User login
Patients planning plastic surgery traditionally were instructed to stop anticoagulants and antiplatelet medications during the perioperative period to avoid bleeding, which could result in flap loss, pain, skin necrosis, and blood transfusions. In the veteran patient population, anticoagulants are prescribed for the prevention of limb- and life-threatening embolic and thrombotic events.1-3 As of June 2021, > 332,000 veterans were prescribed direct oral anticoagulants.1
In 2015, the Malcom Randall Veterans Affairs Medical Center (MRVAMC) in Gainesville, Florida, Plastic Surgery Service began instructing patients planning elective hand surgery to continue their prescription anticoagulants and antiplatelets during the perioperative period. This decision was prompted by a patient who needed carpal tunnel release surgery and was prescribed coumadin for repeated thrombosis of his dialysis grafts. Hand surgery literature at the time suggested allowing patients to continue their anticoagulants and antiplatelets through the perioperative period to avoid life- and limb-threatening events and wide fluctuations in blood anticoagulant levels.4-6 The MRVAMC Plastic Surgery Service chose to accept the risk of perioperative bleeding after shared decision making with the patients rather than risk a cardiac stent obstruction, pulmonary embolism, or embolic stroke in the at-risk patients.
The objective of this study was to determine the postoperative bleeding complication rate over a 7.5-year period in the veteran patients who did not interrupt their prescription blood thinners. This would assist the MRVAMC Plastic Surgery Service with providing data-driven informed consent and determine whether this protocol should continue.
Methods
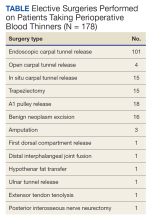
The North Florida/South Georgia Veterans Health System Research Committee and the University of Florida Institutional Review Board approved a retrospective chart review of elective hand cases performed by the MRVAMC Plastic Surgery Service from January 1, 2015, through June 30, 2022. Elective hand cases were identified based on the operation description and included nerve decompressions, tendon releases, trapeziectomy, small-joint fusion, neurectomy, elective amputations, and benign neoplasm removals (Table). Hand surgery included cubital tunnel releases (decompression of the ulnar nerve at the level of the elbow) because hand surgery fellowships, hand surgery training, and hand surgery practices traditionally include a high volume of cubital tunnel releases. We wanted this study to have real-world applications.
Patients’ histories and physicals were reviewed for prescription antithrombotics and for instructions not to interrupt these medications. Postoperative notes were reviewed for 30 days for evidence of postoperative bleeding complications.
The following prescription anticoagulants were included in the study: dabigatran, rivaroxaban, warfarin, edoxaban, and apixaban. In addition, the following prescription antiplatelets were included in the study:
Results
One hundred seventy-eight patients were identified for maintaining prescription blood thinners during their elective hand surgery. There was 1 major complication (0.6%) and 4 minor bleeding complications (2.2%). The major complication occurred when a patient had to return to surgery from the recovery room for emergent control of bleeding. The surgery was for an in situ cubital tunnel release. The patient, aged 48 years, was taking clopidogrel and aspirin and had a personal and family history of cardiovascular disease. The bleeding was controlled with bipolar cautery and Floseal, a topical haemostatic matrix made of bovine gelatin and human thrombin. The minor bleeding complications were treated in the clinic with compression, wound care, or expedited follow-up for reassurance. These included an in situ cubital tunnel release for a patient taking warfarin and aspirin, a digital inclusion cyst for a patient taking apixaban, an endoscopic carpal tunnel for a patient taking aspirin and clopidogrel, and an open carpal tunnel and ulnar tunnel release for a patient taking aspirin and clopidogrel. There were no thrombotic events during the study.
