User login
In clinicians and patients alike, lithium triggers reactions ranging from apprehension and fear about adverse effects and toxicity to confusion over lithium’s usefulness compared with other mood stabilizers that do not require blood monitoring. Research from the 1950s to the 1970s demonstrated that lithium is effective for prophylaxis of mood episodes in patients with bipolar disorder and could reduce the frequency of hospitalization in patients who are depressed.1 For years, lithium was commonly prescribed to treat bipolar disorder, but in recent years its use has fallen out of favor due to concerns about its risks, and the availability of newer medications. This article reviews lithium’s origins (Box1-4), pharmacology, risks, and benefits, and makes a case for why it should remain a first-line therapy for bipolar disorder.
Box
Lithium was initially used in the 1840s to treat gout. William Hammond became the first physician to prescribe lithium bromide for acute mania in 1871, and in 1894, Danish psychiatrist Frederik Lange first used lithium carbonate to treat “melancholic depression.”1 In the 20th century, lithium-containing products were used to treat rheumatologic conditions such as renal calculi and other uric acid diatheses.
Lithium experienced a revival in 1949 when John Cade expanded upon Archibald Garrod’s theory regarding uric acid and gout. As a physician during WWII, Cade observed manic and depressive behaviors among prisoners.2 Theorizing that this was caused by either an excess or lack of a metabolite, he injected urine from patients with mania, depression, and schizophrenia and from healthy individuals into guinea pigs.3 Animals who received urine from patients with mania died faster than those injected with urine from a patient with schizophrenia.2 Concluding that urea was the culprit, Cade substituted the relatively water insoluble uric acid for “the most soluble of urates,” which was lithium urate.2,3 Rather than succumbing to a quicker death, guinea pigs injected with lithium urate became placid, tranquilized, lost their natural timidity, and generally did not respond to stimulation.3
Cade administered lithium carbonate and lithium citrate to himself and, because he did not experience any unwanted effects, began testing the medication on patients. Cade’s landmark 1949 paper4 notes improvement in all 10 patients with mania but little change in 6 patients with schizophrenia and 3 with chronic depression.2
In the United States, interest in lithium did not begin until the 1960s, when Samuel Gershon introduced the medication to a psychiatric hospital in Michigan. Financed by the National Institute of Mental Health, this program bought bulk lithium from a chemical supply store, and a local pharmacy formed it into capsules. Analysis of 4 controlled studies from 1963 to 1971 showed an average response rate to lithium of 78% in 116 patients with mania.1
By the end of the 1960s, many psychiatrists were prescribing lithium. At that time, lithium was not FDA-approved, but it could be prescribed as an investigational new drug by obtaining a special permit. In 1970, the FDA approved lithium for acute mania, and for prophylaxis of mania in 1975. Lithium has not yet been approved for prophylaxis of depression, despite substantial evidence indicating efficacy.1
How lithium works
Lithium has effects on neurotransmitters implicated in mania, such as glutamate, dopamine, and gamma-aminobutyric acid.5 Quiroz et al6 provide a detailed description of lithium’s effects, which can be summarized as modulating neuronal signaling pathways, including B-cell lymphoma 2 (BCL2), cAMP-response element binding protein (CREB), and glycogen synthase kinase-3 (GSK-3). Through these signaling cascades, lithium can curtail progression of neuronal apoptosis caused by the biochemical stress commonly seen in bipolar disorder pathogenesis.6
A wide range of potential adverse effects
Lithium can cause adverse effects in several organ systems. Clinicians must be aware of these effects before prescribing lithium or continuing long-term use. The most commonly documented adverse effects and symptoms of toxicity are:
- tremor
- renal dysfunction, including renal insufficiency and polyuria or polydipsia
- hypothyroidism
- hyperparathyroidism (with subsequent hypercalcemia)
- weight gain
- gastrointestinal (GI) symptoms.
These symptoms tend to occur when lithium serum levels are outside the reference range of 0.6 to 1.2 mEq/L, typically once blood levels reach ≥1.5 mEq/L.7 However, thyroid and renal abnormalities can occur at levels below this value, and might be related to cumulative lithium exposure.7 Adverse effects usually are precipitated by inadequate water intake or inadvertently taking an extra dose. Symptoms of lithium toxicity can be mild, moderate (GI complaints, tremor, weakness, fatigue), or severe (agitation, seizures, autonomic dysregulation, confusion, coma, death).
Lithium adverse effects and toxicity are infrequent. An analysis of 17 years of data in Sweden showed the incidence of moderate to severe lithium intoxication (serum level ≥1.5 mEq/L) was .01 patients per year.8 A recently published US analysis found the prevalence rate of lithium toxicity was 2.2%.9 Results from both groups show that drug interactions were an important cause of increased lithium levels, and specifically that initiating a medication that could interact with lithium was associated with 30-fold higher risk of needing acute care for lithium toxicity.9 Possible drug interactions include nonsteroidal anti-inflammatory drugs, diuretics, and renin-angiotensin-aldosterone system inhibitors.9 Because lithium is eliminated exclusively by the kidneys, impaired or altered renal function can increase the risk of lithium retention, leading to intoxication. Other risk factors include older age, alteration of water-salt homeostasis (fever, diarrhea, vomiting), higher number of treated chronic diseases as measured by Chronic Disease Score (range: 0 to 35; higher scores denotes higher number of treated chronic diseases and increased hospitalization risk), and higher total daily lithium dosage.9
Presentation of lithium intoxication often is mild or nonspecific, and physicians should have a low threshold for checking lithium blood levels.8 Lithium intoxication can be safely managed with volume expansion, forced diuresis, and hemodialysis.
Continue to: Lithium use during pregnancy...
