User login
FDA: REMS for ESAs no longer needed, though risks persist
The US Food and Drug Administration (FDA) has determined that the risk evaluation and mitigation strategy (REMS) for erythropoiesis-stimulating agents (ESAs) is no longer necessary.
The REMS was limited to the use of epoetin alfa (marketed as Epogen and Procrit) and darbepoetin alfa (marketed as Aranesp) to treat patients with anemia due to myelosuppressive chemotherapy.
The FDA said the REMS is no longer necessary to ensure that the benefits of Epogen/Procrit and Aranesp outweigh the risks these drugs pose, which include shortened overall survival and an increased risk of tumor progression or recurrence in patients with cancer.
The FDA has released the REMS requirements for these ESAs and said the risks the drugs pose can be communicated by the current product prescribing information.
The FDA decided the REMS is no longer needed based on its own analyses and an evaluation of the REMS assessment submitted by Amgen, Inc., the company that markets Epogen/Procrit and Aranesp.
Details on the analyses and evaluation are available from the following page on the FDA website: Information on Erythropoiesis-Stimulating Agents (ESA) Epoetin alfa (marketed as Procrit, Epogen), Darbepoetin alfa (marketed as Aranesp). ![]()
The US Food and Drug Administration (FDA) has determined that the risk evaluation and mitigation strategy (REMS) for erythropoiesis-stimulating agents (ESAs) is no longer necessary.
The REMS was limited to the use of epoetin alfa (marketed as Epogen and Procrit) and darbepoetin alfa (marketed as Aranesp) to treat patients with anemia due to myelosuppressive chemotherapy.
The FDA said the REMS is no longer necessary to ensure that the benefits of Epogen/Procrit and Aranesp outweigh the risks these drugs pose, which include shortened overall survival and an increased risk of tumor progression or recurrence in patients with cancer.
The FDA has released the REMS requirements for these ESAs and said the risks the drugs pose can be communicated by the current product prescribing information.
The FDA decided the REMS is no longer needed based on its own analyses and an evaluation of the REMS assessment submitted by Amgen, Inc., the company that markets Epogen/Procrit and Aranesp.
Details on the analyses and evaluation are available from the following page on the FDA website: Information on Erythropoiesis-Stimulating Agents (ESA) Epoetin alfa (marketed as Procrit, Epogen), Darbepoetin alfa (marketed as Aranesp). ![]()
The US Food and Drug Administration (FDA) has determined that the risk evaluation and mitigation strategy (REMS) for erythropoiesis-stimulating agents (ESAs) is no longer necessary.
The REMS was limited to the use of epoetin alfa (marketed as Epogen and Procrit) and darbepoetin alfa (marketed as Aranesp) to treat patients with anemia due to myelosuppressive chemotherapy.
The FDA said the REMS is no longer necessary to ensure that the benefits of Epogen/Procrit and Aranesp outweigh the risks these drugs pose, which include shortened overall survival and an increased risk of tumor progression or recurrence in patients with cancer.
The FDA has released the REMS requirements for these ESAs and said the risks the drugs pose can be communicated by the current product prescribing information.
The FDA decided the REMS is no longer needed based on its own analyses and an evaluation of the REMS assessment submitted by Amgen, Inc., the company that markets Epogen/Procrit and Aranesp.
Details on the analyses and evaluation are available from the following page on the FDA website: Information on Erythropoiesis-Stimulating Agents (ESA) Epoetin alfa (marketed as Procrit, Epogen), Darbepoetin alfa (marketed as Aranesp). ![]()
Study supports use of tPA in stroke patients with SCD
A new study suggests that having sickle cell disease (SCD) should not prevent patients from receiving tissue plasminogen activator (tPA) to treat ischemic stroke if they otherwise qualify for the treatment.
Researchers compared outcomes of tPA treatment in stroke patients with and without SCD and found no significant differences between the groups with regard to serious complications, length of hospital stay, or in-hospital mortality.
“Having sickle cell disease did not adversely affect any of the indicators we measured,” said Robert J. Adams, MD, of the Medical University of South Carolina in Charleston.
The SCD patients did have a higher rate of intracranial hemorrhage (ICH) than patients without SCD, although the rate was not significantly higher. Still, the researchers said further study is needed to look more closely at this outcome.
Dr Adams and his colleagues reported their findings in the journal Stroke.
The team noted that use of tPA has never been contraindicated in SCD, but guidelines recommend acute exchange transfusion for stroke in SCD, rather than tPA.
To gain more insight into the effects of tPA in patients with SCD, the researchers analyzed in-hospital data compiled by the quality improvement program Get With The Guidelines – Stroke.
The data included 2,016,652 stroke patients seen at 1952 participating US hospitals between January 2008 and March 2015. From these patients, the researchers identified 832 with SCD and 3328 age-, sex-, and race-matched controls.
There was no significant difference between the 2 cohorts in the rate of tPA use—8.2% for SCD patients and 9.4% for controls (P=0.3024).
Likewise, there was no significant difference in the timeliness of tPA administration. The median door-to-needle time was 73 minutes for SCD patients and 79 minutes for controls (P=0.3891).
Among patients who received tPA, there was no significant difference in the overall rate of serious complications, which occurred in 6.6% of the SCD patients and 6.0% of controls (P=0.7732).
Serious complications included symptomatic ICH, which occurred in 4.9% of the SCD patients and 3.2% of controls who received tPA (P= 0.4502).
Although this difference was not significant, the researchers said additional studies are needed to track the ICH rate in SCD patients receiving tPA.
The researchers also calculated the odds ratios (ORs) for various outcomes in tPA-treated SCD patients compared to controls.
In an analysis adjusted for multiple covariates, the OR for in-hospital mortality was 1.21 for SCD patients (P=0.4150), the OR for being discharged home was 0.90 (P=0.2686), and the OR for having a hospital stay lasting beyond 4 days was 1.15 (P=0.1151).
“People with sickle cell disease and an acute stroke who would otherwise qualify for tPA did not have worse outcomes than stroke patients who did not have sickle cell disease,” Dr Adams noted.
He and his colleagues said these findings suggest tPA is safe for patients with SCD and could potentially be used as a complementary therapy to red blood cell exchange, the current guideline-recommended frontline therapy for ischemic stroke in patients with SCD.
“These findings suggest that a future randomized trial that compares using red blood cell exchange alone versus combination therapy with tPA and red blood cell exchange should be undertaken to evaluate the outcomes of [ischemic stroke] in patients with sickle cell disease,” said study author Julie Kanter, MD, of the Medical University of South Carolina in Charleston. ![]()
A new study suggests that having sickle cell disease (SCD) should not prevent patients from receiving tissue plasminogen activator (tPA) to treat ischemic stroke if they otherwise qualify for the treatment.
Researchers compared outcomes of tPA treatment in stroke patients with and without SCD and found no significant differences between the groups with regard to serious complications, length of hospital stay, or in-hospital mortality.
“Having sickle cell disease did not adversely affect any of the indicators we measured,” said Robert J. Adams, MD, of the Medical University of South Carolina in Charleston.
The SCD patients did have a higher rate of intracranial hemorrhage (ICH) than patients without SCD, although the rate was not significantly higher. Still, the researchers said further study is needed to look more closely at this outcome.
Dr Adams and his colleagues reported their findings in the journal Stroke.
The team noted that use of tPA has never been contraindicated in SCD, but guidelines recommend acute exchange transfusion for stroke in SCD, rather than tPA.
To gain more insight into the effects of tPA in patients with SCD, the researchers analyzed in-hospital data compiled by the quality improvement program Get With The Guidelines – Stroke.
The data included 2,016,652 stroke patients seen at 1952 participating US hospitals between January 2008 and March 2015. From these patients, the researchers identified 832 with SCD and 3328 age-, sex-, and race-matched controls.
There was no significant difference between the 2 cohorts in the rate of tPA use—8.2% for SCD patients and 9.4% for controls (P=0.3024).
