User login
When thyroid nodules are found clinically or incidentally on imaging, the patient’s thyrotropin level should be measured.1 Ultrasound is the first-line imaging recommended to assess thyroid nodules.1,2 Nodules can then be evaluated by a fine-needle aspiration (FNA) biopsy, which provides cytological information to determine whether the nodule is benign or malignant.1,3,4 Most thyroid nodules pose a low risk of malignancy.1
The American Thyroid Association guidelines on thyroid nodule management do not specify any recommendations for follow-up thyrotropin testing in patients who do not have any history that is known to affect thyroid function.1 Therefore, clinicians have to make decisions regarding follow-up testing in these patients without any evidence-based guidelines. There is a lack of data in the literature on whether thyrotropin levels change over time in this patient population. If thyrotropin levels do not become abnormal over time, then patients would not need thyrotropin monitoring or treatment for hypo- or hyperthyroidism.
The aim of this study was to determine whether thyrotropin levels change over time in patients with thyroid nodules and determine whether repeat thyrotropin testing was required after initial testing. The authors hypothesized that thyrotropin values do not change substantially over time in patients with thyroid nodules, except in patients with a history of hot nodules, autoimmune thyroid disease, thyroid or pituitary surgery, radioactive iodine ablation, neck radiation, or use of medications affecting thyroid function. This study may be able to contribute to the clinical guidelines for thyrotropin testing in patients with thyroid nodules so that clinicians can make evidence-based decisions.
METHODS
This retrospective chart review was conducted using the Computerized Patient Record System at the Veterans Affairs Dayton Healthcare System (VADHS) in Ohio. Patients aged ≥ 18 years who were diagnosed with ≥ 1 thyroid nodule from January 2010 to December 2016 and had a normal thyrotropin level at the time of diagnosis were included in the study. Patients who were found to have thyroid nodules multiple times were included only once from the time of the initial diagnosis. Patients were excluded if they had a medical history known to affect thyroid function. Exclusion criteria included a history of hot thyroid nodules; autoimmune thyroid disease on imaging or blood work; history of thyroid surgery, including pituitary surgery; history of radioactive iodine treatment; history of neck radiation; use of thyroxine before nodule diagnosis; use of amiodarone, programmed cell death-1 inhibitors, programmed cell death ligand-1 inhibitors, or cytotoxic T-lymphocyte-associated protein-4 inhibitors; or 3 consecutive months of steroid use.
Age at nodule diagnosis, sex, race, thyrotropin values at and after the time of nodule diagnosis, and duration from nodule diagnosis to most recent thyrotropin value were retrospectively collected until 100 patients met inclusion criteria for the study. Of note, from 2010 to 2016, the assays used at the VADHS to measure thyrotropin values changed over time, as did the normal reference ranges and the type of sample used for the assays. Normal thyrotropin range at time of diagnosis based on serum or plasma samples and for repeat thyrotropin levels are provided in Table 1, also based on serum or plasma samples. All collected data in the study was de-identified for analysis.
Statistical Analysis
Patients were divided into 2 groups: those who had an abnormal most recent thyrotropin value and those who did not. Mean (SD) of both groups was calculated for continuous variables of age at diagnosis, initial thyrotropin value and most recent thyrotropin value, and time from diagnosis to most recent thyrotropin value. Percentages for both groups were calculated for categorical variables of sex, race, and whether initial and most recent thyrotropin values were based on serum or plasma samples and old or new reference ranges. A 95% CI was determined for the true population rate of patients with an abnormal thyrotropin value at most recent testing. Independent sample t tests were used to compare the continuous variables between the abnormal and normal most recent thyrotropin groups. Categorical variables between the 2 groups were compared using χ2 tests. P < .05 was considered statistically significant. Statistical analyses were completed using IBM SPSS Statistics 27. This study was approved by the Wright State University Institutional Review Board and the VADHS Research and Development Committee.
RESULTS
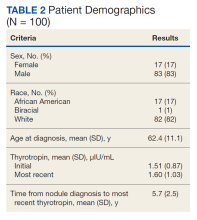
Of 557 patient charts studied, 100 patients were included; the mean (SD) age at nodule diagnosis was 62.4 (11.1) years, and the mean (SD) initial thyrotropin level at nodule diagnosis was 1.51 (0.87) μIU/mL. The mean (SD) most recent thyrotropin level was 1.60 (1.03) μIU/mL after a mean duration of 5.7 (2.5) years postnodule diagnosis (Table 2).
