User login
AYAs struggle socially in early years after cancer diagnosis
A new study indicates that adolescent and young adult (AYA) cancer survivors continue to face social difficulties for more than 2 years after their diagnosis.
The research, published in Cancer, suggests these patients may see some improvement in their social lives during the first year after diagnosis.
However, their social functioning tends to remain constant after that, leaving them socially impaired relative to their cancer-free peers.
Previous studies have shown that AYAs with cancer experience greater challenges in social functioning than their cancer-free peers or even compared to older cancer patients.
But few studies have examined this phenomenon by following the same patients over time.
Olga Husson, PhD, of the Radboud University Medical Center in The Netherlands, and her colleagues set out to examine changes in social functioning among AYAs in the early years after a cancer diagnosis.
The researchers asked AYA cancer patients at 5 US medical institutions to complete a survey about social functioning within 4 months of their diagnosis, 12 months later, and 24 months later.
There were 141 patients (ages 14 to 39 at diagnosis) who completed the surveys.
The researchers found that, when compared to population norms, the cancer patients had inferior social functioning at all the time points studied.
Among the cancer patients, the mean social functioning score from the Medical Outcomes Study Short Form 36 Health Survey (version 2) was 52.0 around the time of cancer diagnosis, 73.1 at the 12-month follow-up, and 69.2 at the 24-month follow-up. In comparison, the population norm (for people ages 18 to 44) is 85.1 (P<0.001 for all time points).
The researchers did note that cancer patients experienced significant improvements in social functioning from baseline to the 12-month follow-up, but there was no further improvement after that.
The researchers also examined the different trajectories of social functioning over time. They found that social functioning improved over time for 47% of the cancer patients but worsened for 13%. In addition, 32% of patients had consistently low social functioning, and 9% had consistently high social functioning.
The cancer patients with consistently low social functioning were more likely to be off treatment at the time of follow-up, report more physical symptoms and higher levels of psychological distress (at both baseline and follow-up), and perceive themselves to receive less social support.
“Reducing physical symptoms and psychological distress and enhancing social support by interventions in the period after treatment may potentially help these young survivors to better reintegrate into society,” Dr Husson said. ![]()
A new study indicates that adolescent and young adult (AYA) cancer survivors continue to face social difficulties for more than 2 years after their diagnosis.
The research, published in Cancer, suggests these patients may see some improvement in their social lives during the first year after diagnosis.
However, their social functioning tends to remain constant after that, leaving them socially impaired relative to their cancer-free peers.
Previous studies have shown that AYAs with cancer experience greater challenges in social functioning than their cancer-free peers or even compared to older cancer patients.
But few studies have examined this phenomenon by following the same patients over time.
Olga Husson, PhD, of the Radboud University Medical Center in The Netherlands, and her colleagues set out to examine changes in social functioning among AYAs in the early years after a cancer diagnosis.
The researchers asked AYA cancer patients at 5 US medical institutions to complete a survey about social functioning within 4 months of their diagnosis, 12 months later, and 24 months later.
There were 141 patients (ages 14 to 39 at diagnosis) who completed the surveys.
The researchers found that, when compared to population norms, the cancer patients had inferior social functioning at all the time points studied.
Among the cancer patients, the mean social functioning score from the Medical Outcomes Study Short Form 36 Health Survey (version 2) was 52.0 around the time of cancer diagnosis, 73.1 at the 12-month follow-up, and 69.2 at the 24-month follow-up. In comparison, the population norm (for people ages 18 to 44) is 85.1 (P<0.001 for all time points).
The researchers did note that cancer patients experienced significant improvements in social functioning from baseline to the 12-month follow-up, but there was no further improvement after that.
The researchers also examined the different trajectories of social functioning over time. They found that social functioning improved over time for 47% of the cancer patients but worsened for 13%. In addition, 32% of patients had consistently low social functioning, and 9% had consistently high social functioning.
The cancer patients with consistently low social functioning were more likely to be off treatment at the time of follow-up, report more physical symptoms and higher levels of psychological distress (at both baseline and follow-up), and perceive themselves to receive less social support.
“Reducing physical symptoms and psychological distress and enhancing social support by interventions in the period after treatment may potentially help these young survivors to better reintegrate into society,” Dr Husson said. ![]()
A new study indicates that adolescent and young adult (AYA) cancer survivors continue to face social difficulties for more than 2 years after their diagnosis.
The research, published in Cancer, suggests these patients may see some improvement in their social lives during the first year after diagnosis.
However, their social functioning tends to remain constant after that, leaving them socially impaired relative to their cancer-free peers.
Previous studies have shown that AYAs with cancer experience greater challenges in social functioning than their cancer-free peers or even compared to older cancer patients.
But few studies have examined this phenomenon by following the same patients over time.
Olga Husson, PhD, of the Radboud University Medical Center in The Netherlands, and her colleagues set out to examine changes in social functioning among AYAs in the early years after a cancer diagnosis.
The researchers asked AYA cancer patients at 5 US medical institutions to complete a survey about social functioning within 4 months of their diagnosis, 12 months later, and 24 months later.
There were 141 patients (ages 14 to 39 at diagnosis) who completed the surveys.
The researchers found that, when compared to population norms, the cancer patients had inferior social functioning at all the time points studied.
Among the cancer patients, the mean social functioning score from the Medical Outcomes Study Short Form 36 Health Survey (version 2) was 52.0 around the time of cancer diagnosis, 73.1 at the 12-month follow-up, and 69.2 at the 24-month follow-up. In comparison, the population norm (for people ages 18 to 44) is 85.1 (P<0.001 for all time points).
The researchers did note that cancer patients experienced significant improvements in social functioning from baseline to the 12-month follow-up, but there was no further improvement after that.
The researchers also examined the different trajectories of social functioning over time. They found that social functioning improved over time for 47% of the cancer patients but worsened for 13%. In addition, 32% of patients had consistently low social functioning, and 9% had consistently high social functioning.
The cancer patients with consistently low social functioning were more likely to be off treatment at the time of follow-up, report more physical symptoms and higher levels of psychological distress (at both baseline and follow-up), and perceive themselves to receive less social support.
“Reducing physical symptoms and psychological distress and enhancing social support by interventions in the period after treatment may potentially help these young survivors to better reintegrate into society,” Dr Husson said. ![]()
Study supports use of rivaroxaban to prevent VTE recurrence
WASHINGTON, DC—Results of the EINSTEIN CHOICE study suggest rivaroxaban is more effective than, and just as safe as, aspirin for long-term anticoagulation in patients with venous thromboembolism (VTE).
In this phase 3 study, patients who had completed 6 to 12 months of anticoagulant therapy were randomized to receive rivaroxaban or aspirin.
Those who received rivaroxaban had a significantly lower risk of recurrent VTE, and the rates of major bleeding were similar between the treatment arms.
“How best to extend anticoagulant use beyond the initial treatment window has been a constant source of debate, with physicians carefully balancing patients’ risk of another VTE with the risk of anticoagulant-related bleeding,” said study investigator Philip S. Wells, MD, of The Ottawa Hospital in Ontario, Canada.
“With EINSTEIN CHOICE, for the first time, we have clinical evidence confirming rivaroxaban is superior to aspirin in reducing recurrent VTE, with no significant impact on safety. These important results have the potential to trigger a paradigm shift in how physicians manage their patients and protect them from VTE recurrence over the long term.”
These results were presented at the American College of Cardiology’s 66th Annual Scientific Session and published in NEJM. The research was funded by Bayer Pharmaceuticals.
The study enrolled 3365 patients with confirmed deep vein thrombosis or pulmonary embolism who were initially treated with anticoagulant therapy for 6 to 12 months.
Patients who required extended anticoagulation at therapeutic doses were not included in this trial, as the objective was to investigate those patients for whom the treating physician was uncertain about the need for continuing anticoagulant therapy.
Patients were randomized in a 1:1:1 ratio to receive a prophylactic dose of rivaroxaban (10 mg once daily), a treatment dose of rivaroxaban (20 mg once daily), or aspirin (100 mg once daily) for up to 12 months. Sixty percent of patients completed the full 12 months of treatment.
Efficacy
Both rivaroxaban doses were superior to aspirin in preventing fatal or non-fatal recurrent VTE, the study’s primary efficacy endpoint.
The rate of recurrent VTE was 1.2% in the 10 mg rivaroxaban arm (hazard ratio [HR]=0.26; 95% CI, 0.14 to 0.47; P<0.001), 1.5% in the 20 mg rivaroxaban arm (HR=0.34; 95% CI, 0.20 to 0.59; P<0.001), and 4.4% in the aspirin arm. Fatal VTE occurred in 0%, 0.2%, and 0.2%, respectively.
For the primary endpoint, the HR for the comparison between the 20 mg and 10 mg rivaroxaban arms was 1.34 (95% CI, 0.65 to 2.75, P=0.42). However, the researchers noted that the comparison of these 2 arms was not powered for significance.
The researchers also found that rivaroxaban reduced patients’ risk of experiencing one of the following events: recurrent VTE, heart attack, ischemic stroke, systemic embolism, or venous thrombosis in another location.
This endpoint occurred in 1.9% of patients in the 10 mg rivaroxaban group (HR=0.33; 95% CI, 0.20 to 0.54; P<0.001), 2.0% of patients in the 20 mg rivaroxaban group (HR=0.35; 95% CI, 0.22 to 0.57; P<0.001), and 5.6% of patients in the aspirin group.
Recurrent VTE or all-cause mortality occurred in 1.3% of patients in the 10 mg rivaroxaban group (HR=0.27; 95% CI, 0.15 to 0.47; P<0.001), 2.1% of patients in the 20 mg rivaroxaban group (HR=0.42; 95% CI, 0.26 to 0.68; P<0.001), and 4.9% of patients in the aspirin group.
Safety
The primary safety endpoint was major bleeding as defined by the International Society on Thrombosis and Haemostasis.
The rate of major bleeding was 0.4% for the 10 mg rivaroxaban group (HR=1.64; 95% CI, 0.39 to 6.84; P=0.50), 0.5% for the 20 mg rivaroxaban group (HR=2.01; 95% CI, 0.50 to 8.04; P=0.32), and 0.3% for the aspirin group.
Rates of clinically relevant non-major bleeding were 2.0%, 2.7%, and 1.8%, respectively (no significant difference).
“We know from previous studies that only about 40% of venous thromboembolism patients are actually on long-term blood thinners,” Dr Wells said.
“We hope that this study, which shows the blood thinner rivaroxaban is as safe as aspirin but much more effective at preventing future clots, will convince patients and their physicians to continue life-long medication that can prevent potentially dangerous blood clots.” ![]()
WASHINGTON, DC—Results of the EINSTEIN CHOICE study suggest rivaroxaban is more effective than, and just as safe as, aspirin for long-term anticoagulation in patients with venous thromboembolism (VTE).
In this phase 3 study, patients who had completed 6 to 12 months of anticoagulant therapy were randomized to receive rivaroxaban or aspirin.
Those who received rivaroxaban had a significantly lower risk of recurrent VTE, and the rates of major bleeding were similar between the treatment arms.
“How best to extend anticoagulant use beyond the initial treatment window has been a constant source of debate, with physicians carefully balancing patients’ risk of another VTE with the risk of anticoagulant-related bleeding,” said study investigator Philip S. Wells, MD, of The Ottawa Hospital in Ontario, Canada.