Likewise, there was no significant difference in the timeliness of tPA administration. The median door-to-needle time was 73 minutes for SCD patients and 79 minutes for controls (P=0.3891).
Among patients who received tPA, there was no significant difference in the overall rate of serious complications, which occurred in 6.6% of the SCD patients and 6.0% of controls (P=0.7732).
Serious complications included symptomatic ICH, which occurred in 4.9% of the SCD patients and 3.2% of controls who received tPA (P= 0.4502).
Although this difference was not significant, the researchers said additional studies are needed to track the ICH rate in SCD patients receiving tPA.
The researchers also calculated the odds ratios (ORs) for various outcomes in tPA-treated SCD patients compared to controls.
In an analysis adjusted for multiple covariates, the OR for in-hospital mortality was 1.21 for SCD patients (P=0.4150), the OR for being discharged home was 0.90 (P=0.2686), and the OR for having a hospital stay lasting beyond 4 days was 1.15 (P=0.1151).
“People with sickle cell disease and an acute stroke who would otherwise qualify for tPA did not have worse outcomes than stroke patients who did not have sickle cell disease,” Dr Adams noted.
He and his colleagues said these findings suggest tPA is safe for patients with SCD and could potentially be used as a complementary therapy to red blood cell exchange, the current guideline-recommended frontline therapy for ischemic stroke in patients with SCD.
“These findings suggest that a future randomized trial that compares using red blood cell exchange alone versus combination therapy with tPA and red blood cell exchange should be undertaken to evaluate the outcomes of [ischemic stroke] in patients with sickle cell disease,” said study author Julie Kanter, MD, of the Medical University of South Carolina in Charleston. ![]()
A new study suggests that having sickle cell disease (SCD) should not prevent patients from receiving tissue plasminogen activator (tPA) to treat ischemic stroke if they otherwise qualify for the treatment.
Researchers compared outcomes of tPA treatment in stroke patients with and without SCD and found no significant differences between the groups with regard to serious complications, length of hospital stay, or in-hospital mortality.
“Having sickle cell disease did not adversely affect any of the indicators we measured,” said Robert J. Adams, MD, of the Medical University of South Carolina in Charleston.
The SCD patients did have a higher rate of intracranial hemorrhage (ICH) than patients without SCD, although the rate was not significantly higher. Still, the researchers said further study is needed to look more closely at this outcome.
Dr Adams and his colleagues reported their findings in the journal Stroke.
The team noted that use of tPA has never been contraindicated in SCD, but guidelines recommend acute exchange transfusion for stroke in SCD, rather than tPA.
To gain more insight into the effects of tPA in patients with SCD, the researchers analyzed in-hospital data compiled by the quality improvement program Get With The Guidelines – Stroke.
The data included 2,016,652 stroke patients seen at 1952 participating US hospitals between January 2008 and March 2015. From these patients, the researchers identified 832 with SCD and 3328 age-, sex-, and race-matched controls.
There was no significant difference between the 2 cohorts in the rate of tPA use—8.2% for SCD patients and 9.4% for controls (P=0.3024).
Likewise, there was no significant difference in the timeliness of tPA administration. The median door-to-needle time was 73 minutes for SCD patients and 79 minutes for controls (P=0.3891).
Among patients who received tPA, there was no significant difference in the overall rate of serious complications, which occurred in 6.6% of the SCD patients and 6.0% of controls (P=0.7732).
Serious complications included symptomatic ICH, which occurred in 4.9% of the SCD patients and 3.2% of controls who received tPA (P= 0.4502).
Although this difference was not significant, the researchers said additional studies are needed to track the ICH rate in SCD patients receiving tPA.
The researchers also calculated the odds ratios (ORs) for various outcomes in tPA-treated SCD patients compared to controls.
In an analysis adjusted for multiple covariates, the OR for in-hospital mortality was 1.21 for SCD patients (P=0.4150), the OR for being discharged home was 0.90 (P=0.2686), and the OR for having a hospital stay lasting beyond 4 days was 1.15 (P=0.1151).
“People with sickle cell disease and an acute stroke who would otherwise qualify for tPA did not have worse outcomes than stroke patients who did not have sickle cell disease,” Dr Adams noted.
He and his colleagues said these findings suggest tPA is safe for patients with SCD and could potentially be used as a complementary therapy to red blood cell exchange, the current guideline-recommended frontline therapy for ischemic stroke in patients with SCD.
“These findings suggest that a future randomized trial that compares using red blood cell exchange alone versus combination therapy with tPA and red blood cell exchange should be undertaken to evaluate the outcomes of [ischemic stroke] in patients with sickle cell disease,” said study author Julie Kanter, MD, of the Medical University of South Carolina in Charleston. ![]()
Drug granted orphan designation for MM
The US Food and Drug Administration (FDA) has granted orphan drug designation for tasquinimod as a treatment for multiple myeloma (MM).
Tasquinimod is an immunomodulatory, anti-metastatic, and anti-angiogenic compound being developed by Active Biotech AB.
The company says tasquinimod works by inhibiting the function of S100A9, a pro-inflammatory protein that is elevated in MM and other malignancies.
S100A9 is believed to aid cancer development by recruiting and activating immune cells such as myeloid-derived suppressor cells.
Active Biotech AB says that, by targeting the S100A9 pathway, tasquinimod interferes with the accumulation and activation of myeloid-derived suppressor cells in the tumor microenvironment, which decreases immune suppression and angiogenesis.
Tasquinimod also inhibits the hypoxic response in the tumor by binding to HDAC4, according to Active Biotech AB.
The company says tasquinimod has produced “robust results” in animal models of MM, and research presented at the AACR Annual Meeting 2015 supports this statement.
Investigators found that tasquinimod reduced tumor growth and improved survival in mouse models of MM. And these effects were associated with reduced angiogenesis in the bone marrow.
Tasquinimod was previously under development as a treatment for prostate cancer, but research suggested the drug did not have a favorable risk-benefit ratio in this patient population.
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent rare diseases/disorders affecting fewer than 200,000 people in the US.
Orphan designation provides companies with certain incentives to develop products for rare diseases. This includes a 50% tax break on research and development, a fee waiver, access to federal grants, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation for tasquinimod as a treatment for multiple myeloma (MM).
Tasquinimod is an immunomodulatory, anti-metastatic, and anti-angiogenic compound being developed by Active Biotech AB.
The company says tasquinimod works by inhibiting the function of S100A9, a pro-inflammatory protein that is elevated in MM and other malignancies.
S100A9 is believed to aid cancer development by recruiting and activating immune cells such as myeloid-derived suppressor cells.
Active Biotech AB says that, by targeting the S100A9 pathway, tasquinimod interferes with the accumulation and activation of myeloid-derived suppressor cells in the tumor microenvironment, which decreases immune suppression and angiogenesis.
Tasquinimod also inhibits the hypoxic response in the tumor by binding to HDAC4, according to Active Biotech AB.
The company says tasquinimod has produced “robust results” in animal models of MM, and research presented at the AACR Annual Meeting 2015 supports this statement.
Investigators found that tasquinimod reduced tumor growth and improved survival in mouse models of MM. And these effects were associated with reduced angiogenesis in the bone marrow.
Tasquinimod was previously under development as a treatment for prostate cancer, but research suggested the drug did not have a favorable risk-benefit ratio in this patient population.
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent rare diseases/disorders affecting fewer than 200,000 people in the US.
Orphan designation provides companies with certain incentives to develop products for rare diseases. This includes a 50% tax break on research and development, a fee waiver, access to federal grants, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation for tasquinimod as a treatment for multiple myeloma (MM).
Tasquinimod is an immunomodulatory, anti-metastatic, and anti-angiogenic compound being developed by Active Biotech AB.
The company says tasquinimod works by inhibiting the function of S100A9, a pro-inflammatory protein that is elevated in MM and other malignancies.
S100A9 is believed to aid cancer development by recruiting and activating immune cells such as myeloid-derived suppressor cells.