Six patients (6%; 95% CI, 2.5%-12.7%) who had a normal thyrotropin level at nodule diagnosis developed an abnormal thyrotropin level in a mean (SD) of 6.9 (3.1) years. These 6 patients had a mean age at nodule diagnosis of 69.2 (11.4) years. Five of the 6 were male, and all were White patients. One patient’s thyrotropin level rose from an initial thyrotropin of 3.38 μIU/mL at nodule diagnosis to a high of 7.76 μIU/mL after 8.5 years. This patient was diagnosed with subclinical hypothyroidism and did not require treatment.
Five patients’ thyrotropin levels dropped below normal in a mean 7 years, with levels ranging from 0.25 to 0.52 μIU/mL. Of these patients, 2 became symptomatic from the nodules, experiencing dysphagia or hoarseness, with 1 diagnosed with hyperthyroidism. This patient was treated with methimazole and radioactive iodine ablation 9 years after diagnosis. The other 3 patients who developed low thyrotropin had no nodule symptoms or treatment. Ninety-four patients maintained thyrotropin values in the normal range for a mean (SD) of 5.7 (2.5) years and had a mean (SD) age at nodule diagnosis of 61.9 (11.0) years.
Both thyrotropin groups were compared. For categorical variables, there were no significant differences for sex (
Of note, the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma and had 3 different normal reference ranges during the 2010 to 2016 period studied. The thyrotropin values fell into 4 categories: serum sample with normal range 0.4 to 5.5 μIU/mL, serum sample with normal range 0.4 to 4.0 μIU/mL, plasma sample with normal range 0.4 to 4.0 μIU/mL, and plasma sample with normal range 0.6 to 4.8 μIU/mL. There were no significant differences between the abnormal and normal most recent thyrotropin groups in sample type for initial or most recent thyrotropin (P = .44 and P = .99, respectively) or in normal range for initial or most recent thyrotropin level (P = .99 and P = .09, respectively).
DISCUSSION
We found no statistically significant change in blood thyrotropin levels over time among patients with thyroid nodules with no history of medical conditions or medications known to affect thyroid hormone levels. Six of 100 patients developed abnormal thyrotropin, but only 2 eventually were treated for thyroid dysfunction: 1 for hypothyroidism and 1 for hyperthyroidism. The other 4 patients who did not receive treatment developed low thyrotropin but had no official diagnosis of hyperthyroidism in their health records, seemingly due to lack of multiple, consistently low thyrotropin values or due to lack of follow-up. Based on these data, monitoring thyrotropin over time may not be necessary in patients without any medical history known to affect thyroid function. The results provide support for the original hypothesis.
Although only thyrotropin values at the time of nodule diagnosis and most recent thyrotropin values were analyzed, thyrotropin trends over time were considered. Some patients did have transient abnormal thyrotropin values; however, a search of the patients’ records showed that these transient abnormalities did not lead to any initiation of hypothyroidism or hyperthyroidism treatment.
Another consideration is that changes in the sample type processed and in the normal thyrotropin ranges over time could have been confounding variables. However, statistical analyses showed that the abnormal and normal most recent thyrotropin groups did not show any significant differences in sample type or reference range for either the initial or most recent thyrotropin values. Hospitals change the laboratory assays they use for clinical tests over time, but these changes likely did not affect the results of this study.
The data from this study showed similar results to previously reported research. This study found that 6% of patients developed abnormal thyrotropin levels over time. A study of 157 patients with nonfunctioning benign thyroid nodules found that 8.3% of patients developed thyroid dysfunction.5 In another follow-up study on patients with thyroid nodules who were otherwise euthyroid, 2 of 118 patients eventually received treatment for hyperthyroidism.6 In the current study, we report that just 1 of 100 included patients had to begin treatment for hyperthyroidism.
The literature also includes research on using thyrotropin and age to predict malignancy in patients with thyroid nodules. One study suggested that a thyrotropin cutoff point of ≥ 2.1 mU/I and an age cutoff point of ≥ 47 years were significantly associated with a diagnosis of malignancy.7 Although the current study did not study malignancy, the results showed that the mean age at nodule diagnosis was higher in patients who had abnormal vs normal most recent thyrotropin levels: 69 vs 62 years, respectively. Future studies could determine whether a certain initial thyrotropin value or age could be used as a cutoff for requiring further thyrotropin monitoring to check for development of hyperthyroidism or hypothyroidism.
Limitations
This study was limited by its small size of 100 subjects. Most patients had to be excluded to focus on the aim of determining whether thyrotropin monitoring is needed in the specific group of patients without medical history that would be expected to affect thyroid function. Another limitation was that 83% of the patients included in the study were male, which does not reflect the general population. Future studies should include a greater number of patients and aim for a balance of 50% male and 50% female patients.
