User login
Neurotransmitter-based diagnosis and treatment: A hypothesis (Part 1)
It is unfortunate that, in some clinical areas, medical conditions are still treated by name and not based on the underlying pathological process. It would be odd in 2022 to treat “dropsy” instead of heart or kidney disease (2 very different causes of edema). Similarly, if the FDA had been approving drugs 150 years ago, we would have medications on label for “dementia praecox,” not schizophrenia or Alzheimer disease. With the help of DSM-5, psychiatry still resides in the descriptive symptomatic world of disorders.
In the United States, thanks to Freud, psychiatric symptoms became separated from medical symptoms, which made it more difficult to associate psychiatric manifestations with the underlying pathophysiology. Though the physical manifestations that parallel emotional symptoms—such as the dry mouth of anxiety, the tremor and leg weakness of fear, the constipation and blurry vision of depression, the breathing difficulty of anger, the abdominal pain of stress, the blushing of shyness, the palpitations of flashbacks, and endless others—are well known, the present classification of psychiatric disorders is blind to it. Neurochemical causes of gastrointestinal spasm or muscle tension are better researched than underlying central neurochemistry, though the same neurotransmitters drive them.
Can the biochemistry of psychiatric symptoms be judged on the basis of peripheral symptoms? Can the mental manifestations be connected to biological causation, and vice versa? Would psychiatrists be better off selecting treatments by recognizing involved neurotransmitters instead of addressing descriptive “depression, anxiety, and psychosis”? Each of these clinical syndromes may be caused by entirely different underlying neuronal mechanisms. Such mechanisms could be suggested if medical symptoms (which are measurable and objective) would become part of the psychiatric diagnosis. Is treating the “cough” sufficient, or would recognition that tuberculosis caused the cough guide better treatment? Is it time to abandon descriptive conditions and replace them with a specific “mechanism-based” viewpoint?
Ample research has shown that serotonin, dopamine, norepinephrine, endorphins, glutamate, and gamma aminobutyric acid (GABA) are the neurotransmitters most responsible in the process of both psychiatric disorders and chronic pain. These neurotransmitters are involved in much more than emotions (including the feeling of pain). An abundance of medical symptom clusters point toward which neurotransmitter dysfunction may be leading in specific cases of distinct types of depression, psychosis, anxiety, or “chronic pain.” Even presently, there are medications available (both for FDA-approved indications and off-label) that can be used to regulate these neurotransmitters, allowing practitioners to target the possible biological underlining of psychiatric or pain pathology. Hopefully, in the not-so-distant future, there will be specific medications for serotonin, dopamine, and noradrenergic depression as well as for GABAergic anxiety, endorphin psychosis, noradrenergic insomnia, and similar conditions.
Numerous neurotransmitters may be connected to both depression and pain in all their forms. These include (but are not limited to) prostaglandins, bradykinins, substance P, potassium, magnesium, calcium, histamine, adenosine triphosphate, calcitonin gene-related peptide (CGRP), nitric oxide (NO), cholecystokinin 7 (CCK7), neurotrophic growth factor (NGF), neurotensin, acetylcholine (Ach), oxytocin, cannabinoids, and others. These have not been researched sufficiently to identify their clinical presentation of excessive or insufficient availability at the sites of neurotransmission. It is difficult to draw conclusions about what kind of clinical symptoms they may cause (outside of pain), and therefore, they are not addressed in this article.
Both high and low levels of certain neurotransmitters may be associated with psychiatric conditions and chronic pain. Too much is as bad as too little.1 This applies to both quantity of neurotransmitters as well as quality of the corresponding receptor activity. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation. Reading indirect signs of bodily functions is a basic clinical skill that should not be forgotten, even in the time of advanced technology.
A different way of viewing psychiatric disorders
In this article, we present 4 hypothetical clinical cases to emphasize a possible way of analyzing symptoms to identify underlying pathology and guide more effective treatment. In no way do these descriptions reflect the entire set of symptoms caused by neurotransmitters; we created them based on what is presently known or suspected, and extensive research is required to confirm or disprove what we describe here.
Continue to: There are no well-recognized...
There are no well-recognized, well-established, reliable, or validated syndromes described in our work. Our goal is to suggest an alternative way of looking at psychiatric disorders by viewing syndromal presentation through the lens of specific neurotransmitters. The collection of symptoms associated with various neurotransmitters as presented in this hypothesis is not complete. We have assembled what is described in the literature as a suggestion for specific future research. We simplified these clinical presentations by omitting scenarios in which a specific neurotransmitter increases in one area but not another. For example, all the symptoms of dopamine excess we describe would not have to occur concurrently in the same patient, but they may develop in certain patients depending on which dopaminergic pathway is exhibiting excess activity. Such distinctions may be established only by exhaustive research not yet conducted.
Our proposal may seem radical, but it truly is not. For example, if we know that dopamine excess may cause seizures, psychosis, and blood pressure elevation, why not consider dopamine excess as an underlying cause in a patient with depression who exhibits these symptoms simultaneously? And why not call it “dopamine excess syndrome”? We already have “serotonin syndrome” for a patient experiencing a serotonin storm. However, using the same logic, it should be called “serotonin excess syndrome.” And if we know of “serotonin excess syndrome,” why not consider “serotonin deficiency syndrome”?
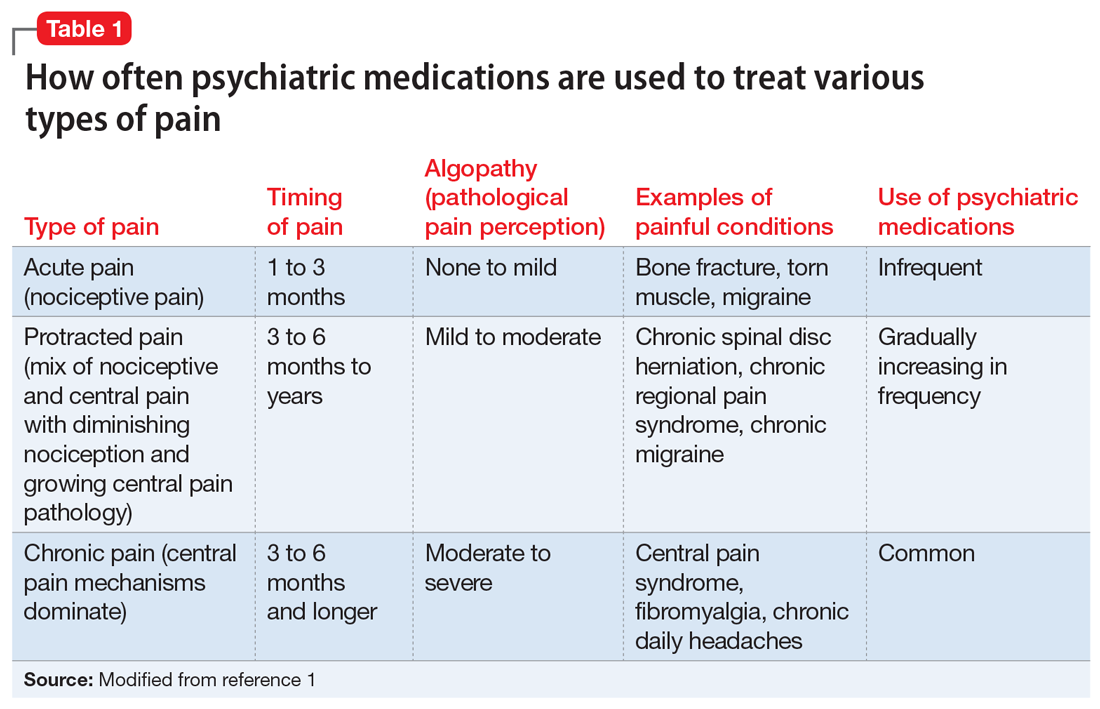
In Part 1 of this article, we discuss serotonin and dopamine. Table 1 outlines medical and psychiatric symptoms that likely reflect serotonin excess2-18 and deficiency,14,19-29 and Table 2 lists symptoms that likely reflect dopamine excess14,30-41 and deficiency.4,14,20,38,40-43 In Part 2 we will touch on endorphins and norepinephrine, and in Part 3 we will conclude by looking at GABA and glutamate.
Serotonin excess (Table 12-18)
On a recent office visit, Ms. H reports that most of the time she does not feel much of anything, but she still experiences panic attacks8,9,13,15 and is easily agitated.6,8 Her mother died recently, and Ms. H is concerned that she did not grieve.15-18 She failed her last semester in college and was indifferent to her failure.18 She sleeps poorly,8 is failing her creative classes, and wonders why she has lost her artistic inclination.16-18 Ms. H has difficulty with amotivation, planning, social interactions, and speech.16,17 All of those symptoms worsened after she was prescribed fluoxetine approximately 1 year ago for her “blues.” Ms. H is obese and continues to gain weight,2 though she frequently has diarrhea,3,4,7,8 loud peristalsis, and abdominal cramps.4,7,8 She sweats easily6-8 and her heart frequently races.8,9 Additionally, Ms. H’s primary care physician told her that she has “borderline diabetes.”2 She is prone to frequent bruising11 and is easy to shake, even when she is experiencing minimal anxiety.6-9 Ms. H had consulted with a neurologist because of unusual electrical “zapping” in her brain and muscle twitches.5,8,9,13 She had experienced a seizure as a child, but this was possibly related to hypernatremia,2 and she has not taken any anticonvulsant medication for several years.8 She exhibits hyperactive deep tendon reflexes and tremors5,7,9 and blinks frequently.6,9 She experiences hot flashes,3,6-8,14 does not tolerate heat, and prefers cooler weather.8,9 Her pains and aches,12,14 to which she has been prone all of her life, have recently become much worse, and she was diagnosed with fibromyalgia in part because she frequently feels stiff all over.10 She complains of strange tingling and prickling sensations in her hands and feet, especially when anxious.7,9,10 Her headaches also worsened and may be precipitated by bright light, as her pupils are usually dilated.5,7,9 Her hypertension is fairly controlled with medication.6,8-10 Ms. H says she experienced a psychotic episode when she was in her mid-teens,6,8 but reassures you that “she is not that bad now,” although she remains hypervigilant.13 Also while in her teens, Ms. H was treated with paroxetine and experienced restlessness, agitation, delirium, tachycardia, fluctuating blood pressure, diaphoresis, diarrhea, and neuromuscular excitation, which prompted discontinuation of the antidepressant.5-7,9,10
Impression. Ms. H exhibits symptoms associated with serotonin hyperactivity. Discontinuing and avoiding selective serotonin reuptake inhibitors (SSRIs) would be prudent; prescribing an anticonvulsant would be reasonable. Using a GABAergic medication to suppress serotonin (eg, baclofen) is likely to help. Avoiding dopaminergic medications is a must. Antidepressive antipsychotics would be logical to use. The use of serotonin-suppressing medications may be considered. One may argue for the use of beta-blockers in such a patient.
Continue to: Serotonin deficiency
Serotonin deficiency (Table 114,19-29)
Mr. A is chronically depressed, hopeless,19 and easily angered.21 He does not believe anyone can help him.19 You are concerned for his safety because he had attempted to end his life by shooting himself in the chest.19,21,22,25 Even when he’s not particularly depressed, Mr. A does not enjoy much of anything.21,26,27 He becomes particularly agitated when he drinks alcohol,25 which unfortunately is common for him.29 He engages in binge eating to feel better; he knows this is not healthy but he cannot control his behavior.20,29 Mr. A is poorly compliant with his medications, even with a blood thinner, which he was prescribed due to an episode of deep vein thrombosis. He complains of chronic daily headaches and episodic migraines.23,24 He rarely blinks,23,28 his skin is dry and cool, his hair is brittle,23 his mouth is dry,14,23,27 and he constantly licks his chapped lips.14,26,27 Mr. A frequently has general body pain26,31 but is dismissive of his body aches and completely stops reporting pain when his depression gets particularly severe. When depressed, he is slow in movement and thinking.14,21,26,27 He is more concerned with anxiety than depression.21 Mr. A is plagued by constipation, abdominal pain, muscle tension, and episodes of shaking.14,26,27 He also frequently complains about chronic tinnitus.28
Impression. Mr. A shows symptoms associated with serotonin hypoactivity. SSRIs and any other antidepressants with serotonin activity would be an obvious choice for treatment. A mood-stabilizing antipsychotic with serotonin activity would be welcome in treatment. Thyroid hormone supplementation may be of value, especially if thyroid stimulating hormone level is high. Light therapy, a diet with food that contains tryptophan, psychotherapy, and exercise are desirable. Avoiding benzodiazepines would be a good idea.
Dopamine excess (Table 214,30-41)
Ms. L presents with complaints of “fibromyalgia” and “daily headaches,”14 and also dissociation (finding herself in places when she does not know how she got there) and “out-of-body experiences.”32 She is odd, and states that people do not understand her and that she is “different.”38 Her friend, who is present at the appointment, elaborates on Ms. L’s bizarreness and oddness in behavior, out-of-context emotions, suspiciousness, paranoia, and possible hallucinations.35,36,38 Ms. L discloses frequent diffuse body pains, headaches, nausea, excessive salivation, and tongue burning, as well as muscle twitching.14 Sex worsens her headaches and body pain. She reports seizures that are not registered on EEG. In the office, she is suspicious, exhibits odd posturing, tends to misinterpret your words, and makes you feel uncomfortable. Anxiety38 and multiple obsessive-compulsive symptoms, especially excessive cleaning and grooming, complicate Ms. L’s life.31,32,34 On examination, she is hypertensive, and she has scars caused by self-cutting and skin picking on her arms.30-32 An electrocardiogram shows an elevated heart rate, widened QRS complex, and ectopic heartbeats.14 Ms. L has experienced trichotillomania since adolescence32-34 and her fingernails are bitten to the skin.34 She has difficulty with impulse control, and thrill-seeking is a prominent part of her life, mainly via gambling, compulsive sex, and compulsive buying.35,36 She also says she experiences indigestion and delayed gastric emptying.37
Impression. Ms. L exhibits multiple symptoms associated with dopamine excess. Dopamine antagonists should be considered and may help not only with her psychiatric symptoms but also with her pain symptoms. Bupropion (as a dopamine agonist), caffeine, and stimulants should be avoided.
Excessive dopamine is, in extreme cases, associated with somatic psychosis, somatic symptom disorder, factitious disorder, pain disorder, and hypochondria.39 It may come with odd and bizarre/peculiar symptoms out of proportion with objectively identified pathology. These symptoms are common in chronic pain and headache patients, and need to be addressed by appropriate use of dopamine antagonizing medications.39
Continue to: Dopamine deficiency
Dopamine deficiency (Table 24,14,20,38,40-43)
Mr. W experiences widespread pain, including chronic back pain, headaches, and abdominal pain. He also has substantial anhedonia, lack of interest, procrastination, and hypersomnia.41,42 He is apathetic and has difficulty getting up in the morning.41,42 Unusual tiredness and weakness drive him to overuse caffeine; he states that 5 Mountain Dews and 4 cups of regular coffee a day make his headaches bearable.38,41-43 Sex also improves his headaches. Since childhood, he has taken stimulants for attention-deficit/hyperactivity disorder. He reports that occasional use of cocaine helps ease his pain and depression. Mr. W’s wife is concerned with her husband’s low sexual drive and alcohol consumption, and discloses that he has periodic trouble with gambling. Mr. W was forced into psychotherapy but never was able to work productively with his therapist.38,41-43 He loves eating and cannot control his weight.40 This contrasts with episodic anorexia he experienced when he was younger.20 His face is usually emotionless.43 Mr. W is prone to constipation.14 His restless leg syndrome and periodic limb movement disorder are so bad that his wife refuses to share a bed with him.14 He is clumsy and has a problem with repetitive motor tasks.43 A paucity of speech, limited eye contact, poor grooming, and difficulty forming therapeutic alliances have long been part of Mr. W’s history.38,42,43 On physical examination, he has a dry mouth; he is stiff, tremulous, and hypotensive.14
Impression. Mr. W shows multiple symptoms associated with dopamine deficiency. Bupropion may be reasonable to consider. Dopamine augmentation via the use of stimulants is warranted in such patients, especially if stimulants had not been tried before (lisdexamfetamine would be a good choice to minimize addictive potential). For a patient with dopamine deficiency, levodopa may improve more than just restless legs. Amantadine may improve dopaminergic signaling through the accelerated dopamine release and decrease in presynaptic uptake, so this medication may be carefully tried.44 Pain treatment would not be successful for Mr. W without simultaneous treatment for his substance use.
Bottom Line
Both high and low levels of serotonin and dopamine may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: How to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Amantadine • Gocovri
Baclofen • Ozobax
Bupropion • Wellbutrin
Fluoxetine • Prozac
Lisdexamfetamine • Vyvanse
Paroxetine • Paxil
1. Stahl SM. Dazzled by the dominions of dopamine: clinical roles of D3, D2, and D1 receptors. CNS Spectr. 2017;22(4):305-311.
2. Young RL, Lumsden AL, Martin AM, et al. Augmented capacity for peripheral serotonin release in human obesity. Int J Obes (Lond). 2018;42(11):1880-1889.
3. Ahlman H. Serotonin and carcinoid tumors. J Cardiovasc Pharmacol. 1985;7(Suppl 7):S79-S85.
4. Terry N, Margolis KG. Serotonergic mechanisms regulating the GI tract: experimental evidence and therapeutic relevance. Handb Exp Pharmacol. 2017;239:319-342.
5. Prakash S, Belani P, Trivedi A. Headache as a presenting feature in patients with serotonin syndrome: a case series. Cephalalgia. 2014;34(2):148-153.
6. van Ewijk CE, Jacobs GE, Girbes ARJ. Unsuspected serotonin toxicity in the ICU. Ann Intensive Care. 2016;6(1):85.
7. Pedavally S, Fugate JE, Rabinstein AA. Serotonin syndrome in the intensive care unit: clinical presentations and precipitating medications. Neurocrit Care. 2014;21(1):108-113.
8. Nguyen H, Pan A, Smollin C, et al. An 11-year retrospective review of cyproheptadine use in serotonin syndrome cases reported to the California Poison Control System. J Clin Pharm Ther. 2019;44(2):327-334.
9. Ansari H, Kouti L. Drug interaction and serotonin toxicity with opioid use: another reason to avoid opioids in headache and migraine treatment. Curr Pain Headache Rep. 2016;20(8):50.
10. Ott M, Mannchen JK, Jamshidi F, et al. Management of severe arterial hypertension associated with serotonin syndrome: a case report analysis based on systematic review techniques. Ther Adv Psychopharmacol. 2019;9:2045125318818814. doi:10.1177/2045125318818814
11. Cerrito F, Lazzaro MP, Gaudio E, et al. 5HT2-receptors and serotonin release: their role in human platelet aggregation. Life Sci. 1993;53(3):209-215.
12. Ohayon MM. Pain sensitivity, depression, and sleep deprivation: links with serotoninergic dysfunction. J Psychiatr Res. 2009;43(16):1243-1245.
13. Maron E, Shlik J. Serotonin function in panic disorder: important, but why? Neuropsychopharmacology. 2006;31(1):1-11.
14. Hall JE, Guyton AC. Textbook of Medical Physiology. 12th ed. Spanish version. Elsevier; 2011:120,199,201-204,730-740.
15. Garland EJ, Baerg EA. Amotivational syndrome associated with selective serotonin reuptake inhibitors in children and adolescents. J Child Adolesc Psychopharmacol. 2001;11(2):181-186.
16. George MS, Trimble MR. A fluvoxamine-induced frontal lobe syndrome in a patient with comorbid Gilles de la Tourette’s syndrome and obsessive compulsive disorder. J Clin Psychiatry. 1992;53(10):379-380.
17. Hoehn-Saric R, Harris GJ, Pearlson GD, et al. A fluoxetine-induced frontal lobe syndrome in an obsessive compulsive patient. J Clin Psychiatry. 1991;52(3):131-133.
18. Hoehn-Saric R, Lipsey JR, McLeod DR. Apathy and indifference in patients on fluvoxamine and fluoxetine. J Clin Psychopharmacol. 1990;10(5):343-345.
19. Samuelsson M, Jokinen J, Nordström AL, et al. CSF 5-HIAA, suicide intent and hopelessness in the prediction of early suicide in male high-risk suicide attempters. Acta Psychiatr Scand. 2006;113(1):44-47.
20. Brewerton TD. Clinical Handbook of Eating Disorders: An Integrated Approach. CRC Press; 2004:257-281.
21. Mann JJ, Oquendo M, Underwood MD, et al. The neurobiology of suicide risk: a review for the clinician. J Clin Psychiatry. 1999;60 Suppl 2:7-116.
22. Mann JJ, Malone KM. Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients. Biol Psychiatry. 1997;41(2):162-171.
23. Joseph R, Welch KM, D’Andrea G. Serotonergic hypofunction in migraine: a synthesis of evidence based on platelet dense body dysfunction. Cephalalgia. 1989;9(4):293-299.
24. Pakalnis A, Splaingard M, Splaingard D, et al. Serotonin effects on sleep and emotional disorders in adolescent migraine. Headache. 2009;49(10):1486-1492.
25. Virkkunen M, Goldman D, Nielsen DA, et al. Low brain serotonin turnover rate (low CSF 5-HIAA) and impulsive violence. J Psychiatry Neurosci. 1995;20(4):271-275.
26. Liu Y, Zhao J, Fan X, et al. Dysfunction in serotonergic and noradrenergic systems and somatic symptoms in psychiatric disorders. Front Psychiatry. 2019;10:286.
27. Ginsburg GS, Riddle MA, Davies M. Somatic symptoms in children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(10):1179-1187.
28. O’Malley PG, Jackson JL, Santoro J, et al. Antidepressant therapy for unexplained symptoms and symptom syndromes. J Fam Pract. 1999;48(12):980-990.
29. Fortuna JL. Sweet preference, sugar addiction and the familial history of alcohol dependence: shared neural pathways and genes. J Psychoactive Drugs. 2010;42(2):147-151.
30. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
31. Graybiel AM, Saka E. A genetic basis for obsessive grooming. Neuron. 2002;33(1):1-2.
32. Tse W, Hälbig TD. Skin picking in Parkinson’s disease: a behavioral side-effect of dopaminergic treatment? Psychiatry Clin Neurosci. 2010;64(2):214.
33. Ayaydın H. Probable emergence of symptoms of trichotillomania by atomoxetine: a case report. Psychiatry and Clinical Psychopharmacology. 2019;29(2)220-222.
34. Paholpak P, Mendez MF. Trichotillomania as a manifestation of dementia. Case Rep Psychiatry. 2016;2016:9782702. doi:10.1155/2016/9782702
35. Clark CA, Dagher A. The role of dopamine in risk taking: a specific look at Parkinson’s disease and gambling. Front Behav Neurosci. 2014;8:196.
36. Norbury A, Husain M. Sensation-seeking: dopaminergic modulation and risk for psychopathology. Behav Brain Res. 2015;288:79-93.
37. Chen TS, Chang FY. Elevated serum dopamine increases while coffee consumption decreases the occurrence of reddish streaks in the intact stomach. J Gastroenterol Hepatol. 2013;28(12):1810-1814.
38. Wong-Riley MT. Neuroscience Secrets. 1st edition. Spanish version. Hanley & Belfus; 1999:420-429.
39. Arbuck DM. Antipsychotics, dopamine, and pain. Current Psychiatry. 2020;19(1):25-29,31.
40. Bello NT, Hajnal A. Dopamine and binge eating behaviors. Pharmacol Biochem Behav. 2010;97(1):25-33.
41. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70 Suppl 4:1-46.
42. Milev P, Ho BC, Arndt S, et al. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162(3):495-506.
43. Gepshtein S, Li X, Snider J, et al. Dopamine function and the efficiency of human movement. J Cogn Neurosci. 2014;26(3):645-657.
