User login
Gene therapy granted fast track status for hemophilia A
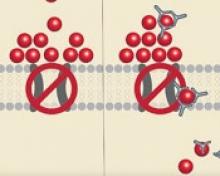
The US Food and Drug Administration (FDA) has granted fast track designation to SB-525, a gene therapy intended for use in patients with hemophilia A.
SB-525 is designed to provide continuous therapeutic expression of factor VIII protein.
SB-525 uses a recombinant adeno-associated virus (AAV) to deliver a human factor VIII complementary DNA construct and synthetic liver-specific promoter to the nucleus of liver cells.
The therapy is being developed by Sangamo Therapeutics, Inc.
In research presented at the 2016 ASH Annual Meeting (abstract 1173), SB-525 induced the expression of human factor VIII in mice and non-human primates (NHPs). SB-525 also corrected the bleeding defect in a mouse model of hemophilia A.
Dosing studies in NHPs demonstrated a robust and reproducible dose response curve, according to researchers. In these animals, mean human factor VIII levels ranged from 5% of normal at the lowest dose to 230% at the highest (AAV doses in the 6 x 1011 – 6 x 1012 vgs/kg range).
The researchers said the peak circulating human factor VIII levels in these experiments exceeded levels previously reported in NHPs. And this could significantly reduce the dose required to achieve therapeutically relevant levels in human subjects.
Sangamo is planning to open a phase 1/2 trial of SB-525 in adults with hemophilia A by the end of the second quarter of 2017. Data from this study are expected in late 2017 or early 2018.
In addition to the fast track designation, SB-525 has orphan drug designation from the FDA.
About fast track designation
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the new drug application or biologic license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted fast track designation to SB-525, a gene therapy intended for use in patients with hemophilia A.
SB-525 is designed to provide continuous therapeutic expression of factor VIII protein.
SB-525 uses a recombinant adeno-associated virus (AAV) to deliver a human factor VIII complementary DNA construct and synthetic liver-specific promoter to the nucleus of liver cells.
The therapy is being developed by Sangamo Therapeutics, Inc.
In research presented at the 2016 ASH Annual Meeting (abstract 1173), SB-525 induced the expression of human factor VIII in mice and non-human primates (NHPs). SB-525 also corrected the bleeding defect in a mouse model of hemophilia A.
Dosing studies in NHPs demonstrated a robust and reproducible dose response curve, according to researchers. In these animals, mean human factor VIII levels ranged from 5% of normal at the lowest dose to 230% at the highest (AAV doses in the 6 x 1011 – 6 x 1012 vgs/kg range).
The researchers said the peak circulating human factor VIII levels in these experiments exceeded levels previously reported in NHPs. And this could significantly reduce the dose required to achieve therapeutically relevant levels in human subjects.
Sangamo is planning to open a phase 1/2 trial of SB-525 in adults with hemophilia A by the end of the second quarter of 2017. Data from this study are expected in late 2017 or early 2018.
In addition to the fast track designation, SB-525 has orphan drug designation from the FDA.
About fast track designation
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the new drug application or biologic license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted fast track designation to SB-525, a gene therapy intended for use in patients with hemophilia A.
SB-525 is designed to provide continuous therapeutic expression of factor VIII protein.
SB-525 uses a recombinant adeno-associated virus (AAV) to deliver a human factor VIII complementary DNA construct and synthetic liver-specific promoter to the nucleus of liver cells.
The therapy is being developed by Sangamo Therapeutics, Inc.
In research presented at the 2016 ASH Annual Meeting (abstract 1173), SB-525 induced the expression of human factor VIII in mice and non-human primates (NHPs). SB-525 also corrected the bleeding defect in a mouse model of hemophilia A.
Dosing studies in NHPs demonstrated a robust and reproducible dose response curve, according to researchers. In these animals, mean human factor VIII levels ranged from 5% of normal at the lowest dose to 230% at the highest (AAV doses in the 6 x 1011 – 6 x 1012 vgs/kg range).
The researchers said the peak circulating human factor VIII levels in these experiments exceeded levels previously reported in NHPs. And this could significantly reduce the dose required to achieve therapeutically relevant levels in human subjects.
Sangamo is planning to open a phase 1/2 trial of SB-525 in adults with hemophilia A by the end of the second quarter of 2017. Data from this study are expected in late 2017 or early 2018.
In addition to the fast track designation, SB-525 has orphan drug designation from the FDA.
About fast track designation
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the new drug application or biologic license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
Intensive chemo upfront means DHL patients can skip HSCT
A new study suggests that patients with double-hit lymphoma (DHL) in first remission only benefit from an autologous hematopoietic stem cell transplant (auto-HSCT) if they received standard frontline chemotherapy.
Researchers looked at long-term outcomes for DHL patients who achieved remission and, overall, found that auto-HSCT did not significantly prolong remission or survival.
However, patients who received standard chemotherapy as frontline treatment did appear to benefit from auto-HSCT, as these patients had worse outcomes than patients who received intensive frontline chemotherapy.
This finding led the researchers to recommend that DHL patients receive intensive chemotherapy upfront and forgo subsequent auto-HSCT.
Daniel J. Landsburg, MD, of the University of Pennsylvania in Philadelphia, and his colleagues made these recommendations in the Journal of Clinical Oncology.
“A major dilemma for oncologists who treat [DHL] was whether or not to recommend the potentially harmful therapy of auto-[H]SCT to patients with this disease as a strategy to help keep them in remission,” Dr Landsburg said.
To gain some insight into the issue, Dr Landsburg and his colleagues looked at data on 159 patients from 19 academic medical centers across the US.
Patients were diagnosed with DHL between 2006 and 2015, and all achieved remission following frontline chemotherapy.
Thirty-five patients received standard frontline therapy—R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone).
The remaining patients received intensive frontline chemotherapy:
- 81 received DA-EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab)
- 32 received R-hyperCVAD (rituximab, cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate, and cytarabine)
- 11 received R-CODOX-M/IVAC (rituximab, cyclophosphamide, doxorubicin, vincristine, methotrexate/ifosfamide, etoposide, high-dose cytarabine).
Sixty-two patients underwent auto-HSCT, and 97 patients did not. There were no significant differences between these 2 patient groups at baseline.
“Our result is not explained by differences in patients’ overall health or disease features,” Dr Landsburg said. “The transplant and non-transplant arms of this study were very well-matched.”
Relapse and survival
For the entire patient cohort, the 3-year relapse-free survival (RFS) rate was 80%, and the 3-year overall survival (OS) rate was 87%.
There was no significant difference in RFS or OS between patients who underwent auto-HSCT and those who did not.
The RFS rate was 89% in patients who underwent auto-HSCT and 75% in patients who did not (P=0.12). The OS rate was 91% and 85%, respectively (P=0.74).
“Once these patients achieve remission, the data show they are likely to stay in remission,” Dr Landsburg said.
“In the absence of a large, randomized, controlled trial, which would be very challenging to carry out in this case, this is the best evidence we have, and it shows there’s no clear benefit to these patients undergoing auto-[H]SCT.”
Impact of frontline therapy
Patients who received R-CHOP upfront had worse RFS and OS than those who received intensive chemotherapy, although the OS difference was not significant.
RFS rates were 56% in patients who received R-CHOP, 88% in those who received DA-EPOCH-R, 87% in those who received R-hyperCVAD, and 91% in those who received R-CODOX-M/IVAC (P=0.003).
OS rates were 77% in patients who received R-CHOP, 87% in those who received DA-EPOCH-R, 90% in those who received R-hyperCVAD, and 100% in those who received R-CODOX-M/IVAC, respectively (P=0.36).
When the 3 intensive regimens were combined, the RFS rate was 88% (vs 56% for R-CHOP, P=0.002), and the OS rate was 90% (vs 77% for R-CHOP, P=0.13).
Frontline therapy and HSCT
Patients who received R-CHOP upfront benefited from auto-HSCT, but patients who received intensive chemotherapy did not.
The RFS was 51% for patients who received R-CHOP and did not undergo auto-HSCT, and it was 75% for patients who received R-CHOP followed by auto-HSCT.
The OS was 75% for patients who received R-CHOP and did not undergo auto-HSCT, and it was 83% for patients who received R-CHOP followed by auto-HSCT.
The RFS was 86% for patients who received intensive chemotherapy and did not undergo auto-HSCT, and it was 91% for patients who received intensive chemotherapy followed by auto-HSCT.
The OS was 89% for patients who received intensive chemotherapy and did not undergo auto-HSCT, and it was 92% for patients who received intensive chemotherapy followed by auto-HSCT.
An intergroup comparison showed a significant difference in RFS (P=0.003), which was driven by a significantly lower rate of RFS for patients who received R-CHOP without auto-HSCT, compared with patients who received intensive chemotherapy without auto-HSCT (P=0.003) or intensive chemotherapy with auto-HSCT (P=0.001).
“[I]f patients do go into remission with R-CHOP, it appears to be less durable, so, in these cases, going forward with auto-[H]SCT may still make sense,” Dr Landsburg said.
On the other hand, there was no significant difference between the groups with regard to OS (P=0.50).
Dr Landsburg said the next step for this research will be to study features of patients who don’t go into remission in order to understand why their disease is resistant to therapy and if that can be overcome with different treatment strategies. He also said it’s important to try to find more effective therapies for DHL patients who relapse. ![]()
A new study suggests that patients with double-hit lymphoma (DHL) in first remission only benefit from an autologous hematopoietic stem cell transplant (auto-HSCT) if they received standard frontline chemotherapy.
Researchers looked at long-term outcomes for DHL patients who achieved remission and, overall, found that auto-HSCT did not significantly prolong remission or survival.
However, patients who received standard chemotherapy as frontline treatment did appear to benefit from auto-HSCT, as these patients had worse outcomes than patients who received intensive frontline chemotherapy.
This finding led the researchers to recommend that DHL patients receive intensive chemotherapy upfront and forgo subsequent auto-HSCT.
