User login
Confronting Uncertainty and Addressing Urgency for Action Through the Establishment of a VA Long COVID Practice-Based Research Network
Confronting Uncertainty and Addressing Urgency for Action Through the Establishment of a VA Long COVID Practice-Based Research Network
Learning health systems (LHS) promote a continuous process that can assist in making sense of uncertainty when confronting emerging complex conditions such as Long COVID. Long COVID is an infection-associated chronic condition that detrimentally impacts veterans, their families, and the communities in which they live. This complex condition is defined by ongoing, new, or returning symptoms following COVID-19 infection that negatively affect return to meaningful participation in social, recreational, and vocational activities.1,2 The clinical uncertainty surrounding Long COVID is amplified by unclear etiology, prognosis, and expected course of symptoms.3,4 Uncertainty surrounding best clinical practices, processes, and policies for Long COVID care has resulted in practice variation despite the emerging evidence base for Long COVID care.4 Failure to address gaps in clinical evidence and care implementation threatens to perpetuate fragmented and unnecessary care.
The context surrounding Long COVID created an urgency to rapidly address clinically relevant questions and make sense of any uncertainty. Thus, the Veterans Health Administration (VHA) funded a Long COVID Practice-Based Research Network (LC-PBRN) to build an infrastructure that supports Long COVID research nationally and promotes interdisciplinary collaboration. The LC-PBRN vision is to centralize Long COVID clinical, research, and operational activities. The research infrastructure of the LC-PBRN is designed with an LHS lens to facilitate feedback loops and integrate knowledge learned while making progress towards this vision.5 This article describes the phases of infrastructure development and network building, as well as associated lessons learned.
Designing the LC-PBRN Infrastructure
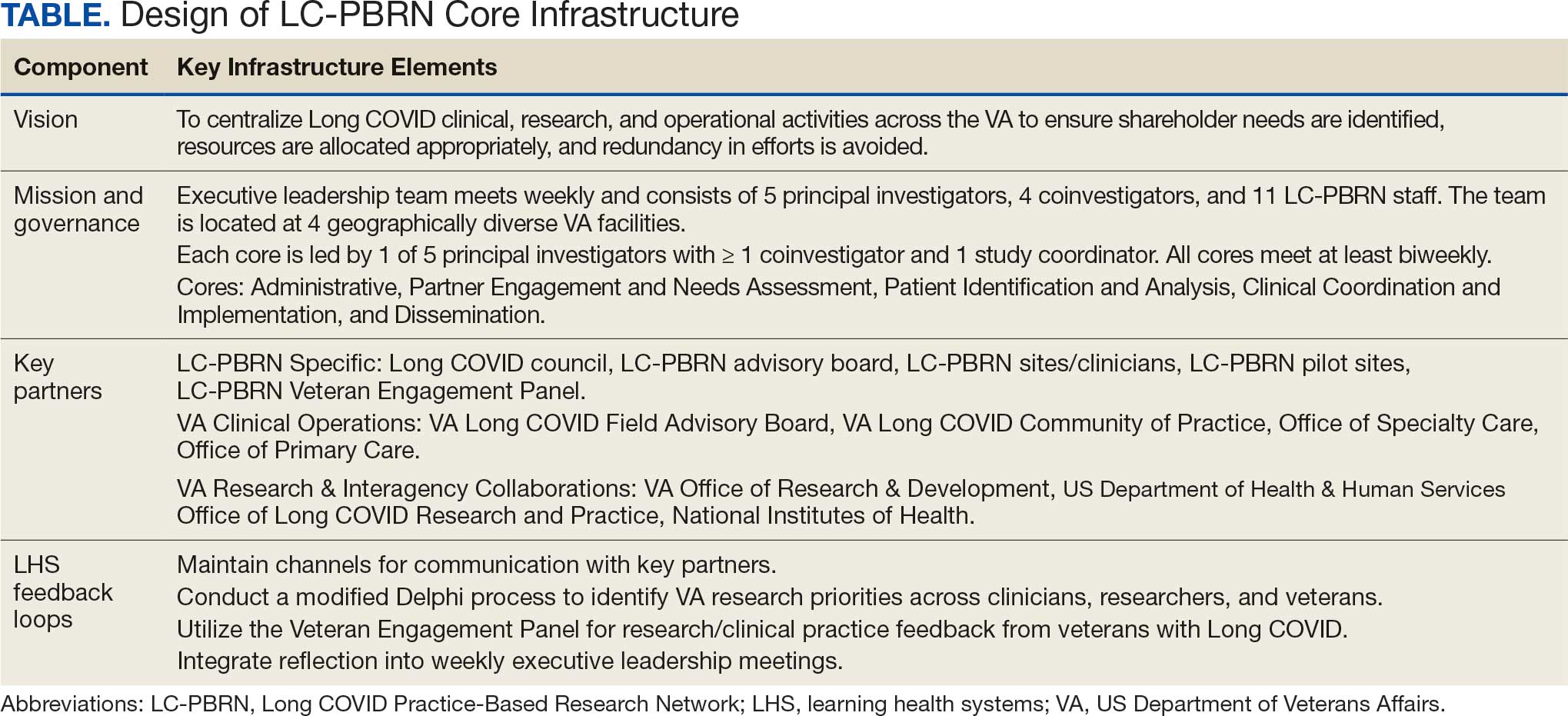
Vision
The LC-PBRN’s vision is to create an infrastructure that integrates an LHS framework by unifying the VA research approach to Long COVID to ensure veteran, clinician, operational, and researcher involvement (Figure 1).
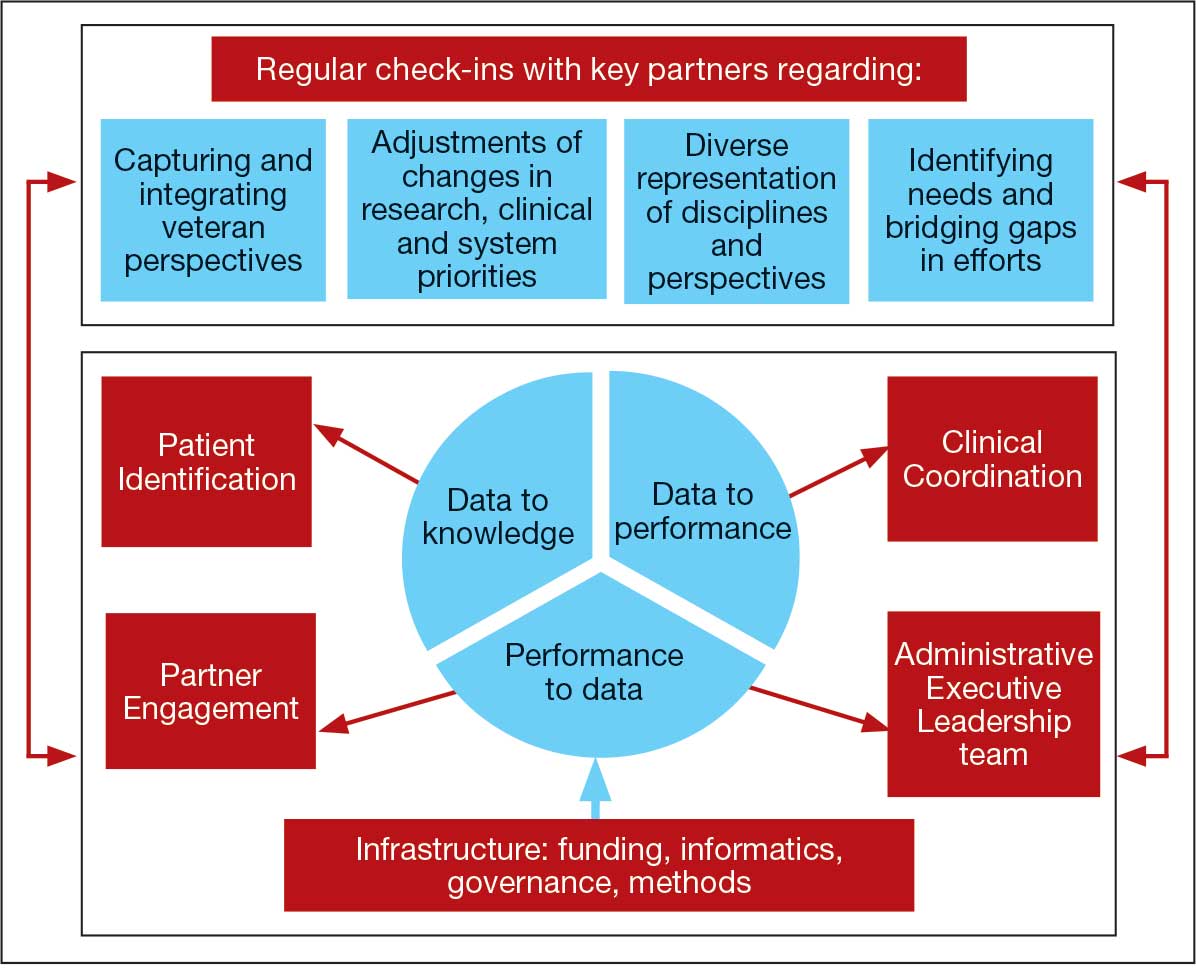
Mission and Governance
The LC-PBRN operates with an executive leadership team and 5 cores. The executive leadership team is responsible for overall LC-PBRN operations, management, and direction setting of the LC-PBRN. The executive leadership team meets weekly to provide oversight of each core, which specializes in different aspects. The cores include: Administrative, Partner Engagement and Needs Assessment, Patient Identification and Analysis, Clinical Coordination and Implementation, and Dissemination (Figure 2).
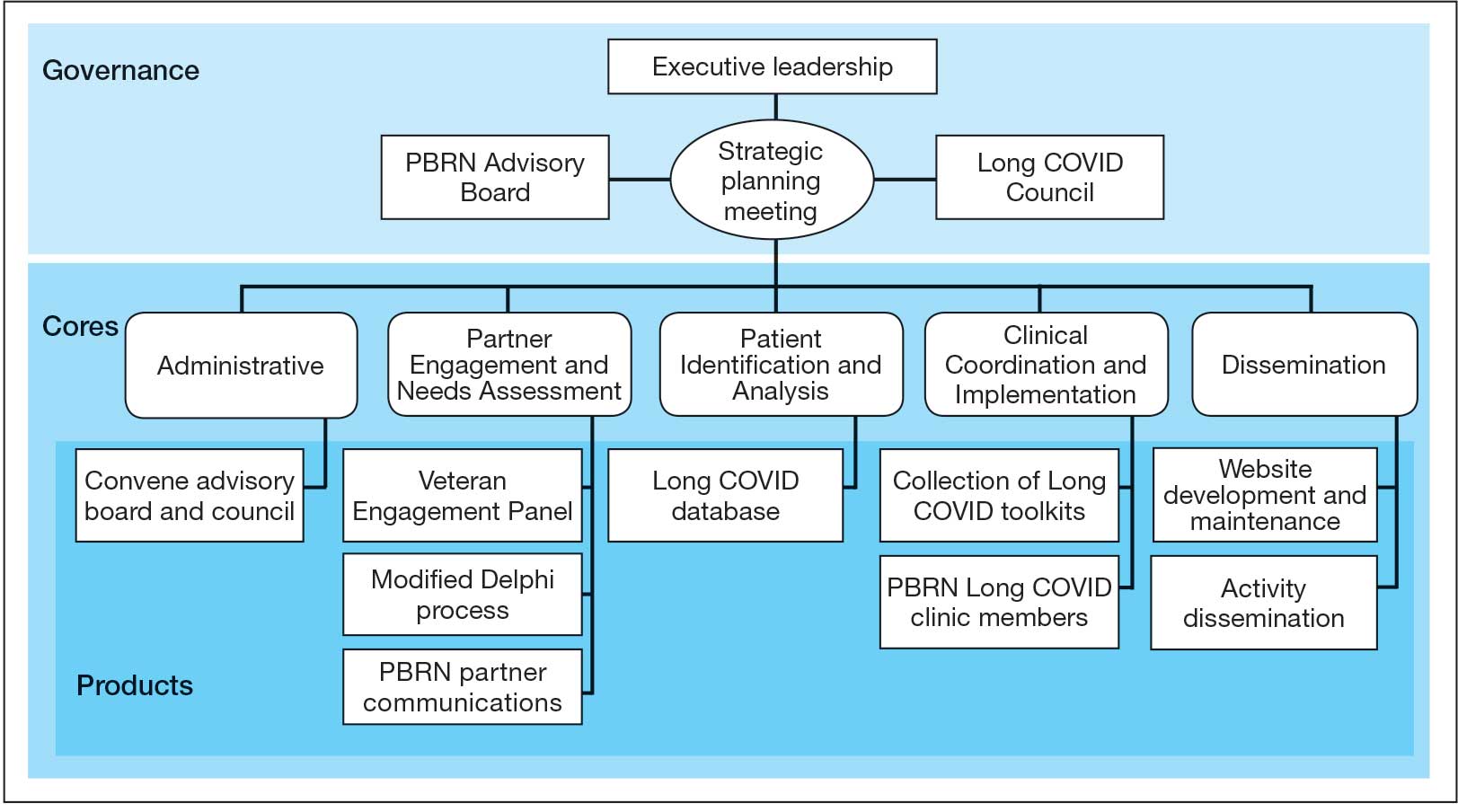
The Administrative core focuses on interagency collaboration to identify and network with key operational and agency leaders to allow for ongoing exploration of funding strategies for Long COVID research. The Administrative core manages 3 teams: an advisory board, Long COVID council, and the strategic planning team. The advisory board meets biannually to oversee achievement of LC-PBRN goals, deliverables, and tactics for meeting these goals. The advisory board includes the LC-PBRN executive leadership team and 13 interagency members from various shareholders (eg, Centers for Disease Control and Prevention, National Institutes of Health, and specialty departments within the VA).
The Long COVID council convenes quarterly to provide scientific input on important overarching issues in Long COVID research, practice, and policy. The council consists of 22 scientific representatives in VA and non-VA contexts, university affiliates, and veteran representatives. The strategic planning team convenes annually to identify how the LC-PBRN and its partners can meet the needs of the broader Long COVID ecosystem and conduct a strengths, opportunities, weaknesses, and threats analysis to identify strategic objectives and expected outcomes. The strategic planning team includes the executive leadership team and key Long COVID shareholders within VHA and affiliated partners. The Partner Engagement and Needs Assessment core aims to solicit feedback from veterans, clinicians, researchers, and operational leadership. Input is gathered through a Veteran Engagement Panel and a modified Delphi consensus process. The panel was formed using a Community Engagement Studio model to engage veterans as consultants on research.7 Currently, 10 members represent a range of ages, genders, racial and ethnic backgrounds, and military experience. All veterans have a history of Long COVID and are paid as consultants. Video conference panel meetings occur quarterly for 1 to 2 hours; the meeting length is shorter than typical engagement studios to accommodate for fatigue-related symptoms that may limit attention and ability to participate in longer meetings. Before each panel, the Partner Engagement and Needs Assessment core helps identify key questions and creates a structured agenda. Each panel begins with a presentation of a research study followed by a group discussion led by a trained facilitator. The modified Delphi consensus process focuses on identifying research priority areas for Long COVID within the VA. Veterans living with Long COVID, as well as clinicians and researchers who work closely with patients who have Long COVID, complete a series of progressive surveys to provide input on research priorities.
The Partner Engagement and Needs Assessment core also actively provides outreach to important partners in research, clinical care, and operational leadership to facilitate introductory meetings to (1) ask partners to describe their 5 largest pain points, (2) find pain points within the scope of LC-PBRN resources, and (3) discuss the strengths and capacity of the PBRN. During introductory meetings, communications preferences and a cadence for subsequent meetings are established. Subsequent engagement meetings aim to provide updates and codevelop solutions to emerging issues. This core maintains a living document to track engagement efforts, points of contact for identified and emerging partners, and ensure all communication is timely.
The Patient Identification and Analysis core develops a database of veterans with confirmed or suspected Long COVID. The goal is for researchers to use the database to identify potential participants for clinical trials and monitor clinical care outcomes. When possible, this core works with existing VA data to facilitate research that aligns with the LC-PBRN mission. The core can also use natural language processing and machine learning to work with researchers conducting clinical trials to help identify patients who may meet eligibility criteria.
The Clinical Coordination and Implementation core gathers information on the best practices for identifying and recruiting veterans for Long COVID research as well as compiles strategies for standardized clinical assessments that can both facilitate ongoing research and the successful implementation of evidence-based care. The Clinical Coordination and Implementation core provides support to pilot and multisite trials in 3 ways. First, it develops toolkits such as best practice strategies for recruiting participants for research, template examples of recruitment materials, and a library of patient-reported outcome measures, standardized clinical note titles and templates in use for Long COVID in the national electronic health record. Second, it partners with the Patient Identification and Analysis core to facilitate access to and use of algorithms that identify Long COVID cases based on electronic health records for recruitment. Finally, it compiles a detailed list of potential collaborating sites. The steps to facilitate patient identification and recruitment inform feasibility assessments and improve efficiency of launching pilot studies and multisite trials. The library of outcome measures, standardized clinical notes, and templates can aid and expedite data collection.
The Dissemination core focuses on developing a website, creating a dissemination plan, and actively disseminating products of the LC-PBRN and its partners. This core’s foundational framework is based on the Agency for Healthcare Research and Quality Quick-Start Guide to Dissemination for PBRNs.8,9 The core built an internal- and external-facing website to connect users with LC-PBRN products, potential outreach contacts, and promote timely updates on LC-PBRN activities. A manual of operating procedures will be drafted to include the development of training for practitioners involved in research projects to learn the processes involved in presenting clinical results for education and training initiatives, presentations, and manuscript preparation. A toolkit will also be developed to support dissemination activities designed to reach a variety of end-users, such as education materials, policy briefings, educational briefs, newsletters, and presentations at local, regional, and national levels.
Key Partners
Key partners exist specific to the LC-PBRN and within the broader VA ecosystem, including VA clinical operations, VA research, and intra-agency collaborations.
LC-PBRN Specific. In addition to the LC-PBRN council, advisory board, and Veteran Engagement Panel discussed earlier,
VA Clinical Operations. To support clinical operations, a Long COVID Field Advisory Board was formed through the VA Office of Specialty Care as an operational effort to develop clinical best practice. The LC-PBRN consults with this group on veteran engagement strategies for input on clinical guides and dissemination of practice guide materials. The LC-PBRN also partners with an existing Long COVID Community of Practice and the Office of Primary Care. The Community of Practice provides a learning space for VA staff interested in advancing Long COVID care and assists with disseminating LC-PBRN to the broader Long COVID clinical community. A member of the Office of Primary Care sits on the PBRN advisory board to provide input on engaging primary care practitioners and ensure their unique needs are considered in LC-PBRN initiatives.
VA Research & Interagency Collaborations. The LC-PBRN engages monthly with an interagency workgroup led by the US Department of Health and Human Services Office of Long COVID Research and Practice. These engagements support identification of research gaps that the VA may help address, monitor emerging funding opportunities, and foster collaborations. LC-PBRN representatives also meet with staff at the National Institutes of Health Researching COVID to Enhance Recovery initiative to identify pathways for veteran recruitment.
LHS Feedback Loops
The LC-PBRN was designed with an LHS approach in mind.10 Throughout development of the LC-PBRN, consideration was given to (1) capture data on new efforts within the Long COVID ecosystem (performance to data), (2) examine performance gaps and identify approaches for best practice (data to knowledge), and (3) implement best practices, develop toolkits, disseminate findings, and measure impacts (knowledge to performance). With this approach, the LC-PBRN is constantly evolving based on new information coming from the internal and external Long COVID ecosystem. Each element was deliberatively considered in relation to how data can be transformed into knowledge, knowledge into performance, and performance into data.
First, an important mechanism for feedback involves establishing clear channels of communication. Regular check-ins with key partners occur through virtual meetings to provide updates, assess needs and challenges, and codevelop action plans. For example, during a check-in with the Long COVID Field Advisory Board, members expressed a desire to incorporate veteran feedback into VA clinical practice recommendations. We provided expertise on different engagement modalities (eg, focus groups vs individual interviews), and collaboration occurred to identify key interview questions for veterans. This process resulted in a published clinician-facing Long COVID Nervous System Clinical Guide (available at longcovid@hhs.gov) that integrated critical feedback from veterans related to neurological symptoms.
Second, weekly executive leadership meetings include dedicated time for reflection on partner feedback, the current state of Long COVID, and contextual changes that impact deliverable priorities and timelines. Outcomes from these discussions are communicated with VHA Health Services Research and, when appropriate, to key partners to ensure alignment. For example, the Patient Identification and Analysis core was originally tasked with identifying a definition of Long COVID. However, as the broader community moved away from a singular definition, efforts were redirected toward higher-priority issues within the VA Long COVID ecosystem, including veteran enrollment in clinical trials.
Third, the Veteran Engagement Panel captures feedback from those with lived experience to inform Long COVID research and clinical efforts. The panel meetings are strategically designed to ask veterans living with Long COVID specific questions related to a given research or clinical topic of interest. For example, panel sessions with the Field Advisory Board focused on concerns articulated by veterans related to the mental health and gastroenterological symptoms associated with Long COVID. Insights from these discussions will inform development of Long COVID mental health and gastroenterological clinical care guides, with several PBRN investigators serving as subject matter experts. This collaborative approach ensures that veteran perspectives are represented in developing Long COVID clinical care processes.
Fourth, research priorities identified through the Delphi consensus process will inform development of VA Request for Funding Proposals related to Long COVID. The initial survey was developed in collaboration with veterans, clinicians, and researchers across the Veteran Engagement Panel, the Field Advisory Board, and the National Research Action Plan on Long COVID.11 The process was launched in October 2024 and concluded in June 2025. The team conducted 3 consensus rounds with veterans and VA clinicians and researchers. Top priority areas included the testing assessments for diagnosing Long COVID, studying subtypes of Long COVID and treatments for each, and finding biomarkers for Long COVID. A formal publication of the results and analysis is the focus of a future publication.
Fifth, ongoing engagement with the Field Advisory Board has supported adoption of a preliminary set of clinical outcome measures. If universally adopted, these instruments may contribute to the development of a standardized data collection process and serve as common data elements collected for epidemiologic, health services, or clinical trial research.
Lessons Learned and Practice Implications
Throughout the development of the LC-PBRN, several decisions were identified that have impacted infrastructure development and implementation.
Include veterans’ voices to ensure network efforts align with patient needs. Given the novelty of Long COVID, practitioners and researchers are learning as they go. It is important to listen to individuals who live with Long COVID. Throughout the development of the LC-PBRN, veteran perspective has proven how vital it is for them to be heard when it comes to their health care. Clinicians similarly highlighted the value of incorporating patient perspectives into the development of tools and treatment strategies. Develop an interdisciplinary leadership team to foster the diverse viewpoints needed to tackle multifaceted problems. It is important to consider as many clinical and research perspectives as possible because Long COVID is a complex condition with symptoms impacting major organ systems.12-15 Therefore, the team spans across a multitude of specialties and locations.
Set clear expectations and goals with partners to uphold timely deliverables and stay within the PBRN’s capacity. When including a multitude of partners, teams should consider each of those partners’ experiences and opinions in decision-making conversations. Expectation setting is important to ensure all partners are on the same page and understand the capacity of the LC-PBRN. This allows the team to focus its efforts, avoid being overwhelmed with requests, and provide quality deliverables.
Build engaging relationships to bridge gaps between internal and external partners. A substantial number of resources focus on building relationships with partners so they can trust the LC-PBRN has their best interests in mind. These relationships are important to ensure the VA avoids duplicate efforts. This includes prioritizing connecting partners who are working on similar efforts to promote collaboration across facilities.
Conclusions
PBRNs provide an important mechanism to use LHS approaches to successfully convene research around complex issues. PBRNs can support integration across the LHS cycle, allowing for multiple feedback loops, and coordinate activities that work to achieve a larger vision. PBRNs offer centralized mechanisms to collaboratively understand and address complex problems, such as Long COVID, where the uncertainty regarding how to treat occurs in tandem with the urgency to treat. The LC-PBRN model described in this article has the potential to transcend Long COVID by building infrastructure necessary to proactively address current or future clinical conditions or populations with a LHS lens. The infrastructure can require cross-system and sector collaborations, expediency, inclusivity, and patient- and family-centeredness. Future efforts will focus on building out a larger network of VHA sites, facilitating recruitment at site and veteran levels into Long COVID trials through case identification, and systematically support the standardization of clinical data for clinical utility and evaluation of quality and/or outcomes across the VHA.
