User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Psychotropic medications for chronic pain
The opioid crisis presents a need to consider alternative options for treating chronic pain. There is significant overlap in neuroanatomical circuits that process pain, emotions, and motivation. Neurotransmitters modulated by psychotropic medications are also involved in regulating the pain pathways.1,2 In light of this, psychotropics can be considered for treating chronic pain in certain patients. The Table1-3 outlines various uses and adverse effects of select psychotropic medications used to treat pain, as well as their psychiatric uses.
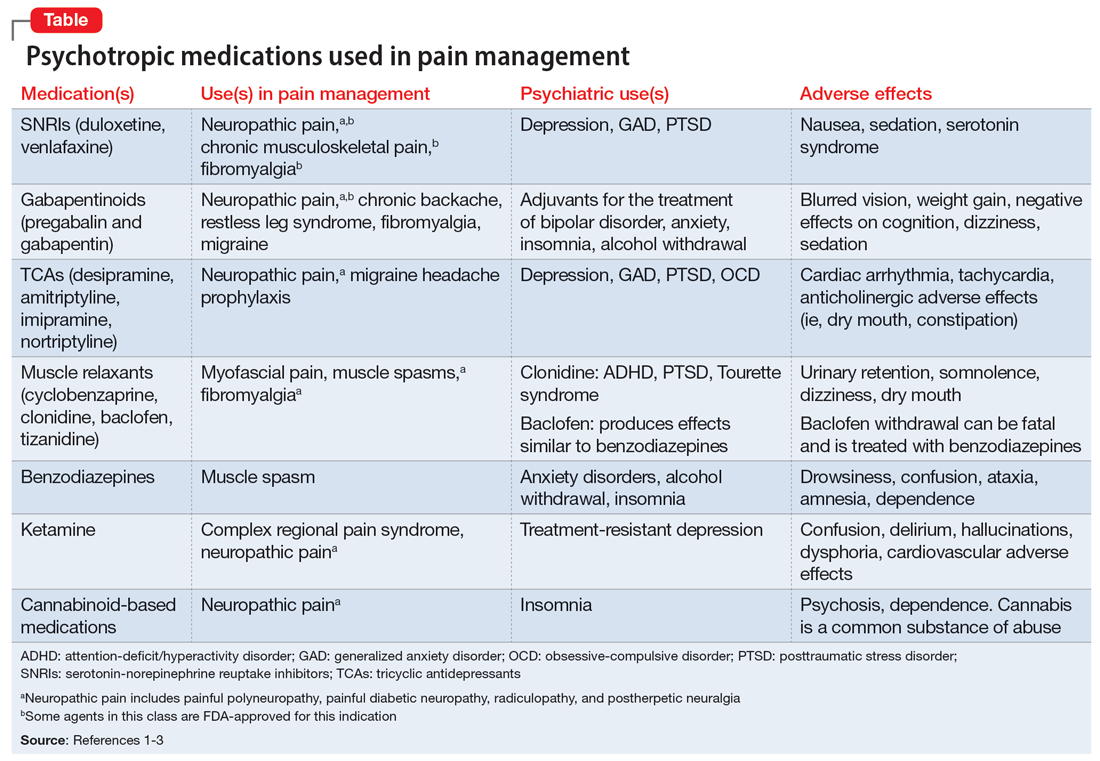
In addition to its psychiatric indications, the serotonin-norepinephrine reuptake inhibitor duloxetine is FDA-approved for treating fibromyalgia and diabetic neuropathic pain. It is often prescribed in the treatment of multiple pain disorders. Tricyclic antidepressants (TCAs) have the largest effect size in the treatment of neuropathic pain.2 Cyclobenzaprine is a TCA used to treat muscle spasms. Gabapentinoids (alpha-2 delta-1 calcium channel inhibition) are FDA-approved for treating postherpetic neuralgia, fibromyalgia, and diabetic neuropathy.1,2
Ketamine is an anesthetic with analgesic and antidepressant properties used as an IV infusion to manage several pain disorders.2 The alpha-2 adrenergic agonists tizanidine and clonidine are muscle relaxants2; the latter is used to treat attention-deficit/hyperactivity disorder and Tourette syndrome. Benzodiazepines (GABA-A agonists) are used for short-term treatment of anxiety disorders, insomnia, and muscle spasms.1,2 Baclofen (GABA-B receptor agonist) is used to treat spasticity.2 Medical cannabis (tetrahydrocannabinol/cannabidiol) is also gaining popularity for treating chronic pain and insomnia.1-3
1. Sutherland AM, Nicholls J, Bao J, et al. Overlaps in pharmacology for the treatment of chronic pain and mental health disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87(Pt B):290-297.
2. Bajwa ZH, Wootton RJ, Warfield CA. Principles and Practice of Pain Medicine. 3rd ed. McGraw Hill; 2016.
3. McDonagh MS, Selph SS, Buckley DI, et al. Nonopioid Pharmacologic Treatments for Chronic Pain. Comparative Effectiveness Review No. 228. Agency for Healthcare Research and Quality; 2020. doi:10.23970/AHRQEPCCER228
The opioid crisis presents a need to consider alternative options for treating chronic pain. There is significant overlap in neuroanatomical circuits that process pain, emotions, and motivation. Neurotransmitters modulated by psychotropic medications are also involved in regulating the pain pathways.1,2 In light of this, psychotropics can be considered for treating chronic pain in certain patients. The Table1-3 outlines various uses and adverse effects of select psychotropic medications used to treat pain, as well as their psychiatric uses.

In addition to its psychiatric indications, the serotonin-norepinephrine reuptake inhibitor duloxetine is FDA-approved for treating fibromyalgia and diabetic neuropathic pain. It is often prescribed in the treatment of multiple pain disorders. Tricyclic antidepressants (TCAs) have the largest effect size in the treatment of neuropathic pain.2 Cyclobenzaprine is a TCA used to treat muscle spasms. Gabapentinoids (alpha-2 delta-1 calcium channel inhibition) are FDA-approved for treating postherpetic neuralgia, fibromyalgia, and diabetic neuropathy.1,2
Ketamine is an anesthetic with analgesic and antidepressant properties used as an IV infusion to manage several pain disorders.2 The alpha-2 adrenergic agonists tizanidine and clonidine are muscle relaxants2; the latter is used to treat attention-deficit/hyperactivity disorder and Tourette syndrome. Benzodiazepines (GABA-A agonists) are used for short-term treatment of anxiety disorders, insomnia, and muscle spasms.1,2 Baclofen (GABA-B receptor agonist) is used to treat spasticity.2 Medical cannabis (tetrahydrocannabinol/cannabidiol) is also gaining popularity for treating chronic pain and insomnia.1-3
The opioid crisis presents a need to consider alternative options for treating chronic pain. There is significant overlap in neuroanatomical circuits that process pain, emotions, and motivation. Neurotransmitters modulated by psychotropic medications are also involved in regulating the pain pathways.1,2 In light of this, psychotropics can be considered for treating chronic pain in certain patients. The Table1-3 outlines various uses and adverse effects of select psychotropic medications used to treat pain, as well as their psychiatric uses.

In addition to its psychiatric indications, the serotonin-norepinephrine reuptake inhibitor duloxetine is FDA-approved for treating fibromyalgia and diabetic neuropathic pain. It is often prescribed in the treatment of multiple pain disorders. Tricyclic antidepressants (TCAs) have the largest effect size in the treatment of neuropathic pain.2 Cyclobenzaprine is a TCA used to treat muscle spasms. Gabapentinoids (alpha-2 delta-1 calcium channel inhibition) are FDA-approved for treating postherpetic neuralgia, fibromyalgia, and diabetic neuropathy.1,2
Ketamine is an anesthetic with analgesic and antidepressant properties used as an IV infusion to manage several pain disorders.2 The alpha-2 adrenergic agonists tizanidine and clonidine are muscle relaxants2; the latter is used to treat attention-deficit/hyperactivity disorder and Tourette syndrome. Benzodiazepines (GABA-A agonists) are used for short-term treatment of anxiety disorders, insomnia, and muscle spasms.1,2 Baclofen (GABA-B receptor agonist) is used to treat spasticity.2 Medical cannabis (tetrahydrocannabinol/cannabidiol) is also gaining popularity for treating chronic pain and insomnia.1-3
1. Sutherland AM, Nicholls J, Bao J, et al. Overlaps in pharmacology for the treatment of chronic pain and mental health disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87(Pt B):290-297.
2. Bajwa ZH, Wootton RJ, Warfield CA. Principles and Practice of Pain Medicine. 3rd ed. McGraw Hill; 2016.
3. McDonagh MS, Selph SS, Buckley DI, et al. Nonopioid Pharmacologic Treatments for Chronic Pain. Comparative Effectiveness Review No. 228. Agency for Healthcare Research and Quality; 2020. doi:10.23970/AHRQEPCCER228
1. Sutherland AM, Nicholls J, Bao J, et al. Overlaps in pharmacology for the treatment of chronic pain and mental health disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87(Pt B):290-297.
2. Bajwa ZH, Wootton RJ, Warfield CA. Principles and Practice of Pain Medicine. 3rd ed. McGraw Hill; 2016.
3. McDonagh MS, Selph SS, Buckley DI, et al. Nonopioid Pharmacologic Treatments for Chronic Pain. Comparative Effectiveness Review No. 228. Agency for Healthcare Research and Quality; 2020. doi:10.23970/AHRQEPCCER228
The light at the end of the tunnel: Reflecting on a 7-year training journey
Throughout my training, a common refrain from more senior colleagues was that training “goes by quickly.” At the risk of sounding cliché, and even after a 7-year journey spanning psychiatry and preventive medicine residencies as well as a consultation-liaison psychiatry fellowship, I agree without reservations that it does indeed go quickly. In the waning days of my training, reflection and nostalgia have become commonplace, as one might expect after such a meaningful pursuit. In sharing my reflections, I hope others progressing through training will also reflect on elements that added meaning to their experience and how they might improve the journey for future trainees.
Residency is a team sport
One realization that quickly struck me was that residency is a team sport, and finding supportive communities is essential to survival. Other residents, colleagues, and mentors played integral roles in making my experience rewarding. Training might be considered a shared traumatic experience, but having peers to commiserate with at each step has been among its greatest rewards. Residency automatically provided a cohort of colleagues who shared and validated my experiences. Additionally, having mentors who have been through it themselves and find ways to improve the training experience made mine superlative. Mentors assisted me in tailoring my training and developing interests that I could integrate into my future practice. The interpersonal connections I made were critical in helping me survive and thrive during training.
See one, do one, teach one
Residency and fellowship programs might be considered “see one, do one, teach one”1 at large scale. Since their inception, these programs—designed to develop junior physicians—have been inherently educational in nature. The structure is elegant, allowing trainees to continue learning while incrementally gaining more autonomy and teaching responsibility.2 Naively, I did not understand that implicit within my education was an expectation to become an educator and hone my teaching skills. Initially, being a newly minted resident receiving brand-new 3rd-year medical students charged me with apprehension. Thoughts I internalized, such as “these students probably know more than me” or “how can I be responsible for patients and students simultaneously,” may have resulted from a paucity of instruction about teaching available during medical school.3,4 I quickly found, though, that teaching was among the most rewarding facets of training. Helping other learners grow became one of my passions and added to my experience.
Iron sharpens iron
Although my experience was enjoyable, I would be remiss without also considering accompanying trials and tribulations. Seemingly interminable night shifts, sleep deprivation, lack of autonomy, and system inefficiencies frustrated me. Eventually, these frustrations seemed less bothersome. These challenges likely had not vanished with time, but perhaps my capacity to tolerate distress improved—likely corresponding with increasing skill and confidence. These challenges allowed me to hone my clinical decision-making abilities while under duress. My struggles and frustrations were not unique but perhaps lessons themselves.
Residency is not meant to be easy. The crucible of residency taught me that I had resilience to draw upon during challenging times. “Iron sharpens iron,” as the adage goes, and I believe adversity ultimately helped me become a better psychiatrist.
Self-reflection is part of completing training
Reminders that my journey is at an end are everywhere. Seeing notes written by past residents or fellows reminds me that soon I too will merely be a name in the chart to future trainees. Perhaps this line of thought is unfair, reducing my training experience to notes I signed—whereas my training experience was defined by connections made with colleagues and mentors, opportunities to teach junior learners, and confidence gained by overcoming adversity.
While becoming an attending psychiatrist fills me with trepidation, fear need not be an inherent aspect of new beginnings. Reflection has been a powerful practice, allowing me to realize what made my experience so meaningful, and that training is meant to be process-oriented rather than outcome-oriented. My reflection has underscored the realization that challenges are inherent in training, although not without purpose. I believe these struggles were meant to allow me to build meaningful relationships with colleagues, discover joy in teaching, and build resiliency.
The purpose of residencies and fellowships should be to produce clinically excellent psychiatrists, but I feel the journey was as important as the destination. Psychiatrists likely understand this better than most, as we were trained to thoughtfully approach the process of termination with patients.5 While the conclusion of our training journeys may seem unceremonious or anticlimactic, the termination process should include self-reflection on meaningful facets of training. For me, this reflection has itself been invaluable, while also making me hopeful to contribute value to the training journeys of future psychiatrists.
1. Gorrindo T, Beresin EV. Is “See one, do one, teach one” dead? Implications for the professionalization of medical educators in the twenty-first century. Acad Psychiatry. 2015;39(6):613-614. doi:10.1007/s40596-015-0424-8
2. Wright Jr. JR, Schachar NS. Necessity is the mother of invention: William Stewart Halsted’s addiction and its influence on the development of residency training in North America. Can J Surg. 2020;63(1):E13-E19. doi:10.1503/cjs.003319
3. Dandavino M, Snell L, Wiseman J. Why medical students should learn how to teach. Med Teach. 2007;29(6):558-565. doi:10.1080/01421590701477449
4. Liu AC, Liu M, Dannaway J, et al. Are Australian medical students being taught to teach? Clin Teach. 2017;14(5):330-335. doi:10.1111/tct.12591
5. Vasquez MJ, Bingham RP, Barnett JE. Psychotherapy termination: clinical and ethical responsibilities. J Clin Psychol. 2008;64(5):653-665. doi:10.1002/jclp.20478
Throughout my training, a common refrain from more senior colleagues was that training “goes by quickly.” At the risk of sounding cliché, and even after a 7-year journey spanning psychiatry and preventive medicine residencies as well as a consultation-liaison psychiatry fellowship, I agree without reservations that it does indeed go quickly. In the waning days of my training, reflection and nostalgia have become commonplace, as one might expect after such a meaningful pursuit. In sharing my reflections, I hope others progressing through training will also reflect on elements that added meaning to their experience and how they might improve the journey for future trainees.
Residency is a team sport
One realization that quickly struck me was that residency is a team sport, and finding supportive communities is essential to survival. Other residents, colleagues, and mentors played integral roles in making my experience rewarding. Training might be considered a shared traumatic experience, but having peers to commiserate with at each step has been among its greatest rewards. Residency automatically provided a cohort of colleagues who shared and validated my experiences. Additionally, having mentors who have been through it themselves and find ways to improve the training experience made mine superlative. Mentors assisted me in tailoring my training and developing interests that I could integrate into my future practice. The interpersonal connections I made were critical in helping me survive and thrive during training.
See one, do one, teach one
Residency and fellowship programs might be considered “see one, do one, teach one”1 at large scale. Since their inception, these programs—designed to develop junior physicians—have been inherently educational in nature. The structure is elegant, allowing trainees to continue learning while incrementally gaining more autonomy and teaching responsibility.2 Naively, I did not understand that implicit within my education was an expectation to become an educator and hone my teaching skills. Initially, being a newly minted resident receiving brand-new 3rd-year medical students charged me with apprehension. Thoughts I internalized, such as “these students probably know more than me” or “how can I be responsible for patients and students simultaneously,” may have resulted from a paucity of instruction about teaching available during medical school.3,4 I quickly found, though, that teaching was among the most rewarding facets of training. Helping other learners grow became one of my passions and added to my experience.
Iron sharpens iron
Although my experience was enjoyable, I would be remiss without also considering accompanying trials and tribulations. Seemingly interminable night shifts, sleep deprivation, lack of autonomy, and system inefficiencies frustrated me. Eventually, these frustrations seemed less bothersome. These challenges likely had not vanished with time, but perhaps my capacity to tolerate distress improved—likely corresponding with increasing skill and confidence. These challenges allowed me to hone my clinical decision-making abilities while under duress. My struggles and frustrations were not unique but perhaps lessons themselves.
Residency is not meant to be easy. The crucible of residency taught me that I had resilience to draw upon during challenging times. “Iron sharpens iron,” as the adage goes, and I believe adversity ultimately helped me become a better psychiatrist.
Self-reflection is part of completing training
Reminders that my journey is at an end are everywhere. Seeing notes written by past residents or fellows reminds me that soon I too will merely be a name in the chart to future trainees. Perhaps this line of thought is unfair, reducing my training experience to notes I signed—whereas my training experience was defined by connections made with colleagues and mentors, opportunities to teach junior learners, and confidence gained by overcoming adversity.
While becoming an attending psychiatrist fills me with trepidation, fear need not be an inherent aspect of new beginnings. Reflection has been a powerful practice, allowing me to realize what made my experience so meaningful, and that training is meant to be process-oriented rather than outcome-oriented. My reflection has underscored the realization that challenges are inherent in training, although not without purpose. I believe these struggles were meant to allow me to build meaningful relationships with colleagues, discover joy in teaching, and build resiliency.
The purpose of residencies and fellowships should be to produce clinically excellent psychiatrists, but I feel the journey was as important as the destination. Psychiatrists likely understand this better than most, as we were trained to thoughtfully approach the process of termination with patients.5 While the conclusion of our training journeys may seem unceremonious or anticlimactic, the termination process should include self-reflection on meaningful facets of training. For me, this reflection has itself been invaluable, while also making me hopeful to contribute value to the training journeys of future psychiatrists.
Throughout my training, a common refrain from more senior colleagues was that training “goes by quickly.” At the risk of sounding cliché, and even after a 7-year journey spanning psychiatry and preventive medicine residencies as well as a consultation-liaison psychiatry fellowship, I agree without reservations that it does indeed go quickly. In the waning days of my training, reflection and nostalgia have become commonplace, as one might expect after such a meaningful pursuit. In sharing my reflections, I hope others progressing through training will also reflect on elements that added meaning to their experience and how they might improve the journey for future trainees.
Residency is a team sport
One realization that quickly struck me was that residency is a team sport, and finding supportive communities is essential to survival. Other residents, colleagues, and mentors played integral roles in making my experience rewarding. Training might be considered a shared traumatic experience, but having peers to commiserate with at each step has been among its greatest rewards. Residency automatically provided a cohort of colleagues who shared and validated my experiences. Additionally, having mentors who have been through it themselves and find ways to improve the training experience made mine superlative. Mentors assisted me in tailoring my training and developing interests that I could integrate into my future practice. The interpersonal connections I made were critical in helping me survive and thrive during training.
See one, do one, teach one
Residency and fellowship programs might be considered “see one, do one, teach one”1 at large scale. Since their inception, these programs—designed to develop junior physicians—have been inherently educational in nature. The structure is elegant, allowing trainees to continue learning while incrementally gaining more autonomy and teaching responsibility.2 Naively, I did not understand that implicit within my education was an expectation to become an educator and hone my teaching skills. Initially, being a newly minted resident receiving brand-new 3rd-year medical students charged me with apprehension. Thoughts I internalized, such as “these students probably know more than me” or “how can I be responsible for patients and students simultaneously,” may have resulted from a paucity of instruction about teaching available during medical school.3,4 I quickly found, though, that teaching was among the most rewarding facets of training. Helping other learners grow became one of my passions and added to my experience.
Iron sharpens iron
Although my experience was enjoyable, I would be remiss without also considering accompanying trials and tribulations. Seemingly interminable night shifts, sleep deprivation, lack of autonomy, and system inefficiencies frustrated me. Eventually, these frustrations seemed less bothersome. These challenges likely had not vanished with time, but perhaps my capacity to tolerate distress improved—likely corresponding with increasing skill and confidence. These challenges allowed me to hone my clinical decision-making abilities while under duress. My struggles and frustrations were not unique but perhaps lessons themselves.
Residency is not meant to be easy. The crucible of residency taught me that I had resilience to draw upon during challenging times. “Iron sharpens iron,” as the adage goes, and I believe adversity ultimately helped me become a better psychiatrist.
Self-reflection is part of completing training
Reminders that my journey is at an end are everywhere. Seeing notes written by past residents or fellows reminds me that soon I too will merely be a name in the chart to future trainees. Perhaps this line of thought is unfair, reducing my training experience to notes I signed—whereas my training experience was defined by connections made with colleagues and mentors, opportunities to teach junior learners, and confidence gained by overcoming adversity.
While becoming an attending psychiatrist fills me with trepidation, fear need not be an inherent aspect of new beginnings. Reflection has been a powerful practice, allowing me to realize what made my experience so meaningful, and that training is meant to be process-oriented rather than outcome-oriented. My reflection has underscored the realization that challenges are inherent in training, although not without purpose. I believe these struggles were meant to allow me to build meaningful relationships with colleagues, discover joy in teaching, and build resiliency.
The purpose of residencies and fellowships should be to produce clinically excellent psychiatrists, but I feel the journey was as important as the destination. Psychiatrists likely understand this better than most, as we were trained to thoughtfully approach the process of termination with patients.5 While the conclusion of our training journeys may seem unceremonious or anticlimactic, the termination process should include self-reflection on meaningful facets of training. For me, this reflection has itself been invaluable, while also making me hopeful to contribute value to the training journeys of future psychiatrists.
