User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
From smiling to smizing: Assessing the affect of a patient wearing a mask
Although the guidelines for masking in hospitals and other health care settings have been revised and face masks are no longer mandatory, it is important to note that some patients and clinicians will choose to continue wearing masks for various personal or clinical reasons. While effective in reducing transmission of the coronavirus, masks have created challenges in assessing patients’ affective states, which impacts the accuracy of diagnosis and treatment. This article discusses strategies for assessing affect in patients wearing face masks.
How masks complicate assessing affect
One obvious challenge masks present is they prevent clinicians from seeing their patients’ facial expressions. Face masks cover the mouth, nose, and cheeks, all of which are involved in communicating emotions. As a result, clinicians may miss important cues that could inform their assessment of a patient’s affect. For example, when a masked patient is smiling, it is difficult to determine whether their smile is genuine or forced. A study that evaluated the interpretation of 6 emotions (angry, disgusted, fearful, happy, neutral, and sad) in masked patients found that emotion recognition was significantly reduced for all emotions except for fearful and neutral faces.1
Another challenge is the potential for misinterpretation. Health care professionals may rely more heavily on nonverbal cues, such as body language, to interpret a patient’s affect. However, these cues can be influenced by other factors, such as cultural differences and individual variations in communication style. Culture is a key component in assessing nonverbal emotion reading cues.2
Strategies to overcome these challenges
There are several strategies clinicians can use to overcome the difficulties of assessing affect while a patient is wearing a mask:
Focus on other nonverbal cues, such as a patient’s posture and hand gestures. Verbal cues—such as tone of voice, choice of words, and voice inflection—can also provide valuable insights. For example, a patient who speaks in a hesitant or monotone voice may be experiencing anxiety or depression. Clinicians can ask open-ended questions, encouraging patients to expand on their emotions and provide further information about their affect.
Maintain eye contact. Eye contact is an essential component of nonverbal communication. The eyes are “the window of the soul” and can convey various emotions including happiness, sadness, fear, anger, surprise, trust, interest, and empathy. Maintaining eye contact is crucial for building positive relationships with patients, and learning to smile with your eyes (smize) can help build rapport.
Take advantage of technology. Clinicians can leverage telemedicine to assess affect. Telemedicine platforms, which have become increasingly popular during the COVID-19 pandemic, allow clinicians to monitor patients remotely and observe nonverbal cues. Virtual reality technology can also help by documenting physiological responses such as heart rate and skin conductance.
Use standardized assessment tools, as these instruments can aid in assessing affect. For example, the Patient Health Questionnaire-9 and Generalized Anxiety Disorder 7-item scale are standardized questionnaires assessing depression and anxiety, respectively. Administering these tools to patients wearing a face mask can provide information about their affective state.
1. Carbon CC. Wearing face masks strongly confuses counterparts in reading emotions. Front Psychol. 2020;11:566886. doi:10.3389/fpsyg.2020.566886
2. Yuki M, Maddux WW, Masuda T. Are the windows to the soul the same in the East and West? Cultural differences in using the eyes and mouth as cues to recognize emotions in Japan and the United States. J Exp Soc Psychol. 2007;43(2):303-311.
Although the guidelines for masking in hospitals and other health care settings have been revised and face masks are no longer mandatory, it is important to note that some patients and clinicians will choose to continue wearing masks for various personal or clinical reasons. While effective in reducing transmission of the coronavirus, masks have created challenges in assessing patients’ affective states, which impacts the accuracy of diagnosis and treatment. This article discusses strategies for assessing affect in patients wearing face masks.
How masks complicate assessing affect
One obvious challenge masks present is they prevent clinicians from seeing their patients’ facial expressions. Face masks cover the mouth, nose, and cheeks, all of which are involved in communicating emotions. As a result, clinicians may miss important cues that could inform their assessment of a patient’s affect. For example, when a masked patient is smiling, it is difficult to determine whether their smile is genuine or forced. A study that evaluated the interpretation of 6 emotions (angry, disgusted, fearful, happy, neutral, and sad) in masked patients found that emotion recognition was significantly reduced for all emotions except for fearful and neutral faces.1
Another challenge is the potential for misinterpretation. Health care professionals may rely more heavily on nonverbal cues, such as body language, to interpret a patient’s affect. However, these cues can be influenced by other factors, such as cultural differences and individual variations in communication style. Culture is a key component in assessing nonverbal emotion reading cues.2
Strategies to overcome these challenges
There are several strategies clinicians can use to overcome the difficulties of assessing affect while a patient is wearing a mask:
Focus on other nonverbal cues, such as a patient’s posture and hand gestures. Verbal cues—such as tone of voice, choice of words, and voice inflection—can also provide valuable insights. For example, a patient who speaks in a hesitant or monotone voice may be experiencing anxiety or depression. Clinicians can ask open-ended questions, encouraging patients to expand on their emotions and provide further information about their affect.
Maintain eye contact. Eye contact is an essential component of nonverbal communication. The eyes are “the window of the soul” and can convey various emotions including happiness, sadness, fear, anger, surprise, trust, interest, and empathy. Maintaining eye contact is crucial for building positive relationships with patients, and learning to smile with your eyes (smize) can help build rapport.
Take advantage of technology. Clinicians can leverage telemedicine to assess affect. Telemedicine platforms, which have become increasingly popular during the COVID-19 pandemic, allow clinicians to monitor patients remotely and observe nonverbal cues. Virtual reality technology can also help by documenting physiological responses such as heart rate and skin conductance.
Use standardized assessment tools, as these instruments can aid in assessing affect. For example, the Patient Health Questionnaire-9 and Generalized Anxiety Disorder 7-item scale are standardized questionnaires assessing depression and anxiety, respectively. Administering these tools to patients wearing a face mask can provide information about their affective state.
Although the guidelines for masking in hospitals and other health care settings have been revised and face masks are no longer mandatory, it is important to note that some patients and clinicians will choose to continue wearing masks for various personal or clinical reasons. While effective in reducing transmission of the coronavirus, masks have created challenges in assessing patients’ affective states, which impacts the accuracy of diagnosis and treatment. This article discusses strategies for assessing affect in patients wearing face masks.
How masks complicate assessing affect
One obvious challenge masks present is they prevent clinicians from seeing their patients’ facial expressions. Face masks cover the mouth, nose, and cheeks, all of which are involved in communicating emotions. As a result, clinicians may miss important cues that could inform their assessment of a patient’s affect. For example, when a masked patient is smiling, it is difficult to determine whether their smile is genuine or forced. A study that evaluated the interpretation of 6 emotions (angry, disgusted, fearful, happy, neutral, and sad) in masked patients found that emotion recognition was significantly reduced for all emotions except for fearful and neutral faces.1
Another challenge is the potential for misinterpretation. Health care professionals may rely more heavily on nonverbal cues, such as body language, to interpret a patient’s affect. However, these cues can be influenced by other factors, such as cultural differences and individual variations in communication style. Culture is a key component in assessing nonverbal emotion reading cues.2
Strategies to overcome these challenges
There are several strategies clinicians can use to overcome the difficulties of assessing affect while a patient is wearing a mask:
Focus on other nonverbal cues, such as a patient’s posture and hand gestures. Verbal cues—such as tone of voice, choice of words, and voice inflection—can also provide valuable insights. For example, a patient who speaks in a hesitant or monotone voice may be experiencing anxiety or depression. Clinicians can ask open-ended questions, encouraging patients to expand on their emotions and provide further information about their affect.
Maintain eye contact. Eye contact is an essential component of nonverbal communication. The eyes are “the window of the soul” and can convey various emotions including happiness, sadness, fear, anger, surprise, trust, interest, and empathy. Maintaining eye contact is crucial for building positive relationships with patients, and learning to smile with your eyes (smize) can help build rapport.
Take advantage of technology. Clinicians can leverage telemedicine to assess affect. Telemedicine platforms, which have become increasingly popular during the COVID-19 pandemic, allow clinicians to monitor patients remotely and observe nonverbal cues. Virtual reality technology can also help by documenting physiological responses such as heart rate and skin conductance.
Use standardized assessment tools, as these instruments can aid in assessing affect. For example, the Patient Health Questionnaire-9 and Generalized Anxiety Disorder 7-item scale are standardized questionnaires assessing depression and anxiety, respectively. Administering these tools to patients wearing a face mask can provide information about their affective state.
1. Carbon CC. Wearing face masks strongly confuses counterparts in reading emotions. Front Psychol. 2020;11:566886. doi:10.3389/fpsyg.2020.566886
2. Yuki M, Maddux WW, Masuda T. Are the windows to the soul the same in the East and West? Cultural differences in using the eyes and mouth as cues to recognize emotions in Japan and the United States. J Exp Soc Psychol. 2007;43(2):303-311.
1. Carbon CC. Wearing face masks strongly confuses counterparts in reading emotions. Front Psychol. 2020;11:566886. doi:10.3389/fpsyg.2020.566886
2. Yuki M, Maddux WW, Masuda T. Are the windows to the soul the same in the East and West? Cultural differences in using the eyes and mouth as cues to recognize emotions in Japan and the United States. J Exp Soc Psychol. 2007;43(2):303-311.
Homelessness in urban areas: The role of mental illness and need for collaboration
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
As an emergency department (ED) psychiatrist with 25 years of experience working in a large city, I am growing increasingly concerned about the escalating number of individuals experiencing homelessness in urban areas.
Homelessness remains a critical issue across the United States. The news reports from major urban areas are startling. In my own practice, I encounter approximately 10,000 patients annually, and at least one-half of them are homeless. Additionally, 75% of these patients who are homeless experience addiction, and many have lost all social support. Due to overcrowding at our area’s shelters, they resort to using the ED as a shelter because most of our shelters are overcrowded. This situation has caused an overwhelming overload in the ED and left staff disheartened and difficult to retain.
The relationship between mental illness and homelessness is complex and multifaceted. Research suggests that up to one-third of individuals who are homeless have serious mental illness.1 Mental illness can contribute to homelessness by impeding individuals’ ability to maintain employment, housing, and social relationships. Conversely, homelessness can worsen mental illness (especially in younger individuals, who are most vulnerable) by exposing individuals to traumatic experiences, substance abuse, and other stressors.2
One approach to effectively address homelessness in urban areas is provide supportive housing that incorporates access to mental health services. Research has demonstrated that offering stable housing and mental health services to individuals experiencing homelessness can significantly improve their mental and physical health and reduce their reliance on costly emergency services.3,4
Collaboration between the health care system and government is also essential. By working together, the health care system and government can develop comprehensive strategies, allocate resources, and implement interventions that address the physical and mental health needs of individuals who are homeless and provide them with the necessary support and services. This collaboration is essential to create sustainable solutions and make a meaningful impact in combating homelessness.5
Addressing homelessness in urban areas requires a comprehensive approach that recognizes the critical role of mental illness and necessity for collaborative solutions. While our ED has implemented certain measures, such as allowing patients to remain on 23-hour holds to prevent immediate re-admission, additional interventions are needed. These include expanding shelters and transitional housing programs, which are currently in short supply, and developing street medicine programs to meet individuals where they are and improve compliance with medications. By implementing these strategies, we can help minimize the impact of homelessness on individuals with mental illness and enhance the health and well-being of individuals experiencing homelessness.
1. Folsom DP, Hawthorne W, Lindamer L, et al. Prevalence and risk factors for homelessness and utilization of mental health services among 10,340 patients with serious mental illness in a large public mental health system. Am J Psychiatry. 2005;162(2):370-376. doi:10.1176/appi.ajp.162.2.370
2. Davis JP, Diguiseppi G, De Leon J, et al. Understanding pathways between PTSD, homelessness, and substance use among adolescents. Psychol Addict Behav. 2019;33(5):467-476. doi:10.1037/adb0000488
3. Larimer ME, Malone DK, Garner MD, et al. Health care and public service use and costs before and after provision of housing for chronically homeless persons with severe alcohol problems. JAMA. 2009;301(13):1349-1357. doi:10.1001/jama.2009.414
4. Wolitski RJ, Kidder DP, Pals SL, et al; Housing and Health Study Team. Randomized trial of the effects of housing assistance on the health and risk behaviors of homeless and unstably housed people living with HIV. AIDS Behav. 2010;14(3):493-503. doi:10.1007/s10461-009-9643-x
5. Sleet DA, Francescutti LH. Homelessness and public health: a focus on strategies and solutions. Int J Environ Res Public Health. 2021;18(21):11660. doi:10.3390/ijerph182111660
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
As an emergency department (ED) psychiatrist with 25 years of experience working in a large city, I am growing increasingly concerned about the escalating number of individuals experiencing homelessness in urban areas.
Homelessness remains a critical issue across the United States. The news reports from major urban areas are startling. In my own practice, I encounter approximately 10,000 patients annually, and at least one-half of them are homeless. Additionally, 75% of these patients who are homeless experience addiction, and many have lost all social support. Due to overcrowding at our area’s shelters, they resort to using the ED as a shelter because most of our shelters are overcrowded. This situation has caused an overwhelming overload in the ED and left staff disheartened and difficult to retain.
The relationship between mental illness and homelessness is complex and multifaceted. Research suggests that up to one-third of individuals who are homeless have serious mental illness.1 Mental illness can contribute to homelessness by impeding individuals’ ability to maintain employment, housing, and social relationships. Conversely, homelessness can worsen mental illness (especially in younger individuals, who are most vulnerable) by exposing individuals to traumatic experiences, substance abuse, and other stressors.2
One approach to effectively address homelessness in urban areas is provide supportive housing that incorporates access to mental health services. Research has demonstrated that offering stable housing and mental health services to individuals experiencing homelessness can significantly improve their mental and physical health and reduce their reliance on costly emergency services.3,4
Collaboration between the health care system and government is also essential. By working together, the health care system and government can develop comprehensive strategies, allocate resources, and implement interventions that address the physical and mental health needs of individuals who are homeless and provide them with the necessary support and services. This collaboration is essential to create sustainable solutions and make a meaningful impact in combating homelessness.5
Addressing homelessness in urban areas requires a comprehensive approach that recognizes the critical role of mental illness and necessity for collaborative solutions. While our ED has implemented certain measures, such as allowing patients to remain on 23-hour holds to prevent immediate re-admission, additional interventions are needed. These include expanding shelters and transitional housing programs, which are currently in short supply, and developing street medicine programs to meet individuals where they are and improve compliance with medications. By implementing these strategies, we can help minimize the impact of homelessness on individuals with mental illness and enhance the health and well-being of individuals experiencing homelessness.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
As an emergency department (ED) psychiatrist with 25 years of experience working in a large city, I am growing increasingly concerned about the escalating number of individuals experiencing homelessness in urban areas.
Homelessness remains a critical issue across the United States. The news reports from major urban areas are startling. In my own practice, I encounter approximately 10,000 patients annually, and at least one-half of them are homeless. Additionally, 75% of these patients who are homeless experience addiction, and many have lost all social support. Due to overcrowding at our area’s shelters, they resort to using the ED as a shelter because most of our shelters are overcrowded. This situation has caused an overwhelming overload in the ED and left staff disheartened and difficult to retain.
The relationship between mental illness and homelessness is complex and multifaceted. Research suggests that up to one-third of individuals who are homeless have serious mental illness.1 Mental illness can contribute to homelessness by impeding individuals’ ability to maintain employment, housing, and social relationships. Conversely, homelessness can worsen mental illness (especially in younger individuals, who are most vulnerable) by exposing individuals to traumatic experiences, substance abuse, and other stressors.2
One approach to effectively address homelessness in urban areas is provide supportive housing that incorporates access to mental health services. Research has demonstrated that offering stable housing and mental health services to individuals experiencing homelessness can significantly improve their mental and physical health and reduce their reliance on costly emergency services.3,4
Collaboration between the health care system and government is also essential. By working together, the health care system and government can develop comprehensive strategies, allocate resources, and implement interventions that address the physical and mental health needs of individuals who are homeless and provide them with the necessary support and services. This collaboration is essential to create sustainable solutions and make a meaningful impact in combating homelessness.5
Addressing homelessness in urban areas requires a comprehensive approach that recognizes the critical role of mental illness and necessity for collaborative solutions. While our ED has implemented certain measures, such as allowing patients to remain on 23-hour holds to prevent immediate re-admission, additional interventions are needed. These include expanding shelters and transitional housing programs, which are currently in short supply, and developing street medicine programs to meet individuals where they are and improve compliance with medications. By implementing these strategies, we can help minimize the impact of homelessness on individuals with mental illness and enhance the health and well-being of individuals experiencing homelessness.
1. Folsom DP, Hawthorne W, Lindamer L, et al. Prevalence and risk factors for homelessness and utilization of mental health services among 10,340 patients with serious mental illness in a large public mental health system. Am J Psychiatry. 2005;162(2):370-376. doi:10.1176/appi.ajp.162.2.370
2. Davis JP, Diguiseppi G, De Leon J, et al. Understanding pathways between PTSD, homelessness, and substance use among adolescents. Psychol Addict Behav. 2019;33(5):467-476. doi:10.1037/adb0000488
3. Larimer ME, Malone DK, Garner MD, et al. Health care and public service use and costs before and after provision of housing for chronically homeless persons with severe alcohol problems. JAMA. 2009;301(13):1349-1357. doi:10.1001/jama.2009.414
4. Wolitski RJ, Kidder DP, Pals SL, et al; Housing and Health Study Team. Randomized trial of the effects of housing assistance on the health and risk behaviors of homeless and unstably housed people living with HIV. AIDS Behav. 2010;14(3):493-503. doi:10.1007/s10461-009-9643-x
5. Sleet DA, Francescutti LH. Homelessness and public health: a focus on strategies and solutions. Int J Environ Res Public Health. 2021;18(21):11660. doi:10.3390/ijerph182111660
1. Folsom DP, Hawthorne W, Lindamer L, et al. Prevalence and risk factors for homelessness and utilization of mental health services among 10,340 patients with serious mental illness in a large public mental health system. Am J Psychiatry. 2005;162(2):370-376. doi:10.1176/appi.ajp.162.2.370
2. Davis JP, Diguiseppi G, De Leon J, et al. Understanding pathways between PTSD, homelessness, and substance use among adolescents. Psychol Addict Behav. 2019;33(5):467-476. doi:10.1037/adb0000488
3. Larimer ME, Malone DK, Garner MD, et al. Health care and public service use and costs before and after provision of housing for chronically homeless persons with severe alcohol problems. JAMA. 2009;301(13):1349-1357. doi:10.1001/jama.2009.414
4. Wolitski RJ, Kidder DP, Pals SL, et al; Housing and Health Study Team. Randomized trial of the effects of housing assistance on the health and risk behaviors of homeless and unstably housed people living with HIV. AIDS Behav. 2010;14(3):493-503. doi:10.1007/s10461-009-9643-x
5. Sleet DA, Francescutti LH. Homelessness and public health: a focus on strategies and solutions. Int J Environ Res Public Health. 2021;18(21):11660. doi:10.3390/ijerph182111660
More on an asymmetric life, transient global amnesia
More on an asymmetric life
I enjoy receiving
Too often, families bear the burden of an individual’s hyperfocused pursuits. I hope your wife has been able to pursue her occupation with the same zeal and commitment. We have all read biographies of driven individuals and, unfortunately, someone pays the price for another’s success. For every Steve Jobs, there is a Lisa Jobs.
If we were surgeons, I would applaud your essay. However, we are psychiatrists. If anything, we balance out the reductionist forces in medicine. When every other physician claims a cure with medications or procedures, we look at all aspects of the patient’s life to find the appropriate treatment. At least that’s what we should be doing.
I was part of the first class of residents to work under the 80-hours-per-week restrictions. I was grateful for the extra time to rest, exercise, and spend time with my wife. The 80-hour restrictions improved resident wellness and had no impact on patient care. There are intangible benefits of diverting the mind from a chosen pursuit (such as creativity).
There is no doubt that becoming number 1 in any field requires a tremendous amount of determination, sacrifice, and effort. But not everyone gets to be first. Our society’s single-minded focus on being the best has had a major impact on mental health, especially for children. I hope you can address that in a future editorial.
Sudhir Nagaraja, DO, MS
Fredericksburg, Virginia
Dr. Nasrallah responds
Thank you for your letter about my editorial. You obviously believe in leading a balanced life, and that’s fine if you so choose. I described why I decided at an early age to lead an intensive, “purpose-driven life,” which requires investing much more time than others do, to achieve my lofty goals and excel in my area of expertise (academic psychiatry). It is really a “calling,” and those who score an extraordinary achievement (a moonshot) in their life, including Olympic gold medalists, entrepreneurs, inventors, or Nobel laureates, must do exactly what I do. I am not urging anyone to do what I have chosen to do in my life. Everyone defines for themselves what constitutes the pursuit of happiness.
You mentioned my wife. Let me assert that she is highly successful as a mother and as a research psychologist. She is my extremely valuable life partner and very supportive of what I do. I am fortunate to have chosen well!
Continue to: More on transient global amnesia
More on transient global amnesia
Your recent article on transient global amnesia (TGA) (“Transient global amnesia: Psychiatric precipitants, features, and comorbidities,”
I witnessed TGA, experienced by my brother, while on a surf trip. After bodyboarding for about an hour in cold water, wearing a full wet suit and hood, he met me on the beach. He recognized me and knew my name but had no idea where we were, how we got there, or other events from earlier that morning. There was no stressor, just the usual surfing excitement. We went to a local emergency department, where the physical examination, usual laboratory tests, and neuroimaging were normal. After approximately 5 hours, he began to fully recall recent events. Ten years later, there has been no recurrence. The only change in his surfing habits has been to avoid using a hood with neck coverage.
In 2022, Papadis et al1 described a case of concurrent Takotsubo cardiomyopathy and TGA, noting that cardiovascular dysfunction and neurologic dysfunction may be provoked by an emotional or stressful situation. The interesting observations of capture myopathy from animal literature appear similar to human reactions to trauma.1-3
Case reports of scopolamine intoxication have been linked to TGA. Severe memory disturbances, characteristics of dry mouth, blurred vision, and tachycardia were evident. Certain South American plant extracts popularly known as “Burundanga” have anticholinergic effects. Severe anterograde amnesia and submissiveness represent the 2 most notorious clinical signs of Burundanga intoxication.4
As one reviews single and groups of case studies, several things stand out. The hallmark of TGA is the sudden inability to make new memories, which resolves in a few hours. The brief and isolated dysfunction is what distinguishes this condition from most episodic disorders, but a clinician should not prognosticate too much without screening for ischemic or metabolic disturbance. Common associated precursors include Valsalva-associated activities, emotional stress with anxiety, acute pain, cold water immersion, static neck posture, and age older than 55.5,6
Neuropsychiatric disorders involve the neuron and its connections. Major reflexes automate the processes of the “neurocardiac” axis. The vasovagal reflex (Barcroft/Edholm reflex), diving reflex, baroreceptor reflex, Cushing reflex, and others depend upon the conversion of a mechanical stimulus to neurotransmission. The reflexes have sensors, afferent paths, a central processing, and efferent paths that lead to events or experiences. CNS processing is complex but the brainstem, amygdala, prefrontal cortex, and some cortical regions are involved. Neurocardiac reactions can come from pathologic events, including ischemia, metabolic disturbance, pain signals, or emotional effects within the axis.7-11
Understanding neurocardiac reflexes may help our progress with challenging clinical conditions, such as chronic pain, trauma, and cognitive impairment. The broad use of vagus nerve stimulation is one indicator of the power of this focus.12-19 Lewis20 suggested increased susceptibility to retrograde jugular venous flow could cause regional brain ischemia, resulting in TGA. The competency of jugular venous valves during the Valsalva maneuver could be assessed with Doppler ultrasound. Abnormalities could be managed, and results assessed.20,21 Vascular shunting from memory regions in the brain to essential neurocardiac control areas should be considered.
Cholinergic processes are active in the parasympathetic nervous system, sustained attention, working memory, executive functions, and mood. Increased central cholinergic activity may lead to depression. Scopolamine, in its therapeutic range, has antidepressant effects but in toxic doses is a dissociative agent.22,23 While cholinesterase inhibitors are used in Alzheimer disease, cholinergic agonists have yet to play a large role in general psychiatry or functional neurology.
TGA continues to provide a window into memory, functional disorders, psychological defenses, and adaptive neurocardiac processes. Continued clinical care and research might include gradual adaptation to cold water immersion, caution with the Valsalva maneuver, cholinergic support, managing the trapped response, avoiding interference with normal jugular flow, and evaluation for jugular venous insufficiency.
Because a variety of medical procedures can trigger TGA, health care professionals in many fields need to understand this symptom complex.24-27 Thanks to the authors for raising the awareness of TGA for psychiatrists.
Mark Chandler, MD
Durham, North Carolina
References
1. Papadis A, Svab S, Brugger N, et al. “Broken heart” and “broken brain”: which connection? Cardiol Res. 2022;13(1):65-70. doi:10.14740/cr1336
2. Blumstein DT, Buckner J, Shah S, et al. The evolution of capture myopathy in hooved mammals: a model for human stress cardiomyopathy? Evol Med Public Health. 2015;2015(1):195-203. doi:10.1093/emph/eov015
3. Seguel M, Paredes E, Pavés H, et al. Capture-induced stress cardiomyopathy in South American fur seal pups (Arctophoca australis gracilis). Marine Mammal Science. 2014;30(3): 1149-1157. https://doi.org/10.1111/mms.12079
4. Ardila A, Moreno C. Scopolamine intoxication as a model of transient global amnesia. Brain Cogn. 1991;15(2):236-245. doi:10.1016/0278-2626(91)90028-7
5. Bartsch T, Deuschl G. Transient global amnesia: functional anatomy and clinical implications. Lancet Neurol. 2010;9(2):205-214. doi:10.1016/S1474-4422(09)70344-8
6. Spiegel DR, Smith J, Wade RR, et al. Transient global amnesia: current perspectives. Neuropsychiatr Dis Treat. 2017;13:2691-2703. doi:10.2147/NDT.S130710
7. Yartsev A. Cardiac reflexes. August 15, 2020. Updated May 19, 2023. Accessed June 12, 2023. https://derangedphysiology.com/main/cicm-primary-exam/required-reading/cardiovascular-system/Chapter%20491/cardiac-reflexes
8. Lemaitre F, Chowdhury T, Schaller B. The trigeminocardiac reflex - a comparison with the diving reflex in humans. Arch Med Sci. 2015;11(2):419-426. doi:10.5114/aoms.2015.50974
9. Lindholm P, Lundgren CE. The physiology and pathophysiology of human breath-hold diving. J Appl Physiol (1985). 2009;106(1):284-292. doi:10.1152/japplphysiol.90991.2008
10. Tansey EA, Johnson CD. Recent advances in thermoregulation. Adv Physiol Educ. 2015;39(3):139-148. doi:10.1152/advan.00126.2014
11. Alboni P, Alboni M. Vasovagal syncope as a manifestation of an evolutionary selected trait. J Atr Fibrillation. 2014;7(2):1035. doi:10.4022/jafib.1035
12. Badran BW, Austelle CW. The future is noninvasive: a brief review of the evolution and clinical utility of vagus nerve stimulation. Focus (Am Psychiatr Publ). 2022;20(1):3-7. doi:10.1176/appi.focus.20210023
13. Suarez-Roca H, Mamoun N, Sigurdson MI, et al. Baroreceptor modulation of the cardiovascular system, pain, consciousness, and cognition. Compr Physiol. 2021;11(2):1373-1423. doi:10.1002/cphy.c190038
14. Pinna T, Edwards DJ. A systematic review of associations between interoception, vagal tone, and emotional regulation: potential applications for mental health, wellbeing, psychological flexibility, and chronic conditions. Front Psychol. 2020;11:1792. doi:10.3389/fpsyg.2020.01792
15. Howland RH. Vagus nerve stimulation. Curr Behav Neurosci Rep. 2014 Jun;1(2):64-73. doi:10.1007/s40473-014-0010-5
16. Panneton WM, Gan Q. The mammalian diving response: inroads to its neural control. Front Neurosci. 2020;14:524. doi:10.3389/fnins.2020.00524
17. Khurana RK, Wu R. The cold face test: a non-baroreflex mediated test of cardiac vagal function. Clin Auton Res. 2006;16(3):202-207. doi:10.1007/s10286-006-0332-9
18. Montirosso R, Provenzi L, Tronick E, et al. Vagal tone as a biomarker of long-term memory for a stressful social event at 4 months. Dev Psychobiol. 2014;56(7):1564-1574. doi:10.1002/dev.21251
19. Hansen AL, Johnsen BH, Thayer JF. Vagal influence on working memory and attention. Int J Psychophysiol. 2003;48(3):263-274. doi:10.1016/s0167-8760(03)00073-4
20. Lewis SL. Aetiology of transient global amnesia. Lancet. 1998;352(9125):397-399. doi:10.1016/S0140-6736(98)01442-1
21. Han K, Chao AC, Chang FC, et al. Obstruction of venous drainage linked to transient global amnesia. PLoS One. 2015;10(7):e0132893. doi:10.1371/journal.pone.0132893
22. Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76(1):116-129. doi:10.1016/j.neuron.2012.08.036
23. Dulawa SC, Janowsky DS. Cholinergic regulation of mood: from basic and clinical studies to emerging therapeutics. Mol Psychiatry. 2019;24(5):694-709. doi:10.1038/s41380-018-0219-x
24. Grande LA, Loeser JD, Samii A. Recurrent transient global amnesia with intrathecal baclofen. Anesth Analg. 2008;106(4):1284-1287. doi:10.1213/ane.0b013e318165e1c6
25. Carrard J, Lambert AC, Genné D. Transient global amnesia following a whole-body cryotherapy session. BMJ Case Rep. 2017;2017:bcr2017221431. doi:10.1136/bcr-2017-221431
26. Jeong M, Kim WS, Kim AR, et al. Medical procedure-related transient global amnesia. Eur Neurol. 2018;80(1-2):42-49. doi:10.1159/000493163
27. Shah B, Hussain MW. Concussion causing transient global amnesia: further insights into pathophysiology? Neurology. 2020;95(20 Suppl 1):S16. doi:10.1212/01.wnl.0000720020.86134.9d
More on an asymmetric life
I enjoy receiving
Too often, families bear the burden of an individual’s hyperfocused pursuits. I hope your wife has been able to pursue her occupation with the same zeal and commitment. We have all read biographies of driven individuals and, unfortunately, someone pays the price for another’s success. For every Steve Jobs, there is a Lisa Jobs.
If we were surgeons, I would applaud your essay. However, we are psychiatrists. If anything, we balance out the reductionist forces in medicine. When every other physician claims a cure with medications or procedures, we look at all aspects of the patient’s life to find the appropriate treatment. At least that’s what we should be doing.
I was part of the first class of residents to work under the 80-hours-per-week restrictions. I was grateful for the extra time to rest, exercise, and spend time with my wife. The 80-hour restrictions improved resident wellness and had no impact on patient care. There are intangible benefits of diverting the mind from a chosen pursuit (such as creativity).
There is no doubt that becoming number 1 in any field requires a tremendous amount of determination, sacrifice, and effort. But not everyone gets to be first. Our society’s single-minded focus on being the best has had a major impact on mental health, especially for children. I hope you can address that in a future editorial.
Sudhir Nagaraja, DO, MS
Fredericksburg, Virginia
Dr. Nasrallah responds
Thank you for your letter about my editorial. You obviously believe in leading a balanced life, and that’s fine if you so choose. I described why I decided at an early age to lead an intensive, “purpose-driven life,” which requires investing much more time than others do, to achieve my lofty goals and excel in my area of expertise (academic psychiatry). It is really a “calling,” and those who score an extraordinary achievement (a moonshot) in their life, including Olympic gold medalists, entrepreneurs, inventors, or Nobel laureates, must do exactly what I do. I am not urging anyone to do what I have chosen to do in my life. Everyone defines for themselves what constitutes the pursuit of happiness.
You mentioned my wife. Let me assert that she is highly successful as a mother and as a research psychologist. She is my extremely valuable life partner and very supportive of what I do. I am fortunate to have chosen well!
Continue to: More on transient global amnesia
More on transient global amnesia
Your recent article on transient global amnesia (TGA) (“Transient global amnesia: Psychiatric precipitants, features, and comorbidities,”
I witnessed TGA, experienced by my brother, while on a surf trip. After bodyboarding for about an hour in cold water, wearing a full wet suit and hood, he met me on the beach. He recognized me and knew my name but had no idea where we were, how we got there, or other events from earlier that morning. There was no stressor, just the usual surfing excitement. We went to a local emergency department, where the physical examination, usual laboratory tests, and neuroimaging were normal. After approximately 5 hours, he began to fully recall recent events. Ten years later, there has been no recurrence. The only change in his surfing habits has been to avoid using a hood with neck coverage.
In 2022, Papadis et al1 described a case of concurrent Takotsubo cardiomyopathy and TGA, noting that cardiovascular dysfunction and neurologic dysfunction may be provoked by an emotional or stressful situation. The interesting observations of capture myopathy from animal literature appear similar to human reactions to trauma.1-3
Case reports of scopolamine intoxication have been linked to TGA. Severe memory disturbances, characteristics of dry mouth, blurred vision, and tachycardia were evident. Certain South American plant extracts popularly known as “Burundanga” have anticholinergic effects. Severe anterograde amnesia and submissiveness represent the 2 most notorious clinical signs of Burundanga intoxication.4
As one reviews single and groups of case studies, several things stand out. The hallmark of TGA is the sudden inability to make new memories, which resolves in a few hours. The brief and isolated dysfunction is what distinguishes this condition from most episodic disorders, but a clinician should not prognosticate too much without screening for ischemic or metabolic disturbance. Common associated precursors include Valsalva-associated activities, emotional stress with anxiety, acute pain, cold water immersion, static neck posture, and age older than 55.5,6
Neuropsychiatric disorders involve the neuron and its connections. Major reflexes automate the processes of the “neurocardiac” axis. The vasovagal reflex (Barcroft/Edholm reflex), diving reflex, baroreceptor reflex, Cushing reflex, and others depend upon the conversion of a mechanical stimulus to neurotransmission. The reflexes have sensors, afferent paths, a central processing, and efferent paths that lead to events or experiences. CNS processing is complex but the brainstem, amygdala, prefrontal cortex, and some cortical regions are involved. Neurocardiac reactions can come from pathologic events, including ischemia, metabolic disturbance, pain signals, or emotional effects within the axis.7-11
Understanding neurocardiac reflexes may help our progress with challenging clinical conditions, such as chronic pain, trauma, and cognitive impairment. The broad use of vagus nerve stimulation is one indicator of the power of this focus.12-19 Lewis20 suggested increased susceptibility to retrograde jugular venous flow could cause regional brain ischemia, resulting in TGA. The competency of jugular venous valves during the Valsalva maneuver could be assessed with Doppler ultrasound. Abnormalities could be managed, and results assessed.20,21 Vascular shunting from memory regions in the brain to essential neurocardiac control areas should be considered.
Cholinergic processes are active in the parasympathetic nervous system, sustained attention, working memory, executive functions, and mood. Increased central cholinergic activity may lead to depression. Scopolamine, in its therapeutic range, has antidepressant effects but in toxic doses is a dissociative agent.22,23 While cholinesterase inhibitors are used in Alzheimer disease, cholinergic agonists have yet to play a large role in general psychiatry or functional neurology.
TGA continues to provide a window into memory, functional disorders, psychological defenses, and adaptive neurocardiac processes. Continued clinical care and research might include gradual adaptation to cold water immersion, caution with the Valsalva maneuver, cholinergic support, managing the trapped response, avoiding interference with normal jugular flow, and evaluation for jugular venous insufficiency.
Because a variety of medical procedures can trigger TGA, health care professionals in many fields need to understand this symptom complex.24-27 Thanks to the authors for raising the awareness of TGA for psychiatrists.
Mark Chandler, MD
Durham, North Carolina
References
1. Papadis A, Svab S, Brugger N, et al. “Broken heart” and “broken brain”: which connection? Cardiol Res. 2022;13(1):65-70. doi:10.14740/cr1336
2. Blumstein DT, Buckner J, Shah S, et al. The evolution of capture myopathy in hooved mammals: a model for human stress cardiomyopathy? Evol Med Public Health. 2015;2015(1):195-203. doi:10.1093/emph/eov015
3. Seguel M, Paredes E, Pavés H, et al. Capture-induced stress cardiomyopathy in South American fur seal pups (Arctophoca australis gracilis). Marine Mammal Science. 2014;30(3): 1149-1157. https://doi.org/10.1111/mms.12079
4. Ardila A, Moreno C. Scopolamine intoxication as a model of transient global amnesia. Brain Cogn. 1991;15(2):236-245. doi:10.1016/0278-2626(91)90028-7
5. Bartsch T, Deuschl G. Transient global amnesia: functional anatomy and clinical implications. Lancet Neurol. 2010;9(2):205-214. doi:10.1016/S1474-4422(09)70344-8
6. Spiegel DR, Smith J, Wade RR, et al. Transient global amnesia: current perspectives. Neuropsychiatr Dis Treat. 2017;13:2691-2703. doi:10.2147/NDT.S130710
7. Yartsev A. Cardiac reflexes. August 15, 2020. Updated May 19, 2023. Accessed June 12, 2023. https://derangedphysiology.com/main/cicm-primary-exam/required-reading/cardiovascular-system/Chapter%20491/cardiac-reflexes
8. Lemaitre F, Chowdhury T, Schaller B. The trigeminocardiac reflex - a comparison with the diving reflex in humans. Arch Med Sci. 2015;11(2):419-426. doi:10.5114/aoms.2015.50974
9. Lindholm P, Lundgren CE. The physiology and pathophysiology of human breath-hold diving. J Appl Physiol (1985). 2009;106(1):284-292. doi:10.1152/japplphysiol.90991.2008
10. Tansey EA, Johnson CD. Recent advances in thermoregulation. Adv Physiol Educ. 2015;39(3):139-148. doi:10.1152/advan.00126.2014
11. Alboni P, Alboni M. Vasovagal syncope as a manifestation of an evolutionary selected trait. J Atr Fibrillation. 2014;7(2):1035. doi:10.4022/jafib.1035
12. Badran BW, Austelle CW. The future is noninvasive: a brief review of the evolution and clinical utility of vagus nerve stimulation. Focus (Am Psychiatr Publ). 2022;20(1):3-7. doi:10.1176/appi.focus.20210023
13. Suarez-Roca H, Mamoun N, Sigurdson MI, et al. Baroreceptor modulation of the cardiovascular system, pain, consciousness, and cognition. Compr Physiol. 2021;11(2):1373-1423. doi:10.1002/cphy.c190038
14. Pinna T, Edwards DJ. A systematic review of associations between interoception, vagal tone, and emotional regulation: potential applications for mental health, wellbeing, psychological flexibility, and chronic conditions. Front Psychol. 2020;11:1792. doi:10.3389/fpsyg.2020.01792
15. Howland RH. Vagus nerve stimulation. Curr Behav Neurosci Rep. 2014 Jun;1(2):64-73. doi:10.1007/s40473-014-0010-5
16. Panneton WM, Gan Q. The mammalian diving response: inroads to its neural control. Front Neurosci. 2020;14:524. doi:10.3389/fnins.2020.00524
17. Khurana RK, Wu R. The cold face test: a non-baroreflex mediated test of cardiac vagal function. Clin Auton Res. 2006;16(3):202-207. doi:10.1007/s10286-006-0332-9
18. Montirosso R, Provenzi L, Tronick E, et al. Vagal tone as a biomarker of long-term memory for a stressful social event at 4 months. Dev Psychobiol. 2014;56(7):1564-1574. doi:10.1002/dev.21251
19. Hansen AL, Johnsen BH, Thayer JF. Vagal influence on working memory and attention. Int J Psychophysiol. 2003;48(3):263-274. doi:10.1016/s0167-8760(03)00073-4
20. Lewis SL. Aetiology of transient global amnesia. Lancet. 1998;352(9125):397-399. doi:10.1016/S0140-6736(98)01442-1
21. Han K, Chao AC, Chang FC, et al. Obstruction of venous drainage linked to transient global amnesia. PLoS One. 2015;10(7):e0132893. doi:10.1371/journal.pone.0132893
22. Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76(1):116-129. doi:10.1016/j.neuron.2012.08.036
23. Dulawa SC, Janowsky DS. Cholinergic regulation of mood: from basic and clinical studies to emerging therapeutics. Mol Psychiatry. 2019;24(5):694-709. doi:10.1038/s41380-018-0219-x
24. Grande LA, Loeser JD, Samii A. Recurrent transient global amnesia with intrathecal baclofen. Anesth Analg. 2008;106(4):1284-1287. doi:10.1213/ane.0b013e318165e1c6
25. Carrard J, Lambert AC, Genné D. Transient global amnesia following a whole-body cryotherapy session. BMJ Case Rep. 2017;2017:bcr2017221431. doi:10.1136/bcr-2017-221431
26. Jeong M, Kim WS, Kim AR, et al. Medical procedure-related transient global amnesia. Eur Neurol. 2018;80(1-2):42-49. doi:10.1159/000493163
27. Shah B, Hussain MW. Concussion causing transient global amnesia: further insights into pathophysiology? Neurology. 2020;95(20 Suppl 1):S16. doi:10.1212/01.wnl.0000720020.86134.9d
More on an asymmetric life
I enjoy receiving
Too often, families bear the burden of an individual’s hyperfocused pursuits. I hope your wife has been able to pursue her occupation with the same zeal and commitment. We have all read biographies of driven individuals and, unfortunately, someone pays the price for another’s success. For every Steve Jobs, there is a Lisa Jobs.
If we were surgeons, I would applaud your essay. However, we are psychiatrists. If anything, we balance out the reductionist forces in medicine. When every other physician claims a cure with medications or procedures, we look at all aspects of the patient’s life to find the appropriate treatment. At least that’s what we should be doing.
I was part of the first class of residents to work under the 80-hours-per-week restrictions. I was grateful for the extra time to rest, exercise, and spend time with my wife. The 80-hour restrictions improved resident wellness and had no impact on patient care. There are intangible benefits of diverting the mind from a chosen pursuit (such as creativity).
There is no doubt that becoming number 1 in any field requires a tremendous amount of determination, sacrifice, and effort. But not everyone gets to be first. Our society’s single-minded focus on being the best has had a major impact on mental health, especially for children. I hope you can address that in a future editorial.
Sudhir Nagaraja, DO, MS
Fredericksburg, Virginia
Dr. Nasrallah responds
Thank you for your letter about my editorial. You obviously believe in leading a balanced life, and that’s fine if you so choose. I described why I decided at an early age to lead an intensive, “purpose-driven life,” which requires investing much more time than others do, to achieve my lofty goals and excel in my area of expertise (academic psychiatry). It is really a “calling,” and those who score an extraordinary achievement (a moonshot) in their life, including Olympic gold medalists, entrepreneurs, inventors, or Nobel laureates, must do exactly what I do. I am not urging anyone to do what I have chosen to do in my life. Everyone defines for themselves what constitutes the pursuit of happiness.
You mentioned my wife. Let me assert that she is highly successful as a mother and as a research psychologist. She is my extremely valuable life partner and very supportive of what I do. I am fortunate to have chosen well!
Continue to: More on transient global amnesia
More on transient global amnesia
Your recent article on transient global amnesia (TGA) (“Transient global amnesia: Psychiatric precipitants, features, and comorbidities,”
I witnessed TGA, experienced by my brother, while on a surf trip. After bodyboarding for about an hour in cold water, wearing a full wet suit and hood, he met me on the beach. He recognized me and knew my name but had no idea where we were, how we got there, or other events from earlier that morning. There was no stressor, just the usual surfing excitement. We went to a local emergency department, where the physical examination, usual laboratory tests, and neuroimaging were normal. After approximately 5 hours, he began to fully recall recent events. Ten years later, there has been no recurrence. The only change in his surfing habits has been to avoid using a hood with neck coverage.
In 2022, Papadis et al1 described a case of concurrent Takotsubo cardiomyopathy and TGA, noting that cardiovascular dysfunction and neurologic dysfunction may be provoked by an emotional or stressful situation. The interesting observations of capture myopathy from animal literature appear similar to human reactions to trauma.1-3
Case reports of scopolamine intoxication have been linked to TGA. Severe memory disturbances, characteristics of dry mouth, blurred vision, and tachycardia were evident. Certain South American plant extracts popularly known as “Burundanga” have anticholinergic effects. Severe anterograde amnesia and submissiveness represent the 2 most notorious clinical signs of Burundanga intoxication.4
As one reviews single and groups of case studies, several things stand out. The hallmark of TGA is the sudden inability to make new memories, which resolves in a few hours. The brief and isolated dysfunction is what distinguishes this condition from most episodic disorders, but a clinician should not prognosticate too much without screening for ischemic or metabolic disturbance. Common associated precursors include Valsalva-associated activities, emotional stress with anxiety, acute pain, cold water immersion, static neck posture, and age older than 55.5,6
Neuropsychiatric disorders involve the neuron and its connections. Major reflexes automate the processes of the “neurocardiac” axis. The vasovagal reflex (Barcroft/Edholm reflex), diving reflex, baroreceptor reflex, Cushing reflex, and others depend upon the conversion of a mechanical stimulus to neurotransmission. The reflexes have sensors, afferent paths, a central processing, and efferent paths that lead to events or experiences. CNS processing is complex but the brainstem, amygdala, prefrontal cortex, and some cortical regions are involved. Neurocardiac reactions can come from pathologic events, including ischemia, metabolic disturbance, pain signals, or emotional effects within the axis.7-11
Understanding neurocardiac reflexes may help our progress with challenging clinical conditions, such as chronic pain, trauma, and cognitive impairment. The broad use of vagus nerve stimulation is one indicator of the power of this focus.12-19 Lewis20 suggested increased susceptibility to retrograde jugular venous flow could cause regional brain ischemia, resulting in TGA. The competency of jugular venous valves during the Valsalva maneuver could be assessed with Doppler ultrasound. Abnormalities could be managed, and results assessed.20,21 Vascular shunting from memory regions in the brain to essential neurocardiac control areas should be considered.
Cholinergic processes are active in the parasympathetic nervous system, sustained attention, working memory, executive functions, and mood. Increased central cholinergic activity may lead to depression. Scopolamine, in its therapeutic range, has antidepressant effects but in toxic doses is a dissociative agent.22,23 While cholinesterase inhibitors are used in Alzheimer disease, cholinergic agonists have yet to play a large role in general psychiatry or functional neurology.
TGA continues to provide a window into memory, functional disorders, psychological defenses, and adaptive neurocardiac processes. Continued clinical care and research might include gradual adaptation to cold water immersion, caution with the Valsalva maneuver, cholinergic support, managing the trapped response, avoiding interference with normal jugular flow, and evaluation for jugular venous insufficiency.
Because a variety of medical procedures can trigger TGA, health care professionals in many fields need to understand this symptom complex.24-27 Thanks to the authors for raising the awareness of TGA for psychiatrists.
Mark Chandler, MD
Durham, North Carolina
References
1. Papadis A, Svab S, Brugger N, et al. “Broken heart” and “broken brain”: which connection? Cardiol Res. 2022;13(1):65-70. doi:10.14740/cr1336
2. Blumstein DT, Buckner J, Shah S, et al. The evolution of capture myopathy in hooved mammals: a model for human stress cardiomyopathy? Evol Med Public Health. 2015;2015(1):195-203. doi:10.1093/emph/eov015
3. Seguel M, Paredes E, Pavés H, et al. Capture-induced stress cardiomyopathy in South American fur seal pups (Arctophoca australis gracilis). Marine Mammal Science. 2014;30(3): 1149-1157. https://doi.org/10.1111/mms.12079
4. Ardila A, Moreno C. Scopolamine intoxication as a model of transient global amnesia. Brain Cogn. 1991;15(2):236-245. doi:10.1016/0278-2626(91)90028-7
5. Bartsch T, Deuschl G. Transient global amnesia: functional anatomy and clinical implications. Lancet Neurol. 2010;9(2):205-214. doi:10.1016/S1474-4422(09)70344-8
6. Spiegel DR, Smith J, Wade RR, et al. Transient global amnesia: current perspectives. Neuropsychiatr Dis Treat. 2017;13:2691-2703. doi:10.2147/NDT.S130710
7. Yartsev A. Cardiac reflexes. August 15, 2020. Updated May 19, 2023. Accessed June 12, 2023. https://derangedphysiology.com/main/cicm-primary-exam/required-reading/cardiovascular-system/Chapter%20491/cardiac-reflexes
8. Lemaitre F, Chowdhury T, Schaller B. The trigeminocardiac reflex - a comparison with the diving reflex in humans. Arch Med Sci. 2015;11(2):419-426. doi:10.5114/aoms.2015.50974
9. Lindholm P, Lundgren CE. The physiology and pathophysiology of human breath-hold diving. J Appl Physiol (1985). 2009;106(1):284-292. doi:10.1152/japplphysiol.90991.2008
10. Tansey EA, Johnson CD. Recent advances in thermoregulation. Adv Physiol Educ. 2015;39(3):139-148. doi:10.1152/advan.00126.2014
11. Alboni P, Alboni M. Vasovagal syncope as a manifestation of an evolutionary selected trait. J Atr Fibrillation. 2014;7(2):1035. doi:10.4022/jafib.1035
12. Badran BW, Austelle CW. The future is noninvasive: a brief review of the evolution and clinical utility of vagus nerve stimulation. Focus (Am Psychiatr Publ). 2022;20(1):3-7. doi:10.1176/appi.focus.20210023
13. Suarez-Roca H, Mamoun N, Sigurdson MI, et al. Baroreceptor modulation of the cardiovascular system, pain, consciousness, and cognition. Compr Physiol. 2021;11(2):1373-1423. doi:10.1002/cphy.c190038
14. Pinna T, Edwards DJ. A systematic review of associations between interoception, vagal tone, and emotional regulation: potential applications for mental health, wellbeing, psychological flexibility, and chronic conditions. Front Psychol. 2020;11:1792. doi:10.3389/fpsyg.2020.01792
15. Howland RH. Vagus nerve stimulation. Curr Behav Neurosci Rep. 2014 Jun;1(2):64-73. doi:10.1007/s40473-014-0010-5
16. Panneton WM, Gan Q. The mammalian diving response: inroads to its neural control. Front Neurosci. 2020;14:524. doi:10.3389/fnins.2020.00524
17. Khurana RK, Wu R. The cold face test: a non-baroreflex mediated test of cardiac vagal function. Clin Auton Res. 2006;16(3):202-207. doi:10.1007/s10286-006-0332-9
18. Montirosso R, Provenzi L, Tronick E, et al. Vagal tone as a biomarker of long-term memory for a stressful social event at 4 months. Dev Psychobiol. 2014;56(7):1564-1574. doi:10.1002/dev.21251
19. Hansen AL, Johnsen BH, Thayer JF. Vagal influence on working memory and attention. Int J Psychophysiol. 2003;48(3):263-274. doi:10.1016/s0167-8760(03)00073-4
20. Lewis SL. Aetiology of transient global amnesia. Lancet. 1998;352(9125):397-399. doi:10.1016/S0140-6736(98)01442-1
21. Han K, Chao AC, Chang FC, et al. Obstruction of venous drainage linked to transient global amnesia. PLoS One. 2015;10(7):e0132893. doi:10.1371/journal.pone.0132893
22. Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76(1):116-129. doi:10.1016/j.neuron.2012.08.036
23. Dulawa SC, Janowsky DS. Cholinergic regulation of mood: from basic and clinical studies to emerging therapeutics. Mol Psychiatry. 2019;24(5):694-709. doi:10.1038/s41380-018-0219-x
24. Grande LA, Loeser JD, Samii A. Recurrent transient global amnesia with intrathecal baclofen. Anesth Analg. 2008;106(4):1284-1287. doi:10.1213/ane.0b013e318165e1c6
25. Carrard J, Lambert AC, Genné D. Transient global amnesia following a whole-body cryotherapy session. BMJ Case Rep. 2017;2017:bcr2017221431. doi:10.1136/bcr-2017-221431
26. Jeong M, Kim WS, Kim AR, et al. Medical procedure-related transient global amnesia. Eur Neurol. 2018;80(1-2):42-49. doi:10.1159/000493163
27. Shah B, Hussain MW. Concussion causing transient global amnesia: further insights into pathophysiology? Neurology. 2020;95(20 Suppl 1):S16. doi:10.1212/01.wnl.0000720020.86134.9d
Optimizing benzodiazepine treatment of anxiety disorders
Though once the main treatment for anxiety disorders—often as monotherapy1—benzodiazepines are now primarily used as adjunctive agents.2-4 Their ability to produce rapid anxiolysis represents a significant therapeutic advantage, but in recent decades their tolerability, class-specific risks, and lack of antidepressant properties contributed to benzodiazepines being largely replaced by selective serotonin reuptake inhibitors (SSRIs) for the pharmacologic treatment of anxiety. This shift within the pharmacologic armamentarium has decreased many clinicians’ familiarity with benzodiazepines.
While benzodiazepines continue to have an important role in managing anxiety disorders, particularly treatment-resistant anxiety,4 clinicians must consider the limitations of these agents. Benzodiazepines can be associated with abuse and dependence, and overdose risk when combined with opiates.5,6 They may cause memory impairment7,8 and conflicting data suggest they may contribute to the risk of developing cognitive disorders.9-11 Benzodiazepines also have been associated with falls and fractures,12 and worse outcomes in patients with posttraumatic stress disorder.13 Some studies of patients with chronic obstructive pulmonary disease (COPD) found benzodiazepines may increase the risk of COPD exacerbations and accidental overdose,14 though others found that was not always the case.15 Benzodiazepines may be associated with an increased risk of spontaneous abortion when used early in pregnancy.16 Prospective research in women who were breastfeeding found benzodiazepines may cause sedation in up to 2% of infants.17
Despite the potential for adverse effects, benzodiazepine use remains common.18 These medications have a rapid onset of action, are useful for breakthrough symptoms, may enhance treatment adherence, and alleviate activating symptoms of SSRIs. Like other commonly used medications, benzodiazepines have the potential for both harm and benefit.19 Similar to other medications with tolerability concerns but established efficacy, particularly in treatment-resistant anxiety disorders, it is important to balance “overprescribing … to patients at risk and underusing these effective medications when indicated.”19 Though the use of benzodiazepines has been discouraged and perceptions have shifted, knowledge of benzodiazepines and benzodiazepine pharmacology also has been degraded contemporaneously.
This article provides a synthesis of the clinically relevant pharmacology of benzodiazepines, with a focus on orally administered benzodiazepines, which are more common in outpatient clinical practice. Specifically, this review describes the pharmacology of benzodiazepines, benzodiazepine medication interactions, the relationship between pharmacologic characteristics and treatment response/tolerability, and selection and dosing of oral benzodiazepines (Table20).
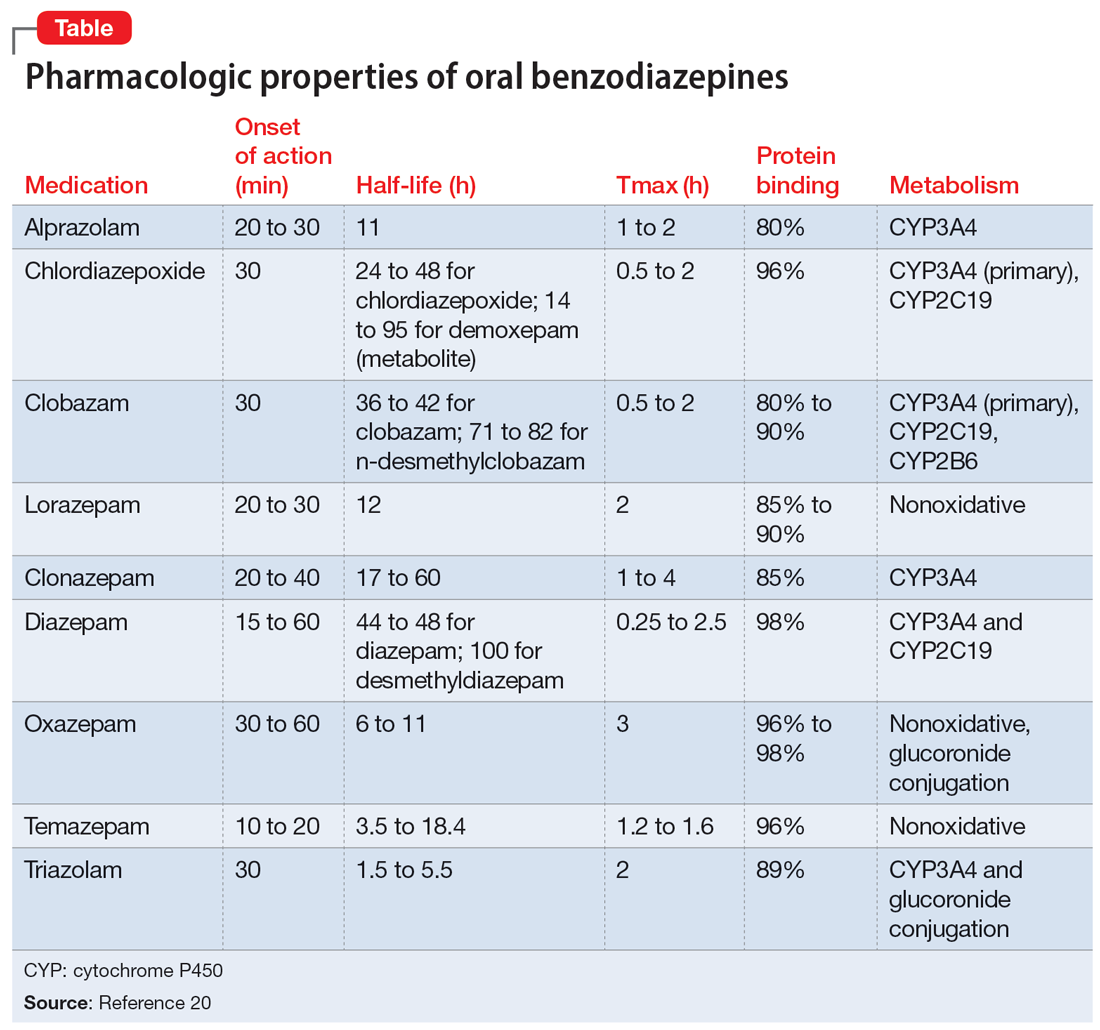
Benzodiazepine pharmacodynamics
Benzodiazepines act at the gamma-aminobutyric acid (GABA)-A receptor complex and bind allosterically.21-23 Comprised of 5 glycoprotein subunits (2 alpha subunits, 2 beta subunits, and 1 gamma subunit), the receptor has 2 distinct sites at which the endogenous inhibitory transmitter GABA binds and 1 benzodiazepine binding site. Benzodiazepines bind within a socket created by the alpha and gamma subunits22 and after binding induce a conformational change in the receptor, which enhances GABA binding. There are 2 types of benzodiazepine receptors: BZ1 and BZ2. The subunits play a critical role in driving the pharmacologic characteristics of the receptor.24 BZ1 and BZ2 receptors bind benzodiazepines, although they are differentially distributed within the brain. Binding at BZ1 receptors—which are distributed in cortical, thalamic, and cerebellar regions—contributes to sedation and deleterious effects of benzodiazepines on memory (eg, anterograde amnesia). BZ2 receptors (which contain gamma-2 subunits) are responsible for anxiolytic and muscle-relaxing effects. They are distributed throughout limbic regions and motor tracts, including motor neurons and neurons in the dorsal horn of the spinal cord.24
Benzodiazepines—positive GABA-A receptor allosteric modulators—produce phasic inhibition, largely through the alpha and gamma subunits discussed above. In contrast, newer positive allosteric modulators (eg, zuranolone) bind at the alpha/beta subunits.25 Mechanistically, endogenous neuroactive steroids and nonbenzodiazepine GABA-A–positive allosteric modulators such as zuranolone and ganaxolone also differ in their regulation of GABA-A (downregulated with benzodiazepines and hypothetically upregulated with zuranolone)26 and their synaptic effects (benzodiazepines synaptically vs endogenous neurosteroids and nonbenzodiazpine positive allosteric modulators extrasynaptically).27
From a developmental perspective, benzodiazepines may have less efficacy for anxiolysis and worse tolerability in some pediatric patients,28 although they generally appear effective for immediate use to treat anxiety in acute settings.29 The differences in efficacy and tolerability may be related to pharmacodynamic differences between pediatric populations and adults. GABA receptor expression and function do not reach adult levels until age 14 to 17½ for subcortical regions and age 18 to 22 for cortical regions, although girls reach adult expression of GABA receptors slightly earlier than boys.30 D
Continue to: Pharmacology and clinical effects
Pharmacology and clinical effects
Benzodiazepine pharmacokinetics are intimately linked with the onset of action and duration of clinical effect and vary based on the route of administration, absorption, and distribution/redistribution.31 In this review, we focus on oral administration as opposed to IV, IM, sublingual, or intranasal administration.
Absorption
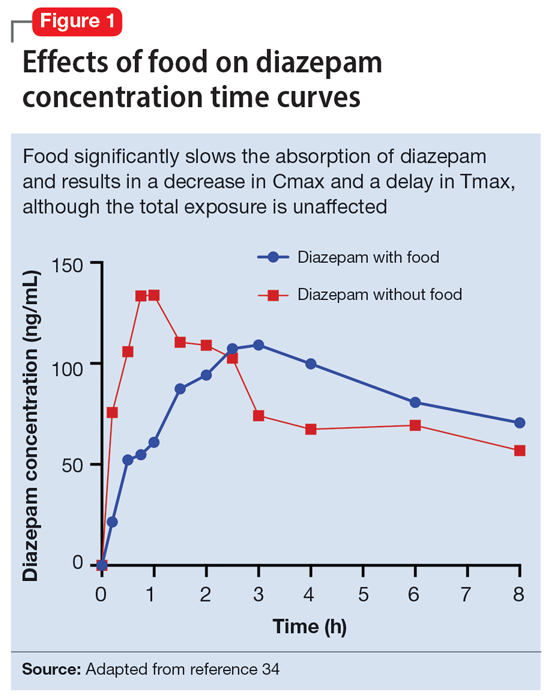
Benzodiazepines are rapidly absorbed after oral administration and quickly enter the systemic circulation. However, absorption rates vary depending on specific aspects of the gastrointestinal milieu and intrinsic properties of the benzodiazepine. For example, alprazolam is more rapidly absorbed than most other benzodiazepines, with a Tmax of 1.8 hours compared to lorazepam, which has a Tmax of approximately 2 hours. These pharmacokinetic effects instantiate differences in tolerability and efficacy. Thus, following single doses of alprazolam and diazepam, self-rated sedating effects and impairment on a task of working memory suggest that effects have a more rapid onset for alprazolam relative to lorazepam.32 Food and concomitant medications can significantly affect benzodiazepine absorption. A single-dose, 3-way crossover study demonstrated that taking diazepam concomitantly with an antacid (eg, aluminum hydroxide) decreased peak concentrations and prolonged absorption by approximately 30 minutes. However, total absorption of the medication was unaffected.33 Additionally, administration of diazepam with food significantly slows absorption from 1 hour 15 minutes to approximately 2 hours 30 minutes and increases benzodiazepine absorption by 25% (Figure 134); the fat content of the meal appears important in moderating this effect.35 The impact of food on alprazolam varies by formulation. For example, when administered in an extended-release (XR) formulation with a high-fat meal, alprazolam absorption increases by one-third, while absorption for administration of the orally disintegrating tablet with a high-fat meal increases from 1 hour 30 minutes to 2 hours. Similarly, for lorazepam, administration with a meal delays absorption by approximately 2 hours; however, this effect does not appear present with the XR formulation. Administering benzodiazepines with food can be clinically leveraged to either accelerate the onset of action or decrease peak-associated adverse effects. Thus, when a highly lipophilic benzodiazepine is needed to treat acute anxiety or prior to an expected anxiogenic stimuli, administering the medication without food may produce a faster onset of action.
CNS penetration
Benzodiazepines enter the CNS by passive diffusion. Because of this, lipophilicity at physiologic pH influences the rate at which a benzodiazepine crosses the blood-brain barrier. The rate at which benzodiazepines enter the CNS influences their clinical effects and the speed at which both efficacy (ie, anxiolysis) and adverse effects (ie, sedation, slowed cognition) are observed. In general, more lipophilic medications initiate their anxiolytic effect more quickly. However, by quickly leaving the CNS (through the same mechanism that allowed them to enter the CNS at such speed), their effects rapidly cease as they redistribute into fat. Thus, highly lipophilic benzodiazepines produce more intense effects compared to less lipophilic benzodiazepines. For these reasons, lipophilicity is more important than half-life for determining the duration of effect in most patients.
Lipophilicity and duration of effect
Benzodiazepines and their metabolites tend to be highly protein-bound and distributed in fat- and lipid-enriched areas such as the CNS. As a result, the more lipophilic agents generally have the highest rates of absorption and the fastest onset of clinical effects. The duration of action for many benzodiazepines is determined by the rate and extent of distribution (a function of lipophilicity) rather than by the rate of elimination. For example, diazepam has a longer half-life than lorazepam, but its duration of action following a single dose is shorter. This is because diazepam is more lipophilic and therefore more extensively distributed (particularly to adipose tissue). This results in it leaving the brain and blood and distributing to other tissues. In turn, its CNS effect (ie, anxiolytic effects) are more quickly terminated.
By contrast, less lipophilic benzodiazepines maintain their CNS concentrations longer; they have a longer duration of action because of their slower redistribution, which culminates in a shorter half-life, and are less extensively distributed to peripheral tissues. In essence, this means that (other things being equal) a less lipophilic benzodiazepine produces a more sustained anxiolytic effect compared to a highly lipophilic benzodiazepine.36 Lipophilicity is also important in predicting some cognitive adverse effects, including amnesia. Benzodiazepines with high lipophilicity have greater absorption and faster onset of action as well as more rapid amnestic effects.37,38 These effects may relate to overall efficacy differences for oral benzodiazepines. A recent meta-analysis by Stimpfl et al36 found that less lipophilic benzodiazepines produced a greater response compared to more lipophilic benzodiazepines.
Continue to: Metabolism
Metabolism
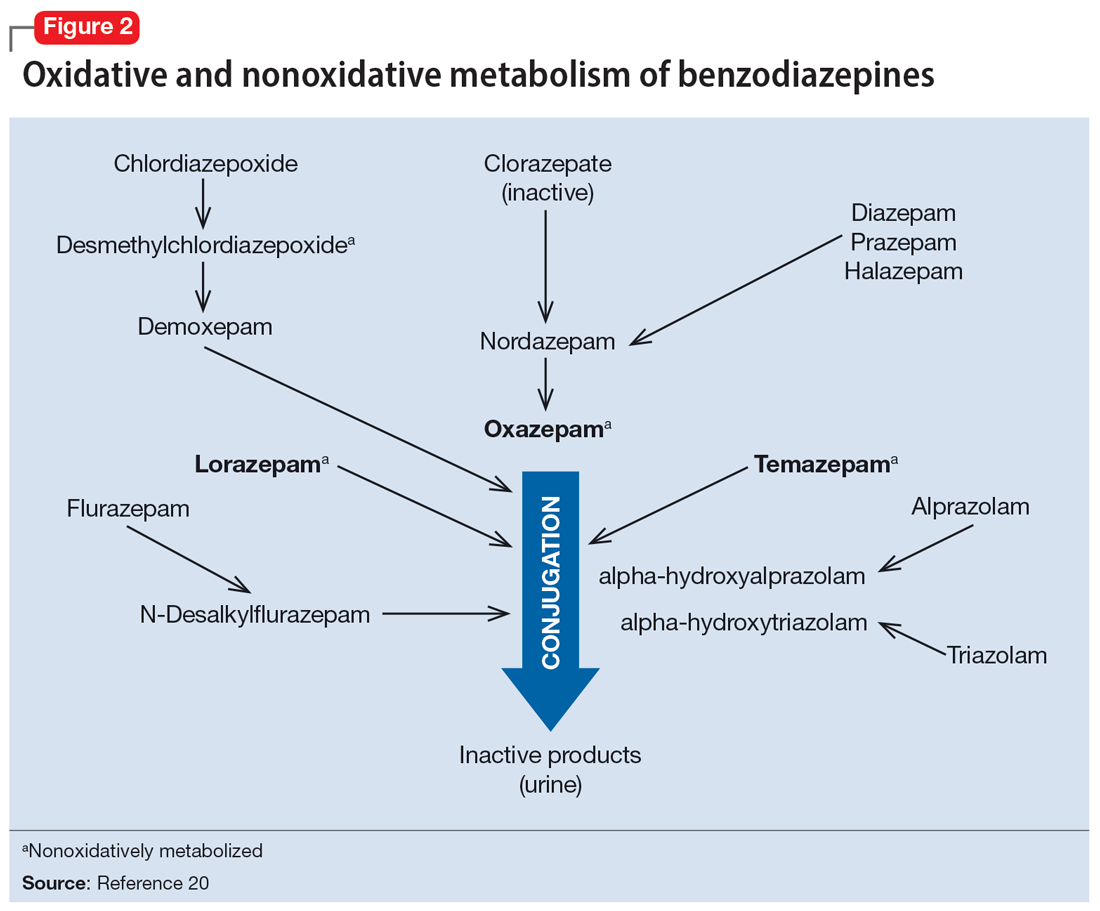
Regarding cytochrome P450 (CYP) metabolism, polymorphic CYP2C19 and CYP3A4/5 are involved in the metabolism of several benzodiazepines39 and CYP2B6 has been recognized as a contributor to diazepam metabolism. CYP3A5 gene polymorphisms may produce variation in alprazolam metabolism; however, the predominant cytochrome involved in the metabolism of oxidatively metabolized benzodiazepines (ie, benzodiazepines other than lorazepam, oxazepam, and temazepam) is primarily CYP3A4, and most effects on CYP3A4 activity are related to concomitant medications and other nongenetic factors.
Drug-drug interactions
Apart from lorazepam,40,41 oxazepam,42,43 and temazepam, most benzodiazepines are metabolized through oxidative mechanisms that involve CYP3A4 (Figure 220).39 As such, their metabolism is influenced by medications that impact CYP3A4, including antifungals (eg, ketoconazole), calcium channel blockers (eg, verapamil, diltiazem), nefazodone, some protease inhibitors, and macrolide antibiotics. Research has examined the impact of low-dose estrogen oral contraceptives (OCPs) on exposure (eg, plasma concentrations) of several benzodiazepines. The mechanism for this interaction is likely complex and putatively involves multiple pathways, including inhibition of CYP3A4 by OCPs. The effects of OCPs on benzodiazepine pharmacokinetics vary based on the metabolism of the benzodiazepine. In general, medications oxidized and nitroreduced (eg, chlordiazepoxide, alprazolam, diazepam, and nitrazepam) have decreased clearance in patients treated with OCPs. Regarding nonoxidatively metabolized benzodiazepines, data are mixed. Research found no OCP-related effects on the pharmacokinetics of nonoxidatively metabolized benzodiazepines44; another study suggested that clearance of these medications—through increased glucuronidation—may be increased.31 The effect of smoking on benzodiazepine concentration has been well documented. Smoking increases the clearance of orally administered diazepam,45 but not IV diazepam, midazolam, or lorazepam, suggesting that this represents a first-pass effect.46 For alprazolam, plasma concentrations are reduced by 15% to 30% in smokers and total body clearance is 24% greater compared to nonsmokers, which results in an approximately 50% increase in half-life in nonsmokers compared to smokers.47 The most notable interaction between benzodiazepines and SSRIs is seen with fluvoxamine. Because fluvoxamine moderately inhibits CYP2C19 and CYP3A4 and potently inhibits CYP1A2,48 the clearance of oxidatively metabolized benzodiazepines is reduced.49 Additionally, the effects of grapefruit juice—a potent inhibitor of CYP3A4—has been evaluated for several benzodiazepines. Yasui et al50 found grapefruit juice did not alter alprazolam plasma concentrations. However, in separate research, grapefruit juice tripled diazepam exposure, increased peak concentrations 1.5-fold, and prolonged absorption.51
Hepatic disease
Exposure to benzodiazepines—other than lorazepam, oxazepam, and temazepam—is influenced by intrinsic hepatic disease and requires dose adjustment in individuals with significant hepatic impairment. The impact of hepatic disease on the clinical pharmacology of benzodiazepines may relate to 2 factors: protein binding and metabolism. In a study of individuals with cirrhosis, lorazepam binding was decreased, although its metabolism and clearance were largely unaffected.40
Aging and benzodiazepine metabolism/clearance
Aging is associated with myriad physiologic changes (eg, decrease in renal clearance after age 40, changes in body fat distribution, changes in activity of cytochromes) that are relevant to benzodiazepine pharmacology. They may underlie differences in the tolerability of benzodiazepines and other clinically relevant characteristics (eg, duration of action, accumulation).
Several studies have evaluated the impact of aging on the clearance and disposition of selected benzodiazepines. The respective half-lives of chlordiazepoxide and diazepam increase from 4- to 6-fold from age 20 to 80. Further, with chronic dosing, highly lipophilic benzodiazepines may require additional attention in geriatric patients. In a study that included individuals up to age 78, steady-state plasma concentrations of diazepam and its metabolite, desmethyldiazepam (DMDZ), were 30% to 35% higher in older patients compared to younger individuals.52 In this study, the half-lives for the young and older patients were 31 hours and 86 hours, respectively, for diazepam, and 40 hours and 80 hours, respectively, for the active metabolite. The half-life of diazepam is increased by “1 hour for each year of age beginning with a half-life of 20 hours at 20 years of age, as the volume of distribution is increased, and clearance is decreased.”52 Clinically, this implies that in older adults, clinicians should expect lower peak concentrations (Cmax), higher trough concentrations (Cmin), and that diazepam will take longer to reach steady-state concentrations. Taken together, these findings raised concern that “slow accumulation and delayed washout of diazepam and DMDZ is probable.”52 These findings—which may have more clinical relevance than those of single-dose studies—suggest that the effects related to diazepam would also take longer to resolve in older patients. Finally, lorazepam clearance or distribution does not appear to be affected by aging, at least in patients age 15 to 73.40 Alprazolam is more slowly cleared in geriatric patients and its effects may be potentiated by reduced protein binding.
Continue to: Obesity
Obesity
The distribution of medications, including benzodiazepines, is altered in patients who are obese because of increased adipose tissue.53,54 This increase in the volume of distribution can attenuate the onset of action, increase medication accumulation in fat, and potentiate the duration of action.55,56
Obesity may also affect hepatic metabolism by induction of CYP1A2, CYP2C9, and CYP2C19, and inhibition of CYP3A4.57 Triazolam, which is metabolized by CYP3A4, is associated with a greater exposure (ie, plasma concentrations) in individuals who are obese.58 However, when considering differences in benzodiazepine pharmacokinetics in patients who are obese, clinicians must remember that elimination half-life depends on both volume of distribution and clearance. In
How quickly do benzodiazepines work?
Benzodiazepines act quickly. Meta-analyses36 suggest that improvement in anxiety symptoms compared to placebo is greatest initially and then the rate of improvement slows over successive weeks. Research on benzodiazepines reveals statistically significant differences between benzodiazepines and placebo within the first week of treatment, with >80% of the expected improvement by Week 8 of treatment emerging by Week 4 (Figure 336). The rapid reduction in anxiety symptoms seen with benzodiazepines has important treatment implications, given that traditional psychotherapeutic and antidepressant treatments are slow to produce improvements. Consistent data suggesting that benzodiazepines work faster than other treatments support that they may have a role during the initiation of other treatments.
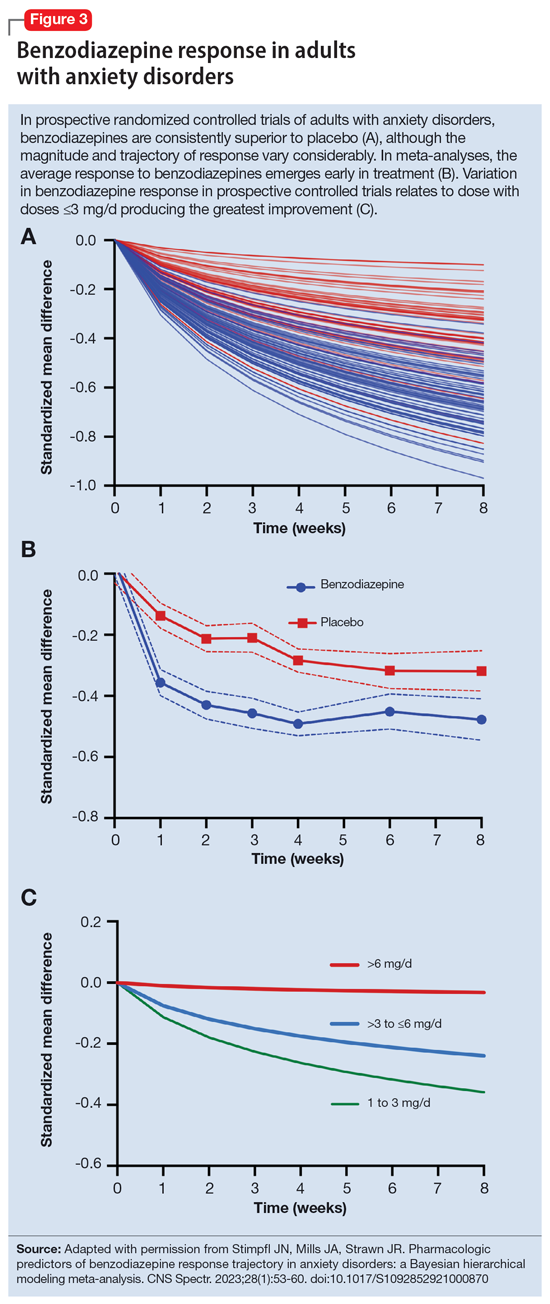
What is the ‘best’ dose?
As seen with other classes of psychotropic medications,4 the relationship between benzodiazepine dose and response is complex. In a recent meta-analysis of 65 placebo-controlled trials of benzodiazepines in adults with anxiety disorders, there was a superior response over time for low-dose benzodiazepines (<3 mg/d in lorazepam equivalents) compared to a medium dose (3 to 6 mg/d; P = .042); high-dose benzodiazepines (>6 mg/d) yielded less improvement compared to medium doses (P = .001).36 A study of adults with panic disorder similarly found the greatest responses with alprazolam plasma concentrations of 20 to 40 ng/mL, with no additional benefit at <20 ng/mL or >40 ng/mL.49 As plasma concentrations increase, adverse effects such as sedation also increase, which may confound the observed loss of a dose-response relationship at higher doses and plasma concentrations.62 This may, in part, account for the observation that higher doses of benzodiazepines are associated with greater depressive symptoms and disrupted sleep.63 As such, low doses may represent a delicate equipoise between efficacy and tolerability, yielding the most optimal clinical response.
Which benzodiazepine should I prescribe?
Comparing benzodiazepines is difficult, given the differences in dosing and disorders studied and differences in how each individual clinical trial was conducted. A meta-analysis by Stimpfl et al36 that used Bayesian hierarchical modeling, which allowed some of this heterogeneity to be addressed, found that relative to the reference benzodiazepine (lorazepam), clonazepam had the greatest trajectory/magnitude of response (other specific benzodiazepines did not statistically differ from lorazepam) (Figure 436).
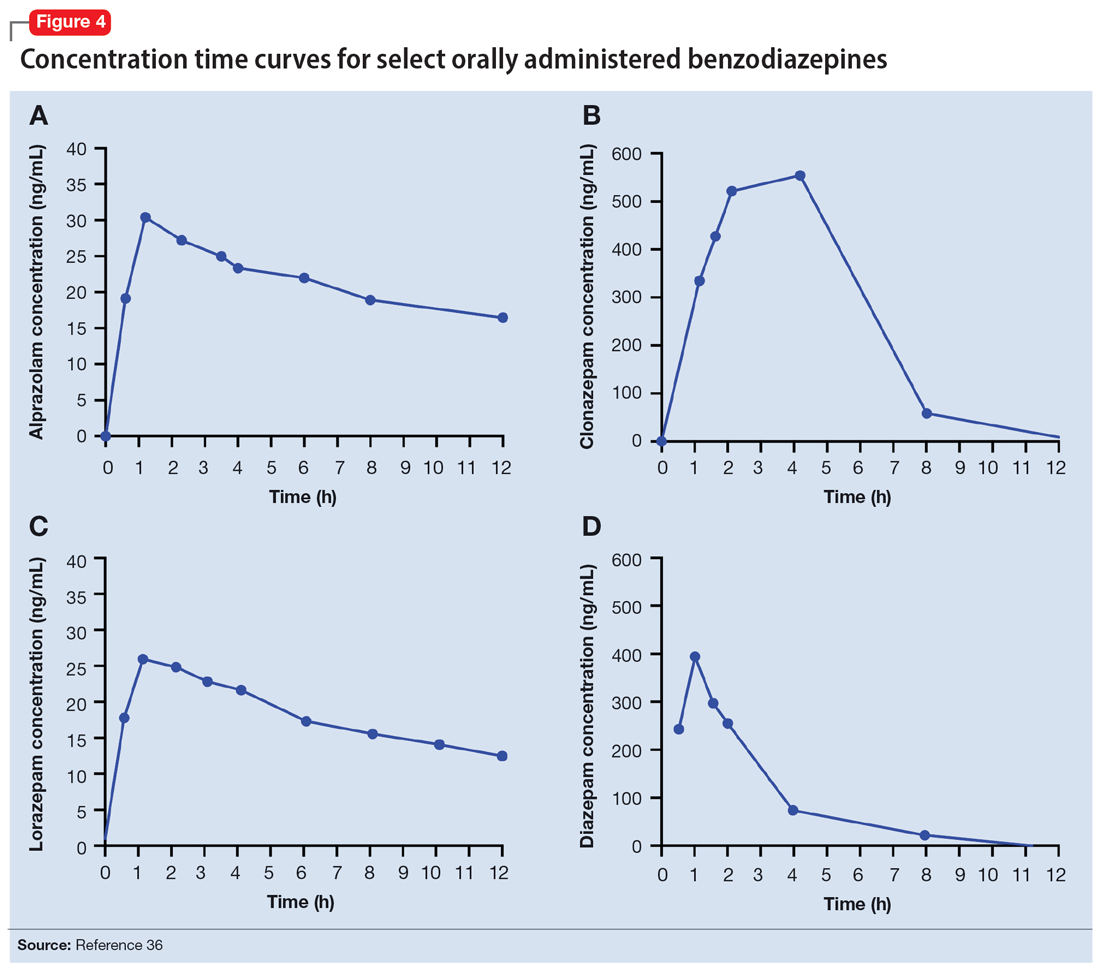
Continue to: Another aspect of the superiority...
Another aspect of the superiority of clonazepam in some research relates to its pharmacokinetic properties, particularly when compared with benzodiazepines that have very short half-lives. Short half-life benzodiazepines have been associated with rebound anxiety, which is defined as “the relative worsening of symptoms on discontinuation of treatment as compared to baseline symptoms” and is distinct from withdrawal.64 While it is difficult to assess this in clinical trials, Herman et al65 provided insight into the contribution of rebound anxiety in a study of patients with panic disorder treated with alprazolam who experienced “interdose anxiety symptoms.” Of the 48 patients in this study, 41 switched to clonazepam, and most who switched (82%) experienced improvement. The improvement was attributed to the decreased frequency of clonazepam (vs alprazolam) administration and lack of interdose anxiety. When selecting an oral benzodiazepine, consider the duration, onset of action, and differences in metabolism that produce varying levels of effectiveness for individual patients. In situations where rapid onset is desired, a short-acting benzodiazepine may be preferable, while a longer-acting benzodiazepine would be preferable in situations where the patient needs sustained effects.
Regarding lipophilicity, differences among benzodiazepines could contribute to differences in psychological dependence and differential utility in some situations. For example, alprazolam rapidly enters the CNS, producing an immediate anxiolytic effect. However, its egress from the CNS is equally rapid, and its anxiolytic effects disappear quickly. This may be desirable for addressing acute, predictable anxiety, but could have unintended consequences in treating chronic anxiety, where it could facilitate psychological dependence.
Practical considerations
When prescribing benzodiazepines, consider a myriad of patient- and medication-specific factors, as these have clinically relevant implications on treatment response. This information, taken together, supports the importance of an individualized approach to benzodiazepine use. Before selecting a benzodiazepine and during treatment, important elements of the patient’s history must be considered, including age, body weight, concomitant medication use (eg, antacids, CYP3A4 inhibitors, OCPs), smoking status, and history of hepatic or renal disease.
Patients age <18 are unlikely to have full expression of GABA receptors in the brain30 and therefore benzodiazepines may not be as efficacious for anxiolysis in this population. Moreover, compared to younger patients, older patients may experience higher steady-state concentrations of benzodiazepines, especially lipophilic agents, due to an increased volume of distribution and decreased clearance. In patients treated with OCPs, some benzodiazepines may take longer to reach steady-state, and dose adjustments may need to be considered. In patients who smoke, clearance of some oral benzodiazepines is also accelerated, potentially decreasing half-life by up to 50%.
When dosing and titrating benzodiazepines, consider the patient’s body weight, particularly if they are obese. The effects of obesity on benzodiazepine pharmacokinetics are complex. For glucuronidated benzodiazepines, clearance is increased in patients who are obese; however, the volume of distribution is also increased in such patients, meaning it will take longer for benzodiazepines to achieve steady-state in these individuals compared to patients who are not obese. These effects suggest it may take longer to achieve a response at a given dose in patients who are obese compared to individuals who are not obese.
Continue to: The properties of individual benzodiazepines...
The properties of individual benzodiazepines should also be considered when selecting a benzodiazepine treatment. If circumstances necessitate rapid symptom relief, a lipophilic benzodiazepine, such as diazepam, may be preferred for quick onset and offset of action. Onset of action may also be hastened by taking the benzodiazepine without food; conversely, if peak adverse effects are problematic, concurrent consumption of a high-fat meal may help decrease peak concentration and prolonging absorption. In other circumstances, such as if sustained anxiolysis is desired, a clinician may opt for a less lipophilic benzodiazepine, such as clonazepam. Finally, in terms of general treatment response, benzodiazepines separate from placebo in the first week of treatment, which supports the idea they may be useful during the introduction of other medications (eg, SSRIs) that take a longer time to achieve clinical effect.
Bottom Line
The pharmacokinetics of benzodiazepines are intimately linked with the onset of action and duration of clinical effect and vary based on individual absorption and distribution/redistribution. Benzodiazepines’ clinical profile derives from their pharmacokinetic differences and is influenced by many factors, including age, body weight, concomitant medication use, smoking status, and hepatic or renal disease. Consider these factors to individualize the approach to using benzodiazepines and optimize tolerability and efficacy.
Related Resources
- Weber SR, Duchemin AM. Benzodiazepines: sensible prescribing in light of the risks. Current Psychiatry. 2018;17(2):22-27.
- Balon R. Benzodiazepines for anxious depression. Current Psychiatry. 2018;17(8):9-12.
Drug Brand Names
Alprazolam • Xanax
Chlordiazepoxide • Librium
Clobazam • Onfi
Clonazepam • Klonopin
Clorazepate • Gen-Xene
Diazepam • Valium
Diltiazem • Cardizem
Fluvoxamine • Luvox
Ganaxolone • Ztalmy
Ketoconazole • Nizoral
Lorazepam • Ativan
Midazolam • Versed
Temazepam • Restoril
Triazolam • Halcion
Verapamil • Calan
1. Rickels K, Moeller HJ. Benzodiazepines in anxiety disorders: reassessment of usefulness and safety. World J Biol Psychiatry. 2019;20(7):514-518. doi:10.1080/15622975.2018.1500031
2. Stevens JC, Pollack MH. Benzodiazepines in clinical practice: consideration of their long-term use and alternative agents. J Clin Psychiatry. 2005;66(Suppl 2):21-27.
3. Pollack MH, van Ameringen M, Simon NM, et al. A double-blind randomized controlled trial of augmentation and switch strategies for refractory social anxiety disorder. Am J Psychiatry. 2014;171(1):44-53. doi:10.1176/appi.ajp.2013.12101353
4. Strawn JR, Geracioti L, Rajdev N, et al. Pharmacotherapy for generalized anxiety disorder in adult and pediatric patients: an evidence-based treatment review. Expert Opin Pharmacother. 2018;19(10):1057-1070. doi:10.1080/14656566.2018.1491966
5. Karaca-Mandic P, Meara E, Morden NE. The growing problem of co-treatment with opioids and benzodiazepines. BMJ. 2017;356:j1224. doi:10.1136/bmj.j1224
6. Bachhuber MA, Hennessy S, Cunningham CO, et al. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996-2013. Am J Public Health. 2016;106(4):686-688. doi:10.2105/AJPH.2016.303061
7. Bentué-Ferrer D, Akwa Y. Benzodiazepines: Effects on memory functioning. In: Pandi-Perumal SR, Verster J, Monti J, et al, eds. Sleep Disorders: Diagnosis and Therapeutics. CRC Press; 2008:104-114. doi:10.3109/9780203091715-15
8. Pomara N, Facelle TM, Roth AE, et al. Dose-dependent retrograde facilitation of verbal memory in healthy elderly after acute oral lorazepam administration.Psychopharmacology (Berl). 2006;185(4):487-494. doi:10.1007/s00213-006-0336-0
9. Gray SL, Dublin S, Yu O, et al. Benzodiazepine use and risk of incident dementia or cognitive decline: prospective population based study. BMJ. 2016;352:i90. doi:10.1136/bmj.i90
10. Biétry FA, Pfeil AM, Reich O, et al. Benzodiazepine use and risk of developing Alzheimer’s disease: a case-control study based on Swiss claims data. CNS Drugs. 2017;31(3):245-251. doi:10.1007/s40263-016-0404-x
11. de Gage SB, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ. 2014;349g5205. doi:10.1136/bmj.g5205
12. Shah R, Raji MA, Westra J, et al. Association of co-prescribing of opioid and benzodiazepine substitutes with incident falls and fractures among older adults: a cohort study. BMJ Open. 2021;11(12):e052057. doi:10.1136/bmjopen-2021-052057
13. Guina J, Rossetter SR, DeRhodes BJ, et al. Benzodiazepines for PTSD: a systematic review and meta-analysis. J Psychiatr Pract. 2015;21(4):281-303.
14. Ekström MP, Bornefalk-Hermansson A, Abernethy AP, et al. Safety of benzodiazepines and opioids in very severe respiratory disease: national prospective study. BMJ. 2014;348:g445. doi:10.1136/bmj.g445
15. Donovan LM, Malte CA, Spece LJ, et al. Center predictors of long-term benzodiazepine use in chronic obstructive pulmonary disease and post-traumatic stress disorder. Ann Am Thorac Soc. 2019;16(9):1151-1157. doi:10.1513/AnnalsATS.201901-048OC
16. Sheehy O, Zhao JP, Bérard A. Association between incident exposure to benzodiazepines in early pregnancy and risk of spontaneous abortion. JAMA Psychiatry. 2019;76(9):948-957. doi:10.1001/jamapsychiatry.2019.0963
17. Kelly LE, Poon S, Madadi P, et al. Neonatal benzodiazepines exposure during breastfeeding. J Pediatr. 2012;161(3):448-451. doi:10.1016/j.jpeds.2012.03.003
18. Agarwal SD, Landon BE. Patterns in outpatient benzodiazepine prescribing in the United States. JAMA Netw Open. 2019;2(1):e187399. doi:10.1001/jamanetworkopen.2018.7399
19. Hirschtritt ME, Olfson M, Kroenke K. Balancing the risks and benefits of benzodiazepines. JAMA. 2021;325(4):347-348. doi:10.1001/jama.2020.22106
20. Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. McGraw-Hill Education; 2018.
21. Nutt DJ, Malizia AL. New insights into the role of the GABA(A)-benzodiazepine receptor in psychiatric disorder. British J Psychiatry. 2001;179:390-396. doi:10.1192/bjp.179.5.390
22. Sigel E. Mapping of the benzodiazepine recognition site on GABA(A) receptors. Curr Top Med Chem. 2002;2(8):833-839. doi:10.2174/1568026023393444
23. Savic
24. Smith TA. Type A gamma-aminobutyric acid (GABAA) receptor subunits and benzodiazepine binding: significance to clinical syndromes and their treatment. Br J Biomed Sci. 2001;58(2):111-121.
25. Althaus AL, Ackley MA, Belfort GM, et al. Preclinical characterization of zuranolone (SAGE-217), a selective neuroactive steroid GABAA receptor positive allosteric modulator. Neuropharmacology. 2020;181:108333. doi:10.1016/j.neuropharm.2020.108333
26. Jacob TC, Michels G, Silayeva L, et al. Benzodiazepine treatment induces subtype-specific changes in GABA(A) receptor trafficking and decreases synaptic inhibition. Proc Natl Acad Sci U S A. 2012;109(45):18595-18600. doi:10.1073/pnas.1204994109
27. Nicholson MW, Sweeney A, Pekle E, et al. Diazepam-induced loss of inhibitory synapses mediated by PLCδ/ Ca2+/calcineurin signalling downstream of GABAA receptors. Mol Psychiatry. 2018;23(9):1851-1867. doi:10.1038/s41380-018-0100-y
28. Dobson ET, Bloch MH, Strawn JR. Efficacy and tolerability of pharmacotherapy for pediatric anxiety disorders: a network meta-analysis. J Clin Psychiatry. 2019;80(1):17r12064. doi:10.4088/JCP.17r12064
29. Kuang H, Johnson JA, Mulqueen JM, et al. The efficacy of benzodiazepines as acute anxiolytics in children: a meta-analysis. Depress Anxiety. 2017;34(10):888-896. doi:10.1002/da.22643
30. Chugani DC, Muzik O, Juhász C, et al. Postnatal maturation of human GABAA receptors measured with positron emission tomography. Ann Neurol. 2001;49(5):618-626. doi:10.1002/ana.1003
31. Jochemsen R, Breimer DD. Pharmacokinetics of benzodiazepines: metabolic pathways and plasma level profiles. Curr Med Res Opin. 1984;8(Suppl 4):60-79. doi:10.1185/03007998409109545
32. Greenblatt DJ, Harmatz JS, Dorsey C, et al. Comparative single-dose kinetics and dynamics of lorazepam, alprazolam, prazepam, and placebo. Clin Pharmacol Ther. 1988;44(3)326-334. doi:10.1038/clpt.1988.158
33. Shader RI, Georgotas A, Greenblatt DJ, et al. Impaired absorption of desmethydiazepam from clorazepate by magnesium aluminum hydroxide. Clin Pharmacol Ther. 1978;24(3):308-315. doi:10.1002/cpt1978243308
34. Greenblatt DJ, Allen MD, MacLaughlin DS, et al. Diazepam absorption: effect of antacids and food. Clin Pharmacol Ther. 1978;24(5):600-609. doi:10.1002/cpt1978245600
35. Yamazaki A, Kumagai Y, Fujita T, et al. Different effects of light food on pharmacokinetics and pharmacodynamics of three benzodiazepines, quazepam, nitrazepam and diazepam. J Clin Pharm Ther. 2007;32(1):31-39. doi:10.1111/j.1365-2710.2007.00795.x
36. Stimpfl J, Mills JA, Strawn JR. Pharmacologic predictors of benzodiazepine response trajectory in anxiety disorders: a Bayesian hierarchical modeling meta-analysis. CNS Spectr. 2023;28(1):53-60. doi:10.1017/S1092852921000870
37. Griffin CE 3rd, Kaye AM, Bueno FR, et al. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214-223.
38. Buffett-Jerrott SE, Stewart SH. Cognitive and sedative effects of benzodiazepine use. Curr Pharm Des. 2005;8(1):45-58. doi:10.2174/1381612023396654
39. Fukasawa T, Suzuki A, Otani K. Effects of genetic polymorphism of cytochrome P450 enzymes on the pharmacokinetics of benzodiazepines. J Clin Pharm Ther. 2007;32(4):333-341. doi:10.1111/j.1365-2710.2007.00829.x
40. Kraus JW, Desmond PV, Marshall JP, et al. Effects of aging and liver disease on disposition of lorazepam. Clin Pharmacol Ther. 1978;24(4):411-419. doi:10.1002/cpt1978244411
41. Greenblatt DJ. Clinical pharmacokinetics of oxazepam and lorazepam. Clin Pharmacokinet. 1981;6(2):89-105. doi:10.2165/00003088-198106020-00001
42. Walkenstein SS, Wiser R, Gudmundsen CH, et al. Absorption, metabolism, and excretion of oxazepam and its succinate half‐ester. J Pharm Sci. 1964;53(10):1181-1186. doi:10.1002/jps.2600531010
43. Shull HJ, Wilkinson GR, Johnson R, et al. Normal disposition of oxazepam in acute viral hepatitis and cirrhosis. Ann Intern Med. 1976;84(4):420-425. doi:10.7326/0003-4819-84-4-420
44. Abernethy DR, Greenblatt DJ, Ochs HR, et al. Lorazepam and oxazepam kinetics in women on low-dose oral contraceptives. Clin Pharmacol Ther. 1983;33(5):628-632. doi:10.1038/clpt.1983.85
45. Greenblatt DJ, Allen MD, Harmatz JS, et al. Diazepam disposition determinants. Clin Pharmacol Ther. 1980;27(3):301-312. doi:10.1038/clpt.1980.40
46. Ochs HR, Greenblatt DJ, Knüchel M. Kinetics of diazepam, midazolam, and lorazepam, in cigarette smokers. Chest. 1985;87(2):223-226. doi:10.1378/chest.87.2.223
47. Smith RB, Gwilt PR, Wright CE 3rd. Single- and multiple-dose pharmacokinetics of oral alprazolam in healthy smoking and nonsmoking men. Clin Pharm. 1983;2(2):139-143.
48. Figgitt DP, McClellan KJ. Fluvoxamine. An updated review of its use in the management of adults with anxiety disorders. Drugs. 2000;60(4):925-954. doi:10.2165/00003495-200060040-00006
49. Greenblatt DJ, Wright CE. Clinical pharmacokinetics of alprazolam. Therapeutic implications. Clin Pharmacokinet. 1993;24(6):453-471. doi:10.2165/00003088-199324060-00003
50. Yasui N, Kondo T, Furukori H, et al. Effects of repeated ingestion of grapefruit juice on the single and multiple oral-dose pharmacokinetics and pharmacodynamics of alprazolam. Psychopharmacology (Berl). 2000;150(2):185-190. doi:10.1007/s002130000438
51. Özdemir M, Aktan Y, Boydagˇ BS, et al. Interaction between grapefruit juice and diazepam in humans. Eur J Drug Metab Pharmacokinet. 1998;23(1):55-59. doi:10.1007/BF03189827
52. Greenblatt DJ, Harmatz JS, Zhang Q, et al. Slow accumulation and elimination of diazepam and its active metabolite with extended treatment in the elderly. J Clin Pharmacol. 2021;61(2):193-203. doi:10.1002/jcph.1726
53. Abernethy DR, Greenblatt DJ. Drug disposition in obese humans: an update. Clin Pharmacokinet. 1986;11(3):199-213. doi:10.2165/00003088-198611030-00002
54. Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49(2):71-87. doi:10.2165/11318100-000000000-00000
55. Bauer LA. Drug Dosing in special populations: renal and hepatic disease, dialysis, heart failure, obesity, and drug interactions. In: Weitz M, Thomas, CM, eds. Applied Clinical Pharmacokinetics. 3rd ed. McGraw-Hill Education; 2014. https://accesspharmacy.mhmedical.com/book.aspx?bookid=1374
56. Kendrick JG, Carr RR, Ensom MHH. Pharmacokinetics and drug dosing in obese children. J Pediatr Pharmacol Ther. 2010;15(2):94-109. doi:10.5863/1551-6776-15.2.94
57. Brill MJE, Diepstraten J, van Rongen A, et al. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51(5):277-304. doi:10.2165/11599410-000000000-00000
58. Derry CL, Kroboth PD, Pittenger AL, et al. Pharmacokinetics and pharmacodynamics of triazolam after two intermittent doses in obese and normal-weight men. J Clin Psychopharmacol. 1995;15(3):197-205. doi:10.1097/00004714-199506000-00008
59. Abernethy DR, Greenblatt DJ, Divoll M, et al. The influence of obesity on the pharmacokinetics of oral alprazolam and triazolam. Clin Pharmacokinet. 1984;9(2):177-183. doi:10.2165/00003088-198409020-00005
60. Abernethy DR, Greenblatt DJ, Divoll M, et al. Prolonged accumulation of diazepam in obesity. J Clin Pharmacol. 1983;23(8-9):369-376. doi:10.1002/j.1552-4604.1983.tb02750.x
61. Abernethy DR, Greenblatt DJ, Divoll M, et al. Enhanced glucuronide conjugation of drugs in obesity: studies of lorazepam, oxazepam, and acetaminophen. J Lab Clin Med. 1983;101(6):873-880.
62. Greenblatt DJ, von Moltke LL, Harmatz JS, et al. Alprazolam pharmacokinetics, metabolism, and plasma levels: clinical implications. J Clin Psychiatry. 1993;54 Suppl:4-11.
63. Chen YT, Liu CY, Chang CM, et al. Perceptions, clinical characteristics, and other factors associated with prolonged and high daily dose of benzodiazepine use among patients with anxiety or depressive disorders. J Affect Disord. 2020;271:215-223. doi:10.1016/j.jad.2020.03.077
64. Herman JB, Brotman AW, Rosenbaum JF. Rebound anxiety in panic disorder patients treated with shorter-acting benzodiazepines. J Clin Psychiatry. 1987;48(Suppl):22-28.
65. Herman JB, Rosenbaum JF, Brotman AW. The alprazolam to clonazepam switch for the treatment of panic disorder. J Clin Psychopharmacol. 1987;7(3):175-178.
Though once the main treatment for anxiety disorders—often as monotherapy1—benzodiazepines are now primarily used as adjunctive agents.2-4 Their ability to produce rapid anxiolysis represents a significant therapeutic advantage, but in recent decades their tolerability, class-specific risks, and lack of antidepressant properties contributed to benzodiazepines being largely replaced by selective serotonin reuptake inhibitors (SSRIs) for the pharmacologic treatment of anxiety. This shift within the pharmacologic armamentarium has decreased many clinicians’ familiarity with benzodiazepines.
While benzodiazepines continue to have an important role in managing anxiety disorders, particularly treatment-resistant anxiety,4 clinicians must consider the limitations of these agents. Benzodiazepines can be associated with abuse and dependence, and overdose risk when combined with opiates.5,6 They may cause memory impairment7,8 and conflicting data suggest they may contribute to the risk of developing cognitive disorders.9-11 Benzodiazepines also have been associated with falls and fractures,12 and worse outcomes in patients with posttraumatic stress disorder.13 Some studies of patients with chronic obstructive pulmonary disease (COPD) found benzodiazepines may increase the risk of COPD exacerbations and accidental overdose,14 though others found that was not always the case.15 Benzodiazepines may be associated with an increased risk of spontaneous abortion when used early in pregnancy.16 Prospective research in women who were breastfeeding found benzodiazepines may cause sedation in up to 2% of infants.17
Despite the potential for adverse effects, benzodiazepine use remains common.18 These medications have a rapid onset of action, are useful for breakthrough symptoms, may enhance treatment adherence, and alleviate activating symptoms of SSRIs. Like other commonly used medications, benzodiazepines have the potential for both harm and benefit.19 Similar to other medications with tolerability concerns but established efficacy, particularly in treatment-resistant anxiety disorders, it is important to balance “overprescribing … to patients at risk and underusing these effective medications when indicated.”19 Though the use of benzodiazepines has been discouraged and perceptions have shifted, knowledge of benzodiazepines and benzodiazepine pharmacology also has been degraded contemporaneously.
This article provides a synthesis of the clinically relevant pharmacology of benzodiazepines, with a focus on orally administered benzodiazepines, which are more common in outpatient clinical practice. Specifically, this review describes the pharmacology of benzodiazepines, benzodiazepine medication interactions, the relationship between pharmacologic characteristics and treatment response/tolerability, and selection and dosing of oral benzodiazepines (Table20).