44. Scarff JR. The ABCDs of treating tardive dyskinesia. Current Psychiatry. 2020;19(4):21,55.
It is unfortunate that, in some clinical areas, medical conditions are still treated by name and not based on the underlying pathological process. It would be odd in 2022 to treat “dropsy” instead of heart or kidney disease (2 very different causes of edema). Similarly, if the FDA had been approving drugs 150 years ago, we would have medications on label for “dementia praecox,” not schizophrenia or Alzheimer disease. With the help of DSM-5, psychiatry still resides in the descriptive symptomatic world of disorders.
In the United States, thanks to Freud, psychiatric symptoms became separated from medical symptoms, which made it more difficult to associate psychiatric manifestations with the underlying pathophysiology. Though the physical manifestations that parallel emotional symptoms—such as the dry mouth of anxiety, the tremor and leg weakness of fear, the constipation and blurry vision of depression, the breathing difficulty of anger, the abdominal pain of stress, the blushing of shyness, the palpitations of flashbacks, and endless others—are well known, the present classification of psychiatric disorders is blind to it. Neurochemical causes of gastrointestinal spasm or muscle tension are better researched than underlying central neurochemistry, though the same neurotransmitters drive them.
Can the biochemistry of psychiatric symptoms be judged on the basis of peripheral symptoms? Can the mental manifestations be connected to biological causation, and vice versa? Would psychiatrists be better off selecting treatments by recognizing involved neurotransmitters instead of addressing descriptive “depression, anxiety, and psychosis”? Each of these clinical syndromes may be caused by entirely different underlying neuronal mechanisms. Such mechanisms could be suggested if medical symptoms (which are measurable and objective) would become part of the psychiatric diagnosis. Is treating the “cough” sufficient, or would recognition that tuberculosis caused the cough guide better treatment? Is it time to abandon descriptive conditions and replace them with a specific “mechanism-based” viewpoint?
Ample research has shown that serotonin, dopamine, norepinephrine, endorphins, glutamate, and gamma aminobutyric acid (GABA) are the neurotransmitters most responsible in the process of both psychiatric disorders and chronic pain. These neurotransmitters are involved in much more than emotions (including the feeling of pain). An abundance of medical symptom clusters point toward which neurotransmitter dysfunction may be leading in specific cases of distinct types of depression, psychosis, anxiety, or “chronic pain.” Even presently, there are medications available (both for FDA-approved indications and off-label) that can be used to regulate these neurotransmitters, allowing practitioners to target the possible biological underlining of psychiatric or pain pathology. Hopefully, in the not-so-distant future, there will be specific medications for serotonin, dopamine, and noradrenergic depression as well as for GABAergic anxiety, endorphin psychosis, noradrenergic insomnia, and similar conditions.
Numerous neurotransmitters may be connected to both depression and pain in all their forms. These include (but are not limited to) prostaglandins, bradykinins, substance P, potassium, magnesium, calcium, histamine, adenosine triphosphate, calcitonin gene-related peptide (CGRP), nitric oxide (NO), cholecystokinin 7 (CCK7), neurotrophic growth factor (NGF), neurotensin, acetylcholine (Ach), oxytocin, cannabinoids, and others. These have not been researched sufficiently to identify their clinical presentation of excessive or insufficient availability at the sites of neurotransmission. It is difficult to draw conclusions about what kind of clinical symptoms they may cause (outside of pain), and therefore, they are not addressed in this article.
Both high and low levels of certain neurotransmitters may be associated with psychiatric conditions and chronic pain. Too much is as bad as too little.1 This applies to both quantity of neurotransmitters as well as quality of the corresponding receptor activity. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation. Reading indirect signs of bodily functions is a basic clinical skill that should not be forgotten, even in the time of advanced technology.
A different way of viewing psychiatric disorders
In this article, we present 4 hypothetical clinical cases to emphasize a possible way of analyzing symptoms to identify underlying pathology and guide more effective treatment. In no way do these descriptions reflect the entire set of symptoms caused by neurotransmitters; we created them based on what is presently known or suspected, and extensive research is required to confirm or disprove what we describe here.
Continue to: There are no well-recognized...
There are no well-recognized, well-established, reliable, or validated syndromes described in our work. Our goal is to suggest an alternative way of looking at psychiatric disorders by viewing syndromal presentation through the lens of specific neurotransmitters. The collection of symptoms associated with various neurotransmitters as presented in this hypothesis is not complete. We have assembled what is described in the literature as a suggestion for specific future research. We simplified these clinical presentations by omitting scenarios in which a specific neurotransmitter increases in one area but not another. For example, all the symptoms of dopamine excess we describe would not have to occur concurrently in the same patient, but they may develop in certain patients depending on which dopaminergic pathway is exhibiting excess activity. Such distinctions may be established only by exhaustive research not yet conducted.
Our proposal may seem radical, but it truly is not. For example, if we know that dopamine excess may cause seizures, psychosis, and blood pressure elevation, why not consider dopamine excess as an underlying cause in a patient with depression who exhibits these symptoms simultaneously? And why not call it “dopamine excess syndrome”? We already have “serotonin syndrome” for a patient experiencing a serotonin storm. However, using the same logic, it should be called “serotonin excess syndrome.” And if we know of “serotonin excess syndrome,” why not consider “serotonin deficiency syndrome”?
In Part 1 of this article, we discuss serotonin and dopamine. Table 1 outlines medical and psychiatric symptoms that likely reflect serotonin excess2-18 and deficiency,14,19-29 and Table 2 lists symptoms that likely reflect dopamine excess14,30-41 and deficiency.4,14,20,38,40-43 In Part 2 we will touch on endorphins and norepinephrine, and in Part 3 we will conclude by looking at GABA and glutamate.
Serotonin excess (Table 12-18)
On a recent office visit, Ms. H reports that most of the time she does not feel much of anything, but she still experiences panic attacks8,9,13,15 and is easily agitated.6,8 Her mother died recently, and Ms. H is concerned that she did not grieve.15-18 She failed her last semester in college and was indifferent to her failure.18 She sleeps poorly,8 is failing her creative classes, and wonders why she has lost her artistic inclination.16-18 Ms. H has difficulty with amotivation, planning, social interactions, and speech.16,17 All of those symptoms worsened after she was prescribed fluoxetine approximately 1 year ago for her “blues.” Ms. H is obese and continues to gain weight,2 though she frequently has diarrhea,3,4,7,8 loud peristalsis, and abdominal cramps.4,7,8 She sweats easily6-8 and her heart frequently races.8,9 Additionally, Ms. H’s primary care physician told her that she has “borderline diabetes.”2 She is prone to frequent bruising11 and is easy to shake, even when she is experiencing minimal anxiety.6-9 Ms. H had consulted with a neurologist because of unusual electrical “zapping” in her brain and muscle twitches.5,8,9,13 She had experienced a seizure as a child, but this was possibly related to hypernatremia,2 and she has not taken any anticonvulsant medication for several years.8 She exhibits hyperactive deep tendon reflexes and tremors5,7,9 and blinks frequently.6,9 She experiences hot flashes,3,6-8,14 does not tolerate heat, and prefers cooler weather.8,9 Her pains and aches,12,14 to which she has been prone all of her life, have recently become much worse, and she was diagnosed with fibromyalgia in part because she frequently feels stiff all over.10 She complains of strange tingling and prickling sensations in her hands and feet, especially when anxious.7,9,10 Her headaches also worsened and may be precipitated by bright light, as her pupils are usually dilated.5,7,9 Her hypertension is fairly controlled with medication.6,8-10 Ms. H says she experienced a psychotic episode when she was in her mid-teens,6,8 but reassures you that “she is not that bad now,” although she remains hypervigilant.13 Also while in her teens, Ms. H was treated with paroxetine and experienced restlessness, agitation, delirium, tachycardia, fluctuating blood pressure, diaphoresis, diarrhea, and neuromuscular excitation, which prompted discontinuation of the antidepressant.5-7,9,10
Impression. Ms. H exhibits symptoms associated with serotonin hyperactivity. Discontinuing and avoiding selective serotonin reuptake inhibitors (SSRIs) would be prudent; prescribing an anticonvulsant would be reasonable. Using a GABAergic medication to suppress serotonin (eg, baclofen) is likely to help. Avoiding dopaminergic medications is a must. Antidepressive antipsychotics would be logical to use. The use of serotonin-suppressing medications may be considered. One may argue for the use of beta-blockers in such a patient.
Continue to: Serotonin deficiency
Serotonin deficiency (Table 114,19-29)
Mr. A is chronically depressed, hopeless,19 and easily angered.21 He does not believe anyone can help him.19 You are concerned for his safety because he had attempted to end his life by shooting himself in the chest.19,21,22,25 Even when he’s not particularly depressed, Mr. A does not enjoy much of anything.21,26,27 He becomes particularly agitated when he drinks alcohol,25 which unfortunately is common for him.29 He engages in binge eating to feel better; he knows this is not healthy but he cannot control his behavior.20,29 Mr. A is poorly compliant with his medications, even with a blood thinner, which he was prescribed due to an episode of deep vein thrombosis. He complains of chronic daily headaches and episodic migraines.23,24 He rarely blinks,23,28 his skin is dry and cool, his hair is brittle,23 his mouth is dry,14,23,27 and he constantly licks his chapped lips.14,26,27 Mr. A frequently has general body pain26,31 but is dismissive of his body aches and completely stops reporting pain when his depression gets particularly severe. When depressed, he is slow in movement and thinking.14,21,26,27 He is more concerned with anxiety than depression.21 Mr. A is plagued by constipation, abdominal pain, muscle tension, and episodes of shaking.14,26,27 He also frequently complains about chronic tinnitus.28
Impression. Mr. A shows symptoms associated with serotonin hypoactivity. SSRIs and any other antidepressants with serotonin activity would be an obvious choice for treatment. A mood-stabilizing antipsychotic with serotonin activity would be welcome in treatment. Thyroid hormone supplementation may be of value, especially if thyroid stimulating hormone level is high. Light therapy, a diet with food that contains tryptophan, psychotherapy, and exercise are desirable. Avoiding benzodiazepines would be a good idea.
Dopamine excess (Table 214,30-41)
Ms. L presents with complaints of “fibromyalgia” and “daily headaches,”14 and also dissociation (finding herself in places when she does not know how she got there) and “out-of-body experiences.”32 She is odd, and states that people do not understand her and that she is “different.”38 Her friend, who is present at the appointment, elaborates on Ms. L’s bizarreness and oddness in behavior, out-of-context emotions, suspiciousness, paranoia, and possible hallucinations.35,36,38 Ms. L discloses frequent diffuse body pains, headaches, nausea, excessive salivation, and tongue burning, as well as muscle twitching.14 Sex worsens her headaches and body pain. She reports seizures that are not registered on EEG. In the office, she is suspicious, exhibits odd posturing, tends to misinterpret your words, and makes you feel uncomfortable. Anxiety38 and multiple obsessive-compulsive symptoms, especially excessive cleaning and grooming, complicate Ms. L’s life.31,32,34 On examination, she is hypertensive, and she has scars caused by self-cutting and skin picking on her arms.30-32 An electrocardiogram shows an elevated heart rate, widened QRS complex, and ectopic heartbeats.14 Ms. L has experienced trichotillomania since adolescence32-34 and her fingernails are bitten to the skin.34 She has difficulty with impulse control, and thrill-seeking is a prominent part of her life, mainly via gambling, compulsive sex, and compulsive buying.35,36 She also says she experiences indigestion and delayed gastric emptying.37
Impression. Ms. L exhibits multiple symptoms associated with dopamine excess. Dopamine antagonists should be considered and may help not only with her psychiatric symptoms but also with her pain symptoms. Bupropion (as a dopamine agonist), caffeine, and stimulants should be avoided.
Excessive dopamine is, in extreme cases, associated with somatic psychosis, somatic symptom disorder, factitious disorder, pain disorder, and hypochondria.39 It may come with odd and bizarre/peculiar symptoms out of proportion with objectively identified pathology. These symptoms are common in chronic pain and headache patients, and need to be addressed by appropriate use of dopamine antagonizing medications.39
Continue to: Dopamine deficiency
Dopamine deficiency (Table 24,14,20,38,40-43)
Mr. W experiences widespread pain, including chronic back pain, headaches, and abdominal pain. He also has substantial anhedonia, lack of interest, procrastination, and hypersomnia.41,42 He is apathetic and has difficulty getting up in the morning.41,42 Unusual tiredness and weakness drive him to overuse caffeine; he states that 5 Mountain Dews and 4 cups of regular coffee a day make his headaches bearable.38,41-43 Sex also improves his headaches. Since childhood, he has taken stimulants for attention-deficit/hyperactivity disorder. He reports that occasional use of cocaine helps ease his pain and depression. Mr. W’s wife is concerned with her husband’s low sexual drive and alcohol consumption, and discloses that he has periodic trouble with gambling. Mr. W was forced into psychotherapy but never was able to work productively with his therapist.38,41-43 He loves eating and cannot control his weight.40 This contrasts with episodic anorexia he experienced when he was younger.20 His face is usually emotionless.43 Mr. W is prone to constipation.14 His restless leg syndrome and periodic limb movement disorder are so bad that his wife refuses to share a bed with him.14 He is clumsy and has a problem with repetitive motor tasks.43 A paucity of speech, limited eye contact, poor grooming, and difficulty forming therapeutic alliances have long been part of Mr. W’s history.38,42,43 On physical examination, he has a dry mouth; he is stiff, tremulous, and hypotensive.14
Impression. Mr. W shows multiple symptoms associated with dopamine deficiency. Bupropion may be reasonable to consider. Dopamine augmentation via the use of stimulants is warranted in such patients, especially if stimulants had not been tried before (lisdexamfetamine would be a good choice to minimize addictive potential). For a patient with dopamine deficiency, levodopa may improve more than just restless legs. Amantadine may improve dopaminergic signaling through the accelerated dopamine release and decrease in presynaptic uptake, so this medication may be carefully tried.44 Pain treatment would not be successful for Mr. W without simultaneous treatment for his substance use.
Bottom Line
Both high and low levels of serotonin and dopamine may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: How to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Amantadine • Gocovri
Baclofen • Ozobax
Bupropion • Wellbutrin
Fluoxetine • Prozac
Lisdexamfetamine • Vyvanse
Paroxetine • Paxil
It is unfortunate that, in some clinical areas, medical conditions are still treated by name and not based on the underlying pathological process. It would be odd in 2022 to treat “dropsy” instead of heart or kidney disease (2 very different causes of edema). Similarly, if the FDA had been approving drugs 150 years ago, we would have medications on label for “dementia praecox,” not schizophrenia or Alzheimer disease. With the help of DSM-5, psychiatry still resides in the descriptive symptomatic world of disorders.
In the United States, thanks to Freud, psychiatric symptoms became separated from medical symptoms, which made it more difficult to associate psychiatric manifestations with the underlying pathophysiology. Though the physical manifestations that parallel emotional symptoms—such as the dry mouth of anxiety, the tremor and leg weakness of fear, the constipation and blurry vision of depression, the breathing difficulty of anger, the abdominal pain of stress, the blushing of shyness, the palpitations of flashbacks, and endless others—are well known, the present classification of psychiatric disorders is blind to it. Neurochemical causes of gastrointestinal spasm or muscle tension are better researched than underlying central neurochemistry, though the same neurotransmitters drive them.
Can the biochemistry of psychiatric symptoms be judged on the basis of peripheral symptoms? Can the mental manifestations be connected to biological causation, and vice versa? Would psychiatrists be better off selecting treatments by recognizing involved neurotransmitters instead of addressing descriptive “depression, anxiety, and psychosis”? Each of these clinical syndromes may be caused by entirely different underlying neuronal mechanisms. Such mechanisms could be suggested if medical symptoms (which are measurable and objective) would become part of the psychiatric diagnosis. Is treating the “cough” sufficient, or would recognition that tuberculosis caused the cough guide better treatment? Is it time to abandon descriptive conditions and replace them with a specific “mechanism-based” viewpoint?
Ample research has shown that serotonin, dopamine, norepinephrine, endorphins, glutamate, and gamma aminobutyric acid (GABA) are the neurotransmitters most responsible in the process of both psychiatric disorders and chronic pain. These neurotransmitters are involved in much more than emotions (including the feeling of pain). An abundance of medical symptom clusters point toward which neurotransmitter dysfunction may be leading in specific cases of distinct types of depression, psychosis, anxiety, or “chronic pain.” Even presently, there are medications available (both for FDA-approved indications and off-label) that can be used to regulate these neurotransmitters, allowing practitioners to target the possible biological underlining of psychiatric or pain pathology. Hopefully, in the not-so-distant future, there will be specific medications for serotonin, dopamine, and noradrenergic depression as well as for GABAergic anxiety, endorphin psychosis, noradrenergic insomnia, and similar conditions.
Numerous neurotransmitters may be connected to both depression and pain in all their forms. These include (but are not limited to) prostaglandins, bradykinins, substance P, potassium, magnesium, calcium, histamine, adenosine triphosphate, calcitonin gene-related peptide (CGRP), nitric oxide (NO), cholecystokinin 7 (CCK7), neurotrophic growth factor (NGF), neurotensin, acetylcholine (Ach), oxytocin, cannabinoids, and others. These have not been researched sufficiently to identify their clinical presentation of excessive or insufficient availability at the sites of neurotransmission. It is difficult to draw conclusions about what kind of clinical symptoms they may cause (outside of pain), and therefore, they are not addressed in this article.
Both high and low levels of certain neurotransmitters may be associated with psychiatric conditions and chronic pain. Too much is as bad as too little.1 This applies to both quantity of neurotransmitters as well as quality of the corresponding receptor activity. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation. Reading indirect signs of bodily functions is a basic clinical skill that should not be forgotten, even in the time of advanced technology.
A different way of viewing psychiatric disorders
In this article, we present 4 hypothetical clinical cases to emphasize a possible way of analyzing symptoms to identify underlying pathology and guide more effective treatment. In no way do these descriptions reflect the entire set of symptoms caused by neurotransmitters; we created them based on what is presently known or suspected, and extensive research is required to confirm or disprove what we describe here.
Continue to: There are no well-recognized...
There are no well-recognized, well-established, reliable, or validated syndromes described in our work. Our goal is to suggest an alternative way of looking at psychiatric disorders by viewing syndromal presentation through the lens of specific neurotransmitters. The collection of symptoms associated with various neurotransmitters as presented in this hypothesis is not complete. We have assembled what is described in the literature as a suggestion for specific future research. We simplified these clinical presentations by omitting scenarios in which a specific neurotransmitter increases in one area but not another. For example, all the symptoms of dopamine excess we describe would not have to occur concurrently in the same patient, but they may develop in certain patients depending on which dopaminergic pathway is exhibiting excess activity. Such distinctions may be established only by exhaustive research not yet conducted.
Our proposal may seem radical, but it truly is not. For example, if we know that dopamine excess may cause seizures, psychosis, and blood pressure elevation, why not consider dopamine excess as an underlying cause in a patient with depression who exhibits these symptoms simultaneously? And why not call it “dopamine excess syndrome”? We already have “serotonin syndrome” for a patient experiencing a serotonin storm. However, using the same logic, it should be called “serotonin excess syndrome.” And if we know of “serotonin excess syndrome,” why not consider “serotonin deficiency syndrome”?
In Part 1 of this article, we discuss serotonin and dopamine. Table 1 outlines medical and psychiatric symptoms that likely reflect serotonin excess2-18 and deficiency,14,19-29 and Table 2 lists symptoms that likely reflect dopamine excess14,30-41 and deficiency.4,14,20,38,40-43 In Part 2 we will touch on endorphins and norepinephrine, and in Part 3 we will conclude by looking at GABA and glutamate.
Serotonin excess (Table 12-18)
On a recent office visit, Ms. H reports that most of the time she does not feel much of anything, but she still experiences panic attacks8,9,13,15 and is easily agitated.6,8 Her mother died recently, and Ms. H is concerned that she did not grieve.15-18 She failed her last semester in college and was indifferent to her failure.18 She sleeps poorly,8 is failing her creative classes, and wonders why she has lost her artistic inclination.16-18 Ms. H has difficulty with amotivation, planning, social interactions, and speech.16,17 All of those symptoms worsened after she was prescribed fluoxetine approximately 1 year ago for her “blues.” Ms. H is obese and continues to gain weight,2 though she frequently has diarrhea,3,4,7,8 loud peristalsis, and abdominal cramps.4,7,8 She sweats easily6-8 and her heart frequently races.8,9 Additionally, Ms. H’s primary care physician told her that she has “borderline diabetes.”2 She is prone to frequent bruising11 and is easy to shake, even when she is experiencing minimal anxiety.6-9 Ms. H had consulted with a neurologist because of unusual electrical “zapping” in her brain and muscle twitches.5,8,9,13 She had experienced a seizure as a child, but this was possibly related to hypernatremia,2 and she has not taken any anticonvulsant medication for several years.8 She exhibits hyperactive deep tendon reflexes and tremors5,7,9 and blinks frequently.6,9 She experiences hot flashes,3,6-8,14 does not tolerate heat, and prefers cooler weather.8,9 Her pains and aches,12,14 to which she has been prone all of her life, have recently become much worse, and she was diagnosed with fibromyalgia in part because she frequently feels stiff all over.10 She complains of strange tingling and prickling sensations in her hands and feet, especially when anxious.7,9,10 Her headaches also worsened and may be precipitated by bright light, as her pupils are usually dilated.5,7,9 Her hypertension is fairly controlled with medication.6,8-10 Ms. H says she experienced a psychotic episode when she was in her mid-teens,6,8 but reassures you that “she is not that bad now,” although she remains hypervigilant.13 Also while in her teens, Ms. H was treated with paroxetine and experienced restlessness, agitation, delirium, tachycardia, fluctuating blood pressure, diaphoresis, diarrhea, and neuromuscular excitation, which prompted discontinuation of the antidepressant.5-7,9,10
Impression. Ms. H exhibits symptoms associated with serotonin hyperactivity. Discontinuing and avoiding selective serotonin reuptake inhibitors (SSRIs) would be prudent; prescribing an anticonvulsant would be reasonable. Using a GABAergic medication to suppress serotonin (eg, baclofen) is likely to help. Avoiding dopaminergic medications is a must. Antidepressive antipsychotics would be logical to use. The use of serotonin-suppressing medications may be considered. One may argue for the use of beta-blockers in such a patient.
Continue to: Serotonin deficiency
Serotonin deficiency (Table 114,19-29)
Mr. A is chronically depressed, hopeless,19 and easily angered.21 He does not believe anyone can help him.19 You are concerned for his safety because he had attempted to end his life by shooting himself in the chest.19,21,22,25 Even when he’s not particularly depressed, Mr. A does not enjoy much of anything.21,26,27 He becomes particularly agitated when he drinks alcohol,25 which unfortunately is common for him.29 He engages in binge eating to feel better; he knows this is not healthy but he cannot control his behavior.20,29 Mr. A is poorly compliant with his medications, even with a blood thinner, which he was prescribed due to an episode of deep vein thrombosis. He complains of chronic daily headaches and episodic migraines.23,24 He rarely blinks,23,28 his skin is dry and cool, his hair is brittle,23 his mouth is dry,14,23,27 and he constantly licks his chapped lips.14,26,27 Mr. A frequently has general body pain26,31 but is dismissive of his body aches and completely stops reporting pain when his depression gets particularly severe. When depressed, he is slow in movement and thinking.14,21,26,27 He is more concerned with anxiety than depression.21 Mr. A is plagued by constipation, abdominal pain, muscle tension, and episodes of shaking.14,26,27 He also frequently complains about chronic tinnitus.28
Impression. Mr. A shows symptoms associated with serotonin hypoactivity. SSRIs and any other antidepressants with serotonin activity would be an obvious choice for treatment. A mood-stabilizing antipsychotic with serotonin activity would be welcome in treatment. Thyroid hormone supplementation may be of value, especially if thyroid stimulating hormone level is high. Light therapy, a diet with food that contains tryptophan, psychotherapy, and exercise are desirable. Avoiding benzodiazepines would be a good idea.
