User login
Developments on the malaria front
Progress is being made in the battle against malaria. From engineering a mosquito-killing fungus to discovering new anti-malaria targets, scientists are making advances on multiple malaria fronts.
And US aid to combat malaria is having a positive impact on reducing childhood mortality in 19 sub-Saharan countries. A few of the recent developments are described here.
Genetic engineering
Scientists developed a genetic technique that disrupts the heme synthesis pathway in Plasmodium berghei parasites, which could be an effective way to target Plasmodium parasites in the liver.
Heme synthesis is essential for P berghei development in mosquitoes that transmit the parasite between rodent hosts. However, the pathway is not essential during a later stage of the parasite’s development in the bloodstream.
So researchers produced P berghei parasites capable of expressing the FC gene. The FC (ferrochelatase) gene allows P berghei to produce heme. The parasites could develop properly in mosquitoes, but produced some FC-deficient parasites once they infected mouse liver cells.
FC-deficient parasites were unable to complete their liver development phase.
The team says this approach would be prophylactic, since malaria symptoms aren't apparent until the parasite leaves the liver and begins its bloodstream phase.
The team published its findings in PLOS Pathogens.
Mosquito-killing fungi
In a report that sounds almost like a science fiction story, researchers genetically engineered a fungus to kill mosquitoes by producing spider and scorpion toxins.
They suggest this method could serve as a highly effective biological control mechanism to fight malaria-carrying mosquitoes.
The researchers isolated genes that express neurotoxins from the venom of scorpions and spiders. They then engineered the genes into the fungus's DNA.
The researchers used the fungus Metarhizium pingshaensei, which is a natural killer of mosquitoes.
The fungus was originally isolated from a mosquito and previous evidence suggests it is specific to disease-carrying mosquito species, including Anopheles gambiae and Aedes aegypti.
When spores of the fungus contact a mosquito's body, the spores germinate and penetrate the insect's exoskeleton, eventually killing the insect host from the inside out.
And the most potent fungal strains, Brian Lovett, a graduate student at the University of Maryland in College Park, explained, “are able to kill mosquitoes with a single spore."
He added that the fungi also stop mosquitoes from blood feeding. Taken together, this means “that our fungal strains are capable of preventing transmission of disease by more than 90 percent of mosquitoes after just 5 days."
The fungus is specific to mosquitoes and does not pose a risk to humans. The study results also suggest the fungus is safe for honeybees and other insects.
The researchers plan to expand on-the-ground testing in Burkina Faso.
For more on this mosquito-killing approach, see their study published in Scientific Reports.
Potential new target
Researchers have described a new protein, the transcription factor PfAP2-I, which they say may turn out to be an effective target to combat drug-resistant malaria parasites.
PfAP2-I regulates genes involved with the parasite's invasion of red blood cells. This is a critical part of the parasite's 3-stage life cycle that could be targeted by new anti-malarial drugs.
“Most multi-celled organisms have hundreds of these regulators,” said lead author Manuel Llinás, PhD, of Penn State University in State College, Pennsylvania, “but it turns out, so far as we can recognize, the [Plasmodium] parasite has a single family of transcription factors called Apicomplexan AP2 proteins. One of these transcription factors is PfAP2-I."
PfAP2-I is the first known regulator of invasion genes in Plasmodium falciparum.
The new study also indicates that PfAP2-I likely recruits another protein, Bromodomain Protein 1 (PfBDP1), which was previously shown to be involved in the invasion of red blood cells.
The two proteins may work together to regulate gene transcription during this critical stage of infection.
For more on this potential new target, see their study published in Cell Host & Microbe.
Parasite diversity
Not all malaria infections result in life-threatening anemia and organ failure, and so a research team led by Matthew B. B. McCall, MD, PhD, of Erasmus Medical Center in Rotterdam, the Netherlands, set out to determine why.
They exposed 23 healthy human volunteers to sets of 5 mosquitoes carrying the NF54, NF135.C10, or NF166.C8 isolates of P falciparum.
All volunteers developed parasitemia, were treated with anti-malarial drugs, and recovered, although some strains caused more severe symptoms.
The investigators found that 3 geographic and genetically diverse forms of the parasite each demonstrated a distinct ability to infect liver cells.
They also observed the degree of infection in human liver cells growing in culture was closely correlated with parasite loads in the bloodstream.
The investigators believe the variability among parasite types suggests that malaria vaccines should use multiple strains.
In addition, the infectivity of different parasite strains could vary in populations previously exposed to malaria.
For more details on parasite diversity, see the team’s findings in Science Translational Medicine.
Malaria test
A new malaria test can diagnose malaria faster and more reliably than current methods, according to a report in the NL Times.
The new test uses an algorithm that can diagnose malaria at a rate of 120 blood tests per hour. It is 97% accurate.
Rather than search for the parasite itself in blood samples, the new test analyzes the effect the infection has on the blood, such as shape and density of red blood cells, hemoglobin level, and 27 other parameters simultaneously.
The developers won the European Inventor Award for the rapid malaria test, which will be further developed by Siemens.
Aid to combat malaria
The US malaria initiative in 19 sub-Saharan African countries has contributed to a 16% reduction in the annual risk of mortality for children under 5 years, according to a new study published in PLOS Medicine.
Thirteen sub-Saharan countries did not receive funding from the initiative, which allowed researchers to compare and analyze the impact of the intervention.
Because the study may have had confounding variables that were not measured, however, the results could not be definitively interpreted as causal evidence of the reduction in child mortality rates.
However, they do indicate an association between the receipt of funding and mortality.
The funding went to support malaria prevention technologies, such as insecticide-treated nets and indoor residual spraying.
The authors believe further investment in these interventions “may translate to additional lives saved, reduced household financial burdens associated with caring for ill household members and lost wages, and less strain on health systems associated with treating malaria cases.”
Countries that received funding included: Angola, Benin, Congo DRC, Ethiopia, Ghana, Guinea, Kenya, Liberia, Madagascar, Malawi, Mali, Mozambique, Nigeria, Rwanda, Senegal, Tanzania, Uganda, Zambia, and Zimbabwe.
Comparison countries include: Burkina Faso, Burundi, Cameroon, Chad, Congo, Cote d’Ivoire, Gabon, Namibia, Niger, Sierra Leone, Swaziland, The Gambia, and Togo. ![]()
Progress is being made in the battle against malaria. From engineering a mosquito-killing fungus to discovering new anti-malaria targets, scientists are making advances on multiple malaria fronts.
And US aid to combat malaria is having a positive impact on reducing childhood mortality in 19 sub-Saharan countries. A few of the recent developments are described here.
Genetic engineering
Scientists developed a genetic technique that disrupts the heme synthesis pathway in Plasmodium berghei parasites, which could be an effective way to target Plasmodium parasites in the liver.
Heme synthesis is essential for P berghei development in mosquitoes that transmit the parasite between rodent hosts. However, the pathway is not essential during a later stage of the parasite’s development in the bloodstream.
So researchers produced P berghei parasites capable of expressing the FC gene. The FC (ferrochelatase) gene allows P berghei to produce heme. The parasites could develop properly in mosquitoes, but produced some FC-deficient parasites once they infected mouse liver cells.
FC-deficient parasites were unable to complete their liver development phase.
The team says this approach would be prophylactic, since malaria symptoms aren't apparent until the parasite leaves the liver and begins its bloodstream phase.
The team published its findings in PLOS Pathogens.
Mosquito-killing fungi
In a report that sounds almost like a science fiction story, researchers genetically engineered a fungus to kill mosquitoes by producing spider and scorpion toxins.
They suggest this method could serve as a highly effective biological control mechanism to fight malaria-carrying mosquitoes.
The researchers isolated genes that express neurotoxins from the venom of scorpions and spiders. They then engineered the genes into the fungus's DNA.
The researchers used the fungus Metarhizium pingshaensei, which is a natural killer of mosquitoes.
The fungus was originally isolated from a mosquito and previous evidence suggests it is specific to disease-carrying mosquito species, including Anopheles gambiae and Aedes aegypti.
When spores of the fungus contact a mosquito's body, the spores germinate and penetrate the insect's exoskeleton, eventually killing the insect host from the inside out.
And the most potent fungal strains, Brian Lovett, a graduate student at the University of Maryland in College Park, explained, “are able to kill mosquitoes with a single spore."
He added that the fungi also stop mosquitoes from blood feeding. Taken together, this means “that our fungal strains are capable of preventing transmission of disease by more than 90 percent of mosquitoes after just 5 days."
The fungus is specific to mosquitoes and does not pose a risk to humans. The study results also suggest the fungus is safe for honeybees and other insects.
The researchers plan to expand on-the-ground testing in Burkina Faso.
For more on this mosquito-killing approach, see their study published in Scientific Reports.
Potential new target
Researchers have described a new protein, the transcription factor PfAP2-I, which they say may turn out to be an effective target to combat drug-resistant malaria parasites.
PfAP2-I regulates genes involved with the parasite's invasion of red blood cells. This is a critical part of the parasite's 3-stage life cycle that could be targeted by new anti-malarial drugs.
“Most multi-celled organisms have hundreds of these regulators,” said lead author Manuel Llinás, PhD, of Penn State University in State College, Pennsylvania, “but it turns out, so far as we can recognize, the [Plasmodium] parasite has a single family of transcription factors called Apicomplexan AP2 proteins. One of these transcription factors is PfAP2-I."
PfAP2-I is the first known regulator of invasion genes in Plasmodium falciparum.
The new study also indicates that PfAP2-I likely recruits another protein, Bromodomain Protein 1 (PfBDP1), which was previously shown to be involved in the invasion of red blood cells.
The two proteins may work together to regulate gene transcription during this critical stage of infection.
For more on this potential new target, see their study published in Cell Host & Microbe.
Parasite diversity
Not all malaria infections result in life-threatening anemia and organ failure, and so a research team led by Matthew B. B. McCall, MD, PhD, of Erasmus Medical Center in Rotterdam, the Netherlands, set out to determine why.
They exposed 23 healthy human volunteers to sets of 5 mosquitoes carrying the NF54, NF135.C10, or NF166.C8 isolates of P falciparum.
All volunteers developed parasitemia, were treated with anti-malarial drugs, and recovered, although some strains caused more severe symptoms.
The investigators found that 3 geographic and genetically diverse forms of the parasite each demonstrated a distinct ability to infect liver cells.
They also observed the degree of infection in human liver cells growing in culture was closely correlated with parasite loads in the bloodstream.
The investigators believe the variability among parasite types suggests that malaria vaccines should use multiple strains.
In addition, the infectivity of different parasite strains could vary in populations previously exposed to malaria.
For more details on parasite diversity, see the team’s findings in Science Translational Medicine.
Malaria test
A new malaria test can diagnose malaria faster and more reliably than current methods, according to a report in the NL Times.
The new test uses an algorithm that can diagnose malaria at a rate of 120 blood tests per hour. It is 97% accurate.
Rather than search for the parasite itself in blood samples, the new test analyzes the effect the infection has on the blood, such as shape and density of red blood cells, hemoglobin level, and 27 other parameters simultaneously.
The developers won the European Inventor Award for the rapid malaria test, which will be further developed by Siemens.
Aid to combat malaria
The US malaria initiative in 19 sub-Saharan African countries has contributed to a 16% reduction in the annual risk of mortality for children under 5 years, according to a new study published in PLOS Medicine.
Thirteen sub-Saharan countries did not receive funding from the initiative, which allowed researchers to compare and analyze the impact of the intervention.
Because the study may have had confounding variables that were not measured, however, the results could not be definitively interpreted as causal evidence of the reduction in child mortality rates.
However, they do indicate an association between the receipt of funding and mortality.
The funding went to support malaria prevention technologies, such as insecticide-treated nets and indoor residual spraying.
The authors believe further investment in these interventions “may translate to additional lives saved, reduced household financial burdens associated with caring for ill household members and lost wages, and less strain on health systems associated with treating malaria cases.”
Countries that received funding included: Angola, Benin, Congo DRC, Ethiopia, Ghana, Guinea, Kenya, Liberia, Madagascar, Malawi, Mali, Mozambique, Nigeria, Rwanda, Senegal, Tanzania, Uganda, Zambia, and Zimbabwe.
Comparison countries include: Burkina Faso, Burundi, Cameroon, Chad, Congo, Cote d’Ivoire, Gabon, Namibia, Niger, Sierra Leone, Swaziland, The Gambia, and Togo. ![]()
Progress is being made in the battle against malaria. From engineering a mosquito-killing fungus to discovering new anti-malaria targets, scientists are making advances on multiple malaria fronts.
And US aid to combat malaria is having a positive impact on reducing childhood mortality in 19 sub-Saharan countries. A few of the recent developments are described here.
Genetic engineering
Scientists developed a genetic technique that disrupts the heme synthesis pathway in Plasmodium berghei parasites, which could be an effective way to target Plasmodium parasites in the liver.
Heme synthesis is essential for P berghei development in mosquitoes that transmit the parasite between rodent hosts. However, the pathway is not essential during a later stage of the parasite’s development in the bloodstream.
So researchers produced P berghei parasites capable of expressing the FC gene. The FC (ferrochelatase) gene allows P berghei to produce heme. The parasites could develop properly in mosquitoes, but produced some FC-deficient parasites once they infected mouse liver cells.
FC-deficient parasites were unable to complete their liver development phase.
The team says this approach would be prophylactic, since malaria symptoms aren't apparent until the parasite leaves the liver and begins its bloodstream phase.
The team published its findings in PLOS Pathogens.
Mosquito-killing fungi
In a report that sounds almost like a science fiction story, researchers genetically engineered a fungus to kill mosquitoes by producing spider and scorpion toxins.
They suggest this method could serve as a highly effective biological control mechanism to fight malaria-carrying mosquitoes.
The researchers isolated genes that express neurotoxins from the venom of scorpions and spiders. They then engineered the genes into the fungus's DNA.
The researchers used the fungus Metarhizium pingshaensei, which is a natural killer of mosquitoes.
The fungus was originally isolated from a mosquito and previous evidence suggests it is specific to disease-carrying mosquito species, including Anopheles gambiae and Aedes aegypti.
When spores of the fungus contact a mosquito's body, the spores germinate and penetrate the insect's exoskeleton, eventually killing the insect host from the inside out.
And the most potent fungal strains, Brian Lovett, a graduate student at the University of Maryland in College Park, explained, “are able to kill mosquitoes with a single spore."
He added that the fungi also stop mosquitoes from blood feeding. Taken together, this means “that our fungal strains are capable of preventing transmission of disease by more than 90 percent of mosquitoes after just 5 days."
