User login
Background
An appropriately feared complication of operations, surgical site infections (SSIs) are infections associated with high economic costs and significantly worse clinical outcomes (1). Defined as infections of the superficial incision site, deep incision space, or organ space, SSIs add additional cost ranging from $2,700 to $26,000 per episode according to CDC’s National Nosocomial Infections Surveillance System. Patients who develop an SSI have hospital lengths of stay (LOS) in excess of 7 days longer and are 60% more likely to spend time in the intensive care unit than are patients without an SSI. A patient with an SSI is five times more likely to be readmitted to the hospital and is twice as likely to die (2).
Unfortunately, surgical site infections are common. Among healthcare-acquired infections, SSIs rank second only to urinary tract infections in frequency, making them more common than bloodstream infections and nosocomial pneumonia (3). There are approximately 30 million operations annually in the United States and an SSI complicates 2–5% of clean extra-abdominal sites. The rate is much higher for intra-abdominal operations, approaching 20% (1). Because most SSIs begin within 2 hours of contamination, the perioperative period is the most crucial for development of an SSI (4). By offering clinical expertise in the practice guidelines that reduce the risk of SSIs, hospital medicine programs can help patients and hospital systems lower morbidity, mortality, and costs associated with this complication. Adherence to best practices will likely require coordinated, multidisciplinary process improvement.
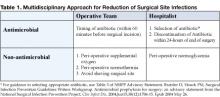
Several important interventions fall directly under the control of the anesthesia and surgical teams, such as administering perioperative oxygen, ensuring perioperative normothermia, and avoiding shaving of the surgical site. In coordinated quality improvement efforts, members of the operative team should assume direct responsibility for the performance of these measures. But the performance of two important interventions in this decisive period is likely to be significantly enhanced by the presence of focused hospitalist surgical co-management: antimicrobial prophylaxis and perioperative glycemic control (Table 1).
Antimicrobial Prophylaxis
Studies overwhelmingly show a marked reduction in the relative risk of SSIs with the use of antibiotic prophylaxis (1). In June 2004, the National Surgical Infection Prevention Project (NSIPP) published an advisory statement on antimicrobial prophylaxis in which it outlined three performance measures for quality improvement in prevention of SSIs:
- The proportion of patients who have parenteral antimicrobial prophylaxis initiated within 1 hour before surgical incision
- The proportion of patients provided with a prophylactic antimicrobial agent that is consistent with currently published guidelines, and
- The proportion of patients whose prophylactic antimicrobial therapy is discontinued within 24 hours after the end of surgery (5)
Pooled data suggest that attention to timing makes a favorable difference in SSI rates (1). Fully administering the appropriate antibiotic within 60 minutes of incision ensures that serum and tissue drug levels exceed the MICs of the most likely contaminating organisms. Dosing the antibiotic immediately prior to the start of surgery also provides the best opportunity to extend therapeutic levels for the duration of the surgery. The fact that anesthesia and surgical teams are in the most practical time-space positions to apply this measure underscores the multi-disciplinary and process-level efforts necessary to reduce SSI rates.
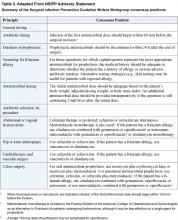
When it comes to the choice of antimicrobial and the duration of its use, hospitalists may find themselves in superior positions of impact. Familiarity with recommendations of the NSIPP advisory statement (summarized in Table 2) promotes evidence-based selection of antibiotic prophylaxis based on patient-specific factors: type of operation and presence of true drug allergies (5). Compared with other members of the surgical co-management team, hospitalists are more likely to be aware of relevant patient-specific risk factors such as the likelihood of colonization with methicillin-resistant Staphylococcus aureus (MRSA). For example, in patients colonized with MRSA, hospitalists might consider vancomycin as the alternative agent for prophylaxis. Free access to the NSIPP advisory statement is available at www.journals.uchicago.edu/CID/journal/issues/v38n12/33257/33257.html.
Antimicrobial prophylaxis after wound closure is unnecessary; published evidence demonstrates the non-inferiority of single dose prophylaxis when compared with multiple dose prophylaxis (5). Further, prolonged use of antimicrobial prophylaxis is associated with the emergence of resistant organisms (6-8). By ensuring that the duration of prophylaxis does not exceed 24 hours past the end of the operation, hospitalists can make valuable contributions to public health and cost containment.