Daniel J. Landsburg, MD, of the University of Pennsylvania in Philadelphia, and his colleagues made these recommendations in the Journal of Clinical Oncology.
“A major dilemma for oncologists who treat [DHL] was whether or not to recommend the potentially harmful therapy of auto-[H]SCT to patients with this disease as a strategy to help keep them in remission,” Dr Landsburg said.
To gain some insight into the issue, Dr Landsburg and his colleagues looked at data on 159 patients from 19 academic medical centers across the US.
Patients were diagnosed with DHL between 2006 and 2015, and all achieved remission following frontline chemotherapy.
Thirty-five patients received standard frontline therapy—R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone).
The remaining patients received intensive frontline chemotherapy:
- 81 received DA-EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab)
- 32 received R-hyperCVAD (rituximab, cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate, and cytarabine)
- 11 received R-CODOX-M/IVAC (rituximab, cyclophosphamide, doxorubicin, vincristine, methotrexate/ifosfamide, etoposide, high-dose cytarabine).
Sixty-two patients underwent auto-HSCT, and 97 patients did not. There were no significant differences between these 2 patient groups at baseline.
“Our result is not explained by differences in patients’ overall health or disease features,” Dr Landsburg said. “The transplant and non-transplant arms of this study were very well-matched.”
Relapse and survival
For the entire patient cohort, the 3-year relapse-free survival (RFS) rate was 80%, and the 3-year overall survival (OS) rate was 87%.
There was no significant difference in RFS or OS between patients who underwent auto-HSCT and those who did not.
The RFS rate was 89% in patients who underwent auto-HSCT and 75% in patients who did not (P=0.12). The OS rate was 91% and 85%, respectively (P=0.74).
“Once these patients achieve remission, the data show they are likely to stay in remission,” Dr Landsburg said.
“In the absence of a large, randomized, controlled trial, which would be very challenging to carry out in this case, this is the best evidence we have, and it shows there’s no clear benefit to these patients undergoing auto-[H]SCT.”
Impact of frontline therapy
Patients who received R-CHOP upfront had worse RFS and OS than those who received intensive chemotherapy, although the OS difference was not significant.
RFS rates were 56% in patients who received R-CHOP, 88% in those who received DA-EPOCH-R, 87% in those who received R-hyperCVAD, and 91% in those who received R-CODOX-M/IVAC (P=0.003).
OS rates were 77% in patients who received R-CHOP, 87% in those who received DA-EPOCH-R, 90% in those who received R-hyperCVAD, and 100% in those who received R-CODOX-M/IVAC, respectively (P=0.36).
When the 3 intensive regimens were combined, the RFS rate was 88% (vs 56% for R-CHOP, P=0.002), and the OS rate was 90% (vs 77% for R-CHOP, P=0.13).
Frontline therapy and HSCT
Patients who received R-CHOP upfront benefited from auto-HSCT, but patients who received intensive chemotherapy did not.
The RFS was 51% for patients who received R-CHOP and did not undergo auto-HSCT, and it was 75% for patients who received R-CHOP followed by auto-HSCT.
The OS was 75% for patients who received R-CHOP and did not undergo auto-HSCT, and it was 83% for patients who received R-CHOP followed by auto-HSCT.
The RFS was 86% for patients who received intensive chemotherapy and did not undergo auto-HSCT, and it was 91% for patients who received intensive chemotherapy followed by auto-HSCT.
The OS was 89% for patients who received intensive chemotherapy and did not undergo auto-HSCT, and it was 92% for patients who received intensive chemotherapy followed by auto-HSCT.
An intergroup comparison showed a significant difference in RFS (P=0.003), which was driven by a significantly lower rate of RFS for patients who received R-CHOP without auto-HSCT, compared with patients who received intensive chemotherapy without auto-HSCT (P=0.003) or intensive chemotherapy with auto-HSCT (P=0.001).
“[I]f patients do go into remission with R-CHOP, it appears to be less durable, so, in these cases, going forward with auto-[H]SCT may still make sense,” Dr Landsburg said.
On the other hand, there was no significant difference between the groups with regard to OS (P=0.50).
Dr Landsburg said the next step for this research will be to study features of patients who don’t go into remission in order to understand why their disease is resistant to therapy and if that can be overcome with different treatment strategies. He also said it’s important to try to find more effective therapies for DHL patients who relapse. ![]()
A new study suggests that patients with double-hit lymphoma (DHL) in first remission only benefit from an autologous hematopoietic stem cell transplant (auto-HSCT) if they received standard frontline chemotherapy.
Researchers looked at long-term outcomes for DHL patients who achieved remission and, overall, found that auto-HSCT did not significantly prolong remission or survival.
However, patients who received standard chemotherapy as frontline treatment did appear to benefit from auto-HSCT, as these patients had worse outcomes than patients who received intensive frontline chemotherapy.
This finding led the researchers to recommend that DHL patients receive intensive chemotherapy upfront and forgo subsequent auto-HSCT.
Daniel J. Landsburg, MD, of the University of Pennsylvania in Philadelphia, and his colleagues made these recommendations in the Journal of Clinical Oncology.
“A major dilemma for oncologists who treat [DHL] was whether or not to recommend the potentially harmful therapy of auto-[H]SCT to patients with this disease as a strategy to help keep them in remission,” Dr Landsburg said.
To gain some insight into the issue, Dr Landsburg and his colleagues looked at data on 159 patients from 19 academic medical centers across the US.
Patients were diagnosed with DHL between 2006 and 2015, and all achieved remission following frontline chemotherapy.
Thirty-five patients received standard frontline therapy—R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone).
The remaining patients received intensive frontline chemotherapy:
- 81 received DA-EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab)
- 32 received R-hyperCVAD (rituximab, cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate, and cytarabine)
- 11 received R-CODOX-M/IVAC (rituximab, cyclophosphamide, doxorubicin, vincristine, methotrexate/ifosfamide, etoposide, high-dose cytarabine).
Sixty-two patients underwent auto-HSCT, and 97 patients did not. There were no significant differences between these 2 patient groups at baseline.
“Our result is not explained by differences in patients’ overall health or disease features,” Dr Landsburg said. “The transplant and non-transplant arms of this study were very well-matched.”
Relapse and survival
For the entire patient cohort, the 3-year relapse-free survival (RFS) rate was 80%, and the 3-year overall survival (OS) rate was 87%.
There was no significant difference in RFS or OS between patients who underwent auto-HSCT and those who did not.
The RFS rate was 89% in patients who underwent auto-HSCT and 75% in patients who did not (P=0.12). The OS rate was 91% and 85%, respectively (P=0.74).
“Once these patients achieve remission, the data show they are likely to stay in remission,” Dr Landsburg said.
“In the absence of a large, randomized, controlled trial, which would be very challenging to carry out in this case, this is the best evidence we have, and it shows there’s no clear benefit to these patients undergoing auto-[H]SCT.”
Impact of frontline therapy
Patients who received R-CHOP upfront had worse RFS and OS than those who received intensive chemotherapy, although the OS difference was not significant.
RFS rates were 56% in patients who received R-CHOP, 88% in those who received DA-EPOCH-R, 87% in those who received R-hyperCVAD, and 91% in those who received R-CODOX-M/IVAC (P=0.003).
OS rates were 77% in patients who received R-CHOP, 87% in those who received DA-EPOCH-R, 90% in those who received R-hyperCVAD, and 100% in those who received R-CODOX-M/IVAC, respectively (P=0.36).
When the 3 intensive regimens were combined, the RFS rate was 88% (vs 56% for R-CHOP, P=0.002), and the OS rate was 90% (vs 77% for R-CHOP, P=0.13).
Frontline therapy and HSCT
Patients who received R-CHOP upfront benefited from auto-HSCT, but patients who received intensive chemotherapy did not.
The RFS was 51% for patients who received R-CHOP and did not undergo auto-HSCT, and it was 75% for patients who received R-CHOP followed by auto-HSCT.
The OS was 75% for patients who received R-CHOP and did not undergo auto-HSCT, and it was 83% for patients who received R-CHOP followed by auto-HSCT.
The RFS was 86% for patients who received intensive chemotherapy and did not undergo auto-HSCT, and it was 91% for patients who received intensive chemotherapy followed by auto-HSCT.
The OS was 89% for patients who received intensive chemotherapy and did not undergo auto-HSCT, and it was 92% for patients who received intensive chemotherapy followed by auto-HSCT.
An intergroup comparison showed a significant difference in RFS (P=0.003), which was driven by a significantly lower rate of RFS for patients who received R-CHOP without auto-HSCT, compared with patients who received intensive chemotherapy without auto-HSCT (P=0.003) or intensive chemotherapy with auto-HSCT (P=0.001).
“[I]f patients do go into remission with R-CHOP, it appears to be less durable, so, in these cases, going forward with auto-[H]SCT may still make sense,” Dr Landsburg said.
On the other hand, there was no significant difference between the groups with regard to OS (P=0.50).
Dr Landsburg said the next step for this research will be to study features of patients who don’t go into remission in order to understand why their disease is resistant to therapy and if that can be overcome with different treatment strategies. He also said it’s important to try to find more effective therapies for DHL patients who relapse. ![]()
Antibody shows potential for treating AML, B-ALL
Endoglin may be a promising therapeutic target in acute myeloid leukemia (AML) and B-cell acute lymphoblastic leukemia (B-ALL), according to researchers.
The group identified endoglin expression on the majority of blasts from patients with AML and B-ALL.
The team also found that an endoglin antibody, TRC105 (carotuximab), exhibited activity against AML and B-ALL in vivo, and combining the drug with chemotherapeutic agents enhanced this activity.
Rita Perlingeiro, PhD, of the University of Minnesota in Minneapolis, and her colleagues reported these findings in Blood.
The researchers first discovered that endoglin, which is also known as CD105, was “highly expressed” in leukemic blasts.
