User login
ONO-4059 makes waves in heavily pretreated CLL
NEW ORLEANS – Early data suggest that the second-generation oral BTK inhibitor ONO-4059 may give ibrutinib a run for its money in chronic lymphocytic leukemia.
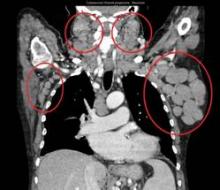
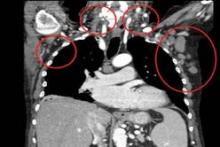
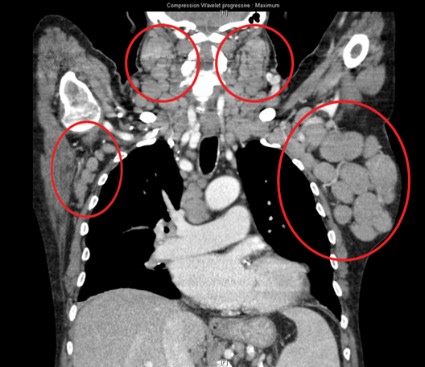
The response rate to ONO-4059 monotherapy was 89% overall and 71% in those with the deleterious 17p deletion among 18 heavily pretreated patients with relapsed/refractory or high-risk CLL in a phase I, dose-escalation study.
Patients had already received a median of three prior therapies, including rituximab (84%) and fludarabine (95%), and had no higher priority therapy available to them, said Dr. Gilles Salles of Hospices Civils de Lyon (France), Universite Claude Bernard Lyon.
All patients had improved hemoglobin and platelet counts after 3 months on treatment and rapid reductions in lymph node size within the first 28-day cycle. Tumor burden was reduced by 50% for most patients, and all but one patient experienced a response that was detectable on a CT scan.
"This was true whatever their FISH status or 17p or 11q deletion status," Dr. Salles said at the annual meeting of the American Society of Hematology.
ONO-4059 is a highly selective Bruton’s tyrosine kinase (BTK) inhibitor with antitumor activity in several preclinical models.
No patients had received prior treatment with a P13 kinase or a BTK inhibitor, including ibrutinib (Imbruvica), which recently gained accelerated approval for previously treated mantle cell lymphoma.
ONO-4059 was given at daily doses ranging from 20 mg to 320 mg for up to 6 months, with the option of additional dosing up to 2 years. Sustained BTK inhibition was established at doses of 40 mg and higher.
Overall, the best response was a partial response in 14 patients, as well as two partial responses with lymphocytosis and one stable disease, he said. No complete responses occurred.
One patient progressed roughly 1 month after showing an initial response and complete disappearance of all palpable disease on physical exam. Richter’s syndrome was suspected.
"It’s very promising efficacy in this highly pretreated population," Dr. Salles said.
Patients with relapsed/refractory mantle cell lymphoma and diffuse large B-cell lymphoma, especially the ABC subtype, also appear sensitive to ONO-4059. Overall response rates were 43% and 75%, respectively, including three complete responses reported from the phase I study in a separate poster presentation at the meeting.
ONO-4059 had a favorable safety profile with a single dose-limiting toxicity observed in a patient who had Waldenstrom’s macroglobulinemia, was on the 320-mg dose, and was intolerant to all prior therapies. The maximum tolerated dose has not yet been reached.
The majority of adverse events in the CLL patients were grades 1 and 2. There were no clinically significant bleeding events or bruising, and there was a low incidence of diarrhea and rash, Dr. Salles said.
ONO-4059–related grade 3-4 events were independent of dose and included one grade 3 neutropenia at 20 mg and two grade 4 events at 20 mg and 320 mg. Four serious adverse events (febrile neutropenia, pyrexia, rash, and neutropenia) occurred in three patients, all of whom are still in the study and showing good clinical response, Dr. Salles said. Of the 30 patients dosed to date, 22 remain in the study.
No other trials are firmly planned, and pharmacokinetics/pharmacodynamics data continue to be explored in order to assess a phase II dosage, he said in an interview.
Dr. Salles reported consulting for and receiving honoraria from Roche. Several coauthors have financial ties, including employment with the study sponsor, Ono Pharmaceutical, which is developing ONO-4059.
NEW ORLEANS – Early data suggest that the second-generation oral BTK inhibitor ONO-4059 may give ibrutinib a run for its money in chronic lymphocytic leukemia.
The response rate to ONO-4059 monotherapy was 89% overall and 71% in those with the deleterious 17p deletion among 18 heavily pretreated patients with relapsed/refractory or high-risk CLL in a phase I, dose-escalation study.
Patients had already received a median of three prior therapies, including rituximab (84%) and fludarabine (95%), and had no higher priority therapy available to them, said Dr. Gilles Salles of Hospices Civils de Lyon (France), Universite Claude Bernard Lyon.
All patients had improved hemoglobin and platelet counts after 3 months on treatment and rapid reductions in lymph node size within the first 28-day cycle. Tumor burden was reduced by 50% for most patients, and all but one patient experienced a response that was detectable on a CT scan.
"This was true whatever their FISH status or 17p or 11q deletion status," Dr. Salles said at the annual meeting of the American Society of Hematology.
ONO-4059 is a highly selective Bruton’s tyrosine kinase (BTK) inhibitor with antitumor activity in several preclinical models.
No patients had received prior treatment with a P13 kinase or a BTK inhibitor, including ibrutinib (Imbruvica), which recently gained accelerated approval for previously treated mantle cell lymphoma.
ONO-4059 was given at daily doses ranging from 20 mg to 320 mg for up to 6 months, with the option of additional dosing up to 2 years. Sustained BTK inhibition was established at doses of 40 mg and higher.
Overall, the best response was a partial response in 14 patients, as well as two partial responses with lymphocytosis and one stable disease, he said. No complete responses occurred.
One patient progressed roughly 1 month after showing an initial response and complete disappearance of all palpable disease on physical exam. Richter’s syndrome was suspected.
"It’s very promising efficacy in this highly pretreated population," Dr. Salles said.
Patients with relapsed/refractory mantle cell lymphoma and diffuse large B-cell lymphoma, especially the ABC subtype, also appear sensitive to ONO-4059. Overall response rates were 43% and 75%, respectively, including three complete responses reported from the phase I study in a separate poster presentation at the meeting.
ONO-4059 had a favorable safety profile with a single dose-limiting toxicity observed in a patient who had Waldenstrom’s macroglobulinemia, was on the 320-mg dose, and was intolerant to all prior therapies. The maximum tolerated dose has not yet been reached.
The majority of adverse events in the CLL patients were grades 1 and 2. There were no clinically significant bleeding events or bruising, and there was a low incidence of diarrhea and rash, Dr. Salles said.
ONO-4059–related grade 3-4 events were independent of dose and included one grade 3 neutropenia at 20 mg and two grade 4 events at 20 mg and 320 mg. Four serious adverse events (febrile neutropenia, pyrexia, rash, and neutropenia) occurred in three patients, all of whom are still in the study and showing good clinical response, Dr. Salles said. Of the 30 patients dosed to date, 22 remain in the study.
No other trials are firmly planned, and pharmacokinetics/pharmacodynamics data continue to be explored in order to assess a phase II dosage, he said in an interview.
Dr. Salles reported consulting for and receiving honoraria from Roche. Several coauthors have financial ties, including employment with the study sponsor, Ono Pharmaceutical, which is developing ONO-4059.
NEW ORLEANS – Early data suggest that the second-generation oral BTK inhibitor ONO-4059 may give ibrutinib a run for its money in chronic lymphocytic leukemia.
The response rate to ONO-4059 monotherapy was 89% overall and 71% in those with the deleterious 17p deletion among 18 heavily pretreated patients with relapsed/refractory or high-risk CLL in a phase I, dose-escalation study.
Patients had already received a median of three prior therapies, including rituximab (84%) and fludarabine (95%), and had no higher priority therapy available to them, said Dr. Gilles Salles of Hospices Civils de Lyon (France), Universite Claude Bernard Lyon.
All patients had improved hemoglobin and platelet counts after 3 months on treatment and rapid reductions in lymph node size within the first 28-day cycle. Tumor burden was reduced by 50% for most patients, and all but one patient experienced a response that was detectable on a CT scan.
"This was true whatever their FISH status or 17p or 11q deletion status," Dr. Salles said at the annual meeting of the American Society of Hematology.
ONO-4059 is a highly selective Bruton’s tyrosine kinase (BTK) inhibitor with antitumor activity in several preclinical models.
No patients had received prior treatment with a P13 kinase or a BTK inhibitor, including ibrutinib (Imbruvica), which recently gained accelerated approval for previously treated mantle cell lymphoma.
ONO-4059 was given at daily doses ranging from 20 mg to 320 mg for up to 6 months, with the option of additional dosing up to 2 years. Sustained BTK inhibition was established at doses of 40 mg and higher.
Overall, the best response was a partial response in 14 patients, as well as two partial responses with lymphocytosis and one stable disease, he said. No complete responses occurred.
One patient progressed roughly 1 month after showing an initial response and complete disappearance of all palpable disease on physical exam. Richter’s syndrome was suspected.
"It’s very promising efficacy in this highly pretreated population," Dr. Salles said.
Patients with relapsed/refractory mantle cell lymphoma and diffuse large B-cell lymphoma, especially the ABC subtype, also appear sensitive to ONO-4059. Overall response rates were 43% and 75%, respectively, including three complete responses reported from the phase I study in a separate poster presentation at the meeting.
ONO-4059 had a favorable safety profile with a single dose-limiting toxicity observed in a patient who had Waldenstrom’s macroglobulinemia, was on the 320-mg dose, and was intolerant to all prior therapies. The maximum tolerated dose has not yet been reached.
The majority of adverse events in the CLL patients were grades 1 and 2. There were no clinically significant bleeding events or bruising, and there was a low incidence of diarrhea and rash, Dr. Salles said.
ONO-4059–related grade 3-4 events were independent of dose and included one grade 3 neutropenia at 20 mg and two grade 4 events at 20 mg and 320 mg. Four serious adverse events (febrile neutropenia, pyrexia, rash, and neutropenia) occurred in three patients, all of whom are still in the study and showing good clinical response, Dr. Salles said. Of the 30 patients dosed to date, 22 remain in the study.
No other trials are firmly planned, and pharmacokinetics/pharmacodynamics data continue to be explored in order to assess a phase II dosage, he said in an interview.
Dr. Salles reported consulting for and receiving honoraria from Roche. Several coauthors have financial ties, including employment with the study sponsor, Ono Pharmaceutical, which is developing ONO-4059.
AT ASH 2013
Major finding: The response rate was 89% overall and 71% for patients with 17p deletion.
Data source: A prospective, phase I dose-escalation study in 18 patients with relapsed/refractory or high-risk CLL.
Disclosures: Dr. Salles reported honoraria from Janssen, Gilead, and Celgene. Several coauthors have financial ties, including employment with the study sponsor, Ono Pharmaceutical, which is developing ONO-4059.
Study reveals RBC function in clot contraction