1. Gorrindo T, Beresin EV. Is “See one, do one, teach one” dead? Implications for the professionalization of medical educators in the twenty-first century. Acad Psychiatry. 2015;39(6):613-614. doi:10.1007/s40596-015-0424-8
2. Wright Jr. JR, Schachar NS. Necessity is the mother of invention: William Stewart Halsted’s addiction and its influence on the development of residency training in North America. Can J Surg. 2020;63(1):E13-E19. doi:10.1503/cjs.003319
3. Dandavino M, Snell L, Wiseman J. Why medical students should learn how to teach. Med Teach. 2007;29(6):558-565. doi:10.1080/01421590701477449
4. Liu AC, Liu M, Dannaway J, et al. Are Australian medical students being taught to teach? Clin Teach. 2017;14(5):330-335. doi:10.1111/tct.12591
5. Vasquez MJ, Bingham RP, Barnett JE. Psychotherapy termination: clinical and ethical responsibilities. J Clin Psychol. 2008;64(5):653-665. doi:10.1002/jclp.20478
1. Gorrindo T, Beresin EV. Is “See one, do one, teach one” dead? Implications for the professionalization of medical educators in the twenty-first century. Acad Psychiatry. 2015;39(6):613-614. doi:10.1007/s40596-015-0424-8
2. Wright Jr. JR, Schachar NS. Necessity is the mother of invention: William Stewart Halsted’s addiction and its influence on the development of residency training in North America. Can J Surg. 2020;63(1):E13-E19. doi:10.1503/cjs.003319
3. Dandavino M, Snell L, Wiseman J. Why medical students should learn how to teach. Med Teach. 2007;29(6):558-565. doi:10.1080/01421590701477449
4. Liu AC, Liu M, Dannaway J, et al. Are Australian medical students being taught to teach? Clin Teach. 2017;14(5):330-335. doi:10.1111/tct.12591
5. Vasquez MJ, Bingham RP, Barnett JE. Psychotherapy termination: clinical and ethical responsibilities. J Clin Psychol. 2008;64(5):653-665. doi:10.1002/jclp.20478
Lamotrigine for bipolar depression?
In reading Dr. Nasrallah's August 2022 editorial (“Reversing depression: A plethora of therapeutic strategies and mechanisms,”
Dr. Nasrallah responds
Thanks for your message. Lamotrigine is not FDA-approved for bipolar or unipolar depression, either as monotherapy or as an adjunctive therapy. It has never been approved for mania, either (no efficacy at all). Its only FDA-approved psychiatric indication is maintenance therapy after a patient with bipolar I disorder emerges from mania with the help of one of the antimanic drugs. Yet many clinicians may perceive lamotrigine as useful for bipolar depression because more than 20 years ago the manufacturer sponsored several small studies (not FDA trials). Two studies that showed efficacy were published, but 4 other studies that failed to show efficacy were not published. As a result, many clinicians got the false impression that lamotrigine is an effective antidepressant. I hope this explains why lamotrigine was not included in the list of antidepressants in my editorial.
In reading Dr. Nasrallah's August 2022 editorial (“Reversing depression: A plethora of therapeutic strategies and mechanisms,”
Dr. Nasrallah responds
Thanks for your message. Lamotrigine is not FDA-approved for bipolar or unipolar depression, either as monotherapy or as an adjunctive therapy. It has never been approved for mania, either (no efficacy at all). Its only FDA-approved psychiatric indication is maintenance therapy after a patient with bipolar I disorder emerges from mania with the help of one of the antimanic drugs. Yet many clinicians may perceive lamotrigine as useful for bipolar depression because more than 20 years ago the manufacturer sponsored several small studies (not FDA trials). Two studies that showed efficacy were published, but 4 other studies that failed to show efficacy were not published. As a result, many clinicians got the false impression that lamotrigine is an effective antidepressant. I hope this explains why lamotrigine was not included in the list of antidepressants in my editorial.
In reading Dr. Nasrallah's August 2022 editorial (“Reversing depression: A plethora of therapeutic strategies and mechanisms,”
Dr. Nasrallah responds
Thanks for your message. Lamotrigine is not FDA-approved for bipolar or unipolar depression, either as monotherapy or as an adjunctive therapy. It has never been approved for mania, either (no efficacy at all). Its only FDA-approved psychiatric indication is maintenance therapy after a patient with bipolar I disorder emerges from mania with the help of one of the antimanic drugs. Yet many clinicians may perceive lamotrigine as useful for bipolar depression because more than 20 years ago the manufacturer sponsored several small studies (not FDA trials). Two studies that showed efficacy were published, but 4 other studies that failed to show efficacy were not published. As a result, many clinicians got the false impression that lamotrigine is an effective antidepressant. I hope this explains why lamotrigine was not included in the list of antidepressants in my editorial.
A gender primer for psychiatrists
Psychiatrists have a long tradition of supporting LGBTQAI+ (lesbian, gay, bisexual, transgender, queer/questioning, asexual, intersex, and others) persons. In professional and public settings, we are educators, role models, and advocates for self-expression and personal empowerment. By better educating ourselves on the topic of gender and its variations, we can become champions of gender-affirming care.
Sex vs gender
A person’s sex is assigned at birth based on their physiological characteristics, including their genitalia and chromosome composition. Male, female, and intersex are a few recognized sexes. Gender or gender identity describe one’s innermost perception of self as a man, a woman, a variation of both, or neither, that may not always be visible to others. When sex and gender identity align, this is known as cisgender.1
Gender identity
Gender identity is best described as a spectrum rather than a binary. Terms that fall under a gender binary include man, woman, trans man, and trans woman. A nonbinary gender identity is one outside the traditional binary of men or women. Being transgender simply means having a gender identity different than the sex assigned at birth. This includes persons whose gender identities cross the gender spectrum, such as trans men or trans women, and those who fall anywhere outside or in between genders. In this way, nonbinary persons are transgender.1
The nonbinary spectrum
The term nonbinary encompasses many gender-nonconforming identities, such as agender, bigender, demigender, genderfluid, genderqueer, intergender, or pangender. Agender people have little connection to gender. Bigender individuals identify as 2 separate genders. Demigender persons feel a partial connection to a gender. Genderfluid individuals have a gender experience that is fluid and can change over time. Genderqueer people have a gender identity that falls in between or outside the binary. Intergender people have a gender identity between genders or identify as a combination of genders. Pangender people identify with a combination of genders. Note that patients may use some of these terms interchangeably or ascribe to them different meanings.2 As the language around gender continues to evolve, psychiatrists should ask patients from a place of nonjudgmental curiosity what gender terms they use, how they define them, and what their gender means to them.
Gender expression and transitioning
Transitioning is what a transgender person does to align their gender identity and expression.3 Gender expression is the external manifestation of gender, including names, pronouns, clothing, haircuts, behaviors, voice, body characteristics, and more.1 Transgender individuals can transition using a combination of social (name, pronouns, dress), legal (changing sex on legal documents, name change), or medical (surgeries, hormone therapies, puberty blockade) means. Transitions often help ease gender dysphoria, which is the clinically significant distress a person experiences when their sex assigned at birth does not align with their gender identity.3 Note that not all transgender persons choose to change their gender expression, and not all transgender individuals experience gender dysphoria. In this case, the proper medical term is gender incongruence, which is simply when someone’s gender identity does not align with their sex assigned at birth.4
Names and pronouns
For many transgender persons, names and pronouns are an important part of their gender transition and expression.2 Most of us have gotten into the habit of assuming pronouns because of socially established gender roles. This assumes that a person’s physical appearance matches their gender identity, which is not always the case.1 To be more affirming, psychiatrists and other health care professionals should try to break the habit of assuming pronouns. Often, an easy way to learn someone’s pronouns is to introduce yourself with yours. For example, “I am Dr. Agapoff. I use they/them/theirs pronouns. It is nice to meet you.” This creates a safe and open space for the other person to share their gender identity if they choose.
Why it’s important
One does not have to be a gender specialist to deliver gender-affirming care. As psychiatrists, having a basic understanding of the differences between sex, gender identity, and gender expression can help us build rapport and support our patients who are transgender. Based on the many kinds of gender identity and expression, judging someone’s gender based solely upon physical appearance is misguided at best and harmful at worst. Even people who are cisgender have many kinds of gender expression. For this reason, psychiatrists should approach gender with the same openness and curiosity as sexual orientation or other important considerations of emotional and physical health. Gender-informed care starts with us.
1. LGBTQIA Resource Center Glossary. UC Davis LGBTQIA Resource Center. Accessed July 19, 2022. https://lgbtqia.ucdavis.edu/educated/glossary
2. Richards C, Bouman WP, Seal L, et al. Non-binary or genderqueer genders. Int Rev Psychiatry. 2016;28(1):95-102. doi:10.3109/09540261.2015.1106446
3. Understanding transitions. TransFamilies.Org. Accessed June 1, 2022. https://transfamilies.org/understanding-transitions/
4. Claahsen-van der Grinten H, Verhaak C, Steensma T, et al. Gender incongruence and gender dysphoria in childhood and adolescence—current insights in diagnostics, management, and follow-up. Eur J Pediatr. 2021;180(5):1349-1357.
Psychiatrists have a long tradition of supporting LGBTQAI+ (lesbian, gay, bisexual, transgender, queer/questioning, asexual, intersex, and others) persons. In professional and public settings, we are educators, role models, and advocates for self-expression and personal empowerment. By better educating ourselves on the topic of gender and its variations, we can become champions of gender-affirming care.
Sex vs gender
A person’s sex is assigned at birth based on their physiological characteristics, including their genitalia and chromosome composition. Male, female, and intersex are a few recognized sexes. Gender or gender identity describe one’s innermost perception of self as a man, a woman, a variation of both, or neither, that may not always be visible to others. When sex and gender identity align, this is known as cisgender.1
Gender identity
Gender identity is best described as a spectrum rather than a binary. Terms that fall under a gender binary include man, woman, trans man, and trans woman. A nonbinary gender identity is one outside the traditional binary of men or women. Being transgender simply means having a gender identity different than the sex assigned at birth. This includes persons whose gender identities cross the gender spectrum, such as trans men or trans women, and those who fall anywhere outside or in between genders. In this way, nonbinary persons are transgender.1
The nonbinary spectrum
The term nonbinary encompasses many gender-nonconforming identities, such as agender, bigender, demigender, genderfluid, genderqueer, intergender, or pangender. Agender people have little connection to gender. Bigender individuals identify as 2 separate genders. Demigender persons feel a partial connection to a gender. Genderfluid individuals have a gender experience that is fluid and can change over time. Genderqueer people have a gender identity that falls in between or outside the binary. Intergender people have a gender identity between genders or identify as a combination of genders. Pangender people identify with a combination of genders. Note that patients may use some of these terms interchangeably or ascribe to them different meanings.2 As the language around gender continues to evolve, psychiatrists should ask patients from a place of nonjudgmental curiosity what gender terms they use, how they define them, and what their gender means to them.
Gender expression and transitioning
Transitioning is what a transgender person does to align their gender identity and expression.3 Gender expression is the external manifestation of gender, including names, pronouns, clothing, haircuts, behaviors, voice, body characteristics, and more.1 Transgender individuals can transition using a combination of social (name, pronouns, dress), legal (changing sex on legal documents, name change), or medical (surgeries, hormone therapies, puberty blockade) means. Transitions often help ease gender dysphoria, which is the clinically significant distress a person experiences when their sex assigned at birth does not align with their gender identity.3 Note that not all transgender persons choose to change their gender expression, and not all transgender individuals experience gender dysphoria. In this case, the proper medical term is gender incongruence, which is simply when someone’s gender identity does not align with their sex assigned at birth.4
Names and pronouns
For many transgender persons, names and pronouns are an important part of their gender transition and expression.2 Most of us have gotten into the habit of assuming pronouns because of socially established gender roles. This assumes that a person’s physical appearance matches their gender identity, which is not always the case.1 To be more affirming, psychiatrists and other health care professionals should try to break the habit of assuming pronouns. Often, an easy way to learn someone’s pronouns is to introduce yourself with yours. For example, “I am Dr. Agapoff. I use they/them/theirs pronouns. It is nice to meet you.” This creates a safe and open space for the other person to share their gender identity if they choose.
Why it’s important
One does not have to be a gender specialist to deliver gender-affirming care. As psychiatrists, having a basic understanding of the differences between sex, gender identity, and gender expression can help us build rapport and support our patients who are transgender. Based on the many kinds of gender identity and expression, judging someone’s gender based solely upon physical appearance is misguided at best and harmful at worst. Even people who are cisgender have many kinds of gender expression. For this reason, psychiatrists should approach gender with the same openness and curiosity as sexual orientation or other important considerations of emotional and physical health. Gender-informed care starts with us.
Psychiatrists have a long tradition of supporting LGBTQAI+ (lesbian, gay, bisexual, transgender, queer/questioning, asexual, intersex, and others) persons. In professional and public settings, we are educators, role models, and advocates for self-expression and personal empowerment. By better educating ourselves on the topic of gender and its variations, we can become champions of gender-affirming care.
Sex vs gender
A person’s sex is assigned at birth based on their physiological characteristics, including their genitalia and chromosome composition. Male, female, and intersex are a few recognized sexes. Gender or gender identity describe one’s innermost perception of self as a man, a woman, a variation of both, or neither, that may not always be visible to others. When sex and gender identity align, this is known as cisgender.1
Gender identity
Gender identity is best described as a spectrum rather than a binary. Terms that fall under a gender binary include man, woman, trans man, and trans woman. A nonbinary gender identity is one outside the traditional binary of men or women. Being transgender simply means having a gender identity different than the sex assigned at birth. This includes persons whose gender identities cross the gender spectrum, such as trans men or trans women, and those who fall anywhere outside or in between genders. In this way, nonbinary persons are transgender.1
The nonbinary spectrum
The term nonbinary encompasses many gender-nonconforming identities, such as agender, bigender, demigender, genderfluid, genderqueer, intergender, or pangender. Agender people have little connection to gender. Bigender individuals identify as 2 separate genders. Demigender persons feel a partial connection to a gender. Genderfluid individuals have a gender experience that is fluid and can change over time. Genderqueer people have a gender identity that falls in between or outside the binary. Intergender people have a gender identity between genders or identify as a combination of genders. Pangender people identify with a combination of genders. Note that patients may use some of these terms interchangeably or ascribe to them different meanings.2 As the language around gender continues to evolve, psychiatrists should ask patients from a place of nonjudgmental curiosity what gender terms they use, how they define them, and what their gender means to them.
Gender expression and transitioning
Transitioning is what a transgender person does to align their gender identity and expression.3 Gender expression is the external manifestation of gender, including names, pronouns, clothing, haircuts, behaviors, voice, body characteristics, and more.1 Transgender individuals can transition using a combination of social (name, pronouns, dress), legal (changing sex on legal documents, name change), or medical (surgeries, hormone therapies, puberty blockade) means. Transitions often help ease gender dysphoria, which is the clinically significant distress a person experiences when their sex assigned at birth does not align with their gender identity.3 Note that not all transgender persons choose to change their gender expression, and not all transgender individuals experience gender dysphoria. In this case, the proper medical term is gender incongruence, which is simply when someone’s gender identity does not align with their sex assigned at birth.4
Names and pronouns
For many transgender persons, names and pronouns are an important part of their gender transition and expression.2 Most of us have gotten into the habit of assuming pronouns because of socially established gender roles. This assumes that a person’s physical appearance matches their gender identity, which is not always the case.1 To be more affirming, psychiatrists and other health care professionals should try to break the habit of assuming pronouns. Often, an easy way to learn someone’s pronouns is to introduce yourself with yours. For example, “I am Dr. Agapoff. I use they/them/theirs pronouns. It is nice to meet you.” This creates a safe and open space for the other person to share their gender identity if they choose.
Why it’s important
One does not have to be a gender specialist to deliver gender-affirming care. As psychiatrists, having a basic understanding of the differences between sex, gender identity, and gender expression can help us build rapport and support our patients who are transgender. Based on the many kinds of gender identity and expression, judging someone’s gender based solely upon physical appearance is misguided at best and harmful at worst. Even people who are cisgender have many kinds of gender expression. For this reason, psychiatrists should approach gender with the same openness and curiosity as sexual orientation or other important considerations of emotional and physical health. Gender-informed care starts with us.
1. LGBTQIA Resource Center Glossary. UC Davis LGBTQIA Resource Center. Accessed July 19, 2022. https://lgbtqia.ucdavis.edu/educated/glossary
2. Richards C, Bouman WP, Seal L, et al. Non-binary or genderqueer genders. Int Rev Psychiatry. 2016;28(1):95-102. doi:10.3109/09540261.2015.1106446
3. Understanding transitions. TransFamilies.Org. Accessed June 1, 2022. https://transfamilies.org/understanding-transitions/
4. Claahsen-van der Grinten H, Verhaak C, Steensma T, et al. Gender incongruence and gender dysphoria in childhood and adolescence—current insights in diagnostics, management, and follow-up. Eur J Pediatr. 2021;180(5):1349-1357.
1. LGBTQIA Resource Center Glossary. UC Davis LGBTQIA Resource Center. Accessed July 19, 2022. https://lgbtqia.ucdavis.edu/educated/glossary
2. Richards C, Bouman WP, Seal L, et al. Non-binary or genderqueer genders. Int Rev Psychiatry. 2016;28(1):95-102. doi:10.3109/09540261.2015.1106446
3. Understanding transitions. TransFamilies.Org. Accessed June 1, 2022. https://transfamilies.org/understanding-transitions/
4. Claahsen-van der Grinten H, Verhaak C, Steensma T, et al. Gender incongruence and gender dysphoria in childhood and adolescence—current insights in diagnostics, management, and follow-up. Eur J Pediatr. 2021;180(5):1349-1357.
Positive psychiatry: An introduction
Historically, psychology and psychiatry have mostly focused on negative emotions and pathological states. However, during the last few decades, new developments in both disciplines have created novel vistas for a more comprehensive understanding of human behavior.1,2 These developments have taken on the names of positive psychology and positive psychiatry, respectively. Positive psychiatry is the science and practice of psychiatry that focuses on psycho-bio-social study and promotion of well-being and health through enhancement of positive psychosocial factors (eg, resilience, optimism, wisdom, social support) in people with illnesses or disabilities as well as in the community at large.3 This new perspective is aimed at enhancing and enriching psychiatric practice and research rather than replacing our stated aim of providing reliable and valid diagnostic categories along with effective therapeutic interventions.
In this issue of
In Part 1, Boardman et al describe positive psychiatry tools to enhance clinical practice through positive interventions in several categories: adopting a positive orientation, harnessing strengths, mobilizing values, cultivating social connections, and optimizing health habits. The authors show how positive psychiatry aims to create a balance between pathogenesis (the study and understanding of diseases) and salutogenesis (the study and creation of health).4
In Part 2, Rettew discusses applying positive psychiatry principles and practices when working with children, adolescents, and their families. The author demonstrates how the principles and practices associated with positive psychiatry represent a natural and highly needed extension of the traditional work within child and adolescent psychiatry, and not a radical transformation of thought or effort. Rettew provides a case example in which he compares traditional and positive psychiatry approaches.
In Part 3, Oughli et al describe resilience in older adults with late-life depression, its clinical and neurocognitive correlates, and associated neurobiological and immunological biomarkers. The authors also narrate resilience-building interventions such as mind-body therapies, which have been reported to enhance resilience through promoting positive perceptions of various experiences and challenges. Evidence suggests that stress reduction, decreased inflammation, and improved emotional regulation may have direct neuroplastic effects on the brain, resulting in greater resilience.
Finally, in Part 4, Hamid Peseschkian summarizes the ideas and practices of positive psychotherapy (PPT) as practiced in Germany since its introduction by Nossrat Peseschkian in 1977. Based on a resource-oriented conception of human beings, PPT combines humanistic, systemic, psychodynamic, and cognitive-behavioral aspects. This short-term method can be readily understood by patients from diverse cultures and social backgrounds.
Taken together, these articles present recent advances in positive psychiatry, especially from an intervention perspective. This is a timely development in view of the evidence of rising global rates of suicide, substance use, anxiety, depression, and perceived stress. By uniting a positive perspective, along with studying its neurobiological underpinnings, and taking a life-long approach, we can now apply these innovations to children, young adults, and older adults, thus providing clinicians with tools to enhance well-being and promote mental health in people with and without mental or physical illnesses.
1. Jeste DV, Palmer BW, Rettew DC, et al. Positive psychiatry: its time has come. J Clin Psychiatry. 2015;76(6):675-683.
2. Jeste DV. A fulfilling year of APA presidency: from DSM-5 to positive psychiatry. Am J Psychiatry. 2013;170(10):1102-1105.
3. Jeste DV. Positive psychiatry comes of age. Int Psychogeriatr. 2018;30(12):1735-1738.
4. Mittelmark MB, Sagy S, Eriksson M, et al (eds). The Handbook of Salutogenesis [Internet]. Springer; 2017.
Historically, psychology and psychiatry have mostly focused on negative emotions and pathological states. However, during the last few decades, new developments in both disciplines have created novel vistas for a more comprehensive understanding of human behavior.1,2 These developments have taken on the names of positive psychology and positive psychiatry, respectively. Positive psychiatry is the science and practice of psychiatry that focuses on psycho-bio-social study and promotion of well-being and health through enhancement of positive psychosocial factors (eg, resilience, optimism, wisdom, social support) in people with illnesses or disabilities as well as in the community at large.3 This new perspective is aimed at enhancing and enriching psychiatric practice and research rather than replacing our stated aim of providing reliable and valid diagnostic categories along with effective therapeutic interventions.