“With EINSTEIN CHOICE, for the first time, we have clinical evidence confirming rivaroxaban is superior to aspirin in reducing recurrent VTE, with no significant impact on safety. These important results have the potential to trigger a paradigm shift in how physicians manage their patients and protect them from VTE recurrence over the long term.”
These results were presented at the American College of Cardiology’s 66th Annual Scientific Session and published in NEJM. The research was funded by Bayer Pharmaceuticals.
The study enrolled 3365 patients with confirmed deep vein thrombosis or pulmonary embolism who were initially treated with anticoagulant therapy for 6 to 12 months.
Patients who required extended anticoagulation at therapeutic doses were not included in this trial, as the objective was to investigate those patients for whom the treating physician was uncertain about the need for continuing anticoagulant therapy.
Patients were randomized in a 1:1:1 ratio to receive a prophylactic dose of rivaroxaban (10 mg once daily), a treatment dose of rivaroxaban (20 mg once daily), or aspirin (100 mg once daily) for up to 12 months. Sixty percent of patients completed the full 12 months of treatment.
Efficacy
Both rivaroxaban doses were superior to aspirin in preventing fatal or non-fatal recurrent VTE, the study’s primary efficacy endpoint.
The rate of recurrent VTE was 1.2% in the 10 mg rivaroxaban arm (hazard ratio [HR]=0.26; 95% CI, 0.14 to 0.47; P<0.001), 1.5% in the 20 mg rivaroxaban arm (HR=0.34; 95% CI, 0.20 to 0.59; P<0.001), and 4.4% in the aspirin arm. Fatal VTE occurred in 0%, 0.2%, and 0.2%, respectively.
For the primary endpoint, the HR for the comparison between the 20 mg and 10 mg rivaroxaban arms was 1.34 (95% CI, 0.65 to 2.75, P=0.42). However, the researchers noted that the comparison of these 2 arms was not powered for significance.
The researchers also found that rivaroxaban reduced patients’ risk of experiencing one of the following events: recurrent VTE, heart attack, ischemic stroke, systemic embolism, or venous thrombosis in another location.
This endpoint occurred in 1.9% of patients in the 10 mg rivaroxaban group (HR=0.33; 95% CI, 0.20 to 0.54; P<0.001), 2.0% of patients in the 20 mg rivaroxaban group (HR=0.35; 95% CI, 0.22 to 0.57; P<0.001), and 5.6% of patients in the aspirin group.
Recurrent VTE or all-cause mortality occurred in 1.3% of patients in the 10 mg rivaroxaban group (HR=0.27; 95% CI, 0.15 to 0.47; P<0.001), 2.1% of patients in the 20 mg rivaroxaban group (HR=0.42; 95% CI, 0.26 to 0.68; P<0.001), and 4.9% of patients in the aspirin group.
Safety
The primary safety endpoint was major bleeding as defined by the International Society on Thrombosis and Haemostasis.
The rate of major bleeding was 0.4% for the 10 mg rivaroxaban group (HR=1.64; 95% CI, 0.39 to 6.84; P=0.50), 0.5% for the 20 mg rivaroxaban group (HR=2.01; 95% CI, 0.50 to 8.04; P=0.32), and 0.3% for the aspirin group.
Rates of clinically relevant non-major bleeding were 2.0%, 2.7%, and 1.8%, respectively (no significant difference).
“We know from previous studies that only about 40% of venous thromboembolism patients are actually on long-term blood thinners,” Dr Wells said.
“We hope that this study, which shows the blood thinner rivaroxaban is as safe as aspirin but much more effective at preventing future clots, will convince patients and their physicians to continue life-long medication that can prevent potentially dangerous blood clots.” ![]()
WASHINGTON, DC—Results of the EINSTEIN CHOICE study suggest rivaroxaban is more effective than, and just as safe as, aspirin for long-term anticoagulation in patients with venous thromboembolism (VTE).
In this phase 3 study, patients who had completed 6 to 12 months of anticoagulant therapy were randomized to receive rivaroxaban or aspirin.
Those who received rivaroxaban had a significantly lower risk of recurrent VTE, and the rates of major bleeding were similar between the treatment arms.
“How best to extend anticoagulant use beyond the initial treatment window has been a constant source of debate, with physicians carefully balancing patients’ risk of another VTE with the risk of anticoagulant-related bleeding,” said study investigator Philip S. Wells, MD, of The Ottawa Hospital in Ontario, Canada.
“With EINSTEIN CHOICE, for the first time, we have clinical evidence confirming rivaroxaban is superior to aspirin in reducing recurrent VTE, with no significant impact on safety. These important results have the potential to trigger a paradigm shift in how physicians manage their patients and protect them from VTE recurrence over the long term.”
These results were presented at the American College of Cardiology’s 66th Annual Scientific Session and published in NEJM. The research was funded by Bayer Pharmaceuticals.
The study enrolled 3365 patients with confirmed deep vein thrombosis or pulmonary embolism who were initially treated with anticoagulant therapy for 6 to 12 months.
Patients who required extended anticoagulation at therapeutic doses were not included in this trial, as the objective was to investigate those patients for whom the treating physician was uncertain about the need for continuing anticoagulant therapy.
Patients were randomized in a 1:1:1 ratio to receive a prophylactic dose of rivaroxaban (10 mg once daily), a treatment dose of rivaroxaban (20 mg once daily), or aspirin (100 mg once daily) for up to 12 months. Sixty percent of patients completed the full 12 months of treatment.
Efficacy
Both rivaroxaban doses were superior to aspirin in preventing fatal or non-fatal recurrent VTE, the study’s primary efficacy endpoint.
The rate of recurrent VTE was 1.2% in the 10 mg rivaroxaban arm (hazard ratio [HR]=0.26; 95% CI, 0.14 to 0.47; P<0.001), 1.5% in the 20 mg rivaroxaban arm (HR=0.34; 95% CI, 0.20 to 0.59; P<0.001), and 4.4% in the aspirin arm. Fatal VTE occurred in 0%, 0.2%, and 0.2%, respectively.
For the primary endpoint, the HR for the comparison between the 20 mg and 10 mg rivaroxaban arms was 1.34 (95% CI, 0.65 to 2.75, P=0.42). However, the researchers noted that the comparison of these 2 arms was not powered for significance.
The researchers also found that rivaroxaban reduced patients’ risk of experiencing one of the following events: recurrent VTE, heart attack, ischemic stroke, systemic embolism, or venous thrombosis in another location.
This endpoint occurred in 1.9% of patients in the 10 mg rivaroxaban group (HR=0.33; 95% CI, 0.20 to 0.54; P<0.001), 2.0% of patients in the 20 mg rivaroxaban group (HR=0.35; 95% CI, 0.22 to 0.57; P<0.001), and 5.6% of patients in the aspirin group.
Recurrent VTE or all-cause mortality occurred in 1.3% of patients in the 10 mg rivaroxaban group (HR=0.27; 95% CI, 0.15 to 0.47; P<0.001), 2.1% of patients in the 20 mg rivaroxaban group (HR=0.42; 95% CI, 0.26 to 0.68; P<0.001), and 4.9% of patients in the aspirin group.
Safety
The primary safety endpoint was major bleeding as defined by the International Society on Thrombosis and Haemostasis.
The rate of major bleeding was 0.4% for the 10 mg rivaroxaban group (HR=1.64; 95% CI, 0.39 to 6.84; P=0.50), 0.5% for the 20 mg rivaroxaban group (HR=2.01; 95% CI, 0.50 to 8.04; P=0.32), and 0.3% for the aspirin group.
Rates of clinically relevant non-major bleeding were 2.0%, 2.7%, and 1.8%, respectively (no significant difference).
“We know from previous studies that only about 40% of venous thromboembolism patients are actually on long-term blood thinners,” Dr Wells said.
“We hope that this study, which shows the blood thinner rivaroxaban is as safe as aspirin but much more effective at preventing future clots, will convince patients and their physicians to continue life-long medication that can prevent potentially dangerous blood clots.” ![]()
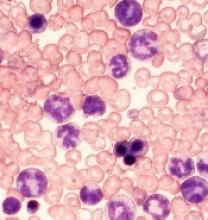
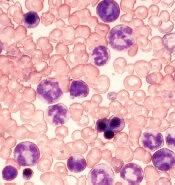
Proteins may be therapeutic targets for TKI-resistant CML, ALL
Researchers say they have identified 2 signaling proteins that enable resistance to tyrosine kinase inhibitors (TKIs) and could be therapeutic targets for acute lymphoblastic leukemia (ALL) and chronic myeloid leukemia (CML).
The team found that by deleting these proteins—c-Fos and Dusp1—they could eradicate BCR-ABL-induced B-cell ALL in mice.
And treatment combining c-Fos and Dusp1 inhibitors with the TKI imatinib was able to cure mice with BCR-ABL-driven CML.
The researchers reported these findings in Nature Medicine.
“We think that, within the next 5 years, our data will change the way people think about cancer development and targeted therapy,” said study author Mohammad Azam, PhD, of Cincinnati Children’s Hospital Medical Center in Ohio.
“This study identifies a potential Achilles’ heel of kinase-driven cancers, and what we propose is intended to be curative, not just treatment.”
The potential Achilles’ heel is a common point of passage in cells—a signaling node—that appears to be required to generate cancer cells. The node is formed by the signaling proteins c-Fos and Dusp1, according to the researchers.
The team identified c-Fos and Dusp1 by conducting global gene-expression analysis of mouse leukemia cells and human CML cells. Analysis of the human cells revealed extremely high levels of c-FOS and DUSP1 in BCR-ABL-positive, TKI-resistant cells.
Dr Azam and his colleagues found that signaling from tyrosine kinase and growth factor proteins that support cell expansion (such as IL-3 and IL-6) converge to elevate c-Fos and Dusp1 levels in leukemia cells.
Working together, these molecules maintain the survival of leukemia stem cells (LSCs), which translates to minimal residual disease (MRD) after treatment.
Dr Azam said Dusp1 and c-Fos support the survival of LSCs by increasing the toxic threshold needed to kill them. This means imatinib and other TKIs cannot eliminate the residual LSCs.
After describing the roles of c-Fos and Dusp1, Dr Azam and his colleagues put their ideas to the test in mouse models of CML.
The team tested several treatments in these mice, including:
- monotherapy with imatinib
- inhibitors of c-Fos and Dusp1
- treatment with imatinib and inhibitors of c-Fos and Dusp1.
As suspected, treatment with imatinib alone initially stopped CML progression, but mice ultimately relapsed.
Treatment with c-Fos and Dusp1 inhibitors significantly slowed CML progression and prolonged survival in a majority of mice, but this treatment wasn’t curative.
However, a month of treatment with c-Fos and Dusp1 inhibitors as well as imatinib cured about 90% of mice with CML, and there were no signs of MRD.
The researchers also found that simply deleting c-Fos and Dusp1 was sufficient to block the development of B-cell ALL in mice.
The team said they are following up this study by testing c-Fos and Dusp1 as treatment targets for different kinase-fueled cancers. ![]()
Researchers say they have identified 2 signaling proteins that enable resistance to tyrosine kinase inhibitors (TKIs) and could be therapeutic targets for acute lymphoblastic leukemia (ALL) and chronic myeloid leukemia (CML).
