User login
Ulcerative Colitis With Background Mucosal Inflammation Signals Poor Survival in Colorectal Cancer
Ulcerative Colitis With Background Mucosal Inflammation Signals Poor Survival in Colorectal Cancer
TOPLINE:
Among patients with ulcerative colitis (UC) who develop colorectal cancer (CRC), greater background mucosal inflammation at the time of CRC diagnosis is associated with progressively worse survival outcomes, with tumors arising within the UC-involved segment having worse prognosis.
METHODOLOGY:
- Patients with UC are at an increased risk for CRC, with risk influenced by the extent and intensity of underlying mucosal inflammation.
- Researchers retrospectively reviewed medical records of patients with UC diagnosed with CRC between 1983 and 2020 at 43 institutions across Japan to determine whether inflammation at cancer diagnosis affected prognosis.
- After endoscopic assessment, tumors were classified as arising inside the UC‑involved segment at diagnosis (within‑area tumors) or outside that segment (outside‑area tumors).
- The Mayo endoscopic score (MES) was used to grade background mucosal inflammation in the within‑area group as inactive (MES 0), mild-moderate (MES 1-2), or severe (MES 3).
- The primary endpoint was 5-year recurrence-free survival, and the secondary endpoint was 5-year cancer-specific survival.
TAKEAWAY:
- Among 723 patients followed for a median of 51 months, 683 had within-area tumors (mean age at CRC diagnosis, 51.8 years; 61.9% male) and 40 had outside-area tumors (mean age at CRC diagnosis, 61.1 years; 60.0% male).
- The within-area group had lower rate of 5-year recurrence-free survival than the outside-area group (75.1% vs 87.6%; P = .022), and lower rate of 5-year cancer-specific survival (81.1% vs 94.3%; P = .038).
- Within-area tumor location independently predicted worse recurrence-free survival (adjusted hazard ratio, 2.99; P = .030).
- In the within‑area group, higher MES was associated with stepwise (although nonsignificant) declines in recurrence‑free survival (inactive, 84.4%; mild-moderate, 79.4%; severe, 73.8%; P = .150). Corresponding cancer‑specific survival rates in these groups declined significantly (89.0%, 84.8%, and 73.8%, respectively; P = .048).
IN PRACTICE:
“These findings shift the clinical focus from inflammation as a risk factor for carcinogenesis to inflammation as a prognostic determinant, highlighting a potential new role for systematic endoscopic assessment of the background mucosa at cancer diagnosis,” the authors wrote.
SOURCE:
This study was led by Akiyoshi Ikebata, Department of Surgery, Keio University School of Medicine, Tokyo, Japan. It was published online in December 2025, in the Journal of Crohn's and Colitis.
LIMITATIONS:
The retrospective design introduced potential for unmeasured confounding and selection bias. The MES was assigned by local physicians without central review, which may have introduced variability. The small size of the outside‑area tumor group increased the risk for baseline imbalances.
DISCLOSURES:
No specific funding source was reported. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Among patients with ulcerative colitis (UC) who develop colorectal cancer (CRC), greater background mucosal inflammation at the time of CRC diagnosis is associated with progressively worse survival outcomes, with tumors arising within the UC-involved segment having worse prognosis.
METHODOLOGY:
- Patients with UC are at an increased risk for CRC, with risk influenced by the extent and intensity of underlying mucosal inflammation.
- Researchers retrospectively reviewed medical records of patients with UC diagnosed with CRC between 1983 and 2020 at 43 institutions across Japan to determine whether inflammation at cancer diagnosis affected prognosis.
- After endoscopic assessment, tumors were classified as arising inside the UC‑involved segment at diagnosis (within‑area tumors) or outside that segment (outside‑area tumors).
- The Mayo endoscopic score (MES) was used to grade background mucosal inflammation in the within‑area group as inactive (MES 0), mild-moderate (MES 1-2), or severe (MES 3).
- The primary endpoint was 5-year recurrence-free survival, and the secondary endpoint was 5-year cancer-specific survival.
TAKEAWAY:
- Among 723 patients followed for a median of 51 months, 683 had within-area tumors (mean age at CRC diagnosis, 51.8 years; 61.9% male) and 40 had outside-area tumors (mean age at CRC diagnosis, 61.1 years; 60.0% male).
- The within-area group had lower rate of 5-year recurrence-free survival than the outside-area group (75.1% vs 87.6%; P = .022), and lower rate of 5-year cancer-specific survival (81.1% vs 94.3%; P = .038).
- Within-area tumor location independently predicted worse recurrence-free survival (adjusted hazard ratio, 2.99; P = .030).
- In the within‑area group, higher MES was associated with stepwise (although nonsignificant) declines in recurrence‑free survival (inactive, 84.4%; mild-moderate, 79.4%; severe, 73.8%; P = .150). Corresponding cancer‑specific survival rates in these groups declined significantly (89.0%, 84.8%, and 73.8%, respectively; P = .048).
IN PRACTICE:
“These findings shift the clinical focus from inflammation as a risk factor for carcinogenesis to inflammation as a prognostic determinant, highlighting a potential new role for systematic endoscopic assessment of the background mucosa at cancer diagnosis,” the authors wrote.
SOURCE:
This study was led by Akiyoshi Ikebata, Department of Surgery, Keio University School of Medicine, Tokyo, Japan. It was published online in December 2025, in the Journal of Crohn's and Colitis.
LIMITATIONS:
The retrospective design introduced potential for unmeasured confounding and selection bias. The MES was assigned by local physicians without central review, which may have introduced variability. The small size of the outside‑area tumor group increased the risk for baseline imbalances.
DISCLOSURES:
No specific funding source was reported. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Among patients with ulcerative colitis (UC) who develop colorectal cancer (CRC), greater background mucosal inflammation at the time of CRC diagnosis is associated with progressively worse survival outcomes, with tumors arising within the UC-involved segment having worse prognosis.
METHODOLOGY:
- Patients with UC are at an increased risk for CRC, with risk influenced by the extent and intensity of underlying mucosal inflammation.
- Researchers retrospectively reviewed medical records of patients with UC diagnosed with CRC between 1983 and 2020 at 43 institutions across Japan to determine whether inflammation at cancer diagnosis affected prognosis.
- After endoscopic assessment, tumors were classified as arising inside the UC‑involved segment at diagnosis (within‑area tumors) or outside that segment (outside‑area tumors).
- The Mayo endoscopic score (MES) was used to grade background mucosal inflammation in the within‑area group as inactive (MES 0), mild-moderate (MES 1-2), or severe (MES 3).
- The primary endpoint was 5-year recurrence-free survival, and the secondary endpoint was 5-year cancer-specific survival.
TAKEAWAY:
- Among 723 patients followed for a median of 51 months, 683 had within-area tumors (mean age at CRC diagnosis, 51.8 years; 61.9% male) and 40 had outside-area tumors (mean age at CRC diagnosis, 61.1 years; 60.0% male).
- The within-area group had lower rate of 5-year recurrence-free survival than the outside-area group (75.1% vs 87.6%; P = .022), and lower rate of 5-year cancer-specific survival (81.1% vs 94.3%; P = .038).
- Within-area tumor location independently predicted worse recurrence-free survival (adjusted hazard ratio, 2.99; P = .030).
- In the within‑area group, higher MES was associated with stepwise (although nonsignificant) declines in recurrence‑free survival (inactive, 84.4%; mild-moderate, 79.4%; severe, 73.8%; P = .150). Corresponding cancer‑specific survival rates in these groups declined significantly (89.0%, 84.8%, and 73.8%, respectively; P = .048).
IN PRACTICE:
“These findings shift the clinical focus from inflammation as a risk factor for carcinogenesis to inflammation as a prognostic determinant, highlighting a potential new role for systematic endoscopic assessment of the background mucosa at cancer diagnosis,” the authors wrote.
SOURCE:
This study was led by Akiyoshi Ikebata, Department of Surgery, Keio University School of Medicine, Tokyo, Japan. It was published online in December 2025, in the Journal of Crohn's and Colitis.
LIMITATIONS:
The retrospective design introduced potential for unmeasured confounding and selection bias. The MES was assigned by local physicians without central review, which may have introduced variability. The small size of the outside‑area tumor group increased the risk for baseline imbalances.
DISCLOSURES:
No specific funding source was reported. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Ulcerative Colitis With Background Mucosal Inflammation Signals Poor Survival in Colorectal Cancer
Ulcerative Colitis With Background Mucosal Inflammation Signals Poor Survival in Colorectal Cancer
Is It Safe to Skip Surgery After Malignant Colorectal Polyp Removal?
Is It Safe to Skip Surgery After Malignant Colorectal Polyp Removal?
TOPLINE:
Among patients with high-risk malignant colorectal polyps, 19% had residual disease, with rates of 25% in the immediate surgery group vs 9% in the nonoperative management group. The rate of rectum and sphincter preservation in the nonoperative surveillance group was over 90%, and all recurrences were successfully treated with salvage surgery or chemoradiotherapy.
METHODOLOGY:
- Although guidelines in the US recommend colorectal resection when a malignant colorectal polyp has high-risk features, some patients choose nonoperative management instead to avoid the associated averse effects and impact on quality of life. The safety of nonoperative management, however, remains unclear.
- A single-center cohort study conducted between 2015 and 2022 included 336 patients who underwent polypectomy in the colon (n = 226) or rectum (n = 110) and had at least one high-risk feature. High-risk features included positive margins, piecemeal resection with unclear margin, lymphovascular invasion, perineural invasion, poor differentiation, and tumor budding.
- The analysis compared rates of residual disease between those who had immediate surgery (62%) and nonoperative management (38%) following the removal of a malignant polyp, 15% of whom (n = 19) received systemic chemotherapy after polypectomy.
- Researchers also assessed the rates of distant metastasis between the two groups and the association between specific high-risk features and residual disease or post-treatment complications.
TAKEAWAY:
- In the overall population, 19% of patients had residual disease (63 of 336). Among the 208 patients who had immediate surgery, 25% (n = 51) had residual disease, including 9% (n = 19) with residual disease in the bowel wall and 19% (n = 39) in locoregional lymph nodes. Postoperative complications occurred in 12% of patients (n = 25) in the immediate surgery group, with 3% (n = 7) having complications considered grade 3 or higher.
- Among the 128 patients who received nonoperative surveillance, 9% (n = 12) developed recurrence during surveillance, 6% (n = 7) in the bowel wall and 4% (n = 5) in locoregional lymph nodes. All recurrences in the nonoperative surveillance group were successfully treated with either salvage surgery (n = 6) or chemoradiotherapy (n = 6).
- Among patients in the nonoperative group with a malignant polyp removed from the rectum, the rate of rectum preservation was 94% (74 of 79 patients); the sphincter preservation rate was 91% for tumors < 5 cm from the anal verge.
- Distant metastases occurred in 2% of all patients across both groups.
IN PRACTICE:
"The risk of residual disease after the removal of a malignant colorectal polyp with [high-risk features] is considerable, but nonoperative management offers the potential for organ preservation, with the availability of effective salvage options if rectal cancer is detected," the authors of the study concluded.
SOURCE:
The study, led by Thikhamporn Tawantanakorn, MD, and Martin R. Weiser, MD, of Memorial Sloan Kettering Cancer Center in New York City, was published online in JCO Oncology Advances.
LIMITATIONS:
The researchers noted several limitations, including variable follow-up among patients and challenges in assessing polypectomy histology, particularly after piecemeal resection, which limited evaluation of certain high-risk features such as tumor budding. Additionally, as the study was conducted at a specialized cancer center with dedicated gastrointestinal pathology and radiology services and readily available office endoscopy, the results may not be fully generalizable to less specialized centers.
DISCLOSURES:
Jinru Shia, MD, reported receiving consulting fees from Paige.AI and research funding through their institution. Andrea Cercek, MD, disclosed consulting roles with multiple pharmaceutical companies, including GlaxoSmithKline, Incyte, Merck, and others, as well as research funding from GlaxoSmithKline and Pfizer. Weiser reported receiving royalties as a section editor for UpToDate. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Among patients with high-risk malignant colorectal polyps, 19% had residual disease, with rates of 25% in the immediate surgery group vs 9% in the nonoperative management group. The rate of rectum and sphincter preservation in the nonoperative surveillance group was over 90%, and all recurrences were successfully treated with salvage surgery or chemoradiotherapy.
METHODOLOGY:
- Although guidelines in the US recommend colorectal resection when a malignant colorectal polyp has high-risk features, some patients choose nonoperative management instead to avoid the associated averse effects and impact on quality of life. The safety of nonoperative management, however, remains unclear.
- A single-center cohort study conducted between 2015 and 2022 included 336 patients who underwent polypectomy in the colon (n = 226) or rectum (n = 110) and had at least one high-risk feature. High-risk features included positive margins, piecemeal resection with unclear margin, lymphovascular invasion, perineural invasion, poor differentiation, and tumor budding.
- The analysis compared rates of residual disease between those who had immediate surgery (62%) and nonoperative management (38%) following the removal of a malignant polyp, 15% of whom (n = 19) received systemic chemotherapy after polypectomy.
- Researchers also assessed the rates of distant metastasis between the two groups and the association between specific high-risk features and residual disease or post-treatment complications.
TAKEAWAY:
- In the overall population, 19% of patients had residual disease (63 of 336). Among the 208 patients who had immediate surgery, 25% (n = 51) had residual disease, including 9% (n = 19) with residual disease in the bowel wall and 19% (n = 39) in locoregional lymph nodes. Postoperative complications occurred in 12% of patients (n = 25) in the immediate surgery group, with 3% (n = 7) having complications considered grade 3 or higher.
- Among the 128 patients who received nonoperative surveillance, 9% (n = 12) developed recurrence during surveillance, 6% (n = 7) in the bowel wall and 4% (n = 5) in locoregional lymph nodes. All recurrences in the nonoperative surveillance group were successfully treated with either salvage surgery (n = 6) or chemoradiotherapy (n = 6).
- Among patients in the nonoperative group with a malignant polyp removed from the rectum, the rate of rectum preservation was 94% (74 of 79 patients); the sphincter preservation rate was 91% for tumors < 5 cm from the anal verge.
- Distant metastases occurred in 2% of all patients across both groups.
IN PRACTICE:
"The risk of residual disease after the removal of a malignant colorectal polyp with [high-risk features] is considerable, but nonoperative management offers the potential for organ preservation, with the availability of effective salvage options if rectal cancer is detected," the authors of the study concluded.
SOURCE:
The study, led by Thikhamporn Tawantanakorn, MD, and Martin R. Weiser, MD, of Memorial Sloan Kettering Cancer Center in New York City, was published online in JCO Oncology Advances.
LIMITATIONS:
The researchers noted several limitations, including variable follow-up among patients and challenges in assessing polypectomy histology, particularly after piecemeal resection, which limited evaluation of certain high-risk features such as tumor budding. Additionally, as the study was conducted at a specialized cancer center with dedicated gastrointestinal pathology and radiology services and readily available office endoscopy, the results may not be fully generalizable to less specialized centers.
DISCLOSURES:
Jinru Shia, MD, reported receiving consulting fees from Paige.AI and research funding through their institution. Andrea Cercek, MD, disclosed consulting roles with multiple pharmaceutical companies, including GlaxoSmithKline, Incyte, Merck, and others, as well as research funding from GlaxoSmithKline and Pfizer. Weiser reported receiving royalties as a section editor for UpToDate. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Among patients with high-risk malignant colorectal polyps, 19% had residual disease, with rates of 25% in the immediate surgery group vs 9% in the nonoperative management group. The rate of rectum and sphincter preservation in the nonoperative surveillance group was over 90%, and all recurrences were successfully treated with salvage surgery or chemoradiotherapy.
METHODOLOGY:
- Although guidelines in the US recommend colorectal resection when a malignant colorectal polyp has high-risk features, some patients choose nonoperative management instead to avoid the associated averse effects and impact on quality of life. The safety of nonoperative management, however, remains unclear.
- A single-center cohort study conducted between 2015 and 2022 included 336 patients who underwent polypectomy in the colon (n = 226) or rectum (n = 110) and had at least one high-risk feature. High-risk features included positive margins, piecemeal resection with unclear margin, lymphovascular invasion, perineural invasion, poor differentiation, and tumor budding.
- The analysis compared rates of residual disease between those who had immediate surgery (62%) and nonoperative management (38%) following the removal of a malignant polyp, 15% of whom (n = 19) received systemic chemotherapy after polypectomy.
- Researchers also assessed the rates of distant metastasis between the two groups and the association between specific high-risk features and residual disease or post-treatment complications.
TAKEAWAY:
- In the overall population, 19% of patients had residual disease (63 of 336). Among the 208 patients who had immediate surgery, 25% (n = 51) had residual disease, including 9% (n = 19) with residual disease in the bowel wall and 19% (n = 39) in locoregional lymph nodes. Postoperative complications occurred in 12% of patients (n = 25) in the immediate surgery group, with 3% (n = 7) having complications considered grade 3 or higher.
- Among the 128 patients who received nonoperative surveillance, 9% (n = 12) developed recurrence during surveillance, 6% (n = 7) in the bowel wall and 4% (n = 5) in locoregional lymph nodes. All recurrences in the nonoperative surveillance group were successfully treated with either salvage surgery (n = 6) or chemoradiotherapy (n = 6).
- Among patients in the nonoperative group with a malignant polyp removed from the rectum, the rate of rectum preservation was 94% (74 of 79 patients); the sphincter preservation rate was 91% for tumors < 5 cm from the anal verge.
- Distant metastases occurred in 2% of all patients across both groups.
IN PRACTICE:
"The risk of residual disease after the removal of a malignant colorectal polyp with [high-risk features] is considerable, but nonoperative management offers the potential for organ preservation, with the availability of effective salvage options if rectal cancer is detected," the authors of the study concluded.
SOURCE:
The study, led by Thikhamporn Tawantanakorn, MD, and Martin R. Weiser, MD, of Memorial Sloan Kettering Cancer Center in New York City, was published online in JCO Oncology Advances.
LIMITATIONS:
The researchers noted several limitations, including variable follow-up among patients and challenges in assessing polypectomy histology, particularly after piecemeal resection, which limited evaluation of certain high-risk features such as tumor budding. Additionally, as the study was conducted at a specialized cancer center with dedicated gastrointestinal pathology and radiology services and readily available office endoscopy, the results may not be fully generalizable to less specialized centers.
DISCLOSURES:
Jinru Shia, MD, reported receiving consulting fees from Paige.AI and research funding through their institution. Andrea Cercek, MD, disclosed consulting roles with multiple pharmaceutical companies, including GlaxoSmithKline, Incyte, Merck, and others, as well as research funding from GlaxoSmithKline and Pfizer. Weiser reported receiving royalties as a section editor for UpToDate. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Is It Safe to Skip Surgery After Malignant Colorectal Polyp Removal?
Is It Safe to Skip Surgery After Malignant Colorectal Polyp Removal?
Marathon Runners May Have Higher Colon Cancer Risk
Marathon Runners May Have Higher Colon Cancer Risk
Intensive long-distance running could be a risk for advanced adenomas (AAs) for the colon, a small prospective study reported this summer at the American Society of Clinical Oncology (ASCO) 2025.
Refined screening strategies for this running population are therefore warranted, and pathologic and epidemiologic evaluations should explore causation and ancillary risk factors in this unique population, according to Timothy L. Cannon, MD, oncologist at Inova Schar Cancer Institute in Fairfax, Virginia, and colleagues.
The full study (NCT 05419531), which is currently being reviewed for publication, looked at colonoscopy results from 100 marathon and ultramarathon runners and found that almost half had polyps, and 15% (95% CI, 7.9-22.4) had confirmed AAs).
The AA rate was higher than the 4.5% to 6% seen in adults in their late 40s in the general population and was higher even than the 12% found in Alaska Natives, who are at heightened risk for colon cancer.
"After meeting 3 extreme endurance athletes — 2 who ran 100-mile ultramarathons and 1 lady who ran dozens of triathlons — with stage IV colon cancer before age 40, I began to be suspicious of a link," Cannon told Medscape Medical News. At least 2 of them said they were told that bleeding after long runs was common, which they took to mean as normal. "I could imagine multiple reasons that endurance runners would be predisposed to cancer, with my initial focus on the inflammation and cell turnover incited by the well-described ischemia and runner's colitis."
Study Details
From October 2022 to December 2024, 100 eligible participants aged 35 to 50 years had colonoscopies. The median age was 42.5 years; 55 participants were female and 45 were male. In terms of endurance eligibility, all had completed at ≥ 2 registered ultramarathons (50 km or longer) or 5 registered marathons (26.2 miles). Patients were excluded if they were known or suspected to have inflammatory bowel disease, familial adenomatous polyposis, or Lynch syndrome (hereditary nonpolyposis colorectal cancer).
The historical 1.2% in average-risk individuals aged 40-49 years was used for the expected rate of AAs, defined as lesions > 10 mm, lesions with 25% tubulovillous features, or high-grade dysplasia.
In other findings, 39 had ≥ 1 adenoma and had ≥ 3 adenomas but did not meet AA criteria and were not included in the 15% with AA.
While no colon cancer was detected in the cohort, Cannon said 30% experienced rectal bleeding after exercise, especially those with AAs compared with those without: 53% vs 22%. "While rectal bleeding had a significant association with finding advanced adenomas on the colonoscopy, there were still many with advanced adenomas who reported no bleeding," he said.
Runner's colitis, or trots, is a common condition thought to be related to ischemia, mechanical stress, or adverse impact on the gut microbiome. "Mechanism is the huge question that I certainly can't answer at this point," Cannon said. "At some distance, blood flow gets diverted from the splanchnic circulation to the legs, and gut ischemia seems to ensue. I envision high rates of disorderly cell turnover and more opportunities for mutagenesis. This needs to be studied, and what I am describing is certainly either an oversimplification or simply not related at all."
The authors noted that exercise-induced gastrointestinal injury is likely associated with reduced blood flow to the intestines during long-distance running, but not evidence has linked this bowel ischemia to carcinogenesis.
Diet could be another factor. "I am fascinated with runners' diets. They seem to consume, on average, a huge amount of ultraprocessed bars and goos. They also may drink from plastic bottles far more than the average person. These are just 2 of many possibilities," Cannon said. "Nearly a third of our participants were vegan or vegetarian. We are planning a second, more detailed, survey or our participants. We will really dig down on these questions as well as specifics regarding their training regimens."
Commenting on the study but not involved in it, Thomas F. Imperiale, MD, professor of gastroenterology and hepatology at Indiana University Indianapolis, said that while the findings are provocative, several methodological issues require consideration in subsequent research.
"First, the comparative benchmark of advanced adenoma prevalence of 1.2% is based on screening colonoscopy data from 25 years ago. At the very least, a concurrent benchmark should be used," he told Medscape Medical News. The second issue is the absence of a control group of persons who may exercise but who do not run marathons. "This addition would strengthen study validity more than using a concurrent comparison."
The case group of long-distance runners and a control group of nonmarathon runners could be compared for prevalence of AAs with adjustment for age, sex, race or ethnicity, family history of colorectal cancer, diet, other physical activity, tobacco use history, BMI or waist circumference, ethanol use, and perhaps other early-life exposures and indication for colonoscopy. "Last, it would be interesting to know whether and how often the 100 participants developed symptoms possibly consistent with colonic ischemia either during or after long-distance runs, which might provide indirect support for the presumptive mechanism of action."
In other comments, Hamed Khalili, MD, MPH, gastroenterologist at Massachusetts General Hospital and associate professor at Harvard Medical School, both in Boston, called the results very preliminary. "The sample size is small, and the comparator group is a historical control, so it's unclear whether the observed differences are just a sampling issue," he said.