Additionally, it is important to note that the changing definition of the normal thyrotropin range was a limitation. It is possible that some patients who were considered normal at the time of a particular thyrotropin measurement may have had an abnormal reading if measured at a different time. Another consideration is that the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma during the time that analyzed thyrotropin values were measured. This could have led to different thyrotropin values and, therefore, different results of this study compared with if the sample type had stayed the same. However, a previous study showed very similar thyrotropin values generated from serum and plasma samples in 17 patients.8 Therefore, possibly the change in sample type in the current study only minimally affected the results.
CONCLUSIONS
Current American Thyroid Association guidelines do not specify recommendations for follow-up thyrotropin testing in patients with thyroid nodules who do not have a history of conditions or medications known to affect thyroid hormone levels.1 This study suggests that repeat thyrotropin monitoring may not be necessary for this group of patients. Individuals who had an abnormal most recent thyrotropin had an older age at thyroid nodule diagnosis compared with patients who had a normal most recent thyrotropin, so it is possible that thyrotropin monitoring may be recommended for people with nodules who are above a certain age. The results of this study as well as future studies could help create new clinical recommendations for thyrotropin monitoring in patients with thyroid nodules that clinicians can use to make evidence-based clinical decisions. There would also be a decreased financial, physical, and time burden on the patients if guidelines specify that they are not required to get continued blood thyrotropin testing.
Acknowledgments
The authors acknowledge Ronald J. Markert, PhD, formerly of Wright State University Boonshoft School of Medicine, for his contributions to the statistical analysis of this research.
1. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. doi:10.1089/thy.2015.0020
2. Chambara N, Liu SYW, Lo X, Ying M. Diagnostic performance evaluation of different TI-RADS using ultrasound computer-aided diagnosis of thyroid nodules: an experience with adjusted settings. PLoS One. 2021;16(1):e0245617. doi:10.1371/journal.pone.0245617
3. Livhits MJ, Zhu CY, Kuo EJ, et al. Effectiveness of molecular testing techniques for diagnosis of indeterminate thyroid nodules: a randomized clinical trial. JAMA Oncol. 2021;7(1):70-77. doi:10.1001/jamaoncol.2020.5935
4. Grani G, Lamartina L, Ascoli V, et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the “right” TIRADS. J Clin Endocrinol Metab. 2019;104(1):95-102. doi:10.1210/jc.2018-01674
5. Memon R, Salgado Nunez Del Prado SR, Lamos EM, et al. Biochemical follow-up of nonfunctioning benign thyroid nodules. Clin Endocrinol (Oxf). 2021;94(2):322-329. doi:10.1111/cen.14303
6. Bajuk Studen K, Gaberscek S, Pirnat E, Zaletel K. Five-year follow-up and clinical outcome in euthyroid patients with thyroid nodules. Radiol Oncol. 2021;55(3):317-322. Published 2021 May 31. doi:10.2478/raon-2021-0025
7. Fernández-Trujillo C, Pérez-Zaballos J, Rodríguez-Pérez CA, et al. TSH level and risk of malignancy in patients with Bethesda category IV thyroid nodules. Horm Cancer. 2020;11(3-4):200-204. doi:10.1007/s12672-020-00384-4
8. Villanger GD, Learner E, Longnecker MP, et al. Effects of sample handling and analytical procedures on thyroid hormone concentrations in pregnant women’s plasma. Epidemiology. 2017;28(3):365-369. doi:10.1097/EDE.0000000000000606
When thyroid nodules are found clinically or incidentally on imaging, the patient’s thyrotropin level should be measured.1 Ultrasound is the first-line imaging recommended to assess thyroid nodules.1,2 Nodules can then be evaluated by a fine-needle aspiration (FNA) biopsy, which provides cytological information to determine whether the nodule is benign or malignant.1,3,4 Most thyroid nodules pose a low risk of malignancy.1
The American Thyroid Association guidelines on thyroid nodule management do not specify any recommendations for follow-up thyrotropin testing in patients who do not have any history that is known to affect thyroid function.1 Therefore, clinicians have to make decisions regarding follow-up testing in these patients without any evidence-based guidelines. There is a lack of data in the literature on whether thyrotropin levels change over time in this patient population. If thyrotropin levels do not become abnormal over time, then patients would not need thyrotropin monitoring or treatment for hypo- or hyperthyroidism.
The aim of this study was to determine whether thyrotropin levels change over time in patients with thyroid nodules and determine whether repeat thyrotropin testing was required after initial testing. The authors hypothesized that thyrotropin values do not change substantially over time in patients with thyroid nodules, except in patients with a history of hot nodules, autoimmune thyroid disease, thyroid or pituitary surgery, radioactive iodine ablation, neck radiation, or use of medications affecting thyroid function. This study may be able to contribute to the clinical guidelines for thyrotropin testing in patients with thyroid nodules so that clinicians can make evidence-based decisions.
