User login
THE CASE
A 30-year-old woman sought care at her rural family physician’s office for progressive dyspnea and peripheral edema, which she had been experiencing for several weeks. She was G1P0 and in her 35th week of gestation.
Her medical history was remarkable for mild preeclampsia, which was being managed observantly by her obstetrician in consultation with a maternal-fetal medicine specialist. She had been evaluated by her local hospital’s labor and delivery department and her maternal-fetal medicine specialist earlier in the week and seen the previous day by her obstetrician for these signs and symptoms. They all reassured her and told her these symptoms were normal during pregnancy. No diagnostic studies were performed. However, she remained concerned and decided to see her family physician for another opinion.
Upon presentation to her family physician, the patient was afebrile. Her blood pressure was 135/98 mm Hg; heart rate, 96 beats/min; and respiration, 20 breaths/min and slightly labored. Edema of 2 to 3+ was noted in her lower extremities, hands, and face. Bibasilar breath sounds were diminished, and her abdomen was nontender.
The family physician suspected left ventricular systolic dysfunction. He worked in a small office that lacked access to a laboratory or radiographic studies. However, he did have an ultrasound machine available, and although he was not skilled in echocardiography to assess cardiac function, he was able to obtain a bedside lung ultrasound.
THE DIAGNOSIS
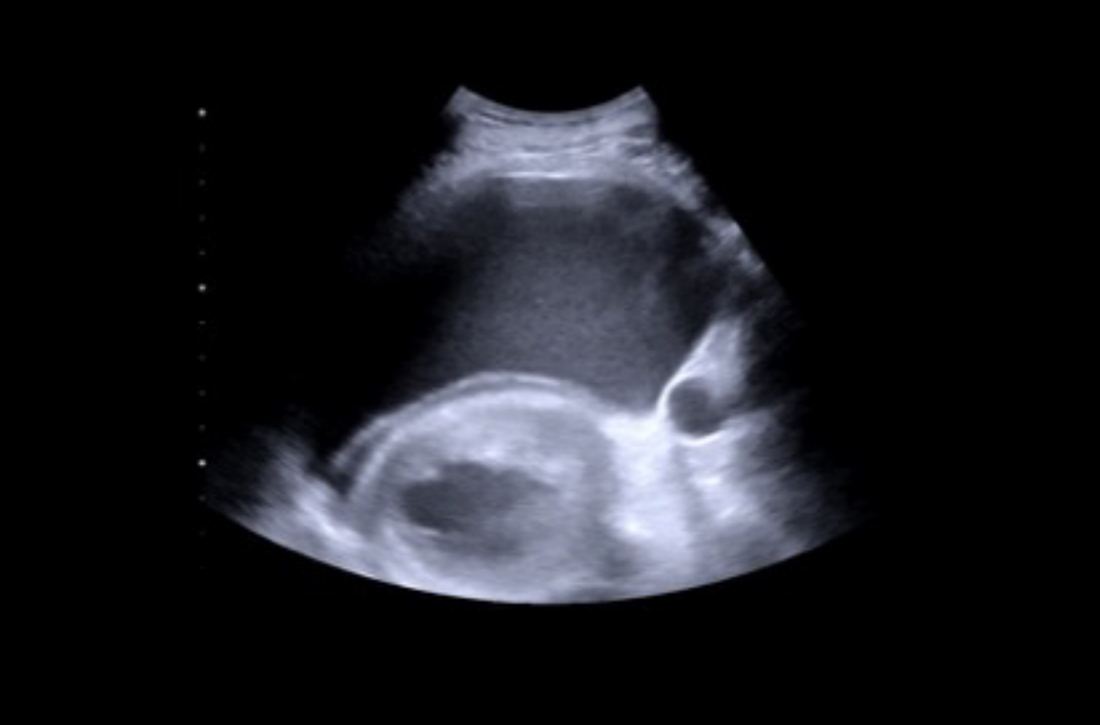
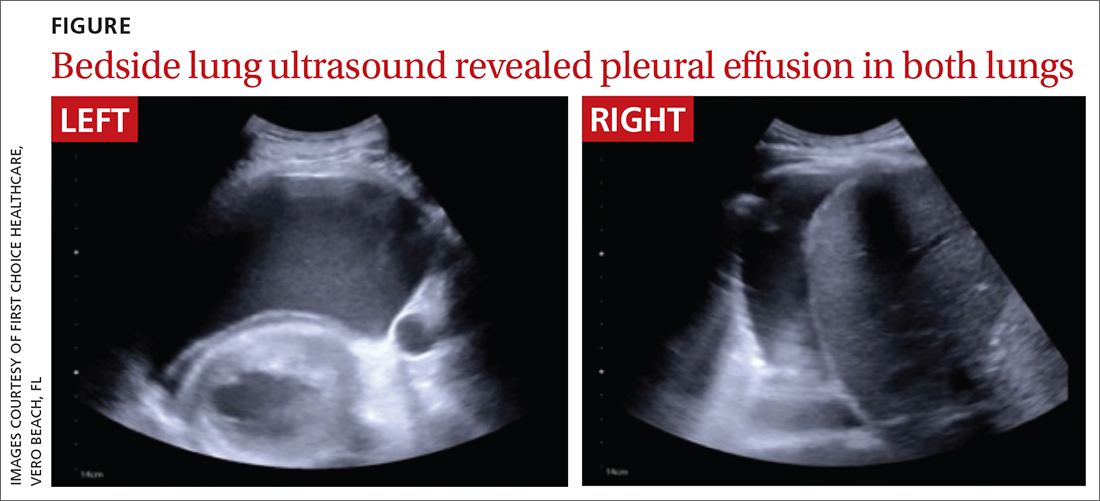
While no B-lines were seen on the lung ultrasound, bilateral plural effusions were noted (FIGURE). This finding, paired with the patient’s signs and symptoms, prompted the family physician to suspect a diagnosis of acute decompensated heart failure with presumptive peripartum cardiomyopathy. The patient was immediately driven to the hospital by her family physician for emergency admission with stat obstetric and cardiology consultations.
