User login
Long-Term Elastic Durability of Polymer Matrix Composite Materials After Repeated Steam Sterilization
Polymer matrix composite materials have been widely promoted for orthopedic use in a variety of settings, including surgical instruments, medical devices, implants, and bone models.1-13 These types of composites are engineered from 2 or more constituent materials with significantly different physical or chemical properties; these materials remain separate and distinct on a macroscopic level within the finished composite structure. As a result of ongoing biomaterial research, polymer matrix composite materials can be engineered with a wide range of physical, mechanical, and surface properties, tailored to their application. Given their advantages (eg, high strength-to-weight ratio, radiolucency), these polymer matrix composite materials have gained popularity over traditional metallic materials.
Sterilization is an essential day-to-day procedure in the health care sector, both for single- and multiple-use devices or instruments, and thus a composite material used in medical components should remain unaffected by the process. The type of sterilization most commonly performed is steam sterilization, which achieves microbiological death by moist heat and pressure. Steam is created in an autoclave at a temperature of 132°C (270°F) in typical hospital settings. Steam sterilization cycles last 5 to 14 minutes based on specific manufacturer recommendations. Most medical-grade plastics used in health care have been designed and formulated to withstand the required sterilization cycles without sacrificing key properties. The structure integrity and overall performance of polymer matrix composites may be strongly influenced by the stability of the fiber/polymer interfacial region in terms of physical, chemical, and mechanical characteristics of the material at different scales.14 Absorption of moisture causes dilatational expansion and induces stresses, which are associated with the moisture-induced expansion resulting in degradation of structure stability.15 Thus, steam sterilization could affect the properties of the polymer matrix composite materials by excessive absorption of moisture by the polymer.
To our knowledge, no one has studied whether polymer matrix material properties degrade from long-term, repeated steam sterilization followed by mechanical loading. We conducted a study to evaluate the structural properties (short-beam strength, SBS) of several composite materials exposed to repeated sterilization as compared with traditional metal materials: SS-316L (stainless steel 316L) and Al-7075-T6 (aluminum 7075-T6).
Materials and Methods
We evaluated 3 types of composite materials: Tepex (Tepex Dynalite 201; HiPer Technology Inc.), CFR-PPS (carbon-fiber–reinforced polyphenylene sulfide, Cetex PPS; TenCate Advanced Composites USA Inc.), and HTN-53 (Zytel HTN53G50HSLR NC010; HiPer Technology Inc.) (Figure 1). Tepex is being used for orthopedic applications (knee braces, orthoses, insoles) and sporting goods applications. The performance of this material is superior to that of unreinforced thermoplastics. CFR-PPS represented the state of the art in composite materials for aerospace applications (eg, airframe structures, engine nacelles, fan casings, floorboards, interior parts). This is a high-performance material with exceptional high temperature and aggressive chemical resistance characteristics. CFR-PPS is also used to make filter fabric for coal boilers, papermaking felts, electrical insulation, specialty membranes, gaskets, and packing. It is not solubilized by any known solvents, even in long-term exposure, at temperatures up to 200°C. In addition, it exhibits exceptional resistance to organic and inorganic solutions, acids and alkali solutions, and a wide array of miscellaneous chemicals. HTN-53 is a 50% glass-reinforced, lubricated, high-performance polyamide resin with improved flow, developed for applications requiring excellent surface appearance with water-heated molds. This material has specifically shown survivability in hot, cold, chemically aggressive, and load-bearing environments. In addition, it has shown superior moisture and temperature resistance. These 3 composite materials were compared with SS-316L and Al-7075-T6. SS-316L is commonly used for implants in orthopedics, and Al-7075-T6 is a relatively radiolucent alternative for medical applications. Two different tests were performed to evaluate and validate these composite materials: (1) radiographic density evaluation and (2) structural property tests (short-beam load-to-failure [LTF] test, short-beam cyclic compression loading [CCL] test) before and after sterilization cycling.
Radiographic Density Evaluation
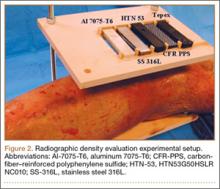
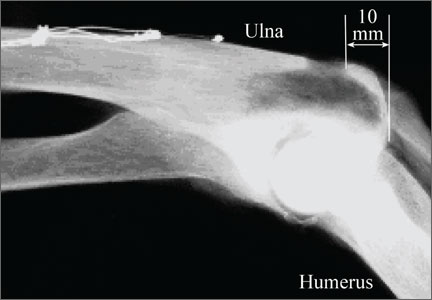
The radiographic density of the 5 materials was evaluated with radiographic images of a cadaveric knee specimen (Figure 2). Radiographic image intensification is the gold standard for repeated radiographic imaging in the operating room. Six different radiographic images were obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). Image-Pro Plus software (Media Cybernetics) was used to measure the radiographic density of the materials from the grayscale of the images.
Structural Properties Testing Before and After Sterilization Cycling
We used a standard SBS testing method to determine whether any degradation of structural properties resulted from standard repeated sterilization. The material geometries of the test specimens were 18.96×6.50×3.37 mm (length × width × thickness). Standard sterilization procedures were performed with steam sterilization using an autoclave at a temperature of 132°C (270°F) for at least 5 minutes (range, 5-14 minutes). Sample interval testing ran at 0, 200, and 400 sterilization cycles for structural properties in terms of SBS and moisture retention, with the structural properties at the 0th sterilization cycle (material before sterilization was performed) used as a baseline for comparison. Materials were subjected to 400 sterilization cycles, which is representative of the number of sterilization cycles per year an instrument or device would be subjected to.
Three structural tests were performed for each sample interval: moisture retention, LTF, and CCL. Moisture retention was investigated before and after repeated sterilization by measuring the weight of the test materials, as steam sterilization is known to affect the amount of moisture that is absorbed by a material. Twelve specimens of each proposed material were weighed at each sample interval, with the structural weight at the 0th sterilization cycle (material before sterilization is performed) serving as a baseline for comparison.
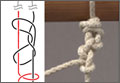
SBS testing was based on the ASTM (American Society for Testing and Materials) D2344 standard16 for LTF and CCL tests (Figure 3). Six samples of material were used for each test at every sample interval, yielding 180 samples. Seven servohydraulic material testing system instruments (1 MTS 810 and 6 MTS 858 Mini Bionix) were used to test the SBS of each material. For LTF testing, each specimen was loaded in compression from 30 N to complete structural failure at a constant displacement rate of 1.0 mm/min (0.05 in/min). Testing was initiated with 5 preconditioning loading cycles from 30 to 100 N at 1 Hz. The load was then applied continuously until failure occurred; force and displacement data were collected every 0.02 second. This procedure was performed for 6 replicates for each sample interval for each test material.
The calculation for SBS, Fsbs (MPa), for the constant loading rate until structural failure is:
Fsbs = 0.75 × Pm
b × h
where Pm (N) is the maximum applied load observed during the test, b is the measured specimen width (mm), and h is the measured specimen thickness (mm).
CCL testing consisted of each test material axially loaded with 100 to 500 N at a frequency of 1 Hz for 100,000 cycles. The maximum load of 500 N was chosen as a standard based on 80% of the minimum ultimate failure load from previous LTF tests. Displacement and force data were collected every 5 cycles at the maximum compressive load. Degradation of the material was calculated using the difference between the deflection of the initial cycle and the deflection of the final cycle (50th cycle and 100,000th cycle). This procedure was performed for 6 replicates for each sample interval for each test material.
Statistical Analysis
LTF and CCL testing data were analyzed for any differences among the test materials using 1-way analysis of variance with the least significant difference multiple comparisons post hoc test method using SPSS Version 16.0, with P < .05 denoting significance. These analyses were used to determine the statistical relevance of the difference between the SBS (LTF and CCL) of each test material. Means and standard deviations were calculated for all tests.
Results
Radiographic Density Evaluation
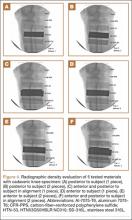
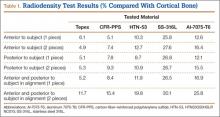
Overall, all the tested composite materials were significantly more radiolucent than either SS-316L or Al-7075-T6. Figure 4 shows the 6 different radiographic images obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). SS-316L can be considered radiopaque, and Al-7075-T6 has been used as a relatively radiolucent alternative. Tepex was statistically more radiolucent than the other 2 tested composite materials (Table 1). Even with 2 pieces placed anterior to the subject and 2 placed posterior, the radiodensity compared to the cortical bone was still lower than 1 piece of Al-7075-T6 either anterior or posterior to the subject.
Structural Properties Testing
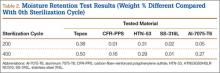
Moisture Retention. Moisture retention was evaluated by weighing the test materials before and after repeated sterilization. There was no significant difference in moisture retention, as weight differences for all the tested materials were less than 0.5 weight percentage compared to the 0th sterilization cycle (Table 2). Therefore, the results of this study showed that all the tested materials exhibited good moisture/temperature resistance after 400 sterilization cycles.
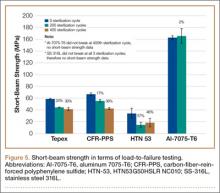
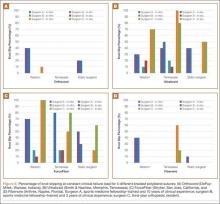
Load to Failure. In the LTF test, significant differences were detected in SBS between all 5 tested materials (P < .05). Figure 5 shows the comparison of the structural properties in terms of SBS between the 5 tested materials, and Figure 6 shows the failure modes for the tested materials. There was no SBS for SS-316L, as the material did not exhibit complete structural failure even after 400 sterilization cycles; however, SS-316L was observed in inelastic deformation failure (Figure 6D). Al-7075-T6 had much higher SBS compared with the other composite materials, and it also resulted in an inelastic deformation failure mode only after 400 sterilization cycles; otherwise, flexure failure modes were observed. Tepex and CFR-PPS exhibited interlaminar shear failure, and HTN-53 exhibited complete structural failure.
Every composite material tested using the short-beam test for LTF showed a decrease in SBS with increased sterilization cycles (Figure 5). This decrease ranged from 17% to 57% compared with the 0th sterilization cycle. SBS was higher for CFR-PPS than for the other 2 composites. No statistically significant difference was found between CFR-PPS and Tepex except at the 200th sterilization cycle. HTN-53 was brittle at the 0th sterilization cycle but performed more like a ductile material at the 200th cycle. In addition, HTN-53 had the lowest SBS in terms of LTF testing when compared with the other 2 composites.
During the complete structural failure test, the failure modes for Tepex and CFR-PPS were visually identified as interlaminar shear failure (Figures 6A, 6B), whereas HTN-53 visually exhibited pure flexure failure (Figure 6C). As for the metals, SS-316L exhibited plastic deformation, and Al-7075-T6 exhibited flexure failure (Figures 6D, 6E).
Cyclic Compression Loading. Tepex was the only material to pass the 100,000 loading cycle without failure (Table 3). HTN-53 had the poorest performance of all: Its failure rates were 33% (2/6 samples) before sterilization (average cycle, 22,213; range, 21,500-22,925), 83% (5/6 samples) at the 200th sterilization cycle (average cycle, 4,210; range, 0-14,360), and 100% after 400 sterilization cycles (average cycle, 12,725; range, 1,190-21,900). CFR-PPS had no failures before the 400th sterilization cycle, and its failure rate after 400 sterilization cycles (average cycle, 50,735; range, 50,270-51,200) was 33% (2/6 samples).
Discussion
The success of a reusable composite material for use in orthopedic surgery depends not only on radiographic density, fabrication methods, and design but also on the ability to withstand repeated sterilization. Over the past 3 decades, investigators have explored several high-performance polymer matrix composite materials for use in orthopedics, especially in trauma, hip stems, and spinal implants.1,3,4,17-34 According to Evans and Gregson,35 composite materials have been widely promoted as possible orthopedic biomaterials but to date have found few successful commercial applications, because of the many challenging problems encountered in fabrication and testing. One of the most important factors in the mechanical properties of many composite materials is the influence of the cooling and loading rates on fiber-matrix interface adhesion.36-38 Our results tended to agree with the findings of Evans and Gregson,35 as some of these composite materials did not withstand repeated sterilizations well.
Guan and colleagues39 evaluated the influence of sterilization treatment on continuous carbon-fiber–reinforced polyolefin composite. Their 3-point bending test results showed that the levels of maximum load of all the specimens undergoing sterilization by autoclave were lower than those of the control group. For these composites, they concluded that autoclave sterilization and Co-60 gamma ray irradiation sterilization should be avoided and that ethylene oxide is the best method. Our results support their findings with a different set of composites.
Although HTN-53 has shown promise in other orthopedic applications because of its superior moisture and temperature resistance, we found that its performance after repeated sterilization was relatively poor. Tepex showed the greatest potential for durability after repeated sterilization; its mechanical properties were stable after 200 steam sterilization cycles.
Clinical Applications
The composite materials investigated in the present study have potential for use in either instrumentation or long-term implantation applications because of their versatility, mechanical strength, fatigue resistance, and biocompatibility. Akay and Aslan40 stated that carbon-fiber–filled composite implants can be designed with more appropriate modulus, strength, toughness, or stiffness by the arrangement of reinforcing fiber volume and orientation, and can provide better fatigue resistance. A notable advantage of using a composite plate with metal screws is that the potential for corrosion of metallic components is eliminated. Another major advantage of composite medical implants (eg, DiPhos-RM) is radiolucency, which allows direct visualization of osseous callus formation as well as monitoring of fracture healing, thereby improving clinical assessment and accuracy.
Numerous studies have documented the successful clinical performance of composite materials in orthopedic, trauma, and spinal surgery applications.41-45 Bagheri and colleagues41 developed a new carbon fiber–flax–epoxy composite plate and biomechanically compared it with a standard clinical metal plate. Their results confirmed that the carbon fiber–flax–epoxy material represents a potential candidate for bone fracture plate applications, as it can simultaneously provide similar mechanical stiffness and lower stress shielding (higher bone stress) compared with a standard clinical metal bone plate. Tarallo and colleagues45 evaluated the clinical results of 40 cases at 12-month follow-up using a new plate made of carbon-fiber–reinforced polyetheretherketon (DiPhos-RM, Lima Corporate) for the treatment of distal radius fractures. They reported good clinical results for this device at early follow-up, and its use allowed maintenance of reduction in complex AO (Arbeitsgemeinschaft für Osteosynthesefragen) fractures.
The main advantage in using composites for surgical instruments is their radiolucency. These materials do not obscure images or radiographs during fluoroscopic visualization. Surgery often requires fluoroscopic visualization of internal organs or bones, which may require temporary removal of radiopaque devices (eg, retractors, clamps, forceps, hooks, distractors). Aside from being inconvenient, removal and subsequent reinsertion consume valuable time and interfere with the smooth flow of an operation.
The shortcomings of using composite materials for surgical instruments involve detectability and sterilization. A significant issue in surgery is the accidental leaving behind of instruments in patients, which can cause serious problems ranging from organ perforation and blood infection to death. Although instrument counting and other safety protocols can reduce the risk of overlooking an instrument, mistakes are bound to happen. The other shortcoming is the influence of repeated sterilization on the mechanical properties of the composite materials, as sterilization is mandatory for surgical instruments used in the operation room. The structural integrity and overall performance of the polymer composite materials—especially the stability of the interface and the interphase zones—are strongly influenced by repeated sterilization.
On the other hand, composite materials have potential advantages that may support their introduction into long-term medical implant applications, as sterilization commonly is performed only once, during packaging. The effects of sterilization by radiation or steam are much less pronounced on composite implants than on composite surgical instruments. However, composite implants require careful consideration with respect to the bioactivity of wear particles that may be produced from articulation. Further, carbon-fiber–reinforced polymer implants are still substantially more difficult to manufacture and more costly than their metallic counterparts.46
Limitations
This study has some limitations. Most important, studies of this nature do not account for biological factors such as corrosion, biological wear, and the soft-tissue attachment effects on structural properties for potential in vivo use. Another limitation was that the study tested only the mechanical properties in terms of SBS and provided no information about other mechanical properties, such as tensile, compression, and torsion strengths. We think SBS testing adequately evaluates challenging scenarios like thin and narrow instruments/devices that are anticipated in application, and information regarding other modes of failure and mechanical properties (compression, tension, torsion) would be a further area of research. An additional limitation was that our model used a relatively small number of samples. A larger study with more samples and varying layout patterns and layers of the carbon fibers may more clearly demonstrate the effect of steam sterilization on composite materials.
