User login
Polymer matrix composite materials have been widely promoted for orthopedic use in a variety of settings, including surgical instruments, medical devices, implants, and bone models.1-13 These types of composites are engineered from 2 or more constituent materials with significantly different physical or chemical properties; these materials remain separate and distinct on a macroscopic level within the finished composite structure. As a result of ongoing biomaterial research, polymer matrix composite materials can be engineered with a wide range of physical, mechanical, and surface properties, tailored to their application. Given their advantages (eg, high strength-to-weight ratio, radiolucency), these polymer matrix composite materials have gained popularity over traditional metallic materials.
Sterilization is an essential day-to-day procedure in the health care sector, both for single- and multiple-use devices or instruments, and thus a composite material used in medical components should remain unaffected by the process. The type of sterilization most commonly performed is steam sterilization, which achieves microbiological death by moist heat and pressure. Steam is created in an autoclave at a temperature of 132°C (270°F) in typical hospital settings. Steam sterilization cycles last 5 to 14 minutes based on specific manufacturer recommendations. Most medical-grade plastics used in health care have been designed and formulated to withstand the required sterilization cycles without sacrificing key properties. The structure integrity and overall performance of polymer matrix composites may be strongly influenced by the stability of the fiber/polymer interfacial region in terms of physical, chemical, and mechanical characteristics of the material at different scales.14 Absorption of moisture causes dilatational expansion and induces stresses, which are associated with the moisture-induced expansion resulting in degradation of structure stability.15 Thus, steam sterilization could affect the properties of the polymer matrix composite materials by excessive absorption of moisture by the polymer.
To our knowledge, no one has studied whether polymer matrix material properties degrade from long-term, repeated steam sterilization followed by mechanical loading. We conducted a study to evaluate the structural properties (short-beam strength, SBS) of several composite materials exposed to repeated sterilization as compared with traditional metal materials: SS-316L (stainless steel 316L) and Al-7075-T6 (aluminum 7075-T6).
Materials and Methods
We evaluated 3 types of composite materials: Tepex (Tepex Dynalite 201; HiPer Technology Inc.), CFR-PPS (carbon-fiber–reinforced polyphenylene sulfide, Cetex PPS; TenCate Advanced Composites USA Inc.), and HTN-53 (Zytel HTN53G50HSLR NC010; HiPer Technology Inc.) (Figure 1). Tepex is being used for orthopedic applications (knee braces, orthoses, insoles) and sporting goods applications. The performance of this material is superior to that of unreinforced thermoplastics. CFR-PPS represented the state of the art in composite materials for aerospace applications (eg, airframe structures, engine nacelles, fan casings, floorboards, interior parts). This is a high-performance material with exceptional high temperature and aggressive chemical resistance characteristics. CFR-PPS is also used to make filter fabric for coal boilers, papermaking felts, electrical insulation, specialty membranes, gaskets, and packing. It is not solubilized by any known solvents, even in long-term exposure, at temperatures up to 200°C. In addition, it exhibits exceptional resistance to organic and inorganic solutions, acids and alkali solutions, and a wide array of miscellaneous chemicals. HTN-53 is a 50% glass-reinforced, lubricated, high-performance polyamide resin with improved flow, developed for applications requiring excellent surface appearance with water-heated molds. This material has specifically shown survivability in hot, cold, chemically aggressive, and load-bearing environments. In addition, it has shown superior moisture and temperature resistance. These 3 composite materials were compared with SS-316L and Al-7075-T6. SS-316L is commonly used for implants in orthopedics, and Al-7075-T6 is a relatively radiolucent alternative for medical applications. Two different tests were performed to evaluate and validate these composite materials: (1) radiographic density evaluation and (2) structural property tests (short-beam load-to-failure [LTF] test, short-beam cyclic compression loading [CCL] test) before and after sterilization cycling.
Radiographic Density Evaluation
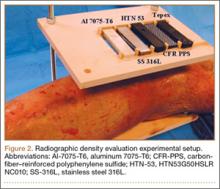
The radiographic density of the 5 materials was evaluated with radiographic images of a cadaveric knee specimen (Figure 2). Radiographic image intensification is the gold standard for repeated radiographic imaging in the operating room. Six different radiographic images were obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). Image-Pro Plus software (Media Cybernetics) was used to measure the radiographic density of the materials from the grayscale of the images.
Structural Properties Testing Before and After Sterilization Cycling
We used a standard SBS testing method to determine whether any degradation of structural properties resulted from standard repeated sterilization. The material geometries of the test specimens were 18.96×6.50×3.37 mm (length × width × thickness). Standard sterilization procedures were performed with steam sterilization using an autoclave at a temperature of 132°C (270°F) for at least 5 minutes (range, 5-14 minutes). Sample interval testing ran at 0, 200, and 400 sterilization cycles for structural properties in terms of SBS and moisture retention, with the structural properties at the 0th sterilization cycle (material before sterilization was performed) used as a baseline for comparison. Materials were subjected to 400 sterilization cycles, which is representative of the number of sterilization cycles per year an instrument or device would be subjected to.
Three structural tests were performed for each sample interval: moisture retention, LTF, and CCL. Moisture retention was investigated before and after repeated sterilization by measuring the weight of the test materials, as steam sterilization is known to affect the amount of moisture that is absorbed by a material. Twelve specimens of each proposed material were weighed at each sample interval, with the structural weight at the 0th sterilization cycle (material before sterilization is performed) serving as a baseline for comparison.
SBS testing was based on the ASTM (American Society for Testing and Materials) D2344 standard16 for LTF and CCL tests (Figure 3). Six samples of material were used for each test at every sample interval, yielding 180 samples. Seven servohydraulic material testing system instruments (1 MTS 810 and 6 MTS 858 Mini Bionix) were used to test the SBS of each material. For LTF testing, each specimen was loaded in compression from 30 N to complete structural failure at a constant displacement rate of 1.0 mm/min (0.05 in/min). Testing was initiated with 5 preconditioning loading cycles from 30 to 100 N at 1 Hz. The load was then applied continuously until failure occurred; force and displacement data were collected every 0.02 second. This procedure was performed for 6 replicates for each sample interval for each test material.
The calculation for SBS, Fsbs (MPa), for the constant loading rate until structural failure is:
Fsbs = 0.75 × Pm
b × h
where Pm (N) is the maximum applied load observed during the test, b is the measured specimen width (mm), and h is the measured specimen thickness (mm).
CCL testing consisted of each test material axially loaded with 100 to 500 N at a frequency of 1 Hz for 100,000 cycles. The maximum load of 500 N was chosen as a standard based on 80% of the minimum ultimate failure load from previous LTF tests. Displacement and force data were collected every 5 cycles at the maximum compressive load. Degradation of the material was calculated using the difference between the deflection of the initial cycle and the deflection of the final cycle (50th cycle and 100,000th cycle). This procedure was performed for 6 replicates for each sample interval for each test material.
Statistical Analysis
LTF and CCL testing data were analyzed for any differences among the test materials using 1-way analysis of variance with the least significant difference multiple comparisons post hoc test method using SPSS Version 16.0, with P < .05 denoting significance. These analyses were used to determine the statistical relevance of the difference between the SBS (LTF and CCL) of each test material. Means and standard deviations were calculated for all tests.
Results
Radiographic Density Evaluation
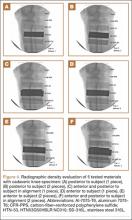
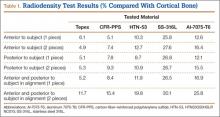
Overall, all the tested composite materials were significantly more radiolucent than either SS-316L or Al-7075-T6. Figure 4 shows the 6 different radiographic images obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). SS-316L can be considered radiopaque, and Al-7075-T6 has been used as a relatively radiolucent alternative. Tepex was statistically more radiolucent than the other 2 tested composite materials (Table 1). Even with 2 pieces placed anterior to the subject and 2 placed posterior, the radiodensity compared to the cortical bone was still lower than 1 piece of Al-7075-T6 either anterior or posterior to the subject.
Structural Properties Testing
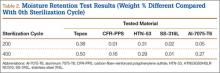
Moisture Retention. Moisture retention was evaluated by weighing the test materials before and after repeated sterilization. There was no significant difference in moisture retention, as weight differences for all the tested materials were less than 0.5 weight percentage compared to the 0th sterilization cycle (Table 2). Therefore, the results of this study showed that all the tested materials exhibited good moisture/temperature resistance after 400 sterilization cycles.
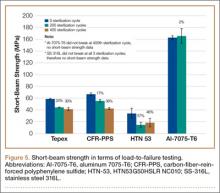
Load to Failure. In the LTF test, significant differences were detected in SBS between all 5 tested materials (P < .05). Figure 5 shows the comparison of the structural properties in terms of SBS between the 5 tested materials, and Figure 6 shows the failure modes for the tested materials. There was no SBS for SS-316L, as the material did not exhibit complete structural failure even after 400 sterilization cycles; however, SS-316L was observed in inelastic deformation failure (Figure 6D). Al-7075-T6 had much higher SBS compared with the other composite materials, and it also resulted in an inelastic deformation failure mode only after 400 sterilization cycles; otherwise, flexure failure modes were observed. Tepex and CFR-PPS exhibited interlaminar shear failure, and HTN-53 exhibited complete structural failure.