The fungus is specific to mosquitoes and does not pose a risk to humans. The study results also suggest the fungus is safe for honeybees and other insects.
The researchers plan to expand on-the-ground testing in Burkina Faso.
For more on this mosquito-killing approach, see their study published in Scientific Reports.
Potential new target
Researchers have described a new protein, the transcription factor PfAP2-I, which they say may turn out to be an effective target to combat drug-resistant malaria parasites.
PfAP2-I regulates genes involved with the parasite's invasion of red blood cells. This is a critical part of the parasite's 3-stage life cycle that could be targeted by new anti-malarial drugs.
“Most multi-celled organisms have hundreds of these regulators,” said lead author Manuel Llinás, PhD, of Penn State University in State College, Pennsylvania, “but it turns out, so far as we can recognize, the [Plasmodium] parasite has a single family of transcription factors called Apicomplexan AP2 proteins. One of these transcription factors is PfAP2-I."
PfAP2-I is the first known regulator of invasion genes in Plasmodium falciparum.
The new study also indicates that PfAP2-I likely recruits another protein, Bromodomain Protein 1 (PfBDP1), which was previously shown to be involved in the invasion of red blood cells.
The two proteins may work together to regulate gene transcription during this critical stage of infection.
For more on this potential new target, see their study published in Cell Host & Microbe.
Parasite diversity
Not all malaria infections result in life-threatening anemia and organ failure, and so a research team led by Matthew B. B. McCall, MD, PhD, of Erasmus Medical Center in Rotterdam, the Netherlands, set out to determine why.
They exposed 23 healthy human volunteers to sets of 5 mosquitoes carrying the NF54, NF135.C10, or NF166.C8 isolates of P falciparum.
All volunteers developed parasitemia, were treated with anti-malarial drugs, and recovered, although some strains caused more severe symptoms.
The investigators found that 3 geographic and genetically diverse forms of the parasite each demonstrated a distinct ability to infect liver cells.
They also observed the degree of infection in human liver cells growing in culture was closely correlated with parasite loads in the bloodstream.
The investigators believe the variability among parasite types suggests that malaria vaccines should use multiple strains.
In addition, the infectivity of different parasite strains could vary in populations previously exposed to malaria.
For more details on parasite diversity, see the team’s findings in Science Translational Medicine.
Malaria test
A new malaria test can diagnose malaria faster and more reliably than current methods, according to a report in the NL Times.
The new test uses an algorithm that can diagnose malaria at a rate of 120 blood tests per hour. It is 97% accurate.
Rather than search for the parasite itself in blood samples, the new test analyzes the effect the infection has on the blood, such as shape and density of red blood cells, hemoglobin level, and 27 other parameters simultaneously.
The developers won the European Inventor Award for the rapid malaria test, which will be further developed by Siemens.
Aid to combat malaria
The US malaria initiative in 19 sub-Saharan African countries has contributed to a 16% reduction in the annual risk of mortality for children under 5 years, according to a new study published in PLOS Medicine.
Thirteen sub-Saharan countries did not receive funding from the initiative, which allowed researchers to compare and analyze the impact of the intervention.
Because the study may have had confounding variables that were not measured, however, the results could not be definitively interpreted as causal evidence of the reduction in child mortality rates.
However, they do indicate an association between the receipt of funding and mortality.
The funding went to support malaria prevention technologies, such as insecticide-treated nets and indoor residual spraying.
The authors believe further investment in these interventions “may translate to additional lives saved, reduced household financial burdens associated with caring for ill household members and lost wages, and less strain on health systems associated with treating malaria cases.”
Countries that received funding included: Angola, Benin, Congo DRC, Ethiopia, Ghana, Guinea, Kenya, Liberia, Madagascar, Malawi, Mali, Mozambique, Nigeria, Rwanda, Senegal, Tanzania, Uganda, Zambia, and Zimbabwe.
Comparison countries include: Burkina Faso, Burundi, Cameroon, Chad, Congo, Cote d’Ivoire, Gabon, Namibia, Niger, Sierra Leone, Swaziland, The Gambia, and Togo. ![]()
New SC rituximab formulation approved, reduces administration time
The US Food and Drug Administration (FDA) approved a new, subcutaneous (SC) formulation of rituximab with hyaluronidase human (Rituxan Hycela™).
The new formulation includes the same monoclonal antibody as intravenous rituximab, but is combined with an enzyme that helps to deliver rituximab under the skin.
The new treatment reduces administration time from 1.5 hours or more for intravenous rituximab to 5 to 7 minutes for the subcutaneous injection.
It is approved for use in adults with previously untreated and relapsed or refractory follicular lymphoma (FL), previously untreated diffuse large B-cell lymphoma (DLBCL), and previously untreated and previously treated chronic lymphocytic leukemia (CLL).
“[P]eople with 3 of the most common blood cancers now have a new treatment option which provides efficacy comparable with intravenous Rituxan and can be delivered under the skin in minutes instead of hours through IV infusion,” said Sandra Horning, MD, chief medical officer of Genentech.
Rituxan Hycela is manufactured by Genentech, Inc, a member of the Roche Group, and jointly marketed by Biogen and Genentech USA, Inc.
“People who benefit from Rituxan may receive years of repeated treatments for their blood cancer, so an option that reduces the administration time can be important,” she noted.
The FDA based its decision on results from 4 clinical studies:
- SABRINA (NCT01200758): Phase 3 combination study with chemotherapy and maintenance study in previously untreated FL
- SAWYER (NCT01292603): Phase 1b study in previously untreated CLL
- MabEase (NCT01649856): Phase 3 study in previously untreated DLBCL
- PrefMab (NCT01724021): Phase 3 patient preference study in previously untreated FL and DLBCL
This last study showed that 77% of patients preferred subcutaneous over intravenous administration, primarily because it reduced administration time.
Together, these trials represented nearly 2,000 people and demonstrated that subcutaneous administration of rituximab/hyaluronidase resulted in non-inferior levels of rituximab in the blood compared to intravenous rituximab.
And the subcutaneous formulation also demonstrated comparable clinical efficacy outcomes to the intravenous formulation.
Patients must have had at least 1 full dose of intravenous rituximab without severe adverse reactions before receiving the subcutaneous injection. There is a higher risk of certain severe adverse reactions during the first infusion.
The safety profile of rituximab/hyaluronidase is also comparable to intravenous rituximab, except for cutaneous reactions.
The most common (≥20%) adverse reactions observed with rituximab/hyaluronidase were:
- In FL, infections, neutropenia, nausea, constipation, cough, and fatigue.
- In DLBCL, infections, neutropenia, alopecia, nausea, and anemia.
- In CLL, infections, neutropenia, nausea, thrombocytopenia, pyrexia, vomiting, and erythema at the injection site.
Rituxan Hycela will be available in the US within 1 to 2 weeks, according to the manufacturer. Intravenous rituximab will continue to be available.
A subcutaneous formulation of rituximab (MabThera) had previously been approved for use in European markets by the European Commission.
For further information on the new US formulation, see the full prescribing information. ![]()
The US Food and Drug Administration (FDA) approved a new, subcutaneous (SC) formulation of rituximab with hyaluronidase human (Rituxan Hycela™).
The new formulation includes the same monoclonal antibody as intravenous rituximab, but is combined with an enzyme that helps to deliver rituximab under the skin.
The new treatment reduces administration time from 1.5 hours or more for intravenous rituximab to 5 to 7 minutes for the subcutaneous injection.
It is approved for use in adults with previously untreated and relapsed or refractory follicular lymphoma (FL), previously untreated diffuse large B-cell lymphoma (DLBCL), and previously untreated and previously treated chronic lymphocytic leukemia (CLL).
“[P]eople with 3 of the most common blood cancers now have a new treatment option which provides efficacy comparable with intravenous Rituxan and can be delivered under the skin in minutes instead of hours through IV infusion,” said Sandra Horning, MD, chief medical officer of Genentech.
Rituxan Hycela is manufactured by Genentech, Inc, a member of the Roche Group, and jointly marketed by Biogen and Genentech USA, Inc.
“People who benefit from Rituxan may receive years of repeated treatments for their blood cancer, so an option that reduces the administration time can be important,” she noted.
The FDA based its decision on results from 4 clinical studies:
- SABRINA (NCT01200758): Phase 3 combination study with chemotherapy and maintenance study in previously untreated FL
- SAWYER (NCT01292603): Phase 1b study in previously untreated CLL
- MabEase (NCT01649856): Phase 3 study in previously untreated DLBCL
- PrefMab (NCT01724021): Phase 3 patient preference study in previously untreated FL and DLBCL
This last study showed that 77% of patients preferred subcutaneous over intravenous administration, primarily because it reduced administration time.
Together, these trials represented nearly 2,000 people and demonstrated that subcutaneous administration of rituximab/hyaluronidase resulted in non-inferior levels of rituximab in the blood compared to intravenous rituximab.
And the subcutaneous formulation also demonstrated comparable clinical efficacy outcomes to the intravenous formulation.
Patients must have had at least 1 full dose of intravenous rituximab without severe adverse reactions before receiving the subcutaneous injection. There is a higher risk of certain severe adverse reactions during the first infusion.
The safety profile of rituximab/hyaluronidase is also comparable to intravenous rituximab, except for cutaneous reactions.
The most common (≥20%) adverse reactions observed with rituximab/hyaluronidase were:
- In FL, infections, neutropenia, nausea, constipation, cough, and fatigue.
- In DLBCL, infections, neutropenia, alopecia, nausea, and anemia.
- In CLL, infections, neutropenia, nausea, thrombocytopenia, pyrexia, vomiting, and erythema at the injection site.
Rituxan Hycela will be available in the US within 1 to 2 weeks, according to the manufacturer. Intravenous rituximab will continue to be available.
A subcutaneous formulation of rituximab (MabThera) had previously been approved for use in European markets by the European Commission.
For further information on the new US formulation, see the full prescribing information. ![]()
The US Food and Drug Administration (FDA) approved a new, subcutaneous (SC) formulation of rituximab with hyaluronidase human (Rituxan Hycela™).
The new formulation includes the same monoclonal antibody as intravenous rituximab, but is combined with an enzyme that helps to deliver rituximab under the skin.
The new treatment reduces administration time from 1.5 hours or more for intravenous rituximab to 5 to 7 minutes for the subcutaneous injection.
It is approved for use in adults with previously untreated and relapsed or refractory follicular lymphoma (FL), previously untreated diffuse large B-cell lymphoma (DLBCL), and previously untreated and previously treated chronic lymphocytic leukemia (CLL).
“[P]eople with 3 of the most common blood cancers now have a new treatment option which provides efficacy comparable with intravenous Rituxan and can be delivered under the skin in minutes instead of hours through IV infusion,” said Sandra Horning, MD, chief medical officer of Genentech.
Rituxan Hycela is manufactured by Genentech, Inc, a member of the Roche Group, and jointly marketed by Biogen and Genentech USA, Inc.
“People who benefit from Rituxan may receive years of repeated treatments for their blood cancer, so an option that reduces the administration time can be important,” she noted.
The FDA based its decision on results from 4 clinical studies:
- SABRINA (NCT01200758): Phase 3 combination study with chemotherapy and maintenance study in previously untreated FL
- SAWYER (NCT01292603): Phase 1b study in previously untreated CLL
- MabEase (NCT01649856): Phase 3 study in previously untreated DLBCL
- PrefMab (NCT01724021): Phase 3 patient preference study in previously untreated FL and DLBCL
This last study showed that 77% of patients preferred subcutaneous over intravenous administration, primarily because it reduced administration time.
Together, these trials represented nearly 2,000 people and demonstrated that subcutaneous administration of rituximab/hyaluronidase resulted in non-inferior levels of rituximab in the blood compared to intravenous rituximab.
And the subcutaneous formulation also demonstrated comparable clinical efficacy outcomes to the intravenous formulation.
Patients must have had at least 1 full dose of intravenous rituximab without severe adverse reactions before receiving the subcutaneous injection. There is a higher risk of certain severe adverse reactions during the first infusion.
The safety profile of rituximab/hyaluronidase is also comparable to intravenous rituximab, except for cutaneous reactions.
The most common (≥20%) adverse reactions observed with rituximab/hyaluronidase were:
- In FL, infections, neutropenia, nausea, constipation, cough, and fatigue.
- In DLBCL, infections, neutropenia, alopecia, nausea, and anemia.
- In CLL, infections, neutropenia, nausea, thrombocytopenia, pyrexia, vomiting, and erythema at the injection site.
Rituxan Hycela will be available in the US within 1 to 2 weeks, according to the manufacturer. Intravenous rituximab will continue to be available.
A subcutaneous formulation of rituximab (MabThera) had previously been approved for use in European markets by the European Commission.
For further information on the new US formulation, see the full prescribing information. ![]()
Kids’ self-reports of symptoms, side effects reliable
A small study of 20 children aged 8 to 18 years with incurable or refractory cancers indicates children are reliable reporters of their symptoms and side effects.
The investigators collected reports from the children, who were enrolled on phase 1/2 clinical trials at 4 cancer centers and undergoing their first courses of chemotherapy.
The team assessed the feasibility and acceptability of collecting symptom, function, and quality of life (QOL) reports from the study participants.
The investigators also evaluated the measurement tool and interview questions at 2 time points.
They contend the youths’ self-reports potentially could be a new trial endpoint.
According to the investigators, only rarely do patient-reported outcomes (PROs) get incorporated into pediatric phase 1 or phase 2 trials.
And because these trials contribute to drug indications and labeling, the researchers decided to assess whether it was feasible to enroll young people and retain them in a PRO endeavor.
The researchers also assessed the reliability, validity, responsiveness, and range of the pediatric measures employed. They used the Patient-Reported Outcomes Measurement Information System (PROMIS) to capture statistically significant and clinically meaningful changes or minimally important differences (MIDs) in PROs.
Pamela S. Hinds, PhD, RN, of George Washington University in Washington, DC, reported the findings on behalf of the Children's National Health System researchers in Cancer, the journal of the American Cancer Society.
"When experimental cancer drugs are studied, researchers collect details about how these promising therapies affect children's organs, but rarely do they ask the children themselves about symptoms they feel or the side effects they experience," Dr Hinds said.
"Without this crucial information, the full impact of the experimental treatment on the pediatric patient is likely underreported and clinicians are hobbled in their ability to effectively manage side effects," she added.
The team recruited children and adolescents enrolled in phase 1 safety or phase 2 efficacy trials at Children's National, Seattle Children's Hospital, Children's Hospital of Philadelphia, and Boston Children's Hospital.
Findings
Sixty percent of the participants were male and 70% were white.
Median age of the participants was 13.6 years: 7 (35%) were age 8 to 12, and 13 (65%) were 13 to 17.