Conclusion
This study provided new information on 3 selected composite materials and their structural properties after repeated steam sterilization. We discovered that these composites were similar in radiographic density and water retention but behaved very differently in terms of mechanical durability after repeated steam sterilization. All selected composites demonstrated deterioration of mechanical properties after repeated steam sterilization. Knowing these results could aid in making decisions about the design and manufacturing of operative instruments and orthopedic biomaterials. Although our preliminary findings are intriguing, further study is warranted to seek specific applications for these composite materials in orthopedic surgery.
1. Ali MS, French TA, Hastings GW, et al. Carbon fibre composite bone plates. Development, evaluation and early clinical experience. J Bone Joint Surg Br. 1990;72(4):586-591.
2. Brooks RA, Jones E, Storer A, Rushton N. Biological evaluation of carbon-fibre–reinforced polybutyleneterephthalate (CFRPBT) employed in a novel acetabular cup. Biomaterials. 2004;25(17):3429-3438.
3. Brown SA, Hastings RS, Mason JJ, Moet A. Characterization of short-fibre reinforced thermoplastics for fracture fixation devices. Biomaterials. 1990;11(8):541-547.
4. Skinner HB. Composite technology for total hip arthroplasty. Clin Orthop Relat Res. 1988;(235):224-236.
5. Field RE, Jones E, Nuijten P, Storer A, Cronin M, Rushton N. Pre-clinical evaluation of the Cambridge acetabular cup. J Mater Sci Mater Med. 2008;19(8):2791-2798.
6. Han N, Ahmed I, Parsons AJ, et al. Influence of screw holes and gamma sterilization on properties of phosphate glass fiber–reinforced composite bone plates. J Biomater Appl. 2013;27(8):990-1002.
7. Losi P, Munaò A, Spiller D, et al. Evaluation of a new composite prosthesis for the repair of abdominal wall defects. J Mater Sci Mater Med. 2007;18(10):1939-1944.
8. Pait TG, Kaufman HH, Voelker JL, McAllister HP, Willison C. Use of a carbon composite radiolucent anterior cervical retractor system. Neurosurgery. 1993;33(5):941-942.
9. Elfar J, Menorca RM, Reed JD, Stanbury S. Composite bone models in orthopaedic surgery research and education. J Am Acad Orthop Surg. 2014;22(2):111-120.
10. Gardner MP, Chong AC, Pollock AG, Wooley PH. Mechanical evaluation of large-size fourth-generation composite femur and tibia models. Ann Biomed Eng. 2010;38(3):613-620.
11. Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284.
12. Dunlap JT, Chong AC, Lucas GL, Cooke FW. Structural properties of a novel design of composite analogue humeri models. Ann Biomed Eng. 2008;36(11):1922-1926.
13. Grover P, Albert C, Wang M, Harris GF. Mechanical characterization of fourth generation composite humerus. Proc Inst Mech Eng H. 2011;225(12):1169-1176.
14. Zheng Q, Morgan RJ. Synergistic thermal-moisture damage mechanisms of epoxies and their carbon fiber composites. J Compos Mater. 1993;27(15):1465-1478.
15. Ray BC. Temperature effect during humid ageing on interfaces of glass and carbon fibers reinforced epoxy composites. J Colloid Interface Sci. 2006;298(1):111-117.
16. Standard test method for short-beam strength of polymer matrix composite materials and their laminates [ASTM specification D2344/D2344M-00]. In: Annual Book of ASTM Standards. Vol 15.03. West Conshohocken, PA: American Society for Testing and Materials; 2006.
17. Bradley JS, Hastings GW, Johnson-Nurse C. Carbon fibre reinforced epoxy as a high strength, low modulus material for internal fixation plates. Biomaterials. 1980;1(1):38-40.
18. McKenna GB, Bradley GW, Dunn HK, Statton WO. Mechanical properties of some fibre reinforced polymer composites after implantation as fracture fixation plates. Biomaterials. 1980;1(4):189-192.
19. Tayton K, Johnson-Nurse C, McKibbin B, Bradley J, Hastings G. The use of semi-rigid carbon-fibre–reinforced plastic plates for fixation of human fractures. Results of preliminary trials. J Bone Joint Surg Br. 1982;64(1):105-111.
20. Tayton K, Bradley J. How stiff should semi-rigid fixation of the human tibia be? A clue to the answer. J Bone Joint Surg Br. 1983;65(3):312-315.
21. Tayton K. Corrosive effect of carbon-fibre reinforced plastic on stainless-steel screws during implantation into man. J Med Eng Technol. 1983;7(1):24-26.
22. Howard CB, Tayton KJ, Gibbs A. The response of human tissues to carbon reinforced epoxy resin. J Bone Joint Surg Br. 1985;67(4):656-658.
23. Skirving AP, Day R, Macdonald W, McLaren R. Carbon fiber reinforced plastic (CFRP) plates versus stainless steel dynamic compression plates in the treatment of fractures of the tibiae in dogs. Clin Orthop Relat Res. 1987;(224):117-124.
24. Prakash R, Marwah S, Goel SC, Tuli SM. Carbon fibre reinforced epoxy implants for bridging large osteoperiosteal gaps. Biomaterials. 1988;9(2):198-202.
25. Pemberton DJ, McKibbin B, Savage R, Tayton K, Stuart D. Carbon-fibre reinforced plates for problem fractures. J Bone Joint Surg Br. 1992;74(1):88-92.
26. Pemberton DJ, Evans PD, Grant A, McKibbin B. Fractures of the distal femur in the elderly treated with a carbon fibre supracondylar plate. Injury. 1994;25(5):317-321.
27. Kelsey DJ, Springer GS, Goodman SB. Composite implant for bone replacement. J Compos Mater. 1997;31(16):1593-1632.
28. Corvelli AA, Biermann PJ, Roberts JC. Design, analysis and fabrication of a composite segmental bone replacement implant. J Adv Mater. 1997;28:2-8.
29. Glassman AH, Crowninshield RD, Schenck R, Herberts P. A low stiffness composite biologically fixed prosthesis. Clin Orthop Relat Res. 2001;(393):128-136.
30. Williams D. New horizons for thermoplastic polymers. Med Device Technol. 2001;12(4):8-9.
31. Al-Shawi AK, Smith SP, Anderson GH. The use of a carbon fiber plate for periprosthetic supracondylar femoral fractures. J Arthroplasty. 2002;17(3):320-324.
32. Baker D, Kadambande SS, Alderman PM. Carbon fibre plates in the treatment of femoral periprosthetic fractures. Injury. 2004;35(6):596-598.
33. Akhavan S, Matthiesen MM, Schulte L, et al. Clinical and histologic results related to a low-modulus composite total hip replacement stem. J Bone Joint Surg Am. 2006;88(6):1308-1314.
34. Toth JM, Wang M, Estes BT, Scifert JL, Seim HB 3rd, Turner AS. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials. 2006;27(3):324-334.
35. Evans SL, Gregson PJ. Composite technology in load-bearing orthopaedic implants. Biomaterials. 1998;19(15):1329-1342.
36. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part I. Crystallinity and interface adhesion. Composites Part A. 2000;31(6):517-530.
37. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part II. Interlaminar fracture toughness. Composites Part A. 2001;32(6):763-774.
38. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part III. Impact damage performance. Composites Part A. 2001;32(6):775-785.
39. Guan SB, Hou CL, Chen AM, Zhang W, Wang JE. Influence of sterilization treatments on continuous carbon-fiber reinforced polyolefin composite. Zhonghua Yi Xue Za Zhi. 2007;87(31):2228-2231.
40. Akay M, Aslan N. An estimation of fatigue life for a carbon fibre/poly ether ether ketone hip joint prosthesis. Proc Inst Mech Eng H. 1995;209(2):93-103.
41. Bagheri ZS, Tavakkoli Awal P, Bougherara H, Aziz MS, Schemitsch EH, Zdero R. Biomechanical analysis of a new carbon fiber/flax/epoxy bone fracture plate shows less stress shielding compared to a standard clinical metal plate. J Biomech Eng. 2014;136(9):091002.
42. Rhee PC, Shin AY. The rate of successful four-corner arthrodesis with a locking, dorsal circular polyether-ether-ketone (PEEK-Optima) plate. J Hand Surg Eur Vol. 2013;38(7):767-773.
43. Nakahara I, Takao M, Bandoh S, Bertollo N, Walsh WR, Sugano N. In vivo implant fixation of carbon fiber–reinforced PEEK hip prostheses in an ovine model. J Orthop Res. 2013;31(3):485-492.
44. Kasliwal MK, O’Toole JE. Clinical experience using polyetheretherketone (PEEK) intervertebral structural cage for anterior cervical corpectomy and fusion. J Clin Neurosci. 2014;21(2):217-220.
45. Tarallo L, Mugnai R, Adani R, Zambianchi F, Catani F. A new volar plate made of carbon-fiber–reinforced polyetheretherketon for distal radius fracture: analysis of 40 cases. J Orthop Traumatol. 2014;15(4):277-283.
46. Cordey J, Perren SM, Steinemann SG. Stress protection due to plates: myth or reality? A parametric analysis made using the composite beam theory. Injury. 2000;31(suppl 3):C1-C13.
Polymer matrix composite materials have been widely promoted for orthopedic use in a variety of settings, including surgical instruments, medical devices, implants, and bone models.1-13 These types of composites are engineered from 2 or more constituent materials with significantly different physical or chemical properties; these materials remain separate and distinct on a macroscopic level within the finished composite structure. As a result of ongoing biomaterial research, polymer matrix composite materials can be engineered with a wide range of physical, mechanical, and surface properties, tailored to their application. Given their advantages (eg, high strength-to-weight ratio, radiolucency), these polymer matrix composite materials have gained popularity over traditional metallic materials.
Sterilization is an essential day-to-day procedure in the health care sector, both for single- and multiple-use devices or instruments, and thus a composite material used in medical components should remain unaffected by the process. The type of sterilization most commonly performed is steam sterilization, which achieves microbiological death by moist heat and pressure. Steam is created in an autoclave at a temperature of 132°C (270°F) in typical hospital settings. Steam sterilization cycles last 5 to 14 minutes based on specific manufacturer recommendations. Most medical-grade plastics used in health care have been designed and formulated to withstand the required sterilization cycles without sacrificing key properties. The structure integrity and overall performance of polymer matrix composites may be strongly influenced by the stability of the fiber/polymer interfacial region in terms of physical, chemical, and mechanical characteristics of the material at different scales.14 Absorption of moisture causes dilatational expansion and induces stresses, which are associated with the moisture-induced expansion resulting in degradation of structure stability.15 Thus, steam sterilization could affect the properties of the polymer matrix composite materials by excessive absorption of moisture by the polymer.
To our knowledge, no one has studied whether polymer matrix material properties degrade from long-term, repeated steam sterilization followed by mechanical loading. We conducted a study to evaluate the structural properties (short-beam strength, SBS) of several composite materials exposed to repeated sterilization as compared with traditional metal materials: SS-316L (stainless steel 316L) and Al-7075-T6 (aluminum 7075-T6).
Materials and Methods
We evaluated 3 types of composite materials: Tepex (Tepex Dynalite 201; HiPer Technology Inc.), CFR-PPS (carbon-fiber–reinforced polyphenylene sulfide, Cetex PPS; TenCate Advanced Composites USA Inc.), and HTN-53 (Zytel HTN53G50HSLR NC010; HiPer Technology Inc.) (Figure 1). Tepex is being used for orthopedic applications (knee braces, orthoses, insoles) and sporting goods applications. The performance of this material is superior to that of unreinforced thermoplastics. CFR-PPS represented the state of the art in composite materials for aerospace applications (eg, airframe structures, engine nacelles, fan casings, floorboards, interior parts). This is a high-performance material with exceptional high temperature and aggressive chemical resistance characteristics. CFR-PPS is also used to make filter fabric for coal boilers, papermaking felts, electrical insulation, specialty membranes, gaskets, and packing. It is not solubilized by any known solvents, even in long-term exposure, at temperatures up to 200°C. In addition, it exhibits exceptional resistance to organic and inorganic solutions, acids and alkali solutions, and a wide array of miscellaneous chemicals. HTN-53 is a 50% glass-reinforced, lubricated, high-performance polyamide resin with improved flow, developed for applications requiring excellent surface appearance with water-heated molds. This material has specifically shown survivability in hot, cold, chemically aggressive, and load-bearing environments. In addition, it has shown superior moisture and temperature resistance. These 3 composite materials were compared with SS-316L and Al-7075-T6. SS-316L is commonly used for implants in orthopedics, and Al-7075-T6 is a relatively radiolucent alternative for medical applications. Two different tests were performed to evaluate and validate these composite materials: (1) radiographic density evaluation and (2) structural property tests (short-beam load-to-failure [LTF] test, short-beam cyclic compression loading [CCL] test) before and after sterilization cycling.
Radiographic Density Evaluation
The radiographic density of the 5 materials was evaluated with radiographic images of a cadaveric knee specimen (Figure 2). Radiographic image intensification is the gold standard for repeated radiographic imaging in the operating room. Six different radiographic images were obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). Image-Pro Plus software (Media Cybernetics) was used to measure the radiographic density of the materials from the grayscale of the images.
Structural Properties Testing Before and After Sterilization Cycling
We used a standard SBS testing method to determine whether any degradation of structural properties resulted from standard repeated sterilization. The material geometries of the test specimens were 18.96×6.50×3.37 mm (length × width × thickness). Standard sterilization procedures were performed with steam sterilization using an autoclave at a temperature of 132°C (270°F) for at least 5 minutes (range, 5-14 minutes). Sample interval testing ran at 0, 200, and 400 sterilization cycles for structural properties in terms of SBS and moisture retention, with the structural properties at the 0th sterilization cycle (material before sterilization was performed) used as a baseline for comparison. Materials were subjected to 400 sterilization cycles, which is representative of the number of sterilization cycles per year an instrument or device would be subjected to.
Three structural tests were performed for each sample interval: moisture retention, LTF, and CCL. Moisture retention was investigated before and after repeated sterilization by measuring the weight of the test materials, as steam sterilization is known to affect the amount of moisture that is absorbed by a material. Twelve specimens of each proposed material were weighed at each sample interval, with the structural weight at the 0th sterilization cycle (material before sterilization is performed) serving as a baseline for comparison.
SBS testing was based on the ASTM (American Society for Testing and Materials) D2344 standard16 for LTF and CCL tests (Figure 3). Six samples of material were used for each test at every sample interval, yielding 180 samples. Seven servohydraulic material testing system instruments (1 MTS 810 and 6 MTS 858 Mini Bionix) were used to test the SBS of each material. For LTF testing, each specimen was loaded in compression from 30 N to complete structural failure at a constant displacement rate of 1.0 mm/min (0.05 in/min). Testing was initiated with 5 preconditioning loading cycles from 30 to 100 N at 1 Hz. The load was then applied continuously until failure occurred; force and displacement data were collected every 0.02 second. This procedure was performed for 6 replicates for each sample interval for each test material.
The calculation for SBS, Fsbs (MPa), for the constant loading rate until structural failure is:
Fsbs = 0.75 × Pm
b × h
where Pm (N) is the maximum applied load observed during the test, b is the measured specimen width (mm), and h is the measured specimen thickness (mm).
CCL testing consisted of each test material axially loaded with 100 to 500 N at a frequency of 1 Hz for 100,000 cycles. The maximum load of 500 N was chosen as a standard based on 80% of the minimum ultimate failure load from previous LTF tests. Displacement and force data were collected every 5 cycles at the maximum compressive load. Degradation of the material was calculated using the difference between the deflection of the initial cycle and the deflection of the final cycle (50th cycle and 100,000th cycle). This procedure was performed for 6 replicates for each sample interval for each test material.