In samples from AML patients, 47.6% to 98.5% of blasts were CD105+. In samples from B-ALL patients, 92.6% to 99% of blasts were CD105+.
“We have been studying the function of endoglin in hematopoiesis for more than a decade, and the consistent expression of this receptor in the majority of acute leukemias was intriguing,” Dr Perlingeiro said.
She and her colleagues also found that CD105+ blasts had superior leukemogenic activity and reduced survival in mice when compared to CD105- blasts.
Mice injected with AML CD105+ blasts had all died at day 110 after injection, but mice injected with CD105- AML blasts survived until day 140.
Mice injected with CD105+ ALL blasts died 3 months after injection, but mice injected with CD105- ALL blasts were still alive and showed no signs of disease at the time of sacrifice, which was 5 months after injection.
TRC105 monotherapy
Several experiments showed that TRC105 could reduce leukemic activity in vivo.
TRC105 reduced blast counts in the peripheral blood of mice that had been injected with AML blasts. The drug also reduced blasts in the bone marrow initially, though blast counts were comparable in treated mice and controls by week 12.
On the other hand, mice treated with TRC105 did not experience the weight loss and splenomegaly observed in control mice. And TRC105 suppressed the ability of AML blasts to give rise to leukemia in secondary recipient mice.
In mice injected with ALL blasts, TRC105 initially decreased blast counts. However, by week 8, blast counts in the peripheral blood, bone marrow, and spleen of treated mice were similar to those observed in controls. The researchers said this suggests that TRC105 only slows the development of ALL.
The team then evaluated the effects of TRC105 after disease had been established. Mice with established AML received TRC105 for 8 weeks, and mice with established ALL received TRC105 for 4 weeks.
In mice with AML, TRC105 reduced blasts in the peripheral blood and spleen but not the bone marrow. The treatment also prevented splenomegaly and weight loss and prolonged survival.
“Our hypothesis that endoglin expression was linked to leukemia-forming activity was proven to be true, and it was even more rewarding to witness the robust anti-leukemogenic effect of blocking endoglin signaling with TRC105, even when leukemia had already been established in the mouse,” Dr Perlingeiro said.
However, in mice with established ALL, TRC105 had no effect on leukemia progression.
The researchers said this could be due to expression of soluble endoglin (sENG), which would titrate the TRC105 antibody, limiting its ability to bind to membrane-bound endoglin on leukemic cells. Results of additional experiments supported this idea.
TRC105 in combination
The researchers also tested TRC105 in combination with chemotherapy in the mouse models. The team combined the antibody with cytarabine to treat AML and cyclophosphamide to treat ALL.
In mice with AML, cytarabine and TRC105 significantly reduced levels of leukemic cells in the peripheral blood.
In mice with ALL, cyclophosphamide and TRC105 suppressed leukemia development more effectively and more quickly than cyclophosphamide alone.
The researchers detected high levels of sENG in untreated mice with ALL, but levels were lower in the TRC105-treated mice. And there was “no significant detection” of sENG in mice that received cyclophosphamide and TRC105 or cyclophosphamide alone.
Dr Perlingeiro and her colleagues said this suggests the inhibitory effects of sENG can be circumvented by suppressing tumor burden, which results in the combination therapy demonstrating potent antileukemic activity. ![]()
Endoglin may be a promising therapeutic target in acute myeloid leukemia (AML) and B-cell acute lymphoblastic leukemia (B-ALL), according to researchers.
The group identified endoglin expression on the majority of blasts from patients with AML and B-ALL.
The team also found that an endoglin antibody, TRC105 (carotuximab), exhibited activity against AML and B-ALL in vivo, and combining the drug with chemotherapeutic agents enhanced this activity.
Rita Perlingeiro, PhD, of the University of Minnesota in Minneapolis, and her colleagues reported these findings in Blood.
The researchers first discovered that endoglin, which is also known as CD105, was “highly expressed” in leukemic blasts.
In samples from AML patients, 47.6% to 98.5% of blasts were CD105+. In samples from B-ALL patients, 92.6% to 99% of blasts were CD105+.
“We have been studying the function of endoglin in hematopoiesis for more than a decade, and the consistent expression of this receptor in the majority of acute leukemias was intriguing,” Dr Perlingeiro said.
She and her colleagues also found that CD105+ blasts had superior leukemogenic activity and reduced survival in mice when compared to CD105- blasts.
Mice injected with AML CD105+ blasts had all died at day 110 after injection, but mice injected with CD105- AML blasts survived until day 140.
Mice injected with CD105+ ALL blasts died 3 months after injection, but mice injected with CD105- ALL blasts were still alive and showed no signs of disease at the time of sacrifice, which was 5 months after injection.
TRC105 monotherapy
Several experiments showed that TRC105 could reduce leukemic activity in vivo.
TRC105 reduced blast counts in the peripheral blood of mice that had been injected with AML blasts. The drug also reduced blasts in the bone marrow initially, though blast counts were comparable in treated mice and controls by week 12.
On the other hand, mice treated with TRC105 did not experience the weight loss and splenomegaly observed in control mice. And TRC105 suppressed the ability of AML blasts to give rise to leukemia in secondary recipient mice.
In mice injected with ALL blasts, TRC105 initially decreased blast counts. However, by week 8, blast counts in the peripheral blood, bone marrow, and spleen of treated mice were similar to those observed in controls. The researchers said this suggests that TRC105 only slows the development of ALL.
The team then evaluated the effects of TRC105 after disease had been established. Mice with established AML received TRC105 for 8 weeks, and mice with established ALL received TRC105 for 4 weeks.
In mice with AML, TRC105 reduced blasts in the peripheral blood and spleen but not the bone marrow. The treatment also prevented splenomegaly and weight loss and prolonged survival.
“Our hypothesis that endoglin expression was linked to leukemia-forming activity was proven to be true, and it was even more rewarding to witness the robust anti-leukemogenic effect of blocking endoglin signaling with TRC105, even when leukemia had already been established in the mouse,” Dr Perlingeiro said.
However, in mice with established ALL, TRC105 had no effect on leukemia progression.
The researchers said this could be due to expression of soluble endoglin (sENG), which would titrate the TRC105 antibody, limiting its ability to bind to membrane-bound endoglin on leukemic cells. Results of additional experiments supported this idea.
TRC105 in combination
The researchers also tested TRC105 in combination with chemotherapy in the mouse models. The team combined the antibody with cytarabine to treat AML and cyclophosphamide to treat ALL.
In mice with AML, cytarabine and TRC105 significantly reduced levels of leukemic cells in the peripheral blood.
In mice with ALL, cyclophosphamide and TRC105 suppressed leukemia development more effectively and more quickly than cyclophosphamide alone.
The researchers detected high levels of sENG in untreated mice with ALL, but levels were lower in the TRC105-treated mice. And there was “no significant detection” of sENG in mice that received cyclophosphamide and TRC105 or cyclophosphamide alone.
Dr Perlingeiro and her colleagues said this suggests the inhibitory effects of sENG can be circumvented by suppressing tumor burden, which results in the combination therapy demonstrating potent antileukemic activity. ![]()
Endoglin may be a promising therapeutic target in acute myeloid leukemia (AML) and B-cell acute lymphoblastic leukemia (B-ALL), according to researchers.
The group identified endoglin expression on the majority of blasts from patients with AML and B-ALL.
The team also found that an endoglin antibody, TRC105 (carotuximab), exhibited activity against AML and B-ALL in vivo, and combining the drug with chemotherapeutic agents enhanced this activity.
Rita Perlingeiro, PhD, of the University of Minnesota in Minneapolis, and her colleagues reported these findings in Blood.
The researchers first discovered that endoglin, which is also known as CD105, was “highly expressed” in leukemic blasts.
In samples from AML patients, 47.6% to 98.5% of blasts were CD105+. In samples from B-ALL patients, 92.6% to 99% of blasts were CD105+.
“We have been studying the function of endoglin in hematopoiesis for more than a decade, and the consistent expression of this receptor in the majority of acute leukemias was intriguing,” Dr Perlingeiro said.
She and her colleagues also found that CD105+ blasts had superior leukemogenic activity and reduced survival in mice when compared to CD105- blasts.
Mice injected with AML CD105+ blasts had all died at day 110 after injection, but mice injected with CD105- AML blasts survived until day 140.
Mice injected with CD105+ ALL blasts died 3 months after injection, but mice injected with CD105- ALL blasts were still alive and showed no signs of disease at the time of sacrifice, which was 5 months after injection.
TRC105 monotherapy
Several experiments showed that TRC105 could reduce leukemic activity in vivo.
TRC105 reduced blast counts in the peripheral blood of mice that had been injected with AML blasts. The drug also reduced blasts in the bone marrow initially, though blast counts were comparable in treated mice and controls by week 12.
On the other hand, mice treated with TRC105 did not experience the weight loss and splenomegaly observed in control mice. And TRC105 suppressed the ability of AML blasts to give rise to leukemia in secondary recipient mice.
In mice injected with ALL blasts, TRC105 initially decreased blast counts. However, by week 8, blast counts in the peripheral blood, bone marrow, and spleen of treated mice were similar to those observed in controls. The researchers said this suggests that TRC105 only slows the development of ALL.
The team then evaluated the effects of TRC105 after disease had been established. Mice with established AML received TRC105 for 8 weeks, and mice with established ALL received TRC105 for 4 weeks.
In mice with AML, TRC105 reduced blasts in the peripheral blood and spleen but not the bone marrow. The treatment also prevented splenomegaly and weight loss and prolonged survival.
“Our hypothesis that endoglin expression was linked to leukemia-forming activity was proven to be true, and it was even more rewarding to witness the robust anti-leukemogenic effect of blocking endoglin signaling with TRC105, even when leukemia had already been established in the mouse,” Dr Perlingeiro said.
However, in mice with established ALL, TRC105 had no effect on leukemia progression.
