User login
FIRST OF 2 PARTS
Autism spectrum disorder (ASD) is a complex, heterogenous neurodevelopmental disorder with genetic and environmental underpinnings, and an onset early in life.1-9 It affects social communication, cognition, and sensory-motor domains, and manifests as deficits in social reciprocity, repetitive behavior, restricted range of interests, and sensory sensitivities.6,10-14 In recent years, the prevalence of ASD has been increasing.3,6,10 A large percentage of individuals with ASD experience significant social deficits in adulthood,10 which often leads to isolation, depressive symptoms, and poor occupational and relationship functioning.15,16 Interventions in early childhood can result in significant and lasting changes in outcome and in functioning of individuals with ASD.
This article provides an update on various aspects of ASD diagnosis, with the goal of equipping clinicians with knowledge to help make an accurate ASD diagnosis at an early stage. Part 1 focuses on early detection and diagnosis, while Part 2 will describe treatment strategies.
Benefits of early detection
Substantial research has established that early intervention confers substantial benefits for outcomes among children with ASD.2,3,5,6,9,13,14,16-22 Earlier age of intervention correlates with greater developmental gain and symptom reduction.21,23 The atypical neural development responsible for ASD likely occurs much earlier than the behavioral manifestations of this disorder, which implies that there is a crucial period to intervene before behavioral features emerge.1 This necessitates early recognition of ASD,9,17 and the need for further research to find novel ways to detect ASD earlier.
In the United States, children with ASD are diagnosed with the disorder on average between age 3 and 4 years.6,24 However, evidence suggests there may be a prodromal phase for ASD during the first several months of life, wherein infants and toddlers exhibit developmentally inadequate communication and social skills and/or unusual behaviors.18 Behavioral signs suggestive of ASD may be evident as early as infancy, and commonly earlier than age 18 months.1,17,19 Problems with sleeping and eating may be evident in early childhood.19 Deficits in joint attention may be evident as early as age 6 months to 8 months. Research suggests that a diagnosis of ASD by trained, expert professionals is likely to be accurate at the age of 2, and even as early as 18 months.6,24
In a prospective study, Anderson et al25 found that 9% of children who were diagnosed with ASD at age 2 no longer met the diagnostic criteria for ASD by adulthood.6 Those who no longer met ASD criteria were more likely to have received early intervention, had a verbal IQ ≥70, and had experienced a larger decrease in repetitive behaviors between ages 2 and 3, compared with other youth in this study who had a verbal IQ ≥70. One of the limitations of this study was a small sample size (85 participants); larger, randomized studies are needed to replicate these findings.25
Continue to: Characteristics of ASD...
Characteristics of ASD
Table 16,8,10,13,15,26-29 outlines various characteristics of ASD, which may manifest in varying degrees among children with the condition.
Speech/language. Speech helps to facilitate bonding between parents and an infant by offering a soothing, pleasurable, and reinforcing experience.30 More than 50% of children with ASD have language delays or deficits that persist throughout adulthood.13 The extent of these language deficits varies; in general, the more severe the speech/language deficits, the more severe the long-term symptoms.13 Language deficits in young children with ASD tend to be of both the expressive and receptive type, with onset in infancy, which suggests that neural processes predate the emergence of behavioral symptoms of ASD, and also that early language deficits/delays could be a marker for or indicator of future risk of ASD.13 Individuals with ASD also have been noted to have limitations in orienting or attending to human voices.13,30
Facial recognition. Evidence has linked ASD with deficits in facial recognition that emerge in the first few months of life.2 Earlier studies have found that lack of attention to others’ faces was the strongest distinguishing factor between 1-year-olds with ASD and typically developing 1-year-olds.2,31 A recent study that used EEG to compare facial emotion recognition in boys with ASD vs typically developing boys found that boys with ASD exhibited significantly lower sensitivity to angry and fearful faces.27
Other features. A 2020 study (N = 37) found that compared with typically developing children, those with ASD show less “interactional synchrony’’ (a dynamic process in which the timing of children and caregivers’ behaviors [specifically, vocalizations and movements] become mutually coordinated) with both familiar and unfamiliar adults.32 These researchers concluded that impairment in interactional synchrony may be linked to social communication deficits in ASD.32
A recent study (N = 98) evaluated “sluggish cognitive tempo” in 3 groups of children: children with attention-deficit/hyperactivity disorder (ADHD), children with ASD, and children with both ADHD and ASD.33 It found that children with ASD exhibited sluggish cognitive tempo at levels similar to those of the other 2 groups, and indicated that sluggish cognitive tempo may be linked with “social and global impairment above and beyond” the impairment associated with ASD.
Understanding early aberrations in neurobiologic processes in ASD can help develop biomarkers for early recognition of ASD, as well as guide the development of targeted interventions and treatments (Box1-3,7-9,12,13,30,35-39).
Box
Compared with individuals who do not have autism spectrum disorder (ASD), individuals with ASD exhibit anatomical differences in the brain that can be seen on MRI.9,35 Brain regions affected in ASD include the frontal gyrus, temporal gyrus, cingulate gyrus, postcentral gyrus, precuneus, caudate, and hippocampus.9 Some studies have found anomalous structural neural characteristics in infants, such as in the uncinate fasciculus, that correlated with later joint attention challenges, while others have found aberrations in the corpus callosum(responsible for transfer of procedural learning between the hemispheres, and oculomotor response)and internal capsule (responsible for sensorimotor function, as well as other functions) in children with ASD.12
Widespread white matter anomalies have been noted in ASD.12,35,36 In a 2-year longitudinal study that used diffusion tensor imaging, Li et al35 found that preschool children with ASD experience overgrowth of the uncinate fasciculus, which is one of the brain regions implicated in socioemotional processing, and concluded that this overgrowth correlated with ASD severity.35 Andrews et al37 used diffusion-weighted MRI to examine white matter in 127 preschool children. They found that compared with typically developing children, children with ASD exhibited altered white matter microstructure.37
Research suggests that developing representations of the reward value of social stimuli may be challenging for children with ASD.2 Abrams et al30 used resting-state functional brain MRI to evaluate children with typical development and children with highfunctioning, “verbally fluent” ASD. They found that the children with ASD exhibited lower functional connectivity between voice-specific left hemisphere posterior superior temporal sulcus and areas representing the reward circuitry.30 This study also found that children with ASD had underconnectivity between the right hemisphere posterior superior temporal sulcus (which deals with speech prosody) and areas known for emotion-linked associative learning, the orbitofrontal cortex and amygdala.30 These findings are thought to align with the social motivation theory of ASD.13,30,38
The extent of underconnectivity between these systems was found to determine the severity of communication challenges in high-functioning children with ASD.30 One MRI study observed lower gray matter volume in the voice-selective bilateral superior temporal sulcus in children age approximately 9 to 11 years with ASD.39
Neural systems responsible for facial recognition (particularly the right fusiform gyrus and other brain areas) have been shown to exist or begin “very early in life,” which suggests that impaired face recognition may be an early marker of ASD.2 In addition to problems with visual scanning, preferential attention to (and visual sensitivity to) biological motion is a forerunner for the development of social interactions in infants, specifically in regard to being able to detect and recognize emotion, which is considered vital for attachment.7,8 Impaired biological motion perception has been found in very young children with ASD.7,8 This presents an important avenue/potential biomarker for further research to better understand neurobiologic processes underlying atypical development at an earlier age.3,8
Early neural biomarkers for ASD
Nonlinear EEG values may serve as an early neurobiomarker for detecting ASD in young children.1 Because it is relatively inexpensive and convenient, EEG may be highly useful for detecting ASD.1 A study that compared EEG results of 99 infants who had siblings with ASD and 89 low-risk controls from age 3 months to 36 months found that nonlinear EEG measurements predicted with high accuracy later diagnosis of ASD, and were strongly correlated with later Autism Diagnostic Observation Schedule scores.1
Continue to: A complex differential diagnosis...
A complex differential diagnosis
The differential diagnosis of ASD warrants careful attention and consideration to rule out other developmental and psychiatric conditions.