Cannon has this advice for physicians: "Please don't dismiss symptoms of runner's colitis as benign. This condition requires investigation," he said. While he hasn't seen any expert recommendation to treat postrunning bleeding any differently from other causes of melena or hematochezia, both of which would normally merit a colonoscopy, in practice many gastroenterologists dismiss this type of bleeding as benign. "If larger studies confirm our findings, I don't think it's out of the question that marathoners will have unique screening recommendations. But this study is not robust enough, of course, to merit such a recommendation."
His group is planning a study on the runner's microbiome and on the proteome of the colonic tissue in this group.
Cannon reported having no relevant conflicts of interest to disclose. Imperiale and Khalili reported having no conflicts of interest relevant to their comments on the study.
A version of this article first appeared on Medscape.com.
Intensive long-distance running could be a risk for advanced adenomas (AAs) for the colon, a small prospective study reported this summer at the American Society of Clinical Oncology (ASCO) 2025.
Refined screening strategies for this running population are therefore warranted, and pathologic and epidemiologic evaluations should explore causation and ancillary risk factors in this unique population, according to Timothy L. Cannon, MD, oncologist at Inova Schar Cancer Institute in Fairfax, Virginia, and colleagues.
The full study (NCT 05419531), which is currently being reviewed for publication, looked at colonoscopy results from 100 marathon and ultramarathon runners and found that almost half had polyps, and 15% (95% CI, 7.9-22.4) had confirmed AAs).
The AA rate was higher than the 4.5% to 6% seen in adults in their late 40s in the general population and was higher even than the 12% found in Alaska Natives, who are at heightened risk for colon cancer.
"After meeting 3 extreme endurance athletes — 2 who ran 100-mile ultramarathons and 1 lady who ran dozens of triathlons — with stage IV colon cancer before age 40, I began to be suspicious of a link," Cannon told Medscape Medical News. At least 2 of them said they were told that bleeding after long runs was common, which they took to mean as normal. "I could imagine multiple reasons that endurance runners would be predisposed to cancer, with my initial focus on the inflammation and cell turnover incited by the well-described ischemia and runner's colitis."
Study Details
From October 2022 to December 2024, 100 eligible participants aged 35 to 50 years had colonoscopies. The median age was 42.5 years; 55 participants were female and 45 were male. In terms of endurance eligibility, all had completed at ≥ 2 registered ultramarathons (50 km or longer) or 5 registered marathons (26.2 miles). Patients were excluded if they were known or suspected to have inflammatory bowel disease, familial adenomatous polyposis, or Lynch syndrome (hereditary nonpolyposis colorectal cancer).
The historical 1.2% in average-risk individuals aged 40-49 years was used for the expected rate of AAs, defined as lesions > 10 mm, lesions with 25% tubulovillous features, or high-grade dysplasia.
In other findings, 39 had ≥ 1 adenoma and had ≥ 3 adenomas but did not meet AA criteria and were not included in the 15% with AA.
While no colon cancer was detected in the cohort, Cannon said 30% experienced rectal bleeding after exercise, especially those with AAs compared with those without: 53% vs 22%. "While rectal bleeding had a significant association with finding advanced adenomas on the colonoscopy, there were still many with advanced adenomas who reported no bleeding," he said.
Runner's colitis, or trots, is a common condition thought to be related to ischemia, mechanical stress, or adverse impact on the gut microbiome. "Mechanism is the huge question that I certainly can't answer at this point," Cannon said. "At some distance, blood flow gets diverted from the splanchnic circulation to the legs, and gut ischemia seems to ensue. I envision high rates of disorderly cell turnover and more opportunities for mutagenesis. This needs to be studied, and what I am describing is certainly either an oversimplification or simply not related at all."
The authors noted that exercise-induced gastrointestinal injury is likely associated with reduced blood flow to the intestines during long-distance running, but not evidence has linked this bowel ischemia to carcinogenesis.
Diet could be another factor. "I am fascinated with runners' diets. They seem to consume, on average, a huge amount of ultraprocessed bars and goos. They also may drink from plastic bottles far more than the average person. These are just 2 of many possibilities," Cannon said. "Nearly a third of our participants were vegan or vegetarian. We are planning a second, more detailed, survey or our participants. We will really dig down on these questions as well as specifics regarding their training regimens."
Commenting on the study but not involved in it, Thomas F. Imperiale, MD, professor of gastroenterology and hepatology at Indiana University Indianapolis, said that while the findings are provocative, several methodological issues require consideration in subsequent research.
"First, the comparative benchmark of advanced adenoma prevalence of 1.2% is based on screening colonoscopy data from 25 years ago. At the very least, a concurrent benchmark should be used," he told Medscape Medical News. The second issue is the absence of a control group of persons who may exercise but who do not run marathons. "This addition would strengthen study validity more than using a concurrent comparison."
The case group of long-distance runners and a control group of nonmarathon runners could be compared for prevalence of AAs with adjustment for age, sex, race or ethnicity, family history of colorectal cancer, diet, other physical activity, tobacco use history, BMI or waist circumference, ethanol use, and perhaps other early-life exposures and indication for colonoscopy. "Last, it would be interesting to know whether and how often the 100 participants developed symptoms possibly consistent with colonic ischemia either during or after long-distance runs, which might provide indirect support for the presumptive mechanism of action."
In other comments, Hamed Khalili, MD, MPH, gastroenterologist at Massachusetts General Hospital and associate professor at Harvard Medical School, both in Boston, called the results very preliminary. "The sample size is small, and the comparator group is a historical control, so it's unclear whether the observed differences are just a sampling issue," he said.
Cannon has this advice for physicians: "Please don't dismiss symptoms of runner's colitis as benign. This condition requires investigation," he said. While he hasn't seen any expert recommendation to treat postrunning bleeding any differently from other causes of melena or hematochezia, both of which would normally merit a colonoscopy, in practice many gastroenterologists dismiss this type of bleeding as benign. "If larger studies confirm our findings, I don't think it's out of the question that marathoners will have unique screening recommendations. But this study is not robust enough, of course, to merit such a recommendation."
His group is planning a study on the runner's microbiome and on the proteome of the colonic tissue in this group.
Cannon reported having no relevant conflicts of interest to disclose. Imperiale and Khalili reported having no conflicts of interest relevant to their comments on the study.
A version of this article first appeared on Medscape.com.
Intensive long-distance running could be a risk for advanced adenomas (AAs) for the colon, a small prospective study reported this summer at the American Society of Clinical Oncology (ASCO) 2025.
Refined screening strategies for this running population are therefore warranted, and pathologic and epidemiologic evaluations should explore causation and ancillary risk factors in this unique population, according to Timothy L. Cannon, MD, oncologist at Inova Schar Cancer Institute in Fairfax, Virginia, and colleagues.
The full study (NCT 05419531), which is currently being reviewed for publication, looked at colonoscopy results from 100 marathon and ultramarathon runners and found that almost half had polyps, and 15% (95% CI, 7.9-22.4) had confirmed AAs).
The AA rate was higher than the 4.5% to 6% seen in adults in their late 40s in the general population and was higher even than the 12% found in Alaska Natives, who are at heightened risk for colon cancer.
"After meeting 3 extreme endurance athletes — 2 who ran 100-mile ultramarathons and 1 lady who ran dozens of triathlons — with stage IV colon cancer before age 40, I began to be suspicious of a link," Cannon told Medscape Medical News. At least 2 of them said they were told that bleeding after long runs was common, which they took to mean as normal. "I could imagine multiple reasons that endurance runners would be predisposed to cancer, with my initial focus on the inflammation and cell turnover incited by the well-described ischemia and runner's colitis."
Study Details
From October 2022 to December 2024, 100 eligible participants aged 35 to 50 years had colonoscopies. The median age was 42.5 years; 55 participants were female and 45 were male. In terms of endurance eligibility, all had completed at ≥ 2 registered ultramarathons (50 km or longer) or 5 registered marathons (26.2 miles). Patients were excluded if they were known or suspected to have inflammatory bowel disease, familial adenomatous polyposis, or Lynch syndrome (hereditary nonpolyposis colorectal cancer).
The historical 1.2% in average-risk individuals aged 40-49 years was used for the expected rate of AAs, defined as lesions > 10 mm, lesions with 25% tubulovillous features, or high-grade dysplasia.
In other findings, 39 had ≥ 1 adenoma and had ≥ 3 adenomas but did not meet AA criteria and were not included in the 15% with AA.
While no colon cancer was detected in the cohort, Cannon said 30% experienced rectal bleeding after exercise, especially those with AAs compared with those without: 53% vs 22%. "While rectal bleeding had a significant association with finding advanced adenomas on the colonoscopy, there were still many with advanced adenomas who reported no bleeding," he said.
Runner's colitis, or trots, is a common condition thought to be related to ischemia, mechanical stress, or adverse impact on the gut microbiome. "Mechanism is the huge question that I certainly can't answer at this point," Cannon said. "At some distance, blood flow gets diverted from the splanchnic circulation to the legs, and gut ischemia seems to ensue. I envision high rates of disorderly cell turnover and more opportunities for mutagenesis. This needs to be studied, and what I am describing is certainly either an oversimplification or simply not related at all."
The authors noted that exercise-induced gastrointestinal injury is likely associated with reduced blood flow to the intestines during long-distance running, but not evidence has linked this bowel ischemia to carcinogenesis.
Diet could be another factor. "I am fascinated with runners' diets. They seem to consume, on average, a huge amount of ultraprocessed bars and goos. They also may drink from plastic bottles far more than the average person. These are just 2 of many possibilities," Cannon said. "Nearly a third of our participants were vegan or vegetarian. We are planning a second, more detailed, survey or our participants. We will really dig down on these questions as well as specifics regarding their training regimens."
Commenting on the study but not involved in it, Thomas F. Imperiale, MD, professor of gastroenterology and hepatology at Indiana University Indianapolis, said that while the findings are provocative, several methodological issues require consideration in subsequent research.
"First, the comparative benchmark of advanced adenoma prevalence of 1.2% is based on screening colonoscopy data from 25 years ago. At the very least, a concurrent benchmark should be used," he told Medscape Medical News. The second issue is the absence of a control group of persons who may exercise but who do not run marathons. "This addition would strengthen study validity more than using a concurrent comparison."
The case group of long-distance runners and a control group of nonmarathon runners could be compared for prevalence of AAs with adjustment for age, sex, race or ethnicity, family history of colorectal cancer, diet, other physical activity, tobacco use history, BMI or waist circumference, ethanol use, and perhaps other early-life exposures and indication for colonoscopy. "Last, it would be interesting to know whether and how often the 100 participants developed symptoms possibly consistent with colonic ischemia either during or after long-distance runs, which might provide indirect support for the presumptive mechanism of action."
In other comments, Hamed Khalili, MD, MPH, gastroenterologist at Massachusetts General Hospital and associate professor at Harvard Medical School, both in Boston, called the results very preliminary. "The sample size is small, and the comparator group is a historical control, so it's unclear whether the observed differences are just a sampling issue," he said.
Cannon has this advice for physicians: "Please don't dismiss symptoms of runner's colitis as benign. This condition requires investigation," he said. While he hasn't seen any expert recommendation to treat postrunning bleeding any differently from other causes of melena or hematochezia, both of which would normally merit a colonoscopy, in practice many gastroenterologists dismiss this type of bleeding as benign. "If larger studies confirm our findings, I don't think it's out of the question that marathoners will have unique screening recommendations. But this study is not robust enough, of course, to merit such a recommendation."
His group is planning a study on the runner's microbiome and on the proteome of the colonic tissue in this group.
Cannon reported having no relevant conflicts of interest to disclose. Imperiale and Khalili reported having no conflicts of interest relevant to their comments on the study.
A version of this article first appeared on Medscape.com.
Marathon Runners May Have Higher Colon Cancer Risk
Marathon Runners May Have Higher Colon Cancer Risk
Single-Incision Robotic Surgery Exhibits Safety, Feasibility in Colorectal Cases
Single-Incision Robotic Surgery Exhibits Safety, Feasibility in Colorectal Cases
TOPLINE: A novel single-incision robotic surgery technique for colorectal procedures demonstrated feasibility with 0% conversion to open surgery rate; only 1 case required additional ports. The technique achieved a 30-day all-severity morbidity rate of 20% and major morbidity of 6%.
METHODOLOGY:
- Researchers conducted a retrospective review to report a unique, single-incision robotic surgery technique that uses a fascial wound protector device and multiport robotic surgical system in colorectal surgery.
- Analysis included 50 patients (60% women) with mean ages of 53.5 years and median BMI of 27.2 kg/m2.
- Study was performed at a single quaternary, urban, academic institution from December 2023 to April 2025.
- Patients aged ≥ 18 years with colorectal indications who underwent robotic single-incision surgery using a Da Vinci multiport robotic platform were included.
TAKEAWAY:
- Conversion to open surgery rate was 0%; 1 case required additional robotic ports.
- The 30-day all-severity morbidity rate was 20%; 30-day major morbidity was 6%.
- Pathologies treated included Crohn's disease (26%), diverticulitis (22%), colon cancer (16%), colostomy status (8%), and rectal cancer (4%).
- Successful procedures included right-sided colectomies (14%), left-sided colectomies (28%), total colectomy (4%), rectal resection (4%), small bowel procedures (22%), and ostomy creation/reversal (18%).
IN PRACTICE: "Our rSIS technique utilizing a multiport robotic system is safe and feasible across a wide spectrum of colorectal procedures," wrote the study authors.
LIMITATIONS: According to the authors, reproducible successful completion of surgeries using this technique may be challenging in populations requiring deep pelvic dissections, especially in narrow male pelvis cases, and in patients with very high BMI and significant intra-abdominal adipose tissue.
DISCLOSURES: The authors report no financial support was received for this study and declare no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
TOPLINE: A novel single-incision robotic surgery technique for colorectal procedures demonstrated feasibility with 0% conversion to open surgery rate; only 1 case required additional ports. The technique achieved a 30-day all-severity morbidity rate of 20% and major morbidity of 6%.
METHODOLOGY:
- Researchers conducted a retrospective review to report a unique, single-incision robotic surgery technique that uses a fascial wound protector device and multiport robotic surgical system in colorectal surgery.
- Analysis included 50 patients (60% women) with mean ages of 53.5 years and median BMI of 27.2 kg/m2.
- Study was performed at a single quaternary, urban, academic institution from December 2023 to April 2025.
- Patients aged ≥ 18 years with colorectal indications who underwent robotic single-incision surgery using a Da Vinci multiport robotic platform were included.
TAKEAWAY:
- Conversion to open surgery rate was 0%; 1 case required additional robotic ports.
- The 30-day all-severity morbidity rate was 20%; 30-day major morbidity was 6%.
- Pathologies treated included Crohn's disease (26%), diverticulitis (22%), colon cancer (16%), colostomy status (8%), and rectal cancer (4%).
- Successful procedures included right-sided colectomies (14%), left-sided colectomies (28%), total colectomy (4%), rectal resection (4%), small bowel procedures (22%), and ostomy creation/reversal (18%).
IN PRACTICE: "Our rSIS technique utilizing a multiport robotic system is safe and feasible across a wide spectrum of colorectal procedures," wrote the study authors.
LIMITATIONS: According to the authors, reproducible successful completion of surgeries using this technique may be challenging in populations requiring deep pelvic dissections, especially in narrow male pelvis cases, and in patients with very high BMI and significant intra-abdominal adipose tissue.
DISCLOSURES: The authors report no financial support was received for this study and declare no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
TOPLINE: A novel single-incision robotic surgery technique for colorectal procedures demonstrated feasibility with 0% conversion to open surgery rate; only 1 case required additional ports. The technique achieved a 30-day all-severity morbidity rate of 20% and major morbidity of 6%.
METHODOLOGY:
- Researchers conducted a retrospective review to report a unique, single-incision robotic surgery technique that uses a fascial wound protector device and multiport robotic surgical system in colorectal surgery.
- Analysis included 50 patients (60% women) with mean ages of 53.5 years and median BMI of 27.2 kg/m2.
- Study was performed at a single quaternary, urban, academic institution from December 2023 to April 2025.
- Patients aged ≥ 18 years with colorectal indications who underwent robotic single-incision surgery using a Da Vinci multiport robotic platform were included.
TAKEAWAY:
- Conversion to open surgery rate was 0%; 1 case required additional robotic ports.
- The 30-day all-severity morbidity rate was 20%; 30-day major morbidity was 6%.
- Pathologies treated included Crohn's disease (26%), diverticulitis (22%), colon cancer (16%), colostomy status (8%), and rectal cancer (4%).
- Successful procedures included right-sided colectomies (14%), left-sided colectomies (28%), total colectomy (4%), rectal resection (4%), small bowel procedures (22%), and ostomy creation/reversal (18%).
IN PRACTICE: "Our rSIS technique utilizing a multiport robotic system is safe and feasible across a wide spectrum of colorectal procedures," wrote the study authors.
LIMITATIONS: According to the authors, reproducible successful completion of surgeries using this technique may be challenging in populations requiring deep pelvic dissections, especially in narrow male pelvis cases, and in patients with very high BMI and significant intra-abdominal adipose tissue.
DISCLOSURES: The authors report no financial support was received for this study and declare no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Single-Incision Robotic Surgery Exhibits Safety, Feasibility in Colorectal Cases
Single-Incision Robotic Surgery Exhibits Safety, Feasibility in Colorectal Cases
LLMs Show High Accuracy in Extracting CRC Data From VA Health Records
TOPLINE: Large Language Models (LLMs) achieve more than 95% accuracy in extracting colorectal cancer and dysplasia diagnoses from Veterans Health Administration (VHA) pathology reports, including patients with Million Veteran Program (MVP) genomic data. The validated approach using publicly available LLMs demonstrates excellent performance across both Inflammatory Bowel Disease (IBD) and non-IBD populations.
METHODOLOGY:
Researchers analyzed 116,373 pathology reports generated in the VHA between 1999 and 2024, utilizing search term filtering followed by simple yes/no question prompts for identifying colorectal dysplasia, high-grade dysplasia and/or colorectal adenocarcinoma, and invasive colorectal cancer.
Results were compared to blinded manual chart review of 200 to 300 pathology reports for each patient cohort and diagnostic task, totaling 3,816 reviewed reports, to validate the LLM approach.
Validation was performed independently in IBD and non-IBD populations using Gemma-2 and Llama-3 LLMs without any task-specific training or fine-tuning.
Performance metrics included F1 scores, positive predictive value, negative predictive value, sensitivity, specificity, and Matthew's correlation coefficient to evaluate accuracy across different tasks.
TAKEAWAY:
In patients with IBD in the MVP, the LLM achieved (F1-score, 96.9%; 95% confidence interval [CI], 94.0%-99.6%) for identifying dysplasia, (F1-score, 93.7%; 95% CI, 88.2%-98.4%) for identifying high-grade dysplasia/colorectal cancer, and (F1-score, 98%; 95% CI, 96.3%-99.4%) for identifying colorectal cancer.
In non-IBD MVP patients, the LLM demonstrated (F1-score, 99.2%; 95% CI, 98.2%-100%) for identifying colorectal dysplasia, (F1-score, 96.5%; 95% CI, 93.0%-99.2%) for high-grade dysplasia/colorectal cancer, and (F1-score, 95%; 95% CI, 92.8%-97.2%) for identifying colorectal cancer.
Agreement between reviewers was excellent across tasks, with (Cohen's kappa, 89%-97%) for main tasks, and (Cohen's kappa, 78.1%-93.1%) for indefinite for dysplasia in IBD cohort.
The LLM approach maintained high accuracy when applied to full pathology reports, with (F1-score, 97.1%; 95% CI, 93.5%-100%) for dysplasia detection in IBD patients.
IN PRACTICE: “We have shown that LLMs are powerful, potentially generalizable tools for accurately extracting important information from clinical semistructured and unstructured text and which require little human-led development.” the authors of the study wrote
SOURCE: The study was based on data from the Million Veteran Program and supported by the Office of Research and Development, Veterans Health Administration, and the US Department of Veterans Affairs Biomedical Laboratory. It was published online in BMJ Open Gastroenterology.
LIMITATIONS: According to the authors, this research may be specific to the VHA system and the LLM models used. The authors did not test larger models. The authors acknowledge that without long-term access to graphics processing units, they could not feasibly test larger models, which may overcome some of the shortcomings seen in smaller models. Additionally, the researchers could not rule out overlap between Million Veteran Program and Corporate Data Warehouse reports, though they state that results in either cohort alone are sufficient validation compared with previously published work.
DISCLOSURES: The study was supported by Merit Review Award from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Service, AGA Research Foundation, National Institutes of Health grants, and the National Library of Medicine Training Grant. Kit Curtius reported receiving an investigator-led research grant from Phathom Pharmaceuticals. Shailja C Shah disclosed being a paid consultant for RedHill Biopharma and Phathom Pharmaceuticals, and an unpaid scientific advisory board member for Ilico Genetics, Inc.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
TOPLINE: Large Language Models (LLMs) achieve more than 95% accuracy in extracting colorectal cancer and dysplasia diagnoses from Veterans Health Administration (VHA) pathology reports, including patients with Million Veteran Program (MVP) genomic data. The validated approach using publicly available LLMs demonstrates excellent performance across both Inflammatory Bowel Disease (IBD) and non-IBD populations.
METHODOLOGY:
Researchers analyzed 116,373 pathology reports generated in the VHA between 1999 and 2024, utilizing search term filtering followed by simple yes/no question prompts for identifying colorectal dysplasia, high-grade dysplasia and/or colorectal adenocarcinoma, and invasive colorectal cancer.
Results were compared to blinded manual chart review of 200 to 300 pathology reports for each patient cohort and diagnostic task, totaling 3,816 reviewed reports, to validate the LLM approach.
Validation was performed independently in IBD and non-IBD populations using Gemma-2 and Llama-3 LLMs without any task-specific training or fine-tuning.
Performance metrics included F1 scores, positive predictive value, negative predictive value, sensitivity, specificity, and Matthew's correlation coefficient to evaluate accuracy across different tasks.
TAKEAWAY:
In patients with IBD in the MVP, the LLM achieved (F1-score, 96.9%; 95% confidence interval [CI], 94.0%-99.6%) for identifying dysplasia, (F1-score, 93.7%; 95% CI, 88.2%-98.4%) for identifying high-grade dysplasia/colorectal cancer, and (F1-score, 98%; 95% CI, 96.3%-99.4%) for identifying colorectal cancer.
In non-IBD MVP patients, the LLM demonstrated (F1-score, 99.2%; 95% CI, 98.2%-100%) for identifying colorectal dysplasia, (F1-score, 96.5%; 95% CI, 93.0%-99.2%) for high-grade dysplasia/colorectal cancer, and (F1-score, 95%; 95% CI, 92.8%-97.2%) for identifying colorectal cancer.
Agreement between reviewers was excellent across tasks, with (Cohen's kappa, 89%-97%) for main tasks, and (Cohen's kappa, 78.1%-93.1%) for indefinite for dysplasia in IBD cohort.
The LLM approach maintained high accuracy when applied to full pathology reports, with (F1-score, 97.1%; 95% CI, 93.5%-100%) for dysplasia detection in IBD patients.
IN PRACTICE: “We have shown that LLMs are powerful, potentially generalizable tools for accurately extracting important information from clinical semistructured and unstructured text and which require little human-led development.” the authors of the study wrote
SOURCE: The study was based on data from the Million Veteran Program and supported by the Office of Research and Development, Veterans Health Administration, and the US Department of Veterans Affairs Biomedical Laboratory. It was published online in BMJ Open Gastroenterology.
LIMITATIONS: According to the authors, this research may be specific to the VHA system and the LLM models used. The authors did not test larger models. The authors acknowledge that without long-term access to graphics processing units, they could not feasibly test larger models, which may overcome some of the shortcomings seen in smaller models. Additionally, the researchers could not rule out overlap between Million Veteran Program and Corporate Data Warehouse reports, though they state that results in either cohort alone are sufficient validation compared with previously published work.