The team found that by deleting these proteins—c-Fos and Dusp1—they could eradicate BCR-ABL-induced B-cell ALL in mice.
And treatment combining c-Fos and Dusp1 inhibitors with the TKI imatinib was able to cure mice with BCR-ABL-driven CML.
The researchers reported these findings in Nature Medicine.
“We think that, within the next 5 years, our data will change the way people think about cancer development and targeted therapy,” said study author Mohammad Azam, PhD, of Cincinnati Children’s Hospital Medical Center in Ohio.
“This study identifies a potential Achilles’ heel of kinase-driven cancers, and what we propose is intended to be curative, not just treatment.”
The potential Achilles’ heel is a common point of passage in cells—a signaling node—that appears to be required to generate cancer cells. The node is formed by the signaling proteins c-Fos and Dusp1, according to the researchers.
The team identified c-Fos and Dusp1 by conducting global gene-expression analysis of mouse leukemia cells and human CML cells. Analysis of the human cells revealed extremely high levels of c-FOS and DUSP1 in BCR-ABL-positive, TKI-resistant cells.
Dr Azam and his colleagues found that signaling from tyrosine kinase and growth factor proteins that support cell expansion (such as IL-3 and IL-6) converge to elevate c-Fos and Dusp1 levels in leukemia cells.
Working together, these molecules maintain the survival of leukemia stem cells (LSCs), which translates to minimal residual disease (MRD) after treatment.
Dr Azam said Dusp1 and c-Fos support the survival of LSCs by increasing the toxic threshold needed to kill them. This means imatinib and other TKIs cannot eliminate the residual LSCs.
After describing the roles of c-Fos and Dusp1, Dr Azam and his colleagues put their ideas to the test in mouse models of CML.
The team tested several treatments in these mice, including:
- monotherapy with imatinib
- inhibitors of c-Fos and Dusp1
- treatment with imatinib and inhibitors of c-Fos and Dusp1.
As suspected, treatment with imatinib alone initially stopped CML progression, but mice ultimately relapsed.
Treatment with c-Fos and Dusp1 inhibitors significantly slowed CML progression and prolonged survival in a majority of mice, but this treatment wasn’t curative.
However, a month of treatment with c-Fos and Dusp1 inhibitors as well as imatinib cured about 90% of mice with CML, and there were no signs of MRD.
The researchers also found that simply deleting c-Fos and Dusp1 was sufficient to block the development of B-cell ALL in mice.
The team said they are following up this study by testing c-Fos and Dusp1 as treatment targets for different kinase-fueled cancers. ![]()
Researchers say they have identified 2 signaling proteins that enable resistance to tyrosine kinase inhibitors (TKIs) and could be therapeutic targets for acute lymphoblastic leukemia (ALL) and chronic myeloid leukemia (CML).
The team found that by deleting these proteins—c-Fos and Dusp1—they could eradicate BCR-ABL-induced B-cell ALL in mice.
And treatment combining c-Fos and Dusp1 inhibitors with the TKI imatinib was able to cure mice with BCR-ABL-driven CML.
The researchers reported these findings in Nature Medicine.
“We think that, within the next 5 years, our data will change the way people think about cancer development and targeted therapy,” said study author Mohammad Azam, PhD, of Cincinnati Children’s Hospital Medical Center in Ohio.
“This study identifies a potential Achilles’ heel of kinase-driven cancers, and what we propose is intended to be curative, not just treatment.”
The potential Achilles’ heel is a common point of passage in cells—a signaling node—that appears to be required to generate cancer cells. The node is formed by the signaling proteins c-Fos and Dusp1, according to the researchers.
The team identified c-Fos and Dusp1 by conducting global gene-expression analysis of mouse leukemia cells and human CML cells. Analysis of the human cells revealed extremely high levels of c-FOS and DUSP1 in BCR-ABL-positive, TKI-resistant cells.
Dr Azam and his colleagues found that signaling from tyrosine kinase and growth factor proteins that support cell expansion (such as IL-3 and IL-6) converge to elevate c-Fos and Dusp1 levels in leukemia cells.
Working together, these molecules maintain the survival of leukemia stem cells (LSCs), which translates to minimal residual disease (MRD) after treatment.
Dr Azam said Dusp1 and c-Fos support the survival of LSCs by increasing the toxic threshold needed to kill them. This means imatinib and other TKIs cannot eliminate the residual LSCs.
After describing the roles of c-Fos and Dusp1, Dr Azam and his colleagues put their ideas to the test in mouse models of CML.
The team tested several treatments in these mice, including:
- monotherapy with imatinib
- inhibitors of c-Fos and Dusp1
- treatment with imatinib and inhibitors of c-Fos and Dusp1.
As suspected, treatment with imatinib alone initially stopped CML progression, but mice ultimately relapsed.
Treatment with c-Fos and Dusp1 inhibitors significantly slowed CML progression and prolonged survival in a majority of mice, but this treatment wasn’t curative.
However, a month of treatment with c-Fos and Dusp1 inhibitors as well as imatinib cured about 90% of mice with CML, and there were no signs of MRD.
The researchers also found that simply deleting c-Fos and Dusp1 was sufficient to block the development of B-cell ALL in mice.
The team said they are following up this study by testing c-Fos and Dusp1 as treatment targets for different kinase-fueled cancers. ![]()
Allo-HSCT cures adult with congenital dyserythropoietic anemia
Physicians have reported what they believe is the first case of an allogeneic hematopoietic stem cell transplant (allo-HSCT) curing an adult with congenital dyserythropoietic anemia (CDA).
The patient, David Levy, was previously transfusion-dependent and suffered from iron overload, severe pain, and other adverse effects of his illness.
Levy was denied a transplant for years, but, in 2014, he received a non-myeloablative allo-HSCT from a matched, unrelated donor.
Now, Levy no longer requires transfusions, iron chelation, or immunosuppression, and says he is able to live a normal life.
Damiano Rondelli, MD, of the University of Illinois at Chicago, and his colleagues described Levy’s case in a letter to Bone Marrow Transplantation.
Levy was diagnosed with CDA at 4 months of age and was treated with regular blood transfusions for most of his life. He was 24 when the pain from his illness became so severe that he had to withdraw from graduate school.
“I spent the following years doing nothing—no work, no school, no social contact—because all I could focus on was managing my pain and getting my health back on track,” Levy said.
By age 32, Levy required transfusions every 2 to 3 weeks, had undergone a splenectomy, had an enlarged liver, and was suffering from fatigue, heart palpitations, and iron overload.
“It was bad,” Levy said. “I had been through enough pain. I was angry and depressed, and I wanted a cure. That’s why I started emailing Dr Rondelli.”
Dr Rondelli said that because of Levy’s range of illnesses and inability to tolerate chemotherapy and radiation, several institutions had denied him the possibility of a transplant.
However, Dr Rondelli and his colleagues had reported success with chemotherapy-free allo-HSCT in patients with sickle cell disease. So Dr Rondelli performed Levy’s transplant in 2014.
Levy received a peripheral blood stem cell transplant from an unrelated donor who was a 10/10 HLA match but ABO incompatible. He received conditioning with rabbit anti-thymocyte globulin, fludarabine, cyclophosphamide, and total body irradiation.
Levy also received graft-vs-host disease (GVHD) prophylaxis consisting of high-dose cyclophosphamide, mycophenolate mofetil, and sirolimus. And he received standard antibacterial, antifungal, antiviral, and anti-Pneumocystis jiroveci prophylaxis.
Levy experienced platelet engraftment on day 20 and neutrophil engraftment on day 21. Whole-blood donor-cell chimerism was 98.7% on day 30 and 100% on day 60 and beyond.
Levy did develop transient hemolytic anemia due to the ABO incompatibility. He was given a total of 10 units of packed red blood cells until day 78.
Levy was tapered off all immunosuppression at 12 months and has shown no signs of acute or chronic GVHD.
At 24 months after HSCT, Levy’s hemoglobin was 13.7 g/dL, and his ferritin was 376 ng/mL. He has had no iron chelation since the transplant.
“The transplant was hard, and I had some complications, but I am back to normal now,” said Levy, who is now 35.
“I still have some pain and some lingering issues from the years my condition was not properly managed, but I can be independent now. That is the most important thing to me.”
Levy is finishing his doctorate in psychology and running group therapy sessions at a behavioral health hospital.
Dr Rondelli said the potential of this treatment approach is promising.
“The use of this transplant protocol may represent a safe therapeutic strategy to treat adult patients with many types of congenital anemias—perhaps the only possible cure,” he said.
“For many adult patients with a blood disorder, treatment options have been limited because they are often not sick enough to qualify for a risky procedure, or they are too sick to tolerate the toxic drugs used alongside a standard transplant. This procedure gives some adults the option of a stem cell transplant, which was not previously available.” ![]()
Physicians have reported what they believe is the first case of an allogeneic hematopoietic stem cell transplant (allo-HSCT) curing an adult with congenital dyserythropoietic anemia (CDA).
The patient, David Levy, was previously transfusion-dependent and suffered from iron overload, severe pain, and other adverse effects of his illness.
Levy was denied a transplant for years, but, in 2014, he received a non-myeloablative allo-HSCT from a matched, unrelated donor.
Now, Levy no longer requires transfusions, iron chelation, or immunosuppression, and says he is able to live a normal life.
Damiano Rondelli, MD, of the University of Illinois at Chicago, and his colleagues described Levy’s case in a letter to Bone Marrow Transplantation.
Levy was diagnosed with CDA at 4 months of age and was treated with regular blood transfusions for most of his life. He was 24 when the pain from his illness became so severe that he had to withdraw from graduate school.
“I spent the following years doing nothing—no work, no school, no social contact—because all I could focus on was managing my pain and getting my health back on track,” Levy said.
By age 32, Levy required transfusions every 2 to 3 weeks, had undergone a splenectomy, had an enlarged liver, and was suffering from fatigue, heart palpitations, and iron overload.
“It was bad,” Levy said. “I had been through enough pain. I was angry and depressed, and I wanted a cure. That’s why I started emailing Dr Rondelli.”
Dr Rondelli said that because of Levy’s range of illnesses and inability to tolerate chemotherapy and radiation, several institutions had denied him the possibility of a transplant.
However, Dr Rondelli and his colleagues had reported success with chemotherapy-free allo-HSCT in patients with sickle cell disease. So Dr Rondelli performed Levy’s transplant in 2014.
Levy received a peripheral blood stem cell transplant from an unrelated donor who was a 10/10 HLA match but ABO incompatible. He received conditioning with rabbit anti-thymocyte globulin, fludarabine, cyclophosphamide, and total body irradiation.
Levy also received graft-vs-host disease (GVHD) prophylaxis consisting of high-dose cyclophosphamide, mycophenolate mofetil, and sirolimus. And he received standard antibacterial, antifungal, antiviral, and anti-Pneumocystis jiroveci prophylaxis.
Levy experienced platelet engraftment on day 20 and neutrophil engraftment on day 21. Whole-blood donor-cell chimerism was 98.7% on day 30 and 100% on day 60 and beyond.
Levy did develop transient hemolytic anemia due to the ABO incompatibility. He was given a total of 10 units of packed red blood cells until day 78.