Red blood cells (RBCs) take on a new shape and perform important functions in contracted blood clots, a new study suggests.
Researchers found that, during clot contraction, RBCs can be compressed into many-sided, closely packed, polyhedral structures.
These polyhedral RBCs form an impermeable seal within the clot to stem bleeding and help prevent vascular obstruction. And the cells may be the reason fibrinolysis is hampered after clot contraction.
John Weisel, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues described these findings in Blood.
The researchers knew that, after a blood clot forms, the actin and myosin in platelets start the contraction process and cause the clot to shrink to about one-third of its original size. RBCs are caught up in the contraction process and get pulled by platelets toward the interior of the clot.
But little was known about the structure of contracted clots or the role RBCs play in the contraction process. So Dr Weisel and his colleagues decided to study clot contraction using a novel magnetic resonance technology.
“We found that contracted blood clots develop a remarkable structure, with a meshwork of fibrin and platelet aggregates on the exterior of the clot and a close-packed, tessellated array of compressed polyhedral erythrocytes within,” Dr Weisel said.
The team saw the same morphology in clots created from human blood reconstituted with its cellular and plasma components, as well as clots made with mouse blood.
The polyhedral erythrocytes, or polyhedrocytes as the researchers named them, were also present in human arterial thrombi taken from patients with myocardial infarctions.
The researchers believe the RBCs take on the polyhedral shape so as to decrease volume, surface energy, or bending energy.
The team said their findings might have clinical implications. It is well known that, with time, thrombi develop resistance to being broken up by thrombolytic agents.
And the nearly impermeable barrier formed by RBCs within the contracted clots may help to explain why. Clot contraction could be a target of intervention to prevent the formation of the closely packed polyhedrocytes. ![]()

Red blood cells (RBCs) take on a new shape and perform important functions in contracted blood clots, a new study suggests.
Researchers found that, during clot contraction, RBCs can be compressed into many-sided, closely packed, polyhedral structures.
These polyhedral RBCs form an impermeable seal within the clot to stem bleeding and help prevent vascular obstruction. And the cells may be the reason fibrinolysis is hampered after clot contraction.
John Weisel, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues described these findings in Blood.
The researchers knew that, after a blood clot forms, the actin and myosin in platelets start the contraction process and cause the clot to shrink to about one-third of its original size. RBCs are caught up in the contraction process and get pulled by platelets toward the interior of the clot.
But little was known about the structure of contracted clots or the role RBCs play in the contraction process. So Dr Weisel and his colleagues decided to study clot contraction using a novel magnetic resonance technology.
“We found that contracted blood clots develop a remarkable structure, with a meshwork of fibrin and platelet aggregates on the exterior of the clot and a close-packed, tessellated array of compressed polyhedral erythrocytes within,” Dr Weisel said.
The team saw the same morphology in clots created from human blood reconstituted with its cellular and plasma components, as well as clots made with mouse blood.
The polyhedral erythrocytes, or polyhedrocytes as the researchers named them, were also present in human arterial thrombi taken from patients with myocardial infarctions.
The researchers believe the RBCs take on the polyhedral shape so as to decrease volume, surface energy, or bending energy.
The team said their findings might have clinical implications. It is well known that, with time, thrombi develop resistance to being broken up by thrombolytic agents.
And the nearly impermeable barrier formed by RBCs within the contracted clots may help to explain why. Clot contraction could be a target of intervention to prevent the formation of the closely packed polyhedrocytes. ![]()

Red blood cells (RBCs) take on a new shape and perform important functions in contracted blood clots, a new study suggests.
Researchers found that, during clot contraction, RBCs can be compressed into many-sided, closely packed, polyhedral structures.
These polyhedral RBCs form an impermeable seal within the clot to stem bleeding and help prevent vascular obstruction. And the cells may be the reason fibrinolysis is hampered after clot contraction.
John Weisel, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues described these findings in Blood.
The researchers knew that, after a blood clot forms, the actin and myosin in platelets start the contraction process and cause the clot to shrink to about one-third of its original size. RBCs are caught up in the contraction process and get pulled by platelets toward the interior of the clot.
But little was known about the structure of contracted clots or the role RBCs play in the contraction process. So Dr Weisel and his colleagues decided to study clot contraction using a novel magnetic resonance technology.
“We found that contracted blood clots develop a remarkable structure, with a meshwork of fibrin and platelet aggregates on the exterior of the clot and a close-packed, tessellated array of compressed polyhedral erythrocytes within,” Dr Weisel said.
The team saw the same morphology in clots created from human blood reconstituted with its cellular and plasma components, as well as clots made with mouse blood.
The polyhedral erythrocytes, or polyhedrocytes as the researchers named them, were also present in human arterial thrombi taken from patients with myocardial infarctions.
The researchers believe the RBCs take on the polyhedral shape so as to decrease volume, surface energy, or bending energy.
The team said their findings might have clinical implications. It is well known that, with time, thrombi develop resistance to being broken up by thrombolytic agents.
And the nearly impermeable barrier formed by RBCs within the contracted clots may help to explain why. Clot contraction could be a target of intervention to prevent the formation of the closely packed polyhedrocytes. ![]()
AML scoring system could optimize treatment