Thirteen participants (65%) had solid tumors, 5 (25%) had brain tumors, and 2 (10%) had lymphoma.
A total of 29 patients were eligible to participate in the trial during 20 months of screening. Five parents and 2 patients declined to participate.
The remaining 22 patients who agreed to participate accounted for a 75.9% enrollment rate. Twenty of them (90.9%) participated at the first data time point, which was at the time of enrollment, and 77.3% participated 3 weeks later at time point 2.
The authors noted that refusals to enroll were more likely to come from parents (17.2%) than the eligible patients (8.3%).
And refusals only occurred when the self-report measures were not embedded in the clinical trial.
Of the 10 protocols represented, 7 patients were enrolled on the same protocol in which the PRO measures were embedded.
The researchers administered the 6-item short-form measures for the scales of Mobility, Pain, Fatigue, Depressive Symptoms, Anxiety, and Peer Relationships.
They asked the 4 open-ended questions—concerning QOL while receiving therapy and acceptability of the patient reporting—at time point 2.
At time point 1, 3 patients did not complete 3 PROMIS measures, for a person-missing rate of 15% and a measure-missing rate of 3.3%.
At the second time point, 2 patients did not complete 1 measure each, for a person-missing rate of 11.8% and a measure-missing rate of 2%.
All but one measure at time point 1 met the reliability criterion and all measures did so at time point 2.
The research team believes their findings support the feasibility and acceptability of completing quantitative and qualitative measures regarding symptom, function, and QOL experiences among children and adolescents with incurable cancer.
The researchers note the small study size and the number of parent refusals are limitations of the trial.
Nevertheless, they recommend embedding PROs in future pediatric oncology phase 1/2 trials. ![]()
A small study of 20 children aged 8 to 18 years with incurable or refractory cancers indicates children are reliable reporters of their symptoms and side effects.
The investigators collected reports from the children, who were enrolled on phase 1/2 clinical trials at 4 cancer centers and undergoing their first courses of chemotherapy.
The team assessed the feasibility and acceptability of collecting symptom, function, and quality of life (QOL) reports from the study participants.
The investigators also evaluated the measurement tool and interview questions at 2 time points.
They contend the youths’ self-reports potentially could be a new trial endpoint.
According to the investigators, only rarely do patient-reported outcomes (PROs) get incorporated into pediatric phase 1 or phase 2 trials.
And because these trials contribute to drug indications and labeling, the researchers decided to assess whether it was feasible to enroll young people and retain them in a PRO endeavor.
The researchers also assessed the reliability, validity, responsiveness, and range of the pediatric measures employed. They used the Patient-Reported Outcomes Measurement Information System (PROMIS) to capture statistically significant and clinically meaningful changes or minimally important differences (MIDs) in PROs.
Pamela S. Hinds, PhD, RN, of George Washington University in Washington, DC, reported the findings on behalf of the Children's National Health System researchers in Cancer, the journal of the American Cancer Society.
"When experimental cancer drugs are studied, researchers collect details about how these promising therapies affect children's organs, but rarely do they ask the children themselves about symptoms they feel or the side effects they experience," Dr Hinds said.
"Without this crucial information, the full impact of the experimental treatment on the pediatric patient is likely underreported and clinicians are hobbled in their ability to effectively manage side effects," she added.
The team recruited children and adolescents enrolled in phase 1 safety or phase 2 efficacy trials at Children's National, Seattle Children's Hospital, Children's Hospital of Philadelphia, and Boston Children's Hospital.
Findings
Sixty percent of the participants were male and 70% were white.
Median age of the participants was 13.6 years: 7 (35%) were age 8 to 12, and 13 (65%) were 13 to 17.
Thirteen participants (65%) had solid tumors, 5 (25%) had brain tumors, and 2 (10%) had lymphoma.
A total of 29 patients were eligible to participate in the trial during 20 months of screening. Five parents and 2 patients declined to participate.
The remaining 22 patients who agreed to participate accounted for a 75.9% enrollment rate. Twenty of them (90.9%) participated at the first data time point, which was at the time of enrollment, and 77.3% participated 3 weeks later at time point 2.
The authors noted that refusals to enroll were more likely to come from parents (17.2%) than the eligible patients (8.3%).
And refusals only occurred when the self-report measures were not embedded in the clinical trial.
Of the 10 protocols represented, 7 patients were enrolled on the same protocol in which the PRO measures were embedded.
The researchers administered the 6-item short-form measures for the scales of Mobility, Pain, Fatigue, Depressive Symptoms, Anxiety, and Peer Relationships.
They asked the 4 open-ended questions—concerning QOL while receiving therapy and acceptability of the patient reporting—at time point 2.
At time point 1, 3 patients did not complete 3 PROMIS measures, for a person-missing rate of 15% and a measure-missing rate of 3.3%.
At the second time point, 2 patients did not complete 1 measure each, for a person-missing rate of 11.8% and a measure-missing rate of 2%.
All but one measure at time point 1 met the reliability criterion and all measures did so at time point 2.
The research team believes their findings support the feasibility and acceptability of completing quantitative and qualitative measures regarding symptom, function, and QOL experiences among children and adolescents with incurable cancer.
The researchers note the small study size and the number of parent refusals are limitations of the trial.
Nevertheless, they recommend embedding PROs in future pediatric oncology phase 1/2 trials. ![]()
A small study of 20 children aged 8 to 18 years with incurable or refractory cancers indicates children are reliable reporters of their symptoms and side effects.
The investigators collected reports from the children, who were enrolled on phase 1/2 clinical trials at 4 cancer centers and undergoing their first courses of chemotherapy.
The team assessed the feasibility and acceptability of collecting symptom, function, and quality of life (QOL) reports from the study participants.
The investigators also evaluated the measurement tool and interview questions at 2 time points.
They contend the youths’ self-reports potentially could be a new trial endpoint.
According to the investigators, only rarely do patient-reported outcomes (PROs) get incorporated into pediatric phase 1 or phase 2 trials.
And because these trials contribute to drug indications and labeling, the researchers decided to assess whether it was feasible to enroll young people and retain them in a PRO endeavor.
The researchers also assessed the reliability, validity, responsiveness, and range of the pediatric measures employed. They used the Patient-Reported Outcomes Measurement Information System (PROMIS) to capture statistically significant and clinically meaningful changes or minimally important differences (MIDs) in PROs.
Pamela S. Hinds, PhD, RN, of George Washington University in Washington, DC, reported the findings on behalf of the Children's National Health System researchers in Cancer, the journal of the American Cancer Society.
"When experimental cancer drugs are studied, researchers collect details about how these promising therapies affect children's organs, but rarely do they ask the children themselves about symptoms they feel or the side effects they experience," Dr Hinds said.
"Without this crucial information, the full impact of the experimental treatment on the pediatric patient is likely underreported and clinicians are hobbled in their ability to effectively manage side effects," she added.
The team recruited children and adolescents enrolled in phase 1 safety or phase 2 efficacy trials at Children's National, Seattle Children's Hospital, Children's Hospital of Philadelphia, and Boston Children's Hospital.
Findings
Sixty percent of the participants were male and 70% were white.
Median age of the participants was 13.6 years: 7 (35%) were age 8 to 12, and 13 (65%) were 13 to 17.
Thirteen participants (65%) had solid tumors, 5 (25%) had brain tumors, and 2 (10%) had lymphoma.
A total of 29 patients were eligible to participate in the trial during 20 months of screening. Five parents and 2 patients declined to participate.
The remaining 22 patients who agreed to participate accounted for a 75.9% enrollment rate. Twenty of them (90.9%) participated at the first data time point, which was at the time of enrollment, and 77.3% participated 3 weeks later at time point 2.
The authors noted that refusals to enroll were more likely to come from parents (17.2%) than the eligible patients (8.3%).
And refusals only occurred when the self-report measures were not embedded in the clinical trial.
Of the 10 protocols represented, 7 patients were enrolled on the same protocol in which the PRO measures were embedded.
The researchers administered the 6-item short-form measures for the scales of Mobility, Pain, Fatigue, Depressive Symptoms, Anxiety, and Peer Relationships.
They asked the 4 open-ended questions—concerning QOL while receiving therapy and acceptability of the patient reporting—at time point 2.
At time point 1, 3 patients did not complete 3 PROMIS measures, for a person-missing rate of 15% and a measure-missing rate of 3.3%.
At the second time point, 2 patients did not complete 1 measure each, for a person-missing rate of 11.8% and a measure-missing rate of 2%.
All but one measure at time point 1 met the reliability criterion and all measures did so at time point 2.
The research team believes their findings support the feasibility and acceptability of completing quantitative and qualitative measures regarding symptom, function, and QOL experiences among children and adolescents with incurable cancer.
The researchers note the small study size and the number of parent refusals are limitations of the trial.
Nevertheless, they recommend embedding PROs in future pediatric oncology phase 1/2 trials. ![]()
Chemo-free triplet produces ‘favorable’ results in advanced disease
LUGANO, SWITZERLAND—A chemotherapy-free combination regimen has demonstrated “favorable” safety and efficacy in patients with advanced chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and non-Hodgkin lymphoma (NHL), according to researchers.
They found that treatment with ublituximab, umbralisib, and ibrutinib produced responses in patients with CLL/SLL, marginal zone lymphoma (MZL), mantle cell lymphoma (MCL), follicular lymphoma (FL), and diffuse large B-cell lymphoma (DLBCL).
Many of these patients are still receiving the combination, some of them beyond 1 year, said Lorretta Nastoupil, MD, of MD Anderson Cancer Center in Houston, Texas.
She presented results with the treatment at the 14th International Conference on Malignant Lymphoma (ICML).
The research was sponsored by TG Therapeutics, the company developing ublituximab (TG-1101) and umbralisib (TGR-1202).
Patients and treatment
Dr Nastoupil presented data on 38 patients—20 with CLL/SLL, and 18 with NHL. Three of the CLL/SLL patients were treatment-naïve. The rest had relapsed/refractory disease.
All NHL patients had relapsed/refractory disease—6 with DLBCL, 6 with FL, 4 with MCL, and 2 with MZL.
For the entire cohort, the median age was 65 (range, 32-85), and most patients (n=29) were male. They had received a median of 3 prior treatment regimens (range, 0-6).
In this trial, the patients received:
- Ublituximab at 900 mg
- Ibrutinib at 420 mg (CLL/SLL) or 560 mg (NHL)
- Umbralisib at 400 mg, 600 mg, or 800 mg.
Eighty-one percent of patients have been on study for more than 6 months. The median time on study is 11.1 months (range, 0.4 to 30+ months).
Safety
There was 1 dose-limiting toxicity in the CLL cohort (umbralisib at 400 mg)—reactivated varicella zoster. And 2 patients discontinued treatment due to an adverse event (AE)—1 due to sepsis and 1 due to pneumonia.
Neutropenia (18%) and pneumonia (11%) were the only grade 3/4 AEs that occurred in more than 10% of patients. Other grade 3/4 AEs included thrombocytopenia (8%), diarrhea (3%), dizziness (3%), pyrexia (3%), rash (3%), anemia (3%), dyspnea (3%), and stomatitis (3%).
The most common AEs of any grade were diarrhea (47%), fatigue (47%), dizziness (37%), insomnia (34%), nausea (34%), neutropenia (32%), cough (32%), and infusion-related reactions (32%).
Efficacy
Thirty-six patients were evaluable for efficacy—19 with CLL/SLL and 17 with NHL patients. Two patients discontinued treatment before the first efficacy assessment—1 due to pneumonia and 1 at investigator discretion.
For the entire cohort, the overall response rate (ORR) was 83%.
In the CLL/SLL cohort, the ORR was 100% (19/19), and the complete response (CR) rate was 32% (n=6). However, 4 of the 6 CRs are pending bone marrow confirmation.
Dr Nastoupil noted that 8 of the CLL patients had a 17p and/or 11q deletion, and 3 had previously received treatment with a BTK and/or PI3Kδ inhibitor.
One patient who was refractory to both idelalisib and ibrutinib achieved a CR with the triplet regimen, and this response has been ongoing for more than 1.5 years.
Among patients with NHL, the ORR was 100% in patients with MZL (2/2) and MCL (4/4). The ORR was 80% (4/5) in FL patients, and 17% (1/6) in DLBCL patients.
The CR rate was 50% in patients with MZL (1/2) and MCL (2/4) and 20% in patients with FL (1/5).
Dr Nastoupil pointed out that the FL patients were heavily pretreated. Two of them had received an autologous stem cell transplant, 1 was refractory to prior ibrutinib treatment, and 1 had received 5 prior lines of rituximab-based therapy.
She also noted that the DLBCL patients had a median of 4 prior therapies, and 4 of these patients had non-GCB DLBCL, including the only patient who responded to the triplet.
“[T]he combination of ublituximab, umbralisib, and ibrutinib in advanced CLL and NHL demonstrated a favorable toxicity profile as well as favorable efficacy,” Dr Nastoupil said in closing.
“[This] suggests umbralisib may be safely combined with other targeted agents to overcome mechanisms of resistance.” ![]()
LUGANO, SWITZERLAND—A chemotherapy-free combination regimen has demonstrated “favorable” safety and efficacy in patients with advanced chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and non-Hodgkin lymphoma (NHL), according to researchers.
They found that treatment with ublituximab, umbralisib, and ibrutinib produced responses in patients with CLL/SLL, marginal zone lymphoma (MZL), mantle cell lymphoma (MCL), follicular lymphoma (FL), and diffuse large B-cell lymphoma (DLBCL).
Many of these patients are still receiving the combination, some of them beyond 1 year, said Lorretta Nastoupil, MD, of MD Anderson Cancer Center in Houston, Texas.
She presented results with the treatment at the 14th International Conference on Malignant Lymphoma (ICML).
The research was sponsored by TG Therapeutics, the company developing ublituximab (TG-1101) and umbralisib (TGR-1202).
Patients and treatment
Dr Nastoupil presented data on 38 patients—20 with CLL/SLL, and 18 with NHL. Three of the CLL/SLL patients were treatment-naïve. The rest had relapsed/refractory disease.
All NHL patients had relapsed/refractory disease—6 with DLBCL, 6 with FL, 4 with MCL, and 2 with MZL.
For the entire cohort, the median age was 65 (range, 32-85), and most patients (n=29) were male. They had received a median of 3 prior treatment regimens (range, 0-6).
In this trial, the patients received:
- Ublituximab at 900 mg
- Ibrutinib at 420 mg (CLL/SLL) or 560 mg (NHL)
- Umbralisib at 400 mg, 600 mg, or 800 mg.