The researchers said this could be due to expression of soluble endoglin (sENG), which would titrate the TRC105 antibody, limiting its ability to bind to membrane-bound endoglin on leukemic cells. Results of additional experiments supported this idea.
TRC105 in combination
The researchers also tested TRC105 in combination with chemotherapy in the mouse models. The team combined the antibody with cytarabine to treat AML and cyclophosphamide to treat ALL.
In mice with AML, cytarabine and TRC105 significantly reduced levels of leukemic cells in the peripheral blood.
In mice with ALL, cyclophosphamide and TRC105 suppressed leukemia development more effectively and more quickly than cyclophosphamide alone.
The researchers detected high levels of sENG in untreated mice with ALL, but levels were lower in the TRC105-treated mice. And there was “no significant detection” of sENG in mice that received cyclophosphamide and TRC105 or cyclophosphamide alone.
Dr Perlingeiro and her colleagues said this suggests the inhibitory effects of sENG can be circumvented by suppressing tumor burden, which results in the combination therapy demonstrating potent antileukemic activity. ![]()
Maternal education can reduce risk of childhood malaria
One way to reduce the risk of malaria infection in children is to educate their mothers, according to a study published in Pathogens and Global Health.
The study suggested that educating mothers can be more effective in preventing childhood malaria than the malaria vaccine candidate RTS,S (Mosquirix).
Researchers said this finding can be explained by the fact that educated mothers know of ways to prevent malaria infection, such as using bed nets and taking their children for treatment if they develop a fever.
For this study, the researchers performed malaria testing in 647 children in the Democratic Republic of Congo (DRC) who were between the ages of 2 months and 5 years.
The team also had the children’s parent or guardian fill out a survey related to demographics, socioeconomic status, maternal education, bed net use, and recent illness involving fever.
Results showed that mothers with a higher education level had children with a lower risk of malaria infection.
“This was not a small effect,” said study author Michael Hawkes, MD, PhD, of the University of Alberta in Edmonton, Alberta, Canada.
“Maternal education had an enormous effect—equivalent to or greater than the leading biomedical vaccine against malaria.”
Overall, 19% of the children studied (123/647) tested positive for malaria.
The prevalence of malaria was 30% in children of mothers with no education, 17% in children of mothers with primary education, and 15% in children of mothers with education beyond primary school (P=0.001).
In a multivariate analysis adjusted for the effect of a child’s age and the study site, maternal education was still a significant predictor of malaria antigenemia.
“It doesn’t take a lot of education to teach a mom how to take simple precautions to prevent malaria in her child,” said study author Cary Ma, a medical student at the University of Alberta.
“All it takes is knowing the importance of using a bed net and knowing the importance of seeking care when your child has a fever. These are fairly straightforward, simple messages in the context of health and hygiene that can easily be conveyed, usually at an elementary or primary school level.”
“The World Health Organization is rolling out a new vaccine [RTS,S] in countries across Africa that has an efficacy of about 30%,” Dr Hawkes added.
“But children whose mothers are educated beyond the primary level have a 53% reduction in their malaria rates. So educating the mom has as profound an effect on childhood malaria as hundreds of millions of dollars spent on a vaccine.”
The researchers said this work builds upon previous studies that have shown the importance of maternal education in reducing child mortality and disease in other countries around the world.
The team noted that maternal education isn’t a magic bullet by itself, but they do believe it is part of the solution. They hope the lessons learned via this study can help lead policymakers to strengthen efforts to educate girls and women in the DRC and other malaria hotspots around the world. ![]()
One way to reduce the risk of malaria infection in children is to educate their mothers, according to a study published in Pathogens and Global Health.
The study suggested that educating mothers can be more effective in preventing childhood malaria than the malaria vaccine candidate RTS,S (Mosquirix).
Researchers said this finding can be explained by the fact that educated mothers know of ways to prevent malaria infection, such as using bed nets and taking their children for treatment if they develop a fever.
For this study, the researchers performed malaria testing in 647 children in the Democratic Republic of Congo (DRC) who were between the ages of 2 months and 5 years.
The team also had the children’s parent or guardian fill out a survey related to demographics, socioeconomic status, maternal education, bed net use, and recent illness involving fever.
Results showed that mothers with a higher education level had children with a lower risk of malaria infection.
“This was not a small effect,” said study author Michael Hawkes, MD, PhD, of the University of Alberta in Edmonton, Alberta, Canada.
“Maternal education had an enormous effect—equivalent to or greater than the leading biomedical vaccine against malaria.”
Overall, 19% of the children studied (123/647) tested positive for malaria.
The prevalence of malaria was 30% in children of mothers with no education, 17% in children of mothers with primary education, and 15% in children of mothers with education beyond primary school (P=0.001).
In a multivariate analysis adjusted for the effect of a child’s age and the study site, maternal education was still a significant predictor of malaria antigenemia.
“It doesn’t take a lot of education to teach a mom how to take simple precautions to prevent malaria in her child,” said study author Cary Ma, a medical student at the University of Alberta.
“All it takes is knowing the importance of using a bed net and knowing the importance of seeking care when your child has a fever. These are fairly straightforward, simple messages in the context of health and hygiene that can easily be conveyed, usually at an elementary or primary school level.”
“The World Health Organization is rolling out a new vaccine [RTS,S] in countries across Africa that has an efficacy of about 30%,” Dr Hawkes added.
“But children whose mothers are educated beyond the primary level have a 53% reduction in their malaria rates. So educating the mom has as profound an effect on childhood malaria as hundreds of millions of dollars spent on a vaccine.”
The researchers said this work builds upon previous studies that have shown the importance of maternal education in reducing child mortality and disease in other countries around the world.
The team noted that maternal education isn’t a magic bullet by itself, but they do believe it is part of the solution. They hope the lessons learned via this study can help lead policymakers to strengthen efforts to educate girls and women in the DRC and other malaria hotspots around the world. ![]()
One way to reduce the risk of malaria infection in children is to educate their mothers, according to a study published in Pathogens and Global Health.
The study suggested that educating mothers can be more effective in preventing childhood malaria than the malaria vaccine candidate RTS,S (Mosquirix).
Researchers said this finding can be explained by the fact that educated mothers know of ways to prevent malaria infection, such as using bed nets and taking their children for treatment if they develop a fever.
For this study, the researchers performed malaria testing in 647 children in the Democratic Republic of Congo (DRC) who were between the ages of 2 months and 5 years.
The team also had the children’s parent or guardian fill out a survey related to demographics, socioeconomic status, maternal education, bed net use, and recent illness involving fever.
Results showed that mothers with a higher education level had children with a lower risk of malaria infection.
“This was not a small effect,” said study author Michael Hawkes, MD, PhD, of the University of Alberta in Edmonton, Alberta, Canada.
“Maternal education had an enormous effect—equivalent to or greater than the leading biomedical vaccine against malaria.”
Overall, 19% of the children studied (123/647) tested positive for malaria.
The prevalence of malaria was 30% in children of mothers with no education, 17% in children of mothers with primary education, and 15% in children of mothers with education beyond primary school (P=0.001).
In a multivariate analysis adjusted for the effect of a child’s age and the study site, maternal education was still a significant predictor of malaria antigenemia.
“It doesn’t take a lot of education to teach a mom how to take simple precautions to prevent malaria in her child,” said study author Cary Ma, a medical student at the University of Alberta.
“All it takes is knowing the importance of using a bed net and knowing the importance of seeking care when your child has a fever. These are fairly straightforward, simple messages in the context of health and hygiene that can easily be conveyed, usually at an elementary or primary school level.”
“The World Health Organization is rolling out a new vaccine [RTS,S] in countries across Africa that has an efficacy of about 30%,” Dr Hawkes added.
“But children whose mothers are educated beyond the primary level have a 53% reduction in their malaria rates. So educating the mom has as profound an effect on childhood malaria as hundreds of millions of dollars spent on a vaccine.”
The researchers said this work builds upon previous studies that have shown the importance of maternal education in reducing child mortality and disease in other countries around the world.
The team noted that maternal education isn’t a magic bullet by itself, but they do believe it is part of the solution. They hope the lessons learned via this study can help lead policymakers to strengthen efforts to educate girls and women in the DRC and other malaria hotspots around the world. ![]()
CMV matching improves survival in HSCT recipients
Matching the cytomegalovirus (CMV) status of the donor and recipient of a hematopoietic stem cell transplant (HSCT) can significantly improve the recipient’s survival, according to a study published in Bone Marrow Transplantation.
In fact, researchers said they found evidence to suggest that CMV matching may abrogate the effect of a human leukocyte antigen (HLA) mismatch.
“This breakthrough will help us discover new and more effective ways to make sure patients in need of a transplant get the best possible match to cure blood cancer and blood disorders,” said study author Steven Marsh, PhD, of Anthony Nolan Research Institute, Royal Free Hospital in London, UK.
Dr Marsh and his colleagues studied 1271 patients who received T-cell-depleted grafts to treat a hematologic disorder, including acute or chronic leukemia, lymphoma, myeloma, myelodysplasia, and “other” disorders.
The 5-year overall survival in these patients was 40.6%.
The researchers found HSCT recipients with a 10/10 HLA-matched donor had significantly better overall survival (OS) and lower non-relapse mortality (NRM) than patients with a mismatched donor.
The 5-year OS was 43.1% for a 10/10 match, 35.6% for a 9/10 match, and 28.4% for a match less than 9/10 (P=0.001). NRM at 1 year was 20.3%, 26.0%, and 33.4%, respectively (P=0.007).
Similarly, HSCT recipients with a CMV-matched donor had significantly better OS and significantly lower NRM than recipients with a CMV-mismatched donor.
The 5-year OS was 44.1% for recipients with a CMV-matched donor and 32.2% for patients with a mismatched donor (P<0.001). NRM at 1 year was 19.1% and 30.4%, respectively (P<0.001)
Most of the associations between CMV/HLA matching and OS/NRM remained significant in multivariate analyses.
