User login
From the University of North Dakota School of Medicine & Health Sciences, Fargo, ND.
Abstract
- Objective: To review the management of community-acquired pneumonia (CAP) in adults.
- Methods: Review of the literature.
- Results: Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually, accounting for significant morbidity and mortality. While numerous studies have previously shown pneumococcus to be the most common causative pathogen, the 2015 EPIC study found that in nearly two-thirds of patients with CAP who required hospitalization, no pathogen was detected. Symptoms and signs of respiratory tract infection are useful in helping to diagnose pneumonia; however, they are less sensitive than chest imaging studies. Laboratory tests used in diagnosing pneumonia include sputum Gram stain and culture, blood culture, urinary antigen, polymerase chain reaction, and biologic markers. In empiric treatment of CAP, both the typical and atypical pathogens should be targeted. Influenza vaccine and pneumococcal polysaccharide and conjugate vaccines should be administered as recommended by the CDC to reduce risk of CAP.
- Conclusion: CAP is a common illness with high rates of morbidity and mortality. Treatment is for the most part empirical; diagnostic testing can be used to identify the causative organism and guide pathogen-specific therapy.
Key words: community-acquired pneumonia; adults; management; vaccines.
Despite advances in medical science, pneumonia remains a major cause of morbidity and mortality. In 2014, 50,620 patients in the United States died from the disease [1]. Pneumonia can be classified as community-acquired, hospital-acquired, or ventilator-associated. Another category, healthcare-associated pneumonia, was included in an earlier American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guideline but was removed from the 2016 guideline because there was no clear evidence that patients diagnosed with healthcare-associated pneumonia were at higher risk for harboring multidrug-resistant pathogens [2]. In this article, we review the epidemiology, microbiology, predisposing factors, diagnosis, treatment, and prevention of community-acquired pneumonia (CAP).
Definition and Epidemiology
CAP is defined as an acute infection of the lungs that develops in patients who have not been hospitalized recently and have not had regular exposure to the health care system [3]. A previously ambulatory patient who is diagnosed with pneumonia within 48 hours after admission also meets the criteria for CAP. Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually [4]. About 25% of CAP patients require hospitalization, and about 5% to 10% of these patients are admitted to the intensive care unit (ICU) [5]. In-hospital mortality is considerable (~10% in population-based studies) [6] and 30-day mortality was found to be as high as 23% in a review by File and Marrie [7]. CAP also confers a high risk of long-term morbidity and mortality compared with the general population who have never had CAP, irrespective of age [8].
Causative Organisms
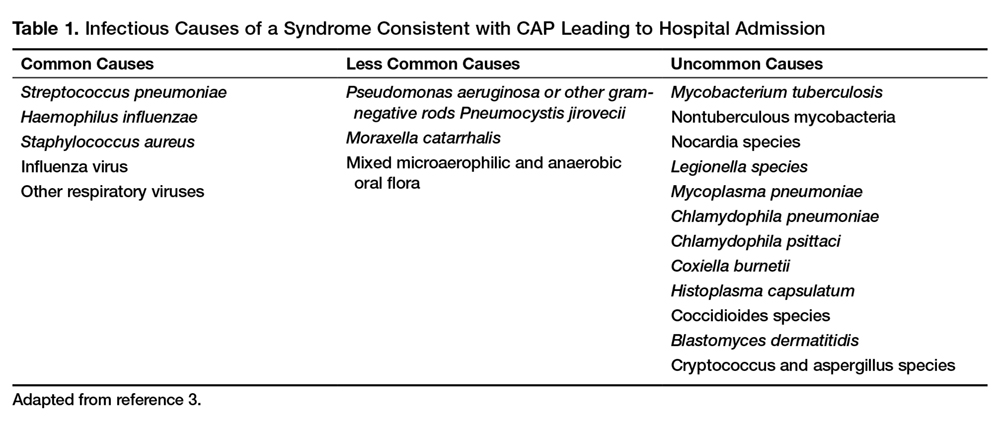
Numerous microorganisms can cause CAP. Common causes and less common causes are delineated in Table 1.
Predisposing Factors
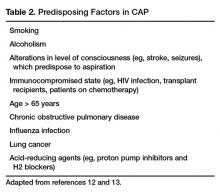
Most people diagnosed with CAP have one or more predisposing factors [12,13] (Table 2).
Clinical Signs and Symptoms
Symptoms of CAP include fever, chills, rigors, fatigue, anorexia, diaphoresis, dyspnea, cough (with or without sputum production), and pleuritic chest pain. There is no individual symptom or cluster of symptoms that can absolutely differentiate pneumonia from other acute respiratory diseases, including upper and lower respiratory infections. However, if a patient presents with the constellation of symptoms of fever ≥ 1000F (37.80C), productive cough, and tachycardia, it is more suggestive of pneumonia [14]. Abnormal vital signs include fever, hypothermia, tachypnea, tachycardia, and oxygen desaturation. Auscultation of the chest reveals crackles or other adventitious breath sounds. Elderly patients with pneumonia report a significantly lower number of both respiratory and nonrespiratory symptoms compared with younger patients. Clinicians should be aware of this phenomenon so it does not lead to delayed diagnosis and treatment [15].
Imaging Evaluation
The presence of a pulmonary consolidation or an infiltrate on chest radiograph is required to diagnose CAP, and a chest radiograph should be obtained when CAP is suspected [16]. It should be noted that there is no pattern of radiographic abnormalities reliable enough to differentiate infectious pneumonia from noninfectious causes [17].
There are case reports and case series demonstrating false-negative plain chest radiographs existing in dehydrated patients [18] or in neutropenic state. However, animal studies have shown that dogs challenged with pneumococcus showed abnormal pulmonary shadow, suggestive of pneumonia, regardless of hydration status [19]. There is also no reliable scientific evidence to support the notion that severe neutropenia can cause false-negative radiographs because of the inability to develop an acute inflammatory reaction in the lungs [20].
A chest CT scan is more sensitive than a plain chest radiograph in detecting pneumonia. Therefore, a chest CT should be performed in a patient with negative plain chest radiograph when pneumonia is still highly suspected [21]. A chest CT scan is also more sensitive in detecting cavitation, adenopathy, interstitial disease and empyema. It also has the advantage of better defining anatomical changes than plain films [22].
Because improvement of pulmonary opacities in patients with CAP lags behind clinical improvement, repeating chest imaging studies is not recommended in patients who demonstrate clinical improvement. Sometimes clearing of pulmonary infiltrate or consolidation can take 6 weeks or longer [23].
Laboratory Evaluation
Generally the etiologic agent of CAP cannot be determined solely on the basis of clinical signs and symptoms or imaging studies. Although routine microbiological testing for patients suspicious for CAP is not necessary for empirical treatment, by determining the etiologic agent of the pneumonia, a clinician will be able to narrow the antibiotics from a broad-spectrum empirical regimen to specific pathogen-directed therapy. Determination of certain etiologic agents causing the pneumonia can have important public health implications (eg, Mycobacterium tuberculosis and influenza virus) [24].
Sputum Gram Stain and Culture
Sputum Gram stain is an inexpensive test that may identify pathogens that cause CAP (eg, S. pneumonia and Haemophilus influenzae). A quality specimen is required. A sputum sample must contain > 25 neutrophils and < 10 squamous epithelial cells/low power field on Gram stain to be considered suitable for culture.
The sensitivity and specificity of sputum Gram stain and culture are highly variable in different clinical settings (eg, outpatient setting, nursing home, ICU). Reed et al’s meta-analysis of patients diagnosed with CAP in the United States showed the sensitivity and specificity of sputum Gram stain (compared with sputum culture) ranged from 15% to 100% and 11% to 100%, respectively [24]. In cases of proven bacteremic pneumococcal pneumonia, positive cultures from sputum samples were positive less than 50% of the time [25].
For patients who cannot provide sputum samples or are intubated, a deep-suction aspirate or bronchoalveolar lavage through a bronchoscopic procedure might be necessary to obtain pulmonary secretion for Gram stain and culture. Besides bacterial culture, sputum samples can also be sent for fungal and mycobacterial cultures and acid-fast stain if deemed clinically necessary.
Blood Culture
Because the positivity rate of blood culture in patients who are suspected to have pneumonia but not exposed to antimicrobial agents is disappointingly low (5%–14%), blood cultures are no longer recommended in patients hospitalized for CAP. Another reason for not recommending blood culture is positive culture rarely leads to changes in antibiotic regimen in patients without underlying diseases [26]. However, high-risk patients, including patients with severe CAP or in immunocompromised patients (eg, patients with neutropenia, asplenia or complement deficiencies) should have a blood culture done [24].
A multinational study published in 2008 examined 125 patients with pneumococcal bacteremic CAP versus 1847 patients with non-bacteremic CAP [27]. Analysis of the data demonstrated no association of pneumococcal bacteremic CAP and time to clinical stability, length of hospital stay, all-cause mortality or CAP-related mortality. The authors concluded that pneumococcal bacteremia does not increase the risk of poor outcomes in patients with CAP compared to non-bacteremic patients, and the presence of pneumococcal bacteremia should not deter de-escalation of therapy in clinically stable patients.
Urinary Antigen Tests
Urinary antigen tests may assist clinicians in narrowing antibiotic therapy when test results are positive. There are 2 U.S. Food and Drug Administration–approved tests available to clinicians for detecting pneumococcal and Legionella antigen in urine. The test for Legionella pneumophila detects disease due to serogroup 1 only, which accounts for 80% of community-acquired Legionnaires disease. The sensitivity and specificity of the Legionella urine antigen test are 90% and 99%, respectively. The pneumococcal urine antigen test is less sensitive and specific than the Legionella urine antigen test (sensitivity 80% and specificity > 90%) [28,29].
Advantages of the urinary antigen tests are that they are easily performed, results are available in less than an hour if done in-house, and results are not affected by prior exposure to antibiotics. However, the tests do not meet Clinical Laboratory Improvements Amendments criteria for waiver and must be performed by a technician in the laboratory.
Polymerase Chain Reaction
There are several FDA-approved polymerase chain reaction (PCR) tests commercially available to assist clinicians in diagnosing pneumonia. PCR test of nasopharyngeal swabs for diagnosing influenza have become standard in many medical U.S. facilities. The great advantage of using PCR to diagnose influenza is its high sensitivity and specificity and rapid turnaround time. PCR can also be used to detect Legionella species, S. pneumonia, Mycoplasma pneumoniae, Chlamydophila pneumonia and mycobacterial species [24].
One limitation of using PCR tests on respiratory specimens is that specimens can be contaminated with oral or upper airway flora, so the results must be interpreted with caution, bearing in mind that some of the pathogens isolated may be colonizers of the oral or upper airway flora [30].
Biologic Markers
Two biologic markers—procalcitonin and C-reactive protein (CRP)—can be used in conjunction with history, physical examination, laboratory tests and imaging studies to assist in the diagnosis and treatment of CAP [24]. Procalcitonin is a peptide precursor of the hormone calcitonin that is released by parenchymal cells into the bloodstream resulting in increased serum level in patients with bacterial infections. In contrast, there is no remarkable proclacitonin level increase with viral or noninfectious inflammation. The reference value of procalcitonin in the blood of an adult individual without infection or inflammation is < 0.15 ng/mL. In the blood, procalcitonin has a half-life of 25 to 30 hours. The quantitative immunoluminometric method (LUMI test, Brahms PCT, Berlin, Germany ) is the preferred test to use because of its high sensitivity [31].
A 2012 Cochrane meta-analysis that involved 4221 patients with acute respiratory infections (with half of the patients diagnosed with CAP) from 14 prospective trials found the use of procalcitonin test for antibiotic use significantly decreased median antibiotic exposure from 8 to 4 days without an increase in treatment failure, mortality rates in any clinical setting (eg, outpatient clinic, emergency room), or length of hospitalization [32]. A prospective study conducted in France on 100 ICU patients showed that increased procalcitonin from day 1 to day 3 has a poor prognosis factor for severe CAP whereas decreasing procalcitonin levels is associated with a favorable outcome [33].
