User login
Factors linked to B-NHL in Palestinians, Israelis
New research has revealed factors that may increase the risk of B-cell non-Hodgkin lymphoma (B-NHL) in Israelis and Palestinians.
This large-scale, epidemiological study indicated that each group had its own unique risk factors.
However, in both groups, recreational sun exposure, black hair-dye use, a history of hospitalization for infection, and having a first-degree relative with a hematopoietic malignancy were all associated with B-NHL.
A team of Palestinian and Israeli researchers reported these findings in PLOS ONE.
The researchers noted that Israelis and Palestinians share the same ecosystem but differ in terms of lifestyle, health behaviors, and medical systems. Yet both populations report high incidences of NHL.
To gain some insight into this phenomenon, the team conducted a study examining risk factors for B-NHL and its subtypes in these two populations.
The researchers looked at medical history, environmental factors, and lifestyle factors in 823 B-NHL patients and 808 healthy controls.
There were 516 Israeli Jews with B-NHL and 307 Palestinian Arabs with B-NHL. The mean age at diagnosis was 60 and 51, respectively.
The proportion of patients with diffuse large B-cell lymphoma was 71% of Palestinian Arabs and 41% of Israeli Jews. The proportion of patients with follicular lymphoma was 14% and 28%, respectively. And the proportion of patients with marginal zone lymphoma was 2% and 14%, respectively.
Using data from questionnaires, pathology review, serology, and genotyping, the researchers uncovered potential risk factors for B-NHL common to both populations and other factors unique to each population.
Results
The data showed that, in both Palestinian Arabs and Israeli Jews, B-NHL was associated with:
- Recreational sun exposure (odds ratio [OR]=1.4)
- Black hair-dye use (OR=1.70)
- A history of hospitalization for infection (OR=1.68)
- Having a first-degree relative with a hematopoietic malignancy (OR=1.69).
Smoking was associated with follicular lymphoma in both populations (OR=1.46). And greater-than-monthly indoor pesticide use was associated with diffuse large B-cell lymphoma in both populations (OR=2.01).
There was an inverse association between alcohol use and B-NHL for both populations (OR=0.46).
Among Palestinian Arabs only, risk factors for B-NHL included gardening (OR=1.93) and a history of herpes (OR=3.73), mononucleosis (OR=6.34), rubella (OR=2.86), and blood transfusion (OR=2.53).
Risk factors that applied to Israeli Jews only included growing fruits and vegetables (OR=1.87) and self-reported autoimmune diseases (OR=1.99).
The researchers said differences in risk factors by ethnicity could reflect differences in lifestyle, medical systems, and reporting patterns, while variations by B-NHL subtypes suggest specific causal factors for different types of disease. However, these findings require further investigation to reveal their mechanisms.
“Apart from the scientific contribution that this research provides in terms of understanding risk factors for NHL, the study entails an important research cooperation among many institutions,” said study author Ora Paltiel, of Hadassah-Hebrew University Medical Organization in Jerusalem, Israel.
“The study provided opportunities for training Palestinian and Israeli researchers and will provide for intellectual interaction for years to come. The data collected will also provide a research platform for the future study of lymphoma. Epidemiologic research has the potential to improve and preserve human health, and it can also serve as a bridge to dialogue among nations.” ![]()
New research has revealed factors that may increase the risk of B-cell non-Hodgkin lymphoma (B-NHL) in Israelis and Palestinians.
This large-scale, epidemiological study indicated that each group had its own unique risk factors.
However, in both groups, recreational sun exposure, black hair-dye use, a history of hospitalization for infection, and having a first-degree relative with a hematopoietic malignancy were all associated with B-NHL.
A team of Palestinian and Israeli researchers reported these findings in PLOS ONE.
The researchers noted that Israelis and Palestinians share the same ecosystem but differ in terms of lifestyle, health behaviors, and medical systems. Yet both populations report high incidences of NHL.
To gain some insight into this phenomenon, the team conducted a study examining risk factors for B-NHL and its subtypes in these two populations.
The researchers looked at medical history, environmental factors, and lifestyle factors in 823 B-NHL patients and 808 healthy controls.
There were 516 Israeli Jews with B-NHL and 307 Palestinian Arabs with B-NHL. The mean age at diagnosis was 60 and 51, respectively.
The proportion of patients with diffuse large B-cell lymphoma was 71% of Palestinian Arabs and 41% of Israeli Jews. The proportion of patients with follicular lymphoma was 14% and 28%, respectively. And the proportion of patients with marginal zone lymphoma was 2% and 14%, respectively.
Using data from questionnaires, pathology review, serology, and genotyping, the researchers uncovered potential risk factors for B-NHL common to both populations and other factors unique to each population.
Results
The data showed that, in both Palestinian Arabs and Israeli Jews, B-NHL was associated with:
- Recreational sun exposure (odds ratio [OR]=1.4)
- Black hair-dye use (OR=1.70)
- A history of hospitalization for infection (OR=1.68)
- Having a first-degree relative with a hematopoietic malignancy (OR=1.69).
Smoking was associated with follicular lymphoma in both populations (OR=1.46). And greater-than-monthly indoor pesticide use was associated with diffuse large B-cell lymphoma in both populations (OR=2.01).
There was an inverse association between alcohol use and B-NHL for both populations (OR=0.46).
Among Palestinian Arabs only, risk factors for B-NHL included gardening (OR=1.93) and a history of herpes (OR=3.73), mononucleosis (OR=6.34), rubella (OR=2.86), and blood transfusion (OR=2.53).
Risk factors that applied to Israeli Jews only included growing fruits and vegetables (OR=1.87) and self-reported autoimmune diseases (OR=1.99).
The researchers said differences in risk factors by ethnicity could reflect differences in lifestyle, medical systems, and reporting patterns, while variations by B-NHL subtypes suggest specific causal factors for different types of disease. However, these findings require further investigation to reveal their mechanisms.
“Apart from the scientific contribution that this research provides in terms of understanding risk factors for NHL, the study entails an important research cooperation among many institutions,” said study author Ora Paltiel, of Hadassah-Hebrew University Medical Organization in Jerusalem, Israel.
“The study provided opportunities for training Palestinian and Israeli researchers and will provide for intellectual interaction for years to come. The data collected will also provide a research platform for the future study of lymphoma. Epidemiologic research has the potential to improve and preserve human health, and it can also serve as a bridge to dialogue among nations.” ![]()
New research has revealed factors that may increase the risk of B-cell non-Hodgkin lymphoma (B-NHL) in Israelis and Palestinians.
This large-scale, epidemiological study indicated that each group had its own unique risk factors.
However, in both groups, recreational sun exposure, black hair-dye use, a history of hospitalization for infection, and having a first-degree relative with a hematopoietic malignancy were all associated with B-NHL.
A team of Palestinian and Israeli researchers reported these findings in PLOS ONE.
The researchers noted that Israelis and Palestinians share the same ecosystem but differ in terms of lifestyle, health behaviors, and medical systems. Yet both populations report high incidences of NHL.
To gain some insight into this phenomenon, the team conducted a study examining risk factors for B-NHL and its subtypes in these two populations.
The researchers looked at medical history, environmental factors, and lifestyle factors in 823 B-NHL patients and 808 healthy controls.
There were 516 Israeli Jews with B-NHL and 307 Palestinian Arabs with B-NHL. The mean age at diagnosis was 60 and 51, respectively.
The proportion of patients with diffuse large B-cell lymphoma was 71% of Palestinian Arabs and 41% of Israeli Jews. The proportion of patients with follicular lymphoma was 14% and 28%, respectively. And the proportion of patients with marginal zone lymphoma was 2% and 14%, respectively.
Using data from questionnaires, pathology review, serology, and genotyping, the researchers uncovered potential risk factors for B-NHL common to both populations and other factors unique to each population.
Results
The data showed that, in both Palestinian Arabs and Israeli Jews, B-NHL was associated with:
- Recreational sun exposure (odds ratio [OR]=1.4)
- Black hair-dye use (OR=1.70)
- A history of hospitalization for infection (OR=1.68)
- Having a first-degree relative with a hematopoietic malignancy (OR=1.69).
Smoking was associated with follicular lymphoma in both populations (OR=1.46). And greater-than-monthly indoor pesticide use was associated with diffuse large B-cell lymphoma in both populations (OR=2.01).
There was an inverse association between alcohol use and B-NHL for both populations (OR=0.46).
Among Palestinian Arabs only, risk factors for B-NHL included gardening (OR=1.93) and a history of herpes (OR=3.73), mononucleosis (OR=6.34), rubella (OR=2.86), and blood transfusion (OR=2.53).
Risk factors that applied to Israeli Jews only included growing fruits and vegetables (OR=1.87) and self-reported autoimmune diseases (OR=1.99).
The researchers said differences in risk factors by ethnicity could reflect differences in lifestyle, medical systems, and reporting patterns, while variations by B-NHL subtypes suggest specific causal factors for different types of disease. However, these findings require further investigation to reveal their mechanisms.