- Ottiger M, Poppele I, Sperling N, et al. Work ability and return-to-work of patients with post-COVID-19: a systematic review and meta-analysis. BMC Public Health. 2024;24:1811. doi:10.1186/s12889-024-19328-6
- Ziauddeen N, Gurdasani D, O’Hara ME, et al. Characteristics and impact of Long Covid: findings from an online survey. PLOS ONE. 2022;17:e0264331. doi:10.1371/journal.pone.0264331
- Graham F. Daily briefing: Answers emerge about long COVID recovery. Nature. Published online June 28, 2023. doi:10.1038/d41586-023-02190-8
- Al-Aly Z, Davis H, McCorkell L, et al. Long COVID science, research and policy. Nat Med. 2024;30:2148-2164. doi:10.1038/s41591-024-03173-6
- Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
- Ely EW, Brown LM, Fineberg HV. Long covid defined. N Engl J Med. 2024;391:1746-1753.doi:10.1056/NEJMsb2408466
- Joosten YA, Israel TL, Williams NA, et al. Community engagement studios: a structured approach to obtaining meaningful input from stakeholders to inform research. Acad Med. 2015;90:1646-1650. doi:10.1097/ACM.0000000000000794
- AHRQ. Quick-start guide to dissemination for practice-based research networks. Revised June 2014. Accessed December 2, 2025. https://www.ahrq.gov/sites/default/files/wysiwyg/ncepcr/resources/dissemination-quick-start-guide.pdf
- Gustavson AM, Morrow CD, Brown RJ, et al. Reimagining how we synthesize information to impact clinical care, policy, and research priorities in real time: examples and lessons learned from COVID-19. J Gen Intern Med. 2024;39:2554-2559. doi:10.1007/s11606-024-08855-y
- University of Minnesota. About the Center for Learning Health System Sciences. Updated December 11, 2025. Accessed December 12, 2025. https://med.umn.edu/clhss/about-us
- AHRQ. National Research Action Plan. Published online 2022. Accessed February 14, 2024. https://www.covid.gov/sites/default/files/documents/National-Research-Action-Plan-on-Long-COVID-08012022.pdf
- Gustavson AM, Eaton TL, Schapira RM, et al. Approaches to long COVID care: the Veterans Health Administration experience in 2021. BMJ Mil Health. 2024;170:179-180. doi:10.1136/military-2022-002185
- Gustavson AM. A learning health system approach to long COVID care. Fed Pract. 2022;39:7. doi:10.12788/fp.0288
- Palacio A, Bast E, Klimas N, et al. Lessons learned in implementing a multidisciplinary long COVID clinic. Am J Med. 2025;138:843-849.doi:10.1016/j.amjmed.2024.05.020
- Prusinski C, Yan D, Klasova J, et al. Multidisciplinary management strategies for long COVID: a narrative review. Cureus. 2024;16:e59478. doi:10.7759/cureus.59478
Learning health systems (LHS) promote a continuous process that can assist in making sense of uncertainty when confronting emerging complex conditions such as Long COVID. Long COVID is an infection-associated chronic condition that detrimentally impacts veterans, their families, and the communities in which they live. This complex condition is defined by ongoing, new, or returning symptoms following COVID-19 infection that negatively affect return to meaningful participation in social, recreational, and vocational activities.1,2 The clinical uncertainty surrounding Long COVID is amplified by unclear etiology, prognosis, and expected course of symptoms.3,4 Uncertainty surrounding best clinical practices, processes, and policies for Long COVID care has resulted in practice variation despite the emerging evidence base for Long COVID care.4 Failure to address gaps in clinical evidence and care implementation threatens to perpetuate fragmented and unnecessary care.
The context surrounding Long COVID created an urgency to rapidly address clinically relevant questions and make sense of any uncertainty. Thus, the Veterans Health Administration (VHA) funded a Long COVID Practice-Based Research Network (LC-PBRN) to build an infrastructure that supports Long COVID research nationally and promotes interdisciplinary collaboration. The LC-PBRN vision is to centralize Long COVID clinical, research, and operational activities. The research infrastructure of the LC-PBRN is designed with an LHS lens to facilitate feedback loops and integrate knowledge learned while making progress towards this vision.5 This article describes the phases of infrastructure development and network building, as well as associated lessons learned.
Designing the LC-PBRN Infrastructure

Vision
The LC-PBRN’s vision is to create an infrastructure that integrates an LHS framework by unifying the VA research approach to Long COVID to ensure veteran, clinician, operational, and researcher involvement (Figure 1).

Mission and Governance
The LC-PBRN operates with an executive leadership team and 5 cores. The executive leadership team is responsible for overall LC-PBRN operations, management, and direction setting of the LC-PBRN. The executive leadership team meets weekly to provide oversight of each core, which specializes in different aspects. The cores include: Administrative, Partner Engagement and Needs Assessment, Patient Identification and Analysis, Clinical Coordination and Implementation, and Dissemination (Figure 2).

The Administrative core focuses on interagency collaboration to identify and network with key operational and agency leaders to allow for ongoing exploration of funding strategies for Long COVID research. The Administrative core manages 3 teams: an advisory board, Long COVID council, and the strategic planning team. The advisory board meets biannually to oversee achievement of LC-PBRN goals, deliverables, and tactics for meeting these goals. The advisory board includes the LC-PBRN executive leadership team and 13 interagency members from various shareholders (eg, Centers for Disease Control and Prevention, National Institutes of Health, and specialty departments within the VA).
The Long COVID council convenes quarterly to provide scientific input on important overarching issues in Long COVID research, practice, and policy. The council consists of 22 scientific representatives in VA and non-VA contexts, university affiliates, and veteran representatives. The strategic planning team convenes annually to identify how the LC-PBRN and its partners can meet the needs of the broader Long COVID ecosystem and conduct a strengths, opportunities, weaknesses, and threats analysis to identify strategic objectives and expected outcomes. The strategic planning team includes the executive leadership team and key Long COVID shareholders within VHA and affiliated partners. The Partner Engagement and Needs Assessment core aims to solicit feedback from veterans, clinicians, researchers, and operational leadership. Input is gathered through a Veteran Engagement Panel and a modified Delphi consensus process. The panel was formed using a Community Engagement Studio model to engage veterans as consultants on research.7 Currently, 10 members represent a range of ages, genders, racial and ethnic backgrounds, and military experience. All veterans have a history of Long COVID and are paid as consultants. Video conference panel meetings occur quarterly for 1 to 2 hours; the meeting length is shorter than typical engagement studios to accommodate for fatigue-related symptoms that may limit attention and ability to participate in longer meetings. Before each panel, the Partner Engagement and Needs Assessment core helps identify key questions and creates a structured agenda. Each panel begins with a presentation of a research study followed by a group discussion led by a trained facilitator. The modified Delphi consensus process focuses on identifying research priority areas for Long COVID within the VA. Veterans living with Long COVID, as well as clinicians and researchers who work closely with patients who have Long COVID, complete a series of progressive surveys to provide input on research priorities.
The Partner Engagement and Needs Assessment core also actively provides outreach to important partners in research, clinical care, and operational leadership to facilitate introductory meetings to (1) ask partners to describe their 5 largest pain points, (2) find pain points within the scope of LC-PBRN resources, and (3) discuss the strengths and capacity of the PBRN. During introductory meetings, communications preferences and a cadence for subsequent meetings are established. Subsequent engagement meetings aim to provide updates and codevelop solutions to emerging issues. This core maintains a living document to track engagement efforts, points of contact for identified and emerging partners, and ensure all communication is timely.
The Patient Identification and Analysis core develops a database of veterans with confirmed or suspected Long COVID. The goal is for researchers to use the database to identify potential participants for clinical trials and monitor clinical care outcomes. When possible, this core works with existing VA data to facilitate research that aligns with the LC-PBRN mission. The core can also use natural language processing and machine learning to work with researchers conducting clinical trials to help identify patients who may meet eligibility criteria.
The Clinical Coordination and Implementation core gathers information on the best practices for identifying and recruiting veterans for Long COVID research as well as compiles strategies for standardized clinical assessments that can both facilitate ongoing research and the successful implementation of evidence-based care. The Clinical Coordination and Implementation core provides support to pilot and multisite trials in 3 ways. First, it develops toolkits such as best practice strategies for recruiting participants for research, template examples of recruitment materials, and a library of patient-reported outcome measures, standardized clinical note titles and templates in use for Long COVID in the national electronic health record. Second, it partners with the Patient Identification and Analysis core to facilitate access to and use of algorithms that identify Long COVID cases based on electronic health records for recruitment. Finally, it compiles a detailed list of potential collaborating sites. The steps to facilitate patient identification and recruitment inform feasibility assessments and improve efficiency of launching pilot studies and multisite trials. The library of outcome measures, standardized clinical notes, and templates can aid and expedite data collection.
The Dissemination core focuses on developing a website, creating a dissemination plan, and actively disseminating products of the LC-PBRN and its partners. This core’s foundational framework is based on the Agency for Healthcare Research and Quality Quick-Start Guide to Dissemination for PBRNs.8,9 The core built an internal- and external-facing website to connect users with LC-PBRN products, potential outreach contacts, and promote timely updates on LC-PBRN activities. A manual of operating procedures will be drafted to include the development of training for practitioners involved in research projects to learn the processes involved in presenting clinical results for education and training initiatives, presentations, and manuscript preparation. A toolkit will also be developed to support dissemination activities designed to reach a variety of end-users, such as education materials, policy briefings, educational briefs, newsletters, and presentations at local, regional, and national levels.
Key Partners
Key partners exist specific to the LC-PBRN and within the broader VA ecosystem, including VA clinical operations, VA research, and intra-agency collaborations.
LC-PBRN Specific. In addition to the LC-PBRN council, advisory board, and Veteran Engagement Panel discussed earlier,
VA Clinical Operations. To support clinical operations, a Long COVID Field Advisory Board was formed through the VA Office of Specialty Care as an operational effort to develop clinical best practice. The LC-PBRN consults with this group on veteran engagement strategies for input on clinical guides and dissemination of practice guide materials. The LC-PBRN also partners with an existing Long COVID Community of Practice and the Office of Primary Care. The Community of Practice provides a learning space for VA staff interested in advancing Long COVID care and assists with disseminating LC-PBRN to the broader Long COVID clinical community. A member of the Office of Primary Care sits on the PBRN advisory board to provide input on engaging primary care practitioners and ensure their unique needs are considered in LC-PBRN initiatives.
VA Research & Interagency Collaborations. The LC-PBRN engages monthly with an interagency workgroup led by the US Department of Health and Human Services Office of Long COVID Research and Practice. These engagements support identification of research gaps that the VA may help address, monitor emerging funding opportunities, and foster collaborations. LC-PBRN representatives also meet with staff at the National Institutes of Health Researching COVID to Enhance Recovery initiative to identify pathways for veteran recruitment.
LHS Feedback Loops
The LC-PBRN was designed with an LHS approach in mind.10 Throughout development of the LC-PBRN, consideration was given to (1) capture data on new efforts within the Long COVID ecosystem (performance to data), (2) examine performance gaps and identify approaches for best practice (data to knowledge), and (3) implement best practices, develop toolkits, disseminate findings, and measure impacts (knowledge to performance). With this approach, the LC-PBRN is constantly evolving based on new information coming from the internal and external Long COVID ecosystem. Each element was deliberatively considered in relation to how data can be transformed into knowledge, knowledge into performance, and performance into data.
First, an important mechanism for feedback involves establishing clear channels of communication. Regular check-ins with key partners occur through virtual meetings to provide updates, assess needs and challenges, and codevelop action plans. For example, during a check-in with the Long COVID Field Advisory Board, members expressed a desire to incorporate veteran feedback into VA clinical practice recommendations. We provided expertise on different engagement modalities (eg, focus groups vs individual interviews), and collaboration occurred to identify key interview questions for veterans. This process resulted in a published clinician-facing Long COVID Nervous System Clinical Guide (available at longcovid@hhs.gov) that integrated critical feedback from veterans related to neurological symptoms.
Second, weekly executive leadership meetings include dedicated time for reflection on partner feedback, the current state of Long COVID, and contextual changes that impact deliverable priorities and timelines. Outcomes from these discussions are communicated with VHA Health Services Research and, when appropriate, to key partners to ensure alignment. For example, the Patient Identification and Analysis core was originally tasked with identifying a definition of Long COVID. However, as the broader community moved away from a singular definition, efforts were redirected toward higher-priority issues within the VA Long COVID ecosystem, including veteran enrollment in clinical trials.
Third, the Veteran Engagement Panel captures feedback from those with lived experience to inform Long COVID research and clinical efforts. The panel meetings are strategically designed to ask veterans living with Long COVID specific questions related to a given research or clinical topic of interest. For example, panel sessions with the Field Advisory Board focused on concerns articulated by veterans related to the mental health and gastroenterological symptoms associated with Long COVID. Insights from these discussions will inform development of Long COVID mental health and gastroenterological clinical care guides, with several PBRN investigators serving as subject matter experts. This collaborative approach ensures that veteran perspectives are represented in developing Long COVID clinical care processes.
Fourth, research priorities identified through the Delphi consensus process will inform development of VA Request for Funding Proposals related to Long COVID. The initial survey was developed in collaboration with veterans, clinicians, and researchers across the Veteran Engagement Panel, the Field Advisory Board, and the National Research Action Plan on Long COVID.11 The process was launched in October 2024 and concluded in June 2025. The team conducted 3 consensus rounds with veterans and VA clinicians and researchers. Top priority areas included the testing assessments for diagnosing Long COVID, studying subtypes of Long COVID and treatments for each, and finding biomarkers for Long COVID. A formal publication of the results and analysis is the focus of a future publication.
Fifth, ongoing engagement with the Field Advisory Board has supported adoption of a preliminary set of clinical outcome measures. If universally adopted, these instruments may contribute to the development of a standardized data collection process and serve as common data elements collected for epidemiologic, health services, or clinical trial research.
Lessons Learned and Practice Implications
Throughout the development of the LC-PBRN, several decisions were identified that have impacted infrastructure development and implementation.
Include veterans’ voices to ensure network efforts align with patient needs. Given the novelty of Long COVID, practitioners and researchers are learning as they go. It is important to listen to individuals who live with Long COVID. Throughout the development of the LC-PBRN, veteran perspective has proven how vital it is for them to be heard when it comes to their health care. Clinicians similarly highlighted the value of incorporating patient perspectives into the development of tools and treatment strategies. Develop an interdisciplinary leadership team to foster the diverse viewpoints needed to tackle multifaceted problems. It is important to consider as many clinical and research perspectives as possible because Long COVID is a complex condition with symptoms impacting major organ systems.12-15 Therefore, the team spans across a multitude of specialties and locations.
Set clear expectations and goals with partners to uphold timely deliverables and stay within the PBRN’s capacity. When including a multitude of partners, teams should consider each of those partners’ experiences and opinions in decision-making conversations. Expectation setting is important to ensure all partners are on the same page and understand the capacity of the LC-PBRN. This allows the team to focus its efforts, avoid being overwhelmed with requests, and provide quality deliverables.
Build engaging relationships to bridge gaps between internal and external partners. A substantial number of resources focus on building relationships with partners so they can trust the LC-PBRN has their best interests in mind. These relationships are important to ensure the VA avoids duplicate efforts. This includes prioritizing connecting partners who are working on similar efforts to promote collaboration across facilities.
Conclusions
PBRNs provide an important mechanism to use LHS approaches to successfully convene research around complex issues. PBRNs can support integration across the LHS cycle, allowing for multiple feedback loops, and coordinate activities that work to achieve a larger vision. PBRNs offer centralized mechanisms to collaboratively understand and address complex problems, such as Long COVID, where the uncertainty regarding how to treat occurs in tandem with the urgency to treat. The LC-PBRN model described in this article has the potential to transcend Long COVID by building infrastructure necessary to proactively address current or future clinical conditions or populations with a LHS lens. The infrastructure can require cross-system and sector collaborations, expediency, inclusivity, and patient- and family-centeredness. Future efforts will focus on building out a larger network of VHA sites, facilitating recruitment at site and veteran levels into Long COVID trials through case identification, and systematically support the standardization of clinical data for clinical utility and evaluation of quality and/or outcomes across the VHA.

Learning health systems (LHS) promote a continuous process that can assist in making sense of uncertainty when confronting emerging complex conditions such as Long COVID. Long COVID is an infection-associated chronic condition that detrimentally impacts veterans, their families, and the communities in which they live. This complex condition is defined by ongoing, new, or returning symptoms following COVID-19 infection that negatively affect return to meaningful participation in social, recreational, and vocational activities.1,2 The clinical uncertainty surrounding Long COVID is amplified by unclear etiology, prognosis, and expected course of symptoms.3,4 Uncertainty surrounding best clinical practices, processes, and policies for Long COVID care has resulted in practice variation despite the emerging evidence base for Long COVID care.4 Failure to address gaps in clinical evidence and care implementation threatens to perpetuate fragmented and unnecessary care.
The context surrounding Long COVID created an urgency to rapidly address clinically relevant questions and make sense of any uncertainty. Thus, the Veterans Health Administration (VHA) funded a Long COVID Practice-Based Research Network (LC-PBRN) to build an infrastructure that supports Long COVID research nationally and promotes interdisciplinary collaboration. The LC-PBRN vision is to centralize Long COVID clinical, research, and operational activities. The research infrastructure of the LC-PBRN is designed with an LHS lens to facilitate feedback loops and integrate knowledge learned while making progress towards this vision.5 This article describes the phases of infrastructure development and network building, as well as associated lessons learned.
Designing the LC-PBRN Infrastructure

Vision
The LC-PBRN’s vision is to create an infrastructure that integrates an LHS framework by unifying the VA research approach to Long COVID to ensure veteran, clinician, operational, and researcher involvement (Figure 1).

Mission and Governance
The LC-PBRN operates with an executive leadership team and 5 cores. The executive leadership team is responsible for overall LC-PBRN operations, management, and direction setting of the LC-PBRN. The executive leadership team meets weekly to provide oversight of each core, which specializes in different aspects. The cores include: Administrative, Partner Engagement and Needs Assessment, Patient Identification and Analysis, Clinical Coordination and Implementation, and Dissemination (Figure 2).

The Administrative core focuses on interagency collaboration to identify and network with key operational and agency leaders to allow for ongoing exploration of funding strategies for Long COVID research. The Administrative core manages 3 teams: an advisory board, Long COVID council, and the strategic planning team. The advisory board meets biannually to oversee achievement of LC-PBRN goals, deliverables, and tactics for meeting these goals. The advisory board includes the LC-PBRN executive leadership team and 13 interagency members from various shareholders (eg, Centers for Disease Control and Prevention, National Institutes of Health, and specialty departments within the VA).
The Long COVID council convenes quarterly to provide scientific input on important overarching issues in Long COVID research, practice, and policy. The council consists of 22 scientific representatives in VA and non-VA contexts, university affiliates, and veteran representatives. The strategic planning team convenes annually to identify how the LC-PBRN and its partners can meet the needs of the broader Long COVID ecosystem and conduct a strengths, opportunities, weaknesses, and threats analysis to identify strategic objectives and expected outcomes. The strategic planning team includes the executive leadership team and key Long COVID shareholders within VHA and affiliated partners. The Partner Engagement and Needs Assessment core aims to solicit feedback from veterans, clinicians, researchers, and operational leadership. Input is gathered through a Veteran Engagement Panel and a modified Delphi consensus process. The panel was formed using a Community Engagement Studio model to engage veterans as consultants on research.7 Currently, 10 members represent a range of ages, genders, racial and ethnic backgrounds, and military experience. All veterans have a history of Long COVID and are paid as consultants. Video conference panel meetings occur quarterly for 1 to 2 hours; the meeting length is shorter than typical engagement studios to accommodate for fatigue-related symptoms that may limit attention and ability to participate in longer meetings. Before each panel, the Partner Engagement and Needs Assessment core helps identify key questions and creates a structured agenda. Each panel begins with a presentation of a research study followed by a group discussion led by a trained facilitator. The modified Delphi consensus process focuses on identifying research priority areas for Long COVID within the VA. Veterans living with Long COVID, as well as clinicians and researchers who work closely with patients who have Long COVID, complete a series of progressive surveys to provide input on research priorities.
The Partner Engagement and Needs Assessment core also actively provides outreach to important partners in research, clinical care, and operational leadership to facilitate introductory meetings to (1) ask partners to describe their 5 largest pain points, (2) find pain points within the scope of LC-PBRN resources, and (3) discuss the strengths and capacity of the PBRN. During introductory meetings, communications preferences and a cadence for subsequent meetings are established. Subsequent engagement meetings aim to provide updates and codevelop solutions to emerging issues. This core maintains a living document to track engagement efforts, points of contact for identified and emerging partners, and ensure all communication is timely.
The Patient Identification and Analysis core develops a database of veterans with confirmed or suspected Long COVID. The goal is for researchers to use the database to identify potential participants for clinical trials and monitor clinical care outcomes. When possible, this core works with existing VA data to facilitate research that aligns with the LC-PBRN mission. The core can also use natural language processing and machine learning to work with researchers conducting clinical trials to help identify patients who may meet eligibility criteria.
The Clinical Coordination and Implementation core gathers information on the best practices for identifying and recruiting veterans for Long COVID research as well as compiles strategies for standardized clinical assessments that can both facilitate ongoing research and the successful implementation of evidence-based care. The Clinical Coordination and Implementation core provides support to pilot and multisite trials in 3 ways. First, it develops toolkits such as best practice strategies for recruiting participants for research, template examples of recruitment materials, and a library of patient-reported outcome measures, standardized clinical note titles and templates in use for Long COVID in the national electronic health record. Second, it partners with the Patient Identification and Analysis core to facilitate access to and use of algorithms that identify Long COVID cases based on electronic health records for recruitment. Finally, it compiles a detailed list of potential collaborating sites. The steps to facilitate patient identification and recruitment inform feasibility assessments and improve efficiency of launching pilot studies and multisite trials. The library of outcome measures, standardized clinical notes, and templates can aid and expedite data collection.
The Dissemination core focuses on developing a website, creating a dissemination plan, and actively disseminating products of the LC-PBRN and its partners. This core’s foundational framework is based on the Agency for Healthcare Research and Quality Quick-Start Guide to Dissemination for PBRNs.8,9 The core built an internal- and external-facing website to connect users with LC-PBRN products, potential outreach contacts, and promote timely updates on LC-PBRN activities. A manual of operating procedures will be drafted to include the development of training for practitioners involved in research projects to learn the processes involved in presenting clinical results for education and training initiatives, presentations, and manuscript preparation. A toolkit will also be developed to support dissemination activities designed to reach a variety of end-users, such as education materials, policy briefings, educational briefs, newsletters, and presentations at local, regional, and national levels.
Key Partners
Key partners exist specific to the LC-PBRN and within the broader VA ecosystem, including VA clinical operations, VA research, and intra-agency collaborations.
LC-PBRN Specific. In addition to the LC-PBRN council, advisory board, and Veteran Engagement Panel discussed earlier,
VA Clinical Operations. To support clinical operations, a Long COVID Field Advisory Board was formed through the VA Office of Specialty Care as an operational effort to develop clinical best practice. The LC-PBRN consults with this group on veteran engagement strategies for input on clinical guides and dissemination of practice guide materials. The LC-PBRN also partners with an existing Long COVID Community of Practice and the Office of Primary Care. The Community of Practice provides a learning space for VA staff interested in advancing Long COVID care and assists with disseminating LC-PBRN to the broader Long COVID clinical community. A member of the Office of Primary Care sits on the PBRN advisory board to provide input on engaging primary care practitioners and ensure their unique needs are considered in LC-PBRN initiatives.
VA Research & Interagency Collaborations. The LC-PBRN engages monthly with an interagency workgroup led by the US Department of Health and Human Services Office of Long COVID Research and Practice. These engagements support identification of research gaps that the VA may help address, monitor emerging funding opportunities, and foster collaborations. LC-PBRN representatives also meet with staff at the National Institutes of Health Researching COVID to Enhance Recovery initiative to identify pathways for veteran recruitment.
LHS Feedback Loops
The LC-PBRN was designed with an LHS approach in mind.10 Throughout development of the LC-PBRN, consideration was given to (1) capture data on new efforts within the Long COVID ecosystem (performance to data), (2) examine performance gaps and identify approaches for best practice (data to knowledge), and (3) implement best practices, develop toolkits, disseminate findings, and measure impacts (knowledge to performance). With this approach, the LC-PBRN is constantly evolving based on new information coming from the internal and external Long COVID ecosystem. Each element was deliberatively considered in relation to how data can be transformed into knowledge, knowledge into performance, and performance into data.
First, an important mechanism for feedback involves establishing clear channels of communication. Regular check-ins with key partners occur through virtual meetings to provide updates, assess needs and challenges, and codevelop action plans. For example, during a check-in with the Long COVID Field Advisory Board, members expressed a desire to incorporate veteran feedback into VA clinical practice recommendations. We provided expertise on different engagement modalities (eg, focus groups vs individual interviews), and collaboration occurred to identify key interview questions for veterans. This process resulted in a published clinician-facing Long COVID Nervous System Clinical Guide (available at longcovid@hhs.gov) that integrated critical feedback from veterans related to neurological symptoms.
Second, weekly executive leadership meetings include dedicated time for reflection on partner feedback, the current state of Long COVID, and contextual changes that impact deliverable priorities and timelines. Outcomes from these discussions are communicated with VHA Health Services Research and, when appropriate, to key partners to ensure alignment. For example, the Patient Identification and Analysis core was originally tasked with identifying a definition of Long COVID. However, as the broader community moved away from a singular definition, efforts were redirected toward higher-priority issues within the VA Long COVID ecosystem, including veteran enrollment in clinical trials.
Third, the Veteran Engagement Panel captures feedback from those with lived experience to inform Long COVID research and clinical efforts. The panel meetings are strategically designed to ask veterans living with Long COVID specific questions related to a given research or clinical topic of interest. For example, panel sessions with the Field Advisory Board focused on concerns articulated by veterans related to the mental health and gastroenterological symptoms associated with Long COVID. Insights from these discussions will inform development of Long COVID mental health and gastroenterological clinical care guides, with several PBRN investigators serving as subject matter experts. This collaborative approach ensures that veteran perspectives are represented in developing Long COVID clinical care processes.
Fourth, research priorities identified through the Delphi consensus process will inform development of VA Request for Funding Proposals related to Long COVID. The initial survey was developed in collaboration with veterans, clinicians, and researchers across the Veteran Engagement Panel, the Field Advisory Board, and the National Research Action Plan on Long COVID.11 The process was launched in October 2024 and concluded in June 2025. The team conducted 3 consensus rounds with veterans and VA clinicians and researchers. Top priority areas included the testing assessments for diagnosing Long COVID, studying subtypes of Long COVID and treatments for each, and finding biomarkers for Long COVID. A formal publication of the results and analysis is the focus of a future publication.
Fifth, ongoing engagement with the Field Advisory Board has supported adoption of a preliminary set of clinical outcome measures. If universally adopted, these instruments may contribute to the development of a standardized data collection process and serve as common data elements collected for epidemiologic, health services, or clinical trial research.
Lessons Learned and Practice Implications
Throughout the development of the LC-PBRN, several decisions were identified that have impacted infrastructure development and implementation.