CRP is an acute phase protein produced by the liver. CRP level in the blood increases in response to acute infection or inflammation. Use of CRP in assisting diagnosis and guiding treatment of CAP is more limited in part due to its poor specificity. A prospective study conducted on 168 consecutive patients presented with cough showed that a CRP > 40 mg/L had a sensitivity and specificity of 70% and 90%, respectively [34].
T reatment
Site of Care Decision
For patients with CAP, the clinician must decide whether the patient will be treated in an outpatient or inpatient setting, and for those in the inpatient setting, whether they can safely be treated on the general medical ward or should be the ICU. Two common scoring systems that can be used to aid the clinician in determining severity of the infection and guiding site-of-care decisions are the Pneumonia Severity Index (PSI) and CURB-65 scores.
The PSI score uses 20 different parameters, including comorbidities, laboratory parameters and radiographic findings to stratify patients into 5 mortality risk classes [35]. On the basis of associated mortality rates, it has been suggested that risk class I and II patients should be treated as outpatients, risk class III patients should be treated in an observation unit or with a short hospitalization, and risk class IV and V patients should be treated as inpatients [35].
The CURB-65 method of risk stratification is based on 5 clinical parameters: confusion, urea level, respiratory rate, systolic blood pressure and age ≥ 65 (Table 3) [36].
Patients with CURB-65 scores of 4 or 5 are considered to have severe pneumonia and admission to the ICU should be considered. Aside from the CURB-65 score, anyone requiring vasopressor support or mechanical ventilation merits admission to the ICU [16]. IDSA/ATS guidelines also recommend the use of “minor criteria” for making ICU admission decisions; these include respiratory rate ≥ 30 breaths / minute, PaO2 fraction ≤ 250, multilobar infiltrates, confusion, blood urea nitrogen ≥ 20 mg/dL, leukopenia, thrombocytopenia, hypothermia and hypotension [16]. These factors are associated with increased mortality due to CAP and admission to an ICU is indicated if 3 of the minor criteria for severe CAP are present.
Similar to CURB-65, another clinical calculator that can be used for assessing severity of CAP is SMART-COP [39]. This scoring system uses 8 weighted criteria to predict which patients will require intensive respiratory or vasopressor support. SMART-COP has a sensitivity of 79% and specificity 64% in predicting ICU admission, whereas CURB-65 had a pooled sensitivity of 57.2% and specificity of 77.2% [40].
Antibiotic Therapy
Antibiotics are the mainstay of treatment for CAP, with the majority of patients with CAP treated empirically taking into account the site of care, likely pathogen, and antimicrobial resistance issues. Patients with pneumonia who are treated as outpatients usually respond well to empiric antibiotic treatment and a causative pathogen is not usually sought. Patients who are hospitalized for treatment of CAP usually receive empiric antibiotic on admission. Once the etiology has been determined by microbiologic or serologic means, antimicrobial therapy should be adjusted accordingly. As noted previously, a CDC study found that the burden of viral etiologies was higher than previously thought, with rhinovirus and influenza accounting for 15% of cases and S. pneumoniae for only 5% [9]. This study highlighted the fact that despite advances in molecular techniques, most patients with pneumonia have no pathogen identified [9]. Given the lack of discernable pathogens in the majority of cases, unless a nonbacterial etiology is found patients should continue to be treated with antibiotics.
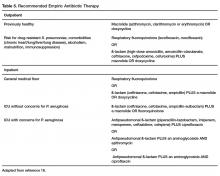
Outpatients without comorbidities or risk factors for drug-resistant S. pneumoniae (Table 4)
As previously mentioned, antibiotic therapy is typically empiric; neither clinical features nor radiographic features are sufficient to include or exclude infectious etiologies. Epidemiologic risk factors should be considered and, in certain cases, expanded antimicrobial coverage to include those entities; for example, treatment of anaerobes in the setting of lung abscess and antipseudomonal antibiotics for patients with bronchiectasis.
Of concern in the treatment of CAP is the increased prevalence of antimicrobial resistance among S. pneumoniae. The IDSA guidelines report that drug-resistant S. pneumoniae is more common in persons aged < 2 or > 65 years, and those with ß-lactam therapy within the previous 3 months, alcoholism, medical comorbidities, immunosuppressive illness or therapy, or exposure to a child who attends a day care center [16].
S. aureus should be considered during influenza outbreaks, with either vancomycin or linezolid being the recommended agents in the setting of methicillin-resistant S. aureus (MRSA). In a study comparing vancomycin versus linezolid for nosocomial pneumonia, the all-cause 60-day mortality was similar for both agents [41]. Datpomycin is another agent used against MRSA; however, its use in the setting of pneumonia is not indicated as daptomycin binds to surfactant, yielding it ineffective in the treatment of pneumonia [42]. Ceftaroline is a newer cephalosporin with activity against MRSA; its role in treatment of community-acquired MRSA pneumonia has not been fully elucidated, but it appears to be a useful agent for this indication [43,44].
A summary of recommended empiric antibiotic therapy is presented in Table 5.
Antibiotic Therapy for Selected Pathogens
S. pneumoniae
Patients with pneumococcal pneumonia who have penicillin-susceptible strains can be treated with intravenous penicillin (2 or 3 million units every 4 hours) or ceftriaxone. Once a patient meets criteria of stability, they can then be transitioned to oral penicillin, amoxicillin, or clarithromycin. Those with strains with reduced susceptibility can still be treated with penicillin but at a higher dose (4 million units IV every 4 hours) or a third-generation cephalosporin. Those whose pneumococcal pneumonia is complicated by bacteremia will benefit from dual therapy if severely ill, requiring ICU monitoring. Those not severely ill can be treated with monotherapy [46].
S. aureus
S. aureus is more commonly associated with hospital-acquired pneumonia but may also be seen during the influenza season and in those with severe necrotizing CAP. Both linezolid and vancomycin can be used to treat MRSA CAP. As noted above, ceftaroline has activity against MRSA and is approved for treatment of CAP, but is not approved by the FDA for MRSA CAP treatment. Similarly, tigecycline is approved for CAP and has activity against MRSA, but is not approved for MRSA CAP. Moreover, the FDA has warned of increased risk of death with tigecycline and has a black box warning to that effect [47].
Legionella
Treatment of legionellosis can be achieved with tetracyclines, macrolides, or fluoroquinolones. For nonimmunosuppressed patients with mild pneumonia, any of the listed antibiotics is considered appropriate. However, patients with severe infection or those with immunosuppression should be treated with either levofloxacin or azithromycin for 7 to 10 days [48].
C. pneumoniae
As with other atypical organisms, C. pneumoniae can be treated with doxycycline, a macrolide, or respiratory fluoroquinolones. However, length of therapy varies by regimen used; whereas treating with doxycycline 100 mg twice daily generally requires 14–21 days, moxifloxacin 400 mg daily only requires 10 days [49].
M. pneumoniae
As with C. pneumoniae, length of therapy of M. pneumoniae varies by antimicrobial used. Shortest courses are seen with the use of macrolides for 5 days, whereas 14 days is considered standard for doxycycline or a respiratory fluoroquinolone [50]. It should be noted that there has been increasingly documented resistance to macrolides, with known resistance of 8.2% in the United States [51].
Duration of Treatment
Most patients with CAP respond within 72 hours to appropriate therapy. IDSA/ATS guidelines recommend that patients be treated for a minimum of 5 days, and before discontinuing antibiotics patients should be afebrile a minimum of 48-72 hours and be clinically stable (Table 6) [16].
Hospitalized patients do not need to be monitored for an additional day once they have reached clinical stability (Table 6), are able to maintain oral intake, and have normal mentation, provided that other comorbidities are stable and social needs have been met [16]. Patients discharged from the hospital with instability have higher risk of readmission or death [55].
Transition to Oral Therapy
IDSA/ATS guidelines [16] recommend that patients should be transitioned from IV to oral antibiotics when they are improving clinically, have stable vital signs, and are able to ingest food/fluids and medications.
Management of Nonresponders
Although the majority of patients respond to antibiotics within 72 hours, treatment failure occurs in up to 15% of patients [45]. Nonresponding pneumonia is generally seen in 2 patterns: worsening of clinical status despite empiric antibiotics OR delay in achieving clinical stability as defined in Table 5 after 72 hours of treatment [13]. Risk factors associated with nonresponding pneumonia [56] are:
- Radiographic: multilobar infiltrates, pleural effusion, cavitation
- Bacteriologic: MRSA, gram-negative or Legionella pneumonia
- Severity index: PSI > 90
- Pharmacologic: incorrect antibiotic choice based on susceptibility
Patients with acute deterioration of clinical status will prompt transfer to a higher level of care and may require mechanical ventilator support. In those with delay in achieving clinical stability, question centers on whether the same antibiotics can be continued while doing further radiographic/microbiologic workup and/or changing antibiotics.
History should be reviewed with particular attention to exposures, travel history, and microbiologic and radiographic data. Clinicians should recall that viral causes account for up to 20% of pneumonias and there are also noninfectious causes that can mimic pyogenic infections [57]. If adequate initial cultures were not obtained, they should be obtained; however, care must be taken in reviewing new sets of cultures while on antibiotics as they may reveal colonization selected out by antibiotics and not a true pathogen. If repeat evaluation is unrevealing, then further evaluation with CT scan and bronchoscopy with bronchoalveolar lavage and biopsy is warranted. CT scans can show pleural effusions, bronchial obstructions or pattern suggestive of cryptogenic pneumonia. A bronchoscopy might yield a microbiologic diagnosis and with biopsy can also evaluate for noninfectious causes.
As with other infections, if escalation of antibiotics is undertaken, clinicians should be mindful to ensure that efforts are being made to elucidate the reason for nonresponse. To simply broaden antimicrobial therapy without attempts at establishing a microbiologic or radiographic cause for nonresponse may lead to inappropriate treatment recurrence of infection. Aside from patients who have bacteremic pneumococcal pneumonia in an ICU setting, there are no published reports pointing to superiority of combination antibiotics [46].
Other Treatment
Because of the inflammatory response associated with pneumonia, several agents have been evaluated as adjunctive treatment of pneumonia to decrease this inflammatory state; namely, steroids, macrolide antibiotics and statins. To date, only the use of steroids (methylprednisolone 0.5 mg/kg every 12 hours for 5 days) in those with severe CAP and high initial anti-inflammatory response (CRP > 150) was shown to decrease treatment failure, decreased risk of ARDS, possibly reduce length of stay, duration of intravenous antibiotics and clinical stability, without effect on mortality or adverse side effects [58,59].
Other adjunctive methods have not been found to have significant impact [16].
Prevention of Pneumonia
Prevention of pneumococcal pneumonia is twofold: prevention of infection caused by S. pneumoniae and prevention of influenza infection. As influenza infection is a risk factor for bacterial infection, specifically with S. pneumoniae, influenza vaccination can prevent bacterial pneumonia [60]. In their most recent recommendations, the CDC continues to recommend routine influenza vaccination for all persons aged greater than 6 months, unless otherwise contraindicated [61].
There are 2 vaccines for prevention of pneumococcal disease: the pneumococcal polysaccharide vaccine (PPSV23) and a conjugate vaccine (PCV13). Following vaccination with PPSV23, 80% of adults develop antibodies against at least 18 of the 23 serotypes [62]. Despite this response, PPSV23 is reported to be protective against invasive pneumococcal infection; yet there is no consensus regarding PPSV23 leading to decreased rates of pneumonia [63]. On the other hand, PCV13 vaccination was associated with prevention of both invasive disease and community-acquired pneumonia in adults 65 years or older [64]. The CDC recommends that all children aged 2 or under receive PCV13, whereas those aged 65 or older should receive PCV13 followed by a dose of PPSV23 [65]. The dose of PPSV23 should be given ≥1 year following the dose of PCV13 [66].Persons < 65 years of age with immunocompromising and certain other conditions should also receive vaccination [67] (Table 7). Full details, many scenarios, and timing of vaccinations can be found at www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule.pdf.