For recipients with more than 1 HLA mismatch, the relative risk (RR) of death was 1.43 (P=0.016), and the RR for NRM was 1.59 (P=0.028), when compared to patients who had received a 10/10 HLA-matched graft.
For recipients with a single mismatch, the RR for death was 1.21 (P=0.042), and the RR for NRM was 1.24 (P=0.14).
For recipients with a CMV mismatched donor, the RR for death was 1.40 (P<0.001), and the RR for NRM was 1.63 (P<0.001).
The researchers also assessed CMV and HLA status together. Compared to fully HLA-matched and CMV-matched recipients, the RRs for death were:
- 1.36 (P=0.003) for HLA matched/CMV mismatched
- 1.22 (P=0.062) for HLA mismatched/CMV matched
- 1.81 (P=0.001) for HLA and CMV mismatched.
The researchers said these results suggest it is possible to improve survival rates for patients with no HLA-matched donor by matching the CMV status of the donor and recipient.
As a result of the findings, experts at Anthony Nolan are exploring how to type donors for CMV when joining the stem cell donor register to allow CMV status to be taken into account when transplant centers are selecting potential donors for a patient.
“[B]y establishing that CMV matching has a significant impact on patient outcomes, we are making it easier for transplant centers to make informed choices about the donors they select for their patients,” Dr Marsh said. ![]()
Matching the cytomegalovirus (CMV) status of the donor and recipient of a hematopoietic stem cell transplant (HSCT) can significantly improve the recipient’s survival, according to a study published in Bone Marrow Transplantation.
In fact, researchers said they found evidence to suggest that CMV matching may abrogate the effect of a human leukocyte antigen (HLA) mismatch.
“This breakthrough will help us discover new and more effective ways to make sure patients in need of a transplant get the best possible match to cure blood cancer and blood disorders,” said study author Steven Marsh, PhD, of Anthony Nolan Research Institute, Royal Free Hospital in London, UK.
Dr Marsh and his colleagues studied 1271 patients who received T-cell-depleted grafts to treat a hematologic disorder, including acute or chronic leukemia, lymphoma, myeloma, myelodysplasia, and “other” disorders.
The 5-year overall survival in these patients was 40.6%.
The researchers found HSCT recipients with a 10/10 HLA-matched donor had significantly better overall survival (OS) and lower non-relapse mortality (NRM) than patients with a mismatched donor.
The 5-year OS was 43.1% for a 10/10 match, 35.6% for a 9/10 match, and 28.4% for a match less than 9/10 (P=0.001). NRM at 1 year was 20.3%, 26.0%, and 33.4%, respectively (P=0.007).
Similarly, HSCT recipients with a CMV-matched donor had significantly better OS and significantly lower NRM than recipients with a CMV-mismatched donor.
The 5-year OS was 44.1% for recipients with a CMV-matched donor and 32.2% for patients with a mismatched donor (P<0.001). NRM at 1 year was 19.1% and 30.4%, respectively (P<0.001)
Most of the associations between CMV/HLA matching and OS/NRM remained significant in multivariate analyses.
For recipients with more than 1 HLA mismatch, the relative risk (RR) of death was 1.43 (P=0.016), and the RR for NRM was 1.59 (P=0.028), when compared to patients who had received a 10/10 HLA-matched graft.
For recipients with a single mismatch, the RR for death was 1.21 (P=0.042), and the RR for NRM was 1.24 (P=0.14).
For recipients with a CMV mismatched donor, the RR for death was 1.40 (P<0.001), and the RR for NRM was 1.63 (P<0.001).
The researchers also assessed CMV and HLA status together. Compared to fully HLA-matched and CMV-matched recipients, the RRs for death were:
- 1.36 (P=0.003) for HLA matched/CMV mismatched
- 1.22 (P=0.062) for HLA mismatched/CMV matched
- 1.81 (P=0.001) for HLA and CMV mismatched.
The researchers said these results suggest it is possible to improve survival rates for patients with no HLA-matched donor by matching the CMV status of the donor and recipient.
As a result of the findings, experts at Anthony Nolan are exploring how to type donors for CMV when joining the stem cell donor register to allow CMV status to be taken into account when transplant centers are selecting potential donors for a patient.
“[B]y establishing that CMV matching has a significant impact on patient outcomes, we are making it easier for transplant centers to make informed choices about the donors they select for their patients,” Dr Marsh said. ![]()
Matching the cytomegalovirus (CMV) status of the donor and recipient of a hematopoietic stem cell transplant (HSCT) can significantly improve the recipient’s survival, according to a study published in Bone Marrow Transplantation.
In fact, researchers said they found evidence to suggest that CMV matching may abrogate the effect of a human leukocyte antigen (HLA) mismatch.
“This breakthrough will help us discover new and more effective ways to make sure patients in need of a transplant get the best possible match to cure blood cancer and blood disorders,” said study author Steven Marsh, PhD, of Anthony Nolan Research Institute, Royal Free Hospital in London, UK.
Dr Marsh and his colleagues studied 1271 patients who received T-cell-depleted grafts to treat a hematologic disorder, including acute or chronic leukemia, lymphoma, myeloma, myelodysplasia, and “other” disorders.
The 5-year overall survival in these patients was 40.6%.
The researchers found HSCT recipients with a 10/10 HLA-matched donor had significantly better overall survival (OS) and lower non-relapse mortality (NRM) than patients with a mismatched donor.
The 5-year OS was 43.1% for a 10/10 match, 35.6% for a 9/10 match, and 28.4% for a match less than 9/10 (P=0.001). NRM at 1 year was 20.3%, 26.0%, and 33.4%, respectively (P=0.007).
Similarly, HSCT recipients with a CMV-matched donor had significantly better OS and significantly lower NRM than recipients with a CMV-mismatched donor.
The 5-year OS was 44.1% for recipients with a CMV-matched donor and 32.2% for patients with a mismatched donor (P<0.001). NRM at 1 year was 19.1% and 30.4%, respectively (P<0.001)
Most of the associations between CMV/HLA matching and OS/NRM remained significant in multivariate analyses.
For recipients with more than 1 HLA mismatch, the relative risk (RR) of death was 1.43 (P=0.016), and the RR for NRM was 1.59 (P=0.028), when compared to patients who had received a 10/10 HLA-matched graft.
For recipients with a single mismatch, the RR for death was 1.21 (P=0.042), and the RR for NRM was 1.24 (P=0.14).
For recipients with a CMV mismatched donor, the RR for death was 1.40 (P<0.001), and the RR for NRM was 1.63 (P<0.001).
The researchers also assessed CMV and HLA status together. Compared to fully HLA-matched and CMV-matched recipients, the RRs for death were:
- 1.36 (P=0.003) for HLA matched/CMV mismatched
- 1.22 (P=0.062) for HLA mismatched/CMV matched
- 1.81 (P=0.001) for HLA and CMV mismatched.
The researchers said these results suggest it is possible to improve survival rates for patients with no HLA-matched donor by matching the CMV status of the donor and recipient.
As a result of the findings, experts at Anthony Nolan are exploring how to type donors for CMV when joining the stem cell donor register to allow CMV status to be taken into account when transplant centers are selecting potential donors for a patient.
“[B]y establishing that CMV matching has a significant impact on patient outcomes, we are making it easier for transplant centers to make informed choices about the donors they select for their patients,” Dr Marsh said. ![]()
Study: Most oncologists don’t discuss exercise with patients
Results of a small, single-center study suggest oncologists may not provide cancer patients with adequate guidance on exercise.
A majority of the patients and oncologists surveyed for this study placed importance on exercise during cancer care, but most of the oncologists failed to give patients recommendations on exercise.
“Our results indicate that exercise is perceived as important to patients with cancer, both from a patient and physician perspective,” said study author Agnes Smaradottir, MD, of Gundersen Health System in La Crosse, Wisconsin.
“However, physicians are reluctant to consistently include [physical activity] recommendations in their patient discussions.”
Dr Smaradottir and her colleagues reported these findings in JNCCN.
The researchers surveyed 20 cancer patients and 9 oncologists for this study.
The patients’ mean age was 64. Ten patients had stage I-III non-metastatic cancer after adjuvant therapy, and 10 had stage IV metastatic disease and were undergoing palliative treatment. Most patients had solid tumor malignancies, but 1 had chronic lymphocytic leukemia.
The oncologists’ mean age was 45, 56% were male, and they had a mean of 12 years of practice. Most (89%) said they exercise on a regular basis.
Discussions
Nineteen (95%) of the patients surveyed felt they benefited from exercise during treatment, but only 3 of the patients recalled being instructed to exercise.
Exercise was felt to be an equally important part of treatment and well-being for patients with early stage cancer treated with curative intent as well as patients receiving palliative therapy.
Although all the oncologists noted that exercise can benefit cancer patients, only 1 of the 9 surveyed documented discussion of exercise in patient charts.
Preferences and concerns
More than 80% of the patients said they would prefer a home-based exercise regimen that could be performed in alignment with their personal schedules and symptoms.
Patients also noted a preference that exercise recommendations come from their oncologists, as they have an established relationship and feel their oncologists best understand the complexities of their personalized treatment plans.
The oncologists, on the other hand, wanted to refer patients to specialist care for exercise recommendations. Reasons for this included the oncologists’ mounting clinic schedules and a lack of education about appropriate physical activity recommendations for patients.
The oncologists also expressed concern about asking patients to be more physically active during chemotherapy and radiation and expressed trepidation about prescribing exercise to frail patients with limited mobility.
“We were surprised by the gap in expectations regarding exercise recommendation between patients and providers,” Dr Smaradottir said. “Many providers, ourselves included, thought patients would prefer to be referred to an exercise center, but they clearly preferred to have a home-based program recommended by their oncologist.”