Eighty-one percent of patients have been on study for more than 6 months. The median time on study is 11.1 months (range, 0.4 to 30+ months).
Safety
There was 1 dose-limiting toxicity in the CLL cohort (umbralisib at 400 mg)—reactivated varicella zoster. And 2 patients discontinued treatment due to an adverse event (AE)—1 due to sepsis and 1 due to pneumonia.
Neutropenia (18%) and pneumonia (11%) were the only grade 3/4 AEs that occurred in more than 10% of patients. Other grade 3/4 AEs included thrombocytopenia (8%), diarrhea (3%), dizziness (3%), pyrexia (3%), rash (3%), anemia (3%), dyspnea (3%), and stomatitis (3%).
The most common AEs of any grade were diarrhea (47%), fatigue (47%), dizziness (37%), insomnia (34%), nausea (34%), neutropenia (32%), cough (32%), and infusion-related reactions (32%).
Efficacy
Thirty-six patients were evaluable for efficacy—19 with CLL/SLL and 17 with NHL patients. Two patients discontinued treatment before the first efficacy assessment—1 due to pneumonia and 1 at investigator discretion.
For the entire cohort, the overall response rate (ORR) was 83%.
In the CLL/SLL cohort, the ORR was 100% (19/19), and the complete response (CR) rate was 32% (n=6). However, 4 of the 6 CRs are pending bone marrow confirmation.
Dr Nastoupil noted that 8 of the CLL patients had a 17p and/or 11q deletion, and 3 had previously received treatment with a BTK and/or PI3Kδ inhibitor.
One patient who was refractory to both idelalisib and ibrutinib achieved a CR with the triplet regimen, and this response has been ongoing for more than 1.5 years.
Among patients with NHL, the ORR was 100% in patients with MZL (2/2) and MCL (4/4). The ORR was 80% (4/5) in FL patients, and 17% (1/6) in DLBCL patients.
The CR rate was 50% in patients with MZL (1/2) and MCL (2/4) and 20% in patients with FL (1/5).
Dr Nastoupil pointed out that the FL patients were heavily pretreated. Two of them had received an autologous stem cell transplant, 1 was refractory to prior ibrutinib treatment, and 1 had received 5 prior lines of rituximab-based therapy.
She also noted that the DLBCL patients had a median of 4 prior therapies, and 4 of these patients had non-GCB DLBCL, including the only patient who responded to the triplet.
“[T]he combination of ublituximab, umbralisib, and ibrutinib in advanced CLL and NHL demonstrated a favorable toxicity profile as well as favorable efficacy,” Dr Nastoupil said in closing.
“[This] suggests umbralisib may be safely combined with other targeted agents to overcome mechanisms of resistance.” ![]()
LUGANO, SWITZERLAND—A chemotherapy-free combination regimen has demonstrated “favorable” safety and efficacy in patients with advanced chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and non-Hodgkin lymphoma (NHL), according to researchers.
They found that treatment with ublituximab, umbralisib, and ibrutinib produced responses in patients with CLL/SLL, marginal zone lymphoma (MZL), mantle cell lymphoma (MCL), follicular lymphoma (FL), and diffuse large B-cell lymphoma (DLBCL).
Many of these patients are still receiving the combination, some of them beyond 1 year, said Lorretta Nastoupil, MD, of MD Anderson Cancer Center in Houston, Texas.
She presented results with the treatment at the 14th International Conference on Malignant Lymphoma (ICML).
The research was sponsored by TG Therapeutics, the company developing ublituximab (TG-1101) and umbralisib (TGR-1202).
Patients and treatment
Dr Nastoupil presented data on 38 patients—20 with CLL/SLL, and 18 with NHL. Three of the CLL/SLL patients were treatment-naïve. The rest had relapsed/refractory disease.
All NHL patients had relapsed/refractory disease—6 with DLBCL, 6 with FL, 4 with MCL, and 2 with MZL.
For the entire cohort, the median age was 65 (range, 32-85), and most patients (n=29) were male. They had received a median of 3 prior treatment regimens (range, 0-6).
In this trial, the patients received:
- Ublituximab at 900 mg
- Ibrutinib at 420 mg (CLL/SLL) or 560 mg (NHL)
- Umbralisib at 400 mg, 600 mg, or 800 mg.
Eighty-one percent of patients have been on study for more than 6 months. The median time on study is 11.1 months (range, 0.4 to 30+ months).
Safety
There was 1 dose-limiting toxicity in the CLL cohort (umbralisib at 400 mg)—reactivated varicella zoster. And 2 patients discontinued treatment due to an adverse event (AE)—1 due to sepsis and 1 due to pneumonia.
Neutropenia (18%) and pneumonia (11%) were the only grade 3/4 AEs that occurred in more than 10% of patients. Other grade 3/4 AEs included thrombocytopenia (8%), diarrhea (3%), dizziness (3%), pyrexia (3%), rash (3%), anemia (3%), dyspnea (3%), and stomatitis (3%).
The most common AEs of any grade were diarrhea (47%), fatigue (47%), dizziness (37%), insomnia (34%), nausea (34%), neutropenia (32%), cough (32%), and infusion-related reactions (32%).
Efficacy
Thirty-six patients were evaluable for efficacy—19 with CLL/SLL and 17 with NHL patients. Two patients discontinued treatment before the first efficacy assessment—1 due to pneumonia and 1 at investigator discretion.
For the entire cohort, the overall response rate (ORR) was 83%.
In the CLL/SLL cohort, the ORR was 100% (19/19), and the complete response (CR) rate was 32% (n=6). However, 4 of the 6 CRs are pending bone marrow confirmation.
Dr Nastoupil noted that 8 of the CLL patients had a 17p and/or 11q deletion, and 3 had previously received treatment with a BTK and/or PI3Kδ inhibitor.
One patient who was refractory to both idelalisib and ibrutinib achieved a CR with the triplet regimen, and this response has been ongoing for more than 1.5 years.
Among patients with NHL, the ORR was 100% in patients with MZL (2/2) and MCL (4/4). The ORR was 80% (4/5) in FL patients, and 17% (1/6) in DLBCL patients.
The CR rate was 50% in patients with MZL (1/2) and MCL (2/4) and 20% in patients with FL (1/5).
Dr Nastoupil pointed out that the FL patients were heavily pretreated. Two of them had received an autologous stem cell transplant, 1 was refractory to prior ibrutinib treatment, and 1 had received 5 prior lines of rituximab-based therapy.
She also noted that the DLBCL patients had a median of 4 prior therapies, and 4 of these patients had non-GCB DLBCL, including the only patient who responded to the triplet.
“[T]he combination of ublituximab, umbralisib, and ibrutinib in advanced CLL and NHL demonstrated a favorable toxicity profile as well as favorable efficacy,” Dr Nastoupil said in closing.
“[This] suggests umbralisib may be safely combined with other targeted agents to overcome mechanisms of resistance.” ![]()
Inhibitor elicits responses in heavily pretreated FL, DLBCL
LUGANO, SWITZERLAND—Interim results of a phase 2 trial suggest tazemetostat can be effective in patients with heavily pretreated, relapsed or refractory non-Hodgkin lymphoma.
The EZH2 inhibitor produced the highest overall response rate in patients with EZH2-mutated follicular lymphoma (FL), followed by EZH2-mutated diffuse large B-cell lymphoma (DLBCL).
However, the drug also produced complete responses in FL and DLBCL patients with wild-type EZH2.
“If we had focused [only] on patients with EZH2 mutations, we would have missed those other complete responders in the wild-type setting,” said study investigator Franck Morschhauser, MD, PhD, of Centre Hospitalier Régional Universitaire de Lille in France.
He presented results of the trial* during the plenary session of the 14th International Conference on Malignant Lymphoma (ICML). The research was sponsored by Epizyme, the company developing tazemetostat.
The trial enrolled patients with relapsed or refractory DLBCL or FL who had received at least 2 prior therapies. The patients received tazemetostat at 800 mg twice daily until disease progression or study withdrawal.
Efficacy in FL
Dr Morschhauser presented efficacy data on 67 patients with FL. Thirteen had EZH2 mutations, and 54 had wild-type EZH2. The median age was 62 in the mutated group and 61 in the wild-type group.
Both groups had a median of 4 prior lines of therapy. Fifty-four percent of EZH2-mutated patients were refractory to their last treatment, as were 48% of wild-type patients.
The median time from diagnosis was 7.4 years in mutated patients and 4.9 years in wild-type patients. The median time from last therapy was 13 weeks and 41.3 weeks, respectively.
The overall response rate was 92% (12/13) in EZH2-mutated patients and 26% (14/54) in wild-type patients. The complete response rates were 8% (n=1) and 6% (n=3), respectively.
The median time to first response was 11.9 weeks and 15.2 weeks, respectively.
None of the EZH2-mutated patients have progressed, but 13 (24%) wild-type patients have.
Forty-eight percent of all FL patients remain on study. One EZH2-mutated patient with stable disease is still on study, as are 23 wild-type patients with stable disease.
Efficacy in DLBCL
Dr Morschhauser presented data on 137 patients with DLBCL, 17 with EZH2 mutations and 120 with wild-type EZH2. The median age was 61 in the mutated group and 69 in the wild-type group.
Both groups had a median of 3 prior lines of therapy. Eighty-two percent of EZH2-mutated patients were refractory to their last treatment, as were 63% of wild-type patients.
The median time from diagnosis was 1 year in mutated patients and 2 years in wild-type patients. The median time from last therapy was 8.6 weeks and 11.6 weeks, respectively.
The overall response rate was 29% (5/17) in EZH2-mutated patients and 15% (18/119) in wild-type patients. The complete response rates were 0% (n=0) and 8% (n=10), respectively.
The median time to first response was 8.3 weeks and 8.5 weeks, respectively.
Six (35%) of the EZH2-mutated patients have progressed, as have 60 (50%) wild-type patients.
Twelve percent of all DLBCL patients remain on study. One EZH2-mutated patient with stable disease is still on therapy, as are 4 wild-type patients with stable disease.
Predictors of response
Dr Morschhauser and his colleagues performed next-generation sequencing of samples from 92 patients in an attempt to identify predictors of response to tazemetostat.
The data suggested that EZH2 and MYD88 activating mutations are positive predictors of response, and negative predictors include MYC, TP53, and HIST1H1E.
Safety
Safety data were available for 210 patients. The overall rate of treatment-related adverse events (AEs) was 59%, the rate of grade 3 or higher treatment-related AEs was 18%, and the rate of serious treatment-related AEs was 10%.
There were treatment-related AEs leading to dose interruption (15%), dose reduction (3%), and discontinuation of tazemetostat (2%).
The most common treatment-related AEs were nausea (14%), thrombocytopenia (13%), anemia (10%), neutropenia (9%), diarrhea (8%), asthenia (8%), and fatigue (7%).
Dr Morschhauser said these results “confirm that tazemetostat is quite safe” in this patient population, and enrollment in this trial is ongoing. ![]()
*Data in the abstract differ from the presentation.
LUGANO, SWITZERLAND—Interim results of a phase 2 trial suggest tazemetostat can be effective in patients with heavily pretreated, relapsed or refractory non-Hodgkin lymphoma.
The EZH2 inhibitor produced the highest overall response rate in patients with EZH2-mutated follicular lymphoma (FL), followed by EZH2-mutated diffuse large B-cell lymphoma (DLBCL).
However, the drug also produced complete responses in FL and DLBCL patients with wild-type EZH2.
“If we had focused [only] on patients with EZH2 mutations, we would have missed those other complete responders in the wild-type setting,” said study investigator Franck Morschhauser, MD, PhD, of Centre Hospitalier Régional Universitaire de Lille in France.
He presented results of the trial* during the plenary session of the 14th International Conference on Malignant Lymphoma (ICML). The research was sponsored by Epizyme, the company developing tazemetostat.
The trial enrolled patients with relapsed or refractory DLBCL or FL who had received at least 2 prior therapies. The patients received tazemetostat at 800 mg twice daily until disease progression or study withdrawal.
Efficacy in FL
Dr Morschhauser presented efficacy data on 67 patients with FL. Thirteen had EZH2 mutations, and 54 had wild-type EZH2. The median age was 62 in the mutated group and 61 in the wild-type group.
Both groups had a median of 4 prior lines of therapy. Fifty-four percent of EZH2-mutated patients were refractory to their last treatment, as were 48% of wild-type patients.
The median time from diagnosis was 7.4 years in mutated patients and 4.9 years in wild-type patients. The median time from last therapy was 13 weeks and 41.3 weeks, respectively.
The overall response rate was 92% (12/13) in EZH2-mutated patients and 26% (14/54) in wild-type patients. The complete response rates were 8% (n=1) and 6% (n=3), respectively.
The median time to first response was 11.9 weeks and 15.2 weeks, respectively.
None of the EZH2-mutated patients have progressed, but 13 (24%) wild-type patients have.
Forty-eight percent of all FL patients remain on study. One EZH2-mutated patient with stable disease is still on study, as are 23 wild-type patients with stable disease.
Efficacy in DLBCL
Dr Morschhauser presented data on 137 patients with DLBCL, 17 with EZH2 mutations and 120 with wild-type EZH2. The median age was 61 in the mutated group and 69 in the wild-type group.
Both groups had a median of 3 prior lines of therapy. Eighty-two percent of EZH2-mutated patients were refractory to their last treatment, as were 63% of wild-type patients.
The median time from diagnosis was 1 year in mutated patients and 2 years in wild-type patients. The median time from last therapy was 8.6 weeks and 11.6 weeks, respectively.
The overall response rate was 29% (5/17) in EZH2-mutated patients and 15% (18/119) in wild-type patients. The complete response rates were 0% (n=0) and 8% (n=10), respectively.
The median time to first response was 8.3 weeks and 8.5 weeks, respectively.
Six (35%) of the EZH2-mutated patients have progressed, as have 60 (50%) wild-type patients.
Twelve percent of all DLBCL patients remain on study. One EZH2-mutated patient with stable disease is still on therapy, as are 4 wild-type patients with stable disease.
Predictors of response
Dr Morschhauser and his colleagues performed next-generation sequencing of samples from 92 patients in an attempt to identify predictors of response to tazemetostat.
The data suggested that EZH2 and MYD88 activating mutations are positive predictors of response, and negative predictors include MYC, TP53, and HIST1H1E.
Safety
Safety data were available for 210 patients. The overall rate of treatment-related adverse events (AEs) was 59%, the rate of grade 3 or higher treatment-related AEs was 18%, and the rate of serious treatment-related AEs was 10%.
There were treatment-related AEs leading to dose interruption (15%), dose reduction (3%), and discontinuation of tazemetostat (2%).