METHODS
This retrospective chart review was conducted using the Computerized Patient Record System at the Veterans Affairs Dayton Healthcare System (VADHS) in Ohio. Patients aged ≥ 18 years who were diagnosed with ≥ 1 thyroid nodule from January 2010 to December 2016 and had a normal thyrotropin level at the time of diagnosis were included in the study. Patients who were found to have thyroid nodules multiple times were included only once from the time of the initial diagnosis. Patients were excluded if they had a medical history known to affect thyroid function. Exclusion criteria included a history of hot thyroid nodules; autoimmune thyroid disease on imaging or blood work; history of thyroid surgery, including pituitary surgery; history of radioactive iodine treatment; history of neck radiation; use of thyroxine before nodule diagnosis; use of amiodarone, programmed cell death-1 inhibitors, programmed cell death ligand-1 inhibitors, or cytotoxic T-lymphocyte-associated protein-4 inhibitors; or 3 consecutive months of steroid use.
Age at nodule diagnosis, sex, race, thyrotropin values at and after the time of nodule diagnosis, and duration from nodule diagnosis to most recent thyrotropin value were retrospectively collected until 100 patients met inclusion criteria for the study. Of note, from 2010 to 2016, the assays used at the VADHS to measure thyrotropin values changed over time, as did the normal reference ranges and the type of sample used for the assays. Normal thyrotropin range at time of diagnosis based on serum or plasma samples and for repeat thyrotropin levels are provided in Table 1, also based on serum or plasma samples. All collected data in the study was de-identified for analysis.
Statistical Analysis
Patients were divided into 2 groups: those who had an abnormal most recent thyrotropin value and those who did not. Mean (SD) of both groups was calculated for continuous variables of age at diagnosis, initial thyrotropin value and most recent thyrotropin value, and time from diagnosis to most recent thyrotropin value. Percentages for both groups were calculated for categorical variables of sex, race, and whether initial and most recent thyrotropin values were based on serum or plasma samples and old or new reference ranges. A 95% CI was determined for the true population rate of patients with an abnormal thyrotropin value at most recent testing. Independent sample t tests were used to compare the continuous variables between the abnormal and normal most recent thyrotropin groups. Categorical variables between the 2 groups were compared using χ2 tests. P < .05 was considered statistically significant. Statistical analyses were completed using IBM SPSS Statistics 27. This study was approved by the Wright State University Institutional Review Board and the VADHS Research and Development Committee.
RESULTS
Of 557 patient charts studied, 100 patients were included; the mean (SD) age at nodule diagnosis was 62.4 (11.1) years, and the mean (SD) initial thyrotropin level at nodule diagnosis was 1.51 (0.87) μIU/mL. The mean (SD) most recent thyrotropin level was 1.60 (1.03) μIU/mL after a mean duration of 5.7 (2.5) years postnodule diagnosis (Table 2).
Six patients (6%; 95% CI, 2.5%-12.7%) who had a normal thyrotropin level at nodule diagnosis developed an abnormal thyrotropin level in a mean (SD) of 6.9 (3.1) years. These 6 patients had a mean age at nodule diagnosis of 69.2 (11.4) years. Five of the 6 were male, and all were White patients. One patient’s thyrotropin level rose from an initial thyrotropin of 3.38 μIU/mL at nodule diagnosis to a high of 7.76 μIU/mL after 8.5 years. This patient was diagnosed with subclinical hypothyroidism and did not require treatment.
Five patients’ thyrotropin levels dropped below normal in a mean 7 years, with levels ranging from 0.25 to 0.52 μIU/mL. Of these patients, 2 became symptomatic from the nodules, experiencing dysphagia or hoarseness, with 1 diagnosed with hyperthyroidism. This patient was treated with methimazole and radioactive iodine ablation 9 years after diagnosis. The other 3 patients who developed low thyrotropin had no nodule symptoms or treatment. Ninety-four patients maintained thyrotropin values in the normal range for a mean (SD) of 5.7 (2.5) years and had a mean (SD) age at nodule diagnosis of 61.9 (11.0) years.
Both thyrotropin groups were compared. For categorical variables, there were no significant differences for sex (
Of note, the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma and had 3 different normal reference ranges during the 2010 to 2016 period studied. The thyrotropin values fell into 4 categories: serum sample with normal range 0.4 to 5.5 μIU/mL, serum sample with normal range 0.4 to 4.0 μIU/mL, plasma sample with normal range 0.4 to 4.0 μIU/mL, and plasma sample with normal range 0.6 to 4.8 μIU/mL. There were no significant differences between the abnormal and normal most recent thyrotropin groups in sample type for initial or most recent thyrotropin (P = .44 and P = .99, respectively) or in normal range for initial or most recent thyrotropin level (P = .99 and P = .09, respectively).
