User login
For more than a century, clinicians have pondered the significance of elevated blood pressure (BP) and its contribution to cardiovascular disease (CVD). While it is widely understood that high BP increases CVD events, and that treatment lowers that risk, the most appropriate BP goal continues to be a subject of debate.
This article briefly summarizes the evidence to support lower BP goals for patients with hypertension who are commonly seen in family practice, including those needing primary prevention, as well as those with, or at high risk for, atherosclerotic cardiovascular disease (ASCVD), patients with diabetes, and those with chronic kidney disease (CKD). Detailed information regarding specific lifestyle and medication treatment recommendations and thresholds for drug therapy is beyond the scope of this review.
A brief history: ACC/AHA guidelines vs JNC 7 and 8
The most recent comprehensive, evidence-based guideline on the prevention, detection, evaluation, and management of high BP in adults was released in late 2017 by the American College of Cardiology (ACC) and the American Heart Association (AHA).1 It was the first comprehensive BP guideline since the Seventh Report of the Joint National Committee (JNC 7) in 2003.2 The new guideline includes several changes, notably in how BP is classified, the threshold for initiation of antihypertensive drug therapy, and target BP.
While widely viewed as positive, the changes in classification, thresholds, and targets for BP therapy have generated controversy and disagreement. Common reasons cited include concern about the data supporting lower thresholds for treatment, the applicability of trial findings to broad patient populations, and the risk of harm with lower BP goals.3 The American Academy of Family Physicians (AAFP) declined to endorse the ACC/AHA guidelines and continues to support the 2014 report by the panel members appointed to the Eighth Joint National Committee (JNC 8) by the National Heart Lung and Blood Institute (NHLBI).4 A primary reason cited for the lack of support for the 2017 guideline is that the majority of recommendations made in the ACC/AHA guideline were not “based on a systematic evidence review.”4 However, there are significant differences in purpose, structure, and scope between the ACC/AHA and JNC 8.
In 2013, the NHLBI announced that it would cease involvement in creating guidelines and transferred responsibility for development to professional organizations.5 Of the 5 guidelines that were in the process of creation (cholesterol, lifestyle intervention, obesity, risk assessment, and high BP), all but the high BP guideline were transferred to the ACC/AHA for completion. The panel members appointed to the JNC 8 elected to publish their recommendations independently and focused only on 3 “critical questions” related to hypertension therapy (eg, therapy initiation, BP goals, and choice of initial agent).6
[polldaddy:10041785]
The JNC 8 report generated significant controversy with the recommendation to relax the BP goal for patients ≥60 years of age to <150/90 mm Hg. Members of the JNC 8 panel who disagreed with this goal published a "minority view" citing concerns about the negative impact the goal would have on CVD and public health, and the "insufficient and inconsistent" evidence supporting relaxed goals.7 The dissenting group cited additional drawbacks of the recommendation, noting that it was highly focused, included data only from randomized controlled trials (RCTs; no meta-analyses or observational data), and did not address or provide guidance on numerous other issues of importance in the care of hypertension.
While the 2017 ACC/AHA guideline also includes formal systematic evidence reviews on major critical questions (ie, optimal BP targets, preferred antihypertensives, the role of home and ambulatory BP monitoring),8 it was designed to be comprehensive and useful for clinicians, providing 106 graded recommendations on commonly encountered questions. It would have been unrealistic to do a formal systematic evidence review and meta-analysis on all clinically relevant questions seen in practice. However, available systematic reviews, meta-analyses, and observational data were scrutinized and used to support the recommendations wherever possible.
Continue to: Say "goodbye" to prehypertension; say "hello" to elevated BP
Say “goodbye” to prehypertension; say “hello” to elevated BP
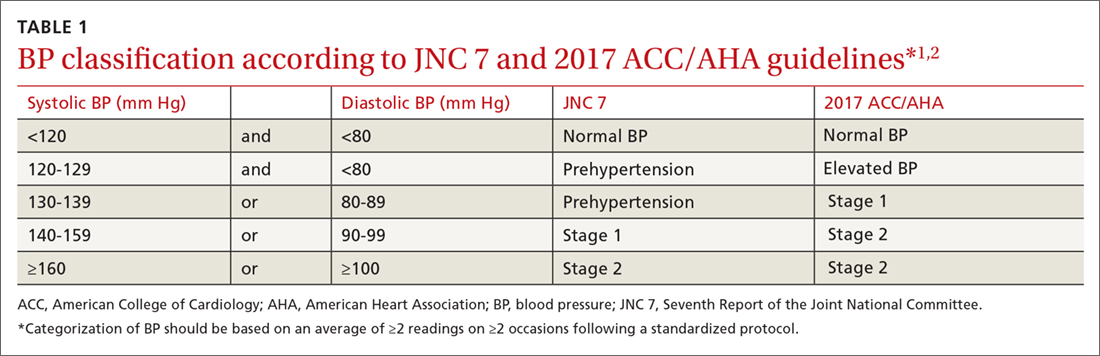
The 2017 ACC/AHA guideline changed the BP classification for adults (TABLE 11,2). While “normal” remained respectively.1 Removal of the “prehypertension” category and use of the term “elevated” instead was meant to better convey the importance of lifestyle interventions to forestall the development of hypertension.
Don’t underestimate the power of BP measurement technique
The importance of appropriate BP measurement technique to confirm the diagnosis of hypertension and assist with medication titration was also emphasized.1 BP measurement technique in usual clinical practice is frequently suboptimal, most commonly resulting in falsely elevated readings.9,10 The guideline recommends the use of out-of-office measurements to confirm elevated clinic readings, screen for white-coat and masked hypertension, and assist in medication adjustment decisions. It is critically important that appropriate BP measurement technique is used, which in many cases, will avoid inappropriate treatment. (See “Getting the hypertension Dx right: Patient positioning matters,” JFP. 2018;67:199-207.)
A look at the evidence supporting lower BP goals
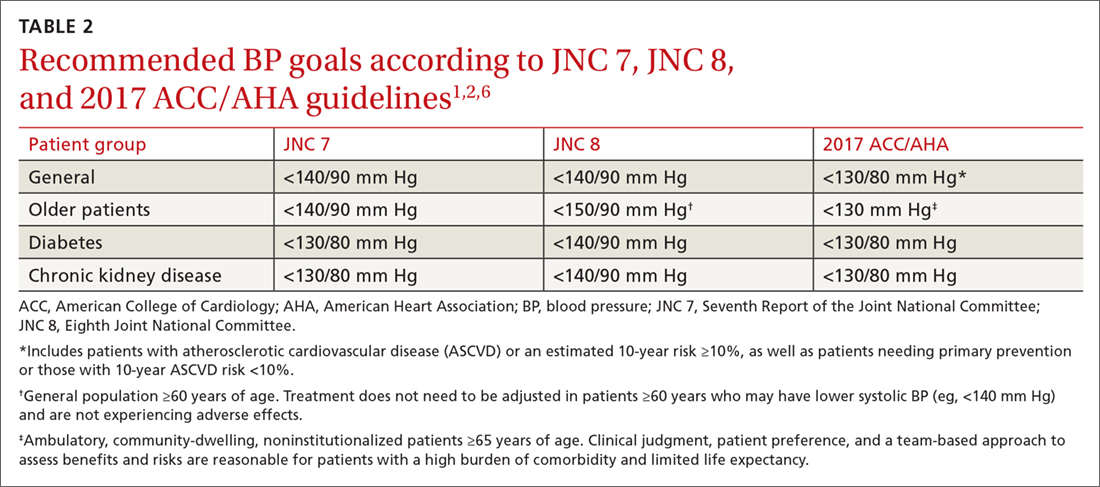
The 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for adults with hypertension commonly seen in clinical practice, including those with CVD or an elevated ASCVD risk (10-year risk ≥10% using the Pooled Cohort Equations11), those with hypertension and low ASCVD risk (10-year risk <10%), and those with hypertension who have concomitant diabetes or CKD.1 The guideline also recommends an SBP goal <130 mm Hg for independently-living, ambulatory older adults (≥65 years) with hypertension.1 TABLE 21,2,6 compares the BP goals in the new 2017 ACC/AHA guidelines to previous recommendations.
