User login
Considerable advances in artificial intelligence (AI) and machine-learning (ML) methodologies have led to the emergence of promising tools in the field of gastrointestinal endoscopy. Computer vision is an application of AI/ML that has been successfully applied for the computer-aided detection (CADe) and computer-aided diagnosis (CADx) of colon polyps and numerous other conditions encountered during GI endoscopy. Outside of computer vision, a wide variety of other AI applications have been applied to gastroenterology, ranging from natural language processing (NLP) to optimize clinical documentation and endoscopy quality reporting to ML techniques that predict disease severity/treatment response and augment clinical decision-making.
In the United States, colonoscopy is the standard for colon cancer screening and prevention; however, precancerous polyps can be missed for various reasons, ranging from subtle surface appearance of the polyp or location behind a colonic fold to operator-dependent reasons such as inadequate mucosal inspection. Though clinical practice guidelines have set adenoma detection rate (ADR) thresholds at 20% for women and 30% for men, studies have shown a 4- to 10-fold variation in ADR among physicians in clinical practice settings,1 with an estimated adenoma miss rate (AMR) of 25% and a false-negative colonoscopy rate of 12%.2 Variability in adenoma detection affects the risk of interval colorectal cancer post colonoscopy.3,4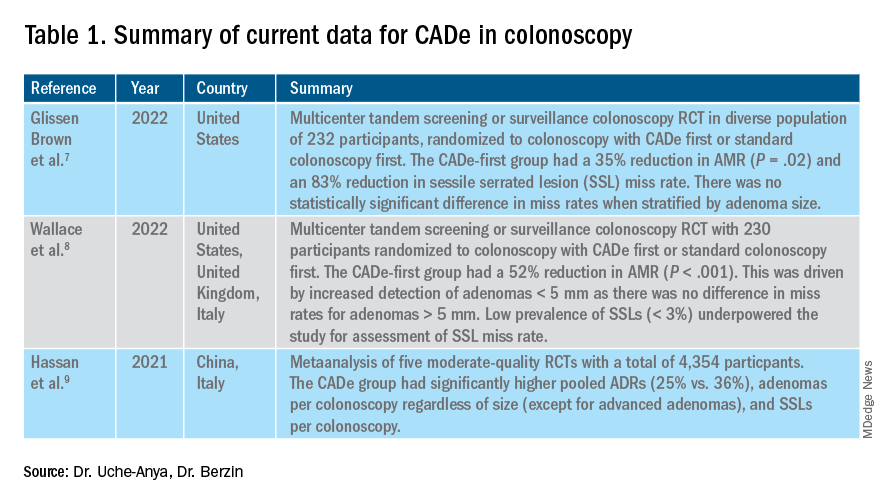
AI provides an opportunity for mitigating this risk. Advances in deep learning and computer vision have led to the development of CADe systems that automatically detect polyps in real time during colonoscopy, resulting in reduced adenoma miss rates (Table 1). In addition to polyp detection, deep-learning technologies are also being used in CADx systems for polyp diagnosis and characterization of malignancy risk. This could aid therapeutic decision-making: Unnecessary resection or histopathologic analysis could be obviated for benign hyperplastic polyps. On the other end of the polyp spectrum, an AI tool that could predict the presence or absence of submucosal invasion could be a powerful tool when evaluating early colon cancers for consideration of endoscopic submucosal dissection vs. surgery. Examples of CADe polyp detection and CADx polyp characterization are shown in Figure 1.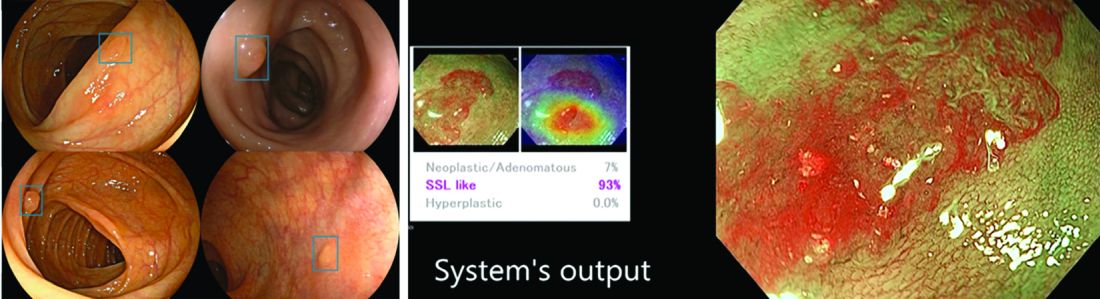
Other potential computer vision applications that may improve colonoscopy quality include tools that help measure adequacy of mucosal exposure, segmental inspection time, and a variety of other parameters associated with polyp detection performance. These are promising areas for future research. Beyond improving colonoscopy technique, natural language processing tools already are being used to optimize clinical documentation as well as extract information from colonoscopy and pathology reports that can facilitate reporting of colonoscopy quality metrics such as ADR, cecal intubation rate, withdrawal time, and bowel preparation adequacy. AI-powered analytics may help unlock large-scale reporting of colonoscopy quality metrics on a health-systems level5 or population-level,6 helping to ensure optimal performance and identifying avenues for colonoscopy quality improvement.