The most common treatment-related AEs were nausea (14%), thrombocytopenia (13%), anemia (10%), neutropenia (9%), diarrhea (8%), asthenia (8%), and fatigue (7%).
Dr Morschhauser said these results “confirm that tazemetostat is quite safe” in this patient population, and enrollment in this trial is ongoing. ![]()
*Data in the abstract differ from the presentation.
LUGANO, SWITZERLAND—Interim results of a phase 2 trial suggest tazemetostat can be effective in patients with heavily pretreated, relapsed or refractory non-Hodgkin lymphoma.
The EZH2 inhibitor produced the highest overall response rate in patients with EZH2-mutated follicular lymphoma (FL), followed by EZH2-mutated diffuse large B-cell lymphoma (DLBCL).
However, the drug also produced complete responses in FL and DLBCL patients with wild-type EZH2.
“If we had focused [only] on patients with EZH2 mutations, we would have missed those other complete responders in the wild-type setting,” said study investigator Franck Morschhauser, MD, PhD, of Centre Hospitalier Régional Universitaire de Lille in France.
He presented results of the trial* during the plenary session of the 14th International Conference on Malignant Lymphoma (ICML). The research was sponsored by Epizyme, the company developing tazemetostat.
The trial enrolled patients with relapsed or refractory DLBCL or FL who had received at least 2 prior therapies. The patients received tazemetostat at 800 mg twice daily until disease progression or study withdrawal.
Efficacy in FL
Dr Morschhauser presented efficacy data on 67 patients with FL. Thirteen had EZH2 mutations, and 54 had wild-type EZH2. The median age was 62 in the mutated group and 61 in the wild-type group.
Both groups had a median of 4 prior lines of therapy. Fifty-four percent of EZH2-mutated patients were refractory to their last treatment, as were 48% of wild-type patients.
The median time from diagnosis was 7.4 years in mutated patients and 4.9 years in wild-type patients. The median time from last therapy was 13 weeks and 41.3 weeks, respectively.
The overall response rate was 92% (12/13) in EZH2-mutated patients and 26% (14/54) in wild-type patients. The complete response rates were 8% (n=1) and 6% (n=3), respectively.
The median time to first response was 11.9 weeks and 15.2 weeks, respectively.
None of the EZH2-mutated patients have progressed, but 13 (24%) wild-type patients have.
Forty-eight percent of all FL patients remain on study. One EZH2-mutated patient with stable disease is still on study, as are 23 wild-type patients with stable disease.
Efficacy in DLBCL
Dr Morschhauser presented data on 137 patients with DLBCL, 17 with EZH2 mutations and 120 with wild-type EZH2. The median age was 61 in the mutated group and 69 in the wild-type group.
Both groups had a median of 3 prior lines of therapy. Eighty-two percent of EZH2-mutated patients were refractory to their last treatment, as were 63% of wild-type patients.
The median time from diagnosis was 1 year in mutated patients and 2 years in wild-type patients. The median time from last therapy was 8.6 weeks and 11.6 weeks, respectively.
The overall response rate was 29% (5/17) in EZH2-mutated patients and 15% (18/119) in wild-type patients. The complete response rates were 0% (n=0) and 8% (n=10), respectively.
The median time to first response was 8.3 weeks and 8.5 weeks, respectively.
Six (35%) of the EZH2-mutated patients have progressed, as have 60 (50%) wild-type patients.
Twelve percent of all DLBCL patients remain on study. One EZH2-mutated patient with stable disease is still on therapy, as are 4 wild-type patients with stable disease.
Predictors of response
Dr Morschhauser and his colleagues performed next-generation sequencing of samples from 92 patients in an attempt to identify predictors of response to tazemetostat.
The data suggested that EZH2 and MYD88 activating mutations are positive predictors of response, and negative predictors include MYC, TP53, and HIST1H1E.
Safety
Safety data were available for 210 patients. The overall rate of treatment-related adverse events (AEs) was 59%, the rate of grade 3 or higher treatment-related AEs was 18%, and the rate of serious treatment-related AEs was 10%.
There were treatment-related AEs leading to dose interruption (15%), dose reduction (3%), and discontinuation of tazemetostat (2%).
The most common treatment-related AEs were nausea (14%), thrombocytopenia (13%), anemia (10%), neutropenia (9%), diarrhea (8%), asthenia (8%), and fatigue (7%).
Dr Morschhauser said these results “confirm that tazemetostat is quite safe” in this patient population, and enrollment in this trial is ongoing. ![]()
*Data in the abstract differ from the presentation.
New frontline treatments needed for Hodgkin lymphoma
In this editorial, Anna Sureda, MD, PhD, details the need for new frontline treatments for patients with Hodgkin lymphoma, including those with advanced stage disease.
Dr Sureda is head of the Hematology Department and Hematopoietic Stem Cell Transplant Programme at the Institut Català d'Oncologia, Hospital Duran i Reynals, in Barcelona, Spain. She has received consultancy fees from Takeda/Millennium Pharmaceuticals, Merck Sharp & Dohme, and Bristol-Myers Squibb.
Hodgkin lymphoma has traditionally been known as a cancer with generally favorable outcomes. Yet, as with any cancer treatment, there is always room for improvement. For Hodgkin lymphoma specifically, there remains a significant unmet need in the frontline setting for patients with advanced disease (Stage III or Stage IV).
Hodgkin lymphoma most commonly affects young adults as well as adults over the age of 55.1 Both age at diagnosis and stage of the disease are significant factors that must be considered when determining treatment plans, as they can affect a patient’s success in achieving long-term remission.
Though early stage patients have demonstrated 5-year survival rates of approximately 90%, this number drops to 70% in patients with advanced stage disease,2-4 underlining the challenges of treating later stage Hodgkin lymphoma.
Additionally, only 50% of patients with relapsed or refractory disease will experience long-term remission with high-dose chemotherapy and an autologous stem cell transplant (ASCT)5-6— a historically and frequently used treatment regimen.
These facts support the importance of successful frontline treatment and highlight a gap with current treatment regimens.7-10
With current frontline Hodgkin lymphoma treatments, it can be a challenge for physicians to balance efficacy with safety. While allowing the patient to achieve long-term remission remains the goal, physicians are also considering the impact of treatment-related side effects including endocrine dysfunction, cardiac dysfunction, lung toxicity, infertility, and an increased risk of secondary cancers when determining the best possible treatment.8-15
Advanced stage vs early stage Hodgkin lymphoma
Stage of disease at diagnosis has a large influence on outcomes, with advanced stage patients having poorer outcomes than earlier stage patients.7,15-16 Advanced Hodgkin lymphoma patients are more likely to progress or relapse,7,15-16 with nearly one third remaining uncured following standard frontline therapy.7-10
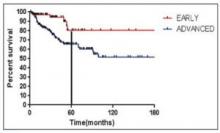
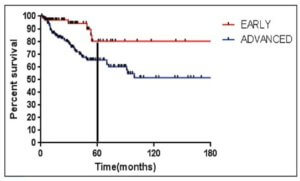
As seen in Figure 1 below, there is a clear difference in progression-free survival for early versus advanced stage Hodgkin lymphoma.16
The difference between early stage and advanced stage patients treated with doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) demonstrates the heightened importance of successful frontline treatment for those with advanced stage disease.16
Unmet needs with current frontline Hodgkin lymphoma treatment
Though current treatments for frontline Hodgkin lymphoma, including ABVD and bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP), have improved outcomes for patients, these standard regimens are more than 20 years old.
ABVD is generally regarded as the treatment of choice based on its efficacy, relative ease of administration, and side effect profile.17
Escalated BEACOPP, on the other hand, was developed to improve outcomes for advanced stage patients but is associated with increased toxicity.8-10,13,18
Positron emission tomography (PET) scans have also been identified as a pathway to help guide further treatment, but patients with advanced stage Hodgkin lymphoma may relapse more often, despite a negative interim PET scan, compared to stage II patients.19
Among current treatments, side effects including lung and cardiotoxicity as well as an increased risk of secondary cancers are a concern for both physicians and their patients.8-10,13-15
Similarly, radiation therapy, often used in conjunction with chemotherapy for patients who have a large tumor burden in one part of the body, usually the chest,20 is also associated with an increased risk of secondary cancers and cardiotoxicity.8-10,21
With these complications in mind, stabilizing the effects between improved efficacy and minimizing the toxicities associated with current frontline treatments needs to be a focus as new therapies are developed.
For young patients specifically, minimizing toxicities is crucial, as many will have a lifetime ahead of them after Hodgkin lymphoma and will want to avoid the risks associated with current treatments including lung disease, heart disease and infertility.8-10,12-15,22
Treating elderly patients can also be challenging due to their reduced ability to tolerate aggressive frontline treatment and multi-agent chemotherapy, which causes inferior survival outcomes when compared to younger patients.23-25 These secondary effects can affect a patient’s quality of life8-9,12,14-15,22,26-28 and exacerbate preexisting conditions commonly experienced by those undergoing treatment, including long-term fatigue, chronic medical and psychosocial complications, and general deterioration in physical well-being.22
Studies have shown that most relapses after ASCT typically occur within 2 years.29 After a relapse, the patient may endure a substantial physical and psychological burden due to the need for additional treatment, impacting quality of life for both the patient and their caregiver.22,26,30
Goals of clinical research
Despite its recognition as a highly treatable cancer, newly diagnosed Hodgkin lymphoma remains incurable in up to 30% of patients with advanced disease.7-10 Though current therapies seek to achieve remission and extend the lives of patients, it is often at the cost of treatment-related toxicities and side effects that can significantly reduce quality of life.
Moving forward, it is critical that these gaps in treatment are addressed in new frontline treatments that aim to benefit patients, including those with advanced stage disease, while reducing short-term and long-term toxicities. ![]()
Acknowledgements: The author would like to acknowledge the W2O Group for their writing support, which was funded by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
______________________________________________________
1American Cancer Society. What Are the Key Statistics About Hodgkin Disease? https://www.cancer.org/cancer/hodgkin-lymphoma/about/key-statistics.html. Accessed February 16, 2017.
2Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J (editors). SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215, Bethesda, MD, 2007.
3American Cancer Society. Survival Rates for Hodgkin Disease by Stage. https://www.cancer.org/cancer/hodgkin-lymphoma/detection-diagnosis-staging/survival-rates.html. Accessed February 16, 2017.
4Fermé C, et al. New Engl J Med, 2007.357:1916–27.
5Sureda A, et al. Ann Oncol, 2005;16: 625–633.
6Majhail NS, et al. Biol Blood Marrow Transplant, 2006;12:1065–1072.
7Gordon LI, et al. J Clin Oncol, 2013;31:684-691.
8Carde P, et al. J Clin Oncol, 2016;34(17):2028-2036.
9Engert A, et al. J Clin Oncol, 2009;27(27):4548-4554
10Viviani S, et al. New Engl J Med, 2011;365(3):203-212.
11Sklar C, et al. J Endocrinology & Metabolism, 2000;85(9):3227-3232
12Behringer K, et al. J Clin Oncol, 2013;31:231-239.
13Borchmann P, et al. J Clin Oncol, 2011;29(32):4234-4242.
14Duggan DB, et al. J Clin Oncol, 2003;21(4):607-614.
15Johnson P, McKenzie H. Blood, 2015;125(11):1717-1723.
16Maddi RN, et al. Indian J Medical and Paediatric Oncology, 2015;36(4):255-260
17Ansell SM. American Journal of Hematology, 2014;89: 771–779.
18Merli F, et al. J Clin Oncol, 34:1175-1181.
19Johnson P, et al. N Engl J Med. 2016;374:2419‑2429
20American Cancer Society. Treating Hodgkin Disease: Radiation Therapy for Hodgkin Disease. https://www.cancer.org/cancer/hodgkin-lymphoma/treating/radiation.html. Accessed January 30, 2017.
21Adams MJ, et al. J Clin Oncol, 2004; 22: 3139–48.
22Khimani N, et al. Ann Oncol, 2013;24(1):226-230.
23Engert A, et al. J Clin Oncol, 2005;23(22):5052-60.
24Evens AM, et al. Br J Haematol, 2013;161: 76–86.
25Janssen-Heijnen ML, et al. Br J Haematol, 2005;129:597-606.
26Ganz PA et al. J Clin Oncol, 2003;21(18):3512-3519.
27Daniels LA, et al. Br J Cancer 2014;110:868-874.
28Loge JH, et al. Ann Oncol. 1999;10:71-77.
29Brusamolino E, Carella AM. Haematologica, 2007;92:6-10
30Consolidation Therapy After ASCT in Hodgkin Lymphoma: Why and Who to Treat? Personalized Medicine in Oncology, 2016. http://www.personalizedmedonc.com/article/consolidation-therapy-after-asct-in-hodgkin-lymphoma-why-and-who-to-treat/. Accessed February 16, 2017.
In this editorial, Anna Sureda, MD, PhD, details the need for new frontline treatments for patients with Hodgkin lymphoma, including those with advanced stage disease.
Dr Sureda is head of the Hematology Department and Hematopoietic Stem Cell Transplant Programme at the Institut Català d'Oncologia, Hospital Duran i Reynals, in Barcelona, Spain. She has received consultancy fees from Takeda/Millennium Pharmaceuticals, Merck Sharp & Dohme, and Bristol-Myers Squibb.
Hodgkin lymphoma has traditionally been known as a cancer with generally favorable outcomes. Yet, as with any cancer treatment, there is always room for improvement. For Hodgkin lymphoma specifically, there remains a significant unmet need in the frontline setting for patients with advanced disease (Stage III or Stage IV).
Hodgkin lymphoma most commonly affects young adults as well as adults over the age of 55.1 Both age at diagnosis and stage of the disease are significant factors that must be considered when determining treatment plans, as they can affect a patient’s success in achieving long-term remission.
Though early stage patients have demonstrated 5-year survival rates of approximately 90%, this number drops to 70% in patients with advanced stage disease,2-4 underlining the challenges of treating later stage Hodgkin lymphoma.
Additionally, only 50% of patients with relapsed or refractory disease will experience long-term remission with high-dose chemotherapy and an autologous stem cell transplant (ASCT)5-6— a historically and frequently used treatment regimen.