SPRINT. Significant new literature has been generated since the publication of JNC 8 that supports these lower BP goals, particularly in patients with CVD or who are at high ASCVD risk.8,12-15 For example, the Systolic Blood Pressure Intervention Trial (SPRINT) was the largest RCT to assess whether lower BP goals decrease the risk of adverse CVD outcomes.16 In SPRINT, 9361 patients with an SBP ≥130 mm Hg and an increased risk of CVD, but without diabetes or a history of stroke, were randomized to intensive BP treatment (SBP goal <120 mm Hg) or standard treatment (SBP goal <140 mm Hg). After a median follow-up of 3.26 years, the study was stopped early due to a decreased risk in the primary composite outcome of myocardial infarction (MI), other acute coronary syndromes (ACS), stroke, heart failure, or death from CV causes (number needed to treat [NNT] to prevent one event=61).
Intensive treatment was also associated with a lower risk of all-cause mortality (NNT=90), heart failure (NNT=123), death from CV causes (NNT=172), and the primary outcome or death (NNT=52
Continue to: Meta-analyses that have been conducted since SPRINT...
Meta-analyses that have been conducted since SPRINT, and that have incorporated SPRINT data, also support lower BP goals. In the systematic review performed for the 2017 ACC/AHA guideline, an SBP <130 mm Hg compared to a higher BP target was associated with a reduced risk of major CV events, stroke, MI, and heart failure, although not all-cause mortality.8 These findings were largely consistent with other recent meta-analyses.12-15 For example, Bundy et al15 reported significant CV benefit with more vs less intensive BP lowering, whether or not the data from SPRINT were included, with the greatest reduction in risk seen in the groups with highest baseline BP.
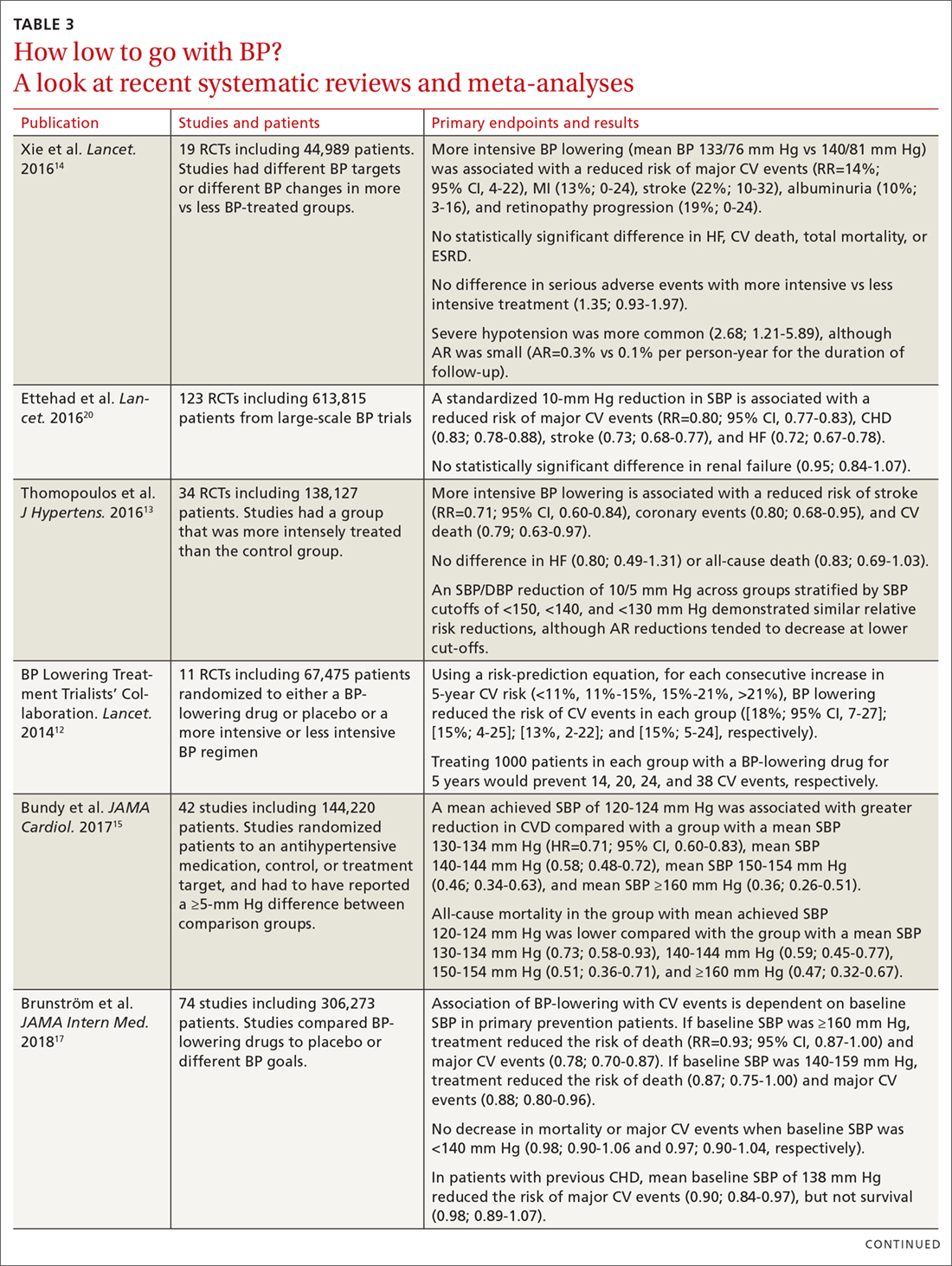
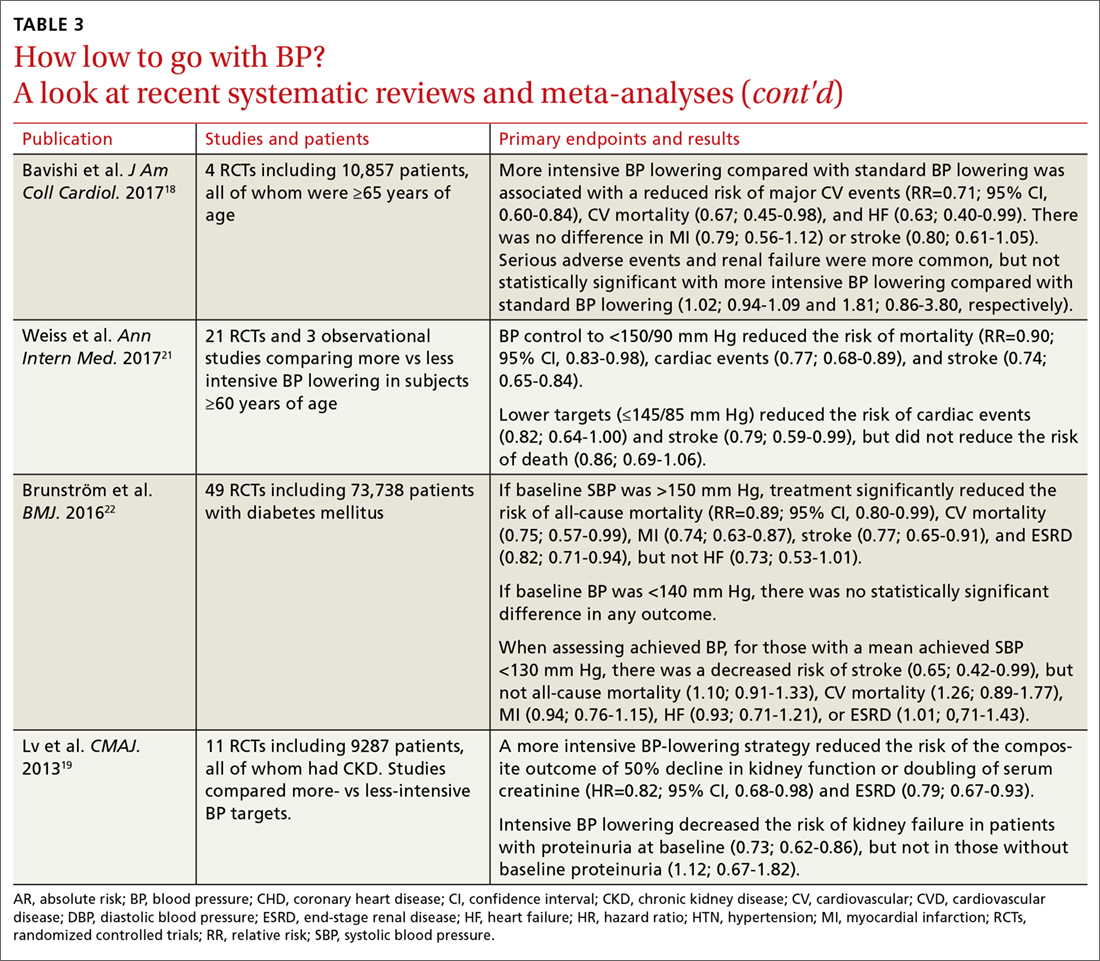
It is important to consider a patient’s baseline level of risk when evaluating the absolute benefit of lower BP targets on CV outcomes. For patients with higher CV risk, the absolute benefit of treatment is greater.12-14 These findings support the 2017 ACC/AHA guideline, which recommends initiating drug therapy, in addition to lifestyle modification, in adults with hypertension and high ASCVD risk when the average BP is >130/80 mm Hg, with a goal of <130/80 mm Hg. TABLE 312-15,17-22 summarizes recent systematic reviews and meta-analyses conducted since the publication of JNC 8 that assess the association between intensity of BP lowering and adverse CV and related outcomes.
