User login
Intimate Partner Violence
The prevalence of intimate partner violence (IPV; defined as mental and/or physical violence directed from 1 person in an intimate relationship to the other) varies widely, depending on the population sampled and method of data collection. In the United States, IPV against women, occurring within the year prior to contact with a healthcare professional, ranges from 2% to 15% in surveys done by telephone, in primary care clinics, or in face‐to‐face home interviews19 and from 10% to 30% in surveys of patients visiting urgent care or emergency departments.1012 The prevalence of IPV occurring at any time during the life of the patient ranges from 18% in the aforementioned settings to as high as 88% in women applying for welfare.1, 2, 4, 5, 10, 1214
Although reports indicate that victims of IPV are more likely to be hospitalized,1517 the only study assessing the prevalence of IPV in hospitalized patients included women on medical, surgical, and obstetrical services and reported 1‐year and lifetime prevalences of only 5% and 23%, respectively.18
We hypothesized that the prevalence of IPV in hospitalized patients would be at least as high as that reported from emergency departments and sought to measure the 1‐year and lifetime prevalences of IPV in women admitted to a general internal medicine service. In addition, because studies done in various outpatient settings have reported that victims of IPV have a variety of somatic complaints and an increased prevalence of chronic and functional illnesses,1923 we also sought to determine whether women with a history of IPV and women without a history of IPV had different numbers or types of positive responses to questions asked on the review of systems.
PATIENTS AND METHODS
This study was approved by the Colorado Multiple Institution Review Board, and informed consent was obtained from all participants.
Women between the ages of 18 and 60 who were admitted to the internal medicine floor service of Denver Health Medical Center (a university‐affiliated public safety‐net hospital) between January 1 and February 28, 2004 and between October 1 and October 30, 2004 were approached to participate. These dates were selected on the basis of the availability of our interviewers. Patients older than 60 were excluded to avoid overlap between IPV and the problem of elder abuse. Women were excluded if they were unable to give informed consent, were pregnant, were incarcerated, were on contact precautions, or spoke a language other than English or Spanish. Although IPV is common in pregnant women and may occur in women who are incarcerated, these are considered vulnerable populations with respect to obtaining approval from internal review boards.
The questionnaire consisted of 23 review‐of‐systems questions,24 4 questions adapted from a previously validated screen for IPV11 (Table 1), and 1 question about attempts to seek help (Table 1). Women were considered to have experienced IPV if they gave positive responses to any of the 4 questions targeting IPV. According to patient preference, the combined questionnaire was either read and filled out by each subject independently or was read to her by a female interviewer who then recorded the subject's verbal responses. All interviewers were women with a shared common concern about, and interest in, IPV. Although none had advanced training in psychology, social work, or other formal discipline that involved interviewing skills, all interviews were scripted so that interactions with subjects and completion of the questionnaires would be uniform. Responses indicating sometimes were considered to be positive. Responses that were not answered, left blank, or marked as not applicable were considered to be negative.
| 1. Have you ever been hit, kicked, punched, or otherwise hurt by someone? If so, by whom? Friend, boyfriend, girlfriend, husband, family member, somebody you do not know, other |
| 2. Within the last year, have you been hit, kicked, or otherwise hurt by someone? If so, by whom? Friend, boyfriend, girlfriend, husband, family member, somebody you do not know, other |
| 3. Do you feel safe in your current relationship? |
| 4. Is there a partner from a previous relationship who is making you feel unsafe now? |
| 5. If you answered yes to any of the above, have you ever asked for help from police, shelter, counselor, physician? If so, how long ago? |
Each patient's medical record was reviewed to determine her age, race, number of previous hospital admissions, visits to the emergency department and walk‐in clinic, visits to primary care and subspecialty physicians, and whether the patient had been screened for IPV as recorded on the admission history and physical template. Admission diagnosis was obtained from the history and physical template, and the discharge diagnosis was obtained from the discharge paperwork. Functional diagnoses were considered to be symptoms (eg, shortness of breath) or problems (eg, constipation) that could not clearly be linked to a specific disease process. All participants were offered a card containing a list of resources for victims of IPV.
Data were analyzed with SAS 8.1 (SAS Institute, Cary, NC) and SPSS 11.5 (SPSS, Chicago, IL). The Student t test was used to compare continuous variables. Data are reported as means standard deviation. Chi‐square analysis was used to test associations between race, primary language, level of education, insurance status, admitting diagnosis, discharge diagnosis, number of previous hospital admissions, visit type, and the presence of IPV. For these, P < 0.05 was considered to be significant. The association of positive review‐of‐systems responses with the presence of IPV was also tested by chi‐square analysis, but P < 0.002 was considered to be significant on the basis of a Bonferroni adjustment for multiple comparisons. A receiver operating characteristic curve was used to assess the relationship between the number of positive responses to the questions included in the review of systems and a history of IPV. The odds ratio and confidence intervals were calculated to test the association between the number of positive responses to the review‐of‐systems questions and a lifetime history of IPV.
RESULTS
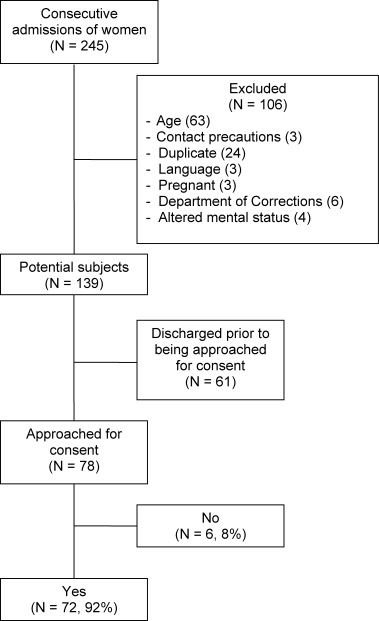
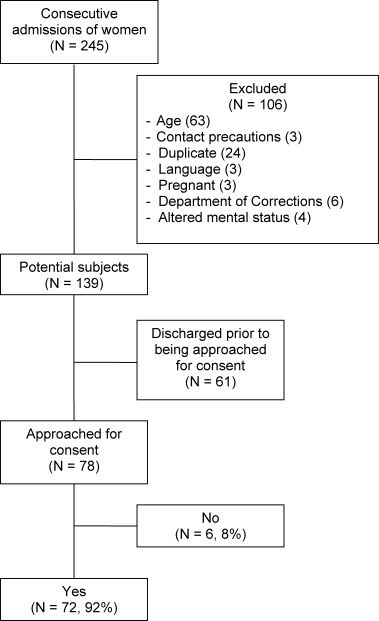
Throughout the dates of the study, 245 women were admitted to the internal medicine service, and 106 were excluded (Figure 1). Of the 139 eligible women, 78 were available to the interviewers and asked to participate, and 72 (92%) agreed. IPV occurring within the year prior to the interview or at any point in the patient's lifetime was reported by 16 (22%) and 44 (61%) subjects, respectively. No significant differences were seen in women who did or did not experience IPV at anytime in their life with respect to age, race, insurance status, education, number of scheduled outpatient, urgent, or emergent visits, or admission or discharge diagnosis even when the diagnoses were grouped into a functional category (although at best our study was powered to detect only >35% differences in prevalences; Tables 2 and 3). Of women reporting a lifetime history of IPV, 26 of 44 (59%) had previously sought help, and 9 of those 26 (35%) said that they sought help from a physician.
| IPV History | No IPV History | |
|---|---|---|
| ||
| Number (%) | 44 (61) | 28 (39) |
| Age (mean standard deviation) | 44 10 | 45 12 |
| Race [n, (%)] | ||
| Caucasian | 18 (41) | 6 (21) |
| Hispanic | 13 (30) | 15 (54) |
| African American | 12 (27) | 6 (21) |
| Other | 1 (2) | 1 (4) |
| Insurance status [n (%)] | ||
| Insured | 12 (27) | 5 (18) |
| Uninsured | 32 (73) | 23 (82) |
| Education [n (%)] | ||
| Grade school | 4 (9) | 3 (11) |
| Some high school | 13 (30) | 5 (18) |
| High school diploma | 15 (34) | 9 (32) |
| Some college | 9 (20) | 7 (25) |
| College degree | 2 (5) | 2 (7) |
| Postgraduate | 1 (2) | 2 (7) |
| Previous visit type (median, IQR) | ||
| Scheduled outpatient (includes primary care and subspecialty) | 2 (8) | 1.5 (7) |
| Emergency department and walk‐in clinic | 2 (3.5) | 1 (3) |
| Previous hospital admissions [n (%)] | ||
| 0 | 24 (55) | 16 (57) |
| 1 | 16 (36) | 4 (14) |
| 2 | 0 (0) | 4 (14) |
| 3 | 2 (5) | 2 (7) |
| >3 | 2 (5) | 2 (7) |
| Admission or Discharge Diagnosis | Admission | Discharge | ||
|---|---|---|---|---|
| IPV (n = 44) | No IPV (n = 28) | IPV (n = 44) | No IPV (n = 28) | |
| ||||
| Cardiovascular | ||||
| Chest pain (%)* | 8 (18) | 5 (18) | 6 (14) | 4 (14) |
| Cardiomyopathy | 0 | 0 | 1 | 0 |
| Cerebrovascular accident | 1 | 0 | 1 | 0 |
| Deep venous thrombosis | 0 | 0 | 1 | 0 |
| Hypertensive emergency | 0 | 0 | 1 | 0 |
| Palpitations* | 0 | 1 | 0 | 1 |
| Valvular disease | 0 | 0 | 1 | 0 |
| Venous stasis | 0 | 1 | 0 | 1 |
| Total (%) | 9 (20) | 7 (25) | 11 (25) | 6 (21) |
| Gastrointestinal | ||||
| Abdominal pain (%)* | 7 (16) | 4 (14) | 2 | 1 |
| Ascites | 0 | 1 | 0 | 0 |
| Constipation* | 0 | 0 | 1 | 0 |
| End‐stage liver disease | 1 | 1 | 1 | 2 |
| Esophagitis | 0 | 0 | 1 | 0 |
| Hepatitis | 1 | 0 | 1 | 0 |
| Nausea/vomiting* | 2 | 0 | 1 | 0 |
| Pancreatitis | 0 | 1 | 3 | 2 |
| Peptic ulcer disease | 1 | 0 | 1 | 0 |
| Upper gastrointestinal bleeding | 2 | 0 | 1 | 0 |
| Total (%) | 14 (32) | 7 (25) | 12 (27) | 5 (18) |
| Hematology/oncology | ||||
| Abdominal mass | 0 | 0 | 0 | 1 |
| Anemia | 1 | 0 | 1 | 0 |
| Breast cancer | 0 | 1 | 0 | 1 |
| Cervical cancer | 1 | 0 | 1 | 0 |
| Colon cancer | 0 | 1 | 0 | 1 |
| Sickle cell anemia | 1 | 0 | 1 | 0 |
| Thrombocytosis | 1 | 0 | 1 | 0 |
| Total (%) | 4 (9) | 2 (7) | 4 (9) | 3 (11) |
| Infectious disease | ||||
| Bacteremia/sepsis | 3 | 0 | 3 | 0 |
| Cellulitis | 1 | 0 | 1 | 1 |
| Cholangitis | 0 | 0 | 1 | 0 |
| Community‐acquired pneumonia | 2 | 2 | 2 | 1 |
| Endocarditis | 1 | 0 | 1 | 0 |
| Fever | 0 | 1 | 0 | 1 |
| Pelvic inflammatory disease | 0 | 0 | 0 | 1 |
| Urinary tract infection | 1 | 0 | 1 | 0 |
| Total (%) | 8 (18) | 3 (11) | 9 (20) | 4 (14) |
| Pulmonary | ||||
| Acute exacerbation of COPD | 0 | 0 | 1 | 0 |
| Asthma exacerbation | 1 | 1 | 1 | 2 |
| Pleuritic chest pain* | 0 | 0 | 1 | 0 |
| Pulmonary embolism | 0 | 0 | 1 | 0 |
| Shortness of breath* | 4 | 0 | 1 | 0 |
| Total (%) | 5 (11) | 1 (4) | 5 (11) | 2 (7) |
| Renal/genitourinary | ||||
| Acute renal failure | 0 | 1 | 0 | 1 |
| End‐stage renal disease | 1 | 2 | 1 | 2 |
| Nephrotic syndrome | 0 | 1 | 0 | 2 |
| Vaginal bleeding | 1 | 0 | 1 | 0 |
| Total (%) | 2 (5) | 4 (14) | 2 (5) | 5 (18) |
| Other | ||||
| Diabetic ketoacidosis | 0 | 1 | 0 | 1 |
| Extremity pain* | 0 | 1 | 0 | 0 |
| Mediastinal thickening | 0 | 0 | 0 | 1 |
| Hyponatremia | 0 | 1 | 0 | 1 |
| Lower extremity swelling | 2 | 1 | 0 | 0 |
| Somatization* | 0 | 0 | 1 | 0 |
| Total (%) | 2 (5) | 4 (14) | 1 (2) | 3 (11) |
| Total functional diagnoses (%) | 21 (48) | 11 (39) | 12 (27) | 6 (21) |
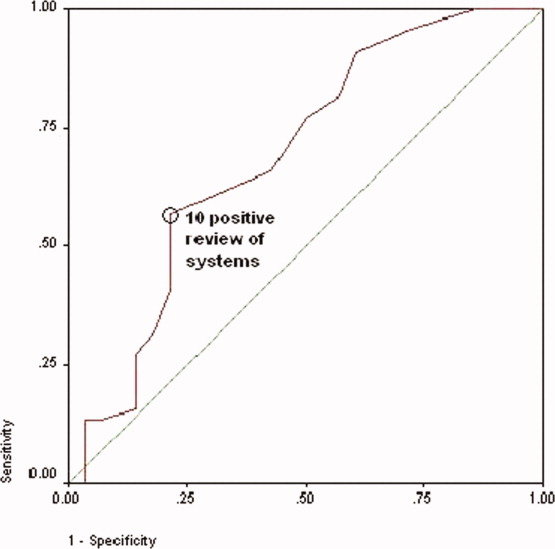
Women with a 1‐year history of IPV and women without a 1‐year history of IPV had 11.4 4.7 and 7.7 5.4 positive responses to the review of systems (P < 0.01), respectively. Women with a lifetime history of IPV and women without a lifetime history of IPV had 10.9 4.4 and 7.7 5.4 positive responses (P < 0.01), respectively. The receiver operating characteristic curve of the number of positive responses versus a lifetime history of IPV is presented in Figure 2. Subjects with 10 or more positive responses were 4.8 times more likely to report a lifetime history of IPV than subjects with 9 or fewer positive responses (confidence interval = 1.614.2, P = 0.003). The c‐statistic indicating the ability of the review of systems to properly classify cases when there were 10 or more positive responses was 0.692.
No differences were observed in the responses to the individual review of systems questions in women who did or did not have a lifetime history of IPV, with the exception that those with a positive history more commonly complained of difficulty sleeping and numbness and tingling in their hands or feet (although at best our study was sufficiently powered to detect only >20% differences in prevalences; Table 4). Although the sensitivity of having problems sleeping or experiencing numbness or tingling in patients with IPV was high, the specificity and positive and negative predictive values were not (Table 5).
| Review‐of‐Systems Questions | IPV History (n = 44) | No IPV History (n = 28) | P Value |
|---|---|---|---|
| |||
| 1. Shortness of breath | 25 (57) | 10 (36) | 0.081 |
| 2. Chest pain/pressure | 19 (43) | 9 (32) | 0.349 |
| 3. Abdominal pain | 17 (39) | 10 (36) | 0.803 |
| 4. Headaches | 24 (55) | 13 (46) | 0.502 |
| 5. Rashes | 15 (34) | 9 (32) | 0.864 |
| 6. Bruising | 32 (73) | 12 (43) | 0.011 |
| 7. Joint pain/stiffness | 27 (61) | 11 (39) | 0.067 |
| 8. Muscle pain/spasms | 22 (50) | 11 (39) | 0.374 |
| 9. Pain with intercourse | 8 (19) | 4 (14) | 0.753 |
| 10. Pelvic pain/cramps | 13 (30) | 5 (18) | 0.264 |
| 11. Nausea/vomiting | 19 (43) | 11 (39) | 0.744 |
| 12. Nervous/anxious | 28 (64) | 14 (50) | 0.253 |
| 13. Sad/crying | 21 (48) | 12 (43) | 0.686 |
| 14. Weight gain/loss | 26 (59) | 17 (61) | 0.891 |
| 15. Trouble sleeping | 37 (84) | 12 (43) | 0.000* |
| 16. Fever/chills | 19 (43) | 6 (21) | 0.059 |
| 17. Frequent/painful urination | 11 (25) | 6 (21) | 0.728 |
| 18. Pounding/emrregular heart beat | 14 (32) | 7 (25) | 0.535 |
| 19. Dizzy/passing out | 13 (30) | 7 (25) | 0.675 |
| 20. Memory problem | 19 (43) | 7 (25) | 0.117 |
| 21. Diarrhea/constipation | 27 (61) | 10 (36) | 0.034 |
| 22. Numbness/tingling | 35 (80) | 9 (32) | <0.0001* |
| 23. Pain chewing/swallowing | 8 (18) | 5 (18) | 0.972 |
| Trouble Sleeping | Numbness/Tingling | |
|---|---|---|
| Sensitivity (%) | 84 | 74 |
| Specificity (%) | 57 | 68 |
| Positive predictive value (%) | 76 | 78 |
| Negative predictive value (%) | 70 | 68 |
The admission history forms filled out by first‐year admitting residents showed that only 18 (25%) of the women were screened for IPV, even though the history and physical examination template used at Denver Health Medical Center includes a prompt in the social history section pertaining to a history of violence as a reminder.
DISCUSSION
The important findings of this study were that women admitted to the internal medicine service of a university‐affiliated public safety‐net hospital had a high prevalence of IPV (22% and 61% 1‐year and lifetime prevalences, respectively), that most women with a history of IPV had previously sought help for the problem, many from physicians, that women were more likely to have a history of IPV if they had >10 positive responses to questions asked in a routine review of systems (particularly problems sleeping and experiencing numbness or tingling in their extremities), and that routine screening for IPV was uncommon at the time of admission.
These conclusions should be interpreted with respect to a number of limitations in our study. First, although our study was designed to be a consecutive series, the interviewers did not have sufficient time to meet with and interview every woman admitted before they were discharged. This occurred in part because the interviewers were available only for a portion of each day, some patients were discharged within 24 hours of admission, and many were out of their rooms for ancillary testing. Within the interviewers' time constraints, however, all hospitalized women meeting entry criteria who were available were approached. Our data could, however, overrepresent the prevalence of IPV if hospitalized women with a history of IPV had longer hospital stays than those who did not or if those experiencing IPV were out of their rooms less frequently (eg, for diagnostic tests). On the other hand, our data could underrepresent the true prevalence of IPV if patients with a history of IPV had shorter hospital stays or if they received more ancillary testing that caused them to be out of their rooms more frequently. Second, none of our interviewers had specific training in interviewing techniques. Accordingly, our data could have underestimated the true prevalence of IPV if interviewers with advanced training in probing sensitive topics had more success in eliciting positive responses. Third, the relationship between a history of IPV and multiple positive responses to the review of systems may be confounded if some of these patients also had a history of adverse childhood experiences or other experiences resulting in posttraumatic stress disorder as these patients also have an increased prevalence of chronic and functional disorders.2527 Finally, as our numbers were small, we were not powered to detect clinically important differences in demographics or specific positive answers on the review of systems.
To the best of our knowledge, the only study presenting IPV prevalence data in patients hospitalized for other than psychiatric problems was performed by McKenzie and colleagues18 in 1997. In their group of 130 patients (61 on internal medicine, 59 on surgery, 7 on obstetrics, and 3 on psychiatry), the 1‐year and lifetime prevalences of IPV were only 5% and 26%, respectively. McKenzie and colleagues used only 1 question to screen for IPV, but that single question incorporated 2 of the 4 questions used in our survey. Forty‐three of our 44 patients (98%) with a history of IPV were discovered on the basis of these 2 questions. The hospitals in which the 2 studies were done were similar, as were the ages and levels of education of the 2 populations studied and the percentage of eligible patients who agreed to participate. The patients in the 2 studies were different with respect to race, language mix, and the percentage who were insured, but neither study found differences in the prevalence of IPV as a function of race or insurance (although others have found an association of IPV with being uninsured1, 3, 4, 12, 23). Our study was conducted in women admitted exclusively to an internal medicine service, whereas nearly half of the patients studied by McKenzie and colleagues were admitted to surgical, gynecologic, or psychiatric services. Although McKenzie and colleagues found no difference in the prevalence of IPV as a function of admitting service, others have suggested that the prevalence of IPV is higher in patients admitted for trauma or psychiatric problems.1517, 28 The percentage of patients who self‐administered the questionnaires was 57% in our study and 77% in the study by McKenzie and colleagues. Neither study, however, found a difference in the percentage of IPV in patients who self‐administered the survey versus those who were interviewed. Women may have become more comfortable discussing this issue in the 10‐year interval between these 2 studies, or the prevalence of IPV may have increased. The only other study of IPV in hospitalized patients of which we are aware reported a 90% 1‐year prevalence in suicidal women admitted to a psychiatric service.28
Several studies have reported that victims of IPV have multiple somatic complaints and an increased prevalence of chronic and functional illnesses.1923 We confirmed that women experiencing IPV have more positive responses to questions posed in a review of systems, but the low specificity and positive and negative predictive values of the responses make this association of little clinical utility.
For only 18 of the 72 patients (25%) in our study was there evidence that they were screened for a history of IPV by the admitting resident. If more women were screened without a response being recorded, or if women were screened only for a current history of violence, our data may not accurately reflect the true rate at which screening occurred; however, the rate of screening that we observed is consistent with a number of other studies.12, 22, 2931 Fourteen of 18 patients who were screened for IPV by the resident gave negative responses. Ten of these, however, gave positive responses to our interviewers. Accordingly, the sensitivity, specificity, and positive and negative predictive values of the information recorded by the admitting resident were 40%, 100%, 100%, and 57%, respectively (assuming that the responses given to the IPV survey represent the gold standard), and this confirms that routine screening underestimates the prevalence of this problem. Accordingly, we identified 2 problems pertaining to screening for IPV: (1) it is not routinely done at the time of hospital admission, and (2) responses reported during routine screening are frequently incorrect. A number of barriers to routine screening have been previously identified, as have interventions designed to increase screening.32 Providing specific screening questions increases the identification of victims of IPV, but simply educating healthcare providers does not.32 Our history and physical templates have a prompt for violence victim to facilitate the screening, but as a result of this study, we are changing our prompting question and indicating what should be done if the response is positive.
The US Preventive Services Task Force and the Canadian Task Force on Preventive Health Care both concluded that there was insufficient evidence to recommend for or against routine screening for IPV.3335 Their rationale was that trials assessing the effectiveness of screening have not been published, that studies designed to assess the effectiveness of any resulting intervention are few in number, focused on pregnant women, and limited by problems in study design, that no studies have determined the accuracy of the screening tools, and that none have addressed the potential harm of screening.3335 The US Preventive Services Task Force did recommend screening if providers were concerned about IPV.34 Our data would suggest that there is little in the admission history that distinguishes women who might be victims of IPV from those who might not. Guidelines published by the American Medical Association, the American Academy of Family Physicians, and the American College of Obstetricians and Gynecologists promote routine screening of all patients.3638 Janssen and colleagues39 support the importance of screening on the basis that IPV is associated with numerous physical and mental health problems (eg, arthritis, migraines and other types of headaches, vaginal bleeding, ulcers, spastic colon, chronic pain, substance abuse, depression, and suicide ideation) and that establishing the link between these conditions and IPV could be important with respect to developing appropriate diagnostic and therapeutic approaches to patients' complaints. Screening also allows physicians to become more knowledgeable about their patients' lives, facilitating their ability to provide a supportive relationship that, in turn, increases women's likelihood of using an intervention method.39 We did not confirm an increased prevalence of any of the complaints noted by Janssen and colleagues in the women experiencing a history of IPV, but we did find an increased prevalence of insomnia and extremity numbness in women admitting to IPV as well as an overall increase in the number of positive responses to the review of systems. Screening identifies women who should receive information about reporting IPV, obtaining available assistance, planning for personal safety, and formal counseling as these have all been shown to reduce the severity of IPV and to improve the quality of life in rather large, randomized controlled trials.4043
As previously observed by others,13, 22, 29, 4446 the large majority of women that we approached welcomed screening for IPV. Over half of those with a history of IPV had previously sought help for the problem, over one‐third of these sought help from physicians, and most took the resource card that we offered, regardless of whether they did or did not have a history of IPV (this suggests either that our data may actually underestimate the true prevalence of IPV or that patients taking the information knew of others experiencing this problem). Accordingly, regardless of whether physicians believe that routine screening is warranted, patients see physicians and other healthcare workers as a resource for this problem.
We have confirmed that a history of IPV is very common in women admitted to an internal medicine service of a university‐affiliated public hospital and that female victims of IPV have more positive responses on the review of systems (particularly difficulty sleeping and extremity numbness or tingling) than those who have not. Although we initially hypothesized that finding numerous somatic complaints might serve as a marker for IPV, thereby identifying patients for whom more careful screening should occur, finding such a high prevalence of IPV argues that screening should be a routine part of the history for all women admitted to internal medicine inpatient services.
Acknowledgements
The authors thank the patients who agreed to participate in this study during their hospitalization. They also thank Cheri Maestas and Debbie Rodriquez for their support and help in interviewing patients.
- ,,.Prevalence and determinants of intimate partner abuse among public hospital primary care patients.J Gen Intern Med.2000;15:811–817.
- ,,,.Women's experiences with violence: a national study.Womens Health Issues.2007;17:3–12.
- ,,,.Multistate analysis of factors associated with intimate partner violence.Am J Prev Med.2002;22:156–164.
- ,,,.Frequency and correlates of intimate partner violence by type: physical, sexual, and psychological battering.Am J Public Health.2000;90:553–559.
- ,,,,.Prevalence of domestic violence among patients in three ambulatory care internal medicine clinics.J Gen Intern Med.1991;6:317–322.
- ,,,.Prevalence of partner violence against 7,443 African American, White and Hispanic women receiving care at urban public primary care clinics.Public Health Nurs.2005;22:98–107.
- ,,,,.Evaluating domestic partner abuse in a family practice clinic.Fam Med.1997;29:492–495.
- ,.Prevalence and predictors of physical partner abuse among Mexican American women.Am J Public Health.2001;91:441–445.
- ,,.Rates of intimate partner violence in the United States.Am J Public Health.1998;88:1702–1704.
- ,,,.Domestic violence against women incidence and prevalence in an emergency department population.JAMA.1995;273:1763–1767.
- ,,, et al.Accuracy of 3 brief screening questions for detecting partner violence in the emergency department.JAMA.1997;277:1357–1361.
- ,,.A prevalence survey of abuse and screening for abuse in urgent care patients.Obstet Gynecol.1998;91:511–514.
- Morbidity and Mortality Weekly Report.Use of medical care, police assistance and restraining orders by women reporting intimate partner violence—Massachusetts, 1996–1997.JAMA.2000;284:558.
- ,,.Interpersonal violence among women seeking welfare: unraveling lives.Am J Public Health.2006;96:1409–1415.
- ,.A 5‐year follow‐up study of 117 battered women.Am J Public Health.1991;81:1486–1488.
- ,,.Rates and relative risk of hospital admission among women in violent intimate partner relationships.Am J Public Health.2000;90:1416–1420.
- ,,,.Intimate partner violence against women: do victims cost health plans more?J Fam Pract.1999;48:439–443.
- ,,,.Prevalence of domestic violence in an inpatient female population.J Gen Intern Med.1998;13:277–279.
- ,,, et al.Intimate partner violence and physical health consequences.Arch Intern Med.2002;162:1157–1163.
- ,,,,.Physical health consequences of physical and psychological intimate partner violence.Arch Fam Med.2000;9:451–457.
- ,,, et al.Sexual and physical abuse in women with functional or organic gastrointestinal disorders.Ann Intern Med.1990;113:828–833.
- ,,.Prevalence of intimate partner violence and health implications for women using emergency departments and primary care clinics.Womens Health Issues.2004;14:19–29.
- ,,, et al.The “battering syndrome”: prevalence and clinical characteristics of domestic violence in primary care internal medicine practices.Ann Intern Med.1995;123:737–746.
- ,.DeGowin and DeGowin's Bedside Diagnostic Examination.5th ed.New York, NY:Macmillan Publishing;1987:18–29.
- ,,, et al.Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study.Am J Prev Med.1998;14:245–258.
- ,,,,,.Posttraumatic stress disorder and health status among female and male medical patients.J Trauma Stress.2004;17:1–9.
- ,,,,.Posttraumatic stress disorder and physical comorbidity among female children and adolescents: results from service‐use data.Pediatrics.2005:116;e767–e776.
- ,,,,.Prevalence and severity of intimate partner violence and associations with family functioning and alcohol abuse in psychiatric inpatients with suicidal intent.J Clin Psychiatry.2006;67:23–29.
- ,,.Intimate partner violence screening and intervention: data from eleven Pennsylvania and California community hospital emergency departments.J Emerg Nurs.2001;27:141–149.
- ,.Missed opportunities: emergency department visits by police‐identified victims of intimate partner violence.Emerg Med.2006;47:190–199.
- ,,, et al.Intimate partner violence and patient screening across medical specialties.Acad Emerg Med.2005;12:712–722.
- ,,,,.Screening for intimate partner violence by health care providers: barriers and interventions.Am J Prev Med.2000;19:230–237.
- ,,,.Screening women and elderly adults for family and intimate partner violence: a review of the evidence for the U.S. Preventive Services Task Force.Ann Intern Med.2004;140:387–396.
- U.S. Preventive Services Task Force.Screening for family and intimate partner violence: recommendation statement.Ann Intern Med.2004;140:382–386.
- ,.Interventions for violence against women: scientific review.JAMA.2003;289:589–600.
- American Medical Association. Policy H‐515.965: family and intimate partner violence. Available at: http://www.ama‐assn.org. Accessed May2007.
- American Academy of Family Physicians. Family and intimate partner violence and abuse. Available at: www.aafp.org/x16506.xml. Accessed May2007.
- Domestic Violence.Washington, DC:American College of Obstetrics and Gynecology;1999. Educational Bulletin Number; No. 257.
- ,,.Assessment for intimate partner violence: where do we stand?J Am Board Fam Med.2006;19:413–415.
- ,,,,,.What happens when health care providers ask about intimate partner violence? A description of consequences from the perspectives of female survivors.JAMA.2003;58:76–81.
- ,,,,,.Assessing intimate partner violence in health care settings leads to women's receipt of interventions and improved health.Public Health Rep.2006;121:435–444.
- ,,.An evaluation of interventions to decrease intimate partner violence to pregnant women.Public Health Nurs.2000;17:443–451.
- ,.Reducing violence using community‐based advocacy for women with abusive partners.J Consult Clin Psychol.1999;67:43–53.
- ,,,,.Help‐seeking for intimate partner violence and forced sex in South Carolina.Am J Prev Med.2000;19:316–320.
- ,,, et al.Women's opinions about domestic violence screening and mandatory reporting.Am J Prev Med.2000;19:279–285.
- ,,,.The factors associated with disclosure of intimate partner abuse to clinicians.J Fam Pract.2001;50:338–344.
The prevalence of intimate partner violence (IPV; defined as mental and/or physical violence directed from 1 person in an intimate relationship to the other) varies widely, depending on the population sampled and method of data collection. In the United States, IPV against women, occurring within the year prior to contact with a healthcare professional, ranges from 2% to 15% in surveys done by telephone, in primary care clinics, or in face‐to‐face home interviews19 and from 10% to 30% in surveys of patients visiting urgent care or emergency departments.1012 The prevalence of IPV occurring at any time during the life of the patient ranges from 18% in the aforementioned settings to as high as 88% in women applying for welfare.1, 2, 4, 5, 10, 1214
Although reports indicate that victims of IPV are more likely to be hospitalized,1517 the only study assessing the prevalence of IPV in hospitalized patients included women on medical, surgical, and obstetrical services and reported 1‐year and lifetime prevalences of only 5% and 23%, respectively.18
We hypothesized that the prevalence of IPV in hospitalized patients would be at least as high as that reported from emergency departments and sought to measure the 1‐year and lifetime prevalences of IPV in women admitted to a general internal medicine service. In addition, because studies done in various outpatient settings have reported that victims of IPV have a variety of somatic complaints and an increased prevalence of chronic and functional illnesses,1923 we also sought to determine whether women with a history of IPV and women without a history of IPV had different numbers or types of positive responses to questions asked on the review of systems.
PATIENTS AND METHODS
This study was approved by the Colorado Multiple Institution Review Board, and informed consent was obtained from all participants.
Women between the ages of 18 and 60 who were admitted to the internal medicine floor service of Denver Health Medical Center (a university‐affiliated public safety‐net hospital) between January 1 and February 28, 2004 and between October 1 and October 30, 2004 were approached to participate. These dates were selected on the basis of the availability of our interviewers. Patients older than 60 were excluded to avoid overlap between IPV and the problem of elder abuse. Women were excluded if they were unable to give informed consent, were pregnant, were incarcerated, were on contact precautions, or spoke a language other than English or Spanish. Although IPV is common in pregnant women and may occur in women who are incarcerated, these are considered vulnerable populations with respect to obtaining approval from internal review boards.
The questionnaire consisted of 23 review‐of‐systems questions,24 4 questions adapted from a previously validated screen for IPV11 (Table 1), and 1 question about attempts to seek help (Table 1). Women were considered to have experienced IPV if they gave positive responses to any of the 4 questions targeting IPV. According to patient preference, the combined questionnaire was either read and filled out by each subject independently or was read to her by a female interviewer who then recorded the subject's verbal responses. All interviewers were women with a shared common concern about, and interest in, IPV. Although none had advanced training in psychology, social work, or other formal discipline that involved interviewing skills, all interviews were scripted so that interactions with subjects and completion of the questionnaires would be uniform. Responses indicating sometimes were considered to be positive. Responses that were not answered, left blank, or marked as not applicable were considered to be negative.
| 1. Have you ever been hit, kicked, punched, or otherwise hurt by someone? If so, by whom? Friend, boyfriend, girlfriend, husband, family member, somebody you do not know, other |
| 2. Within the last year, have you been hit, kicked, or otherwise hurt by someone? If so, by whom? Friend, boyfriend, girlfriend, husband, family member, somebody you do not know, other |
| 3. Do you feel safe in your current relationship? |
| 4. Is there a partner from a previous relationship who is making you feel unsafe now? |
| 5. If you answered yes to any of the above, have you ever asked for help from police, shelter, counselor, physician? If so, how long ago? |
Each patient's medical record was reviewed to determine her age, race, number of previous hospital admissions, visits to the emergency department and walk‐in clinic, visits to primary care and subspecialty physicians, and whether the patient had been screened for IPV as recorded on the admission history and physical template. Admission diagnosis was obtained from the history and physical template, and the discharge diagnosis was obtained from the discharge paperwork. Functional diagnoses were considered to be symptoms (eg, shortness of breath) or problems (eg, constipation) that could not clearly be linked to a specific disease process. All participants were offered a card containing a list of resources for victims of IPV.
Data were analyzed with SAS 8.1 (SAS Institute, Cary, NC) and SPSS 11.5 (SPSS, Chicago, IL). The Student t test was used to compare continuous variables. Data are reported as means standard deviation. Chi‐square analysis was used to test associations between race, primary language, level of education, insurance status, admitting diagnosis, discharge diagnosis, number of previous hospital admissions, visit type, and the presence of IPV. For these, P < 0.05 was considered to be significant. The association of positive review‐of‐systems responses with the presence of IPV was also tested by chi‐square analysis, but P < 0.002 was considered to be significant on the basis of a Bonferroni adjustment for multiple comparisons. A receiver operating characteristic curve was used to assess the relationship between the number of positive responses to the questions included in the review of systems and a history of IPV. The odds ratio and confidence intervals were calculated to test the association between the number of positive responses to the review‐of‐systems questions and a lifetime history of IPV.
RESULTS
Throughout the dates of the study, 245 women were admitted to the internal medicine service, and 106 were excluded (Figure 1). Of the 139 eligible women, 78 were available to the interviewers and asked to participate, and 72 (92%) agreed. IPV occurring within the year prior to the interview or at any point in the patient's lifetime was reported by 16 (22%) and 44 (61%) subjects, respectively. No significant differences were seen in women who did or did not experience IPV at anytime in their life with respect to age, race, insurance status, education, number of scheduled outpatient, urgent, or emergent visits, or admission or discharge diagnosis even when the diagnoses were grouped into a functional category (although at best our study was powered to detect only >35% differences in prevalences; Tables 2 and 3). Of women reporting a lifetime history of IPV, 26 of 44 (59%) had previously sought help, and 9 of those 26 (35%) said that they sought help from a physician.

| IPV History | No IPV History | |
|---|---|---|
| ||
| Number (%) | 44 (61) | 28 (39) |
| Age (mean standard deviation) | 44 10 | 45 12 |
| Race [n, (%)] | ||
| Caucasian | 18 (41) | 6 (21) |
| Hispanic | 13 (30) | 15 (54) |
| African American | 12 (27) | 6 (21) |
| Other | 1 (2) | 1 (4) |
| Insurance status [n (%)] | ||
| Insured | 12 (27) | 5 (18) |
| Uninsured | 32 (73) | 23 (82) |
| Education [n (%)] | ||
| Grade school | 4 (9) | 3 (11) |
| Some high school | 13 (30) | 5 (18) |
| High school diploma | 15 (34) | 9 (32) |
| Some college | 9 (20) | 7 (25) |
| College degree | 2 (5) | 2 (7) |
| Postgraduate | 1 (2) | 2 (7) |
| Previous visit type (median, IQR) | ||
| Scheduled outpatient (includes primary care and subspecialty) | 2 (8) | 1.5 (7) |
| Emergency department and walk‐in clinic | 2 (3.5) | 1 (3) |
| Previous hospital admissions [n (%)] | ||
| 0 | 24 (55) | 16 (57) |
| 1 | 16 (36) | 4 (14) |
| 2 | 0 (0) | 4 (14) |
| 3 | 2 (5) | 2 (7) |
| >3 | 2 (5) | 2 (7) |
| Admission or Discharge Diagnosis | Admission | Discharge | ||
|---|---|---|---|---|
| IPV (n = 44) | No IPV (n = 28) | IPV (n = 44) | No IPV (n = 28) | |
| ||||
| Cardiovascular | ||||
| Chest pain (%)* | 8 (18) | 5 (18) | 6 (14) | 4 (14) |
| Cardiomyopathy | 0 | 0 | 1 | 0 |
| Cerebrovascular accident | 1 | 0 | 1 | 0 |
| Deep venous thrombosis | 0 | 0 | 1 | 0 |
| Hypertensive emergency | 0 | 0 | 1 | 0 |
| Palpitations* | 0 | 1 | 0 | 1 |
| Valvular disease | 0 | 0 | 1 | 0 |
| Venous stasis | 0 | 1 | 0 | 1 |
| Total (%) | 9 (20) | 7 (25) | 11 (25) | 6 (21) |
| Gastrointestinal | ||||
| Abdominal pain (%)* | 7 (16) | 4 (14) | 2 | 1 |
| Ascites | 0 | 1 | 0 | 0 |
| Constipation* | 0 | 0 | 1 | 0 |
| End‐stage liver disease | 1 | 1 | 1 | 2 |
| Esophagitis | 0 | 0 | 1 | 0 |
| Hepatitis | 1 | 0 | 1 | 0 |
| Nausea/vomiting* | 2 | 0 | 1 | 0 |
| Pancreatitis | 0 | 1 | 3 | 2 |
| Peptic ulcer disease | 1 | 0 | 1 | 0 |
| Upper gastrointestinal bleeding | 2 | 0 | 1 | 0 |
| Total (%) | 14 (32) | 7 (25) | 12 (27) | 5 (18) |
| Hematology/oncology | ||||
| Abdominal mass | 0 | 0 | 0 | 1 |
| Anemia | 1 | 0 | 1 | 0 |
| Breast cancer | 0 | 1 | 0 | 1 |
| Cervical cancer | 1 | 0 | 1 | 0 |
| Colon cancer | 0 | 1 | 0 | 1 |
| Sickle cell anemia | 1 | 0 | 1 | 0 |
| Thrombocytosis | 1 | 0 | 1 | 0 |
| Total (%) | 4 (9) | 2 (7) | 4 (9) | 3 (11) |
| Infectious disease | ||||
| Bacteremia/sepsis | 3 | 0 | 3 | 0 |
| Cellulitis | 1 | 0 | 1 | 1 |
| Cholangitis | 0 | 0 | 1 | 0 |
| Community‐acquired pneumonia | 2 | 2 | 2 | 1 |
| Endocarditis | 1 | 0 | 1 | 0 |
| Fever | 0 | 1 | 0 | 1 |
| Pelvic inflammatory disease | 0 | 0 | 0 | 1 |
| Urinary tract infection | 1 | 0 | 1 | 0 |
| Total (%) | 8 (18) | 3 (11) | 9 (20) | 4 (14) |
| Pulmonary | ||||
| Acute exacerbation of COPD | 0 | 0 | 1 | 0 |
| Asthma exacerbation | 1 | 1 | 1 | 2 |
| Pleuritic chest pain* | 0 | 0 | 1 | 0 |
| Pulmonary embolism | 0 | 0 | 1 | 0 |
| Shortness of breath* | 4 | 0 | 1 | 0 |
| Total (%) | 5 (11) | 1 (4) | 5 (11) | 2 (7) |
| Renal/genitourinary | ||||
| Acute renal failure | 0 | 1 | 0 | 1 |
| End‐stage renal disease | 1 | 2 | 1 | 2 |
| Nephrotic syndrome | 0 | 1 | 0 | 2 |
| Vaginal bleeding | 1 | 0 | 1 | 0 |
| Total (%) | 2 (5) | 4 (14) | 2 (5) | 5 (18) |
| Other | ||||
| Diabetic ketoacidosis | 0 | 1 | 0 | 1 |
| Extremity pain* | 0 | 1 | 0 | 0 |
| Mediastinal thickening | 0 | 0 | 0 | 1 |
| Hyponatremia | 0 | 1 | 0 | 1 |
| Lower extremity swelling | 2 | 1 | 0 | 0 |
| Somatization* | 0 | 0 | 1 | 0 |
| Total (%) | 2 (5) | 4 (14) | 1 (2) | 3 (11) |
| Total functional diagnoses (%) | 21 (48) | 11 (39) | 12 (27) | 6 (21) |
Women with a 1‐year history of IPV and women without a 1‐year history of IPV had 11.4 4.7 and 7.7 5.4 positive responses to the review of systems (P < 0.01), respectively. Women with a lifetime history of IPV and women without a lifetime history of IPV had 10.9 4.4 and 7.7 5.4 positive responses (P < 0.01), respectively. The receiver operating characteristic curve of the number of positive responses versus a lifetime history of IPV is presented in Figure 2. Subjects with 10 or more positive responses were 4.8 times more likely to report a lifetime history of IPV than subjects with 9 or fewer positive responses (confidence interval = 1.614.2, P = 0.003). The c‐statistic indicating the ability of the review of systems to properly classify cases when there were 10 or more positive responses was 0.692.

No differences were observed in the responses to the individual review of systems questions in women who did or did not have a lifetime history of IPV, with the exception that those with a positive history more commonly complained of difficulty sleeping and numbness and tingling in their hands or feet (although at best our study was sufficiently powered to detect only >20% differences in prevalences; Table 4). Although the sensitivity of having problems sleeping or experiencing numbness or tingling in patients with IPV was high, the specificity and positive and negative predictive values were not (Table 5).
| Review‐of‐Systems Questions | IPV History (n = 44) | No IPV History (n = 28) | P Value |
|---|---|---|---|
| |||
| 1. Shortness of breath | 25 (57) | 10 (36) | 0.081 |
| 2. Chest pain/pressure | 19 (43) | 9 (32) | 0.349 |
| 3. Abdominal pain | 17 (39) | 10 (36) | 0.803 |
| 4. Headaches | 24 (55) | 13 (46) | 0.502 |
| 5. Rashes | 15 (34) | 9 (32) | 0.864 |
| 6. Bruising | 32 (73) | 12 (43) | 0.011 |
| 7. Joint pain/stiffness | 27 (61) | 11 (39) | 0.067 |
| 8. Muscle pain/spasms | 22 (50) | 11 (39) | 0.374 |
| 9. Pain with intercourse | 8 (19) | 4 (14) | 0.753 |
| 10. Pelvic pain/cramps | 13 (30) | 5 (18) | 0.264 |
| 11. Nausea/vomiting | 19 (43) | 11 (39) | 0.744 |
| 12. Nervous/anxious | 28 (64) | 14 (50) | 0.253 |
| 13. Sad/crying | 21 (48) | 12 (43) | 0.686 |
| 14. Weight gain/loss | 26 (59) | 17 (61) | 0.891 |
| 15. Trouble sleeping | 37 (84) | 12 (43) | 0.000* |
| 16. Fever/chills | 19 (43) | 6 (21) | 0.059 |
| 17. Frequent/painful urination | 11 (25) | 6 (21) | 0.728 |
| 18. Pounding/emrregular heart beat | 14 (32) | 7 (25) | 0.535 |
| 19. Dizzy/passing out | 13 (30) | 7 (25) | 0.675 |
| 20. Memory problem | 19 (43) | 7 (25) | 0.117 |
| 21. Diarrhea/constipation | 27 (61) | 10 (36) | 0.034 |
| 22. Numbness/tingling | 35 (80) | 9 (32) | <0.0001* |
| 23. Pain chewing/swallowing | 8 (18) | 5 (18) | 0.972 |
| Trouble Sleeping | Numbness/Tingling | |
|---|---|---|
| Sensitivity (%) | 84 | 74 |
| Specificity (%) | 57 | 68 |
| Positive predictive value (%) | 76 | 78 |
| Negative predictive value (%) | 70 | 68 |
The admission history forms filled out by first‐year admitting residents showed that only 18 (25%) of the women were screened for IPV, even though the history and physical examination template used at Denver Health Medical Center includes a prompt in the social history section pertaining to a history of violence as a reminder.
DISCUSSION
The important findings of this study were that women admitted to the internal medicine service of a university‐affiliated public safety‐net hospital had a high prevalence of IPV (22% and 61% 1‐year and lifetime prevalences, respectively), that most women with a history of IPV had previously sought help for the problem, many from physicians, that women were more likely to have a history of IPV if they had >10 positive responses to questions asked in a routine review of systems (particularly problems sleeping and experiencing numbness or tingling in their extremities), and that routine screening for IPV was uncommon at the time of admission.
These conclusions should be interpreted with respect to a number of limitations in our study. First, although our study was designed to be a consecutive series, the interviewers did not have sufficient time to meet with and interview every woman admitted before they were discharged. This occurred in part because the interviewers were available only for a portion of each day, some patients were discharged within 24 hours of admission, and many were out of their rooms for ancillary testing. Within the interviewers' time constraints, however, all hospitalized women meeting entry criteria who were available were approached. Our data could, however, overrepresent the prevalence of IPV if hospitalized women with a history of IPV had longer hospital stays than those who did not or if those experiencing IPV were out of their rooms less frequently (eg, for diagnostic tests). On the other hand, our data could underrepresent the true prevalence of IPV if patients with a history of IPV had shorter hospital stays or if they received more ancillary testing that caused them to be out of their rooms more frequently. Second, none of our interviewers had specific training in interviewing techniques. Accordingly, our data could have underestimated the true prevalence of IPV if interviewers with advanced training in probing sensitive topics had more success in eliciting positive responses. Third, the relationship between a history of IPV and multiple positive responses to the review of systems may be confounded if some of these patients also had a history of adverse childhood experiences or other experiences resulting in posttraumatic stress disorder as these patients also have an increased prevalence of chronic and functional disorders.2527 Finally, as our numbers were small, we were not powered to detect clinically important differences in demographics or specific positive answers on the review of systems.
To the best of our knowledge, the only study presenting IPV prevalence data in patients hospitalized for other than psychiatric problems was performed by McKenzie and colleagues18 in 1997. In their group of 130 patients (61 on internal medicine, 59 on surgery, 7 on obstetrics, and 3 on psychiatry), the 1‐year and lifetime prevalences of IPV were only 5% and 26%, respectively. McKenzie and colleagues used only 1 question to screen for IPV, but that single question incorporated 2 of the 4 questions used in our survey. Forty‐three of our 44 patients (98%) with a history of IPV were discovered on the basis of these 2 questions. The hospitals in which the 2 studies were done were similar, as were the ages and levels of education of the 2 populations studied and the percentage of eligible patients who agreed to participate. The patients in the 2 studies were different with respect to race, language mix, and the percentage who were insured, but neither study found differences in the prevalence of IPV as a function of race or insurance (although others have found an association of IPV with being uninsured1, 3, 4, 12, 23). Our study was conducted in women admitted exclusively to an internal medicine service, whereas nearly half of the patients studied by McKenzie and colleagues were admitted to surgical, gynecologic, or psychiatric services. Although McKenzie and colleagues found no difference in the prevalence of IPV as a function of admitting service, others have suggested that the prevalence of IPV is higher in patients admitted for trauma or psychiatric problems.1517, 28 The percentage of patients who self‐administered the questionnaires was 57% in our study and 77% in the study by McKenzie and colleagues. Neither study, however, found a difference in the percentage of IPV in patients who self‐administered the survey versus those who were interviewed. Women may have become more comfortable discussing this issue in the 10‐year interval between these 2 studies, or the prevalence of IPV may have increased. The only other study of IPV in hospitalized patients of which we are aware reported a 90% 1‐year prevalence in suicidal women admitted to a psychiatric service.28
Several studies have reported that victims of IPV have multiple somatic complaints and an increased prevalence of chronic and functional illnesses.1923 We confirmed that women experiencing IPV have more positive responses to questions posed in a review of systems, but the low specificity and positive and negative predictive values of the responses make this association of little clinical utility.
For only 18 of the 72 patients (25%) in our study was there evidence that they were screened for a history of IPV by the admitting resident. If more women were screened without a response being recorded, or if women were screened only for a current history of violence, our data may not accurately reflect the true rate at which screening occurred; however, the rate of screening that we observed is consistent with a number of other studies.12, 22, 2931 Fourteen of 18 patients who were screened for IPV by the resident gave negative responses. Ten of these, however, gave positive responses to our interviewers. Accordingly, the sensitivity, specificity, and positive and negative predictive values of the information recorded by the admitting resident were 40%, 100%, 100%, and 57%, respectively (assuming that the responses given to the IPV survey represent the gold standard), and this confirms that routine screening underestimates the prevalence of this problem. Accordingly, we identified 2 problems pertaining to screening for IPV: (1) it is not routinely done at the time of hospital admission, and (2) responses reported during routine screening are frequently incorrect. A number of barriers to routine screening have been previously identified, as have interventions designed to increase screening.32 Providing specific screening questions increases the identification of victims of IPV, but simply educating healthcare providers does not.32 Our history and physical templates have a prompt for violence victim to facilitate the screening, but as a result of this study, we are changing our prompting question and indicating what should be done if the response is positive.
The US Preventive Services Task Force and the Canadian Task Force on Preventive Health Care both concluded that there was insufficient evidence to recommend for or against routine screening for IPV.3335 Their rationale was that trials assessing the effectiveness of screening have not been published, that studies designed to assess the effectiveness of any resulting intervention are few in number, focused on pregnant women, and limited by problems in study design, that no studies have determined the accuracy of the screening tools, and that none have addressed the potential harm of screening.3335 The US Preventive Services Task Force did recommend screening if providers were concerned about IPV.34 Our data would suggest that there is little in the admission history that distinguishes women who might be victims of IPV from those who might not. Guidelines published by the American Medical Association, the American Academy of Family Physicians, and the American College of Obstetricians and Gynecologists promote routine screening of all patients.3638 Janssen and colleagues39 support the importance of screening on the basis that IPV is associated with numerous physical and mental health problems (eg, arthritis, migraines and other types of headaches, vaginal bleeding, ulcers, spastic colon, chronic pain, substance abuse, depression, and suicide ideation) and that establishing the link between these conditions and IPV could be important with respect to developing appropriate diagnostic and therapeutic approaches to patients' complaints. Screening also allows physicians to become more knowledgeable about their patients' lives, facilitating their ability to provide a supportive relationship that, in turn, increases women's likelihood of using an intervention method.39 We did not confirm an increased prevalence of any of the complaints noted by Janssen and colleagues in the women experiencing a history of IPV, but we did find an increased prevalence of insomnia and extremity numbness in women admitting to IPV as well as an overall increase in the number of positive responses to the review of systems. Screening identifies women who should receive information about reporting IPV, obtaining available assistance, planning for personal safety, and formal counseling as these have all been shown to reduce the severity of IPV and to improve the quality of life in rather large, randomized controlled trials.4043
As previously observed by others,13, 22, 29, 4446 the large majority of women that we approached welcomed screening for IPV. Over half of those with a history of IPV had previously sought help for the problem, over one‐third of these sought help from physicians, and most took the resource card that we offered, regardless of whether they did or did not have a history of IPV (this suggests either that our data may actually underestimate the true prevalence of IPV or that patients taking the information knew of others experiencing this problem). Accordingly, regardless of whether physicians believe that routine screening is warranted, patients see physicians and other healthcare workers as a resource for this problem.
We have confirmed that a history of IPV is very common in women admitted to an internal medicine service of a university‐affiliated public hospital and that female victims of IPV have more positive responses on the review of systems (particularly difficulty sleeping and extremity numbness or tingling) than those who have not. Although we initially hypothesized that finding numerous somatic complaints might serve as a marker for IPV, thereby identifying patients for whom more careful screening should occur, finding such a high prevalence of IPV argues that screening should be a routine part of the history for all women admitted to internal medicine inpatient services.
Acknowledgements
The authors thank the patients who agreed to participate in this study during their hospitalization. They also thank Cheri Maestas and Debbie Rodriquez for their support and help in interviewing patients.
The prevalence of intimate partner violence (IPV; defined as mental and/or physical violence directed from 1 person in an intimate relationship to the other) varies widely, depending on the population sampled and method of data collection. In the United States, IPV against women, occurring within the year prior to contact with a healthcare professional, ranges from 2% to 15% in surveys done by telephone, in primary care clinics, or in face‐to‐face home interviews19 and from 10% to 30% in surveys of patients visiting urgent care or emergency departments.1012 The prevalence of IPV occurring at any time during the life of the patient ranges from 18% in the aforementioned settings to as high as 88% in women applying for welfare.1, 2, 4, 5, 10, 1214
Although reports indicate that victims of IPV are more likely to be hospitalized,1517 the only study assessing the prevalence of IPV in hospitalized patients included women on medical, surgical, and obstetrical services and reported 1‐year and lifetime prevalences of only 5% and 23%, respectively.18
We hypothesized that the prevalence of IPV in hospitalized patients would be at least as high as that reported from emergency departments and sought to measure the 1‐year and lifetime prevalences of IPV in women admitted to a general internal medicine service. In addition, because studies done in various outpatient settings have reported that victims of IPV have a variety of somatic complaints and an increased prevalence of chronic and functional illnesses,1923 we also sought to determine whether women with a history of IPV and women without a history of IPV had different numbers or types of positive responses to questions asked on the review of systems.
PATIENTS AND METHODS
This study was approved by the Colorado Multiple Institution Review Board, and informed consent was obtained from all participants.
Women between the ages of 18 and 60 who were admitted to the internal medicine floor service of Denver Health Medical Center (a university‐affiliated public safety‐net hospital) between January 1 and February 28, 2004 and between October 1 and October 30, 2004 were approached to participate. These dates were selected on the basis of the availability of our interviewers. Patients older than 60 were excluded to avoid overlap between IPV and the problem of elder abuse. Women were excluded if they were unable to give informed consent, were pregnant, were incarcerated, were on contact precautions, or spoke a language other than English or Spanish. Although IPV is common in pregnant women and may occur in women who are incarcerated, these are considered vulnerable populations with respect to obtaining approval from internal review boards.
The questionnaire consisted of 23 review‐of‐systems questions,24 4 questions adapted from a previously validated screen for IPV11 (Table 1), and 1 question about attempts to seek help (Table 1). Women were considered to have experienced IPV if they gave positive responses to any of the 4 questions targeting IPV. According to patient preference, the combined questionnaire was either read and filled out by each subject independently or was read to her by a female interviewer who then recorded the subject's verbal responses. All interviewers were women with a shared common concern about, and interest in, IPV. Although none had advanced training in psychology, social work, or other formal discipline that involved interviewing skills, all interviews were scripted so that interactions with subjects and completion of the questionnaires would be uniform. Responses indicating sometimes were considered to be positive. Responses that were not answered, left blank, or marked as not applicable were considered to be negative.
| 1. Have you ever been hit, kicked, punched, or otherwise hurt by someone? If so, by whom? Friend, boyfriend, girlfriend, husband, family member, somebody you do not know, other |
| 2. Within the last year, have you been hit, kicked, or otherwise hurt by someone? If so, by whom? Friend, boyfriend, girlfriend, husband, family member, somebody you do not know, other |
| 3. Do you feel safe in your current relationship? |
| 4. Is there a partner from a previous relationship who is making you feel unsafe now? |
| 5. If you answered yes to any of the above, have you ever asked for help from police, shelter, counselor, physician? If so, how long ago? |
Each patient's medical record was reviewed to determine her age, race, number of previous hospital admissions, visits to the emergency department and walk‐in clinic, visits to primary care and subspecialty physicians, and whether the patient had been screened for IPV as recorded on the admission history and physical template. Admission diagnosis was obtained from the history and physical template, and the discharge diagnosis was obtained from the discharge paperwork. Functional diagnoses were considered to be symptoms (eg, shortness of breath) or problems (eg, constipation) that could not clearly be linked to a specific disease process. All participants were offered a card containing a list of resources for victims of IPV.
Data were analyzed with SAS 8.1 (SAS Institute, Cary, NC) and SPSS 11.5 (SPSS, Chicago, IL). The Student t test was used to compare continuous variables. Data are reported as means standard deviation. Chi‐square analysis was used to test associations between race, primary language, level of education, insurance status, admitting diagnosis, discharge diagnosis, number of previous hospital admissions, visit type, and the presence of IPV. For these, P < 0.05 was considered to be significant. The association of positive review‐of‐systems responses with the presence of IPV was also tested by chi‐square analysis, but P < 0.002 was considered to be significant on the basis of a Bonferroni adjustment for multiple comparisons. A receiver operating characteristic curve was used to assess the relationship between the number of positive responses to the questions included in the review of systems and a history of IPV. The odds ratio and confidence intervals were calculated to test the association between the number of positive responses to the review‐of‐systems questions and a lifetime history of IPV.
RESULTS
Throughout the dates of the study, 245 women were admitted to the internal medicine service, and 106 were excluded (Figure 1). Of the 139 eligible women, 78 were available to the interviewers and asked to participate, and 72 (92%) agreed. IPV occurring within the year prior to the interview or at any point in the patient's lifetime was reported by 16 (22%) and 44 (61%) subjects, respectively. No significant differences were seen in women who did or did not experience IPV at anytime in their life with respect to age, race, insurance status, education, number of scheduled outpatient, urgent, or emergent visits, or admission or discharge diagnosis even when the diagnoses were grouped into a functional category (although at best our study was powered to detect only >35% differences in prevalences; Tables 2 and 3). Of women reporting a lifetime history of IPV, 26 of 44 (59%) had previously sought help, and 9 of those 26 (35%) said that they sought help from a physician.

| IPV History | No IPV History | |
|---|---|---|
| ||
| Number (%) | 44 (61) | 28 (39) |
| Age (mean standard deviation) | 44 10 | 45 12 |
| Race [n, (%)] | ||
| Caucasian | 18 (41) | 6 (21) |
| Hispanic | 13 (30) | 15 (54) |
| African American | 12 (27) | 6 (21) |
| Other | 1 (2) | 1 (4) |
| Insurance status [n (%)] | ||
| Insured | 12 (27) | 5 (18) |
| Uninsured | 32 (73) | 23 (82) |
| Education [n (%)] | ||
| Grade school | 4 (9) | 3 (11) |
| Some high school | 13 (30) | 5 (18) |
| High school diploma | 15 (34) | 9 (32) |
| Some college | 9 (20) | 7 (25) |
| College degree | 2 (5) | 2 (7) |
| Postgraduate | 1 (2) | 2 (7) |
| Previous visit type (median, IQR) | ||
| Scheduled outpatient (includes primary care and subspecialty) | 2 (8) | 1.5 (7) |
| Emergency department and walk‐in clinic | 2 (3.5) | 1 (3) |
| Previous hospital admissions [n (%)] | ||
| 0 | 24 (55) | 16 (57) |
| 1 | 16 (36) | 4 (14) |
| 2 | 0 (0) | 4 (14) |
| 3 | 2 (5) | 2 (7) |
| >3 | 2 (5) | 2 (7) |
| Admission or Discharge Diagnosis | Admission | Discharge | ||
|---|---|---|---|---|
| IPV (n = 44) | No IPV (n = 28) | IPV (n = 44) | No IPV (n = 28) | |
| ||||
| Cardiovascular | ||||
| Chest pain (%)* | 8 (18) | 5 (18) | 6 (14) | 4 (14) |
| Cardiomyopathy | 0 | 0 | 1 | 0 |
| Cerebrovascular accident | 1 | 0 | 1 | 0 |
| Deep venous thrombosis | 0 | 0 | 1 | 0 |
| Hypertensive emergency | 0 | 0 | 1 | 0 |
| Palpitations* | 0 | 1 | 0 | 1 |
| Valvular disease | 0 | 0 | 1 | 0 |
| Venous stasis | 0 | 1 | 0 | 1 |
| Total (%) | 9 (20) | 7 (25) | 11 (25) | 6 (21) |
| Gastrointestinal | ||||
| Abdominal pain (%)* | 7 (16) | 4 (14) | 2 | 1 |
| Ascites | 0 | 1 | 0 | 0 |
| Constipation* | 0 | 0 | 1 | 0 |
| End‐stage liver disease | 1 | 1 | 1 | 2 |
| Esophagitis | 0 | 0 | 1 | 0 |
| Hepatitis | 1 | 0 | 1 | 0 |
| Nausea/vomiting* | 2 | 0 | 1 | 0 |
| Pancreatitis | 0 | 1 | 3 | 2 |
| Peptic ulcer disease | 1 | 0 | 1 | 0 |
| Upper gastrointestinal bleeding | 2 | 0 | 1 | 0 |
| Total (%) | 14 (32) | 7 (25) | 12 (27) | 5 (18) |
| Hematology/oncology | ||||
| Abdominal mass | 0 | 0 | 0 | 1 |
| Anemia | 1 | 0 | 1 | 0 |
| Breast cancer | 0 | 1 | 0 | 1 |
| Cervical cancer | 1 | 0 | 1 | 0 |
| Colon cancer | 0 | 1 | 0 | 1 |
| Sickle cell anemia | 1 | 0 | 1 | 0 |
| Thrombocytosis | 1 | 0 | 1 | 0 |
| Total (%) | 4 (9) | 2 (7) | 4 (9) | 3 (11) |
| Infectious disease | ||||
| Bacteremia/sepsis | 3 | 0 | 3 | 0 |
| Cellulitis | 1 | 0 | 1 | 1 |
| Cholangitis | 0 | 0 | 1 | 0 |
| Community‐acquired pneumonia | 2 | 2 | 2 | 1 |
| Endocarditis | 1 | 0 | 1 | 0 |
| Fever | 0 | 1 | 0 | 1 |
| Pelvic inflammatory disease | 0 | 0 | 0 | 1 |
| Urinary tract infection | 1 | 0 | 1 | 0 |
| Total (%) | 8 (18) | 3 (11) | 9 (20) | 4 (14) |
| Pulmonary | ||||
| Acute exacerbation of COPD | 0 | 0 | 1 | 0 |
| Asthma exacerbation | 1 | 1 | 1 | 2 |
| Pleuritic chest pain* | 0 | 0 | 1 | 0 |
| Pulmonary embolism | 0 | 0 | 1 | 0 |
| Shortness of breath* | 4 | 0 | 1 | 0 |
| Total (%) | 5 (11) | 1 (4) | 5 (11) | 2 (7) |
| Renal/genitourinary | ||||
| Acute renal failure | 0 | 1 | 0 | 1 |
| End‐stage renal disease | 1 | 2 | 1 | 2 |
| Nephrotic syndrome | 0 | 1 | 0 | 2 |
| Vaginal bleeding | 1 | 0 | 1 | 0 |
| Total (%) | 2 (5) | 4 (14) | 2 (5) | 5 (18) |
| Other | ||||
| Diabetic ketoacidosis | 0 | 1 | 0 | 1 |
| Extremity pain* | 0 | 1 | 0 | 0 |
| Mediastinal thickening | 0 | 0 | 0 | 1 |
| Hyponatremia | 0 | 1 | 0 | 1 |
| Lower extremity swelling | 2 | 1 | 0 | 0 |
| Somatization* | 0 | 0 | 1 | 0 |
| Total (%) | 2 (5) | 4 (14) | 1 (2) | 3 (11) |
| Total functional diagnoses (%) | 21 (48) | 11 (39) | 12 (27) | 6 (21) |
Women with a 1‐year history of IPV and women without a 1‐year history of IPV had 11.4 4.7 and 7.7 5.4 positive responses to the review of systems (P < 0.01), respectively. Women with a lifetime history of IPV and women without a lifetime history of IPV had 10.9 4.4 and 7.7 5.4 positive responses (P < 0.01), respectively. The receiver operating characteristic curve of the number of positive responses versus a lifetime history of IPV is presented in Figure 2. Subjects with 10 or more positive responses were 4.8 times more likely to report a lifetime history of IPV than subjects with 9 or fewer positive responses (confidence interval = 1.614.2, P = 0.003). The c‐statistic indicating the ability of the review of systems to properly classify cases when there were 10 or more positive responses was 0.692.

