User login
Second cancers take greater toll on younger patients
Second cancers take a greater toll on patients under the age of 40, according to research published in JAMA Oncology.
Researchers studied 14 types of cancer occurring in more than 1 million patients.
For nearly all of the cancers studied, 5-year survival rates were much higher if the cancer occurred as a first malignancy rather than a second cancer.
These survival differences were more pronounced in pediatric patients and adolescents and young adults (AYAs) than they were in patients age 40 and older.
Researchers hope these findings will help guide clinicians in providing age-specific recommendations on cancer prevention, screening, treatment, and survivorship, especially among the AYA population.
“Although the increased incidence of second cancers is well known among cancer survivors, less is known about outcomes of these cancers or the influence of age,” said Theresa Keegan, PhD, of the UC Davis Comprehensive Cancer Center in Sacramento, California.
With this in mind, Dr Keegan and her colleagues analyzed data on patients diagnosed with either a single cancer or a first and second malignancy during 1992 through 2008. The researchers used Surveillance, Epidemiology and End Results program data collected from 13 cancer registries.
The team collected data on the 14 most common cancer types that affect AYAs: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), soft tissue sarcoma, and bone sarcoma, as well as female breast, thyroid, testicular, colorectal, central nervous system, cervical, and ovarian cancers.
There were a total of 15,954 pediatric patients (younger than 15 years at diagnosis), 125,750 AYAs (ages 15 to 39), and 878,370 older adult patients (age 40 and older).
Survival rates
For pediatric patients, the 5-year relative survival was 80% for a first cancer and 47% for a second primary malignancy.
For AYAs, the 5-year relative survival was 81% for a first cancer and 60% for a second primary malignancy.
For older adults, the 5-year relative survival was 70% for a first cancer and 61% for a second primary malignancy.
When the researchers looked at 5-year survival by age and individual cancer types, they found striking differences depending on whether it was a first or second malignancy in all but 2 of the 14 cancer types, testicular cancer and melanoma.
“For almost every type of cancer, the AYA population did worse with a secondary cancer,” said study author Melanie Goldfarb, MD, of John Wayne Cancer Institute at Providence Saint John’s Health Center in Santa Monica, California.
“What struck us was that the second cancer caused such an increased risk of death.”
Lymphomas
For pediatric patients with HL, the 5-year relative survival was 95% when patients had HL as a first cancer. There were no data on HL as a second primary malignancy.
For AYAs, the 5-year relative survival was 93% when patients had HL as a first cancer and 72% when they had HL as a second primary malignancy.
For older adults, the 5-year relative survival was 69% when patients had HL as a first cancer and 54% when they had HL as a second primary malignancy.
For pediatric patients with NHL, the 5-year relative survival was 84% when patients had NHL as a first cancer and 63% when they had NHL as a second primary malignancy.
For AYAs, the 5-year relative survival was 64% when patients had NHL as a first cancer and 22% when they had NHL as a second primary malignancy.
For older adults, the 5-year relative survival was 57% when patients had NHL as a first cancer and 54% when they had NHL as a second primary malignancy.
Leukemias
For pediatric patients with ALL, the 5-year relative survival was 87% when patients had ALL as a first cancer and 63% when they had ALL as a second primary malignancy.
For AYAs, the 5-year relative survival was 48% when patients had ALL as a first cancer and 26% when they had ALL as a second primary malignancy.
For older adults, the 5-year relative survival was 17% when patients had ALL as a first cancer and 11% when they had ALL as a second primary malignancy.
For pediatric patients with AML, the 5-year relative survival was 57% when patients had AML as a first cancer and 29% when they had AML as a second primary malignancy.
For AYAs, the 5-year relative survival was 46% when patients had AML as a first cancer and 23% when they had AML as a second primary malignancy.
For older adults, the 5-year relative survival was 12% when patients had AML as a first cancer and 10% when they had AML as a second primary malignancy.
Why younger patients tend to fare worse after a second cancer than older patients is not fully understood or specifically addressed in the current study, the researchers noted.
Now, the team plans to examine how the time between getting a first and second cancer affects survival and whether the type of treatment for the first cancer influences the outcome of a second cancer. ![]()
Second cancers take a greater toll on patients under the age of 40, according to research published in JAMA Oncology.
Researchers studied 14 types of cancer occurring in more than 1 million patients.
For nearly all of the cancers studied, 5-year survival rates were much higher if the cancer occurred as a first malignancy rather than a second cancer.
These survival differences were more pronounced in pediatric patients and adolescents and young adults (AYAs) than they were in patients age 40 and older.
Researchers hope these findings will help guide clinicians in providing age-specific recommendations on cancer prevention, screening, treatment, and survivorship, especially among the AYA population.
“Although the increased incidence of second cancers is well known among cancer survivors, less is known about outcomes of these cancers or the influence of age,” said Theresa Keegan, PhD, of the UC Davis Comprehensive Cancer Center in Sacramento, California.
With this in mind, Dr Keegan and her colleagues analyzed data on patients diagnosed with either a single cancer or a first and second malignancy during 1992 through 2008. The researchers used Surveillance, Epidemiology and End Results program data collected from 13 cancer registries.
The team collected data on the 14 most common cancer types that affect AYAs: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), soft tissue sarcoma, and bone sarcoma, as well as female breast, thyroid, testicular, colorectal, central nervous system, cervical, and ovarian cancers.
There were a total of 15,954 pediatric patients (younger than 15 years at diagnosis), 125,750 AYAs (ages 15 to 39), and 878,370 older adult patients (age 40 and older).
Survival rates
For pediatric patients, the 5-year relative survival was 80% for a first cancer and 47% for a second primary malignancy.
For AYAs, the 5-year relative survival was 81% for a first cancer and 60% for a second primary malignancy.
For older adults, the 5-year relative survival was 70% for a first cancer and 61% for a second primary malignancy.
When the researchers looked at 5-year survival by age and individual cancer types, they found striking differences depending on whether it was a first or second malignancy in all but 2 of the 14 cancer types, testicular cancer and melanoma.
“For almost every type of cancer, the AYA population did worse with a secondary cancer,” said study author Melanie Goldfarb, MD, of John Wayne Cancer Institute at Providence Saint John’s Health Center in Santa Monica, California.
“What struck us was that the second cancer caused such an increased risk of death.”
Lymphomas
For pediatric patients with HL, the 5-year relative survival was 95% when patients had HL as a first cancer. There were no data on HL as a second primary malignancy.
For AYAs, the 5-year relative survival was 93% when patients had HL as a first cancer and 72% when they had HL as a second primary malignancy.
For older adults, the 5-year relative survival was 69% when patients had HL as a first cancer and 54% when they had HL as a second primary malignancy.
For pediatric patients with NHL, the 5-year relative survival was 84% when patients had NHL as a first cancer and 63% when they had NHL as a second primary malignancy.
For AYAs, the 5-year relative survival was 64% when patients had NHL as a first cancer and 22% when they had NHL as a second primary malignancy.
For older adults, the 5-year relative survival was 57% when patients had NHL as a first cancer and 54% when they had NHL as a second primary malignancy.
Leukemias
For pediatric patients with ALL, the 5-year relative survival was 87% when patients had ALL as a first cancer and 63% when they had ALL as a second primary malignancy.
For AYAs, the 5-year relative survival was 48% when patients had ALL as a first cancer and 26% when they had ALL as a second primary malignancy.
For older adults, the 5-year relative survival was 17% when patients had ALL as a first cancer and 11% when they had ALL as a second primary malignancy.
For pediatric patients with AML, the 5-year relative survival was 57% when patients had AML as a first cancer and 29% when they had AML as a second primary malignancy.
For AYAs, the 5-year relative survival was 46% when patients had AML as a first cancer and 23% when they had AML as a second primary malignancy.
For older adults, the 5-year relative survival was 12% when patients had AML as a first cancer and 10% when they had AML as a second primary malignancy.
Why younger patients tend to fare worse after a second cancer than older patients is not fully understood or specifically addressed in the current study, the researchers noted.
Now, the team plans to examine how the time between getting a first and second cancer affects survival and whether the type of treatment for the first cancer influences the outcome of a second cancer. ![]()
Second cancers take a greater toll on patients under the age of 40, according to research published in JAMA Oncology.
Researchers studied 14 types of cancer occurring in more than 1 million patients.
For nearly all of the cancers studied, 5-year survival rates were much higher if the cancer occurred as a first malignancy rather than a second cancer.
These survival differences were more pronounced in pediatric patients and adolescents and young adults (AYAs) than they were in patients age 40 and older.
Researchers hope these findings will help guide clinicians in providing age-specific recommendations on cancer prevention, screening, treatment, and survivorship, especially among the AYA population.
“Although the increased incidence of second cancers is well known among cancer survivors, less is known about outcomes of these cancers or the influence of age,” said Theresa Keegan, PhD, of the UC Davis Comprehensive Cancer Center in Sacramento, California.
With this in mind, Dr Keegan and her colleagues analyzed data on patients diagnosed with either a single cancer or a first and second malignancy during 1992 through 2008. The researchers used Surveillance, Epidemiology and End Results program data collected from 13 cancer registries.
The team collected data on the 14 most common cancer types that affect AYAs: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), soft tissue sarcoma, and bone sarcoma, as well as female breast, thyroid, testicular, colorectal, central nervous system, cervical, and ovarian cancers.
There were a total of 15,954 pediatric patients (younger than 15 years at diagnosis), 125,750 AYAs (ages 15 to 39), and 878,370 older adult patients (age 40 and older).
Survival rates
For pediatric patients, the 5-year relative survival was 80% for a first cancer and 47% for a second primary malignancy.
For AYAs, the 5-year relative survival was 81% for a first cancer and 60% for a second primary malignancy.
For older adults, the 5-year relative survival was 70% for a first cancer and 61% for a second primary malignancy.
When the researchers looked at 5-year survival by age and individual cancer types, they found striking differences depending on whether it was a first or second malignancy in all but 2 of the 14 cancer types, testicular cancer and melanoma.
“For almost every type of cancer, the AYA population did worse with a secondary cancer,” said study author Melanie Goldfarb, MD, of John Wayne Cancer Institute at Providence Saint John’s Health Center in Santa Monica, California.
“What struck us was that the second cancer caused such an increased risk of death.”