Lithium use during pregnancy
When considering lithium for a woman who is pregnant, it is important to weigh the potential teratogenic risks against the benefit of successful management of the mood disorder. Ebstein’s anomaly (abnormal tricuspid valve leaflets) is the most well-known teratogenic risk associated with lithium, with an estimated absolute risk of 1 in 1,000 in patients treated with lithium compared with 1 in 20,000 in controls.10,11 The risk of congenital anomalies is increased in infants exposed to lithium in utero (4% to 12% vs 2% to 4% in controls)12; exposure during the first trimester of pregnancy is associated with increased risk. Lithium levels must be adjusted during pregnancy. Pregnant patients are at higher risk of relapse to mania because renal lithium clearance increases by 30% to 50% during pregnancy, and normalizes shortly after delivery.13
Lithium exposure during pregnancy has been linked to increased risk of miscarriage and preterm delivery; however, more research is needed to define the true risk of noncardiac teratogenicity associated with lithium.11 Because there is a lack of definitive data regarding teratogenicity, and because of lithium’s well-documented effectiveness in mood disorders, lithium should be considered a first-line therapy for pregnant patients with bipolar disorder.10
Prescribing trends
Despite data showing the efficacy and benefits of lithium, there has been a paradoxical decrease in lithium prescribing. This is the result of multiple factors, including fear of adverse effects and lithium toxicity and a shift toward newer medications, such as anticonvulsants and antipsychotics, for treatment and prophylaxis of mania.
A 2011 study examined prescribing trends for bipolar disorder in the United Kingdom.14 Overall, it found increased usage of valproate, carbamazepine, and lamotrigine from 1995 to 2009. During that time, lithium prescribing mostly remained steady at approximately 30%, whereas valproate use increased from 0% to 22.7%. Overall, antipsychotic and valproate prescribing increased relative to lithium.14 A literature review15 analyzed 6 studies of lithium prescribing trends from 1950 to 2010. Four of these studies (2 in the United States, 1 in Canada, and 1 in German-Swiss-Austrian hospitals) found lithium use was declining. The increased use found in Italy and Spain was attributed to multiple factors, including a broader definition of bipolar disorders and the unavailability of valproate in Spain, lithium’s low cost, and mental health reforms in both countries that resulted in overall increased psychotropic prescribing. Decreased lithium use was attributed to increased use of valproate and second-generation antipsychotics, lack of clinician training in lithium therapy, and aggressive marketing of brand-name medications.15
Reduced suicides, possible protection against dementia
A 2013 meta-analysis of 48 randomized controlled trials (RCTs) that included a total of 6,674 patients with mood disorders indicated that compared with placebo, lithium was more effective in reducing suicides and deaths from any cause.16
Large retrospective studies have demonstrated that compared with valproate, lithium has superior anti-suicide properties.17 Researchers found that risk of suicide attempt or completion was 1.5 to 3 times higher during periods of valproate treatment compared with lithium.18 Both short- and long-term lithium use was associated with decreased non-suicide mortality compared with valproate.19 In Denmark, compared with valproate, lithium was associated with fewer psychiatric hospital admissions.19 One RCT, the BALANCE trial, showed that lithium (alone or in combination with valproate) is more likely to prevent relapse in persons with bipolar I disorder than valproate monotherapy.20
Recent research in Denmark suggests that long-term doses of naturally occurring lithium in drinking water might confer some level of protection against dementia.21 Researchers examined the Danish National Patient Register to determine where participants lived and their local water supply. Drinking water lithium levels were assessed, and the mean lithium level for each municipality was calculated. This case-control study selected patients with dementia and 10 age- and sex-matched controls.21
Researchers found that the incidence rate ratio of Alzheimer disease, vascular dementia, and dementia overall was significantly lower among individuals whose drinking water contained lithium, 15.1 to 27.0 µg/L, compared with those whose water had lithium levels 2.0 to 5.0 µg/L.21 Although this study does not prove causality, it opens the door for continued research on lithium as a neuroprotective agent involved in pathways beyond mood stabilization.
Why should you prescribe lithium?
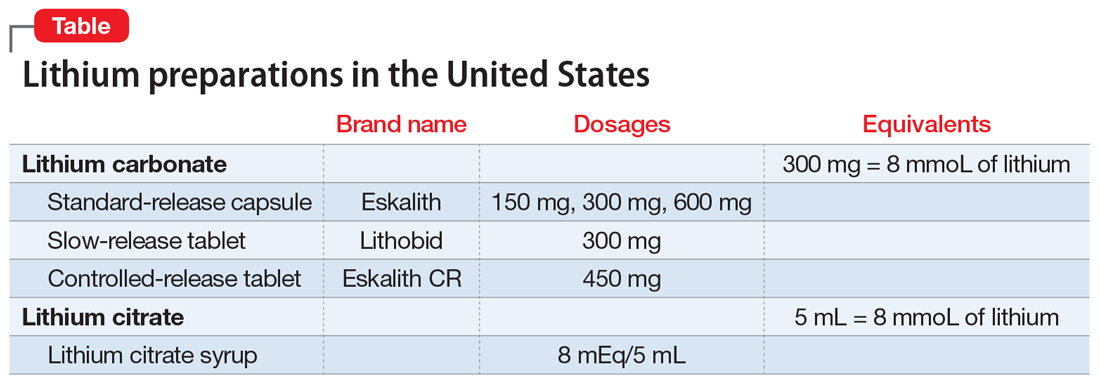
Lithium, which is available in several formulations (Table), should continue to be first-line pharmacotherapy for treating acute mood episodes, prophylaxis, and suicide prevention in bipolar disorder. Although there are many effective medications for treating bipolar disorder—such as second-generation antipsychotics that are available as a long-acting injectable formulation or can be combined with a mood stabilizer—lithium is a thoroughly researched medication with a long history of effectiveness for managing bipolar disorder. As is the case with all psychotropic medications, lithium has adverse effects and necessary precautions, but these are outweighed by its neuroprotective benefits and efficacy. Research has demonstrated that lithium outperforms medications that have largely replaced it, specifically valproate.