Every composite material tested using the short-beam test for LTF showed a decrease in SBS with increased sterilization cycles (Figure 5). This decrease ranged from 17% to 57% compared with the 0th sterilization cycle. SBS was higher for CFR-PPS than for the other 2 composites. No statistically significant difference was found between CFR-PPS and Tepex except at the 200th sterilization cycle. HTN-53 was brittle at the 0th sterilization cycle but performed more like a ductile material at the 200th cycle. In addition, HTN-53 had the lowest SBS in terms of LTF testing when compared with the other 2 composites.
During the complete structural failure test, the failure modes for Tepex and CFR-PPS were visually identified as interlaminar shear failure (Figures 6A, 6B), whereas HTN-53 visually exhibited pure flexure failure (Figure 6C). As for the metals, SS-316L exhibited plastic deformation, and Al-7075-T6 exhibited flexure failure (Figures 6D, 6E).
Cyclic Compression Loading. Tepex was the only material to pass the 100,000 loading cycle without failure (Table 3). HTN-53 had the poorest performance of all: Its failure rates were 33% (2/6 samples) before sterilization (average cycle, 22,213; range, 21,500-22,925), 83% (5/6 samples) at the 200th sterilization cycle (average cycle, 4,210; range, 0-14,360), and 100% after 400 sterilization cycles (average cycle, 12,725; range, 1,190-21,900). CFR-PPS had no failures before the 400th sterilization cycle, and its failure rate after 400 sterilization cycles (average cycle, 50,735; range, 50,270-51,200) was 33% (2/6 samples).
Discussion
The success of a reusable composite material for use in orthopedic surgery depends not only on radiographic density, fabrication methods, and design but also on the ability to withstand repeated sterilization. Over the past 3 decades, investigators have explored several high-performance polymer matrix composite materials for use in orthopedics, especially in trauma, hip stems, and spinal implants.1,3,4,17-34 According to Evans and Gregson,35 composite materials have been widely promoted as possible orthopedic biomaterials but to date have found few successful commercial applications, because of the many challenging problems encountered in fabrication and testing. One of the most important factors in the mechanical properties of many composite materials is the influence of the cooling and loading rates on fiber-matrix interface adhesion.36-38 Our results tended to agree with the findings of Evans and Gregson,35 as some of these composite materials did not withstand repeated sterilizations well.
Guan and colleagues39 evaluated the influence of sterilization treatment on continuous carbon-fiber–reinforced polyolefin composite. Their 3-point bending test results showed that the levels of maximum load of all the specimens undergoing sterilization by autoclave were lower than those of the control group. For these composites, they concluded that autoclave sterilization and Co-60 gamma ray irradiation sterilization should be avoided and that ethylene oxide is the best method. Our results support their findings with a different set of composites.
Although HTN-53 has shown promise in other orthopedic applications because of its superior moisture and temperature resistance, we found that its performance after repeated sterilization was relatively poor. Tepex showed the greatest potential for durability after repeated sterilization; its mechanical properties were stable after 200 steam sterilization cycles.
Clinical Applications
The composite materials investigated in the present study have potential for use in either instrumentation or long-term implantation applications because of their versatility, mechanical strength, fatigue resistance, and biocompatibility. Akay and Aslan40 stated that carbon-fiber–filled composite implants can be designed with more appropriate modulus, strength, toughness, or stiffness by the arrangement of reinforcing fiber volume and orientation, and can provide better fatigue resistance. A notable advantage of using a composite plate with metal screws is that the potential for corrosion of metallic components is eliminated. Another major advantage of composite medical implants (eg, DiPhos-RM) is radiolucency, which allows direct visualization of osseous callus formation as well as monitoring of fracture healing, thereby improving clinical assessment and accuracy.
Numerous studies have documented the successful clinical performance of composite materials in orthopedic, trauma, and spinal surgery applications.41-45 Bagheri and colleagues41 developed a new carbon fiber–flax–epoxy composite plate and biomechanically compared it with a standard clinical metal plate. Their results confirmed that the carbon fiber–flax–epoxy material represents a potential candidate for bone fracture plate applications, as it can simultaneously provide similar mechanical stiffness and lower stress shielding (higher bone stress) compared with a standard clinical metal bone plate. Tarallo and colleagues45 evaluated the clinical results of 40 cases at 12-month follow-up using a new plate made of carbon-fiber–reinforced polyetheretherketon (DiPhos-RM, Lima Corporate) for the treatment of distal radius fractures. They reported good clinical results for this device at early follow-up, and its use allowed maintenance of reduction in complex AO (Arbeitsgemeinschaft für Osteosynthesefragen) fractures.
The main advantage in using composites for surgical instruments is their radiolucency. These materials do not obscure images or radiographs during fluoroscopic visualization. Surgery often requires fluoroscopic visualization of internal organs or bones, which may require temporary removal of radiopaque devices (eg, retractors, clamps, forceps, hooks, distractors). Aside from being inconvenient, removal and subsequent reinsertion consume valuable time and interfere with the smooth flow of an operation.
The shortcomings of using composite materials for surgical instruments involve detectability and sterilization. A significant issue in surgery is the accidental leaving behind of instruments in patients, which can cause serious problems ranging from organ perforation and blood infection to death. Although instrument counting and other safety protocols can reduce the risk of overlooking an instrument, mistakes are bound to happen. The other shortcoming is the influence of repeated sterilization on the mechanical properties of the composite materials, as sterilization is mandatory for surgical instruments used in the operation room. The structural integrity and overall performance of the polymer composite materials—especially the stability of the interface and the interphase zones—are strongly influenced by repeated sterilization.
On the other hand, composite materials have potential advantages that may support their introduction into long-term medical implant applications, as sterilization commonly is performed only once, during packaging. The effects of sterilization by radiation or steam are much less pronounced on composite implants than on composite surgical instruments. However, composite implants require careful consideration with respect to the bioactivity of wear particles that may be produced from articulation. Further, carbon-fiber–reinforced polymer implants are still substantially more difficult to manufacture and more costly than their metallic counterparts.46
Limitations
This study has some limitations. Most important, studies of this nature do not account for biological factors such as corrosion, biological wear, and the soft-tissue attachment effects on structural properties for potential in vivo use. Another limitation was that the study tested only the mechanical properties in terms of SBS and provided no information about other mechanical properties, such as tensile, compression, and torsion strengths. We think SBS testing adequately evaluates challenging scenarios like thin and narrow instruments/devices that are anticipated in application, and information regarding other modes of failure and mechanical properties (compression, tension, torsion) would be a further area of research. An additional limitation was that our model used a relatively small number of samples. A larger study with more samples and varying layout patterns and layers of the carbon fibers may more clearly demonstrate the effect of steam sterilization on composite materials.
Conclusion
This study provided new information on 3 selected composite materials and their structural properties after repeated steam sterilization. We discovered that these composites were similar in radiographic density and water retention but behaved very differently in terms of mechanical durability after repeated steam sterilization. All selected composites demonstrated deterioration of mechanical properties after repeated steam sterilization. Knowing these results could aid in making decisions about the design and manufacturing of operative instruments and orthopedic biomaterials. Although our preliminary findings are intriguing, further study is warranted to seek specific applications for these composite materials in orthopedic surgery.
1. Ali MS, French TA, Hastings GW, et al. Carbon fibre composite bone plates. Development, evaluation and early clinical experience. J Bone Joint Surg Br. 1990;72(4):586-591.
2. Brooks RA, Jones E, Storer A, Rushton N. Biological evaluation of carbon-fibre–reinforced polybutyleneterephthalate (CFRPBT) employed in a novel acetabular cup. Biomaterials. 2004;25(17):3429-3438.
3. Brown SA, Hastings RS, Mason JJ, Moet A. Characterization of short-fibre reinforced thermoplastics for fracture fixation devices. Biomaterials. 1990;11(8):541-547.
4. Skinner HB. Composite technology for total hip arthroplasty. Clin Orthop Relat Res. 1988;(235):224-236.
5. Field RE, Jones E, Nuijten P, Storer A, Cronin M, Rushton N. Pre-clinical evaluation of the Cambridge acetabular cup. J Mater Sci Mater Med. 2008;19(8):2791-2798.
6. Han N, Ahmed I, Parsons AJ, et al. Influence of screw holes and gamma sterilization on properties of phosphate glass fiber–reinforced composite bone plates. J Biomater Appl. 2013;27(8):990-1002.
7. Losi P, Munaò A, Spiller D, et al. Evaluation of a new composite prosthesis for the repair of abdominal wall defects. J Mater Sci Mater Med. 2007;18(10):1939-1944.
8. Pait TG, Kaufman HH, Voelker JL, McAllister HP, Willison C. Use of a carbon composite radiolucent anterior cervical retractor system. Neurosurgery. 1993;33(5):941-942.
9. Elfar J, Menorca RM, Reed JD, Stanbury S. Composite bone models in orthopaedic surgery research and education. J Am Acad Orthop Surg. 2014;22(2):111-120.
10. Gardner MP, Chong AC, Pollock AG, Wooley PH. Mechanical evaluation of large-size fourth-generation composite femur and tibia models. Ann Biomed Eng. 2010;38(3):613-620.
11. Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284.
12. Dunlap JT, Chong AC, Lucas GL, Cooke FW. Structural properties of a novel design of composite analogue humeri models. Ann Biomed Eng. 2008;36(11):1922-1926.