DISCUSSION
We found no statistically significant change in blood thyrotropin levels over time among patients with thyroid nodules with no history of medical conditions or medications known to affect thyroid hormone levels. Six of 100 patients developed abnormal thyrotropin, but only 2 eventually were treated for thyroid dysfunction: 1 for hypothyroidism and 1 for hyperthyroidism. The other 4 patients who did not receive treatment developed low thyrotropin but had no official diagnosis of hyperthyroidism in their health records, seemingly due to lack of multiple, consistently low thyrotropin values or due to lack of follow-up. Based on these data, monitoring thyrotropin over time may not be necessary in patients without any medical history known to affect thyroid function. The results provide support for the original hypothesis.
Although only thyrotropin values at the time of nodule diagnosis and most recent thyrotropin values were analyzed, thyrotropin trends over time were considered. Some patients did have transient abnormal thyrotropin values; however, a search of the patients’ records showed that these transient abnormalities did not lead to any initiation of hypothyroidism or hyperthyroidism treatment.
Another consideration is that changes in the sample type processed and in the normal thyrotropin ranges over time could have been confounding variables. However, statistical analyses showed that the abnormal and normal most recent thyrotropin groups did not show any significant differences in sample type or reference range for either the initial or most recent thyrotropin values. Hospitals change the laboratory assays they use for clinical tests over time, but these changes likely did not affect the results of this study.
The data from this study showed similar results to previously reported research. This study found that 6% of patients developed abnormal thyrotropin levels over time. A study of 157 patients with nonfunctioning benign thyroid nodules found that 8.3% of patients developed thyroid dysfunction.5 In another follow-up study on patients with thyroid nodules who were otherwise euthyroid, 2 of 118 patients eventually received treatment for hyperthyroidism.6 In the current study, we report that just 1 of 100 included patients had to begin treatment for hyperthyroidism.
The literature also includes research on using thyrotropin and age to predict malignancy in patients with thyroid nodules. One study suggested that a thyrotropin cutoff point of ≥ 2.1 mU/I and an age cutoff point of ≥ 47 years were significantly associated with a diagnosis of malignancy.7 Although the current study did not study malignancy, the results showed that the mean age at nodule diagnosis was higher in patients who had abnormal vs normal most recent thyrotropin levels: 69 vs 62 years, respectively. Future studies could determine whether a certain initial thyrotropin value or age could be used as a cutoff for requiring further thyrotropin monitoring to check for development of hyperthyroidism or hypothyroidism.
Limitations
This study was limited by its small size of 100 subjects. Most patients had to be excluded to focus on the aim of determining whether thyrotropin monitoring is needed in the specific group of patients without medical history that would be expected to affect thyroid function. Another limitation was that 83% of the patients included in the study were male, which does not reflect the general population. Future studies should include a greater number of patients and aim for a balance of 50% male and 50% female patients.
Additionally, it is important to note that the changing definition of the normal thyrotropin range was a limitation. It is possible that some patients who were considered normal at the time of a particular thyrotropin measurement may have had an abnormal reading if measured at a different time. Another consideration is that the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma during the time that analyzed thyrotropin values were measured. This could have led to different thyrotropin values and, therefore, different results of this study compared with if the sample type had stayed the same. However, a previous study showed very similar thyrotropin values generated from serum and plasma samples in 17 patients.8 Therefore, possibly the change in sample type in the current study only minimally affected the results.
CONCLUSIONS
Current American Thyroid Association guidelines do not specify recommendations for follow-up thyrotropin testing in patients with thyroid nodules who do not have a history of conditions or medications known to affect thyroid hormone levels.1 This study suggests that repeat thyrotropin monitoring may not be necessary for this group of patients. Individuals who had an abnormal most recent thyrotropin had an older age at thyroid nodule diagnosis compared with patients who had a normal most recent thyrotropin, so it is possible that thyrotropin monitoring may be recommended for people with nodules who are above a certain age. The results of this study as well as future studies could help create new clinical recommendations for thyrotropin monitoring in patients with thyroid nodules that clinicians can use to make evidence-based clinical decisions. There would also be a decreased financial, physical, and time burden on the patients if guidelines specify that they are not required to get continued blood thyrotropin testing.
Acknowledgments
The authors acknowledge Ronald J. Markert, PhD, formerly of Wright State University Boonshoft School of Medicine, for his contributions to the statistical analysis of this research.
