User login
For MD-IQ use only
Latest COVID-19 Shot May Cut Severe Outcomes in Veterans
TOPLINE:
Among US veterans, same-day receipt of both the 2024-2025 COVID19 vaccine and the influenza vaccine was associated with lower risks for emergency department visits, hospitalizations, and deaths compared with receipt of the influenza vaccine alone.
METHODOLOGY:
- Researchers conducted an observational study to assess the effectiveness of the 2024-2025 COVID-19 vaccine by comparing veterans who received both the COVID-19 and influenza vaccines on the same day with those who received only the influenza vaccine between September 3 and December 31, 2024.
- Data on participants (mean age, approximately 71.5 years; approximately 92% men) were sourced from electronic health records of the Department of Veterans Affairs and included 164,132 veterans who received both vaccines vs 131,839 who received only the seasonal influenza vaccine, with a follow-up duration of 180 days.
- The vaccines used were mainly the 2024-2025 mRNA COVID19 vaccines: Moderna mRNA1273, Pfizer BNT162b2, and the highdose trivalent 2024-2025 seasonal influenza vaccine.
- Primary outcomes were COVID-19-associated emergency department visits, hospitalizations, and deaths.
TAKEAWAY:
- Receipt of both the COVID-19 and influenza vaccines was associated with a lower risk for COVID-19-associated emergency department visits compared with receipt of the influenza vaccine alone, resulting in a vaccine effectiveness of 29.3% and a risk difference of 18.3 per 10,000 persons (95% CI, 10.8-27.6).
- Similarly, COVID-19 vaccine effectiveness was 39.2% (95% CI, 21.6-54.5) against COVID-19-associated hospitalizations, with a risk difference of 7.5 per 10,000 persons (95% CI, 3.4-13.0).
- For COVID-19-associated deaths, vaccine effectiveness was 64% (95% CI, 23.0-85.8), with a risk difference of 2.2 per 10,000 persons (95% CI, 0.5-6.9).
- Benefits were consistent across age groups (< 65, 65-75, and > 75 years) and among people with various comorbidities, including cardiovascular disease and immunocompromised status.
IN PRACTICE:
“The evidence may help inform ongoing discussions about the value of COVID-19 vaccines in the current epidemiologic landscape,” the authors wrote.
SOURCE:
The study was led by Miao Cai, PhD , Research and Development Service, Veterans Affairs St. Louis Health Care System, and the Veterans Research and Education Foundation of St. Louis, Missouri. It was published online in The New England Journal of Medicine .
LIMITATIONS:
The demographic composition of the cohort — predominantly older, White, male veterans — may limit the generalizability of the study. Although numerous covariates were adjusted for, residual confounding could not be fully ruled out. Safety and variantspecific effectiveness were not assessed.
DISCLOSURES:
The study was supported by a grant from the Department of Veterans Affairs. Two authors disclosed consulting for Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Among US veterans, same-day receipt of both the 2024-2025 COVID19 vaccine and the influenza vaccine was associated with lower risks for emergency department visits, hospitalizations, and deaths compared with receipt of the influenza vaccine alone.
METHODOLOGY:
- Researchers conducted an observational study to assess the effectiveness of the 2024-2025 COVID-19 vaccine by comparing veterans who received both the COVID-19 and influenza vaccines on the same day with those who received only the influenza vaccine between September 3 and December 31, 2024.
- Data on participants (mean age, approximately 71.5 years; approximately 92% men) were sourced from electronic health records of the Department of Veterans Affairs and included 164,132 veterans who received both vaccines vs 131,839 who received only the seasonal influenza vaccine, with a follow-up duration of 180 days.
- The vaccines used were mainly the 2024-2025 mRNA COVID19 vaccines: Moderna mRNA1273, Pfizer BNT162b2, and the highdose trivalent 2024-2025 seasonal influenza vaccine.
- Primary outcomes were COVID-19-associated emergency department visits, hospitalizations, and deaths.
TAKEAWAY:
- Receipt of both the COVID-19 and influenza vaccines was associated with a lower risk for COVID-19-associated emergency department visits compared with receipt of the influenza vaccine alone, resulting in a vaccine effectiveness of 29.3% and a risk difference of 18.3 per 10,000 persons (95% CI, 10.8-27.6).
- Similarly, COVID-19 vaccine effectiveness was 39.2% (95% CI, 21.6-54.5) against COVID-19-associated hospitalizations, with a risk difference of 7.5 per 10,000 persons (95% CI, 3.4-13.0).
- For COVID-19-associated deaths, vaccine effectiveness was 64% (95% CI, 23.0-85.8), with a risk difference of 2.2 per 10,000 persons (95% CI, 0.5-6.9).
- Benefits were consistent across age groups (< 65, 65-75, and > 75 years) and among people with various comorbidities, including cardiovascular disease and immunocompromised status.
IN PRACTICE:
“The evidence may help inform ongoing discussions about the value of COVID-19 vaccines in the current epidemiologic landscape,” the authors wrote.
SOURCE:
The study was led by Miao Cai, PhD , Research and Development Service, Veterans Affairs St. Louis Health Care System, and the Veterans Research and Education Foundation of St. Louis, Missouri. It was published online in The New England Journal of Medicine .
LIMITATIONS:
The demographic composition of the cohort — predominantly older, White, male veterans — may limit the generalizability of the study. Although numerous covariates were adjusted for, residual confounding could not be fully ruled out. Safety and variantspecific effectiveness were not assessed.
DISCLOSURES:
The study was supported by a grant from the Department of Veterans Affairs. Two authors disclosed consulting for Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Among US veterans, same-day receipt of both the 2024-2025 COVID19 vaccine and the influenza vaccine was associated with lower risks for emergency department visits, hospitalizations, and deaths compared with receipt of the influenza vaccine alone.
METHODOLOGY:
- Researchers conducted an observational study to assess the effectiveness of the 2024-2025 COVID-19 vaccine by comparing veterans who received both the COVID-19 and influenza vaccines on the same day with those who received only the influenza vaccine between September 3 and December 31, 2024.
- Data on participants (mean age, approximately 71.5 years; approximately 92% men) were sourced from electronic health records of the Department of Veterans Affairs and included 164,132 veterans who received both vaccines vs 131,839 who received only the seasonal influenza vaccine, with a follow-up duration of 180 days.
- The vaccines used were mainly the 2024-2025 mRNA COVID19 vaccines: Moderna mRNA1273, Pfizer BNT162b2, and the highdose trivalent 2024-2025 seasonal influenza vaccine.
- Primary outcomes were COVID-19-associated emergency department visits, hospitalizations, and deaths.
TAKEAWAY:
- Receipt of both the COVID-19 and influenza vaccines was associated with a lower risk for COVID-19-associated emergency department visits compared with receipt of the influenza vaccine alone, resulting in a vaccine effectiveness of 29.3% and a risk difference of 18.3 per 10,000 persons (95% CI, 10.8-27.6).
- Similarly, COVID-19 vaccine effectiveness was 39.2% (95% CI, 21.6-54.5) against COVID-19-associated hospitalizations, with a risk difference of 7.5 per 10,000 persons (95% CI, 3.4-13.0).
- For COVID-19-associated deaths, vaccine effectiveness was 64% (95% CI, 23.0-85.8), with a risk difference of 2.2 per 10,000 persons (95% CI, 0.5-6.9).
- Benefits were consistent across age groups (< 65, 65-75, and > 75 years) and among people with various comorbidities, including cardiovascular disease and immunocompromised status.
IN PRACTICE:
“The evidence may help inform ongoing discussions about the value of COVID-19 vaccines in the current epidemiologic landscape,” the authors wrote.
SOURCE:
The study was led by Miao Cai, PhD , Research and Development Service, Veterans Affairs St. Louis Health Care System, and the Veterans Research and Education Foundation of St. Louis, Missouri. It was published online in The New England Journal of Medicine .
LIMITATIONS:
The demographic composition of the cohort — predominantly older, White, male veterans — may limit the generalizability of the study. Although numerous covariates were adjusted for, residual confounding could not be fully ruled out. Safety and variantspecific effectiveness were not assessed.
DISCLOSURES:
The study was supported by a grant from the Department of Veterans Affairs. Two authors disclosed consulting for Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Targeted Osteoporosis Program May Benefit At-Risk Older Men
Efforts to identify older men at risk for osteoporosis and treat those who are eligible received a boost from results reported from a Veterans Affairs (VA) study that showed a significant increase in screening, treatment, and medication adherence.
The cluster randomized trial used a centralized nurse-led intervention to assess men for traditional osteoporosis risk factors, offer bone density testing, and recommend treatment for eligible men. Over 2 years, the intervention group had a higher average femoral neck bone density than patients who underwent usual care.
“We designed this study to see if a risk factor-based approach, which is what most of the guidelines use, made sense and was feasible — that men would be accepting of screening and [the approach] would yield a similar proportion of people who need osteoporosis treatment as screening in women, which is widely recommended and implemented. And sure enough, we found that about 85% of the men in the VA primary care practices in our target age range of between 65 and 85 actually met criteria for screening, and over half of them had low bone mass. They were very accepting of screening, very accepting of treatment, and had excellent compliance rates. So, our study, we believe, supports the idea of identifying men with at least one risk factor for fracture and offering them osteoporosis screening starting at age 65, similar to what we do for women,” Cathleen S. Colón-Emeric, MD, MHS, said in an interview. She is the lead author of the study, a physician in the Durham VA Health Care System, and professor of medicine at Duke University School of Medicine, Durham, North Carolina.
“We were able to see a positive effect on bone density in the bone health group, compared with the usual care group, which suggests that if we followed these folks longer and had enough of them, we would be able to show a fracture reduction benefit,” Colón-Emeric said.
There have been few randomized trials of screening interventions in men, leading to inconsistencies in guidelines, according to the authors of the new study, published online in JAMA Internal Medicine . Both the US Preventive Services Task Force and the Veterans Health Administration National Center for Health Promotion and Disease Prevention consider there to be insufficient evidence to recommend for or against screening in men who have not experienced a fracture. Some professional societies recommend such screening, but there are inconsistencies in the recommended criteria, such as age range or risk factors.
Beyond the age of 50 years, one in five men will experience an osteoporosis-related fracture at some point in their life, according to a 2009 study. Treatment is inexpensive and effective in both men and women, and economic models suggest that screening using dual-energy x-ray absorptiometry (DXA) would be cost-effective. Still, screening is rare among men, with fewer than 10% of men getting screened before having an osteoporosis-related fracture.
“It’s important to screen men at risk for osteoporosis due to the dramatically increased mortality men suffer after a fragility fracture compared with women. Within 1 year of a hip fracture, mortality is as high as 36%. Studies have also shown that osteoporosis in men is undertreated, with only 10%-50% being prescribed antifracture treatment within 1 year of a hip fracture. Most individuals do not regain their prior level of function after a hip fracture,” said Joe C. Huang, MD, who was asked for comment. He is a clinical assistant professor of gerontology and geriatric medicine at Harborview Medical Center Senior Care Clinic and Healthy Bones Clinic in Seattle.
Details of the Intervention
The bone health service (BHS) intervention employed an electronic health record case-finding tool and a nurse care manager who undertook screening and treatment monitoring. They identified potential risk factors that included hyperthyroidism, hyperparathyroidism, rheumatoid arthritis, alcohol dependence, chronic lung disease, chronic liver disease, stroke, parkinsonism, prostate cancer, smoking, diabetes, pernicious anemia, gastrectomy, or high-risk medication use in at least 3 months of the prior 2 years. These medications included traditional antiepileptics, glucocorticoids, and androgen deprivation therapy.
The BHS nurse invited eligible men to be screened using an initial letter, followed by up to three phone calls. After DXA screening, the nurse scheduled an electronic consult with an osteoporosis expert, and patients with a T-score between -1 and -2.4 and an elevated 10-year fracture risk as measured by the Fracture Risk Assessment Tool were recommended for osteoporosis medication, vitamin D, and dietary or supplemental calcium. Following the prescription, the nurse provided patient education over the phone and mailed out written instructions. The nurse also made phone calls at 1 month, 6 months, and 12 months to encourage adherence and address common treatment barriers such as forgetting to take medication or dealing with gastrointestinal effects. The researchers recruited 38 primary care physicians from two VA health systems. The study included 3112 male veterans between the ages of 65 and 85 years (40.4% Black and 56% White). Nearly all participants (85.5%) had at least one indication for screening according to VA undersecretary guidelines, and almost a third (32.1%) had been prescribed androgen deprivation therapy, traditional antiepileptic drugs, or glucocorticoids.
Over a mean follow-up of 1.5 years, there was a much higher screening rate in the BHS group (49.2% vs 2.3%; P < .001), with a similar overall yield of DXA results recommending osteoporosis treatment (22.4% vs 27.2%). In the BHS group, 84.4% of patients who had treatment recommended followed through with treatment initiation. The mean persistence over follow-up was 657 days (SD, 366 days), and adherence was high with a mean proportion of days covered of 91.7%.
It was not possible to statistically compare adherence with the usual-care group because there were too few screened patients found to be eligible for treatment in that group, but the historic mean proportion of days covered at the two participating facilities was 52%.
After 2 years, the mean femoral neck T-score tested randomly in a subset of patients was better in the BHS arm, although it did not meet statistical significance according to the Bonferroni corrected criterion of P < .025 (-0.55 vs -0.70; P = .04). Fracture rates were similar between the two groups (1.8% vs 2.0%; P = .69).
Can the Findings Be Translated Across Clinics?
It remains to be seen how well the model could translate to other healthcare settings, according to Kenny Lin, MD, MPH, who was asked for comment on the study. “Outside of the VA health system and perhaps integrated HMOs [health maintenance organizations] such as Kaiser, Geisinger, etc., it seems unlikely that most primary care docs will have access to a centralized bone health service. Who’s going to pay for it? It leaves unanswered the question of whether it’s more efficient to address [osteoporosis] screening on a practice or population level. I suspect the latter is probably superior, but this study doesn’t provide any empiric evidence that this is so,” said Lin, associate director of the Penn Medicine Lancaster General Hospital’s Family Medicine Residency Program, Lancaster, Pennsylvania. The findings could help sway recommendations to screen men for osteoporosis, according to Susan Ott, MD, who was also asked for comment. Guideline committees “have been trying to be very scientific [about it]. I think they overdo it because they only look at one or two kinds of studies, and there are more kinds of science than just a randomized clinical trial. But they’re kind of stuck on that. The fact that this study was a randomized trial maybe they will finally change their recommendation, because there really shouldn’t be any difference in screening for men and for women. The men are actually discriminated against,” said Ott, emeritus professor of medicine at the University of Washington, Seattle.
In fact, she noted that the risks for men are similar to those for women, except that men tend to develop issues 5-10 years later in life. To screen and treat men, healthcare systems can “do the same thing they do with women. Just change the age range,” Ott said.
Lin sounded a different note, suggesting that the focus should remain on improvement of screening and treatment adherence in women. “We know that up to two thirds of women discontinue osteoporosis drugs within a year, and if we can’t figure out how to improve abysmal adherence in women, it’s unlikely we will persuade enough men to take these drugs to make a difference,” he said.
The study was funded by a grant from the VA Health Systems Research. Colón-Emeric, Lin, Ott, and Huang reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Efforts to identify older men at risk for osteoporosis and treat those who are eligible received a boost from results reported from a Veterans Affairs (VA) study that showed a significant increase in screening, treatment, and medication adherence.
The cluster randomized trial used a centralized nurse-led intervention to assess men for traditional osteoporosis risk factors, offer bone density testing, and recommend treatment for eligible men. Over 2 years, the intervention group had a higher average femoral neck bone density than patients who underwent usual care.
“We designed this study to see if a risk factor-based approach, which is what most of the guidelines use, made sense and was feasible — that men would be accepting of screening and [the approach] would yield a similar proportion of people who need osteoporosis treatment as screening in women, which is widely recommended and implemented. And sure enough, we found that about 85% of the men in the VA primary care practices in our target age range of between 65 and 85 actually met criteria for screening, and over half of them had low bone mass. They were very accepting of screening, very accepting of treatment, and had excellent compliance rates. So, our study, we believe, supports the idea of identifying men with at least one risk factor for fracture and offering them osteoporosis screening starting at age 65, similar to what we do for women,” Cathleen S. Colón-Emeric, MD, MHS, said in an interview. She is the lead author of the study, a physician in the Durham VA Health Care System, and professor of medicine at Duke University School of Medicine, Durham, North Carolina.
“We were able to see a positive effect on bone density in the bone health group, compared with the usual care group, which suggests that if we followed these folks longer and had enough of them, we would be able to show a fracture reduction benefit,” Colón-Emeric said.
There have been few randomized trials of screening interventions in men, leading to inconsistencies in guidelines, according to the authors of the new study, published online in JAMA Internal Medicine . Both the US Preventive Services Task Force and the Veterans Health Administration National Center for Health Promotion and Disease Prevention consider there to be insufficient evidence to recommend for or against screening in men who have not experienced a fracture. Some professional societies recommend such screening, but there are inconsistencies in the recommended criteria, such as age range or risk factors.
Beyond the age of 50 years, one in five men will experience an osteoporosis-related fracture at some point in their life, according to a 2009 study. Treatment is inexpensive and effective in both men and women, and economic models suggest that screening using dual-energy x-ray absorptiometry (DXA) would be cost-effective. Still, screening is rare among men, with fewer than 10% of men getting screened before having an osteoporosis-related fracture.
“It’s important to screen men at risk for osteoporosis due to the dramatically increased mortality men suffer after a fragility fracture compared with women. Within 1 year of a hip fracture, mortality is as high as 36%. Studies have also shown that osteoporosis in men is undertreated, with only 10%-50% being prescribed antifracture treatment within 1 year of a hip fracture. Most individuals do not regain their prior level of function after a hip fracture,” said Joe C. Huang, MD, who was asked for comment. He is a clinical assistant professor of gerontology and geriatric medicine at Harborview Medical Center Senior Care Clinic and Healthy Bones Clinic in Seattle.
Details of the Intervention
The bone health service (BHS) intervention employed an electronic health record case-finding tool and a nurse care manager who undertook screening and treatment monitoring. They identified potential risk factors that included hyperthyroidism, hyperparathyroidism, rheumatoid arthritis, alcohol dependence, chronic lung disease, chronic liver disease, stroke, parkinsonism, prostate cancer, smoking, diabetes, pernicious anemia, gastrectomy, or high-risk medication use in at least 3 months of the prior 2 years. These medications included traditional antiepileptics, glucocorticoids, and androgen deprivation therapy.
The BHS nurse invited eligible men to be screened using an initial letter, followed by up to three phone calls. After DXA screening, the nurse scheduled an electronic consult with an osteoporosis expert, and patients with a T-score between -1 and -2.4 and an elevated 10-year fracture risk as measured by the Fracture Risk Assessment Tool were recommended for osteoporosis medication, vitamin D, and dietary or supplemental calcium. Following the prescription, the nurse provided patient education over the phone and mailed out written instructions. The nurse also made phone calls at 1 month, 6 months, and 12 months to encourage adherence and address common treatment barriers such as forgetting to take medication or dealing with gastrointestinal effects. The researchers recruited 38 primary care physicians from two VA health systems. The study included 3112 male veterans between the ages of 65 and 85 years (40.4% Black and 56% White). Nearly all participants (85.5%) had at least one indication for screening according to VA undersecretary guidelines, and almost a third (32.1%) had been prescribed androgen deprivation therapy, traditional antiepileptic drugs, or glucocorticoids.
Over a mean follow-up of 1.5 years, there was a much higher screening rate in the BHS group (49.2% vs 2.3%; P < .001), with a similar overall yield of DXA results recommending osteoporosis treatment (22.4% vs 27.2%). In the BHS group, 84.4% of patients who had treatment recommended followed through with treatment initiation. The mean persistence over follow-up was 657 days (SD, 366 days), and adherence was high with a mean proportion of days covered of 91.7%.
It was not possible to statistically compare adherence with the usual-care group because there were too few screened patients found to be eligible for treatment in that group, but the historic mean proportion of days covered at the two participating facilities was 52%.
After 2 years, the mean femoral neck T-score tested randomly in a subset of patients was better in the BHS arm, although it did not meet statistical significance according to the Bonferroni corrected criterion of P < .025 (-0.55 vs -0.70; P = .04). Fracture rates were similar between the two groups (1.8% vs 2.0%; P = .69).
Can the Findings Be Translated Across Clinics?
It remains to be seen how well the model could translate to other healthcare settings, according to Kenny Lin, MD, MPH, who was asked for comment on the study. “Outside of the VA health system and perhaps integrated HMOs [health maintenance organizations] such as Kaiser, Geisinger, etc., it seems unlikely that most primary care docs will have access to a centralized bone health service. Who’s going to pay for it? It leaves unanswered the question of whether it’s more efficient to address [osteoporosis] screening on a practice or population level. I suspect the latter is probably superior, but this study doesn’t provide any empiric evidence that this is so,” said Lin, associate director of the Penn Medicine Lancaster General Hospital’s Family Medicine Residency Program, Lancaster, Pennsylvania. The findings could help sway recommendations to screen men for osteoporosis, according to Susan Ott, MD, who was also asked for comment. Guideline committees “have been trying to be very scientific [about it]. I think they overdo it because they only look at one or two kinds of studies, and there are more kinds of science than just a randomized clinical trial. But they’re kind of stuck on that. The fact that this study was a randomized trial maybe they will finally change their recommendation, because there really shouldn’t be any difference in screening for men and for women. The men are actually discriminated against,” said Ott, emeritus professor of medicine at the University of Washington, Seattle.
