User login
Obstipation unresponsive to usual therapeutic maneuvers
A 64-year-old woman came into our emergency department (ED) complaining of constipation and worsening rectal pain. In an attempt to promote her overall health, the patient had recently begun experimenting with healthy alternatives to her regular diet. Three days before her visit, she had ceased having stools and was experiencing intermittent abdominal cramping. She self-administered 2 bisacodyl suppositories, 2 sodium biphosphate enemas, one 10-ounce bottle of magnesium citrate, and 15 senna-containing laxative tablets without improvement.
She sought care at an urgent care clinic where she received 2 additional enemas and a trial of manual disimpaction—without results. She was sent home to rest and asked to return the next morning for another trial of disimpaction. When the patient’s efforts to manually disimpact herself at home were unsuccessful, she contacted her primary care physician, who arranged a house call. When his own protracted disimpaction procedure was unsuccessful, he referred her to our ED.
On presentation, the patient had lower abdominal and rectal discomfort. Her vital signs were normal except for a temperature of 38.8° C. Her abdomen was soft and nontender. Inspection of her perianal area revealed erythema and excoriations. On digital rectal exam (which was poorly tolerated because of pain), we noted a moderate amount of soft, clay-like feces in the rectal vault, with overflow liquid stool expulsion.
Computed tomography (CT) imaging of the abdomen was obtained to rule out rectal injury or colonic perforation (FIGURE 1).
FIGURE 1
CT scan reveals a speckled intraluminal mass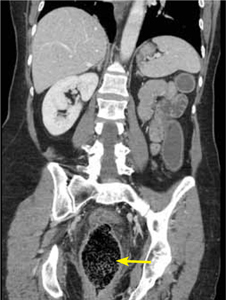
The patient had a markedly distended rectum and distal sigmoid colon caused by an intraluminal mass. Also present: circumferential wall thickening, perirectal edema without extraluminal gas, and generalized proximal colonic wall edema without a drainable collection.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Fecal impaction caused by a proctophytobezoar
CT imaging revealed a proctophytobezoar. On follow-up questioning, the patient recalled consuming approximately 10 ounces of cooked quinoa, a nutritious, gluten-free, high-protein seed, just prior to the onset of her constipation.
Constipation disproportionately affects the elderly and the young.1 Fecal impaction is a sequelae of constipation. Most commonly defined as hard, compacted feces in the rectum, fecal impaction can also include more proximal impactions due to fecal loading or retention.2
Causes of constipation and fecal impaction are similar and include low intake of dietary fiber, dehydration, immobility, alcohol ingestion, laxative abuse, medication adverse effects, depression, dementia, spinal cord dysfunction, diabetes, metabolic imbalances, and hypothyroidism.2,3 Insufficient hydration with consumption of a high-fiber food, as in this case, or with a bulk-forming laxative such as psyllium seed can result in fecal impaction.3
The many causes of a bezoar
A bezoar is a mass of poorly digested material that forms within the gastrointestinal tract—usually in the stomach—and less commonly in the small or large intestine.4 Trichobezoars (hair), lactobezoars (milk curd), phytobezoars (plant fiber), medication bezoars, and lithobezoars (small stones, pebbles, or gravel) are named after their contents. In keeping with this naming tradition, a gummi bear bezoar5 has also been described. Fecal impaction due to phytobezoars primarily composed of seeds has been associated with prickly pears, watermelons, sunflowers, pumpkins, pomegranates,6,7 and sesame seeds.4 Our patient’s experience adds quinoa seeds to this list.
Patients will complain of nausea and rectal urgency
Patients with fecal impaction may complain of nausea, rectal urgency, and rectalgia. A ball-valve effect of the fecal mass may allow paradoxical fecal incontinence and diarrhea.3 Digital rectal exam may demonstrate stool of any consistency, from rock hard pellets to soft clay-like stool.3 Absence of stool in the rectal vault does not rule out fecal impaction, and more proximal impactions may be revealed on plain abdominal radiography as bubbly, speckled masses of stool with associated signs of obstruction, such as colonic dilatation.
Fever, increased leukocyte count, and abdominal tenderness may indicate colonic perforation or ulceration. Signs of generalized peritonitis and free air on abdominal radiography warrant an immediate surgical consult.3
Complications from fecal impaction include bowel obstruction, sigmoid volvulus, and rectal prolapse.2 Stercoral ulceration and perforation due to pressure necrosis from a hard, inspissated fecal mass is an uncommon but life-threatening complication requiring resection of the affected colonic segment.8
What to look for on the CT. When the diagnosis is unclear or signs of complications are present, an abdominal CT is indicated. Concerning CT findings include ulceration, bowel wall enhancement and thickening (FIGURE 2), discontinuity of the bowel wall, presence of fecal material either protruding through the colonic wall or lying free within the intra-abdominal cavity, and extraluminal air.8
FIGURE 2
CT scan shows bowel wall thickening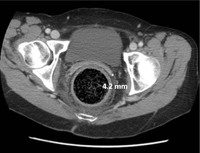
Treatment begins with a pharmacologic approach
By the time a patient with a fecal impaction gets to your office, it’s likely that he or she will have already tried over-the-counter laxatives, stool softeners, and perhaps an enema.
When such pharmacologic management has failed, you’ll need to perform a manual fragmentation and extraction of the fecal mass. Apply topical 2% lidocaine jelly for analgesia and lubrication, and then gently and progressively dilate the anal sphincter with one and then 2 fingers. A scissoring action will fragment the impaction.3
Once fragmentation and partial expulsion has been achieved, you may want to try a lubricating mineral oil enema, bisacodyl suppository, or rectal lavage. If the impaction extends beyond the reach of the fingers, sigmoidoscopic visualization and lavage are indicated.
Adding water-soluble contrast material (Gastrografin) in 20% to 50% solutions directed by fluoroscopy draws water into the lumen, thus lubricating the fecal mass3,9 and helping it to pass spontaneously.
Our patient’s case resolved with a trip to the OR
Since conservative and comprehensive management to improve our patient’s condition failed, she was taken to the operating room for a proctosigmoidoscopic disimpaction. A beveled metal proctoscope was used to disimpact the distal-most 10 cm and then a rigid sigmoidoscope was used to clear the colon of quinoa-laden fecal material to a total distance of 18 cm. Bowel walls were ecchymotic, yet viable and without laceration. She made an uneventful recovery and was discharged on hospital Day 3.
CORRESPONDENCE George L. Higgins, III, MD, Maine Medical Center, Department of Emergency Medicine, 47 Bramhall Street, Portland, ME 04102; higgig@mmc.org
1. Rao SS, Go JT. Update on the management of constipation in the elderly: new treatment options. Clin Interv Aging. 2010;5:163-171.
2. Creason N, Sparks D. Fecal impaction: a review. Nurs Diagn. 2000;11:15-23.
3. Wrenn K. Fecal impaction. N Engl J Med. 1989;321:658-662.
4. Shaw AG, Peacock O, Lund JN, et al. Large bowel obstruction due to sesame seed bezoar: a case report. J Med Case Reports. 2007;1:159.-
5. Barron MM, Steerman P. Gummi bear bezoar: a case report. J Emerg Med. 1989;7:143-144.
6. Eitan A, Bickel A, Katz IM. Fecal impaction in adults: report of 30 cases of seed bezoars in the rectum. Dis Colon Rectum. 2006;49:1768-1771.
7. Eitan A, Katz IM, Sweed Y, et al. Fecal impaction in children: report of 53 cases of rectal seed bezoars. J Pediatr Surg. 2007;42:1114-1117.
8. Kumar P, Pearce O, Higginson A. Imaging manifestations of faecal impaction and stercoral perforation. Clin Radiol. 2011;66:83-88.
9. Brenner BE, Simon RR. Anorectal emergencies. Ann Emerg Med. 1983;12:367-376.
A 64-year-old woman came into our emergency department (ED) complaining of constipation and worsening rectal pain. In an attempt to promote her overall health, the patient had recently begun experimenting with healthy alternatives to her regular diet. Three days before her visit, she had ceased having stools and was experiencing intermittent abdominal cramping. She self-administered 2 bisacodyl suppositories, 2 sodium biphosphate enemas, one 10-ounce bottle of magnesium citrate, and 15 senna-containing laxative tablets without improvement.
She sought care at an urgent care clinic where she received 2 additional enemas and a trial of manual disimpaction—without results. She was sent home to rest and asked to return the next morning for another trial of disimpaction. When the patient’s efforts to manually disimpact herself at home were unsuccessful, she contacted her primary care physician, who arranged a house call. When his own protracted disimpaction procedure was unsuccessful, he referred her to our ED.
On presentation, the patient had lower abdominal and rectal discomfort. Her vital signs were normal except for a temperature of 38.8° C. Her abdomen was soft and nontender. Inspection of her perianal area revealed erythema and excoriations. On digital rectal exam (which was poorly tolerated because of pain), we noted a moderate amount of soft, clay-like feces in the rectal vault, with overflow liquid stool expulsion.
Computed tomography (CT) imaging of the abdomen was obtained to rule out rectal injury or colonic perforation (FIGURE 1).
FIGURE 1
CT scan reveals a speckled intraluminal mass
The patient had a markedly distended rectum and distal sigmoid colon caused by an intraluminal mass. Also present: circumferential wall thickening, perirectal edema without extraluminal gas, and generalized proximal colonic wall edema without a drainable collection.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Fecal impaction caused by a proctophytobezoar
CT imaging revealed a proctophytobezoar. On follow-up questioning, the patient recalled consuming approximately 10 ounces of cooked quinoa, a nutritious, gluten-free, high-protein seed, just prior to the onset of her constipation.
Constipation disproportionately affects the elderly and the young.1 Fecal impaction is a sequelae of constipation. Most commonly defined as hard, compacted feces in the rectum, fecal impaction can also include more proximal impactions due to fecal loading or retention.2
Causes of constipation and fecal impaction are similar and include low intake of dietary fiber, dehydration, immobility, alcohol ingestion, laxative abuse, medication adverse effects, depression, dementia, spinal cord dysfunction, diabetes, metabolic imbalances, and hypothyroidism.2,3 Insufficient hydration with consumption of a high-fiber food, as in this case, or with a bulk-forming laxative such as psyllium seed can result in fecal impaction.3
The many causes of a bezoar
A bezoar is a mass of poorly digested material that forms within the gastrointestinal tract—usually in the stomach—and less commonly in the small or large intestine.4 Trichobezoars (hair), lactobezoars (milk curd), phytobezoars (plant fiber), medication bezoars, and lithobezoars (small stones, pebbles, or gravel) are named after their contents. In keeping with this naming tradition, a gummi bear bezoar5 has also been described. Fecal impaction due to phytobezoars primarily composed of seeds has been associated with prickly pears, watermelons, sunflowers, pumpkins, pomegranates,6,7 and sesame seeds.4 Our patient’s experience adds quinoa seeds to this list.
Patients will complain of nausea and rectal urgency
Patients with fecal impaction may complain of nausea, rectal urgency, and rectalgia. A ball-valve effect of the fecal mass may allow paradoxical fecal incontinence and diarrhea.3 Digital rectal exam may demonstrate stool of any consistency, from rock hard pellets to soft clay-like stool.3 Absence of stool in the rectal vault does not rule out fecal impaction, and more proximal impactions may be revealed on plain abdominal radiography as bubbly, speckled masses of stool with associated signs of obstruction, such as colonic dilatation.
Fever, increased leukocyte count, and abdominal tenderness may indicate colonic perforation or ulceration. Signs of generalized peritonitis and free air on abdominal radiography warrant an immediate surgical consult.3
Complications from fecal impaction include bowel obstruction, sigmoid volvulus, and rectal prolapse.2 Stercoral ulceration and perforation due to pressure necrosis from a hard, inspissated fecal mass is an uncommon but life-threatening complication requiring resection of the affected colonic segment.8
What to look for on the CT. When the diagnosis is unclear or signs of complications are present, an abdominal CT is indicated. Concerning CT findings include ulceration, bowel wall enhancement and thickening (FIGURE 2), discontinuity of the bowel wall, presence of fecal material either protruding through the colonic wall or lying free within the intra-abdominal cavity, and extraluminal air.8
FIGURE 2
CT scan shows bowel wall thickening
Treatment begins with a pharmacologic approach
By the time a patient with a fecal impaction gets to your office, it’s likely that he or she will have already tried over-the-counter laxatives, stool softeners, and perhaps an enema.
When such pharmacologic management has failed, you’ll need to perform a manual fragmentation and extraction of the fecal mass. Apply topical 2% lidocaine jelly for analgesia and lubrication, and then gently and progressively dilate the anal sphincter with one and then 2 fingers. A scissoring action will fragment the impaction.3
Once fragmentation and partial expulsion has been achieved, you may want to try a lubricating mineral oil enema, bisacodyl suppository, or rectal lavage. If the impaction extends beyond the reach of the fingers, sigmoidoscopic visualization and lavage are indicated.
Adding water-soluble contrast material (Gastrografin) in 20% to 50% solutions directed by fluoroscopy draws water into the lumen, thus lubricating the fecal mass3,9 and helping it to pass spontaneously.
Our patient’s case resolved with a trip to the OR
Since conservative and comprehensive management to improve our patient’s condition failed, she was taken to the operating room for a proctosigmoidoscopic disimpaction. A beveled metal proctoscope was used to disimpact the distal-most 10 cm and then a rigid sigmoidoscope was used to clear the colon of quinoa-laden fecal material to a total distance of 18 cm. Bowel walls were ecchymotic, yet viable and without laceration. She made an uneventful recovery and was discharged on hospital Day 3.
CORRESPONDENCE George L. Higgins, III, MD, Maine Medical Center, Department of Emergency Medicine, 47 Bramhall Street, Portland, ME 04102; higgig@mmc.org
A 64-year-old woman came into our emergency department (ED) complaining of constipation and worsening rectal pain. In an attempt to promote her overall health, the patient had recently begun experimenting with healthy alternatives to her regular diet. Three days before her visit, she had ceased having stools and was experiencing intermittent abdominal cramping. She self-administered 2 bisacodyl suppositories, 2 sodium biphosphate enemas, one 10-ounce bottle of magnesium citrate, and 15 senna-containing laxative tablets without improvement.
She sought care at an urgent care clinic where she received 2 additional enemas and a trial of manual disimpaction—without results. She was sent home to rest and asked to return the next morning for another trial of disimpaction. When the patient’s efforts to manually disimpact herself at home were unsuccessful, she contacted her primary care physician, who arranged a house call. When his own protracted disimpaction procedure was unsuccessful, he referred her to our ED.
On presentation, the patient had lower abdominal and rectal discomfort. Her vital signs were normal except for a temperature of 38.8° C. Her abdomen was soft and nontender. Inspection of her perianal area revealed erythema and excoriations. On digital rectal exam (which was poorly tolerated because of pain), we noted a moderate amount of soft, clay-like feces in the rectal vault, with overflow liquid stool expulsion.
Computed tomography (CT) imaging of the abdomen was obtained to rule out rectal injury or colonic perforation (FIGURE 1).
FIGURE 1
CT scan reveals a speckled intraluminal mass
The patient had a markedly distended rectum and distal sigmoid colon caused by an intraluminal mass. Also present: circumferential wall thickening, perirectal edema without extraluminal gas, and generalized proximal colonic wall edema without a drainable collection.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Fecal impaction caused by a proctophytobezoar
CT imaging revealed a proctophytobezoar. On follow-up questioning, the patient recalled consuming approximately 10 ounces of cooked quinoa, a nutritious, gluten-free, high-protein seed, just prior to the onset of her constipation.
Constipation disproportionately affects the elderly and the young.1 Fecal impaction is a sequelae of constipation. Most commonly defined as hard, compacted feces in the rectum, fecal impaction can also include more proximal impactions due to fecal loading or retention.2
Causes of constipation and fecal impaction are similar and include low intake of dietary fiber, dehydration, immobility, alcohol ingestion, laxative abuse, medication adverse effects, depression, dementia, spinal cord dysfunction, diabetes, metabolic imbalances, and hypothyroidism.2,3 Insufficient hydration with consumption of a high-fiber food, as in this case, or with a bulk-forming laxative such as psyllium seed can result in fecal impaction.3
The many causes of a bezoar
A bezoar is a mass of poorly digested material that forms within the gastrointestinal tract—usually in the stomach—and less commonly in the small or large intestine.4 Trichobezoars (hair), lactobezoars (milk curd), phytobezoars (plant fiber), medication bezoars, and lithobezoars (small stones, pebbles, or gravel) are named after their contents. In keeping with this naming tradition, a gummi bear bezoar5 has also been described. Fecal impaction due to phytobezoars primarily composed of seeds has been associated with prickly pears, watermelons, sunflowers, pumpkins, pomegranates,6,7 and sesame seeds.4 Our patient’s experience adds quinoa seeds to this list.
Patients will complain of nausea and rectal urgency
Patients with fecal impaction may complain of nausea, rectal urgency, and rectalgia. A ball-valve effect of the fecal mass may allow paradoxical fecal incontinence and diarrhea.3 Digital rectal exam may demonstrate stool of any consistency, from rock hard pellets to soft clay-like stool.3 Absence of stool in the rectal vault does not rule out fecal impaction, and more proximal impactions may be revealed on plain abdominal radiography as bubbly, speckled masses of stool with associated signs of obstruction, such as colonic dilatation.
Fever, increased leukocyte count, and abdominal tenderness may indicate colonic perforation or ulceration. Signs of generalized peritonitis and free air on abdominal radiography warrant an immediate surgical consult.3
Complications from fecal impaction include bowel obstruction, sigmoid volvulus, and rectal prolapse.2 Stercoral ulceration and perforation due to pressure necrosis from a hard, inspissated fecal mass is an uncommon but life-threatening complication requiring resection of the affected colonic segment.8
What to look for on the CT. When the diagnosis is unclear or signs of complications are present, an abdominal CT is indicated. Concerning CT findings include ulceration, bowel wall enhancement and thickening (FIGURE 2), discontinuity of the bowel wall, presence of fecal material either protruding through the colonic wall or lying free within the intra-abdominal cavity, and extraluminal air.8
FIGURE 2
CT scan shows bowel wall thickening
Treatment begins with a pharmacologic approach
By the time a patient with a fecal impaction gets to your office, it’s likely that he or she will have already tried over-the-counter laxatives, stool softeners, and perhaps an enema.
When such pharmacologic management has failed, you’ll need to perform a manual fragmentation and extraction of the fecal mass. Apply topical 2% lidocaine jelly for analgesia and lubrication, and then gently and progressively dilate the anal sphincter with one and then 2 fingers. A scissoring action will fragment the impaction.3
Once fragmentation and partial expulsion has been achieved, you may want to try a lubricating mineral oil enema, bisacodyl suppository, or rectal lavage. If the impaction extends beyond the reach of the fingers, sigmoidoscopic visualization and lavage are indicated.
Adding water-soluble contrast material (Gastrografin) in 20% to 50% solutions directed by fluoroscopy draws water into the lumen, thus lubricating the fecal mass3,9 and helping it to pass spontaneously.
Our patient’s case resolved with a trip to the OR
Since conservative and comprehensive management to improve our patient’s condition failed, she was taken to the operating room for a proctosigmoidoscopic disimpaction. A beveled metal proctoscope was used to disimpact the distal-most 10 cm and then a rigid sigmoidoscope was used to clear the colon of quinoa-laden fecal material to a total distance of 18 cm. Bowel walls were ecchymotic, yet viable and without laceration. She made an uneventful recovery and was discharged on hospital Day 3.
CORRESPONDENCE George L. Higgins, III, MD, Maine Medical Center, Department of Emergency Medicine, 47 Bramhall Street, Portland, ME 04102; higgig@mmc.org
1. Rao SS, Go JT. Update on the management of constipation in the elderly: new treatment options. Clin Interv Aging. 2010;5:163-171.
2. Creason N, Sparks D. Fecal impaction: a review. Nurs Diagn. 2000;11:15-23.
3. Wrenn K. Fecal impaction. N Engl J Med. 1989;321:658-662.
4. Shaw AG, Peacock O, Lund JN, et al. Large bowel obstruction due to sesame seed bezoar: a case report. J Med Case Reports. 2007;1:159.-
5. Barron MM, Steerman P. Gummi bear bezoar: a case report. J Emerg Med. 1989;7:143-144.
6. Eitan A, Bickel A, Katz IM. Fecal impaction in adults: report of 30 cases of seed bezoars in the rectum. Dis Colon Rectum. 2006;49:1768-1771.
7. Eitan A, Katz IM, Sweed Y, et al. Fecal impaction in children: report of 53 cases of rectal seed bezoars. J Pediatr Surg. 2007;42:1114-1117.
8. Kumar P, Pearce O, Higginson A. Imaging manifestations of faecal impaction and stercoral perforation. Clin Radiol. 2011;66:83-88.
9. Brenner BE, Simon RR. Anorectal emergencies. Ann Emerg Med. 1983;12:367-376.
1. Rao SS, Go JT. Update on the management of constipation in the elderly: new treatment options. Clin Interv Aging. 2010;5:163-171.
2. Creason N, Sparks D. Fecal impaction: a review. Nurs Diagn. 2000;11:15-23.
3. Wrenn K. Fecal impaction. N Engl J Med. 1989;321:658-662.
4. Shaw AG, Peacock O, Lund JN, et al. Large bowel obstruction due to sesame seed bezoar: a case report. J Med Case Reports. 2007;1:159.-
5. Barron MM, Steerman P. Gummi bear bezoar: a case report. J Emerg Med. 1989;7:143-144.
6. Eitan A, Bickel A, Katz IM. Fecal impaction in adults: report of 30 cases of seed bezoars in the rectum. Dis Colon Rectum. 2006;49:1768-1771.
7. Eitan A, Katz IM, Sweed Y, et al. Fecal impaction in children: report of 53 cases of rectal seed bezoars. J Pediatr Surg. 2007;42:1114-1117.
8. Kumar P, Pearce O, Higginson A. Imaging manifestations of faecal impaction and stercoral perforation. Clin Radiol. 2011;66:83-88.
9. Brenner BE, Simon RR. Anorectal emergencies. Ann Emerg Med. 1983;12:367-376.
Acute respiratory tract infection: A practice examines its antibiotic prescribing habits
Purpose We wanted to better understand our practice behaviors by measuring antibiotic prescribing patterns for acute respiratory tract infections (ARTIs), which would perhaps help us delineate goals for quality improvement interventions. We determined (1) the distribution of ARTI final diagnoses in our practice, (2) the frequency and types of antibiotics prescribed, and (3) the factors associated with antibiotic prescribing for patients with ARTI.
Methods We looked at office visits for adults with ARTI symptoms that occurred between December 14, 2009, and March 4, 2010. We compiled a convenience sample of 438 patient visits, collecting historical information, physical examination findings, diagnostic impressions, and treatment decisions.
Results Among the 438 patients, cough was the most common presenting complaint (58%). Acute sinusitis was the most frequently assigned final diagnosis (32%), followed by viral upper respiratory tract infection (29%), and acute bronchitis (24%). Sixty-nine percent of all ARTI patients (304/438) received antibiotic prescriptions, with macrolides being most commonly prescribed (167/304 [55%]). Prescribing antibiotics was associated with a complaint of sinus pain or shortness of breath, duration of illness ≥8 days, and specific abnormal physical exam findings. Prescribing rates did not vary based on patient age or presence of risk factors associated with complication. Variations in prescribing rates were noted between individual providers and groups of providers.
Conclusions We found that we prescribed antibiotics at high rates. Diagnoses of acute sinusitis and bronchitis may have been overused as false justification for antibiotic therapy. We used broad-spectrum antibiotics frequently. We have identified several gaps between current and desired performance to address in practice-based quality improvement interventions.
Most acute respiratory tract infections (ARTIs) are caused by viruses, do not require antibiotics, and resolve spontaneously.1,2 And yet, unnecessary prescribing of antibiotics for ARTIs continues—accounting for approximately half of all such prescriptions2—despite its well-known contribution to antimicrobial resistance, a public health threat as declared by the Institute of Medicine, the Centers for Disease Control and Prevention (CDC), and the World Health Organization (WHO).3-5
Even though the CDC has widely disseminated clinical guidelines for ARTI6-10 and annually publicizes recommendations for ARTI management during “Get Smart About Antibiotics Week,”11 it appears that providers have difficulty implementing the guidelines.12-14 Granted, antibiotic prescription rates in general have declined somewhat, but the use of broad-spectrum antibiotics (macrolides and fluoroquinolones) and antibiotics for older Americans has increased.12
There are several plausible reasons for overprescribing. Patients have expectations for treatment based on prior experience or on a false assumption that their illness is bacterial in origin.14 Providers may be concerned that certain individuals are at risk of complications if not treated. Patient race, health maintenance organization membership, and insurance status have all been implicated as factors related to antimicrobial overutilization.12-16 It can be perceived as time consuming to educate patients about the likely viral nature of their illness and the lack of utility and increased risks in taking unneeded antibiotics.17 Furthermore, attempts at patient and physician education (eg, physician performance feedback) do not always reduce antibiotic overuse.18-20
We wanted to know the state of ARTI antibiotic use in our practice and whether we could identify goals for improvement through quality interventions. We sought to determine the distribution of ARTI final diagnoses in our practice, the frequency and types of antibiotics prescribed, and factors associated with antibiotic prescribing.
Methods
Setting and subjects
Subjects were adult patients seen at Mayo Clinic Family Medicine offices in Arizona between December 14, 2009, and March 4, 2010. We created a convenience sample from visits scheduled for patients with ARTI symptoms. We encouraged, but did not require, clinic staff to use a standardized data collection form to document symptoms, physical examination findings, diagnostic impressions, and prescription decisions that were then entered into an Excel spreadsheet. At one of our 2 sites, clinicians (attending physicians, nurse practitioners, and resident physicians) used the form at the point of care to enroll a portion of the sample population. A retrospective chart audit (with or without use of the form) was the means of selecting the remainder of the sample at this site and the entire sample at our second site. We obtained informed consent from all patients enrolled with the data collection form. The Mayo Foundation Institutional Review Board approved the project.
We defined an ARTI as a new illness occurring within the previous 3 weeks, associated with cough, sinus pain, nasal congestion or rhinorrhea, sore throat, or fever. We excluded patients who had a longer duration of symptoms, a previous evaluation, or a noninfectious diagnosis. We included ARTI patients with concomitant asthma or chronic obstructive pulmonary disease (COPD).
We enrolled 438 patients. Two hundred thirty-one (53%) consented prospectively to data collection with our standardized form; 207 (47%) were reviewed by retrospective chart audit. The mean age of subjects was 54 years (range 18-94, intraquartile range 45-69). Cough was the most frequent chief complaint (58%).
Statistical analysis
We calculated the frequency of each ARTI final diagnosis and its associated antibiotic prescription rate. We also tested for associations between clinical features and the provision of antibiotics. We hypothesized that our providers would be more likely to prescribe antibiotics for patients of advanced age and in the presence of other risk factors for complications.
Results
We determined patient risks for ARTI complication in the prospective data collection group only. Of the 231 patients, 147 (64%) had at least one risk for complication, the most common being age ≥65 (37%). Other risks were employment as a health care worker (12%), asthma (11%), atherosclerotic heart disease (8%), COPD (7%), and tobacco use (5%).
Final diagnoses for all patients appear in TABLE 1. We allowed clinicians to report more than one diagnosis, resulting in 501 final diagnoses reported for 438 patients (63 received 2 final diagnoses). Sinusitis was diagnosed most frequently (32%). Other common diagnoses were viral upper respiratory infection (URI) and acute bronchitis (29% and 24%, respectively).
Antibiotics most often prescribed. Three hundred four ARTI patients (69%) received antibiotic prescriptions. Macrolides were most commonly prescribed (167/304 [55%]). Two hundred eight ARTI patients (68%) received broad-spectrum antibiotics (macrolides or fluoroquinolones); 96 (32%) received narrow-spectrum agents (penicillin, cephalosporin, sulfa, or tetracycline derivatives). TABLE 2 lists the frequency of antibiotic prescription and the antibiotic class most frequently prescribed for each ARTI diagnosis.
Factors associated with increased prescribing included specific history and physical exam findings (TABLE 3). A major determinant of treatment was duration of illness. Those who received antibiotics had a mean duration of illness of 8.3 days, compared with 7.0 days for those not receiving antibiotic therapy (P = .03).
The rate of antibiotic prescribing varied by provider type (TABLE 4). Four resident physicians (all of whom were investigators) prescribed least often, followed by attending physicians, then nurse practitioners. Investigators were significantly less likely to prescribe antimicrobials than noninvestigators (P<.001). We assessed whether use of our standardized data collection form affected prescribing rates. When we excluded patients whose data were entered with this form, no difference in rates was seen.
We also noted wide ranges of prescribing rates between individual providers. While all providers enrolled patients, numbers ranged from one to 51, with a mean of 18. For those who enrolled ≥10 subjects, prescribing rates ranged from a low of 29% (8/28) for a resident physician investigator to 93% (63/68) for 4 noninvestigator attending physicians.
Factors not associated with increased prescribing. We had hypothesized that specific patient characteristics (age and medical complication) would be associated with provision of antimicrobials. However, there was no correlation between patient age and rate of prescribing. The 304 patients who received an antibiotic had a mean age of 54 years (standard deviation [SD]=18), as did the 134 who did not receive one (mean age, 54; SD=20; P=.95). There was a nonsignificant trend for a reduced rate of prescribing for patients younger than age 30. For patients 18 to 29 years old, the rate was 60% (31/52); for those ≥30 years, it was 71% (273/386; odds ratio [OR]=1.64; 95% confidence interval, 0.90-2.97).
Similarly, presence of medical complication did not significantly affect antibiotic prescribing rates. Patients with any risk factor for complication (age >65, diabetes, atherosclerotic heart disease, heart failure, COPD, asthma, tobacco smoking, or active cancer treatment) had a 62% prescription rate (91/147), which was the same as that of patients without such risks (52/84 [62%]; P=1.0).
TABLE 1
Final diagnoses for 438 patients with ARTI
| Diagnosis | n (%)* |
|---|---|
| Acute sinusitis | 141 (32) |
| Viral URI | 125 (29) |
| Acute bronchitis | 104 (24) |
| Asthma | 31 (7) |
| Acute nonstrep pharyngitis | 28 (6) |
| Pneumonia | 17 (4) |
| COPD | 14 (3) |
| Influenza-like illness | 14 (3) |
| Acute otitis media | 14 (3) |
| Strep pharyngitis | 13 (3) |
| ARTI, acute respiratory tract infection; COPD, chronic obstructive pulmonary disease; URI, upper respiratory infection. *Percent total >100% due to 63 patients receiving 2 diagnoses and rounding | |
TABLE 2
Antibiotic use and type prescribed for ARTI varied by diagnosis
| Diagnosis (total) | Antibiotics prescribed* | No antibiotics prescribed | Antibiotic class most frequently prescribed |
|---|---|---|---|
| Acute sinusitis (141) | 139 (99%) | 2 (1%) | Macrolide (53%) |
| Viral URI (125) | 45 (36%) | 80 (64%) | Macrolide (24%) |
| Acute bronchitis (104) | 95 (91%) | 9 (9%) | Macrolide (56%) |
| Acute nonstrep pharyngitis (28) | 16 (57%) | 12 (43%) | Macrolide (36%) |
| Pneumonia (17) | 17 (100%) | 0 | Fluoroquinolone (53%) |
| ARTI, acute respiratory tract infection; URI, upper respiratory infection. *Although 304 patients received prescriptions, some patients received more than one antibiotic. | |||
TABLE 3
Historical features, exam findings associated with antibiotic prescribing
| Historical feature | P value |
|---|---|
| Sinus pain | .0002 |
| Duration of illness >8 days | .0110 |
| Shortness of breath | .0427 |
| Physical exam finding | |
| Abnormal sinus exam | <.0001 |
| Abnormal lung exam | .0005 |
| Abnormal tympanic membrane | .0017 |
| Abnormal pharynx | .0026 |
| Cervical lymphadenopathy | .0141 |
| Abnormal nasal exam | .0363 |
TABLE 4
Antibiotic prescription rates for ARTI varied by provider type, investigator status
| Antibiotic prescription rate | |||
|---|---|---|---|
| Attending physicians | Nurse practitioners | Residents | P value |
| 153/225 (68%) | 97/115 (84%) | 54/98 (55%) | <.001* |
| Investigator | Noninvestigator | P value | |
| 110/192 (57%) | 194/246 (79%) | <.001 | |
| ARTI, acute respiratory tract infection. *The rate for residents is significantly lower than that for attending physicians and nurse practitioners. The rate for attending physicians is significantly lower than that for nurse practitioners. The P value applies to both rate comparisons among provider types. | |||
Discussion
Providers in our practice had surprisingly high rates of antibiotic prescribing for ARTIs (69% overall). By comparison, the overall antibiotic use rate for ARTIs in the most recent National Ambulatory Medical Care Survey (NAMCS) analysis (1995-2006) was 58%.12 The prescribing rate for office settings alone was just 52%. Steinman’s analysis of NAMCS data from 1997-1999 revealed an overall rate of 63%.13
Data analyzed from >4200 Medicare enrollees seen for ARTI visits revealed great variation in prescribing rates by office site: 21% to 88%, with a median rate of 54%.20 The rate varied by final diagnoses: sinusitis, 69%; bronchitis, 59%; pharyngitis, 50%; and URI, 26%. A rate of 77% was recently reported in a Veterans Administration office setting.21 Those with sinusitis and bronchitis similarly received more prescriptions than those with acute pharyngitis and URI.
In addition to our high overall rate, we also diagnosed patients with sinusitis and bronchitis frequently (32% and 24% of all patients, respectively), perhaps as false justification for prescribing antibiotics (provided for 99% and 91%, respectively). Also noteworthy is that more than one-third of URI patients in our practice received antibiotics.
We had expected, but did not see, differences in prescribing rates between older and younger patients, as well as those with and without risk factors for complications. Our expectations were based on NAMCS data, which have demonstrated increasing use of antibiotics in older patients.2
Treatment for those with bronchitis was surprisingly frequent; 91% received antibiotics. A Cochrane systematic review attributes slight symptom benefit to antibiotic use (improvement in cough by about one day).22 This benefit, however, is rarely seen in patients who have been ill for <1 week. The magnitude of this benefit must be weighed against the cost and adverse effects of antibiotics and the potential for promoting antimicrobial resistance. Most patients’ symptoms are mild and self-limited, and risks may exceed benefits.
Guidelines state, “The widespread use of antibiotics for the treatment of acute bronchitis is not justified and vigorous efforts to curtail their use should be encouraged.”23 The CDC agrees, noting that “routine antibiotic treatment of uncomplicated acute bronchitis is not recommended, regardless of duration of cough.”10
As observed in another study,14 a clinical factor associated with prescribing decisions at our practice was the duration of illness. Patients in our practice had been ill, on average, 8 days before presenting to the office. Over time, our encounters with regular patients may have taught them to wait until their symptoms are prolonged or progressive before seeking evaluation.
We saw large differences in prescribing rates between providers, and hope this means there is room for improvement by addressing reasons for variability. Education about individual prescribing behaviors may motivate those with the highest rates of use to improve.
We noted high rates of broad-spectrum antibiotic use. This is consistent with other research findings of a shift away from narrow-spectrum agents.12 We did not determine the frequency of allergies to narrow-spectrum agents. Anecdotally, the opinion of some patients was that narrow-spectrum medicines “just don’t work,” given their experience of persistent cold symptoms when using such agents.
Quality-improvement processes such as DMAIC (Define, Measure, Analyze, Improve, Control) or PDSA (Plan, Do, Study, Act) require collection of baseline data so that interventions can be tailored to meet the root causes identified.24 This project determined preintervention practice behaviors and allowed us to create quality metrics that could define our future success.
Study limitations. One obvious reason for the prescribing variability noted above is that those who helped plan and implement the project knew their practice behaviors were being reviewed and had studied the relevant practice guidelines. Whether non-investigator providers were up to date with recommendations and could carefully select appropriate treatment candidates is unclear.
This study was of our practice alone, and findings may not be generalizable to other practices. We encourage physicians to similarly examine their own prescribing habits in order to set practice-improvement goals.
CORRESPONDENCE Michael L. Grover, DO, Department of Family Medicine, Mayo Clinic, 13737 N 92nd Street, Scottsdale, AZ 85260; grover.michael@mayo.edu
1. Fendrick AM, Monto AS, Nightengale B, et al. The economic burden of non-influenza related viral respiratory tract infection in the United States. Arch Intern Med. 2003;163:487-494.
2. Werner K, Deasy J. Acute respiratory tract infections: when are antibiotics indicated? JAAPA. 2009;22:22–26.
3. US Department of Health and Human Services. Preventing emerging infectious diseases: a strategy for the 21st century. MMWR Morb Mortal Wkly Rep. 1998;47(RR-15). Available at: http://www.cdc.gov/MMWR/pdf/rr/rr4715.pdf. Accessed July 16, 2011.
4. Drug resistance threatens to reverse medical progress [press release]. Geneva, Switzerland: World Health Organization (WHO); June 12, 2000. Available at: http://www.who.int/inf-pr-2000/en/pr2000-41.html. Accessed July 16, 2011.
5. Smolinski MS, Hamburg MA, Lederberg J. eds. Institute of Medicine, Committee on Emerging Microbial Threats to Health in the 21st Century. Microbial Threats to Health: Emergence, Detection, and Response. Washington, DC: National Academies Press; 2003. Available at: http://www.iom.edu/CMS/3783/3919/5381/6146.aspx. Accessed July 16, 2011.
6. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of acute respiratory tract infections in adults: background, specific aims, and methods. Ann Intern Med. 2001;134:479-486.
7. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of nonspecific upper respiratory tract infections in adults: background. Ann Intern Med. 2001;134:490-494.
8. Hickner JM, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Intern Med. 2001;134:498-505.
9. Cooper RJ, Hoffman JR, Bartlett JG, et al. Principles of appropriate antibiotic use for acute pharyngitis in adults: background. Ann Intern Med. 2001;134:509-517.
10. Gonzales R, Bartlett JG, Bessnar RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med. 2001;134:521-529.
11. CDC. Get smart: know when antibiotics work. Adult appropriate antibiotic use summary: physician information sheets (adult). Available at: http://www.cdc.gov/getsmart/campaign-materials/adult-treatment.html. Accessed July 16, 2011.
12. Grijalva CG, Nuorti JP, Griffin M. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758-766.
13. Steinman MA, Landefeld CS, Gonzales R. Predictors of broad spectrum antibiotic prescribing for acute respiratory tract infections in adult primary care. JAMA. 2003;289:719-725.
14. Wigton RS, Darr CA, Corbett KK, et al. How do community practitioners decide whether to prescribe antibiotics for acute respiratory tract infections? J Gen Intern Med. 2008;23:1615-1620.
15. Macfarlane J, Holmes W, Macfarlane R, et al. Influence of patients’ expectations on antibiotic management of acute lower respiratory tract illness in general practice: questionnaire study. BMJ. 1997;315:1211-1214.
16. Colgan R, Powers JH. Appropriate antimicrobial prescribing: approaches that limit antibiotic resistance. Am Fam Physician. 2001;64:999-1004.
17. Coco A, Mainous AG. Relation of time spent in an encounter with the use of antibiotics in pediatric office visits for viral respiratory infections. Arch Pediatr Adolesc Med. 2005;159:1145-1149.
18. Arnold SR, Straus SE. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst Rev 2005;(4):CD003539-
19. Mainous AG, Hueston WJ, Love MM, et al. An evaluation of statewide strategies to reduce antibiotic overuse. Fam Med. 2000;32:22-29.
20. Gonzales R, Sauaia A, Corbett KK, et al. Antibiotic treatment of acute respiratory tract infections in the elderly: effect of a multidimensional educational intervention. J Am Geriatr Soc. 2004;52:39-45.
21. Franck A, Smith R. Antibiotic use for acute respiratory tract infections in a veteran population. J Am Pharm Assoc. 2010;50:726-729.
22. Smucny J, Fahey T, Becker L, et al. Antibiotics for acute bronchitis. Cochrane Database Syst Rev. 2004;(4):CD000245-
23. Bramen SS. Chronic cough due to acute bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129 (1 suppl):95S-103S.
24. Snee RD. Use DMAIC to make improvement part of “the way we work.” Quality Progress Web site. September 2007. Available at: http://asq.org/quality-progress/2007/09/process-managementment/use-dmaic-to-make-improvement-part-of-the-way-we-work.html. Accessed July 16, 2011.
Purpose We wanted to better understand our practice behaviors by measuring antibiotic prescribing patterns for acute respiratory tract infections (ARTIs), which would perhaps help us delineate goals for quality improvement interventions. We determined (1) the distribution of ARTI final diagnoses in our practice, (2) the frequency and types of antibiotics prescribed, and (3) the factors associated with antibiotic prescribing for patients with ARTI.
Methods We looked at office visits for adults with ARTI symptoms that occurred between December 14, 2009, and March 4, 2010. We compiled a convenience sample of 438 patient visits, collecting historical information, physical examination findings, diagnostic impressions, and treatment decisions.
Results Among the 438 patients, cough was the most common presenting complaint (58%). Acute sinusitis was the most frequently assigned final diagnosis (32%), followed by viral upper respiratory tract infection (29%), and acute bronchitis (24%). Sixty-nine percent of all ARTI patients (304/438) received antibiotic prescriptions, with macrolides being most commonly prescribed (167/304 [55%]). Prescribing antibiotics was associated with a complaint of sinus pain or shortness of breath, duration of illness ≥8 days, and specific abnormal physical exam findings. Prescribing rates did not vary based on patient age or presence of risk factors associated with complication. Variations in prescribing rates were noted between individual providers and groups of providers.
Conclusions We found that we prescribed antibiotics at high rates. Diagnoses of acute sinusitis and bronchitis may have been overused as false justification for antibiotic therapy. We used broad-spectrum antibiotics frequently. We have identified several gaps between current and desired performance to address in practice-based quality improvement interventions.
Most acute respiratory tract infections (ARTIs) are caused by viruses, do not require antibiotics, and resolve spontaneously.1,2 And yet, unnecessary prescribing of antibiotics for ARTIs continues—accounting for approximately half of all such prescriptions2—despite its well-known contribution to antimicrobial resistance, a public health threat as declared by the Institute of Medicine, the Centers for Disease Control and Prevention (CDC), and the World Health Organization (WHO).3-5
Even though the CDC has widely disseminated clinical guidelines for ARTI6-10 and annually publicizes recommendations for ARTI management during “Get Smart About Antibiotics Week,”11 it appears that providers have difficulty implementing the guidelines.12-14 Granted, antibiotic prescription rates in general have declined somewhat, but the use of broad-spectrum antibiotics (macrolides and fluoroquinolones) and antibiotics for older Americans has increased.12
There are several plausible reasons for overprescribing. Patients have expectations for treatment based on prior experience or on a false assumption that their illness is bacterial in origin.14 Providers may be concerned that certain individuals are at risk of complications if not treated. Patient race, health maintenance organization membership, and insurance status have all been implicated as factors related to antimicrobial overutilization.12-16 It can be perceived as time consuming to educate patients about the likely viral nature of their illness and the lack of utility and increased risks in taking unneeded antibiotics.17 Furthermore, attempts at patient and physician education (eg, physician performance feedback) do not always reduce antibiotic overuse.18-20
We wanted to know the state of ARTI antibiotic use in our practice and whether we could identify goals for improvement through quality interventions. We sought to determine the distribution of ARTI final diagnoses in our practice, the frequency and types of antibiotics prescribed, and factors associated with antibiotic prescribing.
Methods
Setting and subjects
Subjects were adult patients seen at Mayo Clinic Family Medicine offices in Arizona between December 14, 2009, and March 4, 2010. We created a convenience sample from visits scheduled for patients with ARTI symptoms. We encouraged, but did not require, clinic staff to use a standardized data collection form to document symptoms, physical examination findings, diagnostic impressions, and prescription decisions that were then entered into an Excel spreadsheet. At one of our 2 sites, clinicians (attending physicians, nurse practitioners, and resident physicians) used the form at the point of care to enroll a portion of the sample population. A retrospective chart audit (with or without use of the form) was the means of selecting the remainder of the sample at this site and the entire sample at our second site. We obtained informed consent from all patients enrolled with the data collection form. The Mayo Foundation Institutional Review Board approved the project.
We defined an ARTI as a new illness occurring within the previous 3 weeks, associated with cough, sinus pain, nasal congestion or rhinorrhea, sore throat, or fever. We excluded patients who had a longer duration of symptoms, a previous evaluation, or a noninfectious diagnosis. We included ARTI patients with concomitant asthma or chronic obstructive pulmonary disease (COPD).
We enrolled 438 patients. Two hundred thirty-one (53%) consented prospectively to data collection with our standardized form; 207 (47%) were reviewed by retrospective chart audit. The mean age of subjects was 54 years (range 18-94, intraquartile range 45-69). Cough was the most frequent chief complaint (58%).
Statistical analysis
We calculated the frequency of each ARTI final diagnosis and its associated antibiotic prescription rate. We also tested for associations between clinical features and the provision of antibiotics. We hypothesized that our providers would be more likely to prescribe antibiotics for patients of advanced age and in the presence of other risk factors for complications.
Results
We determined patient risks for ARTI complication in the prospective data collection group only. Of the 231 patients, 147 (64%) had at least one risk for complication, the most common being age ≥65 (37%). Other risks were employment as a health care worker (12%), asthma (11%), atherosclerotic heart disease (8%), COPD (7%), and tobacco use (5%).
Final diagnoses for all patients appear in TABLE 1. We allowed clinicians to report more than one diagnosis, resulting in 501 final diagnoses reported for 438 patients (63 received 2 final diagnoses). Sinusitis was diagnosed most frequently (32%). Other common diagnoses were viral upper respiratory infection (URI) and acute bronchitis (29% and 24%, respectively).
Antibiotics most often prescribed. Three hundred four ARTI patients (69%) received antibiotic prescriptions. Macrolides were most commonly prescribed (167/304 [55%]). Two hundred eight ARTI patients (68%) received broad-spectrum antibiotics (macrolides or fluoroquinolones); 96 (32%) received narrow-spectrum agents (penicillin, cephalosporin, sulfa, or tetracycline derivatives). TABLE 2 lists the frequency of antibiotic prescription and the antibiotic class most frequently prescribed for each ARTI diagnosis.
Factors associated with increased prescribing included specific history and physical exam findings (TABLE 3). A major determinant of treatment was duration of illness. Those who received antibiotics had a mean duration of illness of 8.3 days, compared with 7.0 days for those not receiving antibiotic therapy (P = .03).
The rate of antibiotic prescribing varied by provider type (TABLE 4). Four resident physicians (all of whom were investigators) prescribed least often, followed by attending physicians, then nurse practitioners. Investigators were significantly less likely to prescribe antimicrobials than noninvestigators (P<.001). We assessed whether use of our standardized data collection form affected prescribing rates. When we excluded patients whose data were entered with this form, no difference in rates was seen.
We also noted wide ranges of prescribing rates between individual providers. While all providers enrolled patients, numbers ranged from one to 51, with a mean of 18. For those who enrolled ≥10 subjects, prescribing rates ranged from a low of 29% (8/28) for a resident physician investigator to 93% (63/68) for 4 noninvestigator attending physicians.
Factors not associated with increased prescribing. We had hypothesized that specific patient characteristics (age and medical complication) would be associated with provision of antimicrobials. However, there was no correlation between patient age and rate of prescribing. The 304 patients who received an antibiotic had a mean age of 54 years (standard deviation [SD]=18), as did the 134 who did not receive one (mean age, 54; SD=20; P=.95). There was a nonsignificant trend for a reduced rate of prescribing for patients younger than age 30. For patients 18 to 29 years old, the rate was 60% (31/52); for those ≥30 years, it was 71% (273/386; odds ratio [OR]=1.64; 95% confidence interval, 0.90-2.97).
Similarly, presence of medical complication did not significantly affect antibiotic prescribing rates. Patients with any risk factor for complication (age >65, diabetes, atherosclerotic heart disease, heart failure, COPD, asthma, tobacco smoking, or active cancer treatment) had a 62% prescription rate (91/147), which was the same as that of patients without such risks (52/84 [62%]; P=1.0).
TABLE 1
Final diagnoses for 438 patients with ARTI
| Diagnosis | n (%)* |
|---|---|
| Acute sinusitis | 141 (32) |
| Viral URI | 125 (29) |
| Acute bronchitis | 104 (24) |
| Asthma | 31 (7) |
| Acute nonstrep pharyngitis | 28 (6) |
| Pneumonia | 17 (4) |
| COPD | 14 (3) |
| Influenza-like illness | 14 (3) |
| Acute otitis media | 14 (3) |
| Strep pharyngitis | 13 (3) |
| ARTI, acute respiratory tract infection; COPD, chronic obstructive pulmonary disease; URI, upper respiratory infection. *Percent total >100% due to 63 patients receiving 2 diagnoses and rounding | |
TABLE 2
Antibiotic use and type prescribed for ARTI varied by diagnosis
| Diagnosis (total) | Antibiotics prescribed* | No antibiotics prescribed | Antibiotic class most frequently prescribed |
|---|---|---|---|
| Acute sinusitis (141) | 139 (99%) | 2 (1%) | Macrolide (53%) |
| Viral URI (125) | 45 (36%) | 80 (64%) | Macrolide (24%) |
| Acute bronchitis (104) | 95 (91%) | 9 (9%) | Macrolide (56%) |
| Acute nonstrep pharyngitis (28) | 16 (57%) | 12 (43%) | Macrolide (36%) |
| Pneumonia (17) | 17 (100%) | 0 | Fluoroquinolone (53%) |
| ARTI, acute respiratory tract infection; URI, upper respiratory infection. *Although 304 patients received prescriptions, some patients received more than one antibiotic. | |||
TABLE 3
Historical features, exam findings associated with antibiotic prescribing
| Historical feature | P value |
|---|---|
| Sinus pain | .0002 |
| Duration of illness >8 days | .0110 |
| Shortness of breath | .0427 |
| Physical exam finding | |
| Abnormal sinus exam | <.0001 |
| Abnormal lung exam | .0005 |
| Abnormal tympanic membrane | .0017 |
| Abnormal pharynx | .0026 |
| Cervical lymphadenopathy | .0141 |
| Abnormal nasal exam | .0363 |
TABLE 4
Antibiotic prescription rates for ARTI varied by provider type, investigator status
| Antibiotic prescription rate | |||
|---|---|---|---|
| Attending physicians | Nurse practitioners | Residents | P value |
| 153/225 (68%) | 97/115 (84%) | 54/98 (55%) | <.001* |
| Investigator | Noninvestigator | P value | |
| 110/192 (57%) | 194/246 (79%) | <.001 | |
| ARTI, acute respiratory tract infection. *The rate for residents is significantly lower than that for attending physicians and nurse practitioners. The rate for attending physicians is significantly lower than that for nurse practitioners. The P value applies to both rate comparisons among provider types. | |||
Discussion
Providers in our practice had surprisingly high rates of antibiotic prescribing for ARTIs (69% overall). By comparison, the overall antibiotic use rate for ARTIs in the most recent National Ambulatory Medical Care Survey (NAMCS) analysis (1995-2006) was 58%.12 The prescribing rate for office settings alone was just 52%. Steinman’s analysis of NAMCS data from 1997-1999 revealed an overall rate of 63%.13
Data analyzed from >4200 Medicare enrollees seen for ARTI visits revealed great variation in prescribing rates by office site: 21% to 88%, with a median rate of 54%.20 The rate varied by final diagnoses: sinusitis, 69%; bronchitis, 59%; pharyngitis, 50%; and URI, 26%. A rate of 77% was recently reported in a Veterans Administration office setting.21 Those with sinusitis and bronchitis similarly received more prescriptions than those with acute pharyngitis and URI.
In addition to our high overall rate, we also diagnosed patients with sinusitis and bronchitis frequently (32% and 24% of all patients, respectively), perhaps as false justification for prescribing antibiotics (provided for 99% and 91%, respectively). Also noteworthy is that more than one-third of URI patients in our practice received antibiotics.
We had expected, but did not see, differences in prescribing rates between older and younger patients, as well as those with and without risk factors for complications. Our expectations were based on NAMCS data, which have demonstrated increasing use of antibiotics in older patients.2
Treatment for those with bronchitis was surprisingly frequent; 91% received antibiotics. A Cochrane systematic review attributes slight symptom benefit to antibiotic use (improvement in cough by about one day).22 This benefit, however, is rarely seen in patients who have been ill for <1 week. The magnitude of this benefit must be weighed against the cost and adverse effects of antibiotics and the potential for promoting antimicrobial resistance. Most patients’ symptoms are mild and self-limited, and risks may exceed benefits.
Guidelines state, “The widespread use of antibiotics for the treatment of acute bronchitis is not justified and vigorous efforts to curtail their use should be encouraged.”23 The CDC agrees, noting that “routine antibiotic treatment of uncomplicated acute bronchitis is not recommended, regardless of duration of cough.”10
As observed in another study,14 a clinical factor associated with prescribing decisions at our practice was the duration of illness. Patients in our practice had been ill, on average, 8 days before presenting to the office. Over time, our encounters with regular patients may have taught them to wait until their symptoms are prolonged or progressive before seeking evaluation.
We saw large differences in prescribing rates between providers, and hope this means there is room for improvement by addressing reasons for variability. Education about individual prescribing behaviors may motivate those with the highest rates of use to improve.
We noted high rates of broad-spectrum antibiotic use. This is consistent with other research findings of a shift away from narrow-spectrum agents.12 We did not determine the frequency of allergies to narrow-spectrum agents. Anecdotally, the opinion of some patients was that narrow-spectrum medicines “just don’t work,” given their experience of persistent cold symptoms when using such agents.
Quality-improvement processes such as DMAIC (Define, Measure, Analyze, Improve, Control) or PDSA (Plan, Do, Study, Act) require collection of baseline data so that interventions can be tailored to meet the root causes identified.24 This project determined preintervention practice behaviors and allowed us to create quality metrics that could define our future success.
Study limitations. One obvious reason for the prescribing variability noted above is that those who helped plan and implement the project knew their practice behaviors were being reviewed and had studied the relevant practice guidelines. Whether non-investigator providers were up to date with recommendations and could carefully select appropriate treatment candidates is unclear.
This study was of our practice alone, and findings may not be generalizable to other practices. We encourage physicians to similarly examine their own prescribing habits in order to set practice-improvement goals.
CORRESPONDENCE Michael L. Grover, DO, Department of Family Medicine, Mayo Clinic, 13737 N 92nd Street, Scottsdale, AZ 85260; grover.michael@mayo.edu
Purpose We wanted to better understand our practice behaviors by measuring antibiotic prescribing patterns for acute respiratory tract infections (ARTIs), which would perhaps help us delineate goals for quality improvement interventions. We determined (1) the distribution of ARTI final diagnoses in our practice, (2) the frequency and types of antibiotics prescribed, and (3) the factors associated with antibiotic prescribing for patients with ARTI.
Methods We looked at office visits for adults with ARTI symptoms that occurred between December 14, 2009, and March 4, 2010. We compiled a convenience sample of 438 patient visits, collecting historical information, physical examination findings, diagnostic impressions, and treatment decisions.
Results Among the 438 patients, cough was the most common presenting complaint (58%). Acute sinusitis was the most frequently assigned final diagnosis (32%), followed by viral upper respiratory tract infection (29%), and acute bronchitis (24%). Sixty-nine percent of all ARTI patients (304/438) received antibiotic prescriptions, with macrolides being most commonly prescribed (167/304 [55%]). Prescribing antibiotics was associated with a complaint of sinus pain or shortness of breath, duration of illness ≥8 days, and specific abnormal physical exam findings. Prescribing rates did not vary based on patient age or presence of risk factors associated with complication. Variations in prescribing rates were noted between individual providers and groups of providers.
Conclusions We found that we prescribed antibiotics at high rates. Diagnoses of acute sinusitis and bronchitis may have been overused as false justification for antibiotic therapy. We used broad-spectrum antibiotics frequently. We have identified several gaps between current and desired performance to address in practice-based quality improvement interventions.
Most acute respiratory tract infections (ARTIs) are caused by viruses, do not require antibiotics, and resolve spontaneously.1,2 And yet, unnecessary prescribing of antibiotics for ARTIs continues—accounting for approximately half of all such prescriptions2—despite its well-known contribution to antimicrobial resistance, a public health threat as declared by the Institute of Medicine, the Centers for Disease Control and Prevention (CDC), and the World Health Organization (WHO).3-5
Even though the CDC has widely disseminated clinical guidelines for ARTI6-10 and annually publicizes recommendations for ARTI management during “Get Smart About Antibiotics Week,”11 it appears that providers have difficulty implementing the guidelines.12-14 Granted, antibiotic prescription rates in general have declined somewhat, but the use of broad-spectrum antibiotics (macrolides and fluoroquinolones) and antibiotics for older Americans has increased.12
There are several plausible reasons for overprescribing. Patients have expectations for treatment based on prior experience or on a false assumption that their illness is bacterial in origin.14 Providers may be concerned that certain individuals are at risk of complications if not treated. Patient race, health maintenance organization membership, and insurance status have all been implicated as factors related to antimicrobial overutilization.12-16 It can be perceived as time consuming to educate patients about the likely viral nature of their illness and the lack of utility and increased risks in taking unneeded antibiotics.17 Furthermore, attempts at patient and physician education (eg, physician performance feedback) do not always reduce antibiotic overuse.18-20
We wanted to know the state of ARTI antibiotic use in our practice and whether we could identify goals for improvement through quality interventions. We sought to determine the distribution of ARTI final diagnoses in our practice, the frequency and types of antibiotics prescribed, and factors associated with antibiotic prescribing.
Methods
Setting and subjects
Subjects were adult patients seen at Mayo Clinic Family Medicine offices in Arizona between December 14, 2009, and March 4, 2010. We created a convenience sample from visits scheduled for patients with ARTI symptoms. We encouraged, but did not require, clinic staff to use a standardized data collection form to document symptoms, physical examination findings, diagnostic impressions, and prescription decisions that were then entered into an Excel spreadsheet. At one of our 2 sites, clinicians (attending physicians, nurse practitioners, and resident physicians) used the form at the point of care to enroll a portion of the sample population. A retrospective chart audit (with or without use of the form) was the means of selecting the remainder of the sample at this site and the entire sample at our second site. We obtained informed consent from all patients enrolled with the data collection form. The Mayo Foundation Institutional Review Board approved the project.
We defined an ARTI as a new illness occurring within the previous 3 weeks, associated with cough, sinus pain, nasal congestion or rhinorrhea, sore throat, or fever. We excluded patients who had a longer duration of symptoms, a previous evaluation, or a noninfectious diagnosis. We included ARTI patients with concomitant asthma or chronic obstructive pulmonary disease (COPD).
We enrolled 438 patients. Two hundred thirty-one (53%) consented prospectively to data collection with our standardized form; 207 (47%) were reviewed by retrospective chart audit. The mean age of subjects was 54 years (range 18-94, intraquartile range 45-69). Cough was the most frequent chief complaint (58%).
Statistical analysis
We calculated the frequency of each ARTI final diagnosis and its associated antibiotic prescription rate. We also tested for associations between clinical features and the provision of antibiotics. We hypothesized that our providers would be more likely to prescribe antibiotics for patients of advanced age and in the presence of other risk factors for complications.
Results
We determined patient risks for ARTI complication in the prospective data collection group only. Of the 231 patients, 147 (64%) had at least one risk for complication, the most common being age ≥65 (37%). Other risks were employment as a health care worker (12%), asthma (11%), atherosclerotic heart disease (8%), COPD (7%), and tobacco use (5%).
Final diagnoses for all patients appear in TABLE 1. We allowed clinicians to report more than one diagnosis, resulting in 501 final diagnoses reported for 438 patients (63 received 2 final diagnoses). Sinusitis was diagnosed most frequently (32%). Other common diagnoses were viral upper respiratory infection (URI) and acute bronchitis (29% and 24%, respectively).
Antibiotics most often prescribed. Three hundred four ARTI patients (69%) received antibiotic prescriptions. Macrolides were most commonly prescribed (167/304 [55%]). Two hundred eight ARTI patients (68%) received broad-spectrum antibiotics (macrolides or fluoroquinolones); 96 (32%) received narrow-spectrum agents (penicillin, cephalosporin, sulfa, or tetracycline derivatives). TABLE 2 lists the frequency of antibiotic prescription and the antibiotic class most frequently prescribed for each ARTI diagnosis.
Factors associated with increased prescribing included specific history and physical exam findings (TABLE 3). A major determinant of treatment was duration of illness. Those who received antibiotics had a mean duration of illness of 8.3 days, compared with 7.0 days for those not receiving antibiotic therapy (P = .03).
The rate of antibiotic prescribing varied by provider type (TABLE 4). Four resident physicians (all of whom were investigators) prescribed least often, followed by attending physicians, then nurse practitioners. Investigators were significantly less likely to prescribe antimicrobials than noninvestigators (P<.001). We assessed whether use of our standardized data collection form affected prescribing rates. When we excluded patients whose data were entered with this form, no difference in rates was seen.
We also noted wide ranges of prescribing rates between individual providers. While all providers enrolled patients, numbers ranged from one to 51, with a mean of 18. For those who enrolled ≥10 subjects, prescribing rates ranged from a low of 29% (8/28) for a resident physician investigator to 93% (63/68) for 4 noninvestigator attending physicians.
Factors not associated with increased prescribing. We had hypothesized that specific patient characteristics (age and medical complication) would be associated with provision of antimicrobials. However, there was no correlation between patient age and rate of prescribing. The 304 patients who received an antibiotic had a mean age of 54 years (standard deviation [SD]=18), as did the 134 who did not receive one (mean age, 54; SD=20; P=.95). There was a nonsignificant trend for a reduced rate of prescribing for patients younger than age 30. For patients 18 to 29 years old, the rate was 60% (31/52); for those ≥30 years, it was 71% (273/386; odds ratio [OR]=1.64; 95% confidence interval, 0.90-2.97).
Similarly, presence of medical complication did not significantly affect antibiotic prescribing rates. Patients with any risk factor for complication (age >65, diabetes, atherosclerotic heart disease, heart failure, COPD, asthma, tobacco smoking, or active cancer treatment) had a 62% prescription rate (91/147), which was the same as that of patients without such risks (52/84 [62%]; P=1.0).
TABLE 1
Final diagnoses for 438 patients with ARTI
| Diagnosis | n (%)* |
|---|---|
| Acute sinusitis | 141 (32) |
| Viral URI | 125 (29) |
| Acute bronchitis | 104 (24) |
| Asthma | 31 (7) |
| Acute nonstrep pharyngitis | 28 (6) |
| Pneumonia | 17 (4) |
| COPD | 14 (3) |
| Influenza-like illness | 14 (3) |
| Acute otitis media | 14 (3) |
| Strep pharyngitis | 13 (3) |
| ARTI, acute respiratory tract infection; COPD, chronic obstructive pulmonary disease; URI, upper respiratory infection. *Percent total >100% due to 63 patients receiving 2 diagnoses and rounding | |
TABLE 2
Antibiotic use and type prescribed for ARTI varied by diagnosis
| Diagnosis (total) | Antibiotics prescribed* | No antibiotics prescribed | Antibiotic class most frequently prescribed |
|---|---|---|---|
| Acute sinusitis (141) | 139 (99%) | 2 (1%) | Macrolide (53%) |
| Viral URI (125) | 45 (36%) | 80 (64%) | Macrolide (24%) |
| Acute bronchitis (104) | 95 (91%) | 9 (9%) | Macrolide (56%) |
| Acute nonstrep pharyngitis (28) | 16 (57%) | 12 (43%) | Macrolide (36%) |
| Pneumonia (17) | 17 (100%) | 0 | Fluoroquinolone (53%) |
| ARTI, acute respiratory tract infection; URI, upper respiratory infection. *Although 304 patients received prescriptions, some patients received more than one antibiotic. | |||
TABLE 3
Historical features, exam findings associated with antibiotic prescribing
| Historical feature | P value |
|---|---|
| Sinus pain | .0002 |
| Duration of illness >8 days | .0110 |
| Shortness of breath | .0427 |
| Physical exam finding | |
| Abnormal sinus exam | <.0001 |
| Abnormal lung exam | .0005 |
| Abnormal tympanic membrane | .0017 |
| Abnormal pharynx | .0026 |
| Cervical lymphadenopathy | .0141 |
| Abnormal nasal exam | .0363 |
TABLE 4
Antibiotic prescription rates for ARTI varied by provider type, investigator status
| Antibiotic prescription rate | |||
|---|---|---|---|
| Attending physicians | Nurse practitioners | Residents | P value |
| 153/225 (68%) | 97/115 (84%) | 54/98 (55%) | <.001* |
| Investigator | Noninvestigator | P value | |
| 110/192 (57%) | 194/246 (79%) | <.001 | |
| ARTI, acute respiratory tract infection. *The rate for residents is significantly lower than that for attending physicians and nurse practitioners. The rate for attending physicians is significantly lower than that for nurse practitioners. The P value applies to both rate comparisons among provider types. | |||
Discussion
Providers in our practice had surprisingly high rates of antibiotic prescribing for ARTIs (69% overall). By comparison, the overall antibiotic use rate for ARTIs in the most recent National Ambulatory Medical Care Survey (NAMCS) analysis (1995-2006) was 58%.12 The prescribing rate for office settings alone was just 52%. Steinman’s analysis of NAMCS data from 1997-1999 revealed an overall rate of 63%.13
Data analyzed from >4200 Medicare enrollees seen for ARTI visits revealed great variation in prescribing rates by office site: 21% to 88%, with a median rate of 54%.20 The rate varied by final diagnoses: sinusitis, 69%; bronchitis, 59%; pharyngitis, 50%; and URI, 26%. A rate of 77% was recently reported in a Veterans Administration office setting.21 Those with sinusitis and bronchitis similarly received more prescriptions than those with acute pharyngitis and URI.
In addition to our high overall rate, we also diagnosed patients with sinusitis and bronchitis frequently (32% and 24% of all patients, respectively), perhaps as false justification for prescribing antibiotics (provided for 99% and 91%, respectively). Also noteworthy is that more than one-third of URI patients in our practice received antibiotics.
We had expected, but did not see, differences in prescribing rates between older and younger patients, as well as those with and without risk factors for complications. Our expectations were based on NAMCS data, which have demonstrated increasing use of antibiotics in older patients.2
Treatment for those with bronchitis was surprisingly frequent; 91% received antibiotics. A Cochrane systematic review attributes slight symptom benefit to antibiotic use (improvement in cough by about one day).22 This benefit, however, is rarely seen in patients who have been ill for <1 week. The magnitude of this benefit must be weighed against the cost and adverse effects of antibiotics and the potential for promoting antimicrobial resistance. Most patients’ symptoms are mild and self-limited, and risks may exceed benefits.
Guidelines state, “The widespread use of antibiotics for the treatment of acute bronchitis is not justified and vigorous efforts to curtail their use should be encouraged.”23 The CDC agrees, noting that “routine antibiotic treatment of uncomplicated acute bronchitis is not recommended, regardless of duration of cough.”10
As observed in another study,14 a clinical factor associated with prescribing decisions at our practice was the duration of illness. Patients in our practice had been ill, on average, 8 days before presenting to the office. Over time, our encounters with regular patients may have taught them to wait until their symptoms are prolonged or progressive before seeking evaluation.
We saw large differences in prescribing rates between providers, and hope this means there is room for improvement by addressing reasons for variability. Education about individual prescribing behaviors may motivate those with the highest rates of use to improve.
We noted high rates of broad-spectrum antibiotic use. This is consistent with other research findings of a shift away from narrow-spectrum agents.12 We did not determine the frequency of allergies to narrow-spectrum agents. Anecdotally, the opinion of some patients was that narrow-spectrum medicines “just don’t work,” given their experience of persistent cold symptoms when using such agents.
Quality-improvement processes such as DMAIC (Define, Measure, Analyze, Improve, Control) or PDSA (Plan, Do, Study, Act) require collection of baseline data so that interventions can be tailored to meet the root causes identified.24 This project determined preintervention practice behaviors and allowed us to create quality metrics that could define our future success.
Study limitations. One obvious reason for the prescribing variability noted above is that those who helped plan and implement the project knew their practice behaviors were being reviewed and had studied the relevant practice guidelines. Whether non-investigator providers were up to date with recommendations and could carefully select appropriate treatment candidates is unclear.
This study was of our practice alone, and findings may not be generalizable to other practices. We encourage physicians to similarly examine their own prescribing habits in order to set practice-improvement goals.
CORRESPONDENCE Michael L. Grover, DO, Department of Family Medicine, Mayo Clinic, 13737 N 92nd Street, Scottsdale, AZ 85260; grover.michael@mayo.edu
1. Fendrick AM, Monto AS, Nightengale B, et al. The economic burden of non-influenza related viral respiratory tract infection in the United States. Arch Intern Med. 2003;163:487-494.
2. Werner K, Deasy J. Acute respiratory tract infections: when are antibiotics indicated? JAAPA. 2009;22:22–26.
3. US Department of Health and Human Services. Preventing emerging infectious diseases: a strategy for the 21st century. MMWR Morb Mortal Wkly Rep. 1998;47(RR-15). Available at: http://www.cdc.gov/MMWR/pdf/rr/rr4715.pdf. Accessed July 16, 2011.
4. Drug resistance threatens to reverse medical progress [press release]. Geneva, Switzerland: World Health Organization (WHO); June 12, 2000. Available at: http://www.who.int/inf-pr-2000/en/pr2000-41.html. Accessed July 16, 2011.
5. Smolinski MS, Hamburg MA, Lederberg J. eds. Institute of Medicine, Committee on Emerging Microbial Threats to Health in the 21st Century. Microbial Threats to Health: Emergence, Detection, and Response. Washington, DC: National Academies Press; 2003. Available at: http://www.iom.edu/CMS/3783/3919/5381/6146.aspx. Accessed July 16, 2011.
6. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of acute respiratory tract infections in adults: background, specific aims, and methods. Ann Intern Med. 2001;134:479-486.
7. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of nonspecific upper respiratory tract infections in adults: background. Ann Intern Med. 2001;134:490-494.
8. Hickner JM, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Intern Med. 2001;134:498-505.
9. Cooper RJ, Hoffman JR, Bartlett JG, et al. Principles of appropriate antibiotic use for acute pharyngitis in adults: background. Ann Intern Med. 2001;134:509-517.
10. Gonzales R, Bartlett JG, Bessnar RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med. 2001;134:521-529.
11. CDC. Get smart: know when antibiotics work. Adult appropriate antibiotic use summary: physician information sheets (adult). Available at: http://www.cdc.gov/getsmart/campaign-materials/adult-treatment.html. Accessed July 16, 2011.
12. Grijalva CG, Nuorti JP, Griffin M. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758-766.
13. Steinman MA, Landefeld CS, Gonzales R. Predictors of broad spectrum antibiotic prescribing for acute respiratory tract infections in adult primary care. JAMA. 2003;289:719-725.
14. Wigton RS, Darr CA, Corbett KK, et al. How do community practitioners decide whether to prescribe antibiotics for acute respiratory tract infections? J Gen Intern Med. 2008;23:1615-1620.
15. Macfarlane J, Holmes W, Macfarlane R, et al. Influence of patients’ expectations on antibiotic management of acute lower respiratory tract illness in general practice: questionnaire study. BMJ. 1997;315:1211-1214.
16. Colgan R, Powers JH. Appropriate antimicrobial prescribing: approaches that limit antibiotic resistance. Am Fam Physician. 2001;64:999-1004.
17. Coco A, Mainous AG. Relation of time spent in an encounter with the use of antibiotics in pediatric office visits for viral respiratory infections. Arch Pediatr Adolesc Med. 2005;159:1145-1149.
18. Arnold SR, Straus SE. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst Rev 2005;(4):CD003539-
19. Mainous AG, Hueston WJ, Love MM, et al. An evaluation of statewide strategies to reduce antibiotic overuse. Fam Med. 2000;32:22-29.
20. Gonzales R, Sauaia A, Corbett KK, et al. Antibiotic treatment of acute respiratory tract infections in the elderly: effect of a multidimensional educational intervention. J Am Geriatr Soc. 2004;52:39-45.
21. Franck A, Smith R. Antibiotic use for acute respiratory tract infections in a veteran population. J Am Pharm Assoc. 2010;50:726-729.
22. Smucny J, Fahey T, Becker L, et al. Antibiotics for acute bronchitis. Cochrane Database Syst Rev. 2004;(4):CD000245-
23. Bramen SS. Chronic cough due to acute bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129 (1 suppl):95S-103S.
24. Snee RD. Use DMAIC to make improvement part of “the way we work.” Quality Progress Web site. September 2007. Available at: http://asq.org/quality-progress/2007/09/process-managementment/use-dmaic-to-make-improvement-part-of-the-way-we-work.html. Accessed July 16, 2011.
1. Fendrick AM, Monto AS, Nightengale B, et al. The economic burden of non-influenza related viral respiratory tract infection in the United States. Arch Intern Med. 2003;163:487-494.
2. Werner K, Deasy J. Acute respiratory tract infections: when are antibiotics indicated? JAAPA. 2009;22:22–26.
3. US Department of Health and Human Services. Preventing emerging infectious diseases: a strategy for the 21st century. MMWR Morb Mortal Wkly Rep. 1998;47(RR-15). Available at: http://www.cdc.gov/MMWR/pdf/rr/rr4715.pdf. Accessed July 16, 2011.
4. Drug resistance threatens to reverse medical progress [press release]. Geneva, Switzerland: World Health Organization (WHO); June 12, 2000. Available at: http://www.who.int/inf-pr-2000/en/pr2000-41.html. Accessed July 16, 2011.
5. Smolinski MS, Hamburg MA, Lederberg J. eds. Institute of Medicine, Committee on Emerging Microbial Threats to Health in the 21st Century. Microbial Threats to Health: Emergence, Detection, and Response. Washington, DC: National Academies Press; 2003. Available at: http://www.iom.edu/CMS/3783/3919/5381/6146.aspx. Accessed July 16, 2011.
6. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of acute respiratory tract infections in adults: background, specific aims, and methods. Ann Intern Med. 2001;134:479-486.
7. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of nonspecific upper respiratory tract infections in adults: background. Ann Intern Med. 2001;134:490-494.
8. Hickner JM, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Intern Med. 2001;134:498-505.
9. Cooper RJ, Hoffman JR, Bartlett JG, et al. Principles of appropriate antibiotic use for acute pharyngitis in adults: background. Ann Intern Med. 2001;134:509-517.
10. Gonzales R, Bartlett JG, Bessnar RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med. 2001;134:521-529.
11. CDC. Get smart: know when antibiotics work. Adult appropriate antibiotic use summary: physician information sheets (adult). Available at: http://www.cdc.gov/getsmart/campaign-materials/adult-treatment.html. Accessed July 16, 2011.
12. Grijalva CG, Nuorti JP, Griffin M. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758-766.
13. Steinman MA, Landefeld CS, Gonzales R. Predictors of broad spectrum antibiotic prescribing for acute respiratory tract infections in adult primary care. JAMA. 2003;289:719-725.
14. Wigton RS, Darr CA, Corbett KK, et al. How do community practitioners decide whether to prescribe antibiotics for acute respiratory tract infections? J Gen Intern Med. 2008;23:1615-1620.
15. Macfarlane J, Holmes W, Macfarlane R, et al. Influence of patients’ expectations on antibiotic management of acute lower respiratory tract illness in general practice: questionnaire study. BMJ. 1997;315:1211-1214.
16. Colgan R, Powers JH. Appropriate antimicrobial prescribing: approaches that limit antibiotic resistance. Am Fam Physician. 2001;64:999-1004.
17. Coco A, Mainous AG. Relation of time spent in an encounter with the use of antibiotics in pediatric office visits for viral respiratory infections. Arch Pediatr Adolesc Med. 2005;159:1145-1149.
18. Arnold SR, Straus SE. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst Rev 2005;(4):CD003539-
19. Mainous AG, Hueston WJ, Love MM, et al. An evaluation of statewide strategies to reduce antibiotic overuse. Fam Med. 2000;32:22-29.
20. Gonzales R, Sauaia A, Corbett KK, et al. Antibiotic treatment of acute respiratory tract infections in the elderly: effect of a multidimensional educational intervention. J Am Geriatr Soc. 2004;52:39-45.
21. Franck A, Smith R. Antibiotic use for acute respiratory tract infections in a veteran population. J Am Pharm Assoc. 2010;50:726-729.
22. Smucny J, Fahey T, Becker L, et al. Antibiotics for acute bronchitis. Cochrane Database Syst Rev. 2004;(4):CD000245-
23. Bramen SS. Chronic cough due to acute bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129 (1 suppl):95S-103S.
24. Snee RD. Use DMAIC to make improvement part of “the way we work.” Quality Progress Web site. September 2007. Available at: http://asq.org/quality-progress/2007/09/process-managementment/use-dmaic-to-make-improvement-part-of-the-way-we-work.html. Accessed July 16, 2011.
Undiluted acid used for vulvar surgery … and more
WIDE LOCAL EXCISION was performed on a 42-year-old woman with vulvar intraepithelial neoplasm, VIN II, with moderate dysplasia. Her ObGyn performed the surgery.
Instead of applying a diluted solution of acetic acid wash to delineate the borders of the dysplastic area, a highly concentrated acetic acid or trichloroacetic acid was used. The patient suffered severe chemical burns of the vulva that took several months to heal. She has permanent scarring of the vulvar area, severe tenderness, discoloration, and atrophy of the vaginal opening, with a band of thick scar tissue at the posterior fourchette. The perineum, extending to the anal area, is scarred, including a 2-mm plaque layer.
PATIENT’S CLAIM Sexual intercourse is extremely painful, and therefore impossible. She suffers discomfort at all times. Additional surgery has been recommended to alleviate her condition.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $600,000 Ohio settlement was reached.
Large baby with cervical spine injury
A WOMAN WAS IN LABOR with her third child. Her first baby was born by cesarean delivery. During the vaginal birth of her second child, shoulder dystocia was encountered; this child weighed 8 lb 4 oz at birth.
Using ultrasonography, the ObGyn determined vaginal birth was appropriate. Shoulder dystocia was encountered and the infant suffered injuries to the cervical spine and right arm. The newborn weighed 9 lb 13 oz.
PATIENT’S CLAIM The baby’s weight was grossly underestimated prior to delivery; ultrasonography was not properly performed or evaluated. The mother’s history, large fundal height, estimated fetal weight, and the mother’s request for a cesarean delivery should have resulted in the performance of a cesarean delivery.
PHYSICIAN’S DEFENSE Shoulder dystocia was not reasonably foreseeable. Injuries to the baby were due to the forces of labor.
VERDICT A confidential Texas settlement was reached.
Suture causes nerve damage
PELVIC PROLAPSE RECONSTRUCTION was performed; surgery included a pubovaginal sling procedure with graft, and repairs of Grade 2 cystocele and Grade 3 rectocele. The gynecologist used transvaginal sutures to attach the mesh to the sacrospinous ligament.
The patient immediately reported pain, tingling, and weakness in her buttocks and legs. The gynecologist diagnosed a hematoma and continued conservative treatment while waiting for the hematoma to resorb.
After 10 days, the patient terminated the gynecologist’s services and left the hospital. She saw a neurologist, who diagnosed proximal sciatic nerve irritation secondary to suturing. When a suture was removed from the sacral spinous ligament plexus, many of the patient’s neurologic symptoms immediately resolved. She still has pain and walks with a noticeable limp using a cane.
PATIENT’S CLAIM The gynecologist failed to determine that a suture was causing nerve damage. Removal of the suture within the first 3 days would have avoided neurologic injury.
PHYSICIAN’S DEFENSE Postsurgical care was proper. A neurologist was consulted, and a sonogram had ruled out deep vein thrombosis.
VERDICT A $1.58 million Illinois verdict was returned.
Colon damage after embolization
UTERINE FIBROID EMBOLIZATION was performed on a 51-year-old woman. The next day, she reported severe abdominal pain and was readmitted. A uterine infection was suspected, and she underwent a hysterectomy. Necrosis of the colon was found; a surgeon removed one-third of the colon and performed a colostomy. She underwent several operations, including rectal-vaginal fistula repair, before the colostomy was corrected.
PATIENT’S CLAIM Misdirected embolization injured an artery supplying the colon. She continues to suffer ongoing fecal urgency and frequency.
PHYSICIAN’S DEFENSE An anomalous connection between the patient’s uterine artery and mesenteric artery was impossible for the physician to have known prior to the embolization procedure.
VERDICT A California defense verdict was returned.