Lymphomas
For pediatric patients with HL, the 5-year relative survival was 95% when patients had HL as a first cancer. There were no data on HL as a second primary malignancy.
For AYAs, the 5-year relative survival was 93% when patients had HL as a first cancer and 72% when they had HL as a second primary malignancy.
For older adults, the 5-year relative survival was 69% when patients had HL as a first cancer and 54% when they had HL as a second primary malignancy.
For pediatric patients with NHL, the 5-year relative survival was 84% when patients had NHL as a first cancer and 63% when they had NHL as a second primary malignancy.
For AYAs, the 5-year relative survival was 64% when patients had NHL as a first cancer and 22% when they had NHL as a second primary malignancy.
For older adults, the 5-year relative survival was 57% when patients had NHL as a first cancer and 54% when they had NHL as a second primary malignancy.
Leukemias
For pediatric patients with ALL, the 5-year relative survival was 87% when patients had ALL as a first cancer and 63% when they had ALL as a second primary malignancy.
For AYAs, the 5-year relative survival was 48% when patients had ALL as a first cancer and 26% when they had ALL as a second primary malignancy.
For older adults, the 5-year relative survival was 17% when patients had ALL as a first cancer and 11% when they had ALL as a second primary malignancy.
For pediatric patients with AML, the 5-year relative survival was 57% when patients had AML as a first cancer and 29% when they had AML as a second primary malignancy.
For AYAs, the 5-year relative survival was 46% when patients had AML as a first cancer and 23% when they had AML as a second primary malignancy.
For older adults, the 5-year relative survival was 12% when patients had AML as a first cancer and 10% when they had AML as a second primary malignancy.
Why younger patients tend to fare worse after a second cancer than older patients is not fully understood or specifically addressed in the current study, the researchers noted.
Now, the team plans to examine how the time between getting a first and second cancer affects survival and whether the type of treatment for the first cancer influences the outcome of a second cancer. ![]()
Cord blood product bests standard UCB transplant
The expanded umbilical cord blood (UCB) product NiCord can provide benefits over standard UCB transplant, according to research published in Biology of Blood and Marrow Transplantation.
NiCord is a stand-alone graft derived from a single UCB unit that has been expanded in culture and enriched with stem and progenitor cells.
The study showed that patients transplanted with NiCord had shorter time to engraftment, a lower risk of infection, and shorter hospital stays than patients who received standard UCB transplants.
On the other hand, there was no significant difference between the transplant groups when it came to grade 2-4 acute graft-versus-host disease (GVHD), relapse, or survival within 100 days of transplant.
“Our results indicate that rapid hematopoietic recovery from Gamida Cell’s NiCord transplantation approach is associated with clinical benefit,” said study author Mitchell Horwitz, MD, of Duke University School of Medicine in Durham, North Carolina.
Dr Horwitz receives research support from Gamida Cell Ltd., makers of NiCord.
Patient characteristics
The researchers compared 18 consecutive NiCord-transplanted patients and 86 consecutive patients who received standard UCB transplants. All of the patients received total body irradiation-based myeloablative conditioning.
In both arms, most patients received a double transplant. In the NiCord arm, 61.1% of patients (n=11) received NiCord along with a second unmanipulated UCB unit. In the standard UCB arm, 95.3% of patients (n=82) received a double UCB transplant.
Patients in the NiCord arm were older than patients in the standard UCB arm, with median ages of 45.5 (range, 42-57) and 37.5 (range, 28-51), respectively.
Most patients in both arms had acute leukemia or myelodysplastic syndromes (about 89%), although roughly 11% had lymphoid malignancies.
There were no significant differences between the arms when it came to patient sex, pre-transplant weight, cytomegalovirus serostatus, and Karnofsky Performance Status.
Results
The median time to neutrophil engraftment was 12.5 days (range, 10-18) in the NiCord arm and 27 days (range, 23-28) in the standard UCB arm (P<0.001).
All of the patients studied had at least 1 infection of any severity. However, patients in the NiCord arm had a significantly lower risk of infection than patients in the standard UCB arm.
In an analysis adjusted for age, disease stage, and grade 2-4 acute GVHD, the risk ratios (RRs) for NiCord versus standard UCB transplant were as follows:
- Total infection—RR=0.72, P=0.03
- Grade 2-3 infection—RR=0.38, P=0.001
- Bacterial infection—RR=0.42, P=0.008
- Grade 2-3 bacterial infection—RR=0.23, P=0.006.
Patients in the NiCord arm spent a mean of 72.4 days out of the hospital in the first 100 days after transplant, compared to 48.6 days for the standard UCB arm (P=0.001).
After the researchers adjusted for age and Karnofsky Performance Status, patients in the NiCord arm had a mean of 20.2 more days out of the hospital than their peers who received standard UCB (P=0.005).
The incidence of grade 2-4 acute GHVD was 55.6% in the NiCord arm and 41.9% in the standard UCB arm (P=0.31). The rate of second transplant was 5.6% and 11.6%, respectively (P=0.68).
The rate of relapse was 0% in the NiCord arm and 7% in the standard UCB arm (P=0.59). And the rate of death was 5.6% and 16.3%, respectively (P=0.46). ![]()
The expanded umbilical cord blood (UCB) product NiCord can provide benefits over standard UCB transplant, according to research published in Biology of Blood and Marrow Transplantation.
NiCord is a stand-alone graft derived from a single UCB unit that has been expanded in culture and enriched with stem and progenitor cells.
The study showed that patients transplanted with NiCord had shorter time to engraftment, a lower risk of infection, and shorter hospital stays than patients who received standard UCB transplants.
On the other hand, there was no significant difference between the transplant groups when it came to grade 2-4 acute graft-versus-host disease (GVHD), relapse, or survival within 100 days of transplant.
“Our results indicate that rapid hematopoietic recovery from Gamida Cell’s NiCord transplantation approach is associated with clinical benefit,” said study author Mitchell Horwitz, MD, of Duke University School of Medicine in Durham, North Carolina.
Dr Horwitz receives research support from Gamida Cell Ltd., makers of NiCord.
Patient characteristics
The researchers compared 18 consecutive NiCord-transplanted patients and 86 consecutive patients who received standard UCB transplants. All of the patients received total body irradiation-based myeloablative conditioning.
In both arms, most patients received a double transplant. In the NiCord arm, 61.1% of patients (n=11) received NiCord along with a second unmanipulated UCB unit. In the standard UCB arm, 95.3% of patients (n=82) received a double UCB transplant.
Patients in the NiCord arm were older than patients in the standard UCB arm, with median ages of 45.5 (range, 42-57) and 37.5 (range, 28-51), respectively.
Most patients in both arms had acute leukemia or myelodysplastic syndromes (about 89%), although roughly 11% had lymphoid malignancies.
There were no significant differences between the arms when it came to patient sex, pre-transplant weight, cytomegalovirus serostatus, and Karnofsky Performance Status.
Results
The median time to neutrophil engraftment was 12.5 days (range, 10-18) in the NiCord arm and 27 days (range, 23-28) in the standard UCB arm (P<0.001).
All of the patients studied had at least 1 infection of any severity. However, patients in the NiCord arm had a significantly lower risk of infection than patients in the standard UCB arm.
In an analysis adjusted for age, disease stage, and grade 2-4 acute GVHD, the risk ratios (RRs) for NiCord versus standard UCB transplant were as follows:
- Total infection—RR=0.72, P=0.03
- Grade 2-3 infection—RR=0.38, P=0.001
- Bacterial infection—RR=0.42, P=0.008
- Grade 2-3 bacterial infection—RR=0.23, P=0.006.
Patients in the NiCord arm spent a mean of 72.4 days out of the hospital in the first 100 days after transplant, compared to 48.6 days for the standard UCB arm (P=0.001).
After the researchers adjusted for age and Karnofsky Performance Status, patients in the NiCord arm had a mean of 20.2 more days out of the hospital than their peers who received standard UCB (P=0.005).
The incidence of grade 2-4 acute GHVD was 55.6% in the NiCord arm and 41.9% in the standard UCB arm (P=0.31). The rate of second transplant was 5.6% and 11.6%, respectively (P=0.68).
The rate of relapse was 0% in the NiCord arm and 7% in the standard UCB arm (P=0.59). And the rate of death was 5.6% and 16.3%, respectively (P=0.46). ![]()
The expanded umbilical cord blood (UCB) product NiCord can provide benefits over standard UCB transplant, according to research published in Biology of Blood and Marrow Transplantation.
NiCord is a stand-alone graft derived from a single UCB unit that has been expanded in culture and enriched with stem and progenitor cells.
The study showed that patients transplanted with NiCord had shorter time to engraftment, a lower risk of infection, and shorter hospital stays than patients who received standard UCB transplants.
On the other hand, there was no significant difference between the transplant groups when it came to grade 2-4 acute graft-versus-host disease (GVHD), relapse, or survival within 100 days of transplant.
“Our results indicate that rapid hematopoietic recovery from Gamida Cell’s NiCord transplantation approach is associated with clinical benefit,” said study author Mitchell Horwitz, MD, of Duke University School of Medicine in Durham, North Carolina.
Dr Horwitz receives research support from Gamida Cell Ltd., makers of NiCord.
Patient characteristics
The researchers compared 18 consecutive NiCord-transplanted patients and 86 consecutive patients who received standard UCB transplants. All of the patients received total body irradiation-based myeloablative conditioning.
In both arms, most patients received a double transplant. In the NiCord arm, 61.1% of patients (n=11) received NiCord along with a second unmanipulated UCB unit. In the standard UCB arm, 95.3% of patients (n=82) received a double UCB transplant.
Patients in the NiCord arm were older than patients in the standard UCB arm, with median ages of 45.5 (range, 42-57) and 37.5 (range, 28-51), respectively.
Most patients in both arms had acute leukemia or myelodysplastic syndromes (about 89%), although roughly 11% had lymphoid malignancies.
There were no significant differences between the arms when it came to patient sex, pre-transplant weight, cytomegalovirus serostatus, and Karnofsky Performance Status.
Results
The median time to neutrophil engraftment was 12.5 days (range, 10-18) in the NiCord arm and 27 days (range, 23-28) in the standard UCB arm (P<0.001).
All of the patients studied had at least 1 infection of any severity. However, patients in the NiCord arm had a significantly lower risk of infection than patients in the standard UCB arm.