Benzodiazepine pharmacodynamics
Benzodiazepines act at the gamma-aminobutyric acid (GABA)-A receptor complex and bind allosterically.21-23 Comprised of 5 glycoprotein subunits (2 alpha subunits, 2 beta subunits, and 1 gamma subunit), the receptor has 2 distinct sites at which the endogenous inhibitory transmitter GABA binds and 1 benzodiazepine binding site. Benzodiazepines bind within a socket created by the alpha and gamma subunits22 and after binding induce a conformational change in the receptor, which enhances GABA binding. There are 2 types of benzodiazepine receptors: BZ1 and BZ2. The subunits play a critical role in driving the pharmacologic characteristics of the receptor.24 BZ1 and BZ2 receptors bind benzodiazepines, although they are differentially distributed within the brain. Binding at BZ1 receptors—which are distributed in cortical, thalamic, and cerebellar regions—contributes to sedation and deleterious effects of benzodiazepines on memory (eg, anterograde amnesia). BZ2 receptors (which contain gamma-2 subunits) are responsible for anxiolytic and muscle-relaxing effects. They are distributed throughout limbic regions and motor tracts, including motor neurons and neurons in the dorsal horn of the spinal cord.24
Benzodiazepines—positive GABA-A receptor allosteric modulators—produce phasic inhibition, largely through the alpha and gamma subunits discussed above. In contrast, newer positive allosteric modulators (eg, zuranolone) bind at the alpha/beta subunits.25 Mechanistically, endogenous neuroactive steroids and nonbenzodiazepine GABA-A–positive allosteric modulators such as zuranolone and ganaxolone also differ in their regulation of GABA-A (downregulated with benzodiazepines and hypothetically upregulated with zuranolone)26 and their synaptic effects (benzodiazepines synaptically vs endogenous neurosteroids and nonbenzodiazpine positive allosteric modulators extrasynaptically).27
From a developmental perspective, benzodiazepines may have less efficacy for anxiolysis and worse tolerability in some pediatric patients,28 although they generally appear effective for immediate use to treat anxiety in acute settings.29 The differences in efficacy and tolerability may be related to pharmacodynamic differences between pediatric populations and adults. GABA receptor expression and function do not reach adult levels until age 14 to 17½ for subcortical regions and age 18 to 22 for cortical regions, although girls reach adult expression of GABA receptors slightly earlier than boys.30 D
Continue to: Pharmacology and clinical effects
Pharmacology and clinical effects
Benzodiazepine pharmacokinetics are intimately linked with the onset of action and duration of clinical effect and vary based on the route of administration, absorption, and distribution/redistribution.31 In this review, we focus on oral administration as opposed to IV, IM, sublingual, or intranasal administration.
Absorption
Benzodiazepines are rapidly absorbed after oral administration and quickly enter the systemic circulation. However, absorption rates vary depending on specific aspects of the gastrointestinal milieu and intrinsic properties of the benzodiazepine. For example, alprazolam is more rapidly absorbed than most other benzodiazepines, with a Tmax of 1.8 hours compared to lorazepam, which has a Tmax of approximately 2 hours. These pharmacokinetic effects instantiate differences in tolerability and efficacy. Thus, following single doses of alprazolam and diazepam, self-rated sedating effects and impairment on a task of working memory suggest that effects have a more rapid onset for alprazolam relative to lorazepam.32 Food and concomitant medications can significantly affect benzodiazepine absorption. A single-dose, 3-way crossover study demonstrated that taking diazepam concomitantly with an antacid (eg, aluminum hydroxide) decreased peak concentrations and prolonged absorption by approximately 30 minutes. However, total absorption of the medication was unaffected.33 Additionally, administration of diazepam with food significantly slows absorption from 1 hour 15 minutes to approximately 2 hours 30 minutes and increases benzodiazepine absorption by 25% (Figure 134); the fat content of the meal appears important in moderating this effect.35 The impact of food on alprazolam varies by formulation. For example, when administered in an extended-release (XR) formulation with a high-fat meal, alprazolam absorption increases by one-third, while absorption for administration of the orally disintegrating tablet with a high-fat meal increases from 1 hour 30 minutes to 2 hours. Similarly, for lorazepam, administration with a meal delays absorption by approximately 2 hours; however, this effect does not appear present with the XR formulation. Administering benzodiazepines with food can be clinically leveraged to either accelerate the onset of action or decrease peak-associated adverse effects. Thus, when a highly lipophilic benzodiazepine is needed to treat acute anxiety or prior to an expected anxiogenic stimuli, administering the medication without food may produce a faster onset of action.