No differences were observed in the responses to the individual review of systems questions in women who did or did not have a lifetime history of IPV, with the exception that those with a positive history more commonly complained of difficulty sleeping and numbness and tingling in their hands or feet (although at best our study was sufficiently powered to detect only >20% differences in prevalences; Table 4). Although the sensitivity of having problems sleeping or experiencing numbness or tingling in patients with IPV was high, the specificity and positive and negative predictive values were not (Table 5).
| Review‐of‐Systems Questions | IPV History (n = 44) | No IPV History (n = 28) | P Value |
|---|---|---|---|
| |||
| 1. Shortness of breath | 25 (57) | 10 (36) | 0.081 |
| 2. Chest pain/pressure | 19 (43) | 9 (32) | 0.349 |
| 3. Abdominal pain | 17 (39) | 10 (36) | 0.803 |
| 4. Headaches | 24 (55) | 13 (46) | 0.502 |
| 5. Rashes | 15 (34) | 9 (32) | 0.864 |
| 6. Bruising | 32 (73) | 12 (43) | 0.011 |
| 7. Joint pain/stiffness | 27 (61) | 11 (39) | 0.067 |
| 8. Muscle pain/spasms | 22 (50) | 11 (39) | 0.374 |
| 9. Pain with intercourse | 8 (19) | 4 (14) | 0.753 |
| 10. Pelvic pain/cramps | 13 (30) | 5 (18) | 0.264 |
| 11. Nausea/vomiting | 19 (43) | 11 (39) | 0.744 |
| 12. Nervous/anxious | 28 (64) | 14 (50) | 0.253 |
| 13. Sad/crying | 21 (48) | 12 (43) | 0.686 |
| 14. Weight gain/loss | 26 (59) | 17 (61) | 0.891 |
| 15. Trouble sleeping | 37 (84) | 12 (43) | 0.000* |
| 16. Fever/chills | 19 (43) | 6 (21) | 0.059 |
| 17. Frequent/painful urination | 11 (25) | 6 (21) | 0.728 |
| 18. Pounding/emrregular heart beat | 14 (32) | 7 (25) | 0.535 |
| 19. Dizzy/passing out | 13 (30) | 7 (25) | 0.675 |
| 20. Memory problem | 19 (43) | 7 (25) | 0.117 |
| 21. Diarrhea/constipation | 27 (61) | 10 (36) | 0.034 |
| 22. Numbness/tingling | 35 (80) | 9 (32) | <0.0001* |
| 23. Pain chewing/swallowing | 8 (18) | 5 (18) | 0.972 |
| Trouble Sleeping | Numbness/Tingling | |
|---|---|---|
| Sensitivity (%) | 84 | 74 |
| Specificity (%) | 57 | 68 |
| Positive predictive value (%) | 76 | 78 |
| Negative predictive value (%) | 70 | 68 |
The admission history forms filled out by first‐year admitting residents showed that only 18 (25%) of the women were screened for IPV, even though the history and physical examination template used at Denver Health Medical Center includes a prompt in the social history section pertaining to a history of violence as a reminder.
DISCUSSION
The important findings of this study were that women admitted to the internal medicine service of a university‐affiliated public safety‐net hospital had a high prevalence of IPV (22% and 61% 1‐year and lifetime prevalences, respectively), that most women with a history of IPV had previously sought help for the problem, many from physicians, that women were more likely to have a history of IPV if they had >10 positive responses to questions asked in a routine review of systems (particularly problems sleeping and experiencing numbness or tingling in their extremities), and that routine screening for IPV was uncommon at the time of admission.
These conclusions should be interpreted with respect to a number of limitations in our study. First, although our study was designed to be a consecutive series, the interviewers did not have sufficient time to meet with and interview every woman admitted before they were discharged. This occurred in part because the interviewers were available only for a portion of each day, some patients were discharged within 24 hours of admission, and many were out of their rooms for ancillary testing. Within the interviewers' time constraints, however, all hospitalized women meeting entry criteria who were available were approached. Our data could, however, overrepresent the prevalence of IPV if hospitalized women with a history of IPV had longer hospital stays than those who did not or if those experiencing IPV were out of their rooms less frequently (eg, for diagnostic tests). On the other hand, our data could underrepresent the true prevalence of IPV if patients with a history of IPV had shorter hospital stays or if they received more ancillary testing that caused them to be out of their rooms more frequently. Second, none of our interviewers had specific training in interviewing techniques. Accordingly, our data could have underestimated the true prevalence of IPV if interviewers with advanced training in probing sensitive topics had more success in eliciting positive responses. Third, the relationship between a history of IPV and multiple positive responses to the review of systems may be confounded if some of these patients also had a history of adverse childhood experiences or other experiences resulting in posttraumatic stress disorder as these patients also have an increased prevalence of chronic and functional disorders.2527 Finally, as our numbers were small, we were not powered to detect clinically important differences in demographics or specific positive answers on the review of systems.
To the best of our knowledge, the only study presenting IPV prevalence data in patients hospitalized for other than psychiatric problems was performed by McKenzie and colleagues18 in 1997. In their group of 130 patients (61 on internal medicine, 59 on surgery, 7 on obstetrics, and 3 on psychiatry), the 1‐year and lifetime prevalences of IPV were only 5% and 26%, respectively. McKenzie and colleagues used only 1 question to screen for IPV, but that single question incorporated 2 of the 4 questions used in our survey. Forty‐three of our 44 patients (98%) with a history of IPV were discovered on the basis of these 2 questions. The hospitals in which the 2 studies were done were similar, as were the ages and levels of education of the 2 populations studied and the percentage of eligible patients who agreed to participate. The patients in the 2 studies were different with respect to race, language mix, and the percentage who were insured, but neither study found differences in the prevalence of IPV as a function of race or insurance (although others have found an association of IPV with being uninsured1, 3, 4, 12, 23). Our study was conducted in women admitted exclusively to an internal medicine service, whereas nearly half of the patients studied by McKenzie and colleagues were admitted to surgical, gynecologic, or psychiatric services. Although McKenzie and colleagues found no difference in the prevalence of IPV as a function of admitting service, others have suggested that the prevalence of IPV is higher in patients admitted for trauma or psychiatric problems.1517, 28 The percentage of patients who self‐administered the questionnaires was 57% in our study and 77% in the study by McKenzie and colleagues. Neither study, however, found a difference in the percentage of IPV in patients who self‐administered the survey versus those who were interviewed. Women may have become more comfortable discussing this issue in the 10‐year interval between these 2 studies, or the prevalence of IPV may have increased. The only other study of IPV in hospitalized patients of which we are aware reported a 90% 1‐year prevalence in suicidal women admitted to a psychiatric service.28
Several studies have reported that victims of IPV have multiple somatic complaints and an increased prevalence of chronic and functional illnesses.1923 We confirmed that women experiencing IPV have more positive responses to questions posed in a review of systems, but the low specificity and positive and negative predictive values of the responses make this association of little clinical utility.
For only 18 of the 72 patients (25%) in our study was there evidence that they were screened for a history of IPV by the admitting resident. If more women were screened without a response being recorded, or if women were screened only for a current history of violence, our data may not accurately reflect the true rate at which screening occurred; however, the rate of screening that we observed is consistent with a number of other studies.12, 22, 2931 Fourteen of 18 patients who were screened for IPV by the resident gave negative responses. Ten of these, however, gave positive responses to our interviewers. Accordingly, the sensitivity, specificity, and positive and negative predictive values of the information recorded by the admitting resident were 40%, 100%, 100%, and 57%, respectively (assuming that the responses given to the IPV survey represent the gold standard), and this confirms that routine screening underestimates the prevalence of this problem. Accordingly, we identified 2 problems pertaining to screening for IPV: (1) it is not routinely done at the time of hospital admission, and (2) responses reported during routine screening are frequently incorrect. A number of barriers to routine screening have been previously identified, as have interventions designed to increase screening.32 Providing specific screening questions increases the identification of victims of IPV, but simply educating healthcare providers does not.32 Our history and physical templates have a prompt for violence victim to facilitate the screening, but as a result of this study, we are changing our prompting question and indicating what should be done if the response is positive.
The US Preventive Services Task Force and the Canadian Task Force on Preventive Health Care both concluded that there was insufficient evidence to recommend for or against routine screening for IPV.3335 Their rationale was that trials assessing the effectiveness of screening have not been published, that studies designed to assess the effectiveness of any resulting intervention are few in number, focused on pregnant women, and limited by problems in study design, that no studies have determined the accuracy of the screening tools, and that none have addressed the potential harm of screening.3335 The US Preventive Services Task Force did recommend screening if providers were concerned about IPV.34 Our data would suggest that there is little in the admission history that distinguishes women who might be victims of IPV from those who might not. Guidelines published by the American Medical Association, the American Academy of Family Physicians, and the American College of Obstetricians and Gynecologists promote routine screening of all patients.3638 Janssen and colleagues39 support the importance of screening on the basis that IPV is associated with numerous physical and mental health problems (eg, arthritis, migraines and other types of headaches, vaginal bleeding, ulcers, spastic colon, chronic pain, substance abuse, depression, and suicide ideation) and that establishing the link between these conditions and IPV could be important with respect to developing appropriate diagnostic and therapeutic approaches to patients' complaints. Screening also allows physicians to become more knowledgeable about their patients' lives, facilitating their ability to provide a supportive relationship that, in turn, increases women's likelihood of using an intervention method.39 We did not confirm an increased prevalence of any of the complaints noted by Janssen and colleagues in the women experiencing a history of IPV, but we did find an increased prevalence of insomnia and extremity numbness in women admitting to IPV as well as an overall increase in the number of positive responses to the review of systems. Screening identifies women who should receive information about reporting IPV, obtaining available assistance, planning for personal safety, and formal counseling as these have all been shown to reduce the severity of IPV and to improve the quality of life in rather large, randomized controlled trials.4043
As previously observed by others,13, 22, 29, 4446 the large majority of women that we approached welcomed screening for IPV. Over half of those with a history of IPV had previously sought help for the problem, over one‐third of these sought help from physicians, and most took the resource card that we offered, regardless of whether they did or did not have a history of IPV (this suggests either that our data may actually underestimate the true prevalence of IPV or that patients taking the information knew of others experiencing this problem). Accordingly, regardless of whether physicians believe that routine screening is warranted, patients see physicians and other healthcare workers as a resource for this problem.
We have confirmed that a history of IPV is very common in women admitted to an internal medicine service of a university‐affiliated public hospital and that female victims of IPV have more positive responses on the review of systems (particularly difficulty sleeping and extremity numbness or tingling) than those who have not. Although we initially hypothesized that finding numerous somatic complaints might serve as a marker for IPV, thereby identifying patients for whom more careful screening should occur, finding such a high prevalence of IPV argues that screening should be a routine part of the history for all women admitted to internal medicine inpatient services.
Acknowledgements
The authors thank the patients who agreed to participate in this study during their hospitalization. They also thank Cheri Maestas and Debbie Rodriquez for their support and help in interviewing patients.
- ,,.Prevalence and determinants of intimate partner abuse among public hospital primary care patients.J Gen Intern Med.2000;15:811–817.
- ,,,.Women's experiences with violence: a national study.Womens Health Issues.2007;17:3–12.
- ,,,.Multistate analysis of factors associated with intimate partner violence.Am J Prev Med.2002;22:156–164.
- ,,,.Frequency and correlates of intimate partner violence by type: physical, sexual, and psychological battering.Am J Public Health.2000;90:553–559.
- ,,,,.Prevalence of domestic violence among patients in three ambulatory care internal medicine clinics.J Gen Intern Med.1991;6:317–322.
- ,,,.Prevalence of partner violence against 7,443 African American, White and Hispanic women receiving care at urban public primary care clinics.Public Health Nurs.2005;22:98–107.
- ,,,,.Evaluating domestic partner abuse in a family practice clinic.Fam Med.1997;29:492–495.
- ,.Prevalence and predictors of physical partner abuse among Mexican American women.Am J Public Health.2001;91:441–445.
- ,,.Rates of intimate partner violence in the United States.Am J Public Health.1998;88:1702–1704.
- ,,,.Domestic violence against women incidence and prevalence in an emergency department population.JAMA.1995;273:1763–1767.
- ,,, et al.Accuracy of 3 brief screening questions for detecting partner violence in the emergency department.JAMA.1997;277:1357–1361.
- ,,.A prevalence survey of abuse and screening for abuse in urgent care patients.Obstet Gynecol.1998;91:511–514.
- Morbidity and Mortality Weekly Report.Use of medical care, police assistance and restraining orders by women reporting intimate partner violence—Massachusetts, 1996–1997.JAMA.2000;284:558.
- ,,.Interpersonal violence among women seeking welfare: unraveling lives.Am J Public Health.2006;96:1409–1415.
- ,.A 5‐year follow‐up study of 117 battered women.Am J Public Health.1991;81:1486–1488.
- ,,.Rates and relative risk of hospital admission among women in violent intimate partner relationships.Am J Public Health.2000;90:1416–1420.
- ,,,.Intimate partner violence against women: do victims cost health plans more?J Fam Pract.1999;48:439–443.
- ,,,.Prevalence of domestic violence in an inpatient female population.J Gen Intern Med.1998;13:277–279.
- ,,, et al.Intimate partner violence and physical health consequences.Arch Intern Med.2002;162:1157–1163.
- ,,,,.Physical health consequences of physical and psychological intimate partner violence.Arch Fam Med.2000;9:451–457.
- ,,, et al.Sexual and physical abuse in women with functional or organic gastrointestinal disorders.Ann Intern Med.1990;113:828–833.
- ,,.Prevalence of intimate partner violence and health implications for women using emergency departments and primary care clinics.Womens Health Issues.2004;14:19–29.
- ,,, et al.The “battering syndrome”: prevalence and clinical characteristics of domestic violence in primary care internal medicine practices.Ann Intern Med.1995;123:737–746.
- ,.DeGowin and DeGowin's Bedside Diagnostic Examination.5th ed.New York, NY:Macmillan Publishing;1987:18–29.
- ,,, et al.Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study.Am J Prev Med.1998;14:245–258.
- ,,,,,.Posttraumatic stress disorder and health status among female and male medical patients.J Trauma Stress.2004;17:1–9.
- ,,,,.Posttraumatic stress disorder and physical comorbidity among female children and adolescents: results from service‐use data.Pediatrics.2005:116;e767–e776.
- ,,,,.Prevalence and severity of intimate partner violence and associations with family functioning and alcohol abuse in psychiatric inpatients with suicidal intent.J Clin Psychiatry.2006;67:23–29.
- ,,.Intimate partner violence screening and intervention: data from eleven Pennsylvania and California community hospital emergency departments.J Emerg Nurs.2001;27:141–149.
- ,.Missed opportunities: emergency department visits by police‐identified victims of intimate partner violence.Emerg Med.2006;47:190–199.
- ,,, et al.Intimate partner violence and patient screening across medical specialties.Acad Emerg Med.2005;12:712–722.
- ,,,,.Screening for intimate partner violence by health care providers: barriers and interventions.Am J Prev Med.2000;19:230–237.
- ,,,.Screening women and elderly adults for family and intimate partner violence: a review of the evidence for the U.S. Preventive Services Task Force.Ann Intern Med.2004;140:387–396.
- U.S. Preventive Services Task Force.Screening for family and intimate partner violence: recommendation statement.Ann Intern Med.2004;140:382–386.
- ,.Interventions for violence against women: scientific review.JAMA.2003;289:589–600.
- American Medical Association. Policy H‐515.965: family and intimate partner violence. Available at: http://www.ama‐assn.org. Accessed May2007.
- American Academy of Family Physicians. Family and intimate partner violence and abuse. Available at: www.aafp.org/x16506.xml. Accessed May2007.
- Domestic Violence.Washington, DC:American College of Obstetrics and Gynecology;1999. Educational Bulletin Number; No. 257.
- ,,.Assessment for intimate partner violence: where do we stand?J Am Board Fam Med.2006;19:413–415.
- ,,,,,.What happens when health care providers ask about intimate partner violence? A description of consequences from the perspectives of female survivors.JAMA.2003;58:76–81.
- ,,,,,.Assessing intimate partner violence in health care settings leads to women's receipt of interventions and improved health.Public Health Rep.2006;121:435–444.
- ,,.An evaluation of interventions to decrease intimate partner violence to pregnant women.Public Health Nurs.2000;17:443–451.
- ,.Reducing violence using community‐based advocacy for women with abusive partners.J Consult Clin Psychol.1999;67:43–53.
- ,,,,.Help‐seeking for intimate partner violence and forced sex in South Carolina.Am J Prev Med.2000;19:316–320.
- ,,, et al.Women's opinions about domestic violence screening and mandatory reporting.Am J Prev Med.2000;19:279–285.
- ,,,.The factors associated with disclosure of intimate partner abuse to clinicians.J Fam Pract.2001;50:338–344.
- ,,.Prevalence and determinants of intimate partner abuse among public hospital primary care patients.J Gen Intern Med.2000;15:811–817.
- ,,,.Women's experiences with violence: a national study.Womens Health Issues.2007;17:3–12.
- ,,,.Multistate analysis of factors associated with intimate partner violence.Am J Prev Med.2002;22:156–164.
- ,,,.Frequency and correlates of intimate partner violence by type: physical, sexual, and psychological battering.Am J Public Health.2000;90:553–559.
- ,,,,.Prevalence of domestic violence among patients in three ambulatory care internal medicine clinics.J Gen Intern Med.1991;6:317–322.
- ,,,.Prevalence of partner violence against 7,443 African American, White and Hispanic women receiving care at urban public primary care clinics.Public Health Nurs.2005;22:98–107.
- ,,,,.Evaluating domestic partner abuse in a family practice clinic.Fam Med.1997;29:492–495.
- ,.Prevalence and predictors of physical partner abuse among Mexican American women.Am J Public Health.2001;91:441–445.
- ,,.Rates of intimate partner violence in the United States.Am J Public Health.1998;88:1702–1704.
- ,,,.Domestic violence against women incidence and prevalence in an emergency department population.JAMA.1995;273:1763–1767.
- ,,, et al.Accuracy of 3 brief screening questions for detecting partner violence in the emergency department.JAMA.1997;277:1357–1361.
- ,,.A prevalence survey of abuse and screening for abuse in urgent care patients.Obstet Gynecol.1998;91:511–514.
- Morbidity and Mortality Weekly Report.Use of medical care, police assistance and restraining orders by women reporting intimate partner violence—Massachusetts, 1996–1997.JAMA.2000;284:558.
- ,,.Interpersonal violence among women seeking welfare: unraveling lives.Am J Public Health.2006;96:1409–1415.
- ,.A 5‐year follow‐up study of 117 battered women.Am J Public Health.1991;81:1486–1488.
- ,,.Rates and relative risk of hospital admission among women in violent intimate partner relationships.Am J Public Health.2000;90:1416–1420.
- ,,,.Intimate partner violence against women: do victims cost health plans more?J Fam Pract.1999;48:439–443.
- ,,,.Prevalence of domestic violence in an inpatient female population.J Gen Intern Med.1998;13:277–279.
- ,,, et al.Intimate partner violence and physical health consequences.Arch Intern Med.2002;162:1157–1163.
- ,,,,.Physical health consequences of physical and psychological intimate partner violence.Arch Fam Med.2000;9:451–457.
- ,,, et al.Sexual and physical abuse in women with functional or organic gastrointestinal disorders.Ann Intern Med.1990;113:828–833.
- ,,.Prevalence of intimate partner violence and health implications for women using emergency departments and primary care clinics.Womens Health Issues.2004;14:19–29.
- ,,, et al.The “battering syndrome”: prevalence and clinical characteristics of domestic violence in primary care internal medicine practices.Ann Intern Med.1995;123:737–746.
- ,.DeGowin and DeGowin's Bedside Diagnostic Examination.5th ed.New York, NY:Macmillan Publishing;1987:18–29.
- ,,, et al.Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study.Am J Prev Med.1998;14:245–258.
- ,,,,,.Posttraumatic stress disorder and health status among female and male medical patients.J Trauma Stress.2004;17:1–9.
- ,,,,.Posttraumatic stress disorder and physical comorbidity among female children and adolescents: results from service‐use data.Pediatrics.2005:116;e767–e776.
- ,,,,.Prevalence and severity of intimate partner violence and associations with family functioning and alcohol abuse in psychiatric inpatients with suicidal intent.J Clin Psychiatry.2006;67:23–29.
- ,,.Intimate partner violence screening and intervention: data from eleven Pennsylvania and California community hospital emergency departments.J Emerg Nurs.2001;27:141–149.
- ,.Missed opportunities: emergency department visits by police‐identified victims of intimate partner violence.Emerg Med.2006;47:190–199.
- ,,, et al.Intimate partner violence and patient screening across medical specialties.Acad Emerg Med.2005;12:712–722.
- ,,,,.Screening for intimate partner violence by health care providers: barriers and interventions.Am J Prev Med.2000;19:230–237.
- ,,,.Screening women and elderly adults for family and intimate partner violence: a review of the evidence for the U.S. Preventive Services Task Force.Ann Intern Med.2004;140:387–396.
- U.S. Preventive Services Task Force.Screening for family and intimate partner violence: recommendation statement.Ann Intern Med.2004;140:382–386.
- ,.Interventions for violence against women: scientific review.JAMA.2003;289:589–600.
- American Medical Association. Policy H‐515.965: family and intimate partner violence. Available at: http://www.ama‐assn.org. Accessed May2007.
- American Academy of Family Physicians. Family and intimate partner violence and abuse. Available at: www.aafp.org/x16506.xml. Accessed May2007.
- Domestic Violence.Washington, DC:American College of Obstetrics and Gynecology;1999. Educational Bulletin Number; No. 257.
- ,,.Assessment for intimate partner violence: where do we stand?J Am Board Fam Med.2006;19:413–415.
- ,,,,,.What happens when health care providers ask about intimate partner violence? A description of consequences from the perspectives of female survivors.JAMA.2003;58:76–81.
- ,,,,,.Assessing intimate partner violence in health care settings leads to women's receipt of interventions and improved health.Public Health Rep.2006;121:435–444.
- ,,.An evaluation of interventions to decrease intimate partner violence to pregnant women.Public Health Nurs.2000;17:443–451.
- ,.Reducing violence using community‐based advocacy for women with abusive partners.J Consult Clin Psychol.1999;67:43–53.
- ,,,,.Help‐seeking for intimate partner violence and forced sex in South Carolina.Am J Prev Med.2000;19:316–320.
- ,,, et al.Women's opinions about domestic violence screening and mandatory reporting.Am J Prev Med.2000;19:279–285.
- ,,,.The factors associated with disclosure of intimate partner abuse to clinicians.J Fam Pract.2001;50:338–344.
Copyright © 2008 Society of Hospital Medicine
Editorial
In a minute or two the Caterpillar got down off the mushroom, and crawled away in the grass, merely remarking as it went, One side will make you grow taller, and the other side will make you grow shorter.
One side of WHAT? The other side of WHAT? thought Alice to herself.
Of the mushroom, said the Caterpillar.1
As a hospitalist of about 6 years, I enjoy hospital medicine and hope, over the course of my career, to see it develop into an increasingly respected, diverse, and influential specialty. There is abundant evidence that this is occurring, primarily through the praiseworthy efforts of the leadership and members of the Society of Hospital Medicine (SHM). Efforts to prove our value to inpatient care and align ourselves with quality improvement, as promoted early in the hospitalist movement,2 are coming to fruition. However, I would like to raise a flag of concern; and this is based on my experience working as a hospitalist in 10 community hospitals in 5 states, including positions as a locum tenens hospitalist, staff hospitalist, medical director of a hospitalist group, and full‐time teaching hospitalist for a community hospital residency program. I believe that hospitalists, particularly those working in community hospitals (approximately 80% of all hospitalists),3 are currently at a critical crossroad, with the option of either actively expanding their clinical, administrative, and quality improvement roles or allowing these roles to stagnate or atrophy. As in any career, we are, like Alice, perched on a mushroom, one side of which will make us grow taller and the other side of which will make us grow shorter. Which side are we choosing in our careers as hospitalists?
Hospitalists currently have numerous opportunities to expand their clinical, administrative, and quality improvement roles and responsibilities (Table 1), and these opportunities are in full alignment with the mission statement of SHM: to promote the highest quality of care for all hospitalized patients.4 My concern is that, for one reason or another, hospitalists in some settings are shrinking away from roles that they could or should fill, and this is a trend that I believe could affect our specialty adversely over time and that we, as an organization, should find ways to prevent. Although family medicine and traditional internal medicine physicians who work in the hospital face similar challenges, if we as hospitalists wish to qualify one day as board‐certified hospital medicine specialists, we are obligated to develop knowledge and skill sets that are truly unique to our profession.5 Holding to this goal, we cannot settle into a narrow comfort zone. I believe that the development of the hospital medicine core competencies by SHM6 was an important step in helping us define our intended reach, but even so, what are the specific growth factors or inhibitors that are influencing the expansion or shrinking of hospitalists and hospital medicine groups?
| 1. Quality improvement |
| a. Participating in quality assessments, making and implementing plans for improvement, and assessing effects of interventions |
| b. Assessing patient and family satisfaction with inpatient care and making and implementing plans for improvement |
| c. Assessing primary care physician, emergency room, subspecialist, and hospital staff satisfaction with inpatient care and making and implementing plans for improvement |
| d. Participating in the development and revision of clinical guidelines, pathways, and order sets to improve efficiency and uniformity of care on the basis of current evidence |
| e. Developing multidisciplinary hospitalist rounds to improve the coordination and quality of care |
| 2. Professional development |
| a. Developing new areas of knowledge and skill, such as certification in geriatric or palliative care medicine |
| b. Developing processes of peer review (including chart review or case review) to ensure quality and uniformity of care within the hospitalist group |
| c. Developing a system of continuing medical education for the hospitalist group to keep abreast of the latest evidence‐based guidelines |
| 3. Expansion of services |
| a. Developing an in‐house procedure team to perform bedside procedures for other physicians |
| b. Providing cross‐coverage for intensivists or other subspecialists at night or on weekends |
| c. Developing, participating in, and improving rapid response teams and cardiac arrest teams |
| d. Providing care or coverage for additional clinical areas, such as long‐term acute care hospital units or transitional care units |
| e. Meeting with subspecialist groups to identify any inpatient needs they have that could be filled by hospitalists |
| 4. Teaching |
| a. Participating in the medical education of residents and medical students |
| b. Participating in nursing education efforts |
| c. Promoting hospital medicine topics by speaking at hospital grand rounds or other local continuing medical education venues |
| d. Promoting community health by participating in community education talks or workshops |
| 5. Utilization management |
| a. Participating in utilization management committees |
| b. Evaluating the length of stay and cost per case for specific diagnosis‐related groups and making and implementing plans for improvement |
| c. Demonstrating cost savings and overall value to the hospital |
| d. Reviewing and improving clinical documentation to optimize hospital billing processes |
| 6. Information technology |
| a. Participating in the development and improvement of the electronic medical record system and the computerized physician order entry system |
| 7. Administrative |
| a. Strategically planning with hospital administration to determine areas of highest priority |
| 8. Research |
| a. Performing and publishing clinical research unique to the hospital setting |
On the basis of my observations, I believe that this problem is due in large part to a misalignment of incentives. Specifically, I believe that the expansion of hospitalist roles and responsibilities is often counteraligned with the bottom‐line productivity goals of the group. That is, to maintain high productivity, a hospitalist has a tendency to minimize his or her role in ways that save time. For example, there may be a tendency to overuse subspecialty consultations, which can take away some of the burden of complex clinical decision making, or to quickly transfer patients that are sicker and require more time to a higher level of care (if available). There may also be a tendency to avoid performing inpatient procedures (a significant part of the core competencies) because of time constraints and the demands of a higher census. Excessively rapid rounding results, and this diminishes other claimed benefits of the hospitalist model of care: patient satisfaction, safety, quality, and communication. Length‐of‐stay measures also suffer as productivity exceeds the limits of efficient care. Moreover, in such a productivity‐based environment, there is certainly no incentive for hospitalists to become enthusiastically involved in hospital committees, education, or quality improvement efforts, all of which are critical to the development of hospital medicine as a unique subspecialty. In essence, the incentive to expand one's role as a hospitalist in such a setting is almost completely absent, and I believe that this puts the future influence and reach of our specialty at significant risk.
Particularly as hospitals face increasing scrutiny about their quality and safety, and especially as the costs of hospital care increase and reimbursements threaten to decline, the value of hospitalists to the hospital has become different from that of all other physicians. Their value lies not in sheer productivity but in their ability to improve the cost, quality, efficiency, and safety of inpatient care simultaneously. If hospitalists settle into or are forced into a lesser role, hospital medicine will not be worthy of consideration as a unique subspecialty. Some of the remaining roles of the shrunken hospitalist may, at some point and in some settings, shift to nonphysicians,7 with a decline in the ratio of physicians to mid‐level providers in hospital medicine programs, and the jobs of some hospitalists will be effectively eliminated. Market forces will lead to improved training of mid‐level providers, allowing hospitals to fill inpatient care needs in a more cost‐effective way.
Having worked with some very capable nurse practitioners in 4 different community hospitals, I believe that a well‐trained mid‐level provider, with appropriate physician backup, can effectively manage many of the typical general medical admissions and surgical consultations seen in a community hospital setting. I admit that this may not be the case in larger referral centers or academic medical centers.
In developing and defining this new specialty and also in training new physicians for the field, we do not want to lose this transient opportunity to define ourselves as broadly as possible, pushing beyond traditional internal medicine to new areas of inpatient care and management and managing more complex conditions than a traditional primary care physician would typically manage, conditions that have always fallen within the broad spectrum of inpatient internal medicine (Table 2). If we instead develop a tendency to admit, consult, and walk away and do not have the time or appropriate incentives to expand our roles in other important ways (noted in Table 1) because of a focus on productivity, what is our specialty destined to become?
| Medical Condition | Potential Consult |
|---|---|
| Instructions: For each clinical condition, describe what testing and management of the condition that you, as a hospital medicine specialist, would independently perform before consulting the associated subspecialist. Identify what specific clinical findings would prompt a consultation. Also, ask yourself into which areas you could reasonably expand your clinical practice as a hospitalist with additional experience, training, or study. | |
| Abdominal pain | Gastroenterology |
| Surgery | |
| Abnormal electrocardiogram | Cardiology |
| Abnormal thyroid‐stimulating hormone | Endocrinology |
| Acute renal failure | Nephrology |
| Anemia | Hematology |
| Gastroenterology | |
| Ascites | Gastroenterology |
| Atrial fibrillation, new or uncontrolled | Cardiology |
| Bacteremia | Infectious disease |
| Central venous access | Surgery |
| Anesthesiology | |
| Chest pain | Cardiology |
| Chronic obstructive pulmonary disease | Pulmonary |
| Delirium/mental status change | Neurology |
| Psychiatry | |
| Depression/anxiety | Psychiatry |
| Diabetes, uncontrolled | Endocrinology |
| Diabetic ketoacidosis | Endocrinology |
| Diarrhea | Gastroenterology |
| End‐of‐life care | Palliative care |
| Fever | Infectious disease |
| Gastrointestinal bleed | Gastroenterology |
| Grief | Chaplain |
| Heart murmur | Cardiology |
| Hematuria | Urology |
| Hypercalcemia | Endocrine |
| Hypertension, uncontrolled | Cardiology |
| Nephrology | |
| Hyponatremia | Nephrology |
| Hypoxia/respiratory failure | Pulmonary |
| Infection | Infectious disease |
| Joint effusion | Orthopedics |
| Rheumatology | |
| Kidney stone | Urology |
| Meningitis | Infectious disease |
| Neutropenic fever | Hematology/oncology |
| Nonsustained ventricular tachycardia | Cardiology |
| Nose bleed | Ear, nose, and throat |
| Pain | Pain management |
| Paroxysmal supraventricular tachycardia | Cardiology |
| Pleural effusion | Pulmonary |
| Preoperative clearance | Cardiology |
| Pulmonary | |
| Pulmonary embolism | Pulmonary |
| Hematology | |
| Rash | Dermatology |
| Stroke | Neurology |
| Syncope | Neurology |
| Cardiology | |
| Thrombocytopenia | Hematology |
| Unstable angina | Cardiology |
| Urinary retention | Urology |
| Venous thromboembolism | Hematology |
That said, how can incentives be restructured to encourage hospitalists to expand their universe? Perhaps the simplest way of influencing the incentive structure of hospital medicine programs is more selectivity in the choice of jobs: seeking out jobs that offer us clear incentives (typically financial) to expand our universe by rewarding efforts to improve the quality, safety, and efficiency of inpatient care. According to the SHM 20052006 survey, about two‐thirds of responding hospital medicine programs reimbursed their physicians with a mix of salary and productivity/performance bonuses, with productivity being the dominant incentive (more than 80%). However, bonuses based on quality/efficiency measures were also being rewarded (about 60%), as well as bonuses for committee or project work (about 25%). Of all responding groups, that leaves about 60% of programs with no financial incentives for quality/efficiency measures. There is certainly room for progress in this area, and we can influence the process positively by requesting that such incentives be added to our contract before making a final commitment to a job or by negotiating changes to our current incentive structure at the time of contract renewal. This would be in the best interest of our individual careers as well as our specialty.
As we consider different job opportunities, we may also wish to consider the possible effect of the employment model on the incentive structure. Although it may seem logical that hospital‐employed groups would have broader goals than independent groups and thus might be more motivated to provide proper incentives, I do not believe that this is the case universally. Conversely, private groups who might be expected to focus more on productivity measures may actually offer excellent growth‐promoting incentives. In either case, careful consideration of the incentive structure is warranted when we choose to work in a given employment model.
Perhaps another way of encouraging hospitalists to expand their role would be through a program of national recognition, potentially established by SHM, that would allow individual hospitalists to formally claim specialization in a particular area of hospital medicine and benefit from such distinctions. For example, a hospitalist that was particularly proficient with inpatient procedures could submit documentation of procedures completed in a given time period and subsequently receive a formal designation as a certified procedural hospitalist or something similar. Alternatively, a hospitalist who preferred to focus on quality improvement efforts could submit information regarding his involvement with quality improvement initiatives and results and, on the basis of defined criteria, receive a formal designation as a quality improvement hospitalist. This approach could apply to any area of focus, and more than one designation could be achieved by each hospitalist. As the specialty of hospital medicine matures, these designations (similar to academic rank) could eventually correlate with salary ranges or incentive bonuses as hospitals learned to value the diverse skills of individual hospitalists.
Discouraging overconsultation of subspecialists while concurrently encouraging the broadening of our clinical skills is particularly difficult to address. The only solution to this issue that I can imagine would be to somehow align physician reimbursement more closely to the actual complexity of and time spent in managing patients with multiple comorbidities. Currently, the actual hospitalist physician reimbursement for subsequent visits of patients, with or without subspecialists involved, likely does not vary much. However, if hospitalists knew their extra effort in managing more complex conditions would be reimbursed differently (ie, billing for critical care time), they would certainly tend to broaden their practice to the benefit of their careers and the future of the specialty.
In summary, I believe that misaligned incentives are causing some hospitalists to underestimate their potential; this has the potential to adversely affect the future of the specialty of hospital medicine. I hope that this opinion will serve to generate discussion on the potential origins of and solutions to this problem and ultimately promote the future expansion of our hospital medicine universe, so that we do not find ourselves in Alice's predicament:
Well, I should like to be a LITTLE larger, sir, if you wouldn't mind said Alice: three inches is such a wretched height to be.1
- .Alice's Adventures in Wonderland.London, England:McMillan 1865.
- .Reflections: the hospitalist movement a decade later.J Hosp Med.2006;1:248–252.
- Society of Hospital Medicine. 2005‐2006 SHM Survey: State of the Hospital Medicine Movement. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Surveys22:102–104.
- ,,,,.Core competencies of hospital medicine: development and methodology.J Hosp Med.2006;1:48–56.
- ,,,,.Trends in care by nonphysician clinicians in the United States.N Engl J Med.2003;348(2):130–137.
In a minute or two the Caterpillar got down off the mushroom, and crawled away in the grass, merely remarking as it went, One side will make you grow taller, and the other side will make you grow shorter.
One side of WHAT? The other side of WHAT? thought Alice to herself.
Of the mushroom, said the Caterpillar.1
As a hospitalist of about 6 years, I enjoy hospital medicine and hope, over the course of my career, to see it develop into an increasingly respected, diverse, and influential specialty. There is abundant evidence that this is occurring, primarily through the praiseworthy efforts of the leadership and members of the Society of Hospital Medicine (SHM). Efforts to prove our value to inpatient care and align ourselves with quality improvement, as promoted early in the hospitalist movement,2 are coming to fruition. However, I would like to raise a flag of concern; and this is based on my experience working as a hospitalist in 10 community hospitals in 5 states, including positions as a locum tenens hospitalist, staff hospitalist, medical director of a hospitalist group, and full‐time teaching hospitalist for a community hospital residency program. I believe that hospitalists, particularly those working in community hospitals (approximately 80% of all hospitalists),3 are currently at a critical crossroad, with the option of either actively expanding their clinical, administrative, and quality improvement roles or allowing these roles to stagnate or atrophy. As in any career, we are, like Alice, perched on a mushroom, one side of which will make us grow taller and the other side of which will make us grow shorter. Which side are we choosing in our careers as hospitalists?
Hospitalists currently have numerous opportunities to expand their clinical, administrative, and quality improvement roles and responsibilities (Table 1), and these opportunities are in full alignment with the mission statement of SHM: to promote the highest quality of care for all hospitalized patients.4 My concern is that, for one reason or another, hospitalists in some settings are shrinking away from roles that they could or should fill, and this is a trend that I believe could affect our specialty adversely over time and that we, as an organization, should find ways to prevent. Although family medicine and traditional internal medicine physicians who work in the hospital face similar challenges, if we as hospitalists wish to qualify one day as board‐certified hospital medicine specialists, we are obligated to develop knowledge and skill sets that are truly unique to our profession.5 Holding to this goal, we cannot settle into a narrow comfort zone. I believe that the development of the hospital medicine core competencies by SHM6 was an important step in helping us define our intended reach, but even so, what are the specific growth factors or inhibitors that are influencing the expansion or shrinking of hospitalists and hospital medicine groups?
| 1. Quality improvement |
| a. Participating in quality assessments, making and implementing plans for improvement, and assessing effects of interventions |
| b. Assessing patient and family satisfaction with inpatient care and making and implementing plans for improvement |
| c. Assessing primary care physician, emergency room, subspecialist, and hospital staff satisfaction with inpatient care and making and implementing plans for improvement |
| d. Participating in the development and revision of clinical guidelines, pathways, and order sets to improve efficiency and uniformity of care on the basis of current evidence |
| e. Developing multidisciplinary hospitalist rounds to improve the coordination and quality of care |
| 2. Professional development |
| a. Developing new areas of knowledge and skill, such as certification in geriatric or palliative care medicine |
| b. Developing processes of peer review (including chart review or case review) to ensure quality and uniformity of care within the hospitalist group |
| c. Developing a system of continuing medical education for the hospitalist group to keep abreast of the latest evidence‐based guidelines |
| 3. Expansion of services |
| a. Developing an in‐house procedure team to perform bedside procedures for other physicians |
| b. Providing cross‐coverage for intensivists or other subspecialists at night or on weekends |
| c. Developing, participating in, and improving rapid response teams and cardiac arrest teams |
| d. Providing care or coverage for additional clinical areas, such as long‐term acute care hospital units or transitional care units |
| e. Meeting with subspecialist groups to identify any inpatient needs they have that could be filled by hospitalists |
| 4. Teaching |
| a. Participating in the medical education of residents and medical students |
| b. Participating in nursing education efforts |
| c. Promoting hospital medicine topics by speaking at hospital grand rounds or other local continuing medical education venues |
| d. Promoting community health by participating in community education talks or workshops |
| 5. Utilization management |
| a. Participating in utilization management committees |
| b. Evaluating the length of stay and cost per case for specific diagnosis‐related groups and making and implementing plans for improvement |
| c. Demonstrating cost savings and overall value to the hospital |
| d. Reviewing and improving clinical documentation to optimize hospital billing processes |
| 6. Information technology |
| a. Participating in the development and improvement of the electronic medical record system and the computerized physician order entry system |
| 7. Administrative |
| a. Strategically planning with hospital administration to determine areas of highest priority |
| 8. Research |
| a. Performing and publishing clinical research unique to the hospital setting |
On the basis of my observations, I believe that this problem is due in large part to a misalignment of incentives. Specifically, I believe that the expansion of hospitalist roles and responsibilities is often counteraligned with the bottom‐line productivity goals of the group. That is, to maintain high productivity, a hospitalist has a tendency to minimize his or her role in ways that save time. For example, there may be a tendency to overuse subspecialty consultations, which can take away some of the burden of complex clinical decision making, or to quickly transfer patients that are sicker and require more time to a higher level of care (if available). There may also be a tendency to avoid performing inpatient procedures (a significant part of the core competencies) because of time constraints and the demands of a higher census. Excessively rapid rounding results, and this diminishes other claimed benefits of the hospitalist model of care: patient satisfaction, safety, quality, and communication. Length‐of‐stay measures also suffer as productivity exceeds the limits of efficient care. Moreover, in such a productivity‐based environment, there is certainly no incentive for hospitalists to become enthusiastically involved in hospital committees, education, or quality improvement efforts, all of which are critical to the development of hospital medicine as a unique subspecialty. In essence, the incentive to expand one's role as a hospitalist in such a setting is almost completely absent, and I believe that this puts the future influence and reach of our specialty at significant risk.
Particularly as hospitals face increasing scrutiny about their quality and safety, and especially as the costs of hospital care increase and reimbursements threaten to decline, the value of hospitalists to the hospital has become different from that of all other physicians. Their value lies not in sheer productivity but in their ability to improve the cost, quality, efficiency, and safety of inpatient care simultaneously. If hospitalists settle into or are forced into a lesser role, hospital medicine will not be worthy of consideration as a unique subspecialty. Some of the remaining roles of the shrunken hospitalist may, at some point and in some settings, shift to nonphysicians,7 with a decline in the ratio of physicians to mid‐level providers in hospital medicine programs, and the jobs of some hospitalists will be effectively eliminated. Market forces will lead to improved training of mid‐level providers, allowing hospitals to fill inpatient care needs in a more cost‐effective way.
Having worked with some very capable nurse practitioners in 4 different community hospitals, I believe that a well‐trained mid‐level provider, with appropriate physician backup, can effectively manage many of the typical general medical admissions and surgical consultations seen in a community hospital setting. I admit that this may not be the case in larger referral centers or academic medical centers.
In developing and defining this new specialty and also in training new physicians for the field, we do not want to lose this transient opportunity to define ourselves as broadly as possible, pushing beyond traditional internal medicine to new areas of inpatient care and management and managing more complex conditions than a traditional primary care physician would typically manage, conditions that have always fallen within the broad spectrum of inpatient internal medicine (Table 2). If we instead develop a tendency to admit, consult, and walk away and do not have the time or appropriate incentives to expand our roles in other important ways (noted in Table 1) because of a focus on productivity, what is our specialty destined to become?
| Medical Condition | Potential Consult |
|---|---|
| Instructions: For each clinical condition, describe what testing and management of the condition that you, as a hospital medicine specialist, would independently perform before consulting the associated subspecialist. Identify what specific clinical findings would prompt a consultation. Also, ask yourself into which areas you could reasonably expand your clinical practice as a hospitalist with additional experience, training, or study. | |
| Abdominal pain | Gastroenterology |
| Surgery | |
| Abnormal electrocardiogram | Cardiology |
| Abnormal thyroid‐stimulating hormone | Endocrinology |
| Acute renal failure | Nephrology |
| Anemia | Hematology |
| Gastroenterology | |
| Ascites | Gastroenterology |
| Atrial fibrillation, new or uncontrolled | Cardiology |
| Bacteremia | Infectious disease |
| Central venous access | Surgery |
| Anesthesiology | |
| Chest pain | Cardiology |
| Chronic obstructive pulmonary disease | Pulmonary |
| Delirium/mental status change | Neurology |
| Psychiatry | |
| Depression/anxiety | Psychiatry |
| Diabetes, uncontrolled | Endocrinology |
| Diabetic ketoacidosis | Endocrinology |
| Diarrhea | Gastroenterology |
| End‐of‐life care | Palliative care |
| Fever | Infectious disease |
| Gastrointestinal bleed | Gastroenterology |
| Grief | Chaplain |
| Heart murmur | Cardiology |
| Hematuria | Urology |
| Hypercalcemia | Endocrine |
| Hypertension, uncontrolled | Cardiology |
| Nephrology | |
| Hyponatremia | Nephrology |
| Hypoxia/respiratory failure | Pulmonary |
| Infection | Infectious disease |
| Joint effusion | Orthopedics |
| Rheumatology | |
| Kidney stone | Urology |
| Meningitis | Infectious disease |
| Neutropenic fever | Hematology/oncology |
| Nonsustained ventricular tachycardia | Cardiology |
| Nose bleed | Ear, nose, and throat |
| Pain | Pain management |
| Paroxysmal supraventricular tachycardia | Cardiology |
| Pleural effusion | Pulmonary |
| Preoperative clearance | Cardiology |
| Pulmonary | |
| Pulmonary embolism | Pulmonary |
| Hematology | |
| Rash | Dermatology |
| Stroke | Neurology |
| Syncope | Neurology |
| Cardiology | |
| Thrombocytopenia | Hematology |
| Unstable angina | Cardiology |
| Urinary retention | Urology |
| Venous thromboembolism | Hematology |
That said, how can incentives be restructured to encourage hospitalists to expand their universe? Perhaps the simplest way of influencing the incentive structure of hospital medicine programs is more selectivity in the choice of jobs: seeking out jobs that offer us clear incentives (typically financial) to expand our universe by rewarding efforts to improve the quality, safety, and efficiency of inpatient care. According to the SHM 20052006 survey, about two‐thirds of responding hospital medicine programs reimbursed their physicians with a mix of salary and productivity/performance bonuses, with productivity being the dominant incentive (more than 80%). However, bonuses based on quality/efficiency measures were also being rewarded (about 60%), as well as bonuses for committee or project work (about 25%). Of all responding groups, that leaves about 60% of programs with no financial incentives for quality/efficiency measures. There is certainly room for progress in this area, and we can influence the process positively by requesting that such incentives be added to our contract before making a final commitment to a job or by negotiating changes to our current incentive structure at the time of contract renewal. This would be in the best interest of our individual careers as well as our specialty.
As we consider different job opportunities, we may also wish to consider the possible effect of the employment model on the incentive structure. Although it may seem logical that hospital‐employed groups would have broader goals than independent groups and thus might be more motivated to provide proper incentives, I do not believe that this is the case universally. Conversely, private groups who might be expected to focus more on productivity measures may actually offer excellent growth‐promoting incentives. In either case, careful consideration of the incentive structure is warranted when we choose to work in a given employment model.
Perhaps another way of encouraging hospitalists to expand their role would be through a program of national recognition, potentially established by SHM, that would allow individual hospitalists to formally claim specialization in a particular area of hospital medicine and benefit from such distinctions. For example, a hospitalist that was particularly proficient with inpatient procedures could submit documentation of procedures completed in a given time period and subsequently receive a formal designation as a certified procedural hospitalist or something similar. Alternatively, a hospitalist who preferred to focus on quality improvement efforts could submit information regarding his involvement with quality improvement initiatives and results and, on the basis of defined criteria, receive a formal designation as a quality improvement hospitalist. This approach could apply to any area of focus, and more than one designation could be achieved by each hospitalist. As the specialty of hospital medicine matures, these designations (similar to academic rank) could eventually correlate with salary ranges or incentive bonuses as hospitals learned to value the diverse skills of individual hospitalists.
Discouraging overconsultation of subspecialists while concurrently encouraging the broadening of our clinical skills is particularly difficult to address. The only solution to this issue that I can imagine would be to somehow align physician reimbursement more closely to the actual complexity of and time spent in managing patients with multiple comorbidities. Currently, the actual hospitalist physician reimbursement for subsequent visits of patients, with or without subspecialists involved, likely does not vary much. However, if hospitalists knew their extra effort in managing more complex conditions would be reimbursed differently (ie, billing for critical care time), they would certainly tend to broaden their practice to the benefit of their careers and the future of the specialty.
In summary, I believe that misaligned incentives are causing some hospitalists to underestimate their potential; this has the potential to adversely affect the future of the specialty of hospital medicine. I hope that this opinion will serve to generate discussion on the potential origins of and solutions to this problem and ultimately promote the future expansion of our hospital medicine universe, so that we do not find ourselves in Alice's predicament:
Well, I should like to be a LITTLE larger, sir, if you wouldn't mind said Alice: three inches is such a wretched height to be.1
In a minute or two the Caterpillar got down off the mushroom, and crawled away in the grass, merely remarking as it went, One side will make you grow taller, and the other side will make you grow shorter.
One side of WHAT? The other side of WHAT? thought Alice to herself.
Of the mushroom, said the Caterpillar.1
As a hospitalist of about 6 years, I enjoy hospital medicine and hope, over the course of my career, to see it develop into an increasingly respected, diverse, and influential specialty. There is abundant evidence that this is occurring, primarily through the praiseworthy efforts of the leadership and members of the Society of Hospital Medicine (SHM). Efforts to prove our value to inpatient care and align ourselves with quality improvement, as promoted early in the hospitalist movement,2 are coming to fruition. However, I would like to raise a flag of concern; and this is based on my experience working as a hospitalist in 10 community hospitals in 5 states, including positions as a locum tenens hospitalist, staff hospitalist, medical director of a hospitalist group, and full‐time teaching hospitalist for a community hospital residency program. I believe that hospitalists, particularly those working in community hospitals (approximately 80% of all hospitalists),3 are currently at a critical crossroad, with the option of either actively expanding their clinical, administrative, and quality improvement roles or allowing these roles to stagnate or atrophy. As in any career, we are, like Alice, perched on a mushroom, one side of which will make us grow taller and the other side of which will make us grow shorter. Which side are we choosing in our careers as hospitalists?
Hospitalists currently have numerous opportunities to expand their clinical, administrative, and quality improvement roles and responsibilities (Table 1), and these opportunities are in full alignment with the mission statement of SHM: to promote the highest quality of care for all hospitalized patients.4 My concern is that, for one reason or another, hospitalists in some settings are shrinking away from roles that they could or should fill, and this is a trend that I believe could affect our specialty adversely over time and that we, as an organization, should find ways to prevent. Although family medicine and traditional internal medicine physicians who work in the hospital face similar challenges, if we as hospitalists wish to qualify one day as board‐certified hospital medicine specialists, we are obligated to develop knowledge and skill sets that are truly unique to our profession.5 Holding to this goal, we cannot settle into a narrow comfort zone. I believe that the development of the hospital medicine core competencies by SHM6 was an important step in helping us define our intended reach, but even so, what are the specific growth factors or inhibitors that are influencing the expansion or shrinking of hospitalists and hospital medicine groups?
| 1. Quality improvement |
| a. Participating in quality assessments, making and implementing plans for improvement, and assessing effects of interventions |
| b. Assessing patient and family satisfaction with inpatient care and making and implementing plans for improvement |
| c. Assessing primary care physician, emergency room, subspecialist, and hospital staff satisfaction with inpatient care and making and implementing plans for improvement |
| d. Participating in the development and revision of clinical guidelines, pathways, and order sets to improve efficiency and uniformity of care on the basis of current evidence |
| e. Developing multidisciplinary hospitalist rounds to improve the coordination and quality of care |
| 2. Professional development |
| a. Developing new areas of knowledge and skill, such as certification in geriatric or palliative care medicine |
| b. Developing processes of peer review (including chart review or case review) to ensure quality and uniformity of care within the hospitalist group |
| c. Developing a system of continuing medical education for the hospitalist group to keep abreast of the latest evidence‐based guidelines |
| 3. Expansion of services |
| a. Developing an in‐house procedure team to perform bedside procedures for other physicians |
| b. Providing cross‐coverage for intensivists or other subspecialists at night or on weekends |
| c. Developing, participating in, and improving rapid response teams and cardiac arrest teams |
| d. Providing care or coverage for additional clinical areas, such as long‐term acute care hospital units or transitional care units |
| e. Meeting with subspecialist groups to identify any inpatient needs they have that could be filled by hospitalists |
| 4. Teaching |
| a. Participating in the medical education of residents and medical students |
| b. Participating in nursing education efforts |
| c. Promoting hospital medicine topics by speaking at hospital grand rounds or other local continuing medical education venues |
| d. Promoting community health by participating in community education talks or workshops |
| 5. Utilization management |
| a. Participating in utilization management committees |
| b. Evaluating the length of stay and cost per case for specific diagnosis‐related groups and making and implementing plans for improvement |
| c. Demonstrating cost savings and overall value to the hospital |
| d. Reviewing and improving clinical documentation to optimize hospital billing processes |
| 6. Information technology |
| a. Participating in the development and improvement of the electronic medical record system and the computerized physician order entry system |
| 7. Administrative |
| a. Strategically planning with hospital administration to determine areas of highest priority |
| 8. Research |
| a. Performing and publishing clinical research unique to the hospital setting |
On the basis of my observations, I believe that this problem is due in large part to a misalignment of incentives. Specifically, I believe that the expansion of hospitalist roles and responsibilities is often counteraligned with the bottom‐line productivity goals of the group. That is, to maintain high productivity, a hospitalist has a tendency to minimize his or her role in ways that save time. For example, there may be a tendency to overuse subspecialty consultations, which can take away some of the burden of complex clinical decision making, or to quickly transfer patients that are sicker and require more time to a higher level of care (if available). There may also be a tendency to avoid performing inpatient procedures (a significant part of the core competencies) because of time constraints and the demands of a higher census. Excessively rapid rounding results, and this diminishes other claimed benefits of the hospitalist model of care: patient satisfaction, safety, quality, and communication. Length‐of‐stay measures also suffer as productivity exceeds the limits of efficient care. Moreover, in such a productivity‐based environment, there is certainly no incentive for hospitalists to become enthusiastically involved in hospital committees, education, or quality improvement efforts, all of which are critical to the development of hospital medicine as a unique subspecialty. In essence, the incentive to expand one's role as a hospitalist in such a setting is almost completely absent, and I believe that this puts the future influence and reach of our specialty at significant risk.
Particularly as hospitals face increasing scrutiny about their quality and safety, and especially as the costs of hospital care increase and reimbursements threaten to decline, the value of hospitalists to the hospital has become different from that of all other physicians. Their value lies not in sheer productivity but in their ability to improve the cost, quality, efficiency, and safety of inpatient care simultaneously. If hospitalists settle into or are forced into a lesser role, hospital medicine will not be worthy of consideration as a unique subspecialty. Some of the remaining roles of the shrunken hospitalist may, at some point and in some settings, shift to nonphysicians,7 with a decline in the ratio of physicians to mid‐level providers in hospital medicine programs, and the jobs of some hospitalists will be effectively eliminated. Market forces will lead to improved training of mid‐level providers, allowing hospitals to fill inpatient care needs in a more cost‐effective way.
Having worked with some very capable nurse practitioners in 4 different community hospitals, I believe that a well‐trained mid‐level provider, with appropriate physician backup, can effectively manage many of the typical general medical admissions and surgical consultations seen in a community hospital setting. I admit that this may not be the case in larger referral centers or academic medical centers.
In developing and defining this new specialty and also in training new physicians for the field, we do not want to lose this transient opportunity to define ourselves as broadly as possible, pushing beyond traditional internal medicine to new areas of inpatient care and management and managing more complex conditions than a traditional primary care physician would typically manage, conditions that have always fallen within the broad spectrum of inpatient internal medicine (Table 2). If we instead develop a tendency to admit, consult, and walk away and do not have the time or appropriate incentives to expand our roles in other important ways (noted in Table 1) because of a focus on productivity, what is our specialty destined to become?
| Medical Condition | Potential Consult |
|---|---|
| Instructions: For each clinical condition, describe what testing and management of the condition that you, as a hospital medicine specialist, would independently perform before consulting the associated subspecialist. Identify what specific clinical findings would prompt a consultation. Also, ask yourself into which areas you could reasonably expand your clinical practice as a hospitalist with additional experience, training, or study. | |
| Abdominal pain | Gastroenterology |
| Surgery | |
| Abnormal electrocardiogram | Cardiology |
| Abnormal thyroid‐stimulating hormone | Endocrinology |
| Acute renal failure | Nephrology |
| Anemia | Hematology |
| Gastroenterology | |
| Ascites | Gastroenterology |
| Atrial fibrillation, new or uncontrolled | Cardiology |
| Bacteremia | Infectious disease |
| Central venous access | Surgery |
| Anesthesiology | |
| Chest pain | Cardiology |
| Chronic obstructive pulmonary disease | Pulmonary |
| Delirium/mental status change | Neurology |
| Psychiatry | |
| Depression/anxiety | Psychiatry |
| Diabetes, uncontrolled | Endocrinology |
| Diabetic ketoacidosis | Endocrinology |
| Diarrhea | Gastroenterology |
| End‐of‐life care | Palliative care |
| Fever | Infectious disease |
| Gastrointestinal bleed | Gastroenterology |
| Grief | Chaplain |
| Heart murmur | Cardiology |
| Hematuria | Urology |
| Hypercalcemia | Endocrine |
| Hypertension, uncontrolled | Cardiology |
| Nephrology | |
| Hyponatremia | Nephrology |
| Hypoxia/respiratory failure | Pulmonary |
| Infection | Infectious disease |
| Joint effusion | Orthopedics |
| Rheumatology | |
| Kidney stone | Urology |
| Meningitis | Infectious disease |
| Neutropenic fever | Hematology/oncology |
| Nonsustained ventricular tachycardia | Cardiology |
| Nose bleed | Ear, nose, and throat |
| Pain | Pain management |
| Paroxysmal supraventricular tachycardia | Cardiology |
| Pleural effusion | Pulmonary |
| Preoperative clearance | Cardiology |
| Pulmonary | |
| Pulmonary embolism | Pulmonary |
| Hematology | |
| Rash | Dermatology |
| Stroke | Neurology |
| Syncope | Neurology |
| Cardiology | |
| Thrombocytopenia | Hematology |
| Unstable angina | Cardiology |
| Urinary retention | Urology |
| Venous thromboembolism | Hematology |
That said, how can incentives be restructured to encourage hospitalists to expand their universe? Perhaps the simplest way of influencing the incentive structure of hospital medicine programs is more selectivity in the choice of jobs: seeking out jobs that offer us clear incentives (typically financial) to expand our universe by rewarding efforts to improve the quality, safety, and efficiency of inpatient care. According to the SHM 20052006 survey, about two‐thirds of responding hospital medicine programs reimbursed their physicians with a mix of salary and productivity/performance bonuses, with productivity being the dominant incentive (more than 80%). However, bonuses based on quality/efficiency measures were also being rewarded (about 60%), as well as bonuses for committee or project work (about 25%). Of all responding groups, that leaves about 60% of programs with no financial incentives for quality/efficiency measures. There is certainly room for progress in this area, and we can influence the process positively by requesting that such incentives be added to our contract before making a final commitment to a job or by negotiating changes to our current incentive structure at the time of contract renewal. This would be in the best interest of our individual careers as well as our specialty.
As we consider different job opportunities, we may also wish to consider the possible effect of the employment model on the incentive structure. Although it may seem logical that hospital‐employed groups would have broader goals than independent groups and thus might be more motivated to provide proper incentives, I do not believe that this is the case universally. Conversely, private groups who might be expected to focus more on productivity measures may actually offer excellent growth‐promoting incentives. In either case, careful consideration of the incentive structure is warranted when we choose to work in a given employment model.
Perhaps another way of encouraging hospitalists to expand their role would be through a program of national recognition, potentially established by SHM, that would allow individual hospitalists to formally claim specialization in a particular area of hospital medicine and benefit from such distinctions. For example, a hospitalist that was particularly proficient with inpatient procedures could submit documentation of procedures completed in a given time period and subsequently receive a formal designation as a certified procedural hospitalist or something similar. Alternatively, a hospitalist who preferred to focus on quality improvement efforts could submit information regarding his involvement with quality improvement initiatives and results and, on the basis of defined criteria, receive a formal designation as a quality improvement hospitalist. This approach could apply to any area of focus, and more than one designation could be achieved by each hospitalist. As the specialty of hospital medicine matures, these designations (similar to academic rank) could eventually correlate with salary ranges or incentive bonuses as hospitals learned to value the diverse skills of individual hospitalists.
Discouraging overconsultation of subspecialists while concurrently encouraging the broadening of our clinical skills is particularly difficult to address. The only solution to this issue that I can imagine would be to somehow align physician reimbursement more closely to the actual complexity of and time spent in managing patients with multiple comorbidities. Currently, the actual hospitalist physician reimbursement for subsequent visits of patients, with or without subspecialists involved, likely does not vary much. However, if hospitalists knew their extra effort in managing more complex conditions would be reimbursed differently (ie, billing for critical care time), they would certainly tend to broaden their practice to the benefit of their careers and the future of the specialty.
In summary, I believe that misaligned incentives are causing some hospitalists to underestimate their potential; this has the potential to adversely affect the future of the specialty of hospital medicine. I hope that this opinion will serve to generate discussion on the potential origins of and solutions to this problem and ultimately promote the future expansion of our hospital medicine universe, so that we do not find ourselves in Alice's predicament:
Well, I should like to be a LITTLE larger, sir, if you wouldn't mind said Alice: three inches is such a wretched height to be.1
- .Alice's Adventures in Wonderland.London, England:McMillan 1865.
- .Reflections: the hospitalist movement a decade later.J Hosp Med.2006;1:248–252.
- Society of Hospital Medicine. 2005‐2006 SHM Survey: State of the Hospital Medicine Movement. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Surveys22:102–104.
- ,,,,.Core competencies of hospital medicine: development and methodology.J Hosp Med.2006;1:48–56.
- ,,,,.Trends in care by nonphysician clinicians in the United States.N Engl J Med.2003;348(2):130–137.
- .Alice's Adventures in Wonderland.London, England:McMillan 1865.
- .Reflections: the hospitalist movement a decade later.J Hosp Med.2006;1:248–252.
- Society of Hospital Medicine. 2005‐2006 SHM Survey: State of the Hospital Medicine Movement. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Surveys22:102–104.
- ,,,,.Core competencies of hospital medicine: development and methodology.J Hosp Med.2006;1:48–56.
- ,,,,.Trends in care by nonphysician clinicians in the United States.N Engl J Med.2003;348(2):130–137.
Editorial
Accelerating the development of clinical research in academic hospitalist programs is a worthwhile goal if pursued with clarity, objectivity, and a thorough understanding of the process and its implications. In their articles, Flanders et al.1 and Wright et al.2 identify major barriers to growing academic hospitalist programs. These barriers include the need for protected time, the shortage of trained research faculty, the lack of infrastructure, and the limited availability of senior mentors. Both Flanders et al. and Wright et al. offer smart and innovative ways of addressing these issues. However, building an academic program from the ground up is more complex and challenging than it may seem at first glance. It takes time, patience, creativity, diplomacy, and the ability to recruit collaborators and advocates who are willing to share infrastructure and resources.
Although both articles add significantly to the discussion of strategies for creating an academic hospitalist program, they are unclear about the definition of academic in this context. The term academic is often misunderstood to be synonymous with research. However, research is just one component of an academic program, which also includes education, quality improvement (QI), administration, and program development. It may be helpful, therefore, to replace academic with scholarship, which can be defined as a process that involves peer review and dissemination of ideas at local, regional, and national levels. Scholarship also goes beyond research, encompassing education and other areas such as QI. Although academic programs are not necessarily involved with funded research, there is usually an expectation of peer review, through either presentations at regional and national meetings or publication. For the purposes of this discussion, the term academic hospitalist program will be defined broadly to include any program affiliated with a university that is involved in the teaching of residents and medical students and whose faculty is required to participate in a promotions process.
All members of an academic division should be expected to participate in scholarship, whether it is education, QI projects, or research. If there is a strong expectation that traditional National Institute of Health (NIH) funded research will take place, this expectation must come with sufficient resources. Without infrastructure for research and investment in research faculty, procuring NIH funds for research is not a reasonable expectation. Organizers of hospitalist programs currently within academic divisions of general internal medicine should consider ways to better integrate programs into the existing research infrastructure in their divisions. For either freestanding hospitalist programs or programs within academic divisions of general internal medicine, investments in infrastructure and faculty are needed to nurture this area of research and build an academic focus in hospital medicine. However, if obtaining NIH research funds is not the expectation and resources are not available for hospitalists or for any other division or department at that institution, then academic expectations should focus on other pursuits. Examples include participation in the education and QI initiatives.
For programs with expectations of both funded research and other scholarship, a successful program will most likely include a small core of skilled clinical researchers working closely with well‐trained clinical educators, all of whom are involved in scholarship. Both clinical educators and researchers need to be continuously developing, and to reach their full potential, all should have access to infrastructure that supports these activities, including resources such as MPH‐level project managers, research assistants, database managers, and, most importantly, appropriate mentors.
Clinician educators must be both proficient clinicians and dedicated teachers. Ideally, they should have strong familiarity with educational theory in addition to skills in hands‐on teaching. Their responsibilities include mastering the skills that students need, staying up to the minute in their areas of expertise, and serving as role models in their attitudes toward patients, colleagues, and their work.3 Many hospitalists may not have these skills when they begin, often fresh out of residency, and will need help developing them.
PROTECTED TIME
Protected time is crucial to the success of any academic program. Finding this time presents a challenge for any clinical group, but the challenge is exacerbated for hospitalists, who face tremendous pressure to serve full‐time clinical jobs with little emphasis on academic elements such as education, QI, and participation in funded research. As both Wright et al.2 and Flanders et al.1 point out, to build a group of well‐developed academic clinician educators, academic hospitalists not on track for funding need to be given adequate protected time to participate in committees, sharpen their teaching skills, develop QI projects that can be converted to scholarships, participate in research, and present at national and regional meetings.
The requirements of protected time for researchers are more challenging than those for educators. Building a newly funded research unit within a hospitalist group, as with any group, will entail hiring fellowship trained faculty with significant protected time (approximately 80%) to give them time to obtain funding such as a K award and eventually become independent investigators with RO1 grant funding. Building research units requires support from collaborators with infrastructure and mentors already in place that can be tapped during the incubation stage of the academic program. For most hospitalist programs, infrastructure and mentors will be found in their divisions of general internal medicine.
Protected time should be considered an integral element of academic hospitalist positionsnot a perkas long as the time is used responsibly and productively. As both Wright et al.2 and Flanders et al.1 correctly point out, herein lies the major challenge of creating any kind of academic program: How will the program support protected time for both educators and researchers? In most instances, significant seed money will be needed to support junior faculty over the first few years of their careers. It is noteworthy that building a federally funded hospital medicine research program will be particularly difficult in today's economy because funding levels at the NIH, Agency for Healthcare Research and Quality, Health Resources and Services Administration, and other traditional funders of clinical research either are flat or have been reduced dramatically.
To many Academic Medical Centers (AMC), it may not be immediately obvious why a strong Academic Hospitalist program is in their economic best interest. Hospitalist programs may confront consistently high levels of turnover and a shrinking supply of general internists. The associated high costs of hiring new, junior faculty include the time and effort needed to interview, credential, train, and most importantly build familiarity with the complex systems encountered in maneuvering through a hospital, especially one with widespread dissemination of electronic medical records for documentation and order entry. However, hospitalists provided with opportunities for academic development are more likely to stay on the job longer and perform at a higher level, providing convincing motivation for hospitals to invest in their academic hospitalist programs. Retaining high‐quality hospitalists may be one of the most cost‐efficient methods for an AMC to support a hospital medicine program.
STRATEGIES IN ACTION
Flanders et al.1 point to a shortage of well‐trained clinician investigators with a focus on inpatient research as a barrier to the development of academic hospitalist programming. They describe a strategy of collaboration with specialty groups. This highlights the importance of collaboration with more well‐established research units as a key ingredient to building a new academic unit. Wright et al.2 describe their mentorship program and how they created protected time for scholarship for hospitalists, including supporting mentors' time. These examples highlight another key benchmark of a viable academic program, mentoring, and the importance of ensuring that mentors have time for this essential effort.
However, it is critically important to remember that all politics is local, to quote the late Tip O'Neill, long‐time speaker of the US House of Representatives. What works in one setting may not work as well in a different contexthence the need for creativity and political acumen.
For example, although the specialty‐group collaboration described by Flanders et al.1 may be helpful in one setting, other strategic alliances may work on a larger scale and over a longer time period. Most hospital medicine groups are currently within academic divisions of general internal medicine, where infrastructure and mentoring may already exist for both research and educational scholarship. In those cases, fostering interaction between the division's hospitalists and its researchers would be a critical first step. The programming and growth developed in this way can be leveraged to support ongoing academic activities by hospitalists rather than being limited to a single project.
In the Division of General Internal Medicine at Mount Sinai, which houses the academic hospitalists, building research entailed collaboration with the well‐established Departments of Geriatrics and Health Policy, which had preexisting research infrastructure and mentors. At the same time, we developed a research fellowship program by applying jointly with the Division of General Pediatrics for federal grant support. Such diversity of collaboration enhanced our application.
LOOKING AHEAD
Putting the academic into academic hospitalist programs is the key to the future of hospital medicine. To be successful, one must consider all the issues described in light of available resources and the local and federal political landscape. As Flanders et al.1 and Wright et al.2 emphasize, collaboration will be the main component for success in the current academic landscape.
- ,,,.The University of Michigan Specialist‐Hospitalist Allied Research Program (SHARP): jumpstarting hospital medicine research.J Hosp Med.2008;3(4):308–313.
- , ,,.An innovative approach to supporting hospitalist physicians towards academic success.J Hosp Med.2008;3(4):314–318.
- ,,.The clinician‐educator—present and future roles.J Gen Intern Med.1997;12 (suppl 2):S1–S4.
Accelerating the development of clinical research in academic hospitalist programs is a worthwhile goal if pursued with clarity, objectivity, and a thorough understanding of the process and its implications. In their articles, Flanders et al.1 and Wright et al.2 identify major barriers to growing academic hospitalist programs. These barriers include the need for protected time, the shortage of trained research faculty, the lack of infrastructure, and the limited availability of senior mentors. Both Flanders et al. and Wright et al. offer smart and innovative ways of addressing these issues. However, building an academic program from the ground up is more complex and challenging than it may seem at first glance. It takes time, patience, creativity, diplomacy, and the ability to recruit collaborators and advocates who are willing to share infrastructure and resources.