“Apart from the scientific contribution that this research provides in terms of understanding risk factors for NHL, the study entails an important research cooperation among many institutions,” said study author Ora Paltiel, of Hadassah-Hebrew University Medical Organization in Jerusalem, Israel.
“The study provided opportunities for training Palestinian and Israeli researchers and will provide for intellectual interaction for years to come. The data collected will also provide a research platform for the future study of lymphoma. Epidemiologic research has the potential to improve and preserve human health, and it can also serve as a bridge to dialogue among nations.” ![]()
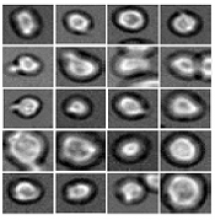
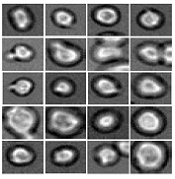
Software predicts HSPC differentiation
Deep learning can be used to determine how murine hematopoietic stem and progenitor cells (HSPCs) will differentiate, according to research published in Nature Methods.
Deep learning algorithms simulate the learning processes in people using artificial neural networks.
Researchers have reported the development of software that uses deep learning to predict which type of cell murine HSPCs will differentiate into, based on microscopy images.
“A hematopoietic stem cell’s decision to become a certain cell type cannot be observed,” said study author Carsten Marr, PhD, of Helmholtz Zentrum München–German Research Center for Environmental Health in Neuherberg, Germany.
“At this time, it is only possible to verify the decision retrospectively with cell surface markers.”
Therefore, Dr Marr and his team set out to develop an algorithm that can predict the decision in advance, and deep learning was key.
“Deep neural networks play a major role in our method,” Dr Marr said. “Our algorithm classifies light microscopic images and videos of individual cells by comparing these data with past experience from the development of such cells. In this way, the algorithm ‘learns’ how certain cells behave.”
Specifically, the researchers examined murine HSPCs filmed under a microscope in the lab. Using information on the cells’ appearance and speed, the software was able to “memorize” the corresponding behavior patterns and then make its prediction.
“Compared to conventional methods, such as fluorescent antibodies against certain surface proteins, we know how the cells will decide 3 cell generations earlier,” said Felix Buggenthin, PhD, of Helmholtz Zentrum München–German Research Center for Environmental Health.
“Since we now know which cells will develop in which way, we can isolate them earlier than before and examine how they differ at a molecular level,” Dr Marr added. “We want to use this information to understand how the choices are made for particular developmental traits.” ![]()
Deep learning can be used to determine how murine hematopoietic stem and progenitor cells (HSPCs) will differentiate, according to research published in Nature Methods.
Deep learning algorithms simulate the learning processes in people using artificial neural networks.
Researchers have reported the development of software that uses deep learning to predict which type of cell murine HSPCs will differentiate into, based on microscopy images.
“A hematopoietic stem cell’s decision to become a certain cell type cannot be observed,” said study author Carsten Marr, PhD, of Helmholtz Zentrum München–German Research Center for Environmental Health in Neuherberg, Germany.
“At this time, it is only possible to verify the decision retrospectively with cell surface markers.”
Therefore, Dr Marr and his team set out to develop an algorithm that can predict the decision in advance, and deep learning was key.
“Deep neural networks play a major role in our method,” Dr Marr said. “Our algorithm classifies light microscopic images and videos of individual cells by comparing these data with past experience from the development of such cells. In this way, the algorithm ‘learns’ how certain cells behave.”
Specifically, the researchers examined murine HSPCs filmed under a microscope in the lab. Using information on the cells’ appearance and speed, the software was able to “memorize” the corresponding behavior patterns and then make its prediction.
“Compared to conventional methods, such as fluorescent antibodies against certain surface proteins, we know how the cells will decide 3 cell generations earlier,” said Felix Buggenthin, PhD, of Helmholtz Zentrum München–German Research Center for Environmental Health.
“Since we now know which cells will develop in which way, we can isolate them earlier than before and examine how they differ at a molecular level,” Dr Marr added. “We want to use this information to understand how the choices are made for particular developmental traits.” ![]()
Deep learning can be used to determine how murine hematopoietic stem and progenitor cells (HSPCs) will differentiate, according to research published in Nature Methods.
Deep learning algorithms simulate the learning processes in people using artificial neural networks.
Researchers have reported the development of software that uses deep learning to predict which type of cell murine HSPCs will differentiate into, based on microscopy images.
“A hematopoietic stem cell’s decision to become a certain cell type cannot be observed,” said study author Carsten Marr, PhD, of Helmholtz Zentrum München–German Research Center for Environmental Health in Neuherberg, Germany.
“At this time, it is only possible to verify the decision retrospectively with cell surface markers.”
Therefore, Dr Marr and his team set out to develop an algorithm that can predict the decision in advance, and deep learning was key.
“Deep neural networks play a major role in our method,” Dr Marr said. “Our algorithm classifies light microscopic images and videos of individual cells by comparing these data with past experience from the development of such cells. In this way, the algorithm ‘learns’ how certain cells behave.”
Specifically, the researchers examined murine HSPCs filmed under a microscope in the lab. Using information on the cells’ appearance and speed, the software was able to “memorize” the corresponding behavior patterns and then make its prediction.
“Compared to conventional methods, such as fluorescent antibodies against certain surface proteins, we know how the cells will decide 3 cell generations earlier,” said Felix Buggenthin, PhD, of Helmholtz Zentrum München–German Research Center for Environmental Health.
“Since we now know which cells will develop in which way, we can isolate them earlier than before and examine how they differ at a molecular level,” Dr Marr added. “We want to use this information to understand how the choices are made for particular developmental traits.” ![]()
European Commission approves rituximab biosimilar
The European Commission has approved a biosimilar rituximab product, Truxima™, for all the same indications as the reference product, MabThera.
This means Truxima (formerly called CT-P10) is approved for use in the European Union to treat patients with non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis (RA), granulomatosis with polyangiitis (GPA), and microscopic polyangiitis (MPA).
Truxima, a product of Celltrion Healthcare Hungary Kft, is the first biosimilar monoclonal antibody approved in an oncology indication worldwide.
The approval is based on data submitted to the European Medicines Agency.
The agency’s Committee for Medicinal Products for Human Use (CHMP) said the evidence suggests Truxima and MabThera are similar in terms of efficacy, safety, immunogenicity, pharmacodynamics, and pharmacokinetics in patients with RA and advanced follicular lymphoma (FL).
Therefore, the European Commission approved Truxima for the following indications.
Non-Hodgkin lymphoma
Truxima is indicated for use in combination with chemotherapy to treat previously untreated patients with stage III-IV FL.
Truxima maintenance therapy is indicated for the treatment of FL patients responding to induction therapy.
Truxima monotherapy is indicated for the treatment of patients with stage III-IV FL who are chemo-resistant or are in their second or subsequent relapse after chemotherapy.
Truxima is indicated for use in combination with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) for the treatment of patients with CD20-positive diffuse large B-cell lymphoma.
Chronic lymphocytic leukemia
Truxima in combination with chemotherapy is indicated for the treatment of patients with previously untreated and relapsed/refractory chronic lymphocytic leukemia.
The CHMP noted that limited efficacy and safety data are available for patients previously treated with monoclonal antibodies, including rituximab, or patients who are refractory to previous rituximab plus chemotherapy.
RA, GPA, and MPA
Truxima in combination with methotrexate is indicated for the treatment of adults with severe, active RA who have had an inadequate response to or cannot tolerate other disease-modifying anti-rheumatic drugs, including one or more tumor necrosis factor inhibitor therapies.
Truxima in combination with glucocorticoids is indicated for the induction of remission in adults with severe, active GPA or MPA.
Truxima studies
There are 3 ongoing, phase 3 trials of Truxima in patients with RA (NCT02149121), advanced FL (NCT02162771), and low-tumor-burden FL (NCT02260804).
Results from the phase 1/3 trial in patients with newly diagnosed, advanced FL suggest that Truxima and the reference rituximab are similar with regard to pharmacokinetics, immunogenicity, and safety (B Coiffier et al. ASH 2016, abstract 1807).
Results from the phase 3 study of RA patients indicate that Truxima is similar to reference products (EU and US-sourced rituximab) with regard to pharmacodynamics, safety, and efficacy for up to 24 weeks (DH Yoo et al. 2016 ACR/ARHP Annual Meeting, abstract 1635). ![]()
The European Commission has approved a biosimilar rituximab product, Truxima™, for all the same indications as the reference product, MabThera.
This means Truxima (formerly called CT-P10) is approved for use in the European Union to treat patients with non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis (RA), granulomatosis with polyangiitis (GPA), and microscopic polyangiitis (MPA).
Truxima, a product of Celltrion Healthcare Hungary Kft, is the first biosimilar monoclonal antibody approved in an oncology indication worldwide.
The approval is based on data submitted to the European Medicines Agency.
The agency’s Committee for Medicinal Products for Human Use (CHMP) said the evidence suggests Truxima and MabThera are similar in terms of efficacy, safety, immunogenicity, pharmacodynamics, and pharmacokinetics in patients with RA and advanced follicular lymphoma (FL).