The majority of AI research in colonoscopy has focused on CADe for colon polyp detection and CADx for polyp diagnosis. Over the last few years, several randomized clinical trials – two in the United States – have shown that CADe significantly improves adenoma detection and reduces adenoma miss rates in comparison to standard colonoscopy. The existing data are summarized in Table 1, focusing on the two U.S. studies and an international meta-analysis.
In comparison, the data landscape for CADx is nascent and currently limited to several retrospective studies dating back to 2009 and a few prospective studies that have shown promising results.10,11 There is an expectation that integrated CADx also may support the adoption of “resect and discard” or “diagnose and leave” strategies for low-risk polyps. About two-thirds of polyps identified on average-risk screening colonoscopies are diminutive polyps (less than 5 mm in size), which rarely have advanced histologic features (about 0.5%) and are sometimes non-neoplastic (30%). Malignancy risk is even lower in the distal colon.12 As routine histopathologic assessment of such polyps is mostly of limited clinical utility and comes with added pathology costs, CADx technologies may offer a more cost-effective approach where polyps that are characterized in real-time as low-risk adenomas or non-neoplastic are “resected and discarded” or “left in” respectively. In 2011, prior to the development of current AI tools, the American Society for Gastrointestinal Endoscopy set performance thresholds for technologies supporting real-time endoscopic assessment of the histology of diminutive colorectal polyps. The ASGE recommended 90% histopathologic concordance for “resect and discard” tools and 90% negative predictive value for adenomatous histology for “diagnose and leave,” tools.13 Narrow-band imaging (NBI), for example, has been shown to meet these benchmarks14,15 with a modeling study suggesting that implementing “resect and discard” strategies with such tools could result in annual savings of $33 million without adversely affecting efficacy, although practical adoption has been limited.16 More recent work has directly explored the feasibility of leveraging CADx to support “leave-in-situ” and “resect-and-discard” strategies.17
Similarly, while CADe use in colonoscopy is associated with additional up-front costs, a modeling study suggests that its associated gains in ADR (as detailed in Table 1) make it a cost-saving strategy for colorectal cancer prevention in the long term.18 There is still uncertainty on whether the incremental CADe-associated gains in adenoma detection will necessarily translate to significant reductions in interval colorectal cancer risk, particularly for endoscopists who are already high-performing polyp detectors. A recent study suggests that, although higher ADRs were associated with lower rates of interval colorectal cancer, the gains in interval colorectal cancer risk reduction appeared to level off with ADRs above 35%-40% (this finding may be limited by statistical power).19 Further, most of the data from CADe trials suggest that gains in adenoma detection are not driven by increased detection of advanced lesions with high malignancy risk but by small polyps with long latency periods of about 5-10 years, which may not significantly alter interval cancer risk. It remains to be determined whether adoption of CADe will have an impact on hard outcomes, most importantly interval colorectal cancer risk, or merely result in increased resource utilization without moving the needle on colorectal cancer prevention. To answer this question, the OperA study – a large-scale randomized clinical trial of 200,000 patients across 18 centers from 13 countries – was launched in 2022. It will investigate the effect of colonoscopy with CADe on a number of critical measures, including long-term interval colon cancer risk.20
Despite commercial availability of regulatory-approved CADe systems and data supporting use for adenoma detection in colonoscopy, mainstream adoption in clinical practice has been sluggish. Physician survey studies have shown that, although there is considerable interest in integrating CADe into clinical practice, there are concerns about access, cost and reimbursement, integration into clinical work-flow, increased procedural times, over-reliance on AI, and algorithmic bias leading to errors.21,22 In addition, without mandatory requirements for ADR reporting or clinical practice guideline recommendations for CADe use, these systems may not be perceived as valuable or ready for prime time even though the evidence suggests otherwise.23,24 For CADe systems to see widespread adoption in clinical practice, it is important that future research studies rigorously investigate and characterize these potential barriers to better inform strategies to address AI hesitancy and implementation challenges. Such efforts can provide an integration framework for future AI applications in gastroenterology beyond colonoscopy, such as CADe of esophageal and gastric premalignant lesions in upper endoscopy, CADx for pancreatic cysts and liver lesions on imaging, NLP tools to optimizing efficient clinical documentation and reporting, and many others.
Dr. Uche-Anya is in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston. Dr. Berzin is with the Center for Advanced Endoscopy, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston. Dr. Berzin is a consultant for Wision AI, Medtronic, Magentiq Eye, RSIP Vision, and Docbot.