Treating patients with low CV risk
The evidence supporting a lower BP goal in patients with low CV risk is less than for patients at elevated risk. There are no large RCTs for this group that have assessed whether an intensive BP lowering strategy decreases CV outcomes more than a standard BP strategy (eg, <140/90 mm Hg). It is likely that absolute benefit is much smaller than for patients with, or at high risk for, ASCVD.
However, epidemiologic observational studies have indicated a significant log-linear increase in CV mortality starting at an SBP of 115 mm Hg.23 A 20-mm Hg increase in SBP above 115 mm Hg is associated with an approximate doubling of stroke and ischemic heart disease mortality risk.23 Decades worth of exposure to “elevated” BP levels would likely result in significant vascular damage, and attenuation of this process would likely be beneficial.24,25 An RCT specifically designed to test this hypothesis, however, would not be pragmatic considering the substantial number of patient-years that would be required.
Due to insufficient data documenting the value of antihypertensive drug therapy for primary prevention in adults with “elevated” BP and stage 1 hypertension at low risk for CVD, the 2017 ACC/AHA guideline recommends that drug therapy be initiated for all adults only when their BP average is ≥140/90 mm Hg.1 In contrast, for patients needing secondary prevention and for those with elevated CVD risk, the guideline recommends medication in addition to lifestyle modifications once the average BP is ≥130/80 mm Hg. The recommendation to withhold drug therapy until the BP is ≥140/90 mm Hg in patients needing primary prevention is supported by a new meta-analysis of 74 trials with 306,273 participants that aimed to assess the association between BP-lowering treatment and death and CVD at various BP levels.17 In this analysis, pharmacologic treatment was associated with a reduced risk of all-cause mortality, major CVD events, and coronary heart disease if the SBP was ≥140 mm Hg.
Continue to: Treating older patients
Treating older patients
Significant controversy has existed regarding the optimal BP goal in older patients, particularly once the JNC 8 recommended relaxing the SBP goal to <150 mm Hg for pateints ≥60 years of age.6,7 This recommendation was consistent with the guideline from the American College of Physicians (ACP)/AAFP,26 which also recommended a lower SBP of <140 mm Hg in patients with a history of stroke or transient ischemic attack and those at high CV risk.26
Evidence is available, however, supporting more intensive BP goals in older independently-living ambulatory adults. A pre-planned subgroup analysis was conducted in 2636 SPRINT participants ≥75 years of age.27 Similar to the overall experience in SPRINT, lower SBP goals were associated with significant reductions in CV events, including the composite CVD primary outcome (NNT=27), heart failure (NNT=63), nonfatal heart failure (NNT=66), and all-cause mortality (NNT=41). In addition, the relative benefits were approximately equal whether the patients were the most fit, non-fit, or frail, with the absolute benefit being greatest in those who were frail (recognizing that the SPRINT participants were independently-living ambulatory adults). While the absolute rate of serious adverse events was higher in the more intensive BP goal group, there was no statistically significant difference in the incidence of hypotension, orthostatic hypotension, syncope, electrolyte abnormalities, or acute kidney injury or renal failure.
Use of lower BP goals than recommended by JNC 8 was also supported by another recent meta-analysis that compared the outcomes of intensive BP lowering (SBP <140 mm Hg) to a standard BP-lowering strategy (SBP <150 mm Hg).18 Using a random-effects model, more intensive BP lowering was associated with a significant reduction in major adverse CV events (29%), CV mortality (33%), and heart failure (37%), with no increase in serious adverse events or renal failure. Findings with the fixed-effects model used to confirm results were largely consistent, with the exception of a possible increase in renal failure.
Although the evidence supporting lower BP goals in older, ambulatory, noninstitutionalized patients is sound, it is important to consider a patient’s overall disease burden. For older adults with multiple comorbidities and limited life expectancy, as well as those who are nonambulatory or institutionalized, decisions on the intensity of BP lowering should be made using a team-based approach, weighing the risks and benefits.1
Continue to: Treating patients with diabetes
Treating patients with diabetes
The most appropriate BP goal for patients with diabetes has been the subject of much debate, with different goals recommended in different guidelines (TABLE 21,2,6). The most recent American Diabetes Association guideline recommends a BP goal <140/90 mm Hg for most patients, with lower targets (<130/80 mm Hg) for patients at high CV risk if it is achievable without undue treatment burden,28 whereas the 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for all adults with diabetes.1
The ACCORD trial. There is limited evidence to suggest which BP goal is most appropriate for patients with diabetes. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial is the only RCT specifically designed to assess the impact of intensive vs standard BP goals in patients with diabetes.29 In ACCORD, 4733 patients with type 2 diabetes were randomized to either an intensive BP-lowering group (SBP <120 mm Hg) or a standard BP-lowering group (SBP <140 mm Hg). After a mean follow-up of 4.7 years, there was no difference in the primary composite endpoint of nonfatal MI, nonfatal stroke, or death from CV causes. However, the risk of stroke was reduced (NNT=89). Interpretation of ACCORD is limited due to its factorial design and because the trial was significantly underpowered.
Systematic reviews and meta-analyses. Literature supporting lower BP goals in patients with diabetes primarily comes from systematic reviews and meta-analyses.30 In the evidence-based review performed for the 2017 ACC/AHA guidelines, more intensive treatment was associated with a decrease in fatal or nonfatal stroke.8 The results from the ACCORD trial and SPRINT are consistent,31 and a sub-study of SPRINT patients with pre-diabetes showed preservation of CV benefit.32 Also, a meta-analysis of subgroups of trial participants with diabetes showed that more intensive BP lowering in patients is associated with a decrease in major CV events.14
Treating patients with chronic kidney disease
As with diabetes and older patients, recommended goals for patients with CKD have varied (TABLE 21,2,6). The Kidney Disease Improving Global Outcomes (KDIGO) 2012 guideline recommended the same target BP as JNC 7 and the 2017 ACC/AHA guideline: ≤130/80 mm Hg in patients with CKD and urine albumin excretion ≥30 mg/24 hours (or equivalent).1,2,33 KDIGO recommended a more relaxed target (≤140/90 mm Hg), however, for patients with CKD and urine albumin excretion <30 mg/24 hours.1,33
Scant data exist from RCTs designed to assess the CV effects of intensive BP targets in patients with CKD. In SPRINT, where 28% of patients had stage 3 or 4 CKD, benefits of more intensive therapy were similar to those observed in the overall cohort.16,34 While some RCTs have assessed the effect of more intensive BP lowering on progression of CKD, they were not specifically designed or powered to address CV outcomes.35,36
Continue to: In recent meta-analyses assessing the effects...
In recent meta-analyses assessing the effects of intensive BP lowering on renal and CV events in patients with CKD, a lower BP strategy was not associated with a decrease in CV events.8,14,19 However, more intensive therapy was associated with a 17% reduced risk of composite kidney failure events and an 18% reduction in end-stage kidney disease.19 The risk of kidney failure with lower BP goals was 27% lower in patients with baseline proteinuria, but was not significant in patients who did not have proteinuria.19
Evidence supports lower BP goals, but guidelines should guide
The lower BP goals advised in the 2017 ACC/AHA guideline are supported by substantial new high-quality evidence that was not available at the time of the JNC 8 report.1 The strongest evidence for lower goals is found in patients with, or at high risk for, CVD, but other patients commonly seen by primary care providers, including those at lower CVD risk, older patients, and those with diabetes or CKD are also likely to benefit.1
Despite the debates, it is important to remember that guidelines are intended to “guide.” As stated in the guideline, “Guidelines are intended to define practices meeting the needs of patients in most, but not all, circumstances and should not replace clinical judgment.”1 They should be easy to understand and apply, and a consistent, evidence-based BP goal of <130/80 mm Hg for most patients facilitates implementation.