Discussion
Higher utilization of anticoagulation has been evidenced by a 30% increase in Medicare claims and a 277% increase in Medicaid anticoagulation claims between 2014 and 2019, driven by more prescriptions for direct oral anticoagulants such as apixaban and rivaroxaban.7 The MRVAMC Plastic Surgery Service began a protocol for managing perioperative anticoagulation in 2015 to avoid the risk of perioperative thrombotic events in veteran patients. Patients who choose elective hand surgery were instructed to continue their prescription blood thinners. Exceptions to this protocol were patients scheduled for a partial fasciectomy (for Dupuytren contracture) or cubital tunnel release with anterior ulnar nerve transposition. A hematoma would increase the risk for skin necrosis in the patients receiving a fasciectomy, resulting from the thin skin flaps and meticulous dissection to identify and protect the digital nerves. Worsening nerve dysfunction could result from hematoma compression and scarring in the ulnar nerve cases. If the risk of holding the blood thinner was felt to be unreasonably high, based on recommendations from the patients’ cardiologist or primary care doctor, we offered an in situ cubital tunnel release for the ulnar nerve patients.
Concerns regarding interrupting chronic anticoagulation involve the increased risk of thromboembolism and the theoretical risk of a rebound hypercoagulable effect.8 Patients prescribed warfarin have been found to unintentionally discontinue this medication after outpatient surgery at more than 1.5 times the rate of the general population.9
A systematic review of 9 published studies looking specifically at elective hand and wrist surgeries demonstrated no significant increase in perioperative bleeding risk with the continuation of anticoagulation and antiplatelet medications.10 Sardenberg and colleagues reviewed 7 studies in which 410 hand and wrist surgeries were performed in patients prescribed warfarin or aspirin and clopidogrel. These patients had a 0.7% serious complication rate, requiring surgical treatment only in patients having complex wrist surgeries (wrist arthrodesis with tenosynovectomy, resection of the distal ulna with tenosynovectomy and tendon transfer, and proximal row carpectomy).11 Bogunovic and colleagues compared 50 hand and wrist patients who were on uninterrupted warfarin with those who were not. They required patients to have an
These and our study are consistent with other disciplines, such as facial plastic surgery, dermatology, and ophthalmology, which do not support routine suspension of anticoagulants.13-16 A review of 30 cutaneous surgery studies involving > 14,000 patients recommended meticulous hemostasis over cessation of blood thinners.15 The University of Massachusetts Dermatology Clinic found a 40 times higher rate of bleeding complications in patients on clopidogrel and warfarin but still recommended continuation of these medications to avoid thrombotic events.16
Limitations
This study is a retrospective chart review and limited by what is already documented in the electronic health record. We can verify that the patients were given instructions to continue their medications up to the day of surgery but cannot be certain whether the instructions were followed. No control group was told to hold their anticoagulants for the same surgery. Once we decided on a protocol, we applied it to all patients. The study approval was for the specific time frame when the protocol was in place.
Our study was designed for elective hand cases because those surgeries can be anticipated, predicted, and patients can be given instructions during the preoperative appointments. We did incidentally find several nonelective hand cases (traumas, infections, and cancers) during the review of patients taking prescription blood thinners that had to be expedited to the operating room. Based on morbidity data during that time period, there were no additional postoperative hand surgery bleeding complications that had to return to the operating room. Future studies are indicated, but we believe our protocol can be applied to urgent and emergent hand surgeries as well as elective cases.
Conclusions
Our study supports continuing prescription anticoagulant and antiplatelet medications during the perioperative period for elective hand surgery. We found this is a safe practice in our veteran population with an acceptably low local bleeding complication rate.
Acknowledgments
This manuscript is the result of work supported with the resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
1. Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Assoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
2. Buck J, Kaboli P, Gage BF, Cram P, Vaughan Sarrazin MS. Trends in antithrombotic therapy for atrial fibrillation: data from the Veterans Health Administration health system. Am Heart J. 2016;179:186-191. doi:10.1016/j.ahj.2016.03.029
3. Kinlay S, Young MM, Sherrod R, Gagnon DR. Long-term outcomes and duration of dual antiplatelet therapy after coronary intervention with second-generation drug-eluting stents: the Veterans Affairs Extended DAPT Study. J Am Heart Assoc. 2023;12(2):e027055.
4. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of antiplatelet medication on hand and wrist surgery. J Hand Surg Am. 2013;38(6):1063-1070. doi:10.1016/j.jhsa.2013.03.034
5. Wallace DL, Latimer MD, Belcher HJ. Stopping warfarin therapy is unnecessary for hand surgery. J Hand Surg Br. 2004;29(3):203-205. doi:10.1016/j.jhsb.2003.12.008
6. Edmunds I, Avakian Z. Hand surgery on anticoagulated patients: a prospective study of 121 operations. Hand Surg. 2010;15(2):109-113. doi:10.1142/S021881041000468
7. Duvalyan A, Pandey A, Vaduganathan M, et al. Trends in anticoagulation prescription spending among Medicare Part D and Medicaid beneficiaries between 2014 and 2019. J Am Heart Assoc. 2021;10(24):e022644. doi:10.1161/JAHA.121.022644
8. Thakur NA, Czerwein JK, Butera JN, Palumbo MA. Perioperative management of chronic anticoagulation in orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(12):729-738. doi:10.5435/00124635-201012000-00003
9. Bell C, Bajca J, Bierman A, Li P, Mamdani M, Urbach D. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Int Med. 2003;166(22):2525-2531.
10. Stone MJ, Wilks DJ, Wade RG. Hand and wrist surgery on anticoagulants and antiplatelets: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2020;73(8):1413-1423.
11. Sardenberg T, Deienno FS, Miranda RF, et al. Hand and wrist surgery without suspending warfarin or oral antiplatelet - systematic review. Rev Bras Ortop. 2017;52(4):390-395. doi:10.1016/j.rboe.2017.07.001
12. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of uninterrupted warfarin on hand and wrist surgery. J Hand Surg Am. 2015;40(11):2133-2140. doi:10.1016/j.jhsa.2015.07.037
13. Kraft CT, Bellile E, Baker SR, Kim JC, Moyer JS. Anticoagulant complications in facial plastic and reconstructive surgery. JAMA Facial Plast Surg. 2015;17(2):103-107. doi:10.1001/jamafacial.2014.1147
14. He X, Chen AF, Nirwan RS, Sridhar J, Kuriyan AE. Perioperative management of anticoagulants in ocular surgeries. Int Ophthalmol Clin. 2020;60(3):3-15. doi:10.1097/IIO.0000000000000316
15. Isted A, Cooper L, Colville RJ. Bleeding on the cutting edge: a systematic review of anticoagulant and antiplatelet continuation in minor cutaneous surgery. J Plast Reconstr Aesthet Surg. 2018;71(4):455-467. doi:10.1016/j.bjps.2017.11.024
16. Bordeaux JS, Martires KJ, Goldberg D, Pattee SF, Fu P, Maloney ME. Prospective evaluation of dermatologic surgery complications including patients on multiple antiplatelet and anticoagulant medications. J Am Acad Dermatol. 2011;65(3):576-583. doi:10.1016/j.jaad.2011.02.012
Patients planning plastic surgery traditionally were instructed to stop anticoagulants and antiplatelet medications during the perioperative period to avoid bleeding, which could result in flap loss, pain, skin necrosis, and blood transfusions. In the veteran patient population, anticoagulants are prescribed for the prevention of limb- and life-threatening embolic and thrombotic events.1-3 As of June 2021, > 332,000 veterans were prescribed direct oral anticoagulants.1
In 2015, the Malcom Randall Veterans Affairs Medical Center (MRVAMC) in Gainesville, Florida, Plastic Surgery Service began instructing patients planning elective hand surgery to continue their prescription anticoagulants and antiplatelets during the perioperative period. This decision was prompted by a patient who needed carpal tunnel release surgery and was prescribed coumadin for repeated thrombosis of his dialysis grafts. Hand surgery literature at the time suggested allowing patients to continue their anticoagulants and antiplatelets through the perioperative period to avoid life- and limb-threatening events and wide fluctuations in blood anticoagulant levels.4-6 The MRVAMC Plastic Surgery Service chose to accept the risk of perioperative bleeding after shared decision making with the patients rather than risk a cardiac stent obstruction, pulmonary embolism, or embolic stroke in the at-risk patients.