An in-hospital echocardiogram revealed severe global hypokinesia with a left ventricular ejection fraction of 25% to 30%, which confirmed the family physician’s suspicions. Laboratory studies were significant for elevated N-terminal pro-brain natriuretic peptide (43,449 pg/mL; normal, < 125 pg/mL), troponin (1.12 ng/mL; normal range, 0-0.10 ng/mL), and white blood cell count (27.6 x 103/µL). She also had evidence of acute renal injury, with blood urea nitrogen of 46 mg/dL (normal range, 7-18 mg/dL), creatinine of 2.0 mg/dL (normal range, 0.5-1.0 mg/dL), and potassium of 7.6 mmol/L (normal range, 3.5-5.1 mmol/L). Emergency delivery was induced by amniotomy, resulting in the birth of a baby girl weighing 5 lb 4 oz (Apgar scores 6, 8, and 9).
Following delivery, the patient was placed on a milrinone infusion and required dialysis. She was emergently transferred to a tertiary care hospital, where she was admitted to the cardiac intensive care unit by the cardiology/heart transplant service with nephrology and obstetric consultations. Hematology and infectious disease specialists were consulted to rule out HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome and sepsis, respectively. Her course of care remained complicated with further testing, including cardiac catheterization and biopsy, which was negative for additional pathology.
Continue to: One week after admission...
One week after admission, she was discharged home with a 24-hour wearable external cardiac defibrillator and a confirmed diagnosis of peripartum cardiomyopathy. Her medication regimen included digoxin (125 µg 3 times/wk), spironolactone (25 mg/d), carvedilol (3.125 mg twice daily), sacubitril/valsartan (24 mg/26 mg twice daily), furosemide (20 mg/d as needed for weight gain > 3-4 lb or leg swelling), magnesium oxide (400 mg twice daily), and ferrous sulfate (325 mg/d).
