User login
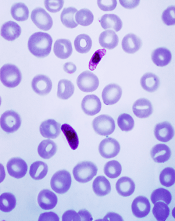
Vaccine can fight different malaria strains
An investigational malaria vaccine can protect healthy adults from infection with a malaria strain different from that contained in the vaccine, according to a phase 1 study published in PNAS.
The vaccine, known as the PfSPZ Vaccine, contains weakened Plasmodium falciparum sporozoites that are able to generate a protective immune response against live malaria infection.
Prior research showed that the PfSPZ Vaccine can provide long-term protection against a single malaria strain matched to the vaccine.
The new study has shown that the PfSPZ Vaccine can protect against a different strain of P falciparum as well.
“An effective malaria vaccine will need to protect people living in endemic areas against multiple strains of the mosquito-borne disease,” said Anthony S. Fauci, MD, of the National Institute of Allergy and Infectious Diseases (NIAID) in Bethesda, Maryland.
“These new findings showing cross-protection with the PfSPZ Vaccine suggest that it may be able to accomplish this goal.”
The PfSPZ Vaccine was developed by Sanaria Inc. The company designed, manufactured, and provided PfSPZ Vaccine and the heterologous challenge mosquitoes for this trial. The NIAID supported the development of the vaccine through several grants.
Study details
The study enrolled 31 healthy, malaria-naive adults ages 19 to 45.
Fifteen subjects were scheduled to receive 3 doses of the PfSPZ Vaccine—9.0 × 105 PfSPZ administered intravenously 3 times at 8-week intervals. The remaining subjects served as controls.
Nineteen weeks after receiving the final dose of the test vaccine, vaccinated subjects and controls were exposed to bites from mosquitoes infected with the same strain of P falciparum parasites (NF54) that were used to manufacture PfSPZ Vaccine.
Nine of the 14 subjects (64%) who received PfSPZ Vaccine demonstrated no evidence of malaria parasites. All 6 of the non-vaccinated subjects who were challenged at the same time had malaria parasites in their blood.
Of the 9 subjects who showed no evidence of malaria, 6 subjects were again exposed to mosquito bites, this time from mosquitoes infected with a different strain of P falciparum (Pf7G8), 33 weeks after the final immunization.
In this group, 5 of the 6 subjects (83%) were protected against malaria infection. None of the 6 control subjects who were challenged were protected.
“Achieving durable protection against a malaria strain different from the vaccine strain, over 8 months after vaccination, is an indication of this vaccine’s potential,” said Robert A. Seder, MD, of NIAID.
“If we can build on these findings with the PfSPZ Vaccine and induce higher efficacy, we may be on our way to a vaccine that could effectively protect people against a variety of malaria parasites where the disease is prevalent.”
The researchers found the PfSPZ Vaccine activated T cells and induced antibody responses in all vaccine recipients. Vaccine-specific T-cell responses were comparable when measured against both malaria challenge strains, providing some insight into how the vaccine was mediating protection.
Ongoing research should determine whether protective efficacy can be improved by changes to the PfSPZ Vaccine dose and number of immunizations.
A phase 2 trial testing 3 different dosages in a 3-dose vaccine regimen is now underway in 5-to 12-month-old infants in Western Kenya to assess safety and efficacy of the vaccine against natural infection. ![]()
An investigational malaria vaccine can protect healthy adults from infection with a malaria strain different from that contained in the vaccine, according to a phase 1 study published in PNAS.
The vaccine, known as the PfSPZ Vaccine, contains weakened Plasmodium falciparum sporozoites that are able to generate a protective immune response against live malaria infection.
Prior research showed that the PfSPZ Vaccine can provide long-term protection against a single malaria strain matched to the vaccine.
The new study has shown that the PfSPZ Vaccine can protect against a different strain of P falciparum as well.
“An effective malaria vaccine will need to protect people living in endemic areas against multiple strains of the mosquito-borne disease,” said Anthony S. Fauci, MD, of the National Institute of Allergy and Infectious Diseases (NIAID) in Bethesda, Maryland.
“These new findings showing cross-protection with the PfSPZ Vaccine suggest that it may be able to accomplish this goal.”
The PfSPZ Vaccine was developed by Sanaria Inc. The company designed, manufactured, and provided PfSPZ Vaccine and the heterologous challenge mosquitoes for this trial. The NIAID supported the development of the vaccine through several grants.
Study details
The study enrolled 31 healthy, malaria-naive adults ages 19 to 45.
Fifteen subjects were scheduled to receive 3 doses of the PfSPZ Vaccine—9.0 × 105 PfSPZ administered intravenously 3 times at 8-week intervals. The remaining subjects served as controls.
Nineteen weeks after receiving the final dose of the test vaccine, vaccinated subjects and controls were exposed to bites from mosquitoes infected with the same strain of P falciparum parasites (NF54) that were used to manufacture PfSPZ Vaccine.
Nine of the 14 subjects (64%) who received PfSPZ Vaccine demonstrated no evidence of malaria parasites. All 6 of the non-vaccinated subjects who were challenged at the same time had malaria parasites in their blood.
Of the 9 subjects who showed no evidence of malaria, 6 subjects were again exposed to mosquito bites, this time from mosquitoes infected with a different strain of P falciparum (Pf7G8), 33 weeks after the final immunization.
In this group, 5 of the 6 subjects (83%) were protected against malaria infection. None of the 6 control subjects who were challenged were protected.
“Achieving durable protection against a malaria strain different from the vaccine strain, over 8 months after vaccination, is an indication of this vaccine’s potential,” said Robert A. Seder, MD, of NIAID.
“If we can build on these findings with the PfSPZ Vaccine and induce higher efficacy, we may be on our way to a vaccine that could effectively protect people against a variety of malaria parasites where the disease is prevalent.”
The researchers found the PfSPZ Vaccine activated T cells and induced antibody responses in all vaccine recipients. Vaccine-specific T-cell responses were comparable when measured against both malaria challenge strains, providing some insight into how the vaccine was mediating protection.
Ongoing research should determine whether protective efficacy can be improved by changes to the PfSPZ Vaccine dose and number of immunizations.
A phase 2 trial testing 3 different dosages in a 3-dose vaccine regimen is now underway in 5-to 12-month-old infants in Western Kenya to assess safety and efficacy of the vaccine against natural infection. ![]()
An investigational malaria vaccine can protect healthy adults from infection with a malaria strain different from that contained in the vaccine, according to a phase 1 study published in PNAS.
The vaccine, known as the PfSPZ Vaccine, contains weakened Plasmodium falciparum sporozoites that are able to generate a protective immune response against live malaria infection.
Prior research showed that the PfSPZ Vaccine can provide long-term protection against a single malaria strain matched to the vaccine.
The new study has shown that the PfSPZ Vaccine can protect against a different strain of P falciparum as well.
“An effective malaria vaccine will need to protect people living in endemic areas against multiple strains of the mosquito-borne disease,” said Anthony S. Fauci, MD, of the National Institute of Allergy and Infectious Diseases (NIAID) in Bethesda, Maryland.
“These new findings showing cross-protection with the PfSPZ Vaccine suggest that it may be able to accomplish this goal.”
The PfSPZ Vaccine was developed by Sanaria Inc. The company designed, manufactured, and provided PfSPZ Vaccine and the heterologous challenge mosquitoes for this trial. The NIAID supported the development of the vaccine through several grants.
Study details
The study enrolled 31 healthy, malaria-naive adults ages 19 to 45.
Fifteen subjects were scheduled to receive 3 doses of the PfSPZ Vaccine—9.0 × 105 PfSPZ administered intravenously 3 times at 8-week intervals. The remaining subjects served as controls.
Nineteen weeks after receiving the final dose of the test vaccine, vaccinated subjects and controls were exposed to bites from mosquitoes infected with the same strain of P falciparum parasites (NF54) that were used to manufacture PfSPZ Vaccine.
Nine of the 14 subjects (64%) who received PfSPZ Vaccine demonstrated no evidence of malaria parasites. All 6 of the non-vaccinated subjects who were challenged at the same time had malaria parasites in their blood.
Of the 9 subjects who showed no evidence of malaria, 6 subjects were again exposed to mosquito bites, this time from mosquitoes infected with a different strain of P falciparum (Pf7G8), 33 weeks after the final immunization.
In this group, 5 of the 6 subjects (83%) were protected against malaria infection. None of the 6 control subjects who were challenged were protected.
“Achieving durable protection against a malaria strain different from the vaccine strain, over 8 months after vaccination, is an indication of this vaccine’s potential,” said Robert A. Seder, MD, of NIAID.
“If we can build on these findings with the PfSPZ Vaccine and induce higher efficacy, we may be on our way to a vaccine that could effectively protect people against a variety of malaria parasites where the disease is prevalent.”