When thyroid nodules are found clinically or incidentally on imaging, the patient’s thyrotropin level should be measured.1 Ultrasound is the first-line imaging recommended to assess thyroid nodules.1,2 Nodules can then be evaluated by a fine-needle aspiration (FNA) biopsy, which provides cytological information to determine whether the nodule is benign or malignant.1,3,4 Most thyroid nodules pose a low risk of malignancy.1
The American Thyroid Association guidelines on thyroid nodule management do not specify any recommendations for follow-up thyrotropin testing in patients who do not have any history that is known to affect thyroid function.1 Therefore, clinicians have to make decisions regarding follow-up testing in these patients without any evidence-based guidelines. There is a lack of data in the literature on whether thyrotropin levels change over time in this patient population. If thyrotropin levels do not become abnormal over time, then patients would not need thyrotropin monitoring or treatment for hypo- or hyperthyroidism.
The aim of this study was to determine whether thyrotropin levels change over time in patients with thyroid nodules and determine whether repeat thyrotropin testing was required after initial testing. The authors hypothesized that thyrotropin values do not change substantially over time in patients with thyroid nodules, except in patients with a history of hot nodules, autoimmune thyroid disease, thyroid or pituitary surgery, radioactive iodine ablation, neck radiation, or use of medications affecting thyroid function. This study may be able to contribute to the clinical guidelines for thyrotropin testing in patients with thyroid nodules so that clinicians can make evidence-based decisions.
METHODS
This retrospective chart review was conducted using the Computerized Patient Record System at the Veterans Affairs Dayton Healthcare System (VADHS) in Ohio. Patients aged ≥ 18 years who were diagnosed with ≥ 1 thyroid nodule from January 2010 to December 2016 and had a normal thyrotropin level at the time of diagnosis were included in the study. Patients who were found to have thyroid nodules multiple times were included only once from the time of the initial diagnosis. Patients were excluded if they had a medical history known to affect thyroid function. Exclusion criteria included a history of hot thyroid nodules; autoimmune thyroid disease on imaging or blood work; history of thyroid surgery, including pituitary surgery; history of radioactive iodine treatment; history of neck radiation; use of thyroxine before nodule diagnosis; use of amiodarone, programmed cell death-1 inhibitors, programmed cell death ligand-1 inhibitors, or cytotoxic T-lymphocyte-associated protein-4 inhibitors; or 3 consecutive months of steroid use.
Age at nodule diagnosis, sex, race, thyrotropin values at and after the time of nodule diagnosis, and duration from nodule diagnosis to most recent thyrotropin value were retrospectively collected until 100 patients met inclusion criteria for the study. Of note, from 2010 to 2016, the assays used at the VADHS to measure thyrotropin values changed over time, as did the normal reference ranges and the type of sample used for the assays. Normal thyrotropin range at time of diagnosis based on serum or plasma samples and for repeat thyrotropin levels are provided in Table 1, also based on serum or plasma samples. All collected data in the study was de-identified for analysis.
Statistical Analysis
Patients were divided into 2 groups: those who had an abnormal most recent thyrotropin value and those who did not. Mean (SD) of both groups was calculated for continuous variables of age at diagnosis, initial thyrotropin value and most recent thyrotropin value, and time from diagnosis to most recent thyrotropin value. Percentages for both groups were calculated for categorical variables of sex, race, and whether initial and most recent thyrotropin values were based on serum or plasma samples and old or new reference ranges. A 95% CI was determined for the true population rate of patients with an abnormal thyrotropin value at most recent testing. Independent sample t tests were used to compare the continuous variables between the abnormal and normal most recent thyrotropin groups. Categorical variables between the 2 groups were compared using χ2 tests. P < .05 was considered statistically significant. Statistical analyses were completed using IBM SPSS Statistics 27. This study was approved by the Wright State University Institutional Review Board and the VADHS Research and Development Committee.
RESULTS
Of 557 patient charts studied, 100 patients were included; the mean (SD) age at nodule diagnosis was 62.4 (11.1) years, and the mean (SD) initial thyrotropin level at nodule diagnosis was 1.51 (0.87) μIU/mL. The mean (SD) most recent thyrotropin level was 1.60 (1.03) μIU/mL after a mean duration of 5.7 (2.5) years postnodule diagnosis (Table 2).
Six patients (6%; 95% CI, 2.5%-12.7%) who had a normal thyrotropin level at nodule diagnosis developed an abnormal thyrotropin level in a mean (SD) of 6.9 (3.1) years. These 6 patients had a mean age at nodule diagnosis of 69.2 (11.4) years. Five of the 6 were male, and all were White patients. One patient’s thyrotropin level rose from an initial thyrotropin of 3.38 μIU/mL at nodule diagnosis to a high of 7.76 μIU/mL after 8.5 years. This patient was diagnosed with subclinical hypothyroidism and did not require treatment.