Dopamine excess (Table 214,30-41)
Ms. L presents with complaints of “fibromyalgia” and “daily headaches,”14 and also dissociation (finding herself in places when she does not know how she got there) and “out-of-body experiences.”32 She is odd, and states that people do not understand her and that she is “different.”38 Her friend, who is present at the appointment, elaborates on Ms. L’s bizarreness and oddness in behavior, out-of-context emotions, suspiciousness, paranoia, and possible hallucinations.35,36,38 Ms. L discloses frequent diffuse body pains, headaches, nausea, excessive salivation, and tongue burning, as well as muscle twitching.14 Sex worsens her headaches and body pain. She reports seizures that are not registered on EEG. In the office, she is suspicious, exhibits odd posturing, tends to misinterpret your words, and makes you feel uncomfortable. Anxiety38 and multiple obsessive-compulsive symptoms, especially excessive cleaning and grooming, complicate Ms. L’s life.31,32,34 On examination, she is hypertensive, and she has scars caused by self-cutting and skin picking on her arms.30-32 An electrocardiogram shows an elevated heart rate, widened QRS complex, and ectopic heartbeats.14 Ms. L has experienced trichotillomania since adolescence32-34 and her fingernails are bitten to the skin.34 She has difficulty with impulse control, and thrill-seeking is a prominent part of her life, mainly via gambling, compulsive sex, and compulsive buying.35,36 She also says she experiences indigestion and delayed gastric emptying.37
Impression. Ms. L exhibits multiple symptoms associated with dopamine excess. Dopamine antagonists should be considered and may help not only with her psychiatric symptoms but also with her pain symptoms. Bupropion (as a dopamine agonist), caffeine, and stimulants should be avoided.
Excessive dopamine is, in extreme cases, associated with somatic psychosis, somatic symptom disorder, factitious disorder, pain disorder, and hypochondria.39 It may come with odd and bizarre/peculiar symptoms out of proportion with objectively identified pathology. These symptoms are common in chronic pain and headache patients, and need to be addressed by appropriate use of dopamine antagonizing medications.39
Continue to: Dopamine deficiency
Dopamine deficiency (Table 24,14,20,38,40-43)
Mr. W experiences widespread pain, including chronic back pain, headaches, and abdominal pain. He also has substantial anhedonia, lack of interest, procrastination, and hypersomnia.41,42 He is apathetic and has difficulty getting up in the morning.41,42 Unusual tiredness and weakness drive him to overuse caffeine; he states that 5 Mountain Dews and 4 cups of regular coffee a day make his headaches bearable.38,41-43 Sex also improves his headaches. Since childhood, he has taken stimulants for attention-deficit/hyperactivity disorder. He reports that occasional use of cocaine helps ease his pain and depression. Mr. W’s wife is concerned with her husband’s low sexual drive and alcohol consumption, and discloses that he has periodic trouble with gambling. Mr. W was forced into psychotherapy but never was able to work productively with his therapist.38,41-43 He loves eating and cannot control his weight.40 This contrasts with episodic anorexia he experienced when he was younger.20 His face is usually emotionless.43 Mr. W is prone to constipation.14 His restless leg syndrome and periodic limb movement disorder are so bad that his wife refuses to share a bed with him.14 He is clumsy and has a problem with repetitive motor tasks.43 A paucity of speech, limited eye contact, poor grooming, and difficulty forming therapeutic alliances have long been part of Mr. W’s history.38,42,43 On physical examination, he has a dry mouth; he is stiff, tremulous, and hypotensive.14
Impression. Mr. W shows multiple symptoms associated with dopamine deficiency. Bupropion may be reasonable to consider. Dopamine augmentation via the use of stimulants is warranted in such patients, especially if stimulants had not been tried before (lisdexamfetamine would be a good choice to minimize addictive potential). For a patient with dopamine deficiency, levodopa may improve more than just restless legs. Amantadine may improve dopaminergic signaling through the accelerated dopamine release and decrease in presynaptic uptake, so this medication may be carefully tried.44 Pain treatment would not be successful for Mr. W without simultaneous treatment for his substance use.
Bottom Line
Both high and low levels of serotonin and dopamine may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: How to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Amantadine • Gocovri
Baclofen • Ozobax
Bupropion • Wellbutrin
Fluoxetine • Prozac
Lisdexamfetamine • Vyvanse
Paroxetine • Paxil
1. Stahl SM. Dazzled by the dominions of dopamine: clinical roles of D3, D2, and D1 receptors. CNS Spectr. 2017;22(4):305-311.
2. Young RL, Lumsden AL, Martin AM, et al. Augmented capacity for peripheral serotonin release in human obesity. Int J Obes (Lond). 2018;42(11):1880-1889.
3. Ahlman H. Serotonin and carcinoid tumors. J Cardiovasc Pharmacol. 1985;7(Suppl 7):S79-S85.
4. Terry N, Margolis KG. Serotonergic mechanisms regulating the GI tract: experimental evidence and therapeutic relevance. Handb Exp Pharmacol. 2017;239:319-342.
5. Prakash S, Belani P, Trivedi A. Headache as a presenting feature in patients with serotonin syndrome: a case series. Cephalalgia. 2014;34(2):148-153.
6. van Ewijk CE, Jacobs GE, Girbes ARJ. Unsuspected serotonin toxicity in the ICU. Ann Intensive Care. 2016;6(1):85.
7. Pedavally S, Fugate JE, Rabinstein AA. Serotonin syndrome in the intensive care unit: clinical presentations and precipitating medications. Neurocrit Care. 2014;21(1):108-113.
8. Nguyen H, Pan A, Smollin C, et al. An 11-year retrospective review of cyproheptadine use in serotonin syndrome cases reported to the California Poison Control System. J Clin Pharm Ther. 2019;44(2):327-334.
9. Ansari H, Kouti L. Drug interaction and serotonin toxicity with opioid use: another reason to avoid opioids in headache and migraine treatment. Curr Pain Headache Rep. 2016;20(8):50.
10. Ott M, Mannchen JK, Jamshidi F, et al. Management of severe arterial hypertension associated with serotonin syndrome: a case report analysis based on systematic review techniques. Ther Adv Psychopharmacol. 2019;9:2045125318818814. doi:10.1177/2045125318818814
11. Cerrito F, Lazzaro MP, Gaudio E, et al. 5HT2-receptors and serotonin release: their role in human platelet aggregation. Life Sci. 1993;53(3):209-215.
12. Ohayon MM. Pain sensitivity, depression, and sleep deprivation: links with serotoninergic dysfunction. J Psychiatr Res. 2009;43(16):1243-1245.
13. Maron E, Shlik J. Serotonin function in panic disorder: important, but why? Neuropsychopharmacology. 2006;31(1):1-11.
14. Hall JE, Guyton AC. Textbook of Medical Physiology. 12th ed. Spanish version. Elsevier; 2011:120,199,201-204,730-740.
15. Garland EJ, Baerg EA. Amotivational syndrome associated with selective serotonin reuptake inhibitors in children and adolescents. J Child Adolesc Psychopharmacol. 2001;11(2):181-186.
16. George MS, Trimble MR. A fluvoxamine-induced frontal lobe syndrome in a patient with comorbid Gilles de la Tourette’s syndrome and obsessive compulsive disorder. J Clin Psychiatry. 1992;53(10):379-380.
17. Hoehn-Saric R, Harris GJ, Pearlson GD, et al. A fluoxetine-induced frontal lobe syndrome in an obsessive compulsive patient. J Clin Psychiatry. 1991;52(3):131-133.
18. Hoehn-Saric R, Lipsey JR, McLeod DR. Apathy and indifference in patients on fluvoxamine and fluoxetine. J Clin Psychopharmacol. 1990;10(5):343-345.
19. Samuelsson M, Jokinen J, Nordström AL, et al. CSF 5-HIAA, suicide intent and hopelessness in the prediction of early suicide in male high-risk suicide attempters. Acta Psychiatr Scand. 2006;113(1):44-47.
20. Brewerton TD. Clinical Handbook of Eating Disorders: An Integrated Approach. CRC Press; 2004:257-281.
21. Mann JJ, Oquendo M, Underwood MD, et al. The neurobiology of suicide risk: a review for the clinician. J Clin Psychiatry. 1999;60 Suppl 2:7-116.
22. Mann JJ, Malone KM. Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients. Biol Psychiatry. 1997;41(2):162-171.
23. Joseph R, Welch KM, D’Andrea G. Serotonergic hypofunction in migraine: a synthesis of evidence based on platelet dense body dysfunction. Cephalalgia. 1989;9(4):293-299.
24. Pakalnis A, Splaingard M, Splaingard D, et al. Serotonin effects on sleep and emotional disorders in adolescent migraine. Headache. 2009;49(10):1486-1492.
25. Virkkunen M, Goldman D, Nielsen DA, et al. Low brain serotonin turnover rate (low CSF 5-HIAA) and impulsive violence. J Psychiatry Neurosci. 1995;20(4):271-275.
26. Liu Y, Zhao J, Fan X, et al. Dysfunction in serotonergic and noradrenergic systems and somatic symptoms in psychiatric disorders. Front Psychiatry. 2019;10:286.
27. Ginsburg GS, Riddle MA, Davies M. Somatic symptoms in children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(10):1179-1187.
28. O’Malley PG, Jackson JL, Santoro J, et al. Antidepressant therapy for unexplained symptoms and symptom syndromes. J Fam Pract. 1999;48(12):980-990.
29. Fortuna JL. Sweet preference, sugar addiction and the familial history of alcohol dependence: shared neural pathways and genes. J Psychoactive Drugs. 2010;42(2):147-151.
30. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
31. Graybiel AM, Saka E. A genetic basis for obsessive grooming. Neuron. 2002;33(1):1-2.
32. Tse W, Hälbig TD. Skin picking in Parkinson’s disease: a behavioral side-effect of dopaminergic treatment? Psychiatry Clin Neurosci. 2010;64(2):214.
33. Ayaydın H. Probable emergence of symptoms of trichotillomania by atomoxetine: a case report. Psychiatry and Clinical Psychopharmacology. 2019;29(2)220-222.
34. Paholpak P, Mendez MF. Trichotillomania as a manifestation of dementia. Case Rep Psychiatry. 2016;2016:9782702. doi:10.1155/2016/9782702
35. Clark CA, Dagher A. The role of dopamine in risk taking: a specific look at Parkinson’s disease and gambling. Front Behav Neurosci. 2014;8:196.
36. Norbury A, Husain M. Sensation-seeking: dopaminergic modulation and risk for psychopathology. Behav Brain Res. 2015;288:79-93.
37. Chen TS, Chang FY. Elevated serum dopamine increases while coffee consumption decreases the occurrence of reddish streaks in the intact stomach. J Gastroenterol Hepatol. 2013;28(12):1810-1814.
38. Wong-Riley MT. Neuroscience Secrets. 1st edition. Spanish version. Hanley & Belfus; 1999:420-429.
39. Arbuck DM. Antipsychotics, dopamine, and pain. Current Psychiatry. 2020;19(1):25-29,31.
40. Bello NT, Hajnal A. Dopamine and binge eating behaviors. Pharmacol Biochem Behav. 2010;97(1):25-33.
41. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70 Suppl 4:1-46.
42. Milev P, Ho BC, Arndt S, et al. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162(3):495-506.
43. Gepshtein S, Li X, Snider J, et al. Dopamine function and the efficiency of human movement. J Cogn Neurosci. 2014;26(3):645-657.
44. Scarff JR. The ABCDs of treating tardive dyskinesia. Current Psychiatry. 2020;19(4):21,55.
1. Stahl SM. Dazzled by the dominions of dopamine: clinical roles of D3, D2, and D1 receptors. CNS Spectr. 2017;22(4):305-311.
2. Young RL, Lumsden AL, Martin AM, et al. Augmented capacity for peripheral serotonin release in human obesity. Int J Obes (Lond). 2018;42(11):1880-1889.
3. Ahlman H. Serotonin and carcinoid tumors. J Cardiovasc Pharmacol. 1985;7(Suppl 7):S79-S85.
4. Terry N, Margolis KG. Serotonergic mechanisms regulating the GI tract: experimental evidence and therapeutic relevance. Handb Exp Pharmacol. 2017;239:319-342.
5. Prakash S, Belani P, Trivedi A. Headache as a presenting feature in patients with serotonin syndrome: a case series. Cephalalgia. 2014;34(2):148-153.
6. van Ewijk CE, Jacobs GE, Girbes ARJ. Unsuspected serotonin toxicity in the ICU. Ann Intensive Care. 2016;6(1):85.
7. Pedavally S, Fugate JE, Rabinstein AA. Serotonin syndrome in the intensive care unit: clinical presentations and precipitating medications. Neurocrit Care. 2014;21(1):108-113.
8. Nguyen H, Pan A, Smollin C, et al. An 11-year retrospective review of cyproheptadine use in serotonin syndrome cases reported to the California Poison Control System. J Clin Pharm Ther. 2019;44(2):327-334.
9. Ansari H, Kouti L. Drug interaction and serotonin toxicity with opioid use: another reason to avoid opioids in headache and migraine treatment. Curr Pain Headache Rep. 2016;20(8):50.
10. Ott M, Mannchen JK, Jamshidi F, et al. Management of severe arterial hypertension associated with serotonin syndrome: a case report analysis based on systematic review techniques. Ther Adv Psychopharmacol. 2019;9:2045125318818814. doi:10.1177/2045125318818814
11. Cerrito F, Lazzaro MP, Gaudio E, et al. 5HT2-receptors and serotonin release: their role in human platelet aggregation. Life Sci. 1993;53(3):209-215.
12. Ohayon MM. Pain sensitivity, depression, and sleep deprivation: links with serotoninergic dysfunction. J Psychiatr Res. 2009;43(16):1243-1245.
13. Maron E, Shlik J. Serotonin function in panic disorder: important, but why? Neuropsychopharmacology. 2006;31(1):1-11.
14. Hall JE, Guyton AC. Textbook of Medical Physiology. 12th ed. Spanish version. Elsevier; 2011:120,199,201-204,730-740.
15. Garland EJ, Baerg EA. Amotivational syndrome associated with selective serotonin reuptake inhibitors in children and adolescents. J Child Adolesc Psychopharmacol. 2001;11(2):181-186.
16. George MS, Trimble MR. A fluvoxamine-induced frontal lobe syndrome in a patient with comorbid Gilles de la Tourette’s syndrome and obsessive compulsive disorder. J Clin Psychiatry. 1992;53(10):379-380.
17. Hoehn-Saric R, Harris GJ, Pearlson GD, et al. A fluoxetine-induced frontal lobe syndrome in an obsessive compulsive patient. J Clin Psychiatry. 1991;52(3):131-133.
18. Hoehn-Saric R, Lipsey JR, McLeod DR. Apathy and indifference in patients on fluvoxamine and fluoxetine. J Clin Psychopharmacol. 1990;10(5):343-345.
19. Samuelsson M, Jokinen J, Nordström AL, et al. CSF 5-HIAA, suicide intent and hopelessness in the prediction of early suicide in male high-risk suicide attempters. Acta Psychiatr Scand. 2006;113(1):44-47.
20. Brewerton TD. Clinical Handbook of Eating Disorders: An Integrated Approach. CRC Press; 2004:257-281.
21. Mann JJ, Oquendo M, Underwood MD, et al. The neurobiology of suicide risk: a review for the clinician. J Clin Psychiatry. 1999;60 Suppl 2:7-116.
22. Mann JJ, Malone KM. Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients. Biol Psychiatry. 1997;41(2):162-171.
23. Joseph R, Welch KM, D’Andrea G. Serotonergic hypofunction in migraine: a synthesis of evidence based on platelet dense body dysfunction. Cephalalgia. 1989;9(4):293-299.
24. Pakalnis A, Splaingard M, Splaingard D, et al. Serotonin effects on sleep and emotional disorders in adolescent migraine. Headache. 2009;49(10):1486-1492.
25. Virkkunen M, Goldman D, Nielsen DA, et al. Low brain serotonin turnover rate (low CSF 5-HIAA) and impulsive violence. J Psychiatry Neurosci. 1995;20(4):271-275.
26. Liu Y, Zhao J, Fan X, et al. Dysfunction in serotonergic and noradrenergic systems and somatic symptoms in psychiatric disorders. Front Psychiatry. 2019;10:286.
27. Ginsburg GS, Riddle MA, Davies M. Somatic symptoms in children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(10):1179-1187.
28. O’Malley PG, Jackson JL, Santoro J, et al. Antidepressant therapy for unexplained symptoms and symptom syndromes. J Fam Pract. 1999;48(12):980-990.
29. Fortuna JL. Sweet preference, sugar addiction and the familial history of alcohol dependence: shared neural pathways and genes. J Psychoactive Drugs. 2010;42(2):147-151.
30. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
31. Graybiel AM, Saka E. A genetic basis for obsessive grooming. Neuron. 2002;33(1):1-2.
32. Tse W, Hälbig TD. Skin picking in Parkinson’s disease: a behavioral side-effect of dopaminergic treatment? Psychiatry Clin Neurosci. 2010;64(2):214.
33. Ayaydın H. Probable emergence of symptoms of trichotillomania by atomoxetine: a case report. Psychiatry and Clinical Psychopharmacology. 2019;29(2)220-222.
34. Paholpak P, Mendez MF. Trichotillomania as a manifestation of dementia. Case Rep Psychiatry. 2016;2016:9782702. doi:10.1155/2016/9782702
35. Clark CA, Dagher A. The role of dopamine in risk taking: a specific look at Parkinson’s disease and gambling. Front Behav Neurosci. 2014;8:196.
36. Norbury A, Husain M. Sensation-seeking: dopaminergic modulation and risk for psychopathology. Behav Brain Res. 2015;288:79-93.
37. Chen TS, Chang FY. Elevated serum dopamine increases while coffee consumption decreases the occurrence of reddish streaks in the intact stomach. J Gastroenterol Hepatol. 2013;28(12):1810-1814.
38. Wong-Riley MT. Neuroscience Secrets. 1st edition. Spanish version. Hanley & Belfus; 1999:420-429.
39. Arbuck DM. Antipsychotics, dopamine, and pain. Current Psychiatry. 2020;19(1):25-29,31.
40. Bello NT, Hajnal A. Dopamine and binge eating behaviors. Pharmacol Biochem Behav. 2010;97(1):25-33.
41. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70 Suppl 4:1-46.
42. Milev P, Ho BC, Arndt S, et al. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162(3):495-506.
43. Gepshtein S, Li X, Snider J, et al. Dopamine function and the efficiency of human movement. J Cogn Neurosci. 2014;26(3):645-657.
44. Scarff JR. The ABCDs of treating tardive dyskinesia. Current Psychiatry. 2020;19(4):21,55.
Could stem cells have a role in treating mental illnesses?
While laboratory studies move forward at full speed, the clinical use of stem cells—undifferentiated cells that can develop into many different types of specialized cells—remains controversial. Presently, only unadulterated stem cells are allowed to be used in patients, and only on an experimental and investigational basis. Stem cells that have been expanded, modified, or enhanced outside of the body are not allowed to be used for clinical application in the United States at this time. In June 2021, the FDA strengthened the language of stem cell regulation, further limiting their clinical application (see https://www.fda.gov/vaccines-blood-biologics/consumers-biologics/important-patient-and-consumer-information-about-regenerative-medicine-therapies). Yet some applications, such as treatment of lymphoma or restorative knee injections, are covered by some health insurance plans, and the acceptance of stem cell treatment is growing.
In this article, I describe the basics of stem cells, and explore the potential therapeutic use of stem cells for treating various mental illnesses.
Stem cells: A primer
Human embryonic stem cells were initially investigated for their healing properties. However, the need to harvest these cells from embryos drew much criticism, and many found the process to be ethically and religiously unacceptable. This was resolved by the Nobel prize–winning discovery that adult somatic cells can be reprogrammed into cells with embryonic stem cell properties by introducing specific transcription factors. These cells have been termed “induced pluripotent stem cells” (iPSCs).1 The use of adult stem cells and stem cells from the umbilical cords of healthy newborns has allowed for wider acceptance of stem cell research and treatment.
Stem cells may be collected from the patient himself or herself; these are autologous stem cells. They may also be harvested from healthy newborn waste, such as the umbilical cord blood and wall; these are allogenic stem cells. Autologous stem cells are present in almost any tissue but are usually collected from the patient’s adipose tissue or from bone marrow. Understandably, younger stem cells possess higher healing properties. Stem cells may be mesenchymal, producing primarily connective and nervous tissue, or hematopoietic, influencing the immune system and blood cell production, though there is a considerable overlap in the function of these types of cells.
Adult somatic stem cells may be turned into stem cells (iPSCs) and then become any tissue, including neurons. This ability of stem cells to physically regenerate the CNS is directly relevant to psychiatry.
In addition to neurogenesis, stem cell transplants can assist in immune and vascular restoration as well as in suppressing inflammation. The ability of stem cells to replace mutated genes may be useful for addressing inheritable neuropsychiatric conditions.
Both autoimmune and inflammatory mechanisms play an important role in most psychiatric illnesses. The more we learn, the more it is clear that brain function is profoundly dependent on more than just its structure, and that structure depends on more than blood supply. Stem cells influence the vascular, nutritional, functional, inflammatory, and immune environment of the brain, potentially assisting in cognitive and emotional rehabilitation.
Stem cells operate in 2 fundamental ways: via direct cell-to-cell interaction, and via the production and release of growth, immune-regulating, and anti-inflammatory factors. Such factors are produced within the cells and then released in the extracellular environment as a content of exosomes. The route of administration is important in the delivery of the stem cells to the target tissue. Unlike their direct introduction into a joint, muscle, or intervertebral disk, injection of stem cells into the brain is more complicated and not routinely feasible. Intrathecal injections may bring stem cells into the CNS, but cerebrospinal fluid does not easily carry stem cells into the brain, and certainly cannot deliver them to an identified target within the brain. Existing technology can allow stem cells to be packaged in such a way that they can penetrate the blood-brain barrier, but this requires stem cell modification, which presently is not permitted in clinical practice in the United States. Alternatively, there is a way to weaken the blood-brain barrier to allow stem cells to travel through the “opened doors,” so to speak, but this allows everything to have access to the CNS, which may be unsafe. IV administration is technologically easy, and it grants stem cells the environment to multiply and produce extracellular factors that can cross the blood-brain barrier, while large cells cannot.
Continue to: Stem cells as a treatment for mental illness...
Stem cells as a treatment for mental illness
Based on our understanding of the function of stem cells, many neurodegenerative-, vascular-, immune-, and inflammation-based psychiatric conditions can be influenced by stem cell treatment. Here I review the potential therapeutic role of stem cells in the treatment of several psychiatric disorders.
Alzheimer’s dementia
Alzheimer’s dementia (AD) is a progressive neurodegenerative pathology based on neuronal and synaptic loss. Repopulation and regeneration of depleted neuronal circuitry by exogenous stem cells may be a rational therapeutic strategy.2 The regeneration of lost neurons has the potential to restore cognitive function. Multiple growth factors that regulate neurogenesis are abundant during child development but dramatically decline with age. The introduction of stem cells—especially those derived from newborn waste—seem to promote recovery from neurodegenerative disease or injury.3
There currently is no cure for AD. Cellular therapy promises new advances in treatment.4 Neurogenesis occurs not only during fetal development but in the adult brain. Neural stem cells reside in the adult CNS of all mammals.5 They are intimately involved in continuous restoration, but age just like the rest of the animal tissue, providing ever-decreasing restorative potential.
The number of studies of stem cells in AD has increased since the early 2000 s,6,7 and research continues to demonstrate robust CNS neurogenesis. In a 2020 study, Zappa Villar et al8 evaluated stem cells as a treatment for rats in which an AD model was induced by the intracerebroventricular injection of streptozotocin (STZ). The STZ-treated rats displayed poor performance in all behavioral tests. Stem cell therapy increased exploratory behavior, decreased anxiety, and improved spatial memory and marble-burying behavior; the latter was representative of daily life activities. Importantly, stem cell therapy ameliorated and restored hippocampal atrophy and some presynaptic protein levels in the rats with AD.8 Animal models cannot be automatically applied to humans, but they shine a light on the areas that need further exploration.