Related Resources
- Ali ZA, El-Mallakh RS. Lithium and kidney disease: Understand the risks. Current Psychiatry. 2021;20(6):34- 38,50. doi:10.12788/cp.0130
- Malhi GS, Gessler D, Outhred T. The use of lithium for the treatment of bipolar disorder: recommendations from clinical practice guidelines. J Affect Disord. 2017;217: 266-280. doi:10.1016/j.jad.2017.03.052
Drug Brand Names
Carbamazepine • Tegretol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Valproate • Depacon, Depakote, Depakene
Bottom Line
Lithium is a well-researched first-line pharmacotherapy for bipolar disorder, with efficacy equivalent to—or superior to—newer pharmacotherapies such as valproate and second-generation antipsychotics. When prescribing lithium, carefully monitor patients for symptoms of adverse effects or toxicity. Despite teratogenic risks, lithium can be considered for pregnant patients with bipolar disorder.
1. Shorter E. The history of lithium therapy. Bipolar Disord. 2009;11 suppl 2(suppl 2):4-9. doi: 10.1111/j.1399-5618.2009.00706.x
2. Cole N, Parker G. Cade’s identification of lithium for manic-depressive illness—the prospector who found a gold nugget. J Nerv Ment Dis. 2012;200(12):1101-1104. doi:10.1097/NMD.0b013e318275d3cb
3. Johnson FN. Lithium research and therapy. Academic Press; 1975.
4. Cade J. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949;2(10):518-520. doi:10.1080/j.1440-1614.1999.06241.x
5. Malhi GS, Tanious M, Das P, et al. The science and practice of lithium therapy. Aust N Z J Psychiatry. 2012;46(3):192-211. doi:10.1177/0004867412437346
6. Quiroz JA, Machado-Vieira R, Zarate CA Jr, et al. Novel insights into lithium’s mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology. 2010;62(1):50-60. doi:10.1159/000314310
7. Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. 2016;4(1):27. doi:10.1186/s40345-016-0068-y
8. Ott M, Stegmayr B, Salander Renberg E, et al. Lithium intoxication: incidence, clinical course and renal function - a population-based retrospective cohort study. J Psychopharmacol. 2016;30(10):1008-1019. doi:10.1177/0269881116652577
9. Heath LJ, Billups SJ, Gaughan KM, et al. Risk factors for utilization of acute care services for lithium toxicity. Psychiatr Serv. 2018;69(6):671-676. doi:10.1176/appi.ps.201700346
10. Raffi ER, Nonacs R, Cohen LS. Safety of psychotropic medications during pregnancy. Clin Perinatol. 2019;46(2):215-234. doi: 10.1016/j.clp.2019.02.004
11. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728. doi:10.1016/S0140-6736(11)61516-X
12. Mohandas E, Rajmohan V. Lithium use in special populations. Indian J Psychiatry. 2007;49(3):211-8. doi: 10.4103/0019-5545.37325
13. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244-55. doi: 10.1097/JCP.0000000000000087
14. Hayes J, Prah P, Nazareth I, et al. Prescribing trends in bipolar disorder: cohort study in the United Kingdom THIN primary care database 1995-2009. PLoS One. 2011;6(12):e28725. doi:10.1371/journal.pone.0028725
15. Netto I, Patil R, Kamble P, et al. Lithium prescribing trends: review. International Journal of Healthcare and Biomedical Research. 2014;2(2):95-103.
16. Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. doi: 10.1136/bmj.f3646
17. Meyer J. Lithium is regaining favor over anticonvulsants. Psychiatric News. October 2, 2015. Accessed October 12, 2021. https://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2015.PP10a6
18. Goodwin FK, Fireman B, Simon GE, et al. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003;290(11):1467-1473. doi:10.1001/jama.290.11.1467
19. Smith EG, Austin KL, Kim HM, et al. Mortality associated with lithium and valproate treatment of US Veterans Health Administration patients with mental disorders. Br J Psychiatry. 2015;207(1):55-63. doi:10.1192/bjp.bp.113.138685
20. Geddes JR, Goodwin GM, Rendell J, et al; BALANCE investigators and collaborators. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet. 2010;375(9712):385-395. doi:10.1016/S0140-6736(09)61828-6
21. Kessing LV, Gerds TA, Knudsen NN, et al. Association of lithium in drinking water with the incidence of dementia. JAMA Psychiatry. 2017;74(10):1005-1010. doi:10.1001/jamapsychiatry.2017.2362
In clinicians and patients alike, lithium triggers reactions ranging from apprehension and fear about adverse effects and toxicity to confusion over lithium’s usefulness compared with other mood stabilizers that do not require blood monitoring. Research from the 1950s to the 1970s demonstrated that lithium is effective for prophylaxis of mood episodes in patients with bipolar disorder and could reduce the frequency of hospitalization in patients who are depressed.1 For years, lithium was commonly prescribed to treat bipolar disorder, but in recent years its use has fallen out of favor due to concerns about its risks, and the availability of newer medications. This article reviews lithium’s origins (Box1-4), pharmacology, risks, and benefits, and makes a case for why it should remain a first-line therapy for bipolar disorder.
Box
Lithium was initially used in the 1840s to treat gout. William Hammond became the first physician to prescribe lithium bromide for acute mania in 1871, and in 1894, Danish psychiatrist Frederik Lange first used lithium carbonate to treat “melancholic depression.”1 In the 20th century, lithium-containing products were used to treat rheumatologic conditions such as renal calculi and other uric acid diatheses.