The researchers found the PfSPZ Vaccine activated T cells and induced antibody responses in all vaccine recipients. Vaccine-specific T-cell responses were comparable when measured against both malaria challenge strains, providing some insight into how the vaccine was mediating protection.
Ongoing research should determine whether protective efficacy can be improved by changes to the PfSPZ Vaccine dose and number of immunizations.
A phase 2 trial testing 3 different dosages in a 3-dose vaccine regimen is now underway in 5-to 12-month-old infants in Western Kenya to assess safety and efficacy of the vaccine against natural infection. ![]()
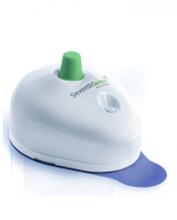
FDA clears push-button blood draw device
The US Food and Drug Administration (FDA) has granted 510(k) clearance for a push-button blood collection device called TAP.
It provides “virtually painless, simple, and fast” blood collection, according to Seventh Sense Biosystems, Inc., the company marketing the device.
The FDA clearance allows healthcare workers to use TAP only for the collection of capillary blood for hemoglobin A1c testing.
However, Seventh Sense Biosystems said it is working with the FDA to expand the use of TAP to add additional tests, as well as at-home blood collection.
How TAP works
The TAP device is placed on a patient’s upper arm, and blood collection starts with the press of a button. The process typically takes 2 to 3 minutes.
When TAP is placed on the arm, the base of the device generates a seal with the skin to create a vacuum. When the user presses the button, TAP deploys microneedles that puncture the skin above the dermis.
The vacuum draws capillary blood out of the micropunctures and through microfluidic channels into a collection chamber prefilled with lithium heparin.
TAP collects up to 100 μL of blood. To transfer the blood to an analyzer, a precision pipette is placed in an access port at the bottom of the device.
Seventh Sense Biosystems said it will launch TAP over the coming months. For up-to-date information, visit www.7sbio.com. ![]()
The US Food and Drug Administration (FDA) has granted 510(k) clearance for a push-button blood collection device called TAP.
It provides “virtually painless, simple, and fast” blood collection, according to Seventh Sense Biosystems, Inc., the company marketing the device.
The FDA clearance allows healthcare workers to use TAP only for the collection of capillary blood for hemoglobin A1c testing.
However, Seventh Sense Biosystems said it is working with the FDA to expand the use of TAP to add additional tests, as well as at-home blood collection.
How TAP works
The TAP device is placed on a patient’s upper arm, and blood collection starts with the press of a button. The process typically takes 2 to 3 minutes.
When TAP is placed on the arm, the base of the device generates a seal with the skin to create a vacuum. When the user presses the button, TAP deploys microneedles that puncture the skin above the dermis.
The vacuum draws capillary blood out of the micropunctures and through microfluidic channels into a collection chamber prefilled with lithium heparin.
TAP collects up to 100 μL of blood. To transfer the blood to an analyzer, a precision pipette is placed in an access port at the bottom of the device.
Seventh Sense Biosystems said it will launch TAP over the coming months. For up-to-date information, visit www.7sbio.com. ![]()
The US Food and Drug Administration (FDA) has granted 510(k) clearance for a push-button blood collection device called TAP.
It provides “virtually painless, simple, and fast” blood collection, according to Seventh Sense Biosystems, Inc., the company marketing the device.
The FDA clearance allows healthcare workers to use TAP only for the collection of capillary blood for hemoglobin A1c testing.
However, Seventh Sense Biosystems said it is working with the FDA to expand the use of TAP to add additional tests, as well as at-home blood collection.
How TAP works
The TAP device is placed on a patient’s upper arm, and blood collection starts with the press of a button. The process typically takes 2 to 3 minutes.
When TAP is placed on the arm, the base of the device generates a seal with the skin to create a vacuum. When the user presses the button, TAP deploys microneedles that puncture the skin above the dermis.
The vacuum draws capillary blood out of the micropunctures and through microfluidic channels into a collection chamber prefilled with lithium heparin.
TAP collects up to 100 μL of blood. To transfer the blood to an analyzer, a precision pipette is placed in an access port at the bottom of the device.
Seventh Sense Biosystems said it will launch TAP over the coming months. For up-to-date information, visit www.7sbio.com. ![]()
Inpatient palliative care improves QOL for HSCT patients
ORLANDO, FL—New research shows that patients who received inpatient palliative care while undergoing hematopoietic stem cell transplant (HSCT) experienced significant improvements in quality of life (QOL), decreases in depression, and reductions in symptom burden compared to patients who received transplant care alone.
Areej R. El-Jawahri, MD, of Harvard Medical School in Boston, Massachusetts, presented these results at the 2017 BMT Tandem Meetings (abstract 49).
She noted that palliative care is rarely used for patients with hematologic malignancies, “in part, because of misperceptions equating palliative care with just end-of-life care.”
So Dr El-Jawahri and her colleagues decided to evaluate palliative care in patients with hematologic malignancies who were scheduled to undergo HSCT.
The researchers enrolled 160 patients on the trial. Eighty-one were randomized to receive inpatient palliative care integrated with transplant care (intervention arm), and 79 were randomized to transplant care alone (control).
The latter group could request palliative care consultations, but only 2 patients did so, Dr El-Jawahri pointed out.
Patients receiving the intervention had at least twice-weekly visits with a palliative care clinician throughout their hospitalization.
“Importantly, palliative care only followed patients during their transplant hospitalization,” Dr El-Jawahri noted. “This was purely an inpatient palliative care intervention.”
Palliative care focused primarily on managing patients’ symptoms, establishing rapport with patients and families, and helping them cope with their illness. The predominant symptoms addressed included pain, nausea, diarrhea, constipation, insomnia, fatigue, depression, and anxiety.
Researchers assessed QOL, symptom burden, and mood at baseline, during hospitalization (Week 2), and at 3 and 6 months using well-validated scales.
They assessed QOL using the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale, mood using the Hospital Anxiety and Depression Scale (HADS) and Patient Health Questionnaire (PHQ-9), and symptom burden using the Edmonton Symptom Assessment Scale (ESAS).
They also measured post-traumatic stress (PTSD) at baseline as well as 3 and 6 months after HSCT using the PTSD checklist.
The primary endpoint of the study was patient-reported QOL at Week 2 of hospitalization. Researchers chose Week 2 because studies have shown the highest symptom burden and QOL deterioration during that period.
Demographics
Patients were a mean age of 57, and a little more than half were female. Most were white, had a college degree or higher, and were married.
Their diagnoses included, for the control and intervention arms, respectively: acute lymphoblastic leukemia (9%, 5%), acute myeloid leukemia/myelodysplastic syndromes (30%, 30%), myelofibrosis/chronic myeloid leukemia (9%, 10%), lymphoma (33%, 23%), and multiple myeloma (19%, 33%).
Results
At baseline, patients in each group had comparable QOL and mood scores.
However, at Week 2, after ANCOVA adjustment for baseline scores, patients in the intervention arm had a clinically and statistically significant effect of the intervention in all areas measured except for the PHQ-9 depression score.
In particular, the HADS depression and anxiety scores were significantly improved, at P=0.008 and P<0.001, respectively, compared to control.
At 3 months, the FACT-BMT (P=0.048), HADS-Depression (P=0.002), PHQ-9-Depression (P=0.002), and PTSD symptom (P=0.002) scores were significantly improved in the intervention group.
And at 6 months, the HADS-Depression assessment (P=0.024), the PHQ-9-Depression assessment (P=0.027), and the PTSD symptom assessment (P=0.013) remained significantly improved. However, there were no significant differences in anxiety between the 2 groups.
The researchers concluded that a relatively brief inpatient care intervention led to “remarkable sustained improvements” in depression and post-traumatic stress symptoms at 3 and 6 months after HSCT.
“This is the first study showing the benefits of palliative care for patients with hematologic malignancies undergoing stem cell transplant,” Dr El-Jawahri said.
“It’s also the first study showing the benefits of palliative care for patients with cancer pursuing curative therapy and extends the potential benefit of palliative care in a population of patients with serious illness. [T]he significant part of what palliative care does is helping patients and families cope with serious and potentially life-threatening illness.”
The researchers recommend future studies to evaluate the impact of early integration of palliative care for this patient population. ![]()
ORLANDO, FL—New research shows that patients who received inpatient palliative care while undergoing hematopoietic stem cell transplant (HSCT) experienced significant improvements in quality of life (QOL), decreases in depression, and reductions in symptom burden compared to patients who received transplant care alone.
Areej R. El-Jawahri, MD, of Harvard Medical School in Boston, Massachusetts, presented these results at the 2017 BMT Tandem Meetings (abstract 49).