Intellectual disability (ID). DSM-5 diagnostic criteria for ASD necessitate that disturbances are not better explained by ID or global developmental delay and that deficits should exceed impairment consistent with the level of intellectual disability.28 Still, ASD is often overdiagnosed in children with ID.28 Research suggests phenotypic and genetic overlap between ID and ASD.28 Social functioning is often impaired in patients with ID; the greater the severity of ID, the greater the degree of social deficits.28 In approximately 30% of cases, ASD and ID are comorbid.6 This overlap and comorbidity can pose a challenge, particularly due to the inherent complexities involved in assessment and differentiation.28 When ID is present in ASD, there is a greater degree of social-communication deficits.6 It may be difficult to assess for ASD symptoms in children with severe ID.28 Although there is no minimum age or developmental level below which ASD should not be diagnosed, some studies have started to use minimum criteria for diagnosis, such as a nonverbal mental age of 18 months.28,40 Commonly used tests for ASD have much lower specificity when used for children with nonverbal age <15 months.28 It would make sense, then, that the presence of ID might significantly affect the results of these diagnostic tests.28
Other conditions that need to be ruled out include language disorders, hearing loss, rare genetic neurodevelopmental disorders (eg, Fragile X syndrome,3 Rett syndrome6), childhood-onset schizophrenia, obsessive-compulsive disorder, attachment disorders, and other conditions.18 ASD may be overdiagnosed in children with genetic disorders such as Angelman syndrome.41 In a systematic review, Moss and Howlin42 recommended caution when evaluating ASD-like behavioral symptoms in children with genetic syndromes and severe ID. On the other hand, some research has observed that individuals with Fragile X syndrome may exhibit symptoms that meet criteria for ASD.6,43 McDuffie et al43 used the Autism Diagnostic Interview-Revised (ADI-R) to compare boys with Fragile X syndrome who also met criteria for ASD with boys with nonsyndromic ASD. Those in the former group had lesser impairment in social smiling, offering, showing, and nonverbal gestures, but had more complex mannerisms, compared with boys in the latter group.43
Milder manifestations of ASD may be more challenging to diagnose,1 particularly in children age <3 and those with above-average cognition.6 Generally, in the case of a patient with ASD, parents find that the child did not have a period of typical development, or unusual behaviors were evident early on.17
ASD can be comorbid with ADHD. The presence of ADHD may mask or delay the diagnosis of ASD in children.6 In children with both ASD and ADHD, studies have found greater reduction in social and adaptive functioning compared with children with ADHD alone.44
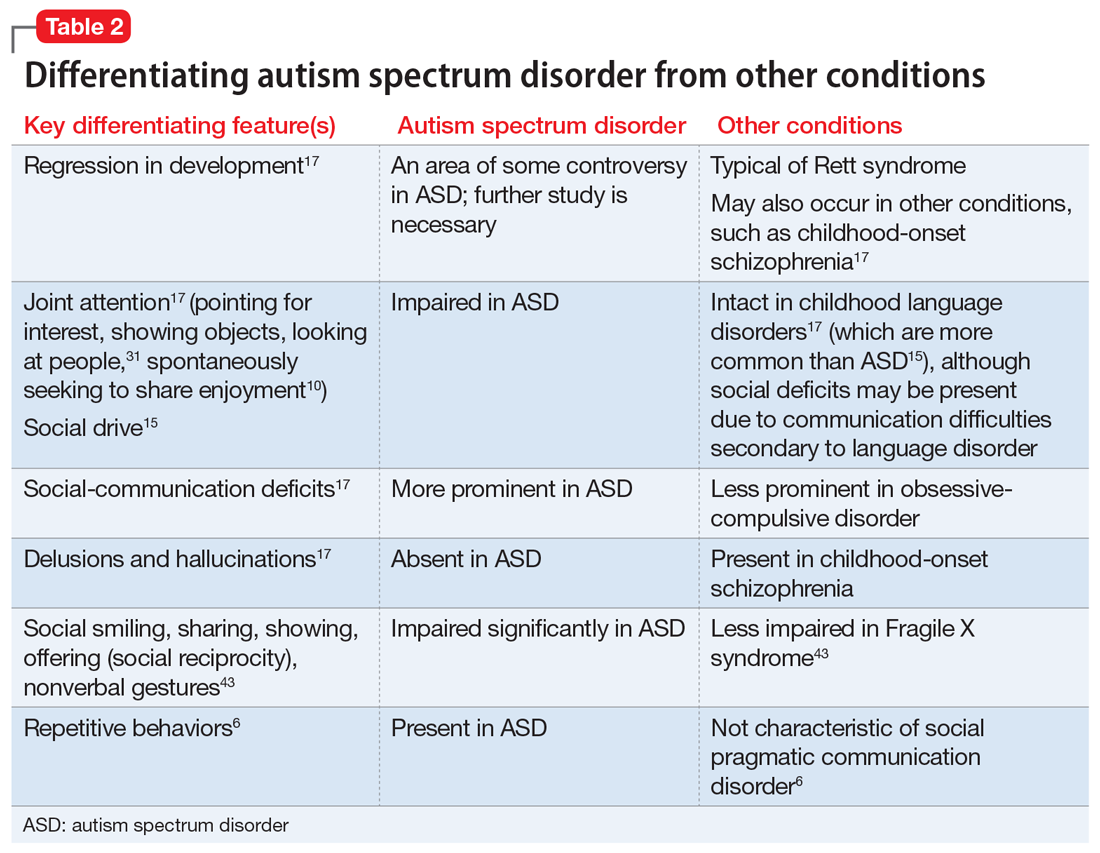
Table 26,10,15,17,31,43 highlights some of the features that can be used to distinguish ASD from other conditions.
Continue to: Screening and diagnosis...
Screening and diagnosis
A medical workup is the first step to rule out other potential conditions that could be masquerading as ASD.17 Obtain a comprehensive history from parents/caregivers, particularly regarding social, behavioral, movement, sensory, and developmental aspects. In addition, audiologic testing is an essential step. Consider genetic testing, particularly if any dysmorphic features and/or ID are present, both of which confer additional risk for a genetic syndrome.6 A physical exam to detect any neurologic anomalies, organ dysfunction, and body dysmorphic features should be conducted.6
The Modified Checklist for Autism in Toddlers–Revised (MCHAT-R) is a commonly used, validated parental screening survey for ASD.5,6 Research has shown that this survey has <50% specificity.5A recent American Academy of Pediatrics Clinical Report recommended universal screening for ASD at pediatric visits at age 18 months and at 24 months, in addition to developmental screening for all children at routine pediatric visits at age 9, 18, and 30 months.6,19
Screening tools such as the Modified Checklist for Autism in Toddlers with Follow-Up (M-CHAT/F) can be integrated into routine primary health care. In a large (N = 25,999) study, Guthrie et al45 used M-CHAT/F to conduct universal, primary care–based screening in young children. They found that the positive predictive value of M-CHAT/F was lower among girls, children of color, and those from lower-income households. There is a need for development of screening tools with higher accuracy and sensitivity for identifying young children with ASD regardless of their ethnic or socioeconomic background, and also for children older than 30 months.5,6,45
Definitive diagnosis of ASD is ideally done by a multidisciplinary team46 using established gold standard measures such as the ADOS (Autism Diagnostic Observation Schedule) and ADI-R.47 Such multidisciplinary teams usually include a child psychiatrist, child psychologist, speech therapist, occupational therapist, school educator, and developmental pediatrician. However, because there are long wait times to receive this type of diagnosis in the United States,6 in the interest of not missing the critical window of early intervention, physicians who suspect a patient may have ASD should refer the child and family for appropriate educational and behavioral interventions as early as possible, rather than waiting for definitive testing.6
ADI-R has limitations in distinguishing ASD from other conditions, especially in very young children, and particularly in distinguishing ASD from childhood-onset schizophrenia.47 Similarly, ADOS, which is a semi-structured, standardized, observation assessment tool, also has limitations, including generating false-positive results, which can make it difficult to distinguish children and adolescents with developmental disabilities from those with ASD.47 However, in combination, these 2 tools are generally efficacious.47 Further research is warranted to develop and fine-tune definitive diagnostic tools with greater sensitivity and specificity.
A newer measure—the Autism Parent Screen for Infants (APSI) questionnaire—has been shown to be effective in detecting early signs predictive of ASD in high-risk infants (eg, siblings of children with ASD), and has potential as an early screening tool.48,49
Part 2 of this article will review nonpharmacologic and pharmacologic treatments for patients with ASD.
1. Bosl WJ, Tager-Flusberg H, Nelson CA. EEG analytics for early detection of autism spectrum disorder: a data-driven approach. Sci Rep. 2018;8(1):6828. doi:10.1038/s41598-018-24318-x
2. Dawson G, Carver L, Meltzoff AN, et al. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Dev. 2002;73(3):700-717. doi:10.1111/1467-8624.00433
3. Frye RE, Vassall S, Kaur G, et al. Emerging biomarkers in autism spectrum disorder: a systematic review. Ann Transl Med. 2019;7(23):792. doi:10.21037/atm.2019.11.5
4. Gordon I, Vander Wyk BC, Bennett RH, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci U S A. 2013;110(52):20953-20958. doi:10.1073/pnas.1312857110
5. Hicks SD, Carpenter RL, Wagner KE, et al. Saliva microRNA differentiates children with autism from peers with typical and atypical development. J Am Acad Child Adolesc Psychiatry. 2020;59(2):296-308.
6. Hyman SL, Levy SE, Myers SM, et al; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145(1):e20193447. doi:10.1542/peds.2019-3447
7. Kaiser MD, Hudac CM, Shultz S, et al. Neural signatures of autism. Proc Natl Acad Sci U S A. 2010;107(49):21223-1228. doi:10.1073/pnas.1010412107
8. Klin A, Lin DJ, Gorrindo P, et al. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459(7244):257-261. doi:10.1038/nature07868
9. Chen T, Chen Y, Yuan M, et al. Towards developing a practical artificial intelligence tool for diagnosing and evaluating autism spectrum disorder: a study using multicenter ABIDE II datasets. JMIR Med Inform. 2020;8(5):e15767. doi:10.2196/15767
10. Maglione MA, Gans D, Das L, et al; Technical Expert Panel, & HRSA Autism Intervention Research – Behavioral (AIR‐B) Network. Nonmedical interventions for children with ASD: recommended guidelines and further research needs. Pediatrics. 2012;30(Suppl 2), S169-S178.