The objective of this study was to determine the postoperative bleeding complication rate over a 7.5-year period in the veteran patients who did not interrupt their prescription blood thinners. This would assist the MRVAMC Plastic Surgery Service with providing data-driven informed consent and determine whether this protocol should continue.
Methods
The North Florida/South Georgia Veterans Health System Research Committee and the University of Florida Institutional Review Board approved a retrospective chart review of elective hand cases performed by the MRVAMC Plastic Surgery Service from January 1, 2015, through June 30, 2022. Elective hand cases were identified based on the operation description and included nerve decompressions, tendon releases, trapeziectomy, small-joint fusion, neurectomy, elective amputations, and benign neoplasm removals (Table). Hand surgery included cubital tunnel releases (decompression of the ulnar nerve at the level of the elbow) because hand surgery fellowships, hand surgery training, and hand surgery practices traditionally include a high volume of cubital tunnel releases. We wanted this study to have real-world applications.
Patients’ histories and physicals were reviewed for prescription antithrombotics and for instructions not to interrupt these medications. Postoperative notes were reviewed for 30 days for evidence of postoperative bleeding complications.
The following prescription anticoagulants were included in the study: dabigatran, rivaroxaban, warfarin, edoxaban, and apixaban. In addition, the following prescription antiplatelets were included in the study:
Results
One hundred seventy-eight patients were identified for maintaining prescription blood thinners during their elective hand surgery. There was 1 major complication (0.6%) and 4 minor bleeding complications (2.2%). The major complication occurred when a patient had to return to surgery from the recovery room for emergent control of bleeding. The surgery was for an in situ cubital tunnel release. The patient, aged 48 years, was taking clopidogrel and aspirin and had a personal and family history of cardiovascular disease. The bleeding was controlled with bipolar cautery and Floseal, a topical haemostatic matrix made of bovine gelatin and human thrombin. The minor bleeding complications were treated in the clinic with compression, wound care, or expedited follow-up for reassurance. These included an in situ cubital tunnel release for a patient taking warfarin and aspirin, a digital inclusion cyst for a patient taking apixaban, an endoscopic carpal tunnel for a patient taking aspirin and clopidogrel, and an open carpal tunnel and ulnar tunnel release for a patient taking aspirin and clopidogrel. There were no thrombotic events during the study.
Discussion
Higher utilization of anticoagulation has been evidenced by a 30% increase in Medicare claims and a 277% increase in Medicaid anticoagulation claims between 2014 and 2019, driven by more prescriptions for direct oral anticoagulants such as apixaban and rivaroxaban.7 The MRVAMC Plastic Surgery Service began a protocol for managing perioperative anticoagulation in 2015 to avoid the risk of perioperative thrombotic events in veteran patients. Patients who choose elective hand surgery were instructed to continue their prescription blood thinners. Exceptions to this protocol were patients scheduled for a partial fasciectomy (for Dupuytren contracture) or cubital tunnel release with anterior ulnar nerve transposition. A hematoma would increase the risk for skin necrosis in the patients receiving a fasciectomy, resulting from the thin skin flaps and meticulous dissection to identify and protect the digital nerves. Worsening nerve dysfunction could result from hematoma compression and scarring in the ulnar nerve cases. If the risk of holding the blood thinner was felt to be unreasonably high, based on recommendations from the patients’ cardiologist or primary care doctor, we offered an in situ cubital tunnel release for the ulnar nerve patients.