In an analysis adjusted for age, disease stage, and grade 2-4 acute GVHD, the risk ratios (RRs) for NiCord versus standard UCB transplant were as follows:
- Total infection—RR=0.72, P=0.03
- Grade 2-3 infection—RR=0.38, P=0.001
- Bacterial infection—RR=0.42, P=0.008
- Grade 2-3 bacterial infection—RR=0.23, P=0.006.
Patients in the NiCord arm spent a mean of 72.4 days out of the hospital in the first 100 days after transplant, compared to 48.6 days for the standard UCB arm (P=0.001).
After the researchers adjusted for age and Karnofsky Performance Status, patients in the NiCord arm had a mean of 20.2 more days out of the hospital than their peers who received standard UCB (P=0.005).
The incidence of grade 2-4 acute GHVD was 55.6% in the NiCord arm and 41.9% in the standard UCB arm (P=0.31). The rate of second transplant was 5.6% and 11.6%, respectively (P=0.68).
The rate of relapse was 0% in the NiCord arm and 7% in the standard UCB arm (P=0.59). And the rate of death was 5.6% and 16.3%, respectively (P=0.46). ![]()
TGA approves therapy for hemophilia A
The Australian Therapeutic Goods Administration (TGA) has approved use of lonoctocog alfa (AFSTYLA®), a recombinant single-chain coagulation factor VIII (FVIII) therapy, in patients with hemophilia A.
The product is indicated for use as routine prophylaxis to prevent or reduce the frequency of bleeding episodes, for control and prevention of bleeding episodes, and for perioperative management.
Lonoctocog alfa is designed to provide long-lasting protection from bleeds with 2- to 3-times weekly dosing, according to CSL Behring, the company developing the product.
The company says lonoctocog alfa uses a covalent bond that forms one structural entity—a single polypeptide chain—to improve the stability of FVIII and provide FVIII activity with the option of twice-weekly dosing.
The TGA’s approval of lonoctocog alfa is based on results from the AFFINITY clinical development program, which includes a trial of children (n=84) and a trial of adolescents and adults (n=175).
Among patients who received lonoctocog alfa prophylactically in these trials, the median annualized bleeding rate was 1.14 in the adults/adolescents and 3.69 in children younger than 12.
In all, there were 1195 bleeding events—848 in the adults/adolescents and 347 in the children.
Ninety-four percent of bleeds in adults/adolescents and 96% of bleeds in pediatric patients were effectively controlled with no more than 2 infusions of lonoctocog alfa weekly.
Eighty-one percent of bleeds in adults/adolescents and 86% of bleeds in pediatric patients were controlled by a single infusion.
Researchers assessed safety in 258 patients from both studies. Adverse reactions occurred in 14 patients and included hypersensitivity (n=4), dizziness (n=2), paresthesia (n=1), rash (n=1), erythema (n=1), pruritus (n=1), pyrexia (n=1), injection-site pain (n=1), chills (n=1), and feeling hot (n=1).
One patient withdrew from treatment due to hypersensitivity.
None of the patients developed neutralizing antibodies to FVIII or antibodies to host cell proteins. There were no reports of anaphylaxis or thrombosis.
Results from the trial of adolescents/adults were published in Blood in August 2016. Results from the trial of children were published in the Journal of Thrombosis and Haemostasis in March 2017. ![]()
The Australian Therapeutic Goods Administration (TGA) has approved use of lonoctocog alfa (AFSTYLA®), a recombinant single-chain coagulation factor VIII (FVIII) therapy, in patients with hemophilia A.
The product is indicated for use as routine prophylaxis to prevent or reduce the frequency of bleeding episodes, for control and prevention of bleeding episodes, and for perioperative management.
Lonoctocog alfa is designed to provide long-lasting protection from bleeds with 2- to 3-times weekly dosing, according to CSL Behring, the company developing the product.
The company says lonoctocog alfa uses a covalent bond that forms one structural entity—a single polypeptide chain—to improve the stability of FVIII and provide FVIII activity with the option of twice-weekly dosing.
The TGA’s approval of lonoctocog alfa is based on results from the AFFINITY clinical development program, which includes a trial of children (n=84) and a trial of adolescents and adults (n=175).
Among patients who received lonoctocog alfa prophylactically in these trials, the median annualized bleeding rate was 1.14 in the adults/adolescents and 3.69 in children younger than 12.
In all, there were 1195 bleeding events—848 in the adults/adolescents and 347 in the children.
Ninety-four percent of bleeds in adults/adolescents and 96% of bleeds in pediatric patients were effectively controlled with no more than 2 infusions of lonoctocog alfa weekly.
Eighty-one percent of bleeds in adults/adolescents and 86% of bleeds in pediatric patients were controlled by a single infusion.
Researchers assessed safety in 258 patients from both studies. Adverse reactions occurred in 14 patients and included hypersensitivity (n=4), dizziness (n=2), paresthesia (n=1), rash (n=1), erythema (n=1), pruritus (n=1), pyrexia (n=1), injection-site pain (n=1), chills (n=1), and feeling hot (n=1).
One patient withdrew from treatment due to hypersensitivity.
None of the patients developed neutralizing antibodies to FVIII or antibodies to host cell proteins. There were no reports of anaphylaxis or thrombosis.
Results from the trial of adolescents/adults were published in Blood in August 2016. Results from the trial of children were published in the Journal of Thrombosis and Haemostasis in March 2017. ![]()
The Australian Therapeutic Goods Administration (TGA) has approved use of lonoctocog alfa (AFSTYLA®), a recombinant single-chain coagulation factor VIII (FVIII) therapy, in patients with hemophilia A.
The product is indicated for use as routine prophylaxis to prevent or reduce the frequency of bleeding episodes, for control and prevention of bleeding episodes, and for perioperative management.
Lonoctocog alfa is designed to provide long-lasting protection from bleeds with 2- to 3-times weekly dosing, according to CSL Behring, the company developing the product.
The company says lonoctocog alfa uses a covalent bond that forms one structural entity—a single polypeptide chain—to improve the stability of FVIII and provide FVIII activity with the option of twice-weekly dosing.
The TGA’s approval of lonoctocog alfa is based on results from the AFFINITY clinical development program, which includes a trial of children (n=84) and a trial of adolescents and adults (n=175).
Among patients who received lonoctocog alfa prophylactically in these trials, the median annualized bleeding rate was 1.14 in the adults/adolescents and 3.69 in children younger than 12.
In all, there were 1195 bleeding events—848 in the adults/adolescents and 347 in the children.
Ninety-four percent of bleeds in adults/adolescents and 96% of bleeds in pediatric patients were effectively controlled with no more than 2 infusions of lonoctocog alfa weekly.
Eighty-one percent of bleeds in adults/adolescents and 86% of bleeds in pediatric patients were controlled by a single infusion.
Researchers assessed safety in 258 patients from both studies. Adverse reactions occurred in 14 patients and included hypersensitivity (n=4), dizziness (n=2), paresthesia (n=1), rash (n=1), erythema (n=1), pruritus (n=1), pyrexia (n=1), injection-site pain (n=1), chills (n=1), and feeling hot (n=1).
One patient withdrew from treatment due to hypersensitivity.
None of the patients developed neutralizing antibodies to FVIII or antibodies to host cell proteins. There were no reports of anaphylaxis or thrombosis.
Results from the trial of adolescents/adults were published in Blood in August 2016. Results from the trial of children were published in the Journal of Thrombosis and Haemostasis in March 2017. ![]()
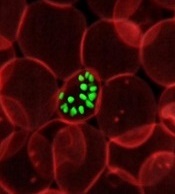
How malaria parasites weaken RBCs’ defenses
Malaria parasites change the properties of red blood cells (RBCs) in a way that helps the parasites achieve cell entry, according to research published in PNAS.
Researchers found that Plasmodium parasites, upon binding to the surface of RBCs, cause the cell membranes to become more pliable, making it easier for the parasites to push inside the cells.
This suggests that differences in RBC stiffness, due to age or increased cholesterol content, could influence the parasites’ ability to invade.
“We have discovered that red cell entry is not just down to the ability of the parasite itself but that parasite-initiated changes to the red blood cells appear to contribute to the process of invasion,” said study author Marion Koch, a PhD student at Imperial College London in the UK.
“This could also mean that naturally more flexible cells would be easier for parasites to invade, which raises some interesting questions. Are parasites choosy about which cells to invade, picking the most deformable? Is susceptibility to malaria modified by fat or cholesterol content, or the age of circulating red blood cells?”
In the PNAS paper, the researchers noted that erythrocyte-binding antigen 175 (EBA175), a protein that’s required for entry in most parasite strains, binds to glycophorin A (GPA) on the RBC surface. However, the function of this binding interaction was unknown.
The team took a closer look at the interaction using real-time deformability cytometry and flicker spectroscopy.
They filmed 1000 RBCs per second passing through a narrow channel. Using this approach, the researchers were able to determine cell deformability by measuring how elongated the cells became during transit through the channel.
The team then measured where this deformation came from. They measured how much the RBCs deviate from their normally circular shape as their membranes naturally fluctuate or flicker.
The researchers found that EBA175 binding to GPA leads to an increase in the cytoskeletal tension of the RBC and a reduction in the bending modulus of the cell’s membrane. (The bending modulus is a measure of how much energy it takes to bend the cell membrane.)
The team then showed that the reduction in the bending modulus was “directly correlated with parasite invasion efficiency.”
The researchers said these results suggest the parasite primes the RBC surface through its binding antigens, altering the cell and reducing a barrier to invasion.
“This suggests we should be investigating not just parasite biology, but also how the body’s own red blood cells respond,” said study author Jake Baum, PhD, of Imperial College London.
“There are therapies developed for diseases like HIV that strengthen the body’s responses in addition to tackling the ‘invader.’ It’s not impossible to imagine something similar for malaria; for example, looking at a host-directed drug target and not just the parasite.” ![]()
Malaria parasites change the properties of red blood cells (RBCs) in a way that helps the parasites achieve cell entry, according to research published in PNAS.
Researchers found that Plasmodium parasites, upon binding to the surface of RBCs, cause the cell membranes to become more pliable, making it easier for the parasites to push inside the cells.
This suggests that differences in RBC stiffness, due to age or increased cholesterol content, could influence the parasites’ ability to invade.