11. Monz BU, Houghton R, Law K, et al. Treatment patterns in children with autism in the United States. Autism Res. 2019;12(3):5170-526. doi:10.1002/aur.2070
12. Shukla DK, Keehn B, Lincoln AJ, et al. White matter compromise of callosal and subcortical fiber tracts in children with autism spectrum disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49(12):1269-1278.e12782. doi:10.1016/j.jaac.2010.08.018
13. Sperdin HF, Schaer M. Aberrant development of speech processing in young children with autism: new insights from neuroimaging biomarkers. Front Neurosci. 2016;10:393. doi: 10.3389/fnins.2016.00393
14. Zwaigenbaum L, Brian JA, Ip A. Early detection for autism spectrum disorder in young children. Paediatr Child Health. 2019;24(7):424-443. doi:10.1093/pch/pxz119
15. Simms MD, Jin XM. Autism, language disorder, and social (pragmatic) communication disorder: DSM-V and differential diagnoses. Pediatr Rev. 2015;36(8):355-363. doi:10.1542/pir.36-8-355
16. Su Maw S, Haga C. Effectiveness of cognitive, developmental, and behavioural interventions for autism spectrum disorder in preschool-aged children: a systematic review and meta-analysis. Heliyon. 2018;4(9):e00763. doi:10.1016/j.heliyon.2018.e00763
17. Volkmar F, Siegel M, Woodbury-Smith M, et al. Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry, 2014;53(2):237-257.
18. Landa RJ. Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int Rev Psychiatry. 2018;30(1):25-39. doi:10.1080/09540261.2018.1432574
19. Lipkin PH, Macias MM; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Promoting optimal development: identifying infants and young children with developmental disorders through developmental surveillance and screening. Pediatrics. 2020;145(1)e20193449. doi:10.1542/peds.2019-3449
20. Pickles A, Le Couteur A, Leadbitter K, et al. Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet. 2016;388:2501-2509.
21. Rogers SJ, Estes A, Lord C, et al. Effects of a brief early start Denver model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1052-1065. doi:10.1016/j.jaac.2012.08.003
22. Schreibman L, Dawson G, Stahmer AC, et al. Naturalistic developmental behavioral interventions: empirically validated treatments for autism spectrum disorder. J Autism Dev Disord. 2015;45(8):2411-2428. doi:10.1007/s10803-015-2407-8
23. Mundy P. A review of joint attention and social-cognitive brain systems in typical development and autism spectrum disorder. Eur J Neurosci. 2018;47(6):497-514.
24. Zwaigenbaum L, Bryson SE, Brian J, et al. Stability of diagnostic assessment for autism spectrum disorder between 18 and 36 months in a high-risk cohort. Autism Res. 2016;9(7):790-800. doi:10.1002/aur.1585
25. Anderson DK, Liang JW, Lord C. Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J Child Psychol Psychiatry. 2014;55(5):485-494. doi:10.1111/jcpp.12178
26. Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Arch Gen Psychiatry. 2008;65(8):946-954. doi:10.1001/archpsyc.65.8.946
27. Van der Donck S, Dzhelyova M, Vettori S, et al. Rapid neural categorization of angry and fearful faces is specifically impaired in boys with autism spectrum disorder. J Child Psychol Psychiatry. 2020;61(9):1019-1029. doi:10.1111/jcpp.13201
28. Thurm A, Farmer C, Salzman E, et al. State of the field: differentiating intellectual disability from autism spectrum disorder. Front Psychiatry. 2019;10:526. doi:10.3389/fpsyt.2019.00526
29. Kuno-Fujita A, Iwabuchi T, Wakusawa K, et al. Sensory processing patterns and fusiform activity during face processing in autism spectrum disorder. Autism Res. 2020;13(5):741-750. doi: 10.1002/aur.2283
30. Abrams DA, Lynch CJ, Cheng KM, et al. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc Natl Acad Sci U S A. 2013;110(29):12060-12065. doi:10.1073/pnas.1302982110
31. Osterling J, Dawson G. Early recognition of children with autism: a study of first birthday home videotapes. J Autism Dev Disord. 1994;24(3):247-257.
32. Zampella CJ, Csumitta KD, Simon E, et al. Interactional synchrony and its association with social and communication ability in children with and without autism spectrum disorder. J Autism Dev Disord. 2020;50(9):3195-3206. doi:10.1007/s10803-020-04412-8
33. McFayden T, Jarrett MA, White SW, et al. Sluggish cognitive tempo in autism spectrum disorder, ADHD, and their comorbidity: implications for impairment. J Clin Child Adolesc Psychol. 2020:1-8. doi:10.1080/15374416.2020.1716365
34. Baribeau DA, Vigod S, Pullenayegum E, et al. Repetitive behavior severity as an early indicator of risk for elevated anxiety symptoms in autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2020;59(7):890-899.e3. doi:10.1016/j.jaac.2019.08.478
35. Li Y, Zhou Z, Chang C, et al. Anomalies in uncinate fasciculus development and social defects in preschoolers with autism spectrum disorder. BMC Psychiatry. 2019;19(1):399. doi:10.1186/s12888-019-2391-1
36. Payabvash S, Palacios EM, Owen JP, et al. White matter connectome edge density in children with autism spectrum disorders: potential imaging biomarkers using machine-learning models. Brain Connect. 2019;9(2):209-220. doi:10.1089/brain.2018.0658
37. Andrews DS, Lee JK, Solomon M, et al. A diffusion-weighted imaging tract-based spatial statistics study of autism spectrum disorder in preschool-aged children. J Neurodev Disord. 2019;11(1):32. doi:10.1186/s11689-019-9291-z
38. Chevallier C, Kohls G, Troiani V, et al. The social motivation theory of autism. Trends Cogn Sci. 2012;16(4):231-239. doi:10.1016/j.tics.2012.02.007
39. Boddaert N, Chabane N, Gervais H, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage. 2004;23(1):364-369. doi:10.1016/j.neuroimage.2004.06.016
40. Lord C, Petkova E, Hus V, et al. A multisite study of the clinical diagnosis of different autism spectrum disorders. Arch Gen Psychiatry. 2012;69(3):306-313. doi:10.1001/archgenpsychiatry.2011.148
41. Trillingsgaard A, ØStergaard JR. Autism in Angelman syndrome: an exploration of comorbidity. Autism. 2004;8(2):163-174.
42. Moss J, Howlin P. Autism spectrum disorders in genetic syndromes: implications for diagnosis, intervention and understanding the wider autism spectrum disorder population. J Intellect Disabil Res. 2009;53(10):852-873. doi:10.1111/j.1365-2788.2009.01197.x
43. McDuffie A, Thurman AJ, Hagerman RJ, et al. Symptoms of autism in males with Fragile X syndrome: a comparison to nonsyndromic ASD using current ADI-R scores. J Autism Dev Disord. 2015;45(7):1925-1937. doi:10.1007/s10803-013-2013-6
44. Ashwood KL, Tye C, Azadi B, et al. Brief report: adaptive functioning in children with ASD, ADHD and ASD + ADHD. J Autism Dev Disord. 2015;45(7):2235-4222. doi:10.1007/s10803-014-2352-y
45. Guthrie W, Wallis K, Bennett A, et al. Accuracy of autism screening in a large pediatric network. Pediatrics. 2019;144(4): e20183963. doi:10.1542/peds.2018-3963
46. Brian JA, Zwaigenbaum L, Ip A. Standards of diagnostic assessment for autism spectrum disorder. Paediatr Child Health. 2019;24(7):444-460. doi:10.1093/pch/pxz117
47. Frigaux A, Evrard R, Lighezzolo-Alnot J. ADI-R and ADOS and the differential diagnosis of autism spectrum disorders: interests, limits and openings. Encephale. 2019;45(5):441-448. doi:10.1016/j.encep.2019.07.002
48. Sacrey LR, Zwaigenbaum L, Bryson S, et al. Screening for behavioral signs of autism spectrum disorder in 9-month-old infant siblings. J Autism Dev Disord. 2021;51(3):839-848. doi:10.1007/s10803-020-04371-0
49. Sacrey LR, Bryson S, Zwaigenbaum L, et al. The autism parent screen for infants: predicting risk of autism spectrum disorder based on parent-reported behavior observed at 6-24 months of age. Autism. 2018;22(3):322-334
FIRST OF 2 PARTS
Autism spectrum disorder (ASD) is a complex, heterogenous neurodevelopmental disorder with genetic and environmental underpinnings, and an onset early in life.1-9 It affects social communication, cognition, and sensory-motor domains, and manifests as deficits in social reciprocity, repetitive behavior, restricted range of interests, and sensory sensitivities.6,10-14 In recent years, the prevalence of ASD has been increasing.3,6,10 A large percentage of individuals with ASD experience significant social deficits in adulthood,10 which often leads to isolation, depressive symptoms, and poor occupational and relationship functioning.15,16 Interventions in early childhood can result in significant and lasting changes in outcome and in functioning of individuals with ASD.
This article provides an update on various aspects of ASD diagnosis, with the goal of equipping clinicians with knowledge to help make an accurate ASD diagnosis at an early stage. Part 1 focuses on early detection and diagnosis, while Part 2 will describe treatment strategies.