She noted that palliative care is rarely used for patients with hematologic malignancies, “in part, because of misperceptions equating palliative care with just end-of-life care.”
So Dr El-Jawahri and her colleagues decided to evaluate palliative care in patients with hematologic malignancies who were scheduled to undergo HSCT.
The researchers enrolled 160 patients on the trial. Eighty-one were randomized to receive inpatient palliative care integrated with transplant care (intervention arm), and 79 were randomized to transplant care alone (control).
The latter group could request palliative care consultations, but only 2 patients did so, Dr El-Jawahri pointed out.
Patients receiving the intervention had at least twice-weekly visits with a palliative care clinician throughout their hospitalization.
“Importantly, palliative care only followed patients during their transplant hospitalization,” Dr El-Jawahri noted. “This was purely an inpatient palliative care intervention.”
Palliative care focused primarily on managing patients’ symptoms, establishing rapport with patients and families, and helping them cope with their illness. The predominant symptoms addressed included pain, nausea, diarrhea, constipation, insomnia, fatigue, depression, and anxiety.
Researchers assessed QOL, symptom burden, and mood at baseline, during hospitalization (Week 2), and at 3 and 6 months using well-validated scales.
They assessed QOL using the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale, mood using the Hospital Anxiety and Depression Scale (HADS) and Patient Health Questionnaire (PHQ-9), and symptom burden using the Edmonton Symptom Assessment Scale (ESAS).
They also measured post-traumatic stress (PTSD) at baseline as well as 3 and 6 months after HSCT using the PTSD checklist.
The primary endpoint of the study was patient-reported QOL at Week 2 of hospitalization. Researchers chose Week 2 because studies have shown the highest symptom burden and QOL deterioration during that period.
Demographics
Patients were a mean age of 57, and a little more than half were female. Most were white, had a college degree or higher, and were married.
Their diagnoses included, for the control and intervention arms, respectively: acute lymphoblastic leukemia (9%, 5%), acute myeloid leukemia/myelodysplastic syndromes (30%, 30%), myelofibrosis/chronic myeloid leukemia (9%, 10%), lymphoma (33%, 23%), and multiple myeloma (19%, 33%).
Results
At baseline, patients in each group had comparable QOL and mood scores.
However, at Week 2, after ANCOVA adjustment for baseline scores, patients in the intervention arm had a clinically and statistically significant effect of the intervention in all areas measured except for the PHQ-9 depression score.
In particular, the HADS depression and anxiety scores were significantly improved, at P=0.008 and P<0.001, respectively, compared to control.
At 3 months, the FACT-BMT (P=0.048), HADS-Depression (P=0.002), PHQ-9-Depression (P=0.002), and PTSD symptom (P=0.002) scores were significantly improved in the intervention group.
And at 6 months, the HADS-Depression assessment (P=0.024), the PHQ-9-Depression assessment (P=0.027), and the PTSD symptom assessment (P=0.013) remained significantly improved. However, there were no significant differences in anxiety between the 2 groups.
The researchers concluded that a relatively brief inpatient care intervention led to “remarkable sustained improvements” in depression and post-traumatic stress symptoms at 3 and 6 months after HSCT.
“This is the first study showing the benefits of palliative care for patients with hematologic malignancies undergoing stem cell transplant,” Dr El-Jawahri said.
“It’s also the first study showing the benefits of palliative care for patients with cancer pursuing curative therapy and extends the potential benefit of palliative care in a population of patients with serious illness. [T]he significant part of what palliative care does is helping patients and families cope with serious and potentially life-threatening illness.”
The researchers recommend future studies to evaluate the impact of early integration of palliative care for this patient population. ![]()
ORLANDO, FL—New research shows that patients who received inpatient palliative care while undergoing hematopoietic stem cell transplant (HSCT) experienced significant improvements in quality of life (QOL), decreases in depression, and reductions in symptom burden compared to patients who received transplant care alone.
Areej R. El-Jawahri, MD, of Harvard Medical School in Boston, Massachusetts, presented these results at the 2017 BMT Tandem Meetings (abstract 49).
She noted that palliative care is rarely used for patients with hematologic malignancies, “in part, because of misperceptions equating palliative care with just end-of-life care.”
So Dr El-Jawahri and her colleagues decided to evaluate palliative care in patients with hematologic malignancies who were scheduled to undergo HSCT.
The researchers enrolled 160 patients on the trial. Eighty-one were randomized to receive inpatient palliative care integrated with transplant care (intervention arm), and 79 were randomized to transplant care alone (control).
The latter group could request palliative care consultations, but only 2 patients did so, Dr El-Jawahri pointed out.
Patients receiving the intervention had at least twice-weekly visits with a palliative care clinician throughout their hospitalization.
“Importantly, palliative care only followed patients during their transplant hospitalization,” Dr El-Jawahri noted. “This was purely an inpatient palliative care intervention.”
Palliative care focused primarily on managing patients’ symptoms, establishing rapport with patients and families, and helping them cope with their illness. The predominant symptoms addressed included pain, nausea, diarrhea, constipation, insomnia, fatigue, depression, and anxiety.
Researchers assessed QOL, symptom burden, and mood at baseline, during hospitalization (Week 2), and at 3 and 6 months using well-validated scales.
They assessed QOL using the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale, mood using the Hospital Anxiety and Depression Scale (HADS) and Patient Health Questionnaire (PHQ-9), and symptom burden using the Edmonton Symptom Assessment Scale (ESAS).
They also measured post-traumatic stress (PTSD) at baseline as well as 3 and 6 months after HSCT using the PTSD checklist.
The primary endpoint of the study was patient-reported QOL at Week 2 of hospitalization. Researchers chose Week 2 because studies have shown the highest symptom burden and QOL deterioration during that period.
Demographics
Patients were a mean age of 57, and a little more than half were female. Most were white, had a college degree or higher, and were married.
Their diagnoses included, for the control and intervention arms, respectively: acute lymphoblastic leukemia (9%, 5%), acute myeloid leukemia/myelodysplastic syndromes (30%, 30%), myelofibrosis/chronic myeloid leukemia (9%, 10%), lymphoma (33%, 23%), and multiple myeloma (19%, 33%).
Results
At baseline, patients in each group had comparable QOL and mood scores.
However, at Week 2, after ANCOVA adjustment for baseline scores, patients in the intervention arm had a clinically and statistically significant effect of the intervention in all areas measured except for the PHQ-9 depression score.
In particular, the HADS depression and anxiety scores were significantly improved, at P=0.008 and P<0.001, respectively, compared to control.
At 3 months, the FACT-BMT (P=0.048), HADS-Depression (P=0.002), PHQ-9-Depression (P=0.002), and PTSD symptom (P=0.002) scores were significantly improved in the intervention group.
And at 6 months, the HADS-Depression assessment (P=0.024), the PHQ-9-Depression assessment (P=0.027), and the PTSD symptom assessment (P=0.013) remained significantly improved. However, there were no significant differences in anxiety between the 2 groups.
The researchers concluded that a relatively brief inpatient care intervention led to “remarkable sustained improvements” in depression and post-traumatic stress symptoms at 3 and 6 months after HSCT.
“This is the first study showing the benefits of palliative care for patients with hematologic malignancies undergoing stem cell transplant,” Dr El-Jawahri said.
“It’s also the first study showing the benefits of palliative care for patients with cancer pursuing curative therapy and extends the potential benefit of palliative care in a population of patients with serious illness. [T]he significant part of what palliative care does is helping patients and families cope with serious and potentially life-threatening illness.”
The researchers recommend future studies to evaluate the impact of early integration of palliative care for this patient population. ![]()
CHMP recommends authorization of antiemetic agent
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for the antiemetic agent rolapitant (Varuby) as a treatment for adults with cancer.
The drug is intended to be used in combination with other antiemetic agents to prevent delayed nausea and vomiting associated with highly and moderately emetogenic chemotherapy.
The CHMP’s recommendation regarding rolapitant has been forwarded to the European Commission, which is expected to make a decision about the drug within 2 months.
If the commission authorizes marketing of rolapitant, the drug will be available as 90 mg film-coated tablets.
The applicant for rolapitant is Tesaro UK Limited.
Rolapitant clinical trials
Results from three phase 3 trials suggested that rolapitant (at 180 mg) in combination with a 5-HT3 receptor antagonist and dexamethasone was more effective than the 5-HT3 receptor antagonist and dexamethasone on their own (active control).
The 3-drug combination demonstrated a significant reduction in episodes of vomiting or use of rescue medication during the 25- to 120-hour period following administration of highly emetogenic and moderately emetogenic chemotherapy regimens.
In addition, patients who received rolapitant reported experiencing less nausea that interfered with normal daily life and fewer episodes of vomiting or retching over multiple cycles of chemotherapy.