Although both articles add significantly to the discussion of strategies for creating an academic hospitalist program, they are unclear about the definition of academic in this context. The term academic is often misunderstood to be synonymous with research. However, research is just one component of an academic program, which also includes education, quality improvement (QI), administration, and program development. It may be helpful, therefore, to replace academic with scholarship, which can be defined as a process that involves peer review and dissemination of ideas at local, regional, and national levels. Scholarship also goes beyond research, encompassing education and other areas such as QI. Although academic programs are not necessarily involved with funded research, there is usually an expectation of peer review, through either presentations at regional and national meetings or publication. For the purposes of this discussion, the term academic hospitalist program will be defined broadly to include any program affiliated with a university that is involved in the teaching of residents and medical students and whose faculty is required to participate in a promotions process.
All members of an academic division should be expected to participate in scholarship, whether it is education, QI projects, or research. If there is a strong expectation that traditional National Institute of Health (NIH) funded research will take place, this expectation must come with sufficient resources. Without infrastructure for research and investment in research faculty, procuring NIH funds for research is not a reasonable expectation. Organizers of hospitalist programs currently within academic divisions of general internal medicine should consider ways to better integrate programs into the existing research infrastructure in their divisions. For either freestanding hospitalist programs or programs within academic divisions of general internal medicine, investments in infrastructure and faculty are needed to nurture this area of research and build an academic focus in hospital medicine. However, if obtaining NIH research funds is not the expectation and resources are not available for hospitalists or for any other division or department at that institution, then academic expectations should focus on other pursuits. Examples include participation in the education and QI initiatives.
For programs with expectations of both funded research and other scholarship, a successful program will most likely include a small core of skilled clinical researchers working closely with well‐trained clinical educators, all of whom are involved in scholarship. Both clinical educators and researchers need to be continuously developing, and to reach their full potential, all should have access to infrastructure that supports these activities, including resources such as MPH‐level project managers, research assistants, database managers, and, most importantly, appropriate mentors.
Clinician educators must be both proficient clinicians and dedicated teachers. Ideally, they should have strong familiarity with educational theory in addition to skills in hands‐on teaching. Their responsibilities include mastering the skills that students need, staying up to the minute in their areas of expertise, and serving as role models in their attitudes toward patients, colleagues, and their work.3 Many hospitalists may not have these skills when they begin, often fresh out of residency, and will need help developing them.
PROTECTED TIME
Protected time is crucial to the success of any academic program. Finding this time presents a challenge for any clinical group, but the challenge is exacerbated for hospitalists, who face tremendous pressure to serve full‐time clinical jobs with little emphasis on academic elements such as education, QI, and participation in funded research. As both Wright et al.2 and Flanders et al.1 point out, to build a group of well‐developed academic clinician educators, academic hospitalists not on track for funding need to be given adequate protected time to participate in committees, sharpen their teaching skills, develop QI projects that can be converted to scholarships, participate in research, and present at national and regional meetings.
The requirements of protected time for researchers are more challenging than those for educators. Building a newly funded research unit within a hospitalist group, as with any group, will entail hiring fellowship trained faculty with significant protected time (approximately 80%) to give them time to obtain funding such as a K award and eventually become independent investigators with RO1 grant funding. Building research units requires support from collaborators with infrastructure and mentors already in place that can be tapped during the incubation stage of the academic program. For most hospitalist programs, infrastructure and mentors will be found in their divisions of general internal medicine.
Protected time should be considered an integral element of academic hospitalist positionsnot a perkas long as the time is used responsibly and productively. As both Wright et al.2 and Flanders et al.1 correctly point out, herein lies the major challenge of creating any kind of academic program: How will the program support protected time for both educators and researchers? In most instances, significant seed money will be needed to support junior faculty over the first few years of their careers. It is noteworthy that building a federally funded hospital medicine research program will be particularly difficult in today's economy because funding levels at the NIH, Agency for Healthcare Research and Quality, Health Resources and Services Administration, and other traditional funders of clinical research either are flat or have been reduced dramatically.
To many Academic Medical Centers (AMC), it may not be immediately obvious why a strong Academic Hospitalist program is in their economic best interest. Hospitalist programs may confront consistently high levels of turnover and a shrinking supply of general internists. The associated high costs of hiring new, junior faculty include the time and effort needed to interview, credential, train, and most importantly build familiarity with the complex systems encountered in maneuvering through a hospital, especially one with widespread dissemination of electronic medical records for documentation and order entry. However, hospitalists provided with opportunities for academic development are more likely to stay on the job longer and perform at a higher level, providing convincing motivation for hospitals to invest in their academic hospitalist programs. Retaining high‐quality hospitalists may be one of the most cost‐efficient methods for an AMC to support a hospital medicine program.
STRATEGIES IN ACTION
Flanders et al.1 point to a shortage of well‐trained clinician investigators with a focus on inpatient research as a barrier to the development of academic hospitalist programming. They describe a strategy of collaboration with specialty groups. This highlights the importance of collaboration with more well‐established research units as a key ingredient to building a new academic unit. Wright et al.2 describe their mentorship program and how they created protected time for scholarship for hospitalists, including supporting mentors' time. These examples highlight another key benchmark of a viable academic program, mentoring, and the importance of ensuring that mentors have time for this essential effort.
However, it is critically important to remember that all politics is local, to quote the late Tip O'Neill, long‐time speaker of the US House of Representatives. What works in one setting may not work as well in a different contexthence the need for creativity and political acumen.
For example, although the specialty‐group collaboration described by Flanders et al.1 may be helpful in one setting, other strategic alliances may work on a larger scale and over a longer time period. Most hospital medicine groups are currently within academic divisions of general internal medicine, where infrastructure and mentoring may already exist for both research and educational scholarship. In those cases, fostering interaction between the division's hospitalists and its researchers would be a critical first step. The programming and growth developed in this way can be leveraged to support ongoing academic activities by hospitalists rather than being limited to a single project.
In the Division of General Internal Medicine at Mount Sinai, which houses the academic hospitalists, building research entailed collaboration with the well‐established Departments of Geriatrics and Health Policy, which had preexisting research infrastructure and mentors. At the same time, we developed a research fellowship program by applying jointly with the Division of General Pediatrics for federal grant support. Such diversity of collaboration enhanced our application.
LOOKING AHEAD
Putting the academic into academic hospitalist programs is the key to the future of hospital medicine. To be successful, one must consider all the issues described in light of available resources and the local and federal political landscape. As Flanders et al.1 and Wright et al.2 emphasize, collaboration will be the main component for success in the current academic landscape.
Accelerating the development of clinical research in academic hospitalist programs is a worthwhile goal if pursued with clarity, objectivity, and a thorough understanding of the process and its implications. In their articles, Flanders et al.1 and Wright et al.2 identify major barriers to growing academic hospitalist programs. These barriers include the need for protected time, the shortage of trained research faculty, the lack of infrastructure, and the limited availability of senior mentors. Both Flanders et al. and Wright et al. offer smart and innovative ways of addressing these issues. However, building an academic program from the ground up is more complex and challenging than it may seem at first glance. It takes time, patience, creativity, diplomacy, and the ability to recruit collaborators and advocates who are willing to share infrastructure and resources.
Although both articles add significantly to the discussion of strategies for creating an academic hospitalist program, they are unclear about the definition of academic in this context. The term academic is often misunderstood to be synonymous with research. However, research is just one component of an academic program, which also includes education, quality improvement (QI), administration, and program development. It may be helpful, therefore, to replace academic with scholarship, which can be defined as a process that involves peer review and dissemination of ideas at local, regional, and national levels. Scholarship also goes beyond research, encompassing education and other areas such as QI. Although academic programs are not necessarily involved with funded research, there is usually an expectation of peer review, through either presentations at regional and national meetings or publication. For the purposes of this discussion, the term academic hospitalist program will be defined broadly to include any program affiliated with a university that is involved in the teaching of residents and medical students and whose faculty is required to participate in a promotions process.
All members of an academic division should be expected to participate in scholarship, whether it is education, QI projects, or research. If there is a strong expectation that traditional National Institute of Health (NIH) funded research will take place, this expectation must come with sufficient resources. Without infrastructure for research and investment in research faculty, procuring NIH funds for research is not a reasonable expectation. Organizers of hospitalist programs currently within academic divisions of general internal medicine should consider ways to better integrate programs into the existing research infrastructure in their divisions. For either freestanding hospitalist programs or programs within academic divisions of general internal medicine, investments in infrastructure and faculty are needed to nurture this area of research and build an academic focus in hospital medicine. However, if obtaining NIH research funds is not the expectation and resources are not available for hospitalists or for any other division or department at that institution, then academic expectations should focus on other pursuits. Examples include participation in the education and QI initiatives.
For programs with expectations of both funded research and other scholarship, a successful program will most likely include a small core of skilled clinical researchers working closely with well‐trained clinical educators, all of whom are involved in scholarship. Both clinical educators and researchers need to be continuously developing, and to reach their full potential, all should have access to infrastructure that supports these activities, including resources such as MPH‐level project managers, research assistants, database managers, and, most importantly, appropriate mentors.
Clinician educators must be both proficient clinicians and dedicated teachers. Ideally, they should have strong familiarity with educational theory in addition to skills in hands‐on teaching. Their responsibilities include mastering the skills that students need, staying up to the minute in their areas of expertise, and serving as role models in their attitudes toward patients, colleagues, and their work.3 Many hospitalists may not have these skills when they begin, often fresh out of residency, and will need help developing them.
PROTECTED TIME
Protected time is crucial to the success of any academic program. Finding this time presents a challenge for any clinical group, but the challenge is exacerbated for hospitalists, who face tremendous pressure to serve full‐time clinical jobs with little emphasis on academic elements such as education, QI, and participation in funded research. As both Wright et al.2 and Flanders et al.1 point out, to build a group of well‐developed academic clinician educators, academic hospitalists not on track for funding need to be given adequate protected time to participate in committees, sharpen their teaching skills, develop QI projects that can be converted to scholarships, participate in research, and present at national and regional meetings.
The requirements of protected time for researchers are more challenging than those for educators. Building a newly funded research unit within a hospitalist group, as with any group, will entail hiring fellowship trained faculty with significant protected time (approximately 80%) to give them time to obtain funding such as a K award and eventually become independent investigators with RO1 grant funding. Building research units requires support from collaborators with infrastructure and mentors already in place that can be tapped during the incubation stage of the academic program. For most hospitalist programs, infrastructure and mentors will be found in their divisions of general internal medicine.
Protected time should be considered an integral element of academic hospitalist positionsnot a perkas long as the time is used responsibly and productively. As both Wright et al.2 and Flanders et al.1 correctly point out, herein lies the major challenge of creating any kind of academic program: How will the program support protected time for both educators and researchers? In most instances, significant seed money will be needed to support junior faculty over the first few years of their careers. It is noteworthy that building a federally funded hospital medicine research program will be particularly difficult in today's economy because funding levels at the NIH, Agency for Healthcare Research and Quality, Health Resources and Services Administration, and other traditional funders of clinical research either are flat or have been reduced dramatically.
To many Academic Medical Centers (AMC), it may not be immediately obvious why a strong Academic Hospitalist program is in their economic best interest. Hospitalist programs may confront consistently high levels of turnover and a shrinking supply of general internists. The associated high costs of hiring new, junior faculty include the time and effort needed to interview, credential, train, and most importantly build familiarity with the complex systems encountered in maneuvering through a hospital, especially one with widespread dissemination of electronic medical records for documentation and order entry. However, hospitalists provided with opportunities for academic development are more likely to stay on the job longer and perform at a higher level, providing convincing motivation for hospitals to invest in their academic hospitalist programs. Retaining high‐quality hospitalists may be one of the most cost‐efficient methods for an AMC to support a hospital medicine program.
STRATEGIES IN ACTION
Flanders et al.1 point to a shortage of well‐trained clinician investigators with a focus on inpatient research as a barrier to the development of academic hospitalist programming. They describe a strategy of collaboration with specialty groups. This highlights the importance of collaboration with more well‐established research units as a key ingredient to building a new academic unit. Wright et al.2 describe their mentorship program and how they created protected time for scholarship for hospitalists, including supporting mentors' time. These examples highlight another key benchmark of a viable academic program, mentoring, and the importance of ensuring that mentors have time for this essential effort.
However, it is critically important to remember that all politics is local, to quote the late Tip O'Neill, long‐time speaker of the US House of Representatives. What works in one setting may not work as well in a different contexthence the need for creativity and political acumen.
For example, although the specialty‐group collaboration described by Flanders et al.1 may be helpful in one setting, other strategic alliances may work on a larger scale and over a longer time period. Most hospital medicine groups are currently within academic divisions of general internal medicine, where infrastructure and mentoring may already exist for both research and educational scholarship. In those cases, fostering interaction between the division's hospitalists and its researchers would be a critical first step. The programming and growth developed in this way can be leveraged to support ongoing academic activities by hospitalists rather than being limited to a single project.
In the Division of General Internal Medicine at Mount Sinai, which houses the academic hospitalists, building research entailed collaboration with the well‐established Departments of Geriatrics and Health Policy, which had preexisting research infrastructure and mentors. At the same time, we developed a research fellowship program by applying jointly with the Division of General Pediatrics for federal grant support. Such diversity of collaboration enhanced our application.
LOOKING AHEAD
Putting the academic into academic hospitalist programs is the key to the future of hospital medicine. To be successful, one must consider all the issues described in light of available resources and the local and federal political landscape. As Flanders et al.1 and Wright et al.2 emphasize, collaboration will be the main component for success in the current academic landscape.
- ,,,.The University of Michigan Specialist‐Hospitalist Allied Research Program (SHARP): jumpstarting hospital medicine research.J Hosp Med.2008;3(4):308–313.
- , ,,.An innovative approach to supporting hospitalist physicians towards academic success.J Hosp Med.2008;3(4):314–318.
- ,,.The clinician‐educator—present and future roles.J Gen Intern Med.1997;12 (suppl 2):S1–S4.
- ,,,.The University of Michigan Specialist‐Hospitalist Allied Research Program (SHARP): jumpstarting hospital medicine research.J Hosp Med.2008;3(4):308–313.
- , ,,.An innovative approach to supporting hospitalist physicians towards academic success.J Hosp Med.2008;3(4):314–318.
- ,,.The clinician‐educator—present and future roles.J Gen Intern Med.1997;12 (suppl 2):S1–S4.
Academic Suppport
Promotion through the ranks is the hallmark of success in academia. The support and infrastructure necessary to develop junior faculty members at academic medical centers may be inadequate.1, 2 Academic hospitalists are particularly vulnerable and at high risk for failure because of their heavy clinical commitment and limited time to pursue scholarly interests. Further, relatively few have pursued fellowship training, which means that many hospitalists must learn research‐related skills and the nuances of academia after joining the faculty.
Top‐notch mentors are believed to be integral to the success of the academic physician.36 Among other responsibilities, mentors (1) direct mentees toward promising opportunities, (2) serve as advocates for mentees, and (3) lend expertise to mentees' studies and scholarship. In general, there is concern that the cadre of talented, committed, and capable mentors is dwindling such that they are insufficient in number to satisfy and support the needs of the faculty.7, 8 In hospital medicine, experienced mentorship is particularly in short supply because the field is relatively new and there has been tremendous growth in the number of academic hospitalists, producing a large demand.
Like many hospitalist groups, our hospitalist division, the Collaborative Inpatient Medicine Service (CIMS), has experienced significant growth. It became apparent that the faculty needed and deserved a well‐designed academic support program to foster the development of skills necessary for academic success. The remainder of this article discusses our approach toward fulfilling these needs and the results to date.
DEVELOPING THE HOSPITALIST ACADEMIC SUPPORT PROGRAM
Problem Identification
Johns Hopkins Bayview Medical Center (JHBMC) is a 700‐bed urban university‐affiliated hospital. The CIMS hospital group is a distinct division separate from the hospitalist group at Johns Hopkins Hospital. All faculty are employed by the Johns Hopkins University School of Medicine (JHUSOM), and there is a single promotion track for the faculty. Specific requirements for promotion may be found in the Johns Hopkins University School of Medicine silver book at
CIMS had been growing in numbers from 4 full‐time equivalent (FTE) physicians in fiscal year (FY) 01 to 11.8 FTE physicians in FY06.
Most had limited training in research.
The physicians had little protected time for skill development and for working on scholarly projects.
Attempts to recruit a professor‐/associate professorlevel hospitalist from another institution to mentor our faculty members had been unsuccessful.
The hospitalists in our group had diverse interests such that we needed to find a flexible mentor who was willing and able to work across a breadth of content areas and methodologies.
Preliminary attempts to link up our hospitalists with clinician‐investigators at our institution were not fruitful.
Needs Assessment
In soliciting input from the hospitalists themselves and other stakeholders (including institutional leadership and leaders in hospital medicine), the following needs were identified:
Each CIMS faculty member must have a body of scholarship to support promotion and long‐term academic success.
Each CIMS faculty member needs appropriate mentorship.
Each CIMS faculty member needs protected time for scholarly work.
The CIMS faculty members need to support one another and be collaborative in their scholarly work.
The scholarly activities of the CIMS faculty need to support the mission of the division.
The mission of our division had been established to value and encourage the diverse interests and talents within the group:
The Collaborative Inpatient Medical Service (CIMS) is dedicated to serving the public trust by advancing the field of Hospital Medicine through the realization of excellence in patient care, education, research, leadership, and systems‐improvement.
Objectives
The objectives of the academic support program were organized into those for the CIMS Division as well as specific individual faculty goals and are outlined below:
Objectives for the division:
To increase the number and quality of peer‐reviewed publications produced by CIMS faculty.
To increase the amount of scholarly time available to CIMS faculty. In addition to external funding sources, we were committed to exploring nontraditional funding sources such as hospital administration and partnerships with other divisions or departments (including information technology) in need of clinically savvy physicians to help with projects.
To augment the leadership roles of the CIMS faculty with our institution and on a national level.
To support the CIMS faculty members such that they can be promoted at Johns Hopkins University School of Medicine (JHUSOM) and thereby retained.
Goals for individuals:
Each CIMS faculty member will advance his or her skill set to be moving toward producing scholarly work independently.
Each faculty member will lead at least 1 scholarly project at all times and will be involved as a team‐member in others.
Each faculty member will understand the criteria for promotion at our institution and will reflect on plans and strategies to realize success.
Strategies for Achieving the Objectives and Goals
Establish a Strong Mentoring System for the CIMS
The CIMS identified a primary mentor for the group, a faculty member within the Division of General Internal Medicine who was an experienced mentor with formidable management skills and an excellent track record in publishing scholarly work. Twenty‐percent of the mentor's time was set aside so he would have sufficient time to spend with CIMS faculty members in developing scholarly activities.
The mentor meets individually with each CIMS faculty member at the beginning of each academic year to identify career objectives; review current activities, interests, and skills; identify career development needs that require additional training or resources; set priorities for scholarly work; identify opportunities for collaboration internally and externally; and identify additional potential mentors to support specific projects. Regular follow‐up meetings are arranged, as needed to review progress and encourage advancing the work. The mentor uses resources to stay abreast of relevant funding opportunities and shares them with the group. The mentor reports regularly to the director of the CIMS regarding progress. The process as outlined remains ongoing.
Investing the Requisite Resources
A major decision was made that CIMS hospitalists would have 30% of their time protected for academic work, without the need for external funding. The expectation that the faculty had to use this time to effectively advance their career goals, which in turn would support the mission of CIMS, was clearly and explicitly expressed. The faculty would also be permitted to decrease their clinical time further on obtaining external funding. Additionally, in conjunction with a specific grant, the group hired a research assistant to permanently support the scholarly work of the faculty.
Leaders in both hospital administration and the Department of Medicine agreed that the only way to maintain a stable group of mature hospitalists who could serve as champions for change and help develop functional quality improvement projects was to support them in their academic efforts, including protected academic time irrespective of external funding.
The funding to protect the scholarly commitment (the mentor, the protected time of CIMS faculty, and the research assistant) has come primarily from divisional funds, although the CIMS budget is subsidized by the Department of Medicine and the medical center.
Recruit Faculty with Fellowship Training
It is our goal to reach a critical mass of hospitalists with experience and advanced training in scholarship. Fellowship‐trained faculty members are best positioned to realize academic success and can impart their knowledge and skills to others. Fellowship‐trained faculty members hired to date have come from either general internal medicine (n = 1) or geriatric (n = 2) fellowship programs, and none have been trained in a hospitalist fellowship program. It is hoped that these fellowship‐trained faculty and some of the other more experienced members of the group will be able to share in the mentoring responsibilities so that mentoring outsourcing can ultimately be replaced by CIMS faculty members.
EVALUATION DATA
In the 2 years since implementation of the scholarly support program, individual faculty in the CIMS have been meeting the above‐mentioned goals. Specifically, with respect to acquiring knowledge and skills, 2 faculty members have completed their master's degrees, and 6 others have made use of select courses to augment their knowledge and skills. All faculty members (100%) have a scholarly project they are leading, and most have reached out to a colleague in the CIMS to assist them, such that nearly all are team members on at least 1 other scholarly project. Through informal mentoring sessions and a once‐yearly formal meeting related to academic promotion, all members (100%) of the faculty are aware of the expectations and requirements for promotion.
Table 1 shows the accomplishment of the 5 faculty members in the academic track who have been division members for 3 years or more. Among the 5 faculty in the academic track, publications and extramural funding are improving. In the 5 years before the initiative, CIMS faculty averaged approximately 0.5 publications per person per year; in the first 2 years of this initiative, that number has increased to 1.3 publications per person per year. The 1 physician who has not yet been published has completed projects and has several article in process. External funding (largely in the form of 3 extramural grants from private foundations) has increased dramatically from an average of 4% per FTE before the intervention to approximately 15% per FTE afterward. In addition, all faculty members have secured a source of additional funding to reduce their clinical efforts since the implementation of this program. One foundation funded project that involved all division members, whose goal was to develop mechanisms to improve the discharge process of elderly patients to their homes, won the award at the SGIM 2007 National Meeting for the best clinical innovation. As illustrated in Table 1, 1 of the founding CIMS members transferred out of the academic track in 2003 in alignment with this physician's personal and professional goals and preferences. Two faculty members have moved up an academic rank, and several others are poised to do so.
| Dr. A* | Dr. B | Dr. C | Dr. D | Dr. E | Dr. F | |
|---|---|---|---|---|---|---|
| ||||||
| Years on faculty | 7 | 7 | 7 | 5 | 3 | 3 |
| Clinical % FTE before ASP | 70% | 60% | 60% | 70% | 70% | 70% |
| Clinical % FTE after ASP | Not applicable | 30% | 60% | 60% | 50% | 45% |
| Number of publications per year before ASP | Not applicable | 0.75 | 0.75 | 0 | 0 | 0 |
| Number of publications per year after ASP | Not applicable | 2.5 | 2 | 1 | 1 | 0 |
| Leadership role and title before ASP: | Not applicable | |||||
| a. within institution | Yes | No | No | No | No | |
| b. national level | No | No | No | No | No | |
| Leadership role and title after ASP: | Not applicable | |||||
| a. within institution | Yes | Yes | Yes | Yes | No | |
| b. national level | Yes | No | No | No | Yes | |
Thus, the divisional objectives (increasing number of publications, securing funding to increase the time devoted to scholarship, new leadership roles, and progression toward promotion) are being met as well.
CONCLUSIONS
Our rapidly growing hospitalist division recognized that several factors threatened the ability of the division and individuals to succeed academically. Divisional, departmental, and medical center leadership was committed to creating a supportive structure that would be available to all hospitalists as opposed to expecting each individual to unearth the necessary resources on their own. The innovative approach to foster individual, and therefore divisional, academic and scholarly success was designed around the following strategies: retention of an expert mentor (who is a not a hospitalist) and securing 20% of his time, investing in scholarship by protecting 30% nonclinical time for academic pursuits, and attempting to seek out fellowship‐trained hospitalists when hiring.
Although quality mentorship, protected time, and recruiting the best‐available talent to fill needs may not seem all that innovative, we believe the systematic approach to the problem and our steadfast application of the strategic plan is unique, innovative, and may present a model to be emulated by other divisions. Some may contend that it is impossible to protect 30% FTE of academic hospitalists indefinitely. Our group has made substantial investment in supporting the academic pursuits of our physicians, and we believe this is essential to maintaining their satisfaction and commitment to scholarship. This amount of protected time is offered to the entire physician faculty and continues even as our division has almost tripled in size. This initiative represents a carefully calculated investment that has influenced our ability to recruit and retain excellent people. Ongoing prospective study of this intervention over time will provide additional perspective on its value and shortcomings. Nonetheless, early data suggest that the plan is indeed working and that our group is satisfied with the return on investment to date.
- ,,,.2001.Status of clinical research in academic health centers: views from the research leadership.JAMA.286:800–806.
- ,,,.Contemporary (post‐Wills) survey of the views of Australian medical researchers: importance of funding, infrastructure and motivators for a research career.Med J Aust.2005;183:604–605.
- ,.Mentors, Advisors, and Role Models in Graduate and Professional Education.Washington DC:Association of Academic Health Centers;1996.
- ,.Characteristics of the successful researcher and implications for faculty development.J Med Educ.1986;61:22–31.
- .On mentoring.J R Soc Med.1997;90:347–349.
- ,,, et al.Junior faculty members' mentoring relationships and their professional development in U.S. medical schools.Acad Med.1998;73:318–323.
- AAMC (Association of American Medical Colleges).For the Health of the Public: Ensuring the Future of Clinical Research.Washington, DC:AAMC;1999.
- .2002.Clinical research career development: the individual perspective.Acad Med.77:1084–1088.
Promotion through the ranks is the hallmark of success in academia. The support and infrastructure necessary to develop junior faculty members at academic medical centers may be inadequate.1, 2 Academic hospitalists are particularly vulnerable and at high risk for failure because of their heavy clinical commitment and limited time to pursue scholarly interests. Further, relatively few have pursued fellowship training, which means that many hospitalists must learn research‐related skills and the nuances of academia after joining the faculty.
Top‐notch mentors are believed to be integral to the success of the academic physician.36 Among other responsibilities, mentors (1) direct mentees toward promising opportunities, (2) serve as advocates for mentees, and (3) lend expertise to mentees' studies and scholarship. In general, there is concern that the cadre of talented, committed, and capable mentors is dwindling such that they are insufficient in number to satisfy and support the needs of the faculty.7, 8 In hospital medicine, experienced mentorship is particularly in short supply because the field is relatively new and there has been tremendous growth in the number of academic hospitalists, producing a large demand.
Like many hospitalist groups, our hospitalist division, the Collaborative Inpatient Medicine Service (CIMS), has experienced significant growth. It became apparent that the faculty needed and deserved a well‐designed academic support program to foster the development of skills necessary for academic success. The remainder of this article discusses our approach toward fulfilling these needs and the results to date.
DEVELOPING THE HOSPITALIST ACADEMIC SUPPORT PROGRAM
Problem Identification
Johns Hopkins Bayview Medical Center (JHBMC) is a 700‐bed urban university‐affiliated hospital. The CIMS hospital group is a distinct division separate from the hospitalist group at Johns Hopkins Hospital. All faculty are employed by the Johns Hopkins University School of Medicine (JHUSOM), and there is a single promotion track for the faculty. Specific requirements for promotion may be found in the Johns Hopkins University School of Medicine silver book at
CIMS had been growing in numbers from 4 full‐time equivalent (FTE) physicians in fiscal year (FY) 01 to 11.8 FTE physicians in FY06.
Most had limited training in research.
The physicians had little protected time for skill development and for working on scholarly projects.
Attempts to recruit a professor‐/associate professorlevel hospitalist from another institution to mentor our faculty members had been unsuccessful.
The hospitalists in our group had diverse interests such that we needed to find a flexible mentor who was willing and able to work across a breadth of content areas and methodologies.
Preliminary attempts to link up our hospitalists with clinician‐investigators at our institution were not fruitful.
Needs Assessment
In soliciting input from the hospitalists themselves and other stakeholders (including institutional leadership and leaders in hospital medicine), the following needs were identified:
Each CIMS faculty member must have a body of scholarship to support promotion and long‐term academic success.
Each CIMS faculty member needs appropriate mentorship.
Each CIMS faculty member needs protected time for scholarly work.
The CIMS faculty members need to support one another and be collaborative in their scholarly work.
The scholarly activities of the CIMS faculty need to support the mission of the division.
The mission of our division had been established to value and encourage the diverse interests and talents within the group:
The Collaborative Inpatient Medical Service (CIMS) is dedicated to serving the public trust by advancing the field of Hospital Medicine through the realization of excellence in patient care, education, research, leadership, and systems‐improvement.
Objectives
The objectives of the academic support program were organized into those for the CIMS Division as well as specific individual faculty goals and are outlined below:
Objectives for the division:
To increase the number and quality of peer‐reviewed publications produced by CIMS faculty.
To increase the amount of scholarly time available to CIMS faculty. In addition to external funding sources, we were committed to exploring nontraditional funding sources such as hospital administration and partnerships with other divisions or departments (including information technology) in need of clinically savvy physicians to help with projects.
To augment the leadership roles of the CIMS faculty with our institution and on a national level.
To support the CIMS faculty members such that they can be promoted at Johns Hopkins University School of Medicine (JHUSOM) and thereby retained.
Goals for individuals:
Each CIMS faculty member will advance his or her skill set to be moving toward producing scholarly work independently.
Each faculty member will lead at least 1 scholarly project at all times and will be involved as a team‐member in others.
Each faculty member will understand the criteria for promotion at our institution and will reflect on plans and strategies to realize success.
Strategies for Achieving the Objectives and Goals
Establish a Strong Mentoring System for the CIMS
The CIMS identified a primary mentor for the group, a faculty member within the Division of General Internal Medicine who was an experienced mentor with formidable management skills and an excellent track record in publishing scholarly work. Twenty‐percent of the mentor's time was set aside so he would have sufficient time to spend with CIMS faculty members in developing scholarly activities.
The mentor meets individually with each CIMS faculty member at the beginning of each academic year to identify career objectives; review current activities, interests, and skills; identify career development needs that require additional training or resources; set priorities for scholarly work; identify opportunities for collaboration internally and externally; and identify additional potential mentors to support specific projects. Regular follow‐up meetings are arranged, as needed to review progress and encourage advancing the work. The mentor uses resources to stay abreast of relevant funding opportunities and shares them with the group. The mentor reports regularly to the director of the CIMS regarding progress. The process as outlined remains ongoing.
Investing the Requisite Resources
A major decision was made that CIMS hospitalists would have 30% of their time protected for academic work, without the need for external funding. The expectation that the faculty had to use this time to effectively advance their career goals, which in turn would support the mission of CIMS, was clearly and explicitly expressed. The faculty would also be permitted to decrease their clinical time further on obtaining external funding. Additionally, in conjunction with a specific grant, the group hired a research assistant to permanently support the scholarly work of the faculty.
Leaders in both hospital administration and the Department of Medicine agreed that the only way to maintain a stable group of mature hospitalists who could serve as champions for change and help develop functional quality improvement projects was to support them in their academic efforts, including protected academic time irrespective of external funding.
The funding to protect the scholarly commitment (the mentor, the protected time of CIMS faculty, and the research assistant) has come primarily from divisional funds, although the CIMS budget is subsidized by the Department of Medicine and the medical center.
Recruit Faculty with Fellowship Training
It is our goal to reach a critical mass of hospitalists with experience and advanced training in scholarship. Fellowship‐trained faculty members are best positioned to realize academic success and can impart their knowledge and skills to others. Fellowship‐trained faculty members hired to date have come from either general internal medicine (n = 1) or geriatric (n = 2) fellowship programs, and none have been trained in a hospitalist fellowship program. It is hoped that these fellowship‐trained faculty and some of the other more experienced members of the group will be able to share in the mentoring responsibilities so that mentoring outsourcing can ultimately be replaced by CIMS faculty members.
EVALUATION DATA
In the 2 years since implementation of the scholarly support program, individual faculty in the CIMS have been meeting the above‐mentioned goals. Specifically, with respect to acquiring knowledge and skills, 2 faculty members have completed their master's degrees, and 6 others have made use of select courses to augment their knowledge and skills. All faculty members (100%) have a scholarly project they are leading, and most have reached out to a colleague in the CIMS to assist them, such that nearly all are team members on at least 1 other scholarly project. Through informal mentoring sessions and a once‐yearly formal meeting related to academic promotion, all members (100%) of the faculty are aware of the expectations and requirements for promotion.
Table 1 shows the accomplishment of the 5 faculty members in the academic track who have been division members for 3 years or more. Among the 5 faculty in the academic track, publications and extramural funding are improving. In the 5 years before the initiative, CIMS faculty averaged approximately 0.5 publications per person per year; in the first 2 years of this initiative, that number has increased to 1.3 publications per person per year. The 1 physician who has not yet been published has completed projects and has several article in process. External funding (largely in the form of 3 extramural grants from private foundations) has increased dramatically from an average of 4% per FTE before the intervention to approximately 15% per FTE afterward. In addition, all faculty members have secured a source of additional funding to reduce their clinical efforts since the implementation of this program. One foundation funded project that involved all division members, whose goal was to develop mechanisms to improve the discharge process of elderly patients to their homes, won the award at the SGIM 2007 National Meeting for the best clinical innovation. As illustrated in Table 1, 1 of the founding CIMS members transferred out of the academic track in 2003 in alignment with this physician's personal and professional goals and preferences. Two faculty members have moved up an academic rank, and several others are poised to do so.
| Dr. A* | Dr. B | Dr. C | Dr. D | Dr. E | Dr. F | |
|---|---|---|---|---|---|---|
| ||||||
| Years on faculty | 7 | 7 | 7 | 5 | 3 | 3 |
| Clinical % FTE before ASP | 70% | 60% | 60% | 70% | 70% | 70% |
| Clinical % FTE after ASP | Not applicable | 30% | 60% | 60% | 50% | 45% |
| Number of publications per year before ASP | Not applicable | 0.75 | 0.75 | 0 | 0 | 0 |
| Number of publications per year after ASP | Not applicable | 2.5 | 2 | 1 | 1 | 0 |
| Leadership role and title before ASP: | Not applicable | |||||
| a. within institution | Yes | No | No | No | No | |
| b. national level | No | No | No | No | No | |
| Leadership role and title after ASP: | Not applicable | |||||
| a. within institution | Yes | Yes | Yes | Yes | No | |
| b. national level | Yes | No | No | No | Yes | |
Thus, the divisional objectives (increasing number of publications, securing funding to increase the time devoted to scholarship, new leadership roles, and progression toward promotion) are being met as well.
CONCLUSIONS
Our rapidly growing hospitalist division recognized that several factors threatened the ability of the division and individuals to succeed academically. Divisional, departmental, and medical center leadership was committed to creating a supportive structure that would be available to all hospitalists as opposed to expecting each individual to unearth the necessary resources on their own. The innovative approach to foster individual, and therefore divisional, academic and scholarly success was designed around the following strategies: retention of an expert mentor (who is a not a hospitalist) and securing 20% of his time, investing in scholarship by protecting 30% nonclinical time for academic pursuits, and attempting to seek out fellowship‐trained hospitalists when hiring.
Although quality mentorship, protected time, and recruiting the best‐available talent to fill needs may not seem all that innovative, we believe the systematic approach to the problem and our steadfast application of the strategic plan is unique, innovative, and may present a model to be emulated by other divisions. Some may contend that it is impossible to protect 30% FTE of academic hospitalists indefinitely. Our group has made substantial investment in supporting the academic pursuits of our physicians, and we believe this is essential to maintaining their satisfaction and commitment to scholarship. This amount of protected time is offered to the entire physician faculty and continues even as our division has almost tripled in size. This initiative represents a carefully calculated investment that has influenced our ability to recruit and retain excellent people. Ongoing prospective study of this intervention over time will provide additional perspective on its value and shortcomings. Nonetheless, early data suggest that the plan is indeed working and that our group is satisfied with the return on investment to date.
Promotion through the ranks is the hallmark of success in academia. The support and infrastructure necessary to develop junior faculty members at academic medical centers may be inadequate.1, 2 Academic hospitalists are particularly vulnerable and at high risk for failure because of their heavy clinical commitment and limited time to pursue scholarly interests. Further, relatively few have pursued fellowship training, which means that many hospitalists must learn research‐related skills and the nuances of academia after joining the faculty.
Top‐notch mentors are believed to be integral to the success of the academic physician.36 Among other responsibilities, mentors (1) direct mentees toward promising opportunities, (2) serve as advocates for mentees, and (3) lend expertise to mentees' studies and scholarship. In general, there is concern that the cadre of talented, committed, and capable mentors is dwindling such that they are insufficient in number to satisfy and support the needs of the faculty.7, 8 In hospital medicine, experienced mentorship is particularly in short supply because the field is relatively new and there has been tremendous growth in the number of academic hospitalists, producing a large demand.
Like many hospitalist groups, our hospitalist division, the Collaborative Inpatient Medicine Service (CIMS), has experienced significant growth. It became apparent that the faculty needed and deserved a well‐designed academic support program to foster the development of skills necessary for academic success. The remainder of this article discusses our approach toward fulfilling these needs and the results to date.
DEVELOPING THE HOSPITALIST ACADEMIC SUPPORT PROGRAM
Problem Identification
Johns Hopkins Bayview Medical Center (JHBMC) is a 700‐bed urban university‐affiliated hospital. The CIMS hospital group is a distinct division separate from the hospitalist group at Johns Hopkins Hospital. All faculty are employed by the Johns Hopkins University School of Medicine (JHUSOM), and there is a single promotion track for the faculty. Specific requirements for promotion may be found in the Johns Hopkins University School of Medicine silver book at
CIMS had been growing in numbers from 4 full‐time equivalent (FTE) physicians in fiscal year (FY) 01 to 11.8 FTE physicians in FY06.
Most had limited training in research.
The physicians had little protected time for skill development and for working on scholarly projects.
Attempts to recruit a professor‐/associate professorlevel hospitalist from another institution to mentor our faculty members had been unsuccessful.
The hospitalists in our group had diverse interests such that we needed to find a flexible mentor who was willing and able to work across a breadth of content areas and methodologies.
Preliminary attempts to link up our hospitalists with clinician‐investigators at our institution were not fruitful.
Needs Assessment
In soliciting input from the hospitalists themselves and other stakeholders (including institutional leadership and leaders in hospital medicine), the following needs were identified:
Each CIMS faculty member must have a body of scholarship to support promotion and long‐term academic success.
Each CIMS faculty member needs appropriate mentorship.
Each CIMS faculty member needs protected time for scholarly work.
The CIMS faculty members need to support one another and be collaborative in their scholarly work.
The scholarly activities of the CIMS faculty need to support the mission of the division.
The mission of our division had been established to value and encourage the diverse interests and talents within the group:
The Collaborative Inpatient Medical Service (CIMS) is dedicated to serving the public trust by advancing the field of Hospital Medicine through the realization of excellence in patient care, education, research, leadership, and systems‐improvement.
Objectives
The objectives of the academic support program were organized into those for the CIMS Division as well as specific individual faculty goals and are outlined below:
Objectives for the division:
To increase the number and quality of peer‐reviewed publications produced by CIMS faculty.
To increase the amount of scholarly time available to CIMS faculty. In addition to external funding sources, we were committed to exploring nontraditional funding sources such as hospital administration and partnerships with other divisions or departments (including information technology) in need of clinically savvy physicians to help with projects.
To augment the leadership roles of the CIMS faculty with our institution and on a national level.
To support the CIMS faculty members such that they can be promoted at Johns Hopkins University School of Medicine (JHUSOM) and thereby retained.
Goals for individuals:
Each CIMS faculty member will advance his or her skill set to be moving toward producing scholarly work independently.
Each faculty member will lead at least 1 scholarly project at all times and will be involved as a team‐member in others.
Each faculty member will understand the criteria for promotion at our institution and will reflect on plans and strategies to realize success.
Strategies for Achieving the Objectives and Goals
Establish a Strong Mentoring System for the CIMS
The CIMS identified a primary mentor for the group, a faculty member within the Division of General Internal Medicine who was an experienced mentor with formidable management skills and an excellent track record in publishing scholarly work. Twenty‐percent of the mentor's time was set aside so he would have sufficient time to spend with CIMS faculty members in developing scholarly activities.
The mentor meets individually with each CIMS faculty member at the beginning of each academic year to identify career objectives; review current activities, interests, and skills; identify career development needs that require additional training or resources; set priorities for scholarly work; identify opportunities for collaboration internally and externally; and identify additional potential mentors to support specific projects. Regular follow‐up meetings are arranged, as needed to review progress and encourage advancing the work. The mentor uses resources to stay abreast of relevant funding opportunities and shares them with the group. The mentor reports regularly to the director of the CIMS regarding progress. The process as outlined remains ongoing.
Investing the Requisite Resources
A major decision was made that CIMS hospitalists would have 30% of their time protected for academic work, without the need for external funding. The expectation that the faculty had to use this time to effectively advance their career goals, which in turn would support the mission of CIMS, was clearly and explicitly expressed. The faculty would also be permitted to decrease their clinical time further on obtaining external funding. Additionally, in conjunction with a specific grant, the group hired a research assistant to permanently support the scholarly work of the faculty.
Leaders in both hospital administration and the Department of Medicine agreed that the only way to maintain a stable group of mature hospitalists who could serve as champions for change and help develop functional quality improvement projects was to support them in their academic efforts, including protected academic time irrespective of external funding.
The funding to protect the scholarly commitment (the mentor, the protected time of CIMS faculty, and the research assistant) has come primarily from divisional funds, although the CIMS budget is subsidized by the Department of Medicine and the medical center.
Recruit Faculty with Fellowship Training
It is our goal to reach a critical mass of hospitalists with experience and advanced training in scholarship. Fellowship‐trained faculty members are best positioned to realize academic success and can impart their knowledge and skills to others. Fellowship‐trained faculty members hired to date have come from either general internal medicine (n = 1) or geriatric (n = 2) fellowship programs, and none have been trained in a hospitalist fellowship program. It is hoped that these fellowship‐trained faculty and some of the other more experienced members of the group will be able to share in the mentoring responsibilities so that mentoring outsourcing can ultimately be replaced by CIMS faculty members.
EVALUATION DATA
In the 2 years since implementation of the scholarly support program, individual faculty in the CIMS have been meeting the above‐mentioned goals. Specifically, with respect to acquiring knowledge and skills, 2 faculty members have completed their master's degrees, and 6 others have made use of select courses to augment their knowledge and skills. All faculty members (100%) have a scholarly project they are leading, and most have reached out to a colleague in the CIMS to assist them, such that nearly all are team members on at least 1 other scholarly project. Through informal mentoring sessions and a once‐yearly formal meeting related to academic promotion, all members (100%) of the faculty are aware of the expectations and requirements for promotion.
Table 1 shows the accomplishment of the 5 faculty members in the academic track who have been division members for 3 years or more. Among the 5 faculty in the academic track, publications and extramural funding are improving. In the 5 years before the initiative, CIMS faculty averaged approximately 0.5 publications per person per year; in the first 2 years of this initiative, that number has increased to 1.3 publications per person per year. The 1 physician who has not yet been published has completed projects and has several article in process. External funding (largely in the form of 3 extramural grants from private foundations) has increased dramatically from an average of 4% per FTE before the intervention to approximately 15% per FTE afterward. In addition, all faculty members have secured a source of additional funding to reduce their clinical efforts since the implementation of this program. One foundation funded project that involved all division members, whose goal was to develop mechanisms to improve the discharge process of elderly patients to their homes, won the award at the SGIM 2007 National Meeting for the best clinical innovation. As illustrated in Table 1, 1 of the founding CIMS members transferred out of the academic track in 2003 in alignment with this physician's personal and professional goals and preferences. Two faculty members have moved up an academic rank, and several others are poised to do so.
| Dr. A* | Dr. B | Dr. C | Dr. D | Dr. E | Dr. F | |
|---|---|---|---|---|---|---|
| ||||||
| Years on faculty | 7 | 7 | 7 | 5 | 3 | 3 |
| Clinical % FTE before ASP | 70% | 60% | 60% | 70% | 70% | 70% |
| Clinical % FTE after ASP | Not applicable | 30% | 60% | 60% | 50% | 45% |
| Number of publications per year before ASP | Not applicable | 0.75 | 0.75 | 0 | 0 | 0 |
| Number of publications per year after ASP | Not applicable | 2.5 | 2 | 1 | 1 | 0 |
| Leadership role and title before ASP: | Not applicable | |||||
| a. within institution | Yes | No | No | No | No | |
| b. national level | No | No | No | No | No | |
| Leadership role and title after ASP: | Not applicable | |||||
| a. within institution | Yes | Yes | Yes | Yes | No | |
| b. national level | Yes | No | No | No | Yes | |
Thus, the divisional objectives (increasing number of publications, securing funding to increase the time devoted to scholarship, new leadership roles, and progression toward promotion) are being met as well.
CONCLUSIONS
Our rapidly growing hospitalist division recognized that several factors threatened the ability of the division and individuals to succeed academically. Divisional, departmental, and medical center leadership was committed to creating a supportive structure that would be available to all hospitalists as opposed to expecting each individual to unearth the necessary resources on their own. The innovative approach to foster individual, and therefore divisional, academic and scholarly success was designed around the following strategies: retention of an expert mentor (who is a not a hospitalist) and securing 20% of his time, investing in scholarship by protecting 30% nonclinical time for academic pursuits, and attempting to seek out fellowship‐trained hospitalists when hiring.
Although quality mentorship, protected time, and recruiting the best‐available talent to fill needs may not seem all that innovative, we believe the systematic approach to the problem and our steadfast application of the strategic plan is unique, innovative, and may present a model to be emulated by other divisions. Some may contend that it is impossible to protect 30% FTE of academic hospitalists indefinitely. Our group has made substantial investment in supporting the academic pursuits of our physicians, and we believe this is essential to maintaining their satisfaction and commitment to scholarship. This amount of protected time is offered to the entire physician faculty and continues even as our division has almost tripled in size. This initiative represents a carefully calculated investment that has influenced our ability to recruit and retain excellent people. Ongoing prospective study of this intervention over time will provide additional perspective on its value and shortcomings. Nonetheless, early data suggest that the plan is indeed working and that our group is satisfied with the return on investment to date.
- ,,,.2001.Status of clinical research in academic health centers: views from the research leadership.JAMA.286:800–806.
- ,,,.Contemporary (post‐Wills) survey of the views of Australian medical researchers: importance of funding, infrastructure and motivators for a research career.Med J Aust.2005;183:604–605.
- ,.Mentors, Advisors, and Role Models in Graduate and Professional Education.Washington DC:Association of Academic Health Centers;1996.
- ,.Characteristics of the successful researcher and implications for faculty development.J Med Educ.1986;61:22–31.
- .On mentoring.J R Soc Med.1997;90:347–349.
- ,,, et al.Junior faculty members' mentoring relationships and their professional development in U.S. medical schools.Acad Med.1998;73:318–323.
- AAMC (Association of American Medical Colleges).For the Health of the Public: Ensuring the Future of Clinical Research.Washington, DC:AAMC;1999.
- .2002.Clinical research career development: the individual perspective.Acad Med.77:1084–1088.
- ,,,.2001.Status of clinical research in academic health centers: views from the research leadership.JAMA.286:800–806.
- ,,,.Contemporary (post‐Wills) survey of the views of Australian medical researchers: importance of funding, infrastructure and motivators for a research career.Med J Aust.2005;183:604–605.
- ,.Mentors, Advisors, and Role Models in Graduate and Professional Education.Washington DC:Association of Academic Health Centers;1996.
- ,.Characteristics of the successful researcher and implications for faculty development.J Med Educ.1986;61:22–31.
- .On mentoring.J R Soc Med.1997;90:347–349.
- ,,, et al.Junior faculty members' mentoring relationships and their professional development in U.S. medical schools.Acad Med.1998;73:318–323.
- AAMC (Association of American Medical Colleges).For the Health of the Public: Ensuring the Future of Clinical Research.Washington, DC:AAMC;1999.
- .2002.Clinical research career development: the individual perspective.Acad Med.77:1084–1088.
Copyright © 2008 Society of Hospital Medicine
Should ACEIs/ARAs Be Continued Before Surgery?
Clinicians commonly use renin‐angiotensin‐aldosterone‐system (RAAS) antagonists such as angiotensin‐converting enzyme inhibitors (ACEIs) and angiotensin II receptor subtype 1 antagonists (ARAs) to treat hypertension, congestive heart failure, and diabetic nephropathy. Hospitalists and other clinicians involved in the preoperative care of patients treated chronically with these agents are faced with the uncertainty of whether to continue these medications immediately prior to surgery.
The concern among those who recommend holding therapy is that pharmacologic suppression of the RAAS in patients undergoing general anesthesia may lead to severe or refractory (to intravenous fluid support) hypotension requiring vasopressors. On the other hand, if complications are no more likely when continuing one of these agents up to the day of surgery, withholding it could represent an unnecessary and potentially harmful intervention (eg, when a clinician caring postoperatively for a patient forgets to restart it). Although several studies have attempted to address this dilemma, a systematic and comprehensive summary of the pertinent evidence has not been published.
In this systematic review and meta‐analysis, we sought to summarize the best available evidence about the relative incidence of patient‐important outcomes1 in patients who do or do not receive ACEI/ARA therapy on the day of their nonemergent surgery.
METHODS
We report this protocol‐driven review in accordance with the Quality of Reporting of Meta‐analyses (QUOROM) standards for reporting systematic reviews of randomized trials.2
Search Strategy
In collaboration with an expert reference librarian (P.J.E.), we designed a search strategy that included the electronic databases MEDLINE, EMBASE, CINAHL, Web of Science, Current Contents, CENTRAL, DARE, and SCOPUS from 1981 (when captopril, the first ACEI, was approved by the FDA) until March 2006. We also reviewed the reference lists of included articles, retrieved articles from our personal files, and consulted with anesthesiologists and hospitalists with an interest in perioperative care in order to identify unpublished studies or studies missed by our strategy.
Study Selection
Eligible studies were prospective cohort studies or randomized controlled trials enrolling adult patients (ie, most patients > 18 years) undergoing nonemergent surgery and using ACEI or ARA chronically and assessing the effect of withdrawing or continuing these agents up to the morning of surgery. Eligible studies measured and reported either events of great patient importance (death, myocardial infarction, transient ischemic attack or stroke, and hepatic or renal failure) or of potentially less importance such as unplanned admission to the intensive care unit or treatment‐requiring hypotension, arrhythmias, or hyperkalemia.
Study Selection
Two reviewers (D.J.R. and F.S.M.) independently screened the titles and abstracts for potential inclusion and retrieved potentially eligible articles for full‐text evaluation. Two reviewers (D.J.R. and M.L.B.) working in duplicate independently selected studies for inclusion. The reviewers were in agreement for full text inclusion 100% of the time.
Data Extraction
Two hospitalists with experience in perioperative care and trained in clinical research (D.J.R. and F.S.M.) working independently and in duplicate extracted data from each eligible article using a standardized structured data extraction form. We extracted information about the study authors and publication, the patients (numbers in each group, indications for chronic ACEI/ARA therapy, type of surgery, agents used for anesthesia), event rates of surgical and perioperative complications (death, stroke, myocardial infarction, unplanned admission to the intensive care unit, treatment‐requiring hypotension, arrhythmias, or hyperkalemia), and relevant periods (e.g., between last dose of ACEI/ARA and surgery, between surgery and clinical end points, total follow‐up). When key information was not available in the published report, we contacted authors by electronic mail. We made 2 attempts to contact authors who failed to respond. Three of the 4 authors contacted responded with the requested information.
Quality Assessment
For randomized trials, we noted whether authors reported adequate allocation concealment, blinding of patients, clinicians, data collectors, data analysts, outcome assessors, and loss to follow‐up. The same reviewers (D.J.R. and F.S.M.) assessed study quality and were in agreement for each article and each domain of quality (kappa statistic in each case was 1.0). For cohort studies we noted details of cohort selection and comparability according to the Newcastle‐Ottawa approach.3
Statistical Analysis
We used a DerSimonian and Laird random effects method4 to conduct meta‐analyses across eligible outcomes. Random effects meta‐analysis incorporates both within‐study and between‐study variability. We chose a random effects approach because of the important degree of clinical heterogeneity expected between the included studies. For rare events we followed the approach by Sweeting et al. for the choice of a continuity correction factor.5 We report the pooled relative risk and the associated 95% confidence interval.
Inconsistency and Subgroup Analyses
To ascertain the magnitude of inconsistency across trials, we measured the I2 statistic, an estimate of the proportion of the overall between‐study variability that is not a result of random error or chance but of true clinical heterogeneity.6 When possible, we explored subgroup analyses to explain heterogeneity, including subgroups defined by type of surgery (cardiovascular versus noncardiovascular), timing of measurement of outcomes (in relation to anesthesia induction postoperatively), and type of agent (ACEI or ARA). We estimated the difference in treatment effects between subgroups by testing the hypothesis of treatmentsubgroup interaction with a nominal significance level of 5%.7
RESULTS
Search Results
The 509 titles reviewed included 410 titles produced by electronic searches and an additional 99 titles from other sources (Fig. 1).
Study Characteristics
Table 1 summarizes the characteristics of the 5 included studies (n = 434 patients). Myocardial infarction was an end point in 3 studies (Brabant, Bertrand, and Comfere); 1 event was reported in the withheld arm of each of these studies (none in the continuing arms). Hypotension requiring vasopressors was reported in all 5 studies. The other end points of interest were reported sparsely. There was considerable heterogeneity across studies regarding follow‐up period, which ranged from ending at incision to ending at dismissal from the hospital.
| Author/Year | Patients (n) | Indication for ACEI/ARA | Type of surgery | End points measured |
|---|---|---|---|---|
| ||||
| Randomized trials | ||||
| Bertrand, 200111 | 19 continued 18 withheld | Hypertension | Elective major vascular | Hypotension, need for vasoactive drugs (at or shortly after induction) |
| Coriat, 19948 | 21 continued 30 withheld | Hypertension | Peripheral vascular (>2 hours) | Systolic blood pressure (at or shortly after induction), plasma ACEI and catecholamine levels |
| Pigott, 199917 | 20 continued 20 withheld | Hypertension (n = 17); previous myocardial infarction (n = 23) | Coronary artery bypass graft | Arterial pressure (at or shortly after induction), cardiac index, systemic vascular resistance, use of vasoactive drugs |
| Observational studies | ||||
| Brabant, 199910 | 12 continued 27 withheld | Previous myocardial infarction (n = 6); diabetes mellitus (n = 6; n with diabetic nephropathy unknown); hypertension (n = unknown) | Elective vascular surgery | Blood pressure (at or shortly after induction) |
| Comfere, 20059 | 144 continued 123 withheld | Hypertension | Noncardiovascular | Blood pressure (at or shortly after induction), unplanned ICU admissions, hemodynamic instability in the PACU (ABP or HR out of range), acute renal impairment, TIA, stroke, myocardial ischemia/emnfarction, and death |
Methodological Quality of Included Studies
Table 2 describes the methodological quality, as reported, of the included studies. Allocation concealment was unclear in 2 of the 3 randomized trials. Details of blinding either were not reported or otherwise were unclear in 2 of these 3 studies. Only 1 study specified the extent of loss to follow‐up.8 In 1 of the observational studies,9 details of cohort selection were generally appropriate. The 12 patients examined in another study10 had been scheduled consecutively for surgery. Both studies controlled for a variety of demographic and other key variables. Duration of follow‐up ranged from 3 days after surgery (for ECG)10 to as long as duration of hospitalization.9
| Randomized trials | |||
|---|---|---|---|
| Allocation concealment | Blinding | Loss to follow‐up | |
| Bertrand, 200111 | Unclear | Unclear | Not reported |
| Coriat, 19948 | Unclear | None | 19% |
| Pigott, 199917 | Adequate | Investigator, cardiac anesthetists, perfusionists, and recovery staff were blinded to allocation. Blinding not reported for other data collectors, assessors of outcome, or data analysts | Not reported |
| Observational studies | |||
| Details of cohort selection | Comparability of cohorts | ||
| Brabant, 199910 | Appropriate Cohort somewhat representative of the adult population undergoing nonemergent surgery. The unexposed cohort was drawn from the same community as the exposed cohort | Similar with 2 exceptions: compared with the ACEI‐withheld group, the ARA‐given group contained more than twice the proportion of patients with previous myocardial revascularization Compared with the ARA‐given group, the ACEI‐withheld group contained more than twice the proportion of patients with diabetes mellitus | |
| Comfere, 20059 | Appropriate Cohort somewhat representative of the adult population undergoing nonemergent surgery (referral center population may not truly represent overall population). The unexposed cohort was drawn from the same community as the exposed cohort. Data were extracted from a secure record | Adequate This study controls for a variety of demographic and other variables | |
Meta‐analyses
Pooled results suggested that patients receiving the immediate preoperative ACEI/ARA dose were more likely (RR 1.51, 95% CI 1.14‐2.01) to develop hypotension requiring vasopressors at or shortly after induction of anesthesia (Fig. 2A). There was important inconsistency between studies (I2 = 59%). The pooled effect derived from randomized trials (RR = 2.26, 95% CI 0.84‐6.12) seemed greater than that derived from the 2 observational studies (RR = 1.33, 95% CI 1.02‐1.73), but the treatment‐study design interaction was not significant (P = .3). Similarly, other subgroup explorations were not contributory.
The incidence of perioperative myocardial infarction was not significantly different between continuing and withheld groups (Fig. 2B); the results were consistent across trials (I2 = 0%) but were imprecise (RR = 0.41, 95% CI 0.07‐2.53). Data were insufficient for subgroup analyses.
DISCUSSION
Statement of Principle Findings
Our systematic review identified 3 randomized trials and 2 observational studies examining the clinical consequences of continuing versus deliberately withholding the immediate preoperative dose of a renin‐angiotensin‐aldosterone system antagonist in patients treated chronically with these agents and scheduled to undergo nonemergent surgery.
Results from pooled estimates suggest that continuing chronic therapy up until surgery may increase the risk of perioperative hypotension requiring vasopressors (Fig. 3). Otherwise, this systematic review did not identify any clinically significant consequences associated either with preoperatively withholding or continuing RAAS antagonists. We do note that all 3 of the myocardial infarctions reported occurred in patients from whom the immediate preoperative ACEI/ARA dose was withheld, although no meaningful conclusion can be inferred from so few data points.
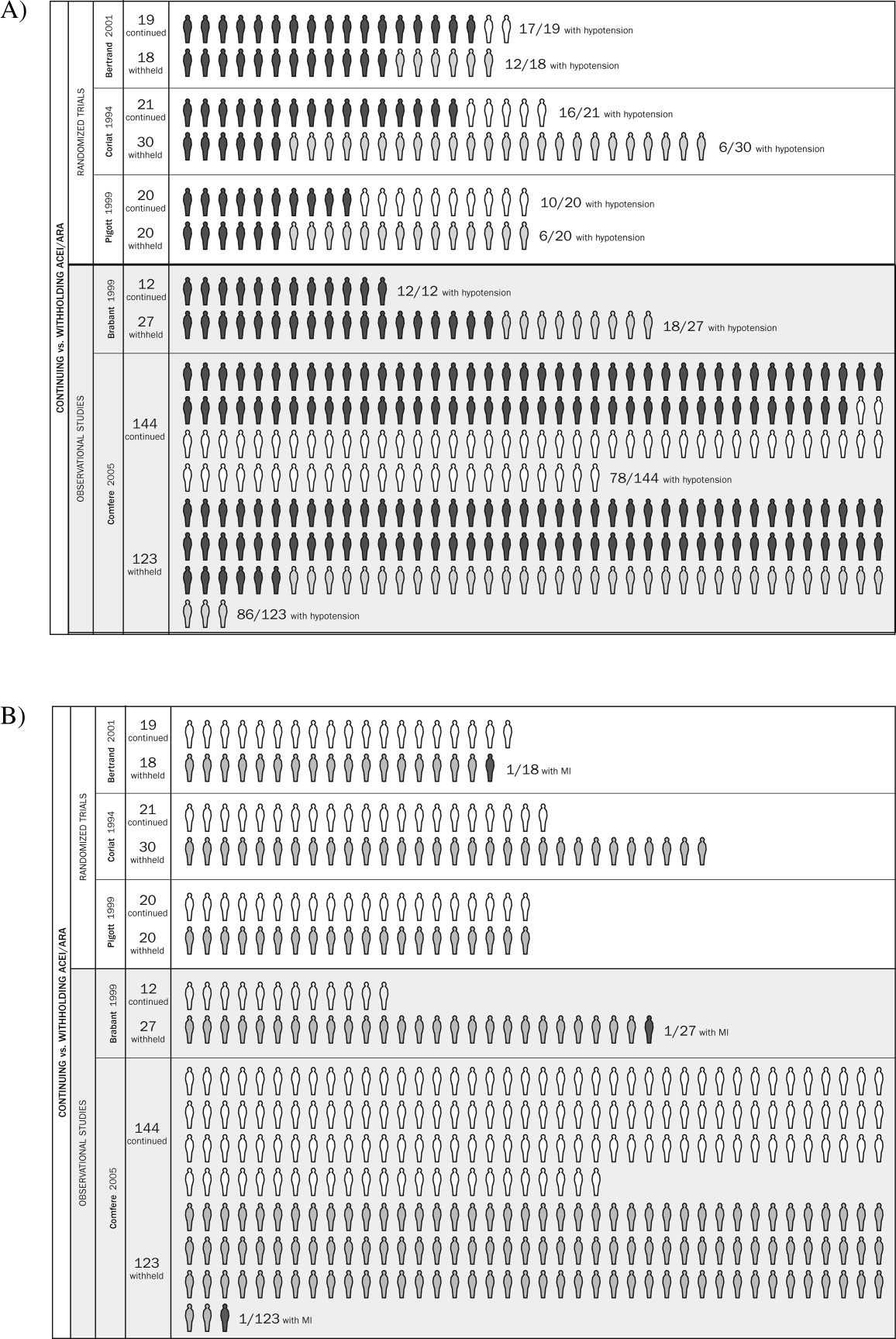
Strengths and Weaknesses of This Review
We observed considerable variation in design quality from study to study. With the exception of hypotension, other end points were not examined uniformly in the studies comprising this review. This was due either to study design (retrospective) or to the belief that the outcomes were not likely. With 1 exception,11 patient‐important end points such as myocardial infarction were noted if they occurred but not explicitly sought. Without active surveillance (serial electrocardiographic and biomarker testing), events such as myocardial infarction may remain undetected. Pain from myocardial ischemia, for example, may be masked by postoperative analgesia. Creatine kinase with muscle and brain subunits (CK‐MB) may be elevated in response to extracardiac injury. Postoperative ECG findings often are nonspecific.12 Furthermore, these studies examined the immediate and short‐term postoperative periods, possibly missing late‐manifesting hypotension‐induced or other end‐organ damage. Thus, truly reliable conclusions regarding the frequency of myocardial infarction, cerebrovascular events, and other patient‐important outcomes cannot be reached. Because this review includes small studies, it is particularly vulnerable to the effects of publication bias. The overall quality of the evidence we summarized makes it likely that larger rigorous trials may fail to confirm our findings.1315 Notably, this is to our knowledge the first systematic review addressing the clinical consequences of continuing or withholding the immediate preoperative dose of ACEI/ARAs.
Meaning of the Study
Evidence exists that perioperative ACEI/ARA therapy can impair the body's already anesthesia‐ suppressed blood pressure regulation system. Patients scheduled to undergo cardiovascular surgery may be at increased risk for the development of perioperative hypotension requiring vasopressors if the immediate preoperative ACEI/ARA dose is given. The results of this reviewa review of studies that were relatively small and generally not powered to observe clinically significant consequencesdo not provide sufficient evidence to support the systematic withholding or the systematic continuation of RAAS antagonists. Patients will be served best by hospitalists and other clinicians involved in perioperative care who take into account situation‐specific details in making this decision. A patient at particularly high risk for the complications of a blood pressure extreme (either hyper‐ or hypotension) represents such an example.
For patients who receive the immediate preoperative ACEI/ARA dose and do develop perioperative hypotension, there is inadequate evidence to determine whether that hypotension leads to patient‐important adverse outcomes. In fact, data from literature presently available are insufficient to reach any conclusion about long‐term clinical consequences of continuing or not continuing chronic ACEI/ARA therapy. The available studies were relatively small, reported few if any events, and were not designed to measure accurately the incidence of patient‐important end points.
Unanswered Questions and Future Research
Large and rigorous randomized trials could help to clarify the relationships suggested in this meta‐analysis and provide valid data about the consequences of continuing versus withholding preoperative ACEI/ARA therapy. Such trials are required before strong evidence‐based recommendations can be formulated.
Acknowledgements
The authors are indebted to James M. Naessens, ScD, David R. Danielson, MD, and David O. Warner, MD, for their advice during the conduct of this study. We also gratefully acknowledge Amanda Ebright, MD, for asking the original question that led to this review and Mr. Matthew Maleska for his design of summary Figure 3.
- ,,,,.Patients at the center: in our practice, and in our use of language.ACP J Club.2004;140:A11–A12.
- ,,,,,.Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement.Lancet.1999;354:1896–1900.
- ,,, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta‐analysis. Ottawa Health Research Institute, University of Ottawa, Ontario, Canada. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed: March 21,2006.
- ,.Meta‐analysis in clinical trials.Control Clin Trials. Sep1986;7:177–188.
- .,..What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data.Stat Med.2004;23:1351–1375.
- ,.Quantifying heterogeneity in a meta‐analysis.Stat Med.2002;21:1539–1558.
- ,.Statistics notes: Interaction revisited: the difference between two estimatesBMJ.2003;326:219
- ,,, et al.Influence of chronic angiotensin‐converting enzyme inhibition on anesthetic induction.Anesthesiology.1994;81:299–307.
- ,,, et al.Angiotensin system inhibitors in a general surgical population.Anesth Analg.2005;100:636–644.
- ,,,,.The hemodynamic effects of anesthetic induction in vascular surgical patients chronically treated with angiotensin II receptor antagonists.Anesth Analg.1999;89:1388–1392.
- ,,,,,.Should the angiotensin II antagonists be discontinued before surgery? [see comment].Anesth Analg.2001;92:26–30.
- Zipes.Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine.7th ed.New York:Saunders, an imprint of Elsevier;2005.
- ,.Summarizing the Evidence: Publication Bias.Chicago:AMA Press;2002.
- ,,, et al.Large trials vsmeta‐analysis of smaller trials: how do their results compare? [see comment].JAMA.1996;276:1332–1338.
- ,,,.Bias in meta‐analysis detected by a simple, graphical test.BMJ.1997;315:629–634.
- .Preoperative evaluation of the patient with hypertension.JAMA.2002;287:2043–2046.
- ,,,,.Effect of omitting regular ACE inhibitor medication before cardiac surgery on haemodynamic variables and vasoactive drug requirements.Br J Anaesth.1999;83:715–720.
Clinicians commonly use renin‐angiotensin‐aldosterone‐system (RAAS) antagonists such as angiotensin‐converting enzyme inhibitors (ACEIs) and angiotensin II receptor subtype 1 antagonists (ARAs) to treat hypertension, congestive heart failure, and diabetic nephropathy. Hospitalists and other clinicians involved in the preoperative care of patients treated chronically with these agents are faced with the uncertainty of whether to continue these medications immediately prior to surgery.
The concern among those who recommend holding therapy is that pharmacologic suppression of the RAAS in patients undergoing general anesthesia may lead to severe or refractory (to intravenous fluid support) hypotension requiring vasopressors. On the other hand, if complications are no more likely when continuing one of these agents up to the day of surgery, withholding it could represent an unnecessary and potentially harmful intervention (eg, when a clinician caring postoperatively for a patient forgets to restart it). Although several studies have attempted to address this dilemma, a systematic and comprehensive summary of the pertinent evidence has not been published.
In this systematic review and meta‐analysis, we sought to summarize the best available evidence about the relative incidence of patient‐important outcomes1 in patients who do or do not receive ACEI/ARA therapy on the day of their nonemergent surgery.
METHODS
We report this protocol‐driven review in accordance with the Quality of Reporting of Meta‐analyses (QUOROM) standards for reporting systematic reviews of randomized trials.2
Search Strategy
In collaboration with an expert reference librarian (P.J.E.), we designed a search strategy that included the electronic databases MEDLINE, EMBASE, CINAHL, Web of Science, Current Contents, CENTRAL, DARE, and SCOPUS from 1981 (when captopril, the first ACEI, was approved by the FDA) until March 2006. We also reviewed the reference lists of included articles, retrieved articles from our personal files, and consulted with anesthesiologists and hospitalists with an interest in perioperative care in order to identify unpublished studies or studies missed by our strategy.
Study Selection
Eligible studies were prospective cohort studies or randomized controlled trials enrolling adult patients (ie, most patients > 18 years) undergoing nonemergent surgery and using ACEI or ARA chronically and assessing the effect of withdrawing or continuing these agents up to the morning of surgery. Eligible studies measured and reported either events of great patient importance (death, myocardial infarction, transient ischemic attack or stroke, and hepatic or renal failure) or of potentially less importance such as unplanned admission to the intensive care unit or treatment‐requiring hypotension, arrhythmias, or hyperkalemia.
Study Selection
Two reviewers (D.J.R. and F.S.M.) independently screened the titles and abstracts for potential inclusion and retrieved potentially eligible articles for full‐text evaluation. Two reviewers (D.J.R. and M.L.B.) working in duplicate independently selected studies for inclusion. The reviewers were in agreement for full text inclusion 100% of the time.
Data Extraction
Two hospitalists with experience in perioperative care and trained in clinical research (D.J.R. and F.S.M.) working independently and in duplicate extracted data from each eligible article using a standardized structured data extraction form. We extracted information about the study authors and publication, the patients (numbers in each group, indications for chronic ACEI/ARA therapy, type of surgery, agents used for anesthesia), event rates of surgical and perioperative complications (death, stroke, myocardial infarction, unplanned admission to the intensive care unit, treatment‐requiring hypotension, arrhythmias, or hyperkalemia), and relevant periods (e.g., between last dose of ACEI/ARA and surgery, between surgery and clinical end points, total follow‐up). When key information was not available in the published report, we contacted authors by electronic mail. We made 2 attempts to contact authors who failed to respond. Three of the 4 authors contacted responded with the requested information.
Quality Assessment
For randomized trials, we noted whether authors reported adequate allocation concealment, blinding of patients, clinicians, data collectors, data analysts, outcome assessors, and loss to follow‐up. The same reviewers (D.J.R. and F.S.M.) assessed study quality and were in agreement for each article and each domain of quality (kappa statistic in each case was 1.0). For cohort studies we noted details of cohort selection and comparability according to the Newcastle‐Ottawa approach.3
Statistical Analysis
We used a DerSimonian and Laird random effects method4 to conduct meta‐analyses across eligible outcomes. Random effects meta‐analysis incorporates both within‐study and between‐study variability. We chose a random effects approach because of the important degree of clinical heterogeneity expected between the included studies. For rare events we followed the approach by Sweeting et al. for the choice of a continuity correction factor.5 We report the pooled relative risk and the associated 95% confidence interval.
Inconsistency and Subgroup Analyses
To ascertain the magnitude of inconsistency across trials, we measured the I2 statistic, an estimate of the proportion of the overall between‐study variability that is not a result of random error or chance but of true clinical heterogeneity.6 When possible, we explored subgroup analyses to explain heterogeneity, including subgroups defined by type of surgery (cardiovascular versus noncardiovascular), timing of measurement of outcomes (in relation to anesthesia induction postoperatively), and type of agent (ACEI or ARA). We estimated the difference in treatment effects between subgroups by testing the hypothesis of treatmentsubgroup interaction with a nominal significance level of 5%.7
RESULTS
Search Results
The 509 titles reviewed included 410 titles produced by electronic searches and an additional 99 titles from other sources (Fig. 1).