Credit: NIGMS
A scoring system that combines genetic and epigenetic changes could help guide therapy for acute myeloid leukemia (AML), according to a study published in the Journal of Clinical Oncology.
The score is based on the presence of 7 mutated genes and DNA methylation.
For each of these genes, lower expression and higher DNA methylation were associated with better patient outcomes.
The investigators therefore believe this scoring system could guide treatment by identifying novel subsets of patients.
“To date, disease classification and prognostication for AML patients have been based largely on chromosomal and genetic markers,” said principal investigator Clara D. Bloomfield, MD, of The Ohio State University in Columbus.
“Epigenetic changes that affect gene expression have not been considered. Here, we show that epigenetic changes in previously recognized and prognostically important mutated genes can identify novel patient subgroups, which might better help guide therapy.”
Creating the score
Dr Bloomfield and her colleagues identified the 7-gene panel in 134 patients who were 60 and older, had cytogenetically normal AML (CN-AML), and had been treated on Cancer and Leukemia Group B/Alliance clinical trials.
The investigators used next-generation sequencing to analyze regions of methylated DNA associated with prognostically important genetic mutations. The 7 genes they identified are CD34, RHOC, SCRN1, F2RL1, FAM92A1, MIR155HG, and VWA8.
The team then developed a summary score based on the number of genes in the panel showing high expression.
And they applied the unweighted score to 126 of the aforementioned patients. Individuals with 1 or no highly expressed genes had a 96% complete remission (CR) rate, a 3-year disease-free survival (DFS) rate of 32%, and a 3-year overall survival (OS) rate of 39%.
Patients with 6 to 7 highly expressed genes, on the other hand, had a 25% CR rate, a 3-year DFS of 0%, and a 3-year OS of 4%.
Validating the system
The investigators also tested the score in 4 validation cohorts: older patients (age 60 and up) with primary AML (n=72), younger patients (59 and under) with primary AML (n=134), older patients with CN-AML (n=65), and younger patients with CN-AML (n=84).
“In both younger and older patients, those who had no highly expressed genes, or had one highly expressed gene, had the best outcomes,” said study author Guido Marcucci, MD, of The Ohio State University Comprehensive Cancer Center.
For the younger patients (with primary or CN-AML), individuals with 1 or no highly expressed genes had a 91% to 100% CR rate, a 3-year DFS of 60% to 65%, and a 3-year OS of 76% to 82%.
But younger patients with 6 to 7 highly expressed genes had a 53% to 71% CR rate, a 3-year DFS of 13% to 17%, and a 3-year OS of 7% to 24%.
For the older patients, individuals with 1 or no highly expressed genes had a 69% to 89% CR rate, a 3-year DFS of 42% (CN-AML only), and a 3-year OS of 44% to 46%.
Older patients with 6 to 7 highly expressed genes had a 50% CR rate (both types of AML), a 3-year DFS of 0% (CN-AML only), and a 3-year OS of 10% to 12%. DFS data were not evaluable for the older patients with primary AML due to the small sample size.
“Overall, our findings suggest that the unweighted summary score is a better model compared with all other prognostic markers and previously reported gene-expression profiles,” Dr Bloomfield concluded. ![]()

Credit: NIGMS
A scoring system that combines genetic and epigenetic changes could help guide therapy for acute myeloid leukemia (AML), according to a study published in the Journal of Clinical Oncology.
The score is based on the presence of 7 mutated genes and DNA methylation.
For each of these genes, lower expression and higher DNA methylation were associated with better patient outcomes.
The investigators therefore believe this scoring system could guide treatment by identifying novel subsets of patients.
“To date, disease classification and prognostication for AML patients have been based largely on chromosomal and genetic markers,” said principal investigator Clara D. Bloomfield, MD, of The Ohio State University in Columbus.
“Epigenetic changes that affect gene expression have not been considered. Here, we show that epigenetic changes in previously recognized and prognostically important mutated genes can identify novel patient subgroups, which might better help guide therapy.”
Creating the score
Dr Bloomfield and her colleagues identified the 7-gene panel in 134 patients who were 60 and older, had cytogenetically normal AML (CN-AML), and had been treated on Cancer and Leukemia Group B/Alliance clinical trials.
The investigators used next-generation sequencing to analyze regions of methylated DNA associated with prognostically important genetic mutations. The 7 genes they identified are CD34, RHOC, SCRN1, F2RL1, FAM92A1, MIR155HG, and VWA8.
The team then developed a summary score based on the number of genes in the panel showing high expression.
And they applied the unweighted score to 126 of the aforementioned patients. Individuals with 1 or no highly expressed genes had a 96% complete remission (CR) rate, a 3-year disease-free survival (DFS) rate of 32%, and a 3-year overall survival (OS) rate of 39%.
Patients with 6 to 7 highly expressed genes, on the other hand, had a 25% CR rate, a 3-year DFS of 0%, and a 3-year OS of 4%.
Validating the system
The investigators also tested the score in 4 validation cohorts: older patients (age 60 and up) with primary AML (n=72), younger patients (59 and under) with primary AML (n=134), older patients with CN-AML (n=65), and younger patients with CN-AML (n=84).
“In both younger and older patients, those who had no highly expressed genes, or had one highly expressed gene, had the best outcomes,” said study author Guido Marcucci, MD, of The Ohio State University Comprehensive Cancer Center.
For the younger patients (with primary or CN-AML), individuals with 1 or no highly expressed genes had a 91% to 100% CR rate, a 3-year DFS of 60% to 65%, and a 3-year OS of 76% to 82%.
But younger patients with 6 to 7 highly expressed genes had a 53% to 71% CR rate, a 3-year DFS of 13% to 17%, and a 3-year OS of 7% to 24%.
For the older patients, individuals with 1 or no highly expressed genes had a 69% to 89% CR rate, a 3-year DFS of 42% (CN-AML only), and a 3-year OS of 44% to 46%.
Older patients with 6 to 7 highly expressed genes had a 50% CR rate (both types of AML), a 3-year DFS of 0% (CN-AML only), and a 3-year OS of 10% to 12%. DFS data were not evaluable for the older patients with primary AML due to the small sample size.
“Overall, our findings suggest that the unweighted summary score is a better model compared with all other prognostic markers and previously reported gene-expression profiles,” Dr Bloomfield concluded. ![]()

Credit: NIGMS
A scoring system that combines genetic and epigenetic changes could help guide therapy for acute myeloid leukemia (AML), according to a study published in the Journal of Clinical Oncology.
The score is based on the presence of 7 mutated genes and DNA methylation.
For each of these genes, lower expression and higher DNA methylation were associated with better patient outcomes.
The investigators therefore believe this scoring system could guide treatment by identifying novel subsets of patients.
“To date, disease classification and prognostication for AML patients have been based largely on chromosomal and genetic markers,” said principal investigator Clara D. Bloomfield, MD, of The Ohio State University in Columbus.
“Epigenetic changes that affect gene expression have not been considered. Here, we show that epigenetic changes in previously recognized and prognostically important mutated genes can identify novel patient subgroups, which might better help guide therapy.”
Creating the score
Dr Bloomfield and her colleagues identified the 7-gene panel in 134 patients who were 60 and older, had cytogenetically normal AML (CN-AML), and had been treated on Cancer and Leukemia Group B/Alliance clinical trials.
The investigators used next-generation sequencing to analyze regions of methylated DNA associated with prognostically important genetic mutations. The 7 genes they identified are CD34, RHOC, SCRN1, F2RL1, FAM92A1, MIR155HG, and VWA8.
The team then developed a summary score based on the number of genes in the panel showing high expression.
And they applied the unweighted score to 126 of the aforementioned patients. Individuals with 1 or no highly expressed genes had a 96% complete remission (CR) rate, a 3-year disease-free survival (DFS) rate of 32%, and a 3-year overall survival (OS) rate of 39%.
Patients with 6 to 7 highly expressed genes, on the other hand, had a 25% CR rate, a 3-year DFS of 0%, and a 3-year OS of 4%.
Validating the system
The investigators also tested the score in 4 validation cohorts: older patients (age 60 and up) with primary AML (n=72), younger patients (59 and under) with primary AML (n=134), older patients with CN-AML (n=65), and younger patients with CN-AML (n=84).
“In both younger and older patients, those who had no highly expressed genes, or had one highly expressed gene, had the best outcomes,” said study author Guido Marcucci, MD, of The Ohio State University Comprehensive Cancer Center.
For the younger patients (with primary or CN-AML), individuals with 1 or no highly expressed genes had a 91% to 100% CR rate, a 3-year DFS of 60% to 65%, and a 3-year OS of 76% to 82%.
But younger patients with 6 to 7 highly expressed genes had a 53% to 71% CR rate, a 3-year DFS of 13% to 17%, and a 3-year OS of 7% to 24%.
For the older patients, individuals with 1 or no highly expressed genes had a 69% to 89% CR rate, a 3-year DFS of 42% (CN-AML only), and a 3-year OS of 44% to 46%.
Older patients with 6 to 7 highly expressed genes had a 50% CR rate (both types of AML), a 3-year DFS of 0% (CN-AML only), and a 3-year OS of 10% to 12%. DFS data were not evaluable for the older patients with primary AML due to the small sample size.
“Overall, our findings suggest that the unweighted summary score is a better model compared with all other prognostic markers and previously reported gene-expression profiles,” Dr Bloomfield concluded. ![]()
Method can detect malaria through the skin
a red blood cell; Credit: St Jude
Children’s Research Hospital
Researchers say they have developed a diagnostic technique that can rapidly detect low levels of malaria infection through the skin.
The approach involves a low-powered laser that creates tiny vapor nanobubbles inside malaria-infected cells.
The bursting bubbles have a unique acoustic signature that allows for a sensitive diagnosis.
This method requires no dyes or diagnostic chemicals, and there is no need to draw blood.
A preclinical study published in PNAS showed that the method could detect a single malaria-infected cell among a million normal cells with 0 false-positive readings.
“Ours is the first through-the-skin method that’s been shown to rapidly and accurately detect malaria in seconds, without the use of blood sampling or reagents,” said lead investigator Dmitri Lapotko, PhD, of Rice University in Houston, Texas.
The transdermal diagnostic method takes advantage of the optical properties and nanosize of hemozoin, a nanoparticle produced by the malaria parasite inside a red blood cell. Hemozoin crystals are not found in normal red blood cells.
Dr Lapotko and his colleagues found that hemozoin absorbs the energy from a short laser pulse and creates a transient vapor nanobubble. This short-lived vapor nanobubble emerges around the hemozoin nanoparticle and is detected both acoustically and optically.
Acoustic detection of nanobubbles made it possible to detect malaria in whole blood and individual red blood cells infected with Plasmodium falciparum. The method also detected malaria infection as low as 0.00034% in mice infected with Plasmodium yoelii.
“The nanobubbles are generated on demand and only by hemozoin,” said study author Ekaterina Lukianova-Hleb, PhD, also of Rice University. “For this reason, we found that our tests never returned a false-positive result . . . .”
To determine the feasibility of this technique in humans, the researchers tested it on human ears.
The laser probe reliably detected capillaries through the skin, located the blood vessel in the ear in less than 10 seconds, and was reproducible in all 4 subjects studied. In addition, the method did not cause any discomfort or morphological damage to the ear skin.
Dr Lapotko said the first clinical trials of this technology are expected to begin in Houston soon.
He and his colleagues have also used nanobubble technology to deliver chemotherapy drugs directly to cancer cells. ![]()