CNS penetration
Benzodiazepines enter the CNS by passive diffusion. Because of this, lipophilicity at physiologic pH influences the rate at which a benzodiazepine crosses the blood-brain barrier. The rate at which benzodiazepines enter the CNS influences their clinical effects and the speed at which both efficacy (ie, anxiolysis) and adverse effects (ie, sedation, slowed cognition) are observed. In general, more lipophilic medications initiate their anxiolytic effect more quickly. However, by quickly leaving the CNS (through the same mechanism that allowed them to enter the CNS at such speed), their effects rapidly cease as they redistribute into fat. Thus, highly lipophilic benzodiazepines produce more intense effects compared to less lipophilic benzodiazepines. For these reasons, lipophilicity is more important than half-life for determining the duration of effect in most patients.
Lipophilicity and duration of effect
Benzodiazepines and their metabolites tend to be highly protein-bound and distributed in fat- and lipid-enriched areas such as the CNS. As a result, the more lipophilic agents generally have the highest rates of absorption and the fastest onset of clinical effects. The duration of action for many benzodiazepines is determined by the rate and extent of distribution (a function of lipophilicity) rather than by the rate of elimination. For example, diazepam has a longer half-life than lorazepam, but its duration of action following a single dose is shorter. This is because diazepam is more lipophilic and therefore more extensively distributed (particularly to adipose tissue). This results in it leaving the brain and blood and distributing to other tissues. In turn, its CNS effect (ie, anxiolytic effects) are more quickly terminated.
By contrast, less lipophilic benzodiazepines maintain their CNS concentrations longer; they have a longer duration of action because of their slower redistribution, which culminates in a shorter half-life, and are less extensively distributed to peripheral tissues. In essence, this means that (other things being equal) a less lipophilic benzodiazepine produces a more sustained anxiolytic effect compared to a highly lipophilic benzodiazepine.36 Lipophilicity is also important in predicting some cognitive adverse effects, including amnesia. Benzodiazepines with high lipophilicity have greater absorption and faster onset of action as well as more rapid amnestic effects.37,38 These effects may relate to overall efficacy differences for oral benzodiazepines. A recent meta-analysis by Stimpfl et al36 found that less lipophilic benzodiazepines produced a greater response compared to more lipophilic benzodiazepines.
Continue to: Metabolism
Metabolism
Regarding cytochrome P450 (CYP) metabolism, polymorphic CYP2C19 and CYP3A4/5 are involved in the metabolism of several benzodiazepines39 and CYP2B6 has been recognized as a contributor to diazepam metabolism. CYP3A5 gene polymorphisms may produce variation in alprazolam metabolism; however, the predominant cytochrome involved in the metabolism of oxidatively metabolized benzodiazepines (ie, benzodiazepines other than lorazepam, oxazepam, and temazepam) is primarily CYP3A4, and most effects on CYP3A4 activity are related to concomitant medications and other nongenetic factors.
Drug-drug interactions
Apart from lorazepam,40,41 oxazepam,42,43 and temazepam, most benzodiazepines are metabolized through oxidative mechanisms that involve CYP3A4 (Figure 220).39 As such, their metabolism is influenced by medications that impact CYP3A4, including antifungals (eg, ketoconazole), calcium channel blockers (eg, verapamil, diltiazem), nefazodone, some protease inhibitors, and macrolide antibiotics. Research has examined the impact of low-dose estrogen oral contraceptives (OCPs) on exposure (eg, plasma concentrations) of several benzodiazepines. The mechanism for this interaction is likely complex and putatively involves multiple pathways, including inhibition of CYP3A4 by OCPs. The effects of OCPs on benzodiazepine pharmacokinetics vary based on the metabolism of the benzodiazepine. In general, medications oxidized and nitroreduced (eg, chlordiazepoxide, alprazolam, diazepam, and nitrazepam) have decreased clearance in patients treated with OCPs. Regarding nonoxidatively metabolized benzodiazepines, data are mixed. Research found no OCP-related effects on the pharmacokinetics of nonoxidatively metabolized benzodiazepines44; another study suggested that clearance of these medications—through increased glucuronidation—may be increased.31 The effect of smoking on benzodiazepine concentration has been well documented. Smoking increases the clearance of orally administered diazepam,45 but not IV diazepam, midazolam, or lorazepam, suggesting that this represents a first-pass effect.46 For alprazolam, plasma concentrations are reduced by 15% to 30% in smokers and total body clearance is 24% greater compared to nonsmokers, which results in an approximately 50% increase in half-life in nonsmokers compared to smokers.47 The most notable interaction between benzodiazepines and SSRIs is seen with fluvoxamine. Because fluvoxamine moderately inhibits CYP2C19 and CYP3A4 and potently inhibits CYP1A2,48 the clearance of oxidatively metabolized benzodiazepines is reduced.49 Additionally, the effects of grapefruit juice—a potent inhibitor of CYP3A4—has been evaluated for several benzodiazepines. Yasui et al50 found grapefruit juice did not alter alprazolam plasma concentrations. However, in separate research, grapefruit juice tripled diazepam exposure, increased peak concentrations 1.5-fold, and prolonged absorption.51

Hepatic disease
Exposure to benzodiazepines—other than lorazepam, oxazepam, and temazepam—is influenced by intrinsic hepatic disease and requires dose adjustment in individuals with significant hepatic impairment. The impact of hepatic disease on the clinical pharmacology of benzodiazepines may relate to 2 factors: protein binding and metabolism. In a study of individuals with cirrhosis, lorazepam binding was decreased, although its metabolism and clearance were largely unaffected.40
Aging and benzodiazepine metabolism/clearance
Aging is associated with myriad physiologic changes (eg, decrease in renal clearance after age 40, changes in body fat distribution, changes in activity of cytochromes) that are relevant to benzodiazepine pharmacology. They may underlie differences in the tolerability of benzodiazepines and other clinically relevant characteristics (eg, duration of action, accumulation).
Several studies have evaluated the impact of aging on the clearance and disposition of selected benzodiazepines. The respective half-lives of chlordiazepoxide and diazepam increase from 4- to 6-fold from age 20 to 80. Further, with chronic dosing, highly lipophilic benzodiazepines may require additional attention in geriatric patients. In a study that included individuals up to age 78, steady-state plasma concentrations of diazepam and its metabolite, desmethyldiazepam (DMDZ), were 30% to 35% higher in older patients compared to younger individuals.52 In this study, the half-lives for the young and older patients were 31 hours and 86 hours, respectively, for diazepam, and 40 hours and 80 hours, respectively, for the active metabolite. The half-life of diazepam is increased by “1 hour for each year of age beginning with a half-life of 20 hours at 20 years of age, as the volume of distribution is increased, and clearance is decreased.”52 Clinically, this implies that in older adults, clinicians should expect lower peak concentrations (Cmax), higher trough concentrations (Cmin), and that diazepam will take longer to reach steady-state concentrations. Taken together, these findings raised concern that “slow accumulation and delayed washout of diazepam and DMDZ is probable.”52 These findings—which may have more clinical relevance than those of single-dose studies—suggest that the effects related to diazepam would also take longer to resolve in older patients. Finally, lorazepam clearance or distribution does not appear to be affected by aging, at least in patients age 15 to 73.40 Alprazolam is more slowly cleared in geriatric patients and its effects may be potentiated by reduced protein binding.
Continue to: Obesity
Obesity
The distribution of medications, including benzodiazepines, is altered in patients who are obese because of increased adipose tissue.53,54 This increase in the volume of distribution can attenuate the onset of action, increase medication accumulation in fat, and potentiate the duration of action.55,56
Obesity may also affect hepatic metabolism by induction of CYP1A2, CYP2C9, and CYP2C19, and inhibition of CYP3A4.57 Triazolam, which is metabolized by CYP3A4, is associated with a greater exposure (ie, plasma concentrations) in individuals who are obese.58 However, when considering differences in benzodiazepine pharmacokinetics in patients who are obese, clinicians must remember that elimination half-life depends on both volume of distribution and clearance. In
How quickly do benzodiazepines work?
Benzodiazepines act quickly. Meta-analyses36 suggest that improvement in anxiety symptoms compared to placebo is greatest initially and then the rate of improvement slows over successive weeks. Research on benzodiazepines reveals statistically significant differences between benzodiazepines and placebo within the first week of treatment, with >80% of the expected improvement by Week 8 of treatment emerging by Week 4 (Figure 336). The rapid reduction in anxiety symptoms seen with benzodiazepines has important treatment implications, given that traditional psychotherapeutic and antidepressant treatments are slow to produce improvements. Consistent data suggesting that benzodiazepines work faster than other treatments support that they may have a role during the initiation of other treatments.

What is the ‘best’ dose?
As seen with other classes of psychotropic medications,4 the relationship between benzodiazepine dose and response is complex. In a recent meta-analysis of 65 placebo-controlled trials of benzodiazepines in adults with anxiety disorders, there was a superior response over time for low-dose benzodiazepines (<3 mg/d in lorazepam equivalents) compared to a medium dose (3 to 6 mg/d; P = .042); high-dose benzodiazepines (>6 mg/d) yielded less improvement compared to medium doses (P = .001).36 A study of adults with panic disorder similarly found the greatest responses with alprazolam plasma concentrations of 20 to 40 ng/mL, with no additional benefit at <20 ng/mL or >40 ng/mL.49 As plasma concentrations increase, adverse effects such as sedation also increase, which may confound the observed loss of a dose-response relationship at higher doses and plasma concentrations.62 This may, in part, account for the observation that higher doses of benzodiazepines are associated with greater depressive symptoms and disrupted sleep.63 As such, low doses may represent a delicate equipoise between efficacy and tolerability, yielding the most optimal clinical response.
Which benzodiazepine should I prescribe?
Comparing benzodiazepines is difficult, given the differences in dosing and disorders studied and differences in how each individual clinical trial was conducted. A meta-analysis by Stimpfl et al36 that used Bayesian hierarchical modeling, which allowed some of this heterogeneity to be addressed, found that relative to the reference benzodiazepine (lorazepam), clonazepam had the greatest trajectory/magnitude of response (other specific benzodiazepines did not statistically differ from lorazepam) (Figure 436).

Continue to: Another aspect of the superiority...
Another aspect of the superiority of clonazepam in some research relates to its pharmacokinetic properties, particularly when compared with benzodiazepines that have very short half-lives. Short half-life benzodiazepines have been associated with rebound anxiety, which is defined as “the relative worsening of symptoms on discontinuation of treatment as compared to baseline symptoms” and is distinct from withdrawal.64 While it is difficult to assess this in clinical trials, Herman et al65 provided insight into the contribution of rebound anxiety in a study of patients with panic disorder treated with alprazolam who experienced “interdose anxiety symptoms.” Of the 48 patients in this study, 41 switched to clonazepam, and most who switched (82%) experienced improvement. The improvement was attributed to the decreased frequency of clonazepam (vs alprazolam) administration and lack of interdose anxiety. When selecting an oral benzodiazepine, consider the duration, onset of action, and differences in metabolism that produce varying levels of effectiveness for individual patients. In situations where rapid onset is desired, a short-acting benzodiazepine may be preferable, while a longer-acting benzodiazepine would be preferable in situations where the patient needs sustained effects.
Regarding lipophilicity, differences among benzodiazepines could contribute to differences in psychological dependence and differential utility in some situations. For example, alprazolam rapidly enters the CNS, producing an immediate anxiolytic effect. However, its egress from the CNS is equally rapid, and its anxiolytic effects disappear quickly. This may be desirable for addressing acute, predictable anxiety, but could have unintended consequences in treating chronic anxiety, where it could facilitate psychological dependence.
Practical considerations
When prescribing benzodiazepines, consider a myriad of patient- and medication-specific factors, as these have clinically relevant implications on treatment response. This information, taken together, supports the importance of an individualized approach to benzodiazepine use. Before selecting a benzodiazepine and during treatment, important elements of the patient’s history must be considered, including age, body weight, concomitant medication use (eg, antacids, CYP3A4 inhibitors, OCPs), smoking status, and history of hepatic or renal disease.
Patients age <18 are unlikely to have full expression of GABA receptors in the brain30 and therefore benzodiazepines may not be as efficacious for anxiolysis in this population. Moreover, compared to younger patients, older patients may experience higher steady-state concentrations of benzodiazepines, especially lipophilic agents, due to an increased volume of distribution and decreased clearance. In patients treated with OCPs, some benzodiazepines may take longer to reach steady-state, and dose adjustments may need to be considered. In patients who smoke, clearance of some oral benzodiazepines is also accelerated, potentially decreasing half-life by up to 50%.
When dosing and titrating benzodiazepines, consider the patient’s body weight, particularly if they are obese. The effects of obesity on benzodiazepine pharmacokinetics are complex. For glucuronidated benzodiazepines, clearance is increased in patients who are obese; however, the volume of distribution is also increased in such patients, meaning it will take longer for benzodiazepines to achieve steady-state in these individuals compared to patients who are not obese. These effects suggest it may take longer to achieve a response at a given dose in patients who are obese compared to individuals who are not obese.
Continue to: The properties of individual benzodiazepines...
The properties of individual benzodiazepines should also be considered when selecting a benzodiazepine treatment. If circumstances necessitate rapid symptom relief, a lipophilic benzodiazepine, such as diazepam, may be preferred for quick onset and offset of action. Onset of action may also be hastened by taking the benzodiazepine without food; conversely, if peak adverse effects are problematic, concurrent consumption of a high-fat meal may help decrease peak concentration and prolonging absorption. In other circumstances, such as if sustained anxiolysis is desired, a clinician may opt for a less lipophilic benzodiazepine, such as clonazepam. Finally, in terms of general treatment response, benzodiazepines separate from placebo in the first week of treatment, which supports the idea they may be useful during the introduction of other medications (eg, SSRIs) that take a longer time to achieve clinical effect.
Bottom Line
The pharmacokinetics of benzodiazepines are intimately linked with the onset of action and duration of clinical effect and vary based on individual absorption and distribution/redistribution. Benzodiazepines’ clinical profile derives from their pharmacokinetic differences and is influenced by many factors, including age, body weight, concomitant medication use, smoking status, and hepatic or renal disease. Consider these factors to individualize the approach to using benzodiazepines and optimize tolerability and efficacy.
Related Resources
- Weber SR, Duchemin AM. Benzodiazepines: sensible prescribing in light of the risks. Current Psychiatry. 2018;17(2):22-27.
- Balon R. Benzodiazepines for anxious depression. Current Psychiatry. 2018;17(8):9-12.
Drug Brand Names
Alprazolam • Xanax
Chlordiazepoxide • Librium
Clobazam • Onfi
Clonazepam • Klonopin
Clorazepate • Gen-Xene
Diazepam • Valium
Diltiazem • Cardizem
Fluvoxamine • Luvox
Ganaxolone • Ztalmy
Ketoconazole • Nizoral
Lorazepam • Ativan
Midazolam • Versed
Temazepam • Restoril
Triazolam • Halcion
Verapamil • Calan
Though once the main treatment for anxiety disorders—often as monotherapy1—benzodiazepines are now primarily used as adjunctive agents.2-4 Their ability to produce rapid anxiolysis represents a significant therapeutic advantage, but in recent decades their tolerability, class-specific risks, and lack of antidepressant properties contributed to benzodiazepines being largely replaced by selective serotonin reuptake inhibitors (SSRIs) for the pharmacologic treatment of anxiety. This shift within the pharmacologic armamentarium has decreased many clinicians’ familiarity with benzodiazepines.
While benzodiazepines continue to have an important role in managing anxiety disorders, particularly treatment-resistant anxiety,4 clinicians must consider the limitations of these agents. Benzodiazepines can be associated with abuse and dependence, and overdose risk when combined with opiates.5,6 They may cause memory impairment7,8 and conflicting data suggest they may contribute to the risk of developing cognitive disorders.9-11 Benzodiazepines also have been associated with falls and fractures,12 and worse outcomes in patients with posttraumatic stress disorder.13 Some studies of patients with chronic obstructive pulmonary disease (COPD) found benzodiazepines may increase the risk of COPD exacerbations and accidental overdose,14 though others found that was not always the case.15 Benzodiazepines may be associated with an increased risk of spontaneous abortion when used early in pregnancy.16 Prospective research in women who were breastfeeding found benzodiazepines may cause sedation in up to 2% of infants.17
Despite the potential for adverse effects, benzodiazepine use remains common.18 These medications have a rapid onset of action, are useful for breakthrough symptoms, may enhance treatment adherence, and alleviate activating symptoms of SSRIs. Like other commonly used medications, benzodiazepines have the potential for both harm and benefit.19 Similar to other medications with tolerability concerns but established efficacy, particularly in treatment-resistant anxiety disorders, it is important to balance “overprescribing … to patients at risk and underusing these effective medications when indicated.”19 Though the use of benzodiazepines has been discouraged and perceptions have shifted, knowledge of benzodiazepines and benzodiazepine pharmacology also has been degraded contemporaneously.
This article provides a synthesis of the clinically relevant pharmacology of benzodiazepines, with a focus on orally administered benzodiazepines, which are more common in outpatient clinical practice. Specifically, this review describes the pharmacology of benzodiazepines, benzodiazepine medication interactions, the relationship between pharmacologic characteristics and treatment response/tolerability, and selection and dosing of oral benzodiazepines (Table20).