Therefore, the European Commission approved Truxima for the following indications.
Non-Hodgkin lymphoma
Truxima is indicated for use in combination with chemotherapy to treat previously untreated patients with stage III-IV FL.
Truxima maintenance therapy is indicated for the treatment of FL patients responding to induction therapy.
Truxima monotherapy is indicated for the treatment of patients with stage III-IV FL who are chemo-resistant or are in their second or subsequent relapse after chemotherapy.
Truxima is indicated for use in combination with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) for the treatment of patients with CD20-positive diffuse large B-cell lymphoma.
Chronic lymphocytic leukemia
Truxima in combination with chemotherapy is indicated for the treatment of patients with previously untreated and relapsed/refractory chronic lymphocytic leukemia.
The CHMP noted that limited efficacy and safety data are available for patients previously treated with monoclonal antibodies, including rituximab, or patients who are refractory to previous rituximab plus chemotherapy.
RA, GPA, and MPA
Truxima in combination with methotrexate is indicated for the treatment of adults with severe, active RA who have had an inadequate response to or cannot tolerate other disease-modifying anti-rheumatic drugs, including one or more tumor necrosis factor inhibitor therapies.
Truxima in combination with glucocorticoids is indicated for the induction of remission in adults with severe, active GPA or MPA.
Truxima studies
There are 3 ongoing, phase 3 trials of Truxima in patients with RA (NCT02149121), advanced FL (NCT02162771), and low-tumor-burden FL (NCT02260804).
Results from the phase 1/3 trial in patients with newly diagnosed, advanced FL suggest that Truxima and the reference rituximab are similar with regard to pharmacokinetics, immunogenicity, and safety (B Coiffier et al. ASH 2016, abstract 1807).
Results from the phase 3 study of RA patients indicate that Truxima is similar to reference products (EU and US-sourced rituximab) with regard to pharmacodynamics, safety, and efficacy for up to 24 weeks (DH Yoo et al. 2016 ACR/ARHP Annual Meeting, abstract 1635). ![]()
The European Commission has approved a biosimilar rituximab product, Truxima™, for all the same indications as the reference product, MabThera.
This means Truxima (formerly called CT-P10) is approved for use in the European Union to treat patients with non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis (RA), granulomatosis with polyangiitis (GPA), and microscopic polyangiitis (MPA).
Truxima, a product of Celltrion Healthcare Hungary Kft, is the first biosimilar monoclonal antibody approved in an oncology indication worldwide.
The approval is based on data submitted to the European Medicines Agency.
The agency’s Committee for Medicinal Products for Human Use (CHMP) said the evidence suggests Truxima and MabThera are similar in terms of efficacy, safety, immunogenicity, pharmacodynamics, and pharmacokinetics in patients with RA and advanced follicular lymphoma (FL).
Therefore, the European Commission approved Truxima for the following indications.
Non-Hodgkin lymphoma
Truxima is indicated for use in combination with chemotherapy to treat previously untreated patients with stage III-IV FL.
Truxima maintenance therapy is indicated for the treatment of FL patients responding to induction therapy.
Truxima monotherapy is indicated for the treatment of patients with stage III-IV FL who are chemo-resistant or are in their second or subsequent relapse after chemotherapy.
Truxima is indicated for use in combination with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) for the treatment of patients with CD20-positive diffuse large B-cell lymphoma.
Chronic lymphocytic leukemia
Truxima in combination with chemotherapy is indicated for the treatment of patients with previously untreated and relapsed/refractory chronic lymphocytic leukemia.
The CHMP noted that limited efficacy and safety data are available for patients previously treated with monoclonal antibodies, including rituximab, or patients who are refractory to previous rituximab plus chemotherapy.
RA, GPA, and MPA
Truxima in combination with methotrexate is indicated for the treatment of adults with severe, active RA who have had an inadequate response to or cannot tolerate other disease-modifying anti-rheumatic drugs, including one or more tumor necrosis factor inhibitor therapies.
Truxima in combination with glucocorticoids is indicated for the induction of remission in adults with severe, active GPA or MPA.
Truxima studies
There are 3 ongoing, phase 3 trials of Truxima in patients with RA (NCT02149121), advanced FL (NCT02162771), and low-tumor-burden FL (NCT02260804).
Results from the phase 1/3 trial in patients with newly diagnosed, advanced FL suggest that Truxima and the reference rituximab are similar with regard to pharmacokinetics, immunogenicity, and safety (B Coiffier et al. ASH 2016, abstract 1807).
Results from the phase 3 study of RA patients indicate that Truxima is similar to reference products (EU and US-sourced rituximab) with regard to pharmacodynamics, safety, and efficacy for up to 24 weeks (DH Yoo et al. 2016 ACR/ARHP Annual Meeting, abstract 1635). ![]()
Costs prompt changes in drug use for cancer survivors
A new analysis indicates that cancer survivors may be more likely than the rest of the US population to change their prescription drug use due to financial concerns.
The study showed that cancer survivors were more likely to delay filling prescriptions, skip medication doses, request cheaper medications from their doctors, and engage in other cost-saving behaviors.
However, this was only true for non-elderly individuals.
There was no significant difference in cost-saving behaviors between elderly (age 65 and older) cancer survivors and elderly individuals in the general population.
Ahmedin Jemal, DVM, PhD, of the American Cancer Society in Atlanta, Georgia, and his colleagues reported these findings in Cancer.
The researchers used 2011-2014 data from the National Health Interview Survey, an annual household interview survey conducted by the US Centers for Disease Control and Prevention.
The survey included 8931 cancer survivors and 126,287 individuals without a cancer history.
Among non-elderly adults, 31.6% of those who had been diagnosed with cancer recently and 27.9% of those who had been diagnosed at least 2 years earlier reported a change in prescription drug use for financial reasons, compared with 21.4% of individuals without a history of cancer (P<0.05).
“Specifically, non-elderly cancer survivors were more likely to skip medication, delay filling a prescription, ask their doctor for lower-cost medication, and use alternative therapies for financial reasons compared with non-elderly individuals without a cancer history,” Dr Jemal said.
On the other hand, changes in prescription drug use for financial reasons were generally similar between elderly cancer survivors and elderly individuals without a cancer history.
The proportion of elderly individuals who changed their drug use for financial reasons was 24.9% among those who had been diagnosed with cancer recently, 21.8% among those who had been diagnosed at least 2 years earlier, and 20.4% among those without a history of cancer.
The researchers said these results could be explained by uniform healthcare coverage through Medicare.
The team also said their findings may have significant policy implications.
“Healthcare reforms addressing the financial burden of cancer among survivors, including the escalating cost of prescription drugs, should consider multiple comorbid conditions and high-deductible health plans, and the working poor,” Dr Jemal said.
“Our findings also have implications for doctor and patient communication about the financial burden of cancer when making treatment decisions, especially on the use of certain drugs that cost hundreds of thousands of dollars but with very small benefit compared with alternative and more affordable drugs.” ![]()
A new analysis indicates that cancer survivors may be more likely than the rest of the US population to change their prescription drug use due to financial concerns.
The study showed that cancer survivors were more likely to delay filling prescriptions, skip medication doses, request cheaper medications from their doctors, and engage in other cost-saving behaviors.
However, this was only true for non-elderly individuals.
There was no significant difference in cost-saving behaviors between elderly (age 65 and older) cancer survivors and elderly individuals in the general population.
Ahmedin Jemal, DVM, PhD, of the American Cancer Society in Atlanta, Georgia, and his colleagues reported these findings in Cancer.
The researchers used 2011-2014 data from the National Health Interview Survey, an annual household interview survey conducted by the US Centers for Disease Control and Prevention.
The survey included 8931 cancer survivors and 126,287 individuals without a cancer history.
Among non-elderly adults, 31.6% of those who had been diagnosed with cancer recently and 27.9% of those who had been diagnosed at least 2 years earlier reported a change in prescription drug use for financial reasons, compared with 21.4% of individuals without a history of cancer (P<0.05).
“Specifically, non-elderly cancer survivors were more likely to skip medication, delay filling a prescription, ask their doctor for lower-cost medication, and use alternative therapies for financial reasons compared with non-elderly individuals without a cancer history,” Dr Jemal said.
On the other hand, changes in prescription drug use for financial reasons were generally similar between elderly cancer survivors and elderly individuals without a cancer history.
The proportion of elderly individuals who changed their drug use for financial reasons was 24.9% among those who had been diagnosed with cancer recently, 21.8% among those who had been diagnosed at least 2 years earlier, and 20.4% among those without a history of cancer.
The researchers said these results could be explained by uniform healthcare coverage through Medicare.
The team also said their findings may have significant policy implications.
“Healthcare reforms addressing the financial burden of cancer among survivors, including the escalating cost of prescription drugs, should consider multiple comorbid conditions and high-deductible health plans, and the working poor,” Dr Jemal said.
“Our findings also have implications for doctor and patient communication about the financial burden of cancer when making treatment decisions, especially on the use of certain drugs that cost hundreds of thousands of dollars but with very small benefit compared with alternative and more affordable drugs.” ![]()
A new analysis indicates that cancer survivors may be more likely than the rest of the US population to change their prescription drug use due to financial concerns.