Corresponding Author: Eugenia Uche-Anya eucheanya@mgh.harvard.edu Twitter: @UcheAnyaMD @tberzin
References
1. Corley DA et al. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc. Sep 2011;74(3):656-65. doi: 10.1016/j.gie.2011.04.017.
2. Zhao S et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: A systematic review and meta-analysis. Gastroenterology. 05 2019;156(6):1661-74.e11. doi: 10.1053/j.gastro.2019.01.260.
3. Kaminski MF et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. May 13 2010;362(19):1795-803. doi: 10.1056/NEJMoa0907667.
4. Corley DA et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. Apr 03 2014;370(14):1298-306. doi: 10.1056/NEJMoa1309086.
5. Laique SN et al. Application of optical character recognition with natural language processing for large-scale quality metric data extraction in colonoscopy reports. Gastrointest Endosc. 03 2021;93(3):750-7. doi: 10.1016/j.gie.2020.08.038.
6. Tinmouth J et al. Validation of a natural language processing algorithm to identify adenomas and measure adenoma detection rates across a health system: a population-level study. Gastrointest Endosc. Jul 14 2022. doi: 10.1016/j.gie.2022.07.009.
7. Glissen Brown JR et al. Deep learning computer-aided polyp detection reduces adenoma miss rate: A United States multi-center randomized tandem colonoscopy study (CADeT-CS Trial). Clin Gastroenterol Hepatol. 07 2022;20(7):1499-1507.e4. doi: 10.1016/j.cgh.2021.09.009.
8. Wallace MB et al. Impact of artificial intelligence on miss rate of colorectal neoplasia. Gastroenterology. 07 2022;163(1):295-304.e5. doi: 10.1053/j.gastro.2022.03.007.
9. Hassan C et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 01 2021;93(1):77-85.e6. doi: 10.1016/j.gie.2020.06.059.
10. Glissen Brown JR and Berzin TM. Adoption of new technologies: Artificial intelligence. Gastrointest Endosc Clin N Am. Oct 2021;31(4):743-58. doi: 10.1016/j.giec.2021.05.010.
11. Larsen SLV and Mori Y. Artificial intelligence in colonoscopy: A review on the current status. DEN open. Apr 2022;2(1):e109. doi: 10.1002/deo2.109.
12. Gupta N et al. Prevalence of advanced histological features in diminutive and small colon polyps. Gastrointest Endosc. May 2012;75(5):1022-30. doi: 10.1016/j.gie.2012.01.020.
13. Rex DK et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. Mar 2011;73(3):419-22. doi: 10.1016/j.gie.2011.01.023.
14. Abu Dayyeh BK et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. Mar 2015;81(3):502.e1-16. doi: 10.1016/j.gie.2014.12.022.
15. Mori Y et al. Real-time use of artificial intelligence in identification of diminutive polyps during colonoscopy: A prospective study. Ann Intern Med. Sep 18 2018;169(6):357-66. doi: 10.7326/M18-0249.
16. Hassan C et al.. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol. Oct 2010;8(10):865-9, 869.e1-3. doi: 10.1016/j.cgh.2010.05.018.
17. Hassan C et al. Artificial intelligence allows leaving-in-situ colorectal polyps. Clin Gastroenterol Hepatol. Nov 2022;20(11):2505-13.e4. doi: 10.1016/j.cgh.2022.04.045.
18. Areia M et al. Cost-effectiveness of artificial intelligence for screening colonoscopy: a modelling study. Lancet Digit Health. 06 2022;4(6):e436-44. doi: 10.1016/S2589-7500(22)00042-5.
19. Schottinger JE et al. Association of physician adenoma detection rates with postcolonoscopy colorectal cancer. JAMA. 2022 Jun 7;327(21):2114-22. doi: 10.1001/jama.2022.6644.
20. Oslo Uo. Optimising colorectal cancer prevention through personalised treatment with artificial intelligence. 2022.
21. Wadhwa V et al. Physician sentiment toward artificial intelligence (AI) in colonoscopic practice: a survey of US gastroenterologists. Endosc Int Open. Oct 2020;8(10):E1379-84. doi: 10.1055/a-1223-1926.
22. Kader R et al. Survey on the perceptions of UK gastroenterologists and endoscopists to artificial intelligence. Frontline Gastroenterol. 2022;13(5):423-9. doi: 10.1136/flgastro-2021-101994.
23. Rex DKet al. Artificial intelligence improves detection at colonoscopy: Why aren’t we all already using it? Gastroenterology. 07 2022;163(1):35-7. doi: 10.1053/j.gastro.2022.04.042.