13. Grover P, Albert C, Wang M, Harris GF. Mechanical characterization of fourth generation composite humerus. Proc Inst Mech Eng H. 2011;225(12):1169-1176.
14. Zheng Q, Morgan RJ. Synergistic thermal-moisture damage mechanisms of epoxies and their carbon fiber composites. J Compos Mater. 1993;27(15):1465-1478.
15. Ray BC. Temperature effect during humid ageing on interfaces of glass and carbon fibers reinforced epoxy composites. J Colloid Interface Sci. 2006;298(1):111-117.
16. Standard test method for short-beam strength of polymer matrix composite materials and their laminates [ASTM specification D2344/D2344M-00]. In: Annual Book of ASTM Standards. Vol 15.03. West Conshohocken, PA: American Society for Testing and Materials; 2006.
17. Bradley JS, Hastings GW, Johnson-Nurse C. Carbon fibre reinforced epoxy as a high strength, low modulus material for internal fixation plates. Biomaterials. 1980;1(1):38-40.
18. McKenna GB, Bradley GW, Dunn HK, Statton WO. Mechanical properties of some fibre reinforced polymer composites after implantation as fracture fixation plates. Biomaterials. 1980;1(4):189-192.
19. Tayton K, Johnson-Nurse C, McKibbin B, Bradley J, Hastings G. The use of semi-rigid carbon-fibre–reinforced plastic plates for fixation of human fractures. Results of preliminary trials. J Bone Joint Surg Br. 1982;64(1):105-111.
20. Tayton K, Bradley J. How stiff should semi-rigid fixation of the human tibia be? A clue to the answer. J Bone Joint Surg Br. 1983;65(3):312-315.
21. Tayton K. Corrosive effect of carbon-fibre reinforced plastic on stainless-steel screws during implantation into man. J Med Eng Technol. 1983;7(1):24-26.
22. Howard CB, Tayton KJ, Gibbs A. The response of human tissues to carbon reinforced epoxy resin. J Bone Joint Surg Br. 1985;67(4):656-658.
23. Skirving AP, Day R, Macdonald W, McLaren R. Carbon fiber reinforced plastic (CFRP) plates versus stainless steel dynamic compression plates in the treatment of fractures of the tibiae in dogs. Clin Orthop Relat Res. 1987;(224):117-124.
24. Prakash R, Marwah S, Goel SC, Tuli SM. Carbon fibre reinforced epoxy implants for bridging large osteoperiosteal gaps. Biomaterials. 1988;9(2):198-202.
25. Pemberton DJ, McKibbin B, Savage R, Tayton K, Stuart D. Carbon-fibre reinforced plates for problem fractures. J Bone Joint Surg Br. 1992;74(1):88-92.
26. Pemberton DJ, Evans PD, Grant A, McKibbin B. Fractures of the distal femur in the elderly treated with a carbon fibre supracondylar plate. Injury. 1994;25(5):317-321.
27. Kelsey DJ, Springer GS, Goodman SB. Composite implant for bone replacement. J Compos Mater. 1997;31(16):1593-1632.
28. Corvelli AA, Biermann PJ, Roberts JC. Design, analysis and fabrication of a composite segmental bone replacement implant. J Adv Mater. 1997;28:2-8.
29. Glassman AH, Crowninshield RD, Schenck R, Herberts P. A low stiffness composite biologically fixed prosthesis. Clin Orthop Relat Res. 2001;(393):128-136.
30. Williams D. New horizons for thermoplastic polymers. Med Device Technol. 2001;12(4):8-9.
31. Al-Shawi AK, Smith SP, Anderson GH. The use of a carbon fiber plate for periprosthetic supracondylar femoral fractures. J Arthroplasty. 2002;17(3):320-324.
32. Baker D, Kadambande SS, Alderman PM. Carbon fibre plates in the treatment of femoral periprosthetic fractures. Injury. 2004;35(6):596-598.
33. Akhavan S, Matthiesen MM, Schulte L, et al. Clinical and histologic results related to a low-modulus composite total hip replacement stem. J Bone Joint Surg Am. 2006;88(6):1308-1314.
34. Toth JM, Wang M, Estes BT, Scifert JL, Seim HB 3rd, Turner AS. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials. 2006;27(3):324-334.
35. Evans SL, Gregson PJ. Composite technology in load-bearing orthopaedic implants. Biomaterials. 1998;19(15):1329-1342.
36. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part I. Crystallinity and interface adhesion. Composites Part A. 2000;31(6):517-530.
37. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part II. Interlaminar fracture toughness. Composites Part A. 2001;32(6):763-774.
38. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part III. Impact damage performance. Composites Part A. 2001;32(6):775-785.
39. Guan SB, Hou CL, Chen AM, Zhang W, Wang JE. Influence of sterilization treatments on continuous carbon-fiber reinforced polyolefin composite. Zhonghua Yi Xue Za Zhi. 2007;87(31):2228-2231.
40. Akay M, Aslan N. An estimation of fatigue life for a carbon fibre/poly ether ether ketone hip joint prosthesis. Proc Inst Mech Eng H. 1995;209(2):93-103.
41. Bagheri ZS, Tavakkoli Awal P, Bougherara H, Aziz MS, Schemitsch EH, Zdero R. Biomechanical analysis of a new carbon fiber/flax/epoxy bone fracture plate shows less stress shielding compared to a standard clinical metal plate. J Biomech Eng. 2014;136(9):091002.
42. Rhee PC, Shin AY. The rate of successful four-corner arthrodesis with a locking, dorsal circular polyether-ether-ketone (PEEK-Optima) plate. J Hand Surg Eur Vol. 2013;38(7):767-773.
43. Nakahara I, Takao M, Bandoh S, Bertollo N, Walsh WR, Sugano N. In vivo implant fixation of carbon fiber–reinforced PEEK hip prostheses in an ovine model. J Orthop Res. 2013;31(3):485-492.
44. Kasliwal MK, O’Toole JE. Clinical experience using polyetheretherketone (PEEK) intervertebral structural cage for anterior cervical corpectomy and fusion. J Clin Neurosci. 2014;21(2):217-220.
45. Tarallo L, Mugnai R, Adani R, Zambianchi F, Catani F. A new volar plate made of carbon-fiber–reinforced polyetheretherketon for distal radius fracture: analysis of 40 cases. J Orthop Traumatol. 2014;15(4):277-283.
46. Cordey J, Perren SM, Steinemann SG. Stress protection due to plates: myth or reality? A parametric analysis made using the composite beam theory. Injury. 2000;31(suppl 3):C1-C13.
Polymer matrix composite materials have been widely promoted for orthopedic use in a variety of settings, including surgical instruments, medical devices, implants, and bone models.1-13 These types of composites are engineered from 2 or more constituent materials with significantly different physical or chemical properties; these materials remain separate and distinct on a macroscopic level within the finished composite structure. As a result of ongoing biomaterial research, polymer matrix composite materials can be engineered with a wide range of physical, mechanical, and surface properties, tailored to their application. Given their advantages (eg, high strength-to-weight ratio, radiolucency), these polymer matrix composite materials have gained popularity over traditional metallic materials.
Sterilization is an essential day-to-day procedure in the health care sector, both for single- and multiple-use devices or instruments, and thus a composite material used in medical components should remain unaffected by the process. The type of sterilization most commonly performed is steam sterilization, which achieves microbiological death by moist heat and pressure. Steam is created in an autoclave at a temperature of 132°C (270°F) in typical hospital settings. Steam sterilization cycles last 5 to 14 minutes based on specific manufacturer recommendations. Most medical-grade plastics used in health care have been designed and formulated to withstand the required sterilization cycles without sacrificing key properties. The structure integrity and overall performance of polymer matrix composites may be strongly influenced by the stability of the fiber/polymer interfacial region in terms of physical, chemical, and mechanical characteristics of the material at different scales.14 Absorption of moisture causes dilatational expansion and induces stresses, which are associated with the moisture-induced expansion resulting in degradation of structure stability.15 Thus, steam sterilization could affect the properties of the polymer matrix composite materials by excessive absorption of moisture by the polymer.
To our knowledge, no one has studied whether polymer matrix material properties degrade from long-term, repeated steam sterilization followed by mechanical loading. We conducted a study to evaluate the structural properties (short-beam strength, SBS) of several composite materials exposed to repeated sterilization as compared with traditional metal materials: SS-316L (stainless steel 316L) and Al-7075-T6 (aluminum 7075-T6).
