User login
Case Report
Clinical Findings
A 65-year-old woman presented with multiple asymptomatic discrete nodules and atrophic plaques on the thighs of 4 years’ duration. The lesions had started as 2 small, asymptomatic, madder red plaques symmetrically located on the anterior aspect of each thigh that had gradually increased in number and size, particularly on the right thigh. Two years later, 2 new atrophic plaques appeared on the anterior aspect of each. The lesions developed slowly but never remitted and had been misdiagnosed as primary macular atrophy of skin by several outpatient clinics. The patient’s general health was good and her personal and family history was unremarkable.
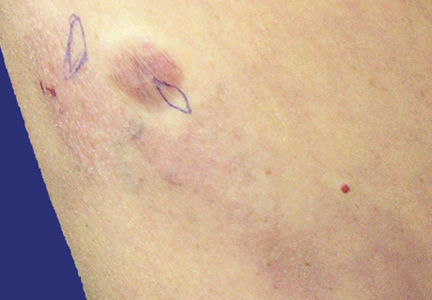
Physical examination revealed multiple madder red plaques and nodules of various shapes and sizes (ie, 1–3 cm in diameter) on the anterior aspect of the right thigh. The lesions were slightly elevated with a waxy surface, firm, and painless to palpation. One similar lesion was noted on the anterior aspect of the left thigh. Two 2-cm, brown-red, atrophic plaques also were noted in a symmetrical distribution on the anterior aspect of each thigh. The plaque surfaces were slightly crinkly and shiny, and anetodermalike lesions produced a buttonhole sign identical to a neurofibroma on palpation (Figure 1).
Histopathologic Findings
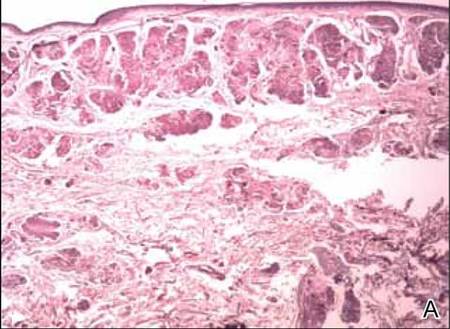
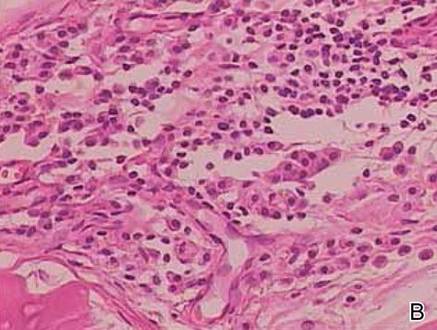
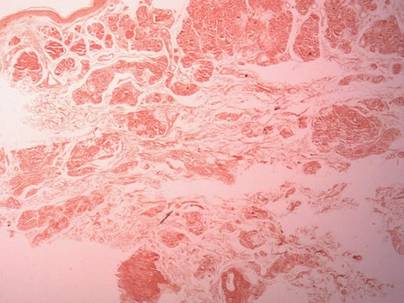
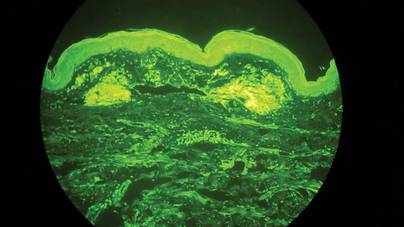
Two biopsy specimens were taken from a nodule and an atrophic plaque on the right thigh. Microscopic examination revealed deposition of homogeneous eosinophilic material in the reticular dermis and subcutis as well as around the fine vessels (Figure 2A). There was mild cellular infiltration of lymphocytes, plasma cells, and giant cells in the dermis, especially adjacent to deposits and around the vessels (Figure 2B). The homogeneous material appeared salmon pink on Congo red staining and bright green by thioflavin T staining using a fluorescent microscope (Figures 3 and 4). These results suggested the characteristic features of cutaneous nodular amyloidosis.