“We have discovered that red cell entry is not just down to the ability of the parasite itself but that parasite-initiated changes to the red blood cells appear to contribute to the process of invasion,” said study author Marion Koch, a PhD student at Imperial College London in the UK.
“This could also mean that naturally more flexible cells would be easier for parasites to invade, which raises some interesting questions. Are parasites choosy about which cells to invade, picking the most deformable? Is susceptibility to malaria modified by fat or cholesterol content, or the age of circulating red blood cells?”
In the PNAS paper, the researchers noted that erythrocyte-binding antigen 175 (EBA175), a protein that’s required for entry in most parasite strains, binds to glycophorin A (GPA) on the RBC surface. However, the function of this binding interaction was unknown.
The team took a closer look at the interaction using real-time deformability cytometry and flicker spectroscopy.
They filmed 1000 RBCs per second passing through a narrow channel. Using this approach, the researchers were able to determine cell deformability by measuring how elongated the cells became during transit through the channel.
The team then measured where this deformation came from. They measured how much the RBCs deviate from their normally circular shape as their membranes naturally fluctuate or flicker.
The researchers found that EBA175 binding to GPA leads to an increase in the cytoskeletal tension of the RBC and a reduction in the bending modulus of the cell’s membrane. (The bending modulus is a measure of how much energy it takes to bend the cell membrane.)
The team then showed that the reduction in the bending modulus was “directly correlated with parasite invasion efficiency.”
The researchers said these results suggest the parasite primes the RBC surface through its binding antigens, altering the cell and reducing a barrier to invasion.
“This suggests we should be investigating not just parasite biology, but also how the body’s own red blood cells respond,” said study author Jake Baum, PhD, of Imperial College London.
“There are therapies developed for diseases like HIV that strengthen the body’s responses in addition to tackling the ‘invader.’ It’s not impossible to imagine something similar for malaria; for example, looking at a host-directed drug target and not just the parasite.” ![]()
Malaria parasites change the properties of red blood cells (RBCs) in a way that helps the parasites achieve cell entry, according to research published in PNAS.
Researchers found that Plasmodium parasites, upon binding to the surface of RBCs, cause the cell membranes to become more pliable, making it easier for the parasites to push inside the cells.
This suggests that differences in RBC stiffness, due to age or increased cholesterol content, could influence the parasites’ ability to invade.
“We have discovered that red cell entry is not just down to the ability of the parasite itself but that parasite-initiated changes to the red blood cells appear to contribute to the process of invasion,” said study author Marion Koch, a PhD student at Imperial College London in the UK.
“This could also mean that naturally more flexible cells would be easier for parasites to invade, which raises some interesting questions. Are parasites choosy about which cells to invade, picking the most deformable? Is susceptibility to malaria modified by fat or cholesterol content, or the age of circulating red blood cells?”
In the PNAS paper, the researchers noted that erythrocyte-binding antigen 175 (EBA175), a protein that’s required for entry in most parasite strains, binds to glycophorin A (GPA) on the RBC surface. However, the function of this binding interaction was unknown.
The team took a closer look at the interaction using real-time deformability cytometry and flicker spectroscopy.
They filmed 1000 RBCs per second passing through a narrow channel. Using this approach, the researchers were able to determine cell deformability by measuring how elongated the cells became during transit through the channel.
The team then measured where this deformation came from. They measured how much the RBCs deviate from their normally circular shape as their membranes naturally fluctuate or flicker.
The researchers found that EBA175 binding to GPA leads to an increase in the cytoskeletal tension of the RBC and a reduction in the bending modulus of the cell’s membrane. (The bending modulus is a measure of how much energy it takes to bend the cell membrane.)
The team then showed that the reduction in the bending modulus was “directly correlated with parasite invasion efficiency.”
The researchers said these results suggest the parasite primes the RBC surface through its binding antigens, altering the cell and reducing a barrier to invasion.
“This suggests we should be investigating not just parasite biology, but also how the body’s own red blood cells respond,” said study author Jake Baum, PhD, of Imperial College London.
“There are therapies developed for diseases like HIV that strengthen the body’s responses in addition to tackling the ‘invader.’ It’s not impossible to imagine something similar for malaria; for example, looking at a host-directed drug target and not just the parasite.” ![]()
Combo improves response rates in treatment-naïve SAA
Adding eltrombopag to immunosuppressive therapy (IST) can produce high rates of response in treatment-naïve severe aplastic anemia (SAA), according to research published in NEJM.
In patients who received eltrombopag for 6 months, the overall response rate (ORR) was 94%, and the complete response (CR) rate was 58%.
Researchers noted that these rates are “markedly higher” than response rates observed in historical controls who received IST alone.
The team also said the safety profile of eltrombopag in this study was consistent with the known safety profile of the drug.
“[E]ltrombopag plus standard immunosuppressive therapy appeared to increase the overall response rate and substantially increase the frequency, speed, and robustness of hematologic recovery in patients with SAA compared to historical controls,” said study author Danielle Townsley, MD, of the National Heart, Lung and Blood Institute (NHLBI).
She and her colleagues at NHLBI conducted this research through a Cooperative Research and Development Agreement with Novartis, the company that markets eltrombopag as Promacta/Revolade.
Patients and treatment
This phase 1/2 trial included 92 patients with treatment-naïve SAA. The patients’ median age was 32 (range, 3-82), and 54% of them were male.
At baseline, the patients’ median neutrophil count was 310/mm3 (range, 0-1810), their median reticulocyte count was 19,950/mm3 (range, 1600-60,400), and their median platelet count was 9000/mm3 (range, 0-37,000). Their median thrombopoietin level was 3163 pg/ml (range, 1806-4955).
All patients received horse antithymocyte globulin on days 1 to 4 and cyclosporine from day 1 to 6 months. The patients also received eltrombopag at an age-dependent dose.
They received eltrombopag at 150 mg daily if they were 12 years or older, 75 mg daily if they were 6 to 11, and 2.5 mg/kg/day if they were 2 to 5 years of age.
Patients were also split into 3 cohorts according to treatment duration:
- Cohort 1 received eltrombopag from day 14 to the 6-month mark.
- Cohort 2 received eltrombopag from day 14 to the 3-month mark.
- Cohort 3 received eltrombopag from day 1 to 6 months.
Response
The study’s primary efficacy endpoint was hematologic CR at 6 months. CR was defined as an absolute neutrophil count of at least 1000/mm3, a hemoglobin level of at least 10 g/dL, and a platelet count of at least 100,000/mm3.
Secondary endpoints included partial response (PR) and ORR, among other endpoints. ORR was the rate of CR plus PR. Patients had a PR if they had blood counts that no longer met the criteria for SAA but they did not meet criteria for CR.
For all cohorts, at 6 months, the ORR was 87%, and the CR rate was 39%.
In cohort 1, the ORR was 80%, and the CR rate was 33%.
In cohort 2, the ORR was 87%, and the CR rate was 26%.
In cohort 3, the ORR was 94%, and the CR rate was 58%.
The researchers noted that ORRs were higher across all cohorts than the ORR observed in a historical cohort (66%). The cohort consisted of 102 patients who had received standard IST while serving as controls in 1 of 2 recent NHLBI clinical trials.
Relapse
Thirty-two percent of responding patients (25/78) relapsed after 6 months.
After the study protocol was amended to allow the continuation of low-dose cyclosporine from 6 months to 2 years, the frequency of relapse decreased.
Fourteen percent of responders receiving low-dose cyclosporine beyond 6 months relapsed (6/43), compared to 54% of patients who stopped cyclosporine at 6 months (19/35).
Restarting full-dose cyclosporine reversed relapse and increased blood counts in 13 of 25 patients. Adding eltrombopag reversed relapse in an additional 10 patients.
Survival
The 2-year overall survival rate was 97% for the entire study population and 99% when data were censored for transplant.
Twelve patients under went transplant after eltrombopag. Six of them had not responded or were still transfusion-dependent, 3 relapsed, and 3 had clonal evolution.
Three patients died—1 while on study and 2 after transplant.
Clonal evolution and PNH
The researchers said the frequency of clonal evolution in this study was similar to that observed in the historical cohort of patients who received standard IST. The rate of clonal evolution at 2 years was 8% in both groups.
In the current study, 7 patients had clonal evolution at 2 years. Five patients had loss of chromosome 7, which was associated with dysplastic bone marrow changes in 3 patients.
One patient with a complex karyotype progressed to acute myeloid leukemia.
Two patients developed hemolytic paroxysmal nocturnal hemoglobinuria (PNH).
Safety
Two severe adverse events (AEs) were attributed to eltrombopag. Both were cutaneous eruptions—a grade 2 and a grade 3 event. Both AEs led to discontinuation of the drug.
Seven patients briefly stopped taking eltrombopag during the first 2 weeks of treatment due to transient elevations in liver enzymes.
AEs not attributed to eltrombopag were neutropenic infections and AEs known to be associated with IST.
The single patient who died on study was a non-responder who died 3 months after starting treatment. The death was due to paraneoplastic encephalopathy, which was attributed to thymoma that predated study entry. ![]()
Adding eltrombopag to immunosuppressive therapy (IST) can produce high rates of response in treatment-naïve severe aplastic anemia (SAA), according to research published in NEJM.
In patients who received eltrombopag for 6 months, the overall response rate (ORR) was 94%, and the complete response (CR) rate was 58%.
Researchers noted that these rates are “markedly higher” than response rates observed in historical controls who received IST alone.
The team also said the safety profile of eltrombopag in this study was consistent with the known safety profile of the drug.
“[E]ltrombopag plus standard immunosuppressive therapy appeared to increase the overall response rate and substantially increase the frequency, speed, and robustness of hematologic recovery in patients with SAA compared to historical controls,” said study author Danielle Townsley, MD, of the National Heart, Lung and Blood Institute (NHLBI).
She and her colleagues at NHLBI conducted this research through a Cooperative Research and Development Agreement with Novartis, the company that markets eltrombopag as Promacta/Revolade.
Patients and treatment
This phase 1/2 trial included 92 patients with treatment-naïve SAA. The patients’ median age was 32 (range, 3-82), and 54% of them were male.