“Our findings highlight the value of examining both patient and provider attitudes and behavioral intentions. While we uncovered barriers to exercise recommendations, questions remain on how to bridge the gap between patient and provider preferences.” ![]()
Results of a small, single-center study suggest oncologists may not provide cancer patients with adequate guidance on exercise.
A majority of the patients and oncologists surveyed for this study placed importance on exercise during cancer care, but most of the oncologists failed to give patients recommendations on exercise.
“Our results indicate that exercise is perceived as important to patients with cancer, both from a patient and physician perspective,” said study author Agnes Smaradottir, MD, of Gundersen Health System in La Crosse, Wisconsin.
“However, physicians are reluctant to consistently include [physical activity] recommendations in their patient discussions.”
Dr Smaradottir and her colleagues reported these findings in JNCCN.
The researchers surveyed 20 cancer patients and 9 oncologists for this study.
The patients’ mean age was 64. Ten patients had stage I-III non-metastatic cancer after adjuvant therapy, and 10 had stage IV metastatic disease and were undergoing palliative treatment. Most patients had solid tumor malignancies, but 1 had chronic lymphocytic leukemia.
The oncologists’ mean age was 45, 56% were male, and they had a mean of 12 years of practice. Most (89%) said they exercise on a regular basis.
Discussions
Nineteen (95%) of the patients surveyed felt they benefited from exercise during treatment, but only 3 of the patients recalled being instructed to exercise.
Exercise was felt to be an equally important part of treatment and well-being for patients with early stage cancer treated with curative intent as well as patients receiving palliative therapy.
Although all the oncologists noted that exercise can benefit cancer patients, only 1 of the 9 surveyed documented discussion of exercise in patient charts.
Preferences and concerns
More than 80% of the patients said they would prefer a home-based exercise regimen that could be performed in alignment with their personal schedules and symptoms.
Patients also noted a preference that exercise recommendations come from their oncologists, as they have an established relationship and feel their oncologists best understand the complexities of their personalized treatment plans.
The oncologists, on the other hand, wanted to refer patients to specialist care for exercise recommendations. Reasons for this included the oncologists’ mounting clinic schedules and a lack of education about appropriate physical activity recommendations for patients.
The oncologists also expressed concern about asking patients to be more physically active during chemotherapy and radiation and expressed trepidation about prescribing exercise to frail patients with limited mobility.
“We were surprised by the gap in expectations regarding exercise recommendation between patients and providers,” Dr Smaradottir said. “Many providers, ourselves included, thought patients would prefer to be referred to an exercise center, but they clearly preferred to have a home-based program recommended by their oncologist.”
“Our findings highlight the value of examining both patient and provider attitudes and behavioral intentions. While we uncovered barriers to exercise recommendations, questions remain on how to bridge the gap between patient and provider preferences.” ![]()
Results of a small, single-center study suggest oncologists may not provide cancer patients with adequate guidance on exercise.
A majority of the patients and oncologists surveyed for this study placed importance on exercise during cancer care, but most of the oncologists failed to give patients recommendations on exercise.
“Our results indicate that exercise is perceived as important to patients with cancer, both from a patient and physician perspective,” said study author Agnes Smaradottir, MD, of Gundersen Health System in La Crosse, Wisconsin.
“However, physicians are reluctant to consistently include [physical activity] recommendations in their patient discussions.”
Dr Smaradottir and her colleagues reported these findings in JNCCN.
The researchers surveyed 20 cancer patients and 9 oncologists for this study.
The patients’ mean age was 64. Ten patients had stage I-III non-metastatic cancer after adjuvant therapy, and 10 had stage IV metastatic disease and were undergoing palliative treatment. Most patients had solid tumor malignancies, but 1 had chronic lymphocytic leukemia.
The oncologists’ mean age was 45, 56% were male, and they had a mean of 12 years of practice. Most (89%) said they exercise on a regular basis.
Discussions
Nineteen (95%) of the patients surveyed felt they benefited from exercise during treatment, but only 3 of the patients recalled being instructed to exercise.
Exercise was felt to be an equally important part of treatment and well-being for patients with early stage cancer treated with curative intent as well as patients receiving palliative therapy.
Although all the oncologists noted that exercise can benefit cancer patients, only 1 of the 9 surveyed documented discussion of exercise in patient charts.
Preferences and concerns
More than 80% of the patients said they would prefer a home-based exercise regimen that could be performed in alignment with their personal schedules and symptoms.
Patients also noted a preference that exercise recommendations come from their oncologists, as they have an established relationship and feel their oncologists best understand the complexities of their personalized treatment plans.
The oncologists, on the other hand, wanted to refer patients to specialist care for exercise recommendations. Reasons for this included the oncologists’ mounting clinic schedules and a lack of education about appropriate physical activity recommendations for patients.
The oncologists also expressed concern about asking patients to be more physically active during chemotherapy and radiation and expressed trepidation about prescribing exercise to frail patients with limited mobility.
“We were surprised by the gap in expectations regarding exercise recommendation between patients and providers,” Dr Smaradottir said. “Many providers, ourselves included, thought patients would prefer to be referred to an exercise center, but they clearly preferred to have a home-based program recommended by their oncologist.”
“Our findings highlight the value of examining both patient and provider attitudes and behavioral intentions. While we uncovered barriers to exercise recommendations, questions remain on how to bridge the gap between patient and provider preferences.” ![]()
System monitors and maintains drug levels in the body
New technology could make it easier to ensure patients receive the correct dose of chemotherapy and other drugs, according to research published in Nature Biomedical Engineering.
Researchers developed a closed-loop system that was able to continuously regulate drug levels in rabbits and rats.
“This is the first time anyone has been able to continuously control the drug levels in the body in real time,” said study author H. Tom Soh, PhD, of Stanford University in California.
“This is a novel concept with big implications because we believe we can adapt our technology to control the levels of a wide range of drugs.”
The researchers’ system has 3 basic components: a real-time biosensor to continuously monitor drug levels in the bloodstream, a control system to calculate the right dose, and a programmable pump that delivers just enough medicine to maintain a desired dose.
The sensor contains aptamers that are specially designed to bind a drug of interest. When the drug is present in the bloodstream, the aptamer changes shape, which an electric sensor detects. The more drug, the more aptamers change shape.
That information, captured every few seconds, is routed through software that controls the pump to deliver additional drugs as needed.
Researchers tested the technology by administering the chemotherapy drug doxorubicin to rabbits and rats.
Despite physiological and metabolic differences among individual animals, the team was able to keep a constant dosage in all the animals, something not possible with current drug delivery methods.
The researchers also tested for acute drug-drug interactions and found the system was able to stabilize drug levels to moderate what might otherwise be a dangerous spike or dip.
Dr Soh and his colleagues believe this technology could be particularly useful in treating pediatric cancer patients, who are notoriously difficult to dose because a child’s metabolism is usually different from an adult’s.
The team plans to miniaturize the system so it can be implanted or worn by the patient.
At present, the technology is an external apparatus, like a smart IV drip. The biosensor is a device about the size of a microscope slide.
The current setup might be suitable for a chemotherapy drug but not for continual use.
The researchers are also adapting the system with different aptamers so it can sense and regulate the levels of other biomolecules in the body. ![]()
New technology could make it easier to ensure patients receive the correct dose of chemotherapy and other drugs, according to research published in Nature Biomedical Engineering.
Researchers developed a closed-loop system that was able to continuously regulate drug levels in rabbits and rats.
“This is the first time anyone has been able to continuously control the drug levels in the body in real time,” said study author H. Tom Soh, PhD, of Stanford University in California.
“This is a novel concept with big implications because we believe we can adapt our technology to control the levels of a wide range of drugs.”
The researchers’ system has 3 basic components: a real-time biosensor to continuously monitor drug levels in the bloodstream, a control system to calculate the right dose, and a programmable pump that delivers just enough medicine to maintain a desired dose.
The sensor contains aptamers that are specially designed to bind a drug of interest. When the drug is present in the bloodstream, the aptamer changes shape, which an electric sensor detects. The more drug, the more aptamers change shape.
That information, captured every few seconds, is routed through software that controls the pump to deliver additional drugs as needed.
Researchers tested the technology by administering the chemotherapy drug doxorubicin to rabbits and rats.
Despite physiological and metabolic differences among individual animals, the team was able to keep a constant dosage in all the animals, something not possible with current drug delivery methods.
The researchers also tested for acute drug-drug interactions and found the system was able to stabilize drug levels to moderate what might otherwise be a dangerous spike or dip.
Dr Soh and his colleagues believe this technology could be particularly useful in treating pediatric cancer patients, who are notoriously difficult to dose because a child’s metabolism is usually different from an adult’s.
The team plans to miniaturize the system so it can be implanted or worn by the patient.
At present, the technology is an external apparatus, like a smart IV drip. The biosensor is a device about the size of a microscope slide.
The current setup might be suitable for a chemotherapy drug but not for continual use.
The researchers are also adapting the system with different aptamers so it can sense and regulate the levels of other biomolecules in the body. ![]()
New technology could make it easier to ensure patients receive the correct dose of chemotherapy and other drugs, according to research published in Nature Biomedical Engineering.
Researchers developed a closed-loop system that was able to continuously regulate drug levels in rabbits and rats.
“This is the first time anyone has been able to continuously control the drug levels in the body in real time,” said study author H. Tom Soh, PhD, of Stanford University in California.
“This is a novel concept with big implications because we believe we can adapt our technology to control the levels of a wide range of drugs.”
The researchers’ system has 3 basic components: a real-time biosensor to continuously monitor drug levels in the bloodstream, a control system to calculate the right dose, and a programmable pump that delivers just enough medicine to maintain a desired dose.
The sensor contains aptamers that are specially designed to bind a drug of interest. When the drug is present in the bloodstream, the aptamer changes shape, which an electric sensor detects. The more drug, the more aptamers change shape.
That information, captured every few seconds, is routed through software that controls the pump to deliver additional drugs as needed.