24. Ahmad OF et al. Establishing key research questions for the implementation of artificial intelligence in colonoscopy: A modified Delphi method. Endoscopy. 09 2021;53(9):893-901. doi: 10.1055/a-1306-7590
Considerable advances in artificial intelligence (AI) and machine-learning (ML) methodologies have led to the emergence of promising tools in the field of gastrointestinal endoscopy. Computer vision is an application of AI/ML that has been successfully applied for the computer-aided detection (CADe) and computer-aided diagnosis (CADx) of colon polyps and numerous other conditions encountered during GI endoscopy. Outside of computer vision, a wide variety of other AI applications have been applied to gastroenterology, ranging from natural language processing (NLP) to optimize clinical documentation and endoscopy quality reporting to ML techniques that predict disease severity/treatment response and augment clinical decision-making.
In the United States, colonoscopy is the standard for colon cancer screening and prevention; however, precancerous polyps can be missed for various reasons, ranging from subtle surface appearance of the polyp or location behind a colonic fold to operator-dependent reasons such as inadequate mucosal inspection. Though clinical practice guidelines have set adenoma detection rate (ADR) thresholds at 20% for women and 30% for men, studies have shown a 4- to 10-fold variation in ADR among physicians in clinical practice settings,1 with an estimated adenoma miss rate (AMR) of 25% and a false-negative colonoscopy rate of 12%.2 Variability in adenoma detection affects the risk of interval colorectal cancer post colonoscopy.3,4
AI provides an opportunity for mitigating this risk. Advances in deep learning and computer vision have led to the development of CADe systems that automatically detect polyps in real time during colonoscopy, resulting in reduced adenoma miss rates (Table 1). In addition to polyp detection, deep-learning technologies are also being used in CADx systems for polyp diagnosis and characterization of malignancy risk. This could aid therapeutic decision-making: Unnecessary resection or histopathologic analysis could be obviated for benign hyperplastic polyps. On the other end of the polyp spectrum, an AI tool that could predict the presence or absence of submucosal invasion could be a powerful tool when evaluating early colon cancers for consideration of endoscopic submucosal dissection vs. surgery. Examples of CADe polyp detection and CADx polyp characterization are shown in Figure 1.
Other potential computer vision applications that may improve colonoscopy quality include tools that help measure adequacy of mucosal exposure, segmental inspection time, and a variety of other parameters associated with polyp detection performance. These are promising areas for future research. Beyond improving colonoscopy technique, natural language processing tools already are being used to optimize clinical documentation as well as extract information from colonoscopy and pathology reports that can facilitate reporting of colonoscopy quality metrics such as ADR, cecal intubation rate, withdrawal time, and bowel preparation adequacy. AI-powered analytics may help unlock large-scale reporting of colonoscopy quality metrics on a health-systems level5 or population-level,6 helping to ensure optimal performance and identifying avenues for colonoscopy quality improvement.
The majority of AI research in colonoscopy has focused on CADe for colon polyp detection and CADx for polyp diagnosis. Over the last few years, several randomized clinical trials – two in the United States – have shown that CADe significantly improves adenoma detection and reduces adenoma miss rates in comparison to standard colonoscopy. The existing data are summarized in Table 1, focusing on the two U.S. studies and an international meta-analysis.
In comparison, the data landscape for CADx is nascent and currently limited to several retrospective studies dating back to 2009 and a few prospective studies that have shown promising results.10,11 There is an expectation that integrated CADx also may support the adoption of “resect and discard” or “diagnose and leave” strategies for low-risk polyps. About two-thirds of polyps identified on average-risk screening colonoscopies are diminutive polyps (less than 5 mm in size), which rarely have advanced histologic features (about 0.5%) and are sometimes non-neoplastic (30%). Malignancy risk is even lower in the distal colon.12 As routine histopathologic assessment of such polyps is mostly of limited clinical utility and comes with added pathology costs, CADx technologies may offer a more cost-effective approach where polyps that are characterized in real-time as low-risk adenomas or non-neoplastic are “resected and discarded” or “left in” respectively. In 2011, prior to the development of current AI tools, the American Society for Gastrointestinal Endoscopy set performance thresholds for technologies supporting real-time endoscopic assessment of the histology of diminutive colorectal polyps. The ASGE recommended 90% histopathologic concordance for “resect and discard” tools and 90% negative predictive value for adenomatous histology for “diagnose and leave,” tools.13 Narrow-band imaging (NBI), for example, has been shown to meet these benchmarks14,15 with a modeling study suggesting that implementing “resect and discard” strategies with such tools could result in annual savings of $33 million without adversely affecting efficacy, although practical adoption has been limited.16 More recent work has directly explored the feasibility of leveraging CADx to support “leave-in-situ” and “resect-and-discard” strategies.17