In humans, elevated cortisol levels during aging predict hippocampal atrophy and memory deficits,9 and this deficiency may be positively influenced by stem cell treatment.
Schizophrenia
Recent research indicates that schizophrenia may begin with abnormal neurogenesis from neural stem cells inside the embryo, and that this process may be particularly vulnerable to numerous genetic and/or environmental disturbances of early brain development.10 Because neurogenesis is not confined to the womb but is a protracted process that continues into postnatal life, adolescence and beyond, influencing this process may be a way to add to the schizophrenia treatment armamentarium.10 Sacco et al11 described links between the alteration of intrauterine and adult neurogenesis and the causes of neuropsychiatric disorders, including schizophrenia. Immune and inflammatory mechanisms are important in the etiology of schizophrenia. By their core function, stem cells address both mechanisms, and may directly modulate this devastating disease.
In addition to clinical hopes, advances in research tools hold the promise of new discoveries. With the advent of iPSC technology, it is possible to generate live neurons in vitro from somatic tissue of patients with schizophrenia. Despite its many limitations, this revolutionary technology has already helped to advance our understanding of schizophrenia.11
Bipolar disorder
Many of the fundamental neurobiological mechanisms of schizophrenia are mirrored in bipolar disorder.12 Though we are not ready to bring stem cells into the day-to-day treatment of this condition, several groups are starting to apply iPSC technology to the study of bipolar disorder.13
Neurodevelopmental factors—particularly pathways related to nervous system development, cell migration, extracellular matrix, methylation, and calcium signaling—have been identified in large gene expression studies as altered in bipolar disorder.14 Stem cell technology opens doorways to reverse engineering of human neurodegenerative disease.15
Continue to: Autism spectrum disorders...
Autism spectrum disorders
Autism spectrum disorders (ASDs) are multiple heterogeneous neurodevelopmental disorders.16 Neuroinflammation and immune dysregulation influence the origin of ASDs. Due to the neurobiologic changes underlying ASD development, cell-based therapies, including the use of mesenchymal stem cells (MSCs), have been applied to ASDs.16 Stem cells show specific immunologic properties that make them promising candidates for treating ASDs.17
The exact mechanisms of action of MSCs to restore function in patients with ASDs are largely unknown, but proposed mechanisms include:
- synthesizing and releasing anti-inflammatory cytokines and survival-promoting growth factors
- integrating into the existing neural and synaptic network
- restoring plasticity.18
In a study of transplantation of human cord blood cells and umbilical cord–derived MSCs for patients with ASDs, Bradstreet et al19 found a statistically significant difference on scores for domains of speech, sociability, sensory, and overall health, as well as reductions in the total scores, in those who received transplants compared to their pretreatment values.
In another study of stem cell therapy for ASDs, Lv et al20 demonstrated the safety and efficacy of combined transplantation of human cord blood cells and umbilical cord–derived MSCs in treating children with ASDs. The transplantations included 4 stem cell IV infusions and intrathecal injections once a week. Statistically significant differences were shown at 24 weeks post-treatment. Although this nonrandomized, open-label, single-center Phase I/II trial cannot be relied on for any definitive conclusions, it suggests an important area of investigation.20
The vascular aspects of ASDs’ pathogenesis should not be overlooked. For example, specific temporal lobe areas associated with facial recognition, social interaction, and language comprehension have been demonstrated to be hypoperfused in children with ASDs, but not in controls. The degree of hypoperfusion and resulting hypoxia correlates with the severity of ASD symptoms. The damage causing hypoperfusion of temporal areas was associated with the onset of autism-like disorders. Damage of the amygdala, hippocampus, or other temporal structures induces permanent or transient autistic-like characteristics, such as unexpressive faces, little eye contact, and motor stereotypes. Clinically, temporal lobe damage by viral and other means has been implicated in the development of ASD in children and adults. Hypoperfusion may contribute to defects, not only by inducing hypoxia, but also by allowing for abnormal metabolite or neurotransmitter accumulation. This is one of the reasons glutamate toxicity has been implicated in ASD. The augmentation of perfusion through stimulation of angiogenesis by stem cells should allow for metabolite clearance and restoration of functionality. Vargas et al21 compared brain autopsy samples from 11 children with ASDs to those of 7 age-matched controls. They demonstrated an active neuroinflammatory process in the cerebral cortex, white matter, and cerebellum of patients with ASDs, both by immunohistochemistry and morphology.21
Multiple studies have confirmed that the systemic administration of cord blood cells is sufficient to induce neuroregeneration.22,23 Angiogenesis has been experimentally demonstrated in peripheral artery disease, myocardial ischemia, and stroke, and has direct implications on brain repair.24 Immune dysregulation25,26 and immune modulation27 also are addressed by stem cell treatment, which provides a promising avenue for battling ASDs.
Like attention-deficit/hyperactivity disorder and obsessive-compulsive disorder, ASDs are neurodevelopmental conditions. Advances based on the use of stem cells hold great promise for understanding, diagnosing and, possibly, treating these psychiatric disorders.28,29
Depression
Neuropsychiatric disorders arise from deviations from the regular differentiation process of the CNS, leading to altered neuronal connectivity. Relatively subtle abnormalities in the size and number of cells in the prefrontal cortex and basal ganglia have been observed in patients with depressive disorder and Tourette syndrome.30 Fibroblast-derived iPSCs generate serotonergic neurons through the exposure of the cells to growth factors and modulators of signaling pathways. If these serotonergic neurons are made from the patients’ own cells, they can be used to screen for new therapeutics and elucidate the unknown mechanisms through which current medications may function.31 This development could lead to the discovery of new medication targets and new insights into the molecular biology of depression.32
Deficiencies of brain-derived neurotrophic factor (BDNF) have a role in depression, anxiety, and other neuropsychiatric illnesses. The acute behavioral effects of selective serotonin reuptake inhibitors and tricyclic antidepressants seem to require BDNF signaling, which suggests that BDNF holds great potential as a therapeutic agent. Cell therapies focused on correcting BDNF deficiencies in mice have had some success.33
Dysregulation of GABAergic neurons has also been implicated in depression and anxiety. Patients with major depressive disorder have reduced gamma aminobutyric acid (GABA) receptors in the parahippocampal and lateral temporal lobes.34
Ultimately, the development of differentiation protocols for serotonergic and GABAergic neuronal populations will pave the way for examining the role of these populations in the pathogenesis of depression and anxiety, and may eventually open the door for cell-based therapies in humans.35
Studies have demonstrated a reduction in the density of pyramidal and nonpyramidal neurons in the anterior cingulate cortex of patients with schizophrenia and bipolar disorder,36 glial reduction in the subgenual prefrontal cortex in mood disorders,37 and morphometric evidence for neuronal and glial prefrontal cell pathology in major depressive disorder.38 The potential for stem cells to repair such pathology may be of clinical benefit to many patients.
Aside from their other suggested clinical uses, iPSCs may be utilized in new pathways for research on the biology and pharmacology of major depressive disorder.39
Continue to: Obsessive-compulsive disorder...
Obsessive-compulsive disorder
Obsessive-compulsive disorder (OCD) is often characterized by excessive behaviors related to cleanliness, including grooming, which is represented across most animal species. In mice, behaviors such as compulsive grooming and hair removal—similar to behaviors in humans with OCD or trichotillomania—are associated with a specific mutation. Chen et al40 reported that the transplantation of bone marrow stem cells into mice with this mutation (bone marrow–derived microglia specifically home to the brain) rescues their pathological phenotype by repairing native neurons.
The autoimmune, inflammatory, and neurodegenerative changes that are prevalent in OCD may be remedied by stem cell treatment in a fashion described throughout this article.
Other conditions
The Box41-50 describes a possible role for stem cells in the treatment or prevention of several types of substance use disorders.
Box
Researchers have begun to explore stem cells as a potential treatment for several substance use disorders, including those involving alcohol, cocaine, and opioids, as well as their interactions with cannabinoids.
Alcohol use disorder. In a 2017 study, Israel et al41 gave intra-cerebral injections of mesenchymal stem cells (MSCs) to rats that were bred to have a high alcohol intake. The MSC injections resulted in drastic reductions in the rats’ alcohol consumption. A single intracerebroventricular MSC administration inhibited relapse-like drinking by up to 85% for 40 days.
It is beyond unlikely that direct brain injections would be used to treat alcohol use disorder in humans. To address this problem, researchers aggregated MSCs into smaller spheroid shapes, which reduced their size up to 75% and allowed them to be injected intravenously to reach the brain in a study conducted in rats.42 Within 48 hours of a single treatment, the rats had reduced their intake of alcohol by 90%. The IV administration of antiinflammatory MSCs in human trials will be the next step to verify these results.
Alcohol research using human stem cells is also being conducted as a model system to understand the neural mechanisms of alcohol use disorder.43
Cocaine use disorder. In a grant proposal, Yadid and Popovtzer44 suggested that cocaine addiction affects neurogenesis, especially in the dentate gyrus, ventral tegmental area, nucleus accumbens, and prefrontal cortex; it damages mitochondrial RNA, brain-derived neurotrophic factor (BDNF), glutamate transporter (excitatory amino acid transporter; EAAT), and interleukin-10. MSCs have a predilection to these areas and influence neurogenesis. Currently, there are no FDAapproved medications for the safe and effective treatment of cocaine addiction. MSCs can home to pathological areas in the brain, release growth factors, and serve as cellular delivery tools in various brain disorders. Moreover, restoration of basal glutamate levels via the EAAT has been proposed as a promising target for treating cocaine dependence. Therefore, MSCs differentiated to express EAATs may have a combined long-term effect that can attenuate cocaine craving and relapse.44
Neural stem cells undergo a series of developmental processes before giving rise to newborn neurons, astrocytes, and oligodendrocytes in adult neurogenesis. During the past decade, studies of adult neurogenesis modulated by addictive drugs have highlighted the role of stem cells. These drugs have been shown to regulate the proliferation, differentiation, and survival of adult cells in different manners, which results in the varying consequences of adult neurogenesis.45 Reversal of these influences by healthy stem cells can be a worthy goal to pursue.
Opioid use disorder. Opiate medications cause a loss of newly born neural progenitors in the subgranular zone of the dentate gyrus by either modulating proliferation or interfering with differentiation and maturation.46 Opiates were the first medications shown to negatively impact neurogenesis in the adult mammalian hippocampus.47,48 The restoration of hippocampal function may positively affect the prognosis of a patient who is addicted.
Cannabinoids. Cannabinoids’ influence on the brain and on stem cells is controversial. On one hand, deteriorated neurogenesis results in reduced long-term potentiation in hippocampal formation. These cellular and physiological alterations lead to decreased short-term spatial memory and increased depressionlike behaviors.49 On the other hand, there is emerging evidence that cannabinoids improve neurogenesis and CNS plasticity, at least in the adult mouse.50 Through normalization of immune function, and restoration of the brain and the body, stem cells may assist in better health and in treatment of cannabis use disorder.
Chronic pain is a neuropsychiatric condition that involves the immune system, inflammation, vascularization, trophic changes, and other aspects of the CNS function in addition to peripheral factors and somatic pain generators. Treatment of painful conditions with the aid of stem cells represents a large and ever-developing field that lies outside of the scope of this article.51
Experimental, but promising
It is not easy to accept revolutionary new approaches in medicine. Endless research and due diligence are needed to prove a concept and then to work out specific applications, safeguards, and limitations for any novel treatments. The stem cell terrain is poorly explored, and one needs to be careful when venturing there. Presently, the FDA appropriately sees treatment with stem cells as experimental and investigational, particularly in the mental health arena. Stem cells are not approved for treatment of any specific condition. At the same time, research and clinical practice suggest stem cell treatment may someday play a more prominent role in health care. Undoubtedly, psychiatry will eventually benefit from the knowledge and application of stem cell research and practice.
Related Resources
- De Los Angeles A, Fernando MB, Hall NAL, et al. Induced pluripotent stem cells in psychiatry: an overview and critical perspective. Biol Psychiatry. 2021;90(6):362-372.
- Heider J, Vogel S, Volkmer H, et al. Human iPSC-derived glia as a tool for neuropsychiatric research and drug development. Int J Mol Sci. 2021;22(19):10254.
Drug Brand Name
Streptozotocin • Zanosar
Bottom Line
Treatment with stem cell transplantation is experimental and not approved for any medical or psychiatric illness. However, based on our growing understanding of the function of stem cells, and preliminary research conducted mainly in animals, many neurodegenerative-, vascular-, immune-, and inflammation-based psychiatric conditions might be beneficially influenced by stem cell treatment.
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861-872.
- Duncan T, Valenzuela M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res Ther. 2017;8(1):111.
- Brinton RD, Wang JM. Therapeutic potential of neurogenesis for prevention and recovery from Alzheimer’s disease: allopregnanolone as a proof of concept neurogenic agent. Curr Alzheimer Res. 2006;3(3):185-190.
- Taupin P. Adult neurogenesis, neural stem cells, and Alzheimer’s disease: developments, limitations, problems, and promises. Curr Alzheimer Res. 2009;6(6):461-470.
- Taupin P. Neurogenesis, NSCs, pathogenesis, and therapies for Alzheimer’s disease. Front Biosci (Schol Ed). 2011;3:178-90.
- Kang JM, Yeon BK, Cho SJ, et al. Stem cell therapy for Alzheimer’s disease: a review of recent clinical trials. J Alzheimers Dis. 2016;54(3):879-889.
- Li M, Guo K, Ikehara S. Stem cell treatment for Alzheimer’s disease. Int J Mol Sci. 2014;15(10):19226-19238.
- Zappa Villar MF, López Hanotte J, Pardo J, et al. Mesenchymal stem cells therapy improved the streptozotocin-induced behavioral and hippocampal impairment in rats. Mol Neurobiol. 2020;57(2):600-615.
- Lupien SJ, de Leon M, de Santi S, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1(1):69-73.
- Iannitelli A, Quartini A, Tirassa P, et al. Schizophrenia and neurogenesis: a stem cell approach. Neurosci Biobehav Rev. 2017;80:414-442.
- Sacco R, Cacci E, Novarino G. Neural stem cells in neuropsychiatric disorders. Curr Opin Neurobiol. 2018; 48:131-138.
- Miller ND, Kelsoe JR. Unraveling the biology of bipolar disorder using induced pluripotent stem-derived neurons. Bipolar Disord. 2017;19(7):544-551.
- O’Shea KS, McInnis MG. Neurodevelopmental origins of bipolar disorder: iPSC models. Mol Cell Neurosci. 2016;73:63-83.
- Jacobs BM. A dangerous method? The use of induced pluripotent stem cells as a model for schizophrenia. Schizophr Res. 2015;168(1-2):563-568.
- Liu Y, Deng W. Reverse engineering human neurodegenerative disease using pluripotent stem cell technology. Brain Res. 2016;1638(Pt A):30-41.
- Siniscalco D, Kannan S, Semprún-Hernández N, et al. Stem cell therapy in autism: recent insights. Stem Cells Cloning. 2018;11:55-67.
- Siniscalco D, Bradstreet JJ, Sych N, et al. Mesenchymal stem cells in treating autism: novel insights. World J Stem Cells. 2014;6(2):173-178.
- Siniscalco D, Sapone A, Cirillo A, et al. Autism spectrum disorders: is mesenchymal stem cell personalized therapy the future? J Biomed Biotechnol. 2012; 2012:480289.
- Bradstreet JJ, Sych N, Antonucci N, et al. Efficacy of fetal stem cell transplantation in autism spectrum disorders: an open-labeled pilot study. Cell Transplant. 2014;23(Suppl 1):S105-S112.
- Lv YT, Zhang Y, Liu M, et al. Transplantation of human cord blood mononuclear cells and umbilical cordderived mesenchymal stem cells in autism. J Transl Med. 2013;11:196.
- Vargas DL, Nascimbene C, Krishnan C, et al. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57(1):67-81.
- Wei L, Keogh CL, Whitaker VR, et al. Angiogenesis and stem cell transplantation as potential treatments of cerebral ischemic stroke. Pathophysiology. 2005;12(1): 47-62.
- Newman MB, Willing AE, Manresa JJ, et al. Cytokines produced by cultured human umbilical cord blood (HUCB) cells: implications for brain repair. Exp Neurol. 2006;199(1):201-218.
- Peterson DA. Umbilical cord blood cells and brain stroke injury: bringing in fresh blood to address an old problem. J Clin Invest. 2004;114(3):312-314.
- Cohly HH, Panja A. Immunological findings in autism. Int Rev Neurobiol. 2005;71:317-341.
- Ashwood P, Van de Water J. Is autism an autoimmune disease? Autoimmun Rev. 2004;3(7-8):557-562.
- Yagi H, Soto-Gutierrez A, Parekkadan B, et al. Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant. 2010;19(6):667-679.
- Vaccarino FM, Urban AE, Stevens HE, et al. Annual Research Review: The promise of stem cell research for neuropsychiatric disorders. J Child Psychol Psychiatry. 2011;52(4):504-516.
- Liu EY, Scott CT. Great expectations: autism spectrum disorder and induced pluripotent stem cell technologies. Stem Cell Rev Rep. 2014;10(2):145-150.
- Richardson-Jones JW, Craige CP, Guiard BP, et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65(1):40-52.
- Saarelainen T, Hendolin P, Lucas G, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23(1):349-357.
- Klumpers UM, Veltman DJ, Drent ML, et al. Reduced parahippocampal and lateral temporal GABAA-[11C] flumazenil binding in major depression: preliminary results. Eur J Nucl Med Mol Imaging. 2010;37(3): 565-574.
- Bremner JD, Narayan M, Anderson ER, et al. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157(1):115-118.
- Bremner JD, Randall P, Scott TM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152(7):973-981.
- Vincent SL, Todtenkopf MS, Benes FM. A comparison of the density of pyramidal and non-pyramidal neurons in the anterior cingulate cortex of schizophrenics and manic depressives. Soc Neurosci Abstr. 1997;23:2199.
- Benes FM, Kwok EW, Vincent SL, et al. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44(2): 88-97.
- Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95(22):13290-13295.
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45(9): 1085-1098.
- Licinio J, Wong ML. Serotonergic neurons derived from induced pluripotent stem cells (iPSCs): a new pathway for research on the biology and pharmacology of major depression. Mol Psychiatry. 2016;21(1):1-2.
- Chen SK, Tvrdik P, Peden E, et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141(5):775-785.
- Israel Y, Ezquer F, Quintanilla ME, et al. Intracerebral stem cell administration inhibits relapse-like alcohol drinking in rats. Alcohol Alcohol. 2017;52(1):1-4.
- Ezquer F, Morales P, Quintanilla ME, et al. Intravenous administration of anti-inflammatory mesenchymal stem cell spheroids reduces chronic alcohol intake and abolishes binge-drinking. Sci Rep. 2018;8(1):4325.
- Scarnati MS, Halikere A, Pang ZP. Using human stem cells as a model system to understand the neural mechanisms of alcohol use disorders: current status and outlook. Alcohol. 2019;74:83-93.
- Yadid GM, Popovtzer R. Nanoparticle-mesenchymal stem cell conjugates for cell therapy in drug addiction. NIH grant application. 2017.
- Xu C, Loh HH, Law PY. Effects of addictive drugs on adult neural stem/progenitor cells. Cell Mol Life Sci. 2016;73(2):327-348.
- Dholakiya SL, Aliberti A, Barile FA. Morphine sulfate concomitantly decreases neuronal differentiation and opioid receptor expression in mouse embryonic stem cells. Toxicol Lett. 2016;247:45-55.
- Zhang Y, Loh HH, Law PY. Effect of opioid on adult hippocampal neurogenesis. Scientific World Journal. 2016;2016:2601264.
- Bortolotto V, Grilli M. Opiate analgesics as negative modulators of adult hippocampal neurogenesis: potential implications in clinical practice. Front Pharmacol. 2017; 8:254.
- Galve-Roperh I, Chiurchiù V, Díaz-Alonso J, et al. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog Lipid Res. 2013; 52(4):633-650.
- Zimmermann T, Maroso M, Beer A, et al. Neural stem cell lineage-specific cannabinoid type-1 receptor regulates neurogenesis and plasticity in the adult mouse hippocampus. Cereb Cortex. 2018;28(12):4454-4471.
- Ren J, Liu N, Sun N, et al. Mesenchymal stem cells and their exosomes: promising therapies for chronic pain. Curr Stem Cell Res Ther. 2019;14(8):644-653.
While laboratory studies move forward at full speed, the clinical use of stem cells—undifferentiated cells that can develop into many different types of specialized cells—remains controversial. Presently, only unadulterated stem cells are allowed to be used in patients, and only on an experimental and investigational basis. Stem cells that have been expanded, modified, or enhanced outside of the body are not allowed to be used for clinical application in the United States at this time. In June 2021, the FDA strengthened the language of stem cell regulation, further limiting their clinical application (see https://www.fda.gov/vaccines-blood-biologics/consumers-biologics/important-patient-and-consumer-information-about-regenerative-medicine-therapies). Yet some applications, such as treatment of lymphoma or restorative knee injections, are covered by some health insurance plans, and the acceptance of stem cell treatment is growing.
In this article, I describe the basics of stem cells, and explore the potential therapeutic use of stem cells for treating various mental illnesses.
Stem cells: A primer
Human embryonic stem cells were initially investigated for their healing properties. However, the need to harvest these cells from embryos drew much criticism, and many found the process to be ethically and religiously unacceptable. This was resolved by the Nobel prize–winning discovery that adult somatic cells can be reprogrammed into cells with embryonic stem cell properties by introducing specific transcription factors. These cells have been termed “induced pluripotent stem cells” (iPSCs).1 The use of adult stem cells and stem cells from the umbilical cords of healthy newborns has allowed for wider acceptance of stem cell research and treatment.
Stem cells may be collected from the patient himself or herself; these are autologous stem cells. They may also be harvested from healthy newborn waste, such as the umbilical cord blood and wall; these are allogenic stem cells. Autologous stem cells are present in almost any tissue but are usually collected from the patient’s adipose tissue or from bone marrow. Understandably, younger stem cells possess higher healing properties. Stem cells may be mesenchymal, producing primarily connective and nervous tissue, or hematopoietic, influencing the immune system and blood cell production, though there is a considerable overlap in the function of these types of cells.
Adult somatic stem cells may be turned into stem cells (iPSCs) and then become any tissue, including neurons. This ability of stem cells to physically regenerate the CNS is directly relevant to psychiatry.
In addition to neurogenesis, stem cell transplants can assist in immune and vascular restoration as well as in suppressing inflammation. The ability of stem cells to replace mutated genes may be useful for addressing inheritable neuropsychiatric conditions.
Both autoimmune and inflammatory mechanisms play an important role in most psychiatric illnesses. The more we learn, the more it is clear that brain function is profoundly dependent on more than just its structure, and that structure depends on more than blood supply. Stem cells influence the vascular, nutritional, functional, inflammatory, and immune environment of the brain, potentially assisting in cognitive and emotional rehabilitation.
Stem cells operate in 2 fundamental ways: via direct cell-to-cell interaction, and via the production and release of growth, immune-regulating, and anti-inflammatory factors. Such factors are produced within the cells and then released in the extracellular environment as a content of exosomes. The route of administration is important in the delivery of the stem cells to the target tissue. Unlike their direct introduction into a joint, muscle, or intervertebral disk, injection of stem cells into the brain is more complicated and not routinely feasible. Intrathecal injections may bring stem cells into the CNS, but cerebrospinal fluid does not easily carry stem cells into the brain, and certainly cannot deliver them to an identified target within the brain. Existing technology can allow stem cells to be packaged in such a way that they can penetrate the blood-brain barrier, but this requires stem cell modification, which presently is not permitted in clinical practice in the United States. Alternatively, there is a way to weaken the blood-brain barrier to allow stem cells to travel through the “opened doors,” so to speak, but this allows everything to have access to the CNS, which may be unsafe. IV administration is technologically easy, and it grants stem cells the environment to multiply and produce extracellular factors that can cross the blood-brain barrier, while large cells cannot.