Five patients’ thyrotropin levels dropped below normal in a mean 7 years, with levels ranging from 0.25 to 0.52 μIU/mL. Of these patients, 2 became symptomatic from the nodules, experiencing dysphagia or hoarseness, with 1 diagnosed with hyperthyroidism. This patient was treated with methimazole and radioactive iodine ablation 9 years after diagnosis. The other 3 patients who developed low thyrotropin had no nodule symptoms or treatment. Ninety-four patients maintained thyrotropin values in the normal range for a mean (SD) of 5.7 (2.5) years and had a mean (SD) age at nodule diagnosis of 61.9 (11.0) years.
Both thyrotropin groups were compared. For categorical variables, there were no significant differences for sex (
Of note, the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma and had 3 different normal reference ranges during the 2010 to 2016 period studied. The thyrotropin values fell into 4 categories: serum sample with normal range 0.4 to 5.5 μIU/mL, serum sample with normal range 0.4 to 4.0 μIU/mL, plasma sample with normal range 0.4 to 4.0 μIU/mL, and plasma sample with normal range 0.6 to 4.8 μIU/mL. There were no significant differences between the abnormal and normal most recent thyrotropin groups in sample type for initial or most recent thyrotropin (P = .44 and P = .99, respectively) or in normal range for initial or most recent thyrotropin level (P = .99 and P = .09, respectively).
DISCUSSION
We found no statistically significant change in blood thyrotropin levels over time among patients with thyroid nodules with no history of medical conditions or medications known to affect thyroid hormone levels. Six of 100 patients developed abnormal thyrotropin, but only 2 eventually were treated for thyroid dysfunction: 1 for hypothyroidism and 1 for hyperthyroidism. The other 4 patients who did not receive treatment developed low thyrotropin but had no official diagnosis of hyperthyroidism in their health records, seemingly due to lack of multiple, consistently low thyrotropin values or due to lack of follow-up. Based on these data, monitoring thyrotropin over time may not be necessary in patients without any medical history known to affect thyroid function. The results provide support for the original hypothesis.
Although only thyrotropin values at the time of nodule diagnosis and most recent thyrotropin values were analyzed, thyrotropin trends over time were considered. Some patients did have transient abnormal thyrotropin values; however, a search of the patients’ records showed that these transient abnormalities did not lead to any initiation of hypothyroidism or hyperthyroidism treatment.
Another consideration is that changes in the sample type processed and in the normal thyrotropin ranges over time could have been confounding variables. However, statistical analyses showed that the abnormal and normal most recent thyrotropin groups did not show any significant differences in sample type or reference range for either the initial or most recent thyrotropin values. Hospitals change the laboratory assays they use for clinical tests over time, but these changes likely did not affect the results of this study.
The data from this study showed similar results to previously reported research. This study found that 6% of patients developed abnormal thyrotropin levels over time. A study of 157 patients with nonfunctioning benign thyroid nodules found that 8.3% of patients developed thyroid dysfunction.5 In another follow-up study on patients with thyroid nodules who were otherwise euthyroid, 2 of 118 patients eventually received treatment for hyperthyroidism.6 In the current study, we report that just 1 of 100 included patients had to begin treatment for hyperthyroidism.
The literature also includes research on using thyrotropin and age to predict malignancy in patients with thyroid nodules. One study suggested that a thyrotropin cutoff point of ≥ 2.1 mU/I and an age cutoff point of ≥ 47 years were significantly associated with a diagnosis of malignancy.7 Although the current study did not study malignancy, the results showed that the mean age at nodule diagnosis was higher in patients who had abnormal vs normal most recent thyrotropin levels: 69 vs 62 years, respectively. Future studies could determine whether a certain initial thyrotropin value or age could be used as a cutoff for requiring further thyrotropin monitoring to check for development of hyperthyroidism or hypothyroidism.
Limitations
This study was limited by its small size of 100 subjects. Most patients had to be excluded to focus on the aim of determining whether thyrotropin monitoring is needed in the specific group of patients without medical history that would be expected to affect thyroid function. Another limitation was that 83% of the patients included in the study were male, which does not reflect the general population. Future studies should include a greater number of patients and aim for a balance of 50% male and 50% female patients.
Additionally, it is important to note that the changing definition of the normal thyrotropin range was a limitation. It is possible that some patients who were considered normal at the time of a particular thyrotropin measurement may have had an abnormal reading if measured at a different time. Another consideration is that the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma during the time that analyzed thyrotropin values were measured. This could have led to different thyrotropin values and, therefore, different results of this study compared with if the sample type had stayed the same. However, a previous study showed very similar thyrotropin values generated from serum and plasma samples in 17 patients.8 Therefore, possibly the change in sample type in the current study only minimally affected the results.