SEVERAL HOURS AFTER A WOMAN’S LABOR BEGAN, fetal bradycardia developed precipitously. The on-call ObGyn arrived after 10 minutes and ordered an immediate cesarean delivery, which occurred 22 minutes later. The child suffered a catastrophic, irreversible brain injury. He lived for 39 days before life support was removed and he died.
ESTATE’S CLAIM The nurses did not report decelerations to the ObGyn, and they were slow to notify him of the fetal bradycardia. The child would not have been injured if the nursing staff had reacted appropriately.
DEFENDANTS’ DEFENSE Isolated heart-rate decelerations during labor are not troubling. A cord accident occurred, which could not be predicted nor avoided. The ObGyn was called promptly; the emergency cesarean delivery was performed quickly. However, the injury already had occurred and was irreparable.
VERDICT A $1.18 million Kentucky verdict was returned. The hospital sought a mistrial because Facebook postings by a juror proved the case had been discussed and prejudged. The court found in favor of the hospital on its post-trial motion.
Bilateral mastectomy: nipples not spared
A 46-YEAR-OLD WOMAN UNDERWENT prophylactic bilateral mastectomy. A plastic surgeon drew presurgical markings on the day of surgery; the breast surgeon removed the nipples.
PATIENT’S CLAIM All parties had agreed the nipples would be spared. The plastic surgeon drew improper markings and failed to remind the breast surgeon prior to surgery that the nipples would be preserved.
PHYSICIAN’S DEFENSE The breast surgeon was at fault for misinterpreting the markings.
VERDICT The patient reached a pretrial settlement with the breast surgeon. The case proceeded against the plastic surgeon. A Maryland defense verdict was returned for the plastic surgeon.
Signs of intrauterine growth restriction; stillborn child
AT 24 WEEKS’ GESTATION, a 17-year-old woman who smoked reported spotting. An ultrasound demonstrated significant fetal growth restriction. The mother was hospitalized to assess the spotting; no testing was ordered to assess fetal growth. When blood was not found in the birth canal, she was discharged. During the next month, she saw the ObGyn three times; testing indicated that the fetus was at least 3 weeks behind the stage of pregnancy. The ObGyn did not order additional testing nor consult a specialist. At 31 weeks’ gestation, ultrasonography found no fetal heart tones. The stillborn was delivered by cesarean section.
ESTATE’S CLAIM A wrongful death suit was filed by the parents, who also claimed lack of informed consent concerning the risk of stillbirth in the presence of intrauterine growth restriction.
PHYSICIANS’ DEFENSE The mother’s smoking was mentioned at trial as a possible explanation of why fetal development was delayed. The ObGyn denied negligence.
VERDICT A $800,000 Maryland verdict was awarded to the parents.
Three BrCa patients share $72.6 M
THREE MENOPAUSAL WOMEN took Premarin (conjugated estrogens) plus Provera (medroxyprogesterone), and/or Prempro (conjugated estrogens/medroxyprogesterone acetate). Each discontinued hormone therapy after being diagnosed with hormone-positive breast cancer.
PATIENTS’ CLAIM The only source of hormonal stimulation for their cancer was the use of estrogen plus progestin.
DEFENDANTS’ DEFENSE Science is currently unable to determine precisely what causes breast cancer. Each plaintiff had risk factors.
VERDICT The three cases were consolidated to a reverse-bifurcated trial, with causation and damages assessed first. The Pennsylvania jury found the Wyeth Pharmaceutical products to be factual causes of the patients’ cancer, and awarded a total of $72.6 million in compensatory damages. The parties settled for confidential amounts before the liability phase began.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.

WIDE LOCAL EXCISION was performed on a 42-year-old woman with vulvar intraepithelial neoplasm, VIN II, with moderate dysplasia. Her ObGyn performed the surgery.
Instead of applying a diluted solution of acetic acid wash to delineate the borders of the dysplastic area, a highly concentrated acetic acid or trichloroacetic acid was used. The patient suffered severe chemical burns of the vulva that took several months to heal. She has permanent scarring of the vulvar area, severe tenderness, discoloration, and atrophy of the vaginal opening, with a band of thick scar tissue at the posterior fourchette. The perineum, extending to the anal area, is scarred, including a 2-mm plaque layer.
PATIENT’S CLAIM Sexual intercourse is extremely painful, and therefore impossible. She suffers discomfort at all times. Additional surgery has been recommended to alleviate her condition.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $600,000 Ohio settlement was reached.
Large baby with cervical spine injury
A WOMAN WAS IN LABOR with her third child. Her first baby was born by cesarean delivery. During the vaginal birth of her second child, shoulder dystocia was encountered; this child weighed 8 lb 4 oz at birth.
Using ultrasonography, the ObGyn determined vaginal birth was appropriate. Shoulder dystocia was encountered and the infant suffered injuries to the cervical spine and right arm. The newborn weighed 9 lb 13 oz.
PATIENT’S CLAIM The baby’s weight was grossly underestimated prior to delivery; ultrasonography was not properly performed or evaluated. The mother’s history, large fundal height, estimated fetal weight, and the mother’s request for a cesarean delivery should have resulted in the performance of a cesarean delivery.
PHYSICIAN’S DEFENSE Shoulder dystocia was not reasonably foreseeable. Injuries to the baby were due to the forces of labor.
VERDICT A confidential Texas settlement was reached.
Suture causes nerve damage
PELVIC PROLAPSE RECONSTRUCTION was performed; surgery included a pubovaginal sling procedure with graft, and repairs of Grade 2 cystocele and Grade 3 rectocele. The gynecologist used transvaginal sutures to attach the mesh to the sacrospinous ligament.
The patient immediately reported pain, tingling, and weakness in her buttocks and legs. The gynecologist diagnosed a hematoma and continued conservative treatment while waiting for the hematoma to resorb.
After 10 days, the patient terminated the gynecologist’s services and left the hospital. She saw a neurologist, who diagnosed proximal sciatic nerve irritation secondary to suturing. When a suture was removed from the sacral spinous ligament plexus, many of the patient’s neurologic symptoms immediately resolved. She still has pain and walks with a noticeable limp using a cane.
PATIENT’S CLAIM The gynecologist failed to determine that a suture was causing nerve damage. Removal of the suture within the first 3 days would have avoided neurologic injury.
PHYSICIAN’S DEFENSE Postsurgical care was proper. A neurologist was consulted, and a sonogram had ruled out deep vein thrombosis.
VERDICT A $1.58 million Illinois verdict was returned.
Colon damage after embolization
UTERINE FIBROID EMBOLIZATION was performed on a 51-year-old woman. The next day, she reported severe abdominal pain and was readmitted. A uterine infection was suspected, and she underwent a hysterectomy. Necrosis of the colon was found; a surgeon removed one-third of the colon and performed a colostomy. She underwent several operations, including rectal-vaginal fistula repair, before the colostomy was corrected.
PATIENT’S CLAIM Misdirected embolization injured an artery supplying the colon. She continues to suffer ongoing fecal urgency and frequency.
PHYSICIAN’S DEFENSE An anomalous connection between the patient’s uterine artery and mesenteric artery was impossible for the physician to have known prior to the embolization procedure.
VERDICT A California defense verdict was returned.

SEVERAL HOURS AFTER A WOMAN’S LABOR BEGAN, fetal bradycardia developed precipitously. The on-call ObGyn arrived after 10 minutes and ordered an immediate cesarean delivery, which occurred 22 minutes later. The child suffered a catastrophic, irreversible brain injury. He lived for 39 days before life support was removed and he died.
ESTATE’S CLAIM The nurses did not report decelerations to the ObGyn, and they were slow to notify him of the fetal bradycardia. The child would not have been injured if the nursing staff had reacted appropriately.
DEFENDANTS’ DEFENSE Isolated heart-rate decelerations during labor are not troubling. A cord accident occurred, which could not be predicted nor avoided. The ObGyn was called promptly; the emergency cesarean delivery was performed quickly. However, the injury already had occurred and was irreparable.
VERDICT A $1.18 million Kentucky verdict was returned. The hospital sought a mistrial because Facebook postings by a juror proved the case had been discussed and prejudged. The court found in favor of the hospital on its post-trial motion.
Bilateral mastectomy: nipples not spared
A 46-YEAR-OLD WOMAN UNDERWENT prophylactic bilateral mastectomy. A plastic surgeon drew presurgical markings on the day of surgery; the breast surgeon removed the nipples.
PATIENT’S CLAIM All parties had agreed the nipples would be spared. The plastic surgeon drew improper markings and failed to remind the breast surgeon prior to surgery that the nipples would be preserved.
PHYSICIAN’S DEFENSE The breast surgeon was at fault for misinterpreting the markings.
VERDICT The patient reached a pretrial settlement with the breast surgeon. The case proceeded against the plastic surgeon. A Maryland defense verdict was returned for the plastic surgeon.
Signs of intrauterine growth restriction; stillborn child
AT 24 WEEKS’ GESTATION, a 17-year-old woman who smoked reported spotting. An ultrasound demonstrated significant fetal growth restriction. The mother was hospitalized to assess the spotting; no testing was ordered to assess fetal growth. When blood was not found in the birth canal, she was discharged. During the next month, she saw the ObGyn three times; testing indicated that the fetus was at least 3 weeks behind the stage of pregnancy. The ObGyn did not order additional testing nor consult a specialist. At 31 weeks’ gestation, ultrasonography found no fetal heart tones. The stillborn was delivered by cesarean section.
ESTATE’S CLAIM A wrongful death suit was filed by the parents, who also claimed lack of informed consent concerning the risk of stillbirth in the presence of intrauterine growth restriction.
PHYSICIANS’ DEFENSE The mother’s smoking was mentioned at trial as a possible explanation of why fetal development was delayed. The ObGyn denied negligence.
VERDICT A $800,000 Maryland verdict was awarded to the parents.
Three BrCa patients share $72.6 M
THREE MENOPAUSAL WOMEN took Premarin (conjugated estrogens) plus Provera (medroxyprogesterone), and/or Prempro (conjugated estrogens/medroxyprogesterone acetate). Each discontinued hormone therapy after being diagnosed with hormone-positive breast cancer.
PATIENTS’ CLAIM The only source of hormonal stimulation for their cancer was the use of estrogen plus progestin.
DEFENDANTS’ DEFENSE Science is currently unable to determine precisely what causes breast cancer. Each plaintiff had risk factors.
VERDICT The three cases were consolidated to a reverse-bifurcated trial, with causation and damages assessed first. The Pennsylvania jury found the Wyeth Pharmaceutical products to be factual causes of the patients’ cancer, and awarded a total of $72.6 million in compensatory damages. The parties settled for confidential amounts before the liability phase began.

WIDE LOCAL EXCISION was performed on a 42-year-old woman with vulvar intraepithelial neoplasm, VIN II, with moderate dysplasia. Her ObGyn performed the surgery.
Instead of applying a diluted solution of acetic acid wash to delineate the borders of the dysplastic area, a highly concentrated acetic acid or trichloroacetic acid was used. The patient suffered severe chemical burns of the vulva that took several months to heal. She has permanent scarring of the vulvar area, severe tenderness, discoloration, and atrophy of the vaginal opening, with a band of thick scar tissue at the posterior fourchette. The perineum, extending to the anal area, is scarred, including a 2-mm plaque layer.
PATIENT’S CLAIM Sexual intercourse is extremely painful, and therefore impossible. She suffers discomfort at all times. Additional surgery has been recommended to alleviate her condition.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $600,000 Ohio settlement was reached.
Large baby with cervical spine injury
A WOMAN WAS IN LABOR with her third child. Her first baby was born by cesarean delivery. During the vaginal birth of her second child, shoulder dystocia was encountered; this child weighed 8 lb 4 oz at birth.
Using ultrasonography, the ObGyn determined vaginal birth was appropriate. Shoulder dystocia was encountered and the infant suffered injuries to the cervical spine and right arm. The newborn weighed 9 lb 13 oz.
PATIENT’S CLAIM The baby’s weight was grossly underestimated prior to delivery; ultrasonography was not properly performed or evaluated. The mother’s history, large fundal height, estimated fetal weight, and the mother’s request for a cesarean delivery should have resulted in the performance of a cesarean delivery.
PHYSICIAN’S DEFENSE Shoulder dystocia was not reasonably foreseeable. Injuries to the baby were due to the forces of labor.
VERDICT A confidential Texas settlement was reached.
Suture causes nerve damage
PELVIC PROLAPSE RECONSTRUCTION was performed; surgery included a pubovaginal sling procedure with graft, and repairs of Grade 2 cystocele and Grade 3 rectocele. The gynecologist used transvaginal sutures to attach the mesh to the sacrospinous ligament.
The patient immediately reported pain, tingling, and weakness in her buttocks and legs. The gynecologist diagnosed a hematoma and continued conservative treatment while waiting for the hematoma to resorb.
After 10 days, the patient terminated the gynecologist’s services and left the hospital. She saw a neurologist, who diagnosed proximal sciatic nerve irritation secondary to suturing. When a suture was removed from the sacral spinous ligament plexus, many of the patient’s neurologic symptoms immediately resolved. She still has pain and walks with a noticeable limp using a cane.
PATIENT’S CLAIM The gynecologist failed to determine that a suture was causing nerve damage. Removal of the suture within the first 3 days would have avoided neurologic injury.
PHYSICIAN’S DEFENSE Postsurgical care was proper. A neurologist was consulted, and a sonogram had ruled out deep vein thrombosis.
VERDICT A $1.58 million Illinois verdict was returned.
Colon damage after embolization
UTERINE FIBROID EMBOLIZATION was performed on a 51-year-old woman. The next day, she reported severe abdominal pain and was readmitted. A uterine infection was suspected, and she underwent a hysterectomy. Necrosis of the colon was found; a surgeon removed one-third of the colon and performed a colostomy. She underwent several operations, including rectal-vaginal fistula repair, before the colostomy was corrected.
PATIENT’S CLAIM Misdirected embolization injured an artery supplying the colon. She continues to suffer ongoing fecal urgency and frequency.
PHYSICIAN’S DEFENSE An anomalous connection between the patient’s uterine artery and mesenteric artery was impossible for the physician to have known prior to the embolization procedure.
VERDICT A California defense verdict was returned.