Benefits of early detection
Substantial research has established that early intervention confers substantial benefits for outcomes among children with ASD.2,3,5,6,9,13,14,16-22 Earlier age of intervention correlates with greater developmental gain and symptom reduction.21,23 The atypical neural development responsible for ASD likely occurs much earlier than the behavioral manifestations of this disorder, which implies that there is a crucial period to intervene before behavioral features emerge.1 This necessitates early recognition of ASD,9,17 and the need for further research to find novel ways to detect ASD earlier.
In the United States, children with ASD are diagnosed with the disorder on average between age 3 and 4 years.6,24 However, evidence suggests there may be a prodromal phase for ASD during the first several months of life, wherein infants and toddlers exhibit developmentally inadequate communication and social skills and/or unusual behaviors.18 Behavioral signs suggestive of ASD may be evident as early as infancy, and commonly earlier than age 18 months.1,17,19 Problems with sleeping and eating may be evident in early childhood.19 Deficits in joint attention may be evident as early as age 6 months to 8 months. Research suggests that a diagnosis of ASD by trained, expert professionals is likely to be accurate at the age of 2, and even as early as 18 months.6,24
In a prospective study, Anderson et al25 found that 9% of children who were diagnosed with ASD at age 2 no longer met the diagnostic criteria for ASD by adulthood.6 Those who no longer met ASD criteria were more likely to have received early intervention, had a verbal IQ ≥70, and had experienced a larger decrease in repetitive behaviors between ages 2 and 3, compared with other youth in this study who had a verbal IQ ≥70. One of the limitations of this study was a small sample size (85 participants); larger, randomized studies are needed to replicate these findings.25
Continue to: Characteristics of ASD...
Characteristics of ASD
Table 16,8,10,13,15,26-29 outlines various characteristics of ASD, which may manifest in varying degrees among children with the condition.
Speech/language. Speech helps to facilitate bonding between parents and an infant by offering a soothing, pleasurable, and reinforcing experience.30 More than 50% of children with ASD have language delays or deficits that persist throughout adulthood.13 The extent of these language deficits varies; in general, the more severe the speech/language deficits, the more severe the long-term symptoms.13 Language deficits in young children with ASD tend to be of both the expressive and receptive type, with onset in infancy, which suggests that neural processes predate the emergence of behavioral symptoms of ASD, and also that early language deficits/delays could be a marker for or indicator of future risk of ASD.13 Individuals with ASD also have been noted to have limitations in orienting or attending to human voices.13,30
Facial recognition. Evidence has linked ASD with deficits in facial recognition that emerge in the first few months of life.2 Earlier studies have found that lack of attention to others’ faces was the strongest distinguishing factor between 1-year-olds with ASD and typically developing 1-year-olds.2,31 A recent study that used EEG to compare facial emotion recognition in boys with ASD vs typically developing boys found that boys with ASD exhibited significantly lower sensitivity to angry and fearful faces.27
Other features. A 2020 study (N = 37) found that compared with typically developing children, those with ASD show less “interactional synchrony’’ (a dynamic process in which the timing of children and caregivers’ behaviors [specifically, vocalizations and movements] become mutually coordinated) with both familiar and unfamiliar adults.32 These researchers concluded that impairment in interactional synchrony may be linked to social communication deficits in ASD.32
A recent study (N = 98) evaluated “sluggish cognitive tempo” in 3 groups of children: children with attention-deficit/hyperactivity disorder (ADHD), children with ASD, and children with both ADHD and ASD.33 It found that children with ASD exhibited sluggish cognitive tempo at levels similar to those of the other 2 groups, and indicated that sluggish cognitive tempo may be linked with “social and global impairment above and beyond” the impairment associated with ASD.
Understanding early aberrations in neurobiologic processes in ASD can help develop biomarkers for early recognition of ASD, as well as guide the development of targeted interventions and treatments (Box1-3,7-9,12,13,30,35-39).
Box
Compared with individuals who do not have autism spectrum disorder (ASD), individuals with ASD exhibit anatomical differences in the brain that can be seen on MRI.9,35 Brain regions affected in ASD include the frontal gyrus, temporal gyrus, cingulate gyrus, postcentral gyrus, precuneus, caudate, and hippocampus.9 Some studies have found anomalous structural neural characteristics in infants, such as in the uncinate fasciculus, that correlated with later joint attention challenges, while others have found aberrations in the corpus callosum(responsible for transfer of procedural learning between the hemispheres, and oculomotor response)and internal capsule (responsible for sensorimotor function, as well as other functions) in children with ASD.12
Widespread white matter anomalies have been noted in ASD.12,35,36 In a 2-year longitudinal study that used diffusion tensor imaging, Li et al35 found that preschool children with ASD experience overgrowth of the uncinate fasciculus, which is one of the brain regions implicated in socioemotional processing, and concluded that this overgrowth correlated with ASD severity.35 Andrews et al37 used diffusion-weighted MRI to examine white matter in 127 preschool children. They found that compared with typically developing children, children with ASD exhibited altered white matter microstructure.37
Research suggests that developing representations of the reward value of social stimuli may be challenging for children with ASD.2 Abrams et al30 used resting-state functional brain MRI to evaluate children with typical development and children with highfunctioning, “verbally fluent” ASD. They found that the children with ASD exhibited lower functional connectivity between voice-specific left hemisphere posterior superior temporal sulcus and areas representing the reward circuitry.30 This study also found that children with ASD had underconnectivity between the right hemisphere posterior superior temporal sulcus (which deals with speech prosody) and areas known for emotion-linked associative learning, the orbitofrontal cortex and amygdala.30 These findings are thought to align with the social motivation theory of ASD.13,30,38
The extent of underconnectivity between these systems was found to determine the severity of communication challenges in high-functioning children with ASD.30 One MRI study observed lower gray matter volume in the voice-selective bilateral superior temporal sulcus in children age approximately 9 to 11 years with ASD.39
Neural systems responsible for facial recognition (particularly the right fusiform gyrus and other brain areas) have been shown to exist or begin “very early in life,” which suggests that impaired face recognition may be an early marker of ASD.2 In addition to problems with visual scanning, preferential attention to (and visual sensitivity to) biological motion is a forerunner for the development of social interactions in infants, specifically in regard to being able to detect and recognize emotion, which is considered vital for attachment.7,8 Impaired biological motion perception has been found in very young children with ASD.7,8 This presents an important avenue/potential biomarker for further research to better understand neurobiologic processes underlying atypical development at an earlier age.3,8
Early neural biomarkers for ASD
Nonlinear EEG values may serve as an early neurobiomarker for detecting ASD in young children.1 Because it is relatively inexpensive and convenient, EEG may be highly useful for detecting ASD.1 A study that compared EEG results of 99 infants who had siblings with ASD and 89 low-risk controls from age 3 months to 36 months found that nonlinear EEG measurements predicted with high accuracy later diagnosis of ASD, and were strongly correlated with later Autism Diagnostic Observation Schedule scores.1
Continue to: A complex differential diagnosis...
A complex differential diagnosis
The differential diagnosis of ASD warrants careful attention and consideration to rule out other developmental and psychiatric conditions.
Intellectual disability (ID). DSM-5 diagnostic criteria for ASD necessitate that disturbances are not better explained by ID or global developmental delay and that deficits should exceed impairment consistent with the level of intellectual disability.28 Still, ASD is often overdiagnosed in children with ID.28 Research suggests phenotypic and genetic overlap between ID and ASD.28 Social functioning is often impaired in patients with ID; the greater the severity of ID, the greater the degree of social deficits.28 In approximately 30% of cases, ASD and ID are comorbid.6 This overlap and comorbidity can pose a challenge, particularly due to the inherent complexities involved in assessment and differentiation.28 When ID is present in ASD, there is a greater degree of social-communication deficits.6 It may be difficult to assess for ASD symptoms in children with severe ID.28 Although there is no minimum age or developmental level below which ASD should not be diagnosed, some studies have started to use minimum criteria for diagnosis, such as a nonverbal mental age of 18 months.28,40 Commonly used tests for ASD have much lower specificity when used for children with nonverbal age <15 months.28 It would make sense, then, that the presence of ID might significantly affect the results of these diagnostic tests.28
Other conditions that need to be ruled out include language disorders, hearing loss, rare genetic neurodevelopmental disorders (eg, Fragile X syndrome,3 Rett syndrome6), childhood-onset schizophrenia, obsessive-compulsive disorder, attachment disorders, and other conditions.18 ASD may be overdiagnosed in children with genetic disorders such as Angelman syndrome.41 In a systematic review, Moss and Howlin42 recommended caution when evaluating ASD-like behavioral symptoms in children with genetic syndromes and severe ID. On the other hand, some research has observed that individuals with Fragile X syndrome may exhibit symptoms that meet criteria for ASD.6,43 McDuffie et al43 used the Autism Diagnostic Interview-Revised (ADI-R) to compare boys with Fragile X syndrome who also met criteria for ASD with boys with nonsyndromic ASD. Those in the former group had lesser impairment in social smiling, offering, showing, and nonverbal gestures, but had more complex mannerisms, compared with boys in the latter group.43
Milder manifestations of ASD may be more challenging to diagnose,1 particularly in children age <3 and those with above-average cognition.6 Generally, in the case of a patient with ASD, parents find that the child did not have a period of typical development, or unusual behaviors were evident early on.17
ASD can be comorbid with ADHD. The presence of ADHD may mask or delay the diagnosis of ASD in children.6 In children with both ASD and ADHD, studies have found greater reduction in social and adaptive functioning compared with children with ADHD alone.44
Table 26,10,15,17,31,43 highlights some of the features that can be used to distinguish ASD from other conditions.