Concerns regarding interrupting chronic anticoagulation involve the increased risk of thromboembolism and the theoretical risk of a rebound hypercoagulable effect.8 Patients prescribed warfarin have been found to unintentionally discontinue this medication after outpatient surgery at more than 1.5 times the rate of the general population.9
A systematic review of 9 published studies looking specifically at elective hand and wrist surgeries demonstrated no significant increase in perioperative bleeding risk with the continuation of anticoagulation and antiplatelet medications.10 Sardenberg and colleagues reviewed 7 studies in which 410 hand and wrist surgeries were performed in patients prescribed warfarin or aspirin and clopidogrel. These patients had a 0.7% serious complication rate, requiring surgical treatment only in patients having complex wrist surgeries (wrist arthrodesis with tenosynovectomy, resection of the distal ulna with tenosynovectomy and tendon transfer, and proximal row carpectomy).11 Bogunovic and colleagues compared 50 hand and wrist patients who were on uninterrupted warfarin with those who were not. They required patients to have an
These and our study are consistent with other disciplines, such as facial plastic surgery, dermatology, and ophthalmology, which do not support routine suspension of anticoagulants.13-16 A review of 30 cutaneous surgery studies involving > 14,000 patients recommended meticulous hemostasis over cessation of blood thinners.15 The University of Massachusetts Dermatology Clinic found a 40 times higher rate of bleeding complications in patients on clopidogrel and warfarin but still recommended continuation of these medications to avoid thrombotic events.16
Limitations
This study is a retrospective chart review and limited by what is already documented in the electronic health record. We can verify that the patients were given instructions to continue their medications up to the day of surgery but cannot be certain whether the instructions were followed. No control group was told to hold their anticoagulants for the same surgery. Once we decided on a protocol, we applied it to all patients. The study approval was for the specific time frame when the protocol was in place.
Our study was designed for elective hand cases because those surgeries can be anticipated, predicted, and patients can be given instructions during the preoperative appointments. We did incidentally find several nonelective hand cases (traumas, infections, and cancers) during the review of patients taking prescription blood thinners that had to be expedited to the operating room. Based on morbidity data during that time period, there were no additional postoperative hand surgery bleeding complications that had to return to the operating room. Future studies are indicated, but we believe our protocol can be applied to urgent and emergent hand surgeries as well as elective cases.
Conclusions
Our study supports continuing prescription anticoagulant and antiplatelet medications during the perioperative period for elective hand surgery. We found this is a safe practice in our veteran population with an acceptably low local bleeding complication rate.
Acknowledgments
This manuscript is the result of work supported with the resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
Patients planning plastic surgery traditionally were instructed to stop anticoagulants and antiplatelet medications during the perioperative period to avoid bleeding, which could result in flap loss, pain, skin necrosis, and blood transfusions. In the veteran patient population, anticoagulants are prescribed for the prevention of limb- and life-threatening embolic and thrombotic events.1-3 As of June 2021, > 332,000 veterans were prescribed direct oral anticoagulants.1
In 2015, the Malcom Randall Veterans Affairs Medical Center (MRVAMC) in Gainesville, Florida, Plastic Surgery Service began instructing patients planning elective hand surgery to continue their prescription anticoagulants and antiplatelets during the perioperative period. This decision was prompted by a patient who needed carpal tunnel release surgery and was prescribed coumadin for repeated thrombosis of his dialysis grafts. Hand surgery literature at the time suggested allowing patients to continue their anticoagulants and antiplatelets through the perioperative period to avoid life- and limb-threatening events and wide fluctuations in blood anticoagulant levels.4-6 The MRVAMC Plastic Surgery Service chose to accept the risk of perioperative bleeding after shared decision making with the patients rather than risk a cardiac stent obstruction, pulmonary embolism, or embolic stroke in the at-risk patients.
The objective of this study was to determine the postoperative bleeding complication rate over a 7.5-year period in the veteran patients who did not interrupt their prescription blood thinners. This would assist the MRVAMC Plastic Surgery Service with providing data-driven informed consent and determine whether this protocol should continue.
Methods
The North Florida/South Georgia Veterans Health System Research Committee and the University of Florida Institutional Review Board approved a retrospective chart review of elective hand cases performed by the MRVAMC Plastic Surgery Service from January 1, 2015, through June 30, 2022. Elective hand cases were identified based on the operation description and included nerve decompressions, tendon releases, trapeziectomy, small-joint fusion, neurectomy, elective amputations, and benign neoplasm removals (Table). Hand surgery included cubital tunnel releases (decompression of the ulnar nerve at the level of the elbow) because hand surgery fellowships, hand surgery training, and hand surgery practices traditionally include a high volume of cubital tunnel releases. We wanted this study to have real-world applications.