Highly emetogenic chemotherapy
The clinical profile of rolapitant in cisplatin-based, highly emetogenic chemotherapy (HEC) was confirmed in two phase 3 studies: HEC1 and HEC2. Results from these trials were published in The Lancet Oncology in August 2015.
Both trials met their primary endpoint of complete response (CR) and demonstrated statistical superiority of the rolapitant combination compared to active control.
In HEC1, 264 patients received the rolapitant combination, and 262 received active control. The proportion of patients achieving a CR was 72.7% and 58.4%, respectively (P<0.001).
In HEC2, 271 patients received the rolapitant combination, and 273 received active control. The proportion of patients achieving a CR was 70.1% and 61.9%, respectively (P=0.043).
The most common adverse events (in the rolapitant and control groups, respectively) were neutropenia (9% and 8%), hiccups (5% and 4%), and abdominal pain (3% and 2%).
Moderately emetogenic chemotherapy
Researchers conducted another phase 3 trial to compare the rolapitant combination with active control in 1332 patients receiving moderately emetogenic chemotherapy. Results from this trial were also published in The Lancet Oncology in August 2015.
This trial met its primary endpoint of CR and demonstrated statistical superiority of the rolapitant combination compared to active control. The proportion of patients achieving a CR was 71.3% and 61.6%, respectively (P<0.001).
The most common adverse events (in the rolapitant and control groups, respectively) were decreased appetite (9% and 7%), neutropenia (7% and 6%), dizziness (6% and 4%), dyspepsia (4% and 2%), urinary tract infection (4% and 3%), stomatitis (4% and 2%), and anemia (3% and 2%). ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for the antiemetic agent rolapitant (Varuby) as a treatment for adults with cancer.
The drug is intended to be used in combination with other antiemetic agents to prevent delayed nausea and vomiting associated with highly and moderately emetogenic chemotherapy.
The CHMP’s recommendation regarding rolapitant has been forwarded to the European Commission, which is expected to make a decision about the drug within 2 months.
If the commission authorizes marketing of rolapitant, the drug will be available as 90 mg film-coated tablets.
The applicant for rolapitant is Tesaro UK Limited.
Rolapitant clinical trials
Results from three phase 3 trials suggested that rolapitant (at 180 mg) in combination with a 5-HT3 receptor antagonist and dexamethasone was more effective than the 5-HT3 receptor antagonist and dexamethasone on their own (active control).
The 3-drug combination demonstrated a significant reduction in episodes of vomiting or use of rescue medication during the 25- to 120-hour period following administration of highly emetogenic and moderately emetogenic chemotherapy regimens.
In addition, patients who received rolapitant reported experiencing less nausea that interfered with normal daily life and fewer episodes of vomiting or retching over multiple cycles of chemotherapy.
Highly emetogenic chemotherapy
The clinical profile of rolapitant in cisplatin-based, highly emetogenic chemotherapy (HEC) was confirmed in two phase 3 studies: HEC1 and HEC2. Results from these trials were published in The Lancet Oncology in August 2015.
Both trials met their primary endpoint of complete response (CR) and demonstrated statistical superiority of the rolapitant combination compared to active control.
In HEC1, 264 patients received the rolapitant combination, and 262 received active control. The proportion of patients achieving a CR was 72.7% and 58.4%, respectively (P<0.001).
In HEC2, 271 patients received the rolapitant combination, and 273 received active control. The proportion of patients achieving a CR was 70.1% and 61.9%, respectively (P=0.043).
The most common adverse events (in the rolapitant and control groups, respectively) were neutropenia (9% and 8%), hiccups (5% and 4%), and abdominal pain (3% and 2%).
Moderately emetogenic chemotherapy
Researchers conducted another phase 3 trial to compare the rolapitant combination with active control in 1332 patients receiving moderately emetogenic chemotherapy. Results from this trial were also published in The Lancet Oncology in August 2015.
This trial met its primary endpoint of CR and demonstrated statistical superiority of the rolapitant combination compared to active control. The proportion of patients achieving a CR was 71.3% and 61.6%, respectively (P<0.001).
The most common adverse events (in the rolapitant and control groups, respectively) were decreased appetite (9% and 7%), neutropenia (7% and 6%), dizziness (6% and 4%), dyspepsia (4% and 2%), urinary tract infection (4% and 3%), stomatitis (4% and 2%), and anemia (3% and 2%). ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for the antiemetic agent rolapitant (Varuby) as a treatment for adults with cancer.
The drug is intended to be used in combination with other antiemetic agents to prevent delayed nausea and vomiting associated with highly and moderately emetogenic chemotherapy.
The CHMP’s recommendation regarding rolapitant has been forwarded to the European Commission, which is expected to make a decision about the drug within 2 months.
If the commission authorizes marketing of rolapitant, the drug will be available as 90 mg film-coated tablets.
The applicant for rolapitant is Tesaro UK Limited.
Rolapitant clinical trials
Results from three phase 3 trials suggested that rolapitant (at 180 mg) in combination with a 5-HT3 receptor antagonist and dexamethasone was more effective than the 5-HT3 receptor antagonist and dexamethasone on their own (active control).
The 3-drug combination demonstrated a significant reduction in episodes of vomiting or use of rescue medication during the 25- to 120-hour period following administration of highly emetogenic and moderately emetogenic chemotherapy regimens.
In addition, patients who received rolapitant reported experiencing less nausea that interfered with normal daily life and fewer episodes of vomiting or retching over multiple cycles of chemotherapy.
Highly emetogenic chemotherapy
The clinical profile of rolapitant in cisplatin-based, highly emetogenic chemotherapy (HEC) was confirmed in two phase 3 studies: HEC1 and HEC2. Results from these trials were published in The Lancet Oncology in August 2015.
Both trials met their primary endpoint of complete response (CR) and demonstrated statistical superiority of the rolapitant combination compared to active control.
In HEC1, 264 patients received the rolapitant combination, and 262 received active control. The proportion of patients achieving a CR was 72.7% and 58.4%, respectively (P<0.001).
In HEC2, 271 patients received the rolapitant combination, and 273 received active control. The proportion of patients achieving a CR was 70.1% and 61.9%, respectively (P=0.043).
The most common adverse events (in the rolapitant and control groups, respectively) were neutropenia (9% and 8%), hiccups (5% and 4%), and abdominal pain (3% and 2%).
Moderately emetogenic chemotherapy
Researchers conducted another phase 3 trial to compare the rolapitant combination with active control in 1332 patients receiving moderately emetogenic chemotherapy. Results from this trial were also published in The Lancet Oncology in August 2015.
This trial met its primary endpoint of CR and demonstrated statistical superiority of the rolapitant combination compared to active control. The proportion of patients achieving a CR was 71.3% and 61.6%, respectively (P<0.001).
The most common adverse events (in the rolapitant and control groups, respectively) were decreased appetite (9% and 7%), neutropenia (7% and 6%), dizziness (6% and 4%), dyspepsia (4% and 2%), urinary tract infection (4% and 3%), stomatitis (4% and 2%), and anemia (3% and 2%). ![]()
CHMP advocates approval of edoxaban product
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended that Roteas receive marketing authorization for the same indications as Lixiana.
Both Roteas and Lixiana are oral factor Xa inhibitors that contain the active ingredient edoxaban.
The CHMP has recommended approving Roteas for the treatment and prevention of deep vein thrombosis (DVT) and pulmonary embolism (PE) in adults.
In addition, the CHMP has recommended approving Roteas for the prevention of stroke and systemic embolism in adults with nonvalvular atrial fibrillation (NVAF) who have 1 or more risk factors, such as congestive heart failure, hypertension, age of 75 or older, diabetes mellitus, prior stroke, or transient ischemic attack.
The European Commission approved Lixiana for these indications in June 2015.
If the European Commission decides to approve Roteas as well, the product will be available as film-coated tablets (15 mg, 30 mg, and 60 mg).
The European Commission is expected to make a decision about Roteas within 67 days from the CHMP’s adoption of its opinion (which occurred on February 23).
The application for Roteas was an informed consent application. In this type of application, reference is made to an authorized medicine if the marketing authorization holder of the reference medicine has given consent to the use of their dossier in the application procedure.
The applicant for Roteas is Daiichi Sankyo Europe GmbH, the company that also developed Lixiana.
Lixiana was approved based on data from a pair of phase 3 trials, ENGAGE AF-TIMI 48 and Hokusai-VTE.
Hokusai-VTE
In the Hokusai-VTE trial, researchers evaluated edoxaban in 4921 patients with DVT and 3319 with PE. Patients received initial treatment with low-molecular-weight heparin and were then randomized to receive edoxaban or warfarin daily for 3 to 12 months.