Although more of the US population is categorized as hypertensive under the new guideline (46% now vs 32% before), only 1.9% more require drug therapy, as the vast majority of the newly classified hypertensives are primary prevention patients for whom only lifestyle modification is recommended.37 However, to attain these goals, greater emphasis will be needed on utilizing team-based care, health information technology including electronic medical records and telehealth, performance measures, quality improvement strategies, and financial incentives.1
Finally, as emphasized in the guidelines, BP monitoring technique matters. Clinicians should not accept flawed BP measurement techniques any more than they would accept flawed results from studies performed incorrectly.
CORRESPONDENCE
Eric J. MacLaughlin, PharmD, BCPS, FASHP, FCCP, Texas Tech University Health Sciences Center,1300 S. Coulter Dr., Amarillo, TX 79106; Eric.MacLaughlin@ttuhsc.edu.
ACKNOWLEDGEMENTS
The authors thank Paul K. Whelton, MB, MD, MSc, FAHA, and Robert M. Carey, MD, FAHA, for their review of this manuscript.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
2. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560-2572.
3. Wilt TJ, Kansagara D, Qaseem A; Clinical Guidelines Committee of the American College of Physicians. Hypertension limbo: balancing benefits, harms, and patient preferences before we lower the bar on blood pressure. Ann Intern Med. 2018;168:369-370.
4. American Academy of Family Physicians. AAFP decides to not endorse AHA/ACC hypertension guideline. Available at: https://www.aafp.org/news/health-of-the-public/20171212notendorseaha-accgdlne.html. Accessed January 9, 2018.
5. Gibbons GH, Shurin SB, Mensah GA, et al. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation. 2013;128:1713-1715.
6. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
7. Wright JT Jr., Fine LJ, Lackland DT, et al. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499-503.
8. Reboussin DM, Allen NB, Griswold ME, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e116-e135.
9. Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation. 2016;134:904-905.
10. Burgess SE, MacLaughlin EJ, Smith PA, et al. Blood pressure rising: differences between current clinical and recommended measurement techniques. J Am Soc Hypertens. 2011;5:484-488.
11. American College of Cardiology. ASCVD Risk Estimator Plus. Available at: http://tools.acc.org/ascvd-risk-estimator-plus/#!/calculate/estimate/. Accessed January 9, 2018.
12. The Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591-598.
13. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels - updated overview and meta-analyses of randomized trials. J Hypertens. 2016;34:613-622.
14. Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435-443.
15. Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2:775-781.
16. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
17. Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:28-36.
18. Bavishi C, Bangalore S, Messerli FH. Outcomes of intensive blood pressure lowering in older hypertensive patients. J Am Coll Cardiol. 2017;69:486-493.
19. Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949-957.
20. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957-967.
21. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med. 2017;166:419-429.
22. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
23. Lewington S, Clarke R, Qizilbash N, et al; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913.
24. Guo X, Zhang X, Guo L, et al. Association between pre-hypertension and cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Curr Hypertens Rep. 2013;15:703-716.
25. Huang Y, Cai X, Li Y, et al. Prehypertension and the risk of stroke: a meta-analysis. Neurology. 2014;82:1153-1161.
26. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430-437.
27. Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673-2682.
28. American Diabetes Association. 9. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S86-S104.
29. The ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
30. Reboldi G, Gentile G, Angeli F, et al. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J Hypertens. 2011;29:1253-1269.
31. Perkovic V, Rodgers A. Redefining blood-pressure targets—SPRINT starts the marathon. N Engl J Med. 2015;373:2175-2178.
32. Bress AP, King JB, Kreider KE, et al. Effect of intensive versus standard blood pressure treatment according to baseline prediabetes status: a post hoc analysis of a randomized trial. Diabetes Care. 2017 Aug 9.
33. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:337-414.
34. Cheung AK, Rahman M, Reboussin DM, et al. Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017;28:2812-2823.
35. Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939-946.
36. Wright JT Jr., Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421-2431.
37. Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 American College of Cardiology/American Heart Association High Blood Pressure Guideline. J Am Coll Cardiol. 2018;71:109-188.
For more than a century, clinicians have pondered the significance of elevated blood pressure (BP) and its contribution to cardiovascular disease (CVD). While it is widely understood that high BP increases CVD events, and that treatment lowers that risk, the most appropriate BP goal continues to be a subject of debate.
This article briefly summarizes the evidence to support lower BP goals for patients with hypertension who are commonly seen in family practice, including those needing primary prevention, as well as those with, or at high risk for, atherosclerotic cardiovascular disease (ASCVD), patients with diabetes, and those with chronic kidney disease (CKD). Detailed information regarding specific lifestyle and medication treatment recommendations and thresholds for drug therapy is beyond the scope of this review.
A brief history: ACC/AHA guidelines vs JNC 7 and 8
The most recent comprehensive, evidence-based guideline on the prevention, detection, evaluation, and management of high BP in adults was released in late 2017 by the American College of Cardiology (ACC) and the American Heart Association (AHA).1 It was the first comprehensive BP guideline since the Seventh Report of the Joint National Committee (JNC 7) in 2003.2 The new guideline includes several changes, notably in how BP is classified, the threshold for initiation of antihypertensive drug therapy, and target BP.
While widely viewed as positive, the changes in classification, thresholds, and targets for BP therapy have generated controversy and disagreement. Common reasons cited include concern about the data supporting lower thresholds for treatment, the applicability of trial findings to broad patient populations, and the risk of harm with lower BP goals.3 The American Academy of Family Physicians (AAFP) declined to endorse the ACC/AHA guidelines and continues to support the 2014 report by the panel members appointed to the Eighth Joint National Committee (JNC 8) by the National Heart Lung and Blood Institute (NHLBI).4 A primary reason cited for the lack of support for the 2017 guideline is that the majority of recommendations made in the ACC/AHA guideline were not “based on a systematic evidence review.”4 However, there are significant differences in purpose, structure, and scope between the ACC/AHA and JNC 8.
In 2013, the NHLBI announced that it would cease involvement in creating guidelines and transferred responsibility for development to professional organizations.5 Of the 5 guidelines that were in the process of creation (cholesterol, lifestyle intervention, obesity, risk assessment, and high BP), all but the high BP guideline were transferred to the ACC/AHA for completion. The panel members appointed to the JNC 8 elected to publish their recommendations independently and focused only on 3 “critical questions” related to hypertension therapy (eg, therapy initiation, BP goals, and choice of initial agent).6
[polldaddy:10041785]
The JNC 8 report generated significant controversy with the recommendation to relax the BP goal for patients ≥60 years of age to <150/90 mm Hg. Members of the JNC 8 panel who disagreed with this goal published a "minority view" citing concerns about the negative impact the goal would have on CVD and public health, and the "insufficient and inconsistent" evidence supporting relaxed goals.7 The dissenting group cited additional drawbacks of the recommendation, noting that it was highly focused, included data only from randomized controlled trials (RCTs; no meta-analyses or observational data), and did not address or provide guidance on numerous other issues of importance in the care of hypertension.
While the 2017 ACC/AHA guideline also includes formal systematic evidence reviews on major critical questions (ie, optimal BP targets, preferred antihypertensives, the role of home and ambulatory BP monitoring),8 it was designed to be comprehensive and useful for clinicians, providing 106 graded recommendations on commonly encountered questions. It would have been unrealistic to do a formal systematic evidence review and meta-analysis on all clinically relevant questions seen in practice. However, available systematic reviews, meta-analyses, and observational data were scrutinized and used to support the recommendations wherever possible.
Continue to: Say "goodbye" to prehypertension; say "hello" to elevated BP
Say “goodbye” to prehypertension; say “hello” to elevated BP
The 2017 ACC/AHA guideline changed the BP classification for adults (TABLE 11,2). While “normal” remained respectively.1 Removal of the “prehypertension” category and use of the term “elevated” instead was meant to better convey the importance of lifestyle interventions to forestall the development of hypertension.

Don’t underestimate the power of BP measurement technique
The importance of appropriate BP measurement technique to confirm the diagnosis of hypertension and assist with medication titration was also emphasized.1 BP measurement technique in usual clinical practice is frequently suboptimal, most commonly resulting in falsely elevated readings.9,10 The guideline recommends the use of out-of-office measurements to confirm elevated clinic readings, screen for white-coat and masked hypertension, and assist in medication adjustment decisions. It is critically important that appropriate BP measurement technique is used, which in many cases, will avoid inappropriate treatment. (See “Getting the hypertension Dx right: Patient positioning matters,” JFP. 2018;67:199-207.)