Include veterans’ voices to ensure network efforts align with patient needs. Given the novelty of Long COVID, practitioners and researchers are learning as they go. It is important to listen to individuals who live with Long COVID. Throughout the development of the LC-PBRN, veteran perspective has proven how vital it is for them to be heard when it comes to their health care. Clinicians similarly highlighted the value of incorporating patient perspectives into the development of tools and treatment strategies. Develop an interdisciplinary leadership team to foster the diverse viewpoints needed to tackle multifaceted problems. It is important to consider as many clinical and research perspectives as possible because Long COVID is a complex condition with symptoms impacting major organ systems.12-15 Therefore, the team spans across a multitude of specialties and locations.
Set clear expectations and goals with partners to uphold timely deliverables and stay within the PBRN’s capacity. When including a multitude of partners, teams should consider each of those partners’ experiences and opinions in decision-making conversations. Expectation setting is important to ensure all partners are on the same page and understand the capacity of the LC-PBRN. This allows the team to focus its efforts, avoid being overwhelmed with requests, and provide quality deliverables.
Build engaging relationships to bridge gaps between internal and external partners. A substantial number of resources focus on building relationships with partners so they can trust the LC-PBRN has their best interests in mind. These relationships are important to ensure the VA avoids duplicate efforts. This includes prioritizing connecting partners who are working on similar efforts to promote collaboration across facilities.
Conclusions
PBRNs provide an important mechanism to use LHS approaches to successfully convene research around complex issues. PBRNs can support integration across the LHS cycle, allowing for multiple feedback loops, and coordinate activities that work to achieve a larger vision. PBRNs offer centralized mechanisms to collaboratively understand and address complex problems, such as Long COVID, where the uncertainty regarding how to treat occurs in tandem with the urgency to treat. The LC-PBRN model described in this article has the potential to transcend Long COVID by building infrastructure necessary to proactively address current or future clinical conditions or populations with a LHS lens. The infrastructure can require cross-system and sector collaborations, expediency, inclusivity, and patient- and family-centeredness. Future efforts will focus on building out a larger network of VHA sites, facilitating recruitment at site and veteran levels into Long COVID trials through case identification, and systematically support the standardization of clinical data for clinical utility and evaluation of quality and/or outcomes across the VHA.

- Ottiger M, Poppele I, Sperling N, et al. Work ability and return-to-work of patients with post-COVID-19: a systematic review and meta-analysis. BMC Public Health. 2024;24:1811. doi:10.1186/s12889-024-19328-6
- Ziauddeen N, Gurdasani D, O’Hara ME, et al. Characteristics and impact of Long Covid: findings from an online survey. PLOS ONE. 2022;17:e0264331. doi:10.1371/journal.pone.0264331
- Graham F. Daily briefing: Answers emerge about long COVID recovery. Nature. Published online June 28, 2023. doi:10.1038/d41586-023-02190-8
- Al-Aly Z, Davis H, McCorkell L, et al. Long COVID science, research and policy. Nat Med. 2024;30:2148-2164. doi:10.1038/s41591-024-03173-6
- Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
- Ely EW, Brown LM, Fineberg HV. Long covid defined. N Engl J Med. 2024;391:1746-1753.doi:10.1056/NEJMsb2408466
- Joosten YA, Israel TL, Williams NA, et al. Community engagement studios: a structured approach to obtaining meaningful input from stakeholders to inform research. Acad Med. 2015;90:1646-1650. doi:10.1097/ACM.0000000000000794
- AHRQ. Quick-start guide to dissemination for practice-based research networks. Revised June 2014. Accessed December 2, 2025. https://www.ahrq.gov/sites/default/files/wysiwyg/ncepcr/resources/dissemination-quick-start-guide.pdf
- Gustavson AM, Morrow CD, Brown RJ, et al. Reimagining how we synthesize information to impact clinical care, policy, and research priorities in real time: examples and lessons learned from COVID-19. J Gen Intern Med. 2024;39:2554-2559. doi:10.1007/s11606-024-08855-y
- University of Minnesota. About the Center for Learning Health System Sciences. Updated December 11, 2025. Accessed December 12, 2025. https://med.umn.edu/clhss/about-us
- AHRQ. National Research Action Plan. Published online 2022. Accessed February 14, 2024. https://www.covid.gov/sites/default/files/documents/National-Research-Action-Plan-on-Long-COVID-08012022.pdf
- Gustavson AM, Eaton TL, Schapira RM, et al. Approaches to long COVID care: the Veterans Health Administration experience in 2021. BMJ Mil Health. 2024;170:179-180. doi:10.1136/military-2022-002185
- Gustavson AM. A learning health system approach to long COVID care. Fed Pract. 2022;39:7. doi:10.12788/fp.0288
- Palacio A, Bast E, Klimas N, et al. Lessons learned in implementing a multidisciplinary long COVID clinic. Am J Med. 2025;138:843-849.doi:10.1016/j.amjmed.2024.05.020
- Prusinski C, Yan D, Klasova J, et al. Multidisciplinary management strategies for long COVID: a narrative review. Cureus. 2024;16:e59478. doi:10.7759/cureus.59478
- Ottiger M, Poppele I, Sperling N, et al. Work ability and return-to-work of patients with post-COVID-19: a systematic review and meta-analysis. BMC Public Health. 2024;24:1811. doi:10.1186/s12889-024-19328-6
- Ziauddeen N, Gurdasani D, O’Hara ME, et al. Characteristics and impact of Long Covid: findings from an online survey. PLOS ONE. 2022;17:e0264331. doi:10.1371/journal.pone.0264331
- Graham F. Daily briefing: Answers emerge about long COVID recovery. Nature. Published online June 28, 2023. doi:10.1038/d41586-023-02190-8
- Al-Aly Z, Davis H, McCorkell L, et al. Long COVID science, research and policy. Nat Med. 2024;30:2148-2164. doi:10.1038/s41591-024-03173-6
- Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
- Ely EW, Brown LM, Fineberg HV. Long covid defined. N Engl J Med. 2024;391:1746-1753.doi:10.1056/NEJMsb2408466
- Joosten YA, Israel TL, Williams NA, et al. Community engagement studios: a structured approach to obtaining meaningful input from stakeholders to inform research. Acad Med. 2015;90:1646-1650. doi:10.1097/ACM.0000000000000794
- AHRQ. Quick-start guide to dissemination for practice-based research networks. Revised June 2014. Accessed December 2, 2025. https://www.ahrq.gov/sites/default/files/wysiwyg/ncepcr/resources/dissemination-quick-start-guide.pdf
- Gustavson AM, Morrow CD, Brown RJ, et al. Reimagining how we synthesize information to impact clinical care, policy, and research priorities in real time: examples and lessons learned from COVID-19. J Gen Intern Med. 2024;39:2554-2559. doi:10.1007/s11606-024-08855-y
- University of Minnesota. About the Center for Learning Health System Sciences. Updated December 11, 2025. Accessed December 12, 2025. https://med.umn.edu/clhss/about-us
- AHRQ. National Research Action Plan. Published online 2022. Accessed February 14, 2024. https://www.covid.gov/sites/default/files/documents/National-Research-Action-Plan-on-Long-COVID-08012022.pdf
- Gustavson AM, Eaton TL, Schapira RM, et al. Approaches to long COVID care: the Veterans Health Administration experience in 2021. BMJ Mil Health. 2024;170:179-180. doi:10.1136/military-2022-002185
- Gustavson AM. A learning health system approach to long COVID care. Fed Pract. 2022;39:7. doi:10.12788/fp.0288
- Palacio A, Bast E, Klimas N, et al. Lessons learned in implementing a multidisciplinary long COVID clinic. Am J Med. 2025;138:843-849.doi:10.1016/j.amjmed.2024.05.020
- Prusinski C, Yan D, Klasova J, et al. Multidisciplinary management strategies for long COVID: a narrative review. Cureus. 2024;16:e59478. doi:10.7759/cureus.59478
Confronting Uncertainty and Addressing Urgency for Action Through the Establishment of a VA Long COVID Practice-Based Research Network
Confronting Uncertainty and Addressing Urgency for Action Through the Establishment of a VA Long COVID Practice-Based Research Network
Management of Children and Adolescents With Long COVID
Current management of children and adolescents with long COVID was the focus of various presentations at the 3rd Long COVID Congress in Berlin in November 2024. The congress aimed to facilitate in-depth discussions on recent research projects, diagnostic procedures, and therapeutic approaches to enhance care for long COVID patients. This year, the focus was on research into long COVID in children and adolescents and how to improve their care.
Uta Behrends, MD, head of the Munich Chronic Fatigue Center, Center for Pediatric and Adolescent Medicine at the Technical University of Munich, Germany, and Nicole Toepfner, MD, a pediatrician at the University Hospital in Dresden, Germany, provided an initial overview.
Prevalence Data Are Limited
Data on the incidence and prevalence of the condition in children and adolescents are limited because most studies have primarily examined adults. A 2022 Swiss study estimated that it affects between 2% and 3.5% of children and adolescents who contract COVID-19. A recent study published in JAMA involving 5367 children and adolescents found that 20% of children aged 6-11 years and 14% of adolescents met the researchers’ criteria for long COVID.
Impaired Mental Health
Initial data from the latest wave of the population-based longitudinal COPSY (Corona and Psyche) study showed that compared with their peer group children and adolescents diagnosed with long COVID exhibit significantly higher rates of psychological issues and depressive symptoms. Although no significant differences were found in anxiety levels, study leader Ulrike Ravens-Sieberer, PhD, from the University Medical Center Hamburg-Eppendorf, Germany, told the congress that those with long COVID do also report more frequent somatic or psychological health complaints and lower health-related quality of life than peers.
Addressing Data Gaps
Another study due to launch in January 2025 and run through to 2028 is the COVYOUTH data study, which aims to better understand the nature, frequency, and risk factors of COVID-related sequelae in children and adolescents.
Study centers include Ruhr University Bochum, University Hospital Cologne, the Paul-Ehrlich-Institut, and University Medical Center Hamburg-Eppendorf. Using routine data from statutory health insurance and newly developed case definitions, researchers aim to investigate:
- Psychological stress caused by COVID-19 measures
- Post-COVID syndrome and myocarditis
- Adverse effects of COVID-19 vaccinations
Specialized Diagnostics and Care
The Post-COVID Kids Bavaria project offers specialized diagnostics and care for children and adolescents, including a day clinic, telemedical follow-ups, and an inpatient pain therapy module providing age-appropriate care as close to patients’ homes as possible.
MOVE-COVID is a model project for patient-focused research on long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) involving university pediatric hospitals in Freiburg, Heidelberg, Tübingen, and Ulm. It also aims to establish a care network across the state of Baden-Württemberg, including the establishment of long COVID outpatient clinics at social pediatric centers in the network hospitals, as well as enhanced telemedical support and standardized diagnostic and treatment protocols. “MOVE-COVID has successfully consolidated competencies and capacities in patient care, health services research, and patient-focused studies across multiple centers,” Behrends said.
Chronic Pain and Fatigue
Post-COVID syndromes in children and adolescents may feature profound fatigue, unrefreshing sleep, post-exertional malaise, cognitive dysfunction, and orthostatic intolerance and overlap with conditions such as ME/CFS. According to the German patient association Fatigatio, Berlin, research and studies for these conditions in children remain limited compared with those in adults. However, the US Centers for Disease Control estimates that around 2% of ME/CFS patients are children or adolescents, with the majority being teenagers.
Two inpatient treatment concepts, SHARK and TIGER, developed by Lea Höfel, PhD, head of the Centre for Pain Therapy for Young People and the Psychological Service at the Children’s Hospital in Garmisch-Partenkirchen, address chronic pain, fatigue, and ME/CFS in young people. These programs integrate structured breaks and flexible access to multiple therapists as needed. The TIGER program focuses on those with post-exertional malaise, while the SHARK program is designed for adolescents without this symptom. Both programs last 4.5-5 weeks and emphasize symptom reduction, education, and energy management.
Preliminary Results
SHARK included 30 participants (7 men; average age, 16 years), of whom 12 had a history of SARS-CoV-2 infection. TIGER involved 100 participants (24 men; average age, 16.7 years), of whom 32 had a SARS-CoV-2 infection as a triggering event. Other triggers included Epstein-Barr virus and other infections.
Preliminary findings from the projects indicate that optimized management with outpatient and follow-up care can yield positive, sometimes lasting effects. No significant differences between SARS-CoV-2 and other triggers emerged, but pain proved more manageable in the SHARK group than in the TIGER group, suggesting they may involve different pathological mechanisms.
Hope for Improved Outcomes
“It’s important to move away from the idea that nothing can be done,” Behrends said. This is a common attitude with children and adolescents displaying these types of symptoms, but it’s simply not true. “Even in pediatrics, we have numerous therapeutic options that may offer relief, from medication to psychosocial interventions,” she concluded.
This story was translated from Medscape’s German edition using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Current management of children and adolescents with long COVID was the focus of various presentations at the 3rd Long COVID Congress in Berlin in November 2024. The congress aimed to facilitate in-depth discussions on recent research projects, diagnostic procedures, and therapeutic approaches to enhance care for long COVID patients. This year, the focus was on research into long COVID in children and adolescents and how to improve their care.
Uta Behrends, MD, head of the Munich Chronic Fatigue Center, Center for Pediatric and Adolescent Medicine at the Technical University of Munich, Germany, and Nicole Toepfner, MD, a pediatrician at the University Hospital in Dresden, Germany, provided an initial overview.
Prevalence Data Are Limited
Data on the incidence and prevalence of the condition in children and adolescents are limited because most studies have primarily examined adults. A 2022 Swiss study estimated that it affects between 2% and 3.5% of children and adolescents who contract COVID-19. A recent study published in JAMA involving 5367 children and adolescents found that 20% of children aged 6-11 years and 14% of adolescents met the researchers’ criteria for long COVID.
Impaired Mental Health
Initial data from the latest wave of the population-based longitudinal COPSY (Corona and Psyche) study showed that compared with their peer group children and adolescents diagnosed with long COVID exhibit significantly higher rates of psychological issues and depressive symptoms. Although no significant differences were found in anxiety levels, study leader Ulrike Ravens-Sieberer, PhD, from the University Medical Center Hamburg-Eppendorf, Germany, told the congress that those with long COVID do also report more frequent somatic or psychological health complaints and lower health-related quality of life than peers.
Addressing Data Gaps
Another study due to launch in January 2025 and run through to 2028 is the COVYOUTH data study, which aims to better understand the nature, frequency, and risk factors of COVID-related sequelae in children and adolescents.
Study centers include Ruhr University Bochum, University Hospital Cologne, the Paul-Ehrlich-Institut, and University Medical Center Hamburg-Eppendorf. Using routine data from statutory health insurance and newly developed case definitions, researchers aim to investigate:
- Psychological stress caused by COVID-19 measures
- Post-COVID syndrome and myocarditis
- Adverse effects of COVID-19 vaccinations
Specialized Diagnostics and Care
The Post-COVID Kids Bavaria project offers specialized diagnostics and care for children and adolescents, including a day clinic, telemedical follow-ups, and an inpatient pain therapy module providing age-appropriate care as close to patients’ homes as possible.
MOVE-COVID is a model project for patient-focused research on long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) involving university pediatric hospitals in Freiburg, Heidelberg, Tübingen, and Ulm. It also aims to establish a care network across the state of Baden-Württemberg, including the establishment of long COVID outpatient clinics at social pediatric centers in the network hospitals, as well as enhanced telemedical support and standardized diagnostic and treatment protocols. “MOVE-COVID has successfully consolidated competencies and capacities in patient care, health services research, and patient-focused studies across multiple centers,” Behrends said.
Chronic Pain and Fatigue
Post-COVID syndromes in children and adolescents may feature profound fatigue, unrefreshing sleep, post-exertional malaise, cognitive dysfunction, and orthostatic intolerance and overlap with conditions such as ME/CFS. According to the German patient association Fatigatio, Berlin, research and studies for these conditions in children remain limited compared with those in adults. However, the US Centers for Disease Control estimates that around 2% of ME/CFS patients are children or adolescents, with the majority being teenagers.
Two inpatient treatment concepts, SHARK and TIGER, developed by Lea Höfel, PhD, head of the Centre for Pain Therapy for Young People and the Psychological Service at the Children’s Hospital in Garmisch-Partenkirchen, address chronic pain, fatigue, and ME/CFS in young people. These programs integrate structured breaks and flexible access to multiple therapists as needed. The TIGER program focuses on those with post-exertional malaise, while the SHARK program is designed for adolescents without this symptom. Both programs last 4.5-5 weeks and emphasize symptom reduction, education, and energy management.
Preliminary Results
SHARK included 30 participants (7 men; average age, 16 years), of whom 12 had a history of SARS-CoV-2 infection. TIGER involved 100 participants (24 men; average age, 16.7 years), of whom 32 had a SARS-CoV-2 infection as a triggering event. Other triggers included Epstein-Barr virus and other infections.
Preliminary findings from the projects indicate that optimized management with outpatient and follow-up care can yield positive, sometimes lasting effects. No significant differences between SARS-CoV-2 and other triggers emerged, but pain proved more manageable in the SHARK group than in the TIGER group, suggesting they may involve different pathological mechanisms.
Hope for Improved Outcomes
“It’s important to move away from the idea that nothing can be done,” Behrends said. This is a common attitude with children and adolescents displaying these types of symptoms, but it’s simply not true. “Even in pediatrics, we have numerous therapeutic options that may offer relief, from medication to psychosocial interventions,” she concluded.
This story was translated from Medscape’s German edition using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Current management of children and adolescents with long COVID was the focus of various presentations at the 3rd Long COVID Congress in Berlin in November 2024. The congress aimed to facilitate in-depth discussions on recent research projects, diagnostic procedures, and therapeutic approaches to enhance care for long COVID patients. This year, the focus was on research into long COVID in children and adolescents and how to improve their care.
Uta Behrends, MD, head of the Munich Chronic Fatigue Center, Center for Pediatric and Adolescent Medicine at the Technical University of Munich, Germany, and Nicole Toepfner, MD, a pediatrician at the University Hospital in Dresden, Germany, provided an initial overview.
Prevalence Data Are Limited
Data on the incidence and prevalence of the condition in children and adolescents are limited because most studies have primarily examined adults. A 2022 Swiss study estimated that it affects between 2% and 3.5% of children and adolescents who contract COVID-19. A recent study published in JAMA involving 5367 children and adolescents found that 20% of children aged 6-11 years and 14% of adolescents met the researchers’ criteria for long COVID.
Impaired Mental Health
Initial data from the latest wave of the population-based longitudinal COPSY (Corona and Psyche) study showed that compared with their peer group children and adolescents diagnosed with long COVID exhibit significantly higher rates of psychological issues and depressive symptoms. Although no significant differences were found in anxiety levels, study leader Ulrike Ravens-Sieberer, PhD, from the University Medical Center Hamburg-Eppendorf, Germany, told the congress that those with long COVID do also report more frequent somatic or psychological health complaints and lower health-related quality of life than peers.
Addressing Data Gaps
Another study due to launch in January 2025 and run through to 2028 is the COVYOUTH data study, which aims to better understand the nature, frequency, and risk factors of COVID-related sequelae in children and adolescents.
Study centers include Ruhr University Bochum, University Hospital Cologne, the Paul-Ehrlich-Institut, and University Medical Center Hamburg-Eppendorf. Using routine data from statutory health insurance and newly developed case definitions, researchers aim to investigate:
- Psychological stress caused by COVID-19 measures
- Post-COVID syndrome and myocarditis
- Adverse effects of COVID-19 vaccinations
Specialized Diagnostics and Care
The Post-COVID Kids Bavaria project offers specialized diagnostics and care for children and adolescents, including a day clinic, telemedical follow-ups, and an inpatient pain therapy module providing age-appropriate care as close to patients’ homes as possible.
MOVE-COVID is a model project for patient-focused research on long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) involving university pediatric hospitals in Freiburg, Heidelberg, Tübingen, and Ulm. It also aims to establish a care network across the state of Baden-Württemberg, including the establishment of long COVID outpatient clinics at social pediatric centers in the network hospitals, as well as enhanced telemedical support and standardized diagnostic and treatment protocols. “MOVE-COVID has successfully consolidated competencies and capacities in patient care, health services research, and patient-focused studies across multiple centers,” Behrends said.
Chronic Pain and Fatigue
Post-COVID syndromes in children and adolescents may feature profound fatigue, unrefreshing sleep, post-exertional malaise, cognitive dysfunction, and orthostatic intolerance and overlap with conditions such as ME/CFS. According to the German patient association Fatigatio, Berlin, research and studies for these conditions in children remain limited compared with those in adults. However, the US Centers for Disease Control estimates that around 2% of ME/CFS patients are children or adolescents, with the majority being teenagers.
Two inpatient treatment concepts, SHARK and TIGER, developed by Lea Höfel, PhD, head of the Centre for Pain Therapy for Young People and the Psychological Service at the Children’s Hospital in Garmisch-Partenkirchen, address chronic pain, fatigue, and ME/CFS in young people. These programs integrate structured breaks and flexible access to multiple therapists as needed. The TIGER program focuses on those with post-exertional malaise, while the SHARK program is designed for adolescents without this symptom. Both programs last 4.5-5 weeks and emphasize symptom reduction, education, and energy management.
Preliminary Results
SHARK included 30 participants (7 men; average age, 16 years), of whom 12 had a history of SARS-CoV-2 infection. TIGER involved 100 participants (24 men; average age, 16.7 years), of whom 32 had a SARS-CoV-2 infection as a triggering event. Other triggers included Epstein-Barr virus and other infections.
Preliminary findings from the projects indicate that optimized management with outpatient and follow-up care can yield positive, sometimes lasting effects. No significant differences between SARS-CoV-2 and other triggers emerged, but pain proved more manageable in the SHARK group than in the TIGER group, suggesting they may involve different pathological mechanisms.
Hope for Improved Outcomes
“It’s important to move away from the idea that nothing can be done,” Behrends said. This is a common attitude with children and adolescents displaying these types of symptoms, but it’s simply not true. “Even in pediatrics, we have numerous therapeutic options that may offer relief, from medication to psychosocial interventions,” she concluded.
This story was translated from Medscape’s German edition using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
FROM THE 3RD LONG COVID CONGRESS
Younger People and Long COVID: Underreported, Undertreated
John Bolecek, 41, of Richmond, Virginia, was diagnosed with long COVID in May of 2022. While his acute infection was mild, once everyone else in his family had recovered, the heavy fatigue he experienced from the start has never lifted.
“When I wake up in the morning, I feel like I haven’t gone to sleep at all,” Bolecek said. “It’s this super fatigue that’s just never gone away.”
The urban planner who once rode his bike to work daily and spent weekends cycling had to quit working and now can barely get through a light walk before long COVID symptoms of post-exertional malaise, an intense fatigue after previously tolerated physical or mental activity, set in. His unrefreshing sleep, fatigue, and dysautonomia — a disruption of the autonomic nervous system that causes dizziness, heart rate changes, and nausea — have made it nearly impossible to share household duties with his wife. She has to do most of the cooking, cleaning, and tending to their two sons, ages 6 and 8 years.
It’s an increasingly familiar story for those hit with long COVID in their prime, a period of life when young and middle-aged adults are the most productive and the busiest, often in the thick of parenting while also taking care of their aging parents. And it’s a group that is among the hardest hit by long COVID both because of the sheer number of patients with the condition and the mental and financial strain that it’s putting on this age group. According to the Centers for Disease Control and Prevention (CDC), 6.9% of adults aged 18-34 years and 8.9% of adults aged 35-49 years have the disorder compared with 4.1% of older adults aged > 65 years who are the least likely to have long COVID.
In a study published recently in Scientific Reports, researchers found that in a population of California residents with long COVID, older individuals (who were sicker to start) had more severe symptoms associated with the condition. But researchers also found that younger people (aged 18-49 years) were more likely to experience symptoms that reduced their productivity and quality of life. They suggested this is both because they have more to do in a given day and because they have a longer life ahead of them living with a chronic condition.
“Much of California’s population falls within the 18-49 age group, [so] we would expect to see the highest overall burden coming from these individuals,” said lead study author Sophie Zhu, a researcher in the Division of Communicable Disease Control at the California Department of Public Health.
The Impact on Work and Life Productivity
Adults and especially those in middle age tend to have a lot of competing stressors during this period of life, said Nisha Viswanathan, MD, director of the UCLA Health Long COVID program. “Patients may need to decrease some of the pressures of life for their health and that can be impossible to do because they have so many other people who are depending on them.”
It’s a different set of circumstances compared with older individuals who may have more severe symptoms because they have underlying conditions. But older Americans are also more likely to be retired and don’t have children who are financially dependent on them. Previous research has shown the burden that long COVID is having on the workforce. A study published in the August 2023 edition of The Lancet Regional Health found that 5.8% of participating patients with long COVID reported occupational changes like moving to part time or remote work, including 1.6% who had completely dropped out of the workforce.
Middle age is also a time of life when patients may not have time to seek the care they need. The chronic nature of long COVID means that treatment can be time consuming and expensive, all of which drains resources from patients who are often supporting spouses, children, and sometimes older parents. A study published in Disability and Health Journal found that patients with long COVID have significantly higher rates of housing instability and financial concerns, such as worries about paying rent or a mortgage, than those without the condition.
The Financial Strain of Long COVID
For those who can’t work, the process of applying for long-term disability can also be complicated. That’s especially true for people whose illness keeps them from doing even basic tasks like filling out paperwork and dealing with disability insurance claims. It requires those applying as a result of their long COVID symptoms to show all records connected to long COVID as well as a medical history, the beginning of their symptoms, and their current treatments.
Even then, many patients complain of having their claims rejected, which can be financially disastrous to families already struggling to get by. Still, experts contend that it’s important to understand that as of July 2021, long COVID is considered a disability under the Americans with Disabilities Act (ADA).
“Long COVID is recognized as a disability under Section 504 of the ADA, and yet day after day, we see violations of the ADA for people with long COVID not getting the accommodations that they need in order to work,” said David Putrino, PhD, the Nash Family director of the Cohen Center for Recovery from Complex Chronic Illness at Mount Sinai in New York City and a renowned expert in long COVID.