Figure 2. Histopathologically, homogeneous eosinophilic material deposited in the reticular dermis and subcutis was noted (A)(H&E, original magnification ×25). Microscopic examination showed lymphocytes and plasma cells infiltrated in the dermis, especially adjacent to deposits and around the vessels (B)(H&E, original magnification ×200). |
 |
|
Laboratory Findings
Laboratory studies showed normal results for complete blood cell count, urinalysis, liver and renal function tests, blood glucose levels, lipid panel, and erythrocyte sedimentation rate. Serum protein electrophoresis was normal and no Bence Jones proteins were detected. Serum IgA, IgG, and IgM levels showed no abnormalities. Electrocardiogram, chest radiography, and abdominal ultrasound were normal.
A diagnosis of primary localized cutaneous nodular amyloidosis (PLCNA) was made based on clinical, histopathologic, and laboratory findings. Although surgical excision in stages was proposed, the patient refused treatment because the lesions were asymptomatic. There was no obvious progression of the skin lesions and no abnormal systemic findings during 2.5 years’ follow-up.
Comment
Amyloidosis is a spectrum of diseases consisting of deposition of amyloid proteins in various tissues. Clinically, amyloidosis is divided into both primary and secondary forms of systemic amyloidosis, hemodialysis-associated amyloidosis, heredofamilial amyloidosis, and cutaneous amyloidosis. Primary cutaneous amyloidosis is localized to the skin without other organ involvement and does not occur in systemic amyloidosis. Secondary cutaneous involvement in systemic amyloidosis is rare. Most cases of primary localized cutaneous amyloidosis (PLCA) are sporadic but approximately 10% of cases may be familial.1 There are 3 main forms of localized cutaneous amyloidosis: macular, lichen, and nodular amyloidosis. Nodular cutaneous amyloidosis is the rarest form of PLCA.
Nodular amyloidosis was first described by Gottron in 1950.2 Its cutaneous lesions may present as single or multiple nodules, occasionally with overlying atrophic plaques. The lesions consist of firm, smooth-surfaced, waxy or rubbery, pink to tan papules, plaques, or nodules measuring up to several centimeters. On some lesions, surface telangiectasia may be seen. Bullous-appearing and anetodermalike lesions have been reported.3 The acral region is the most common location, followed by the legs, head, trunk, arms, and genitalia, respectively.4 In some cases the lesions can spontaneously improve over time. In our patient, the lesions were composed of both multiple nodules and atrophic plaques, which is uncommon.
The pathogenesis of amyloid deposition is still unknown. Cutaneous macular and lichen amyloidosis may originate from degenerated keratinocyte intermediate filaments. Nodular amyloidosis may represent a localized plasma cell dyscrasia that can be associated with a monoclonal gammopathy or multiple myeloma.5 Some components of amyloid in some cases of PLCNA may consist of κ and λ immunoglobulin light chains, with most reported cases being of the l subtype.6 The results of one study indicated that β2-microglobulin was another major component of amyloid fibrils and that β2-microglobulin was partly subjected to the modification of advanced glycation end product in PLCNA.7
The histopathologic examination of PLCNA is characterized by large deposits of amorphous, sometimes fissured, pale, eosinophilic material in the papillary dermis, reticular dermis, and subcutaneous fat. The overlying epidermis may exhibit flattened rete ridges. Amyloid may occur within vessel walls and adnexal structures, sometimes in a ring surrounding individual fat cells. Clinically, the lesions of PLCNA may be indistinguishable from nodular deposits of amyloid occurring in primary systemic amyloidosis or myeloma-associated amyloidosis. Histopathologically, PLCNA usually has a variable infiltrate of plasma cells and lymphocytes at the periphery or within the amyloid deposits,6 but no single stain is highly sensitive and specific. Congo red–stained deposits showed salmon pink amorphous material or apple green birefringence with polarizing microscopy.8 Amyloid derived from immunoglobulin light chains, including cutaneous nodular amyloid, also stain positive for anti-human λ light chain antibody on immunohistochemistry. Additionally, amyloid can stain positively with methyl violet and crystal violet Gram stains, Picrosirius red, thioflavin T, Dylon, and periodic acid–Schiff stains.