The study showed that cancer survivors were more likely to delay filling prescriptions, skip medication doses, request cheaper medications from their doctors, and engage in other cost-saving behaviors.
However, this was only true for non-elderly individuals.
There was no significant difference in cost-saving behaviors between elderly (age 65 and older) cancer survivors and elderly individuals in the general population.
Ahmedin Jemal, DVM, PhD, of the American Cancer Society in Atlanta, Georgia, and his colleagues reported these findings in Cancer.
The researchers used 2011-2014 data from the National Health Interview Survey, an annual household interview survey conducted by the US Centers for Disease Control and Prevention.
The survey included 8931 cancer survivors and 126,287 individuals without a cancer history.
Among non-elderly adults, 31.6% of those who had been diagnosed with cancer recently and 27.9% of those who had been diagnosed at least 2 years earlier reported a change in prescription drug use for financial reasons, compared with 21.4% of individuals without a history of cancer (P<0.05).
“Specifically, non-elderly cancer survivors were more likely to skip medication, delay filling a prescription, ask their doctor for lower-cost medication, and use alternative therapies for financial reasons compared with non-elderly individuals without a cancer history,” Dr Jemal said.
On the other hand, changes in prescription drug use for financial reasons were generally similar between elderly cancer survivors and elderly individuals without a cancer history.
The proportion of elderly individuals who changed their drug use for financial reasons was 24.9% among those who had been diagnosed with cancer recently, 21.8% among those who had been diagnosed at least 2 years earlier, and 20.4% among those without a history of cancer.
The researchers said these results could be explained by uniform healthcare coverage through Medicare.
The team also said their findings may have significant policy implications.
“Healthcare reforms addressing the financial burden of cancer among survivors, including the escalating cost of prescription drugs, should consider multiple comorbid conditions and high-deductible health plans, and the working poor,” Dr Jemal said.
“Our findings also have implications for doctor and patient communication about the financial burden of cancer when making treatment decisions, especially on the use of certain drugs that cost hundreds of thousands of dollars but with very small benefit compared with alternative and more affordable drugs.” ![]()
FDA grants priority review to ALL drug
The US Food and Drug Administration (FDA) has granted priority review for inotuzumab ozogamicin as a treatment for adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10-month period.
The Prescription Drug User Fee Act goal date for inotuzumab ozogamicin is August 2017.
About inotuzumab ozogamicin
Inotuzumab ozogamicin is an antibody-drug conjugate that consists of a monoclonal antibody targeting CD22 and a cytotoxic agent known as calicheamicin.
The product originates from a collaboration between Pfizer and Celltech (now UCB), but Pfizer has sole responsibility for all manufacturing and clinical development activities.
The application for inotuzumab ozogamicin is supported by results from a phase 3 trial, which were published in NEJM in June 2016.
The trial enrolled 326 adult patients with relapsed or refractory B-cell ALL and compared inotuzumab ozogamicin to standard of care chemotherapy.
The rate of complete remission, including incomplete hematologic recovery, was 80.7% in the inotuzumab arm and 29.4% in the chemotherapy arm (P<0.001). The median duration of remission was 4.6 months and 3.1 months, respectively (P=0.03).
Forty-one percent of patients treated with inotuzumab and 11% of those who received chemotherapy proceeded to stem cell transplant directly after treatment (P<0.001).
The median progression-free survival was 5.0 months in the inotuzumab arm and 1.8 months in the chemotherapy arm (P<0.001).
The median overall survival was 7.7 months and 6.7 months, respectively (P=0.04). This did not meet the prespecified boundary of significance (P=0.0208).
Liver-related adverse events were more common in the inotuzumab arm than the chemotherapy arm. The most frequent of these were increased aspartate aminotransferase level (20% vs 10%), hyperbilirubinemia (15% vs 10%), and increased alanine aminotransferase level (14% vs 11%).
Veno-occlusive liver disease occurred in 11% of patients in the inotuzumab arm and 1% in the chemotherapy arm.
There were 17 deaths during treatment in the inotuzumab arm and 11 in the chemotherapy arm. Four deaths were considered related to inotuzumab, and 2 were thought to be related to chemotherapy. ![]()
The US Food and Drug Administration (FDA) has granted priority review for inotuzumab ozogamicin as a treatment for adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10-month period.
The Prescription Drug User Fee Act goal date for inotuzumab ozogamicin is August 2017.
About inotuzumab ozogamicin
Inotuzumab ozogamicin is an antibody-drug conjugate that consists of a monoclonal antibody targeting CD22 and a cytotoxic agent known as calicheamicin.
The product originates from a collaboration between Pfizer and Celltech (now UCB), but Pfizer has sole responsibility for all manufacturing and clinical development activities.
The application for inotuzumab ozogamicin is supported by results from a phase 3 trial, which were published in NEJM in June 2016.
The trial enrolled 326 adult patients with relapsed or refractory B-cell ALL and compared inotuzumab ozogamicin to standard of care chemotherapy.
The rate of complete remission, including incomplete hematologic recovery, was 80.7% in the inotuzumab arm and 29.4% in the chemotherapy arm (P<0.001). The median duration of remission was 4.6 months and 3.1 months, respectively (P=0.03).
Forty-one percent of patients treated with inotuzumab and 11% of those who received chemotherapy proceeded to stem cell transplant directly after treatment (P<0.001).
The median progression-free survival was 5.0 months in the inotuzumab arm and 1.8 months in the chemotherapy arm (P<0.001).
The median overall survival was 7.7 months and 6.7 months, respectively (P=0.04). This did not meet the prespecified boundary of significance (P=0.0208).
Liver-related adverse events were more common in the inotuzumab arm than the chemotherapy arm. The most frequent of these were increased aspartate aminotransferase level (20% vs 10%), hyperbilirubinemia (15% vs 10%), and increased alanine aminotransferase level (14% vs 11%).
Veno-occlusive liver disease occurred in 11% of patients in the inotuzumab arm and 1% in the chemotherapy arm.
There were 17 deaths during treatment in the inotuzumab arm and 11 in the chemotherapy arm. Four deaths were considered related to inotuzumab, and 2 were thought to be related to chemotherapy. ![]()
The US Food and Drug Administration (FDA) has granted priority review for inotuzumab ozogamicin as a treatment for adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10-month period.
The Prescription Drug User Fee Act goal date for inotuzumab ozogamicin is August 2017.
About inotuzumab ozogamicin
Inotuzumab ozogamicin is an antibody-drug conjugate that consists of a monoclonal antibody targeting CD22 and a cytotoxic agent known as calicheamicin.
The product originates from a collaboration between Pfizer and Celltech (now UCB), but Pfizer has sole responsibility for all manufacturing and clinical development activities.
The application for inotuzumab ozogamicin is supported by results from a phase 3 trial, which were published in NEJM in June 2016.
The trial enrolled 326 adult patients with relapsed or refractory B-cell ALL and compared inotuzumab ozogamicin to standard of care chemotherapy.
The rate of complete remission, including incomplete hematologic recovery, was 80.7% in the inotuzumab arm and 29.4% in the chemotherapy arm (P<0.001). The median duration of remission was 4.6 months and 3.1 months, respectively (P=0.03).
Forty-one percent of patients treated with inotuzumab and 11% of those who received chemotherapy proceeded to stem cell transplant directly after treatment (P<0.001).
The median progression-free survival was 5.0 months in the inotuzumab arm and 1.8 months in the chemotherapy arm (P<0.001).
The median overall survival was 7.7 months and 6.7 months, respectively (P=0.04). This did not meet the prespecified boundary of significance (P=0.0208).
Liver-related adverse events were more common in the inotuzumab arm than the chemotherapy arm. The most frequent of these were increased aspartate aminotransferase level (20% vs 10%), hyperbilirubinemia (15% vs 10%), and increased alanine aminotransferase level (14% vs 11%).
Veno-occlusive liver disease occurred in 11% of patients in the inotuzumab arm and 1% in the chemotherapy arm.
There were 17 deaths during treatment in the inotuzumab arm and 11 in the chemotherapy arm. Four deaths were considered related to inotuzumab, and 2 were thought to be related to chemotherapy. ![]()
Tumor suppressor promotes FLT3-ITD AML
New research indicates that RUNX1, a known tumor suppressor, actually cooperates with internal tandem duplications in the FLT3 receptor tyrosine kinase (FLT3-ITD) to induce acute myeloid leukemia (AML).
Investigators say this discovery suggests that blocking RUNX1 activity will “greatly enhance” current therapeutic approaches using FLT3 inhibitors.
The discovery was published in The Journal of Experimental Medicine.
The research began when investigators noticed that many AML patients with FLT3-ITD also showed increased levels of RUNX1.
“This was unexpected because up to 20% of AML patients carry mutations that inactivate RUNX1, which is generally considered to be a tumor suppressor that prevents the formation of leukemias,” said study author Carol Stocking, PhD, of Heinrich-Pette-Institute-Leibniz Institute for Experimental Virology in Hamburg, Germany.