In this issue of
In Part 1, Boardman et al describe positive psychiatry tools to enhance clinical practice through positive interventions in several categories: adopting a positive orientation, harnessing strengths, mobilizing values, cultivating social connections, and optimizing health habits. The authors show how positive psychiatry aims to create a balance between pathogenesis (the study and understanding of diseases) and salutogenesis (the study and creation of health).4
In Part 2, Rettew discusses applying positive psychiatry principles and practices when working with children, adolescents, and their families. The author demonstrates how the principles and practices associated with positive psychiatry represent a natural and highly needed extension of the traditional work within child and adolescent psychiatry, and not a radical transformation of thought or effort. Rettew provides a case example in which he compares traditional and positive psychiatry approaches.
In Part 3, Oughli et al describe resilience in older adults with late-life depression, its clinical and neurocognitive correlates, and associated neurobiological and immunological biomarkers. The authors also narrate resilience-building interventions such as mind-body therapies, which have been reported to enhance resilience through promoting positive perceptions of various experiences and challenges. Evidence suggests that stress reduction, decreased inflammation, and improved emotional regulation may have direct neuroplastic effects on the brain, resulting in greater resilience.
Finally, in Part 4, Hamid Peseschkian summarizes the ideas and practices of positive psychotherapy (PPT) as practiced in Germany since its introduction by Nossrat Peseschkian in 1977. Based on a resource-oriented conception of human beings, PPT combines humanistic, systemic, psychodynamic, and cognitive-behavioral aspects. This short-term method can be readily understood by patients from diverse cultures and social backgrounds.
Taken together, these articles present recent advances in positive psychiatry, especially from an intervention perspective. This is a timely development in view of the evidence of rising global rates of suicide, substance use, anxiety, depression, and perceived stress. By uniting a positive perspective, along with studying its neurobiological underpinnings, and taking a life-long approach, we can now apply these innovations to children, young adults, and older adults, thus providing clinicians with tools to enhance well-being and promote mental health in people with and without mental or physical illnesses.
Historically, psychology and psychiatry have mostly focused on negative emotions and pathological states. However, during the last few decades, new developments in both disciplines have created novel vistas for a more comprehensive understanding of human behavior.1,2 These developments have taken on the names of positive psychology and positive psychiatry, respectively. Positive psychiatry is the science and practice of psychiatry that focuses on psycho-bio-social study and promotion of well-being and health through enhancement of positive psychosocial factors (eg, resilience, optimism, wisdom, social support) in people with illnesses or disabilities as well as in the community at large.3 This new perspective is aimed at enhancing and enriching psychiatric practice and research rather than replacing our stated aim of providing reliable and valid diagnostic categories along with effective therapeutic interventions.
In this issue of
In Part 1, Boardman et al describe positive psychiatry tools to enhance clinical practice through positive interventions in several categories: adopting a positive orientation, harnessing strengths, mobilizing values, cultivating social connections, and optimizing health habits. The authors show how positive psychiatry aims to create a balance between pathogenesis (the study and understanding of diseases) and salutogenesis (the study and creation of health).4
In Part 2, Rettew discusses applying positive psychiatry principles and practices when working with children, adolescents, and their families. The author demonstrates how the principles and practices associated with positive psychiatry represent a natural and highly needed extension of the traditional work within child and adolescent psychiatry, and not a radical transformation of thought or effort. Rettew provides a case example in which he compares traditional and positive psychiatry approaches.
In Part 3, Oughli et al describe resilience in older adults with late-life depression, its clinical and neurocognitive correlates, and associated neurobiological and immunological biomarkers. The authors also narrate resilience-building interventions such as mind-body therapies, which have been reported to enhance resilience through promoting positive perceptions of various experiences and challenges. Evidence suggests that stress reduction, decreased inflammation, and improved emotional regulation may have direct neuroplastic effects on the brain, resulting in greater resilience.
Finally, in Part 4, Hamid Peseschkian summarizes the ideas and practices of positive psychotherapy (PPT) as practiced in Germany since its introduction by Nossrat Peseschkian in 1977. Based on a resource-oriented conception of human beings, PPT combines humanistic, systemic, psychodynamic, and cognitive-behavioral aspects. This short-term method can be readily understood by patients from diverse cultures and social backgrounds.
Taken together, these articles present recent advances in positive psychiatry, especially from an intervention perspective. This is a timely development in view of the evidence of rising global rates of suicide, substance use, anxiety, depression, and perceived stress. By uniting a positive perspective, along with studying its neurobiological underpinnings, and taking a life-long approach, we can now apply these innovations to children, young adults, and older adults, thus providing clinicians with tools to enhance well-being and promote mental health in people with and without mental or physical illnesses.
1. Jeste DV, Palmer BW, Rettew DC, et al. Positive psychiatry: its time has come. J Clin Psychiatry. 2015;76(6):675-683.
2. Jeste DV. A fulfilling year of APA presidency: from DSM-5 to positive psychiatry. Am J Psychiatry. 2013;170(10):1102-1105.
3. Jeste DV. Positive psychiatry comes of age. Int Psychogeriatr. 2018;30(12):1735-1738.
4. Mittelmark MB, Sagy S, Eriksson M, et al (eds). The Handbook of Salutogenesis [Internet]. Springer; 2017.
1. Jeste DV, Palmer BW, Rettew DC, et al. Positive psychiatry: its time has come. J Clin Psychiatry. 2015;76(6):675-683.
2. Jeste DV. A fulfilling year of APA presidency: from DSM-5 to positive psychiatry. Am J Psychiatry. 2013;170(10):1102-1105.
3. Jeste DV. Positive psychiatry comes of age. Int Psychogeriatr. 2018;30(12):1735-1738.
4. Mittelmark MB, Sagy S, Eriksson M, et al (eds). The Handbook of Salutogenesis [Internet]. Springer; 2017.
Using the tools of positive psychiatry to improve clinical practice
FIRST OF 4 PARTS
What does wellness mean to you? A 2018 survey posed this question to more than 6,000 people living with depression and bipolar disorder. In addition to better treatment and greater understanding of their illnesses, other priorities emerged: a longing for better days, a sense of purpose, and a longing to function well and be happy.1 As one respondent explained, “Wellness means stability; well enough to hold a job, well enough to enjoy activities, well enough to feel joy and hope.” Traditional treatment that focuses on alleviating symptoms may not sufficiently address outcomes patients value. When the focus is primarily deficit-based, clinicians and patients may miss opportunities for optimization and transformation.
Positive psychiatry is the science and practice of psychiatry that seeks to enhance and promote well-being and health through the enhancement of positive psychosocial factors such as resilience, optimism, wisdom, and social support in people with illnesses or disabilities as well as those in the community at large.2 It is based on the principles that there is no health without mental health, and that mental health can improve through preventive, therapeutic, and rehabilitative interventions.3
Positive interventions are defined as “treatment methods or intentional activities that aim to cultivate positive feelings, behaviors, or cognitions.”4 They are evidence-based intentional exercises designed to increase well-being and enhance flourishing. Although positive interventions were originally studied as activities for nonclinical populations and for helping healthy people thrive, they are increasingly being valued for their therapeutic role in treating psychopathology.5 By adding positive interventions to their toolbox, psychiatrists can expand the range of treatment options, better engage patients during the treatment process, and bolster positive mental health.
In this article, we provide practical ways to integrate the tools and principles of positive psychiatry into everyday clinical practice. The goal is to broaden how clinicians think about mental health and therapeutic options and, above all, enhance our patients’ everyday well-being. Teaching patients to adopt a positive orientation, harness strengths, mobilize values, cultivate social connections, and optimize healthy habits are strategies clinicians can apply not only to provide a counterweight to the traditional emphasis on illness, but also to enhance the range and richness of their patients’ everyday experience.
Adopt a positive orientation
When a clinician first meets a patient, “What’s wrong?” is a typical conversation starter, and conversations tend to revolve around problems, failures, and negative experiences. Positive psychiatry posits that there is therapeutic benefit to emphasizing and exploring a patient’s positive emotions, experiences, and aspirations. Questions such as “What was your sense of well-being this week? What is your goal for today’s session? What is your goal for the coming week?” can reorient a session towards an individual’s potential and promote exploration of what’s possible.
To promote a positive orientation, clinicians may consider integrating the Savoring and Three Good Things exercises—2 well-studied interventions—into their repertoire to activate and enhance positive emotional states such as gratitude and joy.6 An example of a Savoring activity is taking a 20-minute daily walk while trying to notice as many positive elements as possible. Similarly, the Three Good Things exercise, in which patients are asked to notice and write down 3 positive events and reflect on why they happened, promotes positive reflection and gratitude. A 14-day daily diary study conducted during the COVID-19 pandemic found that higher levels of gratitude were associated with higher levels of positive affect, lower levels of perceived stress related to COVID-19, and better subjective health.7 In addition to coping with life’s negative events, deliberately enhancing the impact of good things is a positive emotion amplifier. As French writer François de La Rochefoucauld argued, “Happiness does not consist in things themselves but in the relish we have of them.”8
Continue to: Harness strengths
Harness strengths
A growing body of evidence suggests that in addition to focusing on a patient’s chief concern, identifying and cultivating an individual’s signature strengths can mitigate stress and enhance well-being. Signature strengths are positive personality qualities that reflect our core identity and are morally valued. The VIA Character Strengths Survey is the most used and validated psychometric instrument to measure and identify signature strengths such as curiosity, self-regulation, honesty, and teamwork.9
To incorporate this tool into clinical practice, ask patients to complete a strengths survey using a validated assessment tool such as the VIA survey (www.viacharacter.org). After a patient identifies their signature strengths, encourage them to explore and apply these strengths in everyday life and in new ways. In addition to becoming aware of and using their signature strengths, encourage patients to “strengths spot” in others. “What strengths did you notice your coworker, family, or friend using today?” is a potential question to explore with patients. A strengths-based approach may be particularly helpful in uncovering motivation and fully engaging patients in treatment. Moreover, integrating strengths into the typically negatively skewed narrative underscores to patients that therapy isn’t only about untwisting distorted thinking, but also about harnessing one’s strengths, talents, and abilities. Strengths expressed through pragmatic actions can boost coping skills as well as enhance well-being.
Mobilize values
Value affirmation exercises have been shown to generate lasting benefits in creating positive feelings and behaviors.10 Encouraging patients to think about what they genuinely value redirects their gaze towards possibility and diverts self-focus. For instance, ask a patient to identify 2 or 3 values and write about why they are important. By reflecting on their values in writing, they affirm their identity and self-worth, thus creating a virtuous cycle of confidence, effort, and achievement. People who put their values front and center are more attuned to the needs of others as well as their own needs, and they make better connections.11 Including a patient’s values in the treatment plan may increase problem-solving skills, boost motivation, and build better stress management skills.
The “life review” is another intervention that facilitates exploration of a patient’s values. This exercise involves asking patients to recount the story of their life and the experiences that were most meaningful to them. This process allows clinicians to gain a deeper understanding of the patient’s values, which can help guide treatment. Meta-analytic evidence has demonstrated these reminiscence-based interventions have significant effects on well-being.6 As Mahatma Gandhi famously said, “Happiness is when what you think, what you say, and what you do are in harmony.” Creating more overlap between a patient’s values and their everyday actions and behaviors bolsters resilience, buffers against stress, and can restore a healthier self-concept.
Cultivate social connections
Social connection is recognized as a core psychological need and essential for well-being. The opposite of connection—social isolation—has negative effects on overall health, including increases in inflammatory markers, depression rates, and even all-cause mortality.12 A 2015 meta-analytic review demonstrated that loneliness increased the likelihood of mortality by 26%—a similar increase as seen with smoking 15 cigarettes a day.13
Continue to: As with any vital sign...
As with any vital sign, exploring a patient’s number of social contacts, quantity of social visits per week, and quality of relationships is an important indicator of health. Giving patients tools to cultivate social connection and deepen their relationships can enhance therapeutic outcomes. Asking patients to perform acts of kindness is one example of a “social prescription.” Feeding a stranger’s parking meter, picking up litter, helping a friend with a chore, providing a meal to a person in need, and volunteering are potential ways for patients to engage in kind deeds. After each act, encourage the patient to write down what they did and how it made them feel.
“Prescribing” positive communication is another way to enhance a patient’s social connections. For instance, teaching them about active constructive responding (ACR)—responding with enthusiasm when another person shares information or good news—has been shown to strengthen bonds with friends and family.14 Making eye contact, giving the other person one’s full attention, inquiring about details, and responding with enthusiasm and interest are simple ways patients can apply ACR in their daily lives. Counseling a patient on increasing social connections, prescribing connections, and inquiring about quantity and quality of social interactions can help them not only add years to their life but also add health and well-being to those years.
Optimize healthy habits
Mounting research demonstrates that exercise, sleep, and nutrition are important for well-being. Evidence shows that therapeutic lifestyle changes can reduce depressive symptoms and boost positive feelings. Numerous meta-analyses have demonstrated the benefits of sleep and exercise interventions for reducing depressive symptoms in psychiatric patients.15,16 Longitudinal studies have provided evidence that healthy diets increase happiness, even after controlling for potential confounders such as socioeconomic factors.17 Other lifestyle factors—including financial stability, pet ownership, decreased social media use, and spending time in nature—have been shown to contribute to well-being.18
Despite the substantial evidence that lifestyle factors can improve health outcomes, few clinicians ask about, focus on, or promote positive habits.19 Positive psychiatry seeks to reorient clinicians towards lifestyle factors that enhance well-being. Clinicians can deploy a variety of strategies to support patients in making healthy and sustainable changes. Assessing readiness for change, motivational interviewing, setting SMART (specific, measurable, assignable, realistic, and time-related) goals, and referring patients to relevant community resources are ways to encourage and promote therapeutic lifestyle changes. Inquiring about a patient’s typical day—such as how they spend their free time, what they eat, when they go to bed, and how much time they spend outdoors—opens conversations about general well-being and shows the patient that therapy is about the whole person, and not only symptom management. Helping patients have better days can empower them to lead more satisfied lives.20
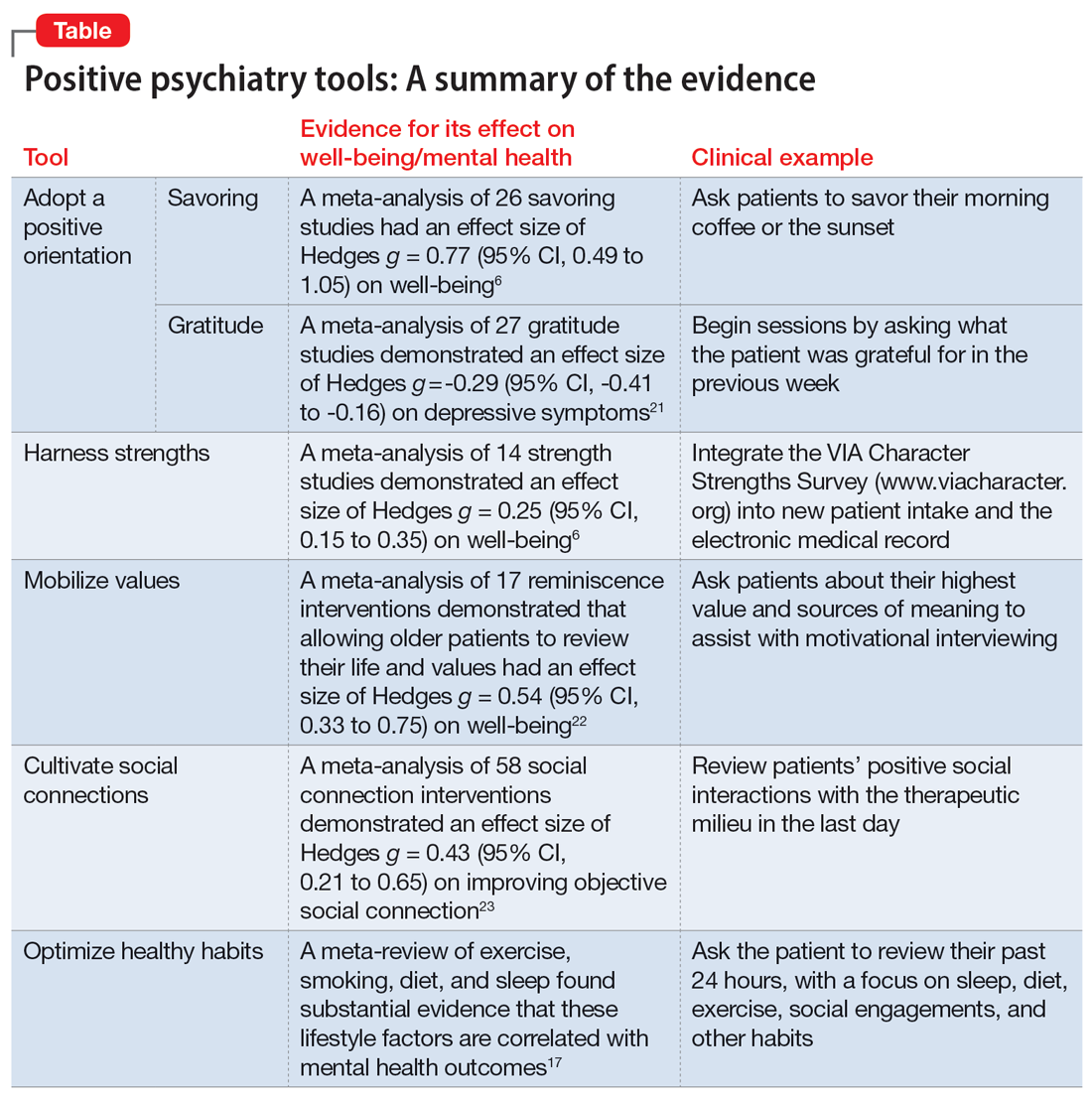
The Table6,17,21-23 summarizes the scientific evidence for the strategies described in this article. The Figure provides a flowchart for using these strategies in clinical practice.
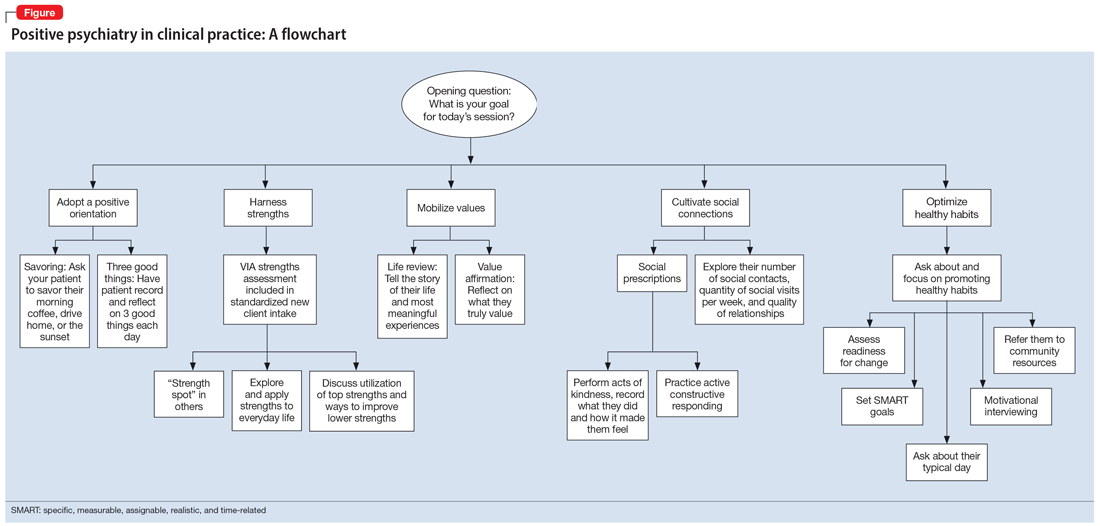
Continue to: Balancing pathogenesis with salutogenesis
Balancing pathogenesis with salutogenesis
By exploring and emphasizing potential and possibility, positive psychiatry aims to create a balance between pathogenesis (the study and understanding of disease) with salutogenesis (the study and creation of health24). Clinicians are well positioned to manage symptoms and bolster positive states. Rather than an either/or approach to well-being, positive psychiatry strives for a both/and approach to well-being. By adding positive interventions to their toolbox, clinicians can expand the range of treatment options, better engage patients in the treatment process, and bolster mental health.
Bottom Line
Clinicians can integrate the tools and principles of positive psychiatry into clinical practice. Teaching patients to adopt a positive orientation, harness strengths, mobilize values, cultivate social connections, and optimize healthy habits can not only provide a counterweight to the traditional emphasis on illness, but also can enhance the range and richness of patients’ everyday experience.
Related Resources
- University of Pennsylvania. Authentic happiness. https://www.authentichappiness.sas.upenn.edu
- Jeste DV, Palmer BW (eds). Positive Psychiatry: A Clinical Handbook. American Psychiatric Publishing; 2015.
- Jeste DV, Palmer BW, Rettew DC, et al. Positive psychiatry: its time has come. J Clin Psychiatry. 2015;76(6):675-683.
1. Morton E, Foxworth P, Dardess P, et al. “Supporting Wellness”: a depression and bipolar support alliance mixed-methods investigation of lived experience perspectives and priorities for mood disorder treatment. J Affect Disord. 2022;299:575-584.