These facts support the importance of successful frontline treatment and highlight a gap with current treatment regimens.7-10
With current frontline Hodgkin lymphoma treatments, it can be a challenge for physicians to balance efficacy with safety. While allowing the patient to achieve long-term remission remains the goal, physicians are also considering the impact of treatment-related side effects including endocrine dysfunction, cardiac dysfunction, lung toxicity, infertility, and an increased risk of secondary cancers when determining the best possible treatment.8-15
Advanced stage vs early stage Hodgkin lymphoma
Stage of disease at diagnosis has a large influence on outcomes, with advanced stage patients having poorer outcomes than earlier stage patients.7,15-16 Advanced Hodgkin lymphoma patients are more likely to progress or relapse,7,15-16 with nearly one third remaining uncured following standard frontline therapy.7-10
As seen in Figure 1 below, there is a clear difference in progression-free survival for early versus advanced stage Hodgkin lymphoma.16
The difference between early stage and advanced stage patients treated with doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) demonstrates the heightened importance of successful frontline treatment for those with advanced stage disease.16
Unmet needs with current frontline Hodgkin lymphoma treatment
Though current treatments for frontline Hodgkin lymphoma, including ABVD and bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP), have improved outcomes for patients, these standard regimens are more than 20 years old.
ABVD is generally regarded as the treatment of choice based on its efficacy, relative ease of administration, and side effect profile.17
Escalated BEACOPP, on the other hand, was developed to improve outcomes for advanced stage patients but is associated with increased toxicity.8-10,13,18
Positron emission tomography (PET) scans have also been identified as a pathway to help guide further treatment, but patients with advanced stage Hodgkin lymphoma may relapse more often, despite a negative interim PET scan, compared to stage II patients.19
Among current treatments, side effects including lung and cardiotoxicity as well as an increased risk of secondary cancers are a concern for both physicians and their patients.8-10,13-15
Similarly, radiation therapy, often used in conjunction with chemotherapy for patients who have a large tumor burden in one part of the body, usually the chest,20 is also associated with an increased risk of secondary cancers and cardiotoxicity.8-10,21
With these complications in mind, stabilizing the effects between improved efficacy and minimizing the toxicities associated with current frontline treatments needs to be a focus as new therapies are developed.
For young patients specifically, minimizing toxicities is crucial, as many will have a lifetime ahead of them after Hodgkin lymphoma and will want to avoid the risks associated with current treatments including lung disease, heart disease and infertility.8-10,12-15,22
Treating elderly patients can also be challenging due to their reduced ability to tolerate aggressive frontline treatment and multi-agent chemotherapy, which causes inferior survival outcomes when compared to younger patients.23-25 These secondary effects can affect a patient’s quality of life8-9,12,14-15,22,26-28 and exacerbate preexisting conditions commonly experienced by those undergoing treatment, including long-term fatigue, chronic medical and psychosocial complications, and general deterioration in physical well-being.22
Studies have shown that most relapses after ASCT typically occur within 2 years.29 After a relapse, the patient may endure a substantial physical and psychological burden due to the need for additional treatment, impacting quality of life for both the patient and their caregiver.22,26,30
Goals of clinical research
Despite its recognition as a highly treatable cancer, newly diagnosed Hodgkin lymphoma remains incurable in up to 30% of patients with advanced disease.7-10 Though current therapies seek to achieve remission and extend the lives of patients, it is often at the cost of treatment-related toxicities and side effects that can significantly reduce quality of life.
Moving forward, it is critical that these gaps in treatment are addressed in new frontline treatments that aim to benefit patients, including those with advanced stage disease, while reducing short-term and long-term toxicities. ![]()
Acknowledgements: The author would like to acknowledge the W2O Group for their writing support, which was funded by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
______________________________________________________
1American Cancer Society. What Are the Key Statistics About Hodgkin Disease? https://www.cancer.org/cancer/hodgkin-lymphoma/about/key-statistics.html. Accessed February 16, 2017.
2Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J (editors). SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215, Bethesda, MD, 2007.
3American Cancer Society. Survival Rates for Hodgkin Disease by Stage. https://www.cancer.org/cancer/hodgkin-lymphoma/detection-diagnosis-staging/survival-rates.html. Accessed February 16, 2017.
4Fermé C, et al. New Engl J Med, 2007.357:1916–27.
5Sureda A, et al. Ann Oncol, 2005;16: 625–633.
6Majhail NS, et al. Biol Blood Marrow Transplant, 2006;12:1065–1072.
7Gordon LI, et al. J Clin Oncol, 2013;31:684-691.
8Carde P, et al. J Clin Oncol, 2016;34(17):2028-2036.
9Engert A, et al. J Clin Oncol, 2009;27(27):4548-4554
10Viviani S, et al. New Engl J Med, 2011;365(3):203-212.
11Sklar C, et al. J Endocrinology & Metabolism, 2000;85(9):3227-3232
12Behringer K, et al. J Clin Oncol, 2013;31:231-239.
13Borchmann P, et al. J Clin Oncol, 2011;29(32):4234-4242.
14Duggan DB, et al. J Clin Oncol, 2003;21(4):607-614.
15Johnson P, McKenzie H. Blood, 2015;125(11):1717-1723.
16Maddi RN, et al. Indian J Medical and Paediatric Oncology, 2015;36(4):255-260
17Ansell SM. American Journal of Hematology, 2014;89: 771–779.
18Merli F, et al. J Clin Oncol, 34:1175-1181.
19Johnson P, et al. N Engl J Med. 2016;374:2419‑2429
20American Cancer Society. Treating Hodgkin Disease: Radiation Therapy for Hodgkin Disease. https://www.cancer.org/cancer/hodgkin-lymphoma/treating/radiation.html. Accessed January 30, 2017.
21Adams MJ, et al. J Clin Oncol, 2004; 22: 3139–48.
22Khimani N, et al. Ann Oncol, 2013;24(1):226-230.
23Engert A, et al. J Clin Oncol, 2005;23(22):5052-60.
24Evens AM, et al. Br J Haematol, 2013;161: 76–86.
25Janssen-Heijnen ML, et al. Br J Haematol, 2005;129:597-606.
26Ganz PA et al. J Clin Oncol, 2003;21(18):3512-3519.
27Daniels LA, et al. Br J Cancer 2014;110:868-874.
28Loge JH, et al. Ann Oncol. 1999;10:71-77.
29Brusamolino E, Carella AM. Haematologica, 2007;92:6-10
30Consolidation Therapy After ASCT in Hodgkin Lymphoma: Why and Who to Treat? Personalized Medicine in Oncology, 2016. http://www.personalizedmedonc.com/article/consolidation-therapy-after-asct-in-hodgkin-lymphoma-why-and-who-to-treat/. Accessed February 16, 2017.
In this editorial, Anna Sureda, MD, PhD, details the need for new frontline treatments for patients with Hodgkin lymphoma, including those with advanced stage disease.
Dr Sureda is head of the Hematology Department and Hematopoietic Stem Cell Transplant Programme at the Institut Català d'Oncologia, Hospital Duran i Reynals, in Barcelona, Spain. She has received consultancy fees from Takeda/Millennium Pharmaceuticals, Merck Sharp & Dohme, and Bristol-Myers Squibb.
Hodgkin lymphoma has traditionally been known as a cancer with generally favorable outcomes. Yet, as with any cancer treatment, there is always room for improvement. For Hodgkin lymphoma specifically, there remains a significant unmet need in the frontline setting for patients with advanced disease (Stage III or Stage IV).
Hodgkin lymphoma most commonly affects young adults as well as adults over the age of 55.1 Both age at diagnosis and stage of the disease are significant factors that must be considered when determining treatment plans, as they can affect a patient’s success in achieving long-term remission.
Though early stage patients have demonstrated 5-year survival rates of approximately 90%, this number drops to 70% in patients with advanced stage disease,2-4 underlining the challenges of treating later stage Hodgkin lymphoma.
Additionally, only 50% of patients with relapsed or refractory disease will experience long-term remission with high-dose chemotherapy and an autologous stem cell transplant (ASCT)5-6— a historically and frequently used treatment regimen.
These facts support the importance of successful frontline treatment and highlight a gap with current treatment regimens.7-10
With current frontline Hodgkin lymphoma treatments, it can be a challenge for physicians to balance efficacy with safety. While allowing the patient to achieve long-term remission remains the goal, physicians are also considering the impact of treatment-related side effects including endocrine dysfunction, cardiac dysfunction, lung toxicity, infertility, and an increased risk of secondary cancers when determining the best possible treatment.8-15
Advanced stage vs early stage Hodgkin lymphoma
Stage of disease at diagnosis has a large influence on outcomes, with advanced stage patients having poorer outcomes than earlier stage patients.7,15-16 Advanced Hodgkin lymphoma patients are more likely to progress or relapse,7,15-16 with nearly one third remaining uncured following standard frontline therapy.7-10
As seen in Figure 1 below, there is a clear difference in progression-free survival for early versus advanced stage Hodgkin lymphoma.16
The difference between early stage and advanced stage patients treated with doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) demonstrates the heightened importance of successful frontline treatment for those with advanced stage disease.16
Unmet needs with current frontline Hodgkin lymphoma treatment
Though current treatments for frontline Hodgkin lymphoma, including ABVD and bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP), have improved outcomes for patients, these standard regimens are more than 20 years old.
ABVD is generally regarded as the treatment of choice based on its efficacy, relative ease of administration, and side effect profile.17
Escalated BEACOPP, on the other hand, was developed to improve outcomes for advanced stage patients but is associated with increased toxicity.8-10,13,18
Positron emission tomography (PET) scans have also been identified as a pathway to help guide further treatment, but patients with advanced stage Hodgkin lymphoma may relapse more often, despite a negative interim PET scan, compared to stage II patients.19
Among current treatments, side effects including lung and cardiotoxicity as well as an increased risk of secondary cancers are a concern for both physicians and their patients.8-10,13-15
Similarly, radiation therapy, often used in conjunction with chemotherapy for patients who have a large tumor burden in one part of the body, usually the chest,20 is also associated with an increased risk of secondary cancers and cardiotoxicity.8-10,21
With these complications in mind, stabilizing the effects between improved efficacy and minimizing the toxicities associated with current frontline treatments needs to be a focus as new therapies are developed.
For young patients specifically, minimizing toxicities is crucial, as many will have a lifetime ahead of them after Hodgkin lymphoma and will want to avoid the risks associated with current treatments including lung disease, heart disease and infertility.8-10,12-15,22
Treating elderly patients can also be challenging due to their reduced ability to tolerate aggressive frontline treatment and multi-agent chemotherapy, which causes inferior survival outcomes when compared to younger patients.23-25 These secondary effects can affect a patient’s quality of life8-9,12,14-15,22,26-28 and exacerbate preexisting conditions commonly experienced by those undergoing treatment, including long-term fatigue, chronic medical and psychosocial complications, and general deterioration in physical well-being.22
Studies have shown that most relapses after ASCT typically occur within 2 years.29 After a relapse, the patient may endure a substantial physical and psychological burden due to the need for additional treatment, impacting quality of life for both the patient and their caregiver.22,26,30
Goals of clinical research
Despite its recognition as a highly treatable cancer, newly diagnosed Hodgkin lymphoma remains incurable in up to 30% of patients with advanced disease.7-10 Though current therapies seek to achieve remission and extend the lives of patients, it is often at the cost of treatment-related toxicities and side effects that can significantly reduce quality of life.
Moving forward, it is critical that these gaps in treatment are addressed in new frontline treatments that aim to benefit patients, including those with advanced stage disease, while reducing short-term and long-term toxicities. ![]()
Acknowledgements: The author would like to acknowledge the W2O Group for their writing support, which was funded by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
______________________________________________________
1American Cancer Society. What Are the Key Statistics About Hodgkin Disease? https://www.cancer.org/cancer/hodgkin-lymphoma/about/key-statistics.html. Accessed February 16, 2017.
2Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J (editors). SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215, Bethesda, MD, 2007.
3American Cancer Society. Survival Rates for Hodgkin Disease by Stage. https://www.cancer.org/cancer/hodgkin-lymphoma/detection-diagnosis-staging/survival-rates.html. Accessed February 16, 2017.
4Fermé C, et al. New Engl J Med, 2007.357:1916–27.
5Sureda A, et al. Ann Oncol, 2005;16: 625–633.
6Majhail NS, et al. Biol Blood Marrow Transplant, 2006;12:1065–1072.
7Gordon LI, et al. J Clin Oncol, 2013;31:684-691.
8Carde P, et al. J Clin Oncol, 2016;34(17):2028-2036.
9Engert A, et al. J Clin Oncol, 2009;27(27):4548-4554
10Viviani S, et al. New Engl J Med, 2011;365(3):203-212.
11Sklar C, et al. J Endocrinology & Metabolism, 2000;85(9):3227-3232
12Behringer K, et al. J Clin Oncol, 2013;31:231-239.
13Borchmann P, et al. J Clin Oncol, 2011;29(32):4234-4242.
14Duggan DB, et al. J Clin Oncol, 2003;21(4):607-614.
15Johnson P, McKenzie H. Blood, 2015;125(11):1717-1723.
16Maddi RN, et al. Indian J Medical and Paediatric Oncology, 2015;36(4):255-260
17Ansell SM. American Journal of Hematology, 2014;89: 771–779.
18Merli F, et al. J Clin Oncol, 34:1175-1181.
19Johnson P, et al. N Engl J Med. 2016;374:2419‑2429
20American Cancer Society. Treating Hodgkin Disease: Radiation Therapy for Hodgkin Disease. https://www.cancer.org/cancer/hodgkin-lymphoma/treating/radiation.html. Accessed January 30, 2017.
21Adams MJ, et al. J Clin Oncol, 2004; 22: 3139–48.
22Khimani N, et al. Ann Oncol, 2013;24(1):226-230.
23Engert A, et al. J Clin Oncol, 2005;23(22):5052-60.
24Evens AM, et al. Br J Haematol, 2013;161: 76–86.
25Janssen-Heijnen ML, et al. Br J Haematol, 2005;129:597-606.
26Ganz PA et al. J Clin Oncol, 2003;21(18):3512-3519.
27Daniels LA, et al. Br J Cancer 2014;110:868-874.
28Loge JH, et al. Ann Oncol. 1999;10:71-77.
29Brusamolino E, Carella AM. Haematologica, 2007;92:6-10
30Consolidation Therapy After ASCT in Hodgkin Lymphoma: Why and Who to Treat? Personalized Medicine in Oncology, 2016. http://www.personalizedmedonc.com/article/consolidation-therapy-after-asct-in-hodgkin-lymphoma-why-and-who-to-treat/. Accessed February 16, 2017.
Tafenoquine reduces relapse risk in patients with P vivax malaria
A single dose of tafenoquine, when given with chloroquine, can significantly reduce the risk of relapse in patients with Plasmodium vivax malaria compared to placebo, as demonstrated by the results of 2 phase 3 studies.
Patients who have had P vivax malaria, even a single infection, can relapse several weeks or even years after the initial occurrence.