DISCLOSURES: The study was supported by Merit Review Award from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Service, AGA Research Foundation, National Institutes of Health grants, and the National Library of Medicine Training Grant. Kit Curtius reported receiving an investigator-led research grant from Phathom Pharmaceuticals. Shailja C Shah disclosed being a paid consultant for RedHill Biopharma and Phathom Pharmaceuticals, and an unpaid scientific advisory board member for Ilico Genetics, Inc.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
TOPLINE: Large Language Models (LLMs) achieve more than 95% accuracy in extracting colorectal cancer and dysplasia diagnoses from Veterans Health Administration (VHA) pathology reports, including patients with Million Veteran Program (MVP) genomic data. The validated approach using publicly available LLMs demonstrates excellent performance across both Inflammatory Bowel Disease (IBD) and non-IBD populations.
METHODOLOGY:
Researchers analyzed 116,373 pathology reports generated in the VHA between 1999 and 2024, utilizing search term filtering followed by simple yes/no question prompts for identifying colorectal dysplasia, high-grade dysplasia and/or colorectal adenocarcinoma, and invasive colorectal cancer.
Results were compared to blinded manual chart review of 200 to 300 pathology reports for each patient cohort and diagnostic task, totaling 3,816 reviewed reports, to validate the LLM approach.
Validation was performed independently in IBD and non-IBD populations using Gemma-2 and Llama-3 LLMs without any task-specific training or fine-tuning.
Performance metrics included F1 scores, positive predictive value, negative predictive value, sensitivity, specificity, and Matthew's correlation coefficient to evaluate accuracy across different tasks.
TAKEAWAY:
In patients with IBD in the MVP, the LLM achieved (F1-score, 96.9%; 95% confidence interval [CI], 94.0%-99.6%) for identifying dysplasia, (F1-score, 93.7%; 95% CI, 88.2%-98.4%) for identifying high-grade dysplasia/colorectal cancer, and (F1-score, 98%; 95% CI, 96.3%-99.4%) for identifying colorectal cancer.
In non-IBD MVP patients, the LLM demonstrated (F1-score, 99.2%; 95% CI, 98.2%-100%) for identifying colorectal dysplasia, (F1-score, 96.5%; 95% CI, 93.0%-99.2%) for high-grade dysplasia/colorectal cancer, and (F1-score, 95%; 95% CI, 92.8%-97.2%) for identifying colorectal cancer.
Agreement between reviewers was excellent across tasks, with (Cohen's kappa, 89%-97%) for main tasks, and (Cohen's kappa, 78.1%-93.1%) for indefinite for dysplasia in IBD cohort.
The LLM approach maintained high accuracy when applied to full pathology reports, with (F1-score, 97.1%; 95% CI, 93.5%-100%) for dysplasia detection in IBD patients.
IN PRACTICE: “We have shown that LLMs are powerful, potentially generalizable tools for accurately extracting important information from clinical semistructured and unstructured text and which require little human-led development.” the authors of the study wrote
SOURCE: The study was based on data from the Million Veteran Program and supported by the Office of Research and Development, Veterans Health Administration, and the US Department of Veterans Affairs Biomedical Laboratory. It was published online in BMJ Open Gastroenterology.
LIMITATIONS: According to the authors, this research may be specific to the VHA system and the LLM models used. The authors did not test larger models. The authors acknowledge that without long-term access to graphics processing units, they could not feasibly test larger models, which may overcome some of the shortcomings seen in smaller models. Additionally, the researchers could not rule out overlap between Million Veteran Program and Corporate Data Warehouse reports, though they state that results in either cohort alone are sufficient validation compared with previously published work.
DISCLOSURES: The study was supported by Merit Review Award from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Service, AGA Research Foundation, National Institutes of Health grants, and the National Library of Medicine Training Grant. Kit Curtius reported receiving an investigator-led research grant from Phathom Pharmaceuticals. Shailja C Shah disclosed being a paid consultant for RedHill Biopharma and Phathom Pharmaceuticals, and an unpaid scientific advisory board member for Ilico Genetics, Inc.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
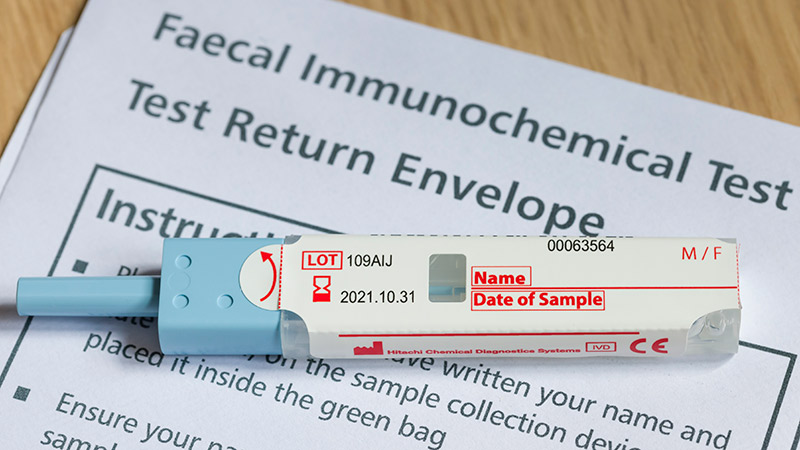
Patients With a Positive FIT Fail to Get Follow-Up Colonoscopies
Patients With a Positive FIT Fail to Get Follow-Up Colonoscopies
PHOENIX -- Patients with or without polyp removal in an index colonoscopy commonly receive follow-up surveillance with a fecal immunochemical test (FIT), yet many of these patients do not receive a recommended colonoscopy after a positive FIT.
"In this large US study, we found interval FITs are frequently performed in patients with and without prior polypectomy," said first author Natalie J. Wilson, MD, of the University of Minnesota in Minneapolis, while presenting the findings this week at the American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
"These findings reinforce the importance of colonoscopy following positive interval FIT, given the high risk of advanced neoplasia and colorectal cancer, regardless of polypectomy history," Wilson said.
Guideline recommendations stress the need for follow-up surveillance with a colonoscopy, particularly in patients who have had a prior polypectomy, due to the higher risk.
Reasons patients may instead turn to FIT include cost or other factors.
To determine just how often that happens, how having a previous polypectomy affects FIT results, and how adherent patients are to follow up if a FIT result is positive, Wilson and her colleagues evaluated data from nearly 4.8 million individuals in the Veterans Health Administration Corporate Data Warehouse who underwent colonoscopy between 2000 and 2004.
Of the patients, 10.9% were found to have subsequently received interval FIT within 10 years of the index colonoscopy, and of those patients, nearly half (49.9%) had received a polypectomy at the index colonoscopy.
The average time from the colonoscopy/polypectomy to the interval FIT was 5.9 years (5.6 years in the polypectomy group vs 6.2 years in the nonpolypectomy group).
Among the FIT screenings, results were positive in 17.2% of postpolypectomy patients and 14.1% of patients who no prior polypectomy, indicating a history of polypectomy to be predictive of positive interval FIT (odds ratio [OR], 1.12; P < .0001).
Notably, while a follow-up colonoscopy is considered essential following a positive FIT result -- and having a previous polypectomy should add further emergency to the matter -- the study showed only 50.4% of those who had an earlier polypectomy went on to receive the recommended follow-up colonoscopy after a positive follow-up FIT, and the rate was 49.3% among those who had not received a polypectomy (P = .001).
For those who did receive a follow-up colonoscopy after a positive FIT, the duration of time to receiving the colonoscopy was longer among those who had a prior polypectomy, at 2.9 months compared with 2.5 months in the nonpolypectomy group (P < .001).
Colonoscopy results following a positive FIT showed higher rates of detections among patients who had prior polypectomies than among those with no prior polypectomy, including tubular adenomas (54.7% vs 45.8%), tubulovillous adenomas (5.6% vs 4.7%), adenomas with high-grade dysplasia (0.8% vs 0.7%), sessile serrated lesions (3.52% vs 2.4%), advanced colorectal neoplasia (9.2% vs 7.9%), and colorectal cancer (3.3% vs 3.0%).
However, a prior polypectomy was not independently predictive of colorectal cancer (OR, 0.96; P = .65) or advanced colorectal neoplasia (OR, 0.97; P = .57) in the postcolonoscopy interval FIT.
The findings underscore that "positive results carried a high risk of advanced neoplasia or cancer, irrespective or prior polypectomy history," Wilson said.
Commenting on the study, William D. Chey, MD, chief of the Division of Gastroenterology & Hepatology at the University of Michigan in Ann Arbor, Michigan, noted that the study "addresses one of the biggest challenges we face as a profession, which is making sure that patients who have a positive stool test get a colonoscopy."
He noted that the low rate of just 50% of recipients of positive FITs going on to receive a colonoscopy is consistent with what is observed in other trials.
"Other data suggest that the rate might even be significantly higher -- at 70% to 80%, depending upon the population and the test," Chey told Medscape Medical News.
Reasons for the failure to receive the follow-up testing range from income restrictions (due to the high cost of a colonoscopy, especially if not covered by insurance), education, speaking a foreign language, and other factors, he said.
The relatively high rates of colon cancers detected by FIT in the study, in those with and without a prior polypectomy, along with findings from other studies "should raise questions about whether there might be a role for FIT testing in addition to colonoscopy." However, much stronger evidence would be needed, Chey noted.
In the meantime, a key issue is "how do we do a better job of making sure that individuals who have a positive FIT test get a colonoscopy," he said.
"I think a lot of this is going to come down to how it's down at the primary care level."
Chey added that in that, and any other setting, "the main message that needs to get out to people who are undergoing stool-based screening is that the stool test is only the first part of the screening process, and if it's positive, a follow-up colonoscopy must be performed.
"Otherwise, the stool-based test is of no value."
Wilson had no disclosures to report. Chey's disclosures include consulting and/or other relationships with Ardelyx, Atmo, Biomerica, Commonwealth Diagnostics International, Corprata, Dieta, Evinature, Food Marble, Gemelli, Kiwi BioScience, Modify Health, Nestle, Phathom, Redhill, Salix/Valean, Takeda, and Vibrant.
A version of this article first appeared on Medscape.com.
PHOENIX -- Patients with or without polyp removal in an index colonoscopy commonly receive follow-up surveillance with a fecal immunochemical test (FIT), yet many of these patients do not receive a recommended colonoscopy after a positive FIT.
"In this large US study, we found interval FITs are frequently performed in patients with and without prior polypectomy," said first author Natalie J. Wilson, MD, of the University of Minnesota in Minneapolis, while presenting the findings this week at the American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
"These findings reinforce the importance of colonoscopy following positive interval FIT, given the high risk of advanced neoplasia and colorectal cancer, regardless of polypectomy history," Wilson said.
Guideline recommendations stress the need for follow-up surveillance with a colonoscopy, particularly in patients who have had a prior polypectomy, due to the higher risk.
Reasons patients may instead turn to FIT include cost or other factors.
To determine just how often that happens, how having a previous polypectomy affects FIT results, and how adherent patients are to follow up if a FIT result is positive, Wilson and her colleagues evaluated data from nearly 4.8 million individuals in the Veterans Health Administration Corporate Data Warehouse who underwent colonoscopy between 2000 and 2004.
Of the patients, 10.9% were found to have subsequently received interval FIT within 10 years of the index colonoscopy, and of those patients, nearly half (49.9%) had received a polypectomy at the index colonoscopy.
The average time from the colonoscopy/polypectomy to the interval FIT was 5.9 years (5.6 years in the polypectomy group vs 6.2 years in the nonpolypectomy group).
Among the FIT screenings, results were positive in 17.2% of postpolypectomy patients and 14.1% of patients who no prior polypectomy, indicating a history of polypectomy to be predictive of positive interval FIT (odds ratio [OR], 1.12; P < .0001).
Notably, while a follow-up colonoscopy is considered essential following a positive FIT result -- and having a previous polypectomy should add further emergency to the matter -- the study showed only 50.4% of those who had an earlier polypectomy went on to receive the recommended follow-up colonoscopy after a positive follow-up FIT, and the rate was 49.3% among those who had not received a polypectomy (P = .001).
For those who did receive a follow-up colonoscopy after a positive FIT, the duration of time to receiving the colonoscopy was longer among those who had a prior polypectomy, at 2.9 months compared with 2.5 months in the nonpolypectomy group (P < .001).
Colonoscopy results following a positive FIT showed higher rates of detections among patients who had prior polypectomies than among those with no prior polypectomy, including tubular adenomas (54.7% vs 45.8%), tubulovillous adenomas (5.6% vs 4.7%), adenomas with high-grade dysplasia (0.8% vs 0.7%), sessile serrated lesions (3.52% vs 2.4%), advanced colorectal neoplasia (9.2% vs 7.9%), and colorectal cancer (3.3% vs 3.0%).
However, a prior polypectomy was not independently predictive of colorectal cancer (OR, 0.96; P = .65) or advanced colorectal neoplasia (OR, 0.97; P = .57) in the postcolonoscopy interval FIT.
The findings underscore that "positive results carried a high risk of advanced neoplasia or cancer, irrespective or prior polypectomy history," Wilson said.
Commenting on the study, William D. Chey, MD, chief of the Division of Gastroenterology & Hepatology at the University of Michigan in Ann Arbor, Michigan, noted that the study "addresses one of the biggest challenges we face as a profession, which is making sure that patients who have a positive stool test get a colonoscopy."
He noted that the low rate of just 50% of recipients of positive FITs going on to receive a colonoscopy is consistent with what is observed in other trials.
"Other data suggest that the rate might even be significantly higher -- at 70% to 80%, depending upon the population and the test," Chey told Medscape Medical News.
Reasons for the failure to receive the follow-up testing range from income restrictions (due to the high cost of a colonoscopy, especially if not covered by insurance), education, speaking a foreign language, and other factors, he said.
The relatively high rates of colon cancers detected by FIT in the study, in those with and without a prior polypectomy, along with findings from other studies "should raise questions about whether there might be a role for FIT testing in addition to colonoscopy." However, much stronger evidence would be needed, Chey noted.
In the meantime, a key issue is "how do we do a better job of making sure that individuals who have a positive FIT test get a colonoscopy," he said.
"I think a lot of this is going to come down to how it's down at the primary care level."
Chey added that in that, and any other setting, "the main message that needs to get out to people who are undergoing stool-based screening is that the stool test is only the first part of the screening process, and if it's positive, a follow-up colonoscopy must be performed.
"Otherwise, the stool-based test is of no value."
Wilson had no disclosures to report. Chey's disclosures include consulting and/or other relationships with Ardelyx, Atmo, Biomerica, Commonwealth Diagnostics International, Corprata, Dieta, Evinature, Food Marble, Gemelli, Kiwi BioScience, Modify Health, Nestle, Phathom, Redhill, Salix/Valean, Takeda, and Vibrant.
A version of this article first appeared on Medscape.com.
PHOENIX -- Patients with or without polyp removal in an index colonoscopy commonly receive follow-up surveillance with a fecal immunochemical test (FIT), yet many of these patients do not receive a recommended colonoscopy after a positive FIT.
"In this large US study, we found interval FITs are frequently performed in patients with and without prior polypectomy," said first author Natalie J. Wilson, MD, of the University of Minnesota in Minneapolis, while presenting the findings this week at the American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
"These findings reinforce the importance of colonoscopy following positive interval FIT, given the high risk of advanced neoplasia and colorectal cancer, regardless of polypectomy history," Wilson said.
Guideline recommendations stress the need for follow-up surveillance with a colonoscopy, particularly in patients who have had a prior polypectomy, due to the higher risk.
Reasons patients may instead turn to FIT include cost or other factors.
To determine just how often that happens, how having a previous polypectomy affects FIT results, and how adherent patients are to follow up if a FIT result is positive, Wilson and her colleagues evaluated data from nearly 4.8 million individuals in the Veterans Health Administration Corporate Data Warehouse who underwent colonoscopy between 2000 and 2004.
Of the patients, 10.9% were found to have subsequently received interval FIT within 10 years of the index colonoscopy, and of those patients, nearly half (49.9%) had received a polypectomy at the index colonoscopy.
The average time from the colonoscopy/polypectomy to the interval FIT was 5.9 years (5.6 years in the polypectomy group vs 6.2 years in the nonpolypectomy group).
Among the FIT screenings, results were positive in 17.2% of postpolypectomy patients and 14.1% of patients who no prior polypectomy, indicating a history of polypectomy to be predictive of positive interval FIT (odds ratio [OR], 1.12; P < .0001).
Notably, while a follow-up colonoscopy is considered essential following a positive FIT result -- and having a previous polypectomy should add further emergency to the matter -- the study showed only 50.4% of those who had an earlier polypectomy went on to receive the recommended follow-up colonoscopy after a positive follow-up FIT, and the rate was 49.3% among those who had not received a polypectomy (P = .001).
For those who did receive a follow-up colonoscopy after a positive FIT, the duration of time to receiving the colonoscopy was longer among those who had a prior polypectomy, at 2.9 months compared with 2.5 months in the nonpolypectomy group (P < .001).
Colonoscopy results following a positive FIT showed higher rates of detections among patients who had prior polypectomies than among those with no prior polypectomy, including tubular adenomas (54.7% vs 45.8%), tubulovillous adenomas (5.6% vs 4.7%), adenomas with high-grade dysplasia (0.8% vs 0.7%), sessile serrated lesions (3.52% vs 2.4%), advanced colorectal neoplasia (9.2% vs 7.9%), and colorectal cancer (3.3% vs 3.0%).
However, a prior polypectomy was not independently predictive of colorectal cancer (OR, 0.96; P = .65) or advanced colorectal neoplasia (OR, 0.97; P = .57) in the postcolonoscopy interval FIT.
The findings underscore that "positive results carried a high risk of advanced neoplasia or cancer, irrespective or prior polypectomy history," Wilson said.
Commenting on the study, William D. Chey, MD, chief of the Division of Gastroenterology & Hepatology at the University of Michigan in Ann Arbor, Michigan, noted that the study "addresses one of the biggest challenges we face as a profession, which is making sure that patients who have a positive stool test get a colonoscopy."
He noted that the low rate of just 50% of recipients of positive FITs going on to receive a colonoscopy is consistent with what is observed in other trials.
"Other data suggest that the rate might even be significantly higher -- at 70% to 80%, depending upon the population and the test," Chey told Medscape Medical News.
Reasons for the failure to receive the follow-up testing range from income restrictions (due to the high cost of a colonoscopy, especially if not covered by insurance), education, speaking a foreign language, and other factors, he said.
The relatively high rates of colon cancers detected by FIT in the study, in those with and without a prior polypectomy, along with findings from other studies "should raise questions about whether there might be a role for FIT testing in addition to colonoscopy." However, much stronger evidence would be needed, Chey noted.
In the meantime, a key issue is "how do we do a better job of making sure that individuals who have a positive FIT test get a colonoscopy," he said.
"I think a lot of this is going to come down to how it's down at the primary care level."
Chey added that in that, and any other setting, "the main message that needs to get out to people who are undergoing stool-based screening is that the stool test is only the first part of the screening process, and if it's positive, a follow-up colonoscopy must be performed.
"Otherwise, the stool-based test is of no value."
Wilson had no disclosures to report. Chey's disclosures include consulting and/or other relationships with Ardelyx, Atmo, Biomerica, Commonwealth Diagnostics International, Corprata, Dieta, Evinature, Food Marble, Gemelli, Kiwi BioScience, Modify Health, Nestle, Phathom, Redhill, Salix/Valean, Takeda, and Vibrant.
A version of this article first appeared on Medscape.com.
Patients With a Positive FIT Fail to Get Follow-Up Colonoscopies
Patients With a Positive FIT Fail to Get Follow-Up Colonoscopies
When in the Treatment Sequence Should Metastatic CRC Be Retreated With an Anti-EGFR?
BERLIN — Re-treatment with an antiepidermal growth factor receptor (EGFR) agent is effective in patients with chemorefractory metastatic colorectal cancer (mCRC) with RAS and BRAF wild-type tumors confirmed on circulating tumor DNA (ctDNA), although the sequencing of therapy does not seem to matter, suggest overall survival results from the crossover trial PARERE.
The findings nevertheless indicate that anti-EGFR rechallenge with panitumumab may prolong progression-free survival (PFS) over the multiple kinase inhibitor regorafenib. This suggests that “the most pragmatic choice” would be to give the anti-EGFR before regorafenib, said study presenter Marco Maria Germani, MD, Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy.
The caveat, however, is in patients who have an anti-EGFR interval since previously receiving the drugs of < 6 months. Those patients appeared to do better if they had regorafenib first and then anti-EGFR rechallenge.
Overall, Germani said that “since [trifluridine/tipiracil] plus bevacizumab is today the third-line standard of care” in this patient population, “anti-EGFR re-treatment might be considered after progression” on that combination.
Germani presented the research on October 18 at the European Society for Medical Oncology (ESMO) Annual Meeting 2025, which was simultaneously published in the Annals of Oncology.
Michel P. Ducreux, MD, PhD, head of the Digestive Cancer Committee at Gustave Roussy, Villejuif, France, and invited discussant for the results, said, despite the study being negative, it is “very important to continue to perform this kind of trial to evaluate the [ideal] sequence in the treatment of our patients.”
He continued that the secondary endpoints in the trial of PFS and objective response and disease control rates were “fairly in favor of the use of rechallenge before regorafenib, and in my opinion, this is really quite convincing.”
Ducreux, who was not involved in PARERE trail, also pointed to the sex difference seen in the study, which suggested that women responded much better to having anti-EGFR retreatment before regorafenib than did men.
Similar findings have been reported in a number of other trials, and previous work has suggested that there are sex differences in the pharmacokinetics of several anticancer drugs. However, while this is “very important,” he said that “we never consider it, because we are not able to really explain [it].”
Overall, he concluded that, on the basis of these results, he would agree with the notion that it is better to propose a rechallenge with anti-EGFR treatment as the fourth-line therapy in this patient population, before administering regorafenib.
Ducreux explained that, after a partial response, tumors acquire resistance to EGFR inhibitors through alterations and mutations that occur during treatment, via nongenetic mechanisms, and through treatment-induced selection for preexisting mutations.
Previous work has shown that mutations, such as in the RAS gene, are detectable early during EGFR inhibitor therapy, but that they then decay exponentially once the drugs are stopped, with the potential that tumors regain their sensitivity to them.
Germani said that this means that ctDNA-guided retreatment with anti-EGFR therapies is a “promising approach” in pretreated patients with RAS and BRAF wild-type mCRC, and that the sequencing of the drugs may be important. Indeed, the REVERCE trial showed that giving regorafenib followed by the anti-EGFR drug cetuzximab was associated with longer overall survival than the other way around in anti-EGFR medication-naive patients.
Methods and Results
For PARERE, the researchers enrolled patients aged at least 18 years with RAS and BRAF wild-type mCRC who were previously treated with a first-line anti-EGFR-containing regimen and had at least a partial response or stable disease for at least 6 months.
The patients were also required to have had at least one intervening anti-EGFR-free line of therapy, and to have previously received treatment with fluoropyrimidine, oxaliplatin, irinotecan, and anti-angiogenics. At least 4 months were required to have passed between the end of anti-EGFR administration and screening for the study.
In all, 428 patients were screened between December 2020 and December 2024, with 213 patients with RAS and BRAF wild-type mCRC, as detected on ctDNA, enrolled. They were randomized to panitumumab or regorafenib until first progression, followed by regorafenib, if they started on panitumumab, or panitumumab, if they started on regorafenib, until second progression.
The median age of the patients was 61 years among those who started on panitumumab and 64 years among those initially given regorafenib in the trial, and 63% and 57%, respectively, were male. The median number of prior lines of therapy was two in both groups, and 65% and 69%, respectively, had received pantitumumab as their first-line anti-EGFR.
Initial findings from the study presented at the 2025 ASCO Annual Meeting indicated that, after a median follow-up of 23.5 months, there was no significant difference in the median first PFS between the two treatment arms.