SEVERAL HOURS AFTER A WOMAN’S LABOR BEGAN, fetal bradycardia developed precipitously. The on-call ObGyn arrived after 10 minutes and ordered an immediate cesarean delivery, which occurred 22 minutes later. The child suffered a catastrophic, irreversible brain injury. He lived for 39 days before life support was removed and he died.
ESTATE’S CLAIM The nurses did not report decelerations to the ObGyn, and they were slow to notify him of the fetal bradycardia. The child would not have been injured if the nursing staff had reacted appropriately.
DEFENDANTS’ DEFENSE Isolated heart-rate decelerations during labor are not troubling. A cord accident occurred, which could not be predicted nor avoided. The ObGyn was called promptly; the emergency cesarean delivery was performed quickly. However, the injury already had occurred and was irreparable.
VERDICT A $1.18 million Kentucky verdict was returned. The hospital sought a mistrial because Facebook postings by a juror proved the case had been discussed and prejudged. The court found in favor of the hospital on its post-trial motion.
Bilateral mastectomy: nipples not spared
A 46-YEAR-OLD WOMAN UNDERWENT prophylactic bilateral mastectomy. A plastic surgeon drew presurgical markings on the day of surgery; the breast surgeon removed the nipples.
PATIENT’S CLAIM All parties had agreed the nipples would be spared. The plastic surgeon drew improper markings and failed to remind the breast surgeon prior to surgery that the nipples would be preserved.
PHYSICIAN’S DEFENSE The breast surgeon was at fault for misinterpreting the markings.
VERDICT The patient reached a pretrial settlement with the breast surgeon. The case proceeded against the plastic surgeon. A Maryland defense verdict was returned for the plastic surgeon.
Signs of intrauterine growth restriction; stillborn child
AT 24 WEEKS’ GESTATION, a 17-year-old woman who smoked reported spotting. An ultrasound demonstrated significant fetal growth restriction. The mother was hospitalized to assess the spotting; no testing was ordered to assess fetal growth. When blood was not found in the birth canal, she was discharged. During the next month, she saw the ObGyn three times; testing indicated that the fetus was at least 3 weeks behind the stage of pregnancy. The ObGyn did not order additional testing nor consult a specialist. At 31 weeks’ gestation, ultrasonography found no fetal heart tones. The stillborn was delivered by cesarean section.
ESTATE’S CLAIM A wrongful death suit was filed by the parents, who also claimed lack of informed consent concerning the risk of stillbirth in the presence of intrauterine growth restriction.
PHYSICIANS’ DEFENSE The mother’s smoking was mentioned at trial as a possible explanation of why fetal development was delayed. The ObGyn denied negligence.
VERDICT A $800,000 Maryland verdict was awarded to the parents.
Three BrCa patients share $72.6 M
THREE MENOPAUSAL WOMEN took Premarin (conjugated estrogens) plus Provera (medroxyprogesterone), and/or Prempro (conjugated estrogens/medroxyprogesterone acetate). Each discontinued hormone therapy after being diagnosed with hormone-positive breast cancer.
PATIENTS’ CLAIM The only source of hormonal stimulation for their cancer was the use of estrogen plus progestin.
DEFENDANTS’ DEFENSE Science is currently unable to determine precisely what causes breast cancer. Each plaintiff had risk factors.
VERDICT The three cases were consolidated to a reverse-bifurcated trial, with causation and damages assessed first. The Pennsylvania jury found the Wyeth Pharmaceutical products to be factual causes of the patients’ cancer, and awarded a total of $72.6 million in compensatory damages. The parties settled for confidential amounts before the liability phase began.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
A stepwise approach to cervical cerclage
Does vaginal progesterone reduce preterm delivery among asymptomatic women who have a short cervix in the midtrimester?
John T. Repke, MD (Examining the Evidence, April 2012)
Update on obstetrics
John T. Repke, MD, and Jaimey M. Pauli, MD (January 2012)
Placement of a suture around an incompetent cervix to prevent premature pregnancy loss was first described more than 50 years ago1,2—but the few randomized studies that have been published (all of them in the past decade) devote very little attention to technique.3-7
Are we to assume, then, that over more than 50 years, no modifications to technique have been devised?
Are we to assume as well that in the two largest randomized studies to date, which involved 266 cerclages inserted in 27 different medical centers over more than 4 years,5,7 all cerclages were inserted in an identical manner using the same technique originated more than half a century ago?
In this article, we lay out five principles to achieve effective cerclage and describe a stepwise approach to technique. This technique is based on our experience with approximately 2,000 cerclages performed in a single medical center. We emphasize anatomic landmarks and surgical principles that are based on the published literature as well as our personal experience.
Five principles of effective cerclage
Place the cerclage as high as possible
In the original paper on cerclage, McDonald emphasized the need to place the suture as high as possible to be as close as possible to the level of the internal cervical os.2
Zilianti and colleagues elegantly described how—in the absence of cerclage—cervical tissue begins to change at the level of the internal os, forming a funnel that advances downward in the shape of the letters “Y,” “V,” and “U.”8 If we accept this notion, then the only way to prevent further shortening from the top down is by placing a high cerclage.
Studies have demonstrated improved pregnancy outcomes after placement of a high cervico-isthmic cerclage following failure of a “conventional” low cerclage.9,10
Place the cerclage adjacent to the cervical stroma
Macroscopic and microscopic visualization of the cervix reveals the following main layers:
- epithelium/mucosa, which covers the deeper connective tissue known as cervical stroma
- cervical stroma, which may be divided into two zones: 1) a superficial, subepithelial zone that appears histologically as loose stromal bands and 2) a deeper, dense collagen layer.
It is the dense collagen layer of the cervical stroma that affords most of the resistance to forces of deformation, whereas the loose stromal layer and the epithelium above it slide easily over the deeper stroma. Including too great a proportion of these “slippery” components within the cerclage could increase the risk of displacement and failure.11,12
Shirodkar was the first to suggest that the mucosa and submucosa be excluded from cerclage,1 and a detailed submucosal cerclage insertion was described by Fahmy more than 30 years ago.13 We support his recommendation that cerclage placement be as close to the inner cervical stroma as possible and that it include as little as possible of the surrounding tissue.
Take three encircling cervical “bites”
In his original publication, McDonald described “five or six bites with the needle” to encircle the cervix.2 Later authors usually described four encircling steps, but no reliable study has challenged the original dogma.
Although we lack science to favor one approach over another, common sense suggests that three bites (versus four or five) offer the following advantages. They:
- produce less penetrating injury
- require less manipulation of the cervix
- are simpler and quicker to perform
- offer less opportunity for the cerclage tape to get twisted (a flat tape provides for better distribution of the load)
- permit a small gap between the two final exit points of the tape (at 5 o’clock and 7 o’clock), which allows for easier cinching and tightening of the cerclage (FIGURE 1).
FIGURE 1 A small gap between the ends of the cerclage tape, which exit at 5 o’clock and 7 o’clock, allows for easier cinching and tightening.
Both Shirodkar1 and McDonald2 described placement of the knot (or approximation of the “ends”) at 12 o’clock, anteriorly. However, this approach can complicate removal if strong pressure is applied to the cerclage or if it is covered by tissue. Attempts to remove the cerclage can result in bladder injury.
For these reasons, we prefer the approach described by Caspi and colleagues, who placed the knot in the back of the cervix at 6 o’clock.14 In that location, the surgeon can reach as high as desired, and there is no risk of organ injury during removal.
That said, it should be noted that removal of a cerclage with a knot at 6 o’clock is more difficult than removal of one with a knot at 12 o’clock—but the convenience of the operator should be secondary to safety and efficacy of the cerclage.
Place a figure of 8 around the cerclage knot
Because of the proximity of the bladder anteriorly, there is a limit to how high one can place the cerclage anteriorly. However, in the back of the cervix, one can place the cerclage much higher without risk of injury. This approach sometimes will result in downward forces on the posterior part (where the knot is) and occasionally may cover the knot with tissue from the posterior vaginal fornix.
For these reasons, we propose securing the knot of the cerclage tape to the posterior surface of the cervical “core” by placing a figure of 8 using bright blue Prolene #1 (Ethicon) to prevent slippage and help call attention to the knot when the time for removal comes.
1. Use a weighted speculum to retract the posterior-inferior vaginal wall. Have an assistant hold one or two right-angle retractors to retract the other aspects of the vaginal wall, including the bladder anteriorly, as needed.
2. Clamp the anterior and posterior lips of the cervix and tug them lightly—at all times—in an outward direction (FIGURE 2).
FIGURE 2 Clamping of the cervix
Clamp the anterior and posterior lips of the cervix and tug them lightly and steadily in an outward direction.
3. Retract and release the bladder several times using a right-angle retractor for more accurate identification of the cervico-vesical fold (FIGURE 3 and FIGURE 4). Note the distance from the external os to the cervico-vesical fold; it should be 2 cm or farther. (If it is less than 2 cm, another type of cerclage may be preferable.)
FIGURE 3 Anatomic landmarks
Cerclage is facilitated by orientation to the following landmarks; A. cervico-vesical fold; B. posterior fornix; C. cervical stroma; D. cervical mucosa.
FIGURE 4 Cervico-vesical fold
The black line indicates the location of the fold.
4. Identify the roof of the posterior fornix (FIGURE 3 and FIGURE 5).
FIGURE 5 Roof of the posterior fornix
This landmark is delineated in black.
5. Using Allis clamps bilaterally, clamp the soft tissue covering the cervical core (stroma), between the cervico-vesical junction anteriorly and the superior point of the posterior fornix posteriorly. This is a cardinal step because it separates the core from the mucosal/ submucosal elements (FIGURE 6). (Helpful hint: To achieve optimal placement of the lateral Allis clamps, place the open clamp ever so slightly to one side of the middle of the cervix. As you close the instrument, let the clamp slide off the cervical core until it is locked adjacent to it. This takes the soft tissue and supporting blood vessels out of the operative field.)
FIGURE 6 Anterolateral view
The pericervical mucosa (black arrow) after application of an Allis clamp.
6. Take three bites, 1 mm in depth, through the cervical core using a 5-mm Mersilene tape and a blunt-tipped needle (RS21; Ethicon). One bite should encompass 12:30 to 11:30 anteriorly. Another bite should go in at 3 o’clock and out at 5 o’clock, and another bite should go in at 9 o’clock and out at 7 o’clock (FIGURE 1). (Helpful hint: Ensure that the direction of the pull always is a direct extension of the passage through tissue in small steps and not an outward direction toward the operator. An instrument such as a curved Mayo clamp should be placed at the point of the needle’s exit to reduce the risk of injury. At the conclusion of the three bites, the Mersilene tape should be the same length on each side, exiting at 5 o’clock and 7 o’clock, as stated earlier [FIGURE 7].)
FIGURE 7 Ensure equal distribution of the tape
After taking three bites of tissue, ensure that the ends of the Mersilene tape are of equal length on each side.
7. Once the tape is of equal length on both sides, closely encircling three sides of the cervical core, empty the bladder with a catheter to ensure the presence of clear urine. Bloody urine could be an indication for cystoscopy to rule out bladder injury.
8. Cut the needles off of the tape and tie the cerclage in three ties, the first one being a surgical tie. After tying the first tie, ensure proper tension by pressing gently with the index finger up and down on the knot; if it is properly tensioned, it will not be displaced by this movement. (Helpful hint: There is no clear indication of how tight a cerclage should be tied. We suggest making the first tie as close as possible to the cervical core to create a visible, and palpable, depression in the soft tissue at the area of the knot [FIGURE 8]).

FIGURE 8 Tying the cerclage
Make the first tie as close as possible to the cervical core so that it creates a visible, and palpable, depression in the soft tissue at the area of the knot. Inset: Cerclage tie secured by a figure of 8.
9. Trim the ends of the tape to 3 cm to facilitate easy identification and manipulation at the time of removal. Place a figure of 8, using bright blue Prolene #1 (Ethicon), around the knot, securing it to the posterior surface of the cervical core (FIGURE 9). The tape should encircle the firm part of the cervix near the internal os, as shown by transvaginal ultrasonography in FIGURE 10. (Helpful hint: Place surgical gauze under pressure around the cervix to support hemostasis after removal of the clamps. Remove the gauze approximately 30 minutes after the procedure.)
FIGURE 9 Mark the cerclage
Place a figure of 8, using bright blue Prolene #1, around the knot of the cerclage, securing it to the posterior surface of the cervical core.
FIGURE 10 Final placement
The cerclage tape should encircle the firm part of the cervix near the internal os, as shown by transvaginal ultrasonography.
Technique is applicable to most cerclage procedures
One potential limitation of this technique is the fact that it is based on surgical experience in a single center, although it includes more than 2,000 operations performed at that center. Therefore, we lack data on the ease of teaching and reproducing this technique. Nevertheless, our approach incorporates various elements that previously were proposed to enhance the effectiveness of cerclage. It likely will be applicable to most cerclage procedures, with the exception of a few unique cases. These unique cases—most of them involving the failure of conventional cerclage—may require more elaborate technique.
As for data on the location of biomechanical stresses on cervical tissue during pregnancy, the literature indicates that the forces of maximum deformation begin internally at the level of the cervico-uterine junction.12 If not successfully resisted, these forces will proceed down along the cervical canal and could lead to premature pregnancy loss.
Although the superiority of a high cerclage has not yet been proven clinically, it appears to be more effective than low placement because it is more likely to provide support at the right location.
As pregnancy progresses, the challenge to the cervix increases—not only because of increasing uterine volume but also because of greater uterine activity. Both raise the risk of cerclage slippage and displacement.15 To address these issues, several investigators proposed an approach that excludes the slippery mucosal layer.1,13,14
The original Shirodkar cerclage and its modifications—but not the McDonald cerclage and its subsequent modifications—included an “anchor” suture attaching the cerclage band to the firm cervical stromal layer as a means to prevent downward slippage and displacement.1 It remains to be seen whether this addition of a figure of 8 using nonabsorbable suture, as proposed here, is indeed effective.
We believe that, if a standardized way to perform effective cerclage can be agreed upon, we also might devise a better way to compare results based on proper patient selection.
We want to hear from you! Tell us what you think.
1. Shirodkar VN. A new method of operative treatment for habitual abortions in the second trimester of pregnancy. Antiseptic. 1955;52:299.-
2. McDonald IA. Suture of the cervix for inevitable miscarriage. J Obstet Gynaecol Br Emp. 1957;64(3):346-350.
3. Rust OA, Atlas RO, Jones KJ, Benham BN, Balducci J. A randomized trial of cerclage versus no cerclage among patients with ultrasonographically detected second-trimester preterm dilatation of the internal os. Am J Obstet Gynecol. 2000;183(4):830-835.
4. Althuisius SM, Dekker GA, Hummel P, Bekedam DJ, van Geijn HP. Final Results of the Cervical Incompetence Prevention Randomized Cerclage Trial (CIPRACT): therapeutic cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol. 2001;185(5):1106-1112.
5. To MS, Alfirevic Z, Heath VC, et al. Fetal Medicine Foundation Second Trimester Screening Group. Cervical cerclage for prevention of preterm delivery in women with short cervix: randomised controlled trial. Lancet. 2004;363(9424):1849-1853.
6. Berghella V, Odibo AO, Tolosa JE. Cerclage for prevention of preterm birth in women with a short cervix found on transvaginal ultrasound examination: a randomized trial. Am J Obstet Gynecol. 2004;191(4):1311-1317.
7. Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201(4):375.e1-8.
8. Zilianti M, Azuaga A, Calderon F, Pagés G, Mendoza G. Monitoring the effacement of the uterine cervix by transperineal sonography: a new perspective. J Ultrasound Med. 1995;14(10):719-724.
9. Herron MA, Parer JT. Transabdominal cerclage for fetal wastage due to cervical incompetence. Obstet Gynecol. 1988;71(6 Pt 1):865-868.
10. Katz M, Abrahams C. Transvaginal placement of cervicoisthmic cerclage: Report on pregnancy outcome. Am J Obstet Gynecol. 2005;192(6):1989-1992.
11. Ferenczy A. Ultrastructure of the uterine cervix. In: Huszar G ed. The Physiology and Biochemistry of the Uterus in Pregnancy and Labor. Boca Raton, FL: CRC Press; 2000: 239–260.
12. Heaps RH, House M, Socrate S, Leppert P, Strauss JF, III. Matrix biology and preterm birth. In: Petraglia F Strauss JF III, Gabbe SG, Weiss G, eds. Preterm Birth: Mechanisms, Mediators, Prediction, Prevention, and Interventions. United Kingdom: Informa; 2007:71–93.
13. Fahmy K. A closed submucous cervical suture for the incompetent cervix. Int Surg. 1978;63(2):77-80.
14. Caspi E, Schneider DF, Mor Z, Langer R, Weinraub Z, Bukovsky I. Cervical internal os cerclage: description of a new technique and comparison with Shirodkar operation. Am J Perinatol. 1990;7(4):347-349.
15. Harger JH. Cerclage and cervical insufficiency: an evidence-based analysis. Obstet Gynecol. 2002;100:1313-1327.
Does vaginal progesterone reduce preterm delivery among asymptomatic women who have a short cervix in the midtrimester?
John T. Repke, MD (Examining the Evidence, April 2012)
Update on obstetrics
John T. Repke, MD, and Jaimey M. Pauli, MD (January 2012)
Placement of a suture around an incompetent cervix to prevent premature pregnancy loss was first described more than 50 years ago1,2—but the few randomized studies that have been published (all of them in the past decade) devote very little attention to technique.3-7
Are we to assume, then, that over more than 50 years, no modifications to technique have been devised?
Are we to assume as well that in the two largest randomized studies to date, which involved 266 cerclages inserted in 27 different medical centers over more than 4 years,5,7 all cerclages were inserted in an identical manner using the same technique originated more than half a century ago?
In this article, we lay out five principles to achieve effective cerclage and describe a stepwise approach to technique. This technique is based on our experience with approximately 2,000 cerclages performed in a single medical center. We emphasize anatomic landmarks and surgical principles that are based on the published literature as well as our personal experience.
Five principles of effective cerclage
Place the cerclage as high as possible
In the original paper on cerclage, McDonald emphasized the need to place the suture as high as possible to be as close as possible to the level of the internal cervical os.2
Zilianti and colleagues elegantly described how—in the absence of cerclage—cervical tissue begins to change at the level of the internal os, forming a funnel that advances downward in the shape of the letters “Y,” “V,” and “U.”8 If we accept this notion, then the only way to prevent further shortening from the top down is by placing a high cerclage.
Studies have demonstrated improved pregnancy outcomes after placement of a high cervico-isthmic cerclage following failure of a “conventional” low cerclage.9,10
Place the cerclage adjacent to the cervical stroma
Macroscopic and microscopic visualization of the cervix reveals the following main layers:
- epithelium/mucosa, which covers the deeper connective tissue known as cervical stroma
- cervical stroma, which may be divided into two zones: 1) a superficial, subepithelial zone that appears histologically as loose stromal bands and 2) a deeper, dense collagen layer.
It is the dense collagen layer of the cervical stroma that affords most of the resistance to forces of deformation, whereas the loose stromal layer and the epithelium above it slide easily over the deeper stroma. Including too great a proportion of these “slippery” components within the cerclage could increase the risk of displacement and failure.11,12
Shirodkar was the first to suggest that the mucosa and submucosa be excluded from cerclage,1 and a detailed submucosal cerclage insertion was described by Fahmy more than 30 years ago.13 We support his recommendation that cerclage placement be as close to the inner cervical stroma as possible and that it include as little as possible of the surrounding tissue.
Take three encircling cervical “bites”
In his original publication, McDonald described “five or six bites with the needle” to encircle the cervix.2 Later authors usually described four encircling steps, but no reliable study has challenged the original dogma.
Although we lack science to favor one approach over another, common sense suggests that three bites (versus four or five) offer the following advantages. They:
- produce less penetrating injury
- require less manipulation of the cervix
- are simpler and quicker to perform
- offer less opportunity for the cerclage tape to get twisted (a flat tape provides for better distribution of the load)
- permit a small gap between the two final exit points of the tape (at 5 o’clock and 7 o’clock), which allows for easier cinching and tightening of the cerclage (FIGURE 1).

FIGURE 1 A small gap between the ends of the cerclage tape, which exit at 5 o’clock and 7 o’clock, allows for easier cinching and tightening.
Both Shirodkar1 and McDonald2 described placement of the knot (or approximation of the “ends”) at 12 o’clock, anteriorly. However, this approach can complicate removal if strong pressure is applied to the cerclage or if it is covered by tissue. Attempts to remove the cerclage can result in bladder injury.
For these reasons, we prefer the approach described by Caspi and colleagues, who placed the knot in the back of the cervix at 6 o’clock.14 In that location, the surgeon can reach as high as desired, and there is no risk of organ injury during removal.
That said, it should be noted that removal of a cerclage with a knot at 6 o’clock is more difficult than removal of one with a knot at 12 o’clock—but the convenience of the operator should be secondary to safety and efficacy of the cerclage.
Place a figure of 8 around the cerclage knot
Because of the proximity of the bladder anteriorly, there is a limit to how high one can place the cerclage anteriorly. However, in the back of the cervix, one can place the cerclage much higher without risk of injury. This approach sometimes will result in downward forces on the posterior part (where the knot is) and occasionally may cover the knot with tissue from the posterior vaginal fornix.
For these reasons, we propose securing the knot of the cerclage tape to the posterior surface of the cervical “core” by placing a figure of 8 using bright blue Prolene #1 (Ethicon) to prevent slippage and help call attention to the knot when the time for removal comes.
1. Use a weighted speculum to retract the posterior-inferior vaginal wall. Have an assistant hold one or two right-angle retractors to retract the other aspects of the vaginal wall, including the bladder anteriorly, as needed.
2. Clamp the anterior and posterior lips of the cervix and tug them lightly—at all times—in an outward direction (FIGURE 2).

FIGURE 2 Clamping of the cervix
Clamp the anterior and posterior lips of the cervix and tug them lightly and steadily in an outward direction.
3. Retract and release the bladder several times using a right-angle retractor for more accurate identification of the cervico-vesical fold (FIGURE 3 and FIGURE 4). Note the distance from the external os to the cervico-vesical fold; it should be 2 cm or farther. (If it is less than 2 cm, another type of cerclage may be preferable.)

FIGURE 3 Anatomic landmarks
Cerclage is facilitated by orientation to the following landmarks; A. cervico-vesical fold; B. posterior fornix; C. cervical stroma; D. cervical mucosa.

FIGURE 4 Cervico-vesical fold
The black line indicates the location of the fold.
4. Identify the roof of the posterior fornix (FIGURE 3 and FIGURE 5).

FIGURE 5 Roof of the posterior fornix
This landmark is delineated in black.
5. Using Allis clamps bilaterally, clamp the soft tissue covering the cervical core (stroma), between the cervico-vesical junction anteriorly and the superior point of the posterior fornix posteriorly. This is a cardinal step because it separates the core from the mucosal/ submucosal elements (FIGURE 6). (Helpful hint: To achieve optimal placement of the lateral Allis clamps, place the open clamp ever so slightly to one side of the middle of the cervix. As you close the instrument, let the clamp slide off the cervical core until it is locked adjacent to it. This takes the soft tissue and supporting blood vessels out of the operative field.)

FIGURE 6 Anterolateral view
The pericervical mucosa (black arrow) after application of an Allis clamp.
6. Take three bites, 1 mm in depth, through the cervical core using a 5-mm Mersilene tape and a blunt-tipped needle (RS21; Ethicon). One bite should encompass 12:30 to 11:30 anteriorly. Another bite should go in at 3 o’clock and out at 5 o’clock, and another bite should go in at 9 o’clock and out at 7 o’clock (FIGURE 1). (Helpful hint: Ensure that the direction of the pull always is a direct extension of the passage through tissue in small steps and not an outward direction toward the operator. An instrument such as a curved Mayo clamp should be placed at the point of the needle’s exit to reduce the risk of injury. At the conclusion of the three bites, the Mersilene tape should be the same length on each side, exiting at 5 o’clock and 7 o’clock, as stated earlier [FIGURE 7].)

FIGURE 7 Ensure equal distribution of the tape
After taking three bites of tissue, ensure that the ends of the Mersilene tape are of equal length on each side.
7. Once the tape is of equal length on both sides, closely encircling three sides of the cervical core, empty the bladder with a catheter to ensure the presence of clear urine. Bloody urine could be an indication for cystoscopy to rule out bladder injury.
8. Cut the needles off of the tape and tie the cerclage in three ties, the first one being a surgical tie. After tying the first tie, ensure proper tension by pressing gently with the index finger up and down on the knot; if it is properly tensioned, it will not be displaced by this movement. (Helpful hint: There is no clear indication of how tight a cerclage should be tied. We suggest making the first tie as close as possible to the cervical core to create a visible, and palpable, depression in the soft tissue at the area of the knot [FIGURE 8]).

FIGURE 8 Tying the cerclage
Make the first tie as close as possible to the cervical core so that it creates a visible, and palpable, depression in the soft tissue at the area of the knot. Inset: Cerclage tie secured by a figure of 8.
9. Trim the ends of the tape to 3 cm to facilitate easy identification and manipulation at the time of removal. Place a figure of 8, using bright blue Prolene #1 (Ethicon), around the knot, securing it to the posterior surface of the cervical core (FIGURE 9). The tape should encircle the firm part of the cervix near the internal os, as shown by transvaginal ultrasonography in FIGURE 10. (Helpful hint: Place surgical gauze under pressure around the cervix to support hemostasis after removal of the clamps. Remove the gauze approximately 30 minutes after the procedure.)

FIGURE 9 Mark the cerclage
Place a figure of 8, using bright blue Prolene #1, around the knot of the cerclage, securing it to the posterior surface of the cervical core.

FIGURE 10 Final placement
The cerclage tape should encircle the firm part of the cervix near the internal os, as shown by transvaginal ultrasonography.
Technique is applicable to most cerclage procedures
One potential limitation of this technique is the fact that it is based on surgical experience in a single center, although it includes more than 2,000 operations performed at that center. Therefore, we lack data on the ease of teaching and reproducing this technique. Nevertheless, our approach incorporates various elements that previously were proposed to enhance the effectiveness of cerclage. It likely will be applicable to most cerclage procedures, with the exception of a few unique cases. These unique cases—most of them involving the failure of conventional cerclage—may require more elaborate technique.
As for data on the location of biomechanical stresses on cervical tissue during pregnancy, the literature indicates that the forces of maximum deformation begin internally at the level of the cervico-uterine junction.12 If not successfully resisted, these forces will proceed down along the cervical canal and could lead to premature pregnancy loss.
Although the superiority of a high cerclage has not yet been proven clinically, it appears to be more effective than low placement because it is more likely to provide support at the right location.
As pregnancy progresses, the challenge to the cervix increases—not only because of increasing uterine volume but also because of greater uterine activity. Both raise the risk of cerclage slippage and displacement.15 To address these issues, several investigators proposed an approach that excludes the slippery mucosal layer.1,13,14
The original Shirodkar cerclage and its modifications—but not the McDonald cerclage and its subsequent modifications—included an “anchor” suture attaching the cerclage band to the firm cervical stromal layer as a means to prevent downward slippage and displacement.1 It remains to be seen whether this addition of a figure of 8 using nonabsorbable suture, as proposed here, is indeed effective.
We believe that, if a standardized way to perform effective cerclage can be agreed upon, we also might devise a better way to compare results based on proper patient selection.
We want to hear from you! Tell us what you think.
Does vaginal progesterone reduce preterm delivery among asymptomatic women who have a short cervix in the midtrimester?
John T. Repke, MD (Examining the Evidence, April 2012)
Update on obstetrics
John T. Repke, MD, and Jaimey M. Pauli, MD (January 2012)
Placement of a suture around an incompetent cervix to prevent premature pregnancy loss was first described more than 50 years ago1,2—but the few randomized studies that have been published (all of them in the past decade) devote very little attention to technique.3-7
Are we to assume, then, that over more than 50 years, no modifications to technique have been devised?
Are we to assume as well that in the two largest randomized studies to date, which involved 266 cerclages inserted in 27 different medical centers over more than 4 years,5,7 all cerclages were inserted in an identical manner using the same technique originated more than half a century ago?
In this article, we lay out five principles to achieve effective cerclage and describe a stepwise approach to technique. This technique is based on our experience with approximately 2,000 cerclages performed in a single medical center. We emphasize anatomic landmarks and surgical principles that are based on the published literature as well as our personal experience.
Five principles of effective cerclage
Place the cerclage as high as possible
In the original paper on cerclage, McDonald emphasized the need to place the suture as high as possible to be as close as possible to the level of the internal cervical os.2
Zilianti and colleagues elegantly described how—in the absence of cerclage—cervical tissue begins to change at the level of the internal os, forming a funnel that advances downward in the shape of the letters “Y,” “V,” and “U.”8 If we accept this notion, then the only way to prevent further shortening from the top down is by placing a high cerclage.
Studies have demonstrated improved pregnancy outcomes after placement of a high cervico-isthmic cerclage following failure of a “conventional” low cerclage.9,10
Place the cerclage adjacent to the cervical stroma
Macroscopic and microscopic visualization of the cervix reveals the following main layers:
- epithelium/mucosa, which covers the deeper connective tissue known as cervical stroma
- cervical stroma, which may be divided into two zones: 1) a superficial, subepithelial zone that appears histologically as loose stromal bands and 2) a deeper, dense collagen layer.
It is the dense collagen layer of the cervical stroma that affords most of the resistance to forces of deformation, whereas the loose stromal layer and the epithelium above it slide easily over the deeper stroma. Including too great a proportion of these “slippery” components within the cerclage could increase the risk of displacement and failure.11,12
Shirodkar was the first to suggest that the mucosa and submucosa be excluded from cerclage,1 and a detailed submucosal cerclage insertion was described by Fahmy more than 30 years ago.13 We support his recommendation that cerclage placement be as close to the inner cervical stroma as possible and that it include as little as possible of the surrounding tissue.
Take three encircling cervical “bites”
In his original publication, McDonald described “five or six bites with the needle” to encircle the cervix.2 Later authors usually described four encircling steps, but no reliable study has challenged the original dogma.
Although we lack science to favor one approach over another, common sense suggests that three bites (versus four or five) offer the following advantages. They:
- produce less penetrating injury
- require less manipulation of the cervix
- are simpler and quicker to perform
- offer less opportunity for the cerclage tape to get twisted (a flat tape provides for better distribution of the load)
- permit a small gap between the two final exit points of the tape (at 5 o’clock and 7 o’clock), which allows for easier cinching and tightening of the cerclage (FIGURE 1).

FIGURE 1 A small gap between the ends of the cerclage tape, which exit at 5 o’clock and 7 o’clock, allows for easier cinching and tightening.
Both Shirodkar1 and McDonald2 described placement of the knot (or approximation of the “ends”) at 12 o’clock, anteriorly. However, this approach can complicate removal if strong pressure is applied to the cerclage or if it is covered by tissue. Attempts to remove the cerclage can result in bladder injury.
For these reasons, we prefer the approach described by Caspi and colleagues, who placed the knot in the back of the cervix at 6 o’clock.14 In that location, the surgeon can reach as high as desired, and there is no risk of organ injury during removal.
That said, it should be noted that removal of a cerclage with a knot at 6 o’clock is more difficult than removal of one with a knot at 12 o’clock—but the convenience of the operator should be secondary to safety and efficacy of the cerclage.
Place a figure of 8 around the cerclage knot
Because of the proximity of the bladder anteriorly, there is a limit to how high one can place the cerclage anteriorly. However, in the back of the cervix, one can place the cerclage much higher without risk of injury. This approach sometimes will result in downward forces on the posterior part (where the knot is) and occasionally may cover the knot with tissue from the posterior vaginal fornix.
For these reasons, we propose securing the knot of the cerclage tape to the posterior surface of the cervical “core” by placing a figure of 8 using bright blue Prolene #1 (Ethicon) to prevent slippage and help call attention to the knot when the time for removal comes.
1. Use a weighted speculum to retract the posterior-inferior vaginal wall. Have an assistant hold one or two right-angle retractors to retract the other aspects of the vaginal wall, including the bladder anteriorly, as needed.
2. Clamp the anterior and posterior lips of the cervix and tug them lightly—at all times—in an outward direction (FIGURE 2).

FIGURE 2 Clamping of the cervix
Clamp the anterior and posterior lips of the cervix and tug them lightly and steadily in an outward direction.
3. Retract and release the bladder several times using a right-angle retractor for more accurate identification of the cervico-vesical fold (FIGURE 3 and FIGURE 4). Note the distance from the external os to the cervico-vesical fold; it should be 2 cm or farther. (If it is less than 2 cm, another type of cerclage may be preferable.)

FIGURE 3 Anatomic landmarks
Cerclage is facilitated by orientation to the following landmarks; A. cervico-vesical fold; B. posterior fornix; C. cervical stroma; D. cervical mucosa.

FIGURE 4 Cervico-vesical fold
The black line indicates the location of the fold.
4. Identify the roof of the posterior fornix (FIGURE 3 and FIGURE 5).

FIGURE 5 Roof of the posterior fornix
This landmark is delineated in black.
5. Using Allis clamps bilaterally, clamp the soft tissue covering the cervical core (stroma), between the cervico-vesical junction anteriorly and the superior point of the posterior fornix posteriorly. This is a cardinal step because it separates the core from the mucosal/ submucosal elements (FIGURE 6). (Helpful hint: To achieve optimal placement of the lateral Allis clamps, place the open clamp ever so slightly to one side of the middle of the cervix. As you close the instrument, let the clamp slide off the cervical core until it is locked adjacent to it. This takes the soft tissue and supporting blood vessels out of the operative field.)

FIGURE 6 Anterolateral view
The pericervical mucosa (black arrow) after application of an Allis clamp.
6. Take three bites, 1 mm in depth, through the cervical core using a 5-mm Mersilene tape and a blunt-tipped needle (RS21; Ethicon). One bite should encompass 12:30 to 11:30 anteriorly. Another bite should go in at 3 o’clock and out at 5 o’clock, and another bite should go in at 9 o’clock and out at 7 o’clock (FIGURE 1). (Helpful hint: Ensure that the direction of the pull always is a direct extension of the passage through tissue in small steps and not an outward direction toward the operator. An instrument such as a curved Mayo clamp should be placed at the point of the needle’s exit to reduce the risk of injury. At the conclusion of the three bites, the Mersilene tape should be the same length on each side, exiting at 5 o’clock and 7 o’clock, as stated earlier [FIGURE 7].)

FIGURE 7 Ensure equal distribution of the tape
After taking three bites of tissue, ensure that the ends of the Mersilene tape are of equal length on each side.
7. Once the tape is of equal length on both sides, closely encircling three sides of the cervical core, empty the bladder with a catheter to ensure the presence of clear urine. Bloody urine could be an indication for cystoscopy to rule out bladder injury.
8. Cut the needles off of the tape and tie the cerclage in three ties, the first one being a surgical tie. After tying the first tie, ensure proper tension by pressing gently with the index finger up and down on the knot; if it is properly tensioned, it will not be displaced by this movement. (Helpful hint: There is no clear indication of how tight a cerclage should be tied. We suggest making the first tie as close as possible to the cervical core to create a visible, and palpable, depression in the soft tissue at the area of the knot [FIGURE 8]).

FIGURE 8 Tying the cerclage
Make the first tie as close as possible to the cervical core so that it creates a visible, and palpable, depression in the soft tissue at the area of the knot. Inset: Cerclage tie secured by a figure of 8.
9. Trim the ends of the tape to 3 cm to facilitate easy identification and manipulation at the time of removal. Place a figure of 8, using bright blue Prolene #1 (Ethicon), around the knot, securing it to the posterior surface of the cervical core (FIGURE 9). The tape should encircle the firm part of the cervix near the internal os, as shown by transvaginal ultrasonography in FIGURE 10. (Helpful hint: Place surgical gauze under pressure around the cervix to support hemostasis after removal of the clamps. Remove the gauze approximately 30 minutes after the procedure.)

FIGURE 9 Mark the cerclage
Place a figure of 8, using bright blue Prolene #1, around the knot of the cerclage, securing it to the posterior surface of the cervical core.