Results of the studies were presented at the 6th International Conference on Plasmodium vivax Research (ICPVR) in Manaus, Brazil.
Tafenoquine, an 8-aminoquinoline derivative, is being developed by GlaxoSmithKline (GSK) in collaboration with Medicines for Malaria Venture (MMV).
“One of the greatest challenges for patients with P vivax malaria,” Patrick Vallance, of GSK, said, “is preventing relapses.”
“Being able to treat patients with a single dose of medicine would be an important step forward in ensuring efficacious treatment,” Vallance noted, “thereby reducing the risk of relapse, particularly in areas with very limited healthcare infrastructure.”
The DETECTIVE and GATHER studies were conducted in malaria-endemic countries in South America, Asia, and Africa.
DETECTIVE (TAF112582) study
This was a double-blind, double-dummy phase 3 study evaluating the efficacy, safety, and tolerability of tafenoquine in 522 patients aged 16 years or older with P vivax malaria.
Patients were randomized to receive placebo, a single dose of 300 mg tafenoquine, or primaquine at 15 mg daily for 14 days in a 1:2:1 ratio.
All patients received a 3-day course of chloroquine to treat the acute blood stage infection.
The study met its primary endpoint. A statistically significant greater proportion of patients treated with tafenoquine (60%) remained relapse-free over the 6-month follow-up period than patients on placebo (26%). The odds ratio for risk of relapse vs placebo given with chloroquine was 0.24, P<0.001.
Treatment with 14 days of primaquine also achieved a statistically significant improvement in relapse-free follow-up, with an odds ratio vs placebo when given with chloroquine of 0.20, P<0.001.
The frequency of adverse events was 63% for the tafenoquine group, 59% for the primaquine group, and 65% for the chloroquine group.
And the frequency of serious adverse events was 8% for the tafenoquine group, 3% for the primaquine group, and 5% for the chloroquine group.
GATHER (TAF116564) study
This study evaluated a single dose of 300 mg tafenoquine on hemoglobin levels compared to a 14-day course of 15 mg primaquine.
Agents in the 8-aminoquinoline drug class are associated with hemolytic anemia in individuals with inherited glucose-6-phosphate dehydrogenase (G6PD) deficiency.
All patients were screened for G6PD deficiency at baseline. Males with G6PD activity of at least 70% of the site median and females with 40% were eligible.
All 251 patients also received the standard 3-day course of chloroquine.
The incidence of decline in hemoglobin was low and similar between the 2 treatment arms, 2.4% for the tafenoquine group and 1.2% for the chloroquine group.
No patient required a blood transfusion.
The companies plan to develop a point-of-care diagnostic for G6PD as a mandatory test prior to treatment with tafenoquine.
“The positive results of the phase 3 trials for single-dose tafenoquine provide great hope that a new, effective drug to stop the relapse of P vivax malaria is in sight,” David Reddy, of MMV, affirmed.
Tafenoquine currently is not approved for use anywhere in the world. GSK plans to submit regulatory filings later in 2017. ![]()
A single dose of tafenoquine, when given with chloroquine, can significantly reduce the risk of relapse in patients with Plasmodium vivax malaria compared to placebo, as demonstrated by the results of 2 phase 3 studies.
Patients who have had P vivax malaria, even a single infection, can relapse several weeks or even years after the initial occurrence.
Results of the studies were presented at the 6th International Conference on Plasmodium vivax Research (ICPVR) in Manaus, Brazil.
Tafenoquine, an 8-aminoquinoline derivative, is being developed by GlaxoSmithKline (GSK) in collaboration with Medicines for Malaria Venture (MMV).
“One of the greatest challenges for patients with P vivax malaria,” Patrick Vallance, of GSK, said, “is preventing relapses.”
“Being able to treat patients with a single dose of medicine would be an important step forward in ensuring efficacious treatment,” Vallance noted, “thereby reducing the risk of relapse, particularly in areas with very limited healthcare infrastructure.”
The DETECTIVE and GATHER studies were conducted in malaria-endemic countries in South America, Asia, and Africa.
DETECTIVE (TAF112582) study
This was a double-blind, double-dummy phase 3 study evaluating the efficacy, safety, and tolerability of tafenoquine in 522 patients aged 16 years or older with P vivax malaria.
Patients were randomized to receive placebo, a single dose of 300 mg tafenoquine, or primaquine at 15 mg daily for 14 days in a 1:2:1 ratio.
All patients received a 3-day course of chloroquine to treat the acute blood stage infection.
The study met its primary endpoint. A statistically significant greater proportion of patients treated with tafenoquine (60%) remained relapse-free over the 6-month follow-up period than patients on placebo (26%). The odds ratio for risk of relapse vs placebo given with chloroquine was 0.24, P<0.001.
Treatment with 14 days of primaquine also achieved a statistically significant improvement in relapse-free follow-up, with an odds ratio vs placebo when given with chloroquine of 0.20, P<0.001.
The frequency of adverse events was 63% for the tafenoquine group, 59% for the primaquine group, and 65% for the chloroquine group.
And the frequency of serious adverse events was 8% for the tafenoquine group, 3% for the primaquine group, and 5% for the chloroquine group.
GATHER (TAF116564) study
This study evaluated a single dose of 300 mg tafenoquine on hemoglobin levels compared to a 14-day course of 15 mg primaquine.
Agents in the 8-aminoquinoline drug class are associated with hemolytic anemia in individuals with inherited glucose-6-phosphate dehydrogenase (G6PD) deficiency.
All patients were screened for G6PD deficiency at baseline. Males with G6PD activity of at least 70% of the site median and females with 40% were eligible.
All 251 patients also received the standard 3-day course of chloroquine.
The incidence of decline in hemoglobin was low and similar between the 2 treatment arms, 2.4% for the tafenoquine group and 1.2% for the chloroquine group.
No patient required a blood transfusion.
The companies plan to develop a point-of-care diagnostic for G6PD as a mandatory test prior to treatment with tafenoquine.
“The positive results of the phase 3 trials for single-dose tafenoquine provide great hope that a new, effective drug to stop the relapse of P vivax malaria is in sight,” David Reddy, of MMV, affirmed.
Tafenoquine currently is not approved for use anywhere in the world. GSK plans to submit regulatory filings later in 2017. ![]()
A single dose of tafenoquine, when given with chloroquine, can significantly reduce the risk of relapse in patients with Plasmodium vivax malaria compared to placebo, as demonstrated by the results of 2 phase 3 studies.
Patients who have had P vivax malaria, even a single infection, can relapse several weeks or even years after the initial occurrence.
Results of the studies were presented at the 6th International Conference on Plasmodium vivax Research (ICPVR) in Manaus, Brazil.
Tafenoquine, an 8-aminoquinoline derivative, is being developed by GlaxoSmithKline (GSK) in collaboration with Medicines for Malaria Venture (MMV).
“One of the greatest challenges for patients with P vivax malaria,” Patrick Vallance, of GSK, said, “is preventing relapses.”
“Being able to treat patients with a single dose of medicine would be an important step forward in ensuring efficacious treatment,” Vallance noted, “thereby reducing the risk of relapse, particularly in areas with very limited healthcare infrastructure.”
The DETECTIVE and GATHER studies were conducted in malaria-endemic countries in South America, Asia, and Africa.
DETECTIVE (TAF112582) study
This was a double-blind, double-dummy phase 3 study evaluating the efficacy, safety, and tolerability of tafenoquine in 522 patients aged 16 years or older with P vivax malaria.
Patients were randomized to receive placebo, a single dose of 300 mg tafenoquine, or primaquine at 15 mg daily for 14 days in a 1:2:1 ratio.
All patients received a 3-day course of chloroquine to treat the acute blood stage infection.
The study met its primary endpoint. A statistically significant greater proportion of patients treated with tafenoquine (60%) remained relapse-free over the 6-month follow-up period than patients on placebo (26%). The odds ratio for risk of relapse vs placebo given with chloroquine was 0.24, P<0.001.
Treatment with 14 days of primaquine also achieved a statistically significant improvement in relapse-free follow-up, with an odds ratio vs placebo when given with chloroquine of 0.20, P<0.001.
The frequency of adverse events was 63% for the tafenoquine group, 59% for the primaquine group, and 65% for the chloroquine group.
And the frequency of serious adverse events was 8% for the tafenoquine group, 3% for the primaquine group, and 5% for the chloroquine group.
GATHER (TAF116564) study
This study evaluated a single dose of 300 mg tafenoquine on hemoglobin levels compared to a 14-day course of 15 mg primaquine.
Agents in the 8-aminoquinoline drug class are associated with hemolytic anemia in individuals with inherited glucose-6-phosphate dehydrogenase (G6PD) deficiency.
All patients were screened for G6PD deficiency at baseline. Males with G6PD activity of at least 70% of the site median and females with 40% were eligible.
All 251 patients also received the standard 3-day course of chloroquine.
The incidence of decline in hemoglobin was low and similar between the 2 treatment arms, 2.4% for the tafenoquine group and 1.2% for the chloroquine group.
No patient required a blood transfusion.
The companies plan to develop a point-of-care diagnostic for G6PD as a mandatory test prior to treatment with tafenoquine.
“The positive results of the phase 3 trials for single-dose tafenoquine provide great hope that a new, effective drug to stop the relapse of P vivax malaria is in sight,” David Reddy, of MMV, affirmed.
Tafenoquine currently is not approved for use anywhere in the world. GSK plans to submit regulatory filings later in 2017.
Fostamatinib under review by FDA for chronic ITP
The US Food and Drug Administration (FDA) has accepted a new drug application (NDA) for the use of fostamatinib disodium (Tavalisse™) in the treatment of patients with chronic/persistent immune thrombocytopenia (ITP).
Fostamatinib is an oral spleen tyrosine kinase (SYK) inhibitor that investigators believe may address an underlying autoimmune cause of ITP by impeding platelet destruction.
Rigel Pharmaceuticals, the drug’s developer, anticipates an action date by the FDA of April 17, 2018.
The FDA previously granted fostamatinib orphan drug designation.
Data from 3 clinical studies support the new drug application. Study 047 and 048 were randomized placebo-controlled trials, and study 049 was an open-label extension study.
Together with an initial proof of concept study, the application included data on 163 ITP patients. Fostamatinib has been evaluated in over 4,600 individuals across all indications.
The patients enrolled in studies 047 and 048 had been diagnosed with persistent or chronic ITP, had failed at least 1 prior therapy for ITP, and had platelet counts consistently below 30,000/uL.
In both studies, patients were randomized in a 2:1 ratio to receive either fostamatinib or placebo orally twice a day for up to 24 weeks.
Study 047
In this study, 51 patients were randomized to fostamatinib and 25 to placebo.
The median platelet counts at baseline were 15,000/uL and 16,000/uL in the fostamatinib and placebo arms, respectively.
The median age was 57 in both treatment arms. The duration of ITP was 7.5 years (range, 0.6-53) in the fostamatinib arm and 5.5 years (range, 0.4-45) in the placebo arm.
The primary efficacy endpoint in both study 047 and 048 was a stable platelet response, defined as achieving platelet counts greater than 50,000/uL for at least 4 of the 6 scheduled clinic visits between weeks 14 and 24 of treatment.
In study 047, the rate of stable platelet response was significantly higher in the fostamatinib arm than the placebo arm—18% (n=9) and 0%, respectively (P=0.026).
Study 048
Fifty patients were randomized to fostamatinib and 24 to placebo.
Patients’ median platelet counts at baseline were 16,000/uL and 21,000/uL in the fostamatinib and placebo arms, respectively.
The median age was 50 in both treatment arms. The duration of ITP was 8.8 years (range, 0.3-50) in the fostamatinib arm and 10.8 years (range, 0.9-29) in the placebo arm.
In this study, however, the difference in stable platelet response between the 2 arms was not significant—18% (n=9) and 4% (n=1), respectively (P=0.152).
However, when the data from study 047 and 048 were combined, the response rate was significantly higher in the fostamatinib arm than the placebo arm—18% (18/101) and 2% (1/49), respectively (P=0.007).
"The FDA acceptance for filing of our NDA is an exciting milestone for Rigel," Raul Rodriguez, Rigel's president and chief executive officer, said.
The approval of fostamatinib “will provide a new treatment option for patients with chronic or persistent ITP,” he affirmed. “We look forward to working closely with the FDA as they review our submission."
The US Food and Drug Administration (FDA) has accepted a new drug application (NDA) for the use of fostamatinib disodium (Tavalisse™) in the treatment of patients with chronic/persistent immune thrombocytopenia (ITP).
Fostamatinib is an oral spleen tyrosine kinase (SYK) inhibitor that investigators believe may address an underlying autoimmune cause of ITP by impeding platelet destruction.
Rigel Pharmaceuticals, the drug’s developer, anticipates an action date by the FDA of April 17, 2018.
The FDA previously granted fostamatinib orphan drug designation.
Data from 3 clinical studies support the new drug application. Study 047 and 048 were randomized placebo-controlled trials, and study 049 was an open-label extension study.
Together with an initial proof of concept study, the application included data on 163 ITP patients. Fostamatinib has been evaluated in over 4,600 individuals across all indications.
The patients enrolled in studies 047 and 048 had been diagnosed with persistent or chronic ITP, had failed at least 1 prior therapy for ITP, and had platelet counts consistently below 30,000/uL.
In both studies, patients were randomized in a 2:1 ratio to receive either fostamatinib or placebo orally twice a day for up to 24 weeks.
Study 047
In this study, 51 patients were randomized to fostamatinib and 25 to placebo.
The median platelet counts at baseline were 15,000/uL and 16,000/uL in the fostamatinib and placebo arms, respectively.
The median age was 57 in both treatment arms. The duration of ITP was 7.5 years (range, 0.6-53) in the fostamatinib arm and 5.5 years (range, 0.4-45) in the placebo arm.
The primary efficacy endpoint in both study 047 and 048 was a stable platelet response, defined as achieving platelet counts greater than 50,000/uL for at least 4 of the 6 scheduled clinic visits between weeks 14 and 24 of treatment.
In study 047, the rate of stable platelet response was significantly higher in the fostamatinib arm than the placebo arm—18% (n=9) and 0%, respectively (P=0.026).
Study 048
Fifty patients were randomized to fostamatinib and 24 to placebo.
Patients’ median platelet counts at baseline were 16,000/uL and 21,000/uL in the fostamatinib and placebo arms, respectively.
The median age was 50 in both treatment arms. The duration of ITP was 8.8 years (range, 0.3-50) in the fostamatinib arm and 10.8 years (range, 0.9-29) in the placebo arm.
In this study, however, the difference in stable platelet response between the 2 arms was not significant—18% (n=9) and 4% (n=1), respectively (P=0.152).