a red blood cell; Credit: St Jude
Children’s Research Hospital
Researchers say they have developed a diagnostic technique that can rapidly detect low levels of malaria infection through the skin.
The approach involves a low-powered laser that creates tiny vapor nanobubbles inside malaria-infected cells.
The bursting bubbles have a unique acoustic signature that allows for a sensitive diagnosis.
This method requires no dyes or diagnostic chemicals, and there is no need to draw blood.
A preclinical study published in PNAS showed that the method could detect a single malaria-infected cell among a million normal cells with 0 false-positive readings.
“Ours is the first through-the-skin method that’s been shown to rapidly and accurately detect malaria in seconds, without the use of blood sampling or reagents,” said lead investigator Dmitri Lapotko, PhD, of Rice University in Houston, Texas.
The transdermal diagnostic method takes advantage of the optical properties and nanosize of hemozoin, a nanoparticle produced by the malaria parasite inside a red blood cell. Hemozoin crystals are not found in normal red blood cells.
Dr Lapotko and his colleagues found that hemozoin absorbs the energy from a short laser pulse and creates a transient vapor nanobubble. This short-lived vapor nanobubble emerges around the hemozoin nanoparticle and is detected both acoustically and optically.
Acoustic detection of nanobubbles made it possible to detect malaria in whole blood and individual red blood cells infected with Plasmodium falciparum. The method also detected malaria infection as low as 0.00034% in mice infected with Plasmodium yoelii.
“The nanobubbles are generated on demand and only by hemozoin,” said study author Ekaterina Lukianova-Hleb, PhD, also of Rice University. “For this reason, we found that our tests never returned a false-positive result . . . .”
To determine the feasibility of this technique in humans, the researchers tested it on human ears.
The laser probe reliably detected capillaries through the skin, located the blood vessel in the ear in less than 10 seconds, and was reproducible in all 4 subjects studied. In addition, the method did not cause any discomfort or morphological damage to the ear skin.
Dr Lapotko said the first clinical trials of this technology are expected to begin in Houston soon.
He and his colleagues have also used nanobubble technology to deliver chemotherapy drugs directly to cancer cells. ![]()

a red blood cell; Credit: St Jude
Children’s Research Hospital
Researchers say they have developed a diagnostic technique that can rapidly detect low levels of malaria infection through the skin.
The approach involves a low-powered laser that creates tiny vapor nanobubbles inside malaria-infected cells.
The bursting bubbles have a unique acoustic signature that allows for a sensitive diagnosis.
This method requires no dyes or diagnostic chemicals, and there is no need to draw blood.
A preclinical study published in PNAS showed that the method could detect a single malaria-infected cell among a million normal cells with 0 false-positive readings.
“Ours is the first through-the-skin method that’s been shown to rapidly and accurately detect malaria in seconds, without the use of blood sampling or reagents,” said lead investigator Dmitri Lapotko, PhD, of Rice University in Houston, Texas.
The transdermal diagnostic method takes advantage of the optical properties and nanosize of hemozoin, a nanoparticle produced by the malaria parasite inside a red blood cell. Hemozoin crystals are not found in normal red blood cells.
Dr Lapotko and his colleagues found that hemozoin absorbs the energy from a short laser pulse and creates a transient vapor nanobubble. This short-lived vapor nanobubble emerges around the hemozoin nanoparticle and is detected both acoustically and optically.
Acoustic detection of nanobubbles made it possible to detect malaria in whole blood and individual red blood cells infected with Plasmodium falciparum. The method also detected malaria infection as low as 0.00034% in mice infected with Plasmodium yoelii.
“The nanobubbles are generated on demand and only by hemozoin,” said study author Ekaterina Lukianova-Hleb, PhD, also of Rice University. “For this reason, we found that our tests never returned a false-positive result . . . .”
To determine the feasibility of this technique in humans, the researchers tested it on human ears.
The laser probe reliably detected capillaries through the skin, located the blood vessel in the ear in less than 10 seconds, and was reproducible in all 4 subjects studied. In addition, the method did not cause any discomfort or morphological damage to the ear skin.
Dr Lapotko said the first clinical trials of this technology are expected to begin in Houston soon.
He and his colleagues have also used nanobubble technology to deliver chemotherapy drugs directly to cancer cells. ![]()
Survey quantifies impact of drug shortages

Credit: Rhoda Baer
Drug shortages remain a serious problem for patient safety, according to a small survey of US pharmacy directors.
Of the nearly 200 directors, 49% said patients received suboptimal treatment as a result of drug shortages.
Fifty-five percent of respondents reported medication errors resulting from shortages. And 45% reported adverse events due to drug shortages, including a small number of disabling events and deaths.
These results appear in the Journal of Managed Care Pharmacy.
“Drug shortages are the first thing I think about when I get up in the morning, and it is the last thing on my mind when I go to bed at night,” said study author Gary Fennessy, of Northwestern Memorial HealthCare in Chicago, Illinois.
“This is not a problem that is going to go away on its own. Healthcare leaders must not lose sight of it as a major contributor to patient harm or consider its adverse effects inevitable.”
With this in mind, Fennessy and his colleagues sent an electronic survey on drug shortages to 1516 directors of pharmacy.
The survey asked respondents to include information on patient demographics, patient complaints, adverse events, medication errors, patient outcomes, and institutional costs related to drug shortages.
Only 193 pharmacy directors responded. The majority were from acute care institutions serving less than 100 patients. The locations were divided evenly among suburban, urban, and rural institutions.
The medications most commonly reported to be in short supply were analgesics/anesthetics (n=176, 92%), anti-emetics (n=171, 89%), and electrolytes/total parenteral nutrition (n=162, 84%).
Respondents said drug shortages contributed to a variety of issues, including medication errors (such as giving the wrong dose, the wrong drug, or the wrong frequency).
Fifty-three percent of respondents reported 1 to 10 medication errors resulting from drug shortages. And 2% reported more than 30 medication errors.
Eighty-five percent of respondents said patients had to use alternative medications due to drug shortages, 71% said patients’ experienced delays in treatment, and 49% said patients received suboptimal treatment.
Thirty-three percent of respondents said drug shortages resulted in an increased stay in the hospital, 16% said drug shortages caused treatment failure, and 12% said shortages caused hospital readmission.
Forty-one percent of respondents reported 1 to 5 possible or probable adverse events related to drug shortages, and 3% reported more than 15 adverse events.
One percent of respondents reported 1 to 5 patient deaths resulting from drug shortages, 2% reported a disabling adverse event in 1 to 5 patients, and 19% reported adverse events requiring intervention in 1 to 5 patients.
Fifty respondents provided numbers on their estimated costs resulting from drug shortages. And 73% of these individuals calculated costs greater than $100,000.
Thirty-eight percent of respondents said their organization had received at least 1 patient complaint related to drug shortages. And of those respondents reporting the actual number of patient complaints, 18% reported more than 10 complaints.
“This survey is the first that we are aware of to describe the effects that drug shortages have on patient complaints,” said study author Despina Kotis, PharmD, also of Northwestern Memorial HealthCare.
“It clearly shows that patients are aware these shortages are happening, and they are upset that their care is being adversely affected by them.” ![]()