Active Biotech AB says that, by targeting the S100A9 pathway, tasquinimod interferes with the accumulation and activation of myeloid-derived suppressor cells in the tumor microenvironment, which decreases immune suppression and angiogenesis.
Tasquinimod also inhibits the hypoxic response in the tumor by binding to HDAC4, according to Active Biotech AB.
The company says tasquinimod has produced “robust results” in animal models of MM, and research presented at the AACR Annual Meeting 2015 supports this statement.
Investigators found that tasquinimod reduced tumor growth and improved survival in mouse models of MM. And these effects were associated with reduced angiogenesis in the bone marrow.
Tasquinimod was previously under development as a treatment for prostate cancer, but research suggested the drug did not have a favorable risk-benefit ratio in this patient population.
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent rare diseases/disorders affecting fewer than 200,000 people in the US.
Orphan designation provides companies with certain incentives to develop products for rare diseases. This includes a 50% tax break on research and development, a fee waiver, access to federal grants, and 7 years of market exclusivity if the product is approved. ![]()
Topical HDAC inhibitor shows activity in MF
Results of a phase 2 trial suggest a topical, skin-directed histone deacetylase (HDAC) inhibitor can elicit responses in patients with early stage mycosis fungoides (MF).
The drug, remetinostat, was designed to be active in the skin but rapidly broken down and inactivated in blood in order to limit the adverse effects associated with systemic exposure to HDAC inhibitors.
The trial included 60 MF patients who were randomized to receive 0.5% remetinostat gel twice daily, 1% remetinostat gel once daily, or 1% remetinostat gel twice daily for between 6 and 12 months.
Results from this trial were recently released by Medivir AB, the company developing remetinostat.
The primary endpoint of the study was the proportion of patients with either a complete or partial confirmed response to therapy, assessed using the Composite Assessment of Index Lesion Severity.
Based on an intent-to-treat analysis, patients receiving the 1% remetinostat gel twice daily arm had the highest proportion of confirmed responses. Eight of 20 patients (40%) responded, which included 1 complete response.
Five of 20 patients (25%) receiving 0.5% remetinostat gel twice daily responded, as did 4 of 20 (20%) patients receiving 1% remetinostat gel once daily. None of these responses were complete responses.
Remetinostat was well-tolerated across all the dose groups, according to Medivir. There were no signs of systemic adverse effects, including those associated with systemic HDAC inhibitors.
Based on these data, Medivir expects to initiate discussions with regulatory authorities with the aim of initiating a phase 3 study later this year, and to present full phase 2 trial data at scientific meetings in the second half of 2017.
“Remetinostat was designed to effectively inhibit HDACs within cutaneous lesions but to be rapidly broken down in the bloodstream, preventing the side effects associated with systemically administered HDAC inhibitors,” said Richard Bethell, Medivir’s chief scientific officer.
“Based on the efficacy and safety data from this phase 2 study, we believe that remetinostat is capable of meeting a very important unmet need in patients with this chronic and poorly treated orphan disease.” ![]()
Results of a phase 2 trial suggest a topical, skin-directed histone deacetylase (HDAC) inhibitor can elicit responses in patients with early stage mycosis fungoides (MF).
The drug, remetinostat, was designed to be active in the skin but rapidly broken down and inactivated in blood in order to limit the adverse effects associated with systemic exposure to HDAC inhibitors.
The trial included 60 MF patients who were randomized to receive 0.5% remetinostat gel twice daily, 1% remetinostat gel once daily, or 1% remetinostat gel twice daily for between 6 and 12 months.
Results from this trial were recently released by Medivir AB, the company developing remetinostat.
The primary endpoint of the study was the proportion of patients with either a complete or partial confirmed response to therapy, assessed using the Composite Assessment of Index Lesion Severity.
Based on an intent-to-treat analysis, patients receiving the 1% remetinostat gel twice daily arm had the highest proportion of confirmed responses. Eight of 20 patients (40%) responded, which included 1 complete response.
Five of 20 patients (25%) receiving 0.5% remetinostat gel twice daily responded, as did 4 of 20 (20%) patients receiving 1% remetinostat gel once daily. None of these responses were complete responses.
Remetinostat was well-tolerated across all the dose groups, according to Medivir. There were no signs of systemic adverse effects, including those associated with systemic HDAC inhibitors.
Based on these data, Medivir expects to initiate discussions with regulatory authorities with the aim of initiating a phase 3 study later this year, and to present full phase 2 trial data at scientific meetings in the second half of 2017.
“Remetinostat was designed to effectively inhibit HDACs within cutaneous lesions but to be rapidly broken down in the bloodstream, preventing the side effects associated with systemically administered HDAC inhibitors,” said Richard Bethell, Medivir’s chief scientific officer.
“Based on the efficacy and safety data from this phase 2 study, we believe that remetinostat is capable of meeting a very important unmet need in patients with this chronic and poorly treated orphan disease.” ![]()
Results of a phase 2 trial suggest a topical, skin-directed histone deacetylase (HDAC) inhibitor can elicit responses in patients with early stage mycosis fungoides (MF).
The drug, remetinostat, was designed to be active in the skin but rapidly broken down and inactivated in blood in order to limit the adverse effects associated with systemic exposure to HDAC inhibitors.
The trial included 60 MF patients who were randomized to receive 0.5% remetinostat gel twice daily, 1% remetinostat gel once daily, or 1% remetinostat gel twice daily for between 6 and 12 months.
Results from this trial were recently released by Medivir AB, the company developing remetinostat.
The primary endpoint of the study was the proportion of patients with either a complete or partial confirmed response to therapy, assessed using the Composite Assessment of Index Lesion Severity.
Based on an intent-to-treat analysis, patients receiving the 1% remetinostat gel twice daily arm had the highest proportion of confirmed responses. Eight of 20 patients (40%) responded, which included 1 complete response.
Five of 20 patients (25%) receiving 0.5% remetinostat gel twice daily responded, as did 4 of 20 (20%) patients receiving 1% remetinostat gel once daily. None of these responses were complete responses.
Remetinostat was well-tolerated across all the dose groups, according to Medivir. There were no signs of systemic adverse effects, including those associated with systemic HDAC inhibitors.
Based on these data, Medivir expects to initiate discussions with regulatory authorities with the aim of initiating a phase 3 study later this year, and to present full phase 2 trial data at scientific meetings in the second half of 2017.
“Remetinostat was designed to effectively inhibit HDACs within cutaneous lesions but to be rapidly broken down in the bloodstream, preventing the side effects associated with systemically administered HDAC inhibitors,” said Richard Bethell, Medivir’s chief scientific officer.
“Based on the efficacy and safety data from this phase 2 study, we believe that remetinostat is capable of meeting a very important unmet need in patients with this chronic and poorly treated orphan disease.” ![]()
Portal allows hemophilia patients to share data with providers
Novo Nordisk has launched a web-based portal that allows hemophilia patients to share real-time data on their treatment and bleeding events with their healthcare providers.
The portal, HemaGo™ XChange, is an extension of Novo Nordisk’s HemaGo™ mobile application and website, which were launched in 2012.
Data a patient enters into the HemaGo™ diary can be shared through the HemaGo™ XChange with the patient’s hemophilia treatment network.
Patients may also choose to have these data entered into the American Thrombosis and Hemostasis Network’s (ATHN) national database of bleeding disorder treatment information.
“We developed HemaGo™ XChange to help drive progress in hemophilia management by turning static data into usable information for people with hemophilia, their care teams, and even researchers,” said John Spera, vice-president of biopharmaceuticals marketing at Novo Nordisk Inc.
“With timely information about the daily experiences of patients, including bleeds, healthcare providers can adjust their care to better fit patient lives.”
Patients using HemaGo™ can:
- Provide information to their healthcare team, including access to treatment and bleed data, in real time through the HemaGo™ XChange web portal
- Choose to email data directly from the app or website at any time
- Opt-in through their hemophilia treatment center to have their data integrated into ATHN’s national database of bleeding disorder treatment information. ATHN will use and share these data with hemophilia treatment centers to foster its mission of advancing knowledge and transforming care for the bleeding and clotting disorders community.