Clinically, some PLCNA lesions can be removed via surgical excision or laser if they are cosmetically disfiguring or symptomatic. Other methods have been attempted to improve the appearance of the lesions, such as intralesional corticosteroids, cryotherapy, and dermabrasion,9 but they usually are not helpful and have a high rate of recurrence. Although PLCNA often is a benign cutaneous disorder and some cases of PLCNA could be reactive diseases rather than neoplastic ones, some patients may develop underlying systemic amyloidosis or even paraproteinemia.10 Northcutt and Vanover11 indicated that systemic amyloidosis may be expected in less than 15% of 47 patients with localized cutaneous amyloidosis during follow-up by reviewing most of the related literature. Some cases may be associated with Sjögren syndrome (SJS), CREST (calcinosis, Raynaud phenomenon, esophageal motility disorders, sclerodactyly, and telangiectasia) syndrome, dermatomyositis, and diabetes mellitus.12-14 Polyclonal immunoglobulin amyloid has been reported only in PLCNA with SJS, which may be due to the fact that a certain population of SJS develops polyclonal B-cell proliferation and hyperglobulinemia.12 Woollons and Black15 estimated the rate of progression of PLCNA to systemic amyloidosis to be only 7%, which is much lower than the rate in the literature by a large clinical follow-up study on PLCNA.16 However, all patients with PLCNA should have a systemic evaluation and should be advised to undergo long-term clinical follow-up to help prevent progression to systemic amyloidosis or plasma cell dyscrasia.
1. Sakuma TH, Hans-Filho G, Arita K, et al. Familial primary localized cutaneous amyloidosis in Brazil. Arch Dermatol. 2009;145:695-699.
2. Rodermund OE. On amyloidosis cutis nodularis atrophicans (Gottron 1950). at the same time a contribution to the classification of amyloidosis [in German]. Arch Klin Exp Dermatol. 1967;230:153-171.
3. Chapel TA, Birmingham DJ, Malinowski YE. Nodular primary localized cutaneous amyloidosis. Arch Dermatol. 1977;113:1248-1249.
4. Criado PR, Silva CS, Vasconcellos C, et al. Extensive nodular cutaneous amyloidosis: an unusual presentation. J Eur Acad Dermatol Venereol. 2005;19:481-483.
5. Touart DM, Sau P. Cutaneous deposition diseases. part I [published correction appears in J Am Acad Dermatol. 1998;39:1042]. J Am Acad Dermatol. 1998;39(2, pt 1):149-171; quiz 172-174.
6. Borrowman TA, Lutz ME, Walsh JS. Cutaneous nodular amyloidosis masquerading as a foot callus. J Am Acad Dermatol. 2003;49:307-310.
7. Fujimoto N, Yajima M, Ohnishi Y, et al. Advanced glycation end product-modified beta2-microglobulin is a component of amyloid fibrils of primary localized cutaneous nodular amyloidosis. J Invest Dermatol. 2002;118:479-484.
8. Clement CG, Truong LD. An evaluation of Congo red fluorescence for the diagnosis of amyloidosis. Hum Pathol. 2014;45:1766-1772.
9. Lien MH, Railan D, Nelson BR. The efficacy of dermabrasion in the treatment of nodular amyloidosis. J Am Acad Dermatol. 1997;36(2, pt 2):315-316.
10. Taylor SC, Baker E, Grossman ME. Nodular vulvar amyloid as a presentation of systemic amyloidosis. J Am Acad Dermatol. 1991;24:139.
11. Northcutt AD, Vanover MJ. Nodular cutaneous amyloidosis involving the vulva. case report and literature review. Arch Dermatol. 1985;121:518-521.
12. Yoneyama K, Tochigi N, Oikawa A, et al. Primary localized cutaneous nodular amyloidosis in a patient with Sjögren’s syndrome: a review of the literature. J Dermatol. 2005;32:120-123.
13. Summers EM, Kendrick CG. Primary localized cutaneous nodular amyloidosis and CREST syndrome: a case report and review of the literature. Cutis. 2008;82:55-59.
14. Taniguchi Y, Horino T, Terada Y. Cutaneous amyloidosis associated with amyopathic dermatomyositis. J Rheumatol. 2009;36:1088-1089.
15. Woollons A, Black MM. Nodular localized primary cutaneous amyloidosis: a long-term follow-up study. Br J Dermatol. 2001;145:105-109.