2. Jeste DV, Palmer BW, Rettew DC, et al. Positive psychiatry: its time has come. J Clin Psychiatry. 2015;76(6):675-683.
3. Jeste DV. Positive psychiatry comes of age. Int Psychogeriatr. 2018;30(12):1735-1738.
4. Sin NL, Lyubomirsky S. Enhancing well-being and alleviating depressive symptoms with positive psychology interventions: a practice-friendly meta-analysis. J Clin Psychol. 2009;65(5):467-487.
5. Seligman MEP, Rashid T, Parks AC. Positive psychotherapy. Am Psychol. 2006;61(8):774-788.
6. Carr A, Cullen K, Keeney C, et al. Effectiveness of positive psychology interventions: a systematic review and meta-analysis. J Posit Psychol. 2021;16(6):749-769.
7. Jiang D. Feeling gratitude is associated with better well-being across the life span: a daily diary study during the COVID-19 outbreak. J Gerontol B Psychol Sci Soc Sci. 2022;77(4):e36-e45.
8. de La Rochefoucauld F. Maxims and moral reflections (1796). Gale ECCO: 2010.
9. Niemiec RM. VIA character strengths: Research and practice (The first 10 years). In: Knoop HH, Fave AD (eds). Well-being and Cultures. Springer;2013:11-29.
10. Cohen GL, Sherman DK. The psychology of change: self-affirmation and social psychological intervention. Annu Rev Psychol. 2014;65:333-371.
11. Thomaes S, Bushman BJ, de Castro BO, et al. Arousing “gentle passions” in young adolescents: sustained experimental effects of value affirmations on prosocial feelings and behaviors. Dev Psychol. 2012;48(1):103-110.
12. Cacioppo JT, Cacioppo S, Capitanio JP, et al. The neuroendocrinology of social isolation. Annu Rev Psychol. 2015;66:733-767.
13. Holt-Lunstad J, Smith TB, Baker M, et al. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci. 2015;10(2):227-237.
14. Gable SL, Reis HT, Impett EA, et al. What do you do when things go right? The intrapersonal and interpersonal benefits of sharing positive events. J Pers Soc Psychol. 2004;87(2):228-245.
15. Gee B, Orchard F, Clarke E, et al. The effect of non-pharmacological sleep interventions on depression symptoms: a meta-analysis of randomised controlled trials. Sleep Med Rev. 2019;43:118-128.
16. Krogh J, Hjorthøj C, Speyer H, et al. Exercise for patients with major depression: a systematic review with meta-analysis and trial sequential analysis. BMJ Open. 2017;7(9):e014820. doi:10.1136/bmjopen-2016-014820
17. Firth J, Solmi M, Wootton RE, et al. A meta-review of “lifestyle psychiatry”: the role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry. 2020;19(3):360-380.
18. Piotrowski MC, Lunsford J, Gaynes BN. Lifestyle psychiatry for depression and anxiety: beyond diet and exercise. Lifestyle Med. 2021;2(1):e21. doi:10.1002/lim2.21
19. Janney CA, Brzoznowski KF, Richardson Cret al. Moving towards wellness: physical activity practices, perspectives, and preferences of users of outpatient mental health service. Gen Hosp Psychiatry. 2017;49:63-66.
20. Walsh R. Lifestyle and mental health. Am Psychol. 2011;66(7):579-592.
21. Cregg DR, Cheavens JS. Gratitude interventions: effective self-help? A meta-analysis of the impact on symptoms of depression and anxiety. J Happiness Stud. 2021;22(1):413-445.
22. Bohlmeijer E, Roemer M, Cuijpers P, et al. The effects of reminiscence on psychological well-being in older adults: a meta-analysis. Aging Ment Health. 2007;11(3):291-300.
23. Zagic D, Wuthrich VM, Rapee RM, et al. Interventions to improve social connections: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2022;57(5):885-906.
24. Mittelmark MB, Sagy S, Eriksson M, et al (eds). The Handbook of Salutogenesis [Internet]. Springer; 2017.
FIRST OF 4 PARTS
What does wellness mean to you? A 2018 survey posed this question to more than 6,000 people living with depression and bipolar disorder. In addition to better treatment and greater understanding of their illnesses, other priorities emerged: a longing for better days, a sense of purpose, and a longing to function well and be happy.1 As one respondent explained, “Wellness means stability; well enough to hold a job, well enough to enjoy activities, well enough to feel joy and hope.” Traditional treatment that focuses on alleviating symptoms may not sufficiently address outcomes patients value. When the focus is primarily deficit-based, clinicians and patients may miss opportunities for optimization and transformation.
Positive psychiatry is the science and practice of psychiatry that seeks to enhance and promote well-being and health through the enhancement of positive psychosocial factors such as resilience, optimism, wisdom, and social support in people with illnesses or disabilities as well as those in the community at large.2 It is based on the principles that there is no health without mental health, and that mental health can improve through preventive, therapeutic, and rehabilitative interventions.3
Positive interventions are defined as “treatment methods or intentional activities that aim to cultivate positive feelings, behaviors, or cognitions.”4 They are evidence-based intentional exercises designed to increase well-being and enhance flourishing. Although positive interventions were originally studied as activities for nonclinical populations and for helping healthy people thrive, they are increasingly being valued for their therapeutic role in treating psychopathology.5 By adding positive interventions to their toolbox, psychiatrists can expand the range of treatment options, better engage patients during the treatment process, and bolster positive mental health.
In this article, we provide practical ways to integrate the tools and principles of positive psychiatry into everyday clinical practice. The goal is to broaden how clinicians think about mental health and therapeutic options and, above all, enhance our patients’ everyday well-being. Teaching patients to adopt a positive orientation, harness strengths, mobilize values, cultivate social connections, and optimize healthy habits are strategies clinicians can apply not only to provide a counterweight to the traditional emphasis on illness, but also to enhance the range and richness of their patients’ everyday experience.
Adopt a positive orientation
When a clinician first meets a patient, “What’s wrong?” is a typical conversation starter, and conversations tend to revolve around problems, failures, and negative experiences. Positive psychiatry posits that there is therapeutic benefit to emphasizing and exploring a patient’s positive emotions, experiences, and aspirations. Questions such as “What was your sense of well-being this week? What is your goal for today’s session? What is your goal for the coming week?” can reorient a session towards an individual’s potential and promote exploration of what’s possible.
To promote a positive orientation, clinicians may consider integrating the Savoring and Three Good Things exercises—2 well-studied interventions—into their repertoire to activate and enhance positive emotional states such as gratitude and joy.6 An example of a Savoring activity is taking a 20-minute daily walk while trying to notice as many positive elements as possible. Similarly, the Three Good Things exercise, in which patients are asked to notice and write down 3 positive events and reflect on why they happened, promotes positive reflection and gratitude. A 14-day daily diary study conducted during the COVID-19 pandemic found that higher levels of gratitude were associated with higher levels of positive affect, lower levels of perceived stress related to COVID-19, and better subjective health.7 In addition to coping with life’s negative events, deliberately enhancing the impact of good things is a positive emotion amplifier. As French writer François de La Rochefoucauld argued, “Happiness does not consist in things themselves but in the relish we have of them.”8
Continue to: Harness strengths
Harness strengths
A growing body of evidence suggests that in addition to focusing on a patient’s chief concern, identifying and cultivating an individual’s signature strengths can mitigate stress and enhance well-being. Signature strengths are positive personality qualities that reflect our core identity and are morally valued. The VIA Character Strengths Survey is the most used and validated psychometric instrument to measure and identify signature strengths such as curiosity, self-regulation, honesty, and teamwork.9
To incorporate this tool into clinical practice, ask patients to complete a strengths survey using a validated assessment tool such as the VIA survey (www.viacharacter.org). After a patient identifies their signature strengths, encourage them to explore and apply these strengths in everyday life and in new ways. In addition to becoming aware of and using their signature strengths, encourage patients to “strengths spot” in others. “What strengths did you notice your coworker, family, or friend using today?” is a potential question to explore with patients. A strengths-based approach may be particularly helpful in uncovering motivation and fully engaging patients in treatment. Moreover, integrating strengths into the typically negatively skewed narrative underscores to patients that therapy isn’t only about untwisting distorted thinking, but also about harnessing one’s strengths, talents, and abilities. Strengths expressed through pragmatic actions can boost coping skills as well as enhance well-being.
Mobilize values
Value affirmation exercises have been shown to generate lasting benefits in creating positive feelings and behaviors.10 Encouraging patients to think about what they genuinely value redirects their gaze towards possibility and diverts self-focus. For instance, ask a patient to identify 2 or 3 values and write about why they are important. By reflecting on their values in writing, they affirm their identity and self-worth, thus creating a virtuous cycle of confidence, effort, and achievement. People who put their values front and center are more attuned to the needs of others as well as their own needs, and they make better connections.11 Including a patient’s values in the treatment plan may increase problem-solving skills, boost motivation, and build better stress management skills.
The “life review” is another intervention that facilitates exploration of a patient’s values. This exercise involves asking patients to recount the story of their life and the experiences that were most meaningful to them. This process allows clinicians to gain a deeper understanding of the patient’s values, which can help guide treatment. Meta-analytic evidence has demonstrated these reminiscence-based interventions have significant effects on well-being.6 As Mahatma Gandhi famously said, “Happiness is when what you think, what you say, and what you do are in harmony.” Creating more overlap between a patient’s values and their everyday actions and behaviors bolsters resilience, buffers against stress, and can restore a healthier self-concept.
Cultivate social connections
Social connection is recognized as a core psychological need and essential for well-being. The opposite of connection—social isolation—has negative effects on overall health, including increases in inflammatory markers, depression rates, and even all-cause mortality.12 A 2015 meta-analytic review demonstrated that loneliness increased the likelihood of mortality by 26%—a similar increase as seen with smoking 15 cigarettes a day.13
Continue to: As with any vital sign...
As with any vital sign, exploring a patient’s number of social contacts, quantity of social visits per week, and quality of relationships is an important indicator of health. Giving patients tools to cultivate social connection and deepen their relationships can enhance therapeutic outcomes. Asking patients to perform acts of kindness is one example of a “social prescription.” Feeding a stranger’s parking meter, picking up litter, helping a friend with a chore, providing a meal to a person in need, and volunteering are potential ways for patients to engage in kind deeds. After each act, encourage the patient to write down what they did and how it made them feel.
“Prescribing” positive communication is another way to enhance a patient’s social connections. For instance, teaching them about active constructive responding (ACR)—responding with enthusiasm when another person shares information or good news—has been shown to strengthen bonds with friends and family.14 Making eye contact, giving the other person one’s full attention, inquiring about details, and responding with enthusiasm and interest are simple ways patients can apply ACR in their daily lives. Counseling a patient on increasing social connections, prescribing connections, and inquiring about quantity and quality of social interactions can help them not only add years to their life but also add health and well-being to those years.
Optimize healthy habits
Mounting research demonstrates that exercise, sleep, and nutrition are important for well-being. Evidence shows that therapeutic lifestyle changes can reduce depressive symptoms and boost positive feelings. Numerous meta-analyses have demonstrated the benefits of sleep and exercise interventions for reducing depressive symptoms in psychiatric patients.15,16 Longitudinal studies have provided evidence that healthy diets increase happiness, even after controlling for potential confounders such as socioeconomic factors.17 Other lifestyle factors—including financial stability, pet ownership, decreased social media use, and spending time in nature—have been shown to contribute to well-being.18
Despite the substantial evidence that lifestyle factors can improve health outcomes, few clinicians ask about, focus on, or promote positive habits.19 Positive psychiatry seeks to reorient clinicians towards lifestyle factors that enhance well-being. Clinicians can deploy a variety of strategies to support patients in making healthy and sustainable changes. Assessing readiness for change, motivational interviewing, setting SMART (specific, measurable, assignable, realistic, and time-related) goals, and referring patients to relevant community resources are ways to encourage and promote therapeutic lifestyle changes. Inquiring about a patient’s typical day—such as how they spend their free time, what they eat, when they go to bed, and how much time they spend outdoors—opens conversations about general well-being and shows the patient that therapy is about the whole person, and not only symptom management. Helping patients have better days can empower them to lead more satisfied lives.20

The Table6,17,21-23 summarizes the scientific evidence for the strategies described in this article. The Figure provides a flowchart for using these strategies in clinical practice.

Continue to: Balancing pathogenesis with salutogenesis
Balancing pathogenesis with salutogenesis
By exploring and emphasizing potential and possibility, positive psychiatry aims to create a balance between pathogenesis (the study and understanding of disease) with salutogenesis (the study and creation of health24). Clinicians are well positioned to manage symptoms and bolster positive states. Rather than an either/or approach to well-being, positive psychiatry strives for a both/and approach to well-being. By adding positive interventions to their toolbox, clinicians can expand the range of treatment options, better engage patients in the treatment process, and bolster mental health.
Bottom Line
Clinicians can integrate the tools and principles of positive psychiatry into clinical practice. Teaching patients to adopt a positive orientation, harness strengths, mobilize values, cultivate social connections, and optimize healthy habits can not only provide a counterweight to the traditional emphasis on illness, but also can enhance the range and richness of patients’ everyday experience.
Related Resources
- University of Pennsylvania. Authentic happiness. https://www.authentichappiness.sas.upenn.edu
- Jeste DV, Palmer BW (eds). Positive Psychiatry: A Clinical Handbook. American Psychiatric Publishing; 2015.
- Jeste DV, Palmer BW, Rettew DC, et al. Positive psychiatry: its time has come. J Clin Psychiatry. 2015;76(6):675-683.
FIRST OF 4 PARTS
What does wellness mean to you? A 2018 survey posed this question to more than 6,000 people living with depression and bipolar disorder. In addition to better treatment and greater understanding of their illnesses, other priorities emerged: a longing for better days, a sense of purpose, and a longing to function well and be happy.1 As one respondent explained, “Wellness means stability; well enough to hold a job, well enough to enjoy activities, well enough to feel joy and hope.” Traditional treatment that focuses on alleviating symptoms may not sufficiently address outcomes patients value. When the focus is primarily deficit-based, clinicians and patients may miss opportunities for optimization and transformation.
Positive psychiatry is the science and practice of psychiatry that seeks to enhance and promote well-being and health through the enhancement of positive psychosocial factors such as resilience, optimism, wisdom, and social support in people with illnesses or disabilities as well as those in the community at large.2 It is based on the principles that there is no health without mental health, and that mental health can improve through preventive, therapeutic, and rehabilitative interventions.3
Positive interventions are defined as “treatment methods or intentional activities that aim to cultivate positive feelings, behaviors, or cognitions.”4 They are evidence-based intentional exercises designed to increase well-being and enhance flourishing. Although positive interventions were originally studied as activities for nonclinical populations and for helping healthy people thrive, they are increasingly being valued for their therapeutic role in treating psychopathology.5 By adding positive interventions to their toolbox, psychiatrists can expand the range of treatment options, better engage patients during the treatment process, and bolster positive mental health.
In this article, we provide practical ways to integrate the tools and principles of positive psychiatry into everyday clinical practice. The goal is to broaden how clinicians think about mental health and therapeutic options and, above all, enhance our patients’ everyday well-being. Teaching patients to adopt a positive orientation, harness strengths, mobilize values, cultivate social connections, and optimize healthy habits are strategies clinicians can apply not only to provide a counterweight to the traditional emphasis on illness, but also to enhance the range and richness of their patients’ everyday experience.
Adopt a positive orientation
When a clinician first meets a patient, “What’s wrong?” is a typical conversation starter, and conversations tend to revolve around problems, failures, and negative experiences. Positive psychiatry posits that there is therapeutic benefit to emphasizing and exploring a patient’s positive emotions, experiences, and aspirations. Questions such as “What was your sense of well-being this week? What is your goal for today’s session? What is your goal for the coming week?” can reorient a session towards an individual’s potential and promote exploration of what’s possible.
To promote a positive orientation, clinicians may consider integrating the Savoring and Three Good Things exercises—2 well-studied interventions—into their repertoire to activate and enhance positive emotional states such as gratitude and joy.6 An example of a Savoring activity is taking a 20-minute daily walk while trying to notice as many positive elements as possible. Similarly, the Three Good Things exercise, in which patients are asked to notice and write down 3 positive events and reflect on why they happened, promotes positive reflection and gratitude. A 14-day daily diary study conducted during the COVID-19 pandemic found that higher levels of gratitude were associated with higher levels of positive affect, lower levels of perceived stress related to COVID-19, and better subjective health.7 In addition to coping with life’s negative events, deliberately enhancing the impact of good things is a positive emotion amplifier. As French writer François de La Rochefoucauld argued, “Happiness does not consist in things themselves but in the relish we have of them.”8
Continue to: Harness strengths
Harness strengths
A growing body of evidence suggests that in addition to focusing on a patient’s chief concern, identifying and cultivating an individual’s signature strengths can mitigate stress and enhance well-being. Signature strengths are positive personality qualities that reflect our core identity and are morally valued. The VIA Character Strengths Survey is the most used and validated psychometric instrument to measure and identify signature strengths such as curiosity, self-regulation, honesty, and teamwork.9
To incorporate this tool into clinical practice, ask patients to complete a strengths survey using a validated assessment tool such as the VIA survey (www.viacharacter.org). After a patient identifies their signature strengths, encourage them to explore and apply these strengths in everyday life and in new ways. In addition to becoming aware of and using their signature strengths, encourage patients to “strengths spot” in others. “What strengths did you notice your coworker, family, or friend using today?” is a potential question to explore with patients. A strengths-based approach may be particularly helpful in uncovering motivation and fully engaging patients in treatment. Moreover, integrating strengths into the typically negatively skewed narrative underscores to patients that therapy isn’t only about untwisting distorted thinking, but also about harnessing one’s strengths, talents, and abilities. Strengths expressed through pragmatic actions can boost coping skills as well as enhance well-being.
Mobilize values
Value affirmation exercises have been shown to generate lasting benefits in creating positive feelings and behaviors.10 Encouraging patients to think about what they genuinely value redirects their gaze towards possibility and diverts self-focus. For instance, ask a patient to identify 2 or 3 values and write about why they are important. By reflecting on their values in writing, they affirm their identity and self-worth, thus creating a virtuous cycle of confidence, effort, and achievement. People who put their values front and center are more attuned to the needs of others as well as their own needs, and they make better connections.11 Including a patient’s values in the treatment plan may increase problem-solving skills, boost motivation, and build better stress management skills.
The “life review” is another intervention that facilitates exploration of a patient’s values. This exercise involves asking patients to recount the story of their life and the experiences that were most meaningful to them. This process allows clinicians to gain a deeper understanding of the patient’s values, which can help guide treatment. Meta-analytic evidence has demonstrated these reminiscence-based interventions have significant effects on well-being.6 As Mahatma Gandhi famously said, “Happiness is when what you think, what you say, and what you do are in harmony.” Creating more overlap between a patient’s values and their everyday actions and behaviors bolsters resilience, buffers against stress, and can restore a healthier self-concept.
Cultivate social connections
Social connection is recognized as a core psychological need and essential for well-being. The opposite of connection—social isolation—has negative effects on overall health, including increases in inflammatory markers, depression rates, and even all-cause mortality.12 A 2015 meta-analytic review demonstrated that loneliness increased the likelihood of mortality by 26%—a similar increase as seen with smoking 15 cigarettes a day.13
Continue to: As with any vital sign...
As with any vital sign, exploring a patient’s number of social contacts, quantity of social visits per week, and quality of relationships is an important indicator of health. Giving patients tools to cultivate social connection and deepen their relationships can enhance therapeutic outcomes. Asking patients to perform acts of kindness is one example of a “social prescription.” Feeding a stranger’s parking meter, picking up litter, helping a friend with a chore, providing a meal to a person in need, and volunteering are potential ways for patients to engage in kind deeds. After each act, encourage the patient to write down what they did and how it made them feel.
“Prescribing” positive communication is another way to enhance a patient’s social connections. For instance, teaching them about active constructive responding (ACR)—responding with enthusiasm when another person shares information or good news—has been shown to strengthen bonds with friends and family.14 Making eye contact, giving the other person one’s full attention, inquiring about details, and responding with enthusiasm and interest are simple ways patients can apply ACR in their daily lives. Counseling a patient on increasing social connections, prescribing connections, and inquiring about quantity and quality of social interactions can help them not only add years to their life but also add health and well-being to those years.
Optimize healthy habits
Mounting research demonstrates that exercise, sleep, and nutrition are important for well-being. Evidence shows that therapeutic lifestyle changes can reduce depressive symptoms and boost positive feelings. Numerous meta-analyses have demonstrated the benefits of sleep and exercise interventions for reducing depressive symptoms in psychiatric patients.15,16 Longitudinal studies have provided evidence that healthy diets increase happiness, even after controlling for potential confounders such as socioeconomic factors.17 Other lifestyle factors—including financial stability, pet ownership, decreased social media use, and spending time in nature—have been shown to contribute to well-being.18
Despite the substantial evidence that lifestyle factors can improve health outcomes, few clinicians ask about, focus on, or promote positive habits.19 Positive psychiatry seeks to reorient clinicians towards lifestyle factors that enhance well-being. Clinicians can deploy a variety of strategies to support patients in making healthy and sustainable changes. Assessing readiness for change, motivational interviewing, setting SMART (specific, measurable, assignable, realistic, and time-related) goals, and referring patients to relevant community resources are ways to encourage and promote therapeutic lifestyle changes. Inquiring about a patient’s typical day—such as how they spend their free time, what they eat, when they go to bed, and how much time they spend outdoors—opens conversations about general well-being and shows the patient that therapy is about the whole person, and not only symptom management. Helping patients have better days can empower them to lead more satisfied lives.20

The Table6,17,21-23 summarizes the scientific evidence for the strategies described in this article. The Figure provides a flowchart for using these strategies in clinical practice.