However, patients who started with panitumumab had a significant improvement in both the objective response and disease control rates (P < .001), as well as a signal for a potentially longer median second PFS, than those who started with regorafenib, particularly on the per-protocol analysis.
Presenting the overall survival results, Germani said that there was no significant difference between the groups on the intention-to-treat analysis, at a stratified hazard ratio of 1.13 (P = .440), or on the per-protocol analysis, at a hazard ratio of 1.07 (P = .730).
“We then ran a subgroup analysis,” he continued, “and we found out that an anti-EGFR-free interval before liquid biopsy shorter than 6 months was associated with less benefit from a panitumumab [first] sequence, which is biologically sound.”
It was also observed that women did significantly better when having panitumumab first, whereas men did not, for which “we do not have a clear biological explanation,” Germani added.
Confining the analysis to so-called “hyperselected” patients, who not only were RAS and BRAF wild type but also had no pathogenic mutations associated with anti-EGFR resistance, did not reveal any significant overall survival differences between the treatment groups.
However, Ducreux took issue with the way in which hyperselection, which is turning up more and more regularly in trials, is defined, as the choice of which mutations to include varies widely. He suggested that a consensus group be assembled to resolve this issue.
Looking more broadly, the researchers were able to show that, in this updated analysis, anti-EGFR re-treatment was superior to regorafenib regardless of the treatment sequence in terms of PFS, at 4.2 months vs 2.4 months (P = .103) when given first in the trial, and 3.9 months vs 2.7 months (P = .019) when given second in the trial, as well as in terms of objective response and disease control rates.
Adverse Events
In terms of safety, the results showed that, as expected, acneiform rash, fatigue, and hypomagnesemia were the most common adverse events associated with panitumumb, while those with regorafenib were fatigue, hand-foot skin reactions, and hypertension.
There were no notable differences in the number of patients receiving a post-study treatment nor in the post-study therapeutic choices, between the study arms.
The study was sponsored by GONO Foundation and partially supported by Amgen and Bayer. Germani declared having relationships with MSD and Amgen. Ducreux declared having relationships with Amgen, Bayer, BeiGene, Incyte, Jazz, Merck KGaA, Merck Serono, Merck Sharp & Dohme, Pierre Fabre, Roche, Servier, Keocyt, AbbVie, Abcely, Arcus, Bayer, BMS, Boehringer, GlaxoSmithKline, Sanofi, Scandion, and Zymeworks.
A version of this article first appeared on Medscape.com.
BERLIN — Re-treatment with an antiepidermal growth factor receptor (EGFR) agent is effective in patients with chemorefractory metastatic colorectal cancer (mCRC) with RAS and BRAF wild-type tumors confirmed on circulating tumor DNA (ctDNA), although the sequencing of therapy does not seem to matter, suggest overall survival results from the crossover trial PARERE.
The findings nevertheless indicate that anti-EGFR rechallenge with panitumumab may prolong progression-free survival (PFS) over the multiple kinase inhibitor regorafenib. This suggests that “the most pragmatic choice” would be to give the anti-EGFR before regorafenib, said study presenter Marco Maria Germani, MD, Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy.
The caveat, however, is in patients who have an anti-EGFR interval since previously receiving the drugs of < 6 months. Those patients appeared to do better if they had regorafenib first and then anti-EGFR rechallenge.
Overall, Germani said that “since [trifluridine/tipiracil] plus bevacizumab is today the third-line standard of care” in this patient population, “anti-EGFR re-treatment might be considered after progression” on that combination.
Germani presented the research on October 18 at the European Society for Medical Oncology (ESMO) Annual Meeting 2025, which was simultaneously published in the Annals of Oncology.
Michel P. Ducreux, MD, PhD, head of the Digestive Cancer Committee at Gustave Roussy, Villejuif, France, and invited discussant for the results, said, despite the study being negative, it is “very important to continue to perform this kind of trial to evaluate the [ideal] sequence in the treatment of our patients.”
He continued that the secondary endpoints in the trial of PFS and objective response and disease control rates were “fairly in favor of the use of rechallenge before regorafenib, and in my opinion, this is really quite convincing.”
Ducreux, who was not involved in PARERE trail, also pointed to the sex difference seen in the study, which suggested that women responded much better to having anti-EGFR retreatment before regorafenib than did men.
Similar findings have been reported in a number of other trials, and previous work has suggested that there are sex differences in the pharmacokinetics of several anticancer drugs. However, while this is “very important,” he said that “we never consider it, because we are not able to really explain [it].”
Overall, he concluded that, on the basis of these results, he would agree with the notion that it is better to propose a rechallenge with anti-EGFR treatment as the fourth-line therapy in this patient population, before administering regorafenib.
Ducreux explained that, after a partial response, tumors acquire resistance to EGFR inhibitors through alterations and mutations that occur during treatment, via nongenetic mechanisms, and through treatment-induced selection for preexisting mutations.
Previous work has shown that mutations, such as in the RAS gene, are detectable early during EGFR inhibitor therapy, but that they then decay exponentially once the drugs are stopped, with the potential that tumors regain their sensitivity to them.
Germani said that this means that ctDNA-guided retreatment with anti-EGFR therapies is a “promising approach” in pretreated patients with RAS and BRAF wild-type mCRC, and that the sequencing of the drugs may be important. Indeed, the REVERCE trial showed that giving regorafenib followed by the anti-EGFR drug cetuzximab was associated with longer overall survival than the other way around in anti-EGFR medication-naive patients.
Methods and Results
For PARERE, the researchers enrolled patients aged at least 18 years with RAS and BRAF wild-type mCRC who were previously treated with a first-line anti-EGFR-containing regimen and had at least a partial response or stable disease for at least 6 months.
The patients were also required to have had at least one intervening anti-EGFR-free line of therapy, and to have previously received treatment with fluoropyrimidine, oxaliplatin, irinotecan, and anti-angiogenics. At least 4 months were required to have passed between the end of anti-EGFR administration and screening for the study.
In all, 428 patients were screened between December 2020 and December 2024, with 213 patients with RAS and BRAF wild-type mCRC, as detected on ctDNA, enrolled. They were randomized to panitumumab or regorafenib until first progression, followed by regorafenib, if they started on panitumumab, or panitumumab, if they started on regorafenib, until second progression.
The median age of the patients was 61 years among those who started on panitumumab and 64 years among those initially given regorafenib in the trial, and 63% and 57%, respectively, were male. The median number of prior lines of therapy was two in both groups, and 65% and 69%, respectively, had received pantitumumab as their first-line anti-EGFR.
Initial findings from the study presented at the 2025 ASCO Annual Meeting indicated that, after a median follow-up of 23.5 months, there was no significant difference in the median first PFS between the two treatment arms.
However, patients who started with panitumumab had a significant improvement in both the objective response and disease control rates (P < .001), as well as a signal for a potentially longer median second PFS, than those who started with regorafenib, particularly on the per-protocol analysis.
Presenting the overall survival results, Germani said that there was no significant difference between the groups on the intention-to-treat analysis, at a stratified hazard ratio of 1.13 (P = .440), or on the per-protocol analysis, at a hazard ratio of 1.07 (P = .730).
“We then ran a subgroup analysis,” he continued, “and we found out that an anti-EGFR-free interval before liquid biopsy shorter than 6 months was associated with less benefit from a panitumumab [first] sequence, which is biologically sound.”
It was also observed that women did significantly better when having panitumumab first, whereas men did not, for which “we do not have a clear biological explanation,” Germani added.
Confining the analysis to so-called “hyperselected” patients, who not only were RAS and BRAF wild type but also had no pathogenic mutations associated with anti-EGFR resistance, did not reveal any significant overall survival differences between the treatment groups.
However, Ducreux took issue with the way in which hyperselection, which is turning up more and more regularly in trials, is defined, as the choice of which mutations to include varies widely. He suggested that a consensus group be assembled to resolve this issue.
Looking more broadly, the researchers were able to show that, in this updated analysis, anti-EGFR re-treatment was superior to regorafenib regardless of the treatment sequence in terms of PFS, at 4.2 months vs 2.4 months (P = .103) when given first in the trial, and 3.9 months vs 2.7 months (P = .019) when given second in the trial, as well as in terms of objective response and disease control rates.
Adverse Events
In terms of safety, the results showed that, as expected, acneiform rash, fatigue, and hypomagnesemia were the most common adverse events associated with panitumumb, while those with regorafenib were fatigue, hand-foot skin reactions, and hypertension.
There were no notable differences in the number of patients receiving a post-study treatment nor in the post-study therapeutic choices, between the study arms.
The study was sponsored by GONO Foundation and partially supported by Amgen and Bayer. Germani declared having relationships with MSD and Amgen. Ducreux declared having relationships with Amgen, Bayer, BeiGene, Incyte, Jazz, Merck KGaA, Merck Serono, Merck Sharp & Dohme, Pierre Fabre, Roche, Servier, Keocyt, AbbVie, Abcely, Arcus, Bayer, BMS, Boehringer, GlaxoSmithKline, Sanofi, Scandion, and Zymeworks.
A version of this article first appeared on Medscape.com.
BERLIN — Re-treatment with an antiepidermal growth factor receptor (EGFR) agent is effective in patients with chemorefractory metastatic colorectal cancer (mCRC) with RAS and BRAF wild-type tumors confirmed on circulating tumor DNA (ctDNA), although the sequencing of therapy does not seem to matter, suggest overall survival results from the crossover trial PARERE.
The findings nevertheless indicate that anti-EGFR rechallenge with panitumumab may prolong progression-free survival (PFS) over the multiple kinase inhibitor regorafenib. This suggests that “the most pragmatic choice” would be to give the anti-EGFR before regorafenib, said study presenter Marco Maria Germani, MD, Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy.
The caveat, however, is in patients who have an anti-EGFR interval since previously receiving the drugs of < 6 months. Those patients appeared to do better if they had regorafenib first and then anti-EGFR rechallenge.
Overall, Germani said that “since [trifluridine/tipiracil] plus bevacizumab is today the third-line standard of care” in this patient population, “anti-EGFR re-treatment might be considered after progression” on that combination.
Germani presented the research on October 18 at the European Society for Medical Oncology (ESMO) Annual Meeting 2025, which was simultaneously published in the Annals of Oncology.
Michel P. Ducreux, MD, PhD, head of the Digestive Cancer Committee at Gustave Roussy, Villejuif, France, and invited discussant for the results, said, despite the study being negative, it is “very important to continue to perform this kind of trial to evaluate the [ideal] sequence in the treatment of our patients.”
He continued that the secondary endpoints in the trial of PFS and objective response and disease control rates were “fairly in favor of the use of rechallenge before regorafenib, and in my opinion, this is really quite convincing.”
Ducreux, who was not involved in PARERE trail, also pointed to the sex difference seen in the study, which suggested that women responded much better to having anti-EGFR retreatment before regorafenib than did men.
Similar findings have been reported in a number of other trials, and previous work has suggested that there are sex differences in the pharmacokinetics of several anticancer drugs. However, while this is “very important,” he said that “we never consider it, because we are not able to really explain [it].”
Overall, he concluded that, on the basis of these results, he would agree with the notion that it is better to propose a rechallenge with anti-EGFR treatment as the fourth-line therapy in this patient population, before administering regorafenib.
Ducreux explained that, after a partial response, tumors acquire resistance to EGFR inhibitors through alterations and mutations that occur during treatment, via nongenetic mechanisms, and through treatment-induced selection for preexisting mutations.
Previous work has shown that mutations, such as in the RAS gene, are detectable early during EGFR inhibitor therapy, but that they then decay exponentially once the drugs are stopped, with the potential that tumors regain their sensitivity to them.
Germani said that this means that ctDNA-guided retreatment with anti-EGFR therapies is a “promising approach” in pretreated patients with RAS and BRAF wild-type mCRC, and that the sequencing of the drugs may be important. Indeed, the REVERCE trial showed that giving regorafenib followed by the anti-EGFR drug cetuzximab was associated with longer overall survival than the other way around in anti-EGFR medication-naive patients.
Methods and Results
For PARERE, the researchers enrolled patients aged at least 18 years with RAS and BRAF wild-type mCRC who were previously treated with a first-line anti-EGFR-containing regimen and had at least a partial response or stable disease for at least 6 months.
The patients were also required to have had at least one intervening anti-EGFR-free line of therapy, and to have previously received treatment with fluoropyrimidine, oxaliplatin, irinotecan, and anti-angiogenics. At least 4 months were required to have passed between the end of anti-EGFR administration and screening for the study.
In all, 428 patients were screened between December 2020 and December 2024, with 213 patients with RAS and BRAF wild-type mCRC, as detected on ctDNA, enrolled. They were randomized to panitumumab or regorafenib until first progression, followed by regorafenib, if they started on panitumumab, or panitumumab, if they started on regorafenib, until second progression.
The median age of the patients was 61 years among those who started on panitumumab and 64 years among those initially given regorafenib in the trial, and 63% and 57%, respectively, were male. The median number of prior lines of therapy was two in both groups, and 65% and 69%, respectively, had received pantitumumab as their first-line anti-EGFR.
Initial findings from the study presented at the 2025 ASCO Annual Meeting indicated that, after a median follow-up of 23.5 months, there was no significant difference in the median first PFS between the two treatment arms.
However, patients who started with panitumumab had a significant improvement in both the objective response and disease control rates (P < .001), as well as a signal for a potentially longer median second PFS, than those who started with regorafenib, particularly on the per-protocol analysis.
Presenting the overall survival results, Germani said that there was no significant difference between the groups on the intention-to-treat analysis, at a stratified hazard ratio of 1.13 (P = .440), or on the per-protocol analysis, at a hazard ratio of 1.07 (P = .730).
“We then ran a subgroup analysis,” he continued, “and we found out that an anti-EGFR-free interval before liquid biopsy shorter than 6 months was associated with less benefit from a panitumumab [first] sequence, which is biologically sound.”
It was also observed that women did significantly better when having panitumumab first, whereas men did not, for which “we do not have a clear biological explanation,” Germani added.
Confining the analysis to so-called “hyperselected” patients, who not only were RAS and BRAF wild type but also had no pathogenic mutations associated with anti-EGFR resistance, did not reveal any significant overall survival differences between the treatment groups.
However, Ducreux took issue with the way in which hyperselection, which is turning up more and more regularly in trials, is defined, as the choice of which mutations to include varies widely. He suggested that a consensus group be assembled to resolve this issue.
Looking more broadly, the researchers were able to show that, in this updated analysis, anti-EGFR re-treatment was superior to regorafenib regardless of the treatment sequence in terms of PFS, at 4.2 months vs 2.4 months (P = .103) when given first in the trial, and 3.9 months vs 2.7 months (P = .019) when given second in the trial, as well as in terms of objective response and disease control rates.
Adverse Events
In terms of safety, the results showed that, as expected, acneiform rash, fatigue, and hypomagnesemia were the most common adverse events associated with panitumumb, while those with regorafenib were fatigue, hand-foot skin reactions, and hypertension.
There were no notable differences in the number of patients receiving a post-study treatment nor in the post-study therapeutic choices, between the study arms.
The study was sponsored by GONO Foundation and partially supported by Amgen and Bayer. Germani declared having relationships with MSD and Amgen. Ducreux declared having relationships with Amgen, Bayer, BeiGene, Incyte, Jazz, Merck KGaA, Merck Serono, Merck Sharp & Dohme, Pierre Fabre, Roche, Servier, Keocyt, AbbVie, Abcely, Arcus, Bayer, BMS, Boehringer, GlaxoSmithKline, Sanofi, Scandion, and Zymeworks.
A version of this article first appeared on Medscape.com.
FROM ENDO 2025
Impact of Retroactive Application of Updated Surveillance Guidelines on Endoscopy Center Capacity at a Large VA Health Care System
Impact of Retroactive Application of Updated Surveillance Guidelines on Endoscopy Center Capacity at a Large VA Health Care System
In 2020, the US Multi-Society Task Force (USMSTF) on Colorectal Cancer (CRC) increased the recommended colon polyp surveillance interval for 1 to 2 subcentimeter tubular adenomas from 5 to 10 years to 7 to 10 years.1 This change was prompted by emerging research indicating that rates of CRC and advanced neoplasia among patients with a history of only 1 to 2 subcentimeter tubular adenomas are lower than initially estimated.2,3 This extension provides an opportunity to increase endoscopy capacity and improve access to colonoscopies by retroactively applying the 2020 guidelines to surveillance interval recommendations made before their introduction. For example, based on the updated guidelines, patients previously recommended to undergo colon polyp surveillance colonoscopy 5 years after an index colonoscopy could extend their surveillance interval by 2 to 5 years. Increasing endoscopic capacity could address the growing demand for colonoscopies from new screening guidelines that reduced the age of initial CRC screening from 50 years to 45 years and the backlog of procedures due to COVID-19 restrictions.4
As part of a project to increase endoscopic capacity at the US Department of Veterans Affairs (VA) Pittsburgh Healthcare System (VAPHS), this study assessed the potential impact of retroactively applying the 2020 USMSTF polyp surveillance guidelines on endoscopic capacity. These results may be informative for other VA and private-sector health care systems seeking to identify strategies to improve endoscopy capacity.
Methods
VAPHS is an integrated health care system in the Veterans Health Administration (VHA) serving 85,000 patients across 8 health care institutions in Pennsylvania, Ohio, and West Virginia. VAPHS manages colorectal screening recommendations for patients receiving medical care in the health care system regardless of whether their prior colonoscopy was performed at VAPHS or external facilities. The VA maintains a national CRC screening and surveillance electronic medical record reminder that prompts health care practitioners to order colon polyp surveillance based on interval recommendations from the index colonoscopy. This study reviewed all patients from the VAPHS panel with a reminder to undergo colonoscopy for screening for CRC or surveillance of colon polyps within 12 months from September 1, 2022.
Among patients with a reminder, 3 investigators reviewed index colonoscopy and pathology reports to identify CRC risk category, colonoscopy indication, procedural quality, and recommended repeat colonoscopy interval. Per the USMSTF guidelines, patients with incomplete colonoscopy or pathology records, high-risk indications (ie, personal history of inflammatory bowel disease, personal history of CRC, or family history of CRC), or inadequate bowel preparation (Boston Bowel Preparation Score < 6) were excluded. Additionally, patients who had CRC screening or surveillance discontinued due to age or comorbidities, had completed a subsequent follow-up colonoscopy, or were deceased at the time of review were excluded.
Retroactive Interval Reclassification
Among eligible patients, this study compared the repeat colonoscopy interval recommended by the prior endoscopist with those from the 2020 USMSTF guidelines. In cases where the interval was documented as a range of years, the lower end was considered the recommendation. Similarly, the lower end of the range from the 2020 USMSTF guidelines was used for the reclassified surveillance interval. Years extended per patient were quantified relative to September 1, 2023 (ie, 1 year after the review date). For example, if the index colonoscopy was completed on September 1, 2016, the initial surveillance recommendation was 5 years, and the reclassified recommendation was 7 years, the interval extension beyond September 1, 2023, was 0 years.
Furthermore, because index surveillance recommendations are not always guideline concordant, the years extended per patient were calculated by harmonizing the index endoscopist’s recommendations with the guidelines at the time of the index colonoscopy.5 For example, if the index colonoscopy was completed on September 1, 2018, and the endoscopist recommended a 5-year follow-up for a patient with average risk for CRC, adequate bowel preparation, and no colorectal polyps, that patient is eligible to extend their colonoscopy to September 1, 2028, based on guideline recommendations at the time of index endoscopy recommending that the next colonoscopy occur in 10 years. In this analysis the 2012 USMSTF guidelines were applied to all index colonoscopies completed in 2021 or earlier to allow time for adoption of the 2020 guidelines.
This project fulfilled a facility mandate to increase capacity to conduct endoscopic procedures. Institutional review board approval was not required by VAPHS policy relating to clinical operations projects. Approval for publication of clinical operations activity was obtained from the VAPHS facility director.
Results
Within 1 year of the September 1, 2022, review date, 637 patients receiving care at VAPHS had clinical reminders for an upcoming colonoscopy. Of these, 54 (8.4%) were already up to date or were deceased at the time of review. Of the 583 eligible patients, 96% were male, the median age was 74 years, the median index colonoscopy year was 2016, and 178 (30.5%) had an average-risk CRC screening indication at the index colonoscopy (Table).
Of the 583 patients due for colonoscopy, 331 (56.7%) had both colonoscopy and pathology reports available. The majority of those with incomplete records had the index colonoscopy completed outside VAPHS. Among these patients, 222 (67.0%) had adequate bowel preparation. Of those with adequate bowel preparation, 43 were not eligible for interval extension because of high-risk conditions and 13 were not eligible because there was no index surveillance interval recommendation from the index endoscopist. Of the patients due for colonoscopy, 166 (28.4%) were potentially eligible for surveillance interval extension (Figure).
Sixty-five (39.2%) of the 166 patients had 1 to 2 subcentimeter tubular adenomas on their index colonoscopy. Sixty-two patients were eligible for interval extension to 7 years, but this only resulted in ≥ 1 year of extension beyond the review date for 36 (6% of all 583 patients due for colonoscopy). The 36 patients were extended 63 years. By harmonizing the index endoscopists’ surveillance interval recommendation with the guideline at the time of the index colonoscopy, 29 additional patients could have their colonoscopy extended by ≥ 1 year. Harmonization extended colonoscopy intervals by 93 years. Retroactively applying the 2020 USMSTF polyp surveillance guidelines and harmonizing recommendations to guidelines extended the time of index colonoscopy by 153 years.
Discussion
With retroactive application of the 2020 USMSTF polyp surveillance guidelines, 6% of patients due for an upcoming colonoscopy could extend their follow-up by ≥ 1 year by extending the surveillance interval for 1 to 2 subcentimeter tubular adenomas to 7 years. An additional 5% of patients could extend their interval by harmonizing the index endoscopist’s interval recommendation with polyp surveillance guidelines at the time of the index colonoscopy. These findings are consistent with the results of 2 studies that demonstrated that about 14% of patients due for colonoscopy could have their interval extended.6,7 The current study enhances those insights by separating the contribution of 2020 USMSTF polyp surveillance guidelines from the contribution of harmonizing surveillance intervals with guidelines for other polyp histologies. This study found that there is an opportunity to improve endoscopic capacity by harmonizing recommendations with guidelines. This complements a 2023 study showing that even when knowledgeable about guidelines, clinicians do not necessarily follow recommendations.8 While this and previous research have identified that 11% to 14% of patients are eligible for extension, these individuals would also have to be willing to have their polyp surveillance intervals extended for there to be a real-world impact on endoscopic capacity. A 2024 study found that only 19% to 37% of patients with 1 to 2 small tubular adenomas were willing to have polyps surveillance interval extension.9 This suggests the actual effect on capacity may be even lower than reported.