16. Brownstein MH, Helwig EB. The cutaneous amyloidoses. I. localized forms. Arch Dermatol. 1970;102:8-19.
Case Report
Clinical Findings
A 65-year-old woman presented with multiple asymptomatic discrete nodules and atrophic plaques on the thighs of 4 years’ duration. The lesions had started as 2 small, asymptomatic, madder red plaques symmetrically located on the anterior aspect of each thigh that had gradually increased in number and size, particularly on the right thigh. Two years later, 2 new atrophic plaques appeared on the anterior aspect of each. The lesions developed slowly but never remitted and had been misdiagnosed as primary macular atrophy of skin by several outpatient clinics. The patient’s general health was good and her personal and family history was unremarkable.
Physical examination revealed multiple madder red plaques and nodules of various shapes and sizes (ie, 1–3 cm in diameter) on the anterior aspect of the right thigh. The lesions were slightly elevated with a waxy surface, firm, and painless to palpation. One similar lesion was noted on the anterior aspect of the left thigh. Two 2-cm, brown-red, atrophic plaques also were noted in a symmetrical distribution on the anterior aspect of each thigh. The plaque surfaces were slightly crinkly and shiny, and anetodermalike lesions produced a buttonhole sign identical to a neurofibroma on palpation (Figure 1).
Histopathologic Findings
Two biopsy specimens were taken from a nodule and an atrophic plaque on the right thigh. Microscopic examination revealed deposition of homogeneous eosinophilic material in the reticular dermis and subcutis as well as around the fine vessels (Figure 2A). There was mild cellular infiltration of lymphocytes, plasma cells, and giant cells in the dermis, especially adjacent to deposits and around the vessels (Figure 2B). The homogeneous material appeared salmon pink on Congo red staining and bright green by thioflavin T staining using a fluorescent microscope (Figures 3 and 4). These results suggested the characteristic features of cutaneous nodular amyloidosis.
Figure 2. Histopathologically, homogeneous eosinophilic material deposited in the reticular dermis and subcutis was noted (A)(H&E, original magnification ×25). Microscopic examination showed lymphocytes and plasma cells infiltrated in the dermis, especially adjacent to deposits and around the vessels (B)(H&E, original magnification ×200). |
 |

|
Laboratory Findings
Laboratory studies showed normal results for complete blood cell count, urinalysis, liver and renal function tests, blood glucose levels, lipid panel, and erythrocyte sedimentation rate. Serum protein electrophoresis was normal and no Bence Jones proteins were detected. Serum IgA, IgG, and IgM levels showed no abnormalities. Electrocardiogram, chest radiography, and abdominal ultrasound were normal.
A diagnosis of primary localized cutaneous nodular amyloidosis (PLCNA) was made based on clinical, histopathologic, and laboratory findings. Although surgical excision in stages was proposed, the patient refused treatment because the lesions were asymptomatic. There was no obvious progression of the skin lesions and no abnormal systemic findings during 2.5 years’ follow-up.
Comment
Amyloidosis is a spectrum of diseases consisting of deposition of amyloid proteins in various tissues. Clinically, amyloidosis is divided into both primary and secondary forms of systemic amyloidosis, hemodialysis-associated amyloidosis, heredofamilial amyloidosis, and cutaneous amyloidosis. Primary cutaneous amyloidosis is localized to the skin without other organ involvement and does not occur in systemic amyloidosis. Secondary cutaneous involvement in systemic amyloidosis is rare. Most cases of primary localized cutaneous amyloidosis (PLCA) are sporadic but approximately 10% of cases may be familial.1 There are 3 main forms of localized cutaneous amyloidosis: macular, lichen, and nodular amyloidosis. Nodular cutaneous amyloidosis is the rarest form of PLCA.
Nodular amyloidosis was first described by Gottron in 1950.2 Its cutaneous lesions may present as single or multiple nodules, occasionally with overlying atrophic plaques. The lesions consist of firm, smooth-surfaced, waxy or rubbery, pink to tan papules, plaques, or nodules measuring up to several centimeters. On some lesions, surface telangiectasia may be seen. Bullous-appearing and anetodermalike lesions have been reported.3 The acral region is the most common location, followed by the legs, head, trunk, arms, and genitalia, respectively.4 In some cases the lesions can spontaneously improve over time. In our patient, the lesions were composed of both multiple nodules and atrophic plaques, which is uncommon.