Continue to: Stem cells as a treatment for mental illness...
Stem cells as a treatment for mental illness
Based on our understanding of the function of stem cells, many neurodegenerative-, vascular-, immune-, and inflammation-based psychiatric conditions can be influenced by stem cell treatment. Here I review the potential therapeutic role of stem cells in the treatment of several psychiatric disorders.
Alzheimer’s dementia
Alzheimer’s dementia (AD) is a progressive neurodegenerative pathology based on neuronal and synaptic loss. Repopulation and regeneration of depleted neuronal circuitry by exogenous stem cells may be a rational therapeutic strategy.2 The regeneration of lost neurons has the potential to restore cognitive function. Multiple growth factors that regulate neurogenesis are abundant during child development but dramatically decline with age. The introduction of stem cells—especially those derived from newborn waste—seem to promote recovery from neurodegenerative disease or injury.3
There currently is no cure for AD. Cellular therapy promises new advances in treatment.4 Neurogenesis occurs not only during fetal development but in the adult brain. Neural stem cells reside in the adult CNS of all mammals.5 They are intimately involved in continuous restoration, but age just like the rest of the animal tissue, providing ever-decreasing restorative potential.
The number of studies of stem cells in AD has increased since the early 2000 s,6,7 and research continues to demonstrate robust CNS neurogenesis. In a 2020 study, Zappa Villar et al8 evaluated stem cells as a treatment for rats in which an AD model was induced by the intracerebroventricular injection of streptozotocin (STZ). The STZ-treated rats displayed poor performance in all behavioral tests. Stem cell therapy increased exploratory behavior, decreased anxiety, and improved spatial memory and marble-burying behavior; the latter was representative of daily life activities. Importantly, stem cell therapy ameliorated and restored hippocampal atrophy and some presynaptic protein levels in the rats with AD.8 Animal models cannot be automatically applied to humans, but they shine a light on the areas that need further exploration.
In humans, elevated cortisol levels during aging predict hippocampal atrophy and memory deficits,9 and this deficiency may be positively influenced by stem cell treatment.
Schizophrenia
Recent research indicates that schizophrenia may begin with abnormal neurogenesis from neural stem cells inside the embryo, and that this process may be particularly vulnerable to numerous genetic and/or environmental disturbances of early brain development.10 Because neurogenesis is not confined to the womb but is a protracted process that continues into postnatal life, adolescence and beyond, influencing this process may be a way to add to the schizophrenia treatment armamentarium.10 Sacco et al11 described links between the alteration of intrauterine and adult neurogenesis and the causes of neuropsychiatric disorders, including schizophrenia. Immune and inflammatory mechanisms are important in the etiology of schizophrenia. By their core function, stem cells address both mechanisms, and may directly modulate this devastating disease.
In addition to clinical hopes, advances in research tools hold the promise of new discoveries. With the advent of iPSC technology, it is possible to generate live neurons in vitro from somatic tissue of patients with schizophrenia. Despite its many limitations, this revolutionary technology has already helped to advance our understanding of schizophrenia.11
Bipolar disorder
Many of the fundamental neurobiological mechanisms of schizophrenia are mirrored in bipolar disorder.12 Though we are not ready to bring stem cells into the day-to-day treatment of this condition, several groups are starting to apply iPSC technology to the study of bipolar disorder.13
Neurodevelopmental factors—particularly pathways related to nervous system development, cell migration, extracellular matrix, methylation, and calcium signaling—have been identified in large gene expression studies as altered in bipolar disorder.14 Stem cell technology opens doorways to reverse engineering of human neurodegenerative disease.15
Continue to: Autism spectrum disorders...
Autism spectrum disorders
Autism spectrum disorders (ASDs) are multiple heterogeneous neurodevelopmental disorders.16 Neuroinflammation and immune dysregulation influence the origin of ASDs. Due to the neurobiologic changes underlying ASD development, cell-based therapies, including the use of mesenchymal stem cells (MSCs), have been applied to ASDs.16 Stem cells show specific immunologic properties that make them promising candidates for treating ASDs.17
The exact mechanisms of action of MSCs to restore function in patients with ASDs are largely unknown, but proposed mechanisms include:
- synthesizing and releasing anti-inflammatory cytokines and survival-promoting growth factors
- integrating into the existing neural and synaptic network
- restoring plasticity.18
In a study of transplantation of human cord blood cells and umbilical cord–derived MSCs for patients with ASDs, Bradstreet et al19 found a statistically significant difference on scores for domains of speech, sociability, sensory, and overall health, as well as reductions in the total scores, in those who received transplants compared to their pretreatment values.
In another study of stem cell therapy for ASDs, Lv et al20 demonstrated the safety and efficacy of combined transplantation of human cord blood cells and umbilical cord–derived MSCs in treating children with ASDs. The transplantations included 4 stem cell IV infusions and intrathecal injections once a week. Statistically significant differences were shown at 24 weeks post-treatment. Although this nonrandomized, open-label, single-center Phase I/II trial cannot be relied on for any definitive conclusions, it suggests an important area of investigation.20
The vascular aspects of ASDs’ pathogenesis should not be overlooked. For example, specific temporal lobe areas associated with facial recognition, social interaction, and language comprehension have been demonstrated to be hypoperfused in children with ASDs, but not in controls. The degree of hypoperfusion and resulting hypoxia correlates with the severity of ASD symptoms. The damage causing hypoperfusion of temporal areas was associated with the onset of autism-like disorders. Damage of the amygdala, hippocampus, or other temporal structures induces permanent or transient autistic-like characteristics, such as unexpressive faces, little eye contact, and motor stereotypes. Clinically, temporal lobe damage by viral and other means has been implicated in the development of ASD in children and adults. Hypoperfusion may contribute to defects, not only by inducing hypoxia, but also by allowing for abnormal metabolite or neurotransmitter accumulation. This is one of the reasons glutamate toxicity has been implicated in ASD. The augmentation of perfusion through stimulation of angiogenesis by stem cells should allow for metabolite clearance and restoration of functionality. Vargas et al21 compared brain autopsy samples from 11 children with ASDs to those of 7 age-matched controls. They demonstrated an active neuroinflammatory process in the cerebral cortex, white matter, and cerebellum of patients with ASDs, both by immunohistochemistry and morphology.21
Multiple studies have confirmed that the systemic administration of cord blood cells is sufficient to induce neuroregeneration.22,23 Angiogenesis has been experimentally demonstrated in peripheral artery disease, myocardial ischemia, and stroke, and has direct implications on brain repair.24 Immune dysregulation25,26 and immune modulation27 also are addressed by stem cell treatment, which provides a promising avenue for battling ASDs.
Like attention-deficit/hyperactivity disorder and obsessive-compulsive disorder, ASDs are neurodevelopmental conditions. Advances based on the use of stem cells hold great promise for understanding, diagnosing and, possibly, treating these psychiatric disorders.28,29
Depression
Neuropsychiatric disorders arise from deviations from the regular differentiation process of the CNS, leading to altered neuronal connectivity. Relatively subtle abnormalities in the size and number of cells in the prefrontal cortex and basal ganglia have been observed in patients with depressive disorder and Tourette syndrome.30 Fibroblast-derived iPSCs generate serotonergic neurons through the exposure of the cells to growth factors and modulators of signaling pathways. If these serotonergic neurons are made from the patients’ own cells, they can be used to screen for new therapeutics and elucidate the unknown mechanisms through which current medications may function.31 This development could lead to the discovery of new medication targets and new insights into the molecular biology of depression.32
Deficiencies of brain-derived neurotrophic factor (BDNF) have a role in depression, anxiety, and other neuropsychiatric illnesses. The acute behavioral effects of selective serotonin reuptake inhibitors and tricyclic antidepressants seem to require BDNF signaling, which suggests that BDNF holds great potential as a therapeutic agent. Cell therapies focused on correcting BDNF deficiencies in mice have had some success.33
Dysregulation of GABAergic neurons has also been implicated in depression and anxiety. Patients with major depressive disorder have reduced gamma aminobutyric acid (GABA) receptors in the parahippocampal and lateral temporal lobes.34
Ultimately, the development of differentiation protocols for serotonergic and GABAergic neuronal populations will pave the way for examining the role of these populations in the pathogenesis of depression and anxiety, and may eventually open the door for cell-based therapies in humans.35
Studies have demonstrated a reduction in the density of pyramidal and nonpyramidal neurons in the anterior cingulate cortex of patients with schizophrenia and bipolar disorder,36 glial reduction in the subgenual prefrontal cortex in mood disorders,37 and morphometric evidence for neuronal and glial prefrontal cell pathology in major depressive disorder.38 The potential for stem cells to repair such pathology may be of clinical benefit to many patients.
Aside from their other suggested clinical uses, iPSCs may be utilized in new pathways for research on the biology and pharmacology of major depressive disorder.39
Continue to: Obsessive-compulsive disorder...
Obsessive-compulsive disorder
Obsessive-compulsive disorder (OCD) is often characterized by excessive behaviors related to cleanliness, including grooming, which is represented across most animal species. In mice, behaviors such as compulsive grooming and hair removal—similar to behaviors in humans with OCD or trichotillomania—are associated with a specific mutation. Chen et al40 reported that the transplantation of bone marrow stem cells into mice with this mutation (bone marrow–derived microglia specifically home to the brain) rescues their pathological phenotype by repairing native neurons.
The autoimmune, inflammatory, and neurodegenerative changes that are prevalent in OCD may be remedied by stem cell treatment in a fashion described throughout this article.
Other conditions
The Box41-50 describes a possible role for stem cells in the treatment or prevention of several types of substance use disorders.
Box
Researchers have begun to explore stem cells as a potential treatment for several substance use disorders, including those involving alcohol, cocaine, and opioids, as well as their interactions with cannabinoids.
Alcohol use disorder. In a 2017 study, Israel et al41 gave intra-cerebral injections of mesenchymal stem cells (MSCs) to rats that were bred to have a high alcohol intake. The MSC injections resulted in drastic reductions in the rats’ alcohol consumption. A single intracerebroventricular MSC administration inhibited relapse-like drinking by up to 85% for 40 days.
It is beyond unlikely that direct brain injections would be used to treat alcohol use disorder in humans. To address this problem, researchers aggregated MSCs into smaller spheroid shapes, which reduced their size up to 75% and allowed them to be injected intravenously to reach the brain in a study conducted in rats.42 Within 48 hours of a single treatment, the rats had reduced their intake of alcohol by 90%. The IV administration of antiinflammatory MSCs in human trials will be the next step to verify these results.
Alcohol research using human stem cells is also being conducted as a model system to understand the neural mechanisms of alcohol use disorder.43
Cocaine use disorder. In a grant proposal, Yadid and Popovtzer44 suggested that cocaine addiction affects neurogenesis, especially in the dentate gyrus, ventral tegmental area, nucleus accumbens, and prefrontal cortex; it damages mitochondrial RNA, brain-derived neurotrophic factor (BDNF), glutamate transporter (excitatory amino acid transporter; EAAT), and interleukin-10. MSCs have a predilection to these areas and influence neurogenesis. Currently, there are no FDAapproved medications for the safe and effective treatment of cocaine addiction. MSCs can home to pathological areas in the brain, release growth factors, and serve as cellular delivery tools in various brain disorders. Moreover, restoration of basal glutamate levels via the EAAT has been proposed as a promising target for treating cocaine dependence. Therefore, MSCs differentiated to express EAATs may have a combined long-term effect that can attenuate cocaine craving and relapse.44
Neural stem cells undergo a series of developmental processes before giving rise to newborn neurons, astrocytes, and oligodendrocytes in adult neurogenesis. During the past decade, studies of adult neurogenesis modulated by addictive drugs have highlighted the role of stem cells. These drugs have been shown to regulate the proliferation, differentiation, and survival of adult cells in different manners, which results in the varying consequences of adult neurogenesis.45 Reversal of these influences by healthy stem cells can be a worthy goal to pursue.
Opioid use disorder. Opiate medications cause a loss of newly born neural progenitors in the subgranular zone of the dentate gyrus by either modulating proliferation or interfering with differentiation and maturation.46 Opiates were the first medications shown to negatively impact neurogenesis in the adult mammalian hippocampus.47,48 The restoration of hippocampal function may positively affect the prognosis of a patient who is addicted.
Cannabinoids. Cannabinoids’ influence on the brain and on stem cells is controversial. On one hand, deteriorated neurogenesis results in reduced long-term potentiation in hippocampal formation. These cellular and physiological alterations lead to decreased short-term spatial memory and increased depressionlike behaviors.49 On the other hand, there is emerging evidence that cannabinoids improve neurogenesis and CNS plasticity, at least in the adult mouse.50 Through normalization of immune function, and restoration of the brain and the body, stem cells may assist in better health and in treatment of cannabis use disorder.
Chronic pain is a neuropsychiatric condition that involves the immune system, inflammation, vascularization, trophic changes, and other aspects of the CNS function in addition to peripheral factors and somatic pain generators. Treatment of painful conditions with the aid of stem cells represents a large and ever-developing field that lies outside of the scope of this article.51
Experimental, but promising
It is not easy to accept revolutionary new approaches in medicine. Endless research and due diligence are needed to prove a concept and then to work out specific applications, safeguards, and limitations for any novel treatments. The stem cell terrain is poorly explored, and one needs to be careful when venturing there. Presently, the FDA appropriately sees treatment with stem cells as experimental and investigational, particularly in the mental health arena. Stem cells are not approved for treatment of any specific condition. At the same time, research and clinical practice suggest stem cell treatment may someday play a more prominent role in health care. Undoubtedly, psychiatry will eventually benefit from the knowledge and application of stem cell research and practice.
Related Resources
- De Los Angeles A, Fernando MB, Hall NAL, et al. Induced pluripotent stem cells in psychiatry: an overview and critical perspective. Biol Psychiatry. 2021;90(6):362-372.
- Heider J, Vogel S, Volkmer H, et al. Human iPSC-derived glia as a tool for neuropsychiatric research and drug development. Int J Mol Sci. 2021;22(19):10254.
Drug Brand Name
Streptozotocin • Zanosar
Bottom Line
Treatment with stem cell transplantation is experimental and not approved for any medical or psychiatric illness. However, based on our growing understanding of the function of stem cells, and preliminary research conducted mainly in animals, many neurodegenerative-, vascular-, immune-, and inflammation-based psychiatric conditions might be beneficially influenced by stem cell treatment.
While laboratory studies move forward at full speed, the clinical use of stem cells—undifferentiated cells that can develop into many different types of specialized cells—remains controversial. Presently, only unadulterated stem cells are allowed to be used in patients, and only on an experimental and investigational basis. Stem cells that have been expanded, modified, or enhanced outside of the body are not allowed to be used for clinical application in the United States at this time. In June 2021, the FDA strengthened the language of stem cell regulation, further limiting their clinical application (see https://www.fda.gov/vaccines-blood-biologics/consumers-biologics/important-patient-and-consumer-information-about-regenerative-medicine-therapies). Yet some applications, such as treatment of lymphoma or restorative knee injections, are covered by some health insurance plans, and the acceptance of stem cell treatment is growing.
In this article, I describe the basics of stem cells, and explore the potential therapeutic use of stem cells for treating various mental illnesses.
Stem cells: A primer
Human embryonic stem cells were initially investigated for their healing properties. However, the need to harvest these cells from embryos drew much criticism, and many found the process to be ethically and religiously unacceptable. This was resolved by the Nobel prize–winning discovery that adult somatic cells can be reprogrammed into cells with embryonic stem cell properties by introducing specific transcription factors. These cells have been termed “induced pluripotent stem cells” (iPSCs).1 The use of adult stem cells and stem cells from the umbilical cords of healthy newborns has allowed for wider acceptance of stem cell research and treatment.
Stem cells may be collected from the patient himself or herself; these are autologous stem cells. They may also be harvested from healthy newborn waste, such as the umbilical cord blood and wall; these are allogenic stem cells. Autologous stem cells are present in almost any tissue but are usually collected from the patient’s adipose tissue or from bone marrow. Understandably, younger stem cells possess higher healing properties. Stem cells may be mesenchymal, producing primarily connective and nervous tissue, or hematopoietic, influencing the immune system and blood cell production, though there is a considerable overlap in the function of these types of cells.
Adult somatic stem cells may be turned into stem cells (iPSCs) and then become any tissue, including neurons. This ability of stem cells to physically regenerate the CNS is directly relevant to psychiatry.
In addition to neurogenesis, stem cell transplants can assist in immune and vascular restoration as well as in suppressing inflammation. The ability of stem cells to replace mutated genes may be useful for addressing inheritable neuropsychiatric conditions.
Both autoimmune and inflammatory mechanisms play an important role in most psychiatric illnesses. The more we learn, the more it is clear that brain function is profoundly dependent on more than just its structure, and that structure depends on more than blood supply. Stem cells influence the vascular, nutritional, functional, inflammatory, and immune environment of the brain, potentially assisting in cognitive and emotional rehabilitation.
Stem cells operate in 2 fundamental ways: via direct cell-to-cell interaction, and via the production and release of growth, immune-regulating, and anti-inflammatory factors. Such factors are produced within the cells and then released in the extracellular environment as a content of exosomes. The route of administration is important in the delivery of the stem cells to the target tissue. Unlike their direct introduction into a joint, muscle, or intervertebral disk, injection of stem cells into the brain is more complicated and not routinely feasible. Intrathecal injections may bring stem cells into the CNS, but cerebrospinal fluid does not easily carry stem cells into the brain, and certainly cannot deliver them to an identified target within the brain. Existing technology can allow stem cells to be packaged in such a way that they can penetrate the blood-brain barrier, but this requires stem cell modification, which presently is not permitted in clinical practice in the United States. Alternatively, there is a way to weaken the blood-brain barrier to allow stem cells to travel through the “opened doors,” so to speak, but this allows everything to have access to the CNS, which may be unsafe. IV administration is technologically easy, and it grants stem cells the environment to multiply and produce extracellular factors that can cross the blood-brain barrier, while large cells cannot.
Continue to: Stem cells as a treatment for mental illness...
Stem cells as a treatment for mental illness
Based on our understanding of the function of stem cells, many neurodegenerative-, vascular-, immune-, and inflammation-based psychiatric conditions can be influenced by stem cell treatment. Here I review the potential therapeutic role of stem cells in the treatment of several psychiatric disorders.
Alzheimer’s dementia
Alzheimer’s dementia (AD) is a progressive neurodegenerative pathology based on neuronal and synaptic loss. Repopulation and regeneration of depleted neuronal circuitry by exogenous stem cells may be a rational therapeutic strategy.2 The regeneration of lost neurons has the potential to restore cognitive function. Multiple growth factors that regulate neurogenesis are abundant during child development but dramatically decline with age. The introduction of stem cells—especially those derived from newborn waste—seem to promote recovery from neurodegenerative disease or injury.3
There currently is no cure for AD. Cellular therapy promises new advances in treatment.4 Neurogenesis occurs not only during fetal development but in the adult brain. Neural stem cells reside in the adult CNS of all mammals.5 They are intimately involved in continuous restoration, but age just like the rest of the animal tissue, providing ever-decreasing restorative potential.
The number of studies of stem cells in AD has increased since the early 2000 s,6,7 and research continues to demonstrate robust CNS neurogenesis. In a 2020 study, Zappa Villar et al8 evaluated stem cells as a treatment for rats in which an AD model was induced by the intracerebroventricular injection of streptozotocin (STZ). The STZ-treated rats displayed poor performance in all behavioral tests. Stem cell therapy increased exploratory behavior, decreased anxiety, and improved spatial memory and marble-burying behavior; the latter was representative of daily life activities. Importantly, stem cell therapy ameliorated and restored hippocampal atrophy and some presynaptic protein levels in the rats with AD.8 Animal models cannot be automatically applied to humans, but they shine a light on the areas that need further exploration.
In humans, elevated cortisol levels during aging predict hippocampal atrophy and memory deficits,9 and this deficiency may be positively influenced by stem cell treatment.
Schizophrenia
Recent research indicates that schizophrenia may begin with abnormal neurogenesis from neural stem cells inside the embryo, and that this process may be particularly vulnerable to numerous genetic and/or environmental disturbances of early brain development.10 Because neurogenesis is not confined to the womb but is a protracted process that continues into postnatal life, adolescence and beyond, influencing this process may be a way to add to the schizophrenia treatment armamentarium.10 Sacco et al11 described links between the alteration of intrauterine and adult neurogenesis and the causes of neuropsychiatric disorders, including schizophrenia. Immune and inflammatory mechanisms are important in the etiology of schizophrenia. By their core function, stem cells address both mechanisms, and may directly modulate this devastating disease.
In addition to clinical hopes, advances in research tools hold the promise of new discoveries. With the advent of iPSC technology, it is possible to generate live neurons in vitro from somatic tissue of patients with schizophrenia. Despite its many limitations, this revolutionary technology has already helped to advance our understanding of schizophrenia.11
Bipolar disorder
Many of the fundamental neurobiological mechanisms of schizophrenia are mirrored in bipolar disorder.12 Though we are not ready to bring stem cells into the day-to-day treatment of this condition, several groups are starting to apply iPSC technology to the study of bipolar disorder.13
Neurodevelopmental factors—particularly pathways related to nervous system development, cell migration, extracellular matrix, methylation, and calcium signaling—have been identified in large gene expression studies as altered in bipolar disorder.14 Stem cell technology opens doorways to reverse engineering of human neurodegenerative disease.15
Continue to: Autism spectrum disorders...
Autism spectrum disorders
Autism spectrum disorders (ASDs) are multiple heterogeneous neurodevelopmental disorders.16 Neuroinflammation and immune dysregulation influence the origin of ASDs. Due to the neurobiologic changes underlying ASD development, cell-based therapies, including the use of mesenchymal stem cells (MSCs), have been applied to ASDs.16 Stem cells show specific immunologic properties that make them promising candidates for treating ASDs.17
The exact mechanisms of action of MSCs to restore function in patients with ASDs are largely unknown, but proposed mechanisms include:
- synthesizing and releasing anti-inflammatory cytokines and survival-promoting growth factors
- integrating into the existing neural and synaptic network
- restoring plasticity.18
In a study of transplantation of human cord blood cells and umbilical cord–derived MSCs for patients with ASDs, Bradstreet et al19 found a statistically significant difference on scores for domains of speech, sociability, sensory, and overall health, as well as reductions in the total scores, in those who received transplants compared to their pretreatment values.
In another study of stem cell therapy for ASDs, Lv et al20 demonstrated the safety and efficacy of combined transplantation of human cord blood cells and umbilical cord–derived MSCs in treating children with ASDs. The transplantations included 4 stem cell IV infusions and intrathecal injections once a week. Statistically significant differences were shown at 24 weeks post-treatment. Although this nonrandomized, open-label, single-center Phase I/II trial cannot be relied on for any definitive conclusions, it suggests an important area of investigation.20
The vascular aspects of ASDs’ pathogenesis should not be overlooked. For example, specific temporal lobe areas associated with facial recognition, social interaction, and language comprehension have been demonstrated to be hypoperfused in children with ASDs, but not in controls. The degree of hypoperfusion and resulting hypoxia correlates with the severity of ASD symptoms. The damage causing hypoperfusion of temporal areas was associated with the onset of autism-like disorders. Damage of the amygdala, hippocampus, or other temporal structures induces permanent or transient autistic-like characteristics, such as unexpressive faces, little eye contact, and motor stereotypes. Clinically, temporal lobe damage by viral and other means has been implicated in the development of ASD in children and adults. Hypoperfusion may contribute to defects, not only by inducing hypoxia, but also by allowing for abnormal metabolite or neurotransmitter accumulation. This is one of the reasons glutamate toxicity has been implicated in ASD. The augmentation of perfusion through stimulation of angiogenesis by stem cells should allow for metabolite clearance and restoration of functionality. Vargas et al21 compared brain autopsy samples from 11 children with ASDs to those of 7 age-matched controls. They demonstrated an active neuroinflammatory process in the cerebral cortex, white matter, and cerebellum of patients with ASDs, both by immunohistochemistry and morphology.21
Multiple studies have confirmed that the systemic administration of cord blood cells is sufficient to induce neuroregeneration.22,23 Angiogenesis has been experimentally demonstrated in peripheral artery disease, myocardial ischemia, and stroke, and has direct implications on brain repair.24 Immune dysregulation25,26 and immune modulation27 also are addressed by stem cell treatment, which provides a promising avenue for battling ASDs.