In fact, she noted that the risks for men are similar to those for women, except that men tend to develop issues 5-10 years later in life. To screen and treat men, healthcare systems can “do the same thing they do with women. Just change the age range,” Ott said.
Lin sounded a different note, suggesting that the focus should remain on improvement of screening and treatment adherence in women. “We know that up to two thirds of women discontinue osteoporosis drugs within a year, and if we can’t figure out how to improve abysmal adherence in women, it’s unlikely we will persuade enough men to take these drugs to make a difference,” he said.
The study was funded by a grant from the VA Health Systems Research. Colón-Emeric, Lin, Ott, and Huang reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Efforts to identify older men at risk for osteoporosis and treat those who are eligible received a boost from results reported from a Veterans Affairs (VA) study that showed a significant increase in screening, treatment, and medication adherence.
The cluster randomized trial used a centralized nurse-led intervention to assess men for traditional osteoporosis risk factors, offer bone density testing, and recommend treatment for eligible men. Over 2 years, the intervention group had a higher average femoral neck bone density than patients who underwent usual care.
“We designed this study to see if a risk factor-based approach, which is what most of the guidelines use, made sense and was feasible — that men would be accepting of screening and [the approach] would yield a similar proportion of people who need osteoporosis treatment as screening in women, which is widely recommended and implemented. And sure enough, we found that about 85% of the men in the VA primary care practices in our target age range of between 65 and 85 actually met criteria for screening, and over half of them had low bone mass. They were very accepting of screening, very accepting of treatment, and had excellent compliance rates. So, our study, we believe, supports the idea of identifying men with at least one risk factor for fracture and offering them osteoporosis screening starting at age 65, similar to what we do for women,” Cathleen S. Colón-Emeric, MD, MHS, said in an interview. She is the lead author of the study, a physician in the Durham VA Health Care System, and professor of medicine at Duke University School of Medicine, Durham, North Carolina.
“We were able to see a positive effect on bone density in the bone health group, compared with the usual care group, which suggests that if we followed these folks longer and had enough of them, we would be able to show a fracture reduction benefit,” Colón-Emeric said.
There have been few randomized trials of screening interventions in men, leading to inconsistencies in guidelines, according to the authors of the new study, published online in JAMA Internal Medicine . Both the US Preventive Services Task Force and the Veterans Health Administration National Center for Health Promotion and Disease Prevention consider there to be insufficient evidence to recommend for or against screening in men who have not experienced a fracture. Some professional societies recommend such screening, but there are inconsistencies in the recommended criteria, such as age range or risk factors.
Beyond the age of 50 years, one in five men will experience an osteoporosis-related fracture at some point in their life, according to a 2009 study. Treatment is inexpensive and effective in both men and women, and economic models suggest that screening using dual-energy x-ray absorptiometry (DXA) would be cost-effective. Still, screening is rare among men, with fewer than 10% of men getting screened before having an osteoporosis-related fracture.
“It’s important to screen men at risk for osteoporosis due to the dramatically increased mortality men suffer after a fragility fracture compared with women. Within 1 year of a hip fracture, mortality is as high as 36%. Studies have also shown that osteoporosis in men is undertreated, with only 10%-50% being prescribed antifracture treatment within 1 year of a hip fracture. Most individuals do not regain their prior level of function after a hip fracture,” said Joe C. Huang, MD, who was asked for comment. He is a clinical assistant professor of gerontology and geriatric medicine at Harborview Medical Center Senior Care Clinic and Healthy Bones Clinic in Seattle.
Details of the Intervention
The bone health service (BHS) intervention employed an electronic health record case-finding tool and a nurse care manager who undertook screening and treatment monitoring. They identified potential risk factors that included hyperthyroidism, hyperparathyroidism, rheumatoid arthritis, alcohol dependence, chronic lung disease, chronic liver disease, stroke, parkinsonism, prostate cancer, smoking, diabetes, pernicious anemia, gastrectomy, or high-risk medication use in at least 3 months of the prior 2 years. These medications included traditional antiepileptics, glucocorticoids, and androgen deprivation therapy.
The BHS nurse invited eligible men to be screened using an initial letter, followed by up to three phone calls. After DXA screening, the nurse scheduled an electronic consult with an osteoporosis expert, and patients with a T-score between -1 and -2.4 and an elevated 10-year fracture risk as measured by the Fracture Risk Assessment Tool were recommended for osteoporosis medication, vitamin D, and dietary or supplemental calcium. Following the prescription, the nurse provided patient education over the phone and mailed out written instructions. The nurse also made phone calls at 1 month, 6 months, and 12 months to encourage adherence and address common treatment barriers such as forgetting to take medication or dealing with gastrointestinal effects. The researchers recruited 38 primary care physicians from two VA health systems. The study included 3112 male veterans between the ages of 65 and 85 years (40.4% Black and 56% White). Nearly all participants (85.5%) had at least one indication for screening according to VA undersecretary guidelines, and almost a third (32.1%) had been prescribed androgen deprivation therapy, traditional antiepileptic drugs, or glucocorticoids.
Over a mean follow-up of 1.5 years, there was a much higher screening rate in the BHS group (49.2% vs 2.3%; P < .001), with a similar overall yield of DXA results recommending osteoporosis treatment (22.4% vs 27.2%). In the BHS group, 84.4% of patients who had treatment recommended followed through with treatment initiation. The mean persistence over follow-up was 657 days (SD, 366 days), and adherence was high with a mean proportion of days covered of 91.7%.
It was not possible to statistically compare adherence with the usual-care group because there were too few screened patients found to be eligible for treatment in that group, but the historic mean proportion of days covered at the two participating facilities was 52%.
After 2 years, the mean femoral neck T-score tested randomly in a subset of patients was better in the BHS arm, although it did not meet statistical significance according to the Bonferroni corrected criterion of P < .025 (-0.55 vs -0.70; P = .04). Fracture rates were similar between the two groups (1.8% vs 2.0%; P = .69).
Can the Findings Be Translated Across Clinics?
It remains to be seen how well the model could translate to other healthcare settings, according to Kenny Lin, MD, MPH, who was asked for comment on the study. “Outside of the VA health system and perhaps integrated HMOs [health maintenance organizations] such as Kaiser, Geisinger, etc., it seems unlikely that most primary care docs will have access to a centralized bone health service. Who’s going to pay for it? It leaves unanswered the question of whether it’s more efficient to address [osteoporosis] screening on a practice or population level. I suspect the latter is probably superior, but this study doesn’t provide any empiric evidence that this is so,” said Lin, associate director of the Penn Medicine Lancaster General Hospital’s Family Medicine Residency Program, Lancaster, Pennsylvania. The findings could help sway recommendations to screen men for osteoporosis, according to Susan Ott, MD, who was also asked for comment. Guideline committees “have been trying to be very scientific [about it]. I think they overdo it because they only look at one or two kinds of studies, and there are more kinds of science than just a randomized clinical trial. But they’re kind of stuck on that. The fact that this study was a randomized trial maybe they will finally change their recommendation, because there really shouldn’t be any difference in screening for men and for women. The men are actually discriminated against,” said Ott, emeritus professor of medicine at the University of Washington, Seattle.
In fact, she noted that the risks for men are similar to those for women, except that men tend to develop issues 5-10 years later in life. To screen and treat men, healthcare systems can “do the same thing they do with women. Just change the age range,” Ott said.
Lin sounded a different note, suggesting that the focus should remain on improvement of screening and treatment adherence in women. “We know that up to two thirds of women discontinue osteoporosis drugs within a year, and if we can’t figure out how to improve abysmal adherence in women, it’s unlikely we will persuade enough men to take these drugs to make a difference,” he said.
The study was funded by a grant from the VA Health Systems Research. Colón-Emeric, Lin, Ott, and Huang reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
U.S. Health Chief Kennedy Targets Vaccine Injury Compensation Program
WASHINGTON (Reuters) - U.S. Health Secretary Robert F. Kennedy Jr. said on July 28 that he will work to “fix” the program that compensates victims of vaccine injuries, the National Vaccine Injury Compensation Program.
Kennedy, a long-time vaccine skeptic and former vaccine injury plaintiff lawyer, accused the program and its so-called “Vaccine Court” of corruption and inefficiency in a post on X. He has long been an outspoken critic of the program.
“I will not allow the VICP to continue to ignore its mandate and fail its mission of quickly and fairly compensating vaccine-injured individuals,” he wrote, adding he was working with Attorney General Pam Bondi. “Together, we will steer the Vaccine Court back to its original congressional intent.”
He said the structure disadvantaged claimants because the Department of Health & Human Services – which he now leads – is the defendant, as opposed to vaccine makers.
Changing the VICP would be the latest in a series of far-reaching actions by Kennedy to reshape U.S. regulation of vaccines, food and medicine.
In June, he fired all 17 members of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, a panel of vaccine experts, replacing them with 7 handpicked members, including known vaccine skeptics.
One of them earned thousands of dollars as an expert witness in litigation against Merck’s, Gardasil vaccine, court records show. Kennedy himself played an instrumental role in organizing mass litigation over the vaccine.
He also is planning to remove all the members of another advisory panel that determines what preventive health measures insurers must cover, the Wall Street Journal reported on July 25. An HHS spokesperson said Kennedy had not yet made a decision regarding the 16-member U.S. Preventive Services Task Force.
Kennedy has for years sown doubt about the safety and efficacy of vaccines. He has a history of clashing with the medical establishment and spreading misinformation about vaccines, including promoting a debunked link between vaccines and autism despite scientific evidence to the contrary.
He has also said the measles vaccine contains cells from aborted fetuses and that the mumps vaccination does not work, comments he made as the U.S. battles one of its worst outbreaks of measles in 25 years.
Kennedy made millions over the years from advocating against vaccines through case referrals, book sales, and consulting fees paid by a nonprofit he founded, according to ethics disclosures.
(Reporting by Ahmed Aboulenein; Additional reporting by Ryan Patrick Jones in Toronto; Editing by Doina Chiacu and Nia Williams)
A version of this article appeared on Medscape.com.
WASHINGTON (Reuters) - U.S. Health Secretary Robert F. Kennedy Jr. said on July 28 that he will work to “fix” the program that compensates victims of vaccine injuries, the National Vaccine Injury Compensation Program.
Kennedy, a long-time vaccine skeptic and former vaccine injury plaintiff lawyer, accused the program and its so-called “Vaccine Court” of corruption and inefficiency in a post on X. He has long been an outspoken critic of the program.
“I will not allow the VICP to continue to ignore its mandate and fail its mission of quickly and fairly compensating vaccine-injured individuals,” he wrote, adding he was working with Attorney General Pam Bondi. “Together, we will steer the Vaccine Court back to its original congressional intent.”
He said the structure disadvantaged claimants because the Department of Health & Human Services – which he now leads – is the defendant, as opposed to vaccine makers.
Changing the VICP would be the latest in a series of far-reaching actions by Kennedy to reshape U.S. regulation of vaccines, food and medicine.
In June, he fired all 17 members of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, a panel of vaccine experts, replacing them with 7 handpicked members, including known vaccine skeptics.
One of them earned thousands of dollars as an expert witness in litigation against Merck’s, Gardasil vaccine, court records show. Kennedy himself played an instrumental role in organizing mass litigation over the vaccine.
He also is planning to remove all the members of another advisory panel that determines what preventive health measures insurers must cover, the Wall Street Journal reported on July 25. An HHS spokesperson said Kennedy had not yet made a decision regarding the 16-member U.S. Preventive Services Task Force.
Kennedy has for years sown doubt about the safety and efficacy of vaccines. He has a history of clashing with the medical establishment and spreading misinformation about vaccines, including promoting a debunked link between vaccines and autism despite scientific evidence to the contrary.
He has also said the measles vaccine contains cells from aborted fetuses and that the mumps vaccination does not work, comments he made as the U.S. battles one of its worst outbreaks of measles in 25 years.
Kennedy made millions over the years from advocating against vaccines through case referrals, book sales, and consulting fees paid by a nonprofit he founded, according to ethics disclosures.
(Reporting by Ahmed Aboulenein; Additional reporting by Ryan Patrick Jones in Toronto; Editing by Doina Chiacu and Nia Williams)
A version of this article appeared on Medscape.com.
WASHINGTON (Reuters) - U.S. Health Secretary Robert F. Kennedy Jr. said on July 28 that he will work to “fix” the program that compensates victims of vaccine injuries, the National Vaccine Injury Compensation Program.
Kennedy, a long-time vaccine skeptic and former vaccine injury plaintiff lawyer, accused the program and its so-called “Vaccine Court” of corruption and inefficiency in a post on X. He has long been an outspoken critic of the program.
“I will not allow the VICP to continue to ignore its mandate and fail its mission of quickly and fairly compensating vaccine-injured individuals,” he wrote, adding he was working with Attorney General Pam Bondi. “Together, we will steer the Vaccine Court back to its original congressional intent.”
He said the structure disadvantaged claimants because the Department of Health & Human Services – which he now leads – is the defendant, as opposed to vaccine makers.
Changing the VICP would be the latest in a series of far-reaching actions by Kennedy to reshape U.S. regulation of vaccines, food and medicine.
In June, he fired all 17 members of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, a panel of vaccine experts, replacing them with 7 handpicked members, including known vaccine skeptics.
One of them earned thousands of dollars as an expert witness in litigation against Merck’s, Gardasil vaccine, court records show. Kennedy himself played an instrumental role in organizing mass litigation over the vaccine.
He also is planning to remove all the members of another advisory panel that determines what preventive health measures insurers must cover, the Wall Street Journal reported on July 25. An HHS spokesperson said Kennedy had not yet made a decision regarding the 16-member U.S. Preventive Services Task Force.
Kennedy has for years sown doubt about the safety and efficacy of vaccines. He has a history of clashing with the medical establishment and spreading misinformation about vaccines, including promoting a debunked link between vaccines and autism despite scientific evidence to the contrary.
He has also said the measles vaccine contains cells from aborted fetuses and that the mumps vaccination does not work, comments he made as the U.S. battles one of its worst outbreaks of measles in 25 years.
Kennedy made millions over the years from advocating against vaccines through case referrals, book sales, and consulting fees paid by a nonprofit he founded, according to ethics disclosures.
(Reporting by Ahmed Aboulenein; Additional reporting by Ryan Patrick Jones in Toronto; Editing by Doina Chiacu and Nia Williams)
A version of this article appeared on Medscape.com.
Rurality and Age May Shape Phone-Only Mental Health Care Access Among Veterans
TOPLINE:
Patients living in rural areas and those aged ≥ 65 y had increased odds of receiving mental health care exclusively by phone.
METHODOLOGY:
- Researchers explored factors linked to receiving phone-only mental health care among patients within the Department of Veterans Affairs.
- They included data for 1,156,146 veteran patients with at least one mental health-specific outpatient encounter between October 2021 and September 2022 and at least one between October 2022 and September 2023.
- Patients were categorized as those who received care through phone only (n = 49,125) and those who received care through other methods (n = 1,107,021. Care was received exclusively through video (6.39%), in-person (6.63%), or a combination of in-person, video, and/or phone (86.98%).
- Demographic and clinical predictors, including rurality, age, sex, race, ethnicity, and the number of mental health diagnoses (< 3 vs ≥ 3), were evaluated.
TAKEAWAY:
- The phone-only group had a mean of 6.27 phone visits, whereas those who received care through other methods had a mean of 4.79 phone visits.
- Highly rural patients had 1.50 times higher odds of receiving phone-only mental health care than their urban counterparts (adjusted odds ratio [aOR], 1.50; P < .0001).
- Patients aged 65 years or older were more than twice as likely to receive phone-only care than those younger than 30 years (aOR, ≥ 2.17; P < .0001).
- Having fewer than three mental health diagnoses and more than 50% of mental health visits conducted by medical providers was associated with higher odds of receiving mental health care exclusively by phone (aORs, 2.03 and 1.87, respectively; P < .0001).
IN PRACTICE:
“The results of this work help to characterize the phone-only patient population and can serve to inform future implementation efforts to ensure that patients are receiving care via the modality that best meets their needs,” the authors wrote.
SOURCE:
This study was led by Samantha L. Connolly, PhD, at the VA Boston Healthcare System in Boston. It was published online in The Journal of Rural Health.
LIMITATIONS:
This study focused on a veteran population which may limit the generalizability of the findings to other groups. Additionally, its cross-sectional design restricted the ability to determine cause-and-effect relationships between factors and phone-only care.
DISCLOSURES:
This study was supported by the US Department of Veterans Affairs. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Patients living in rural areas and those aged ≥ 65 y had increased odds of receiving mental health care exclusively by phone.
METHODOLOGY:
- Researchers explored factors linked to receiving phone-only mental health care among patients within the Department of Veterans Affairs.
- They included data for 1,156,146 veteran patients with at least one mental health-specific outpatient encounter between October 2021 and September 2022 and at least one between October 2022 and September 2023.
- Patients were categorized as those who received care through phone only (n = 49,125) and those who received care through other methods (n = 1,107,021. Care was received exclusively through video (6.39%), in-person (6.63%), or a combination of in-person, video, and/or phone (86.98%).
- Demographic and clinical predictors, including rurality, age, sex, race, ethnicity, and the number of mental health diagnoses (< 3 vs ≥ 3), were evaluated.
TAKEAWAY:
- The phone-only group had a mean of 6.27 phone visits, whereas those who received care through other methods had a mean of 4.79 phone visits.
- Highly rural patients had 1.50 times higher odds of receiving phone-only mental health care than their urban counterparts (adjusted odds ratio [aOR], 1.50; P < .0001).
- Patients aged 65 years or older were more than twice as likely to receive phone-only care than those younger than 30 years (aOR, ≥ 2.17; P < .0001).
- Having fewer than three mental health diagnoses and more than 50% of mental health visits conducted by medical providers was associated with higher odds of receiving mental health care exclusively by phone (aORs, 2.03 and 1.87, respectively; P < .0001).
IN PRACTICE:
“The results of this work help to characterize the phone-only patient population and can serve to inform future implementation efforts to ensure that patients are receiving care via the modality that best meets their needs,” the authors wrote.
SOURCE:
This study was led by Samantha L. Connolly, PhD, at the VA Boston Healthcare System in Boston. It was published online in The Journal of Rural Health.
LIMITATIONS:
This study focused on a veteran population which may limit the generalizability of the findings to other groups. Additionally, its cross-sectional design restricted the ability to determine cause-and-effect relationships between factors and phone-only care.
DISCLOSURES:
This study was supported by the US Department of Veterans Affairs. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Patients living in rural areas and those aged ≥ 65 y had increased odds of receiving mental health care exclusively by phone.
METHODOLOGY:
- Researchers explored factors linked to receiving phone-only mental health care among patients within the Department of Veterans Affairs.
- They included data for 1,156,146 veteran patients with at least one mental health-specific outpatient encounter between October 2021 and September 2022 and at least one between October 2022 and September 2023.
- Patients were categorized as those who received care through phone only (n = 49,125) and those who received care through other methods (n = 1,107,021. Care was received exclusively through video (6.39%), in-person (6.63%), or a combination of in-person, video, and/or phone (86.98%).
- Demographic and clinical predictors, including rurality, age, sex, race, ethnicity, and the number of mental health diagnoses (< 3 vs ≥ 3), were evaluated.
TAKEAWAY:
- The phone-only group had a mean of 6.27 phone visits, whereas those who received care through other methods had a mean of 4.79 phone visits.
- Highly rural patients had 1.50 times higher odds of receiving phone-only mental health care than their urban counterparts (adjusted odds ratio [aOR], 1.50; P < .0001).
- Patients aged 65 years or older were more than twice as likely to receive phone-only care than those younger than 30 years (aOR, ≥ 2.17; P < .0001).
- Having fewer than three mental health diagnoses and more than 50% of mental health visits conducted by medical providers was associated with higher odds of receiving mental health care exclusively by phone (aORs, 2.03 and 1.87, respectively; P < .0001).
IN PRACTICE:
“The results of this work help to characterize the phone-only patient population and can serve to inform future implementation efforts to ensure that patients are receiving care via the modality that best meets their needs,” the authors wrote.
SOURCE:
This study was led by Samantha L. Connolly, PhD, at the VA Boston Healthcare System in Boston. It was published online in The Journal of Rural Health.
LIMITATIONS:
This study focused on a veteran population which may limit the generalizability of the findings to other groups. Additionally, its cross-sectional design restricted the ability to determine cause-and-effect relationships between factors and phone-only care.
DISCLOSURES:
This study was supported by the US Department of Veterans Affairs. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Searching for the Optimal CRC Surveillance Test
About a third of the US population are eligible for colorectal cancer screening but aren’t up to date on screening.
Many patients are reluctant to test for colon cancer for a variety of reasons, said Jeffrey K. Lee, MD, MPH, a research scientist at the Kaiser Permanente Northern California Division of Research and an attending gastroenterologist at Kaiser Permanente San Francisco Medical Center.