DISCUSSION
Peripartum cardiomyopathy is a rare, life-threatening, idiopathic cardiomyopathy that is responsible for one-half to two-thirds of cardiovascular disease–related maternal deaths in the United States.1,2 It manifests in late pregnancy or early in the postpartum period and is characterized by left ventricular systolic dysfunction with resultant heart failure and an ejection fraction of less than 45%.1,2
Recognized as early as the 1800s by Virchow,2,3 the incidence of peripartum cardiomyopathy in the United States ranges from 1 in 1000 to 4000 live births and is increasing worldwide.1,2 While the cause of peripartum cardiomyopathy remains unknown, risk factors include advanced maternal age, African descent, hypertension, preeclampsia, and multiple gestation pregnancy.1,2
Early diagnosis of peripartum cardiomyopathy is imperative for survival of both mother and baby.4 This may be difficult because the signs and symptoms of heart failure—such as dyspnea, edema, orthopnea, cough, and chest and abdominal pain—overlap with those of a typical pregnancy, resulting in it often being missed on evaluation.1,2
Dx with echocardiography; in a pinch, consider lung ultrasound
Usually a diagnosis of peripartum cardiomyopathy is established with echocardiography.1,2 Thus, this case is of significant importance because it illustrates the successful use of lung ultrasound—a simple and easy test—by a rural family doctor to identify this potentially fatal, elusive condition with no additional studies.
Continue to: Use of lung ultrasound...
Use of lung ultrasound in the detection of acute decompensated heart failure is accepted in the medical literature.5-7 Given clinical correlation, a positive scan is defined by the presence of at least 3 B-lines on a longitudinal plane between 2 ribs or, as seen in our case, by the presence of pleural effusion.5-8 Lung ultrasound is readily available worldwide, is completely safe in pregnancy, and is considered one of the easiest studies to perform.7-10
At the patient’s 9-month follow-up visit, she had made a full clinical recovery. Her ejection fraction was 59.8%, and she had stopped all medications. The patient and her child did not experience any continued complications.
THE TAKEAWAY
Family physicians should be aware of peripartum cardiomyopathy—one of the most elusive and life-threatening diseases of pregnancy. When managing a pregnant patient, it is imperative to follow up on complaints such as dyspnea, peripheral edema, and chest and/or abdominal pain. While these symptoms are not unusual during pregnancy, they should always prompt a more thorough evaluation. If peripartum cardiomyopathy is suspected, lung ultrasound is a valuable diagnostic tool for family physicians. Further research is needed before the findings of this case report can be universally applied in the routine prenatal care of women at risk for peripartum cardiomyopathy.
The authors thank their daughter, Nickel Cielo Abarbanell, for her help in the preparation of this manuscript.
CORRESPONDENCE
Neal Robert Abarbanell, MD, First Choice Healthcare, 1867 20th Avenue, Vero Beach, FL 32960; neal.abarbanell@ gmail.com
1. Honigberg MC, Givertz MM. Peripartum cardiomyopathy. BMJ. 2019;364:k5287. doi: 10.1136/bmj.k5287
2. Arany Z, Elkayam U. Peripartum cardiomyopathy. Circulation. 2016;133:1397-1409. doi: 10.1161/CIRCULATIONAHA.115.020491
3. Porak C. De L’influence reciproque de la grossesse et del maladies du Coceur [thesis]. Medical Faculty of Paris, France: 1880.
4. Lewey J, Levine LD, Elovitz MA, et al. Importance of early diagnosis in peripartum cardiomyopathy. Hypertension. 2020;75:91-97. doi: 10.1161/HYPERTENSIONAHA.119.13291
5. Volpicelli G, Caramello V, Cardinale L, et al. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med. 2008;26:585-591. doi: 10.1016/j.ajem.2007.09.014
6. Muniz RT, Mesquita ET, Souza CV Jr, et al. Pulmonary ultrasound in patients with heart failure-systematic review. Arq Bras Cardiol. 2018;110:577-584. doi: 10.5935/abc.20180097
7. Russell FM, Rutz M, Pang PS. Focused ultrasound in the emergency department for patients with acute heart failure. Card Fail Rev. 2015;1:83-86. doi: 10.15420/cfr.2015.1.2.83
8. Gustafsson M, Alehagen U, Johansson P. Imaging congestion with a pocket ultrasound device: prognostic implications in patients with chronic heart failure. J Card Fail. 2015;21:548-554. doi: 10.1016/j.cardfail.2015.02.004
9. Ntusi NA, Samuels P, Moosa S, et al. Diagnosing cardiac disease during pregnancy: imaging modalities. Cardiovasc J Afr. 2016;27:95-103. doi: 10.5830/CVJA-2016-022
10. Kimberly HH, Murray A, Mennicke M, et al. Focused maternal ultrasound by midwives in rural Zambia. Ultrasound Med Biol. 2010;36:1267-1272. doi: 10.1016/j.ultrasmedbio.2010.05.017
THE CASE
A 30-year-old woman sought care at her rural family physician’s office for progressive dyspnea and peripheral edema, which she had been experiencing for several weeks. She was G1P0 and in her 35th week of gestation.