Like attention-deficit/hyperactivity disorder and obsessive-compulsive disorder, ASDs are neurodevelopmental conditions. Advances based on the use of stem cells hold great promise for understanding, diagnosing and, possibly, treating these psychiatric disorders.28,29
Depression
Neuropsychiatric disorders arise from deviations from the regular differentiation process of the CNS, leading to altered neuronal connectivity. Relatively subtle abnormalities in the size and number of cells in the prefrontal cortex and basal ganglia have been observed in patients with depressive disorder and Tourette syndrome.30 Fibroblast-derived iPSCs generate serotonergic neurons through the exposure of the cells to growth factors and modulators of signaling pathways. If these serotonergic neurons are made from the patients’ own cells, they can be used to screen for new therapeutics and elucidate the unknown mechanisms through which current medications may function.31 This development could lead to the discovery of new medication targets and new insights into the molecular biology of depression.32
Deficiencies of brain-derived neurotrophic factor (BDNF) have a role in depression, anxiety, and other neuropsychiatric illnesses. The acute behavioral effects of selective serotonin reuptake inhibitors and tricyclic antidepressants seem to require BDNF signaling, which suggests that BDNF holds great potential as a therapeutic agent. Cell therapies focused on correcting BDNF deficiencies in mice have had some success.33
Dysregulation of GABAergic neurons has also been implicated in depression and anxiety. Patients with major depressive disorder have reduced gamma aminobutyric acid (GABA) receptors in the parahippocampal and lateral temporal lobes.34
Ultimately, the development of differentiation protocols for serotonergic and GABAergic neuronal populations will pave the way for examining the role of these populations in the pathogenesis of depression and anxiety, and may eventually open the door for cell-based therapies in humans.35
Studies have demonstrated a reduction in the density of pyramidal and nonpyramidal neurons in the anterior cingulate cortex of patients with schizophrenia and bipolar disorder,36 glial reduction in the subgenual prefrontal cortex in mood disorders,37 and morphometric evidence for neuronal and glial prefrontal cell pathology in major depressive disorder.38 The potential for stem cells to repair such pathology may be of clinical benefit to many patients.
Aside from their other suggested clinical uses, iPSCs may be utilized in new pathways for research on the biology and pharmacology of major depressive disorder.39
Continue to: Obsessive-compulsive disorder...
Obsessive-compulsive disorder
Obsessive-compulsive disorder (OCD) is often characterized by excessive behaviors related to cleanliness, including grooming, which is represented across most animal species. In mice, behaviors such as compulsive grooming and hair removal—similar to behaviors in humans with OCD or trichotillomania—are associated with a specific mutation. Chen et al40 reported that the transplantation of bone marrow stem cells into mice with this mutation (bone marrow–derived microglia specifically home to the brain) rescues their pathological phenotype by repairing native neurons.
The autoimmune, inflammatory, and neurodegenerative changes that are prevalent in OCD may be remedied by stem cell treatment in a fashion described throughout this article.
Other conditions
The Box41-50 describes a possible role for stem cells in the treatment or prevention of several types of substance use disorders.
Box
Researchers have begun to explore stem cells as a potential treatment for several substance use disorders, including those involving alcohol, cocaine, and opioids, as well as their interactions with cannabinoids.
Alcohol use disorder. In a 2017 study, Israel et al41 gave intra-cerebral injections of mesenchymal stem cells (MSCs) to rats that were bred to have a high alcohol intake. The MSC injections resulted in drastic reductions in the rats’ alcohol consumption. A single intracerebroventricular MSC administration inhibited relapse-like drinking by up to 85% for 40 days.
It is beyond unlikely that direct brain injections would be used to treat alcohol use disorder in humans. To address this problem, researchers aggregated MSCs into smaller spheroid shapes, which reduced their size up to 75% and allowed them to be injected intravenously to reach the brain in a study conducted in rats.42 Within 48 hours of a single treatment, the rats had reduced their intake of alcohol by 90%. The IV administration of antiinflammatory MSCs in human trials will be the next step to verify these results.
Alcohol research using human stem cells is also being conducted as a model system to understand the neural mechanisms of alcohol use disorder.43
Cocaine use disorder. In a grant proposal, Yadid and Popovtzer44 suggested that cocaine addiction affects neurogenesis, especially in the dentate gyrus, ventral tegmental area, nucleus accumbens, and prefrontal cortex; it damages mitochondrial RNA, brain-derived neurotrophic factor (BDNF), glutamate transporter (excitatory amino acid transporter; EAAT), and interleukin-10. MSCs have a predilection to these areas and influence neurogenesis. Currently, there are no FDAapproved medications for the safe and effective treatment of cocaine addiction. MSCs can home to pathological areas in the brain, release growth factors, and serve as cellular delivery tools in various brain disorders. Moreover, restoration of basal glutamate levels via the EAAT has been proposed as a promising target for treating cocaine dependence. Therefore, MSCs differentiated to express EAATs may have a combined long-term effect that can attenuate cocaine craving and relapse.44
Neural stem cells undergo a series of developmental processes before giving rise to newborn neurons, astrocytes, and oligodendrocytes in adult neurogenesis. During the past decade, studies of adult neurogenesis modulated by addictive drugs have highlighted the role of stem cells. These drugs have been shown to regulate the proliferation, differentiation, and survival of adult cells in different manners, which results in the varying consequences of adult neurogenesis.45 Reversal of these influences by healthy stem cells can be a worthy goal to pursue.
Opioid use disorder. Opiate medications cause a loss of newly born neural progenitors in the subgranular zone of the dentate gyrus by either modulating proliferation or interfering with differentiation and maturation.46 Opiates were the first medications shown to negatively impact neurogenesis in the adult mammalian hippocampus.47,48 The restoration of hippocampal function may positively affect the prognosis of a patient who is addicted.
Cannabinoids. Cannabinoids’ influence on the brain and on stem cells is controversial. On one hand, deteriorated neurogenesis results in reduced long-term potentiation in hippocampal formation. These cellular and physiological alterations lead to decreased short-term spatial memory and increased depressionlike behaviors.49 On the other hand, there is emerging evidence that cannabinoids improve neurogenesis and CNS plasticity, at least in the adult mouse.50 Through normalization of immune function, and restoration of the brain and the body, stem cells may assist in better health and in treatment of cannabis use disorder.
Chronic pain is a neuropsychiatric condition that involves the immune system, inflammation, vascularization, trophic changes, and other aspects of the CNS function in addition to peripheral factors and somatic pain generators. Treatment of painful conditions with the aid of stem cells represents a large and ever-developing field that lies outside of the scope of this article.51
Experimental, but promising
It is not easy to accept revolutionary new approaches in medicine. Endless research and due diligence are needed to prove a concept and then to work out specific applications, safeguards, and limitations for any novel treatments. The stem cell terrain is poorly explored, and one needs to be careful when venturing there. Presently, the FDA appropriately sees treatment with stem cells as experimental and investigational, particularly in the mental health arena. Stem cells are not approved for treatment of any specific condition. At the same time, research and clinical practice suggest stem cell treatment may someday play a more prominent role in health care. Undoubtedly, psychiatry will eventually benefit from the knowledge and application of stem cell research and practice.
Related Resources
- De Los Angeles A, Fernando MB, Hall NAL, et al. Induced pluripotent stem cells in psychiatry: an overview and critical perspective. Biol Psychiatry. 2021;90(6):362-372.
- Heider J, Vogel S, Volkmer H, et al. Human iPSC-derived glia as a tool for neuropsychiatric research and drug development. Int J Mol Sci. 2021;22(19):10254.
Drug Brand Name
Streptozotocin • Zanosar
Bottom Line
Treatment with stem cell transplantation is experimental and not approved for any medical or psychiatric illness. However, based on our growing understanding of the function of stem cells, and preliminary research conducted mainly in animals, many neurodegenerative-, vascular-, immune-, and inflammation-based psychiatric conditions might be beneficially influenced by stem cell treatment.
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861-872.
- Duncan T, Valenzuela M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res Ther. 2017;8(1):111.
- Brinton RD, Wang JM. Therapeutic potential of neurogenesis for prevention and recovery from Alzheimer’s disease: allopregnanolone as a proof of concept neurogenic agent. Curr Alzheimer Res. 2006;3(3):185-190.
- Taupin P. Adult neurogenesis, neural stem cells, and Alzheimer’s disease: developments, limitations, problems, and promises. Curr Alzheimer Res. 2009;6(6):461-470.
- Taupin P. Neurogenesis, NSCs, pathogenesis, and therapies for Alzheimer’s disease. Front Biosci (Schol Ed). 2011;3:178-90.
- Kang JM, Yeon BK, Cho SJ, et al. Stem cell therapy for Alzheimer’s disease: a review of recent clinical trials. J Alzheimers Dis. 2016;54(3):879-889.
- Li M, Guo K, Ikehara S. Stem cell treatment for Alzheimer’s disease. Int J Mol Sci. 2014;15(10):19226-19238.
- Zappa Villar MF, López Hanotte J, Pardo J, et al. Mesenchymal stem cells therapy improved the streptozotocin-induced behavioral and hippocampal impairment in rats. Mol Neurobiol. 2020;57(2):600-615.
- Lupien SJ, de Leon M, de Santi S, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1(1):69-73.
- Iannitelli A, Quartini A, Tirassa P, et al. Schizophrenia and neurogenesis: a stem cell approach. Neurosci Biobehav Rev. 2017;80:414-442.
- Sacco R, Cacci E, Novarino G. Neural stem cells in neuropsychiatric disorders. Curr Opin Neurobiol. 2018; 48:131-138.
- Miller ND, Kelsoe JR. Unraveling the biology of bipolar disorder using induced pluripotent stem-derived neurons. Bipolar Disord. 2017;19(7):544-551.
- O’Shea KS, McInnis MG. Neurodevelopmental origins of bipolar disorder: iPSC models. Mol Cell Neurosci. 2016;73:63-83.
- Jacobs BM. A dangerous method? The use of induced pluripotent stem cells as a model for schizophrenia. Schizophr Res. 2015;168(1-2):563-568.
- Liu Y, Deng W. Reverse engineering human neurodegenerative disease using pluripotent stem cell technology. Brain Res. 2016;1638(Pt A):30-41.
- Siniscalco D, Kannan S, Semprún-Hernández N, et al. Stem cell therapy in autism: recent insights. Stem Cells Cloning. 2018;11:55-67.
- Siniscalco D, Bradstreet JJ, Sych N, et al. Mesenchymal stem cells in treating autism: novel insights. World J Stem Cells. 2014;6(2):173-178.
- Siniscalco D, Sapone A, Cirillo A, et al. Autism spectrum disorders: is mesenchymal stem cell personalized therapy the future? J Biomed Biotechnol. 2012; 2012:480289.
- Bradstreet JJ, Sych N, Antonucci N, et al. Efficacy of fetal stem cell transplantation in autism spectrum disorders: an open-labeled pilot study. Cell Transplant. 2014;23(Suppl 1):S105-S112.
- Lv YT, Zhang Y, Liu M, et al. Transplantation of human cord blood mononuclear cells and umbilical cordderived mesenchymal stem cells in autism. J Transl Med. 2013;11:196.
- Vargas DL, Nascimbene C, Krishnan C, et al. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57(1):67-81.
- Wei L, Keogh CL, Whitaker VR, et al. Angiogenesis and stem cell transplantation as potential treatments of cerebral ischemic stroke. Pathophysiology. 2005;12(1): 47-62.
- Newman MB, Willing AE, Manresa JJ, et al. Cytokines produced by cultured human umbilical cord blood (HUCB) cells: implications for brain repair. Exp Neurol. 2006;199(1):201-218.
- Peterson DA. Umbilical cord blood cells and brain stroke injury: bringing in fresh blood to address an old problem. J Clin Invest. 2004;114(3):312-314.
- Cohly HH, Panja A. Immunological findings in autism. Int Rev Neurobiol. 2005;71:317-341.
- Ashwood P, Van de Water J. Is autism an autoimmune disease? Autoimmun Rev. 2004;3(7-8):557-562.
- Yagi H, Soto-Gutierrez A, Parekkadan B, et al. Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant. 2010;19(6):667-679.
- Vaccarino FM, Urban AE, Stevens HE, et al. Annual Research Review: The promise of stem cell research for neuropsychiatric disorders. J Child Psychol Psychiatry. 2011;52(4):504-516.
- Liu EY, Scott CT. Great expectations: autism spectrum disorder and induced pluripotent stem cell technologies. Stem Cell Rev Rep. 2014;10(2):145-150.
- Richardson-Jones JW, Craige CP, Guiard BP, et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65(1):40-52.
- Saarelainen T, Hendolin P, Lucas G, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23(1):349-357.
- Klumpers UM, Veltman DJ, Drent ML, et al. Reduced parahippocampal and lateral temporal GABAA-[11C] flumazenil binding in major depression: preliminary results. Eur J Nucl Med Mol Imaging. 2010;37(3): 565-574.
- Bremner JD, Narayan M, Anderson ER, et al. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157(1):115-118.
- Bremner JD, Randall P, Scott TM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152(7):973-981.
- Vincent SL, Todtenkopf MS, Benes FM. A comparison of the density of pyramidal and non-pyramidal neurons in the anterior cingulate cortex of schizophrenics and manic depressives. Soc Neurosci Abstr. 1997;23:2199.
- Benes FM, Kwok EW, Vincent SL, et al. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44(2): 88-97.
- Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95(22):13290-13295.
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45(9): 1085-1098.
- Licinio J, Wong ML. Serotonergic neurons derived from induced pluripotent stem cells (iPSCs): a new pathway for research on the biology and pharmacology of major depression. Mol Psychiatry. 2016;21(1):1-2.
- Chen SK, Tvrdik P, Peden E, et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141(5):775-785.
- Israel Y, Ezquer F, Quintanilla ME, et al. Intracerebral stem cell administration inhibits relapse-like alcohol drinking in rats. Alcohol Alcohol. 2017;52(1):1-4.
- Ezquer F, Morales P, Quintanilla ME, et al. Intravenous administration of anti-inflammatory mesenchymal stem cell spheroids reduces chronic alcohol intake and abolishes binge-drinking. Sci Rep. 2018;8(1):4325.
- Scarnati MS, Halikere A, Pang ZP. Using human stem cells as a model system to understand the neural mechanisms of alcohol use disorders: current status and outlook. Alcohol. 2019;74:83-93.
- Yadid GM, Popovtzer R. Nanoparticle-mesenchymal stem cell conjugates for cell therapy in drug addiction. NIH grant application. 2017.
- Xu C, Loh HH, Law PY. Effects of addictive drugs on adult neural stem/progenitor cells. Cell Mol Life Sci. 2016;73(2):327-348.
- Dholakiya SL, Aliberti A, Barile FA. Morphine sulfate concomitantly decreases neuronal differentiation and opioid receptor expression in mouse embryonic stem cells. Toxicol Lett. 2016;247:45-55.
- Zhang Y, Loh HH, Law PY. Effect of opioid on adult hippocampal neurogenesis. Scientific World Journal. 2016;2016:2601264.
- Bortolotto V, Grilli M. Opiate analgesics as negative modulators of adult hippocampal neurogenesis: potential implications in clinical practice. Front Pharmacol. 2017; 8:254.
- Galve-Roperh I, Chiurchiù V, Díaz-Alonso J, et al. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog Lipid Res. 2013; 52(4):633-650.
- Zimmermann T, Maroso M, Beer A, et al. Neural stem cell lineage-specific cannabinoid type-1 receptor regulates neurogenesis and plasticity in the adult mouse hippocampus. Cereb Cortex. 2018;28(12):4454-4471.
- Ren J, Liu N, Sun N, et al. Mesenchymal stem cells and their exosomes: promising therapies for chronic pain. Curr Stem Cell Res Ther. 2019;14(8):644-653.
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861-872.
- Duncan T, Valenzuela M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res Ther. 2017;8(1):111.
- Brinton RD, Wang JM. Therapeutic potential of neurogenesis for prevention and recovery from Alzheimer’s disease: allopregnanolone as a proof of concept neurogenic agent. Curr Alzheimer Res. 2006;3(3):185-190.
- Taupin P. Adult neurogenesis, neural stem cells, and Alzheimer’s disease: developments, limitations, problems, and promises. Curr Alzheimer Res. 2009;6(6):461-470.
- Taupin P. Neurogenesis, NSCs, pathogenesis, and therapies for Alzheimer’s disease. Front Biosci (Schol Ed). 2011;3:178-90.
- Kang JM, Yeon BK, Cho SJ, et al. Stem cell therapy for Alzheimer’s disease: a review of recent clinical trials. J Alzheimers Dis. 2016;54(3):879-889.
- Li M, Guo K, Ikehara S. Stem cell treatment for Alzheimer’s disease. Int J Mol Sci. 2014;15(10):19226-19238.
- Zappa Villar MF, López Hanotte J, Pardo J, et al. Mesenchymal stem cells therapy improved the streptozotocin-induced behavioral and hippocampal impairment in rats. Mol Neurobiol. 2020;57(2):600-615.
- Lupien SJ, de Leon M, de Santi S, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1(1):69-73.
- Iannitelli A, Quartini A, Tirassa P, et al. Schizophrenia and neurogenesis: a stem cell approach. Neurosci Biobehav Rev. 2017;80:414-442.
- Sacco R, Cacci E, Novarino G. Neural stem cells in neuropsychiatric disorders. Curr Opin Neurobiol. 2018; 48:131-138.
- Miller ND, Kelsoe JR. Unraveling the biology of bipolar disorder using induced pluripotent stem-derived neurons. Bipolar Disord. 2017;19(7):544-551.
- O’Shea KS, McInnis MG. Neurodevelopmental origins of bipolar disorder: iPSC models. Mol Cell Neurosci. 2016;73:63-83.
- Jacobs BM. A dangerous method? The use of induced pluripotent stem cells as a model for schizophrenia. Schizophr Res. 2015;168(1-2):563-568.
- Liu Y, Deng W. Reverse engineering human neurodegenerative disease using pluripotent stem cell technology. Brain Res. 2016;1638(Pt A):30-41.
- Siniscalco D, Kannan S, Semprún-Hernández N, et al. Stem cell therapy in autism: recent insights. Stem Cells Cloning. 2018;11:55-67.
- Siniscalco D, Bradstreet JJ, Sych N, et al. Mesenchymal stem cells in treating autism: novel insights. World J Stem Cells. 2014;6(2):173-178.
- Siniscalco D, Sapone A, Cirillo A, et al. Autism spectrum disorders: is mesenchymal stem cell personalized therapy the future? J Biomed Biotechnol. 2012; 2012:480289.
- Bradstreet JJ, Sych N, Antonucci N, et al. Efficacy of fetal stem cell transplantation in autism spectrum disorders: an open-labeled pilot study. Cell Transplant. 2014;23(Suppl 1):S105-S112.
- Lv YT, Zhang Y, Liu M, et al. Transplantation of human cord blood mononuclear cells and umbilical cordderived mesenchymal stem cells in autism. J Transl Med. 2013;11:196.
- Vargas DL, Nascimbene C, Krishnan C, et al. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57(1):67-81.
- Wei L, Keogh CL, Whitaker VR, et al. Angiogenesis and stem cell transplantation as potential treatments of cerebral ischemic stroke. Pathophysiology. 2005;12(1): 47-62.
- Newman MB, Willing AE, Manresa JJ, et al. Cytokines produced by cultured human umbilical cord blood (HUCB) cells: implications for brain repair. Exp Neurol. 2006;199(1):201-218.
- Peterson DA. Umbilical cord blood cells and brain stroke injury: bringing in fresh blood to address an old problem. J Clin Invest. 2004;114(3):312-314.
- Cohly HH, Panja A. Immunological findings in autism. Int Rev Neurobiol. 2005;71:317-341.
- Ashwood P, Van de Water J. Is autism an autoimmune disease? Autoimmun Rev. 2004;3(7-8):557-562.
- Yagi H, Soto-Gutierrez A, Parekkadan B, et al. Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant. 2010;19(6):667-679.
- Vaccarino FM, Urban AE, Stevens HE, et al. Annual Research Review: The promise of stem cell research for neuropsychiatric disorders. J Child Psychol Psychiatry. 2011;52(4):504-516.
- Liu EY, Scott CT. Great expectations: autism spectrum disorder and induced pluripotent stem cell technologies. Stem Cell Rev Rep. 2014;10(2):145-150.
- Richardson-Jones JW, Craige CP, Guiard BP, et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65(1):40-52.
- Saarelainen T, Hendolin P, Lucas G, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23(1):349-357.
- Klumpers UM, Veltman DJ, Drent ML, et al. Reduced parahippocampal and lateral temporal GABAA-[11C] flumazenil binding in major depression: preliminary results. Eur J Nucl Med Mol Imaging. 2010;37(3): 565-574.
- Bremner JD, Narayan M, Anderson ER, et al. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157(1):115-118.
- Bremner JD, Randall P, Scott TM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152(7):973-981.
- Vincent SL, Todtenkopf MS, Benes FM. A comparison of the density of pyramidal and non-pyramidal neurons in the anterior cingulate cortex of schizophrenics and manic depressives. Soc Neurosci Abstr. 1997;23:2199.
- Benes FM, Kwok EW, Vincent SL, et al. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44(2): 88-97.
- Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95(22):13290-13295.
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45(9): 1085-1098.
- Licinio J, Wong ML. Serotonergic neurons derived from induced pluripotent stem cells (iPSCs): a new pathway for research on the biology and pharmacology of major depression. Mol Psychiatry. 2016;21(1):1-2.
- Chen SK, Tvrdik P, Peden E, et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141(5):775-785.
- Israel Y, Ezquer F, Quintanilla ME, et al. Intracerebral stem cell administration inhibits relapse-like alcohol drinking in rats. Alcohol Alcohol. 2017;52(1):1-4.
- Ezquer F, Morales P, Quintanilla ME, et al. Intravenous administration of anti-inflammatory mesenchymal stem cell spheroids reduces chronic alcohol intake and abolishes binge-drinking. Sci Rep. 2018;8(1):4325.
- Scarnati MS, Halikere A, Pang ZP. Using human stem cells as a model system to understand the neural mechanisms of alcohol use disorders: current status and outlook. Alcohol. 2019;74:83-93.
- Yadid GM, Popovtzer R. Nanoparticle-mesenchymal stem cell conjugates for cell therapy in drug addiction. NIH grant application. 2017.
- Xu C, Loh HH, Law PY. Effects of addictive drugs on adult neural stem/progenitor cells. Cell Mol Life Sci. 2016;73(2):327-348.
- Dholakiya SL, Aliberti A, Barile FA. Morphine sulfate concomitantly decreases neuronal differentiation and opioid receptor expression in mouse embryonic stem cells. Toxicol Lett. 2016;247:45-55.
- Zhang Y, Loh HH, Law PY. Effect of opioid on adult hippocampal neurogenesis. Scientific World Journal. 2016;2016:2601264.
- Bortolotto V, Grilli M. Opiate analgesics as negative modulators of adult hippocampal neurogenesis: potential implications in clinical practice. Front Pharmacol. 2017; 8:254.