Patients’ histories and physicals were reviewed for prescription antithrombotics and for instructions not to interrupt these medications. Postoperative notes were reviewed for 30 days for evidence of postoperative bleeding complications.
The following prescription anticoagulants were included in the study: dabigatran, rivaroxaban, warfarin, edoxaban, and apixaban. In addition, the following prescription antiplatelets were included in the study:
Results
One hundred seventy-eight patients were identified for maintaining prescription blood thinners during their elective hand surgery. There was 1 major complication (0.6%) and 4 minor bleeding complications (2.2%). The major complication occurred when a patient had to return to surgery from the recovery room for emergent control of bleeding. The surgery was for an in situ cubital tunnel release. The patient, aged 48 years, was taking clopidogrel and aspirin and had a personal and family history of cardiovascular disease. The bleeding was controlled with bipolar cautery and Floseal, a topical haemostatic matrix made of bovine gelatin and human thrombin. The minor bleeding complications were treated in the clinic with compression, wound care, or expedited follow-up for reassurance. These included an in situ cubital tunnel release for a patient taking warfarin and aspirin, a digital inclusion cyst for a patient taking apixaban, an endoscopic carpal tunnel for a patient taking aspirin and clopidogrel, and an open carpal tunnel and ulnar tunnel release for a patient taking aspirin and clopidogrel. There were no thrombotic events during the study.
Discussion
Higher utilization of anticoagulation has been evidenced by a 30% increase in Medicare claims and a 277% increase in Medicaid anticoagulation claims between 2014 and 2019, driven by more prescriptions for direct oral anticoagulants such as apixaban and rivaroxaban.7 The MRVAMC Plastic Surgery Service began a protocol for managing perioperative anticoagulation in 2015 to avoid the risk of perioperative thrombotic events in veteran patients. Patients who choose elective hand surgery were instructed to continue their prescription blood thinners. Exceptions to this protocol were patients scheduled for a partial fasciectomy (for Dupuytren contracture) or cubital tunnel release with anterior ulnar nerve transposition. A hematoma would increase the risk for skin necrosis in the patients receiving a fasciectomy, resulting from the thin skin flaps and meticulous dissection to identify and protect the digital nerves. Worsening nerve dysfunction could result from hematoma compression and scarring in the ulnar nerve cases. If the risk of holding the blood thinner was felt to be unreasonably high, based on recommendations from the patients’ cardiologist or primary care doctor, we offered an in situ cubital tunnel release for the ulnar nerve patients.
Concerns regarding interrupting chronic anticoagulation involve the increased risk of thromboembolism and the theoretical risk of a rebound hypercoagulable effect.8 Patients prescribed warfarin have been found to unintentionally discontinue this medication after outpatient surgery at more than 1.5 times the rate of the general population.9
A systematic review of 9 published studies looking specifically at elective hand and wrist surgeries demonstrated no significant increase in perioperative bleeding risk with the continuation of anticoagulation and antiplatelet medications.10 Sardenberg and colleagues reviewed 7 studies in which 410 hand and wrist surgeries were performed in patients prescribed warfarin or aspirin and clopidogrel. These patients had a 0.7% serious complication rate, requiring surgical treatment only in patients having complex wrist surgeries (wrist arthrodesis with tenosynovectomy, resection of the distal ulna with tenosynovectomy and tendon transfer, and proximal row carpectomy).11 Bogunovic and colleagues compared 50 hand and wrist patients who were on uninterrupted warfarin with those who were not. They required patients to have an
These and our study are consistent with other disciplines, such as facial plastic surgery, dermatology, and ophthalmology, which do not support routine suspension of anticoagulants.13-16 A review of 30 cutaneous surgery studies involving > 14,000 patients recommended meticulous hemostasis over cessation of blood thinners.15 The University of Massachusetts Dermatology Clinic found a 40 times higher rate of bleeding complications in patients on clopidogrel and warfarin but still recommended continuation of these medications to avoid thrombotic events.16
Limitations
This study is a retrospective chart review and limited by what is already documented in the electronic health record. We can verify that the patients were given instructions to continue their medications up to the day of surgery but cannot be certain whether the instructions were followed. No control group was told to hold their anticoagulants for the same surgery. Once we decided on a protocol, we applied it to all patients. The study approval was for the specific time frame when the protocol was in place.