Statistical Analysis
LTF and CCL testing data were analyzed for any differences among the test materials using 1-way analysis of variance with the least significant difference multiple comparisons post hoc test method using SPSS Version 16.0, with P < .05 denoting significance. These analyses were used to determine the statistical relevance of the difference between the SBS (LTF and CCL) of each test material. Means and standard deviations were calculated for all tests.
Results
Radiographic Density Evaluation
Overall, all the tested composite materials were significantly more radiolucent than either SS-316L or Al-7075-T6. Figure 4 shows the 6 different radiographic images obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). SS-316L can be considered radiopaque, and Al-7075-T6 has been used as a relatively radiolucent alternative. Tepex was statistically more radiolucent than the other 2 tested composite materials (Table 1). Even with 2 pieces placed anterior to the subject and 2 placed posterior, the radiodensity compared to the cortical bone was still lower than 1 piece of Al-7075-T6 either anterior or posterior to the subject.
Structural Properties Testing
Moisture Retention. Moisture retention was evaluated by weighing the test materials before and after repeated sterilization. There was no significant difference in moisture retention, as weight differences for all the tested materials were less than 0.5 weight percentage compared to the 0th sterilization cycle (Table 2). Therefore, the results of this study showed that all the tested materials exhibited good moisture/temperature resistance after 400 sterilization cycles.
Load to Failure. In the LTF test, significant differences were detected in SBS between all 5 tested materials (P < .05). Figure 5 shows the comparison of the structural properties in terms of SBS between the 5 tested materials, and Figure 6 shows the failure modes for the tested materials. There was no SBS for SS-316L, as the material did not exhibit complete structural failure even after 400 sterilization cycles; however, SS-316L was observed in inelastic deformation failure (Figure 6D). Al-7075-T6 had much higher SBS compared with the other composite materials, and it also resulted in an inelastic deformation failure mode only after 400 sterilization cycles; otherwise, flexure failure modes were observed. Tepex and CFR-PPS exhibited interlaminar shear failure, and HTN-53 exhibited complete structural failure.
Every composite material tested using the short-beam test for LTF showed a decrease in SBS with increased sterilization cycles (Figure 5). This decrease ranged from 17% to 57% compared with the 0th sterilization cycle. SBS was higher for CFR-PPS than for the other 2 composites. No statistically significant difference was found between CFR-PPS and Tepex except at the 200th sterilization cycle. HTN-53 was brittle at the 0th sterilization cycle but performed more like a ductile material at the 200th cycle. In addition, HTN-53 had the lowest SBS in terms of LTF testing when compared with the other 2 composites.
During the complete structural failure test, the failure modes for Tepex and CFR-PPS were visually identified as interlaminar shear failure (Figures 6A, 6B), whereas HTN-53 visually exhibited pure flexure failure (Figure 6C). As for the metals, SS-316L exhibited plastic deformation, and Al-7075-T6 exhibited flexure failure (Figures 6D, 6E).
Cyclic Compression Loading. Tepex was the only material to pass the 100,000 loading cycle without failure (Table 3). HTN-53 had the poorest performance of all: Its failure rates were 33% (2/6 samples) before sterilization (average cycle, 22,213; range, 21,500-22,925), 83% (5/6 samples) at the 200th sterilization cycle (average cycle, 4,210; range, 0-14,360), and 100% after 400 sterilization cycles (average cycle, 12,725; range, 1,190-21,900). CFR-PPS had no failures before the 400th sterilization cycle, and its failure rate after 400 sterilization cycles (average cycle, 50,735; range, 50,270-51,200) was 33% (2/6 samples).
Discussion
The success of a reusable composite material for use in orthopedic surgery depends not only on radiographic density, fabrication methods, and design but also on the ability to withstand repeated sterilization. Over the past 3 decades, investigators have explored several high-performance polymer matrix composite materials for use in orthopedics, especially in trauma, hip stems, and spinal implants.1,3,4,17-34 According to Evans and Gregson,35 composite materials have been widely promoted as possible orthopedic biomaterials but to date have found few successful commercial applications, because of the many challenging problems encountered in fabrication and testing. One of the most important factors in the mechanical properties of many composite materials is the influence of the cooling and loading rates on fiber-matrix interface adhesion.36-38 Our results tended to agree with the findings of Evans and Gregson,35 as some of these composite materials did not withstand repeated sterilizations well.
Guan and colleagues39 evaluated the influence of sterilization treatment on continuous carbon-fiber–reinforced polyolefin composite. Their 3-point bending test results showed that the levels of maximum load of all the specimens undergoing sterilization by autoclave were lower than those of the control group. For these composites, they concluded that autoclave sterilization and Co-60 gamma ray irradiation sterilization should be avoided and that ethylene oxide is the best method. Our results support their findings with a different set of composites.
Although HTN-53 has shown promise in other orthopedic applications because of its superior moisture and temperature resistance, we found that its performance after repeated sterilization was relatively poor. Tepex showed the greatest potential for durability after repeated sterilization; its mechanical properties were stable after 200 steam sterilization cycles.
Clinical Applications
The composite materials investigated in the present study have potential for use in either instrumentation or long-term implantation applications because of their versatility, mechanical strength, fatigue resistance, and biocompatibility. Akay and Aslan40 stated that carbon-fiber–filled composite implants can be designed with more appropriate modulus, strength, toughness, or stiffness by the arrangement of reinforcing fiber volume and orientation, and can provide better fatigue resistance. A notable advantage of using a composite plate with metal screws is that the potential for corrosion of metallic components is eliminated. Another major advantage of composite medical implants (eg, DiPhos-RM) is radiolucency, which allows direct visualization of osseous callus formation as well as monitoring of fracture healing, thereby improving clinical assessment and accuracy.
Numerous studies have documented the successful clinical performance of composite materials in orthopedic, trauma, and spinal surgery applications.41-45 Bagheri and colleagues41 developed a new carbon fiber–flax–epoxy composite plate and biomechanically compared it with a standard clinical metal plate. Their results confirmed that the carbon fiber–flax–epoxy material represents a potential candidate for bone fracture plate applications, as it can simultaneously provide similar mechanical stiffness and lower stress shielding (higher bone stress) compared with a standard clinical metal bone plate. Tarallo and colleagues45 evaluated the clinical results of 40 cases at 12-month follow-up using a new plate made of carbon-fiber–reinforced polyetheretherketon (DiPhos-RM, Lima Corporate) for the treatment of distal radius fractures. They reported good clinical results for this device at early follow-up, and its use allowed maintenance of reduction in complex AO (Arbeitsgemeinschaft für Osteosynthesefragen) fractures.
The main advantage in using composites for surgical instruments is their radiolucency. These materials do not obscure images or radiographs during fluoroscopic visualization. Surgery often requires fluoroscopic visualization of internal organs or bones, which may require temporary removal of radiopaque devices (eg, retractors, clamps, forceps, hooks, distractors). Aside from being inconvenient, removal and subsequent reinsertion consume valuable time and interfere with the smooth flow of an operation.
The shortcomings of using composite materials for surgical instruments involve detectability and sterilization. A significant issue in surgery is the accidental leaving behind of instruments in patients, which can cause serious problems ranging from organ perforation and blood infection to death. Although instrument counting and other safety protocols can reduce the risk of overlooking an instrument, mistakes are bound to happen. The other shortcoming is the influence of repeated sterilization on the mechanical properties of the composite materials, as sterilization is mandatory for surgical instruments used in the operation room. The structural integrity and overall performance of the polymer composite materials—especially the stability of the interface and the interphase zones—are strongly influenced by repeated sterilization.
On the other hand, composite materials have potential advantages that may support their introduction into long-term medical implant applications, as sterilization commonly is performed only once, during packaging. The effects of sterilization by radiation or steam are much less pronounced on composite implants than on composite surgical instruments. However, composite implants require careful consideration with respect to the bioactivity of wear particles that may be produced from articulation. Further, carbon-fiber–reinforced polymer implants are still substantially more difficult to manufacture and more costly than their metallic counterparts.46
Limitations
This study has some limitations. Most important, studies of this nature do not account for biological factors such as corrosion, biological wear, and the soft-tissue attachment effects on structural properties for potential in vivo use. Another limitation was that the study tested only the mechanical properties in terms of SBS and provided no information about other mechanical properties, such as tensile, compression, and torsion strengths. We think SBS testing adequately evaluates challenging scenarios like thin and narrow instruments/devices that are anticipated in application, and information regarding other modes of failure and mechanical properties (compression, tension, torsion) would be a further area of research. An additional limitation was that our model used a relatively small number of samples. A larger study with more samples and varying layout patterns and layers of the carbon fibers may more clearly demonstrate the effect of steam sterilization on composite materials.
Conclusion
This study provided new information on 3 selected composite materials and their structural properties after repeated steam sterilization. We discovered that these composites were similar in radiographic density and water retention but behaved very differently in terms of mechanical durability after repeated steam sterilization. All selected composites demonstrated deterioration of mechanical properties after repeated steam sterilization. Knowing these results could aid in making decisions about the design and manufacturing of operative instruments and orthopedic biomaterials. Although our preliminary findings are intriguing, further study is warranted to seek specific applications for these composite materials in orthopedic surgery.
Polymer matrix composite materials have been widely promoted for orthopedic use in a variety of settings, including surgical instruments, medical devices, implants, and bone models.1-13 These types of composites are engineered from 2 or more constituent materials with significantly different physical or chemical properties; these materials remain separate and distinct on a macroscopic level within the finished composite structure. As a result of ongoing biomaterial research, polymer matrix composite materials can be engineered with a wide range of physical, mechanical, and surface properties, tailored to their application. Given their advantages (eg, high strength-to-weight ratio, radiolucency), these polymer matrix composite materials have gained popularity over traditional metallic materials.
Sterilization is an essential day-to-day procedure in the health care sector, both for single- and multiple-use devices or instruments, and thus a composite material used in medical components should remain unaffected by the process. The type of sterilization most commonly performed is steam sterilization, which achieves microbiological death by moist heat and pressure. Steam is created in an autoclave at a temperature of 132°C (270°F) in typical hospital settings. Steam sterilization cycles last 5 to 14 minutes based on specific manufacturer recommendations. Most medical-grade plastics used in health care have been designed and formulated to withstand the required sterilization cycles without sacrificing key properties. The structure integrity and overall performance of polymer matrix composites may be strongly influenced by the stability of the fiber/polymer interfacial region in terms of physical, chemical, and mechanical characteristics of the material at different scales.14 Absorption of moisture causes dilatational expansion and induces stresses, which are associated with the moisture-induced expansion resulting in degradation of structure stability.15 Thus, steam sterilization could affect the properties of the polymer matrix composite materials by excessive absorption of moisture by the polymer.
To our knowledge, no one has studied whether polymer matrix material properties degrade from long-term, repeated steam sterilization followed by mechanical loading. We conducted a study to evaluate the structural properties (short-beam strength, SBS) of several composite materials exposed to repeated sterilization as compared with traditional metal materials: SS-316L (stainless steel 316L) and Al-7075-T6 (aluminum 7075-T6).
Materials and Methods
We evaluated 3 types of composite materials: Tepex (Tepex Dynalite 201; HiPer Technology Inc.), CFR-PPS (carbon-fiber–reinforced polyphenylene sulfide, Cetex PPS; TenCate Advanced Composites USA Inc.), and HTN-53 (Zytel HTN53G50HSLR NC010; HiPer Technology Inc.) (Figure 1). Tepex is being used for orthopedic applications (knee braces, orthoses, insoles) and sporting goods applications. The performance of this material is superior to that of unreinforced thermoplastics. CFR-PPS represented the state of the art in composite materials for aerospace applications (eg, airframe structures, engine nacelles, fan casings, floorboards, interior parts). This is a high-performance material with exceptional high temperature and aggressive chemical resistance characteristics. CFR-PPS is also used to make filter fabric for coal boilers, papermaking felts, electrical insulation, specialty membranes, gaskets, and packing. It is not solubilized by any known solvents, even in long-term exposure, at temperatures up to 200°C. In addition, it exhibits exceptional resistance to organic and inorganic solutions, acids and alkali solutions, and a wide array of miscellaneous chemicals. HTN-53 is a 50% glass-reinforced, lubricated, high-performance polyamide resin with improved flow, developed for applications requiring excellent surface appearance with water-heated molds. This material has specifically shown survivability in hot, cold, chemically aggressive, and load-bearing environments. In addition, it has shown superior moisture and temperature resistance. These 3 composite materials were compared with SS-316L and Al-7075-T6. SS-316L is commonly used for implants in orthopedics, and Al-7075-T6 is a relatively radiolucent alternative for medical applications. Two different tests were performed to evaluate and validate these composite materials: (1) radiographic density evaluation and (2) structural property tests (short-beam load-to-failure [LTF] test, short-beam cyclic compression loading [CCL] test) before and after sterilization cycling.
Radiographic Density Evaluation
The radiographic density of the 5 materials was evaluated with radiographic images of a cadaveric knee specimen (Figure 2). Radiographic image intensification is the gold standard for repeated radiographic imaging in the operating room. Six different radiographic images were obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). Image-Pro Plus software (Media Cybernetics) was used to measure the radiographic density of the materials from the grayscale of the images.
Structural Properties Testing Before and After Sterilization Cycling
We used a standard SBS testing method to determine whether any degradation of structural properties resulted from standard repeated sterilization. The material geometries of the test specimens were 18.96×6.50×3.37 mm (length × width × thickness). Standard sterilization procedures were performed with steam sterilization using an autoclave at a temperature of 132°C (270°F) for at least 5 minutes (range, 5-14 minutes). Sample interval testing ran at 0, 200, and 400 sterilization cycles for structural properties in terms of SBS and moisture retention, with the structural properties at the 0th sterilization cycle (material before sterilization was performed) used as a baseline for comparison. Materials were subjected to 400 sterilization cycles, which is representative of the number of sterilization cycles per year an instrument or device would be subjected to.
Three structural tests were performed for each sample interval: moisture retention, LTF, and CCL. Moisture retention was investigated before and after repeated sterilization by measuring the weight of the test materials, as steam sterilization is known to affect the amount of moisture that is absorbed by a material. Twelve specimens of each proposed material were weighed at each sample interval, with the structural weight at the 0th sterilization cycle (material before sterilization is performed) serving as a baseline for comparison.
SBS testing was based on the ASTM (American Society for Testing and Materials) D2344 standard16 for LTF and CCL tests (Figure 3). Six samples of material were used for each test at every sample interval, yielding 180 samples. Seven servohydraulic material testing system instruments (1 MTS 810 and 6 MTS 858 Mini Bionix) were used to test the SBS of each material. For LTF testing, each specimen was loaded in compression from 30 N to complete structural failure at a constant displacement rate of 1.0 mm/min (0.05 in/min). Testing was initiated with 5 preconditioning loading cycles from 30 to 100 N at 1 Hz. The load was then applied continuously until failure occurred; force and displacement data were collected every 0.02 second. This procedure was performed for 6 replicates for each sample interval for each test material.
The calculation for SBS, Fsbs (MPa), for the constant loading rate until structural failure is:
Fsbs = 0.75 × Pm
b × h
where Pm (N) is the maximum applied load observed during the test, b is the measured specimen width (mm), and h is the measured specimen thickness (mm).
CCL testing consisted of each test material axially loaded with 100 to 500 N at a frequency of 1 Hz for 100,000 cycles. The maximum load of 500 N was chosen as a standard based on 80% of the minimum ultimate failure load from previous LTF tests. Displacement and force data were collected every 5 cycles at the maximum compressive load. Degradation of the material was calculated using the difference between the deflection of the initial cycle and the deflection of the final cycle (50th cycle and 100,000th cycle). This procedure was performed for 6 replicates for each sample interval for each test material.
Statistical Analysis
LTF and CCL testing data were analyzed for any differences among the test materials using 1-way analysis of variance with the least significant difference multiple comparisons post hoc test method using SPSS Version 16.0, with P < .05 denoting significance. These analyses were used to determine the statistical relevance of the difference between the SBS (LTF and CCL) of each test material. Means and standard deviations were calculated for all tests.