Providers invited by patients to connect via the HemaGo™ XChange portal can:
- View details about treatments, bleeds, and more for multiple patients
- Track when and how much factor is used; the type of infusion, vial, and dosing amounts; and information about any other medications
- View data on the type, location, duration, frequency, and status of bleeds
- View patient and caregiver life experiences, such as pain and health scores or how a bleeding disorder has affected work, school, or other activities
- Download in-depth reports for all recorded information.
Novo Nordisk does not have access to patient-specific information. The company’s access is restricted to de-identified data in which the individual sources of the data cannot be identified, in accordance with Health Insurance Portability and Accountability Act of 1996 (HIPAA) Privacy and Security Rules.
To download the HemaGo™ app or join the HemaGo™ XChange, visit www.HemaGo.com and www.HGXchange.com. The HemaGo™ app is available for iPhone and Android phones. ![]()
Novo Nordisk has launched a web-based portal that allows hemophilia patients to share real-time data on their treatment and bleeding events with their healthcare providers.
The portal, HemaGo™ XChange, is an extension of Novo Nordisk’s HemaGo™ mobile application and website, which were launched in 2012.
Data a patient enters into the HemaGo™ diary can be shared through the HemaGo™ XChange with the patient’s hemophilia treatment network.
Patients may also choose to have these data entered into the American Thrombosis and Hemostasis Network’s (ATHN) national database of bleeding disorder treatment information.
“We developed HemaGo™ XChange to help drive progress in hemophilia management by turning static data into usable information for people with hemophilia, their care teams, and even researchers,” said John Spera, vice-president of biopharmaceuticals marketing at Novo Nordisk Inc.
“With timely information about the daily experiences of patients, including bleeds, healthcare providers can adjust their care to better fit patient lives.”
Patients using HemaGo™ can:
- Provide information to their healthcare team, including access to treatment and bleed data, in real time through the HemaGo™ XChange web portal
- Choose to email data directly from the app or website at any time
- Opt-in through their hemophilia treatment center to have their data integrated into ATHN’s national database of bleeding disorder treatment information. ATHN will use and share these data with hemophilia treatment centers to foster its mission of advancing knowledge and transforming care for the bleeding and clotting disorders community.
Providers invited by patients to connect via the HemaGo™ XChange portal can:
- View details about treatments, bleeds, and more for multiple patients
- Track when and how much factor is used; the type of infusion, vial, and dosing amounts; and information about any other medications
- View data on the type, location, duration, frequency, and status of bleeds
- View patient and caregiver life experiences, such as pain and health scores or how a bleeding disorder has affected work, school, or other activities
- Download in-depth reports for all recorded information.
Novo Nordisk does not have access to patient-specific information. The company’s access is restricted to de-identified data in which the individual sources of the data cannot be identified, in accordance with Health Insurance Portability and Accountability Act of 1996 (HIPAA) Privacy and Security Rules.
To download the HemaGo™ app or join the HemaGo™ XChange, visit www.HemaGo.com and www.HGXchange.com. The HemaGo™ app is available for iPhone and Android phones. ![]()
Novo Nordisk has launched a web-based portal that allows hemophilia patients to share real-time data on their treatment and bleeding events with their healthcare providers.
The portal, HemaGo™ XChange, is an extension of Novo Nordisk’s HemaGo™ mobile application and website, which were launched in 2012.
Data a patient enters into the HemaGo™ diary can be shared through the HemaGo™ XChange with the patient’s hemophilia treatment network.
Patients may also choose to have these data entered into the American Thrombosis and Hemostasis Network’s (ATHN) national database of bleeding disorder treatment information.
“We developed HemaGo™ XChange to help drive progress in hemophilia management by turning static data into usable information for people with hemophilia, their care teams, and even researchers,” said John Spera, vice-president of biopharmaceuticals marketing at Novo Nordisk Inc.
“With timely information about the daily experiences of patients, including bleeds, healthcare providers can adjust their care to better fit patient lives.”
Patients using HemaGo™ can:
- Provide information to their healthcare team, including access to treatment and bleed data, in real time through the HemaGo™ XChange web portal
- Choose to email data directly from the app or website at any time
- Opt-in through their hemophilia treatment center to have their data integrated into ATHN’s national database of bleeding disorder treatment information. ATHN will use and share these data with hemophilia treatment centers to foster its mission of advancing knowledge and transforming care for the bleeding and clotting disorders community.
Providers invited by patients to connect via the HemaGo™ XChange portal can:
- View details about treatments, bleeds, and more for multiple patients
- Track when and how much factor is used; the type of infusion, vial, and dosing amounts; and information about any other medications
- View data on the type, location, duration, frequency, and status of bleeds
- View patient and caregiver life experiences, such as pain and health scores or how a bleeding disorder has affected work, school, or other activities
- Download in-depth reports for all recorded information.
Novo Nordisk does not have access to patient-specific information. The company’s access is restricted to de-identified data in which the individual sources of the data cannot be identified, in accordance with Health Insurance Portability and Accountability Act of 1996 (HIPAA) Privacy and Security Rules.
To download the HemaGo™ app or join the HemaGo™ XChange, visit www.HemaGo.com and www.HGXchange.com. The HemaGo™ app is available for iPhone and Android phones. ![]()
Study reveals global inequalities in childhood leukemia survival
New research has revealed global inequalities in survival rates for pediatric patients with leukemia.
Investigators analyzed data on nearly 90,000 pediatric leukemia patients treated in 53 countries.
In most countries, patients with lymphoid leukemias or acute myeloid leukemia (AML) saw an increase in 5-year survival between 1995 and 2009.
However, there were wide variations in survival between the countries.
The investigators reported these findings in The Lancet Haematology.
They evaluated data from 89,828 leukemia patients (ages 0 to 14) included in 198 cancer registries in 53 countries.
The team estimated 5-year net survival for patients with AML or lymphoid leukemias (controlling for non-leukemia-related deaths) by calendar period of diagnosis—1995–1999, 2000–2004, and 2005–2009—in each country.
For children diagnosed with lymphoid leukemias between 1995 and 1999, 5-year survival rates ranged from 10.6% (in China) to 86.8% (in Austria). For children diagnosed between 2005 and 2009, the rates ranged from 52.4% (Colombia) to 91.6% (Germany).
For AML, 5-year survival rates ranged from 4.2% (China) to 72.2% (Sweden) in patients diagnosed between 1995 and 1999. For children diagnosed between 2005 and 2009, 5-year survival rates ranged from 33.3% (Bulgaria) to 78.2% (Germany).
The investigators noted that, in some countries, survival for both groups of leukemia patients was consistently high.
In Austria, for example, 5-year survival rates for lymphoid leukemias were 86.8% in 1995-1999 and 91.1% in 2005-2009. For AML, rates were 60.1% and 72.6%, respectively.
Other countries saw substantial increases in survival over time.
In China, the 5-year survival rate for patients with lymphoid leukemias increased from 10.6% in 1995-1999 to 69.2% in 2005-2009. For patients with AML, the rate increased from 4.2% to 41.1%.
“These findings show the extent of worldwide inequalities in access to optimal healthcare for children with cancer,” said study author Audrey Bonaventure, MD, PhD, of the London School of Hygiene & Tropical Medicine in the UK.
“Providing additional resources, alongside evidence-based initiatives such as international collaborations and treatment guidelines, could improve access to efficient treatment and care for all children with leukemia. This would contribute substantially to reducing worldwide inequalities in survival.” ![]()
New research has revealed global inequalities in survival rates for pediatric patients with leukemia.
Investigators analyzed data on nearly 90,000 pediatric leukemia patients treated in 53 countries.
In most countries, patients with lymphoid leukemias or acute myeloid leukemia (AML) saw an increase in 5-year survival between 1995 and 2009.
However, there were wide variations in survival between the countries.
The investigators reported these findings in The Lancet Haematology.