A look at the evidence supporting lower BP goals
The 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for adults with hypertension commonly seen in clinical practice, including those with CVD or an elevated ASCVD risk (10-year risk ≥10% using the Pooled Cohort Equations11), those with hypertension and low ASCVD risk (10-year risk <10%), and those with hypertension who have concomitant diabetes or CKD.1 The guideline also recommends an SBP goal <130 mm Hg for independently-living, ambulatory older adults (≥65 years) with hypertension.1 TABLE 21,2,6 compares the BP goals in the new 2017 ACC/AHA guidelines to previous recommendations.

SPRINT. Significant new literature has been generated since the publication of JNC 8 that supports these lower BP goals, particularly in patients with CVD or who are at high ASCVD risk.8,12-15 For example, the Systolic Blood Pressure Intervention Trial (SPRINT) was the largest RCT to assess whether lower BP goals decrease the risk of adverse CVD outcomes.16 In SPRINT, 9361 patients with an SBP ≥130 mm Hg and an increased risk of CVD, but without diabetes or a history of stroke, were randomized to intensive BP treatment (SBP goal <120 mm Hg) or standard treatment (SBP goal <140 mm Hg). After a median follow-up of 3.26 years, the study was stopped early due to a decreased risk in the primary composite outcome of myocardial infarction (MI), other acute coronary syndromes (ACS), stroke, heart failure, or death from CV causes (number needed to treat [NNT] to prevent one event=61).
Intensive treatment was also associated with a lower risk of all-cause mortality (NNT=90), heart failure (NNT=123), death from CV causes (NNT=172), and the primary outcome or death (NNT=52
Continue to: Meta-analyses that have been conducted since SPRINT...
Meta-analyses that have been conducted since SPRINT, and that have incorporated SPRINT data, also support lower BP goals. In the systematic review performed for the 2017 ACC/AHA guideline, an SBP <130 mm Hg compared to a higher BP target was associated with a reduced risk of major CV events, stroke, MI, and heart failure, although not all-cause mortality.8 These findings were largely consistent with other recent meta-analyses.12-15 For example, Bundy et al15 reported significant CV benefit with more vs less intensive BP lowering, whether or not the data from SPRINT were included, with the greatest reduction in risk seen in the groups with highest baseline BP.
It is important to consider a patient’s baseline level of risk when evaluating the absolute benefit of lower BP targets on CV outcomes. For patients with higher CV risk, the absolute benefit of treatment is greater.12-14 These findings support the 2017 ACC/AHA guideline, which recommends initiating drug therapy, in addition to lifestyle modification, in adults with hypertension and high ASCVD risk when the average BP is >130/80 mm Hg, with a goal of <130/80 mm Hg. TABLE 312-15,17-22 summarizes recent systematic reviews and meta-analyses conducted since the publication of JNC 8 that assess the association between intensity of BP lowering and adverse CV and related outcomes.


Treating patients with low CV risk
The evidence supporting a lower BP goal in patients with low CV risk is less than for patients at elevated risk. There are no large RCTs for this group that have assessed whether an intensive BP lowering strategy decreases CV outcomes more than a standard BP strategy (eg, <140/90 mm Hg). It is likely that absolute benefit is much smaller than for patients with, or at high risk for, ASCVD.
However, epidemiologic observational studies have indicated a significant log-linear increase in CV mortality starting at an SBP of 115 mm Hg.23 A 20-mm Hg increase in SBP above 115 mm Hg is associated with an approximate doubling of stroke and ischemic heart disease mortality risk.23 Decades worth of exposure to “elevated” BP levels would likely result in significant vascular damage, and attenuation of this process would likely be beneficial.24,25 An RCT specifically designed to test this hypothesis, however, would not be pragmatic considering the substantial number of patient-years that would be required.
Due to insufficient data documenting the value of antihypertensive drug therapy for primary prevention in adults with “elevated” BP and stage 1 hypertension at low risk for CVD, the 2017 ACC/AHA guideline recommends that drug therapy be initiated for all adults only when their BP average is ≥140/90 mm Hg.1 In contrast, for patients needing secondary prevention and for those with elevated CVD risk, the guideline recommends medication in addition to lifestyle modifications once the average BP is ≥130/80 mm Hg. The recommendation to withhold drug therapy until the BP is ≥140/90 mm Hg in patients needing primary prevention is supported by a new meta-analysis of 74 trials with 306,273 participants that aimed to assess the association between BP-lowering treatment and death and CVD at various BP levels.17 In this analysis, pharmacologic treatment was associated with a reduced risk of all-cause mortality, major CVD events, and coronary heart disease if the SBP was ≥140 mm Hg.
Continue to: Treating older patients
Treating older patients
Significant controversy has existed regarding the optimal BP goal in older patients, particularly once the JNC 8 recommended relaxing the SBP goal to <150 mm Hg for pateints ≥60 years of age.6,7 This recommendation was consistent with the guideline from the American College of Physicians (ACP)/AAFP,26 which also recommended a lower SBP of <140 mm Hg in patients with a history of stroke or transient ischemic attack and those at high CV risk.26
Evidence is available, however, supporting more intensive BP goals in older independently-living ambulatory adults. A pre-planned subgroup analysis was conducted in 2636 SPRINT participants ≥75 years of age.27 Similar to the overall experience in SPRINT, lower SBP goals were associated with significant reductions in CV events, including the composite CVD primary outcome (NNT=27), heart failure (NNT=63), nonfatal heart failure (NNT=66), and all-cause mortality (NNT=41). In addition, the relative benefits were approximately equal whether the patients were the most fit, non-fit, or frail, with the absolute benefit being greatest in those who were frail (recognizing that the SPRINT participants were independently-living ambulatory adults). While the absolute rate of serious adverse events was higher in the more intensive BP goal group, there was no statistically significant difference in the incidence of hypotension, orthostatic hypotension, syncope, electrolyte abnormalities, or acute kidney injury or renal failure.
Use of lower BP goals than recommended by JNC 8 was also supported by another recent meta-analysis that compared the outcomes of intensive BP lowering (SBP <140 mm Hg) to a standard BP-lowering strategy (SBP <150 mm Hg).18 Using a random-effects model, more intensive BP lowering was associated with a significant reduction in major adverse CV events (29%), CV mortality (33%), and heart failure (37%), with no increase in serious adverse events or renal failure. Findings with the fixed-effects model used to confirm results were largely consistent, with the exception of a possible increase in renal failure.
Although the evidence supporting lower BP goals in older, ambulatory, noninstitutionalized patients is sound, it is important to consider a patient’s overall disease burden. For older adults with multiple comorbidities and limited life expectancy, as well as those who are nonambulatory or institutionalized, decisions on the intensity of BP lowering should be made using a team-based approach, weighing the risks and benefits.1
Continue to: Treating patients with diabetes
Treating patients with diabetes
The most appropriate BP goal for patients with diabetes has been the subject of much debate, with different goals recommended in different guidelines (TABLE 21,2,6). The most recent American Diabetes Association guideline recommends a BP goal <140/90 mm Hg for most patients, with lower targets (<130/80 mm Hg) for patients at high CV risk if it is achievable without undue treatment burden,28 whereas the 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for all adults with diabetes.1
The ACCORD trial. There is limited evidence to suggest which BP goal is most appropriate for patients with diabetes. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial is the only RCT specifically designed to assess the impact of intensive vs standard BP goals in patients with diabetes.29 In ACCORD, 4733 patients with type 2 diabetes were randomized to either an intensive BP-lowering group (SBP <120 mm Hg) or a standard BP-lowering group (SBP <140 mm Hg). After a mean follow-up of 4.7 years, there was no difference in the primary composite endpoint of nonfatal MI, nonfatal stroke, or death from CV causes. However, the risk of stroke was reduced (NNT=89). Interpretation of ACCORD is limited due to its factorial design and because the trial was significantly underpowered.

Systematic reviews and meta-analyses. Literature supporting lower BP goals in patients with diabetes primarily comes from systematic reviews and meta-analyses.30 In the evidence-based review performed for the 2017 ACC/AHA guidelines, more intensive treatment was associated with a decrease in fatal or nonfatal stroke.8 The results from the ACCORD trial and SPRINT are consistent,31 and a sub-study of SPRINT patients with pre-diabetes showed preservation of CV benefit.32 Also, a meta-analysis of subgroups of trial participants with diabetes showed that more intensive BP lowering in patients is associated with a decrease in major CV events.14
Treating patients with chronic kidney disease
As with diabetes and older patients, recommended goals for patients with CKD have varied (TABLE 21,2,6). The Kidney Disease Improving Global Outcomes (KDIGO) 2012 guideline recommended the same target BP as JNC 7 and the 2017 ACC/AHA guideline: ≤130/80 mm Hg in patients with CKD and urine albumin excretion ≥30 mg/24 hours (or equivalent).1,2,33 KDIGO recommended a more relaxed target (≤140/90 mm Hg), however, for patients with CKD and urine albumin excretion <30 mg/24 hours.1,33
Scant data exist from RCTs designed to assess the CV effects of intensive BP targets in patients with CKD. In SPRINT, where 28% of patients had stage 3 or 4 CKD, benefits of more intensive therapy were similar to those observed in the overall cohort.16,34 While some RCTs have assessed the effect of more intensive BP lowering on progression of CKD, they were not specifically designed or powered to address CV outcomes.35,36
Continue to: In recent meta-analyses assessing the effects...