However, when the data from study 047 and 048 were combined, the response rate was significantly higher in the fostamatinib arm than the placebo arm—18% (18/101) and 2% (1/49), respectively (P=0.007).
"The FDA acceptance for filing of our NDA is an exciting milestone for Rigel," Raul Rodriguez, Rigel's president and chief executive officer, said.
The approval of fostamatinib “will provide a new treatment option for patients with chronic or persistent ITP,” he affirmed. “We look forward to working closely with the FDA as they review our submission."
The US Food and Drug Administration (FDA) has accepted a new drug application (NDA) for the use of fostamatinib disodium (Tavalisse™) in the treatment of patients with chronic/persistent immune thrombocytopenia (ITP).
Fostamatinib is an oral spleen tyrosine kinase (SYK) inhibitor that investigators believe may address an underlying autoimmune cause of ITP by impeding platelet destruction.
Rigel Pharmaceuticals, the drug’s developer, anticipates an action date by the FDA of April 17, 2018.
The FDA previously granted fostamatinib orphan drug designation.
Data from 3 clinical studies support the new drug application. Study 047 and 048 were randomized placebo-controlled trials, and study 049 was an open-label extension study.
Together with an initial proof of concept study, the application included data on 163 ITP patients. Fostamatinib has been evaluated in over 4,600 individuals across all indications.
The patients enrolled in studies 047 and 048 had been diagnosed with persistent or chronic ITP, had failed at least 1 prior therapy for ITP, and had platelet counts consistently below 30,000/uL.
In both studies, patients were randomized in a 2:1 ratio to receive either fostamatinib or placebo orally twice a day for up to 24 weeks.
Study 047
In this study, 51 patients were randomized to fostamatinib and 25 to placebo.
The median platelet counts at baseline were 15,000/uL and 16,000/uL in the fostamatinib and placebo arms, respectively.
The median age was 57 in both treatment arms. The duration of ITP was 7.5 years (range, 0.6-53) in the fostamatinib arm and 5.5 years (range, 0.4-45) in the placebo arm.
The primary efficacy endpoint in both study 047 and 048 was a stable platelet response, defined as achieving platelet counts greater than 50,000/uL for at least 4 of the 6 scheduled clinic visits between weeks 14 and 24 of treatment.
In study 047, the rate of stable platelet response was significantly higher in the fostamatinib arm than the placebo arm—18% (n=9) and 0%, respectively (P=0.026).
Study 048
Fifty patients were randomized to fostamatinib and 24 to placebo.
Patients’ median platelet counts at baseline were 16,000/uL and 21,000/uL in the fostamatinib and placebo arms, respectively.
The median age was 50 in both treatment arms. The duration of ITP was 8.8 years (range, 0.3-50) in the fostamatinib arm and 10.8 years (range, 0.9-29) in the placebo arm.
In this study, however, the difference in stable platelet response between the 2 arms was not significant—18% (n=9) and 4% (n=1), respectively (P=0.152).
However, when the data from study 047 and 048 were combined, the response rate was significantly higher in the fostamatinib arm than the placebo arm—18% (18/101) and 2% (1/49), respectively (P=0.007).
"The FDA acceptance for filing of our NDA is an exciting milestone for Rigel," Raul Rodriguez, Rigel's president and chief executive officer, said.
The approval of fostamatinib “will provide a new treatment option for patients with chronic or persistent ITP,” he affirmed. “We look forward to working closely with the FDA as they review our submission."
Company discontinues phase 3 trial of vadastuximab talirine in AML
Update as of June 21: The US Food and Drug Administration (FDA) has placed the Investigational New Drug (IND) application for vadastuximab talirine on hold. No clinical trial may resume under the IND until the FDA lifts the clinical hold.
On the advice of the Independent Data Monitoring Committee, Seattle Genetics is discontinuing the phase 3 CASCADE clinical trial of vadastuximab talirine as frontline treatment in older patients with acute myeloid leukemia (AML).
The company is also suspending patient enrollment and treatment in all its vadastuximab trials, including the ongoing phase 1/2 trial in frontline high-risk myelodysplastic syndromes (MDS).
In December last year, the US Food and Drug Administration (FDA) had placed the trials of vadastuximab on full and partial clinical holds due to the potential risk of hepatotoxicity.
The FDA lifted the hold in March of this year. However, concerns regarding a higher rate of deaths, including fatal infections but not liver toxicity, in the vadastuximab arm compared to control prompted the company to discontinue the phase 3 trial.
Vadastuximab talirene is an antibody-drug conjugate (ADC) targeted to CD33, which is expressed on most AML and MDS blasts. The ADC technology links anti-cancer compounds with targeting antibodies to precisely kill cancer cells and spare healthy ones.
Seattle Genetics’ ADC for Hodgkin lymphoma, brentuximab vedotin, was granted accelerated approval by the FDA in 2011.
The CASCADE trial was evaluating vadastuximab in combination with the hypomethylating agents (HMAs) azacytidine or decitabine compared to an HMA alone in older patients with newly diagnosed AML.
In addition to the MDS trial, the company is stopping enrollment onto the trial of vadastuximab in combination with 7+3 chemotherapy in newly diagnosed, younger AML patients and vadastuximab given prior to or after allogeneic hematopoietic stem cell transplant in AML patients.
Calling the decision “disappointing and unexpected,” Clay Siegall, PhD, president and CEO of Seattle Genetics, said, “Patient safety is our highest priority, and we will closely review the data and evaluate next steps.”
Update as of June 21: The US Food and Drug Administration (FDA) has placed the Investigational New Drug (IND) application for vadastuximab talirine on hold. No clinical trial may resume under the IND until the FDA lifts the clinical hold.
On the advice of the Independent Data Monitoring Committee, Seattle Genetics is discontinuing the phase 3 CASCADE clinical trial of vadastuximab talirine as frontline treatment in older patients with acute myeloid leukemia (AML).
The company is also suspending patient enrollment and treatment in all its vadastuximab trials, including the ongoing phase 1/2 trial in frontline high-risk myelodysplastic syndromes (MDS).
In December last year, the US Food and Drug Administration (FDA) had placed the trials of vadastuximab on full and partial clinical holds due to the potential risk of hepatotoxicity.
The FDA lifted the hold in March of this year. However, concerns regarding a higher rate of deaths, including fatal infections but not liver toxicity, in the vadastuximab arm compared to control prompted the company to discontinue the phase 3 trial.
Vadastuximab talirene is an antibody-drug conjugate (ADC) targeted to CD33, which is expressed on most AML and MDS blasts. The ADC technology links anti-cancer compounds with targeting antibodies to precisely kill cancer cells and spare healthy ones.
Seattle Genetics’ ADC for Hodgkin lymphoma, brentuximab vedotin, was granted accelerated approval by the FDA in 2011.
The CASCADE trial was evaluating vadastuximab in combination with the hypomethylating agents (HMAs) azacytidine or decitabine compared to an HMA alone in older patients with newly diagnosed AML.
In addition to the MDS trial, the company is stopping enrollment onto the trial of vadastuximab in combination with 7+3 chemotherapy in newly diagnosed, younger AML patients and vadastuximab given prior to or after allogeneic hematopoietic stem cell transplant in AML patients.
Calling the decision “disappointing and unexpected,” Clay Siegall, PhD, president and CEO of Seattle Genetics, said, “Patient safety is our highest priority, and we will closely review the data and evaluate next steps.”
Update as of June 21: The US Food and Drug Administration (FDA) has placed the Investigational New Drug (IND) application for vadastuximab talirine on hold. No clinical trial may resume under the IND until the FDA lifts the clinical hold.
On the advice of the Independent Data Monitoring Committee, Seattle Genetics is discontinuing the phase 3 CASCADE clinical trial of vadastuximab talirine as frontline treatment in older patients with acute myeloid leukemia (AML).
The company is also suspending patient enrollment and treatment in all its vadastuximab trials, including the ongoing phase 1/2 trial in frontline high-risk myelodysplastic syndromes (MDS).
In December last year, the US Food and Drug Administration (FDA) had placed the trials of vadastuximab on full and partial clinical holds due to the potential risk of hepatotoxicity.
The FDA lifted the hold in March of this year. However, concerns regarding a higher rate of deaths, including fatal infections but not liver toxicity, in the vadastuximab arm compared to control prompted the company to discontinue the phase 3 trial.
Vadastuximab talirene is an antibody-drug conjugate (ADC) targeted to CD33, which is expressed on most AML and MDS blasts. The ADC technology links anti-cancer compounds with targeting antibodies to precisely kill cancer cells and spare healthy ones.
Seattle Genetics’ ADC for Hodgkin lymphoma, brentuximab vedotin, was granted accelerated approval by the FDA in 2011.
The CASCADE trial was evaluating vadastuximab in combination with the hypomethylating agents (HMAs) azacytidine or decitabine compared to an HMA alone in older patients with newly diagnosed AML.
In addition to the MDS trial, the company is stopping enrollment onto the trial of vadastuximab in combination with 7+3 chemotherapy in newly diagnosed, younger AML patients and vadastuximab given prior to or after allogeneic hematopoietic stem cell transplant in AML patients.
Calling the decision “disappointing and unexpected,” Clay Siegall, PhD, president and CEO of Seattle Genetics, said, “Patient safety is our highest priority, and we will closely review the data and evaluate next steps.”
Biosimilar rituximab approved in Europe
The European Commission (EC) has approved the Sandoz biosimilar rituximab (Rixathon®) for use in the European Economic Area.
Rixathon is approved for all indications of the reference medicine, MabThera®, including follicular lymphoma, diffuse large B-cell lymphoma, chronic lymphocytic leukemia, and immunologic diseases such as rheumatoid arthritis, granulomatosis with polyangiitis, and microscopic polyangiitis.
This approval allows Rixathon to be marketed in the member states of the European Union and Iceland, Liechtenstein, and Norway, members of the European Free Trade Association.
The approval “represents a big win for patients in Europe with blood cancers or immunological diseases,” according to Carol Lynch, global head of Biopharmaceuticals at Sandoz.
“Rixathon will be one of the 5 major launches we plan in the next 4 years,” she said.
Earlier in the year, the European Medicines Agency’s Committee for Medicinal Products for Human Use had recommended marketing authorization for Rixathon.
The EC based its approval on a comprehensive development program generating analytical, preclinical, and clinical data. Clinical studies included ASSIST-RA and ASSIST-FL.
ASSIST-RA demonstrated that the biosimilar product has equivalent pharmacokinetic and pharmacodynamic profiles to the reference medicine, with no clinically meaningful differences in safety, tolerability, or immunogenicity in patients with rheumatoid arthritis.
ASSIST-FL was a phase 3 study confirming efficacy and safety. The study met its primary endpoint of equivalence in overall response rate between the biosimilar product and the reference medicine after 6 months.
ASSIST-FL also confirmed the comparable safety profiles of the 2 medicines.
Sandoz is a division of the Swiss pharmaceutical company Novartis. MabThera is a registered trademark of F. Hoffmann-La-Roche AG.
Another Sandoz biosimilar rituximab has been approved in the EU as Riximyo® under a duplicate marketing authorization.
The European Commission (EC) has approved the Sandoz biosimilar rituximab (Rixathon®) for use in the European Economic Area.
Rixathon is approved for all indications of the reference medicine, MabThera®, including follicular lymphoma, diffuse large B-cell lymphoma, chronic lymphocytic leukemia, and immunologic diseases such as rheumatoid arthritis, granulomatosis with polyangiitis, and microscopic polyangiitis.
This approval allows Rixathon to be marketed in the member states of the European Union and Iceland, Liechtenstein, and Norway, members of the European Free Trade Association.
The approval “represents a big win for patients in Europe with blood cancers or immunological diseases,” according to Carol Lynch, global head of Biopharmaceuticals at Sandoz.
“Rixathon will be one of the 5 major launches we plan in the next 4 years,” she said.
Earlier in the year, the European Medicines Agency’s Committee for Medicinal Products for Human Use had recommended marketing authorization for Rixathon.
The EC based its approval on a comprehensive development program generating analytical, preclinical, and clinical data. Clinical studies included ASSIST-RA and ASSIST-FL.
ASSIST-RA demonstrated that the biosimilar product has equivalent pharmacokinetic and pharmacodynamic profiles to the reference medicine, with no clinically meaningful differences in safety, tolerability, or immunogenicity in patients with rheumatoid arthritis.
ASSIST-FL was a phase 3 study confirming efficacy and safety. The study met its primary endpoint of equivalence in overall response rate between the biosimilar product and the reference medicine after 6 months.
ASSIST-FL also confirmed the comparable safety profiles of the 2 medicines.
Sandoz is a division of the Swiss pharmaceutical company Novartis. MabThera is a registered trademark of F. Hoffmann-La-Roche AG.
Another Sandoz biosimilar rituximab has been approved in the EU as Riximyo® under a duplicate marketing authorization.
The European Commission (EC) has approved the Sandoz biosimilar rituximab (Rixathon®) for use in the European Economic Area.
Rixathon is approved for all indications of the reference medicine, MabThera®, including follicular lymphoma, diffuse large B-cell lymphoma, chronic lymphocytic leukemia, and immunologic diseases such as rheumatoid arthritis, granulomatosis with polyangiitis, and microscopic polyangiitis.
This approval allows Rixathon to be marketed in the member states of the European Union and Iceland, Liechtenstein, and Norway, members of the European Free Trade Association.
The approval “represents a big win for patients in Europe with blood cancers or immunological diseases,” according to Carol Lynch, global head of Biopharmaceuticals at Sandoz.
“Rixathon will be one of the 5 major launches we plan in the next 4 years,” she said.
Earlier in the year, the European Medicines Agency’s Committee for Medicinal Products for Human Use had recommended marketing authorization for Rixathon.
The EC based its approval on a comprehensive development program generating analytical, preclinical, and clinical data. Clinical studies included ASSIST-RA and ASSIST-FL.
ASSIST-RA demonstrated that the biosimilar product has equivalent pharmacokinetic and pharmacodynamic profiles to the reference medicine, with no clinically meaningful differences in safety, tolerability, or immunogenicity in patients with rheumatoid arthritis.
ASSIST-FL was a phase 3 study confirming efficacy and safety. The study met its primary endpoint of equivalence in overall response rate between the biosimilar product and the reference medicine after 6 months.
ASSIST-FL also confirmed the comparable safety profiles of the 2 medicines.
Sandoz is a division of the Swiss pharmaceutical company Novartis. MabThera is a registered trademark of F. Hoffmann-La-Roche AG.
Another Sandoz biosimilar rituximab has been approved in the EU as Riximyo® under a duplicate marketing authorization.