Her medical history was remarkable for mild preeclampsia, which was being managed observantly by her obstetrician in consultation with a maternal-fetal medicine specialist. She had been evaluated by her local hospital’s labor and delivery department and her maternal-fetal medicine specialist earlier in the week and seen the previous day by her obstetrician for these signs and symptoms. They all reassured her and told her these symptoms were normal during pregnancy. No diagnostic studies were performed. However, she remained concerned and decided to see her family physician for another opinion.
Upon presentation to her family physician, the patient was afebrile. Her blood pressure was 135/98 mm Hg; heart rate, 96 beats/min; and respiration, 20 breaths/min and slightly labored. Edema of 2 to 3+ was noted in her lower extremities, hands, and face. Bibasilar breath sounds were diminished, and her abdomen was nontender.
The family physician suspected left ventricular systolic dysfunction. He worked in a small office that lacked access to a laboratory or radiographic studies. However, he did have an ultrasound machine available, and although he was not skilled in echocardiography to assess cardiac function, he was able to obtain a bedside lung ultrasound.
THE DIAGNOSIS
While no B-lines were seen on the lung ultrasound, bilateral plural effusions were noted (FIGURE). This finding, paired with the patient’s signs and symptoms, prompted the family physician to suspect a diagnosis of acute decompensated heart failure with presumptive peripartum cardiomyopathy. The patient was immediately driven to the hospital by her family physician for emergency admission with stat obstetric and cardiology consultations.

An in-hospital echocardiogram revealed severe global hypokinesia with a left ventricular ejection fraction of 25% to 30%, which confirmed the family physician’s suspicions. Laboratory studies were significant for elevated N-terminal pro-brain natriuretic peptide (43,449 pg/mL; normal, < 125 pg/mL), troponin (1.12 ng/mL; normal range, 0-0.10 ng/mL), and white blood cell count (27.6 x 103/µL). She also had evidence of acute renal injury, with blood urea nitrogen of 46 mg/dL (normal range, 7-18 mg/dL), creatinine of 2.0 mg/dL (normal range, 0.5-1.0 mg/dL), and potassium of 7.6 mmol/L (normal range, 3.5-5.1 mmol/L). Emergency delivery was induced by amniotomy, resulting in the birth of a baby girl weighing 5 lb 4 oz (Apgar scores 6, 8, and 9).
Following delivery, the patient was placed on a milrinone infusion and required dialysis. She was emergently transferred to a tertiary care hospital, where she was admitted to the cardiac intensive care unit by the cardiology/heart transplant service with nephrology and obstetric consultations. Hematology and infectious disease specialists were consulted to rule out HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome and sepsis, respectively. Her course of care remained complicated with further testing, including cardiac catheterization and biopsy, which was negative for additional pathology.
Continue to: One week after admission...
One week after admission, she was discharged home with a 24-hour wearable external cardiac defibrillator and a confirmed diagnosis of peripartum cardiomyopathy. Her medication regimen included digoxin (125 µg 3 times/wk), spironolactone (25 mg/d), carvedilol (3.125 mg twice daily), sacubitril/valsartan (24 mg/26 mg twice daily), furosemide (20 mg/d as needed for weight gain > 3-4 lb or leg swelling), magnesium oxide (400 mg twice daily), and ferrous sulfate (325 mg/d).