He added that short- and long-term disability claims are sometimes denied because of a lack of diagnostic testing to prove a patient has the condition. “This is nonsensical and absurd because the CDC does not require a blood test for the diagnosis of long COVID. It’s at your physician’s discretion,” Putrino said.
Viswanathan agreed. She said that for many of her patients, getting long-term disability has been particularly challenging because there’s no blood test for long COVID to prove patients have the condition. “As a result, for many of our patients, especially when they’re young, they may have to return to work in one form or another,” Viswanathan said.
The Impact of Long COVID on Quality of Life
What’s worse, the full impact is yet unknown because this is likely an underestimated cohort as many of these patients had mild cases of acute COVID-19 and fewer underlying conditions. For others, their long COVID is undiagnosed.
“Much of the impact on productivity and quality of life for this group remains hidden,” said Ziyad Al-Aly, MD, a global expert on long COVID and chief of research and development at the Veterans Affairs St. Louis Health Care System in Missouri.
Unfortunately, the impact on Bolecek’s life isn’t so hidden. He can’t work, which has been a financial stressor on the family. He spends much of the day in bed so that he can help with a few things when his wife gets home from work. He can’t cycle anymore and, as a result, has lost many of the friends associated with his favorite hobby.
But he remains hopeful, and more than anything else, he’s thankful for his family. His wife and kids have given him the strength to push on even when the days are hard. “I just don’t know where I’d be without them,” Bolecek said.
A version of this article first appeared on Medscape.com.
John Bolecek, 41, of Richmond, Virginia, was diagnosed with long COVID in May of 2022. While his acute infection was mild, once everyone else in his family had recovered, the heavy fatigue he experienced from the start has never lifted.
“When I wake up in the morning, I feel like I haven’t gone to sleep at all,” Bolecek said. “It’s this super fatigue that’s just never gone away.”
The urban planner who once rode his bike to work daily and spent weekends cycling had to quit working and now can barely get through a light walk before long COVID symptoms of post-exertional malaise, an intense fatigue after previously tolerated physical or mental activity, set in. His unrefreshing sleep, fatigue, and dysautonomia — a disruption of the autonomic nervous system that causes dizziness, heart rate changes, and nausea — have made it nearly impossible to share household duties with his wife. She has to do most of the cooking, cleaning, and tending to their two sons, ages 6 and 8 years.
It’s an increasingly familiar story for those hit with long COVID in their prime, a period of life when young and middle-aged adults are the most productive and the busiest, often in the thick of parenting while also taking care of their aging parents. And it’s a group that is among the hardest hit by long COVID both because of the sheer number of patients with the condition and the mental and financial strain that it’s putting on this age group. According to the Centers for Disease Control and Prevention (CDC), 6.9% of adults aged 18-34 years and 8.9% of adults aged 35-49 years have the disorder compared with 4.1% of older adults aged > 65 years who are the least likely to have long COVID.
In a study published recently in Scientific Reports, researchers found that in a population of California residents with long COVID, older individuals (who were sicker to start) had more severe symptoms associated with the condition. But researchers also found that younger people (aged 18-49 years) were more likely to experience symptoms that reduced their productivity and quality of life. They suggested this is both because they have more to do in a given day and because they have a longer life ahead of them living with a chronic condition.
“Much of California’s population falls within the 18-49 age group, [so] we would expect to see the highest overall burden coming from these individuals,” said lead study author Sophie Zhu, a researcher in the Division of Communicable Disease Control at the California Department of Public Health.
The Impact on Work and Life Productivity
Adults and especially those in middle age tend to have a lot of competing stressors during this period of life, said Nisha Viswanathan, MD, director of the UCLA Health Long COVID program. “Patients may need to decrease some of the pressures of life for their health and that can be impossible to do because they have so many other people who are depending on them.”
It’s a different set of circumstances compared with older individuals who may have more severe symptoms because they have underlying conditions. But older Americans are also more likely to be retired and don’t have children who are financially dependent on them. Previous research has shown the burden that long COVID is having on the workforce. A study published in the August 2023 edition of The Lancet Regional Health found that 5.8% of participating patients with long COVID reported occupational changes like moving to part time or remote work, including 1.6% who had completely dropped out of the workforce.
Middle age is also a time of life when patients may not have time to seek the care they need. The chronic nature of long COVID means that treatment can be time consuming and expensive, all of which drains resources from patients who are often supporting spouses, children, and sometimes older parents. A study published in Disability and Health Journal found that patients with long COVID have significantly higher rates of housing instability and financial concerns, such as worries about paying rent or a mortgage, than those without the condition.
The Financial Strain of Long COVID
For those who can’t work, the process of applying for long-term disability can also be complicated. That’s especially true for people whose illness keeps them from doing even basic tasks like filling out paperwork and dealing with disability insurance claims. It requires those applying as a result of their long COVID symptoms to show all records connected to long COVID as well as a medical history, the beginning of their symptoms, and their current treatments.
Even then, many patients complain of having their claims rejected, which can be financially disastrous to families already struggling to get by. Still, experts contend that it’s important to understand that as of July 2021, long COVID is considered a disability under the Americans with Disabilities Act (ADA).
“Long COVID is recognized as a disability under Section 504 of the ADA, and yet day after day, we see violations of the ADA for people with long COVID not getting the accommodations that they need in order to work,” said David Putrino, PhD, the Nash Family director of the Cohen Center for Recovery from Complex Chronic Illness at Mount Sinai in New York City and a renowned expert in long COVID.
He added that short- and long-term disability claims are sometimes denied because of a lack of diagnostic testing to prove a patient has the condition. “This is nonsensical and absurd because the CDC does not require a blood test for the diagnosis of long COVID. It’s at your physician’s discretion,” Putrino said.
Viswanathan agreed. She said that for many of her patients, getting long-term disability has been particularly challenging because there’s no blood test for long COVID to prove patients have the condition. “As a result, for many of our patients, especially when they’re young, they may have to return to work in one form or another,” Viswanathan said.
The Impact of Long COVID on Quality of Life
What’s worse, the full impact is yet unknown because this is likely an underestimated cohort as many of these patients had mild cases of acute COVID-19 and fewer underlying conditions. For others, their long COVID is undiagnosed.
“Much of the impact on productivity and quality of life for this group remains hidden,” said Ziyad Al-Aly, MD, a global expert on long COVID and chief of research and development at the Veterans Affairs St. Louis Health Care System in Missouri.
Unfortunately, the impact on Bolecek’s life isn’t so hidden. He can’t work, which has been a financial stressor on the family. He spends much of the day in bed so that he can help with a few things when his wife gets home from work. He can’t cycle anymore and, as a result, has lost many of the friends associated with his favorite hobby.
But he remains hopeful, and more than anything else, he’s thankful for his family. His wife and kids have given him the strength to push on even when the days are hard. “I just don’t know where I’d be without them,” Bolecek said.
A version of this article first appeared on Medscape.com.
John Bolecek, 41, of Richmond, Virginia, was diagnosed with long COVID in May of 2022. While his acute infection was mild, once everyone else in his family had recovered, the heavy fatigue he experienced from the start has never lifted.
“When I wake up in the morning, I feel like I haven’t gone to sleep at all,” Bolecek said. “It’s this super fatigue that’s just never gone away.”
The urban planner who once rode his bike to work daily and spent weekends cycling had to quit working and now can barely get through a light walk before long COVID symptoms of post-exertional malaise, an intense fatigue after previously tolerated physical or mental activity, set in. His unrefreshing sleep, fatigue, and dysautonomia — a disruption of the autonomic nervous system that causes dizziness, heart rate changes, and nausea — have made it nearly impossible to share household duties with his wife. She has to do most of the cooking, cleaning, and tending to their two sons, ages 6 and 8 years.
It’s an increasingly familiar story for those hit with long COVID in their prime, a period of life when young and middle-aged adults are the most productive and the busiest, often in the thick of parenting while also taking care of their aging parents. And it’s a group that is among the hardest hit by long COVID both because of the sheer number of patients with the condition and the mental and financial strain that it’s putting on this age group. According to the Centers for Disease Control and Prevention (CDC), 6.9% of adults aged 18-34 years and 8.9% of adults aged 35-49 years have the disorder compared with 4.1% of older adults aged > 65 years who are the least likely to have long COVID.
In a study published recently in Scientific Reports, researchers found that in a population of California residents with long COVID, older individuals (who were sicker to start) had more severe symptoms associated with the condition. But researchers also found that younger people (aged 18-49 years) were more likely to experience symptoms that reduced their productivity and quality of life. They suggested this is both because they have more to do in a given day and because they have a longer life ahead of them living with a chronic condition.
“Much of California’s population falls within the 18-49 age group, [so] we would expect to see the highest overall burden coming from these individuals,” said lead study author Sophie Zhu, a researcher in the Division of Communicable Disease Control at the California Department of Public Health.
The Impact on Work and Life Productivity
Adults and especially those in middle age tend to have a lot of competing stressors during this period of life, said Nisha Viswanathan, MD, director of the UCLA Health Long COVID program. “Patients may need to decrease some of the pressures of life for their health and that can be impossible to do because they have so many other people who are depending on them.”
It’s a different set of circumstances compared with older individuals who may have more severe symptoms because they have underlying conditions. But older Americans are also more likely to be retired and don’t have children who are financially dependent on them. Previous research has shown the burden that long COVID is having on the workforce. A study published in the August 2023 edition of The Lancet Regional Health found that 5.8% of participating patients with long COVID reported occupational changes like moving to part time or remote work, including 1.6% who had completely dropped out of the workforce.
Middle age is also a time of life when patients may not have time to seek the care they need. The chronic nature of long COVID means that treatment can be time consuming and expensive, all of which drains resources from patients who are often supporting spouses, children, and sometimes older parents. A study published in Disability and Health Journal found that patients with long COVID have significantly higher rates of housing instability and financial concerns, such as worries about paying rent or a mortgage, than those without the condition.
The Financial Strain of Long COVID
For those who can’t work, the process of applying for long-term disability can also be complicated. That’s especially true for people whose illness keeps them from doing even basic tasks like filling out paperwork and dealing with disability insurance claims. It requires those applying as a result of their long COVID symptoms to show all records connected to long COVID as well as a medical history, the beginning of their symptoms, and their current treatments.
Even then, many patients complain of having their claims rejected, which can be financially disastrous to families already struggling to get by. Still, experts contend that it’s important to understand that as of July 2021, long COVID is considered a disability under the Americans with Disabilities Act (ADA).
“Long COVID is recognized as a disability under Section 504 of the ADA, and yet day after day, we see violations of the ADA for people with long COVID not getting the accommodations that they need in order to work,” said David Putrino, PhD, the Nash Family director of the Cohen Center for Recovery from Complex Chronic Illness at Mount Sinai in New York City and a renowned expert in long COVID.
He added that short- and long-term disability claims are sometimes denied because of a lack of diagnostic testing to prove a patient has the condition. “This is nonsensical and absurd because the CDC does not require a blood test for the diagnosis of long COVID. It’s at your physician’s discretion,” Putrino said.
Viswanathan agreed. She said that for many of her patients, getting long-term disability has been particularly challenging because there’s no blood test for long COVID to prove patients have the condition. “As a result, for many of our patients, especially when they’re young, they may have to return to work in one form or another,” Viswanathan said.
The Impact of Long COVID on Quality of Life
What’s worse, the full impact is yet unknown because this is likely an underestimated cohort as many of these patients had mild cases of acute COVID-19 and fewer underlying conditions. For others, their long COVID is undiagnosed.
“Much of the impact on productivity and quality of life for this group remains hidden,” said Ziyad Al-Aly, MD, a global expert on long COVID and chief of research and development at the Veterans Affairs St. Louis Health Care System in Missouri.
Unfortunately, the impact on Bolecek’s life isn’t so hidden. He can’t work, which has been a financial stressor on the family. He spends much of the day in bed so that he can help with a few things when his wife gets home from work. He can’t cycle anymore and, as a result, has lost many of the friends associated with his favorite hobby.
But he remains hopeful, and more than anything else, he’s thankful for his family. His wife and kids have given him the strength to push on even when the days are hard. “I just don’t know where I’d be without them,” Bolecek said.
A version of this article first appeared on Medscape.com.
Management of Children and Adolescents With Long COVID
Current management of children and adolescents with long COVID was the focus of various presentations at the 3rd Long COVID Congress in Berlin in November. In 2024, the focus was on research into long COVID in children and adolescents and how to improve their care.
Uta Behrends, MD, head of the Munich Chronic Fatigue Center, Center for Pediatric and Adolescent Medicine at the Technical University of Munich in Germany, and Nicole Toepfner, MD, a pediatrician at the University Hospital in Dresden, Germany, provided an initial overview.
Prevalence Data Are Limited
Data on the incidence and prevalence of the condition in children and adolescents are limited because most studies have primarily examined adults. A 2022 Swiss study estimated that it affects between 2% and 3.5% of children and adolescents who contract COVID-19. A recent study published in JAMA involving 5367 children and adolescents found that 20% of children aged 6-11 years and 14% of adolescents met the researchers’ criteria for long COVID.
Impaired Mental Health
Initial data from the latest wave of the population-based longitudinal COPSY (Corona and Psyche) study showed that, compared with their peer group, children and adolescents diagnosed with long COVID exhibit significantly higher rates of psychological issues and depressive symptoms. Although no significant differences were found in anxiety levels, study leader Ulrike Ravens-Sieberer, PhD, from the University Medical Center Hamburg-Eppendorf, Germany, told the congress that those with long COVID also report more frequent somatic or psychological health complaints and lower health-related quality of life than peers.
Addressing Data Gaps
Another study due to launch in January 2025 and run through to 2028 is the COVYOUTH data study, which aims to better understand the nature, frequency, and risk factors of COVID-related sequelae in children and adolescents.
Study centers include Ruhr University Bochum, University Hospital Cologne, the Paul-Ehrlich-Institut, and University Medical Center Hamburg-Eppendorf. Using routine data from statutory health insurance and newly developed case definitions, researchers aim to investigate psychological stress caused by COVID-19 measures, post-COVID syndrome and myocarditis, and adverse effects of COVID-19 vaccinations.
Specialized Diagnostics and Care
The Post-COVID Kids Bavaria project offers specialized diagnostics and care for children and adolescents, including a day clinic, telemedical follow-ups, and an inpatient pain therapy module providing age-appropriate care as close to patients’ homes as possible.
MOVE-COVID is a model project for patient-focused research on long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) involving university pediatric hospitals in Freiburg, Heidelberg, Tübingen, and Ulm. It also aims to establish a care network across the state of Baden-Württemberg, including the establishment of long COVID outpatient clinics at social pediatric centers in the network hospitals, as well as enhanced telemedical support and standardized diagnostic and treatment protocols. “MOVE-COVID has successfully consolidated competencies and capacities in patient care, health services research, and patient-focused studies across multiple centers,” Behrends said.
Chronic Pain and Fatigue
Post-COVID syndromes in children and adolescents may feature profound fatigue, unrefreshing sleep, post-exertional malaise, cognitive dysfunction, and orthostatic intolerance and overlap with conditions such as ME/CFS. According to the German patient association Fatigatio, Berlin, research and studies for these conditions in children remain limited compared with those in adults. However, the US Centers for Disease Control estimates that around 2% of ME/CFS patients are children or adolescents, with the majority being teenagers.
Two inpatient treatment concepts, SHARK and TIGER, developed by Lea Höfel, PhD, head of the Centre for Pain Therapy for Young People and the Psychological Service at the Children’s Hospital in Garmisch-Partenkirchen, address chronic pain, fatigue, and ME/CFS in young people. These programs integrate structured breaks and flexible access to multiple therapists as needed. The TIGER program focuses on those with post-exertional malaise, while the SHARK program is designed for adolescents without this symptom. Both programs last 4.5 to 5 weeks and emphasize symptom reduction, education, and energy management.
Preliminary Results
SHARK included 30 participants (7 men; average age, 16 years), of whom 12 had a history of SARS-CoV-2 infection. TIGER involved 100 participants (24 men; average age, 16.7 years), of whom 32 had a SARS-CoV-2 infection as a triggering event. Other triggers included Epstein-Barr virus and other infections.
Preliminary findings from the projects indicate that optimized management with outpatient and follow-up care can yield positive, sometimes lasting effects. No significant differences between SARS-CoV-2 and other triggers emerged, but pain proved more manageable in the SHARK group than in the TIGER group, suggesting they may involve different pathological mechanisms.
Hope for Improved Outcomes
“It’s important to move away from the idea that nothing can be done,” Behrends said. This is a common attitude with children and adolescents displaying these types of symptoms, but it’s simply not true. “Even in pediatrics, we have numerous therapeutic options that may offer relief, from medication to psychosocial interventions.”
This story was translated from Medscape’s German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Current management of children and adolescents with long COVID was the focus of various presentations at the 3rd Long COVID Congress in Berlin in November. In 2024, the focus was on research into long COVID in children and adolescents and how to improve their care.
Uta Behrends, MD, head of the Munich Chronic Fatigue Center, Center for Pediatric and Adolescent Medicine at the Technical University of Munich in Germany, and Nicole Toepfner, MD, a pediatrician at the University Hospital in Dresden, Germany, provided an initial overview.
Prevalence Data Are Limited
Data on the incidence and prevalence of the condition in children and adolescents are limited because most studies have primarily examined adults. A 2022 Swiss study estimated that it affects between 2% and 3.5% of children and adolescents who contract COVID-19. A recent study published in JAMA involving 5367 children and adolescents found that 20% of children aged 6-11 years and 14% of adolescents met the researchers’ criteria for long COVID.
Impaired Mental Health
Initial data from the latest wave of the population-based longitudinal COPSY (Corona and Psyche) study showed that, compared with their peer group, children and adolescents diagnosed with long COVID exhibit significantly higher rates of psychological issues and depressive symptoms. Although no significant differences were found in anxiety levels, study leader Ulrike Ravens-Sieberer, PhD, from the University Medical Center Hamburg-Eppendorf, Germany, told the congress that those with long COVID also report more frequent somatic or psychological health complaints and lower health-related quality of life than peers.
Addressing Data Gaps
Another study due to launch in January 2025 and run through to 2028 is the COVYOUTH data study, which aims to better understand the nature, frequency, and risk factors of COVID-related sequelae in children and adolescents.
Study centers include Ruhr University Bochum, University Hospital Cologne, the Paul-Ehrlich-Institut, and University Medical Center Hamburg-Eppendorf. Using routine data from statutory health insurance and newly developed case definitions, researchers aim to investigate psychological stress caused by COVID-19 measures, post-COVID syndrome and myocarditis, and adverse effects of COVID-19 vaccinations.
Specialized Diagnostics and Care
The Post-COVID Kids Bavaria project offers specialized diagnostics and care for children and adolescents, including a day clinic, telemedical follow-ups, and an inpatient pain therapy module providing age-appropriate care as close to patients’ homes as possible.
MOVE-COVID is a model project for patient-focused research on long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) involving university pediatric hospitals in Freiburg, Heidelberg, Tübingen, and Ulm. It also aims to establish a care network across the state of Baden-Württemberg, including the establishment of long COVID outpatient clinics at social pediatric centers in the network hospitals, as well as enhanced telemedical support and standardized diagnostic and treatment protocols. “MOVE-COVID has successfully consolidated competencies and capacities in patient care, health services research, and patient-focused studies across multiple centers,” Behrends said.
Chronic Pain and Fatigue
Post-COVID syndromes in children and adolescents may feature profound fatigue, unrefreshing sleep, post-exertional malaise, cognitive dysfunction, and orthostatic intolerance and overlap with conditions such as ME/CFS. According to the German patient association Fatigatio, Berlin, research and studies for these conditions in children remain limited compared with those in adults. However, the US Centers for Disease Control estimates that around 2% of ME/CFS patients are children or adolescents, with the majority being teenagers.
Two inpatient treatment concepts, SHARK and TIGER, developed by Lea Höfel, PhD, head of the Centre for Pain Therapy for Young People and the Psychological Service at the Children’s Hospital in Garmisch-Partenkirchen, address chronic pain, fatigue, and ME/CFS in young people. These programs integrate structured breaks and flexible access to multiple therapists as needed. The TIGER program focuses on those with post-exertional malaise, while the SHARK program is designed for adolescents without this symptom. Both programs last 4.5 to 5 weeks and emphasize symptom reduction, education, and energy management.
Preliminary Results
SHARK included 30 participants (7 men; average age, 16 years), of whom 12 had a history of SARS-CoV-2 infection. TIGER involved 100 participants (24 men; average age, 16.7 years), of whom 32 had a SARS-CoV-2 infection as a triggering event. Other triggers included Epstein-Barr virus and other infections.
Preliminary findings from the projects indicate that optimized management with outpatient and follow-up care can yield positive, sometimes lasting effects. No significant differences between SARS-CoV-2 and other triggers emerged, but pain proved more manageable in the SHARK group than in the TIGER group, suggesting they may involve different pathological mechanisms.
Hope for Improved Outcomes
“It’s important to move away from the idea that nothing can be done,” Behrends said. This is a common attitude with children and adolescents displaying these types of symptoms, but it’s simply not true. “Even in pediatrics, we have numerous therapeutic options that may offer relief, from medication to psychosocial interventions.”
This story was translated from Medscape’s German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Current management of children and adolescents with long COVID was the focus of various presentations at the 3rd Long COVID Congress in Berlin in November. In 2024, the focus was on research into long COVID in children and adolescents and how to improve their care.
Uta Behrends, MD, head of the Munich Chronic Fatigue Center, Center for Pediatric and Adolescent Medicine at the Technical University of Munich in Germany, and Nicole Toepfner, MD, a pediatrician at the University Hospital in Dresden, Germany, provided an initial overview.
Prevalence Data Are Limited
Data on the incidence and prevalence of the condition in children and adolescents are limited because most studies have primarily examined adults. A 2022 Swiss study estimated that it affects between 2% and 3.5% of children and adolescents who contract COVID-19. A recent study published in JAMA involving 5367 children and adolescents found that 20% of children aged 6-11 years and 14% of adolescents met the researchers’ criteria for long COVID.
Impaired Mental Health
Initial data from the latest wave of the population-based longitudinal COPSY (Corona and Psyche) study showed that, compared with their peer group, children and adolescents diagnosed with long COVID exhibit significantly higher rates of psychological issues and depressive symptoms. Although no significant differences were found in anxiety levels, study leader Ulrike Ravens-Sieberer, PhD, from the University Medical Center Hamburg-Eppendorf, Germany, told the congress that those with long COVID also report more frequent somatic or psychological health complaints and lower health-related quality of life than peers.
Addressing Data Gaps
Another study due to launch in January 2025 and run through to 2028 is the COVYOUTH data study, which aims to better understand the nature, frequency, and risk factors of COVID-related sequelae in children and adolescents.
Study centers include Ruhr University Bochum, University Hospital Cologne, the Paul-Ehrlich-Institut, and University Medical Center Hamburg-Eppendorf. Using routine data from statutory health insurance and newly developed case definitions, researchers aim to investigate psychological stress caused by COVID-19 measures, post-COVID syndrome and myocarditis, and adverse effects of COVID-19 vaccinations.
Specialized Diagnostics and Care
The Post-COVID Kids Bavaria project offers specialized diagnostics and care for children and adolescents, including a day clinic, telemedical follow-ups, and an inpatient pain therapy module providing age-appropriate care as close to patients’ homes as possible.
MOVE-COVID is a model project for patient-focused research on long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) involving university pediatric hospitals in Freiburg, Heidelberg, Tübingen, and Ulm. It also aims to establish a care network across the state of Baden-Württemberg, including the establishment of long COVID outpatient clinics at social pediatric centers in the network hospitals, as well as enhanced telemedical support and standardized diagnostic and treatment protocols. “MOVE-COVID has successfully consolidated competencies and capacities in patient care, health services research, and patient-focused studies across multiple centers,” Behrends said.
Chronic Pain and Fatigue
Post-COVID syndromes in children and adolescents may feature profound fatigue, unrefreshing sleep, post-exertional malaise, cognitive dysfunction, and orthostatic intolerance and overlap with conditions such as ME/CFS. According to the German patient association Fatigatio, Berlin, research and studies for these conditions in children remain limited compared with those in adults. However, the US Centers for Disease Control estimates that around 2% of ME/CFS patients are children or adolescents, with the majority being teenagers.
Two inpatient treatment concepts, SHARK and TIGER, developed by Lea Höfel, PhD, head of the Centre for Pain Therapy for Young People and the Psychological Service at the Children’s Hospital in Garmisch-Partenkirchen, address chronic pain, fatigue, and ME/CFS in young people. These programs integrate structured breaks and flexible access to multiple therapists as needed. The TIGER program focuses on those with post-exertional malaise, while the SHARK program is designed for adolescents without this symptom. Both programs last 4.5 to 5 weeks and emphasize symptom reduction, education, and energy management.
Preliminary Results
SHARK included 30 participants (7 men; average age, 16 years), of whom 12 had a history of SARS-CoV-2 infection. TIGER involved 100 participants (24 men; average age, 16.7 years), of whom 32 had a SARS-CoV-2 infection as a triggering event. Other triggers included Epstein-Barr virus and other infections.
Preliminary findings from the projects indicate that optimized management with outpatient and follow-up care can yield positive, sometimes lasting effects. No significant differences between SARS-CoV-2 and other triggers emerged, but pain proved more manageable in the SHARK group than in the TIGER group, suggesting they may involve different pathological mechanisms.
Hope for Improved Outcomes
“It’s important to move away from the idea that nothing can be done,” Behrends said. This is a common attitude with children and adolescents displaying these types of symptoms, but it’s simply not true. “Even in pediatrics, we have numerous therapeutic options that may offer relief, from medication to psychosocial interventions.”
This story was translated from Medscape’s German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Post-Exertional Malaise in Fatiguing Diseases: What to Know to Avoid Harmful Exercise
Identifying the phenomenon of post-exertional malaise (PEM) in patients with fatiguing conditions is critical because it necessitates a far more cautious approach to exercise, experts said.
PEM is a defining feature of the condition myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), and it is present in many people with long COVID. It is characterized by a worsening of fatigue and of other symptoms after previously tolerated physical or mental exertion, typically emerging 24-72 hours after the exertion and lasting days or weeks thereafter. The experience is often called a “crash.”