Limitations
The overall impact of the 2020 USMSTF polyp surveillance guidelines on endoscopic capacity was blunted by the high prevalence of incomplete index colonoscopy records among the study population. Without data on bowel preparation quality or procedure indications, this study could not assess whether 43% of patients were eligible for surveillance interval extension. Most index colonoscopies with incomplete documentation were completed at community-care gastroenterology facilities. This high rate of incomplete documentation is likely generalizable to other VA health care systems—especially in the era of the Veterans Access, Choice, and Accountability Act of 2014, which increased veteran access to non-VA community care.10 Veterans due for colon polyp surveillance colonoscopies are more likely to have had their prior colonoscopy in community care compared with prior eras.11 Furthermore, because the VHA is among the most established integrated health care systems offering primary and subspecialty care in the US, private sector health care systems may have even greater rates of care fragmentation for longitudinal CRC screening and colon polyp surveillance, as these systems have only begun to regionally integrate recently.12,13
Another limitation is that nearly one-third of the individuals with documentation had inadequate bowel preparation for surveillance recommendations. This results in shorter surveillance follow-up colonoscopies and increases downstream demand for future colonoscopies. The low yield of extending colon polyp surveillance interval in this study emphasizes that improved efforts to obtain colonoscopy and pathology reports from community care, right-sizing the colon polyp surveillance intervals recommended by endoscopists, and improving quality of bowel preparation could have downstream health care system benefits in the future. These efforts could increase colonoscopy capacity at VA health care systems, thereby shortening colonoscopy wait times, decreasing fragmentation of care, and increasing the number of veterans who receive high-quality colonoscopies at VA health care systems.14
Conclusions
Eleven percent of patients in this study due for a colonoscopy could extend their follow-up by ≥ 1 year. About half of these extensions were directly due to the 2020 USMSTF polyp surveillance interval extension for 1 to 2 subcentimeter tubular adenomas. The rest resulted from harmonizing recommendations with guidelines at the time of the procedure. To determine whether retroactively applying polyp surveillance guidelines to follow-up interval recommendations will result in improved endoscopic capacity, health care system administrators should consider the degree of CRC screening care fragmentation in their patient population. Greater long-term gains in endoscopic capacity may be achieved by proactively supporting endoscopists in making guideline-concordant screening recommendations at the time of colonoscopy.
Gupta S, Lieberman D, Anderson JC, et al. Recommendations for follow-up after colonoscopy and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2020;91:463-485. doi:10.1016/j.gie.2020.01.014
Dubé C, Yakubu M, McCurdy BR, et al. Risk of advanced adenoma, colorectal cancer, and colorectal cancer mortality in people with low-risk adenomas at baseline colonoscopy: a systematic review and meta-analysis. Am J Gastroenterol. 2017;112:1790-1801. doi:10.1038/ajg.2017.360
Click B, Pinsky PF, Hickey T, Doroudi M, Shoen RE. Association of colonoscopy adenoma findings with long-term colorectal cancer incidence. JAMA. 2018;319:2021-2031. doi:10.1001/jama.2018.5809
US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977. doi:10.1001/jama.2021.6238
Djinbachian R, Dubé AJ, Durand M, et al. Adherence to post-polypectomy surveillance guidelines: a systematic review and meta-analysis. Endoscopy. 2019;51:673-683. doi:10.1055/a-0865-2082
Gawron AJ, Kaltenbach T, Dominitz JA. The impact of the coronavirus disease-19 pandemic on access to endoscopy procedures in the VA healthcare system. Gastroenterology. 2020;159:1216-1220.e1. doi:10.1053/j.gastro.2020.07.033
Xiao AH, Chang SY, Stevoff CG, Komanduri S, Pandolfino JE, Keswani RN. Adoption of multi-society guidelines facilitates value-based reduction in screening and surveillance colonoscopy volume during COVID-19 pandemic. Dig Dis Sci. 2021;66:2578-2584. doi:10.1007/s10620-020-06539-1
Dong J, Wang LF, Ardolino E, Feuerstein JD. Real-world compliance with the 2020 U.S. Multi-Society Task Force on Colorectal Cancer polypectomy surveillance guidelines: an observational study. Gastrointest Endosc. 2023;97:350-356.e3. doi:10.1016/j.gie.2022.08.020
Lee JK, Koripella PC, Jensen CD, et al. Randomized trial of patient outreach approaches to de-implement outdated colonoscopy surveillance intervals. Clin Gastroenterol Hepatol. 2024;22:1315-1322.e7. doi:10.1016/j.cgh.2023.12.027
Veterans Access, Choice, and Accountability Act of 2014, HR 3230, 113th Cong (2014). Accessed September 8, 2025. https://www.congress.gov/bill/113th-congress/house-bill/3230
Dueker JM, Khalid A. Performance of the Veterans Choice Program for improving access to colonoscopy at a tertiary VA facility. Fed Pract. 2020;37:224-228.
Oliver A. The Veterans Health Administration: an American success story? Milbank Q. 2007;85:5-35. doi:10.1111/j.1468-0009.2007.00475.x
Furukawa MF, Machta RM, Barrett KA, et al. Landscape of health systems in the United States. Med Care Res Rev. 2020;77:357-366. doi:10.1177/1077558718823130
Petros V, Tsambikos E, Madhoun M, Tierney WM. Impact of community referral on colonoscopy quality metrics in a Veterans Affairs Medical Center. Clin Transl Gastroenterol. 2022;13:e00460. doi:10.14309/ctg.0000000000000460
In 2020, the US Multi-Society Task Force (USMSTF) on Colorectal Cancer (CRC) increased the recommended colon polyp surveillance interval for 1 to 2 subcentimeter tubular adenomas from 5 to 10 years to 7 to 10 years.1 This change was prompted by emerging research indicating that rates of CRC and advanced neoplasia among patients with a history of only 1 to 2 subcentimeter tubular adenomas are lower than initially estimated.2,3 This extension provides an opportunity to increase endoscopy capacity and improve access to colonoscopies by retroactively applying the 2020 guidelines to surveillance interval recommendations made before their introduction. For example, based on the updated guidelines, patients previously recommended to undergo colon polyp surveillance colonoscopy 5 years after an index colonoscopy could extend their surveillance interval by 2 to 5 years. Increasing endoscopic capacity could address the growing demand for colonoscopies from new screening guidelines that reduced the age of initial CRC screening from 50 years to 45 years and the backlog of procedures due to COVID-19 restrictions.4
As part of a project to increase endoscopic capacity at the US Department of Veterans Affairs (VA) Pittsburgh Healthcare System (VAPHS), this study assessed the potential impact of retroactively applying the 2020 USMSTF polyp surveillance guidelines on endoscopic capacity. These results may be informative for other VA and private-sector health care systems seeking to identify strategies to improve endoscopy capacity.
Methods
VAPHS is an integrated health care system in the Veterans Health Administration (VHA) serving 85,000 patients across 8 health care institutions in Pennsylvania, Ohio, and West Virginia. VAPHS manages colorectal screening recommendations for patients receiving medical care in the health care system regardless of whether their prior colonoscopy was performed at VAPHS or external facilities. The VA maintains a national CRC screening and surveillance electronic medical record reminder that prompts health care practitioners to order colon polyp surveillance based on interval recommendations from the index colonoscopy. This study reviewed all patients from the VAPHS panel with a reminder to undergo colonoscopy for screening for CRC or surveillance of colon polyps within 12 months from September 1, 2022.
Among patients with a reminder, 3 investigators reviewed index colonoscopy and pathology reports to identify CRC risk category, colonoscopy indication, procedural quality, and recommended repeat colonoscopy interval. Per the USMSTF guidelines, patients with incomplete colonoscopy or pathology records, high-risk indications (ie, personal history of inflammatory bowel disease, personal history of CRC, or family history of CRC), or inadequate bowel preparation (Boston Bowel Preparation Score < 6) were excluded. Additionally, patients who had CRC screening or surveillance discontinued due to age or comorbidities, had completed a subsequent follow-up colonoscopy, or were deceased at the time of review were excluded.
Retroactive Interval Reclassification
Among eligible patients, this study compared the repeat colonoscopy interval recommended by the prior endoscopist with those from the 2020 USMSTF guidelines. In cases where the interval was documented as a range of years, the lower end was considered the recommendation. Similarly, the lower end of the range from the 2020 USMSTF guidelines was used for the reclassified surveillance interval. Years extended per patient were quantified relative to September 1, 2023 (ie, 1 year after the review date). For example, if the index colonoscopy was completed on September 1, 2016, the initial surveillance recommendation was 5 years, and the reclassified recommendation was 7 years, the interval extension beyond September 1, 2023, was 0 years.
Furthermore, because index surveillance recommendations are not always guideline concordant, the years extended per patient were calculated by harmonizing the index endoscopist’s recommendations with the guidelines at the time of the index colonoscopy.5 For example, if the index colonoscopy was completed on September 1, 2018, and the endoscopist recommended a 5-year follow-up for a patient with average risk for CRC, adequate bowel preparation, and no colorectal polyps, that patient is eligible to extend their colonoscopy to September 1, 2028, based on guideline recommendations at the time of index endoscopy recommending that the next colonoscopy occur in 10 years. In this analysis the 2012 USMSTF guidelines were applied to all index colonoscopies completed in 2021 or earlier to allow time for adoption of the 2020 guidelines.
This project fulfilled a facility mandate to increase capacity to conduct endoscopic procedures. Institutional review board approval was not required by VAPHS policy relating to clinical operations projects. Approval for publication of clinical operations activity was obtained from the VAPHS facility director.
Results
Within 1 year of the September 1, 2022, review date, 637 patients receiving care at VAPHS had clinical reminders for an upcoming colonoscopy. Of these, 54 (8.4%) were already up to date or were deceased at the time of review. Of the 583 eligible patients, 96% were male, the median age was 74 years, the median index colonoscopy year was 2016, and 178 (30.5%) had an average-risk CRC screening indication at the index colonoscopy (Table).
Of the 583 patients due for colonoscopy, 331 (56.7%) had both colonoscopy and pathology reports available. The majority of those with incomplete records had the index colonoscopy completed outside VAPHS. Among these patients, 222 (67.0%) had adequate bowel preparation. Of those with adequate bowel preparation, 43 were not eligible for interval extension because of high-risk conditions and 13 were not eligible because there was no index surveillance interval recommendation from the index endoscopist. Of the patients due for colonoscopy, 166 (28.4%) were potentially eligible for surveillance interval extension (Figure).
Sixty-five (39.2%) of the 166 patients had 1 to 2 subcentimeter tubular adenomas on their index colonoscopy. Sixty-two patients were eligible for interval extension to 7 years, but this only resulted in ≥ 1 year of extension beyond the review date for 36 (6% of all 583 patients due for colonoscopy). The 36 patients were extended 63 years. By harmonizing the index endoscopists’ surveillance interval recommendation with the guideline at the time of the index colonoscopy, 29 additional patients could have their colonoscopy extended by ≥ 1 year. Harmonization extended colonoscopy intervals by 93 years. Retroactively applying the 2020 USMSTF polyp surveillance guidelines and harmonizing recommendations to guidelines extended the time of index colonoscopy by 153 years.
Discussion
With retroactive application of the 2020 USMSTF polyp surveillance guidelines, 6% of patients due for an upcoming colonoscopy could extend their follow-up by ≥ 1 year by extending the surveillance interval for 1 to 2 subcentimeter tubular adenomas to 7 years. An additional 5% of patients could extend their interval by harmonizing the index endoscopist’s interval recommendation with polyp surveillance guidelines at the time of the index colonoscopy. These findings are consistent with the results of 2 studies that demonstrated that about 14% of patients due for colonoscopy could have their interval extended.6,7 The current study enhances those insights by separating the contribution of 2020 USMSTF polyp surveillance guidelines from the contribution of harmonizing surveillance intervals with guidelines for other polyp histologies. This study found that there is an opportunity to improve endoscopic capacity by harmonizing recommendations with guidelines. This complements a 2023 study showing that even when knowledgeable about guidelines, clinicians do not necessarily follow recommendations.8 While this and previous research have identified that 11% to 14% of patients are eligible for extension, these individuals would also have to be willing to have their polyp surveillance intervals extended for there to be a real-world impact on endoscopic capacity. A 2024 study found that only 19% to 37% of patients with 1 to 2 small tubular adenomas were willing to have polyps surveillance interval extension.9 This suggests the actual effect on capacity may be even lower than reported.
Limitations
The overall impact of the 2020 USMSTF polyp surveillance guidelines on endoscopic capacity was blunted by the high prevalence of incomplete index colonoscopy records among the study population. Without data on bowel preparation quality or procedure indications, this study could not assess whether 43% of patients were eligible for surveillance interval extension. Most index colonoscopies with incomplete documentation were completed at community-care gastroenterology facilities. This high rate of incomplete documentation is likely generalizable to other VA health care systems—especially in the era of the Veterans Access, Choice, and Accountability Act of 2014, which increased veteran access to non-VA community care.10 Veterans due for colon polyp surveillance colonoscopies are more likely to have had their prior colonoscopy in community care compared with prior eras.11 Furthermore, because the VHA is among the most established integrated health care systems offering primary and subspecialty care in the US, private sector health care systems may have even greater rates of care fragmentation for longitudinal CRC screening and colon polyp surveillance, as these systems have only begun to regionally integrate recently.12,13
Another limitation is that nearly one-third of the individuals with documentation had inadequate bowel preparation for surveillance recommendations. This results in shorter surveillance follow-up colonoscopies and increases downstream demand for future colonoscopies. The low yield of extending colon polyp surveillance interval in this study emphasizes that improved efforts to obtain colonoscopy and pathology reports from community care, right-sizing the colon polyp surveillance intervals recommended by endoscopists, and improving quality of bowel preparation could have downstream health care system benefits in the future. These efforts could increase colonoscopy capacity at VA health care systems, thereby shortening colonoscopy wait times, decreasing fragmentation of care, and increasing the number of veterans who receive high-quality colonoscopies at VA health care systems.14
Conclusions
Eleven percent of patients in this study due for a colonoscopy could extend their follow-up by ≥ 1 year. About half of these extensions were directly due to the 2020 USMSTF polyp surveillance interval extension for 1 to 2 subcentimeter tubular adenomas. The rest resulted from harmonizing recommendations with guidelines at the time of the procedure. To determine whether retroactively applying polyp surveillance guidelines to follow-up interval recommendations will result in improved endoscopic capacity, health care system administrators should consider the degree of CRC screening care fragmentation in their patient population. Greater long-term gains in endoscopic capacity may be achieved by proactively supporting endoscopists in making guideline-concordant screening recommendations at the time of colonoscopy.
In 2020, the US Multi-Society Task Force (USMSTF) on Colorectal Cancer (CRC) increased the recommended colon polyp surveillance interval for 1 to 2 subcentimeter tubular adenomas from 5 to 10 years to 7 to 10 years.1 This change was prompted by emerging research indicating that rates of CRC and advanced neoplasia among patients with a history of only 1 to 2 subcentimeter tubular adenomas are lower than initially estimated.2,3 This extension provides an opportunity to increase endoscopy capacity and improve access to colonoscopies by retroactively applying the 2020 guidelines to surveillance interval recommendations made before their introduction. For example, based on the updated guidelines, patients previously recommended to undergo colon polyp surveillance colonoscopy 5 years after an index colonoscopy could extend their surveillance interval by 2 to 5 years. Increasing endoscopic capacity could address the growing demand for colonoscopies from new screening guidelines that reduced the age of initial CRC screening from 50 years to 45 years and the backlog of procedures due to COVID-19 restrictions.4
As part of a project to increase endoscopic capacity at the US Department of Veterans Affairs (VA) Pittsburgh Healthcare System (VAPHS), this study assessed the potential impact of retroactively applying the 2020 USMSTF polyp surveillance guidelines on endoscopic capacity. These results may be informative for other VA and private-sector health care systems seeking to identify strategies to improve endoscopy capacity.
Methods
VAPHS is an integrated health care system in the Veterans Health Administration (VHA) serving 85,000 patients across 8 health care institutions in Pennsylvania, Ohio, and West Virginia. VAPHS manages colorectal screening recommendations for patients receiving medical care in the health care system regardless of whether their prior colonoscopy was performed at VAPHS or external facilities. The VA maintains a national CRC screening and surveillance electronic medical record reminder that prompts health care practitioners to order colon polyp surveillance based on interval recommendations from the index colonoscopy. This study reviewed all patients from the VAPHS panel with a reminder to undergo colonoscopy for screening for CRC or surveillance of colon polyps within 12 months from September 1, 2022.
Among patients with a reminder, 3 investigators reviewed index colonoscopy and pathology reports to identify CRC risk category, colonoscopy indication, procedural quality, and recommended repeat colonoscopy interval. Per the USMSTF guidelines, patients with incomplete colonoscopy or pathology records, high-risk indications (ie, personal history of inflammatory bowel disease, personal history of CRC, or family history of CRC), or inadequate bowel preparation (Boston Bowel Preparation Score < 6) were excluded. Additionally, patients who had CRC screening or surveillance discontinued due to age or comorbidities, had completed a subsequent follow-up colonoscopy, or were deceased at the time of review were excluded.
Retroactive Interval Reclassification
Among eligible patients, this study compared the repeat colonoscopy interval recommended by the prior endoscopist with those from the 2020 USMSTF guidelines. In cases where the interval was documented as a range of years, the lower end was considered the recommendation. Similarly, the lower end of the range from the 2020 USMSTF guidelines was used for the reclassified surveillance interval. Years extended per patient were quantified relative to September 1, 2023 (ie, 1 year after the review date). For example, if the index colonoscopy was completed on September 1, 2016, the initial surveillance recommendation was 5 years, and the reclassified recommendation was 7 years, the interval extension beyond September 1, 2023, was 0 years.
Furthermore, because index surveillance recommendations are not always guideline concordant, the years extended per patient were calculated by harmonizing the index endoscopist’s recommendations with the guidelines at the time of the index colonoscopy.5 For example, if the index colonoscopy was completed on September 1, 2018, and the endoscopist recommended a 5-year follow-up for a patient with average risk for CRC, adequate bowel preparation, and no colorectal polyps, that patient is eligible to extend their colonoscopy to September 1, 2028, based on guideline recommendations at the time of index endoscopy recommending that the next colonoscopy occur in 10 years. In this analysis the 2012 USMSTF guidelines were applied to all index colonoscopies completed in 2021 or earlier to allow time for adoption of the 2020 guidelines.
This project fulfilled a facility mandate to increase capacity to conduct endoscopic procedures. Institutional review board approval was not required by VAPHS policy relating to clinical operations projects. Approval for publication of clinical operations activity was obtained from the VAPHS facility director.
Results
Within 1 year of the September 1, 2022, review date, 637 patients receiving care at VAPHS had clinical reminders for an upcoming colonoscopy. Of these, 54 (8.4%) were already up to date or were deceased at the time of review. Of the 583 eligible patients, 96% were male, the median age was 74 years, the median index colonoscopy year was 2016, and 178 (30.5%) had an average-risk CRC screening indication at the index colonoscopy (Table).
Of the 583 patients due for colonoscopy, 331 (56.7%) had both colonoscopy and pathology reports available. The majority of those with incomplete records had the index colonoscopy completed outside VAPHS. Among these patients, 222 (67.0%) had adequate bowel preparation. Of those with adequate bowel preparation, 43 were not eligible for interval extension because of high-risk conditions and 13 were not eligible because there was no index surveillance interval recommendation from the index endoscopist. Of the patients due for colonoscopy, 166 (28.4%) were potentially eligible for surveillance interval extension (Figure).
Sixty-five (39.2%) of the 166 patients had 1 to 2 subcentimeter tubular adenomas on their index colonoscopy. Sixty-two patients were eligible for interval extension to 7 years, but this only resulted in ≥ 1 year of extension beyond the review date for 36 (6% of all 583 patients due for colonoscopy). The 36 patients were extended 63 years. By harmonizing the index endoscopists’ surveillance interval recommendation with the guideline at the time of the index colonoscopy, 29 additional patients could have their colonoscopy extended by ≥ 1 year. Harmonization extended colonoscopy intervals by 93 years. Retroactively applying the 2020 USMSTF polyp surveillance guidelines and harmonizing recommendations to guidelines extended the time of index colonoscopy by 153 years.
Discussion
With retroactive application of the 2020 USMSTF polyp surveillance guidelines, 6% of patients due for an upcoming colonoscopy could extend their follow-up by ≥ 1 year by extending the surveillance interval for 1 to 2 subcentimeter tubular adenomas to 7 years. An additional 5% of patients could extend their interval by harmonizing the index endoscopist’s interval recommendation with polyp surveillance guidelines at the time of the index colonoscopy. These findings are consistent with the results of 2 studies that demonstrated that about 14% of patients due for colonoscopy could have their interval extended.6,7 The current study enhances those insights by separating the contribution of 2020 USMSTF polyp surveillance guidelines from the contribution of harmonizing surveillance intervals with guidelines for other polyp histologies. This study found that there is an opportunity to improve endoscopic capacity by harmonizing recommendations with guidelines. This complements a 2023 study showing that even when knowledgeable about guidelines, clinicians do not necessarily follow recommendations.8 While this and previous research have identified that 11% to 14% of patients are eligible for extension, these individuals would also have to be willing to have their polyp surveillance intervals extended for there to be a real-world impact on endoscopic capacity. A 2024 study found that only 19% to 37% of patients with 1 to 2 small tubular adenomas were willing to have polyps surveillance interval extension.9 This suggests the actual effect on capacity may be even lower than reported.
Limitations
The overall impact of the 2020 USMSTF polyp surveillance guidelines on endoscopic capacity was blunted by the high prevalence of incomplete index colonoscopy records among the study population. Without data on bowel preparation quality or procedure indications, this study could not assess whether 43% of patients were eligible for surveillance interval extension. Most index colonoscopies with incomplete documentation were completed at community-care gastroenterology facilities. This high rate of incomplete documentation is likely generalizable to other VA health care systems—especially in the era of the Veterans Access, Choice, and Accountability Act of 2014, which increased veteran access to non-VA community care.10 Veterans due for colon polyp surveillance colonoscopies are more likely to have had their prior colonoscopy in community care compared with prior eras.11 Furthermore, because the VHA is among the most established integrated health care systems offering primary and subspecialty care in the US, private sector health care systems may have even greater rates of care fragmentation for longitudinal CRC screening and colon polyp surveillance, as these systems have only begun to regionally integrate recently.12,13
Another limitation is that nearly one-third of the individuals with documentation had inadequate bowel preparation for surveillance recommendations. This results in shorter surveillance follow-up colonoscopies and increases downstream demand for future colonoscopies. The low yield of extending colon polyp surveillance interval in this study emphasizes that improved efforts to obtain colonoscopy and pathology reports from community care, right-sizing the colon polyp surveillance intervals recommended by endoscopists, and improving quality of bowel preparation could have downstream health care system benefits in the future. These efforts could increase colonoscopy capacity at VA health care systems, thereby shortening colonoscopy wait times, decreasing fragmentation of care, and increasing the number of veterans who receive high-quality colonoscopies at VA health care systems.14
Conclusions
Eleven percent of patients in this study due for a colonoscopy could extend their follow-up by ≥ 1 year. About half of these extensions were directly due to the 2020 USMSTF polyp surveillance interval extension for 1 to 2 subcentimeter tubular adenomas. The rest resulted from harmonizing recommendations with guidelines at the time of the procedure. To determine whether retroactively applying polyp surveillance guidelines to follow-up interval recommendations will result in improved endoscopic capacity, health care system administrators should consider the degree of CRC screening care fragmentation in their patient population. Greater long-term gains in endoscopic capacity may be achieved by proactively supporting endoscopists in making guideline-concordant screening recommendations at the time of colonoscopy.
