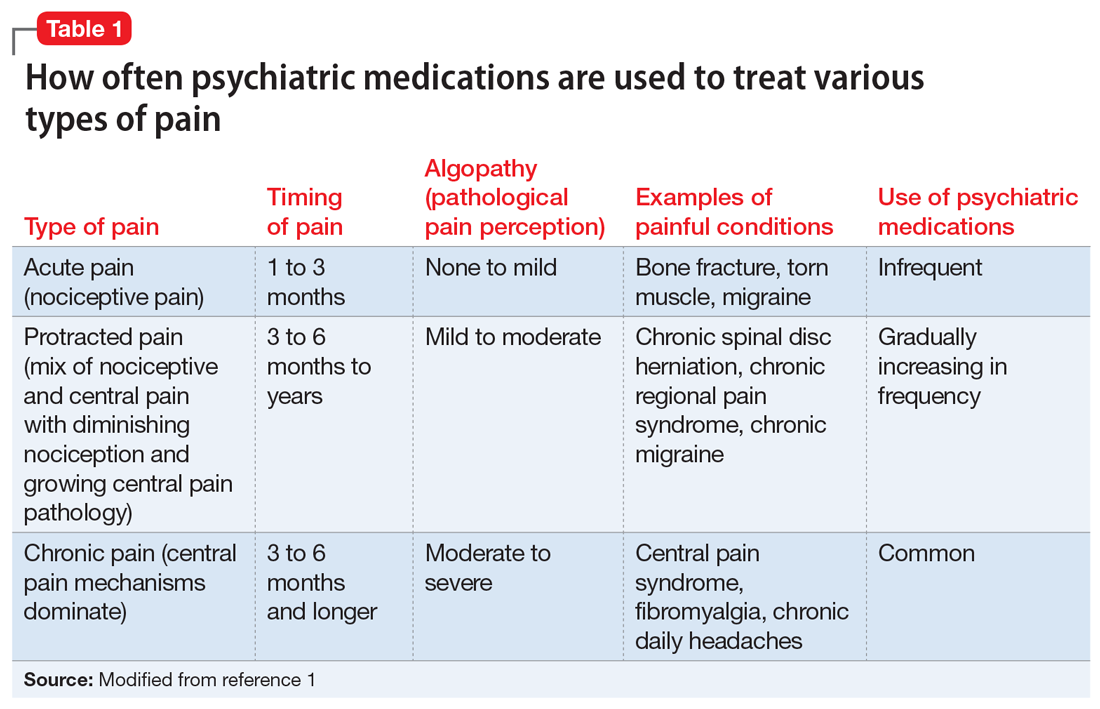
With the diminishing emphasis on opioid medications, researchers are exploring other pharmacologic modalities for treating pain. Many nonopioid psychiatric medications are used off-label for the treatment of pain. Psychiatric medications play a larger role in the management of pain as pain becomes more chronic (Table 11). For simplicity, acute pain may be seen as nociception colored by emotions, and chronic pain as emotions colored by nociception. Protracted pain connects those extremes with a diminishing role of nociception and an increasing role of emotion,1 which may increase the potential role of psychiatric medications, including antipsychotics.
In this article, I discuss the potential role of dopamine in the perception of pain, and review the potential use of first- and second-generation antipsychotics for treating various pain syndromes.
Role of dopamine in pain
There is increasing interest in exploring antipsychotics to treat chronic pain2 because dopamine dysfunction is part of pathological pain perception. Excess dopamine is associated with headaches (dopamine hypersensitivity hypothesis3,4) and dopamine dysfunction is a part of posttraumatic stress disorder (PTSD),5 dissociation,6 paranoia,7 and catastrophizing.8 Somatic psychosis, like any psychosis, can be based on dopamine pathology. Dopaminergic neurons affect nociceptive function in the spinal dorsal horn,9 and dopamine receptors are altered in atypical facial pain,10 burning mouth syndrome,11 and fibromyalgia.12
In normal circumstances, dopamine is fundamentally a protective neurotransmitter. In acute pain, dopamine is powerfully released, making the pain bearable. A patient may describe acute pain as seeming “like it was not happening to me” or “it was like a dream”; both are examples of dopamine-caused dissociation and a possible prediction of subsequent chronification. In chronic pain, pathological mechanisms settle in and take root; therefore, keeping protective dopamine levels high becomes a priority. This is especially common in patients who have experienced abuse or PTSD. The only natural way to keep dopamine up for prolonged periods of time is to decrease pain and stress thresholds. Both phenomena are readily observed in patients with pain. In extreme cases, self-mutilation and involvement in conflicts become pathologically gratifying.
The dopaminergic system is essential for pain control with a tissue injury.13 It becomes pathologically stimulated and increasingly dysfunctional as algopathy (a pathological pain perception) develops. At the same time, a flood or drought of any neurotransmitter is equally bad and may produce similar clinical pictures. Both a lack of and excess of dopamine are associated with pain.14 This is why opposite treatments may be beneficial in different patients with chronic pain. As an example, the use of stimulants15 and bupropion16 has been reported in the treatment of abdominal pain. And, reversely, antipsychotics, especially first-generation agents, may be associated with chronic (tardive) pain, including orofacial and genital pain.17
First-generation antipsychotics
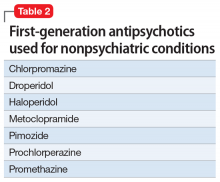
First-generation antipsychotics (FGAs) have been used to treat various nonpsychiatric conditions (Table 2). Although they are powerful D2 receptor inhibitors, FGAs lack the intrinsic ability to counteract the unwanted adverse effects of strong inhibition. As a result, movement disorders and prolactinemia are commonly induced by FGAs. The most dangerous consequence of treatment with these agents is neuroleptic malignant syndrome (NMS).
Continue to: Haloperidol
Haloperidol is prescribed widely by nonpsychiatrists, primarily to treat agitation. Intravenous haloperidol has been used for the abortive treatment of headaches.18 Paradoxically, IV haloperidol is less likely to induce extrapyramidal symptoms (EPS) than the oral formulation because of a more pronounced anticholinergic action in IV use. Haloperidol can help relieve gastroparesis and nausea, especially in IV administration,19 but prolonged oral administration is associated with unwanted movement problems and should be avoided.20
Chlorpromazine is more anticholinergic than haloperidol. It can be used in the abortive treatment of headaches (preferably via IV and IM administration), nausea, hiccups, porphyria, and serotonin syndrome, but it is very sedating and frequently produces hypotension, dangerous QT prolongation, and sensations of thought-blocking.21
Pimozide is reported to help with skin picking, trichotillomania, and somatic hallucinations.22
Droperidol, promethazine, and prochlorperazine are used off-label to treat nausea and headaches. Primary care clinicians may not be aware that these commonly used medications are antipsychotics. Similar to other FGAs, these 3 agents may produce NMS and tardive dyskinesia (TD). The same applies to the prokinetic drug metoclopramide.