“As a gastroenterologist, I strongly believe we should emphasize the importance of colorectal cancer screening. And there’s many tests available, not just a colonoscopy, to help reduce your chances of developing colorectal cancer and even dying from colorectal cancer,” said Dr. Lee.
Many patients prefer a test that’s more convenient, that doesn’t require them to take time out of their busy schedules. “We must educate our patients that there are some noninvasive screening options that are helpful, and to be able to share with them some of the benefits, but also some of the drawbacks compared to colonoscopy and allow them to have a choice,” he advised.
He is a recipient of the AGA Research Scholar Award, and has in turn supported other researchers by contributing to the AGA Research Foundation. In 2012, Dr. Lee received a grant from the Sylvia Allison Kaplan Clinical Research Fund to fund a study on long-term colorectal cancer risk in patients with normal colonoscopy results.
The findings, published in JAMA Internal Medicine, determined that 10 years after a negative colonoscopy, Kaiser Permanente members had a 46% lower risk of being diagnosed with CRC and were 88% less likely to die from disease compared with patients who didn’t undergo screening.
“Furthermore, the reduced risk of developing colorectal cancer, even dying from it, persisted for more than 12 years after the examination compared with an unscreened population,” said Dr. Lee. “I firmly believe our study really supports the ten-year screening interval after a normal colonoscopy, as currently recommended by our guidelines.”
In an interview, he discussed his research efforts to find the best detection regimens for CRC, and the mentors who guided his career path as a GI scientist.
Q: Why did you choose GI?
During medical school I was fortunate to work in the lab of Dr. John M. Carethers at UC San Diego. He introduced me to GI and inspired me to choose GI as a career. His mentorship was invaluable because he not only solidified my interest in GI, but also inspired me to become a physician scientist, focusing on colorectal cancer prevention and control. His amazing mentorship drew me to this field.
Q: One of your clinical focus areas is hereditary gastrointestinal cancer syndromes. How did you become interested in this area of GI medicine?
My interest in hereditary GI cancer syndromes stemmed from my work as a medical student in Dr. Carethers’ lab. One of my research projects was looking at certain gene mutations among patients with hereditary GI cancer syndromes, specifically, familial hamartomatous polyposis syndrome. It was through these research projects and seeing how these genetic mutations impacted their risk of developing colorectal cancer, inspired me to care for patients with hereditary GI cancer syndromes.
Q: Have you been doing any research on the reasons why more young people are getting colon cancer?
We recently published work looking at the potential factors that may be driving the rising rates of early onset colorectal cancer. One hypothesis that’s been floating around is antibiotic exposure in early adulthood or childhood because of its effect on the microbiome. Using our large database at Kaiser Permanente Northern California, we did not find an association between oral antibiotic use during early adulthood and the risk of early-onset colorectal cancer.
You have the usual suspects like obesity and diabetes, but it’s not explaining all that risk. While familial colorectal cancer syndromes contribute to a small proportion of early-onset colorectal, these syndromes are not increasing across generations. I really do feel it’s something in the diet or how foods are processed and environmental factors that’s driving some of the risk of early onset colorectal cancer and this should be explored further.
Q: In 2018, you issued a landmark study which found an association between a 10-year follow-up after negative colonoscopy and reduced risk of disease and mortality. Has there been any updates to these findings over the last 6 years?
We recently saw a study in JAMA Oncology of a Swedish cohort that showed a negative colonoscopy result was associated with a reduced risk of developing and even dying from colorectal cancer 15 years from that examination, compared to the general population of Sweden. I think there’s some things that we need to be cautious about regarding that study. We have to think about the comparison group that they used and the lack of information regarding the indication of the colonoscopy and the quality of the examination. So, it remains uncertain whether future guidelines are going to stretch out that 10-year interval to 15 years.
Q: What other CRC studies are you working on now?
We have several studies that we are working on right now. One is called the PREVENT CRC study, which is looking at whether a polygenic risk score can improve risk stratification following adenoma removal for colorectal cancer prevention and tailoring post-polypectomy surveillance. This is a large observational cohort study that we have teamed up with the Fred Hutchinson Cancer Center, Erasmus University, and Kaiser Permanente Northwest to answer this important question that may have implications for personalized medicine.
Then there’s the COOP study, funded by the Patient-Centered Outcomes Research Institute. This is looking at the best surveillance test to use among older adults 65 years and older with a history of polyps. The trial is randomizing them to either getting a colonoscopy for surveillance or annual fecal immunochemical test (FIT) for surveillance. This is to see which test is best for detecting colorectal cancer among older adults with a history of polyps.
Q: Do you think FIT tests could eventually replace colonoscopy, given that it’s less invasive?
Although FIT and other stool-based tests are less invasive and have been shown to have high accuracy for detecting colorectal cancer, I personally do not think they are going to replace colonoscopy as the most popular screening modality in the United States. Colonoscopy remains the gold standard for detecting and removing precancerous polyps and has the highest accuracy for detecting colorectal cancer.
Q: Besides Dr. Carethers, what teacher or mentor had the greatest impact on you?
Clinically it’s been Dr. Jonathan Terdiman from UCSF, who taught me everything I know about clinical GI, and the art of colonoscopy. In addition, Douglas A. Corley, MD, PhD, the Permanente Medical Group’s chief research officer, has made the greatest impact on my research career. He’s really taught me how to rigorously design a research study to answer important clinically relevant questions, and has given me the skill set to write NIH grants. I would not be here without these mentors who are truly giants in the field of GI.
Q: When you’re not being a GI, how do you spend your free weekend afternoons? Are you still a “Cal Bears” fan at your alma mater, UC Berkeley?
I spend a lot of time taking my kids to their activities on the weekends. I just took my son to a Cal Bears Game Day, which was hosted by ESPN at Berkeley.
It was an incredible experience hearing sports analyst Pat McAfee lead all the Cal chants, seeing Nick Saban from the University of Alabama take off his red tie and replace it with a Cal Bears tie, and watching a Cal student win a hundred thousand dollars by kicking a football through the goal posts wearing checkered vans.
Lightning Round
Texting or talking?
Text
Favorite breakfast?
Taiwanese breakfast
Place you most want to travel to?
Japan
Favorite junk food?
Trader Joe’s chili lime chips
Favorite season?
Springtime, baseball season
Favorite ice cream flavor?
Mint chocolate chip
How many cups of coffee do you drink per day?
2-3
Last movie you watched?
Oppenheimer
Best place you ever went on vacation?
Hawaii
If you weren’t a gastroenterologist, what would you be?
Barber
Best Halloween costume you ever wore?
SpongeBob SquarePants
Favorite sport?
Tennis
What song do you have to sing along with when you hear it?
Any classic 80s song
Introvert or extrovert?
Introvert
About a third of the US population are eligible for colorectal cancer screening but aren’t up to date on screening.
Many patients are reluctant to test for colon cancer for a variety of reasons, said Jeffrey K. Lee, MD, MPH, a research scientist at the Kaiser Permanente Northern California Division of Research and an attending gastroenterologist at Kaiser Permanente San Francisco Medical Center.
“As a gastroenterologist, I strongly believe we should emphasize the importance of colorectal cancer screening. And there’s many tests available, not just a colonoscopy, to help reduce your chances of developing colorectal cancer and even dying from colorectal cancer,” said Dr. Lee.
Many patients prefer a test that’s more convenient, that doesn’t require them to take time out of their busy schedules. “We must educate our patients that there are some noninvasive screening options that are helpful, and to be able to share with them some of the benefits, but also some of the drawbacks compared to colonoscopy and allow them to have a choice,” he advised.
He is a recipient of the AGA Research Scholar Award, and has in turn supported other researchers by contributing to the AGA Research Foundation. In 2012, Dr. Lee received a grant from the Sylvia Allison Kaplan Clinical Research Fund to fund a study on long-term colorectal cancer risk in patients with normal colonoscopy results.
The findings, published in JAMA Internal Medicine, determined that 10 years after a negative colonoscopy, Kaiser Permanente members had a 46% lower risk of being diagnosed with CRC and were 88% less likely to die from disease compared with patients who didn’t undergo screening.
“Furthermore, the reduced risk of developing colorectal cancer, even dying from it, persisted for more than 12 years after the examination compared with an unscreened population,” said Dr. Lee. “I firmly believe our study really supports the ten-year screening interval after a normal colonoscopy, as currently recommended by our guidelines.”
In an interview, he discussed his research efforts to find the best detection regimens for CRC, and the mentors who guided his career path as a GI scientist.
Q: Why did you choose GI?
During medical school I was fortunate to work in the lab of Dr. John M. Carethers at UC San Diego. He introduced me to GI and inspired me to choose GI as a career. His mentorship was invaluable because he not only solidified my interest in GI, but also inspired me to become a physician scientist, focusing on colorectal cancer prevention and control. His amazing mentorship drew me to this field.
Q: One of your clinical focus areas is hereditary gastrointestinal cancer syndromes. How did you become interested in this area of GI medicine?
My interest in hereditary GI cancer syndromes stemmed from my work as a medical student in Dr. Carethers’ lab. One of my research projects was looking at certain gene mutations among patients with hereditary GI cancer syndromes, specifically, familial hamartomatous polyposis syndrome. It was through these research projects and seeing how these genetic mutations impacted their risk of developing colorectal cancer, inspired me to care for patients with hereditary GI cancer syndromes.
Q: Have you been doing any research on the reasons why more young people are getting colon cancer?
We recently published work looking at the potential factors that may be driving the rising rates of early onset colorectal cancer. One hypothesis that’s been floating around is antibiotic exposure in early adulthood or childhood because of its effect on the microbiome. Using our large database at Kaiser Permanente Northern California, we did not find an association between oral antibiotic use during early adulthood and the risk of early-onset colorectal cancer.
You have the usual suspects like obesity and diabetes, but it’s not explaining all that risk. While familial colorectal cancer syndromes contribute to a small proportion of early-onset colorectal, these syndromes are not increasing across generations. I really do feel it’s something in the diet or how foods are processed and environmental factors that’s driving some of the risk of early onset colorectal cancer and this should be explored further.
Q: In 2018, you issued a landmark study which found an association between a 10-year follow-up after negative colonoscopy and reduced risk of disease and mortality. Has there been any updates to these findings over the last 6 years?
We recently saw a study in JAMA Oncology of a Swedish cohort that showed a negative colonoscopy result was associated with a reduced risk of developing and even dying from colorectal cancer 15 years from that examination, compared to the general population of Sweden. I think there’s some things that we need to be cautious about regarding that study. We have to think about the comparison group that they used and the lack of information regarding the indication of the colonoscopy and the quality of the examination. So, it remains uncertain whether future guidelines are going to stretch out that 10-year interval to 15 years.
Q: What other CRC studies are you working on now?
We have several studies that we are working on right now. One is called the PREVENT CRC study, which is looking at whether a polygenic risk score can improve risk stratification following adenoma removal for colorectal cancer prevention and tailoring post-polypectomy surveillance. This is a large observational cohort study that we have teamed up with the Fred Hutchinson Cancer Center, Erasmus University, and Kaiser Permanente Northwest to answer this important question that may have implications for personalized medicine.
Then there’s the COOP study, funded by the Patient-Centered Outcomes Research Institute. This is looking at the best surveillance test to use among older adults 65 years and older with a history of polyps. The trial is randomizing them to either getting a colonoscopy for surveillance or annual fecal immunochemical test (FIT) for surveillance. This is to see which test is best for detecting colorectal cancer among older adults with a history of polyps.
Q: Do you think FIT tests could eventually replace colonoscopy, given that it’s less invasive?
Although FIT and other stool-based tests are less invasive and have been shown to have high accuracy for detecting colorectal cancer, I personally do not think they are going to replace colonoscopy as the most popular screening modality in the United States. Colonoscopy remains the gold standard for detecting and removing precancerous polyps and has the highest accuracy for detecting colorectal cancer.
Q: Besides Dr. Carethers, what teacher or mentor had the greatest impact on you?
Clinically it’s been Dr. Jonathan Terdiman from UCSF, who taught me everything I know about clinical GI, and the art of colonoscopy. In addition, Douglas A. Corley, MD, PhD, the Permanente Medical Group’s chief research officer, has made the greatest impact on my research career. He’s really taught me how to rigorously design a research study to answer important clinically relevant questions, and has given me the skill set to write NIH grants. I would not be here without these mentors who are truly giants in the field of GI.
Q: When you’re not being a GI, how do you spend your free weekend afternoons? Are you still a “Cal Bears” fan at your alma mater, UC Berkeley?
I spend a lot of time taking my kids to their activities on the weekends. I just took my son to a Cal Bears Game Day, which was hosted by ESPN at Berkeley.
It was an incredible experience hearing sports analyst Pat McAfee lead all the Cal chants, seeing Nick Saban from the University of Alabama take off his red tie and replace it with a Cal Bears tie, and watching a Cal student win a hundred thousand dollars by kicking a football through the goal posts wearing checkered vans.
Lightning Round
Texting or talking?
Text
Favorite breakfast?
Taiwanese breakfast
Place you most want to travel to?
Japan
Favorite junk food?
Trader Joe’s chili lime chips
Favorite season?
Springtime, baseball season
Favorite ice cream flavor?
Mint chocolate chip
How many cups of coffee do you drink per day?
2-3
Last movie you watched?
Oppenheimer
Best place you ever went on vacation?
Hawaii
If you weren’t a gastroenterologist, what would you be?
Barber
Best Halloween costume you ever wore?
SpongeBob SquarePants
Favorite sport?
Tennis
What song do you have to sing along with when you hear it?
Any classic 80s song
Introvert or extrovert?
Introvert
About a third of the US population are eligible for colorectal cancer screening but aren’t up to date on screening.
Many patients are reluctant to test for colon cancer for a variety of reasons, said Jeffrey K. Lee, MD, MPH, a research scientist at the Kaiser Permanente Northern California Division of Research and an attending gastroenterologist at Kaiser Permanente San Francisco Medical Center.
“As a gastroenterologist, I strongly believe we should emphasize the importance of colorectal cancer screening. And there’s many tests available, not just a colonoscopy, to help reduce your chances of developing colorectal cancer and even dying from colorectal cancer,” said Dr. Lee.
Many patients prefer a test that’s more convenient, that doesn’t require them to take time out of their busy schedules. “We must educate our patients that there are some noninvasive screening options that are helpful, and to be able to share with them some of the benefits, but also some of the drawbacks compared to colonoscopy and allow them to have a choice,” he advised.
He is a recipient of the AGA Research Scholar Award, and has in turn supported other researchers by contributing to the AGA Research Foundation. In 2012, Dr. Lee received a grant from the Sylvia Allison Kaplan Clinical Research Fund to fund a study on long-term colorectal cancer risk in patients with normal colonoscopy results.
The findings, published in JAMA Internal Medicine, determined that 10 years after a negative colonoscopy, Kaiser Permanente members had a 46% lower risk of being diagnosed with CRC and were 88% less likely to die from disease compared with patients who didn’t undergo screening.
“Furthermore, the reduced risk of developing colorectal cancer, even dying from it, persisted for more than 12 years after the examination compared with an unscreened population,” said Dr. Lee. “I firmly believe our study really supports the ten-year screening interval after a normal colonoscopy, as currently recommended by our guidelines.”
In an interview, he discussed his research efforts to find the best detection regimens for CRC, and the mentors who guided his career path as a GI scientist.
Q: Why did you choose GI?
During medical school I was fortunate to work in the lab of Dr. John M. Carethers at UC San Diego. He introduced me to GI and inspired me to choose GI as a career. His mentorship was invaluable because he not only solidified my interest in GI, but also inspired me to become a physician scientist, focusing on colorectal cancer prevention and control. His amazing mentorship drew me to this field.
Q: One of your clinical focus areas is hereditary gastrointestinal cancer syndromes. How did you become interested in this area of GI medicine?
My interest in hereditary GI cancer syndromes stemmed from my work as a medical student in Dr. Carethers’ lab. One of my research projects was looking at certain gene mutations among patients with hereditary GI cancer syndromes, specifically, familial hamartomatous polyposis syndrome. It was through these research projects and seeing how these genetic mutations impacted their risk of developing colorectal cancer, inspired me to care for patients with hereditary GI cancer syndromes.
Q: Have you been doing any research on the reasons why more young people are getting colon cancer?
We recently published work looking at the potential factors that may be driving the rising rates of early onset colorectal cancer. One hypothesis that’s been floating around is antibiotic exposure in early adulthood or childhood because of its effect on the microbiome. Using our large database at Kaiser Permanente Northern California, we did not find an association between oral antibiotic use during early adulthood and the risk of early-onset colorectal cancer.
You have the usual suspects like obesity and diabetes, but it’s not explaining all that risk. While familial colorectal cancer syndromes contribute to a small proportion of early-onset colorectal, these syndromes are not increasing across generations. I really do feel it’s something in the diet or how foods are processed and environmental factors that’s driving some of the risk of early onset colorectal cancer and this should be explored further.
Q: In 2018, you issued a landmark study which found an association between a 10-year follow-up after negative colonoscopy and reduced risk of disease and mortality. Has there been any updates to these findings over the last 6 years?
We recently saw a study in JAMA Oncology of a Swedish cohort that showed a negative colonoscopy result was associated with a reduced risk of developing and even dying from colorectal cancer 15 years from that examination, compared to the general population of Sweden. I think there’s some things that we need to be cautious about regarding that study. We have to think about the comparison group that they used and the lack of information regarding the indication of the colonoscopy and the quality of the examination. So, it remains uncertain whether future guidelines are going to stretch out that 10-year interval to 15 years.
Q: What other CRC studies are you working on now?
We have several studies that we are working on right now. One is called the PREVENT CRC study, which is looking at whether a polygenic risk score can improve risk stratification following adenoma removal for colorectal cancer prevention and tailoring post-polypectomy surveillance. This is a large observational cohort study that we have teamed up with the Fred Hutchinson Cancer Center, Erasmus University, and Kaiser Permanente Northwest to answer this important question that may have implications for personalized medicine.
Then there’s the COOP study, funded by the Patient-Centered Outcomes Research Institute. This is looking at the best surveillance test to use among older adults 65 years and older with a history of polyps. The trial is randomizing them to either getting a colonoscopy for surveillance or annual fecal immunochemical test (FIT) for surveillance. This is to see which test is best for detecting colorectal cancer among older adults with a history of polyps.
Q: Do you think FIT tests could eventually replace colonoscopy, given that it’s less invasive?
Although FIT and other stool-based tests are less invasive and have been shown to have high accuracy for detecting colorectal cancer, I personally do not think they are going to replace colonoscopy as the most popular screening modality in the United States. Colonoscopy remains the gold standard for detecting and removing precancerous polyps and has the highest accuracy for detecting colorectal cancer.
Q: Besides Dr. Carethers, what teacher or mentor had the greatest impact on you?
Clinically it’s been Dr. Jonathan Terdiman from UCSF, who taught me everything I know about clinical GI, and the art of colonoscopy. In addition, Douglas A. Corley, MD, PhD, the Permanente Medical Group’s chief research officer, has made the greatest impact on my research career. He’s really taught me how to rigorously design a research study to answer important clinically relevant questions, and has given me the skill set to write NIH grants. I would not be here without these mentors who are truly giants in the field of GI.
Q: When you’re not being a GI, how do you spend your free weekend afternoons? Are you still a “Cal Bears” fan at your alma mater, UC Berkeley?
I spend a lot of time taking my kids to their activities on the weekends. I just took my son to a Cal Bears Game Day, which was hosted by ESPN at Berkeley.
It was an incredible experience hearing sports analyst Pat McAfee lead all the Cal chants, seeing Nick Saban from the University of Alabama take off his red tie and replace it with a Cal Bears tie, and watching a Cal student win a hundred thousand dollars by kicking a football through the goal posts wearing checkered vans.
Lightning Round
Texting or talking?
Text
Favorite breakfast?
Taiwanese breakfast
Place you most want to travel to?
Japan
Favorite junk food?
Trader Joe’s chili lime chips
Favorite season?
Springtime, baseball season
Favorite ice cream flavor?
Mint chocolate chip
How many cups of coffee do you drink per day?
2-3
Last movie you watched?
Oppenheimer
Best place you ever went on vacation?
Hawaii
If you weren’t a gastroenterologist, what would you be?
Barber
Best Halloween costume you ever wore?
SpongeBob SquarePants
Favorite sport?
Tennis
What song do you have to sing along with when you hear it?
Any classic 80s song
Introvert or extrovert?
Introvert

Impact of a Museum-Based Retreat on the Clinical Skills and Well-Being of Dermatology Residents and Faculty
Impact of a Museum-Based Retreat on the Clinical Skills and Well-Being of Dermatology Residents and Faculty
Prior research has demonstrated that museum-based programming decreases resident burnout and depersonalization.1 A partnership between the Museum of Fine Arts Boston and the Harvard Combined Dermatology Residency Program was well received by residents and resulted in improvement of their observational skills.2 The impact of museum-based programming on the clinical practice skills and well-being of Duke dermatology residents and faculty has not been previously assessed.
In this study, our objective was to evaluate the impact of a 3-part museum-based arts retreat on arts appreciation, clinical practice skills, and well-being among dermatology resident and faculty participants. Surveys administered before and after the retreat were used to assess the value that participants attributed to the arts in various areas of clinical practice.