Credit: Rhoda Baer
Drug shortages remain a serious problem for patient safety, according to a small survey of US pharmacy directors.
Of the nearly 200 directors, 49% said patients received suboptimal treatment as a result of drug shortages.
Fifty-five percent of respondents reported medication errors resulting from shortages. And 45% reported adverse events due to drug shortages, including a small number of disabling events and deaths.
These results appear in the Journal of Managed Care Pharmacy.
“Drug shortages are the first thing I think about when I get up in the morning, and it is the last thing on my mind when I go to bed at night,” said study author Gary Fennessy, of Northwestern Memorial HealthCare in Chicago, Illinois.
“This is not a problem that is going to go away on its own. Healthcare leaders must not lose sight of it as a major contributor to patient harm or consider its adverse effects inevitable.”
With this in mind, Fennessy and his colleagues sent an electronic survey on drug shortages to 1516 directors of pharmacy.
The survey asked respondents to include information on patient demographics, patient complaints, adverse events, medication errors, patient outcomes, and institutional costs related to drug shortages.
Only 193 pharmacy directors responded. The majority were from acute care institutions serving less than 100 patients. The locations were divided evenly among suburban, urban, and rural institutions.
The medications most commonly reported to be in short supply were analgesics/anesthetics (n=176, 92%), anti-emetics (n=171, 89%), and electrolytes/total parenteral nutrition (n=162, 84%).
Respondents said drug shortages contributed to a variety of issues, including medication errors (such as giving the wrong dose, the wrong drug, or the wrong frequency).
Fifty-three percent of respondents reported 1 to 10 medication errors resulting from drug shortages. And 2% reported more than 30 medication errors.
Eighty-five percent of respondents said patients had to use alternative medications due to drug shortages, 71% said patients’ experienced delays in treatment, and 49% said patients received suboptimal treatment.
Thirty-three percent of respondents said drug shortages resulted in an increased stay in the hospital, 16% said drug shortages caused treatment failure, and 12% said shortages caused hospital readmission.
Forty-one percent of respondents reported 1 to 5 possible or probable adverse events related to drug shortages, and 3% reported more than 15 adverse events.
One percent of respondents reported 1 to 5 patient deaths resulting from drug shortages, 2% reported a disabling adverse event in 1 to 5 patients, and 19% reported adverse events requiring intervention in 1 to 5 patients.
Fifty respondents provided numbers on their estimated costs resulting from drug shortages. And 73% of these individuals calculated costs greater than $100,000.
Thirty-eight percent of respondents said their organization had received at least 1 patient complaint related to drug shortages. And of those respondents reporting the actual number of patient complaints, 18% reported more than 10 complaints.
“This survey is the first that we are aware of to describe the effects that drug shortages have on patient complaints,” said study author Despina Kotis, PharmD, also of Northwestern Memorial HealthCare.
“It clearly shows that patients are aware these shortages are happening, and they are upset that their care is being adversely affected by them.” ![]()

Credit: Rhoda Baer
Drug shortages remain a serious problem for patient safety, according to a small survey of US pharmacy directors.
Of the nearly 200 directors, 49% said patients received suboptimal treatment as a result of drug shortages.
Fifty-five percent of respondents reported medication errors resulting from shortages. And 45% reported adverse events due to drug shortages, including a small number of disabling events and deaths.
These results appear in the Journal of Managed Care Pharmacy.
“Drug shortages are the first thing I think about when I get up in the morning, and it is the last thing on my mind when I go to bed at night,” said study author Gary Fennessy, of Northwestern Memorial HealthCare in Chicago, Illinois.
“This is not a problem that is going to go away on its own. Healthcare leaders must not lose sight of it as a major contributor to patient harm or consider its adverse effects inevitable.”
With this in mind, Fennessy and his colleagues sent an electronic survey on drug shortages to 1516 directors of pharmacy.
The survey asked respondents to include information on patient demographics, patient complaints, adverse events, medication errors, patient outcomes, and institutional costs related to drug shortages.
Only 193 pharmacy directors responded. The majority were from acute care institutions serving less than 100 patients. The locations were divided evenly among suburban, urban, and rural institutions.
The medications most commonly reported to be in short supply were analgesics/anesthetics (n=176, 92%), anti-emetics (n=171, 89%), and electrolytes/total parenteral nutrition (n=162, 84%).
Respondents said drug shortages contributed to a variety of issues, including medication errors (such as giving the wrong dose, the wrong drug, or the wrong frequency).
Fifty-three percent of respondents reported 1 to 10 medication errors resulting from drug shortages. And 2% reported more than 30 medication errors.
Eighty-five percent of respondents said patients had to use alternative medications due to drug shortages, 71% said patients’ experienced delays in treatment, and 49% said patients received suboptimal treatment.
Thirty-three percent of respondents said drug shortages resulted in an increased stay in the hospital, 16% said drug shortages caused treatment failure, and 12% said shortages caused hospital readmission.
Forty-one percent of respondents reported 1 to 5 possible or probable adverse events related to drug shortages, and 3% reported more than 15 adverse events.
One percent of respondents reported 1 to 5 patient deaths resulting from drug shortages, 2% reported a disabling adverse event in 1 to 5 patients, and 19% reported adverse events requiring intervention in 1 to 5 patients.
Fifty respondents provided numbers on their estimated costs resulting from drug shortages. And 73% of these individuals calculated costs greater than $100,000.
Thirty-eight percent of respondents said their organization had received at least 1 patient complaint related to drug shortages. And of those respondents reporting the actual number of patient complaints, 18% reported more than 10 complaints.
“This survey is the first that we are aware of to describe the effects that drug shortages have on patient complaints,” said study author Despina Kotis, PharmD, also of Northwestern Memorial HealthCare.
“It clearly shows that patients are aware these shortages are happening, and they are upset that their care is being adversely affected by them.” ![]()
MCL-1 proves critical in MYC-driven lymphomas

Walter and Eliza Hall Institute
Results of preclinical research suggest the prosurvival protein MCL-1 is the BCL-2 family member most important for the growth and survival of MYC-driven lymphomas.
Investigators found that MYC-driven lymphoma growth in mice and human cell lines was significantly more dependent upon MCL-1 than BCL-XL.
And mutations in p53 could diminish but not counteract this dependency.
The team described this research is Genes & Development.
The work built on more than 3 decades of research into how MYC drives cancer development, according to study author Gemma Kelly, PhD, of the Walter and Eliza Hall Institute in Victoria, Australia.
“For many years, we have known that proteins from the BCL-2 protein family enhance cell survival and cooperate with MYC to accelerate the development of cancer,” she said. “Until now, it was not known which specific BCL-2 family protein was most important for the survival and growth of MYC-driven cancers.”
To investigate, Dr Kelly and her colleagues first generated mice in which they could delete Mcl-1 or Bcl-x in c-MYC-driven lymphoma cells.
The researchers found that homozygous loss of Bcl-x slightly impaired lymphoma growth. Four percent of Bcl-x-deleted mice had complete lymphoma regression. The rest experienced a modest delay in tumor expansion and slightly prolonged survival compared to controls (P=0.0367).
On the other hand, homozygous Mcl-1 deletion prompted complete lymphoma regression in 30% of mice, and it significantly improved overall survival compared to controls (P<0.0001). Even heterozygous Mcl-1 deletion substantially impaired lymphoma growth.
The investigators also conducted experiments on human Burkitt lymphoma cell lines. And they found evidence suggesting the survival and growth of Burkitt lymphoma cells is largely dependent on MCL-1. In fact, sustained growth and survival may not depend on BCL-XL at all.
Finally, the researchers investigated the role p53 mutations play in MCL-1 dependency. The results showed that mutations in p53 can reduce but not ablate lymphomas’ dependency on MCL-1.
These findings suggest MCL-1 could be an attractive therapeutic target for MYC-driven cancers, the investigators said, particularly because the loss of a single Mcl-1 allele is well-tolerated in healthy tissues.
“Anticancer agents that target the protein BCL-2, which is closely related to MCL-1, are already showing promise in clinical trials . . . ,” said study author Andreas Strasser, PhD, of the Walter and Eliza Hall Institute.
“We are hopeful that inhibitors of MCL-1 will soon become available for clinical testing. We will be very interested in determining whether these compounds could be used to treat MYC-driven cancers.” ![]()