Continue to: Screening and diagnosis...
Screening and diagnosis
A medical workup is the first step to rule out other potential conditions that could be masquerading as ASD.17 Obtain a comprehensive history from parents/caregivers, particularly regarding social, behavioral, movement, sensory, and developmental aspects. In addition, audiologic testing is an essential step. Consider genetic testing, particularly if any dysmorphic features and/or ID are present, both of which confer additional risk for a genetic syndrome.6 A physical exam to detect any neurologic anomalies, organ dysfunction, and body dysmorphic features should be conducted.6
The Modified Checklist for Autism in Toddlers–Revised (MCHAT-R) is a commonly used, validated parental screening survey for ASD.5,6 Research has shown that this survey has <50% specificity.5A recent American Academy of Pediatrics Clinical Report recommended universal screening for ASD at pediatric visits at age 18 months and at 24 months, in addition to developmental screening for all children at routine pediatric visits at age 9, 18, and 30 months.6,19
Screening tools such as the Modified Checklist for Autism in Toddlers with Follow-Up (M-CHAT/F) can be integrated into routine primary health care. In a large (N = 25,999) study, Guthrie et al45 used M-CHAT/F to conduct universal, primary care–based screening in young children. They found that the positive predictive value of M-CHAT/F was lower among girls, children of color, and those from lower-income households. There is a need for development of screening tools with higher accuracy and sensitivity for identifying young children with ASD regardless of their ethnic or socioeconomic background, and also for children older than 30 months.5,6,45
Definitive diagnosis of ASD is ideally done by a multidisciplinary team46 using established gold standard measures such as the ADOS (Autism Diagnostic Observation Schedule) and ADI-R.47 Such multidisciplinary teams usually include a child psychiatrist, child psychologist, speech therapist, occupational therapist, school educator, and developmental pediatrician. However, because there are long wait times to receive this type of diagnosis in the United States,6 in the interest of not missing the critical window of early intervention, physicians who suspect a patient may have ASD should refer the child and family for appropriate educational and behavioral interventions as early as possible, rather than waiting for definitive testing.6
ADI-R has limitations in distinguishing ASD from other conditions, especially in very young children, and particularly in distinguishing ASD from childhood-onset schizophrenia.47 Similarly, ADOS, which is a semi-structured, standardized, observation assessment tool, also has limitations, including generating false-positive results, which can make it difficult to distinguish children and adolescents with developmental disabilities from those with ASD.47 However, in combination, these 2 tools are generally efficacious.47 Further research is warranted to develop and fine-tune definitive diagnostic tools with greater sensitivity and specificity.
A newer measure—the Autism Parent Screen for Infants (APSI) questionnaire—has been shown to be effective in detecting early signs predictive of ASD in high-risk infants (eg, siblings of children with ASD), and has potential as an early screening tool.48,49
Part 2 of this article will review nonpharmacologic and pharmacologic treatments for patients with ASD.
FIRST OF 2 PARTS
Autism spectrum disorder (ASD) is a complex, heterogenous neurodevelopmental disorder with genetic and environmental underpinnings, and an onset early in life.1-9 It affects social communication, cognition, and sensory-motor domains, and manifests as deficits in social reciprocity, repetitive behavior, restricted range of interests, and sensory sensitivities.6,10-14 In recent years, the prevalence of ASD has been increasing.3,6,10 A large percentage of individuals with ASD experience significant social deficits in adulthood,10 which often leads to isolation, depressive symptoms, and poor occupational and relationship functioning.15,16 Interventions in early childhood can result in significant and lasting changes in outcome and in functioning of individuals with ASD.
This article provides an update on various aspects of ASD diagnosis, with the goal of equipping clinicians with knowledge to help make an accurate ASD diagnosis at an early stage. Part 1 focuses on early detection and diagnosis, while Part 2 will describe treatment strategies.
Benefits of early detection
Substantial research has established that early intervention confers substantial benefits for outcomes among children with ASD.2,3,5,6,9,13,14,16-22 Earlier age of intervention correlates with greater developmental gain and symptom reduction.21,23 The atypical neural development responsible for ASD likely occurs much earlier than the behavioral manifestations of this disorder, which implies that there is a crucial period to intervene before behavioral features emerge.1 This necessitates early recognition of ASD,9,17 and the need for further research to find novel ways to detect ASD earlier.
In the United States, children with ASD are diagnosed with the disorder on average between age 3 and 4 years.6,24 However, evidence suggests there may be a prodromal phase for ASD during the first several months of life, wherein infants and toddlers exhibit developmentally inadequate communication and social skills and/or unusual behaviors.18 Behavioral signs suggestive of ASD may be evident as early as infancy, and commonly earlier than age 18 months.1,17,19 Problems with sleeping and eating may be evident in early childhood.19 Deficits in joint attention may be evident as early as age 6 months to 8 months. Research suggests that a diagnosis of ASD by trained, expert professionals is likely to be accurate at the age of 2, and even as early as 18 months.6,24
In a prospective study, Anderson et al25 found that 9% of children who were diagnosed with ASD at age 2 no longer met the diagnostic criteria for ASD by adulthood.6 Those who no longer met ASD criteria were more likely to have received early intervention, had a verbal IQ ≥70, and had experienced a larger decrease in repetitive behaviors between ages 2 and 3, compared with other youth in this study who had a verbal IQ ≥70. One of the limitations of this study was a small sample size (85 participants); larger, randomized studies are needed to replicate these findings.25
Continue to: Characteristics of ASD...
Characteristics of ASD
Table 16,8,10,13,15,26-29 outlines various characteristics of ASD, which may manifest in varying degrees among children with the condition.
Speech/language. Speech helps to facilitate bonding between parents and an infant by offering a soothing, pleasurable, and reinforcing experience.30 More than 50% of children with ASD have language delays or deficits that persist throughout adulthood.13 The extent of these language deficits varies; in general, the more severe the speech/language deficits, the more severe the long-term symptoms.13 Language deficits in young children with ASD tend to be of both the expressive and receptive type, with onset in infancy, which suggests that neural processes predate the emergence of behavioral symptoms of ASD, and also that early language deficits/delays could be a marker for or indicator of future risk of ASD.13 Individuals with ASD also have been noted to have limitations in orienting or attending to human voices.13,30
Facial recognition. Evidence has linked ASD with deficits in facial recognition that emerge in the first few months of life.2 Earlier studies have found that lack of attention to others’ faces was the strongest distinguishing factor between 1-year-olds with ASD and typically developing 1-year-olds.2,31 A recent study that used EEG to compare facial emotion recognition in boys with ASD vs typically developing boys found that boys with ASD exhibited significantly lower sensitivity to angry and fearful faces.27
Other features. A 2020 study (N = 37) found that compared with typically developing children, those with ASD show less “interactional synchrony’’ (a dynamic process in which the timing of children and caregivers’ behaviors [specifically, vocalizations and movements] become mutually coordinated) with both familiar and unfamiliar adults.32 These researchers concluded that impairment in interactional synchrony may be linked to social communication deficits in ASD.32
A recent study (N = 98) evaluated “sluggish cognitive tempo” in 3 groups of children: children with attention-deficit/hyperactivity disorder (ADHD), children with ASD, and children with both ADHD and ASD.33 It found that children with ASD exhibited sluggish cognitive tempo at levels similar to those of the other 2 groups, and indicated that sluggish cognitive tempo may be linked with “social and global impairment above and beyond” the impairment associated with ASD.
Understanding early aberrations in neurobiologic processes in ASD can help develop biomarkers for early recognition of ASD, as well as guide the development of targeted interventions and treatments (Box1-3,7-9,12,13,30,35-39).