Results
Radiographic Density Evaluation
Overall, all the tested composite materials were significantly more radiolucent than either SS-316L or Al-7075-T6. Figure 4 shows the 6 different radiographic images obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). SS-316L can be considered radiopaque, and Al-7075-T6 has been used as a relatively radiolucent alternative. Tepex was statistically more radiolucent than the other 2 tested composite materials (Table 1). Even with 2 pieces placed anterior to the subject and 2 placed posterior, the radiodensity compared to the cortical bone was still lower than 1 piece of Al-7075-T6 either anterior or posterior to the subject.
Structural Properties Testing
Moisture Retention. Moisture retention was evaluated by weighing the test materials before and after repeated sterilization. There was no significant difference in moisture retention, as weight differences for all the tested materials were less than 0.5 weight percentage compared to the 0th sterilization cycle (Table 2). Therefore, the results of this study showed that all the tested materials exhibited good moisture/temperature resistance after 400 sterilization cycles.
Load to Failure. In the LTF test, significant differences were detected in SBS between all 5 tested materials (P < .05). Figure 5 shows the comparison of the structural properties in terms of SBS between the 5 tested materials, and Figure 6 shows the failure modes for the tested materials. There was no SBS for SS-316L, as the material did not exhibit complete structural failure even after 400 sterilization cycles; however, SS-316L was observed in inelastic deformation failure (Figure 6D). Al-7075-T6 had much higher SBS compared with the other composite materials, and it also resulted in an inelastic deformation failure mode only after 400 sterilization cycles; otherwise, flexure failure modes were observed. Tepex and CFR-PPS exhibited interlaminar shear failure, and HTN-53 exhibited complete structural failure.
Every composite material tested using the short-beam test for LTF showed a decrease in SBS with increased sterilization cycles (Figure 5). This decrease ranged from 17% to 57% compared with the 0th sterilization cycle. SBS was higher for CFR-PPS than for the other 2 composites. No statistically significant difference was found between CFR-PPS and Tepex except at the 200th sterilization cycle. HTN-53 was brittle at the 0th sterilization cycle but performed more like a ductile material at the 200th cycle. In addition, HTN-53 had the lowest SBS in terms of LTF testing when compared with the other 2 composites.
During the complete structural failure test, the failure modes for Tepex and CFR-PPS were visually identified as interlaminar shear failure (Figures 6A, 6B), whereas HTN-53 visually exhibited pure flexure failure (Figure 6C). As for the metals, SS-316L exhibited plastic deformation, and Al-7075-T6 exhibited flexure failure (Figures 6D, 6E).
Cyclic Compression Loading. Tepex was the only material to pass the 100,000 loading cycle without failure (Table 3). HTN-53 had the poorest performance of all: Its failure rates were 33% (2/6 samples) before sterilization (average cycle, 22,213; range, 21,500-22,925), 83% (5/6 samples) at the 200th sterilization cycle (average cycle, 4,210; range, 0-14,360), and 100% after 400 sterilization cycles (average cycle, 12,725; range, 1,190-21,900). CFR-PPS had no failures before the 400th sterilization cycle, and its failure rate after 400 sterilization cycles (average cycle, 50,735; range, 50,270-51,200) was 33% (2/6 samples).
Discussion
The success of a reusable composite material for use in orthopedic surgery depends not only on radiographic density, fabrication methods, and design but also on the ability to withstand repeated sterilization. Over the past 3 decades, investigators have explored several high-performance polymer matrix composite materials for use in orthopedics, especially in trauma, hip stems, and spinal implants.1,3,4,17-34 According to Evans and Gregson,35 composite materials have been widely promoted as possible orthopedic biomaterials but to date have found few successful commercial applications, because of the many challenging problems encountered in fabrication and testing. One of the most important factors in the mechanical properties of many composite materials is the influence of the cooling and loading rates on fiber-matrix interface adhesion.36-38 Our results tended to agree with the findings of Evans and Gregson,35 as some of these composite materials did not withstand repeated sterilizations well.
Guan and colleagues39 evaluated the influence of sterilization treatment on continuous carbon-fiber–reinforced polyolefin composite. Their 3-point bending test results showed that the levels of maximum load of all the specimens undergoing sterilization by autoclave were lower than those of the control group. For these composites, they concluded that autoclave sterilization and Co-60 gamma ray irradiation sterilization should be avoided and that ethylene oxide is the best method. Our results support their findings with a different set of composites.
Although HTN-53 has shown promise in other orthopedic applications because of its superior moisture and temperature resistance, we found that its performance after repeated sterilization was relatively poor. Tepex showed the greatest potential for durability after repeated sterilization; its mechanical properties were stable after 200 steam sterilization cycles.
Clinical Applications
The composite materials investigated in the present study have potential for use in either instrumentation or long-term implantation applications because of their versatility, mechanical strength, fatigue resistance, and biocompatibility. Akay and Aslan40 stated that carbon-fiber–filled composite implants can be designed with more appropriate modulus, strength, toughness, or stiffness by the arrangement of reinforcing fiber volume and orientation, and can provide better fatigue resistance. A notable advantage of using a composite plate with metal screws is that the potential for corrosion of metallic components is eliminated. Another major advantage of composite medical implants (eg, DiPhos-RM) is radiolucency, which allows direct visualization of osseous callus formation as well as monitoring of fracture healing, thereby improving clinical assessment and accuracy.
Numerous studies have documented the successful clinical performance of composite materials in orthopedic, trauma, and spinal surgery applications.41-45 Bagheri and colleagues41 developed a new carbon fiber–flax–epoxy composite plate and biomechanically compared it with a standard clinical metal plate. Their results confirmed that the carbon fiber–flax–epoxy material represents a potential candidate for bone fracture plate applications, as it can simultaneously provide similar mechanical stiffness and lower stress shielding (higher bone stress) compared with a standard clinical metal bone plate. Tarallo and colleagues45 evaluated the clinical results of 40 cases at 12-month follow-up using a new plate made of carbon-fiber–reinforced polyetheretherketon (DiPhos-RM, Lima Corporate) for the treatment of distal radius fractures. They reported good clinical results for this device at early follow-up, and its use allowed maintenance of reduction in complex AO (Arbeitsgemeinschaft für Osteosynthesefragen) fractures.
The main advantage in using composites for surgical instruments is their radiolucency. These materials do not obscure images or radiographs during fluoroscopic visualization. Surgery often requires fluoroscopic visualization of internal organs or bones, which may require temporary removal of radiopaque devices (eg, retractors, clamps, forceps, hooks, distractors). Aside from being inconvenient, removal and subsequent reinsertion consume valuable time and interfere with the smooth flow of an operation.
The shortcomings of using composite materials for surgical instruments involve detectability and sterilization. A significant issue in surgery is the accidental leaving behind of instruments in patients, which can cause serious problems ranging from organ perforation and blood infection to death. Although instrument counting and other safety protocols can reduce the risk of overlooking an instrument, mistakes are bound to happen. The other shortcoming is the influence of repeated sterilization on the mechanical properties of the composite materials, as sterilization is mandatory for surgical instruments used in the operation room. The structural integrity and overall performance of the polymer composite materials—especially the stability of the interface and the interphase zones—are strongly influenced by repeated sterilization.
On the other hand, composite materials have potential advantages that may support their introduction into long-term medical implant applications, as sterilization commonly is performed only once, during packaging. The effects of sterilization by radiation or steam are much less pronounced on composite implants than on composite surgical instruments. However, composite implants require careful consideration with respect to the bioactivity of wear particles that may be produced from articulation. Further, carbon-fiber–reinforced polymer implants are still substantially more difficult to manufacture and more costly than their metallic counterparts.46
Limitations
This study has some limitations. Most important, studies of this nature do not account for biological factors such as corrosion, biological wear, and the soft-tissue attachment effects on structural properties for potential in vivo use. Another limitation was that the study tested only the mechanical properties in terms of SBS and provided no information about other mechanical properties, such as tensile, compression, and torsion strengths. We think SBS testing adequately evaluates challenging scenarios like thin and narrow instruments/devices that are anticipated in application, and information regarding other modes of failure and mechanical properties (compression, tension, torsion) would be a further area of research. An additional limitation was that our model used a relatively small number of samples. A larger study with more samples and varying layout patterns and layers of the carbon fibers may more clearly demonstrate the effect of steam sterilization on composite materials.
Conclusion
This study provided new information on 3 selected composite materials and their structural properties after repeated steam sterilization. We discovered that these composites were similar in radiographic density and water retention but behaved very differently in terms of mechanical durability after repeated steam sterilization. All selected composites demonstrated deterioration of mechanical properties after repeated steam sterilization. Knowing these results could aid in making decisions about the design and manufacturing of operative instruments and orthopedic biomaterials. Although our preliminary findings are intriguing, further study is warranted to seek specific applications for these composite materials in orthopedic surgery.
1. Ali MS, French TA, Hastings GW, et al. Carbon fibre composite bone plates. Development, evaluation and early clinical experience. J Bone Joint Surg Br. 1990;72(4):586-591.
2. Brooks RA, Jones E, Storer A, Rushton N. Biological evaluation of carbon-fibre–reinforced polybutyleneterephthalate (CFRPBT) employed in a novel acetabular cup. Biomaterials. 2004;25(17):3429-3438.
3. Brown SA, Hastings RS, Mason JJ, Moet A. Characterization of short-fibre reinforced thermoplastics for fracture fixation devices. Biomaterials. 1990;11(8):541-547.
4. Skinner HB. Composite technology for total hip arthroplasty. Clin Orthop Relat Res. 1988;(235):224-236.
5. Field RE, Jones E, Nuijten P, Storer A, Cronin M, Rushton N. Pre-clinical evaluation of the Cambridge acetabular cup. J Mater Sci Mater Med. 2008;19(8):2791-2798.
6. Han N, Ahmed I, Parsons AJ, et al. Influence of screw holes and gamma sterilization on properties of phosphate glass fiber–reinforced composite bone plates. J Biomater Appl. 2013;27(8):990-1002.
7. Losi P, Munaò A, Spiller D, et al. Evaluation of a new composite prosthesis for the repair of abdominal wall defects. J Mater Sci Mater Med. 2007;18(10):1939-1944.
8. Pait TG, Kaufman HH, Voelker JL, McAllister HP, Willison C. Use of a carbon composite radiolucent anterior cervical retractor system. Neurosurgery. 1993;33(5):941-942.
9. Elfar J, Menorca RM, Reed JD, Stanbury S. Composite bone models in orthopaedic surgery research and education. J Am Acad Orthop Surg. 2014;22(2):111-120.
10. Gardner MP, Chong AC, Pollock AG, Wooley PH. Mechanical evaluation of large-size fourth-generation composite femur and tibia models. Ann Biomed Eng. 2010;38(3):613-620.
11. Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284.
12. Dunlap JT, Chong AC, Lucas GL, Cooke FW. Structural properties of a novel design of composite analogue humeri models. Ann Biomed Eng. 2008;36(11):1922-1926.
13. Grover P, Albert C, Wang M, Harris GF. Mechanical characterization of fourth generation composite humerus. Proc Inst Mech Eng H. 2011;225(12):1169-1176.
14. Zheng Q, Morgan RJ. Synergistic thermal-moisture damage mechanisms of epoxies and their carbon fiber composites. J Compos Mater. 1993;27(15):1465-1478.
15. Ray BC. Temperature effect during humid ageing on interfaces of glass and carbon fibers reinforced epoxy composites. J Colloid Interface Sci. 2006;298(1):111-117.
16. Standard test method for short-beam strength of polymer matrix composite materials and their laminates [ASTM specification D2344/D2344M-00]. In: Annual Book of ASTM Standards. Vol 15.03. West Conshohocken, PA: American Society for Testing and Materials; 2006.
17. Bradley JS, Hastings GW, Johnson-Nurse C. Carbon fibre reinforced epoxy as a high strength, low modulus material for internal fixation plates. Biomaterials. 1980;1(1):38-40.
18. McKenna GB, Bradley GW, Dunn HK, Statton WO. Mechanical properties of some fibre reinforced polymer composites after implantation as fracture fixation plates. Biomaterials. 1980;1(4):189-192.
19. Tayton K, Johnson-Nurse C, McKibbin B, Bradley J, Hastings G. The use of semi-rigid carbon-fibre–reinforced plastic plates for fixation of human fractures. Results of preliminary trials. J Bone Joint Surg Br. 1982;64(1):105-111.
20. Tayton K, Bradley J. How stiff should semi-rigid fixation of the human tibia be? A clue to the answer. J Bone Joint Surg Br. 1983;65(3):312-315.
21. Tayton K. Corrosive effect of carbon-fibre reinforced plastic on stainless-steel screws during implantation into man. J Med Eng Technol. 1983;7(1):24-26.
22. Howard CB, Tayton KJ, Gibbs A. The response of human tissues to carbon reinforced epoxy resin. J Bone Joint Surg Br. 1985;67(4):656-658.
23. Skirving AP, Day R, Macdonald W, McLaren R. Carbon fiber reinforced plastic (CFRP) plates versus stainless steel dynamic compression plates in the treatment of fractures of the tibiae in dogs. Clin Orthop Relat Res. 1987;(224):117-124.
24. Prakash R, Marwah S, Goel SC, Tuli SM. Carbon fibre reinforced epoxy implants for bridging large osteoperiosteal gaps. Biomaterials. 1988;9(2):198-202.
25. Pemberton DJ, McKibbin B, Savage R, Tayton K, Stuart D. Carbon-fibre reinforced plates for problem fractures. J Bone Joint Surg Br. 1992;74(1):88-92.
26. Pemberton DJ, Evans PD, Grant A, McKibbin B. Fractures of the distal femur in the elderly treated with a carbon fibre supracondylar plate. Injury. 1994;25(5):317-321.
27. Kelsey DJ, Springer GS, Goodman SB. Composite implant for bone replacement. J Compos Mater. 1997;31(16):1593-1632.
28. Corvelli AA, Biermann PJ, Roberts JC. Design, analysis and fabrication of a composite segmental bone replacement implant. J Adv Mater. 1997;28:2-8.
29. Glassman AH, Crowninshield RD, Schenck R, Herberts P. A low stiffness composite biologically fixed prosthesis. Clin Orthop Relat Res. 2001;(393):128-136.
30. Williams D. New horizons for thermoplastic polymers. Med Device Technol. 2001;12(4):8-9.
31. Al-Shawi AK, Smith SP, Anderson GH. The use of a carbon fiber plate for periprosthetic supracondylar femoral fractures. J Arthroplasty. 2002;17(3):320-324.
32. Baker D, Kadambande SS, Alderman PM. Carbon fibre plates in the treatment of femoral periprosthetic fractures. Injury. 2004;35(6):596-598.
33. Akhavan S, Matthiesen MM, Schulte L, et al. Clinical and histologic results related to a low-modulus composite total hip replacement stem. J Bone Joint Surg Am. 2006;88(6):1308-1314.
34. Toth JM, Wang M, Estes BT, Scifert JL, Seim HB 3rd, Turner AS. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials. 2006;27(3):324-334.
35. Evans SL, Gregson PJ. Composite technology in load-bearing orthopaedic implants. Biomaterials. 1998;19(15):1329-1342.
36. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part I. Crystallinity and interface adhesion. Composites Part A. 2000;31(6):517-530.
37. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part II. Interlaminar fracture toughness. Composites Part A. 2001;32(6):763-774.
38. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part III. Impact damage performance. Composites Part A. 2001;32(6):775-785.
39. Guan SB, Hou CL, Chen AM, Zhang W, Wang JE. Influence of sterilization treatments on continuous carbon-fiber reinforced polyolefin composite. Zhonghua Yi Xue Za Zhi. 2007;87(31):2228-2231.
40. Akay M, Aslan N. An estimation of fatigue life for a carbon fibre/poly ether ether ketone hip joint prosthesis. Proc Inst Mech Eng H. 1995;209(2):93-103.
41. Bagheri ZS, Tavakkoli Awal P, Bougherara H, Aziz MS, Schemitsch EH, Zdero R. Biomechanical analysis of a new carbon fiber/flax/epoxy bone fracture plate shows less stress shielding compared to a standard clinical metal plate. J Biomech Eng. 2014;136(9):091002.