Researchers tested the technology by administering the chemotherapy drug doxorubicin to rabbits and rats.
Despite physiological and metabolic differences among individual animals, the team was able to keep a constant dosage in all the animals, something not possible with current drug delivery methods.
The researchers also tested for acute drug-drug interactions and found the system was able to stabilize drug levels to moderate what might otherwise be a dangerous spike or dip.
Dr Soh and his colleagues believe this technology could be particularly useful in treating pediatric cancer patients, who are notoriously difficult to dose because a child’s metabolism is usually different from an adult’s.
The team plans to miniaturize the system so it can be implanted or worn by the patient.
At present, the technology is an external apparatus, like a smart IV drip. The biosensor is a device about the size of a microscope slide.
The current setup might be suitable for a chemotherapy drug but not for continual use.
The researchers are also adapting the system with different aptamers so it can sense and regulate the levels of other biomolecules in the body.
Iron-transporting molecule could treat anemia, iron overload
Researchers say they have identified a small molecule that can transport iron when typical transport routes are mutated or absent.
The molecule, hinokitiol, was able to move iron into or out of cells by wrapping around iron atoms and shuttling them across the membrane layer.
Hinokitiol promoted gut iron absorption in rats and mice deficient in iron transport complexes, and it promoted hemoglobin production in zebrafish that otherwise couldn’t transport iron effectively.
The researchers believe these findings, published in Science, may lead to new treatments for disorders associated with iron metabolism, such as anemias and hemochromatosis.
“The long-term therapeutic implications of our work with hinokitiol points to potentially using this chemical to correct anemias caused by genetic deficiencies of iron transporters required for normal red cell formation,” said study author Barry Paw, MD, PhD, of Brigham and Women’s Hospital in Boston, Massachusetts.
“At the same time, hinokitiol has the potential to correct iron-overload syndromes, such as hemochromatosis. More extensive clinical trials are necessary to work out the full potential of hinokitiol and to identify potential toxicities that we have not identified using preclinical models.”
Dr Paw and his colleagues discovered the potential of hinokitiol when screening for a molecule that could restore growth to yeast lacking an iron transporter complex. Hinokitiol is a natural product originally isolated from the Taiwanese hinoki tree.
The researchers found that hinokitiol could transport iron across the yeast cellular membrane in mutant yeasts lacking their major iron uptake transporters. Three hinokitiol molecules can wrap around an iron atom and transport it directly across the membrane where the missing protein should be.
The team also tested hinokitiol in mice, rats, and zebrafish that were missing iron-transport proteins.
Orally administered hinokitiol restored iron uptake in the guts of ferroportin-deficient mice and DMT1-deficient rats, and adding hinokitiol to a tank housing DMT1- and mitoferrin-deficient zebrafish prompted hemoglobin production in the fish.
The researchers also found that hinokitiol restored iron transport in human cells taken from the lining of the gut.
“We found that hinokitiol can restore iron transport within cells, out of cells, or both,” Dr Paw said. “It can also promote iron gut absorption and the creation of hemoglobin in some of our models. These findings suggest that small molecules like hinokitiol that can mimic the biological function of a missing protein may have potential for treating human diseases.”
Researchers say they have identified a small molecule that can transport iron when typical transport routes are mutated or absent.
The molecule, hinokitiol, was able to move iron into or out of cells by wrapping around iron atoms and shuttling them across the membrane layer.
Hinokitiol promoted gut iron absorption in rats and mice deficient in iron transport complexes, and it promoted hemoglobin production in zebrafish that otherwise couldn’t transport iron effectively.
The researchers believe these findings, published in Science, may lead to new treatments for disorders associated with iron metabolism, such as anemias and hemochromatosis.
“The long-term therapeutic implications of our work with hinokitiol points to potentially using this chemical to correct anemias caused by genetic deficiencies of iron transporters required for normal red cell formation,” said study author Barry Paw, MD, PhD, of Brigham and Women’s Hospital in Boston, Massachusetts.
“At the same time, hinokitiol has the potential to correct iron-overload syndromes, such as hemochromatosis. More extensive clinical trials are necessary to work out the full potential of hinokitiol and to identify potential toxicities that we have not identified using preclinical models.”
Dr Paw and his colleagues discovered the potential of hinokitiol when screening for a molecule that could restore growth to yeast lacking an iron transporter complex. Hinokitiol is a natural product originally isolated from the Taiwanese hinoki tree.
The researchers found that hinokitiol could transport iron across the yeast cellular membrane in mutant yeasts lacking their major iron uptake transporters. Three hinokitiol molecules can wrap around an iron atom and transport it directly across the membrane where the missing protein should be.
The team also tested hinokitiol in mice, rats, and zebrafish that were missing iron-transport proteins.
Orally administered hinokitiol restored iron uptake in the guts of ferroportin-deficient mice and DMT1-deficient rats, and adding hinokitiol to a tank housing DMT1- and mitoferrin-deficient zebrafish prompted hemoglobin production in the fish.
The researchers also found that hinokitiol restored iron transport in human cells taken from the lining of the gut.
“We found that hinokitiol can restore iron transport within cells, out of cells, or both,” Dr Paw said. “It can also promote iron gut absorption and the creation of hemoglobin in some of our models. These findings suggest that small molecules like hinokitiol that can mimic the biological function of a missing protein may have potential for treating human diseases.”
Researchers say they have identified a small molecule that can transport iron when typical transport routes are mutated or absent.
The molecule, hinokitiol, was able to move iron into or out of cells by wrapping around iron atoms and shuttling them across the membrane layer.
Hinokitiol promoted gut iron absorption in rats and mice deficient in iron transport complexes, and it promoted hemoglobin production in zebrafish that otherwise couldn’t transport iron effectively.
The researchers believe these findings, published in Science, may lead to new treatments for disorders associated with iron metabolism, such as anemias and hemochromatosis.
“The long-term therapeutic implications of our work with hinokitiol points to potentially using this chemical to correct anemias caused by genetic deficiencies of iron transporters required for normal red cell formation,” said study author Barry Paw, MD, PhD, of Brigham and Women’s Hospital in Boston, Massachusetts.
“At the same time, hinokitiol has the potential to correct iron-overload syndromes, such as hemochromatosis. More extensive clinical trials are necessary to work out the full potential of hinokitiol and to identify potential toxicities that we have not identified using preclinical models.”
Dr Paw and his colleagues discovered the potential of hinokitiol when screening for a molecule that could restore growth to yeast lacking an iron transporter complex. Hinokitiol is a natural product originally isolated from the Taiwanese hinoki tree.
The researchers found that hinokitiol could transport iron across the yeast cellular membrane in mutant yeasts lacking their major iron uptake transporters. Three hinokitiol molecules can wrap around an iron atom and transport it directly across the membrane where the missing protein should be.
The team also tested hinokitiol in mice, rats, and zebrafish that were missing iron-transport proteins.
Orally administered hinokitiol restored iron uptake in the guts of ferroportin-deficient mice and DMT1-deficient rats, and adding hinokitiol to a tank housing DMT1- and mitoferrin-deficient zebrafish prompted hemoglobin production in the fish.
The researchers also found that hinokitiol restored iron transport in human cells taken from the lining of the gut.
“We found that hinokitiol can restore iron transport within cells, out of cells, or both,” Dr Paw said. “It can also promote iron gut absorption and the creation of hemoglobin in some of our models. These findings suggest that small molecules like hinokitiol that can mimic the biological function of a missing protein may have potential for treating human diseases.”
Videos reduce need for anesthesia in kids undergoing radiotherapy
VIENNA, AUSTRIA—Children with cancer may not require general anesthesia prior to radiotherapy if they can watch videos during their treatment, according to research presented at the ESTRO 36 conference (abstract OC-0546).
Allowing children to watch videos during radiotherapy reduced but did not completely eliminate the use of anesthesia in this small study.
The use of videos proved less traumatic than anesthesia for children and their families, as well as making each treatment quicker and more cost-effective, according to study investigator Catia Aguas, of the Cliniques Universitaires Saint Luc in Brussels, Belgium.
“Being treated with radiotherapy means coming in for a treatment every weekday for 4 to 6 weeks,” Aguas noted. “The children need to remain motionless during treatment, and, on the whole, that means a general anesthesia. That, in turn, means they have to keep their stomach empty for 6 hours before the treatment.”
“We wanted to see if installing a projector and letting children watch a video of their choice would allow them to keep still enough that we would not need to give them anesthesia.”
The study included 12 children, ages 1.5 to 6 years, who were treated with radiotherapy using a Tomotherapy® treatment unit at the university hospital. Six children were treated before a video projector was installed in 2014, and 6 were treated after.
Before the video was available, general anesthesia was needed for 83.3% of children’s treatments. Once the projector was installed, anesthesia was needed in 33.3% of treatments.
“Radiotherapy can be very scary for children,” Aguas noted. “It’s a huge room full of machines and strange noises, and the worst part is that they’re in the room alone during their treatment. Before their radiotherapy treatment, they have already been through a series of tests and treatments, some of them painful, so when they arrive for radiotherapy, they don’t really feel very safe or confident.”
“Since we started using videos, children are a lot less anxious. Now they know that they’re going to watch a movie of their choice, they’re more relaxed, and, once the movie starts, it’s as though they travel to another world. Sponge Bob, Cars, and Barbie have been popular movie choices with our patients.”
The research also showed that treatments that used to take 1 hour or more now take around 15 to 20 minutes. This is partly because of the time saved by not having to prepare and administer anesthesia, but it is also because the children who know they are going to watch videos are more cooperative.
“Now, in our clinic, video has almost completely replaced anesthesia, resulting in reduced treatment times and reduction of stress for the young patients and their families,” Aguas said.
She also noted that the projector was inexpensive and simple to install.
“In radiotherapy, everything is usually very expensive, but, in this case, it was not,” Aguas said. “We bought a projector, and, with the help of college students, we created a support to fix the device to the patient couch. Using video is saving money and resources by reducing the need for anesthesia.”