Box
Compared with individuals who do not have autism spectrum disorder (ASD), individuals with ASD exhibit anatomical differences in the brain that can be seen on MRI.9,35 Brain regions affected in ASD include the frontal gyrus, temporal gyrus, cingulate gyrus, postcentral gyrus, precuneus, caudate, and hippocampus.9 Some studies have found anomalous structural neural characteristics in infants, such as in the uncinate fasciculus, that correlated with later joint attention challenges, while others have found aberrations in the corpus callosum(responsible for transfer of procedural learning between the hemispheres, and oculomotor response)and internal capsule (responsible for sensorimotor function, as well as other functions) in children with ASD.12
Widespread white matter anomalies have been noted in ASD.12,35,36 In a 2-year longitudinal study that used diffusion tensor imaging, Li et al35 found that preschool children with ASD experience overgrowth of the uncinate fasciculus, which is one of the brain regions implicated in socioemotional processing, and concluded that this overgrowth correlated with ASD severity.35 Andrews et al37 used diffusion-weighted MRI to examine white matter in 127 preschool children. They found that compared with typically developing children, children with ASD exhibited altered white matter microstructure.37
Research suggests that developing representations of the reward value of social stimuli may be challenging for children with ASD.2 Abrams et al30 used resting-state functional brain MRI to evaluate children with typical development and children with highfunctioning, “verbally fluent” ASD. They found that the children with ASD exhibited lower functional connectivity between voice-specific left hemisphere posterior superior temporal sulcus and areas representing the reward circuitry.30 This study also found that children with ASD had underconnectivity between the right hemisphere posterior superior temporal sulcus (which deals with speech prosody) and areas known for emotion-linked associative learning, the orbitofrontal cortex and amygdala.30 These findings are thought to align with the social motivation theory of ASD.13,30,38
The extent of underconnectivity between these systems was found to determine the severity of communication challenges in high-functioning children with ASD.30 One MRI study observed lower gray matter volume in the voice-selective bilateral superior temporal sulcus in children age approximately 9 to 11 years with ASD.39
Neural systems responsible for facial recognition (particularly the right fusiform gyrus and other brain areas) have been shown to exist or begin “very early in life,” which suggests that impaired face recognition may be an early marker of ASD.2 In addition to problems with visual scanning, preferential attention to (and visual sensitivity to) biological motion is a forerunner for the development of social interactions in infants, specifically in regard to being able to detect and recognize emotion, which is considered vital for attachment.7,8 Impaired biological motion perception has been found in very young children with ASD.7,8 This presents an important avenue/potential biomarker for further research to better understand neurobiologic processes underlying atypical development at an earlier age.3,8
Early neural biomarkers for ASD
Nonlinear EEG values may serve as an early neurobiomarker for detecting ASD in young children.1 Because it is relatively inexpensive and convenient, EEG may be highly useful for detecting ASD.1 A study that compared EEG results of 99 infants who had siblings with ASD and 89 low-risk controls from age 3 months to 36 months found that nonlinear EEG measurements predicted with high accuracy later diagnosis of ASD, and were strongly correlated with later Autism Diagnostic Observation Schedule scores.1
Continue to: A complex differential diagnosis...
A complex differential diagnosis
The differential diagnosis of ASD warrants careful attention and consideration to rule out other developmental and psychiatric conditions.
Intellectual disability (ID). DSM-5 diagnostic criteria for ASD necessitate that disturbances are not better explained by ID or global developmental delay and that deficits should exceed impairment consistent with the level of intellectual disability.28 Still, ASD is often overdiagnosed in children with ID.28 Research suggests phenotypic and genetic overlap between ID and ASD.28 Social functioning is often impaired in patients with ID; the greater the severity of ID, the greater the degree of social deficits.28 In approximately 30% of cases, ASD and ID are comorbid.6 This overlap and comorbidity can pose a challenge, particularly due to the inherent complexities involved in assessment and differentiation.28 When ID is present in ASD, there is a greater degree of social-communication deficits.6 It may be difficult to assess for ASD symptoms in children with severe ID.28 Although there is no minimum age or developmental level below which ASD should not be diagnosed, some studies have started to use minimum criteria for diagnosis, such as a nonverbal mental age of 18 months.28,40 Commonly used tests for ASD have much lower specificity when used for children with nonverbal age <15 months.28 It would make sense, then, that the presence of ID might significantly affect the results of these diagnostic tests.28
Other conditions that need to be ruled out include language disorders, hearing loss, rare genetic neurodevelopmental disorders (eg, Fragile X syndrome,3 Rett syndrome6), childhood-onset schizophrenia, obsessive-compulsive disorder, attachment disorders, and other conditions.18 ASD may be overdiagnosed in children with genetic disorders such as Angelman syndrome.41 In a systematic review, Moss and Howlin42 recommended caution when evaluating ASD-like behavioral symptoms in children with genetic syndromes and severe ID. On the other hand, some research has observed that individuals with Fragile X syndrome may exhibit symptoms that meet criteria for ASD.6,43 McDuffie et al43 used the Autism Diagnostic Interview-Revised (ADI-R) to compare boys with Fragile X syndrome who also met criteria for ASD with boys with nonsyndromic ASD. Those in the former group had lesser impairment in social smiling, offering, showing, and nonverbal gestures, but had more complex mannerisms, compared with boys in the latter group.43
Milder manifestations of ASD may be more challenging to diagnose,1 particularly in children age <3 and those with above-average cognition.6 Generally, in the case of a patient with ASD, parents find that the child did not have a period of typical development, or unusual behaviors were evident early on.17
ASD can be comorbid with ADHD. The presence of ADHD may mask or delay the diagnosis of ASD in children.6 In children with both ASD and ADHD, studies have found greater reduction in social and adaptive functioning compared with children with ADHD alone.44
Table 26,10,15,17,31,43 highlights some of the features that can be used to distinguish ASD from other conditions.
Continue to: Screening and diagnosis...
Screening and diagnosis
A medical workup is the first step to rule out other potential conditions that could be masquerading as ASD.17 Obtain a comprehensive history from parents/caregivers, particularly regarding social, behavioral, movement, sensory, and developmental aspects. In addition, audiologic testing is an essential step. Consider genetic testing, particularly if any dysmorphic features and/or ID are present, both of which confer additional risk for a genetic syndrome.6 A physical exam to detect any neurologic anomalies, organ dysfunction, and body dysmorphic features should be conducted.6
The Modified Checklist for Autism in Toddlers–Revised (MCHAT-R) is a commonly used, validated parental screening survey for ASD.5,6 Research has shown that this survey has <50% specificity.5A recent American Academy of Pediatrics Clinical Report recommended universal screening for ASD at pediatric visits at age 18 months and at 24 months, in addition to developmental screening for all children at routine pediatric visits at age 9, 18, and 30 months.6,19
Screening tools such as the Modified Checklist for Autism in Toddlers with Follow-Up (M-CHAT/F) can be integrated into routine primary health care. In a large (N = 25,999) study, Guthrie et al45 used M-CHAT/F to conduct universal, primary care–based screening in young children. They found that the positive predictive value of M-CHAT/F was lower among girls, children of color, and those from lower-income households. There is a need for development of screening tools with higher accuracy and sensitivity for identifying young children with ASD regardless of their ethnic or socioeconomic background, and also for children older than 30 months.5,6,45
Definitive diagnosis of ASD is ideally done by a multidisciplinary team46 using established gold standard measures such as the ADOS (Autism Diagnostic Observation Schedule) and ADI-R.47 Such multidisciplinary teams usually include a child psychiatrist, child psychologist, speech therapist, occupational therapist, school educator, and developmental pediatrician. However, because there are long wait times to receive this type of diagnosis in the United States,6 in the interest of not missing the critical window of early intervention, physicians who suspect a patient may have ASD should refer the child and family for appropriate educational and behavioral interventions as early as possible, rather than waiting for definitive testing.6
ADI-R has limitations in distinguishing ASD from other conditions, especially in very young children, and particularly in distinguishing ASD from childhood-onset schizophrenia.47 Similarly, ADOS, which is a semi-structured, standardized, observation assessment tool, also has limitations, including generating false-positive results, which can make it difficult to distinguish children and adolescents with developmental disabilities from those with ASD.47 However, in combination, these 2 tools are generally efficacious.47 Further research is warranted to develop and fine-tune definitive diagnostic tools with greater sensitivity and specificity.
A newer measure—the Autism Parent Screen for Infants (APSI) questionnaire—has been shown to be effective in detecting early signs predictive of ASD in high-risk infants (eg, siblings of children with ASD), and has potential as an early screening tool.48,49
Part 2 of this article will review nonpharmacologic and pharmacologic treatments for patients with ASD.
1. Bosl WJ, Tager-Flusberg H, Nelson CA. EEG analytics for early detection of autism spectrum disorder: a data-driven approach. Sci Rep. 2018;8(1):6828. doi:10.1038/s41598-018-24318-x
2. Dawson G, Carver L, Meltzoff AN, et al. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Dev. 2002;73(3):700-717. doi:10.1111/1467-8624.00433
3. Frye RE, Vassall S, Kaur G, et al. Emerging biomarkers in autism spectrum disorder: a systematic review. Ann Transl Med. 2019;7(23):792. doi:10.21037/atm.2019.11.5
4. Gordon I, Vander Wyk BC, Bennett RH, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci U S A. 2013;110(52):20953-20958. doi:10.1073/pnas.1312857110
5. Hicks SD, Carpenter RL, Wagner KE, et al. Saliva microRNA differentiates children with autism from peers with typical and atypical development. J Am Acad Child Adolesc Psychiatry. 2020;59(2):296-308.
6. Hyman SL, Levy SE, Myers SM, et al; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145(1):e20193447. doi:10.1542/peds.2019-3447
7. Kaiser MD, Hudac CM, Shultz S, et al. Neural signatures of autism. Proc Natl Acad Sci U S A. 2010;107(49):21223-1228. doi:10.1073/pnas.1010412107
8. Klin A, Lin DJ, Gorrindo P, et al. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459(7244):257-261. doi:10.1038/nature07868
9. Chen T, Chen Y, Yuan M, et al. Towards developing a practical artificial intelligence tool for diagnosing and evaluating autism spectrum disorder: a study using multicenter ABIDE II datasets. JMIR Med Inform. 2020;8(5):e15767. doi:10.2196/15767
10. Maglione MA, Gans D, Das L, et al; Technical Expert Panel, & HRSA Autism Intervention Research – Behavioral (AIR‐B) Network. Nonmedical interventions for children with ASD: recommended guidelines and further research needs. Pediatrics. 2012;30(Suppl 2), S169-S178.