Lithium experienced a revival in 1949 when John Cade expanded upon Archibald Garrod’s theory regarding uric acid and gout. As a physician during WWII, Cade observed manic and depressive behaviors among prisoners.2 Theorizing that this was caused by either an excess or lack of a metabolite, he injected urine from patients with mania, depression, and schizophrenia and from healthy individuals into guinea pigs.3 Animals who received urine from patients with mania died faster than those injected with urine from a patient with schizophrenia.2 Concluding that urea was the culprit, Cade substituted the relatively water insoluble uric acid for “the most soluble of urates,” which was lithium urate.2,3 Rather than succumbing to a quicker death, guinea pigs injected with lithium urate became placid, tranquilized, lost their natural timidity, and generally did not respond to stimulation.3
Cade administered lithium carbonate and lithium citrate to himself and, because he did not experience any unwanted effects, began testing the medication on patients. Cade’s landmark 1949 paper4 notes improvement in all 10 patients with mania but little change in 6 patients with schizophrenia and 3 with chronic depression.2
In the United States, interest in lithium did not begin until the 1960s, when Samuel Gershon introduced the medication to a psychiatric hospital in Michigan. Financed by the National Institute of Mental Health, this program bought bulk lithium from a chemical supply store, and a local pharmacy formed it into capsules. Analysis of 4 controlled studies from 1963 to 1971 showed an average response rate to lithium of 78% in 116 patients with mania.1
By the end of the 1960s, many psychiatrists were prescribing lithium. At that time, lithium was not FDA-approved, but it could be prescribed as an investigational new drug by obtaining a special permit. In 1970, the FDA approved lithium for acute mania, and for prophylaxis of mania in 1975. Lithium has not yet been approved for prophylaxis of depression, despite substantial evidence indicating efficacy.1
How lithium works
Lithium has effects on neurotransmitters implicated in mania, such as glutamate, dopamine, and gamma-aminobutyric acid.5 Quiroz et al6 provide a detailed description of lithium’s effects, which can be summarized as modulating neuronal signaling pathways, including B-cell lymphoma 2 (BCL2), cAMP-response element binding protein (CREB), and glycogen synthase kinase-3 (GSK-3). Through these signaling cascades, lithium can curtail progression of neuronal apoptosis caused by the biochemical stress commonly seen in bipolar disorder pathogenesis.6
A wide range of potential adverse effects
Lithium can cause adverse effects in several organ systems. Clinicians must be aware of these effects before prescribing lithium or continuing long-term use. The most commonly documented adverse effects and symptoms of toxicity are:
- tremor
- renal dysfunction, including renal insufficiency and polyuria or polydipsia
- hypothyroidism
- hyperparathyroidism (with subsequent hypercalcemia)
- weight gain
- gastrointestinal (GI) symptoms.
These symptoms tend to occur when lithium serum levels are outside the reference range of 0.6 to 1.2 mEq/L, typically once blood levels reach ≥1.5 mEq/L.7 However, thyroid and renal abnormalities can occur at levels below this value, and might be related to cumulative lithium exposure.7 Adverse effects usually are precipitated by inadequate water intake or inadvertently taking an extra dose. Symptoms of lithium toxicity can be mild, moderate (GI complaints, tremor, weakness, fatigue), or severe (agitation, seizures, autonomic dysregulation, confusion, coma, death).
Lithium adverse effects and toxicity are infrequent. An analysis of 17 years of data in Sweden showed the incidence of moderate to severe lithium intoxication (serum level ≥1.5 mEq/L) was .01 patients per year.8 A recently published US analysis found the prevalence rate of lithium toxicity was 2.2%.9 Results from both groups show that drug interactions were an important cause of increased lithium levels, and specifically that initiating a medication that could interact with lithium was associated with 30-fold higher risk of needing acute care for lithium toxicity.9 Possible drug interactions include nonsteroidal anti-inflammatory drugs, diuretics, and renin-angiotensin-aldosterone system inhibitors.9 Because lithium is eliminated exclusively by the kidneys, impaired or altered renal function can increase the risk of lithium retention, leading to intoxication. Other risk factors include older age, alteration of water-salt homeostasis (fever, diarrhea, vomiting), higher number of treated chronic diseases as measured by Chronic Disease Score (range: 0 to 35; higher scores denotes higher number of treated chronic diseases and increased hospitalization risk), and higher total daily lithium dosage.9
Presentation of lithium intoxication often is mild or nonspecific, and physicians should have a low threshold for checking lithium blood levels.8 Lithium intoxication can be safely managed with volume expansion, forced diuresis, and hemodialysis.
Continue to: Lithium use during pregnancy...
Lithium use during pregnancy
When considering lithium for a woman who is pregnant, it is important to weigh the potential teratogenic risks against the benefit of successful management of the mood disorder. Ebstein’s anomaly (abnormal tricuspid valve leaflets) is the most well-known teratogenic risk associated with lithium, with an estimated absolute risk of 1 in 1,000 in patients treated with lithium compared with 1 in 20,000 in controls.10,11 The risk of congenital anomalies is increased in infants exposed to lithium in utero (4% to 12% vs 2% to 4% in controls)12; exposure during the first trimester of pregnancy is associated with increased risk. Lithium levels must be adjusted during pregnancy. Pregnant patients are at higher risk of relapse to mania because renal lithium clearance increases by 30% to 50% during pregnancy, and normalizes shortly after delivery.13
Lithium exposure during pregnancy has been linked to increased risk of miscarriage and preterm delivery; however, more research is needed to define the true risk of noncardiac teratogenicity associated with lithium.11 Because there is a lack of definitive data regarding teratogenicity, and because of lithium’s well-documented effectiveness in mood disorders, lithium should be considered a first-line therapy for pregnant patients with bipolar disorder.10
Prescribing trends
Despite data showing the efficacy and benefits of lithium, there has been a paradoxical decrease in lithium prescribing. This is the result of multiple factors, including fear of adverse effects and lithium toxicity and a shift toward newer medications, such as anticonvulsants and antipsychotics, for treatment and prophylaxis of mania.