Gupta S, Lieberman D, Anderson JC, et al. Recommendations for follow-up after colonoscopy and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2020;91:463-485. doi:10.1016/j.gie.2020.01.014
Dubé C, Yakubu M, McCurdy BR, et al. Risk of advanced adenoma, colorectal cancer, and colorectal cancer mortality in people with low-risk adenomas at baseline colonoscopy: a systematic review and meta-analysis. Am J Gastroenterol. 2017;112:1790-1801. doi:10.1038/ajg.2017.360
Click B, Pinsky PF, Hickey T, Doroudi M, Shoen RE. Association of colonoscopy adenoma findings with long-term colorectal cancer incidence. JAMA. 2018;319:2021-2031. doi:10.1001/jama.2018.5809
US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977. doi:10.1001/jama.2021.6238
Djinbachian R, Dubé AJ, Durand M, et al. Adherence to post-polypectomy surveillance guidelines: a systematic review and meta-analysis. Endoscopy. 2019;51:673-683. doi:10.1055/a-0865-2082
Gawron AJ, Kaltenbach T, Dominitz JA. The impact of the coronavirus disease-19 pandemic on access to endoscopy procedures in the VA healthcare system. Gastroenterology. 2020;159:1216-1220.e1. doi:10.1053/j.gastro.2020.07.033
Xiao AH, Chang SY, Stevoff CG, Komanduri S, Pandolfino JE, Keswani RN. Adoption of multi-society guidelines facilitates value-based reduction in screening and surveillance colonoscopy volume during COVID-19 pandemic. Dig Dis Sci. 2021;66:2578-2584. doi:10.1007/s10620-020-06539-1
Dong J, Wang LF, Ardolino E, Feuerstein JD. Real-world compliance with the 2020 U.S. Multi-Society Task Force on Colorectal Cancer polypectomy surveillance guidelines: an observational study. Gastrointest Endosc. 2023;97:350-356.e3. doi:10.1016/j.gie.2022.08.020
Lee JK, Koripella PC, Jensen CD, et al. Randomized trial of patient outreach approaches to de-implement outdated colonoscopy surveillance intervals. Clin Gastroenterol Hepatol. 2024;22:1315-1322.e7. doi:10.1016/j.cgh.2023.12.027
Veterans Access, Choice, and Accountability Act of 2014, HR 3230, 113th Cong (2014). Accessed September 8, 2025. https://www.congress.gov/bill/113th-congress/house-bill/3230
Dueker JM, Khalid A. Performance of the Veterans Choice Program for improving access to colonoscopy at a tertiary VA facility. Fed Pract. 2020;37:224-228.
Oliver A. The Veterans Health Administration: an American success story? Milbank Q. 2007;85:5-35. doi:10.1111/j.1468-0009.2007.00475.x
Furukawa MF, Machta RM, Barrett KA, et al. Landscape of health systems in the United States. Med Care Res Rev. 2020;77:357-366. doi:10.1177/1077558718823130
Petros V, Tsambikos E, Madhoun M, Tierney WM. Impact of community referral on colonoscopy quality metrics in a Veterans Affairs Medical Center. Clin Transl Gastroenterol. 2022;13:e00460. doi:10.14309/ctg.0000000000000460
Gupta S, Lieberman D, Anderson JC, et al. Recommendations for follow-up after colonoscopy and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2020;91:463-485. doi:10.1016/j.gie.2020.01.014
Dubé C, Yakubu M, McCurdy BR, et al. Risk of advanced adenoma, colorectal cancer, and colorectal cancer mortality in people with low-risk adenomas at baseline colonoscopy: a systematic review and meta-analysis. Am J Gastroenterol. 2017;112:1790-1801. doi:10.1038/ajg.2017.360
Click B, Pinsky PF, Hickey T, Doroudi M, Shoen RE. Association of colonoscopy adenoma findings with long-term colorectal cancer incidence. JAMA. 2018;319:2021-2031. doi:10.1001/jama.2018.5809
US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977. doi:10.1001/jama.2021.6238
Djinbachian R, Dubé AJ, Durand M, et al. Adherence to post-polypectomy surveillance guidelines: a systematic review and meta-analysis. Endoscopy. 2019;51:673-683. doi:10.1055/a-0865-2082
Gawron AJ, Kaltenbach T, Dominitz JA. The impact of the coronavirus disease-19 pandemic on access to endoscopy procedures in the VA healthcare system. Gastroenterology. 2020;159:1216-1220.e1. doi:10.1053/j.gastro.2020.07.033
Xiao AH, Chang SY, Stevoff CG, Komanduri S, Pandolfino JE, Keswani RN. Adoption of multi-society guidelines facilitates value-based reduction in screening and surveillance colonoscopy volume during COVID-19 pandemic. Dig Dis Sci. 2021;66:2578-2584. doi:10.1007/s10620-020-06539-1
Dong J, Wang LF, Ardolino E, Feuerstein JD. Real-world compliance with the 2020 U.S. Multi-Society Task Force on Colorectal Cancer polypectomy surveillance guidelines: an observational study. Gastrointest Endosc. 2023;97:350-356.e3. doi:10.1016/j.gie.2022.08.020
Lee JK, Koripella PC, Jensen CD, et al. Randomized trial of patient outreach approaches to de-implement outdated colonoscopy surveillance intervals. Clin Gastroenterol Hepatol. 2024;22:1315-1322.e7. doi:10.1016/j.cgh.2023.12.027
Veterans Access, Choice, and Accountability Act of 2014, HR 3230, 113th Cong (2014). Accessed September 8, 2025. https://www.congress.gov/bill/113th-congress/house-bill/3230
Dueker JM, Khalid A. Performance of the Veterans Choice Program for improving access to colonoscopy at a tertiary VA facility. Fed Pract. 2020;37:224-228.
Oliver A. The Veterans Health Administration: an American success story? Milbank Q. 2007;85:5-35. doi:10.1111/j.1468-0009.2007.00475.x
Furukawa MF, Machta RM, Barrett KA, et al. Landscape of health systems in the United States. Med Care Res Rev. 2020;77:357-366. doi:10.1177/1077558718823130
Petros V, Tsambikos E, Madhoun M, Tierney WM. Impact of community referral on colonoscopy quality metrics in a Veterans Affairs Medical Center. Clin Transl Gastroenterol. 2022;13:e00460. doi:10.14309/ctg.0000000000000460
Impact of Retroactive Application of Updated Surveillance Guidelines on Endoscopy Center Capacity at a Large VA Health Care System
Impact of Retroactive Application of Updated Surveillance Guidelines on Endoscopy Center Capacity at a Large VA Health Care System
Streamlined Testosterone Order Template to Improve the Diagnosis and Evaluation of Hypogonadism in Veterans
Streamlined Testosterone Order Template to Improve the Diagnosis and Evaluation of Hypogonadism in Veterans
Testosterone therapy is administered following pragmatic diagnostic evaluation and workup to assess whether an adult male is hypogonadal, based on symptoms consistent with androgen deficiency and low morning serum testosterone concentrations on ≥ 2 occasions. Effects of testosterone administration include the development or maintenance of secondary sexual characteristics and increases in libido, muscle strength, fat-free mass, and bone density.
Testosterone prescriptions have markedly increased in the past 20 years, including within the US Department of Veterans Affairs (VA) health care system.1-3 This trend may be influenced by various factors, including patient perceptions of benefit, an increase in marketing, and the availability of more user-friendly formulations.
Since 2006, evidence-based clinical practice guidelines have recommended specific clinical and laboratory evaluation and counseling prior to starting testosterone replacement therapy (TRT).4-8 However, research has shown poor adherence to these recommendations, including at the VA, which raises concerns about inappropriate TRT initiation without proper diagnostic evaluation.9,10 Observational research has suggested a possible link between testosterone therapy and increased risk of cardiovascular (CV) events. The US Food and Drug Administration prescribing information includes boxed warnings about potential risks of high blood pressure, myocardial infarction, stroke, and CV-related mortality with testosterone treatment, contact transfer of transdermal testosterone, and pulmonary oil microembolism with testosterone undecanoate injections.11-15
A VA Office of Inspector General (OIG) review of VA clinician adherence to clinical and laboratory evaluation guidelines for testosterone deficiency found poor adherence among VA practitioners and made recommendations for improvement.4,15 These focused on establishing clinical signs and symptoms consistent with testosterone deficiency, confirming hypogonadism by repeated testosterone testing, determining the etiology of hypogonadism by measuring gonadotropins, initiating a discussion of risks and benefits of TRT, and assessing clinical improvement and obtaining an updated hematocrit test within 3 to 6 months of initiation.
The VA Puget Sound Health Care System (VAPSHCS) developed a local prior authorization template to assist health care practitioners (HCPs) to address the OIG recommendations. This testosterone order template (TOT) aimed to improve the diagnosis, evaluation, and monitoring of TRT in males with hypogonadism, combined with existing VA pharmacy criteria for the use of testosterone based on Endocrine Society guidelines. A version of the VAPSHCS TOT was approved as the national VA Computerized Patient Record System (CPRS) template.
Preliminary evaluation of the TOT suggested improved short-term adherence to guideline recommendations following implementation.16 This quality improvement study sought to assess the long-term effectiveness of the TOT with respect to clinical practice guideline adherence. The OIG did not address prostate-specific antigen (PSA) monitoring because understanding of the relationship between TRT and the risks of elevated PSA levels remains incomplete.6,17 This project hypothesized that implementation of a pharmacy-managed TOT incorporated into CPRS would result in higher adherence rates to guideline-recommended clinical and laboratory evaluation, in addition to counseling of men with hypogonadism prior to initiation of TRT.
Methods
Eligible participants were cisgender males who received a new testosterone prescription, had ≥ 2 clinic visits at VAPSHCS, and no previous testosterone prescription in the previous 2 years. Individuals were excluded if they had testosterone administered at VAPSHCS; were prescribed testosterone at another facility (VA or community-based); pilot tested an initial version of the TOT prior to November 30, 2019; or had an International Classification of Diseases, Tenth Revision codes for hypopituitarism, gender identity disorder, history of sexual assignment, or Klinefelter syndrome for which testosterone therapy was already approved. Patients who met the inclusion criteria were identified by an algorithm developed by the VAPSHCS pharmacoeconomist.
This quality improvement project used a retrospective, pre-post experimental design. Electronic chart review and systematic manual review of all eligible patient charts were performed for the pretemplate period (December 1, 2018, to November 30, 2019) and after the template implementation, (December 1, 2021, to November 30, 2022).
An initial version of the TOT was implemented on July 1, 2019, but was not fully integrated into CPRS until early 2020; individuals in whom the TOT was used prior to November 30, 2019, were excluded. Data from the initial period of the COVID-19 pandemic were avoided because of alterations in clinic and prescribing practices. As a quality improvement project, the TOT evaluation was exempt from formal review by the VAPSHCS Institutional Review Board, as determined by the Director of the Office of Transformation/Quality/Safety/Value.
Interventions
Testosterone is a Schedule III controlled substance with potential risks and a propensity for varied prescribing practices. It was designated as a restricted drug requiring a prior authorization drug request (PADR) for which a specific TOT was developed, approved by the VAPSHCS Pharmacy and Therapeutics Committee, and incorporated into CPRS. A team of pharmacists, primary care physicians, geriatricians, endocrinologists, and health informatics experts created and developed the TOT. Pharmacists managed and monitored its completion.
The process for prescribing testosterone via the TOT is outlined in the eAppendix. When an HCP orders testosterone in CPRS, reminders prompt them to use the TOT and indicate required laboratory measurements (an order set is provided). Completion of TOT is not necessary to order testosterone for patients with an existing diagnosis of an organic cause of hypogonadism (eg, Klinefelter syndrome or hypopituitarism) or transgender women (assigned male at birth). In the TOT, the prescriber must also indicate signs and symptoms of testosterone deficiency; required laboratory tests; and counseling regarding potential risks and benefits of TRT. A pharmacist reviews the TOT and either approves or rejects the testosterone prescription and provides follow-up guidance to the prescriber. The completed TOT serves as documentation of guideline adherence in CPRS. The TOT also includes sections for first renewal testosterone prescriptions, addressing guideline recommendations for follow-up laboratory evaluation and clinical response to TRT. Due to limited completion of this section in the posttemplate period, evaluating adherence to follow-up recommendations was not feasible.
Measures
This project assessed the percentage of patients in the posttemplate period vs pretemplate period with an approved PADR. Documentation of specific guideline-recommended measures was assessed: signs and symptoms of testosterone deficiency; ≥ 2 serum testosterone measurements (≥ 2 total, free and total, or 2 free testosterone levels, and ≥ 1 testosterone level before 10
The project also assessed the proportion of patients in the posttemplate period vs pretemplate period who had all hormone tests (≥ 2 serum testosterone and LH and FSH concentrations), all laboratory tests (hormone tests and hematocrit), and all 5 guideline-recommended measures.
Analysis
Statistical comparisons between the proportions of patients in the pretemplate and posttemplate periods for each measure were performed using a χ2 test, without correction for multiple comparisons. All analyses were conducted using Stata version 10.0. A P value < .05 was considered significant for all comparisons.
Results
Chart review identified 189 patients in the pretemplate period and 113 patients in the posttemplate period with a new testosterone prescription (Figure). After exclusions, 91 and 49 patients, respectively, met eligibility criteria (Table 1). Fifty-six patients (62%) pretemplate and 40 patients (82%) posttemplate (P = .015) had approved PADRs and comprised the groups that were analyzed (Table 2).

The mean age and body mass index were similar in the pretemplate and posttemplate periods, but there was variation in the proportions of patients aged < 70 years and those with a body mass index < 30 between the groups. The most common diagnosis in both groups was testicular hypofunction, and the most common comorbidity was type 2 diabetes mellitus. Concomitant use of opioids or glucocorticoids that can lower testosterone levels was rare. Most testosterone prescriptions originated from primary care clinics in both periods: 68 (75%) in the pretemplate period and 35 (71%) in the posttemplate period. Most testosterone treatment was delivered by intramuscular injection.
In the posttemplate period vs pretemplate period, the proportion of patients with an approved PADR (82% vs 62%, P = .02), and documentation of signs and symptoms of hypogonadism (93% vs 71%, P = .002) prior to starting TRT were higher, while the percentage of patients having ≥ 2 testosterone measurements (85% vs 89%, P = .53), ≥ 1 testosterone level before 10 AM (78% vs 75%, P = .70), and hematocrit measured (95% vs 91%, P = .47) were similar. Rates of LH and FSH testing were higher in the posttemplate period (80%) vs the pretemplate period (63%) but did not achieve statistical significance (P = .07), and discussion of the risks and benefits of TRT was higher in the posttemplate period (58%) vs the pretemplate period (34%) (P = .02). The percentage of patients who had all hormone measurements (total and/or free testosterone, LH, and FSH) was higher in the posttemplate period (78%) vs the pretemplate period (59%) but did not achieve statistical significance (P = .06). The rates of all guideline-recommended laboratory test orders were higher in the posttemplate period (78%) vs the pretemplate period (55%) (P = .03), and all 5 guideline-recommended clinical and laboratory measures were higher in the posttemplate period (45%) vs the pretemplate period (18%) (P = .004).
Discussion
The implementation of a pharmacy-managed TOT in CPRS demonstrated higher adherence to evidence-based guidelines for diagnosing and evaluating hypogonadism before TRT. After TOT implementation, a higher proportion of patients had documented signs and symptoms of testosterone deficiency, underwent all recommended laboratory tests, and had discussions about the risks and benefits of TRT. Adherence to 5 clinical and laboratory measures recommended by Endocrine Society guidelines was higher after TOT implementation, indicating improved prescribing practices.4
The requirement for TOT completion before testosterone prescription and its management by trained pharmacists likely contributed to higher adherence to guideline recommendations than previously reported. Integration of the TOT into CPRS with pharmacy oversight may have enhanced adherence by summarizing and codifying evidence-based guideline recommendations for clinical and biochemical evaluation prior to TRT initiation, offering relevant education to clinicians and pharmacists, automatically importing pertinent clinical information and laboratory results, and generating CPRS documentation to reduce clinician burden during patient care.
The proportion of patients with documented signs and symptoms of testosterone deficiency before TRT increased from the pretemplate period (71%) to the posttemplate period (93%), indicating that most patients receiving TRT had clinical manifestations of hypogonadism. This aligns with Endocrine Society guidelines, which define hypogonadism as a clinical disorder characterized by clinical manifestations of testosterone deficiency and persistently low serum testosterone levels on ≥ 2 separate occasions.4,6 However, recent trends in direct-to-consumer advertising for testosterone and the rise of “low T” clinics may contribute to increased testing, varied practices, and inappropriate testosterone therapy initiation (eg, in men with low testosterone levels who lack symptoms of hypogonadism).18 Improved adherence in documenting clinical hypogonadism with implementation of the TOT reinforces the value of incorporating educational material, as previously reported.11
Adherence to guideline recommendations following implementation of the TOT in this project was higher than those previously reported. In a study of 111,631 outpatient veterans prescribed testosterone from 2009 to 2012, only 18.3% had ≥ 2 testosterone prescriptions, and 3.5% had ≥ 2 testosterone, LH, and FSH levels measured prior to the initiation of a TRT.9 In a report of 63,534 insured patients who received TRT from 2010 to 2012, 40.3% had ≥ 2 testosterone prescriptions, and 12% had LH and/or FSH measured prior to the initiation.8
Low rates of guideline-recommended laboratory tests prior to initiation of testosterone treatment were reported in prior non-VA studies.19,20 Poor guideline adherence reinforces the need for clinician education or other methods to improve TRT and ensure appropriate prescribing practices across health care systems. The TOT described in this project is a sustainable clinical tool with the potential to improve testosterone prescribing practices.
The high rates of adherence to guideline recommendations at VAPSHCS likely stem from local endocrine expertise and ongoing educational initiatives, as well as the requirement for template completion before testosterone prescription. However, most testosterone prescriptions were initiated by primary care and monitored by pharmacists with varying degrees of training and clinical experience in hypogonadism and TRT.
However, adherence to guideline recommendations was modest, suggesting there is still an opportunity for improvement. The decision to initiate therapy should be made only after appropriate counseling with patients regarding its potential benefits and risks. Reports on the CV risk of TRT have been mixed. The 2023 TRAVERSE study found no increase in major adverse CV events among older men with hypogonadism and pre-existing CV risks undergoing TRT, but noted higher instances of pulmonary embolism, atrial fibrillation, and acute kidney injury.21 This highlights the need for clinicians to continue to engage in informed decision-making with patients. Effective pretreatment counseling is important but time-consuming; future TOT monitoring and modifications could consider mandatory checkboxes to document counseling on TRT risks and benefits.
The TOT described in this study could be adapted and incorporated into the prescribing process and electronic health record of larger health care systems. Use of an electronic template allows for automatic real-time dashboard monitoring of organization performance. The TOT described could be modified or simplified for specialty or primary care clinics or individual practitioners to improve adherence to evidence-based guideline recommendations and quality of care.
Strengths
A strength of this study is the multidisciplinary team (composed of stakeholders with experience in VA health care system and subject matter experts in hypogonadism) that developed and incorporated a user-friendly template for testosterone prescriptions; the use of evidence-based guideline recommendations; and the use of a structured chart review permitted accurate assessment of adherence to recommendations to document signs and symptoms of testosterone deficiency and a discussion of potential risks and benefits prior to TRT. To our knowledge, these recommendations have not been assessed in previous reports.
Limitations
The retrospective pre-post design of this study precludes a conclusion that implementation of the TOT caused the increase in adherence to guideline recommendations. Improved adherence could have resulted from the ongoing development of the preauthorization process for testosterone prescriptions or other changes over time. However, the preauthorization process had already been established for many years prior to template implementation. Forty-nine patients had new prescriptions for testosterone in the posttemplate period compared to 91 in the pretemplate period, but TRT was initiated in accordance with guideline recommendations more appropriately in the posttemplate period. The study’s sample size was small, and many eligible patients were excluded; however, exclusions were necessary to evaluate men who had new testosterone prescriptions for which the template was designed. Most men excluded were already taking testosterone.
Conclusions
The implementation of a CPRS-based TOT improved adherence to evidence-based guidelines for the diagnosis, evaluation, and counseling of patients with hypogonadism before starting TRT. While there were improvements in adherence with the TOT, the relatively low proportion of patients with documentation of TRT risks and benefits and all guideline recommendations highlights the need for additional efforts to further strengthen adherence to guideline recommendations and ensure appropriate evaluation, counseling, and prescribing practices before initiating TRT.
- Layton JB, Li D, Meier CR, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab. 2014;99:835-842. doi:10.1210/jc.2013-3570
- Baillargeon J, Kuo YF, Westra JR, et al. Testosterone prescribing in the United States, 2002-2016. JAMA. 2018;320:200-202. doi:10.1001/jama.2018.7999
- Jasuja GK, Bhasin S, Rose AJ. Patterns of testosterone prescription overuse. Curr Opin Endocrinol Diabetes Obes. 2017;24:240-245. doi:10.1097/MED.0000000000000336
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995-2010. doi:10.1210/jc.2005-2847
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-2559. doi:10.1210/jc.2009-2354
- Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1715-1744. doi:10.1210/jc.2018-00229
- Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. 2018;200:423-432. doi:10.1016/j.juro.2018.03.115
- Muram D, Zhang X, Cui Z, et al. Use of hormone testing for the diagnosis and evaluation of male hypogonadism and monitoring of testosterone therapy: application of hormone testing guideline recommendations in clinical practice. J Sex Med. 2015;12:1886-1894. doi:10.1111/jsm.12968
- Jasuja GK, Bhasin S, Reisman JI, et al. Ascertainment of testosterone prescribing practices in the VA. Med Care. 2015;53:746-752. doi:10.1097/MLR.0000000000000398?
- Jasuja GK, Bhasin S, Reisman JI, et al. Who gets testosterone? Patient characteristics associated with testosterone prescribing in the Veteran Affairs system: a cross-sectional study. J Gen Intern Med. 2017;32:304-311. doi:10.1007/s11606-016-3940-7
- Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109-122. doi:10.1056/NEJMoa1000485
- Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829-1836. doi:10.1001/jama.2013.280386
- Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9:e85805. doi:10.1371/journal.pone.0085805
- US Food and Drug Administration. FDA Drug Safety Communication: FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke with use. FDA.gov. March 3, 2015. Updated February 28, 2025. Accessed July 8, 2025. http://www.fda.gov/Drugs/DrugSafety/ucm436259.htm
- US Dept of Veterans Affairs, Office of Inspector General. Healthcare inspection – testosterone replacement therapy initiation and follow-up evaluation in VA male patients. April 11, 2018. Accessed July 8, 2025. https://www.vaoig.gov/reports/national-healthcare-review/healthcare-inspection-testosterone-replacement-therapy
- Narla R, Mobley D, Nguyen EHK, et al. Preliminary evaluation of an order template to improve diagnosis and testosterone therapy of hypogonadism in veterans. Fed Pract. 2021;38:121-127. doi:10.12788/fp.0103
- Bhasin S, Travison TG, Pencina KM, et al. Prostate safety events during testosterone replacement therapy in men with hypogonadism: a randomized clinical trial. JAMA Netw Open. 2023;6:e2348692. doi:10.1001/jamanetworkopen.2023.48692
- Dubin JM, Jesse E, Fantus RJ, et al. Guideline-discordant care among direct-to-consumer testosterone therapy platforms. JAMA Intern Med. 2022;182:1321-1323. doi:10.1001/jamainternmed.2022.4928
- Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465-1466. doi:10.1001/jamainternmed.2013.6895
- Locke JA, Flannigan R, Günther OP, et al. Testosterone therapy: prescribing and monitoring patterns of practice in British Columbia. Can Urol Assoc J. 2021;15:e110-e117. doi:10.5489/cuaj.6586
- Lincoff AM, Bhasin S, Flevaris P, et al. Cardiovascular safety of testosterone-replacement therapy. N Engl J Med. 2023;389:107-117. doi:10.1056/NEJMoa2215025
Testosterone therapy is administered following pragmatic diagnostic evaluation and workup to assess whether an adult male is hypogonadal, based on symptoms consistent with androgen deficiency and low morning serum testosterone concentrations on ≥ 2 occasions. Effects of testosterone administration include the development or maintenance of secondary sexual characteristics and increases in libido, muscle strength, fat-free mass, and bone density.
Testosterone prescriptions have markedly increased in the past 20 years, including within the US Department of Veterans Affairs (VA) health care system.1-3 This trend may be influenced by various factors, including patient perceptions of benefit, an increase in marketing, and the availability of more user-friendly formulations.