11. Monz BU, Houghton R, Law K, et al. Treatment patterns in children with autism in the United States. Autism Res. 2019;12(3):5170-526. doi:10.1002/aur.2070
12. Shukla DK, Keehn B, Lincoln AJ, et al. White matter compromise of callosal and subcortical fiber tracts in children with autism spectrum disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49(12):1269-1278.e12782. doi:10.1016/j.jaac.2010.08.018
13. Sperdin HF, Schaer M. Aberrant development of speech processing in young children with autism: new insights from neuroimaging biomarkers. Front Neurosci. 2016;10:393. doi: 10.3389/fnins.2016.00393
14. Zwaigenbaum L, Brian JA, Ip A. Early detection for autism spectrum disorder in young children. Paediatr Child Health. 2019;24(7):424-443. doi:10.1093/pch/pxz119
15. Simms MD, Jin XM. Autism, language disorder, and social (pragmatic) communication disorder: DSM-V and differential diagnoses. Pediatr Rev. 2015;36(8):355-363. doi:10.1542/pir.36-8-355
16. Su Maw S, Haga C. Effectiveness of cognitive, developmental, and behavioural interventions for autism spectrum disorder in preschool-aged children: a systematic review and meta-analysis. Heliyon. 2018;4(9):e00763. doi:10.1016/j.heliyon.2018.e00763
17. Volkmar F, Siegel M, Woodbury-Smith M, et al. Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry, 2014;53(2):237-257.
18. Landa RJ. Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int Rev Psychiatry. 2018;30(1):25-39. doi:10.1080/09540261.2018.1432574
19. Lipkin PH, Macias MM; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Promoting optimal development: identifying infants and young children with developmental disorders through developmental surveillance and screening. Pediatrics. 2020;145(1)e20193449. doi:10.1542/peds.2019-3449
20. Pickles A, Le Couteur A, Leadbitter K, et al. Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet. 2016;388:2501-2509.
21. Rogers SJ, Estes A, Lord C, et al. Effects of a brief early start Denver model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1052-1065. doi:10.1016/j.jaac.2012.08.003
22. Schreibman L, Dawson G, Stahmer AC, et al. Naturalistic developmental behavioral interventions: empirically validated treatments for autism spectrum disorder. J Autism Dev Disord. 2015;45(8):2411-2428. doi:10.1007/s10803-015-2407-8
23. Mundy P. A review of joint attention and social-cognitive brain systems in typical development and autism spectrum disorder. Eur J Neurosci. 2018;47(6):497-514.
24. Zwaigenbaum L, Bryson SE, Brian J, et al. Stability of diagnostic assessment for autism spectrum disorder between 18 and 36 months in a high-risk cohort. Autism Res. 2016;9(7):790-800. doi:10.1002/aur.1585
25. Anderson DK, Liang JW, Lord C. Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J Child Psychol Psychiatry. 2014;55(5):485-494. doi:10.1111/jcpp.12178
26. Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Arch Gen Psychiatry. 2008;65(8):946-954. doi:10.1001/archpsyc.65.8.946
27. Van der Donck S, Dzhelyova M, Vettori S, et al. Rapid neural categorization of angry and fearful faces is specifically impaired in boys with autism spectrum disorder. J Child Psychol Psychiatry. 2020;61(9):1019-1029. doi:10.1111/jcpp.13201
28. Thurm A, Farmer C, Salzman E, et al. State of the field: differentiating intellectual disability from autism spectrum disorder. Front Psychiatry. 2019;10:526. doi:10.3389/fpsyt.2019.00526
29. Kuno-Fujita A, Iwabuchi T, Wakusawa K, et al. Sensory processing patterns and fusiform activity during face processing in autism spectrum disorder. Autism Res. 2020;13(5):741-750. doi: 10.1002/aur.2283
30. Abrams DA, Lynch CJ, Cheng KM, et al. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc Natl Acad Sci U S A. 2013;110(29):12060-12065. doi:10.1073/pnas.1302982110
31. Osterling J, Dawson G. Early recognition of children with autism: a study of first birthday home videotapes. J Autism Dev Disord. 1994;24(3):247-257.
32. Zampella CJ, Csumitta KD, Simon E, et al. Interactional synchrony and its association with social and communication ability in children with and without autism spectrum disorder. J Autism Dev Disord. 2020;50(9):3195-3206. doi:10.1007/s10803-020-04412-8
33. McFayden T, Jarrett MA, White SW, et al. Sluggish cognitive tempo in autism spectrum disorder, ADHD, and their comorbidity: implications for impairment. J Clin Child Adolesc Psychol. 2020:1-8. doi:10.1080/15374416.2020.1716365
34. Baribeau DA, Vigod S, Pullenayegum E, et al. Repetitive behavior severity as an early indicator of risk for elevated anxiety symptoms in autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2020;59(7):890-899.e3. doi:10.1016/j.jaac.2019.08.478
35. Li Y, Zhou Z, Chang C, et al. Anomalies in uncinate fasciculus development and social defects in preschoolers with autism spectrum disorder. BMC Psychiatry. 2019;19(1):399. doi:10.1186/s12888-019-2391-1
36. Payabvash S, Palacios EM, Owen JP, et al. White matter connectome edge density in children with autism spectrum disorders: potential imaging biomarkers using machine-learning models. Brain Connect. 2019;9(2):209-220. doi:10.1089/brain.2018.0658
37. Andrews DS, Lee JK, Solomon M, et al. A diffusion-weighted imaging tract-based spatial statistics study of autism spectrum disorder in preschool-aged children. J Neurodev Disord. 2019;11(1):32. doi:10.1186/s11689-019-9291-z
38. Chevallier C, Kohls G, Troiani V, et al. The social motivation theory of autism. Trends Cogn Sci. 2012;16(4):231-239. doi:10.1016/j.tics.2012.02.007
39. Boddaert N, Chabane N, Gervais H, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage. 2004;23(1):364-369. doi:10.1016/j.neuroimage.2004.06.016
40. Lord C, Petkova E, Hus V, et al. A multisite study of the clinical diagnosis of different autism spectrum disorders. Arch Gen Psychiatry. 2012;69(3):306-313. doi:10.1001/archgenpsychiatry.2011.148
41. Trillingsgaard A, ØStergaard JR. Autism in Angelman syndrome: an exploration of comorbidity. Autism. 2004;8(2):163-174.
42. Moss J, Howlin P. Autism spectrum disorders in genetic syndromes: implications for diagnosis, intervention and understanding the wider autism spectrum disorder population. J Intellect Disabil Res. 2009;53(10):852-873. doi:10.1111/j.1365-2788.2009.01197.x
43. McDuffie A, Thurman AJ, Hagerman RJ, et al. Symptoms of autism in males with Fragile X syndrome: a comparison to nonsyndromic ASD using current ADI-R scores. J Autism Dev Disord. 2015;45(7):1925-1937. doi:10.1007/s10803-013-2013-6
44. Ashwood KL, Tye C, Azadi B, et al. Brief report: adaptive functioning in children with ASD, ADHD and ASD + ADHD. J Autism Dev Disord. 2015;45(7):2235-4222. doi:10.1007/s10803-014-2352-y
45. Guthrie W, Wallis K, Bennett A, et al. Accuracy of autism screening in a large pediatric network. Pediatrics. 2019;144(4): e20183963. doi:10.1542/peds.2018-3963
46. Brian JA, Zwaigenbaum L, Ip A. Standards of diagnostic assessment for autism spectrum disorder. Paediatr Child Health. 2019;24(7):444-460. doi:10.1093/pch/pxz117
47. Frigaux A, Evrard R, Lighezzolo-Alnot J. ADI-R and ADOS and the differential diagnosis of autism spectrum disorders: interests, limits and openings. Encephale. 2019;45(5):441-448. doi:10.1016/j.encep.2019.07.002
48. Sacrey LR, Zwaigenbaum L, Bryson S, et al. Screening for behavioral signs of autism spectrum disorder in 9-month-old infant siblings. J Autism Dev Disord. 2021;51(3):839-848. doi:10.1007/s10803-020-04371-0
49. Sacrey LR, Bryson S, Zwaigenbaum L, et al. The autism parent screen for infants: predicting risk of autism spectrum disorder based on parent-reported behavior observed at 6-24 months of age. Autism. 2018;22(3):322-334
1. Bosl WJ, Tager-Flusberg H, Nelson CA. EEG analytics for early detection of autism spectrum disorder: a data-driven approach. Sci Rep. 2018;8(1):6828. doi:10.1038/s41598-018-24318-x
2. Dawson G, Carver L, Meltzoff AN, et al. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Dev. 2002;73(3):700-717. doi:10.1111/1467-8624.00433
3. Frye RE, Vassall S, Kaur G, et al. Emerging biomarkers in autism spectrum disorder: a systematic review. Ann Transl Med. 2019;7(23):792. doi:10.21037/atm.2019.11.5
4. Gordon I, Vander Wyk BC, Bennett RH, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci U S A. 2013;110(52):20953-20958. doi:10.1073/pnas.1312857110
5. Hicks SD, Carpenter RL, Wagner KE, et al. Saliva microRNA differentiates children with autism from peers with typical and atypical development. J Am Acad Child Adolesc Psychiatry. 2020;59(2):296-308.