A 2011 study examined prescribing trends for bipolar disorder in the United Kingdom.14 Overall, it found increased usage of valproate, carbamazepine, and lamotrigine from 1995 to 2009. During that time, lithium prescribing mostly remained steady at approximately 30%, whereas valproate use increased from 0% to 22.7%. Overall, antipsychotic and valproate prescribing increased relative to lithium.14 A literature review15 analyzed 6 studies of lithium prescribing trends from 1950 to 2010. Four of these studies (2 in the United States, 1 in Canada, and 1 in German-Swiss-Austrian hospitals) found lithium use was declining. The increased use found in Italy and Spain was attributed to multiple factors, including a broader definition of bipolar disorders and the unavailability of valproate in Spain, lithium’s low cost, and mental health reforms in both countries that resulted in overall increased psychotropic prescribing. Decreased lithium use was attributed to increased use of valproate and second-generation antipsychotics, lack of clinician training in lithium therapy, and aggressive marketing of brand-name medications.15
Reduced suicides, possible protection against dementia
A 2013 meta-analysis of 48 randomized controlled trials (RCTs) that included a total of 6,674 patients with mood disorders indicated that compared with placebo, lithium was more effective in reducing suicides and deaths from any cause.16
Large retrospective studies have demonstrated that compared with valproate, lithium has superior anti-suicide properties.17 Researchers found that risk of suicide attempt or completion was 1.5 to 3 times higher during periods of valproate treatment compared with lithium.18 Both short- and long-term lithium use was associated with decreased non-suicide mortality compared with valproate.19 In Denmark, compared with valproate, lithium was associated with fewer psychiatric hospital admissions.19 One RCT, the BALANCE trial, showed that lithium (alone or in combination with valproate) is more likely to prevent relapse in persons with bipolar I disorder than valproate monotherapy.20
Recent research in Denmark suggests that long-term doses of naturally occurring lithium in drinking water might confer some level of protection against dementia.21 Researchers examined the Danish National Patient Register to determine where participants lived and their local water supply. Drinking water lithium levels were assessed, and the mean lithium level for each municipality was calculated. This case-control study selected patients with dementia and 10 age- and sex-matched controls.21
Researchers found that the incidence rate ratio of Alzheimer disease, vascular dementia, and dementia overall was significantly lower among individuals whose drinking water contained lithium, 15.1 to 27.0 µg/L, compared with those whose water had lithium levels 2.0 to 5.0 µg/L.21 Although this study does not prove causality, it opens the door for continued research on lithium as a neuroprotective agent involved in pathways beyond mood stabilization.
Why should you prescribe lithium?
Lithium, which is available in several formulations (Table), should continue to be first-line pharmacotherapy for treating acute mood episodes, prophylaxis, and suicide prevention in bipolar disorder. Although there are many effective medications for treating bipolar disorder—such as second-generation antipsychotics that are available as a long-acting injectable formulation or can be combined with a mood stabilizer—lithium is a thoroughly researched medication with a long history of effectiveness for managing bipolar disorder. As is the case with all psychotropic medications, lithium has adverse effects and necessary precautions, but these are outweighed by its neuroprotective benefits and efficacy. Research has demonstrated that lithium outperforms medications that have largely replaced it, specifically valproate.

Related Resources
- Ali ZA, El-Mallakh RS. Lithium and kidney disease: Understand the risks. Current Psychiatry. 2021;20(6):34- 38,50. doi:10.12788/cp.0130
- Malhi GS, Gessler D, Outhred T. The use of lithium for the treatment of bipolar disorder: recommendations from clinical practice guidelines. J Affect Disord. 2017;217: 266-280. doi:10.1016/j.jad.2017.03.052
Drug Brand Names
Carbamazepine • Tegretol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Valproate • Depacon, Depakote, Depakene
Bottom Line
Lithium is a well-researched first-line pharmacotherapy for bipolar disorder, with efficacy equivalent to—or superior to—newer pharmacotherapies such as valproate and second-generation antipsychotics. When prescribing lithium, carefully monitor patients for symptoms of adverse effects or toxicity. Despite teratogenic risks, lithium can be considered for pregnant patients with bipolar disorder.
In clinicians and patients alike, lithium triggers reactions ranging from apprehension and fear about adverse effects and toxicity to confusion over lithium’s usefulness compared with other mood stabilizers that do not require blood monitoring. Research from the 1950s to the 1970s demonstrated that lithium is effective for prophylaxis of mood episodes in patients with bipolar disorder and could reduce the frequency of hospitalization in patients who are depressed.1 For years, lithium was commonly prescribed to treat bipolar disorder, but in recent years its use has fallen out of favor due to concerns about its risks, and the availability of newer medications. This article reviews lithium’s origins (Box1-4), pharmacology, risks, and benefits, and makes a case for why it should remain a first-line therapy for bipolar disorder.
Box
Lithium was initially used in the 1840s to treat gout. William Hammond became the first physician to prescribe lithium bromide for acute mania in 1871, and in 1894, Danish psychiatrist Frederik Lange first used lithium carbonate to treat “melancholic depression.”1 In the 20th century, lithium-containing products were used to treat rheumatologic conditions such as renal calculi and other uric acid diatheses.