Walter and Eliza Hall Institute
Results of preclinical research suggest the prosurvival protein MCL-1 is the BCL-2 family member most important for the growth and survival of MYC-driven lymphomas.
Investigators found that MYC-driven lymphoma growth in mice and human cell lines was significantly more dependent upon MCL-1 than BCL-XL.
And mutations in p53 could diminish but not counteract this dependency.
The team described this research is Genes & Development.
The work built on more than 3 decades of research into how MYC drives cancer development, according to study author Gemma Kelly, PhD, of the Walter and Eliza Hall Institute in Victoria, Australia.
“For many years, we have known that proteins from the BCL-2 protein family enhance cell survival and cooperate with MYC to accelerate the development of cancer,” she said. “Until now, it was not known which specific BCL-2 family protein was most important for the survival and growth of MYC-driven cancers.”
To investigate, Dr Kelly and her colleagues first generated mice in which they could delete Mcl-1 or Bcl-x in c-MYC-driven lymphoma cells.
The researchers found that homozygous loss of Bcl-x slightly impaired lymphoma growth. Four percent of Bcl-x-deleted mice had complete lymphoma regression. The rest experienced a modest delay in tumor expansion and slightly prolonged survival compared to controls (P=0.0367).
On the other hand, homozygous Mcl-1 deletion prompted complete lymphoma regression in 30% of mice, and it significantly improved overall survival compared to controls (P<0.0001). Even heterozygous Mcl-1 deletion substantially impaired lymphoma growth.
The investigators also conducted experiments on human Burkitt lymphoma cell lines. And they found evidence suggesting the survival and growth of Burkitt lymphoma cells is largely dependent on MCL-1. In fact, sustained growth and survival may not depend on BCL-XL at all.
Finally, the researchers investigated the role p53 mutations play in MCL-1 dependency. The results showed that mutations in p53 can reduce but not ablate lymphomas’ dependency on MCL-1.
These findings suggest MCL-1 could be an attractive therapeutic target for MYC-driven cancers, the investigators said, particularly because the loss of a single Mcl-1 allele is well-tolerated in healthy tissues.
“Anticancer agents that target the protein BCL-2, which is closely related to MCL-1, are already showing promise in clinical trials . . . ,” said study author Andreas Strasser, PhD, of the Walter and Eliza Hall Institute.
“We are hopeful that inhibitors of MCL-1 will soon become available for clinical testing. We will be very interested in determining whether these compounds could be used to treat MYC-driven cancers.” ![]()

Walter and Eliza Hall Institute
Results of preclinical research suggest the prosurvival protein MCL-1 is the BCL-2 family member most important for the growth and survival of MYC-driven lymphomas.
Investigators found that MYC-driven lymphoma growth in mice and human cell lines was significantly more dependent upon MCL-1 than BCL-XL.
And mutations in p53 could diminish but not counteract this dependency.
The team described this research is Genes & Development.
The work built on more than 3 decades of research into how MYC drives cancer development, according to study author Gemma Kelly, PhD, of the Walter and Eliza Hall Institute in Victoria, Australia.
“For many years, we have known that proteins from the BCL-2 protein family enhance cell survival and cooperate with MYC to accelerate the development of cancer,” she said. “Until now, it was not known which specific BCL-2 family protein was most important for the survival and growth of MYC-driven cancers.”
To investigate, Dr Kelly and her colleagues first generated mice in which they could delete Mcl-1 or Bcl-x in c-MYC-driven lymphoma cells.
The researchers found that homozygous loss of Bcl-x slightly impaired lymphoma growth. Four percent of Bcl-x-deleted mice had complete lymphoma regression. The rest experienced a modest delay in tumor expansion and slightly prolonged survival compared to controls (P=0.0367).
On the other hand, homozygous Mcl-1 deletion prompted complete lymphoma regression in 30% of mice, and it significantly improved overall survival compared to controls (P<0.0001). Even heterozygous Mcl-1 deletion substantially impaired lymphoma growth.
The investigators also conducted experiments on human Burkitt lymphoma cell lines. And they found evidence suggesting the survival and growth of Burkitt lymphoma cells is largely dependent on MCL-1. In fact, sustained growth and survival may not depend on BCL-XL at all.
Finally, the researchers investigated the role p53 mutations play in MCL-1 dependency. The results showed that mutations in p53 can reduce but not ablate lymphomas’ dependency on MCL-1.
These findings suggest MCL-1 could be an attractive therapeutic target for MYC-driven cancers, the investigators said, particularly because the loss of a single Mcl-1 allele is well-tolerated in healthy tissues.
“Anticancer agents that target the protein BCL-2, which is closely related to MCL-1, are already showing promise in clinical trials . . . ,” said study author Andreas Strasser, PhD, of the Walter and Eliza Hall Institute.
“We are hopeful that inhibitors of MCL-1 will soon become available for clinical testing. We will be very interested in determining whether these compounds could be used to treat MYC-driven cancers.” ![]()
Leukemia is leading cause of cancer death among young Americans

receiving chemotherapy
Credit: Rhoda Baer
Leukemia is the leading cause of cancer death in the US for men under 40 and women aged 20 and younger, according to a report by the American Cancer Society.
Non-Hodgkin lymphoma (NHL) is also among the 5 leading causes of cancer death for men under 40 and for women age 80 and older.
These data appear in “Cancer Statistics, 2014,” a report published in CA: A Cancer Journal for Clinicians.
The report includes statistics on cancer incidence and death from 1975 to 2010, as well as projections for 2014.
In the latest data (from 2010), NHL was the fifth leading cause of cancer death for men under 20 and for women over 79. It was the fourth leading cause of cancer death for men ages 20 to 39.
And leukemia was the third leading cause of cancer death for women ages 20 to 39, in addition to being the leading cause of cancer death for women under 21 and men under 40.
However, of all cancer types, leukemia and NHL have seen the largest improvements in survival, according to data comparing 5-year survival rates between 1975-1977 and 2003-2009.
Five-year survival rates for leukemia were 34% for 1975-1977 and 59% for 2003-2009 (P<0.05). For NHL, 5-year survival rates were 47% for 1975-1977 and 71% for 2003-2009 (P<0.05).
Projections for 2014
The report authors took past data into account to make estimates on cancer incidence and death for 2014. They projected that 1,665,540 patients will be diagnosed with cancer this year, and 585,720 patients will die of cancer.
Roughly 79,990 patients will be diagnosed with lymphoma—9190 with Hodgkin lymphoma and 70,800 with NHL. Approximately 18,990 patients will die of NHL, and 1180 will die of Hodgkin lymphoma.
There will be 24,050 new cases of myeloma in 2014 and 11,090 myeloma deaths, the authors said.
This year will see 52,380 patients diagnosed with leukemias—6020 with acute lymphocytic leukemia (ALL), 15,720 with chronic lymphocytic leukemia (CLL), 18,860 with acute myeloid leukemia (AML), 5980 with chronic myeloid leukemia (CML), and 5800 with other types of leukemia.
And there will be 24,090 leukemia deaths—1440 from ALL, 4600 from CLL, 10,460 from AML, 810 from CML, and 6780 from other leukemias.
For more information, see the complete report. ![]()

receiving chemotherapy
Credit: Rhoda Baer
Leukemia is the leading cause of cancer death in the US for men under 40 and women aged 20 and younger, according to a report by the American Cancer Society.
Non-Hodgkin lymphoma (NHL) is also among the 5 leading causes of cancer death for men under 40 and for women age 80 and older.
These data appear in “Cancer Statistics, 2014,” a report published in CA: A Cancer Journal for Clinicians.
The report includes statistics on cancer incidence and death from 1975 to 2010, as well as projections for 2014.
In the latest data (from 2010), NHL was the fifth leading cause of cancer death for men under 20 and for women over 79. It was the fourth leading cause of cancer death for men ages 20 to 39.
And leukemia was the third leading cause of cancer death for women ages 20 to 39, in addition to being the leading cause of cancer death for women under 21 and men under 40.
However, of all cancer types, leukemia and NHL have seen the largest improvements in survival, according to data comparing 5-year survival rates between 1975-1977 and 2003-2009.
Five-year survival rates for leukemia were 34% for 1975-1977 and 59% for 2003-2009 (P<0.05). For NHL, 5-year survival rates were 47% for 1975-1977 and 71% for 2003-2009 (P<0.05).
Projections for 2014
The report authors took past data into account to make estimates on cancer incidence and death for 2014. They projected that 1,665,540 patients will be diagnosed with cancer this year, and 585,720 patients will die of cancer.
Roughly 79,990 patients will be diagnosed with lymphoma—9190 with Hodgkin lymphoma and 70,800 with NHL. Approximately 18,990 patients will die of NHL, and 1180 will die of Hodgkin lymphoma.
There will be 24,050 new cases of myeloma in 2014 and 11,090 myeloma deaths, the authors said.
This year will see 52,380 patients diagnosed with leukemias—6020 with acute lymphocytic leukemia (ALL), 15,720 with chronic lymphocytic leukemia (CLL), 18,860 with acute myeloid leukemia (AML), 5980 with chronic myeloid leukemia (CML), and 5800 with other types of leukemia.
And there will be 24,090 leukemia deaths—1440 from ALL, 4600 from CLL, 10,460 from AML, 810 from CML, and 6780 from other leukemias.
For more information, see the complete report. ![]()