Second-generation antipsychotics
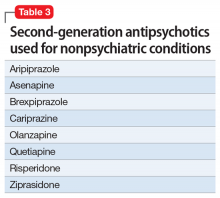
Second-generation antipsychotics (SGAs) work with various serotonin receptors, offsetting and enhancing the antipsychotic function of dopamine blockade. This diminishes but does not eliminate EPS and the risk of TD. Fortunately, the risk of NMS is lower with SGAs than with FGAs. Many SGAs are FDA-approved for treating schizophrenia and other psychiatric disorders, and some have relevance for pain management (Table 3). Many SGAs help with depressive symptoms and are powerful mood stabilizers. As such, they may diminish central over-firing of dopaminergic and serotonergic neurons involved in the pain cascade, which in turn decreases pain transmission and perception. The downside is that in general, SGAs increase the risk of diabetes and hyperlipidemia.
Continue to: Risperidone
Risperidone was the second FDA-approved SGA. Pain practitioners primarily prescribe it for treatmeant-resistant headaches, but patients with fibromyalgia and those with phantom and thalamic pain also may respond. Because risperidone’s properties are similar to that of many FGAs, it may potently cause EPS, TD, and prolactinemia. Neuroleptic malignant syndrome also has been reported.23
Ziprasidone is frequently overlooked by clinicians who treat pain. Although ziprasidone may be sedating, it is powerful as both a preventive and abortive (in an IM formulation) agent for treatment-resistant headaches. This might be attributed to its effects on the 5HT9 receptor. It is approved for treating bipolar depression and has been prescribed to effectively treat anxiety. For patients receiving ziprasidone, QT prolongation needs to be monitored closely.24
Olanzapine was modeled after clozapine and is effective as a mood stabilizer and an antianxiety, antipsychotic, and sleep-promoting medication. It has a useful “mellowing” effect and helps with central pain syndrome management. Patients with fibromyalgia respond well; in some cases, patients with phantom and thalamic pain also respond. Among SGAs prescribed to treat chronic pain, olanzapine has the most published studies. However, the downside is the risk of severe weight gain and diabetes. Usually, if a patient is already overweight, they gain less, but these patients typically are concerned about any additional weight gain.25
Aripiprazole is a partial dopamine agonist. It increases dopamine function in the prefrontal cortex, and by doing so it possibly improves cognition, mental acuity, goal-oriented activity, and attention. At the same time, it decreases dopamine activity in the basal ganglia and limbic system, improving catastrophizing, paranoia, abnormal pain perception, and multiple homeostasis functions. This combination of effects can be invaluable for some patients, but depending on individual susceptibility, aripiprazole might be too activating (causing agitation and akathisia) or too sedating.26
Brexpiprazole is a relative of aripiprazole, but for some patients it is better tolerated, and compliance with this medication usually is good. It partially antagonizes the D2 and 5HT1A receptors while antagonizing the 5HT2A receptors (which decreases the dopamine release in the striatum) and mimics the mechanism of action of an antidepressant. Through alpha-1-adrenergic receptor antagonism, it reduces EPS. All these effects are also part of the mechanisms of action of quetiapine, clozapine, and iloperidone, but brexpiprazole is considered to be the most alpha-1 antagonistic, which is a mechanism of action of other potential pain-controlling medications such as clonidine and tizanidine. In patients with pain who have an overactive noradrenergic system, this property may be beneficial. Its major problem stems from cytochrome P450 2D6 (CYP2D6) enzyme-dependent metabolism, which causes an approximately 5-fold increase in brexpiprazole blood level in poor CYP2D6 metabolizers. Therefore, combining brexpiprazole with CYP2D6 inhibitors such as fluoxetine, paroxetine, and duloxetine would be unwise. Aripiprazole and brexpiprazole are less associated with diabetes and sexual adverse effects than many other SGAs.27
Continue to: Asenapine
Asenapine is an underutilized antipsychotic. Its mechanism of action spans multiple receptors and is less specific in individual receptor activity than other dopamine blockers. It is administered under the tongue due to poor absorption when swallowed, and its molecule has an anesthetic property that causes mouth and tongue numbness/paresthesia. This function may help patients with orofacial pain. Significant somnolence and weight gain (although less than with olanzapine) limit its use. Some patients cannot tolerate the taste.28
Quetiapine is prescribed rather frequently due to its significant antianxiety effect. It is also reported to be beneficial in pain control.29 Weight gain may be severe. In doses smaller than typically administered to patients with bipolar disorder or schizophrenia, quetiapine is widely prescribed off-label for sleep. In lower doses, it acts primarily as an antihistamine (hence the sedation), but at an increased dose it activates the adrenergic system, which offsets sedation. Quetiapine antagonizes H1 histamine and 5HT2
Cariprazine is typically well tolerated because of its benign metabolic profile. It does not increase the QT interval and is not sedating. Cariprazine is a D2 and D3 partial receptor agonist. This allows the medication to inhibit overstimulated dopamine receptors (a desirable effect in pain management) and induces them when the endogenous dopamine level is low (helping with cognition, volition, and attention). Pro-cognitive effects are always beneficial for patients with pain. Cariprazine produces less EPS due to more ventral striatum vs dorsal striatum activity. Mood improvement caused by this medication is attributed to its 5HT2A, 5HT2B, and 5HT2C inverse agonism, which modulates the serotonergic system. Cariprazine will likely have a positive future in pain management because it has shown efficacy in the chronic stress model.33
A complex condition
No single medication or group of medications may be exclusively relied on for treating patients with chronic pain. Identifying alternatives to opioids for treating pain brings more attention to centrally-acting medications that may aid in the stabilization of the nervous system, which can decrease pathological pain perception and help patients cope with chronic painful conditions.
Bottom Line
Antipsychotics may be a valuable asset in the treatment of chronic pain, offering a potential alternative to prescribing opioids for pain. More research is needed to identify specific ways of using dopamine blockade or dopamine enhancement to help patients with chronic pain.