Methods
A 3-part museum-based retreat held on February 7, 2024, was developed with a Nasher Museum of Art (Durham, North Carolina) curator (E.R.). Part 1 was a personal response tour in which 15 residents and 3 faculty members were given individualized prompts and asked to identify an art piece in the museum that encapsulated their response; they then were asked to explain to the group why they chose that particular piece. Participants were given 10 minutes to explore the museum galleries to choose their piece, followed by 15 minutes to share their selected work in groups of 3 to 4.
Part 2 encompassed visual-thinking strategies, a research-based method that uses art to teach visual literacy, thinking, and communication skills.2 Using this method, facilitators follow a specific protocol to guide participants in the exploration of an art piece through sharing observations and interpretations.4 Participants were divided into 2 groups led by trained museum educators (including E.R.) to analyze and ascribe meaning to a chosen art piece. Three questions were asked: What’s going on in this picture? What do you see that makes you say that? What else can we find?
Part 3 involved back-to-back drawing, in which participants were paired up and tasked with recreating an art piece in the museum based solely on their partner’s verbal description. In each pair, both participants took turns as the describer and the drawer.
After each part of the retreat, 5 to 10 minutes were dedicated to debriefing in small groups about how each activity may connect to the role of a clinician. A total of 15 participants completed pre- and post-retreat surveys to assess the value they attributed to the arts and identify in which aspects of clinical practice they believe the arts play a role.
Results
Seventy-three percent of participants (11/15) found the museum-based retreat “extremely useful” or “very useful.” There was a 20% increase in those who attributed at least moderate value to the arts as a clinician after compared to before the retreat (13/15 [87%] vs 8/15 [53%]), and 100% of the participants desired to participate in future arts-based programming. Following the retreat, a greater percentage of participants believed the arts have a role in the following aspects of clinical practice: education, observation, listening, communication, empathy, compassion, forming connections, cultural sensitivity, tolerance for ambiguity, reflection, mindfulness, stress reduction, preventing burnout, bias prevention, mental wellness, spiritual wellness, and physical wellness (eTable). Qualitative feedback compiled from the participants’ responses to survey questions following the retreat about their thoughts on each activity and overall feedback was used to create a word cloud (eFigure).
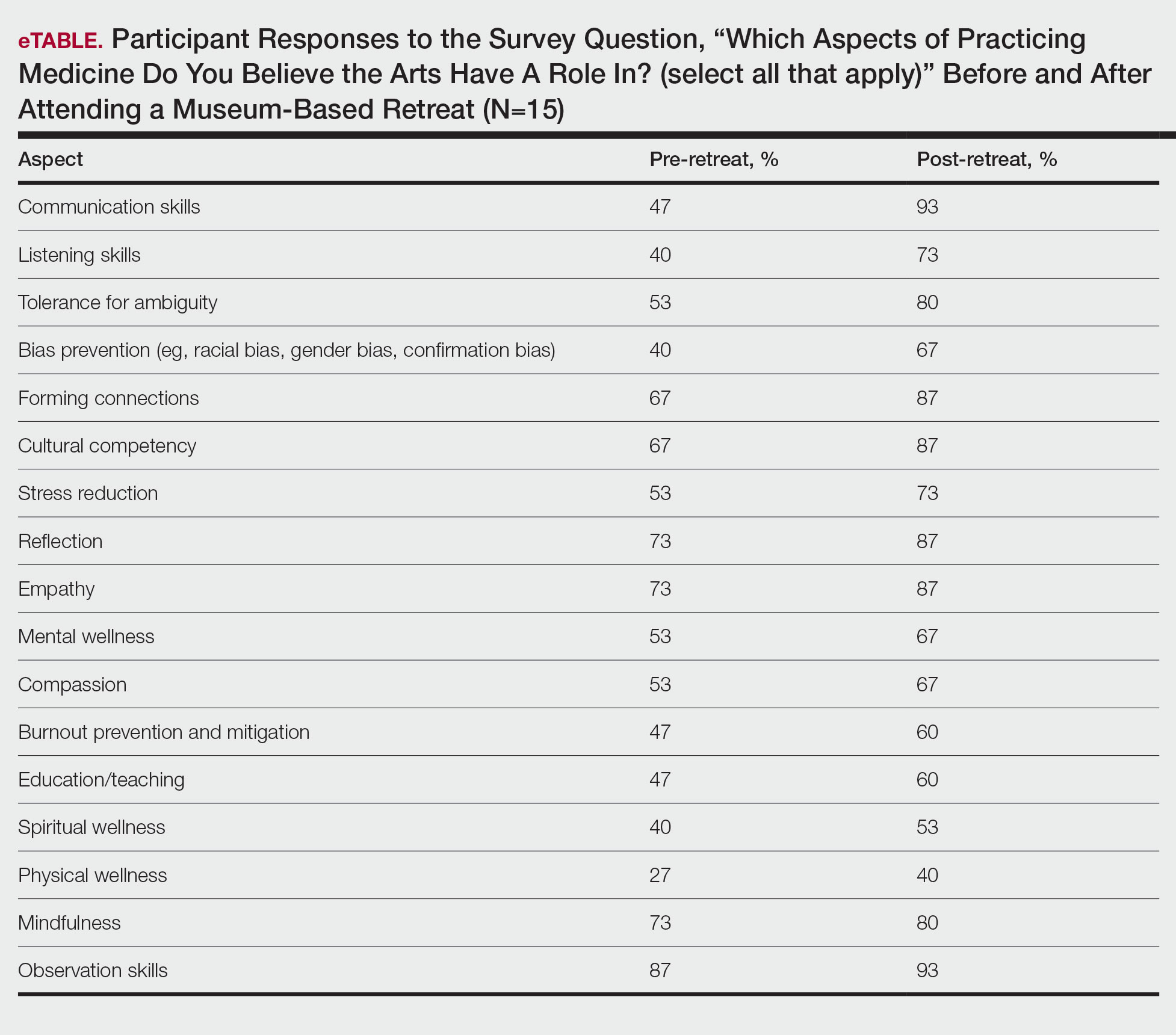

Comment
The importance of arts and humanities integration into medical education previously has been described.5 Our survey results suggest that museum-based programming increases dermatology resident and faculty appreciation for the arts and encourages participation in future arts-based programming. Our results also demonstrate that arts-based programming positively impacts important resident competencies in the practice of medicine including tolerance for ambiguity, bias prevention, and cultural competency, and that the incorporation of arts-based programming can enhance residents’ well-being (physical, mental, and spiritual) as well as their ability to be better clinicians by addressing skills in communication, listening, and observation. The structure of our 3-part museum-based retreat offers practical implementation strategies for integrating the humanities into dermatology residency curricula and easily can be modified to meet the needs of different dermatology residency programs.
Orr AR, Moghbeli N, Swain A, et al. The Fostering Resilience through Art in Medical Education (FRAME) workshop: a partnership with the Philadelphia Museum of Art. Adv Med Educ Pract. 2019;10:361-369. doi:10.2147/AMEP.S194575
Zimmermann C, Huang JT, Buzney EA. Refining the eye: dermatology and visual literacy. J Museum Ed. 2016;41:116-122.
Yenawine P. Visual Thinking Strategies: Using Art to Deepen Learning Across School Disciplines. Harvard Education Press; 2013.
Hailey D, Miller A, Yenawine P. Understanding visual literacy: the visual thinking strategies approach. In: Baylen DM, D’Alba A. Essentials of Teaching and Integrating Visual and Media Literacy: Visualizing Learning. Springer Cham; 2015:49-73. doi:10.1007/978-3-319-05837-5
Howley L, Gaufberg E, King BE. The Fundamental Role of the Arts and Humanities in Medical Education. Association of American Medical Colleges; 2020. Accessed December 18, 2025. https://store.aamc.org/the-fundamental-role-of-the-arts-and-humanities-in-medical-education.html
Prior research has demonstrated that museum-based programming decreases resident burnout and depersonalization.1 A partnership between the Museum of Fine Arts Boston and the Harvard Combined Dermatology Residency Program was well received by residents and resulted in improvement of their observational skills.2 The impact of museum-based programming on the clinical practice skills and well-being of Duke dermatology residents and faculty has not been previously assessed.
In this study, our objective was to evaluate the impact of a 3-part museum-based arts retreat on arts appreciation, clinical practice skills, and well-being among dermatology resident and faculty participants. Surveys administered before and after the retreat were used to assess the value that participants attributed to the arts in various areas of clinical practice.
Methods
A 3-part museum-based retreat held on February 7, 2024, was developed with a Nasher Museum of Art (Durham, North Carolina) curator (E.R.). Part 1 was a personal response tour in which 15 residents and 3 faculty members were given individualized prompts and asked to identify an art piece in the museum that encapsulated their response; they then were asked to explain to the group why they chose that particular piece. Participants were given 10 minutes to explore the museum galleries to choose their piece, followed by 15 minutes to share their selected work in groups of 3 to 4.
Part 2 encompassed visual-thinking strategies, a research-based method that uses art to teach visual literacy, thinking, and communication skills.2 Using this method, facilitators follow a specific protocol to guide participants in the exploration of an art piece through sharing observations and interpretations.4 Participants were divided into 2 groups led by trained museum educators (including E.R.) to analyze and ascribe meaning to a chosen art piece. Three questions were asked: What’s going on in this picture? What do you see that makes you say that? What else can we find?
Part 3 involved back-to-back drawing, in which participants were paired up and tasked with recreating an art piece in the museum based solely on their partner’s verbal description. In each pair, both participants took turns as the describer and the drawer.
After each part of the retreat, 5 to 10 minutes were dedicated to debriefing in small groups about how each activity may connect to the role of a clinician. A total of 15 participants completed pre- and post-retreat surveys to assess the value they attributed to the arts and identify in which aspects of clinical practice they believe the arts play a role.
Results
Seventy-three percent of participants (11/15) found the museum-based retreat “extremely useful” or “very useful.” There was a 20% increase in those who attributed at least moderate value to the arts as a clinician after compared to before the retreat (13/15 [87%] vs 8/15 [53%]), and 100% of the participants desired to participate in future arts-based programming. Following the retreat, a greater percentage of participants believed the arts have a role in the following aspects of clinical practice: education, observation, listening, communication, empathy, compassion, forming connections, cultural sensitivity, tolerance for ambiguity, reflection, mindfulness, stress reduction, preventing burnout, bias prevention, mental wellness, spiritual wellness, and physical wellness (eTable). Qualitative feedback compiled from the participants’ responses to survey questions following the retreat about their thoughts on each activity and overall feedback was used to create a word cloud (eFigure).


Comment
The importance of arts and humanities integration into medical education previously has been described.5 Our survey results suggest that museum-based programming increases dermatology resident and faculty appreciation for the arts and encourages participation in future arts-based programming. Our results also demonstrate that arts-based programming positively impacts important resident competencies in the practice of medicine including tolerance for ambiguity, bias prevention, and cultural competency, and that the incorporation of arts-based programming can enhance residents’ well-being (physical, mental, and spiritual) as well as their ability to be better clinicians by addressing skills in communication, listening, and observation. The structure of our 3-part museum-based retreat offers practical implementation strategies for integrating the humanities into dermatology residency curricula and easily can be modified to meet the needs of different dermatology residency programs.
Prior research has demonstrated that museum-based programming decreases resident burnout and depersonalization.1 A partnership between the Museum of Fine Arts Boston and the Harvard Combined Dermatology Residency Program was well received by residents and resulted in improvement of their observational skills.2 The impact of museum-based programming on the clinical practice skills and well-being of Duke dermatology residents and faculty has not been previously assessed.
In this study, our objective was to evaluate the impact of a 3-part museum-based arts retreat on arts appreciation, clinical practice skills, and well-being among dermatology resident and faculty participants. Surveys administered before and after the retreat were used to assess the value that participants attributed to the arts in various areas of clinical practice.
Methods
A 3-part museum-based retreat held on February 7, 2024, was developed with a Nasher Museum of Art (Durham, North Carolina) curator (E.R.). Part 1 was a personal response tour in which 15 residents and 3 faculty members were given individualized prompts and asked to identify an art piece in the museum that encapsulated their response; they then were asked to explain to the group why they chose that particular piece. Participants were given 10 minutes to explore the museum galleries to choose their piece, followed by 15 minutes to share their selected work in groups of 3 to 4.
Part 2 encompassed visual-thinking strategies, a research-based method that uses art to teach visual literacy, thinking, and communication skills.2 Using this method, facilitators follow a specific protocol to guide participants in the exploration of an art piece through sharing observations and interpretations.4 Participants were divided into 2 groups led by trained museum educators (including E.R.) to analyze and ascribe meaning to a chosen art piece. Three questions were asked: What’s going on in this picture? What do you see that makes you say that? What else can we find?
Part 3 involved back-to-back drawing, in which participants were paired up and tasked with recreating an art piece in the museum based solely on their partner’s verbal description. In each pair, both participants took turns as the describer and the drawer.
After each part of the retreat, 5 to 10 minutes were dedicated to debriefing in small groups about how each activity may connect to the role of a clinician. A total of 15 participants completed pre- and post-retreat surveys to assess the value they attributed to the arts and identify in which aspects of clinical practice they believe the arts play a role.
Results
Seventy-three percent of participants (11/15) found the museum-based retreat “extremely useful” or “very useful.” There was a 20% increase in those who attributed at least moderate value to the arts as a clinician after compared to before the retreat (13/15 [87%] vs 8/15 [53%]), and 100% of the participants desired to participate in future arts-based programming. Following the retreat, a greater percentage of participants believed the arts have a role in the following aspects of clinical practice: education, observation, listening, communication, empathy, compassion, forming connections, cultural sensitivity, tolerance for ambiguity, reflection, mindfulness, stress reduction, preventing burnout, bias prevention, mental wellness, spiritual wellness, and physical wellness (eTable). Qualitative feedback compiled from the participants’ responses to survey questions following the retreat about their thoughts on each activity and overall feedback was used to create a word cloud (eFigure).


Comment
The importance of arts and humanities integration into medical education previously has been described.5 Our survey results suggest that museum-based programming increases dermatology resident and faculty appreciation for the arts and encourages participation in future arts-based programming. Our results also demonstrate that arts-based programming positively impacts important resident competencies in the practice of medicine including tolerance for ambiguity, bias prevention, and cultural competency, and that the incorporation of arts-based programming can enhance residents’ well-being (physical, mental, and spiritual) as well as their ability to be better clinicians by addressing skills in communication, listening, and observation. The structure of our 3-part museum-based retreat offers practical implementation strategies for integrating the humanities into dermatology residency curricula and easily can be modified to meet the needs of different dermatology residency programs.
Orr AR, Moghbeli N, Swain A, et al. The Fostering Resilience through Art in Medical Education (FRAME) workshop: a partnership with the Philadelphia Museum of Art. Adv Med Educ Pract. 2019;10:361-369. doi:10.2147/AMEP.S194575
Zimmermann C, Huang JT, Buzney EA. Refining the eye: dermatology and visual literacy. J Museum Ed. 2016;41:116-122.
Yenawine P. Visual Thinking Strategies: Using Art to Deepen Learning Across School Disciplines. Harvard Education Press; 2013.
Hailey D, Miller A, Yenawine P. Understanding visual literacy: the visual thinking strategies approach. In: Baylen DM, D’Alba A. Essentials of Teaching and Integrating Visual and Media Literacy: Visualizing Learning. Springer Cham; 2015:49-73. doi:10.1007/978-3-319-05837-5
Howley L, Gaufberg E, King BE. The Fundamental Role of the Arts and Humanities in Medical Education. Association of American Medical Colleges; 2020. Accessed December 18, 2025. https://store.aamc.org/the-fundamental-role-of-the-arts-and-humanities-in-medical-education.html
Orr AR, Moghbeli N, Swain A, et al. The Fostering Resilience through Art in Medical Education (FRAME) workshop: a partnership with the Philadelphia Museum of Art. Adv Med Educ Pract. 2019;10:361-369. doi:10.2147/AMEP.S194575
Zimmermann C, Huang JT, Buzney EA. Refining the eye: dermatology and visual literacy. J Museum Ed. 2016;41:116-122.
Yenawine P. Visual Thinking Strategies: Using Art to Deepen Learning Across School Disciplines. Harvard Education Press; 2013.
Hailey D, Miller A, Yenawine P. Understanding visual literacy: the visual thinking strategies approach. In: Baylen DM, D’Alba A. Essentials of Teaching and Integrating Visual and Media Literacy: Visualizing Learning. Springer Cham; 2015:49-73. doi:10.1007/978-3-319-05837-5
Howley L, Gaufberg E, King BE. The Fundamental Role of the Arts and Humanities in Medical Education. Association of American Medical Colleges; 2020. Accessed December 18, 2025. https://store.aamc.org/the-fundamental-role-of-the-arts-and-humanities-in-medical-education.html
Impact of a Museum-Based Retreat on the Clinical Skills and Well-Being of Dermatology Residents and Faculty
Impact of a Museum-Based Retreat on the Clinical Skills and Well-Being of Dermatology Residents and Faculty
Practice Points
- Arts-based programming positively impacts resident competencies that are important to the practice of medicine.
- Incorporating arts-based programming in the dermatology residency curriculum can enhance resident well-being and the ability to be better clinicians.
Dark-Brown Macule on the Periumbilical Skin
Dark-Brown Macule on the Periumbilical Skin
THE DIAGNOSIS: Seborrheic Keratosis
Histopathology revealed epidermal hyperplasia and hyperkeratosis with no notation of atypical melanocytic activity (Figure). There were no Kamino bodies, junctional nesting, or cytologic atypia. Based on these features as well as the clinical and dermoscopic findings, a diagnosis of an inflamed seborrheic keratosis (SK) was made. No further treatment was required following the shave biopsy, and the patient was reassured regarding the benign nature of the lesion.
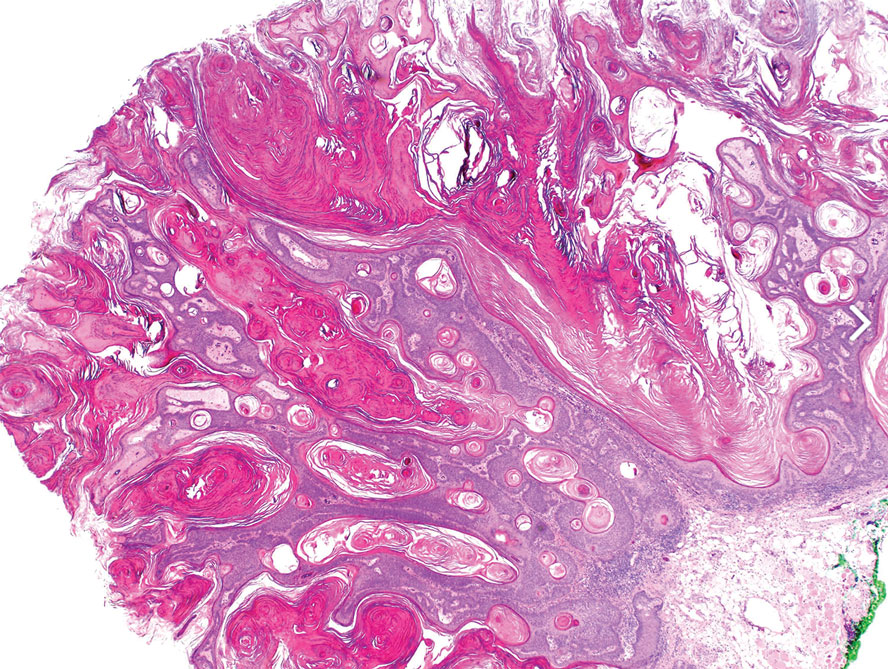
Seborrheic keratoses are benign epidermal growths that can manifest on any area of the skin except the palms and soles. They present clinically as tan, yellow, gray, brown, or black with a smooth, waxy, or verrucous surface. They range from 1 mm to several centimeters in diameter. Although SKs traditionally manifest more frequently in individuals with lighter skin tones, pigmented variants, such as dermatosis papulosa nigra, have been reported to occur more commonly and at younger ages in patients with skin of color.1
Dermoscopy of SK in patients with skin of color can present diagnostic challenges, as these lesions may display atypical pigmented patterns that overlap with melanocytic lesions, including Spitz nevi, particularly when starburstlike or globular structures are present.2 What sets inflamed SKs apart from other SKs is the lack of a heavily keratinized surface on both clinical and dermoscopic evaluation. Common histopathologic diagnostic criteria for Spitz nevi include Kamino bodies, uniform nuclear enlargement, and spindled or epithelioid nevus cells, which were not noted in our patient.3 Therefore, in presentations such as this, histopathology remains the gold standard for diagnosis.
The differential diagnosis in this case included benign nevus, dysplastic nevus, melanoma, and Spitz nevus. Benign nevi typically demonstrate uniform pigmentation and symmetric dermoscopic patterns. Dysplastic nevi may show architectural disorder and cytologic atypia but lack invasive features.3 Melanoma often exhibits asymmetry, atypical network patterns, and irregular pigmentation.4 Spitz nevi characteristically demonstrate large epithelioid or spindle cells with Kamino bodies on histopathology, which were absent in our patient.
- Greco MJ, Bhutta BS. Seborrheic keratosis. StatPearls [Internet]. StatPearls Publishing; 2025. Updated May 6, 2024. Accessed December 19, 2025. https://www.ncbi.nlm.nih.gov/books/NBK545285/
- Emanuel P, Cheng, H. Spitz naevus pathology. Accessed November 25, 2025. https://dermnetnz.org/topics/spitz-naevus-pathology.