The pathogenesis of amyloid deposition is still unknown. Cutaneous macular and lichen amyloidosis may originate from degenerated keratinocyte intermediate filaments. Nodular amyloidosis may represent a localized plasma cell dyscrasia that can be associated with a monoclonal gammopathy or multiple myeloma.5 Some components of amyloid in some cases of PLCNA may consist of κ and λ immunoglobulin light chains, with most reported cases being of the l subtype.6 The results of one study indicated that β2-microglobulin was another major component of amyloid fibrils and that β2-microglobulin was partly subjected to the modification of advanced glycation end product in PLCNA.7
The histopathologic examination of PLCNA is characterized by large deposits of amorphous, sometimes fissured, pale, eosinophilic material in the papillary dermis, reticular dermis, and subcutaneous fat. The overlying epidermis may exhibit flattened rete ridges. Amyloid may occur within vessel walls and adnexal structures, sometimes in a ring surrounding individual fat cells. Clinically, the lesions of PLCNA may be indistinguishable from nodular deposits of amyloid occurring in primary systemic amyloidosis or myeloma-associated amyloidosis. Histopathologically, PLCNA usually has a variable infiltrate of plasma cells and lymphocytes at the periphery or within the amyloid deposits,6 but no single stain is highly sensitive and specific. Congo red–stained deposits showed salmon pink amorphous material or apple green birefringence with polarizing microscopy.8 Amyloid derived from immunoglobulin light chains, including cutaneous nodular amyloid, also stain positive for anti-human λ light chain antibody on immunohistochemistry. Additionally, amyloid can stain positively with methyl violet and crystal violet Gram stains, Picrosirius red, thioflavin T, Dylon, and periodic acid–Schiff stains.
Clinically, some PLCNA lesions can be removed via surgical excision or laser if they are cosmetically disfiguring or symptomatic. Other methods have been attempted to improve the appearance of the lesions, such as intralesional corticosteroids, cryotherapy, and dermabrasion,9 but they usually are not helpful and have a high rate of recurrence. Although PLCNA often is a benign cutaneous disorder and some cases of PLCNA could be reactive diseases rather than neoplastic ones, some patients may develop underlying systemic amyloidosis or even paraproteinemia.10 Northcutt and Vanover11 indicated that systemic amyloidosis may be expected in less than 15% of 47 patients with localized cutaneous amyloidosis during follow-up by reviewing most of the related literature. Some cases may be associated with Sjögren syndrome (SJS), CREST (calcinosis, Raynaud phenomenon, esophageal motility disorders, sclerodactyly, and telangiectasia) syndrome, dermatomyositis, and diabetes mellitus.12-14 Polyclonal immunoglobulin amyloid has been reported only in PLCNA with SJS, which may be due to the fact that a certain population of SJS develops polyclonal B-cell proliferation and hyperglobulinemia.12 Woollons and Black15 estimated the rate of progression of PLCNA to systemic amyloidosis to be only 7%, which is much lower than the rate in the literature by a large clinical follow-up study on PLCNA.16 However, all patients with PLCNA should have a systemic evaluation and should be advised to undergo long-term clinical follow-up to help prevent progression to systemic amyloidosis or plasma cell dyscrasia.
Case Report
Clinical Findings
A 65-year-old woman presented with multiple asymptomatic discrete nodules and atrophic plaques on the thighs of 4 years’ duration. The lesions had started as 2 small, asymptomatic, madder red plaques symmetrically located on the anterior aspect of each thigh that had gradually increased in number and size, particularly on the right thigh. Two years later, 2 new atrophic plaques appeared on the anterior aspect of each. The lesions developed slowly but never remitted and had been misdiagnosed as primary macular atrophy of skin by several outpatient clinics. The patient’s general health was good and her personal and family history was unremarkable.
Physical examination revealed multiple madder red plaques and nodules of various shapes and sizes (ie, 1–3 cm in diameter) on the anterior aspect of the right thigh. The lesions were slightly elevated with a waxy surface, firm, and painless to palpation. One similar lesion was noted on the anterior aspect of the left thigh. Two 2-cm, brown-red, atrophic plaques also were noted in a symmetrical distribution on the anterior aspect of each thigh. The plaque surfaces were slightly crinkly and shiny, and anetodermalike lesions produced a buttonhole sign identical to a neurofibroma on palpation (Figure 1).