Similarly, while CADe use in colonoscopy is associated with additional up-front costs, a modeling study suggests that its associated gains in ADR (as detailed in Table 1) make it a cost-saving strategy for colorectal cancer prevention in the long term.18 There is still uncertainty on whether the incremental CADe-associated gains in adenoma detection will necessarily translate to significant reductions in interval colorectal cancer risk, particularly for endoscopists who are already high-performing polyp detectors. A recent study suggests that, although higher ADRs were associated with lower rates of interval colorectal cancer, the gains in interval colorectal cancer risk reduction appeared to level off with ADRs above 35%-40% (this finding may be limited by statistical power).19 Further, most of the data from CADe trials suggest that gains in adenoma detection are not driven by increased detection of advanced lesions with high malignancy risk but by small polyps with long latency periods of about 5-10 years, which may not significantly alter interval cancer risk. It remains to be determined whether adoption of CADe will have an impact on hard outcomes, most importantly interval colorectal cancer risk, or merely result in increased resource utilization without moving the needle on colorectal cancer prevention. To answer this question, the OperA study – a large-scale randomized clinical trial of 200,000 patients across 18 centers from 13 countries – was launched in 2022. It will investigate the effect of colonoscopy with CADe on a number of critical measures, including long-term interval colon cancer risk.20
Despite commercial availability of regulatory-approved CADe systems and data supporting use for adenoma detection in colonoscopy, mainstream adoption in clinical practice has been sluggish. Physician survey studies have shown that, although there is considerable interest in integrating CADe into clinical practice, there are concerns about access, cost and reimbursement, integration into clinical work-flow, increased procedural times, over-reliance on AI, and algorithmic bias leading to errors.21,22 In addition, without mandatory requirements for ADR reporting or clinical practice guideline recommendations for CADe use, these systems may not be perceived as valuable or ready for prime time even though the evidence suggests otherwise.23,24 For CADe systems to see widespread adoption in clinical practice, it is important that future research studies rigorously investigate and characterize these potential barriers to better inform strategies to address AI hesitancy and implementation challenges. Such efforts can provide an integration framework for future AI applications in gastroenterology beyond colonoscopy, such as CADe of esophageal and gastric premalignant lesions in upper endoscopy, CADx for pancreatic cysts and liver lesions on imaging, NLP tools to optimizing efficient clinical documentation and reporting, and many others.
Dr. Uche-Anya is in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston. Dr. Berzin is with the Center for Advanced Endoscopy, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston. Dr. Berzin is a consultant for Wision AI, Medtronic, Magentiq Eye, RSIP Vision, and Docbot.
Corresponding Author: Eugenia Uche-Anya eucheanya@mgh.harvard.edu Twitter: @UcheAnyaMD @tberzin
References
1. Corley DA et al. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc. Sep 2011;74(3):656-65. doi: 10.1016/j.gie.2011.04.017.
2. Zhao S et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: A systematic review and meta-analysis. Gastroenterology. 05 2019;156(6):1661-74.e11. doi: 10.1053/j.gastro.2019.01.260.
3. Kaminski MF et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. May 13 2010;362(19):1795-803. doi: 10.1056/NEJMoa0907667.
4. Corley DA et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. Apr 03 2014;370(14):1298-306. doi: 10.1056/NEJMoa1309086.
5. Laique SN et al. Application of optical character recognition with natural language processing for large-scale quality metric data extraction in colonoscopy reports. Gastrointest Endosc. 03 2021;93(3):750-7. doi: 10.1016/j.gie.2020.08.038.
6. Tinmouth J et al. Validation of a natural language processing algorithm to identify adenomas and measure adenoma detection rates across a health system: a population-level study. Gastrointest Endosc. Jul 14 2022. doi: 10.1016/j.gie.2022.07.009.
7. Glissen Brown JR et al. Deep learning computer-aided polyp detection reduces adenoma miss rate: A United States multi-center randomized tandem colonoscopy study (CADeT-CS Trial). Clin Gastroenterol Hepatol. 07 2022;20(7):1499-1507.e4. doi: 10.1016/j.cgh.2021.09.009.
8. Wallace MB et al. Impact of artificial intelligence on miss rate of colorectal neoplasia. Gastroenterology. 07 2022;163(1):295-304.e5. doi: 10.1053/j.gastro.2022.03.007.
9. Hassan C et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 01 2021;93(1):77-85.e6. doi: 10.1016/j.gie.2020.06.059.
10. Glissen Brown JR and Berzin TM. Adoption of new technologies: Artificial intelligence. Gastrointest Endosc Clin N Am. Oct 2021;31(4):743-58. doi: 10.1016/j.giec.2021.05.010.
11. Larsen SLV and Mori Y. Artificial intelligence in colonoscopy: A review on the current status. DEN open. Apr 2022;2(1):e109. doi: 10.1002/deo2.109.