In recent meta-analyses assessing the effects of intensive BP lowering on renal and CV events in patients with CKD, a lower BP strategy was not associated with a decrease in CV events.8,14,19 However, more intensive therapy was associated with a 17% reduced risk of composite kidney failure events and an 18% reduction in end-stage kidney disease.19 The risk of kidney failure with lower BP goals was 27% lower in patients with baseline proteinuria, but was not significant in patients who did not have proteinuria.19
Evidence supports lower BP goals, but guidelines should guide
The lower BP goals advised in the 2017 ACC/AHA guideline are supported by substantial new high-quality evidence that was not available at the time of the JNC 8 report.1 The strongest evidence for lower goals is found in patients with, or at high risk for, CVD, but other patients commonly seen by primary care providers, including those at lower CVD risk, older patients, and those with diabetes or CKD are also likely to benefit.1
Despite the debates, it is important to remember that guidelines are intended to “guide.” As stated in the guideline, “Guidelines are intended to define practices meeting the needs of patients in most, but not all, circumstances and should not replace clinical judgment.”1 They should be easy to understand and apply, and a consistent, evidence-based BP goal of <130/80 mm Hg for most patients facilitates implementation.
Although more of the US population is categorized as hypertensive under the new guideline (46% now vs 32% before), only 1.9% more require drug therapy, as the vast majority of the newly classified hypertensives are primary prevention patients for whom only lifestyle modification is recommended.37 However, to attain these goals, greater emphasis will be needed on utilizing team-based care, health information technology including electronic medical records and telehealth, performance measures, quality improvement strategies, and financial incentives.1
Finally, as emphasized in the guidelines, BP monitoring technique matters. Clinicians should not accept flawed BP measurement techniques any more than they would accept flawed results from studies performed incorrectly.
CORRESPONDENCE
Eric J. MacLaughlin, PharmD, BCPS, FASHP, FCCP, Texas Tech University Health Sciences Center,1300 S. Coulter Dr., Amarillo, TX 79106; Eric.MacLaughlin@ttuhsc.edu.
ACKNOWLEDGEMENTS
The authors thank Paul K. Whelton, MB, MD, MSc, FAHA, and Robert M. Carey, MD, FAHA, for their review of this manuscript.
For more than a century, clinicians have pondered the significance of elevated blood pressure (BP) and its contribution to cardiovascular disease (CVD). While it is widely understood that high BP increases CVD events, and that treatment lowers that risk, the most appropriate BP goal continues to be a subject of debate.
This article briefly summarizes the evidence to support lower BP goals for patients with hypertension who are commonly seen in family practice, including those needing primary prevention, as well as those with, or at high risk for, atherosclerotic cardiovascular disease (ASCVD), patients with diabetes, and those with chronic kidney disease (CKD). Detailed information regarding specific lifestyle and medication treatment recommendations and thresholds for drug therapy is beyond the scope of this review.
A brief history: ACC/AHA guidelines vs JNC 7 and 8
The most recent comprehensive, evidence-based guideline on the prevention, detection, evaluation, and management of high BP in adults was released in late 2017 by the American College of Cardiology (ACC) and the American Heart Association (AHA).1 It was the first comprehensive BP guideline since the Seventh Report of the Joint National Committee (JNC 7) in 2003.2 The new guideline includes several changes, notably in how BP is classified, the threshold for initiation of antihypertensive drug therapy, and target BP.
While widely viewed as positive, the changes in classification, thresholds, and targets for BP therapy have generated controversy and disagreement. Common reasons cited include concern about the data supporting lower thresholds for treatment, the applicability of trial findings to broad patient populations, and the risk of harm with lower BP goals.3 The American Academy of Family Physicians (AAFP) declined to endorse the ACC/AHA guidelines and continues to support the 2014 report by the panel members appointed to the Eighth Joint National Committee (JNC 8) by the National Heart Lung and Blood Institute (NHLBI).4 A primary reason cited for the lack of support for the 2017 guideline is that the majority of recommendations made in the ACC/AHA guideline were not “based on a systematic evidence review.”4 However, there are significant differences in purpose, structure, and scope between the ACC/AHA and JNC 8.
In 2013, the NHLBI announced that it would cease involvement in creating guidelines and transferred responsibility for development to professional organizations.5 Of the 5 guidelines that were in the process of creation (cholesterol, lifestyle intervention, obesity, risk assessment, and high BP), all but the high BP guideline were transferred to the ACC/AHA for completion. The panel members appointed to the JNC 8 elected to publish their recommendations independently and focused only on 3 “critical questions” related to hypertension therapy (eg, therapy initiation, BP goals, and choice of initial agent).6
[polldaddy:10041785]
The JNC 8 report generated significant controversy with the recommendation to relax the BP goal for patients ≥60 years of age to <150/90 mm Hg. Members of the JNC 8 panel who disagreed with this goal published a "minority view" citing concerns about the negative impact the goal would have on CVD and public health, and the "insufficient and inconsistent" evidence supporting relaxed goals.7 The dissenting group cited additional drawbacks of the recommendation, noting that it was highly focused, included data only from randomized controlled trials (RCTs; no meta-analyses or observational data), and did not address or provide guidance on numerous other issues of importance in the care of hypertension.
While the 2017 ACC/AHA guideline also includes formal systematic evidence reviews on major critical questions (ie, optimal BP targets, preferred antihypertensives, the role of home and ambulatory BP monitoring),8 it was designed to be comprehensive and useful for clinicians, providing 106 graded recommendations on commonly encountered questions. It would have been unrealistic to do a formal systematic evidence review and meta-analysis on all clinically relevant questions seen in practice. However, available systematic reviews, meta-analyses, and observational data were scrutinized and used to support the recommendations wherever possible.
Continue to: Say "goodbye" to prehypertension; say "hello" to elevated BP
Say “goodbye” to prehypertension; say “hello” to elevated BP
The 2017 ACC/AHA guideline changed the BP classification for adults (TABLE 11,2). While “normal” remained respectively.1 Removal of the “prehypertension” category and use of the term “elevated” instead was meant to better convey the importance of lifestyle interventions to forestall the development of hypertension.

Don’t underestimate the power of BP measurement technique
The importance of appropriate BP measurement technique to confirm the diagnosis of hypertension and assist with medication titration was also emphasized.1 BP measurement technique in usual clinical practice is frequently suboptimal, most commonly resulting in falsely elevated readings.9,10 The guideline recommends the use of out-of-office measurements to confirm elevated clinic readings, screen for white-coat and masked hypertension, and assist in medication adjustment decisions. It is critically important that appropriate BP measurement technique is used, which in many cases, will avoid inappropriate treatment. (See “Getting the hypertension Dx right: Patient positioning matters,” JFP. 2018;67:199-207.)
A look at the evidence supporting lower BP goals
The 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for adults with hypertension commonly seen in clinical practice, including those with CVD or an elevated ASCVD risk (10-year risk ≥10% using the Pooled Cohort Equations11), those with hypertension and low ASCVD risk (10-year risk <10%), and those with hypertension who have concomitant diabetes or CKD.1 The guideline also recommends an SBP goal <130 mm Hg for independently-living, ambulatory older adults (≥65 years) with hypertension.1 TABLE 21,2,6 compares the BP goals in the new 2017 ACC/AHA guidelines to previous recommendations.

SPRINT. Significant new literature has been generated since the publication of JNC 8 that supports these lower BP goals, particularly in patients with CVD or who are at high ASCVD risk.8,12-15 For example, the Systolic Blood Pressure Intervention Trial (SPRINT) was the largest RCT to assess whether lower BP goals decrease the risk of adverse CVD outcomes.16 In SPRINT, 9361 patients with an SBP ≥130 mm Hg and an increased risk of CVD, but without diabetes or a history of stroke, were randomized to intensive BP treatment (SBP goal <120 mm Hg) or standard treatment (SBP goal <140 mm Hg). After a median follow-up of 3.26 years, the study was stopped early due to a decreased risk in the primary composite outcome of myocardial infarction (MI), other acute coronary syndromes (ACS), stroke, heart failure, or death from CV causes (number needed to treat [NNT] to prevent one event=61).