Aguas and her colleagues continue to study children who have been treated since the projector was installed, and the team is extending the project to include adult patients who are claustrophobic or anxious.
VIENNA, AUSTRIA—Children with cancer may not require general anesthesia prior to radiotherapy if they can watch videos during their treatment, according to research presented at the ESTRO 36 conference (abstract OC-0546).
Allowing children to watch videos during radiotherapy reduced but did not completely eliminate the use of anesthesia in this small study.
The use of videos proved less traumatic than anesthesia for children and their families, as well as making each treatment quicker and more cost-effective, according to study investigator Catia Aguas, of the Cliniques Universitaires Saint Luc in Brussels, Belgium.
“Being treated with radiotherapy means coming in for a treatment every weekday for 4 to 6 weeks,” Aguas noted. “The children need to remain motionless during treatment, and, on the whole, that means a general anesthesia. That, in turn, means they have to keep their stomach empty for 6 hours before the treatment.”
“We wanted to see if installing a projector and letting children watch a video of their choice would allow them to keep still enough that we would not need to give them anesthesia.”
The study included 12 children, ages 1.5 to 6 years, who were treated with radiotherapy using a Tomotherapy® treatment unit at the university hospital. Six children were treated before a video projector was installed in 2014, and 6 were treated after.
Before the video was available, general anesthesia was needed for 83.3% of children’s treatments. Once the projector was installed, anesthesia was needed in 33.3% of treatments.
“Radiotherapy can be very scary for children,” Aguas noted. “It’s a huge room full of machines and strange noises, and the worst part is that they’re in the room alone during their treatment. Before their radiotherapy treatment, they have already been through a series of tests and treatments, some of them painful, so when they arrive for radiotherapy, they don’t really feel very safe or confident.”
“Since we started using videos, children are a lot less anxious. Now they know that they’re going to watch a movie of their choice, they’re more relaxed, and, once the movie starts, it’s as though they travel to another world. Sponge Bob, Cars, and Barbie have been popular movie choices with our patients.”
The research also showed that treatments that used to take 1 hour or more now take around 15 to 20 minutes. This is partly because of the time saved by not having to prepare and administer anesthesia, but it is also because the children who know they are going to watch videos are more cooperative.
“Now, in our clinic, video has almost completely replaced anesthesia, resulting in reduced treatment times and reduction of stress for the young patients and their families,” Aguas said.
She also noted that the projector was inexpensive and simple to install.
“In radiotherapy, everything is usually very expensive, but, in this case, it was not,” Aguas said. “We bought a projector, and, with the help of college students, we created a support to fix the device to the patient couch. Using video is saving money and resources by reducing the need for anesthesia.”
Aguas and her colleagues continue to study children who have been treated since the projector was installed, and the team is extending the project to include adult patients who are claustrophobic or anxious.
VIENNA, AUSTRIA—Children with cancer may not require general anesthesia prior to radiotherapy if they can watch videos during their treatment, according to research presented at the ESTRO 36 conference (abstract OC-0546).
Allowing children to watch videos during radiotherapy reduced but did not completely eliminate the use of anesthesia in this small study.
The use of videos proved less traumatic than anesthesia for children and their families, as well as making each treatment quicker and more cost-effective, according to study investigator Catia Aguas, of the Cliniques Universitaires Saint Luc in Brussels, Belgium.
“Being treated with radiotherapy means coming in for a treatment every weekday for 4 to 6 weeks,” Aguas noted. “The children need to remain motionless during treatment, and, on the whole, that means a general anesthesia. That, in turn, means they have to keep their stomach empty for 6 hours before the treatment.”
“We wanted to see if installing a projector and letting children watch a video of their choice would allow them to keep still enough that we would not need to give them anesthesia.”
The study included 12 children, ages 1.5 to 6 years, who were treated with radiotherapy using a Tomotherapy® treatment unit at the university hospital. Six children were treated before a video projector was installed in 2014, and 6 were treated after.
Before the video was available, general anesthesia was needed for 83.3% of children’s treatments. Once the projector was installed, anesthesia was needed in 33.3% of treatments.
“Radiotherapy can be very scary for children,” Aguas noted. “It’s a huge room full of machines and strange noises, and the worst part is that they’re in the room alone during their treatment. Before their radiotherapy treatment, they have already been through a series of tests and treatments, some of them painful, so when they arrive for radiotherapy, they don’t really feel very safe or confident.”
“Since we started using videos, children are a lot less anxious. Now they know that they’re going to watch a movie of their choice, they’re more relaxed, and, once the movie starts, it’s as though they travel to another world. Sponge Bob, Cars, and Barbie have been popular movie choices with our patients.”
The research also showed that treatments that used to take 1 hour or more now take around 15 to 20 minutes. This is partly because of the time saved by not having to prepare and administer anesthesia, but it is also because the children who know they are going to watch videos are more cooperative.
“Now, in our clinic, video has almost completely replaced anesthesia, resulting in reduced treatment times and reduction of stress for the young patients and their families,” Aguas said.
She also noted that the projector was inexpensive and simple to install.
“In radiotherapy, everything is usually very expensive, but, in this case, it was not,” Aguas said. “We bought a projector, and, with the help of college students, we created a support to fix the device to the patient couch. Using video is saving money and resources by reducing the need for anesthesia.”
Aguas and her colleagues continue to study children who have been treated since the projector was installed, and the team is extending the project to include adult patients who are claustrophobic or anxious.
Company stops developing hemophilia therapy
Dimension Therapeutics, Inc. has announced its decision to stop development of DTX101, a gene therapy product intended to treat moderate/severe to severe hemophilia B.
The decision was based on “disappointing” results from the phase 1/2 study of DTX101, according to Annalisa Jenkins, MBBS, chief executive officer of Dimension.
“[O]ur phase 1/2, open-label clinical study did not demonstrate an ability to achieve a minimum target product profile for continued development or future commercialization,” Dr Jenkins said.
Dimension previously reported results from this 6-patient study in February.
At that time, the data showed that DTX101 could increase factor IX (FIX) expression in adults with hemophilia B, allowing 3 of them to forgo prophylactic and on-demand treatment.
However, 5 of the 6 patients in the trial had alanine aminotransferase elevations, and 1 patient had a grade 4 adverse event as a result.
Dimension said it plans to present full results from this study, including results from ongoing immune and biomarker analyses, at a future scientific conference.
“[D]imension remains committed to the hemophilia community through continued investment in the company’s ongoing [investigational new drug]-enabling activities for DTX201 for hemophilia A, in collaboration with Bayer, and the follow-up of the 6 patients dosed with DTX101 in the phase 1/2 clinical trial through an extension study that will monitor all patients for a total of 5 years,” Dr Jenkins noted.
DTX101 was designed to deliver stable expression of FIX in patients with hemophilia B. It is a non-replicating, recombinant adeno-associated viral vector, AAVrh10, with a codon-optimized FIX gene expressing wild-type FIX protein.
Dimension Therapeutics, Inc. has announced its decision to stop development of DTX101, a gene therapy product intended to treat moderate/severe to severe hemophilia B.
The decision was based on “disappointing” results from the phase 1/2 study of DTX101, according to Annalisa Jenkins, MBBS, chief executive officer of Dimension.
“[O]ur phase 1/2, open-label clinical study did not demonstrate an ability to achieve a minimum target product profile for continued development or future commercialization,” Dr Jenkins said.
Dimension previously reported results from this 6-patient study in February.
At that time, the data showed that DTX101 could increase factor IX (FIX) expression in adults with hemophilia B, allowing 3 of them to forgo prophylactic and on-demand treatment.
However, 5 of the 6 patients in the trial had alanine aminotransferase elevations, and 1 patient had a grade 4 adverse event as a result.
Dimension said it plans to present full results from this study, including results from ongoing immune and biomarker analyses, at a future scientific conference.
“[D]imension remains committed to the hemophilia community through continued investment in the company’s ongoing [investigational new drug]-enabling activities for DTX201 for hemophilia A, in collaboration with Bayer, and the follow-up of the 6 patients dosed with DTX101 in the phase 1/2 clinical trial through an extension study that will monitor all patients for a total of 5 years,” Dr Jenkins noted.
DTX101 was designed to deliver stable expression of FIX in patients with hemophilia B. It is a non-replicating, recombinant adeno-associated viral vector, AAVrh10, with a codon-optimized FIX gene expressing wild-type FIX protein.
Dimension Therapeutics, Inc. has announced its decision to stop development of DTX101, a gene therapy product intended to treat moderate/severe to severe hemophilia B.
The decision was based on “disappointing” results from the phase 1/2 study of DTX101, according to Annalisa Jenkins, MBBS, chief executive officer of Dimension.
“[O]ur phase 1/2, open-label clinical study did not demonstrate an ability to achieve a minimum target product profile for continued development or future commercialization,” Dr Jenkins said.
Dimension previously reported results from this 6-patient study in February.
At that time, the data showed that DTX101 could increase factor IX (FIX) expression in adults with hemophilia B, allowing 3 of them to forgo prophylactic and on-demand treatment.
However, 5 of the 6 patients in the trial had alanine aminotransferase elevations, and 1 patient had a grade 4 adverse event as a result.
Dimension said it plans to present full results from this study, including results from ongoing immune and biomarker analyses, at a future scientific conference.
“[D]imension remains committed to the hemophilia community through continued investment in the company’s ongoing [investigational new drug]-enabling activities for DTX201 for hemophilia A, in collaboration with Bayer, and the follow-up of the 6 patients dosed with DTX101 in the phase 1/2 clinical trial through an extension study that will monitor all patients for a total of 5 years,” Dr Jenkins noted.
DTX101 was designed to deliver stable expression of FIX in patients with hemophilia B. It is a non-replicating, recombinant adeno-associated viral vector, AAVrh10, with a codon-optimized FIX gene expressing wild-type FIX protein.