Materials and Methods
We evaluated 3 types of composite materials: Tepex (Tepex Dynalite 201; HiPer Technology Inc.), CFR-PPS (carbon-fiber–reinforced polyphenylene sulfide, Cetex PPS; TenCate Advanced Composites USA Inc.), and HTN-53 (Zytel HTN53G50HSLR NC010; HiPer Technology Inc.) (Figure 1). Tepex is being used for orthopedic applications (knee braces, orthoses, insoles) and sporting goods applications. The performance of this material is superior to that of unreinforced thermoplastics. CFR-PPS represented the state of the art in composite materials for aerospace applications (eg, airframe structures, engine nacelles, fan casings, floorboards, interior parts). This is a high-performance material with exceptional high temperature and aggressive chemical resistance characteristics. CFR-PPS is also used to make filter fabric for coal boilers, papermaking felts, electrical insulation, specialty membranes, gaskets, and packing. It is not solubilized by any known solvents, even in long-term exposure, at temperatures up to 200°C. In addition, it exhibits exceptional resistance to organic and inorganic solutions, acids and alkali solutions, and a wide array of miscellaneous chemicals. HTN-53 is a 50% glass-reinforced, lubricated, high-performance polyamide resin with improved flow, developed for applications requiring excellent surface appearance with water-heated molds. This material has specifically shown survivability in hot, cold, chemically aggressive, and load-bearing environments. In addition, it has shown superior moisture and temperature resistance. These 3 composite materials were compared with SS-316L and Al-7075-T6. SS-316L is commonly used for implants in orthopedics, and Al-7075-T6 is a relatively radiolucent alternative for medical applications. Two different tests were performed to evaluate and validate these composite materials: (1) radiographic density evaluation and (2) structural property tests (short-beam load-to-failure [LTF] test, short-beam cyclic compression loading [CCL] test) before and after sterilization cycling.
Radiographic Density Evaluation
The radiographic density of the 5 materials was evaluated with radiographic images of a cadaveric knee specimen (Figure 2). Radiographic image intensification is the gold standard for repeated radiographic imaging in the operating room. Six different radiographic images were obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). Image-Pro Plus software (Media Cybernetics) was used to measure the radiographic density of the materials from the grayscale of the images.
Structural Properties Testing Before and After Sterilization Cycling
We used a standard SBS testing method to determine whether any degradation of structural properties resulted from standard repeated sterilization. The material geometries of the test specimens were 18.96×6.50×3.37 mm (length × width × thickness). Standard sterilization procedures were performed with steam sterilization using an autoclave at a temperature of 132°C (270°F) for at least 5 minutes (range, 5-14 minutes). Sample interval testing ran at 0, 200, and 400 sterilization cycles for structural properties in terms of SBS and moisture retention, with the structural properties at the 0th sterilization cycle (material before sterilization was performed) used as a baseline for comparison. Materials were subjected to 400 sterilization cycles, which is representative of the number of sterilization cycles per year an instrument or device would be subjected to.
Three structural tests were performed for each sample interval: moisture retention, LTF, and CCL. Moisture retention was investigated before and after repeated sterilization by measuring the weight of the test materials, as steam sterilization is known to affect the amount of moisture that is absorbed by a material. Twelve specimens of each proposed material were weighed at each sample interval, with the structural weight at the 0th sterilization cycle (material before sterilization is performed) serving as a baseline for comparison.
SBS testing was based on the ASTM (American Society for Testing and Materials) D2344 standard16 for LTF and CCL tests (Figure 3). Six samples of material were used for each test at every sample interval, yielding 180 samples. Seven servohydraulic material testing system instruments (1 MTS 810 and 6 MTS 858 Mini Bionix) were used to test the SBS of each material. For LTF testing, each specimen was loaded in compression from 30 N to complete structural failure at a constant displacement rate of 1.0 mm/min (0.05 in/min). Testing was initiated with 5 preconditioning loading cycles from 30 to 100 N at 1 Hz. The load was then applied continuously until failure occurred; force and displacement data were collected every 0.02 second. This procedure was performed for 6 replicates for each sample interval for each test material.
The calculation for SBS, Fsbs (MPa), for the constant loading rate until structural failure is:
Fsbs = 0.75 × Pm
b × h
where Pm (N) is the maximum applied load observed during the test, b is the measured specimen width (mm), and h is the measured specimen thickness (mm).
CCL testing consisted of each test material axially loaded with 100 to 500 N at a frequency of 1 Hz for 100,000 cycles. The maximum load of 500 N was chosen as a standard based on 80% of the minimum ultimate failure load from previous LTF tests. Displacement and force data were collected every 5 cycles at the maximum compressive load. Degradation of the material was calculated using the difference between the deflection of the initial cycle and the deflection of the final cycle (50th cycle and 100,000th cycle). This procedure was performed for 6 replicates for each sample interval for each test material.
Statistical Analysis
LTF and CCL testing data were analyzed for any differences among the test materials using 1-way analysis of variance with the least significant difference multiple comparisons post hoc test method using SPSS Version 16.0, with P < .05 denoting significance. These analyses were used to determine the statistical relevance of the difference between the SBS (LTF and CCL) of each test material. Means and standard deviations were calculated for all tests.
Results
Radiographic Density Evaluation
Overall, all the tested composite materials were significantly more radiolucent than either SS-316L or Al-7075-T6. Figure 4 shows the 6 different radiographic images obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). SS-316L can be considered radiopaque, and Al-7075-T6 has been used as a relatively radiolucent alternative. Tepex was statistically more radiolucent than the other 2 tested composite materials (Table 1). Even with 2 pieces placed anterior to the subject and 2 placed posterior, the radiodensity compared to the cortical bone was still lower than 1 piece of Al-7075-T6 either anterior or posterior to the subject.
Structural Properties Testing
Moisture Retention. Moisture retention was evaluated by weighing the test materials before and after repeated sterilization. There was no significant difference in moisture retention, as weight differences for all the tested materials were less than 0.5 weight percentage compared to the 0th sterilization cycle (Table 2). Therefore, the results of this study showed that all the tested materials exhibited good moisture/temperature resistance after 400 sterilization cycles.
Load to Failure. In the LTF test, significant differences were detected in SBS between all 5 tested materials (P < .05). Figure 5 shows the comparison of the structural properties in terms of SBS between the 5 tested materials, and Figure 6 shows the failure modes for the tested materials. There was no SBS for SS-316L, as the material did not exhibit complete structural failure even after 400 sterilization cycles; however, SS-316L was observed in inelastic deformation failure (Figure 6D). Al-7075-T6 had much higher SBS compared with the other composite materials, and it also resulted in an inelastic deformation failure mode only after 400 sterilization cycles; otherwise, flexure failure modes were observed. Tepex and CFR-PPS exhibited interlaminar shear failure, and HTN-53 exhibited complete structural failure.
Every composite material tested using the short-beam test for LTF showed a decrease in SBS with increased sterilization cycles (Figure 5). This decrease ranged from 17% to 57% compared with the 0th sterilization cycle. SBS was higher for CFR-PPS than for the other 2 composites. No statistically significant difference was found between CFR-PPS and Tepex except at the 200th sterilization cycle. HTN-53 was brittle at the 0th sterilization cycle but performed more like a ductile material at the 200th cycle. In addition, HTN-53 had the lowest SBS in terms of LTF testing when compared with the other 2 composites.
During the complete structural failure test, the failure modes for Tepex and CFR-PPS were visually identified as interlaminar shear failure (Figures 6A, 6B), whereas HTN-53 visually exhibited pure flexure failure (Figure 6C). As for the metals, SS-316L exhibited plastic deformation, and Al-7075-T6 exhibited flexure failure (Figures 6D, 6E).
Cyclic Compression Loading. Tepex was the only material to pass the 100,000 loading cycle without failure (Table 3). HTN-53 had the poorest performance of all: Its failure rates were 33% (2/6 samples) before sterilization (average cycle, 22,213; range, 21,500-22,925), 83% (5/6 samples) at the 200th sterilization cycle (average cycle, 4,210; range, 0-14,360), and 100% after 400 sterilization cycles (average cycle, 12,725; range, 1,190-21,900). CFR-PPS had no failures before the 400th sterilization cycle, and its failure rate after 400 sterilization cycles (average cycle, 50,735; range, 50,270-51,200) was 33% (2/6 samples).
Discussion
The success of a reusable composite material for use in orthopedic surgery depends not only on radiographic density, fabrication methods, and design but also on the ability to withstand repeated sterilization. Over the past 3 decades, investigators have explored several high-performance polymer matrix composite materials for use in orthopedics, especially in trauma, hip stems, and spinal implants.1,3,4,17-34 According to Evans and Gregson,35 composite materials have been widely promoted as possible orthopedic biomaterials but to date have found few successful commercial applications, because of the many challenging problems encountered in fabrication and testing. One of the most important factors in the mechanical properties of many composite materials is the influence of the cooling and loading rates on fiber-matrix interface adhesion.36-38 Our results tended to agree with the findings of Evans and Gregson,35 as some of these composite materials did not withstand repeated sterilizations well.
Guan and colleagues39 evaluated the influence of sterilization treatment on continuous carbon-fiber–reinforced polyolefin composite. Their 3-point bending test results showed that the levels of maximum load of all the specimens undergoing sterilization by autoclave were lower than those of the control group. For these composites, they concluded that autoclave sterilization and Co-60 gamma ray irradiation sterilization should be avoided and that ethylene oxide is the best method. Our results support their findings with a different set of composites.
Although HTN-53 has shown promise in other orthopedic applications because of its superior moisture and temperature resistance, we found that its performance after repeated sterilization was relatively poor. Tepex showed the greatest potential for durability after repeated sterilization; its mechanical properties were stable after 200 steam sterilization cycles.