42. Rhee PC, Shin AY. The rate of successful four-corner arthrodesis with a locking, dorsal circular polyether-ether-ketone (PEEK-Optima) plate. J Hand Surg Eur Vol. 2013;38(7):767-773.
43. Nakahara I, Takao M, Bandoh S, Bertollo N, Walsh WR, Sugano N. In vivo implant fixation of carbon fiber–reinforced PEEK hip prostheses in an ovine model. J Orthop Res. 2013;31(3):485-492.
44. Kasliwal MK, O’Toole JE. Clinical experience using polyetheretherketone (PEEK) intervertebral structural cage for anterior cervical corpectomy and fusion. J Clin Neurosci. 2014;21(2):217-220.
45. Tarallo L, Mugnai R, Adani R, Zambianchi F, Catani F. A new volar plate made of carbon-fiber–reinforced polyetheretherketon for distal radius fracture: analysis of 40 cases. J Orthop Traumatol. 2014;15(4):277-283.
46. Cordey J, Perren SM, Steinemann SG. Stress protection due to plates: myth or reality? A parametric analysis made using the composite beam theory. Injury. 2000;31(suppl 3):C1-C13.
1. Ali MS, French TA, Hastings GW, et al. Carbon fibre composite bone plates. Development, evaluation and early clinical experience. J Bone Joint Surg Br. 1990;72(4):586-591.
2. Brooks RA, Jones E, Storer A, Rushton N. Biological evaluation of carbon-fibre–reinforced polybutyleneterephthalate (CFRPBT) employed in a novel acetabular cup. Biomaterials. 2004;25(17):3429-3438.
3. Brown SA, Hastings RS, Mason JJ, Moet A. Characterization of short-fibre reinforced thermoplastics for fracture fixation devices. Biomaterials. 1990;11(8):541-547.
4. Skinner HB. Composite technology for total hip arthroplasty. Clin Orthop Relat Res. 1988;(235):224-236.
5. Field RE, Jones E, Nuijten P, Storer A, Cronin M, Rushton N. Pre-clinical evaluation of the Cambridge acetabular cup. J Mater Sci Mater Med. 2008;19(8):2791-2798.
6. Han N, Ahmed I, Parsons AJ, et al. Influence of screw holes and gamma sterilization on properties of phosphate glass fiber–reinforced composite bone plates. J Biomater Appl. 2013;27(8):990-1002.
7. Losi P, Munaò A, Spiller D, et al. Evaluation of a new composite prosthesis for the repair of abdominal wall defects. J Mater Sci Mater Med. 2007;18(10):1939-1944.
8. Pait TG, Kaufman HH, Voelker JL, McAllister HP, Willison C. Use of a carbon composite radiolucent anterior cervical retractor system. Neurosurgery. 1993;33(5):941-942.
9. Elfar J, Menorca RM, Reed JD, Stanbury S. Composite bone models in orthopaedic surgery research and education. J Am Acad Orthop Surg. 2014;22(2):111-120.
10. Gardner MP, Chong AC, Pollock AG, Wooley PH. Mechanical evaluation of large-size fourth-generation composite femur and tibia models. Ann Biomed Eng. 2010;38(3):613-620.
11. Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284.
12. Dunlap JT, Chong AC, Lucas GL, Cooke FW. Structural properties of a novel design of composite analogue humeri models. Ann Biomed Eng. 2008;36(11):1922-1926.
13. Grover P, Albert C, Wang M, Harris GF. Mechanical characterization of fourth generation composite humerus. Proc Inst Mech Eng H. 2011;225(12):1169-1176.
14. Zheng Q, Morgan RJ. Synergistic thermal-moisture damage mechanisms of epoxies and their carbon fiber composites. J Compos Mater. 1993;27(15):1465-1478.
15. Ray BC. Temperature effect during humid ageing on interfaces of glass and carbon fibers reinforced epoxy composites. J Colloid Interface Sci. 2006;298(1):111-117.
16. Standard test method for short-beam strength of polymer matrix composite materials and their laminates [ASTM specification D2344/D2344M-00]. In: Annual Book of ASTM Standards. Vol 15.03. West Conshohocken, PA: American Society for Testing and Materials; 2006.
17. Bradley JS, Hastings GW, Johnson-Nurse C. Carbon fibre reinforced epoxy as a high strength, low modulus material for internal fixation plates. Biomaterials. 1980;1(1):38-40.
18. McKenna GB, Bradley GW, Dunn HK, Statton WO. Mechanical properties of some fibre reinforced polymer composites after implantation as fracture fixation plates. Biomaterials. 1980;1(4):189-192.
19. Tayton K, Johnson-Nurse C, McKibbin B, Bradley J, Hastings G. The use of semi-rigid carbon-fibre–reinforced plastic plates for fixation of human fractures. Results of preliminary trials. J Bone Joint Surg Br. 1982;64(1):105-111.
20. Tayton K, Bradley J. How stiff should semi-rigid fixation of the human tibia be? A clue to the answer. J Bone Joint Surg Br. 1983;65(3):312-315.
21. Tayton K. Corrosive effect of carbon-fibre reinforced plastic on stainless-steel screws during implantation into man. J Med Eng Technol. 1983;7(1):24-26.
22. Howard CB, Tayton KJ, Gibbs A. The response of human tissues to carbon reinforced epoxy resin. J Bone Joint Surg Br. 1985;67(4):656-658.
23. Skirving AP, Day R, Macdonald W, McLaren R. Carbon fiber reinforced plastic (CFRP) plates versus stainless steel dynamic compression plates in the treatment of fractures of the tibiae in dogs. Clin Orthop Relat Res. 1987;(224):117-124.
24. Prakash R, Marwah S, Goel SC, Tuli SM. Carbon fibre reinforced epoxy implants for bridging large osteoperiosteal gaps. Biomaterials. 1988;9(2):198-202.
25. Pemberton DJ, McKibbin B, Savage R, Tayton K, Stuart D. Carbon-fibre reinforced plates for problem fractures. J Bone Joint Surg Br. 1992;74(1):88-92.
26. Pemberton DJ, Evans PD, Grant A, McKibbin B. Fractures of the distal femur in the elderly treated with a carbon fibre supracondylar plate. Injury. 1994;25(5):317-321.
27. Kelsey DJ, Springer GS, Goodman SB. Composite implant for bone replacement. J Compos Mater. 1997;31(16):1593-1632.
28. Corvelli AA, Biermann PJ, Roberts JC. Design, analysis and fabrication of a composite segmental bone replacement implant. J Adv Mater. 1997;28:2-8.
29. Glassman AH, Crowninshield RD, Schenck R, Herberts P. A low stiffness composite biologically fixed prosthesis. Clin Orthop Relat Res. 2001;(393):128-136.
30. Williams D. New horizons for thermoplastic polymers. Med Device Technol. 2001;12(4):8-9.
31. Al-Shawi AK, Smith SP, Anderson GH. The use of a carbon fiber plate for periprosthetic supracondylar femoral fractures. J Arthroplasty. 2002;17(3):320-324.
32. Baker D, Kadambande SS, Alderman PM. Carbon fibre plates in the treatment of femoral periprosthetic fractures. Injury. 2004;35(6):596-598.
33. Akhavan S, Matthiesen MM, Schulte L, et al. Clinical and histologic results related to a low-modulus composite total hip replacement stem. J Bone Joint Surg Am. 2006;88(6):1308-1314.
34. Toth JM, Wang M, Estes BT, Scifert JL, Seim HB 3rd, Turner AS. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials. 2006;27(3):324-334.
35. Evans SL, Gregson PJ. Composite technology in load-bearing orthopaedic implants. Biomaterials. 1998;19(15):1329-1342.
36. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part I. Crystallinity and interface adhesion. Composites Part A. 2000;31(6):517-530.
37. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part II. Interlaminar fracture toughness. Composites Part A. 2001;32(6):763-774.
38. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part III. Impact damage performance. Composites Part A. 2001;32(6):775-785.
39. Guan SB, Hou CL, Chen AM, Zhang W, Wang JE. Influence of sterilization treatments on continuous carbon-fiber reinforced polyolefin composite. Zhonghua Yi Xue Za Zhi. 2007;87(31):2228-2231.
40. Akay M, Aslan N. An estimation of fatigue life for a carbon fibre/poly ether ether ketone hip joint prosthesis. Proc Inst Mech Eng H. 1995;209(2):93-103.
41. Bagheri ZS, Tavakkoli Awal P, Bougherara H, Aziz MS, Schemitsch EH, Zdero R. Biomechanical analysis of a new carbon fiber/flax/epoxy bone fracture plate shows less stress shielding compared to a standard clinical metal plate. J Biomech Eng. 2014;136(9):091002.
42. Rhee PC, Shin AY. The rate of successful four-corner arthrodesis with a locking, dorsal circular polyether-ether-ketone (PEEK-Optima) plate. J Hand Surg Eur Vol. 2013;38(7):767-773.
43. Nakahara I, Takao M, Bandoh S, Bertollo N, Walsh WR, Sugano N. In vivo implant fixation of carbon fiber–reinforced PEEK hip prostheses in an ovine model. J Orthop Res. 2013;31(3):485-492.
44. Kasliwal MK, O’Toole JE. Clinical experience using polyetheretherketone (PEEK) intervertebral structural cage for anterior cervical corpectomy and fusion. J Clin Neurosci. 2014;21(2):217-220.
45. Tarallo L, Mugnai R, Adani R, Zambianchi F, Catani F. A new volar plate made of carbon-fiber–reinforced polyetheretherketon for distal radius fracture: analysis of 40 cases. J Orthop Traumatol. 2014;15(4):277-283.
46. Cordey J, Perren SM, Steinemann SG. Stress protection due to plates: myth or reality? A parametric analysis made using the composite beam theory. Injury. 2000;31(suppl 3):C1-C13.
In Vitro and In Situ Characterization of Arthroscopic Loop Security and Knot Security of Braided Polyblend Sutures: A Biomechanical Study
Open-surgery knot tying is easily learned and performed, but knot tying during arthroscopic procedures can be both challenging and frustrating. According to Burkhart and colleagues,1,2 knot security is defined as the effectiveness of the knot in resisting slippage when load is applied, whereas loop security is the effectiveness in maintaining a tight suture loop while a knot is being tied. Arthroscopic knots commonly begin with an initial slipknot locked in place with a series of half-hitches. During arthroscopic surgery, the surgeon usually must tie an arthroscopic knot to obtain secure tissue fixation, an essential component of soft-tissue repair. A secure knot provides optimal tissue apposition for healing, which will ultimately improve functional outcome. For a knot to be effective, it must have both knot security and loop security. Knot security depends on knot configuration, the coefficient of friction, ductility, handling properties, solubility and diameter of suture material, internal interference, slack between throws, and surgeon experience. Tissue fluid and tissue reaction to suture material may affect knot and loop security.
The ideal knot would be easy to tie and reproducible and would not slip or stretch before tissue is healed. The ideal suture material should provide adequate strength to hold soft tissue in an anatomically correct position until healing can occur. It should also be easily and efficiently manipulated by arthroscopic means when tissues are being secured with knots and secure suture loops. Studies have been conducted to evaluate the security of knots tied with arthroscopic techniques, knot configurations, and suture materials, and these investigations have often evaluated knot performance under single load-to-failure (LTF) test scenarios and cyclic loading in vitro (dry environment) in a room-temperature environment.2-10 To our knowledge, few if any attempts have been made to simulate in situ conditions at body temperature when testing knot security. The fluid environment and the temperature could potentially affect the effectiveness of knots, as knot security depends on friction, internal interference, and slack between throws.1
We conducted a study to evaluate biomechanical performance (knot security, loop security) during destructive testing of several different suture materials with various arthroscopic knot configurations. The study was performed under in vitro (dry environment) and in situ (wet environment) conditions by surgeons with different levels of experience.
Materials and Methods
This investigation was conducted at the Orthopaedic Research Institute at Via Christi Health in Wichita, Kansas. The study compared 4 different suture materials tied with 3 different commonly used arthroscopic knots by 3 surgeons with different levels of experience. The 4 types of braided polyblend polyethylene sutures were Fiberwire (Arthrex, Naples, Florida), ForceFiber (Stryker, San Jose, California), Orthocord (DePuy-Mitek, Warsaw, Indiana), and Ultrabraid (Smith & Nephew, Memphis, Tennessee). Each suture material was tied with 3 arthroscopic knots—static surgeon’s knot, Weston knot,11 Tennessee slider12—and a series of 3 reversing half-hitches on alternating posts (RHAPs) (Figure 1). These knots were chosen based on studies showing they have a higher maximum force to failure when combined with 3 RHAPs.1,2,5,9,13-17
We evaluated performer variability with the help of 3 investigator-surgeons who differed in their level of experience tying arthroscopic knots. This experience was defined on the basis of total number of arthroscopies performed—one of the most important factors predicting basic arthroscopic skills. Our surgeon A was a sports medicine fellowship–trained surgeon with 10 years of experience and a significant number of arthroscopies performed annually (350); surgeon B was a sports medicine fellowship–trained surgeon with 3 years of experience and an annual arthroscopy volume of more than 250 procedures; and surgeon C was a third-year orthopedic resident with about 100 arthroscopies performed.
All knots were tied on a standardized post 30 mm in circumference, which provided a consistent starting circumference for each knot and replicated the suture loop created during arthroscopic rotator cuff repair. All knots were tied using standard arthroscopic techniques, with a standard knot pusher and a modified arthroscopic cannula, in a dry environment (Figure 2). Servohydraulic materials testing system instruments (model 810; MTS Systems, Eden Prairie, Minnesota) were used to test the knot security and loop security of each combination of knots and suture types. Two round hooks (diameter, 3.9 mm) were attached to the actuator and the load cell (Figure 3). Loops were preloaded to 6 N to avoid potential errors caused by slack in the loops or by stretching of suture materials and to provide a well-defined starting point for data recording.
LTF testing was performed for both in vitro and in situ conditions using 10 samples of each suture–knot configuration for each mechanical testing. Each type of testing was conducted for a total of 240 suture–knot combinations per investigator. For the in vitro condition, each suture loop was initiated with 5 preconditioning loading cycles, from 6 N to 30 N at 1 Hz. The load was then applied continuously at a crosshead speed of 1 mm/s until “clinical failure” (3 mm crosshead displacement). We used this criterion for clinical failure, as studies have indicated that 3 mm is the point at which tissue apposition is lost.15,18-21 After the crosshead reached the 3-mm displacement, the loads (under load control) were held for 5 minutes at maximum load, and then load was applied continuously at a crosshead speed of 1 mm/s until complete structure failure. Load and displacement data were collected at a frequency of 20 Hz.
For the in situ condition, the same test parameters were used, except that each combination of the suture loop was preloaded to 6 N and soaked in physiologic solution bath (human blood plasma) at 37°C (body temperature) for 24 hours before testing in an effort to simulate the aqueous medium in vivo after surgery. The in situ tests were performed under physiologic solution maintained at 37°C to approximate postoperative physical conditions.
Statistical Analysis
Means and standard deviations of the knot security and loop security achieved by the surgeons (different experience levels) were calculated for each test configuration and each test condition. These values were used to determine the statistical relevance of the difference in arthroscopic loop security and knot security in each configuration. One-way analysis of variance (ANOVA) performed with SPSS Version 19.0 software (SPSS, Chicago, Illinois) with the least significant difference (LSD) multiple comparisons post hoc analysis was used to determine if any observed differences between the types of braided polyblend sutures, the types of sliding knots, the test conditions (in vitro, in situ), and the levels of surgeon experience were significant for each knot configuration. The level of significance of differences was set at P < .001.
Results
Figure 4 shows the mean maximum clinical failure load (3 mm of displacement) of different arthroscopic knot configurations for different braided polyblend sutures by surgeons of different levels of experience. In the comparison of biomechanical performance (knot and loop security) under in vitro and in situ conditions, no significant difference was detected when Ultrabraid suture material was used, regardless of surgeon experience, for all knot configurations. For surgeon B, there was no significant difference between in vitro and in situ conditions for any knot configurations or suture materials. When Orthocord suture material was used, Weston knots tied by surgeon A, and static surgeon’s knots by surgeons A and C, resulted in a significant difference between the in vitro and in situ conditions. When ForceFiber suture material was used, only Weston knots and Tennessee slider knots by surgeon A had a significant difference between in vitro and in situ conditions. Weston knots by surgeon A exhibited a significant difference between in vitro and in situ conditions, except when Ultrabraid suture material was used.