They evaluated data from 89,828 leukemia patients (ages 0 to 14) included in 198 cancer registries in 53 countries.
The team estimated 5-year net survival for patients with AML or lymphoid leukemias (controlling for non-leukemia-related deaths) by calendar period of diagnosis—1995–1999, 2000–2004, and 2005–2009—in each country.
For children diagnosed with lymphoid leukemias between 1995 and 1999, 5-year survival rates ranged from 10.6% (in China) to 86.8% (in Austria). For children diagnosed between 2005 and 2009, the rates ranged from 52.4% (Colombia) to 91.6% (Germany).
For AML, 5-year survival rates ranged from 4.2% (China) to 72.2% (Sweden) in patients diagnosed between 1995 and 1999. For children diagnosed between 2005 and 2009, 5-year survival rates ranged from 33.3% (Bulgaria) to 78.2% (Germany).
The investigators noted that, in some countries, survival for both groups of leukemia patients was consistently high.
In Austria, for example, 5-year survival rates for lymphoid leukemias were 86.8% in 1995-1999 and 91.1% in 2005-2009. For AML, rates were 60.1% and 72.6%, respectively.
Other countries saw substantial increases in survival over time.
In China, the 5-year survival rate for patients with lymphoid leukemias increased from 10.6% in 1995-1999 to 69.2% in 2005-2009. For patients with AML, the rate increased from 4.2% to 41.1%.
“These findings show the extent of worldwide inequalities in access to optimal healthcare for children with cancer,” said study author Audrey Bonaventure, MD, PhD, of the London School of Hygiene & Tropical Medicine in the UK.
“Providing additional resources, alongside evidence-based initiatives such as international collaborations and treatment guidelines, could improve access to efficient treatment and care for all children with leukemia. This would contribute substantially to reducing worldwide inequalities in survival.” ![]()
New research has revealed global inequalities in survival rates for pediatric patients with leukemia.
Investigators analyzed data on nearly 90,000 pediatric leukemia patients treated in 53 countries.
In most countries, patients with lymphoid leukemias or acute myeloid leukemia (AML) saw an increase in 5-year survival between 1995 and 2009.
However, there were wide variations in survival between the countries.
The investigators reported these findings in The Lancet Haematology.
They evaluated data from 89,828 leukemia patients (ages 0 to 14) included in 198 cancer registries in 53 countries.
The team estimated 5-year net survival for patients with AML or lymphoid leukemias (controlling for non-leukemia-related deaths) by calendar period of diagnosis—1995–1999, 2000–2004, and 2005–2009—in each country.
For children diagnosed with lymphoid leukemias between 1995 and 1999, 5-year survival rates ranged from 10.6% (in China) to 86.8% (in Austria). For children diagnosed between 2005 and 2009, the rates ranged from 52.4% (Colombia) to 91.6% (Germany).
For AML, 5-year survival rates ranged from 4.2% (China) to 72.2% (Sweden) in patients diagnosed between 1995 and 1999. For children diagnosed between 2005 and 2009, 5-year survival rates ranged from 33.3% (Bulgaria) to 78.2% (Germany).
The investigators noted that, in some countries, survival for both groups of leukemia patients was consistently high.
In Austria, for example, 5-year survival rates for lymphoid leukemias were 86.8% in 1995-1999 and 91.1% in 2005-2009. For AML, rates were 60.1% and 72.6%, respectively.
Other countries saw substantial increases in survival over time.
In China, the 5-year survival rate for patients with lymphoid leukemias increased from 10.6% in 1995-1999 to 69.2% in 2005-2009. For patients with AML, the rate increased from 4.2% to 41.1%.
“These findings show the extent of worldwide inequalities in access to optimal healthcare for children with cancer,” said study author Audrey Bonaventure, MD, PhD, of the London School of Hygiene & Tropical Medicine in the UK.
“Providing additional resources, alongside evidence-based initiatives such as international collaborations and treatment guidelines, could improve access to efficient treatment and care for all children with leukemia. This would contribute substantially to reducing worldwide inequalities in survival.” ![]()
FDA clears test for individual WBD platelet units
The US Food and Drug Administration (FDA) has granted new clearance for Verax Biomedical’s Platelet PGD® Test.
This qualitative immunoassay is designed to detect aerobic and anaerobic Gram-positive and Gram-negative bacteria in platelets.
The test is now cleared for use on single units of leukocyte-reduced or non-leukocyte-reduced whole blood-derived (WBD) platelets in plasma.
The Platelet PGD Test was previously cleared by the FDA as a safety measure to be used following testing with a growth-based, quality control (QC) test for platelet components that is cleared by the FDA.
For this indication, the Platelet PGD Test can be used within 24 hours of transfusion on:
- Leukocyte-reduced apheresis platelets suspended in plasma
- Leukocyte-reduced apheresis platelets suspended in platelet additive solution C and plasma
- Pre-storage pools of up to 6 leukocyte-reduced WBD platelets suspended in plasma.
When used as a safety measure, the Platelet PGD Test can extend the dating of apheresis platelets in plasma from 5 to 7 days.
The Platelet PGD Test also has FDA clearance as a QC test for use on pools of up to 6 units of leukocyte-reduced and non-leukocyte-reduced WBD platelets suspended in plasma that are pooled within 4 hours of transfusion.
The latest FDA clearance extends this use to individual units of WBD platelets in plasma.
“[The new clearance] has been requested by current users of PGD as well as being outlined as a need in pending FDA Draft Guidance to address the risk of bacterial contamination in platelets,” said Jim Lousararian, chief executive officer of Verax Biomedical.
According to the company, the new clearance is intended to help reduce the risk of bacterial contamination for pediatric patients receiving platelet transfusions.
“Pediatric patients pose unique challenges in transfusion medicine,” said Paul Mintz, MD, chief medical officer of Verax Biomedical.
“They require small platelet doses and possess fragile immune systems. PGD testing individual WBD units for transfusion makes it practical to provide bacterially tested platelets to this most vulnerable group of patients.”
Multicenter study
The Verax PGD® test was evaluated in a 2-year study including 18 US hospitals. The results were published in Transfusion in 2011.
The objective of the study was to evaluate the test’s ability to detect bacterially contaminated units in the US apheresis inventory that tested negative for contamination by existing growth-based QC tests.
A total of 9 contaminated units were detected by PGD and confirmed as bacterially contaminated in a population of 27,620 leukocyte-reduced apheresis units (1:3,069 doses tested).
All 9 units had previously tested negative by growth-based QC methods applied earlier in unit life in conformance with all applicable AABB and CAP standards for bacterial testing.
Researchers said the study clearly demonstrated the ability of the Platelet PGD Test to detect and interdict contaminated units missed by current QC testing methods. ![]()
The US Food and Drug Administration (FDA) has granted new clearance for Verax Biomedical’s Platelet PGD® Test.
This qualitative immunoassay is designed to detect aerobic and anaerobic Gram-positive and Gram-negative bacteria in platelets.
The test is now cleared for use on single units of leukocyte-reduced or non-leukocyte-reduced whole blood-derived (WBD) platelets in plasma.
The Platelet PGD Test was previously cleared by the FDA as a safety measure to be used following testing with a growth-based, quality control (QC) test for platelet components that is cleared by the FDA.
For this indication, the Platelet PGD Test can be used within 24 hours of transfusion on:
- Leukocyte-reduced apheresis platelets suspended in plasma
- Leukocyte-reduced apheresis platelets suspended in platelet additive solution C and plasma
- Pre-storage pools of up to 6 leukocyte-reduced WBD platelets suspended in plasma.
When used as a safety measure, the Platelet PGD Test can extend the dating of apheresis platelets in plasma from 5 to 7 days.
The Platelet PGD Test also has FDA clearance as a QC test for use on pools of up to 6 units of leukocyte-reduced and non-leukocyte-reduced WBD platelets suspended in plasma that are pooled within 4 hours of transfusion.
The latest FDA clearance extends this use to individual units of WBD platelets in plasma.