Continue to: Related Resource
Related Resource
- Tripathi A. Antipsychotics for migraines, cluster headaches, and nausea. Current Psychiatry. 2013;12(2):E1-E4.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Bupropion • Wellbutrin, Zyban
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Clonidine • Catapres
Clozapine • Clozaril
Droperidol • Inapsine
Duloxetine • Cymbalta
Fluoxetine • Prozac
Haloperidol • Haldol
Iloperidone • Fanapt
Metoclopramide • Reglan
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimozide • Orap
Prochlorperazine • Compazine
Promethazine • Phenergan
Quetiapine • Seroquel
Risperidone • Risperdal
Tizanidine • Zanaflex
Ziprasidone • Geodon
1. Arbuck D, Pergolizzi J. Algopathy—acknowledging the pathological process of pain chronification. Pract Pain Manag. 2017;17(4):4,26-32.
2. Shin SW, Lee JS, Abdi S, et al. Antipsychotics for patients with pain. Korean J Pain. 2019;32(1):3-11.
3. D’Andrea G, Leone M, Bussone G, et al. Abnormal tyrosine metabolism in chronic cluster headache. Cephalalgia. 2017;37(2):148-153.
4. D’Andrea G, Granella F, Perini F, et al. Platelet levels of dopamine are increased in migraine and cluster headache. Headache. 2006;46(4):585-591.
5. Wolf EJ, Mitchell KS, Logue MW, et al. The dopamine D3 receptor gene, and posttraumatic stress disorder. J Trauma Stress. 2014;27(4):379-387.
6. den Ouden HEM, Daw ND, Fernandez G, et al. Dissociable effects of dopamine and serotonin on reversal learning. Neuron. 2013;80(4):1090-1100.
7. Nour MM, Dahoun T, Schwartenbeck P, et al. Dopaminergic basis for signaling belief updates, but not surprise, and the link to paranoia. Proc Natl Acad Sci U S A. 2018;115(43):E10167-E10176.
8. Zhu H, Clemens S, Sawchuk M, et al. Expression and distribution of all dopamine receptor subtypes (D(1)-D(5)) in the mouse lumbar spinal cord: a real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience. 2007;149:885-897.
9. Wood PB, Schweinhardt P, Jaeger E, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576-3582.
10. Hagelberg N, Fossell H, Aalto S, et al. Altered dopamine D2 receptor binding in atypical facial pain. Pain. 2003;106(1-2):43-48.
11. Hagelberg N, Fossell H, Rinne JD, et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003;101(1-2):149-154.
12. Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron. 2016;89(1):11-36.
13. Siahposht-Khachaki A, Pourreza P, Ezzatpanah S, et al. Nucleus accumbens dopamine receptors mediate hypothalamus-induced antinociception in the rat formalin test. Eur J Pain. 2017;21(7):1285-1294.
14. Thompson T, Gallop K, Correll CU, et al. Pain perception in Parkinson’s disease: a systematic review and meta-analysis of experimental studies. Aging Res Rev. 2017;35:74-86.
15. Check JH. Chronic unremitting lower abdominal pain quickly abrogated following treatment with amphetamine. Clin Exp Obstet Gynecol. 2016;43(1):109-111.
16. Wilkes S. Bupropion. Drugs Today (Barc). 2006;42(10):671-681.
17. Frei K, Truong DD, Fahn S, et al. The nosology of tardive syndromes. J Neurol Sci. 2018;389:10-16.
18. Honkaniemi J, Liimatainen S, Rainesalo S, et al. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787.
19. Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;(11):CD006271.
20. Gaffigan ME, Bruner DI, Wason C, et al. A randomized controlled trial of intravenous haloperidol vs. intravenous metoclopramide for acute migraine therapy in the emergency department. J Emerg Med. 2015;49(3):326-334.
21. Weinman D, Nicastro O, Akala O, et al. Parenteral treatment of episodic tension-type headache: a systematic review. Headache. 2014;54(2):260-268.
22. Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology, and approaches to treatment. CNS Drugs. 2001;15(5):351-359.
23. Khouzam HR. Psychopharmacology of chronic pain: a focus on antidepressants and atypical antipsychotics. Postgrad Med. 2016;128(3):323-330.
24. Landsness EC, Wang LH, Bucelli RC. Ziprasidone as a potential abortive therapy for status migrainosus. Neurohospitalist. 2016;6(4):151-156.
25. Jimenez XF, Sundararajan T, Covington EC. A systematic review of atypical antipsychotics in chronic pain management: olanzapine demonstrates potential in central sensitization, fibromyalgia, and headache/migraine. Clin J Pain. 2018;34(6):585-591.
26. Fei L, Abrardi L, Mediati RD. Unexpected effect of aripiprazole on nociceptive pain. Ther Adv Psychopharmacol. 2012;2(5):211-212.
27. Markovic M, Gallipani A, Patel KH, et al. Brexpiprazole. Ann Pharmacother. 2017;51(4):315-322.
28. Gerrits M, de Greef R, Peeters P. Effect of absorption site on the pharmacokinetics of sublingual asenapine in healthy male subjects. Biopharm Drug Dispos. 2010;31(5-6):351-357.
29. Heo MH, Kim JY, Hwang I, et al. Analgesic effect of quetiapine in a mouse model of cancer-induced bone pain. Korean J Intern Med. 2017;32(6):1069-1074.
30. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Am Acad Psychiatry Law. 2012;40(4):502-508.
31. Fountoulakis KN, Iacovides A, Kaprinis SG, et al. Diffuse muscle pain with quetiapine. Br J Psychiatry. 2003;182:81.
32. Shintani F. Diminished pain perception in schizophrenia. Lancet. 2010;376(9735):87.
33. Duric V, Banasr M, Franklin T, et al. Cariprazine exhibits anxiolytic and dopamine D3 receptor-dependent antidepressant effects in the chronic stress model. Int J Neuropsychopharmacol. 2017;20(10):788-796
Our understanding of pain mechanisms continues to evolve and, accordingly, so do our treatment strategies. The fundamental differences between acute and chronic pain were only recently recognized; this lack of recognition led to the application of acute pain treatments to chronic pain, contributing to the opioid epidemic in the United States.