DISCUSSION
Peripartum cardiomyopathy is a rare, life-threatening, idiopathic cardiomyopathy that is responsible for one-half to two-thirds of cardiovascular disease–related maternal deaths in the United States.1,2 It manifests in late pregnancy or early in the postpartum period and is characterized by left ventricular systolic dysfunction with resultant heart failure and an ejection fraction of less than 45%.1,2
Recognized as early as the 1800s by Virchow,2,3 the incidence of peripartum cardiomyopathy in the United States ranges from 1 in 1000 to 4000 live births and is increasing worldwide.1,2 While the cause of peripartum cardiomyopathy remains unknown, risk factors include advanced maternal age, African descent, hypertension, preeclampsia, and multiple gestation pregnancy.1,2
Early diagnosis of peripartum cardiomyopathy is imperative for survival of both mother and baby.4 This may be difficult because the signs and symptoms of heart failure—such as dyspnea, edema, orthopnea, cough, and chest and abdominal pain—overlap with those of a typical pregnancy, resulting in it often being missed on evaluation.1,2
Dx with echocardiography; in a pinch, consider lung ultrasound
Usually a diagnosis of peripartum cardiomyopathy is established with echocardiography.1,2 Thus, this case is of significant importance because it illustrates the successful use of lung ultrasound—a simple and easy test—by a rural family doctor to identify this potentially fatal, elusive condition with no additional studies.
Continue to: Use of lung ultrasound...
Use of lung ultrasound in the detection of acute decompensated heart failure is accepted in the medical literature.5-7 Given clinical correlation, a positive scan is defined by the presence of at least 3 B-lines on a longitudinal plane between 2 ribs or, as seen in our case, by the presence of pleural effusion.5-8 Lung ultrasound is readily available worldwide, is completely safe in pregnancy, and is considered one of the easiest studies to perform.7-10
At the patient’s 9-month follow-up visit, she had made a full clinical recovery. Her ejection fraction was 59.8%, and she had stopped all medications. The patient and her child did not experience any continued complications.
THE TAKEAWAY
Family physicians should be aware of peripartum cardiomyopathy—one of the most elusive and life-threatening diseases of pregnancy. When managing a pregnant patient, it is imperative to follow up on complaints such as dyspnea, peripheral edema, and chest and/or abdominal pain. While these symptoms are not unusual during pregnancy, they should always prompt a more thorough evaluation. If peripartum cardiomyopathy is suspected, lung ultrasound is a valuable diagnostic tool for family physicians. Further research is needed before the findings of this case report can be universally applied in the routine prenatal care of women at risk for peripartum cardiomyopathy.
The authors thank their daughter, Nickel Cielo Abarbanell, for her help in the preparation of this manuscript.
CORRESPONDENCE
Neal Robert Abarbanell, MD, First Choice Healthcare, 1867 20th Avenue, Vero Beach, FL 32960; neal.abarbanell@ gmail.com
THE CASE
A 30-year-old woman sought care at her rural family physician’s office for progressive dyspnea and peripheral edema, which she had been experiencing for several weeks. She was G1P0 and in her 35th week of gestation.
Her medical history was remarkable for mild preeclampsia, which was being managed observantly by her obstetrician in consultation with a maternal-fetal medicine specialist. She had been evaluated by her local hospital’s labor and delivery department and her maternal-fetal medicine specialist earlier in the week and seen the previous day by her obstetrician for these signs and symptoms. They all reassured her and told her these symptoms were normal during pregnancy. No diagnostic studies were performed. However, she remained concerned and decided to see her family physician for another opinion.
Upon presentation to her family physician, the patient was afebrile. Her blood pressure was 135/98 mm Hg; heart rate, 96 beats/min; and respiration, 20 breaths/min and slightly labored. Edema of 2 to 3+ was noted in her lower extremities, hands, and face. Bibasilar breath sounds were diminished, and her abdomen was nontender.
The family physician suspected left ventricular systolic dysfunction. He worked in a small office that lacked access to a laboratory or radiographic studies. However, he did have an ultrasound machine available, and although he was not skilled in echocardiography to assess cardiac function, he was able to obtain a bedside lung ultrasound.
THE DIAGNOSIS
While no B-lines were seen on the lung ultrasound, bilateral plural effusions were noted (FIGURE). This finding, paired with the patient’s signs and symptoms, prompted the family physician to suspect a diagnosis of acute decompensated heart failure with presumptive peripartum cardiomyopathy. The patient was immediately driven to the hospital by her family physician for emergency admission with stat obstetric and cardiology consultations.