Benzodiazepine pharmacodynamics
Benzodiazepines act at the gamma-aminobutyric acid (GABA)-A receptor complex and bind allosterically.21-23 Comprised of 5 glycoprotein subunits (2 alpha subunits, 2 beta subunits, and 1 gamma subunit), the receptor has 2 distinct sites at which the endogenous inhibitory transmitter GABA binds and 1 benzodiazepine binding site. Benzodiazepines bind within a socket created by the alpha and gamma subunits22 and after binding induce a conformational change in the receptor, which enhances GABA binding. There are 2 types of benzodiazepine receptors: BZ1 and BZ2. The subunits play a critical role in driving the pharmacologic characteristics of the receptor.24 BZ1 and BZ2 receptors bind benzodiazepines, although they are differentially distributed within the brain. Binding at BZ1 receptors—which are distributed in cortical, thalamic, and cerebellar regions—contributes to sedation and deleterious effects of benzodiazepines on memory (eg, anterograde amnesia). BZ2 receptors (which contain gamma-2 subunits) are responsible for anxiolytic and muscle-relaxing effects. They are distributed throughout limbic regions and motor tracts, including motor neurons and neurons in the dorsal horn of the spinal cord.24
Benzodiazepines—positive GABA-A receptor allosteric modulators—produce phasic inhibition, largely through the alpha and gamma subunits discussed above. In contrast, newer positive allosteric modulators (eg, zuranolone) bind at the alpha/beta subunits.25 Mechanistically, endogenous neuroactive steroids and nonbenzodiazepine GABA-A–positive allosteric modulators such as zuranolone and ganaxolone also differ in their regulation of GABA-A (downregulated with benzodiazepines and hypothetically upregulated with zuranolone)26 and their synaptic effects (benzodiazepines synaptically vs endogenous neurosteroids and nonbenzodiazpine positive allosteric modulators extrasynaptically).27
From a developmental perspective, benzodiazepines may have less efficacy for anxiolysis and worse tolerability in some pediatric patients,28 although they generally appear effective for immediate use to treat anxiety in acute settings.29 The differences in efficacy and tolerability may be related to pharmacodynamic differences between pediatric populations and adults. GABA receptor expression and function do not reach adult levels until age 14 to 17½ for subcortical regions and age 18 to 22 for cortical regions, although girls reach adult expression of GABA receptors slightly earlier than boys.30 D
Continue to: Pharmacology and clinical effects
Pharmacology and clinical effects
Benzodiazepine pharmacokinetics are intimately linked with the onset of action and duration of clinical effect and vary based on the route of administration, absorption, and distribution/redistribution.31 In this review, we focus on oral administration as opposed to IV, IM, sublingual, or intranasal administration.
Absorption
Benzodiazepines are rapidly absorbed after oral administration and quickly enter the systemic circulation. However, absorption rates vary depending on specific aspects of the gastrointestinal milieu and intrinsic properties of the benzodiazepine. For example, alprazolam is more rapidly absorbed than most other benzodiazepines, with a Tmax of 1.8 hours compared to lorazepam, which has a Tmax of approximately 2 hours. These pharmacokinetic effects instantiate differences in tolerability and efficacy. Thus, following single doses of alprazolam and diazepam, self-rated sedating effects and impairment on a task of working memory suggest that effects have a more rapid onset for alprazolam relative to lorazepam.32 Food and concomitant medications can significantly affect benzodiazepine absorption. A single-dose, 3-way crossover study demonstrated that taking diazepam concomitantly with an antacid (eg, aluminum hydroxide) decreased peak concentrations and prolonged absorption by approximately 30 minutes. However, total absorption of the medication was unaffected.33 Additionally, administration of diazepam with food significantly slows absorption from 1 hour 15 minutes to approximately 2 hours 30 minutes and increases benzodiazepine absorption by 25% (Figure 134); the fat content of the meal appears important in moderating this effect.35 The impact of food on alprazolam varies by formulation. For example, when administered in an extended-release (XR) formulation with a high-fat meal, alprazolam absorption increases by one-third, while absorption for administration of the orally disintegrating tablet with a high-fat meal increases from 1 hour 30 minutes to 2 hours. Similarly, for lorazepam, administration with a meal delays absorption by approximately 2 hours; however, this effect does not appear present with the XR formulation. Administering benzodiazepines with food can be clinically leveraged to either accelerate the onset of action or decrease peak-associated adverse effects. Thus, when a highly lipophilic benzodiazepine is needed to treat acute anxiety or prior to an expected anxiogenic stimuli, administering the medication without food may produce a faster onset of action.

CNS penetration
Benzodiazepines enter the CNS by passive diffusion. Because of this, lipophilicity at physiologic pH influences the rate at which a benzodiazepine crosses the blood-brain barrier. The rate at which benzodiazepines enter the CNS influences their clinical effects and the speed at which both efficacy (ie, anxiolysis) and adverse effects (ie, sedation, slowed cognition) are observed. In general, more lipophilic medications initiate their anxiolytic effect more quickly. However, by quickly leaving the CNS (through the same mechanism that allowed them to enter the CNS at such speed), their effects rapidly cease as they redistribute into fat. Thus, highly lipophilic benzodiazepines produce more intense effects compared to less lipophilic benzodiazepines. For these reasons, lipophilicity is more important than half-life for determining the duration of effect in most patients.
Lipophilicity and duration of effect
Benzodiazepines and their metabolites tend to be highly protein-bound and distributed in fat- and lipid-enriched areas such as the CNS. As a result, the more lipophilic agents generally have the highest rates of absorption and the fastest onset of clinical effects. The duration of action for many benzodiazepines is determined by the rate and extent of distribution (a function of lipophilicity) rather than by the rate of elimination. For example, diazepam has a longer half-life than lorazepam, but its duration of action following a single dose is shorter. This is because diazepam is more lipophilic and therefore more extensively distributed (particularly to adipose tissue). This results in it leaving the brain and blood and distributing to other tissues. In turn, its CNS effect (ie, anxiolytic effects) are more quickly terminated.
By contrast, less lipophilic benzodiazepines maintain their CNS concentrations longer; they have a longer duration of action because of their slower redistribution, which culminates in a shorter half-life, and are less extensively distributed to peripheral tissues. In essence, this means that (other things being equal) a less lipophilic benzodiazepine produces a more sustained anxiolytic effect compared to a highly lipophilic benzodiazepine.36 Lipophilicity is also important in predicting some cognitive adverse effects, including amnesia. Benzodiazepines with high lipophilicity have greater absorption and faster onset of action as well as more rapid amnestic effects.37,38 These effects may relate to overall efficacy differences for oral benzodiazepines. A recent meta-analysis by Stimpfl et al36 found that less lipophilic benzodiazepines produced a greater response compared to more lipophilic benzodiazepines.
Continue to: Metabolism
Metabolism
Regarding cytochrome P450 (CYP) metabolism, polymorphic CYP2C19 and CYP3A4/5 are involved in the metabolism of several benzodiazepines39 and CYP2B6 has been recognized as a contributor to diazepam metabolism. CYP3A5 gene polymorphisms may produce variation in alprazolam metabolism; however, the predominant cytochrome involved in the metabolism of oxidatively metabolized benzodiazepines (ie, benzodiazepines other than lorazepam, oxazepam, and temazepam) is primarily CYP3A4, and most effects on CYP3A4 activity are related to concomitant medications and other nongenetic factors.
Drug-drug interactions
Apart from lorazepam,40,41 oxazepam,42,43 and temazepam, most benzodiazepines are metabolized through oxidative mechanisms that involve CYP3A4 (Figure 220).39 As such, their metabolism is influenced by medications that impact CYP3A4, including antifungals (eg, ketoconazole), calcium channel blockers (eg, verapamil, diltiazem), nefazodone, some protease inhibitors, and macrolide antibiotics. Research has examined the impact of low-dose estrogen oral contraceptives (OCPs) on exposure (eg, plasma concentrations) of several benzodiazepines. The mechanism for this interaction is likely complex and putatively involves multiple pathways, including inhibition of CYP3A4 by OCPs. The effects of OCPs on benzodiazepine pharmacokinetics vary based on the metabolism of the benzodiazepine. In general, medications oxidized and nitroreduced (eg, chlordiazepoxide, alprazolam, diazepam, and nitrazepam) have decreased clearance in patients treated with OCPs. Regarding nonoxidatively metabolized benzodiazepines, data are mixed. Research found no OCP-related effects on the pharmacokinetics of nonoxidatively metabolized benzodiazepines44; another study suggested that clearance of these medications—through increased glucuronidation—may be increased.31 The effect of smoking on benzodiazepine concentration has been well documented. Smoking increases the clearance of orally administered diazepam,45 but not IV diazepam, midazolam, or lorazepam, suggesting that this represents a first-pass effect.46 For alprazolam, plasma concentrations are reduced by 15% to 30% in smokers and total body clearance is 24% greater compared to nonsmokers, which results in an approximately 50% increase in half-life in nonsmokers compared to smokers.47 The most notable interaction between benzodiazepines and SSRIs is seen with fluvoxamine. Because fluvoxamine moderately inhibits CYP2C19 and CYP3A4 and potently inhibits CYP1A2,48 the clearance of oxidatively metabolized benzodiazepines is reduced.49 Additionally, the effects of grapefruit juice—a potent inhibitor of CYP3A4—has been evaluated for several benzodiazepines. Yasui et al50 found grapefruit juice did not alter alprazolam plasma concentrations. However, in separate research, grapefruit juice tripled diazepam exposure, increased peak concentrations 1.5-fold, and prolonged absorption.51

Hepatic disease
Exposure to benzodiazepines—other than lorazepam, oxazepam, and temazepam—is influenced by intrinsic hepatic disease and requires dose adjustment in individuals with significant hepatic impairment. The impact of hepatic disease on the clinical pharmacology of benzodiazepines may relate to 2 factors: protein binding and metabolism. In a study of individuals with cirrhosis, lorazepam binding was decreased, although its metabolism and clearance were largely unaffected.40
Aging and benzodiazepine metabolism/clearance
Aging is associated with myriad physiologic changes (eg, decrease in renal clearance after age 40, changes in body fat distribution, changes in activity of cytochromes) that are relevant to benzodiazepine pharmacology. They may underlie differences in the tolerability of benzodiazepines and other clinically relevant characteristics (eg, duration of action, accumulation).
Several studies have evaluated the impact of aging on the clearance and disposition of selected benzodiazepines. The respective half-lives of chlordiazepoxide and diazepam increase from 4- to 6-fold from age 20 to 80. Further, with chronic dosing, highly lipophilic benzodiazepines may require additional attention in geriatric patients. In a study that included individuals up to age 78, steady-state plasma concentrations of diazepam and its metabolite, desmethyldiazepam (DMDZ), were 30% to 35% higher in older patients compared to younger individuals.52 In this study, the half-lives for the young and older patients were 31 hours and 86 hours, respectively, for diazepam, and 40 hours and 80 hours, respectively, for the active metabolite. The half-life of diazepam is increased by “1 hour for each year of age beginning with a half-life of 20 hours at 20 years of age, as the volume of distribution is increased, and clearance is decreased.”52 Clinically, this implies that in older adults, clinicians should expect lower peak concentrations (Cmax), higher trough concentrations (Cmin), and that diazepam will take longer to reach steady-state concentrations. Taken together, these findings raised concern that “slow accumulation and delayed washout of diazepam and DMDZ is probable.”52 These findings—which may have more clinical relevance than those of single-dose studies—suggest that the effects related to diazepam would also take longer to resolve in older patients. Finally, lorazepam clearance or distribution does not appear to be affected by aging, at least in patients age 15 to 73.40 Alprazolam is more slowly cleared in geriatric patients and its effects may be potentiated by reduced protein binding.
Continue to: Obesity
Obesity
The distribution of medications, including benzodiazepines, is altered in patients who are obese because of increased adipose tissue.53,54 This increase in the volume of distribution can attenuate the onset of action, increase medication accumulation in fat, and potentiate the duration of action.55,56
Obesity may also affect hepatic metabolism by induction of CYP1A2, CYP2C9, and CYP2C19, and inhibition of CYP3A4.57 Triazolam, which is metabolized by CYP3A4, is associated with a greater exposure (ie, plasma concentrations) in individuals who are obese.58 However, when considering differences in benzodiazepine pharmacokinetics in patients who are obese, clinicians must remember that elimination half-life depends on both volume of distribution and clearance. In
How quickly do benzodiazepines work?
Benzodiazepines act quickly. Meta-analyses36 suggest that improvement in anxiety symptoms compared to placebo is greatest initially and then the rate of improvement slows over successive weeks. Research on benzodiazepines reveals statistically significant differences between benzodiazepines and placebo within the first week of treatment, with >80% of the expected improvement by Week 8 of treatment emerging by Week 4 (Figure 336). The rapid reduction in anxiety symptoms seen with benzodiazepines has important treatment implications, given that traditional psychotherapeutic and antidepressant treatments are slow to produce improvements. Consistent data suggesting that benzodiazepines work faster than other treatments support that they may have a role during the initiation of other treatments.

What is the ‘best’ dose?
As seen with other classes of psychotropic medications,4 the relationship between benzodiazepine dose and response is complex. In a recent meta-analysis of 65 placebo-controlled trials of benzodiazepines in adults with anxiety disorders, there was a superior response over time for low-dose benzodiazepines (<3 mg/d in lorazepam equivalents) compared to a medium dose (3 to 6 mg/d; P = .042); high-dose benzodiazepines (>6 mg/d) yielded less improvement compared to medium doses (P = .001).36 A study of adults with panic disorder similarly found the greatest responses with alprazolam plasma concentrations of 20 to 40 ng/mL, with no additional benefit at <20 ng/mL or >40 ng/mL.49 As plasma concentrations increase, adverse effects such as sedation also increase, which may confound the observed loss of a dose-response relationship at higher doses and plasma concentrations.62 This may, in part, account for the observation that higher doses of benzodiazepines are associated with greater depressive symptoms and disrupted sleep.63 As such, low doses may represent a delicate equipoise between efficacy and tolerability, yielding the most optimal clinical response.
Which benzodiazepine should I prescribe?
Comparing benzodiazepines is difficult, given the differences in dosing and disorders studied and differences in how each individual clinical trial was conducted. A meta-analysis by Stimpfl et al36 that used Bayesian hierarchical modeling, which allowed some of this heterogeneity to be addressed, found that relative to the reference benzodiazepine (lorazepam), clonazepam had the greatest trajectory/magnitude of response (other specific benzodiazepines did not statistically differ from lorazepam) (Figure 436).

Continue to: Another aspect of the superiority...
Another aspect of the superiority of clonazepam in some research relates to its pharmacokinetic properties, particularly when compared with benzodiazepines that have very short half-lives. Short half-life benzodiazepines have been associated with rebound anxiety, which is defined as “the relative worsening of symptoms on discontinuation of treatment as compared to baseline symptoms” and is distinct from withdrawal.64 While it is difficult to assess this in clinical trials, Herman et al65 provided insight into the contribution of rebound anxiety in a study of patients with panic disorder treated with alprazolam who experienced “interdose anxiety symptoms.” Of the 48 patients in this study, 41 switched to clonazepam, and most who switched (82%) experienced improvement. The improvement was attributed to the decreased frequency of clonazepam (vs alprazolam) administration and lack of interdose anxiety. When selecting an oral benzodiazepine, consider the duration, onset of action, and differences in metabolism that produce varying levels of effectiveness for individual patients. In situations where rapid onset is desired, a short-acting benzodiazepine may be preferable, while a longer-acting benzodiazepine would be preferable in situations where the patient needs sustained effects.
Regarding lipophilicity, differences among benzodiazepines could contribute to differences in psychological dependence and differential utility in some situations. For example, alprazolam rapidly enters the CNS, producing an immediate anxiolytic effect. However, its egress from the CNS is equally rapid, and its anxiolytic effects disappear quickly. This may be desirable for addressing acute, predictable anxiety, but could have unintended consequences in treating chronic anxiety, where it could facilitate psychological dependence.
Practical considerations
When prescribing benzodiazepines, consider a myriad of patient- and medication-specific factors, as these have clinically relevant implications on treatment response. This information, taken together, supports the importance of an individualized approach to benzodiazepine use. Before selecting a benzodiazepine and during treatment, important elements of the patient’s history must be considered, including age, body weight, concomitant medication use (eg, antacids, CYP3A4 inhibitors, OCPs), smoking status, and history of hepatic or renal disease.
Patients age <18 are unlikely to have full expression of GABA receptors in the brain30 and therefore benzodiazepines may not be as efficacious for anxiolysis in this population. Moreover, compared to younger patients, older patients may experience higher steady-state concentrations of benzodiazepines, especially lipophilic agents, due to an increased volume of distribution and decreased clearance. In patients treated with OCPs, some benzodiazepines may take longer to reach steady-state, and dose adjustments may need to be considered. In patients who smoke, clearance of some oral benzodiazepines is also accelerated, potentially decreasing half-life by up to 50%.
When dosing and titrating benzodiazepines, consider the patient’s body weight, particularly if they are obese. The effects of obesity on benzodiazepine pharmacokinetics are complex. For glucuronidated benzodiazepines, clearance is increased in patients who are obese; however, the volume of distribution is also increased in such patients, meaning it will take longer for benzodiazepines to achieve steady-state in these individuals compared to patients who are not obese. These effects suggest it may take longer to achieve a response at a given dose in patients who are obese compared to individuals who are not obese.
Continue to: The properties of individual benzodiazepines...
The properties of individual benzodiazepines should also be considered when selecting a benzodiazepine treatment. If circumstances necessitate rapid symptom relief, a lipophilic benzodiazepine, such as diazepam, may be preferred for quick onset and offset of action. Onset of action may also be hastened by taking the benzodiazepine without food; conversely, if peak adverse effects are problematic, concurrent consumption of a high-fat meal may help decrease peak concentration and prolonging absorption. In other circumstances, such as if sustained anxiolysis is desired, a clinician may opt for a less lipophilic benzodiazepine, such as clonazepam. Finally, in terms of general treatment response, benzodiazepines separate from placebo in the first week of treatment, which supports the idea they may be useful during the introduction of other medications (eg, SSRIs) that take a longer time to achieve clinical effect.
Bottom Line
The pharmacokinetics of benzodiazepines are intimately linked with the onset of action and duration of clinical effect and vary based on individual absorption and distribution/redistribution. Benzodiazepines’ clinical profile derives from their pharmacokinetic differences and is influenced by many factors, including age, body weight, concomitant medication use, smoking status, and hepatic or renal disease. Consider these factors to individualize the approach to using benzodiazepines and optimize tolerability and efficacy.
Related Resources
- Weber SR, Duchemin AM. Benzodiazepines: sensible prescribing in light of the risks. Current Psychiatry. 2018;17(2):22-27.
- Balon R. Benzodiazepines for anxious depression. Current Psychiatry. 2018;17(8):9-12.
Drug Brand Names
Alprazolam • Xanax
Chlordiazepoxide • Librium
Clobazam • Onfi
Clonazepam • Klonopin
Clorazepate • Gen-Xene
Diazepam • Valium
Diltiazem • Cardizem
Fluvoxamine • Luvox
Ganaxolone • Ztalmy
Ketoconazole • Nizoral
Lorazepam • Ativan
Midazolam • Versed
Temazepam • Restoril
Triazolam • Halcion
Verapamil • Calan
1. Rickels K, Moeller HJ. Benzodiazepines in anxiety disorders: reassessment of usefulness and safety. World J Biol Psychiatry. 2019;20(7):514-518. doi:10.1080/15622975.2018.1500031
2. Stevens JC, Pollack MH. Benzodiazepines in clinical practice: consideration of their long-term use and alternative agents. J Clin Psychiatry. 2005;66(Suppl 2):21-27.
3. Pollack MH, van Ameringen M, Simon NM, et al. A double-blind randomized controlled trial of augmentation and switch strategies for refractory social anxiety disorder. Am J Psychiatry. 2014;171(1):44-53. doi:10.1176/appi.ajp.2013.12101353
4. Strawn JR, Geracioti L, Rajdev N, et al. Pharmacotherapy for generalized anxiety disorder in adult and pediatric patients: an evidence-based treatment review. Expert Opin Pharmacother. 2018;19(10):1057-1070. doi:10.1080/14656566.2018.1491966
5. Karaca-Mandic P, Meara E, Morden NE. The growing problem of co-treatment with opioids and benzodiazepines. BMJ. 2017;356:j1224. doi:10.1136/bmj.j1224
6. Bachhuber MA, Hennessy S, Cunningham CO, et al. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996-2013. Am J Public Health. 2016;106(4):686-688. doi:10.2105/AJPH.2016.303061
7. Bentué-Ferrer D, Akwa Y. Benzodiazepines: Effects on memory functioning. In: Pandi-Perumal SR, Verster J, Monti J, et al, eds. Sleep Disorders: Diagnosis and Therapeutics. CRC Press; 2008:104-114. doi:10.3109/9780203091715-15
8. Pomara N, Facelle TM, Roth AE, et al. Dose-dependent retrograde facilitation of verbal memory in healthy elderly after acute oral lorazepam administration.Psychopharmacology (Berl). 2006;185(4):487-494. doi:10.1007/s00213-006-0336-0
9. Gray SL, Dublin S, Yu O, et al. Benzodiazepine use and risk of incident dementia or cognitive decline: prospective population based study. BMJ. 2016;352:i90. doi:10.1136/bmj.i90
10. Biétry FA, Pfeil AM, Reich O, et al. Benzodiazepine use and risk of developing Alzheimer’s disease: a case-control study based on Swiss claims data. CNS Drugs. 2017;31(3):245-251. doi:10.1007/s40263-016-0404-x
11. de Gage SB, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ. 2014;349g5205. doi:10.1136/bmj.g5205
12. Shah R, Raji MA, Westra J, et al. Association of co-prescribing of opioid and benzodiazepine substitutes with incident falls and fractures among older adults: a cohort study. BMJ Open. 2021;11(12):e052057. doi:10.1136/bmjopen-2021-052057
13. Guina J, Rossetter SR, DeRhodes BJ, et al. Benzodiazepines for PTSD: a systematic review and meta-analysis. J Psychiatr Pract. 2015;21(4):281-303.
14. Ekström MP, Bornefalk-Hermansson A, Abernethy AP, et al. Safety of benzodiazepines and opioids in very severe respiratory disease: national prospective study. BMJ. 2014;348:g445. doi:10.1136/bmj.g445
15. Donovan LM, Malte CA, Spece LJ, et al. Center predictors of long-term benzodiazepine use in chronic obstructive pulmonary disease and post-traumatic stress disorder. Ann Am Thorac Soc. 2019;16(9):1151-1157. doi:10.1513/AnnalsATS.201901-048OC
16. Sheehy O, Zhao JP, Bérard A. Association between incident exposure to benzodiazepines in early pregnancy and risk of spontaneous abortion. JAMA Psychiatry. 2019;76(9):948-957. doi:10.1001/jamapsychiatry.2019.0963
17. Kelly LE, Poon S, Madadi P, et al. Neonatal benzodiazepines exposure during breastfeeding. J Pediatr. 2012;161(3):448-451. doi:10.1016/j.jpeds.2012.03.003
18. Agarwal SD, Landon BE. Patterns in outpatient benzodiazepine prescribing in the United States. JAMA Netw Open. 2019;2(1):e187399. doi:10.1001/jamanetworkopen.2018.7399
19. Hirschtritt ME, Olfson M, Kroenke K. Balancing the risks and benefits of benzodiazepines. JAMA. 2021;325(4):347-348. doi:10.1001/jama.2020.22106
20. Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. McGraw-Hill Education; 2018.
21. Nutt DJ, Malizia AL. New insights into the role of the GABA(A)-benzodiazepine receptor in psychiatric disorder. British J Psychiatry. 2001;179:390-396. doi:10.1192/bjp.179.5.390
22. Sigel E. Mapping of the benzodiazepine recognition site on GABA(A) receptors. Curr Top Med Chem. 2002;2(8):833-839. doi:10.2174/1568026023393444
23. Savic
24. Smith TA. Type A gamma-aminobutyric acid (GABAA) receptor subunits and benzodiazepine binding: significance to clinical syndromes and their treatment. Br J Biomed Sci. 2001;58(2):111-121.
25. Althaus AL, Ackley MA, Belfort GM, et al. Preclinical characterization of zuranolone (SAGE-217), a selective neuroactive steroid GABAA receptor positive allosteric modulator. Neuropharmacology. 2020;181:108333. doi:10.1016/j.neuropharm.2020.108333
26. Jacob TC, Michels G, Silayeva L, et al. Benzodiazepine treatment induces subtype-specific changes in GABA(A) receptor trafficking and decreases synaptic inhibition. Proc Natl Acad Sci U S A. 2012;109(45):18595-18600. doi:10.1073/pnas.1204994109
27. Nicholson MW, Sweeney A, Pekle E, et al. Diazepam-induced loss of inhibitory synapses mediated by PLCδ/ Ca2+/calcineurin signalling downstream of GABAA receptors. Mol Psychiatry. 2018;23(9):1851-1867. doi:10.1038/s41380-018-0100-y
28. Dobson ET, Bloch MH, Strawn JR. Efficacy and tolerability of pharmacotherapy for pediatric anxiety disorders: a network meta-analysis. J Clin Psychiatry. 2019;80(1):17r12064. doi:10.4088/JCP.17r12064
29. Kuang H, Johnson JA, Mulqueen JM, et al. The efficacy of benzodiazepines as acute anxiolytics in children: a meta-analysis. Depress Anxiety. 2017;34(10):888-896. doi:10.1002/da.22643
30. Chugani DC, Muzik O, Juhász C, et al. Postnatal maturation of human GABAA receptors measured with positron emission tomography. Ann Neurol. 2001;49(5):618-626. doi:10.1002/ana.1003
31. Jochemsen R, Breimer DD. Pharmacokinetics of benzodiazepines: metabolic pathways and plasma level profiles. Curr Med Res Opin. 1984;8(Suppl 4):60-79. doi:10.1185/03007998409109545
32. Greenblatt DJ, Harmatz JS, Dorsey C, et al. Comparative single-dose kinetics and dynamics of lorazepam, alprazolam, prazepam, and placebo. Clin Pharmacol Ther. 1988;44(3)326-334. doi:10.1038/clpt.1988.158
33. Shader RI, Georgotas A, Greenblatt DJ, et al. Impaired absorption of desmethydiazepam from clorazepate by magnesium aluminum hydroxide. Clin Pharmacol Ther. 1978;24(3):308-315. doi:10.1002/cpt1978243308
34. Greenblatt DJ, Allen MD, MacLaughlin DS, et al. Diazepam absorption: effect of antacids and food. Clin Pharmacol Ther. 1978;24(5):600-609. doi:10.1002/cpt1978245600
35. Yamazaki A, Kumagai Y, Fujita T, et al. Different effects of light food on pharmacokinetics and pharmacodynamics of three benzodiazepines, quazepam, nitrazepam and diazepam. J Clin Pharm Ther. 2007;32(1):31-39. doi:10.1111/j.1365-2710.2007.00795.x
36. Stimpfl J, Mills JA, Strawn JR. Pharmacologic predictors of benzodiazepine response trajectory in anxiety disorders: a Bayesian hierarchical modeling meta-analysis. CNS Spectr. 2023;28(1):53-60. doi:10.1017/S1092852921000870
37. Griffin CE 3rd, Kaye AM, Bueno FR, et al. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214-223.
38. Buffett-Jerrott SE, Stewart SH. Cognitive and sedative effects of benzodiazepine use. Curr Pharm Des. 2005;8(1):45-58. doi:10.2174/1381612023396654
39. Fukasawa T, Suzuki A, Otani K. Effects of genetic polymorphism of cytochrome P450 enzymes on the pharmacokinetics of benzodiazepines. J Clin Pharm Ther. 2007;32(4):333-341. doi:10.1111/j.1365-2710.2007.00829.x
40. Kraus JW, Desmond PV, Marshall JP, et al. Effects of aging and liver disease on disposition of lorazepam. Clin Pharmacol Ther. 1978;24(4):411-419. doi:10.1002/cpt1978244411
41. Greenblatt DJ. Clinical pharmacokinetics of oxazepam and lorazepam. Clin Pharmacokinet. 1981;6(2):89-105. doi:10.2165/00003088-198106020-00001
42. Walkenstein SS, Wiser R, Gudmundsen CH, et al. Absorption, metabolism, and excretion of oxazepam and its succinate half‐ester. J Pharm Sci. 1964;53(10):1181-1186. doi:10.1002/jps.2600531010
43. Shull HJ, Wilkinson GR, Johnson R, et al. Normal disposition of oxazepam in acute viral hepatitis and cirrhosis. Ann Intern Med. 1976;84(4):420-425. doi:10.7326/0003-4819-84-4-420
44. Abernethy DR, Greenblatt DJ, Ochs HR, et al. Lorazepam and oxazepam kinetics in women on low-dose oral contraceptives. Clin Pharmacol Ther. 1983;33(5):628-632. doi:10.1038/clpt.1983.85
45. Greenblatt DJ, Allen MD, Harmatz JS, et al. Diazepam disposition determinants. Clin Pharmacol Ther. 1980;27(3):301-312. doi:10.1038/clpt.1980.40
46. Ochs HR, Greenblatt DJ, Knüchel M. Kinetics of diazepam, midazolam, and lorazepam, in cigarette smokers. Chest. 1985;87(2):223-226. doi:10.1378/chest.87.2.223
47. Smith RB, Gwilt PR, Wright CE 3rd. Single- and multiple-dose pharmacokinetics of oral alprazolam in healthy smoking and nonsmoking men. Clin Pharm. 1983;2(2):139-143.
48. Figgitt DP, McClellan KJ. Fluvoxamine. An updated review of its use in the management of adults with anxiety disorders. Drugs. 2000;60(4):925-954. doi:10.2165/00003495-200060040-00006
49. Greenblatt DJ, Wright CE. Clinical pharmacokinetics of alprazolam. Therapeutic implications. Clin Pharmacokinet. 1993;24(6):453-471. doi:10.2165/00003088-199324060-00003
50. Yasui N, Kondo T, Furukori H, et al. Effects of repeated ingestion of grapefruit juice on the single and multiple oral-dose pharmacokinetics and pharmacodynamics of alprazolam. Psychopharmacology (Berl). 2000;150(2):185-190. doi:10.1007/s002130000438
51. Özdemir M, Aktan Y, Boydagˇ BS, et al. Interaction between grapefruit juice and diazepam in humans. Eur J Drug Metab Pharmacokinet. 1998;23(1):55-59. doi:10.1007/BF03189827
52. Greenblatt DJ, Harmatz JS, Zhang Q, et al. Slow accumulation and elimination of diazepam and its active metabolite with extended treatment in the elderly. J Clin Pharmacol. 2021;61(2):193-203. doi:10.1002/jcph.1726
53. Abernethy DR, Greenblatt DJ. Drug disposition in obese humans: an update. Clin Pharmacokinet. 1986;11(3):199-213. doi:10.2165/00003088-198611030-00002
54. Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49(2):71-87. doi:10.2165/11318100-000000000-00000
55. Bauer LA. Drug Dosing in special populations: renal and hepatic disease, dialysis, heart failure, obesity, and drug interactions. In: Weitz M, Thomas, CM, eds. Applied Clinical Pharmacokinetics. 3rd ed. McGraw-Hill Education; 2014. https://accesspharmacy.mhmedical.com/book.aspx?bookid=1374
56. Kendrick JG, Carr RR, Ensom MHH. Pharmacokinetics and drug dosing in obese children. J Pediatr Pharmacol Ther. 2010;15(2):94-109. doi:10.5863/1551-6776-15.2.94
57. Brill MJE, Diepstraten J, van Rongen A, et al. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51(5):277-304. doi:10.2165/11599410-000000000-00000
58. Derry CL, Kroboth PD, Pittenger AL, et al. Pharmacokinetics and pharmacodynamics of triazolam after two intermittent doses in obese and normal-weight men. J Clin Psychopharmacol. 1995;15(3):197-205. doi:10.1097/00004714-199506000-00008
59. Abernethy DR, Greenblatt DJ, Divoll M, et al. The influence of obesity on the pharmacokinetics of oral alprazolam and triazolam. Clin Pharmacokinet. 1984;9(2):177-183. doi:10.2165/00003088-198409020-00005
60. Abernethy DR, Greenblatt DJ, Divoll M, et al. Prolonged accumulation of diazepam in obesity. J Clin Pharmacol. 1983;23(8-9):369-376. doi:10.1002/j.1552-4604.1983.tb02750.x
61. Abernethy DR, Greenblatt DJ, Divoll M, et al. Enhanced glucuronide conjugation of drugs in obesity: studies of lorazepam, oxazepam, and acetaminophen. J Lab Clin Med. 1983;101(6):873-880.
62. Greenblatt DJ, von Moltke LL, Harmatz JS, et al. Alprazolam pharmacokinetics, metabolism, and plasma levels: clinical implications. J Clin Psychiatry. 1993;54 Suppl:4-11.
63. Chen YT, Liu CY, Chang CM, et al. Perceptions, clinical characteristics, and other factors associated with prolonged and high daily dose of benzodiazepine use among patients with anxiety or depressive disorders. J Affect Disord. 2020;271:215-223. doi:10.1016/j.jad.2020.03.077
64. Herman JB, Brotman AW, Rosenbaum JF. Rebound anxiety in panic disorder patients treated with shorter-acting benzodiazepines. J Clin Psychiatry. 1987;48(Suppl):22-28.
65. Herman JB, Rosenbaum JF, Brotman AW. The alprazolam to clonazepam switch for the treatment of panic disorder. J Clin Psychopharmacol. 1987;7(3):175-178.
1. Rickels K, Moeller HJ. Benzodiazepines in anxiety disorders: reassessment of usefulness and safety. World J Biol Psychiatry. 2019;20(7):514-518. doi:10.1080/15622975.2018.1500031
2. Stevens JC, Pollack MH. Benzodiazepines in clinical practice: consideration of their long-term use and alternative agents. J Clin Psychiatry. 2005;66(Suppl 2):21-27.
3. Pollack MH, van Ameringen M, Simon NM, et al. A double-blind randomized controlled trial of augmentation and switch strategies for refractory social anxiety disorder. Am J Psychiatry. 2014;171(1):44-53. doi:10.1176/appi.ajp.2013.12101353
4. Strawn JR, Geracioti L, Rajdev N, et al. Pharmacotherapy for generalized anxiety disorder in adult and pediatric patients: an evidence-based treatment review. Expert Opin Pharmacother. 2018;19(10):1057-1070. doi:10.1080/14656566.2018.1491966
5. Karaca-Mandic P, Meara E, Morden NE. The growing problem of co-treatment with opioids and benzodiazepines. BMJ. 2017;356:j1224. doi:10.1136/bmj.j1224
6. Bachhuber MA, Hennessy S, Cunningham CO, et al. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996-2013. Am J Public Health. 2016;106(4):686-688. doi:10.2105/AJPH.2016.303061
7. Bentué-Ferrer D, Akwa Y. Benzodiazepines: Effects on memory functioning. In: Pandi-Perumal SR, Verster J, Monti J, et al, eds. Sleep Disorders: Diagnosis and Therapeutics. CRC Press; 2008:104-114. doi:10.3109/9780203091715-15
8. Pomara N, Facelle TM, Roth AE, et al. Dose-dependent retrograde facilitation of verbal memory in healthy elderly after acute oral lorazepam administration.Psychopharmacology (Berl). 2006;185(4):487-494. doi:10.1007/s00213-006-0336-0
9. Gray SL, Dublin S, Yu O, et al. Benzodiazepine use and risk of incident dementia or cognitive decline: prospective population based study. BMJ. 2016;352:i90. doi:10.1136/bmj.i90
10. Biétry FA, Pfeil AM, Reich O, et al. Benzodiazepine use and risk of developing Alzheimer’s disease: a case-control study based on Swiss claims data. CNS Drugs. 2017;31(3):245-251. doi:10.1007/s40263-016-0404-x
11. de Gage SB, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ. 2014;349g5205. doi:10.1136/bmj.g5205
12. Shah R, Raji MA, Westra J, et al. Association of co-prescribing of opioid and benzodiazepine substitutes with incident falls and fractures among older adults: a cohort study. BMJ Open. 2021;11(12):e052057. doi:10.1136/bmjopen-2021-052057
13. Guina J, Rossetter SR, DeRhodes BJ, et al. Benzodiazepines for PTSD: a systematic review and meta-analysis. J Psychiatr Pract. 2015;21(4):281-303.
14. Ekström MP, Bornefalk-Hermansson A, Abernethy AP, et al. Safety of benzodiazepines and opioids in very severe respiratory disease: national prospective study. BMJ. 2014;348:g445. doi:10.1136/bmj.g445
15. Donovan LM, Malte CA, Spece LJ, et al. Center predictors of long-term benzodiazepine use in chronic obstructive pulmonary disease and post-traumatic stress disorder. Ann Am Thorac Soc. 2019;16(9):1151-1157. doi:10.1513/AnnalsATS.201901-048OC
16. Sheehy O, Zhao JP, Bérard A. Association between incident exposure to benzodiazepines in early pregnancy and risk of spontaneous abortion. JAMA Psychiatry. 2019;76(9):948-957. doi:10.1001/jamapsychiatry.2019.0963
17. Kelly LE, Poon S, Madadi P, et al. Neonatal benzodiazepines exposure during breastfeeding. J Pediatr. 2012;161(3):448-451. doi:10.1016/j.jpeds.2012.03.003
18. Agarwal SD, Landon BE. Patterns in outpatient benzodiazepine prescribing in the United States. JAMA Netw Open. 2019;2(1):e187399. doi:10.1001/jamanetworkopen.2018.7399
19. Hirschtritt ME, Olfson M, Kroenke K. Balancing the risks and benefits of benzodiazepines. JAMA. 2021;325(4):347-348. doi:10.1001/jama.2020.22106
20. Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. McGraw-Hill Education; 2018.
21. Nutt DJ, Malizia AL. New insights into the role of the GABA(A)-benzodiazepine receptor in psychiatric disorder. British J Psychiatry. 2001;179:390-396. doi:10.1192/bjp.179.5.390
22. Sigel E. Mapping of the benzodiazepine recognition site on GABA(A) receptors. Curr Top Med Chem. 2002;2(8):833-839. doi:10.2174/1568026023393444
23. Savic
24. Smith TA. Type A gamma-aminobutyric acid (GABAA) receptor subunits and benzodiazepine binding: significance to clinical syndromes and their treatment. Br J Biomed Sci. 2001;58(2):111-121.
25. Althaus AL, Ackley MA, Belfort GM, et al. Preclinical characterization of zuranolone (SAGE-217), a selective neuroactive steroid GABAA receptor positive allosteric modulator. Neuropharmacology. 2020;181:108333. doi:10.1016/j.neuropharm.2020.108333
26. Jacob TC, Michels G, Silayeva L, et al. Benzodiazepine treatment induces subtype-specific changes in GABA(A) receptor trafficking and decreases synaptic inhibition. Proc Natl Acad Sci U S A. 2012;109(45):18595-18600. doi:10.1073/pnas.1204994109
27. Nicholson MW, Sweeney A, Pekle E, et al. Diazepam-induced loss of inhibitory synapses mediated by PLCδ/ Ca2+/calcineurin signalling downstream of GABAA receptors. Mol Psychiatry. 2018;23(9):1851-1867. doi:10.1038/s41380-018-0100-y
28. Dobson ET, Bloch MH, Strawn JR. Efficacy and tolerability of pharmacotherapy for pediatric anxiety disorders: a network meta-analysis. J Clin Psychiatry. 2019;80(1):17r12064. doi:10.4088/JCP.17r12064
29. Kuang H, Johnson JA, Mulqueen JM, et al. The efficacy of benzodiazepines as acute anxiolytics in children: a meta-analysis. Depress Anxiety. 2017;34(10):888-896. doi:10.1002/da.22643
30. Chugani DC, Muzik O, Juhász C, et al. Postnatal maturation of human GABAA receptors measured with positron emission tomography. Ann Neurol. 2001;49(5):618-626. doi:10.1002/ana.1003
31. Jochemsen R, Breimer DD. Pharmacokinetics of benzodiazepines: metabolic pathways and plasma level profiles. Curr Med Res Opin. 1984;8(Suppl 4):60-79. doi:10.1185/03007998409109545
32. Greenblatt DJ, Harmatz JS, Dorsey C, et al. Comparative single-dose kinetics and dynamics of lorazepam, alprazolam, prazepam, and placebo. Clin Pharmacol Ther. 1988;44(3)326-334. doi:10.1038/clpt.1988.158
33. Shader RI, Georgotas A, Greenblatt DJ, et al. Impaired absorption of desmethydiazepam from clorazepate by magnesium aluminum hydroxide. Clin Pharmacol Ther. 1978;24(3):308-315. doi:10.1002/cpt1978243308
34. Greenblatt DJ, Allen MD, MacLaughlin DS, et al. Diazepam absorption: effect of antacids and food. Clin Pharmacol Ther. 1978;24(5):600-609. doi:10.1002/cpt1978245600
35. Yamazaki A, Kumagai Y, Fujita T, et al. Different effects of light food on pharmacokinetics and pharmacodynamics of three benzodiazepines, quazepam, nitrazepam and diazepam. J Clin Pharm Ther. 2007;32(1):31-39. doi:10.1111/j.1365-2710.2007.00795.x
36. Stimpfl J, Mills JA, Strawn JR. Pharmacologic predictors of benzodiazepine response trajectory in anxiety disorders: a Bayesian hierarchical modeling meta-analysis. CNS Spectr. 2023;28(1):53-60. doi:10.1017/S1092852921000870
37. Griffin CE 3rd, Kaye AM, Bueno FR, et al. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214-223.
38. Buffett-Jerrott SE, Stewart SH. Cognitive and sedative effects of benzodiazepine use. Curr Pharm Des. 2005;8(1):45-58. doi:10.2174/1381612023396654
39. Fukasawa T, Suzuki A, Otani K. Effects of genetic polymorphism of cytochrome P450 enzymes on the pharmacokinetics of benzodiazepines. J Clin Pharm Ther. 2007;32(4):333-341. doi:10.1111/j.1365-2710.2007.00829.x
40. Kraus JW, Desmond PV, Marshall JP, et al. Effects of aging and liver disease on disposition of lorazepam. Clin Pharmacol Ther. 1978;24(4):411-419. doi:10.1002/cpt1978244411
41. Greenblatt DJ. Clinical pharmacokinetics of oxazepam and lorazepam. Clin Pharmacokinet. 1981;6(2):89-105. doi:10.2165/00003088-198106020-00001
42. Walkenstein SS, Wiser R, Gudmundsen CH, et al. Absorption, metabolism, and excretion of oxazepam and its succinate half‐ester. J Pharm Sci. 1964;53(10):1181-1186. doi:10.1002/jps.2600531010
43. Shull HJ, Wilkinson GR, Johnson R, et al. Normal disposition of oxazepam in acute viral hepatitis and cirrhosis. Ann Intern Med. 1976;84(4):420-425. doi:10.7326/0003-4819-84-4-420
44. Abernethy DR, Greenblatt DJ, Ochs HR, et al. Lorazepam and oxazepam kinetics in women on low-dose oral contraceptives. Clin Pharmacol Ther. 1983;33(5):628-632. doi:10.1038/clpt.1983.85
45. Greenblatt DJ, Allen MD, Harmatz JS, et al. Diazepam disposition determinants. Clin Pharmacol Ther. 1980;27(3):301-312. doi:10.1038/clpt.1980.40
46. Ochs HR, Greenblatt DJ, Knüchel M. Kinetics of diazepam, midazolam, and lorazepam, in cigarette smokers. Chest. 1985;87(2):223-226. doi:10.1378/chest.87.2.223
47. Smith RB, Gwilt PR, Wright CE 3rd. Single- and multiple-dose pharmacokinetics of oral alprazolam in healthy smoking and nonsmoking men. Clin Pharm. 1983;2(2):139-143.
48. Figgitt DP, McClellan KJ. Fluvoxamine. An updated review of its use in the management of adults with anxiety disorders. Drugs. 2000;60(4):925-954. doi:10.2165/00003495-200060040-00006
49. Greenblatt DJ, Wright CE. Clinical pharmacokinetics of alprazolam. Therapeutic implications. Clin Pharmacokinet. 1993;24(6):453-471. doi:10.2165/00003088-199324060-00003
50. Yasui N, Kondo T, Furukori H, et al. Effects of repeated ingestion of grapefruit juice on the single and multiple oral-dose pharmacokinetics and pharmacodynamics of alprazolam. Psychopharmacology (Berl). 2000;150(2):185-190. doi:10.1007/s002130000438
51. Özdemir M, Aktan Y, Boydagˇ BS, et al. Interaction between grapefruit juice and diazepam in humans. Eur J Drug Metab Pharmacokinet. 1998;23(1):55-59. doi:10.1007/BF03189827
52. Greenblatt DJ, Harmatz JS, Zhang Q, et al. Slow accumulation and elimination of diazepam and its active metabolite with extended treatment in the elderly. J Clin Pharmacol. 2021;61(2):193-203. doi:10.1002/jcph.1726
53. Abernethy DR, Greenblatt DJ. Drug disposition in obese humans: an update. Clin Pharmacokinet. 1986;11(3):199-213. doi:10.2165/00003088-198611030-00002
54. Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49(2):71-87. doi:10.2165/11318100-000000000-00000
55. Bauer LA. Drug Dosing in special populations: renal and hepatic disease, dialysis, heart failure, obesity, and drug interactions. In: Weitz M, Thomas, CM, eds. Applied Clinical Pharmacokinetics. 3rd ed. McGraw-Hill Education; 2014. https://accesspharmacy.mhmedical.com/book.aspx?bookid=1374
56. Kendrick JG, Carr RR, Ensom MHH. Pharmacokinetics and drug dosing in obese children. J Pediatr Pharmacol Ther. 2010;15(2):94-109. doi:10.5863/1551-6776-15.2.94
57. Brill MJE, Diepstraten J, van Rongen A, et al. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51(5):277-304. doi:10.2165/11599410-000000000-00000
58. Derry CL, Kroboth PD, Pittenger AL, et al. Pharmacokinetics and pharmacodynamics of triazolam after two intermittent doses in obese and normal-weight men. J Clin Psychopharmacol. 1995;15(3):197-205. doi:10.1097/00004714-199506000-00008
59. Abernethy DR, Greenblatt DJ, Divoll M, et al. The influence of obesity on the pharmacokinetics of oral alprazolam and triazolam. Clin Pharmacokinet. 1984;9(2):177-183. doi:10.2165/00003088-198409020-00005
60. Abernethy DR, Greenblatt DJ, Divoll M, et al. Prolonged accumulation of diazepam in obesity. J Clin Pharmacol. 1983;23(8-9):369-376. doi:10.1002/j.1552-4604.1983.tb02750.x
61. Abernethy DR, Greenblatt DJ, Divoll M, et al. Enhanced glucuronide conjugation of drugs in obesity: studies of lorazepam, oxazepam, and acetaminophen. J Lab Clin Med. 1983;101(6):873-880.
62. Greenblatt DJ, von Moltke LL, Harmatz JS, et al. Alprazolam pharmacokinetics, metabolism, and plasma levels: clinical implications. J Clin Psychiatry. 1993;54 Suppl:4-11.
63. Chen YT, Liu CY, Chang CM, et al. Perceptions, clinical characteristics, and other factors associated with prolonged and high daily dose of benzodiazepine use among patients with anxiety or depressive disorders. J Affect Disord. 2020;271:215-223. doi:10.1016/j.jad.2020.03.077
64. Herman JB, Brotman AW, Rosenbaum JF. Rebound anxiety in panic disorder patients treated with shorter-acting benzodiazepines. J Clin Psychiatry. 1987;48(Suppl):22-28.
65. Herman JB, Rosenbaum JF, Brotman AW. The alprazolam to clonazepam switch for the treatment of panic disorder. J Clin Psychopharmacol. 1987;7(3):175-178.
Child murder by parents: Toward prevention
Deaths of children who are killed by their parents often make the news. Cases of maternal infanticide may be particularly shocking, since women are expected to be selfless nurturers. Yet when a child is murdered, the most common perpetrator is their parent, and mothers and fathers kill at similar rates.1
As psychiatrists, we may see these cases in the news and worry about the risks of our own patients killing their children. In approximately 500 cases annually, an American parent is arrested for the homicide of their child.2 This is not even the entire story, since a large percentage of such cases end in suicide—and no arrest. This article reviews the reasons parents kill their children, and considers common characteristics of these parents, dispelling some myths, before discussing the importance of prevention efforts.
Types of child murder by parents
Child murder by parents is termed filicide. Infanticide has various meanings but often refers to the murder of a child younger than age 1. Approximately 2 dozen nations (but not the United States) have Infanticide Acts that decrease the penalty for mothers who kill their young child.3 Neonaticide refers to murder of the infant at birth or in the first day of life.4
Epidemiology and common characteristics
Approximately 15%—or 1 in 7 murders with an arrest—is a filicide.2 The younger the child, the greater the risk, but older children are killed as well.2 Internationally, fathers and mothers are found to kill at similar rates. For other types of homicide, offenders are overwhelmingly male. This makes child murder by parents the singular type of murder in which women and men perpetrate in equal numbers. Fathers are more likely than mothers to also commit suicide after they kill their children.5 The “Cinderella effect” refers to the elevated risk of a stepchild being killed compared to the risk for a biological child.6
In the general international population, mothers who commit filicide tend to have multiple stressors and limited resources. They may be socially isolated and may be victims themselves as well as potentially experiencing substance abuse.1 Some mothers view the child they killed as abnormal.
Less research has been conducted about fathers who kill. Fathers are more likely to also commit partner homicide.5,7 They are more likely to complete filicide-suicide and use firearms or other violent means.5,7-9 Fathers may have a history of violence, substance abuse, and/or mental illness.7
Neonaticide
Mothers are the most common perpetrator of neonaticide.4 It is unusual for a father to be involved in a neonaticide, or for the father and mother to perpetrate the act together. Rates of neonaticide are considered underestimates because of the number of hidden pregnancies, hidden corpses, and the difficulty that forensic pathologists may have in determining whether a baby was born alive or dead.
Continue to: Perpetrators of neonaticide...
Perpetrators of neonaticide tend to be single, relatively young women acting alone. They often live with their parents and are fearful of the repercussions of being pregnant. Pregnancies are often hidden, with no prenatal care. This includes both denial and concealment of pregnancy.4 Perpetrators of neonaticide commonly lack a premorbid serious mental illness, though after the homicide they may develop anxiety, depression, posttraumatic stress disorder (PTSD), or adjustment disorder.4 (Individuals who unwittingly find a murdered baby’s corpse may also be at risk of PTSD.)
Hidden pregnancies may be due to concealment or denial of pregnancy.10,11 Concealment of pregnancy involves a woman knowing she is pregnant, but purposely hiding from others. Concealment may occur after a period of denial of pregnancy. Denial of pregnancy has several subtypes: pervasive denial, affective denial, and psychotic denial. In cases of pervasive denial, the existence of the pregnancy and the pregnancy’s emotional significance is outside the woman’s awareness. Alternatively, in affective denial, she is intellectually aware that she is pregnant but makes little emotional or physical preparation. In the rarest form, psychotic denial, a woman with a psychotic disorder such as schizophrenia may intermittently deny her pregnancy. This may be correlated with a history of custody loss.10,11 Unlike denial of other medical conditions, in cases of denial of pregnancy, there will exist a very specific point in time (delivery) when the reality of the baby confronts the woman. Risks in cases of hidden pregnancies include those from lack of prenatal care and an assisted delivery as well as neonaticide. An FBI study12 of law enforcement files found most neonaticide offenders were single young women with no criminal or psychological history. A caveat is that in the rare cases in which a woman with psychotic illness commits neonaticide, she may have different characteristics from those generally reported.13
Motives
Fathers and mothers have a similar set of motives for killing their child (Table 113-15). Motives are critical to understand not only within forensics, but also for prevention. In performing assessments after a filicide, forensic psychiatrists must be mindful of gender bias.7,16 Resnick15 initially described 5 motives based on his 1969 review of the world literature. Our work5,17 has subsequently further explored these motives.