With the diminishing emphasis on opioid medications, researchers are exploring other pharmacologic modalities for treating pain. Many nonopioid psychiatric medications are used off-label for the treatment of pain. Psychiatric medications play a larger role in the management of pain as pain becomes more chronic (Table 11). For simplicity, acute pain may be seen as nociception colored by emotions, and chronic pain as emotions colored by nociception. Protracted pain connects those extremes with a diminishing role of nociception and an increasing role of emotion,1 which may increase the potential role of psychiatric medications, including antipsychotics.
In this article, I discuss the potential role of dopamine in the perception of pain, and review the potential use of first- and second-generation antipsychotics for treating various pain syndromes.
Role of dopamine in pain
There is increasing interest in exploring antipsychotics to treat chronic pain2 because dopamine dysfunction is part of pathological pain perception. Excess dopamine is associated with headaches (dopamine hypersensitivity hypothesis3,4) and dopamine dysfunction is a part of posttraumatic stress disorder (PTSD),5 dissociation,6 paranoia,7 and catastrophizing.8 Somatic psychosis, like any psychosis, can be based on dopamine pathology. Dopaminergic neurons affect nociceptive function in the spinal dorsal horn,9 and dopamine receptors are altered in atypical facial pain,10 burning mouth syndrome,11 and fibromyalgia.12
In normal circumstances, dopamine is fundamentally a protective neurotransmitter. In acute pain, dopamine is powerfully released, making the pain bearable. A patient may describe acute pain as seeming “like it was not happening to me” or “it was like a dream”; both are examples of dopamine-caused dissociation and a possible prediction of subsequent chronification. In chronic pain, pathological mechanisms settle in and take root; therefore, keeping protective dopamine levels high becomes a priority. This is especially common in patients who have experienced abuse or PTSD. The only natural way to keep dopamine up for prolonged periods of time is to decrease pain and stress thresholds. Both phenomena are readily observed in patients with pain. In extreme cases, self-mutilation and involvement in conflicts become pathologically gratifying.
The dopaminergic system is essential for pain control with a tissue injury.13 It becomes pathologically stimulated and increasingly dysfunctional as algopathy (a pathological pain perception) develops. At the same time, a flood or drought of any neurotransmitter is equally bad and may produce similar clinical pictures. Both a lack of and excess of dopamine are associated with pain.14 This is why opposite treatments may be beneficial in different patients with chronic pain. As an example, the use of stimulants15 and bupropion16 has been reported in the treatment of abdominal pain. And, reversely, antipsychotics, especially first-generation agents, may be associated with chronic (tardive) pain, including orofacial and genital pain.17
First-generation antipsychotics
First-generation antipsychotics (FGAs) have been used to treat various nonpsychiatric conditions (Table 2). Although they are powerful D2 receptor inhibitors, FGAs lack the intrinsic ability to counteract the unwanted adverse effects of strong inhibition. As a result, movement disorders and prolactinemia are commonly induced by FGAs. The most dangerous consequence of treatment with these agents is neuroleptic malignant syndrome (NMS).
Continue to: Haloperidol
Haloperidol is prescribed widely by nonpsychiatrists, primarily to treat agitation. Intravenous haloperidol has been used for the abortive treatment of headaches.18 Paradoxically, IV haloperidol is less likely to induce extrapyramidal symptoms (EPS) than the oral formulation because of a more pronounced anticholinergic action in IV use. Haloperidol can help relieve gastroparesis and nausea, especially in IV administration,19 but prolonged oral administration is associated with unwanted movement problems and should be avoided.20
Chlorpromazine is more anticholinergic than haloperidol. It can be used in the abortive treatment of headaches (preferably via IV and IM administration), nausea, hiccups, porphyria, and serotonin syndrome, but it is very sedating and frequently produces hypotension, dangerous QT prolongation, and sensations of thought-blocking.21
Pimozide is reported to help with skin picking, trichotillomania, and somatic hallucinations.22
Droperidol, promethazine, and prochlorperazine are used off-label to treat nausea and headaches. Primary care clinicians may not be aware that these commonly used medications are antipsychotics. Similar to other FGAs, these 3 agents may produce NMS and tardive dyskinesia (TD). The same applies to the prokinetic drug metoclopramide.
Second-generation antipsychotics
Second-generation antipsychotics (SGAs) work with various serotonin receptors, offsetting and enhancing the antipsychotic function of dopamine blockade. This diminishes but does not eliminate EPS and the risk of TD. Fortunately, the risk of NMS is lower with SGAs than with FGAs. Many SGAs are FDA-approved for treating schizophrenia and other psychiatric disorders, and some have relevance for pain management (Table 3). Many SGAs help with depressive symptoms and are powerful mood stabilizers. As such, they may diminish central over-firing of dopaminergic and serotonergic neurons involved in the pain cascade, which in turn decreases pain transmission and perception. The downside is that in general, SGAs increase the risk of diabetes and hyperlipidemia.