Cigarette smoking increases the risk of respiratory infections as evidenced by smokers accounting for almost half of all patients with invasive pneumococcal disease [11]. As this is a modifiable risk factor it should be a goal of a comprehensive approach towards prevention of pneumonia.
Summary
CAP remains a leading cause of hospitalization and death in the 21st century. Traditionally, pneumococcus has been considered the major pathogen causing CAP; however, the 2015 EPIC study found that in only 5% of patients diagnosed with CAP was S. pneumoniae detected. Despite the new findings, it is still recommended that empiric treatment for CAP target common typical bacteria (pneumococcus, H. influenzae, Moraxella catarrhalis) and atypical bacteria (M. pneumonia, C. pneumoniae, L. pneumophila).
Because diagnosing pneumonia through history and clinical examination is less than 50% sensitive, a chest imaging study (a plain chest radiograph or a chest CT scan) is usually required to make the diagnosis. Laboratory tests, such as sputum Gram stain/culture, blood culture, urinary antigen tests, PCR test, procalcitonin, and CRP are important adjunctive diagnostic modalities to assist in the diagnosis and management of CAP. However, no single test is sensitive and specific enough to be a stand-alone test. They should be used in conjunction with history, physical examination, and imaging studies. Because vaccination (PPSV23, PCV13, and influenza vaccine) remains the most effective tool in preventing the development of CAP, clinicians, should strive for 100% vaccination rates in appropriate persons.
Corresponding author: Tze Shein Lo, MD, University of North Dakota, 1919 Elm Street, Fargo, ND 58102, tzeshein.lo@med.und.edu.
Financial disclosures: None.
Author contributions: drafting of article, PM, TSL; critical revision of the article, PM, TSL.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. FastStats - Pneumonia. Accessed 6 Oct 2016 at www.cdc.gov/nchs/fastats/pneumonia.htm.
2. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61-e111.
3. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med 2014;371:1619–28.
4. Mandell LA. Epidemiology and etiology of community-acquired pneumonia. Infect Dis
5. Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ 2006;332:1077–9.
6. Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med 2007;167:1938–43
7. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med 2010;122:130–41.
8. Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-year mortality after community-acquired pneumonia. a prospective cohort. Am J Respir Crit Care Med 2015;192:597-604.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015;373:415–27.
10. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013;369:155–63.
11. Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med 2000;342:681–9.
12. Almirall J, Serra-Prat M, Bolíbar I, BalassoV. Risk factors for community-acquired pneumonia in adults: a systemic review of observational studies. Respiration 2017;94:299–311.
13. Janoff EM.Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s principles and practice of infectioius diseases. 8th ed. Philadelphia: Sauders; 2015: 2310–27.
14. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough--a statistical approach. J Chronic Dis 1984;37:215–25.
15. Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med 1997;157:1453–9.
16. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44 Suppl 2:S27–72.
17. Jartti A, Rauvala E, Kauma H, et al. Chest imaging findings in hospitalized patients with H1N1 influenza. Acta Radiol 2011;52:297–304.
18. Basi SK, Marrie TJ, Huang JQ, Majumdar SR. Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med 2004;117:305–11.
19. Caldwell A, Glauser FL, Smith WR, et al. The effects of dehydration on the radiologic and pathologic appearance of experimental canine segmental pneumonia. Am Rev Respir Dis 1975;112:651–6.
20. Bartlett JG. Pneumonia. In: Barlett JG, editor. Management of respiratory tract infections. Philadelphia: Lippincott, Williams & Wilkins; 2001: 1–122.
21. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med 2015;192:974–82.
22. Wheeler JH, Fishman EK. Computed tomography in the management of chest infections: current status. Clin Infect Dis 1996;23:232–40.
23. Chesnutt MP. Pulmonary disorders. In: Papadakis MM, editor. Current medical diagnosis and treatment. New York: McGraw-Hill; 2016: 242–320.
24. Mandell LW. Pneumonia. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 188–201.
25. Reed WW, Byrd GS, Gates RH Jr, et al. Sputum gram’s stain in community-acquired pneumococcal pneumonia. A meta-analysis. West J Med 1996;165:197–204.
26. Chalasani NP, Valdecanas MA, Gopal AK, et al. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest 1995;108:932–6.
27. Bordon J, Peyrani P, Brock GN, et al. The presence of pneumococcal bacteremia does not influence clinical outcomes in patients with community-acquired pneumonia: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort study. Chest 2008;133:618–24.
28. Helbig JH, Uldum SA, Bernander S, et al. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires’ disease. J Clin Microbiol 2003;41:838–40.
29. Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol 2003;41:2810–3.
30. Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis 2010;50:202–9.
31. Gilbert DN. Procalcitonin as a biomarker in respiratory tract infection. Clin Infect Dis 2011;52 Suppl 4:S346–50.
32. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2012;(9):CD007498.
33. Boussekey N, Leroy O, Alfandari S, et al. Procalcitonin kinetics in the prognosis of severe community-acquired pneumonia. Intensive Care Med 2006;32:469–72.
34. Flanders SA, Stein J, Shochat G, et al. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am J Med 2004;116:529–35.
35. Fine MJ, et al A prediction rule to identify low-risk patients with community-acquired pneumonia.N Engl J Med.1997;336:243-50.
36. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003;58:377–82.
37. Arnold FW, Ramirez JA, McDonald LC, Xia EL. Hospitalization for community-acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest 2003;124:121–4.
38. Aujesky D, McCausland JB, Whittle J, et al. Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumonia. Clin Infect Dis 2009;49:e100–8.
39. Charles PG, Wolfe R, Whitby M, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis 2008;47:375–84.
40. Marti C, Garin N, Grosgurin O, et al. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Crit Care 2012;16:R141.
41. Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 2012;54:621–9.
42. Silverman JA, Mortin LI, Vanpraagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis 2005;191:2149–52.
43. El Hajj MS, Turgeon RD, Wilby KJ. Ceftaroline fosamil for community-acquired pneumonia and skin and skin structure infections: a systematic review. Int J Clin Pharm 2017 Jan 5.
44. Taboada M, Melnick D, Iaconis JP, et al. Ceftaroline fosamil versus ceftriaxone for the treatment of community-acquired pneumonia: individual patient data meta-analysis of randomized controlled trials. J Antimicrob Chemother 2016;71:862–70.
45. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011;52:285–92.
46. Baddour LM, Yu VL, Klugman KP, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med 2004;170:440–4.
47. FDA Drug Safety Communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new boxed warning [Internet]. 15 Jan 2016. Available at www.fda.gov/Drugs/DrugSafety/ucm369580.htm.
48. Edelstein PR, CR. Legionnaires’ Disease and Pontiac Fever. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2633.
49. Hammerschlag MR, Kohlhoff SA, Gaydos, CA. Chlamydia pneumoniae. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2174.
50. Holzman RS, MS. Mycoplasma pneumoniae and Atypical Pneumonia. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2183.
51. Yamada M, Buller R, Bledsoe S, Storch GA. Rising rates of macrolide-resistant Mycoplasma pneumoniae in the central United States. Pediatr Infect Dis J 2012;31:409–10.
52. Yi SH, Hatfield KM, Baggs J, et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis 2017 Nov 6.
53. Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis 2011;52:1232–40.
54. Akram AR, Chalmers JD, Taylor JK, et al. An evaluation of clinical stability criteria to predict hospital course in community-acquired pneumonia. Clin Microbiol Infect 2013;19:1174–80.
55. Halm EA, Fine MJ, Kapoor WN, et al. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med 2002;162:1278–84.
56. Roson B, Carratala J, Fernandez-Sabe N, et al. Causes and factors associated with early failure in hospitalized patients with community-acquired pneumonia. Arch Intern Med 2004;164:502–8.
57. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med 2003;167:1650–4.
58. Wan YD, Sun TW, Liu ZQ, et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest 2016;149:209–19.
59. Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 2015;313:677–86.
60. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 2006;19:571–82.
61. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines. MMWR Recomm Rep 2016;65:1–54.
62. Rubins JB, Alter M, Loch J, Janoff EN. Determination of antibody responses of elderly adults to all 23 capsular polysaccharides after pneumococcal vaccination. Infect Immun 1999;67:5979–84.
63. Centers for Disease Control. Vaccines and preventable diseases [Internet]. 22 Nov 2016. Available at www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html.
64. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015;372:1114–25.
65. Centers for Disease Control. Recommended adult immunization schedule -- United States -- 2016 [Internet]. 2016. Available at www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule.pdf.
66. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2015;64:944–7.
67. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012;61:816–9.
68. Aujesky D, Auble TE, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med 2005;118:384–92.
From the University of North Dakota School of Medicine & Health Sciences, Fargo, ND.
Abstract
- Objective: To review the management of community-acquired pneumonia (CAP) in adults.
- Methods: Review of the literature.
- Results: Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually, accounting for significant morbidity and mortality. While numerous studies have previously shown pneumococcus to be the most common causative pathogen, the 2015 EPIC study found that in nearly two-thirds of patients with CAP who required hospitalization, no pathogen was detected. Symptoms and signs of respiratory tract infection are useful in helping to diagnose pneumonia; however, they are less sensitive than chest imaging studies. Laboratory tests used in diagnosing pneumonia include sputum Gram stain and culture, blood culture, urinary antigen, polymerase chain reaction, and biologic markers. In empiric treatment of CAP, both the typical and atypical pathogens should be targeted. Influenza vaccine and pneumococcal polysaccharide and conjugate vaccines should be administered as recommended by the CDC to reduce risk of CAP.
- Conclusion: CAP is a common illness with high rates of morbidity and mortality. Treatment is for the most part empirical; diagnostic testing can be used to identify the causative organism and guide pathogen-specific therapy.
Key words: community-acquired pneumonia; adults; management; vaccines.
Despite advances in medical science, pneumonia remains a major cause of morbidity and mortality. In 2014, 50,620 patients in the United States died from the disease [1]. Pneumonia can be classified as community-acquired, hospital-acquired, or ventilator-associated. Another category, healthcare-associated pneumonia, was included in an earlier American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guideline but was removed from the 2016 guideline because there was no clear evidence that patients diagnosed with healthcare-associated pneumonia were at higher risk for harboring multidrug-resistant pathogens [2]. In this article, we review the epidemiology, microbiology, predisposing factors, diagnosis, treatment, and prevention of community-acquired pneumonia (CAP).
Definition and Epidemiology
CAP is defined as an acute infection of the lungs that develops in patients who have not been hospitalized recently and have not had regular exposure to the health care system [3]. A previously ambulatory patient who is diagnosed with pneumonia within 48 hours after admission also meets the criteria for CAP. Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually [4]. About 25% of CAP patients require hospitalization, and about 5% to 10% of these patients are admitted to the intensive care unit (ICU) [5]. In-hospital mortality is considerable (~10% in population-based studies) [6] and 30-day mortality was found to be as high as 23% in a review by File and Marrie [7]. CAP also confers a high risk of long-term morbidity and mortality compared with the general population who have never had CAP, irrespective of age [8].
Causative Organisms
Numerous microorganisms can cause CAP. Common causes and less common causes are delineated in Table 1.
Predisposing Factors
Most people diagnosed with CAP have one or more predisposing factors [12,13] (Table 2).