Clinical Applications
The composite materials investigated in the present study have potential for use in either instrumentation or long-term implantation applications because of their versatility, mechanical strength, fatigue resistance, and biocompatibility. Akay and Aslan40 stated that carbon-fiber–filled composite implants can be designed with more appropriate modulus, strength, toughness, or stiffness by the arrangement of reinforcing fiber volume and orientation, and can provide better fatigue resistance. A notable advantage of using a composite plate with metal screws is that the potential for corrosion of metallic components is eliminated. Another major advantage of composite medical implants (eg, DiPhos-RM) is radiolucency, which allows direct visualization of osseous callus formation as well as monitoring of fracture healing, thereby improving clinical assessment and accuracy.
Numerous studies have documented the successful clinical performance of composite materials in orthopedic, trauma, and spinal surgery applications.41-45 Bagheri and colleagues41 developed a new carbon fiber–flax–epoxy composite plate and biomechanically compared it with a standard clinical metal plate. Their results confirmed that the carbon fiber–flax–epoxy material represents a potential candidate for bone fracture plate applications, as it can simultaneously provide similar mechanical stiffness and lower stress shielding (higher bone stress) compared with a standard clinical metal bone plate. Tarallo and colleagues45 evaluated the clinical results of 40 cases at 12-month follow-up using a new plate made of carbon-fiber–reinforced polyetheretherketon (DiPhos-RM, Lima Corporate) for the treatment of distal radius fractures. They reported good clinical results for this device at early follow-up, and its use allowed maintenance of reduction in complex AO (Arbeitsgemeinschaft für Osteosynthesefragen) fractures.
The main advantage in using composites for surgical instruments is their radiolucency. These materials do not obscure images or radiographs during fluoroscopic visualization. Surgery often requires fluoroscopic visualization of internal organs or bones, which may require temporary removal of radiopaque devices (eg, retractors, clamps, forceps, hooks, distractors). Aside from being inconvenient, removal and subsequent reinsertion consume valuable time and interfere with the smooth flow of an operation.
The shortcomings of using composite materials for surgical instruments involve detectability and sterilization. A significant issue in surgery is the accidental leaving behind of instruments in patients, which can cause serious problems ranging from organ perforation and blood infection to death. Although instrument counting and other safety protocols can reduce the risk of overlooking an instrument, mistakes are bound to happen. The other shortcoming is the influence of repeated sterilization on the mechanical properties of the composite materials, as sterilization is mandatory for surgical instruments used in the operation room. The structural integrity and overall performance of the polymer composite materials—especially the stability of the interface and the interphase zones—are strongly influenced by repeated sterilization.
On the other hand, composite materials have potential advantages that may support their introduction into long-term medical implant applications, as sterilization commonly is performed only once, during packaging. The effects of sterilization by radiation or steam are much less pronounced on composite implants than on composite surgical instruments. However, composite implants require careful consideration with respect to the bioactivity of wear particles that may be produced from articulation. Further, carbon-fiber–reinforced polymer implants are still substantially more difficult to manufacture and more costly than their metallic counterparts.46
Limitations
This study has some limitations. Most important, studies of this nature do not account for biological factors such as corrosion, biological wear, and the soft-tissue attachment effects on structural properties for potential in vivo use. Another limitation was that the study tested only the mechanical properties in terms of SBS and provided no information about other mechanical properties, such as tensile, compression, and torsion strengths. We think SBS testing adequately evaluates challenging scenarios like thin and narrow instruments/devices that are anticipated in application, and information regarding other modes of failure and mechanical properties (compression, tension, torsion) would be a further area of research. An additional limitation was that our model used a relatively small number of samples. A larger study with more samples and varying layout patterns and layers of the carbon fibers may more clearly demonstrate the effect of steam sterilization on composite materials.
Conclusion
This study provided new information on 3 selected composite materials and their structural properties after repeated steam sterilization. We discovered that these composites were similar in radiographic density and water retention but behaved very differently in terms of mechanical durability after repeated steam sterilization. All selected composites demonstrated deterioration of mechanical properties after repeated steam sterilization. Knowing these results could aid in making decisions about the design and manufacturing of operative instruments and orthopedic biomaterials. Although our preliminary findings are intriguing, further study is warranted to seek specific applications for these composite materials in orthopedic surgery.
Polymer matrix composite materials have been widely promoted for orthopedic use in a variety of settings, including surgical instruments, medical devices, implants, and bone models.1-13 These types of composites are engineered from 2 or more constituent materials with significantly different physical or chemical properties; these materials remain separate and distinct on a macroscopic level within the finished composite structure. As a result of ongoing biomaterial research, polymer matrix composite materials can be engineered with a wide range of physical, mechanical, and surface properties, tailored to their application. Given their advantages (eg, high strength-to-weight ratio, radiolucency), these polymer matrix composite materials have gained popularity over traditional metallic materials.
Sterilization is an essential day-to-day procedure in the health care sector, both for single- and multiple-use devices or instruments, and thus a composite material used in medical components should remain unaffected by the process. The type of sterilization most commonly performed is steam sterilization, which achieves microbiological death by moist heat and pressure. Steam is created in an autoclave at a temperature of 132°C (270°F) in typical hospital settings. Steam sterilization cycles last 5 to 14 minutes based on specific manufacturer recommendations. Most medical-grade plastics used in health care have been designed and formulated to withstand the required sterilization cycles without sacrificing key properties. The structure integrity and overall performance of polymer matrix composites may be strongly influenced by the stability of the fiber/polymer interfacial region in terms of physical, chemical, and mechanical characteristics of the material at different scales.14 Absorption of moisture causes dilatational expansion and induces stresses, which are associated with the moisture-induced expansion resulting in degradation of structure stability.15 Thus, steam sterilization could affect the properties of the polymer matrix composite materials by excessive absorption of moisture by the polymer.
To our knowledge, no one has studied whether polymer matrix material properties degrade from long-term, repeated steam sterilization followed by mechanical loading. We conducted a study to evaluate the structural properties (short-beam strength, SBS) of several composite materials exposed to repeated sterilization as compared with traditional metal materials: SS-316L (stainless steel 316L) and Al-7075-T6 (aluminum 7075-T6).
Materials and Methods
We evaluated 3 types of composite materials: Tepex (Tepex Dynalite 201; HiPer Technology Inc.), CFR-PPS (carbon-fiber–reinforced polyphenylene sulfide, Cetex PPS; TenCate Advanced Composites USA Inc.), and HTN-53 (Zytel HTN53G50HSLR NC010; HiPer Technology Inc.) (Figure 1). Tepex is being used for orthopedic applications (knee braces, orthoses, insoles) and sporting goods applications. The performance of this material is superior to that of unreinforced thermoplastics. CFR-PPS represented the state of the art in composite materials for aerospace applications (eg, airframe structures, engine nacelles, fan casings, floorboards, interior parts). This is a high-performance material with exceptional high temperature and aggressive chemical resistance characteristics. CFR-PPS is also used to make filter fabric for coal boilers, papermaking felts, electrical insulation, specialty membranes, gaskets, and packing. It is not solubilized by any known solvents, even in long-term exposure, at temperatures up to 200°C. In addition, it exhibits exceptional resistance to organic and inorganic solutions, acids and alkali solutions, and a wide array of miscellaneous chemicals. HTN-53 is a 50% glass-reinforced, lubricated, high-performance polyamide resin with improved flow, developed for applications requiring excellent surface appearance with water-heated molds. This material has specifically shown survivability in hot, cold, chemically aggressive, and load-bearing environments. In addition, it has shown superior moisture and temperature resistance. These 3 composite materials were compared with SS-316L and Al-7075-T6. SS-316L is commonly used for implants in orthopedics, and Al-7075-T6 is a relatively radiolucent alternative for medical applications. Two different tests were performed to evaluate and validate these composite materials: (1) radiographic density evaluation and (2) structural property tests (short-beam load-to-failure [LTF] test, short-beam cyclic compression loading [CCL] test) before and after sterilization cycling.
Radiographic Density Evaluation
The radiographic density of the 5 materials was evaluated with radiographic images of a cadaveric knee specimen (Figure 2). Radiographic image intensification is the gold standard for repeated radiographic imaging in the operating room. Six different radiographic images were obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). Image-Pro Plus software (Media Cybernetics) was used to measure the radiographic density of the materials from the grayscale of the images.
Structural Properties Testing Before and After Sterilization Cycling
We used a standard SBS testing method to determine whether any degradation of structural properties resulted from standard repeated sterilization. The material geometries of the test specimens were 18.96×6.50×3.37 mm (length × width × thickness). Standard sterilization procedures were performed with steam sterilization using an autoclave at a temperature of 132°C (270°F) for at least 5 minutes (range, 5-14 minutes). Sample interval testing ran at 0, 200, and 400 sterilization cycles for structural properties in terms of SBS and moisture retention, with the structural properties at the 0th sterilization cycle (material before sterilization was performed) used as a baseline for comparison. Materials were subjected to 400 sterilization cycles, which is representative of the number of sterilization cycles per year an instrument or device would be subjected to.
Three structural tests were performed for each sample interval: moisture retention, LTF, and CCL. Moisture retention was investigated before and after repeated sterilization by measuring the weight of the test materials, as steam sterilization is known to affect the amount of moisture that is absorbed by a material. Twelve specimens of each proposed material were weighed at each sample interval, with the structural weight at the 0th sterilization cycle (material before sterilization is performed) serving as a baseline for comparison.