FIGURE 10 Final placement
The cerclage tape should encircle the firm part of the cervix near the internal os, as shown by transvaginal ultrasonography.
Technique is applicable to most cerclage procedures
One potential limitation of this technique is the fact that it is based on surgical experience in a single center, although it includes more than 2,000 operations performed at that center. Therefore, we lack data on the ease of teaching and reproducing this technique. Nevertheless, our approach incorporates various elements that previously were proposed to enhance the effectiveness of cerclage. It likely will be applicable to most cerclage procedures, with the exception of a few unique cases. These unique cases—most of them involving the failure of conventional cerclage—may require more elaborate technique.
As for data on the location of biomechanical stresses on cervical tissue during pregnancy, the literature indicates that the forces of maximum deformation begin internally at the level of the cervico-uterine junction.12 If not successfully resisted, these forces will proceed down along the cervical canal and could lead to premature pregnancy loss.
Although the superiority of a high cerclage has not yet been proven clinically, it appears to be more effective than low placement because it is more likely to provide support at the right location.
As pregnancy progresses, the challenge to the cervix increases—not only because of increasing uterine volume but also because of greater uterine activity. Both raise the risk of cerclage slippage and displacement.15 To address these issues, several investigators proposed an approach that excludes the slippery mucosal layer.1,13,14
The original Shirodkar cerclage and its modifications—but not the McDonald cerclage and its subsequent modifications—included an “anchor” suture attaching the cerclage band to the firm cervical stromal layer as a means to prevent downward slippage and displacement.1 It remains to be seen whether this addition of a figure of 8 using nonabsorbable suture, as proposed here, is indeed effective.
We believe that, if a standardized way to perform effective cerclage can be agreed upon, we also might devise a better way to compare results based on proper patient selection.
We want to hear from you! Tell us what you think.
1. Shirodkar VN. A new method of operative treatment for habitual abortions in the second trimester of pregnancy. Antiseptic. 1955;52:299.-
2. McDonald IA. Suture of the cervix for inevitable miscarriage. J Obstet Gynaecol Br Emp. 1957;64(3):346-350.
3. Rust OA, Atlas RO, Jones KJ, Benham BN, Balducci J. A randomized trial of cerclage versus no cerclage among patients with ultrasonographically detected second-trimester preterm dilatation of the internal os. Am J Obstet Gynecol. 2000;183(4):830-835.
4. Althuisius SM, Dekker GA, Hummel P, Bekedam DJ, van Geijn HP. Final Results of the Cervical Incompetence Prevention Randomized Cerclage Trial (CIPRACT): therapeutic cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol. 2001;185(5):1106-1112.
5. To MS, Alfirevic Z, Heath VC, et al. Fetal Medicine Foundation Second Trimester Screening Group. Cervical cerclage for prevention of preterm delivery in women with short cervix: randomised controlled trial. Lancet. 2004;363(9424):1849-1853.
6. Berghella V, Odibo AO, Tolosa JE. Cerclage for prevention of preterm birth in women with a short cervix found on transvaginal ultrasound examination: a randomized trial. Am J Obstet Gynecol. 2004;191(4):1311-1317.
7. Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201(4):375.e1-8.
8. Zilianti M, Azuaga A, Calderon F, Pagés G, Mendoza G. Monitoring the effacement of the uterine cervix by transperineal sonography: a new perspective. J Ultrasound Med. 1995;14(10):719-724.
9. Herron MA, Parer JT. Transabdominal cerclage for fetal wastage due to cervical incompetence. Obstet Gynecol. 1988;71(6 Pt 1):865-868.
10. Katz M, Abrahams C. Transvaginal placement of cervicoisthmic cerclage: Report on pregnancy outcome. Am J Obstet Gynecol. 2005;192(6):1989-1992.
11. Ferenczy A. Ultrastructure of the uterine cervix. In: Huszar G ed. The Physiology and Biochemistry of the Uterus in Pregnancy and Labor. Boca Raton, FL: CRC Press; 2000: 239–260.
12. Heaps RH, House M, Socrate S, Leppert P, Strauss JF, III. Matrix biology and preterm birth. In: Petraglia F Strauss JF III, Gabbe SG, Weiss G, eds. Preterm Birth: Mechanisms, Mediators, Prediction, Prevention, and Interventions. United Kingdom: Informa; 2007:71–93.
13. Fahmy K. A closed submucous cervical suture for the incompetent cervix. Int Surg. 1978;63(2):77-80.
14. Caspi E, Schneider DF, Mor Z, Langer R, Weinraub Z, Bukovsky I. Cervical internal os cerclage: description of a new technique and comparison with Shirodkar operation. Am J Perinatol. 1990;7(4):347-349.
15. Harger JH. Cerclage and cervical insufficiency: an evidence-based analysis. Obstet Gynecol. 2002;100:1313-1327.
1. Shirodkar VN. A new method of operative treatment for habitual abortions in the second trimester of pregnancy. Antiseptic. 1955;52:299.-
2. McDonald IA. Suture of the cervix for inevitable miscarriage. J Obstet Gynaecol Br Emp. 1957;64(3):346-350.
3. Rust OA, Atlas RO, Jones KJ, Benham BN, Balducci J. A randomized trial of cerclage versus no cerclage among patients with ultrasonographically detected second-trimester preterm dilatation of the internal os. Am J Obstet Gynecol. 2000;183(4):830-835.
4. Althuisius SM, Dekker GA, Hummel P, Bekedam DJ, van Geijn HP. Final Results of the Cervical Incompetence Prevention Randomized Cerclage Trial (CIPRACT): therapeutic cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol. 2001;185(5):1106-1112.
5. To MS, Alfirevic Z, Heath VC, et al. Fetal Medicine Foundation Second Trimester Screening Group. Cervical cerclage for prevention of preterm delivery in women with short cervix: randomised controlled trial. Lancet. 2004;363(9424):1849-1853.
6. Berghella V, Odibo AO, Tolosa JE. Cerclage for prevention of preterm birth in women with a short cervix found on transvaginal ultrasound examination: a randomized trial. Am J Obstet Gynecol. 2004;191(4):1311-1317.
7. Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201(4):375.e1-8.
8. Zilianti M, Azuaga A, Calderon F, Pagés G, Mendoza G. Monitoring the effacement of the uterine cervix by transperineal sonography: a new perspective. J Ultrasound Med. 1995;14(10):719-724.
9. Herron MA, Parer JT. Transabdominal cerclage for fetal wastage due to cervical incompetence. Obstet Gynecol. 1988;71(6 Pt 1):865-868.
10. Katz M, Abrahams C. Transvaginal placement of cervicoisthmic cerclage: Report on pregnancy outcome. Am J Obstet Gynecol. 2005;192(6):1989-1992.
11. Ferenczy A. Ultrastructure of the uterine cervix. In: Huszar G ed. The Physiology and Biochemistry of the Uterus in Pregnancy and Labor. Boca Raton, FL: CRC Press; 2000: 239–260.
12. Heaps RH, House M, Socrate S, Leppert P, Strauss JF, III. Matrix biology and preterm birth. In: Petraglia F Strauss JF III, Gabbe SG, Weiss G, eds. Preterm Birth: Mechanisms, Mediators, Prediction, Prevention, and Interventions. United Kingdom: Informa; 2007:71–93.
13. Fahmy K. A closed submucous cervical suture for the incompetent cervix. Int Surg. 1978;63(2):77-80.
14. Caspi E, Schneider DF, Mor Z, Langer R, Weinraub Z, Bukovsky I. Cervical internal os cerclage: description of a new technique and comparison with Shirodkar operation. Am J Perinatol. 1990;7(4):347-349.
15. Harger JH. Cerclage and cervical insufficiency: an evidence-based analysis. Obstet Gynecol. 2002;100:1313-1327.
In women who have stress incontinence and intrinsic sphincter deficiency, which midurethral sling produces the best long-term results?
- Which sling for which SUI patient?
Mark D. Walters, MD, and Anne M. Weber, MD (Surgical Techniques, May 2012)
![]()
These videos were selected by Dr. Walters and presented courtesy of the International Academy of Pelvic Surgery (IAPS)
When ISD is present, the urethra cannot coaptate and loses its ability to maintain a watertight seal. Women who have this condition often are severely incontinent, leaking urine at low volumes and pressures and with minimal exertion.
In this randomized trial, Schierlitz and colleagues hypothesized that TOT would produce higher objective and subjective failure rates than the TVT. This was confirmed by 6-month data published in 2008.
Details of the trial
Women who had SUI were included in the trial if they had ISD based on urodynamic findings (i.e., maximum urethral closure pressure ≤20 cm H2O or Valsalva leak-point pressure ≤60 cm H2O, or both) and were randomly assigned to TVT or TOT. The primary endpoint was symptomatic SUI (confirmed by repeat urodynamic testing) that required a second procedure upon patient request.
Participants were followed for 3 years. If a patient reported symptoms, urodynamic testing was repeated. In addition, the patient was offered another surgery, usually involving placement of a TVT sling.
Schierlitz and colleagues concluded that, if TVT were used in all patients, repeat surgery would be avoided in one in every six patients. The risk of repeat surgery was 15 times greater for TOT, compared with the TVT sling. The median time to failure was 15.6 months for the TOT sling, compared with 43.7 months for the TVT.
Of the 16 patients who underwent repeat surgery, 56% were cured, 25% reported minimal leakage, and 19% remained unchanged.
Quality-of-life scores were similar between groups at the 6-month follow-up.
Why did the TVT outperform the TOT in this population?
Investigators theorized that there is a difference in sling axis, with the TVT placed at a more acute angle than the TOT sling. In addition, the location of the TOT sling is more distal than that of the TVT, based on ultrasonographic imaging. As a result, more effective urethral kinking and support are likely with the TVT sling, improving continence rates.
Strengths and limitations of the trial
The randomization of participants and long-term follow-up bolster the trial’s credibility.
Weaknesses include unblinded participation and postoperative surgical assessment.
Although the sample size was underpowered, there was a significant difference in the primary outcome between the two groups.
Long-term success is more likely with placement of a TVT sling in women who have SUI with ISD.
Urodynamic assessment still serves an important role in the diagnosis of ISD, and aids in preoperative planning.
LADIN A. YURTERI-KAPLAN, MD, AND AMY J. PARK, MD
We want to hear from you! Tell us what you think.
- Which sling for which SUI patient?
Mark D. Walters, MD, and Anne M. Weber, MD (Surgical Techniques, May 2012)
![]()
These videos were selected by Dr. Walters and presented courtesy of the International Academy of Pelvic Surgery (IAPS)
When ISD is present, the urethra cannot coaptate and loses its ability to maintain a watertight seal. Women who have this condition often are severely incontinent, leaking urine at low volumes and pressures and with minimal exertion.
In this randomized trial, Schierlitz and colleagues hypothesized that TOT would produce higher objective and subjective failure rates than the TVT. This was confirmed by 6-month data published in 2008.
Details of the trial
Women who had SUI were included in the trial if they had ISD based on urodynamic findings (i.e., maximum urethral closure pressure ≤20 cm H2O or Valsalva leak-point pressure ≤60 cm H2O, or both) and were randomly assigned to TVT or TOT. The primary endpoint was symptomatic SUI (confirmed by repeat urodynamic testing) that required a second procedure upon patient request.
Participants were followed for 3 years. If a patient reported symptoms, urodynamic testing was repeated. In addition, the patient was offered another surgery, usually involving placement of a TVT sling.
Schierlitz and colleagues concluded that, if TVT were used in all patients, repeat surgery would be avoided in one in every six patients. The risk of repeat surgery was 15 times greater for TOT, compared with the TVT sling. The median time to failure was 15.6 months for the TOT sling, compared with 43.7 months for the TVT.
Of the 16 patients who underwent repeat surgery, 56% were cured, 25% reported minimal leakage, and 19% remained unchanged.
Quality-of-life scores were similar between groups at the 6-month follow-up.
Why did the TVT outperform the TOT in this population?
Investigators theorized that there is a difference in sling axis, with the TVT placed at a more acute angle than the TOT sling. In addition, the location of the TOT sling is more distal than that of the TVT, based on ultrasonographic imaging. As a result, more effective urethral kinking and support are likely with the TVT sling, improving continence rates.
Strengths and limitations of the trial
The randomization of participants and long-term follow-up bolster the trial’s credibility.
Weaknesses include unblinded participation and postoperative surgical assessment.
Although the sample size was underpowered, there was a significant difference in the primary outcome between the two groups.
Long-term success is more likely with placement of a TVT sling in women who have SUI with ISD.
Urodynamic assessment still serves an important role in the diagnosis of ISD, and aids in preoperative planning.
LADIN A. YURTERI-KAPLAN, MD, AND AMY J. PARK, MD
We want to hear from you! Tell us what you think.
- Which sling for which SUI patient?
Mark D. Walters, MD, and Anne M. Weber, MD (Surgical Techniques, May 2012)
![]()
These videos were selected by Dr. Walters and presented courtesy of the International Academy of Pelvic Surgery (IAPS)
When ISD is present, the urethra cannot coaptate and loses its ability to maintain a watertight seal. Women who have this condition often are severely incontinent, leaking urine at low volumes and pressures and with minimal exertion.
In this randomized trial, Schierlitz and colleagues hypothesized that TOT would produce higher objective and subjective failure rates than the TVT. This was confirmed by 6-month data published in 2008.
Details of the trial
Women who had SUI were included in the trial if they had ISD based on urodynamic findings (i.e., maximum urethral closure pressure ≤20 cm H2O or Valsalva leak-point pressure ≤60 cm H2O, or both) and were randomly assigned to TVT or TOT. The primary endpoint was symptomatic SUI (confirmed by repeat urodynamic testing) that required a second procedure upon patient request.
Participants were followed for 3 years. If a patient reported symptoms, urodynamic testing was repeated. In addition, the patient was offered another surgery, usually involving placement of a TVT sling.
Schierlitz and colleagues concluded that, if TVT were used in all patients, repeat surgery would be avoided in one in every six patients. The risk of repeat surgery was 15 times greater for TOT, compared with the TVT sling. The median time to failure was 15.6 months for the TOT sling, compared with 43.7 months for the TVT.
Of the 16 patients who underwent repeat surgery, 56% were cured, 25% reported minimal leakage, and 19% remained unchanged.
Quality-of-life scores were similar between groups at the 6-month follow-up.
Why did the TVT outperform the TOT in this population?
Investigators theorized that there is a difference in sling axis, with the TVT placed at a more acute angle than the TOT sling. In addition, the location of the TOT sling is more distal than that of the TVT, based on ultrasonographic imaging. As a result, more effective urethral kinking and support are likely with the TVT sling, improving continence rates.
Strengths and limitations of the trial
The randomization of participants and long-term follow-up bolster the trial’s credibility.
Weaknesses include unblinded participation and postoperative surgical assessment.
Although the sample size was underpowered, there was a significant difference in the primary outcome between the two groups.
Long-term success is more likely with placement of a TVT sling in women who have SUI with ISD.
Urodynamic assessment still serves an important role in the diagnosis of ISD, and aids in preoperative planning.
LADIN A. YURTERI-KAPLAN, MD, AND AMY J. PARK, MD
We want to hear from you! Tell us what you think.
PTSD nightmares: Prazosin and atypical antipsychotics
• Prazosin is recommended as a first-line therapy for nighttime PTSD symptoms, such as nightmares or sleep disturbances—especially among veterans—because of superior long-term effectiveness.
• Risk of metabolic syndrome, which has been reported with low-dose atypical antipsychotics used for treating insomnia, limits their use for PTSD-related nightmares.
Mr. S, a 45-year-old veteran, was diagnosed with posttraumatic stress disorder (PTSD) 18 years ago after a tour of duty in the Persian Gulf. He had combat-related flashbacks triggered by the smell of gasoline or smoke from a fire, was easily startled, and began to isolate himself socially. However, his symptoms improved when he started volunteering at his local Veterans Affairs Medical Center. After he lost his job 3 years ago, Mr. S started experiencing flashbacks. He was irritable, easily startled, and avoided things that reminded him of his time in the Persian Gulf. His psychiatrist prescribed sertraline, titrated to 200 mg/d. The drug reduced the severity of his avoidance and hyperarousal symptoms and improved his mood.
During a clinic visit, Mr. S says he is doing well and can fall asleep at night but is having recurring nightmares about traumatic events that occurred during combat. These nightmares wake him up and have become more frequent, occurring once per night for the past month. Mr. S says he has been watching more news programs about conflicts in Afghanistan and Iraq since the nightmares began. His psychiatrist starts quetiapine, 50 mg at bedtime for 7 nights then 100 mg at bedtime, but after 6 weeks Mr. S says his nightmares continue.
PTSD occurs in approximately 19% of Vietnam war combat veterans1 and 14% of service members returning from Iraq and Afghanistan.2 PTSD symptoms are classified into clusters: intrusive/re-experiencing; avoidant/numbing; and hyperarousal.3 Nightmares are part of the intrusive/re-experiencing cluster, which is Criterion B in DSM-IV-TR. See Table 1 for a description of DSM-IV-TR PTSD criteria. Among PTSD patients, 50% to 70% report PTSD-associated nightmares.4 Despite adequate treatment targeted to improve PTSD’s core symptoms, symptoms such as sleep disturbances or nightmares often persist.
Table 1
DSM-IV-TR diagnostic criteria for posttraumatic stress disorder
|
| Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 |
Nightmares and other sleep disturbances are associated with significant distress and daytime impairment and can interfere with PTSD recovery4-8 by disrupting sleep-dependent processing of emotional experiences and causing repeated resensitization to trauma cues (Table 2).8
Table 2
Psychosocial consequences of sleep disruption in PTSD
| Increased reactivity to emotional cues |
| Compromised ability to function in social and occupational roles |
| Negative psychiatric outcomes, including suicidal ideation or worsening of depression or psychosis |
| Interference of natural recovery from trauma exposure |
| Repeated resensitization to trauma cues |
| Neurocognitive deficits |
| Neuroendocrine abnormalities |
| PTSD: posttraumatic stress disorder Source: Adapted from reference 8 |
Few randomized controlled medication trials specifically address PTSD-related nightmares. Most PTSD studies do not examine sleep outcomes as a primary measure, and comprehensive literature reviews could not offer evidence-based recommendations.9,10 The American Academy of Sleep Medicine (AASM) also noted a paucity of PTSD studies that identified nightmares as a primary outcome measure.11 See Table 3 for a list of recommended medication options for PTSD-associated nightmares.
Table 3
Recommended medication treatments for PTSD-associated nightmares
| Evidence level | Medication | Evidence |
|---|---|---|
| Recommended for treating PTSD-associated nightmares | ||
| 1, 4 | Prazosin | In 3 level 1 studies, adding prazosin (mean dose 3 mg/d) significantly decreased trauma-related nightmares according to the CAPS “recurrent distressing dreams” item after 3 to 9 weeks of treatment vs placebo in veteran and civilian patients (N = 57) |
| Not suggested for treating PTSD-associated nightmares | ||
| 1 | Venlafaxine | No difference between extended-release venlafaxine (37.5 to 300 mg/d) and placebo in the CAPS-SX17 “distressing dreams” item at 12 weeks in 340 PTSD patients |
| May be considered for treating PTSD-associated nightmares | ||
| 4 | Clonidine | Reduced the number of nightmares in 11 of 13 refugees for 2 weeks to 3 months (dose: 0.2 to 0.6 mg/d) |
| May be considered for treating PTSD-associated nightmares, but data are low grade and sparse | ||
| 4 | Trazodone | Although trazodone (25 to 600 mg) significantly decreased nightmare frequency in veteran patients during an 8-week hospital stay (N = 60), 19% discontinued therapy because of side effects |
| 4 | Olanzapine | Adjunctive olanzapine (10 to 20 mg) rapidly improved sleep in a case series of combat-related PTSD patients resistant to SSRIs and benzodiazepines (N = 5) |
| 4 | Risperidone | In case series, risperidone (0.5 to 3 mg) significantly decreased CAPS scores for recurrent distressing dreams and proportion of traumatic dreams documented in diaries of combat veterans over 6 weeks (N = 17), and improved nightmares in adult burn patients taking pain medications after 1 to 2 days (N = 10) |
| 4 | Aripiprazole | In a case series, aripiprazole (15 to 30 mg at bedtime) with CBT or sertraline significantly improved nightmares in 4 of 5 combat-related PTSD patients |
| 4 | Topiramate | Topiramate reduced nightmares in 79% of civilians with PTSD and fully suppressed nightmares in 50% of patients in a case series (N = 35) |
| 4 | Low-dose cortisol | Significant decrease in frequency but not intensity of nightmares with low-dose cortisol (10 mg/d) in civilians with PTSD (N = 3) |
| 4 | Fluvoxamine | In 2 case series, fluvoxamine (up to 300 mg/d) significantly decreased the IES-R level of “dreams about combat trauma” but not the SRRS “bad dreams” rating at 10 weeks (N = 21). During 4 to 12 weeks of follow-up there was a qualitative decrease in reported nightmares in veteran patients (n = 12) |
| 2 | Triazolam/nitrazepam | Limited data showed triazolam (0.5 mg) and nitrazepam (5 mg) provide equal efficacy in decreasing the number of patients who experience unpleasant dreams over 1 night |
| 4 | Phenelzine | One study showed phenelzine monotherapy (30 to 90 mg) resulted in elimination of nightmares within 1 month (N = 5); another reported “moderately reduced traumatic dreams” (N = 21) in veterans. Therapy was discontinued because of short-lived efficacy or plateau effect |
| 4 | Gabapentin | Adjunctive gabapentin (300 to 3,600 mg/d) improved insomnia and decreased nightmare frequency and/or intensity over 1 to 36 months in 30 veterans with PTSD |
| 4 | Cyproheptadine | Conflicting data ranges from eliminating nightmares to no changes in the presence or intensity of nightmares |
| 4 | TCAs | Among 10 Cambodian concentration camp survivors treated with TCAs, 4 reported their nightmares ceased and 4 reported improvement after 1-year follow-up |
| 4 | Nefazodone | Reduced nightmare occurrence in 3 open-label studies as monotherapy (386 to 600 mg/d). Not recommended first line because of hepatotoxicity risk |
| No recommendation because of sparse data | ||
| 2 | Clonazepam | Clonazepam (1 to 2 mg/d) was ineffective in decreasing frequency or intensity of combat-related PTSD nightmares in veterans (N = 6) |
Evidence levels:
| ||
| CAPS: Clinician-Administered PTSD Scale; CAPS-SX17: 17-item Clinician-Administered PTSD Scale; CBT: cognitive-behavioral therapy; IES-R: Impact of Event Scale-Revised; PTSD: posttraumatic stress disorder; SRRS: Stress Response Rating Scale; SSRI: selective serotonin reuptake inhibitor; TCAs: tricyclic antidepressants Source: Adapted from Aurora RN, Zak RS, Auerbach SH, et al. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389-401 | ||
CASE CONTINUED: Medication change, improvement
After reviewing AASM’s treatment recommendations, we prescribe prazosin, 1 mg at bedtime for 7 nights, then increase by 1 mg at bedtime each week until Mr. S’s nightmares improve. He reports a substantial improvement in nightmare severity and frequency after a few weeks of treatment with prazosin, 5 mg at bedtime.
Prazosin
Prazosin is an α1-adrenergic receptor antagonist with good CNS penetrability. The rationale for reducing adrenergic activity to address intrusive PTSD symptoms has been well documented.12,13 In open-label trials,14-18 a chart review,19 and placebo-controlled trials,20-22prazosin reduced trauma nightmares and improved sleep quality and global clinical status more than placebo (Table 4). In these studies, prazosin doses ranged from 1 to 20 mg/d, with an average of 3 mg at bedtime and a starting dose of 1 mg. Prazosin is the only agent recommended in the AASM’s Best Practice Guide for treating PTSD-related nightmares.11
Table 4
RCTs of prazosin for trauma-related nightmares
| Study | Design | Patients | Results |
|---|---|---|---|
| Raskind et al, 200320 | 20-week, double-blind, placebo-controlled, crossover study (mean dose 9.5 mg/d at bedtime) | 10 Vietnam veterans with chronic PTSD and severe trauma-related nightmares | Prazosin was superior to placebo on scores on the recurrent distressing dreams item and difficulty falling/staying asleep item of the CAPS and change in PTSD severity and functional status on the CGI-C |
| Raskind et al, 200721 | 8-week, placebo-controlled, parallel study (mean dose 13.3 ± 3 mg/d in the evening) | 40 veterans with chronic PTSD, distressing trauma nightmares, and sleep disturbance | Prazosin was superior to placebo in reducing trauma nightmares and improving sleep quality and global clinical status; prazosin also shifted dream characteristics of trauma-related nightmares to those typical of normal dreams |
| Taylor et al, 200822 | 7-week, randomized, placebo-controlled, crossover trial (mean dose 3.1 ± 1.3 mg) | 13 outpatients with chronic civilian trauma PTSD, frequent nightmares, and sleep disturbance | Prazosin significantly increased total sleep time and REM sleep time; reduced trauma-related nightmares, distressed awakenings, and total PCL-C scores; improved CGI-I scores; and changed PDRS scores toward normal dreaming |
| CAPS: Clinician-Administered PTSD Scale; CGI-C: Clinical Global Impression of Change; CGI-I: Clinical Global Impression of Improvement; PCL-C: PTSD Checklist-Civilian; PDRS: PTSD Dream Rating Scale; PTSD: posttraumatic stress disorder; RCTs: randomized controlled trials; REM: rapid eye movement | |||
Atypical antipsychotics
Atypical antipsychotics have been used to reduce nightmares in PTSD; however, most of the evidence from studies evaluated in the AASM’s Best Practice Guide were considered to be low quality.11 Quetiapine and ziprasidone were not included in the AASM review. See (Table 5) for a review of the evidence for atypical antipsychotics for treating PTSD nightmares.
Table 5
Combat-related nightmares: Evidence for atypical antipsychotics
| Study | Design | Patients/dosage | Results |
|---|---|---|---|
| Aripiprazole | |||
| Lambert, 2006 a | Case report | 4 veterans with combat-related PTSD (3 male, 1 female; age 22 to 24); dose: 15 to 30 mg; concurrent treatment sertraline or CBT | Decreased frequency of weekly nightmares and agitated sleep by at least 50% |
| Olanzapine | |||
| Stein et al, 2002 b | 8-week, double-blind, placebo-controlled study | 19 male veterans with combat-related PTSD (olanzapine group mean age: 55.2 ± 6.6; placebo group 51.1 ± 8.1); mean dose: 15 mg/d | Significantly greater reduction in sleep disturbances (PSQI: -3.29 vs 1.57; P = .01); significantly higher weight gain (13.2 lbs vs -3 lbs; P = .001) |
| Jakovljevic et al, 2003 c | Case reports | 5 veterans with combat-related PTSD for 6 to 7 years (age: 28 to 50); dose: 10 to 20 mg; adjunct treatment | Decreased frequency of nightmares within 3 days |
| Labbate et al, 2000 d | Case report | 1 male veteran (age: 58) with a 20-year history of combat-related PTSD; dose: 5 mg at bedtime; concurrent treatment with sertraline (200 mg/d), bupropion (150 mg/d), and diazepam (15 mg/d) | Eliminated nightmares after 1 week and improved sleep quality |
| Quetiapine | |||
| Ahearn et al, 2006 e | 8-week, open-label trial | 15 PTSD patients (8 male; 7 female; 5 with combat-related PTSD; mean age: 49); mean dose: 216 mg/d (100 to 400 mg/d) | Significantly improved re-experiencing (CAPS: 10 vs 23; P = .0012) and sleep (PSQI: 17.5 vs 30; P = .0044) at 8 weeks compared with baseline |
| Robert et al, 2005 f | 6-week, open-label trial | 19 combat veterans; mean dose: 100 ± 70 mg/d (25 to 300 mg/d); adjunct treatment | Significantly improved sleep quality (PSQI: 1.67 vs 2.41; P = .006), latency (PSQI: 1.5 vs 2.65; P = .002), duration (PSQI: 1.31 vs 2.71; P < .001), and sleep disturbances (PSQI: 1.22 vs 1.71; P = .034) and decreased terror episodes (PSQI-A: 0.73 vs 0.91; P = .040) and acting out dreams (PSQI-A: 1.07 vs 1.35; P = .013); however, no difference in nightmares caused by trauma (PSQI-A: 1.53 vs 2.06) |
| Sokolski et al, 2003 g | Retrospective chart review | 68 male Vietnam War combat veterans (mean age: 55 ± 3.5); mean dose: 155 ± 130 mg (25 to 700 mg); adjunct treatment | Improved sleep disturbances in 62% and nightmares in 25% of patients |
| Ahearn et al, 2003 h | Case report | 2 male patients with combat-related PTSD (age 53, 72); dose: 25 to 50 mg; adjunct to SSRI therapy | Decreased frequency of nightmares with increased sleep duration |
| Risperidone | |||
| David et al, 2006 i | 6-week, open-label trial | 17 male veterans with combat-related PTSD (mean age: 53.7 ± 3.8); mean maximum dose: 2.3 ± 0.6 mg (range: 1 to 3 mg) | Improved recurrent distressing dreams (CAPS B-2: 3.8 vs 5.4; P = .04), but not with the PSQI subscale (PSQI bad dreams: 2.5 vs 2.7; NS). Decreased nighttime awakenings (1.9 vs 2.8; P = .003) and trauma dreams (19% vs 38%; P = .04) |
| Leyba et al, 1998 j | Case reports | 3 male patients (age 43 to 46); dose: 1 to 3 mg; adjunct therapy | Decreased occurrence of nightmares |
| Ziprasidone | |||
| Siddiqui et al, 2005 k | Case report | 1 male veteran with chronic combat-related PTSD (age 55); dose: 80 to 120 mg/d; adjunct with trazodone (100 mg) and topiramate | Improved occurrence of nightmares up to 4 months |
CAPS: Clinician-Administered PTSD Scale; CAPS B-2: Clinician-Administered PTSD Scale B-2 (recurrent distressing dreams of the event); CBT: cognitive-behavioral therapy; PSQI: Pittsburgh Sleep Quality Index; PSQI-A: Pittsburgh Sleep Quality Index Addendum for PTSD; NS: not significant; PTSD: posttraumatic stress disorder; SSRI: selective serotonin reuptake inhibitor References
| |||
Comparing prazosin and quetiapine. A historical prospective cohort study of 237 veterans with PTSD receiving prazosin or quetiapine for nighttime PTSD symptoms demonstrated that although the 2 drugs have similar efficacy (defined as symptomatic improvement) for short-term, 6-month treatment (61% vs 62%; P=.54), a higher percentage of patients continued prazosin long-term (3 to 6 years) than those taking quetiapine (48% vs 24%; P < .001).23 Twenty-five percent of patients taking quetiapine switched to prazosin during the study, and approximately one-half of these patients remained on prazosin until the study’s end. Only 8% of prazosin patients switched to quetiapine, and none continued this therapy until study end.23 Patients in the quetiapine group were more likely to discontinue the drug because of lack of efficacy (13% vs 3%; P=.03) and adverse effects (35% vs 18%; P=.008), specifically sedation (21% vs 2%; P < .001) and metabolic effects (9% vs 0%; P=.014), compared with prazosin. Although this trial may be the only published comparison study of prazosin and quetiapine, its methodological quality has been questioned, which makes it difficult to draw definitive conclusions.
Metabolic syndrome—elevated diastolic blood pressure, increased waist circumference, and low high-density lipoprotein cholesterol—is common among PTSD patients treated with antipsychotics.24 This syndrome may be caused by medications, lifestyle factors, or long-term overactivation of stress-response pathways. A retrospective chart review at a community mental health center revealed that patients taking even low doses of quetiapine for insomnia gained an average of 5 lbs (P=.037).25 Another retrospective chart review at 2 military hospitals reported that patients receiving low-dose quetiapine (≤100 mg/d) gained an average of slightly less than 1 lb per month, which adds up to approximately 10 lbs per year (P < .001).26 The benefit of using atypical antipsychotics may be outweighed by metabolic risks such as obesity, new-onset diabetes, and dyslipidemia.27
Prazosin is considered a first-line treatment for sleep disturbances and nightmares in PTSD because of its superior long-term efficacy and decreased adverse effects compared with quetiapine.
Related Resources
- American Psychiatric Association. Practice guidelines for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Arlington, VA: American Psychiatric Publishing, Inc.; 2004.
- Veterans Affairs/Department of Defense clinical practice guidelines. Management of traumatic stress disorder and acute stress reaction. www.healthquality.va.gov/Post_Traumatic_Stress_Disorder_PTSD.asp.
Drug Brand Names
- Prazosin • Minipress
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Dohrenwend BP, Turner JB, Turse NA, et al. The psychological risks of Vietnam for U.S. veterans: a revisit with new data and methods. Science. 2006;313(5789):979-982.
2. Tanielian T, Jaycox L. eds. Invisible wounds of war: psychological and cognitive injuries, their consequences, and services to assist recovery. Santa Monica, CA: RAND Corporation; 2008.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
4. Wittmann L, Schredl M, Kramer M. Dreaming in posttraumatic stress disorder: a critical review of phenomenology psychophysiology and treatment. Psychother Psychosom. 2007;76(1):25-39.
5. Clum GA, Nishith P, Resick PA. Trauma-related sleep disturbance and self-reported physical health symptoms in treatment-seeking female rape victims. J Nerv Ment Dis. 2001;189(9):618-622.
6. Kramer TL, Booth BM, Han X, et al. Service utilization and outcomes in medically ill veterans with posttraumatic stress and depressive disorders. J Trauma Stress. 2003;16(3):211-219.
7. Neylan TC, Marmar CR, Metzler TJ, et al. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam veterans. Am J Psychiatry. 1998;155(7):929-933.
8. Nappi CM, Drummond SP, Hall JM. Treating nightmares and insomnia in posttraumatic stress disorder: a review of current evidence. Neuropharmacology. 2012;62(2):576-585.
9. Maher MJ, Rego SA, Asnis GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology impact and approaches to management. CNS Drugs. 2006;20(7):567-590.
10. van Liempt S, Vermetten E, Geuze E, et al. Pharmacotherapy for disordered sleep in post-traumatic stress disorder: a systematic review. Int Clin Psychopharmacol. 2006;21(4):193-202.
11. Aurora RN, Zak RS, Auerbach SH, et al. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389-401.
12. Boehnlein JK, Kinzie JD. Pharmacologic reduction of CNS noradrenergic activity in PTSD: the case for clonidine and prazosin. J Psychiatr Pract. 2007;13(2):72-78.
13. Strawn JR, Geracioti TD, Jr. Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25(3):260-271.
14. Calohan J, Peterson K, Peskind ER, et al. Prazosin treatment of trauma nightmares and sleep disturbance in soldiers deployed in Iraq. J Trauma Stress. 2010;23(5):645-648.
15. Daly CM, Doyle ME, Radkind M, et al. Clinical case series: the use of Prazosin for combat-related recurrent nightmares among Operation Iraqi Freedom combat veterans. Mil Med. 2005;170(6):513-515.
16. Peskind ER, Bonner LT, Hoff DJ, et al. Prazosin reduces trauma-related nightmares in older men with chronic posttraumatic stress disorder. J Geriatr Psychiatry Neurol. 2003;16(3):165-171.
17. Raskind MA, Dobie DJ, Kanter ED, et al. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases. J Clin Psychiatry. 2000;61(2):129-133.
18. Taylor F, Raskind MA. The alpha1-adrenergic antagonist prazosin improves sleep and nightmares in civilian trauma posttraumatic stress disorder. J Clin Psychopharmacol. 2002;22(1):82-85.
19. Raskind MA, Thompson C, Petrie EC, et al. Prazosin reduces nightmares in combat veterans with posttraumatic stress disorder. J Clin Psychiatry. 2002;63(7):565-568.
20. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373.
21. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
22. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63(6):629-632.
23. Byers MG, Allison KM, Wendel CS, et al. Prazosin versus quetiapine for nighttime posttraumatic stress disorder symptoms in veterans: an assessment of long-term comparative effectiveness and safety. J Clin Psychopharmacol. 2010;30(3):225-229.
24. Jin H, Lanouette NM, Mudaliar S, et al. Association of posttraumatic stress disorder with increased prevalence of metabolic syndrome. J Clin Psychopharmacol. 2009;29(3):210-215.
25. Cates ME, Jackson CW, Feldman JM, et al. Metabolic consequences of using low-dose quetiapine for insomnia in psychiatric patients. Community Ment Health J. 2009;45(4):251-254.
26. Williams SG, Alinejad NA, Williams JA, et al. Statistically significant increase in weight caused by low-dose quetiapine. Pharmacotherapy. 2010;30(10):1011-1015.
27. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65(2):267-272.
• Prazosin is recommended as a first-line therapy for nighttime PTSD symptoms, such as nightmares or sleep disturbances—especially among veterans—because of superior long-term effectiveness.
• Risk of metabolic syndrome, which has been reported with low-dose atypical antipsychotics used for treating insomnia, limits their use for PTSD-related nightmares.
Mr. S, a 45-year-old veteran, was diagnosed with posttraumatic stress disorder (PTSD) 18 years ago after a tour of duty in the Persian Gulf. He had combat-related flashbacks triggered by the smell of gasoline or smoke from a fire, was easily startled, and began to isolate himself socially. However, his symptoms improved when he started volunteering at his local Veterans Affairs Medical Center. After he lost his job 3 years ago, Mr. S started experiencing flashbacks. He was irritable, easily startled, and avoided things that reminded him of his time in the Persian Gulf. His psychiatrist prescribed sertraline, titrated to 200 mg/d. The drug reduced the severity of his avoidance and hyperarousal symptoms and improved his mood.
During a clinic visit, Mr. S says he is doing well and can fall asleep at night but is having recurring nightmares about traumatic events that occurred during combat. These nightmares wake him up and have become more frequent, occurring once per night for the past month. Mr. S says he has been watching more news programs about conflicts in Afghanistan and Iraq since the nightmares began. His psychiatrist starts quetiapine, 50 mg at bedtime for 7 nights then 100 mg at bedtime, but after 6 weeks Mr. S says his nightmares continue.
PTSD occurs in approximately 19% of Vietnam war combat veterans1 and 14% of service members returning from Iraq and Afghanistan.2 PTSD symptoms are classified into clusters: intrusive/re-experiencing; avoidant/numbing; and hyperarousal.3 Nightmares are part of the intrusive/re-experiencing cluster, which is Criterion B in DSM-IV-TR. See Table 1 for a description of DSM-IV-TR PTSD criteria. Among PTSD patients, 50% to 70% report PTSD-associated nightmares.4 Despite adequate treatment targeted to improve PTSD’s core symptoms, symptoms such as sleep disturbances or nightmares often persist.
Table 1
DSM-IV-TR diagnostic criteria for posttraumatic stress disorder
|
| Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 |
Nightmares and other sleep disturbances are associated with significant distress and daytime impairment and can interfere with PTSD recovery4-8 by disrupting sleep-dependent processing of emotional experiences and causing repeated resensitization to trauma cues (Table 2).8
Table 2
Psychosocial consequences of sleep disruption in PTSD
| Increased reactivity to emotional cues |
| Compromised ability to function in social and occupational roles |
| Negative psychiatric outcomes, including suicidal ideation or worsening of depression or psychosis |
| Interference of natural recovery from trauma exposure |
| Repeated resensitization to trauma cues |
| Neurocognitive deficits |
| Neuroendocrine abnormalities |
| PTSD: posttraumatic stress disorder Source: Adapted from reference 8 |
Few randomized controlled medication trials specifically address PTSD-related nightmares. Most PTSD studies do not examine sleep outcomes as a primary measure, and comprehensive literature reviews could not offer evidence-based recommendations.9,10 The American Academy of Sleep Medicine (AASM) also noted a paucity of PTSD studies that identified nightmares as a primary outcome measure.11 See Table 3 for a list of recommended medication options for PTSD-associated nightmares.
Table 3
Recommended medication treatments for PTSD-associated nightmares
| Evidence level | Medication | Evidence |
|---|---|---|
| Recommended for treating PTSD-associated nightmares | ||
| 1, 4 | Prazosin | In 3 level 1 studies, adding prazosin (mean dose 3 mg/d) significantly decreased trauma-related nightmares according to the CAPS “recurrent distressing dreams” item after 3 to 9 weeks of treatment vs placebo in veteran and civilian patients (N = 57) |
| Not suggested for treating PTSD-associated nightmares | ||
| 1 | Venlafaxine | No difference between extended-release venlafaxine (37.5 to 300 mg/d) and placebo in the CAPS-SX17 “distressing dreams” item at 12 weeks in 340 PTSD patients |
| May be considered for treating PTSD-associated nightmares | ||
| 4 | Clonidine | Reduced the number of nightmares in 11 of 13 refugees for 2 weeks to 3 months (dose: 0.2 to 0.6 mg/d) |
| May be considered for treating PTSD-associated nightmares, but data are low grade and sparse | ||
| 4 | Trazodone | Although trazodone (25 to 600 mg) significantly decreased nightmare frequency in veteran patients during an 8-week hospital stay (N = 60), 19% discontinued therapy because of side effects |
| 4 | Olanzapine | Adjunctive olanzapine (10 to 20 mg) rapidly improved sleep in a case series of combat-related PTSD patients resistant to SSRIs and benzodiazepines (N = 5) |
| 4 | Risperidone | In case series, risperidone (0.5 to 3 mg) significantly decreased CAPS scores for recurrent distressing dreams and proportion of traumatic dreams documented in diaries of combat veterans over 6 weeks (N = 17), and improved nightmares in adult burn patients taking pain medications after 1 to 2 days (N = 10) |
| 4 | Aripiprazole | In a case series, aripiprazole (15 to 30 mg at bedtime) with CBT or sertraline significantly improved nightmares in 4 of 5 combat-related PTSD patients |
| 4 | Topiramate | Topiramate reduced nightmares in 79% of civilians with PTSD and fully suppressed nightmares in 50% of patients in a case series (N = 35) |
| 4 | Low-dose cortisol | Significant decrease in frequency but not intensity of nightmares with low-dose cortisol (10 mg/d) in civilians with PTSD (N = 3) |
| 4 | Fluvoxamine | In 2 case series, fluvoxamine (up to 300 mg/d) significantly decreased the IES-R level of “dreams about combat trauma” but not the SRRS “bad dreams” rating at 10 weeks (N = 21). During 4 to 12 weeks of follow-up there was a qualitative decrease in reported nightmares in veteran patients (n = 12) |
| 2 | Triazolam/nitrazepam | Limited data showed triazolam (0.5 mg) and nitrazepam (5 mg) provide equal efficacy in decreasing the number of patients who experience unpleasant dreams over 1 night |
| 4 | Phenelzine | One study showed phenelzine monotherapy (30 to 90 mg) resulted in elimination of nightmares within 1 month (N = 5); another reported “moderately reduced traumatic dreams” (N = 21) in veterans. Therapy was discontinued because of short-lived efficacy or plateau effect |
| 4 | Gabapentin | Adjunctive gabapentin (300 to 3,600 mg/d) improved insomnia and decreased nightmare frequency and/or intensity over 1 to 36 months in 30 veterans with PTSD |
| 4 | Cyproheptadine | Conflicting data ranges from eliminating nightmares to no changes in the presence or intensity of nightmares |
| 4 | TCAs | Among 10 Cambodian concentration camp survivors treated with TCAs, 4 reported their nightmares ceased and 4 reported improvement after 1-year follow-up |
| 4 | Nefazodone | Reduced nightmare occurrence in 3 open-label studies as monotherapy (386 to 600 mg/d). Not recommended first line because of hepatotoxicity risk |
| No recommendation because of sparse data | ||
| 2 | Clonazepam | Clonazepam (1 to 2 mg/d) was ineffective in decreasing frequency or intensity of combat-related PTSD nightmares in veterans (N = 6) |
Evidence levels:
| ||
| CAPS: Clinician-Administered PTSD Scale; CAPS-SX17: 17-item Clinician-Administered PTSD Scale; CBT: cognitive-behavioral therapy; IES-R: Impact of Event Scale-Revised; PTSD: posttraumatic stress disorder; SRRS: Stress Response Rating Scale; SSRI: selective serotonin reuptake inhibitor; TCAs: tricyclic antidepressants Source: Adapted from Aurora RN, Zak RS, Auerbach SH, et al. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389-401 | ||
CASE CONTINUED: Medication change, improvement
After reviewing AASM’s treatment recommendations, we prescribe prazosin, 1 mg at bedtime for 7 nights, then increase by 1 mg at bedtime each week until Mr. S’s nightmares improve. He reports a substantial improvement in nightmare severity and frequency after a few weeks of treatment with prazosin, 5 mg at bedtime.
Prazosin
Prazosin is an α1-adrenergic receptor antagonist with good CNS penetrability. The rationale for reducing adrenergic activity to address intrusive PTSD symptoms has been well documented.12,13 In open-label trials,14-18 a chart review,19 and placebo-controlled trials,20-22prazosin reduced trauma nightmares and improved sleep quality and global clinical status more than placebo (Table 4). In these studies, prazosin doses ranged from 1 to 20 mg/d, with an average of 3 mg at bedtime and a starting dose of 1 mg. Prazosin is the only agent recommended in the AASM’s Best Practice Guide for treating PTSD-related nightmares.11
Table 4
RCTs of prazosin for trauma-related nightmares
| Study | Design | Patients | Results |
|---|---|---|---|
| Raskind et al, 200320 | 20-week, double-blind, placebo-controlled, crossover study (mean dose 9.5 mg/d at bedtime) | 10 Vietnam veterans with chronic PTSD and severe trauma-related nightmares | Prazosin was superior to placebo on scores on the recurrent distressing dreams item and difficulty falling/staying asleep item of the CAPS and change in PTSD severity and functional status on the CGI-C |
| Raskind et al, 200721 | 8-week, placebo-controlled, parallel study (mean dose 13.3 ± 3 mg/d in the evening) | 40 veterans with chronic PTSD, distressing trauma nightmares, and sleep disturbance | Prazosin was superior to placebo in reducing trauma nightmares and improving sleep quality and global clinical status; prazosin also shifted dream characteristics of trauma-related nightmares to those typical of normal dreams |
| Taylor et al, 200822 | 7-week, randomized, placebo-controlled, crossover trial (mean dose 3.1 ± 1.3 mg) | 13 outpatients with chronic civilian trauma PTSD, frequent nightmares, and sleep disturbance | Prazosin significantly increased total sleep time and REM sleep time; reduced trauma-related nightmares, distressed awakenings, and total PCL-C scores; improved CGI-I scores; and changed PDRS scores toward normal dreaming |
| CAPS: Clinician-Administered PTSD Scale; CGI-C: Clinical Global Impression of Change; CGI-I: Clinical Global Impression of Improvement; PCL-C: PTSD Checklist-Civilian; PDRS: PTSD Dream Rating Scale; PTSD: posttraumatic stress disorder; RCTs: randomized controlled trials; REM: rapid eye movement | |||
Atypical antipsychotics
Atypical antipsychotics have been used to reduce nightmares in PTSD; however, most of the evidence from studies evaluated in the AASM’s Best Practice Guide were considered to be low quality.11 Quetiapine and ziprasidone were not included in the AASM review. See (Table 5) for a review of the evidence for atypical antipsychotics for treating PTSD nightmares.
Table 5
Combat-related nightmares: Evidence for atypical antipsychotics
| Study | Design | Patients/dosage | Results |
|---|---|---|---|
| Aripiprazole | |||
| Lambert, 2006 a | Case report | 4 veterans with combat-related PTSD (3 male, 1 female; age 22 to 24); dose: 15 to 30 mg; concurrent treatment sertraline or CBT | Decreased frequency of weekly nightmares and agitated sleep by at least 50% |
| Olanzapine | |||
| Stein et al, 2002 b | 8-week, double-blind, placebo-controlled study | 19 male veterans with combat-related PTSD (olanzapine group mean age: 55.2 ± 6.6; placebo group 51.1 ± 8.1); mean dose: 15 mg/d | Significantly greater reduction in sleep disturbances (PSQI: -3.29 vs 1.57; P = .01); significantly higher weight gain (13.2 lbs vs -3 lbs; P = .001) |
| Jakovljevic et al, 2003 c | Case reports | 5 veterans with combat-related PTSD for 6 to 7 years (age: 28 to 50); dose: 10 to 20 mg; adjunct treatment | Decreased frequency of nightmares within 3 days |
| Labbate et al, 2000 d | Case report | 1 male veteran (age: 58) with a 20-year history of combat-related PTSD; dose: 5 mg at bedtime; concurrent treatment with sertraline (200 mg/d), bupropion (150 mg/d), and diazepam (15 mg/d) | Eliminated nightmares after 1 week and improved sleep quality |
| Quetiapine | |||
| Ahearn et al, 2006 e | 8-week, open-label trial | 15 PTSD patients (8 male; 7 female; 5 with combat-related PTSD; mean age: 49); mean dose: 216 mg/d (100 to 400 mg/d) | Significantly improved re-experiencing (CAPS: 10 vs 23; P = .0012) and sleep (PSQI: 17.5 vs 30; P = .0044) at 8 weeks compared with baseline |
| Robert et al, 2005 f | 6-week, open-label trial | 19 combat veterans; mean dose: 100 ± 70 mg/d (25 to 300 mg/d); adjunct treatment | Significantly improved sleep quality (PSQI: 1.67 vs 2.41; P = .006), latency (PSQI: 1.5 vs 2.65; P = .002), duration (PSQI: 1.31 vs 2.71; P < .001), and sleep disturbances (PSQI: 1.22 vs 1.71; P = .034) and decreased terror episodes (PSQI-A: 0.73 vs 0.91; P = .040) and acting out dreams (PSQI-A: 1.07 vs 1.35; P = .013); however, no difference in nightmares caused by trauma (PSQI-A: 1.53 vs 2.06) |
| Sokolski et al, 2003 g | Retrospective chart review | 68 male Vietnam War combat veterans (mean age: 55 ± 3.5); mean dose: 155 ± 130 mg (25 to 700 mg); adjunct treatment | Improved sleep disturbances in 62% and nightmares in 25% of patients |
| Ahearn et al, 2003 h | Case report | 2 male patients with combat-related PTSD (age 53, 72); dose: 25 to 50 mg; adjunct to SSRI therapy | Decreased frequency of nightmares with increased sleep duration |
| Risperidone | |||
| David et al, 2006 i | 6-week, open-label trial | 17 male veterans with combat-related PTSD (mean age: 53.7 ± 3.8); mean maximum dose: 2.3 ± 0.6 mg (range: 1 to 3 mg) | Improved recurrent distressing dreams (CAPS B-2: 3.8 vs 5.4; P = .04), but not with the PSQI subscale (PSQI bad dreams: 2.5 vs 2.7; NS). Decreased nighttime awakenings (1.9 vs 2.8; P = .003) and trauma dreams (19% vs 38%; P = .04) |
| Leyba et al, 1998 j | Case reports | 3 male patients (age 43 to 46); dose: 1 to 3 mg; adjunct therapy | Decreased occurrence of nightmares |
| Ziprasidone | |||
| Siddiqui et al, 2005 k | Case report | 1 male veteran with chronic combat-related PTSD (age 55); dose: 80 to 120 mg/d; adjunct with trazodone (100 mg) and topiramate | Improved occurrence of nightmares up to 4 months |
CAPS: Clinician-Administered PTSD Scale; CAPS B-2: Clinician-Administered PTSD Scale B-2 (recurrent distressing dreams of the event); CBT: cognitive-behavioral therapy; PSQI: Pittsburgh Sleep Quality Index; PSQI-A: Pittsburgh Sleep Quality Index Addendum for PTSD; NS: not significant; PTSD: posttraumatic stress disorder; SSRI: selective serotonin reuptake inhibitor References
| |||
Comparing prazosin and quetiapine. A historical prospective cohort study of 237 veterans with PTSD receiving prazosin or quetiapine for nighttime PTSD symptoms demonstrated that although the 2 drugs have similar efficacy (defined as symptomatic improvement) for short-term, 6-month treatment (61% vs 62%; P=.54), a higher percentage of patients continued prazosin long-term (3 to 6 years) than those taking quetiapine (48% vs 24%; P < .001).23 Twenty-five percent of patients taking quetiapine switched to prazosin during the study, and approximately one-half of these patients remained on prazosin until the study’s end. Only 8% of prazosin patients switched to quetiapine, and none continued this therapy until study end.23 Patients in the quetiapine group were more likely to discontinue the drug because of lack of efficacy (13% vs 3%; P=.03) and adverse effects (35% vs 18%; P=.008), specifically sedation (21% vs 2%; P < .001) and metabolic effects (9% vs 0%; P=.014), compared with prazosin. Although this trial may be the only published comparison study of prazosin and quetiapine, its methodological quality has been questioned, which makes it difficult to draw definitive conclusions.