- Wensley KE, Zito PM. Atypical mole. StatPearls [Internet]. StatPearls Publishing; 2025. Updated July 3, 2023. Accessed December 19, 2025. https://www.ncbi.nlm.nih.gov/books/NBK560606/
- Valenzuela FI, Hohnadel M. Dermatoscopic characteristics of melanoma versus benign lesions and nonmelanoma cancers. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 10, 2024. Accessed December 19, 2025. https://www.ncbi.nlm .nih.gov/books/NBK606113/
THE DIAGNOSIS: Seborrheic Keratosis
Histopathology revealed epidermal hyperplasia and hyperkeratosis with no notation of atypical melanocytic activity (Figure). There were no Kamino bodies, junctional nesting, or cytologic atypia. Based on these features as well as the clinical and dermoscopic findings, a diagnosis of an inflamed seborrheic keratosis (SK) was made. No further treatment was required following the shave biopsy, and the patient was reassured regarding the benign nature of the lesion.

Seborrheic keratoses are benign epidermal growths that can manifest on any area of the skin except the palms and soles. They present clinically as tan, yellow, gray, brown, or black with a smooth, waxy, or verrucous surface. They range from 1 mm to several centimeters in diameter. Although SKs traditionally manifest more frequently in individuals with lighter skin tones, pigmented variants, such as dermatosis papulosa nigra, have been reported to occur more commonly and at younger ages in patients with skin of color.1
Dermoscopy of SK in patients with skin of color can present diagnostic challenges, as these lesions may display atypical pigmented patterns that overlap with melanocytic lesions, including Spitz nevi, particularly when starburstlike or globular structures are present.2 What sets inflamed SKs apart from other SKs is the lack of a heavily keratinized surface on both clinical and dermoscopic evaluation. Common histopathologic diagnostic criteria for Spitz nevi include Kamino bodies, uniform nuclear enlargement, and spindled or epithelioid nevus cells, which were not noted in our patient.3 Therefore, in presentations such as this, histopathology remains the gold standard for diagnosis.
The differential diagnosis in this case included benign nevus, dysplastic nevus, melanoma, and Spitz nevus. Benign nevi typically demonstrate uniform pigmentation and symmetric dermoscopic patterns. Dysplastic nevi may show architectural disorder and cytologic atypia but lack invasive features.3 Melanoma often exhibits asymmetry, atypical network patterns, and irregular pigmentation.4 Spitz nevi characteristically demonstrate large epithelioid or spindle cells with Kamino bodies on histopathology, which were absent in our patient.
THE DIAGNOSIS: Seborrheic Keratosis
Histopathology revealed epidermal hyperplasia and hyperkeratosis with no notation of atypical melanocytic activity (Figure). There were no Kamino bodies, junctional nesting, or cytologic atypia. Based on these features as well as the clinical and dermoscopic findings, a diagnosis of an inflamed seborrheic keratosis (SK) was made. No further treatment was required following the shave biopsy, and the patient was reassured regarding the benign nature of the lesion.

Seborrheic keratoses are benign epidermal growths that can manifest on any area of the skin except the palms and soles. They present clinically as tan, yellow, gray, brown, or black with a smooth, waxy, or verrucous surface. They range from 1 mm to several centimeters in diameter. Although SKs traditionally manifest more frequently in individuals with lighter skin tones, pigmented variants, such as dermatosis papulosa nigra, have been reported to occur more commonly and at younger ages in patients with skin of color.1
Dermoscopy of SK in patients with skin of color can present diagnostic challenges, as these lesions may display atypical pigmented patterns that overlap with melanocytic lesions, including Spitz nevi, particularly when starburstlike or globular structures are present.2 What sets inflamed SKs apart from other SKs is the lack of a heavily keratinized surface on both clinical and dermoscopic evaluation. Common histopathologic diagnostic criteria for Spitz nevi include Kamino bodies, uniform nuclear enlargement, and spindled or epithelioid nevus cells, which were not noted in our patient.3 Therefore, in presentations such as this, histopathology remains the gold standard for diagnosis.
The differential diagnosis in this case included benign nevus, dysplastic nevus, melanoma, and Spitz nevus. Benign nevi typically demonstrate uniform pigmentation and symmetric dermoscopic patterns. Dysplastic nevi may show architectural disorder and cytologic atypia but lack invasive features.3 Melanoma often exhibits asymmetry, atypical network patterns, and irregular pigmentation.4 Spitz nevi characteristically demonstrate large epithelioid or spindle cells with Kamino bodies on histopathology, which were absent in our patient.
- Greco MJ, Bhutta BS. Seborrheic keratosis. StatPearls [Internet]. StatPearls Publishing; 2025. Updated May 6, 2024. Accessed December 19, 2025. https://www.ncbi.nlm.nih.gov/books/NBK545285/
- Emanuel P, Cheng, H. Spitz naevus pathology. Accessed November 25, 2025. https://dermnetnz.org/topics/spitz-naevus-pathology.
- Wensley KE, Zito PM. Atypical mole. StatPearls [Internet]. StatPearls Publishing; 2025. Updated July 3, 2023. Accessed December 19, 2025. https://www.ncbi.nlm.nih.gov/books/NBK560606/
- Valenzuela FI, Hohnadel M. Dermatoscopic characteristics of melanoma versus benign lesions and nonmelanoma cancers. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 10, 2024. Accessed December 19, 2025. https://www.ncbi.nlm .nih.gov/books/NBK606113/
- Greco MJ, Bhutta BS. Seborrheic keratosis. StatPearls [Internet]. StatPearls Publishing; 2025. Updated May 6, 2024. Accessed December 19, 2025. https://www.ncbi.nlm.nih.gov/books/NBK545285/
- Emanuel P, Cheng, H. Spitz naevus pathology. Accessed November 25, 2025. https://dermnetnz.org/topics/spitz-naevus-pathology.
- Wensley KE, Zito PM. Atypical mole. StatPearls [Internet]. StatPearls Publishing; 2025. Updated July 3, 2023. Accessed December 19, 2025. https://www.ncbi.nlm.nih.gov/books/NBK560606/
- Valenzuela FI, Hohnadel M. Dermatoscopic characteristics of melanoma versus benign lesions and nonmelanoma cancers. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 10, 2024. Accessed December 19, 2025. https://www.ncbi.nlm .nih.gov/books/NBK606113/
Dark-Brown Macule on the Periumbilical Skin
Dark-Brown Macule on the Periumbilical Skin
A 33-year-old man with moderately to deeply pigmented skin presented to the dermatology department with a dark-brown macule in the periumbilical area of more than 1 year’s duration. The patient was otherwise healthy and reported no personal or family history of atypical nevi, nonmelanoma skin cancer, or melanoma. Dermoscopy of the lesion showed a dark brown macule less than 2 mm in diameter with a starburst like pattern and a blue-hued border. A shave biopsy of the lesion was performed.
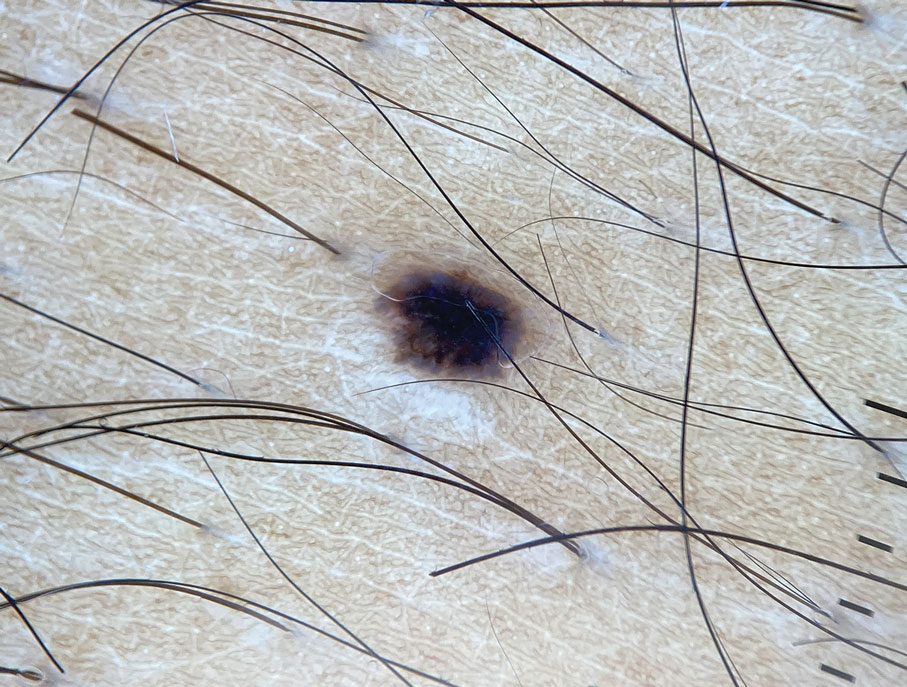
Illuminating the Role of Visible Light in Dermatology
Illuminating the Role of Visible Light in Dermatology
Visible light is part of the electromagnetic spectrum and is confined to a range of 400 to 700 nm. Visible light phototherapy can be delivered across various wavelengths within this spectrum, with most research focusing on blue light (BL)(400-500 nm) and red light (RL)(600-700 nm). Blue light commonly is used to treat acne as well as actinic keratosis and other inflammatory disorders,1,2 while RL largely targets signs of skin aging and fibrosis.2,3 Because of its shorter wavelength, the clinically meaningful skin penetration of BL reaches up to1 mm and is confined to the epidermis; in contrast, RL can access the dermal adnexa due to its penetration depth of more than 2 mm.4 Therapeutically, visible light can be utilized alone (eg, photobiomodulation [PBM]) or in combination with a photosensitizing agent (eg, photodynamic therapy [PDT]).5,6
Our laboratory’s prior research has contributed to a greater understanding of the safety profile of visible light at various wavelengths.1,3 Specifically, our work has shown that BL (417 nm [range, 412-422 nm]) and RL (633 nm [range, 627-639 nm]) demonstrated no evidence of DNA damage—via no formation of cyclobutane pyrimidine dimers and/or 6-4 photoproducts, the hallmark photolesions caused by UV exposure—in human dermal fibroblasts following visible light exposure at all fluences tested.1,3 This evidence reinforces the safety of visible light at clinically relevant wavelengths, supporting its integration into dermatologic practice. In this editorial, we highlight the key clinical applications of PBM and PDT and outline safety considerations for visible light-based therapies in dermatologic practice.
Photobiomodulation
Photobiomodulation is a noninvasive treatment in which low-level lasers or light-emitting diodes deliver photons from a nonionizing light source to endogenous photoreceptors, primarily cytochrome C oxidase.7-9 On the visible light spectrum, PBM primarily encompasses RL.7-9 Photoactivation leads to production of reactive oxygen species as well as mitochondrial alterations, with resulting modulation of cellular activity.7-9 Upregulation of cellular activity generally occurs at lower fluences (ie, energy delivered per unit area) of light, whereas higher fluences cause downregulation of cellular activity.5
Recent consensus guidelines, established with expert colleagues, define additional key parameters that are crucial to optimizing PBM treatment, including distance from the light source, area of the light beam, wavelength, length of treatment time, and number of treatments.5 Understanding the effects of different parameter combinations is essential for clinicians to select the best treatment regimen for each patient. Our laboratory has conducted National Institutes of Health–funded phase 1 and phase 2 clinical trials to determine the safety and efficacy of red-light PBM.10-13 Additionally, we completed several pilot phase 2 clinical studies with commercially available light-emitting diode face masks using PBM technology, which demonstrated a favorable safety profile and high patient satisfaction across multiple self-reported measures.14,15 These findings highlight PBM as a reliable and well-tolerated therapeutic approach that can be administered in clinical settings or by patients at home.
Adverse effects of PBM therapy generally are mild and transient, most commonly manifesting as slight irritation and erythema.5 Overall, PBM is widely regarded as safe with a favorable and nontoxic profile across treatment settings. Growing evidence supports the role of PBM in managing wound healing, acne, alopecia, and skin aging, among other dermatologic concerns.8
Photodynamic Therapy
Photodynamic therapy is a noninvasive procedure during which a photosensitizer—typically 5-aminolevulinic acid (5-ALA) or a derivative, methyl aminolevulinate—reacts with a light source and oxygen, resulting in reactive oxygen species.6,16 This reaction ultimately triggers targeted cellular destruction of the intended lesional skin but with negligible effects on adjacent nonlesional tissue.6 The efficacy of PDT is determined by several parameters, including composition and concentration of the photosensitizer, photosensitizer incubation temperature, and incubation time with the photosensitizer. Methyl aminolevulinate is a lipophilic molecule and may promote greater skin penetration and cellular uptake than 5-ALA, which is a hydrophilic molecule.6
Our research further demonstrated that apoptosis increases in a dose- and temperature-dependent manner following 5-ALA exposure, both in cutaneous and mucosal squamous cell carcinoma cells and in human dermal fibroblasts.17,18 Our mechanistic insights have clinical relevance, as evidenced by an independent pilot study demonstrating that temperature-modulated PDT significantly improved actinic keratosis lesion clearance rates (P<.0001).19 Additionally, we determined that even short periods of incubation with 5-ALA (ie, 15-30 minutes) result in statistically significant increases in apoptosis (P<.05).20 Thus, these findings highlight that the choice of photosensitizing agent and the administration parameters are critical in determining PDT efficacy as well as the need to optimize clinical protocols.
Photodynamic therapy also has demonstrated general clinical and genotoxic safety, with the most common potential adverse events limited to temporary inflammation, erythema, and discomfort.21 A study in murine skin and human keratinocytes revealed that 5-ALA PDT had a photoprotective effect against previous irradiation with UVB (a known inducer of DNA damage) via removal of cyclobutane pyrimidine dimers.22 Thus, PDT has been recognized as a safe and effective therapeutic modality with broad applications in dermatology, including treatment of actinic keratosis and nonmelanoma skin cancers.16
Clinical Safety, Photoprotection, and Precautions
While visible light has shown substantial therapeutic potential in dermatology, there are several safety measures and precautions to be aware of. Visible light constitutes approximately 44% of the solar output; therefore, precautions against both UV and visible light are recommended for the general population.23 Cumulative exposure to visible light has been shown to trigger melanogenesis, resulting in persistent erythema, hyperpigmentation, and uneven skin tones across all Fitzpatrick skin types.24 Individuals with skin of color are more photosensitive to visible light due to increased baseline melanin levels.24 Similarly, patients with pigmentary conditions such as melasma and postinflammatory hyperpigmentation may experience worsening of their dermatologic symptoms due to underlying visible light photosensitivity.25
Patients undergoing PBM or PDT could benefit from visible light protection. The primary form of photoprotection against visible light is tinted sunscreen, which contains iron oxides and titanium dioxide.26 Iron (III) oxide is capable of blocking nearly all visible light damage.26 Use of physical barriers such as wavelength-specific sunglasses and wide-brimmed hats also is important for preventing photodamage from visible light.26
Final Thoughts
Visible light has a role in the treatment of a variety of skin conditions, including actinic keratosis, nonmelanoma skin cancers, acne, wound healing, skin fibrosis, and photodamage. Photobiomodulation and PDT represent 2 noninvasive phototherapeutic options that utilize visible light to enact cellular changes necessary to improve skin health. Integrating visible light phototherapy into standard clinical practice is important for enhancing patient outcomes. Clinicians should remain mindful of the rare pigmentary risks associated with visible light therapy devices. Future research should prioritize optimization of standardized protocols and expansion of clinical indications for visible light phototherapy.
- Kabakova M, Wang J, Stolyar J, et al. Visible blue light does not induce DNA damage in human dermal fibroblasts. J Biophotonics. 2025;18:E202400510. doi:10.1002/jbio.202400510
- Wan MT, Lin JY. Current evidence and applications of photodynamic therapy in dermatology. Clin Cosmet Investig Dermatol. 2014;7:145-163. doi:10.2147/CCID.S35334
- Wang JY, Austin E, Jagdeo J. Visible red light does not induce DNA damage in human dermal fibroblasts. J Biophotonics. 2022;15:E202200023. doi:10.1002/jbio.202200023
- Opel DR, Hagstrom E, Pace AK, et al. Light-emitting diodes: a brief review and clinical experience. J Clin Aesthet Dermatol. 2015;8:36-44.
- Maghfour J, Mineroff J, Ozog DM, et al. Evidence-based consensus on the clinical application of photobiomodulation. J Am Acad Dermatol. 2025;93:429-443. doi:10.1016/j.jaad.2025.04.031
- Ozog DM, Rkein AM, Fabi SG, et al. Photodynamic therapy: a clinical consensus guide. Dermatol Surg. 2016;42:804-827. doi:10.1097/DSS.0000000000000800
- Maghfour J, Ozog DM, Mineroff J, et al. Photobiomodulation CME part I: overview and mechanism of action. J Am Acad Dermatol. 2024;91:793-802. doi:10.1016/j.jaad.2023.10.073
- Mineroff J, Maghfour J, Ozog DM, et al. Photobiomodulation CME part II: clinical applications in dermatology. J Am Acad Dermatol. 2024;91:805-815. doi:10.1016/j.jaad.2023.10.074
- Mamalis A, Siegel D, Jagdeo J. Visible red light emitting diode photobiomodulation for skin fibrosis: key molecular pathways. Curr Dermatol Rep. 2016;5:121-128. doi:10.1007/s13671-016-0141-x
- Kurtti A, Nguyen JK, Weedon J, et al. Light emitting diode-red light for reduction of post-surgical scarring: results from a dose-ranging, split-face, randomized controlled trial. J Biophotonics. 2021;14:E202100073. doi:10.1002/jbio.202100073
- Nguyen JK, Weedon J, Jakus J, et al. A dose-ranging, parallel group, split-face, single-blind phase II study of light emitting diode-red light (LED-RL) for skin scarring prevention: study protocol for a randomized controlled trial. Trials. 2019;20:432. doi:10.1186/s13063-019-3546-6
- Ho D, Kraeva E, Wun T, et al. A single-blind, dose escalation, phase I study of high-fluence light-emitting diode-red light (LED-RL) on human skin: study protocol for a randomized controlled trial. Trials. 2016;17:385. doi:10.1186/s13063-016-1518-7
- Wang EB, Kaur R, Nguyen J, et al. A single-blind, dose-escalation, phase I study of high-fluence light-emitting diode-red light on Caucasian non-Hispanic skin: study protocol for a randomized controlled trial. Trials. 2019;20:177. doi:10.1186/s13063-019-3278-7
- Wang JY, Kabakova M, Patel P, et al. Outstanding user reported satisfaction for light emitting diodes under-eye rejuvenation. Arch Dermatol Res. 2024;316:511. doi:10.1007/s00403-024-03254-z
- Mineroff J, Austin E, Feit E, et al. Male facial rejuvenation using a combination 633, 830, and 1072 nm LED face mask. Arch Dermatol Res. 2023;315:2605-2611. doi:10.1007/s00403-023-02663-w
- Wang JY, Zeitouni N, Austin E, et al. Photodynamic therapy: clinical applications in dermatology. J Am Acad Dermatol. Published online February 20, 2025. doi:10.1016/j.jaad.2024.12.050
- Austin E, Koo E, Jagdeo J. Thermal photodynamic therapy increases apoptosis and reactive oxygen species generation in cutaneous and mucosal squamous cell carcinoma cells. Sci Rep. 2018;8:12599. doi:10.1038/s41598-018-30908-6
- Mamalis A, Koo E, Sckisel GD, et al. Temperature-dependent impact of thermal aminolaevulinic acid photodynamic therapy on apoptosis and reactive oxygen species generation in human dermal fibroblasts. Br J Dermatol. 2016;175:512-519. doi:10.1111/bjd.14509
- Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40:1094-1102. doi:10.1097/01.DSS.0000452662.69539.57
- Koo E, Austin E, Mamalis A, et al. Efficacy of ultra short sub-30 minute incubation of 5-aminolevulinic acid photodynamic therapy in vitro. Lasers Surg Med. 2017;49:592-598. doi:10.1002/lsm.22648
- Austin E, Wang JY, Ozog DM, et al. Photodynamic therapy: overview and mechanism of action. J Am Acad Dermatol. Published online February 20, 2025. doi:10.1016/j.jaad.2025.02.037
- Hua H, Cheng JW, Bu WB, et al. 5-aminolaevulinic acid-based photodynamic therapy inhibits ultraviolet B-induced skin photodamage. Int J Biol Sci. 2019;15:2100-2109. doi:10.7150/ijbs.31583
- Liebel F, Kaur S, Ruvolo E, et al. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J Invest Dermatol. 2012;132:1901-1907. doi:10.1038/jid.2011.476
- Austin E, Geisler AN, Nguyen J, et al. Visible light. part I: properties and cutaneous effects of visible light. J Am Acad Dermatol. 2021;84:1219-1231. doi:10.1016/j.jaad.2021.02.048
- Fatima S, Braunberger T, Mohammad TF, et al. The role of sunscreen in melasma and postinflammatory hyperpigmentation. Indian J Dermatol. 2020;65:5-10. doi:10.4103/ijd.IJD_295_18
- Geisler AN, Austin E, Nguyen J, et al. Visible light. part II: photoprotection against visible and ultraviolet light. J Am Acad Dermatol. 2021;84:1233-1244. doi:10.1016/j.jaad.2020.11.074
Visible light is part of the electromagnetic spectrum and is confined to a range of 400 to 700 nm. Visible light phototherapy can be delivered across various wavelengths within this spectrum, with most research focusing on blue light (BL)(400-500 nm) and red light (RL)(600-700 nm). Blue light commonly is used to treat acne as well as actinic keratosis and other inflammatory disorders,1,2 while RL largely targets signs of skin aging and fibrosis.2,3 Because of its shorter wavelength, the clinically meaningful skin penetration of BL reaches up to1 mm and is confined to the epidermis; in contrast, RL can access the dermal adnexa due to its penetration depth of more than 2 mm.4 Therapeutically, visible light can be utilized alone (eg, photobiomodulation [PBM]) or in combination with a photosensitizing agent (eg, photodynamic therapy [PDT]).5,6
Our laboratory’s prior research has contributed to a greater understanding of the safety profile of visible light at various wavelengths.1,3 Specifically, our work has shown that BL (417 nm [range, 412-422 nm]) and RL (633 nm [range, 627-639 nm]) demonstrated no evidence of DNA damage—via no formation of cyclobutane pyrimidine dimers and/or 6-4 photoproducts, the hallmark photolesions caused by UV exposure—in human dermal fibroblasts following visible light exposure at all fluences tested.1,3 This evidence reinforces the safety of visible light at clinically relevant wavelengths, supporting its integration into dermatologic practice. In this editorial, we highlight the key clinical applications of PBM and PDT and outline safety considerations for visible light-based therapies in dermatologic practice.