Continue to: Risperidone
Risperidone was the second FDA-approved SGA. Pain practitioners primarily prescribe it for treatmeant-resistant headaches, but patients with fibromyalgia and those with phantom and thalamic pain also may respond. Because risperidone’s properties are similar to that of many FGAs, it may potently cause EPS, TD, and prolactinemia. Neuroleptic malignant syndrome also has been reported.23
Ziprasidone is frequently overlooked by clinicians who treat pain. Although ziprasidone may be sedating, it is powerful as both a preventive and abortive (in an IM formulation) agent for treatment-resistant headaches. This might be attributed to its effects on the 5HT9 receptor. It is approved for treating bipolar depression and has been prescribed to effectively treat anxiety. For patients receiving ziprasidone, QT prolongation needs to be monitored closely.24
Olanzapine was modeled after clozapine and is effective as a mood stabilizer and an antianxiety, antipsychotic, and sleep-promoting medication. It has a useful “mellowing” effect and helps with central pain syndrome management. Patients with fibromyalgia respond well; in some cases, patients with phantom and thalamic pain also respond. Among SGAs prescribed to treat chronic pain, olanzapine has the most published studies. However, the downside is the risk of severe weight gain and diabetes. Usually, if a patient is already overweight, they gain less, but these patients typically are concerned about any additional weight gain.25
Aripiprazole is a partial dopamine agonist. It increases dopamine function in the prefrontal cortex, and by doing so it possibly improves cognition, mental acuity, goal-oriented activity, and attention. At the same time, it decreases dopamine activity in the basal ganglia and limbic system, improving catastrophizing, paranoia, abnormal pain perception, and multiple homeostasis functions. This combination of effects can be invaluable for some patients, but depending on individual susceptibility, aripiprazole might be too activating (causing agitation and akathisia) or too sedating.26
Brexpiprazole is a relative of aripiprazole, but for some patients it is better tolerated, and compliance with this medication usually is good. It partially antagonizes the D2 and 5HT1A receptors while antagonizing the 5HT2A receptors (which decreases the dopamine release in the striatum) and mimics the mechanism of action of an antidepressant. Through alpha-1-adrenergic receptor antagonism, it reduces EPS. All these effects are also part of the mechanisms of action of quetiapine, clozapine, and iloperidone, but brexpiprazole is considered to be the most alpha-1 antagonistic, which is a mechanism of action of other potential pain-controlling medications such as clonidine and tizanidine. In patients with pain who have an overactive noradrenergic system, this property may be beneficial. Its major problem stems from cytochrome P450 2D6 (CYP2D6) enzyme-dependent metabolism, which causes an approximately 5-fold increase in brexpiprazole blood level in poor CYP2D6 metabolizers. Therefore, combining brexpiprazole with CYP2D6 inhibitors such as fluoxetine, paroxetine, and duloxetine would be unwise. Aripiprazole and brexpiprazole are less associated with diabetes and sexual adverse effects than many other SGAs.27
Continue to: Asenapine
Asenapine is an underutilized antipsychotic. Its mechanism of action spans multiple receptors and is less specific in individual receptor activity than other dopamine blockers. It is administered under the tongue due to poor absorption when swallowed, and its molecule has an anesthetic property that causes mouth and tongue numbness/paresthesia. This function may help patients with orofacial pain. Significant somnolence and weight gain (although less than with olanzapine) limit its use. Some patients cannot tolerate the taste.28
Quetiapine is prescribed rather frequently due to its significant antianxiety effect. It is also reported to be beneficial in pain control.29 Weight gain may be severe. In doses smaller than typically administered to patients with bipolar disorder or schizophrenia, quetiapine is widely prescribed off-label for sleep. In lower doses, it acts primarily as an antihistamine (hence the sedation), but at an increased dose it activates the adrenergic system, which offsets sedation. Quetiapine antagonizes H1 histamine and 5HT2
Cariprazine is typically well tolerated because of its benign metabolic profile. It does not increase the QT interval and is not sedating. Cariprazine is a D2 and D3 partial receptor agonist. This allows the medication to inhibit overstimulated dopamine receptors (a desirable effect in pain management) and induces them when the endogenous dopamine level is low (helping with cognition, volition, and attention). Pro-cognitive effects are always beneficial for patients with pain. Cariprazine produces less EPS due to more ventral striatum vs dorsal striatum activity. Mood improvement caused by this medication is attributed to its 5HT2A, 5HT2B, and 5HT2C inverse agonism, which modulates the serotonergic system. Cariprazine will likely have a positive future in pain management because it has shown efficacy in the chronic stress model.33
A complex condition
No single medication or group of medications may be exclusively relied on for treating patients with chronic pain. Identifying alternatives to opioids for treating pain brings more attention to centrally-acting medications that may aid in the stabilization of the nervous system, which can decrease pathological pain perception and help patients cope with chronic painful conditions.
Bottom Line
Antipsychotics may be a valuable asset in the treatment of chronic pain, offering a potential alternative to prescribing opioids for pain. More research is needed to identify specific ways of using dopamine blockade or dopamine enhancement to help patients with chronic pain.
Continue to: Related Resource
Related Resource
- Tripathi A. Antipsychotics for migraines, cluster headaches, and nausea. Current Psychiatry. 2013;12(2):E1-E4.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Bupropion • Wellbutrin, Zyban
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Clonidine • Catapres
Clozapine • Clozaril
Droperidol • Inapsine
Duloxetine • Cymbalta
Fluoxetine • Prozac
Haloperidol • Haldol
Iloperidone • Fanapt
Metoclopramide • Reglan
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimozide • Orap
Prochlorperazine • Compazine
Promethazine • Phenergan
Quetiapine • Seroquel
Risperidone • Risperdal
Tizanidine • Zanaflex
Ziprasidone • Geodon
Our understanding of pain mechanisms continues to evolve and, accordingly, so do our treatment strategies. The fundamental differences between acute and chronic pain were only recently recognized; this lack of recognition led to the application of acute pain treatments to chronic pain, contributing to the opioid epidemic in the United States.
With the diminishing emphasis on opioid medications, researchers are exploring other pharmacologic modalities for treating pain. Many nonopioid psychiatric medications are used off-label for the treatment of pain. Psychiatric medications play a larger role in the management of pain as pain becomes more chronic (Table 11). For simplicity, acute pain may be seen as nociception colored by emotions, and chronic pain as emotions colored by nociception. Protracted pain connects those extremes with a diminishing role of nociception and an increasing role of emotion,1 which may increase the potential role of psychiatric medications, including antipsychotics.
In this article, I discuss the potential role of dopamine in the perception of pain, and review the potential use of first- and second-generation antipsychotics for treating various pain syndromes.
Role of dopamine in pain
There is increasing interest in exploring antipsychotics to treat chronic pain2 because dopamine dysfunction is part of pathological pain perception. Excess dopamine is associated with headaches (dopamine hypersensitivity hypothesis3,4) and dopamine dysfunction is a part of posttraumatic stress disorder (PTSD),5 dissociation,6 paranoia,7 and catastrophizing.8 Somatic psychosis, like any psychosis, can be based on dopamine pathology. Dopaminergic neurons affect nociceptive function in the spinal dorsal horn,9 and dopamine receptors are altered in atypical facial pain,10 burning mouth syndrome,11 and fibromyalgia.12
In normal circumstances, dopamine is fundamentally a protective neurotransmitter. In acute pain, dopamine is powerfully released, making the pain bearable. A patient may describe acute pain as seeming “like it was not happening to me” or “it was like a dream”; both are examples of dopamine-caused dissociation and a possible prediction of subsequent chronification. In chronic pain, pathological mechanisms settle in and take root; therefore, keeping protective dopamine levels high becomes a priority. This is especially common in patients who have experienced abuse or PTSD. The only natural way to keep dopamine up for prolonged periods of time is to decrease pain and stress thresholds. Both phenomena are readily observed in patients with pain. In extreme cases, self-mutilation and involvement in conflicts become pathologically gratifying.