6. Hyman SL, Levy SE, Myers SM, et al; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145(1):e20193447. doi:10.1542/peds.2019-3447
7. Kaiser MD, Hudac CM, Shultz S, et al. Neural signatures of autism. Proc Natl Acad Sci U S A. 2010;107(49):21223-1228. doi:10.1073/pnas.1010412107
8. Klin A, Lin DJ, Gorrindo P, et al. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459(7244):257-261. doi:10.1038/nature07868
9. Chen T, Chen Y, Yuan M, et al. Towards developing a practical artificial intelligence tool for diagnosing and evaluating autism spectrum disorder: a study using multicenter ABIDE II datasets. JMIR Med Inform. 2020;8(5):e15767. doi:10.2196/15767
10. Maglione MA, Gans D, Das L, et al; Technical Expert Panel, & HRSA Autism Intervention Research – Behavioral (AIR‐B) Network. Nonmedical interventions for children with ASD: recommended guidelines and further research needs. Pediatrics. 2012;30(Suppl 2), S169-S178.
11. Monz BU, Houghton R, Law K, et al. Treatment patterns in children with autism in the United States. Autism Res. 2019;12(3):5170-526. doi:10.1002/aur.2070
12. Shukla DK, Keehn B, Lincoln AJ, et al. White matter compromise of callosal and subcortical fiber tracts in children with autism spectrum disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49(12):1269-1278.e12782. doi:10.1016/j.jaac.2010.08.018
13. Sperdin HF, Schaer M. Aberrant development of speech processing in young children with autism: new insights from neuroimaging biomarkers. Front Neurosci. 2016;10:393. doi: 10.3389/fnins.2016.00393
14. Zwaigenbaum L, Brian JA, Ip A. Early detection for autism spectrum disorder in young children. Paediatr Child Health. 2019;24(7):424-443. doi:10.1093/pch/pxz119
15. Simms MD, Jin XM. Autism, language disorder, and social (pragmatic) communication disorder: DSM-V and differential diagnoses. Pediatr Rev. 2015;36(8):355-363. doi:10.1542/pir.36-8-355
16. Su Maw S, Haga C. Effectiveness of cognitive, developmental, and behavioural interventions for autism spectrum disorder in preschool-aged children: a systematic review and meta-analysis. Heliyon. 2018;4(9):e00763. doi:10.1016/j.heliyon.2018.e00763
17. Volkmar F, Siegel M, Woodbury-Smith M, et al. Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry, 2014;53(2):237-257.
18. Landa RJ. Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int Rev Psychiatry. 2018;30(1):25-39. doi:10.1080/09540261.2018.1432574
19. Lipkin PH, Macias MM; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Promoting optimal development: identifying infants and young children with developmental disorders through developmental surveillance and screening. Pediatrics. 2020;145(1)e20193449. doi:10.1542/peds.2019-3449
20. Pickles A, Le Couteur A, Leadbitter K, et al. Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet. 2016;388:2501-2509.
21. Rogers SJ, Estes A, Lord C, et al. Effects of a brief early start Denver model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1052-1065. doi:10.1016/j.jaac.2012.08.003
22. Schreibman L, Dawson G, Stahmer AC, et al. Naturalistic developmental behavioral interventions: empirically validated treatments for autism spectrum disorder. J Autism Dev Disord. 2015;45(8):2411-2428. doi:10.1007/s10803-015-2407-8
23. Mundy P. A review of joint attention and social-cognitive brain systems in typical development and autism spectrum disorder. Eur J Neurosci. 2018;47(6):497-514.
24. Zwaigenbaum L, Bryson SE, Brian J, et al. Stability of diagnostic assessment for autism spectrum disorder between 18 and 36 months in a high-risk cohort. Autism Res. 2016;9(7):790-800. doi:10.1002/aur.1585
25. Anderson DK, Liang JW, Lord C. Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J Child Psychol Psychiatry. 2014;55(5):485-494. doi:10.1111/jcpp.12178
26. Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Arch Gen Psychiatry. 2008;65(8):946-954. doi:10.1001/archpsyc.65.8.946
27. Van der Donck S, Dzhelyova M, Vettori S, et al. Rapid neural categorization of angry and fearful faces is specifically impaired in boys with autism spectrum disorder. J Child Psychol Psychiatry. 2020;61(9):1019-1029. doi:10.1111/jcpp.13201
28. Thurm A, Farmer C, Salzman E, et al. State of the field: differentiating intellectual disability from autism spectrum disorder. Front Psychiatry. 2019;10:526. doi:10.3389/fpsyt.2019.00526
29. Kuno-Fujita A, Iwabuchi T, Wakusawa K, et al. Sensory processing patterns and fusiform activity during face processing in autism spectrum disorder. Autism Res. 2020;13(5):741-750. doi: 10.1002/aur.2283
30. Abrams DA, Lynch CJ, Cheng KM, et al. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc Natl Acad Sci U S A. 2013;110(29):12060-12065. doi:10.1073/pnas.1302982110
31. Osterling J, Dawson G. Early recognition of children with autism: a study of first birthday home videotapes. J Autism Dev Disord. 1994;24(3):247-257.
32. Zampella CJ, Csumitta KD, Simon E, et al. Interactional synchrony and its association with social and communication ability in children with and without autism spectrum disorder. J Autism Dev Disord. 2020;50(9):3195-3206. doi:10.1007/s10803-020-04412-8
33. McFayden T, Jarrett MA, White SW, et al. Sluggish cognitive tempo in autism spectrum disorder, ADHD, and their comorbidity: implications for impairment. J Clin Child Adolesc Psychol. 2020:1-8. doi:10.1080/15374416.2020.1716365
34. Baribeau DA, Vigod S, Pullenayegum E, et al. Repetitive behavior severity as an early indicator of risk for elevated anxiety symptoms in autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2020;59(7):890-899.e3. doi:10.1016/j.jaac.2019.08.478
35. Li Y, Zhou Z, Chang C, et al. Anomalies in uncinate fasciculus development and social defects in preschoolers with autism spectrum disorder. BMC Psychiatry. 2019;19(1):399. doi:10.1186/s12888-019-2391-1
36. Payabvash S, Palacios EM, Owen JP, et al. White matter connectome edge density in children with autism spectrum disorders: potential imaging biomarkers using machine-learning models. Brain Connect. 2019;9(2):209-220. doi:10.1089/brain.2018.0658
37. Andrews DS, Lee JK, Solomon M, et al. A diffusion-weighted imaging tract-based spatial statistics study of autism spectrum disorder in preschool-aged children. J Neurodev Disord. 2019;11(1):32. doi:10.1186/s11689-019-9291-z
38. Chevallier C, Kohls G, Troiani V, et al. The social motivation theory of autism. Trends Cogn Sci. 2012;16(4):231-239. doi:10.1016/j.tics.2012.02.007
39. Boddaert N, Chabane N, Gervais H, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage. 2004;23(1):364-369. doi:10.1016/j.neuroimage.2004.06.016
40. Lord C, Petkova E, Hus V, et al. A multisite study of the clinical diagnosis of different autism spectrum disorders. Arch Gen Psychiatry. 2012;69(3):306-313. doi:10.1001/archgenpsychiatry.2011.148
41. Trillingsgaard A, ØStergaard JR. Autism in Angelman syndrome: an exploration of comorbidity. Autism. 2004;8(2):163-174.
42. Moss J, Howlin P. Autism spectrum disorders in genetic syndromes: implications for diagnosis, intervention and understanding the wider autism spectrum disorder population. J Intellect Disabil Res. 2009;53(10):852-873. doi:10.1111/j.1365-2788.2009.01197.x
43. McDuffie A, Thurman AJ, Hagerman RJ, et al. Symptoms of autism in males with Fragile X syndrome: a comparison to nonsyndromic ASD using current ADI-R scores. J Autism Dev Disord. 2015;45(7):1925-1937. doi:10.1007/s10803-013-2013-6
44. Ashwood KL, Tye C, Azadi B, et al. Brief report: adaptive functioning in children with ASD, ADHD and ASD + ADHD. J Autism Dev Disord. 2015;45(7):2235-4222. doi:10.1007/s10803-014-2352-y
45. Guthrie W, Wallis K, Bennett A, et al. Accuracy of autism screening in a large pediatric network. Pediatrics. 2019;144(4): e20183963. doi:10.1542/peds.2018-3963
46. Brian JA, Zwaigenbaum L, Ip A. Standards of diagnostic assessment for autism spectrum disorder. Paediatr Child Health. 2019;24(7):444-460. doi:10.1093/pch/pxz117
47. Frigaux A, Evrard R, Lighezzolo-Alnot J. ADI-R and ADOS and the differential diagnosis of autism spectrum disorders: interests, limits and openings. Encephale. 2019;45(5):441-448. doi:10.1016/j.encep.2019.07.002
48. Sacrey LR, Zwaigenbaum L, Bryson S, et al. Screening for behavioral signs of autism spectrum disorder in 9-month-old infant siblings. J Autism Dev Disord. 2021;51(3):839-848. doi:10.1007/s10803-020-04371-0
49. Sacrey LR, Bryson S, Zwaigenbaum L, et al. The autism parent screen for infants: predicting risk of autism spectrum disorder based on parent-reported behavior observed at 6-24 months of age. Autism. 2018;22(3):322-334