Non-Antimicrobial Measures
Several non-antimicrobial measures also significantly reduce SSI rates. Those that fall outside the domain of the hospitalist and into the direct purview of the operative team include high levels of inspired oxygen, maintenance of perioperative normothermia, and use of clippers rather than a razor when hair removal is necessary. The risk of SSIs is directly related to tissue oxygenation. Bacterial infectivity is enhanced and cellular immunity is compromised in hypoperfused, poorly oxygenated tissue (9). The practice of administering perioperative supplemental oxygen (at least 80% FIO2 in intubated patients) reduces the risk of SSI by nearly one-half (1). For non-intubated patients, oxygen at 12 L/min by non-rebreathing face mask applied intra-operatively and for at least 2 hours following surgery leads to similar reductions of SSI rates. Besides being effective, this intervention is inexpensive, has no recognized adverse effects, and carries the added benefit of significantly reducing post-operative nausea and vomiting (4).
Hypothermia also predisposes the surgical wound to infection. Even mild perioperative hypothermia (i.e., core temperature 35-36.5°C) typically occurs in the absence of specific measures to prevent net heat loss. Perioperative hypothermia is the combined result of exposure and anesthetic-induced thermo-dysregulation, with redistribution of core body heat to the periphery (4). Even mild hypothermia causes vasoconstriction which diminishes perfusion, dropping tissue oxygen tension which impairs phagocytosis and oxidative killing by neutrophils (10). Hypothermia also blunts scar formation which further diminishes wound integrity. Active warming of the patient to maintain a core temperature near 36.5°C constitutes the intra-operative standard of care and is effective at reducing the risk of SSIs by as much as two-thirds (1).
Hyperglycemia, an established independent risk factor for an array of adverse outcomes in hospitalized patients, is also an independent risk factor for SSIs across a range of surgical patients (1). Short-term hyperglycemia depresses immune function through nonenzymatic glycosylation of immunoglobulin and by impairing normal leukocyte performance (11). Among diabetic cardiac surgery patients, reduction of hyperglycemia with an intravenous insulin infusion lowered the incidence of deep sternal wound infection by as much as two-thirds (12). While the value of achieving glycemic targets has already been established for a variety of important endpoints and across a range of inpatient populations, hospitalists should stay tuned. As high quality studies emerge proving that glycemic control lowers SSIs among non-cardiac surgical subpopulations, hospitalists may increasingly be relied upon to achieve strict glycemic targets.
By recognizing and coordinating practices known to reduce SSIs, hospitalists can elevate the level of care provided for surgical patients. At the same time, hospitalists can help lower costs and keep the hospital system mindful of public health goals, such as prevention of antimicrobial resistance. While individual hospitalists have key roles to play, the overall approach to SSI reduction calls for a coordinated, multidisciplinary team approach with process and system-level efforts.
Dr. Stein can be contacted at Jason_Stein@emoryhealthcare.org.
References
- Auerbach AD. Prevention of surgical site infections. In: Shojania KG, Duncan BW, McDonald KM, et al., eds. Making health care safer: a critical analysis of patient safety practices. Evidence report/technology assessment no. 43. AHRQ publication no. 01-E058. Rockville, MD: Agency for Healthcare Research and Quality, 20 July 2001:221-44.
- Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-30.
- National Nosocomial Infections Surveillance (NNIS) report, data summary from October 1986-April 1996, issued May 1996: a report from the National Nosocomial Infections Surveillance (NNIS) system. Am J Infect Control. 1996;24:380-8.
- Sessler DI, Akca O. Nonpharmacologic prevention of surgical wound infections. Clin Infect Dis. 2002;35:1397 404.
- Bratzler D, Houck PM. Surgical Infection Prevention Guidelines Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis. 2004;Jun15;38(12):1706-15. E-pub 2004 May 26.
- Harbarth S, Samore MH, Lichtenberg D, Carmeli Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation. 2000;101:2916-21.
- Eggimann P, Pittet D. Infection control in the ICU. Chest. 2001;120:2059-93.
- Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163:972-8.
- Hopf HW, Hunt TK, West JM, et al. Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Arch Surg. 1997;132:997-1005.
- Kurz A, Sessler DI, Lenhardt RA. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;334:1209-15.
- Garber AJ, Moghissi ES, Bransome ED Jr, et al. American College of Endocrinology Task Force on Inpatient Diabetes Metabolic Control. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;Mar-Apr;10Suppl2:4-9.