Histopathologic Findings
Two biopsy specimens were taken from a nodule and an atrophic plaque on the right thigh. Microscopic examination revealed deposition of homogeneous eosinophilic material in the reticular dermis and subcutis as well as around the fine vessels (Figure 2A). There was mild cellular infiltration of lymphocytes, plasma cells, and giant cells in the dermis, especially adjacent to deposits and around the vessels (Figure 2B). The homogeneous material appeared salmon pink on Congo red staining and bright green by thioflavin T staining using a fluorescent microscope (Figures 3 and 4). These results suggested the characteristic features of cutaneous nodular amyloidosis.
Figure 2. Histopathologically, homogeneous eosinophilic material deposited in the reticular dermis and subcutis was noted (A)(H&E, original magnification ×25). Microscopic examination showed lymphocytes and plasma cells infiltrated in the dermis, especially adjacent to deposits and around the vessels (B)(H&E, original magnification ×200). |
 |

|
Laboratory Findings
Laboratory studies showed normal results for complete blood cell count, urinalysis, liver and renal function tests, blood glucose levels, lipid panel, and erythrocyte sedimentation rate. Serum protein electrophoresis was normal and no Bence Jones proteins were detected. Serum IgA, IgG, and IgM levels showed no abnormalities. Electrocardiogram, chest radiography, and abdominal ultrasound were normal.
A diagnosis of primary localized cutaneous nodular amyloidosis (PLCNA) was made based on clinical, histopathologic, and laboratory findings. Although surgical excision in stages was proposed, the patient refused treatment because the lesions were asymptomatic. There was no obvious progression of the skin lesions and no abnormal systemic findings during 2.5 years’ follow-up.
Comment
Amyloidosis is a spectrum of diseases consisting of deposition of amyloid proteins in various tissues. Clinically, amyloidosis is divided into both primary and secondary forms of systemic amyloidosis, hemodialysis-associated amyloidosis, heredofamilial amyloidosis, and cutaneous amyloidosis. Primary cutaneous amyloidosis is localized to the skin without other organ involvement and does not occur in systemic amyloidosis. Secondary cutaneous involvement in systemic amyloidosis is rare. Most cases of primary localized cutaneous amyloidosis (PLCA) are sporadic but approximately 10% of cases may be familial.1 There are 3 main forms of localized cutaneous amyloidosis: macular, lichen, and nodular amyloidosis. Nodular cutaneous amyloidosis is the rarest form of PLCA.
Nodular amyloidosis was first described by Gottron in 1950.2 Its cutaneous lesions may present as single or multiple nodules, occasionally with overlying atrophic plaques. The lesions consist of firm, smooth-surfaced, waxy or rubbery, pink to tan papules, plaques, or nodules measuring up to several centimeters. On some lesions, surface telangiectasia may be seen. Bullous-appearing and anetodermalike lesions have been reported.3 The acral region is the most common location, followed by the legs, head, trunk, arms, and genitalia, respectively.4 In some cases the lesions can spontaneously improve over time. In our patient, the lesions were composed of both multiple nodules and atrophic plaques, which is uncommon.
The pathogenesis of amyloid deposition is still unknown. Cutaneous macular and lichen amyloidosis may originate from degenerated keratinocyte intermediate filaments. Nodular amyloidosis may represent a localized plasma cell dyscrasia that can be associated with a monoclonal gammopathy or multiple myeloma.5 Some components of amyloid in some cases of PLCNA may consist of κ and λ immunoglobulin light chains, with most reported cases being of the l subtype.6 The results of one study indicated that β2-microglobulin was another major component of amyloid fibrils and that β2-microglobulin was partly subjected to the modification of advanced glycation end product in PLCNA.7
The histopathologic examination of PLCNA is characterized by large deposits of amorphous, sometimes fissured, pale, eosinophilic material in the papillary dermis, reticular dermis, and subcutaneous fat. The overlying epidermis may exhibit flattened rete ridges. Amyloid may occur within vessel walls and adnexal structures, sometimes in a ring surrounding individual fat cells. Clinically, the lesions of PLCNA may be indistinguishable from nodular deposits of amyloid occurring in primary systemic amyloidosis or myeloma-associated amyloidosis. Histopathologically, PLCNA usually has a variable infiltrate of plasma cells and lymphocytes at the periphery or within the amyloid deposits,6 but no single stain is highly sensitive and specific. Congo red–stained deposits showed salmon pink amorphous material or apple green birefringence with polarizing microscopy.8 Amyloid derived from immunoglobulin light chains, including cutaneous nodular amyloid, also stain positive for anti-human λ light chain antibody on immunohistochemistry. Additionally, amyloid can stain positively with methyl violet and crystal violet Gram stains, Picrosirius red, thioflavin T, Dylon, and periodic acid–Schiff stains.