Levy was tapered off all immunosuppression at 12 months and has shown no signs of acute or chronic GVHD.
At 24 months after HSCT, Levy’s hemoglobin was 13.7 g/dL, and his ferritin was 376 ng/mL. He has had no iron chelation since the transplant.
“The transplant was hard, and I had some complications, but I am back to normal now,” said Levy, who is now 35.
“I still have some pain and some lingering issues from the years my condition was not properly managed, but I can be independent now. That is the most important thing to me.”
Levy is finishing his doctorate in psychology and running group therapy sessions at a behavioral health hospital.
Dr Rondelli said the potential of this treatment approach is promising.
“The use of this transplant protocol may represent a safe therapeutic strategy to treat adult patients with many types of congenital anemias—perhaps the only possible cure,” he said.
“For many adult patients with a blood disorder, treatment options have been limited because they are often not sick enough to qualify for a risky procedure, or they are too sick to tolerate the toxic drugs used alongside a standard transplant. This procedure gives some adults the option of a stem cell transplant, which was not previously available.” ![]()
Physicians have reported what they believe is the first case of an allogeneic hematopoietic stem cell transplant (allo-HSCT) curing an adult with congenital dyserythropoietic anemia (CDA).
The patient, David Levy, was previously transfusion-dependent and suffered from iron overload, severe pain, and other adverse effects of his illness.
Levy was denied a transplant for years, but, in 2014, he received a non-myeloablative allo-HSCT from a matched, unrelated donor.
Now, Levy no longer requires transfusions, iron chelation, or immunosuppression, and says he is able to live a normal life.
Damiano Rondelli, MD, of the University of Illinois at Chicago, and his colleagues described Levy’s case in a letter to Bone Marrow Transplantation.
Levy was diagnosed with CDA at 4 months of age and was treated with regular blood transfusions for most of his life. He was 24 when the pain from his illness became so severe that he had to withdraw from graduate school.
“I spent the following years doing nothing—no work, no school, no social contact—because all I could focus on was managing my pain and getting my health back on track,” Levy said.
By age 32, Levy required transfusions every 2 to 3 weeks, had undergone a splenectomy, had an enlarged liver, and was suffering from fatigue, heart palpitations, and iron overload.
“It was bad,” Levy said. “I had been through enough pain. I was angry and depressed, and I wanted a cure. That’s why I started emailing Dr Rondelli.”
Dr Rondelli said that because of Levy’s range of illnesses and inability to tolerate chemotherapy and radiation, several institutions had denied him the possibility of a transplant.
However, Dr Rondelli and his colleagues had reported success with chemotherapy-free allo-HSCT in patients with sickle cell disease. So Dr Rondelli performed Levy’s transplant in 2014.
Levy received a peripheral blood stem cell transplant from an unrelated donor who was a 10/10 HLA match but ABO incompatible. He received conditioning with rabbit anti-thymocyte globulin, fludarabine, cyclophosphamide, and total body irradiation.
Levy also received graft-vs-host disease (GVHD) prophylaxis consisting of high-dose cyclophosphamide, mycophenolate mofetil, and sirolimus. And he received standard antibacterial, antifungal, antiviral, and anti-Pneumocystis jiroveci prophylaxis.
Levy experienced platelet engraftment on day 20 and neutrophil engraftment on day 21. Whole-blood donor-cell chimerism was 98.7% on day 30 and 100% on day 60 and beyond.
Levy did develop transient hemolytic anemia due to the ABO incompatibility. He was given a total of 10 units of packed red blood cells until day 78.
Levy was tapered off all immunosuppression at 12 months and has shown no signs of acute or chronic GVHD.
At 24 months after HSCT, Levy’s hemoglobin was 13.7 g/dL, and his ferritin was 376 ng/mL. He has had no iron chelation since the transplant.
“The transplant was hard, and I had some complications, but I am back to normal now,” said Levy, who is now 35.
“I still have some pain and some lingering issues from the years my condition was not properly managed, but I can be independent now. That is the most important thing to me.”
Levy is finishing his doctorate in psychology and running group therapy sessions at a behavioral health hospital.
Dr Rondelli said the potential of this treatment approach is promising.
“The use of this transplant protocol may represent a safe therapeutic strategy to treat adult patients with many types of congenital anemias—perhaps the only possible cure,” he said.
“For many adult patients with a blood disorder, treatment options have been limited because they are often not sick enough to qualify for a risky procedure, or they are too sick to tolerate the toxic drugs used alongside a standard transplant. This procedure gives some adults the option of a stem cell transplant, which was not previously available.” ![]()
New insight into high-hyperdiploid ALL
New research appears to explain how 10q21.2 influences the risk of high-hyperdiploid acute lymphoblastic leukemia (HD-ALL).
Previous research indicated that variation in the gene ARID5B at 10q21.2 is associated with HD-ALL.
Now, researchers have reported that the 10q21.2 risk locus for HD-ALL is mediated through the single nucleotide polymorphism (SNP) rs7090445, which disrupts RUNX3 transcription factor binding.
Specifically, the rs7090445-C allele confers an increased risk of HD-ALL through reduced RUNX3-mediated expression of ARID5B.
The researchers described these findings in Nature Communications.
“This study expands our understanding of how genetic risk factors can influence the development of acute lymphoblastic leukemia . . .,” said study author Richard Houlston, MD, PhD, of The Institute of Cancer Research in London, UK.
Dr Houlston and his colleagues focused this research on 10q21.2 because it had previously been implicated in HD-ALL, but it wasn’t clear how the region affects the risk of HD-ALL.
The team said they found that a SNP in the region, rs7090445, is “highly associated” with HD-ALL.
Further investigation revealed that variation at rs7090445 disrupts RUNX3 binding and reduces the expression of ARID5B, as RUNX3 regulates ARID5B expression.
The researchers also discovered that the rs7090445-C risk allele, which is associated with reduced ARID5B expression, is amplified in HD-ALL. The risk allele is “preferentially retained” on additional copies of chromosome 10 in HD-ALL blasts.
“We implicate reduced expression of a gene called ARID5B in the production and release of the immature ‘blast’ cells that characterize [HD-ALL],” Dr Houlston said. “Our study gives a new insight into the causes of the disease and may open up new strategies for prevention.” ![]()
New research appears to explain how 10q21.2 influences the risk of high-hyperdiploid acute lymphoblastic leukemia (HD-ALL).
Previous research indicated that variation in the gene ARID5B at 10q21.2 is associated with HD-ALL.
Now, researchers have reported that the 10q21.2 risk locus for HD-ALL is mediated through the single nucleotide polymorphism (SNP) rs7090445, which disrupts RUNX3 transcription factor binding.
Specifically, the rs7090445-C allele confers an increased risk of HD-ALL through reduced RUNX3-mediated expression of ARID5B.
The researchers described these findings in Nature Communications.
“This study expands our understanding of how genetic risk factors can influence the development of acute lymphoblastic leukemia . . .,” said study author Richard Houlston, MD, PhD, of The Institute of Cancer Research in London, UK.
Dr Houlston and his colleagues focused this research on 10q21.2 because it had previously been implicated in HD-ALL, but it wasn’t clear how the region affects the risk of HD-ALL.
The team said they found that a SNP in the region, rs7090445, is “highly associated” with HD-ALL.
Further investigation revealed that variation at rs7090445 disrupts RUNX3 binding and reduces the expression of ARID5B, as RUNX3 regulates ARID5B expression.
The researchers also discovered that the rs7090445-C risk allele, which is associated with reduced ARID5B expression, is amplified in HD-ALL. The risk allele is “preferentially retained” on additional copies of chromosome 10 in HD-ALL blasts.
“We implicate reduced expression of a gene called ARID5B in the production and release of the immature ‘blast’ cells that characterize [HD-ALL],” Dr Houlston said. “Our study gives a new insight into the causes of the disease and may open up new strategies for prevention.” ![]()
New research appears to explain how 10q21.2 influences the risk of high-hyperdiploid acute lymphoblastic leukemia (HD-ALL).
Previous research indicated that variation in the gene ARID5B at 10q21.2 is associated with HD-ALL.
Now, researchers have reported that the 10q21.2 risk locus for HD-ALL is mediated through the single nucleotide polymorphism (SNP) rs7090445, which disrupts RUNX3 transcription factor binding.
Specifically, the rs7090445-C allele confers an increased risk of HD-ALL through reduced RUNX3-mediated expression of ARID5B.
The researchers described these findings in Nature Communications.
“This study expands our understanding of how genetic risk factors can influence the development of acute lymphoblastic leukemia . . .,” said study author Richard Houlston, MD, PhD, of The Institute of Cancer Research in London, UK.
Dr Houlston and his colleagues focused this research on 10q21.2 because it had previously been implicated in HD-ALL, but it wasn’t clear how the region affects the risk of HD-ALL.
The team said they found that a SNP in the region, rs7090445, is “highly associated” with HD-ALL.
Further investigation revealed that variation at rs7090445 disrupts RUNX3 binding and reduces the expression of ARID5B, as RUNX3 regulates ARID5B expression.
The researchers also discovered that the rs7090445-C risk allele, which is associated with reduced ARID5B expression, is amplified in HD-ALL. The risk allele is “preferentially retained” on additional copies of chromosome 10 in HD-ALL blasts.
“We implicate reduced expression of a gene called ARID5B in the production and release of the immature ‘blast’ cells that characterize [HD-ALL],” Dr Houlston said. “Our study gives a new insight into the causes of the disease and may open up new strategies for prevention.” ![]()
Apixaban bests warfarin in real-world analysis
WASHINGTON, DC—An analysis of real-world data suggests elderly patients with non-valvular atrial fibrillation (NVAF) have a lower risk of stroke or systemic embolism and a lower risk of major bleeding if they receive apixaban rather than warfarin.
The study also indicates that elderly NVAF patients who receive dabigatran have a similar risk of stroke/systemic embolism as those on warfarin, but the risk of major bleeding is lower with dabigatran.
And rivaroxaban poses a lower risk of stroke/systemic embolism than warfarin but a higher risk of major bleeding.
However, the researchers who conducted this analysis stressed that it cannot be used as stand-alone evidence to validate the efficacy and/or safety of any of the drugs studied.
The results of the analysis were presented at the American College of Cardiology’s 66th Annual Scientific Session (abstract 1134M-13).
The researchers also presented data on the financial costs of major bleeding associated with apixaban, dabigatran, rivaroxaban, and warfarin (abstract 1189-085/085).
The research was conducted by employees from Bristol-Myers Squibb Company and Pfizer Inc., the companies marketing apixaban, as well as other researchers who have relationships with the companies.
The team evaluated medical and pharmacy claims from the US Medicare fee-for-service database. They looked at NVAF patients age 65 and older who were newly prescribed apixaban, dabigatran, rivaroxaban, or warfarin between January 1, 2013, and December 31, 2014.
The researchers compared each of the direct oral anticoagulants to warfarin using 1:1 propensity score matching methodology to balance select demographic and clinical characteristics between the groups.
The team used Cox proportional hazards models to estimate the hazard ratio (HR) of stroke/systemic embolism and major bleeding.
Results
In a comparison of apixaban and warfarin (20,803 patients in each treatment group), apixaban was associated with a significantly lower risk of stroke or systemic embolism than warfarin (HR: 0.40, 95% CI: 0.31-0.53; P<0.0001).