Study Characteristics
Table 1 summarizes the characteristics of the 5 included studies (n = 434 patients). Myocardial infarction was an end point in 3 studies (Brabant, Bertrand, and Comfere); 1 event was reported in the withheld arm of each of these studies (none in the continuing arms). Hypotension requiring vasopressors was reported in all 5 studies. The other end points of interest were reported sparsely. There was considerable heterogeneity across studies regarding follow‐up period, which ranged from ending at incision to ending at dismissal from the hospital.
| Author/Year | Patients (n) | Indication for ACEI/ARA | Type of surgery | End points measured |
|---|---|---|---|---|
| ||||
| Randomized trials | ||||
| Bertrand, 200111 | 19 continued 18 withheld | Hypertension | Elective major vascular | Hypotension, need for vasoactive drugs (at or shortly after induction) |
| Coriat, 19948 | 21 continued 30 withheld | Hypertension | Peripheral vascular (>2 hours) | Systolic blood pressure (at or shortly after induction), plasma ACEI and catecholamine levels |
| Pigott, 199917 | 20 continued 20 withheld | Hypertension (n = 17); previous myocardial infarction (n = 23) | Coronary artery bypass graft | Arterial pressure (at or shortly after induction), cardiac index, systemic vascular resistance, use of vasoactive drugs |
| Observational studies | ||||
| Brabant, 199910 | 12 continued 27 withheld | Previous myocardial infarction (n = 6); diabetes mellitus (n = 6; n with diabetic nephropathy unknown); hypertension (n = unknown) | Elective vascular surgery | Blood pressure (at or shortly after induction) |
| Comfere, 20059 | 144 continued 123 withheld | Hypertension | Noncardiovascular | Blood pressure (at or shortly after induction), unplanned ICU admissions, hemodynamic instability in the PACU (ABP or HR out of range), acute renal impairment, TIA, stroke, myocardial ischemia/emnfarction, and death |
Methodological Quality of Included Studies
Table 2 describes the methodological quality, as reported, of the included studies. Allocation concealment was unclear in 2 of the 3 randomized trials. Details of blinding either were not reported or otherwise were unclear in 2 of these 3 studies. Only 1 study specified the extent of loss to follow‐up.8 In 1 of the observational studies,9 details of cohort selection were generally appropriate. The 12 patients examined in another study10 had been scheduled consecutively for surgery. Both studies controlled for a variety of demographic and other key variables. Duration of follow‐up ranged from 3 days after surgery (for ECG)10 to as long as duration of hospitalization.9
| Randomized trials | |||
|---|---|---|---|
| Allocation concealment | Blinding | Loss to follow‐up | |
| Bertrand, 200111 | Unclear | Unclear | Not reported |
| Coriat, 19948 | Unclear | None | 19% |
| Pigott, 199917 | Adequate | Investigator, cardiac anesthetists, perfusionists, and recovery staff were blinded to allocation. Blinding not reported for other data collectors, assessors of outcome, or data analysts | Not reported |
| Observational studies | |||
| Details of cohort selection | Comparability of cohorts | ||
| Brabant, 199910 | Appropriate Cohort somewhat representative of the adult population undergoing nonemergent surgery. The unexposed cohort was drawn from the same community as the exposed cohort | Similar with 2 exceptions: compared with the ACEI‐withheld group, the ARA‐given group contained more than twice the proportion of patients with previous myocardial revascularization Compared with the ARA‐given group, the ACEI‐withheld group contained more than twice the proportion of patients with diabetes mellitus | |
| Comfere, 20059 | Appropriate Cohort somewhat representative of the adult population undergoing nonemergent surgery (referral center population may not truly represent overall population). The unexposed cohort was drawn from the same community as the exposed cohort. Data were extracted from a secure record | Adequate This study controls for a variety of demographic and other variables | |
Meta‐analyses
Pooled results suggested that patients receiving the immediate preoperative ACEI/ARA dose were more likely (RR 1.51, 95% CI 1.14‐2.01) to develop hypotension requiring vasopressors at or shortly after induction of anesthesia (Fig. 2A). There was important inconsistency between studies (I2 = 59%). The pooled effect derived from randomized trials (RR = 2.26, 95% CI 0.84‐6.12) seemed greater than that derived from the 2 observational studies (RR = 1.33, 95% CI 1.02‐1.73), but the treatment‐study design interaction was not significant (P = .3). Similarly, other subgroup explorations were not contributory.