Lithium experienced a revival in 1949 when John Cade expanded upon Archibald Garrod’s theory regarding uric acid and gout. As a physician during WWII, Cade observed manic and depressive behaviors among prisoners.2 Theorizing that this was caused by either an excess or lack of a metabolite, he injected urine from patients with mania, depression, and schizophrenia and from healthy individuals into guinea pigs.3 Animals who received urine from patients with mania died faster than those injected with urine from a patient with schizophrenia.2 Concluding that urea was the culprit, Cade substituted the relatively water insoluble uric acid for “the most soluble of urates,” which was lithium urate.2,3 Rather than succumbing to a quicker death, guinea pigs injected with lithium urate became placid, tranquilized, lost their natural timidity, and generally did not respond to stimulation.3
Cade administered lithium carbonate and lithium citrate to himself and, because he did not experience any unwanted effects, began testing the medication on patients. Cade’s landmark 1949 paper4 notes improvement in all 10 patients with mania but little change in 6 patients with schizophrenia and 3 with chronic depression.2
In the United States, interest in lithium did not begin until the 1960s, when Samuel Gershon introduced the medication to a psychiatric hospital in Michigan. Financed by the National Institute of Mental Health, this program bought bulk lithium from a chemical supply store, and a local pharmacy formed it into capsules. Analysis of 4 controlled studies from 1963 to 1971 showed an average response rate to lithium of 78% in 116 patients with mania.1
By the end of the 1960s, many psychiatrists were prescribing lithium. At that time, lithium was not FDA-approved, but it could be prescribed as an investigational new drug by obtaining a special permit. In 1970, the FDA approved lithium for acute mania, and for prophylaxis of mania in 1975. Lithium has not yet been approved for prophylaxis of depression, despite substantial evidence indicating efficacy.1
How lithium works
Lithium has effects on neurotransmitters implicated in mania, such as glutamate, dopamine, and gamma-aminobutyric acid.5 Quiroz et al6 provide a detailed description of lithium’s effects, which can be summarized as modulating neuronal signaling pathways, including B-cell lymphoma 2 (BCL2), cAMP-response element binding protein (CREB), and glycogen synthase kinase-3 (GSK-3). Through these signaling cascades, lithium can curtail progression of neuronal apoptosis caused by the biochemical stress commonly seen in bipolar disorder pathogenesis.6
A wide range of potential adverse effects
Lithium can cause adverse effects in several organ systems. Clinicians must be aware of these effects before prescribing lithium or continuing long-term use. The most commonly documented adverse effects and symptoms of toxicity are:
- tremor
- renal dysfunction, including renal insufficiency and polyuria or polydipsia
- hypothyroidism
- hyperparathyroidism (with subsequent hypercalcemia)
- weight gain
- gastrointestinal (GI) symptoms.
These symptoms tend to occur when lithium serum levels are outside the reference range of 0.6 to 1.2 mEq/L, typically once blood levels reach ≥1.5 mEq/L.7 However, thyroid and renal abnormalities can occur at levels below this value, and might be related to cumulative lithium exposure.7 Adverse effects usually are precipitated by inadequate water intake or inadvertently taking an extra dose. Symptoms of lithium toxicity can be mild, moderate (GI complaints, tremor, weakness, fatigue), or severe (agitation, seizures, autonomic dysregulation, confusion, coma, death).
Lithium adverse effects and toxicity are infrequent. An analysis of 17 years of data in Sweden showed the incidence of moderate to severe lithium intoxication (serum level ≥1.5 mEq/L) was .01 patients per year.8 A recently published US analysis found the prevalence rate of lithium toxicity was 2.2%.9 Results from both groups show that drug interactions were an important cause of increased lithium levels, and specifically that initiating a medication that could interact with lithium was associated with 30-fold higher risk of needing acute care for lithium toxicity.9 Possible drug interactions include nonsteroidal anti-inflammatory drugs, diuretics, and renin-angiotensin-aldosterone system inhibitors.9 Because lithium is eliminated exclusively by the kidneys, impaired or altered renal function can increase the risk of lithium retention, leading to intoxication. Other risk factors include older age, alteration of water-salt homeostasis (fever, diarrhea, vomiting), higher number of treated chronic diseases as measured by Chronic Disease Score (range: 0 to 35; higher scores denotes higher number of treated chronic diseases and increased hospitalization risk), and higher total daily lithium dosage.9
Presentation of lithium intoxication often is mild or nonspecific, and physicians should have a low threshold for checking lithium blood levels.8 Lithium intoxication can be safely managed with volume expansion, forced diuresis, and hemodialysis.
Continue to: Lithium use during pregnancy...
Lithium use during pregnancy
When considering lithium for a woman who is pregnant, it is important to weigh the potential teratogenic risks against the benefit of successful management of the mood disorder. Ebstein’s anomaly (abnormal tricuspid valve leaflets) is the most well-known teratogenic risk associated with lithium, with an estimated absolute risk of 1 in 1,000 in patients treated with lithium compared with 1 in 20,000 in controls.10,11 The risk of congenital anomalies is increased in infants exposed to lithium in utero (4% to 12% vs 2% to 4% in controls)12; exposure during the first trimester of pregnancy is associated with increased risk. Lithium levels must be adjusted during pregnancy. Pregnant patients are at higher risk of relapse to mania because renal lithium clearance increases by 30% to 50% during pregnancy, and normalizes shortly after delivery.13
Lithium exposure during pregnancy has been linked to increased risk of miscarriage and preterm delivery; however, more research is needed to define the true risk of noncardiac teratogenicity associated with lithium.11 Because there is a lack of definitive data regarding teratogenicity, and because of lithium’s well-documented effectiveness in mood disorders, lithium should be considered a first-line therapy for pregnant patients with bipolar disorder.10
Prescribing trends
Despite data showing the efficacy and benefits of lithium, there has been a paradoxical decrease in lithium prescribing. This is the result of multiple factors, including fear of adverse effects and lithium toxicity and a shift toward newer medications, such as anticonvulsants and antipsychotics, for treatment and prophylaxis of mania.