Clinically, some PLCNA lesions can be removed via surgical excision or laser if they are cosmetically disfiguring or symptomatic. Other methods have been attempted to improve the appearance of the lesions, such as intralesional corticosteroids, cryotherapy, and dermabrasion,9 but they usually are not helpful and have a high rate of recurrence. Although PLCNA often is a benign cutaneous disorder and some cases of PLCNA could be reactive diseases rather than neoplastic ones, some patients may develop underlying systemic amyloidosis or even paraproteinemia.10 Northcutt and Vanover11 indicated that systemic amyloidosis may be expected in less than 15% of 47 patients with localized cutaneous amyloidosis during follow-up by reviewing most of the related literature. Some cases may be associated with Sjögren syndrome (SJS), CREST (calcinosis, Raynaud phenomenon, esophageal motility disorders, sclerodactyly, and telangiectasia) syndrome, dermatomyositis, and diabetes mellitus.12-14 Polyclonal immunoglobulin amyloid has been reported only in PLCNA with SJS, which may be due to the fact that a certain population of SJS develops polyclonal B-cell proliferation and hyperglobulinemia.12 Woollons and Black15 estimated the rate of progression of PLCNA to systemic amyloidosis to be only 7%, which is much lower than the rate in the literature by a large clinical follow-up study on PLCNA.16 However, all patients with PLCNA should have a systemic evaluation and should be advised to undergo long-term clinical follow-up to help prevent progression to systemic amyloidosis or plasma cell dyscrasia.
1. Sakuma TH, Hans-Filho G, Arita K, et al. Familial primary localized cutaneous amyloidosis in Brazil. Arch Dermatol. 2009;145:695-699.
2. Rodermund OE. On amyloidosis cutis nodularis atrophicans (Gottron 1950). at the same time a contribution to the classification of amyloidosis [in German]. Arch Klin Exp Dermatol. 1967;230:153-171.
3. Chapel TA, Birmingham DJ, Malinowski YE. Nodular primary localized cutaneous amyloidosis. Arch Dermatol. 1977;113:1248-1249.
4. Criado PR, Silva CS, Vasconcellos C, et al. Extensive nodular cutaneous amyloidosis: an unusual presentation. J Eur Acad Dermatol Venereol. 2005;19:481-483.
5. Touart DM, Sau P. Cutaneous deposition diseases. part I [published correction appears in J Am Acad Dermatol. 1998;39:1042]. J Am Acad Dermatol. 1998;39(2, pt 1):149-171; quiz 172-174.
6. Borrowman TA, Lutz ME, Walsh JS. Cutaneous nodular amyloidosis masquerading as a foot callus. J Am Acad Dermatol. 2003;49:307-310.
7. Fujimoto N, Yajima M, Ohnishi Y, et al. Advanced glycation end product-modified beta2-microglobulin is a component of amyloid fibrils of primary localized cutaneous nodular amyloidosis. J Invest Dermatol. 2002;118:479-484.
8. Clement CG, Truong LD. An evaluation of Congo red fluorescence for the diagnosis of amyloidosis. Hum Pathol. 2014;45:1766-1772.
9. Lien MH, Railan D, Nelson BR. The efficacy of dermabrasion in the treatment of nodular amyloidosis. J Am Acad Dermatol. 1997;36(2, pt 2):315-316.
10. Taylor SC, Baker E, Grossman ME. Nodular vulvar amyloid as a presentation of systemic amyloidosis. J Am Acad Dermatol. 1991;24:139.
11. Northcutt AD, Vanover MJ. Nodular cutaneous amyloidosis involving the vulva. case report and literature review. Arch Dermatol. 1985;121:518-521.
12. Yoneyama K, Tochigi N, Oikawa A, et al. Primary localized cutaneous nodular amyloidosis in a patient with Sjögren’s syndrome: a review of the literature. J Dermatol. 2005;32:120-123.