“[The new clearance] has been requested by current users of PGD as well as being outlined as a need in pending FDA Draft Guidance to address the risk of bacterial contamination in platelets,” said Jim Lousararian, chief executive officer of Verax Biomedical.
According to the company, the new clearance is intended to help reduce the risk of bacterial contamination for pediatric patients receiving platelet transfusions.
“Pediatric patients pose unique challenges in transfusion medicine,” said Paul Mintz, MD, chief medical officer of Verax Biomedical.
“They require small platelet doses and possess fragile immune systems. PGD testing individual WBD units for transfusion makes it practical to provide bacterially tested platelets to this most vulnerable group of patients.”
Multicenter study
The Verax PGD® test was evaluated in a 2-year study including 18 US hospitals. The results were published in Transfusion in 2011.
The objective of the study was to evaluate the test’s ability to detect bacterially contaminated units in the US apheresis inventory that tested negative for contamination by existing growth-based QC tests.
A total of 9 contaminated units were detected by PGD and confirmed as bacterially contaminated in a population of 27,620 leukocyte-reduced apheresis units (1:3,069 doses tested).
All 9 units had previously tested negative by growth-based QC methods applied earlier in unit life in conformance with all applicable AABB and CAP standards for bacterial testing.
Researchers said the study clearly demonstrated the ability of the Platelet PGD Test to detect and interdict contaminated units missed by current QC testing methods. ![]()
The US Food and Drug Administration (FDA) has granted new clearance for Verax Biomedical’s Platelet PGD® Test.
This qualitative immunoassay is designed to detect aerobic and anaerobic Gram-positive and Gram-negative bacteria in platelets.
The test is now cleared for use on single units of leukocyte-reduced or non-leukocyte-reduced whole blood-derived (WBD) platelets in plasma.
The Platelet PGD Test was previously cleared by the FDA as a safety measure to be used following testing with a growth-based, quality control (QC) test for platelet components that is cleared by the FDA.
For this indication, the Platelet PGD Test can be used within 24 hours of transfusion on:
- Leukocyte-reduced apheresis platelets suspended in plasma
- Leukocyte-reduced apheresis platelets suspended in platelet additive solution C and plasma
- Pre-storage pools of up to 6 leukocyte-reduced WBD platelets suspended in plasma.
When used as a safety measure, the Platelet PGD Test can extend the dating of apheresis platelets in plasma from 5 to 7 days.
The Platelet PGD Test also has FDA clearance as a QC test for use on pools of up to 6 units of leukocyte-reduced and non-leukocyte-reduced WBD platelets suspended in plasma that are pooled within 4 hours of transfusion.
The latest FDA clearance extends this use to individual units of WBD platelets in plasma.
“[The new clearance] has been requested by current users of PGD as well as being outlined as a need in pending FDA Draft Guidance to address the risk of bacterial contamination in platelets,” said Jim Lousararian, chief executive officer of Verax Biomedical.
According to the company, the new clearance is intended to help reduce the risk of bacterial contamination for pediatric patients receiving platelet transfusions.
“Pediatric patients pose unique challenges in transfusion medicine,” said Paul Mintz, MD, chief medical officer of Verax Biomedical.
“They require small platelet doses and possess fragile immune systems. PGD testing individual WBD units for transfusion makes it practical to provide bacterially tested platelets to this most vulnerable group of patients.”
Multicenter study
The Verax PGD® test was evaluated in a 2-year study including 18 US hospitals. The results were published in Transfusion in 2011.
The objective of the study was to evaluate the test’s ability to detect bacterially contaminated units in the US apheresis inventory that tested negative for contamination by existing growth-based QC tests.
A total of 9 contaminated units were detected by PGD and confirmed as bacterially contaminated in a population of 27,620 leukocyte-reduced apheresis units (1:3,069 doses tested).
All 9 units had previously tested negative by growth-based QC methods applied earlier in unit life in conformance with all applicable AABB and CAP standards for bacterial testing.
Researchers said the study clearly demonstrated the ability of the Platelet PGD Test to detect and interdict contaminated units missed by current QC testing methods.
FDA authorizes use of first fully automated Zika IgM test
The US Food and Drug Administration (FDA) has granted emergency use authorization (EUA) for DiaSorin Incorporated’s LIAISON® XL Zika Capture IgM assay, the first fully automated serology assay for the detection of Zika virus infection.
The LIAISON® XL Zika Capture IgM assay is intended for the presumptive qualitative detection of Zika virus IgM antibodies in human sera.
The FDA’s decision to grant an EUA means the LIAISON® XL Zika Capture IgM assay can be used to test serum samples collected from individuals meeting criteria for Zika virus testing set forth by the US Centers for Disease Control and Prevention.
This includes clinical criteria—such as a history of clinical signs and symptoms associated with Zika virus infection—and/or epidemiological criteria—such as a history of residence in or travel to a geographic region with active Zika transmission.
Specimens used with the LIAISON® XL Zika Capture IgM assay should be collected between 8 days and 10 weeks after the onset of symptoms or risk of exposure to Zika.
The assay is intended for use in US laboratories that are certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, to perform high-complexity tests, or by similarly qualified non-US laboratories, pursuant to section 564 of the Federal Food, Drug, and Cosmetic Act (21 U.S.C. § 360bbb-3).
Where there are presumptive Zika IgM positive and presumptive recent Zika positive results from the LIAISON® XL Zika Capture IgM assay, confirmation of the presence of anti-Zika IgM antibodies requires additional testing and/or consideration alongside test results for other patient-matched specimens using the latest CDC testing algorithms for the diagnosis of Zika virus infection.
More information on the LIAISON® XL Zika Capture IgM assay and other Zika assays granted EUAs can be found on the FDA’s EUA page.
Funding for the LIAISON® XL Zika Capture IgM assay was provided by the US Department of Health and Human Services, which granted DiaSorin a $2.6 million contract in the fall of 2016.
About the EUA
The EUA does not mean the LIAISON® XL Zika Capture IgM assay is FDA cleared or approved.
An EUA allows for the use of unapproved medical products or unapproved uses of approved medical products in an emergency.
The products must be used to diagnose, treat, or prevent serious or life-threatening conditions caused by chemical, biological, radiological, or nuclear threat agents, when there are no adequate alternatives.
This means the LIAISON® XL Zika Capture IgM assay is only authorized as long as circumstances exist to justify the emergency use of in vitro diagnostics for the detection of Zika virus, unless the authorization is terminated or revoked sooner.
The US Food and Drug Administration (FDA) has granted emergency use authorization (EUA) for DiaSorin Incorporated’s LIAISON® XL Zika Capture IgM assay, the first fully automated serology assay for the detection of Zika virus infection.
The LIAISON® XL Zika Capture IgM assay is intended for the presumptive qualitative detection of Zika virus IgM antibodies in human sera.
The FDA’s decision to grant an EUA means the LIAISON® XL Zika Capture IgM assay can be used to test serum samples collected from individuals meeting criteria for Zika virus testing set forth by the US Centers for Disease Control and Prevention.
This includes clinical criteria—such as a history of clinical signs and symptoms associated with Zika virus infection—and/or epidemiological criteria—such as a history of residence in or travel to a geographic region with active Zika transmission.
Specimens used with the LIAISON® XL Zika Capture IgM assay should be collected between 8 days and 10 weeks after the onset of symptoms or risk of exposure to Zika.
The assay is intended for use in US laboratories that are certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, to perform high-complexity tests, or by similarly qualified non-US laboratories, pursuant to section 564 of the Federal Food, Drug, and Cosmetic Act (21 U.S.C. § 360bbb-3).
Where there are presumptive Zika IgM positive and presumptive recent Zika positive results from the LIAISON® XL Zika Capture IgM assay, confirmation of the presence of anti-Zika IgM antibodies requires additional testing and/or consideration alongside test results for other patient-matched specimens using the latest CDC testing algorithms for the diagnosis of Zika virus infection.
More information on the LIAISON® XL Zika Capture IgM assay and other Zika assays granted EUAs can be found on the FDA’s EUA page.