Continue to: Balancing pathogenesis with salutogenesis
Balancing pathogenesis with salutogenesis
By exploring and emphasizing potential and possibility, positive psychiatry aims to create a balance between pathogenesis (the study and understanding of disease) with salutogenesis (the study and creation of health24). Clinicians are well positioned to manage symptoms and bolster positive states. Rather than an either/or approach to well-being, positive psychiatry strives for a both/and approach to well-being. By adding positive interventions to their toolbox, clinicians can expand the range of treatment options, better engage patients in the treatment process, and bolster mental health.
Bottom Line
Clinicians can integrate the tools and principles of positive psychiatry into clinical practice. Teaching patients to adopt a positive orientation, harness strengths, mobilize values, cultivate social connections, and optimize healthy habits can not only provide a counterweight to the traditional emphasis on illness, but also can enhance the range and richness of patients’ everyday experience.
Related Resources
- University of Pennsylvania. Authentic happiness. https://www.authentichappiness.sas.upenn.edu
- Jeste DV, Palmer BW (eds). Positive Psychiatry: A Clinical Handbook. American Psychiatric Publishing; 2015.
- Jeste DV, Palmer BW, Rettew DC, et al. Positive psychiatry: its time has come. J Clin Psychiatry. 2015;76(6):675-683.
1. Morton E, Foxworth P, Dardess P, et al. “Supporting Wellness”: a depression and bipolar support alliance mixed-methods investigation of lived experience perspectives and priorities for mood disorder treatment. J Affect Disord. 2022;299:575-584.
2. Jeste DV, Palmer BW, Rettew DC, et al. Positive psychiatry: its time has come. J Clin Psychiatry. 2015;76(6):675-683.
3. Jeste DV. Positive psychiatry comes of age. Int Psychogeriatr. 2018;30(12):1735-1738.
4. Sin NL, Lyubomirsky S. Enhancing well-being and alleviating depressive symptoms with positive psychology interventions: a practice-friendly meta-analysis. J Clin Psychol. 2009;65(5):467-487.
5. Seligman MEP, Rashid T, Parks AC. Positive psychotherapy. Am Psychol. 2006;61(8):774-788.
6. Carr A, Cullen K, Keeney C, et al. Effectiveness of positive psychology interventions: a systematic review and meta-analysis. J Posit Psychol. 2021;16(6):749-769.
7. Jiang D. Feeling gratitude is associated with better well-being across the life span: a daily diary study during the COVID-19 outbreak. J Gerontol B Psychol Sci Soc Sci. 2022;77(4):e36-e45.
8. de La Rochefoucauld F. Maxims and moral reflections (1796). Gale ECCO: 2010.
9. Niemiec RM. VIA character strengths: Research and practice (The first 10 years). In: Knoop HH, Fave AD (eds). Well-being and Cultures. Springer;2013:11-29.
10. Cohen GL, Sherman DK. The psychology of change: self-affirmation and social psychological intervention. Annu Rev Psychol. 2014;65:333-371.
11. Thomaes S, Bushman BJ, de Castro BO, et al. Arousing “gentle passions” in young adolescents: sustained experimental effects of value affirmations on prosocial feelings and behaviors. Dev Psychol. 2012;48(1):103-110.
12. Cacioppo JT, Cacioppo S, Capitanio JP, et al. The neuroendocrinology of social isolation. Annu Rev Psychol. 2015;66:733-767.
13. Holt-Lunstad J, Smith TB, Baker M, et al. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci. 2015;10(2):227-237.
14. Gable SL, Reis HT, Impett EA, et al. What do you do when things go right? The intrapersonal and interpersonal benefits of sharing positive events. J Pers Soc Psychol. 2004;87(2):228-245.
15. Gee B, Orchard F, Clarke E, et al. The effect of non-pharmacological sleep interventions on depression symptoms: a meta-analysis of randomised controlled trials. Sleep Med Rev. 2019;43:118-128.
16. Krogh J, Hjorthøj C, Speyer H, et al. Exercise for patients with major depression: a systematic review with meta-analysis and trial sequential analysis. BMJ Open. 2017;7(9):e014820. doi:10.1136/bmjopen-2016-014820
17. Firth J, Solmi M, Wootton RE, et al. A meta-review of “lifestyle psychiatry”: the role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry. 2020;19(3):360-380.
18. Piotrowski MC, Lunsford J, Gaynes BN. Lifestyle psychiatry for depression and anxiety: beyond diet and exercise. Lifestyle Med. 2021;2(1):e21. doi:10.1002/lim2.21
19. Janney CA, Brzoznowski KF, Richardson Cret al. Moving towards wellness: physical activity practices, perspectives, and preferences of users of outpatient mental health service. Gen Hosp Psychiatry. 2017;49:63-66.
20. Walsh R. Lifestyle and mental health. Am Psychol. 2011;66(7):579-592.
21. Cregg DR, Cheavens JS. Gratitude interventions: effective self-help? A meta-analysis of the impact on symptoms of depression and anxiety. J Happiness Stud. 2021;22(1):413-445.
22. Bohlmeijer E, Roemer M, Cuijpers P, et al. The effects of reminiscence on psychological well-being in older adults: a meta-analysis. Aging Ment Health. 2007;11(3):291-300.
23. Zagic D, Wuthrich VM, Rapee RM, et al. Interventions to improve social connections: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2022;57(5):885-906.
24. Mittelmark MB, Sagy S, Eriksson M, et al (eds). The Handbook of Salutogenesis [Internet]. Springer; 2017.
1. Morton E, Foxworth P, Dardess P, et al. “Supporting Wellness”: a depression and bipolar support alliance mixed-methods investigation of lived experience perspectives and priorities for mood disorder treatment. J Affect Disord. 2022;299:575-584.
2. Jeste DV, Palmer BW, Rettew DC, et al. Positive psychiatry: its time has come. J Clin Psychiatry. 2015;76(6):675-683.
3. Jeste DV. Positive psychiatry comes of age. Int Psychogeriatr. 2018;30(12):1735-1738.
4. Sin NL, Lyubomirsky S. Enhancing well-being and alleviating depressive symptoms with positive psychology interventions: a practice-friendly meta-analysis. J Clin Psychol. 2009;65(5):467-487.
5. Seligman MEP, Rashid T, Parks AC. Positive psychotherapy. Am Psychol. 2006;61(8):774-788.
6. Carr A, Cullen K, Keeney C, et al. Effectiveness of positive psychology interventions: a systematic review and meta-analysis. J Posit Psychol. 2021;16(6):749-769.
7. Jiang D. Feeling gratitude is associated with better well-being across the life span: a daily diary study during the COVID-19 outbreak. J Gerontol B Psychol Sci Soc Sci. 2022;77(4):e36-e45.
8. de La Rochefoucauld F. Maxims and moral reflections (1796). Gale ECCO: 2010.
9. Niemiec RM. VIA character strengths: Research and practice (The first 10 years). In: Knoop HH, Fave AD (eds). Well-being and Cultures. Springer;2013:11-29.
10. Cohen GL, Sherman DK. The psychology of change: self-affirmation and social psychological intervention. Annu Rev Psychol. 2014;65:333-371.
11. Thomaes S, Bushman BJ, de Castro BO, et al. Arousing “gentle passions” in young adolescents: sustained experimental effects of value affirmations on prosocial feelings and behaviors. Dev Psychol. 2012;48(1):103-110.
12. Cacioppo JT, Cacioppo S, Capitanio JP, et al. The neuroendocrinology of social isolation. Annu Rev Psychol. 2015;66:733-767.
13. Holt-Lunstad J, Smith TB, Baker M, et al. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci. 2015;10(2):227-237.
14. Gable SL, Reis HT, Impett EA, et al. What do you do when things go right? The intrapersonal and interpersonal benefits of sharing positive events. J Pers Soc Psychol. 2004;87(2):228-245.
15. Gee B, Orchard F, Clarke E, et al. The effect of non-pharmacological sleep interventions on depression symptoms: a meta-analysis of randomised controlled trials. Sleep Med Rev. 2019;43:118-128.
16. Krogh J, Hjorthøj C, Speyer H, et al. Exercise for patients with major depression: a systematic review with meta-analysis and trial sequential analysis. BMJ Open. 2017;7(9):e014820. doi:10.1136/bmjopen-2016-014820
17. Firth J, Solmi M, Wootton RE, et al. A meta-review of “lifestyle psychiatry”: the role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry. 2020;19(3):360-380.
18. Piotrowski MC, Lunsford J, Gaynes BN. Lifestyle psychiatry for depression and anxiety: beyond diet and exercise. Lifestyle Med. 2021;2(1):e21. doi:10.1002/lim2.21
19. Janney CA, Brzoznowski KF, Richardson Cret al. Moving towards wellness: physical activity practices, perspectives, and preferences of users of outpatient mental health service. Gen Hosp Psychiatry. 2017;49:63-66.
20. Walsh R. Lifestyle and mental health. Am Psychol. 2011;66(7):579-592.
21. Cregg DR, Cheavens JS. Gratitude interventions: effective self-help? A meta-analysis of the impact on symptoms of depression and anxiety. J Happiness Stud. 2021;22(1):413-445.
22. Bohlmeijer E, Roemer M, Cuijpers P, et al. The effects of reminiscence on psychological well-being in older adults: a meta-analysis. Aging Ment Health. 2007;11(3):291-300.
23. Zagic D, Wuthrich VM, Rapee RM, et al. Interventions to improve social connections: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2022;57(5):885-906.
24. Mittelmark MB, Sagy S, Eriksson M, et al (eds). The Handbook of Salutogenesis [Internet]. Springer; 2017.
The accelerating societal entropy undermines mental health
According to the second law of thermodynamics, it is inevitable that entropy will continue to increase over time.1 Entropy is a measure of disorder, which can eventuate in chaos and lead to profound uncertainty, with serious psychological consequences.
The increase in entropy is usually gradual. It took hundreds of years for powerful empires and civilizations to collapse and disappear. Inanimate objects such as a house, a piece of furniture, or a piece of equipment eventually deteriorate and break down over time. Tidy offices will become messy, cluttered, and dirty unless attended to regularly. Living organisms, including humans, inevitably undergo an aging process with cellular senescence, atrophy, and loss of cerebral, muscle, and bone tissue, ending in death. Even human relationships will eventually fracture, wither, and end. The passage of time ruthlessly increases the entropy of everything in life. Even the 13-billion-year-old universe, which currently looks formidable and permanent to us, is inexorably expanding and hurtling towards a calamitous end a few billion years from now.
To slow down, halt, or reverse entropy, work and energy must be invested. A house requires regular maintenance for all its components to avoid deteriorating and becoming uninhabitable (very high entropy). Humans require massive amounts of work during fetal life, infancy, childhood, adolescence, adulthood, and throughout old age. This includes work by parents, teachers, friends, physicians, farmers, and manufacturers of food, clothing, and sundry supplies, all targeted to maintain an individual and slow the rate of entropy. But death is inevitable as the final stage of human entropy.
The brain is an entropic organ.2 Psychiatric disorders can be conceptualized as a neurobiologic consequence of a major rise in brain entropy. The chaos created by high brain entropy will lead to a disruption of basic mental functions such as thought, mood, affect, impulses, behavior, and cognition. Brain entropy increases can be due to genetics or the environment, but most often are due an interaction of both (G x E).
Societal entropy and our patients
Psychiatric patients are deeply influenced by the context in which they live (society). The entropy of contemporary society is rising at an alarming rate, which means that order is rapidly degenerating into disorder at an unprecedented pace. When the COVID-19 pandemic abruptly emerged in early 2020, it was a major public health shock that drastically changed the lives of all citizens and dramatically increased societal entropy. The pandemic led to lockdowns, fear of death, gut-wrenching uncertainty (especially for a whole year before vaccines were developed, but even after), loss of socialization and sexual intimacy, loss of employment, financial straits, and an inability to access routine medical or surgical procedures. Everyone in society developed anxiety and acute stress reaction, but those with pre-existing psychiatric disorders suffered the most with an intensification of their symptoms.
The unforeseen, sudden, and traumatically life-altering pandemic triggered various degrees of posttraumatic stress disorder across all age groups, and painful death in medically compromised individuals and older adults. Both physical and psychological entropy skyrocketed and the “order” of life as we knew it rapidly disintegrated into shambles and disorder. The abrupt traumatic jolt triggered various degrees of deleterious impacts on the brains of all who experienced it in real time. The rise in the psychobiological entropy was unprecedented across the structures of society, especially the population, its vulnerable human component.
But even as the worst of the pandemic is in our rearview mirror and life again has a semblance of normality, the rise of entropy continues to accelerate because we continue to be surrounded and engulfed by countless stressful events in contemporary society. Those nagging stresses continue to transmute order to chaos and metamorphose comforting predictability to entrenched uncertainty:
- Toxic political hyperpartisanship, with intense animus and visceral bidirectional hatred
- Racial tensions, with overt bias across groups
- Economic turmoil, with inflation and threats of recession
- Actual wars and threats of war
- Social media that spreads bad news and distorts facts
- An opioid crisis, with hundreds of thousands of deaths
- Skyrocketing crime, with a decline in policing and quick release of criminals without bail
- A ruthless and arbitrary “cancel culture” that doesn’t even spare the previously revered founders of the republic
- Cognitive dissonance of disparaging Abraham Lincoln despite his major achievement of eliminating slavery by waging a civil war
- The social and medical strife regarding access to abortion.
Continue to: I also would include...
(I also would include some “entropy pet peeves” of mine: Torn clothes as a fashion statement, transforming tattoos from an oddity to a fad, nose rings that disfigure pretty faces, and banishing neckties for men.)
Our role in this scenario
As psychiatrists, we must step up to intensify the work needed to slow down and even reverse the dangerously rising brain entropy in our patients. But that is not an easy task given the implosion of societal norms and traditional values, along with the radicalization of beliefs, with utter intolerance of others’ beliefs. We also face the challenge of maintaining a modicum of resilience and wellness in ourselves, which can be antidotes to entropy.
It’s impossible to stop the inevitability of rising entropy, both physical and psychological, but psychiatrists and other mental health professionals must invest their skills and talents now more than ever to at least slow down the pace of entropy among our patients. Otherwise, psychological chaos and disorder will be quite damaging to their lives, and worsen their outcomes.
1. Ben-Naim A. Entropy Demystified. World Scientific; 2007.
2. Carhart-Harris RL. The entropic brain - revisited. Neuropharmacology. 2018;142:167-178. doi: 10.1016/j.neuropharm.2018.03.010
According to the second law of thermodynamics, it is inevitable that entropy will continue to increase over time.1 Entropy is a measure of disorder, which can eventuate in chaos and lead to profound uncertainty, with serious psychological consequences.
The increase in entropy is usually gradual. It took hundreds of years for powerful empires and civilizations to collapse and disappear. Inanimate objects such as a house, a piece of furniture, or a piece of equipment eventually deteriorate and break down over time. Tidy offices will become messy, cluttered, and dirty unless attended to regularly. Living organisms, including humans, inevitably undergo an aging process with cellular senescence, atrophy, and loss of cerebral, muscle, and bone tissue, ending in death. Even human relationships will eventually fracture, wither, and end. The passage of time ruthlessly increases the entropy of everything in life. Even the 13-billion-year-old universe, which currently looks formidable and permanent to us, is inexorably expanding and hurtling towards a calamitous end a few billion years from now.
To slow down, halt, or reverse entropy, work and energy must be invested. A house requires regular maintenance for all its components to avoid deteriorating and becoming uninhabitable (very high entropy). Humans require massive amounts of work during fetal life, infancy, childhood, adolescence, adulthood, and throughout old age. This includes work by parents, teachers, friends, physicians, farmers, and manufacturers of food, clothing, and sundry supplies, all targeted to maintain an individual and slow the rate of entropy. But death is inevitable as the final stage of human entropy.
The brain is an entropic organ.2 Psychiatric disorders can be conceptualized as a neurobiologic consequence of a major rise in brain entropy. The chaos created by high brain entropy will lead to a disruption of basic mental functions such as thought, mood, affect, impulses, behavior, and cognition. Brain entropy increases can be due to genetics or the environment, but most often are due an interaction of both (G x E).
Societal entropy and our patients
Psychiatric patients are deeply influenced by the context in which they live (society). The entropy of contemporary society is rising at an alarming rate, which means that order is rapidly degenerating into disorder at an unprecedented pace. When the COVID-19 pandemic abruptly emerged in early 2020, it was a major public health shock that drastically changed the lives of all citizens and dramatically increased societal entropy. The pandemic led to lockdowns, fear of death, gut-wrenching uncertainty (especially for a whole year before vaccines were developed, but even after), loss of socialization and sexual intimacy, loss of employment, financial straits, and an inability to access routine medical or surgical procedures. Everyone in society developed anxiety and acute stress reaction, but those with pre-existing psychiatric disorders suffered the most with an intensification of their symptoms.
The unforeseen, sudden, and traumatically life-altering pandemic triggered various degrees of posttraumatic stress disorder across all age groups, and painful death in medically compromised individuals and older adults. Both physical and psychological entropy skyrocketed and the “order” of life as we knew it rapidly disintegrated into shambles and disorder. The abrupt traumatic jolt triggered various degrees of deleterious impacts on the brains of all who experienced it in real time. The rise in the psychobiological entropy was unprecedented across the structures of society, especially the population, its vulnerable human component.
But even as the worst of the pandemic is in our rearview mirror and life again has a semblance of normality, the rise of entropy continues to accelerate because we continue to be surrounded and engulfed by countless stressful events in contemporary society. Those nagging stresses continue to transmute order to chaos and metamorphose comforting predictability to entrenched uncertainty:
- Toxic political hyperpartisanship, with intense animus and visceral bidirectional hatred
- Racial tensions, with overt bias across groups
- Economic turmoil, with inflation and threats of recession
- Actual wars and threats of war
- Social media that spreads bad news and distorts facts
- An opioid crisis, with hundreds of thousands of deaths
- Skyrocketing crime, with a decline in policing and quick release of criminals without bail
- A ruthless and arbitrary “cancel culture” that doesn’t even spare the previously revered founders of the republic
- Cognitive dissonance of disparaging Abraham Lincoln despite his major achievement of eliminating slavery by waging a civil war
- The social and medical strife regarding access to abortion.
Continue to: I also would include...
(I also would include some “entropy pet peeves” of mine: Torn clothes as a fashion statement, transforming tattoos from an oddity to a fad, nose rings that disfigure pretty faces, and banishing neckties for men.)
Our role in this scenario
As psychiatrists, we must step up to intensify the work needed to slow down and even reverse the dangerously rising brain entropy in our patients. But that is not an easy task given the implosion of societal norms and traditional values, along with the radicalization of beliefs, with utter intolerance of others’ beliefs. We also face the challenge of maintaining a modicum of resilience and wellness in ourselves, which can be antidotes to entropy.
It’s impossible to stop the inevitability of rising entropy, both physical and psychological, but psychiatrists and other mental health professionals must invest their skills and talents now more than ever to at least slow down the pace of entropy among our patients. Otherwise, psychological chaos and disorder will be quite damaging to their lives, and worsen their outcomes.
According to the second law of thermodynamics, it is inevitable that entropy will continue to increase over time.1 Entropy is a measure of disorder, which can eventuate in chaos and lead to profound uncertainty, with serious psychological consequences.
The increase in entropy is usually gradual. It took hundreds of years for powerful empires and civilizations to collapse and disappear. Inanimate objects such as a house, a piece of furniture, or a piece of equipment eventually deteriorate and break down over time. Tidy offices will become messy, cluttered, and dirty unless attended to regularly. Living organisms, including humans, inevitably undergo an aging process with cellular senescence, atrophy, and loss of cerebral, muscle, and bone tissue, ending in death. Even human relationships will eventually fracture, wither, and end. The passage of time ruthlessly increases the entropy of everything in life. Even the 13-billion-year-old universe, which currently looks formidable and permanent to us, is inexorably expanding and hurtling towards a calamitous end a few billion years from now.
To slow down, halt, or reverse entropy, work and energy must be invested. A house requires regular maintenance for all its components to avoid deteriorating and becoming uninhabitable (very high entropy). Humans require massive amounts of work during fetal life, infancy, childhood, adolescence, adulthood, and throughout old age. This includes work by parents, teachers, friends, physicians, farmers, and manufacturers of food, clothing, and sundry supplies, all targeted to maintain an individual and slow the rate of entropy. But death is inevitable as the final stage of human entropy.
The brain is an entropic organ.2 Psychiatric disorders can be conceptualized as a neurobiologic consequence of a major rise in brain entropy. The chaos created by high brain entropy will lead to a disruption of basic mental functions such as thought, mood, affect, impulses, behavior, and cognition. Brain entropy increases can be due to genetics or the environment, but most often are due an interaction of both (G x E).
Societal entropy and our patients
Psychiatric patients are deeply influenced by the context in which they live (society). The entropy of contemporary society is rising at an alarming rate, which means that order is rapidly degenerating into disorder at an unprecedented pace. When the COVID-19 pandemic abruptly emerged in early 2020, it was a major public health shock that drastically changed the lives of all citizens and dramatically increased societal entropy. The pandemic led to lockdowns, fear of death, gut-wrenching uncertainty (especially for a whole year before vaccines were developed, but even after), loss of socialization and sexual intimacy, loss of employment, financial straits, and an inability to access routine medical or surgical procedures. Everyone in society developed anxiety and acute stress reaction, but those with pre-existing psychiatric disorders suffered the most with an intensification of their symptoms.