Photobiomodulation
Photobiomodulation is a noninvasive treatment in which low-level lasers or light-emitting diodes deliver photons from a nonionizing light source to endogenous photoreceptors, primarily cytochrome C oxidase.7-9 On the visible light spectrum, PBM primarily encompasses RL.7-9 Photoactivation leads to production of reactive oxygen species as well as mitochondrial alterations, with resulting modulation of cellular activity.7-9 Upregulation of cellular activity generally occurs at lower fluences (ie, energy delivered per unit area) of light, whereas higher fluences cause downregulation of cellular activity.5
Recent consensus guidelines, established with expert colleagues, define additional key parameters that are crucial to optimizing PBM treatment, including distance from the light source, area of the light beam, wavelength, length of treatment time, and number of treatments.5 Understanding the effects of different parameter combinations is essential for clinicians to select the best treatment regimen for each patient. Our laboratory has conducted National Institutes of Health–funded phase 1 and phase 2 clinical trials to determine the safety and efficacy of red-light PBM.10-13 Additionally, we completed several pilot phase 2 clinical studies with commercially available light-emitting diode face masks using PBM technology, which demonstrated a favorable safety profile and high patient satisfaction across multiple self-reported measures.14,15 These findings highlight PBM as a reliable and well-tolerated therapeutic approach that can be administered in clinical settings or by patients at home.
Adverse effects of PBM therapy generally are mild and transient, most commonly manifesting as slight irritation and erythema.5 Overall, PBM is widely regarded as safe with a favorable and nontoxic profile across treatment settings. Growing evidence supports the role of PBM in managing wound healing, acne, alopecia, and skin aging, among other dermatologic concerns.8
Photodynamic Therapy
Photodynamic therapy is a noninvasive procedure during which a photosensitizer—typically 5-aminolevulinic acid (5-ALA) or a derivative, methyl aminolevulinate—reacts with a light source and oxygen, resulting in reactive oxygen species.6,16 This reaction ultimately triggers targeted cellular destruction of the intended lesional skin but with negligible effects on adjacent nonlesional tissue.6 The efficacy of PDT is determined by several parameters, including composition and concentration of the photosensitizer, photosensitizer incubation temperature, and incubation time with the photosensitizer. Methyl aminolevulinate is a lipophilic molecule and may promote greater skin penetration and cellular uptake than 5-ALA, which is a hydrophilic molecule.6
Our research further demonstrated that apoptosis increases in a dose- and temperature-dependent manner following 5-ALA exposure, both in cutaneous and mucosal squamous cell carcinoma cells and in human dermal fibroblasts.17,18 Our mechanistic insights have clinical relevance, as evidenced by an independent pilot study demonstrating that temperature-modulated PDT significantly improved actinic keratosis lesion clearance rates (P<.0001).19 Additionally, we determined that even short periods of incubation with 5-ALA (ie, 15-30 minutes) result in statistically significant increases in apoptosis (P<.05).20 Thus, these findings highlight that the choice of photosensitizing agent and the administration parameters are critical in determining PDT efficacy as well as the need to optimize clinical protocols.
Photodynamic therapy also has demonstrated general clinical and genotoxic safety, with the most common potential adverse events limited to temporary inflammation, erythema, and discomfort.21 A study in murine skin and human keratinocytes revealed that 5-ALA PDT had a photoprotective effect against previous irradiation with UVB (a known inducer of DNA damage) via removal of cyclobutane pyrimidine dimers.22 Thus, PDT has been recognized as a safe and effective therapeutic modality with broad applications in dermatology, including treatment of actinic keratosis and nonmelanoma skin cancers.16
Clinical Safety, Photoprotection, and Precautions
While visible light has shown substantial therapeutic potential in dermatology, there are several safety measures and precautions to be aware of. Visible light constitutes approximately 44% of the solar output; therefore, precautions against both UV and visible light are recommended for the general population.23 Cumulative exposure to visible light has been shown to trigger melanogenesis, resulting in persistent erythema, hyperpigmentation, and uneven skin tones across all Fitzpatrick skin types.24 Individuals with skin of color are more photosensitive to visible light due to increased baseline melanin levels.24 Similarly, patients with pigmentary conditions such as melasma and postinflammatory hyperpigmentation may experience worsening of their dermatologic symptoms due to underlying visible light photosensitivity.25
Patients undergoing PBM or PDT could benefit from visible light protection. The primary form of photoprotection against visible light is tinted sunscreen, which contains iron oxides and titanium dioxide.26 Iron (III) oxide is capable of blocking nearly all visible light damage.26 Use of physical barriers such as wavelength-specific sunglasses and wide-brimmed hats also is important for preventing photodamage from visible light.26
Final Thoughts
Visible light has a role in the treatment of a variety of skin conditions, including actinic keratosis, nonmelanoma skin cancers, acne, wound healing, skin fibrosis, and photodamage. Photobiomodulation and PDT represent 2 noninvasive phototherapeutic options that utilize visible light to enact cellular changes necessary to improve skin health. Integrating visible light phototherapy into standard clinical practice is important for enhancing patient outcomes. Clinicians should remain mindful of the rare pigmentary risks associated with visible light therapy devices. Future research should prioritize optimization of standardized protocols and expansion of clinical indications for visible light phototherapy.
Visible light is part of the electromagnetic spectrum and is confined to a range of 400 to 700 nm. Visible light phototherapy can be delivered across various wavelengths within this spectrum, with most research focusing on blue light (BL)(400-500 nm) and red light (RL)(600-700 nm). Blue light commonly is used to treat acne as well as actinic keratosis and other inflammatory disorders,1,2 while RL largely targets signs of skin aging and fibrosis.2,3 Because of its shorter wavelength, the clinically meaningful skin penetration of BL reaches up to1 mm and is confined to the epidermis; in contrast, RL can access the dermal adnexa due to its penetration depth of more than 2 mm.4 Therapeutically, visible light can be utilized alone (eg, photobiomodulation [PBM]) or in combination with a photosensitizing agent (eg, photodynamic therapy [PDT]).5,6
Our laboratory’s prior research has contributed to a greater understanding of the safety profile of visible light at various wavelengths.1,3 Specifically, our work has shown that BL (417 nm [range, 412-422 nm]) and RL (633 nm [range, 627-639 nm]) demonstrated no evidence of DNA damage—via no formation of cyclobutane pyrimidine dimers and/or 6-4 photoproducts, the hallmark photolesions caused by UV exposure—in human dermal fibroblasts following visible light exposure at all fluences tested.1,3 This evidence reinforces the safety of visible light at clinically relevant wavelengths, supporting its integration into dermatologic practice. In this editorial, we highlight the key clinical applications of PBM and PDT and outline safety considerations for visible light-based therapies in dermatologic practice.
Photobiomodulation
Photobiomodulation is a noninvasive treatment in which low-level lasers or light-emitting diodes deliver photons from a nonionizing light source to endogenous photoreceptors, primarily cytochrome C oxidase.7-9 On the visible light spectrum, PBM primarily encompasses RL.7-9 Photoactivation leads to production of reactive oxygen species as well as mitochondrial alterations, with resulting modulation of cellular activity.7-9 Upregulation of cellular activity generally occurs at lower fluences (ie, energy delivered per unit area) of light, whereas higher fluences cause downregulation of cellular activity.5
Recent consensus guidelines, established with expert colleagues, define additional key parameters that are crucial to optimizing PBM treatment, including distance from the light source, area of the light beam, wavelength, length of treatment time, and number of treatments.5 Understanding the effects of different parameter combinations is essential for clinicians to select the best treatment regimen for each patient. Our laboratory has conducted National Institutes of Health–funded phase 1 and phase 2 clinical trials to determine the safety and efficacy of red-light PBM.10-13 Additionally, we completed several pilot phase 2 clinical studies with commercially available light-emitting diode face masks using PBM technology, which demonstrated a favorable safety profile and high patient satisfaction across multiple self-reported measures.14,15 These findings highlight PBM as a reliable and well-tolerated therapeutic approach that can be administered in clinical settings or by patients at home.
Adverse effects of PBM therapy generally are mild and transient, most commonly manifesting as slight irritation and erythema.5 Overall, PBM is widely regarded as safe with a favorable and nontoxic profile across treatment settings. Growing evidence supports the role of PBM in managing wound healing, acne, alopecia, and skin aging, among other dermatologic concerns.8
Photodynamic Therapy
Photodynamic therapy is a noninvasive procedure during which a photosensitizer—typically 5-aminolevulinic acid (5-ALA) or a derivative, methyl aminolevulinate—reacts with a light source and oxygen, resulting in reactive oxygen species.6,16 This reaction ultimately triggers targeted cellular destruction of the intended lesional skin but with negligible effects on adjacent nonlesional tissue.6 The efficacy of PDT is determined by several parameters, including composition and concentration of the photosensitizer, photosensitizer incubation temperature, and incubation time with the photosensitizer. Methyl aminolevulinate is a lipophilic molecule and may promote greater skin penetration and cellular uptake than 5-ALA, which is a hydrophilic molecule.6
Our research further demonstrated that apoptosis increases in a dose- and temperature-dependent manner following 5-ALA exposure, both in cutaneous and mucosal squamous cell carcinoma cells and in human dermal fibroblasts.17,18 Our mechanistic insights have clinical relevance, as evidenced by an independent pilot study demonstrating that temperature-modulated PDT significantly improved actinic keratosis lesion clearance rates (P<.0001).19 Additionally, we determined that even short periods of incubation with 5-ALA (ie, 15-30 minutes) result in statistically significant increases in apoptosis (P<.05).20 Thus, these findings highlight that the choice of photosensitizing agent and the administration parameters are critical in determining PDT efficacy as well as the need to optimize clinical protocols.
Photodynamic therapy also has demonstrated general clinical and genotoxic safety, with the most common potential adverse events limited to temporary inflammation, erythema, and discomfort.21 A study in murine skin and human keratinocytes revealed that 5-ALA PDT had a photoprotective effect against previous irradiation with UVB (a known inducer of DNA damage) via removal of cyclobutane pyrimidine dimers.22 Thus, PDT has been recognized as a safe and effective therapeutic modality with broad applications in dermatology, including treatment of actinic keratosis and nonmelanoma skin cancers.16
Clinical Safety, Photoprotection, and Precautions
While visible light has shown substantial therapeutic potential in dermatology, there are several safety measures and precautions to be aware of. Visible light constitutes approximately 44% of the solar output; therefore, precautions against both UV and visible light are recommended for the general population.23 Cumulative exposure to visible light has been shown to trigger melanogenesis, resulting in persistent erythema, hyperpigmentation, and uneven skin tones across all Fitzpatrick skin types.24 Individuals with skin of color are more photosensitive to visible light due to increased baseline melanin levels.24 Similarly, patients with pigmentary conditions such as melasma and postinflammatory hyperpigmentation may experience worsening of their dermatologic symptoms due to underlying visible light photosensitivity.25
Patients undergoing PBM or PDT could benefit from visible light protection. The primary form of photoprotection against visible light is tinted sunscreen, which contains iron oxides and titanium dioxide.26 Iron (III) oxide is capable of blocking nearly all visible light damage.26 Use of physical barriers such as wavelength-specific sunglasses and wide-brimmed hats also is important for preventing photodamage from visible light.26
Final Thoughts
Visible light has a role in the treatment of a variety of skin conditions, including actinic keratosis, nonmelanoma skin cancers, acne, wound healing, skin fibrosis, and photodamage. Photobiomodulation and PDT represent 2 noninvasive phototherapeutic options that utilize visible light to enact cellular changes necessary to improve skin health. Integrating visible light phototherapy into standard clinical practice is important for enhancing patient outcomes. Clinicians should remain mindful of the rare pigmentary risks associated with visible light therapy devices. Future research should prioritize optimization of standardized protocols and expansion of clinical indications for visible light phototherapy.
- Kabakova M, Wang J, Stolyar J, et al. Visible blue light does not induce DNA damage in human dermal fibroblasts. J Biophotonics. 2025;18:E202400510. doi:10.1002/jbio.202400510
- Wan MT, Lin JY. Current evidence and applications of photodynamic therapy in dermatology. Clin Cosmet Investig Dermatol. 2014;7:145-163. doi:10.2147/CCID.S35334
- Wang JY, Austin E, Jagdeo J. Visible red light does not induce DNA damage in human dermal fibroblasts. J Biophotonics. 2022;15:E202200023. doi:10.1002/jbio.202200023
- Opel DR, Hagstrom E, Pace AK, et al. Light-emitting diodes: a brief review and clinical experience. J Clin Aesthet Dermatol. 2015;8:36-44.
- Maghfour J, Mineroff J, Ozog DM, et al. Evidence-based consensus on the clinical application of photobiomodulation. J Am Acad Dermatol. 2025;93:429-443. doi:10.1016/j.jaad.2025.04.031
- Ozog DM, Rkein AM, Fabi SG, et al. Photodynamic therapy: a clinical consensus guide. Dermatol Surg. 2016;42:804-827. doi:10.1097/DSS.0000000000000800
- Maghfour J, Ozog DM, Mineroff J, et al. Photobiomodulation CME part I: overview and mechanism of action. J Am Acad Dermatol. 2024;91:793-802. doi:10.1016/j.jaad.2023.10.073
- Mineroff J, Maghfour J, Ozog DM, et al. Photobiomodulation CME part II: clinical applications in dermatology. J Am Acad Dermatol. 2024;91:805-815. doi:10.1016/j.jaad.2023.10.074
- Mamalis A, Siegel D, Jagdeo J. Visible red light emitting diode photobiomodulation for skin fibrosis: key molecular pathways. Curr Dermatol Rep. 2016;5:121-128. doi:10.1007/s13671-016-0141-x
- Kurtti A, Nguyen JK, Weedon J, et al. Light emitting diode-red light for reduction of post-surgical scarring: results from a dose-ranging, split-face, randomized controlled trial. J Biophotonics. 2021;14:E202100073. doi:10.1002/jbio.202100073
- Nguyen JK, Weedon J, Jakus J, et al. A dose-ranging, parallel group, split-face, single-blind phase II study of light emitting diode-red light (LED-RL) for skin scarring prevention: study protocol for a randomized controlled trial. Trials. 2019;20:432. doi:10.1186/s13063-019-3546-6
- Ho D, Kraeva E, Wun T, et al. A single-blind, dose escalation, phase I study of high-fluence light-emitting diode-red light (LED-RL) on human skin: study protocol for a randomized controlled trial. Trials. 2016;17:385. doi:10.1186/s13063-016-1518-7
- Wang EB, Kaur R, Nguyen J, et al. A single-blind, dose-escalation, phase I study of high-fluence light-emitting diode-red light on Caucasian non-Hispanic skin: study protocol for a randomized controlled trial. Trials. 2019;20:177. doi:10.1186/s13063-019-3278-7
- Wang JY, Kabakova M, Patel P, et al. Outstanding user reported satisfaction for light emitting diodes under-eye rejuvenation. Arch Dermatol Res. 2024;316:511. doi:10.1007/s00403-024-03254-z
- Mineroff J, Austin E, Feit E, et al. Male facial rejuvenation using a combination 633, 830, and 1072 nm LED face mask. Arch Dermatol Res. 2023;315:2605-2611. doi:10.1007/s00403-023-02663-w
- Wang JY, Zeitouni N, Austin E, et al. Photodynamic therapy: clinical applications in dermatology. J Am Acad Dermatol. Published online February 20, 2025. doi:10.1016/j.jaad.2024.12.050
- Austin E, Koo E, Jagdeo J. Thermal photodynamic therapy increases apoptosis and reactive oxygen species generation in cutaneous and mucosal squamous cell carcinoma cells. Sci Rep. 2018;8:12599. doi:10.1038/s41598-018-30908-6
- Mamalis A, Koo E, Sckisel GD, et al. Temperature-dependent impact of thermal aminolaevulinic acid photodynamic therapy on apoptosis and reactive oxygen species generation in human dermal fibroblasts. Br J Dermatol. 2016;175:512-519. doi:10.1111/bjd.14509
- Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40:1094-1102. doi:10.1097/01.DSS.0000452662.69539.57
- Koo E, Austin E, Mamalis A, et al. Efficacy of ultra short sub-30 minute incubation of 5-aminolevulinic acid photodynamic therapy in vitro. Lasers Surg Med. 2017;49:592-598. doi:10.1002/lsm.22648
- Austin E, Wang JY, Ozog DM, et al. Photodynamic therapy: overview and mechanism of action. J Am Acad Dermatol. Published online February 20, 2025. doi:10.1016/j.jaad.2025.02.037
- Hua H, Cheng JW, Bu WB, et al. 5-aminolaevulinic acid-based photodynamic therapy inhibits ultraviolet B-induced skin photodamage. Int J Biol Sci. 2019;15:2100-2109. doi:10.7150/ijbs.31583
- Liebel F, Kaur S, Ruvolo E, et al. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J Invest Dermatol. 2012;132:1901-1907. doi:10.1038/jid.2011.476
- Austin E, Geisler AN, Nguyen J, et al. Visible light. part I: properties and cutaneous effects of visible light. J Am Acad Dermatol. 2021;84:1219-1231. doi:10.1016/j.jaad.2021.02.048
- Fatima S, Braunberger T, Mohammad TF, et al. The role of sunscreen in melasma and postinflammatory hyperpigmentation. Indian J Dermatol. 2020;65:5-10. doi:10.4103/ijd.IJD_295_18
- Geisler AN, Austin E, Nguyen J, et al. Visible light. part II: photoprotection against visible and ultraviolet light. J Am Acad Dermatol. 2021;84:1233-1244. doi:10.1016/j.jaad.2020.11.074
- Kabakova M, Wang J, Stolyar J, et al. Visible blue light does not induce DNA damage in human dermal fibroblasts. J Biophotonics. 2025;18:E202400510. doi:10.1002/jbio.202400510
- Wan MT, Lin JY. Current evidence and applications of photodynamic therapy in dermatology. Clin Cosmet Investig Dermatol. 2014;7:145-163. doi:10.2147/CCID.S35334
- Wang JY, Austin E, Jagdeo J. Visible red light does not induce DNA damage in human dermal fibroblasts. J Biophotonics. 2022;15:E202200023. doi:10.1002/jbio.202200023
- Opel DR, Hagstrom E, Pace AK, et al. Light-emitting diodes: a brief review and clinical experience. J Clin Aesthet Dermatol. 2015;8:36-44.