Since 2006, evidence-based clinical practice guidelines have recommended specific clinical and laboratory evaluation and counseling prior to starting testosterone replacement therapy (TRT).4-8 However, research has shown poor adherence to these recommendations, including at the VA, which raises concerns about inappropriate TRT initiation without proper diagnostic evaluation.9,10 Observational research has suggested a possible link between testosterone therapy and increased risk of cardiovascular (CV) events. The US Food and Drug Administration prescribing information includes boxed warnings about potential risks of high blood pressure, myocardial infarction, stroke, and CV-related mortality with testosterone treatment, contact transfer of transdermal testosterone, and pulmonary oil microembolism with testosterone undecanoate injections.11-15
A VA Office of Inspector General (OIG) review of VA clinician adherence to clinical and laboratory evaluation guidelines for testosterone deficiency found poor adherence among VA practitioners and made recommendations for improvement.4,15 These focused on establishing clinical signs and symptoms consistent with testosterone deficiency, confirming hypogonadism by repeated testosterone testing, determining the etiology of hypogonadism by measuring gonadotropins, initiating a discussion of risks and benefits of TRT, and assessing clinical improvement and obtaining an updated hematocrit test within 3 to 6 months of initiation.
The VA Puget Sound Health Care System (VAPSHCS) developed a local prior authorization template to assist health care practitioners (HCPs) to address the OIG recommendations. This testosterone order template (TOT) aimed to improve the diagnosis, evaluation, and monitoring of TRT in males with hypogonadism, combined with existing VA pharmacy criteria for the use of testosterone based on Endocrine Society guidelines. A version of the VAPSHCS TOT was approved as the national VA Computerized Patient Record System (CPRS) template.
Preliminary evaluation of the TOT suggested improved short-term adherence to guideline recommendations following implementation.16 This quality improvement study sought to assess the long-term effectiveness of the TOT with respect to clinical practice guideline adherence. The OIG did not address prostate-specific antigen (PSA) monitoring because understanding of the relationship between TRT and the risks of elevated PSA levels remains incomplete.6,17 This project hypothesized that implementation of a pharmacy-managed TOT incorporated into CPRS would result in higher adherence rates to guideline-recommended clinical and laboratory evaluation, in addition to counseling of men with hypogonadism prior to initiation of TRT.
Methods
Eligible participants were cisgender males who received a new testosterone prescription, had ≥ 2 clinic visits at VAPSHCS, and no previous testosterone prescription in the previous 2 years. Individuals were excluded if they had testosterone administered at VAPSHCS; were prescribed testosterone at another facility (VA or community-based); pilot tested an initial version of the TOT prior to November 30, 2019; or had an International Classification of Diseases, Tenth Revision codes for hypopituitarism, gender identity disorder, history of sexual assignment, or Klinefelter syndrome for which testosterone therapy was already approved. Patients who met the inclusion criteria were identified by an algorithm developed by the VAPSHCS pharmacoeconomist.
This quality improvement project used a retrospective, pre-post experimental design. Electronic chart review and systematic manual review of all eligible patient charts were performed for the pretemplate period (December 1, 2018, to November 30, 2019) and after the template implementation, (December 1, 2021, to November 30, 2022).
An initial version of the TOT was implemented on July 1, 2019, but was not fully integrated into CPRS until early 2020; individuals in whom the TOT was used prior to November 30, 2019, were excluded. Data from the initial period of the COVID-19 pandemic were avoided because of alterations in clinic and prescribing practices. As a quality improvement project, the TOT evaluation was exempt from formal review by the VAPSHCS Institutional Review Board, as determined by the Director of the Office of Transformation/Quality/Safety/Value.
Interventions
Testosterone is a Schedule III controlled substance with potential risks and a propensity for varied prescribing practices. It was designated as a restricted drug requiring a prior authorization drug request (PADR) for which a specific TOT was developed, approved by the VAPSHCS Pharmacy and Therapeutics Committee, and incorporated into CPRS. A team of pharmacists, primary care physicians, geriatricians, endocrinologists, and health informatics experts created and developed the TOT. Pharmacists managed and monitored its completion.
The process for prescribing testosterone via the TOT is outlined in the eAppendix. When an HCP orders testosterone in CPRS, reminders prompt them to use the TOT and indicate required laboratory measurements (an order set is provided). Completion of TOT is not necessary to order testosterone for patients with an existing diagnosis of an organic cause of hypogonadism (eg, Klinefelter syndrome or hypopituitarism) or transgender women (assigned male at birth). In the TOT, the prescriber must also indicate signs and symptoms of testosterone deficiency; required laboratory tests; and counseling regarding potential risks and benefits of TRT. A pharmacist reviews the TOT and either approves or rejects the testosterone prescription and provides follow-up guidance to the prescriber. The completed TOT serves as documentation of guideline adherence in CPRS. The TOT also includes sections for first renewal testosterone prescriptions, addressing guideline recommendations for follow-up laboratory evaluation and clinical response to TRT. Due to limited completion of this section in the posttemplate period, evaluating adherence to follow-up recommendations was not feasible.
Measures
This project assessed the percentage of patients in the posttemplate period vs pretemplate period with an approved PADR. Documentation of specific guideline-recommended measures was assessed: signs and symptoms of testosterone deficiency; ≥ 2 serum testosterone measurements (≥ 2 total, free and total, or 2 free testosterone levels, and ≥ 1 testosterone level before 10
The project also assessed the proportion of patients in the posttemplate period vs pretemplate period who had all hormone tests (≥ 2 serum testosterone and LH and FSH concentrations), all laboratory tests (hormone tests and hematocrit), and all 5 guideline-recommended measures.
Analysis
Statistical comparisons between the proportions of patients in the pretemplate and posttemplate periods for each measure were performed using a χ2 test, without correction for multiple comparisons. All analyses were conducted using Stata version 10.0. A P value < .05 was considered significant for all comparisons.
Results
Chart review identified 189 patients in the pretemplate period and 113 patients in the posttemplate period with a new testosterone prescription (Figure). After exclusions, 91 and 49 patients, respectively, met eligibility criteria (Table 1). Fifty-six patients (62%) pretemplate and 40 patients (82%) posttemplate (P = .015) had approved PADRs and comprised the groups that were analyzed (Table 2).



The mean age and body mass index were similar in the pretemplate and posttemplate periods, but there was variation in the proportions of patients aged < 70 years and those with a body mass index < 30 between the groups. The most common diagnosis in both groups was testicular hypofunction, and the most common comorbidity was type 2 diabetes mellitus. Concomitant use of opioids or glucocorticoids that can lower testosterone levels was rare. Most testosterone prescriptions originated from primary care clinics in both periods: 68 (75%) in the pretemplate period and 35 (71%) in the posttemplate period. Most testosterone treatment was delivered by intramuscular injection.
In the posttemplate period vs pretemplate period, the proportion of patients with an approved PADR (82% vs 62%, P = .02), and documentation of signs and symptoms of hypogonadism (93% vs 71%, P = .002) prior to starting TRT were higher, while the percentage of patients having ≥ 2 testosterone measurements (85% vs 89%, P = .53), ≥ 1 testosterone level before 10 AM (78% vs 75%, P = .70), and hematocrit measured (95% vs 91%, P = .47) were similar. Rates of LH and FSH testing were higher in the posttemplate period (80%) vs the pretemplate period (63%) but did not achieve statistical significance (P = .07), and discussion of the risks and benefits of TRT was higher in the posttemplate period (58%) vs the pretemplate period (34%) (P = .02). The percentage of patients who had all hormone measurements (total and/or free testosterone, LH, and FSH) was higher in the posttemplate period (78%) vs the pretemplate period (59%) but did not achieve statistical significance (P = .06). The rates of all guideline-recommended laboratory test orders were higher in the posttemplate period (78%) vs the pretemplate period (55%) (P = .03), and all 5 guideline-recommended clinical and laboratory measures were higher in the posttemplate period (45%) vs the pretemplate period (18%) (P = .004).
Discussion
The implementation of a pharmacy-managed TOT in CPRS demonstrated higher adherence to evidence-based guidelines for diagnosing and evaluating hypogonadism before TRT. After TOT implementation, a higher proportion of patients had documented signs and symptoms of testosterone deficiency, underwent all recommended laboratory tests, and had discussions about the risks and benefits of TRT. Adherence to 5 clinical and laboratory measures recommended by Endocrine Society guidelines was higher after TOT implementation, indicating improved prescribing practices.4
The requirement for TOT completion before testosterone prescription and its management by trained pharmacists likely contributed to higher adherence to guideline recommendations than previously reported. Integration of the TOT into CPRS with pharmacy oversight may have enhanced adherence by summarizing and codifying evidence-based guideline recommendations for clinical and biochemical evaluation prior to TRT initiation, offering relevant education to clinicians and pharmacists, automatically importing pertinent clinical information and laboratory results, and generating CPRS documentation to reduce clinician burden during patient care.
The proportion of patients with documented signs and symptoms of testosterone deficiency before TRT increased from the pretemplate period (71%) to the posttemplate period (93%), indicating that most patients receiving TRT had clinical manifestations of hypogonadism. This aligns with Endocrine Society guidelines, which define hypogonadism as a clinical disorder characterized by clinical manifestations of testosterone deficiency and persistently low serum testosterone levels on ≥ 2 separate occasions.4,6 However, recent trends in direct-to-consumer advertising for testosterone and the rise of “low T” clinics may contribute to increased testing, varied practices, and inappropriate testosterone therapy initiation (eg, in men with low testosterone levels who lack symptoms of hypogonadism).18 Improved adherence in documenting clinical hypogonadism with implementation of the TOT reinforces the value of incorporating educational material, as previously reported.11
Adherence to guideline recommendations following implementation of the TOT in this project was higher than those previously reported. In a study of 111,631 outpatient veterans prescribed testosterone from 2009 to 2012, only 18.3% had ≥ 2 testosterone prescriptions, and 3.5% had ≥ 2 testosterone, LH, and FSH levels measured prior to the initiation of a TRT.9 In a report of 63,534 insured patients who received TRT from 2010 to 2012, 40.3% had ≥ 2 testosterone prescriptions, and 12% had LH and/or FSH measured prior to the initiation.8
Low rates of guideline-recommended laboratory tests prior to initiation of testosterone treatment were reported in prior non-VA studies.19,20 Poor guideline adherence reinforces the need for clinician education or other methods to improve TRT and ensure appropriate prescribing practices across health care systems. The TOT described in this project is a sustainable clinical tool with the potential to improve testosterone prescribing practices.
The high rates of adherence to guideline recommendations at VAPSHCS likely stem from local endocrine expertise and ongoing educational initiatives, as well as the requirement for template completion before testosterone prescription. However, most testosterone prescriptions were initiated by primary care and monitored by pharmacists with varying degrees of training and clinical experience in hypogonadism and TRT.
However, adherence to guideline recommendations was modest, suggesting there is still an opportunity for improvement. The decision to initiate therapy should be made only after appropriate counseling with patients regarding its potential benefits and risks. Reports on the CV risk of TRT have been mixed. The 2023 TRAVERSE study found no increase in major adverse CV events among older men with hypogonadism and pre-existing CV risks undergoing TRT, but noted higher instances of pulmonary embolism, atrial fibrillation, and acute kidney injury.21 This highlights the need for clinicians to continue to engage in informed decision-making with patients. Effective pretreatment counseling is important but time-consuming; future TOT monitoring and modifications could consider mandatory checkboxes to document counseling on TRT risks and benefits.
The TOT described in this study could be adapted and incorporated into the prescribing process and electronic health record of larger health care systems. Use of an electronic template allows for automatic real-time dashboard monitoring of organization performance. The TOT described could be modified or simplified for specialty or primary care clinics or individual practitioners to improve adherence to evidence-based guideline recommendations and quality of care.
Strengths
A strength of this study is the multidisciplinary team (composed of stakeholders with experience in VA health care system and subject matter experts in hypogonadism) that developed and incorporated a user-friendly template for testosterone prescriptions; the use of evidence-based guideline recommendations; and the use of a structured chart review permitted accurate assessment of adherence to recommendations to document signs and symptoms of testosterone deficiency and a discussion of potential risks and benefits prior to TRT. To our knowledge, these recommendations have not been assessed in previous reports.
Limitations
The retrospective pre-post design of this study precludes a conclusion that implementation of the TOT caused the increase in adherence to guideline recommendations. Improved adherence could have resulted from the ongoing development of the preauthorization process for testosterone prescriptions or other changes over time. However, the preauthorization process had already been established for many years prior to template implementation. Forty-nine patients had new prescriptions for testosterone in the posttemplate period compared to 91 in the pretemplate period, but TRT was initiated in accordance with guideline recommendations more appropriately in the posttemplate period. The study’s sample size was small, and many eligible patients were excluded; however, exclusions were necessary to evaluate men who had new testosterone prescriptions for which the template was designed. Most men excluded were already taking testosterone.
Conclusions
The implementation of a CPRS-based TOT improved adherence to evidence-based guidelines for the diagnosis, evaluation, and counseling of patients with hypogonadism before starting TRT. While there were improvements in adherence with the TOT, the relatively low proportion of patients with documentation of TRT risks and benefits and all guideline recommendations highlights the need for additional efforts to further strengthen adherence to guideline recommendations and ensure appropriate evaluation, counseling, and prescribing practices before initiating TRT.
Testosterone therapy is administered following pragmatic diagnostic evaluation and workup to assess whether an adult male is hypogonadal, based on symptoms consistent with androgen deficiency and low morning serum testosterone concentrations on ≥ 2 occasions. Effects of testosterone administration include the development or maintenance of secondary sexual characteristics and increases in libido, muscle strength, fat-free mass, and bone density.
Testosterone prescriptions have markedly increased in the past 20 years, including within the US Department of Veterans Affairs (VA) health care system.1-3 This trend may be influenced by various factors, including patient perceptions of benefit, an increase in marketing, and the availability of more user-friendly formulations.
Since 2006, evidence-based clinical practice guidelines have recommended specific clinical and laboratory evaluation and counseling prior to starting testosterone replacement therapy (TRT).4-8 However, research has shown poor adherence to these recommendations, including at the VA, which raises concerns about inappropriate TRT initiation without proper diagnostic evaluation.9,10 Observational research has suggested a possible link between testosterone therapy and increased risk of cardiovascular (CV) events. The US Food and Drug Administration prescribing information includes boxed warnings about potential risks of high blood pressure, myocardial infarction, stroke, and CV-related mortality with testosterone treatment, contact transfer of transdermal testosterone, and pulmonary oil microembolism with testosterone undecanoate injections.11-15
A VA Office of Inspector General (OIG) review of VA clinician adherence to clinical and laboratory evaluation guidelines for testosterone deficiency found poor adherence among VA practitioners and made recommendations for improvement.4,15 These focused on establishing clinical signs and symptoms consistent with testosterone deficiency, confirming hypogonadism by repeated testosterone testing, determining the etiology of hypogonadism by measuring gonadotropins, initiating a discussion of risks and benefits of TRT, and assessing clinical improvement and obtaining an updated hematocrit test within 3 to 6 months of initiation.
The VA Puget Sound Health Care System (VAPSHCS) developed a local prior authorization template to assist health care practitioners (HCPs) to address the OIG recommendations. This testosterone order template (TOT) aimed to improve the diagnosis, evaluation, and monitoring of TRT in males with hypogonadism, combined with existing VA pharmacy criteria for the use of testosterone based on Endocrine Society guidelines. A version of the VAPSHCS TOT was approved as the national VA Computerized Patient Record System (CPRS) template.
Preliminary evaluation of the TOT suggested improved short-term adherence to guideline recommendations following implementation.16 This quality improvement study sought to assess the long-term effectiveness of the TOT with respect to clinical practice guideline adherence. The OIG did not address prostate-specific antigen (PSA) monitoring because understanding of the relationship between TRT and the risks of elevated PSA levels remains incomplete.6,17 This project hypothesized that implementation of a pharmacy-managed TOT incorporated into CPRS would result in higher adherence rates to guideline-recommended clinical and laboratory evaluation, in addition to counseling of men with hypogonadism prior to initiation of TRT.
Methods
Eligible participants were cisgender males who received a new testosterone prescription, had ≥ 2 clinic visits at VAPSHCS, and no previous testosterone prescription in the previous 2 years. Individuals were excluded if they had testosterone administered at VAPSHCS; were prescribed testosterone at another facility (VA or community-based); pilot tested an initial version of the TOT prior to November 30, 2019; or had an International Classification of Diseases, Tenth Revision codes for hypopituitarism, gender identity disorder, history of sexual assignment, or Klinefelter syndrome for which testosterone therapy was already approved. Patients who met the inclusion criteria were identified by an algorithm developed by the VAPSHCS pharmacoeconomist.
This quality improvement project used a retrospective, pre-post experimental design. Electronic chart review and systematic manual review of all eligible patient charts were performed for the pretemplate period (December 1, 2018, to November 30, 2019) and after the template implementation, (December 1, 2021, to November 30, 2022).
An initial version of the TOT was implemented on July 1, 2019, but was not fully integrated into CPRS until early 2020; individuals in whom the TOT was used prior to November 30, 2019, were excluded. Data from the initial period of the COVID-19 pandemic were avoided because of alterations in clinic and prescribing practices. As a quality improvement project, the TOT evaluation was exempt from formal review by the VAPSHCS Institutional Review Board, as determined by the Director of the Office of Transformation/Quality/Safety/Value.
Interventions
Testosterone is a Schedule III controlled substance with potential risks and a propensity for varied prescribing practices. It was designated as a restricted drug requiring a prior authorization drug request (PADR) for which a specific TOT was developed, approved by the VAPSHCS Pharmacy and Therapeutics Committee, and incorporated into CPRS. A team of pharmacists, primary care physicians, geriatricians, endocrinologists, and health informatics experts created and developed the TOT. Pharmacists managed and monitored its completion.
The process for prescribing testosterone via the TOT is outlined in the eAppendix. When an HCP orders testosterone in CPRS, reminders prompt them to use the TOT and indicate required laboratory measurements (an order set is provided). Completion of TOT is not necessary to order testosterone for patients with an existing diagnosis of an organic cause of hypogonadism (eg, Klinefelter syndrome or hypopituitarism) or transgender women (assigned male at birth). In the TOT, the prescriber must also indicate signs and symptoms of testosterone deficiency; required laboratory tests; and counseling regarding potential risks and benefits of TRT. A pharmacist reviews the TOT and either approves or rejects the testosterone prescription and provides follow-up guidance to the prescriber. The completed TOT serves as documentation of guideline adherence in CPRS. The TOT also includes sections for first renewal testosterone prescriptions, addressing guideline recommendations for follow-up laboratory evaluation and clinical response to TRT. Due to limited completion of this section in the posttemplate period, evaluating adherence to follow-up recommendations was not feasible.
Measures
This project assessed the percentage of patients in the posttemplate period vs pretemplate period with an approved PADR. Documentation of specific guideline-recommended measures was assessed: signs and symptoms of testosterone deficiency; ≥ 2 serum testosterone measurements (≥ 2 total, free and total, or 2 free testosterone levels, and ≥ 1 testosterone level before 10
The project also assessed the proportion of patients in the posttemplate period vs pretemplate period who had all hormone tests (≥ 2 serum testosterone and LH and FSH concentrations), all laboratory tests (hormone tests and hematocrit), and all 5 guideline-recommended measures.
Analysis
Statistical comparisons between the proportions of patients in the pretemplate and posttemplate periods for each measure were performed using a χ2 test, without correction for multiple comparisons. All analyses were conducted using Stata version 10.0. A P value < .05 was considered significant for all comparisons.
Results
Chart review identified 189 patients in the pretemplate period and 113 patients in the posttemplate period with a new testosterone prescription (Figure). After exclusions, 91 and 49 patients, respectively, met eligibility criteria (Table 1). Fifty-six patients (62%) pretemplate and 40 patients (82%) posttemplate (P = .015) had approved PADRs and comprised the groups that were analyzed (Table 2).



The mean age and body mass index were similar in the pretemplate and posttemplate periods, but there was variation in the proportions of patients aged < 70 years and those with a body mass index < 30 between the groups. The most common diagnosis in both groups was testicular hypofunction, and the most common comorbidity was type 2 diabetes mellitus. Concomitant use of opioids or glucocorticoids that can lower testosterone levels was rare. Most testosterone prescriptions originated from primary care clinics in both periods: 68 (75%) in the pretemplate period and 35 (71%) in the posttemplate period. Most testosterone treatment was delivered by intramuscular injection.
In the posttemplate period vs pretemplate period, the proportion of patients with an approved PADR (82% vs 62%, P = .02), and documentation of signs and symptoms of hypogonadism (93% vs 71%, P = .002) prior to starting TRT were higher, while the percentage of patients having ≥ 2 testosterone measurements (85% vs 89%, P = .53), ≥ 1 testosterone level before 10 AM (78% vs 75%, P = .70), and hematocrit measured (95% vs 91%, P = .47) were similar. Rates of LH and FSH testing were higher in the posttemplate period (80%) vs the pretemplate period (63%) but did not achieve statistical significance (P = .07), and discussion of the risks and benefits of TRT was higher in the posttemplate period (58%) vs the pretemplate period (34%) (P = .02). The percentage of patients who had all hormone measurements (total and/or free testosterone, LH, and FSH) was higher in the posttemplate period (78%) vs the pretemplate period (59%) but did not achieve statistical significance (P = .06). The rates of all guideline-recommended laboratory test orders were higher in the posttemplate period (78%) vs the pretemplate period (55%) (P = .03), and all 5 guideline-recommended clinical and laboratory measures were higher in the posttemplate period (45%) vs the pretemplate period (18%) (P = .004).
Discussion
The implementation of a pharmacy-managed TOT in CPRS demonstrated higher adherence to evidence-based guidelines for diagnosing and evaluating hypogonadism before TRT. After TOT implementation, a higher proportion of patients had documented signs and symptoms of testosterone deficiency, underwent all recommended laboratory tests, and had discussions about the risks and benefits of TRT. Adherence to 5 clinical and laboratory measures recommended by Endocrine Society guidelines was higher after TOT implementation, indicating improved prescribing practices.4
The requirement for TOT completion before testosterone prescription and its management by trained pharmacists likely contributed to higher adherence to guideline recommendations than previously reported. Integration of the TOT into CPRS with pharmacy oversight may have enhanced adherence by summarizing and codifying evidence-based guideline recommendations for clinical and biochemical evaluation prior to TRT initiation, offering relevant education to clinicians and pharmacists, automatically importing pertinent clinical information and laboratory results, and generating CPRS documentation to reduce clinician burden during patient care.
The proportion of patients with documented signs and symptoms of testosterone deficiency before TRT increased from the pretemplate period (71%) to the posttemplate period (93%), indicating that most patients receiving TRT had clinical manifestations of hypogonadism. This aligns with Endocrine Society guidelines, which define hypogonadism as a clinical disorder characterized by clinical manifestations of testosterone deficiency and persistently low serum testosterone levels on ≥ 2 separate occasions.4,6 However, recent trends in direct-to-consumer advertising for testosterone and the rise of “low T” clinics may contribute to increased testing, varied practices, and inappropriate testosterone therapy initiation (eg, in men with low testosterone levels who lack symptoms of hypogonadism).18 Improved adherence in documenting clinical hypogonadism with implementation of the TOT reinforces the value of incorporating educational material, as previously reported.11
Adherence to guideline recommendations following implementation of the TOT in this project was higher than those previously reported. In a study of 111,631 outpatient veterans prescribed testosterone from 2009 to 2012, only 18.3% had ≥ 2 testosterone prescriptions, and 3.5% had ≥ 2 testosterone, LH, and FSH levels measured prior to the initiation of a TRT.9 In a report of 63,534 insured patients who received TRT from 2010 to 2012, 40.3% had ≥ 2 testosterone prescriptions, and 12% had LH and/or FSH measured prior to the initiation.8
Low rates of guideline-recommended laboratory tests prior to initiation of testosterone treatment were reported in prior non-VA studies.19,20 Poor guideline adherence reinforces the need for clinician education or other methods to improve TRT and ensure appropriate prescribing practices across health care systems. The TOT described in this project is a sustainable clinical tool with the potential to improve testosterone prescribing practices.