Metabolic syndrome—elevated diastolic blood pressure, increased waist circumference, and low high-density lipoprotein cholesterol—is common among PTSD patients treated with antipsychotics.24 This syndrome may be caused by medications, lifestyle factors, or long-term overactivation of stress-response pathways. A retrospective chart review at a community mental health center revealed that patients taking even low doses of quetiapine for insomnia gained an average of 5 lbs (P=.037).25 Another retrospective chart review at 2 military hospitals reported that patients receiving low-dose quetiapine (≤100 mg/d) gained an average of slightly less than 1 lb per month, which adds up to approximately 10 lbs per year (P < .001).26 The benefit of using atypical antipsychotics may be outweighed by metabolic risks such as obesity, new-onset diabetes, and dyslipidemia.27
Prazosin is considered a first-line treatment for sleep disturbances and nightmares in PTSD because of its superior long-term efficacy and decreased adverse effects compared with quetiapine.
Related Resources
- American Psychiatric Association. Practice guidelines for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Arlington, VA: American Psychiatric Publishing, Inc.; 2004.
- Veterans Affairs/Department of Defense clinical practice guidelines. Management of traumatic stress disorder and acute stress reaction. www.healthquality.va.gov/Post_Traumatic_Stress_Disorder_PTSD.asp.
Drug Brand Names
- Prazosin • Minipress
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
• Prazosin is recommended as a first-line therapy for nighttime PTSD symptoms, such as nightmares or sleep disturbances—especially among veterans—because of superior long-term effectiveness.
• Risk of metabolic syndrome, which has been reported with low-dose atypical antipsychotics used for treating insomnia, limits their use for PTSD-related nightmares.
Mr. S, a 45-year-old veteran, was diagnosed with posttraumatic stress disorder (PTSD) 18 years ago after a tour of duty in the Persian Gulf. He had combat-related flashbacks triggered by the smell of gasoline or smoke from a fire, was easily startled, and began to isolate himself socially. However, his symptoms improved when he started volunteering at his local Veterans Affairs Medical Center. After he lost his job 3 years ago, Mr. S started experiencing flashbacks. He was irritable, easily startled, and avoided things that reminded him of his time in the Persian Gulf. His psychiatrist prescribed sertraline, titrated to 200 mg/d. The drug reduced the severity of his avoidance and hyperarousal symptoms and improved his mood.
During a clinic visit, Mr. S says he is doing well and can fall asleep at night but is having recurring nightmares about traumatic events that occurred during combat. These nightmares wake him up and have become more frequent, occurring once per night for the past month. Mr. S says he has been watching more news programs about conflicts in Afghanistan and Iraq since the nightmares began. His psychiatrist starts quetiapine, 50 mg at bedtime for 7 nights then 100 mg at bedtime, but after 6 weeks Mr. S says his nightmares continue.
PTSD occurs in approximately 19% of Vietnam war combat veterans1 and 14% of service members returning from Iraq and Afghanistan.2 PTSD symptoms are classified into clusters: intrusive/re-experiencing; avoidant/numbing; and hyperarousal.3 Nightmares are part of the intrusive/re-experiencing cluster, which is Criterion B in DSM-IV-TR. See Table 1 for a description of DSM-IV-TR PTSD criteria. Among PTSD patients, 50% to 70% report PTSD-associated nightmares.4 Despite adequate treatment targeted to improve PTSD’s core symptoms, symptoms such as sleep disturbances or nightmares often persist.
Table 1
DSM-IV-TR diagnostic criteria for posttraumatic stress disorder
|
| Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 |
Nightmares and other sleep disturbances are associated with significant distress and daytime impairment and can interfere with PTSD recovery4-8 by disrupting sleep-dependent processing of emotional experiences and causing repeated resensitization to trauma cues (Table 2).8
Table 2
Psychosocial consequences of sleep disruption in PTSD
| Increased reactivity to emotional cues |
| Compromised ability to function in social and occupational roles |
| Negative psychiatric outcomes, including suicidal ideation or worsening of depression or psychosis |
| Interference of natural recovery from trauma exposure |
| Repeated resensitization to trauma cues |
| Neurocognitive deficits |
| Neuroendocrine abnormalities |
| PTSD: posttraumatic stress disorder Source: Adapted from reference 8 |
Few randomized controlled medication trials specifically address PTSD-related nightmares. Most PTSD studies do not examine sleep outcomes as a primary measure, and comprehensive literature reviews could not offer evidence-based recommendations.9,10 The American Academy of Sleep Medicine (AASM) also noted a paucity of PTSD studies that identified nightmares as a primary outcome measure.11 See Table 3 for a list of recommended medication options for PTSD-associated nightmares.
Table 3
Recommended medication treatments for PTSD-associated nightmares
| Evidence level | Medication | Evidence |
|---|---|---|
| Recommended for treating PTSD-associated nightmares | ||
| 1, 4 | Prazosin | In 3 level 1 studies, adding prazosin (mean dose 3 mg/d) significantly decreased trauma-related nightmares according to the CAPS “recurrent distressing dreams” item after 3 to 9 weeks of treatment vs placebo in veteran and civilian patients (N = 57) |
| Not suggested for treating PTSD-associated nightmares | ||
| 1 | Venlafaxine | No difference between extended-release venlafaxine (37.5 to 300 mg/d) and placebo in the CAPS-SX17 “distressing dreams” item at 12 weeks in 340 PTSD patients |
| May be considered for treating PTSD-associated nightmares | ||
| 4 | Clonidine | Reduced the number of nightmares in 11 of 13 refugees for 2 weeks to 3 months (dose: 0.2 to 0.6 mg/d) |
| May be considered for treating PTSD-associated nightmares, but data are low grade and sparse | ||
| 4 | Trazodone | Although trazodone (25 to 600 mg) significantly decreased nightmare frequency in veteran patients during an 8-week hospital stay (N = 60), 19% discontinued therapy because of side effects |
| 4 | Olanzapine | Adjunctive olanzapine (10 to 20 mg) rapidly improved sleep in a case series of combat-related PTSD patients resistant to SSRIs and benzodiazepines (N = 5) |
| 4 | Risperidone | In case series, risperidone (0.5 to 3 mg) significantly decreased CAPS scores for recurrent distressing dreams and proportion of traumatic dreams documented in diaries of combat veterans over 6 weeks (N = 17), and improved nightmares in adult burn patients taking pain medications after 1 to 2 days (N = 10) |
| 4 | Aripiprazole | In a case series, aripiprazole (15 to 30 mg at bedtime) with CBT or sertraline significantly improved nightmares in 4 of 5 combat-related PTSD patients |
| 4 | Topiramate | Topiramate reduced nightmares in 79% of civilians with PTSD and fully suppressed nightmares in 50% of patients in a case series (N = 35) |
| 4 | Low-dose cortisol | Significant decrease in frequency but not intensity of nightmares with low-dose cortisol (10 mg/d) in civilians with PTSD (N = 3) |
| 4 | Fluvoxamine | In 2 case series, fluvoxamine (up to 300 mg/d) significantly decreased the IES-R level of “dreams about combat trauma” but not the SRRS “bad dreams” rating at 10 weeks (N = 21). During 4 to 12 weeks of follow-up there was a qualitative decrease in reported nightmares in veteran patients (n = 12) |
| 2 | Triazolam/nitrazepam | Limited data showed triazolam (0.5 mg) and nitrazepam (5 mg) provide equal efficacy in decreasing the number of patients who experience unpleasant dreams over 1 night |
| 4 | Phenelzine | One study showed phenelzine monotherapy (30 to 90 mg) resulted in elimination of nightmares within 1 month (N = 5); another reported “moderately reduced traumatic dreams” (N = 21) in veterans. Therapy was discontinued because of short-lived efficacy or plateau effect |
| 4 | Gabapentin | Adjunctive gabapentin (300 to 3,600 mg/d) improved insomnia and decreased nightmare frequency and/or intensity over 1 to 36 months in 30 veterans with PTSD |
| 4 | Cyproheptadine | Conflicting data ranges from eliminating nightmares to no changes in the presence or intensity of nightmares |
| 4 | TCAs | Among 10 Cambodian concentration camp survivors treated with TCAs, 4 reported their nightmares ceased and 4 reported improvement after 1-year follow-up |
| 4 | Nefazodone | Reduced nightmare occurrence in 3 open-label studies as monotherapy (386 to 600 mg/d). Not recommended first line because of hepatotoxicity risk |
| No recommendation because of sparse data | ||
| 2 | Clonazepam | Clonazepam (1 to 2 mg/d) was ineffective in decreasing frequency or intensity of combat-related PTSD nightmares in veterans (N = 6) |
Evidence levels:
| ||
| CAPS: Clinician-Administered PTSD Scale; CAPS-SX17: 17-item Clinician-Administered PTSD Scale; CBT: cognitive-behavioral therapy; IES-R: Impact of Event Scale-Revised; PTSD: posttraumatic stress disorder; SRRS: Stress Response Rating Scale; SSRI: selective serotonin reuptake inhibitor; TCAs: tricyclic antidepressants Source: Adapted from Aurora RN, Zak RS, Auerbach SH, et al. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389-401 | ||
CASE CONTINUED: Medication change, improvement
After reviewing AASM’s treatment recommendations, we prescribe prazosin, 1 mg at bedtime for 7 nights, then increase by 1 mg at bedtime each week until Mr. S’s nightmares improve. He reports a substantial improvement in nightmare severity and frequency after a few weeks of treatment with prazosin, 5 mg at bedtime.
Prazosin
Prazosin is an α1-adrenergic receptor antagonist with good CNS penetrability. The rationale for reducing adrenergic activity to address intrusive PTSD symptoms has been well documented.12,13 In open-label trials,14-18 a chart review,19 and placebo-controlled trials,20-22prazosin reduced trauma nightmares and improved sleep quality and global clinical status more than placebo (Table 4). In these studies, prazosin doses ranged from 1 to 20 mg/d, with an average of 3 mg at bedtime and a starting dose of 1 mg. Prazosin is the only agent recommended in the AASM’s Best Practice Guide for treating PTSD-related nightmares.11
Table 4
RCTs of prazosin for trauma-related nightmares
| Study | Design | Patients | Results |
|---|---|---|---|
| Raskind et al, 200320 | 20-week, double-blind, placebo-controlled, crossover study (mean dose 9.5 mg/d at bedtime) | 10 Vietnam veterans with chronic PTSD and severe trauma-related nightmares | Prazosin was superior to placebo on scores on the recurrent distressing dreams item and difficulty falling/staying asleep item of the CAPS and change in PTSD severity and functional status on the CGI-C |
| Raskind et al, 200721 | 8-week, placebo-controlled, parallel study (mean dose 13.3 ± 3 mg/d in the evening) | 40 veterans with chronic PTSD, distressing trauma nightmares, and sleep disturbance | Prazosin was superior to placebo in reducing trauma nightmares and improving sleep quality and global clinical status; prazosin also shifted dream characteristics of trauma-related nightmares to those typical of normal dreams |
| Taylor et al, 200822 | 7-week, randomized, placebo-controlled, crossover trial (mean dose 3.1 ± 1.3 mg) | 13 outpatients with chronic civilian trauma PTSD, frequent nightmares, and sleep disturbance | Prazosin significantly increased total sleep time and REM sleep time; reduced trauma-related nightmares, distressed awakenings, and total PCL-C scores; improved CGI-I scores; and changed PDRS scores toward normal dreaming |
| CAPS: Clinician-Administered PTSD Scale; CGI-C: Clinical Global Impression of Change; CGI-I: Clinical Global Impression of Improvement; PCL-C: PTSD Checklist-Civilian; PDRS: PTSD Dream Rating Scale; PTSD: posttraumatic stress disorder; RCTs: randomized controlled trials; REM: rapid eye movement | |||
Atypical antipsychotics
Atypical antipsychotics have been used to reduce nightmares in PTSD; however, most of the evidence from studies evaluated in the AASM’s Best Practice Guide were considered to be low quality.11 Quetiapine and ziprasidone were not included in the AASM review. See (Table 5) for a review of the evidence for atypical antipsychotics for treating PTSD nightmares.
Table 5
Combat-related nightmares: Evidence for atypical antipsychotics
| Study | Design | Patients/dosage | Results |
|---|---|---|---|
| Aripiprazole | |||
| Lambert, 2006 a | Case report | 4 veterans with combat-related PTSD (3 male, 1 female; age 22 to 24); dose: 15 to 30 mg; concurrent treatment sertraline or CBT | Decreased frequency of weekly nightmares and agitated sleep by at least 50% |
| Olanzapine | |||
| Stein et al, 2002 b | 8-week, double-blind, placebo-controlled study | 19 male veterans with combat-related PTSD (olanzapine group mean age: 55.2 ± 6.6; placebo group 51.1 ± 8.1); mean dose: 15 mg/d | Significantly greater reduction in sleep disturbances (PSQI: -3.29 vs 1.57; P = .01); significantly higher weight gain (13.2 lbs vs -3 lbs; P = .001) |
| Jakovljevic et al, 2003 c | Case reports | 5 veterans with combat-related PTSD for 6 to 7 years (age: 28 to 50); dose: 10 to 20 mg; adjunct treatment | Decreased frequency of nightmares within 3 days |
| Labbate et al, 2000 d | Case report | 1 male veteran (age: 58) with a 20-year history of combat-related PTSD; dose: 5 mg at bedtime; concurrent treatment with sertraline (200 mg/d), bupropion (150 mg/d), and diazepam (15 mg/d) | Eliminated nightmares after 1 week and improved sleep quality |
| Quetiapine | |||
| Ahearn et al, 2006 e | 8-week, open-label trial | 15 PTSD patients (8 male; 7 female; 5 with combat-related PTSD; mean age: 49); mean dose: 216 mg/d (100 to 400 mg/d) | Significantly improved re-experiencing (CAPS: 10 vs 23; P = .0012) and sleep (PSQI: 17.5 vs 30; P = .0044) at 8 weeks compared with baseline |
| Robert et al, 2005 f | 6-week, open-label trial | 19 combat veterans; mean dose: 100 ± 70 mg/d (25 to 300 mg/d); adjunct treatment | Significantly improved sleep quality (PSQI: 1.67 vs 2.41; P = .006), latency (PSQI: 1.5 vs 2.65; P = .002), duration (PSQI: 1.31 vs 2.71; P < .001), and sleep disturbances (PSQI: 1.22 vs 1.71; P = .034) and decreased terror episodes (PSQI-A: 0.73 vs 0.91; P = .040) and acting out dreams (PSQI-A: 1.07 vs 1.35; P = .013); however, no difference in nightmares caused by trauma (PSQI-A: 1.53 vs 2.06) |
| Sokolski et al, 2003 g | Retrospective chart review | 68 male Vietnam War combat veterans (mean age: 55 ± 3.5); mean dose: 155 ± 130 mg (25 to 700 mg); adjunct treatment | Improved sleep disturbances in 62% and nightmares in 25% of patients |
| Ahearn et al, 2003 h | Case report | 2 male patients with combat-related PTSD (age 53, 72); dose: 25 to 50 mg; adjunct to SSRI therapy | Decreased frequency of nightmares with increased sleep duration |
| Risperidone | |||
| David et al, 2006 i | 6-week, open-label trial | 17 male veterans with combat-related PTSD (mean age: 53.7 ± 3.8); mean maximum dose: 2.3 ± 0.6 mg (range: 1 to 3 mg) | Improved recurrent distressing dreams (CAPS B-2: 3.8 vs 5.4; P = .04), but not with the PSQI subscale (PSQI bad dreams: 2.5 vs 2.7; NS). Decreased nighttime awakenings (1.9 vs 2.8; P = .003) and trauma dreams (19% vs 38%; P = .04) |
| Leyba et al, 1998 j | Case reports | 3 male patients (age 43 to 46); dose: 1 to 3 mg; adjunct therapy | Decreased occurrence of nightmares |
| Ziprasidone | |||
| Siddiqui et al, 2005 k | Case report | 1 male veteran with chronic combat-related PTSD (age 55); dose: 80 to 120 mg/d; adjunct with trazodone (100 mg) and topiramate | Improved occurrence of nightmares up to 4 months |
CAPS: Clinician-Administered PTSD Scale; CAPS B-2: Clinician-Administered PTSD Scale B-2 (recurrent distressing dreams of the event); CBT: cognitive-behavioral therapy; PSQI: Pittsburgh Sleep Quality Index; PSQI-A: Pittsburgh Sleep Quality Index Addendum for PTSD; NS: not significant; PTSD: posttraumatic stress disorder; SSRI: selective serotonin reuptake inhibitor References
| |||
Comparing prazosin and quetiapine. A historical prospective cohort study of 237 veterans with PTSD receiving prazosin or quetiapine for nighttime PTSD symptoms demonstrated that although the 2 drugs have similar efficacy (defined as symptomatic improvement) for short-term, 6-month treatment (61% vs 62%; P=.54), a higher percentage of patients continued prazosin long-term (3 to 6 years) than those taking quetiapine (48% vs 24%; P < .001).23 Twenty-five percent of patients taking quetiapine switched to prazosin during the study, and approximately one-half of these patients remained on prazosin until the study’s end. Only 8% of prazosin patients switched to quetiapine, and none continued this therapy until study end.23 Patients in the quetiapine group were more likely to discontinue the drug because of lack of efficacy (13% vs 3%; P=.03) and adverse effects (35% vs 18%; P=.008), specifically sedation (21% vs 2%; P < .001) and metabolic effects (9% vs 0%; P=.014), compared with prazosin. Although this trial may be the only published comparison study of prazosin and quetiapine, its methodological quality has been questioned, which makes it difficult to draw definitive conclusions.
Metabolic syndrome—elevated diastolic blood pressure, increased waist circumference, and low high-density lipoprotein cholesterol—is common among PTSD patients treated with antipsychotics.24 This syndrome may be caused by medications, lifestyle factors, or long-term overactivation of stress-response pathways. A retrospective chart review at a community mental health center revealed that patients taking even low doses of quetiapine for insomnia gained an average of 5 lbs (P=.037).25 Another retrospective chart review at 2 military hospitals reported that patients receiving low-dose quetiapine (≤100 mg/d) gained an average of slightly less than 1 lb per month, which adds up to approximately 10 lbs per year (P < .001).26 The benefit of using atypical antipsychotics may be outweighed by metabolic risks such as obesity, new-onset diabetes, and dyslipidemia.27
Prazosin is considered a first-line treatment for sleep disturbances and nightmares in PTSD because of its superior long-term efficacy and decreased adverse effects compared with quetiapine.
Related Resources
- American Psychiatric Association. Practice guidelines for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Arlington, VA: American Psychiatric Publishing, Inc.; 2004.
- Veterans Affairs/Department of Defense clinical practice guidelines. Management of traumatic stress disorder and acute stress reaction. www.healthquality.va.gov/Post_Traumatic_Stress_Disorder_PTSD.asp.
Drug Brand Names
- Prazosin • Minipress
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Dohrenwend BP, Turner JB, Turse NA, et al. The psychological risks of Vietnam for U.S. veterans: a revisit with new data and methods. Science. 2006;313(5789):979-982.
2. Tanielian T, Jaycox L. eds. Invisible wounds of war: psychological and cognitive injuries, their consequences, and services to assist recovery. Santa Monica, CA: RAND Corporation; 2008.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
4. Wittmann L, Schredl M, Kramer M. Dreaming in posttraumatic stress disorder: a critical review of phenomenology psychophysiology and treatment. Psychother Psychosom. 2007;76(1):25-39.
5. Clum GA, Nishith P, Resick PA. Trauma-related sleep disturbance and self-reported physical health symptoms in treatment-seeking female rape victims. J Nerv Ment Dis. 2001;189(9):618-622.
6. Kramer TL, Booth BM, Han X, et al. Service utilization and outcomes in medically ill veterans with posttraumatic stress and depressive disorders. J Trauma Stress. 2003;16(3):211-219.
7. Neylan TC, Marmar CR, Metzler TJ, et al. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam veterans. Am J Psychiatry. 1998;155(7):929-933.
8. Nappi CM, Drummond SP, Hall JM. Treating nightmares and insomnia in posttraumatic stress disorder: a review of current evidence. Neuropharmacology. 2012;62(2):576-585.
9. Maher MJ, Rego SA, Asnis GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology impact and approaches to management. CNS Drugs. 2006;20(7):567-590.
10. van Liempt S, Vermetten E, Geuze E, et al. Pharmacotherapy for disordered sleep in post-traumatic stress disorder: a systematic review. Int Clin Psychopharmacol. 2006;21(4):193-202.
11. Aurora RN, Zak RS, Auerbach SH, et al. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389-401.
12. Boehnlein JK, Kinzie JD. Pharmacologic reduction of CNS noradrenergic activity in PTSD: the case for clonidine and prazosin. J Psychiatr Pract. 2007;13(2):72-78.
13. Strawn JR, Geracioti TD, Jr. Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25(3):260-271.
14. Calohan J, Peterson K, Peskind ER, et al. Prazosin treatment of trauma nightmares and sleep disturbance in soldiers deployed in Iraq. J Trauma Stress. 2010;23(5):645-648.
15. Daly CM, Doyle ME, Radkind M, et al. Clinical case series: the use of Prazosin for combat-related recurrent nightmares among Operation Iraqi Freedom combat veterans. Mil Med. 2005;170(6):513-515.
16. Peskind ER, Bonner LT, Hoff DJ, et al. Prazosin reduces trauma-related nightmares in older men with chronic posttraumatic stress disorder. J Geriatr Psychiatry Neurol. 2003;16(3):165-171.
17. Raskind MA, Dobie DJ, Kanter ED, et al. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases. J Clin Psychiatry. 2000;61(2):129-133.
18. Taylor F, Raskind MA. The alpha1-adrenergic antagonist prazosin improves sleep and nightmares in civilian trauma posttraumatic stress disorder. J Clin Psychopharmacol. 2002;22(1):82-85.
19. Raskind MA, Thompson C, Petrie EC, et al. Prazosin reduces nightmares in combat veterans with posttraumatic stress disorder. J Clin Psychiatry. 2002;63(7):565-568.
20. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373.
21. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
22. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63(6):629-632.
23. Byers MG, Allison KM, Wendel CS, et al. Prazosin versus quetiapine for nighttime posttraumatic stress disorder symptoms in veterans: an assessment of long-term comparative effectiveness and safety. J Clin Psychopharmacol. 2010;30(3):225-229.
24. Jin H, Lanouette NM, Mudaliar S, et al. Association of posttraumatic stress disorder with increased prevalence of metabolic syndrome. J Clin Psychopharmacol. 2009;29(3):210-215.
25. Cates ME, Jackson CW, Feldman JM, et al. Metabolic consequences of using low-dose quetiapine for insomnia in psychiatric patients. Community Ment Health J. 2009;45(4):251-254.
26. Williams SG, Alinejad NA, Williams JA, et al. Statistically significant increase in weight caused by low-dose quetiapine. Pharmacotherapy. 2010;30(10):1011-1015.
27. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65(2):267-272.
1. Dohrenwend BP, Turner JB, Turse NA, et al. The psychological risks of Vietnam for U.S. veterans: a revisit with new data and methods. Science. 2006;313(5789):979-982.
2. Tanielian T, Jaycox L. eds. Invisible wounds of war: psychological and cognitive injuries, their consequences, and services to assist recovery. Santa Monica, CA: RAND Corporation; 2008.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
4. Wittmann L, Schredl M, Kramer M. Dreaming in posttraumatic stress disorder: a critical review of phenomenology psychophysiology and treatment. Psychother Psychosom. 2007;76(1):25-39.
5. Clum GA, Nishith P, Resick PA. Trauma-related sleep disturbance and self-reported physical health symptoms in treatment-seeking female rape victims. J Nerv Ment Dis. 2001;189(9):618-622.
6. Kramer TL, Booth BM, Han X, et al. Service utilization and outcomes in medically ill veterans with posttraumatic stress and depressive disorders. J Trauma Stress. 2003;16(3):211-219.
7. Neylan TC, Marmar CR, Metzler TJ, et al. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam veterans. Am J Psychiatry. 1998;155(7):929-933.
8. Nappi CM, Drummond SP, Hall JM. Treating nightmares and insomnia in posttraumatic stress disorder: a review of current evidence. Neuropharmacology. 2012;62(2):576-585.
9. Maher MJ, Rego SA, Asnis GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology impact and approaches to management. CNS Drugs. 2006;20(7):567-590.
10. van Liempt S, Vermetten E, Geuze E, et al. Pharmacotherapy for disordered sleep in post-traumatic stress disorder: a systematic review. Int Clin Psychopharmacol. 2006;21(4):193-202.
11. Aurora RN, Zak RS, Auerbach SH, et al. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389-401.
12. Boehnlein JK, Kinzie JD. Pharmacologic reduction of CNS noradrenergic activity in PTSD: the case for clonidine and prazosin. J Psychiatr Pract. 2007;13(2):72-78.
13. Strawn JR, Geracioti TD, Jr. Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25(3):260-271.
14. Calohan J, Peterson K, Peskind ER, et al. Prazosin treatment of trauma nightmares and sleep disturbance in soldiers deployed in Iraq. J Trauma Stress. 2010;23(5):645-648.
15. Daly CM, Doyle ME, Radkind M, et al. Clinical case series: the use of Prazosin for combat-related recurrent nightmares among Operation Iraqi Freedom combat veterans. Mil Med. 2005;170(6):513-515.
16. Peskind ER, Bonner LT, Hoff DJ, et al. Prazosin reduces trauma-related nightmares in older men with chronic posttraumatic stress disorder. J Geriatr Psychiatry Neurol. 2003;16(3):165-171.
17. Raskind MA, Dobie DJ, Kanter ED, et al. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases. J Clin Psychiatry. 2000;61(2):129-133.
18. Taylor F, Raskind MA. The alpha1-adrenergic antagonist prazosin improves sleep and nightmares in civilian trauma posttraumatic stress disorder. J Clin Psychopharmacol. 2002;22(1):82-85.
19. Raskind MA, Thompson C, Petrie EC, et al. Prazosin reduces nightmares in combat veterans with posttraumatic stress disorder. J Clin Psychiatry. 2002;63(7):565-568.
20. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373.
21. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
22. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63(6):629-632.
23. Byers MG, Allison KM, Wendel CS, et al. Prazosin versus quetiapine for nighttime posttraumatic stress disorder symptoms in veterans: an assessment of long-term comparative effectiveness and safety. J Clin Psychopharmacol. 2010;30(3):225-229.
24. Jin H, Lanouette NM, Mudaliar S, et al. Association of posttraumatic stress disorder with increased prevalence of metabolic syndrome. J Clin Psychopharmacol. 2009;29(3):210-215.
25. Cates ME, Jackson CW, Feldman JM, et al. Metabolic consequences of using low-dose quetiapine for insomnia in psychiatric patients. Community Ment Health J. 2009;45(4):251-254.
26. Williams SG, Alinejad NA, Williams JA, et al. Statistically significant increase in weight caused by low-dose quetiapine. Pharmacotherapy. 2010;30(10):1011-1015.
27. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65(2):267-272.
‘Curbside’ consults: Know your liability
Discuss this article at www.facebook.com/CurrentPsychiatry
Dear Dr. Mossman:
Could providing a “curbside” consultation to a colleague leave me medico legally vulnerable if an adverse event leads to a malpractice lawsuit? If so, what can I do to address this risk?—Submitted by “Dr. W”
Medicine is a collaborative profession. Surgeons often combine skills to perform complex operations together, and specialists pool their expertise when they collectively manage patients with several medical problems. Doctors share their knowledge when they give lectures to medical audiences, write reports to referring physicians, or respond verbally to colleagues’ requests for information or advice.1
Doctors use the phrase “curbside consult” to refer (with humor and self-deprecation) to informal conversations with colleagues about patients’ medical management—advice-seeking that falls short of asking a colleague to make recommendations based on a formal, personal examination. Many physicians seek or provide curbside advice several times a month.2 Curbside consults transmit knowledge and cement professional bonds among physicians, making them “an integral part of our medical culture.”3
More than a dozen legal decisions mention curbside consultations. Judges think informal information-sharing improves medical practice and don’t want doctors to stop soliciting ideas or offering suggestions because they fear lawsuits.4,5 However, courts have found that, under certain conditions, giving advice can create liability for a bad outcome, even though the doctor never met the patient who was harmed.
In this article, we’ll look at:
- when such liability might occur, and
- what you can do to minimize it.
A doctor-patient relationship?
Legally, doctors are obligated to provide competent care for just 1 group of people: their patients. Therefore, to decide if plaintiffs could pursue malpractice claims in cases where doctors offered comments about patients they did not personally examine, courts have asked whether the circumstances, actions undertaken, or nature of information that was exchanged created a professional relationship.
Reynolds v Decatur Memorial Hospital4 describes an informal consultation that did not create a physician-patient relationship. In this case, a boy was admitted to a hospital after he had fallen. The treating pediatrician telephoned a neurosurgeon, who asked whether the boy’s neck was stiff, discussed diagnostic possibilities with the pediatrician, and suggested doing a lumbar puncture. The neurosurgeon offered to see the boy if requested, but he never did, and he did not bill for the telephone consultation. Guillain-Barré syndrome was first suspected, but a spinal cord injury was discovered after the boy—who developed quadriplegia—was transferred to another hospital.
In a subsequent lawsuit, the boy’s mother claimed her son’s paralysis resulted from negligence by the first hospital and its doctors, but the trial court dismissed the case against the neurosurgeon. Affirming the trial court’s ruling, an Illinois appeals court explained that the neurosurgeon had not been asked to provide medical services, conduct tests, or interpret test results. “A doctor who gives an informal opinion at the request of a treating physician does not owe a duty of care to the patient whose case was discussed,” the Reynolds court said.
Campbell v Haber6 describes circumstances that differed slightly from those described in the Reynolds decision but appeared to create a doctor-patient relationship. Campbell concerned a patient who came to an emergency room (ER) complaining of chest pain. The ER physician’s findings indicated possible heart muscle damage, so he telephoned a cardiologist (whom the ER doctor believed was “on call”) and described the patient’s symptoms and test results. The cardiologist thought the test results were not consistent with a cardiac event. The ER physician told the patient and his wife about the cardiologist’s opinion and, relying on what the cardiologist said, discharged the patient. Shortly after, the patient had a heart attack.
The patient sued not just the ER physician, but the cardiologist, who sought dismissal from the suit because he never saw the patient, had no treatment relationship with him, and never billed for services. However, the trial judge ruled that the patient could sue the cardiologist and the appellate court agreed, saying that a jury had to decide whether the cardiologist had incurred a doctor-patient relationship and might be liable. “An implied physician-patient relationship may arise when a physician gives advice to a patient,” the appeals court said, “even if that advice is communicated through another health care professional.”
Telling the difference
So what differentiates a no-liability curbside consult from a medical discussion that creates a doctor-patient duty and potential for liability for adverse results?
You create a physician-patient relationship when you assume responsibility to diagnose or treat someone.7 Although typically this requires an in-person encounter with a patient, it can happen indirectly—electronically (through e-mail), by telephone, or through a family member or another professional. But if you do nothing that implies consent to act for the patient’s benefit, you should have no actual malpractice liability if something goes wrong.3,8 As a Kansas Supreme Court decision explains, you “cannot be liable for medical malpractice” if you “merely consult with a treating physician and [do] nothing more.”5
Several legal cases discuss doctors’ efforts to extricate themselves from lawsuits arising from clinical encounters that the doctors mistakenly thought were just curbside consults. Table 18-12 lists situations in which talking about patients goes beyond just being “curbsided.”
Table 1
When it’s not a ‘curbside consultation’
| Situation | Why it’s not a curbside consultation |
|---|---|
| On call | If you are “on call” for an emergency room, get called about a patient with an emergency condition, and discuss the patient’s symptoms, possible diagnosis, or treatment, you have a relationship with the patient that entails a duty of care8,9 |
| Covering | If you have agreed to “cover” patients for a colleague, you have assumed a duty to properly care for the colleague’s patients: they’re your patients during the colleague’s absence. Getting asked questions about managing those patients is not a curbside consultation, even if you’ve never met or spoken to the patient10,11 |
| Supervising | Physician assistants, residents in training, and nurse practitioners do not practice independently of their supervising physicians. If you’re a supervisor and get a call about managing a patient, you may bear vicarious liability for adverse results12 |
| Specifics and reliance | If responding to the informal consult requires you to give specific advice that the consulting colleague will rely on to make a diagnosis or select treatment, you are participating in the patient’s care11 |
How to respond
Should you decline to provide curbside consultations to keep yourself out of lawsuits? Some authors think so, pointing out that informally transmitted clinical data may be faulty, which means you may give bad advice based on incomplete information or a verbal misunderstanding.13-16 These authors suggest that if you’re curbsided you should ask to see the patient for a formal consultation, decline to give informal advice, or provide a response that lacks specifics.
Other authors feel that these approaches are needlessly cautious and would harm patients by impeding doctors’ ability to help and learn from each other.3,17 These authors think the risk of incurring liability from a curbside consult is low. Also, getting advice from a colleague is a valuable risk management strategy; it helps you make sure you’re on the right track, and it shows you are a thoughtful clinician whose patients benefit from your own and your colleagues’ medical expertise.
Even if you’re comfortable soliciting and providing curbside advice, sometimes circumstances make it wise to follow-up an informal initial inquiry with a formal consultation. Table 23,17 lists examples of when you should follow-up with a formal consultation.
Table 2
Considerations that favor formal consultation
| Complicated diagnostic situations |
| The consulted or requesting physician feels that giving good advice requires a personal examination |
| Advice is based on a detailed discussion and is specific to a patient’s situation |
| The patient requested the consultation |
| The consultant will make a report for the patient’s record |
| The consult bills for the consultation |
Documentation
Experts disagree about whether the requesting or receiving physician should document a curbside consultation, and if so, how. On one hand, making a notation in a patient’s record documents the treating doctor’s diligence and may provide a measure of liability protection in a malpractice action. Doing this, however, exposes the identity of the consultant, who might be named among the defendants in a lawsuit.
One commonly recommended strategy is to request the consultant’s permission before identifying him or her in the record,13,16,17 a position that is defensible on grounds of courtesy alone. But omitting a consultant’s name from record does not guarantee that the consultant’s involvement won’t be discovered in the course of litigation.3 For example, treating doctors who get sued often are asked during their depositions about whether they talked with anyone about the case, and they have to answer honestly.
If a consulted doctor makes written notes, it might suggest that the consultation was more than the sort of informal information-sharing implied by the term “curbside.” However, in the unlikely event that a lawsuit arose and included the consultant as a defendant, documentation of advice given would help the consultant recall and defend what was said.
Related Resources
- Grant-Kels JM, Kels BD. The curbside consultation: legal, moral, and ethical considerations. J Am Acad Dermatol. 2012;66(5):827-829.
- Kreichelt R, Hilbert ML, Shinn D. Minimizing the legal risk with ‘curbside’ consultation. J Healthc Risk Manag. 2008;28(1):27-29.
- Atkinson L. Curbside consults: what is your liability risk? Iowa Med. 2003;93(4):15.
Disclosure
Dr. Mossman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Perley CM. Physician use of the curbside consultation to address information needs: report on a collective case study. J Med Libr Assoc. 2006;94(2):137-144.
2. Kuo D, Gifford DR, Stein MD. Curbside consultation practices and attitudes among primary care physicians and medical subspecialists. JAMA. 1998;280(10):905-909.
3. Cotton VR. Legal risks of “curbside” consults. Am J Cardiol. 2010;106(1):135-138.
4. Reynolds v Decatur Memorial Hospital, 277 Ill App 3d 80 (Ill App Ct 4th Dist 1996).
5. Irvin v Smith, 272 Kan 112 (Kan 2001).
6. Campbell v Haber, 274 A.D.2d 946 (NY App Div 4th Dep’t 2000).
7. Sterling v Johns Hopkins Hospital, 802 A.2d 440 (Md Ct Spec App 2002), cert den, 808 A.2d 808 (Md 2002).
8. Emergency Medical Treatment and Active Labor Act, 42 USC § 1395DD.
9. Lownsbury v VanBuren, 94 Ohio St 3d 231, 762 NE 2d 354 (2002).
10. Blazo v McLaren Regional Medical Center, 2002 Mich App LEXIS 752 (Mich Ct App 2002).
11. Kelley v Middle Tennessee Emergency Physicians, PC, 133 SW3d 587 (Tenn 2004).
12. Hammonds v Jewish Hospital, 899 SW2d 527 (Mo Ct App 1995).
13. MAG Mutual Insurance Company, Inc. Curbing the curbside consult—a risk management perspective. J Med Assoc Ga. 2008;97(1):50.-
14. Burns CD. Death of the curbside consult? J Ky Med Assoc. 2006;104(1):27.-
15. Hendel T. Informal consultations: do new risks exist with this age-old tradition? J Med Pract Manage. 2002;17(6):308-311.
16. Manian FA, Janssen DA. Curbside consultations. A closer look at a common practice. JAMA. 1996;275(2):145-147.
17. Curbside consultations. Psychiatry (Edgmont). 2010;7(5):51-53.
Discuss this article at www.facebook.com/CurrentPsychiatry
Dear Dr. Mossman:
Could providing a “curbside” consultation to a colleague leave me medico legally vulnerable if an adverse event leads to a malpractice lawsuit? If so, what can I do to address this risk?—Submitted by “Dr. W”
Medicine is a collaborative profession. Surgeons often combine skills to perform complex operations together, and specialists pool their expertise when they collectively manage patients with several medical problems. Doctors share their knowledge when they give lectures to medical audiences, write reports to referring physicians, or respond verbally to colleagues’ requests for information or advice.1
Doctors use the phrase “curbside consult” to refer (with humor and self-deprecation) to informal conversations with colleagues about patients’ medical management—advice-seeking that falls short of asking a colleague to make recommendations based on a formal, personal examination. Many physicians seek or provide curbside advice several times a month.2 Curbside consults transmit knowledge and cement professional bonds among physicians, making them “an integral part of our medical culture.”3
More than a dozen legal decisions mention curbside consultations. Judges think informal information-sharing improves medical practice and don’t want doctors to stop soliciting ideas or offering suggestions because they fear lawsuits.4,5 However, courts have found that, under certain conditions, giving advice can create liability for a bad outcome, even though the doctor never met the patient who was harmed.
In this article, we’ll look at:
- when such liability might occur, and
- what you can do to minimize it.
A doctor-patient relationship?
Legally, doctors are obligated to provide competent care for just 1 group of people: their patients. Therefore, to decide if plaintiffs could pursue malpractice claims in cases where doctors offered comments about patients they did not personally examine, courts have asked whether the circumstances, actions undertaken, or nature of information that was exchanged created a professional relationship.
Reynolds v Decatur Memorial Hospital4 describes an informal consultation that did not create a physician-patient relationship. In this case, a boy was admitted to a hospital after he had fallen. The treating pediatrician telephoned a neurosurgeon, who asked whether the boy’s neck was stiff, discussed diagnostic possibilities with the pediatrician, and suggested doing a lumbar puncture. The neurosurgeon offered to see the boy if requested, but he never did, and he did not bill for the telephone consultation. Guillain-Barré syndrome was first suspected, but a spinal cord injury was discovered after the boy—who developed quadriplegia—was transferred to another hospital.
In a subsequent lawsuit, the boy’s mother claimed her son’s paralysis resulted from negligence by the first hospital and its doctors, but the trial court dismissed the case against the neurosurgeon. Affirming the trial court’s ruling, an Illinois appeals court explained that the neurosurgeon had not been asked to provide medical services, conduct tests, or interpret test results. “A doctor who gives an informal opinion at the request of a treating physician does not owe a duty of care to the patient whose case was discussed,” the Reynolds court said.
Campbell v Haber6 describes circumstances that differed slightly from those described in the Reynolds decision but appeared to create a doctor-patient relationship. Campbell concerned a patient who came to an emergency room (ER) complaining of chest pain. The ER physician’s findings indicated possible heart muscle damage, so he telephoned a cardiologist (whom the ER doctor believed was “on call”) and described the patient’s symptoms and test results. The cardiologist thought the test results were not consistent with a cardiac event. The ER physician told the patient and his wife about the cardiologist’s opinion and, relying on what the cardiologist said, discharged the patient. Shortly after, the patient had a heart attack.
The patient sued not just the ER physician, but the cardiologist, who sought dismissal from the suit because he never saw the patient, had no treatment relationship with him, and never billed for services. However, the trial judge ruled that the patient could sue the cardiologist and the appellate court agreed, saying that a jury had to decide whether the cardiologist had incurred a doctor-patient relationship and might be liable. “An implied physician-patient relationship may arise when a physician gives advice to a patient,” the appeals court said, “even if that advice is communicated through another health care professional.”
Telling the difference
So what differentiates a no-liability curbside consult from a medical discussion that creates a doctor-patient duty and potential for liability for adverse results?
You create a physician-patient relationship when you assume responsibility to diagnose or treat someone.7 Although typically this requires an in-person encounter with a patient, it can happen indirectly—electronically (through e-mail), by telephone, or through a family member or another professional. But if you do nothing that implies consent to act for the patient’s benefit, you should have no actual malpractice liability if something goes wrong.3,8 As a Kansas Supreme Court decision explains, you “cannot be liable for medical malpractice” if you “merely consult with a treating physician and [do] nothing more.”5
Several legal cases discuss doctors’ efforts to extricate themselves from lawsuits arising from clinical encounters that the doctors mistakenly thought were just curbside consults. Table 18-12 lists situations in which talking about patients goes beyond just being “curbsided.”
Table 1
When it’s not a ‘curbside consultation’
| Situation | Why it’s not a curbside consultation |
|---|---|
| On call | If you are “on call” for an emergency room, get called about a patient with an emergency condition, and discuss the patient’s symptoms, possible diagnosis, or treatment, you have a relationship with the patient that entails a duty of care8,9 |
| Covering | If you have agreed to “cover” patients for a colleague, you have assumed a duty to properly care for the colleague’s patients: they’re your patients during the colleague’s absence. Getting asked questions about managing those patients is not a curbside consultation, even if you’ve never met or spoken to the patient10,11 |
| Supervising | Physician assistants, residents in training, and nurse practitioners do not practice independently of their supervising physicians. If you’re a supervisor and get a call about managing a patient, you may bear vicarious liability for adverse results12 |
| Specifics and reliance | If responding to the informal consult requires you to give specific advice that the consulting colleague will rely on to make a diagnosis or select treatment, you are participating in the patient’s care11 |
How to respond
Should you decline to provide curbside consultations to keep yourself out of lawsuits? Some authors think so, pointing out that informally transmitted clinical data may be faulty, which means you may give bad advice based on incomplete information or a verbal misunderstanding.13-16 These authors suggest that if you’re curbsided you should ask to see the patient for a formal consultation, decline to give informal advice, or provide a response that lacks specifics.
Other authors feel that these approaches are needlessly cautious and would harm patients by impeding doctors’ ability to help and learn from each other.3,17 These authors think the risk of incurring liability from a curbside consult is low. Also, getting advice from a colleague is a valuable risk management strategy; it helps you make sure you’re on the right track, and it shows you are a thoughtful clinician whose patients benefit from your own and your colleagues’ medical expertise.
Even if you’re comfortable soliciting and providing curbside advice, sometimes circumstances make it wise to follow-up an informal initial inquiry with a formal consultation. Table 23,17 lists examples of when you should follow-up with a formal consultation.
Table 2
Considerations that favor formal consultation
| Complicated diagnostic situations |
| The consulted or requesting physician feels that giving good advice requires a personal examination |
| Advice is based on a detailed discussion and is specific to a patient’s situation |
| The patient requested the consultation |
| The consultant will make a report for the patient’s record |
| The consult bills for the consultation |
Documentation
Experts disagree about whether the requesting or receiving physician should document a curbside consultation, and if so, how. On one hand, making a notation in a patient’s record documents the treating doctor’s diligence and may provide a measure of liability protection in a malpractice action. Doing this, however, exposes the identity of the consultant, who might be named among the defendants in a lawsuit.
One commonly recommended strategy is to request the consultant’s permission before identifying him or her in the record,13,16,17 a position that is defensible on grounds of courtesy alone. But omitting a consultant’s name from record does not guarantee that the consultant’s involvement won’t be discovered in the course of litigation.3 For example, treating doctors who get sued often are asked during their depositions about whether they talked with anyone about the case, and they have to answer honestly.
If a consulted doctor makes written notes, it might suggest that the consultation was more than the sort of informal information-sharing implied by the term “curbside.” However, in the unlikely event that a lawsuit arose and included the consultant as a defendant, documentation of advice given would help the consultant recall and defend what was said.
Related Resources
- Grant-Kels JM, Kels BD. The curbside consultation: legal, moral, and ethical considerations. J Am Acad Dermatol. 2012;66(5):827-829.
- Kreichelt R, Hilbert ML, Shinn D. Minimizing the legal risk with ‘curbside’ consultation. J Healthc Risk Manag. 2008;28(1):27-29.
- Atkinson L. Curbside consults: what is your liability risk? Iowa Med. 2003;93(4):15.
Disclosure
Dr. Mossman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Dear Dr. Mossman:
Could providing a “curbside” consultation to a colleague leave me medico legally vulnerable if an adverse event leads to a malpractice lawsuit? If so, what can I do to address this risk?—Submitted by “Dr. W”
Medicine is a collaborative profession. Surgeons often combine skills to perform complex operations together, and specialists pool their expertise when they collectively manage patients with several medical problems. Doctors share their knowledge when they give lectures to medical audiences, write reports to referring physicians, or respond verbally to colleagues’ requests for information or advice.1
Doctors use the phrase “curbside consult” to refer (with humor and self-deprecation) to informal conversations with colleagues about patients’ medical management—advice-seeking that falls short of asking a colleague to make recommendations based on a formal, personal examination. Many physicians seek or provide curbside advice several times a month.2 Curbside consults transmit knowledge and cement professional bonds among physicians, making them “an integral part of our medical culture.”3
More than a dozen legal decisions mention curbside consultations. Judges think informal information-sharing improves medical practice and don’t want doctors to stop soliciting ideas or offering suggestions because they fear lawsuits.4,5 However, courts have found that, under certain conditions, giving advice can create liability for a bad outcome, even though the doctor never met the patient who was harmed.
In this article, we’ll look at:
- when such liability might occur, and
- what you can do to minimize it.
A doctor-patient relationship?
Legally, doctors are obligated to provide competent care for just 1 group of people: their patients. Therefore, to decide if plaintiffs could pursue malpractice claims in cases where doctors offered comments about patients they did not personally examine, courts have asked whether the circumstances, actions undertaken, or nature of information that was exchanged created a professional relationship.
Reynolds v Decatur Memorial Hospital4 describes an informal consultation that did not create a physician-patient relationship. In this case, a boy was admitted to a hospital after he had fallen. The treating pediatrician telephoned a neurosurgeon, who asked whether the boy’s neck was stiff, discussed diagnostic possibilities with the pediatrician, and suggested doing a lumbar puncture. The neurosurgeon offered to see the boy if requested, but he never did, and he did not bill for the telephone consultation. Guillain-Barré syndrome was first suspected, but a spinal cord injury was discovered after the boy—who developed quadriplegia—was transferred to another hospital.
In a subsequent lawsuit, the boy’s mother claimed her son’s paralysis resulted from negligence by the first hospital and its doctors, but the trial court dismissed the case against the neurosurgeon. Affirming the trial court’s ruling, an Illinois appeals court explained that the neurosurgeon had not been asked to provide medical services, conduct tests, or interpret test results. “A doctor who gives an informal opinion at the request of a treating physician does not owe a duty of care to the patient whose case was discussed,” the Reynolds court said.