Our study was designed for elective hand cases because those surgeries can be anticipated, predicted, and patients can be given instructions during the preoperative appointments. We did incidentally find several nonelective hand cases (traumas, infections, and cancers) during the review of patients taking prescription blood thinners that had to be expedited to the operating room. Based on morbidity data during that time period, there were no additional postoperative hand surgery bleeding complications that had to return to the operating room. Future studies are indicated, but we believe our protocol can be applied to urgent and emergent hand surgeries as well as elective cases.
Conclusions
Our study supports continuing prescription anticoagulant and antiplatelet medications during the perioperative period for elective hand surgery. We found this is a safe practice in our veteran population with an acceptably low local bleeding complication rate.
Acknowledgments
This manuscript is the result of work supported with the resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
1. Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Assoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
2. Buck J, Kaboli P, Gage BF, Cram P, Vaughan Sarrazin MS. Trends in antithrombotic therapy for atrial fibrillation: data from the Veterans Health Administration health system. Am Heart J. 2016;179:186-191. doi:10.1016/j.ahj.2016.03.029
3. Kinlay S, Young MM, Sherrod R, Gagnon DR. Long-term outcomes and duration of dual antiplatelet therapy after coronary intervention with second-generation drug-eluting stents: the Veterans Affairs Extended DAPT Study. J Am Heart Assoc. 2023;12(2):e027055.
4. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of antiplatelet medication on hand and wrist surgery. J Hand Surg Am. 2013;38(6):1063-1070. doi:10.1016/j.jhsa.2013.03.034
5. Wallace DL, Latimer MD, Belcher HJ. Stopping warfarin therapy is unnecessary for hand surgery. J Hand Surg Br. 2004;29(3):203-205. doi:10.1016/j.jhsb.2003.12.008
6. Edmunds I, Avakian Z. Hand surgery on anticoagulated patients: a prospective study of 121 operations. Hand Surg. 2010;15(2):109-113. doi:10.1142/S021881041000468
7. Duvalyan A, Pandey A, Vaduganathan M, et al. Trends in anticoagulation prescription spending among Medicare Part D and Medicaid beneficiaries between 2014 and 2019. J Am Heart Assoc. 2021;10(24):e022644. doi:10.1161/JAHA.121.022644
8. Thakur NA, Czerwein JK, Butera JN, Palumbo MA. Perioperative management of chronic anticoagulation in orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(12):729-738. doi:10.5435/00124635-201012000-00003
9. Bell C, Bajca J, Bierman A, Li P, Mamdani M, Urbach D. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Int Med. 2003;166(22):2525-2531.
10. Stone MJ, Wilks DJ, Wade RG. Hand and wrist surgery on anticoagulants and antiplatelets: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2020;73(8):1413-1423.