The high rates of adherence to guideline recommendations at VAPSHCS likely stem from local endocrine expertise and ongoing educational initiatives, as well as the requirement for template completion before testosterone prescription. However, most testosterone prescriptions were initiated by primary care and monitored by pharmacists with varying degrees of training and clinical experience in hypogonadism and TRT.
However, adherence to guideline recommendations was modest, suggesting there is still an opportunity for improvement. The decision to initiate therapy should be made only after appropriate counseling with patients regarding its potential benefits and risks. Reports on the CV risk of TRT have been mixed. The 2023 TRAVERSE study found no increase in major adverse CV events among older men with hypogonadism and pre-existing CV risks undergoing TRT, but noted higher instances of pulmonary embolism, atrial fibrillation, and acute kidney injury.21 This highlights the need for clinicians to continue to engage in informed decision-making with patients. Effective pretreatment counseling is important but time-consuming; future TOT monitoring and modifications could consider mandatory checkboxes to document counseling on TRT risks and benefits.
The TOT described in this study could be adapted and incorporated into the prescribing process and electronic health record of larger health care systems. Use of an electronic template allows for automatic real-time dashboard monitoring of organization performance. The TOT described could be modified or simplified for specialty or primary care clinics or individual practitioners to improve adherence to evidence-based guideline recommendations and quality of care.
Strengths
A strength of this study is the multidisciplinary team (composed of stakeholders with experience in VA health care system and subject matter experts in hypogonadism) that developed and incorporated a user-friendly template for testosterone prescriptions; the use of evidence-based guideline recommendations; and the use of a structured chart review permitted accurate assessment of adherence to recommendations to document signs and symptoms of testosterone deficiency and a discussion of potential risks and benefits prior to TRT. To our knowledge, these recommendations have not been assessed in previous reports.
Limitations
The retrospective pre-post design of this study precludes a conclusion that implementation of the TOT caused the increase in adherence to guideline recommendations. Improved adherence could have resulted from the ongoing development of the preauthorization process for testosterone prescriptions or other changes over time. However, the preauthorization process had already been established for many years prior to template implementation. Forty-nine patients had new prescriptions for testosterone in the posttemplate period compared to 91 in the pretemplate period, but TRT was initiated in accordance with guideline recommendations more appropriately in the posttemplate period. The study’s sample size was small, and many eligible patients were excluded; however, exclusions were necessary to evaluate men who had new testosterone prescriptions for which the template was designed. Most men excluded were already taking testosterone.
Conclusions
The implementation of a CPRS-based TOT improved adherence to evidence-based guidelines for the diagnosis, evaluation, and counseling of patients with hypogonadism before starting TRT. While there were improvements in adherence with the TOT, the relatively low proportion of patients with documentation of TRT risks and benefits and all guideline recommendations highlights the need for additional efforts to further strengthen adherence to guideline recommendations and ensure appropriate evaluation, counseling, and prescribing practices before initiating TRT.
- Layton JB, Li D, Meier CR, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab. 2014;99:835-842. doi:10.1210/jc.2013-3570
- Baillargeon J, Kuo YF, Westra JR, et al. Testosterone prescribing in the United States, 2002-2016. JAMA. 2018;320:200-202. doi:10.1001/jama.2018.7999
- Jasuja GK, Bhasin S, Rose AJ. Patterns of testosterone prescription overuse. Curr Opin Endocrinol Diabetes Obes. 2017;24:240-245. doi:10.1097/MED.0000000000000336
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995-2010. doi:10.1210/jc.2005-2847
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-2559. doi:10.1210/jc.2009-2354
- Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1715-1744. doi:10.1210/jc.2018-00229
- Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. 2018;200:423-432. doi:10.1016/j.juro.2018.03.115
- Muram D, Zhang X, Cui Z, et al. Use of hormone testing for the diagnosis and evaluation of male hypogonadism and monitoring of testosterone therapy: application of hormone testing guideline recommendations in clinical practice. J Sex Med. 2015;12:1886-1894. doi:10.1111/jsm.12968
- Jasuja GK, Bhasin S, Reisman JI, et al. Ascertainment of testosterone prescribing practices in the VA. Med Care. 2015;53:746-752. doi:10.1097/MLR.0000000000000398?
- Jasuja GK, Bhasin S, Reisman JI, et al. Who gets testosterone? Patient characteristics associated with testosterone prescribing in the Veteran Affairs system: a cross-sectional study. J Gen Intern Med. 2017;32:304-311. doi:10.1007/s11606-016-3940-7
- Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109-122. doi:10.1056/NEJMoa1000485
- Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829-1836. doi:10.1001/jama.2013.280386
- Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9:e85805. doi:10.1371/journal.pone.0085805
- US Food and Drug Administration. FDA Drug Safety Communication: FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke with use. FDA.gov. March 3, 2015. Updated February 28, 2025. Accessed July 8, 2025. http://www.fda.gov/Drugs/DrugSafety/ucm436259.htm
- US Dept of Veterans Affairs, Office of Inspector General. Healthcare inspection – testosterone replacement therapy initiation and follow-up evaluation in VA male patients. April 11, 2018. Accessed July 8, 2025. https://www.vaoig.gov/reports/national-healthcare-review/healthcare-inspection-testosterone-replacement-therapy
- Narla R, Mobley D, Nguyen EHK, et al. Preliminary evaluation of an order template to improve diagnosis and testosterone therapy of hypogonadism in veterans. Fed Pract. 2021;38:121-127. doi:10.12788/fp.0103
- Bhasin S, Travison TG, Pencina KM, et al. Prostate safety events during testosterone replacement therapy in men with hypogonadism: a randomized clinical trial. JAMA Netw Open. 2023;6:e2348692. doi:10.1001/jamanetworkopen.2023.48692
- Dubin JM, Jesse E, Fantus RJ, et al. Guideline-discordant care among direct-to-consumer testosterone therapy platforms. JAMA Intern Med. 2022;182:1321-1323. doi:10.1001/jamainternmed.2022.4928
- Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465-1466. doi:10.1001/jamainternmed.2013.6895
- Locke JA, Flannigan R, Günther OP, et al. Testosterone therapy: prescribing and monitoring patterns of practice in British Columbia. Can Urol Assoc J. 2021;15:e110-e117. doi:10.5489/cuaj.6586
- Lincoff AM, Bhasin S, Flevaris P, et al. Cardiovascular safety of testosterone-replacement therapy. N Engl J Med. 2023;389:107-117. doi:10.1056/NEJMoa2215025
- Layton JB, Li D, Meier CR, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab. 2014;99:835-842. doi:10.1210/jc.2013-3570
- Baillargeon J, Kuo YF, Westra JR, et al. Testosterone prescribing in the United States, 2002-2016. JAMA. 2018;320:200-202. doi:10.1001/jama.2018.7999
- Jasuja GK, Bhasin S, Rose AJ. Patterns of testosterone prescription overuse. Curr Opin Endocrinol Diabetes Obes. 2017;24:240-245. doi:10.1097/MED.0000000000000336
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995-2010. doi:10.1210/jc.2005-2847
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-2559. doi:10.1210/jc.2009-2354
- Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1715-1744. doi:10.1210/jc.2018-00229
- Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. 2018;200:423-432. doi:10.1016/j.juro.2018.03.115
- Muram D, Zhang X, Cui Z, et al. Use of hormone testing for the diagnosis and evaluation of male hypogonadism and monitoring of testosterone therapy: application of hormone testing guideline recommendations in clinical practice. J Sex Med. 2015;12:1886-1894. doi:10.1111/jsm.12968
- Jasuja GK, Bhasin S, Reisman JI, et al. Ascertainment of testosterone prescribing practices in the VA. Med Care. 2015;53:746-752. doi:10.1097/MLR.0000000000000398?
- Jasuja GK, Bhasin S, Reisman JI, et al. Who gets testosterone? Patient characteristics associated with testosterone prescribing in the Veteran Affairs system: a cross-sectional study. J Gen Intern Med. 2017;32:304-311. doi:10.1007/s11606-016-3940-7
- Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109-122. doi:10.1056/NEJMoa1000485
- Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829-1836. doi:10.1001/jama.2013.280386
- Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9:e85805. doi:10.1371/journal.pone.0085805
- US Food and Drug Administration. FDA Drug Safety Communication: FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke with use. FDA.gov. March 3, 2015. Updated February 28, 2025. Accessed July 8, 2025. http://www.fda.gov/Drugs/DrugSafety/ucm436259.htm
- US Dept of Veterans Affairs, Office of Inspector General. Healthcare inspection – testosterone replacement therapy initiation and follow-up evaluation in VA male patients. April 11, 2018. Accessed July 8, 2025. https://www.vaoig.gov/reports/national-healthcare-review/healthcare-inspection-testosterone-replacement-therapy
- Narla R, Mobley D, Nguyen EHK, et al. Preliminary evaluation of an order template to improve diagnosis and testosterone therapy of hypogonadism in veterans. Fed Pract. 2021;38:121-127. doi:10.12788/fp.0103
- Bhasin S, Travison TG, Pencina KM, et al. Prostate safety events during testosterone replacement therapy in men with hypogonadism: a randomized clinical trial. JAMA Netw Open. 2023;6:e2348692. doi:10.1001/jamanetworkopen.2023.48692
- Dubin JM, Jesse E, Fantus RJ, et al. Guideline-discordant care among direct-to-consumer testosterone therapy platforms. JAMA Intern Med. 2022;182:1321-1323. doi:10.1001/jamainternmed.2022.4928
- Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465-1466. doi:10.1001/jamainternmed.2013.6895
- Locke JA, Flannigan R, Günther OP, et al. Testosterone therapy: prescribing and monitoring patterns of practice in British Columbia. Can Urol Assoc J. 2021;15:e110-e117. doi:10.5489/cuaj.6586
- Lincoff AM, Bhasin S, Flevaris P, et al. Cardiovascular safety of testosterone-replacement therapy. N Engl J Med. 2023;389:107-117. doi:10.1056/NEJMoa2215025
Streamlined Testosterone Order Template to Improve the Diagnosis and Evaluation of Hypogonadism in Veterans
Streamlined Testosterone Order Template to Improve the Diagnosis and Evaluation of Hypogonadism in Veterans
Mental Health Practitioners Continue to Decrease Despite Aging Vet Population
This article has been updated with a response from the US Department of Veterans Affairs.
The number of US Department of Veterans Affairs (VA) geriatric mental health professionals is failing to keep pace with a growing population of older veterans: nearly 8 million are aged ≥ 65 years. VA psychologists may treat older veterans in primary care settings or community living centers, but many lack formal training in geropsychology.
Some psychologists with the proper training to treat this population are leaving the workforce; a survey by the VA Office of Inspector General found psychology was the most frequently reported severe clinical occupational staffing shortage and the most frequently reported Hybrid Title 38 severe shortage occupation, with 57% of 139 facilities reporting it as a shortage. According to the September Workforce Dashboard, the VA has lost > 200 psychologists in 2025.
Veterans aged ≥ 65 years have higher rates of combined medical and mental health diagnoses than younger veterans and older nonveterans. Nearly 1 of 5 older veterans enrolled in US Department of Veterans Affairs (VA) health care services have confirmed mental health diagnoses, and another 26% have documented mental health concerns without a formal diagnosis in their health record.
Older veterans also tend to have more complex mental health issues than younger adults. Posttraumatic stress nearly doubles their risk of dementia, and their psychiatric diagnoses may be complicated by co-occurring delirium, social isolation/loneliness, and polypharmacy.
According to reporting by The War Horse, the VA has been instituting limits on one-on-one mental health therapy and transitioning veterans to lower levels of treatment after having been told to stop treating them for long, indeterminate periods prior to referring them to group therapy, primary care, or discharging them altogether. In a statement to Federal Practitioner, VA Press Secretary Pete Kasperowicz refuted the reporting from The War Horse.
"The War Horse story is false. VA does not put caps on one-on-one mental health sessions for veterans with clinical care needs," he told Federal Practitioner. "VA works with veterans over an initial eight to 15 mental health sessions, and collaboratively plans any needed follow-on care. As part of this process, veterans and their health care team decide together how to address ongoing needs, including whether to step down to other types of care and self-maintenance, or continue with VA therapy."
The smaller pool of qualified mental health practitioners also may be due to medical students not knowing enough about the category. A study of 136 medical students and 61 internal medicine residents at an academic health center evaluated their beliefs and attitudes regarding 25 content areas essential to the primary care of older adults. Students and residents expressed similar beliefs about the importance of content areas, and attitudes toward aging did not appreciably differ. However, students rated lower in knowledge in areas surrounding general primary care, such as chronic conditions and medications. Residents reported larger gap scores in areas that reflected specialists’ expertise (eg, driving risk, cognition, and psychiatric symptoms).
VA does have channels for filling the gap in geriatric health care. Established in 1975, Geriatric Research, Education, and Clinical Centers (GRECCs), are the department’s centers of excellence focused on aging. Currently, there are 20 GRECCs across the country, each connected with a major research university. Studies focus on aging, for example, examining the effects of Alzheimer’s disease or traumatic brain injuries.
Geriatric Scholars
To specifically fill the gap in mental health care, the Geriatric Scholars Program (GSP) was developed in 2008. Initially focused on primary care physicians, nurse practitioners, physician assistants, and pharmacists, the program later expanded to include other disciplines, including psychiatrists. In 2013, the GSP–Psychology Track (GSP-P) was developed because there were no commercially available training in geropsychology for licensed psychologists. GSP-P is based on an evidence-based educational model for the VA primary care workforce and includes a stepwise curriculum design, pilot implementation, and program evaluation.
A recent survey that assessed the track’s effectiveness found respondents “strongly agreed” that participation in the program improved their geropsychology knowledge and skills. That positive reaction led to shifts in practice that had a positive impact on VA organizational goals. Several GSP-P graduates have become board certified in geropsychology and many proceed to supervise geropsychology-focused clinical rotations for psychology practicum students, predoctoral interns, and postdoctoral fellows.
Whether programs such as GSP-P can adequately address the dwindling number of VA mental health care professionals remains to be seen. More than 160 doctors, psychologists, nurses, and researchers sent a letter to VA Secretary Doug Collins, the VA inspector general, and congressional leaders on Sept. 24 warning that workforce reductions and moves to outsource care will harm veterans.
“We have witnessed these ongoing harms and can provide evidence and testimony of their impacts,” the letter read. By the next day, the number of signees had increased to 350.
Though these shortages may impact their mental health care, older veterans could have an edge in mental resilience. While research in younger adults has found positive linear associations between physical health difficulties and severity of psychiatric symptoms, older veterans may benefit from what researchers have called an “aging paradox,” in which mental health improves later in life despite declining physical and cognitive function. A 2021 study suggests that prevention and treatment strategies designed to foster attachment security, mindfulness, and purpose in life may help enhance psychological resilience to physical health difficulties in older veterans.
This article has been updated with a response from the US Department of Veterans Affairs.
The number of US Department of Veterans Affairs (VA) geriatric mental health professionals is failing to keep pace with a growing population of older veterans: nearly 8 million are aged ≥ 65 years. VA psychologists may treat older veterans in primary care settings or community living centers, but many lack formal training in geropsychology.
Some psychologists with the proper training to treat this population are leaving the workforce; a survey by the VA Office of Inspector General found psychology was the most frequently reported severe clinical occupational staffing shortage and the most frequently reported Hybrid Title 38 severe shortage occupation, with 57% of 139 facilities reporting it as a shortage. According to the September Workforce Dashboard, the VA has lost > 200 psychologists in 2025.
Veterans aged ≥ 65 years have higher rates of combined medical and mental health diagnoses than younger veterans and older nonveterans. Nearly 1 of 5 older veterans enrolled in US Department of Veterans Affairs (VA) health care services have confirmed mental health diagnoses, and another 26% have documented mental health concerns without a formal diagnosis in their health record.
Older veterans also tend to have more complex mental health issues than younger adults. Posttraumatic stress nearly doubles their risk of dementia, and their psychiatric diagnoses may be complicated by co-occurring delirium, social isolation/loneliness, and polypharmacy.
According to reporting by The War Horse, the VA has been instituting limits on one-on-one mental health therapy and transitioning veterans to lower levels of treatment after having been told to stop treating them for long, indeterminate periods prior to referring them to group therapy, primary care, or discharging them altogether. In a statement to Federal Practitioner, VA Press Secretary Pete Kasperowicz refuted the reporting from The War Horse.
"The War Horse story is false. VA does not put caps on one-on-one mental health sessions for veterans with clinical care needs," he told Federal Practitioner. "VA works with veterans over an initial eight to 15 mental health sessions, and collaboratively plans any needed follow-on care. As part of this process, veterans and their health care team decide together how to address ongoing needs, including whether to step down to other types of care and self-maintenance, or continue with VA therapy."
The smaller pool of qualified mental health practitioners also may be due to medical students not knowing enough about the category. A study of 136 medical students and 61 internal medicine residents at an academic health center evaluated their beliefs and attitudes regarding 25 content areas essential to the primary care of older adults. Students and residents expressed similar beliefs about the importance of content areas, and attitudes toward aging did not appreciably differ. However, students rated lower in knowledge in areas surrounding general primary care, such as chronic conditions and medications. Residents reported larger gap scores in areas that reflected specialists’ expertise (eg, driving risk, cognition, and psychiatric symptoms).
VA does have channels for filling the gap in geriatric health care. Established in 1975, Geriatric Research, Education, and Clinical Centers (GRECCs), are the department’s centers of excellence focused on aging. Currently, there are 20 GRECCs across the country, each connected with a major research university. Studies focus on aging, for example, examining the effects of Alzheimer’s disease or traumatic brain injuries.
Geriatric Scholars
To specifically fill the gap in mental health care, the Geriatric Scholars Program (GSP) was developed in 2008. Initially focused on primary care physicians, nurse practitioners, physician assistants, and pharmacists, the program later expanded to include other disciplines, including psychiatrists. In 2013, the GSP–Psychology Track (GSP-P) was developed because there were no commercially available training in geropsychology for licensed psychologists. GSP-P is based on an evidence-based educational model for the VA primary care workforce and includes a stepwise curriculum design, pilot implementation, and program evaluation.
A recent survey that assessed the track’s effectiveness found respondents “strongly agreed” that participation in the program improved their geropsychology knowledge and skills. That positive reaction led to shifts in practice that had a positive impact on VA organizational goals. Several GSP-P graduates have become board certified in geropsychology and many proceed to supervise geropsychology-focused clinical rotations for psychology practicum students, predoctoral interns, and postdoctoral fellows.
Whether programs such as GSP-P can adequately address the dwindling number of VA mental health care professionals remains to be seen. More than 160 doctors, psychologists, nurses, and researchers sent a letter to VA Secretary Doug Collins, the VA inspector general, and congressional leaders on Sept. 24 warning that workforce reductions and moves to outsource care will harm veterans.
“We have witnessed these ongoing harms and can provide evidence and testimony of their impacts,” the letter read. By the next day, the number of signees had increased to 350.
Though these shortages may impact their mental health care, older veterans could have an edge in mental resilience. While research in younger adults has found positive linear associations between physical health difficulties and severity of psychiatric symptoms, older veterans may benefit from what researchers have called an “aging paradox,” in which mental health improves later in life despite declining physical and cognitive function. A 2021 study suggests that prevention and treatment strategies designed to foster attachment security, mindfulness, and purpose in life may help enhance psychological resilience to physical health difficulties in older veterans.
This article has been updated with a response from the US Department of Veterans Affairs.
The number of US Department of Veterans Affairs (VA) geriatric mental health professionals is failing to keep pace with a growing population of older veterans: nearly 8 million are aged ≥ 65 years. VA psychologists may treat older veterans in primary care settings or community living centers, but many lack formal training in geropsychology.
Some psychologists with the proper training to treat this population are leaving the workforce; a survey by the VA Office of Inspector General found psychology was the most frequently reported severe clinical occupational staffing shortage and the most frequently reported Hybrid Title 38 severe shortage occupation, with 57% of 139 facilities reporting it as a shortage. According to the September Workforce Dashboard, the VA has lost > 200 psychologists in 2025.
Veterans aged ≥ 65 years have higher rates of combined medical and mental health diagnoses than younger veterans and older nonveterans. Nearly 1 of 5 older veterans enrolled in US Department of Veterans Affairs (VA) health care services have confirmed mental health diagnoses, and another 26% have documented mental health concerns without a formal diagnosis in their health record.
Older veterans also tend to have more complex mental health issues than younger adults. Posttraumatic stress nearly doubles their risk of dementia, and their psychiatric diagnoses may be complicated by co-occurring delirium, social isolation/loneliness, and polypharmacy.
According to reporting by The War Horse, the VA has been instituting limits on one-on-one mental health therapy and transitioning veterans to lower levels of treatment after having been told to stop treating them for long, indeterminate periods prior to referring them to group therapy, primary care, or discharging them altogether. In a statement to Federal Practitioner, VA Press Secretary Pete Kasperowicz refuted the reporting from The War Horse.
"The War Horse story is false. VA does not put caps on one-on-one mental health sessions for veterans with clinical care needs," he told Federal Practitioner. "VA works with veterans over an initial eight to 15 mental health sessions, and collaboratively plans any needed follow-on care. As part of this process, veterans and their health care team decide together how to address ongoing needs, including whether to step down to other types of care and self-maintenance, or continue with VA therapy."
The smaller pool of qualified mental health practitioners also may be due to medical students not knowing enough about the category. A study of 136 medical students and 61 internal medicine residents at an academic health center evaluated their beliefs and attitudes regarding 25 content areas essential to the primary care of older adults. Students and residents expressed similar beliefs about the importance of content areas, and attitudes toward aging did not appreciably differ. However, students rated lower in knowledge in areas surrounding general primary care, such as chronic conditions and medications. Residents reported larger gap scores in areas that reflected specialists’ expertise (eg, driving risk, cognition, and psychiatric symptoms).
VA does have channels for filling the gap in geriatric health care. Established in 1975, Geriatric Research, Education, and Clinical Centers (GRECCs), are the department’s centers of excellence focused on aging. Currently, there are 20 GRECCs across the country, each connected with a major research university. Studies focus on aging, for example, examining the effects of Alzheimer’s disease or traumatic brain injuries.
Geriatric Scholars
To specifically fill the gap in mental health care, the Geriatric Scholars Program (GSP) was developed in 2008. Initially focused on primary care physicians, nurse practitioners, physician assistants, and pharmacists, the program later expanded to include other disciplines, including psychiatrists. In 2013, the GSP–Psychology Track (GSP-P) was developed because there were no commercially available training in geropsychology for licensed psychologists. GSP-P is based on an evidence-based educational model for the VA primary care workforce and includes a stepwise curriculum design, pilot implementation, and program evaluation.
A recent survey that assessed the track’s effectiveness found respondents “strongly agreed” that participation in the program improved their geropsychology knowledge and skills. That positive reaction led to shifts in practice that had a positive impact on VA organizational goals. Several GSP-P graduates have become board certified in geropsychology and many proceed to supervise geropsychology-focused clinical rotations for psychology practicum students, predoctoral interns, and postdoctoral fellows.
Whether programs such as GSP-P can adequately address the dwindling number of VA mental health care professionals remains to be seen. More than 160 doctors, psychologists, nurses, and researchers sent a letter to VA Secretary Doug Collins, the VA inspector general, and congressional leaders on Sept. 24 warning that workforce reductions and moves to outsource care will harm veterans.
“We have witnessed these ongoing harms and can provide evidence and testimony of their impacts,” the letter read. By the next day, the number of signees had increased to 350.
Though these shortages may impact their mental health care, older veterans could have an edge in mental resilience. While research in younger adults has found positive linear associations between physical health difficulties and severity of psychiatric symptoms, older veterans may benefit from what researchers have called an “aging paradox,” in which mental health improves later in life despite declining physical and cognitive function. A 2021 study suggests that prevention and treatment strategies designed to foster attachment security, mindfulness, and purpose in life may help enhance psychological resilience to physical health difficulties in older veterans.