A 2011 study examined prescribing trends for bipolar disorder in the United Kingdom.14 Overall, it found increased usage of valproate, carbamazepine, and lamotrigine from 1995 to 2009. During that time, lithium prescribing mostly remained steady at approximately 30%, whereas valproate use increased from 0% to 22.7%. Overall, antipsychotic and valproate prescribing increased relative to lithium.14 A literature review15 analyzed 6 studies of lithium prescribing trends from 1950 to 2010. Four of these studies (2 in the United States, 1 in Canada, and 1 in German-Swiss-Austrian hospitals) found lithium use was declining. The increased use found in Italy and Spain was attributed to multiple factors, including a broader definition of bipolar disorders and the unavailability of valproate in Spain, lithium’s low cost, and mental health reforms in both countries that resulted in overall increased psychotropic prescribing. Decreased lithium use was attributed to increased use of valproate and second-generation antipsychotics, lack of clinician training in lithium therapy, and aggressive marketing of brand-name medications.15
Reduced suicides, possible protection against dementia
A 2013 meta-analysis of 48 randomized controlled trials (RCTs) that included a total of 6,674 patients with mood disorders indicated that compared with placebo, lithium was more effective in reducing suicides and deaths from any cause.16
Large retrospective studies have demonstrated that compared with valproate, lithium has superior anti-suicide properties.17 Researchers found that risk of suicide attempt or completion was 1.5 to 3 times higher during periods of valproate treatment compared with lithium.18 Both short- and long-term lithium use was associated with decreased non-suicide mortality compared with valproate.19 In Denmark, compared with valproate, lithium was associated with fewer psychiatric hospital admissions.19 One RCT, the BALANCE trial, showed that lithium (alone or in combination with valproate) is more likely to prevent relapse in persons with bipolar I disorder than valproate monotherapy.20
Recent research in Denmark suggests that long-term doses of naturally occurring lithium in drinking water might confer some level of protection against dementia.21 Researchers examined the Danish National Patient Register to determine where participants lived and their local water supply. Drinking water lithium levels were assessed, and the mean lithium level for each municipality was calculated. This case-control study selected patients with dementia and 10 age- and sex-matched controls.21
Researchers found that the incidence rate ratio of Alzheimer disease, vascular dementia, and dementia overall was significantly lower among individuals whose drinking water contained lithium, 15.1 to 27.0 µg/L, compared with those whose water had lithium levels 2.0 to 5.0 µg/L.21 Although this study does not prove causality, it opens the door for continued research on lithium as a neuroprotective agent involved in pathways beyond mood stabilization.
Why should you prescribe lithium?
Lithium, which is available in several formulations (Table), should continue to be first-line pharmacotherapy for treating acute mood episodes, prophylaxis, and suicide prevention in bipolar disorder. Although there are many effective medications for treating bipolar disorder—such as second-generation antipsychotics that are available as a long-acting injectable formulation or can be combined with a mood stabilizer—lithium is a thoroughly researched medication with a long history of effectiveness for managing bipolar disorder. As is the case with all psychotropic medications, lithium has adverse effects and necessary precautions, but these are outweighed by its neuroprotective benefits and efficacy. Research has demonstrated that lithium outperforms medications that have largely replaced it, specifically valproate.

Related Resources
- Ali ZA, El-Mallakh RS. Lithium and kidney disease: Understand the risks. Current Psychiatry. 2021;20(6):34- 38,50. doi:10.12788/cp.0130
- Malhi GS, Gessler D, Outhred T. The use of lithium for the treatment of bipolar disorder: recommendations from clinical practice guidelines. J Affect Disord. 2017;217: 266-280. doi:10.1016/j.jad.2017.03.052
Drug Brand Names
Carbamazepine • Tegretol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Valproate • Depacon, Depakote, Depakene
Bottom Line
Lithium is a well-researched first-line pharmacotherapy for bipolar disorder, with efficacy equivalent to—or superior to—newer pharmacotherapies such as valproate and second-generation antipsychotics. When prescribing lithium, carefully monitor patients for symptoms of adverse effects or toxicity. Despite teratogenic risks, lithium can be considered for pregnant patients with bipolar disorder.
1. Shorter E. The history of lithium therapy. Bipolar Disord. 2009;11 suppl 2(suppl 2):4-9. doi: 10.1111/j.1399-5618.2009.00706.x
2. Cole N, Parker G. Cade’s identification of lithium for manic-depressive illness—the prospector who found a gold nugget. J Nerv Ment Dis. 2012;200(12):1101-1104. doi:10.1097/NMD.0b013e318275d3cb