Apixaban was also associated with a significantly lower risk of major bleeding than warfarin (HR: 0.51, 95% CI: 0.44-0.58; P<0.0001). The cost of major bleeding per patient per month was $286 with apixaban and $537 with warfarin (P<0.0001).
In a comparison of dabigatran and warfarin (16,731 patients in each treatment group), the risk of stroke or systemic embolism was similar between the cohorts (HR: 0.94, 95% CI: 0.74-1.21; P=0.647).
However, the risk of major bleeding was significantly lower with dabigatran than with warfarin (HR: 0.79, 95% CI: 0.69-0.91; P=0.001). The cost of major bleeding per patient per month was $367 with dabigatran and $452 with warfarin (P=0.032).
In a comparison of rivaroxaban and warfarin (52,476 patients in each group), rivaroxaban was associated with a significantly lower risk of stroke or systemic embolism than warfarin (HR: 0.72, 95% CI: 0.63-0.83; P<0.0001).
However, the risk of major bleeding was significantly higher with rivaroxaban than with warfarin (HR: 1.17, 95% CI: 1.10-1.26; P<0.0001). The cost of major bleeding per patient per month was $524 with rivaroxaban and $500 with warfarin (P=0.154).
Limitations
The researchers noted that this analysis has several limitations. For instance, information on laboratory results and time in therapeutic range were not available. Diagnoses were identified through ICD-9 codes, and drug prescriptions were identified through prescription claims.
Propensity score matching was used to mimic randomization by balancing pre-defined demographic and clinical characteristics at baseline for both treatment cohorts. However, unobserved confounders (such as laboratory values and patient preferences) may exist.
As with any real-world data analysis, missing values, coding errors, and a lack of clinical accuracy may have introduced bias.
The researchers stressed that, due to such limitations, real-world data analyses cannot be used as stand-alone evidence to validate the efficacy and/or safety of a treatment. However, these analyses add to information provided by randomized clinical trials.
“Studies such as this large US Medicare database analysis supplement pivotal trials by broadening and deepening our scientific knowledge of how patients respond to direct oral anticoagulants in everyday clinical practice,” said Alpesh Amin, MD, principal investigator and professor of medicine at the University of California, Irvine.
“Given the diversity of patients with non-valvular atrial fibrillation, analyses of real-world data provide further information that adds to data generated in randomized clinical trials.” ![]()
WASHINGTON, DC—An analysis of real-world data suggests elderly patients with non-valvular atrial fibrillation (NVAF) have a lower risk of stroke or systemic embolism and a lower risk of major bleeding if they receive apixaban rather than warfarin.
The study also indicates that elderly NVAF patients who receive dabigatran have a similar risk of stroke/systemic embolism as those on warfarin, but the risk of major bleeding is lower with dabigatran.
And rivaroxaban poses a lower risk of stroke/systemic embolism than warfarin but a higher risk of major bleeding.
However, the researchers who conducted this analysis stressed that it cannot be used as stand-alone evidence to validate the efficacy and/or safety of any of the drugs studied.
The results of the analysis were presented at the American College of Cardiology’s 66th Annual Scientific Session (abstract 1134M-13).
The researchers also presented data on the financial costs of major bleeding associated with apixaban, dabigatran, rivaroxaban, and warfarin (abstract 1189-085/085).
The research was conducted by employees from Bristol-Myers Squibb Company and Pfizer Inc., the companies marketing apixaban, as well as other researchers who have relationships with the companies.
The team evaluated medical and pharmacy claims from the US Medicare fee-for-service database. They looked at NVAF patients age 65 and older who were newly prescribed apixaban, dabigatran, rivaroxaban, or warfarin between January 1, 2013, and December 31, 2014.
The researchers compared each of the direct oral anticoagulants to warfarin using 1:1 propensity score matching methodology to balance select demographic and clinical characteristics between the groups.
The team used Cox proportional hazards models to estimate the hazard ratio (HR) of stroke/systemic embolism and major bleeding.
Results
In a comparison of apixaban and warfarin (20,803 patients in each treatment group), apixaban was associated with a significantly lower risk of stroke or systemic embolism than warfarin (HR: 0.40, 95% CI: 0.31-0.53; P<0.0001).
Apixaban was also associated with a significantly lower risk of major bleeding than warfarin (HR: 0.51, 95% CI: 0.44-0.58; P<0.0001). The cost of major bleeding per patient per month was $286 with apixaban and $537 with warfarin (P<0.0001).
In a comparison of dabigatran and warfarin (16,731 patients in each treatment group), the risk of stroke or systemic embolism was similar between the cohorts (HR: 0.94, 95% CI: 0.74-1.21; P=0.647).
However, the risk of major bleeding was significantly lower with dabigatran than with warfarin (HR: 0.79, 95% CI: 0.69-0.91; P=0.001). The cost of major bleeding per patient per month was $367 with dabigatran and $452 with warfarin (P=0.032).
In a comparison of rivaroxaban and warfarin (52,476 patients in each group), rivaroxaban was associated with a significantly lower risk of stroke or systemic embolism than warfarin (HR: 0.72, 95% CI: 0.63-0.83; P<0.0001).
However, the risk of major bleeding was significantly higher with rivaroxaban than with warfarin (HR: 1.17, 95% CI: 1.10-1.26; P<0.0001). The cost of major bleeding per patient per month was $524 with rivaroxaban and $500 with warfarin (P=0.154).
Limitations
The researchers noted that this analysis has several limitations. For instance, information on laboratory results and time in therapeutic range were not available. Diagnoses were identified through ICD-9 codes, and drug prescriptions were identified through prescription claims.
Propensity score matching was used to mimic randomization by balancing pre-defined demographic and clinical characteristics at baseline for both treatment cohorts. However, unobserved confounders (such as laboratory values and patient preferences) may exist.
As with any real-world data analysis, missing values, coding errors, and a lack of clinical accuracy may have introduced bias.
The researchers stressed that, due to such limitations, real-world data analyses cannot be used as stand-alone evidence to validate the efficacy and/or safety of a treatment. However, these analyses add to information provided by randomized clinical trials.
“Studies such as this large US Medicare database analysis supplement pivotal trials by broadening and deepening our scientific knowledge of how patients respond to direct oral anticoagulants in everyday clinical practice,” said Alpesh Amin, MD, principal investigator and professor of medicine at the University of California, Irvine.
“Given the diversity of patients with non-valvular atrial fibrillation, analyses of real-world data provide further information that adds to data generated in randomized clinical trials.” ![]()
WASHINGTON, DC—An analysis of real-world data suggests elderly patients with non-valvular atrial fibrillation (NVAF) have a lower risk of stroke or systemic embolism and a lower risk of major bleeding if they receive apixaban rather than warfarin.
The study also indicates that elderly NVAF patients who receive dabigatran have a similar risk of stroke/systemic embolism as those on warfarin, but the risk of major bleeding is lower with dabigatran.
And rivaroxaban poses a lower risk of stroke/systemic embolism than warfarin but a higher risk of major bleeding.
However, the researchers who conducted this analysis stressed that it cannot be used as stand-alone evidence to validate the efficacy and/or safety of any of the drugs studied.
The results of the analysis were presented at the American College of Cardiology’s 66th Annual Scientific Session (abstract 1134M-13).
The researchers also presented data on the financial costs of major bleeding associated with apixaban, dabigatran, rivaroxaban, and warfarin (abstract 1189-085/085).
The research was conducted by employees from Bristol-Myers Squibb Company and Pfizer Inc., the companies marketing apixaban, as well as other researchers who have relationships with the companies.
The team evaluated medical and pharmacy claims from the US Medicare fee-for-service database. They looked at NVAF patients age 65 and older who were newly prescribed apixaban, dabigatran, rivaroxaban, or warfarin between January 1, 2013, and December 31, 2014.
The researchers compared each of the direct oral anticoagulants to warfarin using 1:1 propensity score matching methodology to balance select demographic and clinical characteristics between the groups.
The team used Cox proportional hazards models to estimate the hazard ratio (HR) of stroke/systemic embolism and major bleeding.
Results
In a comparison of apixaban and warfarin (20,803 patients in each treatment group), apixaban was associated with a significantly lower risk of stroke or systemic embolism than warfarin (HR: 0.40, 95% CI: 0.31-0.53; P<0.0001).
Apixaban was also associated with a significantly lower risk of major bleeding than warfarin (HR: 0.51, 95% CI: 0.44-0.58; P<0.0001). The cost of major bleeding per patient per month was $286 with apixaban and $537 with warfarin (P<0.0001).
In a comparison of dabigatran and warfarin (16,731 patients in each treatment group), the risk of stroke or systemic embolism was similar between the cohorts (HR: 0.94, 95% CI: 0.74-1.21; P=0.647).
However, the risk of major bleeding was significantly lower with dabigatran than with warfarin (HR: 0.79, 95% CI: 0.69-0.91; P=0.001). The cost of major bleeding per patient per month was $367 with dabigatran and $452 with warfarin (P=0.032).
In a comparison of rivaroxaban and warfarin (52,476 patients in each group), rivaroxaban was associated with a significantly lower risk of stroke or systemic embolism than warfarin (HR: 0.72, 95% CI: 0.63-0.83; P<0.0001).
However, the risk of major bleeding was significantly higher with rivaroxaban than with warfarin (HR: 1.17, 95% CI: 1.10-1.26; P<0.0001). The cost of major bleeding per patient per month was $524 with rivaroxaban and $500 with warfarin (P=0.154).
Limitations
The researchers noted that this analysis has several limitations. For instance, information on laboratory results and time in therapeutic range were not available. Diagnoses were identified through ICD-9 codes, and drug prescriptions were identified through prescription claims.
Propensity score matching was used to mimic randomization by balancing pre-defined demographic and clinical characteristics at baseline for both treatment cohorts. However, unobserved confounders (such as laboratory values and patient preferences) may exist.
As with any real-world data analysis, missing values, coding errors, and a lack of clinical accuracy may have introduced bias.
The researchers stressed that, due to such limitations, real-world data analyses cannot be used as stand-alone evidence to validate the efficacy and/or safety of a treatment. However, these analyses add to information provided by randomized clinical trials.
“Studies such as this large US Medicare database analysis supplement pivotal trials by broadening and deepening our scientific knowledge of how patients respond to direct oral anticoagulants in everyday clinical practice,” said Alpesh Amin, MD, principal investigator and professor of medicine at the University of California, Irvine.
“Given the diversity of patients with non-valvular atrial fibrillation, analyses of real-world data provide further information that adds to data generated in randomized clinical trials.” ![]()
Major bleeding lower with dabigatran than warfarin
WASHINGTON, DC—Results from the RE-CIRCUIT trial suggest that uninterrupted dabigatran poses a lower risk of major bleeding than uninterrupted warfarin in patients with non-valvular atrial fibrillation (NVAF) who are undergoing catheter ablation.
Patients who received dabigatran also had fewer serious and severe adverse events (AEs).
The incidence of minor bleeding was similar between the treatment groups, and the incidence of AEs leading to treatment discontinuation was higher with dabigatran.
These results were presented at the American College of Cardiology’s 66th Annual Scientific Session and simultaneously published in NEJM.
This study was sponsored by Boehringer Ingelheim Pharmaceuticals, Inc.