In a study presented at the American College of Rheumatology (ACR) 2024 Annual Meeting, PEM was also identified in people with various rheumatologic conditions, ranging from 4% in those with osteoarthritis to 20% in those with fibromyalgia. The presence of PEM was also associated with worse pain, sleep, cognition, and other symptoms that are also characteristic of ME/CFS and many cases of long COVID.
“PEM assessment is becoming more important in those with long COVID, as we are assisting more of those with long durations of this condition. ... This is the first study we know of presenting PEM rates in a rheumatologic disease population,” Kaleb Michaud, PhD, director of FORWARD — The National Databank for Rheumatic Diseases and professor of rheumatology and immunology, University of Nebraska Medical Center, Omaha, said at the meeting.
During the discussion period, study investigator Leonard H. Calabrese, DO, head of the Section of Clinical Immunology, Cleveland Clinic, Ohio, commented, “PEM is seen with numerous post-acute infectious sequelae. It segregates with that population of patients who meet the diagnostic criteria for ME/CFS, of which 50%-70% of people will also meet criteria for fibromyalgia…This is a first step, but it has big ramifications regarding exercise.”
In an interview with this news organization, Calabrese said, “We recommend exercise to virtually everyone with fibromyalgia who doesn’t have ME/CFS,” but that the assessment tool used in the study, the 5-item DePaul Symptoms Questionnaire, isn’t adequate for assessing true PEM that would preclude exercise, despite being validated. “That instrument is inexact and lacks specificity. ... It just shows where the field is. We need better biomarkers.”
In Those With PEM, Exercise May Harm
Asked to comment, Brayden P. Yellman, MD, a rheumatologist at the Bateman Horne Center, Salt Lake City, Utah, told this news organization, “if there is an infection-associated chronic condition that meets criteria for what we would call ME/CFS or long COVID, and if there’s true post-exertional malaise, any graded exercise that ultimately leads to post-exertional malaise is harmful. ... There is a subset of people who have milder disease, who can sometimes do very mild exercise that does not trigger PEM, and they do see benefits over time very slowly with really carefully curated, carefully monitored exercise. But we have to be really careful.”
For the majority, however, the approach is to teach patients to pace their activities in order to avoid PEM, also referred to as staying within their “energy envelope.” Clinician resources are available on the Bateman Horne Center’s website.
This isn’t typically included in rheumatology training, Yellman noted. “Having completed an entire rheumatology fellowship and working in rheumatology, I was not taught at all about [then-termed] chronic fatigue syndrome. It was lumped under fibromyalgia. And of course, they teach about fibromyalgia because it’s a great mimic of a lot of inflammatory, rheumatologic conditions, but the idea of [PEM], that pathognomonic feature that we see in infection-associated chronic conditions, was not once mentioned when I trained, in 2014 to 2016.”
Nonetheless, he added, “rheumatologists are definitely seeing this in their fibromyalgia patients and some of their other patients at a high rate, and I’m sure that they’re missing it, along with other comorbidities like orthostatic hypotension.”
Another expert asked to weigh in, Todd Davenport, PT, DPT, PhD, professor and chair of the Department of Physical Therapy at the University of the Pacific, Stockton, California, told this news organization: “Our experience is that the body’s responses to short bouts of exercise are abnormal, and graded exercise is unsuccessful and makes people worse. ... Clinicians should be particularly on the lookout for PEM in patients who are already reporting fatigue, such as with fibromyalgia and rheumatologic conditions that can have some diagnostic overlaps with ME/CFS, because you can get fooled into thinking that your well-meaning exercise program intended to help give them a little more juice during their daily activities actually might be harmful.”
There are several lines of evidence for abnormal responses to exercise in people with PEM, Davenport said. These include muscle worsening, cardiac preload failure and impaired systemic oxygen extraction, metabolic dysregulation, and abnormal immunologic and neurologic changes.
Several studies show impaired recovery after 2-day cardiopulmonary exercise testing, with the largest to date published in July 2024. Patients with PEM have also reported harm from prescribed exercise.
Yellman commented: “We think of PEM like an injury, where you need to recover. If you keep stacking injuries on top of it, that injury is never going to heal the same way again…We are still trying to understand the pathophysiology of ME/CFS in general, and of PEM. But if you think of it as a neuroinflammatory injury, and there’s some evidence suggesting neuroinflammation, you can kind of understand the approach of needing to heal and to recover.”
How Prevalent Is PEM in Rheumatologic Conditions?
For the study presented at the ACR meeting, data of people with confirmed rheumatic diseases were taken from the ongoing longitudinal US-based research database FORWARD. Participants completed biannual self-reported questionnaires during January–June 2024 that included the 5-item PEM subscale from the validated DePaul Symptoms Questionnaire.
Questions relate to frequency and severity of each of the five items: “Dead, heavy feeling after starting to exercise,” “next-day soreness or fatigue after nonstrenuous, everyday activities,” “mentally tired after the slightest effort,” “minimum exercise makes you physically tired,” and “physically drained or sick after mild activity.” Participants are asked to rate each item on a scale from 0 if not present to 1 (mild/a little of the time) up to 4 (very severe/all of the time).
A positive PEM result was defined as a frequency of at least two and simultaneous severity of at least two on any survey item. Additional questions asked about recent and previous SARS-CoV-2 infections, long COVID diagnoses, and comorbidities.
Of 1158 individuals who completed the PEM questionnaire, 7.5% overall met PEM criteria. By individual condition, the proportions were 4.4% with osteoarthritis, 7.4% with rheumatoid arthritis, 12.2% with systemic lupus erythematosus, 13.8% with fibromyalgia diagnosed by rheumatologists, and 20.3% with fibromyalgia based on the 2016 revised ACR criteria.
The overall PEM prevalence was 8.3% among those reporting ever having COVID-19 and 9.5% among those who had COVID-19 during July–December 2023. The PEM prevalence increased more dramatically with more severe COVID-19 — 17.2% among those who had been hospitalized for COVID-19, 22.0% of those ever diagnosed with long COVID, and 28.1% with a long COVID diagnosis in January 2024.
By diagnosis, 50% of individuals who met the ACR’s 2016 fibromyalgia criteria and currently had long COVID scored positively for PEM.
Measures of pain, fatigue, sleep, patient global assessment, activity score, polysymptomatic distress, disability, depression, anxiety, and other functional scores were all significantly worse among those scoring positive for PEM (P < .001), Michaud reported.
Better Tools Are Available
The developer of the DePaul questionnaire, Leonard Jason, PhD, director of the Center for Community Research and professor of psychology at DePaul College of Science and Health, Chicago, Illinois, told this news organization that an updated 10-item screening tool specifically designed to screen for PEM adds some important elements missing from the 5-item version.
Here, patients are initially asked two questions: “Do you experience a worsening of your fatigue/energy related illness after engaging in minimal physical effort?” and “Do you experience a worsening of your fatigue/energy related illness after engaging in mental effort?” If they answer “yes” to either, the next question is “If you feel worse after activities, how long does this last?” Answers are coded from 0 to 6 (24 hours or more).
The fourth additional question then asks how quickly patients recover, while a fifth question asks whether the person is avoiding activity because it makes them feel worse (thereby potentially creating a false negative).
For those scoring positive on the 10-item screen, a more comprehensive measure could be used, such as this online screening tool, Jason said.
Yellman said that the Bateman Horne Center uses a “good day, bad day” questionnaire to tease out some of the same information. In addition, he noted that it’s important to capture the timeframe between the exertion and the onset of symptoms because PEM doesn’t start during or immediately after activity. “If somebody is mowing the lawn and they start feeling symptoms immediately, they’re probably, at least in ME/CFS, experiencing orthostatic intolerance. Post-exertional malaise occurs 12-72 hours later, when their function is severely reduced as compared to baseline.”
And of course, Davenport noted, listening to patients is key. “Patients will tell you wildly unusual responses to activity before you even do the work of trying to figure out what the activity was. They’ll tell you things like they can’t think as well, that they have to be in bed for 3 days to a week to 2 weeks, depending on the level of exertion.”
Yellman, Davenport, and several other colleagues are currently working on a paper that will explain the differences between pacing and graded exercise, define PEM, and provide guidelines. They aim to submit it in time for publication early in 2025. In the meantime, the Bateman Horne Center’s website provides numerous resources for healthcare professionals and patients.
Yellman is also working to define minimum quality of care standards for infection-associated chronic conditions for state medical boards and to provide continuing medical education for clinicians on those standards. These would include recognizing and evaluating patients for PEM, as well as orthostatic intolerance, cognitive impairment, and other associated comorbidities.
Importantly, he said, the standards will include the principles of teaching people with PEM how to pace and will emphasize not prescribing them graded exercise as first- or even second-line therapy. “We need people to do some basic things. And the first thing is do no harm.”
None of the individuals quoted for this article had relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Identifying the phenomenon of post-exertional malaise (PEM) in patients with fatiguing conditions is critical because it necessitates a far more cautious approach to exercise, experts said.
PEM is a defining feature of the condition myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), and it is present in many people with long COVID. It is characterized by a worsening of fatigue and of other symptoms after previously tolerated physical or mental exertion, typically emerging 24-72 hours after the exertion and lasting days or weeks thereafter. The experience is often called a “crash.”
In a study presented at the American College of Rheumatology (ACR) 2024 Annual Meeting, PEM was also identified in people with various rheumatologic conditions, ranging from 4% in those with osteoarthritis to 20% in those with fibromyalgia. The presence of PEM was also associated with worse pain, sleep, cognition, and other symptoms that are also characteristic of ME/CFS and many cases of long COVID.
“PEM assessment is becoming more important in those with long COVID, as we are assisting more of those with long durations of this condition. ... This is the first study we know of presenting PEM rates in a rheumatologic disease population,” Kaleb Michaud, PhD, director of FORWARD — The National Databank for Rheumatic Diseases and professor of rheumatology and immunology, University of Nebraska Medical Center, Omaha, said at the meeting.
During the discussion period, study investigator Leonard H. Calabrese, DO, head of the Section of Clinical Immunology, Cleveland Clinic, Ohio, commented, “PEM is seen with numerous post-acute infectious sequelae. It segregates with that population of patients who meet the diagnostic criteria for ME/CFS, of which 50%-70% of people will also meet criteria for fibromyalgia…This is a first step, but it has big ramifications regarding exercise.”
In an interview with this news organization, Calabrese said, “We recommend exercise to virtually everyone with fibromyalgia who doesn’t have ME/CFS,” but that the assessment tool used in the study, the 5-item DePaul Symptoms Questionnaire, isn’t adequate for assessing true PEM that would preclude exercise, despite being validated. “That instrument is inexact and lacks specificity. ... It just shows where the field is. We need better biomarkers.”
In Those With PEM, Exercise May Harm
Asked to comment, Brayden P. Yellman, MD, a rheumatologist at the Bateman Horne Center, Salt Lake City, Utah, told this news organization, “if there is an infection-associated chronic condition that meets criteria for what we would call ME/CFS or long COVID, and if there’s true post-exertional malaise, any graded exercise that ultimately leads to post-exertional malaise is harmful. ... There is a subset of people who have milder disease, who can sometimes do very mild exercise that does not trigger PEM, and they do see benefits over time very slowly with really carefully curated, carefully monitored exercise. But we have to be really careful.”
For the majority, however, the approach is to teach patients to pace their activities in order to avoid PEM, also referred to as staying within their “energy envelope.” Clinician resources are available on the Bateman Horne Center’s website.
This isn’t typically included in rheumatology training, Yellman noted. “Having completed an entire rheumatology fellowship and working in rheumatology, I was not taught at all about [then-termed] chronic fatigue syndrome. It was lumped under fibromyalgia. And of course, they teach about fibromyalgia because it’s a great mimic of a lot of inflammatory, rheumatologic conditions, but the idea of [PEM], that pathognomonic feature that we see in infection-associated chronic conditions, was not once mentioned when I trained, in 2014 to 2016.”
Nonetheless, he added, “rheumatologists are definitely seeing this in their fibromyalgia patients and some of their other patients at a high rate, and I’m sure that they’re missing it, along with other comorbidities like orthostatic hypotension.”
Another expert asked to weigh in, Todd Davenport, PT, DPT, PhD, professor and chair of the Department of Physical Therapy at the University of the Pacific, Stockton, California, told this news organization: “Our experience is that the body’s responses to short bouts of exercise are abnormal, and graded exercise is unsuccessful and makes people worse. ... Clinicians should be particularly on the lookout for PEM in patients who are already reporting fatigue, such as with fibromyalgia and rheumatologic conditions that can have some diagnostic overlaps with ME/CFS, because you can get fooled into thinking that your well-meaning exercise program intended to help give them a little more juice during their daily activities actually might be harmful.”
There are several lines of evidence for abnormal responses to exercise in people with PEM, Davenport said. These include muscle worsening, cardiac preload failure and impaired systemic oxygen extraction, metabolic dysregulation, and abnormal immunologic and neurologic changes.
Several studies show impaired recovery after 2-day cardiopulmonary exercise testing, with the largest to date published in July 2024. Patients with PEM have also reported harm from prescribed exercise.
Yellman commented: “We think of PEM like an injury, where you need to recover. If you keep stacking injuries on top of it, that injury is never going to heal the same way again…We are still trying to understand the pathophysiology of ME/CFS in general, and of PEM. But if you think of it as a neuroinflammatory injury, and there’s some evidence suggesting neuroinflammation, you can kind of understand the approach of needing to heal and to recover.”
How Prevalent Is PEM in Rheumatologic Conditions?
For the study presented at the ACR meeting, data of people with confirmed rheumatic diseases were taken from the ongoing longitudinal US-based research database FORWARD. Participants completed biannual self-reported questionnaires during January–June 2024 that included the 5-item PEM subscale from the validated DePaul Symptoms Questionnaire.
Questions relate to frequency and severity of each of the five items: “Dead, heavy feeling after starting to exercise,” “next-day soreness or fatigue after nonstrenuous, everyday activities,” “mentally tired after the slightest effort,” “minimum exercise makes you physically tired,” and “physically drained or sick after mild activity.” Participants are asked to rate each item on a scale from 0 if not present to 1 (mild/a little of the time) up to 4 (very severe/all of the time).
A positive PEM result was defined as a frequency of at least two and simultaneous severity of at least two on any survey item. Additional questions asked about recent and previous SARS-CoV-2 infections, long COVID diagnoses, and comorbidities.
Of 1158 individuals who completed the PEM questionnaire, 7.5% overall met PEM criteria. By individual condition, the proportions were 4.4% with osteoarthritis, 7.4% with rheumatoid arthritis, 12.2% with systemic lupus erythematosus, 13.8% with fibromyalgia diagnosed by rheumatologists, and 20.3% with fibromyalgia based on the 2016 revised ACR criteria.
The overall PEM prevalence was 8.3% among those reporting ever having COVID-19 and 9.5% among those who had COVID-19 during July–December 2023. The PEM prevalence increased more dramatically with more severe COVID-19 — 17.2% among those who had been hospitalized for COVID-19, 22.0% of those ever diagnosed with long COVID, and 28.1% with a long COVID diagnosis in January 2024.
By diagnosis, 50% of individuals who met the ACR’s 2016 fibromyalgia criteria and currently had long COVID scored positively for PEM.
Measures of pain, fatigue, sleep, patient global assessment, activity score, polysymptomatic distress, disability, depression, anxiety, and other functional scores were all significantly worse among those scoring positive for PEM (P < .001), Michaud reported.
Better Tools Are Available
The developer of the DePaul questionnaire, Leonard Jason, PhD, director of the Center for Community Research and professor of psychology at DePaul College of Science and Health, Chicago, Illinois, told this news organization that an updated 10-item screening tool specifically designed to screen for PEM adds some important elements missing from the 5-item version.
Here, patients are initially asked two questions: “Do you experience a worsening of your fatigue/energy related illness after engaging in minimal physical effort?” and “Do you experience a worsening of your fatigue/energy related illness after engaging in mental effort?” If they answer “yes” to either, the next question is “If you feel worse after activities, how long does this last?” Answers are coded from 0 to 6 (24 hours or more).
The fourth additional question then asks how quickly patients recover, while a fifth question asks whether the person is avoiding activity because it makes them feel worse (thereby potentially creating a false negative).
For those scoring positive on the 10-item screen, a more comprehensive measure could be used, such as this online screening tool, Jason said.
Yellman said that the Bateman Horne Center uses a “good day, bad day” questionnaire to tease out some of the same information. In addition, he noted that it’s important to capture the timeframe between the exertion and the onset of symptoms because PEM doesn’t start during or immediately after activity. “If somebody is mowing the lawn and they start feeling symptoms immediately, they’re probably, at least in ME/CFS, experiencing orthostatic intolerance. Post-exertional malaise occurs 12-72 hours later, when their function is severely reduced as compared to baseline.”
And of course, Davenport noted, listening to patients is key. “Patients will tell you wildly unusual responses to activity before you even do the work of trying to figure out what the activity was. They’ll tell you things like they can’t think as well, that they have to be in bed for 3 days to a week to 2 weeks, depending on the level of exertion.”
Yellman, Davenport, and several other colleagues are currently working on a paper that will explain the differences between pacing and graded exercise, define PEM, and provide guidelines. They aim to submit it in time for publication early in 2025. In the meantime, the Bateman Horne Center’s website provides numerous resources for healthcare professionals and patients.
Yellman is also working to define minimum quality of care standards for infection-associated chronic conditions for state medical boards and to provide continuing medical education for clinicians on those standards. These would include recognizing and evaluating patients for PEM, as well as orthostatic intolerance, cognitive impairment, and other associated comorbidities.
Importantly, he said, the standards will include the principles of teaching people with PEM how to pace and will emphasize not prescribing them graded exercise as first- or even second-line therapy. “We need people to do some basic things. And the first thing is do no harm.”
None of the individuals quoted for this article had relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Identifying the phenomenon of post-exertional malaise (PEM) in patients with fatiguing conditions is critical because it necessitates a far more cautious approach to exercise, experts said.
PEM is a defining feature of the condition myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), and it is present in many people with long COVID. It is characterized by a worsening of fatigue and of other symptoms after previously tolerated physical or mental exertion, typically emerging 24-72 hours after the exertion and lasting days or weeks thereafter. The experience is often called a “crash.”
In a study presented at the American College of Rheumatology (ACR) 2024 Annual Meeting, PEM was also identified in people with various rheumatologic conditions, ranging from 4% in those with osteoarthritis to 20% in those with fibromyalgia. The presence of PEM was also associated with worse pain, sleep, cognition, and other symptoms that are also characteristic of ME/CFS and many cases of long COVID.
“PEM assessment is becoming more important in those with long COVID, as we are assisting more of those with long durations of this condition. ... This is the first study we know of presenting PEM rates in a rheumatologic disease population,” Kaleb Michaud, PhD, director of FORWARD — The National Databank for Rheumatic Diseases and professor of rheumatology and immunology, University of Nebraska Medical Center, Omaha, said at the meeting.
During the discussion period, study investigator Leonard H. Calabrese, DO, head of the Section of Clinical Immunology, Cleveland Clinic, Ohio, commented, “PEM is seen with numerous post-acute infectious sequelae. It segregates with that population of patients who meet the diagnostic criteria for ME/CFS, of which 50%-70% of people will also meet criteria for fibromyalgia…This is a first step, but it has big ramifications regarding exercise.”
In an interview with this news organization, Calabrese said, “We recommend exercise to virtually everyone with fibromyalgia who doesn’t have ME/CFS,” but that the assessment tool used in the study, the 5-item DePaul Symptoms Questionnaire, isn’t adequate for assessing true PEM that would preclude exercise, despite being validated. “That instrument is inexact and lacks specificity. ... It just shows where the field is. We need better biomarkers.”
In Those With PEM, Exercise May Harm
Asked to comment, Brayden P. Yellman, MD, a rheumatologist at the Bateman Horne Center, Salt Lake City, Utah, told this news organization, “if there is an infection-associated chronic condition that meets criteria for what we would call ME/CFS or long COVID, and if there’s true post-exertional malaise, any graded exercise that ultimately leads to post-exertional malaise is harmful. ... There is a subset of people who have milder disease, who can sometimes do very mild exercise that does not trigger PEM, and they do see benefits over time very slowly with really carefully curated, carefully monitored exercise. But we have to be really careful.”
For the majority, however, the approach is to teach patients to pace their activities in order to avoid PEM, also referred to as staying within their “energy envelope.” Clinician resources are available on the Bateman Horne Center’s website.
This isn’t typically included in rheumatology training, Yellman noted. “Having completed an entire rheumatology fellowship and working in rheumatology, I was not taught at all about [then-termed] chronic fatigue syndrome. It was lumped under fibromyalgia. And of course, they teach about fibromyalgia because it’s a great mimic of a lot of inflammatory, rheumatologic conditions, but the idea of [PEM], that pathognomonic feature that we see in infection-associated chronic conditions, was not once mentioned when I trained, in 2014 to 2016.”
Nonetheless, he added, “rheumatologists are definitely seeing this in their fibromyalgia patients and some of their other patients at a high rate, and I’m sure that they’re missing it, along with other comorbidities like orthostatic hypotension.”
Another expert asked to weigh in, Todd Davenport, PT, DPT, PhD, professor and chair of the Department of Physical Therapy at the University of the Pacific, Stockton, California, told this news organization: “Our experience is that the body’s responses to short bouts of exercise are abnormal, and graded exercise is unsuccessful and makes people worse. ... Clinicians should be particularly on the lookout for PEM in patients who are already reporting fatigue, such as with fibromyalgia and rheumatologic conditions that can have some diagnostic overlaps with ME/CFS, because you can get fooled into thinking that your well-meaning exercise program intended to help give them a little more juice during their daily activities actually might be harmful.”
There are several lines of evidence for abnormal responses to exercise in people with PEM, Davenport said. These include muscle worsening, cardiac preload failure and impaired systemic oxygen extraction, metabolic dysregulation, and abnormal immunologic and neurologic changes.
Several studies show impaired recovery after 2-day cardiopulmonary exercise testing, with the largest to date published in July 2024. Patients with PEM have also reported harm from prescribed exercise.
Yellman commented: “We think of PEM like an injury, where you need to recover. If you keep stacking injuries on top of it, that injury is never going to heal the same way again…We are still trying to understand the pathophysiology of ME/CFS in general, and of PEM. But if you think of it as a neuroinflammatory injury, and there’s some evidence suggesting neuroinflammation, you can kind of understand the approach of needing to heal and to recover.”
How Prevalent Is PEM in Rheumatologic Conditions?
For the study presented at the ACR meeting, data of people with confirmed rheumatic diseases were taken from the ongoing longitudinal US-based research database FORWARD. Participants completed biannual self-reported questionnaires during January–June 2024 that included the 5-item PEM subscale from the validated DePaul Symptoms Questionnaire.
Questions relate to frequency and severity of each of the five items: “Dead, heavy feeling after starting to exercise,” “next-day soreness or fatigue after nonstrenuous, everyday activities,” “mentally tired after the slightest effort,” “minimum exercise makes you physically tired,” and “physically drained or sick after mild activity.” Participants are asked to rate each item on a scale from 0 if not present to 1 (mild/a little of the time) up to 4 (very severe/all of the time).
A positive PEM result was defined as a frequency of at least two and simultaneous severity of at least two on any survey item. Additional questions asked about recent and previous SARS-CoV-2 infections, long COVID diagnoses, and comorbidities.
Of 1158 individuals who completed the PEM questionnaire, 7.5% overall met PEM criteria. By individual condition, the proportions were 4.4% with osteoarthritis, 7.4% with rheumatoid arthritis, 12.2% with systemic lupus erythematosus, 13.8% with fibromyalgia diagnosed by rheumatologists, and 20.3% with fibromyalgia based on the 2016 revised ACR criteria.
The overall PEM prevalence was 8.3% among those reporting ever having COVID-19 and 9.5% among those who had COVID-19 during July–December 2023. The PEM prevalence increased more dramatically with more severe COVID-19 — 17.2% among those who had been hospitalized for COVID-19, 22.0% of those ever diagnosed with long COVID, and 28.1% with a long COVID diagnosis in January 2024.
By diagnosis, 50% of individuals who met the ACR’s 2016 fibromyalgia criteria and currently had long COVID scored positively for PEM.
Measures of pain, fatigue, sleep, patient global assessment, activity score, polysymptomatic distress, disability, depression, anxiety, and other functional scores were all significantly worse among those scoring positive for PEM (P < .001), Michaud reported.
Better Tools Are Available
The developer of the DePaul questionnaire, Leonard Jason, PhD, director of the Center for Community Research and professor of psychology at DePaul College of Science and Health, Chicago, Illinois, told this news organization that an updated 10-item screening tool specifically designed to screen for PEM adds some important elements missing from the 5-item version.
Here, patients are initially asked two questions: “Do you experience a worsening of your fatigue/energy related illness after engaging in minimal physical effort?” and “Do you experience a worsening of your fatigue/energy related illness after engaging in mental effort?” If they answer “yes” to either, the next question is “If you feel worse after activities, how long does this last?” Answers are coded from 0 to 6 (24 hours or more).
The fourth additional question then asks how quickly patients recover, while a fifth question asks whether the person is avoiding activity because it makes them feel worse (thereby potentially creating a false negative).
For those scoring positive on the 10-item screen, a more comprehensive measure could be used, such as this online screening tool, Jason said.
Yellman said that the Bateman Horne Center uses a “good day, bad day” questionnaire to tease out some of the same information. In addition, he noted that it’s important to capture the timeframe between the exertion and the onset of symptoms because PEM doesn’t start during or immediately after activity. “If somebody is mowing the lawn and they start feeling symptoms immediately, they’re probably, at least in ME/CFS, experiencing orthostatic intolerance. Post-exertional malaise occurs 12-72 hours later, when their function is severely reduced as compared to baseline.”
And of course, Davenport noted, listening to patients is key. “Patients will tell you wildly unusual responses to activity before you even do the work of trying to figure out what the activity was. They’ll tell you things like they can’t think as well, that they have to be in bed for 3 days to a week to 2 weeks, depending on the level of exertion.”