CONCLUSIONS
Current American Thyroid Association guidelines do not specify recommendations for follow-up thyrotropin testing in patients with thyroid nodules who do not have a history of conditions or medications known to affect thyroid hormone levels.1 This study suggests that repeat thyrotropin monitoring may not be necessary for this group of patients. Individuals who had an abnormal most recent thyrotropin had an older age at thyroid nodule diagnosis compared with patients who had a normal most recent thyrotropin, so it is possible that thyrotropin monitoring may be recommended for people with nodules who are above a certain age. The results of this study as well as future studies could help create new clinical recommendations for thyrotropin monitoring in patients with thyroid nodules that clinicians can use to make evidence-based clinical decisions. There would also be a decreased financial, physical, and time burden on the patients if guidelines specify that they are not required to get continued blood thyrotropin testing.
Acknowledgments
The authors acknowledge Ronald J. Markert, PhD, formerly of Wright State University Boonshoft School of Medicine, for his contributions to the statistical analysis of this research.
1. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. doi:10.1089/thy.2015.0020
2. Chambara N, Liu SYW, Lo X, Ying M. Diagnostic performance evaluation of different TI-RADS using ultrasound computer-aided diagnosis of thyroid nodules: an experience with adjusted settings. PLoS One. 2021;16(1):e0245617. doi:10.1371/journal.pone.0245617
3. Livhits MJ, Zhu CY, Kuo EJ, et al. Effectiveness of molecular testing techniques for diagnosis of indeterminate thyroid nodules: a randomized clinical trial. JAMA Oncol. 2021;7(1):70-77. doi:10.1001/jamaoncol.2020.5935
4. Grani G, Lamartina L, Ascoli V, et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the “right” TIRADS. J Clin Endocrinol Metab. 2019;104(1):95-102. doi:10.1210/jc.2018-01674
5. Memon R, Salgado Nunez Del Prado SR, Lamos EM, et al. Biochemical follow-up of nonfunctioning benign thyroid nodules. Clin Endocrinol (Oxf). 2021;94(2):322-329. doi:10.1111/cen.14303
6. Bajuk Studen K, Gaberscek S, Pirnat E, Zaletel K. Five-year follow-up and clinical outcome in euthyroid patients with thyroid nodules. Radiol Oncol. 2021;55(3):317-322. Published 2021 May 31. doi:10.2478/raon-2021-0025
7. Fernández-Trujillo C, Pérez-Zaballos J, Rodríguez-Pérez CA, et al. TSH level and risk of malignancy in patients with Bethesda category IV thyroid nodules. Horm Cancer. 2020;11(3-4):200-204. doi:10.1007/s12672-020-00384-4
8. Villanger GD, Learner E, Longnecker MP, et al. Effects of sample handling and analytical procedures on thyroid hormone concentrations in pregnant women’s plasma. Epidemiology. 2017;28(3):365-369. doi:10.1097/EDE.0000000000000606
1. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. doi:10.1089/thy.2015.0020
2. Chambara N, Liu SYW, Lo X, Ying M. Diagnostic performance evaluation of different TI-RADS using ultrasound computer-aided diagnosis of thyroid nodules: an experience with adjusted settings. PLoS One. 2021;16(1):e0245617. doi:10.1371/journal.pone.0245617
3. Livhits MJ, Zhu CY, Kuo EJ, et al. Effectiveness of molecular testing techniques for diagnosis of indeterminate thyroid nodules: a randomized clinical trial. JAMA Oncol. 2021;7(1):70-77. doi:10.1001/jamaoncol.2020.5935
4. Grani G, Lamartina L, Ascoli V, et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the “right” TIRADS. J Clin Endocrinol Metab. 2019;104(1):95-102. doi:10.1210/jc.2018-01674
5. Memon R, Salgado Nunez Del Prado SR, Lamos EM, et al. Biochemical follow-up of nonfunctioning benign thyroid nodules. Clin Endocrinol (Oxf). 2021;94(2):322-329. doi:10.1111/cen.14303
6. Bajuk Studen K, Gaberscek S, Pirnat E, Zaletel K. Five-year follow-up and clinical outcome in euthyroid patients with thyroid nodules. Radiol Oncol. 2021;55(3):317-322. Published 2021 May 31. doi:10.2478/raon-2021-0025
7. Fernández-Trujillo C, Pérez-Zaballos J, Rodríguez-Pérez CA, et al. TSH level and risk of malignancy in patients with Bethesda category IV thyroid nodules. Horm Cancer. 2020;11(3-4):200-204. doi:10.1007/s12672-020-00384-4
8. Villanger GD, Learner E, Longnecker MP, et al. Effects of sample handling and analytical procedures on thyroid hormone concentrations in pregnant women’s plasma. Epidemiology. 2017;28(3):365-369. doi:10.1097/EDE.0000000000000606