To investigate this finding, she and her colleagues injected mice with human AML cells expressing FLT3-ITD. The team found that reducing RUNX1 levels attenuated the cells’ ability to form tumors, but elevated RUNX1 levels worked with FLT3-ITD to induce AML.
Mouse hematopoietic stem cells expressing FLT3-ITD were highly proliferative, and co-expression of RUNX1 blocked their differentiation, allowing them to give rise to AML.
Mutant FLT3 appears to stabilize and activate RUNX1 by promoting the transcription factor’s phosphorylation. Active RUNX1 then blocks differentiation, at least in part, by upregulation of another transcription factor, Hhex.
The investigators found that hematopoietic stem cells expressing both Hhex and FLT3-ITD gave rise to AML.
RUNX1 may therefore suppress the initiation of AML but, after being activated by mutant FLT3, block differentiation and promote tumor development.
“Therapies that can reverse this differentiation block may offer significant therapeutic efficacy in AML patients with FLT3 mutations,” Dr Stocking said. “Ablating RUNX1 is toxic to leukemic cells but not to normal hematopoietic stem cells, so inhibiting RUNX1 may be a promising target in combination with FLT3 inhibitors.” ![]()
New research indicates that RUNX1, a known tumor suppressor, actually cooperates with internal tandem duplications in the FLT3 receptor tyrosine kinase (FLT3-ITD) to induce acute myeloid leukemia (AML).
Investigators say this discovery suggests that blocking RUNX1 activity will “greatly enhance” current therapeutic approaches using FLT3 inhibitors.
The discovery was published in The Journal of Experimental Medicine.
The research began when investigators noticed that many AML patients with FLT3-ITD also showed increased levels of RUNX1.
“This was unexpected because up to 20% of AML patients carry mutations that inactivate RUNX1, which is generally considered to be a tumor suppressor that prevents the formation of leukemias,” said study author Carol Stocking, PhD, of Heinrich-Pette-Institute-Leibniz Institute for Experimental Virology in Hamburg, Germany.
To investigate this finding, she and her colleagues injected mice with human AML cells expressing FLT3-ITD. The team found that reducing RUNX1 levels attenuated the cells’ ability to form tumors, but elevated RUNX1 levels worked with FLT3-ITD to induce AML.
Mouse hematopoietic stem cells expressing FLT3-ITD were highly proliferative, and co-expression of RUNX1 blocked their differentiation, allowing them to give rise to AML.
Mutant FLT3 appears to stabilize and activate RUNX1 by promoting the transcription factor’s phosphorylation. Active RUNX1 then blocks differentiation, at least in part, by upregulation of another transcription factor, Hhex.
The investigators found that hematopoietic stem cells expressing both Hhex and FLT3-ITD gave rise to AML.
RUNX1 may therefore suppress the initiation of AML but, after being activated by mutant FLT3, block differentiation and promote tumor development.
“Therapies that can reverse this differentiation block may offer significant therapeutic efficacy in AML patients with FLT3 mutations,” Dr Stocking said. “Ablating RUNX1 is toxic to leukemic cells but not to normal hematopoietic stem cells, so inhibiting RUNX1 may be a promising target in combination with FLT3 inhibitors.” ![]()
New research indicates that RUNX1, a known tumor suppressor, actually cooperates with internal tandem duplications in the FLT3 receptor tyrosine kinase (FLT3-ITD) to induce acute myeloid leukemia (AML).
Investigators say this discovery suggests that blocking RUNX1 activity will “greatly enhance” current therapeutic approaches using FLT3 inhibitors.
The discovery was published in The Journal of Experimental Medicine.
The research began when investigators noticed that many AML patients with FLT3-ITD also showed increased levels of RUNX1.
“This was unexpected because up to 20% of AML patients carry mutations that inactivate RUNX1, which is generally considered to be a tumor suppressor that prevents the formation of leukemias,” said study author Carol Stocking, PhD, of Heinrich-Pette-Institute-Leibniz Institute for Experimental Virology in Hamburg, Germany.
To investigate this finding, she and her colleagues injected mice with human AML cells expressing FLT3-ITD. The team found that reducing RUNX1 levels attenuated the cells’ ability to form tumors, but elevated RUNX1 levels worked with FLT3-ITD to induce AML.
Mouse hematopoietic stem cells expressing FLT3-ITD were highly proliferative, and co-expression of RUNX1 blocked their differentiation, allowing them to give rise to AML.
Mutant FLT3 appears to stabilize and activate RUNX1 by promoting the transcription factor’s phosphorylation. Active RUNX1 then blocks differentiation, at least in part, by upregulation of another transcription factor, Hhex.
The investigators found that hematopoietic stem cells expressing both Hhex and FLT3-ITD gave rise to AML.
RUNX1 may therefore suppress the initiation of AML but, after being activated by mutant FLT3, block differentiation and promote tumor development.
“Therapies that can reverse this differentiation block may offer significant therapeutic efficacy in AML patients with FLT3 mutations,” Dr Stocking said. “Ablating RUNX1 is toxic to leukemic cells but not to normal hematopoietic stem cells, so inhibiting RUNX1 may be a promising target in combination with FLT3 inhibitors.” ![]()
Immunotherapy receives fast track designation
The US Food and Drug Administration (FDA) has granted fast track designation to CMD-003 (baltaleucel-T) for patients with relapsed/refractory lymphoma and post-transplant lymphoproliferative disease associated with Epstein-Barr virus (EBV).
CMD-003 consists of patient-derived T cells that have been activated to kill malignant cells expressing antigens associated with EBV.
The T cells specifically target 4 EBV epitopes—LMP1, LMP2, EBNA, and BARF1.
CMD-003 is being developed by Cell Medica and the Baylor College of Medicine with funding provided, in part, by the Cancer Prevention and Research Institute of Texas.
About fast track designation
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the FDA’s fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the biologic license application or new drug application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
CMD-003-related research
CMD-003 is currently under investigation in the phase 2 CITADEL trial for patients with extranodal natural killer T-cell lymphoma and the phase 2 CIVIC trial for patients with EBV-associated diffuse large B-cell lymphoma, Hodgkin lymphoma, and post-transplant lymphoproliferative disease.
Researchers have not published results from any trials of CMD-003, but they have published results with EBV-specific T-cell products related to CMD-003.
In one study, published in the Journal of Clinical Oncology in 2014, researchers administered cytotoxic T lymphocytes (CTLs) in 50 patients with EBV-associated Hodgkin or non-Hodgkin lymphoma.
Twenty-nine of the patients were in remission when they received CTL infusions, but they were at a high risk of relapse. The remaining 21 patients had relapsed or refractory disease at the time of CTL infusion.
Twenty-seven of the patients who received CTLs as an adjuvant treatment remained in remission at 3.1 years after treatment.
Their 2-year event-free survival rate was 82%. None of the patients died of lymphoma, but 9 died from complications associated with the chemotherapy and radiation they had received.
Of the 21 patients with relapsed or refractory disease, 13 responded to CTL infusions, and 11 patients achieved a complete response. In this group, the 2-year event-free survival rate was about 50%.
The researchers said there were no toxicities that were definitively related to CTL infusion.
One patient had central nervous system deterioration 2 weeks after infusion. This was attributed to disease progression but could possibly have been treatment-related.
Another patient developed respiratory complications about 4 weeks after a second CTL infusion that may have been treatment-related. However, the researchers attributed it to an intercurrent infection, and the patient made a complete recovery.
The US Food and Drug Administration (FDA) has granted fast track designation to CMD-003 (baltaleucel-T) for patients with relapsed/refractory lymphoma and post-transplant lymphoproliferative disease associated with Epstein-Barr virus (EBV).
CMD-003 consists of patient-derived T cells that have been activated to kill malignant cells expressing antigens associated with EBV.
The T cells specifically target 4 EBV epitopes—LMP1, LMP2, EBNA, and BARF1.
CMD-003 is being developed by Cell Medica and the Baylor College of Medicine with funding provided, in part, by the Cancer Prevention and Research Institute of Texas.
About fast track designation
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the FDA’s fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the biologic license application or new drug application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
CMD-003-related research
CMD-003 is currently under investigation in the phase 2 CITADEL trial for patients with extranodal natural killer T-cell lymphoma and the phase 2 CIVIC trial for patients with EBV-associated diffuse large B-cell lymphoma, Hodgkin lymphoma, and post-transplant lymphoproliferative disease.
Researchers have not published results from any trials of CMD-003, but they have published results with EBV-specific T-cell products related to CMD-003.
In one study, published in the Journal of Clinical Oncology in 2014, researchers administered cytotoxic T lymphocytes (CTLs) in 50 patients with EBV-associated Hodgkin or non-Hodgkin lymphoma.
Twenty-nine of the patients were in remission when they received CTL infusions, but they were at a high risk of relapse. The remaining 21 patients had relapsed or refractory disease at the time of CTL infusion.
Twenty-seven of the patients who received CTLs as an adjuvant treatment remained in remission at 3.1 years after treatment.
Their 2-year event-free survival rate was 82%. None of the patients died of lymphoma, but 9 died from complications associated with the chemotherapy and radiation they had received.