Overall, edoxaban proved as effective as warfarin. Recurrent, symptomatic DVT/PE occurred in 3.2% and 3.5% of patients, respectively (P<0.001 for non-inferiority).
In addition, the incidence of clinically relevant bleeding was significantly lower in the edoxaban arm than the warfarin arm—8.5% and 10.3%, respectively (P=0.004 for superiority).
ENGAGE-AF TIMI 48
In the ENGAGE AF-TIMI 48 trial, researchers compared edoxaban and warfarin as prophylaxis for stroke or systemic embolism in patients with NVAF.
The trial included 21,105 patients who were randomized to receive warfarin (n=7036), edoxaban at 60 mg (n=7035), or edoxaban at 30 mg (n=7034).
Edoxaban was at least non-inferior to warfarin with regard to efficacy. The annual incidence of stroke or systemic embolism was 1.50% with warfarin, 1.18% with edoxaban at 60 mg (P<0.001 for non-inferiority), and 1.61% with edoxaban at 30 mg (P=0.005 for non-inferiority).
In addition, edoxaban was associated with a significantly lower rate of major and fatal bleeding. The annual incidence of major bleeding was 3.43% with warfarin, 2.75% with edoxaban at 60 mg (P<0.001), and 1.61% with edoxaban at 30 mg (P<0.001).
Fatal bleeds occurred at an annual rate of 0.38% with warfarin, 0.21% with edoxaban at 60 mg (P=0.006), and 0.13% with edoxaban at 30 mg (P<0.001). ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended that Roteas receive marketing authorization for the same indications as Lixiana.
Both Roteas and Lixiana are oral factor Xa inhibitors that contain the active ingredient edoxaban.
The CHMP has recommended approving Roteas for the treatment and prevention of deep vein thrombosis (DVT) and pulmonary embolism (PE) in adults.
In addition, the CHMP has recommended approving Roteas for the prevention of stroke and systemic embolism in adults with nonvalvular atrial fibrillation (NVAF) who have 1 or more risk factors, such as congestive heart failure, hypertension, age of 75 or older, diabetes mellitus, prior stroke, or transient ischemic attack.
The European Commission approved Lixiana for these indications in June 2015.
If the European Commission decides to approve Roteas as well, the product will be available as film-coated tablets (15 mg, 30 mg, and 60 mg).
The European Commission is expected to make a decision about Roteas within 67 days from the CHMP’s adoption of its opinion (which occurred on February 23).
The application for Roteas was an informed consent application. In this type of application, reference is made to an authorized medicine if the marketing authorization holder of the reference medicine has given consent to the use of their dossier in the application procedure.
The applicant for Roteas is Daiichi Sankyo Europe GmbH, the company that also developed Lixiana.
Lixiana was approved based on data from a pair of phase 3 trials, ENGAGE AF-TIMI 48 and Hokusai-VTE.
Hokusai-VTE
In the Hokusai-VTE trial, researchers evaluated edoxaban in 4921 patients with DVT and 3319 with PE. Patients received initial treatment with low-molecular-weight heparin and were then randomized to receive edoxaban or warfarin daily for 3 to 12 months.
Overall, edoxaban proved as effective as warfarin. Recurrent, symptomatic DVT/PE occurred in 3.2% and 3.5% of patients, respectively (P<0.001 for non-inferiority).
In addition, the incidence of clinically relevant bleeding was significantly lower in the edoxaban arm than the warfarin arm—8.5% and 10.3%, respectively (P=0.004 for superiority).
ENGAGE-AF TIMI 48
In the ENGAGE AF-TIMI 48 trial, researchers compared edoxaban and warfarin as prophylaxis for stroke or systemic embolism in patients with NVAF.
The trial included 21,105 patients who were randomized to receive warfarin (n=7036), edoxaban at 60 mg (n=7035), or edoxaban at 30 mg (n=7034).
Edoxaban was at least non-inferior to warfarin with regard to efficacy. The annual incidence of stroke or systemic embolism was 1.50% with warfarin, 1.18% with edoxaban at 60 mg (P<0.001 for non-inferiority), and 1.61% with edoxaban at 30 mg (P=0.005 for non-inferiority).
In addition, edoxaban was associated with a significantly lower rate of major and fatal bleeding. The annual incidence of major bleeding was 3.43% with warfarin, 2.75% with edoxaban at 60 mg (P<0.001), and 1.61% with edoxaban at 30 mg (P<0.001).
Fatal bleeds occurred at an annual rate of 0.38% with warfarin, 0.21% with edoxaban at 60 mg (P=0.006), and 0.13% with edoxaban at 30 mg (P<0.001). ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended that Roteas receive marketing authorization for the same indications as Lixiana.
Both Roteas and Lixiana are oral factor Xa inhibitors that contain the active ingredient edoxaban.
The CHMP has recommended approving Roteas for the treatment and prevention of deep vein thrombosis (DVT) and pulmonary embolism (PE) in adults.
In addition, the CHMP has recommended approving Roteas for the prevention of stroke and systemic embolism in adults with nonvalvular atrial fibrillation (NVAF) who have 1 or more risk factors, such as congestive heart failure, hypertension, age of 75 or older, diabetes mellitus, prior stroke, or transient ischemic attack.
The European Commission approved Lixiana for these indications in June 2015.
If the European Commission decides to approve Roteas as well, the product will be available as film-coated tablets (15 mg, 30 mg, and 60 mg).
The European Commission is expected to make a decision about Roteas within 67 days from the CHMP’s adoption of its opinion (which occurred on February 23).
The application for Roteas was an informed consent application. In this type of application, reference is made to an authorized medicine if the marketing authorization holder of the reference medicine has given consent to the use of their dossier in the application procedure.
The applicant for Roteas is Daiichi Sankyo Europe GmbH, the company that also developed Lixiana.
Lixiana was approved based on data from a pair of phase 3 trials, ENGAGE AF-TIMI 48 and Hokusai-VTE.
Hokusai-VTE
In the Hokusai-VTE trial, researchers evaluated edoxaban in 4921 patients with DVT and 3319 with PE. Patients received initial treatment with low-molecular-weight heparin and were then randomized to receive edoxaban or warfarin daily for 3 to 12 months.
Overall, edoxaban proved as effective as warfarin. Recurrent, symptomatic DVT/PE occurred in 3.2% and 3.5% of patients, respectively (P<0.001 for non-inferiority).
In addition, the incidence of clinically relevant bleeding was significantly lower in the edoxaban arm than the warfarin arm—8.5% and 10.3%, respectively (P=0.004 for superiority).
ENGAGE-AF TIMI 48
In the ENGAGE AF-TIMI 48 trial, researchers compared edoxaban and warfarin as prophylaxis for stroke or systemic embolism in patients with NVAF.
The trial included 21,105 patients who were randomized to receive warfarin (n=7036), edoxaban at 60 mg (n=7035), or edoxaban at 30 mg (n=7034).
Edoxaban was at least non-inferior to warfarin with regard to efficacy. The annual incidence of stroke or systemic embolism was 1.50% with warfarin, 1.18% with edoxaban at 60 mg (P<0.001 for non-inferiority), and 1.61% with edoxaban at 30 mg (P=0.005 for non-inferiority).
In addition, edoxaban was associated with a significantly lower rate of major and fatal bleeding. The annual incidence of major bleeding was 3.43% with warfarin, 2.75% with edoxaban at 60 mg (P<0.001), and 1.61% with edoxaban at 30 mg (P<0.001).
Fatal bleeds occurred at an annual rate of 0.38% with warfarin, 0.21% with edoxaban at 60 mg (P=0.006), and 0.13% with edoxaban at 30 mg (P<0.001). ![]()
CHMP recommends new indication for daratumumab
The CHMP recommended approving daratumumab in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone as treatment for adults with multiple myeloma (MM) who have received at least 1 prior therapy.
The CHMP’s recommendation has been forwarded to the European Commission, which is expected to make its decision on daratumumab within 2 months.
Daratumumab is a human IgG1k monoclonal antibody that binds to CD38, which is highly expressed on the surface of MM cells.
Daratumumab is being developed by Janssen Biotech, Inc. under an exclusive worldwide license from Genmab.
Phase 3 trials
The CHMP’s recommendation regarding daratumumab was based on data from the phase 3 POLLUX and CASTOR trials.
In the POLLUX trial, researchers compared treatment with lenalidomide and dexamethasone to treatment with daratumumab, lenalidomide, and dexamethasone in patients with relapsed or refractory MM.
Patients who received daratumumab in combination had a significantly higher response rate and longer progression-free survival than patients who received the 2-drug combination.
However, treatment with daratumumab was associated with infusion-related reactions and a higher incidence of neutropenia.
