User login
A 47-year-old obese male with type 2 diabetes has been on metformin for the last 2 years with good effect (hemoglobin A1c of 6.8), and with exercise has been able to lose 5-10 pounds. His last two blood tests show creatinine levels of 1.5 and 1.6. What do you recommend?
A) Continue with metformin.
B) Stop metformin, start sulfonylurea.
C) Stop metformin, begin glargine.
D) Stop metformin, begin pioglitazone.
Myth: Metformin should not be used in patients with mild to moderate renal insufficiency because of an increased risk of lactic acidosis.
Metformin is the most commonly used oral agent for the treatment of type 2 diabetes in the United States, but the FDA-approved drug label states that it is contraindicated in patients with an abnormal creatinine clearance or serum creatinine of 1.4 in women and 1.5 in men.<sup/>The concern is for development of lactic acidosis in patients because the renally excreted metformin may build up as a result of decreased renal function.
Metformin was approved for use in the United States in 1995, many years after the drug was introduced in Europe. The first drug in its class, phenformin, was removed from the United States and most European markets in 1977 because of a high incidence of lactic acidosis occurring at therapeutic doses. One in 4,000 patients taking phenformin develops lactic acidosis (J. Emerg. Med. 1998;16:881-6). Phenformin has been shown to cause type B lactic acidosis, without evidence of hypoxia or hypoperfusion, and lactic acidosis because of phenformin carried a 50% mortality rate.
Deep concern for the possibility of a similar problem with metformin played an important role in its delay of availability in the United States. It isn’t clear, however, that diabetes patients on metformin have a higher risk of developing lactic acidosis than diabetes patients who are not on metformin.
A Cochrane review of 347 studies, including 70,490 person-years of metformin use, compared with 55,451 person-years in the nonmetformin group, showed no cases of fatal or nonfatal lactic acidosis in either group (Cochrane Database Syst. Rev. 2010 Apr 14:CD002967). More than half the studies included (53%) allowed for the inclusion of patients with creatinine levels greater than 1.5. There were no differences in lactate levels between metformin-treated patients and patients who did not receive metformin.
In a study using the Saskatchewan Health administrative database, which involved 11,797 patients with 22,296 years of metformin exposure, there were two cases of lactic acidosis (Diabetes Care 1999;22:925-7). This calculates to a rate of 9 cases per 100,000 person years, the same rate as in patients with diabetes who are not taking metformin (9.7 cases per 100,000) (Diabetes Care 1998;21:1659-63).
A recent study looked at the incidence of lactic acidosis in patients taking metformin with and without abnormalities in renal function (Diabetes Care 2014;37:2291-5). There was no statistically significant difference in the rates of lactic acidosis in patients who were on metformin with normal renal function, compared with those with varying degrees of renal insufficiency. The overall rate of lactic acidosis was 10.3 per 100,000 patient years, which is almost identical to the rates in the other studies mentioned, and there were no fatalities.
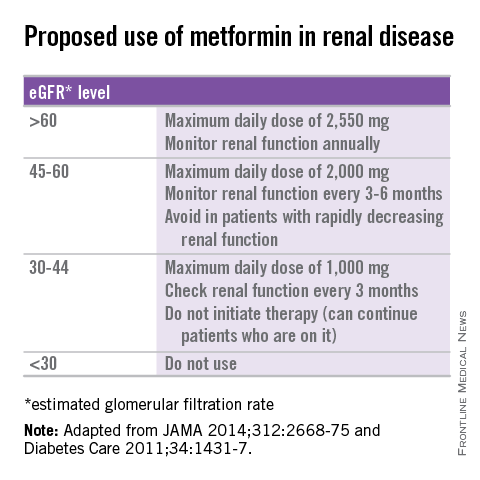
Several recommendations for using metformin in patients with renal insufficiency have been published (see table) (JAMA 2014;312:2668-75; Diabetes Care 2011;34:1431-7). Metformin has shown cardiovascular mortality benefits, compared with sulfonylureas, in the treatment of diabetes (Diabetes Care 2013;36:1304-11). Avoiding its use in patients with mild to moderate renal insufficiency in favor of other treatments that may not be as beneficial and may well lead to worse outcomes.