SBS testing was based on the ASTM (American Society for Testing and Materials) D2344 standard16 for LTF and CCL tests (Figure 3). Six samples of material were used for each test at every sample interval, yielding 180 samples. Seven servohydraulic material testing system instruments (1 MTS 810 and 6 MTS 858 Mini Bionix) were used to test the SBS of each material. For LTF testing, each specimen was loaded in compression from 30 N to complete structural failure at a constant displacement rate of 1.0 mm/min (0.05 in/min). Testing was initiated with 5 preconditioning loading cycles from 30 to 100 N at 1 Hz. The load was then applied continuously until failure occurred; force and displacement data were collected every 0.02 second. This procedure was performed for 6 replicates for each sample interval for each test material.
The calculation for SBS, Fsbs (MPa), for the constant loading rate until structural failure is:
Fsbs = 0.75 × Pm
b × h
where Pm (N) is the maximum applied load observed during the test, b is the measured specimen width (mm), and h is the measured specimen thickness (mm).
CCL testing consisted of each test material axially loaded with 100 to 500 N at a frequency of 1 Hz for 100,000 cycles. The maximum load of 500 N was chosen as a standard based on 80% of the minimum ultimate failure load from previous LTF tests. Displacement and force data were collected every 5 cycles at the maximum compressive load. Degradation of the material was calculated using the difference between the deflection of the initial cycle and the deflection of the final cycle (50th cycle and 100,000th cycle). This procedure was performed for 6 replicates for each sample interval for each test material.
Statistical Analysis
LTF and CCL testing data were analyzed for any differences among the test materials using 1-way analysis of variance with the least significant difference multiple comparisons post hoc test method using SPSS Version 16.0, with P < .05 denoting significance. These analyses were used to determine the statistical relevance of the difference between the SBS (LTF and CCL) of each test material. Means and standard deviations were calculated for all tests.
Results
Radiographic Density Evaluation
Overall, all the tested composite materials were significantly more radiolucent than either SS-316L or Al-7075-T6. Figure 4 shows the 6 different radiographic images obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). SS-316L can be considered radiopaque, and Al-7075-T6 has been used as a relatively radiolucent alternative. Tepex was statistically more radiolucent than the other 2 tested composite materials (Table 1). Even with 2 pieces placed anterior to the subject and 2 placed posterior, the radiodensity compared to the cortical bone was still lower than 1 piece of Al-7075-T6 either anterior or posterior to the subject.
Structural Properties Testing
Moisture Retention. Moisture retention was evaluated by weighing the test materials before and after repeated sterilization. There was no significant difference in moisture retention, as weight differences for all the tested materials were less than 0.5 weight percentage compared to the 0th sterilization cycle (Table 2). Therefore, the results of this study showed that all the tested materials exhibited good moisture/temperature resistance after 400 sterilization cycles.
Load to Failure. In the LTF test, significant differences were detected in SBS between all 5 tested materials (P < .05). Figure 5 shows the comparison of the structural properties in terms of SBS between the 5 tested materials, and Figure 6 shows the failure modes for the tested materials. There was no SBS for SS-316L, as the material did not exhibit complete structural failure even after 400 sterilization cycles; however, SS-316L was observed in inelastic deformation failure (Figure 6D). Al-7075-T6 had much higher SBS compared with the other composite materials, and it also resulted in an inelastic deformation failure mode only after 400 sterilization cycles; otherwise, flexure failure modes were observed. Tepex and CFR-PPS exhibited interlaminar shear failure, and HTN-53 exhibited complete structural failure.
Every composite material tested using the short-beam test for LTF showed a decrease in SBS with increased sterilization cycles (Figure 5). This decrease ranged from 17% to 57% compared with the 0th sterilization cycle. SBS was higher for CFR-PPS than for the other 2 composites. No statistically significant difference was found between CFR-PPS and Tepex except at the 200th sterilization cycle. HTN-53 was brittle at the 0th sterilization cycle but performed more like a ductile material at the 200th cycle. In addition, HTN-53 had the lowest SBS in terms of LTF testing when compared with the other 2 composites.
During the complete structural failure test, the failure modes for Tepex and CFR-PPS were visually identified as interlaminar shear failure (Figures 6A, 6B), whereas HTN-53 visually exhibited pure flexure failure (Figure 6C). As for the metals, SS-316L exhibited plastic deformation, and Al-7075-T6 exhibited flexure failure (Figures 6D, 6E).
Cyclic Compression Loading. Tepex was the only material to pass the 100,000 loading cycle without failure (Table 3). HTN-53 had the poorest performance of all: Its failure rates were 33% (2/6 samples) before sterilization (average cycle, 22,213; range, 21,500-22,925), 83% (5/6 samples) at the 200th sterilization cycle (average cycle, 4,210; range, 0-14,360), and 100% after 400 sterilization cycles (average cycle, 12,725; range, 1,190-21,900). CFR-PPS had no failures before the 400th sterilization cycle, and its failure rate after 400 sterilization cycles (average cycle, 50,735; range, 50,270-51,200) was 33% (2/6 samples).
Discussion
The success of a reusable composite material for use in orthopedic surgery depends not only on radiographic density, fabrication methods, and design but also on the ability to withstand repeated sterilization. Over the past 3 decades, investigators have explored several high-performance polymer matrix composite materials for use in orthopedics, especially in trauma, hip stems, and spinal implants.1,3,4,17-34 According to Evans and Gregson,35 composite materials have been widely promoted as possible orthopedic biomaterials but to date have found few successful commercial applications, because of the many challenging problems encountered in fabrication and testing. One of the most important factors in the mechanical properties of many composite materials is the influence of the cooling and loading rates on fiber-matrix interface adhesion.36-38 Our results tended to agree with the findings of Evans and Gregson,35 as some of these composite materials did not withstand repeated sterilizations well.
Guan and colleagues39 evaluated the influence of sterilization treatment on continuous carbon-fiber–reinforced polyolefin composite. Their 3-point bending test results showed that the levels of maximum load of all the specimens undergoing sterilization by autoclave were lower than those of the control group. For these composites, they concluded that autoclave sterilization and Co-60 gamma ray irradiation sterilization should be avoided and that ethylene oxide is the best method. Our results support their findings with a different set of composites.
Although HTN-53 has shown promise in other orthopedic applications because of its superior moisture and temperature resistance, we found that its performance after repeated sterilization was relatively poor. Tepex showed the greatest potential for durability after repeated sterilization; its mechanical properties were stable after 200 steam sterilization cycles.
Clinical Applications
The composite materials investigated in the present study have potential for use in either instrumentation or long-term implantation applications because of their versatility, mechanical strength, fatigue resistance, and biocompatibility. Akay and Aslan40 stated that carbon-fiber–filled composite implants can be designed with more appropriate modulus, strength, toughness, or stiffness by the arrangement of reinforcing fiber volume and orientation, and can provide better fatigue resistance. A notable advantage of using a composite plate with metal screws is that the potential for corrosion of metallic components is eliminated. Another major advantage of composite medical implants (eg, DiPhos-RM) is radiolucency, which allows direct visualization of osseous callus formation as well as monitoring of fracture healing, thereby improving clinical assessment and accuracy.
Numerous studies have documented the successful clinical performance of composite materials in orthopedic, trauma, and spinal surgery applications.41-45 Bagheri and colleagues41 developed a new carbon fiber–flax–epoxy composite plate and biomechanically compared it with a standard clinical metal plate. Their results confirmed that the carbon fiber–flax–epoxy material represents a potential candidate for bone fracture plate applications, as it can simultaneously provide similar mechanical stiffness and lower stress shielding (higher bone stress) compared with a standard clinical metal bone plate. Tarallo and colleagues45 evaluated the clinical results of 40 cases at 12-month follow-up using a new plate made of carbon-fiber–reinforced polyetheretherketon (DiPhos-RM, Lima Corporate) for the treatment of distal radius fractures. They reported good clinical results for this device at early follow-up, and its use allowed maintenance of reduction in complex AO (Arbeitsgemeinschaft für Osteosynthesefragen) fractures.
The main advantage in using composites for surgical instruments is their radiolucency. These materials do not obscure images or radiographs during fluoroscopic visualization. Surgery often requires fluoroscopic visualization of internal organs or bones, which may require temporary removal of radiopaque devices (eg, retractors, clamps, forceps, hooks, distractors). Aside from being inconvenient, removal and subsequent reinsertion consume valuable time and interfere with the smooth flow of an operation.
The shortcomings of using composite materials for surgical instruments involve detectability and sterilization. A significant issue in surgery is the accidental leaving behind of instruments in patients, which can cause serious problems ranging from organ perforation and blood infection to death. Although instrument counting and other safety protocols can reduce the risk of overlooking an instrument, mistakes are bound to happen. The other shortcoming is the influence of repeated sterilization on the mechanical properties of the composite materials, as sterilization is mandatory for surgical instruments used in the operation room. The structural integrity and overall performance of the polymer composite materials—especially the stability of the interface and the interphase zones—are strongly influenced by repeated sterilization.