Results from this trial were published in NEJM in October 2016.
In the CASTOR trial, researchers compared treatment with bortezomib and dexamethasone to treatment with daratumumab, bortezomib, and dexamethasone in patients with previously treated MM.
Patients who received the 3-drug combination had a higher response rate, longer progression-free survival, and a higher incidence of grade 3/4 adverse events than those who received the 2-drug combination.
Results from this trial were published in NEJM in August 2016. ![]()
The CHMP recommended approving daratumumab in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone as treatment for adults with multiple myeloma (MM) who have received at least 1 prior therapy.
The CHMP’s recommendation has been forwarded to the European Commission, which is expected to make its decision on daratumumab within 2 months.
Daratumumab is a human IgG1k monoclonal antibody that binds to CD38, which is highly expressed on the surface of MM cells.
Daratumumab is being developed by Janssen Biotech, Inc. under an exclusive worldwide license from Genmab.
Phase 3 trials
The CHMP’s recommendation regarding daratumumab was based on data from the phase 3 POLLUX and CASTOR trials.
In the POLLUX trial, researchers compared treatment with lenalidomide and dexamethasone to treatment with daratumumab, lenalidomide, and dexamethasone in patients with relapsed or refractory MM.
Patients who received daratumumab in combination had a significantly higher response rate and longer progression-free survival than patients who received the 2-drug combination.
However, treatment with daratumumab was associated with infusion-related reactions and a higher incidence of neutropenia.
Results from this trial were published in NEJM in October 2016.
In the CASTOR trial, researchers compared treatment with bortezomib and dexamethasone to treatment with daratumumab, bortezomib, and dexamethasone in patients with previously treated MM.
Patients who received the 3-drug combination had a higher response rate, longer progression-free survival, and a higher incidence of grade 3/4 adverse events than those who received the 2-drug combination.
Results from this trial were published in NEJM in August 2016. ![]()
The CHMP recommended approving daratumumab in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone as treatment for adults with multiple myeloma (MM) who have received at least 1 prior therapy.
The CHMP’s recommendation has been forwarded to the European Commission, which is expected to make its decision on daratumumab within 2 months.
Daratumumab is a human IgG1k monoclonal antibody that binds to CD38, which is highly expressed on the surface of MM cells.
Daratumumab is being developed by Janssen Biotech, Inc. under an exclusive worldwide license from Genmab.
Phase 3 trials
The CHMP’s recommendation regarding daratumumab was based on data from the phase 3 POLLUX and CASTOR trials.
In the POLLUX trial, researchers compared treatment with lenalidomide and dexamethasone to treatment with daratumumab, lenalidomide, and dexamethasone in patients with relapsed or refractory MM.
Patients who received daratumumab in combination had a significantly higher response rate and longer progression-free survival than patients who received the 2-drug combination.
However, treatment with daratumumab was associated with infusion-related reactions and a higher incidence of neutropenia.
Results from this trial were published in NEJM in October 2016.
In the CASTOR trial, researchers compared treatment with bortezomib and dexamethasone to treatment with daratumumab, bortezomib, and dexamethasone in patients with previously treated MM.
Patients who received the 3-drug combination had a higher response rate, longer progression-free survival, and a higher incidence of grade 3/4 adverse events than those who received the 2-drug combination.
Results from this trial were published in NEJM in August 2016. ![]()
Docs create guideline to aid workup of acute leukemia
Two physician groups have published an evidence-based guideline that addresses the initial workup of acute leukemia.
The guideline includes 27 recommendations intended to aid treating physicians and pathologists involved in the diagnostic and prognostic evaluation of acute leukemia samples, including those from patients with acute lymphoblastic leukemia, acute myeloid leukemia, and acute leukemias of ambiguous lineage.
The guideline, which was developed through a collaboration between the College of American Pathologists (CAP) and the American Society of Hematology (ASH), has been published in the Archives of Pathology and Laboratory Medicine.
The recommendations in the guideline will also be available in a pocket guide and via the ASH Pocket Guides app in March.
“With its multidisciplinary perspective, this guideline reflects contemporary, integrated cancer care, and, therefore, it will also help providers realize efficiencies in test management,” said ASH guideline co-chair James W. Vardiman, MD, of the University of Chicago in Illinois.
To create this guideline, Dr Vardiman and his colleagues sought and reviewed relevant published evidence.
The group set out to answer 6 questions for the initial diagnosis of acute leukemias:
1) What clinical and laboratory information should be available?
2) What samples and specimen types should be evaluated?
3) What tests are required for all patients during the initial evaluation?
4) What tests are required for only a subset of patients?
5) Where should laboratory testing be performed?
6) How should the results be reported?
The authors say the guideline’s 27 recommendations answer these questions, providing a framework for the steps involved in the evaluation of acute leukemia samples.
In particular, the guideline includes steps to coordinate and communicate across clinical teams, specifying information that must be shared—particularly among treating physicians and pathologists—for optimal patient outcomes and to avoid duplicative testing.
“The laboratory testing to diagnose acute leukemia and inform treatment is increasingly complex, making this guideline essential,” said CAP guideline co-chair Daniel A. Arber, MD, of the University of Chicago.
“New gene mutations and protein expressions have been described over the last decade in all types of acute leukemia, and many of them impact diagnosis or inform prognosis.” ![]()
Two physician groups have published an evidence-based guideline that addresses the initial workup of acute leukemia.
The guideline includes 27 recommendations intended to aid treating physicians and pathologists involved in the diagnostic and prognostic evaluation of acute leukemia samples, including those from patients with acute lymphoblastic leukemia, acute myeloid leukemia, and acute leukemias of ambiguous lineage.
The guideline, which was developed through a collaboration between the College of American Pathologists (CAP) and the American Society of Hematology (ASH), has been published in the Archives of Pathology and Laboratory Medicine.
The recommendations in the guideline will also be available in a pocket guide and via the ASH Pocket Guides app in March.
“With its multidisciplinary perspective, this guideline reflects contemporary, integrated cancer care, and, therefore, it will also help providers realize efficiencies in test management,” said ASH guideline co-chair James W. Vardiman, MD, of the University of Chicago in Illinois.
To create this guideline, Dr Vardiman and his colleagues sought and reviewed relevant published evidence.
The group set out to answer 6 questions for the initial diagnosis of acute leukemias:
1) What clinical and laboratory information should be available?
2) What samples and specimen types should be evaluated?
3) What tests are required for all patients during the initial evaluation?
4) What tests are required for only a subset of patients?
5) Where should laboratory testing be performed?
6) How should the results be reported?
The authors say the guideline’s 27 recommendations answer these questions, providing a framework for the steps involved in the evaluation of acute leukemia samples.
In particular, the guideline includes steps to coordinate and communicate across clinical teams, specifying information that must be shared—particularly among treating physicians and pathologists—for optimal patient outcomes and to avoid duplicative testing.
“The laboratory testing to diagnose acute leukemia and inform treatment is increasingly complex, making this guideline essential,” said CAP guideline co-chair Daniel A. Arber, MD, of the University of Chicago.
“New gene mutations and protein expressions have been described over the last decade in all types of acute leukemia, and many of them impact diagnosis or inform prognosis.” ![]()
Two physician groups have published an evidence-based guideline that addresses the initial workup of acute leukemia.
The guideline includes 27 recommendations intended to aid treating physicians and pathologists involved in the diagnostic and prognostic evaluation of acute leukemia samples, including those from patients with acute lymphoblastic leukemia, acute myeloid leukemia, and acute leukemias of ambiguous lineage.
The guideline, which was developed through a collaboration between the College of American Pathologists (CAP) and the American Society of Hematology (ASH), has been published in the Archives of Pathology and Laboratory Medicine.
The recommendations in the guideline will also be available in a pocket guide and via the ASH Pocket Guides app in March.
“With its multidisciplinary perspective, this guideline reflects contemporary, integrated cancer care, and, therefore, it will also help providers realize efficiencies in test management,” said ASH guideline co-chair James W. Vardiman, MD, of the University of Chicago in Illinois.
To create this guideline, Dr Vardiman and his colleagues sought and reviewed relevant published evidence.
The group set out to answer 6 questions for the initial diagnosis of acute leukemias:
1) What clinical and laboratory information should be available?
2) What samples and specimen types should be evaluated?
3) What tests are required for all patients during the initial evaluation?
4) What tests are required for only a subset of patients?
5) Where should laboratory testing be performed?
6) How should the results be reported?
The authors say the guideline’s 27 recommendations answer these questions, providing a framework for the steps involved in the evaluation of acute leukemia samples.
In particular, the guideline includes steps to coordinate and communicate across clinical teams, specifying information that must be shared—particularly among treating physicians and pathologists—for optimal patient outcomes and to avoid duplicative testing.