The incidence of perioperative myocardial infarction was not significantly different between continuing and withheld groups (Fig. 2B); the results were consistent across trials (I2 = 0%) but were imprecise (RR = 0.41, 95% CI 0.07‐2.53). Data were insufficient for subgroup analyses.
DISCUSSION
Statement of Principle Findings
Our systematic review identified 3 randomized trials and 2 observational studies examining the clinical consequences of continuing versus deliberately withholding the immediate preoperative dose of a renin‐angiotensin‐aldosterone system antagonist in patients treated chronically with these agents and scheduled to undergo nonemergent surgery.
Results from pooled estimates suggest that continuing chronic therapy up until surgery may increase the risk of perioperative hypotension requiring vasopressors (Fig. 3). Otherwise, this systematic review did not identify any clinically significant consequences associated either with preoperatively withholding or continuing RAAS antagonists. We do note that all 3 of the myocardial infarctions reported occurred in patients from whom the immediate preoperative ACEI/ARA dose was withheld, although no meaningful conclusion can be inferred from so few data points.

Strengths and Weaknesses of This Review
We observed considerable variation in design quality from study to study. With the exception of hypotension, other end points were not examined uniformly in the studies comprising this review. This was due either to study design (retrospective) or to the belief that the outcomes were not likely. With 1 exception,11 patient‐important end points such as myocardial infarction were noted if they occurred but not explicitly sought. Without active surveillance (serial electrocardiographic and biomarker testing), events such as myocardial infarction may remain undetected. Pain from myocardial ischemia, for example, may be masked by postoperative analgesia. Creatine kinase with muscle and brain subunits (CK‐MB) may be elevated in response to extracardiac injury. Postoperative ECG findings often are nonspecific.12 Furthermore, these studies examined the immediate and short‐term postoperative periods, possibly missing late‐manifesting hypotension‐induced or other end‐organ damage. Thus, truly reliable conclusions regarding the frequency of myocardial infarction, cerebrovascular events, and other patient‐important outcomes cannot be reached. Because this review includes small studies, it is particularly vulnerable to the effects of publication bias. The overall quality of the evidence we summarized makes it likely that larger rigorous trials may fail to confirm our findings.1315 Notably, this is to our knowledge the first systematic review addressing the clinical consequences of continuing or withholding the immediate preoperative dose of ACEI/ARAs.
Meaning of the Study
Evidence exists that perioperative ACEI/ARA therapy can impair the body's already anesthesia‐ suppressed blood pressure regulation system. Patients scheduled to undergo cardiovascular surgery may be at increased risk for the development of perioperative hypotension requiring vasopressors if the immediate preoperative ACEI/ARA dose is given. The results of this reviewa review of studies that were relatively small and generally not powered to observe clinically significant consequencesdo not provide sufficient evidence to support the systematic withholding or the systematic continuation of RAAS antagonists. Patients will be served best by hospitalists and other clinicians involved in perioperative care who take into account situation‐specific details in making this decision. A patient at particularly high risk for the complications of a blood pressure extreme (either hyper‐ or hypotension) represents such an example.
For patients who receive the immediate preoperative ACEI/ARA dose and do develop perioperative hypotension, there is inadequate evidence to determine whether that hypotension leads to patient‐important adverse outcomes. In fact, data from literature presently available are insufficient to reach any conclusion about long‐term clinical consequences of continuing or not continuing chronic ACEI/ARA therapy. The available studies were relatively small, reported few if any events, and were not designed to measure accurately the incidence of patient‐important end points.
Unanswered Questions and Future Research
Large and rigorous randomized trials could help to clarify the relationships suggested in this meta‐analysis and provide valid data about the consequences of continuing versus withholding preoperative ACEI/ARA therapy. Such trials are required before strong evidence‐based recommendations can be formulated.
Acknowledgements
The authors are indebted to James M. Naessens, ScD, David R. Danielson, MD, and David O. Warner, MD, for their advice during the conduct of this study. We also gratefully acknowledge Amanda Ebright, MD, for asking the original question that led to this review and Mr. Matthew Maleska for his design of summary Figure 3.
Clinicians commonly use renin‐angiotensin‐aldosterone‐system (RAAS) antagonists such as angiotensin‐converting enzyme inhibitors (ACEIs) and angiotensin II receptor subtype 1 antagonists (ARAs) to treat hypertension, congestive heart failure, and diabetic nephropathy. Hospitalists and other clinicians involved in the preoperative care of patients treated chronically with these agents are faced with the uncertainty of whether to continue these medications immediately prior to surgery.
The concern among those who recommend holding therapy is that pharmacologic suppression of the RAAS in patients undergoing general anesthesia may lead to severe or refractory (to intravenous fluid support) hypotension requiring vasopressors. On the other hand, if complications are no more likely when continuing one of these agents up to the day of surgery, withholding it could represent an unnecessary and potentially harmful intervention (eg, when a clinician caring postoperatively for a patient forgets to restart it). Although several studies have attempted to address this dilemma, a systematic and comprehensive summary of the pertinent evidence has not been published.
In this systematic review and meta‐analysis, we sought to summarize the best available evidence about the relative incidence of patient‐important outcomes1 in patients who do or do not receive ACEI/ARA therapy on the day of their nonemergent surgery.
METHODS
We report this protocol‐driven review in accordance with the Quality of Reporting of Meta‐analyses (QUOROM) standards for reporting systematic reviews of randomized trials.2
Search Strategy
In collaboration with an expert reference librarian (P.J.E.), we designed a search strategy that included the electronic databases MEDLINE, EMBASE, CINAHL, Web of Science, Current Contents, CENTRAL, DARE, and SCOPUS from 1981 (when captopril, the first ACEI, was approved by the FDA) until March 2006. We also reviewed the reference lists of included articles, retrieved articles from our personal files, and consulted with anesthesiologists and hospitalists with an interest in perioperative care in order to identify unpublished studies or studies missed by our strategy.
Study Selection
Eligible studies were prospective cohort studies or randomized controlled trials enrolling adult patients (ie, most patients > 18 years) undergoing nonemergent surgery and using ACEI or ARA chronically and assessing the effect of withdrawing or continuing these agents up to the morning of surgery. Eligible studies measured and reported either events of great patient importance (death, myocardial infarction, transient ischemic attack or stroke, and hepatic or renal failure) or of potentially less importance such as unplanned admission to the intensive care unit or treatment‐requiring hypotension, arrhythmias, or hyperkalemia.
Study Selection
Two reviewers (D.J.R. and F.S.M.) independently screened the titles and abstracts for potential inclusion and retrieved potentially eligible articles for full‐text evaluation. Two reviewers (D.J.R. and M.L.B.) working in duplicate independently selected studies for inclusion. The reviewers were in agreement for full text inclusion 100% of the time.
Data Extraction
Two hospitalists with experience in perioperative care and trained in clinical research (D.J.R. and F.S.M.) working independently and in duplicate extracted data from each eligible article using a standardized structured data extraction form. We extracted information about the study authors and publication, the patients (numbers in each group, indications for chronic ACEI/ARA therapy, type of surgery, agents used for anesthesia), event rates of surgical and perioperative complications (death, stroke, myocardial infarction, unplanned admission to the intensive care unit, treatment‐requiring hypotension, arrhythmias, or hyperkalemia), and relevant periods (e.g., between last dose of ACEI/ARA and surgery, between surgery and clinical end points, total follow‐up). When key information was not available in the published report, we contacted authors by electronic mail. We made 2 attempts to contact authors who failed to respond. Three of the 4 authors contacted responded with the requested information.
Quality Assessment
For randomized trials, we noted whether authors reported adequate allocation concealment, blinding of patients, clinicians, data collectors, data analysts, outcome assessors, and loss to follow‐up. The same reviewers (D.J.R. and F.S.M.) assessed study quality and were in agreement for each article and each domain of quality (kappa statistic in each case was 1.0). For cohort studies we noted details of cohort selection and comparability according to the Newcastle‐Ottawa approach.3
Statistical Analysis
We used a DerSimonian and Laird random effects method4 to conduct meta‐analyses across eligible outcomes. Random effects meta‐analysis incorporates both within‐study and between‐study variability. We chose a random effects approach because of the important degree of clinical heterogeneity expected between the included studies. For rare events we followed the approach by Sweeting et al. for the choice of a continuity correction factor.5 We report the pooled relative risk and the associated 95% confidence interval.
Inconsistency and Subgroup Analyses
To ascertain the magnitude of inconsistency across trials, we measured the I2 statistic, an estimate of the proportion of the overall between‐study variability that is not a result of random error or chance but of true clinical heterogeneity.6 When possible, we explored subgroup analyses to explain heterogeneity, including subgroups defined by type of surgery (cardiovascular versus noncardiovascular), timing of measurement of outcomes (in relation to anesthesia induction postoperatively), and type of agent (ACEI or ARA). We estimated the difference in treatment effects between subgroups by testing the hypothesis of treatmentsubgroup interaction with a nominal significance level of 5%.7
RESULTS
Search Results
The 509 titles reviewed included 410 titles produced by electronic searches and an additional 99 titles from other sources (Fig. 1).

Study Characteristics
Table 1 summarizes the characteristics of the 5 included studies (n = 434 patients). Myocardial infarction was an end point in 3 studies (Brabant, Bertrand, and Comfere); 1 event was reported in the withheld arm of each of these studies (none in the continuing arms). Hypotension requiring vasopressors was reported in all 5 studies. The other end points of interest were reported sparsely. There was considerable heterogeneity across studies regarding follow‐up period, which ranged from ending at incision to ending at dismissal from the hospital.
| Author/Year | Patients (n) | Indication for ACEI/ARA | Type of surgery | End points measured |
|---|---|---|---|---|
| ||||
| Randomized trials | ||||
| Bertrand, 200111 | 19 continued 18 withheld | Hypertension | Elective major vascular | Hypotension, need for vasoactive drugs (at or shortly after induction) |
| Coriat, 19948 | 21 continued 30 withheld | Hypertension | Peripheral vascular (>2 hours) | Systolic blood pressure (at or shortly after induction), plasma ACEI and catecholamine levels |
| Pigott, 199917 | 20 continued 20 withheld | Hypertension (n = 17); previous myocardial infarction (n = 23) | Coronary artery bypass graft | Arterial pressure (at or shortly after induction), cardiac index, systemic vascular resistance, use of vasoactive drugs |
| Observational studies | ||||
| Brabant, 199910 | 12 continued 27 withheld | Previous myocardial infarction (n = 6); diabetes mellitus (n = 6; n with diabetic nephropathy unknown); hypertension (n = unknown) | Elective vascular surgery | Blood pressure (at or shortly after induction) |
| Comfere, 20059 | 144 continued 123 withheld | Hypertension | Noncardiovascular | Blood pressure (at or shortly after induction), unplanned ICU admissions, hemodynamic instability in the PACU (ABP or HR out of range), acute renal impairment, TIA, stroke, myocardial ischemia/emnfarction, and death |
Methodological Quality of Included Studies
Table 2 describes the methodological quality, as reported, of the included studies. Allocation concealment was unclear in 2 of the 3 randomized trials. Details of blinding either were not reported or otherwise were unclear in 2 of these 3 studies. Only 1 study specified the extent of loss to follow‐up.8 In 1 of the observational studies,9 details of cohort selection were generally appropriate. The 12 patients examined in another study10 had been scheduled consecutively for surgery. Both studies controlled for a variety of demographic and other key variables. Duration of follow‐up ranged from 3 days after surgery (for ECG)10 to as long as duration of hospitalization.9
| Randomized trials | |||
|---|---|---|---|
| Allocation concealment | Blinding | Loss to follow‐up | |
| Bertrand, 200111 | Unclear | Unclear | Not reported |
| Coriat, 19948 | Unclear | None | 19% |
| Pigott, 199917 | Adequate | Investigator, cardiac anesthetists, perfusionists, and recovery staff were blinded to allocation. Blinding not reported for other data collectors, assessors of outcome, or data analysts | Not reported |
| Observational studies | |||
| Details of cohort selection | Comparability of cohorts | ||
| Brabant, 199910 | Appropriate Cohort somewhat representative of the adult population undergoing nonemergent surgery. The unexposed cohort was drawn from the same community as the exposed cohort | Similar with 2 exceptions: compared with the ACEI‐withheld group, the ARA‐given group contained more than twice the proportion of patients with previous myocardial revascularization Compared with the ARA‐given group, the ACEI‐withheld group contained more than twice the proportion of patients with diabetes mellitus | |
| Comfere, 20059 | Appropriate Cohort somewhat representative of the adult population undergoing nonemergent surgery (referral center population may not truly represent overall population). The unexposed cohort was drawn from the same community as the exposed cohort. Data were extracted from a secure record | Adequate This study controls for a variety of demographic and other variables | |
Meta‐analyses
Pooled results suggested that patients receiving the immediate preoperative ACEI/ARA dose were more likely (RR 1.51, 95% CI 1.14‐2.01) to develop hypotension requiring vasopressors at or shortly after induction of anesthesia (Fig. 2A). There was important inconsistency between studies (I2 = 59%). The pooled effect derived from randomized trials (RR = 2.26, 95% CI 0.84‐6.12) seemed greater than that derived from the 2 observational studies (RR = 1.33, 95% CI 1.02‐1.73), but the treatment‐study design interaction was not significant (P = .3). Similarly, other subgroup explorations were not contributory.

The incidence of perioperative myocardial infarction was not significantly different between continuing and withheld groups (Fig. 2B); the results were consistent across trials (I2 = 0%) but were imprecise (RR = 0.41, 95% CI 0.07‐2.53). Data were insufficient for subgroup analyses.
DISCUSSION
Statement of Principle Findings
Our systematic review identified 3 randomized trials and 2 observational studies examining the clinical consequences of continuing versus deliberately withholding the immediate preoperative dose of a renin‐angiotensin‐aldosterone system antagonist in patients treated chronically with these agents and scheduled to undergo nonemergent surgery.
Results from pooled estimates suggest that continuing chronic therapy up until surgery may increase the risk of perioperative hypotension requiring vasopressors (Fig. 3). Otherwise, this systematic review did not identify any clinically significant consequences associated either with preoperatively withholding or continuing RAAS antagonists. We do note that all 3 of the myocardial infarctions reported occurred in patients from whom the immediate preoperative ACEI/ARA dose was withheld, although no meaningful conclusion can be inferred from so few data points.