The dopaminergic system is essential for pain control with a tissue injury.13 It becomes pathologically stimulated and increasingly dysfunctional as algopathy (a pathological pain perception) develops. At the same time, a flood or drought of any neurotransmitter is equally bad and may produce similar clinical pictures. Both a lack of and excess of dopamine are associated with pain.14 This is why opposite treatments may be beneficial in different patients with chronic pain. As an example, the use of stimulants15 and bupropion16 has been reported in the treatment of abdominal pain. And, reversely, antipsychotics, especially first-generation agents, may be associated with chronic (tardive) pain, including orofacial and genital pain.17
First-generation antipsychotics
First-generation antipsychotics (FGAs) have been used to treat various nonpsychiatric conditions (Table 2). Although they are powerful D2 receptor inhibitors, FGAs lack the intrinsic ability to counteract the unwanted adverse effects of strong inhibition. As a result, movement disorders and prolactinemia are commonly induced by FGAs. The most dangerous consequence of treatment with these agents is neuroleptic malignant syndrome (NMS).
Continue to: Haloperidol
Haloperidol is prescribed widely by nonpsychiatrists, primarily to treat agitation. Intravenous haloperidol has been used for the abortive treatment of headaches.18 Paradoxically, IV haloperidol is less likely to induce extrapyramidal symptoms (EPS) than the oral formulation because of a more pronounced anticholinergic action in IV use. Haloperidol can help relieve gastroparesis and nausea, especially in IV administration,19 but prolonged oral administration is associated with unwanted movement problems and should be avoided.20
Chlorpromazine is more anticholinergic than haloperidol. It can be used in the abortive treatment of headaches (preferably via IV and IM administration), nausea, hiccups, porphyria, and serotonin syndrome, but it is very sedating and frequently produces hypotension, dangerous QT prolongation, and sensations of thought-blocking.21
Pimozide is reported to help with skin picking, trichotillomania, and somatic hallucinations.22
Droperidol, promethazine, and prochlorperazine are used off-label to treat nausea and headaches. Primary care clinicians may not be aware that these commonly used medications are antipsychotics. Similar to other FGAs, these 3 agents may produce NMS and tardive dyskinesia (TD). The same applies to the prokinetic drug metoclopramide.
Second-generation antipsychotics
Second-generation antipsychotics (SGAs) work with various serotonin receptors, offsetting and enhancing the antipsychotic function of dopamine blockade. This diminishes but does not eliminate EPS and the risk of TD. Fortunately, the risk of NMS is lower with SGAs than with FGAs. Many SGAs are FDA-approved for treating schizophrenia and other psychiatric disorders, and some have relevance for pain management (Table 3). Many SGAs help with depressive symptoms and are powerful mood stabilizers. As such, they may diminish central over-firing of dopaminergic and serotonergic neurons involved in the pain cascade, which in turn decreases pain transmission and perception. The downside is that in general, SGAs increase the risk of diabetes and hyperlipidemia.
Continue to: Risperidone
Risperidone was the second FDA-approved SGA. Pain practitioners primarily prescribe it for treatmeant-resistant headaches, but patients with fibromyalgia and those with phantom and thalamic pain also may respond. Because risperidone’s properties are similar to that of many FGAs, it may potently cause EPS, TD, and prolactinemia. Neuroleptic malignant syndrome also has been reported.23
Ziprasidone is frequently overlooked by clinicians who treat pain. Although ziprasidone may be sedating, it is powerful as both a preventive and abortive (in an IM formulation) agent for treatment-resistant headaches. This might be attributed to its effects on the 5HT9 receptor. It is approved for treating bipolar depression and has been prescribed to effectively treat anxiety. For patients receiving ziprasidone, QT prolongation needs to be monitored closely.24
Olanzapine was modeled after clozapine and is effective as a mood stabilizer and an antianxiety, antipsychotic, and sleep-promoting medication. It has a useful “mellowing” effect and helps with central pain syndrome management. Patients with fibromyalgia respond well; in some cases, patients with phantom and thalamic pain also respond. Among SGAs prescribed to treat chronic pain, olanzapine has the most published studies. However, the downside is the risk of severe weight gain and diabetes. Usually, if a patient is already overweight, they gain less, but these patients typically are concerned about any additional weight gain.25
Aripiprazole is a partial dopamine agonist. It increases dopamine function in the prefrontal cortex, and by doing so it possibly improves cognition, mental acuity, goal-oriented activity, and attention. At the same time, it decreases dopamine activity in the basal ganglia and limbic system, improving catastrophizing, paranoia, abnormal pain perception, and multiple homeostasis functions. This combination of effects can be invaluable for some patients, but depending on individual susceptibility, aripiprazole might be too activating (causing agitation and akathisia) or too sedating.26
Brexpiprazole is a relative of aripiprazole, but for some patients it is better tolerated, and compliance with this medication usually is good. It partially antagonizes the D2 and 5HT1A receptors while antagonizing the 5HT2A receptors (which decreases the dopamine release in the striatum) and mimics the mechanism of action of an antidepressant. Through alpha-1-adrenergic receptor antagonism, it reduces EPS. All these effects are also part of the mechanisms of action of quetiapine, clozapine, and iloperidone, but brexpiprazole is considered to be the most alpha-1 antagonistic, which is a mechanism of action of other potential pain-controlling medications such as clonidine and tizanidine. In patients with pain who have an overactive noradrenergic system, this property may be beneficial. Its major problem stems from cytochrome P450 2D6 (CYP2D6) enzyme-dependent metabolism, which causes an approximately 5-fold increase in brexpiprazole blood level in poor CYP2D6 metabolizers. Therefore, combining brexpiprazole with CYP2D6 inhibitors such as fluoxetine, paroxetine, and duloxetine would be unwise. Aripiprazole and brexpiprazole are less associated with diabetes and sexual adverse effects than many other SGAs.27
Continue to: Asenapine
Asenapine is an underutilized antipsychotic. Its mechanism of action spans multiple receptors and is less specific in individual receptor activity than other dopamine blockers. It is administered under the tongue due to poor absorption when swallowed, and its molecule has an anesthetic property that causes mouth and tongue numbness/paresthesia. This function may help patients with orofacial pain. Significant somnolence and weight gain (although less than with olanzapine) limit its use. Some patients cannot tolerate the taste.28
Quetiapine is prescribed rather frequently due to its significant antianxiety effect. It is also reported to be beneficial in pain control.29 Weight gain may be severe. In doses smaller than typically administered to patients with bipolar disorder or schizophrenia, quetiapine is widely prescribed off-label for sleep. In lower doses, it acts primarily as an antihistamine (hence the sedation), but at an increased dose it activates the adrenergic system, which offsets sedation. Quetiapine antagonizes H1 histamine and 5HT2
Cariprazine is typically well tolerated because of its benign metabolic profile. It does not increase the QT interval and is not sedating. Cariprazine is a D2 and D3 partial receptor agonist. This allows the medication to inhibit overstimulated dopamine receptors (a desirable effect in pain management) and induces them when the endogenous dopamine level is low (helping with cognition, volition, and attention). Pro-cognitive effects are always beneficial for patients with pain. Cariprazine produces less EPS due to more ventral striatum vs dorsal striatum activity. Mood improvement caused by this medication is attributed to its 5HT2A, 5HT2B, and 5HT2C inverse agonism, which modulates the serotonergic system. Cariprazine will likely have a positive future in pain management because it has shown efficacy in the chronic stress model.33
A complex condition
No single medication or group of medications may be exclusively relied on for treating patients with chronic pain. Identifying alternatives to opioids for treating pain brings more attention to centrally-acting medications that may aid in the stabilization of the nervous system, which can decrease pathological pain perception and help patients cope with chronic painful conditions.