At baseline, the patients’ median neutrophil count was 310/mm3 (range, 0-1810), their median reticulocyte count was 19,950/mm3 (range, 1600-60,400), and their median platelet count was 9000/mm3 (range, 0-37,000). Their median thrombopoietin level was 3163 pg/ml (range, 1806-4955).
All patients received horse antithymocyte globulin on days 1 to 4 and cyclosporine from day 1 to 6 months. The patients also received eltrombopag at an age-dependent dose.
They received eltrombopag at 150 mg daily if they were 12 years or older, 75 mg daily if they were 6 to 11, and 2.5 mg/kg/day if they were 2 to 5 years of age.
Patients were also split into 3 cohorts according to treatment duration:
- Cohort 1 received eltrombopag from day 14 to the 6-month mark.
- Cohort 2 received eltrombopag from day 14 to the 3-month mark.
- Cohort 3 received eltrombopag from day 1 to 6 months.
Response
The study’s primary efficacy endpoint was hematologic CR at 6 months. CR was defined as an absolute neutrophil count of at least 1000/mm3, a hemoglobin level of at least 10 g/dL, and a platelet count of at least 100,000/mm3.
Secondary endpoints included partial response (PR) and ORR, among other endpoints. ORR was the rate of CR plus PR. Patients had a PR if they had blood counts that no longer met the criteria for SAA but they did not meet criteria for CR.
For all cohorts, at 6 months, the ORR was 87%, and the CR rate was 39%.
In cohort 1, the ORR was 80%, and the CR rate was 33%.
In cohort 2, the ORR was 87%, and the CR rate was 26%.
In cohort 3, the ORR was 94%, and the CR rate was 58%.
The researchers noted that ORRs were higher across all cohorts than the ORR observed in a historical cohort (66%). The cohort consisted of 102 patients who had received standard IST while serving as controls in 1 of 2 recent NHLBI clinical trials.
Relapse
Thirty-two percent of responding patients (25/78) relapsed after 6 months.
After the study protocol was amended to allow the continuation of low-dose cyclosporine from 6 months to 2 years, the frequency of relapse decreased.
Fourteen percent of responders receiving low-dose cyclosporine beyond 6 months relapsed (6/43), compared to 54% of patients who stopped cyclosporine at 6 months (19/35).
Restarting full-dose cyclosporine reversed relapse and increased blood counts in 13 of 25 patients. Adding eltrombopag reversed relapse in an additional 10 patients.
Survival
The 2-year overall survival rate was 97% for the entire study population and 99% when data were censored for transplant.
Twelve patients under went transplant after eltrombopag. Six of them had not responded or were still transfusion-dependent, 3 relapsed, and 3 had clonal evolution.
Three patients died—1 while on study and 2 after transplant.
Clonal evolution and PNH
The researchers said the frequency of clonal evolution in this study was similar to that observed in the historical cohort of patients who received standard IST. The rate of clonal evolution at 2 years was 8% in both groups.
In the current study, 7 patients had clonal evolution at 2 years. Five patients had loss of chromosome 7, which was associated with dysplastic bone marrow changes in 3 patients.
One patient with a complex karyotype progressed to acute myeloid leukemia.
Two patients developed hemolytic paroxysmal nocturnal hemoglobinuria (PNH).
Safety
Two severe adverse events (AEs) were attributed to eltrombopag. Both were cutaneous eruptions—a grade 2 and a grade 3 event. Both AEs led to discontinuation of the drug.
Seven patients briefly stopped taking eltrombopag during the first 2 weeks of treatment due to transient elevations in liver enzymes.
AEs not attributed to eltrombopag were neutropenic infections and AEs known to be associated with IST.
The single patient who died on study was a non-responder who died 3 months after starting treatment. The death was due to paraneoplastic encephalopathy, which was attributed to thymoma that predated study entry. ![]()
Adding eltrombopag to immunosuppressive therapy (IST) can produce high rates of response in treatment-naïve severe aplastic anemia (SAA), according to research published in NEJM.
In patients who received eltrombopag for 6 months, the overall response rate (ORR) was 94%, and the complete response (CR) rate was 58%.
Researchers noted that these rates are “markedly higher” than response rates observed in historical controls who received IST alone.
The team also said the safety profile of eltrombopag in this study was consistent with the known safety profile of the drug.
“[E]ltrombopag plus standard immunosuppressive therapy appeared to increase the overall response rate and substantially increase the frequency, speed, and robustness of hematologic recovery in patients with SAA compared to historical controls,” said study author Danielle Townsley, MD, of the National Heart, Lung and Blood Institute (NHLBI).
She and her colleagues at NHLBI conducted this research through a Cooperative Research and Development Agreement with Novartis, the company that markets eltrombopag as Promacta/Revolade.
Patients and treatment
This phase 1/2 trial included 92 patients with treatment-naïve SAA. The patients’ median age was 32 (range, 3-82), and 54% of them were male.
At baseline, the patients’ median neutrophil count was 310/mm3 (range, 0-1810), their median reticulocyte count was 19,950/mm3 (range, 1600-60,400), and their median platelet count was 9000/mm3 (range, 0-37,000). Their median thrombopoietin level was 3163 pg/ml (range, 1806-4955).
All patients received horse antithymocyte globulin on days 1 to 4 and cyclosporine from day 1 to 6 months. The patients also received eltrombopag at an age-dependent dose.
They received eltrombopag at 150 mg daily if they were 12 years or older, 75 mg daily if they were 6 to 11, and 2.5 mg/kg/day if they were 2 to 5 years of age.
Patients were also split into 3 cohorts according to treatment duration:
- Cohort 1 received eltrombopag from day 14 to the 6-month mark.
- Cohort 2 received eltrombopag from day 14 to the 3-month mark.
- Cohort 3 received eltrombopag from day 1 to 6 months.
Response
The study’s primary efficacy endpoint was hematologic CR at 6 months. CR was defined as an absolute neutrophil count of at least 1000/mm3, a hemoglobin level of at least 10 g/dL, and a platelet count of at least 100,000/mm3.
Secondary endpoints included partial response (PR) and ORR, among other endpoints. ORR was the rate of CR plus PR. Patients had a PR if they had blood counts that no longer met the criteria for SAA but they did not meet criteria for CR.
For all cohorts, at 6 months, the ORR was 87%, and the CR rate was 39%.
In cohort 1, the ORR was 80%, and the CR rate was 33%.
In cohort 2, the ORR was 87%, and the CR rate was 26%.
In cohort 3, the ORR was 94%, and the CR rate was 58%.
The researchers noted that ORRs were higher across all cohorts than the ORR observed in a historical cohort (66%). The cohort consisted of 102 patients who had received standard IST while serving as controls in 1 of 2 recent NHLBI clinical trials.
Relapse
Thirty-two percent of responding patients (25/78) relapsed after 6 months.
After the study protocol was amended to allow the continuation of low-dose cyclosporine from 6 months to 2 years, the frequency of relapse decreased.
Fourteen percent of responders receiving low-dose cyclosporine beyond 6 months relapsed (6/43), compared to 54% of patients who stopped cyclosporine at 6 months (19/35).
Restarting full-dose cyclosporine reversed relapse and increased blood counts in 13 of 25 patients. Adding eltrombopag reversed relapse in an additional 10 patients.
Survival
The 2-year overall survival rate was 97% for the entire study population and 99% when data were censored for transplant.
Twelve patients under went transplant after eltrombopag. Six of them had not responded or were still transfusion-dependent, 3 relapsed, and 3 had clonal evolution.
Three patients died—1 while on study and 2 after transplant.
Clonal evolution and PNH
The researchers said the frequency of clonal evolution in this study was similar to that observed in the historical cohort of patients who received standard IST. The rate of clonal evolution at 2 years was 8% in both groups.
In the current study, 7 patients had clonal evolution at 2 years. Five patients had loss of chromosome 7, which was associated with dysplastic bone marrow changes in 3 patients.
One patient with a complex karyotype progressed to acute myeloid leukemia.
Two patients developed hemolytic paroxysmal nocturnal hemoglobinuria (PNH).
Safety
Two severe adverse events (AEs) were attributed to eltrombopag. Both were cutaneous eruptions—a grade 2 and a grade 3 event. Both AEs led to discontinuation of the drug.
Seven patients briefly stopped taking eltrombopag during the first 2 weeks of treatment due to transient elevations in liver enzymes.
AEs not attributed to eltrombopag were neutropenic infections and AEs known to be associated with IST.
The single patient who died on study was a non-responder who died 3 months after starting treatment. The death was due to paraneoplastic encephalopathy, which was attributed to thymoma that predated study entry. ![]()
Cutting amino acids from diet could fight lymphoma
Cutting certain amino acids from the diet slows tumor growth and prolongs survival in mouse models of malignancy, according to research published in Nature.
Researchers found that removing 2 non-essential amino acids—serine and glycine (SG)—from the diet of mice slowed the development of lymphoma and intestinal cancer.
The SG-free diet also made lymphoma more susceptible to reactive oxygen species (ROS).
As chemotherapy and radiotherapy boost levels of ROS, the researchers believe an SG-free diet could make conventional cancer treatments more effective.
“Our findings suggest that restricting specific amino acids through a controlled diet plan could be an additional part of treatment for some cancer patients in future, helping to make other treatments more effective,” said study author Oliver Maddocks, PhD, of the University of Glasgow in the UK.
He and his colleagues tested the SG-free diet in mouse models of lymphoma (Eμ-Myc) and observed decreased tumor volume and lymphoma cell numbers as well as a significant improvement in survival compared to controls.
The median survival (from the time of diet change at day 60) was 192 days for mice on the SG-free diet and 59 days for mice on the control diet (P=0.0367).
To test whether increasing ROS could enhance the anticancer effects of the SG-free diet, the researchers crossed Eμ-Myc mice with mice that don’t express Tigar, a protein that can support tumor development by limiting ROS.
The researchers found that Tigar -/- mice on the SG-free diet had significantly better survival than Tigar -/- mice on the control diet (P=0.0451).
The median survival was 50.5 days for Tigar +/+ mice on the control diet, 80 days for Tigar +/+ mice on the SG-free diet, 107 days for Tigar -/- mice on the control diet, and 226 days for Tigar -/- mice on the SG-free diet.
The researchers also found the SG-free diet was less effective in cancers with an activated Kras gene (such as prostate cancer) because the faulty gene boosted the cancer cells’ ability to make their own serine and glycine. The team said this could help in selecting which tumors could be best targeted by diet therapy.