- Galve-Roperh I, Chiurchiù V, Díaz-Alonso J, et al. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog Lipid Res. 2013; 52(4):633-650.
- Zimmermann T, Maroso M, Beer A, et al. Neural stem cell lineage-specific cannabinoid type-1 receptor regulates neurogenesis and plasticity in the adult mouse hippocampus. Cereb Cortex. 2018;28(12):4454-4471.
- Ren J, Liu N, Sun N, et al. Mesenchymal stem cells and their exosomes: promising therapies for chronic pain. Curr Stem Cell Res Ther. 2019;14(8):644-653.
Antipsychotics, dopamine, and pain
Our understanding of pain mechanisms continues to evolve and, accordingly, so do our treatment strategies. The fundamental differences between acute and chronic pain were only recently recognized; this lack of recognition led to the application of acute pain treatments to chronic pain, contributing to the opioid epidemic in the United States.
With the diminishing emphasis on opioid medications, researchers are exploring other pharmacologic modalities for treating pain. Many nonopioid psychiatric medications are used off-label for the treatment of pain. Psychiatric medications play a larger role in the management of pain as pain becomes more chronic (Table 11). For simplicity, acute pain may be seen as nociception colored by emotions, and chronic pain as emotions colored by nociception. Protracted pain connects those extremes with a diminishing role of nociception and an increasing role of emotion,1 which may increase the potential role of psychiatric medications, including antipsychotics.
In this article, I discuss the potential role of dopamine in the perception of pain, and review the potential use of first- and second-generation antipsychotics for treating various pain syndromes.
Role of dopamine in pain
There is increasing interest in exploring antipsychotics to treat chronic pain2 because dopamine dysfunction is part of pathological pain perception. Excess dopamine is associated with headaches (dopamine hypersensitivity hypothesis3,4) and dopamine dysfunction is a part of posttraumatic stress disorder (PTSD),5 dissociation,6 paranoia,7 and catastrophizing.8 Somatic psychosis, like any psychosis, can be based on dopamine pathology. Dopaminergic neurons affect nociceptive function in the spinal dorsal horn,9 and dopamine receptors are altered in atypical facial pain,10 burning mouth syndrome,11 and fibromyalgia.12
In normal circumstances, dopamine is fundamentally a protective neurotransmitter. In acute pain, dopamine is powerfully released, making the pain bearable. A patient may describe acute pain as seeming “like it was not happening to me” or “it was like a dream”; both are examples of dopamine-caused dissociation and a possible prediction of subsequent chronification. In chronic pain, pathological mechanisms settle in and take root; therefore, keeping protective dopamine levels high becomes a priority. This is especially common in patients who have experienced abuse or PTSD. The only natural way to keep dopamine up for prolonged periods of time is to decrease pain and stress thresholds. Both phenomena are readily observed in patients with pain. In extreme cases, self-mutilation and involvement in conflicts become pathologically gratifying.
The dopaminergic system is essential for pain control with a tissue injury.13 It becomes pathologically stimulated and increasingly dysfunctional as algopathy (a pathological pain perception) develops. At the same time, a flood or drought of any neurotransmitter is equally bad and may produce similar clinical pictures. Both a lack of and excess of dopamine are associated with pain.14 This is why opposite treatments may be beneficial in different patients with chronic pain. As an example, the use of stimulants15 and bupropion16 has been reported in the treatment of abdominal pain. And, reversely, antipsychotics, especially first-generation agents, may be associated with chronic (tardive) pain, including orofacial and genital pain.17
First-generation antipsychotics
First-generation antipsychotics (FGAs) have been used to treat various nonpsychiatric conditions (Table 2). Although they are powerful D2 receptor inhibitors, FGAs lack the intrinsic ability to counteract the unwanted adverse effects of strong inhibition. As a result, movement disorders and prolactinemia are commonly induced by FGAs. The most dangerous consequence of treatment with these agents is neuroleptic malignant syndrome (NMS).
Continue to: Haloperidol
Haloperidol is prescribed widely by nonpsychiatrists, primarily to treat agitation. Intravenous haloperidol has been used for the abortive treatment of headaches.18 Paradoxically, IV haloperidol is less likely to induce extrapyramidal symptoms (EPS) than the oral formulation because of a more pronounced anticholinergic action in IV use. Haloperidol can help relieve gastroparesis and nausea, especially in IV administration,19 but prolonged oral administration is associated with unwanted movement problems and should be avoided.20
Chlorpromazine is more anticholinergic than haloperidol. It can be used in the abortive treatment of headaches (preferably via IV and IM administration), nausea, hiccups, porphyria, and serotonin syndrome, but it is very sedating and frequently produces hypotension, dangerous QT prolongation, and sensations of thought-blocking.21
Pimozide is reported to help with skin picking, trichotillomania, and somatic hallucinations.22
Droperidol, promethazine, and prochlorperazine are used off-label to treat nausea and headaches. Primary care clinicians may not be aware that these commonly used medications are antipsychotics. Similar to other FGAs, these 3 agents may produce NMS and tardive dyskinesia (TD). The same applies to the prokinetic drug metoclopramide.
Second-generation antipsychotics
Second-generation antipsychotics (SGAs) work with various serotonin receptors, offsetting and enhancing the antipsychotic function of dopamine blockade. This diminishes but does not eliminate EPS and the risk of TD. Fortunately, the risk of NMS is lower with SGAs than with FGAs. Many SGAs are FDA-approved for treating schizophrenia and other psychiatric disorders, and some have relevance for pain management (Table 3). Many SGAs help with depressive symptoms and are powerful mood stabilizers. As such, they may diminish central over-firing of dopaminergic and serotonergic neurons involved in the pain cascade, which in turn decreases pain transmission and perception. The downside is that in general, SGAs increase the risk of diabetes and hyperlipidemia.
Continue to: Risperidone
Risperidone was the second FDA-approved SGA. Pain practitioners primarily prescribe it for treatmeant-resistant headaches, but patients with fibromyalgia and those with phantom and thalamic pain also may respond. Because risperidone’s properties are similar to that of many FGAs, it may potently cause EPS, TD, and prolactinemia. Neuroleptic malignant syndrome also has been reported.23
Ziprasidone is frequently overlooked by clinicians who treat pain. Although ziprasidone may be sedating, it is powerful as both a preventive and abortive (in an IM formulation) agent for treatment-resistant headaches. This might be attributed to its effects on the 5HT9 receptor. It is approved for treating bipolar depression and has been prescribed to effectively treat anxiety. For patients receiving ziprasidone, QT prolongation needs to be monitored closely.24
Olanzapine was modeled after clozapine and is effective as a mood stabilizer and an antianxiety, antipsychotic, and sleep-promoting medication. It has a useful “mellowing” effect and helps with central pain syndrome management. Patients with fibromyalgia respond well; in some cases, patients with phantom and thalamic pain also respond. Among SGAs prescribed to treat chronic pain, olanzapine has the most published studies. However, the downside is the risk of severe weight gain and diabetes. Usually, if a patient is already overweight, they gain less, but these patients typically are concerned about any additional weight gain.25
Aripiprazole is a partial dopamine agonist. It increases dopamine function in the prefrontal cortex, and by doing so it possibly improves cognition, mental acuity, goal-oriented activity, and attention. At the same time, it decreases dopamine activity in the basal ganglia and limbic system, improving catastrophizing, paranoia, abnormal pain perception, and multiple homeostasis functions. This combination of effects can be invaluable for some patients, but depending on individual susceptibility, aripiprazole might be too activating (causing agitation and akathisia) or too sedating.26
Brexpiprazole is a relative of aripiprazole, but for some patients it is better tolerated, and compliance with this medication usually is good. It partially antagonizes the D2 and 5HT1A receptors while antagonizing the 5HT2A receptors (which decreases the dopamine release in the striatum) and mimics the mechanism of action of an antidepressant. Through alpha-1-adrenergic receptor antagonism, it reduces EPS. All these effects are also part of the mechanisms of action of quetiapine, clozapine, and iloperidone, but brexpiprazole is considered to be the most alpha-1 antagonistic, which is a mechanism of action of other potential pain-controlling medications such as clonidine and tizanidine. In patients with pain who have an overactive noradrenergic system, this property may be beneficial. Its major problem stems from cytochrome P450 2D6 (CYP2D6) enzyme-dependent metabolism, which causes an approximately 5-fold increase in brexpiprazole blood level in poor CYP2D6 metabolizers. Therefore, combining brexpiprazole with CYP2D6 inhibitors such as fluoxetine, paroxetine, and duloxetine would be unwise. Aripiprazole and brexpiprazole are less associated with diabetes and sexual adverse effects than many other SGAs.27
Continue to: Asenapine
Asenapine is an underutilized antipsychotic. Its mechanism of action spans multiple receptors and is less specific in individual receptor activity than other dopamine blockers. It is administered under the tongue due to poor absorption when swallowed, and its molecule has an anesthetic property that causes mouth and tongue numbness/paresthesia. This function may help patients with orofacial pain. Significant somnolence and weight gain (although less than with olanzapine) limit its use. Some patients cannot tolerate the taste.28
Quetiapine is prescribed rather frequently due to its significant antianxiety effect. It is also reported to be beneficial in pain control.29 Weight gain may be severe. In doses smaller than typically administered to patients with bipolar disorder or schizophrenia, quetiapine is widely prescribed off-label for sleep. In lower doses, it acts primarily as an antihistamine (hence the sedation), but at an increased dose it activates the adrenergic system, which offsets sedation. Quetiapine antagonizes H1 histamine and 5HT2
Cariprazine is typically well tolerated because of its benign metabolic profile. It does not increase the QT interval and is not sedating. Cariprazine is a D2 and D3 partial receptor agonist. This allows the medication to inhibit overstimulated dopamine receptors (a desirable effect in pain management) and induces them when the endogenous dopamine level is low (helping with cognition, volition, and attention). Pro-cognitive effects are always beneficial for patients with pain. Cariprazine produces less EPS due to more ventral striatum vs dorsal striatum activity. Mood improvement caused by this medication is attributed to its 5HT2A, 5HT2B, and 5HT2C inverse agonism, which modulates the serotonergic system. Cariprazine will likely have a positive future in pain management because it has shown efficacy in the chronic stress model.33
A complex condition
No single medication or group of medications may be exclusively relied on for treating patients with chronic pain. Identifying alternatives to opioids for treating pain brings more attention to centrally-acting medications that may aid in the stabilization of the nervous system, which can decrease pathological pain perception and help patients cope with chronic painful conditions.
Bottom Line
Antipsychotics may be a valuable asset in the treatment of chronic pain, offering a potential alternative to prescribing opioids for pain. More research is needed to identify specific ways of using dopamine blockade or dopamine enhancement to help patients with chronic pain.
Continue to: Related Resource
Related Resource
- Tripathi A. Antipsychotics for migraines, cluster headaches, and nausea. Current Psychiatry. 2013;12(2):E1-E4.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Bupropion • Wellbutrin, Zyban
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Clonidine • Catapres
Clozapine • Clozaril
Droperidol • Inapsine
Duloxetine • Cymbalta
Fluoxetine • Prozac
Haloperidol • Haldol
Iloperidone • Fanapt
Metoclopramide • Reglan
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimozide • Orap
Prochlorperazine • Compazine
Promethazine • Phenergan
Quetiapine • Seroquel
Risperidone • Risperdal
Tizanidine • Zanaflex
Ziprasidone • Geodon
1. Arbuck D, Pergolizzi J. Algopathy—acknowledging the pathological process of pain chronification. Pract Pain Manag. 2017;17(4):4,26-32.
2. Shin SW, Lee JS, Abdi S, et al. Antipsychotics for patients with pain. Korean J Pain. 2019;32(1):3-11.
3. D’Andrea G, Leone M, Bussone G, et al. Abnormal tyrosine metabolism in chronic cluster headache. Cephalalgia. 2017;37(2):148-153.
4. D’Andrea G, Granella F, Perini F, et al. Platelet levels of dopamine are increased in migraine and cluster headache. Headache. 2006;46(4):585-591.
5. Wolf EJ, Mitchell KS, Logue MW, et al. The dopamine D3 receptor gene, and posttraumatic stress disorder. J Trauma Stress. 2014;27(4):379-387.
6. den Ouden HEM, Daw ND, Fernandez G, et al. Dissociable effects of dopamine and serotonin on reversal learning. Neuron. 2013;80(4):1090-1100.
7. Nour MM, Dahoun T, Schwartenbeck P, et al. Dopaminergic basis for signaling belief updates, but not surprise, and the link to paranoia. Proc Natl Acad Sci U S A. 2018;115(43):E10167-E10176.
8. Zhu H, Clemens S, Sawchuk M, et al. Expression and distribution of all dopamine receptor subtypes (D(1)-D(5)) in the mouse lumbar spinal cord: a real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience. 2007;149:885-897.
9. Wood PB, Schweinhardt P, Jaeger E, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576-3582.
10. Hagelberg N, Fossell H, Aalto S, et al. Altered dopamine D2 receptor binding in atypical facial pain. Pain. 2003;106(1-2):43-48.
11. Hagelberg N, Fossell H, Rinne JD, et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003;101(1-2):149-154.
12. Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron. 2016;89(1):11-36.
13. Siahposht-Khachaki A, Pourreza P, Ezzatpanah S, et al. Nucleus accumbens dopamine receptors mediate hypothalamus-induced antinociception in the rat formalin test. Eur J Pain. 2017;21(7):1285-1294.
14. Thompson T, Gallop K, Correll CU, et al. Pain perception in Parkinson’s disease: a systematic review and meta-analysis of experimental studies. Aging Res Rev. 2017;35:74-86.
15. Check JH. Chronic unremitting lower abdominal pain quickly abrogated following treatment with amphetamine. Clin Exp Obstet Gynecol. 2016;43(1):109-111.
16. Wilkes S. Bupropion. Drugs Today (Barc). 2006;42(10):671-681.
17. Frei K, Truong DD, Fahn S, et al. The nosology of tardive syndromes. J Neurol Sci. 2018;389:10-16.
18. Honkaniemi J, Liimatainen S, Rainesalo S, et al. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787.
19. Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;(11):CD006271.
20. Gaffigan ME, Bruner DI, Wason C, et al. A randomized controlled trial of intravenous haloperidol vs. intravenous metoclopramide for acute migraine therapy in the emergency department. J Emerg Med. 2015;49(3):326-334.
21. Weinman D, Nicastro O, Akala O, et al. Parenteral treatment of episodic tension-type headache: a systematic review. Headache. 2014;54(2):260-268.
22. Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology, and approaches to treatment. CNS Drugs. 2001;15(5):351-359.
23. Khouzam HR. Psychopharmacology of chronic pain: a focus on antidepressants and atypical antipsychotics. Postgrad Med. 2016;128(3):323-330.
24. Landsness EC, Wang LH, Bucelli RC. Ziprasidone as a potential abortive therapy for status migrainosus. Neurohospitalist. 2016;6(4):151-156.
25. Jimenez XF, Sundararajan T, Covington EC. A systematic review of atypical antipsychotics in chronic pain management: olanzapine demonstrates potential in central sensitization, fibromyalgia, and headache/migraine. Clin J Pain. 2018;34(6):585-591.
26. Fei L, Abrardi L, Mediati RD. Unexpected effect of aripiprazole on nociceptive pain. Ther Adv Psychopharmacol. 2012;2(5):211-212.
27. Markovic M, Gallipani A, Patel KH, et al. Brexpiprazole. Ann Pharmacother. 2017;51(4):315-322.
28. Gerrits M, de Greef R, Peeters P. Effect of absorption site on the pharmacokinetics of sublingual asenapine in healthy male subjects. Biopharm Drug Dispos. 2010;31(5-6):351-357.
29. Heo MH, Kim JY, Hwang I, et al. Analgesic effect of quetiapine in a mouse model of cancer-induced bone pain. Korean J Intern Med. 2017;32(6):1069-1074.
30. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Am Acad Psychiatry Law. 2012;40(4):502-508.
31. Fountoulakis KN, Iacovides A, Kaprinis SG, et al. Diffuse muscle pain with quetiapine. Br J Psychiatry. 2003;182:81.
32. Shintani F. Diminished pain perception in schizophrenia. Lancet. 2010;376(9735):87.
33. Duric V, Banasr M, Franklin T, et al. Cariprazine exhibits anxiolytic and dopamine D3 receptor-dependent antidepressant effects in the chronic stress model. Int J Neuropsychopharmacol. 2017;20(10):788-796
Our understanding of pain mechanisms continues to evolve and, accordingly, so do our treatment strategies. The fundamental differences between acute and chronic pain were only recently recognized; this lack of recognition led to the application of acute pain treatments to chronic pain, contributing to the opioid epidemic in the United States.
With the diminishing emphasis on opioid medications, researchers are exploring other pharmacologic modalities for treating pain. Many nonopioid psychiatric medications are used off-label for the treatment of pain. Psychiatric medications play a larger role in the management of pain as pain becomes more chronic (Table 11). For simplicity, acute pain may be seen as nociception colored by emotions, and chronic pain as emotions colored by nociception. Protracted pain connects those extremes with a diminishing role of nociception and an increasing role of emotion,1 which may increase the potential role of psychiatric medications, including antipsychotics.
In this article, I discuss the potential role of dopamine in the perception of pain, and review the potential use of first- and second-generation antipsychotics for treating various pain syndromes.
Role of dopamine in pain
There is increasing interest in exploring antipsychotics to treat chronic pain2 because dopamine dysfunction is part of pathological pain perception. Excess dopamine is associated with headaches (dopamine hypersensitivity hypothesis3,4) and dopamine dysfunction is a part of posttraumatic stress disorder (PTSD),5 dissociation,6 paranoia,7 and catastrophizing.8 Somatic psychosis, like any psychosis, can be based on dopamine pathology. Dopaminergic neurons affect nociceptive function in the spinal dorsal horn,9 and dopamine receptors are altered in atypical facial pain,10 burning mouth syndrome,11 and fibromyalgia.12
In normal circumstances, dopamine is fundamentally a protective neurotransmitter. In acute pain, dopamine is powerfully released, making the pain bearable. A patient may describe acute pain as seeming “like it was not happening to me” or “it was like a dream”; both are examples of dopamine-caused dissociation and a possible prediction of subsequent chronification. In chronic pain, pathological mechanisms settle in and take root; therefore, keeping protective dopamine levels high becomes a priority. This is especially common in patients who have experienced abuse or PTSD. The only natural way to keep dopamine up for prolonged periods of time is to decrease pain and stress thresholds. Both phenomena are readily observed in patients with pain. In extreme cases, self-mutilation and involvement in conflicts become pathologically gratifying.
The dopaminergic system is essential for pain control with a tissue injury.13 It becomes pathologically stimulated and increasingly dysfunctional as algopathy (a pathological pain perception) develops. At the same time, a flood or drought of any neurotransmitter is equally bad and may produce similar clinical pictures. Both a lack of and excess of dopamine are associated with pain.14 This is why opposite treatments may be beneficial in different patients with chronic pain. As an example, the use of stimulants15 and bupropion16 has been reported in the treatment of abdominal pain. And, reversely, antipsychotics, especially first-generation agents, may be associated with chronic (tardive) pain, including orofacial and genital pain.17
First-generation antipsychotics
First-generation antipsychotics (FGAs) have been used to treat various nonpsychiatric conditions (Table 2). Although they are powerful D2 receptor inhibitors, FGAs lack the intrinsic ability to counteract the unwanted adverse effects of strong inhibition. As a result, movement disorders and prolactinemia are commonly induced by FGAs. The most dangerous consequence of treatment with these agents is neuroleptic malignant syndrome (NMS).
Continue to: Haloperidol
Haloperidol is prescribed widely by nonpsychiatrists, primarily to treat agitation. Intravenous haloperidol has been used for the abortive treatment of headaches.18 Paradoxically, IV haloperidol is less likely to induce extrapyramidal symptoms (EPS) than the oral formulation because of a more pronounced anticholinergic action in IV use. Haloperidol can help relieve gastroparesis and nausea, especially in IV administration,19 but prolonged oral administration is associated with unwanted movement problems and should be avoided.20
Chlorpromazine is more anticholinergic than haloperidol. It can be used in the abortive treatment of headaches (preferably via IV and IM administration), nausea, hiccups, porphyria, and serotonin syndrome, but it is very sedating and frequently produces hypotension, dangerous QT prolongation, and sensations of thought-blocking.21
Pimozide is reported to help with skin picking, trichotillomania, and somatic hallucinations.22
Droperidol, promethazine, and prochlorperazine are used off-label to treat nausea and headaches. Primary care clinicians may not be aware that these commonly used medications are antipsychotics. Similar to other FGAs, these 3 agents may produce NMS and tardive dyskinesia (TD). The same applies to the prokinetic drug metoclopramide.
Second-generation antipsychotics
Second-generation antipsychotics (SGAs) work with various serotonin receptors, offsetting and enhancing the antipsychotic function of dopamine blockade. This diminishes but does not eliminate EPS and the risk of TD. Fortunately, the risk of NMS is lower with SGAs than with FGAs. Many SGAs are FDA-approved for treating schizophrenia and other psychiatric disorders, and some have relevance for pain management (Table 3). Many SGAs help with depressive symptoms and are powerful mood stabilizers. As such, they may diminish central over-firing of dopaminergic and serotonergic neurons involved in the pain cascade, which in turn decreases pain transmission and perception. The downside is that in general, SGAs increase the risk of diabetes and hyperlipidemia.
Continue to: Risperidone
Risperidone was the second FDA-approved SGA. Pain practitioners primarily prescribe it for treatmeant-resistant headaches, but patients with fibromyalgia and those with phantom and thalamic pain also may respond. Because risperidone’s properties are similar to that of many FGAs, it may potently cause EPS, TD, and prolactinemia. Neuroleptic malignant syndrome also has been reported.23
Ziprasidone is frequently overlooked by clinicians who treat pain. Although ziprasidone may be sedating, it is powerful as both a preventive and abortive (in an IM formulation) agent for treatment-resistant headaches. This might be attributed to its effects on the 5HT9 receptor. It is approved for treating bipolar depression and has been prescribed to effectively treat anxiety. For patients receiving ziprasidone, QT prolongation needs to be monitored closely.24
Olanzapine was modeled after clozapine and is effective as a mood stabilizer and an antianxiety, antipsychotic, and sleep-promoting medication. It has a useful “mellowing” effect and helps with central pain syndrome management. Patients with fibromyalgia respond well; in some cases, patients with phantom and thalamic pain also respond. Among SGAs prescribed to treat chronic pain, olanzapine has the most published studies. However, the downside is the risk of severe weight gain and diabetes. Usually, if a patient is already overweight, they gain less, but these patients typically are concerned about any additional weight gain.25
Aripiprazole is a partial dopamine agonist. It increases dopamine function in the prefrontal cortex, and by doing so it possibly improves cognition, mental acuity, goal-oriented activity, and attention. At the same time, it decreases dopamine activity in the basal ganglia and limbic system, improving catastrophizing, paranoia, abnormal pain perception, and multiple homeostasis functions. This combination of effects can be invaluable for some patients, but depending on individual susceptibility, aripiprazole might be too activating (causing agitation and akathisia) or too sedating.26
Brexpiprazole is a relative of aripiprazole, but for some patients it is better tolerated, and compliance with this medication usually is good. It partially antagonizes the D2 and 5HT1A receptors while antagonizing the 5HT2A receptors (which decreases the dopamine release in the striatum) and mimics the mechanism of action of an antidepressant. Through alpha-1-adrenergic receptor antagonism, it reduces EPS. All these effects are also part of the mechanisms of action of quetiapine, clozapine, and iloperidone, but brexpiprazole is considered to be the most alpha-1 antagonistic, which is a mechanism of action of other potential pain-controlling medications such as clonidine and tizanidine. In patients with pain who have an overactive noradrenergic system, this property may be beneficial. Its major problem stems from cytochrome P450 2D6 (CYP2D6) enzyme-dependent metabolism, which causes an approximately 5-fold increase in brexpiprazole blood level in poor CYP2D6 metabolizers. Therefore, combining brexpiprazole with CYP2D6 inhibitors such as fluoxetine, paroxetine, and duloxetine would be unwise. Aripiprazole and brexpiprazole are less associated with diabetes and sexual adverse effects than many other SGAs.27
Continue to: Asenapine
Asenapine is an underutilized antipsychotic. Its mechanism of action spans multiple receptors and is less specific in individual receptor activity than other dopamine blockers. It is administered under the tongue due to poor absorption when swallowed, and its molecule has an anesthetic property that causes mouth and tongue numbness/paresthesia. This function may help patients with orofacial pain. Significant somnolence and weight gain (although less than with olanzapine) limit its use. Some patients cannot tolerate the taste.28
Quetiapine is prescribed rather frequently due to its significant antianxiety effect. It is also reported to be beneficial in pain control.29 Weight gain may be severe. In doses smaller than typically administered to patients with bipolar disorder or schizophrenia, quetiapine is widely prescribed off-label for sleep. In lower doses, it acts primarily as an antihistamine (hence the sedation), but at an increased dose it activates the adrenergic system, which offsets sedation. Quetiapine antagonizes H1 histamine and 5HT2
Cariprazine is typically well tolerated because of its benign metabolic profile. It does not increase the QT interval and is not sedating. Cariprazine is a D2 and D3 partial receptor agonist. This allows the medication to inhibit overstimulated dopamine receptors (a desirable effect in pain management) and induces them when the endogenous dopamine level is low (helping with cognition, volition, and attention). Pro-cognitive effects are always beneficial for patients with pain. Cariprazine produces less EPS due to more ventral striatum vs dorsal striatum activity. Mood improvement caused by this medication is attributed to its 5HT2A, 5HT2B, and 5HT2C inverse agonism, which modulates the serotonergic system. Cariprazine will likely have a positive future in pain management because it has shown efficacy in the chronic stress model.33
A complex condition
No single medication or group of medications may be exclusively relied on for treating patients with chronic pain. Identifying alternatives to opioids for treating pain brings more attention to centrally-acting medications that may aid in the stabilization of the nervous system, which can decrease pathological pain perception and help patients cope with chronic painful conditions.