11. Sardenberg T, Deienno FS, Miranda RF, et al. Hand and wrist surgery without suspending warfarin or oral antiplatelet - systematic review. Rev Bras Ortop. 2017;52(4):390-395. doi:10.1016/j.rboe.2017.07.001
12. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of uninterrupted warfarin on hand and wrist surgery. J Hand Surg Am. 2015;40(11):2133-2140. doi:10.1016/j.jhsa.2015.07.037
13. Kraft CT, Bellile E, Baker SR, Kim JC, Moyer JS. Anticoagulant complications in facial plastic and reconstructive surgery. JAMA Facial Plast Surg. 2015;17(2):103-107. doi:10.1001/jamafacial.2014.1147
14. He X, Chen AF, Nirwan RS, Sridhar J, Kuriyan AE. Perioperative management of anticoagulants in ocular surgeries. Int Ophthalmol Clin. 2020;60(3):3-15. doi:10.1097/IIO.0000000000000316
15. Isted A, Cooper L, Colville RJ. Bleeding on the cutting edge: a systematic review of anticoagulant and antiplatelet continuation in minor cutaneous surgery. J Plast Reconstr Aesthet Surg. 2018;71(4):455-467. doi:10.1016/j.bjps.2017.11.024
16. Bordeaux JS, Martires KJ, Goldberg D, Pattee SF, Fu P, Maloney ME. Prospective evaluation of dermatologic surgery complications including patients on multiple antiplatelet and anticoagulant medications. J Am Acad Dermatol. 2011;65(3):576-583. doi:10.1016/j.jaad.2011.02.012
1. Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Assoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
2. Buck J, Kaboli P, Gage BF, Cram P, Vaughan Sarrazin MS. Trends in antithrombotic therapy for atrial fibrillation: data from the Veterans Health Administration health system. Am Heart J. 2016;179:186-191. doi:10.1016/j.ahj.2016.03.029
3. Kinlay S, Young MM, Sherrod R, Gagnon DR. Long-term outcomes and duration of dual antiplatelet therapy after coronary intervention with second-generation drug-eluting stents: the Veterans Affairs Extended DAPT Study. J Am Heart Assoc. 2023;12(2):e027055.
4. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of antiplatelet medication on hand and wrist surgery. J Hand Surg Am. 2013;38(6):1063-1070. doi:10.1016/j.jhsa.2013.03.034
5. Wallace DL, Latimer MD, Belcher HJ. Stopping warfarin therapy is unnecessary for hand surgery. J Hand Surg Br. 2004;29(3):203-205. doi:10.1016/j.jhsb.2003.12.008
6. Edmunds I, Avakian Z. Hand surgery on anticoagulated patients: a prospective study of 121 operations. Hand Surg. 2010;15(2):109-113. doi:10.1142/S021881041000468
7. Duvalyan A, Pandey A, Vaduganathan M, et al. Trends in anticoagulation prescription spending among Medicare Part D and Medicaid beneficiaries between 2014 and 2019. J Am Heart Assoc. 2021;10(24):e022644. doi:10.1161/JAHA.121.022644
8. Thakur NA, Czerwein JK, Butera JN, Palumbo MA. Perioperative management of chronic anticoagulation in orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(12):729-738. doi:10.5435/00124635-201012000-00003
9. Bell C, Bajca J, Bierman A, Li P, Mamdani M, Urbach D. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Int Med. 2003;166(22):2525-2531.
10. Stone MJ, Wilks DJ, Wade RG. Hand and wrist surgery on anticoagulants and antiplatelets: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2020;73(8):1413-1423.
11. Sardenberg T, Deienno FS, Miranda RF, et al. Hand and wrist surgery without suspending warfarin or oral antiplatelet - systematic review. Rev Bras Ortop. 2017;52(4):390-395. doi:10.1016/j.rboe.2017.07.001
12. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of uninterrupted warfarin on hand and wrist surgery. J Hand Surg Am. 2015;40(11):2133-2140. doi:10.1016/j.jhsa.2015.07.037
13. Kraft CT, Bellile E, Baker SR, Kim JC, Moyer JS. Anticoagulant complications in facial plastic and reconstructive surgery. JAMA Facial Plast Surg. 2015;17(2):103-107. doi:10.1001/jamafacial.2014.1147
14. He X, Chen AF, Nirwan RS, Sridhar J, Kuriyan AE. Perioperative management of anticoagulants in ocular surgeries. Int Ophthalmol Clin. 2020;60(3):3-15. doi:10.1097/IIO.0000000000000316
15. Isted A, Cooper L, Colville RJ. Bleeding on the cutting edge: a systematic review of anticoagulant and antiplatelet continuation in minor cutaneous surgery. J Plast Reconstr Aesthet Surg. 2018;71(4):455-467. doi:10.1016/j.bjps.2017.11.024
16. Bordeaux JS, Martires KJ, Goldberg D, Pattee SF, Fu P, Maloney ME. Prospective evaluation of dermatologic surgery complications including patients on multiple antiplatelet and anticoagulant medications. J Am Acad Dermatol. 2011;65(3):576-583. doi:10.1016/j.jaad.2011.02.012