Strengths and Weaknesses of This Review
We observed considerable variation in design quality from study to study. With the exception of hypotension, other end points were not examined uniformly in the studies comprising this review. This was due either to study design (retrospective) or to the belief that the outcomes were not likely. With 1 exception,11 patient‐important end points such as myocardial infarction were noted if they occurred but not explicitly sought. Without active surveillance (serial electrocardiographic and biomarker testing), events such as myocardial infarction may remain undetected. Pain from myocardial ischemia, for example, may be masked by postoperative analgesia. Creatine kinase with muscle and brain subunits (CK‐MB) may be elevated in response to extracardiac injury. Postoperative ECG findings often are nonspecific.12 Furthermore, these studies examined the immediate and short‐term postoperative periods, possibly missing late‐manifesting hypotension‐induced or other end‐organ damage. Thus, truly reliable conclusions regarding the frequency of myocardial infarction, cerebrovascular events, and other patient‐important outcomes cannot be reached. Because this review includes small studies, it is particularly vulnerable to the effects of publication bias. The overall quality of the evidence we summarized makes it likely that larger rigorous trials may fail to confirm our findings.1315 Notably, this is to our knowledge the first systematic review addressing the clinical consequences of continuing or withholding the immediate preoperative dose of ACEI/ARAs.
Meaning of the Study
Evidence exists that perioperative ACEI/ARA therapy can impair the body's already anesthesia‐ suppressed blood pressure regulation system. Patients scheduled to undergo cardiovascular surgery may be at increased risk for the development of perioperative hypotension requiring vasopressors if the immediate preoperative ACEI/ARA dose is given. The results of this reviewa review of studies that were relatively small and generally not powered to observe clinically significant consequencesdo not provide sufficient evidence to support the systematic withholding or the systematic continuation of RAAS antagonists. Patients will be served best by hospitalists and other clinicians involved in perioperative care who take into account situation‐specific details in making this decision. A patient at particularly high risk for the complications of a blood pressure extreme (either hyper‐ or hypotension) represents such an example.
For patients who receive the immediate preoperative ACEI/ARA dose and do develop perioperative hypotension, there is inadequate evidence to determine whether that hypotension leads to patient‐important adverse outcomes. In fact, data from literature presently available are insufficient to reach any conclusion about long‐term clinical consequences of continuing or not continuing chronic ACEI/ARA therapy. The available studies were relatively small, reported few if any events, and were not designed to measure accurately the incidence of patient‐important end points.
Unanswered Questions and Future Research
Large and rigorous randomized trials could help to clarify the relationships suggested in this meta‐analysis and provide valid data about the consequences of continuing versus withholding preoperative ACEI/ARA therapy. Such trials are required before strong evidence‐based recommendations can be formulated.
Acknowledgements
The authors are indebted to James M. Naessens, ScD, David R. Danielson, MD, and David O. Warner, MD, for their advice during the conduct of this study. We also gratefully acknowledge Amanda Ebright, MD, for asking the original question that led to this review and Mr. Matthew Maleska for his design of summary Figure 3.
- ,,,,.Patients at the center: in our practice, and in our use of language.ACP J Club.2004;140:A11–A12.
- ,,,,,.Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement.Lancet.1999;354:1896–1900.
- ,,, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta‐analysis. Ottawa Health Research Institute, University of Ottawa, Ontario, Canada. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed: March 21,2006.
- ,.Meta‐analysis in clinical trials.Control Clin Trials. Sep1986;7:177–188.
- .,..What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data.Stat Med.2004;23:1351–1375.
- ,.Quantifying heterogeneity in a meta‐analysis.Stat Med.2002;21:1539–1558.
- ,.Statistics notes: Interaction revisited: the difference between two estimatesBMJ.2003;326:219
- ,,, et al.Influence of chronic angiotensin‐converting enzyme inhibition on anesthetic induction.Anesthesiology.1994;81:299–307.
- ,,, et al.Angiotensin system inhibitors in a general surgical population.Anesth Analg.2005;100:636–644.
- ,,,,.The hemodynamic effects of anesthetic induction in vascular surgical patients chronically treated with angiotensin II receptor antagonists.Anesth Analg.1999;89:1388–1392.
- ,,,,,.Should the angiotensin II antagonists be discontinued before surgery? [see comment].Anesth Analg.2001;92:26–30.
- Zipes.Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine.7th ed.New York:Saunders, an imprint of Elsevier;2005.
- ,.Summarizing the Evidence: Publication Bias.Chicago:AMA Press;2002.
- ,,, et al.Large trials vsmeta‐analysis of smaller trials: how do their results compare? [see comment].JAMA.1996;276:1332–1338.
- ,,,.Bias in meta‐analysis detected by a simple, graphical test.BMJ.1997;315:629–634.
- .Preoperative evaluation of the patient with hypertension.JAMA.2002;287:2043–2046.
- ,,,,.Effect of omitting regular ACE inhibitor medication before cardiac surgery on haemodynamic variables and vasoactive drug requirements.Br J Anaesth.1999;83:715–720.
- ,,,,.Patients at the center: in our practice, and in our use of language.ACP J Club.2004;140:A11–A12.
- ,,,,,.Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement.Lancet.1999;354:1896–1900.
- ,,, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta‐analysis. Ottawa Health Research Institute, University of Ottawa, Ontario, Canada. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed: March 21,2006.
- ,.Meta‐analysis in clinical trials.Control Clin Trials. Sep1986;7:177–188.
- .,..What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data.Stat Med.2004;23:1351–1375.
- ,.Quantifying heterogeneity in a meta‐analysis.Stat Med.2002;21:1539–1558.
- ,.Statistics notes: Interaction revisited: the difference between two estimatesBMJ.2003;326:219
- ,,, et al.Influence of chronic angiotensin‐converting enzyme inhibition on anesthetic induction.Anesthesiology.1994;81:299–307.
- ,,, et al.Angiotensin system inhibitors in a general surgical population.Anesth Analg.2005;100:636–644.
- ,,,,.The hemodynamic effects of anesthetic induction in vascular surgical patients chronically treated with angiotensin II receptor antagonists.Anesth Analg.1999;89:1388–1392.
- ,,,,,.Should the angiotensin II antagonists be discontinued before surgery? [see comment].Anesth Analg.2001;92:26–30.
- Zipes.Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine.7th ed.New York:Saunders, an imprint of Elsevier;2005.
- ,.Summarizing the Evidence: Publication Bias.Chicago:AMA Press;2002.
- ,,, et al.Large trials vsmeta‐analysis of smaller trials: how do their results compare? [see comment].JAMA.1996;276:1332–1338.
- ,,,.Bias in meta‐analysis detected by a simple, graphical test.BMJ.1997;315:629–634.
- .Preoperative evaluation of the patient with hypertension.JAMA.2002;287:2043–2046.
- ,,,,.Effect of omitting regular ACE inhibitor medication before cardiac surgery on haemodynamic variables and vasoactive drug requirements.Br J Anaesth.1999;83:715–720.
Pediatric Hospitalist Variation in Care
Reduction of undesirable variation in care has been a major focus of systematic efforts to improve the quality of the healthcare system.13 The emergence of hospitalists, physicians specializing in the care of hospitalized patients, was spurred by a desire to streamline care and reduce variability in hospital management of common diseases.4, 5 Over the past decade, hospitalist systems have become a leading vehicle for care delivery.4, 6, 7 It remains unclear, however, whether implementation of hospitalist systems has lessened undesirable variation in the inpatient management of common diseases.
While systematic reviews have found costs and hospital length of stay to be 10‐15% lower in both pediatric and internal medicine hospitalist systems, few studies have adequately assessed the processes or quality of care in hospitalist systems.8, 9 Two internal medicine studies have found decreased mortality in hospitalist systems, but the mechanism by which hospitalists apparently achieved these gains is unclear.10, 11 Even less is known about care processes or quality in pediatric hospitalist systems. Death is a rare occurrence in pediatric ward settings, and the seven studies conducted to date comparing pediatric hospitalist and traditional systems have been universally underpowered to detect differences in mortality.9, 1218 There is a need to better understand care processes as a first step in understanding and improving quality of care in hospitalist systems.19
The Pediatric Research in Inpatient Settings (PRIS) Network was formed to improve the quality of care for hospitalized children through collaborative clinical research. In this study, we sought to study variation in the care of common pediatric conditions among a cohort of pediatric hospitalists. We have previously reported that less variability exists in hospitalists' reported management of inpatient conditions than in the reported management of these same conditions by community‐based pediatricians,20 but we were concerned that substantial undesirable variation (ie, variation in practice due to uncertainty or unsubstantiated local practice traditions, rather than justified variation in care based on different risks of harms or benefits in different patients) may still exist among hospitalists. We therefore conducted a study: 1) to investigate variation in hospitalists' reported use of common inpatient therapies, and 2) to test the hypothesis that greater variation exists in hospitalists' reported use of inpatient therapies of unproven benefit than in those therapies proven to be beneficial.
METHODS
Survey Design and Administration
In 2003, we designed the PRIS Survey to collect data on hospitalists' backgrounds, practices, and training needs, as well as their management of common pediatric conditions. For the current study, we chose a priori to evaluate hospitalists' use of 14 therapies in the management of 4 common conditions: asthma, bronchiolitis, gastroenteritis, and gastro‐esophageal reflux disease (GERD) (Table 1). These four conditions were chosen for study because they were among the top discharge diagnoses (primary and secondary) from the inpatient services at 2 of the authors' institutions (Children's Hospital Boston and Children's Hospital San Diego) during the year before administration of the survey, and because a discrete set of therapeutic agents are commonly used in their management. Respondents were asked to report the frequency with which they used each of the 14 therapies of interest on 5‐point Likert scales (from 1=never to 5=almost always). The survey initially developed was piloted with a small group of hospitalists and pediatricians, and a final version incorporating revisions was subsequently administered to all pediatric hospitalists in the US and Canada identified through any of 3 sources: 1) the Pediatric Research in Inpatient Settings (PRIS) list of participants; 2) the Society for Hospital Medicine (SHM) pediatric hospital medicine e‐mail listserv; and 3) the list of all attendees of the first national pediatric hospitalist conference sponsored by the Ambulatory Pediatrics Association (APA), SHM, and American Academy of Pediatrics (AAP); this meeting was held in San Antonio, Texas, USA in November 2003. Individuals identified through more than 1 of these groups were counted only once. Potential participants were assured that individual responses would be kept confidential, and were e‐mailed an access code to participate in the online survey, using a secure web‐based interface; a paper‐based version was also made available to those who preferred to respond in this manner. Regular reminder notices were sent to all non‐responders. Further details regarding PRIS Survey recruitment and study methods have been published previously.20
| Condition | Therapy | BMJ clinical evidence Treatment effect categorization* | Study classification |
|---|---|---|---|
| |||
| Asthma | Inhaled albuterol | Beneficial | Proven |
| Systemic corticosteroids | Beneficial | Proven | |
| Inhaled ipratropium in the first 24 hours of hospitalization | Beneficial | Proven | |
| Inhaled ipratropium after the first 24 hours of hospitalization | Unknown effectiveness | Unproven | |
| Bronchiolitis | Inhaled albuterol | Unknown effectiveness | Unproven |
| Inhaled epinephrine | Unknown effectiveness | Unproven | |
| Systemic corticosteroids | Unknown effectiveness | Unproven | |
| Gastroenteritis | Intravenous hydration | Beneficial | Proven |
| Lactobacillus | Not assessed | Unproven | |
| Ondansetron | Not assessed | Unproven | |
| Gastro‐Esophageal Reflux Disease (GERD) | H2 histamine‐receptor antagonists | Unknown effectiveness | Unproven |
| Thickened feeds | Unknown effectiveness Likely to be beneficial | Unproven Proven | |
| Metoclopramide | Unknown effectiveness | Unproven | |
| Proton‐pump inhibitors | Unknown effectiveness | Unproven | |
DefinitionsReference Responses and Percent Variation
To measure variation in reported management, we first sought to determine a reference response for each therapy of interest. Since the evidence base for most of the therapies we studied is weak, it was not possible to determine a gold standard response for each therapy. Instead, we sought to measure the degree of divergence from a reference response for each therapy in the following manner. First, to simplify analyses, we collapsed our five‐category Likert scale into three categories (never/rarely, sometimes, and often/almost always). We then defined the reference response for each therapy to be never/rarely or often/almost always, whichever of the 2 was more frequently selected by respondents; sometimes was not used as a reference category, as reporting use of a particular therapy sometimes indicated substantial variability even within an individual's own practice.
Classification of therapies as proven or unproven.
To classify each of the 14 studied therapies as being of proven or unproven, we used the British Medical Journal's publication Clinical Evidence.19 We chose to use Clinical Evidence as an evidence‐based reference because it provides rigorously developed, systematic analyses of therapeutic management options for multiple common pediatric conditions, and organizes recommendations in a straightforward manner. Four of the 14 therapies had been determined on systematic review to be proven beneficial at the time of study design: systemic corticosteroids, inhaled albuterol, and ipratropium (in the first 24 h) in the care of children with asthma; and IV hydration in the care of children with acute gastroenteritis. The remaining 10 therapies were either considered to be of unknown effectiveness or had not been formally evaluated by Clinical Evidence, and were hence considered unproven for this study (Table 1). Of note, the use of thickened feeds in the treatment of children with GERD had been determined to be of unknown effectiveness at the time of study design, but was reclassified as likely to be beneficial during the course of the study.
Analyses
Descriptive statistics were used to report respondents' demographic characteristics and work environments, as well as variation in their reported use of each of the 14 therapies. Variation in hospitalists' use of proven versus unproven therapies was compared using the Wilcoxon rank sum test, as it was distributed non‐normally. For our primary analysis, the use of thickened feeds in GERD was considered unproven, but a sensitivity analysis was conducted reclassifying it as proven in light of the evolving literature on its use and its consequent reclassification in Clinical Evidence.(SAS Version 9.1, Cary, NC) was used for statistical analyses.
RESULTS
213 of the 320 individuals identified through the 3 lists of pediatric hospitalists (67%) responded to the survey. Of these, 198 (93%) identified themselves as hospitalists and were therefore included. As previously reported,20 53% of respondents were male, 55% worked in academic training environments, and 47% had completed advanced training (fellowship) beyond their core pediatric training (residency training); respondents reported completing residency training 11 9 (mean, standard deviation) years prior to the survey, and spending 176 72 days per year in the care of hospitalized patients.
Variation in reported management: asthma
(Figure 1, Panel A). Relatively little variation existed in reported use of the 4 asthma therapies studied. Only 4.4% (95% CI, 1.4‐7.4%) of respondents did not provide the reference response of using inhaled albuterol often or almost always in the care of inpatients with asthma, and only 6.0% (2.5‐9.5%) of respondents did not report using systemic corticosteroids often or almost always. Variation in reported use of ipratropium was somewhat higher.
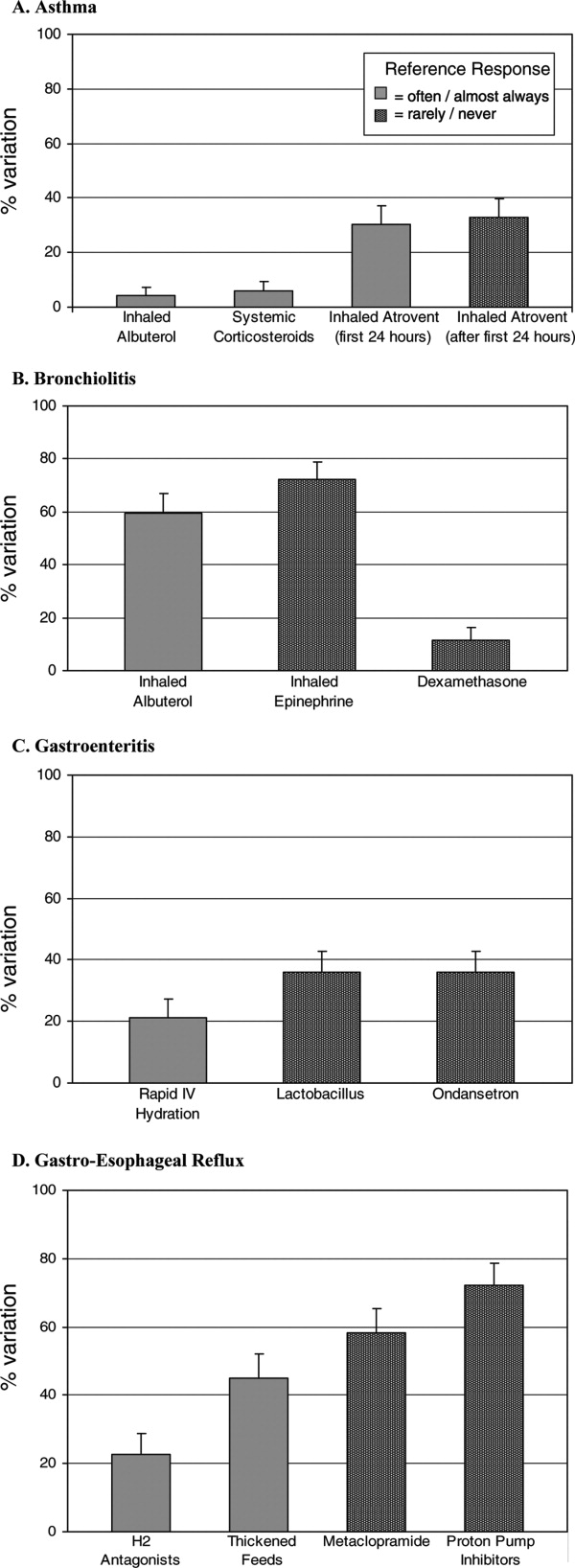
Bronchiolitis
(Figure 1B). By contrast, variation in reported use of inhaled therapies for bronchiolitis was high, with many respondents reporting that they often or always used inhaled albuterol or epinephrine, while many others reported rarely or never using them. There was 59.6% (52.4‐66.8%) variation from the reference response of often/almost always using inhaled albuterol, and 72.2% (65.6‐78.8%) variation from the reference response of never/rarely using inhaled epinephrine. Only 11.6% (6.9‐16.3%) of respondents, however, varied from the reference response of using dexamethasone more than rarely in the care of children with bronchiolitis.
Gastroenteritis
(Figure 1C). Moderate variability existed in the reported use of the 3 studied therapies for children hospitalized with gastroenteritis. 21.1% (15.1‐27.1%) of respondents did not provide the reference response of often/almost always using IV hydration; 35.9% (28.9‐42.9%) did not provide the reference response of never or rarely using lactobacillus; likewise, 35.9% (28.9‐42.9%) did not provide the reference response of never or rarely using ondansetron.
Gastro‐Esophageal Reflux Disease
(Figure 1, Panel D). There was moderate to high variability in the reported management of GERD. 22.8% (16.7‐28.9%) of respondents did not provide the reference response of often/almost always using H2 antagonists, and 44.9% (37.6‐52.2%) did not report often/almost always using thickened feeds in the care of these children. 58.3% (51.1‐65.5%) and 72.1% (65.5‐78.7%) of respondents did not provide the reference response of never/rarely using metoclopramide and proton pump inhibitors, respectively.
Proven vs. Unproven Therapies
(Figure 2). Variation in reported use of therapies of unproven benefit was significantly higher than variation in reported use of the 4 proven therapies (albuterol, corticosteroids, and ipratropium in the first 24 h for asthma; IV re‐hydration for gastroenteritis). The mean variation in reported use of unproven therapies was 44.6 20.5%, compared with 15.5 12.5% variation in reported use of therapies of proven benefit (p = 0.02).
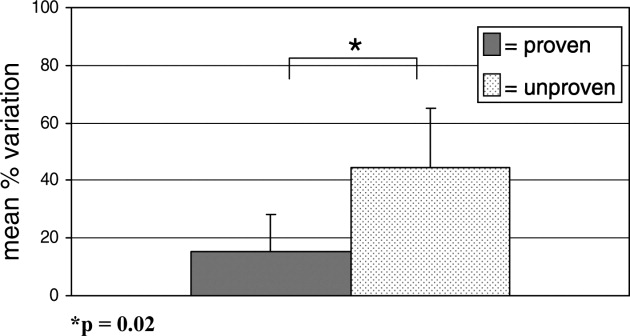
As a sensitivity analysis, the use of thickened feeds as a therapy for GERD was re‐categorized as proven and the above analysis repeated, for the reasons outlined in the methods section. This did not alter the identified relationship between variability and the evidence base fundamentally; hospitalists' reported variation in use of therapies of unproven benefit in this sensitivity analysis was 44.6 21.7%, compared with 21.4 17.0% variation in reported use of proven therapies (p = 0.05).
DISCUSSION
Substantial variation exists in the inpatient management of common pediatric diseases. Although we have previously found less reported variability in pediatric hospitalists' practices than in those of community‐based pediatricians,20 the current study demonstrates a high degree of reported variation even among a cohort of inpatient specialists. Importantly, however, reported variation was found to be significantly less for those inpatient therapies supported by a robust evidence base.
Bronchiolitis, gastroenteritis, asthma, and GERD are extremely common causes of pediatric hospitalization throughout the developed world.2125 Our finding of high reported variability in the routine care of all of these conditions except asthma is concerning, as it suggests that experts do not agree on how to manage children hospitalized with even the most common childhood diseases. While we hypothesized that there would be some variation in the use of therapies whose benefit has not been well established, the high degree of variation observed is of concern because it indicates that an insufficient evidentiary base exists to support much of our day‐to‐day practice. Some variation in practice in response to differing clinical presentations is both expected and desirable, but it is remarkable that variance in practice was significantly less for the most evidence‐based therapies than for those grounded less firmly in science, suggesting that the variation identified here is not justifiable variation based on appropriate responses to atypical clinical presentations, but uncertainty in the absence of clear data. Such undesired variability may decrease system reliability (introducing avoidable opportunity for error),26 and lead to under‐use of needed therapies as well as overuse of unnecessary therapies.1
Our work extends prior research that has identified wide variation in patterns of hospital admission, use of hospital resources, and processes of inpatient care,2732 by documenting reported variation in the use of common inpatient therapies. Rates of hospital admission may vary by as much as 7‐fold across regions.33 Our study demonstrates that wide variation exists not only in admission rates, but in reported inpatient care processes for some of the most common diseases seen in pediatric hospitals. Our study also supports the hypothesis that variation in care may be driven by gaps in knowledge.32 Among hospitalists, we found the strength of the evidence base to be a major determinant of reported variability.
Our study has several limitations. First, the data presented here are derived from provider self‐reports, which may not fully reflect actual practice. In the case of the few proven therapies studied, reporting bias could lead to an over‐reporting of adherence to evidence‐based standards of care. Like our study, however, prior studies have found that hospital‐based providers fairly consistently comply with evidence‐based practice recommendations for acute asthma care,34, 35 supporting our finding that variation in acute asthma care (which represented 3 of our 4 proven therapies) is low in this setting.
Another limitation is that classifications of therapies as proven or unproven change as the evidence base evolves. Of particular relevance to this study, the use of thickened feeds as a therapy for GERD, originally classified as being of unknown effectiveness, was reclassified by Clinical Evidence during the course of the study as likely to be beneficial. The relationship we identified between proven therapies and degree of variability in care did not change when we conducted a sensitivity analysis re‐categorizing this therapy as proven, but precisely quantifying variation is complicated by continuous changes in the state of the evidence.
Pediatric hospitalist systems have been found consistently to improve the efficiency of care,9 yet this study suggests that considerable variation in hospitalists' management of key conditions remains. The Pediatric Research in Inpatient Settings (PRIS) Network was formed in 2002 to improve the care of hospitalized children and the quality of inpatient practice by developing an evidence base for inpatient pediatric care. Ongoing multi‐center research efforts through PRIS and other research networks are beginning to critically evaluate therapies used in the management of common pediatric conditions. Rigorous studies of the processes and outcomes of pediatric hospital care will inform inpatient pediatric practice, and ultimately improve the care of hospitalized children. The current study strongly affirms the urgent need to establish such an evidence base. Without data to inform optimal care, efforts to reduce undesirable variation in care and improve care quality cannot be fully realized.
Acknowledgements
The authors would like to extend their thanks to the hospitalists and members of the Pediatric Research in Inpatient Settings Network who participated in this research, as well as the Children's National Medical Center and Children's Hospital Boston Inpatient Pediatrics Services, who provided funding to support this study. Special thanks to the Ambulatory Pediatrics Association (APA), for its core support of the PRIS Network. Dr. Landrigan is the recipient of a career development award from the Agency for Healthcare Research and Quality (AHRQ K08 HS13333). Dr. Conway is the recipient of a Robert Wood Johnson Clinical Scholars Grant. All researchers were independent from the funding agencies; the academic medical centers named above, APA, and AHRQ had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
- Institute of Medicine: Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, D.C.:National Academic Press,2001.
- ..Reducing variation in surgical care.BMJ2005;330:1401–1402.
- ,,,.Variation in use of video assisted thoracic surgery in the United Kingdom.BMJ2004;329:1011–1012.
- ,..The emerging role of “hospitalists” in the American health care system.N. Engl J Med1996;335:514–517.
- ,..Hospitalism in the USA.Lancet1999;353:1902.
- Society of Hospital Medicine. Growth of Hospital Medicine Nationwide. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNationwide/Growth_of_Hospital_M.htm. Accessed April 11,2007.
- .The changing face of hospital practice.Med Econ2002;79:72–79.
- ,..The hospitalist movement 5 years later.JAMA2002;287:487–494.
- ,,,.Pediatric hospitalists: a systematic review of the literature.Pediatrics2006;117:1736–1744.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med2002;137:859–865.
- ,,,,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med2002;137:866–874.
- ,.Evaluation of a pediatric hospitalist service: impact on length of stay and hospital charges.Pediatrics2000;105:478–484.
- ,,,,,,, and .Impact of an HMO hospitalist system in academic pediatrics.Pediatrics2002;110:720–728.
- ,, and .Evaluation of a pediatric hospitalist service by APR‐DRG's: impact on length of stay and hospital charges.Pediatr Research2001;49(suppl),691.
- ,,.Pediatric hospitalists: quality care for the underserved?Am J Med Qual2001;16:174–180.
- ,,,,,.Restructuring an academic pediatric inpatient service using concepts developed by hospitalists.Clin Pediatr (Phila)2001;40:653–660.
- ,,, and .Hospitalist care of medically complex children.Pediatr Research2004;55(suppl),1789.
- ,,.Hospital‐based and community pediatricians: comparing outcomes for asthma and bronchiolitis.J Clin Outcomes Manage1997;4:21–24.
- Godlee F,Tovey D,Bedford M, et al., eds.Clinical Evidence: The International Source of the Best Available Evidence for Effective Health Care.London, United Kingdom:BMJ Publishing Group;2004.
- ,,,,,.Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians.Pediatrics2006;118:441–447.
- ,,,,.Contribution of RSV to bronchiolitis and pneumonia‐associated hospitalizations in English children, April 1995‐March 1998.Epidemiol Infect2002;129:99–106.
- ,,.Direct medical costs of bronchiolitis hospitalizations in the United States.Pediatrics2006;118:2418–2423.
- ,,,,,.Multicenter Prospective Study of the Burden of Rotavirus Acute Gastroenteritis in Europe, 2004‐2005: The REVEAL Study.J Infect Dis2007;195Suppl 1:S4–S16.
- .The state of childhood asthma, United States, 1980‐2005.Adv.Data.2006;1–24.
- ,.Gastroesophageal reflux in children: pathogenesis, prevalence, diagnosis, and role of proton pump inhibitors in treatment.Paediatr Drugs2002;4:673–685.
- ,,,.Reliability science and patient safety.Pediatr Clin North Am2006;53:1121–1133.
- Wennberg JE and McAndrew Cooper M, eds.The Dartmouth Atlas of Health Care in the United States.Hanover, NH, USA:Health Forum, Inc.,1999.
- ,,,,,.Variations in rates of hospitalization of children in three urban communities.N Engl J Med1989;320:1183–1187.
- ,,,,,.Use of hospitals, physician visits, and hospice care during last six months of life among cohorts loyal to highly respected hospitals in the United States.BMJ2004;328:607.
- ,,,,,, et al.Variations in practice and outcomes in the Canadian NICU network: 1996‐1997.Pediatrics2000;106:1070–1079.
- ,,,.Evaluation of febrile children with petechial rashes: is there consensus among pediatricians?Pediatr Infect Dis J1998;17:1135–1140.
- ,,,,,, et al.Practice variation among pediatric emergency departments in the treatment of bronchiolitis.Acad Emerg Med2004;11:353–360.
- ,,,.Paediatric inpatient utilisation in a district general hospital.Arch Dis Child1994;70:488–492.
- ,,,.Emergency department asthma: compliance with an evidence‐based management algorithm.Ann Acad Med Singapore2002;31:419–424.
- ,,.Is the practice of paediatric inpatient medicine evidence‐based?J Paediatr Child Health2002;38:347–351.
Reduction of undesirable variation in care has been a major focus of systematic efforts to improve the quality of the healthcare system.13 The emergence of hospitalists, physicians specializing in the care of hospitalized patients, was spurred by a desire to streamline care and reduce variability in hospital management of common diseases.4, 5 Over the past decade, hospitalist systems have become a leading vehicle for care delivery.4, 6, 7 It remains unclear, however, whether implementation of hospitalist systems has lessened undesirable variation in the inpatient management of common diseases.
While systematic reviews have found costs and hospital length of stay to be 10‐15% lower in both pediatric and internal medicine hospitalist systems, few studies have adequately assessed the processes or quality of care in hospitalist systems.8, 9 Two internal medicine studies have found decreased mortality in hospitalist systems, but the mechanism by which hospitalists apparently achieved these gains is unclear.10, 11 Even less is known about care processes or quality in pediatric hospitalist systems. Death is a rare occurrence in pediatric ward settings, and the seven studies conducted to date comparing pediatric hospitalist and traditional systems have been universally underpowered to detect differences in mortality.9, 1218 There is a need to better understand care processes as a first step in understanding and improving quality of care in hospitalist systems.19
The Pediatric Research in Inpatient Settings (PRIS) Network was formed to improve the quality of care for hospitalized children through collaborative clinical research. In this study, we sought to study variation in the care of common pediatric conditions among a cohort of pediatric hospitalists. We have previously reported that less variability exists in hospitalists' reported management of inpatient conditions than in the reported management of these same conditions by community‐based pediatricians,20 but we were concerned that substantial undesirable variation (ie, variation in practice due to uncertainty or unsubstantiated local practice traditions, rather than justified variation in care based on different risks of harms or benefits in different patients) may still exist among hospitalists. We therefore conducted a study: 1) to investigate variation in hospitalists' reported use of common inpatient therapies, and 2) to test the hypothesis that greater variation exists in hospitalists' reported use of inpatient therapies of unproven benefit than in those therapies proven to be beneficial.
METHODS
Survey Design and Administration
In 2003, we designed the PRIS Survey to collect data on hospitalists' backgrounds, practices, and training needs, as well as their management of common pediatric conditions. For the current study, we chose a priori to evaluate hospitalists' use of 14 therapies in the management of 4 common conditions: asthma, bronchiolitis, gastroenteritis, and gastro‐esophageal reflux disease (GERD) (Table 1). These four conditions were chosen for study because they were among the top discharge diagnoses (primary and secondary) from the inpatient services at 2 of the authors' institutions (Children's Hospital Boston and Children's Hospital San Diego) during the year before administration of the survey, and because a discrete set of therapeutic agents are commonly used in their management. Respondents were asked to report the frequency with which they used each of the 14 therapies of interest on 5‐point Likert scales (from 1=never to 5=almost always). The survey initially developed was piloted with a small group of hospitalists and pediatricians, and a final version incorporating revisions was subsequently administered to all pediatric hospitalists in the US and Canada identified through any of 3 sources: 1) the Pediatric Research in Inpatient Settings (PRIS) list of participants; 2) the Society for Hospital Medicine (SHM) pediatric hospital medicine e‐mail listserv; and 3) the list of all attendees of the first national pediatric hospitalist conference sponsored by the Ambulatory Pediatrics Association (APA), SHM, and American Academy of Pediatrics (AAP); this meeting was held in San Antonio, Texas, USA in November 2003. Individuals identified through more than 1 of these groups were counted only once. Potential participants were assured that individual responses would be kept confidential, and were e‐mailed an access code to participate in the online survey, using a secure web‐based interface; a paper‐based version was also made available to those who preferred to respond in this manner. Regular reminder notices were sent to all non‐responders. Further details regarding PRIS Survey recruitment and study methods have been published previously.20
| Condition | Therapy | BMJ clinical evidence Treatment effect categorization* | Study classification |
|---|---|---|---|
| |||
| Asthma | Inhaled albuterol | Beneficial | Proven |
| Systemic corticosteroids | Beneficial | Proven | |
| Inhaled ipratropium in the first 24 hours of hospitalization | Beneficial | Proven | |
| Inhaled ipratropium after the first 24 hours of hospitalization | Unknown effectiveness | Unproven | |
| Bronchiolitis | Inhaled albuterol | Unknown effectiveness | Unproven |
| Inhaled epinephrine | Unknown effectiveness | Unproven | |
| Systemic corticosteroids | Unknown effectiveness | Unproven | |
| Gastroenteritis | Intravenous hydration | Beneficial | Proven |
| Lactobacillus | Not assessed | Unproven | |
| Ondansetron | Not assessed | Unproven | |
| Gastro‐Esophageal Reflux Disease (GERD) | H2 histamine‐receptor antagonists | Unknown effectiveness | Unproven |
| Thickened feeds | Unknown effectiveness Likely to be beneficial | Unproven Proven | |
| Metoclopramide | Unknown effectiveness | Unproven | |
| Proton‐pump inhibitors | Unknown effectiveness | Unproven | |
DefinitionsReference Responses and Percent Variation
To measure variation in reported management, we first sought to determine a reference response for each therapy of interest. Since the evidence base for most of the therapies we studied is weak, it was not possible to determine a gold standard response for each therapy. Instead, we sought to measure the degree of divergence from a reference response for each therapy in the following manner. First, to simplify analyses, we collapsed our five‐category Likert scale into three categories (never/rarely, sometimes, and often/almost always). We then defined the reference response for each therapy to be never/rarely or often/almost always, whichever of the 2 was more frequently selected by respondents; sometimes was not used as a reference category, as reporting use of a particular therapy sometimes indicated substantial variability even within an individual's own practice.
Classification of therapies as proven or unproven.
To classify each of the 14 studied therapies as being of proven or unproven, we used the British Medical Journal's publication Clinical Evidence.19 We chose to use Clinical Evidence as an evidence‐based reference because it provides rigorously developed, systematic analyses of therapeutic management options for multiple common pediatric conditions, and organizes recommendations in a straightforward manner. Four of the 14 therapies had been determined on systematic review to be proven beneficial at the time of study design: systemic corticosteroids, inhaled albuterol, and ipratropium (in the first 24 h) in the care of children with asthma; and IV hydration in the care of children with acute gastroenteritis. The remaining 10 therapies were either considered to be of unknown effectiveness or had not been formally evaluated by Clinical Evidence, and were hence considered unproven for this study (Table 1). Of note, the use of thickened feeds in the treatment of children with GERD had been determined to be of unknown effectiveness at the time of study design, but was reclassified as likely to be beneficial during the course of the study.
Analyses
Descriptive statistics were used to report respondents' demographic characteristics and work environments, as well as variation in their reported use of each of the 14 therapies. Variation in hospitalists' use of proven versus unproven therapies was compared using the Wilcoxon rank sum test, as it was distributed non‐normally. For our primary analysis, the use of thickened feeds in GERD was considered unproven, but a sensitivity analysis was conducted reclassifying it as proven in light of the evolving literature on its use and its consequent reclassification in Clinical Evidence.(SAS Version 9.1, Cary, NC) was used for statistical analyses.
RESULTS
213 of the 320 individuals identified through the 3 lists of pediatric hospitalists (67%) responded to the survey. Of these, 198 (93%) identified themselves as hospitalists and were therefore included. As previously reported,20 53% of respondents were male, 55% worked in academic training environments, and 47% had completed advanced training (fellowship) beyond their core pediatric training (residency training); respondents reported completing residency training 11 9 (mean, standard deviation) years prior to the survey, and spending 176 72 days per year in the care of hospitalized patients.
Variation in reported management: asthma
(Figure 1, Panel A). Relatively little variation existed in reported use of the 4 asthma therapies studied. Only 4.4% (95% CI, 1.4‐7.4%) of respondents did not provide the reference response of using inhaled albuterol often or almost always in the care of inpatients with asthma, and only 6.0% (2.5‐9.5%) of respondents did not report using systemic corticosteroids often or almost always. Variation in reported use of ipratropium was somewhat higher.

Bronchiolitis
(Figure 1B). By contrast, variation in reported use of inhaled therapies for bronchiolitis was high, with many respondents reporting that they often or always used inhaled albuterol or epinephrine, while many others reported rarely or never using them. There was 59.6% (52.4‐66.8%) variation from the reference response of often/almost always using inhaled albuterol, and 72.2% (65.6‐78.8%) variation from the reference response of never/rarely using inhaled epinephrine. Only 11.6% (6.9‐16.3%) of respondents, however, varied from the reference response of using dexamethasone more than rarely in the care of children with bronchiolitis.
Gastroenteritis
(Figure 1C). Moderate variability existed in the reported use of the 3 studied therapies for children hospitalized with gastroenteritis. 21.1% (15.1‐27.1%) of respondents did not provide the reference response of often/almost always using IV hydration; 35.9% (28.9‐42.9%) did not provide the reference response of never or rarely using lactobacillus; likewise, 35.9% (28.9‐42.9%) did not provide the reference response of never or rarely using ondansetron.
Gastro‐Esophageal Reflux Disease
(Figure 1, Panel D). There was moderate to high variability in the reported management of GERD. 22.8% (16.7‐28.9%) of respondents did not provide the reference response of often/almost always using H2 antagonists, and 44.9% (37.6‐52.2%) did not report often/almost always using thickened feeds in the care of these children. 58.3% (51.1‐65.5%) and 72.1% (65.5‐78.7%) of respondents did not provide the reference response of never/rarely using metoclopramide and proton pump inhibitors, respectively.
Proven vs. Unproven Therapies
(Figure 2). Variation in reported use of therapies of unproven benefit was significantly higher than variation in reported use of the 4 proven therapies (albuterol, corticosteroids, and ipratropium in the first 24 h for asthma; IV re‐hydration for gastroenteritis). The mean variation in reported use of unproven therapies was 44.6 20.5%, compared with 15.5 12.5% variation in reported use of therapies of proven benefit (p = 0.02).

As a sensitivity analysis, the use of thickened feeds as a therapy for GERD was re‐categorized as proven and the above analysis repeated, for the reasons outlined in the methods section. This did not alter the identified relationship between variability and the evidence base fundamentally; hospitalists' reported variation in use of therapies of unproven benefit in this sensitivity analysis was 44.6 21.7%, compared with 21.4 17.0% variation in reported use of proven therapies (p = 0.05).
DISCUSSION
Substantial variation exists in the inpatient management of common pediatric diseases. Although we have previously found less reported variability in pediatric hospitalists' practices than in those of community‐based pediatricians,20 the current study demonstrates a high degree of reported variation even among a cohort of inpatient specialists. Importantly, however, reported variation was found to be significantly less for those inpatient therapies supported by a robust evidence base.
Bronchiolitis, gastroenteritis, asthma, and GERD are extremely common causes of pediatric hospitalization throughout the developed world.2125 Our finding of high reported variability in the routine care of all of these conditions except asthma is concerning, as it suggests that experts do not agree on how to manage children hospitalized with even the most common childhood diseases. While we hypothesized that there would be some variation in the use of therapies whose benefit has not been well established, the high degree of variation observed is of concern because it indicates that an insufficient evidentiary base exists to support much of our day‐to‐day practice. Some variation in practice in response to differing clinical presentations is both expected and desirable, but it is remarkable that variance in practice was significantly less for the most evidence‐based therapies than for those grounded less firmly in science, suggesting that the variation identified here is not justifiable variation based on appropriate responses to atypical clinical presentations, but uncertainty in the absence of clear data. Such undesired variability may decrease system reliability (introducing avoidable opportunity for error),26 and lead to under‐use of needed therapies as well as overuse of unnecessary therapies.1
Our work extends prior research that has identified wide variation in patterns of hospital admission, use of hospital resources, and processes of inpatient care,2732 by documenting reported variation in the use of common inpatient therapies. Rates of hospital admission may vary by as much as 7‐fold across regions.33 Our study demonstrates that wide variation exists not only in admission rates, but in reported inpatient care processes for some of the most common diseases seen in pediatric hospitals. Our study also supports the hypothesis that variation in care may be driven by gaps in knowledge.32 Among hospitalists, we found the strength of the evidence base to be a major determinant of reported variability.
Our study has several limitations. First, the data presented here are derived from provider self‐reports, which may not fully reflect actual practice. In the case of the few proven therapies studied, reporting bias could lead to an over‐reporting of adherence to evidence‐based standards of care. Like our study, however, prior studies have found that hospital‐based providers fairly consistently comply with evidence‐based practice recommendations for acute asthma care,34, 35 supporting our finding that variation in acute asthma care (which represented 3 of our 4 proven therapies) is low in this setting.
Another limitation is that classifications of therapies as proven or unproven change as the evidence base evolves. Of particular relevance to this study, the use of thickened feeds as a therapy for GERD, originally classified as being of unknown effectiveness, was reclassified by Clinical Evidence during the course of the study as likely to be beneficial. The relationship we identified between proven therapies and degree of variability in care did not change when we conducted a sensitivity analysis re‐categorizing this therapy as proven, but precisely quantifying variation is complicated by continuous changes in the state of the evidence.
Pediatric hospitalist systems have been found consistently to improve the efficiency of care,9 yet this study suggests that considerable variation in hospitalists' management of key conditions remains. The Pediatric Research in Inpatient Settings (PRIS) Network was formed in 2002 to improve the care of hospitalized children and the quality of inpatient practice by developing an evidence base for inpatient pediatric care. Ongoing multi‐center research efforts through PRIS and other research networks are beginning to critically evaluate therapies used in the management of common pediatric conditions. Rigorous studies of the processes and outcomes of pediatric hospital care will inform inpatient pediatric practice, and ultimately improve the care of hospitalized children. The current study strongly affirms the urgent need to establish such an evidence base. Without data to inform optimal care, efforts to reduce undesirable variation in care and improve care quality cannot be fully realized.
Acknowledgements
The authors would like to extend their thanks to the hospitalists and members of the Pediatric Research in Inpatient Settings Network who participated in this research, as well as the Children's National Medical Center and Children's Hospital Boston Inpatient Pediatrics Services, who provided funding to support this study. Special thanks to the Ambulatory Pediatrics Association (APA), for its core support of the PRIS Network. Dr. Landrigan is the recipient of a career development award from the Agency for Healthcare Research and Quality (AHRQ K08 HS13333). Dr. Conway is the recipient of a Robert Wood Johnson Clinical Scholars Grant. All researchers were independent from the funding agencies; the academic medical centers named above, APA, and AHRQ had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Reduction of undesirable variation in care has been a major focus of systematic efforts to improve the quality of the healthcare system.13 The emergence of hospitalists, physicians specializing in the care of hospitalized patients, was spurred by a desire to streamline care and reduce variability in hospital management of common diseases.4, 5 Over the past decade, hospitalist systems have become a leading vehicle for care delivery.4, 6, 7 It remains unclear, however, whether implementation of hospitalist systems has lessened undesirable variation in the inpatient management of common diseases.
While systematic reviews have found costs and hospital length of stay to be 10‐15% lower in both pediatric and internal medicine hospitalist systems, few studies have adequately assessed the processes or quality of care in hospitalist systems.8, 9 Two internal medicine studies have found decreased mortality in hospitalist systems, but the mechanism by which hospitalists apparently achieved these gains is unclear.10, 11 Even less is known about care processes or quality in pediatric hospitalist systems. Death is a rare occurrence in pediatric ward settings, and the seven studies conducted to date comparing pediatric hospitalist and traditional systems have been universally underpowered to detect differences in mortality.9, 1218 There is a need to better understand care processes as a first step in understanding and improving quality of care in hospitalist systems.19
The Pediatric Research in Inpatient Settings (PRIS) Network was formed to improve the quality of care for hospitalized children through collaborative clinical research. In this study, we sought to study variation in the care of common pediatric conditions among a cohort of pediatric hospitalists. We have previously reported that less variability exists in hospitalists' reported management of inpatient conditions than in the reported management of these same conditions by community‐based pediatricians,20 but we were concerned that substantial undesirable variation (ie, variation in practice due to uncertainty or unsubstantiated local practice traditions, rather than justified variation in care based on different risks of harms or benefits in different patients) may still exist among hospitalists. We therefore conducted a study: 1) to investigate variation in hospitalists' reported use of common inpatient therapies, and 2) to test the hypothesis that greater variation exists in hospitalists' reported use of inpatient therapies of unproven benefit than in those therapies proven to be beneficial.
METHODS
Survey Design and Administration
In 2003, we designed the PRIS Survey to collect data on hospitalists' backgrounds, practices, and training needs, as well as their management of common pediatric conditions. For the current study, we chose a priori to evaluate hospitalists' use of 14 therapies in the management of 4 common conditions: asthma, bronchiolitis, gastroenteritis, and gastro‐esophageal reflux disease (GERD) (Table 1). These four conditions were chosen for study because they were among the top discharge diagnoses (primary and secondary) from the inpatient services at 2 of the authors' institutions (Children's Hospital Boston and Children's Hospital San Diego) during the year before administration of the survey, and because a discrete set of therapeutic agents are commonly used in their management. Respondents were asked to report the frequency with which they used each of the 14 therapies of interest on 5‐point Likert scales (from 1=never to 5=almost always). The survey initially developed was piloted with a small group of hospitalists and pediatricians, and a final version incorporating revisions was subsequently administered to all pediatric hospitalists in the US and Canada identified through any of 3 sources: 1) the Pediatric Research in Inpatient Settings (PRIS) list of participants; 2) the Society for Hospital Medicine (SHM) pediatric hospital medicine e‐mail listserv; and 3) the list of all attendees of the first national pediatric hospitalist conference sponsored by the Ambulatory Pediatrics Association (APA), SHM, and American Academy of Pediatrics (AAP); this meeting was held in San Antonio, Texas, USA in November 2003. Individuals identified through more than 1 of these groups were counted only once. Potential participants were assured that individual responses would be kept confidential, and were e‐mailed an access code to participate in the online survey, using a secure web‐based interface; a paper‐based version was also made available to those who preferred to respond in this manner. Regular reminder notices were sent to all non‐responders. Further details regarding PRIS Survey recruitment and study methods have been published previously.20
| Condition | Therapy | BMJ clinical evidence Treatment effect categorization* | Study classification |
|---|---|---|---|
| |||
| Asthma | Inhaled albuterol | Beneficial | Proven |
| Systemic corticosteroids | Beneficial | Proven | |
| Inhaled ipratropium in the first 24 hours of hospitalization | Beneficial | Proven | |
| Inhaled ipratropium after the first 24 hours of hospitalization | Unknown effectiveness | Unproven | |
| Bronchiolitis | Inhaled albuterol | Unknown effectiveness | Unproven |
| Inhaled epinephrine | Unknown effectiveness | Unproven | |
| Systemic corticosteroids | Unknown effectiveness | Unproven | |
| Gastroenteritis | Intravenous hydration | Beneficial | Proven |
| Lactobacillus | Not assessed | Unproven | |
| Ondansetron | Not assessed | Unproven | |
| Gastro‐Esophageal Reflux Disease (GERD) | H2 histamine‐receptor antagonists | Unknown effectiveness | Unproven |
| Thickened feeds | Unknown effectiveness Likely to be beneficial | Unproven Proven | |
| Metoclopramide | Unknown effectiveness | Unproven | |
| Proton‐pump inhibitors | Unknown effectiveness | Unproven | |
DefinitionsReference Responses and Percent Variation
To measure variation in reported management, we first sought to determine a reference response for each therapy of interest. Since the evidence base for most of the therapies we studied is weak, it was not possible to determine a gold standard response for each therapy. Instead, we sought to measure the degree of divergence from a reference response for each therapy in the following manner. First, to simplify analyses, we collapsed our five‐category Likert scale into three categories (never/rarely, sometimes, and often/almost always). We then defined the reference response for each therapy to be never/rarely or often/almost always, whichever of the 2 was more frequently selected by respondents; sometimes was not used as a reference category, as reporting use of a particular therapy sometimes indicated substantial variability even within an individual's own practice.
Classification of therapies as proven or unproven.
To classify each of the 14 studied therapies as being of proven or unproven, we used the British Medical Journal's publication Clinical Evidence.19 We chose to use Clinical Evidence as an evidence‐based reference because it provides rigorously developed, systematic analyses of therapeutic management options for multiple common pediatric conditions, and organizes recommendations in a straightforward manner. Four of the 14 therapies had been determined on systematic review to be proven beneficial at the time of study design: systemic corticosteroids, inhaled albuterol, and ipratropium (in the first 24 h) in the care of children with asthma; and IV hydration in the care of children with acute gastroenteritis. The remaining 10 therapies were either considered to be of unknown effectiveness or had not been formally evaluated by Clinical Evidence, and were hence considered unproven for this study (Table 1). Of note, the use of thickened feeds in the treatment of children with GERD had been determined to be of unknown effectiveness at the time of study design, but was reclassified as likely to be beneficial during the course of the study.
Analyses
Descriptive statistics were used to report respondents' demographic characteristics and work environments, as well as variation in their reported use of each of the 14 therapies. Variation in hospitalists' use of proven versus unproven therapies was compared using the Wilcoxon rank sum test, as it was distributed non‐normally. For our primary analysis, the use of thickened feeds in GERD was considered unproven, but a sensitivity analysis was conducted reclassifying it as proven in light of the evolving literature on its use and its consequent reclassification in Clinical Evidence.(SAS Version 9.1, Cary, NC) was used for statistical analyses.
RESULTS
213 of the 320 individuals identified through the 3 lists of pediatric hospitalists (67%) responded to the survey. Of these, 198 (93%) identified themselves as hospitalists and were therefore included. As previously reported,20 53% of respondents were male, 55% worked in academic training environments, and 47% had completed advanced training (fellowship) beyond their core pediatric training (residency training); respondents reported completing residency training 11 9 (mean, standard deviation) years prior to the survey, and spending 176 72 days per year in the care of hospitalized patients.
Variation in reported management: asthma
(Figure 1, Panel A). Relatively little variation existed in reported use of the 4 asthma therapies studied. Only 4.4% (95% CI, 1.4‐7.4%) of respondents did not provide the reference response of using inhaled albuterol often or almost always in the care of inpatients with asthma, and only 6.0% (2.5‐9.5%) of respondents did not report using systemic corticosteroids often or almost always. Variation in reported use of ipratropium was somewhat higher.

Bronchiolitis
(Figure 1B). By contrast, variation in reported use of inhaled therapies for bronchiolitis was high, with many respondents reporting that they often or always used inhaled albuterol or epinephrine, while many others reported rarely or never using them. There was 59.6% (52.4‐66.8%) variation from the reference response of often/almost always using inhaled albuterol, and 72.2% (65.6‐78.8%) variation from the reference response of never/rarely using inhaled epinephrine. Only 11.6% (6.9‐16.3%) of respondents, however, varied from the reference response of using dexamethasone more than rarely in the care of children with bronchiolitis.
Gastroenteritis
(Figure 1C). Moderate variability existed in the reported use of the 3 studied therapies for children hospitalized with gastroenteritis. 21.1% (15.1‐27.1%) of respondents did not provide the reference response of often/almost always using IV hydration; 35.9% (28.9‐42.9%) did not provide the reference response of never or rarely using lactobacillus; likewise, 35.9% (28.9‐42.9%) did not provide the reference response of never or rarely using ondansetron.
Gastro‐Esophageal Reflux Disease
(Figure 1, Panel D). There was moderate to high variability in the reported management of GERD. 22.8% (16.7‐28.9%) of respondents did not provide the reference response of often/almost always using H2 antagonists, and 44.9% (37.6‐52.2%) did not report often/almost always using thickened feeds in the care of these children. 58.3% (51.1‐65.5%) and 72.1% (65.5‐78.7%) of respondents did not provide the reference response of never/rarely using metoclopramide and proton pump inhibitors, respectively.
Proven vs. Unproven Therapies
(Figure 2). Variation in reported use of therapies of unproven benefit was significantly higher than variation in reported use of the 4 proven therapies (albuterol, corticosteroids, and ipratropium in the first 24 h for asthma; IV re‐hydration for gastroenteritis). The mean variation in reported use of unproven therapies was 44.6 20.5%, compared with 15.5 12.5% variation in reported use of therapies of proven benefit (p = 0.02).