Of the 21 patients with relapsed or refractory disease, 13 responded to CTL infusions, and 11 patients achieved a complete response. In this group, the 2-year event-free survival rate was about 50%.
The researchers said there were no toxicities that were definitively related to CTL infusion.
One patient had central nervous system deterioration 2 weeks after infusion. This was attributed to disease progression but could possibly have been treatment-related.
Another patient developed respiratory complications about 4 weeks after a second CTL infusion that may have been treatment-related. However, the researchers attributed it to an intercurrent infection, and the patient made a complete recovery.
The US Food and Drug Administration (FDA) has granted fast track designation to CMD-003 (baltaleucel-T) for patients with relapsed/refractory lymphoma and post-transplant lymphoproliferative disease associated with Epstein-Barr virus (EBV).
CMD-003 consists of patient-derived T cells that have been activated to kill malignant cells expressing antigens associated with EBV.
The T cells specifically target 4 EBV epitopes—LMP1, LMP2, EBNA, and BARF1.
CMD-003 is being developed by Cell Medica and the Baylor College of Medicine with funding provided, in part, by the Cancer Prevention and Research Institute of Texas.
About fast track designation
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the FDA’s fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the biologic license application or new drug application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
CMD-003-related research
CMD-003 is currently under investigation in the phase 2 CITADEL trial for patients with extranodal natural killer T-cell lymphoma and the phase 2 CIVIC trial for patients with EBV-associated diffuse large B-cell lymphoma, Hodgkin lymphoma, and post-transplant lymphoproliferative disease.
Researchers have not published results from any trials of CMD-003, but they have published results with EBV-specific T-cell products related to CMD-003.
In one study, published in the Journal of Clinical Oncology in 2014, researchers administered cytotoxic T lymphocytes (CTLs) in 50 patients with EBV-associated Hodgkin or non-Hodgkin lymphoma.
Twenty-nine of the patients were in remission when they received CTL infusions, but they were at a high risk of relapse. The remaining 21 patients had relapsed or refractory disease at the time of CTL infusion.
Twenty-seven of the patients who received CTLs as an adjuvant treatment remained in remission at 3.1 years after treatment.
Their 2-year event-free survival rate was 82%. None of the patients died of lymphoma, but 9 died from complications associated with the chemotherapy and radiation they had received.
Of the 21 patients with relapsed or refractory disease, 13 responded to CTL infusions, and 11 patients achieved a complete response. In this group, the 2-year event-free survival rate was about 50%.
The researchers said there were no toxicities that were definitively related to CTL infusion.
One patient had central nervous system deterioration 2 weeks after infusion. This was attributed to disease progression but could possibly have been treatment-related.
Another patient developed respiratory complications about 4 weeks after a second CTL infusion that may have been treatment-related. However, the researchers attributed it to an intercurrent infection, and the patient made a complete recovery.
How long Zika remains in body fluids
A study published in NEJM provides evidence that Zika virus RNA remain longer in blood and semen than in other body fluids, which suggests these may be superior diagnostic specimens.
This is the first study in which researchers examined multiple body fluids for the presence of Zika virus over a length of time.
The team sought to determine the frequency and duration of detectable Zika virus RNA in serum, saliva, urine, semen, and vaginal secretions.
They collected such specimens from 150 men and women in Puerto Rico who initially tested positive for Zika virus in urine or blood. The specimens were collected weekly for the first month and then at 2 months, 4 months, and 6 months.
The researchers tested all specimens using the Trioplex RT-PCR assay, a test that can be used to detect dengue, chikungunya, and Zika virus RNA. There have been allegations that this assay is less effective than a test used to detect Zika virus alone.
The researchers also performed validation analyses for the use of the Trioplex RT-PCR assay in semen. And they tested serum using the Zika IgM Antibody Capture Enzyme-Linked Immunosorbent Assay (Zika MAC-ELISA).
The US Centers for Disease Control and Prevention (CDC) developed both Zika MAC-ELISA and the Trioplex RT-PCR assay. This research was supported by the CDC.
After sample testing was complete, the researchers used parametric Weibull regression models to estimate the time to the loss of Zika virus RNA, which they reported in medians and 95th percentiles.
Results
The researchers said 88% of subjects (132/150) had detectable Zika virus RNA in at least 1 serum specimen. The median time to the loss of RNA detection in serum was 14 days (95% confidence interval [CI], 11 to 17), and the 95th percentile of time was 54 days (95% CI, 43 to 64).
The team also found that 61.7% of eligible subjects (92/149) had detectable Zika virus RNA in at least 1 urine specimen. The median time to the loss of RNA detection in urine was 8 days (95% CI, 6 to 10), and the 95th percentile of time was 39 days (95% CI, 31 to 47).
Fifteen subjects (10.1%) had detectable Zika virus RNA in urine but not serum, and 55 (36.7%) had RNA in serum but not urine.
Fifty-six percent of eligible male subjects (31/55) had Zika virus RNA in at least 1 semen specimen. The median time to loss of RNA detection in semen was 34 days (95% CI, 28 to 41), and the 95th percentile of time was 81 days (95% CI, 64 to 98).
The researchers noted that 11 of the 55 subjects had Zika virus RNA in their semen at their last visit and were still being followed at the time the NEJM article was written. The maximum duration of RNA detection was 125 days after the onset of symptoms.
Zika virus RNA levels were detectable in few saliva samples, with 10.2% of eligible subjects (15/147) having detectable levels in at least 1 saliva specimen.
Only 1 of 50 women (2%) had detectable Zika virus RNA in vaginal secretions.
“The findings of this study are important for both diagnostic and prevention purposes,” said study author Eli Rosenberg, PhD, of Emory University in Atlanta, Georgia.
“The results fully support current CDC sexual transmission recommendations but also provide critical information to help in understanding how often and how long evidence of Zika virus can be found in different body fluids. This knowledge is key to improving accuracy and effectiveness of testing methods while providing important baseline information for future research.” ![]()
A study published in NEJM provides evidence that Zika virus RNA remain longer in blood and semen than in other body fluids, which suggests these may be superior diagnostic specimens.
This is the first study in which researchers examined multiple body fluids for the presence of Zika virus over a length of time.
The team sought to determine the frequency and duration of detectable Zika virus RNA in serum, saliva, urine, semen, and vaginal secretions.
They collected such specimens from 150 men and women in Puerto Rico who initially tested positive for Zika virus in urine or blood. The specimens were collected weekly for the first month and then at 2 months, 4 months, and 6 months.
The researchers tested all specimens using the Trioplex RT-PCR assay, a test that can be used to detect dengue, chikungunya, and Zika virus RNA. There have been allegations that this assay is less effective than a test used to detect Zika virus alone.
The researchers also performed validation analyses for the use of the Trioplex RT-PCR assay in semen. And they tested serum using the Zika IgM Antibody Capture Enzyme-Linked Immunosorbent Assay (Zika MAC-ELISA).
The US Centers for Disease Control and Prevention (CDC) developed both Zika MAC-ELISA and the Trioplex RT-PCR assay. This research was supported by the CDC.
After sample testing was complete, the researchers used parametric Weibull regression models to estimate the time to the loss of Zika virus RNA, which they reported in medians and 95th percentiles.
Results
The researchers said 88% of subjects (132/150) had detectable Zika virus RNA in at least 1 serum specimen. The median time to the loss of RNA detection in serum was 14 days (95% confidence interval [CI], 11 to 17), and the 95th percentile of time was 54 days (95% CI, 43 to 64).
The team also found that 61.7% of eligible subjects (92/149) had detectable Zika virus RNA in at least 1 urine specimen. The median time to the loss of RNA detection in urine was 8 days (95% CI, 6 to 10), and the 95th percentile of time was 39 days (95% CI, 31 to 47).
Fifteen subjects (10.1%) had detectable Zika virus RNA in urine but not serum, and 55 (36.7%) had RNA in serum but not urine.
Fifty-six percent of eligible male subjects (31/55) had Zika virus RNA in at least 1 semen specimen. The median time to loss of RNA detection in semen was 34 days (95% CI, 28 to 41), and the 95th percentile of time was 81 days (95% CI, 64 to 98).
The researchers noted that 11 of the 55 subjects had Zika virus RNA in their semen at their last visit and were still being followed at the time the NEJM article was written. The maximum duration of RNA detection was 125 days after the onset of symptoms.
Zika virus RNA levels were detectable in few saliva samples, with 10.2% of eligible subjects (15/147) having detectable levels in at least 1 saliva specimen.
Only 1 of 50 women (2%) had detectable Zika virus RNA in vaginal secretions.
“The findings of this study are important for both diagnostic and prevention purposes,” said study author Eli Rosenberg, PhD, of Emory University in Atlanta, Georgia.
“The results fully support current CDC sexual transmission recommendations but also provide critical information to help in understanding how often and how long evidence of Zika virus can be found in different body fluids. This knowledge is key to improving accuracy and effectiveness of testing methods while providing important baseline information for future research.” ![]()
A study published in NEJM provides evidence that Zika virus RNA remain longer in blood and semen than in other body fluids, which suggests these may be superior diagnostic specimens.