Yellman, Davenport, and several other colleagues are currently working on a paper that will explain the differences between pacing and graded exercise, define PEM, and provide guidelines. They aim to submit it in time for publication early in 2025. In the meantime, the Bateman Horne Center’s website provides numerous resources for healthcare professionals and patients.
Yellman is also working to define minimum quality of care standards for infection-associated chronic conditions for state medical boards and to provide continuing medical education for clinicians on those standards. These would include recognizing and evaluating patients for PEM, as well as orthostatic intolerance, cognitive impairment, and other associated comorbidities.
Importantly, he said, the standards will include the principles of teaching people with PEM how to pace and will emphasize not prescribing them graded exercise as first- or even second-line therapy. “We need people to do some basic things. And the first thing is do no harm.”
None of the individuals quoted for this article had relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM ACR 2024
Managing Return-to-Work Barriers for People With Long COVID
Although some patients experience symptoms so severe that they cannot work under any conditions, medical providers and employers can help ensure many patients with long COVID can stay in the workforce.
Long COVID is an infection-associated chronic condition that occurs after SARS-CoV-2 infection and is present for at least 3 months as a continuous, relapsing and remitting, or progressive disease state that affects one or more organ systems. By the end of 2023, at least 400 million people worldwide were estimated to have long COVID.
As members of the Patient-Led Research Collaborative, an international group of more than 60 researchers and health advocates living with long COVID and other infection-associated chronic conditions, we have published one of the first research studies of people with long COVID and their desire to work, the specific needs they have, and what doctors and employers can do to create a path for returning to the workforce.
In our recent paper, we document the barriers and facilitators that individuals living with long COVID experience when attempting to return to work. Our recommendations are based on these findings and include recommendations for both medical providers and employers.
If you are a medical provider:
- Ensure you adequately document your patients’ COVID cases, any long COVID diagnoses, and the functional impairment that long COVID causes. Remember that you can diagnose a patient with long COVID on the basis of their symptoms, and clinical guidelines do not require a record of a positive COVID-19 test, which many patients lack owing to testing barriers.
- Keep up to date on research on long COVID and related infection-associated chronic conditions — for example, through the Project ECHO Long COVID and Fatiguing Illness Recovery Program — and learn about pacing and other treatment options.
If you are an employer:
- Utilize a return-to-work model in which any worker with suspected or confirmed COVID discusses support they may need with their employer when they return to work, with additional check-in dates scheduled to reevaluate supports as needed. Planning for this collaborative and iterative evaluation of return-to-work supports for all workers with COVID-19 is important because it may not be immediately clear to a worker whether they have developed long COVID or are generally recuperating from the illness.
- Do not require medical documentation of a SARS-CoV-2 infection or a Long COVID diagnosis to access accommodations — this is owing to disparities in accessing documentation.
- Tailor job responsibilities, provide remote options, allow flexible hours, and provide longer-range deadlines to account for symptoms for people with long COVID and other infection-associated chronic conditions.
- Provide accommodations to any caregivers of people with long COVID in your workplace.
- If requiring in-person work, make the workplace as safe as possible through ventilation and masking requirements, which will help ensure fewer of your workers develop long COVID, and those already with infection-associated chronic conditions will not get worse.
Our findings and recommendations are specific to long COVID, but they can and should apply to other disabilities. Given that our study’s sample was predominately White and working in jobs that did not require substantial physical labor, additional recommendations may be needed for other populations and workers who have labor-intensive jobs.
510 Study Participants
Long COVID is characterized as a relapsing-remitting illness, often described as episodic, in which an individual’s symptoms may fluctuate. Symptoms can become more or less severe depending on tasks, exertion, and social support in addition to physiologic processes and medical intervention. In our paper, we illustrate how the long COVID return-to-work experience and individuals’ symptoms can be shaped by workplace, home, and medical environments.
We randomly selected 510 participants from a global survey of people living with long COVID and systematically analyzed their open-ended responses using established qualitative analysis methods. In this study, we specifically analyzed what patients wrote about their return-to-work experiences, considering how work experiences and relapsing and remitting long COVID symptoms intersected with personal lives and medical care.
Most of the study participants identified as White, were 30-60 years old (ie, in their key earning years), and had at least a baccalaureate degree. Participants lived in the United States (38%), United Kingdom (25%), continental Europe (8%), Canada (4%), or other countries (25%). Most participants worked in professions that did not require substantial physical labor, and individuals in those fields may experience even greater return-to-work barriers than are reported in this study.
Key Findings
Through our qualitative analysis, we identified four primary return-to-work themes:
1. People living with long COVID have a strong desire and financial need to return to work.
The participants in our study described how they had experienced financial hardship because they could not successfully return to work and may have incurred new expenses with long COVID. They also often wrote how they wanted to return to work because their jobs provided meaning and structure for their lives. Some people in this study shared how they had tried to return to their jobs but relapsed. As a result, they considered leaving the workforce.
2. Workers’ long COVID symptoms intersect with organization of work and home life.
Most of the people in our study were employed in positions that did not require substantial physical labor. Even so, workers described how their long COVID symptoms were exacerbated by some job tasks. Computer screen time; reading dense material or writing (including emails); and conversations and meetings, regardless of whether they were in-person or via phone or video conferencing, could trigger or make symptoms worse. Workers who needed to stand for long periods of time, such as teachers and healthcare workers, and workers who needed to do lifting as part of their jobs described how these requirements were too taxing and could lead to relapses.
Because of the relapsing and remitting nature of many long COVID symptoms, people reported how it could be difficult to predict how job tasks, long hours, or pressing deadlines may exacerbate symptoms, which would require them to take time off work. For these participants, “pushing through” symptoms only made the symptoms worse. However, people in the study who were allowed to work from home reported how pacing, elevating their legs, and conserving energy (especially by not commuting) was key to doing their jobs well.
Some people in the study described how they were only able to return to work because they had substantial support from family or partners at home. These individuals shared how the people they lived with did most of the cooking, cleaning, and other household tasks so that the person living with long COVID could conserve their energy for work. This reorganization of home life notably shifted household tasks and caregiving to other people in the household, but without this shift, the individual’s long COVID symptoms may be too severe to work.
3. People with long COVID experience disbelief and stigma at work and healthcare settings.
Some people in our study described how their colleagues, supervisors, and human resource managers insinuated that they were fabricating or exaggerating their symptoms. This made it hard for workers to communicate what support they needed and could limit access to necessary work accommodations.
Many people in our study also described how medical providers did not believe that they had long COVID despite experiencing debilitating symptoms, often because they did not have a positive COVID-19 test to prove they had had an acute infection. Many people with long COVID may not have a positive COVID-19 test because:
- They could not access a test because testing access was limited at the start of the COVID-19 pandemic, there are transportation and cost barriers to tests, many health insurance providers no longer cover tests; and there are fewer public testing sites since the World Health Organization declared an end to the public health emergency;
- There is a high probability of false-negative results for viral and antibody tests (especially during the first wave of the pandemic and for individuals with limited immune response); and
- People can develop long COVID after asymptomatic acute infection.
Although healthcare providers can provide a long COVID diagnosis without a positive COVID-19 test on the basis of a patient’s presentation of symptoms and clinical history, many people in our study said that their providers would not provide this diagnosis, which restricted access to worker’s compensation, paid time off, and job accommodations.
Many people in the study also reported that their medical providers misdiagnosed them with a mental health disorder, such as anxiety, instead of long COVID. Although some people with long COVID may experience poor mental health as a natural consequence of dealing with a debilitating medical condition or may have neuropsychiatric symptoms as part of their long COVID, long COVID is not caused by an underlying psychiatric illness.
4. Support of medical providers is key to successful return to work for people living with long COVID.
Some people in our study described how they were able to get workplace accommodations or access workers’ compensation or sick leave because their medical providers recognized they had long COVID and provided them with this documentation. Some of these participants did not have a positive COVID-19 test, but their medical providers were able to diagnose them with long COVID on the basis of symptom presentation and clinical history. This documentation was critical for helping workers remain financially stable and able to return to work.
Conclusion
While we continue to search for treatment and cures for long COVID and work to provide a robust social safety net, it is crucial to address the stigma, inaccessibility, and lack of support often experienced by patients in their workplaces. Disabled people have long faced these issues; long COVID may be an opportunity to revolutionize the workplace to ensure an inclusive and accessible environment that can improve the lives of all workers.
For more on how to best be inclusive of employees with long COVID, read Harvard Business Review’s “Long Covid at Work: A Manager’s Guide” and visit the Job Accommodation Network webpage dedicated to long COVID.
Additional discussion about our study and applying the findings to improve work and medical care can be found by listening to the Healthy Work podcast episode titled “Supporting Long COVID at Work.”
Elisabeth Stelson, Gina Assaf, and Lisa McCorkell are members of the Patient-Led Research Collaborative, an international group of more than 60 researchers. Dr Stelson, Postdoctoral Research Fellow, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, has disclosed no relevant financial relationships. Gina Assaf is Research Lead, Patient-Led Research Collaborative, Washington, DC. Lisa McCorkell is a long COVID patient; Cofounder, Team Lead, Researcher, Patient-Led Research Collaborative, Washington, DC.
A version of this article appeared on Medscape.com.
Although some patients experience symptoms so severe that they cannot work under any conditions, medical providers and employers can help ensure many patients with long COVID can stay in the workforce.
Long COVID is an infection-associated chronic condition that occurs after SARS-CoV-2 infection and is present for at least 3 months as a continuous, relapsing and remitting, or progressive disease state that affects one or more organ systems. By the end of 2023, at least 400 million people worldwide were estimated to have long COVID.
As members of the Patient-Led Research Collaborative, an international group of more than 60 researchers and health advocates living with long COVID and other infection-associated chronic conditions, we have published one of the first research studies of people with long COVID and their desire to work, the specific needs they have, and what doctors and employers can do to create a path for returning to the workforce.
In our recent paper, we document the barriers and facilitators that individuals living with long COVID experience when attempting to return to work. Our recommendations are based on these findings and include recommendations for both medical providers and employers.
If you are a medical provider:
- Ensure you adequately document your patients’ COVID cases, any long COVID diagnoses, and the functional impairment that long COVID causes. Remember that you can diagnose a patient with long COVID on the basis of their symptoms, and clinical guidelines do not require a record of a positive COVID-19 test, which many patients lack owing to testing barriers.
- Keep up to date on research on long COVID and related infection-associated chronic conditions — for example, through the Project ECHO Long COVID and Fatiguing Illness Recovery Program — and learn about pacing and other treatment options.
If you are an employer:
- Utilize a return-to-work model in which any worker with suspected or confirmed COVID discusses support they may need with their employer when they return to work, with additional check-in dates scheduled to reevaluate supports as needed. Planning for this collaborative and iterative evaluation of return-to-work supports for all workers with COVID-19 is important because it may not be immediately clear to a worker whether they have developed long COVID or are generally recuperating from the illness.
- Do not require medical documentation of a SARS-CoV-2 infection or a Long COVID diagnosis to access accommodations — this is owing to disparities in accessing documentation.
- Tailor job responsibilities, provide remote options, allow flexible hours, and provide longer-range deadlines to account for symptoms for people with long COVID and other infection-associated chronic conditions.
- Provide accommodations to any caregivers of people with long COVID in your workplace.
- If requiring in-person work, make the workplace as safe as possible through ventilation and masking requirements, which will help ensure fewer of your workers develop long COVID, and those already with infection-associated chronic conditions will not get worse.
Our findings and recommendations are specific to long COVID, but they can and should apply to other disabilities. Given that our study’s sample was predominately White and working in jobs that did not require substantial physical labor, additional recommendations may be needed for other populations and workers who have labor-intensive jobs.
510 Study Participants
Long COVID is characterized as a relapsing-remitting illness, often described as episodic, in which an individual’s symptoms may fluctuate. Symptoms can become more or less severe depending on tasks, exertion, and social support in addition to physiologic processes and medical intervention. In our paper, we illustrate how the long COVID return-to-work experience and individuals’ symptoms can be shaped by workplace, home, and medical environments.
We randomly selected 510 participants from a global survey of people living with long COVID and systematically analyzed their open-ended responses using established qualitative analysis methods. In this study, we specifically analyzed what patients wrote about their return-to-work experiences, considering how work experiences and relapsing and remitting long COVID symptoms intersected with personal lives and medical care.
Most of the study participants identified as White, were 30-60 years old (ie, in their key earning years), and had at least a baccalaureate degree. Participants lived in the United States (38%), United Kingdom (25%), continental Europe (8%), Canada (4%), or other countries (25%). Most participants worked in professions that did not require substantial physical labor, and individuals in those fields may experience even greater return-to-work barriers than are reported in this study.
Key Findings
Through our qualitative analysis, we identified four primary return-to-work themes:
1. People living with long COVID have a strong desire and financial need to return to work.
The participants in our study described how they had experienced financial hardship because they could not successfully return to work and may have incurred new expenses with long COVID. They also often wrote how they wanted to return to work because their jobs provided meaning and structure for their lives. Some people in this study shared how they had tried to return to their jobs but relapsed. As a result, they considered leaving the workforce.
2. Workers’ long COVID symptoms intersect with organization of work and home life.
Most of the people in our study were employed in positions that did not require substantial physical labor. Even so, workers described how their long COVID symptoms were exacerbated by some job tasks. Computer screen time; reading dense material or writing (including emails); and conversations and meetings, regardless of whether they were in-person or via phone or video conferencing, could trigger or make symptoms worse. Workers who needed to stand for long periods of time, such as teachers and healthcare workers, and workers who needed to do lifting as part of their jobs described how these requirements were too taxing and could lead to relapses.
Because of the relapsing and remitting nature of many long COVID symptoms, people reported how it could be difficult to predict how job tasks, long hours, or pressing deadlines may exacerbate symptoms, which would require them to take time off work. For these participants, “pushing through” symptoms only made the symptoms worse. However, people in the study who were allowed to work from home reported how pacing, elevating their legs, and conserving energy (especially by not commuting) was key to doing their jobs well.
Some people in the study described how they were only able to return to work because they had substantial support from family or partners at home. These individuals shared how the people they lived with did most of the cooking, cleaning, and other household tasks so that the person living with long COVID could conserve their energy for work. This reorganization of home life notably shifted household tasks and caregiving to other people in the household, but without this shift, the individual’s long COVID symptoms may be too severe to work.
3. People with long COVID experience disbelief and stigma at work and healthcare settings.
Some people in our study described how their colleagues, supervisors, and human resource managers insinuated that they were fabricating or exaggerating their symptoms. This made it hard for workers to communicate what support they needed and could limit access to necessary work accommodations.
Many people in our study also described how medical providers did not believe that they had long COVID despite experiencing debilitating symptoms, often because they did not have a positive COVID-19 test to prove they had had an acute infection. Many people with long COVID may not have a positive COVID-19 test because:
- They could not access a test because testing access was limited at the start of the COVID-19 pandemic, there are transportation and cost barriers to tests, many health insurance providers no longer cover tests; and there are fewer public testing sites since the World Health Organization declared an end to the public health emergency;
- There is a high probability of false-negative results for viral and antibody tests (especially during the first wave of the pandemic and for individuals with limited immune response); and
- People can develop long COVID after asymptomatic acute infection.
Although healthcare providers can provide a long COVID diagnosis without a positive COVID-19 test on the basis of a patient’s presentation of symptoms and clinical history, many people in our study said that their providers would not provide this diagnosis, which restricted access to worker’s compensation, paid time off, and job accommodations.
Many people in the study also reported that their medical providers misdiagnosed them with a mental health disorder, such as anxiety, instead of long COVID. Although some people with long COVID may experience poor mental health as a natural consequence of dealing with a debilitating medical condition or may have neuropsychiatric symptoms as part of their long COVID, long COVID is not caused by an underlying psychiatric illness.
4. Support of medical providers is key to successful return to work for people living with long COVID.
Some people in our study described how they were able to get workplace accommodations or access workers’ compensation or sick leave because their medical providers recognized they had long COVID and provided them with this documentation. Some of these participants did not have a positive COVID-19 test, but their medical providers were able to diagnose them with long COVID on the basis of symptom presentation and clinical history. This documentation was critical for helping workers remain financially stable and able to return to work.
Conclusion
While we continue to search for treatment and cures for long COVID and work to provide a robust social safety net, it is crucial to address the stigma, inaccessibility, and lack of support often experienced by patients in their workplaces. Disabled people have long faced these issues; long COVID may be an opportunity to revolutionize the workplace to ensure an inclusive and accessible environment that can improve the lives of all workers.
For more on how to best be inclusive of employees with long COVID, read Harvard Business Review’s “Long Covid at Work: A Manager’s Guide” and visit the Job Accommodation Network webpage dedicated to long COVID.
Additional discussion about our study and applying the findings to improve work and medical care can be found by listening to the Healthy Work podcast episode titled “Supporting Long COVID at Work.”
Elisabeth Stelson, Gina Assaf, and Lisa McCorkell are members of the Patient-Led Research Collaborative, an international group of more than 60 researchers. Dr Stelson, Postdoctoral Research Fellow, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, has disclosed no relevant financial relationships. Gina Assaf is Research Lead, Patient-Led Research Collaborative, Washington, DC. Lisa McCorkell is a long COVID patient; Cofounder, Team Lead, Researcher, Patient-Led Research Collaborative, Washington, DC.
A version of this article appeared on Medscape.com.
Although some patients experience symptoms so severe that they cannot work under any conditions, medical providers and employers can help ensure many patients with long COVID can stay in the workforce.
Long COVID is an infection-associated chronic condition that occurs after SARS-CoV-2 infection and is present for at least 3 months as a continuous, relapsing and remitting, or progressive disease state that affects one or more organ systems. By the end of 2023, at least 400 million people worldwide were estimated to have long COVID.
As members of the Patient-Led Research Collaborative, an international group of more than 60 researchers and health advocates living with long COVID and other infection-associated chronic conditions, we have published one of the first research studies of people with long COVID and their desire to work, the specific needs they have, and what doctors and employers can do to create a path for returning to the workforce.
In our recent paper, we document the barriers and facilitators that individuals living with long COVID experience when attempting to return to work. Our recommendations are based on these findings and include recommendations for both medical providers and employers.
If you are a medical provider:
- Ensure you adequately document your patients’ COVID cases, any long COVID diagnoses, and the functional impairment that long COVID causes. Remember that you can diagnose a patient with long COVID on the basis of their symptoms, and clinical guidelines do not require a record of a positive COVID-19 test, which many patients lack owing to testing barriers.
- Keep up to date on research on long COVID and related infection-associated chronic conditions — for example, through the Project ECHO Long COVID and Fatiguing Illness Recovery Program — and learn about pacing and other treatment options.
If you are an employer:
- Utilize a return-to-work model in which any worker with suspected or confirmed COVID discusses support they may need with their employer when they return to work, with additional check-in dates scheduled to reevaluate supports as needed. Planning for this collaborative and iterative evaluation of return-to-work supports for all workers with COVID-19 is important because it may not be immediately clear to a worker whether they have developed long COVID or are generally recuperating from the illness.
- Do not require medical documentation of a SARS-CoV-2 infection or a Long COVID diagnosis to access accommodations — this is owing to disparities in accessing documentation.
- Tailor job responsibilities, provide remote options, allow flexible hours, and provide longer-range deadlines to account for symptoms for people with long COVID and other infection-associated chronic conditions.
- Provide accommodations to any caregivers of people with long COVID in your workplace.
- If requiring in-person work, make the workplace as safe as possible through ventilation and masking requirements, which will help ensure fewer of your workers develop long COVID, and those already with infection-associated chronic conditions will not get worse.
Our findings and recommendations are specific to long COVID, but they can and should apply to other disabilities. Given that our study’s sample was predominately White and working in jobs that did not require substantial physical labor, additional recommendations may be needed for other populations and workers who have labor-intensive jobs.
510 Study Participants
Long COVID is characterized as a relapsing-remitting illness, often described as episodic, in which an individual’s symptoms may fluctuate. Symptoms can become more or less severe depending on tasks, exertion, and social support in addition to physiologic processes and medical intervention. In our paper, we illustrate how the long COVID return-to-work experience and individuals’ symptoms can be shaped by workplace, home, and medical environments.
We randomly selected 510 participants from a global survey of people living with long COVID and systematically analyzed their open-ended responses using established qualitative analysis methods. In this study, we specifically analyzed what patients wrote about their return-to-work experiences, considering how work experiences and relapsing and remitting long COVID symptoms intersected with personal lives and medical care.
Most of the study participants identified as White, were 30-60 years old (ie, in their key earning years), and had at least a baccalaureate degree. Participants lived in the United States (38%), United Kingdom (25%), continental Europe (8%), Canada (4%), or other countries (25%). Most participants worked in professions that did not require substantial physical labor, and individuals in those fields may experience even greater return-to-work barriers than are reported in this study.
Key Findings
Through our qualitative analysis, we identified four primary return-to-work themes:
1. People living with long COVID have a strong desire and financial need to return to work.
The participants in our study described how they had experienced financial hardship because they could not successfully return to work and may have incurred new expenses with long COVID. They also often wrote how they wanted to return to work because their jobs provided meaning and structure for their lives. Some people in this study shared how they had tried to return to their jobs but relapsed. As a result, they considered leaving the workforce.
2. Workers’ long COVID symptoms intersect with organization of work and home life.
Most of the people in our study were employed in positions that did not require substantial physical labor. Even so, workers described how their long COVID symptoms were exacerbated by some job tasks. Computer screen time; reading dense material or writing (including emails); and conversations and meetings, regardless of whether they were in-person or via phone or video conferencing, could trigger or make symptoms worse. Workers who needed to stand for long periods of time, such as teachers and healthcare workers, and workers who needed to do lifting as part of their jobs described how these requirements were too taxing and could lead to relapses.
Because of the relapsing and remitting nature of many long COVID symptoms, people reported how it could be difficult to predict how job tasks, long hours, or pressing deadlines may exacerbate symptoms, which would require them to take time off work. For these participants, “pushing through” symptoms only made the symptoms worse. However, people in the study who were allowed to work from home reported how pacing, elevating their legs, and conserving energy (especially by not commuting) was key to doing their jobs well.
Some people in the study described how they were only able to return to work because they had substantial support from family or partners at home. These individuals shared how the people they lived with did most of the cooking, cleaning, and other household tasks so that the person living with long COVID could conserve their energy for work. This reorganization of home life notably shifted household tasks and caregiving to other people in the household, but without this shift, the individual’s long COVID symptoms may be too severe to work.
3. People with long COVID experience disbelief and stigma at work and healthcare settings.
Some people in our study described how their colleagues, supervisors, and human resource managers insinuated that they were fabricating or exaggerating their symptoms. This made it hard for workers to communicate what support they needed and could limit access to necessary work accommodations.
Many people in our study also described how medical providers did not believe that they had long COVID despite experiencing debilitating symptoms, often because they did not have a positive COVID-19 test to prove they had had an acute infection. Many people with long COVID may not have a positive COVID-19 test because:
- They could not access a test because testing access was limited at the start of the COVID-19 pandemic, there are transportation and cost barriers to tests, many health insurance providers no longer cover tests; and there are fewer public testing sites since the World Health Organization declared an end to the public health emergency;
- There is a high probability of false-negative results for viral and antibody tests (especially during the first wave of the pandemic and for individuals with limited immune response); and
- People can develop long COVID after asymptomatic acute infection.
Although healthcare providers can provide a long COVID diagnosis without a positive COVID-19 test on the basis of a patient’s presentation of symptoms and clinical history, many people in our study said that their providers would not provide this diagnosis, which restricted access to worker’s compensation, paid time off, and job accommodations.
Many people in the study also reported that their medical providers misdiagnosed them with a mental health disorder, such as anxiety, instead of long COVID. Although some people with long COVID may experience poor mental health as a natural consequence of dealing with a debilitating medical condition or may have neuropsychiatric symptoms as part of their long COVID, long COVID is not caused by an underlying psychiatric illness.
4. Support of medical providers is key to successful return to work for people living with long COVID.
Some people in our study described how they were able to get workplace accommodations or access workers’ compensation or sick leave because their medical providers recognized they had long COVID and provided them with this documentation. Some of these participants did not have a positive COVID-19 test, but their medical providers were able to diagnose them with long COVID on the basis of symptom presentation and clinical history. This documentation was critical for helping workers remain financially stable and able to return to work.
Conclusion
While we continue to search for treatment and cures for long COVID and work to provide a robust social safety net, it is crucial to address the stigma, inaccessibility, and lack of support often experienced by patients in their workplaces. Disabled people have long faced these issues; long COVID may be an opportunity to revolutionize the workplace to ensure an inclusive and accessible environment that can improve the lives of all workers.
For more on how to best be inclusive of employees with long COVID, read Harvard Business Review’s “Long Covid at Work: A Manager’s Guide” and visit the Job Accommodation Network webpage dedicated to long COVID.
Additional discussion about our study and applying the findings to improve work and medical care can be found by listening to the Healthy Work podcast episode titled “Supporting Long COVID at Work.”
Elisabeth Stelson, Gina Assaf, and Lisa McCorkell are members of the Patient-Led Research Collaborative, an international group of more than 60 researchers. Dr Stelson, Postdoctoral Research Fellow, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, has disclosed no relevant financial relationships. Gina Assaf is Research Lead, Patient-Led Research Collaborative, Washington, DC. Lisa McCorkell is a long COVID patient; Cofounder, Team Lead, Researcher, Patient-Led Research Collaborative, Washington, DC.
A version of this article appeared on Medscape.com.
Communicating the Benefits of Prenatal Vaccination to Patients
Vaccines recommended by the Advisory Committee on Immunization Practices (ACIP) offer important protection against severe illness for pregnant people and their babies.1 However, vaccination coverage estimates among pregnant people remain suboptimal.2-5 Additionally, some measures indicate that vaccine hesitancy among pregnant people is increasing; for example, 17.5% of surveyed pregnant women reported being very hesitant about influenza vaccination during pregnancy in 2019-2020, compared with 24.7% in 2022-2023.6 Explore updated provider toolkits and prenatal vaccination patient education resources, including fact sheets, social media assets, posters, and short videos on respiratory syncytial virus (RSV), Tdap, COVID-19, influenza, and hepatitis B.