Clinical Signs and Symptoms
Symptoms of CAP include fever, chills, rigors, fatigue, anorexia, diaphoresis, dyspnea, cough (with or without sputum production), and pleuritic chest pain. There is no individual symptom or cluster of symptoms that can absolutely differentiate pneumonia from other acute respiratory diseases, including upper and lower respiratory infections. However, if a patient presents with the constellation of symptoms of fever ≥ 1000F (37.80C), productive cough, and tachycardia, it is more suggestive of pneumonia [14]. Abnormal vital signs include fever, hypothermia, tachypnea, tachycardia, and oxygen desaturation. Auscultation of the chest reveals crackles or other adventitious breath sounds. Elderly patients with pneumonia report a significantly lower number of both respiratory and nonrespiratory symptoms compared with younger patients. Clinicians should be aware of this phenomenon so it does not lead to delayed diagnosis and treatment [15].
Imaging Evaluation
The presence of a pulmonary consolidation or an infiltrate on chest radiograph is required to diagnose CAP, and a chest radiograph should be obtained when CAP is suspected [16]. It should be noted that there is no pattern of radiographic abnormalities reliable enough to differentiate infectious pneumonia from noninfectious causes [17].
There are case reports and case series demonstrating false-negative plain chest radiographs existing in dehydrated patients [18] or in neutropenic state. However, animal studies have shown that dogs challenged with pneumococcus showed abnormal pulmonary shadow, suggestive of pneumonia, regardless of hydration status [19]. There is also no reliable scientific evidence to support the notion that severe neutropenia can cause false-negative radiographs because of the inability to develop an acute inflammatory reaction in the lungs [20].
A chest CT scan is more sensitive than a plain chest radiograph in detecting pneumonia. Therefore, a chest CT should be performed in a patient with negative plain chest radiograph when pneumonia is still highly suspected [21]. A chest CT scan is also more sensitive in detecting cavitation, adenopathy, interstitial disease and empyema. It also has the advantage of better defining anatomical changes than plain films [22].
Because improvement of pulmonary opacities in patients with CAP lags behind clinical improvement, repeating chest imaging studies is not recommended in patients who demonstrate clinical improvement. Sometimes clearing of pulmonary infiltrate or consolidation can take 6 weeks or longer [23].
Laboratory Evaluation
Generally the etiologic agent of CAP cannot be determined solely on the basis of clinical signs and symptoms or imaging studies. Although routine microbiological testing for patients suspicious for CAP is not necessary for empirical treatment, by determining the etiologic agent of the pneumonia, a clinician will be able to narrow the antibiotics from a broad-spectrum empirical regimen to specific pathogen-directed therapy. Determination of certain etiologic agents causing the pneumonia can have important public health implications (eg, Mycobacterium tuberculosis and influenza virus) [24].
Sputum Gram Stain and Culture
Sputum Gram stain is an inexpensive test that may identify pathogens that cause CAP (eg, S. pneumonia and Haemophilus influenzae). A quality specimen is required. A sputum sample must contain > 25 neutrophils and < 10 squamous epithelial cells/low power field on Gram stain to be considered suitable for culture.
The sensitivity and specificity of sputum Gram stain and culture are highly variable in different clinical settings (eg, outpatient setting, nursing home, ICU). Reed et al’s meta-analysis of patients diagnosed with CAP in the United States showed the sensitivity and specificity of sputum Gram stain (compared with sputum culture) ranged from 15% to 100% and 11% to 100%, respectively [24]. In cases of proven bacteremic pneumococcal pneumonia, positive cultures from sputum samples were positive less than 50% of the time [25].
For patients who cannot provide sputum samples or are intubated, a deep-suction aspirate or bronchoalveolar lavage through a bronchoscopic procedure might be necessary to obtain pulmonary secretion for Gram stain and culture. Besides bacterial culture, sputum samples can also be sent for fungal and mycobacterial cultures and acid-fast stain if deemed clinically necessary.
Blood Culture
Because the positivity rate of blood culture in patients who are suspected to have pneumonia but not exposed to antimicrobial agents is disappointingly low (5%–14%), blood cultures are no longer recommended in patients hospitalized for CAP. Another reason for not recommending blood culture is positive culture rarely leads to changes in antibiotic regimen in patients without underlying diseases [26]. However, high-risk patients, including patients with severe CAP or in immunocompromised patients (eg, patients with neutropenia, asplenia or complement deficiencies) should have a blood culture done [24].
A multinational study published in 2008 examined 125 patients with pneumococcal bacteremic CAP versus 1847 patients with non-bacteremic CAP [27]. Analysis of the data demonstrated no association of pneumococcal bacteremic CAP and time to clinical stability, length of hospital stay, all-cause mortality or CAP-related mortality. The authors concluded that pneumococcal bacteremia does not increase the risk of poor outcomes in patients with CAP compared to non-bacteremic patients, and the presence of pneumococcal bacteremia should not deter de-escalation of therapy in clinically stable patients.
Urinary Antigen Tests
Urinary antigen tests may assist clinicians in narrowing antibiotic therapy when test results are positive. There are 2 U.S. Food and Drug Administration–approved tests available to clinicians for detecting pneumococcal and Legionella antigen in urine. The test for Legionella pneumophila detects disease due to serogroup 1 only, which accounts for 80% of community-acquired Legionnaires disease. The sensitivity and specificity of the Legionella urine antigen test are 90% and 99%, respectively. The pneumococcal urine antigen test is less sensitive and specific than the Legionella urine antigen test (sensitivity 80% and specificity > 90%) [28,29].
Advantages of the urinary antigen tests are that they are easily performed, results are available in less than an hour if done in-house, and results are not affected by prior exposure to antibiotics. However, the tests do not meet Clinical Laboratory Improvements Amendments criteria for waiver and must be performed by a technician in the laboratory.
Polymerase Chain Reaction
There are several FDA-approved polymerase chain reaction (PCR) tests commercially available to assist clinicians in diagnosing pneumonia. PCR test of nasopharyngeal swabs for diagnosing influenza have become standard in many medical U.S. facilities. The great advantage of using PCR to diagnose influenza is its high sensitivity and specificity and rapid turnaround time. PCR can also be used to detect Legionella species, S. pneumonia, Mycoplasma pneumoniae, Chlamydophila pneumonia and mycobacterial species [24].
One limitation of using PCR tests on respiratory specimens is that specimens can be contaminated with oral or upper airway flora, so the results must be interpreted with caution, bearing in mind that some of the pathogens isolated may be colonizers of the oral or upper airway flora [30].
Biologic Markers
Two biologic markers—procalcitonin and C-reactive protein (CRP)—can be used in conjunction with history, physical examination, laboratory tests and imaging studies to assist in the diagnosis and treatment of CAP [24]. Procalcitonin is a peptide precursor of the hormone calcitonin that is released by parenchymal cells into the bloodstream resulting in increased serum level in patients with bacterial infections. In contrast, there is no remarkable proclacitonin level increase with viral or noninfectious inflammation. The reference value of procalcitonin in the blood of an adult individual without infection or inflammation is < 0.15 ng/mL. In the blood, procalcitonin has a half-life of 25 to 30 hours. The quantitative immunoluminometric method (LUMI test, Brahms PCT, Berlin, Germany ) is the preferred test to use because of its high sensitivity [31].
A 2012 Cochrane meta-analysis that involved 4221 patients with acute respiratory infections (with half of the patients diagnosed with CAP) from 14 prospective trials found the use of procalcitonin test for antibiotic use significantly decreased median antibiotic exposure from 8 to 4 days without an increase in treatment failure, mortality rates in any clinical setting (eg, outpatient clinic, emergency room), or length of hospitalization [32]. A prospective study conducted in France on 100 ICU patients showed that increased procalcitonin from day 1 to day 3 has a poor prognosis factor for severe CAP whereas decreasing procalcitonin levels is associated with a favorable outcome [33].
CRP is an acute phase protein produced by the liver. CRP level in the blood increases in response to acute infection or inflammation. Use of CRP in assisting diagnosis and guiding treatment of CAP is more limited in part due to its poor specificity. A prospective study conducted on 168 consecutive patients presented with cough showed that a CRP > 40 mg/L had a sensitivity and specificity of 70% and 90%, respectively [34].
T reatment
Site of Care Decision
For patients with CAP, the clinician must decide whether the patient will be treated in an outpatient or inpatient setting, and for those in the inpatient setting, whether they can safely be treated on the general medical ward or should be the ICU. Two common scoring systems that can be used to aid the clinician in determining severity of the infection and guiding site-of-care decisions are the Pneumonia Severity Index (PSI) and CURB-65 scores.
The PSI score uses 20 different parameters, including comorbidities, laboratory parameters and radiographic findings to stratify patients into 5 mortality risk classes [35]. On the basis of associated mortality rates, it has been suggested that risk class I and II patients should be treated as outpatients, risk class III patients should be treated in an observation unit or with a short hospitalization, and risk class IV and V patients should be treated as inpatients [35].
The CURB-65 method of risk stratification is based on 5 clinical parameters: confusion, urea level, respiratory rate, systolic blood pressure and age ≥ 65 (Table 3) [36].
Patients with CURB-65 scores of 4 or 5 are considered to have severe pneumonia and admission to the ICU should be considered. Aside from the CURB-65 score, anyone requiring vasopressor support or mechanical ventilation merits admission to the ICU [16]. IDSA/ATS guidelines also recommend the use of “minor criteria” for making ICU admission decisions; these include respiratory rate ≥ 30 breaths / minute, PaO2 fraction ≤ 250, multilobar infiltrates, confusion, blood urea nitrogen ≥ 20 mg/dL, leukopenia, thrombocytopenia, hypothermia and hypotension [16]. These factors are associated with increased mortality due to CAP and admission to an ICU is indicated if 3 of the minor criteria for severe CAP are present.
Similar to CURB-65, another clinical calculator that can be used for assessing severity of CAP is SMART-COP [39]. This scoring system uses 8 weighted criteria to predict which patients will require intensive respiratory or vasopressor support. SMART-COP has a sensitivity of 79% and specificity 64% in predicting ICU admission, whereas CURB-65 had a pooled sensitivity of 57.2% and specificity of 77.2% [40].
Antibiotic Therapy
Antibiotics are the mainstay of treatment for CAP, with the majority of patients with CAP treated empirically taking into account the site of care, likely pathogen, and antimicrobial resistance issues. Patients with pneumonia who are treated as outpatients usually respond well to empiric antibiotic treatment and a causative pathogen is not usually sought. Patients who are hospitalized for treatment of CAP usually receive empiric antibiotic on admission. Once the etiology has been determined by microbiologic or serologic means, antimicrobial therapy should be adjusted accordingly. As noted previously, a CDC study found that the burden of viral etiologies was higher than previously thought, with rhinovirus and influenza accounting for 15% of cases and S. pneumoniae for only 5% [9]. This study highlighted the fact that despite advances in molecular techniques, most patients with pneumonia have no pathogen identified [9]. Given the lack of discernable pathogens in the majority of cases, unless a nonbacterial etiology is found patients should continue to be treated with antibiotics.
Outpatients without comorbidities or risk factors for drug-resistant S. pneumoniae (Table 4)
As previously mentioned, antibiotic therapy is typically empiric; neither clinical features nor radiographic features are sufficient to include or exclude infectious etiologies. Epidemiologic risk factors should be considered and, in certain cases, expanded antimicrobial coverage to include those entities; for example, treatment of anaerobes in the setting of lung abscess and antipseudomonal antibiotics for patients with bronchiectasis.
Of concern in the treatment of CAP is the increased prevalence of antimicrobial resistance among S. pneumoniae. The IDSA guidelines report that drug-resistant S. pneumoniae is more common in persons aged < 2 or > 65 years, and those with ß-lactam therapy within the previous 3 months, alcoholism, medical comorbidities, immunosuppressive illness or therapy, or exposure to a child who attends a day care center [16].