In child homicides from “fatal maltreatment,” the child has often been a chronic victim of abuse or neglect. National American data indicate that approximately 2 per 100,000 children are killed from child maltreatment annually. Of note in conceptualizing prevention, out of the same population of 100,000, there will be 471 referrals to Child Protective Services and 91 substantiated cases.18 However, only a minority of children who die from maltreatment had previous Child Protective Services involvement. While a child may be killed by fatal maltreatment at any age, one-half are younger than age 1, and three-quarters are younger than age 3.18 In rare cases, a parent who engages in medical child abuse (including factitious disorder imposed upon another) kills the child. Depending on the location and whether or not the death appeared to be intended, parents who kill because of fatal maltreatment might face charges of various levels of murder or manslaughter.
“Unwanted child” homicides occur when the parent has determined that they do not want to have the child, especially in comparison to another need or want. Unwanted child motive is the most common in neonaticide cases, occurring after a hidden pregnancy.4
Continue to: In "partner revenge" cases...
In “partner revenge” cases, parenting disputes, a custody battle, infidelity, or a difficult relationship breakup is often present. The parent wants to make the other parent suffer, and does so by killing their child. A parent may make statements such as “If I can’t have [the child], no one can,” and the child is used as a pawn.
In the final 2 motives—“altruistic” and “acutely psychotic”—mental illness is common. These are the populations we tend to find in samples of filicide-suicide cases where the parent has killed themselves and their child, and those found not guilty by reason of insanity.5,17 Altruistic filicide has been described as “murder out of love.” How can a parent kill their child out of love? Our research has shown several ways. First, the parent may be severely depressed and suicidal. They may be planning their own suicide, and as a parent who loves their child, they plan to take their child with them in death and not leave them alone in the “cruel world” that they themselves are departing. Or the parent may believe they are killing the child out of love to prevent or relieve the child’s suffering. The psychotic parent may believe that a terrible fate will befall their child, and they are killing them “gently.” For example, the parent may believe the child will be tortured or sex trafficked. Some parents may believe that their child has a devastating disease and think they would be better off dead. (Similar thinking of misguided altruism is seen in some cases of intimate partner homicide among older adults.19)
Alternatively, in rare cases of acutely psychotic filicide, parents with psychosis kill their child with no comprehensible motive. For example, they may be in a postictal state or may hear a command hallucination from God in the context of their psychosis.15
Myths vs realities of filicide
Common myths vs the realities of filicide are noted in Table 2. There are issues with believing these myths. For example, if we believe that most parents who kill their child have mental illness, this conflates mental illness and child homicide in our minds as well as the mind of the public. This can lead to further stigmatization of mental illness, and a lack of help-seeking behaviors because parents experiencing psychiatric symptoms may be afraid that if they report their symptoms, their child will be removed by Child Protective Services. However, treated mental illness decreases the risks of child abuse, similar to how treating mental illness decreases risks of other types of violence.20,21
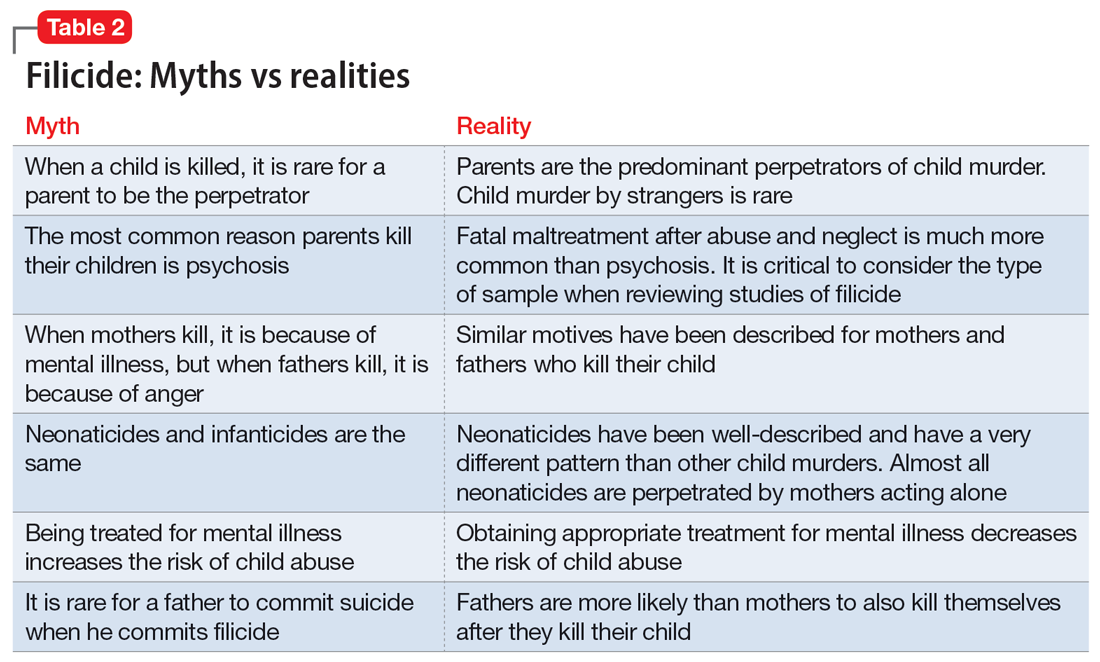
Focusing on prevention
On a local level, we need to understand these tragedies to better understand prevention. To this end, across the United States, counties have Child Fatality Review teams.22 These teams are a partnership across sectors and disciplines, including professionals from health services, law enforcement, and social services—among others—working together to understand cases and consider preventive strategies and additional services needed within our communities.
Continue to: When conceptualizing prevention...
When conceptualizing prevention of child murder by parents, we can think of primary, secondary, and tertiary prevention. This means we want to encourage healthy families and healthy relationships within the family, as well as screening for risk and targeting interventions for families that have experienced difficulties, as well as for parents who have mental illness or substance use disorders.
Understanding the motive behind an individual committing filicide is also critical so that we do not conflate filicide and mental illness. Conflating these concepts leads to increased stigmatization and less help-seeking behavior.
Table 33,4,7,18,22,23 describes the importance of understanding the motives for child murder by a parent in order to conceptualize appropriate prevention. Prevention efforts for 1 type of child murder will not necessarily help prevent murders that occur due to the other motives. Regarding prevention for fatal maltreatment cases, poor parenting skills, including inappropriate expressions of discipline, anger, and frustration, are common. In some cases, substance abuse is involved or the parent was acutely mentally unwell. Reporting to Child Protective Services can be helpful, but as previously noted, it is difficult to ascertain which cases will lead to a homicide. Recommendations from Child Fatality Review teams also are valuable.
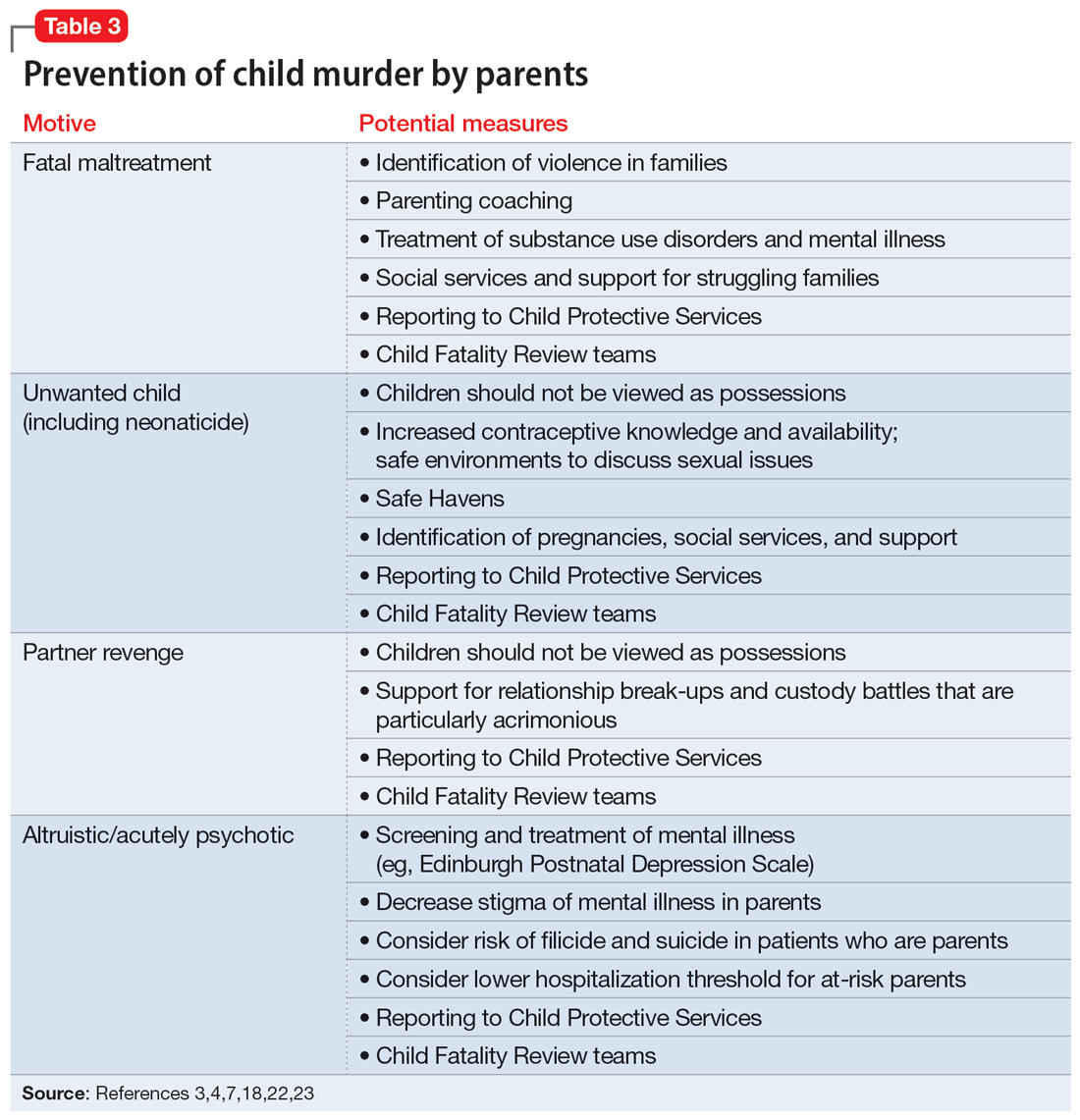
Though many parents have frustrations with their children or thoughts of child harm, the act of filicide is rare, and individual cases may be difficult to predict. Regarding prediction, some mothers who committed filicide saw their psychiatrist within days to weeks before the murders.17 A small New Zealand study found that psychotic mothers reported no plans for killing their children in advance, whereas depressed mothers had contemplated the killing for days to weeks.24
Several studies have asked mothers about thoughts of harming their child. Among mothers with colicky infants, 70% reported “explicit aggressive thoughts and fantasies” while 26% had “infanticidal thoughts” during a colic episode.25 Another study26 found that among depressed mothers of infants and toddlers, 41% revealed thoughts of harming their child. Women with postpartum depression preferred not to share infanticidal thoughts with their doctor but were more likely to disclose that they were having suicidal thoughts in order to get needed help.27 Psychiatrists need to feel comfortable asking mothers about their coping skills, their suicidal thoughts, and their filicidal thoughts.14,23,28 Screening and treatment of mental illness is critical. Postpartum psychosis is well-known to pose an elevated risk of filicide and suicide.23 Obsessive-compulsive disorder may cause a parent to ruminate over ego-dystonic child harm but should be treated and the risk conceptualized very differently than in postpartum psychosis.23,29 Screening for postpartum depression and appropriate treatment of depression during pregnancy and the postpartum period decrease risk.30
Continue to: Regarding prevention of neonaticide...
Regarding prevention of neonaticide, Safe Haven laws, baby boxes, anonymous birth options, and increased contraceptive information and availability can help decrease the risk of this well-defined type of homicide.4 Safe Haven laws originated from Child Fatality Review teams.24 Though each state has its own variation, in general, parents can drop off an unharmed unwanted infant into Safe Havens in their state, which may include hospitals, police stations, or fire stations. In general, the mother remains anonymous and has immunity from prosecution for (safe) abandonment. There are drawbacks, such as lack of information regarding adoption and paternal rights. Safe Haven laws do not prevent all deaths and all unsafe abandonments. Baby boxes and baby hatches are used in various nations, including in Europe, and in some places have been used for centuries. In anonymous birth options, such as in France, a mother is not identified but is able to give birth at a hospital. This can decrease the risk from unattended delivery, but many women with denial of pregnancy report that they did not realize when they were about to give birth.4
Bottom Line
Knowledge about the intersection of mental illness and filicide can help in prevention. Parents who experience mental health concerns should be encouraged to obtain needed treatment, which aids prevention. However, many other factors elevate the risk of child murder by parents.
Related Resources
- National Center for Fatality Review and Prevention. https://ncfrp.org/
- Child Welfare Information Gateway. https://www.childwelfare.gov/topics/preventing/overview/federal-agencies/
1. Friedman SH, Horwitz SM, Resnick PJ. Child murder by mothers: a critical analysis of the current state of knowledge and a research agenda. Am J Psych. 2005;162(9):1578-1587.
2. Mariano TY, Chan HC, Myers WC. Toward a more holistic understanding of filicide: a multidisciplinary analysis of 32 years of US arrest data [published corrections appears in Forensic Sci Int. 2014;245:92-94]. Forensic Sci Int. 2014;236:46-53.
3. Hatters Friedman S, Resnick PJ. Child murder by mothers: patterns and prevention. World Psychiatry. 2007;6(3):137-141.
4. Friedman SH, Resnick PJ. Neonaticide: phenomenology and considerations for prevention. Int J Law Psychiatry. 2009;32(1):43-47.
5. Hatters Friedman S, Hrouda DR, Holden CE, et al. Filicide-suicide: common factors in parents who kill their children and themselves. J Am Acad Psychiatry Law. 2005;33(4):496-504.
6. Daly M, Wilson M. Is the “Cinderella effect” controversial? A case study of evolution-minded research and critiques thereof. In: Crawford C, Krebs D, eds. Foundations of Evolutionary Psychology. Taylor & Francis Group/Lawrence Erlbaum Associates; 2008:383-400.
7. Friedman SH. Fathers and filicide: Mental illness and outcomes. In: Wong G, Parnham G, eds. Infanticide and Filicide: Foundations in Maternal Mental Health Forensics. 1st ed. American Psychiatric Association Publishing; 2020:85-107.
8. West SG, Friedman SH, Resnick PJ. Fathers who kill their children: an analysis of the literature. J Forensic Sci. 2009;54(2):463-468.
9. Putkonen H, Amon S, Eronen M, et al. Gender differences in filicide offense characteristics--a comprehensive register-based study of child murder in two European countries. Child Abuse Neglect. 2011;35(5):319-328.
10. Miller LJ. Denial of pregnancy. In: Spinelli MG, ed. Infanticide: Psychosocial and Legal Perspectives on Mothers Who Kill. American Psychiatric Association Publishing; 2003:81-104.
11. Friedman SH, Heneghan A, Rosenthal M. Characteristics of women who deny or conceal pregnancy. Psychosomatics. 2007;48(2):117-122.
12. Beyer K, Mack SM, Shelton JL. Investigative analysis of neonaticide: an exploratory study. Criminal Justice and Behavior. 2008;35(4):522-535.
13. Putkonen H, Weizmann-Henelius G, Collander J, et al. Neonaticides may be more preventable and heterogeneous than previously thought--neonaticides in Finland 1980-2000. Arch Womens Ment Health. 2007;10(1):15-23.
14. Friedman SH, Resnick PJ. Child murder and mental illness in parents: implications for psychiatrists. J Clin Psychiatry. 2011;72(5):587-588.
15. Resnick PJ. Child murder by parents: a psychiatric review of filicide. Am J Psychiatry. 1969;126(3):325-334.
16. Friedman SH. Searching for the whole truth: considering culture and gender in forensic psychiatric practice. J Am Acad Psychiatry Law. 2023;51(1):23-34.
17. Friedman SH, Hrouda DR, Holden CE, et al. Child murder committed by severely mentally ill mothers: an examination of mothers found not guilty by reason of insanity. J Forensic Sci. 2005;50(6):1466-1471.
18. Ash P. Fatal maltreatment and child abuse turned to murder. In: Friedman SH, ed. Family Murder: Pathologies of Love and Hate. Group for the Advancement Psychiatry; 2018.
19. Friedman SH, Appel JM. Murder in the family: intimate partner homicide in the elderly. Psychiatric News. 2018. Accessed April 8, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.12a21
20. Friedman SH, McEwan MV. Treated mental illness and the risk of child abuse perpetration. Psychiatr Serv. 2018;69(2):211-216.
21. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psychiatr Clin North Am. 2016;39(4):691-700.
22. Hatters Friedman S, Beaman JW, Friedman JB. Fatality review and the role of the forensic psychiatrist. J Am Acad Psychiatry Law. 2021;49(3):396-405.
23. Friedman SH, Prakash C, Nagle-Yang S. Postpartum psychosis: protecting mother and infant. Current Psychiatry. 2019;18(4):12-21.
24. Stanton J, Simpson AI, Wouldes T. A qualitative study of filicide by mentally ill mothers. Child Abuse Negl. 2000;24(11):1451-1460.
25. Levitzky S, Cooper R. Infant colic syndrome—maternal fantasies of aggression and infanticide. Clin Pediatr (Phila). 2000;39(7):395-400.
26. Jennings KD, Ross S, Popper S, et al. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54(1-2):21-28.
27. Barr JA, Beck CT. Infanticide secrets: qualitative study on postpartum depression. Can Fam Physician. 2008;54(12):1716-1717.e5.
28. Friedman SH, Sorrentino RM, Stankowski JE, et al. Psychiatrists’ knowledge about maternal filicidal thoughts. Compr Psychiatry. 2008;49(1):106-110.
29. Booth BD, Friedman SH, Curry S, et al. Obsessions of child murder: underrecognized manifestations of obsessive-compulsive disorder. J Am Acad Psychiatry Law. 2014;42(1):66-74.
30. Friedman SH, Hall RCW. Avoiding malpractice while treating depression in pregnant women. Current Psychiatry. 2021;20(8):30-36.
Deaths of children who are killed by their parents often make the news. Cases of maternal infanticide may be particularly shocking, since women are expected to be selfless nurturers. Yet when a child is murdered, the most common perpetrator is their parent, and mothers and fathers kill at similar rates.1
As psychiatrists, we may see these cases in the news and worry about the risks of our own patients killing their children. In approximately 500 cases annually, an American parent is arrested for the homicide of their child.2 This is not even the entire story, since a large percentage of such cases end in suicide—and no arrest. This article reviews the reasons parents kill their children, and considers common characteristics of these parents, dispelling some myths, before discussing the importance of prevention efforts.
Types of child murder by parents
Child murder by parents is termed filicide. Infanticide has various meanings but often refers to the murder of a child younger than age 1. Approximately 2 dozen nations (but not the United States) have Infanticide Acts that decrease the penalty for mothers who kill their young child.3 Neonaticide refers to murder of the infant at birth or in the first day of life.4
Epidemiology and common characteristics
Approximately 15%—or 1 in 7 murders with an arrest—is a filicide.2 The younger the child, the greater the risk, but older children are killed as well.2 Internationally, fathers and mothers are found to kill at similar rates. For other types of homicide, offenders are overwhelmingly male. This makes child murder by parents the singular type of murder in which women and men perpetrate in equal numbers. Fathers are more likely than mothers to also commit suicide after they kill their children.5 The “Cinderella effect” refers to the elevated risk of a stepchild being killed compared to the risk for a biological child.6
In the general international population, mothers who commit filicide tend to have multiple stressors and limited resources. They may be socially isolated and may be victims themselves as well as potentially experiencing substance abuse.1 Some mothers view the child they killed as abnormal.
Less research has been conducted about fathers who kill. Fathers are more likely to also commit partner homicide.5,7 They are more likely to complete filicide-suicide and use firearms or other violent means.5,7-9 Fathers may have a history of violence, substance abuse, and/or mental illness.7
Neonaticide
Mothers are the most common perpetrator of neonaticide.4 It is unusual for a father to be involved in a neonaticide, or for the father and mother to perpetrate the act together. Rates of neonaticide are considered underestimates because of the number of hidden pregnancies, hidden corpses, and the difficulty that forensic pathologists may have in determining whether a baby was born alive or dead.
Continue to: Perpetrators of neonaticide...
Perpetrators of neonaticide tend to be single, relatively young women acting alone. They often live with their parents and are fearful of the repercussions of being pregnant. Pregnancies are often hidden, with no prenatal care. This includes both denial and concealment of pregnancy.4 Perpetrators of neonaticide commonly lack a premorbid serious mental illness, though after the homicide they may develop anxiety, depression, posttraumatic stress disorder (PTSD), or adjustment disorder.4 (Individuals who unwittingly find a murdered baby’s corpse may also be at risk of PTSD.)
Hidden pregnancies may be due to concealment or denial of pregnancy.10,11 Concealment of pregnancy involves a woman knowing she is pregnant, but purposely hiding from others. Concealment may occur after a period of denial of pregnancy. Denial of pregnancy has several subtypes: pervasive denial, affective denial, and psychotic denial. In cases of pervasive denial, the existence of the pregnancy and the pregnancy’s emotional significance is outside the woman’s awareness. Alternatively, in affective denial, she is intellectually aware that she is pregnant but makes little emotional or physical preparation. In the rarest form, psychotic denial, a woman with a psychotic disorder such as schizophrenia may intermittently deny her pregnancy. This may be correlated with a history of custody loss.10,11 Unlike denial of other medical conditions, in cases of denial of pregnancy, there will exist a very specific point in time (delivery) when the reality of the baby confronts the woman. Risks in cases of hidden pregnancies include those from lack of prenatal care and an assisted delivery as well as neonaticide. An FBI study12 of law enforcement files found most neonaticide offenders were single young women with no criminal or psychological history. A caveat is that in the rare cases in which a woman with psychotic illness commits neonaticide, she may have different characteristics from those generally reported.13
Motives
Fathers and mothers have a similar set of motives for killing their child (Table 113-15). Motives are critical to understand not only within forensics, but also for prevention. In performing assessments after a filicide, forensic psychiatrists must be mindful of gender bias.7,16 Resnick15 initially described 5 motives based on his 1969 review of the world literature. Our work5,17 has subsequently further explored these motives.

In child homicides from “fatal maltreatment,” the child has often been a chronic victim of abuse or neglect. National American data indicate that approximately 2 per 100,000 children are killed from child maltreatment annually. Of note in conceptualizing prevention, out of the same population of 100,000, there will be 471 referrals to Child Protective Services and 91 substantiated cases.18 However, only a minority of children who die from maltreatment had previous Child Protective Services involvement. While a child may be killed by fatal maltreatment at any age, one-half are younger than age 1, and three-quarters are younger than age 3.18 In rare cases, a parent who engages in medical child abuse (including factitious disorder imposed upon another) kills the child. Depending on the location and whether or not the death appeared to be intended, parents who kill because of fatal maltreatment might face charges of various levels of murder or manslaughter.
“Unwanted child” homicides occur when the parent has determined that they do not want to have the child, especially in comparison to another need or want. Unwanted child motive is the most common in neonaticide cases, occurring after a hidden pregnancy.4
Continue to: In "partner revenge" cases...
In “partner revenge” cases, parenting disputes, a custody battle, infidelity, or a difficult relationship breakup is often present. The parent wants to make the other parent suffer, and does so by killing their child. A parent may make statements such as “If I can’t have [the child], no one can,” and the child is used as a pawn.
In the final 2 motives—“altruistic” and “acutely psychotic”—mental illness is common. These are the populations we tend to find in samples of filicide-suicide cases where the parent has killed themselves and their child, and those found not guilty by reason of insanity.5,17 Altruistic filicide has been described as “murder out of love.” How can a parent kill their child out of love? Our research has shown several ways. First, the parent may be severely depressed and suicidal. They may be planning their own suicide, and as a parent who loves their child, they plan to take their child with them in death and not leave them alone in the “cruel world” that they themselves are departing. Or the parent may believe they are killing the child out of love to prevent or relieve the child’s suffering. The psychotic parent may believe that a terrible fate will befall their child, and they are killing them “gently.” For example, the parent may believe the child will be tortured or sex trafficked. Some parents may believe that their child has a devastating disease and think they would be better off dead. (Similar thinking of misguided altruism is seen in some cases of intimate partner homicide among older adults.19)
Alternatively, in rare cases of acutely psychotic filicide, parents with psychosis kill their child with no comprehensible motive. For example, they may be in a postictal state or may hear a command hallucination from God in the context of their psychosis.15
Myths vs realities of filicide
Common myths vs the realities of filicide are noted in Table 2. There are issues with believing these myths. For example, if we believe that most parents who kill their child have mental illness, this conflates mental illness and child homicide in our minds as well as the mind of the public. This can lead to further stigmatization of mental illness, and a lack of help-seeking behaviors because parents experiencing psychiatric symptoms may be afraid that if they report their symptoms, their child will be removed by Child Protective Services. However, treated mental illness decreases the risks of child abuse, similar to how treating mental illness decreases risks of other types of violence.20,21

Focusing on prevention
On a local level, we need to understand these tragedies to better understand prevention. To this end, across the United States, counties have Child Fatality Review teams.22 These teams are a partnership across sectors and disciplines, including professionals from health services, law enforcement, and social services—among others—working together to understand cases and consider preventive strategies and additional services needed within our communities.
Continue to: When conceptualizing prevention...
When conceptualizing prevention of child murder by parents, we can think of primary, secondary, and tertiary prevention. This means we want to encourage healthy families and healthy relationships within the family, as well as screening for risk and targeting interventions for families that have experienced difficulties, as well as for parents who have mental illness or substance use disorders.
Understanding the motive behind an individual committing filicide is also critical so that we do not conflate filicide and mental illness. Conflating these concepts leads to increased stigmatization and less help-seeking behavior.
Table 33,4,7,18,22,23 describes the importance of understanding the motives for child murder by a parent in order to conceptualize appropriate prevention. Prevention efforts for 1 type of child murder will not necessarily help prevent murders that occur due to the other motives. Regarding prevention for fatal maltreatment cases, poor parenting skills, including inappropriate expressions of discipline, anger, and frustration, are common. In some cases, substance abuse is involved or the parent was acutely mentally unwell. Reporting to Child Protective Services can be helpful, but as previously noted, it is difficult to ascertain which cases will lead to a homicide. Recommendations from Child Fatality Review teams also are valuable.