Bottom Line
Antipsychotics may be a valuable asset in the treatment of chronic pain, offering a potential alternative to prescribing opioids for pain. More research is needed to identify specific ways of using dopamine blockade or dopamine enhancement to help patients with chronic pain.
Continue to: Related Resource
Related Resource
- Tripathi A. Antipsychotics for migraines, cluster headaches, and nausea. Current Psychiatry. 2013;12(2):E1-E4.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Bupropion • Wellbutrin, Zyban
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Clonidine • Catapres
Clozapine • Clozaril
Droperidol • Inapsine
Duloxetine • Cymbalta
Fluoxetine • Prozac
Haloperidol • Haldol
Iloperidone • Fanapt
Metoclopramide • Reglan
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimozide • Orap
Prochlorperazine • Compazine
Promethazine • Phenergan
Quetiapine • Seroquel
Risperidone • Risperdal
Tizanidine • Zanaflex
Ziprasidone • Geodon
Our understanding of pain mechanisms continues to evolve and, accordingly, so do our treatment strategies. The fundamental differences between acute and chronic pain were only recently recognized; this lack of recognition led to the application of acute pain treatments to chronic pain, contributing to the opioid epidemic in the United States.
With the diminishing emphasis on opioid medications, researchers are exploring other pharmacologic modalities for treating pain. Many nonopioid psychiatric medications are used off-label for the treatment of pain. Psychiatric medications play a larger role in the management of pain as pain becomes more chronic (Table 11). For simplicity, acute pain may be seen as nociception colored by emotions, and chronic pain as emotions colored by nociception. Protracted pain connects those extremes with a diminishing role of nociception and an increasing role of emotion,1 which may increase the potential role of psychiatric medications, including antipsychotics.
In this article, I discuss the potential role of dopamine in the perception of pain, and review the potential use of first- and second-generation antipsychotics for treating various pain syndromes.
Role of dopamine in pain
There is increasing interest in exploring antipsychotics to treat chronic pain2 because dopamine dysfunction is part of pathological pain perception. Excess dopamine is associated with headaches (dopamine hypersensitivity hypothesis3,4) and dopamine dysfunction is a part of posttraumatic stress disorder (PTSD),5 dissociation,6 paranoia,7 and catastrophizing.8 Somatic psychosis, like any psychosis, can be based on dopamine pathology. Dopaminergic neurons affect nociceptive function in the spinal dorsal horn,9 and dopamine receptors are altered in atypical facial pain,10 burning mouth syndrome,11 and fibromyalgia.12
In normal circumstances, dopamine is fundamentally a protective neurotransmitter. In acute pain, dopamine is powerfully released, making the pain bearable. A patient may describe acute pain as seeming “like it was not happening to me” or “it was like a dream”; both are examples of dopamine-caused dissociation and a possible prediction of subsequent chronification. In chronic pain, pathological mechanisms settle in and take root; therefore, keeping protective dopamine levels high becomes a priority. This is especially common in patients who have experienced abuse or PTSD. The only natural way to keep dopamine up for prolonged periods of time is to decrease pain and stress thresholds. Both phenomena are readily observed in patients with pain. In extreme cases, self-mutilation and involvement in conflicts become pathologically gratifying.
The dopaminergic system is essential for pain control with a tissue injury.13 It becomes pathologically stimulated and increasingly dysfunctional as algopathy (a pathological pain perception) develops. At the same time, a flood or drought of any neurotransmitter is equally bad and may produce similar clinical pictures. Both a lack of and excess of dopamine are associated with pain.14 This is why opposite treatments may be beneficial in different patients with chronic pain. As an example, the use of stimulants15 and bupropion16 has been reported in the treatment of abdominal pain. And, reversely, antipsychotics, especially first-generation agents, may be associated with chronic (tardive) pain, including orofacial and genital pain.17
First-generation antipsychotics
First-generation antipsychotics (FGAs) have been used to treat various nonpsychiatric conditions (Table 2). Although they are powerful D2 receptor inhibitors, FGAs lack the intrinsic ability to counteract the unwanted adverse effects of strong inhibition. As a result, movement disorders and prolactinemia are commonly induced by FGAs. The most dangerous consequence of treatment with these agents is neuroleptic malignant syndrome (NMS).
Continue to: Haloperidol
Haloperidol is prescribed widely by nonpsychiatrists, primarily to treat agitation. Intravenous haloperidol has been used for the abortive treatment of headaches.18 Paradoxically, IV haloperidol is less likely to induce extrapyramidal symptoms (EPS) than the oral formulation because of a more pronounced anticholinergic action in IV use. Haloperidol can help relieve gastroparesis and nausea, especially in IV administration,19 but prolonged oral administration is associated with unwanted movement problems and should be avoided.20
Chlorpromazine is more anticholinergic than haloperidol. It can be used in the abortive treatment of headaches (preferably via IV and IM administration), nausea, hiccups, porphyria, and serotonin syndrome, but it is very sedating and frequently produces hypotension, dangerous QT prolongation, and sensations of thought-blocking.21
Pimozide is reported to help with skin picking, trichotillomania, and somatic hallucinations.22
Droperidol, promethazine, and prochlorperazine are used off-label to treat nausea and headaches. Primary care clinicians may not be aware that these commonly used medications are antipsychotics. Similar to other FGAs, these 3 agents may produce NMS and tardive dyskinesia (TD). The same applies to the prokinetic drug metoclopramide.
Second-generation antipsychotics
Second-generation antipsychotics (SGAs) work with various serotonin receptors, offsetting and enhancing the antipsychotic function of dopamine blockade. This diminishes but does not eliminate EPS and the risk of TD. Fortunately, the risk of NMS is lower with SGAs than with FGAs. Many SGAs are FDA-approved for treating schizophrenia and other psychiatric disorders, and some have relevance for pain management (Table 3). Many SGAs help with depressive symptoms and are powerful mood stabilizers. As such, they may diminish central over-firing of dopaminergic and serotonergic neurons involved in the pain cascade, which in turn decreases pain transmission and perception. The downside is that in general, SGAs increase the risk of diabetes and hyperlipidemia.
Continue to: Risperidone
Risperidone was the second FDA-approved SGA. Pain practitioners primarily prescribe it for treatmeant-resistant headaches, but patients with fibromyalgia and those with phantom and thalamic pain also may respond. Because risperidone’s properties are similar to that of many FGAs, it may potently cause EPS, TD, and prolactinemia. Neuroleptic malignant syndrome also has been reported.23
Ziprasidone is frequently overlooked by clinicians who treat pain. Although ziprasidone may be sedating, it is powerful as both a preventive and abortive (in an IM formulation) agent for treatment-resistant headaches. This might be attributed to its effects on the 5HT9 receptor. It is approved for treating bipolar depression and has been prescribed to effectively treat anxiety. For patients receiving ziprasidone, QT prolongation needs to be monitored closely.24
Olanzapine was modeled after clozapine and is effective as a mood stabilizer and an antianxiety, antipsychotic, and sleep-promoting medication. It has a useful “mellowing” effect and helps with central pain syndrome management. Patients with fibromyalgia respond well; in some cases, patients with phantom and thalamic pain also respond. Among SGAs prescribed to treat chronic pain, olanzapine has the most published studies. However, the downside is the risk of severe weight gain and diabetes. Usually, if a patient is already overweight, they gain less, but these patients typically are concerned about any additional weight gain.25
Aripiprazole is a partial dopamine agonist. It increases dopamine function in the prefrontal cortex, and by doing so it possibly improves cognition, mental acuity, goal-oriented activity, and attention. At the same time, it decreases dopamine activity in the basal ganglia and limbic system, improving catastrophizing, paranoia, abnormal pain perception, and multiple homeostasis functions. This combination of effects can be invaluable for some patients, but depending on individual susceptibility, aripiprazole might be too activating (causing agitation and akathisia) or too sedating.26
Brexpiprazole is a relative of aripiprazole, but for some patients it is better tolerated, and compliance with this medication usually is good. It partially antagonizes the D2 and 5HT1A receptors while antagonizing the 5HT2A receptors (which decreases the dopamine release in the striatum) and mimics the mechanism of action of an antidepressant. Through alpha-1-adrenergic receptor antagonism, it reduces EPS. All these effects are also part of the mechanisms of action of quetiapine, clozapine, and iloperidone, but brexpiprazole is considered to be the most alpha-1 antagonistic, which is a mechanism of action of other potential pain-controlling medications such as clonidine and tizanidine. In patients with pain who have an overactive noradrenergic system, this property may be beneficial. Its major problem stems from cytochrome P450 2D6 (CYP2D6) enzyme-dependent metabolism, which causes an approximately 5-fold increase in brexpiprazole blood level in poor CYP2D6 metabolizers. Therefore, combining brexpiprazole with CYP2D6 inhibitors such as fluoxetine, paroxetine, and duloxetine would be unwise. Aripiprazole and brexpiprazole are less associated with diabetes and sexual adverse effects than many other SGAs.27
Continue to: Asenapine
Asenapine is an underutilized antipsychotic. Its mechanism of action spans multiple receptors and is less specific in individual receptor activity than other dopamine blockers. It is administered under the tongue due to poor absorption when swallowed, and its molecule has an anesthetic property that causes mouth and tongue numbness/paresthesia. This function may help patients with orofacial pain. Significant somnolence and weight gain (although less than with olanzapine) limit its use. Some patients cannot tolerate the taste.28
Quetiapine is prescribed rather frequently due to its significant antianxiety effect. It is also reported to be beneficial in pain control.29 Weight gain may be severe. In doses smaller than typically administered to patients with bipolar disorder or schizophrenia, quetiapine is widely prescribed off-label for sleep. In lower doses, it acts primarily as an antihistamine (hence the sedation), but at an increased dose it activates the adrenergic system, which offsets sedation. Quetiapine antagonizes H1 histamine and 5HT2
Cariprazine is typically well tolerated because of its benign metabolic profile. It does not increase the QT interval and is not sedating. Cariprazine is a D2 and D3 partial receptor agonist. This allows the medication to inhibit overstimulated dopamine receptors (a desirable effect in pain management) and induces them when the endogenous dopamine level is low (helping with cognition, volition, and attention). Pro-cognitive effects are always beneficial for patients with pain. Cariprazine produces less EPS due to more ventral striatum vs dorsal striatum activity. Mood improvement caused by this medication is attributed to its 5HT2A, 5HT2B, and 5HT2C inverse agonism, which modulates the serotonergic system. Cariprazine will likely have a positive future in pain management because it has shown efficacy in the chronic stress model.33
A complex condition
No single medication or group of medications may be exclusively relied on for treating patients with chronic pain. Identifying alternatives to opioids for treating pain brings more attention to centrally-acting medications that may aid in the stabilization of the nervous system, which can decrease pathological pain perception and help patients cope with chronic painful conditions.
Bottom Line
Antipsychotics may be a valuable asset in the treatment of chronic pain, offering a potential alternative to prescribing opioids for pain. More research is needed to identify specific ways of using dopamine blockade or dopamine enhancement to help patients with chronic pain.
Continue to: Related Resource
Related Resource
- Tripathi A. Antipsychotics for migraines, cluster headaches, and nausea. Current Psychiatry. 2013;12(2):E1-E4.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Bupropion • Wellbutrin, Zyban
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Clonidine • Catapres
Clozapine • Clozaril
Droperidol • Inapsine
Duloxetine • Cymbalta
Fluoxetine • Prozac
Haloperidol • Haldol
Iloperidone • Fanapt
Metoclopramide • Reglan
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimozide • Orap
Prochlorperazine • Compazine
Promethazine • Phenergan
Quetiapine • Seroquel
Risperidone • Risperdal
Tizanidine • Zanaflex
Ziprasidone • Geodon
1. Arbuck D, Pergolizzi J. Algopathy—acknowledging the pathological process of pain chronification. Pract Pain Manag. 2017;17(4):4,26-32.
2. Shin SW, Lee JS, Abdi S, et al. Antipsychotics for patients with pain. Korean J Pain. 2019;32(1):3-11.
3. D’Andrea G, Leone M, Bussone G, et al. Abnormal tyrosine metabolism in chronic cluster headache. Cephalalgia. 2017;37(2):148-153.
4. D’Andrea G, Granella F, Perini F, et al. Platelet levels of dopamine are increased in migraine and cluster headache. Headache. 2006;46(4):585-591.
5. Wolf EJ, Mitchell KS, Logue MW, et al. The dopamine D3 receptor gene, and posttraumatic stress disorder. J Trauma Stress. 2014;27(4):379-387.
6. den Ouden HEM, Daw ND, Fernandez G, et al. Dissociable effects of dopamine and serotonin on reversal learning. Neuron. 2013;80(4):1090-1100.
7. Nour MM, Dahoun T, Schwartenbeck P, et al. Dopaminergic basis for signaling belief updates, but not surprise, and the link to paranoia. Proc Natl Acad Sci U S A. 2018;115(43):E10167-E10176.
8. Zhu H, Clemens S, Sawchuk M, et al. Expression and distribution of all dopamine receptor subtypes (D(1)-D(5)) in the mouse lumbar spinal cord: a real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience. 2007;149:885-897.
9. Wood PB, Schweinhardt P, Jaeger E, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576-3582.
10. Hagelberg N, Fossell H, Aalto S, et al. Altered dopamine D2 receptor binding in atypical facial pain. Pain. 2003;106(1-2):43-48.
11. Hagelberg N, Fossell H, Rinne JD, et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003;101(1-2):149-154.
12. Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron. 2016;89(1):11-36.
13. Siahposht-Khachaki A, Pourreza P, Ezzatpanah S, et al. Nucleus accumbens dopamine receptors mediate hypothalamus-induced antinociception in the rat formalin test. Eur J Pain. 2017;21(7):1285-1294.
14. Thompson T, Gallop K, Correll CU, et al. Pain perception in Parkinson’s disease: a systematic review and meta-analysis of experimental studies. Aging Res Rev. 2017;35:74-86.
15. Check JH. Chronic unremitting lower abdominal pain quickly abrogated following treatment with amphetamine. Clin Exp Obstet Gynecol. 2016;43(1):109-111.
16. Wilkes S. Bupropion. Drugs Today (Barc). 2006;42(10):671-681.
17. Frei K, Truong DD, Fahn S, et al. The nosology of tardive syndromes. J Neurol Sci. 2018;389:10-16.
18. Honkaniemi J, Liimatainen S, Rainesalo S, et al. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787.
19. Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;(11):CD006271.
20. Gaffigan ME, Bruner DI, Wason C, et al. A randomized controlled trial of intravenous haloperidol vs. intravenous metoclopramide for acute migraine therapy in the emergency department. J Emerg Med. 2015;49(3):326-334.
21. Weinman D, Nicastro O, Akala O, et al. Parenteral treatment of episodic tension-type headache: a systematic review. Headache. 2014;54(2):260-268.
22. Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology, and approaches to treatment. CNS Drugs. 2001;15(5):351-359.
23. Khouzam HR. Psychopharmacology of chronic pain: a focus on antidepressants and atypical antipsychotics. Postgrad Med. 2016;128(3):323-330.
24. Landsness EC, Wang LH, Bucelli RC. Ziprasidone as a potential abortive therapy for status migrainosus. Neurohospitalist. 2016;6(4):151-156.
25. Jimenez XF, Sundararajan T, Covington EC. A systematic review of atypical antipsychotics in chronic pain management: olanzapine demonstrates potential in central sensitization, fibromyalgia, and headache/migraine. Clin J Pain. 2018;34(6):585-591.
26. Fei L, Abrardi L, Mediati RD. Unexpected effect of aripiprazole on nociceptive pain. Ther Adv Psychopharmacol. 2012;2(5):211-212.
27. Markovic M, Gallipani A, Patel KH, et al. Brexpiprazole. Ann Pharmacother. 2017;51(4):315-322.
28. Gerrits M, de Greef R, Peeters P. Effect of absorption site on the pharmacokinetics of sublingual asenapine in healthy male subjects. Biopharm Drug Dispos. 2010;31(5-6):351-357.
29. Heo MH, Kim JY, Hwang I, et al. Analgesic effect of quetiapine in a mouse model of cancer-induced bone pain. Korean J Intern Med. 2017;32(6):1069-1074.
30. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Am Acad Psychiatry Law. 2012;40(4):502-508.
31. Fountoulakis KN, Iacovides A, Kaprinis SG, et al. Diffuse muscle pain with quetiapine. Br J Psychiatry. 2003;182:81.
32. Shintani F. Diminished pain perception in schizophrenia. Lancet. 2010;376(9735):87.
33. Duric V, Banasr M, Franklin T, et al. Cariprazine exhibits anxiolytic and dopamine D3 receptor-dependent antidepressant effects in the chronic stress model. Int J Neuropsychopharmacol. 2017;20(10):788-796
1. Arbuck D, Pergolizzi J. Algopathy—acknowledging the pathological process of pain chronification. Pract Pain Manag. 2017;17(4):4,26-32.
2. Shin SW, Lee JS, Abdi S, et al. Antipsychotics for patients with pain. Korean J Pain. 2019;32(1):3-11.
3. D’Andrea G, Leone M, Bussone G, et al. Abnormal tyrosine metabolism in chronic cluster headache. Cephalalgia. 2017;37(2):148-153.
4. D’Andrea G, Granella F, Perini F, et al. Platelet levels of dopamine are increased in migraine and cluster headache. Headache. 2006;46(4):585-591.
5. Wolf EJ, Mitchell KS, Logue MW, et al. The dopamine D3 receptor gene, and posttraumatic stress disorder. J Trauma Stress. 2014;27(4):379-387.
6. den Ouden HEM, Daw ND, Fernandez G, et al. Dissociable effects of dopamine and serotonin on reversal learning. Neuron. 2013;80(4):1090-1100.
7. Nour MM, Dahoun T, Schwartenbeck P, et al. Dopaminergic basis for signaling belief updates, but not surprise, and the link to paranoia. Proc Natl Acad Sci U S A. 2018;115(43):E10167-E10176.
8. Zhu H, Clemens S, Sawchuk M, et al. Expression and distribution of all dopamine receptor subtypes (D(1)-D(5)) in the mouse lumbar spinal cord: a real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience. 2007;149:885-897.
9. Wood PB, Schweinhardt P, Jaeger E, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576-3582.
10. Hagelberg N, Fossell H, Aalto S, et al. Altered dopamine D2 receptor binding in atypical facial pain. Pain. 2003;106(1-2):43-48.
11. Hagelberg N, Fossell H, Rinne JD, et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003;101(1-2):149-154.
12. Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron. 2016;89(1):11-36.
13. Siahposht-Khachaki A, Pourreza P, Ezzatpanah S, et al. Nucleus accumbens dopamine receptors mediate hypothalamus-induced antinociception in the rat formalin test. Eur J Pain. 2017;21(7):1285-1294.
14. Thompson T, Gallop K, Correll CU, et al. Pain perception in Parkinson’s disease: a systematic review and meta-analysis of experimental studies. Aging Res Rev. 2017;35:74-86.
15. Check JH. Chronic unremitting lower abdominal pain quickly abrogated following treatment with amphetamine. Clin Exp Obstet Gynecol. 2016;43(1):109-111.
16. Wilkes S. Bupropion. Drugs Today (Barc). 2006;42(10):671-681.
17. Frei K, Truong DD, Fahn S, et al. The nosology of tardive syndromes. J Neurol Sci. 2018;389:10-16.
18. Honkaniemi J, Liimatainen S, Rainesalo S, et al. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787.
19. Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;(11):CD006271.
20. Gaffigan ME, Bruner DI, Wason C, et al. A randomized controlled trial of intravenous haloperidol vs. intravenous metoclopramide for acute migraine therapy in the emergency department. J Emerg Med. 2015;49(3):326-334.
21. Weinman D, Nicastro O, Akala O, et al. Parenteral treatment of episodic tension-type headache: a systematic review. Headache. 2014;54(2):260-268.
22. Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology, and approaches to treatment. CNS Drugs. 2001;15(5):351-359.
23. Khouzam HR. Psychopharmacology of chronic pain: a focus on antidepressants and atypical antipsychotics. Postgrad Med. 2016;128(3):323-330.
24. Landsness EC, Wang LH, Bucelli RC. Ziprasidone as a potential abortive therapy for status migrainosus. Neurohospitalist. 2016;6(4):151-156.
25. Jimenez XF, Sundararajan T, Covington EC. A systematic review of atypical antipsychotics in chronic pain management: olanzapine demonstrates potential in central sensitization, fibromyalgia, and headache/migraine. Clin J Pain. 2018;34(6):585-591.
26. Fei L, Abrardi L, Mediati RD. Unexpected effect of aripiprazole on nociceptive pain. Ther Adv Psychopharmacol. 2012;2(5):211-212.
27. Markovic M, Gallipani A, Patel KH, et al. Brexpiprazole. Ann Pharmacother. 2017;51(4):315-322.
28. Gerrits M, de Greef R, Peeters P. Effect of absorption site on the pharmacokinetics of sublingual asenapine in healthy male subjects. Biopharm Drug Dispos. 2010;31(5-6):351-357.
29. Heo MH, Kim JY, Hwang I, et al. Analgesic effect of quetiapine in a mouse model of cancer-induced bone pain. Korean J Intern Med. 2017;32(6):1069-1074.
30. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Am Acad Psychiatry Law. 2012;40(4):502-508.
31. Fountoulakis KN, Iacovides A, Kaprinis SG, et al. Diffuse muscle pain with quetiapine. Br J Psychiatry. 2003;182:81.
32. Shintani F. Diminished pain perception in schizophrenia. Lancet. 2010;376(9735):87.
33. Duric V, Banasr M, Franklin T, et al. Cariprazine exhibits anxiolytic and dopamine D3 receptor-dependent antidepressant effects in the chronic stress model. Int J Neuropsychopharmacol. 2017;20(10):788-796