12. Gupta N et al. Prevalence of advanced histological features in diminutive and small colon polyps. Gastrointest Endosc. May 2012;75(5):1022-30. doi: 10.1016/j.gie.2012.01.020.
13. Rex DK et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. Mar 2011;73(3):419-22. doi: 10.1016/j.gie.2011.01.023.
14. Abu Dayyeh BK et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. Mar 2015;81(3):502.e1-16. doi: 10.1016/j.gie.2014.12.022.
15. Mori Y et al. Real-time use of artificial intelligence in identification of diminutive polyps during colonoscopy: A prospective study. Ann Intern Med. Sep 18 2018;169(6):357-66. doi: 10.7326/M18-0249.
16. Hassan C et al.. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol. Oct 2010;8(10):865-9, 869.e1-3. doi: 10.1016/j.cgh.2010.05.018.
17. Hassan C et al. Artificial intelligence allows leaving-in-situ colorectal polyps. Clin Gastroenterol Hepatol. Nov 2022;20(11):2505-13.e4. doi: 10.1016/j.cgh.2022.04.045.
18. Areia M et al. Cost-effectiveness of artificial intelligence for screening colonoscopy: a modelling study. Lancet Digit Health. 06 2022;4(6):e436-44. doi: 10.1016/S2589-7500(22)00042-5.
19. Schottinger JE et al. Association of physician adenoma detection rates with postcolonoscopy colorectal cancer. JAMA. 2022 Jun 7;327(21):2114-22. doi: 10.1001/jama.2022.6644.
20. Oslo Uo. Optimising colorectal cancer prevention through personalised treatment with artificial intelligence. 2022.
21. Wadhwa V et al. Physician sentiment toward artificial intelligence (AI) in colonoscopic practice: a survey of US gastroenterologists. Endosc Int Open. Oct 2020;8(10):E1379-84. doi: 10.1055/a-1223-1926.
22. Kader R et al. Survey on the perceptions of UK gastroenterologists and endoscopists to artificial intelligence. Frontline Gastroenterol. 2022;13(5):423-9. doi: 10.1136/flgastro-2021-101994.
23. Rex DKet al. Artificial intelligence improves detection at colonoscopy: Why aren’t we all already using it? Gastroenterology. 07 2022;163(1):35-7. doi: 10.1053/j.gastro.2022.04.042.
24. Ahmad OF et al. Establishing key research questions for the implementation of artificial intelligence in colonoscopy: A modified Delphi method. Endoscopy. 09 2021;53(9):893-901. doi: 10.1055/a-1306-7590
Considerable advances in artificial intelligence (AI) and machine-learning (ML) methodologies have led to the emergence of promising tools in the field of gastrointestinal endoscopy. Computer vision is an application of AI/ML that has been successfully applied for the computer-aided detection (CADe) and computer-aided diagnosis (CADx) of colon polyps and numerous other conditions encountered during GI endoscopy. Outside of computer vision, a wide variety of other AI applications have been applied to gastroenterology, ranging from natural language processing (NLP) to optimize clinical documentation and endoscopy quality reporting to ML techniques that predict disease severity/treatment response and augment clinical decision-making.
In the United States, colonoscopy is the standard for colon cancer screening and prevention; however, precancerous polyps can be missed for various reasons, ranging from subtle surface appearance of the polyp or location behind a colonic fold to operator-dependent reasons such as inadequate mucosal inspection. Though clinical practice guidelines have set adenoma detection rate (ADR) thresholds at 20% for women and 30% for men, studies have shown a 4- to 10-fold variation in ADR among physicians in clinical practice settings,1 with an estimated adenoma miss rate (AMR) of 25% and a false-negative colonoscopy rate of 12%.2 Variability in adenoma detection affects the risk of interval colorectal cancer post colonoscopy.3,4
AI provides an opportunity for mitigating this risk. Advances in deep learning and computer vision have led to the development of CADe systems that automatically detect polyps in real time during colonoscopy, resulting in reduced adenoma miss rates (Table 1). In addition to polyp detection, deep-learning technologies are also being used in CADx systems for polyp diagnosis and characterization of malignancy risk. This could aid therapeutic decision-making: Unnecessary resection or histopathologic analysis could be obviated for benign hyperplastic polyps. On the other end of the polyp spectrum, an AI tool that could predict the presence or absence of submucosal invasion could be a powerful tool when evaluating early colon cancers for consideration of endoscopic submucosal dissection vs. surgery. Examples of CADe polyp detection and CADx polyp characterization are shown in Figure 1.
Other potential computer vision applications that may improve colonoscopy quality include tools that help measure adequacy of mucosal exposure, segmental inspection time, and a variety of other parameters associated with polyp detection performance. These are promising areas for future research. Beyond improving colonoscopy technique, natural language processing tools already are being used to optimize clinical documentation as well as extract information from colonoscopy and pathology reports that can facilitate reporting of colonoscopy quality metrics such as ADR, cecal intubation rate, withdrawal time, and bowel preparation adequacy. AI-powered analytics may help unlock large-scale reporting of colonoscopy quality metrics on a health-systems level5 or population-level,6 helping to ensure optimal performance and identifying avenues for colonoscopy quality improvement.