Though many parents have frustrations with their children or thoughts of child harm, the act of filicide is rare, and individual cases may be difficult to predict. Regarding prediction, some mothers who committed filicide saw their psychiatrist within days to weeks before the murders.17 A small New Zealand study found that psychotic mothers reported no plans for killing their children in advance, whereas depressed mothers had contemplated the killing for days to weeks.24
Several studies have asked mothers about thoughts of harming their child. Among mothers with colicky infants, 70% reported “explicit aggressive thoughts and fantasies” while 26% had “infanticidal thoughts” during a colic episode.25 Another study26 found that among depressed mothers of infants and toddlers, 41% revealed thoughts of harming their child. Women with postpartum depression preferred not to share infanticidal thoughts with their doctor but were more likely to disclose that they were having suicidal thoughts in order to get needed help.27 Psychiatrists need to feel comfortable asking mothers about their coping skills, their suicidal thoughts, and their filicidal thoughts.14,23,28 Screening and treatment of mental illness is critical. Postpartum psychosis is well-known to pose an elevated risk of filicide and suicide.23 Obsessive-compulsive disorder may cause a parent to ruminate over ego-dystonic child harm but should be treated and the risk conceptualized very differently than in postpartum psychosis.23,29 Screening for postpartum depression and appropriate treatment of depression during pregnancy and the postpartum period decrease risk.30
Continue to: Regarding prevention of neonaticide...
Regarding prevention of neonaticide, Safe Haven laws, baby boxes, anonymous birth options, and increased contraceptive information and availability can help decrease the risk of this well-defined type of homicide.4 Safe Haven laws originated from Child Fatality Review teams.24 Though each state has its own variation, in general, parents can drop off an unharmed unwanted infant into Safe Havens in their state, which may include hospitals, police stations, or fire stations. In general, the mother remains anonymous and has immunity from prosecution for (safe) abandonment. There are drawbacks, such as lack of information regarding adoption and paternal rights. Safe Haven laws do not prevent all deaths and all unsafe abandonments. Baby boxes and baby hatches are used in various nations, including in Europe, and in some places have been used for centuries. In anonymous birth options, such as in France, a mother is not identified but is able to give birth at a hospital. This can decrease the risk from unattended delivery, but many women with denial of pregnancy report that they did not realize when they were about to give birth.4
Bottom Line
Knowledge about the intersection of mental illness and filicide can help in prevention. Parents who experience mental health concerns should be encouraged to obtain needed treatment, which aids prevention. However, many other factors elevate the risk of child murder by parents.
Related Resources
- National Center for Fatality Review and Prevention. https://ncfrp.org/
- Child Welfare Information Gateway. https://www.childwelfare.gov/topics/preventing/overview/federal-agencies/
Deaths of children who are killed by their parents often make the news. Cases of maternal infanticide may be particularly shocking, since women are expected to be selfless nurturers. Yet when a child is murdered, the most common perpetrator is their parent, and mothers and fathers kill at similar rates.1
As psychiatrists, we may see these cases in the news and worry about the risks of our own patients killing their children. In approximately 500 cases annually, an American parent is arrested for the homicide of their child.2 This is not even the entire story, since a large percentage of such cases end in suicide—and no arrest. This article reviews the reasons parents kill their children, and considers common characteristics of these parents, dispelling some myths, before discussing the importance of prevention efforts.
Types of child murder by parents
Child murder by parents is termed filicide. Infanticide has various meanings but often refers to the murder of a child younger than age 1. Approximately 2 dozen nations (but not the United States) have Infanticide Acts that decrease the penalty for mothers who kill their young child.3 Neonaticide refers to murder of the infant at birth or in the first day of life.4
Epidemiology and common characteristics
Approximately 15%—or 1 in 7 murders with an arrest—is a filicide.2 The younger the child, the greater the risk, but older children are killed as well.2 Internationally, fathers and mothers are found to kill at similar rates. For other types of homicide, offenders are overwhelmingly male. This makes child murder by parents the singular type of murder in which women and men perpetrate in equal numbers. Fathers are more likely than mothers to also commit suicide after they kill their children.5 The “Cinderella effect” refers to the elevated risk of a stepchild being killed compared to the risk for a biological child.6
In the general international population, mothers who commit filicide tend to have multiple stressors and limited resources. They may be socially isolated and may be victims themselves as well as potentially experiencing substance abuse.1 Some mothers view the child they killed as abnormal.
Less research has been conducted about fathers who kill. Fathers are more likely to also commit partner homicide.5,7 They are more likely to complete filicide-suicide and use firearms or other violent means.5,7-9 Fathers may have a history of violence, substance abuse, and/or mental illness.7
Neonaticide
Mothers are the most common perpetrator of neonaticide.4 It is unusual for a father to be involved in a neonaticide, or for the father and mother to perpetrate the act together. Rates of neonaticide are considered underestimates because of the number of hidden pregnancies, hidden corpses, and the difficulty that forensic pathologists may have in determining whether a baby was born alive or dead.
Continue to: Perpetrators of neonaticide...
Perpetrators of neonaticide tend to be single, relatively young women acting alone. They often live with their parents and are fearful of the repercussions of being pregnant. Pregnancies are often hidden, with no prenatal care. This includes both denial and concealment of pregnancy.4 Perpetrators of neonaticide commonly lack a premorbid serious mental illness, though after the homicide they may develop anxiety, depression, posttraumatic stress disorder (PTSD), or adjustment disorder.4 (Individuals who unwittingly find a murdered baby’s corpse may also be at risk of PTSD.)
Hidden pregnancies may be due to concealment or denial of pregnancy.10,11 Concealment of pregnancy involves a woman knowing she is pregnant, but purposely hiding from others. Concealment may occur after a period of denial of pregnancy. Denial of pregnancy has several subtypes: pervasive denial, affective denial, and psychotic denial. In cases of pervasive denial, the existence of the pregnancy and the pregnancy’s emotional significance is outside the woman’s awareness. Alternatively, in affective denial, she is intellectually aware that she is pregnant but makes little emotional or physical preparation. In the rarest form, psychotic denial, a woman with a psychotic disorder such as schizophrenia may intermittently deny her pregnancy. This may be correlated with a history of custody loss.10,11 Unlike denial of other medical conditions, in cases of denial of pregnancy, there will exist a very specific point in time (delivery) when the reality of the baby confronts the woman. Risks in cases of hidden pregnancies include those from lack of prenatal care and an assisted delivery as well as neonaticide. An FBI study12 of law enforcement files found most neonaticide offenders were single young women with no criminal or psychological history. A caveat is that in the rare cases in which a woman with psychotic illness commits neonaticide, she may have different characteristics from those generally reported.13
Motives
Fathers and mothers have a similar set of motives for killing their child (Table 113-15). Motives are critical to understand not only within forensics, but also for prevention. In performing assessments after a filicide, forensic psychiatrists must be mindful of gender bias.7,16 Resnick15 initially described 5 motives based on his 1969 review of the world literature. Our work5,17 has subsequently further explored these motives.

In child homicides from “fatal maltreatment,” the child has often been a chronic victim of abuse or neglect. National American data indicate that approximately 2 per 100,000 children are killed from child maltreatment annually. Of note in conceptualizing prevention, out of the same population of 100,000, there will be 471 referrals to Child Protective Services and 91 substantiated cases.18 However, only a minority of children who die from maltreatment had previous Child Protective Services involvement. While a child may be killed by fatal maltreatment at any age, one-half are younger than age 1, and three-quarters are younger than age 3.18 In rare cases, a parent who engages in medical child abuse (including factitious disorder imposed upon another) kills the child. Depending on the location and whether or not the death appeared to be intended, parents who kill because of fatal maltreatment might face charges of various levels of murder or manslaughter.
“Unwanted child” homicides occur when the parent has determined that they do not want to have the child, especially in comparison to another need or want. Unwanted child motive is the most common in neonaticide cases, occurring after a hidden pregnancy.4
Continue to: In "partner revenge" cases...
In “partner revenge” cases, parenting disputes, a custody battle, infidelity, or a difficult relationship breakup is often present. The parent wants to make the other parent suffer, and does so by killing their child. A parent may make statements such as “If I can’t have [the child], no one can,” and the child is used as a pawn.
In the final 2 motives—“altruistic” and “acutely psychotic”—mental illness is common. These are the populations we tend to find in samples of filicide-suicide cases where the parent has killed themselves and their child, and those found not guilty by reason of insanity.5,17 Altruistic filicide has been described as “murder out of love.” How can a parent kill their child out of love? Our research has shown several ways. First, the parent may be severely depressed and suicidal. They may be planning their own suicide, and as a parent who loves their child, they plan to take their child with them in death and not leave them alone in the “cruel world” that they themselves are departing. Or the parent may believe they are killing the child out of love to prevent or relieve the child’s suffering. The psychotic parent may believe that a terrible fate will befall their child, and they are killing them “gently.” For example, the parent may believe the child will be tortured or sex trafficked. Some parents may believe that their child has a devastating disease and think they would be better off dead. (Similar thinking of misguided altruism is seen in some cases of intimate partner homicide among older adults.19)
Alternatively, in rare cases of acutely psychotic filicide, parents with psychosis kill their child with no comprehensible motive. For example, they may be in a postictal state or may hear a command hallucination from God in the context of their psychosis.15
Myths vs realities of filicide
Common myths vs the realities of filicide are noted in Table 2. There are issues with believing these myths. For example, if we believe that most parents who kill their child have mental illness, this conflates mental illness and child homicide in our minds as well as the mind of the public. This can lead to further stigmatization of mental illness, and a lack of help-seeking behaviors because parents experiencing psychiatric symptoms may be afraid that if they report their symptoms, their child will be removed by Child Protective Services. However, treated mental illness decreases the risks of child abuse, similar to how treating mental illness decreases risks of other types of violence.20,21

Focusing on prevention
On a local level, we need to understand these tragedies to better understand prevention. To this end, across the United States, counties have Child Fatality Review teams.22 These teams are a partnership across sectors and disciplines, including professionals from health services, law enforcement, and social services—among others—working together to understand cases and consider preventive strategies and additional services needed within our communities.
Continue to: When conceptualizing prevention...
When conceptualizing prevention of child murder by parents, we can think of primary, secondary, and tertiary prevention. This means we want to encourage healthy families and healthy relationships within the family, as well as screening for risk and targeting interventions for families that have experienced difficulties, as well as for parents who have mental illness or substance use disorders.
Understanding the motive behind an individual committing filicide is also critical so that we do not conflate filicide and mental illness. Conflating these concepts leads to increased stigmatization and less help-seeking behavior.
Table 33,4,7,18,22,23 describes the importance of understanding the motives for child murder by a parent in order to conceptualize appropriate prevention. Prevention efforts for 1 type of child murder will not necessarily help prevent murders that occur due to the other motives. Regarding prevention for fatal maltreatment cases, poor parenting skills, including inappropriate expressions of discipline, anger, and frustration, are common. In some cases, substance abuse is involved or the parent was acutely mentally unwell. Reporting to Child Protective Services can be helpful, but as previously noted, it is difficult to ascertain which cases will lead to a homicide. Recommendations from Child Fatality Review teams also are valuable.

Though many parents have frustrations with their children or thoughts of child harm, the act of filicide is rare, and individual cases may be difficult to predict. Regarding prediction, some mothers who committed filicide saw their psychiatrist within days to weeks before the murders.17 A small New Zealand study found that psychotic mothers reported no plans for killing their children in advance, whereas depressed mothers had contemplated the killing for days to weeks.24
Several studies have asked mothers about thoughts of harming their child. Among mothers with colicky infants, 70% reported “explicit aggressive thoughts and fantasies” while 26% had “infanticidal thoughts” during a colic episode.25 Another study26 found that among depressed mothers of infants and toddlers, 41% revealed thoughts of harming their child. Women with postpartum depression preferred not to share infanticidal thoughts with their doctor but were more likely to disclose that they were having suicidal thoughts in order to get needed help.27 Psychiatrists need to feel comfortable asking mothers about their coping skills, their suicidal thoughts, and their filicidal thoughts.14,23,28 Screening and treatment of mental illness is critical. Postpartum psychosis is well-known to pose an elevated risk of filicide and suicide.23 Obsessive-compulsive disorder may cause a parent to ruminate over ego-dystonic child harm but should be treated and the risk conceptualized very differently than in postpartum psychosis.23,29 Screening for postpartum depression and appropriate treatment of depression during pregnancy and the postpartum period decrease risk.30
Continue to: Regarding prevention of neonaticide...
Regarding prevention of neonaticide, Safe Haven laws, baby boxes, anonymous birth options, and increased contraceptive information and availability can help decrease the risk of this well-defined type of homicide.4 Safe Haven laws originated from Child Fatality Review teams.24 Though each state has its own variation, in general, parents can drop off an unharmed unwanted infant into Safe Havens in their state, which may include hospitals, police stations, or fire stations. In general, the mother remains anonymous and has immunity from prosecution for (safe) abandonment. There are drawbacks, such as lack of information regarding adoption and paternal rights. Safe Haven laws do not prevent all deaths and all unsafe abandonments. Baby boxes and baby hatches are used in various nations, including in Europe, and in some places have been used for centuries. In anonymous birth options, such as in France, a mother is not identified but is able to give birth at a hospital. This can decrease the risk from unattended delivery, but many women with denial of pregnancy report that they did not realize when they were about to give birth.4
Bottom Line
Knowledge about the intersection of mental illness and filicide can help in prevention. Parents who experience mental health concerns should be encouraged to obtain needed treatment, which aids prevention. However, many other factors elevate the risk of child murder by parents.
Related Resources
- National Center for Fatality Review and Prevention. https://ncfrp.org/
- Child Welfare Information Gateway. https://www.childwelfare.gov/topics/preventing/overview/federal-agencies/
1. Friedman SH, Horwitz SM, Resnick PJ. Child murder by mothers: a critical analysis of the current state of knowledge and a research agenda. Am J Psych. 2005;162(9):1578-1587.
2. Mariano TY, Chan HC, Myers WC. Toward a more holistic understanding of filicide: a multidisciplinary analysis of 32 years of US arrest data [published corrections appears in Forensic Sci Int. 2014;245:92-94]. Forensic Sci Int. 2014;236:46-53.
3. Hatters Friedman S, Resnick PJ. Child murder by mothers: patterns and prevention. World Psychiatry. 2007;6(3):137-141.
4. Friedman SH, Resnick PJ. Neonaticide: phenomenology and considerations for prevention. Int J Law Psychiatry. 2009;32(1):43-47.
5. Hatters Friedman S, Hrouda DR, Holden CE, et al. Filicide-suicide: common factors in parents who kill their children and themselves. J Am Acad Psychiatry Law. 2005;33(4):496-504.
6. Daly M, Wilson M. Is the “Cinderella effect” controversial? A case study of evolution-minded research and critiques thereof. In: Crawford C, Krebs D, eds. Foundations of Evolutionary Psychology. Taylor & Francis Group/Lawrence Erlbaum Associates; 2008:383-400.
7. Friedman SH. Fathers and filicide: Mental illness and outcomes. In: Wong G, Parnham G, eds. Infanticide and Filicide: Foundations in Maternal Mental Health Forensics. 1st ed. American Psychiatric Association Publishing; 2020:85-107.
8. West SG, Friedman SH, Resnick PJ. Fathers who kill their children: an analysis of the literature. J Forensic Sci. 2009;54(2):463-468.
9. Putkonen H, Amon S, Eronen M, et al. Gender differences in filicide offense characteristics--a comprehensive register-based study of child murder in two European countries. Child Abuse Neglect. 2011;35(5):319-328.
10. Miller LJ. Denial of pregnancy. In: Spinelli MG, ed. Infanticide: Psychosocial and Legal Perspectives on Mothers Who Kill. American Psychiatric Association Publishing; 2003:81-104.
11. Friedman SH, Heneghan A, Rosenthal M. Characteristics of women who deny or conceal pregnancy. Psychosomatics. 2007;48(2):117-122.
12. Beyer K, Mack SM, Shelton JL. Investigative analysis of neonaticide: an exploratory study. Criminal Justice and Behavior. 2008;35(4):522-535.
13. Putkonen H, Weizmann-Henelius G, Collander J, et al. Neonaticides may be more preventable and heterogeneous than previously thought--neonaticides in Finland 1980-2000. Arch Womens Ment Health. 2007;10(1):15-23.
14. Friedman SH, Resnick PJ. Child murder and mental illness in parents: implications for psychiatrists. J Clin Psychiatry. 2011;72(5):587-588.
15. Resnick PJ. Child murder by parents: a psychiatric review of filicide. Am J Psychiatry. 1969;126(3):325-334.
16. Friedman SH. Searching for the whole truth: considering culture and gender in forensic psychiatric practice. J Am Acad Psychiatry Law. 2023;51(1):23-34.
17. Friedman SH, Hrouda DR, Holden CE, et al. Child murder committed by severely mentally ill mothers: an examination of mothers found not guilty by reason of insanity. J Forensic Sci. 2005;50(6):1466-1471.
18. Ash P. Fatal maltreatment and child abuse turned to murder. In: Friedman SH, ed. Family Murder: Pathologies of Love and Hate. Group for the Advancement Psychiatry; 2018.
19. Friedman SH, Appel JM. Murder in the family: intimate partner homicide in the elderly. Psychiatric News. 2018. Accessed April 8, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.12a21
20. Friedman SH, McEwan MV. Treated mental illness and the risk of child abuse perpetration. Psychiatr Serv. 2018;69(2):211-216.
21. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psychiatr Clin North Am. 2016;39(4):691-700.
22. Hatters Friedman S, Beaman JW, Friedman JB. Fatality review and the role of the forensic psychiatrist. J Am Acad Psychiatry Law. 2021;49(3):396-405.
23. Friedman SH, Prakash C, Nagle-Yang S. Postpartum psychosis: protecting mother and infant. Current Psychiatry. 2019;18(4):12-21.
24. Stanton J, Simpson AI, Wouldes T. A qualitative study of filicide by mentally ill mothers. Child Abuse Negl. 2000;24(11):1451-1460.
25. Levitzky S, Cooper R. Infant colic syndrome—maternal fantasies of aggression and infanticide. Clin Pediatr (Phila). 2000;39(7):395-400.
26. Jennings KD, Ross S, Popper S, et al. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54(1-2):21-28.
27. Barr JA, Beck CT. Infanticide secrets: qualitative study on postpartum depression. Can Fam Physician. 2008;54(12):1716-1717.e5.
28. Friedman SH, Sorrentino RM, Stankowski JE, et al. Psychiatrists’ knowledge about maternal filicidal thoughts. Compr Psychiatry. 2008;49(1):106-110.
29. Booth BD, Friedman SH, Curry S, et al. Obsessions of child murder: underrecognized manifestations of obsessive-compulsive disorder. J Am Acad Psychiatry Law. 2014;42(1):66-74.
30. Friedman SH, Hall RCW. Avoiding malpractice while treating depression in pregnant women. Current Psychiatry. 2021;20(8):30-36.
1. Friedman SH, Horwitz SM, Resnick PJ. Child murder by mothers: a critical analysis of the current state of knowledge and a research agenda. Am J Psych. 2005;162(9):1578-1587.
2. Mariano TY, Chan HC, Myers WC. Toward a more holistic understanding of filicide: a multidisciplinary analysis of 32 years of US arrest data [published corrections appears in Forensic Sci Int. 2014;245:92-94]. Forensic Sci Int. 2014;236:46-53.
3. Hatters Friedman S, Resnick PJ. Child murder by mothers: patterns and prevention. World Psychiatry. 2007;6(3):137-141.
4. Friedman SH, Resnick PJ. Neonaticide: phenomenology and considerations for prevention. Int J Law Psychiatry. 2009;32(1):43-47.
5. Hatters Friedman S, Hrouda DR, Holden CE, et al. Filicide-suicide: common factors in parents who kill their children and themselves. J Am Acad Psychiatry Law. 2005;33(4):496-504.
6. Daly M, Wilson M. Is the “Cinderella effect” controversial? A case study of evolution-minded research and critiques thereof. In: Crawford C, Krebs D, eds. Foundations of Evolutionary Psychology. Taylor & Francis Group/Lawrence Erlbaum Associates; 2008:383-400.
7. Friedman SH. Fathers and filicide: Mental illness and outcomes. In: Wong G, Parnham G, eds. Infanticide and Filicide: Foundations in Maternal Mental Health Forensics. 1st ed. American Psychiatric Association Publishing; 2020:85-107.
8. West SG, Friedman SH, Resnick PJ. Fathers who kill their children: an analysis of the literature. J Forensic Sci. 2009;54(2):463-468.
9. Putkonen H, Amon S, Eronen M, et al. Gender differences in filicide offense characteristics--a comprehensive register-based study of child murder in two European countries. Child Abuse Neglect. 2011;35(5):319-328.
10. Miller LJ. Denial of pregnancy. In: Spinelli MG, ed. Infanticide: Psychosocial and Legal Perspectives on Mothers Who Kill. American Psychiatric Association Publishing; 2003:81-104.
11. Friedman SH, Heneghan A, Rosenthal M. Characteristics of women who deny or conceal pregnancy. Psychosomatics. 2007;48(2):117-122.
12. Beyer K, Mack SM, Shelton JL. Investigative analysis of neonaticide: an exploratory study. Criminal Justice and Behavior. 2008;35(4):522-535.
13. Putkonen H, Weizmann-Henelius G, Collander J, et al. Neonaticides may be more preventable and heterogeneous than previously thought--neonaticides in Finland 1980-2000. Arch Womens Ment Health. 2007;10(1):15-23.
14. Friedman SH, Resnick PJ. Child murder and mental illness in parents: implications for psychiatrists. J Clin Psychiatry. 2011;72(5):587-588.
15. Resnick PJ. Child murder by parents: a psychiatric review of filicide. Am J Psychiatry. 1969;126(3):325-334.
16. Friedman SH. Searching for the whole truth: considering culture and gender in forensic psychiatric practice. J Am Acad Psychiatry Law. 2023;51(1):23-34.
17. Friedman SH, Hrouda DR, Holden CE, et al. Child murder committed by severely mentally ill mothers: an examination of mothers found not guilty by reason of insanity. J Forensic Sci. 2005;50(6):1466-1471.
18. Ash P. Fatal maltreatment and child abuse turned to murder. In: Friedman SH, ed. Family Murder: Pathologies of Love and Hate. Group for the Advancement Psychiatry; 2018.
19. Friedman SH, Appel JM. Murder in the family: intimate partner homicide in the elderly. Psychiatric News. 2018. Accessed April 8, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.12a21
20. Friedman SH, McEwan MV. Treated mental illness and the risk of child abuse perpetration. Psychiatr Serv. 2018;69(2):211-216.
21. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psychiatr Clin North Am. 2016;39(4):691-700.
22. Hatters Friedman S, Beaman JW, Friedman JB. Fatality review and the role of the forensic psychiatrist. J Am Acad Psychiatry Law. 2021;49(3):396-405.
23. Friedman SH, Prakash C, Nagle-Yang S. Postpartum psychosis: protecting mother and infant. Current Psychiatry. 2019;18(4):12-21.
24. Stanton J, Simpson AI, Wouldes T. A qualitative study of filicide by mentally ill mothers. Child Abuse Negl. 2000;24(11):1451-1460.
25. Levitzky S, Cooper R. Infant colic syndrome—maternal fantasies of aggression and infanticide. Clin Pediatr (Phila). 2000;39(7):395-400.
26. Jennings KD, Ross S, Popper S, et al. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54(1-2):21-28.
27. Barr JA, Beck CT. Infanticide secrets: qualitative study on postpartum depression. Can Fam Physician. 2008;54(12):1716-1717.e5.
28. Friedman SH, Sorrentino RM, Stankowski JE, et al. Psychiatrists’ knowledge about maternal filicidal thoughts. Compr Psychiatry. 2008;49(1):106-110.
29. Booth BD, Friedman SH, Curry S, et al. Obsessions of child murder: underrecognized manifestations of obsessive-compulsive disorder. J Am Acad Psychiatry Law. 2014;42(1):66-74.
30. Friedman SH, Hall RCW. Avoiding malpractice while treating depression in pregnant women. Current Psychiatry. 2021;20(8):30-36.
Is the contemporary mental health crisis among youth due to DMN disruption?
The advent of unprecedented technologies drastically altering the behavior of children and adolescents, compounded by prolonged isolation from a once-in-a-century pandemic, may have negatively impacted the normal connectivity of the human brain among youth, leading to the current alarming increase of depression, anxiety, and suicidality among this population.
The human brain is comprised of multiple large-scale networks that are functionally connected and control feelings, thoughts, and behaviors. As clinical neuroscientists, psychiatrists must consider the profound impact of a massive societal shift in human behavior on the functional connectivity of brain networks in health and disease. The advent of smartphones, social media, and video game addiction may have disrupted the developing brain networks in children and adolescents, leading to the current escalating epidemic of mental disorders in youth.
The major networks in the human brain include the default mode network (DMN), the salience network, the limbic system, the dorsal attention network, the central executive network, and the visual system.1 Each network connects several brain regions. Researchers can use functional MRI to detect the connectivity of those networks. When blood flow increases concurrently across 2 or 3 networks, this indicates those networks are functionally connected.
There was an old “dogma” that brain regions use energy only when activated and being used. Hans Berger, who developed the EEG in 1929, noticed electrical activity at rest and proposed that the brain is constantly busy, but his neurology peers did not take him seriously.2 In the 1950s, Louis Sokoloff noticed that brain metabolism was the same whether a person is at rest or doing math. In the 1970s, David Ingvar discovered that the highest blood flow in the frontal lobe occurred when a person was at rest.3 Finally, in 2007, Raichle et al4 used positron emission tomography scans to confirm that the frontal lobe is most active when a person is not doing anything. He labeled this phenomenon the DMN, comprising the medial fronto-parietal cortex, the posterior cingulate gyrus, the precuneus, and the angular gyrus. Interestingly, the number of publications about the DMN has skyrocketed since 2007.
The many roles of the DMN
Ongoing research has revealed that the DMN is most active at rest, and its anatomical hubs mediate several key functions5:
- Posterior cingulate gyrus (the central core of the DMN): remembering the past and thinking about the future
- Medial prefrontal cortex: autobiographical memories, future goals and events, reflecting on one’s emotional self, and considering decisions about family members
- Dorsal medial subsystem: thinking about others, determining and inferring the purpose of other people’s actions
- Temporo-parietal junction: reflecting on the beliefs and emotions of others (known as “theory of mind”6)
- Lateral parietal junction: retrieval of social and conceptual knowledge
- Hippocampus: forming new memories, remembering the past, imagining the future
- Posterior-inferior parietal lobe: junction of auditory, visual, and somatic sensory information and attention
- Precuneus: Visual, sensory-motor, and attention.
Many terms have been used to describe the function of the DMN, including “daydreaming,” “auto-pilot,” “mind-wondering,” “reminiscing,” “contemplating,” “self-reflection,” “the neurological basis of the self,” and “seat of literary creativity.”
Psychiatric consequences of DMN deactivation
When another brain network, the attention network (which is also referred to as the task-positive network), is activated consciously and volitionally to perform a task that demands focus (such as text messaging, playing video games, or continuously interacting with social media sites), DMN activity declines.
Continue to: The DMN does not exist...
The DMN does not exist in infants, but starts to develop in childhood.7 It is enhanced by exercise, daydreaming, and sleep, activities that are common in childhood but have declined drastically with the widespread use of smartphones, video games, and social media, which for many youth occupy the bulk of their waking hours. Those tasks, which require continuous attention, deactivate the DMN. In fact, research has shown that addictive behavior decreases the connectivity of the DMN and suppresses its activity.8 Most children and adolescents can be regarded as essentially addicted to social media, text messaging, and video games. Unsurprisingly, serious psychiatric consequences follow.9
DMN dysfunction has been reported in several psychiatric conditions, including depression, posttraumatic stress disorder, autism, schizophrenia, anxiety, obsessive-compulsive disorder, and substance use.10-12 Impaired social interactions and communications, negative ruminations, suicidal ideas, and impaired encoding of long-term memories are some of the adverse effects of DMN dysfunction. The good news is that the DMN’s connectivity and functioning can be modulated and restored by meditation, mentalizing, exercise, psychotherapy, antidepressants, and psychedelics.13,14
The lockdown and stress of the COVID-19 pandemic added insult to injury and exacerbated mental illness in children by isolating them from each other and intensifying their technological addiction to fill the void of isolation. This crisis in youth mental health continues unabated, and calls for action to prevent grim outcomes. DMN dysfunction in youth can be reversed with treatment, but access to mental health care has become more challenging due to workforce shortages and insurance restrictions. Psychiatrists and parents must work diligently to treat psychiatrically affected youth, which has become a DaMN serious problem…
1. Yao Z, Hu B, Xie Y, et al. A review of structural and functional brain networks: small world and atlas. Brain Inform. 2015;2(1):45-52. doi:10.1007/s40708-015-0009-z
2. Raichle ME. The brain’s dark energy. Sci Am. 2010;302(3):44-49. doi:10.1038/scientific american0310-44
3. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1-38. doi:10.1196/annals.1440.011
4. Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37(4):1083-1090; discussion 1097-1099. doi:10.1016/j.neuroimage.2007.02.041
5. Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18(3):251-270. doi:10.1177/1073858411403316
6. Tsoukalas I. Theory of mind: towards an evolutionary theory. Evolutionary Psychological Science. 2018;4(1):38-66. https://doi.org/10.1007/s40806-017-0112-x
7. Broyd SJ, Demanuele C, Debener S, et al. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33(3):279-296. doi:10.1016/j.neubiorev.2008.09.002
8. Zhang R, Volkow ND. Brain default-mode network dysfunction in addiction. Neuroimage. 2019;200:313-331. doi:10.1016/j.neuroimage.2019.06.036
9. Bommersbach TJ, McKean AJ, Olfson M, et al. National trends in mental health-related emergency department visits among youth, 2011-2020. JAMA. 2023;329(17):1469-1477. doi:10.1001/jama.2023.4809
10. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49-76. doi:10.1146/annurev-clinpsy-032511-143049
11. Akiki TJ, Averill CL, Wrocklage KM, et al. Default mode network abnormalities in posttraumatic stress disorder: a novel network-restricted topology approach. Neuroimage. 2018;176:489-498. doi:10.1016/j.neuroimage.2018.05.005
12. Nagata JM, Chu J, Zamora G, et al. Screen time and obsessive-compulsive disorder among children 9-10 years old: a prospective cohort study. J Adolesc Health. 2023;72(3):390-396. doi:10.1016/j.jadohealth.2022.10.023
13. Fox KC, Nijeboer S, Dixon ML, et al. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci Biobehav Rev. 2014;43:48-73. doi:10.1016/j.neubiorev.2014.03.016
14. Gattuso JJ, Perkins D, Ruffell S, et al. Default mode network modulation by psychedelics: a systematic review. Int J Neuropsychopharmacol. 2023;26(3):155-188. doi:10.1093/ijnp/pyac074
The advent of unprecedented technologies drastically altering the behavior of children and adolescents, compounded by prolonged isolation from a once-in-a-century pandemic, may have negatively impacted the normal connectivity of the human brain among youth, leading to the current alarming increase of depression, anxiety, and suicidality among this population.
The human brain is comprised of multiple large-scale networks that are functionally connected and control feelings, thoughts, and behaviors. As clinical neuroscientists, psychiatrists must consider the profound impact of a massive societal shift in human behavior on the functional connectivity of brain networks in health and disease. The advent of smartphones, social media, and video game addiction may have disrupted the developing brain networks in children and adolescents, leading to the current escalating epidemic of mental disorders in youth.
The major networks in the human brain include the default mode network (DMN), the salience network, the limbic system, the dorsal attention network, the central executive network, and the visual system.1 Each network connects several brain regions. Researchers can use functional MRI to detect the connectivity of those networks. When blood flow increases concurrently across 2 or 3 networks, this indicates those networks are functionally connected.
There was an old “dogma” that brain regions use energy only when activated and being used. Hans Berger, who developed the EEG in 1929, noticed electrical activity at rest and proposed that the brain is constantly busy, but his neurology peers did not take him seriously.2 In the 1950s, Louis Sokoloff noticed that brain metabolism was the same whether a person is at rest or doing math. In the 1970s, David Ingvar discovered that the highest blood flow in the frontal lobe occurred when a person was at rest.3 Finally, in 2007, Raichle et al4 used positron emission tomography scans to confirm that the frontal lobe is most active when a person is not doing anything. He labeled this phenomenon the DMN, comprising the medial fronto-parietal cortex, the posterior cingulate gyrus, the precuneus, and the angular gyrus. Interestingly, the number of publications about the DMN has skyrocketed since 2007.
The many roles of the DMN
Ongoing research has revealed that the DMN is most active at rest, and its anatomical hubs mediate several key functions5:
- Posterior cingulate gyrus (the central core of the DMN): remembering the past and thinking about the future
- Medial prefrontal cortex: autobiographical memories, future goals and events, reflecting on one’s emotional self, and considering decisions about family members
- Dorsal medial subsystem: thinking about others, determining and inferring the purpose of other people’s actions
- Temporo-parietal junction: reflecting on the beliefs and emotions of others (known as “theory of mind”6)
- Lateral parietal junction: retrieval of social and conceptual knowledge
- Hippocampus: forming new memories, remembering the past, imagining the future
- Posterior-inferior parietal lobe: junction of auditory, visual, and somatic sensory information and attention
- Precuneus: Visual, sensory-motor, and attention.
Many terms have been used to describe the function of the DMN, including “daydreaming,” “auto-pilot,” “mind-wondering,” “reminiscing,” “contemplating,” “self-reflection,” “the neurological basis of the self,” and “seat of literary creativity.”
Psychiatric consequences of DMN deactivation
When another brain network, the attention network (which is also referred to as the task-positive network), is activated consciously and volitionally to perform a task that demands focus (such as text messaging, playing video games, or continuously interacting with social media sites), DMN activity declines.
Continue to: The DMN does not exist...
The DMN does not exist in infants, but starts to develop in childhood.7 It is enhanced by exercise, daydreaming, and sleep, activities that are common in childhood but have declined drastically with the widespread use of smartphones, video games, and social media, which for many youth occupy the bulk of their waking hours. Those tasks, which require continuous attention, deactivate the DMN. In fact, research has shown that addictive behavior decreases the connectivity of the DMN and suppresses its activity.8 Most children and adolescents can be regarded as essentially addicted to social media, text messaging, and video games. Unsurprisingly, serious psychiatric consequences follow.9
DMN dysfunction has been reported in several psychiatric conditions, including depression, posttraumatic stress disorder, autism, schizophrenia, anxiety, obsessive-compulsive disorder, and substance use.10-12 Impaired social interactions and communications, negative ruminations, suicidal ideas, and impaired encoding of long-term memories are some of the adverse effects of DMN dysfunction. The good news is that the DMN’s connectivity and functioning can be modulated and restored by meditation, mentalizing, exercise, psychotherapy, antidepressants, and psychedelics.13,14
The lockdown and stress of the COVID-19 pandemic added insult to injury and exacerbated mental illness in children by isolating them from each other and intensifying their technological addiction to fill the void of isolation. This crisis in youth mental health continues unabated, and calls for action to prevent grim outcomes. DMN dysfunction in youth can be reversed with treatment, but access to mental health care has become more challenging due to workforce shortages and insurance restrictions. Psychiatrists and parents must work diligently to treat psychiatrically affected youth, which has become a DaMN serious problem…
The advent of unprecedented technologies drastically altering the behavior of children and adolescents, compounded by prolonged isolation from a once-in-a-century pandemic, may have negatively impacted the normal connectivity of the human brain among youth, leading to the current alarming increase of depression, anxiety, and suicidality among this population.
The human brain is comprised of multiple large-scale networks that are functionally connected and control feelings, thoughts, and behaviors. As clinical neuroscientists, psychiatrists must consider the profound impact of a massive societal shift in human behavior on the functional connectivity of brain networks in health and disease. The advent of smartphones, social media, and video game addiction may have disrupted the developing brain networks in children and adolescents, leading to the current escalating epidemic of mental disorders in youth.
The major networks in the human brain include the default mode network (DMN), the salience network, the limbic system, the dorsal attention network, the central executive network, and the visual system.1 Each network connects several brain regions. Researchers can use functional MRI to detect the connectivity of those networks. When blood flow increases concurrently across 2 or 3 networks, this indicates those networks are functionally connected.
There was an old “dogma” that brain regions use energy only when activated and being used. Hans Berger, who developed the EEG in 1929, noticed electrical activity at rest and proposed that the brain is constantly busy, but his neurology peers did not take him seriously.2 In the 1950s, Louis Sokoloff noticed that brain metabolism was the same whether a person is at rest or doing math. In the 1970s, David Ingvar discovered that the highest blood flow in the frontal lobe occurred when a person was at rest.3 Finally, in 2007, Raichle et al4 used positron emission tomography scans to confirm that the frontal lobe is most active when a person is not doing anything. He labeled this phenomenon the DMN, comprising the medial fronto-parietal cortex, the posterior cingulate gyrus, the precuneus, and the angular gyrus. Interestingly, the number of publications about the DMN has skyrocketed since 2007.
The many roles of the DMN
Ongoing research has revealed that the DMN is most active at rest, and its anatomical hubs mediate several key functions5:
- Posterior cingulate gyrus (the central core of the DMN): remembering the past and thinking about the future
- Medial prefrontal cortex: autobiographical memories, future goals and events, reflecting on one’s emotional self, and considering decisions about family members
- Dorsal medial subsystem: thinking about others, determining and inferring the purpose of other people’s actions
- Temporo-parietal junction: reflecting on the beliefs and emotions of others (known as “theory of mind”6)
- Lateral parietal junction: retrieval of social and conceptual knowledge
- Hippocampus: forming new memories, remembering the past, imagining the future
- Posterior-inferior parietal lobe: junction of auditory, visual, and somatic sensory information and attention
- Precuneus: Visual, sensory-motor, and attention.
Many terms have been used to describe the function of the DMN, including “daydreaming,” “auto-pilot,” “mind-wondering,” “reminiscing,” “contemplating,” “self-reflection,” “the neurological basis of the self,” and “seat of literary creativity.”
Psychiatric consequences of DMN deactivation
When another brain network, the attention network (which is also referred to as the task-positive network), is activated consciously and volitionally to perform a task that demands focus (such as text messaging, playing video games, or continuously interacting with social media sites), DMN activity declines.
Continue to: The DMN does not exist...
The DMN does not exist in infants, but starts to develop in childhood.7 It is enhanced by exercise, daydreaming, and sleep, activities that are common in childhood but have declined drastically with the widespread use of smartphones, video games, and social media, which for many youth occupy the bulk of their waking hours. Those tasks, which require continuous attention, deactivate the DMN. In fact, research has shown that addictive behavior decreases the connectivity of the DMN and suppresses its activity.8 Most children and adolescents can be regarded as essentially addicted to social media, text messaging, and video games. Unsurprisingly, serious psychiatric consequences follow.9
DMN dysfunction has been reported in several psychiatric conditions, including depression, posttraumatic stress disorder, autism, schizophrenia, anxiety, obsessive-compulsive disorder, and substance use.10-12 Impaired social interactions and communications, negative ruminations, suicidal ideas, and impaired encoding of long-term memories are some of the adverse effects of DMN dysfunction. The good news is that the DMN’s connectivity and functioning can be modulated and restored by meditation, mentalizing, exercise, psychotherapy, antidepressants, and psychedelics.13,14
The lockdown and stress of the COVID-19 pandemic added insult to injury and exacerbated mental illness in children by isolating them from each other and intensifying their technological addiction to fill the void of isolation. This crisis in youth mental health continues unabated, and calls for action to prevent grim outcomes. DMN dysfunction in youth can be reversed with treatment, but access to mental health care has become more challenging due to workforce shortages and insurance restrictions. Psychiatrists and parents must work diligently to treat psychiatrically affected youth, which has become a DaMN serious problem…
1. Yao Z, Hu B, Xie Y, et al. A review of structural and functional brain networks: small world and atlas. Brain Inform. 2015;2(1):45-52. doi:10.1007/s40708-015-0009-z
2. Raichle ME. The brain’s dark energy. Sci Am. 2010;302(3):44-49. doi:10.1038/scientific american0310-44
3. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1-38. doi:10.1196/annals.1440.011
4. Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37(4):1083-1090; discussion 1097-1099. doi:10.1016/j.neuroimage.2007.02.041
5. Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18(3):251-270. doi:10.1177/1073858411403316
6. Tsoukalas I. Theory of mind: towards an evolutionary theory. Evolutionary Psychological Science. 2018;4(1):38-66. https://doi.org/10.1007/s40806-017-0112-x
7. Broyd SJ, Demanuele C, Debener S, et al. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33(3):279-296. doi:10.1016/j.neubiorev.2008.09.002
8. Zhang R, Volkow ND. Brain default-mode network dysfunction in addiction. Neuroimage. 2019;200:313-331. doi:10.1016/j.neuroimage.2019.06.036
9. Bommersbach TJ, McKean AJ, Olfson M, et al. National trends in mental health-related emergency department visits among youth, 2011-2020. JAMA. 2023;329(17):1469-1477. doi:10.1001/jama.2023.4809
10. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49-76. doi:10.1146/annurev-clinpsy-032511-143049
11. Akiki TJ, Averill CL, Wrocklage KM, et al. Default mode network abnormalities in posttraumatic stress disorder: a novel network-restricted topology approach. Neuroimage. 2018;176:489-498. doi:10.1016/j.neuroimage.2018.05.005
12. Nagata JM, Chu J, Zamora G, et al. Screen time and obsessive-compulsive disorder among children 9-10 years old: a prospective cohort study. J Adolesc Health. 2023;72(3):390-396. doi:10.1016/j.jadohealth.2022.10.023
13. Fox KC, Nijeboer S, Dixon ML, et al. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci Biobehav Rev. 2014;43:48-73. doi:10.1016/j.neubiorev.2014.03.016
14. Gattuso JJ, Perkins D, Ruffell S, et al. Default mode network modulation by psychedelics: a systematic review. Int J Neuropsychopharmacol. 2023;26(3):155-188. doi:10.1093/ijnp/pyac074
1. Yao Z, Hu B, Xie Y, et al. A review of structural and functional brain networks: small world and atlas. Brain Inform. 2015;2(1):45-52. doi:10.1007/s40708-015-0009-z
2. Raichle ME. The brain’s dark energy. Sci Am. 2010;302(3):44-49. doi:10.1038/scientific american0310-44
3. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1-38. doi:10.1196/annals.1440.011
4. Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37(4):1083-1090; discussion 1097-1099. doi:10.1016/j.neuroimage.2007.02.041
5. Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18(3):251-270. doi:10.1177/1073858411403316
6. Tsoukalas I. Theory of mind: towards an evolutionary theory. Evolutionary Psychological Science. 2018;4(1):38-66. https://doi.org/10.1007/s40806-017-0112-x
7. Broyd SJ, Demanuele C, Debener S, et al. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33(3):279-296. doi:10.1016/j.neubiorev.2008.09.002
8. Zhang R, Volkow ND. Brain default-mode network dysfunction in addiction. Neuroimage. 2019;200:313-331. doi:10.1016/j.neuroimage.2019.06.036
9. Bommersbach TJ, McKean AJ, Olfson M, et al. National trends in mental health-related emergency department visits among youth, 2011-2020. JAMA. 2023;329(17):1469-1477. doi:10.1001/jama.2023.4809
10. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49-76. doi:10.1146/annurev-clinpsy-032511-143049
11. Akiki TJ, Averill CL, Wrocklage KM, et al. Default mode network abnormalities in posttraumatic stress disorder: a novel network-restricted topology approach. Neuroimage. 2018;176:489-498. doi:10.1016/j.neuroimage.2018.05.005
12. Nagata JM, Chu J, Zamora G, et al. Screen time and obsessive-compulsive disorder among children 9-10 years old: a prospective cohort study. J Adolesc Health. 2023;72(3):390-396. doi:10.1016/j.jadohealth.2022.10.023
13. Fox KC, Nijeboer S, Dixon ML, et al. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci Biobehav Rev. 2014;43:48-73. doi:10.1016/j.neubiorev.2014.03.016
14. Gattuso JJ, Perkins D, Ruffell S, et al. Default mode network modulation by psychedelics: a systematic review. Int J Neuropsychopharmacol. 2023;26(3):155-188. doi:10.1093/ijnp/pyac074
High-dose stimulants for adult ADHD
Ms. H, age 30, presents to the outpatient clinic for a follow-up visit, where she reports difficulty paying attention to conversations, starting and completing tasks, and meeting deadlines. These challenges occur at work and home. Her psychiatric history includes attention-deficit/hyperactivity disorder (ADHD), major depressive disorder, and generalized anxiety disorder. Approximately 10 years ago, she underwent Roux-en-Y gastric bypass surgery. Following surgery, Ms. H’s care team prescribed liquid formulations of medications whenever possible to minimize malabsorption. Ms. H may be a rapid metabolizer; she says the effects of her prescribed stimulants only last briefly, so she has to frequently redose. As a result, she often runs out of her monthly stimulant allotment earlier than expected.
Ms. H’s current medications include dextroamphetamine/amphetamine immediate-release (IR) 30 mg 3 times daily, atenolol 50 mg/d, and escitalopram oral solution 10 mg/d. Previous unsuccessful medication trials for her ADHD include methylphenidate IR 20 mg 3 times daily and lisdexamfetamine 70 mg/d. Ms. H reports that when her responsibilities increased at work or home, she took methylphenidate IR 20 mg up to 6 times daily to relieve her symptoms.
In the United States, ADHD affects an estimated 4.4% of adults age 18 to 44.1 The actual rate may be higher, however, as recent research has called into question the hypothesis that approximately 50% of cases of childhood ADHD remit by adulthood.2 Prevalence estimates relying on DSM-IV criteria (which were designed with children in mind) can underestimate this condition in adults. Newer data suggest that up to 90% of individuals with ADHD in childhood continue to experience significant ADHD symptoms into adulthood.2
Unless contraindications are present, methylphenidate or amphetamine-based stimulants are the medications of choice for treating adult ADHD.3 Many formulations of both medications are available,4 which allows clinicians to better tailor therapy to each patient’s pharmacokinetics and daily schedule. Although there can be differences in response and tolerability, methylphenidate and amphetamine offer comparable efficacy and a similar adverse effect profile.5
Because amphetamine is more potent than methylphenidate, clinicians commonly start treatment with an amphetamine dose that is one-half to two-thirds the dose of methylphenidate.6 While both classes of stimulants inhibit the reuptake of dopamine and norepinephrine into presynaptic neurons, amphetamines also promote the release of dopamine and norepinephrine from their storage sites in presynaptic nerve terminals.3
Methylphenidate
Methylphenidate IR has an average onset of action of 30 to 45 minutes and its effects last approximately 3 to 4 hours. The extended-release (XR) formulations have varying onsets of action, with durations of action up to 12 hours (Table 13,7).4 The XR products usually immediately release a certain percentage of the medication, eliminating the need for an additional IR tablet. One methylphenidate XR product (Jornay) as well as serdexmethylphenidate/dexmethylphenidate (Azstarys) offer durations of action of 24 to 36 hours. Methylphenidate is primarily metabolized by carboxylesterase 1 (CES1) to the inactive metabolite ritalinic acid. Most of the medication (60% to 80%) is excreted in the urine as ritalinic acid.4 Theoretically, genetic variations in the CES1 and concomitant use of medications that compete with or alter this pathway may impact methylphenidate pharmacokinetics.8 However, plasma levels have not yet shown to be helpful in guiding treatment selection or dosing.4