Campbell v Haber6 describes circumstances that differed slightly from those described in the Reynolds decision but appeared to create a doctor-patient relationship. Campbell concerned a patient who came to an emergency room (ER) complaining of chest pain. The ER physician’s findings indicated possible heart muscle damage, so he telephoned a cardiologist (whom the ER doctor believed was “on call”) and described the patient’s symptoms and test results. The cardiologist thought the test results were not consistent with a cardiac event. The ER physician told the patient and his wife about the cardiologist’s opinion and, relying on what the cardiologist said, discharged the patient. Shortly after, the patient had a heart attack.
The patient sued not just the ER physician, but the cardiologist, who sought dismissal from the suit because he never saw the patient, had no treatment relationship with him, and never billed for services. However, the trial judge ruled that the patient could sue the cardiologist and the appellate court agreed, saying that a jury had to decide whether the cardiologist had incurred a doctor-patient relationship and might be liable. “An implied physician-patient relationship may arise when a physician gives advice to a patient,” the appeals court said, “even if that advice is communicated through another health care professional.”
Telling the difference
So what differentiates a no-liability curbside consult from a medical discussion that creates a doctor-patient duty and potential for liability for adverse results?
You create a physician-patient relationship when you assume responsibility to diagnose or treat someone.7 Although typically this requires an in-person encounter with a patient, it can happen indirectly—electronically (through e-mail), by telephone, or through a family member or another professional. But if you do nothing that implies consent to act for the patient’s benefit, you should have no actual malpractice liability if something goes wrong.3,8 As a Kansas Supreme Court decision explains, you “cannot be liable for medical malpractice” if you “merely consult with a treating physician and [do] nothing more.”5
Several legal cases discuss doctors’ efforts to extricate themselves from lawsuits arising from clinical encounters that the doctors mistakenly thought were just curbside consults. Table 18-12 lists situations in which talking about patients goes beyond just being “curbsided.”
Table 1
When it’s not a ‘curbside consultation’
| Situation | Why it’s not a curbside consultation |
|---|---|
| On call | If you are “on call” for an emergency room, get called about a patient with an emergency condition, and discuss the patient’s symptoms, possible diagnosis, or treatment, you have a relationship with the patient that entails a duty of care8,9 |
| Covering | If you have agreed to “cover” patients for a colleague, you have assumed a duty to properly care for the colleague’s patients: they’re your patients during the colleague’s absence. Getting asked questions about managing those patients is not a curbside consultation, even if you’ve never met or spoken to the patient10,11 |
| Supervising | Physician assistants, residents in training, and nurse practitioners do not practice independently of their supervising physicians. If you’re a supervisor and get a call about managing a patient, you may bear vicarious liability for adverse results12 |
| Specifics and reliance | If responding to the informal consult requires you to give specific advice that the consulting colleague will rely on to make a diagnosis or select treatment, you are participating in the patient’s care11 |
How to respond
Should you decline to provide curbside consultations to keep yourself out of lawsuits? Some authors think so, pointing out that informally transmitted clinical data may be faulty, which means you may give bad advice based on incomplete information or a verbal misunderstanding.13-16 These authors suggest that if you’re curbsided you should ask to see the patient for a formal consultation, decline to give informal advice, or provide a response that lacks specifics.
Other authors feel that these approaches are needlessly cautious and would harm patients by impeding doctors’ ability to help and learn from each other.3,17 These authors think the risk of incurring liability from a curbside consult is low. Also, getting advice from a colleague is a valuable risk management strategy; it helps you make sure you’re on the right track, and it shows you are a thoughtful clinician whose patients benefit from your own and your colleagues’ medical expertise.
Even if you’re comfortable soliciting and providing curbside advice, sometimes circumstances make it wise to follow-up an informal initial inquiry with a formal consultation. Table 23,17 lists examples of when you should follow-up with a formal consultation.
Table 2
Considerations that favor formal consultation
| Complicated diagnostic situations |
| The consulted or requesting physician feels that giving good advice requires a personal examination |
| Advice is based on a detailed discussion and is specific to a patient’s situation |
| The patient requested the consultation |
| The consultant will make a report for the patient’s record |
| The consult bills for the consultation |
Documentation
Experts disagree about whether the requesting or receiving physician should document a curbside consultation, and if so, how. On one hand, making a notation in a patient’s record documents the treating doctor’s diligence and may provide a measure of liability protection in a malpractice action. Doing this, however, exposes the identity of the consultant, who might be named among the defendants in a lawsuit.
One commonly recommended strategy is to request the consultant’s permission before identifying him or her in the record,13,16,17 a position that is defensible on grounds of courtesy alone. But omitting a consultant’s name from record does not guarantee that the consultant’s involvement won’t be discovered in the course of litigation.3 For example, treating doctors who get sued often are asked during their depositions about whether they talked with anyone about the case, and they have to answer honestly.
If a consulted doctor makes written notes, it might suggest that the consultation was more than the sort of informal information-sharing implied by the term “curbside.” However, in the unlikely event that a lawsuit arose and included the consultant as a defendant, documentation of advice given would help the consultant recall and defend what was said.
Related Resources
- Grant-Kels JM, Kels BD. The curbside consultation: legal, moral, and ethical considerations. J Am Acad Dermatol. 2012;66(5):827-829.
- Kreichelt R, Hilbert ML, Shinn D. Minimizing the legal risk with ‘curbside’ consultation. J Healthc Risk Manag. 2008;28(1):27-29.
- Atkinson L. Curbside consults: what is your liability risk? Iowa Med. 2003;93(4):15.
Disclosure
Dr. Mossman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Perley CM. Physician use of the curbside consultation to address information needs: report on a collective case study. J Med Libr Assoc. 2006;94(2):137-144.
2. Kuo D, Gifford DR, Stein MD. Curbside consultation practices and attitudes among primary care physicians and medical subspecialists. JAMA. 1998;280(10):905-909.
3. Cotton VR. Legal risks of “curbside” consults. Am J Cardiol. 2010;106(1):135-138.
4. Reynolds v Decatur Memorial Hospital, 277 Ill App 3d 80 (Ill App Ct 4th Dist 1996).
5. Irvin v Smith, 272 Kan 112 (Kan 2001).
6. Campbell v Haber, 274 A.D.2d 946 (NY App Div 4th Dep’t 2000).
7. Sterling v Johns Hopkins Hospital, 802 A.2d 440 (Md Ct Spec App 2002), cert den, 808 A.2d 808 (Md 2002).
8. Emergency Medical Treatment and Active Labor Act, 42 USC § 1395DD.
9. Lownsbury v VanBuren, 94 Ohio St 3d 231, 762 NE 2d 354 (2002).
10. Blazo v McLaren Regional Medical Center, 2002 Mich App LEXIS 752 (Mich Ct App 2002).
11. Kelley v Middle Tennessee Emergency Physicians, PC, 133 SW3d 587 (Tenn 2004).
12. Hammonds v Jewish Hospital, 899 SW2d 527 (Mo Ct App 1995).
13. MAG Mutual Insurance Company, Inc. Curbing the curbside consult—a risk management perspective. J Med Assoc Ga. 2008;97(1):50.-
14. Burns CD. Death of the curbside consult? J Ky Med Assoc. 2006;104(1):27.-
15. Hendel T. Informal consultations: do new risks exist with this age-old tradition? J Med Pract Manage. 2002;17(6):308-311.
16. Manian FA, Janssen DA. Curbside consultations. A closer look at a common practice. JAMA. 1996;275(2):145-147.
17. Curbside consultations. Psychiatry (Edgmont). 2010;7(5):51-53.
1. Perley CM. Physician use of the curbside consultation to address information needs: report on a collective case study. J Med Libr Assoc. 2006;94(2):137-144.
2. Kuo D, Gifford DR, Stein MD. Curbside consultation practices and attitudes among primary care physicians and medical subspecialists. JAMA. 1998;280(10):905-909.
3. Cotton VR. Legal risks of “curbside” consults. Am J Cardiol. 2010;106(1):135-138.
4. Reynolds v Decatur Memorial Hospital, 277 Ill App 3d 80 (Ill App Ct 4th Dist 1996).
5. Irvin v Smith, 272 Kan 112 (Kan 2001).
6. Campbell v Haber, 274 A.D.2d 946 (NY App Div 4th Dep’t 2000).
7. Sterling v Johns Hopkins Hospital, 802 A.2d 440 (Md Ct Spec App 2002), cert den, 808 A.2d 808 (Md 2002).
8. Emergency Medical Treatment and Active Labor Act, 42 USC § 1395DD.
9. Lownsbury v VanBuren, 94 Ohio St 3d 231, 762 NE 2d 354 (2002).
10. Blazo v McLaren Regional Medical Center, 2002 Mich App LEXIS 752 (Mich Ct App 2002).
11. Kelley v Middle Tennessee Emergency Physicians, PC, 133 SW3d 587 (Tenn 2004).
12. Hammonds v Jewish Hospital, 899 SW2d 527 (Mo Ct App 1995).
13. MAG Mutual Insurance Company, Inc. Curbing the curbside consult—a risk management perspective. J Med Assoc Ga. 2008;97(1):50.-
14. Burns CD. Death of the curbside consult? J Ky Med Assoc. 2006;104(1):27.-
15. Hendel T. Informal consultations: do new risks exist with this age-old tradition? J Med Pract Manage. 2002;17(6):308-311.
16. Manian FA, Janssen DA. Curbside consultations. A closer look at a common practice. JAMA. 1996;275(2):145-147.
17. Curbside consultations. Psychiatry (Edgmont). 2010;7(5):51-53.
High-dose donepezil or memantine: Next step for Alzheimer’s disease?
Although cholinesterase inhibitors (ChEIs) and memantine at standard doses may slow the progression of Alzheimer’s disease (AD) as assessed by cognitive, functional, and global measures, this effect is relatively modest. For the estimated 5.4 million Americans with AD1—more than one-half of whom have moderate to severe disease2—there is a great need for new approaches to slow AD progression.
High doses of donepezil or memantine may be the next step in achieving better results than standard pharmacologic treatments for AD. This article presents the possible benefits and indications for high doses of donepezil (23 mg/d) and memantine (28 mg/d) for managing moderate to severe AD and their safety and tolerability profiles.
Current treatments offer modest benefits
AD treatments comprise 2 categories: ChEIs (donepezil, rivastigmine, and galantamine) and the N-methyl-D-aspartate (NMDA) receptor antagonist memantine (Table 1).3,4 All ChEIs are FDA-approved for mild to moderate AD; donepezil also is approved for severe AD. Memantine is approved for moderate to severe AD, either alone or in combination with ChEIs. Until recently, the maximum FDA-approved doses were donepezil, 10 mg/d, and memantine, 20 mg/d. However, these dosages are associated with only modest beneficial effects in managing cognitive deterioration in patients with moderate to severe dementia.5,6 Studies have reported that combining a ChEI, such as donepezil, and memantine is well tolerated and may result in synergistic benefits by affecting different neurotransmitters in patients with moderate to severe AD.7,8
Recently, the FDA approved higher daily doses of donepezil (23 mg) and memantine (28 mg) for moderate to severe AD on the basis of positive phase III trial results.9-11 Donepezil, 23 mg/d, currently is marketed in the United States; the availability date for memantine, 28 mg/d, was undetermined at press time.
Table 1
FDA-approved treatments for Alzheimer’s disease
| Drug | Maximum daily dose | Mechanism of action | Indication | Common side effects/comments |
|---|---|---|---|---|
| Tacrine | 160 mg/d | ChEI | Mild to moderate AD | Nausea, vomiting, loss of appetite, diarrhea. First ChEI to be approved, but rarely used because of associated possible hepatotoxicity |
| Donepezil | 10 mg/d | ChEI | All stages of AD | Nausea, vomiting, loss of appetite, diarrhea, sleep disturbance |
| Rivastigmine | 12 mg/d | ChEI | Mild to moderate AD | Nausea, vomiting, diarrhea, weight loss, loss of appetite |
| Galantamine | 24 mg/d | ChEI | Mild to moderate AD | Nausea, vomiting, diarrhea, weight loss, loss of appetite |
| Memantine | 20 mg/d | NMDA receptor antagonist | Moderate to severe AD | Dizziness, headache, constipation, confusion |
| Galantamine ER | 24 mg/d | ChEI | Mild to moderate AD | Nausea, vomiting, diarrhea, weight loss, loss of appetite |
| Rivastigmine transdermal system | 9.5 mg/d | ChEI | Mild to moderate AD | Nausea, vomiting, diarrhea, weight loss, loss of appetite |
| Donepezil 23 | 23 mg/d | ChEI | Moderate to severe AD | Nausea, vomiting, diarrhea |
| Memantine ER | 28 mg/d | NMDA receptor antagonist | Moderate to severe AD | Dizziness, headache, constipation, confusion |
| AD: Alzheimer’s disease; ChEI: cholinesterase inhibitor; ER: extended release; NMDA: N-methyl-D-aspartate Source: References 3,4 | ||||
High-dose donepezil (23 mg/d)
Cognitive decline with AD has been associated with increasing loss of cholinergic neurons and cholinergic activities, particularly in areas associated with memory/cognition and learning, including cortical areas involving the temporal lobe, hippocampus, and nucleus basalis of Meynert.12-14 In addition, evidence suggests that increasing levels of acetylcholine by using ChEIs can enhance cognitive function.13,15
Donepezil is a selective, reversible ChEI believed to enhance central cholinergic function.15 Randomized clinical trials assessing dose-response with donepezil, 5 mg/d and 10 mg/d, have demonstrated more benefit in cognition with either dose than placebo. The 10 mg/d dose was more effective than 5 mg/d in patients with mild to moderate and severe AD.16-18 In patients with advanced AD who are stable on 5 mg/d, increasing to 10 mg/d could slow the progression of cognitive decline.18
Rationale for higher doses. Positron emission tomography studies have shown that at stable doses of donepezil, 5 mg/d or 10 mg/d, average cortical acetylcholinesterase (AChE) inhibition was <30%.19,20 Based on these findings, researchers thought that cortical AChE inhibition may be suboptimal with donepezil, 10 mg/d, and that higher doses of ChEI may be required in patients with more advanced AD—and therefore more cholinergic loss—for adequate cholinesterase inhibition. In a pilot study of patients with mild to moderate AD, higher doses of donepezil (15 mg/d and 20 mg/d) were reported to be safe and well tolerated.21
The 23-mg/d donepezil formulation was developed to provide a higher dose administered once daily without a sharp rise in peak concentration. The FDA approved donepezil, 23 mg/d, for patients with moderate to severe AD on the basis of phase III trial results.9,22 In a randomized, double-blind, multicenter, head-to-head clinical trial, >1,400 patients with moderate to severe AD (Mini-Mental State Exam [MMSE]: 0 to 20) on a stable dose of donepezil, 10 mg/d, for ≥3 months were randomly assigned to receive high-dose donepezil (23 mg/d) or standard-dose donepezil (10 mg/d) for 24 weeks.9,22 Patients in the 23-mg/d group showed a statistically significant improvement in cognition compared with the 10-mg/d group. The difference between groups on a measure of global improvement was not significant.9,22 However, in a post-hoc analysis, it was demonstrated that a subgroup of patients with more severe cognitive impairment (baseline MMSE: 0 to 16), showed significant improvement in cognition as well as global functioning.9
Overall, treatment-emergent adverse events (TEAEs) during the study were higher in patients receiving 23 mg/d (74%) than those receiving 10 mg/d (64%). The most common TEAEs in the 23-mg/d and 10-mg/d groups were nausea (12% vs 3%, respectively), vomiting (9% vs 3%), and diarrhea (8% vs 5%) (Table 2).22 These gastrointestinal adverse effects were more frequent during the first month of treatment and were relatively infrequent beyond 1 month. Serious TEAEs, such as falls, urinary tract infection, pneumonia, syncope, aggression, and confusional state, were noted in a similar proportion of patients in the 23-mg/d and 10-mg/d groups; most of these were considered unrelated to treatment. No drug-related deaths occurred during the study. High-dose (23 mg/d) donepezil generally was well tolerated, with a typical ChEI safety profile but superior efficacy.
A recent commentary discussed the issue of effect size and whether a 2.2-point difference on a 100-point scale (the Severe Impairment Battery [SIB]) is clinically meaningful.23 As with all anti-dementia therapies, in any cohort some patients will gain considerably more than 2.2 points on the SIB, which is clinically significant. A 6-month trial is recommended to identify these optimal responders.
Table 2
High-dose vs standard-dose donepezil: Treatment-emergent adverse events
| Adverse event | Donepezil, 23 mg/d | Donepezil,10 mg/d |
|---|---|---|
| Nausea | 12% | 3% |
| Vomiting | 9% | 3% |
| Diarrhea | 8% | 5% |
| Anorexia | 5% | 2% |
| Dizziness | 5% | 3% |
| Weight decrease | 5% | 3% |
| Headache | 4% | 3% |
| Insomnia | 3% | 2% |
| Urinary incontinence | 3% | 1% |
| Fatigue | 2% | 1% |
| Weakness | 2% | 1% |
| Somnolence | 2% | 1% |
| Contusion | 2% | 0% |
| Source: Reference 22 | ||
High-dose memantine
Memantine is an NMDA receptor antagonist, which works on glutamate, an ubiquitous neurotransmitter in the brain that serves many functions. For reasons that are not fully understood, in AD glutamate becomes excitotoxic and causes neuronal death.
Some researchers have hypothesized that if safe and well tolerated, a memantine dose >20 mg/d may have better efficacy than a lower dose. Memantine’s manufacturer has developed an extended-release (ER), once-daily formulation of memantine, 28 mg/d, to improve adherence and possibly increase efficacy.10,11 Because of memantine ER’s relatively slow absorption rate and longer median Tmax, of 12 hours, there is minimal fluctuation in plasma levels during steady-state dosing intervals compared with the immediate-release (IR) formulation.10
In a phase I study of 24 healthy volunteers that investigated the safety, tolerability, and pharmacokinetics of memantine ER, 28 mg/d, TEAEs were mild; the most common were headache, somnolence, and dizziness.10 During memantine treatment, there were no serious adverse events, potential significant changes in patients’ vital signs, or deaths.
Memantine ER plus ChEI. A multicenter, multinational, randomized, double-blind study compared memantine ER, 28 mg/d, and placebo in patients with moderate to severe AD (MMSE: 3 to 14).11 All patients were receiving concurrent, stable ChEI treatment (donepezil, rivastigmine, or galantamine) for ≥3 months before the study. Patients treated with memantine ER, 28 mg/d, and ChEI (n = 342) showed a significant improvement compared with the placebo/ChEI group (n = 335) in cognition and global functioning. Patients receiving memantine/ChEI also showed statistically significant benefits on behavior and verbal fluency testing compared with patients receiving placebo/ChEI. Memantine was well tolerated; most adverse events were mild or moderate. The most common adverse events in the memantine/ChEI group that occurred at a higher rate relative to the placebo/ChEI group were headache (5.6% vs 5.1%, respectively), diarrhea (5.0% vs 3.9%), and dizziness (4.7% vs 1.5%). There were no deaths related to memantine (Table 3).11
Memantine ER, 28 mg/d, may be tolerated better than the IR formulation because of less plasma level fluctuation during the steady-state dosing interval. Also, memantine ER, 28 mg/d, may offer better efficacy over memantine IR, 20 mg/d, because of dose-dependent cognitive, global, and behavioral effects. In addition, once-daily dosing of memantine ER may improve adherence compared with the IR formulation.24
In patients with severe renal impairment, dosage of memantine IR should be reduced from 20 mg/d to 10 mg/d.25 However, there is no available information regarding the dosing, safety, and tolerability of memantine ER, 28 mg/d, in patients with renal disease.
Table3
High-dose memantine: Treatment-emergent adverse eventsa
| Adverse event | Placebo (n = 335) | Memantine ER (n = 341) |
|---|---|---|
| Any TEAE | 214 (63.9%) | 214 (62.8%) |
| Fall | 26 (7.8%) | 19 (5.6%) |
| Urinary tract infection | 24 (7.2%) | 19 (5.6%) |
| Headache | 17 (5.1%) | 19 (5.6%) |
| Diarrhea | 13 (3.9%) | 17 (5.0%) |
| Dizziness | 5 (1.5%) | 16 (4.7%) |
| Influenza | 9 (2.7%) | 15 (4.4%) |
| Insomnia | 16 (4.8%) | 14 (4.1%) |
| Agitation | 15 (4.5%) | 14 (4.1%) |
| Hypertension | 8 (2.4%) | 13 (3.8%) |
| Anxiety | 9 (2.7%) | 12 (3.5%) |
| Depression | 5 (1.5%) | 11 (3.2%) |
| Weight increased | 3 (0.9%) | 11 (3.2%) |
| Constipation | 4 (1.2%) | 10 (2.9%) |
| Somnolence | 4 (1.2%) | 10 (2.9%) |
| Back pain | 2 (0.6%) | 9 (2.6%) |
| Aggression | 5 (1.5%) | 8 (2.3%) |
| Hypotension | 5 (1.5%) | 7 (2.1%) |
| Vomiting | 4 (1.2%) | 7 (2.1%) |
| Abdominal pain | 2 (0.6%) | 7 (2.1%) |
| Nasopharyngitis | 10 (3.0%) | 6 (1.8%) |
| Confusional state | 7 (2.1%) | 6 (1.8%) |
| Weight decreased | 11 (3.3%) | 5 (1.5%) |
| Nausea | 7 (2.1%) | 5 (1.5%) |
| Irritability | 8 (2.4%) | 4 (1.2%) |
| Cough | 8 (2.4%) | 3 (0.9%) |
| aData [n (%)] include all adverse events experienced by ≥2% patients in either group (safety population). Adverse events that were experienced at twice the rate in 1 group compared with the other are indicated by bold type ER: extended-release (28 mg); TEAE: treatment-emergent adverse event Source: Reference 11 | ||
Recommendations
Because there are few FDA-approved treatments for AD, higher doses of donepezil or memantine may be an option for patients who have “maxed out” on their AD therapy or no longer respond to lower doses. Higher doses of donepezil (23 mg/d) and memantine (28 mg/d) could improve medication adherence because both are once-daily preparations. In clinical trials, donepezil, 23 mg/d, was more effective than donepezil, 10 mg/d.9 Whether memantine ER, 28 mg/d, is superior to memantine IR, 20 mg/d, needs to be investigated in head-to-head, double-blind, controlled studies.
For patients with moderate to severe AD, donepezil, 23 mg, is associated with greater benefits in cognition compared with donepezil, 10 mg/d.9 Similarly, because of potentially superior efficacy because of a higher dose, memantine ER, 28 mg, might best help patients with moderate to severe AD, specifically those who either don’t respond or lose response to memantine IR, 20 mg/d. Combining a ChEI, such as donepezil, with memantine is associated with slower cognitive decline and short and long-term benefits on measures of cognition, activities of daily living, global outcome, and behavior.7,26 However, additional clinical trials are needed to assess the safety, tolerability, and efficacy of combination therapy with higher doses of donepezil and memantine ER.
Related Resources
- Alzheimer’s Disease Education and Referral Center. www.nia.nih.gov/Alzheimers.
- Lleó A, Greenberg SM, Growdon JH. Current pharmacotherapy for Alzheimer’s disease. Annu Rev Med. 2006;57:513-533.
Drug Brand Names
- Donepezil • Aricept
- Galantamine • Razadyne
- Memantine • Namenda
- Rivastigmine • Exelon
- Tacrine • Cognex
Disclosures
Dr. Grossberg’s academic department has received research funding from Forest Pharmaceuticals and Pfizer Inc. Dr. Grossberg has received grant/research support from Baxter BioScience, Forest Pharmaceuticals, Janssen, the National Institutes of Health, Novartis, and Pfizer, Inc.; is a consultant to Baxter BioScience, Forest Pharmaceuticals, Merck, Novartis, and Otsuka; and is on the Safety Monitoring Committee for Merck.
Dr. Singh reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Alzheimer’s Association, Thies W, Bleiler L. 2011 Alzheimer’s disease facts and figures. Alzheimers Dement. 2011;7(2):208-244.
2. Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60(8):1119-1122.
3. Alzheimer’s Disease Education and Referral Center. Alzheimer’s disease medications. http://www.nia.nih.gov/alzheimers/publication/alzheimers-disease-medications-fact-sheet. Accessed May 10 2012.
4. Osborn GG, Saunders AV. Current treatments for patients with Alzheimer disease. J Am Osteopath Assoc. 2010;110(9 suppl 8):S16-S26.
5. Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148(5):379-397.
6. Cummings JL. Alzheimer’s disease. N Engl J Med. 2004;351(1):56-67.
7. Tariot PN, Farlow MR, Grossberg GT, et al. Memantine Study Group. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317-324.
8. Xiong G, Doraiswamy PM. Combination drug therapy for Alzheimer’s disease: what is evidence-based and what is not? Geriatrics. 2005;60(6):22-26.
9. Farlow MR, Salloway S, Tariot PN, et al. Effectiveness and tolerability of high (23 mg/d) versus standard-dose (10 mg/d) donepezil in moderate to severe Alzheimer’s disease: a 24-week, randomized, double-blind study. Clin Ther. 2010;32(7):1234-1251.
10. Periclou A, Hu Y. Extended-release memantine capsule (28 mg once daily): a multiple dose, open-label study evaluating steady-state pharmacokinetics in healthy volunteers. Poster presented at 11th International Conference on Alzheimer’s Disease; July 26-31, 2008; Chicago, IL.
11. Grossberg GT, Manes F, Allegri R, et al. A multinational, randomized, double-blind, placebo-controlled, parallel-group trial of memantine extended-release capsule (28 mg, once daily) in patients with moderate to severe Alzheimer’s disease. Poster presented at 11th International Conference on Alzheimer’s Disease; July 26-31, 2008; Chicago, IL.
12. Geula C, Mesulam MM. Systematic regional variations in the loss of cortical cholinergic fibers in Alzheimer’s disease. Cereb Cortex. 1996;6(2):165-177.
13. Whitehouse PJ. The cholinergic deficit in Alzheimer’s disease. J Clin Psychiatry. 1998;59(suppl 13):19-22.
14. Teipel SJ, Flatz WH, Heinsen H, et al. Measurement of basal forebrain atrophy in Alzheimer’s disease using MRI. Brain. 2005;128(11):2626-2644.
15. Shintani EY, Uchida KM. Donepezil: an anticholinesterase inhibitor for Alzheimer’s disease. Am J Health Syst Pharm. 1997;54(24):2805-2810.
16. Homma A, Imai Y, Tago H, et al. Donepezil treatment of patients with severe Alzheimer’s disease in a Japanese population: results from a 24-week, double-blind, placebo-controlled, randomized trial. Dement Geriatr Cogn Disord. 2008;25(5):399-407.
17. Whitehead A, Perdomo C, Pratt RD, et al. Donepezil for the symptomatic treatment of patients with mild to moderate Alzheimer’s disease: a meta-analysis of individual patient data from randomised controlled trials. Int J Geriatr Psychiatry. 2004;19(7):624-633.
18. Nozawa M, Ichimiya Y, Nozawa E, et al. Clinical effects of high oral dose of donepezil for patients with Alzheimer’s disease in Japan. Psychogeriatrics. 2009;9(2):50-55.
19. Kuhl DE, Minoshima S, Frey KA, et al. Limited donepezil inhibition of acetylcholinesterase measured with positron emission tomography in living Alzheimer cerebral cortex. Ann Neurol. 2000;48(3):391-395.
20. Bohnen NI, Kaufer DI, Hendrickson R, et al. Degree of inhibition of cortical acetylcholinesterase activity and cognitive effects by donepezil treatment in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2005;76(3):315-319.
21. Doody RS, Corey-Bloom J, Zhang R, et al. Safety and tolerability of donepezil at doses up to 20 mg/day: results from a pilot study in patients with Alzheimer’s disease. Drugs Aging. 2008;25(2):163-174.
22. Aricept [package insert]. Woodcliff Lake NJ: Eisai Co.; 2012.
23. Schwartz LM, Woloshin S. How the FDA forgot the evidence: the case of donepezil 23 mg. BMJ. 2012;344:e1086.-doi: 10.1136/bmj.e1086.
24. Saini SD, Schoenfeld P, Kaulback K, et al. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009;15(6):e22-e33.
25. Periclou A, Ventura D, Rao N, et al. Pharmacokinetic study of memantine in healthy and renally impaired subjects. Clin Pharmacol Ther. 2006;79(1):134-143.
26. Atri A, Shaughnessy LW, Locascio JJ, et al. Long-term course and effectiveness of combination therapy in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22(3):209-221.
Although cholinesterase inhibitors (ChEIs) and memantine at standard doses may slow the progression of Alzheimer’s disease (AD) as assessed by cognitive, functional, and global measures, this effect is relatively modest. For the estimated 5.4 million Americans with AD1—more than one-half of whom have moderate to severe disease2—there is a great need for new approaches to slow AD progression.
High doses of donepezil or memantine may be the next step in achieving better results than standard pharmacologic treatments for AD. This article presents the possible benefits and indications for high doses of donepezil (23 mg/d) and memantine (28 mg/d) for managing moderate to severe AD and their safety and tolerability profiles.
Current treatments offer modest benefits
AD treatments comprise 2 categories: ChEIs (donepezil, rivastigmine, and galantamine) and the N-methyl-D-aspartate (NMDA) receptor antagonist memantine (Table 1).3,4 All ChEIs are FDA-approved for mild to moderate AD; donepezil also is approved for severe AD. Memantine is approved for moderate to severe AD, either alone or in combination with ChEIs. Until recently, the maximum FDA-approved doses were donepezil, 10 mg/d, and memantine, 20 mg/d. However, these dosages are associated with only modest beneficial effects in managing cognitive deterioration in patients with moderate to severe dementia.5,6 Studies have reported that combining a ChEI, such as donepezil, and memantine is well tolerated and may result in synergistic benefits by affecting different neurotransmitters in patients with moderate to severe AD.7,8
Recently, the FDA approved higher daily doses of donepezil (23 mg) and memantine (28 mg) for moderate to severe AD on the basis of positive phase III trial results.9-11 Donepezil, 23 mg/d, currently is marketed in the United States; the availability date for memantine, 28 mg/d, was undetermined at press time.
Table 1
FDA-approved treatments for Alzheimer’s disease
| Drug | Maximum daily dose | Mechanism of action | Indication | Common side effects/comments |
|---|---|---|---|---|
| Tacrine | 160 mg/d | ChEI | Mild to moderate AD | Nausea, vomiting, loss of appetite, diarrhea. First ChEI to be approved, but rarely used because of associated possible hepatotoxicity |
| Donepezil | 10 mg/d | ChEI | All stages of AD | Nausea, vomiting, loss of appetite, diarrhea, sleep disturbance |
| Rivastigmine | 12 mg/d | ChEI | Mild to moderate AD | Nausea, vomiting, diarrhea, weight loss, loss of appetite |
| Galantamine | 24 mg/d | ChEI | Mild to moderate AD | Nausea, vomiting, diarrhea, weight loss, loss of appetite |
| Memantine | 20 mg/d | NMDA receptor antagonist | Moderate to severe AD | Dizziness, headache, constipation, confusion |
| Galantamine ER | 24 mg/d | ChEI | Mild to moderate AD | Nausea, vomiting, diarrhea, weight loss, loss of appetite |
| Rivastigmine transdermal system | 9.5 mg/d | ChEI | Mild to moderate AD | Nausea, vomiting, diarrhea, weight loss, loss of appetite |
| Donepezil 23 | 23 mg/d | ChEI | Moderate to severe AD | Nausea, vomiting, diarrhea |
| Memantine ER | 28 mg/d | NMDA receptor antagonist | Moderate to severe AD | Dizziness, headache, constipation, confusion |
| AD: Alzheimer’s disease; ChEI: cholinesterase inhibitor; ER: extended release; NMDA: N-methyl-D-aspartate Source: References 3,4 | ||||
High-dose donepezil (23 mg/d)
Cognitive decline with AD has been associated with increasing loss of cholinergic neurons and cholinergic activities, particularly in areas associated with memory/cognition and learning, including cortical areas involving the temporal lobe, hippocampus, and nucleus basalis of Meynert.12-14 In addition, evidence suggests that increasing levels of acetylcholine by using ChEIs can enhance cognitive function.13,15
Donepezil is a selective, reversible ChEI believed to enhance central cholinergic function.15 Randomized clinical trials assessing dose-response with donepezil, 5 mg/d and 10 mg/d, have demonstrated more benefit in cognition with either dose than placebo. The 10 mg/d dose was more effective than 5 mg/d in patients with mild to moderate and severe AD.16-18 In patients with advanced AD who are stable on 5 mg/d, increasing to 10 mg/d could slow the progression of cognitive decline.18
Rationale for higher doses. Positron emission tomography studies have shown that at stable doses of donepezil, 5 mg/d or 10 mg/d, average cortical acetylcholinesterase (AChE) inhibition was <30%.19,20 Based on these findings, researchers thought that cortical AChE inhibition may be suboptimal with donepezil, 10 mg/d, and that higher doses of ChEI may be required in patients with more advanced AD—and therefore more cholinergic loss—for adequate cholinesterase inhibition. In a pilot study of patients with mild to moderate AD, higher doses of donepezil (15 mg/d and 20 mg/d) were reported to be safe and well tolerated.21
The 23-mg/d donepezil formulation was developed to provide a higher dose administered once daily without a sharp rise in peak concentration. The FDA approved donepezil, 23 mg/d, for patients with moderate to severe AD on the basis of phase III trial results.9,22 In a randomized, double-blind, multicenter, head-to-head clinical trial, >1,400 patients with moderate to severe AD (Mini-Mental State Exam [MMSE]: 0 to 20) on a stable dose of donepezil, 10 mg/d, for ≥3 months were randomly assigned to receive high-dose donepezil (23 mg/d) or standard-dose donepezil (10 mg/d) for 24 weeks.9,22 Patients in the 23-mg/d group showed a statistically significant improvement in cognition compared with the 10-mg/d group. The difference between groups on a measure of global improvement was not significant.9,22 However, in a post-hoc analysis, it was demonstrated that a subgroup of patients with more severe cognitive impairment (baseline MMSE: 0 to 16), showed significant improvement in cognition as well as global functioning.9
Overall, treatment-emergent adverse events (TEAEs) during the study were higher in patients receiving 23 mg/d (74%) than those receiving 10 mg/d (64%). The most common TEAEs in the 23-mg/d and 10-mg/d groups were nausea (12% vs 3%, respectively), vomiting (9% vs 3%), and diarrhea (8% vs 5%) (Table 2).22 These gastrointestinal adverse effects were more frequent during the first month of treatment and were relatively infrequent beyond 1 month. Serious TEAEs, such as falls, urinary tract infection, pneumonia, syncope, aggression, and confusional state, were noted in a similar proportion of patients in the 23-mg/d and 10-mg/d groups; most of these were considered unrelated to treatment. No drug-related deaths occurred during the study. High-dose (23 mg/d) donepezil generally was well tolerated, with a typical ChEI safety profile but superior efficacy.
A recent commentary discussed the issue of effect size and whether a 2.2-point difference on a 100-point scale (the Severe Impairment Battery [SIB]) is clinically meaningful.23 As with all anti-dementia therapies, in any cohort some patients will gain considerably more than 2.2 points on the SIB, which is clinically significant. A 6-month trial is recommended to identify these optimal responders.
Table 2
High-dose vs standard-dose donepezil: Treatment-emergent adverse events
| Adverse event | Donepezil, 23 mg/d | Donepezil,10 mg/d |
|---|---|---|
| Nausea | 12% | 3% |
| Vomiting | 9% | 3% |
| Diarrhea | 8% | 5% |
| Anorexia | 5% | 2% |
| Dizziness | 5% | 3% |
| Weight decrease | 5% | 3% |
| Headache | 4% | 3% |
| Insomnia | 3% | 2% |
| Urinary incontinence | 3% | 1% |
| Fatigue | 2% | 1% |
| Weakness | 2% | 1% |
| Somnolence | 2% | 1% |
| Contusion | 2% | 0% |
| Source: Reference 22 | ||
High-dose memantine
Memantine is an NMDA receptor antagonist, which works on glutamate, an ubiquitous neurotransmitter in the brain that serves many functions. For reasons that are not fully understood, in AD glutamate becomes excitotoxic and causes neuronal death.
Some researchers have hypothesized that if safe and well tolerated, a memantine dose >20 mg/d may have better efficacy than a lower dose. Memantine’s manufacturer has developed an extended-release (ER), once-daily formulation of memantine, 28 mg/d, to improve adherence and possibly increase efficacy.10,11 Because of memantine ER’s relatively slow absorption rate and longer median Tmax, of 12 hours, there is minimal fluctuation in plasma levels during steady-state dosing intervals compared with the immediate-release (IR) formulation.10
In a phase I study of 24 healthy volunteers that investigated the safety, tolerability, and pharmacokinetics of memantine ER, 28 mg/d, TEAEs were mild; the most common were headache, somnolence, and dizziness.10 During memantine treatment, there were no serious adverse events, potential significant changes in patients’ vital signs, or deaths.
Memantine ER plus ChEI. A multicenter, multinational, randomized, double-blind study compared memantine ER, 28 mg/d, and placebo in patients with moderate to severe AD (MMSE: 3 to 14).11 All patients were receiving concurrent, stable ChEI treatment (donepezil, rivastigmine, or galantamine) for ≥3 months before the study. Patients treated with memantine ER, 28 mg/d, and ChEI (n = 342) showed a significant improvement compared with the placebo/ChEI group (n = 335) in cognition and global functioning. Patients receiving memantine/ChEI also showed statistically significant benefits on behavior and verbal fluency testing compared with patients receiving placebo/ChEI. Memantine was well tolerated; most adverse events were mild or moderate. The most common adverse events in the memantine/ChEI group that occurred at a higher rate relative to the placebo/ChEI group were headache (5.6% vs 5.1%, respectively), diarrhea (5.0% vs 3.9%), and dizziness (4.7% vs 1.5%). There were no deaths related to memantine (Table 3).11
Memantine ER, 28 mg/d, may be tolerated better than the IR formulation because of less plasma level fluctuation during the steady-state dosing interval. Also, memantine ER, 28 mg/d, may offer better efficacy over memantine IR, 20 mg/d, because of dose-dependent cognitive, global, and behavioral effects. In addition, once-daily dosing of memantine ER may improve adherence compared with the IR formulation.24
In patients with severe renal impairment, dosage of memantine IR should be reduced from 20 mg/d to 10 mg/d.25 However, there is no available information regarding the dosing, safety, and tolerability of memantine ER, 28 mg/d, in patients with renal disease.
Table3
High-dose memantine: Treatment-emergent adverse eventsa
| Adverse event | Placebo (n = 335) | Memantine ER (n = 341) |
|---|---|---|
| Any TEAE | 214 (63.9%) | 214 (62.8%) |
| Fall | 26 (7.8%) | 19 (5.6%) |
| Urinary tract infection | 24 (7.2%) | 19 (5.6%) |
| Headache | 17 (5.1%) | 19 (5.6%) |
| Diarrhea | 13 (3.9%) | 17 (5.0%) |
| Dizziness | 5 (1.5%) | 16 (4.7%) |
| Influenza | 9 (2.7%) | 15 (4.4%) |
| Insomnia | 16 (4.8%) | 14 (4.1%) |
| Agitation | 15 (4.5%) | 14 (4.1%) |
| Hypertension | 8 (2.4%) | 13 (3.8%) |
| Anxiety | 9 (2.7%) | 12 (3.5%) |
| Depression | 5 (1.5%) | 11 (3.2%) |
| Weight increased | 3 (0.9%) | 11 (3.2%) |
| Constipation | 4 (1.2%) | 10 (2.9%) |
| Somnolence | 4 (1.2%) | 10 (2.9%) |
| Back pain | 2 (0.6%) | 9 (2.6%) |
| Aggression | 5 (1.5%) | 8 (2.3%) |
| Hypotension | 5 (1.5%) | 7 (2.1%) |
| Vomiting | 4 (1.2%) | 7 (2.1%) |
| Abdominal pain | 2 (0.6%) | 7 (2.1%) |
| Nasopharyngitis | 10 (3.0%) | 6 (1.8%) |
| Confusional state | 7 (2.1%) | 6 (1.8%) |
| Weight decreased | 11 (3.3%) | 5 (1.5%) |
| Nausea | 7 (2.1%) | 5 (1.5%) |
| Irritability | 8 (2.4%) | 4 (1.2%) |
| Cough | 8 (2.4%) | 3 (0.9%) |
| aData [n (%)] include all adverse events experienced by ≥2% patients in either group (safety population). Adverse events that were experienced at twice the rate in 1 group compared with the other are indicated by bold type ER: extended-release (28 mg); TEAE: treatment-emergent adverse event Source: Reference 11 | ||
Recommendations
Because there are few FDA-approved treatments for AD, higher doses of donepezil or memantine may be an option for patients who have “maxed out” on their AD therapy or no longer respond to lower doses. Higher doses of donepezil (23 mg/d) and memantine (28 mg/d) could improve medication adherence because both are once-daily preparations. In clinical trials, donepezil, 23 mg/d, was more effective than donepezil, 10 mg/d.9 Whether memantine ER, 28 mg/d, is superior to memantine IR, 20 mg/d, needs to be investigated in head-to-head, double-blind, controlled studies.
For patients with moderate to severe AD, donepezil, 23 mg, is associated with greater benefits in cognition compared with donepezil, 10 mg/d.9 Similarly, because of potentially superior efficacy because of a higher dose, memantine ER, 28 mg, might best help patients with moderate to severe AD, specifically those who either don’t respond or lose response to memantine IR, 20 mg/d. Combining a ChEI, such as donepezil, with memantine is associated with slower cognitive decline and short and long-term benefits on measures of cognition, activities of daily living, global outcome, and behavior.7,26 However, additional clinical trials are needed to assess the safety, tolerability, and efficacy of combination therapy with higher doses of donepezil and memantine ER.
Related Resources
- Alzheimer’s Disease Education and Referral Center. www.nia.nih.gov/Alzheimers.
- Lleó A, Greenberg SM, Growdon JH. Current pharmacotherapy for Alzheimer’s disease. Annu Rev Med. 2006;57:513-533.
Drug Brand Names
- Donepezil • Aricept
- Galantamine • Razadyne
- Memantine • Namenda
- Rivastigmine • Exelon
- Tacrine • Cognex
Disclosures
Dr. Grossberg’s academic department has received research funding from Forest Pharmaceuticals and Pfizer Inc. Dr. Grossberg has received grant/research support from Baxter BioScience, Forest Pharmaceuticals, Janssen, the National Institutes of Health, Novartis, and Pfizer, Inc.; is a consultant to Baxter BioScience, Forest Pharmaceuticals, Merck, Novartis, and Otsuka; and is on the Safety Monitoring Committee for Merck.
Dr. Singh reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Although cholinesterase inhibitors (ChEIs) and memantine at standard doses may slow the progression of Alzheimer’s disease (AD) as assessed by cognitive, functional, and global measures, this effect is relatively modest. For the estimated 5.4 million Americans with AD1—more than one-half of whom have moderate to severe disease2—there is a great need for new approaches to slow AD progression.
High doses of donepezil or memantine may be the next step in achieving better results than standard pharmacologic treatments for AD. This article presents the possible benefits and indications for high doses of donepezil (23 mg/d) and memantine (28 mg/d) for managing moderate to severe AD and their safety and tolerability profiles.
Current treatments offer modest benefits
AD treatments comprise 2 categories: ChEIs (donepezil, rivastigmine, and galantamine) and the N-methyl-D-aspartate (NMDA) receptor antagonist memantine (Table 1).3,4 All ChEIs are FDA-approved for mild to moderate AD; donepezil also is approved for severe AD. Memantine is approved for moderate to severe AD, either alone or in combination with ChEIs. Until recently, the maximum FDA-approved doses were donepezil, 10 mg/d, and memantine, 20 mg/d. However, these dosages are associated with only modest beneficial effects in managing cognitive deterioration in patients with moderate to severe dementia.5,6 Studies have reported that combining a ChEI, such as donepezil, and memantine is well tolerated and may result in synergistic benefits by affecting different neurotransmitters in patients with moderate to severe AD.7,8
Recently, the FDA approved higher daily doses of donepezil (23 mg) and memantine (28 mg) for moderate to severe AD on the basis of positive phase III trial results.9-11 Donepezil, 23 mg/d, currently is marketed in the United States; the availability date for memantine, 28 mg/d, was undetermined at press time.
Table 1
FDA-approved treatments for Alzheimer’s disease
| Drug | Maximum daily dose | Mechanism of action | Indication | Common side effects/comments |
|---|---|---|---|---|
| Tacrine | 160 mg/d | ChEI | Mild to moderate AD | Nausea, vomiting, loss of appetite, diarrhea. First ChEI to be approved, but rarely used because of associated possible hepatotoxicity |
| Donepezil | 10 mg/d | ChEI | All stages of AD | Nausea, vomiting, loss of appetite, diarrhea, sleep disturbance |
| Rivastigmine | 12 mg/d | ChEI | Mild to moderate AD | Nausea, vomiting, diarrhea, weight loss, loss of appetite |
| Galantamine | 24 mg/d | ChEI | Mild to moderate AD | Nausea, vomiting, diarrhea, weight loss, loss of appetite |
| Memantine | 20 mg/d | NMDA receptor antagonist | Moderate to severe AD | Dizziness, headache, constipation, confusion |
| Galantamine ER | 24 mg/d | ChEI | Mild to moderate AD | Nausea, vomiting, diarrhea, weight loss, loss of appetite |
| Rivastigmine transdermal system | 9.5 mg/d | ChEI | Mild to moderate AD | Nausea, vomiting, diarrhea, weight loss, loss of appetite |
| Donepezil 23 | 23 mg/d | ChEI | Moderate to severe AD | Nausea, vomiting, diarrhea |
| Memantine ER | 28 mg/d | NMDA receptor antagonist | Moderate to severe AD | Dizziness, headache, constipation, confusion |
| AD: Alzheimer’s disease; ChEI: cholinesterase inhibitor; ER: extended release; NMDA: N-methyl-D-aspartate Source: References 3,4 | ||||
High-dose donepezil (23 mg/d)
Cognitive decline with AD has been associated with increasing loss of cholinergic neurons and cholinergic activities, particularly in areas associated with memory/cognition and learning, including cortical areas involving the temporal lobe, hippocampus, and nucleus basalis of Meynert.12-14 In addition, evidence suggests that increasing levels of acetylcholine by using ChEIs can enhance cognitive function.13,15
Donepezil is a selective, reversible ChEI believed to enhance central cholinergic function.15 Randomized clinical trials assessing dose-response with donepezil, 5 mg/d and 10 mg/d, have demonstrated more benefit in cognition with either dose than placebo. The 10 mg/d dose was more effective than 5 mg/d in patients with mild to moderate and severe AD.16-18 In patients with advanced AD who are stable on 5 mg/d, increasing to 10 mg/d could slow the progression of cognitive decline.18
Rationale for higher doses. Positron emission tomography studies have shown that at stable doses of donepezil, 5 mg/d or 10 mg/d, average cortical acetylcholinesterase (AChE) inhibition was <30%.19,20 Based on these findings, researchers thought that cortical AChE inhibition may be suboptimal with donepezil, 10 mg/d, and that higher doses of ChEI may be required in patients with more advanced AD—and therefore more cholinergic loss—for adequate cholinesterase inhibition. In a pilot study of patients with mild to moderate AD, higher doses of donepezil (15 mg/d and 20 mg/d) were reported to be safe and well tolerated.21
The 23-mg/d donepezil formulation was developed to provide a higher dose administered once daily without a sharp rise in peak concentration. The FDA approved donepezil, 23 mg/d, for patients with moderate to severe AD on the basis of phase III trial results.9,22 In a randomized, double-blind, multicenter, head-to-head clinical trial, >1,400 patients with moderate to severe AD (Mini-Mental State Exam [MMSE]: 0 to 20) on a stable dose of donepezil, 10 mg/d, for ≥3 months were randomly assigned to receive high-dose donepezil (23 mg/d) or standard-dose donepezil (10 mg/d) for 24 weeks.9,22 Patients in the 23-mg/d group showed a statistically significant improvement in cognition compared with the 10-mg/d group. The difference between groups on a measure of global improvement was not significant.9,22 However, in a post-hoc analysis, it was demonstrated that a subgroup of patients with more severe cognitive impairment (baseline MMSE: 0 to 16), showed significant improvement in cognition as well as global functioning.9
Overall, treatment-emergent adverse events (TEAEs) during the study were higher in patients receiving 23 mg/d (74%) than those receiving 10 mg/d (64%). The most common TEAEs in the 23-mg/d and 10-mg/d groups were nausea (12% vs 3%, respectively), vomiting (9% vs 3%), and diarrhea (8% vs 5%) (Table 2).22 These gastrointestinal adverse effects were more frequent during the first month of treatment and were relatively infrequent beyond 1 month. Serious TEAEs, such as falls, urinary tract infection, pneumonia, syncope, aggression, and confusional state, were noted in a similar proportion of patients in the 23-mg/d and 10-mg/d groups; most of these were considered unrelated to treatment. No drug-related deaths occurred during the study. High-dose (23 mg/d) donepezil generally was well tolerated, with a typical ChEI safety profile but superior efficacy.
A recent commentary discussed the issue of effect size and whether a 2.2-point difference on a 100-point scale (the Severe Impairment Battery [SIB]) is clinically meaningful.23 As with all anti-dementia therapies, in any cohort some patients will gain considerably more than 2.2 points on the SIB, which is clinically significant. A 6-month trial is recommended to identify these optimal responders.
Table 2
High-dose vs standard-dose donepezil: Treatment-emergent adverse events
| Adverse event | Donepezil, 23 mg/d | Donepezil,10 mg/d |
|---|---|---|
| Nausea | 12% | 3% |
| Vomiting | 9% | 3% |
| Diarrhea | 8% | 5% |
| Anorexia | 5% | 2% |
| Dizziness | 5% | 3% |
| Weight decrease | 5% | 3% |
| Headache | 4% | 3% |
| Insomnia | 3% | 2% |
| Urinary incontinence | 3% | 1% |
| Fatigue | 2% | 1% |
| Weakness | 2% | 1% |
| Somnolence | 2% | 1% |
| Contusion | 2% | 0% |
| Source: Reference 22 | ||
High-dose memantine
Memantine is an NMDA receptor antagonist, which works on glutamate, an ubiquitous neurotransmitter in the brain that serves many functions. For reasons that are not fully understood, in AD glutamate becomes excitotoxic and causes neuronal death.
Some researchers have hypothesized that if safe and well tolerated, a memantine dose >20 mg/d may have better efficacy than a lower dose. Memantine’s manufacturer has developed an extended-release (ER), once-daily formulation of memantine, 28 mg/d, to improve adherence and possibly increase efficacy.10,11 Because of memantine ER’s relatively slow absorption rate and longer median Tmax, of 12 hours, there is minimal fluctuation in plasma levels during steady-state dosing intervals compared with the immediate-release (IR) formulation.10
In a phase I study of 24 healthy volunteers that investigated the safety, tolerability, and pharmacokinetics of memantine ER, 28 mg/d, TEAEs were mild; the most common were headache, somnolence, and dizziness.10 During memantine treatment, there were no serious adverse events, potential significant changes in patients’ vital signs, or deaths.
Memantine ER plus ChEI. A multicenter, multinational, randomized, double-blind study compared memantine ER, 28 mg/d, and placebo in patients with moderate to severe AD (MMSE: 3 to 14).11 All patients were receiving concurrent, stable ChEI treatment (donepezil, rivastigmine, or galantamine) for ≥3 months before the study. Patients treated with memantine ER, 28 mg/d, and ChEI (n = 342) showed a significant improvement compared with the placebo/ChEI group (n = 335) in cognition and global functioning. Patients receiving memantine/ChEI also showed statistically significant benefits on behavior and verbal fluency testing compared with patients receiving placebo/ChEI. Memantine was well tolerated; most adverse events were mild or moderate. The most common adverse events in the memantine/ChEI group that occurred at a higher rate relative to the placebo/ChEI group were headache (5.6% vs 5.1%, respectively), diarrhea (5.0% vs 3.9%), and dizziness (4.7% vs 1.5%). There were no deaths related to memantine (Table 3).11
Memantine ER, 28 mg/d, may be tolerated better than the IR formulation because of less plasma level fluctuation during the steady-state dosing interval. Also, memantine ER, 28 mg/d, may offer better efficacy over memantine IR, 20 mg/d, because of dose-dependent cognitive, global, and behavioral effects. In addition, once-daily dosing of memantine ER may improve adherence compared with the IR formulation.24
In patients with severe renal impairment, dosage of memantine IR should be reduced from 20 mg/d to 10 mg/d.25 However, there is no available information regarding the dosing, safety, and tolerability of memantine ER, 28 mg/d, in patients with renal disease.
Table3
High-dose memantine: Treatment-emergent adverse eventsa
| Adverse event | Placebo (n = 335) | Memantine ER (n = 341) |
|---|---|---|
| Any TEAE | 214 (63.9%) | 214 (62.8%) |
| Fall | 26 (7.8%) | 19 (5.6%) |