There is no evidence that metformin increases the lactic acidosis risk in patients with diabetes, but until there is a change in the FDA labeling, physicians will likely continue to be hesitant to use it in patients with renal insufficiency.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is the Rathmann Family Foundation Chair in Patient-Centered Clinical Education. Contact Dr. Paauw at dpaauw@uw.edu.
A 47-year-old obese male with type 2 diabetes has been on metformin for the last 2 years with good effect (hemoglobin A1c of 6.8), and with exercise has been able to lose 5-10 pounds. His last two blood tests show creatinine levels of 1.5 and 1.6. What do you recommend?
A) Continue with metformin.
B) Stop metformin, start sulfonylurea.
C) Stop metformin, begin glargine.
D) Stop metformin, begin pioglitazone.
Myth: Metformin should not be used in patients with mild to moderate renal insufficiency because of an increased risk of lactic acidosis.
Metformin is the most commonly used oral agent for the treatment of type 2 diabetes in the United States, but the FDA-approved drug label states that it is contraindicated in patients with an abnormal creatinine clearance or serum creatinine of 1.4 in women and 1.5 in men.<sup/>The concern is for development of lactic acidosis in patients because the renally excreted metformin may build up as a result of decreased renal function.
Metformin was approved for use in the United States in 1995, many years after the drug was introduced in Europe. The first drug in its class, phenformin, was removed from the United States and most European markets in 1977 because of a high incidence of lactic acidosis occurring at therapeutic doses. One in 4,000 patients taking phenformin develops lactic acidosis (J. Emerg. Med. 1998;16:881-6). Phenformin has been shown to cause type B lactic acidosis, without evidence of hypoxia or hypoperfusion, and lactic acidosis because of phenformin carried a 50% mortality rate.
Deep concern for the possibility of a similar problem with metformin played an important role in its delay of availability in the United States. It isn’t clear, however, that diabetes patients on metformin have a higher risk of developing lactic acidosis than diabetes patients who are not on metformin.
A Cochrane review of 347 studies, including 70,490 person-years of metformin use, compared with 55,451 person-years in the nonmetformin group, showed no cases of fatal or nonfatal lactic acidosis in either group (Cochrane Database Syst. Rev. 2010 Apr 14:CD002967). More than half the studies included (53%) allowed for the inclusion of patients with creatinine levels greater than 1.5. There were no differences in lactate levels between metformin-treated patients and patients who did not receive metformin.
In a study using the Saskatchewan Health administrative database, which involved 11,797 patients with 22,296 years of metformin exposure, there were two cases of lactic acidosis (Diabetes Care 1999;22:925-7). This calculates to a rate of 9 cases per 100,000 person years, the same rate as in patients with diabetes who are not taking metformin (9.7 cases per 100,000) (Diabetes Care 1998;21:1659-63).
A recent study looked at the incidence of lactic acidosis in patients taking metformin with and without abnormalities in renal function (Diabetes Care 2014;37:2291-5). There was no statistically significant difference in the rates of lactic acidosis in patients who were on metformin with normal renal function, compared with those with varying degrees of renal insufficiency. The overall rate of lactic acidosis was 10.3 per 100,000 patient years, which is almost identical to the rates in the other studies mentioned, and there were no fatalities.
Several recommendations for using metformin in patients with renal insufficiency have been published (see table) (JAMA 2014;312:2668-75; Diabetes Care 2011;34:1431-7). Metformin has shown cardiovascular mortality benefits, compared with sulfonylureas, in the treatment of diabetes (Diabetes Care 2013;36:1304-11). Avoiding its use in patients with mild to moderate renal insufficiency in favor of other treatments that may not be as beneficial and may well lead to worse outcomes.