In an interview, CDC’s Haben Debessai, MD, an adjunct instructor in obstetrics and gynecology at Emory School of Medicine, Atlanta, Georgia, contextualizes the data to help healthcare professionals communicate effectively with their pregnant patients.
What can practitioners communicate to patients about why it is important to get vaccinated during their pregnancy?
When communicating with their patients, practitioners can consider opportunities to discuss how vaccines work during pregnancy, emphasizing that prenatal vaccinations are beneficial for both the pregnant person and the fetus. It can be helpful to educate patients on how a pregnant person’s immune system can develop antibodies that will then pass to the fetus during the pregnancy and confer protection during the infant’s early months of life — when they are highly susceptible to illnesses that can be severe, such as RSV-associated lower respiratory tract infections. It can also be useful to discuss pregnancy’s impact on the immune system, which contributes to pregnant people being at higher risk for severe illness from infections like COVID-19 and flu, if contracted. The outcomes of severe illness can be dire for both the pregnant person and their pregnancy, which is why vaccination is the best mitigation option. It can also be beneficial to share with patients that some vaccines, like RSV and Tdap, are specifically for neonatal benefit, which could help patients understand why some vaccines are recommended at a specific gestational age and in each pregnancy or subsequent pregnancies.
What is known about pregnant populations that experience disparities in vaccination coverage?
While vaccination coverage among pregnant people is suboptimal, coverage estimates are often lowest among Black pregnant people, some of whom report experiencing mistreatment and discrimination during pregnancy and delivery.7 It is important to recognize that there are many intersecting factors that may impact vaccination coverage. Systemic and structural factors may prohibit some patient populations from accessing vaccinations (eg, transportation barriers, difficulty accessing adequate healthcare for those on government assistance, language barriers). To be responsive to the intersectional lived realities of each of these communities, the medical and public health community continually strives to increase trustworthiness, which can lead to increased uptake of vaccinations in these populations.
What vaccines are available and recommended for pregnant people?
Four vaccines are routinely recommended during pregnancy: Tdap, COVID-19, influenza (seasonal), and RSV (seasonal). CDC recommends getting a Tdap vaccine between the 27th and 36th week of each pregnancy, preferably during the earlier part of this time period. CDC recommends that everyone 6 months or older in the United States, including pregnant people, stay up to date on COVID-19 vaccines. A COVID-19 vaccine can be given during any trimester of pregnancy. CDC recommends an annual flu vaccine during each flu season (fall/winter) for everyone 6 months or older in the United States, including pregnant people. A flu vaccine can be given during any trimester of pregnancy. For individuals who will be between 32 and 36 weeks pregnant during September through January, CDC recommends getting an RSV vaccine. RSV season and timing of vaccination may vary depending on geography. If a pregnant patient does not get the RSV vaccine during their pregnancy, CDC recommends that their baby receive an RSV monoclonal antibody (nirsevimab) to provide additional protection during the infant’s first RSV season, if they are younger than 8 months. At this time, pregnant people who received an RSV vaccine during a previous pregnancy (last year) are not recommended to receive another RSV vaccine during pregnancy. The current recommendation is for babies born during subsequent pregnancies to receive nirsevimab. Some pregnant people may also need other vaccines, such as hepatitis B.
How can practitioners approach conversations about vaccination during pregnancy amid increasing vaccine hesitancy?
Many pregnant people who do get vaccinated describe their provider’s recommendation as an important motivator toward vaccination.8-11 Communications research suggests that practitioners can further increase trustworthiness by openly discussing potential side effects of prenatal vaccinations and providing patients with a rationale for why each vaccine is recommended. Practitioners can also utilize opportunities to communicate that the risk for severe illness from whooping cough, COVID-19, flu, and RSV in pregnancy and among neonates in the first few months of life is often higher than the risk for an adverse reaction from receiving ACIP-recommended vaccines. Finally, practitioners can consider sharing tested and refined patient education resources at least one appointment prior to the recommended administration of each vaccine, providing individuals with time to process the information they need to facilitate their vaccine decision-making process.
Some patients may be more comfortable with older, well-known prenatal vaccinations but have skepticism about newer vaccines like COVID-19 and RSV. How can practitioners respond to these concerns?
As pregnant people navigate the challenges of making health decisions that could impact their developing baby, practitioners can build trust through empathetically responding to safety concerns and questions, particularly with respect to newly authorized vaccines. Vaccine confidence may be strengthened by communicating to patients that all recommended vaccinations, including those that have been newly authorized, have been rigorously tested prior to being recommended for pregnant people. Additionally, in my clinical practice, I see that patients are often more comfortable accepting vaccines when the benefit for the baby is clearly communicated. I have been pleasantly surprised that most patients I have counseled on the new maternal RSV vaccine have been receptive, making statements like, “If this will help protect my baby from getting sick, then yes, I will get it.”
As you and your staff care for pregnant patients during fall and winter virus season, remember that a provider recommendation remains one of the strongest known predictors of vaccination uptake.12 As a trusted source of information about prenatal vaccination, consider further incorporating patient education resources to help communicate how prenatal vaccination helps pregnant people share important protection against severe illnesses with their babies.
Haben Debessai, MD, is a Gilstrap Fellow at the CDC Foundation. Debessai also serves as an Emory Obstetrics/Gynecology Adjunct Instructor at Grady Health System in Atlanta, Georgia. She disclosed no relevant conflicts of interest.
References
1. ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol. 2018;131:e214-e217. doi:10.1097/AOG.0000000000002662
2. Centers for Disease Control and Prevention. Flu, Tdap, and COVID-19 vaccination coverage among pregnant women – United States, April 2024. 2024 Sep 23. 3. Centers for Disease Control and Prevention. Respiratory syncytial virus (rsv) vaccination coverage, pregnant persons. 2024 Nov 19. 4. Centers for Disease Control and Prevention. COVID-19 vaccination coverage, pregnant persons. 2024 Nov 19. 5. Centers for Disease Control and Prevention. Influenza vaccination coverage, pregnant persons. 2024 Nov 19.6. Razzaghi H et al. IMMWR Morb Mortal Wkly Rep. 2023;72:1065-1071. Published 2023 Sep 29. doi: 10.15585/mmwr.mm7239a4
7. Mohamoud YA et al. MMWR Morb Mortal Wkly Rep 2023;72:961-967. doi: https://dx.doi.org/10.15585/mmwr.mm7235e1.
8. Kiefer MK et al. Am J Obstet Gynecol MFM. 2022;4:100603. doi: 10.1016/j.ajogmf.2022.100603
9. Spires B et al. Obstet Gynecol Clin North Am. 2023;50:401-419. doi: 10.1016/j.ogc.2023.02.013
10. Wales DP et al. Public Health. 2020;179:38-44. doi: 10.1016/j.puhe.2019.10.001
11. Zimmerman M et al. J Natl Med Assoc. 2023;115:362-376. doi:10.1016/j.jnma.2023.04.003
12. Castillo E et al. Best Pract Res Clin Obstet Gynaecol. 2021;76:83-95. doi:10.1016/j.bpobgyn.2021.03.008
Vaccines recommended by the Advisory Committee on Immunization Practices (ACIP) offer important protection against severe illness for pregnant people and their babies.1 However, vaccination coverage estimates among pregnant people remain suboptimal.2-5 Additionally, some measures indicate that vaccine hesitancy among pregnant people is increasing; for example, 17.5% of surveyed pregnant women reported being very hesitant about influenza vaccination during pregnancy in 2019-2020, compared with 24.7% in 2022-2023.6 Explore updated provider toolkits and prenatal vaccination patient education resources, including fact sheets, social media assets, posters, and short videos on respiratory syncytial virus (RSV), Tdap, COVID-19, influenza, and hepatitis B.
In an interview, CDC’s Haben Debessai, MD, an adjunct instructor in obstetrics and gynecology at Emory School of Medicine, Atlanta, Georgia, contextualizes the data to help healthcare professionals communicate effectively with their pregnant patients.
What can practitioners communicate to patients about why it is important to get vaccinated during their pregnancy?
When communicating with their patients, practitioners can consider opportunities to discuss how vaccines work during pregnancy, emphasizing that prenatal vaccinations are beneficial for both the pregnant person and the fetus. It can be helpful to educate patients on how a pregnant person’s immune system can develop antibodies that will then pass to the fetus during the pregnancy and confer protection during the infant’s early months of life — when they are highly susceptible to illnesses that can be severe, such as RSV-associated lower respiratory tract infections. It can also be useful to discuss pregnancy’s impact on the immune system, which contributes to pregnant people being at higher risk for severe illness from infections like COVID-19 and flu, if contracted. The outcomes of severe illness can be dire for both the pregnant person and their pregnancy, which is why vaccination is the best mitigation option. It can also be beneficial to share with patients that some vaccines, like RSV and Tdap, are specifically for neonatal benefit, which could help patients understand why some vaccines are recommended at a specific gestational age and in each pregnancy or subsequent pregnancies.
What is known about pregnant populations that experience disparities in vaccination coverage?
While vaccination coverage among pregnant people is suboptimal, coverage estimates are often lowest among Black pregnant people, some of whom report experiencing mistreatment and discrimination during pregnancy and delivery.7 It is important to recognize that there are many intersecting factors that may impact vaccination coverage. Systemic and structural factors may prohibit some patient populations from accessing vaccinations (eg, transportation barriers, difficulty accessing adequate healthcare for those on government assistance, language barriers). To be responsive to the intersectional lived realities of each of these communities, the medical and public health community continually strives to increase trustworthiness, which can lead to increased uptake of vaccinations in these populations.
What vaccines are available and recommended for pregnant people?
Four vaccines are routinely recommended during pregnancy: Tdap, COVID-19, influenza (seasonal), and RSV (seasonal). CDC recommends getting a Tdap vaccine between the 27th and 36th week of each pregnancy, preferably during the earlier part of this time period. CDC recommends that everyone 6 months or older in the United States, including pregnant people, stay up to date on COVID-19 vaccines. A COVID-19 vaccine can be given during any trimester of pregnancy. CDC recommends an annual flu vaccine during each flu season (fall/winter) for everyone 6 months or older in the United States, including pregnant people. A flu vaccine can be given during any trimester of pregnancy. For individuals who will be between 32 and 36 weeks pregnant during September through January, CDC recommends getting an RSV vaccine. RSV season and timing of vaccination may vary depending on geography. If a pregnant patient does not get the RSV vaccine during their pregnancy, CDC recommends that their baby receive an RSV monoclonal antibody (nirsevimab) to provide additional protection during the infant’s first RSV season, if they are younger than 8 months. At this time, pregnant people who received an RSV vaccine during a previous pregnancy (last year) are not recommended to receive another RSV vaccine during pregnancy. The current recommendation is for babies born during subsequent pregnancies to receive nirsevimab. Some pregnant people may also need other vaccines, such as hepatitis B.
How can practitioners approach conversations about vaccination during pregnancy amid increasing vaccine hesitancy?
Many pregnant people who do get vaccinated describe their provider’s recommendation as an important motivator toward vaccination.8-11 Communications research suggests that practitioners can further increase trustworthiness by openly discussing potential side effects of prenatal vaccinations and providing patients with a rationale for why each vaccine is recommended. Practitioners can also utilize opportunities to communicate that the risk for severe illness from whooping cough, COVID-19, flu, and RSV in pregnancy and among neonates in the first few months of life is often higher than the risk for an adverse reaction from receiving ACIP-recommended vaccines. Finally, practitioners can consider sharing tested and refined patient education resources at least one appointment prior to the recommended administration of each vaccine, providing individuals with time to process the information they need to facilitate their vaccine decision-making process.
Some patients may be more comfortable with older, well-known prenatal vaccinations but have skepticism about newer vaccines like COVID-19 and RSV. How can practitioners respond to these concerns?
As pregnant people navigate the challenges of making health decisions that could impact their developing baby, practitioners can build trust through empathetically responding to safety concerns and questions, particularly with respect to newly authorized vaccines. Vaccine confidence may be strengthened by communicating to patients that all recommended vaccinations, including those that have been newly authorized, have been rigorously tested prior to being recommended for pregnant people. Additionally, in my clinical practice, I see that patients are often more comfortable accepting vaccines when the benefit for the baby is clearly communicated. I have been pleasantly surprised that most patients I have counseled on the new maternal RSV vaccine have been receptive, making statements like, “If this will help protect my baby from getting sick, then yes, I will get it.”
As you and your staff care for pregnant patients during fall and winter virus season, remember that a provider recommendation remains one of the strongest known predictors of vaccination uptake.12 As a trusted source of information about prenatal vaccination, consider further incorporating patient education resources to help communicate how prenatal vaccination helps pregnant people share important protection against severe illnesses with their babies.
Haben Debessai, MD, is a Gilstrap Fellow at the CDC Foundation. Debessai also serves as an Emory Obstetrics/Gynecology Adjunct Instructor at Grady Health System in Atlanta, Georgia. She disclosed no relevant conflicts of interest.
References
1. ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol. 2018;131:e214-e217. doi:10.1097/AOG.0000000000002662
2. Centers for Disease Control and Prevention. Flu, Tdap, and COVID-19 vaccination coverage among pregnant women – United States, April 2024. 2024 Sep 23. 3. Centers for Disease Control and Prevention. Respiratory syncytial virus (rsv) vaccination coverage, pregnant persons. 2024 Nov 19. 4. Centers for Disease Control and Prevention. COVID-19 vaccination coverage, pregnant persons. 2024 Nov 19. 5. Centers for Disease Control and Prevention. Influenza vaccination coverage, pregnant persons. 2024 Nov 19.6. Razzaghi H et al. IMMWR Morb Mortal Wkly Rep. 2023;72:1065-1071. Published 2023 Sep 29. doi: 10.15585/mmwr.mm7239a4
7. Mohamoud YA et al. MMWR Morb Mortal Wkly Rep 2023;72:961-967. doi: https://dx.doi.org/10.15585/mmwr.mm7235e1.
8. Kiefer MK et al. Am J Obstet Gynecol MFM. 2022;4:100603. doi: 10.1016/j.ajogmf.2022.100603
9. Spires B et al. Obstet Gynecol Clin North Am. 2023;50:401-419. doi: 10.1016/j.ogc.2023.02.013
10. Wales DP et al. Public Health. 2020;179:38-44. doi: 10.1016/j.puhe.2019.10.001
11. Zimmerman M et al. J Natl Med Assoc. 2023;115:362-376. doi:10.1016/j.jnma.2023.04.003
12. Castillo E et al. Best Pract Res Clin Obstet Gynaecol. 2021;76:83-95. doi:10.1016/j.bpobgyn.2021.03.008
Vaccines recommended by the Advisory Committee on Immunization Practices (ACIP) offer important protection against severe illness for pregnant people and their babies.1 However, vaccination coverage estimates among pregnant people remain suboptimal.2-5 Additionally, some measures indicate that vaccine hesitancy among pregnant people is increasing; for example, 17.5% of surveyed pregnant women reported being very hesitant about influenza vaccination during pregnancy in 2019-2020, compared with 24.7% in 2022-2023.6 Explore updated provider toolkits and prenatal vaccination patient education resources, including fact sheets, social media assets, posters, and short videos on respiratory syncytial virus (RSV), Tdap, COVID-19, influenza, and hepatitis B.
In an interview, CDC’s Haben Debessai, MD, an adjunct instructor in obstetrics and gynecology at Emory School of Medicine, Atlanta, Georgia, contextualizes the data to help healthcare professionals communicate effectively with their pregnant patients.
What can practitioners communicate to patients about why it is important to get vaccinated during their pregnancy?
When communicating with their patients, practitioners can consider opportunities to discuss how vaccines work during pregnancy, emphasizing that prenatal vaccinations are beneficial for both the pregnant person and the fetus. It can be helpful to educate patients on how a pregnant person’s immune system can develop antibodies that will then pass to the fetus during the pregnancy and confer protection during the infant’s early months of life — when they are highly susceptible to illnesses that can be severe, such as RSV-associated lower respiratory tract infections. It can also be useful to discuss pregnancy’s impact on the immune system, which contributes to pregnant people being at higher risk for severe illness from infections like COVID-19 and flu, if contracted. The outcomes of severe illness can be dire for both the pregnant person and their pregnancy, which is why vaccination is the best mitigation option. It can also be beneficial to share with patients that some vaccines, like RSV and Tdap, are specifically for neonatal benefit, which could help patients understand why some vaccines are recommended at a specific gestational age and in each pregnancy or subsequent pregnancies.
What is known about pregnant populations that experience disparities in vaccination coverage?
While vaccination coverage among pregnant people is suboptimal, coverage estimates are often lowest among Black pregnant people, some of whom report experiencing mistreatment and discrimination during pregnancy and delivery.7 It is important to recognize that there are many intersecting factors that may impact vaccination coverage. Systemic and structural factors may prohibit some patient populations from accessing vaccinations (eg, transportation barriers, difficulty accessing adequate healthcare for those on government assistance, language barriers). To be responsive to the intersectional lived realities of each of these communities, the medical and public health community continually strives to increase trustworthiness, which can lead to increased uptake of vaccinations in these populations.
What vaccines are available and recommended for pregnant people?
Four vaccines are routinely recommended during pregnancy: Tdap, COVID-19, influenza (seasonal), and RSV (seasonal). CDC recommends getting a Tdap vaccine between the 27th and 36th week of each pregnancy, preferably during the earlier part of this time period. CDC recommends that everyone 6 months or older in the United States, including pregnant people, stay up to date on COVID-19 vaccines. A COVID-19 vaccine can be given during any trimester of pregnancy. CDC recommends an annual flu vaccine during each flu season (fall/winter) for everyone 6 months or older in the United States, including pregnant people. A flu vaccine can be given during any trimester of pregnancy. For individuals who will be between 32 and 36 weeks pregnant during September through January, CDC recommends getting an RSV vaccine. RSV season and timing of vaccination may vary depending on geography. If a pregnant patient does not get the RSV vaccine during their pregnancy, CDC recommends that their baby receive an RSV monoclonal antibody (nirsevimab) to provide additional protection during the infant’s first RSV season, if they are younger than 8 months. At this time, pregnant people who received an RSV vaccine during a previous pregnancy (last year) are not recommended to receive another RSV vaccine during pregnancy. The current recommendation is for babies born during subsequent pregnancies to receive nirsevimab. Some pregnant people may also need other vaccines, such as hepatitis B.
How can practitioners approach conversations about vaccination during pregnancy amid increasing vaccine hesitancy?
Many pregnant people who do get vaccinated describe their provider’s recommendation as an important motivator toward vaccination.8-11 Communications research suggests that practitioners can further increase trustworthiness by openly discussing potential side effects of prenatal vaccinations and providing patients with a rationale for why each vaccine is recommended. Practitioners can also utilize opportunities to communicate that the risk for severe illness from whooping cough, COVID-19, flu, and RSV in pregnancy and among neonates in the first few months of life is often higher than the risk for an adverse reaction from receiving ACIP-recommended vaccines. Finally, practitioners can consider sharing tested and refined patient education resources at least one appointment prior to the recommended administration of each vaccine, providing individuals with time to process the information they need to facilitate their vaccine decision-making process.
Some patients may be more comfortable with older, well-known prenatal vaccinations but have skepticism about newer vaccines like COVID-19 and RSV. How can practitioners respond to these concerns?
As pregnant people navigate the challenges of making health decisions that could impact their developing baby, practitioners can build trust through empathetically responding to safety concerns and questions, particularly with respect to newly authorized vaccines. Vaccine confidence may be strengthened by communicating to patients that all recommended vaccinations, including those that have been newly authorized, have been rigorously tested prior to being recommended for pregnant people. Additionally, in my clinical practice, I see that patients are often more comfortable accepting vaccines when the benefit for the baby is clearly communicated. I have been pleasantly surprised that most patients I have counseled on the new maternal RSV vaccine have been receptive, making statements like, “If this will help protect my baby from getting sick, then yes, I will get it.”
As you and your staff care for pregnant patients during fall and winter virus season, remember that a provider recommendation remains one of the strongest known predictors of vaccination uptake.12 As a trusted source of information about prenatal vaccination, consider further incorporating patient education resources to help communicate how prenatal vaccination helps pregnant people share important protection against severe illnesses with their babies.
Haben Debessai, MD, is a Gilstrap Fellow at the CDC Foundation. Debessai also serves as an Emory Obstetrics/Gynecology Adjunct Instructor at Grady Health System in Atlanta, Georgia. She disclosed no relevant conflicts of interest.
References
1. ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol. 2018;131:e214-e217. doi:10.1097/AOG.0000000000002662
2. Centers for Disease Control and Prevention. Flu, Tdap, and COVID-19 vaccination coverage among pregnant women – United States, April 2024. 2024 Sep 23. 3. Centers for Disease Control and Prevention. Respiratory syncytial virus (rsv) vaccination coverage, pregnant persons. 2024 Nov 19. 4. Centers for Disease Control and Prevention. COVID-19 vaccination coverage, pregnant persons. 2024 Nov 19. 5. Centers for Disease Control and Prevention. Influenza vaccination coverage, pregnant persons. 2024 Nov 19.6. Razzaghi H et al. IMMWR Morb Mortal Wkly Rep. 2023;72:1065-1071. Published 2023 Sep 29. doi: 10.15585/mmwr.mm7239a4
7. Mohamoud YA et al. MMWR Morb Mortal Wkly Rep 2023;72:961-967. doi: https://dx.doi.org/10.15585/mmwr.mm7235e1.
8. Kiefer MK et al. Am J Obstet Gynecol MFM. 2022;4:100603. doi: 10.1016/j.ajogmf.2022.100603
9. Spires B et al. Obstet Gynecol Clin North Am. 2023;50:401-419. doi: 10.1016/j.ogc.2023.02.013
10. Wales DP et al. Public Health. 2020;179:38-44. doi: 10.1016/j.puhe.2019.10.001
11. Zimmerman M et al. J Natl Med Assoc. 2023;115:362-376. doi:10.1016/j.jnma.2023.04.003
12. Castillo E et al. Best Pract Res Clin Obstet Gynaecol. 2021;76:83-95. doi:10.1016/j.bpobgyn.2021.03.008
Most US Adults Plan to Skip Annual COVID Vaccines
Most US adults continue to plan on skipping an annual COVID vaccine.
according to results of a new survey from the Pew Research Center.
When asked why people wouldn’t get an updated COVID vaccine, 61% said a major reason was that they don’t think they need it, and 60% said a major reason is that they are concerned about side effects. Cost was a factor for 14% of people, and 46% of people said they don’t get vaccines in general.
There were some differences in intention to get vaccinated based on a person’s age. Among people ages 65 and older, 27% said they had already gotten the vaccine, and another 27% said they probably will get the shot, leaving 45% who said they probably won’t roll up their sleeves. People ages 30-49 years old were the least likely to plan on getting a COVID shot – 66% said they probably won’t get one.
Public health officials say everyone should get an annual COVID vaccine, just as they should get a flu shot, because the vaccines are formulated each year to target virus strains predicted to be in wide circulation. Also, immunity – either from past vaccination or past infection – wanes over time.
Research shows that the vaccines reduce the likelihood of hospitalization or death caused by severe illness, particularly among people who have risk factors, like being over age 65 or having health issues that are becoming increasingly common in the United States, like diabetes, heart problems, and lung conditions.
The survey included 9,593 adults who were asked about their COVID vaccine intentions with this question: “Public health officials recently recommended an updated vaccine for COVID-19. Do you think you will probably get an updated vaccine, probably not get an updated vaccine, or have you already received an updated vaccine?” The survey was done online and by telephone from October 21 to October 27.
So far in 2024, the CDC’s ongoing immunization survey shows that 17% of adults say that, as of November 2, they have gotten vaccinated for COVID-19 this season, and 14% said they will definitely get vaccinated. The Pew Research Center survey found that 15% of people said they’ve already gotten the shot this season.
Reports of positive COVID tests, emergency room visits, and hospitalizations remain very low. About 3.6% of test results shared with the CDC were positive for COVID the week ending November 9. Less than 1% of ER visits involve a COVID diagnosis, and hospitalizations are well below the rate seen at this time last year. Last year, COVID activity in the United States began rising around Thanksgiving and continued upward, peaking in early January.
The protection from a COVID-19 vaccination usually fully kicks in about 2 weeks after you get the shot, and the vaccines are most effective for the following 3 months.
A version of this article first appeared on WebMD.com.
Most US adults continue to plan on skipping an annual COVID vaccine.
according to results of a new survey from the Pew Research Center.
When asked why people wouldn’t get an updated COVID vaccine, 61% said a major reason was that they don’t think they need it, and 60% said a major reason is that they are concerned about side effects. Cost was a factor for 14% of people, and 46% of people said they don’t get vaccines in general.
There were some differences in intention to get vaccinated based on a person’s age. Among people ages 65 and older, 27% said they had already gotten the vaccine, and another 27% said they probably will get the shot, leaving 45% who said they probably won’t roll up their sleeves. People ages 30-49 years old were the least likely to plan on getting a COVID shot – 66% said they probably won’t get one.
Public health officials say everyone should get an annual COVID vaccine, just as they should get a flu shot, because the vaccines are formulated each year to target virus strains predicted to be in wide circulation. Also, immunity – either from past vaccination or past infection – wanes over time.
Research shows that the vaccines reduce the likelihood of hospitalization or death caused by severe illness, particularly among people who have risk factors, like being over age 65 or having health issues that are becoming increasingly common in the United States, like diabetes, heart problems, and lung conditions.
The survey included 9,593 adults who were asked about their COVID vaccine intentions with this question: “Public health officials recently recommended an updated vaccine for COVID-19. Do you think you will probably get an updated vaccine, probably not get an updated vaccine, or have you already received an updated vaccine?” The survey was done online and by telephone from October 21 to October 27.