On the other hand, composite materials have potential advantages that may support their introduction into long-term medical implant applications, as sterilization commonly is performed only once, during packaging. The effects of sterilization by radiation or steam are much less pronounced on composite implants than on composite surgical instruments. However, composite implants require careful consideration with respect to the bioactivity of wear particles that may be produced from articulation. Further, carbon-fiber–reinforced polymer implants are still substantially more difficult to manufacture and more costly than their metallic counterparts.46
Limitations
This study has some limitations. Most important, studies of this nature do not account for biological factors such as corrosion, biological wear, and the soft-tissue attachment effects on structural properties for potential in vivo use. Another limitation was that the study tested only the mechanical properties in terms of SBS and provided no information about other mechanical properties, such as tensile, compression, and torsion strengths. We think SBS testing adequately evaluates challenging scenarios like thin and narrow instruments/devices that are anticipated in application, and information regarding other modes of failure and mechanical properties (compression, tension, torsion) would be a further area of research. An additional limitation was that our model used a relatively small number of samples. A larger study with more samples and varying layout patterns and layers of the carbon fibers may more clearly demonstrate the effect of steam sterilization on composite materials.
Conclusion
This study provided new information on 3 selected composite materials and their structural properties after repeated steam sterilization. We discovered that these composites were similar in radiographic density and water retention but behaved very differently in terms of mechanical durability after repeated steam sterilization. All selected composites demonstrated deterioration of mechanical properties after repeated steam sterilization. Knowing these results could aid in making decisions about the design and manufacturing of operative instruments and orthopedic biomaterials. Although our preliminary findings are intriguing, further study is warranted to seek specific applications for these composite materials in orthopedic surgery.
1. Ali MS, French TA, Hastings GW, et al. Carbon fibre composite bone plates. Development, evaluation and early clinical experience. J Bone Joint Surg Br. 1990;72(4):586-591.
2. Brooks RA, Jones E, Storer A, Rushton N. Biological evaluation of carbon-fibre–reinforced polybutyleneterephthalate (CFRPBT) employed in a novel acetabular cup. Biomaterials. 2004;25(17):3429-3438.
3. Brown SA, Hastings RS, Mason JJ, Moet A. Characterization of short-fibre reinforced thermoplastics for fracture fixation devices. Biomaterials. 1990;11(8):541-547.
4. Skinner HB. Composite technology for total hip arthroplasty. Clin Orthop Relat Res. 1988;(235):224-236.
5. Field RE, Jones E, Nuijten P, Storer A, Cronin M, Rushton N. Pre-clinical evaluation of the Cambridge acetabular cup. J Mater Sci Mater Med. 2008;19(8):2791-2798.
6. Han N, Ahmed I, Parsons AJ, et al. Influence of screw holes and gamma sterilization on properties of phosphate glass fiber–reinforced composite bone plates. J Biomater Appl. 2013;27(8):990-1002.
7. Losi P, Munaò A, Spiller D, et al. Evaluation of a new composite prosthesis for the repair of abdominal wall defects. J Mater Sci Mater Med. 2007;18(10):1939-1944.
8. Pait TG, Kaufman HH, Voelker JL, McAllister HP, Willison C. Use of a carbon composite radiolucent anterior cervical retractor system. Neurosurgery. 1993;33(5):941-942.
9. Elfar J, Menorca RM, Reed JD, Stanbury S. Composite bone models in orthopaedic surgery research and education. J Am Acad Orthop Surg. 2014;22(2):111-120.
10. Gardner MP, Chong AC, Pollock AG, Wooley PH. Mechanical evaluation of large-size fourth-generation composite femur and tibia models. Ann Biomed Eng. 2010;38(3):613-620.
11. Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284.
12. Dunlap JT, Chong AC, Lucas GL, Cooke FW. Structural properties of a novel design of composite analogue humeri models. Ann Biomed Eng. 2008;36(11):1922-1926.
13. Grover P, Albert C, Wang M, Harris GF. Mechanical characterization of fourth generation composite humerus. Proc Inst Mech Eng H. 2011;225(12):1169-1176.
14. Zheng Q, Morgan RJ. Synergistic thermal-moisture damage mechanisms of epoxies and their carbon fiber composites. J Compos Mater. 1993;27(15):1465-1478.
15. Ray BC. Temperature effect during humid ageing on interfaces of glass and carbon fibers reinforced epoxy composites. J Colloid Interface Sci. 2006;298(1):111-117.
16. Standard test method for short-beam strength of polymer matrix composite materials and their laminates [ASTM specification D2344/D2344M-00]. In: Annual Book of ASTM Standards. Vol 15.03. West Conshohocken, PA: American Society for Testing and Materials; 2006.
17. Bradley JS, Hastings GW, Johnson-Nurse C. Carbon fibre reinforced epoxy as a high strength, low modulus material for internal fixation plates. Biomaterials. 1980;1(1):38-40.
18. McKenna GB, Bradley GW, Dunn HK, Statton WO. Mechanical properties of some fibre reinforced polymer composites after implantation as fracture fixation plates. Biomaterials. 1980;1(4):189-192.
19. Tayton K, Johnson-Nurse C, McKibbin B, Bradley J, Hastings G. The use of semi-rigid carbon-fibre–reinforced plastic plates for fixation of human fractures. Results of preliminary trials. J Bone Joint Surg Br. 1982;64(1):105-111.
20. Tayton K, Bradley J. How stiff should semi-rigid fixation of the human tibia be? A clue to the answer. J Bone Joint Surg Br. 1983;65(3):312-315.
21. Tayton K. Corrosive effect of carbon-fibre reinforced plastic on stainless-steel screws during implantation into man. J Med Eng Technol. 1983;7(1):24-26.
22. Howard CB, Tayton KJ, Gibbs A. The response of human tissues to carbon reinforced epoxy resin. J Bone Joint Surg Br. 1985;67(4):656-658.
23. Skirving AP, Day R, Macdonald W, McLaren R. Carbon fiber reinforced plastic (CFRP) plates versus stainless steel dynamic compression plates in the treatment of fractures of the tibiae in dogs. Clin Orthop Relat Res. 1987;(224):117-124.
24. Prakash R, Marwah S, Goel SC, Tuli SM. Carbon fibre reinforced epoxy implants for bridging large osteoperiosteal gaps. Biomaterials. 1988;9(2):198-202.
25. Pemberton DJ, McKibbin B, Savage R, Tayton K, Stuart D. Carbon-fibre reinforced plates for problem fractures. J Bone Joint Surg Br. 1992;74(1):88-92.
26. Pemberton DJ, Evans PD, Grant A, McKibbin B. Fractures of the distal femur in the elderly treated with a carbon fibre supracondylar plate. Injury. 1994;25(5):317-321.
27. Kelsey DJ, Springer GS, Goodman SB. Composite implant for bone replacement. J Compos Mater. 1997;31(16):1593-1632.
28. Corvelli AA, Biermann PJ, Roberts JC. Design, analysis and fabrication of a composite segmental bone replacement implant. J Adv Mater. 1997;28:2-8.
29. Glassman AH, Crowninshield RD, Schenck R, Herberts P. A low stiffness composite biologically fixed prosthesis. Clin Orthop Relat Res. 2001;(393):128-136.
30. Williams D. New horizons for thermoplastic polymers. Med Device Technol. 2001;12(4):8-9.
31. Al-Shawi AK, Smith SP, Anderson GH. The use of a carbon fiber plate for periprosthetic supracondylar femoral fractures. J Arthroplasty. 2002;17(3):320-324.
32. Baker D, Kadambande SS, Alderman PM. Carbon fibre plates in the treatment of femoral periprosthetic fractures. Injury. 2004;35(6):596-598.
33. Akhavan S, Matthiesen MM, Schulte L, et al. Clinical and histologic results related to a low-modulus composite total hip replacement stem. J Bone Joint Surg Am. 2006;88(6):1308-1314.
34. Toth JM, Wang M, Estes BT, Scifert JL, Seim HB 3rd, Turner AS. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials. 2006;27(3):324-334.
35. Evans SL, Gregson PJ. Composite technology in load-bearing orthopaedic implants. Biomaterials. 1998;19(15):1329-1342.
36. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part I. Crystallinity and interface adhesion. Composites Part A. 2000;31(6):517-530.
37. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part II. Interlaminar fracture toughness. Composites Part A. 2001;32(6):763-774.
38. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part III. Impact damage performance. Composites Part A. 2001;32(6):775-785.
39. Guan SB, Hou CL, Chen AM, Zhang W, Wang JE. Influence of sterilization treatments on continuous carbon-fiber reinforced polyolefin composite. Zhonghua Yi Xue Za Zhi. 2007;87(31):2228-2231.
40. Akay M, Aslan N. An estimation of fatigue life for a carbon fibre/poly ether ether ketone hip joint prosthesis. Proc Inst Mech Eng H. 1995;209(2):93-103.
41. Bagheri ZS, Tavakkoli Awal P, Bougherara H, Aziz MS, Schemitsch EH, Zdero R. Biomechanical analysis of a new carbon fiber/flax/epoxy bone fracture plate shows less stress shielding compared to a standard clinical metal plate. J Biomech Eng. 2014;136(9):091002.
42. Rhee PC, Shin AY. The rate of successful four-corner arthrodesis with a locking, dorsal circular polyether-ether-ketone (PEEK-Optima) plate. J Hand Surg Eur Vol. 2013;38(7):767-773.
43. Nakahara I, Takao M, Bandoh S, Bertollo N, Walsh WR, Sugano N. In vivo implant fixation of carbon fiber–reinforced PEEK hip prostheses in an ovine model. J Orthop Res. 2013;31(3):485-492.