Funding for the LIAISON® XL Zika Capture IgM assay was provided by the US Department of Health and Human Services, which granted DiaSorin a $2.6 million contract in the fall of 2016.
About the EUA
The EUA does not mean the LIAISON® XL Zika Capture IgM assay is FDA cleared or approved.
An EUA allows for the use of unapproved medical products or unapproved uses of approved medical products in an emergency.
The products must be used to diagnose, treat, or prevent serious or life-threatening conditions caused by chemical, biological, radiological, or nuclear threat agents, when there are no adequate alternatives.
This means the LIAISON® XL Zika Capture IgM assay is only authorized as long as circumstances exist to justify the emergency use of in vitro diagnostics for the detection of Zika virus, unless the authorization is terminated or revoked sooner.
The US Food and Drug Administration (FDA) has granted emergency use authorization (EUA) for DiaSorin Incorporated’s LIAISON® XL Zika Capture IgM assay, the first fully automated serology assay for the detection of Zika virus infection.
The LIAISON® XL Zika Capture IgM assay is intended for the presumptive qualitative detection of Zika virus IgM antibodies in human sera.
The FDA’s decision to grant an EUA means the LIAISON® XL Zika Capture IgM assay can be used to test serum samples collected from individuals meeting criteria for Zika virus testing set forth by the US Centers for Disease Control and Prevention.
This includes clinical criteria—such as a history of clinical signs and symptoms associated with Zika virus infection—and/or epidemiological criteria—such as a history of residence in or travel to a geographic region with active Zika transmission.
Specimens used with the LIAISON® XL Zika Capture IgM assay should be collected between 8 days and 10 weeks after the onset of symptoms or risk of exposure to Zika.
The assay is intended for use in US laboratories that are certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, to perform high-complexity tests, or by similarly qualified non-US laboratories, pursuant to section 564 of the Federal Food, Drug, and Cosmetic Act (21 U.S.C. § 360bbb-3).
Where there are presumptive Zika IgM positive and presumptive recent Zika positive results from the LIAISON® XL Zika Capture IgM assay, confirmation of the presence of anti-Zika IgM antibodies requires additional testing and/or consideration alongside test results for other patient-matched specimens using the latest CDC testing algorithms for the diagnosis of Zika virus infection.
More information on the LIAISON® XL Zika Capture IgM assay and other Zika assays granted EUAs can be found on the FDA’s EUA page.
Funding for the LIAISON® XL Zika Capture IgM assay was provided by the US Department of Health and Human Services, which granted DiaSorin a $2.6 million contract in the fall of 2016.
About the EUA
The EUA does not mean the LIAISON® XL Zika Capture IgM assay is FDA cleared or approved.
An EUA allows for the use of unapproved medical products or unapproved uses of approved medical products in an emergency.
The products must be used to diagnose, treat, or prevent serious or life-threatening conditions caused by chemical, biological, radiological, or nuclear threat agents, when there are no adequate alternatives.
This means the LIAISON® XL Zika Capture IgM assay is only authorized as long as circumstances exist to justify the emergency use of in vitro diagnostics for the detection of Zika virus, unless the authorization is terminated or revoked sooner.
FDA issues EUA for test to detect Zika virus RNA
The US Food and Drug Administration (FDA) has issued an emergency use authorization (EUA) for Nanobiosym Diagnostics Inc.’s Gene-RADAR® Zika Virus Test.
The Gene-RADAR® Zika Virus Test is authorized for the qualitative detection of RNA from Zika virus in human serum.
The test should be used on serum samples collected from individuals meeting the US Centers for Disease Control and Prevention’s (CDC) criteria for Zika virus testing.
This includes clinical criteria—such as a history of clinical signs and symptoms associated with Zika virus infection—and/or epidemiological criteria—such as a history of residence in or travel to a geographic region with active Zika transmission.
The Gene-RADAR® Zika Virus Test is intended for use in US laboratories that are certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, to perform high-complexity tests, or by similarly qualified non-US laboratories, pursuant to section 564 of the Federal Food, Drug, and Cosmetic Act (21 U.S.C. § 360bbb-3).
The Gene-RADAR® Zika Virus Test should be performed according to the CDC’s algorithm for Zika testing (see http://www.cdc.gov/zika/laboratories/lab-guidance.html).
According to the CDC, Zika virus RNA has been detected in serum up to 13 days post-symptom onset in non-pregnant patients, up to 62 days post-symptom onset in pregnant patients, and up to 53 days after the last known possible exposure in an asymptomatic pregnant woman.
About the EUA
The EUA does not mean the Gene-RADAR® Zika Virus Test is FDA cleared or approved.
An EUA allows for the use of unapproved medical products or unapproved uses of approved medical products in an emergency.
The products must be used to diagnose, treat, or prevent serious or life-threatening conditions caused by chemical, biological, radiological, or nuclear threat agents, when there are no adequate alternatives.
The EUA for the Gene-RADAR® Zika Virus Test means the test is only authorized as long as circumstances exist to justify the emergency use of in vitro diagnostics for the detection of Zika virus, unless the authorization is terminated or revoked sooner.
More information on the Gene-RADAR® Zika Virus Test and other Zika tests granted EUAs can be found on the FDA’s EUA page.
The US Food and Drug Administration (FDA) has issued an emergency use authorization (EUA) for Nanobiosym Diagnostics Inc.’s Gene-RADAR® Zika Virus Test.
The Gene-RADAR® Zika Virus Test is authorized for the qualitative detection of RNA from Zika virus in human serum.
The test should be used on serum samples collected from individuals meeting the US Centers for Disease Control and Prevention’s (CDC) criteria for Zika virus testing.
This includes clinical criteria—such as a history of clinical signs and symptoms associated with Zika virus infection—and/or epidemiological criteria—such as a history of residence in or travel to a geographic region with active Zika transmission.
The Gene-RADAR® Zika Virus Test is intended for use in US laboratories that are certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, to perform high-complexity tests, or by similarly qualified non-US laboratories, pursuant to section 564 of the Federal Food, Drug, and Cosmetic Act (21 U.S.C. § 360bbb-3).
The Gene-RADAR® Zika Virus Test should be performed according to the CDC’s algorithm for Zika testing (see http://www.cdc.gov/zika/laboratories/lab-guidance.html).
According to the CDC, Zika virus RNA has been detected in serum up to 13 days post-symptom onset in non-pregnant patients, up to 62 days post-symptom onset in pregnant patients, and up to 53 days after the last known possible exposure in an asymptomatic pregnant woman.
About the EUA
The EUA does not mean the Gene-RADAR® Zika Virus Test is FDA cleared or approved.
An EUA allows for the use of unapproved medical products or unapproved uses of approved medical products in an emergency.
The products must be used to diagnose, treat, or prevent serious or life-threatening conditions caused by chemical, biological, radiological, or nuclear threat agents, when there are no adequate alternatives.
The EUA for the Gene-RADAR® Zika Virus Test means the test is only authorized as long as circumstances exist to justify the emergency use of in vitro diagnostics for the detection of Zika virus, unless the authorization is terminated or revoked sooner.
More information on the Gene-RADAR® Zika Virus Test and other Zika tests granted EUAs can be found on the FDA’s EUA page.
The US Food and Drug Administration (FDA) has issued an emergency use authorization (EUA) for Nanobiosym Diagnostics Inc.’s Gene-RADAR® Zika Virus Test.
The Gene-RADAR® Zika Virus Test is authorized for the qualitative detection of RNA from Zika virus in human serum.
The test should be used on serum samples collected from individuals meeting the US Centers for Disease Control and Prevention’s (CDC) criteria for Zika virus testing.
This includes clinical criteria—such as a history of clinical signs and symptoms associated with Zika virus infection—and/or epidemiological criteria—such as a history of residence in or travel to a geographic region with active Zika transmission.