The majority of AI research in colonoscopy has focused on CADe for colon polyp detection and CADx for polyp diagnosis. Over the last few years, several randomized clinical trials – two in the United States – have shown that CADe significantly improves adenoma detection and reduces adenoma miss rates in comparison to standard colonoscopy. The existing data are summarized in Table 1, focusing on the two U.S. studies and an international meta-analysis.
In comparison, the data landscape for CADx is nascent and currently limited to several retrospective studies dating back to 2009 and a few prospective studies that have shown promising results.10,11 There is an expectation that integrated CADx also may support the adoption of “resect and discard” or “diagnose and leave” strategies for low-risk polyps. About two-thirds of polyps identified on average-risk screening colonoscopies are diminutive polyps (less than 5 mm in size), which rarely have advanced histologic features (about 0.5%) and are sometimes non-neoplastic (30%). Malignancy risk is even lower in the distal colon.12 As routine histopathologic assessment of such polyps is mostly of limited clinical utility and comes with added pathology costs, CADx technologies may offer a more cost-effective approach where polyps that are characterized in real-time as low-risk adenomas or non-neoplastic are “resected and discarded” or “left in” respectively. In 2011, prior to the development of current AI tools, the American Society for Gastrointestinal Endoscopy set performance thresholds for technologies supporting real-time endoscopic assessment of the histology of diminutive colorectal polyps. The ASGE recommended 90% histopathologic concordance for “resect and discard” tools and 90% negative predictive value for adenomatous histology for “diagnose and leave,” tools.13 Narrow-band imaging (NBI), for example, has been shown to meet these benchmarks14,15 with a modeling study suggesting that implementing “resect and discard” strategies with such tools could result in annual savings of $33 million without adversely affecting efficacy, although practical adoption has been limited.16 More recent work has directly explored the feasibility of leveraging CADx to support “leave-in-situ” and “resect-and-discard” strategies.17
Similarly, while CADe use in colonoscopy is associated with additional up-front costs, a modeling study suggests that its associated gains in ADR (as detailed in Table 1) make it a cost-saving strategy for colorectal cancer prevention in the long term.18 There is still uncertainty on whether the incremental CADe-associated gains in adenoma detection will necessarily translate to significant reductions in interval colorectal cancer risk, particularly for endoscopists who are already high-performing polyp detectors. A recent study suggests that, although higher ADRs were associated with lower rates of interval colorectal cancer, the gains in interval colorectal cancer risk reduction appeared to level off with ADRs above 35%-40% (this finding may be limited by statistical power).19 Further, most of the data from CADe trials suggest that gains in adenoma detection are not driven by increased detection of advanced lesions with high malignancy risk but by small polyps with long latency periods of about 5-10 years, which may not significantly alter interval cancer risk. It remains to be determined whether adoption of CADe will have an impact on hard outcomes, most importantly interval colorectal cancer risk, or merely result in increased resource utilization without moving the needle on colorectal cancer prevention. To answer this question, the OperA study – a large-scale randomized clinical trial of 200,000 patients across 18 centers from 13 countries – was launched in 2022. It will investigate the effect of colonoscopy with CADe on a number of critical measures, including long-term interval colon cancer risk.20
Despite commercial availability of regulatory-approved CADe systems and data supporting use for adenoma detection in colonoscopy, mainstream adoption in clinical practice has been sluggish. Physician survey studies have shown that, although there is considerable interest in integrating CADe into clinical practice, there are concerns about access, cost and reimbursement, integration into clinical work-flow, increased procedural times, over-reliance on AI, and algorithmic bias leading to errors.21,22 In addition, without mandatory requirements for ADR reporting or clinical practice guideline recommendations for CADe use, these systems may not be perceived as valuable or ready for prime time even though the evidence suggests otherwise.23,24 For CADe systems to see widespread adoption in clinical practice, it is important that future research studies rigorously investigate and characterize these potential barriers to better inform strategies to address AI hesitancy and implementation challenges. Such efforts can provide an integration framework for future AI applications in gastroenterology beyond colonoscopy, such as CADe of esophageal and gastric premalignant lesions in upper endoscopy, CADx for pancreatic cysts and liver lesions on imaging, NLP tools to optimizing efficient clinical documentation and reporting, and many others.
Dr. Uche-Anya is in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston. Dr. Berzin is with the Center for Advanced Endoscopy, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston. Dr. Berzin is a consultant for Wision AI, Medtronic, Magentiq Eye, RSIP Vision, and Docbot.