This is the first study in which researchers examined multiple body fluids for the presence of Zika virus over a length of time.
The team sought to determine the frequency and duration of detectable Zika virus RNA in serum, saliva, urine, semen, and vaginal secretions.
They collected such specimens from 150 men and women in Puerto Rico who initially tested positive for Zika virus in urine or blood. The specimens were collected weekly for the first month and then at 2 months, 4 months, and 6 months.
The researchers tested all specimens using the Trioplex RT-PCR assay, a test that can be used to detect dengue, chikungunya, and Zika virus RNA. There have been allegations that this assay is less effective than a test used to detect Zika virus alone.
The researchers also performed validation analyses for the use of the Trioplex RT-PCR assay in semen. And they tested serum using the Zika IgM Antibody Capture Enzyme-Linked Immunosorbent Assay (Zika MAC-ELISA).
The US Centers for Disease Control and Prevention (CDC) developed both Zika MAC-ELISA and the Trioplex RT-PCR assay. This research was supported by the CDC.
After sample testing was complete, the researchers used parametric Weibull regression models to estimate the time to the loss of Zika virus RNA, which they reported in medians and 95th percentiles.
Results
The researchers said 88% of subjects (132/150) had detectable Zika virus RNA in at least 1 serum specimen. The median time to the loss of RNA detection in serum was 14 days (95% confidence interval [CI], 11 to 17), and the 95th percentile of time was 54 days (95% CI, 43 to 64).
The team also found that 61.7% of eligible subjects (92/149) had detectable Zika virus RNA in at least 1 urine specimen. The median time to the loss of RNA detection in urine was 8 days (95% CI, 6 to 10), and the 95th percentile of time was 39 days (95% CI, 31 to 47).
Fifteen subjects (10.1%) had detectable Zika virus RNA in urine but not serum, and 55 (36.7%) had RNA in serum but not urine.
Fifty-six percent of eligible male subjects (31/55) had Zika virus RNA in at least 1 semen specimen. The median time to loss of RNA detection in semen was 34 days (95% CI, 28 to 41), and the 95th percentile of time was 81 days (95% CI, 64 to 98).
The researchers noted that 11 of the 55 subjects had Zika virus RNA in their semen at their last visit and were still being followed at the time the NEJM article was written. The maximum duration of RNA detection was 125 days after the onset of symptoms.
Zika virus RNA levels were detectable in few saliva samples, with 10.2% of eligible subjects (15/147) having detectable levels in at least 1 saliva specimen.
Only 1 of 50 women (2%) had detectable Zika virus RNA in vaginal secretions.
“The findings of this study are important for both diagnostic and prevention purposes,” said study author Eli Rosenberg, PhD, of Emory University in Atlanta, Georgia.
“The results fully support current CDC sexual transmission recommendations but also provide critical information to help in understanding how often and how long evidence of Zika virus can be found in different body fluids. This knowledge is key to improving accuracy and effectiveness of testing methods while providing important baseline information for future research.” ![]()
Model illustrates progression to MDS, AML
Researchers say they have created a model that shows the step-by-step progression from normal blood cells to acute myeloid leukemia (AML).
The team generated induced pluripotent stem cell (iPSC) lines capturing disease stages that included preleukemia, low-risk myelodysplastic syndrome (MDS), high-risk MDS, and AML.
The researchers then used CRISPR/Cas9 genome editing to induce disease progression and reversal.
And they used the iPSCs to uncover disease-stage-specific effects of 2 drugs.
Eirini P. Papapetrou, MD, PhD, of the Icahn School of Medicine at Mount Sinai in New York, New York, and her colleagues described this work in Cell Stem Cell.
The researchers first explained how they generated patient-derived iPSCs that represented familial predisposition to myeloid malignancy, low-risk and high-risk MDS, and AML.
By studying these iPSC lines, the team uncovered “a phenotypic road map of disease progression” that led to a “serially transplantable leukemia.”
“We are encouraged by the discovery that it was possible to generate potent, engraftable leukemia derived from AML induced pluripotent stem cells,” said study author Michael G. Kharas, PhD, of the Icahn School of Medicine at Mount Sinai.
The researchers also showed that they could revert a high-risk MDS iPSC line to a premalignant state by correcting a chromosome 7q deletion.
And they could force progression in a preleukemic iPSC line. The team induced progression to low-risk MDS by inactivating the second GATA2 allele and progression to high-risk MDS by deleting chromosome 7q.
“This work shows that integrated patient cell reprogramming and cancer genetics is a powerful way to dissect cancer progression,” Dr Kharas said.
The researchers reported that, ultimately, they were able to model the stepwise progression of normal cells to preleukemia and MDS by sequentially introducing genetic lesions associated with earlier and later disease stages (ASXL1 truncation and chromosome 7q deletion, respectively).
“The new model will empower investigation into the cellular and molecular events underlying the development of leukemia in ways that were not possible before,” Dr Papapetrou said.
She added that the group’s findings provide a framework to aid investigation into disease mechanisms, events driving progression, and drug responses.
In fact, the researchers did use hematopoietic progenitor cells (HPCs) derived from their iPSCs to analyze the disease-stage-specific effects of 2 drugs—5-azacytidine and rigosertib.
The team said they found evidence to suggest that 5-azacytidine may work in low-risk MDS by affecting differentiation, and the drug’s main therapeutic action in high-risk MDS might be mediated through selective inhibition of the MDS clone.
The researchers tested rigosertib in HPCs derived from 2 AML lines (from the same patient) that captured 2 different disease stages. One line was derived from the dominant clone (del 7q), and the other was derived from a KRAS-mutated subclone.
The team found that HPCs derived from the KRAS-mutated line demonstrated “marked sensitivity” to rigosertib, but the other HPCs were “marginally affected.” ![]()
Researchers say they have created a model that shows the step-by-step progression from normal blood cells to acute myeloid leukemia (AML).
The team generated induced pluripotent stem cell (iPSC) lines capturing disease stages that included preleukemia, low-risk myelodysplastic syndrome (MDS), high-risk MDS, and AML.
The researchers then used CRISPR/Cas9 genome editing to induce disease progression and reversal.
And they used the iPSCs to uncover disease-stage-specific effects of 2 drugs.
Eirini P. Papapetrou, MD, PhD, of the Icahn School of Medicine at Mount Sinai in New York, New York, and her colleagues described this work in Cell Stem Cell.
The researchers first explained how they generated patient-derived iPSCs that represented familial predisposition to myeloid malignancy, low-risk and high-risk MDS, and AML.
By studying these iPSC lines, the team uncovered “a phenotypic road map of disease progression” that led to a “serially transplantable leukemia.”
“We are encouraged by the discovery that it was possible to generate potent, engraftable leukemia derived from AML induced pluripotent stem cells,” said study author Michael G. Kharas, PhD, of the Icahn School of Medicine at Mount Sinai.
The researchers also showed that they could revert a high-risk MDS iPSC line to a premalignant state by correcting a chromosome 7q deletion.
And they could force progression in a preleukemic iPSC line. The team induced progression to low-risk MDS by inactivating the second GATA2 allele and progression to high-risk MDS by deleting chromosome 7q.
“This work shows that integrated patient cell reprogramming and cancer genetics is a powerful way to dissect cancer progression,” Dr Kharas said.
The researchers reported that, ultimately, they were able to model the stepwise progression of normal cells to preleukemia and MDS by sequentially introducing genetic lesions associated with earlier and later disease stages (ASXL1 truncation and chromosome 7q deletion, respectively).
“The new model will empower investigation into the cellular and molecular events underlying the development of leukemia in ways that were not possible before,” Dr Papapetrou said.
She added that the group’s findings provide a framework to aid investigation into disease mechanisms, events driving progression, and drug responses.
In fact, the researchers did use hematopoietic progenitor cells (HPCs) derived from their iPSCs to analyze the disease-stage-specific effects of 2 drugs—5-azacytidine and rigosertib.
The team said they found evidence to suggest that 5-azacytidine may work in low-risk MDS by affecting differentiation, and the drug’s main therapeutic action in high-risk MDS might be mediated through selective inhibition of the MDS clone.
The researchers tested rigosertib in HPCs derived from 2 AML lines (from the same patient) that captured 2 different disease stages. One line was derived from the dominant clone (del 7q), and the other was derived from a KRAS-mutated subclone.
The team found that HPCs derived from the KRAS-mutated line demonstrated “marked sensitivity” to rigosertib, but the other HPCs were “marginally affected.” ![]()
Researchers say they have created a model that shows the step-by-step progression from normal blood cells to acute myeloid leukemia (AML).
The team generated induced pluripotent stem cell (iPSC) lines capturing disease stages that included preleukemia, low-risk myelodysplastic syndrome (MDS), high-risk MDS, and AML.
The researchers then used CRISPR/Cas9 genome editing to induce disease progression and reversal.
And they used the iPSCs to uncover disease-stage-specific effects of 2 drugs.