As a sensitivity analysis, the use of thickened feeds as a therapy for GERD was re‐categorized as proven and the above analysis repeated, for the reasons outlined in the methods section. This did not alter the identified relationship between variability and the evidence base fundamentally; hospitalists' reported variation in use of therapies of unproven benefit in this sensitivity analysis was 44.6 21.7%, compared with 21.4 17.0% variation in reported use of proven therapies (p = 0.05).
DISCUSSION
Substantial variation exists in the inpatient management of common pediatric diseases. Although we have previously found less reported variability in pediatric hospitalists' practices than in those of community‐based pediatricians,20 the current study demonstrates a high degree of reported variation even among a cohort of inpatient specialists. Importantly, however, reported variation was found to be significantly less for those inpatient therapies supported by a robust evidence base.
Bronchiolitis, gastroenteritis, asthma, and GERD are extremely common causes of pediatric hospitalization throughout the developed world.2125 Our finding of high reported variability in the routine care of all of these conditions except asthma is concerning, as it suggests that experts do not agree on how to manage children hospitalized with even the most common childhood diseases. While we hypothesized that there would be some variation in the use of therapies whose benefit has not been well established, the high degree of variation observed is of concern because it indicates that an insufficient evidentiary base exists to support much of our day‐to‐day practice. Some variation in practice in response to differing clinical presentations is both expected and desirable, but it is remarkable that variance in practice was significantly less for the most evidence‐based therapies than for those grounded less firmly in science, suggesting that the variation identified here is not justifiable variation based on appropriate responses to atypical clinical presentations, but uncertainty in the absence of clear data. Such undesired variability may decrease system reliability (introducing avoidable opportunity for error),26 and lead to under‐use of needed therapies as well as overuse of unnecessary therapies.1
Our work extends prior research that has identified wide variation in patterns of hospital admission, use of hospital resources, and processes of inpatient care,2732 by documenting reported variation in the use of common inpatient therapies. Rates of hospital admission may vary by as much as 7‐fold across regions.33 Our study demonstrates that wide variation exists not only in admission rates, but in reported inpatient care processes for some of the most common diseases seen in pediatric hospitals. Our study also supports the hypothesis that variation in care may be driven by gaps in knowledge.32 Among hospitalists, we found the strength of the evidence base to be a major determinant of reported variability.
Our study has several limitations. First, the data presented here are derived from provider self‐reports, which may not fully reflect actual practice. In the case of the few proven therapies studied, reporting bias could lead to an over‐reporting of adherence to evidence‐based standards of care. Like our study, however, prior studies have found that hospital‐based providers fairly consistently comply with evidence‐based practice recommendations for acute asthma care,34, 35 supporting our finding that variation in acute asthma care (which represented 3 of our 4 proven therapies) is low in this setting.
Another limitation is that classifications of therapies as proven or unproven change as the evidence base evolves. Of particular relevance to this study, the use of thickened feeds as a therapy for GERD, originally classified as being of unknown effectiveness, was reclassified by Clinical Evidence during the course of the study as likely to be beneficial. The relationship we identified between proven therapies and degree of variability in care did not change when we conducted a sensitivity analysis re‐categorizing this therapy as proven, but precisely quantifying variation is complicated by continuous changes in the state of the evidence.
Pediatric hospitalist systems have been found consistently to improve the efficiency of care,9 yet this study suggests that considerable variation in hospitalists' management of key conditions remains. The Pediatric Research in Inpatient Settings (PRIS) Network was formed in 2002 to improve the care of hospitalized children and the quality of inpatient practice by developing an evidence base for inpatient pediatric care. Ongoing multi‐center research efforts through PRIS and other research networks are beginning to critically evaluate therapies used in the management of common pediatric conditions. Rigorous studies of the processes and outcomes of pediatric hospital care will inform inpatient pediatric practice, and ultimately improve the care of hospitalized children. The current study strongly affirms the urgent need to establish such an evidence base. Without data to inform optimal care, efforts to reduce undesirable variation in care and improve care quality cannot be fully realized.
Acknowledgements
The authors would like to extend their thanks to the hospitalists and members of the Pediatric Research in Inpatient Settings Network who participated in this research, as well as the Children's National Medical Center and Children's Hospital Boston Inpatient Pediatrics Services, who provided funding to support this study. Special thanks to the Ambulatory Pediatrics Association (APA), for its core support of the PRIS Network. Dr. Landrigan is the recipient of a career development award from the Agency for Healthcare Research and Quality (AHRQ K08 HS13333). Dr. Conway is the recipient of a Robert Wood Johnson Clinical Scholars Grant. All researchers were independent from the funding agencies; the academic medical centers named above, APA, and AHRQ had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
- Institute of Medicine: Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, D.C.:National Academic Press,2001.
- ..Reducing variation in surgical care.BMJ2005;330:1401–1402.
- ,,,.Variation in use of video assisted thoracic surgery in the United Kingdom.BMJ2004;329:1011–1012.
- ,..The emerging role of “hospitalists” in the American health care system.N. Engl J Med1996;335:514–517.
- ,..Hospitalism in the USA.Lancet1999;353:1902.
- Society of Hospital Medicine. Growth of Hospital Medicine Nationwide. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNationwide/Growth_of_Hospital_M.htm. Accessed April 11,2007.
- .The changing face of hospital practice.Med Econ2002;79:72–79.
- ,..The hospitalist movement 5 years later.JAMA2002;287:487–494.
- ,,,.Pediatric hospitalists: a systematic review of the literature.Pediatrics2006;117:1736–1744.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med2002;137:859–865.
- ,,,,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med2002;137:866–874.
- ,.Evaluation of a pediatric hospitalist service: impact on length of stay and hospital charges.Pediatrics2000;105:478–484.
- ,,,,,,, and .Impact of an HMO hospitalist system in academic pediatrics.Pediatrics2002;110:720–728.
- ,, and .Evaluation of a pediatric hospitalist service by APR‐DRG's: impact on length of stay and hospital charges.Pediatr Research2001;49(suppl),691.
- ,,.Pediatric hospitalists: quality care for the underserved?Am J Med Qual2001;16:174–180.
- ,,,,,.Restructuring an academic pediatric inpatient service using concepts developed by hospitalists.Clin Pediatr (Phila)2001;40:653–660.
- ,,, and .Hospitalist care of medically complex children.Pediatr Research2004;55(suppl),1789.
- ,,.Hospital‐based and community pediatricians: comparing outcomes for asthma and bronchiolitis.J Clin Outcomes Manage1997;4:21–24.
- Godlee F,Tovey D,Bedford M, et al., eds.Clinical Evidence: The International Source of the Best Available Evidence for Effective Health Care.London, United Kingdom:BMJ Publishing Group;2004.
- ,,,,,.Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians.Pediatrics2006;118:441–447.
- ,,,,.Contribution of RSV to bronchiolitis and pneumonia‐associated hospitalizations in English children, April 1995‐March 1998.Epidemiol Infect2002;129:99–106.
- ,,.Direct medical costs of bronchiolitis hospitalizations in the United States.Pediatrics2006;118:2418–2423.
- ,,,,,.Multicenter Prospective Study of the Burden of Rotavirus Acute Gastroenteritis in Europe, 2004‐2005: The REVEAL Study.J Infect Dis2007;195Suppl 1:S4–S16.
- .The state of childhood asthma, United States, 1980‐2005.Adv.Data.2006;1–24.
- ,.Gastroesophageal reflux in children: pathogenesis, prevalence, diagnosis, and role of proton pump inhibitors in treatment.Paediatr Drugs2002;4:673–685.
- ,,,.Reliability science and patient safety.Pediatr Clin North Am2006;53:1121–1133.
- Wennberg JE and McAndrew Cooper M, eds.The Dartmouth Atlas of Health Care in the United States.Hanover, NH, USA:Health Forum, Inc.,1999.
- ,,,,,.Variations in rates of hospitalization of children in three urban communities.N Engl J Med1989;320:1183–1187.
- ,,,,,.Use of hospitals, physician visits, and hospice care during last six months of life among cohorts loyal to highly respected hospitals in the United States.BMJ2004;328:607.
- ,,,,,, et al.Variations in practice and outcomes in the Canadian NICU network: 1996‐1997.Pediatrics2000;106:1070–1079.
- ,,,.Evaluation of febrile children with petechial rashes: is there consensus among pediatricians?Pediatr Infect Dis J1998;17:1135–1140.
- ,,,,,, et al.Practice variation among pediatric emergency departments in the treatment of bronchiolitis.Acad Emerg Med2004;11:353–360.
- ,,,.Paediatric inpatient utilisation in a district general hospital.Arch Dis Child1994;70:488–492.
- ,,,.Emergency department asthma: compliance with an evidence‐based management algorithm.Ann Acad Med Singapore2002;31:419–424.
- ,,.Is the practice of paediatric inpatient medicine evidence‐based?J Paediatr Child Health2002;38:347–351.
- Institute of Medicine: Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, D.C.:National Academic Press,2001.
- ..Reducing variation in surgical care.BMJ2005;330:1401–1402.
- ,,,.Variation in use of video assisted thoracic surgery in the United Kingdom.BMJ2004;329:1011–1012.
- ,..The emerging role of “hospitalists” in the American health care system.N. Engl J Med1996;335:514–517.
- ,..Hospitalism in the USA.Lancet1999;353:1902.
- Society of Hospital Medicine. Growth of Hospital Medicine Nationwide. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNationwide/Growth_of_Hospital_M.htm. Accessed April 11,2007.
- .The changing face of hospital practice.Med Econ2002;79:72–79.
- ,..The hospitalist movement 5 years later.JAMA2002;287:487–494.
- ,,,.Pediatric hospitalists: a systematic review of the literature.Pediatrics2006;117:1736–1744.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med2002;137:859–865.
- ,,,,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med2002;137:866–874.
- ,.Evaluation of a pediatric hospitalist service: impact on length of stay and hospital charges.Pediatrics2000;105:478–484.
- ,,,,,,, and .Impact of an HMO hospitalist system in academic pediatrics.Pediatrics2002;110:720–728.
- ,, and .Evaluation of a pediatric hospitalist service by APR‐DRG's: impact on length of stay and hospital charges.Pediatr Research2001;49(suppl),691.
- ,,.Pediatric hospitalists: quality care for the underserved?Am J Med Qual2001;16:174–180.
- ,,,,,.Restructuring an academic pediatric inpatient service using concepts developed by hospitalists.Clin Pediatr (Phila)2001;40:653–660.
- ,,, and .Hospitalist care of medically complex children.Pediatr Research2004;55(suppl),1789.
- ,,.Hospital‐based and community pediatricians: comparing outcomes for asthma and bronchiolitis.J Clin Outcomes Manage1997;4:21–24.
- Godlee F,Tovey D,Bedford M, et al., eds.Clinical Evidence: The International Source of the Best Available Evidence for Effective Health Care.London, United Kingdom:BMJ Publishing Group;2004.
- ,,,,,.Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians.Pediatrics2006;118:441–447.
- ,,,,.Contribution of RSV to bronchiolitis and pneumonia‐associated hospitalizations in English children, April 1995‐March 1998.Epidemiol Infect2002;129:99–106.
- ,,.Direct medical costs of bronchiolitis hospitalizations in the United States.Pediatrics2006;118:2418–2423.
- ,,,,,.Multicenter Prospective Study of the Burden of Rotavirus Acute Gastroenteritis in Europe, 2004‐2005: The REVEAL Study.J Infect Dis2007;195Suppl 1:S4–S16.
- .The state of childhood asthma, United States, 1980‐2005.Adv.Data.2006;1–24.
- ,.Gastroesophageal reflux in children: pathogenesis, prevalence, diagnosis, and role of proton pump inhibitors in treatment.Paediatr Drugs2002;4:673–685.
- ,,,.Reliability science and patient safety.Pediatr Clin North Am2006;53:1121–1133.
- Wennberg JE and McAndrew Cooper M, eds.The Dartmouth Atlas of Health Care in the United States.Hanover, NH, USA:Health Forum, Inc.,1999.
- ,,,,,.Variations in rates of hospitalization of children in three urban communities.N Engl J Med1989;320:1183–1187.
- ,,,,,.Use of hospitals, physician visits, and hospice care during last six months of life among cohorts loyal to highly respected hospitals in the United States.BMJ2004;328:607.
- ,,,,,, et al.Variations in practice and outcomes in the Canadian NICU network: 1996‐1997.Pediatrics2000;106:1070–1079.
- ,,,.Evaluation of febrile children with petechial rashes: is there consensus among pediatricians?Pediatr Infect Dis J1998;17:1135–1140.
- ,,,,,, et al.Practice variation among pediatric emergency departments in the treatment of bronchiolitis.Acad Emerg Med2004;11:353–360.
- ,,,.Paediatric inpatient utilisation in a district general hospital.Arch Dis Child1994;70:488–492.
- ,,,.Emergency department asthma: compliance with an evidence‐based management algorithm.Ann Acad Med Singapore2002;31:419–424.
- ,,.Is the practice of paediatric inpatient medicine evidence‐based?J Paediatr Child Health2002;38:347–351.
Copyright © 2008 Society of Hospital Medicine
Nerves of Steal
A 60 year‐old woman with advanced kidney disease presented with one month of progressively worsening, sharp burning pain and decreased sensation in her left hand. Cold air exacerbated the pain. She noted decreasing ability to utilize her left fingers, a weakened grip and that the muscles in her hand looked smaller.
Localized sensory and motor symptoms in a discrete region of a single limb suggest neuropathy. The lack of symptoms in the face or ipsilateral lower extremity would dissuade a clinician from considering central etiologies; the presence of neuropathic pain is uncommon for cortical lesions. The involvement of motor and sensory nerves indicates peripheral nerve involvement.
The general approach to patients with peripheral neuropathy begins with identifying the neuropathy as a mononeuropathy (involving a single nerve), a polyneuropathy (symmetric involvement of multiple nerves) or a mononeuropathy multiplex (asymmetric involvement of multiple nerves). The patient, in this case, described subacute neuropathic pain, sensory loss, and weakness in her left hand in a distribution consistent with mononeuropathy or mononeuropathy multiplex.
This patient could have carpal tunnel syndrome given its prevalence in patients with advanced renal disease. The differential diagnosis is broad, however, and includes ulnar mononeuropathy, nerve ischemia due to vasculitis or vasculopathy, lower cervical radiculopathy (though the patient does not describe neck or radicular pain), lower brachial plexopathy, and complex regional pain syndrome.
The patient was diagnosed with advanced kidney disease one year ago when biopsy revealed focal segmental glomerulosclerosis secondary to lithium. Since her diagnosis, two grafts were placed in the left upper arm in anticipation of dialysis: the first, placed seven months prior to this admission, failed to mature; the second, placed one month prior to this admission, was complicated by bleeding at the fistula site and was not yet mature. Prior to this admission she had not required hemodialysis. Her past history included hypertension, dyslipidemia, hypothyroidism, secondary hyperparathyroidism, a remote history of cervical cancer (stage unknown, recent PAP smear negative), microcytosis and schizoaffective disorder. Her medications were furosemide, amlodipine, lisinopril, atenolol, atorvastatin, pantoprazole, olanzapine, levothyroxine, iron, darbepoetin, sevelamer, multivitamin and docusate.
Given the history of procedures in the left arm one should consider ischemic injury to the left median nerve. Other local complications could include compressive lesions such as an abscess or hematoma or direct nerve injury from the procedure. Carpal tunnel syndrome remains high on the differential due to its prevalence and because renal failure and hypothyroidism increase the risk of carpal tunnel syndrome.
A careful physical examination would localize the nerve or nerves involved. Examination findings that would be consistent with carpal tunnel syndrome include sensory loss in the distribution of the left median nerve, weakness of muscles innervated by the median nerve, including the abductor pollicus brevis and opponens muscles, and a Tinels and Phalens sign of the left wrist. A proximal median neuropathy resulting from ischemia or compression might also involve median‐innervated forearm muscle such as the pronator teres (forearm pronation) and flexor carpi radialis (hand flexion and abduction) muscles. Complex regional pain syndrome can be seen after a traumatic injury or surgery and is typified by severe neuropathic pain in a limb, often in combination with trophic changes in the affected extremity. A cervical radiculopathy would affect muscles supplied by the injured nerve root. For example, a C8 radiculopathy would affect all of the intrinsic hand muscles, the wrist and finger extensors, and the triceps brachii. A lower brachial plexopathy would present similarly to a lower cervical radiculopathy on clinical examination; electrodiagnostic evaluation would be necessary to distinguish these two disorders.
The patient appeared fatigued and her left hand was wrapped in blankets. Her vital signs were stable. There was no thyroid enlargement or lymphadenopathy. There was marked thenar, hypothenar and forearm atrophy on the left. Strength testing of her left hand demonstrated a grip strength of 2/5, finger extension and interosseous strength of 1/5, left wrist flexion and extension of 3/5; left biceps and triceps were 5/5. Sensation was mildly decreased to light touch, temperature, pain and proprioception throughout the left hand. Strength and sensation were intact in the right upper and bilateral lower extremities. Reflexes were 2+ throughout. The left radial pulse was diminished compared to the left ulnar that was 1+. The left hand was dry and cool. Laboratory evaluation revealed an elevated white blood cell count of 15,300/mm3, a hematocrit of 33 percent, mean corpuscular volume of 73 fL, and a normal platelet count. Electrolytes were consistent with advanced renal disease. The thyroid stimulating hormone level was normal.
The patient's examination reveals an injury to the sensory and motor components of the left ulnar, median, and distal radial nerves. The volar forearm wasting suggests proximal median motor nerve injury in the forearm. Because the triceps is spared, the weakness of finger and wrist extension implies distal radial motor nerve injury. The interosseous weakness is consistent with injury to the ulnar motor nerve. Weakness of grip and wrist flexion is less specific and it may be explained by injury to either the median or ulnar motor nerves. The diffuse sensory loss over the palmar and dorsal aspect of the left hand is consistent with neuropathic injury to the left median, ulnar, and radial sensory nerves. The preserved deep tendon reflexes are controlled by the musculocutaneous nerve (the biceps reflex) and branches arising from the proximal radial nerve (the triceps and brachioradialis reflexes), both of which are proximal to the apparent level of neuropathic insult.
Carpal tunnel syndrome is excluded since abnormalities extend beyond the median nerve distribution. The presentation is consistent with a mononeuropathy multiplex. Axonal etiologies of mononeuropathy multiplexincluding vasculitis, ischemia, neoplastic infiltration, and infectious etiologies such as Lyme diseaseare more common than demyelinating causes. Vasculitic neuropathy commonly involves the lower extremities and may have systemic symptoms, not present in this case. Neoplasm or other compressive lesions such as hematoma could explain these findings. Abscess must be considered given the leukocytosis. A lower brachial plexopathy, technically a mononeuropathy multiplex involving the proximal arm at the level of the brachial plexus, is also in the differential diagnosis. Another axonal disorder to consider would be neuralgic amyotrophy, an idiopathic form of acute brachial plexopathy associated with pain that is a complication of surgery that typically presents within a few hours to weeks of the procedure.
Demyelinating causes of mononeuropathy multiplex are less likely and include a variant of chronic inflammatory demyelinating polyneuropathy (Lewis‐Sumner syndrome) and hereditary neuropathy with liability to pressure palsies. Both processes are typically indolent and usually not painful, though the latter may present with fulminant numbness and weakness.
Nerve conduction and needle electromyography of the arm and cervical paraspinal muscles would differentiate axonal degeneration from demyelination. It would identify the affected nerves and any nerve root involvement. Given the concern for abscess or hematoma, MR neurography focused on the surgical site would also be important.
Electromyography and nerve conduction velocities demonstrated severe axonal loss of the left median, ulnar and distal radial sensory nerves consistent with acute denervation. A magnetic resonance neurogram following the course of these nerves revealed enlarged ulnar and median nerves with abnormal signal, but no compressive lesion (Fig. 1).
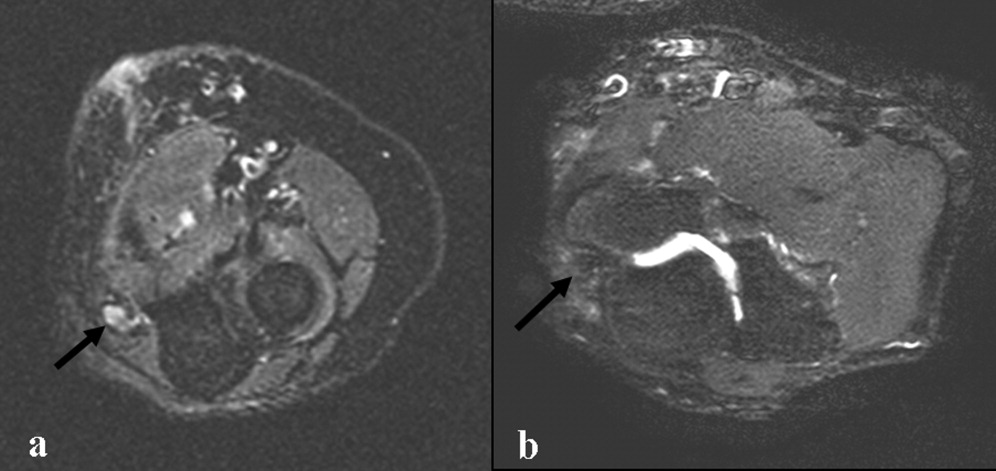
The electrodiagnostic testing is consistent with severe acute axonal injury to the left ulnar, median and radial nerves. This supports a diagnosis of mononeuropathy multiplex with axonal injury. Demyelinating causes are excluded at this point.
The MR neurogram demonstrates nonspecific nerve enlargement, which may be seen in ischemia, neoplastic processes (primary or metastatic), demyelinating disease, or, rarely, amyloidosis. Neoplastic involvement is unlikely in this case given the absence of a compressive mass lesion and the long segmental involvement of both the median and ulnar nerves. Compression from an abscess or hematoma is excluded. Neuralgic amyotrophy does not typically cause nerve enlargement.
Ischemia is the most likely diagnosis. Laboratory evaluation for vasculitis would be reasonable. Vasculitides that could present in this fashion include: polyarteritis nodosa, mixed connective tissue disease, Wegner's granulomatosis, Churg‐Strauss angiitis, Sjogren's, hepatitis C with serum cryoglobulinemia and possibly rheumatoid arthritis. Given the history of fistula placement in the affected limb, vascular sufficiency must be assessed.
Anti‐nuclear antibodies and anti‐neutrophilic cytoplasmic antibodies were negative, and a C‐ reactive protein was 5.9 mg/L (normal range, 0 to 10 mg/L). The erythrocyte sedimentation rate was 32mm/hr (normal range, 0 to 20) and serologies for hepatitis B and C were negative. There was no evidence of serum cryoglobulins.
A modestly elevated sedimentation rate and normal C‐reactive protein argue against a diagnosis of vasculitis. The negative ANA, ANCA, hepatitis serologies and cryoglobulin tests render unlikely the diagnoses of polyarteritis nodosa, Wegner's granulomatosis, Churg‐Strauss angiitis, or hepatitis related cryoglobulinemia. Eosinophilia (present in Churg‐Strauss), ENA (positive in mixed connective tissue disease), anti‐SSA and SSB (positive in Sjogren's) and a rheumatoid factor would round out this evaluation for vasculitis. A left radial sensory nerve biopsy could also be of value in diagnosing vasculitic neuropathy in this patient.
Given the evidence against vasculitis, the possibility of ischemia due to vascular insufficiency is concerning. Two ischemic complications of hemodialysis are known to cause distal multiple mononeuropathies. The first, ischemic monomelic neuropathy syndrome is seen almost exclusively in diabetics. It is characterized by the development of acute pain, weakness of the forearm and hand muscles, and sensory loss within minutes or hours of AV graft placement. Transient occlusion of the blood supply to the nerves of the forearm and hand induces nerve ischemia, but does not cause necrosis of other tissues. The nerve conduction findings in this patient are consistent with ischemic monomelic neuropathy syndrome. The delayed onset of her symptoms, however, makes this diagnosis unlikely.
The second ischemic complication of hemodialysis, and the likelier diagnosis, is vascular steal syndrome. This has a similar clinical and electrodiagnostic presentation to ischemic monomelic neuropathy syndrome, but has a latency period after surgery of days to months. Vascular steal occurs when a reversal of blood flow into the fistula steals flow from the palmar arch arteries and induces ischemia of the vasa nervorum. Vascular studies should be obtained urgently when this diagnosis is considered.
Evaluation of the arteriovenous graft and vascular surgery consultation were sought. Digital photoplethysmography revealed diminished waveforms in all fingers of the left hand. Arterial Doppler evaluation of the left upper extremity confirmed low‐velocity flow in the radial and ulnar arteries and failed to confirm flow in the brachial artery distal to the arteriovenous fistula. The patient underwent an angiogram of the left axillary and brachial arteries. There was normal flow until the level of the arteriovenous fistula but minimal flow distal to the fistula (Fig. 2).
The diminished waveforms on digital photoplethysmography are consistent with poor perfusion distally. The angiogram suggests that the multiple mononeuropathies are a consequence of ischemia from impaired blood flow.
Consulting the vascular surgeons in this setting is essential because restoring adequate blood flow to the affected nerves can prevent further loss of function. Prognosis is dependent on many factors, including the severity of the functional loss and the duration of the symptoms prior to the restoration of blood flow. The patient's severe weakness and substantial muscle atrophy, manifestations of axonal degeneration, imply a poorer prognosis for recovery of function.
Embolization of the arteriovenous fistula was performed by interventional radiology. Post embolization angiograms demonstrated improved peripheral arterial flow (Fig. 3). One day later, the patient's finger flexion and extension improved. She reported mildly decreased dysesthesias and on examination her fingers were warmer to the touch. One month after discharge, her strength continued to be impaired, though improved and she still experienced pain.
COMMENTARY
Hospitalists must be equipped to recognize urgent and potentially reversible causes of neuropathy. The hospitalist should maintain a high index of suspicion for ischemia (either due to vasculitis or vascular compromise), traumatic nerve injury, nerve compression or entrapment, lymphoma or metastatic infiltration, hepatitis C with cryoglobulinemia, Guillain‐Barre syndrome and toxic exposures. Table 1 highlights important causes of mononeuropathy multiplex and summarizes associated findings and indicated diagnostic tests for specific evaluation.
| Diagnosis | Associated features | Specific evaluation |
|---|---|---|
| Axonal neuropathies | ||
| Ischemia (including vascular steal) | Poor arterial pulses, history of vascular surgery | Digital photoplethysmography, Doppler, angiography |
| Nerve compression and trauma | History of traumatic injury, mass, infection/abscess | MR neurography |
| Lymphoma or metastatic infiltration | History of known cancer, weight loss | PET, whole body CT, bone marrow biopsy |
| Vasculitis | Waxing and waning symptoms, association with connective tissue diseases, painful | CRP, ESR, Hepatitis C, cryoglobulins, ANA, ANCA, antibodies to SSA/SSB, ENA, eosinophil count, serum complement, SPEP/UPEP, RF, nerve biopsy |
| Neurosarcoidosis | Hilar lymphadenopathy, chronic cough | Chest CT, ACE, nerve biopsy |
| Lyme | Tick bite, erythema chronicum migrans | Lyme serology |
| Leprosy | Resident of southeast Asia, skin lesions | Skin smear for acid fast bacilli (mycobacterium), nerve biopsy |
| Demyelinating neuropathies | ||
| Lewis‐Sumner syndrome (i.e. asymmetric CIDP) | Relapsing remitting or chronic progressive course, areflexia | Lumbar puncture (increased spinal fluid protein common) |
| Hereditary neuropathy with liability to pressure palsy | Family history, recurrent episodes of entrapment/compression neuropathies | Genetic testing (deletion in the gene for peripheral myelin protein‐22) |
| Multifocal motor neuropathy with conduction block | Multifocal weakness in the distal arms/legs without sensory symptoms | Only motor abnormalities on nerve conduction including conduction block |
Another challenge for hospitalists is efficient evaluation of neuropathy. A systematic framework for creating a differential diagnosis and familiarity with available diagnostic tests is crucial. Hospitalists should be aware of three broad categories of neuropathy: mononeuropathy, polyneuropathy and mononeuropathy multiplex. Electrodiagnostic testing is essential to confirm the involved nerves and distinguishes axonal from demyelinating etiologies. Ultrasound, MR neurogram and, when indicated, nerve biopsy may be useful. Table 2 reviews these diagnostic tools as well as their indications and limitations.
| Test | Indications | Limitations |
|---|---|---|
| Electrodiagnostic Testing7 | Any peripheral neuropathy, muscle or neuromuscular junction disorder | Concomitant disease can reduce accuracy |
| Detects severity, chronicity, axonal v. demyelinating, diffuse v. focal, asymmetric v. symmetric | ||
| Electromyography (EMG) | EMG | EMG |
| Monopolar/concentric needle electrode inserted into the muscle belly | Differentiates axonal v. muscle damage; sensitive for even mild axon degeneration; localizes lesions. | Patient discomfort |
| Evaluates only motor fibers | ||
| Measures action potential at rest vs. during voluntary activation | Does not detect demyelination | |
| Might not be positive in first 21 days of symptoms | ||
| Nerve Conduction Studies (NCS) | NCS | NCS |
| Sensory | High sensitivity to differentiate axon loss from demyelination; localizes lesions. | Certain sensory responses lost with aging |
| Recording electrode placed over sensory nerve | ||
| Sensory nerve stimulated distally | Sensory localizes lesion to proximal vs. distal or to dorsal root ganglion | Less sensitive for mild axonal loss |
| Measures stimulus at proximal site | ||
| Motor | Motor amount of axonal loss | |
| Recording electrode placed over muscle belly | ||
| Motor nerve stimulated proximally | ||
| Measures stimulus at muscle | ||
| Specialized NCS tests | Specialized testing can identify radiculopathy, peripheral neuropathy, myasthenia gravis | |
| Ultrasound8 | ||
| Performed with typical ultrasound equipment | Suspected nerve entrapment | Doesn't show pathologic changes within nerves |
| Clinician must localize lesion for technician and explicitly guide test process | Evidence strongest for evaluation of median and ulnar nerves and Morton's neuroma | Difficult to visualize deep nerves or nerves surrounded by fat |
| Normal nerves appear tubular with linear echoes on a longitudinal scan; honeycomb on transverse scan | Detects lesions, nerve thickening, decreased echogenicity | Small field of view unless reconstructed |
| Results operator dependent | ||
| Less accurate than MRI for tumors | ||
| MRI9, 10 MR neurography | ||
| Standard MRI equipment | Concern regarding entrapment, trauma or mass lesions | Expense |
| Optimizes nerve resolution compared with surrounding tissues | To narrow differential when clinical and electrodiagnostic studies are inconclusive | Time (1560 minutes depending on scan requested) |
| When carpal tunnel syndrome does not respond to conservative management | ||
| Detects mass lesions compressing nerves, nerve enlargement and abnormal signal (neuritis, infiltration), increased signal in denervated muscle groups (once strength is 3 of 5). These changes can be seen as early at 4 days post trauma compared to 23 weeks on EMG. | ||
| Nerve Biopsy11 | ||
| Biopsy a nerve in the region of sensory loss or of a sensory nerve demonstrating electrophysiological abnormalities (decrease risk of adverse effects and to increase the likelihood of diagnosis | Rarely necessary | Painful, often for months |
| Concomitant muscle biopsy increases likelihood of diagnosing vasculitis or sarcoidosis | Use as last resort when evaluation not definitive | Risk of bleeding and infection |
| Greatest yield in multifocal neuropathies, or suspected amyloidotic polyneuropathy, vasculitis, sarcoidosis, lepromatous neuropathy, or rare hereditary disease where no genetic testing exists | ||
| Detects inflammation, amyloid deposits, tumor infiltration | ||
| Commonly targeted nerves include: LE sural, superficial peroneal, UE superficial radial |
Ischemic steal syndrome should be considered when neuropathy develops in a limb subsequent to arterio‐venous access procedures. Any vascular network, including the vertebral, carotid and coronary arteries, is at risk for steal. A feature common to all steal syndromes is the diversion of blood away from its original destination toward a lower pressure alternative. In some cases, this leads to a reversal of arterial flow and ischemia. Ischemic complications from AV access occur in 1‐9% of patients.1 Symptoms of steal can be mild, such as self‐limited dialysis induced pain, coldness and numbness, or severe, including severe pain, sensory and motor loss.2 If vascular compromise is sufficient, gangrene can ensue. Sensory deficits usually precede motor loss and the radial pulse is commonly absent or diminished. Other findings can include pallor of the fingers, muscle atrophy, resorption of the nail bed, and gangrene or ulcerations of the fingers. Risk factors for steal include atherosclerotic disease, female gender, age greater than 60 years, diabetes mellitus, previous surgery on the same arm, and use of the brachial artery as a donor.3 Symptoms of ischemic steal typically present within the first month after surgery, but can also be delayed; there is one report of a patient presenting one year postoperatively.4
Imaging studies such as doppler and angiography can be helpful in diagnosing ischemic steal syndrome. Fistulagrams may reveal a reversal of blood flow in the distal arm and hand, but these are reserved for cases with suspected proximal obstructive arterial disease.5 Vascular imaging studies can be misleading, however, as many patients will have physiologic but asymptomatic reversal of flow. Thus, a functional assessment such as digital plethysmography is recommended, especially in cases where clinical symptoms are vague. Digital pressures less than 60mmHg demonstrated 100% sensitivity and 87% specificity in one case control study of 40 patients.6 Treatment of ischemic steal syndrome is aimed at decreasing flow through the access shunt.
In conclusion, this case highlights the importance of timely and systematic evaluation of peripheral neuropathy in the hospital setting. Neuropathy with rapid progression and high potential for permanent damage necessitates early neurologic, or in this case, vascular consultation. Hospitalists should be facile in evaluating peripheral neuropathies and recognizing the appropriate indications for diagnostic tests and procedures.
- .Upper limb ischemia after vascular access surgery: differential diagnosis and management.Sem Dial2000;13:312–315.
- ,,,,,.Steal syndrome complicating hemodialysis access.Cardiovascular Surg (London, England)1997;5:648–653.
- ,,,,,.Onset of arterial ‘steal’ following proximal angioaccess: immediate and delayed types.Nephrol Dial Transplant2003;18:2387–2390.
- ,,,,.Incidence and characteristics of patients with hand ischemia after hemodialysis access procedure.J Surg Res1998;74:8–10
- ,,,,,.Ischemic steal syndrome: a case series and review of current management.Curr Surg2006;63:130–135.
- ,,,.Use of digital pressure measurements for the diagnosis of AV access‐induced hand ischemia.Vasc Med2006;11:227–231.
- ,.Electrodiagnostic testing of nerves and muscles: when, why, and how to order.Cleve Clin J Med2005;72:37–48.
- ,.High‐resolution sonography of the peripheral nervous system—a review of the literature.Eur J Neurol2004;11:305–314.
- ,,, et al.Role of magnetic resonance imaging in entrapment and compressive neuropathy‐what, where, and how to see the peripheral nerves on the musculoskeletal magnetic resonance image: part 2. Upper extremity.Eur Radiol2007;17:509–522.
- ,,,,,.The utility of magnetic resonance imaging in evaluating peripheral nerve disorders.Muscle Nerve2002;25:314–331.
- .Indications and usefulness of nerve biopsy.Arch Neurol2002;59:1532–1535.
A 60 year‐old woman with advanced kidney disease presented with one month of progressively worsening, sharp burning pain and decreased sensation in her left hand. Cold air exacerbated the pain. She noted decreasing ability to utilize her left fingers, a weakened grip and that the muscles in her hand looked smaller.
Localized sensory and motor symptoms in a discrete region of a single limb suggest neuropathy. The lack of symptoms in the face or ipsilateral lower extremity would dissuade a clinician from considering central etiologies; the presence of neuropathic pain is uncommon for cortical lesions. The involvement of motor and sensory nerves indicates peripheral nerve involvement.
The general approach to patients with peripheral neuropathy begins with identifying the neuropathy as a mononeuropathy (involving a single nerve), a polyneuropathy (symmetric involvement of multiple nerves) or a mononeuropathy multiplex (asymmetric involvement of multiple nerves). The patient, in this case, described subacute neuropathic pain, sensory loss, and weakness in her left hand in a distribution consistent with mononeuropathy or mononeuropathy multiplex.
This patient could have carpal tunnel syndrome given its prevalence in patients with advanced renal disease. The differential diagnosis is broad, however, and includes ulnar mononeuropathy, nerve ischemia due to vasculitis or vasculopathy, lower cervical radiculopathy (though the patient does not describe neck or radicular pain), lower brachial plexopathy, and complex regional pain syndrome.
The patient was diagnosed with advanced kidney disease one year ago when biopsy revealed focal segmental glomerulosclerosis secondary to lithium. Since her diagnosis, two grafts were placed in the left upper arm in anticipation of dialysis: the first, placed seven months prior to this admission, failed to mature; the second, placed one month prior to this admission, was complicated by bleeding at the fistula site and was not yet mature. Prior to this admission she had not required hemodialysis. Her past history included hypertension, dyslipidemia, hypothyroidism, secondary hyperparathyroidism, a remote history of cervical cancer (stage unknown, recent PAP smear negative), microcytosis and schizoaffective disorder. Her medications were furosemide, amlodipine, lisinopril, atenolol, atorvastatin, pantoprazole, olanzapine, levothyroxine, iron, darbepoetin, sevelamer, multivitamin and docusate.
Given the history of procedures in the left arm one should consider ischemic injury to the left median nerve. Other local complications could include compressive lesions such as an abscess or hematoma or direct nerve injury from the procedure. Carpal tunnel syndrome remains high on the differential due to its prevalence and because renal failure and hypothyroidism increase the risk of carpal tunnel syndrome.
A careful physical examination would localize the nerve or nerves involved. Examination findings that would be consistent with carpal tunnel syndrome include sensory loss in the distribution of the left median nerve, weakness of muscles innervated by the median nerve, including the abductor pollicus brevis and opponens muscles, and a Tinels and Phalens sign of the left wrist. A proximal median neuropathy resulting from ischemia or compression might also involve median‐innervated forearm muscle such as the pronator teres (forearm pronation) and flexor carpi radialis (hand flexion and abduction) muscles. Complex regional pain syndrome can be seen after a traumatic injury or surgery and is typified by severe neuropathic pain in a limb, often in combination with trophic changes in the affected extremity. A cervical radiculopathy would affect muscles supplied by the injured nerve root. For example, a C8 radiculopathy would affect all of the intrinsic hand muscles, the wrist and finger extensors, and the triceps brachii. A lower brachial plexopathy would present similarly to a lower cervical radiculopathy on clinical examination; electrodiagnostic evaluation would be necessary to distinguish these two disorders.
The patient appeared fatigued and her left hand was wrapped in blankets. Her vital signs were stable. There was no thyroid enlargement or lymphadenopathy. There was marked thenar, hypothenar and forearm atrophy on the left. Strength testing of her left hand demonstrated a grip strength of 2/5, finger extension and interosseous strength of 1/5, left wrist flexion and extension of 3/5; left biceps and triceps were 5/5. Sensation was mildly decreased to light touch, temperature, pain and proprioception throughout the left hand. Strength and sensation were intact in the right upper and bilateral lower extremities. Reflexes were 2+ throughout. The left radial pulse was diminished compared to the left ulnar that was 1+. The left hand was dry and cool. Laboratory evaluation revealed an elevated white blood cell count of 15,300/mm3, a hematocrit of 33 percent, mean corpuscular volume of 73 fL, and a normal platelet count. Electrolytes were consistent with advanced renal disease. The thyroid stimulating hormone level was normal.
The patient's examination reveals an injury to the sensory and motor components of the left ulnar, median, and distal radial nerves. The volar forearm wasting suggests proximal median motor nerve injury in the forearm. Because the triceps is spared, the weakness of finger and wrist extension implies distal radial motor nerve injury. The interosseous weakness is consistent with injury to the ulnar motor nerve. Weakness of grip and wrist flexion is less specific and it may be explained by injury to either the median or ulnar motor nerves. The diffuse sensory loss over the palmar and dorsal aspect of the left hand is consistent with neuropathic injury to the left median, ulnar, and radial sensory nerves. The preserved deep tendon reflexes are controlled by the musculocutaneous nerve (the biceps reflex) and branches arising from the proximal radial nerve (the triceps and brachioradialis reflexes), both of which are proximal to the apparent level of neuropathic insult.
Carpal tunnel syndrome is excluded since abnormalities extend beyond the median nerve distribution. The presentation is consistent with a mononeuropathy multiplex. Axonal etiologies of mononeuropathy multiplexincluding vasculitis, ischemia, neoplastic infiltration, and infectious etiologies such as Lyme diseaseare more common than demyelinating causes. Vasculitic neuropathy commonly involves the lower extremities and may have systemic symptoms, not present in this case. Neoplasm or other compressive lesions such as hematoma could explain these findings. Abscess must be considered given the leukocytosis. A lower brachial plexopathy, technically a mononeuropathy multiplex involving the proximal arm at the level of the brachial plexus, is also in the differential diagnosis. Another axonal disorder to consider would be neuralgic amyotrophy, an idiopathic form of acute brachial plexopathy associated with pain that is a complication of surgery that typically presents within a few hours to weeks of the procedure.
Demyelinating causes of mononeuropathy multiplex are less likely and include a variant of chronic inflammatory demyelinating polyneuropathy (Lewis‐Sumner syndrome) and hereditary neuropathy with liability to pressure palsies. Both processes are typically indolent and usually not painful, though the latter may present with fulminant numbness and weakness.
Nerve conduction and needle electromyography of the arm and cervical paraspinal muscles would differentiate axonal degeneration from demyelination. It would identify the affected nerves and any nerve root involvement. Given the concern for abscess or hematoma, MR neurography focused on the surgical site would also be important.
Electromyography and nerve conduction velocities demonstrated severe axonal loss of the left median, ulnar and distal radial sensory nerves consistent with acute denervation. A magnetic resonance neurogram following the course of these nerves revealed enlarged ulnar and median nerves with abnormal signal, but no compressive lesion (Fig. 1).

The electrodiagnostic testing is consistent with severe acute axonal injury to the left ulnar, median and radial nerves. This supports a diagnosis of mononeuropathy multiplex with axonal injury. Demyelinating causes are excluded at this point.
The MR neurogram demonstrates nonspecific nerve enlargement, which may be seen in ischemia, neoplastic processes (primary or metastatic), demyelinating disease, or, rarely, amyloidosis. Neoplastic involvement is unlikely in this case given the absence of a compressive mass lesion and the long segmental involvement of both the median and ulnar nerves. Compression from an abscess or hematoma is excluded. Neuralgic amyotrophy does not typically cause nerve enlargement.
Ischemia is the most likely diagnosis. Laboratory evaluation for vasculitis would be reasonable. Vasculitides that could present in this fashion include: polyarteritis nodosa, mixed connective tissue disease, Wegner's granulomatosis, Churg‐Strauss angiitis, Sjogren's, hepatitis C with serum cryoglobulinemia and possibly rheumatoid arthritis. Given the history of fistula placement in the affected limb, vascular sufficiency must be assessed.
Anti‐nuclear antibodies and anti‐neutrophilic cytoplasmic antibodies were negative, and a C‐ reactive protein was 5.9 mg/L (normal range, 0 to 10 mg/L). The erythrocyte sedimentation rate was 32mm/hr (normal range, 0 to 20) and serologies for hepatitis B and C were negative. There was no evidence of serum cryoglobulins.
A modestly elevated sedimentation rate and normal C‐reactive protein argue against a diagnosis of vasculitis. The negative ANA, ANCA, hepatitis serologies and cryoglobulin tests render unlikely the diagnoses of polyarteritis nodosa, Wegner's granulomatosis, Churg‐Strauss angiitis, or hepatitis related cryoglobulinemia. Eosinophilia (present in Churg‐Strauss), ENA (positive in mixed connective tissue disease), anti‐SSA and SSB (positive in Sjogren's) and a rheumatoid factor would round out this evaluation for vasculitis. A left radial sensory nerve biopsy could also be of value in diagnosing vasculitic neuropathy in this patient.
Given the evidence against vasculitis, the possibility of ischemia due to vascular insufficiency is concerning. Two ischemic complications of hemodialysis are known to cause distal multiple mononeuropathies. The first, ischemic monomelic neuropathy syndrome is seen almost exclusively in diabetics. It is characterized by the development of acute pain, weakness of the forearm and hand muscles, and sensory loss within minutes or hours of AV graft placement. Transient occlusion of the blood supply to the nerves of the forearm and hand induces nerve ischemia, but does not cause necrosis of other tissues. The nerve conduction findings in this patient are consistent with ischemic monomelic neuropathy syndrome. The delayed onset of her symptoms, however, makes this diagnosis unlikely.
The second ischemic complication of hemodialysis, and the likelier diagnosis, is vascular steal syndrome. This has a similar clinical and electrodiagnostic presentation to ischemic monomelic neuropathy syndrome, but has a latency period after surgery of days to months. Vascular steal occurs when a reversal of blood flow into the fistula steals flow from the palmar arch arteries and induces ischemia of the vasa nervorum. Vascular studies should be obtained urgently when this diagnosis is considered.
Evaluation of the arteriovenous graft and vascular surgery consultation were sought. Digital photoplethysmography revealed diminished waveforms in all fingers of the left hand. Arterial Doppler evaluation of the left upper extremity confirmed low‐velocity flow in the radial and ulnar arteries and failed to confirm flow in the brachial artery distal to the arteriovenous fistula. The patient underwent an angiogram of the left axillary and brachial arteries. There was normal flow until the level of the arteriovenous fistula but minimal flow distal to the fistula (Fig. 2).

The diminished waveforms on digital photoplethysmography are consistent with poor perfusion distally. The angiogram suggests that the multiple mononeuropathies are a consequence of ischemia from impaired blood flow.
Consulting the vascular surgeons in this setting is essential because restoring adequate blood flow to the affected nerves can prevent further loss of function. Prognosis is dependent on many factors, including the severity of the functional loss and the duration of the symptoms prior to the restoration of blood flow. The patient's severe weakness and substantial muscle atrophy, manifestations of axonal degeneration, imply a poorer prognosis for recovery of function.
Embolization of the arteriovenous fistula was performed by interventional radiology. Post embolization angiograms demonstrated improved peripheral arterial flow (Fig. 3). One day later, the patient's finger flexion and extension improved. She reported mildly decreased dysesthesias and on examination her fingers were warmer to the touch. One month after discharge, her strength continued to be impaired, though improved and she still experienced pain.

COMMENTARY
Hospitalists must be equipped to recognize urgent and potentially reversible causes of neuropathy. The hospitalist should maintain a high index of suspicion for ischemia (either due to vasculitis or vascular compromise), traumatic nerve injury, nerve compression or entrapment, lymphoma or metastatic infiltration, hepatitis C with cryoglobulinemia, Guillain‐Barre syndrome and toxic exposures. Table 1 highlights important causes of mononeuropathy multiplex and summarizes associated findings and indicated diagnostic tests for specific evaluation.
| Diagnosis | Associated features | Specific evaluation |
|---|---|---|
| Axonal neuropathies | ||
| Ischemia (including vascular steal) | Poor arterial pulses, history of vascular surgery | Digital photoplethysmography, Doppler, angiography |
| Nerve compression and trauma | History of traumatic injury, mass, infection/abscess | MR neurography |
| Lymphoma or metastatic infiltration | History of known cancer, weight loss | PET, whole body CT, bone marrow biopsy |
| Vasculitis | Waxing and waning symptoms, association with connective tissue diseases, painful | CRP, ESR, Hepatitis C, cryoglobulins, ANA, ANCA, antibodies to SSA/SSB, ENA, eosinophil count, serum complement, SPEP/UPEP, RF, nerve biopsy |
| Neurosarcoidosis | Hilar lymphadenopathy, chronic cough | Chest CT, ACE, nerve biopsy |
| Lyme | Tick bite, erythema chronicum migrans | Lyme serology |
| Leprosy | Resident of southeast Asia, skin lesions | Skin smear for acid fast bacilli (mycobacterium), nerve biopsy |
| Demyelinating neuropathies | ||
| Lewis‐Sumner syndrome (i.e. asymmetric CIDP) | Relapsing remitting or chronic progressive course, areflexia | Lumbar puncture (increased spinal fluid protein common) |
| Hereditary neuropathy with liability to pressure palsy | Family history, recurrent episodes of entrapment/compression neuropathies | Genetic testing (deletion in the gene for peripheral myelin protein‐22) |
| Multifocal motor neuropathy with conduction block | Multifocal weakness in the distal arms/legs without sensory symptoms | Only motor abnormalities on nerve conduction including conduction block |
Another challenge for hospitalists is efficient evaluation of neuropathy. A systematic framework for creating a differential diagnosis and familiarity with available diagnostic tests is crucial. Hospitalists should be aware of three broad categories of neuropathy: mononeuropathy, polyneuropathy and mononeuropathy multiplex. Electrodiagnostic testing is essential to confirm the involved nerves and distinguishes axonal from demyelinating etiologies. Ultrasound, MR neurogram and, when indicated, nerve biopsy may be useful. Table 2 reviews these diagnostic tools as well as their indications and limitations.
| Test | Indications | Limitations |
|---|---|---|
| Electrodiagnostic Testing7 | Any peripheral neuropathy, muscle or neuromuscular junction disorder | Concomitant disease can reduce accuracy |
| Detects severity, chronicity, axonal v. demyelinating, diffuse v. focal, asymmetric v. symmetric | ||
| Electromyography (EMG) | EMG | EMG |
| Monopolar/concentric needle electrode inserted into the muscle belly | Differentiates axonal v. muscle damage; sensitive for even mild axon degeneration; localizes lesions. | Patient discomfort |
| Evaluates only motor fibers | ||
| Measures action potential at rest vs. during voluntary activation | Does not detect demyelination | |
| Might not be positive in first 21 days of symptoms | ||
| Nerve Conduction Studies (NCS) | NCS | NCS |
| Sensory | High sensitivity to differentiate axon loss from demyelination; localizes lesions. | Certain sensory responses lost with aging |
| Recording electrode placed over sensory nerve | ||
| Sensory nerve stimulated distally | Sensory localizes lesion to proximal vs. distal or to dorsal root ganglion | Less sensitive for mild axonal loss |
| Measures stimulus at proximal site | ||
| Motor | Motor amount of axonal loss | |
| Recording electrode placed over muscle belly | ||
| Motor nerve stimulated proximally | ||
| Measures stimulus at muscle | ||
| Specialized NCS tests | Specialized testing can identify radiculopathy, peripheral neuropathy, myasthenia gravis | |
| Ultrasound8 | ||
| Performed with typical ultrasound equipment | Suspected nerve entrapment | Doesn't show pathologic changes within nerves |
| Clinician must localize lesion for technician and explicitly guide test process | Evidence strongest for evaluation of median and ulnar nerves and Morton's neuroma | Difficult to visualize deep nerves or nerves surrounded by fat |
| Normal nerves appear tubular with linear echoes on a longitudinal scan; honeycomb on transverse scan | Detects lesions, nerve thickening, decreased echogenicity | Small field of view unless reconstructed |
| Results operator dependent | ||
| Less accurate than MRI for tumors | ||
| MRI9, 10 MR neurography | ||
| Standard MRI equipment | Concern regarding entrapment, trauma or mass lesions | Expense |
| Optimizes nerve resolution compared with surrounding tissues | To narrow differential when clinical and electrodiagnostic studies are inconclusive | Time (1560 minutes depending on scan requested) |
| When carpal tunnel syndrome does not respond to conservative management | ||
| Detects mass lesions compressing nerves, nerve enlargement and abnormal signal (neuritis, infiltration), increased signal in denervated muscle groups (once strength is 3 of 5). These changes can be seen as early at 4 days post trauma compared to 23 weeks on EMG. | ||
| Nerve Biopsy11 | ||
| Biopsy a nerve in the region of sensory loss or of a sensory nerve demonstrating electrophysiological abnormalities (decrease risk of adverse effects and to increase the likelihood of diagnosis | Rarely necessary | Painful, often for months |
| Concomitant muscle biopsy increases likelihood of diagnosing vasculitis or sarcoidosis | Use as last resort when evaluation not definitive | Risk of bleeding and infection |
| Greatest yield in multifocal neuropathies, or suspected amyloidotic polyneuropathy, vasculitis, sarcoidosis, lepromatous neuropathy, or rare hereditary disease where no genetic testing exists | ||
| Detects inflammation, amyloid deposits, tumor infiltration | ||
| Commonly targeted nerves include: LE sural, superficial peroneal, UE superficial radial |
Ischemic steal syndrome should be considered when neuropathy develops in a limb subsequent to arterio‐venous access procedures. Any vascular network, including the vertebral, carotid and coronary arteries, is at risk for steal. A feature common to all steal syndromes is the diversion of blood away from its original destination toward a lower pressure alternative. In some cases, this leads to a reversal of arterial flow and ischemia. Ischemic complications from AV access occur in 1‐9% of patients.1 Symptoms of steal can be mild, such as self‐limited dialysis induced pain, coldness and numbness, or severe, including severe pain, sensory and motor loss.2 If vascular compromise is sufficient, gangrene can ensue. Sensory deficits usually precede motor loss and the radial pulse is commonly absent or diminished. Other findings can include pallor of the fingers, muscle atrophy, resorption of the nail bed, and gangrene or ulcerations of the fingers. Risk factors for steal include atherosclerotic disease, female gender, age greater than 60 years, diabetes mellitus, previous surgery on the same arm, and use of the brachial artery as a donor.3 Symptoms of ischemic steal typically present within the first month after surgery, but can also be delayed; there is one report of a patient presenting one year postoperatively.4
Imaging studies such as doppler and angiography can be helpful in diagnosing ischemic steal syndrome. Fistulagrams may reveal a reversal of blood flow in the distal arm and hand, but these are reserved for cases with suspected proximal obstructive arterial disease.5 Vascular imaging studies can be misleading, however, as many patients will have physiologic but asymptomatic reversal of flow. Thus, a functional assessment such as digital plethysmography is recommended, especially in cases where clinical symptoms are vague. Digital pressures less than 60mmHg demonstrated 100% sensitivity and 87% specificity in one case control study of 40 patients.6 Treatment of ischemic steal syndrome is aimed at decreasing flow through the access shunt.
In conclusion, this case highlights the importance of timely and systematic evaluation of peripheral neuropathy in the hospital setting. Neuropathy with rapid progression and high potential for permanent damage necessitates early neurologic, or in this case, vascular consultation. Hospitalists should be facile in evaluating peripheral neuropathies and recognizing the appropriate indications for diagnostic tests and procedures.
A 60 year‐old woman with advanced kidney disease presented with one month of progressively worsening, sharp burning pain and decreased sensation in her left hand. Cold air exacerbated the pain. She noted decreasing ability to utilize her left fingers, a weakened grip and that the muscles in her hand looked smaller.
Localized sensory and motor symptoms in a discrete region of a single limb suggest neuropathy. The lack of symptoms in the face or ipsilateral lower extremity would dissuade a clinician from considering central etiologies; the presence of neuropathic pain is uncommon for cortical lesions. The involvement of motor and sensory nerves indicates peripheral nerve involvement.
The general approach to patients with peripheral neuropathy begins with identifying the neuropathy as a mononeuropathy (involving a single nerve), a polyneuropathy (symmetric involvement of multiple nerves) or a mononeuropathy multiplex (asymmetric involvement of multiple nerves). The patient, in this case, described subacute neuropathic pain, sensory loss, and weakness in her left hand in a distribution consistent with mononeuropathy or mononeuropathy multiplex.
This patient could have carpal tunnel syndrome given its prevalence in patients with advanced renal disease. The differential diagnosis is broad, however, and includes ulnar mononeuropathy, nerve ischemia due to vasculitis or vasculopathy, lower cervical radiculopathy (though the patient does not describe neck or radicular pain), lower brachial plexopathy, and complex regional pain syndrome.
The patient was diagnosed with advanced kidney disease one year ago when biopsy revealed focal segmental glomerulosclerosis secondary to lithium. Since her diagnosis, two grafts were placed in the left upper arm in anticipation of dialysis: the first, placed seven months prior to this admission, failed to mature; the second, placed one month prior to this admission, was complicated by bleeding at the fistula site and was not yet mature. Prior to this admission she had not required hemodialysis. Her past history included hypertension, dyslipidemia, hypothyroidism, secondary hyperparathyroidism, a remote history of cervical cancer (stage unknown, recent PAP smear negative), microcytosis and schizoaffective disorder. Her medications were furosemide, amlodipine, lisinopril, atenolol, atorvastatin, pantoprazole, olanzapine, levothyroxine, iron, darbepoetin, sevelamer, multivitamin and docusate.
Given the history of procedures in the left arm one should consider ischemic injury to the left median nerve. Other local complications could include compressive lesions such as an abscess or hematoma or direct nerve injury from the procedure. Carpal tunnel syndrome remains high on the differential due to its prevalence and because renal failure and hypothyroidism increase the risk of carpal tunnel syndrome.
A careful physical examination would localize the nerve or nerves involved. Examination findings that would be consistent with carpal tunnel syndrome include sensory loss in the distribution of the left median nerve, weakness of muscles innervated by the median nerve, including the abductor pollicus brevis and opponens muscles, and a Tinels and Phalens sign of the left wrist. A proximal median neuropathy resulting from ischemia or compression might also involve median‐innervated forearm muscle such as the pronator teres (forearm pronation) and flexor carpi radialis (hand flexion and abduction) muscles. Complex regional pain syndrome can be seen after a traumatic injury or surgery and is typified by severe neuropathic pain in a limb, often in combination with trophic changes in the affected extremity. A cervical radiculopathy would affect muscles supplied by the injured nerve root. For example, a C8 radiculopathy would affect all of the intrinsic hand muscles, the wrist and finger extensors, and the triceps brachii. A lower brachial plexopathy would present similarly to a lower cervical radiculopathy on clinical examination; electrodiagnostic evaluation would be necessary to distinguish these two disorders.
The patient appeared fatigued and her left hand was wrapped in blankets. Her vital signs were stable. There was no thyroid enlargement or lymphadenopathy. There was marked thenar, hypothenar and forearm atrophy on the left. Strength testing of her left hand demonstrated a grip strength of 2/5, finger extension and interosseous strength of 1/5, left wrist flexion and extension of 3/5; left biceps and triceps were 5/5. Sensation was mildly decreased to light touch, temperature, pain and proprioception throughout the left hand. Strength and sensation were intact in the right upper and bilateral lower extremities. Reflexes were 2+ throughout. The left radial pulse was diminished compared to the left ulnar that was 1+. The left hand was dry and cool. Laboratory evaluation revealed an elevated white blood cell count of 15,300/mm3, a hematocrit of 33 percent, mean corpuscular volume of 73 fL, and a normal platelet count. Electrolytes were consistent with advanced renal disease. The thyroid stimulating hormone level was normal.
The patient's examination reveals an injury to the sensory and motor components of the left ulnar, median, and distal radial nerves. The volar forearm wasting suggests proximal median motor nerve injury in the forearm. Because the triceps is spared, the weakness of finger and wrist extension implies distal radial motor nerve injury. The interosseous weakness is consistent with injury to the ulnar motor nerve. Weakness of grip and wrist flexion is less specific and it may be explained by injury to either the median or ulnar motor nerves. The diffuse sensory loss over the palmar and dorsal aspect of the left hand is consistent with neuropathic injury to the left median, ulnar, and radial sensory nerves. The preserved deep tendon reflexes are controlled by the musculocutaneous nerve (the biceps reflex) and branches arising from the proximal radial nerve (the triceps and brachioradialis reflexes), both of which are proximal to the apparent level of neuropathic insult.
Carpal tunnel syndrome is excluded since abnormalities extend beyond the median nerve distribution. The presentation is consistent with a mononeuropathy multiplex. Axonal etiologies of mononeuropathy multiplexincluding vasculitis, ischemia, neoplastic infiltration, and infectious etiologies such as Lyme diseaseare more common than demyelinating causes. Vasculitic neuropathy commonly involves the lower extremities and may have systemic symptoms, not present in this case. Neoplasm or other compressive lesions such as hematoma could explain these findings. Abscess must be considered given the leukocytosis. A lower brachial plexopathy, technically a mononeuropathy multiplex involving the proximal arm at the level of the brachial plexus, is also in the differential diagnosis. Another axonal disorder to consider would be neuralgic amyotrophy, an idiopathic form of acute brachial plexopathy associated with pain that is a complication of surgery that typically presents within a few hours to weeks of the procedure.
Demyelinating causes of mononeuropathy multiplex are less likely and include a variant of chronic inflammatory demyelinating polyneuropathy (Lewis‐Sumner syndrome) and hereditary neuropathy with liability to pressure palsies. Both processes are typically indolent and usually not painful, though the latter may present with fulminant numbness and weakness.
Nerve conduction and needle electromyography of the arm and cervical paraspinal muscles would differentiate axonal degeneration from demyelination. It would identify the affected nerves and any nerve root involvement. Given the concern for abscess or hematoma, MR neurography focused on the surgical site would also be important.
Electromyography and nerve conduction velocities demonstrated severe axonal loss of the left median, ulnar and distal radial sensory nerves consistent with acute denervation. A magnetic resonance neurogram following the course of these nerves revealed enlarged ulnar and median nerves with abnormal signal, but no compressive lesion (Fig. 1).

The electrodiagnostic testing is consistent with severe acute axonal injury to the left ulnar, median and radial nerves. This supports a diagnosis of mononeuropathy multiplex with axonal injury. Demyelinating causes are excluded at this point.
The MR neurogram demonstrates nonspecific nerve enlargement, which may be seen in ischemia, neoplastic processes (primary or metastatic), demyelinating disease, or, rarely, amyloidosis. Neoplastic involvement is unlikely in this case given the absence of a compressive mass lesion and the long segmental involvement of both the median and ulnar nerves. Compression from an abscess or hematoma is excluded. Neuralgic amyotrophy does not typically cause nerve enlargement.
Ischemia is the most likely diagnosis. Laboratory evaluation for vasculitis would be reasonable. Vasculitides that could present in this fashion include: polyarteritis nodosa, mixed connective tissue disease, Wegner's granulomatosis, Churg‐Strauss angiitis, Sjogren's, hepatitis C with serum cryoglobulinemia and possibly rheumatoid arthritis. Given the history of fistula placement in the affected limb, vascular sufficiency must be assessed.
Anti‐nuclear antibodies and anti‐neutrophilic cytoplasmic antibodies were negative, and a C‐ reactive protein was 5.9 mg/L (normal range, 0 to 10 mg/L). The erythrocyte sedimentation rate was 32mm/hr (normal range, 0 to 20) and serologies for hepatitis B and C were negative. There was no evidence of serum cryoglobulins.
A modestly elevated sedimentation rate and normal C‐reactive protein argue against a diagnosis of vasculitis. The negative ANA, ANCA, hepatitis serologies and cryoglobulin tests render unlikely the diagnoses of polyarteritis nodosa, Wegner's granulomatosis, Churg‐Strauss angiitis, or hepatitis related cryoglobulinemia. Eosinophilia (present in Churg‐Strauss), ENA (positive in mixed connective tissue disease), anti‐SSA and SSB (positive in Sjogren's) and a rheumatoid factor would round out this evaluation for vasculitis. A left radial sensory nerve biopsy could also be of value in diagnosing vasculitic neuropathy in this patient.
Given the evidence against vasculitis, the possibility of ischemia due to vascular insufficiency is concerning. Two ischemic complications of hemodialysis are known to cause distal multiple mononeuropathies. The first, ischemic monomelic neuropathy syndrome is seen almost exclusively in diabetics. It is characterized by the development of acute pain, weakness of the forearm and hand muscles, and sensory loss within minutes or hours of AV graft placement. Transient occlusion of the blood supply to the nerves of the forearm and hand induces nerve ischemia, but does not cause necrosis of other tissues. The nerve conduction findings in this patient are consistent with ischemic monomelic neuropathy syndrome. The delayed onset of her symptoms, however, makes this diagnosis unlikely.
The second ischemic complication of hemodialysis, and the likelier diagnosis, is vascular steal syndrome. This has a similar clinical and electrodiagnostic presentation to ischemic monomelic neuropathy syndrome, but has a latency period after surgery of days to months. Vascular steal occurs when a reversal of blood flow into the fistula steals flow from the palmar arch arteries and induces ischemia of the vasa nervorum. Vascular studies should be obtained urgently when this diagnosis is considered.
Evaluation of the arteriovenous graft and vascular surgery consultation were sought. Digital photoplethysmography revealed diminished waveforms in all fingers of the left hand. Arterial Doppler evaluation of the left upper extremity confirmed low‐velocity flow in the radial and ulnar arteries and failed to confirm flow in the brachial artery distal to the arteriovenous fistula. The patient underwent an angiogram of the left axillary and brachial arteries. There was normal flow until the level of the arteriovenous fistula but minimal flow distal to the fistula (Fig. 2).