Bottom Line
Antipsychotics may be a valuable asset in the treatment of chronic pain, offering a potential alternative to prescribing opioids for pain. More research is needed to identify specific ways of using dopamine blockade or dopamine enhancement to help patients with chronic pain.
Continue to: Related Resource
Related Resource
- Tripathi A. Antipsychotics for migraines, cluster headaches, and nausea. Current Psychiatry. 2013;12(2):E1-E4.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Bupropion • Wellbutrin, Zyban
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Clonidine • Catapres
Clozapine • Clozaril
Droperidol • Inapsine
Duloxetine • Cymbalta
Fluoxetine • Prozac
Haloperidol • Haldol
Iloperidone • Fanapt
Metoclopramide • Reglan
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimozide • Orap
Prochlorperazine • Compazine
Promethazine • Phenergan
Quetiapine • Seroquel
Risperidone • Risperdal
Tizanidine • Zanaflex
Ziprasidone • Geodon
1. Arbuck D, Pergolizzi J. Algopathy—acknowledging the pathological process of pain chronification. Pract Pain Manag. 2017;17(4):4,26-32.
2. Shin SW, Lee JS, Abdi S, et al. Antipsychotics for patients with pain. Korean J Pain. 2019;32(1):3-11.
3. D’Andrea G, Leone M, Bussone G, et al. Abnormal tyrosine metabolism in chronic cluster headache. Cephalalgia. 2017;37(2):148-153.
4. D’Andrea G, Granella F, Perini F, et al. Platelet levels of dopamine are increased in migraine and cluster headache. Headache. 2006;46(4):585-591.
5. Wolf EJ, Mitchell KS, Logue MW, et al. The dopamine D3 receptor gene, and posttraumatic stress disorder. J Trauma Stress. 2014;27(4):379-387.
6. den Ouden HEM, Daw ND, Fernandez G, et al. Dissociable effects of dopamine and serotonin on reversal learning. Neuron. 2013;80(4):1090-1100.
7. Nour MM, Dahoun T, Schwartenbeck P, et al. Dopaminergic basis for signaling belief updates, but not surprise, and the link to paranoia. Proc Natl Acad Sci U S A. 2018;115(43):E10167-E10176.
8. Zhu H, Clemens S, Sawchuk M, et al. Expression and distribution of all dopamine receptor subtypes (D(1)-D(5)) in the mouse lumbar spinal cord: a real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience. 2007;149:885-897.
9. Wood PB, Schweinhardt P, Jaeger E, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576-3582.
10. Hagelberg N, Fossell H, Aalto S, et al. Altered dopamine D2 receptor binding in atypical facial pain. Pain. 2003;106(1-2):43-48.
11. Hagelberg N, Fossell H, Rinne JD, et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003;101(1-2):149-154.
12. Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron. 2016;89(1):11-36.
13. Siahposht-Khachaki A, Pourreza P, Ezzatpanah S, et al. Nucleus accumbens dopamine receptors mediate hypothalamus-induced antinociception in the rat formalin test. Eur J Pain. 2017;21(7):1285-1294.
14. Thompson T, Gallop K, Correll CU, et al. Pain perception in Parkinson’s disease: a systematic review and meta-analysis of experimental studies. Aging Res Rev. 2017;35:74-86.
15. Check JH. Chronic unremitting lower abdominal pain quickly abrogated following treatment with amphetamine. Clin Exp Obstet Gynecol. 2016;43(1):109-111.
16. Wilkes S. Bupropion. Drugs Today (Barc). 2006;42(10):671-681.
17. Frei K, Truong DD, Fahn S, et al. The nosology of tardive syndromes. J Neurol Sci. 2018;389:10-16.
18. Honkaniemi J, Liimatainen S, Rainesalo S, et al. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787.
19. Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;(11):CD006271.
20. Gaffigan ME, Bruner DI, Wason C, et al. A randomized controlled trial of intravenous haloperidol vs. intravenous metoclopramide for acute migraine therapy in the emergency department. J Emerg Med. 2015;49(3):326-334.
21. Weinman D, Nicastro O, Akala O, et al. Parenteral treatment of episodic tension-type headache: a systematic review. Headache. 2014;54(2):260-268.
22. Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology, and approaches to treatment. CNS Drugs. 2001;15(5):351-359.
23. Khouzam HR. Psychopharmacology of chronic pain: a focus on antidepressants and atypical antipsychotics. Postgrad Med. 2016;128(3):323-330.
24. Landsness EC, Wang LH, Bucelli RC. Ziprasidone as a potential abortive therapy for status migrainosus. Neurohospitalist. 2016;6(4):151-156.
25. Jimenez XF, Sundararajan T, Covington EC. A systematic review of atypical antipsychotics in chronic pain management: olanzapine demonstrates potential in central sensitization, fibromyalgia, and headache/migraine. Clin J Pain. 2018;34(6):585-591.
26. Fei L, Abrardi L, Mediati RD. Unexpected effect of aripiprazole on nociceptive pain. Ther Adv Psychopharmacol. 2012;2(5):211-212.