- Furnary AP, Zerr K, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures [with discussion]. Ann Thorac Surg. 1999;67:352-62.
Background
An appropriately feared complication of operations, surgical site infections (SSIs) are infections associated with high economic costs and significantly worse clinical outcomes (1). Defined as infections of the superficial incision site, deep incision space, or organ space, SSIs add additional cost ranging from $2,700 to $26,000 per episode according to CDC’s National Nosocomial Infections Surveillance System. Patients who develop an SSI have hospital lengths of stay (LOS) in excess of 7 days longer and are 60% more likely to spend time in the intensive care unit than are patients without an SSI. A patient with an SSI is five times more likely to be readmitted to the hospital and is twice as likely to die (2).
Unfortunately, surgical site infections are common. Among healthcare-acquired infections, SSIs rank second only to urinary tract infections in frequency, making them more common than bloodstream infections and nosocomial pneumonia (3). There are approximately 30 million operations annually in the United States and an SSI complicates 2–5% of clean extra-abdominal sites. The rate is much higher for intra-abdominal operations, approaching 20% (1). Because most SSIs begin within 2 hours of contamination, the perioperative period is the most crucial for development of an SSI (4). By offering clinical expertise in the practice guidelines that reduce the risk of SSIs, hospital medicine programs can help patients and hospital systems lower morbidity, mortality, and costs associated with this complication. Adherence to best practices will likely require coordinated, multidisciplinary process improvement.
Several important interventions fall directly under the control of the anesthesia and surgical teams, such as administering perioperative oxygen, ensuring perioperative normothermia, and avoiding shaving of the surgical site. In coordinated quality improvement efforts, members of the operative team should assume direct responsibility for the performance of these measures. But the performance of two important interventions in this decisive period is likely to be significantly enhanced by the presence of focused hospitalist surgical co-management: antimicrobial prophylaxis and perioperative glycemic control (Table 1).
Antimicrobial Prophylaxis
Studies overwhelmingly show a marked reduction in the relative risk of SSIs with the use of antibiotic prophylaxis (1). In June 2004, the National Surgical Infection Prevention Project (NSIPP) published an advisory statement on antimicrobial prophylaxis in which it outlined three performance measures for quality improvement in prevention of SSIs:
- The proportion of patients who have parenteral antimicrobial prophylaxis initiated within 1 hour before surgical incision
- The proportion of patients provided with a prophylactic antimicrobial agent that is consistent with currently published guidelines, and
- The proportion of patients whose prophylactic antimicrobial therapy is discontinued within 24 hours after the end of surgery (5)
Pooled data suggest that attention to timing makes a favorable difference in SSI rates (1). Fully administering the appropriate antibiotic within 60 minutes of incision ensures that serum and tissue drug levels exceed the MICs of the most likely contaminating organisms. Dosing the antibiotic immediately prior to the start of surgery also provides the best opportunity to extend therapeutic levels for the duration of the surgery. The fact that anesthesia and surgical teams are in the most practical time-space positions to apply this measure underscores the multi-disciplinary and process-level efforts necessary to reduce SSI rates.
When it comes to the choice of antimicrobial and the duration of its use, hospitalists may find themselves in superior positions of impact. Familiarity with recommendations of the NSIPP advisory statement (summarized in Table 2) promotes evidence-based selection of antibiotic prophylaxis based on patient-specific factors: type of operation and presence of true drug allergies (5). Compared with other members of the surgical co-management team, hospitalists are more likely to be aware of relevant patient-specific risk factors such as the likelihood of colonization with methicillin-resistant Staphylococcus aureus (MRSA). For example, in patients colonized with MRSA, hospitalists might consider vancomycin as the alternative agent for prophylaxis. Free access to the NSIPP advisory statement is available at www.journals.uchicago.edu/CID/journal/issues/v38n12/33257/33257.html.
Antimicrobial prophylaxis after wound closure is unnecessary; published evidence demonstrates the non-inferiority of single dose prophylaxis when compared with multiple dose prophylaxis (5). Further, prolonged use of antimicrobial prophylaxis is associated with the emergence of resistant organisms (6-8). By ensuring that the duration of prophylaxis does not exceed 24 hours past the end of the operation, hospitalists can make valuable contributions to public health and cost containment.