- Maghfour J, Mineroff J, Ozog DM, et al. Evidence-based consensus on the clinical application of photobiomodulation. J Am Acad Dermatol. 2025;93:429-443. doi:10.1016/j.jaad.2025.04.031
- Ozog DM, Rkein AM, Fabi SG, et al. Photodynamic therapy: a clinical consensus guide. Dermatol Surg. 2016;42:804-827. doi:10.1097/DSS.0000000000000800
- Maghfour J, Ozog DM, Mineroff J, et al. Photobiomodulation CME part I: overview and mechanism of action. J Am Acad Dermatol. 2024;91:793-802. doi:10.1016/j.jaad.2023.10.073
- Mineroff J, Maghfour J, Ozog DM, et al. Photobiomodulation CME part II: clinical applications in dermatology. J Am Acad Dermatol. 2024;91:805-815. doi:10.1016/j.jaad.2023.10.074
- Mamalis A, Siegel D, Jagdeo J. Visible red light emitting diode photobiomodulation for skin fibrosis: key molecular pathways. Curr Dermatol Rep. 2016;5:121-128. doi:10.1007/s13671-016-0141-x
- Kurtti A, Nguyen JK, Weedon J, et al. Light emitting diode-red light for reduction of post-surgical scarring: results from a dose-ranging, split-face, randomized controlled trial. J Biophotonics. 2021;14:E202100073. doi:10.1002/jbio.202100073
- Nguyen JK, Weedon J, Jakus J, et al. A dose-ranging, parallel group, split-face, single-blind phase II study of light emitting diode-red light (LED-RL) for skin scarring prevention: study protocol for a randomized controlled trial. Trials. 2019;20:432. doi:10.1186/s13063-019-3546-6
- Ho D, Kraeva E, Wun T, et al. A single-blind, dose escalation, phase I study of high-fluence light-emitting diode-red light (LED-RL) on human skin: study protocol for a randomized controlled trial. Trials. 2016;17:385. doi:10.1186/s13063-016-1518-7
- Wang EB, Kaur R, Nguyen J, et al. A single-blind, dose-escalation, phase I study of high-fluence light-emitting diode-red light on Caucasian non-Hispanic skin: study protocol for a randomized controlled trial. Trials. 2019;20:177. doi:10.1186/s13063-019-3278-7
- Wang JY, Kabakova M, Patel P, et al. Outstanding user reported satisfaction for light emitting diodes under-eye rejuvenation. Arch Dermatol Res. 2024;316:511. doi:10.1007/s00403-024-03254-z
- Mineroff J, Austin E, Feit E, et al. Male facial rejuvenation using a combination 633, 830, and 1072 nm LED face mask. Arch Dermatol Res. 2023;315:2605-2611. doi:10.1007/s00403-023-02663-w
- Wang JY, Zeitouni N, Austin E, et al. Photodynamic therapy: clinical applications in dermatology. J Am Acad Dermatol. Published online February 20, 2025. doi:10.1016/j.jaad.2024.12.050
- Austin E, Koo E, Jagdeo J. Thermal photodynamic therapy increases apoptosis and reactive oxygen species generation in cutaneous and mucosal squamous cell carcinoma cells. Sci Rep. 2018;8:12599. doi:10.1038/s41598-018-30908-6
- Mamalis A, Koo E, Sckisel GD, et al. Temperature-dependent impact of thermal aminolaevulinic acid photodynamic therapy on apoptosis and reactive oxygen species generation in human dermal fibroblasts. Br J Dermatol. 2016;175:512-519. doi:10.1111/bjd.14509
- Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40:1094-1102. doi:10.1097/01.DSS.0000452662.69539.57
- Koo E, Austin E, Mamalis A, et al. Efficacy of ultra short sub-30 minute incubation of 5-aminolevulinic acid photodynamic therapy in vitro. Lasers Surg Med. 2017;49:592-598. doi:10.1002/lsm.22648
- Austin E, Wang JY, Ozog DM, et al. Photodynamic therapy: overview and mechanism of action. J Am Acad Dermatol. Published online February 20, 2025. doi:10.1016/j.jaad.2025.02.037
- Hua H, Cheng JW, Bu WB, et al. 5-aminolaevulinic acid-based photodynamic therapy inhibits ultraviolet B-induced skin photodamage. Int J Biol Sci. 2019;15:2100-2109. doi:10.7150/ijbs.31583
- Liebel F, Kaur S, Ruvolo E, et al. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J Invest Dermatol. 2012;132:1901-1907. doi:10.1038/jid.2011.476
- Austin E, Geisler AN, Nguyen J, et al. Visible light. part I: properties and cutaneous effects of visible light. J Am Acad Dermatol. 2021;84:1219-1231. doi:10.1016/j.jaad.2021.02.048
- Fatima S, Braunberger T, Mohammad TF, et al. The role of sunscreen in melasma and postinflammatory hyperpigmentation. Indian J Dermatol. 2020;65:5-10. doi:10.4103/ijd.IJD_295_18
- Geisler AN, Austin E, Nguyen J, et al. Visible light. part II: photoprotection against visible and ultraviolet light. J Am Acad Dermatol. 2021;84:1233-1244. doi:10.1016/j.jaad.2020.11.074
Illuminating the Role of Visible Light in Dermatology
Illuminating the Role of Visible Light in Dermatology
The Habit of Curiosity: How Writing Shapes Clinical Thinking in Medical Training
The Habit of Curiosity: How Writing Shapes Clinical Thinking in Medical Training
I was accepted into my fellowship almost 1 year ago: major milestones on my curriculum vitae are now met, fellowship application materials are complete, and the stress of the match is long gone. At the start of my fellowship, I had 2 priorities: (1) to learn as much as I could about dermatologic surgery and (2) to be the best dad possible to my newborn son, Jay. However, most nights I still find myself up late editing a manuscript draft or chasing down references, long after the “need” to publish has passed. Recently, my wife asked me why—what’s left to prove?
I’ll be the first to admit it: early on, publishing felt almost purely transactional. Each project was little more than a line on an application or a way to stand out or meet a new mentor. I have reflected before on how easily that mindset can slip into a kind of research arms race, in which productivity overshadows purpose.1 This time, I wanted to explore the other side of that equation: the “why” behind it all.
I have learned that writing forces me to slow down and actually think about what I am seeing every day. It turns routine work into something I must understand well enough to explain. Even a small write-up can make me notice details I would otherwise skim past in clinic or surgery. These days, most of my projects start small: a case that taught me something, an observation that made me pause and think. Those seemingly small questions are what eventually grow into bigger ones. The clinical trial I am designing now did not begin as a grand plan—it started because I could not stop thinking about how we manage pain and analgesia after Mohs surgery. That curiosity, shaped by the experience of writing those earlier “smaller” papers, evolved into a study that might actually help improve patient care one day. Still, most of what I write will not revolutionize the field. It is not cutting-edge science or paradigm-shifting data; it is mostly modest analyses with a few interesting conclusions or surgical pearls that might cut down on a patient’s procedural time or save a dermatologist somewhere a few sutures. But it still feels worth doing.
While rotating with Dr. Anna Bar at Oregon Health & Science University, Portland, I noticed a poster hanging on the wall titled, “Top 10 Reasons Why Our Faculty Are Dedicated to Academics and Teaching,” based on the wisdom of Dr. Jane M. Grant-Kels.2 My favorite line on the poster reads, “Residents make us better by asking questions.” I think this philosophy is the main reason why I still write. Even though I am not a resident anymore, I am still asking questions. But if I had to sum up my “why” into a neat list, here is what it might look like:
Because asking questions keeps your brain wired for curiosity. Even small projects train us to remain curious, and this curiosity can mean the difference between just doing your job and continuing to evolve within it. As Dr. Rodolfo Neirotti reminds us, “Questions are useful tools—they open communication, improve understanding, and drive scientific research. In medicine, doing things without knowing why is risky.”3
Because the small stuff builds the culture. Dermatology is a small world. Even short case series, pearls, or “how we do it” pieces can shape how we practice. They may not change paradigms, but they can refine them. Over time, those small practical contributions become part of the field’s collective muscle memory.
Because it preserves perspective. Residency, fellowship, and early practice can blur together. A tiny project can become a timestamp of what you were learning or caring about at that specific moment. Years later, you may remember the case through the paper.
Because the act of writing is the point. Writing forces clarity. You cannot hide behind saying, “That’s just how I do things,” when you have to explain it to others. The discipline of organizing your thoughts sharpens your clinical reasoning and keeps you honest about what you actually know.
Because sometimes it is simply about participating. Publishing, even small pieces, is a way of staying in touch with your field. It says, “I’m still here. I’m still paying attention.”
I think about how Dr. Frederic Mohs developed the technique that now bears his name while he was still a medical student.4 He could have said, “I already made it into medical school. That’s enough.” But he did not. I guess my point is not that we are all on the verge of inventing something revolutionary; it is that innovation happens only when curiosity keeps moving us forward. So no, I do not write to check boxes anymore. I write because it keeps me curious, and I have realized that curiosity is a habit I never want to outgrow.
Or maybe it’s because Jay keeps me up at night, and I have nothing better to do.
- Jeha GM. A roadmap to research opportunities for dermatology residents. Cutis. 2024;114:E53-E56.
- Grant-Kels J. The gift that keeps on giving. UConn Health Dermatology. Accessed November 24, 2025. https://health.uconn.edu/dermatology/education/
- Neirotti RA. The importance of asking questions and doing things for a reason. Braz J Cardiovasc Surg. 2021;36:I-II.
- Trost LB, Bailin PL. History of Mohs surgery. Dermatol Clin. 2011;29:135-139, vii.
I was accepted into my fellowship almost 1 year ago: major milestones on my curriculum vitae are now met, fellowship application materials are complete, and the stress of the match is long gone. At the start of my fellowship, I had 2 priorities: (1) to learn as much as I could about dermatologic surgery and (2) to be the best dad possible to my newborn son, Jay. However, most nights I still find myself up late editing a manuscript draft or chasing down references, long after the “need” to publish has passed. Recently, my wife asked me why—what’s left to prove?
I’ll be the first to admit it: early on, publishing felt almost purely transactional. Each project was little more than a line on an application or a way to stand out or meet a new mentor. I have reflected before on how easily that mindset can slip into a kind of research arms race, in which productivity overshadows purpose.1 This time, I wanted to explore the other side of that equation: the “why” behind it all.
I have learned that writing forces me to slow down and actually think about what I am seeing every day. It turns routine work into something I must understand well enough to explain. Even a small write-up can make me notice details I would otherwise skim past in clinic or surgery. These days, most of my projects start small: a case that taught me something, an observation that made me pause and think. Those seemingly small questions are what eventually grow into bigger ones. The clinical trial I am designing now did not begin as a grand plan—it started because I could not stop thinking about how we manage pain and analgesia after Mohs surgery. That curiosity, shaped by the experience of writing those earlier “smaller” papers, evolved into a study that might actually help improve patient care one day. Still, most of what I write will not revolutionize the field. It is not cutting-edge science or paradigm-shifting data; it is mostly modest analyses with a few interesting conclusions or surgical pearls that might cut down on a patient’s procedural time or save a dermatologist somewhere a few sutures. But it still feels worth doing.
While rotating with Dr. Anna Bar at Oregon Health & Science University, Portland, I noticed a poster hanging on the wall titled, “Top 10 Reasons Why Our Faculty Are Dedicated to Academics and Teaching,” based on the wisdom of Dr. Jane M. Grant-Kels.2 My favorite line on the poster reads, “Residents make us better by asking questions.” I think this philosophy is the main reason why I still write. Even though I am not a resident anymore, I am still asking questions. But if I had to sum up my “why” into a neat list, here is what it might look like:
Because asking questions keeps your brain wired for curiosity. Even small projects train us to remain curious, and this curiosity can mean the difference between just doing your job and continuing to evolve within it. As Dr. Rodolfo Neirotti reminds us, “Questions are useful tools—they open communication, improve understanding, and drive scientific research. In medicine, doing things without knowing why is risky.”3
Because the small stuff builds the culture. Dermatology is a small world. Even short case series, pearls, or “how we do it” pieces can shape how we practice. They may not change paradigms, but they can refine them. Over time, those small practical contributions become part of the field’s collective muscle memory.
Because it preserves perspective. Residency, fellowship, and early practice can blur together. A tiny project can become a timestamp of what you were learning or caring about at that specific moment. Years later, you may remember the case through the paper.
Because the act of writing is the point. Writing forces clarity. You cannot hide behind saying, “That’s just how I do things,” when you have to explain it to others. The discipline of organizing your thoughts sharpens your clinical reasoning and keeps you honest about what you actually know.
Because sometimes it is simply about participating. Publishing, even small pieces, is a way of staying in touch with your field. It says, “I’m still here. I’m still paying attention.”
I think about how Dr. Frederic Mohs developed the technique that now bears his name while he was still a medical student.4 He could have said, “I already made it into medical school. That’s enough.” But he did not. I guess my point is not that we are all on the verge of inventing something revolutionary; it is that innovation happens only when curiosity keeps moving us forward. So no, I do not write to check boxes anymore. I write because it keeps me curious, and I have realized that curiosity is a habit I never want to outgrow.
Or maybe it’s because Jay keeps me up at night, and I have nothing better to do.
I was accepted into my fellowship almost 1 year ago: major milestones on my curriculum vitae are now met, fellowship application materials are complete, and the stress of the match is long gone. At the start of my fellowship, I had 2 priorities: (1) to learn as much as I could about dermatologic surgery and (2) to be the best dad possible to my newborn son, Jay. However, most nights I still find myself up late editing a manuscript draft or chasing down references, long after the “need” to publish has passed. Recently, my wife asked me why—what’s left to prove?
I’ll be the first to admit it: early on, publishing felt almost purely transactional. Each project was little more than a line on an application or a way to stand out or meet a new mentor. I have reflected before on how easily that mindset can slip into a kind of research arms race, in which productivity overshadows purpose.1 This time, I wanted to explore the other side of that equation: the “why” behind it all.
I have learned that writing forces me to slow down and actually think about what I am seeing every day. It turns routine work into something I must understand well enough to explain. Even a small write-up can make me notice details I would otherwise skim past in clinic or surgery. These days, most of my projects start small: a case that taught me something, an observation that made me pause and think. Those seemingly small questions are what eventually grow into bigger ones. The clinical trial I am designing now did not begin as a grand plan—it started because I could not stop thinking about how we manage pain and analgesia after Mohs surgery. That curiosity, shaped by the experience of writing those earlier “smaller” papers, evolved into a study that might actually help improve patient care one day. Still, most of what I write will not revolutionize the field. It is not cutting-edge science or paradigm-shifting data; it is mostly modest analyses with a few interesting conclusions or surgical pearls that might cut down on a patient’s procedural time or save a dermatologist somewhere a few sutures. But it still feels worth doing.
While rotating with Dr. Anna Bar at Oregon Health & Science University, Portland, I noticed a poster hanging on the wall titled, “Top 10 Reasons Why Our Faculty Are Dedicated to Academics and Teaching,” based on the wisdom of Dr. Jane M. Grant-Kels.2 My favorite line on the poster reads, “Residents make us better by asking questions.” I think this philosophy is the main reason why I still write. Even though I am not a resident anymore, I am still asking questions. But if I had to sum up my “why” into a neat list, here is what it might look like:
Because asking questions keeps your brain wired for curiosity. Even small projects train us to remain curious, and this curiosity can mean the difference between just doing your job and continuing to evolve within it. As Dr. Rodolfo Neirotti reminds us, “Questions are useful tools—they open communication, improve understanding, and drive scientific research. In medicine, doing things without knowing why is risky.”3
Because the small stuff builds the culture. Dermatology is a small world. Even short case series, pearls, or “how we do it” pieces can shape how we practice. They may not change paradigms, but they can refine them. Over time, those small practical contributions become part of the field’s collective muscle memory.
Because it preserves perspective. Residency, fellowship, and early practice can blur together. A tiny project can become a timestamp of what you were learning or caring about at that specific moment. Years later, you may remember the case through the paper.
Because the act of writing is the point. Writing forces clarity. You cannot hide behind saying, “That’s just how I do things,” when you have to explain it to others. The discipline of organizing your thoughts sharpens your clinical reasoning and keeps you honest about what you actually know.
Because sometimes it is simply about participating. Publishing, even small pieces, is a way of staying in touch with your field. It says, “I’m still here. I’m still paying attention.”
I think about how Dr. Frederic Mohs developed the technique that now bears his name while he was still a medical student.4 He could have said, “I already made it into medical school. That’s enough.” But he did not. I guess my point is not that we are all on the verge of inventing something revolutionary; it is that innovation happens only when curiosity keeps moving us forward. So no, I do not write to check boxes anymore. I write because it keeps me curious, and I have realized that curiosity is a habit I never want to outgrow.
Or maybe it’s because Jay keeps me up at night, and I have nothing better to do.
- Jeha GM. A roadmap to research opportunities for dermatology residents. Cutis. 2024;114:E53-E56.
- Grant-Kels J. The gift that keeps on giving. UConn Health Dermatology. Accessed November 24, 2025. https://health.uconn.edu/dermatology/education/
- Neirotti RA. The importance of asking questions and doing things for a reason. Braz J Cardiovasc Surg. 2021;36:I-II.
- Trost LB, Bailin PL. History of Mohs surgery. Dermatol Clin. 2011;29:135-139, vii.
- Jeha GM. A roadmap to research opportunities for dermatology residents. Cutis. 2024;114:E53-E56.
- Grant-Kels J. The gift that keeps on giving. UConn Health Dermatology. Accessed November 24, 2025. https://health.uconn.edu/dermatology/education/
- Neirotti RA. The importance of asking questions and doing things for a reason. Braz J Cardiovasc Surg. 2021;36:I-II.
- Trost LB, Bailin PL. History of Mohs surgery. Dermatol Clin. 2011;29:135-139, vii.
The Habit of Curiosity: How Writing Shapes Clinical Thinking in Medical Training
The Habit of Curiosity: How Writing Shapes Clinical Thinking in Medical Training
Practice Points
- Writing about everyday clinical experiences forces trainees to slow down, think more carefully, and better understand why they do what they do. Being able to write clearly about a clinical scenario reflects true understanding.
- The act of writing sharpens clinical judgment by requiring clarity, honesty, and reflection rather than relying on habit or routine.
- Writing fosters habits of curiosity that support continued professional growth and ongoing engagement with one’s field beyond formal training milestones.
Cobblestonelike Papules on the Neck
The Diagnosis: Fibroelastolytic Papulosis
Histopathology demonstrated decreased density and fragmentation of elastic fibers in the superficial reticular and papillary dermis consistent with an elastolytic disease process (Figure). Of note, elastolysis typically is visualized with Verhoeff-van Gieson stain but cannot be visualized well with standard hematoxylin and eosin staining. Additional staining with Congo red was negative for amyloid, and colloidal iron did not show any increase in dermal mucin, ruling out amyloidosis and scleromyxedema, respectively. Based on the histopathologic findings and the clinical history, a diagnosis of fibroelastolytic papulosis (FP) was made. Given the benign nature of the condition, the patient was prescribed a topical steroid (clobetasol 0.05%) for symptomatic relief.
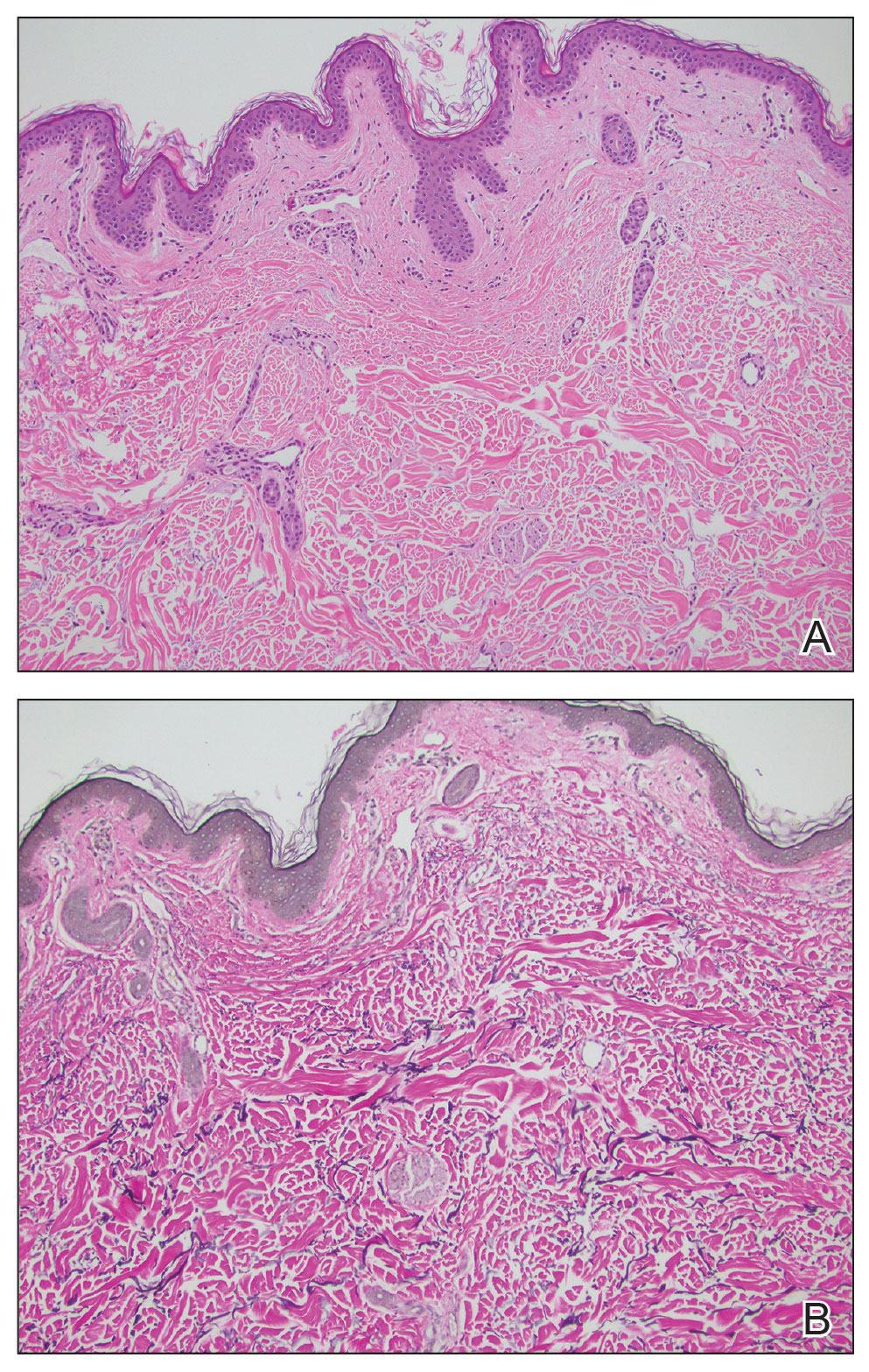
Cutaneous conditions can arise from abnormalities in the elastin composition of connective tissue due to abnormal elastin formation or degradation (elastolysis).1 Fibroelastolytic papulosis is a distinct elastolytic disorder diagnosed histologically by a notable loss of elastic fibers localized to the papillary dermis.2 Fibroelastolytic papulosis is an acquired condition linked to exposure to UV radiation, abnormal elastogenesis, and hormonal factors that commonly involves the neck, supraclavicular area, and upper back.1-3 Predominantly affecting elderly women, FP is characterized by soft white papules that often coalesce into a cobblestonelike plaque.2 Because the condition rarely is seen in men, there is speculation that it may involve genetic, hereditary, and hormonal factors that have yet to be identified.1
Fibroelastolytic papulosis can be classified as either pseudoxanthoma elasticum–like papillary dermal elastolysis or white fibrous papulosis.2,3 White fibrous papulosis manifests with haphazardly arranged collagen fibers in the reticular and deep dermis with papillary dermal elastolysis and most commonly develops on the neck.3 Although our patient’s lesion was on the neck, the absence of thickened collagen bands on histology supported classification as the pseudoxanthoma elasticum– like papillary dermal elastolysis subtype.