So far in 2024, the CDC’s ongoing immunization survey shows that 17% of adults say that, as of November 2, they have gotten vaccinated for COVID-19 this season, and 14% said they will definitely get vaccinated. The Pew Research Center survey found that 15% of people said they’ve already gotten the shot this season.
Reports of positive COVID tests, emergency room visits, and hospitalizations remain very low. About 3.6% of test results shared with the CDC were positive for COVID the week ending November 9. Less than 1% of ER visits involve a COVID diagnosis, and hospitalizations are well below the rate seen at this time last year. Last year, COVID activity in the United States began rising around Thanksgiving and continued upward, peaking in early January.
The protection from a COVID-19 vaccination usually fully kicks in about 2 weeks after you get the shot, and the vaccines are most effective for the following 3 months.
A version of this article first appeared on WebMD.com.
Most US adults continue to plan on skipping an annual COVID vaccine.
according to results of a new survey from the Pew Research Center.
When asked why people wouldn’t get an updated COVID vaccine, 61% said a major reason was that they don’t think they need it, and 60% said a major reason is that they are concerned about side effects. Cost was a factor for 14% of people, and 46% of people said they don’t get vaccines in general.
There were some differences in intention to get vaccinated based on a person’s age. Among people ages 65 and older, 27% said they had already gotten the vaccine, and another 27% said they probably will get the shot, leaving 45% who said they probably won’t roll up their sleeves. People ages 30-49 years old were the least likely to plan on getting a COVID shot – 66% said they probably won’t get one.
Public health officials say everyone should get an annual COVID vaccine, just as they should get a flu shot, because the vaccines are formulated each year to target virus strains predicted to be in wide circulation. Also, immunity – either from past vaccination or past infection – wanes over time.
Research shows that the vaccines reduce the likelihood of hospitalization or death caused by severe illness, particularly among people who have risk factors, like being over age 65 or having health issues that are becoming increasingly common in the United States, like diabetes, heart problems, and lung conditions.
The survey included 9,593 adults who were asked about their COVID vaccine intentions with this question: “Public health officials recently recommended an updated vaccine for COVID-19. Do you think you will probably get an updated vaccine, probably not get an updated vaccine, or have you already received an updated vaccine?” The survey was done online and by telephone from October 21 to October 27.
So far in 2024, the CDC’s ongoing immunization survey shows that 17% of adults say that, as of November 2, they have gotten vaccinated for COVID-19 this season, and 14% said they will definitely get vaccinated. The Pew Research Center survey found that 15% of people said they’ve already gotten the shot this season.
Reports of positive COVID tests, emergency room visits, and hospitalizations remain very low. About 3.6% of test results shared with the CDC were positive for COVID the week ending November 9. Less than 1% of ER visits involve a COVID diagnosis, and hospitalizations are well below the rate seen at this time last year. Last year, COVID activity in the United States began rising around Thanksgiving and continued upward, peaking in early January.
The protection from a COVID-19 vaccination usually fully kicks in about 2 weeks after you get the shot, and the vaccines are most effective for the following 3 months.
A version of this article first appeared on WebMD.com.
BCG Vaccine May Protect Against Long COVID Symptoms
TOPLINE:
METHODOLOGY:
- A phase 3 clinical trial initiated in early 2020 investigated the effect of the BCG vaccine injected during active infection on COVID-19 progression in adults with mild or moderate COVID-19. The current study summarizes the 6- and 12-month follow-up data with a focus on long-COVID symptoms.
- Patients who tested positive for severe acute respiratory syndrome coronavirus 2 were randomly assigned to receive either 0.1 mL of intradermal BCG (n = 191) or 0.9% saline placebo (n = 202) within 14 days of symptom onset and were followed up at 7, 14, 21, and 45 days and at 6 and 12 months postinjection.
- Overall, 157 BCG (median age, 40 years; 54.1% women) and 142 placebo (median age, 41 years; 65.5% women) recipients completed the 6-month follow-up, and 97 BCG (median age, 37 years; 49.5% women) and 95 placebo (median age, 40 years; 67.4% women) recipients completed the 12-month follow-up.
- The researchers primarily assessed the effect of the BCG vaccine on the development of the symptoms of long COVID at 6 and 12 months.
TAKEAWAY:
- Hearing problems were less frequent among BCG recipients at 6 months compared with those who received placebo (odds ratio [OR], 0.26; 95% CI, 0.045-1.0; P = .044).
- At 12 months, participants who received the BCG vaccine exhibited fewer issues with sleeping (P = .027), concentration (P = .009), memory (P = .009), and vision (P = .022) along with a lower long-COVID score (one-sided Wilcoxon test, P = .002) than those who received placebo.
- At 6 months, BCG demonstrated a sex-specific paradoxical effect on hair loss, decreasing it in men (P = .031), while causing a slight, though statistically nonsignificant, increase in women.
- Male sex was the strongest predictive factor for long COVID, cognitive dysfunction, and cardiopulmonary scores at both follow-up assessments.
IN PRACTICE:
“[The study] findings suggest that BCG immunotherapy for an existing ailment may be superior to prophylaxis in healthy individuals,” the authors wrote.
SOURCE:
The study was led by Mehrsa Jalalizadeh and Keini Buosi, UroScience, State University of Campinas, Unicamp, São Paulo, Brazil. It was published online on November 19, 2024, in the Journal of Internal Medicine.
LIMITATIONS:
Previous mycobacterial exposure was not tested among the study participants. A notable loss to follow-up, particularly at 12 months, may have introduced bias into the results.
DISCLOSURES:
The study was supported by the Coordination for the Improvement of Higher Education Personnel, Federal Government of Brazil, the General Coordination of the National Immunization Program, Ministry of Health (Brazil), and the National Council for Scientific and Technological Development-Research Productivity. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A phase 3 clinical trial initiated in early 2020 investigated the effect of the BCG vaccine injected during active infection on COVID-19 progression in adults with mild or moderate COVID-19. The current study summarizes the 6- and 12-month follow-up data with a focus on long-COVID symptoms.
- Patients who tested positive for severe acute respiratory syndrome coronavirus 2 were randomly assigned to receive either 0.1 mL of intradermal BCG (n = 191) or 0.9% saline placebo (n = 202) within 14 days of symptom onset and were followed up at 7, 14, 21, and 45 days and at 6 and 12 months postinjection.
- Overall, 157 BCG (median age, 40 years; 54.1% women) and 142 placebo (median age, 41 years; 65.5% women) recipients completed the 6-month follow-up, and 97 BCG (median age, 37 years; 49.5% women) and 95 placebo (median age, 40 years; 67.4% women) recipients completed the 12-month follow-up.
- The researchers primarily assessed the effect of the BCG vaccine on the development of the symptoms of long COVID at 6 and 12 months.
TAKEAWAY:
- Hearing problems were less frequent among BCG recipients at 6 months compared with those who received placebo (odds ratio [OR], 0.26; 95% CI, 0.045-1.0; P = .044).
- At 12 months, participants who received the BCG vaccine exhibited fewer issues with sleeping (P = .027), concentration (P = .009), memory (P = .009), and vision (P = .022) along with a lower long-COVID score (one-sided Wilcoxon test, P = .002) than those who received placebo.
- At 6 months, BCG demonstrated a sex-specific paradoxical effect on hair loss, decreasing it in men (P = .031), while causing a slight, though statistically nonsignificant, increase in women.
- Male sex was the strongest predictive factor for long COVID, cognitive dysfunction, and cardiopulmonary scores at both follow-up assessments.
IN PRACTICE:
“[The study] findings suggest that BCG immunotherapy for an existing ailment may be superior to prophylaxis in healthy individuals,” the authors wrote.
SOURCE:
The study was led by Mehrsa Jalalizadeh and Keini Buosi, UroScience, State University of Campinas, Unicamp, São Paulo, Brazil. It was published online on November 19, 2024, in the Journal of Internal Medicine.
LIMITATIONS:
Previous mycobacterial exposure was not tested among the study participants. A notable loss to follow-up, particularly at 12 months, may have introduced bias into the results.
DISCLOSURES:
The study was supported by the Coordination for the Improvement of Higher Education Personnel, Federal Government of Brazil, the General Coordination of the National Immunization Program, Ministry of Health (Brazil), and the National Council for Scientific and Technological Development-Research Productivity. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A phase 3 clinical trial initiated in early 2020 investigated the effect of the BCG vaccine injected during active infection on COVID-19 progression in adults with mild or moderate COVID-19. The current study summarizes the 6- and 12-month follow-up data with a focus on long-COVID symptoms.
- Patients who tested positive for severe acute respiratory syndrome coronavirus 2 were randomly assigned to receive either 0.1 mL of intradermal BCG (n = 191) or 0.9% saline placebo (n = 202) within 14 days of symptom onset and were followed up at 7, 14, 21, and 45 days and at 6 and 12 months postinjection.
- Overall, 157 BCG (median age, 40 years; 54.1% women) and 142 placebo (median age, 41 years; 65.5% women) recipients completed the 6-month follow-up, and 97 BCG (median age, 37 years; 49.5% women) and 95 placebo (median age, 40 years; 67.4% women) recipients completed the 12-month follow-up.
- The researchers primarily assessed the effect of the BCG vaccine on the development of the symptoms of long COVID at 6 and 12 months.
TAKEAWAY:
- Hearing problems were less frequent among BCG recipients at 6 months compared with those who received placebo (odds ratio [OR], 0.26; 95% CI, 0.045-1.0; P = .044).
- At 12 months, participants who received the BCG vaccine exhibited fewer issues with sleeping (P = .027), concentration (P = .009), memory (P = .009), and vision (P = .022) along with a lower long-COVID score (one-sided Wilcoxon test, P = .002) than those who received placebo.
- At 6 months, BCG demonstrated a sex-specific paradoxical effect on hair loss, decreasing it in men (P = .031), while causing a slight, though statistically nonsignificant, increase in women.
- Male sex was the strongest predictive factor for long COVID, cognitive dysfunction, and cardiopulmonary scores at both follow-up assessments.
IN PRACTICE:
“[The study] findings suggest that BCG immunotherapy for an existing ailment may be superior to prophylaxis in healthy individuals,” the authors wrote.
SOURCE:
The study was led by Mehrsa Jalalizadeh and Keini Buosi, UroScience, State University of Campinas, Unicamp, São Paulo, Brazil. It was published online on November 19, 2024, in the Journal of Internal Medicine.
LIMITATIONS:
Previous mycobacterial exposure was not tested among the study participants. A notable loss to follow-up, particularly at 12 months, may have introduced bias into the results.
DISCLOSURES:
The study was supported by the Coordination for the Improvement of Higher Education Personnel, Federal Government of Brazil, the General Coordination of the National Immunization Program, Ministry of Health (Brazil), and the National Council for Scientific and Technological Development-Research Productivity. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
New Data: The Most Promising Treatments for Long COVID
Long COVID is a symptom-driven disease, meaning that with no cure, physicians primarily treat the symptoms their patients are experiencing. But as 2024 winds down, researchers have begun to pinpoint a number of treatments that are bringing relief to the 17 million Americans diagnosed with long COVID.
Here’s a current look at what research has identified as some of the most promising treatments.
Low-Dose Naltrexone
Some research suggests that low-dose naltrexone may be helpful for patients suffering from brain fog, pain, sleep issues, and fatigue, said Ziyad Al-Aly, MD, a global expert on long COVID and chief of research and development at the Veterans Affairs St Louis Health Care System in Missouri.
Low-dose naltrexone is an anti-inflammatory agent currently approved by the Food and Drug Administration for the treatment of alcohol and opioid dependence.
“We don’t know the mechanism for how the medication works, and for that matter, we don’t really understand what causes brain fog. But perhaps its anti-inflammatory properties seem to help, and for some patients, low-dose naltrexone has been helpful,” said Al-Aly.
A March 2024 study found that both fatigue and pain were improved in patients taking low-dose naltrexone. In another study, published in the June 2024 issue of Frontiers in Medicine, researchers found that low-dose naltrexone was associated with improvement of several clinical symptoms related to long COVID such as fatigue, poor sleep quality, brain fog, post-exertional malaise, and headache.
Selective Serotonin Reuptake Inhibitors (SSRIs) and Antidepressants
In 2023, University of Pennsylvania researchers uncovered a link between long COVID and lower levels of serotonin in the body. This helped point to the potential treatment of using SSRIs to treat the condition.
For patients who have overlapping psychiatric issues that go along with brain fog, SSRIs prescribed to treat depression and other mental health conditions, as well as the antidepressant Wellbutrin, have been shown effective at dealing with concentration issues, brain fog, and depression, said Nisha Viswanathan, MD, director of the University of California, Los Angeles (UCLA) Long COVID Program at UCLA Health.
A study published in the November 2023 issue of the journal Scientific Reports found that SSRIs led to a “considerable reduction of symptoms,” especially brain fog, fatigue, sensory overload, and overall improved functioning. Low-dose Abilify, which contains aripiprazole, an antipsychotic medication, has also been found to be effective for cognitive issues caused by long COVID.
“Abilify is traditionally used for the treatment of schizophrenia or other psychotic disorders, but in a low-dose format, there is some data to suggest that it can also be anti-inflammatory and helpful for cognitive issues like brain fog,” said Viswanathan.
Modafinil
Modafinil, a medication previously used for managing narcolepsy, has also been shown effective for the treatment of fatigue and neurocognitive deficits caused by long COVID, said Viswanathan, adding that it’s another medication that she’s found useful for a number of her patients.
It’s thought that these cognitive symptoms are caused by an inflammatory cytokine release that leads to excessive stimulation of neurotransmitters in the body. According to a June 2024 article in the American Journal of Psychiatry, “Modafinil can therapeutically act on these pathways, which possibly contributed to the symptomatic improvement.” But the medication has not been studied widely in patients with long COVID and has been shown to have interactions with other medications.
Metformin
Some research has shown that metformin, a well-known diabetes medication, reduces instances of long COVID when taken during the illness’s acute phase. It seems to boost metabolic function in patients.
“It makes sense that it would work because it seems to have anti-inflammatory effects on the body,” said Grace McComsey, MD, who leads one of the 15 nationwide long COVID centers funded by the federal RECOVER (Researching COVID to Enhance Recovery) Initiative in Cleveland, Ohio. McComsey added that it may reduce the viral persistence that causes some forms of long COVID.
A study published in the October 2023 issue of the journal The Lancet Infectious Diseases found that metformin seemed to reduce instances of long COVID in patients who took it after being diagnosed with acute COVID. It seems less effective in patients who already have long COVID.
Antihistamines
Other data suggest that some patients with long COVID showed improvement after taking antihistamines. Research has shown that long COVID symptoms improved in 29% of patients with long COVID.
While researchers aren’t sure why antihistamines work to quell long COVID, the thought is that, when mast cells, a white blood cell that’s part of the immune system, shed granules and cause an inflammatory reaction, they release a lot of histamines. Antihistamine medications like famotidine block histamine receptors in the body, improving symptoms like brain fog, difficulty breathing, and elevated heart rate in patients.
“For some patients, these can be a lifesaver,” said David Putrino, the Nash Family Director of the Cohen Center for Recovery from Complex Chronic Illness and a national leader in the treatment of long COVID.
Putrino cautions patients toward taking these and other medications haphazardly without fully understanding that all treatments have risks, especially if they’re taking a number of them.
“Often patients are told that there’s no risk to trying something, but physicians should be counseling their patients and reminding them that there is a risk that includes medication sensitivities and medication interactions,” said Putrino.
The good news is that doctors have begun to identify some treatments that seem to be working in their patients, but we still don’t have the large-scale clinical trials to identify which treatments will work for certain patients and why.
There’s still so much we don’t know, and for physicians on the front lines of treating long COVID, it’s still largely a guessing game. “This is a constellation of symptoms; it’s not just one thing,” said Al-Aly. And while a treatment might be wildly effective for one patient, it might be ineffective or worse, problematic, for another.
A version of this article first appeared on Medscape.com.
Long COVID is a symptom-driven disease, meaning that with no cure, physicians primarily treat the symptoms their patients are experiencing. But as 2024 winds down, researchers have begun to pinpoint a number of treatments that are bringing relief to the 17 million Americans diagnosed with long COVID.
Here’s a current look at what research has identified as some of the most promising treatments.
Low-Dose Naltrexone
Some research suggests that low-dose naltrexone may be helpful for patients suffering from brain fog, pain, sleep issues, and fatigue, said Ziyad Al-Aly, MD, a global expert on long COVID and chief of research and development at the Veterans Affairs St Louis Health Care System in Missouri.
Low-dose naltrexone is an anti-inflammatory agent currently approved by the Food and Drug Administration for the treatment of alcohol and opioid dependence.
“We don’t know the mechanism for how the medication works, and for that matter, we don’t really understand what causes brain fog. But perhaps its anti-inflammatory properties seem to help, and for some patients, low-dose naltrexone has been helpful,” said Al-Aly.
A March 2024 study found that both fatigue and pain were improved in patients taking low-dose naltrexone. In another study, published in the June 2024 issue of Frontiers in Medicine, researchers found that low-dose naltrexone was associated with improvement of several clinical symptoms related to long COVID such as fatigue, poor sleep quality, brain fog, post-exertional malaise, and headache.
Selective Serotonin Reuptake Inhibitors (SSRIs) and Antidepressants
In 2023, University of Pennsylvania researchers uncovered a link between long COVID and lower levels of serotonin in the body. This helped point to the potential treatment of using SSRIs to treat the condition.
For patients who have overlapping psychiatric issues that go along with brain fog, SSRIs prescribed to treat depression and other mental health conditions, as well as the antidepressant Wellbutrin, have been shown effective at dealing with concentration issues, brain fog, and depression, said Nisha Viswanathan, MD, director of the University of California, Los Angeles (UCLA) Long COVID Program at UCLA Health.
A study published in the November 2023 issue of the journal Scientific Reports found that SSRIs led to a “considerable reduction of symptoms,” especially brain fog, fatigue, sensory overload, and overall improved functioning. Low-dose Abilify, which contains aripiprazole, an antipsychotic medication, has also been found to be effective for cognitive issues caused by long COVID.
“Abilify is traditionally used for the treatment of schizophrenia or other psychotic disorders, but in a low-dose format, there is some data to suggest that it can also be anti-inflammatory and helpful for cognitive issues like brain fog,” said Viswanathan.
Modafinil
Modafinil, a medication previously used for managing narcolepsy, has also been shown effective for the treatment of fatigue and neurocognitive deficits caused by long COVID, said Viswanathan, adding that it’s another medication that she’s found useful for a number of her patients.
It’s thought that these cognitive symptoms are caused by an inflammatory cytokine release that leads to excessive stimulation of neurotransmitters in the body. According to a June 2024 article in the American Journal of Psychiatry, “Modafinil can therapeutically act on these pathways, which possibly contributed to the symptomatic improvement.” But the medication has not been studied widely in patients with long COVID and has been shown to have interactions with other medications.
Metformin
Some research has shown that metformin, a well-known diabetes medication, reduces instances of long COVID when taken during the illness’s acute phase. It seems to boost metabolic function in patients.
“It makes sense that it would work because it seems to have anti-inflammatory effects on the body,” said Grace McComsey, MD, who leads one of the 15 nationwide long COVID centers funded by the federal RECOVER (Researching COVID to Enhance Recovery) Initiative in Cleveland, Ohio. McComsey added that it may reduce the viral persistence that causes some forms of long COVID.
A study published in the October 2023 issue of the journal The Lancet Infectious Diseases found that metformin seemed to reduce instances of long COVID in patients who took it after being diagnosed with acute COVID. It seems less effective in patients who already have long COVID.
Antihistamines
Other data suggest that some patients with long COVID showed improvement after taking antihistamines. Research has shown that long COVID symptoms improved in 29% of patients with long COVID.
While researchers aren’t sure why antihistamines work to quell long COVID, the thought is that, when mast cells, a white blood cell that’s part of the immune system, shed granules and cause an inflammatory reaction, they release a lot of histamines. Antihistamine medications like famotidine block histamine receptors in the body, improving symptoms like brain fog, difficulty breathing, and elevated heart rate in patients.
“For some patients, these can be a lifesaver,” said David Putrino, the Nash Family Director of the Cohen Center for Recovery from Complex Chronic Illness and a national leader in the treatment of long COVID.
Putrino cautions patients toward taking these and other medications haphazardly without fully understanding that all treatments have risks, especially if they’re taking a number of them.
“Often patients are told that there’s no risk to trying something, but physicians should be counseling their patients and reminding them that there is a risk that includes medication sensitivities and medication interactions,” said Putrino.
The good news is that doctors have begun to identify some treatments that seem to be working in their patients, but we still don’t have the large-scale clinical trials to identify which treatments will work for certain patients and why.
There’s still so much we don’t know, and for physicians on the front lines of treating long COVID, it’s still largely a guessing game. “This is a constellation of symptoms; it’s not just one thing,” said Al-Aly. And while a treatment might be wildly effective for one patient, it might be ineffective or worse, problematic, for another.
A version of this article first appeared on Medscape.com.
Long COVID is a symptom-driven disease, meaning that with no cure, physicians primarily treat the symptoms their patients are experiencing. But as 2024 winds down, researchers have begun to pinpoint a number of treatments that are bringing relief to the 17 million Americans diagnosed with long COVID.
Here’s a current look at what research has identified as some of the most promising treatments.
Low-Dose Naltrexone
Some research suggests that low-dose naltrexone may be helpful for patients suffering from brain fog, pain, sleep issues, and fatigue, said Ziyad Al-Aly, MD, a global expert on long COVID and chief of research and development at the Veterans Affairs St Louis Health Care System in Missouri.
Low-dose naltrexone is an anti-inflammatory agent currently approved by the Food and Drug Administration for the treatment of alcohol and opioid dependence.
“We don’t know the mechanism for how the medication works, and for that matter, we don’t really understand what causes brain fog. But perhaps its anti-inflammatory properties seem to help, and for some patients, low-dose naltrexone has been helpful,” said Al-Aly.
A March 2024 study found that both fatigue and pain were improved in patients taking low-dose naltrexone. In another study, published in the June 2024 issue of Frontiers in Medicine, researchers found that low-dose naltrexone was associated with improvement of several clinical symptoms related to long COVID such as fatigue, poor sleep quality, brain fog, post-exertional malaise, and headache.
Selective Serotonin Reuptake Inhibitors (SSRIs) and Antidepressants
In 2023, University of Pennsylvania researchers uncovered a link between long COVID and lower levels of serotonin in the body. This helped point to the potential treatment of using SSRIs to treat the condition.
For patients who have overlapping psychiatric issues that go along with brain fog, SSRIs prescribed to treat depression and other mental health conditions, as well as the antidepressant Wellbutrin, have been shown effective at dealing with concentration issues, brain fog, and depression, said Nisha Viswanathan, MD, director of the University of California, Los Angeles (UCLA) Long COVID Program at UCLA Health.
A study published in the November 2023 issue of the journal Scientific Reports found that SSRIs led to a “considerable reduction of symptoms,” especially brain fog, fatigue, sensory overload, and overall improved functioning. Low-dose Abilify, which contains aripiprazole, an antipsychotic medication, has also been found to be effective for cognitive issues caused by long COVID.
“Abilify is traditionally used for the treatment of schizophrenia or other psychotic disorders, but in a low-dose format, there is some data to suggest that it can also be anti-inflammatory and helpful for cognitive issues like brain fog,” said Viswanathan.
Modafinil
Modafinil, a medication previously used for managing narcolepsy, has also been shown effective for the treatment of fatigue and neurocognitive deficits caused by long COVID, said Viswanathan, adding that it’s another medication that she’s found useful for a number of her patients.
It’s thought that these cognitive symptoms are caused by an inflammatory cytokine release that leads to excessive stimulation of neurotransmitters in the body. According to a June 2024 article in the American Journal of Psychiatry, “Modafinil can therapeutically act on these pathways, which possibly contributed to the symptomatic improvement.” But the medication has not been studied widely in patients with long COVID and has been shown to have interactions with other medications.
Metformin
Some research has shown that metformin, a well-known diabetes medication, reduces instances of long COVID when taken during the illness’s acute phase. It seems to boost metabolic function in patients.
“It makes sense that it would work because it seems to have anti-inflammatory effects on the body,” said Grace McComsey, MD, who leads one of the 15 nationwide long COVID centers funded by the federal RECOVER (Researching COVID to Enhance Recovery) Initiative in Cleveland, Ohio. McComsey added that it may reduce the viral persistence that causes some forms of long COVID.
A study published in the October 2023 issue of the journal The Lancet Infectious Diseases found that metformin seemed to reduce instances of long COVID in patients who took it after being diagnosed with acute COVID. It seems less effective in patients who already have long COVID.
Antihistamines
Other data suggest that some patients with long COVID showed improvement after taking antihistamines. Research has shown that long COVID symptoms improved in 29% of patients with long COVID.
While researchers aren’t sure why antihistamines work to quell long COVID, the thought is that, when mast cells, a white blood cell that’s part of the immune system, shed granules and cause an inflammatory reaction, they release a lot of histamines. Antihistamine medications like famotidine block histamine receptors in the body, improving symptoms like brain fog, difficulty breathing, and elevated heart rate in patients.
“For some patients, these can be a lifesaver,” said David Putrino, the Nash Family Director of the Cohen Center for Recovery from Complex Chronic Illness and a national leader in the treatment of long COVID.
Putrino cautions patients toward taking these and other medications haphazardly without fully understanding that all treatments have risks, especially if they’re taking a number of them.
“Often patients are told that there’s no risk to trying something, but physicians should be counseling their patients and reminding them that there is a risk that includes medication sensitivities and medication interactions,” said Putrino.
The good news is that doctors have begun to identify some treatments that seem to be working in their patients, but we still don’t have the large-scale clinical trials to identify which treatments will work for certain patients and why.
There’s still so much we don’t know, and for physicians on the front lines of treating long COVID, it’s still largely a guessing game. “This is a constellation of symptoms; it’s not just one thing,” said Al-Aly. And while a treatment might be wildly effective for one patient, it might be ineffective or worse, problematic, for another.
A version of this article first appeared on Medscape.com.