Non-Antimicrobial Measures
Several non-antimicrobial measures also significantly reduce SSI rates. Those that fall outside the domain of the hospitalist and into the direct purview of the operative team include high levels of inspired oxygen, maintenance of perioperative normothermia, and use of clippers rather than a razor when hair removal is necessary. The risk of SSIs is directly related to tissue oxygenation. Bacterial infectivity is enhanced and cellular immunity is compromised in hypoperfused, poorly oxygenated tissue (9). The practice of administering perioperative supplemental oxygen (at least 80% FIO2 in intubated patients) reduces the risk of SSI by nearly one-half (1). For non-intubated patients, oxygen at 12 L/min by non-rebreathing face mask applied intra-operatively and for at least 2 hours following surgery leads to similar reductions of SSI rates. Besides being effective, this intervention is inexpensive, has no recognized adverse effects, and carries the added benefit of significantly reducing post-operative nausea and vomiting (4).
Hypothermia also predisposes the surgical wound to infection. Even mild perioperative hypothermia (i.e., core temperature 35-36.5°C) typically occurs in the absence of specific measures to prevent net heat loss. Perioperative hypothermia is the combined result of exposure and anesthetic-induced thermo-dysregulation, with redistribution of core body heat to the periphery (4). Even mild hypothermia causes vasoconstriction which diminishes perfusion, dropping tissue oxygen tension which impairs phagocytosis and oxidative killing by neutrophils (10). Hypothermia also blunts scar formation which further diminishes wound integrity. Active warming of the patient to maintain a core temperature near 36.5°C constitutes the intra-operative standard of care and is effective at reducing the risk of SSIs by as much as two-thirds (1).
Hyperglycemia, an established independent risk factor for an array of adverse outcomes in hospitalized patients, is also an independent risk factor for SSIs across a range of surgical patients (1). Short-term hyperglycemia depresses immune function through nonenzymatic glycosylation of immunoglobulin and by impairing normal leukocyte performance (11). Among diabetic cardiac surgery patients, reduction of hyperglycemia with an intravenous insulin infusion lowered the incidence of deep sternal wound infection by as much as two-thirds (12). While the value of achieving glycemic targets has already been established for a variety of important endpoints and across a range of inpatient populations, hospitalists should stay tuned. As high quality studies emerge proving that glycemic control lowers SSIs among non-cardiac surgical subpopulations, hospitalists may increasingly be relied upon to achieve strict glycemic targets.
By recognizing and coordinating practices known to reduce SSIs, hospitalists can elevate the level of care provided for surgical patients. At the same time, hospitalists can help lower costs and keep the hospital system mindful of public health goals, such as prevention of antimicrobial resistance. While individual hospitalists have key roles to play, the overall approach to SSI reduction calls for a coordinated, multidisciplinary team approach with process and system-level efforts.
Dr. Stein can be contacted at Jason_Stein@emoryhealthcare.org.
References
- Auerbach AD. Prevention of surgical site infections. In: Shojania KG, Duncan BW, McDonald KM, et al., eds. Making health care safer: a critical analysis of patient safety practices. Evidence report/technology assessment no. 43. AHRQ publication no. 01-E058. Rockville, MD: Agency for Healthcare Research and Quality, 20 July 2001:221-44.
- Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-30.
- National Nosocomial Infections Surveillance (NNIS) report, data summary from October 1986-April 1996, issued May 1996: a report from the National Nosocomial Infections Surveillance (NNIS) system. Am J Infect Control. 1996;24:380-8.
- Sessler DI, Akca O. Nonpharmacologic prevention of surgical wound infections. Clin Infect Dis. 2002;35:1397 404.
- Bratzler D, Houck PM. Surgical Infection Prevention Guidelines Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis. 2004;Jun15;38(12):1706-15. E-pub 2004 May 26.
- Harbarth S, Samore MH, Lichtenberg D, Carmeli Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation. 2000;101:2916-21.
- Eggimann P, Pittet D. Infection control in the ICU. Chest. 2001;120:2059-93.
- Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163:972-8.
- Hopf HW, Hunt TK, West JM, et al. Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Arch Surg. 1997;132:997-1005.
- Kurz A, Sessler DI, Lenhardt RA. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;334:1209-15.
- Garber AJ, Moghissi ES, Bransome ED Jr, et al. American College of Endocrinology Task Force on Inpatient Diabetes Metabolic Control. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;Mar-Apr;10Suppl2:4-9.