Fibroelastolytic papulosis can be distinguished from other elastic abnormalities by its characteristic clinical appearance, demographic distribution, and associated histopathologic findings. The differential diagnosis of FP includes pseudoxanthoma elasticum (PXE), anetoderma, scleromyxedema, and lichen amyloidosis.
Pseudoxanthoma elasticum is a hereditary or acquired multisystem disease characterized by fragmentation and calcification of elastic fibers in the mid dermis.1,4 Its clinical presentation resembles that of FP, appearing as small, asymptomatic, yellowish or flesh-colored papules in a reticular pattern that progressively coalesce into larger plaques with a cobblestonelike appearance.1 Like FP, PXE commonly affects the flexural creases in women but in contrast may manifest earlier (ie, second or third decades of life). Additionally, the pathogenesis of PXE is not related to UV radiation exposure. The hereditary form develops due to a gene variation, whereas the acquired form may be due to conditions associated with physiologic and/or mechanical stress.1
Anetoderma, also known as macular atrophy, is another condition that demonstrates elastic tissue loss in the dermis on histopathology.1 Anetoderma commonly is seen in younger patients and can be differentiated from FP by the antecedent presence of an inflammatory process. Anetoderma is classified as primary or secondary. Primary anetoderma is associated with prothrombotic abnormalities, while secondary anetoderma is associated with systemic disease including but not limited to sarcoidosis, systemic lupus erythematous, and Graves disease.1
Neither lichen myxedematosus (LM) nor lichen amyloidosis (LA) are true elastolytic conditions. Lichen myxedematosus is considered in the differential diagnosis of FP due to the associated loss of elastin observed with disease progression. An idiopathic cutaneous mucinosis, LM is a localized form of scleromyxedema, which is characterized by small, firm, waxy papules; mucin deposition in the skin; fibroblast proliferation; and fibrosis. On histologic analysis, typical findings of LM include irregularly arranged fibroblasts, diffuse mucin deposition within the upper and mid reticular dermis, increased collagen deposition, and a decrease in elastin fibers.5
Lichen amyloidosis is a subtype of primary localized cutaneous amyloidosis, a rare condition characterized by the extracellular deposition of amyloid proteins in the skin and a lack of systemic involvement. Although it is not an elastolytic condition, LA is clinically similar to FP, often manifesting as multiple localized, pruritic, hyperpigmented papules that can coalesce into larger plaques; it tends to develop on the shins, calves, ankles, and thighs.6,7 The condition commonly manifests in the fifth and sixth decades of life; however, in contrast to FP, LA is more prevalent in men and individuals from Central and South American as well as Middle Eastern and non-Chinese Asian populations.8 Lichen amyloidosis is a keratin-derived amyloidosis with cytokeratin-based amyloid precursors that only deposit in the dermis.6 Histopathology reveals colloid bodies due to the presence of apoptotic basal keratinocytes. The etiology of LA is unknown, but on rare occasions it has been associated with multiple endocrine neoplasia 2A rearranged during transfection mutations.6
In summary, FP is an uncommonly diagnosed elastolytic condition that often is asymptomatic or associated with mild pruritus. Biopsy is warranted to help differentiate it from mimicker conditions that may be associated with systemic disease. Currently, there is no established therapy that provides successful treatment. Research suggests unsatisfactory results with the use of topical tretinoin or topical antioxidants.3 More recently, nonablative fractional resurfacing lasers have been evaluated as a possible therapeutic strategy of promise for elastic disorders.9
- Andrés-Ramos I, Alegría-Landa V, Gimeno I, et al. Cutaneous elastic tissue anomalies. Am J Dermatopathol. 2019;41:85-117. doi:10.1097/DAD.0000000000001275
- Valbuena V, Assaad D, Yeung J. Pseudoxanthoma elasticum-like papillary dermal elastolysis: a single case report. J Cutan Med Surg. 2017;21:345-347. doi:10.1177/1203475417699407
- Dokic Y, Tschen J. White fibrous papulosis of the axillae and neck. Cureus. 2020;12:E7635. doi:10.7759/cureus.7635
- Recio-Monescillo M, Torre-Castro J, Manzanas C, et al. Papillary dermal elastolysis histopathology mimicking folliculotropic mycosis fungoides. J Cutan Pathol. 2023;50:430-433. doi:10.1111/cup.14402
- Cokonis Georgakis CD, Falasca G, Georgakis A, et al. Scleromyxedema. Clin Dermatol. 2006;24:493-497. doi:10.1016/j.clindermatol.2006.07.011
- Weidner T, Illing T, Elsner P. Primary localized cutaneous amyloidosis: a systematic treatment review. Am J Clin Dermatol. 2017;18:629-642. doi:10.1007/s40257-017-0278-9
- Ladizinski B, Lee KC. Lichen amyloidosis. CMAJ. 2014;186:532. doi:10.1503/cmaj.130698
- Chen JF, Chen YF. Answer: can you identify this condition? Can Fam Physician. 2012;58:1234-1235.
- Foering K, Torbeck RL, Frank MP, et al. Treatment of pseudoxanthoma elasticum-like papillary dermal elastolysis with nonablative fractional resurfacing laser resulting in clinical and histologic improvement in elastin and collagen. J Cosmet Laser Ther. 2018;20:382-384. doi:10.1080/14764172.2017.1358457
The Diagnosis: Fibroelastolytic Papulosis
Histopathology demonstrated decreased density and fragmentation of elastic fibers in the superficial reticular and papillary dermis consistent with an elastolytic disease process (Figure). Of note, elastolysis typically is visualized with Verhoeff-van Gieson stain but cannot be visualized well with standard hematoxylin and eosin staining. Additional staining with Congo red was negative for amyloid, and colloidal iron did not show any increase in dermal mucin, ruling out amyloidosis and scleromyxedema, respectively. Based on the histopathologic findings and the clinical history, a diagnosis of fibroelastolytic papulosis (FP) was made. Given the benign nature of the condition, the patient was prescribed a topical steroid (clobetasol 0.05%) for symptomatic relief.

Cutaneous conditions can arise from abnormalities in the elastin composition of connective tissue due to abnormal elastin formation or degradation (elastolysis).1 Fibroelastolytic papulosis is a distinct elastolytic disorder diagnosed histologically by a notable loss of elastic fibers localized to the papillary dermis.2 Fibroelastolytic papulosis is an acquired condition linked to exposure to UV radiation, abnormal elastogenesis, and hormonal factors that commonly involves the neck, supraclavicular area, and upper back.1-3 Predominantly affecting elderly women, FP is characterized by soft white papules that often coalesce into a cobblestonelike plaque.2 Because the condition rarely is seen in men, there is speculation that it may involve genetic, hereditary, and hormonal factors that have yet to be identified.1
Fibroelastolytic papulosis can be classified as either pseudoxanthoma elasticum–like papillary dermal elastolysis or white fibrous papulosis.2,3 White fibrous papulosis manifests with haphazardly arranged collagen fibers in the reticular and deep dermis with papillary dermal elastolysis and most commonly develops on the neck.3 Although our patient’s lesion was on the neck, the absence of thickened collagen bands on histology supported classification as the pseudoxanthoma elasticum– like papillary dermal elastolysis subtype.
Fibroelastolytic papulosis can be distinguished from other elastic abnormalities by its characteristic clinical appearance, demographic distribution, and associated histopathologic findings. The differential diagnosis of FP includes pseudoxanthoma elasticum (PXE), anetoderma, scleromyxedema, and lichen amyloidosis.
Pseudoxanthoma elasticum is a hereditary or acquired multisystem disease characterized by fragmentation and calcification of elastic fibers in the mid dermis.1,4 Its clinical presentation resembles that of FP, appearing as small, asymptomatic, yellowish or flesh-colored papules in a reticular pattern that progressively coalesce into larger plaques with a cobblestonelike appearance.1 Like FP, PXE commonly affects the flexural creases in women but in contrast may manifest earlier (ie, second or third decades of life). Additionally, the pathogenesis of PXE is not related to UV radiation exposure. The hereditary form develops due to a gene variation, whereas the acquired form may be due to conditions associated with physiologic and/or mechanical stress.1
Anetoderma, also known as macular atrophy, is another condition that demonstrates elastic tissue loss in the dermis on histopathology.1 Anetoderma commonly is seen in younger patients and can be differentiated from FP by the antecedent presence of an inflammatory process. Anetoderma is classified as primary or secondary. Primary anetoderma is associated with prothrombotic abnormalities, while secondary anetoderma is associated with systemic disease including but not limited to sarcoidosis, systemic lupus erythematous, and Graves disease.1
Neither lichen myxedematosus (LM) nor lichen amyloidosis (LA) are true elastolytic conditions. Lichen myxedematosus is considered in the differential diagnosis of FP due to the associated loss of elastin observed with disease progression. An idiopathic cutaneous mucinosis, LM is a localized form of scleromyxedema, which is characterized by small, firm, waxy papules; mucin deposition in the skin; fibroblast proliferation; and fibrosis. On histologic analysis, typical findings of LM include irregularly arranged fibroblasts, diffuse mucin deposition within the upper and mid reticular dermis, increased collagen deposition, and a decrease in elastin fibers.5
Lichen amyloidosis is a subtype of primary localized cutaneous amyloidosis, a rare condition characterized by the extracellular deposition of amyloid proteins in the skin and a lack of systemic involvement. Although it is not an elastolytic condition, LA is clinically similar to FP, often manifesting as multiple localized, pruritic, hyperpigmented papules that can coalesce into larger plaques; it tends to develop on the shins, calves, ankles, and thighs.6,7 The condition commonly manifests in the fifth and sixth decades of life; however, in contrast to FP, LA is more prevalent in men and individuals from Central and South American as well as Middle Eastern and non-Chinese Asian populations.8 Lichen amyloidosis is a keratin-derived amyloidosis with cytokeratin-based amyloid precursors that only deposit in the dermis.6 Histopathology reveals colloid bodies due to the presence of apoptotic basal keratinocytes. The etiology of LA is unknown, but on rare occasions it has been associated with multiple endocrine neoplasia 2A rearranged during transfection mutations.6
In summary, FP is an uncommonly diagnosed elastolytic condition that often is asymptomatic or associated with mild pruritus. Biopsy is warranted to help differentiate it from mimicker conditions that may be associated with systemic disease. Currently, there is no established therapy that provides successful treatment. Research suggests unsatisfactory results with the use of topical tretinoin or topical antioxidants.3 More recently, nonablative fractional resurfacing lasers have been evaluated as a possible therapeutic strategy of promise for elastic disorders.9
The Diagnosis: Fibroelastolytic Papulosis
Histopathology demonstrated decreased density and fragmentation of elastic fibers in the superficial reticular and papillary dermis consistent with an elastolytic disease process (Figure). Of note, elastolysis typically is visualized with Verhoeff-van Gieson stain but cannot be visualized well with standard hematoxylin and eosin staining. Additional staining with Congo red was negative for amyloid, and colloidal iron did not show any increase in dermal mucin, ruling out amyloidosis and scleromyxedema, respectively. Based on the histopathologic findings and the clinical history, a diagnosis of fibroelastolytic papulosis (FP) was made. Given the benign nature of the condition, the patient was prescribed a topical steroid (clobetasol 0.05%) for symptomatic relief.

Cutaneous conditions can arise from abnormalities in the elastin composition of connective tissue due to abnormal elastin formation or degradation (elastolysis).1 Fibroelastolytic papulosis is a distinct elastolytic disorder diagnosed histologically by a notable loss of elastic fibers localized to the papillary dermis.2 Fibroelastolytic papulosis is an acquired condition linked to exposure to UV radiation, abnormal elastogenesis, and hormonal factors that commonly involves the neck, supraclavicular area, and upper back.1-3 Predominantly affecting elderly women, FP is characterized by soft white papules that often coalesce into a cobblestonelike plaque.2 Because the condition rarely is seen in men, there is speculation that it may involve genetic, hereditary, and hormonal factors that have yet to be identified.1
Fibroelastolytic papulosis can be classified as either pseudoxanthoma elasticum–like papillary dermal elastolysis or white fibrous papulosis.2,3 White fibrous papulosis manifests with haphazardly arranged collagen fibers in the reticular and deep dermis with papillary dermal elastolysis and most commonly develops on the neck.3 Although our patient’s lesion was on the neck, the absence of thickened collagen bands on histology supported classification as the pseudoxanthoma elasticum– like papillary dermal elastolysis subtype.
Fibroelastolytic papulosis can be distinguished from other elastic abnormalities by its characteristic clinical appearance, demographic distribution, and associated histopathologic findings. The differential diagnosis of FP includes pseudoxanthoma elasticum (PXE), anetoderma, scleromyxedema, and lichen amyloidosis.
Pseudoxanthoma elasticum is a hereditary or acquired multisystem disease characterized by fragmentation and calcification of elastic fibers in the mid dermis.1,4 Its clinical presentation resembles that of FP, appearing as small, asymptomatic, yellowish or flesh-colored papules in a reticular pattern that progressively coalesce into larger plaques with a cobblestonelike appearance.1 Like FP, PXE commonly affects the flexural creases in women but in contrast may manifest earlier (ie, second or third decades of life). Additionally, the pathogenesis of PXE is not related to UV radiation exposure. The hereditary form develops due to a gene variation, whereas the acquired form may be due to conditions associated with physiologic and/or mechanical stress.1
Anetoderma, also known as macular atrophy, is another condition that demonstrates elastic tissue loss in the dermis on histopathology.1 Anetoderma commonly is seen in younger patients and can be differentiated from FP by the antecedent presence of an inflammatory process. Anetoderma is classified as primary or secondary. Primary anetoderma is associated with prothrombotic abnormalities, while secondary anetoderma is associated with systemic disease including but not limited to sarcoidosis, systemic lupus erythematous, and Graves disease.1
Neither lichen myxedematosus (LM) nor lichen amyloidosis (LA) are true elastolytic conditions. Lichen myxedematosus is considered in the differential diagnosis of FP due to the associated loss of elastin observed with disease progression. An idiopathic cutaneous mucinosis, LM is a localized form of scleromyxedema, which is characterized by small, firm, waxy papules; mucin deposition in the skin; fibroblast proliferation; and fibrosis. On histologic analysis, typical findings of LM include irregularly arranged fibroblasts, diffuse mucin deposition within the upper and mid reticular dermis, increased collagen deposition, and a decrease in elastin fibers.5
Lichen amyloidosis is a subtype of primary localized cutaneous amyloidosis, a rare condition characterized by the extracellular deposition of amyloid proteins in the skin and a lack of systemic involvement. Although it is not an elastolytic condition, LA is clinically similar to FP, often manifesting as multiple localized, pruritic, hyperpigmented papules that can coalesce into larger plaques; it tends to develop on the shins, calves, ankles, and thighs.6,7 The condition commonly manifests in the fifth and sixth decades of life; however, in contrast to FP, LA is more prevalent in men and individuals from Central and South American as well as Middle Eastern and non-Chinese Asian populations.8 Lichen amyloidosis is a keratin-derived amyloidosis with cytokeratin-based amyloid precursors that only deposit in the dermis.6 Histopathology reveals colloid bodies due to the presence of apoptotic basal keratinocytes. The etiology of LA is unknown, but on rare occasions it has been associated with multiple endocrine neoplasia 2A rearranged during transfection mutations.6
In summary, FP is an uncommonly diagnosed elastolytic condition that often is asymptomatic or associated with mild pruritus. Biopsy is warranted to help differentiate it from mimicker conditions that may be associated with systemic disease. Currently, there is no established therapy that provides successful treatment. Research suggests unsatisfactory results with the use of topical tretinoin or topical antioxidants.3 More recently, nonablative fractional resurfacing lasers have been evaluated as a possible therapeutic strategy of promise for elastic disorders.9
- Andrés-Ramos I, Alegría-Landa V, Gimeno I, et al. Cutaneous elastic tissue anomalies. Am J Dermatopathol. 2019;41:85-117. doi:10.1097/DAD.0000000000001275
- Valbuena V, Assaad D, Yeung J. Pseudoxanthoma elasticum-like papillary dermal elastolysis: a single case report. J Cutan Med Surg. 2017;21:345-347. doi:10.1177/1203475417699407
- Dokic Y, Tschen J. White fibrous papulosis of the axillae and neck. Cureus. 2020;12:E7635. doi:10.7759/cureus.7635
- Recio-Monescillo M, Torre-Castro J, Manzanas C, et al. Papillary dermal elastolysis histopathology mimicking folliculotropic mycosis fungoides. J Cutan Pathol. 2023;50:430-433. doi:10.1111/cup.14402
- Cokonis Georgakis CD, Falasca G, Georgakis A, et al. Scleromyxedema. Clin Dermatol. 2006;24:493-497. doi:10.1016/j.clindermatol.2006.07.011
- Weidner T, Illing T, Elsner P. Primary localized cutaneous amyloidosis: a systematic treatment review. Am J Clin Dermatol. 2017;18:629-642. doi:10.1007/s40257-017-0278-9
- Ladizinski B, Lee KC. Lichen amyloidosis. CMAJ. 2014;186:532. doi:10.1503/cmaj.130698
- Chen JF, Chen YF. Answer: can you identify this condition? Can Fam Physician. 2012;58:1234-1235.
- Foering K, Torbeck RL, Frank MP, et al. Treatment of pseudoxanthoma elasticum-like papillary dermal elastolysis with nonablative fractional resurfacing laser resulting in clinical and histologic improvement in elastin and collagen. J Cosmet Laser Ther. 2018;20:382-384. doi:10.1080/14764172.2017.1358457
- Andrés-Ramos I, Alegría-Landa V, Gimeno I, et al. Cutaneous elastic tissue anomalies. Am J Dermatopathol. 2019;41:85-117. doi:10.1097/DAD.0000000000001275
- Valbuena V, Assaad D, Yeung J. Pseudoxanthoma elasticum-like papillary dermal elastolysis: a single case report. J Cutan Med Surg. 2017;21:345-347. doi:10.1177/1203475417699407
- Dokic Y, Tschen J. White fibrous papulosis of the axillae and neck. Cureus. 2020;12:E7635. doi:10.7759/cureus.7635
- Recio-Monescillo M, Torre-Castro J, Manzanas C, et al. Papillary dermal elastolysis histopathology mimicking folliculotropic mycosis fungoides. J Cutan Pathol. 2023;50:430-433. doi:10.1111/cup.14402
- Cokonis Georgakis CD, Falasca G, Georgakis A, et al. Scleromyxedema. Clin Dermatol. 2006;24:493-497. doi:10.1016/j.clindermatol.2006.07.011
- Weidner T, Illing T, Elsner P. Primary localized cutaneous amyloidosis: a systematic treatment review. Am J Clin Dermatol. 2017;18:629-642. doi:10.1007/s40257-017-0278-9
- Ladizinski B, Lee KC. Lichen amyloidosis. CMAJ. 2014;186:532. doi:10.1503/cmaj.130698
- Chen JF, Chen YF. Answer: can you identify this condition? Can Fam Physician. 2012;58:1234-1235.
- Foering K, Torbeck RL, Frank MP, et al. Treatment of pseudoxanthoma elasticum-like papillary dermal elastolysis with nonablative fractional resurfacing laser resulting in clinical and histologic improvement in elastin and collagen. J Cosmet Laser Ther. 2018;20:382-384. doi:10.1080/14764172.2017.1358457
A 76-year-old woman presented to the dermatology clinic for evaluation of a pruritic rash on the posterior lateral neck of several years’ duration. The rash had been slowly worsening and was intermittently symptomatic. Physical examination revealed monomorphous flesh-colored papules coalescing on the neck, yielding a cobblestonelike texture. The patient had been treated previously by dermatology with topical steroids, but symptoms persisted. A punch biopsy of the left lateral neck was performed.