S. aureus should be considered during influenza outbreaks, with either vancomycin or linezolid being the recommended agents in the setting of methicillin-resistant S. aureus (MRSA). In a study comparing vancomycin versus linezolid for nosocomial pneumonia, the all-cause 60-day mortality was similar for both agents [41]. Datpomycin is another agent used against MRSA; however, its use in the setting of pneumonia is not indicated as daptomycin binds to surfactant, yielding it ineffective in the treatment of pneumonia [42]. Ceftaroline is a newer cephalosporin with activity against MRSA; its role in treatment of community-acquired MRSA pneumonia has not been fully elucidated, but it appears to be a useful agent for this indication [43,44].
A summary of recommended empiric antibiotic therapy is presented in Table 5.
Antibiotic Therapy for Selected Pathogens
S. pneumoniae
Patients with pneumococcal pneumonia who have penicillin-susceptible strains can be treated with intravenous penicillin (2 or 3 million units every 4 hours) or ceftriaxone. Once a patient meets criteria of stability, they can then be transitioned to oral penicillin, amoxicillin, or clarithromycin. Those with strains with reduced susceptibility can still be treated with penicillin but at a higher dose (4 million units IV every 4 hours) or a third-generation cephalosporin. Those whose pneumococcal pneumonia is complicated by bacteremia will benefit from dual therapy if severely ill, requiring ICU monitoring. Those not severely ill can be treated with monotherapy [46].
S. aureus
S. aureus is more commonly associated with hospital-acquired pneumonia but may also be seen during the influenza season and in those with severe necrotizing CAP. Both linezolid and vancomycin can be used to treat MRSA CAP. As noted above, ceftaroline has activity against MRSA and is approved for treatment of CAP, but is not approved by the FDA for MRSA CAP treatment. Similarly, tigecycline is approved for CAP and has activity against MRSA, but is not approved for MRSA CAP. Moreover, the FDA has warned of increased risk of death with tigecycline and has a black box warning to that effect [47].
Legionella
Treatment of legionellosis can be achieved with tetracyclines, macrolides, or fluoroquinolones. For nonimmunosuppressed patients with mild pneumonia, any of the listed antibiotics is considered appropriate. However, patients with severe infection or those with immunosuppression should be treated with either levofloxacin or azithromycin for 7 to 10 days [48].
C. pneumoniae
As with other atypical organisms, C. pneumoniae can be treated with doxycycline, a macrolide, or respiratory fluoroquinolones. However, length of therapy varies by regimen used; whereas treating with doxycycline 100 mg twice daily generally requires 14–21 days, moxifloxacin 400 mg daily only requires 10 days [49].
M. pneumoniae
As with C. pneumoniae, length of therapy of M. pneumoniae varies by antimicrobial used. Shortest courses are seen with the use of macrolides for 5 days, whereas 14 days is considered standard for doxycycline or a respiratory fluoroquinolone [50]. It should be noted that there has been increasingly documented resistance to macrolides, with known resistance of 8.2% in the United States [51].
Duration of Treatment
Most patients with CAP respond within 72 hours to appropriate therapy. IDSA/ATS guidelines recommend that patients be treated for a minimum of 5 days, and before discontinuing antibiotics patients should be afebrile a minimum of 48-72 hours and be clinically stable (Table 6) [16].
Hospitalized patients do not need to be monitored for an additional day once they have reached clinical stability (Table 6), are able to maintain oral intake, and have normal mentation, provided that other comorbidities are stable and social needs have been met [16]. Patients discharged from the hospital with instability have higher risk of readmission or death [55].
Transition to Oral Therapy
IDSA/ATS guidelines [16] recommend that patients should be transitioned from IV to oral antibiotics when they are improving clinically, have stable vital signs, and are able to ingest food/fluids and medications.
Management of Nonresponders
Although the majority of patients respond to antibiotics within 72 hours, treatment failure occurs in up to 15% of patients [45]. Nonresponding pneumonia is generally seen in 2 patterns: worsening of clinical status despite empiric antibiotics OR delay in achieving clinical stability as defined in Table 5 after 72 hours of treatment [13]. Risk factors associated with nonresponding pneumonia [56] are:
- Radiographic: multilobar infiltrates, pleural effusion, cavitation
- Bacteriologic: MRSA, gram-negative or Legionella pneumonia
- Severity index: PSI > 90
- Pharmacologic: incorrect antibiotic choice based on susceptibility
Patients with acute deterioration of clinical status will prompt transfer to a higher level of care and may require mechanical ventilator support. In those with delay in achieving clinical stability, question centers on whether the same antibiotics can be continued while doing further radiographic/microbiologic workup and/or changing antibiotics.
History should be reviewed with particular attention to exposures, travel history, and microbiologic and radiographic data. Clinicians should recall that viral causes account for up to 20% of pneumonias and there are also noninfectious causes that can mimic pyogenic infections [57]. If adequate initial cultures were not obtained, they should be obtained; however, care must be taken in reviewing new sets of cultures while on antibiotics as they may reveal colonization selected out by antibiotics and not a true pathogen. If repeat evaluation is unrevealing, then further evaluation with CT scan and bronchoscopy with bronchoalveolar lavage and biopsy is warranted. CT scans can show pleural effusions, bronchial obstructions or pattern suggestive of cryptogenic pneumonia. A bronchoscopy might yield a microbiologic diagnosis and with biopsy can also evaluate for noninfectious causes.
As with other infections, if escalation of antibiotics is undertaken, clinicians should be mindful to ensure that efforts are being made to elucidate the reason for nonresponse. To simply broaden antimicrobial therapy without attempts at establishing a microbiologic or radiographic cause for nonresponse may lead to inappropriate treatment recurrence of infection. Aside from patients who have bacteremic pneumococcal pneumonia in an ICU setting, there are no published reports pointing to superiority of combination antibiotics [46].
Other Treatment
Because of the inflammatory response associated with pneumonia, several agents have been evaluated as adjunctive treatment of pneumonia to decrease this inflammatory state; namely, steroids, macrolide antibiotics and statins. To date, only the use of steroids (methylprednisolone 0.5 mg/kg every 12 hours for 5 days) in those with severe CAP and high initial anti-inflammatory response (CRP > 150) was shown to decrease treatment failure, decreased risk of ARDS, possibly reduce length of stay, duration of intravenous antibiotics and clinical stability, without effect on mortality or adverse side effects [58,59].
Other adjunctive methods have not been found to have significant impact [16].
Prevention of Pneumonia
Prevention of pneumococcal pneumonia is twofold: prevention of infection caused by S. pneumoniae and prevention of influenza infection. As influenza infection is a risk factor for bacterial infection, specifically with S. pneumoniae, influenza vaccination can prevent bacterial pneumonia [60]. In their most recent recommendations, the CDC continues to recommend routine influenza vaccination for all persons aged greater than 6 months, unless otherwise contraindicated [61].
There are 2 vaccines for prevention of pneumococcal disease: the pneumococcal polysaccharide vaccine (PPSV23) and a conjugate vaccine (PCV13). Following vaccination with PPSV23, 80% of adults develop antibodies against at least 18 of the 23 serotypes [62]. Despite this response, PPSV23 is reported to be protective against invasive pneumococcal infection; yet there is no consensus regarding PPSV23 leading to decreased rates of pneumonia [63]. On the other hand, PCV13 vaccination was associated with prevention of both invasive disease and community-acquired pneumonia in adults 65 years or older [64]. The CDC recommends that all children aged 2 or under receive PCV13, whereas those aged 65 or older should receive PCV13 followed by a dose of PPSV23 [65]. The dose of PPSV23 should be given ≥1 year following the dose of PCV13 [66].Persons < 65 years of age with immunocompromising and certain other conditions should also receive vaccination [67] (Table 7). Full details, many scenarios, and timing of vaccinations can be found at www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule.pdf.
Cigarette smoking increases the risk of respiratory infections as evidenced by smokers accounting for almost half of all patients with invasive pneumococcal disease [11]. As this is a modifiable risk factor it should be a goal of a comprehensive approach towards prevention of pneumonia.
Summary
CAP remains a leading cause of hospitalization and death in the 21st century. Traditionally, pneumococcus has been considered the major pathogen causing CAP; however, the 2015 EPIC study found that in only 5% of patients diagnosed with CAP was S. pneumoniae detected. Despite the new findings, it is still recommended that empiric treatment for CAP target common typical bacteria (pneumococcus, H. influenzae, Moraxella catarrhalis) and atypical bacteria (M. pneumonia, C. pneumoniae, L. pneumophila).
Because diagnosing pneumonia through history and clinical examination is less than 50% sensitive, a chest imaging study (a plain chest radiograph or a chest CT scan) is usually required to make the diagnosis. Laboratory tests, such as sputum Gram stain/culture, blood culture, urinary antigen tests, PCR test, procalcitonin, and CRP are important adjunctive diagnostic modalities to assist in the diagnosis and management of CAP. However, no single test is sensitive and specific enough to be a stand-alone test. They should be used in conjunction with history, physical examination, and imaging studies. Because vaccination (PPSV23, PCV13, and influenza vaccine) remains the most effective tool in preventing the development of CAP, clinicians, should strive for 100% vaccination rates in appropriate persons.
Corresponding author: Tze Shein Lo, MD, University of North Dakota, 1919 Elm Street, Fargo, ND 58102, tzeshein.lo@med.und.edu.
Financial disclosures: None.
Author contributions: drafting of article, PM, TSL; critical revision of the article, PM, TSL.
From the University of North Dakota School of Medicine & Health Sciences, Fargo, ND.
Abstract
- Objective: To review the management of community-acquired pneumonia (CAP) in adults.
- Methods: Review of the literature.
- Results: Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually, accounting for significant morbidity and mortality. While numerous studies have previously shown pneumococcus to be the most common causative pathogen, the 2015 EPIC study found that in nearly two-thirds of patients with CAP who required hospitalization, no pathogen was detected. Symptoms and signs of respiratory tract infection are useful in helping to diagnose pneumonia; however, they are less sensitive than chest imaging studies. Laboratory tests used in diagnosing pneumonia include sputum Gram stain and culture, blood culture, urinary antigen, polymerase chain reaction, and biologic markers. In empiric treatment of CAP, both the typical and atypical pathogens should be targeted. Influenza vaccine and pneumococcal polysaccharide and conjugate vaccines should be administered as recommended by the CDC to reduce risk of CAP.
- Conclusion: CAP is a common illness with high rates of morbidity and mortality. Treatment is for the most part empirical; diagnostic testing can be used to identify the causative organism and guide pathogen-specific therapy.
Key words: community-acquired pneumonia; adults; management; vaccines.
Despite advances in medical science, pneumonia remains a major cause of morbidity and mortality. In 2014, 50,620 patients in the United States died from the disease [1]. Pneumonia can be classified as community-acquired, hospital-acquired, or ventilator-associated. Another category, healthcare-associated pneumonia, was included in an earlier American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guideline but was removed from the 2016 guideline because there was no clear evidence that patients diagnosed with healthcare-associated pneumonia were at higher risk for harboring multidrug-resistant pathogens [2]. In this article, we review the epidemiology, microbiology, predisposing factors, diagnosis, treatment, and prevention of community-acquired pneumonia (CAP).
Definition and Epidemiology
CAP is defined as an acute infection of the lungs that develops in patients who have not been hospitalized recently and have not had regular exposure to the health care system [3]. A previously ambulatory patient who is diagnosed with pneumonia within 48 hours after admission also meets the criteria for CAP. Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually [4]. About 25% of CAP patients require hospitalization, and about 5% to 10% of these patients are admitted to the intensive care unit (ICU) [5]. In-hospital mortality is considerable (~10% in population-based studies) [6] and 30-day mortality was found to be as high as 23% in a review by File and Marrie [7]. CAP also confers a high risk of long-term morbidity and mortality compared with the general population who have never had CAP, irrespective of age [8].