“The laboratory testing to diagnose acute leukemia and inform treatment is increasingly complex, making this guideline essential,” said CAP guideline co-chair Daniel A. Arber, MD, of the University of Chicago.
“New gene mutations and protein expressions have been described over the last decade in all types of acute leukemia, and many of them impact diagnosis or inform prognosis.”
First case of artemisinin resistance in Africa
Researchers have identified the first known case of artemisinin-resistant malaria originating in Africa, according to a letter published in NEJM.
Resistant Plasmodium falciparum parasites were detected in a Chinese man who had travelled from Equatorial Guinea to China.
The finding means Africa has joined Southeast Asia in hosting parasites that are partially resistant to the first-line antimalaria drug, artemisinin.
Researchers were able to confirm that the parasites in the current case carried a new mutation in the Kelch13 (K13) gene, the main driver for artemisinin resistance in Asia.
Then, the team set out to determine whether the parasite originated from Africa or Southeast Asia.
“We used whole-genome sequencing and bioinformatics tools we had previously developed—like detectives trying to link the culprit parasite to the crime scene,” explained Arnab Pain, PhD, of King Abdullah University of Science and Technology in Thuwal, Saudi Arabia.
Sequencing and analysis of P falciparum DNA unveiled its origin by disclosing the single nucleotide polymorphisms that vary according to the geographical source of the strain.
The researchers used the nuclear DNA, as well as the one present in 2 organelles of the parasite—the mitochondrium and the apicoplast.
Both methods independently validated the origin of the parasite as West African, confirming the first case of artemisinin resistance mediated by a K13 gene mutation on the African continent.
“The spread of artemisinin resistance in Africa would be a major setback in the fight against malaria, as ACT [artemisinin-based combination therapy] is the only effective and widely used antimalarial treatment at the moment,” Dr Pain said. “Therefore, it is very important to regularly monitor artemisinin resistance worldwide.”
Researchers have identified the first known case of artemisinin-resistant malaria originating in Africa, according to a letter published in NEJM.
Resistant Plasmodium falciparum parasites were detected in a Chinese man who had travelled from Equatorial Guinea to China.
The finding means Africa has joined Southeast Asia in hosting parasites that are partially resistant to the first-line antimalaria drug, artemisinin.
Researchers were able to confirm that the parasites in the current case carried a new mutation in the Kelch13 (K13) gene, the main driver for artemisinin resistance in Asia.
Then, the team set out to determine whether the parasite originated from Africa or Southeast Asia.
“We used whole-genome sequencing and bioinformatics tools we had previously developed—like detectives trying to link the culprit parasite to the crime scene,” explained Arnab Pain, PhD, of King Abdullah University of Science and Technology in Thuwal, Saudi Arabia.
Sequencing and analysis of P falciparum DNA unveiled its origin by disclosing the single nucleotide polymorphisms that vary according to the geographical source of the strain.
The researchers used the nuclear DNA, as well as the one present in 2 organelles of the parasite—the mitochondrium and the apicoplast.
Both methods independently validated the origin of the parasite as West African, confirming the first case of artemisinin resistance mediated by a K13 gene mutation on the African continent.
“The spread of artemisinin resistance in Africa would be a major setback in the fight against malaria, as ACT [artemisinin-based combination therapy] is the only effective and widely used antimalarial treatment at the moment,” Dr Pain said. “Therefore, it is very important to regularly monitor artemisinin resistance worldwide.”
Researchers have identified the first known case of artemisinin-resistant malaria originating in Africa, according to a letter published in NEJM.
Resistant Plasmodium falciparum parasites were detected in a Chinese man who had travelled from Equatorial Guinea to China.
The finding means Africa has joined Southeast Asia in hosting parasites that are partially resistant to the first-line antimalaria drug, artemisinin.
Researchers were able to confirm that the parasites in the current case carried a new mutation in the Kelch13 (K13) gene, the main driver for artemisinin resistance in Asia.
Then, the team set out to determine whether the parasite originated from Africa or Southeast Asia.
“We used whole-genome sequencing and bioinformatics tools we had previously developed—like detectives trying to link the culprit parasite to the crime scene,” explained Arnab Pain, PhD, of King Abdullah University of Science and Technology in Thuwal, Saudi Arabia.
Sequencing and analysis of P falciparum DNA unveiled its origin by disclosing the single nucleotide polymorphisms that vary according to the geographical source of the strain.
The researchers used the nuclear DNA, as well as the one present in 2 organelles of the parasite—the mitochondrium and the apicoplast.
Both methods independently validated the origin of the parasite as West African, confirming the first case of artemisinin resistance mediated by a K13 gene mutation on the African continent.
“The spread of artemisinin resistance in Africa would be a major setback in the fight against malaria, as ACT [artemisinin-based combination therapy] is the only effective and widely used antimalarial treatment at the moment,” Dr Pain said. “Therefore, it is very important to regularly monitor artemisinin resistance worldwide.”
Oncolytic virus can eradicate MM in mice
Myxoma virus (MYXV), a nonhuman oncolytic agent, has demonstrated efficacy in mouse models of multiple myeloma (MM), according to research published in Molecular Therapy—Oncolytics.
MYXV significantly improved overall survival in mice with MM, providing a modest delay in disease progression for about two-thirds of the mice and completely eradicating the disease in a quarter of them.
“[W]e could actually get rid of disease, and it didn’t appear to ever come back,” said study author Eric C. Bartee, PhD, of the Medical University of South Carolina in Charleston.
For the past several years, Dr Bartee has been using MYXV to treat MM in cell culture. He and his colleagues previously showed that MYXV was able to kill human MM cells.
The team found that treatment with MYXV could eradicate MM cells in patient stem cell samples prior to transplant, thereby preventing relapse of MM.
In the current study, Dr Bartee and his colleagues took this one step further by assessing whether treatment with MYXV also has a benefit on disease outside the context of transplantation.
Using a mouse model of MM, the researchers showed that systemic treatment with MYXV reduced tumor burden and led to a modest decrease in disease progression (about 6 days) in 66% of mice.
In 25% of mice, there was complete eradication of disease with no evidence of relapse.
Since MYXV does not replicate in MM cells, the researchers postulated that eradication was caused by the host’s immune system. Investigation of the bone marrow showed that it was unaffected by treatment with MYXV.
This suggested that the immune system remained functional and could combat MM. Indeed, treatment with MYXV led to an increase in CD8+ T cells in the bone marrow, indicating a strong antitumor response.
The researchers noted that, although these results are promising, there are hurdles that must be overcome before this treatment can be brought to the clinic. One hurdle is large-scale production of clinical-grade virus. Another is demonstrating a high response rate.
“I think the major next question is ‘How do you get that response rate from 25% to 50% to 80% to 100%?’” Dr Bartee said. “How do you define the patients in which it works?”
Myxoma virus (MYXV), a nonhuman oncolytic agent, has demonstrated efficacy in mouse models of multiple myeloma (MM), according to research published in Molecular Therapy—Oncolytics.
MYXV significantly improved overall survival in mice with MM, providing a modest delay in disease progression for about two-thirds of the mice and completely eradicating the disease in a quarter of them.
“[W]e could actually get rid of disease, and it didn’t appear to ever come back,” said study author Eric C. Bartee, PhD, of the Medical University of South Carolina in Charleston.
For the past several years, Dr Bartee has been using MYXV to treat MM in cell culture. He and his colleagues previously showed that MYXV was able to kill human MM cells.
The team found that treatment with MYXV could eradicate MM cells in patient stem cell samples prior to transplant, thereby preventing relapse of MM.
In the current study, Dr Bartee and his colleagues took this one step further by assessing whether treatment with MYXV also has a benefit on disease outside the context of transplantation.
Using a mouse model of MM, the researchers showed that systemic treatment with MYXV reduced tumor burden and led to a modest decrease in disease progression (about 6 days) in 66% of mice.
In 25% of mice, there was complete eradication of disease with no evidence of relapse.
Since MYXV does not replicate in MM cells, the researchers postulated that eradication was caused by the host’s immune system. Investigation of the bone marrow showed that it was unaffected by treatment with MYXV.
This suggested that the immune system remained functional and could combat MM. Indeed, treatment with MYXV led to an increase in CD8+ T cells in the bone marrow, indicating a strong antitumor response.
The researchers noted that, although these results are promising, there are hurdles that must be overcome before this treatment can be brought to the clinic. One hurdle is large-scale production of clinical-grade virus. Another is demonstrating a high response rate.
“I think the major next question is ‘How do you get that response rate from 25% to 50% to 80% to 100%?’” Dr Bartee said. “How do you define the patients in which it works?”