13. Summers EM, Kendrick CG. Primary localized cutaneous nodular amyloidosis and CREST syndrome: a case report and review of the literature. Cutis. 2008;82:55-59.
14. Taniguchi Y, Horino T, Terada Y. Cutaneous amyloidosis associated with amyopathic dermatomyositis. J Rheumatol. 2009;36:1088-1089.
15. Woollons A, Black MM. Nodular localized primary cutaneous amyloidosis: a long-term follow-up study. Br J Dermatol. 2001;145:105-109.
16. Brownstein MH, Helwig EB. The cutaneous amyloidoses. I. localized forms. Arch Dermatol. 1970;102:8-19.
1. Sakuma TH, Hans-Filho G, Arita K, et al. Familial primary localized cutaneous amyloidosis in Brazil. Arch Dermatol. 2009;145:695-699.
2. Rodermund OE. On amyloidosis cutis nodularis atrophicans (Gottron 1950). at the same time a contribution to the classification of amyloidosis [in German]. Arch Klin Exp Dermatol. 1967;230:153-171.
3. Chapel TA, Birmingham DJ, Malinowski YE. Nodular primary localized cutaneous amyloidosis. Arch Dermatol. 1977;113:1248-1249.
4. Criado PR, Silva CS, Vasconcellos C, et al. Extensive nodular cutaneous amyloidosis: an unusual presentation. J Eur Acad Dermatol Venereol. 2005;19:481-483.
5. Touart DM, Sau P. Cutaneous deposition diseases. part I [published correction appears in J Am Acad Dermatol. 1998;39:1042]. J Am Acad Dermatol. 1998;39(2, pt 1):149-171; quiz 172-174.
6. Borrowman TA, Lutz ME, Walsh JS. Cutaneous nodular amyloidosis masquerading as a foot callus. J Am Acad Dermatol. 2003;49:307-310.
7. Fujimoto N, Yajima M, Ohnishi Y, et al. Advanced glycation end product-modified beta2-microglobulin is a component of amyloid fibrils of primary localized cutaneous nodular amyloidosis. J Invest Dermatol. 2002;118:479-484.
8. Clement CG, Truong LD. An evaluation of Congo red fluorescence for the diagnosis of amyloidosis. Hum Pathol. 2014;45:1766-1772.
9. Lien MH, Railan D, Nelson BR. The efficacy of dermabrasion in the treatment of nodular amyloidosis. J Am Acad Dermatol. 1997;36(2, pt 2):315-316.
10. Taylor SC, Baker E, Grossman ME. Nodular vulvar amyloid as a presentation of systemic amyloidosis. J Am Acad Dermatol. 1991;24:139.
11. Northcutt AD, Vanover MJ. Nodular cutaneous amyloidosis involving the vulva. case report and literature review. Arch Dermatol. 1985;121:518-521.
12. Yoneyama K, Tochigi N, Oikawa A, et al. Primary localized cutaneous nodular amyloidosis in a patient with Sjögren’s syndrome: a review of the literature. J Dermatol. 2005;32:120-123.
13. Summers EM, Kendrick CG. Primary localized cutaneous nodular amyloidosis and CREST syndrome: a case report and review of the literature. Cutis. 2008;82:55-59.
14. Taniguchi Y, Horino T, Terada Y. Cutaneous amyloidosis associated with amyopathic dermatomyositis. J Rheumatol. 2009;36:1088-1089.
15. Woollons A, Black MM. Nodular localized primary cutaneous amyloidosis: a long-term follow-up study. Br J Dermatol. 2001;145:105-109.
16. Brownstein MH, Helwig EB. The cutaneous amyloidoses. I. localized forms. Arch Dermatol. 1970;102:8-19.
Practice Points
- The cutaneous lesions of primary localized cutaneous nodular amyloidosis (PLCNA) may present as single or multiple nodules, occasionally with overlying atrophic plaques and some with surface telangiectasia.
- Primary localized cutaneous nodular amyloidosis may represent a localized plasma cell dyscrasia that can be associated with a monoclonal gammopathy or multiple myeloma.
- Although PLCNA often is a benign cutaneous disorder, some patients can develop underlying systemic amyloidosis or even paraproteinemia.