Intensive treatment was also associated with a lower risk of all-cause mortality (NNT=90), heart failure (NNT=123), death from CV causes (NNT=172), and the primary outcome or death (NNT=52
Continue to: Meta-analyses that have been conducted since SPRINT...
Meta-analyses that have been conducted since SPRINT, and that have incorporated SPRINT data, also support lower BP goals. In the systematic review performed for the 2017 ACC/AHA guideline, an SBP <130 mm Hg compared to a higher BP target was associated with a reduced risk of major CV events, stroke, MI, and heart failure, although not all-cause mortality.8 These findings were largely consistent with other recent meta-analyses.12-15 For example, Bundy et al15 reported significant CV benefit with more vs less intensive BP lowering, whether or not the data from SPRINT were included, with the greatest reduction in risk seen in the groups with highest baseline BP.
It is important to consider a patient’s baseline level of risk when evaluating the absolute benefit of lower BP targets on CV outcomes. For patients with higher CV risk, the absolute benefit of treatment is greater.12-14 These findings support the 2017 ACC/AHA guideline, which recommends initiating drug therapy, in addition to lifestyle modification, in adults with hypertension and high ASCVD risk when the average BP is >130/80 mm Hg, with a goal of <130/80 mm Hg. TABLE 312-15,17-22 summarizes recent systematic reviews and meta-analyses conducted since the publication of JNC 8 that assess the association between intensity of BP lowering and adverse CV and related outcomes.


Treating patients with low CV risk
The evidence supporting a lower BP goal in patients with low CV risk is less than for patients at elevated risk. There are no large RCTs for this group that have assessed whether an intensive BP lowering strategy decreases CV outcomes more than a standard BP strategy (eg, <140/90 mm Hg). It is likely that absolute benefit is much smaller than for patients with, or at high risk for, ASCVD.
However, epidemiologic observational studies have indicated a significant log-linear increase in CV mortality starting at an SBP of 115 mm Hg.23 A 20-mm Hg increase in SBP above 115 mm Hg is associated with an approximate doubling of stroke and ischemic heart disease mortality risk.23 Decades worth of exposure to “elevated” BP levels would likely result in significant vascular damage, and attenuation of this process would likely be beneficial.24,25 An RCT specifically designed to test this hypothesis, however, would not be pragmatic considering the substantial number of patient-years that would be required.
Due to insufficient data documenting the value of antihypertensive drug therapy for primary prevention in adults with “elevated” BP and stage 1 hypertension at low risk for CVD, the 2017 ACC/AHA guideline recommends that drug therapy be initiated for all adults only when their BP average is ≥140/90 mm Hg.1 In contrast, for patients needing secondary prevention and for those with elevated CVD risk, the guideline recommends medication in addition to lifestyle modifications once the average BP is ≥130/80 mm Hg. The recommendation to withhold drug therapy until the BP is ≥140/90 mm Hg in patients needing primary prevention is supported by a new meta-analysis of 74 trials with 306,273 participants that aimed to assess the association between BP-lowering treatment and death and CVD at various BP levels.17 In this analysis, pharmacologic treatment was associated with a reduced risk of all-cause mortality, major CVD events, and coronary heart disease if the SBP was ≥140 mm Hg.
Continue to: Treating older patients
Treating older patients
Significant controversy has existed regarding the optimal BP goal in older patients, particularly once the JNC 8 recommended relaxing the SBP goal to <150 mm Hg for pateints ≥60 years of age.6,7 This recommendation was consistent with the guideline from the American College of Physicians (ACP)/AAFP,26 which also recommended a lower SBP of <140 mm Hg in patients with a history of stroke or transient ischemic attack and those at high CV risk.26
Evidence is available, however, supporting more intensive BP goals in older independently-living ambulatory adults. A pre-planned subgroup analysis was conducted in 2636 SPRINT participants ≥75 years of age.27 Similar to the overall experience in SPRINT, lower SBP goals were associated with significant reductions in CV events, including the composite CVD primary outcome (NNT=27), heart failure (NNT=63), nonfatal heart failure (NNT=66), and all-cause mortality (NNT=41). In addition, the relative benefits were approximately equal whether the patients were the most fit, non-fit, or frail, with the absolute benefit being greatest in those who were frail (recognizing that the SPRINT participants were independently-living ambulatory adults). While the absolute rate of serious adverse events was higher in the more intensive BP goal group, there was no statistically significant difference in the incidence of hypotension, orthostatic hypotension, syncope, electrolyte abnormalities, or acute kidney injury or renal failure.
Use of lower BP goals than recommended by JNC 8 was also supported by another recent meta-analysis that compared the outcomes of intensive BP lowering (SBP <140 mm Hg) to a standard BP-lowering strategy (SBP <150 mm Hg).18 Using a random-effects model, more intensive BP lowering was associated with a significant reduction in major adverse CV events (29%), CV mortality (33%), and heart failure (37%), with no increase in serious adverse events or renal failure. Findings with the fixed-effects model used to confirm results were largely consistent, with the exception of a possible increase in renal failure.
Although the evidence supporting lower BP goals in older, ambulatory, noninstitutionalized patients is sound, it is important to consider a patient’s overall disease burden. For older adults with multiple comorbidities and limited life expectancy, as well as those who are nonambulatory or institutionalized, decisions on the intensity of BP lowering should be made using a team-based approach, weighing the risks and benefits.1
Continue to: Treating patients with diabetes
Treating patients with diabetes
The most appropriate BP goal for patients with diabetes has been the subject of much debate, with different goals recommended in different guidelines (TABLE 21,2,6). The most recent American Diabetes Association guideline recommends a BP goal <140/90 mm Hg for most patients, with lower targets (<130/80 mm Hg) for patients at high CV risk if it is achievable without undue treatment burden,28 whereas the 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for all adults with diabetes.1
The ACCORD trial. There is limited evidence to suggest which BP goal is most appropriate for patients with diabetes. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial is the only RCT specifically designed to assess the impact of intensive vs standard BP goals in patients with diabetes.29 In ACCORD, 4733 patients with type 2 diabetes were randomized to either an intensive BP-lowering group (SBP <120 mm Hg) or a standard BP-lowering group (SBP <140 mm Hg). After a mean follow-up of 4.7 years, there was no difference in the primary composite endpoint of nonfatal MI, nonfatal stroke, or death from CV causes. However, the risk of stroke was reduced (NNT=89). Interpretation of ACCORD is limited due to its factorial design and because the trial was significantly underpowered.

Systematic reviews and meta-analyses. Literature supporting lower BP goals in patients with diabetes primarily comes from systematic reviews and meta-analyses.30 In the evidence-based review performed for the 2017 ACC/AHA guidelines, more intensive treatment was associated with a decrease in fatal or nonfatal stroke.8 The results from the ACCORD trial and SPRINT are consistent,31 and a sub-study of SPRINT patients with pre-diabetes showed preservation of CV benefit.32 Also, a meta-analysis of subgroups of trial participants with diabetes showed that more intensive BP lowering in patients is associated with a decrease in major CV events.14
Treating patients with chronic kidney disease
As with diabetes and older patients, recommended goals for patients with CKD have varied (TABLE 21,2,6). The Kidney Disease Improving Global Outcomes (KDIGO) 2012 guideline recommended the same target BP as JNC 7 and the 2017 ACC/AHA guideline: ≤130/80 mm Hg in patients with CKD and urine albumin excretion ≥30 mg/24 hours (or equivalent).1,2,33 KDIGO recommended a more relaxed target (≤140/90 mm Hg), however, for patients with CKD and urine albumin excretion <30 mg/24 hours.1,33
Scant data exist from RCTs designed to assess the CV effects of intensive BP targets in patients with CKD. In SPRINT, where 28% of patients had stage 3 or 4 CKD, benefits of more intensive therapy were similar to those observed in the overall cohort.16,34 While some RCTs have assessed the effect of more intensive BP lowering on progression of CKD, they were not specifically designed or powered to address CV outcomes.35,36
Continue to: In recent meta-analyses assessing the effects...