Amphetamine
Dextroamphetamine/amphetamine IR has an average onset of action of 30 to 45 minutes and its effects last approximately 4 to 6 hours. XR formulations have varying onsets of action, with durations of action up to 13 hours (Table 23,7,9).4 One XR product, mixed salts of single amphetamine entity (Mydayis), has a duration of action of 16 hours. In XR formulations, a certain percentage of the medication is typically released immediately, eliminating the need for an additional IR tablet. Amphetamine is primarily metabolized by cytochrome P450 (CYP) 2D6 hydroxylation and oxidative deamination. Genetic variability in amphetamine metabolism may be relevant due to CYP2D6 polymorphisms. Ultra-rapid metabolizers might need higher doses, while poor metabolizers might require smaller amounts and may be more susceptible to adverse effects.4 However, there is currently insufficient data supporting gene/medication concentration relationships. As is the case with methylphenidate, plasma levels have not yet shown to be helpful in guiding treatment selection or dosing.6

Continue to: Impaired medication absorption after bariatric surgery
Impaired medication absorption after bariatric surgery
Medication malabsorption following bariatric surgery is a significant concern. In a systematic review of 22 studies, Padwal et al10 found that in one-third of these studies, decreased absorption following bariatric surgery may be present in patients taking medications that have poor absorption, high lipophilicity, or enterohepatic recirculation. Childress et al11 found that methylphenidate IR and dextroamphetamine/amphetamine are both well absorbed, with bioavailability percentages of 100% and 90%, respectively. Additional research shows both stimulants have rapid absorption rates but relatively poor bioavailability.12 In one study analyzing the dissolution of common psychiatric medications, methylphenidate was shown to dissolve slightly more in the Roux-en-Y gastric bypass surgery model (80 mg) compared to controls (70 mg).13 One case indicated potential methylphenidate toxicity following Roux-en-Y gastric bypass surgery,14 while another suggested impaired absorption following the same procedure.15 A case-control design study assessing the impact of Roux-en-Y gastric bypass surgery on the pharmacokinetic properties of lisdexamfetamine found no significant differences between the Roux-en-Y group (n = 10) and nonsurgical controls (n = 10). The investigators concluded that while data suggest adjusting lisdexamfetamine dosing following Roux-en-Y gastric bypass surgery is unnecessary, there may be interindividual differences, and individualized dosing regimens may be needed.16
When managing patients who might be experiencing medication malabsorption, it may be helpful to use dosage forms that avoid disintegration, acidic environments, and slow dissolution. Because they are more rapidly absorbed and not susceptible to disintegration and dissolution, liquid formulations are recommended.17 For medications that are not available as a liquid, an IR formulation is recommended.18
Using nonoral routes of administration that avoid the anatomical changes of the gastrointestinal tract should be considered for patients who have undergone Roux-en-Y gastric bypass surgery.17 The methylphenidate transdermal patch, a medication delivery system that avoids gut and hepatic first-pass metabolism, can improve medication bioavailability, reduce dose frequency, and stabilize medication delivery. It is available in 4 sizes/dosages: 10 mg/9 hours, 15 mg/9 hours, 20 mg/9 hours, and 30 mg/9 hours. Methylphenidate is delivered at a steady rate based upon patch size. The onset of action of the patch is approximately 2 hours, and patients should wear the patch for 9 hours, then remove it. Methylphenidate will still be absorbed up to 2 to 3 hours after patch removal. Appropriate application and removal of the patch is important for optimal effectiveness and to avoid adverse effects.4
In March 2022, t
CASE CONTINUED
Ms. H emphasizes her desire to maintain functionality in all areas of life, while her care team reiterates the risks of continuing to take high-dose stimulants. Both Ms. H and her care team acknowledge that stimulant usage could be worsening her anxiety, and that Roux-en-Y gastric bypass surgery may be a possible explanation for her dosing challenges.
Continue to: Following consultation with the pharmacist...
Following consultation with the pharmacist, the care team explains the possible pharmacokinetic benefits of using the methylphenidate transdermal patch. After completing the prior authorization paperwork, Ms. H is started on the 30 mg/d patch. This dose was selected because she previously tolerated high-dose stimulants, including methylphenidate IR 20 mg up to 6 times daily. At a follow-up visit 1 month after starting the patch, Ms. H reports an improvement in her ADHD symptoms and says she is not experiencing any adverse effects.
Related Resources
- DeMarco R, Rana R, Powell K, et al. How bariatric surgery affects psychotropic drug absorption. Current Psychiatry. 2022;21(8):39-44. doi:10.12788/cp.0271
- Santos MG, Majarwitz DJ, Saeed SA. Adult ADHD: 6 studies of pharmacologic interventions. Current Psychiatry. 2023;22(4):16-27. doi:10.12788/cp.0344
Drug Brand Names
Amphetamine sulfate • Adzenys ER, Adzenys XR-ODT, Dyanavel XR, Evekeo
Atenolol • Tenormin
Dexmethylphenidate • Focalin, Focalin XR
Dextroamphetamine transdermal • Xelstrym
Dextroamphetamine • Dexedrine, Dexedrine Spansule, ProCentra, Zenzedi
Escitalopram • Lexapro
Lisdexamfetamine • Vyvanse
Methylphenidate • Aptensio XR, Adhansia XR, Concerta, Cotempla, Jornay PM, Metadate CD, Metadate ER, Methylin, Qullichew ER, Quillivant XR, Relexxii, Ritalin, Ritalin LA
Methylphenidate transdermal • Daytrana
Mixed amphetamine salts • Adderall, Adderall XR
Mixed salts of a single-entity amphetamine • Mydayis
Serdexmethylphenidate and dexmethylphenidate • Azstarys
1. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716-723. doi:10.1176/ajp.2006.163.4.716
2. Sibley MH, Arnold LE, Swanson JM, et al. Variable patterns of remission from ADHD in the multimodal treatment study of ADHD. Am J Psychiatry. 2022;179(2):142-151. doi:10.1176/appi.ajp.2021.21010032
3. Cleveland KW, Boyle J, Robinson RF. Attention-deficit/hyperactivity disorder. In: Chisholm-Burns MA, Schwinghammer TL, Malone PM, et al, eds. Pharmacotherapy Principles & Practice. 6th ed. McGraw Hill; 2022. Accessed December 1, 2022. https://ppp.mhmedical.com/content.aspx?bookid=3114§ionid=261474885
4. Steingard R, Taskiran S, Connor DF, et al. New formulations of stimulants: an update for clinicians. J Child Adolesc Psychopharmacol. 2019;29(5):324-339. doi:10.1089/cap.2019.0043
5. Faraone SV. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. 2018;87:255-270. doi:10.1016/j.neubiorev.2018.02.001
6. Markowitz JS, Patrick KS. The clinical pharmacokinetics of amphetamines utilized in the treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2017;27(8):678-689. doi:10.1089/cap.2017.0071
7. Mullen S. Medication Table 2: Attention Deficit Hyperactivity Disorder. In: English C, ed. CPNP Psychiatric Pharmacotherapy Review Course. 2022-2023 ed. College of Psychiatric and Neurologic Pharmacists; 2022.
8. Zhu HJ, Patrick KS, Yuan HJ, et al. Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: clinical significance and molecular basis. Am J Hum Genet. 2008;82(6):1241-1248. doi:10.1016/j.ajhg.2008.04.015
9. Xelstrym [package insert]. Miami, FL: Noven Pharmaceuticals, Inc.; 2022.
10. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50. doi:10.1111/j.1467-789X.2009.00614.x
11. Childress AC, Komolova M, Sallee FR. An update on the pharmacokinetic considerations in the treatment of ADHD with long-acting methylphenidate and amphetamine formulations. Expert Opin Drug Metab Toxicol. 2019;15(11):937-974. doi:10.1080/17425255.2019.1675636
12. Markowitz JS, Melchert PW. The pharmacokinetics and pharmacogenomics of psychostimulants. Child Adolesc Psychiatr Clin N Am. 2022;31(3):393-416. doi:10.1016/j.chc.2022.03.003
13. Seaman JS, Bowers SP, Dixon P, et al. Dissolution of common psychiatric medications in a Roux-en-Y gastric bypass model. Psychosomatics. 2005;46(3):250-253. doi:10.1176/appi.psy.46.3.250
14. Ludvigsson M, Haenni A. Methylphenidate toxicity after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;12(5):e55-e57. doi:10.1016/j.soard.2016.03.015
15. Azran C, Langguth P, Dahan A. Impaired oral absorption of methylphenidate after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2017;13(7):1245-1247. doi:10.1016/j.soard.2017.03.003
16. Steffen KJ, Mohammad AS, Roerig JL, et al. Lisdexamfetamine pharmacokinetic comparison between patients who underwent Roux-en-Y gastric bypass and nonsurgical controls. Obes Surg. 2021;31(10):4289-4294. doi:10.1007/s11695-020-04969-4
17. Buxton ILO. Pharmacokinetics: the dynamics of drug absorption, distribution, metabolism, and elimination. In: Brunton LL, Knollmann BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 14th ed. McGraw Hill; 2023. Accessed December 1, 2022. https://accesspharmacy.mhmedical.com/content.aspx?bookid=2189§ionid=166182905
18. DeMarco R, Rana R, Powell K, et al. How bariatric surgery affects psychotropic drug absorption. Current Psychiatry. 2022;21(8):39-44. doi:10.12788/cp.0271
Ms. H, age 30, presents to the outpatient clinic for a follow-up visit, where she reports difficulty paying attention to conversations, starting and completing tasks, and meeting deadlines. These challenges occur at work and home. Her psychiatric history includes attention-deficit/hyperactivity disorder (ADHD), major depressive disorder, and generalized anxiety disorder. Approximately 10 years ago, she underwent Roux-en-Y gastric bypass surgery. Following surgery, Ms. H’s care team prescribed liquid formulations of medications whenever possible to minimize malabsorption. Ms. H may be a rapid metabolizer; she says the effects of her prescribed stimulants only last briefly, so she has to frequently redose. As a result, she often runs out of her monthly stimulant allotment earlier than expected.
Ms. H’s current medications include dextroamphetamine/amphetamine immediate-release (IR) 30 mg 3 times daily, atenolol 50 mg/d, and escitalopram oral solution 10 mg/d. Previous unsuccessful medication trials for her ADHD include methylphenidate IR 20 mg 3 times daily and lisdexamfetamine 70 mg/d. Ms. H reports that when her responsibilities increased at work or home, she took methylphenidate IR 20 mg up to 6 times daily to relieve her symptoms.
In the United States, ADHD affects an estimated 4.4% of adults age 18 to 44.1 The actual rate may be higher, however, as recent research has called into question the hypothesis that approximately 50% of cases of childhood ADHD remit by adulthood.2 Prevalence estimates relying on DSM-IV criteria (which were designed with children in mind) can underestimate this condition in adults. Newer data suggest that up to 90% of individuals with ADHD in childhood continue to experience significant ADHD symptoms into adulthood.2
Unless contraindications are present, methylphenidate or amphetamine-based stimulants are the medications of choice for treating adult ADHD.3 Many formulations of both medications are available,4 which allows clinicians to better tailor therapy to each patient’s pharmacokinetics and daily schedule. Although there can be differences in response and tolerability, methylphenidate and amphetamine offer comparable efficacy and a similar adverse effect profile.5
Because amphetamine is more potent than methylphenidate, clinicians commonly start treatment with an amphetamine dose that is one-half to two-thirds the dose of methylphenidate.6 While both classes of stimulants inhibit the reuptake of dopamine and norepinephrine into presynaptic neurons, amphetamines also promote the release of dopamine and norepinephrine from their storage sites in presynaptic nerve terminals.3
Methylphenidate
Methylphenidate IR has an average onset of action of 30 to 45 minutes and its effects last approximately 3 to 4 hours. The extended-release (XR) formulations have varying onsets of action, with durations of action up to 12 hours (Table 13,7).4 The XR products usually immediately release a certain percentage of the medication, eliminating the need for an additional IR tablet. One methylphenidate XR product (Jornay) as well as serdexmethylphenidate/dexmethylphenidate (Azstarys) offer durations of action of 24 to 36 hours. Methylphenidate is primarily metabolized by carboxylesterase 1 (CES1) to the inactive metabolite ritalinic acid. Most of the medication (60% to 80%) is excreted in the urine as ritalinic acid.4 Theoretically, genetic variations in the CES1 and concomitant use of medications that compete with or alter this pathway may impact methylphenidate pharmacokinetics.8 However, plasma levels have not yet shown to be helpful in guiding treatment selection or dosing.4

Amphetamine
Dextroamphetamine/amphetamine IR has an average onset of action of 30 to 45 minutes and its effects last approximately 4 to 6 hours. XR formulations have varying onsets of action, with durations of action up to 13 hours (Table 23,7,9).4 One XR product, mixed salts of single amphetamine entity (Mydayis), has a duration of action of 16 hours. In XR formulations, a certain percentage of the medication is typically released immediately, eliminating the need for an additional IR tablet. Amphetamine is primarily metabolized by cytochrome P450 (CYP) 2D6 hydroxylation and oxidative deamination. Genetic variability in amphetamine metabolism may be relevant due to CYP2D6 polymorphisms. Ultra-rapid metabolizers might need higher doses, while poor metabolizers might require smaller amounts and may be more susceptible to adverse effects.4 However, there is currently insufficient data supporting gene/medication concentration relationships. As is the case with methylphenidate, plasma levels have not yet shown to be helpful in guiding treatment selection or dosing.6

Continue to: Impaired medication absorption after bariatric surgery
Impaired medication absorption after bariatric surgery
Medication malabsorption following bariatric surgery is a significant concern. In a systematic review of 22 studies, Padwal et al10 found that in one-third of these studies, decreased absorption following bariatric surgery may be present in patients taking medications that have poor absorption, high lipophilicity, or enterohepatic recirculation. Childress et al11 found that methylphenidate IR and dextroamphetamine/amphetamine are both well absorbed, with bioavailability percentages of 100% and 90%, respectively. Additional research shows both stimulants have rapid absorption rates but relatively poor bioavailability.12 In one study analyzing the dissolution of common psychiatric medications, methylphenidate was shown to dissolve slightly more in the Roux-en-Y gastric bypass surgery model (80 mg) compared to controls (70 mg).13 One case indicated potential methylphenidate toxicity following Roux-en-Y gastric bypass surgery,14 while another suggested impaired absorption following the same procedure.15 A case-control design study assessing the impact of Roux-en-Y gastric bypass surgery on the pharmacokinetic properties of lisdexamfetamine found no significant differences between the Roux-en-Y group (n = 10) and nonsurgical controls (n = 10). The investigators concluded that while data suggest adjusting lisdexamfetamine dosing following Roux-en-Y gastric bypass surgery is unnecessary, there may be interindividual differences, and individualized dosing regimens may be needed.16
When managing patients who might be experiencing medication malabsorption, it may be helpful to use dosage forms that avoid disintegration, acidic environments, and slow dissolution. Because they are more rapidly absorbed and not susceptible to disintegration and dissolution, liquid formulations are recommended.17 For medications that are not available as a liquid, an IR formulation is recommended.18
Using nonoral routes of administration that avoid the anatomical changes of the gastrointestinal tract should be considered for patients who have undergone Roux-en-Y gastric bypass surgery.17 The methylphenidate transdermal patch, a medication delivery system that avoids gut and hepatic first-pass metabolism, can improve medication bioavailability, reduce dose frequency, and stabilize medication delivery. It is available in 4 sizes/dosages: 10 mg/9 hours, 15 mg/9 hours, 20 mg/9 hours, and 30 mg/9 hours. Methylphenidate is delivered at a steady rate based upon patch size. The onset of action of the patch is approximately 2 hours, and patients should wear the patch for 9 hours, then remove it. Methylphenidate will still be absorbed up to 2 to 3 hours after patch removal. Appropriate application and removal of the patch is important for optimal effectiveness and to avoid adverse effects.4
In March 2022, t
CASE CONTINUED
Ms. H emphasizes her desire to maintain functionality in all areas of life, while her care team reiterates the risks of continuing to take high-dose stimulants. Both Ms. H and her care team acknowledge that stimulant usage could be worsening her anxiety, and that Roux-en-Y gastric bypass surgery may be a possible explanation for her dosing challenges.
Continue to: Following consultation with the pharmacist...
Following consultation with the pharmacist, the care team explains the possible pharmacokinetic benefits of using the methylphenidate transdermal patch. After completing the prior authorization paperwork, Ms. H is started on the 30 mg/d patch. This dose was selected because she previously tolerated high-dose stimulants, including methylphenidate IR 20 mg up to 6 times daily. At a follow-up visit 1 month after starting the patch, Ms. H reports an improvement in her ADHD symptoms and says she is not experiencing any adverse effects.
Related Resources
- DeMarco R, Rana R, Powell K, et al. How bariatric surgery affects psychotropic drug absorption. Current Psychiatry. 2022;21(8):39-44. doi:10.12788/cp.0271
- Santos MG, Majarwitz DJ, Saeed SA. Adult ADHD: 6 studies of pharmacologic interventions. Current Psychiatry. 2023;22(4):16-27. doi:10.12788/cp.0344
Drug Brand Names
Amphetamine sulfate • Adzenys ER, Adzenys XR-ODT, Dyanavel XR, Evekeo
Atenolol • Tenormin
Dexmethylphenidate • Focalin, Focalin XR
Dextroamphetamine transdermal • Xelstrym
Dextroamphetamine • Dexedrine, Dexedrine Spansule, ProCentra, Zenzedi
Escitalopram • Lexapro
Lisdexamfetamine • Vyvanse
Methylphenidate • Aptensio XR, Adhansia XR, Concerta, Cotempla, Jornay PM, Metadate CD, Metadate ER, Methylin, Qullichew ER, Quillivant XR, Relexxii, Ritalin, Ritalin LA
Methylphenidate transdermal • Daytrana
Mixed amphetamine salts • Adderall, Adderall XR
Mixed salts of a single-entity amphetamine • Mydayis
Serdexmethylphenidate and dexmethylphenidate • Azstarys
Ms. H, age 30, presents to the outpatient clinic for a follow-up visit, where she reports difficulty paying attention to conversations, starting and completing tasks, and meeting deadlines. These challenges occur at work and home. Her psychiatric history includes attention-deficit/hyperactivity disorder (ADHD), major depressive disorder, and generalized anxiety disorder. Approximately 10 years ago, she underwent Roux-en-Y gastric bypass surgery. Following surgery, Ms. H’s care team prescribed liquid formulations of medications whenever possible to minimize malabsorption. Ms. H may be a rapid metabolizer; she says the effects of her prescribed stimulants only last briefly, so she has to frequently redose. As a result, she often runs out of her monthly stimulant allotment earlier than expected.
Ms. H’s current medications include dextroamphetamine/amphetamine immediate-release (IR) 30 mg 3 times daily, atenolol 50 mg/d, and escitalopram oral solution 10 mg/d. Previous unsuccessful medication trials for her ADHD include methylphenidate IR 20 mg 3 times daily and lisdexamfetamine 70 mg/d. Ms. H reports that when her responsibilities increased at work or home, she took methylphenidate IR 20 mg up to 6 times daily to relieve her symptoms.
In the United States, ADHD affects an estimated 4.4% of adults age 18 to 44.1 The actual rate may be higher, however, as recent research has called into question the hypothesis that approximately 50% of cases of childhood ADHD remit by adulthood.2 Prevalence estimates relying on DSM-IV criteria (which were designed with children in mind) can underestimate this condition in adults. Newer data suggest that up to 90% of individuals with ADHD in childhood continue to experience significant ADHD symptoms into adulthood.2
Unless contraindications are present, methylphenidate or amphetamine-based stimulants are the medications of choice for treating adult ADHD.3 Many formulations of both medications are available,4 which allows clinicians to better tailor therapy to each patient’s pharmacokinetics and daily schedule. Although there can be differences in response and tolerability, methylphenidate and amphetamine offer comparable efficacy and a similar adverse effect profile.5
Because amphetamine is more potent than methylphenidate, clinicians commonly start treatment with an amphetamine dose that is one-half to two-thirds the dose of methylphenidate.6 While both classes of stimulants inhibit the reuptake of dopamine and norepinephrine into presynaptic neurons, amphetamines also promote the release of dopamine and norepinephrine from their storage sites in presynaptic nerve terminals.3
Methylphenidate
Methylphenidate IR has an average onset of action of 30 to 45 minutes and its effects last approximately 3 to 4 hours. The extended-release (XR) formulations have varying onsets of action, with durations of action up to 12 hours (Table 13,7).4 The XR products usually immediately release a certain percentage of the medication, eliminating the need for an additional IR tablet. One methylphenidate XR product (Jornay) as well as serdexmethylphenidate/dexmethylphenidate (Azstarys) offer durations of action of 24 to 36 hours. Methylphenidate is primarily metabolized by carboxylesterase 1 (CES1) to the inactive metabolite ritalinic acid. Most of the medication (60% to 80%) is excreted in the urine as ritalinic acid.4 Theoretically, genetic variations in the CES1 and concomitant use of medications that compete with or alter this pathway may impact methylphenidate pharmacokinetics.8 However, plasma levels have not yet shown to be helpful in guiding treatment selection or dosing.4

Amphetamine
Dextroamphetamine/amphetamine IR has an average onset of action of 30 to 45 minutes and its effects last approximately 4 to 6 hours. XR formulations have varying onsets of action, with durations of action up to 13 hours (Table 23,7,9).4 One XR product, mixed salts of single amphetamine entity (Mydayis), has a duration of action of 16 hours. In XR formulations, a certain percentage of the medication is typically released immediately, eliminating the need for an additional IR tablet. Amphetamine is primarily metabolized by cytochrome P450 (CYP) 2D6 hydroxylation and oxidative deamination. Genetic variability in amphetamine metabolism may be relevant due to CYP2D6 polymorphisms. Ultra-rapid metabolizers might need higher doses, while poor metabolizers might require smaller amounts and may be more susceptible to adverse effects.4 However, there is currently insufficient data supporting gene/medication concentration relationships. As is the case with methylphenidate, plasma levels have not yet shown to be helpful in guiding treatment selection or dosing.6

Continue to: Impaired medication absorption after bariatric surgery
Impaired medication absorption after bariatric surgery
Medication malabsorption following bariatric surgery is a significant concern. In a systematic review of 22 studies, Padwal et al10 found that in one-third of these studies, decreased absorption following bariatric surgery may be present in patients taking medications that have poor absorption, high lipophilicity, or enterohepatic recirculation. Childress et al11 found that methylphenidate IR and dextroamphetamine/amphetamine are both well absorbed, with bioavailability percentages of 100% and 90%, respectively. Additional research shows both stimulants have rapid absorption rates but relatively poor bioavailability.12 In one study analyzing the dissolution of common psychiatric medications, methylphenidate was shown to dissolve slightly more in the Roux-en-Y gastric bypass surgery model (80 mg) compared to controls (70 mg).13 One case indicated potential methylphenidate toxicity following Roux-en-Y gastric bypass surgery,14 while another suggested impaired absorption following the same procedure.15 A case-control design study assessing the impact of Roux-en-Y gastric bypass surgery on the pharmacokinetic properties of lisdexamfetamine found no significant differences between the Roux-en-Y group (n = 10) and nonsurgical controls (n = 10). The investigators concluded that while data suggest adjusting lisdexamfetamine dosing following Roux-en-Y gastric bypass surgery is unnecessary, there may be interindividual differences, and individualized dosing regimens may be needed.16
When managing patients who might be experiencing medication malabsorption, it may be helpful to use dosage forms that avoid disintegration, acidic environments, and slow dissolution. Because they are more rapidly absorbed and not susceptible to disintegration and dissolution, liquid formulations are recommended.17 For medications that are not available as a liquid, an IR formulation is recommended.18
Using nonoral routes of administration that avoid the anatomical changes of the gastrointestinal tract should be considered for patients who have undergone Roux-en-Y gastric bypass surgery.17 The methylphenidate transdermal patch, a medication delivery system that avoids gut and hepatic first-pass metabolism, can improve medication bioavailability, reduce dose frequency, and stabilize medication delivery. It is available in 4 sizes/dosages: 10 mg/9 hours, 15 mg/9 hours, 20 mg/9 hours, and 30 mg/9 hours. Methylphenidate is delivered at a steady rate based upon patch size. The onset of action of the patch is approximately 2 hours, and patients should wear the patch for 9 hours, then remove it. Methylphenidate will still be absorbed up to 2 to 3 hours after patch removal. Appropriate application and removal of the patch is important for optimal effectiveness and to avoid adverse effects.4
In March 2022, t
CASE CONTINUED
Ms. H emphasizes her desire to maintain functionality in all areas of life, while her care team reiterates the risks of continuing to take high-dose stimulants. Both Ms. H and her care team acknowledge that stimulant usage could be worsening her anxiety, and that Roux-en-Y gastric bypass surgery may be a possible explanation for her dosing challenges.
Continue to: Following consultation with the pharmacist...
Following consultation with the pharmacist, the care team explains the possible pharmacokinetic benefits of using the methylphenidate transdermal patch. After completing the prior authorization paperwork, Ms. H is started on the 30 mg/d patch. This dose was selected because she previously tolerated high-dose stimulants, including methylphenidate IR 20 mg up to 6 times daily. At a follow-up visit 1 month after starting the patch, Ms. H reports an improvement in her ADHD symptoms and says she is not experiencing any adverse effects.
Related Resources
- DeMarco R, Rana R, Powell K, et al. How bariatric surgery affects psychotropic drug absorption. Current Psychiatry. 2022;21(8):39-44. doi:10.12788/cp.0271
- Santos MG, Majarwitz DJ, Saeed SA. Adult ADHD: 6 studies of pharmacologic interventions. Current Psychiatry. 2023;22(4):16-27. doi:10.12788/cp.0344
Drug Brand Names
Amphetamine sulfate • Adzenys ER, Adzenys XR-ODT, Dyanavel XR, Evekeo
Atenolol • Tenormin
Dexmethylphenidate • Focalin, Focalin XR
Dextroamphetamine transdermal • Xelstrym
Dextroamphetamine • Dexedrine, Dexedrine Spansule, ProCentra, Zenzedi
Escitalopram • Lexapro
Lisdexamfetamine • Vyvanse
Methylphenidate • Aptensio XR, Adhansia XR, Concerta, Cotempla, Jornay PM, Metadate CD, Metadate ER, Methylin, Qullichew ER, Quillivant XR, Relexxii, Ritalin, Ritalin LA
Methylphenidate transdermal • Daytrana
Mixed amphetamine salts • Adderall, Adderall XR
Mixed salts of a single-entity amphetamine • Mydayis
Serdexmethylphenidate and dexmethylphenidate • Azstarys
1. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716-723. doi:10.1176/ajp.2006.163.4.716
2. Sibley MH, Arnold LE, Swanson JM, et al. Variable patterns of remission from ADHD in the multimodal treatment study of ADHD. Am J Psychiatry. 2022;179(2):142-151. doi:10.1176/appi.ajp.2021.21010032
3. Cleveland KW, Boyle J, Robinson RF. Attention-deficit/hyperactivity disorder. In: Chisholm-Burns MA, Schwinghammer TL, Malone PM, et al, eds. Pharmacotherapy Principles & Practice. 6th ed. McGraw Hill; 2022. Accessed December 1, 2022. https://ppp.mhmedical.com/content.aspx?bookid=3114§ionid=261474885
4. Steingard R, Taskiran S, Connor DF, et al. New formulations of stimulants: an update for clinicians. J Child Adolesc Psychopharmacol. 2019;29(5):324-339. doi:10.1089/cap.2019.0043
5. Faraone SV. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. 2018;87:255-270. doi:10.1016/j.neubiorev.2018.02.001
6. Markowitz JS, Patrick KS. The clinical pharmacokinetics of amphetamines utilized in the treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2017;27(8):678-689. doi:10.1089/cap.2017.0071
7. Mullen S. Medication Table 2: Attention Deficit Hyperactivity Disorder. In: English C, ed. CPNP Psychiatric Pharmacotherapy Review Course. 2022-2023 ed. College of Psychiatric and Neurologic Pharmacists; 2022.
8. Zhu HJ, Patrick KS, Yuan HJ, et al. Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: clinical significance and molecular basis. Am J Hum Genet. 2008;82(6):1241-1248. doi:10.1016/j.ajhg.2008.04.015
9. Xelstrym [package insert]. Miami, FL: Noven Pharmaceuticals, Inc.; 2022.
10. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50. doi:10.1111/j.1467-789X.2009.00614.x
11. Childress AC, Komolova M, Sallee FR. An update on the pharmacokinetic considerations in the treatment of ADHD with long-acting methylphenidate and amphetamine formulations. Expert Opin Drug Metab Toxicol. 2019;15(11):937-974. doi:10.1080/17425255.2019.1675636
12. Markowitz JS, Melchert PW. The pharmacokinetics and pharmacogenomics of psychostimulants. Child Adolesc Psychiatr Clin N Am. 2022;31(3):393-416. doi:10.1016/j.chc.2022.03.003
13. Seaman JS, Bowers SP, Dixon P, et al. Dissolution of common psychiatric medications in a Roux-en-Y gastric bypass model. Psychosomatics. 2005;46(3):250-253. doi:10.1176/appi.psy.46.3.250
14. Ludvigsson M, Haenni A. Methylphenidate toxicity after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;12(5):e55-e57. doi:10.1016/j.soard.2016.03.015
15. Azran C, Langguth P, Dahan A. Impaired oral absorption of methylphenidate after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2017;13(7):1245-1247. doi:10.1016/j.soard.2017.03.003
16. Steffen KJ, Mohammad AS, Roerig JL, et al. Lisdexamfetamine pharmacokinetic comparison between patients who underwent Roux-en-Y gastric bypass and nonsurgical controls. Obes Surg. 2021;31(10):4289-4294. doi:10.1007/s11695-020-04969-4
17. Buxton ILO. Pharmacokinetics: the dynamics of drug absorption, distribution, metabolism, and elimination. In: Brunton LL, Knollmann BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 14th ed. McGraw Hill; 2023. Accessed December 1, 2022. https://accesspharmacy.mhmedical.com/content.aspx?bookid=2189§ionid=166182905
18. DeMarco R, Rana R, Powell K, et al. How bariatric surgery affects psychotropic drug absorption. Current Psychiatry. 2022;21(8):39-44. doi:10.12788/cp.0271
1. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716-723. doi:10.1176/ajp.2006.163.4.716
2. Sibley MH, Arnold LE, Swanson JM, et al. Variable patterns of remission from ADHD in the multimodal treatment study of ADHD. Am J Psychiatry. 2022;179(2):142-151. doi:10.1176/appi.ajp.2021.21010032
3. Cleveland KW, Boyle J, Robinson RF. Attention-deficit/hyperactivity disorder. In: Chisholm-Burns MA, Schwinghammer TL, Malone PM, et al, eds. Pharmacotherapy Principles & Practice. 6th ed. McGraw Hill; 2022. Accessed December 1, 2022. https://ppp.mhmedical.com/content.aspx?bookid=3114§ionid=261474885
4. Steingard R, Taskiran S, Connor DF, et al. New formulations of stimulants: an update for clinicians. J Child Adolesc Psychopharmacol. 2019;29(5):324-339. doi:10.1089/cap.2019.0043
5. Faraone SV. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. 2018;87:255-270. doi:10.1016/j.neubiorev.2018.02.001
6. Markowitz JS, Patrick KS. The clinical pharmacokinetics of amphetamines utilized in the treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2017;27(8):678-689. doi:10.1089/cap.2017.0071
7. Mullen S. Medication Table 2: Attention Deficit Hyperactivity Disorder. In: English C, ed. CPNP Psychiatric Pharmacotherapy Review Course. 2022-2023 ed. College of Psychiatric and Neurologic Pharmacists; 2022.
8. Zhu HJ, Patrick KS, Yuan HJ, et al. Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: clinical significance and molecular basis. Am J Hum Genet. 2008;82(6):1241-1248. doi:10.1016/j.ajhg.2008.04.015
9. Xelstrym [package insert]. Miami, FL: Noven Pharmaceuticals, Inc.; 2022.
10. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50. doi:10.1111/j.1467-789X.2009.00614.x
11. Childress AC, Komolova M, Sallee FR. An update on the pharmacokinetic considerations in the treatment of ADHD with long-acting methylphenidate and amphetamine formulations. Expert Opin Drug Metab Toxicol. 2019;15(11):937-974. doi:10.1080/17425255.2019.1675636
12. Markowitz JS, Melchert PW. The pharmacokinetics and pharmacogenomics of psychostimulants. Child Adolesc Psychiatr Clin N Am. 2022;31(3):393-416. doi:10.1016/j.chc.2022.03.003
13. Seaman JS, Bowers SP, Dixon P, et al. Dissolution of common psychiatric medications in a Roux-en-Y gastric bypass model. Psychosomatics. 2005;46(3):250-253. doi:10.1176/appi.psy.46.3.250
14. Ludvigsson M, Haenni A. Methylphenidate toxicity after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;12(5):e55-e57. doi:10.1016/j.soard.2016.03.015
15. Azran C, Langguth P, Dahan A. Impaired oral absorption of methylphenidate after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2017;13(7):1245-1247. doi:10.1016/j.soard.2017.03.003
16. Steffen KJ, Mohammad AS, Roerig JL, et al. Lisdexamfetamine pharmacokinetic comparison between patients who underwent Roux-en-Y gastric bypass and nonsurgical controls. Obes Surg. 2021;31(10):4289-4294. doi:10.1007/s11695-020-04969-4
17. Buxton ILO. Pharmacokinetics: the dynamics of drug absorption, distribution, metabolism, and elimination. In: Brunton LL, Knollmann BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 14th ed. McGraw Hill; 2023. Accessed December 1, 2022. https://accesspharmacy.mhmedical.com/content.aspx?bookid=2189§ionid=166182905
18. DeMarco R, Rana R, Powell K, et al. How bariatric surgery affects psychotropic drug absorption. Current Psychiatry. 2022;21(8):39-44. doi:10.12788/cp.0271
Serious complications due to ‘huffing’
CASE A relapse and crisis
Ms. G, age 32, is brought to the emergency department (ED) by police after being found in a stupor-like state in a public restroom. The consultation-liaison (CL) psychiatry team assesses her for concerns of self-harm and suicide behavior. Ms. G discloses that she “huffs” an average of 4 canisters of air dusters daily to cope with psychosocial stressors and achieve a euphoric state. She recently lost her job, which led to homelessness, financial difficulties, a relapse to aerosol use after 2 years of abstinence, and stealing aerosol cans. The latest incident follows 2 prior arrests, which led officers to bring her to the ED for medical evaluation. Ms. G has a history of bipolar disorder (BD), generalized anxiety disorder (GAD), insomnia, and inhalant use disorder.
HISTORY Inhalant abuse and suicide attempt
Ms. G reports a longstanding history of severe inhalant abuse, primarily with air dusters due to their accessibility and low cost. She previously underwent inpatient rehab for inhalant abuse, and received inpatient psychiatry treatment 5 years ago for a suicide attempt by overdose linked to psychosocial stressors. In addition to BD, GAD, insomnia, and inhalant use disorder, Ms. G has a history of neuropathy, seizures, and recurrent hypokalemia. She is single and does not have insurance.
[polldaddy:12318871]
The authors’ observations
Inhalant abuse is the intentional inhalation of volatile substances to achieve an altered mental state. Inhalants are commercially available products that can produce intoxication if inhaled, such as glue, toluene, spray paint, gasoline, and lighter fluid (Table 11).
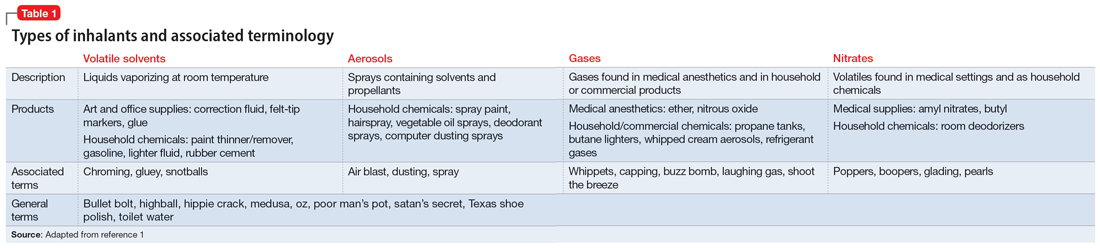
The epidemiology of inhalant abuse is difficult to accurately report due to a lack of recognition and social stigma. Due to inhalants’ ease of access and low cost, this form of substance abuse is popular among adolescents, adults of low socioeconomic status, individuals who live in rural areas, and those living in institutions. Inhalants act as reinforcers, producing a euphoric state. Rapid pulmonary absorption and lipid solubility of the substance rapidly alters the brain. Inhalant abuse can result in chemical and thermal burns, withdrawal symptoms, persistent mental illness, and catastrophic medical emergencies such as ventricular arrhythmias leading to disruptive myocardial electrical propagation. Chronic abuse can cause irreversible neurological and neuropsychological effects, cardiomyopathy, rapid airway compromise, pulmonary debilitations, renal tubular acidosis, bone marrow toxicity, reduced immunity, and peripheral neuropathy.2 Ms. G’s diagnosis of inhalant use disorder was based on her mental state and history of severe inhalant misuse, specifically with air dusters. Several additional factors further support this diagnosis, including the fact she survived a suicide attempt by overdose 5 years ago, had an inpatient rehabilitation placement for inhalant abuse, experiences insomnia, and was attempting to self-treat a depressive episode relapse with inhalants.
EVALUATION Depressed but cooperative
After being monitored in the ED for several hours, Ms. G is no longer in a stupor-like state. She has poor body habitus, appears older than her stated age, and is unkempt in appearance/attire. She is mildly distressed but relatively cooperative and engaged during the interview. Ms. G has a depressed mood and is anxious, with mood-congruent affect, and is tearful at times, especially when discussing recent stressors. She denies suicidality, homicidality, paranoia, delusions, and hallucinations. Her thought process is linear, goal-directed, and logical. She has fair insight, but relatively poor and impulsive judgment. The nursing staff expresses concerns that Ms. G was possibly responding to internal stimuli and behaving bizarrely during her initial presentation; this was not evident upon examination.
Ms. G reports having acute-on-chronic headaches, intermittent myalgias and weakness in her lower extremities (acute), and polyneuropathy (chronic). She denies a history of manic episodes or psychosis but reports previous relative hypomanic episodes that vacillated with periods of recurrent depressive episodes. Ms. G denies using illicit substances other than tobacco and inhalants. She says she had adhered to her outpatient psychiatric management services and medication regimen (duloxetine 60 mg/d at bedtime for mood/migraines, trazodone 150 mg/d at bedtime for insomnia, ziprasidone 40 mg/d at bedtime for BD, carbamazepine 200 mg twice daily for neuropathy/migraines, gabapentin 400 mg 3 times daily for neuropathy migraines/anxiety, and propranolol 10 mg 3 times daily for anxiety/tremors/migraine prophylaxis) until 4 days before her current presentation to the ED, when she used inhalants and was arrested.
Ms. G’s vitals are mostly unremarkable, but her heart rate is 116 beats per minute. There are no acute findings on physical examination. She is not pregnant, and her creatinine, glomerular filtration rate, complete blood count, and thyroid-stimulating hormone are all within normal limits. Her blood sugar is high (120 mg/dL; reference range 70 to 100 mg/dL). She has slight transaminitis with high aspartate aminotransferase (93 U/L; reference range 17 to 59 U/L) and high alanine aminotransferase (69 U/L; reference range 20 to 35 U/L); chronic hypokalemia (2.4 mmol/L; reference range 3.5 to 5.2 mmol/L), which leads the primary team to initiate a potassium replacement protocol; lactic acidosis (2.2 mmol/L; normal levels <2 mmol/L); and creatine kinase (CK) 5,930 U/L.
[polldaddy:12318873]
Continue to: The authors' observations
The authors’ observations
Efforts to improve the laboratory diagnosis of inhalant abuse are ongoing, but they have not yet been widely implemented. Systemic screening and assessment of inhalant use can help prevent and treat complications. For Ms. G, we considered several possible complications, including hypoglycemia. Although the classic triad of myalgia, weakness, and myoglobinuria (tea-colored urine) was not present, elevated CK levels in the context of Ms. G’s intermittent myalgia and lower extremity weakness led us to suspect she was experiencing moderate rhabdomyolysis (Table 23).
Rhabdomyolysis can be caused by several factors, including drug abuse, trauma, neuromuscular syndrome, and immobility. Treatment is mainly supportive, with a focus on preserving the ABCs (airway, breathing, circulation) and renal function through vigorous rehydration.4 We postulated Ms. G’s rhabdomyolysis was caused by muscle damage directly resulting from inhalant abuse and compounded by her remaining in prolonged fixed position on the ground after overdosing on inhalants.
TREATMENT Rehydration and psychotropics
The treatment team initiates IV fluid hydration of chloride 0.9% 150 mL/h and monitors Ms. G until she is stable and the trajectory of her CK levels begins to decline. On hospital Day 2, Ms. G’s CK decreases to 2,475 U/L and her lactic acid levels normalize. Ms. G restarts her regimen of duloxetine 60 mg/d, trazodone 150 mg/d, ziprasidone 40 mg/d, carbamazepine 200 mg twice daily, gabapentin 400 mg 3 times daily, and propranolol 10 mg 3 times daily. The team adds quetiapine 25 mg as needed for hallucinations, paranoia, and/or anxiety. Ms. G is closely monitored due to the potential risk of toxicity-induced or withdrawal-induced psychotic symptoms.
[polldaddy:12318869]
The authors’ observations
Presently, there are no effective treatments for acute inhalant intoxication or withdrawal, which makes supportive care and vigilant monitoring the only options.5 Although clinical research has not led to any FDA-approved treatments for chronic inhalant use disorder, a multipronged biopsychosocial treatment approach is critical in light of the negative consequences of inhalant abuse, including poor academic performance, criminal behavior, abuse of other substances, social maladjustment, low self-esteem, and suicidality.6
Ms. G had a moderate form of rhabdomyolysis, which was managed with IV fluid rehydration. Education and counseling were crucial to help Ms. G understand the unintended complications and potentially life-threatening consequences of inhalant abuse, with rehabilitation services to encourage abstinence. Ms. G had previously undergone successful inpatient rehabilitation and was willing to start such services again. She reported success with gabapentin for her polyneuropathy and migraines, which may be long-term consequences of prolonged inhalant abuse with neurological lesions. Ziprasidone may have mitigated some of the impulsivity and hypomanic symptoms of her BD that could make her more likely to engage in risky self-harm behaviors.
Continue to: After extensive discussion...
After extensive discussion on the long-term complications of inhalant abuse, Ms. G was motivated, cooperative, and sought care to return to rehabilitation services. The CL psychiatry team collaborated with the social work team to address the psychosocial components of Ms. G’s homelessness and facilitated an application for a local resource to obtain rehabilitation placement and living assistance. Her years of abstinence from inhalant use and success with rehabilitation demonstrate the need for a multimodal approach to manage and treat inhalant use disorder. Outpatient follow-up arrangements were made with local mental health resources.
OUTCOME Improved outlook and discharge
Ms. G reports improved mood and willingness to change her substance use habits. The treatment team counsels her on the acute risk of fatal arrhythmias and end-organ complications of inhalant abuse. They warn her about the potential long-term effects of mood alterations, neurological lesions, and polyneuropathy that could possibly worsen with substance abuse. Ms. G expresses appreciation for this counseling, the help associated with her aftercare, and the referral to restart the 30-day inpatient rehabilitation services. The team arranges follow-up with outpatient psychiatry and outpatient therapy services to enhance Ms. G’s coping skills and mitigate her reliance on inhalants to regulate her mood.
Bottom Line
Inhalant use is a poorly understood form of substance abuse that disproportionately affects vulnerable populations. It can lead to life-threatening medical emergencies such as rhabdomyolysis. Clinicians need to be able to identify and manage inhalant abuse and associated complications, as well as provide appropriate education and counseling to prevent further misuse.
Related Resources
- Gude J, Bisen V, Fujii K. Medication-induced rhabdomyolysis. Current Psychiatry. 2023;22(2):39-40. doi:10.12788/cp.0332
- Waldman W, Kabata PM, Dines AM, et al. Rhabdomyolysis related to acute recreational drug toxicity--a Euro-DEN study. PLoS One. 2021;16(3):e0246297. doi:10.1371/journal. pone.0246297
Drug Brand Names
Carbamazepine • Tegretol
Duloxetine • Cymbalta
Gabapentin • Neurontin
Propranolol • Inderal
Quetiapine • Seroquel
Trazodone • Oleptro
Ziprasidone • Geodon
1. Ahern NR, Falsafi N. Inhalant abuse: youth at risk. J Psychosoc Nurs Ment Health Serv. 2013;51(8):19-24. doi:10.3928/02793695-20130612-02
2. Howard MO, Bowen SE, Garland EL, et al. Inhalant use and inhalant use disorders in the United States. Addict Sci Clin Prac. 2011;6(1):18-31.
3. Farkas J. Rhabdomyolysis. Internet Book of Critical Care. June 25, 2021. Accessed February 24, 2023. https://emcrit.org/ibcc/rhabdo/
4. Torres PA, Helmstetter JA, Kaye AM, et al. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15(1):58-69.
5. Muller AA, Muller GF. Inhalant abuse. J Emerg Nurs. 2006;32(5):447-448. doi:10.1016/j.jen.2006.05.018
6. Kozel N, Sloboda Z, De La Rosa M, eds. Epidemiology of Inhalant Abuse: An International Perspective; Nida Research Monograph 148. National Institute on Drug Abuse Research, US Dept of Health and Human Services; 1995. Accessed April 20, 2023. https://archives.nida.nih.gov/sites/default/files/monograph148.pdf
CASE A relapse and crisis
Ms. G, age 32, is brought to the emergency department (ED) by police after being found in a stupor-like state in a public restroom. The consultation-liaison (CL) psychiatry team assesses her for concerns of self-harm and suicide behavior. Ms. G discloses that she “huffs” an average of 4 canisters of air dusters daily to cope with psychosocial stressors and achieve a euphoric state. She recently lost her job, which led to homelessness, financial difficulties, a relapse to aerosol use after 2 years of abstinence, and stealing aerosol cans. The latest incident follows 2 prior arrests, which led officers to bring her to the ED for medical evaluation. Ms. G has a history of bipolar disorder (BD), generalized anxiety disorder (GAD), insomnia, and inhalant use disorder.
HISTORY Inhalant abuse and suicide attempt
Ms. G reports a longstanding history of severe inhalant abuse, primarily with air dusters due to their accessibility and low cost. She previously underwent inpatient rehab for inhalant abuse, and received inpatient psychiatry treatment 5 years ago for a suicide attempt by overdose linked to psychosocial stressors. In addition to BD, GAD, insomnia, and inhalant use disorder, Ms. G has a history of neuropathy, seizures, and recurrent hypokalemia. She is single and does not have insurance.
[polldaddy:12318871]
The authors’ observations
Inhalant abuse is the intentional inhalation of volatile substances to achieve an altered mental state. Inhalants are commercially available products that can produce intoxication if inhaled, such as glue, toluene, spray paint, gasoline, and lighter fluid (Table 11).