The RE-CIRCUIT trial enrolled 704 patients with paroxysmal or persistent NVAF, and 635 of them ultimately underwent catheter ablation with uninterrupted anticoagulation.
The patients were randomized to receive dabigatran at 150 mg twice daily or warfarin (with a target international normalized ratio [INR] of 2.0–3.0) in a 1:1 ratio and remained on this treatment for the duration of the trial.
The mean adherence to dabigatran was 97.6%, and patients receiving warfarin were within the guideline-defined target INR range 66% of the time. The majority of patients in both groups (86.1% in the dabigatran group and 84.3% in the warfarin group) received study treatment for at least 8 weeks after ablation.
Results
The study’s primary endpoint was the incidence of major bleeding events, as defined by the International Society on Thrombosis and Hemostasis, during the ablation procedure and up to 2 months after.
There was a significantly lower incidence of major bleeding with uninterrupted dabigatran than with uninterrupted warfarin—1.6% (n=5) and 6.9% (n=22), respectively (P<0.001).
Major bleeding events (in the dabigatran and warfarin groups, respectively) included pericardial tamponade (1 and 6), pericardial effusion (1 and 0), groin bleeds (2 in both), groin hematomas (0 and 8), gastrointestinal bleeds (1 and 2), intracranial bleeds (0 and 2), pseudoaneurysm (0 and 1), and hematomas (0 and 2).
The incidence of minor bleeding was similar between the treatment groups—18.6% (n=59) in the dabigatran group and 17.0% (n=54) in the warfarin group.
The incidence of serious AEs was 18.6% in the dabigatran group and 22.2% in the warfarin group. The most frequent serious AEs were atrial flutter (5.9% and 5.6%, respectively), atrial fibrillation (1.8% and 3.8%, respectively), and cardiac tamponade (0.3% and 1.2%, respectively).
The incidence of severe AEs was 3.3% in the dabigatran group and 6.2% in the warfarin group. The incidence of AEs leading to treatment discontinuation was 5.6% and 2.4%, respectively.
There were no cases of stroke, systemic embolism, or transient ischemic attack in the dabigatran group. However, there was a transient ischemic attack in the warfarin group.
“These results are exciting news for the medical community,” said study investigator Hugh Calkins, MD, of Johns Hopkins Hospital in Baltimore, Maryland.
“During an ablation procedure, patients are at risk of potential major complications, including stroke and bleeding. Therefore, anticoagulation management at the time of AFib ablation is critically important. In RE-CIRCUIT, we have seen that uninterrupted anticoagulation with dabigatran showed significantly lower major bleeding complications than warfarin in atrial fibrillation patients undergoing cardiac ablation.” ![]()
WASHINGTON, DC—Results from the RE-CIRCUIT trial suggest that uninterrupted dabigatran poses a lower risk of major bleeding than uninterrupted warfarin in patients with non-valvular atrial fibrillation (NVAF) who are undergoing catheter ablation.
Patients who received dabigatran also had fewer serious and severe adverse events (AEs).
The incidence of minor bleeding was similar between the treatment groups, and the incidence of AEs leading to treatment discontinuation was higher with dabigatran.
These results were presented at the American College of Cardiology’s 66th Annual Scientific Session and simultaneously published in NEJM.
This study was sponsored by Boehringer Ingelheim Pharmaceuticals, Inc.
The RE-CIRCUIT trial enrolled 704 patients with paroxysmal or persistent NVAF, and 635 of them ultimately underwent catheter ablation with uninterrupted anticoagulation.
The patients were randomized to receive dabigatran at 150 mg twice daily or warfarin (with a target international normalized ratio [INR] of 2.0–3.0) in a 1:1 ratio and remained on this treatment for the duration of the trial.
The mean adherence to dabigatran was 97.6%, and patients receiving warfarin were within the guideline-defined target INR range 66% of the time. The majority of patients in both groups (86.1% in the dabigatran group and 84.3% in the warfarin group) received study treatment for at least 8 weeks after ablation.
Results
The study’s primary endpoint was the incidence of major bleeding events, as defined by the International Society on Thrombosis and Hemostasis, during the ablation procedure and up to 2 months after.
There was a significantly lower incidence of major bleeding with uninterrupted dabigatran than with uninterrupted warfarin—1.6% (n=5) and 6.9% (n=22), respectively (P<0.001).
Major bleeding events (in the dabigatran and warfarin groups, respectively) included pericardial tamponade (1 and 6), pericardial effusion (1 and 0), groin bleeds (2 in both), groin hematomas (0 and 8), gastrointestinal bleeds (1 and 2), intracranial bleeds (0 and 2), pseudoaneurysm (0 and 1), and hematomas (0 and 2).
The incidence of minor bleeding was similar between the treatment groups—18.6% (n=59) in the dabigatran group and 17.0% (n=54) in the warfarin group.
The incidence of serious AEs was 18.6% in the dabigatran group and 22.2% in the warfarin group. The most frequent serious AEs were atrial flutter (5.9% and 5.6%, respectively), atrial fibrillation (1.8% and 3.8%, respectively), and cardiac tamponade (0.3% and 1.2%, respectively).
The incidence of severe AEs was 3.3% in the dabigatran group and 6.2% in the warfarin group. The incidence of AEs leading to treatment discontinuation was 5.6% and 2.4%, respectively.
There were no cases of stroke, systemic embolism, or transient ischemic attack in the dabigatran group. However, there was a transient ischemic attack in the warfarin group.
“These results are exciting news for the medical community,” said study investigator Hugh Calkins, MD, of Johns Hopkins Hospital in Baltimore, Maryland.
“During an ablation procedure, patients are at risk of potential major complications, including stroke and bleeding. Therefore, anticoagulation management at the time of AFib ablation is critically important. In RE-CIRCUIT, we have seen that uninterrupted anticoagulation with dabigatran showed significantly lower major bleeding complications than warfarin in atrial fibrillation patients undergoing cardiac ablation.” ![]()
WASHINGTON, DC—Results from the RE-CIRCUIT trial suggest that uninterrupted dabigatran poses a lower risk of major bleeding than uninterrupted warfarin in patients with non-valvular atrial fibrillation (NVAF) who are undergoing catheter ablation.
Patients who received dabigatran also had fewer serious and severe adverse events (AEs).
The incidence of minor bleeding was similar between the treatment groups, and the incidence of AEs leading to treatment discontinuation was higher with dabigatran.
These results were presented at the American College of Cardiology’s 66th Annual Scientific Session and simultaneously published in NEJM.
This study was sponsored by Boehringer Ingelheim Pharmaceuticals, Inc.
The RE-CIRCUIT trial enrolled 704 patients with paroxysmal or persistent NVAF, and 635 of them ultimately underwent catheter ablation with uninterrupted anticoagulation.
The patients were randomized to receive dabigatran at 150 mg twice daily or warfarin (with a target international normalized ratio [INR] of 2.0–3.0) in a 1:1 ratio and remained on this treatment for the duration of the trial.
The mean adherence to dabigatran was 97.6%, and patients receiving warfarin were within the guideline-defined target INR range 66% of the time. The majority of patients in both groups (86.1% in the dabigatran group and 84.3% in the warfarin group) received study treatment for at least 8 weeks after ablation.
Results
The study’s primary endpoint was the incidence of major bleeding events, as defined by the International Society on Thrombosis and Hemostasis, during the ablation procedure and up to 2 months after.
There was a significantly lower incidence of major bleeding with uninterrupted dabigatran than with uninterrupted warfarin—1.6% (n=5) and 6.9% (n=22), respectively (P<0.001).
Major bleeding events (in the dabigatran and warfarin groups, respectively) included pericardial tamponade (1 and 6), pericardial effusion (1 and 0), groin bleeds (2 in both), groin hematomas (0 and 8), gastrointestinal bleeds (1 and 2), intracranial bleeds (0 and 2), pseudoaneurysm (0 and 1), and hematomas (0 and 2).
The incidence of minor bleeding was similar between the treatment groups—18.6% (n=59) in the dabigatran group and 17.0% (n=54) in the warfarin group.
The incidence of serious AEs was 18.6% in the dabigatran group and 22.2% in the warfarin group. The most frequent serious AEs were atrial flutter (5.9% and 5.6%, respectively), atrial fibrillation (1.8% and 3.8%, respectively), and cardiac tamponade (0.3% and 1.2%, respectively).
The incidence of severe AEs was 3.3% in the dabigatran group and 6.2% in the warfarin group. The incidence of AEs leading to treatment discontinuation was 5.6% and 2.4%, respectively.
There were no cases of stroke, systemic embolism, or transient ischemic attack in the dabigatran group. However, there was a transient ischemic attack in the warfarin group.
“These results are exciting news for the medical community,” said study investigator Hugh Calkins, MD, of Johns Hopkins Hospital in Baltimore, Maryland.
“During an ablation procedure, patients are at risk of potential major complications, including stroke and bleeding. Therefore, anticoagulation management at the time of AFib ablation is critically important. In RE-CIRCUIT, we have seen that uninterrupted anticoagulation with dabigatran showed significantly lower major bleeding complications than warfarin in atrial fibrillation patients undergoing cardiac ablation.”
Drugs demonstrate similar safety profile in ACS trial
WASHINGTON, DC—Results of a phase 2 trial suggest rivaroxaban and aspirin have similar safety profiles in patients with acute coronary syndrome (ACS) who are also taking a P2Y12 inhibitor.
Researchers found that, overall, the risk of bleeding was similar whether patients received aspirin or rivaroxaban.
There was no significant difference between the groups when the researchers used TIMI, GUSTO, or BARC bleeding definitions.
However, patients who received rivaroxaban did have a significantly increased risk of bleeding according to the ISTH major bleeding definition.
There was no significant difference between the treatment groups when it came to the study’s efficacy endpoints, although the researchers said the study was not adequately powered to assess efficacy.
These results were presented at the American College of Cardiology’s 66th Annual Scientific Session and published simultaneously in The Lancet.
The study, known as GEMINI-ACS-1, was funded by Janssen Research & Development and Bayer AG.
In this trial, researchers compared rivaroxaban (given at 2.5 mg twice daily) and aspirin (at 100 mg once daily) in patients with ACS who were also receiving clopidogrel or ticagrelor for the secondary prevention of cardiovascular events.
The trial included 3037 adults with unstable angina, non-ST segment elevation myocardial infarction, or ST segment elevation myocardial infarction. Patients had positive cardiac biomarkers and either ischemic electrocardiographic changes or an atherosclerotic culprit lesion identified during angiography.
Within 10 days of hospital admission for ACS, the patients were randomized to receive aspirin (n=1518) or rivaroxaban (n=1519). Patients also received clopidogrel (n=1333) or ticagrelor (n=1704) based on investigator preference.
Patients received a minimum of 180 days of study treatment. The median treatment duration was 291 days.
Safety
The study’s primary endpoint was TIMI clinically significant bleeding not related to coronary artery bypass grafting (CABG) up to day 390.
This type of bleeding occurred in 5.3% of patients in the rivaroxaban group and 4.9% of patients in the aspirin group. The hazard ratio (HR) was 1.09 (P=0.5840).
The researchers also assessed other types of bleeding and used other bleeding definitions. The HRs for these endpoints (with aspirin as the reference) were as follows.