| Urinary tract infection | 24 (7.2%) | 19 (5.6%) |
| Headache | 17 (5.1%) | 19 (5.6%) |
| Diarrhea | 13 (3.9%) | 17 (5.0%) |
| Dizziness | 5 (1.5%) | 16 (4.7%) |
| Influenza | 9 (2.7%) | 15 (4.4%) |
| Insomnia | 16 (4.8%) | 14 (4.1%) |
| Agitation | 15 (4.5%) | 14 (4.1%) |
| Hypertension | 8 (2.4%) | 13 (3.8%) |
| Anxiety | 9 (2.7%) | 12 (3.5%) |
| Depression | 5 (1.5%) | 11 (3.2%) |
| Weight increased | 3 (0.9%) | 11 (3.2%) |
| Constipation | 4 (1.2%) | 10 (2.9%) |
| Somnolence | 4 (1.2%) | 10 (2.9%) |
| Back pain | 2 (0.6%) | 9 (2.6%) |
| Aggression | 5 (1.5%) | 8 (2.3%) |
| Hypotension | 5 (1.5%) | 7 (2.1%) |
| Vomiting | 4 (1.2%) | 7 (2.1%) |
| Abdominal pain | 2 (0.6%) | 7 (2.1%) |
| Nasopharyngitis | 10 (3.0%) | 6 (1.8%) |
| Confusional state | 7 (2.1%) | 6 (1.8%) |
| Weight decreased | 11 (3.3%) | 5 (1.5%) |
| Nausea | 7 (2.1%) | 5 (1.5%) |
| Irritability | 8 (2.4%) | 4 (1.2%) |
| Cough | 8 (2.4%) | 3 (0.9%) |
| aData [n (%)] include all adverse events experienced by ≥2% patients in either group (safety population). Adverse events that were experienced at twice the rate in 1 group compared with the other are indicated by bold type ER: extended-release (28 mg); TEAE: treatment-emergent adverse event Source: Reference 11 | ||
Recommendations
Because there are few FDA-approved treatments for AD, higher doses of donepezil or memantine may be an option for patients who have “maxed out” on their AD therapy or no longer respond to lower doses. Higher doses of donepezil (23 mg/d) and memantine (28 mg/d) could improve medication adherence because both are once-daily preparations. In clinical trials, donepezil, 23 mg/d, was more effective than donepezil, 10 mg/d.9 Whether memantine ER, 28 mg/d, is superior to memantine IR, 20 mg/d, needs to be investigated in head-to-head, double-blind, controlled studies.
For patients with moderate to severe AD, donepezil, 23 mg, is associated with greater benefits in cognition compared with donepezil, 10 mg/d.9 Similarly, because of potentially superior efficacy because of a higher dose, memantine ER, 28 mg, might best help patients with moderate to severe AD, specifically those who either don’t respond or lose response to memantine IR, 20 mg/d. Combining a ChEI, such as donepezil, with memantine is associated with slower cognitive decline and short and long-term benefits on measures of cognition, activities of daily living, global outcome, and behavior.7,26 However, additional clinical trials are needed to assess the safety, tolerability, and efficacy of combination therapy with higher doses of donepezil and memantine ER.
Related Resources
- Alzheimer’s Disease Education and Referral Center. www.nia.nih.gov/Alzheimers.
- Lleó A, Greenberg SM, Growdon JH. Current pharmacotherapy for Alzheimer’s disease. Annu Rev Med. 2006;57:513-533.
Drug Brand Names
- Donepezil • Aricept
- Galantamine • Razadyne
- Memantine • Namenda
- Rivastigmine • Exelon
- Tacrine • Cognex
Disclosures
Dr. Grossberg’s academic department has received research funding from Forest Pharmaceuticals and Pfizer Inc. Dr. Grossberg has received grant/research support from Baxter BioScience, Forest Pharmaceuticals, Janssen, the National Institutes of Health, Novartis, and Pfizer, Inc.; is a consultant to Baxter BioScience, Forest Pharmaceuticals, Merck, Novartis, and Otsuka; and is on the Safety Monitoring Committee for Merck.
Dr. Singh reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Alzheimer’s Association, Thies W, Bleiler L. 2011 Alzheimer’s disease facts and figures. Alzheimers Dement. 2011;7(2):208-244.
2. Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60(8):1119-1122.
3. Alzheimer’s Disease Education and Referral Center. Alzheimer’s disease medications. http://www.nia.nih.gov/alzheimers/publication/alzheimers-disease-medications-fact-sheet. Accessed May 10 2012.
4. Osborn GG, Saunders AV. Current treatments for patients with Alzheimer disease. J Am Osteopath Assoc. 2010;110(9 suppl 8):S16-S26.
5. Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148(5):379-397.
6. Cummings JL. Alzheimer’s disease. N Engl J Med. 2004;351(1):56-67.
7. Tariot PN, Farlow MR, Grossberg GT, et al. Memantine Study Group. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317-324.
8. Xiong G, Doraiswamy PM. Combination drug therapy for Alzheimer’s disease: what is evidence-based and what is not? Geriatrics. 2005;60(6):22-26.
9. Farlow MR, Salloway S, Tariot PN, et al. Effectiveness and tolerability of high (23 mg/d) versus standard-dose (10 mg/d) donepezil in moderate to severe Alzheimer’s disease: a 24-week, randomized, double-blind study. Clin Ther. 2010;32(7):1234-1251.
10. Periclou A, Hu Y. Extended-release memantine capsule (28 mg once daily): a multiple dose, open-label study evaluating steady-state pharmacokinetics in healthy volunteers. Poster presented at 11th International Conference on Alzheimer’s Disease; July 26-31, 2008; Chicago, IL.
11. Grossberg GT, Manes F, Allegri R, et al. A multinational, randomized, double-blind, placebo-controlled, parallel-group trial of memantine extended-release capsule (28 mg, once daily) in patients with moderate to severe Alzheimer’s disease. Poster presented at 11th International Conference on Alzheimer’s Disease; July 26-31, 2008; Chicago, IL.
12. Geula C, Mesulam MM. Systematic regional variations in the loss of cortical cholinergic fibers in Alzheimer’s disease. Cereb Cortex. 1996;6(2):165-177.
13. Whitehouse PJ. The cholinergic deficit in Alzheimer’s disease. J Clin Psychiatry. 1998;59(suppl 13):19-22.
14. Teipel SJ, Flatz WH, Heinsen H, et al. Measurement of basal forebrain atrophy in Alzheimer’s disease using MRI. Brain. 2005;128(11):2626-2644.
15. Shintani EY, Uchida KM. Donepezil: an anticholinesterase inhibitor for Alzheimer’s disease. Am J Health Syst Pharm. 1997;54(24):2805-2810.
16. Homma A, Imai Y, Tago H, et al. Donepezil treatment of patients with severe Alzheimer’s disease in a Japanese population: results from a 24-week, double-blind, placebo-controlled, randomized trial. Dement Geriatr Cogn Disord. 2008;25(5):399-407.
17. Whitehead A, Perdomo C, Pratt RD, et al. Donepezil for the symptomatic treatment of patients with mild to moderate Alzheimer’s disease: a meta-analysis of individual patient data from randomised controlled trials. Int J Geriatr Psychiatry. 2004;19(7):624-633.
18. Nozawa M, Ichimiya Y, Nozawa E, et al. Clinical effects of high oral dose of donepezil for patients with Alzheimer’s disease in Japan. Psychogeriatrics. 2009;9(2):50-55.
19. Kuhl DE, Minoshima S, Frey KA, et al. Limited donepezil inhibition of acetylcholinesterase measured with positron emission tomography in living Alzheimer cerebral cortex. Ann Neurol. 2000;48(3):391-395.
20. Bohnen NI, Kaufer DI, Hendrickson R, et al. Degree of inhibition of cortical acetylcholinesterase activity and cognitive effects by donepezil treatment in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2005;76(3):315-319.
21. Doody RS, Corey-Bloom J, Zhang R, et al. Safety and tolerability of donepezil at doses up to 20 mg/day: results from a pilot study in patients with Alzheimer’s disease. Drugs Aging. 2008;25(2):163-174.
22. Aricept [package insert]. Woodcliff Lake NJ: Eisai Co.; 2012.
23. Schwartz LM, Woloshin S. How the FDA forgot the evidence: the case of donepezil 23 mg. BMJ. 2012;344:e1086.-doi: 10.1136/bmj.e1086.
24. Saini SD, Schoenfeld P, Kaulback K, et al. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009;15(6):e22-e33.
25. Periclou A, Ventura D, Rao N, et al. Pharmacokinetic study of memantine in healthy and renally impaired subjects. Clin Pharmacol Ther. 2006;79(1):134-143.
26. Atri A, Shaughnessy LW, Locascio JJ, et al. Long-term course and effectiveness of combination therapy in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22(3):209-221.
1. Alzheimer’s Association, Thies W, Bleiler L. 2011 Alzheimer’s disease facts and figures. Alzheimers Dement. 2011;7(2):208-244.
2. Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60(8):1119-1122.
3. Alzheimer’s Disease Education and Referral Center. Alzheimer’s disease medications. http://www.nia.nih.gov/alzheimers/publication/alzheimers-disease-medications-fact-sheet. Accessed May 10 2012.
4. Osborn GG, Saunders AV. Current treatments for patients with Alzheimer disease. J Am Osteopath Assoc. 2010;110(9 suppl 8):S16-S26.
5. Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148(5):379-397.
6. Cummings JL. Alzheimer’s disease. N Engl J Med. 2004;351(1):56-67.
7. Tariot PN, Farlow MR, Grossberg GT, et al. Memantine Study Group. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317-324.
8. Xiong G, Doraiswamy PM. Combination drug therapy for Alzheimer’s disease: what is evidence-based and what is not? Geriatrics. 2005;60(6):22-26.
9. Farlow MR, Salloway S, Tariot PN, et al. Effectiveness and tolerability of high (23 mg/d) versus standard-dose (10 mg/d) donepezil in moderate to severe Alzheimer’s disease: a 24-week, randomized, double-blind study. Clin Ther. 2010;32(7):1234-1251.
10. Periclou A, Hu Y. Extended-release memantine capsule (28 mg once daily): a multiple dose, open-label study evaluating steady-state pharmacokinetics in healthy volunteers. Poster presented at 11th International Conference on Alzheimer’s Disease; July 26-31, 2008; Chicago, IL.
11. Grossberg GT, Manes F, Allegri R, et al. A multinational, randomized, double-blind, placebo-controlled, parallel-group trial of memantine extended-release capsule (28 mg, once daily) in patients with moderate to severe Alzheimer’s disease. Poster presented at 11th International Conference on Alzheimer’s Disease; July 26-31, 2008; Chicago, IL.
12. Geula C, Mesulam MM. Systematic regional variations in the loss of cortical cholinergic fibers in Alzheimer’s disease. Cereb Cortex. 1996;6(2):165-177.
13. Whitehouse PJ. The cholinergic deficit in Alzheimer’s disease. J Clin Psychiatry. 1998;59(suppl 13):19-22.
14. Teipel SJ, Flatz WH, Heinsen H, et al. Measurement of basal forebrain atrophy in Alzheimer’s disease using MRI. Brain. 2005;128(11):2626-2644.
15. Shintani EY, Uchida KM. Donepezil: an anticholinesterase inhibitor for Alzheimer’s disease. Am J Health Syst Pharm. 1997;54(24):2805-2810.
16. Homma A, Imai Y, Tago H, et al. Donepezil treatment of patients with severe Alzheimer’s disease in a Japanese population: results from a 24-week, double-blind, placebo-controlled, randomized trial. Dement Geriatr Cogn Disord. 2008;25(5):399-407.
17. Whitehead A, Perdomo C, Pratt RD, et al. Donepezil for the symptomatic treatment of patients with mild to moderate Alzheimer’s disease: a meta-analysis of individual patient data from randomised controlled trials. Int J Geriatr Psychiatry. 2004;19(7):624-633.
18. Nozawa M, Ichimiya Y, Nozawa E, et al. Clinical effects of high oral dose of donepezil for patients with Alzheimer’s disease in Japan. Psychogeriatrics. 2009;9(2):50-55.
19. Kuhl DE, Minoshima S, Frey KA, et al. Limited donepezil inhibition of acetylcholinesterase measured with positron emission tomography in living Alzheimer cerebral cortex. Ann Neurol. 2000;48(3):391-395.
20. Bohnen NI, Kaufer DI, Hendrickson R, et al. Degree of inhibition of cortical acetylcholinesterase activity and cognitive effects by donepezil treatment in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2005;76(3):315-319.
21. Doody RS, Corey-Bloom J, Zhang R, et al. Safety and tolerability of donepezil at doses up to 20 mg/day: results from a pilot study in patients with Alzheimer’s disease. Drugs Aging. 2008;25(2):163-174.
22. Aricept [package insert]. Woodcliff Lake NJ: Eisai Co.; 2012.
23. Schwartz LM, Woloshin S. How the FDA forgot the evidence: the case of donepezil 23 mg. BMJ. 2012;344:e1086.-doi: 10.1136/bmj.e1086.
24. Saini SD, Schoenfeld P, Kaulback K, et al. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009;15(6):e22-e33.
25. Periclou A, Ventura D, Rao N, et al. Pharmacokinetic study of memantine in healthy and renally impaired subjects. Clin Pharmacol Ther. 2006;79(1):134-143.
26. Atri A, Shaughnessy LW, Locascio JJ, et al. Long-term course and effectiveness of combination therapy in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22(3):209-221.
Does bupropion exacerbate anxiety?
For many clinicians, bupropion is the “go-to” medication for treating depressed patients who smoke, have concerns about sexual dysfunction side effects, and/or worry about weight gain. Bupropion is FDA-approved for preventing seasonal major depressive episodes in patients with seasonal affective disorder and is indicated as a smoking cessation aid.
“Anxious depression”—defined as depression with high levels of anxiety—is associated with poorer outcomes than “non-anxious” depression.1 Prescribing medications for these patients can be challenging. Some clinicians believe that bupropion exacerbates anxiety and should not be used to treat patients who experience both anxiety and depression.
Reports from our patients and our cumulative clinical experience are key factors in developing expertise in selecting appropriate medications. When informing our patients about what to expect from medications, however, it can be useful to combine anecdotal evidence with knowledge of the facts or lack thereof. Are there data to support or contradict the idea that bupropion can cause anxiety while treating depression?
What the research shows
The drug manufacturer reports a “substantial proportion of patients treated with Wellbutrin experience some degree of increased restlessness, agitation, anxiety, and insomnia, especially shortly after initiation of treatment.”2
In 2001, Rush et al3 published the results of a 16-week study (n=248) assessing pre-treatment anxiety levels and response to sertraline or bupropion. The authors concluded that anxious and depressed patients who received sertraline didn’t experience a superior anxiolytic or antidepressant response compared with bupropion.3 The same authors came to similar conclusions in a retrospective analysis of a pair of 8-week randomized, controlled, double-blind trials of selective serotonin reuptake inhibitors (SSRIs) and bupropion.4
In 2001, Nieuwstraten et al5 compared bupropion with SSRIs for treating depression by reviewing several randomized, double-blind, controlled trials. The relative risk of developing “anxiety/agitation” was 1.32 (95% confidence interval, 0.85 to 2.04), which was not statistically significant.
In a 2008 meta-analysis, Papakostas et al6 pooled individual patient data from 10 randomized, double-blind, placebo-controlled trials. Their aim was to compare the efficacy of bupropion to SSRIs in treating “anxious depression.” They found no difference in timing or degree of improvement in anxiety symptoms between groups based on Hamilton Anxiety Scale or Hamilton Depression Rating Scale—Anxiety-Somatization (HDRS-AS) scores. The authors recommended that antidepressant choice should not be based on concerns about worsening anxiety symptoms in depressed patients.6
Another meta-analysis by Papakostas et al7 of the same 10 randomized, double-blind, placebo-controlled trials suggested SSRIs may confer an advantage over bupropion in treating a subset of patients with “anxious depression,” which they defined as a HDRS-AS score ≥7. The authors noted the advantage was statistically significant, although “modest.”
Other smaller studies suggest that bupropion does not increase anxiety.8,9 A pilot study (N = 24, no placebo control) concluded that bupropion XL was comparable to escitalopram in treating anxiety in outpatients with generalized anxiety disorder.8
Because designing and executing drug trials can be expensive, it is not surprising that most of the evidence cited above derives from pharmaceutical company-sponsored or industry-affiliated work. As such, we should evaluate available evidence within the context of what we hear from and observe in our patients.
Our opinion
When assessing patients with depression and anxiety, we must carefully evaluate symptoms to distinguish between depression with associated anxiety symptoms and depression with a comorbid anxiety disorder.
If a patient suffers from depression with associated anxiety symptoms (“anxious depression”), keep in mind that although some data demonstrate a superior response to SSRIs, other studies show no difference in effect. Some research—albeit smaller, less compelling studies—suggests that bupropion may decrease anxiety.
If your patient suffers from comorbid depression and an anxiety disorder, bupropion would not be a first-line choice because it is not FDA-approved to treat anxiety disorders. Although it is possible that anxiety/agitation could result from bupropion use, there is not sufficient data to support its reputation as ”anxiogenic.”
What is your experience?
Do you agree with the authors? Send comments to letters@currentpsychiatry.com or share your thoughts on http://www.facebook.com/CurrentPsychiatry.
Related Resource
- American Psychiatric Association. Mixed anxiety-depressive disorder. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000:780-781.
Drug Brand Names
- Bupropion • Wellbutrin, Zyban
- Escitalopram • Lexapro
- Sertraline • Zoloft
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342-351.
2. Wellbutrin [package insert]. Research Triangle Park NC: GlaxoSmithKline; 2008.
3. Rush AJ, Trivedi MH, Carmody TJ, et al. Response in relation to baseline anxiety levels in major depressive disorder treated with bupropion sustained release or sertraline. Neuropsychopharmacology. 2001;25(1):131-138.
4. Trivedi MH, Rush AJ, Carmody TJ, et al. Do bupropion SR and sertraline differ in their effects on anxiety in depressed patients? J Clin Psychiatry. 2001;62(10):776-781.
5. Nieuwstraten CE, Dolovich LR. Bupropion versus selective serotonin-reuptake inhibitors for treatment of depression. Ann Pharmacother. 2001;35(12):1608-1613.
6. Papakostas GI, Trivedi MH, Alpert JE, et al. Efficacy of bupropion and the selective serotonin reuptake inhibitors in the treatment of anxiety symptoms in major depressive disorder: a meta-analysis of individual patient data from 10 double-blind, randomized clinical trials. J Psychiatr Res. 2008;42(2):134-140.
7. Papakostas GI, Stahl SM, Krishen A, et al. Efficacy of bupropion and the selective serotonin reuptake inhibitors in the treatment of major depressive disorder with high levels of anxiety (anxious depression): a pooled analysis of 10 studies. J Clin Psychiatry. 2008;69(8):1287-1292.
8. Bystritsky A, Kerwin L, Feusner JD, et al. A pilot controlled trial of bupropion XL versus escitalopram in generalized anxiety disorder. Psychopharmacol Bull. 2008;41(1):46-51.
9. Feighner JP, Gardner EA, Johnston JA, et al. Double-blind comparison of bupropion and fluoxetine in depressed outpatients. J Clin Psychiatry. 1991;52(8):329-335.
For many clinicians, bupropion is the “go-to” medication for treating depressed patients who smoke, have concerns about sexual dysfunction side effects, and/or worry about weight gain. Bupropion is FDA-approved for preventing seasonal major depressive episodes in patients with seasonal affective disorder and is indicated as a smoking cessation aid.
“Anxious depression”—defined as depression with high levels of anxiety—is associated with poorer outcomes than “non-anxious” depression.1 Prescribing medications for these patients can be challenging. Some clinicians believe that bupropion exacerbates anxiety and should not be used to treat patients who experience both anxiety and depression.
Reports from our patients and our cumulative clinical experience are key factors in developing expertise in selecting appropriate medications. When informing our patients about what to expect from medications, however, it can be useful to combine anecdotal evidence with knowledge of the facts or lack thereof. Are there data to support or contradict the idea that bupropion can cause anxiety while treating depression?
What the research shows
The drug manufacturer reports a “substantial proportion of patients treated with Wellbutrin experience some degree of increased restlessness, agitation, anxiety, and insomnia, especially shortly after initiation of treatment.”2
In 2001, Rush et al3 published the results of a 16-week study (n=248) assessing pre-treatment anxiety levels and response to sertraline or bupropion. The authors concluded that anxious and depressed patients who received sertraline didn’t experience a superior anxiolytic or antidepressant response compared with bupropion.3 The same authors came to similar conclusions in a retrospective analysis of a pair of 8-week randomized, controlled, double-blind trials of selective serotonin reuptake inhibitors (SSRIs) and bupropion.4
In 2001, Nieuwstraten et al5 compared bupropion with SSRIs for treating depression by reviewing several randomized, double-blind, controlled trials. The relative risk of developing “anxiety/agitation” was 1.32 (95% confidence interval, 0.85 to 2.04), which was not statistically significant.
In a 2008 meta-analysis, Papakostas et al6 pooled individual patient data from 10 randomized, double-blind, placebo-controlled trials. Their aim was to compare the efficacy of bupropion to SSRIs in treating “anxious depression.” They found no difference in timing or degree of improvement in anxiety symptoms between groups based on Hamilton Anxiety Scale or Hamilton Depression Rating Scale—Anxiety-Somatization (HDRS-AS) scores. The authors recommended that antidepressant choice should not be based on concerns about worsening anxiety symptoms in depressed patients.6
Another meta-analysis by Papakostas et al7 of the same 10 randomized, double-blind, placebo-controlled trials suggested SSRIs may confer an advantage over bupropion in treating a subset of patients with “anxious depression,” which they defined as a HDRS-AS score ≥7. The authors noted the advantage was statistically significant, although “modest.”
Other smaller studies suggest that bupropion does not increase anxiety.8,9 A pilot study (N = 24, no placebo control) concluded that bupropion XL was comparable to escitalopram in treating anxiety in outpatients with generalized anxiety disorder.8
Because designing and executing drug trials can be expensive, it is not surprising that most of the evidence cited above derives from pharmaceutical company-sponsored or industry-affiliated work. As such, we should evaluate available evidence within the context of what we hear from and observe in our patients.
Our opinion
When assessing patients with depression and anxiety, we must carefully evaluate symptoms to distinguish between depression with associated anxiety symptoms and depression with a comorbid anxiety disorder.
If a patient suffers from depression with associated anxiety symptoms (“anxious depression”), keep in mind that although some data demonstrate a superior response to SSRIs, other studies show no difference in effect. Some research—albeit smaller, less compelling studies—suggests that bupropion may decrease anxiety.
If your patient suffers from comorbid depression and an anxiety disorder, bupropion would not be a first-line choice because it is not FDA-approved to treat anxiety disorders. Although it is possible that anxiety/agitation could result from bupropion use, there is not sufficient data to support its reputation as ”anxiogenic.”
What is your experience?
Do you agree with the authors? Send comments to letters@currentpsychiatry.com or share your thoughts on http://www.facebook.com/CurrentPsychiatry.
Related Resource
- American Psychiatric Association. Mixed anxiety-depressive disorder. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000:780-781.
Drug Brand Names
- Bupropion • Wellbutrin, Zyban
- Escitalopram • Lexapro
- Sertraline • Zoloft
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
For many clinicians, bupropion is the “go-to” medication for treating depressed patients who smoke, have concerns about sexual dysfunction side effects, and/or worry about weight gain. Bupropion is FDA-approved for preventing seasonal major depressive episodes in patients with seasonal affective disorder and is indicated as a smoking cessation aid.
“Anxious depression”—defined as depression with high levels of anxiety—is associated with poorer outcomes than “non-anxious” depression.1 Prescribing medications for these patients can be challenging. Some clinicians believe that bupropion exacerbates anxiety and should not be used to treat patients who experience both anxiety and depression.
Reports from our patients and our cumulative clinical experience are key factors in developing expertise in selecting appropriate medications. When informing our patients about what to expect from medications, however, it can be useful to combine anecdotal evidence with knowledge of the facts or lack thereof. Are there data to support or contradict the idea that bupropion can cause anxiety while treating depression?
What the research shows
The drug manufacturer reports a “substantial proportion of patients treated with Wellbutrin experience some degree of increased restlessness, agitation, anxiety, and insomnia, especially shortly after initiation of treatment.”2
In 2001, Rush et al3 published the results of a 16-week study (n=248) assessing pre-treatment anxiety levels and response to sertraline or bupropion. The authors concluded that anxious and depressed patients who received sertraline didn’t experience a superior anxiolytic or antidepressant response compared with bupropion.3 The same authors came to similar conclusions in a retrospective analysis of a pair of 8-week randomized, controlled, double-blind trials of selective serotonin reuptake inhibitors (SSRIs) and bupropion.4
In 2001, Nieuwstraten et al5 compared bupropion with SSRIs for treating depression by reviewing several randomized, double-blind, controlled trials. The relative risk of developing “anxiety/agitation” was 1.32 (95% confidence interval, 0.85 to 2.04), which was not statistically significant.
In a 2008 meta-analysis, Papakostas et al6 pooled individual patient data from 10 randomized, double-blind, placebo-controlled trials. Their aim was to compare the efficacy of bupropion to SSRIs in treating “anxious depression.” They found no difference in timing or degree of improvement in anxiety symptoms between groups based on Hamilton Anxiety Scale or Hamilton Depression Rating Scale—Anxiety-Somatization (HDRS-AS) scores. The authors recommended that antidepressant choice should not be based on concerns about worsening anxiety symptoms in depressed patients.6
Another meta-analysis by Papakostas et al7 of the same 10 randomized, double-blind, placebo-controlled trials suggested SSRIs may confer an advantage over bupropion in treating a subset of patients with “anxious depression,” which they defined as a HDRS-AS score ≥7. The authors noted the advantage was statistically significant, although “modest.”
Other smaller studies suggest that bupropion does not increase anxiety.8,9 A pilot study (N = 24, no placebo control) concluded that bupropion XL was comparable to escitalopram in treating anxiety in outpatients with generalized anxiety disorder.8
Because designing and executing drug trials can be expensive, it is not surprising that most of the evidence cited above derives from pharmaceutical company-sponsored or industry-affiliated work. As such, we should evaluate available evidence within the context of what we hear from and observe in our patients.
Our opinion
When assessing patients with depression and anxiety, we must carefully evaluate symptoms to distinguish between depression with associated anxiety symptoms and depression with a comorbid anxiety disorder.
If a patient suffers from depression with associated anxiety symptoms (“anxious depression”), keep in mind that although some data demonstrate a superior response to SSRIs, other studies show no difference in effect. Some research—albeit smaller, less compelling studies—suggests that bupropion may decrease anxiety.
If your patient suffers from comorbid depression and an anxiety disorder, bupropion would not be a first-line choice because it is not FDA-approved to treat anxiety disorders. Although it is possible that anxiety/agitation could result from bupropion use, there is not sufficient data to support its reputation as ”anxiogenic.”
What is your experience?
Do you agree with the authors? Send comments to letters@currentpsychiatry.com or share your thoughts on http://www.facebook.com/CurrentPsychiatry.
Related Resource
- American Psychiatric Association. Mixed anxiety-depressive disorder. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000:780-781.
Drug Brand Names
- Bupropion • Wellbutrin, Zyban
- Escitalopram • Lexapro
- Sertraline • Zoloft
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342-351.
2. Wellbutrin [package insert]. Research Triangle Park NC: GlaxoSmithKline; 2008.
3. Rush AJ, Trivedi MH, Carmody TJ, et al. Response in relation to baseline anxiety levels in major depressive disorder treated with bupropion sustained release or sertraline. Neuropsychopharmacology. 2001;25(1):131-138.
4. Trivedi MH, Rush AJ, Carmody TJ, et al. Do bupropion SR and sertraline differ in their effects on anxiety in depressed patients? J Clin Psychiatry. 2001;62(10):776-781.
5. Nieuwstraten CE, Dolovich LR. Bupropion versus selective serotonin-reuptake inhibitors for treatment of depression. Ann Pharmacother. 2001;35(12):1608-1613.
6. Papakostas GI, Trivedi MH, Alpert JE, et al. Efficacy of bupropion and the selective serotonin reuptake inhibitors in the treatment of anxiety symptoms in major depressive disorder: a meta-analysis of individual patient data from 10 double-blind, randomized clinical trials. J Psychiatr Res. 2008;42(2):134-140.
7. Papakostas GI, Stahl SM, Krishen A, et al. Efficacy of bupropion and the selective serotonin reuptake inhibitors in the treatment of major depressive disorder with high levels of anxiety (anxious depression): a pooled analysis of 10 studies. J Clin Psychiatry. 2008;69(8):1287-1292.
8. Bystritsky A, Kerwin L, Feusner JD, et al. A pilot controlled trial of bupropion XL versus escitalopram in generalized anxiety disorder. Psychopharmacol Bull. 2008;41(1):46-51.
9. Feighner JP, Gardner EA, Johnston JA, et al. Double-blind comparison of bupropion and fluoxetine in depressed outpatients. J Clin Psychiatry. 1991;52(8):329-335.
1. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342-351.
2. Wellbutrin [package insert]. Research Triangle Park NC: GlaxoSmithKline; 2008.
3. Rush AJ, Trivedi MH, Carmody TJ, et al. Response in relation to baseline anxiety levels in major depressive disorder treated with bupropion sustained release or sertraline. Neuropsychopharmacology. 2001;25(1):131-138.
4. Trivedi MH, Rush AJ, Carmody TJ, et al. Do bupropion SR and sertraline differ in their effects on anxiety in depressed patients? J Clin Psychiatry. 2001;62(10):776-781.
5. Nieuwstraten CE, Dolovich LR. Bupropion versus selective serotonin-reuptake inhibitors for treatment of depression. Ann Pharmacother. 2001;35(12):1608-1613.
6. Papakostas GI, Trivedi MH, Alpert JE, et al. Efficacy of bupropion and the selective serotonin reuptake inhibitors in the treatment of anxiety symptoms in major depressive disorder: a meta-analysis of individual patient data from 10 double-blind, randomized clinical trials. J Psychiatr Res. 2008;42(2):134-140.
7. Papakostas GI, Stahl SM, Krishen A, et al. Efficacy of bupropion and the selective serotonin reuptake inhibitors in the treatment of major depressive disorder with high levels of anxiety (anxious depression): a pooled analysis of 10 studies. J Clin Psychiatry. 2008;69(8):1287-1292.
8. Bystritsky A, Kerwin L, Feusner JD, et al. A pilot controlled trial of bupropion XL versus escitalopram in generalized anxiety disorder. Psychopharmacol Bull. 2008;41(1):46-51.
9. Feighner JP, Gardner EA, Johnston JA, et al. Double-blind comparison of bupropion and fluoxetine in depressed outpatients. J Clin Psychiatry. 1991;52(8):329-335.
Managing Type 2 Diabetes in Men
Managing the Multiple Symptoms of Benign Prostatic Hyperplasia — CME
Managing Type 2 Diabetes in Men
Meeting New Challenges with Antiplatelet Therapy in Primary Care
Dr. Aguilar has disclosed that he has ongoing relationships with the following companies: Amylin Pharmaceuticals; Eli Lilly; Janssen Pharmaceuticals, Inc; Novo Nordisk, Inc; and Takeda Pharmaceuticals USA, Inc.
SUPPORT
This program is sponsored by the PCEC and is supported by funding from Novo Nordisk, Inc.
The prevalence of type 2 diabetes mellitus (T2DM) is similar in men and women (11.8% vs 10.8%, respectively), however there are gender differences that should be considered when developing a treatment plan (eg, cardiovascular risk, psychosocial factors, coping strategies, and the perception of benefit from self-care) when managing those diagnosed with this disease and those at risk for developing it.1 This article describes these differences in the context of two patients—one at risk for T2DM being seen by his health care provider for a routine physical examination, and one who has been treated for several years for T2DM and is being seen for a follow-up office visit. For each patient, the implications for treatment are discussed.
Men at Risk for Type 2 Diabetes Mellitus
JW is a 48-year-old white male being seen for a routine physical examination; he last saw a physician 6 years ago, also for a routine physical. He has no complaints and is taking no medications. Having divorced 7 years ago, he lives alone in an apartment and eats many of his meals at fast food restaurants. JW drinks 2 to 3 beers a night several times a week and more when he socializes with his friends 2 to 3 evenings per week. He smokes socially. His father has a 12-year history of T2DM. His mother has a 4-year history of essential hypertension and a 9-year history of chronic obstructive pulmonary disease.
Physical examination shows that JW is 5’11” tall, weighs 207 pounds (body mass index (BMI), 29 kg/m2), and has a 41” waist circumference; his blood pressure (BP) is 138/86 mm Hg and respiratory rate is 17 breaths/min. The remainder of his physical examination, including eye and neurologic exams, is normal. Laboratory results, including a screening glycated homoglobin (A1C), are pending.
Key Risk Factors for Type 2 Diabetes Mellitus in Men
This case is not an uncommon presentation of a middle-aged male who has several risk factors for diabetes (see Case Study 1 continued ). JW also has key risk factors for T2DM in men. The Monitoring of Trends and Determinants in Cardiovascular Disease (MONICA) Augsburg surveys identified 128 men and 85 women with T2DM.2 Increasing age and BMI, positive parental history of T2DM, and a low high-density lipoprotein cholesterol (HDL-C) level were independent risk factors predicting the development of T2DM in both men and women. However, several other factors posed a higher risk in men relative to women, including systolic BP (hazard ratio [HR], 1.16 per 10-mm Hg increase), regular smoking (HR, 1.75), and alcohol intake ≥ 40 g/d (HR, 1.95). (Note: 1 fluid ounce 80 proof alcohol ≈ 11 g ethanol; 12 fluid ounces beer [~5% alcohol] ≈ 14 g ethanol). After adjusting for these factors, a separate analysis (4424 men, 4380 women) showed that men who lived alone were more likely to develop T2DM than either men or women who did not live alone (HR, 1.69 in men vs 0.85 in women; P = .006).3 While the number of people with T2DM in MONICA was small, the results suggest that measuring BP, particularly systolic BP, and taking a smoking and alcohol history may be especially important in men.
With respect to alcohol intake, epidemiologic and randomized clinical trials have generally demonstrated an inverse relationship between moderate alcohol consumption (20 to 30 g/d) and the long-term risk of T2DM.2,4-7 Differences among studies in how patients were grouped preclude determination of the daily alcohol consumption that confers the greatest risk benefit, although one recent study conducted over 4 years indicates that the greatest benefit in diabetes risk reduction may occur when men who previously consumed 8
Other nutrition and lifestyle patterns also seem to be particularly beneficial in reducing the risk of T2DM in men. Survey data involving 22,921 Japanese men and 29,759 Japanese women followed over 5 years showed that fish and seafood intake was significantly associated with a decreased risk of T2DM in men but not in women.9 The odds ratio of developing T2DM for the highest quartile versus the lowest quartile of fish and seafood intake was 0.73 (P = .04 for trend). Additional analysis did not identify any significant association with the fat content of fish.
Results of the Health Professionals Follow-up Study provide evidence of benefit in lowering the risk of T2DM in men who consume high amounts of low-fat dairy products, whole grains, and magnesium ( TABLE 1 ). With respect to dairy food consumption, after 12 years of follow-up involving 1243 incident cases of T2DM, the relative risk (RR) of developing T2DM in men in the top quintile of dairy intake was 0.77 compared with those in the lowest quintile (P = .003 for trend).10 Men in the highest quintile consumed 4.1 servings of dairy food per day compared with 0.5 servings per day in the lowest quintile. Each serving-per-day increase in total dairy intake was associated with a 9% lower risk for T2DM, with a lower risk seen with consumption of low-fat vs high-fat dairy food. With respect to whole-grain intake, the RR of developing T2DM was 0.58 in men in the upper vs lower quintiles (3.2 vs 0.4 servings/d), although the effect was attenuated with BMI (P = .0006 for trend).11 Similar observations were made with respect to magnesium consumption; a RR of 0.76 for T2DM was observed in men with a median magnesium consumption of 457 mg/d compared with those who consumed 270 mg/d.12
TABLE 1
Suggestions for Men Who Are at Risk of or Have Been Diagnosed with Type 2 Diabetes Mellitus (T2DM)*
For men who are at risk:
|
For men who have been diagnosed:
|
| BP, blood pressure; HDL-C, high-density lipoprotein cholesterol. *These suggestions are in addition to developing and fostering a collaborative, patient-centered approach. |
JW has the following risk factors for T2DM:
- Overweight with central adiposity
- Physical inactivity
- First-degree relative with T2DM
- Possible cardiovascular disease (CVD; hypertension, smoking)
- High daily alcohol intake (10 to 20 g alcohol/beer x 2-3 beers/d = 20 to 60 g alcohol/d)
- Poor nutrition
- Lives alone
Plan:
- Discuss above risk factors with JW
- Repeat BP measurement at next visit; implement treatment if BP >140/90 mm Hg (130/80 mm Hg if T2DM is diagnosed)
- Consider evaluation for alcohol/substance abuse
- Evaluate for smoking cessation program
- Nutrition referral for lifestyle and dietary management intervention
Working with men to avoid the development of T2DM is an important objective for family physicians. It is essential to identify men who are at increased risk, including those with prediabetes, provide education about the disease and its risk factors, and implement appropriate risk reduction strategies. Risk reduction strategies should focus on modifiable factors, such as body weight, physical activity, BP, blood lipids, blood glucose, and smoking. With JW, his motivation to “get back into shape” will help move the conversation toward achievable goals that can be set and modified over time. Other strategies that may be helpful in reducing the risk of developing T2DM in men include a moderate daily alcohol intake and a diet high in fish and seafood, low-fat dairy products, whole grains, and magnesium ( TABLE 1 ).
Once diagnosed with T2DM, there are risk management strategies that can be particularly helpful in men. These include strategies that target cardiovascular health, as well as those that consider the psychosocial and coping behaviors of men.
Risk of Complications in Men With Type 2 Diabetes Mellitus
Physical examination: BP, 126/78 mm Hg; body weight, 183 pounds (a 13 to 17 pound increase since the knee injury); waist circumference, 38” (BMI, 28 kg/m2); grade 1 retinopathy bilaterally; neurologic exam normal.
Laboratory: A1C, 7.8%; lipids normal except triglyceride level, 219 mg/dL; creatinine clearance (calculated), 69 mL/min; urine, 45 mg albumin/g creatinine.
MR’s self-measured fasting plasma glucose (FPG) has ranged from 121 to 143 mg/dL over the past month; isolated postprandial glucose (PPG) measurements show 194 to 258 mg/dL.
MR works as a vocational teacher at the local high school, and he teaches driver education after school. Review of his pharmacy records suggests his adherence over the past year has been: metformin (88%), hydrochlorothiazide (72%), and lisinopril (72%).
Assessment:
- A1C level of 7.8% indicates an estimated average glucose (eAG) of 177 mg/dL13
- –Mildly elevated FPG and PPG
- –Evidence of microvascular disease (retinopathy, nephropathy)
- –Creatinine clearance 69 mL/min and microalbuminuria indicate stage 2 chronic kidney disease14
In addition to referring MR for physical rehabilitation of his knee, you discuss with MR the need and options for intensifying his diabetes therapy.
Does the fact that MR is male affect your management plan?
In people diagnosed with T2DM, there are differences between men and women with respect to risk for cardiovascular and other comorbid diseases, as well as in their psychosocial well-being and coping strategies.
Risk for Cardiovascular Disease in Type 2 Diabetes Mellitus
A systematic literature review shows that men with T2DM generally fare better than women with T2DM regarding their risk for CVD. Men with T2DM have a 2- to 3-fold increase in the risk of developing coronary heart disease (CHD) compared with men without T2DM, whereas women with T2DM have a 4- to 6-fold increase in risk compared with women without T2DM.15 Compared with women with T2DM, men with T2DM also have a better prognosis after myocardial infarction (MI) and a lower risk of death overall from CVD. Possible reasons for these differences include a lower risk of hypertension, a less severe form of dyslipidemia, and a lower prevalence of obesity in men with T2DM compared with women with T2DM.15 These same reasons for observed differences between men and women were seen in a meta-analysis of 29 studies, where the RR of fatal MI in men with T2DM compared with women with T2DM was 0.68.16 Similar findings were seen in the Skaraborg Project, which involved 1116 Swedish patients with hypertension and/or T2DM.17 Compared with a healthy population, the age-adjusted HR for fatal MI was 1.9 for men with T2DM and 5.0 for women with T2DM over 8.1 years of follow-up (RR, 0.38 for men vs women). Analysis of the data indicated that these results were not explained by the more favorable survival rate in women without T2DM than in men without T2DM.17
Somewhat different results have been reported by the Italian Diabetes and Informatics Study Group in a slightly different T2DM population. This investigation involved men and women with T2DM (N = 11,644) who could have microvascular but not macrovascular disease.18 After 4 years of follow-up, the age-adjusted incident rates for first CHD event (composite of acute MI, coronary artery bypass grafting, percutaneous transluminal coronary angioplasty) were 28.8 per 1000 person-years in men and 23.3 per 1000 person-years in women. Incident rates (per 1000 person-years) of acute MI (10.3 vs 4.7), major CHD events (13.1 vs 5.8), and fatal CHD (2.6 vs 0.6) were all significantly more frequent in men than in women, respectively. Multivariate analysis showed that hypertension and A1C were additional risk factors for CHD in men; for each 20% increment above the A1C upper limit of normal, there was a 14% risk increase for CHD. The presence of microvascular complications increased risk by 20% in men and 35% in women. In this analysis, glycemic control and hypertension were found to be the predominant risk factors in men, while high triglyceride levels, low HDL-C levels, and microangiopathy were predominant in women.
Additional multivariate analyses provide greater insight into specific factors that affect the risk of CVD and outcomes in men with T2DM. One investigation compared men and women with T2DM who were normotensive without evidence of CVD but with microalbuminuria. After 4.7 years of follow-up, men were found to be at lower risk (RR, 0.12) for a composite of death, acute MI, unstable angina, coronary interventions, heart failure, cerebral ischemic stroke or transient ischemic attack, and peripheral artery disease.19 Other investigators have reported a lower risk of stroke, including fatal stroke, in men with T2DM compared with women with T2DM.20,21 For example, analysis of the General Practice Research Database identified 22,178 men and 19,621 women with T2DM between the ages of 35 and 89 years.20 The stroke rate per 1000 person-years across all ages was 10.82 (95% confidence interval (CI), 10.17-11.51) in men and 13.16 (95% CI, 12.40-13.97) in women. In men, the rate per 1000 person-years rose from 1.81 in the 35 to 44 year age group to 28.35 in men 85 years of age or older. Although the rate of stroke per 1000 person-years was lower in women than men in the 35 to 44 year age group (1.53 vs 1.81), the rate in women exceeded that of men in the 85 years of age or older group (32.20 vs 28.35).
Other Chronic Complications
Kidney disease is affected by blood lipids, specifically HDL-C, in men with T2DM. An investigation in men and women with T2DM with normoalbuminuria or microalbuminuria at baseline showed that a low HDL-C level was an independent predictor of progression to a more advanced stage of albuminuria over 4.3 years of follow-up (HR, 0.391 for men with normal HDL-C compared with men with low HDL-C). In women, no lipid parameters were associated with progression of albuminuria.22
While these investigations do not provide a clear picture of the differences regarding cardiovascular risk between men and women with T2DM, they suggest that men with T2DM have a lower risk of nonfatal and fatal CVD and stroke than do women with T2DM. However, the lower risk seen in men may be affected by the cardiovascular endpoints measured and the presence of microvascular disease. Possible independent risk factors for CVD in men with T2DM include hypertension, poor glycemic control, and low HDL-C.
Risk factors that place MR at greater risk for CVD compared with a woman with T2DM and therefore serve as key treatment targets include:
- Hypertension—although controlled (126/78 mm Hg) with hydrochlorothiazide and lisinopril
- Poor glycemic control—A1C, 7.8% (eAG, 177mg/dL)
- –Increase physical activity—refer for knee rehabilitation
- –Intensify glucose-lowering therapy by adding an additional glucose-lowering agent (eg, dipeptidyl peptidase-4 inhibitor, glucagon-like peptide-1 receptor agonist, thiazolidinedione, α-glucosidase inhibitor, sulfonylurea, glinide, or basal insulin)
- Microalbuminuria (45 mg urinary albumin/g creatinine)—encourage better adherence to lisinopril; monitor renal function
- Hypertriglyceridemia—initiate omega-3 fatty acid or extended-release niacin
Psychosocial Well-Being, Benefit of Self-Care, and Coping Strategies
Type 2 diabetes mellitus is a chronic disease with glycemic control largely determined by patient self-management, and the attitudes and beliefs of patients with T2DM are important factors to consider from diagnosis onward.23 There are important differences between men and women with T2DM regarding attitudes and beliefs. Published investigations provide some, although not entirely consistent, insight into these psychosocial differences between men and women with T2DM. These differences are summarized in TABLE 2 .24-32 Taking these differences into account when planning treatment and when communicating with and educating the patient is essential for improved patient self-management.
TABLE 2
Psychosocial and Coping Characteristics of Men with Type 2 Diabetes Mellitus (T2DM)24-32
Compared with women with T2DM, generally, men with T2DM:
|
Key interventions for MR:
- Maintain a dialogue and enhance collaboration with MR
- Establish shared goals that are customized to incorporate MR’s personal goals
- Problem solve with MR to identify ways he can better integrate the diabetes self-care objectives of dietary changes and blood glucose self-monitoring into his daily life
- Emphasize that enhanced or greater disease control can be achieved by good self-management, including better adherence to the management plan
- Remind MR that T2DM is a progressive disease that requires intermittent medication adjustments to keep pace with its progression
- Build upon the belief that T2DM can be controlled by reminding MR that the disease was well controlled before his knee injury
- –Focus on the importance of rehabilitating his knee
- –Develop a rehabilitation plan
- Provide informational support regarding options for intensifying diabetes therapy (eg, dipeptidyl peptidase-4 inhibitor, thiazolidinedione, glucagon-like peptide-1 receptor agonist, sulfonylurea, or insulin)
- –Discuss MR’s needs and concerns, as well as barriers for each treatment option, particularly hypoglycemia and weight gain
- –Provide instruction or educational materials regarding injection devices
- –Involve the healthcare team, as appropriate
- Keep the treatment regimen as simple as possible; consider pill combinations where appropriate
Summary
The growing epidemic of T2DM requires intervention to assist patients who have been diagnosed to better manage the disease, to reduce the risk of developing the disease in those who have not yet been diagnosed, and to manage the associated complications. In addition to individualizing interventions based on a patient’s needs, concerns, and capabilities, taking gender into account is necessary. In otherwise healthy people, several independent factors appear to pose a higher risk of T2DM in men relative to women, including systolic hypertension, regular smoking, and alcohol intake ≥ 40 g/d. At the same time, men achieve greater risk reduction from moderate daily alcohol intake and a diet high in fish and seafood, low-fat dairy products, whole grains, and magnesium.
Once diagnosed with T2DM, men generally fare better than women regarding the risk for CVD; they also have a better prognosis after MI and a lower risk of death overall from CVD. Possible independent risk factors for CVD in men with T2DM that are especially important may include hypertension, poor glycemic control, and low HDL-C levels. Psychosocial complications, such as depression, are less likely in men with T2DM. However, men expend less effort coping, are less likely to utilize healthcare services, and are less informed about treatment options. Although men have a lower expectation of the benefit of self-management, they find support from family and friends more helpful than do women, but they are fearful of losing control of their disease.
Taking these gender differences into account should prove helpful as family care physicians work with men to reduce their risk of developing T2DM and in helping men diagnosed with T2DM to better self-manage their disease.
1. Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Published 2011. Accessed May 2, 2011.
2. Meisinger C, Thorand B, Schneider A, Stieber J, Doring A, Lowel H. Sex differences in risk factors for incident type 2 diabetes mellitus: the MONICA Augsburg cohort study. Arch Intern Med. 2002;162(1):82-89.
3. Meisinger C, Kandler U, Ladwig KH. Living alone is associated with an increased risk of type 2 diabetes mellitus in men but not women from the general population: the MONICA/KORA Augsburg Cohort Study. Psychosom Med. 2009;71(7):784-788.
4. Baliunas DO, Taylor BJ, Irving H, et al. Alcohol as a risk factor for type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2009;32(11):2123-2132.
5. Mozaffarian D, Kamineni A, Carnethon M, Djoussé L, Mukamal KJ, Siscovick D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: the cardiovascular health study. Arch Intern Med. 2009;169(8):798-807.
6. Joosten MM, Grobbee DE, van der A DL, Verschuren WM, Hendriks HF, Beulens JW. Combined effect of alcohol consumption and lifestyle behaviors on risk of type 2 diabetes. Am J Clin Nutr. 2010;91(6):1777-1783.
7. Gigleux I, Gagnon J, St-Pierre A, et al. Moderate alcohol consumption is more cardioprotective in men with the metabolic syndrome. J Nutr. 2006;136(12):3027-3032.
8. Joosten MM, Chiuve SE, Mukamal KJ, Hu FB, Hendriks HF, Rimm EB. Changes in alcohol consumption and subsequent risk of type 2 diabetes in men. Diabetes. 2011;60(1):74-79.
9. Nanri A, Mizoue T, Noda M, et al. Fish intake and type 2 diabetes in Japanese men and women: the Japan Public Health Center-based Prospective Study. Am J Clin Nutr. 2011;94(3):884-891.
10. Choi HK, Willett WC, Stampfer MJ, Rimm E, Hu FB. Dairy consumption and risk of type 2 diabetes mellitus in men: a prospective study. Arch Intern Med. 2005;165(9):997-1003.
11. Fung TT, Hu FB, Pereira MA, et al. Whole-grain intake and the risk of type 2 diabetes: a prospective study in men. Am J Clin Nutr. 2002;76(3):535-540.
12. Lopez-Ridaura R, Willett WC, Rimm EB, et al. Magnesium intake and risk of type 2 diabetes in men and women. Diabetes Care. 2004;27(1):134-140.
13. Nathan DM, Kuenen J, Borg R, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31(8):1473-1478.
14. Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137-147.
15. Legato MJ, Gelzer A, Goland R, et al. Gender-specific care of the patient with diabetes: review and recommendations. Gend Med. 2006;3(2):131-158.
16. Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332(7533):73-78.
17. Larsson CA, Gullberg B, Merlo J, Rastam L, Lindblad U. Female advantage in AMI mortality is reversed in patients with type 2 diabetes in the Skaraborg Project. Diabetes Care. 2005;28(9):2246-2248.
18. Avogaro A, Giorda C, Maggini M, et al. Incidence of coronary heart disease in type 2 diabetic men and women: impact of microvascular complications, treatment, and geographic location. Diabetes Care. 2007;30(5):1241-1247.
19. Zandbergen AA, Sijbrands EJ, Lamberts SW, Bootsma AH. Normotensive women with type 2 diabetes and microalbuminuria are at high risk for macrovascular disease. Diabetes Care. 2006;29(8):1851-1855.
20. Mulnier HE, Seaman HE, Raleigh VS, et al. Risk of stroke in people with type 2 diabetes in the UK: a study using the General Practice Research Database. Diabetologia. 2006;49(12):2859-2865.
21. Tuomilehto J, Rastenyte D, Jousilahti P, Sarti C, Vartiainen E. Diabetes mellitus as a risk factor for death from stroke. Prospective study of the middle-aged Finnish population. Stroke. 1996;27(2):210-215.
22. Hanai K, Babazono T, Yoshida N, et al. Gender differences in the association between HDL cholesterol and the progression of diabetic kidney disease in type 2 diabetic patients. Nephrol Dial Transplant. 2012;27(3):1070-1075.
23. Tuerk PW, Mueller M, Egede LE. Estimating physician effects on glycemic control in the treatment of diabetes: methods, effects sizes, and implications for treatment policy. Diabetes Care. 2008;31(5):869-873.
24. Rubin RR, Peyrot M, Siminerio LM. Health care and patient-reported outcomes: results of the cross-national Diabetes Attitudes, Wishes and Needs (DAWN) study. Diabetes Care. 2006;29(6):1249-1255.
25. McCollum M, Hansen LB, Ghushchyan V, Sullivan PW. Inconsistent health perceptions for US women and men with diabetes. J Womens Health (Larchmt). 2007;16(10):1421-1428.
26. Gucciardi E, Wang SC, DeMelo M, Amaral L, Stewart DE. Characteristics of men and women with diabetes: observations during patients’ initial visit to a diabetes education centre. Can Fam Physician. 2008;54(2):219-227.
27. Chiu CJ, Wray LA. Physical disability trajectories in older Americans with and without diabetes: the role of age, gender, race or ethnicity, and education. Gerontologist. 2011;51(1):51-63.
28. Nielsen AB, de Fine Olivarius N, Gannik D, Hindsberger C, Hollnagel H. Structured personal diabetes care in primary health care affects only women’s HbA1c. Diabetes Care. 2006;29(5):963-969.
29. Shalev V, Chodick G, Heymann AD, Kokia E. Gender differences in healthcare utilization and medical indicators among patients with diabetes. Public Health. 2005;119(1):45-49.
30. Kacerovsky-Bielesz G, Lienhardt S, Hagenhofer M, et al. Sex-related psychological effects on metabolic control in type 2 diabetes mellitus. Diabetologia. 2009;52(5):781-788.
31. Brown SA, Harrist RB, Villagomez ET, Segura M, Barton SA, Hanis CL. Gender and treatment differences in knowledge, health beliefs, and metabolic control in Mexican Americans with type 2 diabetes. Diabetes Educ. 2000;26(3):425-438.
32. Liburd LC, Namageyo-Funa A, Jack L, Jr. Understanding “masculinity” and the challenges of managing type-2 diabetes among African-American men. J Natl Med Assoc. 2007;99(5):550-552, 554–558.
Managing the Multiple Symptoms of Benign Prostatic Hyperplasia — CME
Managing Type 2 Diabetes in Men
Meeting New Challenges with Antiplatelet Therapy in Primary Care
Dr. Aguilar has disclosed that he has ongoing relationships with the following companies: Amylin Pharmaceuticals; Eli Lilly; Janssen Pharmaceuticals, Inc; Novo Nordisk, Inc; and Takeda Pharmaceuticals USA, Inc.
SUPPORT
This program is sponsored by the PCEC and is supported by funding from Novo Nordisk, Inc.
The prevalence of type 2 diabetes mellitus (T2DM) is similar in men and women (11.8% vs 10.8%, respectively), however there are gender differences that should be considered when developing a treatment plan (eg, cardiovascular risk, psychosocial factors, coping strategies, and the perception of benefit from self-care) when managing those diagnosed with this disease and those at risk for developing it.1 This article describes these differences in the context of two patients—one at risk for T2DM being seen by his health care provider for a routine physical examination, and one who has been treated for several years for T2DM and is being seen for a follow-up office visit. For each patient, the implications for treatment are discussed.
Men at Risk for Type 2 Diabetes Mellitus
JW is a 48-year-old white male being seen for a routine physical examination; he last saw a physician 6 years ago, also for a routine physical. He has no complaints and is taking no medications. Having divorced 7 years ago, he lives alone in an apartment and eats many of his meals at fast food restaurants. JW drinks 2 to 3 beers a night several times a week and more when he socializes with his friends 2 to 3 evenings per week. He smokes socially. His father has a 12-year history of T2DM. His mother has a 4-year history of essential hypertension and a 9-year history of chronic obstructive pulmonary disease.
Physical examination shows that JW is 5’11” tall, weighs 207 pounds (body mass index (BMI), 29 kg/m2), and has a 41” waist circumference; his blood pressure (BP) is 138/86 mm Hg and respiratory rate is 17 breaths/min. The remainder of his physical examination, including eye and neurologic exams, is normal. Laboratory results, including a screening glycated homoglobin (A1C), are pending.
Key Risk Factors for Type 2 Diabetes Mellitus in Men
This case is not an uncommon presentation of a middle-aged male who has several risk factors for diabetes (see Case Study 1 continued ). JW also has key risk factors for T2DM in men. The Monitoring of Trends and Determinants in Cardiovascular Disease (MONICA) Augsburg surveys identified 128 men and 85 women with T2DM.2 Increasing age and BMI, positive parental history of T2DM, and a low high-density lipoprotein cholesterol (HDL-C) level were independent risk factors predicting the development of T2DM in both men and women. However, several other factors posed a higher risk in men relative to women, including systolic BP (hazard ratio [HR], 1.16 per 10-mm Hg increase), regular smoking (HR, 1.75), and alcohol intake ≥ 40 g/d (HR, 1.95). (Note: 1 fluid ounce 80 proof alcohol ≈ 11 g ethanol; 12 fluid ounces beer [~5% alcohol] ≈ 14 g ethanol). After adjusting for these factors, a separate analysis (4424 men, 4380 women) showed that men who lived alone were more likely to develop T2DM than either men or women who did not live alone (HR, 1.69 in men vs 0.85 in women; P = .006).3 While the number of people with T2DM in MONICA was small, the results suggest that measuring BP, particularly systolic BP, and taking a smoking and alcohol history may be especially important in men.
With respect to alcohol intake, epidemiologic and randomized clinical trials have generally demonstrated an inverse relationship between moderate alcohol consumption (20 to 30 g/d) and the long-term risk of T2DM.2,4-7 Differences among studies in how patients were grouped preclude determination of the daily alcohol consumption that confers the greatest risk benefit, although one recent study conducted over 4 years indicates that the greatest benefit in diabetes risk reduction may occur when men who previously consumed 8
Other nutrition and lifestyle patterns also seem to be particularly beneficial in reducing the risk of T2DM in men. Survey data involving 22,921 Japanese men and 29,759 Japanese women followed over 5 years showed that fish and seafood intake was significantly associated with a decreased risk of T2DM in men but not in women.9 The odds ratio of developing T2DM for the highest quartile versus the lowest quartile of fish and seafood intake was 0.73 (P = .04 for trend). Additional analysis did not identify any significant association with the fat content of fish.
Results of the Health Professionals Follow-up Study provide evidence of benefit in lowering the risk of T2DM in men who consume high amounts of low-fat dairy products, whole grains, and magnesium ( TABLE 1 ). With respect to dairy food consumption, after 12 years of follow-up involving 1243 incident cases of T2DM, the relative risk (RR) of developing T2DM in men in the top quintile of dairy intake was 0.77 compared with those in the lowest quintile (P = .003 for trend).10 Men in the highest quintile consumed 4.1 servings of dairy food per day compared with 0.5 servings per day in the lowest quintile. Each serving-per-day increase in total dairy intake was associated with a 9% lower risk for T2DM, with a lower risk seen with consumption of low-fat vs high-fat dairy food. With respect to whole-grain intake, the RR of developing T2DM was 0.58 in men in the upper vs lower quintiles (3.2 vs 0.4 servings/d), although the effect was attenuated with BMI (P = .0006 for trend).11 Similar observations were made with respect to magnesium consumption; a RR of 0.76 for T2DM was observed in men with a median magnesium consumption of 457 mg/d compared with those who consumed 270 mg/d.12
TABLE 1
Suggestions for Men Who Are at Risk of or Have Been Diagnosed with Type 2 Diabetes Mellitus (T2DM)*
For men who are at risk:
|
For men who have been diagnosed:
|
| BP, blood pressure; HDL-C, high-density lipoprotein cholesterol. *These suggestions are in addition to developing and fostering a collaborative, patient-centered approach. |
JW has the following risk factors for T2DM:
- Overweight with central adiposity
- Physical inactivity
- First-degree relative with T2DM
- Possible cardiovascular disease (CVD; hypertension, smoking)
- High daily alcohol intake (10 to 20 g alcohol/beer x 2-3 beers/d = 20 to 60 g alcohol/d)
- Poor nutrition
- Lives alone
Plan:
- Discuss above risk factors with JW
- Repeat BP measurement at next visit; implement treatment if BP >140/90 mm Hg (130/80 mm Hg if T2DM is diagnosed)
- Consider evaluation for alcohol/substance abuse
- Evaluate for smoking cessation program
- Nutrition referral for lifestyle and dietary management intervention
Working with men to avoid the development of T2DM is an important objective for family physicians. It is essential to identify men who are at increased risk, including those with prediabetes, provide education about the disease and its risk factors, and implement appropriate risk reduction strategies. Risk reduction strategies should focus on modifiable factors, such as body weight, physical activity, BP, blood lipids, blood glucose, and smoking. With JW, his motivation to “get back into shape” will help move the conversation toward achievable goals that can be set and modified over time. Other strategies that may be helpful in reducing the risk of developing T2DM in men include a moderate daily alcohol intake and a diet high in fish and seafood, low-fat dairy products, whole grains, and magnesium ( TABLE 1 ).
Once diagnosed with T2DM, there are risk management strategies that can be particularly helpful in men. These include strategies that target cardiovascular health, as well as those that consider the psychosocial and coping behaviors of men.
Risk of Complications in Men With Type 2 Diabetes Mellitus
Physical examination: BP, 126/78 mm Hg; body weight, 183 pounds (a 13 to 17 pound increase since the knee injury); waist circumference, 38” (BMI, 28 kg/m2); grade 1 retinopathy bilaterally; neurologic exam normal.
Laboratory: A1C, 7.8%; lipids normal except triglyceride level, 219 mg/dL; creatinine clearance (calculated), 69 mL/min; urine, 45 mg albumin/g creatinine.
MR’s self-measured fasting plasma glucose (FPG) has ranged from 121 to 143 mg/dL over the past month; isolated postprandial glucose (PPG) measurements show 194 to 258 mg/dL.
MR works as a vocational teacher at the local high school, and he teaches driver education after school. Review of his pharmacy records suggests his adherence over the past year has been: metformin (88%), hydrochlorothiazide (72%), and lisinopril (72%).
Assessment:
- A1C level of 7.8% indicates an estimated average glucose (eAG) of 177 mg/dL13
- –Mildly elevated FPG and PPG
- –Evidence of microvascular disease (retinopathy, nephropathy)
- –Creatinine clearance 69 mL/min and microalbuminuria indicate stage 2 chronic kidney disease14
In addition to referring MR for physical rehabilitation of his knee, you discuss with MR the need and options for intensifying his diabetes therapy.
Does the fact that MR is male affect your management plan?
In people diagnosed with T2DM, there are differences between men and women with respect to risk for cardiovascular and other comorbid diseases, as well as in their psychosocial well-being and coping strategies.
Risk for Cardiovascular Disease in Type 2 Diabetes Mellitus
A systematic literature review shows that men with T2DM generally fare better than women with T2DM regarding their risk for CVD. Men with T2DM have a 2- to 3-fold increase in the risk of developing coronary heart disease (CHD) compared with men without T2DM, whereas women with T2DM have a 4- to 6-fold increase in risk compared with women without T2DM.15 Compared with women with T2DM, men with T2DM also have a better prognosis after myocardial infarction (MI) and a lower risk of death overall from CVD. Possible reasons for these differences include a lower risk of hypertension, a less severe form of dyslipidemia, and a lower prevalence of obesity in men with T2DM compared with women with T2DM.15 These same reasons for observed differences between men and women were seen in a meta-analysis of 29 studies, where the RR of fatal MI in men with T2DM compared with women with T2DM was 0.68.16 Similar findings were seen in the Skaraborg Project, which involved 1116 Swedish patients with hypertension and/or T2DM.17 Compared with a healthy population, the age-adjusted HR for fatal MI was 1.9 for men with T2DM and 5.0 for women with T2DM over 8.1 years of follow-up (RR, 0.38 for men vs women). Analysis of the data indicated that these results were not explained by the more favorable survival rate in women without T2DM than in men without T2DM.17
Somewhat different results have been reported by the Italian Diabetes and Informatics Study Group in a slightly different T2DM population. This investigation involved men and women with T2DM (N = 11,644) who could have microvascular but not macrovascular disease.18 After 4 years of follow-up, the age-adjusted incident rates for first CHD event (composite of acute MI, coronary artery bypass grafting, percutaneous transluminal coronary angioplasty) were 28.8 per 1000 person-years in men and 23.3 per 1000 person-years in women. Incident rates (per 1000 person-years) of acute MI (10.3 vs 4.7), major CHD events (13.1 vs 5.8), and fatal CHD (2.6 vs 0.6) were all significantly more frequent in men than in women, respectively. Multivariate analysis showed that hypertension and A1C were additional risk factors for CHD in men; for each 20% increment above the A1C upper limit of normal, there was a 14% risk increase for CHD. The presence of microvascular complications increased risk by 20% in men and 35% in women. In this analysis, glycemic control and hypertension were found to be the predominant risk factors in men, while high triglyceride levels, low HDL-C levels, and microangiopathy were predominant in women.
Additional multivariate analyses provide greater insight into specific factors that affect the risk of CVD and outcomes in men with T2DM. One investigation compared men and women with T2DM who were normotensive without evidence of CVD but with microalbuminuria. After 4.7 years of follow-up, men were found to be at lower risk (RR, 0.12) for a composite of death, acute MI, unstable angina, coronary interventions, heart failure, cerebral ischemic stroke or transient ischemic attack, and peripheral artery disease.19 Other investigators have reported a lower risk of stroke, including fatal stroke, in men with T2DM compared with women with T2DM.20,21 For example, analysis of the General Practice Research Database identified 22,178 men and 19,621 women with T2DM between the ages of 35 and 89 years.20 The stroke rate per 1000 person-years across all ages was 10.82 (95% confidence interval (CI), 10.17-11.51) in men and 13.16 (95% CI, 12.40-13.97) in women. In men, the rate per 1000 person-years rose from 1.81 in the 35 to 44 year age group to 28.35 in men 85 years of age or older. Although the rate of stroke per 1000 person-years was lower in women than men in the 35 to 44 year age group (1.53 vs 1.81), the rate in women exceeded that of men in the 85 years of age or older group (32.20 vs 28.35).
Other Chronic Complications
Kidney disease is affected by blood lipids, specifically HDL-C, in men with T2DM. An investigation in men and women with T2DM with normoalbuminuria or microalbuminuria at baseline showed that a low HDL-C level was an independent predictor of progression to a more advanced stage of albuminuria over 4.3 years of follow-up (HR, 0.391 for men with normal HDL-C compared with men with low HDL-C). In women, no lipid parameters were associated with progression of albuminuria.22
While these investigations do not provide a clear picture of the differences regarding cardiovascular risk between men and women with T2DM, they suggest that men with T2DM have a lower risk of nonfatal and fatal CVD and stroke than do women with T2DM. However, the lower risk seen in men may be affected by the cardiovascular endpoints measured and the presence of microvascular disease. Possible independent risk factors for CVD in men with T2DM include hypertension, poor glycemic control, and low HDL-C.
Risk factors that place MR at greater risk for CVD compared with a woman with T2DM and therefore serve as key treatment targets include:
- Hypertension—although controlled (126/78 mm Hg) with hydrochlorothiazide and lisinopril
- Poor glycemic control—A1C, 7.8% (eAG, 177mg/dL)
- –Increase physical activity—refer for knee rehabilitation
- –Intensify glucose-lowering therapy by adding an additional glucose-lowering agent (eg, dipeptidyl peptidase-4 inhibitor, glucagon-like peptide-1 receptor agonist, thiazolidinedione, α-glucosidase inhibitor, sulfonylurea, glinide, or basal insulin)
- Microalbuminuria (45 mg urinary albumin/g creatinine)—encourage better adherence to lisinopril; monitor renal function
- Hypertriglyceridemia—initiate omega-3 fatty acid or extended-release niacin
Psychosocial Well-Being, Benefit of Self-Care, and Coping Strategies
Type 2 diabetes mellitus is a chronic disease with glycemic control largely determined by patient self-management, and the attitudes and beliefs of patients with T2DM are important factors to consider from diagnosis onward.23 There are important differences between men and women with T2DM regarding attitudes and beliefs. Published investigations provide some, although not entirely consistent, insight into these psychosocial differences between men and women with T2DM. These differences are summarized in TABLE 2 .24-32 Taking these differences into account when planning treatment and when communicating with and educating the patient is essential for improved patient self-management.
TABLE 2
Psychosocial and Coping Characteristics of Men with Type 2 Diabetes Mellitus (T2DM)24-32
Compared with women with T2DM, generally, men with T2DM:
|
Key interventions for MR:
- Maintain a dialogue and enhance collaboration with MR
- Establish shared goals that are customized to incorporate MR’s personal goals
- Problem solve with MR to identify ways he can better integrate the diabetes self-care objectives of dietary changes and blood glucose self-monitoring into his daily life
- Emphasize that enhanced or greater disease control can be achieved by good self-management, including better adherence to the management plan
- Remind MR that T2DM is a progressive disease that requires intermittent medication adjustments to keep pace with its progression
- Build upon the belief that T2DM can be controlled by reminding MR that the disease was well controlled before his knee injury
- –Focus on the importance of rehabilitating his knee
- –Develop a rehabilitation plan
- Provide informational support regarding options for intensifying diabetes therapy (eg, dipeptidyl peptidase-4 inhibitor, thiazolidinedione, glucagon-like peptide-1 receptor agonist, sulfonylurea, or insulin)
- –Discuss MR’s needs and concerns, as well as barriers for each treatment option, particularly hypoglycemia and weight gain
- –Provide instruction or educational materials regarding injection devices
- –Involve the healthcare team, as appropriate
- Keep the treatment regimen as simple as possible; consider pill combinations where appropriate
Summary
The growing epidemic of T2DM requires intervention to assist patients who have been diagnosed to better manage the disease, to reduce the risk of developing the disease in those who have not yet been diagnosed, and to manage the associated complications. In addition to individualizing interventions based on a patient’s needs, concerns, and capabilities, taking gender into account is necessary. In otherwise healthy people, several independent factors appear to pose a higher risk of T2DM in men relative to women, including systolic hypertension, regular smoking, and alcohol intake ≥ 40 g/d. At the same time, men achieve greater risk reduction from moderate daily alcohol intake and a diet high in fish and seafood, low-fat dairy products, whole grains, and magnesium.
Once diagnosed with T2DM, men generally fare better than women regarding the risk for CVD; they also have a better prognosis after MI and a lower risk of death overall from CVD. Possible independent risk factors for CVD in men with T2DM that are especially important may include hypertension, poor glycemic control, and low HDL-C levels. Psychosocial complications, such as depression, are less likely in men with T2DM. However, men expend less effort coping, are less likely to utilize healthcare services, and are less informed about treatment options. Although men have a lower expectation of the benefit of self-management, they find support from family and friends more helpful than do women, but they are fearful of losing control of their disease.
Taking these gender differences into account should prove helpful as family care physicians work with men to reduce their risk of developing T2DM and in helping men diagnosed with T2DM to better self-manage their disease.
Managing the Multiple Symptoms of Benign Prostatic Hyperplasia — CME
Managing Type 2 Diabetes in Men
Meeting New Challenges with Antiplatelet Therapy in Primary Care
Dr. Aguilar has disclosed that he has ongoing relationships with the following companies: Amylin Pharmaceuticals; Eli Lilly; Janssen Pharmaceuticals, Inc; Novo Nordisk, Inc; and Takeda Pharmaceuticals USA, Inc.
SUPPORT
This program is sponsored by the PCEC and is supported by funding from Novo Nordisk, Inc.
The prevalence of type 2 diabetes mellitus (T2DM) is similar in men and women (11.8% vs 10.8%, respectively), however there are gender differences that should be considered when developing a treatment plan (eg, cardiovascular risk, psychosocial factors, coping strategies, and the perception of benefit from self-care) when managing those diagnosed with this disease and those at risk for developing it.1 This article describes these differences in the context of two patients—one at risk for T2DM being seen by his health care provider for a routine physical examination, and one who has been treated for several years for T2DM and is being seen for a follow-up office visit. For each patient, the implications for treatment are discussed.
Men at Risk for Type 2 Diabetes Mellitus
JW is a 48-year-old white male being seen for a routine physical examination; he last saw a physician 6 years ago, also for a routine physical. He has no complaints and is taking no medications. Having divorced 7 years ago, he lives alone in an apartment and eats many of his meals at fast food restaurants. JW drinks 2 to 3 beers a night several times a week and more when he socializes with his friends 2 to 3 evenings per week. He smokes socially. His father has a 12-year history of T2DM. His mother has a 4-year history of essential hypertension and a 9-year history of chronic obstructive pulmonary disease.
Physical examination shows that JW is 5’11” tall, weighs 207 pounds (body mass index (BMI), 29 kg/m2), and has a 41” waist circumference; his blood pressure (BP) is 138/86 mm Hg and respiratory rate is 17 breaths/min. The remainder of his physical examination, including eye and neurologic exams, is normal. Laboratory results, including a screening glycated homoglobin (A1C), are pending.
Key Risk Factors for Type 2 Diabetes Mellitus in Men
This case is not an uncommon presentation of a middle-aged male who has several risk factors for diabetes (see Case Study 1 continued ). JW also has key risk factors for T2DM in men. The Monitoring of Trends and Determinants in Cardiovascular Disease (MONICA) Augsburg surveys identified 128 men and 85 women with T2DM.2 Increasing age and BMI, positive parental history of T2DM, and a low high-density lipoprotein cholesterol (HDL-C) level were independent risk factors predicting the development of T2DM in both men and women. However, several other factors posed a higher risk in men relative to women, including systolic BP (hazard ratio [HR], 1.16 per 10-mm Hg increase), regular smoking (HR, 1.75), and alcohol intake ≥ 40 g/d (HR, 1.95). (Note: 1 fluid ounce 80 proof alcohol ≈ 11 g ethanol; 12 fluid ounces beer [~5% alcohol] ≈ 14 g ethanol). After adjusting for these factors, a separate analysis (4424 men, 4380 women) showed that men who lived alone were more likely to develop T2DM than either men or women who did not live alone (HR, 1.69 in men vs 0.85 in women; P = .006).3 While the number of people with T2DM in MONICA was small, the results suggest that measuring BP, particularly systolic BP, and taking a smoking and alcohol history may be especially important in men.
With respect to alcohol intake, epidemiologic and randomized clinical trials have generally demonstrated an inverse relationship between moderate alcohol consumption (20 to 30 g/d) and the long-term risk of T2DM.2,4-7 Differences among studies in how patients were grouped preclude determination of the daily alcohol consumption that confers the greatest risk benefit, although one recent study conducted over 4 years indicates that the greatest benefit in diabetes risk reduction may occur when men who previously consumed 8
Other nutrition and lifestyle patterns also seem to be particularly beneficial in reducing the risk of T2DM in men. Survey data involving 22,921 Japanese men and 29,759 Japanese women followed over 5 years showed that fish and seafood intake was significantly associated with a decreased risk of T2DM in men but not in women.9 The odds ratio of developing T2DM for the highest quartile versus the lowest quartile of fish and seafood intake was 0.73 (P = .04 for trend). Additional analysis did not identify any significant association with the fat content of fish.
Results of the Health Professionals Follow-up Study provide evidence of benefit in lowering the risk of T2DM in men who consume high amounts of low-fat dairy products, whole grains, and magnesium ( TABLE 1 ). With respect to dairy food consumption, after 12 years of follow-up involving 1243 incident cases of T2DM, the relative risk (RR) of developing T2DM in men in the top quintile of dairy intake was 0.77 compared with those in the lowest quintile (P = .003 for trend).10 Men in the highest quintile consumed 4.1 servings of dairy food per day compared with 0.5 servings per day in the lowest quintile. Each serving-per-day increase in total dairy intake was associated with a 9% lower risk for T2DM, with a lower risk seen with consumption of low-fat vs high-fat dairy food. With respect to whole-grain intake, the RR of developing T2DM was 0.58 in men in the upper vs lower quintiles (3.2 vs 0.4 servings/d), although the effect was attenuated with BMI (P = .0006 for trend).11 Similar observations were made with respect to magnesium consumption; a RR of 0.76 for T2DM was observed in men with a median magnesium consumption of 457 mg/d compared with those who consumed 270 mg/d.12
TABLE 1
Suggestions for Men Who Are at Risk of or Have Been Diagnosed with Type 2 Diabetes Mellitus (T2DM)*
For men who are at risk:
|
For men who have been diagnosed:
|
| BP, blood pressure; HDL-C, high-density lipoprotein cholesterol. *These suggestions are in addition to developing and fostering a collaborative, patient-centered approach. |
JW has the following risk factors for T2DM:
- Overweight with central adiposity
- Physical inactivity
- First-degree relative with T2DM
- Possible cardiovascular disease (CVD; hypertension, smoking)
- High daily alcohol intake (10 to 20 g alcohol/beer x 2-3 beers/d = 20 to 60 g alcohol/d)
- Poor nutrition
- Lives alone
Plan:
- Discuss above risk factors with JW
- Repeat BP measurement at next visit; implement treatment if BP >140/90 mm Hg (130/80 mm Hg if T2DM is diagnosed)
- Consider evaluation for alcohol/substance abuse
- Evaluate for smoking cessation program
- Nutrition referral for lifestyle and dietary management intervention
Working with men to avoid the development of T2DM is an important objective for family physicians. It is essential to identify men who are at increased risk, including those with prediabetes, provide education about the disease and its risk factors, and implement appropriate risk reduction strategies. Risk reduction strategies should focus on modifiable factors, such as body weight, physical activity, BP, blood lipids, blood glucose, and smoking. With JW, his motivation to “get back into shape” will help move the conversation toward achievable goals that can be set and modified over time. Other strategies that may be helpful in reducing the risk of developing T2DM in men include a moderate daily alcohol intake and a diet high in fish and seafood, low-fat dairy products, whole grains, and magnesium ( TABLE 1 ).
Once diagnosed with T2DM, there are risk management strategies that can be particularly helpful in men. These include strategies that target cardiovascular health, as well as those that consider the psychosocial and coping behaviors of men.
Risk of Complications in Men With Type 2 Diabetes Mellitus
Physical examination: BP, 126/78 mm Hg; body weight, 183 pounds (a 13 to 17 pound increase since the knee injury); waist circumference, 38” (BMI, 28 kg/m2); grade 1 retinopathy bilaterally; neurologic exam normal.
Laboratory: A1C, 7.8%; lipids normal except triglyceride level, 219 mg/dL; creatinine clearance (calculated), 69 mL/min; urine, 45 mg albumin/g creatinine.
MR’s self-measured fasting plasma glucose (FPG) has ranged from 121 to 143 mg/dL over the past month; isolated postprandial glucose (PPG) measurements show 194 to 258 mg/dL.
MR works as a vocational teacher at the local high school, and he teaches driver education after school. Review of his pharmacy records suggests his adherence over the past year has been: metformin (88%), hydrochlorothiazide (72%), and lisinopril (72%).
Assessment:
- A1C level of 7.8% indicates an estimated average glucose (eAG) of 177 mg/dL13
- –Mildly elevated FPG and PPG
- –Evidence of microvascular disease (retinopathy, nephropathy)
- –Creatinine clearance 69 mL/min and microalbuminuria indicate stage 2 chronic kidney disease14
In addition to referring MR for physical rehabilitation of his knee, you discuss with MR the need and options for intensifying his diabetes therapy.
Does the fact that MR is male affect your management plan?
In people diagnosed with T2DM, there are differences between men and women with respect to risk for cardiovascular and other comorbid diseases, as well as in their psychosocial well-being and coping strategies.
Risk for Cardiovascular Disease in Type 2 Diabetes Mellitus
A systematic literature review shows that men with T2DM generally fare better than women with T2DM regarding their risk for CVD. Men with T2DM have a 2- to 3-fold increase in the risk of developing coronary heart disease (CHD) compared with men without T2DM, whereas women with T2DM have a 4- to 6-fold increase in risk compared with women without T2DM.15 Compared with women with T2DM, men with T2DM also have a better prognosis after myocardial infarction (MI) and a lower risk of death overall from CVD. Possible reasons for these differences include a lower risk of hypertension, a less severe form of dyslipidemia, and a lower prevalence of obesity in men with T2DM compared with women with T2DM.15 These same reasons for observed differences between men and women were seen in a meta-analysis of 29 studies, where the RR of fatal MI in men with T2DM compared with women with T2DM was 0.68.16 Similar findings were seen in the Skaraborg Project, which involved 1116 Swedish patients with hypertension and/or T2DM.17 Compared with a healthy population, the age-adjusted HR for fatal MI was 1.9 for men with T2DM and 5.0 for women with T2DM over 8.1 years of follow-up (RR, 0.38 for men vs women). Analysis of the data indicated that these results were not explained by the more favorable survival rate in women without T2DM than in men without T2DM.17
Somewhat different results have been reported by the Italian Diabetes and Informatics Study Group in a slightly different T2DM population. This investigation involved men and women with T2DM (N = 11,644) who could have microvascular but not macrovascular disease.18 After 4 years of follow-up, the age-adjusted incident rates for first CHD event (composite of acute MI, coronary artery bypass grafting, percutaneous transluminal coronary angioplasty) were 28.8 per 1000 person-years in men and 23.3 per 1000 person-years in women. Incident rates (per 1000 person-years) of acute MI (10.3 vs 4.7), major CHD events (13.1 vs 5.8), and fatal CHD (2.6 vs 0.6) were all significantly more frequent in men than in women, respectively. Multivariate analysis showed that hypertension and A1C were additional risk factors for CHD in men; for each 20% increment above the A1C upper limit of normal, there was a 14% risk increase for CHD. The presence of microvascular complications increased risk by 20% in men and 35% in women. In this analysis, glycemic control and hypertension were found to be the predominant risk factors in men, while high triglyceride levels, low HDL-C levels, and microangiopathy were predominant in women.
Additional multivariate analyses provide greater insight into specific factors that affect the risk of CVD and outcomes in men with T2DM. One investigation compared men and women with T2DM who were normotensive without evidence of CVD but with microalbuminuria. After 4.7 years of follow-up, men were found to be at lower risk (RR, 0.12) for a composite of death, acute MI, unstable angina, coronary interventions, heart failure, cerebral ischemic stroke or transient ischemic attack, and peripheral artery disease.19 Other investigators have reported a lower risk of stroke, including fatal stroke, in men with T2DM compared with women with T2DM.20,21 For example, analysis of the General Practice Research Database identified 22,178 men and 19,621 women with T2DM between the ages of 35 and 89 years.20 The stroke rate per 1000 person-years across all ages was 10.82 (95% confidence interval (CI), 10.17-11.51) in men and 13.16 (95% CI, 12.40-13.97) in women. In men, the rate per 1000 person-years rose from 1.81 in the 35 to 44 year age group to 28.35 in men 85 years of age or older. Although the rate of stroke per 1000 person-years was lower in women than men in the 35 to 44 year age group (1.53 vs 1.81), the rate in women exceeded that of men in the 85 years of age or older group (32.20 vs 28.35).
Other Chronic Complications
Kidney disease is affected by blood lipids, specifically HDL-C, in men with T2DM. An investigation in men and women with T2DM with normoalbuminuria or microalbuminuria at baseline showed that a low HDL-C level was an independent predictor of progression to a more advanced stage of albuminuria over 4.3 years of follow-up (HR, 0.391 for men with normal HDL-C compared with men with low HDL-C). In women, no lipid parameters were associated with progression of albuminuria.22
While these investigations do not provide a clear picture of the differences regarding cardiovascular risk between men and women with T2DM, they suggest that men with T2DM have a lower risk of nonfatal and fatal CVD and stroke than do women with T2DM. However, the lower risk seen in men may be affected by the cardiovascular endpoints measured and the presence of microvascular disease. Possible independent risk factors for CVD in men with T2DM include hypertension, poor glycemic control, and low HDL-C.
Risk factors that place MR at greater risk for CVD compared with a woman with T2DM and therefore serve as key treatment targets include:
- Hypertension—although controlled (126/78 mm Hg) with hydrochlorothiazide and lisinopril
- Poor glycemic control—A1C, 7.8% (eAG, 177mg/dL)
- –Increase physical activity—refer for knee rehabilitation
- –Intensify glucose-lowering therapy by adding an additional glucose-lowering agent (eg, dipeptidyl peptidase-4 inhibitor, glucagon-like peptide-1 receptor agonist, thiazolidinedione, α-glucosidase inhibitor, sulfonylurea, glinide, or basal insulin)
- Microalbuminuria (45 mg urinary albumin/g creatinine)—encourage better adherence to lisinopril; monitor renal function
- Hypertriglyceridemia—initiate omega-3 fatty acid or extended-release niacin
Psychosocial Well-Being, Benefit of Self-Care, and Coping Strategies
Type 2 diabetes mellitus is a chronic disease with glycemic control largely determined by patient self-management, and the attitudes and beliefs of patients with T2DM are important factors to consider from diagnosis onward.23 There are important differences between men and women with T2DM regarding attitudes and beliefs. Published investigations provide some, although not entirely consistent, insight into these psychosocial differences between men and women with T2DM. These differences are summarized in TABLE 2 .24-32 Taking these differences into account when planning treatment and when communicating with and educating the patient is essential for improved patient self-management.
TABLE 2
Psychosocial and Coping Characteristics of Men with Type 2 Diabetes Mellitus (T2DM)24-32
Compared with women with T2DM, generally, men with T2DM:
|
Key interventions for MR:
- Maintain a dialogue and enhance collaboration with MR
- Establish shared goals that are customized to incorporate MR’s personal goals
- Problem solve with MR to identify ways he can better integrate the diabetes self-care objectives of dietary changes and blood glucose self-monitoring into his daily life
- Emphasize that enhanced or greater disease control can be achieved by good self-management, including better adherence to the management plan
- Remind MR that T2DM is a progressive disease that requires intermittent medication adjustments to keep pace with its progression
- Build upon the belief that T2DM can be controlled by reminding MR that the disease was well controlled before his knee injury
- –Focus on the importance of rehabilitating his knee
- –Develop a rehabilitation plan
- Provide informational support regarding options for intensifying diabetes therapy (eg, dipeptidyl peptidase-4 inhibitor, thiazolidinedione, glucagon-like peptide-1 receptor agonist, sulfonylurea, or insulin)
- –Discuss MR’s needs and concerns, as well as barriers for each treatment option, particularly hypoglycemia and weight gain
- –Provide instruction or educational materials regarding injection devices
- –Involve the healthcare team, as appropriate
- Keep the treatment regimen as simple as possible; consider pill combinations where appropriate
Summary
The growing epidemic of T2DM requires intervention to assist patients who have been diagnosed to better manage the disease, to reduce the risk of developing the disease in those who have not yet been diagnosed, and to manage the associated complications. In addition to individualizing interventions based on a patient’s needs, concerns, and capabilities, taking gender into account is necessary. In otherwise healthy people, several independent factors appear to pose a higher risk of T2DM in men relative to women, including systolic hypertension, regular smoking, and alcohol intake ≥ 40 g/d. At the same time, men achieve greater risk reduction from moderate daily alcohol intake and a diet high in fish and seafood, low-fat dairy products, whole grains, and magnesium.
Once diagnosed with T2DM, men generally fare better than women regarding the risk for CVD; they also have a better prognosis after MI and a lower risk of death overall from CVD. Possible independent risk factors for CVD in men with T2DM that are especially important may include hypertension, poor glycemic control, and low HDL-C levels. Psychosocial complications, such as depression, are less likely in men with T2DM. However, men expend less effort coping, are less likely to utilize healthcare services, and are less informed about treatment options. Although men have a lower expectation of the benefit of self-management, they find support from family and friends more helpful than do women, but they are fearful of losing control of their disease.
Taking these gender differences into account should prove helpful as family care physicians work with men to reduce their risk of developing T2DM and in helping men diagnosed with T2DM to better self-manage their disease.
1. Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Published 2011. Accessed May 2, 2011.
2. Meisinger C, Thorand B, Schneider A, Stieber J, Doring A, Lowel H. Sex differences in risk factors for incident type 2 diabetes mellitus: the MONICA Augsburg cohort study. Arch Intern Med. 2002;162(1):82-89.
3. Meisinger C, Kandler U, Ladwig KH. Living alone is associated with an increased risk of type 2 diabetes mellitus in men but not women from the general population: the MONICA/KORA Augsburg Cohort Study. Psychosom Med. 2009;71(7):784-788.
4. Baliunas DO, Taylor BJ, Irving H, et al. Alcohol as a risk factor for type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2009;32(11):2123-2132.
5. Mozaffarian D, Kamineni A, Carnethon M, Djoussé L, Mukamal KJ, Siscovick D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: the cardiovascular health study. Arch Intern Med. 2009;169(8):798-807.
6. Joosten MM, Grobbee DE, van der A DL, Verschuren WM, Hendriks HF, Beulens JW. Combined effect of alcohol consumption and lifestyle behaviors on risk of type 2 diabetes. Am J Clin Nutr. 2010;91(6):1777-1783.
7. Gigleux I, Gagnon J, St-Pierre A, et al. Moderate alcohol consumption is more cardioprotective in men with the metabolic syndrome. J Nutr. 2006;136(12):3027-3032.
8. Joosten MM, Chiuve SE, Mukamal KJ, Hu FB, Hendriks HF, Rimm EB. Changes in alcohol consumption and subsequent risk of type 2 diabetes in men. Diabetes. 2011;60(1):74-79.
9. Nanri A, Mizoue T, Noda M, et al. Fish intake and type 2 diabetes in Japanese men and women: the Japan Public Health Center-based Prospective Study. Am J Clin Nutr. 2011;94(3):884-891.
10. Choi HK, Willett WC, Stampfer MJ, Rimm E, Hu FB. Dairy consumption and risk of type 2 diabetes mellitus in men: a prospective study. Arch Intern Med. 2005;165(9):997-1003.
11. Fung TT, Hu FB, Pereira MA, et al. Whole-grain intake and the risk of type 2 diabetes: a prospective study in men. Am J Clin Nutr. 2002;76(3):535-540.
12. Lopez-Ridaura R, Willett WC, Rimm EB, et al. Magnesium intake and risk of type 2 diabetes in men and women. Diabetes Care. 2004;27(1):134-140.
13. Nathan DM, Kuenen J, Borg R, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31(8):1473-1478.
14. Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137-147.
15. Legato MJ, Gelzer A, Goland R, et al. Gender-specific care of the patient with diabetes: review and recommendations. Gend Med. 2006;3(2):131-158.
16. Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332(7533):73-78.
17. Larsson CA, Gullberg B, Merlo J, Rastam L, Lindblad U. Female advantage in AMI mortality is reversed in patients with type 2 diabetes in the Skaraborg Project. Diabetes Care. 2005;28(9):2246-2248.
18. Avogaro A, Giorda C, Maggini M, et al. Incidence of coronary heart disease in type 2 diabetic men and women: impact of microvascular complications, treatment, and geographic location. Diabetes Care. 2007;30(5):1241-1247.
19. Zandbergen AA, Sijbrands EJ, Lamberts SW, Bootsma AH. Normotensive women with type 2 diabetes and microalbuminuria are at high risk for macrovascular disease. Diabetes Care. 2006;29(8):1851-1855.
20. Mulnier HE, Seaman HE, Raleigh VS, et al. Risk of stroke in people with type 2 diabetes in the UK: a study using the General Practice Research Database. Diabetologia. 2006;49(12):2859-2865.
21. Tuomilehto J, Rastenyte D, Jousilahti P, Sarti C, Vartiainen E. Diabetes mellitus as a risk factor for death from stroke. Prospective study of the middle-aged Finnish population. Stroke. 1996;27(2):210-215.
22. Hanai K, Babazono T, Yoshida N, et al. Gender differences in the association between HDL cholesterol and the progression of diabetic kidney disease in type 2 diabetic patients. Nephrol Dial Transplant. 2012;27(3):1070-1075.
23. Tuerk PW, Mueller M, Egede LE. Estimating physician effects on glycemic control in the treatment of diabetes: methods, effects sizes, and implications for treatment policy. Diabetes Care. 2008;31(5):869-873.
24. Rubin RR, Peyrot M, Siminerio LM. Health care and patient-reported outcomes: results of the cross-national Diabetes Attitudes, Wishes and Needs (DAWN) study. Diabetes Care. 2006;29(6):1249-1255.
25. McCollum M, Hansen LB, Ghushchyan V, Sullivan PW. Inconsistent health perceptions for US women and men with diabetes. J Womens Health (Larchmt). 2007;16(10):1421-1428.
26. Gucciardi E, Wang SC, DeMelo M, Amaral L, Stewart DE. Characteristics of men and women with diabetes: observations during patients’ initial visit to a diabetes education centre. Can Fam Physician. 2008;54(2):219-227.
27. Chiu CJ, Wray LA. Physical disability trajectories in older Americans with and without diabetes: the role of age, gender, race or ethnicity, and education. Gerontologist. 2011;51(1):51-63.
28. Nielsen AB, de Fine Olivarius N, Gannik D, Hindsberger C, Hollnagel H. Structured personal diabetes care in primary health care affects only women’s HbA1c. Diabetes Care. 2006;29(5):963-969.
29. Shalev V, Chodick G, Heymann AD, Kokia E. Gender differences in healthcare utilization and medical indicators among patients with diabetes. Public Health. 2005;119(1):45-49.
30. Kacerovsky-Bielesz G, Lienhardt S, Hagenhofer M, et al. Sex-related psychological effects on metabolic control in type 2 diabetes mellitus. Diabetologia. 2009;52(5):781-788.
31. Brown SA, Harrist RB, Villagomez ET, Segura M, Barton SA, Hanis CL. Gender and treatment differences in knowledge, health beliefs, and metabolic control in Mexican Americans with type 2 diabetes. Diabetes Educ. 2000;26(3):425-438.
32. Liburd LC, Namageyo-Funa A, Jack L, Jr. Understanding “masculinity” and the challenges of managing type-2 diabetes among African-American men. J Natl Med Assoc. 2007;99(5):550-552, 554–558.
1. Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Published 2011. Accessed May 2, 2011.
2. Meisinger C, Thorand B, Schneider A, Stieber J, Doring A, Lowel H. Sex differences in risk factors for incident type 2 diabetes mellitus: the MONICA Augsburg cohort study. Arch Intern Med. 2002;162(1):82-89.
3. Meisinger C, Kandler U, Ladwig KH. Living alone is associated with an increased risk of type 2 diabetes mellitus in men but not women from the general population: the MONICA/KORA Augsburg Cohort Study. Psychosom Med. 2009;71(7):784-788.
4. Baliunas DO, Taylor BJ, Irving H, et al. Alcohol as a risk factor for type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2009;32(11):2123-2132.
5. Mozaffarian D, Kamineni A, Carnethon M, Djoussé L, Mukamal KJ, Siscovick D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: the cardiovascular health study. Arch Intern Med. 2009;169(8):798-807.
6. Joosten MM, Grobbee DE, van der A DL, Verschuren WM, Hendriks HF, Beulens JW. Combined effect of alcohol consumption and lifestyle behaviors on risk of type 2 diabetes. Am J Clin Nutr. 2010;91(6):1777-1783.
7. Gigleux I, Gagnon J, St-Pierre A, et al. Moderate alcohol consumption is more cardioprotective in men with the metabolic syndrome. J Nutr. 2006;136(12):3027-3032.
8. Joosten MM, Chiuve SE, Mukamal KJ, Hu FB, Hendriks HF, Rimm EB. Changes in alcohol consumption and subsequent risk of type 2 diabetes in men. Diabetes. 2011;60(1):74-79.
9. Nanri A, Mizoue T, Noda M, et al. Fish intake and type 2 diabetes in Japanese men and women: the Japan Public Health Center-based Prospective Study. Am J Clin Nutr. 2011;94(3):884-891.
10. Choi HK, Willett WC, Stampfer MJ, Rimm E, Hu FB. Dairy consumption and risk of type 2 diabetes mellitus in men: a prospective study. Arch Intern Med. 2005;165(9):997-1003.
11. Fung TT, Hu FB, Pereira MA, et al. Whole-grain intake and the risk of type 2 diabetes: a prospective study in men. Am J Clin Nutr. 2002;76(3):535-540.
12. Lopez-Ridaura R, Willett WC, Rimm EB, et al. Magnesium intake and risk of type 2 diabetes in men and women. Diabetes Care. 2004;27(1):134-140.
13. Nathan DM, Kuenen J, Borg R, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31(8):1473-1478.
14. Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137-147.
15. Legato MJ, Gelzer A, Goland R, et al. Gender-specific care of the patient with diabetes: review and recommendations. Gend Med. 2006;3(2):131-158.
16. Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332(7533):73-78.
17. Larsson CA, Gullberg B, Merlo J, Rastam L, Lindblad U. Female advantage in AMI mortality is reversed in patients with type 2 diabetes in the Skaraborg Project. Diabetes Care. 2005;28(9):2246-2248.
18. Avogaro A, Giorda C, Maggini M, et al. Incidence of coronary heart disease in type 2 diabetic men and women: impact of microvascular complications, treatment, and geographic location. Diabetes Care. 2007;30(5):1241-1247.
19. Zandbergen AA, Sijbrands EJ, Lamberts SW, Bootsma AH. Normotensive women with type 2 diabetes and microalbuminuria are at high risk for macrovascular disease. Diabetes Care. 2006;29(8):1851-1855.
20. Mulnier HE, Seaman HE, Raleigh VS, et al. Risk of stroke in people with type 2 diabetes in the UK: a study using the General Practice Research Database. Diabetologia. 2006;49(12):2859-2865.
21. Tuomilehto J, Rastenyte D, Jousilahti P, Sarti C, Vartiainen E. Diabetes mellitus as a risk factor for death from stroke. Prospective study of the middle-aged Finnish population. Stroke. 1996;27(2):210-215.
22. Hanai K, Babazono T, Yoshida N, et al. Gender differences in the association between HDL cholesterol and the progression of diabetic kidney disease in type 2 diabetic patients. Nephrol Dial Transplant. 2012;27(3):1070-1075.
23. Tuerk PW, Mueller M, Egede LE. Estimating physician effects on glycemic control in the treatment of diabetes: methods, effects sizes, and implications for treatment policy. Diabetes Care. 2008;31(5):869-873.
24. Rubin RR, Peyrot M, Siminerio LM. Health care and patient-reported outcomes: results of the cross-national Diabetes Attitudes, Wishes and Needs (DAWN) study. Diabetes Care. 2006;29(6):1249-1255.
25. McCollum M, Hansen LB, Ghushchyan V, Sullivan PW. Inconsistent health perceptions for US women and men with diabetes. J Womens Health (Larchmt). 2007;16(10):1421-1428.
26. Gucciardi E, Wang SC, DeMelo M, Amaral L, Stewart DE. Characteristics of men and women with diabetes: observations during patients’ initial visit to a diabetes education centre. Can Fam Physician. 2008;54(2):219-227.
27. Chiu CJ, Wray LA. Physical disability trajectories in older Americans with and without diabetes: the role of age, gender, race or ethnicity, and education. Gerontologist. 2011;51(1):51-63.
28. Nielsen AB, de Fine Olivarius N, Gannik D, Hindsberger C, Hollnagel H. Structured personal diabetes care in primary health care affects only women’s HbA1c. Diabetes Care. 2006;29(5):963-969.
29. Shalev V, Chodick G, Heymann AD, Kokia E. Gender differences in healthcare utilization and medical indicators among patients with diabetes. Public Health. 2005;119(1):45-49.
30. Kacerovsky-Bielesz G, Lienhardt S, Hagenhofer M, et al. Sex-related psychological effects on metabolic control in type 2 diabetes mellitus. Diabetologia. 2009;52(5):781-788.
31. Brown SA, Harrist RB, Villagomez ET, Segura M, Barton SA, Hanis CL. Gender and treatment differences in knowledge, health beliefs, and metabolic control in Mexican Americans with type 2 diabetes. Diabetes Educ. 2000;26(3):425-438.
32. Liburd LC, Namageyo-Funa A, Jack L, Jr. Understanding “masculinity” and the challenges of managing type-2 diabetes among African-American men. J Natl Med Assoc. 2007;99(5):550-552, 554–558.