The unforeseen, sudden, and traumatically life-altering pandemic triggered various degrees of posttraumatic stress disorder across all age groups, and painful death in medically compromised individuals and older adults. Both physical and psychological entropy skyrocketed and the “order” of life as we knew it rapidly disintegrated into shambles and disorder. The abrupt traumatic jolt triggered various degrees of deleterious impacts on the brains of all who experienced it in real time. The rise in the psychobiological entropy was unprecedented across the structures of society, especially the population, its vulnerable human component.
But even as the worst of the pandemic is in our rearview mirror and life again has a semblance of normality, the rise of entropy continues to accelerate because we continue to be surrounded and engulfed by countless stressful events in contemporary society. Those nagging stresses continue to transmute order to chaos and metamorphose comforting predictability to entrenched uncertainty:
- Toxic political hyperpartisanship, with intense animus and visceral bidirectional hatred
- Racial tensions, with overt bias across groups
- Economic turmoil, with inflation and threats of recession
- Actual wars and threats of war
- Social media that spreads bad news and distorts facts
- An opioid crisis, with hundreds of thousands of deaths
- Skyrocketing crime, with a decline in policing and quick release of criminals without bail
- A ruthless and arbitrary “cancel culture” that doesn’t even spare the previously revered founders of the republic
- Cognitive dissonance of disparaging Abraham Lincoln despite his major achievement of eliminating slavery by waging a civil war
- The social and medical strife regarding access to abortion.
Continue to: I also would include...
(I also would include some “entropy pet peeves” of mine: Torn clothes as a fashion statement, transforming tattoos from an oddity to a fad, nose rings that disfigure pretty faces, and banishing neckties for men.)
Our role in this scenario
As psychiatrists, we must step up to intensify the work needed to slow down and even reverse the dangerously rising brain entropy in our patients. But that is not an easy task given the implosion of societal norms and traditional values, along with the radicalization of beliefs, with utter intolerance of others’ beliefs. We also face the challenge of maintaining a modicum of resilience and wellness in ourselves, which can be antidotes to entropy.
It’s impossible to stop the inevitability of rising entropy, both physical and psychological, but psychiatrists and other mental health professionals must invest their skills and talents now more than ever to at least slow down the pace of entropy among our patients. Otherwise, psychological chaos and disorder will be quite damaging to their lives, and worsen their outcomes.
1. Ben-Naim A. Entropy Demystified. World Scientific; 2007.
2. Carhart-Harris RL. The entropic brain - revisited. Neuropharmacology. 2018;142:167-178. doi: 10.1016/j.neuropharm.2018.03.010
1. Ben-Naim A. Entropy Demystified. World Scientific; 2007.
2. Carhart-Harris RL. The entropic brain - revisited. Neuropharmacology. 2018;142:167-178. doi: 10.1016/j.neuropharm.2018.03.010
More on varenicline
Murray et al have written a timely, thoughtful, and useful article (“Smoking cessation: Varenicline and the risk of neuropsychiatric adverse events,”
Just a few caveats regarding Murray et al’s excellent summary:
• The article did not address that nicotine is consumed in multiple ways, such as vaping, snuff, chewing tobacco, and hookah
• The safety of varenicline appears fair when psychiatric illness is well controlled but can be problematic (and even severely detrimental) when mental illness is not well controlled. This should not be glossed over, especially since it was the reason for the original black-box warning (for risks including behavioral impulsivity, suicidality, severe insomnia, and nightmares) that was removed in 2016
• Patients with severe mental illness may not fully understand the risks, benefits, and priorities of the treatment intervention. The importance of psychiatric and internal medicine in addition to pharmacy follow-up is critical and needs to be documented.
Varenicline has been contextualized in its current role as a first-line treatment for smoking cessation. By bypassing a sizeable population of patients who have unstable psychiatric illness (especially bipolar I disorder), the path has been opened for risky “off-label” varenicline prescribing to this population by internists, who should be very cautious and prudent about prescribing for such patients. This alone is probably a good reason to reinstate the black-box warning.
Interestingly, one review found that only 1 of 11 patients receiving varenicline stopped smoking.1 Not dramatically beneficial for a first-line treatment! Decreasing smoking occurs as well and is more robust with combinational use with bupropion, nicotine replacement therapy, and cognitive-behavioral therapy.
If we are focusing on patients with unstable mental illness—who are seen primarily by psychiatrists—adherence, urgency of intervention, and context regarding acute safety for this population must be seen as top priorities.
So-called “second-line” treatment options must also be considered. Sandiego et al3 make excellent points regarding the role of alpha-adrenergic agonists such as guanfacine, which have been shown to be helpful in smoking cessation. They work by decreasing cortical dopamine release and their calming effects on the noradrenergic system, which may decrease smoking precipitated by stress. For the particularly challenging subpopulation of unstable smokers, the combination of varenicline plus guanfacine ER may turn out to be a game-changer.
Varenicline has not proven itself to be useful in patients who are severely mentally ill, and due to its low success rate, expectations should remain tempered, pragmatically realistic, and safety-based.4,5 The bottom line is that in an unstable psychiatrically ill patient, interventions other than varenicline should be first-line.
1. Crawford P, Cieslak D. Varenicline for smoking cessation. Am Fam Physician. 2017;96(5).
2. Beard E, Jackson SE, Anthenelli RM, et al. Estimation of risk of neuropsychiatric adverse events from varenicline, bupropion and nicotine patch versus placebo: secondary analysis of results from the EAGLES trial using Bayes factors. Addiction. 2021;116(10):2816-2824.
3. Sandiego CM, Matuskey D, Lavery M, et al. The effect of treatment with guanfacine, an alpha2 adrenergic agonist, on dopaminergic tone in tobacco smokers: an [11C]FLB457 PET study. Neuropsychopharmacology. 2018;43(5):1052-1058.
4. Sharma R, Alla K, Pfeffer D, et al. An appraisal of practice guidelines for smoking cessation in people with severe mental illness. Aust N Z J Psychiatry. 2017;51(11):1106-1120.
5. Tofler IR. Varenicline for smoking cessation in the bipolar patient. J Clin Psychiatry. 2015;76(5):625.
Murray et al have written a timely, thoughtful, and useful article (“Smoking cessation: Varenicline and the risk of neuropsychiatric adverse events,”
Just a few caveats regarding Murray et al’s excellent summary:
• The article did not address that nicotine is consumed in multiple ways, such as vaping, snuff, chewing tobacco, and hookah
• The safety of varenicline appears fair when psychiatric illness is well controlled but can be problematic (and even severely detrimental) when mental illness is not well controlled. This should not be glossed over, especially since it was the reason for the original black-box warning (for risks including behavioral impulsivity, suicidality, severe insomnia, and nightmares) that was removed in 2016
• Patients with severe mental illness may not fully understand the risks, benefits, and priorities of the treatment intervention. The importance of psychiatric and internal medicine in addition to pharmacy follow-up is critical and needs to be documented.
Varenicline has been contextualized in its current role as a first-line treatment for smoking cessation. By bypassing a sizeable population of patients who have unstable psychiatric illness (especially bipolar I disorder), the path has been opened for risky “off-label” varenicline prescribing to this population by internists, who should be very cautious and prudent about prescribing for such patients. This alone is probably a good reason to reinstate the black-box warning.
Interestingly, one review found that only 1 of 11 patients receiving varenicline stopped smoking.1 Not dramatically beneficial for a first-line treatment! Decreasing smoking occurs as well and is more robust with combinational use with bupropion, nicotine replacement therapy, and cognitive-behavioral therapy.
If we are focusing on patients with unstable mental illness—who are seen primarily by psychiatrists—adherence, urgency of intervention, and context regarding acute safety for this population must be seen as top priorities.
So-called “second-line” treatment options must also be considered. Sandiego et al3 make excellent points regarding the role of alpha-adrenergic agonists such as guanfacine, which have been shown to be helpful in smoking cessation. They work by decreasing cortical dopamine release and their calming effects on the noradrenergic system, which may decrease smoking precipitated by stress. For the particularly challenging subpopulation of unstable smokers, the combination of varenicline plus guanfacine ER may turn out to be a game-changer.
Varenicline has not proven itself to be useful in patients who are severely mentally ill, and due to its low success rate, expectations should remain tempered, pragmatically realistic, and safety-based.4,5 The bottom line is that in an unstable psychiatrically ill patient, interventions other than varenicline should be first-line.
Murray et al have written a timely, thoughtful, and useful article (“Smoking cessation: Varenicline and the risk of neuropsychiatric adverse events,”
Just a few caveats regarding Murray et al’s excellent summary:
• The article did not address that nicotine is consumed in multiple ways, such as vaping, snuff, chewing tobacco, and hookah
• The safety of varenicline appears fair when psychiatric illness is well controlled but can be problematic (and even severely detrimental) when mental illness is not well controlled. This should not be glossed over, especially since it was the reason for the original black-box warning (for risks including behavioral impulsivity, suicidality, severe insomnia, and nightmares) that was removed in 2016
• Patients with severe mental illness may not fully understand the risks, benefits, and priorities of the treatment intervention. The importance of psychiatric and internal medicine in addition to pharmacy follow-up is critical and needs to be documented.
Varenicline has been contextualized in its current role as a first-line treatment for smoking cessation. By bypassing a sizeable population of patients who have unstable psychiatric illness (especially bipolar I disorder), the path has been opened for risky “off-label” varenicline prescribing to this population by internists, who should be very cautious and prudent about prescribing for such patients. This alone is probably a good reason to reinstate the black-box warning.
Interestingly, one review found that only 1 of 11 patients receiving varenicline stopped smoking.1 Not dramatically beneficial for a first-line treatment! Decreasing smoking occurs as well and is more robust with combinational use with bupropion, nicotine replacement therapy, and cognitive-behavioral therapy.
If we are focusing on patients with unstable mental illness—who are seen primarily by psychiatrists—adherence, urgency of intervention, and context regarding acute safety for this population must be seen as top priorities.
So-called “second-line” treatment options must also be considered. Sandiego et al3 make excellent points regarding the role of alpha-adrenergic agonists such as guanfacine, which have been shown to be helpful in smoking cessation. They work by decreasing cortical dopamine release and their calming effects on the noradrenergic system, which may decrease smoking precipitated by stress. For the particularly challenging subpopulation of unstable smokers, the combination of varenicline plus guanfacine ER may turn out to be a game-changer.
Varenicline has not proven itself to be useful in patients who are severely mentally ill, and due to its low success rate, expectations should remain tempered, pragmatically realistic, and safety-based.4,5 The bottom line is that in an unstable psychiatrically ill patient, interventions other than varenicline should be first-line.
1. Crawford P, Cieslak D. Varenicline for smoking cessation. Am Fam Physician. 2017;96(5).
2. Beard E, Jackson SE, Anthenelli RM, et al. Estimation of risk of neuropsychiatric adverse events from varenicline, bupropion and nicotine patch versus placebo: secondary analysis of results from the EAGLES trial using Bayes factors. Addiction. 2021;116(10):2816-2824.
3. Sandiego CM, Matuskey D, Lavery M, et al. The effect of treatment with guanfacine, an alpha2 adrenergic agonist, on dopaminergic tone in tobacco smokers: an [11C]FLB457 PET study. Neuropsychopharmacology. 2018;43(5):1052-1058.
4. Sharma R, Alla K, Pfeffer D, et al. An appraisal of practice guidelines for smoking cessation in people with severe mental illness. Aust N Z J Psychiatry. 2017;51(11):1106-1120.
5. Tofler IR. Varenicline for smoking cessation in the bipolar patient. J Clin Psychiatry. 2015;76(5):625.
1. Crawford P, Cieslak D. Varenicline for smoking cessation. Am Fam Physician. 2017;96(5).
2. Beard E, Jackson SE, Anthenelli RM, et al. Estimation of risk of neuropsychiatric adverse events from varenicline, bupropion and nicotine patch versus placebo: secondary analysis of results from the EAGLES trial using Bayes factors. Addiction. 2021;116(10):2816-2824.
3. Sandiego CM, Matuskey D, Lavery M, et al. The effect of treatment with guanfacine, an alpha2 adrenergic agonist, on dopaminergic tone in tobacco smokers: an [11C]FLB457 PET study. Neuropsychopharmacology. 2018;43(5):1052-1058.
4. Sharma R, Alla K, Pfeffer D, et al. An appraisal of practice guidelines for smoking cessation in people with severe mental illness. Aust N Z J Psychiatry. 2017;51(11):1106-1120.
5. Tofler IR. Varenicline for smoking cessation in the bipolar patient. J Clin Psychiatry. 2015;76(5):625.
Faulty fences: Blood-brain barrier dysfunction in schizophrenia
The blood-brain barrier (BBB) is an essential barrier of closely spaced cells that regulates entry into the CNS. What passes should be highly regulated to protect the brain from potentially harmful peripheral cells or molecules from the rest of the body. However, research has revealed that the BBB is pathologically permeable in several disease states, including schizophrenia, epilepsy, traumatic brain injury, autism, and DiGeorge syndrome (22q11.2 deletion syndrome, which often presents with symptoms of schizophrenia).1,2 In this article, we discuss potential markers of BBB dysfunction, the consequences of a porous BBB, the effect of BBB permeability on microglial activation, and possible treatment implications.
Detecting a BBB leak
The BBB is composed of microvascular endothelial cell units. Adherens junctions, astrocyte endfeet, and pericytes are all part of these units, but tight junctions have the most significant role in BBB barrier function. Tight junction protein composition varies depending on the location of the endothelium. In the BBB, they are primarily composed of claudin-5, occludin, zonulin, and junction adhesion molecules (JAMs) (Figure). Claudins and occludins are especially important components of the tight junction because they span plasma membranes.3
Researchers began to suspect tight junction permeability in schizophrenia while searching for schizophrenia biomarkers. For example, S100B is a marker of astrocytic reactivity to damage. It is increased in schizophrenia, major depressive disorder, and bipolar disorder.4 Studies found elevated S100B specifically in drug-free patients with schizophrenia,5 which prompted research suggesting it could predict the severity of negative symptoms.6 The accuracy of S100B as a biomarker was later complicated by the finding that adipose tissue also secretes S100B. This is problematic due to the high rates of comorbid obesity in psychiatric populations.2
Perhaps a better biomarker is the ratio of albumin in the CSF vs that in peripheral serum. The CSF-to-blood albumin ratio (Q-Alb) is widely considered an acceptable marker of BBB dysfunction because albumin must cross the BBB to alter the ratio. Studies have found a high Q-Alb in neurodegenerative disorders such as multiple sclerosis as well as in schizophrenia, which suggests that some level of BBB dysfunction is occurring. Although the Q-Alb may change slightly when confounded by antipsychotic use or with CSF flow changes,2,4 both S100B and Q-Alb elevation are sufficient for further investigation into tight junction alteration in schizophrenia.
Claudin-5 is a promising factor in detecting BBB permeability. Claudin-5 is deleted in DiGeorge syndrome, which is highly comorbid with schizophrenia and psychosis.1 Mouse knockdown studies show that full suppression of claudin-5 results in psychotic symptoms before fatal seizures,2,7 but a partial absence may enable psychotic symptoms. The same study showed that normally continuous claudin-5 was patchy along blood vessels in the affected sample.7 Follow-up experiments suggest that loss of claudin-5 in schizophrenia is especially prominent in the hippocampus, and there is mixed evidence of a decrease in the prefrontal cortex.8
Outside of claudin-5 alone, JAM-A plays a more regulatory role. It is upstream from an enhancer protein gene that serves as a transcription factor for the claudin-5 promoter, so when JAM-A is deleted, there is less claudin-5.9 However, while this decrease in claudin-5 may be pathological, there could still be various upstream changes that lead to schizophrenia.
What are the consequences of a porous BBB?
Although it is well established that the BBB passes small molecules and solutes, there is significant evidence of inflammatory trafficking in disease states. The BBB moves proinflammatory cytokines, alters transporters, and may even let white blood cells (WBCs) pass through. Immune cell infiltration has different requirements depending on the cell type. T cells rely on integrins, vascular cell adhesion molecule 1 (vCAM1), and intercellular adhesion molecule 1 (iCAM1) for binding, rolling, adhering, crossing, and migration to sites of inflammation.10,11 Both iCAM1 and vCAM1 are elevated in schizophrenia compared to other psychiatric disorders (such as unipolar depression) and correlate with other biomarkers. For example, vCAM1, responsible for recruitment and crossing, is correlated with a high Q-Alb.12 Primarily produced by astrocytes and endothelial cells, iCAM1 plays the largest role in crossing the BBB and migration. Postmortem tissue demonstrates that cytokines upregulate iCAM1 mRNA at the BBB in schizophrenia.13 Increased cytokines are well documented in the inflammatory model of schizophrenia. Interestingly, decreasing claudin-5 also upregulates iCAM1 production.14 Therefore, low baseline claudin-5 may contribute to additional inflammation and symptoms.
Continue to: BBB permeability also results...
BBB permeability also results in a certain pattern of leukocyte and cytokine activity. Interleukin-1 (IL-1), IL-6, and tumor necrosis factor–alpha can all cross the BBB during neuroimmune inflammation,10 but there are abnormal heightened and sustained responses of these molecules in schizophrenia. IL-6 is a proinflammatory cytokine in both acute and chronic inflammation that is expressed by astrocytes, endothelial cells, and microglia.15 IL-6 and its soluble receptor are both elevated in schizophrenia and are associated with white matter degeneration16,17 and an increase in vCAM1.15 This implies that while neuroinflammation in schizophrenia is occurring, additional leukocytes are being recruited and secreting their own cytokines in a chronic destructive positive feedback loop. Meanwhile, atypical IL-10 levels can no longer maintain balanced levels of inflammatory molecules,16 which leads to reduced control of inflammation.
Genetics and immunohistochemistry suggest that the BBB allows the passage of excess B cells and T cells in schizophrenia. Cytokines from WBCs or the BBB during inflammation recruit these additional infiltrating lymphocytes. In gene-wide association studies, there are several genes in schizophrenia important for B cells and T cells in addition to inflammation that interact in a proinflammatory network.16 These cells are also diffusely found in the white matter18 and hippocampal tissue19 of patients with schizophrenia. Taken together, an increase in adhesion molecules, WBCs, and cytokine crosstalk supports a leaky BBB as an important component of the inflammatory model of schizophrenia.
The role of microglia in BBB dysfunction
The effect of BBB permeability on microglial activation is an important caveat in the current research. Although several reports have linked neuroinflammation to confirmed microglial activation in schizophrenia, there is not enough evidence to claim that the BBB alone is the missing link between these theories. Some research suggests that chronic release of cytokines such as IL-6 from macrophages and T cells could increase migration across the BBB for microglial activation.16,20 However, positron emission tomography has shown mixed results at best. Translocator protein (TSPO) is expressed by microglia that are actively secreting cytokines.21 Researchers tracking TSPO changes in relation to BBB alteration have not seen elevated binding in schizophrenia, change due to stage of disease course, or differentiation from low-grade inflammation.21-24 Moreover, TSPO may be confounded by antipsychotic use25 and microglial expression did not correlate with any changes in adhesion molecules.13 TSPO is not an ideal indicator of microglial activation due to BBB breakdown, but that does not bar the possibility of at least a partial contribution to the development of schizophrenia.
Corsi-Zuelli et al26 created a model that attempts to merge BBB permeability and microglial activation through a different medium—T regulatory cells (TRegs). They write that if TRegs mediate interactions between astrocytes and microglia, their hypofunction would impose a prolonged T cell response. The increased access to a high level of IL-6 and its soluble receptors may keep the TRegs hypofunctional in schizophrenia and promote T cell conversion to inflammatory cell types. Experimentally, TReg induction reversed some psychotic symptoms, and greater TReg expression was associated with fewer negative symptoms.26 In an already insufficient BBB, more access to cytokines and leukocytes would sustain inflammation and microglial secretions.
In addition to the issues described regarding the BBB, the blood-CSF barrier at the choroid plexus may also be insufficient in schizophrenia (Box27-31).
Box
The choroid plexus’ primary role is to make CSF, but it also secretes cytokines and to some extent serves as a barrier. Unlike the blood-brain barrier (BBB), the blood-CSF barrier is composed of endothelial cells with fenestrations as well as tight junctions, which make the blood-CSF barrier overall more permeable.27,28 The most unusual finding regarding the choroid plexus in schizophrenia is size. The choroid plexus is physically larger in patients with schizophrenia, and to a lesser extent, in their first-degree relatives.29 A larger choroid plexus is correlated with more severe cognitive symptoms, increased risk for psychosis via biological stress, and significantly higher interleukin-6 (IL-6).27,29 The increased thickness could be an attempt to compensate for hyperactivity and toxic processes in a permeable environment. More circulating cytokines such as IL-6 and tumor necrosis factor–alpha from microglia can trigger an increase in intercellular adhesion molecule 1, resulting in leukocyte attachment and entry.30 Less claudin-5 at the choroid plexus in schizophrenia implicates similar permissive effects as seen at the BBB.31 Although the contribution of blood-CSF barrier dysfunction to schizophrenia requires further study, reduced barrier function outside the BBB is a viable line of inquiry.
Continue to: Caveats about this research
Caveats about this research
There are 3 important points to note about the current research concerning abnormal BBB permeability:
1. BBB dysfunction may exist only in a subset of people diagnosed with schizophrenia. In most human studies, only some patients with schizophrenia demonstrated alterations that suggested pathological BBB permeability. In addition, even when there is BBB dysfunction, it could be a secondary phenomenon, rather than a primary etiologic process.
2. Patient demographics across studies have not always been adequately described. Potential confounds such as obesity, smoking, or antipsychotic use were not consistently recorded or examined as a possible factor.