- Furnary AP, Zerr K, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures [with discussion]. Ann Thorac Surg. 1999;67:352-62.
Background
An appropriately feared complication of operations, surgical site infections (SSIs) are infections associated with high economic costs and significantly worse clinical outcomes (1). Defined as infections of the superficial incision site, deep incision space, or organ space, SSIs add additional cost ranging from $2,700 to $26,000 per episode according to CDC’s National Nosocomial Infections Surveillance System. Patients who develop an SSI have hospital lengths of stay (LOS) in excess of 7 days longer and are 60% more likely to spend time in the intensive care unit than are patients without an SSI. A patient with an SSI is five times more likely to be readmitted to the hospital and is twice as likely to die (2).
Unfortunately, surgical site infections are common. Among healthcare-acquired infections, SSIs rank second only to urinary tract infections in frequency, making them more common than bloodstream infections and nosocomial pneumonia (3). There are approximately 30 million operations annually in the United States and an SSI complicates 2–5% of clean extra-abdominal sites. The rate is much higher for intra-abdominal operations, approaching 20% (1). Because most SSIs begin within 2 hours of contamination, the perioperative period is the most crucial for development of an SSI (4). By offering clinical expertise in the practice guidelines that reduce the risk of SSIs, hospital medicine programs can help patients and hospital systems lower morbidity, mortality, and costs associated with this complication. Adherence to best practices will likely require coordinated, multidisciplinary process improvement.
Several important interventions fall directly under the control of the anesthesia and surgical teams, such as administering perioperative oxygen, ensuring perioperative normothermia, and avoiding shaving of the surgical site. In coordinated quality improvement efforts, members of the operative team should assume direct responsibility for the performance of these measures. But the performance of two important interventions in this decisive period is likely to be significantly enhanced by the presence of focused hospitalist surgical co-management: antimicrobial prophylaxis and perioperative glycemic control (Table 1).
Antimicrobial Prophylaxis
Studies overwhelmingly show a marked reduction in the relative risk of SSIs with the use of antibiotic prophylaxis (1). In June 2004, the National Surgical Infection Prevention Project (NSIPP) published an advisory statement on antimicrobial prophylaxis in which it outlined three performance measures for quality improvement in prevention of SSIs:
- The proportion of patients who have parenteral antimicrobial prophylaxis initiated within 1 hour before surgical incision
- The proportion of patients provided with a prophylactic antimicrobial agent that is consistent with currently published guidelines, and
- The proportion of patients whose prophylactic antimicrobial therapy is discontinued within 24 hours after the end of surgery (5)
Pooled data suggest that attention to timing makes a favorable difference in SSI rates (1). Fully administering the appropriate antibiotic within 60 minutes of incision ensures that serum and tissue drug levels exceed the MICs of the most likely contaminating organisms. Dosing the antibiotic immediately prior to the start of surgery also provides the best opportunity to extend therapeutic levels for the duration of the surgery. The fact that anesthesia and surgical teams are in the most practical time-space positions to apply this measure underscores the multi-disciplinary and process-level efforts necessary to reduce SSI rates.
When it comes to the choice of antimicrobial and the duration of its use, hospitalists may find themselves in superior positions of impact. Familiarity with recommendations of the NSIPP advisory statement (summarized in Table 2) promotes evidence-based selection of antibiotic prophylaxis based on patient-specific factors: type of operation and presence of true drug allergies (5). Compared with other members of the surgical co-management team, hospitalists are more likely to be aware of relevant patient-specific risk factors such as the likelihood of colonization with methicillin-resistant Staphylococcus aureus (MRSA). For example, in patients colonized with MRSA, hospitalists might consider vancomycin as the alternative agent for prophylaxis. Free access to the NSIPP advisory statement is available at www.journals.uchicago.edu/CID/journal/issues/v38n12/33257/33257.html.
Antimicrobial prophylaxis after wound closure is unnecessary; published evidence demonstrates the non-inferiority of single dose prophylaxis when compared with multiple dose prophylaxis (5). Further, prolonged use of antimicrobial prophylaxis is associated with the emergence of resistant organisms (6-8). By ensuring that the duration of prophylaxis does not exceed 24 hours past the end of the operation, hospitalists can make valuable contributions to public health and cost containment.