There is no evidence that metformin increases the lactic acidosis risk in patients with diabetes, but until there is a change in the FDA labeling, physicians will likely continue to be hesitant to use it in patients with renal insufficiency.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is the Rathmann Family Foundation Chair in Patient-Centered Clinical Education. Contact Dr. Paauw at dpaauw@uw.edu.
A 47-year-old obese male with type 2 diabetes has been on metformin for the last 2 years with good effect (hemoglobin A1c of 6.8), and with exercise has been able to lose 5-10 pounds. His last two blood tests show creatinine levels of 1.5 and 1.6. What do you recommend?
A) Continue with metformin.
B) Stop metformin, start sulfonylurea.
C) Stop metformin, begin glargine.
D) Stop metformin, begin pioglitazone.
Myth: Metformin should not be used in patients with mild to moderate renal insufficiency because of an increased risk of lactic acidosis.
Metformin is the most commonly used oral agent for the treatment of type 2 diabetes in the United States, but the FDA-approved drug label states that it is contraindicated in patients with an abnormal creatinine clearance or serum creatinine of 1.4 in women and 1.5 in men.<sup/>The concern is for development of lactic acidosis in patients because the renally excreted metformin may build up as a result of decreased renal function.
Metformin was approved for use in the United States in 1995, many years after the drug was introduced in Europe. The first drug in its class, phenformin, was removed from the United States and most European markets in 1977 because of a high incidence of lactic acidosis occurring at therapeutic doses. One in 4,000 patients taking phenformin develops lactic acidosis (J. Emerg. Med. 1998;16:881-6). Phenformin has been shown to cause type B lactic acidosis, without evidence of hypoxia or hypoperfusion, and lactic acidosis because of phenformin carried a 50% mortality rate.
Deep concern for the possibility of a similar problem with metformin played an important role in its delay of availability in the United States. It isn’t clear, however, that diabetes patients on metformin have a higher risk of developing lactic acidosis than diabetes patients who are not on metformin.
A Cochrane review of 347 studies, including 70,490 person-years of metformin use, compared with 55,451 person-years in the nonmetformin group, showed no cases of fatal or nonfatal lactic acidosis in either group (Cochrane Database Syst. Rev. 2010 Apr 14:CD002967). More than half the studies included (53%) allowed for the inclusion of patients with creatinine levels greater than 1.5. There were no differences in lactate levels between metformin-treated patients and patients who did not receive metformin.
In a study using the Saskatchewan Health administrative database, which involved 11,797 patients with 22,296 years of metformin exposure, there were two cases of lactic acidosis (Diabetes Care 1999;22:925-7). This calculates to a rate of 9 cases per 100,000 person years, the same rate as in patients with diabetes who are not taking metformin (9.7 cases per 100,000) (Diabetes Care 1998;21:1659-63).
A recent study looked at the incidence of lactic acidosis in patients taking metformin with and without abnormalities in renal function (Diabetes Care 2014;37:2291-5). There was no statistically significant difference in the rates of lactic acidosis in patients who were on metformin with normal renal function, compared with those with varying degrees of renal insufficiency. The overall rate of lactic acidosis was 10.3 per 100,000 patient years, which is almost identical to the rates in the other studies mentioned, and there were no fatalities.
Several recommendations for using metformin in patients with renal insufficiency have been published (see table) (JAMA 2014;312:2668-75; Diabetes Care 2011;34:1431-7). Metformin has shown cardiovascular mortality benefits, compared with sulfonylureas, in the treatment of diabetes (Diabetes Care 2013;36:1304-11). Avoiding its use in patients with mild to moderate renal insufficiency in favor of other treatments that may not be as beneficial and may well lead to worse outcomes.
There is no evidence that metformin increases the lactic acidosis risk in patients with diabetes, but until there is a change in the FDA labeling, physicians will likely continue to be hesitant to use it in patients with renal insufficiency.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is the Rathmann Family Foundation Chair in Patient-Centered Clinical Education. Contact Dr. Paauw at dpaauw@uw.edu.