receiving chemotherapy
Credit: Rhoda Baer
Leukemia is the leading cause of cancer death in the US for men under 40 and women aged 20 and younger, according to a report by the American Cancer Society.
Non-Hodgkin lymphoma (NHL) is also among the 5 leading causes of cancer death for men under 40 and for women age 80 and older.
These data appear in “Cancer Statistics, 2014,” a report published in CA: A Cancer Journal for Clinicians.
The report includes statistics on cancer incidence and death from 1975 to 2010, as well as projections for 2014.
In the latest data (from 2010), NHL was the fifth leading cause of cancer death for men under 20 and for women over 79. It was the fourth leading cause of cancer death for men ages 20 to 39.
And leukemia was the third leading cause of cancer death for women ages 20 to 39, in addition to being the leading cause of cancer death for women under 21 and men under 40.
However, of all cancer types, leukemia and NHL have seen the largest improvements in survival, according to data comparing 5-year survival rates between 1975-1977 and 2003-2009.
Five-year survival rates for leukemia were 34% for 1975-1977 and 59% for 2003-2009 (P<0.05). For NHL, 5-year survival rates were 47% for 1975-1977 and 71% for 2003-2009 (P<0.05).
Projections for 2014
The report authors took past data into account to make estimates on cancer incidence and death for 2014. They projected that 1,665,540 patients will be diagnosed with cancer this year, and 585,720 patients will die of cancer.
Roughly 79,990 patients will be diagnosed with lymphoma—9190 with Hodgkin lymphoma and 70,800 with NHL. Approximately 18,990 patients will die of NHL, and 1180 will die of Hodgkin lymphoma.
There will be 24,050 new cases of myeloma in 2014 and 11,090 myeloma deaths, the authors said.
This year will see 52,380 patients diagnosed with leukemias—6020 with acute lymphocytic leukemia (ALL), 15,720 with chronic lymphocytic leukemia (CLL), 18,860 with acute myeloid leukemia (AML), 5980 with chronic myeloid leukemia (CML), and 5800 with other types of leukemia.
And there will be 24,090 leukemia deaths—1440 from ALL, 4600 from CLL, 10,460 from AML, 810 from CML, and 6780 from other leukemias.
For more information, see the complete report. ![]()
Team identifies highly mutagenic compounds

Credit: Heather Luis
Researchers say they have discovered compounds that are hundreds of times more mutagenic than known carcinogens.
These compounds are produced by certain types of chemical reactions, such as those found in vehicle exhaust or the reactions that take place when meat is grilled over a flame.
The discovery of these compounds raises additional concerns about the health impacts of heavily polluted urban air and dietary exposure to carcinogens, according to the researchers.
Their findings were published in Environmental Science and Technology.
“Some of the compounds that we’ve discovered are far more mutagenic than we previously understood and may exist in the environment as a result of heavy air pollution from vehicles or some types of food preparation,” said Staci Simonich, PhD, of Oregon State University College of Agricultural Sciences.
“We don’t know at this point what levels may be present and will explore that in continued research.”
The parent compounds involved in this research are polycyclic aromatic hydrocarbons (PAHs), which are formed naturally as the result of almost any type of combustion.
Many PAHs, such as benzo[a]pyrene, have been shown to induce leukemias, lymphomas, and other cancers. PAHs are now believed to be more of a health concern than we thought in the past and are the subject of extensive research around the world.
PAHs can become even more of a problem when they chemically interact with nitrogen to become nitrated (NPAHs), according to scientists.
The newly discovered compounds are NPAHs that were unknown to this point. The researchers found the direct mutagenicity of the NPAHs with 1 nitrogen group can be 6 to 432 times more than the parent compound. NPAHs based on 2 nitrogen groups can be 272 to 467 times more mutagenic.
And the team said the mutagenic assays they used may actually have understated the increase in toxicity. It could be even higher. ![]()

Credit: Heather Luis
Researchers say they have discovered compounds that are hundreds of times more mutagenic than known carcinogens.
These compounds are produced by certain types of chemical reactions, such as those found in vehicle exhaust or the reactions that take place when meat is grilled over a flame.
The discovery of these compounds raises additional concerns about the health impacts of heavily polluted urban air and dietary exposure to carcinogens, according to the researchers.
Their findings were published in Environmental Science and Technology.
“Some of the compounds that we’ve discovered are far more mutagenic than we previously understood and may exist in the environment as a result of heavy air pollution from vehicles or some types of food preparation,” said Staci Simonich, PhD, of Oregon State University College of Agricultural Sciences.
“We don’t know at this point what levels may be present and will explore that in continued research.”
The parent compounds involved in this research are polycyclic aromatic hydrocarbons (PAHs), which are formed naturally as the result of almost any type of combustion.
Many PAHs, such as benzo[a]pyrene, have been shown to induce leukemias, lymphomas, and other cancers. PAHs are now believed to be more of a health concern than we thought in the past and are the subject of extensive research around the world.
PAHs can become even more of a problem when they chemically interact with nitrogen to become nitrated (NPAHs), according to scientists.
The newly discovered compounds are NPAHs that were unknown to this point. The researchers found the direct mutagenicity of the NPAHs with 1 nitrogen group can be 6 to 432 times more than the parent compound. NPAHs based on 2 nitrogen groups can be 272 to 467 times more mutagenic.
And the team said the mutagenic assays they used may actually have understated the increase in toxicity. It could be even higher. ![]()

Credit: Heather Luis
Researchers say they have discovered compounds that are hundreds of times more mutagenic than known carcinogens.
These compounds are produced by certain types of chemical reactions, such as those found in vehicle exhaust or the reactions that take place when meat is grilled over a flame.
The discovery of these compounds raises additional concerns about the health impacts of heavily polluted urban air and dietary exposure to carcinogens, according to the researchers.
Their findings were published in Environmental Science and Technology.
“Some of the compounds that we’ve discovered are far more mutagenic than we previously understood and may exist in the environment as a result of heavy air pollution from vehicles or some types of food preparation,” said Staci Simonich, PhD, of Oregon State University College of Agricultural Sciences.
“We don’t know at this point what levels may be present and will explore that in continued research.”
The parent compounds involved in this research are polycyclic aromatic hydrocarbons (PAHs), which are formed naturally as the result of almost any type of combustion.
Many PAHs, such as benzo[a]pyrene, have been shown to induce leukemias, lymphomas, and other cancers. PAHs are now believed to be more of a health concern than we thought in the past and are the subject of extensive research around the world.
PAHs can become even more of a problem when they chemically interact with nitrogen to become nitrated (NPAHs), according to scientists.
The newly discovered compounds are NPAHs that were unknown to this point. The researchers found the direct mutagenicity of the NPAHs with 1 nitrogen group can be 6 to 432 times more than the parent compound. NPAHs based on 2 nitrogen groups can be 272 to 467 times more mutagenic.
And the team said the mutagenic assays they used may actually have understated the increase in toxicity. It could be even higher.
Chemo patients may have higher VTE risk than trials suggest

Credit: Andre E.X. Brown
A large-scale analysis assessing the real-world risk of venous thromboembolism (VTE) among chemotherapy patients showed a greater occurrence of VTE than that identified in clinical trials.
The data also revealed a progressively increased risk during the year following treatment initiation.
The study, published in The Oncologist, showed that outpatients receiving chemotherapy were at high risk of both VTE and major bleeding complications.
The risks were particularly high for patients with pancreas, stomach, and lung cancer.
Gary H. Lyman, MD, of the Duke University School of Medicine in Durham, North Carolina, and his colleagues conducted this research, analyzing data from the United States IMPACT healthcare claims database.
Their sample included 27,479 patients with solid tumor malignancies who had undergone chemotherapy. The researchers retrospectively evaluated the patients’ VTE risk, as well as their risk of bleeding and the economic burden borne by the patient as a result of the disease.
The team used 3 definitions of VTE. Definition A included patients who had 1 or more VTE claim. Definition B included patients with 2 or more VTE claims 30 days or more apart, 1 inpatient claim, or 1 outpatient claim in which they received an anticoagulant within 90 days.
Definition C excluded from definition B any VTE events within 90 days of any major or invasive surgery. Codes relating to esophageal, renal, and uterine cancer were excluded.
VTE risk
The risk of VTE increased over time, with a greater percentage of patients developing the complication at 12 months after initiation of chemotherapy than at 3.5 months. This held true across the 3 definitions of VTE considered.
According to definition A, the overall VTE incidence was 7.3% (4.6%-11.6%) at 3.5 months and 13.5% (9.8%-21.3%) at a year.
Rates of VTE were similar according to definitions B and C. At 3.5 months, the rates were 3.4%-9.6% and 3.2%-8.7%, respectively. At 12 months, they were 7.1%-17.7% and 6.7%-16.6%, respectively.
According to definition A, VTE was most frequently observed in cancers of the pancreas (11.6%), lung (8.5%), and stomach (8.3%).
Major bleeding
Patients with VTE had a higher risk of major bleeding events in the year following chemotherapy initiation.
Individuals receiving outpatient chemotherapy who developed VTE according to definition A at 3.5 months had a higher risk for major bleeding complications within 3.5 months than patients who were not receiving outpatient chemotherapy—11.0% and 3.8%, respectively.
The incidence of major bleeding within 12 months after the index date was 19.8% in patients with VTE (according to definition A) and 9.6% in patients without VTE.
Healthcare costs
While the baseline healthcare costs of patients who would develop VTE were comparable to those of patients who would not, the costs soared for VTE patients over the year following chemotherapy initiation.
On average, in the first 12 months after the index date, patients with VTE had $110,719 worth of healthcare costs, compared with $76,804 for patients without VTE. This difference was primarily accounted for by VTE-related inpatient, outpatient, and emergency room expenses.
Claims data vs trial data
The researchers noted that the incidence of VTE reported in this study is inconsistent with data previously reported in randomized clinical trials, which may be due to the selection of lower-risk patients for participation in these studies.
“Importantly, this observational study suggests that the observed rates of symptomatic VTE in real-world practice are considerably greater than reported in patients eligible for randomized clinical trials,” Dr Lyman said.
“Clinical oncologists need to be aware of the increased risk of this serious complication of cancer and cancer treatment, and, when the risk is sufficiently great, and the balance of benefits and harms acceptable, oncologists should consider prophylactic anticoagulation.”
Dr Lyman and his colleagues hypothesized that there is a definable, high-risk cohort of patients who would benefit from thromboprophylaxis.
And the scope of this risk warrants consideration for the use of treatments such as low- and ultra-low-molecular-weight heparins, which recent studies have found to be safe and effective thromboprophylaxis for chemotherapy patients.