“This is a really interesting look at how cutting off the supply of nutrients essential to cancer cell growth and division could help restrain tumors,” said Emma Smith, PhD, of Cancer Research UK, which funded this study.
“The next steps are clinical trials in people to see if giving a specialized diet that lacks these amino acids is safe and helps slow tumor growth as seen in mice. We’d also need to work out which patients are most likely to benefit, depending on the characteristics of their cancer.” ![]()
Cutting certain amino acids from the diet slows tumor growth and prolongs survival in mouse models of malignancy, according to research published in Nature.
Researchers found that removing 2 non-essential amino acids—serine and glycine (SG)—from the diet of mice slowed the development of lymphoma and intestinal cancer.
The SG-free diet also made lymphoma more susceptible to reactive oxygen species (ROS).
As chemotherapy and radiotherapy boost levels of ROS, the researchers believe an SG-free diet could make conventional cancer treatments more effective.
“Our findings suggest that restricting specific amino acids through a controlled diet plan could be an additional part of treatment for some cancer patients in future, helping to make other treatments more effective,” said study author Oliver Maddocks, PhD, of the University of Glasgow in the UK.
He and his colleagues tested the SG-free diet in mouse models of lymphoma (Eμ-Myc) and observed decreased tumor volume and lymphoma cell numbers as well as a significant improvement in survival compared to controls.
The median survival (from the time of diet change at day 60) was 192 days for mice on the SG-free diet and 59 days for mice on the control diet (P=0.0367).
To test whether increasing ROS could enhance the anticancer effects of the SG-free diet, the researchers crossed Eμ-Myc mice with mice that don’t express Tigar, a protein that can support tumor development by limiting ROS.
The researchers found that Tigar -/- mice on the SG-free diet had significantly better survival than Tigar -/- mice on the control diet (P=0.0451).
The median survival was 50.5 days for Tigar +/+ mice on the control diet, 80 days for Tigar +/+ mice on the SG-free diet, 107 days for Tigar -/- mice on the control diet, and 226 days for Tigar -/- mice on the SG-free diet.
The researchers also found the SG-free diet was less effective in cancers with an activated Kras gene (such as prostate cancer) because the faulty gene boosted the cancer cells’ ability to make their own serine and glycine. The team said this could help in selecting which tumors could be best targeted by diet therapy.
“This is a really interesting look at how cutting off the supply of nutrients essential to cancer cell growth and division could help restrain tumors,” said Emma Smith, PhD, of Cancer Research UK, which funded this study.
“The next steps are clinical trials in people to see if giving a specialized diet that lacks these amino acids is safe and helps slow tumor growth as seen in mice. We’d also need to work out which patients are most likely to benefit, depending on the characteristics of their cancer.” ![]()
Cutting certain amino acids from the diet slows tumor growth and prolongs survival in mouse models of malignancy, according to research published in Nature.
Researchers found that removing 2 non-essential amino acids—serine and glycine (SG)—from the diet of mice slowed the development of lymphoma and intestinal cancer.
The SG-free diet also made lymphoma more susceptible to reactive oxygen species (ROS).
As chemotherapy and radiotherapy boost levels of ROS, the researchers believe an SG-free diet could make conventional cancer treatments more effective.
“Our findings suggest that restricting specific amino acids through a controlled diet plan could be an additional part of treatment for some cancer patients in future, helping to make other treatments more effective,” said study author Oliver Maddocks, PhD, of the University of Glasgow in the UK.
He and his colleagues tested the SG-free diet in mouse models of lymphoma (Eμ-Myc) and observed decreased tumor volume and lymphoma cell numbers as well as a significant improvement in survival compared to controls.
The median survival (from the time of diet change at day 60) was 192 days for mice on the SG-free diet and 59 days for mice on the control diet (P=0.0367).
To test whether increasing ROS could enhance the anticancer effects of the SG-free diet, the researchers crossed Eμ-Myc mice with mice that don’t express Tigar, a protein that can support tumor development by limiting ROS.
The researchers found that Tigar -/- mice on the SG-free diet had significantly better survival than Tigar -/- mice on the control diet (P=0.0451).
The median survival was 50.5 days for Tigar +/+ mice on the control diet, 80 days for Tigar +/+ mice on the SG-free diet, 107 days for Tigar -/- mice on the control diet, and 226 days for Tigar -/- mice on the SG-free diet.
The researchers also found the SG-free diet was less effective in cancers with an activated Kras gene (such as prostate cancer) because the faulty gene boosted the cancer cells’ ability to make their own serine and glycine. The team said this could help in selecting which tumors could be best targeted by diet therapy.
“This is a really interesting look at how cutting off the supply of nutrients essential to cancer cell growth and division could help restrain tumors,” said Emma Smith, PhD, of Cancer Research UK, which funded this study.
“The next steps are clinical trials in people to see if giving a specialized diet that lacks these amino acids is safe and helps slow tumor growth as seen in mice. We’d also need to work out which patients are most likely to benefit, depending on the characteristics of their cancer.” ![]()
Hemoglobin nanoparticles could serve as blood substitute
A new type of hemoglobin-based oxygen carrier has shown early promise as a potential blood substitute, according to researchers.
The team created nanoparticles consisting of bovine hemoglobin coated with the polymer polydopamine (PDA).
Lab tests showed that hemoglobin-PDA nanoparticles effectively carried oxygen, caused minimal cell damage, and acted as antioxidants, scavenging for potentially damaging free radicals and reactive oxygen species (ROS).
Hong Zhou, PhD, of the Beijing Institute of Transfusion Medicine in China, and colleagues reported these results in Biomacromolecules.
Past attempts to develop chemically modified hemoglobin as a blood substitute have been hindered by the formation of methemoglobin.
Methemoglobin doesn’t bind oxygen and therefore decreases the amount of oxygen blood delivers in the body. In addition, the generation of methemoglobin produces hydrogen peroxide, which leads to cell damage.
Dr Zhou and colleagues wanted to see if packaging hemoglobin in a benign envelope could prevent these problems. So the team developed a one-step method for wrapping hemoglobin—extracted from bovine red cells—in PDA.
The researchers said their simple preparation method meant the nanoparticles maintained the integrity of hemoglobin’s chemical structure without introducing any toxic reagent.
In addition, the PDA coating allowed hemoglobin to maintain its oxygen-carrying ability by preventing the formation of methemoglobin.
The researchers found the hemoglobin-PDA nanoparticles to have “excellent antioxidant capacity,” as they scavenged free radicals. The team said the ABTS+ radical scavenging rate reached 89%, and the DPPH radical scavenging rate reached 49%.
The hemoglobin-PDA nanoparticles also demonstrated antioxidant capacity by suppressing ROS generation. They reduced hydrogen peroxide-induced ROS generation in H9c2 cells.
Furthermore, the hemoglobin-PDA nanoparticles had little effect on blood constituents and cell viability.
The researchers found the nanoparticles had minimal effects on platelet aggregation, hemolysis in red blood cells, and coagulation processes.
And the nanoparticles had no significant cytotoxic effects on human umbilical vein endothelial cells.
The researchers therefore concluded that their hemoglobin-PDA nanoparticles represent “promising progress” for hemoglobin-based oxygen carriers. ![]()
A new type of hemoglobin-based oxygen carrier has shown early promise as a potential blood substitute, according to researchers.
The team created nanoparticles consisting of bovine hemoglobin coated with the polymer polydopamine (PDA).
Lab tests showed that hemoglobin-PDA nanoparticles effectively carried oxygen, caused minimal cell damage, and acted as antioxidants, scavenging for potentially damaging free radicals and reactive oxygen species (ROS).
Hong Zhou, PhD, of the Beijing Institute of Transfusion Medicine in China, and colleagues reported these results in Biomacromolecules.
Past attempts to develop chemically modified hemoglobin as a blood substitute have been hindered by the formation of methemoglobin.
Methemoglobin doesn’t bind oxygen and therefore decreases the amount of oxygen blood delivers in the body. In addition, the generation of methemoglobin produces hydrogen peroxide, which leads to cell damage.
Dr Zhou and colleagues wanted to see if packaging hemoglobin in a benign envelope could prevent these problems. So the team developed a one-step method for wrapping hemoglobin—extracted from bovine red cells—in PDA.
The researchers said their simple preparation method meant the nanoparticles maintained the integrity of hemoglobin’s chemical structure without introducing any toxic reagent.
In addition, the PDA coating allowed hemoglobin to maintain its oxygen-carrying ability by preventing the formation of methemoglobin.
The researchers found the hemoglobin-PDA nanoparticles to have “excellent antioxidant capacity,” as they scavenged free radicals. The team said the ABTS+ radical scavenging rate reached 89%, and the DPPH radical scavenging rate reached 49%.
The hemoglobin-PDA nanoparticles also demonstrated antioxidant capacity by suppressing ROS generation. They reduced hydrogen peroxide-induced ROS generation in H9c2 cells.
Furthermore, the hemoglobin-PDA nanoparticles had little effect on blood constituents and cell viability.
The researchers found the nanoparticles had minimal effects on platelet aggregation, hemolysis in red blood cells, and coagulation processes.
And the nanoparticles had no significant cytotoxic effects on human umbilical vein endothelial cells.
The researchers therefore concluded that their hemoglobin-PDA nanoparticles represent “promising progress” for hemoglobin-based oxygen carriers. ![]()
A new type of hemoglobin-based oxygen carrier has shown early promise as a potential blood substitute, according to researchers.
The team created nanoparticles consisting of bovine hemoglobin coated with the polymer polydopamine (PDA).
Lab tests showed that hemoglobin-PDA nanoparticles effectively carried oxygen, caused minimal cell damage, and acted as antioxidants, scavenging for potentially damaging free radicals and reactive oxygen species (ROS).
Hong Zhou, PhD, of the Beijing Institute of Transfusion Medicine in China, and colleagues reported these results in Biomacromolecules.
Past attempts to develop chemically modified hemoglobin as a blood substitute have been hindered by the formation of methemoglobin.
Methemoglobin doesn’t bind oxygen and therefore decreases the amount of oxygen blood delivers in the body. In addition, the generation of methemoglobin produces hydrogen peroxide, which leads to cell damage.