In recent meta-analyses assessing the effects of intensive BP lowering on renal and CV events in patients with CKD, a lower BP strategy was not associated with a decrease in CV events.8,14,19 However, more intensive therapy was associated with a 17% reduced risk of composite kidney failure events and an 18% reduction in end-stage kidney disease.19 The risk of kidney failure with lower BP goals was 27% lower in patients with baseline proteinuria, but was not significant in patients who did not have proteinuria.19
Evidence supports lower BP goals, but guidelines should guide
The lower BP goals advised in the 2017 ACC/AHA guideline are supported by substantial new high-quality evidence that was not available at the time of the JNC 8 report.1 The strongest evidence for lower goals is found in patients with, or at high risk for, CVD, but other patients commonly seen by primary care providers, including those at lower CVD risk, older patients, and those with diabetes or CKD are also likely to benefit.1
Despite the debates, it is important to remember that guidelines are intended to “guide.” As stated in the guideline, “Guidelines are intended to define practices meeting the needs of patients in most, but not all, circumstances and should not replace clinical judgment.”1 They should be easy to understand and apply, and a consistent, evidence-based BP goal of <130/80 mm Hg for most patients facilitates implementation.
Although more of the US population is categorized as hypertensive under the new guideline (46% now vs 32% before), only 1.9% more require drug therapy, as the vast majority of the newly classified hypertensives are primary prevention patients for whom only lifestyle modification is recommended.37 However, to attain these goals, greater emphasis will be needed on utilizing team-based care, health information technology including electronic medical records and telehealth, performance measures, quality improvement strategies, and financial incentives.1
Finally, as emphasized in the guidelines, BP monitoring technique matters. Clinicians should not accept flawed BP measurement techniques any more than they would accept flawed results from studies performed incorrectly.
CORRESPONDENCE
Eric J. MacLaughlin, PharmD, BCPS, FASHP, FCCP, Texas Tech University Health Sciences Center,1300 S. Coulter Dr., Amarillo, TX 79106; Eric.MacLaughlin@ttuhsc.edu.
ACKNOWLEDGEMENTS
The authors thank Paul K. Whelton, MB, MD, MSc, FAHA, and Robert M. Carey, MD, FAHA, for their review of this manuscript.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
2. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560-2572.
3. Wilt TJ, Kansagara D, Qaseem A; Clinical Guidelines Committee of the American College of Physicians. Hypertension limbo: balancing benefits, harms, and patient preferences before we lower the bar on blood pressure. Ann Intern Med. 2018;168:369-370.
4. American Academy of Family Physicians. AAFP decides to not endorse AHA/ACC hypertension guideline. Available at: https://www.aafp.org/news/health-of-the-public/20171212notendorseaha-accgdlne.html. Accessed January 9, 2018.
5. Gibbons GH, Shurin SB, Mensah GA, et al. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation. 2013;128:1713-1715.
6. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
7. Wright JT Jr., Fine LJ, Lackland DT, et al. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499-503.
8. Reboussin DM, Allen NB, Griswold ME, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e116-e135.
9. Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation. 2016;134:904-905.
10. Burgess SE, MacLaughlin EJ, Smith PA, et al. Blood pressure rising: differences between current clinical and recommended measurement techniques. J Am Soc Hypertens. 2011;5:484-488.
11. American College of Cardiology. ASCVD Risk Estimator Plus. Available at: http://tools.acc.org/ascvd-risk-estimator-plus/#!/calculate/estimate/. Accessed January 9, 2018.
12. The Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591-598.
13. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels - updated overview and meta-analyses of randomized trials. J Hypertens. 2016;34:613-622.
14. Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435-443.
15. Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2:775-781.
16. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
17. Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:28-36.
18. Bavishi C, Bangalore S, Messerli FH. Outcomes of intensive blood pressure lowering in older hypertensive patients. J Am Coll Cardiol. 2017;69:486-493.
19. Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949-957.
20. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957-967.
21. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med. 2017;166:419-429.
22. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
23. Lewington S, Clarke R, Qizilbash N, et al; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913.
24. Guo X, Zhang X, Guo L, et al. Association between pre-hypertension and cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Curr Hypertens Rep. 2013;15:703-716.
25. Huang Y, Cai X, Li Y, et al. Prehypertension and the risk of stroke: a meta-analysis. Neurology. 2014;82:1153-1161.
26. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430-437.
27. Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673-2682.
28. American Diabetes Association. 9. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S86-S104.
29. The ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
30. Reboldi G, Gentile G, Angeli F, et al. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J Hypertens. 2011;29:1253-1269.
31. Perkovic V, Rodgers A. Redefining blood-pressure targets—SPRINT starts the marathon. N Engl J Med. 2015;373:2175-2178.
32. Bress AP, King JB, Kreider KE, et al. Effect of intensive versus standard blood pressure treatment according to baseline prediabetes status: a post hoc analysis of a randomized trial. Diabetes Care. 2017 Aug 9.
33. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:337-414.
34. Cheung AK, Rahman M, Reboussin DM, et al. Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017;28:2812-2823.
35. Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939-946.
36. Wright JT Jr., Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421-2431.
37. Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 American College of Cardiology/American Heart Association High Blood Pressure Guideline. J Am Coll Cardiol. 2018;71:109-188.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
2. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560-2572.
3. Wilt TJ, Kansagara D, Qaseem A; Clinical Guidelines Committee of the American College of Physicians. Hypertension limbo: balancing benefits, harms, and patient preferences before we lower the bar on blood pressure. Ann Intern Med. 2018;168:369-370.
4. American Academy of Family Physicians. AAFP decides to not endorse AHA/ACC hypertension guideline. Available at: https://www.aafp.org/news/health-of-the-public/20171212notendorseaha-accgdlne.html. Accessed January 9, 2018.
5. Gibbons GH, Shurin SB, Mensah GA, et al. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation. 2013;128:1713-1715.
6. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
7. Wright JT Jr., Fine LJ, Lackland DT, et al. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499-503.
8. Reboussin DM, Allen NB, Griswold ME, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e116-e135.
9. Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation. 2016;134:904-905.
10. Burgess SE, MacLaughlin EJ, Smith PA, et al. Blood pressure rising: differences between current clinical and recommended measurement techniques. J Am Soc Hypertens. 2011;5:484-488.
11. American College of Cardiology. ASCVD Risk Estimator Plus. Available at: http://tools.acc.org/ascvd-risk-estimator-plus/#!/calculate/estimate/. Accessed January 9, 2018.
12. The Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591-598.
13. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels - updated overview and meta-analyses of randomized trials. J Hypertens. 2016;34:613-622.
14. Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435-443.
15. Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2:775-781.
16. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
17. Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:28-36.
18. Bavishi C, Bangalore S, Messerli FH. Outcomes of intensive blood pressure lowering in older hypertensive patients. J Am Coll Cardiol. 2017;69:486-493.
19. Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949-957.
20. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957-967.
21. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med. 2017;166:419-429.
22. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
23. Lewington S, Clarke R, Qizilbash N, et al; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913.
24. Guo X, Zhang X, Guo L, et al. Association between pre-hypertension and cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Curr Hypertens Rep. 2013;15:703-716.
25. Huang Y, Cai X, Li Y, et al. Prehypertension and the risk of stroke: a meta-analysis. Neurology. 2014;82:1153-1161.
26. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430-437.
27. Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673-2682.
28. American Diabetes Association. 9. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S86-S104.
29. The ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
30. Reboldi G, Gentile G, Angeli F, et al. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J Hypertens. 2011;29:1253-1269.
31. Perkovic V, Rodgers A. Redefining blood-pressure targets—SPRINT starts the marathon. N Engl J Med. 2015;373:2175-2178.
32. Bress AP, King JB, Kreider KE, et al. Effect of intensive versus standard blood pressure treatment according to baseline prediabetes status: a post hoc analysis of a randomized trial. Diabetes Care. 2017 Aug 9.
33. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:337-414.
34. Cheung AK, Rahman M, Reboussin DM, et al. Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017;28:2812-2823.
35. Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939-946.
36. Wright JT Jr., Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421-2431.
37. Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 American College of Cardiology/American Heart Association High Blood Pressure Guideline. J Am Coll Cardiol. 2018;71:109-188.
PRACTICE RECOMMENDATIONS
› Treat adults with hypertension and cardiovascular disease or those at high risk (≥10%) of an atherosclerotic cardiovascular disease (ASCVD) event to a blood pressure (BP) goal <130/80 mm Hg. A for systolic BP goal; C for diastolic BP goal.
› Treat adults with hypertension and a low risk of a cardiovascular event (ie, primary prevention and ASCVD <10%) to a BP goal <130/80 mm Hg. B for systolic BP goal; C for diastolic BP goal.
› Treat ambulatory, community-dwelling, noninstitutionalized older patients to a systolic BP goal <130 mm Hg. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series