- TIMI major bleeding, HR=1.25 (P=0.6341)
- TIMI non-CABG major bleeding, HR=1.25 (P=0.6341)
- TIMI minor bleeding, HR=2.25 (P=0.1664)
- TIMI bleeding requiring medical attention, HR=1.01 (P=0.9581)
- TIMI insignificant bleeding, HR=0.84 (P=0.5504)
- GUSTO life-threatening or severe bleeding, HR=1.50 (P=0.6571)
- GUSTO life-threatening, severe, or moderate bleeding, HR=1.58 (P=0.3395)
- GUSTO life-threatening, severe, moderate, or mild bleeding, HR=1.04 (P=0.7869)
- BARC 3a and higher bleeding, HR=1.70 (P=0.1263)
- BARC 3b and higher bleeding, HR=1.38 (P=0.4882)
- ISTH major bleeding, HR=1.83 (P=0.0420)
Efficacy
Researchers also examined several exploratory efficacy endpoints. For the composite efficacy endpoint (which included cardiovascular death, myocardial infarction, stroke, and stent thrombosis), the 2 treatment groups had similar rates.
Five percent of patients in the rivaroxaban group and 4.7% of patients in the aspirin group experienced one of these cardiovascular events. The HR was 1.06 (P=0.7316).
There was no significant difference between the treatment arms for the individual components of the efficacy endpoint or for all-cause death.
The HRs were 1.12 (P=0.7401) for cardiovascular death, 1.15 (P=0.4872) for myocardial infarction, 0.58 (P=0.2506) for stroke, 1.37 (P=0.4917) for definite stent thrombosis, and 0.95 (P=0.8771) for all-cause death.
WASHINGTON, DC—Results of a phase 2 trial suggest rivaroxaban and aspirin have similar safety profiles in patients with acute coronary syndrome (ACS) who are also taking a P2Y12 inhibitor.
Researchers found that, overall, the risk of bleeding was similar whether patients received aspirin or rivaroxaban.
There was no significant difference between the groups when the researchers used TIMI, GUSTO, or BARC bleeding definitions.
However, patients who received rivaroxaban did have a significantly increased risk of bleeding according to the ISTH major bleeding definition.
There was no significant difference between the treatment groups when it came to the study’s efficacy endpoints, although the researchers said the study was not adequately powered to assess efficacy.
These results were presented at the American College of Cardiology’s 66th Annual Scientific Session and published simultaneously in The Lancet.
The study, known as GEMINI-ACS-1, was funded by Janssen Research & Development and Bayer AG.
In this trial, researchers compared rivaroxaban (given at 2.5 mg twice daily) and aspirin (at 100 mg once daily) in patients with ACS who were also receiving clopidogrel or ticagrelor for the secondary prevention of cardiovascular events.
The trial included 3037 adults with unstable angina, non-ST segment elevation myocardial infarction, or ST segment elevation myocardial infarction. Patients had positive cardiac biomarkers and either ischemic electrocardiographic changes or an atherosclerotic culprit lesion identified during angiography.
Within 10 days of hospital admission for ACS, the patients were randomized to receive aspirin (n=1518) or rivaroxaban (n=1519). Patients also received clopidogrel (n=1333) or ticagrelor (n=1704) based on investigator preference.
Patients received a minimum of 180 days of study treatment. The median treatment duration was 291 days.
Safety
The study’s primary endpoint was TIMI clinically significant bleeding not related to coronary artery bypass grafting (CABG) up to day 390.
This type of bleeding occurred in 5.3% of patients in the rivaroxaban group and 4.9% of patients in the aspirin group. The hazard ratio (HR) was 1.09 (P=0.5840).
The researchers also assessed other types of bleeding and used other bleeding definitions. The HRs for these endpoints (with aspirin as the reference) were as follows.
- TIMI major bleeding, HR=1.25 (P=0.6341)
- TIMI non-CABG major bleeding, HR=1.25 (P=0.6341)
- TIMI minor bleeding, HR=2.25 (P=0.1664)
- TIMI bleeding requiring medical attention, HR=1.01 (P=0.9581)
- TIMI insignificant bleeding, HR=0.84 (P=0.5504)
- GUSTO life-threatening or severe bleeding, HR=1.50 (P=0.6571)
- GUSTO life-threatening, severe, or moderate bleeding, HR=1.58 (P=0.3395)
- GUSTO life-threatening, severe, moderate, or mild bleeding, HR=1.04 (P=0.7869)
- BARC 3a and higher bleeding, HR=1.70 (P=0.1263)
- BARC 3b and higher bleeding, HR=1.38 (P=0.4882)
- ISTH major bleeding, HR=1.83 (P=0.0420)
Efficacy
Researchers also examined several exploratory efficacy endpoints. For the composite efficacy endpoint (which included cardiovascular death, myocardial infarction, stroke, and stent thrombosis), the 2 treatment groups had similar rates.
Five percent of patients in the rivaroxaban group and 4.7% of patients in the aspirin group experienced one of these cardiovascular events. The HR was 1.06 (P=0.7316).
There was no significant difference between the treatment arms for the individual components of the efficacy endpoint or for all-cause death.
The HRs were 1.12 (P=0.7401) for cardiovascular death, 1.15 (P=0.4872) for myocardial infarction, 0.58 (P=0.2506) for stroke, 1.37 (P=0.4917) for definite stent thrombosis, and 0.95 (P=0.8771) for all-cause death.
WASHINGTON, DC—Results of a phase 2 trial suggest rivaroxaban and aspirin have similar safety profiles in patients with acute coronary syndrome (ACS) who are also taking a P2Y12 inhibitor.
Researchers found that, overall, the risk of bleeding was similar whether patients received aspirin or rivaroxaban.
There was no significant difference between the groups when the researchers used TIMI, GUSTO, or BARC bleeding definitions.
However, patients who received rivaroxaban did have a significantly increased risk of bleeding according to the ISTH major bleeding definition.
There was no significant difference between the treatment groups when it came to the study’s efficacy endpoints, although the researchers said the study was not adequately powered to assess efficacy.
These results were presented at the American College of Cardiology’s 66th Annual Scientific Session and published simultaneously in The Lancet.
The study, known as GEMINI-ACS-1, was funded by Janssen Research & Development and Bayer AG.
In this trial, researchers compared rivaroxaban (given at 2.5 mg twice daily) and aspirin (at 100 mg once daily) in patients with ACS who were also receiving clopidogrel or ticagrelor for the secondary prevention of cardiovascular events.
The trial included 3037 adults with unstable angina, non-ST segment elevation myocardial infarction, or ST segment elevation myocardial infarction. Patients had positive cardiac biomarkers and either ischemic electrocardiographic changes or an atherosclerotic culprit lesion identified during angiography.
Within 10 days of hospital admission for ACS, the patients were randomized to receive aspirin (n=1518) or rivaroxaban (n=1519). Patients also received clopidogrel (n=1333) or ticagrelor (n=1704) based on investigator preference.
Patients received a minimum of 180 days of study treatment. The median treatment duration was 291 days.
Safety
The study’s primary endpoint was TIMI clinically significant bleeding not related to coronary artery bypass grafting (CABG) up to day 390.
This type of bleeding occurred in 5.3% of patients in the rivaroxaban group and 4.9% of patients in the aspirin group. The hazard ratio (HR) was 1.09 (P=0.5840).
The researchers also assessed other types of bleeding and used other bleeding definitions. The HRs for these endpoints (with aspirin as the reference) were as follows.
- TIMI major bleeding, HR=1.25 (P=0.6341)
- TIMI non-CABG major bleeding, HR=1.25 (P=0.6341)
- TIMI minor bleeding, HR=2.25 (P=0.1664)
- TIMI bleeding requiring medical attention, HR=1.01 (P=0.9581)
- TIMI insignificant bleeding, HR=0.84 (P=0.5504)
- GUSTO life-threatening or severe bleeding, HR=1.50 (P=0.6571)
- GUSTO life-threatening, severe, or moderate bleeding, HR=1.58 (P=0.3395)
- GUSTO life-threatening, severe, moderate, or mild bleeding, HR=1.04 (P=0.7869)
- BARC 3a and higher bleeding, HR=1.70 (P=0.1263)
- BARC 3b and higher bleeding, HR=1.38 (P=0.4882)
- ISTH major bleeding, HR=1.83 (P=0.0420)
Efficacy
Researchers also examined several exploratory efficacy endpoints. For the composite efficacy endpoint (which included cardiovascular death, myocardial infarction, stroke, and stent thrombosis), the 2 treatment groups had similar rates.
Five percent of patients in the rivaroxaban group and 4.7% of patients in the aspirin group experienced one of these cardiovascular events. The HR was 1.06 (P=0.7316).
There was no significant difference between the treatment arms for the individual components of the efficacy endpoint or for all-cause death.
The HRs were 1.12 (P=0.7401) for cardiovascular death, 1.15 (P=0.4872) for myocardial infarction, 0.58 (P=0.2506) for stroke, 1.37 (P=0.4917) for definite stent thrombosis, and 0.95 (P=0.8771) for all-cause death.
PE patients rarely receive CDT or ST, study shows
WASHINGTON, DC—New research suggests US patients with pulmonary embolism (PE) rarely receive catheter-directed thrombolysis (CDT) or systemic thrombolysis (ST).
Investigators analyzed data from more than 100,000 patients who were hospitalized for PE and found that roughly 2% received CDT or ST.
These findings were presented at the American College of Cardiology’s 66th Annual Scientific Session (abstract 904-12).
“For years, ST and CDT have been available for use in patients with PE,” said study investigator Srinath Adusumalli, MD, of the University of Pennsylvania in Philadelphia.
“However, there has been little research done to understand how these therapies are being utilized in the real world. Our initial data suggest that, in fact, both ST and CDT are used infrequently to treat PE, including in young, critically ill patients who may experience the highest clinical benefit from those therapies.”
Dr Adusumalli and his colleagues performed a retrospective study in which they collected data from the OptumInsight national commercial insurance claims database.
The team identified 100,744 patients who had been hospitalized with PE during a 10-year period (2004-2014). About 2% of these patients (2.2%, n=2175) received either CDT (n=761) or ST (n=1414).
During the period studied, the number of PE hospitalizations increased by 306% (P<0.001), while the number of patients treated with CDT increased by 197% (P=0.001) and the number treated with ST increased by 514% (P<0.001).
The investigators said these findings are clinically useful and could impact decisions about patient care, but more research is needed.
“This study is the first in a 2-step research plan,” noted Bram Geller, MD, of the University of Pennsylvania.
“[O]ur next phase will be to actually evaluate the safety and clinical effectiveness of CDT versus ST by exploring patient outcomes in the OptumInsight commercial insurance claims database.”
WASHINGTON, DC—New research suggests US patients with pulmonary embolism (PE) rarely receive catheter-directed thrombolysis (CDT) or systemic thrombolysis (ST).
Investigators analyzed data from more than 100,000 patients who were hospitalized for PE and found that roughly 2% received CDT or ST.
These findings were presented at the American College of Cardiology’s 66th Annual Scientific Session (abstract 904-12).
“For years, ST and CDT have been available for use in patients with PE,” said study investigator Srinath Adusumalli, MD, of the University of Pennsylvania in Philadelphia.