Dr Zhou and colleagues wanted to see if packaging hemoglobin in a benign envelope could prevent these problems. So the team developed a one-step method for wrapping hemoglobin—extracted from bovine red cells—in PDA.
The researchers said their simple preparation method meant the nanoparticles maintained the integrity of hemoglobin’s chemical structure without introducing any toxic reagent.
In addition, the PDA coating allowed hemoglobin to maintain its oxygen-carrying ability by preventing the formation of methemoglobin.
The researchers found the hemoglobin-PDA nanoparticles to have “excellent antioxidant capacity,” as they scavenged free radicals. The team said the ABTS+ radical scavenging rate reached 89%, and the DPPH radical scavenging rate reached 49%.
The hemoglobin-PDA nanoparticles also demonstrated antioxidant capacity by suppressing ROS generation. They reduced hydrogen peroxide-induced ROS generation in H9c2 cells.
Furthermore, the hemoglobin-PDA nanoparticles had little effect on blood constituents and cell viability.
The researchers found the nanoparticles had minimal effects on platelet aggregation, hemolysis in red blood cells, and coagulation processes.
And the nanoparticles had no significant cytotoxic effects on human umbilical vein endothelial cells.
The researchers therefore concluded that their hemoglobin-PDA nanoparticles represent “promising progress” for hemoglobin-based oxygen carriers.
Method could prevent GVHD while preserving GVL effect
Researchers believe they have found a way to prevent graft-versus-host disease (GVHD) after hematopoietic stem cell transplant (HSCT) while preserving a strong graft-versus-leukemia (GVL) effect.
In experiments with mice, the team found that temporary in vivo depletion of CD4+ T cells soon after HSCT prevented GVHD without inhibiting GVL effects.
The depletion of CD4+ cells essentially caused CD8+ cells to become exhausted in their quest to destroy normal tissue but strengthened in their fight against leukemia, which meant the CD8+ cells could eliminate leukemic cells without causing GVHD.
Defu Zeng, MD, of City of Hope in Duarte, California, and his colleagues recounted these findings in the Journal of Clinical Investigation.
The researchers were able to achieve temporary in vivo depletion of CD4+ T cells by injecting mice with an anti-CD4 monoclonal antibody (mAb).
The team found that a single injection of the mAb given immediately after HSCT prevented acute but not chronic GVHD. Three injections of the mAb—given on days 0, 14, and 28—prevented both types of GVHD.
The researchers said their results suggest GVHD is more effectively prevented by temporary in vivo depletion of donor CD4+ T cells early after HSCT than by ex vivo depletion of donor CD4+ T cells.
This is because treatment with an anti-CD4 mAb temporarily depletes both the injected mature CD4+ T cells and the CD4+ T cells generated de novo from the marrow progenitors early after HSCT.
In explaining the mechanism behind their observations, the researchers noted that the interaction between PD-L1 and CD80 has been shown to exacerbate GVHD, but costimulation of CD80 and PD-1 ameliorates GVHD.
In their experiments, the team found that depleting CD4+ T cells increased serum IFN-γ and reduced IL-2 concentrations. And this led to upregulation of PD-L1 expression by recipient tissues and donor CD8+ T cells.
The researchers said that, in GVHD target tissues, the interactions of PD-L1 and PD-1 on donor CD8+ T cells caused anergy, exhaustion, and apoptosis. These effects prevented the development of GVHD.
On the other hand, in lymphoid tissues, the interactions of PD-L1 and CD80 augmented CD8+ T-cell expansion without increasing anergy, exhaustion, or apoptosis. This allowed for the preservation of GVL effects.
“If successfully translated into clinical application, this [CD4+ T-cell-depleting] regimen may represent one of the novel approaches that allow strong GVL effects without causing GVHD,” Dr Zeng said.
“This kind of regimen has the potential to promote widespread application of allogenic [HSCT] as a curative therapy for hematological malignancies.”
Researchers believe they have found a way to prevent graft-versus-host disease (GVHD) after hematopoietic stem cell transplant (HSCT) while preserving a strong graft-versus-leukemia (GVL) effect.
In experiments with mice, the team found that temporary in vivo depletion of CD4+ T cells soon after HSCT prevented GVHD without inhibiting GVL effects.
The depletion of CD4+ cells essentially caused CD8+ cells to become exhausted in their quest to destroy normal tissue but strengthened in their fight against leukemia, which meant the CD8+ cells could eliminate leukemic cells without causing GVHD.
Defu Zeng, MD, of City of Hope in Duarte, California, and his colleagues recounted these findings in the Journal of Clinical Investigation.
The researchers were able to achieve temporary in vivo depletion of CD4+ T cells by injecting mice with an anti-CD4 monoclonal antibody (mAb).
The team found that a single injection of the mAb given immediately after HSCT prevented acute but not chronic GVHD. Three injections of the mAb—given on days 0, 14, and 28—prevented both types of GVHD.
The researchers said their results suggest GVHD is more effectively prevented by temporary in vivo depletion of donor CD4+ T cells early after HSCT than by ex vivo depletion of donor CD4+ T cells.
This is because treatment with an anti-CD4 mAb temporarily depletes both the injected mature CD4+ T cells and the CD4+ T cells generated de novo from the marrow progenitors early after HSCT.
In explaining the mechanism behind their observations, the researchers noted that the interaction between PD-L1 and CD80 has been shown to exacerbate GVHD, but costimulation of CD80 and PD-1 ameliorates GVHD.
In their experiments, the team found that depleting CD4+ T cells increased serum IFN-γ and reduced IL-2 concentrations. And this led to upregulation of PD-L1 expression by recipient tissues and donor CD8+ T cells.
The researchers said that, in GVHD target tissues, the interactions of PD-L1 and PD-1 on donor CD8+ T cells caused anergy, exhaustion, and apoptosis. These effects prevented the development of GVHD.
On the other hand, in lymphoid tissues, the interactions of PD-L1 and CD80 augmented CD8+ T-cell expansion without increasing anergy, exhaustion, or apoptosis. This allowed for the preservation of GVL effects.
“If successfully translated into clinical application, this [CD4+ T-cell-depleting] regimen may represent one of the novel approaches that allow strong GVL effects without causing GVHD,” Dr Zeng said.
“This kind of regimen has the potential to promote widespread application of allogenic [HSCT] as a curative therapy for hematological malignancies.”
Researchers believe they have found a way to prevent graft-versus-host disease (GVHD) after hematopoietic stem cell transplant (HSCT) while preserving a strong graft-versus-leukemia (GVL) effect.
In experiments with mice, the team found that temporary in vivo depletion of CD4+ T cells soon after HSCT prevented GVHD without inhibiting GVL effects.
The depletion of CD4+ cells essentially caused CD8+ cells to become exhausted in their quest to destroy normal tissue but strengthened in their fight against leukemia, which meant the CD8+ cells could eliminate leukemic cells without causing GVHD.
Defu Zeng, MD, of City of Hope in Duarte, California, and his colleagues recounted these findings in the Journal of Clinical Investigation.
The researchers were able to achieve temporary in vivo depletion of CD4+ T cells by injecting mice with an anti-CD4 monoclonal antibody (mAb).
The team found that a single injection of the mAb given immediately after HSCT prevented acute but not chronic GVHD. Three injections of the mAb—given on days 0, 14, and 28—prevented both types of GVHD.
The researchers said their results suggest GVHD is more effectively prevented by temporary in vivo depletion of donor CD4+ T cells early after HSCT than by ex vivo depletion of donor CD4+ T cells.
This is because treatment with an anti-CD4 mAb temporarily depletes both the injected mature CD4+ T cells and the CD4+ T cells generated de novo from the marrow progenitors early after HSCT.
In explaining the mechanism behind their observations, the researchers noted that the interaction between PD-L1 and CD80 has been shown to exacerbate GVHD, but costimulation of CD80 and PD-1 ameliorates GVHD.
In their experiments, the team found that depleting CD4+ T cells increased serum IFN-γ and reduced IL-2 concentrations. And this led to upregulation of PD-L1 expression by recipient tissues and donor CD8+ T cells.
The researchers said that, in GVHD target tissues, the interactions of PD-L1 and PD-1 on donor CD8+ T cells caused anergy, exhaustion, and apoptosis. These effects prevented the development of GVHD.
On the other hand, in lymphoid tissues, the interactions of PD-L1 and CD80 augmented CD8+ T-cell expansion without increasing anergy, exhaustion, or apoptosis. This allowed for the preservation of GVL effects.
“If successfully translated into clinical application, this [CD4+ T-cell-depleting] regimen may represent one of the novel approaches that allow strong GVL effects without causing GVHD,” Dr Zeng said.
“This kind of regimen has the potential to promote widespread application of allogenic [HSCT] as a curative therapy for hematological malignancies.”
CAR T-cell therapy receives breakthrough designation
The US Food and Drug Administration (FDA) has granted another breakthrough therapy designation to CTL019 (tisagenlecleucel-T), an investigational chimeric antigen receptor (CAR) T-cell therapy.
CTL019 now has breakthrough designation as a treatment for adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have failed 2 or more prior therapies.
The FDA previously granted CTL019 breakthrough designation as a treatment for relapsed/refractory B-cell acute lymphoblastic leukemia (ALL) in pediatric and young adult patients.
Breakthrough designation is intended to expedite the development and review of new medicines that treat serious or life-threatening conditions, if those medicines have demonstrated substantial improvement over available therapies.
The breakthrough designation for CTL019 in DLBCL is based on data from the phase 2 JULIET trial (NCT02445248), in which researchers are evaluating the efficacy and safety of CTL019 in adults with relapsed/refractory DLBCL.
Findings from JULIET are expected to be presented at an upcoming medical meeting.
About CTL019
CTL019 consists of autologous T cells expressing a CD19-specific CAR. The therapy was first developed by the University of Pennsylvania.
In 2012, the university and Novartis entered into a global collaboration to further research, develop, and commercialize CAR-T cell therapies, including CTL019. Novartis holds the worldwide rights to CARs developed through the collaboration.
According to Novartis, regulatory submissions for CTL019 in the treatment of relapsed/refractory DLBCL are expected to be filed by the end of the year.
The company has already filed a biologics license application with the FDA for CTL019 as a treatment for patients with relapsed/refractory B-cell ALL. The FDA granted that application priority review.