The epidemiology of inhalant abuse is difficult to accurately report due to a lack of recognition and social stigma. Due to inhalants’ ease of access and low cost, this form of substance abuse is popular among adolescents, adults of low socioeconomic status, individuals who live in rural areas, and those living in institutions. Inhalants act as reinforcers, producing a euphoric state. Rapid pulmonary absorption and lipid solubility of the substance rapidly alters the brain. Inhalant abuse can result in chemical and thermal burns, withdrawal symptoms, persistent mental illness, and catastrophic medical emergencies such as ventricular arrhythmias leading to disruptive myocardial electrical propagation. Chronic abuse can cause irreversible neurological and neuropsychological effects, cardiomyopathy, rapid airway compromise, pulmonary debilitations, renal tubular acidosis, bone marrow toxicity, reduced immunity, and peripheral neuropathy.2 Ms. G’s diagnosis of inhalant use disorder was based on her mental state and history of severe inhalant misuse, specifically with air dusters. Several additional factors further support this diagnosis, including the fact she survived a suicide attempt by overdose 5 years ago, had an inpatient rehabilitation placement for inhalant abuse, experiences insomnia, and was attempting to self-treat a depressive episode relapse with inhalants.
EVALUATION Depressed but cooperative
After being monitored in the ED for several hours, Ms. G is no longer in a stupor-like state. She has poor body habitus, appears older than her stated age, and is unkempt in appearance/attire. She is mildly distressed but relatively cooperative and engaged during the interview. Ms. G has a depressed mood and is anxious, with mood-congruent affect, and is tearful at times, especially when discussing recent stressors. She denies suicidality, homicidality, paranoia, delusions, and hallucinations. Her thought process is linear, goal-directed, and logical. She has fair insight, but relatively poor and impulsive judgment. The nursing staff expresses concerns that Ms. G was possibly responding to internal stimuli and behaving bizarrely during her initial presentation; this was not evident upon examination.
Ms. G reports having acute-on-chronic headaches, intermittent myalgias and weakness in her lower extremities (acute), and polyneuropathy (chronic). She denies a history of manic episodes or psychosis but reports previous relative hypomanic episodes that vacillated with periods of recurrent depressive episodes. Ms. G denies using illicit substances other than tobacco and inhalants. She says she had adhered to her outpatient psychiatric management services and medication regimen (duloxetine 60 mg/d at bedtime for mood/migraines, trazodone 150 mg/d at bedtime for insomnia, ziprasidone 40 mg/d at bedtime for BD, carbamazepine 200 mg twice daily for neuropathy/migraines, gabapentin 400 mg 3 times daily for neuropathy migraines/anxiety, and propranolol 10 mg 3 times daily for anxiety/tremors/migraine prophylaxis) until 4 days before her current presentation to the ED, when she used inhalants and was arrested.
Ms. G’s vitals are mostly unremarkable, but her heart rate is 116 beats per minute. There are no acute findings on physical examination. She is not pregnant, and her creatinine, glomerular filtration rate, complete blood count, and thyroid-stimulating hormone are all within normal limits. Her blood sugar is high (120 mg/dL; reference range 70 to 100 mg/dL). She has slight transaminitis with high aspartate aminotransferase (93 U/L; reference range 17 to 59 U/L) and high alanine aminotransferase (69 U/L; reference range 20 to 35 U/L); chronic hypokalemia (2.4 mmol/L; reference range 3.5 to 5.2 mmol/L), which leads the primary team to initiate a potassium replacement protocol; lactic acidosis (2.2 mmol/L; normal levels <2 mmol/L); and creatine kinase (CK) 5,930 U/L.
[polldaddy:12318873]
Continue to: The authors' observations
The authors’ observations
Efforts to improve the laboratory diagnosis of inhalant abuse are ongoing, but they have not yet been widely implemented. Systemic screening and assessment of inhalant use can help prevent and treat complications. For Ms. G, we considered several possible complications, including hypoglycemia. Although the classic triad of myalgia, weakness, and myoglobinuria (tea-colored urine) was not present, elevated CK levels in the context of Ms. G’s intermittent myalgia and lower extremity weakness led us to suspect she was experiencing moderate rhabdomyolysis (Table 23).

Rhabdomyolysis can be caused by several factors, including drug abuse, trauma, neuromuscular syndrome, and immobility. Treatment is mainly supportive, with a focus on preserving the ABCs (airway, breathing, circulation) and renal function through vigorous rehydration.4 We postulated Ms. G’s rhabdomyolysis was caused by muscle damage directly resulting from inhalant abuse and compounded by her remaining in prolonged fixed position on the ground after overdosing on inhalants.
TREATMENT Rehydration and psychotropics
The treatment team initiates IV fluid hydration of chloride 0.9% 150 mL/h and monitors Ms. G until she is stable and the trajectory of her CK levels begins to decline. On hospital Day 2, Ms. G’s CK decreases to 2,475 U/L and her lactic acid levels normalize. Ms. G restarts her regimen of duloxetine 60 mg/d, trazodone 150 mg/d, ziprasidone 40 mg/d, carbamazepine 200 mg twice daily, gabapentin 400 mg 3 times daily, and propranolol 10 mg 3 times daily. The team adds quetiapine 25 mg as needed for hallucinations, paranoia, and/or anxiety. Ms. G is closely monitored due to the potential risk of toxicity-induced or withdrawal-induced psychotic symptoms.
[polldaddy:12318869]
The authors’ observations
Presently, there are no effective treatments for acute inhalant intoxication or withdrawal, which makes supportive care and vigilant monitoring the only options.5 Although clinical research has not led to any FDA-approved treatments for chronic inhalant use disorder, a multipronged biopsychosocial treatment approach is critical in light of the negative consequences of inhalant abuse, including poor academic performance, criminal behavior, abuse of other substances, social maladjustment, low self-esteem, and suicidality.6
Ms. G had a moderate form of rhabdomyolysis, which was managed with IV fluid rehydration. Education and counseling were crucial to help Ms. G understand the unintended complications and potentially life-threatening consequences of inhalant abuse, with rehabilitation services to encourage abstinence. Ms. G had previously undergone successful inpatient rehabilitation and was willing to start such services again. She reported success with gabapentin for her polyneuropathy and migraines, which may be long-term consequences of prolonged inhalant abuse with neurological lesions. Ziprasidone may have mitigated some of the impulsivity and hypomanic symptoms of her BD that could make her more likely to engage in risky self-harm behaviors.
Continue to: After extensive discussion...
After extensive discussion on the long-term complications of inhalant abuse, Ms. G was motivated, cooperative, and sought care to return to rehabilitation services. The CL psychiatry team collaborated with the social work team to address the psychosocial components of Ms. G’s homelessness and facilitated an application for a local resource to obtain rehabilitation placement and living assistance. Her years of abstinence from inhalant use and success with rehabilitation demonstrate the need for a multimodal approach to manage and treat inhalant use disorder. Outpatient follow-up arrangements were made with local mental health resources.
OUTCOME Improved outlook and discharge
Ms. G reports improved mood and willingness to change her substance use habits. The treatment team counsels her on the acute risk of fatal arrhythmias and end-organ complications of inhalant abuse. They warn her about the potential long-term effects of mood alterations, neurological lesions, and polyneuropathy that could possibly worsen with substance abuse. Ms. G expresses appreciation for this counseling, the help associated with her aftercare, and the referral to restart the 30-day inpatient rehabilitation services. The team arranges follow-up with outpatient psychiatry and outpatient therapy services to enhance Ms. G’s coping skills and mitigate her reliance on inhalants to regulate her mood.
Bottom Line
Inhalant use is a poorly understood form of substance abuse that disproportionately affects vulnerable populations. It can lead to life-threatening medical emergencies such as rhabdomyolysis. Clinicians need to be able to identify and manage inhalant abuse and associated complications, as well as provide appropriate education and counseling to prevent further misuse.
Related Resources
- Gude J, Bisen V, Fujii K. Medication-induced rhabdomyolysis. Current Psychiatry. 2023;22(2):39-40. doi:10.12788/cp.0332
- Waldman W, Kabata PM, Dines AM, et al. Rhabdomyolysis related to acute recreational drug toxicity--a Euro-DEN study. PLoS One. 2021;16(3):e0246297. doi:10.1371/journal. pone.0246297
Drug Brand Names
Carbamazepine • Tegretol
Duloxetine • Cymbalta
Gabapentin • Neurontin
Propranolol • Inderal
Quetiapine • Seroquel
Trazodone • Oleptro
Ziprasidone • Geodon
CASE A relapse and crisis
Ms. G, age 32, is brought to the emergency department (ED) by police after being found in a stupor-like state in a public restroom. The consultation-liaison (CL) psychiatry team assesses her for concerns of self-harm and suicide behavior. Ms. G discloses that she “huffs” an average of 4 canisters of air dusters daily to cope with psychosocial stressors and achieve a euphoric state. She recently lost her job, which led to homelessness, financial difficulties, a relapse to aerosol use after 2 years of abstinence, and stealing aerosol cans. The latest incident follows 2 prior arrests, which led officers to bring her to the ED for medical evaluation. Ms. G has a history of bipolar disorder (BD), generalized anxiety disorder (GAD), insomnia, and inhalant use disorder.
HISTORY Inhalant abuse and suicide attempt
Ms. G reports a longstanding history of severe inhalant abuse, primarily with air dusters due to their accessibility and low cost. She previously underwent inpatient rehab for inhalant abuse, and received inpatient psychiatry treatment 5 years ago for a suicide attempt by overdose linked to psychosocial stressors. In addition to BD, GAD, insomnia, and inhalant use disorder, Ms. G has a history of neuropathy, seizures, and recurrent hypokalemia. She is single and does not have insurance.
[polldaddy:12318871]
The authors’ observations
Inhalant abuse is the intentional inhalation of volatile substances to achieve an altered mental state. Inhalants are commercially available products that can produce intoxication if inhaled, such as glue, toluene, spray paint, gasoline, and lighter fluid (Table 11).

The epidemiology of inhalant abuse is difficult to accurately report due to a lack of recognition and social stigma. Due to inhalants’ ease of access and low cost, this form of substance abuse is popular among adolescents, adults of low socioeconomic status, individuals who live in rural areas, and those living in institutions. Inhalants act as reinforcers, producing a euphoric state. Rapid pulmonary absorption and lipid solubility of the substance rapidly alters the brain. Inhalant abuse can result in chemical and thermal burns, withdrawal symptoms, persistent mental illness, and catastrophic medical emergencies such as ventricular arrhythmias leading to disruptive myocardial electrical propagation. Chronic abuse can cause irreversible neurological and neuropsychological effects, cardiomyopathy, rapid airway compromise, pulmonary debilitations, renal tubular acidosis, bone marrow toxicity, reduced immunity, and peripheral neuropathy.2 Ms. G’s diagnosis of inhalant use disorder was based on her mental state and history of severe inhalant misuse, specifically with air dusters. Several additional factors further support this diagnosis, including the fact she survived a suicide attempt by overdose 5 years ago, had an inpatient rehabilitation placement for inhalant abuse, experiences insomnia, and was attempting to self-treat a depressive episode relapse with inhalants.
EVALUATION Depressed but cooperative
After being monitored in the ED for several hours, Ms. G is no longer in a stupor-like state. She has poor body habitus, appears older than her stated age, and is unkempt in appearance/attire. She is mildly distressed but relatively cooperative and engaged during the interview. Ms. G has a depressed mood and is anxious, with mood-congruent affect, and is tearful at times, especially when discussing recent stressors. She denies suicidality, homicidality, paranoia, delusions, and hallucinations. Her thought process is linear, goal-directed, and logical. She has fair insight, but relatively poor and impulsive judgment. The nursing staff expresses concerns that Ms. G was possibly responding to internal stimuli and behaving bizarrely during her initial presentation; this was not evident upon examination.
Ms. G reports having acute-on-chronic headaches, intermittent myalgias and weakness in her lower extremities (acute), and polyneuropathy (chronic). She denies a history of manic episodes or psychosis but reports previous relative hypomanic episodes that vacillated with periods of recurrent depressive episodes. Ms. G denies using illicit substances other than tobacco and inhalants. She says she had adhered to her outpatient psychiatric management services and medication regimen (duloxetine 60 mg/d at bedtime for mood/migraines, trazodone 150 mg/d at bedtime for insomnia, ziprasidone 40 mg/d at bedtime for BD, carbamazepine 200 mg twice daily for neuropathy/migraines, gabapentin 400 mg 3 times daily for neuropathy migraines/anxiety, and propranolol 10 mg 3 times daily for anxiety/tremors/migraine prophylaxis) until 4 days before her current presentation to the ED, when she used inhalants and was arrested.
Ms. G’s vitals are mostly unremarkable, but her heart rate is 116 beats per minute. There are no acute findings on physical examination. She is not pregnant, and her creatinine, glomerular filtration rate, complete blood count, and thyroid-stimulating hormone are all within normal limits. Her blood sugar is high (120 mg/dL; reference range 70 to 100 mg/dL). She has slight transaminitis with high aspartate aminotransferase (93 U/L; reference range 17 to 59 U/L) and high alanine aminotransferase (69 U/L; reference range 20 to 35 U/L); chronic hypokalemia (2.4 mmol/L; reference range 3.5 to 5.2 mmol/L), which leads the primary team to initiate a potassium replacement protocol; lactic acidosis (2.2 mmol/L; normal levels <2 mmol/L); and creatine kinase (CK) 5,930 U/L.
[polldaddy:12318873]
Continue to: The authors' observations
The authors’ observations
Efforts to improve the laboratory diagnosis of inhalant abuse are ongoing, but they have not yet been widely implemented. Systemic screening and assessment of inhalant use can help prevent and treat complications. For Ms. G, we considered several possible complications, including hypoglycemia. Although the classic triad of myalgia, weakness, and myoglobinuria (tea-colored urine) was not present, elevated CK levels in the context of Ms. G’s intermittent myalgia and lower extremity weakness led us to suspect she was experiencing moderate rhabdomyolysis (Table 23).

Rhabdomyolysis can be caused by several factors, including drug abuse, trauma, neuromuscular syndrome, and immobility. Treatment is mainly supportive, with a focus on preserving the ABCs (airway, breathing, circulation) and renal function through vigorous rehydration.4 We postulated Ms. G’s rhabdomyolysis was caused by muscle damage directly resulting from inhalant abuse and compounded by her remaining in prolonged fixed position on the ground after overdosing on inhalants.
TREATMENT Rehydration and psychotropics
The treatment team initiates IV fluid hydration of chloride 0.9% 150 mL/h and monitors Ms. G until she is stable and the trajectory of her CK levels begins to decline. On hospital Day 2, Ms. G’s CK decreases to 2,475 U/L and her lactic acid levels normalize. Ms. G restarts her regimen of duloxetine 60 mg/d, trazodone 150 mg/d, ziprasidone 40 mg/d, carbamazepine 200 mg twice daily, gabapentin 400 mg 3 times daily, and propranolol 10 mg 3 times daily. The team adds quetiapine 25 mg as needed for hallucinations, paranoia, and/or anxiety. Ms. G is closely monitored due to the potential risk of toxicity-induced or withdrawal-induced psychotic symptoms.
[polldaddy:12318869]
The authors’ observations
Presently, there are no effective treatments for acute inhalant intoxication or withdrawal, which makes supportive care and vigilant monitoring the only options.5 Although clinical research has not led to any FDA-approved treatments for chronic inhalant use disorder, a multipronged biopsychosocial treatment approach is critical in light of the negative consequences of inhalant abuse, including poor academic performance, criminal behavior, abuse of other substances, social maladjustment, low self-esteem, and suicidality.6
Ms. G had a moderate form of rhabdomyolysis, which was managed with IV fluid rehydration. Education and counseling were crucial to help Ms. G understand the unintended complications and potentially life-threatening consequences of inhalant abuse, with rehabilitation services to encourage abstinence. Ms. G had previously undergone successful inpatient rehabilitation and was willing to start such services again. She reported success with gabapentin for her polyneuropathy and migraines, which may be long-term consequences of prolonged inhalant abuse with neurological lesions. Ziprasidone may have mitigated some of the impulsivity and hypomanic symptoms of her BD that could make her more likely to engage in risky self-harm behaviors.
Continue to: After extensive discussion...
After extensive discussion on the long-term complications of inhalant abuse, Ms. G was motivated, cooperative, and sought care to return to rehabilitation services. The CL psychiatry team collaborated with the social work team to address the psychosocial components of Ms. G’s homelessness and facilitated an application for a local resource to obtain rehabilitation placement and living assistance. Her years of abstinence from inhalant use and success with rehabilitation demonstrate the need for a multimodal approach to manage and treat inhalant use disorder. Outpatient follow-up arrangements were made with local mental health resources.
OUTCOME Improved outlook and discharge
Ms. G reports improved mood and willingness to change her substance use habits. The treatment team counsels her on the acute risk of fatal arrhythmias and end-organ complications of inhalant abuse. They warn her about the potential long-term effects of mood alterations, neurological lesions, and polyneuropathy that could possibly worsen with substance abuse. Ms. G expresses appreciation for this counseling, the help associated with her aftercare, and the referral to restart the 30-day inpatient rehabilitation services. The team arranges follow-up with outpatient psychiatry and outpatient therapy services to enhance Ms. G’s coping skills and mitigate her reliance on inhalants to regulate her mood.
Bottom Line
Inhalant use is a poorly understood form of substance abuse that disproportionately affects vulnerable populations. It can lead to life-threatening medical emergencies such as rhabdomyolysis. Clinicians need to be able to identify and manage inhalant abuse and associated complications, as well as provide appropriate education and counseling to prevent further misuse.
Related Resources
- Gude J, Bisen V, Fujii K. Medication-induced rhabdomyolysis. Current Psychiatry. 2023;22(2):39-40. doi:10.12788/cp.0332
- Waldman W, Kabata PM, Dines AM, et al. Rhabdomyolysis related to acute recreational drug toxicity--a Euro-DEN study. PLoS One. 2021;16(3):e0246297. doi:10.1371/journal. pone.0246297
Drug Brand Names
Carbamazepine • Tegretol
Duloxetine • Cymbalta
Gabapentin • Neurontin
Propranolol • Inderal
Quetiapine • Seroquel
Trazodone • Oleptro
Ziprasidone • Geodon
1. Ahern NR, Falsafi N. Inhalant abuse: youth at risk. J Psychosoc Nurs Ment Health Serv. 2013;51(8):19-24. doi:10.3928/02793695-20130612-02
2. Howard MO, Bowen SE, Garland EL, et al. Inhalant use and inhalant use disorders in the United States. Addict Sci Clin Prac. 2011;6(1):18-31.
3. Farkas J. Rhabdomyolysis. Internet Book of Critical Care. June 25, 2021. Accessed February 24, 2023. https://emcrit.org/ibcc/rhabdo/
4. Torres PA, Helmstetter JA, Kaye AM, et al. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15(1):58-69.
5. Muller AA, Muller GF. Inhalant abuse. J Emerg Nurs. 2006;32(5):447-448. doi:10.1016/j.jen.2006.05.018
6. Kozel N, Sloboda Z, De La Rosa M, eds. Epidemiology of Inhalant Abuse: An International Perspective; Nida Research Monograph 148. National Institute on Drug Abuse Research, US Dept of Health and Human Services; 1995. Accessed April 20, 2023. https://archives.nida.nih.gov/sites/default/files/monograph148.pdf
1. Ahern NR, Falsafi N. Inhalant abuse: youth at risk. J Psychosoc Nurs Ment Health Serv. 2013;51(8):19-24. doi:10.3928/02793695-20130612-02
2. Howard MO, Bowen SE, Garland EL, et al. Inhalant use and inhalant use disorders in the United States. Addict Sci Clin Prac. 2011;6(1):18-31.
3. Farkas J. Rhabdomyolysis. Internet Book of Critical Care. June 25, 2021. Accessed February 24, 2023. https://emcrit.org/ibcc/rhabdo/
4. Torres PA, Helmstetter JA, Kaye AM, et al. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15(1):58-69.
5. Muller AA, Muller GF. Inhalant abuse. J Emerg Nurs. 2006;32(5):447-448. doi:10.1016/j.jen.2006.05.018
6. Kozel N, Sloboda Z, De La Rosa M, eds. Epidemiology of Inhalant Abuse: An International Perspective; Nida Research Monograph 148. National Institute on Drug Abuse Research, US Dept of Health and Human Services; 1995. Accessed April 20, 2023. https://archives.nida.nih.gov/sites/default/files/monograph148.pdf
When a patient wants to stop taking their antipsychotic: Be ‘A SPORT’
For patients with schizophrenia, adherence to antipsychotic treatment reduces the rate of relapse of psychosis, lowers the rate of rehospitalization, and reduces the severity of illness.1 Despite this, patients may want to discontinue their medications for multiple reasons, including limited insight, adverse effects, or a negative attitude toward medication.1 Understanding a patient’s reason for wanting to discontinue their antipsychotic is critical to providing patient-centered care, building the therapeutic alliance, and offering potential solutions.
Clinicians can recall the mnemonic “A SPORT” (Table) to help ensure they have a thorough discussion with patients about the risks of discontinuation and potential solutions.

Points to cover
First, explore and acknowledge if a patient is experiencing adverse effects from their antipsychotic, which may be causing them to have a negative attitude toward medications. If a patient is experiencing adverse effects from their antipsychotic, offer interventions to mitigate those effects, such as adding an anticholinergic agent to address extrapyramidal symptoms. Decreasing the antipsychotic dosage might reduce the adverse effects burden while still optimizing the benefits from the antipsychotic. Additionally, switching to an alternate medication with a more favorable adverse effect profile may be an option. Whether the patient is experiencing intolerable adverse effects or just has a negative view of their prescribed antipsychotic, it is important to discuss switching medications.
Identifying patient attitudes and their general perspective toward their medication and illness is key. Similarly, a patient’s impaired insight into their mental illness has been associated with treatment discontinuation.2 A strong therapeutic alliance with your patient is of the utmost importance in these situations.
Long-acting injectable antipsychotics (LAIs) are useful clinical tools for patients who struggle to adhere to oral medications. Educating patients and caregivers about other formulations—namely LAIs—can help clarify any misconceptions they may have. One study found that patients who were prescribed oral antipsychotics thought LAIs would be painful, have worse adverse effects, and would not be beneficial in preventing relapse.3 In addition to LAIs, other formulations of antipsychotic medications, such as patches, sublingual tablets, or liquids, may be an option.
For patients to be able to provide informed consent regarding the decision to discontinue their antipsychotic, it is important to educate them about the risks of not taking an antipsychotic, such as an increased risk of relapse, hospitalization, and poor outcomes. Explain that patients with first-episode psychosis who achieve remission of symptoms while taking an antipsychotic can remain in remission with continued treatment, but there is a 5-fold increased risk of relapse when discontinuing an antipsychotic during first-episode psychosis.4
Lastly, despite discussing the risks and benefits, if a patient is determined to discontinue their antipsychotic, we recommend a slow taper of medication rather than abrupt discontinuation. Research has shown that more than one-half of patients who abruptly discontinue an antipsychotic experience withdrawal symptoms, including (but not limited to) nausea, vomiting, abdominal pain, and headaches, as well as anxiety, restlessness, and insomnia.5 These symptoms may occur within 4 weeks after discontinuation.5 While there are no clear guidelines on deprescribing antipsychotics, it is best to individualize the taper based on patient response. Family and caregiver involvement, close follow-up, and symptom monitoring should be integrated into the tapering process.6
1. Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherenc. 2017;11:449-468. doi:10.2147/PPA.S124658
2. Kim J, Ozzoude M, Nakajima S, et al. Insight and medication adherence in schizophrenia: an analysis of the CATIE trial. Neuropharmacology. 2020;168:107634. doi:10.1016/j.neuropharm.2019.05.011
3. Sugawara N, Kudo S, Ishioka M, et al. Attitudes toward long-acting injectable antipsychotics among patients with schizophrenia in Japan. Neuropsychiatr Dis Treat. 2019;15:205-211. doi:10.2147/NDT.S188337
4. Winton-Brown TT, Elanjithara T, Power P, et al. Five-fold increased risk of relapse following breaks in antipsychotic treatment of first episode psychosis. Schizophr Res. 2017;179:50-56. doi:10.1016/j.schres.2016.09.029
5. Brandt L, Bschor T, Henssler J, et al. Antipsychotic withdrawal symptoms: a systematic review and meta-analysis. Front Psychiatry. 2020;11:569912. doi:10.3389/fpsyt.2020.569912
6. Gupta S, Cahill JD, Miller R. Deprescribing antipsychotics: a guide for clinicians. BJPsych Advances. 2018;24(5):295-302. doi:10.1192/bja.2018.2
For patients with schizophrenia, adherence to antipsychotic treatment reduces the rate of relapse of psychosis, lowers the rate of rehospitalization, and reduces the severity of illness.1 Despite this, patients may want to discontinue their medications for multiple reasons, including limited insight, adverse effects, or a negative attitude toward medication.1 Understanding a patient’s reason for wanting to discontinue their antipsychotic is critical to providing patient-centered care, building the therapeutic alliance, and offering potential solutions.
Clinicians can recall the mnemonic “A SPORT” (Table) to help ensure they have a thorough discussion with patients about the risks of discontinuation and potential solutions.

Points to cover
First, explore and acknowledge if a patient is experiencing adverse effects from their antipsychotic, which may be causing them to have a negative attitude toward medications. If a patient is experiencing adverse effects from their antipsychotic, offer interventions to mitigate those effects, such as adding an anticholinergic agent to address extrapyramidal symptoms. Decreasing the antipsychotic dosage might reduce the adverse effects burden while still optimizing the benefits from the antipsychotic. Additionally, switching to an alternate medication with a more favorable adverse effect profile may be an option. Whether the patient is experiencing intolerable adverse effects or just has a negative view of their prescribed antipsychotic, it is important to discuss switching medications.
Identifying patient attitudes and their general perspective toward their medication and illness is key. Similarly, a patient’s impaired insight into their mental illness has been associated with treatment discontinuation.2 A strong therapeutic alliance with your patient is of the utmost importance in these situations.
Long-acting injectable antipsychotics (LAIs) are useful clinical tools for patients who struggle to adhere to oral medications. Educating patients and caregivers about other formulations—namely LAIs—can help clarify any misconceptions they may have. One study found that patients who were prescribed oral antipsychotics thought LAIs would be painful, have worse adverse effects, and would not be beneficial in preventing relapse.3 In addition to LAIs, other formulations of antipsychotic medications, such as patches, sublingual tablets, or liquids, may be an option.
For patients to be able to provide informed consent regarding the decision to discontinue their antipsychotic, it is important to educate them about the risks of not taking an antipsychotic, such as an increased risk of relapse, hospitalization, and poor outcomes. Explain that patients with first-episode psychosis who achieve remission of symptoms while taking an antipsychotic can remain in remission with continued treatment, but there is a 5-fold increased risk of relapse when discontinuing an antipsychotic during first-episode psychosis.4
Lastly, despite discussing the risks and benefits, if a patient is determined to discontinue their antipsychotic, we recommend a slow taper of medication rather than abrupt discontinuation. Research has shown that more than one-half of patients who abruptly discontinue an antipsychotic experience withdrawal symptoms, including (but not limited to) nausea, vomiting, abdominal pain, and headaches, as well as anxiety, restlessness, and insomnia.5 These symptoms may occur within 4 weeks after discontinuation.5 While there are no clear guidelines on deprescribing antipsychotics, it is best to individualize the taper based on patient response. Family and caregiver involvement, close follow-up, and symptom monitoring should be integrated into the tapering process.6
For patients with schizophrenia, adherence to antipsychotic treatment reduces the rate of relapse of psychosis, lowers the rate of rehospitalization, and reduces the severity of illness.1 Despite this, patients may want to discontinue their medications for multiple reasons, including limited insight, adverse effects, or a negative attitude toward medication.1 Understanding a patient’s reason for wanting to discontinue their antipsychotic is critical to providing patient-centered care, building the therapeutic alliance, and offering potential solutions.
Clinicians can recall the mnemonic “A SPORT” (Table) to help ensure they have a thorough discussion with patients about the risks of discontinuation and potential solutions.

Points to cover
First, explore and acknowledge if a patient is experiencing adverse effects from their antipsychotic, which may be causing them to have a negative attitude toward medications. If a patient is experiencing adverse effects from their antipsychotic, offer interventions to mitigate those effects, such as adding an anticholinergic agent to address extrapyramidal symptoms. Decreasing the antipsychotic dosage might reduce the adverse effects burden while still optimizing the benefits from the antipsychotic. Additionally, switching to an alternate medication with a more favorable adverse effect profile may be an option. Whether the patient is experiencing intolerable adverse effects or just has a negative view of their prescribed antipsychotic, it is important to discuss switching medications.
Identifying patient attitudes and their general perspective toward their medication and illness is key. Similarly, a patient’s impaired insight into their mental illness has been associated with treatment discontinuation.2 A strong therapeutic alliance with your patient is of the utmost importance in these situations.
Long-acting injectable antipsychotics (LAIs) are useful clinical tools for patients who struggle to adhere to oral medications. Educating patients and caregivers about other formulations—namely LAIs—can help clarify any misconceptions they may have. One study found that patients who were prescribed oral antipsychotics thought LAIs would be painful, have worse adverse effects, and would not be beneficial in preventing relapse.3 In addition to LAIs, other formulations of antipsychotic medications, such as patches, sublingual tablets, or liquids, may be an option.
For patients to be able to provide informed consent regarding the decision to discontinue their antipsychotic, it is important to educate them about the risks of not taking an antipsychotic, such as an increased risk of relapse, hospitalization, and poor outcomes. Explain that patients with first-episode psychosis who achieve remission of symptoms while taking an antipsychotic can remain in remission with continued treatment, but there is a 5-fold increased risk of relapse when discontinuing an antipsychotic during first-episode psychosis.4
Lastly, despite discussing the risks and benefits, if a patient is determined to discontinue their antipsychotic, we recommend a slow taper of medication rather than abrupt discontinuation. Research has shown that more than one-half of patients who abruptly discontinue an antipsychotic experience withdrawal symptoms, including (but not limited to) nausea, vomiting, abdominal pain, and headaches, as well as anxiety, restlessness, and insomnia.5 These symptoms may occur within 4 weeks after discontinuation.5 While there are no clear guidelines on deprescribing antipsychotics, it is best to individualize the taper based on patient response. Family and caregiver involvement, close follow-up, and symptom monitoring should be integrated into the tapering process.6
1. Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherenc. 2017;11:449-468. doi:10.2147/PPA.S124658
2. Kim J, Ozzoude M, Nakajima S, et al. Insight and medication adherence in schizophrenia: an analysis of the CATIE trial. Neuropharmacology. 2020;168:107634. doi:10.1016/j.neuropharm.2019.05.011
3. Sugawara N, Kudo S, Ishioka M, et al. Attitudes toward long-acting injectable antipsychotics among patients with schizophrenia in Japan. Neuropsychiatr Dis Treat. 2019;15:205-211. doi:10.2147/NDT.S188337
4. Winton-Brown TT, Elanjithara T, Power P, et al. Five-fold increased risk of relapse following breaks in antipsychotic treatment of first episode psychosis. Schizophr Res. 2017;179:50-56. doi:10.1016/j.schres.2016.09.029
5. Brandt L, Bschor T, Henssler J, et al. Antipsychotic withdrawal symptoms: a systematic review and meta-analysis. Front Psychiatry. 2020;11:569912. doi:10.3389/fpsyt.2020.569912
6. Gupta S, Cahill JD, Miller R. Deprescribing antipsychotics: a guide for clinicians. BJPsych Advances. 2018;24(5):295-302. doi:10.1192/bja.2018.2
1. Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherenc. 2017;11:449-468. doi:10.2147/PPA.S124658
2. Kim J, Ozzoude M, Nakajima S, et al. Insight and medication adherence in schizophrenia: an analysis of the CATIE trial. Neuropharmacology. 2020;168:107634. doi:10.1016/j.neuropharm.2019.05.011
3. Sugawara N, Kudo S, Ishioka M, et al. Attitudes toward long-acting injectable antipsychotics among patients with schizophrenia in Japan. Neuropsychiatr Dis Treat. 2019;15:205-211. doi:10.2147/NDT.S188337
4. Winton-Brown TT, Elanjithara T, Power P, et al. Five-fold increased risk of relapse following breaks in antipsychotic treatment of first episode psychosis. Schizophr Res. 2017;179:50-56. doi:10.1016/j.schres.2016.09.029
5. Brandt L, Bschor T, Henssler J, et al. Antipsychotic withdrawal symptoms: a systematic review and meta-analysis. Front Psychiatry. 2020;11:569912. doi:10.3389/fpsyt.2020.569912
6. Gupta S, Cahill JD, Miller R. Deprescribing antipsychotics: a guide for clinicians. BJPsych Advances. 2018;24(5):295-302. doi:10.1192/bja.2018.2