Eirini P. Papapetrou, MD, PhD, of the Icahn School of Medicine at Mount Sinai in New York, New York, and her colleagues described this work in Cell Stem Cell.
The researchers first explained how they generated patient-derived iPSCs that represented familial predisposition to myeloid malignancy, low-risk and high-risk MDS, and AML.
By studying these iPSC lines, the team uncovered “a phenotypic road map of disease progression” that led to a “serially transplantable leukemia.”
“We are encouraged by the discovery that it was possible to generate potent, engraftable leukemia derived from AML induced pluripotent stem cells,” said study author Michael G. Kharas, PhD, of the Icahn School of Medicine at Mount Sinai.
The researchers also showed that they could revert a high-risk MDS iPSC line to a premalignant state by correcting a chromosome 7q deletion.
And they could force progression in a preleukemic iPSC line. The team induced progression to low-risk MDS by inactivating the second GATA2 allele and progression to high-risk MDS by deleting chromosome 7q.
“This work shows that integrated patient cell reprogramming and cancer genetics is a powerful way to dissect cancer progression,” Dr Kharas said.
The researchers reported that, ultimately, they were able to model the stepwise progression of normal cells to preleukemia and MDS by sequentially introducing genetic lesions associated with earlier and later disease stages (ASXL1 truncation and chromosome 7q deletion, respectively).
“The new model will empower investigation into the cellular and molecular events underlying the development of leukemia in ways that were not possible before,” Dr Papapetrou said.
She added that the group’s findings provide a framework to aid investigation into disease mechanisms, events driving progression, and drug responses.
In fact, the researchers did use hematopoietic progenitor cells (HPCs) derived from their iPSCs to analyze the disease-stage-specific effects of 2 drugs—5-azacytidine and rigosertib.
The team said they found evidence to suggest that 5-azacytidine may work in low-risk MDS by affecting differentiation, and the drug’s main therapeutic action in high-risk MDS might be mediated through selective inhibition of the MDS clone.
The researchers tested rigosertib in HPCs derived from 2 AML lines (from the same patient) that captured 2 different disease stages. One line was derived from the dominant clone (del 7q), and the other was derived from a KRAS-mutated subclone.
The team found that HPCs derived from the KRAS-mutated line demonstrated “marked sensitivity” to rigosertib, but the other HPCs were “marginally affected.” ![]()
Walking can benefit advanced cancer patients
Walking for 30 minutes 3 times a week can improve quality of life for patients with advanced cancer, according to research published in BMJ Open.
The study indicated that some patients with advanced cancer may not be able to commit to weekly walks with a group of fellow patients.
However, some patients enjoyed walking in groups, and most reported benefits from regular walks, whether taken alone or with others.
“Findings from this important study show that exercise is valued by, suitable for, and beneficial to people with advanced cancer,” said study author Emma Ream, RN, PhD, of the University of Surrey in the UK.
“Rather than shying away from exercise, people with advanced disease should be encouraged to be more active and incorporate exercise into their daily lives where possible.”
One hundred and ten patients with advanced cancer were eligible to participate in this study, but 49 (47%) declined, primarily because of work commitments. Patients said they could not commit to a weekly walking group.
The 42 patients who did participate in this study were divided into 2 groups.
Group 1 (n=21) received coaching, which included a short motivational interview, as well as the recommendation to walk for at least 30 minutes on alternate days and attend a volunteer-led group walk weekly.
Patients in group 2 (n=21) were encouraged to maintain their current level of activity.
Nineteen participants (45%) withdrew from the study—11 in group 1 and 8 in group 2. In general, patients did not provide reasons for withdrawal. However, 2 patients were too unwell to participate, and 2 patients died during the study.
At 6, 12, and 24 weeks, scores on quality of life questionnaires were not significantly different between groups 1 and 2.
However, in interviews, patients in group 1 said they felt walking provided physical, emotional, and psychological benefits, as well as improvements in social well-being and lifestyle.
At 24 weeks, 8 of 9 participants in group 1 said they found the walking intervention useful, and 7 participants said they were satisfied with it.
Some patients said walking improved their attitude toward their illness and spoke of the social benefits of participating in group walks.
But other patients were dissatisfied with the walking groups. They reported accessibility issues and a dislike of group activities. One younger individual felt the group was more appropriate for older patients.
“This study is a first step towards exploring how walking can help people living with advanced cancer,” said study author Jo Armes, RGN, PhD, of King’s College London in the UK.
“Walking is a free and accessible form of physical activity, and patients reported that it made a real difference to their quality of life. Further research is needed with a larger number of people to provide definitive evidence that walking improves both health outcomes and social and emotional wellbeing in this group of people.” ![]()
Walking for 30 minutes 3 times a week can improve quality of life for patients with advanced cancer, according to research published in BMJ Open.
The study indicated that some patients with advanced cancer may not be able to commit to weekly walks with a group of fellow patients.
However, some patients enjoyed walking in groups, and most reported benefits from regular walks, whether taken alone or with others.
“Findings from this important study show that exercise is valued by, suitable for, and beneficial to people with advanced cancer,” said study author Emma Ream, RN, PhD, of the University of Surrey in the UK.
“Rather than shying away from exercise, people with advanced disease should be encouraged to be more active and incorporate exercise into their daily lives where possible.”
One hundred and ten patients with advanced cancer were eligible to participate in this study, but 49 (47%) declined, primarily because of work commitments. Patients said they could not commit to a weekly walking group.
The 42 patients who did participate in this study were divided into 2 groups.
Group 1 (n=21) received coaching, which included a short motivational interview, as well as the recommendation to walk for at least 30 minutes on alternate days and attend a volunteer-led group walk weekly.
Patients in group 2 (n=21) were encouraged to maintain their current level of activity.
Nineteen participants (45%) withdrew from the study—11 in group 1 and 8 in group 2. In general, patients did not provide reasons for withdrawal. However, 2 patients were too unwell to participate, and 2 patients died during the study.
At 6, 12, and 24 weeks, scores on quality of life questionnaires were not significantly different between groups 1 and 2.
However, in interviews, patients in group 1 said they felt walking provided physical, emotional, and psychological benefits, as well as improvements in social well-being and lifestyle.
At 24 weeks, 8 of 9 participants in group 1 said they found the walking intervention useful, and 7 participants said they were satisfied with it.
Some patients said walking improved their attitude toward their illness and spoke of the social benefits of participating in group walks.
But other patients were dissatisfied with the walking groups. They reported accessibility issues and a dislike of group activities. One younger individual felt the group was more appropriate for older patients.
“This study is a first step towards exploring how walking can help people living with advanced cancer,” said study author Jo Armes, RGN, PhD, of King’s College London in the UK.
“Walking is a free and accessible form of physical activity, and patients reported that it made a real difference to their quality of life. Further research is needed with a larger number of people to provide definitive evidence that walking improves both health outcomes and social and emotional wellbeing in this group of people.” ![]()
Walking for 30 minutes 3 times a week can improve quality of life for patients with advanced cancer, according to research published in BMJ Open.
The study indicated that some patients with advanced cancer may not be able to commit to weekly walks with a group of fellow patients.
However, some patients enjoyed walking in groups, and most reported benefits from regular walks, whether taken alone or with others.
“Findings from this important study show that exercise is valued by, suitable for, and beneficial to people with advanced cancer,” said study author Emma Ream, RN, PhD, of the University of Surrey in the UK.
“Rather than shying away from exercise, people with advanced disease should be encouraged to be more active and incorporate exercise into their daily lives where possible.”
One hundred and ten patients with advanced cancer were eligible to participate in this study, but 49 (47%) declined, primarily because of work commitments. Patients said they could not commit to a weekly walking group.
The 42 patients who did participate in this study were divided into 2 groups.
Group 1 (n=21) received coaching, which included a short motivational interview, as well as the recommendation to walk for at least 30 minutes on alternate days and attend a volunteer-led group walk weekly.
Patients in group 2 (n=21) were encouraged to maintain their current level of activity.
Nineteen participants (45%) withdrew from the study—11 in group 1 and 8 in group 2. In general, patients did not provide reasons for withdrawal. However, 2 patients were too unwell to participate, and 2 patients died during the study.
At 6, 12, and 24 weeks, scores on quality of life questionnaires were not significantly different between groups 1 and 2.
However, in interviews, patients in group 1 said they felt walking provided physical, emotional, and psychological benefits, as well as improvements in social well-being and lifestyle.
At 24 weeks, 8 of 9 participants in group 1 said they found the walking intervention useful, and 7 participants said they were satisfied with it.
Some patients said walking improved their attitude toward their illness and spoke of the social benefits of participating in group walks.
But other patients were dissatisfied with the walking groups. They reported accessibility issues and a dislike of group activities. One younger individual felt the group was more appropriate for older patients.
“This study is a first step towards exploring how walking can help people living with advanced cancer,” said study author Jo Armes, RGN, PhD, of King’s College London in the UK.
“Walking is a free and accessible form of physical activity, and patients reported that it made a real difference to their quality of life. Further research is needed with a larger number of people to provide definitive evidence that walking improves both health outcomes and social and emotional wellbeing in this group of people.”