The US Food and Drug Administration (FDA) has granted another breakthrough therapy designation to CTL019 (tisagenlecleucel-T), an investigational chimeric antigen receptor (CAR) T-cell therapy.
CTL019 now has breakthrough designation as a treatment for adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have failed 2 or more prior therapies.
The FDA previously granted CTL019 breakthrough designation as a treatment for relapsed/refractory B-cell acute lymphoblastic leukemia (ALL) in pediatric and young adult patients.
Breakthrough designation is intended to expedite the development and review of new medicines that treat serious or life-threatening conditions, if those medicines have demonstrated substantial improvement over available therapies.
The breakthrough designation for CTL019 in DLBCL is based on data from the phase 2 JULIET trial (NCT02445248), in which researchers are evaluating the efficacy and safety of CTL019 in adults with relapsed/refractory DLBCL.
Findings from JULIET are expected to be presented at an upcoming medical meeting.
About CTL019
CTL019 consists of autologous T cells expressing a CD19-specific CAR. The therapy was first developed by the University of Pennsylvania.
In 2012, the university and Novartis entered into a global collaboration to further research, develop, and commercialize CAR-T cell therapies, including CTL019. Novartis holds the worldwide rights to CARs developed through the collaboration.
According to Novartis, regulatory submissions for CTL019 in the treatment of relapsed/refractory DLBCL are expected to be filed by the end of the year.
The company has already filed a biologics license application with the FDA for CTL019 as a treatment for patients with relapsed/refractory B-cell ALL. The FDA granted that application priority review.
The US Food and Drug Administration (FDA) has granted another breakthrough therapy designation to CTL019 (tisagenlecleucel-T), an investigational chimeric antigen receptor (CAR) T-cell therapy.
CTL019 now has breakthrough designation as a treatment for adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have failed 2 or more prior therapies.
The FDA previously granted CTL019 breakthrough designation as a treatment for relapsed/refractory B-cell acute lymphoblastic leukemia (ALL) in pediatric and young adult patients.
Breakthrough designation is intended to expedite the development and review of new medicines that treat serious or life-threatening conditions, if those medicines have demonstrated substantial improvement over available therapies.
The breakthrough designation for CTL019 in DLBCL is based on data from the phase 2 JULIET trial (NCT02445248), in which researchers are evaluating the efficacy and safety of CTL019 in adults with relapsed/refractory DLBCL.
Findings from JULIET are expected to be presented at an upcoming medical meeting.
About CTL019
CTL019 consists of autologous T cells expressing a CD19-specific CAR. The therapy was first developed by the University of Pennsylvania.
In 2012, the university and Novartis entered into a global collaboration to further research, develop, and commercialize CAR-T cell therapies, including CTL019. Novartis holds the worldwide rights to CARs developed through the collaboration.
According to Novartis, regulatory submissions for CTL019 in the treatment of relapsed/refractory DLBCL are expected to be filed by the end of the year.
The company has already filed a biologics license application with the FDA for CTL019 as a treatment for patients with relapsed/refractory B-cell ALL. The FDA granted that application priority review.
Study correlates genetic mutations with AML subgroups
A large study of adults with acute myeloid leukemia (AML) correlates cancer-related genetic mutations with subtypes of AML defined by the presence of specific cytogenetic abnormalities.
Researchers combined the cytogenetic abnormalities that define each of 34 AML subgroups with the mutation status of 80 cancer-related genes to produce an “oncoprint,” a tabular summary of the mutations associated with each cytogenetic group.
This allowed the researchers to identify genetic differences among the 34 subgroups and unexpected associations between the AML subsets and specific genetic mutations as well as gene functional groups.
The researchers believe their findings, published in Leukemia, might help guide mutation testing and treatment decisions in the future.
The study involved 1603 newly diagnosed adult AML patients who were treated on Cancer and Leukemia Group B/Alliance for Clinical Trials in Oncology trials in multiple centers across the US. Most patients (n=1080) were under age 60.
The researchers classified the patients into 34 karyotype subgroups within 5 major cytogenetic groups—cytogenetically normal (CN) AML, complex karyotype (CK) AML, core-binding factor (CBF) AML, AML with balanced translocations or inversions other than those associated with CBF-AML, and AML with unbalanced chromosomal abnormalities detected in non-complex karyotypes.
The team sequenced pretreatment bone marrow or peripheral blood samples from each patient to learn the mutational status of 80 cancer- and leukemia-related genes.
The mutations were assigned to 1 of 9 categories based on the gene’s biological function—for example, methylation-related, cohesin complex, chromatin remodeling, and tumor-suppressor genes.
The researchers identified 4390 mutations, with a median of 3 mutations per patient.
Overall, the most frequently mutated genes that contributed to AML in this cohort belonged to the methylation group.
The researchers observed a high incidence of mutations in methylation-related genes in patients with CN-AML, CK-AML, or unbalanced chromosomal abnormalities.
In contrast, mutations in methylation-related genes were almost absent in CBF-AML patients and were rather rare in patients with non-CBF-AML-related balanced translocations or inversions.
Mutations in spliceosome genes were frequent in patients with unbalanced chromosomal abnormalities, especially those with sole trisomy of chromosomes 4, 8, 11, 13, or 21.
“Our study summarizes cytogenetic and mutational information of 1603 AML patients in a single image,” said Ann-Kathrin Eisfeld, MD, of The Ohio State University Comprehensive Cancer Center in Columbus.
“The identification of key mutational features of each subgroup may help us to better understand the pathogenesis of the different AML types and provides a wealth of information for ongoing and future research.”
A large study of adults with acute myeloid leukemia (AML) correlates cancer-related genetic mutations with subtypes of AML defined by the presence of specific cytogenetic abnormalities.
Researchers combined the cytogenetic abnormalities that define each of 34 AML subgroups with the mutation status of 80 cancer-related genes to produce an “oncoprint,” a tabular summary of the mutations associated with each cytogenetic group.
This allowed the researchers to identify genetic differences among the 34 subgroups and unexpected associations between the AML subsets and specific genetic mutations as well as gene functional groups.
The researchers believe their findings, published in Leukemia, might help guide mutation testing and treatment decisions in the future.
The study involved 1603 newly diagnosed adult AML patients who were treated on Cancer and Leukemia Group B/Alliance for Clinical Trials in Oncology trials in multiple centers across the US. Most patients (n=1080) were under age 60.
The researchers classified the patients into 34 karyotype subgroups within 5 major cytogenetic groups—cytogenetically normal (CN) AML, complex karyotype (CK) AML, core-binding factor (CBF) AML, AML with balanced translocations or inversions other than those associated with CBF-AML, and AML with unbalanced chromosomal abnormalities detected in non-complex karyotypes.
The team sequenced pretreatment bone marrow or peripheral blood samples from each patient to learn the mutational status of 80 cancer- and leukemia-related genes.
The mutations were assigned to 1 of 9 categories based on the gene’s biological function—for example, methylation-related, cohesin complex, chromatin remodeling, and tumor-suppressor genes.
The researchers identified 4390 mutations, with a median of 3 mutations per patient.
Overall, the most frequently mutated genes that contributed to AML in this cohort belonged to the methylation group.
The researchers observed a high incidence of mutations in methylation-related genes in patients with CN-AML, CK-AML, or unbalanced chromosomal abnormalities.
In contrast, mutations in methylation-related genes were almost absent in CBF-AML patients and were rather rare in patients with non-CBF-AML-related balanced translocations or inversions.
Mutations in spliceosome genes were frequent in patients with unbalanced chromosomal abnormalities, especially those with sole trisomy of chromosomes 4, 8, 11, 13, or 21.
“Our study summarizes cytogenetic and mutational information of 1603 AML patients in a single image,” said Ann-Kathrin Eisfeld, MD, of The Ohio State University Comprehensive Cancer Center in Columbus.
“The identification of key mutational features of each subgroup may help us to better understand the pathogenesis of the different AML types and provides a wealth of information for ongoing and future research.”
A large study of adults with acute myeloid leukemia (AML) correlates cancer-related genetic mutations with subtypes of AML defined by the presence of specific cytogenetic abnormalities.
Researchers combined the cytogenetic abnormalities that define each of 34 AML subgroups with the mutation status of 80 cancer-related genes to produce an “oncoprint,” a tabular summary of the mutations associated with each cytogenetic group.
This allowed the researchers to identify genetic differences among the 34 subgroups and unexpected associations between the AML subsets and specific genetic mutations as well as gene functional groups.
The researchers believe their findings, published in Leukemia, might help guide mutation testing and treatment decisions in the future.
The study involved 1603 newly diagnosed adult AML patients who were treated on Cancer and Leukemia Group B/Alliance for Clinical Trials in Oncology trials in multiple centers across the US. Most patients (n=1080) were under age 60.
The researchers classified the patients into 34 karyotype subgroups within 5 major cytogenetic groups—cytogenetically normal (CN) AML, complex karyotype (CK) AML, core-binding factor (CBF) AML, AML with balanced translocations or inversions other than those associated with CBF-AML, and AML with unbalanced chromosomal abnormalities detected in non-complex karyotypes.
The team sequenced pretreatment bone marrow or peripheral blood samples from each patient to learn the mutational status of 80 cancer- and leukemia-related genes.
The mutations were assigned to 1 of 9 categories based on the gene’s biological function—for example, methylation-related, cohesin complex, chromatin remodeling, and tumor-suppressor genes.
The researchers identified 4390 mutations, with a median of 3 mutations per patient.
Overall, the most frequently mutated genes that contributed to AML in this cohort belonged to the methylation group.
The researchers observed a high incidence of mutations in methylation-related genes in patients with CN-AML, CK-AML, or unbalanced chromosomal abnormalities.
In contrast, mutations in methylation-related genes were almost absent in CBF-AML patients and were rather rare in patients with non-CBF-AML-related balanced translocations or inversions.
Mutations in spliceosome genes were frequent in patients with unbalanced chromosomal abnormalities, especially those with sole trisomy of chromosomes 4, 8, 11, 13, or 21.
“Our study summarizes cytogenetic and mutational information of 1603 AML patients in a single image,” said Ann-Kathrin Eisfeld, MD, of The Ohio State University Comprehensive Cancer Center in Columbus.
“The identification of key mutational features of each subgroup may help us to better understand the pathogenesis of the different AML types and provides a wealth of information for ongoing and future research.”