“However, there has been little research done to understand how these therapies are being utilized in the real world. Our initial data suggest that, in fact, both ST and CDT are used infrequently to treat PE, including in young, critically ill patients who may experience the highest clinical benefit from those therapies.”
Dr Adusumalli and his colleagues performed a retrospective study in which they collected data from the OptumInsight national commercial insurance claims database.
The team identified 100,744 patients who had been hospitalized with PE during a 10-year period (2004-2014). About 2% of these patients (2.2%, n=2175) received either CDT (n=761) or ST (n=1414).
During the period studied, the number of PE hospitalizations increased by 306% (P<0.001), while the number of patients treated with CDT increased by 197% (P=0.001) and the number treated with ST increased by 514% (P<0.001).
The investigators said these findings are clinically useful and could impact decisions about patient care, but more research is needed.
“This study is the first in a 2-step research plan,” noted Bram Geller, MD, of the University of Pennsylvania.
“[O]ur next phase will be to actually evaluate the safety and clinical effectiveness of CDT versus ST by exploring patient outcomes in the OptumInsight commercial insurance claims database.”
WASHINGTON, DC—New research suggests US patients with pulmonary embolism (PE) rarely receive catheter-directed thrombolysis (CDT) or systemic thrombolysis (ST).
Investigators analyzed data from more than 100,000 patients who were hospitalized for PE and found that roughly 2% received CDT or ST.
These findings were presented at the American College of Cardiology’s 66th Annual Scientific Session (abstract 904-12).
“For years, ST and CDT have been available for use in patients with PE,” said study investigator Srinath Adusumalli, MD, of the University of Pennsylvania in Philadelphia.
“However, there has been little research done to understand how these therapies are being utilized in the real world. Our initial data suggest that, in fact, both ST and CDT are used infrequently to treat PE, including in young, critically ill patients who may experience the highest clinical benefit from those therapies.”
Dr Adusumalli and his colleagues performed a retrospective study in which they collected data from the OptumInsight national commercial insurance claims database.
The team identified 100,744 patients who had been hospitalized with PE during a 10-year period (2004-2014). About 2% of these patients (2.2%, n=2175) received either CDT (n=761) or ST (n=1414).
During the period studied, the number of PE hospitalizations increased by 306% (P<0.001), while the number of patients treated with CDT increased by 197% (P=0.001) and the number treated with ST increased by 514% (P<0.001).
The investigators said these findings are clinically useful and could impact decisions about patient care, but more research is needed.
“This study is the first in a 2-step research plan,” noted Bram Geller, MD, of the University of Pennsylvania.
“[O]ur next phase will be to actually evaluate the safety and clinical effectiveness of CDT versus ST by exploring patient outcomes in the OptumInsight commercial insurance claims database.”
Therapy can produce durable CRs in NHL
When given after low-dose chemotherapy, an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy can produce durable complete responses (CRs) in patients with relapsed/refractory non-Hodgkin lymphoma (NHL), according to research published in the Journal of Clinical Oncology.
In this phase 1 study, the overall response rate was 73%, and 50% of patients had an ongoing CR at last follow-up.
Fifty-five percent of patients experienced grade 3/4 neurologic toxicities, though these events eventually resolved.
This research was conducted under a cooperative research and development agreement between the National Cancer Institute and Kite Pharma, Inc.
Kite is developing the CAR T-cell therapy axicabtagene ciloleucel (formerly known as KTE-C19), and the therapy tested in this trial has the same CAR construct as axicabtagene ciloleucel.
Results from this study (NCT00924326) were previously published in the Journal of Clinical Oncology in 2014.
The current report included 22 patients with relapsed/refractory NHL. Seventeen patients had diffuse large B-cell lymphoma (DLBCL), 2 had primary mediastinal B-cell lymphoma (PMBCL), 2 had follicular lymphoma (FL), and 1 had mantle cell lymphoma (MCL).
Patients received a single dose of CAR T cells 2 days after a low-dose chemotherapy conditioning regimen consisting of cyclophosphamide and fludarabine.
Response
The overall response rate was 73% (16/22), with a CR rate of 55% (n=12) and a partial response (PR) rate of 18% (n=4).
Among patients with DLBCL, there were 9 CRs, 4 PRs, 1 patient with stable disease, and 3 patients with progressive disease.
Both FL patients achieved a CR, as did the patient with MCL. One patient with PMBCL had stable disease, and the other progressed.
Eleven of the 12 CRs are ongoing, with durations ranging from more than 7 months to more than 24 months. The median duration of CR is 12.5 months.
The researchers found that serum IL-15 levels and CAR T-cell expansion correlated with treatment response (CR or PR).
The median peak blood CAR+ cell level was 98/μL in patients who achieved a response and 15/μL in those who did not (P=0.027).
High serum IL-15 levels were significantly associated with high peak blood CAR+ cell levels (P=0.001) and response (P<0.001).
Toxicity
Fifty-five percent of patients had grade 3 or 4 neurologic toxicities, the most common of which were dysphasia (n=9) and confusion (n=8).
The researchers said all acute toxicities resolved completely, and none of the patients died as a result of toxicity.
One patient experienced vision loss 3 months after receiving CAR T-cell therapy. The researchers said they could not confirm the cause of the vision loss, but it is consistent with fludarabine toxicity.
One patient developed myelodysplastic syndrome, which was thought to be related to prior therapy.
The researchers noted that patients who experienced grade 3/4 neurologic toxicity had significantly higher levels of blood CAR+ cells than patients who had neurologic toxicities of a lower grade (P=0.003).
In addition, peak levels of serum IL-10 and IL-15 were higher in patients with grade 3/4 neurologic toxicities (P=0.006 and 0.014, respectively).
When given after low-dose chemotherapy, an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy can produce durable complete responses (CRs) in patients with relapsed/refractory non-Hodgkin lymphoma (NHL), according to research published in the Journal of Clinical Oncology.
In this phase 1 study, the overall response rate was 73%, and 50% of patients had an ongoing CR at last follow-up.
Fifty-five percent of patients experienced grade 3/4 neurologic toxicities, though these events eventually resolved.
This research was conducted under a cooperative research and development agreement between the National Cancer Institute and Kite Pharma, Inc.
Kite is developing the CAR T-cell therapy axicabtagene ciloleucel (formerly known as KTE-C19), and the therapy tested in this trial has the same CAR construct as axicabtagene ciloleucel.
Results from this study (NCT00924326) were previously published in the Journal of Clinical Oncology in 2014.
The current report included 22 patients with relapsed/refractory NHL. Seventeen patients had diffuse large B-cell lymphoma (DLBCL), 2 had primary mediastinal B-cell lymphoma (PMBCL), 2 had follicular lymphoma (FL), and 1 had mantle cell lymphoma (MCL).
Patients received a single dose of CAR T cells 2 days after a low-dose chemotherapy conditioning regimen consisting of cyclophosphamide and fludarabine.
Response
The overall response rate was 73% (16/22), with a CR rate of 55% (n=12) and a partial response (PR) rate of 18% (n=4).
Among patients with DLBCL, there were 9 CRs, 4 PRs, 1 patient with stable disease, and 3 patients with progressive disease.
Both FL patients achieved a CR, as did the patient with MCL. One patient with PMBCL had stable disease, and the other progressed.
Eleven of the 12 CRs are ongoing, with durations ranging from more than 7 months to more than 24 months. The median duration of CR is 12.5 months.
The researchers found that serum IL-15 levels and CAR T-cell expansion correlated with treatment response (CR or PR).
The median peak blood CAR+ cell level was 98/μL in patients who achieved a response and 15/μL in those who did not (P=0.027).
High serum IL-15 levels were significantly associated with high peak blood CAR+ cell levels (P=0.001) and response (P<0.001).
Toxicity
Fifty-five percent of patients had grade 3 or 4 neurologic toxicities, the most common of which were dysphasia (n=9) and confusion (n=8).
The researchers said all acute toxicities resolved completely, and none of the patients died as a result of toxicity.
One patient experienced vision loss 3 months after receiving CAR T-cell therapy. The researchers said they could not confirm the cause of the vision loss, but it is consistent with fludarabine toxicity.
One patient developed myelodysplastic syndrome, which was thought to be related to prior therapy.
The researchers noted that patients who experienced grade 3/4 neurologic toxicity had significantly higher levels of blood CAR+ cells than patients who had neurologic toxicities of a lower grade (P=0.003).
In addition, peak levels of serum IL-10 and IL-15 were higher in patients with grade 3/4 neurologic toxicities (P=0.006 and 0.014, respectively).
When given after low-dose chemotherapy, an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy can produce durable complete responses (CRs) in patients with relapsed/refractory non-Hodgkin lymphoma (NHL), according to research published in the Journal of Clinical Oncology.
In this phase 1 study, the overall response rate was 73%, and 50% of patients had an ongoing CR at last follow-up.
Fifty-five percent of patients experienced grade 3/4 neurologic toxicities, though these events eventually resolved.
This research was conducted under a cooperative research and development agreement between the National Cancer Institute and Kite Pharma, Inc.
Kite is developing the CAR T-cell therapy axicabtagene ciloleucel (formerly known as KTE-C19), and the therapy tested in this trial has the same CAR construct as axicabtagene ciloleucel.
Results from this study (NCT00924326) were previously published in the Journal of Clinical Oncology in 2014.
The current report included 22 patients with relapsed/refractory NHL. Seventeen patients had diffuse large B-cell lymphoma (DLBCL), 2 had primary mediastinal B-cell lymphoma (PMBCL), 2 had follicular lymphoma (FL), and 1 had mantle cell lymphoma (MCL).
Patients received a single dose of CAR T cells 2 days after a low-dose chemotherapy conditioning regimen consisting of cyclophosphamide and fludarabine.
Response
The overall response rate was 73% (16/22), with a CR rate of 55% (n=12) and a partial response (PR) rate of 18% (n=4).
Among patients with DLBCL, there were 9 CRs, 4 PRs, 1 patient with stable disease, and 3 patients with progressive disease.
Both FL patients achieved a CR, as did the patient with MCL. One patient with PMBCL had stable disease, and the other progressed.
Eleven of the 12 CRs are ongoing, with durations ranging from more than 7 months to more than 24 months. The median duration of CR is 12.5 months.
The researchers found that serum IL-15 levels and CAR T-cell expansion correlated with treatment response (CR or PR).
The median peak blood CAR+ cell level was 98/μL in patients who achieved a response and 15/μL in those who did not (P=0.027).
High serum IL-15 levels were significantly associated with high peak blood CAR+ cell levels (P=0.001) and response (P<0.001).
Toxicity
Fifty-five percent of patients had grade 3 or 4 neurologic toxicities, the most common of which were dysphasia (n=9) and confusion (n=8).
The researchers said all acute toxicities resolved completely, and none of the patients died as a result of toxicity.
One patient experienced vision loss 3 months after receiving CAR T-cell therapy. The researchers said they could not confirm the cause of the vision loss, but it is consistent with fludarabine toxicity.
One patient developed myelodysplastic syndrome, which was thought to be related to prior therapy.
The researchers noted that patients who experienced grade 3/4 neurologic toxicity had significantly higher levels of blood CAR+ cells than patients who had neurologic toxicities of a lower grade (P=0.003).
In addition, peak levels of serum IL-10 and IL-15 were higher in patients with grade 3/4 neurologic toxicities (P=0.006 and 0.014, respectively).