Credit: Andre E.X. Brown
A large-scale analysis assessing the real-world risk of venous thromboembolism (VTE) among chemotherapy patients showed a greater occurrence of VTE than that identified in clinical trials.
The data also revealed a progressively increased risk during the year following treatment initiation.
The study, published in The Oncologist, showed that outpatients receiving chemotherapy were at high risk of both VTE and major bleeding complications.
The risks were particularly high for patients with pancreas, stomach, and lung cancer.
Gary H. Lyman, MD, of the Duke University School of Medicine in Durham, North Carolina, and his colleagues conducted this research, analyzing data from the United States IMPACT healthcare claims database.
Their sample included 27,479 patients with solid tumor malignancies who had undergone chemotherapy. The researchers retrospectively evaluated the patients’ VTE risk, as well as their risk of bleeding and the economic burden borne by the patient as a result of the disease.
The team used 3 definitions of VTE. Definition A included patients who had 1 or more VTE claim. Definition B included patients with 2 or more VTE claims 30 days or more apart, 1 inpatient claim, or 1 outpatient claim in which they received an anticoagulant within 90 days.
Definition C excluded from definition B any VTE events within 90 days of any major or invasive surgery. Codes relating to esophageal, renal, and uterine cancer were excluded.
VTE risk
The risk of VTE increased over time, with a greater percentage of patients developing the complication at 12 months after initiation of chemotherapy than at 3.5 months. This held true across the 3 definitions of VTE considered.
According to definition A, the overall VTE incidence was 7.3% (4.6%-11.6%) at 3.5 months and 13.5% (9.8%-21.3%) at a year.
Rates of VTE were similar according to definitions B and C. At 3.5 months, the rates were 3.4%-9.6% and 3.2%-8.7%, respectively. At 12 months, they were 7.1%-17.7% and 6.7%-16.6%, respectively.
According to definition A, VTE was most frequently observed in cancers of the pancreas (11.6%), lung (8.5%), and stomach (8.3%).
Major bleeding
Patients with VTE had a higher risk of major bleeding events in the year following chemotherapy initiation.
Individuals receiving outpatient chemotherapy who developed VTE according to definition A at 3.5 months had a higher risk for major bleeding complications within 3.5 months than patients who were not receiving outpatient chemotherapy—11.0% and 3.8%, respectively.
The incidence of major bleeding within 12 months after the index date was 19.8% in patients with VTE (according to definition A) and 9.6% in patients without VTE.
Healthcare costs
While the baseline healthcare costs of patients who would develop VTE were comparable to those of patients who would not, the costs soared for VTE patients over the year following chemotherapy initiation.
On average, in the first 12 months after the index date, patients with VTE had $110,719 worth of healthcare costs, compared with $76,804 for patients without VTE. This difference was primarily accounted for by VTE-related inpatient, outpatient, and emergency room expenses.
Claims data vs trial data
The researchers noted that the incidence of VTE reported in this study is inconsistent with data previously reported in randomized clinical trials, which may be due to the selection of lower-risk patients for participation in these studies.
“Importantly, this observational study suggests that the observed rates of symptomatic VTE in real-world practice are considerably greater than reported in patients eligible for randomized clinical trials,” Dr Lyman said.
“Clinical oncologists need to be aware of the increased risk of this serious complication of cancer and cancer treatment, and, when the risk is sufficiently great, and the balance of benefits and harms acceptable, oncologists should consider prophylactic anticoagulation.”
Dr Lyman and his colleagues hypothesized that there is a definable, high-risk cohort of patients who would benefit from thromboprophylaxis.
And the scope of this risk warrants consideration for the use of treatments such as low- and ultra-low-molecular-weight heparins, which recent studies have found to be safe and effective thromboprophylaxis for chemotherapy patients.

Credit: Andre E.X. Brown
A large-scale analysis assessing the real-world risk of venous thromboembolism (VTE) among chemotherapy patients showed a greater occurrence of VTE than that identified in clinical trials.
The data also revealed a progressively increased risk during the year following treatment initiation.
The study, published in The Oncologist, showed that outpatients receiving chemotherapy were at high risk of both VTE and major bleeding complications.
The risks were particularly high for patients with pancreas, stomach, and lung cancer.
Gary H. Lyman, MD, of the Duke University School of Medicine in Durham, North Carolina, and his colleagues conducted this research, analyzing data from the United States IMPACT healthcare claims database.
Their sample included 27,479 patients with solid tumor malignancies who had undergone chemotherapy. The researchers retrospectively evaluated the patients’ VTE risk, as well as their risk of bleeding and the economic burden borne by the patient as a result of the disease.
The team used 3 definitions of VTE. Definition A included patients who had 1 or more VTE claim. Definition B included patients with 2 or more VTE claims 30 days or more apart, 1 inpatient claim, or 1 outpatient claim in which they received an anticoagulant within 90 days.
Definition C excluded from definition B any VTE events within 90 days of any major or invasive surgery. Codes relating to esophageal, renal, and uterine cancer were excluded.
VTE risk
The risk of VTE increased over time, with a greater percentage of patients developing the complication at 12 months after initiation of chemotherapy than at 3.5 months. This held true across the 3 definitions of VTE considered.
According to definition A, the overall VTE incidence was 7.3% (4.6%-11.6%) at 3.5 months and 13.5% (9.8%-21.3%) at a year.
Rates of VTE were similar according to definitions B and C. At 3.5 months, the rates were 3.4%-9.6% and 3.2%-8.7%, respectively. At 12 months, they were 7.1%-17.7% and 6.7%-16.6%, respectively.
According to definition A, VTE was most frequently observed in cancers of the pancreas (11.6%), lung (8.5%), and stomach (8.3%).
Major bleeding
Patients with VTE had a higher risk of major bleeding events in the year following chemotherapy initiation.
Individuals receiving outpatient chemotherapy who developed VTE according to definition A at 3.5 months had a higher risk for major bleeding complications within 3.5 months than patients who were not receiving outpatient chemotherapy—11.0% and 3.8%, respectively.
The incidence of major bleeding within 12 months after the index date was 19.8% in patients with VTE (according to definition A) and 9.6% in patients without VTE.
Healthcare costs
While the baseline healthcare costs of patients who would develop VTE were comparable to those of patients who would not, the costs soared for VTE patients over the year following chemotherapy initiation.
On average, in the first 12 months after the index date, patients with VTE had $110,719 worth of healthcare costs, compared with $76,804 for patients without VTE. This difference was primarily accounted for by VTE-related inpatient, outpatient, and emergency room expenses.
Claims data vs trial data
The researchers noted that the incidence of VTE reported in this study is inconsistent with data previously reported in randomized clinical trials, which may be due to the selection of lower-risk patients for participation in these studies.
“Importantly, this observational study suggests that the observed rates of symptomatic VTE in real-world practice are considerably greater than reported in patients eligible for randomized clinical trials,” Dr Lyman said.
“Clinical oncologists need to be aware of the increased risk of this serious complication of cancer and cancer treatment, and, when the risk is sufficiently great, and the balance of benefits and harms acceptable, oncologists should consider prophylactic anticoagulation.”
Dr Lyman and his colleagues hypothesized that there is a definable, high-risk cohort of patients who would benefit from thromboprophylaxis.
And the scope of this risk warrants consideration for the use of treatments such as low- and ultra-low-molecular-weight heparins, which recent studies have found to be safe and effective thromboprophylaxis for chemotherapy patients.