An in-hospital echocardiogram revealed severe global hypokinesia with a left ventricular ejection fraction of 25% to 30%, which confirmed the family physician’s suspicions. Laboratory studies were significant for elevated N-terminal pro-brain natriuretic peptide (43,449 pg/mL; normal, < 125 pg/mL), troponin (1.12 ng/mL; normal range, 0-0.10 ng/mL), and white blood cell count (27.6 x 103/µL). She also had evidence of acute renal injury, with blood urea nitrogen of 46 mg/dL (normal range, 7-18 mg/dL), creatinine of 2.0 mg/dL (normal range, 0.5-1.0 mg/dL), and potassium of 7.6 mmol/L (normal range, 3.5-5.1 mmol/L). Emergency delivery was induced by amniotomy, resulting in the birth of a baby girl weighing 5 lb 4 oz (Apgar scores 6, 8, and 9).
Following delivery, the patient was placed on a milrinone infusion and required dialysis. She was emergently transferred to a tertiary care hospital, where she was admitted to the cardiac intensive care unit by the cardiology/heart transplant service with nephrology and obstetric consultations. Hematology and infectious disease specialists were consulted to rule out HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome and sepsis, respectively. Her course of care remained complicated with further testing, including cardiac catheterization and biopsy, which was negative for additional pathology.
Continue to: One week after admission...
One week after admission, she was discharged home with a 24-hour wearable external cardiac defibrillator and a confirmed diagnosis of peripartum cardiomyopathy. Her medication regimen included digoxin (125 µg 3 times/wk), spironolactone (25 mg/d), carvedilol (3.125 mg twice daily), sacubitril/valsartan (24 mg/26 mg twice daily), furosemide (20 mg/d as needed for weight gain > 3-4 lb or leg swelling), magnesium oxide (400 mg twice daily), and ferrous sulfate (325 mg/d).
DISCUSSION
Peripartum cardiomyopathy is a rare, life-threatening, idiopathic cardiomyopathy that is responsible for one-half to two-thirds of cardiovascular disease–related maternal deaths in the United States.1,2 It manifests in late pregnancy or early in the postpartum period and is characterized by left ventricular systolic dysfunction with resultant heart failure and an ejection fraction of less than 45%.1,2
Recognized as early as the 1800s by Virchow,2,3 the incidence of peripartum cardiomyopathy in the United States ranges from 1 in 1000 to 4000 live births and is increasing worldwide.1,2 While the cause of peripartum cardiomyopathy remains unknown, risk factors include advanced maternal age, African descent, hypertension, preeclampsia, and multiple gestation pregnancy.1,2
Early diagnosis of peripartum cardiomyopathy is imperative for survival of both mother and baby.4 This may be difficult because the signs and symptoms of heart failure—such as dyspnea, edema, orthopnea, cough, and chest and abdominal pain—overlap with those of a typical pregnancy, resulting in it often being missed on evaluation.1,2
Dx with echocardiography; in a pinch, consider lung ultrasound
Usually a diagnosis of peripartum cardiomyopathy is established with echocardiography.1,2 Thus, this case is of significant importance because it illustrates the successful use of lung ultrasound—a simple and easy test—by a rural family doctor to identify this potentially fatal, elusive condition with no additional studies.
Continue to: Use of lung ultrasound...
Use of lung ultrasound in the detection of acute decompensated heart failure is accepted in the medical literature.5-7 Given clinical correlation, a positive scan is defined by the presence of at least 3 B-lines on a longitudinal plane between 2 ribs or, as seen in our case, by the presence of pleural effusion.5-8 Lung ultrasound is readily available worldwide, is completely safe in pregnancy, and is considered one of the easiest studies to perform.7-10
At the patient’s 9-month follow-up visit, she had made a full clinical recovery. Her ejection fraction was 59.8%, and she had stopped all medications. The patient and her child did not experience any continued complications.
THE TAKEAWAY
Family physicians should be aware of peripartum cardiomyopathy—one of the most elusive and life-threatening diseases of pregnancy. When managing a pregnant patient, it is imperative to follow up on complaints such as dyspnea, peripheral edema, and chest and/or abdominal pain. While these symptoms are not unusual during pregnancy, they should always prompt a more thorough evaluation. If peripartum cardiomyopathy is suspected, lung ultrasound is a valuable diagnostic tool for family physicians. Further research is needed before the findings of this case report can be universally applied in the routine prenatal care of women at risk for peripartum cardiomyopathy.
The authors thank their daughter, Nickel Cielo Abarbanell, for her help in the preparation of this manuscript.