The diminished waveforms on digital photoplethysmography are consistent with poor perfusion distally. The angiogram suggests that the multiple mononeuropathies are a consequence of ischemia from impaired blood flow.
Consulting the vascular surgeons in this setting is essential because restoring adequate blood flow to the affected nerves can prevent further loss of function. Prognosis is dependent on many factors, including the severity of the functional loss and the duration of the symptoms prior to the restoration of blood flow. The patient's severe weakness and substantial muscle atrophy, manifestations of axonal degeneration, imply a poorer prognosis for recovery of function.
Embolization of the arteriovenous fistula was performed by interventional radiology. Post embolization angiograms demonstrated improved peripheral arterial flow (Fig. 3). One day later, the patient's finger flexion and extension improved. She reported mildly decreased dysesthesias and on examination her fingers were warmer to the touch. One month after discharge, her strength continued to be impaired, though improved and she still experienced pain.

COMMENTARY
Hospitalists must be equipped to recognize urgent and potentially reversible causes of neuropathy. The hospitalist should maintain a high index of suspicion for ischemia (either due to vasculitis or vascular compromise), traumatic nerve injury, nerve compression or entrapment, lymphoma or metastatic infiltration, hepatitis C with cryoglobulinemia, Guillain‐Barre syndrome and toxic exposures. Table 1 highlights important causes of mononeuropathy multiplex and summarizes associated findings and indicated diagnostic tests for specific evaluation.
| Diagnosis | Associated features | Specific evaluation |
|---|---|---|
| Axonal neuropathies | ||
| Ischemia (including vascular steal) | Poor arterial pulses, history of vascular surgery | Digital photoplethysmography, Doppler, angiography |
| Nerve compression and trauma | History of traumatic injury, mass, infection/abscess | MR neurography |
| Lymphoma or metastatic infiltration | History of known cancer, weight loss | PET, whole body CT, bone marrow biopsy |
| Vasculitis | Waxing and waning symptoms, association with connective tissue diseases, painful | CRP, ESR, Hepatitis C, cryoglobulins, ANA, ANCA, antibodies to SSA/SSB, ENA, eosinophil count, serum complement, SPEP/UPEP, RF, nerve biopsy |
| Neurosarcoidosis | Hilar lymphadenopathy, chronic cough | Chest CT, ACE, nerve biopsy |
| Lyme | Tick bite, erythema chronicum migrans | Lyme serology |
| Leprosy | Resident of southeast Asia, skin lesions | Skin smear for acid fast bacilli (mycobacterium), nerve biopsy |
| Demyelinating neuropathies | ||
| Lewis‐Sumner syndrome (i.e. asymmetric CIDP) | Relapsing remitting or chronic progressive course, areflexia | Lumbar puncture (increased spinal fluid protein common) |
| Hereditary neuropathy with liability to pressure palsy | Family history, recurrent episodes of entrapment/compression neuropathies | Genetic testing (deletion in the gene for peripheral myelin protein‐22) |
| Multifocal motor neuropathy with conduction block | Multifocal weakness in the distal arms/legs without sensory symptoms | Only motor abnormalities on nerve conduction including conduction block |
Another challenge for hospitalists is efficient evaluation of neuropathy. A systematic framework for creating a differential diagnosis and familiarity with available diagnostic tests is crucial. Hospitalists should be aware of three broad categories of neuropathy: mononeuropathy, polyneuropathy and mononeuropathy multiplex. Electrodiagnostic testing is essential to confirm the involved nerves and distinguishes axonal from demyelinating etiologies. Ultrasound, MR neurogram and, when indicated, nerve biopsy may be useful. Table 2 reviews these diagnostic tools as well as their indications and limitations.
| Test | Indications | Limitations |
|---|---|---|
| Electrodiagnostic Testing7 | Any peripheral neuropathy, muscle or neuromuscular junction disorder | Concomitant disease can reduce accuracy |
| Detects severity, chronicity, axonal v. demyelinating, diffuse v. focal, asymmetric v. symmetric | ||
| Electromyography (EMG) | EMG | EMG |
| Monopolar/concentric needle electrode inserted into the muscle belly | Differentiates axonal v. muscle damage; sensitive for even mild axon degeneration; localizes lesions. | Patient discomfort |
| Evaluates only motor fibers | ||
| Measures action potential at rest vs. during voluntary activation | Does not detect demyelination | |
| Might not be positive in first 21 days of symptoms | ||
| Nerve Conduction Studies (NCS) | NCS | NCS |
| Sensory | High sensitivity to differentiate axon loss from demyelination; localizes lesions. | Certain sensory responses lost with aging |
| Recording electrode placed over sensory nerve | ||
| Sensory nerve stimulated distally | Sensory localizes lesion to proximal vs. distal or to dorsal root ganglion | Less sensitive for mild axonal loss |
| Measures stimulus at proximal site | ||
| Motor | Motor amount of axonal loss | |
| Recording electrode placed over muscle belly | ||
| Motor nerve stimulated proximally | ||
| Measures stimulus at muscle | ||
| Specialized NCS tests | Specialized testing can identify radiculopathy, peripheral neuropathy, myasthenia gravis | |
| Ultrasound8 | ||
| Performed with typical ultrasound equipment | Suspected nerve entrapment | Doesn't show pathologic changes within nerves |
| Clinician must localize lesion for technician and explicitly guide test process | Evidence strongest for evaluation of median and ulnar nerves and Morton's neuroma | Difficult to visualize deep nerves or nerves surrounded by fat |
| Normal nerves appear tubular with linear echoes on a longitudinal scan; honeycomb on transverse scan | Detects lesions, nerve thickening, decreased echogenicity | Small field of view unless reconstructed |
| Results operator dependent | ||
| Less accurate than MRI for tumors | ||
| MRI9, 10 MR neurography | ||
| Standard MRI equipment | Concern regarding entrapment, trauma or mass lesions | Expense |
| Optimizes nerve resolution compared with surrounding tissues | To narrow differential when clinical and electrodiagnostic studies are inconclusive | Time (1560 minutes depending on scan requested) |
| When carpal tunnel syndrome does not respond to conservative management | ||
| Detects mass lesions compressing nerves, nerve enlargement and abnormal signal (neuritis, infiltration), increased signal in denervated muscle groups (once strength is 3 of 5). These changes can be seen as early at 4 days post trauma compared to 23 weeks on EMG. | ||
| Nerve Biopsy11 | ||
| Biopsy a nerve in the region of sensory loss or of a sensory nerve demonstrating electrophysiological abnormalities (decrease risk of adverse effects and to increase the likelihood of diagnosis | Rarely necessary | Painful, often for months |
| Concomitant muscle biopsy increases likelihood of diagnosing vasculitis or sarcoidosis | Use as last resort when evaluation not definitive | Risk of bleeding and infection |
| Greatest yield in multifocal neuropathies, or suspected amyloidotic polyneuropathy, vasculitis, sarcoidosis, lepromatous neuropathy, or rare hereditary disease where no genetic testing exists | ||
| Detects inflammation, amyloid deposits, tumor infiltration | ||
| Commonly targeted nerves include: LE sural, superficial peroneal, UE superficial radial |
Ischemic steal syndrome should be considered when neuropathy develops in a limb subsequent to arterio‐venous access procedures. Any vascular network, including the vertebral, carotid and coronary arteries, is at risk for steal. A feature common to all steal syndromes is the diversion of blood away from its original destination toward a lower pressure alternative. In some cases, this leads to a reversal of arterial flow and ischemia. Ischemic complications from AV access occur in 1‐9% of patients.1 Symptoms of steal can be mild, such as self‐limited dialysis induced pain, coldness and numbness, or severe, including severe pain, sensory and motor loss.2 If vascular compromise is sufficient, gangrene can ensue. Sensory deficits usually precede motor loss and the radial pulse is commonly absent or diminished. Other findings can include pallor of the fingers, muscle atrophy, resorption of the nail bed, and gangrene or ulcerations of the fingers. Risk factors for steal include atherosclerotic disease, female gender, age greater than 60 years, diabetes mellitus, previous surgery on the same arm, and use of the brachial artery as a donor.3 Symptoms of ischemic steal typically present within the first month after surgery, but can also be delayed; there is one report of a patient presenting one year postoperatively.4
Imaging studies such as doppler and angiography can be helpful in diagnosing ischemic steal syndrome. Fistulagrams may reveal a reversal of blood flow in the distal arm and hand, but these are reserved for cases with suspected proximal obstructive arterial disease.5 Vascular imaging studies can be misleading, however, as many patients will have physiologic but asymptomatic reversal of flow. Thus, a functional assessment such as digital plethysmography is recommended, especially in cases where clinical symptoms are vague. Digital pressures less than 60mmHg demonstrated 100% sensitivity and 87% specificity in one case control study of 40 patients.6 Treatment of ischemic steal syndrome is aimed at decreasing flow through the access shunt.
In conclusion, this case highlights the importance of timely and systematic evaluation of peripheral neuropathy in the hospital setting. Neuropathy with rapid progression and high potential for permanent damage necessitates early neurologic, or in this case, vascular consultation. Hospitalists should be facile in evaluating peripheral neuropathies and recognizing the appropriate indications for diagnostic tests and procedures.
- .Upper limb ischemia after vascular access surgery: differential diagnosis and management.Sem Dial2000;13:312–315.
- ,,,,,.Steal syndrome complicating hemodialysis access.Cardiovascular Surg (London, England)1997;5:648–653.
- ,,,,,.Onset of arterial ‘steal’ following proximal angioaccess: immediate and delayed types.Nephrol Dial Transplant2003;18:2387–2390.
- ,,,,.Incidence and characteristics of patients with hand ischemia after hemodialysis access procedure.J Surg Res1998;74:8–10
- ,,,,,.Ischemic steal syndrome: a case series and review of current management.Curr Surg2006;63:130–135.
- ,,,.Use of digital pressure measurements for the diagnosis of AV access‐induced hand ischemia.Vasc Med2006;11:227–231.
- ,.Electrodiagnostic testing of nerves and muscles: when, why, and how to order.Cleve Clin J Med2005;72:37–48.
- ,.High‐resolution sonography of the peripheral nervous system—a review of the literature.Eur J Neurol2004;11:305–314.
- ,,, et al.Role of magnetic resonance imaging in entrapment and compressive neuropathy‐what, where, and how to see the peripheral nerves on the musculoskeletal magnetic resonance image: part 2. Upper extremity.Eur Radiol2007;17:509–522.
- ,,,,,.The utility of magnetic resonance imaging in evaluating peripheral nerve disorders.Muscle Nerve2002;25:314–331.
- .Indications and usefulness of nerve biopsy.Arch Neurol2002;59:1532–1535.
- .Upper limb ischemia after vascular access surgery: differential diagnosis and management.Sem Dial2000;13:312–315.
- ,,,,,.Steal syndrome complicating hemodialysis access.Cardiovascular Surg (London, England)1997;5:648–653.
- ,,,,,.Onset of arterial ‘steal’ following proximal angioaccess: immediate and delayed types.Nephrol Dial Transplant2003;18:2387–2390.
- ,,,,.Incidence and characteristics of patients with hand ischemia after hemodialysis access procedure.J Surg Res1998;74:8–10
- ,,,,,.Ischemic steal syndrome: a case series and review of current management.Curr Surg2006;63:130–135.
- ,,,.Use of digital pressure measurements for the diagnosis of AV access‐induced hand ischemia.Vasc Med2006;11:227–231.
- ,.Electrodiagnostic testing of nerves and muscles: when, why, and how to order.Cleve Clin J Med2005;72:37–48.
- ,.High‐resolution sonography of the peripheral nervous system—a review of the literature.Eur J Neurol2004;11:305–314.
- ,,, et al.Role of magnetic resonance imaging in entrapment and compressive neuropathy‐what, where, and how to see the peripheral nerves on the musculoskeletal magnetic resonance image: part 2. Upper extremity.Eur Radiol2007;17:509–522.
- ,,,,,.The utility of magnetic resonance imaging in evaluating peripheral nerve disorders.Muscle Nerve2002;25:314–331.
- .Indications and usefulness of nerve biopsy.Arch Neurol2002;59:1532–1535.
Handoffs
To the seasoned intensivist, discussions with family members of critically ill patients in the intensive care unit (ICU) can be very predictable. However, this does not imply that these dialogues are straightforward or simple. Each day, we spend a significant amount of time meeting with family members at different stages of their loved one's ICU stay. Some family members are satisfied with these exchanges while others leave them distraught or in emotional shambles. At times, intensivists do not always effectively communicate with family members.13 Both the team and the families come to the ICU table with different sets of perspectives, expectations, conversational skill sets, life experiences, and tolerances for stress. These differences are magnified when the ICU course turns rocky.
We thought it useful to illustrate ICU dialogues with family members as we perceive them. We focus on the potential checkpoints where miscommunication and misunderstanding may occur throughout the roller coaster ICU experience. To this end, over the past few years, our Critical Care Medicine group has been collecting thought‐provoking comments from various family conferences. Herein, we present the dynamic phases of an ICU encounter contextually inserting relevant quotes.
The specter of a loved one lying helplessly in an ICU bed, attached to imposing machines, with tubes coming out on all sides can be quite numbing and frightening. No amount of schooling or training can really prepare a person for this emotionally taxing situation. Disbelief reigns! We frequently hear, How can a person go from being fine one day to being so sick the next? or He was shoveling snow just last week! or, She was just fine after surgery, talking, walking, and eating.
Not only is the ICU a strange and scary place, but oftentimes, in the midst of our first meeting with family members of newly admitted ICU patients, we quickly realize that they are not really sure who we are or what we do. It seems to us that Critical Care Medicine as a medical specialty suffers from a lack of brand recognition. Often the family members say, You're a what? An intensivist? We've never heard of an intensivist. Do you also work in the Emergency Room? I heard someone mention critical care, is that the same as intensive care? Or sometimes we get whacked, What about getting a real doctor, like a cardiologist or a pulmonologist!
Family members immediately find different ways to let us know how much the patient means to them. They try to impress upon us the vitality and unique nature of their loved ones in the hope that this will make us all work harder. He's a real fighter and never gives up. Or He's a young and healthy 90. Or You have to take extra care of her, she's very special, she's the mother of eight children. Sometimes political or social connections are used to further incentivize or push the ICU team. He's best friends with Mr. Z who is on the Board of Trustees, or She's friends with this or that politician.
But, Google has really altered the nature of our family discussions; everyone, it seems, can now be a doctor. We used to hear I'm not a doctor, but Now, the inevitable internet search leads to I've been doing some reading on the web about this new drug and I've heard that it's a wonder drug. Why isn't my mother getting it? Or What is the APACHE III score of my sister and how are you using this value?
Just as intensivists regularly look at reams of lab data and calculate all types of organ failure and prognostication scores, family members similarly reframe the ICU discussion to a numbers game. This approach to seemingly getting our arms around the complicated big picture is used in many aspects of our lives, whether tracking our retirement portfolios or determining the odds of next week's football game. Doctor, what are her chances for improvement ‐ 50/50, 30/70 or 80/20? Even one in a million? Over time, family members even become experts at looking at the bedside monitors and devices. I've been watching the numbers, I see that the heart rate is down and you were able to decrease the oxygen on the respirator to 80%, and the blood oxygen is still over 90%, so my father must be better, right?
As the days go by, some families become more desperate. They seek good news or even any news from every person they meet. And the ICU environment certainly offers family members a myriad of people with whom to converse. Unfortunately, this frantic search for information leads them to receive conflicting and unreliable data. Frustration results, Why do we keep getting mixed messages? Or, Doctor, we like the hospital, but why can't all of you get the story straight? As family members gather together with other patients' families in the waiting room day and night, they often share their ICU stories with each other. We'll overhear someone say, What about the new antibiotic the patient in Bed 8 is getting? Shouldn't my husband be getting that too? I see everyone is gowning up, I keep hearing about that bad Staph bug going around, my father better not catch it.
Occasionally we become concerned, even perplexed, when we cannot successfully convey our message to the family despite our best intentions and efforts. Some families are just in denial; the reality of no progress and/or likely poor outcome cannot be heard, much less accepted. Doctor, I have been badgered with the truth enough. I just don't want to believe it. Or, Doctor, don't you ever have any good news to report to us? The insatiable need for prolonged and repetitive family conferences may deflect time away from the care of other patients and meeting with other families.
Unfortunately, some patients get stuck in the ICU, either not improving, or just steadily deteriorating despite aggressive care. We broach treatment limitation or end of life care with the family as we realize that further ICU care is not going to be beneficial. Sometimes this news is greeted with stunned silence. The family often pleads for their loved one to be able to stay a little bit longer in the ICU, Let's just see how he does for a few more days or over the weekend. Or, We just need to buy some more time until everything turns around. Or other approaches are used to postpone the inevitable ICU transfer. Doctor, our 50th wedding anniversary is in two weeks, so let's continue to keep him in the ICU. Or, You can't discharge my mother, she averages 14.5 alarms per hour, how can the ward ever take care of her?
And then comes the quest for the miracle, Don't you believe in miracles? Haven't you ever seen someone in this condition get better? Are you giving up? We'll never give up! Or, ignoring the express wishes of the patient, Oh, Doctor, my father did have an advanced directive, but I'm not sure whether I want to give it to you. Or, the message now gets personal What would you do if this was your mother or father?
Such questions highlight the existential dichotomy of critical care. As intensivists, we sometimes have to reconcile the family members' unrealistic view of prognosis, overly hopeful expectations, and desire for endless futile ICU care with our own understandings of prognosis, goals of care, and appropriate use of ICU beds. Where, and when should we draw the line? How do we all let go?4
This collection of comments and thoughts reflects a synthesis of many different discussions conducted under diverse conditions. Thankfully, not all of the individual elements of the scenario described above occur with each patient. Family members and intensivists commonly have amicable discourse resulting in an acceptable degree of understanding and consensus regarding the prognosis and care plan. However, on occasion, things just don't go as well as hoped for, neither in clinical outcomes nor in our discussions. While effective ICU communication strategies have been designed and studied,512 even the best of these may not prevent conflict and disagreement. Nevertheless, our challenge as critical care practitioners is to ensure that our dialogues with family members are honest and direct and that we communicate in a timely, consistent and empathetic manner.
Well, onto the next family meeting!
Acknowledgements
The authors thank the current and past critical care fellows for their contributions to this manuscript. We are particularly indebted to the ICU nurses, patient representatives and social workers of the Memorial Sloan‐Kettering Cancer Center, New York, New York who provide daily clinical and emotional support to our ICU patients and their families and to the CCM attending team.
- ,,, et al.Half the families of intensive care unit patients experience inadequate communication with physicians.Crit Care Med2000;28(8):3044–3049.
- ,,, et al.Missed opportunities during family conferences about end‐of‐life care in the intensive care unit.Am J Respir Crit Care Med2005;171:844–849.
- ,.Impact on family satisfaction: The Critical Care Family Assistance Program.Chest2005;128:76S–80S.
- .The art of letting go.NEJM2007;357:3–5.
- ,,, et al.The family conference as a focus to improve communication about end‐of‐life care in the intensive care unit: opportunities for improvement.Crit Care Med2001;29:suppl 2:N26–N33.
- ,,, et al.A communication strategy and brochure for relatives of patients dying in the ICU.N Engl J Med2007:356:469–478.
- ,.The healing power of listening in the ICU.N Engl J Med2007;356:513–515.
- ,.The pressure to withhold or withdraw life‐sustaining therapy from critically ill patients in the United States.Am J Respir Crit Care Med2007;175(11):1104–1108.
- ,,, et al.An intensive communication intervention for the critically ill.Am J Med2000;109:469–475.
- ,,.Improving family communications at the end of life: implications for length of stay in the intensive care unit and resource use.Am J Crit Care2003;12(4)317–323.
- ,,, et al.Conflict associated with decisions to limit life‐sustaining treatment in intensive care units.J Gen Intern Med2001;16(5)339–341.
- ,,, et al.Families looking back: one year after discussion of withdrawal or withholding of life‐sustaining support.Crit Care Med2001;29(1)197–201.
To the seasoned intensivist, discussions with family members of critically ill patients in the intensive care unit (ICU) can be very predictable. However, this does not imply that these dialogues are straightforward or simple. Each day, we spend a significant amount of time meeting with family members at different stages of their loved one's ICU stay. Some family members are satisfied with these exchanges while others leave them distraught or in emotional shambles. At times, intensivists do not always effectively communicate with family members.13 Both the team and the families come to the ICU table with different sets of perspectives, expectations, conversational skill sets, life experiences, and tolerances for stress. These differences are magnified when the ICU course turns rocky.
We thought it useful to illustrate ICU dialogues with family members as we perceive them. We focus on the potential checkpoints where miscommunication and misunderstanding may occur throughout the roller coaster ICU experience. To this end, over the past few years, our Critical Care Medicine group has been collecting thought‐provoking comments from various family conferences. Herein, we present the dynamic phases of an ICU encounter contextually inserting relevant quotes.
The specter of a loved one lying helplessly in an ICU bed, attached to imposing machines, with tubes coming out on all sides can be quite numbing and frightening. No amount of schooling or training can really prepare a person for this emotionally taxing situation. Disbelief reigns! We frequently hear, How can a person go from being fine one day to being so sick the next? or He was shoveling snow just last week! or, She was just fine after surgery, talking, walking, and eating.
Not only is the ICU a strange and scary place, but oftentimes, in the midst of our first meeting with family members of newly admitted ICU patients, we quickly realize that they are not really sure who we are or what we do. It seems to us that Critical Care Medicine as a medical specialty suffers from a lack of brand recognition. Often the family members say, You're a what? An intensivist? We've never heard of an intensivist. Do you also work in the Emergency Room? I heard someone mention critical care, is that the same as intensive care? Or sometimes we get whacked, What about getting a real doctor, like a cardiologist or a pulmonologist!
Family members immediately find different ways to let us know how much the patient means to them. They try to impress upon us the vitality and unique nature of their loved ones in the hope that this will make us all work harder. He's a real fighter and never gives up. Or He's a young and healthy 90. Or You have to take extra care of her, she's very special, she's the mother of eight children. Sometimes political or social connections are used to further incentivize or push the ICU team. He's best friends with Mr. Z who is on the Board of Trustees, or She's friends with this or that politician.
But, Google has really altered the nature of our family discussions; everyone, it seems, can now be a doctor. We used to hear I'm not a doctor, but Now, the inevitable internet search leads to I've been doing some reading on the web about this new drug and I've heard that it's a wonder drug. Why isn't my mother getting it? Or What is the APACHE III score of my sister and how are you using this value?
Just as intensivists regularly look at reams of lab data and calculate all types of organ failure and prognostication scores, family members similarly reframe the ICU discussion to a numbers game. This approach to seemingly getting our arms around the complicated big picture is used in many aspects of our lives, whether tracking our retirement portfolios or determining the odds of next week's football game. Doctor, what are her chances for improvement ‐ 50/50, 30/70 or 80/20? Even one in a million? Over time, family members even become experts at looking at the bedside monitors and devices. I've been watching the numbers, I see that the heart rate is down and you were able to decrease the oxygen on the respirator to 80%, and the blood oxygen is still over 90%, so my father must be better, right?
As the days go by, some families become more desperate. They seek good news or even any news from every person they meet. And the ICU environment certainly offers family members a myriad of people with whom to converse. Unfortunately, this frantic search for information leads them to receive conflicting and unreliable data. Frustration results, Why do we keep getting mixed messages? Or, Doctor, we like the hospital, but why can't all of you get the story straight? As family members gather together with other patients' families in the waiting room day and night, they often share their ICU stories with each other. We'll overhear someone say, What about the new antibiotic the patient in Bed 8 is getting? Shouldn't my husband be getting that too? I see everyone is gowning up, I keep hearing about that bad Staph bug going around, my father better not catch it.
Occasionally we become concerned, even perplexed, when we cannot successfully convey our message to the family despite our best intentions and efforts. Some families are just in denial; the reality of no progress and/or likely poor outcome cannot be heard, much less accepted. Doctor, I have been badgered with the truth enough. I just don't want to believe it. Or, Doctor, don't you ever have any good news to report to us? The insatiable need for prolonged and repetitive family conferences may deflect time away from the care of other patients and meeting with other families.
Unfortunately, some patients get stuck in the ICU, either not improving, or just steadily deteriorating despite aggressive care. We broach treatment limitation or end of life care with the family as we realize that further ICU care is not going to be beneficial. Sometimes this news is greeted with stunned silence. The family often pleads for their loved one to be able to stay a little bit longer in the ICU, Let's just see how he does for a few more days or over the weekend. Or, We just need to buy some more time until everything turns around. Or other approaches are used to postpone the inevitable ICU transfer. Doctor, our 50th wedding anniversary is in two weeks, so let's continue to keep him in the ICU. Or, You can't discharge my mother, she averages 14.5 alarms per hour, how can the ward ever take care of her?
And then comes the quest for the miracle, Don't you believe in miracles? Haven't you ever seen someone in this condition get better? Are you giving up? We'll never give up! Or, ignoring the express wishes of the patient, Oh, Doctor, my father did have an advanced directive, but I'm not sure whether I want to give it to you. Or, the message now gets personal What would you do if this was your mother or father?
Such questions highlight the existential dichotomy of critical care. As intensivists, we sometimes have to reconcile the family members' unrealistic view of prognosis, overly hopeful expectations, and desire for endless futile ICU care with our own understandings of prognosis, goals of care, and appropriate use of ICU beds. Where, and when should we draw the line? How do we all let go?4
This collection of comments and thoughts reflects a synthesis of many different discussions conducted under diverse conditions. Thankfully, not all of the individual elements of the scenario described above occur with each patient. Family members and intensivists commonly have amicable discourse resulting in an acceptable degree of understanding and consensus regarding the prognosis and care plan. However, on occasion, things just don't go as well as hoped for, neither in clinical outcomes nor in our discussions. While effective ICU communication strategies have been designed and studied,512 even the best of these may not prevent conflict and disagreement. Nevertheless, our challenge as critical care practitioners is to ensure that our dialogues with family members are honest and direct and that we communicate in a timely, consistent and empathetic manner.
Well, onto the next family meeting!
Acknowledgements
The authors thank the current and past critical care fellows for their contributions to this manuscript. We are particularly indebted to the ICU nurses, patient representatives and social workers of the Memorial Sloan‐Kettering Cancer Center, New York, New York who provide daily clinical and emotional support to our ICU patients and their families and to the CCM attending team.
To the seasoned intensivist, discussions with family members of critically ill patients in the intensive care unit (ICU) can be very predictable. However, this does not imply that these dialogues are straightforward or simple. Each day, we spend a significant amount of time meeting with family members at different stages of their loved one's ICU stay. Some family members are satisfied with these exchanges while others leave them distraught or in emotional shambles. At times, intensivists do not always effectively communicate with family members.13 Both the team and the families come to the ICU table with different sets of perspectives, expectations, conversational skill sets, life experiences, and tolerances for stress. These differences are magnified when the ICU course turns rocky.
We thought it useful to illustrate ICU dialogues with family members as we perceive them. We focus on the potential checkpoints where miscommunication and misunderstanding may occur throughout the roller coaster ICU experience. To this end, over the past few years, our Critical Care Medicine group has been collecting thought‐provoking comments from various family conferences. Herein, we present the dynamic phases of an ICU encounter contextually inserting relevant quotes.
The specter of a loved one lying helplessly in an ICU bed, attached to imposing machines, with tubes coming out on all sides can be quite numbing and frightening. No amount of schooling or training can really prepare a person for this emotionally taxing situation. Disbelief reigns! We frequently hear, How can a person go from being fine one day to being so sick the next? or He was shoveling snow just last week! or, She was just fine after surgery, talking, walking, and eating.
Not only is the ICU a strange and scary place, but oftentimes, in the midst of our first meeting with family members of newly admitted ICU patients, we quickly realize that they are not really sure who we are or what we do. It seems to us that Critical Care Medicine as a medical specialty suffers from a lack of brand recognition. Often the family members say, You're a what? An intensivist? We've never heard of an intensivist. Do you also work in the Emergency Room? I heard someone mention critical care, is that the same as intensive care? Or sometimes we get whacked, What about getting a real doctor, like a cardiologist or a pulmonologist!
Family members immediately find different ways to let us know how much the patient means to them. They try to impress upon us the vitality and unique nature of their loved ones in the hope that this will make us all work harder. He's a real fighter and never gives up. Or He's a young and healthy 90. Or You have to take extra care of her, she's very special, she's the mother of eight children. Sometimes political or social connections are used to further incentivize or push the ICU team. He's best friends with Mr. Z who is on the Board of Trustees, or She's friends with this or that politician.
But, Google has really altered the nature of our family discussions; everyone, it seems, can now be a doctor. We used to hear I'm not a doctor, but Now, the inevitable internet search leads to I've been doing some reading on the web about this new drug and I've heard that it's a wonder drug. Why isn't my mother getting it? Or What is the APACHE III score of my sister and how are you using this value?
Just as intensivists regularly look at reams of lab data and calculate all types of organ failure and prognostication scores, family members similarly reframe the ICU discussion to a numbers game. This approach to seemingly getting our arms around the complicated big picture is used in many aspects of our lives, whether tracking our retirement portfolios or determining the odds of next week's football game. Doctor, what are her chances for improvement ‐ 50/50, 30/70 or 80/20? Even one in a million? Over time, family members even become experts at looking at the bedside monitors and devices. I've been watching the numbers, I see that the heart rate is down and you were able to decrease the oxygen on the respirator to 80%, and the blood oxygen is still over 90%, so my father must be better, right?
As the days go by, some families become more desperate. They seek good news or even any news from every person they meet. And the ICU environment certainly offers family members a myriad of people with whom to converse. Unfortunately, this frantic search for information leads them to receive conflicting and unreliable data. Frustration results, Why do we keep getting mixed messages? Or, Doctor, we like the hospital, but why can't all of you get the story straight? As family members gather together with other patients' families in the waiting room day and night, they often share their ICU stories with each other. We'll overhear someone say, What about the new antibiotic the patient in Bed 8 is getting? Shouldn't my husband be getting that too? I see everyone is gowning up, I keep hearing about that bad Staph bug going around, my father better not catch it.
Occasionally we become concerned, even perplexed, when we cannot successfully convey our message to the family despite our best intentions and efforts. Some families are just in denial; the reality of no progress and/or likely poor outcome cannot be heard, much less accepted. Doctor, I have been badgered with the truth enough. I just don't want to believe it. Or, Doctor, don't you ever have any good news to report to us? The insatiable need for prolonged and repetitive family conferences may deflect time away from the care of other patients and meeting with other families.
Unfortunately, some patients get stuck in the ICU, either not improving, or just steadily deteriorating despite aggressive care. We broach treatment limitation or end of life care with the family as we realize that further ICU care is not going to be beneficial. Sometimes this news is greeted with stunned silence. The family often pleads for their loved one to be able to stay a little bit longer in the ICU, Let's just see how he does for a few more days or over the weekend. Or, We just need to buy some more time until everything turns around. Or other approaches are used to postpone the inevitable ICU transfer. Doctor, our 50th wedding anniversary is in two weeks, so let's continue to keep him in the ICU. Or, You can't discharge my mother, she averages 14.5 alarms per hour, how can the ward ever take care of her?
And then comes the quest for the miracle, Don't you believe in miracles? Haven't you ever seen someone in this condition get better? Are you giving up? We'll never give up! Or, ignoring the express wishes of the patient, Oh, Doctor, my father did have an advanced directive, but I'm not sure whether I want to give it to you. Or, the message now gets personal What would you do if this was your mother or father?
Such questions highlight the existential dichotomy of critical care. As intensivists, we sometimes have to reconcile the family members' unrealistic view of prognosis, overly hopeful expectations, and desire for endless futile ICU care with our own understandings of prognosis, goals of care, and appropriate use of ICU beds. Where, and when should we draw the line? How do we all let go?4
This collection of comments and thoughts reflects a synthesis of many different discussions conducted under diverse conditions. Thankfully, not all of the individual elements of the scenario described above occur with each patient. Family members and intensivists commonly have amicable discourse resulting in an acceptable degree of understanding and consensus regarding the prognosis and care plan. However, on occasion, things just don't go as well as hoped for, neither in clinical outcomes nor in our discussions. While effective ICU communication strategies have been designed and studied,512 even the best of these may not prevent conflict and disagreement. Nevertheless, our challenge as critical care practitioners is to ensure that our dialogues with family members are honest and direct and that we communicate in a timely, consistent and empathetic manner.
Well, onto the next family meeting!
Acknowledgements
The authors thank the current and past critical care fellows for their contributions to this manuscript. We are particularly indebted to the ICU nurses, patient representatives and social workers of the Memorial Sloan‐Kettering Cancer Center, New York, New York who provide daily clinical and emotional support to our ICU patients and their families and to the CCM attending team.
- ,,, et al.Half the families of intensive care unit patients experience inadequate communication with physicians.Crit Care Med2000;28(8):3044–3049.
- ,,, et al.Missed opportunities during family conferences about end‐of‐life care in the intensive care unit.Am J Respir Crit Care Med2005;171:844–849.
- ,.Impact on family satisfaction: The Critical Care Family Assistance Program.Chest2005;128:76S–80S.
- .The art of letting go.NEJM2007;357:3–5.
- ,,, et al.The family conference as a focus to improve communication about end‐of‐life care in the intensive care unit: opportunities for improvement.Crit Care Med2001;29:suppl 2:N26–N33.
- ,,, et al.A communication strategy and brochure for relatives of patients dying in the ICU.N Engl J Med2007:356:469–478.
- ,.The healing power of listening in the ICU.N Engl J Med2007;356:513–515.
- ,.The pressure to withhold or withdraw life‐sustaining therapy from critically ill patients in the United States.Am J Respir Crit Care Med2007;175(11):1104–1108.
- ,,, et al.An intensive communication intervention for the critically ill.Am J Med2000;109:469–475.
- ,,.Improving family communications at the end of life: implications for length of stay in the intensive care unit and resource use.Am J Crit Care2003;12(4)317–323.
- ,,, et al.Conflict associated with decisions to limit life‐sustaining treatment in intensive care units.J Gen Intern Med2001;16(5)339–341.
- ,,, et al.Families looking back: one year after discussion of withdrawal or withholding of life‐sustaining support.Crit Care Med2001;29(1)197–201.
- ,,, et al.Half the families of intensive care unit patients experience inadequate communication with physicians.Crit Care Med2000;28(8):3044–3049.
- ,,, et al.Missed opportunities during family conferences about end‐of‐life care in the intensive care unit.Am J Respir Crit Care Med2005;171:844–849.
- ,.Impact on family satisfaction: The Critical Care Family Assistance Program.Chest2005;128:76S–80S.
- .The art of letting go.NEJM2007;357:3–5.
- ,,, et al.The family conference as a focus to improve communication about end‐of‐life care in the intensive care unit: opportunities for improvement.Crit Care Med2001;29:suppl 2:N26–N33.
- ,,, et al.A communication strategy and brochure for relatives of patients dying in the ICU.N Engl J Med2007:356:469–478.
- ,.The healing power of listening in the ICU.N Engl J Med2007;356:513–515.
- ,.The pressure to withhold or withdraw life‐sustaining therapy from critically ill patients in the United States.Am J Respir Crit Care Med2007;175(11):1104–1108.
- ,,, et al.An intensive communication intervention for the critically ill.Am J Med2000;109:469–475.
- ,,.Improving family communications at the end of life: implications for length of stay in the intensive care unit and resource use.Am J Crit Care2003;12(4)317–323.
- ,,, et al.Conflict associated with decisions to limit life‐sustaining treatment in intensive care units.J Gen Intern Med2001;16(5)339–341.
- ,,, et al.Families looking back: one year after discussion of withdrawal or withholding of life‐sustaining support.Crit Care Med2001;29(1)197–201.
Case Report: Failure at the Transition of Care
The patient is an 86‐year‐old woman with a history of mild dementia, major depression with psychotic features, congestive heart failure, hypertension, hyperlipidemia, osteoporosis, and hypothyroidism. She presented to her primary care physician (PCP) complaining of 4 days of bilateral lower extremity edema and dyspnea on exertion. She was admitted to the hospitalist service for exacerbation of congestive heart failure.
MEDICATIONS
Donepezil, olanzapine, mirtazapine, sertraline, spironolactone, triamterene/hydrochlorothizide, simvastatin, alendronate, levothyroxine, multivitamin.
SOCIAL HISTORY
She lived alone in an independent‐living retirement apartment that provided meals but not medical care, and she was able to function independently in her activities of daily living. Her pharmacy delivered her medications via courier service, whereas visiting home nurses filled her medication box and checked on her status weekly.
HOSPITAL COURSE
Admission vitals were: heart rate, 83; blood pressure, 158/84; respiratory rate, 20; temperature, 36.4, and saturation, 95% on room air. Echocardiogram revealed intact ejection fraction, left ventricular hypertrophy, and impaired relaxation. A TSH of 6.6 demonstrated undertreated hypothyroidism. Telemetry monitoring was significant for frequent short bursts of narrow‐complex tachycardia without clear atrial activity. The etiology of her heart failure exacerbation was presumed to be paroxysmal atrial fibrillation in the setting of diastolic dysfunction. Given her mild hyperkalemia (5.1), her diuretics were changed to monotherapy with furosemide. Low‐dose beta blockade and antithrombotic therapy were started as well as increased supplementation of levothyroxine. After several days of diuresis, her potassium had normalized, she tolerated initiation of a new ACE inhibitor, and her dyspnea had resolved. On the last hospital day, her dentures were accidentally discarded with her breakfast tray, causing her great distress.
On discharge she was on 4 new medications, 2 old medications had been stopped, and 1 prior medication's dose had been increased. During medication reconciliation, the patient reported that she had not been taking olanzapine for weeks, and thus this was omitted from her home health medication orders, with instructions to discuss with her PCP on first follow‐up within the week. The patient was provided with congestive heart failure instructions and a complete medication list. Unfortunately, the day of discharge was the first day of a holiday weekend.
Case management was unavailable on the weekend; her out‐of‐state family member was unable to be reached by phone, and her usual pharmacy courier service was closed. As she did not have a friend or family member to pick up her prescriptions from an alternate pharmacy, her prescriptions were provided as handwritten scripts, called in to her pharmacy's voice mail, and written on the home health orders. The patient was discharged to her home with communication to her PCP via telephone, e‐mail, and electronic discharge summary.
POSTDISCHARGE
Medications were not delivered to the patient until the third postdischarge day. Three days after discharge, the daughter from out of state left a message for the PCP expressing concern that the patient was failingnot eating or taking any of her medications. An expedited home nursing visit was arranged. Five days after discharge, the pharmacist called the PCP stating he had not received a prescription for the beta‐blocker. Her PCP saw the patient in clinic 6 days after discharge and reconciled the medication list, restarting the olanzapine that the patient had stopped a few weeks before and the mirtazapine, which had not been restarted despite its presence on the discharge orders and patient instructions. She continued to have poor appetite and mood and was taking her medications only with great effort from her visiting nurse and staff at the retirement community. A major cause of this decline was the significant worsening of her depression brought on by hospitalization, lapses in her psychiatric medications, and emotional distress induced by the loss of her dentures during her hospital stay. She was readmitted 10 days after her discharge because she was unable to care for herself.
DISCUSSION
This case demonstrates numerous pitfalls in the transition process. Despite communication between the hospitalists and the PCP and a common electronic medical record, this patient failed the transition from the acute care hospital to the ambulatory setting. On the holiday weekend, ancillary support services were unavailable, including case management to contact her home care agency and her pharmacy to fill and deliver her new prescriptions. Despite efforts by the discharging physician, the out‐of‐town family could not be contacted. Thus, an elderly woman with cognitive impairment was left to process a new diagnosis and 7 medication changes with an unreliable mechanism to obtain her new medications.
With the rise of hospital medicine, it has increasingly been recognized that transitions represent a point of vulnerability in the care of geriatric patients. A change in physical location of care and handoffs between caregivers create the potential for error and loss of information. Prior research has demonstrated frequent quantitative and qualitative deficiencies in the information conveyed between inpatient and outpatient physicians, with direct communication occurring less than 20% of the time.1 In this case, communication occurred between the hospitalists and the outpatient physician, demonstrating that communication is just one element of successful transitions.
Components of effective care transitions have been described in the literature, including: preparation of the patient and caregiver for the transition, medication reconciliation, instructions to patient and caregiver about symptoms and signs of worsening, and an explicit follow‐up plan for tests and appointments.2 Optimally, there is interactive discussion between the hospitalist and the receiving clinician with a summary of events including an updated medication, allergy, and problem list, current advance directives, and a common plan of care.2 This case illustrates that these elements are necessary but may not be sufficient.
Some interventions have been found to be effective. A nurse‐led multidisciplinary approach to the discharge of elderly patients with congestive heart failure led to decreased readmission rates after 90 days and was found to be a cost‐saving measure.3 Similar results have been seen in geriatric patients with a variety of diagnoses in trials using advanced‐practice nurses to bridge the vulnerable period of discharge or by interventions to improve the ability of family caregivers to handle the challenges of the transition.46 Individualized attention to the unique needs of each patient and members of their social support structure, and investment in resources to do so, has the ability to decrease readmissions.
Medication errors, medication omissions, or the inability to fill medications on discharge represent a patient safety challenge. There has been increasing emphasis on medication reconciliation at admission and discharge, but in some cases the gold standard medication list is hard to determine. Electronic medical records would seem to be a natural solution to this problem, but as this case illustrates, the electronic record may not reflect the reality of patient adherence. As in this case, clarification may require another visit with the primary provider, leaving a period of time with an uncertain medication list and therefore a vulnerable patient. Access to medications after discharge was also a problem in this case, but this is not rare. A 2001 study found that 2 days after discharge from a general medicine hospital service, 1 in 5 patients had been unable to obtain all discharge medications.7 A pharmacist‐led medication reconciliation intervention in nursing home patients led to decreases in length of stay and discrepancy‐related adverse drug events. Furthermore, a follow‐up call allowed for clarification of medication questions in 25% of cases.7, 8
Patient characteristics such as depression and cognitive dysfunction have been found to affect readmission rates and are important to assess in addressing risk for poor outcomes after discharge. A 2000 study comparing readmission rates after discharge from a geriatric rehabilitation hospital found that patients with depression had an odds ratio of 3.5 for readmission compared with those without depression.9 Inadequate health literacy is also associated with decreased ability to self‐manage chronic disease and is associated with increased risk of mortality in community‐dwelling elderly such as the patient in this case.10 Asking patients or their proxies to explain their own understanding of the discharge plan can unmask comprehension issues that otherwise may go undetected.
Discharge on a weekend presents a period of critical vulnerability. Early recognition that a transition is susceptible to failure allows the events necessary for success to occur during the week when services are available. An example in the present case might include having had the pharmacy fill the prescriptions prior to the day of discharge. This does introduce a new opportunity for error in cases in which the plan of care changes but would have solved the inability to have prescriptions filled once the holiday weekend had begun.
In cases in which the usual mechanisms break down, increased effort on the part of the hospitalist can usually create a unique solution to the problem. Examples of creative solutions that did not occur in this case might include contacting the manager of the patient's retirement apartment to determine if this individual might be willing to fill prescriptions at an alternate pharmacy. A better alternative would be to change the system such that the solution is readily accessible and time efficient. Weekend availability of case management would be one such step. A means for the hospital's inpatient pharmacy to provide 2‐3 days of bridging medications would prevent weekend prescription access from affecting timely discharges of multiple patients over the course of a year. The hospitalist is in a unique position to take a leadership role in effecting system change to address these issues.
Ultimately, it is the duty of the hospitalist to take responsibility for the safety and well‐being of the patient, and if no solution can be found, it may be necessary to hold discharge or find an alternate disposition until logistical hurdles have been overcome. Indeed, for patients admitted for myocardial infarction, discharges were less likely to occur on weekends, presumably because of lack of ancillary services.11 With foresight, creative problem solving, and systems improvement, this should rarely be necessary.
Transitions continue to be a difficult time for the most vulnerable patients. Intense efforts have improved outcomes in selected populations but have not been broadly applied. Identification of patients at the highest risk, such as those with depression, poor social support, and cognitive limitations, would allow anticipation of difficult transitions and potential utilization of proven interventions, such as advanced‐practice nurses or follow‐up pharmacy contact. Processes such as these might have prevented some of the problems in this patient's discharge. Appreciation of the weekend discharge as a time of particular challenges allows barriers to be identified and solutions created during the week, when resources are still available. Attention to all elements of effective transitions should become part of the growing culture of patient safety.
- ,,, et al.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- .Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs.J Am Geriatr Soc.2003;51:549–555.
- ,,, et al.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333:1190–1195.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281:613–620.
- ,,, et al.Comprehensive discharge planning for the hospitalized elderly: a randomized clinical trial.Ann Intern Med.1994;120:999–1006.
- ,,, et al.Preparing patients and caregivers to participate in care delivered across settings: the care transitions intervention.J Am Geriatr Soc.2004;52:1817–1825.
- ,,, et al.The impact of follow‐up telephone calls to patients after hospitalization.Am J Med.2001;111:26S–30S.
- ,,, et al.Medication reconciliation for reducing drug discrepancy adverse events.Am J Geriatr Pharmacother.2006;4:236–243.
- ,,, et al.Depression and activities of daily living predict rehospitalization within 6 months of discharge from geriatric rehabilitation.Rehabil Psychol.2004;49:219–223.
- ,,, et al.Health literacy and mortality among elderly persons.Arch Intern Med.2007;167:1503–1509.
- ,,, et al.Restricted weekend service inappropriately delays discharge after acute myocardial infarction.Heart.2002;87:216–219.
The patient is an 86‐year‐old woman with a history of mild dementia, major depression with psychotic features, congestive heart failure, hypertension, hyperlipidemia, osteoporosis, and hypothyroidism. She presented to her primary care physician (PCP) complaining of 4 days of bilateral lower extremity edema and dyspnea on exertion. She was admitted to the hospitalist service for exacerbation of congestive heart failure.
MEDICATIONS
Donepezil, olanzapine, mirtazapine, sertraline, spironolactone, triamterene/hydrochlorothizide, simvastatin, alendronate, levothyroxine, multivitamin.
SOCIAL HISTORY
She lived alone in an independent‐living retirement apartment that provided meals but not medical care, and she was able to function independently in her activities of daily living. Her pharmacy delivered her medications via courier service, whereas visiting home nurses filled her medication box and checked on her status weekly.
HOSPITAL COURSE
Admission vitals were: heart rate, 83; blood pressure, 158/84; respiratory rate, 20; temperature, 36.4, and saturation, 95% on room air. Echocardiogram revealed intact ejection fraction, left ventricular hypertrophy, and impaired relaxation. A TSH of 6.6 demonstrated undertreated hypothyroidism. Telemetry monitoring was significant for frequent short bursts of narrow‐complex tachycardia without clear atrial activity. The etiology of her heart failure exacerbation was presumed to be paroxysmal atrial fibrillation in the setting of diastolic dysfunction. Given her mild hyperkalemia (5.1), her diuretics were changed to monotherapy with furosemide. Low‐dose beta blockade and antithrombotic therapy were started as well as increased supplementation of levothyroxine. After several days of diuresis, her potassium had normalized, she tolerated initiation of a new ACE inhibitor, and her dyspnea had resolved. On the last hospital day, her dentures were accidentally discarded with her breakfast tray, causing her great distress.
On discharge she was on 4 new medications, 2 old medications had been stopped, and 1 prior medication's dose had been increased. During medication reconciliation, the patient reported that she had not been taking olanzapine for weeks, and thus this was omitted from her home health medication orders, with instructions to discuss with her PCP on first follow‐up within the week. The patient was provided with congestive heart failure instructions and a complete medication list. Unfortunately, the day of discharge was the first day of a holiday weekend.
Case management was unavailable on the weekend; her out‐of‐state family member was unable to be reached by phone, and her usual pharmacy courier service was closed. As she did not have a friend or family member to pick up her prescriptions from an alternate pharmacy, her prescriptions were provided as handwritten scripts, called in to her pharmacy's voice mail, and written on the home health orders. The patient was discharged to her home with communication to her PCP via telephone, e‐mail, and electronic discharge summary.
POSTDISCHARGE
Medications were not delivered to the patient until the third postdischarge day. Three days after discharge, the daughter from out of state left a message for the PCP expressing concern that the patient was failingnot eating or taking any of her medications. An expedited home nursing visit was arranged. Five days after discharge, the pharmacist called the PCP stating he had not received a prescription for the beta‐blocker. Her PCP saw the patient in clinic 6 days after discharge and reconciled the medication list, restarting the olanzapine that the patient had stopped a few weeks before and the mirtazapine, which had not been restarted despite its presence on the discharge orders and patient instructions. She continued to have poor appetite and mood and was taking her medications only with great effort from her visiting nurse and staff at the retirement community. A major cause of this decline was the significant worsening of her depression brought on by hospitalization, lapses in her psychiatric medications, and emotional distress induced by the loss of her dentures during her hospital stay. She was readmitted 10 days after her discharge because she was unable to care for herself.
DISCUSSION
This case demonstrates numerous pitfalls in the transition process. Despite communication between the hospitalists and the PCP and a common electronic medical record, this patient failed the transition from the acute care hospital to the ambulatory setting. On the holiday weekend, ancillary support services were unavailable, including case management to contact her home care agency and her pharmacy to fill and deliver her new prescriptions. Despite efforts by the discharging physician, the out‐of‐town family could not be contacted. Thus, an elderly woman with cognitive impairment was left to process a new diagnosis and 7 medication changes with an unreliable mechanism to obtain her new medications.
With the rise of hospital medicine, it has increasingly been recognized that transitions represent a point of vulnerability in the care of geriatric patients. A change in physical location of care and handoffs between caregivers create the potential for error and loss of information. Prior research has demonstrated frequent quantitative and qualitative deficiencies in the information conveyed between inpatient and outpatient physicians, with direct communication occurring less than 20% of the time.1 In this case, communication occurred between the hospitalists and the outpatient physician, demonstrating that communication is just one element of successful transitions.
Components of effective care transitions have been described in the literature, including: preparation of the patient and caregiver for the transition, medication reconciliation, instructions to patient and caregiver about symptoms and signs of worsening, and an explicit follow‐up plan for tests and appointments.2 Optimally, there is interactive discussion between the hospitalist and the receiving clinician with a summary of events including an updated medication, allergy, and problem list, current advance directives, and a common plan of care.2 This case illustrates that these elements are necessary but may not be sufficient.
Some interventions have been found to be effective. A nurse‐led multidisciplinary approach to the discharge of elderly patients with congestive heart failure led to decreased readmission rates after 90 days and was found to be a cost‐saving measure.3 Similar results have been seen in geriatric patients with a variety of diagnoses in trials using advanced‐practice nurses to bridge the vulnerable period of discharge or by interventions to improve the ability of family caregivers to handle the challenges of the transition.46 Individualized attention to the unique needs of each patient and members of their social support structure, and investment in resources to do so, has the ability to decrease readmissions.
Medication errors, medication omissions, or the inability to fill medications on discharge represent a patient safety challenge. There has been increasing emphasis on medication reconciliation at admission and discharge, but in some cases the gold standard medication list is hard to determine. Electronic medical records would seem to be a natural solution to this problem, but as this case illustrates, the electronic record may not reflect the reality of patient adherence. As in this case, clarification may require another visit with the primary provider, leaving a period of time with an uncertain medication list and therefore a vulnerable patient. Access to medications after discharge was also a problem in this case, but this is not rare. A 2001 study found that 2 days after discharge from a general medicine hospital service, 1 in 5 patients had been unable to obtain all discharge medications.7 A pharmacist‐led medication reconciliation intervention in nursing home patients led to decreases in length of stay and discrepancy‐related adverse drug events. Furthermore, a follow‐up call allowed for clarification of medication questions in 25% of cases.7, 8
Patient characteristics such as depression and cognitive dysfunction have been found to affect readmission rates and are important to assess in addressing risk for poor outcomes after discharge. A 2000 study comparing readmission rates after discharge from a geriatric rehabilitation hospital found that patients with depression had an odds ratio of 3.5 for readmission compared with those without depression.9 Inadequate health literacy is also associated with decreased ability to self‐manage chronic disease and is associated with increased risk of mortality in community‐dwelling elderly such as the patient in this case.10 Asking patients or their proxies to explain their own understanding of the discharge plan can unmask comprehension issues that otherwise may go undetected.
Discharge on a weekend presents a period of critical vulnerability. Early recognition that a transition is susceptible to failure allows the events necessary for success to occur during the week when services are available. An example in the present case might include having had the pharmacy fill the prescriptions prior to the day of discharge. This does introduce a new opportunity for error in cases in which the plan of care changes but would have solved the inability to have prescriptions filled once the holiday weekend had begun.
In cases in which the usual mechanisms break down, increased effort on the part of the hospitalist can usually create a unique solution to the problem. Examples of creative solutions that did not occur in this case might include contacting the manager of the patient's retirement apartment to determine if this individual might be willing to fill prescriptions at an alternate pharmacy. A better alternative would be to change the system such that the solution is readily accessible and time efficient. Weekend availability of case management would be one such step. A means for the hospital's inpatient pharmacy to provide 2‐3 days of bridging medications would prevent weekend prescription access from affecting timely discharges of multiple patients over the course of a year. The hospitalist is in a unique position to take a leadership role in effecting system change to address these issues.
Ultimately, it is the duty of the hospitalist to take responsibility for the safety and well‐being of the patient, and if no solution can be found, it may be necessary to hold discharge or find an alternate disposition until logistical hurdles have been overcome. Indeed, for patients admitted for myocardial infarction, discharges were less likely to occur on weekends, presumably because of lack of ancillary services.11 With foresight, creative problem solving, and systems improvement, this should rarely be necessary.
Transitions continue to be a difficult time for the most vulnerable patients. Intense efforts have improved outcomes in selected populations but have not been broadly applied. Identification of patients at the highest risk, such as those with depression, poor social support, and cognitive limitations, would allow anticipation of difficult transitions and potential utilization of proven interventions, such as advanced‐practice nurses or follow‐up pharmacy contact. Processes such as these might have prevented some of the problems in this patient's discharge. Appreciation of the weekend discharge as a time of particular challenges allows barriers to be identified and solutions created during the week, when resources are still available. Attention to all elements of effective transitions should become part of the growing culture of patient safety.
The patient is an 86‐year‐old woman with a history of mild dementia, major depression with psychotic features, congestive heart failure, hypertension, hyperlipidemia, osteoporosis, and hypothyroidism. She presented to her primary care physician (PCP) complaining of 4 days of bilateral lower extremity edema and dyspnea on exertion. She was admitted to the hospitalist service for exacerbation of congestive heart failure.
MEDICATIONS
Donepezil, olanzapine, mirtazapine, sertraline, spironolactone, triamterene/hydrochlorothizide, simvastatin, alendronate, levothyroxine, multivitamin.
SOCIAL HISTORY
She lived alone in an independent‐living retirement apartment that provided meals but not medical care, and she was able to function independently in her activities of daily living. Her pharmacy delivered her medications via courier service, whereas visiting home nurses filled her medication box and checked on her status weekly.
HOSPITAL COURSE
Admission vitals were: heart rate, 83; blood pressure, 158/84; respiratory rate, 20; temperature, 36.4, and saturation, 95% on room air. Echocardiogram revealed intact ejection fraction, left ventricular hypertrophy, and impaired relaxation. A TSH of 6.6 demonstrated undertreated hypothyroidism. Telemetry monitoring was significant for frequent short bursts of narrow‐complex tachycardia without clear atrial activity. The etiology of her heart failure exacerbation was presumed to be paroxysmal atrial fibrillation in the setting of diastolic dysfunction. Given her mild hyperkalemia (5.1), her diuretics were changed to monotherapy with furosemide. Low‐dose beta blockade and antithrombotic therapy were started as well as increased supplementation of levothyroxine. After several days of diuresis, her potassium had normalized, she tolerated initiation of a new ACE inhibitor, and her dyspnea had resolved. On the last hospital day, her dentures were accidentally discarded with her breakfast tray, causing her great distress.
On discharge she was on 4 new medications, 2 old medications had been stopped, and 1 prior medication's dose had been increased. During medication reconciliation, the patient reported that she had not been taking olanzapine for weeks, and thus this was omitted from her home health medication orders, with instructions to discuss with her PCP on first follow‐up within the week. The patient was provided with congestive heart failure instructions and a complete medication list. Unfortunately, the day of discharge was the first day of a holiday weekend.
Case management was unavailable on the weekend; her out‐of‐state family member was unable to be reached by phone, and her usual pharmacy courier service was closed. As she did not have a friend or family member to pick up her prescriptions from an alternate pharmacy, her prescriptions were provided as handwritten scripts, called in to her pharmacy's voice mail, and written on the home health orders. The patient was discharged to her home with communication to her PCP via telephone, e‐mail, and electronic discharge summary.
POSTDISCHARGE
Medications were not delivered to the patient until the third postdischarge day. Three days after discharge, the daughter from out of state left a message for the PCP expressing concern that the patient was failingnot eating or taking any of her medications. An expedited home nursing visit was arranged. Five days after discharge, the pharmacist called the PCP stating he had not received a prescription for the beta‐blocker. Her PCP saw the patient in clinic 6 days after discharge and reconciled the medication list, restarting the olanzapine that the patient had stopped a few weeks before and the mirtazapine, which had not been restarted despite its presence on the discharge orders and patient instructions. She continued to have poor appetite and mood and was taking her medications only with great effort from her visiting nurse and staff at the retirement community. A major cause of this decline was the significant worsening of her depression brought on by hospitalization, lapses in her psychiatric medications, and emotional distress induced by the loss of her dentures during her hospital stay. She was readmitted 10 days after her discharge because she was unable to care for herself.
DISCUSSION
This case demonstrates numerous pitfalls in the transition process. Despite communication between the hospitalists and the PCP and a common electronic medical record, this patient failed the transition from the acute care hospital to the ambulatory setting. On the holiday weekend, ancillary support services were unavailable, including case management to contact her home care agency and her pharmacy to fill and deliver her new prescriptions. Despite efforts by the discharging physician, the out‐of‐town family could not be contacted. Thus, an elderly woman with cognitive impairment was left to process a new diagnosis and 7 medication changes with an unreliable mechanism to obtain her new medications.
With the rise of hospital medicine, it has increasingly been recognized that transitions represent a point of vulnerability in the care of geriatric patients. A change in physical location of care and handoffs between caregivers create the potential for error and loss of information. Prior research has demonstrated frequent quantitative and qualitative deficiencies in the information conveyed between inpatient and outpatient physicians, with direct communication occurring less than 20% of the time.1 In this case, communication occurred between the hospitalists and the outpatient physician, demonstrating that communication is just one element of successful transitions.
Components of effective care transitions have been described in the literature, including: preparation of the patient and caregiver for the transition, medication reconciliation, instructions to patient and caregiver about symptoms and signs of worsening, and an explicit follow‐up plan for tests and appointments.2 Optimally, there is interactive discussion between the hospitalist and the receiving clinician with a summary of events including an updated medication, allergy, and problem list, current advance directives, and a common plan of care.2 This case illustrates that these elements are necessary but may not be sufficient.
Some interventions have been found to be effective. A nurse‐led multidisciplinary approach to the discharge of elderly patients with congestive heart failure led to decreased readmission rates after 90 days and was found to be a cost‐saving measure.3 Similar results have been seen in geriatric patients with a variety of diagnoses in trials using advanced‐practice nurses to bridge the vulnerable period of discharge or by interventions to improve the ability of family caregivers to handle the challenges of the transition.46 Individualized attention to the unique needs of each patient and members of their social support structure, and investment in resources to do so, has the ability to decrease readmissions.
Medication errors, medication omissions, or the inability to fill medications on discharge represent a patient safety challenge. There has been increasing emphasis on medication reconciliation at admission and discharge, but in some cases the gold standard medication list is hard to determine. Electronic medical records would seem to be a natural solution to this problem, but as this case illustrates, the electronic record may not reflect the reality of patient adherence. As in this case, clarification may require another visit with the primary provider, leaving a period of time with an uncertain medication list and therefore a vulnerable patient. Access to medications after discharge was also a problem in this case, but this is not rare. A 2001 study found that 2 days after discharge from a general medicine hospital service, 1 in 5 patients had been unable to obtain all discharge medications.7 A pharmacist‐led medication reconciliation intervention in nursing home patients led to decreases in length of stay and discrepancy‐related adverse drug events. Furthermore, a follow‐up call allowed for clarification of medication questions in 25% of cases.7, 8
Patient characteristics such as depression and cognitive dysfunction have been found to affect readmission rates and are important to assess in addressing risk for poor outcomes after discharge. A 2000 study comparing readmission rates after discharge from a geriatric rehabilitation hospital found that patients with depression had an odds ratio of 3.5 for readmission compared with those without depression.9 Inadequate health literacy is also associated with decreased ability to self‐manage chronic disease and is associated with increased risk of mortality in community‐dwelling elderly such as the patient in this case.10 Asking patients or their proxies to explain their own understanding of the discharge plan can unmask comprehension issues that otherwise may go undetected.
Discharge on a weekend presents a period of critical vulnerability. Early recognition that a transition is susceptible to failure allows the events necessary for success to occur during the week when services are available. An example in the present case might include having had the pharmacy fill the prescriptions prior to the day of discharge. This does introduce a new opportunity for error in cases in which the plan of care changes but would have solved the inability to have prescriptions filled once the holiday weekend had begun.
In cases in which the usual mechanisms break down, increased effort on the part of the hospitalist can usually create a unique solution to the problem. Examples of creative solutions that did not occur in this case might include contacting the manager of the patient's retirement apartment to determine if this individual might be willing to fill prescriptions at an alternate pharmacy. A better alternative would be to change the system such that the solution is readily accessible and time efficient. Weekend availability of case management would be one such step. A means for the hospital's inpatient pharmacy to provide 2‐3 days of bridging medications would prevent weekend prescription access from affecting timely discharges of multiple patients over the course of a year. The hospitalist is in a unique position to take a leadership role in effecting system change to address these issues.
Ultimately, it is the duty of the hospitalist to take responsibility for the safety and well‐being of the patient, and if no solution can be found, it may be necessary to hold discharge or find an alternate disposition until logistical hurdles have been overcome. Indeed, for patients admitted for myocardial infarction, discharges were less likely to occur on weekends, presumably because of lack of ancillary services.11 With foresight, creative problem solving, and systems improvement, this should rarely be necessary.
Transitions continue to be a difficult time for the most vulnerable patients. Intense efforts have improved outcomes in selected populations but have not been broadly applied. Identification of patients at the highest risk, such as those with depression, poor social support, and cognitive limitations, would allow anticipation of difficult transitions and potential utilization of proven interventions, such as advanced‐practice nurses or follow‐up pharmacy contact. Processes such as these might have prevented some of the problems in this patient's discharge. Appreciation of the weekend discharge as a time of particular challenges allows barriers to be identified and solutions created during the week, when resources are still available. Attention to all elements of effective transitions should become part of the growing culture of patient safety.
- ,,, et al.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- .Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs.J Am Geriatr Soc.2003;51:549–555.
- ,,, et al.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333:1190–1195.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281:613–620.
- ,,, et al.Comprehensive discharge planning for the hospitalized elderly: a randomized clinical trial.Ann Intern Med.1994;120:999–1006.
- ,,, et al.Preparing patients and caregivers to participate in care delivered across settings: the care transitions intervention.J Am Geriatr Soc.2004;52:1817–1825.
- ,,, et al.The impact of follow‐up telephone calls to patients after hospitalization.Am J Med.2001;111:26S–30S.
- ,,, et al.Medication reconciliation for reducing drug discrepancy adverse events.Am J Geriatr Pharmacother.2006;4:236–243.
- ,,, et al.Depression and activities of daily living predict rehospitalization within 6 months of discharge from geriatric rehabilitation.Rehabil Psychol.2004;49:219–223.
- ,,, et al.Health literacy and mortality among elderly persons.Arch Intern Med.2007;167:1503–1509.
- ,,, et al.Restricted weekend service inappropriately delays discharge after acute myocardial infarction.Heart.2002;87:216–219.
- ,,, et al.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- .Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs.J Am Geriatr Soc.2003;51:549–555.
- ,,, et al.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333:1190–1195.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281:613–620.
- ,,, et al.Comprehensive discharge planning for the hospitalized elderly: a randomized clinical trial.Ann Intern Med.1994;120:999–1006.
- ,,, et al.Preparing patients and caregivers to participate in care delivered across settings: the care transitions intervention.J Am Geriatr Soc.2004;52:1817–1825.
- ,,, et al.The impact of follow‐up telephone calls to patients after hospitalization.Am J Med.2001;111:26S–30S.
- ,,, et al.Medication reconciliation for reducing drug discrepancy adverse events.Am J Geriatr Pharmacother.2006;4:236–243.
- ,,, et al.Depression and activities of daily living predict rehospitalization within 6 months of discharge from geriatric rehabilitation.Rehabil Psychol.2004;49:219–223.
- ,,, et al.Health literacy and mortality among elderly persons.Arch Intern Med.2007;167:1503–1509.
- ,,, et al.Restricted weekend service inappropriately delays discharge after acute myocardial infarction.Heart.2002;87:216–219.
Jumpstarting Hospital Medicine Research
Dramatic changes in the organization, financing, and delivery of hospital care that began a decade ago continue to accelerate. One of the most important changes has been the emergence of hospitalists as providers of inpatient care.1 Hospitalists are physicians, usually general internists, whose clinical focus is the hospitalized patient. As patient illnesses have become more severe and complex, physicians have found it difficult to balance inpatient and outpatient care and have focused on one of the two.25 It is estimated that there are currently 15,000 practicing hospitalists nationally, and projections suggest that this number may exceed 30,000 by 2010, which is equal to the number of cardiologists currently practicing in the United States.6 A 2003 survey from the American Hospital Association showed that more than 30% of the nation's 4900 community hospitals have hospital medicine groups.7 Furthermore, more than 70% of the nation's largest hospitals (>500 beds) and 66% of major teaching hospitals use hospitalists.7
The transition to a hospitalist model generates multiple new research questions about the best approach to caring for the hospitalized patient. Additionally, hospitalists may spawn new areas of clinical research by tackling clinical issues that formerly lacked a large number of specialist investigators. Examples include implementation‐based studies,8, 9 inpatient safety practices,1012 quasi‐experimental studies focusing on common inpatient issues,13, 14 and the evaluation of new methods for reducing resource utilization within various inpatient care delivery structures.15, 16
Similarly, if future clinical trials are to be carried out in real‐world settings, by necessity these will require the participation of hospitalists. Clinical research performed by hospitalists and hospital medicine programs, however, remains underdeveloped. Although this has been attributed to several variables, including the youth of the field, a paucity of fellowship‐trained hospitalist researchers, and a lack of a hospitalist‐oriented national funding source, we also believe that additional barriers exist which could be overcome if hospitalists actively partnered with specialists to perform hospital‐based clinical and translational research.
Hospitalists lack clinical expertise in many clinical issues. In both academic and nonacademic settings, the diagnostic approach, individual treatment decisions, and follow‐up of complex patients occur with frequent consultation of specialists. Specialists often provide a deeper understanding of both the pathophysiologic concepts and scientific principles underlying important clinical questions and are more likely to have had fellowship training that included clinical research experience. Specialists also have more access to extramural funding for disease‐based investigation, and thus their involvement in hospital‐based clinical research would likely enhance funding opportunities, improve project feasibility, and increase dissemination of the results. A successful clinical research program will therefore be one that combines specialists and hospitalists working collaboratively to determine the best way to care for inpatients. With that in mind, we created the University of Michigan SpecialistHospitalist Allied Research Program (SHARP).
METHODS
Setting
The University of Michigan Medical Center includes a 900‐bed teaching hospital with more than 44,000 yearly inpatient discharges, and the Department of Internal Medicine manages nearly 15,000 annual discharges. The University of Michigan Hospital Medicine Program has grown dramatically over the past few years and now includes more than 30 hospitalists. These hospitalists will manage nearly 8000 admissions in the upcoming year, which represent more than half of all the patients admitted to the Department of Internal Medicine. Five years ago, these 8000 admissions would have been cared for by 3 to 4 times as many providers, most of whom would have been specialists. Currently, specialists consult regularly on patients cared for by hospitalists, and as a result, a few loosely formed research collaborations developed spontaneously but lacked resources or infrastructure to facilitate their completion. SHARP was intended to organize these clinical research pilot studies and jumpstart hospital‐based clinical and translational research.
The SHARP Intervention
Objectives
In 2006, hospitalists and specialists with an interest in expanding clinical and translational research aimed at caring for inpatients were brought together for the SHARP intervention. This intervention had several objectives:
To develop a clinical research infrastructure within the University of Michigan Hospital Medicine Program to facilitate patient participation.
To foster increased specialisthospitalist collaboration for addressing common inpatient problems.
To facilitate pilot projects and preliminary data collection that enhance the ability to obtain subsequent extramural funding for collaborative research projects.
To facilitate multicenter investigation led by the University of Michigan by allowing the SHARP investigators to use an existing hospitalist consortium to expand the scope of research projects.
Ultimately, to develop the ability to perform multicenter intervention‐based clinical trials.
Structure
The key to SHARP's infrastructure is its personnel and governance structure. At the head of SHARP is an academic hospitalist as principal investigator (PI) and an academic cardiologist with health services research training serving as coprincipal investigator (Co‐PI). Key personnel also include a hospitalist investigator, a masters‐level research associate, a PhD clinical epidemiologist, and the hospitalists and subspecialists who serve as investigators. Although the program leadership has research experience, many of the hospitalist and specialist investigators are junior faculty without extensive prior research experience. Thus, SHARP was specifically designed to build the capacity to enhance inpatient clinical and translational research and to remove barriers for new investigators developing their academic careers.
It is critical that oversight provides direction for the research program, assists with project identification and selection, and facilitates collaborations that tie diverse projects together. We believe that this is best accomplished by the creation of a steering committee chaired by both the PI and Co‐PI. The steering committee also includes key individuals such as the Vice Chair of the Department of Medicine and the Associate Dean for Clinical and Translational Research at the University of Michigan. The 2 cochairs are responsible for overseeing the program and reporting the progress of SHARP to the University of Michigan Department of Internal Medicine. They will help identify and produce viable research proposals that can be brought to the full committee. To help the program understand and overcome bureaucratic obstacles, we have also included a former high‐level administrator on the steering committee as a consultant. Given the initial scope of the program, the SHARP steering committee has had a small number of key individuals. As the program grows and increases its number of ongoing collaborative projects, we will likely need to expand committee membership.
SHARP leadership meets regularly to plan projects, discuss grant ideas, make hiring decisions, and troubleshoot problems in existing projects. The entire steering committee meets quarterly to help chart the overall course of the program. A more thorough description of the program and its structure can be found on the SHARP Web site (
SHARP Funding
SHARP could not exist without resources. The funding for the program comes from the Department of Internal Medicine and uses revenue from the hospital medicine program that flows to the department. To garner support for the program, SHARP leadership sought buy‐in from the Chair of Medicine, all the division chiefs, and key faculty active in clinical research. The fact that the program has the potential to benefit not just hospitalists but also other department faculty such as specialists facilitated departmental funding. The program is funded for 3 years with an 18‐month program review to gauge progress. Funding is used to build clinical research infrastructure and facilitate collection of pilot data. SHARP resources support a portion of the salaries of key personnel for the 3‐year duration of the project (research associate, 50%; PI, 10%; Co‐PI, 5%; and epidemiologist, 5%), after which time intramural funding ends. Every SHARP project is, therefore, expected to apply for extramural funding with the goal of full extramural programmatic support after 3 years.
SHARP Performance Metrics
Measuring the accomplishments of SHARP is clearly important. As the program is intended to jumpstart collaborative inpatient clinical research, the number of such projects is important to track. An additional goal is to support work that leads to extramural funding. As the program started from scratch, it is unrealistic to have completed peer‐reviewed manuscripts or successful extramural grants as the sole metrics by which the program is judged, especially early in its initiation. In a yearly report to the department chair, we will report on primary and secondary outcomes (see Table 1).
| Primary outcomes |
|---|
|
| 1. Number of ongoing research projects involving SHARP support and a brief description of the aims and status of each |
| 2. Number of extramural grants submitted in which SHARP is mentioned or involved |
| 3. Extramural grants received (total and direct dollars) |
| 4. Peer‐reviewed publications authored by SHARP investigators |
| Secondary outcomes |
| 1. Abstracts accepted for presentation at national or international scientific meetings |
| 2. Non‐peer‐reviewed publications related to SHARP |
| 3. Invited presentations by SHARP investigators |
| 4. People who have visited the University of Michigan in conjunction with SHARP work (eg, visiting professors) |
Initial SHARP Projects
SHARP has a formal process for evaluating potential projects. A steering committee ultimately decides how best to use SHARP‐related resources. Key components in this decision are related to the proposal's innovation, feasibility, and importance as well as the extent of specialisthospitalist collaboration. The 2 projects described next are our initial areas of focus and exemplify these concepts. One project partners hospitalists with infectious disease specialists, whereas the second pairs hospitalists with geriatricians and clinical pharmacists.
Reducing False Positive Blood Cultures
The blood culture is an important tool for the diagnosis and management of bloodstream infections. As a result, physicians have a low threshold for obtaining blood cultures. Unfortunately, up to half of all positive blood cultures are positive because of contamination. These false positive cultures lead to additional diagnostic testing, unnecessary antibiotics, and increased healthcare costs.17 A variety of antiseptic agents and techniques are used to prevent falsely positive cultures. However, a recent evidence‐based systematic review performed by University of Michigan investigators found no clear evidence to suggest which antiseptic agent should be routinely used. They concluded that a randomized controlled trial was urgently needed.18
SHARP and its infrastructure have begun a cluster‐randomized crossover trial at the university hospital. The trial compares the effects of a variety of skin antiseptic agents on peripheral blood culture contamination rates. The study population includes hospitalized patients undergoing venipuncture for peripheral blood cultures on 3 general medicine and surgery floors. The trial will include over 12,000 blood culture sets and will have 85% power to detect a 0.5% difference in effectiveness between antiseptic agents. Key outcomes will be rates of positive blood cultures (true positive versus false positive), quantity of additional diagnostic testing generated by positive cultures, resource use (including antibiotics), and associated costs. Clinical outcomes such as length of stay and inpatient mortality will also be measured as secondary outcomes.
Pharmacist‐Facilitated Hospital Discharge
Hospital discharge is a complex process in which patients must be transferred from the care of an inpatient team to that of an outpatient provider. During most hospitalizations, a patient will have new medications added, a chronic medication stopped, or a change in medication dosage. Studies have revealed that the most common adverse events that have an impact on patients after discharge are related to medications.1921 In our experience at the University of Michigan, patients frequently have medication‐related adverse events after discharge because they do not understand what medications they should be taking, what they are used for, how to manage side effects, or whom to call with problems. In addition, predictable medication‐related issues (such as the ability to pay for a medicine or expected serum electrolyte changes with newly added medications) are not universally anticipated. The frail elderly are especially vulnerable to medication‐related adverse events.
Building on the work of others in the field, we proposed studying the impact of an inpatient clinical pharmacist to address medication misadventures related to hospital discharge in our elderly population.22 The study uses an interrupted time series design (the pharmacist will alternate months at a nonresident hospitalist service and a resident general medicine service) to measure the impact of the clinical pharmacist. The pharmacist will focus on patients over the age of 65 meeting criteria that identify them to be at high risk for an adverse medication event after discharge. These factors include any new medication started in the hospital, medication noncompliance or an adverse medication event that led to the admission, or use of a high‐risk medication (eg, anticoagulants, narcotics, diuretics, diabetic agents, and immunosuppressives). The pharmacist and inpatient physicians will identify high‐risk patients who will receive predischarge medication counseling. This process will identify problem medications and needed follow‐up (eg, laboratory testing) and assess compliance issues. After discharge, patients will be contacted by the pharmacist both within 72 hours and at 30 days. Standardized questions will be asked of patients to troubleshoot medication issues, assess them for problems with medications or follow‐up, and identify patients who may need more urgent access to a healthcare provider to address medication‐related problems.
Key outcomes will include the pharmacist's actions at discharge (eg, dose changes made, medication class switches, and side‐effect monitoring implemented). In addition, we will track types of medication issues identified after discharge and interventions made. Important clinical outcomes will include return to the emergency department after discharge, 30‐day readmission rates, and healthcare‐related costs.
DISCUSSION AND NEXT STEPS
SHARP is a novel clinical research program partnering hospitalists with specialists. Its current focus targets single‐institution studies that generate pilot data leading to larger projects. The ultimate goal is to develop the ability to do larger multicenter investigator‐initiated projects. The SHARP program will also have the ability to perform observational studies to identify predictors and risk factors and the ability to carry out implementation studies that show how best to translate results from published articles to direct patient care.
A specialisthospitalist collaboration overcomes barriers that we feel may impede hospital medicine research at an academic medical center. For a similar program to succeed at other institutions, key components from our program will have to be replicated. First, senior, fellowship‐trained researchers are required to mentor junior investigators (who may or may not have additional fellowship training), help guide project selection, oversee grant and manuscript submissions, and troubleshoot problems that arise in the course of any clinical research project. In our institution, this comes from within our hospitalist program and from our specialist collaborators. In institutions lacking hospitalists with research experience, this guidance could come from within a division of general medicine, internal medicine specialty divisions, internal medicine department leadership, or even noninternal medicine departments (eg, emergency medicine, neurology, and surgery) that have traditionally been involved in clinical research programs.
A second key component that must be considered is funding. An initial investment is necessary to fund key personnel dedicated to getting projects started on the right track, collecting pilot data, and ensuring project completion and dissemination of the results. The positive margin generated by our hospitalist program facilitated the initial investment. In the absence of a positive margin, resources could come directly from the hospital, the medical school, the department of internal medicine, or perhaps a foundation. The case would need to be made that an initial short‐term investment would enhance the academic standing of the institution, enhance the careers of young investigators, and over time lead to a self‐sustaining program through investigator‐initiated grants and extramural funding. In addition to experienced leadership and funding, we created an oversight committee, but we feel that this is not a critical component. A potential concern with a program that partners with specialists might be that research topics become too disease‐specific or specialty‐oriented. We specifically created the oversight committee to protect against this possibility, and other institutions might need similar safeguards.
Our next step includes leveraging existing hospitalist collaboratives that reach beyond academic medical centers to expand further the reach of SHARP. Ultimately, any new therapy, clinical tool, diagnostic paradigm, or implementation strategy that is developed or evaluated bythe SHARP program would need to be tested in a real‐world setting to assess external validity. With support from the Blue Cross Blue Shield of Michigan Foundation, we have created a multihospital patient safety consortium, the Hospitalists as Emerging Leaders in Patient Safety Consortium, which includes academic, government, urban, rural, teaching, and nonteaching hospitals.23 Although the initial focus is patient safety, our goal for the consortium is to develop it into a multihospital clinical research program that could take pilot projects developed by SHARP and test them in real‐world settings. We believe that full‐scale multihospital studies based on SHARP pilot data will be very attractive to external funding agencies and will help SHARP become financially self‐sufficient after the initial 3‐year start‐up.
Hospital medicine research is desperately needed.24, 25 Unfortunately, the clinical research capabilities of most hospital medicine programs are quite underdeveloped. We believe that partnering hospitalists with specialists can facilitate collaborative research to identify the best way to care for inpatients. If successful, we believe that variations of this model can be replicated at other institutions and will be a critical factor in jumpstarting hospital medicine clinical research.
Acknowledgements
The authors thank Dr. Marc E. Lippman, Dr. Robert F. Todd, Dr. Larry McMahon, Dr. Timothy J. Laing, and Mr. Lindsay J. Graham, whose support made this program possible.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- ,,,,,.What effect does increasing inpatient time have on outpatient‐oriented internist satisfaction?J Gen Intern Med.2003;18:725–729.
- ,,,,.Characteristics of general internists who practice only outpatient medicine: results from the physician worklife study.Semin Med Pract.2002;5:5–11.
- ,.Hospitalists in teaching hospitals: opportunities but not without danger.J Gen Intern Med.2004;19:392–393.
- ,.Hospitalists: the new model of inpatient medical care in the United States.Eur J Intern Med.2003;14:65–70.
- ,,,,.The potential size of the hospitalist workforce in the United States.Am J Med.1999;106:441–445.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,,, et al.Translating infection prevention evidence into practice using quantitative and qualitative research.Am J Infect Control.2006;34:507–512.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,,,.Making health care safer: a critical analysis of patient safety practices.Evid Rep Technol Assess (Summ).2001;(43):i–x,1–668.
- ,,,.Safe but sound: patient safety meets evidence‐based medicine.JAMA.2002;288:508–513.
- ,,,.Clinical pharmacists and inpatient medical care: a systematic review.Arch Intern Med.2006;166:955–964.
- ,,, et al.Are antiseptic‐coated central venous catheters effective in a real‐world setting?Am J Infect Control.2006;34:388–393.
- ,,,,.Effectiveness of ceftriaxone plus doxycycline in the treatment of patients hospitalized with community‐acquired pneumonia.J Hosp Med.2006;1:7–12.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,.What effect does physician “profiling” have on inpatient physician satisfaction and hospital length of stay?BMC Health Serv Res.2006;6:45.
- ,,.Contaminant blood cultures and resource utilization. The true consequences of false‐positive results.JAMA.1991;265:365–369.
- ,,,,,.Review of clinical trials of skin antiseptic agents used to reduce blood culture contamination.Infect Control Hosp Epidemiol.2007;28:892–895.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20:317–323.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170:345–349.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- ,,.Hospitalists as emerging leaders in patient safety: targeting a few to affect many.JPatient Saf.2005;1:78–82.
- .The future of hospital medicine: evolution or revolution?Am J Med.2004;117:446–450.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:72 e1–7.
Dramatic changes in the organization, financing, and delivery of hospital care that began a decade ago continue to accelerate. One of the most important changes has been the emergence of hospitalists as providers of inpatient care.1 Hospitalists are physicians, usually general internists, whose clinical focus is the hospitalized patient. As patient illnesses have become more severe and complex, physicians have found it difficult to balance inpatient and outpatient care and have focused on one of the two.25 It is estimated that there are currently 15,000 practicing hospitalists nationally, and projections suggest that this number may exceed 30,000 by 2010, which is equal to the number of cardiologists currently practicing in the United States.6 A 2003 survey from the American Hospital Association showed that more than 30% of the nation's 4900 community hospitals have hospital medicine groups.7 Furthermore, more than 70% of the nation's largest hospitals (>500 beds) and 66% of major teaching hospitals use hospitalists.7
The transition to a hospitalist model generates multiple new research questions about the best approach to caring for the hospitalized patient. Additionally, hospitalists may spawn new areas of clinical research by tackling clinical issues that formerly lacked a large number of specialist investigators. Examples include implementation‐based studies,8, 9 inpatient safety practices,1012 quasi‐experimental studies focusing on common inpatient issues,13, 14 and the evaluation of new methods for reducing resource utilization within various inpatient care delivery structures.15, 16
Similarly, if future clinical trials are to be carried out in real‐world settings, by necessity these will require the participation of hospitalists. Clinical research performed by hospitalists and hospital medicine programs, however, remains underdeveloped. Although this has been attributed to several variables, including the youth of the field, a paucity of fellowship‐trained hospitalist researchers, and a lack of a hospitalist‐oriented national funding source, we also believe that additional barriers exist which could be overcome if hospitalists actively partnered with specialists to perform hospital‐based clinical and translational research.
Hospitalists lack clinical expertise in many clinical issues. In both academic and nonacademic settings, the diagnostic approach, individual treatment decisions, and follow‐up of complex patients occur with frequent consultation of specialists. Specialists often provide a deeper understanding of both the pathophysiologic concepts and scientific principles underlying important clinical questions and are more likely to have had fellowship training that included clinical research experience. Specialists also have more access to extramural funding for disease‐based investigation, and thus their involvement in hospital‐based clinical research would likely enhance funding opportunities, improve project feasibility, and increase dissemination of the results. A successful clinical research program will therefore be one that combines specialists and hospitalists working collaboratively to determine the best way to care for inpatients. With that in mind, we created the University of Michigan SpecialistHospitalist Allied Research Program (SHARP).
METHODS
Setting
The University of Michigan Medical Center includes a 900‐bed teaching hospital with more than 44,000 yearly inpatient discharges, and the Department of Internal Medicine manages nearly 15,000 annual discharges. The University of Michigan Hospital Medicine Program has grown dramatically over the past few years and now includes more than 30 hospitalists. These hospitalists will manage nearly 8000 admissions in the upcoming year, which represent more than half of all the patients admitted to the Department of Internal Medicine. Five years ago, these 8000 admissions would have been cared for by 3 to 4 times as many providers, most of whom would have been specialists. Currently, specialists consult regularly on patients cared for by hospitalists, and as a result, a few loosely formed research collaborations developed spontaneously but lacked resources or infrastructure to facilitate their completion. SHARP was intended to organize these clinical research pilot studies and jumpstart hospital‐based clinical and translational research.
The SHARP Intervention
Objectives
In 2006, hospitalists and specialists with an interest in expanding clinical and translational research aimed at caring for inpatients were brought together for the SHARP intervention. This intervention had several objectives:
To develop a clinical research infrastructure within the University of Michigan Hospital Medicine Program to facilitate patient participation.
To foster increased specialisthospitalist collaboration for addressing common inpatient problems.
To facilitate pilot projects and preliminary data collection that enhance the ability to obtain subsequent extramural funding for collaborative research projects.
To facilitate multicenter investigation led by the University of Michigan by allowing the SHARP investigators to use an existing hospitalist consortium to expand the scope of research projects.
Ultimately, to develop the ability to perform multicenter intervention‐based clinical trials.
Structure
The key to SHARP's infrastructure is its personnel and governance structure. At the head of SHARP is an academic hospitalist as principal investigator (PI) and an academic cardiologist with health services research training serving as coprincipal investigator (Co‐PI). Key personnel also include a hospitalist investigator, a masters‐level research associate, a PhD clinical epidemiologist, and the hospitalists and subspecialists who serve as investigators. Although the program leadership has research experience, many of the hospitalist and specialist investigators are junior faculty without extensive prior research experience. Thus, SHARP was specifically designed to build the capacity to enhance inpatient clinical and translational research and to remove barriers for new investigators developing their academic careers.
It is critical that oversight provides direction for the research program, assists with project identification and selection, and facilitates collaborations that tie diverse projects together. We believe that this is best accomplished by the creation of a steering committee chaired by both the PI and Co‐PI. The steering committee also includes key individuals such as the Vice Chair of the Department of Medicine and the Associate Dean for Clinical and Translational Research at the University of Michigan. The 2 cochairs are responsible for overseeing the program and reporting the progress of SHARP to the University of Michigan Department of Internal Medicine. They will help identify and produce viable research proposals that can be brought to the full committee. To help the program understand and overcome bureaucratic obstacles, we have also included a former high‐level administrator on the steering committee as a consultant. Given the initial scope of the program, the SHARP steering committee has had a small number of key individuals. As the program grows and increases its number of ongoing collaborative projects, we will likely need to expand committee membership.
SHARP leadership meets regularly to plan projects, discuss grant ideas, make hiring decisions, and troubleshoot problems in existing projects. The entire steering committee meets quarterly to help chart the overall course of the program. A more thorough description of the program and its structure can be found on the SHARP Web site (
SHARP Funding
SHARP could not exist without resources. The funding for the program comes from the Department of Internal Medicine and uses revenue from the hospital medicine program that flows to the department. To garner support for the program, SHARP leadership sought buy‐in from the Chair of Medicine, all the division chiefs, and key faculty active in clinical research. The fact that the program has the potential to benefit not just hospitalists but also other department faculty such as specialists facilitated departmental funding. The program is funded for 3 years with an 18‐month program review to gauge progress. Funding is used to build clinical research infrastructure and facilitate collection of pilot data. SHARP resources support a portion of the salaries of key personnel for the 3‐year duration of the project (research associate, 50%; PI, 10%; Co‐PI, 5%; and epidemiologist, 5%), after which time intramural funding ends. Every SHARP project is, therefore, expected to apply for extramural funding with the goal of full extramural programmatic support after 3 years.
SHARP Performance Metrics
Measuring the accomplishments of SHARP is clearly important. As the program is intended to jumpstart collaborative inpatient clinical research, the number of such projects is important to track. An additional goal is to support work that leads to extramural funding. As the program started from scratch, it is unrealistic to have completed peer‐reviewed manuscripts or successful extramural grants as the sole metrics by which the program is judged, especially early in its initiation. In a yearly report to the department chair, we will report on primary and secondary outcomes (see Table 1).
| Primary outcomes |
|---|
|
| 1. Number of ongoing research projects involving SHARP support and a brief description of the aims and status of each |
| 2. Number of extramural grants submitted in which SHARP is mentioned or involved |
| 3. Extramural grants received (total and direct dollars) |
| 4. Peer‐reviewed publications authored by SHARP investigators |
| Secondary outcomes |
| 1. Abstracts accepted for presentation at national or international scientific meetings |
| 2. Non‐peer‐reviewed publications related to SHARP |
| 3. Invited presentations by SHARP investigators |
| 4. People who have visited the University of Michigan in conjunction with SHARP work (eg, visiting professors) |
Initial SHARP Projects
SHARP has a formal process for evaluating potential projects. A steering committee ultimately decides how best to use SHARP‐related resources. Key components in this decision are related to the proposal's innovation, feasibility, and importance as well as the extent of specialisthospitalist collaboration. The 2 projects described next are our initial areas of focus and exemplify these concepts. One project partners hospitalists with infectious disease specialists, whereas the second pairs hospitalists with geriatricians and clinical pharmacists.
Reducing False Positive Blood Cultures
The blood culture is an important tool for the diagnosis and management of bloodstream infections. As a result, physicians have a low threshold for obtaining blood cultures. Unfortunately, up to half of all positive blood cultures are positive because of contamination. These false positive cultures lead to additional diagnostic testing, unnecessary antibiotics, and increased healthcare costs.17 A variety of antiseptic agents and techniques are used to prevent falsely positive cultures. However, a recent evidence‐based systematic review performed by University of Michigan investigators found no clear evidence to suggest which antiseptic agent should be routinely used. They concluded that a randomized controlled trial was urgently needed.18
SHARP and its infrastructure have begun a cluster‐randomized crossover trial at the university hospital. The trial compares the effects of a variety of skin antiseptic agents on peripheral blood culture contamination rates. The study population includes hospitalized patients undergoing venipuncture for peripheral blood cultures on 3 general medicine and surgery floors. The trial will include over 12,000 blood culture sets and will have 85% power to detect a 0.5% difference in effectiveness between antiseptic agents. Key outcomes will be rates of positive blood cultures (true positive versus false positive), quantity of additional diagnostic testing generated by positive cultures, resource use (including antibiotics), and associated costs. Clinical outcomes such as length of stay and inpatient mortality will also be measured as secondary outcomes.
Pharmacist‐Facilitated Hospital Discharge
Hospital discharge is a complex process in which patients must be transferred from the care of an inpatient team to that of an outpatient provider. During most hospitalizations, a patient will have new medications added, a chronic medication stopped, or a change in medication dosage. Studies have revealed that the most common adverse events that have an impact on patients after discharge are related to medications.1921 In our experience at the University of Michigan, patients frequently have medication‐related adverse events after discharge because they do not understand what medications they should be taking, what they are used for, how to manage side effects, or whom to call with problems. In addition, predictable medication‐related issues (such as the ability to pay for a medicine or expected serum electrolyte changes with newly added medications) are not universally anticipated. The frail elderly are especially vulnerable to medication‐related adverse events.
Building on the work of others in the field, we proposed studying the impact of an inpatient clinical pharmacist to address medication misadventures related to hospital discharge in our elderly population.22 The study uses an interrupted time series design (the pharmacist will alternate months at a nonresident hospitalist service and a resident general medicine service) to measure the impact of the clinical pharmacist. The pharmacist will focus on patients over the age of 65 meeting criteria that identify them to be at high risk for an adverse medication event after discharge. These factors include any new medication started in the hospital, medication noncompliance or an adverse medication event that led to the admission, or use of a high‐risk medication (eg, anticoagulants, narcotics, diuretics, diabetic agents, and immunosuppressives). The pharmacist and inpatient physicians will identify high‐risk patients who will receive predischarge medication counseling. This process will identify problem medications and needed follow‐up (eg, laboratory testing) and assess compliance issues. After discharge, patients will be contacted by the pharmacist both within 72 hours and at 30 days. Standardized questions will be asked of patients to troubleshoot medication issues, assess them for problems with medications or follow‐up, and identify patients who may need more urgent access to a healthcare provider to address medication‐related problems.
Key outcomes will include the pharmacist's actions at discharge (eg, dose changes made, medication class switches, and side‐effect monitoring implemented). In addition, we will track types of medication issues identified after discharge and interventions made. Important clinical outcomes will include return to the emergency department after discharge, 30‐day readmission rates, and healthcare‐related costs.
DISCUSSION AND NEXT STEPS
SHARP is a novel clinical research program partnering hospitalists with specialists. Its current focus targets single‐institution studies that generate pilot data leading to larger projects. The ultimate goal is to develop the ability to do larger multicenter investigator‐initiated projects. The SHARP program will also have the ability to perform observational studies to identify predictors and risk factors and the ability to carry out implementation studies that show how best to translate results from published articles to direct patient care.
A specialisthospitalist collaboration overcomes barriers that we feel may impede hospital medicine research at an academic medical center. For a similar program to succeed at other institutions, key components from our program will have to be replicated. First, senior, fellowship‐trained researchers are required to mentor junior investigators (who may or may not have additional fellowship training), help guide project selection, oversee grant and manuscript submissions, and troubleshoot problems that arise in the course of any clinical research project. In our institution, this comes from within our hospitalist program and from our specialist collaborators. In institutions lacking hospitalists with research experience, this guidance could come from within a division of general medicine, internal medicine specialty divisions, internal medicine department leadership, or even noninternal medicine departments (eg, emergency medicine, neurology, and surgery) that have traditionally been involved in clinical research programs.
A second key component that must be considered is funding. An initial investment is necessary to fund key personnel dedicated to getting projects started on the right track, collecting pilot data, and ensuring project completion and dissemination of the results. The positive margin generated by our hospitalist program facilitated the initial investment. In the absence of a positive margin, resources could come directly from the hospital, the medical school, the department of internal medicine, or perhaps a foundation. The case would need to be made that an initial short‐term investment would enhance the academic standing of the institution, enhance the careers of young investigators, and over time lead to a self‐sustaining program through investigator‐initiated grants and extramural funding. In addition to experienced leadership and funding, we created an oversight committee, but we feel that this is not a critical component. A potential concern with a program that partners with specialists might be that research topics become too disease‐specific or specialty‐oriented. We specifically created the oversight committee to protect against this possibility, and other institutions might need similar safeguards.
Our next step includes leveraging existing hospitalist collaboratives that reach beyond academic medical centers to expand further the reach of SHARP. Ultimately, any new therapy, clinical tool, diagnostic paradigm, or implementation strategy that is developed or evaluated bythe SHARP program would need to be tested in a real‐world setting to assess external validity. With support from the Blue Cross Blue Shield of Michigan Foundation, we have created a multihospital patient safety consortium, the Hospitalists as Emerging Leaders in Patient Safety Consortium, which includes academic, government, urban, rural, teaching, and nonteaching hospitals.23 Although the initial focus is patient safety, our goal for the consortium is to develop it into a multihospital clinical research program that could take pilot projects developed by SHARP and test them in real‐world settings. We believe that full‐scale multihospital studies based on SHARP pilot data will be very attractive to external funding agencies and will help SHARP become financially self‐sufficient after the initial 3‐year start‐up.
Hospital medicine research is desperately needed.24, 25 Unfortunately, the clinical research capabilities of most hospital medicine programs are quite underdeveloped. We believe that partnering hospitalists with specialists can facilitate collaborative research to identify the best way to care for inpatients. If successful, we believe that variations of this model can be replicated at other institutions and will be a critical factor in jumpstarting hospital medicine clinical research.
Acknowledgements
The authors thank Dr. Marc E. Lippman, Dr. Robert F. Todd, Dr. Larry McMahon, Dr. Timothy J. Laing, and Mr. Lindsay J. Graham, whose support made this program possible.
Dramatic changes in the organization, financing, and delivery of hospital care that began a decade ago continue to accelerate. One of the most important changes has been the emergence of hospitalists as providers of inpatient care.1 Hospitalists are physicians, usually general internists, whose clinical focus is the hospitalized patient. As patient illnesses have become more severe and complex, physicians have found it difficult to balance inpatient and outpatient care and have focused on one of the two.25 It is estimated that there are currently 15,000 practicing hospitalists nationally, and projections suggest that this number may exceed 30,000 by 2010, which is equal to the number of cardiologists currently practicing in the United States.6 A 2003 survey from the American Hospital Association showed that more than 30% of the nation's 4900 community hospitals have hospital medicine groups.7 Furthermore, more than 70% of the nation's largest hospitals (>500 beds) and 66% of major teaching hospitals use hospitalists.7
The transition to a hospitalist model generates multiple new research questions about the best approach to caring for the hospitalized patient. Additionally, hospitalists may spawn new areas of clinical research by tackling clinical issues that formerly lacked a large number of specialist investigators. Examples include implementation‐based studies,8, 9 inpatient safety practices,1012 quasi‐experimental studies focusing on common inpatient issues,13, 14 and the evaluation of new methods for reducing resource utilization within various inpatient care delivery structures.15, 16
Similarly, if future clinical trials are to be carried out in real‐world settings, by necessity these will require the participation of hospitalists. Clinical research performed by hospitalists and hospital medicine programs, however, remains underdeveloped. Although this has been attributed to several variables, including the youth of the field, a paucity of fellowship‐trained hospitalist researchers, and a lack of a hospitalist‐oriented national funding source, we also believe that additional barriers exist which could be overcome if hospitalists actively partnered with specialists to perform hospital‐based clinical and translational research.
Hospitalists lack clinical expertise in many clinical issues. In both academic and nonacademic settings, the diagnostic approach, individual treatment decisions, and follow‐up of complex patients occur with frequent consultation of specialists. Specialists often provide a deeper understanding of both the pathophysiologic concepts and scientific principles underlying important clinical questions and are more likely to have had fellowship training that included clinical research experience. Specialists also have more access to extramural funding for disease‐based investigation, and thus their involvement in hospital‐based clinical research would likely enhance funding opportunities, improve project feasibility, and increase dissemination of the results. A successful clinical research program will therefore be one that combines specialists and hospitalists working collaboratively to determine the best way to care for inpatients. With that in mind, we created the University of Michigan SpecialistHospitalist Allied Research Program (SHARP).
METHODS
Setting
The University of Michigan Medical Center includes a 900‐bed teaching hospital with more than 44,000 yearly inpatient discharges, and the Department of Internal Medicine manages nearly 15,000 annual discharges. The University of Michigan Hospital Medicine Program has grown dramatically over the past few years and now includes more than 30 hospitalists. These hospitalists will manage nearly 8000 admissions in the upcoming year, which represent more than half of all the patients admitted to the Department of Internal Medicine. Five years ago, these 8000 admissions would have been cared for by 3 to 4 times as many providers, most of whom would have been specialists. Currently, specialists consult regularly on patients cared for by hospitalists, and as a result, a few loosely formed research collaborations developed spontaneously but lacked resources or infrastructure to facilitate their completion. SHARP was intended to organize these clinical research pilot studies and jumpstart hospital‐based clinical and translational research.
The SHARP Intervention
Objectives
In 2006, hospitalists and specialists with an interest in expanding clinical and translational research aimed at caring for inpatients were brought together for the SHARP intervention. This intervention had several objectives:
To develop a clinical research infrastructure within the University of Michigan Hospital Medicine Program to facilitate patient participation.
To foster increased specialisthospitalist collaboration for addressing common inpatient problems.
To facilitate pilot projects and preliminary data collection that enhance the ability to obtain subsequent extramural funding for collaborative research projects.
To facilitate multicenter investigation led by the University of Michigan by allowing the SHARP investigators to use an existing hospitalist consortium to expand the scope of research projects.
Ultimately, to develop the ability to perform multicenter intervention‐based clinical trials.
Structure
The key to SHARP's infrastructure is its personnel and governance structure. At the head of SHARP is an academic hospitalist as principal investigator (PI) and an academic cardiologist with health services research training serving as coprincipal investigator (Co‐PI). Key personnel also include a hospitalist investigator, a masters‐level research associate, a PhD clinical epidemiologist, and the hospitalists and subspecialists who serve as investigators. Although the program leadership has research experience, many of the hospitalist and specialist investigators are junior faculty without extensive prior research experience. Thus, SHARP was specifically designed to build the capacity to enhance inpatient clinical and translational research and to remove barriers for new investigators developing their academic careers.
It is critical that oversight provides direction for the research program, assists with project identification and selection, and facilitates collaborations that tie diverse projects together. We believe that this is best accomplished by the creation of a steering committee chaired by both the PI and Co‐PI. The steering committee also includes key individuals such as the Vice Chair of the Department of Medicine and the Associate Dean for Clinical and Translational Research at the University of Michigan. The 2 cochairs are responsible for overseeing the program and reporting the progress of SHARP to the University of Michigan Department of Internal Medicine. They will help identify and produce viable research proposals that can be brought to the full committee. To help the program understand and overcome bureaucratic obstacles, we have also included a former high‐level administrator on the steering committee as a consultant. Given the initial scope of the program, the SHARP steering committee has had a small number of key individuals. As the program grows and increases its number of ongoing collaborative projects, we will likely need to expand committee membership.
SHARP leadership meets regularly to plan projects, discuss grant ideas, make hiring decisions, and troubleshoot problems in existing projects. The entire steering committee meets quarterly to help chart the overall course of the program. A more thorough description of the program and its structure can be found on the SHARP Web site (
SHARP Funding
SHARP could not exist without resources. The funding for the program comes from the Department of Internal Medicine and uses revenue from the hospital medicine program that flows to the department. To garner support for the program, SHARP leadership sought buy‐in from the Chair of Medicine, all the division chiefs, and key faculty active in clinical research. The fact that the program has the potential to benefit not just hospitalists but also other department faculty such as specialists facilitated departmental funding. The program is funded for 3 years with an 18‐month program review to gauge progress. Funding is used to build clinical research infrastructure and facilitate collection of pilot data. SHARP resources support a portion of the salaries of key personnel for the 3‐year duration of the project (research associate, 50%; PI, 10%; Co‐PI, 5%; and epidemiologist, 5%), after which time intramural funding ends. Every SHARP project is, therefore, expected to apply for extramural funding with the goal of full extramural programmatic support after 3 years.
SHARP Performance Metrics
Measuring the accomplishments of SHARP is clearly important. As the program is intended to jumpstart collaborative inpatient clinical research, the number of such projects is important to track. An additional goal is to support work that leads to extramural funding. As the program started from scratch, it is unrealistic to have completed peer‐reviewed manuscripts or successful extramural grants as the sole metrics by which the program is judged, especially early in its initiation. In a yearly report to the department chair, we will report on primary and secondary outcomes (see Table 1).
| Primary outcomes |
|---|
|
| 1. Number of ongoing research projects involving SHARP support and a brief description of the aims and status of each |
| 2. Number of extramural grants submitted in which SHARP is mentioned or involved |
| 3. Extramural grants received (total and direct dollars) |
| 4. Peer‐reviewed publications authored by SHARP investigators |
| Secondary outcomes |
| 1. Abstracts accepted for presentation at national or international scientific meetings |
| 2. Non‐peer‐reviewed publications related to SHARP |
| 3. Invited presentations by SHARP investigators |
| 4. People who have visited the University of Michigan in conjunction with SHARP work (eg, visiting professors) |
Initial SHARP Projects
SHARP has a formal process for evaluating potential projects. A steering committee ultimately decides how best to use SHARP‐related resources. Key components in this decision are related to the proposal's innovation, feasibility, and importance as well as the extent of specialisthospitalist collaboration. The 2 projects described next are our initial areas of focus and exemplify these concepts. One project partners hospitalists with infectious disease specialists, whereas the second pairs hospitalists with geriatricians and clinical pharmacists.
Reducing False Positive Blood Cultures
The blood culture is an important tool for the diagnosis and management of bloodstream infections. As a result, physicians have a low threshold for obtaining blood cultures. Unfortunately, up to half of all positive blood cultures are positive because of contamination. These false positive cultures lead to additional diagnostic testing, unnecessary antibiotics, and increased healthcare costs.17 A variety of antiseptic agents and techniques are used to prevent falsely positive cultures. However, a recent evidence‐based systematic review performed by University of Michigan investigators found no clear evidence to suggest which antiseptic agent should be routinely used. They concluded that a randomized controlled trial was urgently needed.18
SHARP and its infrastructure have begun a cluster‐randomized crossover trial at the university hospital. The trial compares the effects of a variety of skin antiseptic agents on peripheral blood culture contamination rates. The study population includes hospitalized patients undergoing venipuncture for peripheral blood cultures on 3 general medicine and surgery floors. The trial will include over 12,000 blood culture sets and will have 85% power to detect a 0.5% difference in effectiveness between antiseptic agents. Key outcomes will be rates of positive blood cultures (true positive versus false positive), quantity of additional diagnostic testing generated by positive cultures, resource use (including antibiotics), and associated costs. Clinical outcomes such as length of stay and inpatient mortality will also be measured as secondary outcomes.
Pharmacist‐Facilitated Hospital Discharge
Hospital discharge is a complex process in which patients must be transferred from the care of an inpatient team to that of an outpatient provider. During most hospitalizations, a patient will have new medications added, a chronic medication stopped, or a change in medication dosage. Studies have revealed that the most common adverse events that have an impact on patients after discharge are related to medications.1921 In our experience at the University of Michigan, patients frequently have medication‐related adverse events after discharge because they do not understand what medications they should be taking, what they are used for, how to manage side effects, or whom to call with problems. In addition, predictable medication‐related issues (such as the ability to pay for a medicine or expected serum electrolyte changes with newly added medications) are not universally anticipated. The frail elderly are especially vulnerable to medication‐related adverse events.
Building on the work of others in the field, we proposed studying the impact of an inpatient clinical pharmacist to address medication misadventures related to hospital discharge in our elderly population.22 The study uses an interrupted time series design (the pharmacist will alternate months at a nonresident hospitalist service and a resident general medicine service) to measure the impact of the clinical pharmacist. The pharmacist will focus on patients over the age of 65 meeting criteria that identify them to be at high risk for an adverse medication event after discharge. These factors include any new medication started in the hospital, medication noncompliance or an adverse medication event that led to the admission, or use of a high‐risk medication (eg, anticoagulants, narcotics, diuretics, diabetic agents, and immunosuppressives). The pharmacist and inpatient physicians will identify high‐risk patients who will receive predischarge medication counseling. This process will identify problem medications and needed follow‐up (eg, laboratory testing) and assess compliance issues. After discharge, patients will be contacted by the pharmacist both within 72 hours and at 30 days. Standardized questions will be asked of patients to troubleshoot medication issues, assess them for problems with medications or follow‐up, and identify patients who may need more urgent access to a healthcare provider to address medication‐related problems.
Key outcomes will include the pharmacist's actions at discharge (eg, dose changes made, medication class switches, and side‐effect monitoring implemented). In addition, we will track types of medication issues identified after discharge and interventions made. Important clinical outcomes will include return to the emergency department after discharge, 30‐day readmission rates, and healthcare‐related costs.
DISCUSSION AND NEXT STEPS
SHARP is a novel clinical research program partnering hospitalists with specialists. Its current focus targets single‐institution studies that generate pilot data leading to larger projects. The ultimate goal is to develop the ability to do larger multicenter investigator‐initiated projects. The SHARP program will also have the ability to perform observational studies to identify predictors and risk factors and the ability to carry out implementation studies that show how best to translate results from published articles to direct patient care.
A specialisthospitalist collaboration overcomes barriers that we feel may impede hospital medicine research at an academic medical center. For a similar program to succeed at other institutions, key components from our program will have to be replicated. First, senior, fellowship‐trained researchers are required to mentor junior investigators (who may or may not have additional fellowship training), help guide project selection, oversee grant and manuscript submissions, and troubleshoot problems that arise in the course of any clinical research project. In our institution, this comes from within our hospitalist program and from our specialist collaborators. In institutions lacking hospitalists with research experience, this guidance could come from within a division of general medicine, internal medicine specialty divisions, internal medicine department leadership, or even noninternal medicine departments (eg, emergency medicine, neurology, and surgery) that have traditionally been involved in clinical research programs.
A second key component that must be considered is funding. An initial investment is necessary to fund key personnel dedicated to getting projects started on the right track, collecting pilot data, and ensuring project completion and dissemination of the results. The positive margin generated by our hospitalist program facilitated the initial investment. In the absence of a positive margin, resources could come directly from the hospital, the medical school, the department of internal medicine, or perhaps a foundation. The case would need to be made that an initial short‐term investment would enhance the academic standing of the institution, enhance the careers of young investigators, and over time lead to a self‐sustaining program through investigator‐initiated grants and extramural funding. In addition to experienced leadership and funding, we created an oversight committee, but we feel that this is not a critical component. A potential concern with a program that partners with specialists might be that research topics become too disease‐specific or specialty‐oriented. We specifically created the oversight committee to protect against this possibility, and other institutions might need similar safeguards.
Our next step includes leveraging existing hospitalist collaboratives that reach beyond academic medical centers to expand further the reach of SHARP. Ultimately, any new therapy, clinical tool, diagnostic paradigm, or implementation strategy that is developed or evaluated bythe SHARP program would need to be tested in a real‐world setting to assess external validity. With support from the Blue Cross Blue Shield of Michigan Foundation, we have created a multihospital patient safety consortium, the Hospitalists as Emerging Leaders in Patient Safety Consortium, which includes academic, government, urban, rural, teaching, and nonteaching hospitals.23 Although the initial focus is patient safety, our goal for the consortium is to develop it into a multihospital clinical research program that could take pilot projects developed by SHARP and test them in real‐world settings. We believe that full‐scale multihospital studies based on SHARP pilot data will be very attractive to external funding agencies and will help SHARP become financially self‐sufficient after the initial 3‐year start‐up.
Hospital medicine research is desperately needed.24, 25 Unfortunately, the clinical research capabilities of most hospital medicine programs are quite underdeveloped. We believe that partnering hospitalists with specialists can facilitate collaborative research to identify the best way to care for inpatients. If successful, we believe that variations of this model can be replicated at other institutions and will be a critical factor in jumpstarting hospital medicine clinical research.
Acknowledgements
The authors thank Dr. Marc E. Lippman, Dr. Robert F. Todd, Dr. Larry McMahon, Dr. Timothy J. Laing, and Mr. Lindsay J. Graham, whose support made this program possible.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- ,,,,,.What effect does increasing inpatient time have on outpatient‐oriented internist satisfaction?J Gen Intern Med.2003;18:725–729.
- ,,,,.Characteristics of general internists who practice only outpatient medicine: results from the physician worklife study.Semin Med Pract.2002;5:5–11.
- ,.Hospitalists in teaching hospitals: opportunities but not without danger.J Gen Intern Med.2004;19:392–393.
- ,.Hospitalists: the new model of inpatient medical care in the United States.Eur J Intern Med.2003;14:65–70.
- ,,,,.The potential size of the hospitalist workforce in the United States.Am J Med.1999;106:441–445.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,,, et al.Translating infection prevention evidence into practice using quantitative and qualitative research.Am J Infect Control.2006;34:507–512.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,,,.Making health care safer: a critical analysis of patient safety practices.Evid Rep Technol Assess (Summ).2001;(43):i–x,1–668.
- ,,,.Safe but sound: patient safety meets evidence‐based medicine.JAMA.2002;288:508–513.
- ,,,.Clinical pharmacists and inpatient medical care: a systematic review.Arch Intern Med.2006;166:955–964.
- ,,, et al.Are antiseptic‐coated central venous catheters effective in a real‐world setting?Am J Infect Control.2006;34:388–393.
- ,,,,.Effectiveness of ceftriaxone plus doxycycline in the treatment of patients hospitalized with community‐acquired pneumonia.J Hosp Med.2006;1:7–12.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,.What effect does physician “profiling” have on inpatient physician satisfaction and hospital length of stay?BMC Health Serv Res.2006;6:45.
- ,,.Contaminant blood cultures and resource utilization. The true consequences of false‐positive results.JAMA.1991;265:365–369.
- ,,,,,.Review of clinical trials of skin antiseptic agents used to reduce blood culture contamination.Infect Control Hosp Epidemiol.2007;28:892–895.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20:317–323.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170:345–349.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- ,,.Hospitalists as emerging leaders in patient safety: targeting a few to affect many.JPatient Saf.2005;1:78–82.
- .The future of hospital medicine: evolution or revolution?Am J Med.2004;117:446–450.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:72 e1–7.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- ,,,,,.What effect does increasing inpatient time have on outpatient‐oriented internist satisfaction?J Gen Intern Med.2003;18:725–729.
- ,,,,.Characteristics of general internists who practice only outpatient medicine: results from the physician worklife study.Semin Med Pract.2002;5:5–11.
- ,.Hospitalists in teaching hospitals: opportunities but not without danger.J Gen Intern Med.2004;19:392–393.
- ,.Hospitalists: the new model of inpatient medical care in the United States.Eur J Intern Med.2003;14:65–70.
- ,,,,.The potential size of the hospitalist workforce in the United States.Am J Med.1999;106:441–445.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,,, et al.Translating infection prevention evidence into practice using quantitative and qualitative research.Am J Infect Control.2006;34:507–512.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,,,.Making health care safer: a critical analysis of patient safety practices.Evid Rep Technol Assess (Summ).2001;(43):i–x,1–668.
- ,,,.Safe but sound: patient safety meets evidence‐based medicine.JAMA.2002;288:508–513.
- ,,,.Clinical pharmacists and inpatient medical care: a systematic review.Arch Intern Med.2006;166:955–964.
- ,,, et al.Are antiseptic‐coated central venous catheters effective in a real‐world setting?Am J Infect Control.2006;34:388–393.
- ,,,,.Effectiveness of ceftriaxone plus doxycycline in the treatment of patients hospitalized with community‐acquired pneumonia.J Hosp Med.2006;1:7–12.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,.What effect does physician “profiling” have on inpatient physician satisfaction and hospital length of stay?BMC Health Serv Res.2006;6:45.
- ,,.Contaminant blood cultures and resource utilization. The true consequences of false‐positive results.JAMA.1991;265:365–369.
- ,,,,,.Review of clinical trials of skin antiseptic agents used to reduce blood culture contamination.Infect Control Hosp Epidemiol.2007;28:892–895.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20:317–323.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170:345–349.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- ,,.Hospitalists as emerging leaders in patient safety: targeting a few to affect many.JPatient Saf.2005;1:78–82.
- .The future of hospital medicine: evolution or revolution?Am J Med.2004;117:446–450.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:72 e1–7.
Copyright © 2008 Society of Hospital Medicine