Non-Antimicrobial Measures
Several non-antimicrobial measures also significantly reduce SSI rates. Those that fall outside the domain of the hospitalist and into the direct purview of the operative team include high levels of inspired oxygen, maintenance of perioperative normothermia, and use of clippers rather than a razor when hair removal is necessary. The risk of SSIs is directly related to tissue oxygenation. Bacterial infectivity is enhanced and cellular immunity is compromised in hypoperfused, poorly oxygenated tissue (9). The practice of administering perioperative supplemental oxygen (at least 80% FIO2 in intubated patients) reduces the risk of SSI by nearly one-half (1). For non-intubated patients, oxygen at 12 L/min by non-rebreathing face mask applied intra-operatively and for at least 2 hours following surgery leads to similar reductions of SSI rates. Besides being effective, this intervention is inexpensive, has no recognized adverse effects, and carries the added benefit of significantly reducing post-operative nausea and vomiting (4).
Hypothermia also predisposes the surgical wound to infection. Even mild perioperative hypothermia (i.e., core temperature 35-36.5°C) typically occurs in the absence of specific measures to prevent net heat loss. Perioperative hypothermia is the combined result of exposure and anesthetic-induced thermo-dysregulation, with redistribution of core body heat to the periphery (4). Even mild hypothermia causes vasoconstriction which diminishes perfusion, dropping tissue oxygen tension which impairs phagocytosis and oxidative killing by neutrophils (10). Hypothermia also blunts scar formation which further diminishes wound integrity. Active warming of the patient to maintain a core temperature near 36.5°C constitutes the intra-operative standard of care and is effective at reducing the risk of SSIs by as much as two-thirds (1).
Hyperglycemia, an established independent risk factor for an array of adverse outcomes in hospitalized patients, is also an independent risk factor for SSIs across a range of surgical patients (1). Short-term hyperglycemia depresses immune function through nonenzymatic glycosylation of immunoglobulin and by impairing normal leukocyte performance (11). Among diabetic cardiac surgery patients, reduction of hyperglycemia with an intravenous insulin infusion lowered the incidence of deep sternal wound infection by as much as two-thirds (12). While the value of achieving glycemic targets has already been established for a variety of important endpoints and across a range of inpatient populations, hospitalists should stay tuned. As high quality studies emerge proving that glycemic control lowers SSIs among non-cardiac surgical subpopulations, hospitalists may increasingly be relied upon to achieve strict glycemic targets.
By recognizing and coordinating practices known to reduce SSIs, hospitalists can elevate the level of care provided for surgical patients. At the same time, hospitalists can help lower costs and keep the hospital system mindful of public health goals, such as prevention of antimicrobial resistance. While individual hospitalists have key roles to play, the overall approach to SSI reduction calls for a coordinated, multidisciplinary team approach with process and system-level efforts.
Dr. Stein can be contacted at Jason_Stein@emoryhealthcare.org.
References
- Auerbach AD. Prevention of surgical site infections. In: Shojania KG, Duncan BW, McDonald KM, et al., eds. Making health care safer: a critical analysis of patient safety practices. Evidence report/technology assessment no. 43. AHRQ publication no. 01-E058. Rockville, MD: Agency for Healthcare Research and Quality, 20 July 2001:221-44.
- Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-30.
- National Nosocomial Infections Surveillance (NNIS) report, data summary from October 1986-April 1996, issued May 1996: a report from the National Nosocomial Infections Surveillance (NNIS) system. Am J Infect Control. 1996;24:380-8.
- Sessler DI, Akca O. Nonpharmacologic prevention of surgical wound infections. Clin Infect Dis. 2002;35:1397 404.
- Bratzler D, Houck PM. Surgical Infection Prevention Guidelines Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis. 2004;Jun15;38(12):1706-15. E-pub 2004 May 26.
- Harbarth S, Samore MH, Lichtenberg D, Carmeli Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation. 2000;101:2916-21.
- Eggimann P, Pittet D. Infection control in the ICU. Chest. 2001;120:2059-93.
- Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163:972-8.
- Hopf HW, Hunt TK, West JM, et al. Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Arch Surg. 1997;132:997-1005.
- Kurz A, Sessler DI, Lenhardt RA. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;334:1209-15.
- Garber AJ, Moghissi ES, Bransome ED Jr, et al. American College of Endocrinology Task Force on Inpatient Diabetes Metabolic Control. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;Mar-Apr;10Suppl2:4-9.
- Furnary AP, Zerr K, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures [with discussion]. Ann Thorac Surg. 1999;67:352-62.