CORRESPONDENCE
Neal Robert Abarbanell, MD, First Choice Healthcare, 1867 20th Avenue, Vero Beach, FL 32960; neal.abarbanell@ gmail.com
1. Honigberg MC, Givertz MM. Peripartum cardiomyopathy. BMJ. 2019;364:k5287. doi: 10.1136/bmj.k5287
2. Arany Z, Elkayam U. Peripartum cardiomyopathy. Circulation. 2016;133:1397-1409. doi: 10.1161/CIRCULATIONAHA.115.020491
3. Porak C. De L’influence reciproque de la grossesse et del maladies du Coceur [thesis]. Medical Faculty of Paris, France: 1880.
4. Lewey J, Levine LD, Elovitz MA, et al. Importance of early diagnosis in peripartum cardiomyopathy. Hypertension. 2020;75:91-97. doi: 10.1161/HYPERTENSIONAHA.119.13291
5. Volpicelli G, Caramello V, Cardinale L, et al. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med. 2008;26:585-591. doi: 10.1016/j.ajem.2007.09.014
6. Muniz RT, Mesquita ET, Souza CV Jr, et al. Pulmonary ultrasound in patients with heart failure-systematic review. Arq Bras Cardiol. 2018;110:577-584. doi: 10.5935/abc.20180097
7. Russell FM, Rutz M, Pang PS. Focused ultrasound in the emergency department for patients with acute heart failure. Card Fail Rev. 2015;1:83-86. doi: 10.15420/cfr.2015.1.2.83
8. Gustafsson M, Alehagen U, Johansson P. Imaging congestion with a pocket ultrasound device: prognostic implications in patients with chronic heart failure. J Card Fail. 2015;21:548-554. doi: 10.1016/j.cardfail.2015.02.004
9. Ntusi NA, Samuels P, Moosa S, et al. Diagnosing cardiac disease during pregnancy: imaging modalities. Cardiovasc J Afr. 2016;27:95-103. doi: 10.5830/CVJA-2016-022
10. Kimberly HH, Murray A, Mennicke M, et al. Focused maternal ultrasound by midwives in rural Zambia. Ultrasound Med Biol. 2010;36:1267-1272. doi: 10.1016/j.ultrasmedbio.2010.05.017
1. Honigberg MC, Givertz MM. Peripartum cardiomyopathy. BMJ. 2019;364:k5287. doi: 10.1136/bmj.k5287
2. Arany Z, Elkayam U. Peripartum cardiomyopathy. Circulation. 2016;133:1397-1409. doi: 10.1161/CIRCULATIONAHA.115.020491
3. Porak C. De L’influence reciproque de la grossesse et del maladies du Coceur [thesis]. Medical Faculty of Paris, France: 1880.
4. Lewey J, Levine LD, Elovitz MA, et al. Importance of early diagnosis in peripartum cardiomyopathy. Hypertension. 2020;75:91-97. doi: 10.1161/HYPERTENSIONAHA.119.13291
5. Volpicelli G, Caramello V, Cardinale L, et al. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med. 2008;26:585-591. doi: 10.1016/j.ajem.2007.09.014
6. Muniz RT, Mesquita ET, Souza CV Jr, et al. Pulmonary ultrasound in patients with heart failure-systematic review. Arq Bras Cardiol. 2018;110:577-584. doi: 10.5935/abc.20180097
7. Russell FM, Rutz M, Pang PS. Focused ultrasound in the emergency department for patients with acute heart failure. Card Fail Rev. 2015;1:83-86. doi: 10.15420/cfr.2015.1.2.83
8. Gustafsson M, Alehagen U, Johansson P. Imaging congestion with a pocket ultrasound device: prognostic implications in patients with chronic heart failure. J Card Fail. 2015;21:548-554. doi: 10.1016/j.cardfail.2015.02.004
9. Ntusi NA, Samuels P, Moosa S, et al. Diagnosing cardiac disease during pregnancy: imaging modalities. Cardiovasc J Afr. 2016;27:95-103. doi: 10.5830/CVJA-2016-022
10. Kimberly HH, Murray A, Mennicke M, et al. Focused maternal ultrasound by midwives in rural Zambia. Ultrasound Med Biol. 2010;36:1267-1272. doi: 10.1016/j.ultrasmedbio.2010.05.017
► Progressive dyspnea and peripheral edema
► 35th week of gestation with a history of mild preeclampsia