3. Currently available biomarkers are not perfect. Cytokine elevation, S100B, and Q-Alb are indirect measures of BBB disruption and are found in other disorders. Therefore, they only support the theory of BBB dysfunction in schizophrenia, rather than prove it. They are also not reliable markers for schizophrenia alone. Researchers have pointed out that these markers and proteins work in concert, which necessitates a network analysis approach.16 More research regarding the details of permeability is required to establish more reliable biomarkers and tailored treatment.
Treatment implications
One of the first treatment directions that comes to mind is managing the gaps in the BBB via tight junctions. Presently, there are no FDA-approved medications for altering tight junction proteins, but researchers are exploring potential agents that can induce claudin-5 and reduce inflammation.14 While we wait for such a medication, patients may benefit from existing anti-inflammatory treatments to control immune infiltration and its products. Various anti-inflammatory agents—including cyclooxygenase inhibitors,
Bottom Line
Recent research has revealed that the blood-brain barrier (BBB) is pathologically permeable in several disease states, including schizophrenia. Better characterization of the leaky BBB in schizophrenia has enormous potential in helping us understand how current theories fit together and could serve as a missing puzzle piece in treating schizophrenia.
Related Resources
- Levine A, Strawn JR. The brain’s Twitter system: neuronal extracellular vesicles. Current Psychiatry. 2022;21(6):9-11, 17-19,27. doi:10.12788/cp.0257
Drug Brand Names
Minocycline • Dynacin, Minocin
1. Li Y, Xia Y, Zhu H, et al. Investigation of neurodevelopmental deficits of 22 q11.2 deletion syndrome with a patient-iPSC-derived blood-brain barrier model. Cells. 2021;10(10):2576. doi:10.3390/cells10102576
2. Kealy J, Greene C, Campbell M. Blood-brain barrier regulation in psychiatric disorders. Neurosci Lett. 2020;726:133664. doi:10.1016/j.neulet.2018.06.033
3. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1-13. doi:10.1016/j.nbd.2003.12.016
4. Futtrup J, Margolinsky R, Benros ME, et al. Blood-brain barrier pathology in patients with severe mental disorders: a systematic review and meta-analysis of biomarkers in case-control studies. Brain Behav Immun Health. 2020;6:100102. doi:10.1016/j.bbih.2020.100102
5. Chen S, Tian L, Chen N, et al. Cognitive dysfunction correlates with elevated serum S100B concentration in drug-free acutely relapsed patients with schizophrenia. Psychiatry Res. 2017;247:6-11. doi:10.1016/j.psychres.2016.09.029
6. Wu YF, Sytwu HK, Lung FW. Human aquaporin 4 gene polymorphisms and haplotypes are associated with serum S100B level and negative symptoms of schizophrenia in a southern Chinese Han population. Front Psychiatry. 2018;9:657. doi:10.3389/fpsyt.2018.00657
7. Greene C, Kealy J, Humphries MM, et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol Psychiatry. 2018;23(11):2156-2166. doi:10.1038/MP.2017.156
8. Greene C, Hanley N, Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry. 2020;10(1):373. doi:10.1038/s41398-020-01054-3
9. Kakogiannos N, Ferrari L, Giampietro C, et al. JAM-A acts via C/EBP-α to promote claudin-5 expression and enhance endothelial barrier function. Circ Res. 2020:1056-1073. doi:10.1161/CIRCRESAHA.120.316742
10. Erickson MA, Dohi K, Banks WA. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation. 2012;19(2):121-130. doi:10.1159/000330247
11. Ao LY, Yan YY, Zhou L, et al. Immune cells after ischemic stroke onset: roles, migration, and target intervention. J Mol Neurosci. 2018;66(3):342-355. doi:10.1007/s12031-018-1173-4
12. Meixensberger S, Kuzior H, Fiebich BL, et al. Upregulation of sICAM-1 and sVCAM-1 levels in the cerebrospinal fluid of patients with schizophrenia spectrum disorders. Diagnostics (Basel). 2021;11(7):1134. doi:10.3390diagnostics11071134
13. Cai HQ, Catts VS, Webster MJ, et al. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatry. 2020;25(4):761-775. doi:10.1038/s41380-018-0235-x
14. Greene C, Hanley N, Reschke CR, et al. Microvascular stabilization via blood-brain barrier regulation prevents seizure activity. Nat Commun. 2022;13(1):2003. doi:10.1038/s41467-022-29657-y
15. García-Juárez M, Camacho-Morales A. Defining the role of anti- and pro-inflammatory outcomes of interleukin-6 in mental health. Neuroscience. 2022;492:32-46. doi:10.1016/j.neuroscience.2022.03.020
16. Pong S, Karmacharya R, Sofman M, et al. The role of brain microvascular endothelial cell and blood-brain barrier dysfunction in schizophrenia. Complex Psychiatry. 2020;6(1-2):30-46. doi:10.1159/000511552
17. Patel A, Zhu Y, Kuzhikandathil EV, et al. Soluble interleukin-6 receptor induces motor stereotypies and co-localizes with gp130 in regions linked to cortico-striato-thalamo-cortical circuits. PLoS One. 2012;7(7): e41623. doi:10.1371/journal.pone.0041623
18. Schlaaff K, Dobrowolny H, Frodl T, et al. Increased densities of T and B lymphocytes indicate neuroinflammation in subgroups of schizophrenia and mood disorder patients. Brain Behav Immun. 2020;88:497-506. doi:10.1016/j.bbi.2020.04.021
19. Busse S, Busse M, Schiltz K, et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun. 2012;26(8):1273-1279. doi:10.1016/j.bbi.2012.08.005
20. Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277-286. doi:10.1016/j.pnpbp.2012.10.022
21. Conen S, Gregory CJ, Hinz R, et al. Neuroinflammation as measured by positron emission tomography in patients with recent onset and established schizophrenia: implications for immune pathogenesis. Mol Psychiatry. 2021;26(9):5398-5406. doi:10.1038/S41380-020-0829-Y
22. Najjar S, Pahlajani S, De Sanctis V, et al. Neurovascular unit dysfunction and blood-brain barrier hyperpermeability contribute to schizophrenia neurobiology: a theoretical integration of clinical and experimental evidence. Front Psychiatry. 2017;8:83. doi:10.3389/fpsyt.2017.00083
23. Pinjari OF, Dasgupta SK, Okusaga OO. Plasma soluble P-selectin, interleukin-6 and S100B protein in patients with schizophrenia: a pilot study. Psychiatr Q. 2022;93(1):335-345. doi:10.1007/s11126-021-09954-3
24. Di Biase MA, Zalesky A, O’keefe G, et al. PET imaging of putative microglial activation in individuals at ultra-high risk for psychosis, recently diagnosed and chronically ill with schizophrenia. Transl Psychiatry. 2017;7(8):e1225. doi:10.1038/tp.2017.193
25. Holmes SE, Hinz R, Drake RJ, et al. In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C](R)-PK11195 positron emission tomography study. Mol Psychiatry. 2016;21(12):1672-1679. doi:10.1038/mp.2016.180
26. Corsi-Zuelli F, Deakin B, de Lima MHF, et al. T regulatory cells as a potential therapeutic target in psychosis? Current challenges and future perspectives. Brain Behav Immun Health. 2021;17:100330. doi:10.1016/j.bbih.2021.100330
27. Bannai D, Lutz O, Lizano P. Neuroimaging considerations when investigating choroid plexus morphology in idiopathic psychosis. Schizophr Res. 2020;224:19-21. doi:10.1016/j.schres.2020.07.013
28. Hladky SB, Barrand MA. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS. 2016;13(1):19. doi:10.1186/s12987-016-0040-3
29. Lizano P, Lutz O, Ling G, et al. Association of choroid plexus enlargement with cognitive, inflammatory, and structural phenotypes across the psychosis spectrum. Am J Psychiatry. 2019;176(7):564-572. doi:10.1176/appi.ajp.2019.18070825
30. Castellani G, Contarini G, Mereu M, et al. Dopamine-mediated immunomodulation affects choroid plexus function. Brain Behav Immun. 2019;81:138-150. doi:10.1016/j.bbi.2019.06.006
31. Bitanihirwe BKY, Lizano P, Woo TW. Deconstructing the functional neuroanatomy of the choroid plexus: an ontogenetic perspective for studying neurodevelopmental and neuropsychiatric disorders. Mol Psychiatry. 2022;1-10. doi:10.1038/s41380-022-01623-6
The blood-brain barrier (BBB) is an essential barrier of closely spaced cells that regulates entry into the CNS. What passes should be highly regulated to protect the brain from potentially harmful peripheral cells or molecules from the rest of the body. However, research has revealed that the BBB is pathologically permeable in several disease states, including schizophrenia, epilepsy, traumatic brain injury, autism, and DiGeorge syndrome (22q11.2 deletion syndrome, which often presents with symptoms of schizophrenia).1,2 In this article, we discuss potential markers of BBB dysfunction, the consequences of a porous BBB, the effect of BBB permeability on microglial activation, and possible treatment implications.
Detecting a BBB leak
The BBB is composed of microvascular endothelial cell units. Adherens junctions, astrocyte endfeet, and pericytes are all part of these units, but tight junctions have the most significant role in BBB barrier function. Tight junction protein composition varies depending on the location of the endothelium. In the BBB, they are primarily composed of claudin-5, occludin, zonulin, and junction adhesion molecules (JAMs) (Figure). Claudins and occludins are especially important components of the tight junction because they span plasma membranes.3

Researchers began to suspect tight junction permeability in schizophrenia while searching for schizophrenia biomarkers. For example, S100B is a marker of astrocytic reactivity to damage. It is increased in schizophrenia, major depressive disorder, and bipolar disorder.4 Studies found elevated S100B specifically in drug-free patients with schizophrenia,5 which prompted research suggesting it could predict the severity of negative symptoms.6 The accuracy of S100B as a biomarker was later complicated by the finding that adipose tissue also secretes S100B. This is problematic due to the high rates of comorbid obesity in psychiatric populations.2
Perhaps a better biomarker is the ratio of albumin in the CSF vs that in peripheral serum. The CSF-to-blood albumin ratio (Q-Alb) is widely considered an acceptable marker of BBB dysfunction because albumin must cross the BBB to alter the ratio. Studies have found a high Q-Alb in neurodegenerative disorders such as multiple sclerosis as well as in schizophrenia, which suggests that some level of BBB dysfunction is occurring. Although the Q-Alb may change slightly when confounded by antipsychotic use or with CSF flow changes,2,4 both S100B and Q-Alb elevation are sufficient for further investigation into tight junction alteration in schizophrenia.
Claudin-5 is a promising factor in detecting BBB permeability. Claudin-5 is deleted in DiGeorge syndrome, which is highly comorbid with schizophrenia and psychosis.1 Mouse knockdown studies show that full suppression of claudin-5 results in psychotic symptoms before fatal seizures,2,7 but a partial absence may enable psychotic symptoms. The same study showed that normally continuous claudin-5 was patchy along blood vessels in the affected sample.7 Follow-up experiments suggest that loss of claudin-5 in schizophrenia is especially prominent in the hippocampus, and there is mixed evidence of a decrease in the prefrontal cortex.8
Outside of claudin-5 alone, JAM-A plays a more regulatory role. It is upstream from an enhancer protein gene that serves as a transcription factor for the claudin-5 promoter, so when JAM-A is deleted, there is less claudin-5.9 However, while this decrease in claudin-5 may be pathological, there could still be various upstream changes that lead to schizophrenia.
What are the consequences of a porous BBB?
Although it is well established that the BBB passes small molecules and solutes, there is significant evidence of inflammatory trafficking in disease states. The BBB moves proinflammatory cytokines, alters transporters, and may even let white blood cells (WBCs) pass through. Immune cell infiltration has different requirements depending on the cell type. T cells rely on integrins, vascular cell adhesion molecule 1 (vCAM1), and intercellular adhesion molecule 1 (iCAM1) for binding, rolling, adhering, crossing, and migration to sites of inflammation.10,11 Both iCAM1 and vCAM1 are elevated in schizophrenia compared to other psychiatric disorders (such as unipolar depression) and correlate with other biomarkers. For example, vCAM1, responsible for recruitment and crossing, is correlated with a high Q-Alb.12 Primarily produced by astrocytes and endothelial cells, iCAM1 plays the largest role in crossing the BBB and migration. Postmortem tissue demonstrates that cytokines upregulate iCAM1 mRNA at the BBB in schizophrenia.13 Increased cytokines are well documented in the inflammatory model of schizophrenia. Interestingly, decreasing claudin-5 also upregulates iCAM1 production.14 Therefore, low baseline claudin-5 may contribute to additional inflammation and symptoms.
Continue to: BBB permeability also results...
BBB permeability also results in a certain pattern of leukocyte and cytokine activity. Interleukin-1 (IL-1), IL-6, and tumor necrosis factor–alpha can all cross the BBB during neuroimmune inflammation,10 but there are abnormal heightened and sustained responses of these molecules in schizophrenia. IL-6 is a proinflammatory cytokine in both acute and chronic inflammation that is expressed by astrocytes, endothelial cells, and microglia.15 IL-6 and its soluble receptor are both elevated in schizophrenia and are associated with white matter degeneration16,17 and an increase in vCAM1.15 This implies that while neuroinflammation in schizophrenia is occurring, additional leukocytes are being recruited and secreting their own cytokines in a chronic destructive positive feedback loop. Meanwhile, atypical IL-10 levels can no longer maintain balanced levels of inflammatory molecules,16 which leads to reduced control of inflammation.
Genetics and immunohistochemistry suggest that the BBB allows the passage of excess B cells and T cells in schizophrenia. Cytokines from WBCs or the BBB during inflammation recruit these additional infiltrating lymphocytes. In gene-wide association studies, there are several genes in schizophrenia important for B cells and T cells in addition to inflammation that interact in a proinflammatory network.16 These cells are also diffusely found in the white matter18 and hippocampal tissue19 of patients with schizophrenia. Taken together, an increase in adhesion molecules, WBCs, and cytokine crosstalk supports a leaky BBB as an important component of the inflammatory model of schizophrenia.
The role of microglia in BBB dysfunction
The effect of BBB permeability on microglial activation is an important caveat in the current research. Although several reports have linked neuroinflammation to confirmed microglial activation in schizophrenia, there is not enough evidence to claim that the BBB alone is the missing link between these theories. Some research suggests that chronic release of cytokines such as IL-6 from macrophages and T cells could increase migration across the BBB for microglial activation.16,20 However, positron emission tomography has shown mixed results at best. Translocator protein (TSPO) is expressed by microglia that are actively secreting cytokines.21 Researchers tracking TSPO changes in relation to BBB alteration have not seen elevated binding in schizophrenia, change due to stage of disease course, or differentiation from low-grade inflammation.21-24 Moreover, TSPO may be confounded by antipsychotic use25 and microglial expression did not correlate with any changes in adhesion molecules.13 TSPO is not an ideal indicator of microglial activation due to BBB breakdown, but that does not bar the possibility of at least a partial contribution to the development of schizophrenia.
Corsi-Zuelli et al26 created a model that attempts to merge BBB permeability and microglial activation through a different medium—T regulatory cells (TRegs). They write that if TRegs mediate interactions between astrocytes and microglia, their hypofunction would impose a prolonged T cell response. The increased access to a high level of IL-6 and its soluble receptors may keep the TRegs hypofunctional in schizophrenia and promote T cell conversion to inflammatory cell types. Experimentally, TReg induction reversed some psychotic symptoms, and greater TReg expression was associated with fewer negative symptoms.26 In an already insufficient BBB, more access to cytokines and leukocytes would sustain inflammation and microglial secretions.
In addition to the issues described regarding the BBB, the blood-CSF barrier at the choroid plexus may also be insufficient in schizophrenia (Box27-31).
Box
The choroid plexus’ primary role is to make CSF, but it also secretes cytokines and to some extent serves as a barrier. Unlike the blood-brain barrier (BBB), the blood-CSF barrier is composed of endothelial cells with fenestrations as well as tight junctions, which make the blood-CSF barrier overall more permeable.27,28 The most unusual finding regarding the choroid plexus in schizophrenia is size. The choroid plexus is physically larger in patients with schizophrenia, and to a lesser extent, in their first-degree relatives.29 A larger choroid plexus is correlated with more severe cognitive symptoms, increased risk for psychosis via biological stress, and significantly higher interleukin-6 (IL-6).27,29 The increased thickness could be an attempt to compensate for hyperactivity and toxic processes in a permeable environment. More circulating cytokines such as IL-6 and tumor necrosis factor–alpha from microglia can trigger an increase in intercellular adhesion molecule 1, resulting in leukocyte attachment and entry.30 Less claudin-5 at the choroid plexus in schizophrenia implicates similar permissive effects as seen at the BBB.31 Although the contribution of blood-CSF barrier dysfunction to schizophrenia requires further study, reduced barrier function outside the BBB is a viable line of inquiry.
Continue to: Caveats about this research
Caveats about this research
There are 3 important points to note about the current research concerning abnormal BBB permeability:
1. BBB dysfunction may exist only in a subset of people diagnosed with schizophrenia. In most human studies, only some patients with schizophrenia demonstrated alterations that suggested pathological BBB permeability. In addition, even when there is BBB dysfunction, it could be a secondary phenomenon, rather than a primary etiologic process.
2. Patient demographics across studies have not always been adequately described. Potential confounds such as obesity, smoking, or antipsychotic use were not consistently recorded or examined as a possible factor.
3. Currently available biomarkers are not perfect. Cytokine elevation, S100B, and Q-Alb are indirect measures of BBB disruption and are found in other disorders. Therefore, they only support the theory of BBB dysfunction in schizophrenia, rather than prove it. They are also not reliable markers for schizophrenia alone. Researchers have pointed out that these markers and proteins work in concert, which necessitates a network analysis approach.16 More research regarding the details of permeability is required to establish more reliable biomarkers and tailored treatment.
Treatment implications
One of the first treatment directions that comes to mind is managing the gaps in the BBB via tight junctions. Presently, there are no FDA-approved medications for altering tight junction proteins, but researchers are exploring potential agents that can induce claudin-5 and reduce inflammation.14 While we wait for such a medication, patients may benefit from existing anti-inflammatory treatments to control immune infiltration and its products. Various anti-inflammatory agents—including cyclooxygenase inhibitors,
Bottom Line
Recent research has revealed that the blood-brain barrier (BBB) is pathologically permeable in several disease states, including schizophrenia. Better characterization of the leaky BBB in schizophrenia has enormous potential in helping us understand how current theories fit together and could serve as a missing puzzle piece in treating schizophrenia.
Related Resources
- Levine A, Strawn JR. The brain’s Twitter system: neuronal extracellular vesicles. Current Psychiatry. 2022;21(6):9-11, 17-19,27. doi:10.12788/cp.0257
Drug Brand Names
Minocycline • Dynacin, Minocin
The blood-brain barrier (BBB) is an essential barrier of closely spaced cells that regulates entry into the CNS. What passes should be highly regulated to protect the brain from potentially harmful peripheral cells or molecules from the rest of the body. However, research has revealed that the BBB is pathologically permeable in several disease states, including schizophrenia, epilepsy, traumatic brain injury, autism, and DiGeorge syndrome (22q11.2 deletion syndrome, which often presents with symptoms of schizophrenia).1,2 In this article, we discuss potential markers of BBB dysfunction, the consequences of a porous BBB, the effect of BBB permeability on microglial activation, and possible treatment implications.
Detecting a BBB leak
The BBB is composed of microvascular endothelial cell units. Adherens junctions, astrocyte endfeet, and pericytes are all part of these units, but tight junctions have the most significant role in BBB barrier function. Tight junction protein composition varies depending on the location of the endothelium. In the BBB, they are primarily composed of claudin-5, occludin, zonulin, and junction adhesion molecules (JAMs) (Figure). Claudins and occludins are especially important components of the tight junction because they span plasma membranes.3

Researchers began to suspect tight junction permeability in schizophrenia while searching for schizophrenia biomarkers. For example, S100B is a marker of astrocytic reactivity to damage. It is increased in schizophrenia, major depressive disorder, and bipolar disorder.4 Studies found elevated S100B specifically in drug-free patients with schizophrenia,5 which prompted research suggesting it could predict the severity of negative symptoms.6 The accuracy of S100B as a biomarker was later complicated by the finding that adipose tissue also secretes S100B. This is problematic due to the high rates of comorbid obesity in psychiatric populations.2
Perhaps a better biomarker is the ratio of albumin in the CSF vs that in peripheral serum. The CSF-to-blood albumin ratio (Q-Alb) is widely considered an acceptable marker of BBB dysfunction because albumin must cross the BBB to alter the ratio. Studies have found a high Q-Alb in neurodegenerative disorders such as multiple sclerosis as well as in schizophrenia, which suggests that some level of BBB dysfunction is occurring. Although the Q-Alb may change slightly when confounded by antipsychotic use or with CSF flow changes,2,4 both S100B and Q-Alb elevation are sufficient for further investigation into tight junction alteration in schizophrenia.
Claudin-5 is a promising factor in detecting BBB permeability. Claudin-5 is deleted in DiGeorge syndrome, which is highly comorbid with schizophrenia and psychosis.1 Mouse knockdown studies show that full suppression of claudin-5 results in psychotic symptoms before fatal seizures,2,7 but a partial absence may enable psychotic symptoms. The same study showed that normally continuous claudin-5 was patchy along blood vessels in the affected sample.7 Follow-up experiments suggest that loss of claudin-5 in schizophrenia is especially prominent in the hippocampus, and there is mixed evidence of a decrease in the prefrontal cortex.8
Outside of claudin-5 alone, JAM-A plays a more regulatory role. It is upstream from an enhancer protein gene that serves as a transcription factor for the claudin-5 promoter, so when JAM-A is deleted, there is less claudin-5.9 However, while this decrease in claudin-5 may be pathological, there could still be various upstream changes that lead to schizophrenia.