Surgeon C’s Tennessee slider knots with all polyblend sutures showed significantly lower loads at clinical failure compared with all the other knot configurations and with knots tied by the other 2 surgeons under both in vitro and in situ conditions. Overall, knots tied by surgeon B had higher clinical failure load than knots tied by the other 2 surgeons.
Figure 5 shows the mean ultimate failure load (complete structural failure) of different arthroscopic knot configurations for different braided polyblend sutures by surgeons of different levels of experience. Knots tied with Orthocord suture material had the overall lower ultimate failure load compared with other suture materials, whereas knots tied with Ultrabraid suture material had the overall highest ultimate failure load. However, the ultimate failure loads for all the knots tied using any suture material, regardless of surgeon experience, were more than 61 N, which is the estimated minimum required ultimate load per suture during a maximum muscle contraction.1
Figure 6 shows the percentage of knot slipping at constant clinical failure load. Orthocord and Fiberwire suture materials had the lowest incidence of knot slippage. Surgeon C had complete knot slippage at constant clinical failure load using ForceFiber with the Weston knot and Ultrabraid with the Tennessee slider knot. When using Ultrabraid or ForceFiber, surgeons A and C had at least 2 knots slip for all knot configurations.
Discussion
Optimization of knot security for any given knot configuration, suture material, and surgeon experience level during arthroscopic knot tying is crucial.1-10 Our study results showed that, under single LTF test scenarios, there was a significant difference between in vitro and in situ conditions with respect to both knot configuration and surgeon experience level, except when Ultrabraid suture material was used. Arthroscopic sliding knots are lockable or nonlockable.7,12 With lockable sliding knots, slippage may be prevented by tensioning the wrapping limb, which distorts the post in the distal part of the knot, resulting in a kink in the post, thereby increasing the internal interference that increases the resistance of the knot from backing off. With nonlockable sliding knots, slippage may be prevented by the tight grip of the wrappings around the initial post.7 The static surgeon’s knot and the Tennessee slider knot are nonlockable, whereas the Weston knot is a distal lockable sliding knot. Compared with nonlockable sliding knots, lockable sliding knots cause less suture loop enlargement. In 1976, Tera and Aberg22 studied the strength of knotted thread for 12 different types of suture knots combined with 11 types of suture material. They conducted their study 1 week after suture material was inserted into the subcutaneous tissue of rabbits. Their results show a greater propensity for certain suture materials to slip when tested in an aqueous environment. In 1998, Babetty and colleagues23 used Wistar rats to compare the in vivo strength, knot efficiency, and knot security of 4 types of sliding knots and to assess tissue reaction as a result of knot configuration, knot volume, and suture size. They found that 4/0 knots lost more strength than 2/0 knots did, and they concluded that the tissue response to all the knots, except 2/0 nylon, was similar. They indicated that the inflammatory sheath volume varied with knot volume, suture size, and knot configuration. Our results agree with observations that exposure to an aqueous environment alters the force to clinical failure of comparable suture and knot configurations.
In addition, our findings indicate that surgeon familiarity with certain knots has a major effect on knot security. The difference in our 3 surgeons’ levels of familiarity with certain knots was somewhat minimized by the knot tying they practiced before submitting knots for testing. The findings contrast with those of Milia and colleagues,24 who conducted a biomechanical study to determine the effect of experience level on knot security. They compared an experienced arthroscopic shoulder surgeon with a junior-level orthopedic resident surgeon and concluded that experience did not affect knot security. However, the knots in their study were tied by hand, not through an arthroscopic cannula with instruments. Our findings suggest that both experienced and less experienced orthopedic residents should be encouraged to practice arthroscopic knot tying in a nonsurgical environment in order to become comfortable tying arthroscopic knots.
Braided nonabsorbable polyester suture traditionally has been found to be stronger than monofilament absorbable polydioxanone (PDS) and to have less slippage potential.8,9,25 Several studies have determined that the braided polyblend sutures now commonly used for arthroscopic knots have better strength profiles over more traditional materials.12,26,27 Orthocord has a dyed absorbable core (PDS, 68%), an undyed nonabsorbable ultrahigh-molecular-weight polyethylene (UHMWPE, 32%) sleeve, and a polyglactin coating.9,10 Both Ultrabraid and ForceFiber are made with braided UHMWPE and have just a few variations in weave patterns. Fiberwire has a multifiber UHMWPE core covered with braided polyester suture material. Several biomechanical studies25,26,28 have evaluated different arthroscopic sliding knot configurations with different suture materials, and all concluded that a surgeon who is choosing an arthroscopic repair technique should know the differences in suture materials and the knot strengths afforded by different knot configurations, as suture material is an important aspect of loop security. Our findings agree with their findings, that suture materials have a major effect on knot security, even with a series of 3 RHAPs, as in theory the RHAPs should minimize suture friction, internal interference, and slack between knot loops—emphasizing the effect of material selection. Furthermore, our findings also indicated that suture materials with a core in their design (Fiberwire, Orthocord) tend to have the lowest incidence of knot slippage. We had suspected that suture surface characteristics and suture construction could be important factors in knot slippage.
Our experimental design had its limitations. First, although we simulated factors such as temperature, plasma environment, and surgeon experience, tying a knot on a standardized post (30 mm in circumference) differed from what is typically done clinically. Second, the metal hooks used in this study were not compressible and did not interpose in the substance of the knot as soft tissue does in the clinical setting. Third, knots were tied with no tension against the sutures, whereas clinically knots are tied under tension as tissues are pulled together in reconstructions. Fourth, it was assumed that soaking in a physiologic solution bath (human blood plasma) at 37°C (body temperature) for 24 hours before testing was sufficient to simulate the aqueous medium in vivo after surgery, but these parameters may not represent conditions in a patient who has just undergone an arthroscopic shoulder repair and adheres to a passive motion protocol. Fifth, there was no blinding of knot type, and there was no randomization of tying order or testing order. Sixth, only a single LTF test was performed, and incremental cyclic loading can be more useful, as it has long been recognized as a leading source of failure in orthopedic repairs.
Conclusion
These study results advance our overall understanding of the biomechanics of the different knot configurations and loop security levels of the different braided polyblend sutures used in arthroscopic procedures through LTF in both in vitro and in situ conditions. Overall, no suture material was superior to any other in a fluid environment, as the combination of aqueous environment and surgeon level of experience with arthroscopic knot tying has a major effect on knot security under single LTF test scenarios. However, our data showed that Ultrabraid suture material had no effect on knot effectiveness over the fluid environment and the temperature. Furthermore, the study showed that the Tennessee slider knot had the steepest learning curve. This study may provide an alternative arthroscopic knots option for soft-tissue repair in which use of certain suture materials is limited.
1. Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Knot security in simple sliding knots and its relationship to rotator cuff repair: how secure must the knot be? Arthroscopy. 2000;16(2):202-207.
2. Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Loop security as a determinant of tissue fixation security. Arthroscopy. 1998;14(7):773-776.
3. Elkousy H, Hammerman SM, Edwards TB, et al. The arthroscopic square knot: a biomechanical comparison with open and arthroscopic knots. Arthroscopy. 2006;22(7):736-741.
4. Elkousy HA, Sekiya JK, Stabile KJ, McMahon PJ. A biomechanical comparison of arthroscopic sliding and sliding-locking knots. Arthroscopy. 2005;21(2):204-210.
5. Ilahi OA, Younas SA, Alexander J, Noble PC. Cyclic testing of arthroscopic knot security. Arthroscopy. 2004;20(1):62-68.
6. Loutzenheiser TD, Harryman DT 2nd, Ziegler DW, Yung SW. Optimizing arthroscopic knots using braided or monofilament suture. Arthroscopy. 1998;14(1):57-65.
7. Chan KC, Burkhart SS, Thiagarajan P, Goh JC. Optimization of stacked half-hitch knots for arthroscopic surgery. Arthroscopy. 2001;17(7):752-759.
8. Lee TQ, Matsuura PA, Fogolin RP, Lin AC, Kim D, McMahon PJ. Arthroscopic suture tying: a comparison of knot types and suture materials. Arthroscopy. 2001;17(4):348-352.
9. Mishra DK, Cannon WD Jr, Lucas DJ, Belzer JP. Elongation of arthroscopically tied knots. Am J Sports Med. 1997;25(1):113-117.
10. Kim SH, Ha KI, Kim SH, Kim JS. Significance of the internal locking mechanism for loop security enhancement in the arthroscopic knot. Arthroscopy. 2001;17(8):850-855.
11. Weston PV. A new clinch knot. Obstet Gynecol. 1991;78(1):144-147.
12. Lo IK, Burkhart SS, Chan KC, Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489-502.
13. Lo IK, Burkhart SS, Athanasiou K. Abrasion resistance of two types of nonabsorbable braided suture. Arthroscopy. 2004;20(4):407-413.
14. De Beer JF, van Rooyen K, Boezaart AP. Nicky’s knot—a new slip knot for arthroscopic surgery. Arthroscopy. 1998;14(1):109-110.
15. Loutzenheiser TD, Harryman DT 2nd, Yung SW, France MP, Sidles JA. Optimizing arthroscopic knots. Arthroscopy. 1995;11(2):199-206.
16. Wetzler MJ, Bartolozzi AR, Gillespie MJ, et al. Fatigue properties of suture anchors in anterior shoulder reconstructions: Mitek GII. Arthroscopy. 1996;12(6):687-693.
17. Barber FA, Herbert MA, Beavis RC. Cyclic load and failure behavior of arthroscopic knots and high strength sutures. Arthroscopy. 2009;25(2):192-199.
18. Richmond JC. A comparison of ultrasonic suture welding and traditional knot tying. Am J Sports Med. 200;29(3):297-299.
19. James JD, Wu MM, Batra EK, Rodeheaver GT, Edlich RF. Technical considerations in manual and instrument tying techniques. J Emerg Med. 1992;10(4):469-480.
20. Batra EK, Franz DA, Towler MA, et al. Influence of emergency physician’s tying technique on knot security. J Emerg Med. 1992;10(3):309-316.
21. Livermore RW, Chong AC, Prohaska DJ, Cooke FW, Jones TL. Knot security, loop security and elongation of braided polyblend sutures used for arthroscopic knots. Am J Orthop. 2010;39(12):569-576.
22. Tera H, Aberg C. The strength of suture knots after one week in vivo. Acta Chir Scand. 1976;142(4):301-307.
23. Babetty Z, Sümer A, Altintaş S, Ergüney S, Göksel S. Changes in knot-holding capacity of sliding knots in vivo and tissue reaction. Arch Surg. 1998;133(7):727-734.
24. Milia MJ, Peindl RD, Connor PM. Arthroscopic knot tying: the role of instrumentation in achieving knot security. Arthroscopy. 2005;21(1):69-76.
25. Lieurance RK, Pflaster DS, Abbott D, Nottage WM. Failure characteristics of various arthroscopically tied knots. Clin Orthop. 2003;(408):311-318.
26. Abbi G, Espinoza L, Odell T, Mahar A, Pedowitz R. Evaluation of 5 knots and 2 suture materials for arthroscopic rotator cuff repair: very strong sutures can still slip. Arthroscopy. 2006;22(1):38-43.
27. Wüst DM, Meyer DC, Favre P, Gerber C. Mechanical and handling properties of braided polyblend polyethylene sutures in comparison to braided polyester and monofilament polydioxanone sutures. Arthroscopy. 2006;22(11):1146-1153.
28. Mahar AT, Moezzi DM, Serra-Hsu F, Pedowitz RA. Comparison and performance characteristics of 3 different knots when tied with 2 suture materials used for shoulder arthroscopy. Arthroscopy. 2006;22(6):614.e1-e2.
Open-surgery knot tying is easily learned and performed, but knot tying during arthroscopic procedures can be both challenging and frustrating. According to Burkhart and colleagues,1,2 knot security is defined as the effectiveness of the knot in resisting slippage when load is applied, whereas loop security is the effectiveness in maintaining a tight suture loop while a knot is being tied. Arthroscopic knots commonly begin with an initial slipknot locked in place with a series of half-hitches. During arthroscopic surgery, the surgeon usually must tie an arthroscopic knot to obtain secure tissue fixation, an essential component of soft-tissue repair. A secure knot provides optimal tissue apposition for healing, which will ultimately improve functional outcome. For a knot to be effective, it must have both knot security and loop security. Knot security depends on knot configuration, the coefficient of friction, ductility, handling properties, solubility and diameter of suture material, internal interference, slack between throws, and surgeon experience. Tissue fluid and tissue reaction to suture material may affect knot and loop security.
The ideal knot would be easy to tie and reproducible and would not slip or stretch before tissue is healed. The ideal suture material should provide adequate strength to hold soft tissue in an anatomically correct position until healing can occur. It should also be easily and efficiently manipulated by arthroscopic means when tissues are being secured with knots and secure suture loops. Studies have been conducted to evaluate the security of knots tied with arthroscopic techniques, knot configurations, and suture materials, and these investigations have often evaluated knot performance under single load-to-failure (LTF) test scenarios and cyclic loading in vitro (dry environment) in a room-temperature environment.2-10 To our knowledge, few if any attempts have been made to simulate in situ conditions at body temperature when testing knot security. The fluid environment and the temperature could potentially affect the effectiveness of knots, as knot security depends on friction, internal interference, and slack between throws.1
We conducted a study to evaluate biomechanical performance (knot security, loop security) during destructive testing of several different suture materials with various arthroscopic knot configurations. The study was performed under in vitro (dry environment) and in situ (wet environment) conditions by surgeons with different levels of experience.
Materials and Methods
This investigation was conducted at the Orthopaedic Research Institute at Via Christi Health in Wichita, Kansas. The study compared 4 different suture materials tied with 3 different commonly used arthroscopic knots by 3 surgeons with different levels of experience. The 4 types of braided polyblend polyethylene sutures were Fiberwire (Arthrex, Naples, Florida), ForceFiber (Stryker, San Jose, California), Orthocord (DePuy-Mitek, Warsaw, Indiana), and Ultrabraid (Smith & Nephew, Memphis, Tennessee). Each suture material was tied with 3 arthroscopic knots—static surgeon’s knot, Weston knot,11 Tennessee slider12—and a series of 3 reversing half-hitches on alternating posts (RHAPs) (Figure 1). These knots were chosen based on studies showing they have a higher maximum force to failure when combined with 3 RHAPs.1,2,5,9,13-17
We evaluated performer variability with the help of 3 investigator-surgeons who differed in their level of experience tying arthroscopic knots. This experience was defined on the basis of total number of arthroscopies performed—one of the most important factors predicting basic arthroscopic skills. Our surgeon A was a sports medicine fellowship–trained surgeon with 10 years of experience and a significant number of arthroscopies performed annually (350); surgeon B was a sports medicine fellowship–trained surgeon with 3 years of experience and an annual arthroscopy volume of more than 250 procedures; and surgeon C was a third-year orthopedic resident with about 100 arthroscopies performed.
All knots were tied on a standardized post 30 mm in circumference, which provided a consistent starting circumference for each knot and replicated the suture loop created during arthroscopic rotator cuff repair. All knots were tied using standard arthroscopic techniques, with a standard knot pusher and a modified arthroscopic cannula, in a dry environment (Figure 2). Servohydraulic materials testing system instruments (model 810; MTS Systems, Eden Prairie, Minnesota) were used to test the knot security and loop security of each combination of knots and suture types. Two round hooks (diameter, 3.9 mm) were attached to the actuator and the load cell (Figure 3). Loops were preloaded to 6 N to avoid potential errors caused by slack in the loops or by stretching of suture materials and to provide a well-defined starting point for data recording.