44. Kasliwal MK, O’Toole JE. Clinical experience using polyetheretherketone (PEEK) intervertebral structural cage for anterior cervical corpectomy and fusion. J Clin Neurosci. 2014;21(2):217-220.
45. Tarallo L, Mugnai R, Adani R, Zambianchi F, Catani F. A new volar plate made of carbon-fiber–reinforced polyetheretherketon for distal radius fracture: analysis of 40 cases. J Orthop Traumatol. 2014;15(4):277-283.
46. Cordey J, Perren SM, Steinemann SG. Stress protection due to plates: myth or reality? A parametric analysis made using the composite beam theory. Injury. 2000;31(suppl 3):C1-C13.
1. Ali MS, French TA, Hastings GW, et al. Carbon fibre composite bone plates. Development, evaluation and early clinical experience. J Bone Joint Surg Br. 1990;72(4):586-591.
2. Brooks RA, Jones E, Storer A, Rushton N. Biological evaluation of carbon-fibre–reinforced polybutyleneterephthalate (CFRPBT) employed in a novel acetabular cup. Biomaterials. 2004;25(17):3429-3438.
3. Brown SA, Hastings RS, Mason JJ, Moet A. Characterization of short-fibre reinforced thermoplastics for fracture fixation devices. Biomaterials. 1990;11(8):541-547.
4. Skinner HB. Composite technology for total hip arthroplasty. Clin Orthop Relat Res. 1988;(235):224-236.
5. Field RE, Jones E, Nuijten P, Storer A, Cronin M, Rushton N. Pre-clinical evaluation of the Cambridge acetabular cup. J Mater Sci Mater Med. 2008;19(8):2791-2798.
6. Han N, Ahmed I, Parsons AJ, et al. Influence of screw holes and gamma sterilization on properties of phosphate glass fiber–reinforced composite bone plates. J Biomater Appl. 2013;27(8):990-1002.
7. Losi P, Munaò A, Spiller D, et al. Evaluation of a new composite prosthesis for the repair of abdominal wall defects. J Mater Sci Mater Med. 2007;18(10):1939-1944.
8. Pait TG, Kaufman HH, Voelker JL, McAllister HP, Willison C. Use of a carbon composite radiolucent anterior cervical retractor system. Neurosurgery. 1993;33(5):941-942.
9. Elfar J, Menorca RM, Reed JD, Stanbury S. Composite bone models in orthopaedic surgery research and education. J Am Acad Orthop Surg. 2014;22(2):111-120.
10. Gardner MP, Chong AC, Pollock AG, Wooley PH. Mechanical evaluation of large-size fourth-generation composite femur and tibia models. Ann Biomed Eng. 2010;38(3):613-620.
11. Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284.
12. Dunlap JT, Chong AC, Lucas GL, Cooke FW. Structural properties of a novel design of composite analogue humeri models. Ann Biomed Eng. 2008;36(11):1922-1926.
13. Grover P, Albert C, Wang M, Harris GF. Mechanical characterization of fourth generation composite humerus. Proc Inst Mech Eng H. 2011;225(12):1169-1176.
14. Zheng Q, Morgan RJ. Synergistic thermal-moisture damage mechanisms of epoxies and their carbon fiber composites. J Compos Mater. 1993;27(15):1465-1478.
15. Ray BC. Temperature effect during humid ageing on interfaces of glass and carbon fibers reinforced epoxy composites. J Colloid Interface Sci. 2006;298(1):111-117.
16. Standard test method for short-beam strength of polymer matrix composite materials and their laminates [ASTM specification D2344/D2344M-00]. In: Annual Book of ASTM Standards. Vol 15.03. West Conshohocken, PA: American Society for Testing and Materials; 2006.
17. Bradley JS, Hastings GW, Johnson-Nurse C. Carbon fibre reinforced epoxy as a high strength, low modulus material for internal fixation plates. Biomaterials. 1980;1(1):38-40.
18. McKenna GB, Bradley GW, Dunn HK, Statton WO. Mechanical properties of some fibre reinforced polymer composites after implantation as fracture fixation plates. Biomaterials. 1980;1(4):189-192.
19. Tayton K, Johnson-Nurse C, McKibbin B, Bradley J, Hastings G. The use of semi-rigid carbon-fibre–reinforced plastic plates for fixation of human fractures. Results of preliminary trials. J Bone Joint Surg Br. 1982;64(1):105-111.
20. Tayton K, Bradley J. How stiff should semi-rigid fixation of the human tibia be? A clue to the answer. J Bone Joint Surg Br. 1983;65(3):312-315.
21. Tayton K. Corrosive effect of carbon-fibre reinforced plastic on stainless-steel screws during implantation into man. J Med Eng Technol. 1983;7(1):24-26.
22. Howard CB, Tayton KJ, Gibbs A. The response of human tissues to carbon reinforced epoxy resin. J Bone Joint Surg Br. 1985;67(4):656-658.
23. Skirving AP, Day R, Macdonald W, McLaren R. Carbon fiber reinforced plastic (CFRP) plates versus stainless steel dynamic compression plates in the treatment of fractures of the tibiae in dogs. Clin Orthop Relat Res. 1987;(224):117-124.
24. Prakash R, Marwah S, Goel SC, Tuli SM. Carbon fibre reinforced epoxy implants for bridging large osteoperiosteal gaps. Biomaterials. 1988;9(2):198-202.
25. Pemberton DJ, McKibbin B, Savage R, Tayton K, Stuart D. Carbon-fibre reinforced plates for problem fractures. J Bone Joint Surg Br. 1992;74(1):88-92.
26. Pemberton DJ, Evans PD, Grant A, McKibbin B. Fractures of the distal femur in the elderly treated with a carbon fibre supracondylar plate. Injury. 1994;25(5):317-321.
27. Kelsey DJ, Springer GS, Goodman SB. Composite implant for bone replacement. J Compos Mater. 1997;31(16):1593-1632.
28. Corvelli AA, Biermann PJ, Roberts JC. Design, analysis and fabrication of a composite segmental bone replacement implant. J Adv Mater. 1997;28:2-8.
29. Glassman AH, Crowninshield RD, Schenck R, Herberts P. A low stiffness composite biologically fixed prosthesis. Clin Orthop Relat Res. 2001;(393):128-136.
30. Williams D. New horizons for thermoplastic polymers. Med Device Technol. 2001;12(4):8-9.
31. Al-Shawi AK, Smith SP, Anderson GH. The use of a carbon fiber plate for periprosthetic supracondylar femoral fractures. J Arthroplasty. 2002;17(3):320-324.
32. Baker D, Kadambande SS, Alderman PM. Carbon fibre plates in the treatment of femoral periprosthetic fractures. Injury. 2004;35(6):596-598.
33. Akhavan S, Matthiesen MM, Schulte L, et al. Clinical and histologic results related to a low-modulus composite total hip replacement stem. J Bone Joint Surg Am. 2006;88(6):1308-1314.
34. Toth JM, Wang M, Estes BT, Scifert JL, Seim HB 3rd, Turner AS. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials. 2006;27(3):324-334.
35. Evans SL, Gregson PJ. Composite technology in load-bearing orthopaedic implants. Biomaterials. 1998;19(15):1329-1342.
36. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part I. Crystallinity and interface adhesion. Composites Part A. 2000;31(6):517-530.
37. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part II. Interlaminar fracture toughness. Composites Part A. 2001;32(6):763-774.
38. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part III. Impact damage performance. Composites Part A. 2001;32(6):775-785.
39. Guan SB, Hou CL, Chen AM, Zhang W, Wang JE. Influence of sterilization treatments on continuous carbon-fiber reinforced polyolefin composite. Zhonghua Yi Xue Za Zhi. 2007;87(31):2228-2231.
40. Akay M, Aslan N. An estimation of fatigue life for a carbon fibre/poly ether ether ketone hip joint prosthesis. Proc Inst Mech Eng H. 1995;209(2):93-103.
41. Bagheri ZS, Tavakkoli Awal P, Bougherara H, Aziz MS, Schemitsch EH, Zdero R. Biomechanical analysis of a new carbon fiber/flax/epoxy bone fracture plate shows less stress shielding compared to a standard clinical metal plate. J Biomech Eng. 2014;136(9):091002.
42. Rhee PC, Shin AY. The rate of successful four-corner arthrodesis with a locking, dorsal circular polyether-ether-ketone (PEEK-Optima) plate. J Hand Surg Eur Vol. 2013;38(7):767-773.
43. Nakahara I, Takao M, Bandoh S, Bertollo N, Walsh WR, Sugano N. In vivo implant fixation of carbon fiber–reinforced PEEK hip prostheses in an ovine model. J Orthop Res. 2013;31(3):485-492.
44. Kasliwal MK, O’Toole JE. Clinical experience using polyetheretherketone (PEEK) intervertebral structural cage for anterior cervical corpectomy and fusion. J Clin Neurosci. 2014;21(2):217-220.
45. Tarallo L, Mugnai R, Adani R, Zambianchi F, Catani F. A new volar plate made of carbon-fiber–reinforced polyetheretherketon for distal radius fracture: analysis of 40 cases. J Orthop Traumatol. 2014;15(4):277-283.
46. Cordey J, Perren SM, Steinemann SG. Stress protection due to plates: myth or reality? A parametric analysis made using the composite beam theory. Injury. 2000;31(suppl 3):C1-C13.