27. Markovic M, Gallipani A, Patel KH, et al. Brexpiprazole. Ann Pharmacother. 2017;51(4):315-322.
28. Gerrits M, de Greef R, Peeters P. Effect of absorption site on the pharmacokinetics of sublingual asenapine in healthy male subjects. Biopharm Drug Dispos. 2010;31(5-6):351-357.
29. Heo MH, Kim JY, Hwang I, et al. Analgesic effect of quetiapine in a mouse model of cancer-induced bone pain. Korean J Intern Med. 2017;32(6):1069-1074.
30. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Am Acad Psychiatry Law. 2012;40(4):502-508.
31. Fountoulakis KN, Iacovides A, Kaprinis SG, et al. Diffuse muscle pain with quetiapine. Br J Psychiatry. 2003;182:81.
32. Shintani F. Diminished pain perception in schizophrenia. Lancet. 2010;376(9735):87.
33. Duric V, Banasr M, Franklin T, et al. Cariprazine exhibits anxiolytic and dopamine D3 receptor-dependent antidepressant effects in the chronic stress model. Int J Neuropsychopharmacol. 2017;20(10):788-796
1. Arbuck D, Pergolizzi J. Algopathy—acknowledging the pathological process of pain chronification. Pract Pain Manag. 2017;17(4):4,26-32.
2. Shin SW, Lee JS, Abdi S, et al. Antipsychotics for patients with pain. Korean J Pain. 2019;32(1):3-11.
3. D’Andrea G, Leone M, Bussone G, et al. Abnormal tyrosine metabolism in chronic cluster headache. Cephalalgia. 2017;37(2):148-153.
4. D’Andrea G, Granella F, Perini F, et al. Platelet levels of dopamine are increased in migraine and cluster headache. Headache. 2006;46(4):585-591.
5. Wolf EJ, Mitchell KS, Logue MW, et al. The dopamine D3 receptor gene, and posttraumatic stress disorder. J Trauma Stress. 2014;27(4):379-387.
6. den Ouden HEM, Daw ND, Fernandez G, et al. Dissociable effects of dopamine and serotonin on reversal learning. Neuron. 2013;80(4):1090-1100.
7. Nour MM, Dahoun T, Schwartenbeck P, et al. Dopaminergic basis for signaling belief updates, but not surprise, and the link to paranoia. Proc Natl Acad Sci U S A. 2018;115(43):E10167-E10176.
8. Zhu H, Clemens S, Sawchuk M, et al. Expression and distribution of all dopamine receptor subtypes (D(1)-D(5)) in the mouse lumbar spinal cord: a real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience. 2007;149:885-897.
9. Wood PB, Schweinhardt P, Jaeger E, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576-3582.
10. Hagelberg N, Fossell H, Aalto S, et al. Altered dopamine D2 receptor binding in atypical facial pain. Pain. 2003;106(1-2):43-48.
11. Hagelberg N, Fossell H, Rinne JD, et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003;101(1-2):149-154.
12. Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron. 2016;89(1):11-36.
13. Siahposht-Khachaki A, Pourreza P, Ezzatpanah S, et al. Nucleus accumbens dopamine receptors mediate hypothalamus-induced antinociception in the rat formalin test. Eur J Pain. 2017;21(7):1285-1294.
14. Thompson T, Gallop K, Correll CU, et al. Pain perception in Parkinson’s disease: a systematic review and meta-analysis of experimental studies. Aging Res Rev. 2017;35:74-86.
15. Check JH. Chronic unremitting lower abdominal pain quickly abrogated following treatment with amphetamine. Clin Exp Obstet Gynecol. 2016;43(1):109-111.
16. Wilkes S. Bupropion. Drugs Today (Barc). 2006;42(10):671-681.
17. Frei K, Truong DD, Fahn S, et al. The nosology of tardive syndromes. J Neurol Sci. 2018;389:10-16.
18. Honkaniemi J, Liimatainen S, Rainesalo S, et al. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787.
19. Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;(11):CD006271.
20. Gaffigan ME, Bruner DI, Wason C, et al. A randomized controlled trial of intravenous haloperidol vs. intravenous metoclopramide for acute migraine therapy in the emergency department. J Emerg Med. 2015;49(3):326-334.
21. Weinman D, Nicastro O, Akala O, et al. Parenteral treatment of episodic tension-type headache: a systematic review. Headache. 2014;54(2):260-268.
22. Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology, and approaches to treatment. CNS Drugs. 2001;15(5):351-359.
23. Khouzam HR. Psychopharmacology of chronic pain: a focus on antidepressants and atypical antipsychotics. Postgrad Med. 2016;128(3):323-330.
24. Landsness EC, Wang LH, Bucelli RC. Ziprasidone as a potential abortive therapy for status migrainosus. Neurohospitalist. 2016;6(4):151-156.
25. Jimenez XF, Sundararajan T, Covington EC. A systematic review of atypical antipsychotics in chronic pain management: olanzapine demonstrates potential in central sensitization, fibromyalgia, and headache/migraine. Clin J Pain. 2018;34(6):585-591.
26. Fei L, Abrardi L, Mediati RD. Unexpected effect of aripiprazole on nociceptive pain. Ther Adv Psychopharmacol. 2012;2(5):211-212.
27. Markovic M, Gallipani A, Patel KH, et al. Brexpiprazole. Ann Pharmacother. 2017;51(4):315-322.
28. Gerrits M, de Greef R, Peeters P. Effect of absorption site on the pharmacokinetics of sublingual asenapine in healthy male subjects. Biopharm Drug Dispos. 2010;31(5-6):351-357.
29. Heo MH, Kim JY, Hwang I, et al. Analgesic effect of quetiapine in a mouse model of cancer-induced bone pain. Korean J Intern Med. 2017;32(6):1069-1074.
30. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Am Acad Psychiatry Law. 2012;40(4):502-508.
31. Fountoulakis KN, Iacovides A, Kaprinis SG, et al. Diffuse muscle pain with quetiapine. Br J Psychiatry. 2003;182:81.
32. Shintani F. Diminished pain perception in schizophrenia. Lancet. 2010;376(9735):87.
33. Duric V, Banasr M, Franklin T, et al. Cariprazine exhibits anxiolytic and dopamine D3 receptor-dependent antidepressant effects in the chronic stress model. Int J Neuropsychopharmacol. 2017;20(10):788-796