3. Johnson FN. Lithium research and therapy. Academic Press; 1975.
4. Cade J. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949;2(10):518-520. doi:10.1080/j.1440-1614.1999.06241.x
5. Malhi GS, Tanious M, Das P, et al. The science and practice of lithium therapy. Aust N Z J Psychiatry. 2012;46(3):192-211. doi:10.1177/0004867412437346
6. Quiroz JA, Machado-Vieira R, Zarate CA Jr, et al. Novel insights into lithium’s mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology. 2010;62(1):50-60. doi:10.1159/000314310
7. Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. 2016;4(1):27. doi:10.1186/s40345-016-0068-y
8. Ott M, Stegmayr B, Salander Renberg E, et al. Lithium intoxication: incidence, clinical course and renal function - a population-based retrospective cohort study. J Psychopharmacol. 2016;30(10):1008-1019. doi:10.1177/0269881116652577
9. Heath LJ, Billups SJ, Gaughan KM, et al. Risk factors for utilization of acute care services for lithium toxicity. Psychiatr Serv. 2018;69(6):671-676. doi:10.1176/appi.ps.201700346
10. Raffi ER, Nonacs R, Cohen LS. Safety of psychotropic medications during pregnancy. Clin Perinatol. 2019;46(2):215-234. doi: 10.1016/j.clp.2019.02.004
11. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728. doi:10.1016/S0140-6736(11)61516-X
12. Mohandas E, Rajmohan V. Lithium use in special populations. Indian J Psychiatry. 2007;49(3):211-8. doi: 10.4103/0019-5545.37325
13. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244-55. doi: 10.1097/JCP.0000000000000087
14. Hayes J, Prah P, Nazareth I, et al. Prescribing trends in bipolar disorder: cohort study in the United Kingdom THIN primary care database 1995-2009. PLoS One. 2011;6(12):e28725. doi:10.1371/journal.pone.0028725
15. Netto I, Patil R, Kamble P, et al. Lithium prescribing trends: review. International Journal of Healthcare and Biomedical Research. 2014;2(2):95-103.
16. Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. doi: 10.1136/bmj.f3646
17. Meyer J. Lithium is regaining favor over anticonvulsants. Psychiatric News. October 2, 2015. Accessed October 12, 2021. https://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2015.PP10a6
18. Goodwin FK, Fireman B, Simon GE, et al. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003;290(11):1467-1473. doi:10.1001/jama.290.11.1467
19. Smith EG, Austin KL, Kim HM, et al. Mortality associated with lithium and valproate treatment of US Veterans Health Administration patients with mental disorders. Br J Psychiatry. 2015;207(1):55-63. doi:10.1192/bjp.bp.113.138685
20. Geddes JR, Goodwin GM, Rendell J, et al; BALANCE investigators and collaborators. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet. 2010;375(9712):385-395. doi:10.1016/S0140-6736(09)61828-6
21. Kessing LV, Gerds TA, Knudsen NN, et al. Association of lithium in drinking water with the incidence of dementia. JAMA Psychiatry. 2017;74(10):1005-1010. doi:10.1001/jamapsychiatry.2017.2362
1. Shorter E. The history of lithium therapy. Bipolar Disord. 2009;11 suppl 2(suppl 2):4-9. doi: 10.1111/j.1399-5618.2009.00706.x
2. Cole N, Parker G. Cade’s identification of lithium for manic-depressive illness—the prospector who found a gold nugget. J Nerv Ment Dis. 2012;200(12):1101-1104. doi:10.1097/NMD.0b013e318275d3cb
3. Johnson FN. Lithium research and therapy. Academic Press; 1975.
4. Cade J. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949;2(10):518-520. doi:10.1080/j.1440-1614.1999.06241.x
5. Malhi GS, Tanious M, Das P, et al. The science and practice of lithium therapy. Aust N Z J Psychiatry. 2012;46(3):192-211. doi:10.1177/0004867412437346
6. Quiroz JA, Machado-Vieira R, Zarate CA Jr, et al. Novel insights into lithium’s mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology. 2010;62(1):50-60. doi:10.1159/000314310
7. Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. 2016;4(1):27. doi:10.1186/s40345-016-0068-y
8. Ott M, Stegmayr B, Salander Renberg E, et al. Lithium intoxication: incidence, clinical course and renal function - a population-based retrospective cohort study. J Psychopharmacol. 2016;30(10):1008-1019. doi:10.1177/0269881116652577
9. Heath LJ, Billups SJ, Gaughan KM, et al. Risk factors for utilization of acute care services for lithium toxicity. Psychiatr Serv. 2018;69(6):671-676. doi:10.1176/appi.ps.201700346
10. Raffi ER, Nonacs R, Cohen LS. Safety of psychotropic medications during pregnancy. Clin Perinatol. 2019;46(2):215-234. doi: 10.1016/j.clp.2019.02.004
11. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728. doi:10.1016/S0140-6736(11)61516-X
12. Mohandas E, Rajmohan V. Lithium use in special populations. Indian J Psychiatry. 2007;49(3):211-8. doi: 10.4103/0019-5545.37325
13. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244-55. doi: 10.1097/JCP.0000000000000087
14. Hayes J, Prah P, Nazareth I, et al. Prescribing trends in bipolar disorder: cohort study in the United Kingdom THIN primary care database 1995-2009. PLoS One. 2011;6(12):e28725. doi:10.1371/journal.pone.0028725
15. Netto I, Patil R, Kamble P, et al. Lithium prescribing trends: review. International Journal of Healthcare and Biomedical Research. 2014;2(2):95-103.
16. Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. doi: 10.1136/bmj.f3646
17. Meyer J. Lithium is regaining favor over anticonvulsants. Psychiatric News. October 2, 2015. Accessed October 12, 2021. https://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2015.PP10a6
18. Goodwin FK, Fireman B, Simon GE, et al. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003;290(11):1467-1473. doi:10.1001/jama.290.11.1467
19. Smith EG, Austin KL, Kim HM, et al. Mortality associated with lithium and valproate treatment of US Veterans Health Administration patients with mental disorders. Br J Psychiatry. 2015;207(1):55-63. doi:10.1192/bjp.bp.113.138685
20. Geddes JR, Goodwin GM, Rendell J, et al; BALANCE investigators and collaborators. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet. 2010;375(9712):385-395. doi:10.1016/S0140-6736(09)61828-6
21. Kessing LV, Gerds TA, Knudsen NN, et al. Association of lithium in drinking water with the incidence of dementia. JAMA Psychiatry. 2017;74(10):1005-1010. doi:10.1001/jamapsychiatry.2017.2362