Myxoma virus (MYXV), a nonhuman oncolytic agent, has demonstrated efficacy in mouse models of multiple myeloma (MM), according to research published in Molecular Therapy—Oncolytics.
MYXV significantly improved overall survival in mice with MM, providing a modest delay in disease progression for about two-thirds of the mice and completely eradicating the disease in a quarter of them.
“[W]e could actually get rid of disease, and it didn’t appear to ever come back,” said study author Eric C. Bartee, PhD, of the Medical University of South Carolina in Charleston.
For the past several years, Dr Bartee has been using MYXV to treat MM in cell culture. He and his colleagues previously showed that MYXV was able to kill human MM cells.
The team found that treatment with MYXV could eradicate MM cells in patient stem cell samples prior to transplant, thereby preventing relapse of MM.
In the current study, Dr Bartee and his colleagues took this one step further by assessing whether treatment with MYXV also has a benefit on disease outside the context of transplantation.
Using a mouse model of MM, the researchers showed that systemic treatment with MYXV reduced tumor burden and led to a modest decrease in disease progression (about 6 days) in 66% of mice.
In 25% of mice, there was complete eradication of disease with no evidence of relapse.
Since MYXV does not replicate in MM cells, the researchers postulated that eradication was caused by the host’s immune system. Investigation of the bone marrow showed that it was unaffected by treatment with MYXV.
This suggested that the immune system remained functional and could combat MM. Indeed, treatment with MYXV led to an increase in CD8+ T cells in the bone marrow, indicating a strong antitumor response.
The researchers noted that, although these results are promising, there are hurdles that must be overcome before this treatment can be brought to the clinic. One hurdle is large-scale production of clinical-grade virus. Another is demonstrating a high response rate.
“I think the major next question is ‘How do you get that response rate from 25% to 50% to 80% to 100%?’” Dr Bartee said. “How do you define the patients in which it works?”
Drug granted orphan status for follicular lymphoma
The US Food and Drug Administration (FDA) has granted orphan designation to G100 for the treatment of follicular lymphoma.
G100 is a synthetic small-molecule toll-like receptor-4 agonist, glucopyranosyl lipid A, formulated in a stable emulsion.
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent rare diseases/disorders affecting fewer than 200,000 people in the US.
Orphan designation provides companies with certain incentives to develop products for rare diseases. This includes a 50% tax break on research and development, a fee waiver, access to federal grants, and 7 years of market exclusivity if the product is approved.
G100 is being developed by Immune Design. The company says G100 works by leveraging the activation of innate and adaptive immunity in the tumor microenvironment to create an immune response against the tumor’s pre-existing antigens.
According to Immune Design, clinical and preclinical data have demonstrated G100’s ability to activate tumor-infiltrating lymphocytes, macrophages, and dendritic cells, and promote antigen-presentation and the recruitment of T cells to the tumor.
The ensuing induction of local and systemic immune responses has been shown to result in local and abscopal tumor control in preclinical studies.
In fact, G100, when combined with local radiation, demonstrated efficacy against A20 lymphoma in mice. This research was presented in a poster at the 2016 ASH Annual Meeting (abstract 4166).
In this study, investigators evaluated the immune response and therapeutic effects of intratumoral G100 alone, local radiation alone, and concomitant G100 and local radiation in mice with A20 lymphoma.
The investigators said the combination therapy demonstrated:
- Synergistic antitumor effects in both injected as well as uninjected tumors (abscopal effects)
- Synergistic induction of pro-inflammatory cytokine and chemokine environment, as well as induction of genes governing antigen processing and presentation
- Increased infiltration of T cells, including CD4 and CD8 T cells, in treated tumors.
In contrast, tumors that received only radiation had significantly lower T-cell levels than untreated tumors.
“These findings highlight the potential beneficial effect that immunotherapy with G100 could provide when given with radiation by modulating the tumor microenvironment to generate a systemic, durable, T-cell anti-tumor response,” said study investigator Ramesh Rengan, MD, of the University of Washington in Seattle.
“As shown in this model, G100 may hold potential as a treatment for lymphoma patients.”
To test that theory, Immune Design is conducting a phase 1/2 trial of G100 given with local radiation or the anti-PD-1 agent pembrolizumab to patients with follicular lymphoma.
The US Food and Drug Administration (FDA) has granted orphan designation to G100 for the treatment of follicular lymphoma.
G100 is a synthetic small-molecule toll-like receptor-4 agonist, glucopyranosyl lipid A, formulated in a stable emulsion.
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent rare diseases/disorders affecting fewer than 200,000 people in the US.
Orphan designation provides companies with certain incentives to develop products for rare diseases. This includes a 50% tax break on research and development, a fee waiver, access to federal grants, and 7 years of market exclusivity if the product is approved.
G100 is being developed by Immune Design. The company says G100 works by leveraging the activation of innate and adaptive immunity in the tumor microenvironment to create an immune response against the tumor’s pre-existing antigens.
According to Immune Design, clinical and preclinical data have demonstrated G100’s ability to activate tumor-infiltrating lymphocytes, macrophages, and dendritic cells, and promote antigen-presentation and the recruitment of T cells to the tumor.
The ensuing induction of local and systemic immune responses has been shown to result in local and abscopal tumor control in preclinical studies.
In fact, G100, when combined with local radiation, demonstrated efficacy against A20 lymphoma in mice. This research was presented in a poster at the 2016 ASH Annual Meeting (abstract 4166).
In this study, investigators evaluated the immune response and therapeutic effects of intratumoral G100 alone, local radiation alone, and concomitant G100 and local radiation in mice with A20 lymphoma.
The investigators said the combination therapy demonstrated:
- Synergistic antitumor effects in both injected as well as uninjected tumors (abscopal effects)
- Synergistic induction of pro-inflammatory cytokine and chemokine environment, as well as induction of genes governing antigen processing and presentation
- Increased infiltration of T cells, including CD4 and CD8 T cells, in treated tumors.
In contrast, tumors that received only radiation had significantly lower T-cell levels than untreated tumors.
“These findings highlight the potential beneficial effect that immunotherapy with G100 could provide when given with radiation by modulating the tumor microenvironment to generate a systemic, durable, T-cell anti-tumor response,” said study investigator Ramesh Rengan, MD, of the University of Washington in Seattle.
“As shown in this model, G100 may hold potential as a treatment for lymphoma patients.”
To test that theory, Immune Design is conducting a phase 1/2 trial of G100 given with local radiation or the anti-PD-1 agent pembrolizumab to patients with follicular lymphoma.
The US Food and Drug Administration (FDA) has granted orphan designation to G100 for the treatment of follicular lymphoma.
G100 is a synthetic small-molecule toll-like receptor-4 agonist, glucopyranosyl lipid A, formulated in a stable emulsion.
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent rare diseases/disorders affecting fewer than 200,000 people in the US.
Orphan designation provides companies with certain incentives to develop products for rare diseases. This includes a 50% tax break on research and development, a fee waiver, access to federal grants, and 7 years of market exclusivity if the product is approved.
G100 is being developed by Immune Design. The company says G100 works by leveraging the activation of innate and adaptive immunity in the tumor microenvironment to create an immune response against the tumor’s pre-existing antigens.
According to Immune Design, clinical and preclinical data have demonstrated G100’s ability to activate tumor-infiltrating lymphocytes, macrophages, and dendritic cells, and promote antigen-presentation and the recruitment of T cells to the tumor.
The ensuing induction of local and systemic immune responses has been shown to result in local and abscopal tumor control in preclinical studies.
In fact, G100, when combined with local radiation, demonstrated efficacy against A20 lymphoma in mice. This research was presented in a poster at the 2016 ASH Annual Meeting (abstract 4166).
In this study, investigators evaluated the immune response and therapeutic effects of intratumoral G100 alone, local radiation alone, and concomitant G100 and local radiation in mice with A20 lymphoma.
The investigators said the combination therapy demonstrated:
- Synergistic antitumor effects in both injected as well as uninjected tumors (abscopal effects)
- Synergistic induction of pro-inflammatory cytokine and chemokine environment, as well as induction of genes governing antigen processing and presentation
- Increased infiltration of T cells, including CD4 and CD8 T cells, in treated tumors.
In contrast, tumors that received only radiation had significantly lower T-cell levels than untreated tumors.
“These findings highlight the potential beneficial effect that immunotherapy with G100 could provide when given with radiation by modulating the tumor microenvironment to generate a systemic, durable, T-cell anti-tumor response,” said study investigator Ramesh Rengan, MD, of the University of Washington in Seattle.
“As shown in this model, G100 may hold potential as a treatment for lymphoma patients.”
To test that theory, Immune Design is conducting a phase 1/2 trial of G100 given with local radiation or the anti-PD-1 agent pembrolizumab to patients with follicular lymphoma.