What are the consequences of a porous BBB?
Although it is well established that the BBB passes small molecules and solutes, there is significant evidence of inflammatory trafficking in disease states. The BBB moves proinflammatory cytokines, alters transporters, and may even let white blood cells (WBCs) pass through. Immune cell infiltration has different requirements depending on the cell type. T cells rely on integrins, vascular cell adhesion molecule 1 (vCAM1), and intercellular adhesion molecule 1 (iCAM1) for binding, rolling, adhering, crossing, and migration to sites of inflammation.10,11 Both iCAM1 and vCAM1 are elevated in schizophrenia compared to other psychiatric disorders (such as unipolar depression) and correlate with other biomarkers. For example, vCAM1, responsible for recruitment and crossing, is correlated with a high Q-Alb.12 Primarily produced by astrocytes and endothelial cells, iCAM1 plays the largest role in crossing the BBB and migration. Postmortem tissue demonstrates that cytokines upregulate iCAM1 mRNA at the BBB in schizophrenia.13 Increased cytokines are well documented in the inflammatory model of schizophrenia. Interestingly, decreasing claudin-5 also upregulates iCAM1 production.14 Therefore, low baseline claudin-5 may contribute to additional inflammation and symptoms.
Continue to: BBB permeability also results...
BBB permeability also results in a certain pattern of leukocyte and cytokine activity. Interleukin-1 (IL-1), IL-6, and tumor necrosis factor–alpha can all cross the BBB during neuroimmune inflammation,10 but there are abnormal heightened and sustained responses of these molecules in schizophrenia. IL-6 is a proinflammatory cytokine in both acute and chronic inflammation that is expressed by astrocytes, endothelial cells, and microglia.15 IL-6 and its soluble receptor are both elevated in schizophrenia and are associated with white matter degeneration16,17 and an increase in vCAM1.15 This implies that while neuroinflammation in schizophrenia is occurring, additional leukocytes are being recruited and secreting their own cytokines in a chronic destructive positive feedback loop. Meanwhile, atypical IL-10 levels can no longer maintain balanced levels of inflammatory molecules,16 which leads to reduced control of inflammation.
Genetics and immunohistochemistry suggest that the BBB allows the passage of excess B cells and T cells in schizophrenia. Cytokines from WBCs or the BBB during inflammation recruit these additional infiltrating lymphocytes. In gene-wide association studies, there are several genes in schizophrenia important for B cells and T cells in addition to inflammation that interact in a proinflammatory network.16 These cells are also diffusely found in the white matter18 and hippocampal tissue19 of patients with schizophrenia. Taken together, an increase in adhesion molecules, WBCs, and cytokine crosstalk supports a leaky BBB as an important component of the inflammatory model of schizophrenia.
The role of microglia in BBB dysfunction
The effect of BBB permeability on microglial activation is an important caveat in the current research. Although several reports have linked neuroinflammation to confirmed microglial activation in schizophrenia, there is not enough evidence to claim that the BBB alone is the missing link between these theories. Some research suggests that chronic release of cytokines such as IL-6 from macrophages and T cells could increase migration across the BBB for microglial activation.16,20 However, positron emission tomography has shown mixed results at best. Translocator protein (TSPO) is expressed by microglia that are actively secreting cytokines.21 Researchers tracking TSPO changes in relation to BBB alteration have not seen elevated binding in schizophrenia, change due to stage of disease course, or differentiation from low-grade inflammation.21-24 Moreover, TSPO may be confounded by antipsychotic use25 and microglial expression did not correlate with any changes in adhesion molecules.13 TSPO is not an ideal indicator of microglial activation due to BBB breakdown, but that does not bar the possibility of at least a partial contribution to the development of schizophrenia.
Corsi-Zuelli et al26 created a model that attempts to merge BBB permeability and microglial activation through a different medium—T regulatory cells (TRegs). They write that if TRegs mediate interactions between astrocytes and microglia, their hypofunction would impose a prolonged T cell response. The increased access to a high level of IL-6 and its soluble receptors may keep the TRegs hypofunctional in schizophrenia and promote T cell conversion to inflammatory cell types. Experimentally, TReg induction reversed some psychotic symptoms, and greater TReg expression was associated with fewer negative symptoms.26 In an already insufficient BBB, more access to cytokines and leukocytes would sustain inflammation and microglial secretions.
In addition to the issues described regarding the BBB, the blood-CSF barrier at the choroid plexus may also be insufficient in schizophrenia (Box27-31).
Box
The choroid plexus’ primary role is to make CSF, but it also secretes cytokines and to some extent serves as a barrier. Unlike the blood-brain barrier (BBB), the blood-CSF barrier is composed of endothelial cells with fenestrations as well as tight junctions, which make the blood-CSF barrier overall more permeable.27,28 The most unusual finding regarding the choroid plexus in schizophrenia is size. The choroid plexus is physically larger in patients with schizophrenia, and to a lesser extent, in their first-degree relatives.29 A larger choroid plexus is correlated with more severe cognitive symptoms, increased risk for psychosis via biological stress, and significantly higher interleukin-6 (IL-6).27,29 The increased thickness could be an attempt to compensate for hyperactivity and toxic processes in a permeable environment. More circulating cytokines such as IL-6 and tumor necrosis factor–alpha from microglia can trigger an increase in intercellular adhesion molecule 1, resulting in leukocyte attachment and entry.30 Less claudin-5 at the choroid plexus in schizophrenia implicates similar permissive effects as seen at the BBB.31 Although the contribution of blood-CSF barrier dysfunction to schizophrenia requires further study, reduced barrier function outside the BBB is a viable line of inquiry.
Continue to: Caveats about this research
Caveats about this research
There are 3 important points to note about the current research concerning abnormal BBB permeability:
1. BBB dysfunction may exist only in a subset of people diagnosed with schizophrenia. In most human studies, only some patients with schizophrenia demonstrated alterations that suggested pathological BBB permeability. In addition, even when there is BBB dysfunction, it could be a secondary phenomenon, rather than a primary etiologic process.
2. Patient demographics across studies have not always been adequately described. Potential confounds such as obesity, smoking, or antipsychotic use were not consistently recorded or examined as a possible factor.
3. Currently available biomarkers are not perfect. Cytokine elevation, S100B, and Q-Alb are indirect measures of BBB disruption and are found in other disorders. Therefore, they only support the theory of BBB dysfunction in schizophrenia, rather than prove it. They are also not reliable markers for schizophrenia alone. Researchers have pointed out that these markers and proteins work in concert, which necessitates a network analysis approach.16 More research regarding the details of permeability is required to establish more reliable biomarkers and tailored treatment.
Treatment implications
One of the first treatment directions that comes to mind is managing the gaps in the BBB via tight junctions. Presently, there are no FDA-approved medications for altering tight junction proteins, but researchers are exploring potential agents that can induce claudin-5 and reduce inflammation.14 While we wait for such a medication, patients may benefit from existing anti-inflammatory treatments to control immune infiltration and its products. Various anti-inflammatory agents—including cyclooxygenase inhibitors,
Bottom Line
Recent research has revealed that the blood-brain barrier (BBB) is pathologically permeable in several disease states, including schizophrenia. Better characterization of the leaky BBB in schizophrenia has enormous potential in helping us understand how current theories fit together and could serve as a missing puzzle piece in treating schizophrenia.
Related Resources
- Levine A, Strawn JR. The brain’s Twitter system: neuronal extracellular vesicles. Current Psychiatry. 2022;21(6):9-11, 17-19,27. doi:10.12788/cp.0257
Drug Brand Names
Minocycline • Dynacin, Minocin
1. Li Y, Xia Y, Zhu H, et al. Investigation of neurodevelopmental deficits of 22 q11.2 deletion syndrome with a patient-iPSC-derived blood-brain barrier model. Cells. 2021;10(10):2576. doi:10.3390/cells10102576
2. Kealy J, Greene C, Campbell M. Blood-brain barrier regulation in psychiatric disorders. Neurosci Lett. 2020;726:133664. doi:10.1016/j.neulet.2018.06.033
3. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1-13. doi:10.1016/j.nbd.2003.12.016
4. Futtrup J, Margolinsky R, Benros ME, et al. Blood-brain barrier pathology in patients with severe mental disorders: a systematic review and meta-analysis of biomarkers in case-control studies. Brain Behav Immun Health. 2020;6:100102. doi:10.1016/j.bbih.2020.100102
5. Chen S, Tian L, Chen N, et al. Cognitive dysfunction correlates with elevated serum S100B concentration in drug-free acutely relapsed patients with schizophrenia. Psychiatry Res. 2017;247:6-11. doi:10.1016/j.psychres.2016.09.029
6. Wu YF, Sytwu HK, Lung FW. Human aquaporin 4 gene polymorphisms and haplotypes are associated with serum S100B level and negative symptoms of schizophrenia in a southern Chinese Han population. Front Psychiatry. 2018;9:657. doi:10.3389/fpsyt.2018.00657
7. Greene C, Kealy J, Humphries MM, et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol Psychiatry. 2018;23(11):2156-2166. doi:10.1038/MP.2017.156
8. Greene C, Hanley N, Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry. 2020;10(1):373. doi:10.1038/s41398-020-01054-3
9. Kakogiannos N, Ferrari L, Giampietro C, et al. JAM-A acts via C/EBP-α to promote claudin-5 expression and enhance endothelial barrier function. Circ Res. 2020:1056-1073. doi:10.1161/CIRCRESAHA.120.316742
10. Erickson MA, Dohi K, Banks WA. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation. 2012;19(2):121-130. doi:10.1159/000330247
11. Ao LY, Yan YY, Zhou L, et al. Immune cells after ischemic stroke onset: roles, migration, and target intervention. J Mol Neurosci. 2018;66(3):342-355. doi:10.1007/s12031-018-1173-4
12. Meixensberger S, Kuzior H, Fiebich BL, et al. Upregulation of sICAM-1 and sVCAM-1 levels in the cerebrospinal fluid of patients with schizophrenia spectrum disorders. Diagnostics (Basel). 2021;11(7):1134. doi:10.3390diagnostics11071134
13. Cai HQ, Catts VS, Webster MJ, et al. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatry. 2020;25(4):761-775. doi:10.1038/s41380-018-0235-x
14. Greene C, Hanley N, Reschke CR, et al. Microvascular stabilization via blood-brain barrier regulation prevents seizure activity. Nat Commun. 2022;13(1):2003. doi:10.1038/s41467-022-29657-y
15. García-Juárez M, Camacho-Morales A. Defining the role of anti- and pro-inflammatory outcomes of interleukin-6 in mental health. Neuroscience. 2022;492:32-46. doi:10.1016/j.neuroscience.2022.03.020
16. Pong S, Karmacharya R, Sofman M, et al. The role of brain microvascular endothelial cell and blood-brain barrier dysfunction in schizophrenia. Complex Psychiatry. 2020;6(1-2):30-46. doi:10.1159/000511552
17. Patel A, Zhu Y, Kuzhikandathil EV, et al. Soluble interleukin-6 receptor induces motor stereotypies and co-localizes with gp130 in regions linked to cortico-striato-thalamo-cortical circuits. PLoS One. 2012;7(7): e41623. doi:10.1371/journal.pone.0041623
18. Schlaaff K, Dobrowolny H, Frodl T, et al. Increased densities of T and B lymphocytes indicate neuroinflammation in subgroups of schizophrenia and mood disorder patients. Brain Behav Immun. 2020;88:497-506. doi:10.1016/j.bbi.2020.04.021
19. Busse S, Busse M, Schiltz K, et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun. 2012;26(8):1273-1279. doi:10.1016/j.bbi.2012.08.005
20. Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277-286. doi:10.1016/j.pnpbp.2012.10.022
21. Conen S, Gregory CJ, Hinz R, et al. Neuroinflammation as measured by positron emission tomography in patients with recent onset and established schizophrenia: implications for immune pathogenesis. Mol Psychiatry. 2021;26(9):5398-5406. doi:10.1038/S41380-020-0829-Y
22. Najjar S, Pahlajani S, De Sanctis V, et al. Neurovascular unit dysfunction and blood-brain barrier hyperpermeability contribute to schizophrenia neurobiology: a theoretical integration of clinical and experimental evidence. Front Psychiatry. 2017;8:83. doi:10.3389/fpsyt.2017.00083
23. Pinjari OF, Dasgupta SK, Okusaga OO. Plasma soluble P-selectin, interleukin-6 and S100B protein in patients with schizophrenia: a pilot study. Psychiatr Q. 2022;93(1):335-345. doi:10.1007/s11126-021-09954-3
24. Di Biase MA, Zalesky A, O’keefe G, et al. PET imaging of putative microglial activation in individuals at ultra-high risk for psychosis, recently diagnosed and chronically ill with schizophrenia. Transl Psychiatry. 2017;7(8):e1225. doi:10.1038/tp.2017.193
25. Holmes SE, Hinz R, Drake RJ, et al. In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C](R)-PK11195 positron emission tomography study. Mol Psychiatry. 2016;21(12):1672-1679. doi:10.1038/mp.2016.180
26. Corsi-Zuelli F, Deakin B, de Lima MHF, et al. T regulatory cells as a potential therapeutic target in psychosis? Current challenges and future perspectives. Brain Behav Immun Health. 2021;17:100330. doi:10.1016/j.bbih.2021.100330
27. Bannai D, Lutz O, Lizano P. Neuroimaging considerations when investigating choroid plexus morphology in idiopathic psychosis. Schizophr Res. 2020;224:19-21. doi:10.1016/j.schres.2020.07.013
28. Hladky SB, Barrand MA. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS. 2016;13(1):19. doi:10.1186/s12987-016-0040-3
29. Lizano P, Lutz O, Ling G, et al. Association of choroid plexus enlargement with cognitive, inflammatory, and structural phenotypes across the psychosis spectrum. Am J Psychiatry. 2019;176(7):564-572. doi:10.1176/appi.ajp.2019.18070825
30. Castellani G, Contarini G, Mereu M, et al. Dopamine-mediated immunomodulation affects choroid plexus function. Brain Behav Immun. 2019;81:138-150. doi:10.1016/j.bbi.2019.06.006
31. Bitanihirwe BKY, Lizano P, Woo TW. Deconstructing the functional neuroanatomy of the choroid plexus: an ontogenetic perspective for studying neurodevelopmental and neuropsychiatric disorders. Mol Psychiatry. 2022;1-10. doi:10.1038/s41380-022-01623-6
1. Li Y, Xia Y, Zhu H, et al. Investigation of neurodevelopmental deficits of 22 q11.2 deletion syndrome with a patient-iPSC-derived blood-brain barrier model. Cells. 2021;10(10):2576. doi:10.3390/cells10102576
2. Kealy J, Greene C, Campbell M. Blood-brain barrier regulation in psychiatric disorders. Neurosci Lett. 2020;726:133664. doi:10.1016/j.neulet.2018.06.033
3. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1-13. doi:10.1016/j.nbd.2003.12.016
4. Futtrup J, Margolinsky R, Benros ME, et al. Blood-brain barrier pathology in patients with severe mental disorders: a systematic review and meta-analysis of biomarkers in case-control studies. Brain Behav Immun Health. 2020;6:100102. doi:10.1016/j.bbih.2020.100102
5. Chen S, Tian L, Chen N, et al. Cognitive dysfunction correlates with elevated serum S100B concentration in drug-free acutely relapsed patients with schizophrenia. Psychiatry Res. 2017;247:6-11. doi:10.1016/j.psychres.2016.09.029
6. Wu YF, Sytwu HK, Lung FW. Human aquaporin 4 gene polymorphisms and haplotypes are associated with serum S100B level and negative symptoms of schizophrenia in a southern Chinese Han population. Front Psychiatry. 2018;9:657. doi:10.3389/fpsyt.2018.00657
7. Greene C, Kealy J, Humphries MM, et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol Psychiatry. 2018;23(11):2156-2166. doi:10.1038/MP.2017.156
8. Greene C, Hanley N, Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry. 2020;10(1):373. doi:10.1038/s41398-020-01054-3
9. Kakogiannos N, Ferrari L, Giampietro C, et al. JAM-A acts via C/EBP-α to promote claudin-5 expression and enhance endothelial barrier function. Circ Res. 2020:1056-1073. doi:10.1161/CIRCRESAHA.120.316742
10. Erickson MA, Dohi K, Banks WA. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation. 2012;19(2):121-130. doi:10.1159/000330247
11. Ao LY, Yan YY, Zhou L, et al. Immune cells after ischemic stroke onset: roles, migration, and target intervention. J Mol Neurosci. 2018;66(3):342-355. doi:10.1007/s12031-018-1173-4
12. Meixensberger S, Kuzior H, Fiebich BL, et al. Upregulation of sICAM-1 and sVCAM-1 levels in the cerebrospinal fluid of patients with schizophrenia spectrum disorders. Diagnostics (Basel). 2021;11(7):1134. doi:10.3390diagnostics11071134
13. Cai HQ, Catts VS, Webster MJ, et al. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatry. 2020;25(4):761-775. doi:10.1038/s41380-018-0235-x
14. Greene C, Hanley N, Reschke CR, et al. Microvascular stabilization via blood-brain barrier regulation prevents seizure activity. Nat Commun. 2022;13(1):2003. doi:10.1038/s41467-022-29657-y
15. García-Juárez M, Camacho-Morales A. Defining the role of anti- and pro-inflammatory outcomes of interleukin-6 in mental health. Neuroscience. 2022;492:32-46. doi:10.1016/j.neuroscience.2022.03.020
16. Pong S, Karmacharya R, Sofman M, et al. The role of brain microvascular endothelial cell and blood-brain barrier dysfunction in schizophrenia. Complex Psychiatry. 2020;6(1-2):30-46. doi:10.1159/000511552
17. Patel A, Zhu Y, Kuzhikandathil EV, et al. Soluble interleukin-6 receptor induces motor stereotypies and co-localizes with gp130 in regions linked to cortico-striato-thalamo-cortical circuits. PLoS One. 2012;7(7): e41623. doi:10.1371/journal.pone.0041623
18. Schlaaff K, Dobrowolny H, Frodl T, et al. Increased densities of T and B lymphocytes indicate neuroinflammation in subgroups of schizophrenia and mood disorder patients. Brain Behav Immun. 2020;88:497-506. doi:10.1016/j.bbi.2020.04.021
19. Busse S, Busse M, Schiltz K, et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun. 2012;26(8):1273-1279. doi:10.1016/j.bbi.2012.08.005
20. Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277-286. doi:10.1016/j.pnpbp.2012.10.022
21. Conen S, Gregory CJ, Hinz R, et al. Neuroinflammation as measured by positron emission tomography in patients with recent onset and established schizophrenia: implications for immune pathogenesis. Mol Psychiatry. 2021;26(9):5398-5406. doi:10.1038/S41380-020-0829-Y
22. Najjar S, Pahlajani S, De Sanctis V, et al. Neurovascular unit dysfunction and blood-brain barrier hyperpermeability contribute to schizophrenia neurobiology: a theoretical integration of clinical and experimental evidence. Front Psychiatry. 2017;8:83. doi:10.3389/fpsyt.2017.00083
23. Pinjari OF, Dasgupta SK, Okusaga OO. Plasma soluble P-selectin, interleukin-6 and S100B protein in patients with schizophrenia: a pilot study. Psychiatr Q. 2022;93(1):335-345. doi:10.1007/s11126-021-09954-3
24. Di Biase MA, Zalesky A, O’keefe G, et al. PET imaging of putative microglial activation in individuals at ultra-high risk for psychosis, recently diagnosed and chronically ill with schizophrenia. Transl Psychiatry. 2017;7(8):e1225. doi:10.1038/tp.2017.193
25. Holmes SE, Hinz R, Drake RJ, et al. In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C](R)-PK11195 positron emission tomography study. Mol Psychiatry. 2016;21(12):1672-1679. doi:10.1038/mp.2016.180
26. Corsi-Zuelli F, Deakin B, de Lima MHF, et al. T regulatory cells as a potential therapeutic target in psychosis? Current challenges and future perspectives. Brain Behav Immun Health. 2021;17:100330. doi:10.1016/j.bbih.2021.100330
27. Bannai D, Lutz O, Lizano P. Neuroimaging considerations when investigating choroid plexus morphology in idiopathic psychosis. Schizophr Res. 2020;224:19-21. doi:10.1016/j.schres.2020.07.013
28. Hladky SB, Barrand MA. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS. 2016;13(1):19. doi:10.1186/s12987-016-0040-3
29. Lizano P, Lutz O, Ling G, et al. Association of choroid plexus enlargement with cognitive, inflammatory, and structural phenotypes across the psychosis spectrum. Am J Psychiatry. 2019;176(7):564-572. doi:10.1176/appi.ajp.2019.18070825
30. Castellani G, Contarini G, Mereu M, et al. Dopamine-mediated immunomodulation affects choroid plexus function. Brain Behav Immun. 2019;81:138-150. doi:10.1016/j.bbi.2019.06.006
31. Bitanihirwe BKY, Lizano P, Woo TW. Deconstructing the functional neuroanatomy of the choroid plexus: an ontogenetic perspective for studying neurodevelopmental and neuropsychiatric disorders. Mol Psychiatry. 2022;1-10. doi:10.1038/s41380-022-01623-6