Corresponding Author: Eugenia Uche-Anya eucheanya@mgh.harvard.edu Twitter: @UcheAnyaMD @tberzin
References
1. Corley DA et al. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc. Sep 2011;74(3):656-65. doi: 10.1016/j.gie.2011.04.017.
2. Zhao S et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: A systematic review and meta-analysis. Gastroenterology. 05 2019;156(6):1661-74.e11. doi: 10.1053/j.gastro.2019.01.260.
3. Kaminski MF et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. May 13 2010;362(19):1795-803. doi: 10.1056/NEJMoa0907667.
4. Corley DA et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. Apr 03 2014;370(14):1298-306. doi: 10.1056/NEJMoa1309086.
5. Laique SN et al. Application of optical character recognition with natural language processing for large-scale quality metric data extraction in colonoscopy reports. Gastrointest Endosc. 03 2021;93(3):750-7. doi: 10.1016/j.gie.2020.08.038.
6. Tinmouth J et al. Validation of a natural language processing algorithm to identify adenomas and measure adenoma detection rates across a health system: a population-level study. Gastrointest Endosc. Jul 14 2022. doi: 10.1016/j.gie.2022.07.009.
7. Glissen Brown JR et al. Deep learning computer-aided polyp detection reduces adenoma miss rate: A United States multi-center randomized tandem colonoscopy study (CADeT-CS Trial). Clin Gastroenterol Hepatol. 07 2022;20(7):1499-1507.e4. doi: 10.1016/j.cgh.2021.09.009.
8. Wallace MB et al. Impact of artificial intelligence on miss rate of colorectal neoplasia. Gastroenterology. 07 2022;163(1):295-304.e5. doi: 10.1053/j.gastro.2022.03.007.
9. Hassan C et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 01 2021;93(1):77-85.e6. doi: 10.1016/j.gie.2020.06.059.
10. Glissen Brown JR and Berzin TM. Adoption of new technologies: Artificial intelligence. Gastrointest Endosc Clin N Am. Oct 2021;31(4):743-58. doi: 10.1016/j.giec.2021.05.010.
11. Larsen SLV and Mori Y. Artificial intelligence in colonoscopy: A review on the current status. DEN open. Apr 2022;2(1):e109. doi: 10.1002/deo2.109.
12. Gupta N et al. Prevalence of advanced histological features in diminutive and small colon polyps. Gastrointest Endosc. May 2012;75(5):1022-30. doi: 10.1016/j.gie.2012.01.020.
13. Rex DK et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. Mar 2011;73(3):419-22. doi: 10.1016/j.gie.2011.01.023.
14. Abu Dayyeh BK et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. Mar 2015;81(3):502.e1-16. doi: 10.1016/j.gie.2014.12.022.
15. Mori Y et al. Real-time use of artificial intelligence in identification of diminutive polyps during colonoscopy: A prospective study. Ann Intern Med. Sep 18 2018;169(6):357-66. doi: 10.7326/M18-0249.
16. Hassan C et al.. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol. Oct 2010;8(10):865-9, 869.e1-3. doi: 10.1016/j.cgh.2010.05.018.
17. Hassan C et al. Artificial intelligence allows leaving-in-situ colorectal polyps. Clin Gastroenterol Hepatol. Nov 2022;20(11):2505-13.e4. doi: 10.1016/j.cgh.2022.04.045.
18. Areia M et al. Cost-effectiveness of artificial intelligence for screening colonoscopy: a modelling study. Lancet Digit Health. 06 2022;4(6):e436-44. doi: 10.1016/S2589-7500(22)00042-5.
19. Schottinger JE et al. Association of physician adenoma detection rates with postcolonoscopy colorectal cancer. JAMA. 2022 Jun 7;327(21):2114-22. doi: 10.1001/jama.2022.6644.
20. Oslo Uo. Optimising colorectal cancer prevention through personalised treatment with artificial intelligence. 2022.
21. Wadhwa V et al. Physician sentiment toward artificial intelligence (AI) in colonoscopic practice: a survey of US gastroenterologists. Endosc Int Open. Oct 2020;8(10):E1379-84. doi: 10.1055/a-1223-1926.
22. Kader R et al. Survey on the perceptions of UK gastroenterologists and endoscopists to artificial intelligence. Frontline Gastroenterol. 2022;13(5):423-9. doi: 10.1136/flgastro-2021-101994.
23. Rex DKet al. Artificial intelligence improves detection at colonoscopy: Why aren’t we all already using it? Gastroenterology. 07 2022;163(1):35-7. doi: 10.1053/j.gastro.2022.04.042.
24. Ahmad OF et al. Establishing key research questions for the implementation of artificial intelligence in colonoscopy: A modified Delphi method. Endoscopy. 09 2021;53(9):893-901. doi: 10.1055/a-1306-7590