Causative Organisms
Numerous microorganisms can cause CAP. Common causes and less common causes are delineated in Table 1.
Predisposing Factors
Most people diagnosed with CAP have one or more predisposing factors [12,13] (Table 2).
Clinical Signs and Symptoms
Symptoms of CAP include fever, chills, rigors, fatigue, anorexia, diaphoresis, dyspnea, cough (with or without sputum production), and pleuritic chest pain. There is no individual symptom or cluster of symptoms that can absolutely differentiate pneumonia from other acute respiratory diseases, including upper and lower respiratory infections. However, if a patient presents with the constellation of symptoms of fever ≥ 1000F (37.80C), productive cough, and tachycardia, it is more suggestive of pneumonia [14]. Abnormal vital signs include fever, hypothermia, tachypnea, tachycardia, and oxygen desaturation. Auscultation of the chest reveals crackles or other adventitious breath sounds. Elderly patients with pneumonia report a significantly lower number of both respiratory and nonrespiratory symptoms compared with younger patients. Clinicians should be aware of this phenomenon so it does not lead to delayed diagnosis and treatment [15].
Imaging Evaluation
The presence of a pulmonary consolidation or an infiltrate on chest radiograph is required to diagnose CAP, and a chest radiograph should be obtained when CAP is suspected [16]. It should be noted that there is no pattern of radiographic abnormalities reliable enough to differentiate infectious pneumonia from noninfectious causes [17].
There are case reports and case series demonstrating false-negative plain chest radiographs existing in dehydrated patients [18] or in neutropenic state. However, animal studies have shown that dogs challenged with pneumococcus showed abnormal pulmonary shadow, suggestive of pneumonia, regardless of hydration status [19]. There is also no reliable scientific evidence to support the notion that severe neutropenia can cause false-negative radiographs because of the inability to develop an acute inflammatory reaction in the lungs [20].
A chest CT scan is more sensitive than a plain chest radiograph in detecting pneumonia. Therefore, a chest CT should be performed in a patient with negative plain chest radiograph when pneumonia is still highly suspected [21]. A chest CT scan is also more sensitive in detecting cavitation, adenopathy, interstitial disease and empyema. It also has the advantage of better defining anatomical changes than plain films [22].
Because improvement of pulmonary opacities in patients with CAP lags behind clinical improvement, repeating chest imaging studies is not recommended in patients who demonstrate clinical improvement. Sometimes clearing of pulmonary infiltrate or consolidation can take 6 weeks or longer [23].
Laboratory Evaluation
Generally the etiologic agent of CAP cannot be determined solely on the basis of clinical signs and symptoms or imaging studies. Although routine microbiological testing for patients suspicious for CAP is not necessary for empirical treatment, by determining the etiologic agent of the pneumonia, a clinician will be able to narrow the antibiotics from a broad-spectrum empirical regimen to specific pathogen-directed therapy. Determination of certain etiologic agents causing the pneumonia can have important public health implications (eg, Mycobacterium tuberculosis and influenza virus) [24].
Sputum Gram Stain and Culture
Sputum Gram stain is an inexpensive test that may identify pathogens that cause CAP (eg, S. pneumonia and Haemophilus influenzae). A quality specimen is required. A sputum sample must contain > 25 neutrophils and < 10 squamous epithelial cells/low power field on Gram stain to be considered suitable for culture.
The sensitivity and specificity of sputum Gram stain and culture are highly variable in different clinical settings (eg, outpatient setting, nursing home, ICU). Reed et al’s meta-analysis of patients diagnosed with CAP in the United States showed the sensitivity and specificity of sputum Gram stain (compared with sputum culture) ranged from 15% to 100% and 11% to 100%, respectively [24]. In cases of proven bacteremic pneumococcal pneumonia, positive cultures from sputum samples were positive less than 50% of the time [25].
For patients who cannot provide sputum samples or are intubated, a deep-suction aspirate or bronchoalveolar lavage through a bronchoscopic procedure might be necessary to obtain pulmonary secretion for Gram stain and culture. Besides bacterial culture, sputum samples can also be sent for fungal and mycobacterial cultures and acid-fast stain if deemed clinically necessary.
Blood Culture
Because the positivity rate of blood culture in patients who are suspected to have pneumonia but not exposed to antimicrobial agents is disappointingly low (5%–14%), blood cultures are no longer recommended in patients hospitalized for CAP. Another reason for not recommending blood culture is positive culture rarely leads to changes in antibiotic regimen in patients without underlying diseases [26]. However, high-risk patients, including patients with severe CAP or in immunocompromised patients (eg, patients with neutropenia, asplenia or complement deficiencies) should have a blood culture done [24].
A multinational study published in 2008 examined 125 patients with pneumococcal bacteremic CAP versus 1847 patients with non-bacteremic CAP [27]. Analysis of the data demonstrated no association of pneumococcal bacteremic CAP and time to clinical stability, length of hospital stay, all-cause mortality or CAP-related mortality. The authors concluded that pneumococcal bacteremia does not increase the risk of poor outcomes in patients with CAP compared to non-bacteremic patients, and the presence of pneumococcal bacteremia should not deter de-escalation of therapy in clinically stable patients.
Urinary Antigen Tests
Urinary antigen tests may assist clinicians in narrowing antibiotic therapy when test results are positive. There are 2 U.S. Food and Drug Administration–approved tests available to clinicians for detecting pneumococcal and Legionella antigen in urine. The test for Legionella pneumophila detects disease due to serogroup 1 only, which accounts for 80% of community-acquired Legionnaires disease. The sensitivity and specificity of the Legionella urine antigen test are 90% and 99%, respectively. The pneumococcal urine antigen test is less sensitive and specific than the Legionella urine antigen test (sensitivity 80% and specificity > 90%) [28,29].
Advantages of the urinary antigen tests are that they are easily performed, results are available in less than an hour if done in-house, and results are not affected by prior exposure to antibiotics. However, the tests do not meet Clinical Laboratory Improvements Amendments criteria for waiver and must be performed by a technician in the laboratory.
Polymerase Chain Reaction
There are several FDA-approved polymerase chain reaction (PCR) tests commercially available to assist clinicians in diagnosing pneumonia. PCR test of nasopharyngeal swabs for diagnosing influenza have become standard in many medical U.S. facilities. The great advantage of using PCR to diagnose influenza is its high sensitivity and specificity and rapid turnaround time. PCR can also be used to detect Legionella species, S. pneumonia, Mycoplasma pneumoniae, Chlamydophila pneumonia and mycobacterial species [24].
One limitation of using PCR tests on respiratory specimens is that specimens can be contaminated with oral or upper airway flora, so the results must be interpreted with caution, bearing in mind that some of the pathogens isolated may be colonizers of the oral or upper airway flora [30].
Biologic Markers
Two biologic markers—procalcitonin and C-reactive protein (CRP)—can be used in conjunction with history, physical examination, laboratory tests and imaging studies to assist in the diagnosis and treatment of CAP [24]. Procalcitonin is a peptide precursor of the hormone calcitonin that is released by parenchymal cells into the bloodstream resulting in increased serum level in patients with bacterial infections. In contrast, there is no remarkable proclacitonin level increase with viral or noninfectious inflammation. The reference value of procalcitonin in the blood of an adult individual without infection or inflammation is < 0.15 ng/mL. In the blood, procalcitonin has a half-life of 25 to 30 hours. The quantitative immunoluminometric method (LUMI test, Brahms PCT, Berlin, Germany ) is the preferred test to use because of its high sensitivity [31].
A 2012 Cochrane meta-analysis that involved 4221 patients with acute respiratory infections (with half of the patients diagnosed with CAP) from 14 prospective trials found the use of procalcitonin test for antibiotic use significantly decreased median antibiotic exposure from 8 to 4 days without an increase in treatment failure, mortality rates in any clinical setting (eg, outpatient clinic, emergency room), or length of hospitalization [32]. A prospective study conducted in France on 100 ICU patients showed that increased procalcitonin from day 1 to day 3 has a poor prognosis factor for severe CAP whereas decreasing procalcitonin levels is associated with a favorable outcome [33].
CRP is an acute phase protein produced by the liver. CRP level in the blood increases in response to acute infection or inflammation. Use of CRP in assisting diagnosis and guiding treatment of CAP is more limited in part due to its poor specificity. A prospective study conducted on 168 consecutive patients presented with cough showed that a CRP > 40 mg/L had a sensitivity and specificity of 70% and 90%, respectively [34].
T reatment
Site of Care Decision
For patients with CAP, the clinician must decide whether the patient will be treated in an outpatient or inpatient setting, and for those in the inpatient setting, whether they can safely be treated on the general medical ward or should be the ICU. Two common scoring systems that can be used to aid the clinician in determining severity of the infection and guiding site-of-care decisions are the Pneumonia Severity Index (PSI) and CURB-65 scores.
The PSI score uses 20 different parameters, including comorbidities, laboratory parameters and radiographic findings to stratify patients into 5 mortality risk classes [35]. On the basis of associated mortality rates, it has been suggested that risk class I and II patients should be treated as outpatients, risk class III patients should be treated in an observation unit or with a short hospitalization, and risk class IV and V patients should be treated as inpatients [35].
The CURB-65 method of risk stratification is based on 5 clinical parameters: confusion, urea level, respiratory rate, systolic blood pressure and age ≥ 65 (Table 3) [36].
Patients with CURB-65 scores of 4 or 5 are considered to have severe pneumonia and admission to the ICU should be considered. Aside from the CURB-65 score, anyone requiring vasopressor support or mechanical ventilation merits admission to the ICU [16]. IDSA/ATS guidelines also recommend the use of “minor criteria” for making ICU admission decisions; these include respiratory rate ≥ 30 breaths / minute, PaO2 fraction ≤ 250, multilobar infiltrates, confusion, blood urea nitrogen ≥ 20 mg/dL, leukopenia, thrombocytopenia, hypothermia and hypotension [16]. These factors are associated with increased mortality due to CAP and admission to an ICU is indicated if 3 of the minor criteria for severe CAP are present.
Similar to CURB-65, another clinical calculator that can be used for assessing severity of CAP is SMART-COP [39]. This scoring system uses 8 weighted criteria to predict which patients will require intensive respiratory or vasopressor support. SMART-COP has a sensitivity of 79% and specificity 64% in predicting ICU admission, whereas CURB-65 had a pooled sensitivity of 57.2% and specificity of 77.2% [40].
Antibiotic Therapy
Antibiotics are the mainstay of treatment for CAP, with the majority of patients with CAP treated empirically taking into account the site of care, likely pathogen, and antimicrobial resistance issues. Patients with pneumonia who are treated as outpatients usually respond well to empiric antibiotic treatment and a causative pathogen is not usually sought. Patients who are hospitalized for treatment of CAP usually receive empiric antibiotic on admission. Once the etiology has been determined by microbiologic or serologic means, antimicrobial therapy should be adjusted accordingly. As noted previously, a CDC study found that the burden of viral etiologies was higher than previously thought, with rhinovirus and influenza accounting for 15% of cases and S. pneumoniae for only 5% [9]. This study highlighted the fact that despite advances in molecular techniques, most patients with pneumonia have no pathogen identified [9]. Given the lack of discernable pathogens in the majority of cases, unless a nonbacterial etiology is found patients should continue to be treated with antibiotics.
Outpatients without comorbidities or risk factors for drug-resistant S. pneumoniae (Table 4)
As previously mentioned, antibiotic therapy is typically empiric; neither clinical features nor radiographic features are sufficient to include or exclude infectious etiologies. Epidemiologic risk factors should be considered and, in certain cases, expanded antimicrobial coverage to include those entities; for example, treatment of anaerobes in the setting of lung abscess and antipseudomonal antibiotics for patients with bronchiectasis.