LTF testing was performed for both in vitro and in situ conditions using 10 samples of each suture–knot configuration for each mechanical testing. Each type of testing was conducted for a total of 240 suture–knot combinations per investigator. For the in vitro condition, each suture loop was initiated with 5 preconditioning loading cycles, from 6 N to 30 N at 1 Hz. The load was then applied continuously at a crosshead speed of 1 mm/s until “clinical failure” (3 mm crosshead displacement). We used this criterion for clinical failure, as studies have indicated that 3 mm is the point at which tissue apposition is lost.15,18-21 After the crosshead reached the 3-mm displacement, the loads (under load control) were held for 5 minutes at maximum load, and then load was applied continuously at a crosshead speed of 1 mm/s until complete structure failure. Load and displacement data were collected at a frequency of 20 Hz.
For the in situ condition, the same test parameters were used, except that each combination of the suture loop was preloaded to 6 N and soaked in physiologic solution bath (human blood plasma) at 37°C (body temperature) for 24 hours before testing in an effort to simulate the aqueous medium in vivo after surgery. The in situ tests were performed under physiologic solution maintained at 37°C to approximate postoperative physical conditions.
Statistical Analysis
Means and standard deviations of the knot security and loop security achieved by the surgeons (different experience levels) were calculated for each test configuration and each test condition. These values were used to determine the statistical relevance of the difference in arthroscopic loop security and knot security in each configuration. One-way analysis of variance (ANOVA) performed with SPSS Version 19.0 software (SPSS, Chicago, Illinois) with the least significant difference (LSD) multiple comparisons post hoc analysis was used to determine if any observed differences between the types of braided polyblend sutures, the types of sliding knots, the test conditions (in vitro, in situ), and the levels of surgeon experience were significant for each knot configuration. The level of significance of differences was set at P < .001.
Results
Figure 4 shows the mean maximum clinical failure load (3 mm of displacement) of different arthroscopic knot configurations for different braided polyblend sutures by surgeons of different levels of experience. In the comparison of biomechanical performance (knot and loop security) under in vitro and in situ conditions, no significant difference was detected when Ultrabraid suture material was used, regardless of surgeon experience, for all knot configurations. For surgeon B, there was no significant difference between in vitro and in situ conditions for any knot configurations or suture materials. When Orthocord suture material was used, Weston knots tied by surgeon A, and static surgeon’s knots by surgeons A and C, resulted in a significant difference between the in vitro and in situ conditions. When ForceFiber suture material was used, only Weston knots and Tennessee slider knots by surgeon A had a significant difference between in vitro and in situ conditions. Weston knots by surgeon A exhibited a significant difference between in vitro and in situ conditions, except when Ultrabraid suture material was used.
Surgeon C’s Tennessee slider knots with all polyblend sutures showed significantly lower loads at clinical failure compared with all the other knot configurations and with knots tied by the other 2 surgeons under both in vitro and in situ conditions. Overall, knots tied by surgeon B had higher clinical failure load than knots tied by the other 2 surgeons.
Figure 5 shows the mean ultimate failure load (complete structural failure) of different arthroscopic knot configurations for different braided polyblend sutures by surgeons of different levels of experience. Knots tied with Orthocord suture material had the overall lower ultimate failure load compared with other suture materials, whereas knots tied with Ultrabraid suture material had the overall highest ultimate failure load. However, the ultimate failure loads for all the knots tied using any suture material, regardless of surgeon experience, were more than 61 N, which is the estimated minimum required ultimate load per suture during a maximum muscle contraction.1
Figure 6 shows the percentage of knot slipping at constant clinical failure load. Orthocord and Fiberwire suture materials had the lowest incidence of knot slippage. Surgeon C had complete knot slippage at constant clinical failure load using ForceFiber with the Weston knot and Ultrabraid with the Tennessee slider knot. When using Ultrabraid or ForceFiber, surgeons A and C had at least 2 knots slip for all knot configurations.
Discussion
Optimization of knot security for any given knot configuration, suture material, and surgeon experience level during arthroscopic knot tying is crucial.1-10 Our study results showed that, under single LTF test scenarios, there was a significant difference between in vitro and in situ conditions with respect to both knot configuration and surgeon experience level, except when Ultrabraid suture material was used. Arthroscopic sliding knots are lockable or nonlockable.7,12 With lockable sliding knots, slippage may be prevented by tensioning the wrapping limb, which distorts the post in the distal part of the knot, resulting in a kink in the post, thereby increasing the internal interference that increases the resistance of the knot from backing off. With nonlockable sliding knots, slippage may be prevented by the tight grip of the wrappings around the initial post.7 The static surgeon’s knot and the Tennessee slider knot are nonlockable, whereas the Weston knot is a distal lockable sliding knot. Compared with nonlockable sliding knots, lockable sliding knots cause less suture loop enlargement. In 1976, Tera and Aberg22 studied the strength of knotted thread for 12 different types of suture knots combined with 11 types of suture material. They conducted their study 1 week after suture material was inserted into the subcutaneous tissue of rabbits. Their results show a greater propensity for certain suture materials to slip when tested in an aqueous environment. In 1998, Babetty and colleagues23 used Wistar rats to compare the in vivo strength, knot efficiency, and knot security of 4 types of sliding knots and to assess tissue reaction as a result of knot configuration, knot volume, and suture size. They found that 4/0 knots lost more strength than 2/0 knots did, and they concluded that the tissue response to all the knots, except 2/0 nylon, was similar. They indicated that the inflammatory sheath volume varied with knot volume, suture size, and knot configuration. Our results agree with observations that exposure to an aqueous environment alters the force to clinical failure of comparable suture and knot configurations.
In addition, our findings indicate that surgeon familiarity with certain knots has a major effect on knot security. The difference in our 3 surgeons’ levels of familiarity with certain knots was somewhat minimized by the knot tying they practiced before submitting knots for testing. The findings contrast with those of Milia and colleagues,24 who conducted a biomechanical study to determine the effect of experience level on knot security. They compared an experienced arthroscopic shoulder surgeon with a junior-level orthopedic resident surgeon and concluded that experience did not affect knot security. However, the knots in their study were tied by hand, not through an arthroscopic cannula with instruments. Our findings suggest that both experienced and less experienced orthopedic residents should be encouraged to practice arthroscopic knot tying in a nonsurgical environment in order to become comfortable tying arthroscopic knots.
Braided nonabsorbable polyester suture traditionally has been found to be stronger than monofilament absorbable polydioxanone (PDS) and to have less slippage potential.8,9,25 Several studies have determined that the braided polyblend sutures now commonly used for arthroscopic knots have better strength profiles over more traditional materials.12,26,27 Orthocord has a dyed absorbable core (PDS, 68%), an undyed nonabsorbable ultrahigh-molecular-weight polyethylene (UHMWPE, 32%) sleeve, and a polyglactin coating.9,10 Both Ultrabraid and ForceFiber are made with braided UHMWPE and have just a few variations in weave patterns. Fiberwire has a multifiber UHMWPE core covered with braided polyester suture material. Several biomechanical studies25,26,28 have evaluated different arthroscopic sliding knot configurations with different suture materials, and all concluded that a surgeon who is choosing an arthroscopic repair technique should know the differences in suture materials and the knot strengths afforded by different knot configurations, as suture material is an important aspect of loop security. Our findings agree with their findings, that suture materials have a major effect on knot security, even with a series of 3 RHAPs, as in theory the RHAPs should minimize suture friction, internal interference, and slack between knot loops—emphasizing the effect of material selection. Furthermore, our findings also indicated that suture materials with a core in their design (Fiberwire, Orthocord) tend to have the lowest incidence of knot slippage. We had suspected that suture surface characteristics and suture construction could be important factors in knot slippage.
Our experimental design had its limitations. First, although we simulated factors such as temperature, plasma environment, and surgeon experience, tying a knot on a standardized post (30 mm in circumference) differed from what is typically done clinically. Second, the metal hooks used in this study were not compressible and did not interpose in the substance of the knot as soft tissue does in the clinical setting. Third, knots were tied with no tension against the sutures, whereas clinically knots are tied under tension as tissues are pulled together in reconstructions. Fourth, it was assumed that soaking in a physiologic solution bath (human blood plasma) at 37°C (body temperature) for 24 hours before testing was sufficient to simulate the aqueous medium in vivo after surgery, but these parameters may not represent conditions in a patient who has just undergone an arthroscopic shoulder repair and adheres to a passive motion protocol. Fifth, there was no blinding of knot type, and there was no randomization of tying order or testing order. Sixth, only a single LTF test was performed, and incremental cyclic loading can be more useful, as it has long been recognized as a leading source of failure in orthopedic repairs.
Conclusion
These study results advance our overall understanding of the biomechanics of the different knot configurations and loop security levels of the different braided polyblend sutures used in arthroscopic procedures through LTF in both in vitro and in situ conditions. Overall, no suture material was superior to any other in a fluid environment, as the combination of aqueous environment and surgeon level of experience with arthroscopic knot tying has a major effect on knot security under single LTF test scenarios. However, our data showed that Ultrabraid suture material had no effect on knot effectiveness over the fluid environment and the temperature. Furthermore, the study showed that the Tennessee slider knot had the steepest learning curve. This study may provide an alternative arthroscopic knots option for soft-tissue repair in which use of certain suture materials is limited.
Open-surgery knot tying is easily learned and performed, but knot tying during arthroscopic procedures can be both challenging and frustrating. According to Burkhart and colleagues,1,2 knot security is defined as the effectiveness of the knot in resisting slippage when load is applied, whereas loop security is the effectiveness in maintaining a tight suture loop while a knot is being tied. Arthroscopic knots commonly begin with an initial slipknot locked in place with a series of half-hitches. During arthroscopic surgery, the surgeon usually must tie an arthroscopic knot to obtain secure tissue fixation, an essential component of soft-tissue repair. A secure knot provides optimal tissue apposition for healing, which will ultimately improve functional outcome. For a knot to be effective, it must have both knot security and loop security. Knot security depends on knot configuration, the coefficient of friction, ductility, handling properties, solubility and diameter of suture material, internal interference, slack between throws, and surgeon experience. Tissue fluid and tissue reaction to suture material may affect knot and loop security.
The ideal knot would be easy to tie and reproducible and would not slip or stretch before tissue is healed. The ideal suture material should provide adequate strength to hold soft tissue in an anatomically correct position until healing can occur. It should also be easily and efficiently manipulated by arthroscopic means when tissues are being secured with knots and secure suture loops. Studies have been conducted to evaluate the security of knots tied with arthroscopic techniques, knot configurations, and suture materials, and these investigations have often evaluated knot performance under single load-to-failure (LTF) test scenarios and cyclic loading in vitro (dry environment) in a room-temperature environment.2-10 To our knowledge, few if any attempts have been made to simulate in situ conditions at body temperature when testing knot security. The fluid environment and the temperature could potentially affect the effectiveness of knots, as knot security depends on friction, internal interference, and slack between throws.1
We conducted a study to evaluate biomechanical performance (knot security, loop security) during destructive testing of several different suture materials with various arthroscopic knot configurations. The study was performed under in vitro (dry environment) and in situ (wet environment) conditions by surgeons with different levels of experience.
Materials and Methods
This investigation was conducted at the Orthopaedic Research Institute at Via Christi Health in Wichita, Kansas. The study compared 4 different suture materials tied with 3 different commonly used arthroscopic knots by 3 surgeons with different levels of experience. The 4 types of braided polyblend polyethylene sutures were Fiberwire (Arthrex, Naples, Florida), ForceFiber (Stryker, San Jose, California), Orthocord (DePuy-Mitek, Warsaw, Indiana), and Ultrabraid (Smith & Nephew, Memphis, Tennessee). Each suture material was tied with 3 arthroscopic knots—static surgeon’s knot, Weston knot,11 Tennessee slider12—and a series of 3 reversing half-hitches on alternating posts (RHAPs) (Figure 1). These knots were chosen based on studies showing they have a higher maximum force to failure when combined with 3 RHAPs.1,2,5,9,13-17
We evaluated performer variability with the help of 3 investigator-surgeons who differed in their level of experience tying arthroscopic knots. This experience was defined on the basis of total number of arthroscopies performed—one of the most important factors predicting basic arthroscopic skills. Our surgeon A was a sports medicine fellowship–trained surgeon with 10 years of experience and a significant number of arthroscopies performed annually (350); surgeon B was a sports medicine fellowship–trained surgeon with 3 years of experience and an annual arthroscopy volume of more than 250 procedures; and surgeon C was a third-year orthopedic resident with about 100 arthroscopies performed.
All knots were tied on a standardized post 30 mm in circumference, which provided a consistent starting circumference for each knot and replicated the suture loop created during arthroscopic rotator cuff repair. All knots were tied using standard arthroscopic techniques, with a standard knot pusher and a modified arthroscopic cannula, in a dry environment (Figure 2). Servohydraulic materials testing system instruments (model 810; MTS Systems, Eden Prairie, Minnesota) were used to test the knot security and loop security of each combination of knots and suture types. Two round hooks (diameter, 3.9 mm) were attached to the actuator and the load cell (Figure 3). Loops were preloaded to 6 N to avoid potential errors caused by slack in the loops or by stretching of suture materials and to provide a well-defined starting point for data recording.
LTF testing was performed for both in vitro and in situ conditions using 10 samples of each suture–knot configuration for each mechanical testing. Each type of testing was conducted for a total of 240 suture–knot combinations per investigator. For the in vitro condition, each suture loop was initiated with 5 preconditioning loading cycles, from 6 N to 30 N at 1 Hz. The load was then applied continuously at a crosshead speed of 1 mm/s until “clinical failure” (3 mm crosshead displacement). We used this criterion for clinical failure, as studies have indicated that 3 mm is the point at which tissue apposition is lost.15,18-21 After the crosshead reached the 3-mm displacement, the loads (under load control) were held for 5 minutes at maximum load, and then load was applied continuously at a crosshead speed of 1 mm/s until complete structure failure. Load and displacement data were collected at a frequency of 20 Hz.
For the in situ condition, the same test parameters were used, except that each combination of the suture loop was preloaded to 6 N and soaked in physiologic solution bath (human blood plasma) at 37°C (body temperature) for 24 hours before testing in an effort to simulate the aqueous medium in vivo after surgery. The in situ tests were performed under physiologic solution maintained at 37°C to approximate postoperative physical conditions.
Statistical Analysis
Means and standard deviations of the knot security and loop security achieved by the surgeons (different experience levels) were calculated for each test configuration and each test condition. These values were used to determine the statistical relevance of the difference in arthroscopic loop security and knot security in each configuration. One-way analysis of variance (ANOVA) performed with SPSS Version 19.0 software (SPSS, Chicago, Illinois) with the least significant difference (LSD) multiple comparisons post hoc analysis was used to determine if any observed differences between the types of braided polyblend sutures, the types of sliding knots, the test conditions (in vitro, in situ), and the levels of surgeon experience were significant for each knot configuration. The level of significance of differences was set at P < .001.
Results
Figure 4 shows the mean maximum clinical failure load (3 mm of displacement) of different arthroscopic knot configurations for different braided polyblend sutures by surgeons of different levels of experience. In the comparison of biomechanical performance (knot and loop security) under in vitro and in situ conditions, no significant difference was detected when Ultrabraid suture material was used, regardless of surgeon experience, for all knot configurations. For surgeon B, there was no significant difference between in vitro and in situ conditions for any knot configurations or suture materials. When Orthocord suture material was used, Weston knots tied by surgeon A, and static surgeon’s knots by surgeons A and C, resulted in a significant difference between the in vitro and in situ conditions. When ForceFiber suture material was used, only Weston knots and Tennessee slider knots by surgeon A had a significant difference between in vitro and in situ conditions. Weston knots by surgeon A exhibited a significant difference between in vitro and in situ conditions, except when Ultrabraid suture material was used.
Surgeon C’s Tennessee slider knots with all polyblend sutures showed significantly lower loads at clinical failure compared with all the other knot configurations and with knots tied by the other 2 surgeons under both in vitro and in situ conditions. Overall, knots tied by surgeon B had higher clinical failure load than knots tied by the other 2 surgeons.