The Gene-RADAR® Zika Virus Test is intended for use in US laboratories that are certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, to perform high-complexity tests, or by similarly qualified non-US laboratories, pursuant to section 564 of the Federal Food, Drug, and Cosmetic Act (21 U.S.C. § 360bbb-3).
The Gene-RADAR® Zika Virus Test should be performed according to the CDC’s algorithm for Zika testing (see http://www.cdc.gov/zika/laboratories/lab-guidance.html).
According to the CDC, Zika virus RNA has been detected in serum up to 13 days post-symptom onset in non-pregnant patients, up to 62 days post-symptom onset in pregnant patients, and up to 53 days after the last known possible exposure in an asymptomatic pregnant woman.
About the EUA
The EUA does not mean the Gene-RADAR® Zika Virus Test is FDA cleared or approved.
An EUA allows for the use of unapproved medical products or unapproved uses of approved medical products in an emergency.
The products must be used to diagnose, treat, or prevent serious or life-threatening conditions caused by chemical, biological, radiological, or nuclear threat agents, when there are no adequate alternatives.
The EUA for the Gene-RADAR® Zika Virus Test means the test is only authorized as long as circumstances exist to justify the emergency use of in vitro diagnostics for the detection of Zika virus, unless the authorization is terminated or revoked sooner.
More information on the Gene-RADAR® Zika Virus Test and other Zika tests granted EUAs can be found on the FDA’s EUA page.
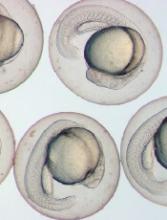
Cells are key to specification of HSCs, team says
Experiments in zebrafish embryos have suggested that trunk neural crest cells play a key role in the specification of hematopoietic stem cells (HSCs).
Researchers believe this finding could be used to aid the creation of HSCs in the lab and ultimately help improve access to HSC transplant.
“The research will likely open new avenues of investigation in stem cell biology and blood development and provide insight to aid efforts to make transplantable hematopoietic stem cells in the lab,” said Wilson Clements, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Dr Clements and Erich W. Damm, PhD, also of St. Jude, described this research in Nature Cell Biology.
The researchers noted that scientists have yet to achieve in vitro specification of normal HSCs with a high level of engraftment and normal multilineage potential. And this suggests key specification signals remain unknown.
The pair pointed out that, in all vertebrates, HSCs arise from hemogenic endothelium in the ventral floor of the dorsal aorta. And stromal cells residing in stem cell microenvironments often act as a niche to contribute cues that regulate behavior.
“Researchers have speculated that the endothelial cells that give rise to blood-forming stem cells are surrounded by a support niche of other cells whose identity and origins were unknown,” Dr Damm said. “Our results support the existence of a niche and identify trunk neural crest cells as an occupant.”
Trunk neural crest cells are made in the developing spinal cord and migrate throughout the embryo. The cells eventually give rise to a variety of adult cells, including neurons and glial cells in the sympathetic and parasympathetic nervous system.
Using time-lapse video, Drs Clements and Damm tracked the migration of neural crest cells in the transparent embryos of zebrafish. (Zebrafish and humans share nearly identical blood systems.)
After about 20 hours, the neural crest cells had reached the developing aorta. After hour 24, the migrating cells had cozied up to the endothelial cells in the aorta, which then turned on genes, such as runx1, indicating their conversion to HSCs.
Additional experiments revealed that migration of neural crest cells to the dorsal aorta is dependent on platelet-derived growth factor signaling, and this signaling is required for HSC specification.
Likewise, the physical association of neural crest cells with the pre-hematopoietic dorsal aorta is required for initiation of the hematopoietic program and HSC specification.
Drs Clements and Damm said these results suggest neural crest cells are key cellular components of the HSC specification niche that can be profiled to identify unknown HSC specification signals.
Experiments in zebrafish embryos have suggested that trunk neural crest cells play a key role in the specification of hematopoietic stem cells (HSCs).
Researchers believe this finding could be used to aid the creation of HSCs in the lab and ultimately help improve access to HSC transplant.
“The research will likely open new avenues of investigation in stem cell biology and blood development and provide insight to aid efforts to make transplantable hematopoietic stem cells in the lab,” said Wilson Clements, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Dr Clements and Erich W. Damm, PhD, also of St. Jude, described this research in Nature Cell Biology.
The researchers noted that scientists have yet to achieve in vitro specification of normal HSCs with a high level of engraftment and normal multilineage potential. And this suggests key specification signals remain unknown.
The pair pointed out that, in all vertebrates, HSCs arise from hemogenic endothelium in the ventral floor of the dorsal aorta. And stromal cells residing in stem cell microenvironments often act as a niche to contribute cues that regulate behavior.
“Researchers have speculated that the endothelial cells that give rise to blood-forming stem cells are surrounded by a support niche of other cells whose identity and origins were unknown,” Dr Damm said. “Our results support the existence of a niche and identify trunk neural crest cells as an occupant.”
Trunk neural crest cells are made in the developing spinal cord and migrate throughout the embryo. The cells eventually give rise to a variety of adult cells, including neurons and glial cells in the sympathetic and parasympathetic nervous system.
Using time-lapse video, Drs Clements and Damm tracked the migration of neural crest cells in the transparent embryos of zebrafish. (Zebrafish and humans share nearly identical blood systems.)
After about 20 hours, the neural crest cells had reached the developing aorta. After hour 24, the migrating cells had cozied up to the endothelial cells in the aorta, which then turned on genes, such as runx1, indicating their conversion to HSCs.
Additional experiments revealed that migration of neural crest cells to the dorsal aorta is dependent on platelet-derived growth factor signaling, and this signaling is required for HSC specification.
Likewise, the physical association of neural crest cells with the pre-hematopoietic dorsal aorta is required for initiation of the hematopoietic program and HSC specification.
Drs Clements and Damm said these results suggest neural crest cells are key cellular components of the HSC specification niche that can be profiled to identify unknown HSC specification signals.
Experiments in zebrafish embryos have suggested that trunk neural crest cells play a key role in the specification of hematopoietic stem cells (HSCs).
Researchers believe this finding could be used to aid the creation of HSCs in the lab and ultimately help improve access to HSC transplant.
“The research will likely open new avenues of investigation in stem cell biology and blood development and provide insight to aid efforts to make transplantable hematopoietic stem cells in the lab,” said Wilson Clements, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Dr Clements and Erich W. Damm, PhD, also of St. Jude, described this research in Nature Cell Biology.
The researchers noted that scientists have yet to achieve in vitro specification of normal HSCs with a high level of engraftment and normal multilineage potential. And this suggests key specification signals remain unknown.
The pair pointed out that, in all vertebrates, HSCs arise from hemogenic endothelium in the ventral floor of the dorsal aorta. And stromal cells residing in stem cell microenvironments often act as a niche to contribute cues that regulate behavior.
“Researchers have speculated that the endothelial cells that give rise to blood-forming stem cells are surrounded by a support niche of other cells whose identity and origins were unknown,” Dr Damm said. “Our results support the existence of a niche and identify trunk neural crest cells as an occupant.”
Trunk neural crest cells are made in the developing spinal cord and migrate throughout the embryo. The cells eventually give rise to a variety of adult cells, including neurons and glial cells in the sympathetic and parasympathetic nervous system.
Using time-lapse video, Drs Clements and Damm tracked the migration of neural crest cells in the transparent embryos of zebrafish. (Zebrafish and humans share nearly identical blood systems.)
After about 20 hours, the neural crest cells had reached the developing aorta. After hour 24, the migrating cells had cozied up to the endothelial cells in the aorta, which then turned on genes, such as runx1, indicating their conversion to HSCs.
Additional experiments revealed that migration of neural crest cells to the dorsal aorta is dependent on platelet-derived growth factor signaling, and this signaling is required for HSC specification.
Likewise, the physical association of neural crest cells with the pre-hematopoietic dorsal aorta is required for initiation of the hematopoietic program and HSC specification.
Drs Clements and Damm said these results suggest neural crest cells are key cellular components of the HSC specification niche that can be profiled to identify unknown HSC specification signals.