Of concern in the treatment of CAP is the increased prevalence of antimicrobial resistance among S. pneumoniae. The IDSA guidelines report that drug-resistant S. pneumoniae is more common in persons aged < 2 or > 65 years, and those with ß-lactam therapy within the previous 3 months, alcoholism, medical comorbidities, immunosuppressive illness or therapy, or exposure to a child who attends a day care center [16].
S. aureus should be considered during influenza outbreaks, with either vancomycin or linezolid being the recommended agents in the setting of methicillin-resistant S. aureus (MRSA). In a study comparing vancomycin versus linezolid for nosocomial pneumonia, the all-cause 60-day mortality was similar for both agents [41]. Datpomycin is another agent used against MRSA; however, its use in the setting of pneumonia is not indicated as daptomycin binds to surfactant, yielding it ineffective in the treatment of pneumonia [42]. Ceftaroline is a newer cephalosporin with activity against MRSA; its role in treatment of community-acquired MRSA pneumonia has not been fully elucidated, but it appears to be a useful agent for this indication [43,44].
A summary of recommended empiric antibiotic therapy is presented in Table 5.
Antibiotic Therapy for Selected Pathogens
S. pneumoniae
Patients with pneumococcal pneumonia who have penicillin-susceptible strains can be treated with intravenous penicillin (2 or 3 million units every 4 hours) or ceftriaxone. Once a patient meets criteria of stability, they can then be transitioned to oral penicillin, amoxicillin, or clarithromycin. Those with strains with reduced susceptibility can still be treated with penicillin but at a higher dose (4 million units IV every 4 hours) or a third-generation cephalosporin. Those whose pneumococcal pneumonia is complicated by bacteremia will benefit from dual therapy if severely ill, requiring ICU monitoring. Those not severely ill can be treated with monotherapy [46].
S. aureus
S. aureus is more commonly associated with hospital-acquired pneumonia but may also be seen during the influenza season and in those with severe necrotizing CAP. Both linezolid and vancomycin can be used to treat MRSA CAP. As noted above, ceftaroline has activity against MRSA and is approved for treatment of CAP, but is not approved by the FDA for MRSA CAP treatment. Similarly, tigecycline is approved for CAP and has activity against MRSA, but is not approved for MRSA CAP. Moreover, the FDA has warned of increased risk of death with tigecycline and has a black box warning to that effect [47].
Legionella
Treatment of legionellosis can be achieved with tetracyclines, macrolides, or fluoroquinolones. For nonimmunosuppressed patients with mild pneumonia, any of the listed antibiotics is considered appropriate. However, patients with severe infection or those with immunosuppression should be treated with either levofloxacin or azithromycin for 7 to 10 days [48].
C. pneumoniae
As with other atypical organisms, C. pneumoniae can be treated with doxycycline, a macrolide, or respiratory fluoroquinolones. However, length of therapy varies by regimen used; whereas treating with doxycycline 100 mg twice daily generally requires 14–21 days, moxifloxacin 400 mg daily only requires 10 days [49].
M. pneumoniae
As with C. pneumoniae, length of therapy of M. pneumoniae varies by antimicrobial used. Shortest courses are seen with the use of macrolides for 5 days, whereas 14 days is considered standard for doxycycline or a respiratory fluoroquinolone [50]. It should be noted that there has been increasingly documented resistance to macrolides, with known resistance of 8.2% in the United States [51].
Duration of Treatment
Most patients with CAP respond within 72 hours to appropriate therapy. IDSA/ATS guidelines recommend that patients be treated for a minimum of 5 days, and before discontinuing antibiotics patients should be afebrile a minimum of 48-72 hours and be clinically stable (Table 6) [16].
Hospitalized patients do not need to be monitored for an additional day once they have reached clinical stability (Table 6), are able to maintain oral intake, and have normal mentation, provided that other comorbidities are stable and social needs have been met [16]. Patients discharged from the hospital with instability have higher risk of readmission or death [55].
Transition to Oral Therapy
IDSA/ATS guidelines [16] recommend that patients should be transitioned from IV to oral antibiotics when they are improving clinically, have stable vital signs, and are able to ingest food/fluids and medications.
Management of Nonresponders
Although the majority of patients respond to antibiotics within 72 hours, treatment failure occurs in up to 15% of patients [45]. Nonresponding pneumonia is generally seen in 2 patterns: worsening of clinical status despite empiric antibiotics OR delay in achieving clinical stability as defined in Table 5 after 72 hours of treatment [13]. Risk factors associated with nonresponding pneumonia [56] are:
- Radiographic: multilobar infiltrates, pleural effusion, cavitation
- Bacteriologic: MRSA, gram-negative or Legionella pneumonia
- Severity index: PSI > 90
- Pharmacologic: incorrect antibiotic choice based on susceptibility
Patients with acute deterioration of clinical status will prompt transfer to a higher level of care and may require mechanical ventilator support. In those with delay in achieving clinical stability, question centers on whether the same antibiotics can be continued while doing further radiographic/microbiologic workup and/or changing antibiotics.
History should be reviewed with particular attention to exposures, travel history, and microbiologic and radiographic data. Clinicians should recall that viral causes account for up to 20% of pneumonias and there are also noninfectious causes that can mimic pyogenic infections [57]. If adequate initial cultures were not obtained, they should be obtained; however, care must be taken in reviewing new sets of cultures while on antibiotics as they may reveal colonization selected out by antibiotics and not a true pathogen. If repeat evaluation is unrevealing, then further evaluation with CT scan and bronchoscopy with bronchoalveolar lavage and biopsy is warranted. CT scans can show pleural effusions, bronchial obstructions or pattern suggestive of cryptogenic pneumonia. A bronchoscopy might yield a microbiologic diagnosis and with biopsy can also evaluate for noninfectious causes.
As with other infections, if escalation of antibiotics is undertaken, clinicians should be mindful to ensure that efforts are being made to elucidate the reason for nonresponse. To simply broaden antimicrobial therapy without attempts at establishing a microbiologic or radiographic cause for nonresponse may lead to inappropriate treatment recurrence of infection. Aside from patients who have bacteremic pneumococcal pneumonia in an ICU setting, there are no published reports pointing to superiority of combination antibiotics [46].
Other Treatment
Because of the inflammatory response associated with pneumonia, several agents have been evaluated as adjunctive treatment of pneumonia to decrease this inflammatory state; namely, steroids, macrolide antibiotics and statins. To date, only the use of steroids (methylprednisolone 0.5 mg/kg every 12 hours for 5 days) in those with severe CAP and high initial anti-inflammatory response (CRP > 150) was shown to decrease treatment failure, decreased risk of ARDS, possibly reduce length of stay, duration of intravenous antibiotics and clinical stability, without effect on mortality or adverse side effects [58,59].
Other adjunctive methods have not been found to have significant impact [16].
Prevention of Pneumonia
Prevention of pneumococcal pneumonia is twofold: prevention of infection caused by S. pneumoniae and prevention of influenza infection. As influenza infection is a risk factor for bacterial infection, specifically with S. pneumoniae, influenza vaccination can prevent bacterial pneumonia [60]. In their most recent recommendations, the CDC continues to recommend routine influenza vaccination for all persons aged greater than 6 months, unless otherwise contraindicated [61].
There are 2 vaccines for prevention of pneumococcal disease: the pneumococcal polysaccharide vaccine (PPSV23) and a conjugate vaccine (PCV13). Following vaccination with PPSV23, 80% of adults develop antibodies against at least 18 of the 23 serotypes [62]. Despite this response, PPSV23 is reported to be protective against invasive pneumococcal infection; yet there is no consensus regarding PPSV23 leading to decreased rates of pneumonia [63]. On the other hand, PCV13 vaccination was associated with prevention of both invasive disease and community-acquired pneumonia in adults 65 years or older [64]. The CDC recommends that all children aged 2 or under receive PCV13, whereas those aged 65 or older should receive PCV13 followed by a dose of PPSV23 [65]. The dose of PPSV23 should be given ≥1 year following the dose of PCV13 [66].Persons < 65 years of age with immunocompromising and certain other conditions should also receive vaccination [67] (Table 7). Full details, many scenarios, and timing of vaccinations can be found at www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule.pdf.
Cigarette smoking increases the risk of respiratory infections as evidenced by smokers accounting for almost half of all patients with invasive pneumococcal disease [11]. As this is a modifiable risk factor it should be a goal of a comprehensive approach towards prevention of pneumonia.
Summary
CAP remains a leading cause of hospitalization and death in the 21st century. Traditionally, pneumococcus has been considered the major pathogen causing CAP; however, the 2015 EPIC study found that in only 5% of patients diagnosed with CAP was S. pneumoniae detected. Despite the new findings, it is still recommended that empiric treatment for CAP target common typical bacteria (pneumococcus, H. influenzae, Moraxella catarrhalis) and atypical bacteria (M. pneumonia, C. pneumoniae, L. pneumophila).
Because diagnosing pneumonia through history and clinical examination is less than 50% sensitive, a chest imaging study (a plain chest radiograph or a chest CT scan) is usually required to make the diagnosis. Laboratory tests, such as sputum Gram stain/culture, blood culture, urinary antigen tests, PCR test, procalcitonin, and CRP are important adjunctive diagnostic modalities to assist in the diagnosis and management of CAP. However, no single test is sensitive and specific enough to be a stand-alone test. They should be used in conjunction with history, physical examination, and imaging studies. Because vaccination (PPSV23, PCV13, and influenza vaccine) remains the most effective tool in preventing the development of CAP, clinicians, should strive for 100% vaccination rates in appropriate persons.
Corresponding author: Tze Shein Lo, MD, University of North Dakota, 1919 Elm Street, Fargo, ND 58102, tzeshein.lo@med.und.edu.
Financial disclosures: None.
Author contributions: drafting of article, PM, TSL; critical revision of the article, PM, TSL.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. FastStats - Pneumonia. Accessed 6 Oct 2016 at www.cdc.gov/nchs/fastats/pneumonia.htm.
2. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61-e111.
3. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med 2014;371:1619–28.
4. Mandell LA. Epidemiology and etiology of community-acquired pneumonia. Infect Dis
5. Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ 2006;332:1077–9.
6. Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med 2007;167:1938–43
7. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med 2010;122:130–41.
8. Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-year mortality after community-acquired pneumonia. a prospective cohort. Am J Respir Crit Care Med 2015;192:597-604.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015;373:415–27.
10. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013;369:155–63.
11. Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med 2000;342:681–9.
12. Almirall J, Serra-Prat M, Bolíbar I, BalassoV. Risk factors for community-acquired pneumonia in adults: a systemic review of observational studies. Respiration 2017;94:299–311.
13. Janoff EM.Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s principles and practice of infectioius diseases. 8th ed. Philadelphia: Sauders; 2015: 2310–27.
14. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough--a statistical approach. J Chronic Dis 1984;37:215–25.
15. Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med 1997;157:1453–9.
16. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44 Suppl 2:S27–72.
17. Jartti A, Rauvala E, Kauma H, et al. Chest imaging findings in hospitalized patients with H1N1 influenza. Acta Radiol 2011;52:297–304.
18. Basi SK, Marrie TJ, Huang JQ, Majumdar SR. Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med 2004;117:305–11.
19. Caldwell A, Glauser FL, Smith WR, et al. The effects of dehydration on the radiologic and pathologic appearance of experimental canine segmental pneumonia. Am Rev Respir Dis 1975;112:651–6.
20. Bartlett JG. Pneumonia. In: Barlett JG, editor. Management of respiratory tract infections. Philadelphia: Lippincott, Williams & Wilkins; 2001: 1–122.
21. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med 2015;192:974–82.
22. Wheeler JH, Fishman EK. Computed tomography in the management of chest infections: current status. Clin Infect Dis 1996;23:232–40.