Figure 5 shows the mean ultimate failure load (complete structural failure) of different arthroscopic knot configurations for different braided polyblend sutures by surgeons of different levels of experience. Knots tied with Orthocord suture material had the overall lower ultimate failure load compared with other suture materials, whereas knots tied with Ultrabraid suture material had the overall highest ultimate failure load. However, the ultimate failure loads for all the knots tied using any suture material, regardless of surgeon experience, were more than 61 N, which is the estimated minimum required ultimate load per suture during a maximum muscle contraction.1
Figure 6 shows the percentage of knot slipping at constant clinical failure load. Orthocord and Fiberwire suture materials had the lowest incidence of knot slippage. Surgeon C had complete knot slippage at constant clinical failure load using ForceFiber with the Weston knot and Ultrabraid with the Tennessee slider knot. When using Ultrabraid or ForceFiber, surgeons A and C had at least 2 knots slip for all knot configurations.
Discussion
Optimization of knot security for any given knot configuration, suture material, and surgeon experience level during arthroscopic knot tying is crucial.1-10 Our study results showed that, under single LTF test scenarios, there was a significant difference between in vitro and in situ conditions with respect to both knot configuration and surgeon experience level, except when Ultrabraid suture material was used. Arthroscopic sliding knots are lockable or nonlockable.7,12 With lockable sliding knots, slippage may be prevented by tensioning the wrapping limb, which distorts the post in the distal part of the knot, resulting in a kink in the post, thereby increasing the internal interference that increases the resistance of the knot from backing off. With nonlockable sliding knots, slippage may be prevented by the tight grip of the wrappings around the initial post.7 The static surgeon’s knot and the Tennessee slider knot are nonlockable, whereas the Weston knot is a distal lockable sliding knot. Compared with nonlockable sliding knots, lockable sliding knots cause less suture loop enlargement. In 1976, Tera and Aberg22 studied the strength of knotted thread for 12 different types of suture knots combined with 11 types of suture material. They conducted their study 1 week after suture material was inserted into the subcutaneous tissue of rabbits. Their results show a greater propensity for certain suture materials to slip when tested in an aqueous environment. In 1998, Babetty and colleagues23 used Wistar rats to compare the in vivo strength, knot efficiency, and knot security of 4 types of sliding knots and to assess tissue reaction as a result of knot configuration, knot volume, and suture size. They found that 4/0 knots lost more strength than 2/0 knots did, and they concluded that the tissue response to all the knots, except 2/0 nylon, was similar. They indicated that the inflammatory sheath volume varied with knot volume, suture size, and knot configuration. Our results agree with observations that exposure to an aqueous environment alters the force to clinical failure of comparable suture and knot configurations.
In addition, our findings indicate that surgeon familiarity with certain knots has a major effect on knot security. The difference in our 3 surgeons’ levels of familiarity with certain knots was somewhat minimized by the knot tying they practiced before submitting knots for testing. The findings contrast with those of Milia and colleagues,24 who conducted a biomechanical study to determine the effect of experience level on knot security. They compared an experienced arthroscopic shoulder surgeon with a junior-level orthopedic resident surgeon and concluded that experience did not affect knot security. However, the knots in their study were tied by hand, not through an arthroscopic cannula with instruments. Our findings suggest that both experienced and less experienced orthopedic residents should be encouraged to practice arthroscopic knot tying in a nonsurgical environment in order to become comfortable tying arthroscopic knots.
Braided nonabsorbable polyester suture traditionally has been found to be stronger than monofilament absorbable polydioxanone (PDS) and to have less slippage potential.8,9,25 Several studies have determined that the braided polyblend sutures now commonly used for arthroscopic knots have better strength profiles over more traditional materials.12,26,27 Orthocord has a dyed absorbable core (PDS, 68%), an undyed nonabsorbable ultrahigh-molecular-weight polyethylene (UHMWPE, 32%) sleeve, and a polyglactin coating.9,10 Both Ultrabraid and ForceFiber are made with braided UHMWPE and have just a few variations in weave patterns. Fiberwire has a multifiber UHMWPE core covered with braided polyester suture material. Several biomechanical studies25,26,28 have evaluated different arthroscopic sliding knot configurations with different suture materials, and all concluded that a surgeon who is choosing an arthroscopic repair technique should know the differences in suture materials and the knot strengths afforded by different knot configurations, as suture material is an important aspect of loop security. Our findings agree with their findings, that suture materials have a major effect on knot security, even with a series of 3 RHAPs, as in theory the RHAPs should minimize suture friction, internal interference, and slack between knot loops—emphasizing the effect of material selection. Furthermore, our findings also indicated that suture materials with a core in their design (Fiberwire, Orthocord) tend to have the lowest incidence of knot slippage. We had suspected that suture surface characteristics and suture construction could be important factors in knot slippage.
Our experimental design had its limitations. First, although we simulated factors such as temperature, plasma environment, and surgeon experience, tying a knot on a standardized post (30 mm in circumference) differed from what is typically done clinically. Second, the metal hooks used in this study were not compressible and did not interpose in the substance of the knot as soft tissue does in the clinical setting. Third, knots were tied with no tension against the sutures, whereas clinically knots are tied under tension as tissues are pulled together in reconstructions. Fourth, it was assumed that soaking in a physiologic solution bath (human blood plasma) at 37°C (body temperature) for 24 hours before testing was sufficient to simulate the aqueous medium in vivo after surgery, but these parameters may not represent conditions in a patient who has just undergone an arthroscopic shoulder repair and adheres to a passive motion protocol. Fifth, there was no blinding of knot type, and there was no randomization of tying order or testing order. Sixth, only a single LTF test was performed, and incremental cyclic loading can be more useful, as it has long been recognized as a leading source of failure in orthopedic repairs.
Conclusion
These study results advance our overall understanding of the biomechanics of the different knot configurations and loop security levels of the different braided polyblend sutures used in arthroscopic procedures through LTF in both in vitro and in situ conditions. Overall, no suture material was superior to any other in a fluid environment, as the combination of aqueous environment and surgeon level of experience with arthroscopic knot tying has a major effect on knot security under single LTF test scenarios. However, our data showed that Ultrabraid suture material had no effect on knot effectiveness over the fluid environment and the temperature. Furthermore, the study showed that the Tennessee slider knot had the steepest learning curve. This study may provide an alternative arthroscopic knots option for soft-tissue repair in which use of certain suture materials is limited.
1. Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Knot security in simple sliding knots and its relationship to rotator cuff repair: how secure must the knot be? Arthroscopy. 2000;16(2):202-207.
2. Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Loop security as a determinant of tissue fixation security. Arthroscopy. 1998;14(7):773-776.
3. Elkousy H, Hammerman SM, Edwards TB, et al. The arthroscopic square knot: a biomechanical comparison with open and arthroscopic knots. Arthroscopy. 2006;22(7):736-741.
4. Elkousy HA, Sekiya JK, Stabile KJ, McMahon PJ. A biomechanical comparison of arthroscopic sliding and sliding-locking knots. Arthroscopy. 2005;21(2):204-210.
5. Ilahi OA, Younas SA, Alexander J, Noble PC. Cyclic testing of arthroscopic knot security. Arthroscopy. 2004;20(1):62-68.
6. Loutzenheiser TD, Harryman DT 2nd, Ziegler DW, Yung SW. Optimizing arthroscopic knots using braided or monofilament suture. Arthroscopy. 1998;14(1):57-65.
7. Chan KC, Burkhart SS, Thiagarajan P, Goh JC. Optimization of stacked half-hitch knots for arthroscopic surgery. Arthroscopy. 2001;17(7):752-759.
8. Lee TQ, Matsuura PA, Fogolin RP, Lin AC, Kim D, McMahon PJ. Arthroscopic suture tying: a comparison of knot types and suture materials. Arthroscopy. 2001;17(4):348-352.
9. Mishra DK, Cannon WD Jr, Lucas DJ, Belzer JP. Elongation of arthroscopically tied knots. Am J Sports Med. 1997;25(1):113-117.
10. Kim SH, Ha KI, Kim SH, Kim JS. Significance of the internal locking mechanism for loop security enhancement in the arthroscopic knot. Arthroscopy. 2001;17(8):850-855.
11. Weston PV. A new clinch knot. Obstet Gynecol. 1991;78(1):144-147.
12. Lo IK, Burkhart SS, Chan KC, Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489-502.
13. Lo IK, Burkhart SS, Athanasiou K. Abrasion resistance of two types of nonabsorbable braided suture. Arthroscopy. 2004;20(4):407-413.
14. De Beer JF, van Rooyen K, Boezaart AP. Nicky’s knot—a new slip knot for arthroscopic surgery. Arthroscopy. 1998;14(1):109-110.
15. Loutzenheiser TD, Harryman DT 2nd, Yung SW, France MP, Sidles JA. Optimizing arthroscopic knots. Arthroscopy. 1995;11(2):199-206.
16. Wetzler MJ, Bartolozzi AR, Gillespie MJ, et al. Fatigue properties of suture anchors in anterior shoulder reconstructions: Mitek GII. Arthroscopy. 1996;12(6):687-693.
17. Barber FA, Herbert MA, Beavis RC. Cyclic load and failure behavior of arthroscopic knots and high strength sutures. Arthroscopy. 2009;25(2):192-199.
18. Richmond JC. A comparison of ultrasonic suture welding and traditional knot tying. Am J Sports Med. 200;29(3):297-299.
19. James JD, Wu MM, Batra EK, Rodeheaver GT, Edlich RF. Technical considerations in manual and instrument tying techniques. J Emerg Med. 1992;10(4):469-480.
20. Batra EK, Franz DA, Towler MA, et al. Influence of emergency physician’s tying technique on knot security. J Emerg Med. 1992;10(3):309-316.
21. Livermore RW, Chong AC, Prohaska DJ, Cooke FW, Jones TL. Knot security, loop security and elongation of braided polyblend sutures used for arthroscopic knots. Am J Orthop. 2010;39(12):569-576.
22. Tera H, Aberg C. The strength of suture knots after one week in vivo. Acta Chir Scand. 1976;142(4):301-307.
23. Babetty Z, Sümer A, Altintaş S, Ergüney S, Göksel S. Changes in knot-holding capacity of sliding knots in vivo and tissue reaction. Arch Surg. 1998;133(7):727-734.
24. Milia MJ, Peindl RD, Connor PM. Arthroscopic knot tying: the role of instrumentation in achieving knot security. Arthroscopy. 2005;21(1):69-76.
25. Lieurance RK, Pflaster DS, Abbott D, Nottage WM. Failure characteristics of various arthroscopically tied knots. Clin Orthop. 2003;(408):311-318.
26. Abbi G, Espinoza L, Odell T, Mahar A, Pedowitz R. Evaluation of 5 knots and 2 suture materials for arthroscopic rotator cuff repair: very strong sutures can still slip. Arthroscopy. 2006;22(1):38-43.
27. Wüst DM, Meyer DC, Favre P, Gerber C. Mechanical and handling properties of braided polyblend polyethylene sutures in comparison to braided polyester and monofilament polydioxanone sutures. Arthroscopy. 2006;22(11):1146-1153.
28. Mahar AT, Moezzi DM, Serra-Hsu F, Pedowitz RA. Comparison and performance characteristics of 3 different knots when tied with 2 suture materials used for shoulder arthroscopy. Arthroscopy. 2006;22(6):614.e1-e2.
1. Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Knot security in simple sliding knots and its relationship to rotator cuff repair: how secure must the knot be? Arthroscopy. 2000;16(2):202-207.
2. Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Loop security as a determinant of tissue fixation security. Arthroscopy. 1998;14(7):773-776.
3. Elkousy H, Hammerman SM, Edwards TB, et al. The arthroscopic square knot: a biomechanical comparison with open and arthroscopic knots. Arthroscopy. 2006;22(7):736-741.
4. Elkousy HA, Sekiya JK, Stabile KJ, McMahon PJ. A biomechanical comparison of arthroscopic sliding and sliding-locking knots. Arthroscopy. 2005;21(2):204-210.
5. Ilahi OA, Younas SA, Alexander J, Noble PC. Cyclic testing of arthroscopic knot security. Arthroscopy. 2004;20(1):62-68.
6. Loutzenheiser TD, Harryman DT 2nd, Ziegler DW, Yung SW. Optimizing arthroscopic knots using braided or monofilament suture. Arthroscopy. 1998;14(1):57-65.
7. Chan KC, Burkhart SS, Thiagarajan P, Goh JC. Optimization of stacked half-hitch knots for arthroscopic surgery. Arthroscopy. 2001;17(7):752-759.
8. Lee TQ, Matsuura PA, Fogolin RP, Lin AC, Kim D, McMahon PJ. Arthroscopic suture tying: a comparison of knot types and suture materials. Arthroscopy. 2001;17(4):348-352.
9. Mishra DK, Cannon WD Jr, Lucas DJ, Belzer JP. Elongation of arthroscopically tied knots. Am J Sports Med. 1997;25(1):113-117.
10. Kim SH, Ha KI, Kim SH, Kim JS. Significance of the internal locking mechanism for loop security enhancement in the arthroscopic knot. Arthroscopy. 2001;17(8):850-855.
11. Weston PV. A new clinch knot. Obstet Gynecol. 1991;78(1):144-147.
12. Lo IK, Burkhart SS, Chan KC, Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489-502.
13. Lo IK, Burkhart SS, Athanasiou K. Abrasion resistance of two types of nonabsorbable braided suture. Arthroscopy. 2004;20(4):407-413.
14. De Beer JF, van Rooyen K, Boezaart AP. Nicky’s knot—a new slip knot for arthroscopic surgery. Arthroscopy. 1998;14(1):109-110.
15. Loutzenheiser TD, Harryman DT 2nd, Yung SW, France MP, Sidles JA. Optimizing arthroscopic knots. Arthroscopy. 1995;11(2):199-206.
16. Wetzler MJ, Bartolozzi AR, Gillespie MJ, et al. Fatigue properties of suture anchors in anterior shoulder reconstructions: Mitek GII. Arthroscopy. 1996;12(6):687-693.
17. Barber FA, Herbert MA, Beavis RC. Cyclic load and failure behavior of arthroscopic knots and high strength sutures. Arthroscopy. 2009;25(2):192-199.
18. Richmond JC. A comparison of ultrasonic suture welding and traditional knot tying. Am J Sports Med. 200;29(3):297-299.
19. James JD, Wu MM, Batra EK, Rodeheaver GT, Edlich RF. Technical considerations in manual and instrument tying techniques. J Emerg Med. 1992;10(4):469-480.
20. Batra EK, Franz DA, Towler MA, et al. Influence of emergency physician’s tying technique on knot security. J Emerg Med. 1992;10(3):309-316.
21. Livermore RW, Chong AC, Prohaska DJ, Cooke FW, Jones TL. Knot security, loop security and elongation of braided polyblend sutures used for arthroscopic knots. Am J Orthop. 2010;39(12):569-576.
22. Tera H, Aberg C. The strength of suture knots after one week in vivo. Acta Chir Scand. 1976;142(4):301-307.
23. Babetty Z, Sümer A, Altintaş S, Ergüney S, Göksel S. Changes in knot-holding capacity of sliding knots in vivo and tissue reaction. Arch Surg. 1998;133(7):727-734.
24. Milia MJ, Peindl RD, Connor PM. Arthroscopic knot tying: the role of instrumentation in achieving knot security. Arthroscopy. 2005;21(1):69-76.
25. Lieurance RK, Pflaster DS, Abbott D, Nottage WM. Failure characteristics of various arthroscopically tied knots. Clin Orthop. 2003;(408):311-318.
26. Abbi G, Espinoza L, Odell T, Mahar A, Pedowitz R. Evaluation of 5 knots and 2 suture materials for arthroscopic rotator cuff repair: very strong sutures can still slip. Arthroscopy. 2006;22(1):38-43.
27. Wüst DM, Meyer DC, Favre P, Gerber C. Mechanical and handling properties of braided polyblend polyethylene sutures in comparison to braided polyester and monofilament polydioxanone sutures. Arthroscopy. 2006;22(11):1146-1153.
28. Mahar AT, Moezzi DM, Serra-Hsu F, Pedowitz RA. Comparison and performance characteristics of 3 different knots when tied with 2 suture materials used for shoulder arthroscopy. Arthroscopy. 2006;22(6):614.e1-e2.