23. Chesnutt MP. Pulmonary disorders. In: Papadakis MM, editor. Current medical diagnosis and treatment. New York: McGraw-Hill; 2016: 242–320.
24. Mandell LW. Pneumonia. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 188–201.
25. Reed WW, Byrd GS, Gates RH Jr, et al. Sputum gram’s stain in community-acquired pneumococcal pneumonia. A meta-analysis. West J Med 1996;165:197–204.
26. Chalasani NP, Valdecanas MA, Gopal AK, et al. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest 1995;108:932–6.
27. Bordon J, Peyrani P, Brock GN, et al. The presence of pneumococcal bacteremia does not influence clinical outcomes in patients with community-acquired pneumonia: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort study. Chest 2008;133:618–24.
28. Helbig JH, Uldum SA, Bernander S, et al. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires’ disease. J Clin Microbiol 2003;41:838–40.
29. Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol 2003;41:2810–3.
30. Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis 2010;50:202–9.
31. Gilbert DN. Procalcitonin as a biomarker in respiratory tract infection. Clin Infect Dis 2011;52 Suppl 4:S346–50.
32. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2012;(9):CD007498.
33. Boussekey N, Leroy O, Alfandari S, et al. Procalcitonin kinetics in the prognosis of severe community-acquired pneumonia. Intensive Care Med 2006;32:469–72.
34. Flanders SA, Stein J, Shochat G, et al. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am J Med 2004;116:529–35.
35. Fine MJ, et al A prediction rule to identify low-risk patients with community-acquired pneumonia.N Engl J Med.1997;336:243-50.
36. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003;58:377–82.
37. Arnold FW, Ramirez JA, McDonald LC, Xia EL. Hospitalization for community-acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest 2003;124:121–4.
38. Aujesky D, McCausland JB, Whittle J, et al. Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumonia. Clin Infect Dis 2009;49:e100–8.
39. Charles PG, Wolfe R, Whitby M, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis 2008;47:375–84.
40. Marti C, Garin N, Grosgurin O, et al. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Crit Care 2012;16:R141.
41. Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 2012;54:621–9.
42. Silverman JA, Mortin LI, Vanpraagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis 2005;191:2149–52.
43. El Hajj MS, Turgeon RD, Wilby KJ. Ceftaroline fosamil for community-acquired pneumonia and skin and skin structure infections: a systematic review. Int J Clin Pharm 2017 Jan 5.
44. Taboada M, Melnick D, Iaconis JP, et al. Ceftaroline fosamil versus ceftriaxone for the treatment of community-acquired pneumonia: individual patient data meta-analysis of randomized controlled trials. J Antimicrob Chemother 2016;71:862–70.
45. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011;52:285–92.
46. Baddour LM, Yu VL, Klugman KP, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med 2004;170:440–4.
47. FDA Drug Safety Communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new boxed warning [Internet]. 15 Jan 2016. Available at www.fda.gov/Drugs/DrugSafety/ucm369580.htm.
48. Edelstein PR, CR. Legionnaires’ Disease and Pontiac Fever. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2633.
49. Hammerschlag MR, Kohlhoff SA, Gaydos, CA. Chlamydia pneumoniae. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2174.
50. Holzman RS, MS. Mycoplasma pneumoniae and Atypical Pneumonia. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2183.
51. Yamada M, Buller R, Bledsoe S, Storch GA. Rising rates of macrolide-resistant Mycoplasma pneumoniae in the central United States. Pediatr Infect Dis J 2012;31:409–10.
52. Yi SH, Hatfield KM, Baggs J, et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis 2017 Nov 6.
53. Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis 2011;52:1232–40.
54. Akram AR, Chalmers JD, Taylor JK, et al. An evaluation of clinical stability criteria to predict hospital course in community-acquired pneumonia. Clin Microbiol Infect 2013;19:1174–80.
55. Halm EA, Fine MJ, Kapoor WN, et al. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med 2002;162:1278–84.
56. Roson B, Carratala J, Fernandez-Sabe N, et al. Causes and factors associated with early failure in hospitalized patients with community-acquired pneumonia. Arch Intern Med 2004;164:502–8.
57. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med 2003;167:1650–4.
58. Wan YD, Sun TW, Liu ZQ, et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest 2016;149:209–19.
59. Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 2015;313:677–86.
60. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 2006;19:571–82.
61. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines. MMWR Recomm Rep 2016;65:1–54.
62. Rubins JB, Alter M, Loch J, Janoff EN. Determination of antibody responses of elderly adults to all 23 capsular polysaccharides after pneumococcal vaccination. Infect Immun 1999;67:5979–84.
63. Centers for Disease Control. Vaccines and preventable diseases [Internet]. 22 Nov 2016. Available at www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html.
64. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015;372:1114–25.
65. Centers for Disease Control. Recommended adult immunization schedule -- United States -- 2016 [Internet]. 2016. Available at www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule.pdf.
66. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2015;64:944–7.
67. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012;61:816–9.
68. Aujesky D, Auble TE, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med 2005;118:384–92.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. FastStats - Pneumonia. Accessed 6 Oct 2016 at www.cdc.gov/nchs/fastats/pneumonia.htm.
2. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61-e111.
3. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med 2014;371:1619–28.
4. Mandell LA. Epidemiology and etiology of community-acquired pneumonia. Infect Dis
5. Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ 2006;332:1077–9.
6. Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med 2007;167:1938–43
7. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med 2010;122:130–41.
8. Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-year mortality after community-acquired pneumonia. a prospective cohort. Am J Respir Crit Care Med 2015;192:597-604.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015;373:415–27.
10. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013;369:155–63.
11. Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med 2000;342:681–9.
12. Almirall J, Serra-Prat M, Bolíbar I, BalassoV. Risk factors for community-acquired pneumonia in adults: a systemic review of observational studies. Respiration 2017;94:299–311.
13. Janoff EM.Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s principles and practice of infectioius diseases. 8th ed. Philadelphia: Sauders; 2015: 2310–27.
14. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough--a statistical approach. J Chronic Dis 1984;37:215–25.
15. Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med 1997;157:1453–9.
16. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44 Suppl 2:S27–72.
17. Jartti A, Rauvala E, Kauma H, et al. Chest imaging findings in hospitalized patients with H1N1 influenza. Acta Radiol 2011;52:297–304.
18. Basi SK, Marrie TJ, Huang JQ, Majumdar SR. Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med 2004;117:305–11.
19. Caldwell A, Glauser FL, Smith WR, et al. The effects of dehydration on the radiologic and pathologic appearance of experimental canine segmental pneumonia. Am Rev Respir Dis 1975;112:651–6.
20. Bartlett JG. Pneumonia. In: Barlett JG, editor. Management of respiratory tract infections. Philadelphia: Lippincott, Williams & Wilkins; 2001: 1–122.
21. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med 2015;192:974–82.
22. Wheeler JH, Fishman EK. Computed tomography in the management of chest infections: current status. Clin Infect Dis 1996;23:232–40.
23. Chesnutt MP. Pulmonary disorders. In: Papadakis MM, editor. Current medical diagnosis and treatment. New York: McGraw-Hill; 2016: 242–320.
24. Mandell LW. Pneumonia. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 188–201.
25. Reed WW, Byrd GS, Gates RH Jr, et al. Sputum gram’s stain in community-acquired pneumococcal pneumonia. A meta-analysis. West J Med 1996;165:197–204.
26. Chalasani NP, Valdecanas MA, Gopal AK, et al. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest 1995;108:932–6.
27. Bordon J, Peyrani P, Brock GN, et al. The presence of pneumococcal bacteremia does not influence clinical outcomes in patients with community-acquired pneumonia: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort study. Chest 2008;133:618–24.
28. Helbig JH, Uldum SA, Bernander S, et al. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires’ disease. J Clin Microbiol 2003;41:838–40.
29. Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol 2003;41:2810–3.
30. Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis 2010;50:202–9.
31. Gilbert DN. Procalcitonin as a biomarker in respiratory tract infection. Clin Infect Dis 2011;52 Suppl 4:S346–50.
32. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2012;(9):CD007498.
33. Boussekey N, Leroy O, Alfandari S, et al. Procalcitonin kinetics in the prognosis of severe community-acquired pneumonia. Intensive Care Med 2006;32:469–72.
34. Flanders SA, Stein J, Shochat G, et al. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am J Med 2004;116:529–35.
35. Fine MJ, et al A prediction rule to identify low-risk patients with community-acquired pneumonia.N Engl J Med.1997;336:243-50.
36. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003;58:377–82.
37. Arnold FW, Ramirez JA, McDonald LC, Xia EL. Hospitalization for community-acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest 2003;124:121–4.
38. Aujesky D, McCausland JB, Whittle J, et al. Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumonia. Clin Infect Dis 2009;49:e100–8.
39. Charles PG, Wolfe R, Whitby M, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis 2008;47:375–84.
40. Marti C, Garin N, Grosgurin O, et al. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Crit Care 2012;16:R141.
41. Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 2012;54:621–9.
42. Silverman JA, Mortin LI, Vanpraagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis 2005;191:2149–52.
43. El Hajj MS, Turgeon RD, Wilby KJ. Ceftaroline fosamil for community-acquired pneumonia and skin and skin structure infections: a systematic review. Int J Clin Pharm 2017 Jan 5.
44. Taboada M, Melnick D, Iaconis JP, et al. Ceftaroline fosamil versus ceftriaxone for the treatment of community-acquired pneumonia: individual patient data meta-analysis of randomized controlled trials. J Antimicrob Chemother 2016;71:862–70.
45. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011;52:285–92.
46. Baddour LM, Yu VL, Klugman KP, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med 2004;170:440–4.
47. FDA Drug Safety Communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new boxed warning [Internet]. 15 Jan 2016. Available at www.fda.gov/Drugs/DrugSafety/ucm369580.htm.
48. Edelstein PR, CR. Legionnaires’ Disease and Pontiac Fever. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2633.
49. Hammerschlag MR, Kohlhoff SA, Gaydos, CA. Chlamydia pneumoniae. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2174.
50. Holzman RS, MS. Mycoplasma pneumoniae and Atypical Pneumonia. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2183.
51. Yamada M, Buller R, Bledsoe S, Storch GA. Rising rates of macrolide-resistant Mycoplasma pneumoniae in the central United States. Pediatr Infect Dis J 2012;31:409–10.
52. Yi SH, Hatfield KM, Baggs J, et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis 2017 Nov 6.
53. Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis 2011;52:1232–40.
54. Akram AR, Chalmers JD, Taylor JK, et al. An evaluation of clinical stability criteria to predict hospital course in community-acquired pneumonia. Clin Microbiol Infect 2013;19:1174–80.
55. Halm EA, Fine MJ, Kapoor WN, et al. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med 2002;162:1278–84.
56. Roson B, Carratala J, Fernandez-Sabe N, et al. Causes and factors associated with early failure in hospitalized patients with community-acquired pneumonia. Arch Intern Med 2004;164:502–8.
57. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med 2003;167:1650–4.
58. Wan YD, Sun TW, Liu ZQ, et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest 2016;149:209–19.
59. Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 2015;313:677–86.
60. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 2006;19:571–82.
61. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines. MMWR Recomm Rep 2016;65:1–54.
62. Rubins JB, Alter M, Loch J, Janoff EN. Determination of antibody responses of elderly adults to all 23 capsular polysaccharides after pneumococcal vaccination. Infect Immun 1999;67:5979–84.
63. Centers for Disease Control. Vaccines and preventable diseases [Internet]. 22 Nov 2016. Available at www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html.
64. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015;372:1114–25.
65. Centers for Disease Control. Recommended adult immunization schedule -- United States -- 2016 [Internet]. 2016. Available at www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule.pdf.
66. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2015;64:944–7.
67. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012;61:816–9.
68. Aujesky D, Auble TE, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med 2005;118:384–92.